As filed with the Securities and Exchange Commission on June 13, 2012April 12, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,Washington, D.C. 20549
For the Quarterly Period Ended March 31, 2012FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ORGANOVO HOLDINGS, INC.Organovo Holdings, Inc.
(Exact name of registrant as specified in its charter)
| | | | |
Delaware
|
| 2836 | | 27-1488943
|
Delaware | 2836 | 27-1488943 |
(State or other jurisdiction of
incorporation or organization)
| | (Primary Standard IndustrialClassification Classification Code Number)
| | (I.R.S. Employer
Identification Number)
|
5871 Oberlin Drive, 11555 Sorrento Valley Road, Suite 150,100
San Diego California, CA 92121
Phone: (858) 550-9994(858) 224-1000
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Keith Murphy
Chairman, Chief Executive Officer and PresidentChairman
5871 Oberlin Drive, Organovo Holdings, Inc.
11555 Sorrento Valley Road, Suite 150,100
San Diego California , CA92121
Phone: (858) 550-9994(858) 224-1000
(Name, address including zip code, and telephone number, including area code, of agent for service)
Copies to:
Jeff C. Thacker,With copies to:
Jeffrey T. Hartlin, Esq.
DLA PiperSamantha H. Eldredge, Esq.
Paul Hastings LLP (US)
4365 Executive Drive, Suite 11001117 S. California Avenue
San Diego, California 92121Palo Alto, CA 94304
Tel: (858) 677-1400(650) 320-1800
Fax: (858) 677-1401
Approximate date of commencement of proposed sale to public:the public: As soon as practicable after the effectivenesseffective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. x☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”filer,” “smaller reporting company” and “smaller reporting“emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
|
|
|
|
|
|
|
Large accelerated filer |
| ¨☐ |
| Accelerated filer |
| ¨☐ |
|
|
|
|
Non-accelerated filer |
| ¨☒ |
| Smaller reporting company |
| x☒ |
|
|
|
|
|
|
|
| Emerging growth company |
| ☐ |
CalculationIf an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of Securities Act. ☐
The Registrant hereby amends this Registration FeeStatement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
| | | | | | | | |
|
Title of Each Class of Securities to be Registered | | Amount to be Registered (1) | | Proposed Maximum Offering Price Per Unit | | Proposed Maximum Aggregate Offering Price | | Amount of Registration Fee |
Common Stock, $0.001 par value per share (2) | | 15,247,987 | | $5.24(3) | | $79,899,452(3) | | $9,157(3) |
Common Stock, $0.001 par value per share (4) | | 15,247,987 | | $1.00(6) | | $15,247,987(6) | | $1,748 (6) |
Common Stock, $0.001 par value per share (5) | | 1,500,000 | | $1.00(6) | | $1,500,000(6) | | $172 (6) |
Total | | 31,995,974 | | N/A | | $96,647,439 | | $11,077 |
|
|
(1) | In the event of a stock split, stock dividend or similar transaction involving our common stock, the number of shares registered shall automatically be increased to cover the additional shares of common stock issuable pursuant to Rule 416 under the Securities Act of 1933, as amended. |
(2) | Represents shares of common stock issued to the selling security holders in the registrant’s private placement (the “Offering”) of units consisting of (i) one share of the registrant’s common stock and (ii) one warrant to purchase one share of the registrant’s common stock at an exercise price of $1.00 per share (the “Units”). Closings of the Offering occurred on each of February 8, 2012 (the “Initial Closing”), February 29, 2012 and March 16, 2012. Also represents shares of common stock issued to certain of the selling security holders on the date of the Initial Closing of the Offering in connection with the conversion of the registrant’s $1,500,000 in principal amount of 6% convertible promissory notes due March 31, 2012 (the “Bridge Notes”) into 1,525,387 Units. |
(3) | Fee calculated in accordance with Rule 457(c) of the Securities Act based on the average of the high and low price for our common stock on the OTCQB as of June 11, 2012. |
(4) | Represents shares of common stock issuable upon the exercise of warrants issued to the selling security holders in the Offering of Units and shares of common stock issuable upon the exercise of warrants issued to certain of the selling security holders on the date of the Initial Closing of the Offering in connection with the conversion of the Bridge Notes into 1,525,387 Units. |
(5) | Represents shares of common stock issuable upon the exercise of warrants issued to certain selling security holders in connection with the original issuance of the registrant’s Bridge Notes that were converted into 1,500,000 new warrants on the date of the Initial Closing, each exercisable at a price of $1.00 per share of the registrant’s common stock. |
(6) | Fee calculated in accordance with Rule 457(g), based upon the highest exercise price of the warrants held by the selling security holders at the time of registration. |
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT, OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(A), MAY DETERMINE.
The information in this prospectus is not complete and may be changed. The selling security holdersThese securities may not sell these securitiesbe sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offeroffers to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JUNE 13, 2012APRIL 12, 2024
PRELIMINARY PROSPECTUS
ORGANOVO HOLDINGS, INC.
15,247,987 shares
Up to [_______] Shares of Common Stock
16,747,987 sharesUp to [_______] Pre-Funded Warrants to Purchase [_______] Shares of Common Stock issuable upon
Up to [________] Shares of Common Stock Underlying the exercise ofPre-Funded Warrants
This prospectus relates to the resale by certain selling security holders of Organovo Holdings, Inc. ofWe are offering up to 31,995,974 shares of our common stock in connection with the resale of:
up to 15,247,987 shares of our common stock which were issued in our private placement (the “Offering”) of units consisting of (i) one share of our common stock and (ii) one warrant to purchase one share of our common stock at an exercise price of $1.00 per share (the “Units”), with closings of the Offering occurring on each of February 8, 2012 (the “Initial Closing”), February 29, 2012 and March 16, 2012 and[________] shares of common stock, issued to certain of the selling security holdersbased on the date of the Initial Closing of the Offering in connection with the conversion of our $1,500,000 in principal amount of 6% convertible promissory notes due March 31, 2012 (the “Bridge Notes”) into 1,525,387 Units;
up to 15,247,987 shares of our common stock issuable upon the exercise of warrants issued to the selling security holders in our Offering of Units (excluding warrants issued to our placement agents in the Offering) and shares of common stock issuable upon the exercise of warrants issued to certain of the selling security holders on the date of the Initial Closing of the Offering in connection with the conversion of the Bridge Notes into 1,525,387 Units; and
up to 1,500,000 shares of our common stock issuable upon the exercise of warrants issued to certain selling security holders in connection with the original issuance of our Bridge Notes that where converted into 1,500,000 new warrants on the date of the Initial Closing, each exercisable at aan assumed public offering price of $1.00$[___] per share, of our common stock.
The selling security holders may offer to sell the shares of common stock being offered in this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices, or at negotiated prices. We do not know when or in what amount the selling security holders may offer the securities for sale. The selling security holders may sell any, all or none of the securities offered by this prospectus.
We will not receive proceeds from the sale of shares by the selling security holders. Any proceeds received by us from the exercise of warrants by the selling security holders will be used for general corporate purposes. The selling security holders and any brokers executing sell orders on behalf of the selling security holders may be deemed to be “underwriters” within the meaning of the Securities Act of 1933, as amended (the “Securities Act”). Commissions received by a broker executing sell orders may be deemed to be underwriting commissions under the Securities Act.
Our common stock is traded on the OTCQB under the symbol “ONVO.” On June 11, 2012, the closinglast reported sale price of our common stock on the OTCQBNasdaq Capital Market on [________], 2024.
We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants, in lieu of shares of common stock that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. The public offering price of each pre-funded warrant will be equal to the price at which one share of common stock is sold to the public in this offering, minus $0.001, and the exercise price of each pre-funded warrant will be $0.001 per share. The pre-funded warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis.
This offering will terminate on [________], 2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one closing for all the securities purchased in this offering.
Our common shares are listed on the Nasdaq Capital Market under the symbol “ONVO.” On [________], 2024, the last reported sale price of our common stock was $5.48$[___]. The public offering price per share.share of common stock and per pre-funded warrant will be determined between us and investors based on market conditions at the time of pricing, and may be at a discount to the then current market price of our common stock. The recent market price used throughout this prospectus may not be indicative of the actual offering price. The actual public offering price may be based upon a number of factors, including our history and our prospects, the industry in which we operate, our past and present operating results, the previous experience of our executive officers and the general condition of the securities markets at the time of this offering. There is no established public trading market for the pre-funded warrants and we do not expect a market to develop. Without an active trading market, the liquidity of the pre-funded will be limited. In addition, we do not intend to list the pre-funded warrants on the Nasdaq Capital Market, any other national securities exchange or any other trading system.
We have engaged JonesTrading Institutional Services LLC (the “placement agent”) to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific number of securities or dollar amount. We have agreed to pay to the placement agent the placement agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. Since we will deliver the securities to be issued in this offering upon our receipt of investor funds, there is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum offering requirement as a condition of closing of this offering. Because there is no minimum offering amount required as a condition to closing this offering, we may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to pursue our business goals described in this prospectus. In addition, because there is no escrow account and no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill all of our contemplated objectives due to a lack of interest in this offering. Further, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. See the section entitled “Risk Factors” for more information. We will bear all costs associated with the offering. See “Plan of Distribution” on page 75 of this prospectus for more information regarding these arrangements.
Investing in our securities involves significant risks. Seerisks that are described in the “Risk Factors” section beginning on page 6.10 of this prospectus.
We may amend
| | | | | | | | | | | | |
|
| Per Share |
|
| Per Pre-Funded Warrant |
|
| Total |
|
Public offering price |
| $ |
|
|
| $ |
|
|
| $ |
|
|
Placement agent fees |
| $ |
|
|
| $ |
|
|
| $ |
|
|
Proceeds, before expenses, to us |
| $ |
|
|
| $ |
|
|
| $ |
|
|
The placement agent expects to deliver the securities on or supplement this prospectus from timeabout [_______], 2024, subject to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements carefully before you make your investment decision.satisfaction of customary closing conditions.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.

The date of thethis prospectus is [_______], 2012.
2024
TABLE OF CONTENTS
Organovo Holdings, Inc.
Table of Contents
ORGANOVO HOLDINGS, INC. HAS NOT REGISTERED THE SHARES OF COMMON STOCK THAT MAY BE SOLD BY THE SELLING SECURITY HOLDERS UNDER THE SECURITIES LAWS OF ANY STATE. SELLING SECURITY HOLDERS, AND ANY BROKERS OR DEALERS, EFFECTING TRANSACTIONS IN THE SHARES SHOULD CONFIRM THAT THE SHARES HAVE BEEN REGISTERED UNDER THE SECURITIES LAWS OF THE STATE OR STATES IN WHICH SALES OF THE SHARES OCCUR AS OF THE TIME OF SUCH SALES, OR THAT THERE IS AN AVAILABLE EXEMPTION FROM THE REGISTRATION REQUIREMENTS OF THE SECURITIES LAWS OF SUCH STATES.
THIS PROSPECTUS IS NOT AN OFFER TO SELL ANY SECURITIES OTHER THAN THE SHARES OF COMMON STOCK FOR SALE BY THE SELLING SECURITY HOLDERS. THIS PROSPECTUS IS NOT AN OFFER TO SELL SECURITIES IN ANY CIRCUMSTANCES IN WHICH SUCH AN OFFER IS UNLAWFUL.
About this Prospectus
You should rely only on the information contained in this prospectus. Neither we nor the selling security holdersWe have not authorized anyone to provide you with information other than the information that is different from that containedwe have provided or incorporated by reference in this prospectus. Weprospectus and the selling security holders take no responsibility for,your reliance on any unauthorized information or representation is at your own risk. This prospectus may be used only in jurisdictions where offers and can provide no assurance as to the reliabilitysales of any other information that others may give you. We. If anyone provides you with different information, you should not rely on it. Neither we nor the selling security holders are making an offer to sell these securities in any jurisdiction where the offer or sale is notare permitted. You should assume that the information containedappearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Neitherprospectus, regardless of the time of delivery of this prospectus, noror any sale madeof our common stock. Our business, financial condition and results of operations may have changed since those dates.
The information appearing in this prospectus and any free writing prospectus that we have authorized for use in connection with this offering is accurate only as of its respective date, regardless of the time of delivery of the respective document or of any sale of securities covered by this prospectus. You should not assume that the information contained in this prospectus, shall, underor in any circumstances, createfree writing prospectus that we have authorized for use in connection with this offering, is accurate as of any implicationdate other than the respective dates thereof.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
To the extent there has been no changeis a conflict between the information contained in our affairs sincethis prospectus, on the one hand, and the information contained in any document incorporated by reference filed with the U.S. Securities and Exchange Commission (the “SEC”) before the date of this prospectus, or thaton the other hand, you should rely on the information containedin this prospectus. If any statement in a document incorporated by reference to this prospectus is correct as of any time after its date.
In this prospectus, “Organovo,” “the Company,” “we,” “us,” and “our” refer to Organovo Holdings, Inc.,inconsistent with a Delaware corporation, unlessstatement in another document incorporated by reference having a later date, the context otherwise requires.
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements as that term is definedstatement in the Private Securities Litigation Reform Act of 1995. These statements relate to anticipated future events, future results of operationsdocument having the later date modifies or future financial performance. These forward-looking statements include, but are not limited to, statements relating to our ability to raise sufficient capital to finance our planned operations, market acceptance of our technology and product offerings, our ability to attract and retain key personnel, our ability to protect our intellectual property, and estimates of our cash expenditures forsupersedes the next 12 to 36 months. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “intends,” “expects,” “plans,” “goals,” “projects,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,”earlier statement.
Neither we nor the placement agent have done anything that would permit this offering or “continue”possession or the negative of these terms or other comparable terminology.
These forward-looking statements are only predictions, are uncertain and involve substantial known and unknown risks, uncertainties and other factors which may cause our (or our industry’s) actual results, levels of activity or performance to be materially different from any future results, levels of activity or performance expressed or implied by these forward-looking statements. The “Risk Factors” sectiondistribution of this prospectus sets forth detailed risks, uncertainties and cautionary statements regarding our business and these forward-looking statements.
We cannot guarantee future results, levels of activity or performance. You should not place undue reliance on these forward-looking statements, which speak only as of the datein any jurisdiction where action for that they were made. These cautionary statements should be considered with any written or oral forward-looking statements that we may issuepurpose is required, other than in the future. Except as required by applicable law, including the securities lawsUnited States. Persons who come into possession of this prospectus and any free writing prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus and any free writing prospectus applicable to that jurisdiction.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not intendguarantee the accuracy or completeness of such information. Industry publications and third-party research, surveys and studies often indicate that their information has been obtained from sources believed to update anybe reliable, although they do not guarantee the accuracy or completeness of the forward-looking statements to conform these statements to reflect actual results, later events or circumstances or to reflect the occurrence of unanticipated events.such information and such information is inherently imprecise.
PROSPECTUS SUMMARY
The following
This summary highlights selected information contained elsewhere in this prospectus. Because it is aThis summary it does not contain all of the information you should consider before making an investment decision. Before making an investment decision, youinvesting in our common stock. You should read thethis entire prospectus carefully, including the sections of this prospectus titled “Risk Factors” section, theFactors,” “Special Note Regarding Forward-Looking Statements,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus, before making an investment decision. Unless otherwise indicated, all references in this prospectus to “Organovo,” the financial statements.“company,” “we,” “our,” “us” or similar terms refer to Organovo Holdings, Inc. and its wholly owned subsidiaries, including Organovo, Inc. and Opal Merger Sub, Inc.
Overview
Overview
Organovo Holdings, Inc. (Nasdaq: ONVO), together with its wholly owned subsidiaries (collectively, “Organovo”, “we”, “us” and “our”), is a clinical stage biotechnology company that is focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in our established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC.
Our current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). We have developedplan to start a Phase 2a clinical trial in UC in the calendar year 2024. We are exploring the potential for combination therapies using FXR314 and are commercializing a platformcurrently approved mechanisms in preclinical animal studies and our IBD disease models.
Our second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. We use our proprietary technology for the generation of three-dimensional (3D)to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. We believe these attributes can enable critical complex, multicellular disease models that can be employedused to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, we are initially focusing on the intestine and have ongoing 3D tissue development efforts in drug discoveryhuman tissue models of UC and development, biological research,CD. We use these models to identify new molecular targets responsible for driving the disease and as therapeutic implants forto explore the treatmentmechanism of damaged or degenerating tissuesaction of known drugs including FXR314 and organs.related molecules. We intend to introduce a paradigm shiftinitiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Our current understanding of intestinal tissue models and IBD disease models leads us to believe that we can create models that provide greater insight into the biology of these diseases than are generally currently available. We are creating high fidelity disease models, leveraging our prior work including the work found in our peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Our advances include cell type-specific compartments, prevalent intercellular tight junctions, and the approachformation of microvascular structures.
Using these disease models, we intend to identify and validate novel therapeutic targets. After finding therapeutic drug targets, we intend to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the generationdisease, and advance these novel drug candidates towards an Investigational New Drug filing and potential future clinical trials.
We expect to broaden our work into additional therapeutic areas over time and are currently exploring specific tissues for development. In our work to identify the areas of three-dimensional human tissues, by creation of constructs ininterest, we evaluate areas that might be better served with 3D that havedisease models than currently available models as well as the potential commercial opportunity. In line with these plans, we are building upon both our external and in house scientific expertise, which will be essential to replicate native human biology. We can improve on previous technologies by moving away from monolayer 2D cell cultures and by enabling all or partour drug development effort.
Recent Developments
In February 2024, we formed our Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain of the tissuesprimary human cells we create to be constructed solely of cells.utilize in our research and development efforts. We believe Mosaic can help us optimize our expertisesupply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials we use are of the highest quality and are derived from tissues that are ethically sourced in printing small-diameter, fully cellular human blood vessels in vitro provides a strong foundation upon which other tissues can be built to replicate human biologyfull compliance with state and human disease.federal guidelines. We believe that our broad and exclusive commercial rights to patented and patent-pending 3D bioprinting technology, combined with strengths in engineering and biology, put us in an ideal positionintend for Mosaic to provide a wide array of productsus with qualified human cells for use in our clinical research drug discovery and regenerative medicine therapies.
Our foundational proprietary technology derives from research led by Dr. Gabor Forgacs, a Professor of Biophysics at the University of Missouri. We have a broad portfolio of intellectual property rights covering principles, enabling instrumentation applications and methods of cell based printing, including exclusive licensesdevelopment programs. In addition to certain patented and patent pending technologies from the University of Missouri-Columbia and Clemson University, and outright ownership of six pending patent applications (the patents and patent rights described in this paragraph are sometimes collectively referred to as the “Intellectual Property Rights”). See “Description of Business—Intellectual Property”. We believe that our portfolio of Intellectual Property Rights provides a strong and defensible market position for the commercialization of 3D bioprinting technology.
We believe we have the potential to build and maintain a sustainable business by leveraging our core technology platform across a variety of applications. As part of our business strategysupplying us with primary human cells, we intend for Mosaic to pursue collaboration agreements with drug development companiesoffer human cells for sale to life science customers, both directly and through distribution partners, which we expect to offset costs and over time become a profit center that will allow us to further develop our 3D bioprinting technology and the potential uses of the cellular structures and tissues that can be produced with our technology. We also plan to develop research products with our 3D bioprinting technology that can be offered to third parties involved in drug discovery. We currently have collaborative research agreements currently in effect with Pfizer, Inc. (“Pfizer”) and United Therapeutic Corporation (“Unither”). offsets overall R&D spending by Organovo.
Financial Update (Unaudited)
As of March 31, 2012,2024, we have also secured five federal grants in the aggregate amounthad cash and cash equivalents of approximately $955,000 including Small Business Innovation Research grants$2.9 million.
The estimated preliminary cash and developed the NovoGen MMX Bioprinter™ (our first-generation 3D bioprinter) – within two and one half years of opening our first facilities. We believe these corporate achievements provide strong validation for the commercial viability of our technology.
Ascash equivalents number presented above as of March 31, 2012, we had devoted substantially all2024 is preliminary and unaudited and may change, is based on information available to management as of the date of this prospectus, and is subject to the completion of the Company’s financial closing procedures and the audit for the year ended March 31, 2024. There can be no assurance that such financial information as of March 31, 2024 will not differ from these estimates, including as a result of year-end closing and the audit and any such changes could be material. We have not yet completed our normal audit procedures as of and for the year ended March 31, 2024.
The foregoing preliminary financial data has been prepared by, and is the responsibility of, our effortsmanagement. This data has not been audited, is subject to product development, raising capital and building infrastructure. We did not, as of that date, realize significant revenues from our planned principal operations. Accordingly, we are considered to be in the development stage.
The Technology
Our technology is centered around a core 3D bioprinting method, represented by our bioprinting instrument, the NovoGen MMX Bioprinter™. The 3D bioprinting technology enables a wide array of tissue compositions and architectures to be created, using combinations of cellular ‘bio-ink’ (building blocks comprised solely of cells), hydrogel (building blocks comprised of biocompatible gels), or hybrid ‘bio-ink’ (building blocks comprised of a mixture of cells and material such as hydrogel). A key distinguishing featurechange upon completion of our bioprinting platform isongoing audit and could change as a result of further review. Complete annual results will be included in our Annual Report on Form 10-K for the ability to generate three-dimensional constructs that have all or some of their components comprised entirely of cells. The fully-cellular featureyear ended March 31, 2024.
Corporate Information
We were incorporated in Delaware under the name Organovo Holdings, Inc. in January 2012. We are operating the business of our technology enables architecturally- and compositionally-defined 3D human tissues to be generated for in vitro use in drug discovery and development to potentially replicate the functional biology of a solid, fully cellular tissue. Furthermore, fully cellular constructs may offer specific advantages for regenerative medicine applications where bioactive cells are required and three-dimensional configuration is necessary, such as augmenting or replacing functional mass in tissues and organs that have sustained acute or chronic damage.
We plan to develop research products with our 3D bioprinting technology that can be offered to third parties involved in drug discovery. We intend to deliver the following products to the market:
Three-dimensional models of human tissue for utilization in traditional absorption, distribution, metabolism, excretion (ADME) / toxicology (TOX) / and drug metabolism and pharmacokinetics (DMPK) testing in drug development.
Specific models of human biology or pathophysiology, in the form of three-dimensional human tissues, and for use in drug discovery, development, and delivery.
Three-dimensional human tissues for use as therapeutic regenerative medicine products, such as blood vessels for bypass grafting, nerve grafts for nerve damage repair and cardiac patches for treatment of heart disease.
3D bioprinters for use in medical research.
A portfolio of consumables for use in 3D bioprinting.
As part of our business strategy we intend to pursue collaboration agreements with drug development companies that will allow us to further develop our 3D bioprinting technology and the potential uses of the cellular structures and tissues that can be produced with our technology. We currently have a collaborative research agreement with Pfizer to develop specific three-dimensional tissue models. We are engaged in the development of specific 3D human tissues to aid Pfizer in discovery of successful therapies in two areas of interest. In addition, in October 2011, we entered into a research agreement with Unither to establish and conduct a research program to discover treatments for pulmonary hypertension using our NovoGen MMX Bioprinter™ technology.
Market Opportunity
We believe that our bioprinting technology is uniquely positioned to provide three-dimensional human tissues for use in drug discovery and development as well as a broad array of tissues suitable for therapeutic use in regenerative medicine applications. While there are rapid-prototyping printers currently available that build three-dimensional structures out of polymers (often used for prototyping of plastic parts for tools or devices), these instruments are not specifically designed or intended for use with purely cellular inks in building biologic tissues and we do not believe that the firms working on these instruments have the required biology expertise to create tissues using these instruments at this time. There are multiple markets addressable by our technology platform:
| 1) | Specialized Models for Drug Discovery and Development: Our NovoGen MMX Bioprinter™ can produce highly specialized three-dimensional human tissues that can be utilized to model a specific tissue physiology or pathophysiology. Our bioprinting technology has demonstrated the ability to create human blood vessel constructs, and to create fully human tissue containing capillary structures. These capabilities are anticipated to broaden the scope and scale of 3D tissues that can be generated, and to facilitate the development of disease models in such areas as cardiovascular disease, oncology, and fibrosis. |
| 2) | Biological Research Tools: Absorption, distribution, metabolism, excretion (ADME) testing is used to determine which factors enhance or inhibit how a potential drug compound reaches the blood stream. Distribution of a compound can be affected by binding to plasma proteins; age, genetics, and other factors can influence metabolism of a compound; and the presence of certain disease states can have effects on excretion of a compound. Many companies perform ADME studies utilizing various cell-based assays or automated bioanalytical techniques. Drug metabolism and pharmacokinetics (DMPK) testing is a subset of ADME. Determining the DMPK properties of a drug helps the drug developer to understand its safety and efficacy. Toxicology (TOX) testing is a further requirement to determine the detrimental effects of a particular drug on specific tissues. We believe that the NovoGen MMX Bioprinter™ is positioned to deliver highly differentiated products for use in traditional cell-based ADME / TOX / DMPK studies. Products in this arena may replace or complement traditional cell-based assays that typically employ primary hepatocytes, intestinal cell lines, renal epithelial cells and cell lines grown in a traditional two-dimensional format. Importantly, the combination of tissue-like three-dimensionality and human cellular components is believed to provide an advantage over non-human animal systems toward predicting in vivo human outcomes. |
| 3) | Regenerative Medicine: The field of regenerative medicine is advancing via multiple strategic approaches in development and practice, including cell therapies and scaffold-based products (+/- cells). The architectural precision and flexibility of our technology may facilitate the optimization, development, and clinical use of three-dimensional tissue constructs. Importantly, our technology offers a next-generation strategy whereby three-dimensional structures can be generated without the use of scaffolding or biomaterial components. The ultimate goal is to enable fully cellular constructs to be generated in a configuration compatible with surgical modes of delivery, thereby enabling restoration of significant functional mass to a damaged tissue or organ. |
We believe that our technology can capitalize, via strategic partnerships, on additional market opportunities in the provision of enabling tools for drug discovery and development as well as the discovery and development of therapeutic implants that augment or replace damaged tissues and organs. There are multiple short- and long-term revenue opportunities for us in these areas,subsidiaries, including direct sales of 3D human tissue constructs for drug screening and development, licensing fees for commercial access to our technology, and royalties from product enablement, particularly in the area of therapeutic products for regenerative medicine.
Corporate Background
Real Estate Restoration and Rental,Organovo, Inc. (“RERR”), our predecessor company,wholly-owned subsidiary, which we acquired in February 2012. Organovo, Inc. was incorporated in 2007Delaware in the state of Nevada. On December 28, 2011, RERR entered into an Agreement and Plan of Merger pursuant to which RERR merged with its newly formed, wholly owned subsidiary, Organovo Holdings, Inc. (“Merger Sub”), a Nevada corporation (the “RERR Merger”). Upon the consummation of the RERR Merger, the separate existence of Merger Sub ceased and RERR, the surviving corporation in the RERR Merger, became known as Organovo Holdings, Inc. (“Holdings-Nevada”).
As permitted by Chapter 92A.180 of Nevada Revised Statutes, the sole purpose of the RERR Merger was to effect a change of RERR’s name. Upon the filing of Articles of Merger with the Secretary of State of Nevada on December 28, 2011 to effect the RERR Merger, RERR’s articles of incorporation were deemed amended to reflect the change in RERR’s corporate name.
On January 30, 2012, Holdings-Nevada entered into an Agreement and Plan of Merger pursuant to which Holdings-Nevada merged with and into its newly formed, wholly owned subsidiary, Organovo Holdings, Inc. (“Holdings-Delaware” or “Pubco”), a Delaware corporation (the “Reincorporation Merger”). Upon the consummation of the Reincorporation Merger, the separate existence of Holdings-Nevada ceased and Holdings-Delaware was the surviving corporation in the Reincorporation Merger. The sole purpose of the Reincorporation Merger was to change the domicile of Pubco from Nevada to Delaware.
On February 8, 2012, Organovo Acquisition Corp. (“Acquisition Corp.”), a wholly-owned subsidiary of Pubco, merged (the “Merger”) with and into Organovo, Inc., a Delaware corporation (“Organovo”). Organovo was the surviving corporation of that Merger. As a result of the Merger, Pubco acquired the business of Organovo, and will continue the existing business operations of Organovo.
Risks Associated withApril 2007. Our Business
Investing in our common stock involves substantial risk. Before participating in this offering, you should carefully consider all ofhas traded on the information in this prospectus, includingNasdaq Capital Market under the risks discussed in “Risk Factors” immediately following this summary. In particular:
We have a limited operating historysymbol “ONVO” since December 27, 2019. Prior to that time, it had traded on the Nasdaq Global Market under the symbol “ONVO” since August 8, 2016 and a history of operating losses, and expect to incur significant additional operating losses;
We need to secure additional financing to support our planned operations;
We are an early-stage company with an unproven business strategy and may never achieve commercialization of our research tools and therapeutic products or profitability;
Our success and our collaborators’ ability to sell therapeutic products will depend to a large extent upon reimbursement from health care insurance companies;
Our research tools are new and unproven and may not allow us or our collaborators to develop successful commercial products;
Our proprietary tissue creation technology, drug discovery and research tools are subject to the risks associated with new and rapidly evolving technologies.
The commercialization of therapeutic or other life science products developed using our research tools is subject to a variety of risks of failure inherent in their development or commercial viability, including the possibility that any such products will (i) fail to be found through the use of research tools; (ii) be found to be toxic or ineffective; (iii) fail to receive necessary regulatory approvals; (iv) be difficult or impossible to manufacture on a large scale; (v) be economically infeasible to market; (vi) fail to be developed prior to that it traded on the successful marketing of similar products by competitors; or (vii) be impossible to market because they infringeNYSE MKT under the proprietary rights of third parties or compete with superior products marketed by third parties;
If we are unable to enter into or maintain strategic collaborations with third parties, we may have difficulty selling our research tools and therapeutic products and we may not generate sufficient revenue to achieve or maintain profitability; and
We cannot control our collaborators’ allocation of resources or the amount of time that our collaborators devote to developing our programs or potential products, which may have a material adverse effect on our business.
We will depend on our patent portfolio, our licensed technology and other trade secrets in the conduct of our business and must ensure that we do not violate the patent or intellectual property rights of others.
Corporate Information
Our principal executive offices are located at 5871 Oberlin Drive,11555 Sorrento Valley Road, Suite 150,100, San Diego, California 92121. OurCA 92121, and our telephone number is (858) 550-9994.224-1000. Our website can be found ataddress is www.organovo.com. TheAny information contained inon, or that can be accessed through, our website is not incorporated by reference into, nor is it in any way part of this prospectus.
The Offering
Key Factsprospectus and should not be relied upon in connection with making any decision with respect to an investment in our securities. We are required to file annual, quarterly and current reports, proxy statements and other information with the SEC. You may obtain any of the Offeringdocuments filed by us with the SEC at no cost from the SEC’s website at http://www.sec.gov.
We are a “smaller reporting company” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies in this prospectus as well as our filings under the Exchange Act.
The Offering
| | |
Common stock beingSecurities offered by the selling security holders:us
| | 15,247,987 |
| |
TotalUp to [_______] shares of common stock outstanding: (1) | | 43,693,241 |
| |
Numberor pre-funded warrants to purchase shares of common stock. We are also registering [_______] shares of common stock issuable upon the exercise of warrants held by the selling security holders registered on this prospectus: | | 16,747,987pre-funded warrants |
Pre-funded warrants offered by us in this offering | Each purchaser whose purchase of shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, has the opportunity to purchase, if the purchaser so chooses, pre-funded warrants (each pre-funded warrant to purchase one share of our common stock) in lieu of shares that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding common stock (or, at the election of the purchaser, 9.99%). The purchase price of each pre-funded warrant will equal the price at which one share of common stock is being sold to the public in this offering, minus $0.001, and the exercise price of each pre-funded warrant will be $0.001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised in full. For each pre-funded warrant we sell, the number of shares we are offering will be decreased on a one-for-one basis. |
Term of the offering | This offering will terminate on [_______], 2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. |
Common stock to be outstanding immediately after this offering | [_______] shares, assuming no sale of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis |
Use of Proceeds:proceeds | | We will not receive any ofintend to use the net proceeds from the sale of our shares by the selling security holders. Any proceeds received by us from the exercise of warrants by the selling security holders will be usedthis offering for working capital and general corporate purposes.purposes, which could include capital expenditures, research and development expenditures, regulatory affairs expenditures, clinical trial expenditures, legal expenditures, including intellectual property protection and maintenance expenditures, acquisitions of new technologies and investments, business combinations and the repurchase of capital stock. See “Use of Proceeds” on page 32 for more information. |
Risk factors | |
OTCQB Symbol: | | ONVO |
| |
Risk Factors: | | Investing in our securities involves a high degree of risk and purchasersrisk. As an investor, you should be able to bear a complete loss of our securities may lose their entireyour investment. See “Risk Factors” below and the other information included elsewhere in this prospectus for a discussion of factors youYou should carefully consider before decidingthe information set forth in the “Risk Factors” section beginning on page 10. |
Nasdaq Capital Market trading symbol | Our common stock is listed on the Nasdaq Capital Market under the symbol “ONVO.” There is no established trading market for the pre-funded warrants, and we do not expect a trading market to invest our securities.develop. We do not intend to list the pre-funded warrants on any securities exchange or other trading market. Without a |
| |
| trading market, the liquidity of the pre-funded warrants will be extremely limited. |
(1) | The number of shares of our common stock outstanding is based on the number of shares of our common stock outstanding as of March 31, 2012, including the shares of common stock held by the selling security holders. This number does not include: |
The number of shares immediately outstanding following this offering is based on 9,838,755 shares of common stock outstanding as of December 31, 2023 and also gives effect to 237,712 shares of our common sold and issued through an “at the market” offering”
pursuant to a Sales Agreement that we entered into with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC
on March 16, 2018 (the "Sales Agreement") and excludes:
24,256,932•
695,459 shares of common stock issuable upon the exercise of stock options outstanding warrants at a weighted average exercise price of $1.00approximately $4.35 per share, including the warrants held by the selling security holders;share;
896,256•
123,892 shares of common stock issuable upon exercisethe vesting and settlement of outstanding options, at a weighted average exercise pricerestricted stock units;
•1,000 shares of $0.08 per share, which were issued under our 2008common stock available for issuance pursuant to the 2021 Inducement Equity Plan;
•1,643,798 shares of common stock available for issuance pursuant to the 2022 Equity Incentive Plan prior to this offering;Plan; and
6,553,986•
45,000 shares of our common stock which remain available for grant and possible subsequent issuance under our 2012 Equity Incentivepursuant to the 2023 Employee Stock Purchase Plan.
Unless otherwise indicated, all information in this prospectus assumes that no exercise of options warrants or sharesunder our equity incentive plans and no exercise of common stock were issued afterpre-funded warrants.
Summary Financial Data
The following tables set forth our summary statements of operations and comprehensive loss data for the years ended March 31, 2012,2023 and no outstanding options or warrants were exercised after March 31, 2012. In addition, unless otherwise indicated, all information in this prospectus assumes that2022 and the warrants issued in connection with this offering to the investors in the Units and our placement agents and financial advisor have not been exercised.
Summary Financial Data
The following summary audited financial information for the fiscal yearsnine months ended December 31, 20112023 and 2010, includes2022, and our summary balance sheet and statementdata as of December 31, 2023. The statements of operations and comprehensive loss data for the years ended March 31, 2023 and 2022 have been derived from our audited financial statements included elsewhere in this prospectus. The financial informationWe derived our summary statements of operations and comprehensive loss data for the nine months ended December 31, 2023 and 2022 and the summary balance sheet data as of MarchDecember 31, 2012, and for the three months ended March 31, 2012 and 2011 is derived2023 from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. The information containedprospectus, which have been prepared on a basis consistent with our audited financial statements and, in this tablethe opinion of management, contain all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of such interim financial statements. Our historical results are not necessarily indicative of the results that may be expected for any period in the future, and our interim results are not necessarily indicative of the results that may be expected for the full year or any other period. You should be read in conjunctionthe following summary financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus. The summary financial data included in this section are not intended to replace the financial statements and accompanyingare qualified in their entirety by our financial statements and the related notes included elsewhere in this prospectus.
Statement of Operations and comprehensive loss for the years ended March 31, 2023 and 2022
| | | | | | | | |
| | Year Ended | | | Year Ended | |
| | March 31, 2023 | | | March 31, 2022 | |
Revenues | | | | | | |
Royalty revenue | | $ | 370 | | | $ | 1,500 | |
Total Revenues | | | 370 | | | | 1,500 | |
Research and development expenses | | | 8,885 | | | | 3,320 | |
Selling, general, and administrative expenses | | | 9,216 | | | | 9,659 | |
Total costs and expenses | | | 18,101 | |
| | 12,979 | |
Loss from Operations | | | (17,731 | ) | | | (11,479 | ) |
Other Income (Expense) | | | | | | |
Loss on fixed asset disposals | | | (9 | ) | | | — | |
Gain on investment in equity securities | | | 29 | | | | — | |
Interest income | | | 454 | | | | 8 | |
Other income | | | — | | | | 25 | |
Total Other Income | | | 474 | | | | 33 | |
Income Tax Expense | | | (2 | ) | | | (2 | ) |
Net Loss | | $ | (17,259 | ) | | $ | (11,448 | ) |
Other comprehensive income: | | | | | | |
Unrealized gain on available-for-sale debt securities | | | 2 | | | | — | |
Comprehensive loss | | $ | (17,257 | ) | | $ | (11,448 | ) |
Net loss per common share—basic and diluted | | $ | (1.98 | ) | | $ | (1.32 | ) |
Weighted average shares used in computing net loss per common share—basic
and diluted | | | 8,713,032 | | | | 8,703,596 | |
Statement of Operations and comprehensive loss for the nine months ended December 31, 2023 and 2022 (unaudited)
| | | | | | | | |
| | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023 | | | December 31, 2022 | |
Revenues | | | | | | |
Royalty revenue | | $ | 80 | | | $ | 208 | |
Total Revenues | | | 80 | | | | 208 | |
Research and development expenses | | | 4,435 | | | | 3,436 | |
Selling, general and administrative expenses | | | 7,635 | | | | 6,724 | |
Total costs and expenses | | | 12,070 | | | | 10,160 | |
Loss from Operations | | | (11,990 | ) | | | (9,952 | ) |
Other Income (Expense) | | | | | | |
(Loss) gain on investment in equity securities | | | 12 | | | | (123 | ) |
Interest income | | | 354 | | | | 277 | |
Total Other Income | | | 366 | | | | 154 | |
Income Tax Expense | | | (2 | ) | | | (2 | ) |
Net Loss | | $ | (11,626 | ) | | $ | (9,800 | ) |
Other Comprehensive Loss: | | | | | | |
Unrealized gain (loss) on available-for-sale debt
securities | | $ | (1 | ) | | $ | 3 | |
Comprehensive Loss | | $ | (11,627 | ) | | $ | (9,797 | ) |
Net loss per common share—basic and diluted | | $ | (1.31 | ) | | $ | (1.13 | ) |
Weighted average shares used in computing
net loss per common share—basic and
diluted | | | 8,850,881 | | | | 8,712,294 | |
Balance Sheet as of March 31, 2023 and 2022
| | | | | | | | |
| | March 31, 2023 | | | March 31, 2022 | |
Assets | | | | | | |
Current Assets | | | | | | |
Cash and cash equivalents | | $ | 15,301 | | | $ | 28,675 | |
Accounts receivable | | | 152 | | | | — | |
Investment in equity securities | | | 706 | | | | — | |
Prepaid expenses and other current assets | | | 889 | | | | 858 | |
Total current assets | | | 17,048 | | | | 29,533 | |
Fixed assets, net | | | 902 | | | | 662 | |
Restricted cash | | | 143 | | | | 143 | |
Operating lease right-of-use assets | | | 1,705 | | | | 2,153 | |
Prepaid expenses and other assets, net | | | 515 | | | | 805 | |
Total assets | | $ | 20,313 | | | $ | 33,296 | |
Liabilities and Stockholders' Equity | | | | | | |
Current Liabilities | | | | | | |
Accounts payable | | $ | 331 | | | $ | 415 | |
Accrued expenses | | | 2,848 | | | | 489 | |
Operating lease liability, current portion | | | 492 | | | | 479 | |
Total current liabilities | | | 3,671 | | | | 1,383 | |
Operating lease liability, net of current portion | | | 1,313 | | | | 1,704 | |
Total liabilities | | | 4,984 | | | | 3,087 | |
Commitments and Contingencies | | | | | | |
Stockholders' Equity | | | | | | |
Common stock, $0.001 par value; 200,000,000 shares authorized,
8,716,906 and 8,710,627 shares issued and outstanding at
March 31, 2023 and 2022, respectively | | | 9 | | | | 9 | |
Additional paid-in capital | | | 340,317 | | | | 337,940 | |
Accumulated deficit | | | (324,998 | ) | | | (307,739 | ) |
Accumulated other comprehensive income | | | 2 | | | | — | |
Treasury stock, 46 shares at cost | | | (1 | ) | | | (1 | ) |
Total stockholders' equity | | | 15,329 | | | | 30,209 | |
Total Liabilities and Stockholders' Equity | | $ | 20,313 | | | $ | 33,296 | |
Balance Sheet as of the nine months ended December 31, 2023 and 2022 (unaudited)
| | | | | | | | |
| | December 31, 2023 | | | December 31, 2022 | |
| | (Unaudited) | | | (Unaudited) | |
Assets | | | | | | |
Current Assets | | | | | | |
Cash and cash equivalents | | $ | 5,295 | | | $ | 20,196 | |
Accounts receivable | | | 33 | | | | 76 | |
Investment in equity securities | | | — | | | | 554 | |
Prepaid expenses and other current assets | | | 913 | | | | 788 | |
Total current assets | | | 6,241 | | | | 21,614 | |
Fixed assets, net | | | 739 | | | | 742 | |
Restricted cash | | | 143 | | | | 143 | |
Operating lease right-of-use assets | | | 1,403 | | | | 1,803 | |
Prepaid expenses and other assets, net | | | 355 | | | | 777 | |
Total assets | | $ | 8,881 | | | $ | 25,079 | |
Liabilities and Stockholders’ Equity | | | | | | |
Current Liabilities | | | | | | |
Accounts payable | | $ | 471 | | | $ | 242 | |
Accrued expenses | | | 727 | | | | 646 | |
Operating lease liability, current portion | | | 502 | | | | 488 | |
Total current liabilities | | | 1,700 | | | | 1,376 | |
Operating lease liability, net of current portion | | | 999 | | | | 1,414 | |
Total liabilities | | | 2,699 | | | | 2,790 | |
Commitments and Contingencies (Note 7) | | | | | | |
Stockholders’ Equity | | | | | | |
Common stock, $0.001 par value; 200,000,000 shares authorized, 9,838,755
and 8,714,590 shares issued and outstanding at December 31, 2023 and
December 31, 2022, respectively | | | 10 | | | | 9 | |
Additional paid-in capital | | | 342,796 | | | | 339,817 | |
Accumulated deficit | | | (336,624 | ) | | | (317,539 | ) |
Accumulated other comprehensive income | | | 1 | | | | 3 | |
Treasury stock, 46 shares at cost | | | (1 | ) | | | (1 | ) |
Total stockholders’ equity | | | 6,182 | | | | 22,289 | |
Total Liabilities and Stockholders’ Equity | | $ | 8,881 | | | $ | 25,079 | |
Special Note Regarding Forward-Looking Statements
This prospectus and any accompanying prospectus supplement may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Exchange Act, about Organovo. These forward-looking statements are intended to be covered by the safe harbor for forward-looking statements provided by the Private Securities Litigation Reform Act of 1995. Forward-looking statements are not statements of historical fact, and can be identified by the use of forward-looking terminology such as “believes”, “expects”, “may”, “will”, “could”, “should”, “projects”, “plans”, “goal”, “targets”, “potential”, “estimates”, “pro forma”, “seeks”, “intends” or “anticipates” or the negative thereof or comparable terminology. Forward-looking statements include discussions of strategy, financial projections, guidance and estimates (including their underlying assumptions), statements regarding plans, objectives, expectations or consequences of various transactions, and statements about the future performance, operations, products and services of Organovo. We caution our stockholders and other readers not to place undue reliance on such statements.
You should read this prospectus, any accompanying prospectus supplement and the documents incorporated by reference completely and with the understanding that our actual future results may be materially different from what we currently expect. Our business and operations are and will be subject to a variety of risks, uncertainties and other factors. Consequently, actual results and experience may materially differ from those contained in any forward-looking statements. Such risks, uncertainties and other factors that could cause actual results and experience to differ from those projected include, but are not limited to, the risk factors described in the “Risk Factors” section beginning on page 10 of this prospectus.
You should assume that the information appearing in this prospectus, any accompanying prospectus supplement and any related free writing prospectus is accurate as of its date only. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. All written or oral forward-looking statements attributable to us or any person acting on our behalf made after the date of this prospectus are expressly qualified in their entirety by the risk factors and cautionary statements contained in and incorporated by reference into this prospectus. Unless legally required, we do not undertake any obligation to release publicly any revisions to such forward-looking statements to reflect events or circumstances after the date of this prospectus or to reflect the occurrence of unanticipated events.
Market, Industry and Other Data
Unless otherwise indicated, we have based the information concerning economic conditions, our industry and our market contained in this prospectus on a variety of sources, including information from third-party industry analysts and publications and our own estimates and research. This information involves a number of assumptions, estimates and limitations. The industry publications, surveys and forecasts and other public information generally indicate or suggest that their information has been obtained from sources believed to be reliable. None of the third-party industry publications used in this prospectus were prepared on our behalf. The industries in which we operate are subject to a high degree of uncertainty and risk due to a variety of factors, and are subject to change based on various factors, including those discussed in the “Risk Factors” section of this prospectus and in the other information contained in this prospectus. InThese and other factors could cause the opinioninformation concerning our industry to differ materially from those expressed in this prospectus and incorporated by reference herein.
Dividend Policy
We have never declared or paid cash dividends on our common stock. We currently intend to retain our future earnings, if any, for use in our business and therefore do not anticipate paying cash dividends in the foreseeable future. Payment of management,future dividends, if any, will be at the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, consisting of only normal recurring adjustments, necessary for a fair presentationdiscretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and financial positionanticipated cash needs and plans for those periods and as of such dates. The results for any interim period are not necessarily indicative of the results that may be expected for a full year.expansion.
| | | | | | | | | | | | | | | | |
| | | Organovo Holdings, Inc.
For the Three Months Ended
March 31,
(unaudited) | | | Organovo Holdings, Inc.
For the Year Ended
December 31, | |
Statement of Operations Data: | | 2012 | | | 2011 | | | 2011 | | | 2010 | |
Revenues | | $ | 120,000 | | | $ | 200,789 | | | $ | 968,513 | | | $ | 603,412 | |
Research and Development Expense | | | 547,287 | | | | 398,664 | | | | 1,419,718 | | | | 1,203,716 | |
General and Administrative Expense | | | 901,843 | | | | 243,494 | | | | 1,705,171 | | | | 577,914 | |
Income (loss) from Operations | | | (1,329,130 | ) | | | (491,953 | ) | | | (2,289,983 | ) | | | (1,178,218 | ) |
Change in fair value of warrants | | | (13,505,819 | ) | | | — | | | | 6,569 | | | | — | |
Net Income (loss) | | | (37,080,582 | ) | | | (546,585 | ) | | | (4,383,262 | ) | | | (1,338,694 | ) |
Income (loss) per Share | | $ | (1.17 | ) | | $ | (0.04 | ) | | $ | (0.02 | ) | | $ | (0.09 | ) |
| | | | | | | | | | | | |
| | | Organovo Holdings, Inc. For the Three Months Ended March 31, (unaudited) | | | Organovo Holdings, Inc.
For the Year Ended
December 31, | |
Balance Sheet Data: | | 2012 | | | 2011 | | | 2010 | |
Working Capital | | $ | 9,723,755 | | | $ | (945,543 | ) | | $ | (749,142 | ) |
Total Assets | | | 11,240,550 | | | | 1,408,832 | | | | 760,398 | |
Current Liabilities | | | 1,110,948 | | | | 1,975,748 | | | | 1,173,258 | |
| | | |
Total Stockholders’ Equity (Deficit) | | $ | (37,385,108 | ) | | $ | (1,833,785 | ) | | $ | (2,300,360 | ) |
RISK FACTORS
Any investmentInvestment in our common stock involves a highsubstantial degree of risk. Yourisk and should consider carefullybe regarded as speculative. As a result, the following information about these risks, together with the other information contained in this prospectus, before you decide to buypurchase of our common stock. The risksstock should be considered only by persons who can reasonably afford to lose their entire investment. Before you elect to purchase our common stock, you should carefully consider the risk and uncertainties described below are notin addition to the only ones we face.other information incorporated herein by reference. Additional risks and uncertainties not presently known to usof which we are unaware or thatwhich we currently deembelieve are immaterial maycould also impairmaterially adversely affect our business, financial condition or results of operations. If any of the following risks actuallyor uncertainties discussed in this prospectus occur, our business, would likely sufferprospects, liquidity, financial condition and results of operations could be materially and adversely affected, in which case the trading price of our common stock could decline, and you maycould lose all or part of your investment.
Risk factors marked with an asterisk (*) below include a substantive change from or an update to the money you paid to buyrisk factors included in our Annual Report on Form 10-K for the fiscal year ended March 31, 2023, filed with the SEC on July 14, 2023.
Risk Factor Summary
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below and should be carefully considered, together with other information in this prospectus and our other filings with the Securities and Exchange Commission before making investment decisions regarding our common stock.
Risks related•We will incur substantial additional operating losses over the next several years as our research and development activities increase.
•Using our platform technology to develop human tissues and disease models for drug discovery and development is new and unproven.
•As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our development activities.
•We may require substantial additional funding. Raising additional capital would cause dilution to our Businessexisting stockholders and may restrict our Industryoperations or require us to relinquish rights to our technologies or to a product candidate.
•Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes, and results of earlier studies and trials may not be predictive of future results.
•The near and long-term viability of our drug discovery and development efforts will depend on our ability to successfully establish strategic relationships.
•Current and future legislation may increase the difficulty and cost of commercializing our drug candidates and may affect the prices we may obtain if our drug candidates are approved for commercialization.
•Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern.
•Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations.
•We have
a limited operating history and a history of operating losses and expect to incur significant additional operating losses.
We were incorporated
•There is no assurance that an active market in 2007, opened our laboratoriescommon stock will continue at present levels or increase in San Diego in January, 2009 and have only a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluationthe future.
•The price of our performance. Our prospects mustcommon stock may continue to be consideredvolatile, which could lead to losses by investors and costly securities litigation.
•Patents covering our products could be found invalid or unenforceable if challenged in lightcourt or before administrative bodies in the United States or abroad.
•We may be involved in lawsuits or other proceedings to protect or enforce our patents or the patents of the uncertainties, risks, expenses,our licensors, which could be expensive, time-consuming and difficulties frequently encountered by companiesunsuccessful.
Risks related to this Offering
*We need to raise capital in their early stages ofthis offering to support our operations. If we are unable to raise capital in this offering, our financial position will be materially adversely impacted.
We have generated operatingincurred substantial losses since our inception, and we beganexpect to continue to incur additional losses for the next several years. For the three months ended December 31, 2023, we had net losses of $3.6 million. From our inception through December 31, 2023, we had an accumulated deficit of $336.6 million. We believe that current cash on hand, prior to the receipt of any proceeds from this offering, is not sufficient to fund operations including $1,338,694, $3,964,610beyond June 2024. If we were to receive net proceeds of $[___] million from this offering, we believe that the net proceeds from this offering, together with our existing cash and $1,329,130cash equivalents, will meet our capital needs into [___]. If we receive the foregoing net proceeds of $[___] million in this offering, and if we raise an additional $[___] million in net proceeds through the sale of securities or otherwise throughout 2024, we believe that we will then meet our capital needs through the end of [___]. If we were to receive net proceeds of $[___] million from this offering, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will meet our capital needs through [__]. In addition, the report of our independent registered public accounting firm on our financial statements for the year ended March 31, 2023 contains explanatory language that substantial doubt exists about our ability to continue as a going concern. If we do not have access to sufficient cash and liquidity to finance our business operations as currently contemplated, we would be compelled to reduce general and administrative expenses and delay research and development projects, including the purchase of scientific equipment and supplies, until we are able to obtain sufficient financing. We have no additional committed sources of capital and may find it difficult to raise money on terms favorable to us or at all. The failure to obtain sufficient capital to support our operations would have a material adverse effect on our business, financial condition and results of operations. If such sufficient financing is not received timely, we would then need to pursue a plan to license or sell assets, seek to be acquired by another entity, cease operations and/or seek bankruptcy protection.
*Purchasers who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase agreement.
In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including: (i) timely delivery of shares; (ii) agreement to not enter into variable rate financings for [____] from closing, subject to certain exceptions; (iii) agreement to not enter into any financings for [__] days from closing; and (iv) indemnification for breach of contract.
*This is a best efforts offering, no minimum amount of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business plans, including our near-term business plans.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum
amounts set forth herein. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term and may need to raise additional funds to complete such short-term operations. Such additional fundraises may not be available or available on terms acceptable to us.
*Our management has broad discretion as to the use of the net proceeds from this offering.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds.” Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment, and the failure by our management to apply these funds effectively could harm our business. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected results, which could cause our common stock price to decline.
*If you purchase shares of our common stock in this offering, you will experience immediate and substantial dilution and may experience additional dilution in the future.
Investors purchasing shares of our common stock in this offering will pay a price per share that substantially exceeds the as adjusted net tangible book value per share. As a result, investors purchasing our common stock in this offering will incur immediate dilution of $[____________] per share, representing the difference between the assumed public offering price of $[____________] per share, which is the last reported sale price of our common stock on the Nasdaq Capital Market on [________], 2024, and our as adjusted net tangible book value as of December 31, 20102023. For more information on the dilution you may suffer as a result of investing in this offering, see “Dilution.”
*Because there is no minimum required for the offering to close, investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to pursue the business goals outlined in this prospectus.
We have not specified a minimum offering amount nor have or will we establish an escrow account in connection with this offering. Because there is no escrow account and 2011no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill our objectives due to a lack of interest in this offering. Further, because there is no escrow account in operation and no minimum investment amount, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. Investor funds will not be returned under any circumstances whether during or after the offering.
*We may issue additional equity or convertible debt securities in the future, which may result in additional dilution to you.
In order to raise additional capital, we expect to in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock or other securities convertible into or exchangeable for our common stock in future transactions may be higher or lower than the price per share in this offering.
*Future sales of shares of our common stock in the public market, or the perception that such sales could occur, could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market, or the perception that these sales could occur, following this offering could cause the market price of our common stock to decline. A substantial majority of the outstanding shares of our common stock are, and the three months endedshares of common stock sold in this offering upon issuance will be, freely tradable without restriction or further registration under the Securities Act. We may sell large quantities of our common stock at any time pursuant to one or more separate offerings in the future, including through “at the market offerings” pursuant to a Sales Agreement that we entered into with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC on March 31, 2012, respectively,16, 2018. We cannot predict the effect that future sales of common stock or other equity-related securities would have on the market price of our common stock.
*There is no public market for the pre-funded warrants to purchase shares of our common stock being offered by us in this offering.
There is no established public trading market for the pre-funded warrants to purchase shares of our common stock that are being offered as part of this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system, including the Nasdaq Capital Market. Without an active market, the liquidity of the pre-funded warrants will be limited.
*The pre-funded warrants are speculative in nature.
The pre-funded warrants do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of March 31, 2012,common stock at a fixed price. Holders of pre-funded warrants have identical rights, except that the pre-funded warrants have an exercise price of $0.001 and do not expire until exercised in full. Moreover, following this offering, the market value of the pre-funded warrants, if any, is uncertain and there can be no assurance that the market value of the pre-funded warrants will equal or exceed their imputed offering price.
*Holders of the pre-funded warrants offered hereby will have no rights as common stockholders with respect to the shares our common stock underlying the pre-funded warrants until such holders exercise their pre-funded warrants and acquire our common stock, except as otherwise provided in the pre-funded warrants.
Until holders of the pre-funded warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our common stock underlying such warrants. Upon exercise of the pre-funded warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
Risks Related to our Business
We are a clinical stage biotechnology company focusing on clinical drug development of the farnesoid X receptor (“FXR”) agonist FXR314, which involves a substantial degree of uncertainty, and on 3D bioprinting technology to develop human tissues and disease models for drug discovery and development, which is an unproven business strategy that may never achieve profitability.
We are a clinical stage biotechnology company focusing clinical drug development of the FXR agonist FXR314. Our secondary focus isbuilding high fidelity, 3D tissues that recapitulate key aspects of human disease. Our success will depend upon our ability to advance the development of FXR314, our ability to determine the appropriate clinical focus for FXR314, our ability to identify additional drug candidates to pursue and the viability of our platform technology and any disease models we haddevelop. Our success will also depend on our ability to select an accumulated operating loss of $43,772,138.appropriate development strategy for FXR314 and any other drug candidates we may identify, including internal development or partnering or licensing arrangements with pharmaceutical companies. We expectmay not be able to partner or license our drug candidates. We may never achieve profitability, or even if we achieve profitability, we may not be able to maintain or increase our profitability.
We will incur substantial additional operating expenseslosses over the next several years as our research and development activities increase.
We will incur substantial additional operating losses over the next several years as our research and commercialdevelopment activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things, things:
•successfully developing human tissues and disease models for drug discovery and development that enable us to identify drug candidates;
•successfully outsourcing certain portions of our development efforts;
•entering into customer relationshipspartnering or licensing arrangements with strategic partners, successful completion of the preclinicalpharmaceutical companies to further develop and conduct clinical development of our partners’ product candidates; trials for any drug candidates we identify;
•obtaining any necessary regulatory approvals by our partners or us from the FDAapproval for any drug candidates we identify; and international regulatory agencies; successful manufacturing, sales, and marketing arrangements; and
•raising sufficient funds to finance our activities. activities and long-term business plan.
We might not succeed at any of these undertakings. If we are unsuccessful at someone or allmore of these undertakings, our business, prospects, and results of operations maywill be materially adversely affected.
We will needUsing our platform technology to secure additional financing to support our planned operations.
We will require additional fundsdevelop human tissues and disease models for our anticipated operationsdrug discovery and if we are not successful in securing additional financing, we may be required to delay significantly, reduce the scope of or eliminate one or more of our research or development programs, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency, including arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies, product candidates or products.
We are an early-stage company with an unproven business strategy and may never achieve commercialization of our research tools and therapeutic products or profitability.
Our strategy of using our research tools for the collaborative development of therapeutic products is unproven. Our success will depend upon our ability to enter into additional collaboration agreements on favorable terms, to determine which research tools and therapeutic products have potential value, and to select an appropriate commercialization strategy for each research tool and potential therapeutic product we or our collaborators choose to pursue. If we are not successful in implementing our strategy to commercialize our research tools and potential therapeutic products, we may never achieve, maintain or increase profitability.
Our success and our collaborators’ ability to sell therapeutic products will depend to a large extent upon reimbursement from health care insurance companies.
Our success may depend, in part, on the extent to which reimbursement for the costs of therapeutic products and related treatments will be available from third-party payers such as government health administration authorities, private health insurers, managed care programs, and other organizations. Over the past decade, the cost of health care has risen significantly, and there have been numerous proposals by legislators, regulators, and third-party health care payers to curb these costs. Some of these proposals have involved limitations on the amount of reimbursement for certain products. Similar federal or state health care legislation may be adopted in the future and any products that we or our collaborators seek to commercialize may not be considered cost-effective. Adequate third-party insurance coverage may not be available for us or our collaborative partners to establish and maintain price levels that are sufficient for realization of an appropriate return on investment in product development.
Our research tools are new and unproven and may not allow us orunproven.
Utilizing our collaborators3D bioprinting platform technology to develop successful commercial products
Our research toolshuman tissues and disease models for drug discovery and development will involve new and unproven approaches. Wetechnologies, disease models and approaches, each of which is subject to the risk associated with new
and evolving technologies. To date, we have not provenidentified or developed any drug candidates utilizing our new business model. Our future success will depend on our ability to utilize our 3D bioprinting platform to develop human tissues and disease models that our research tools will enable us or our collaborators to identify therapeutic products with commercial potential, or toand develop or commercialize such therapeutic products. Even if we or our collaborators are successful in identifying therapeutic products based on discoveries made using our research tools, we or our collaborators may not be able to discover or develop commercially viable products. To date, no one has developed or commercialized any therapeutic or other life science product based on our research tools. If our research tools do not assist in the discovery and development of such therapeutic products, our current and potential collaborators may lose confidence in us and our research tools and our business may suffer as a result.
If our collaborators, licensees and customers do not successfully develop or commercialize therapeutic or other life science products using our research tools, we may not generate revenues from those customers. In addition, wedrug candidates. We may experience unforeseen technical complications, unrecognized defects and limitations in the productions of our research tools.technology or our ability to develop disease models or identify viable drug candidates. These complications could materially delay or limit the use of those tools, substantially increase the anticipated cost of manufacturing them or prevent us from implementing research projects at high efficiency levels.
Our productscosts and services are subjecttime to the risks associated with newidentify and rapidly evolving technologies.
Our proprietary tissue creation technology,develop viable drug discovery and research tools are subject to the risks associated with new, rapidly evolving technologies. In addition, the process of developing new technologies and products is complex, and if we are unable to develop enhancements to, and new features for, our existing products or acceptable new products that keep pace with technological developments or industry standards, our products may become obsolete, less marketable and less competitive.
The commercialization of therapeutic or other life science products developed using our research tools is subject to a variety of risks.
Development of therapeutic and other life science products based on our or our collaborators’ use of our technologies will be subject to risks of failure inherent in their development or commercial viability. These risks include the possibility that any such products will:
fail to be found through the use of research tools;
be found to be ineffective;
fail to receive necessary regulatory approvals;
be difficult or impossible to manufacture on a large scale;
be economically infeasible to market;
fail to be developed prior to the successful marketing of similar products by competitors; or
be impossible to market because they infringe the proprietary rights of third parties or compete with superior products marketed by third parties.
We expect that our drug discovery collaborative partners or other clients that utilize our research tools will be required to submit their research for regulatory review in order to proceed with human testing of drug candidates. This review by the FDA and other regulatory agencies may result in timeline setbacks or complete rejection of an application to begin human studies, such as an Investigative New Drug (IND) application. Should our collaborative partners or other clients face such setbacks, wecandidates, which would be at risk of not being paid if there were agreed upon milestone and royalty payments. The risks of non-approval for our partners or other clients will include the inherent risks of unfavorable regulator opinion of a drug candidate’s safety or efficacy, as well as the risk that the data generated by our platform technology is not found to be suitable to support the safety or efficacy of the drug. In addition, our platform technology is subject to the requirements of Good Laboratory Practice (GLP) to provide suitable data for INDs and other regulatory filings; no regulatory review of data from this platform has yet been conducted and there is no guarantee that our technology will be acceptable under GLP.
If we are unable to enter into or maintain strategic collaborations with third parties, we may have difficulty selling our research tools and therapeutic products and we may not generate sufficient revenue to achieve or maintain profitability.
Since we do not currently possess the resources necessary to develop, obtain approvals for or commercialize potential therapeutic products based on our technology, we must enter into collaborative arrangements to develop and commercialize these products. If we are not able to enter into these arrangements or implement our strategy to develop and commercialize therapeutic and other life science products based upon our research tools, we may not generate sufficient revenues to achieve or maintain profitability. Additionally, we may not be able to negotiate future collaborative arrangements on acceptable terms, if at all.
We cannot control our collaborators’ allocation of resources or the amount of time that our collaborators devote to developing our programs or potential products, which may have a material adverse effect on our business.
We have collaborative research agreements with Pfizer and Unither, and will seek to enter into additional collaborations. Our agreements with our collaborators typically allow them significant discretion in electing whether to pursue product development, regulatory approval, manufacturing and marketing of the products they may develop with the help of our technology. We cannot control the amount and timing of resources our collaborators may devote to our programs or potential products. As a result, we cannot be certain that our collaborators will choose to develop and commercialize these products or that we will realize any milestone payments, royalties and other payments to which we may become entitled. In addition, if a partner is involved in a business combination, such as a merger or acquisition, or if a partner changes its business focus, its performance pursuant to its agreement with us may suffer and, as a result, we may not generate any revenues from royalty, milestone and similar provisions that may be included in our collaborative agreement with that partner.
Any termination or breach by or conflict with our collaborators or licensees could harm our business.
If we or any of our collaborators or licensees fail to renew or terminate any of our collaboration or license agreements or if either party fails to satisfy its obligations under any of our collaboration or license agreements or complete them in a timely manner, we could lose significant sources of revenue, which could result in volatility in our future revenue.
In addition, our agreements with our collaborators and licensees may have provisions that give rise to disputes regarding the rights and obligations of the parties. These and other possible disagreements could lead to termination of the agreement or delays in collaborative research, development, supply or commercialization of certain products, or could require or result in litigation or arbitration. Moreover, disagreements could arise with our collaborators over rights to our intellectual property or our rights to share in any of the future revenues of products developed by our collaborators. These kinds of disagreements could result in costly and time-consuming litigation. Any such conflicts with our collaborators could reduce our ability to obtain future collaboration agreements and could have a negative impact on our relationship with existing collaborators, adversely affecting our business and revenues. Finally, any of our collaborations or license agreements may prove to be unsuccessful.
Our collaborators could develop competing research, reducing the available pool of potential collaborators and increasing competition, which may adversely affect our business and revenues.
Our collaborators and potential collaborators could develop research tools similar to our own, reducing our pool of possible collaborative parties and increasing competition. Any of these developments could harm our product and technology development efforts, which could seriously harm our business. In addition, we may pursue opportunities in fields that could conflict with those of our collaborators. Developing products that compete with our collaborators’ or potential collaborators’ products could preclude us from entering into future collaborations with our collaborators or potential collaborators. Any of these developments could harm our product development efforts and could adversely affect our business and revenues.
If restrictions on reimbursements and health care reform limit our collaborators’ actual or potential financial returns on therapeutic products that they develop based on our platform technology, our collaborators may reduce or terminate their collaborations with us.
Our collaborators’ abilities to commercialize therapeutic and other life science products that are developed through the research tools or services that we provide may depend in part on the extent to which coverage and adequate payments for these products will be available from government payors, such as Medicare and Medicaid, private health insurers, including managed care organizations, and other third-party payors. These payors are increasingly challenging the price of medical products and services. Significant uncertainty exists as to the reimbursement status of newly approved therapeutic and other life science products, and coverage and adequate payments may not be available for these products.
In recent years, officials have made numerous proposals to change the health care system in the U.S. These proposals included measures to limit or eliminate payments for some medical procedures and treatments or subject the pricing of pharmaceuticals and other medical products to government control. Government and other third-party payors increasingly attempt to contain health care costs by limiting both coverage and the level of payments of newly approved health care products. In some cases, they may also refuse to provide any coverage of uses of approved products for disease indications other than those for which the FDA has granted marketing approval. Governments may adopt future legislative proposals and federal, state or private payors for healthcare goods and services may take action to limit their payments for goods and services. Any of these events could limit our ability to form collaborations or collaborators’condition and our ability to commercialize therapeutic products successfully.continue operations.
We and our collaborators are subject to extensive and uncertain regulatory requirements, which could adversely affect our ability to obtain regulatory approval in a timely manner, or at all, for products that we identify or develop.
Therapeutic and other life science products are subject to an extensive and uncertain regulatory approval process by the Food and Drug Administration (FDA) and comparable agencies in other countries. The regulation of new products is extensive, and the required process of laboratory testing and human studies is lengthy and expensive. The burden of these regulations will fall on our collaborating partners, or may be shared with us, to the extent that we are developing proprietary products that are the result of a collaboration effort. The burden of these regulations will fall on us to the extent we are developing proprietary products on our own.
We may not be able to obtain FDA approvals for those products in a timely manner, or at all. We may encounter significant delays or excessive costs in our efforts to secure necessary approvals or licenses. Even if we obtain FDA regulatory approvals, the FDA extensively regulates manufacturing, labeling, distributing, marketing, promotion and advertising after product approval. Moreover, several of our product development areas may involve relatively new technology and have not been the subject of extensive product testing in humans. The regulatory requirements governing these products and related clinical procedures remain uncertain and the products themselves may be subject to substantial review by foreign governmental regulatory authorities that could prevent or delay approval in those countries. Regulatory requirements ultimately imposed on our products could limit our ability to test, manufacture and, ultimately, commercialize our products and thereby could adversely affect our financial condition and results of operations.
Our business depends upon the success of our research tools as alternatives to current research tools.
Our success depends on commercial acceptance of our research tools. We believe that adoption of our research tools by our current and future collaborators will be essential for commercial acceptance of our research tools. We cannot assure you that our research tools will be adopted, or if adopted, that they will be broadly accepted by pharmaceutical, biotechnology and diagnostic companies or various academic institutions.
We believe that recommendations by health care professionals and health care payors will be essential for commercial acceptance of our collaborators’ or our products. We cannot assure you that the products we or our collaborators develop will achieve commercial acceptance among patients, physicians or third-party payors. Failure to achieve commercial acceptance would materially adversely affect our business, financial condition and results of operations.
We face intense competition which could result in reduced acceptance and demand for our research tools and products.drug discovery efforts.
The biotechnology and pharmaceutical industry is subject to intense competition and rapid and significant technological change. We haveThere are many potential competitors for the disease indications we may pursue, including major drug companies, specialized biotechnology firms, academic institutions, government agencies and private and public research institutions. Many of these competitors have significantly greater financial and technical resources, experience and expertise in the following areas than we have, including:
•research and technology development;
•development preclinical testing, designingof or access to disease models;
•identification and implementing clinical trials; development of drug candidates;
•regulatory processes and approvals; productionand
•identifying and manufacturing; and sales and marketing of approved products than we have. entering into agreements with potential collaborators.
Principal competitive factors in our industry includeinclude: the quality, scientific and breadth of an organization’s technology;technical support, management of the organization and the execution of the organization’s strategy; thedrug development and regulatory approval strategies; skill and experience of an organization’s employees, and itsincluding the ability to recruit and retain skilled, and experienced employees; an organization’s intellectual property portfolio; the range of capabilities, from targetincluding drug identification, development and validation to drug and device discovery and development to manufacturing and marketing;regulatory approval; and the availability of substantial capital resources to fund discovery, development and commercializationthese activities.
Large and established companies compete in the biotech market. In particular, these companies have greater experience and expertise than we have in securing government contracts and grants to support their research and development efforts, conducting testing and clinical trials, obtaining regulatory approvals to market products, manufacturing such products on a broad scale and marketing approved products than we have.
Smaller or early-stage companies and research institutions may also prove to be significant competitors, particularly through collaborative arrangements with large and established biotech or other companies, or the obtaining of substantial private financing. We will also face competition from these parties in recruiting and retaining qualified scientific and management personnel.
In order to effectively compete, we will havemay need to make substantial investments in our research and technology development, drug candidate identification and development, testing manufacturing and salesregulatory approval and marketing or partner with one or more established companies.licensing and business development activities. There is no assurance that we or our collaborators will be successful in commercializing and gaining significant market share for any of products developed in part through use ofdiscovering effective drug candidates using our technology.3D bioprinted tissues or disease models. Our technologies products and servicesdrug development plans also may be rendered obsolete or noncompetitive as a result of drugs, intellectual property, technologies, products and services introduced by our competitors.
We may have product liability exposure from the sale Any of our research tools and therapeutic products or the services we provide.
We may have exposure to claims for product liability. Product liability coverage is expensive and sometimes difficult to obtain. Given our operations to date, we currently do not maintain any product liability insurance coverage. At such point that we determine it is prudent to obtain this insurance, we may not be able to obtain or maintain insurance at a reasonable cost. There can be no assurance that existing insurance coverage will extend to other products in the future. Any product liability insurance coverage may not be sufficient to satisfy all liabilities resulting from product liability claims. A successful claimthese risks may prevent us from building a successful drug discovery business or entering into a strategic partnership or collaboration related to, any drug candidates we identify on favorable terms, or at all.
As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our development activities.
As we pursue drug development through 3D tissues and disease models, we will require access to a constant, steady, reliable supply of human cells to support our 3D tissue development activities. We purchase human cells from selected third-party suppliers based on quality assurance, cost effectiveness, and regulatory requirements. We need to continue to identify additional sources of qualified human cells and there can be no guarantee that we will be able to access the quantity and quality of raw materials needed at a cost-effective price. Any failure to obtain a reliable supply of sufficient human cells or a supply at cost effective prices would harm our business and our results of operations and could cause us to be unable to support our drug development efforts.
We may not be successful in establishing our Mosaic Cell Sciences division (“Mosaic”) as a profitable commercial business.
We formed Mosaic to serve as a key source of certain of the primary human cells we utilize in our research and development efforts. In addition to supplying human cells for our business requirements, we believe there is an opportunity for Mosaic to operate as a commercial business by selling human cells to other pharmaceutical, biotech and research organizations. We intend for Mosaic to
begin selling its human cell offerings to end users both directly and through distribution partners during fiscal 2025. Operating and developing Mosaic’s business is subject to a number of risks and uncertainties, including:
•failing to source a sufficient supply of high-quality human organs or cells;
•failing to achieve market acceptance for its human cell offerings;
•failing to demonstrate the quality and reliability of its human cell offerings; • failing to be both cost effective and competitive with the products offered by third parties;
•failing to obtain any necessary regulatory approvals;
•failing to be able to produce its human cell offerings on a large enough scale;
•failing to establish and maintain distribution relationships with reliable third parties;
•failing to hire and retain qualified personnel; and
•infringing the proprietary rights of third parties or failing to protect our own intellectual property.
If any of these or any other risks and uncertainties occur, our efforts to establish Mosaic as a commercial business may be unsuccessful, which would harm our business and results of operations.
Our business will be adversely impacted if we are unable to successfully attract, hire and integrate key additional employees or contractors.
Our future success depends in part on our ability to successfully attract and then retain key additional executive officers and other key employees and contractors to support our drug discovery plans. Recruiting and retaining qualified scientific and clinical personnel are critical to our success. Competition to hire qualified personnel in our industry is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. If we are unable to attract and retain high quality personnel, our ability to pursue our drug discovery business will be limited, and our business, prospects, financial condition and results of operations may be adversely affected.
*We may require substantial additional funding. Raising additional capital would cause dilution to our existing stockholders and may restrict our operations or require us to relinquish rights to our technologies or to a product candidate.
We currently do not have any committed external source of funds and do not expect to generate any meaningful revenue in the foreseeable future. If our board of directors decides that we should pursue further research and development activities than already proposed, we will require substantial additional funding to operate our proposed business, including expanding our facilities and hiring additional qualified personnel, and we would expect to finance these cash needs through a combination of equity offerings, debt financings, government or other third-party funding and licensing or collaboration arrangements.
To the extent that we raise additional capital through the sale of equity or convertible debt, the ownership interests of our stockholders will be diluted. In addition, the terms of any equity or convertible debt we agree to issue may include liquidation or other preferences that adversely affect the rights of our stockholders. Convertible debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, and declaring dividends, and may impose limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Moreover, we have the ability to sell up to $2.6 million of additional shares of our common stock to the public through an “at the market offering” pursuant to a Sales Agreement that we entered into with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC on March 16, 2018 (the “Sales Agreement”). Any shares of common stock issued in the “at the market offering” (“ATM offering”) will result in dilution to our existing stockholders.
We currently have an effective shelf registration statement on Form S-3 filed with the Securities and Exchange Commission (the “SEC”), which we may use to offer from time to time any combination of debt securities, common and preferred stock and warrants. On March 16, 2018, we entered into the Sales Agreement pursuant to which we have the ability to sell an aggregate of $50.0 million of shares of our common stock to the public through an ATM offering. As of March 31, 2024, we have issued and sold an aggregate of 4,018,818 shares of our common stock for gross proceeds of approximately $45.2 million. However, in the event that the aggregate market value of our common stock held by non-affiliates (“public float”) is less than $75.0 million, the amount we can raise through primary public offerings of securities, including sales under the Sales Agreement, in any twelve-month period using shelf registration statements is limited to an aggregate of one-third of our public float. As of April 12, 2024, our public float was less than $75.0 million, and therefore we are limited to an aggregate of one-third of our public float in the amount we could raise through primary public
offerings of securities in any twelve-month period using shelf registration statements, or $2,157,419. Although we would still maintain the ability to raise funds through other means, such as through the filing of a registration statement on Form S-1 or in private placements, the rules and regulations of the SEC or any other regulatory agencies may restrict our ability to conduct certain types of financing activities, or may affect the timing of and amounts we can raise by undertaking such activities.
Further, additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations. Raising additional funding through debt or equity financing is likely to be difficult or unavailable altogether given the early stage of our technology and any drug candidates we identify. Furthermore, the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline further and existing stockholders may not agree with our financing plans or the terms of such financings.
*Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes, and results of earlier studies and trials may not be predictive of future results.
Before obtaining adequatemarketing approval from regulatory authorities for the sale of any drug candidates we identify, any such drug candidates must undergo extensive clinical trials to demonstrate the safety and efficacy of the drug candidates in humans. Human clinical testing is expensive and can take many years to complete, and we cannot be certain that any clinical trials will be conducted as planned or completed on schedule, if at all. We may elect to complete this testing, or some portion thereof, internally or enter into a partnering or development agreement with a pharmaceutical company to complete these trials. Our inability, or the inability of any third party with whom we enter into a partnering or development agreement, to successfully complete preclinical and clinical development could result in additional costs to us and negatively impact our ability to generate revenues or receive development or milestone payments. Our future success is dependent on our ability, or the ability of any pharmaceutical company with whom we enter into a partnering or development agreement, to successfully develop, obtain regulatory approval for, and then successfully commercialize any drug candidates we identify.
Any drug candidates we identify will require additional clinical development, management of clinical, preclinical and manufacturing activities, regulatory approval in applicable jurisdictions, achieving and maintaining commercial-scale supply, building of a commercial organization, substantial investment and significant marketing efforts. We are not permitted to market or promote any of our drug candidates before we receive regulatory approval from the U.S. Food and Drug Administration (“FDA”) or comparable foreign regulatory authorities, and we may never receive such regulatory approval for any of our drug candidates.
We, or any third party with whom we enter into a partnering or development agreement, may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to earn development or milestone payments or for any drug candidates to obtain regulatory approval, including:
•delays in or failure to reach agreement on acceptable terms with prospective contract research organizations (“CROs”) and clinical sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
•failure to obtain sufficient enrollment in clinical trials or participants may fail to complete clinical trials;
•clinical trials of our drug candidates that may produce negative or inconclusive results, and as a result we, or any pharmaceutical company with who we enter into a partnering or development agreement, may decide, or regulators may require, additional clinical trials;
•suspension or termination of clinical research, either by us, any third party with whom we enter into a partnering or development agreement, regulators or institutional review boards, for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks;
•additional or unanticipated clinical trials required by regulators or institutional review boards to obtain approval or any drug candidates may be subject to additional post-marketing testing requirements to maintain regulatory approval;
•regulators may revise the requirements for approving any drug candidates, or such requirements may not be as anticipated;
•the cost of clinical trials for any drug candidates may be greater than anticipated;
•the supply or quality of any drug candidates or other materials necessary to conduct clinical trials of our drug candidates may be insufficient or inadequate or may be delayed; and
•regulatory authorities may suspend or withdraw their approval of a product liability insuranceor impose restrictions on its distribution;
If we, or any third party with whom we enter into a partnering or development agreement, experience delays in the completion of, or termination of, any clinical trial of any drug candidates that we develop, or are unable to achieve clinical endpoints due to unforeseen events, the commercial prospects of our drug candidates will be harmed, and our ability to develop milestones, development fees or product revenues from any of these drug candidates will be delayed.
We will rely upon third-party contractors and service providers for the execution of critical aspects of any future development programs. Failure of these collaborators to provide services of a suitable quality and within acceptable timeframes may cause the delay or failure of any future development programs.
We plan to outsource certain functions, tests and services to CROs, medical institutions and collaborators as well as outsource manufacturing to collaborators and/or contract manufacturers, and we will rely on third parties for quality assurance, clinical monitoring, clinical data management and regulatory expertise. We may elect, in the future, to engage a CRO to run all aspects of a clinical trial on commercially desirable items, if at all. Even ifour behalf. There is no assurance that such individuals or organizations will be able to provide the functions, tests, biologic supply or services as agreed upon or in a claim is not successful, defending such a claim would be time-consumingquality fashion and expensive, may damage our reputationwe could suffer significant delays in the marketplace,development of our drug candidates or development programs.
In some cases, there may be only one or few providers of such services, including clinical data management or manufacturing services. In addition, the cost of such services could be significantly increased over time. We may rely on third parties and would likely divert management’s attention.collaborators to enroll qualified patients and conduct, supervise and monitor our clinical trials. Our reliance on these third parties and collaborators for clinical development activities reduces our control over these activities. Our reliance on these parties, however, does not relieve us of our regulatory responsibilities, including ensuring that our clinical trials are conducted in accordance with Good Clinical Practice (“GCP”) regulations and the investigational plan and protocols contained in the regulatory agency applications. In addition, these third parties may not complete activities on schedule or may not manufacture under Current Good Manufacturing Practice (“cGMP”) conditions. Preclinical or clinical studies may not be performed or completed in accordance with Good Laboratory Practices (“GLP”) regulatory requirements or our trial design. If these third parties or collaborators do not successfully carry out their contractual duties or meet expected deadlines, obtaining regulatory approval for manufacturing and commercialization of our drug candidates may be delayed or prevented. We may rely substantially on third-party data managers for our clinical trial data. There is no assurance that these third parties will not make errors in the design, management or retention of our data or data systems. There is no assurance these third parties will pass FDA or regulatory audits, which could delay or prohibit regulatory approval.
In addition, we will exercise limited control over our third-party partners and vendors, which makes us vulnerable to any errors, interruptions or delays in their operations. If these third parties experience any service disruptions, financial distress or other business disruption, or difficulties meeting our requirements or standards, it could make it difficult for us to operate some aspects of our business.
The near and long-term viability of our productsdrug discovery and servicesdevelopment efforts will depend on our ability to successfully establish strategic relationships.
The near and long-term viability of our productsdrug discovery and services willdevelopment efforts depend in part on our ability to successfully establish new strategic collaborationspartnering, collaboration and licensing arrangements with biotechnology companies, pharmaceutical companies, universities, hospitals, insurance companies and or government agencies. Establishing strategic collaborationsrelationships is difficult and time-consuming. Potential partners and collaborators may reject collaborationsnot enter into relationships with us based upon their assessment of our technology or drug candidates or our financial, regulatory or intellectual property position. If we fail to establish a sufficient number of collaborationsstrategic relationships on acceptable terms, we may not be able to commercializedevelop and obtain regulatory approval for our productsdrug candidates or generate sufficient revenue to fund further research and development efforts.
Even if we establish new collaborations,strategic relationships, these relationships may never result in the successful development or commercializationregulatory approval for any drug candidates we identify for a number of any product or service candidates for several reasons both within and outside of our control.
Although our current focus is on providing drug discovery services and research tools in the research setting, we may develop tissue therapeutic products and seek approval to sell them as medical care. Before we could begin commercial manufacturing of anyInvestors’ expectations of our product candidates, we or performance relating to environmental, social and governance factors may impose additional costs and expose us to new risks.
There is an increasing focus from certain investors, employees, regulators and other stakeholders concerning corporate responsibility, specifically related to environmental, social and governance (“ESG”) factors. Some investors and investor advocacy groups may use these factors to guide investment strategies and, in some cases, investors may choose not to invest inour manufacturers must passcompany if they believe our policies relating to corporate responsibility are inadequate. Third-party providers of corporate responsibility ratings and reports on companies have increased to meet growing investor demand for measurement of corporate responsibility performance, and a pre-approval inspection byvariety of organizations currently measure the FDAperformance of companies on such ESG topics, and comply with the FDA’s current Good Manufacturing Practices. If our manufacturers failresults of these assessments are widely publicized. Investors, particularly institutional investors, use these ratings to comply with these requirements, our product candidates would not be approved. If our collaborators fail to comply with these requirements after approval, we would be subject to possible regulatory actionbenchmark companies against their peers and may be limited in the jurisdictions in whichif we are permitted to sell products.
We will be dependent on third-party research organizations to conduct some of our future laboratory testing, animal and human studies.
We will be dependent on third-party research organizations to conduct some of our laboratory testing, animal and human studiesperceived as lagging with respect to therapeutic tissues ESG initiatives, certain investors may engage with us to improve ESG disclosures or performance
and may also make voting decisions, or take other life science productsactions, to hold us and our board of directors accountable. In addition, the criteria by which our corporate responsibility practices are assessed may change, which could result in greater expectations of us and cause us to undertake costly initiatives to satisfy such new criteria. If we elect not to or are unable to satisfy such new criteria, investors may conclude that weour policies with respect to corporate responsibility are inadequate. We may developface reputational damage in the future. Ifevent that our corporate responsibility procedures or standards do not meet the standards set by various constituencies.
We may face reputational damage in the event our corporate responsibility initiatives or objectives do not meet the standards set by our investors, stockholders, lawmakers, listing exchanges or other constituencies, or if we are unable to obtainachieve an acceptable ESG or sustainability rating from third-party rating services. A low ESG or sustainability rating by a third-party rating service could also result in the exclusion of our common stock from consideration by certain investors who may elect to invest with our competition instead. Ongoing focus on corporate responsibility matters by investors and other parties as described above may impose additional costs or expose us to new risks. Any failure or perceived failure by us in this regard could have a material adverse effect on our reputation and on our business, share price, financial condition, or results of operations, including the sustainability of our business over time.
*Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and share price.
Our business, financial condition and share price could be adversely affected by general conditions in the global economy and in the global financial markets. As widely reported, in the past several years, global credit and financial markets have experienced volatility and disruptions, and especially in 2020, 2021 and 2022 due to the impacts of the COVID-19 pandemic, and, more recently, the ongoing conflict between Ukraine and Russia and the global impact of restrictions and sanctions imposed on Russia, including, for example, severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. Moreover, the global impacts of the Israel-Hamas war are still unknown. There can be no assurances that further deterioration in credit and financial markets and confidence in economic conditions will not occur. For example, U.S. debt ceiling and budget deficit concerns have increased the possibility of additional credit-rating downgrades and economic slowdowns, or a recession in the United States. Although U.S. lawmakers passed legislation to raise the federal debt ceiling on multiple occasions, including a suspension of the federal debt ceiling in June 2023, ratings agencies have lowered or threatened to lower the long-term sovereign credit rating on the United States. The impact of this or any further downgrades to the U.S. government’s sovereign credit rating or its perceived creditworthiness could adversely affect the U.S. and global financial markets and economic conditions. Absent further quantitative easing by the Federal Reserve, these developments could cause interest rates and borrowing costs to rise, which may negatively impact our results of operations or financial condition.
Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make any necessary testing services on acceptable terms, we may not complete our product development effortsdebt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner. If we relymanner and on third parties for laboratory testing and/or animalfavorable terms could have a material adverse effect on our growth strategy, financial performance and human studies, we may lose some control over these activitiesshare price and become too dependent upon these parties. These third parties may not complete testing activities on schedule or when we so request. We may not be able to secure and maintain suitable research organizations to conduct our laboratory testing and/or animal and human studies. We are responsible for confirming that each of our clinical trials is conducted in accordance with our general plan and protocol. Moreover, the FDA and foreign regulatory agenciescould require us to comply with regulationsdelay or abandon clinical development plans. Any of the foregoing could harm our business and standards, commonly referredwe cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
*The impact of the Russian invasion of Ukraine and the Israel-Hamas War on the global economy, energy supplies and raw materials is uncertain, but may prove to as good clinical practices, for conducting, recordingnegatively impact our business and reportingoperations.
The short and long-term implications of Russia’s invasion of Ukraine and the results of clinical trialsIsrael-Hamas war are difficult to assure that data and reported results are credible and accurate andpredict at this time. We continue to monitor any adverse impact that the trial participants are adequately protected. Our relianceoutbreak of war in Ukraine and the subsequent institution of sanctions against Russia by the United States and several European and Asian countries and the Israel-Hamas war may have on the global economy in general, on our business and operations and on the businesses and operations of our suppliers and other third parties does not relieve uswith which we conduct business. For example, prolonged conflict in Ukraine or Israel has resulted and may result, respectively, in increased inflation, escalating energy prices and constrained availability, and thus increasing costs, of these responsibilitiesraw materials. We will continue to monitor this fluid situation and requirements. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties needdevelop contingency plans as necessary to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhereaddress any disruptions to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our future product candidates.
We may require access to a constant, steady, reliable supply of products.
business operations as they develop. To the extent that we develop products for sale, wethe war in Ukraine or Israel may be required to complete clinical trials before we can offer such products for sale. Commercializationadversely affect our business as discussed above, it may also have the effect of products will require access to, or development of, facilities to manufacture a sufficient supply of our product candidates. If we are unable to manufacture our products in commercial quantities, then we will need to rely on third parties. These third-party manufacturers must also receive FDA approval before they can produce clinical material or commercial products. Our products may be in competition with other products for access to these facilities and may be subject to delays in manufacture if third parties give other products greater priority. In addition, we may not be able to enter into any necessary third-party manufacturing arrangements on acceptable terms, or on a timely basis. Furthermore, we would likely have to enter into a technical transfer agreement and share our know-how with the third party manufacturer.
We may rely on third-party suppliers for some our materials.
We may rely on third-party suppliers and vendors for someheightening many of the materials we requireother risks described herein. Such risks include, but are not limited to, adverse effects on macroeconomic conditions, including inflation; disruptions to our technology infrastructure, including through cyberattack, ransom attack, or cyber-intrusion; adverse changes in our drug discoveryinternational trade policies and research tool businesses as well as for the manufacture of any product candidates that we may developrelations; disruptions in global supply chains; and constraints, volatility, or disruption in the future. Any significant problem experienced by onecapital markets, any of our suppliers could result in a delay or interruption in the supply of materials to us until such supplier resolves the problem or an alternative source of supply is located. Any delay or interruptionwhich could negatively affect our operations.business and financial condition.
Risks Related to Government Regulation
Violation of government regulations or quality programs could harm demand for our products or services,In the past, we have used hazardous chemicals, biological materials and the evolving nature of government regulations could have an adverse impact oninfectious agents in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
ToOur product manufacturing, research and development, and testing activities have involved the extent that our collaboratorscontrolled use of hazardous materials, including chemicals, biological materials and infectious disease agents. We cannot eliminate the risks of accidental contamination or customers use our products in the manufacturingaccidental spread or testing processes for their drug and medical device products,discharge of these materials, or any resulting injury from such end-products or servicesan event. We may be regulated by the FDA under Quality System Regulations (QSR)sued for any injury or contamination that results from our use or the Centers for Medicare & Medicaid Services (CMS) under Clinical Laboratory Improvement Amendmentsuse by third parties of 1988 (CLIA’88) regulations. The customer is ultimately responsible for QSR, CLIA’88 and other compliance requirements for their products; however, we may agree to comply with certain requirements, and, if we fail to do so, we could lose sales and customers and be exposed to product liability claims.
Products that are intended for the diagnosis or treatment of disease are subject to government regulation. Our drug discovery and research tool offerings are currently intended for research or investigational uses. Research uses are not subject to FDA or premarket approval or other regulatory requirements. Investigational uses are not subject to FDA premarket approval or most regulatory requirements, but are subject to limited regulatory controls for entities conducting investigational studies.
As we continue to adapt and develop parts of our product line in the future, including tissue-based products in the field of regenerative medicine, the manufacture and marketing of our products will become subject to government regulation in the United States and other countries. In the United States and most foreign countries, we will be required to complete rigorous preclinical testing and extensive human clinical trials that demonstrate the safety and efficacy of a product in order to apply for regulatory approval to market the product.
The steps required by the FDA before our proposed products may be marketed in the United States include performance of preclinical (animal and laboratory) tests; submissions to the FDA of an IDE (Investigational Device Exemption), NDA (New Drug Application), or BLA (Biologic License Application) which must become effective before human clinical trials may commence; performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product in the intended target population; performance of a consistent and reproducible manufacturing process intended for commercial use; Pre-Market Approval Application (“ PMA “); and FDA approval of the PMA before any commercial sale or shipment of the product.
The processes are expensive and can take many years to complete, and we may not be able to demonstrate the safety and efficacy of our products to the satisfaction of such regulatory authorities. The start of clinical trials can be delayed or take longer than anticipated for many and varied reasons, many of which are outside of our control. Safety concerns may emerge that could lengthen the ongoing trials or require additional trials to be conducted. Regulatory authorities may also require additional testing, and we may be required to demonstrate that our proposed products represent an improved form of treatment over existing therapies, which we may be unable to do without conducting further clinical studies. Moreover, if the FDA grants regulatory approval of a product, the approval may be limited to specific indications or limited with respect to our distribution. Expanded or additional indications for approved devices or drugs may not be approved, which could limit our revenues. Foreign regulatory authorities may apply similar limitations or may refuse to grant any approval. Consequently, even if we believe that preclinical and clinical data are sufficient to support regulatory approval for our product candidates, the FDA and foreign regulatory authorities may not ultimately grant approval for commercial sale in any jurisdiction. If our products are not approved, our ability to generate revenues will be limitedthese materials, and our business will be adversely affected.
Even if a product gains regulatory approval, such approval is likely to limitliability may exceed our insurance coverage and our total assets. Federal, state and local laws and regulations govern the indicated uses for which it may be marketed,use, manufacture, storage, handling and the product and the manufacturer of the product will be subject to continuing regulatory review, including adverse event reporting requirements and the FDA’s general prohibition against promoting products for unapproved uses. Failure to comply with any post-approval requirements can, among other things, result in warning letters, product seizures, recalls, substantial fines, injunctions, suspensions or revocations of marketing licenses, operating restrictions and criminal prosecutions. Anydisposal of these enforcement actions, any unanticipated changes in existing regulatory requirements orhazardous materials and specified waste products, as well as the adoptiondischarge of new requirements, or any safety issues that arise with any approved products, could adversely affect our ability to market productspollutants into the environment and generate revenues and thus adversely affect our ability to continue our business.
We also may be restricted or prohibited from marketing or manufacturing a product, even after obtaining product approval, if previously unknown problems with the product or our manufacture are subsequently discovered and we cannot provide assurance that newly discovered or developed safety issues will not arise following any regulatory approval. With the use of any treatment by a wide patient population, serious adverse events may occur from time to time that initially do not appear to relate to the treatment itself, and only if the specific event occurs with some regularity over a period of time does the treatment become suspect as having a causal relationship to the adverse event. Any safety issues could cause us to suspend or cease marketing of our approved products, possibly subject us to substantial liabilities, and adversely affect our ability to generate revenues.
We are subject to various environmental,human health and safety laws.
matters. We arewere also subject to various laws and regulations relating to safe working conditions, laboratory and manufacturing practices, and the experimental use of animals, emissionsanimals. Our operations may have required that environmental permits and wastewater discharges,approvals be issued by applicable government agencies. If we failed to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance.
If we fail to obtain and sustain an adequate level of reimbursement for our potential products by third-party payors, potential future sales would be materially adversely affected.
There will be no viable commercial market for our drug candidates, if approved, without reimbursement from third-party payors. Reimbursement policies may be affected by future healthcare reform measures. We cannot be certain that reimbursement will be available for our current drug candidates or any other drug candidate we may develop. Additionally, even if there is a viable commercial market, if the level of reimbursement is below our expectations, our anticipated revenue and gross margins will be adversely affected.
Third-party payors, such as government or private healthcare insurers, carefully review and increasingly question and challenge the coverage of and the prices charged for drugs. Reimbursement rates from private health insurance companies vary depending on the Company, the insurance plan and other factors. Reimbursement rates may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. There is a current trend in the U.S. healthcare industry toward cost containment.
Large public and private payors, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and disposalreimbursement levels for, particular treatments. Such third-party payors, including Medicare, may question the coverage of, hazardousand challenge the prices charged for, medical products and services, and many third-party payors limit coverage of or potentially hazardous substances usedreimbursement for newly approved healthcare products. In particular, third-party payors may limit the covered indications. Cost-control initiatives could decrease the price we might establish for products, which could result in product revenues being lower than anticipated. We believe our drugs will be priced significantly higher than existing generic drugs and consistent with current branded drugs. If we are unable to show a significant benefit relative to existing generic drugs, Medicare, Medicaid and private payors may not be willing to provide reimbursement for our drugs, which would significantly reduce the likelihood of our products gaining market acceptance.
We expect that private insurers will consider the efficacy, cost-effectiveness, safety and tolerability of our potential products in determining whether to approve reimbursement for such products and at what level. Obtaining these approvals can be a time consuming and expensive process. Our business, financial condition and results of operations would be materially adversely affected if we do not receive approval for reimbursement of our potential products from private insurers on a timely or satisfactory basis. Limitations on coverage could also be imposed at the local Medicare carrier level or by fiscal intermediaries. Medicare Part D, which provides a pharmacy benefit to Medicare patients as discussed below, does not require participating prescription drug plans to cover all drugs within a class of products. Our business, financial condition and results of operations could be materially adversely affected if Part D prescription drug plans were to limit access to, or deny or limit reimbursement of, our drug candidates or other potential products.
Reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis. In many countries, the product cannot be commercially launched until reimbursement is approved. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. The negotiation process in some countries can exceed 12 months. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our products to other available therapies.
If the prices for our potential products are reduced or if governmental and other third-party payors do not provide adequate coverage and reimbursement of our drugs, our future revenue, cash flows and prospects for profitability will suffer.
Current and future legislation may increase the difficulty and cost of commercializing our drug candidates and may affect the prices we may obtain if our drug candidates are approved for commercialization.
In the U.S. and some foreign jurisdictions, there have been a number of adopted and proposed legislative and regulatory changes regarding the healthcare system that could prevent or delay regulatory approval of our drug candidates, restrict or regulate post-marketing activities and affect our ability to profitably sell any of our drug candidates for which we obtain regulatory approval.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“MMA”) changed the way Medicare covers and pays for pharmaceutical products. Cost reduction initiatives and other provisions of this legislation could limit the coverage and reimbursement rate that we receive for any of our approved products. While the MMA only applies to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
In addition, on August 16, 2022, President Biden signed into law the Inflation Reduction Act of 2022, which, among other things, includes policies that are designed to have a direct impact on drug prices and reduce drug spending by the federal government, which shall take effect in 2023. Under the Inflation Reduction Act of 2022, Congress authorized Medicare beginning in 2026 to negotiate lower prices for certain costly single-source drug and biologic products that do not have competing generics or biosimilars. This provision is limited in terms of the number of pharmaceuticals whose prices can be negotiated in any given year and it only applies to drug products that have been approved for at least 9 years and biologics that have been licensed for 13 years. Drugs and biologics that have been approved for a single rare disease or condition are categorically excluded from price negotiation. Further, the new legislation provides that if pharmaceutical companies raise prices in Medicare faster than the rate of inflation, they must pay rebates back to the government for the difference. The new law also caps Medicare out-of-pocket drug costs at an estimated $4,000 a year in 2024 and, thereafter beginning in 2025, at $2,000 a year.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively the “PPACA”), was enacted. The PPACA was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The PPACA increased manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate amount for both branded and generic drugs and revised the definition of “average manufacturer price”, which may also increase the amount of Medicaid drug rebates manufacturers are required to pay to states. The legislation also expanded Medicaid drug rebates and created an alternative rebate formula for certain new formulations of certain existing products that is intended to increase the rebates due on those drugs. The Centers for Medicare & Medicaid Services (“CMS”), which administers the Medicaid Drug Rebate Program, also has proposed to expand Medicaid rebates to the utilization that occurs in the territories of the U.S., such as Puerto Rico and the Virgin Islands. Further, beginning in 2011, the PPACA imposed a significant annual fee on companies that manufacture or import branded prescription drug products and required manufacturers to provide a 50% discount off the negotiated price of prescriptions filled by beneficiaries in the Medicare Part D coverage gap, referred to as the “donut hole.” Legislative and regulatory proposals have been introduced at both the state and federal level to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products.
There have been public announcements by members of the U.S. Congress, regarding plans to repeal and replace the PPACA and Medicare. For example, on December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act of 2017, which, among other things, eliminated the individual mandate requiring most Americans (other than those who qualify for a hardship exemption) to carry a minimum level of health coverage, effective January 1, 2019. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, or the Texas District Court Judge, ruled that the individual mandate is a critical and inseverable feature of the PPACA, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act of 2017, the remaining provisions of the PPACA are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the Fifth Circuit upheld the District Court’s ruling with respect to the individual mandate but remanded the case to the District Court to consider whether other parts of the law can remain in effect. While the Texas District Court Judge has stated that the ruling will have no immediate effect, it is unclear how this decision, subsequent appeals, and other efforts to repeal and replace the PPACA will impact the law and our business. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our drug candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing approval testing and other requirements.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under government payor programs, and review the relationship between pricing and manufacturer patient programs. The U.S. Department of Health and Human Services has started soliciting feedback on some of these measures and, at the same time, is implementing others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. While any proposed measures will require authorization through additional legislation to become effective, Congress has indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our drug candidates, if approved for commercialization.
In Europe, the United Kingdom formally withdrew from the European Union on January 31, 2020, and entered into a transition period that ended on December 31, 2020. A significant portion of the regulatory framework in the United Kingdom is derived from the regulations of the European Union. We cannot predict what consequences the recent withdrawal of the United Kingdom from the European Union will have on the regulatory frameworks of the United Kingdom or the European Union, or on our future operations, if any, in these jurisdictions, and the United Kingdom is in the process of negotiating trade deals with other countries. Additionally, the United Kingdom’s withdrawal from the European Union may increase the possibility that other countries may decide to leave the European Union again.
*Actions that we have taken to restructure our business to align our cost structure with our strategic priorities may not have the anticipated effects.
In August 2023, we announced a plan to reduce our workforce by six employees, which represented approximately 24% of our employees as of August 18, 2023. The decision to reduce our workforce was made in order to focus spending on our clinical program for FXR314, reduce ongoing operating expenses not related to clinical expenses, and extend our cash runway. As a result of the reduction in force, we expect to incur approximately $0.4 million of cash expenditures in connection with the reduction in force, which relate to severance pay, which are expected to be incurred through the quarter ending March 31, 2024. We may incur additional expenses not currently contemplated due to events associated with the reduction in force; for example, the reduction in force may have a future impact on other areas of our research, including infectious disease agents. We also cannot accurately predictliabilities and obligations, which could result in losses in future periods. Moreover, we may not realize, in full or in part, the extentanticipated benefits and savings from this restructuring due to unforeseen difficulties, delays or unexpected costs. If we are unable to realize the expected operational efficiencies and cost savings from the restructuring, our operating results and financial condition would be adversely affected. In addition, we may need to undertake additional workforce reductions or restructuring activities in the future.
Risks Related to Our Capital Requirements, Finances and Operations
*Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern.
Our financial statements as of regulationsDecember 31, 2023 have been prepared under the assumption that we will continue as a going concern for the next twelve months. Management has performed an analysis and concluded that substantial doubt exists about our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, obtain government grants, reduce expenditures and generate significant revenue. Our financial statements as of December 31, 2023 do not include any adjustments that might result from anythe outcome of this uncertainty. The reaction of investors to the inclusion of a going concern statement by management and our auditors, and our potential inability to continue as a going concern, in future legislativeyears could materially adversely affect our share price and our ability to raise new capital or administrative action.enter into strategic alliances.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to curtail or cease our operations.
There can be no assurance that we will be able to raise sufficient additional capital on acceptable terms or at all. Raising additional funding through debt or equity financing is likely to be difficult or unavailable altogether given the early stage of our therapeutic candidates. If such additional financing is not available on satisfactory terms, or is not available in sufficient amounts, we may be required to delay, limit or eliminate the development of business opportunities and our ability to achieve our business objectives, our competitiveness, and our business, financial condition and results of operations will be materially adversely affected. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing stockholders, increased fixed payment obligations and the existence of securities with rights that may be senior to those of our common stock. If we
incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these lawsevents could significantly harm our business, financial condition and prospects. Furthermore, the issuance of additional securities, whether equity or regulationsdebt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline further and existing stockholders may not agree with our financing plans or the terms of such financings. In addition, if we seek funds through arrangements with collaborative partners, these arrangements may require us to relinquish rights to our technology or potential future product candidates or otherwise agree to terms unfavorable to us.
We have a history of operating losses and expect to incur significant additional operating losses.
We have generated operating losses each year since we began operations, including $12.0 million and $10.0 million for the nine months ended December 31, 2023 and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $336.6 million. We expect to incur substantial additional operating losses over the next several years as our research and development activities increase.
The amount of future losses and when, if ever, we will achieve profitability are uncertain. Our ability to generate revenue and achieve profitability will depend on, among other things:
•successfully developing and advancing FXR314 and our FXR program generally;
•successfully developing human tissues and disease models for drug discovery and development that enable us to identify drug candidates;
•successfully outsourcing certain portions of our development efforts;
•entering into collaboration or licensing arrangements with pharmaceutical companies to further develop and conduct clinical trials for any drug candidates we identify;
•obtaining any necessary regulatory approvals for any drug candidates we identify; and
•raising sufficient funds to finance our activities and long-term business plan.
We might not succeed at any of these undertakings. If we are unsuccessful at one or more of these undertakings, our business, prospects, and results of operations will be materially adversely affected. We may never generate significant revenue, and even if we do generate significant revenue, we may never achieve profitability.
Our quarterly operating results may vary, which could negatively affect the market price of our common stock.
Our results of operations in any quarter may vary from quarter to quarter and are influenced by such factors as expenses related to:
•evaluating and implementing strategic alternatives, technology licensing opportunities, potential collaborations, and other strategic transactions;
•research and development expenditures, including commencement of preclinical studies and clinical trials;
•the timing of the hiring of new employees, which may require payments of signing, retention or similar bonuses; and
•changes in costs related to the general global economy.
We believe that operating results for any particular quarter are not necessarily a meaningful indication of future results. Nonetheless, fluctuations in our quarterly operating results could negatively affect the market price of our common stock.
We may identify material weaknesses in the future that may cause us to incur additional expensefail to meet our reporting obligations or restrictresult in material misstatements of our operations. Compliancefinancial statements.
Our management team is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with environmental lawsU.S. generally accepted accounting principles. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis.
We cannot assure you that we will not have material weaknesses or significant deficiencies in our internal control over financial reporting. If we identify any material weaknesses or significant deficiencies that may exist, the accuracy and regulationstiming of our financial reporting may be expensive,adversely affected, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, and current or future environmental regulationsour stock price may impairdecline materially as a result.
Future strategic investments could negatively affect our research, development or production efforts.
We will dependbusiness, financial condition and results of operations if we fail to achieve the desired returns on our patent portfolio, our licensed technology and other trade secrets in the conduct of our business and must ensure that we do not violate the patent or intellectual rights of others.investment.
Our success in large partability to benefit from future external strategic investments depends on our ability to maintainsuccessfully conduct due diligence, evaluate prospective opportunities, and buy the proprietary natureequity of our technologytarget investments at acceptable market prices. Our failure in any of these tasks could result in unforeseen losses associated with the strategic investments.
We may also discover deficiencies in internal controls, data adequacy and integrity, product quality, regulatory compliance, product liabilities or other trade secrets. To do so,undisclosed liabilities that we anddid not uncover prior to our licensors must prosecute and maintain existing patents, obtain new patents and pursue trade secret and other intellectual property protection. We also must operate without infringing the proprietary rightsinvestment, which could result in us becoming subject to asset impairments, including potential loss of third parties or allowing third parties infringe our rights. Our research, development and commercialization activities, including any product candidates or products resulting from these activities, may infringe or be claimed to infringe patents owned by third parties and as to whichinvestment capital. In addition, if we do not hold licensesachieve the anticipated benefits of an external investment as rapidly as expected, or other rights. Thereat all, investors or analysts may be rightsdowngrade our stock.
We also expect to continue to carry out strategic investments that we believe are necessary to expand our business. There are no assurances that such initiatives will yield favorable results for us. Accordingly, if these initiatives are not awaresuccessful, our business, financial condition and results of including applications that have been filed but not published that, when issued,operations could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, weadversely affected. If these risks materialize, our stock price could be forced to stop or delay research, development, manufacturing or sales of the product or biologic treatment candidate that is the subject of the suit.
In addition, competitors may infringe our patents or the patents of our collaborators or licensors. As a result, we may be required to file infringement claims to counter infringement for unauthorized use. This can be expensive and time-consuming. In addition,materially adversely affected. Any difficulties in an infringement proceeding, a court may decide that a patent owned by us is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover our technology. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at the risk of not issuing.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
A significant portion of our sales are dependent upon our customers’ capital spending policies and research and development budgets, and government funding of research and development programs at universities and other organizations, which are each subject to significant and unexpected decrease.
Our prospective customers include pharmaceutical and biotechnology companies, academic institutions, government laboratories, and private research foundations. Fluctuations in the research and development budgets at these organizationssuch investments could have a significantmaterial adverse effect on the demand for our productsbusiness, financial condition and services. Research and development budgets fluctuate due to changes in available resources, patent expirations, mergersresults of pharmaceutical and biotechnology companies, spending priorities, general economic conditions, and institutional and governmental budgetary policies, including but not limited to reductions in grants for research by educational institutions. In addition, ouroperations.
Our business could be seriously damagedadversely impacted if we are unable to retain our executive officers and other key personnel.
Our future success will depend to a significant degree upon the continued contributions of our key personnel, especially our executive officers. We do not currently have long-term employment agreements with our executive officers or our other key personnel, and there is no guarantee that our executive officers or key personnel will remain employed with us. Moreover, we have not obtained key man life insurance that would provide us with proceeds in the event of the death, disability or incapacity of any of our executive officers or other key personnel. Further, the process of attracting and retaining suitable replacements for any executive officers and other key personnel we lose in the future would result in transition costs and would divert the attention of other members of our senior management from our existing operations. Additionally, such a loss could be negatively perceived in the capital markets. Finally, our Executive Chairman also provides services to Viscient Biosciences, Inc. (“Viscient”). Executives that provide services to us and Viscient do not dedicate all of their time to us, as disclosed in our filings, and we may therefore compete with Viscient for the time commitments of our Executive Chairman from time to time.
We may be subject to security breaches or other cybersecurity incidents that could compromise our information and expose us to liability.
We routinely collect and store sensitive data (such as intellectual property, proprietary business information and personally identifiable information) for ourselves, our employees and our suppliers and customers. We make significant efforts to maintain the security and integrity of our computer systems and networks and to protect this information. However, like other companies in our industry, our networks and infrastructure may be vulnerable to cyber-attacks or intrusions, including by anycomputer hackers, foreign governments, foreign companies or competitors, or may be breached by employee error, malfeasance or other disruption. Any such breach could result in unauthorized access to (or disclosure of) sensitive, proprietary or confidential information of ours, our employees or our suppliers or customers, and/or loss or damage to our data. Any such unauthorized access, disclosure, or loss of information could cause competitive harm, result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and/or cause reputational harm.
*Compliance with global privacy and data security requirements could result in additional costs and liabilities to us or inhibit our ability to collect and process data globally, and the failure to comply with such requirements could subject us to significant decreasefines and penalties, which may have a material adverse effect on our business, financial condition and results of operations.
The regulatory framework for the collection, use, safeguarding, sharing, transfer, and other processing of information worldwide is rapidly evolving and is likely to remain uncertain for the foreseeable future. Globally, virtually every jurisdiction in life sciences researchwhich we operate has established its own data security and development expenditures by pharmaceutical and biotechnology companies, academic institutions, government laboratories,privacy frameworks with which we must comply. For example, the collection, use, disclosure, transfer, or private foundations.
The timing and amountother processing of revenues from customers that rely on government funding of research may vary significantly due to factors that can be difficult to forecast. Research funding for life science research has increased more slowly duringpersonal data regarding individuals in the past several years compared to the previous years and has declined in some countries, and some grants have been frozen for extended periods of time or otherwise become unavailable to various institutions, sometimes without advance notice. Government funding of research and developmentEuropean Union, including personal health data, is subject to the politicalEU General Data Protection Regulation (the “GDPR”), which took effect across all member states of the European
Economic Area (the “EEA”) in May 2018. The GDPR is wide-ranging in scope and imposes numerous requirements on companies that process which is inherently fluidpersonal data, including requirements relating to processing health and unpredictable. Other programs, such as homelandother sensitive data, obtaining consent of the individuals to whom the personal data relates, providing information to individuals regarding data processing activities, implementing safeguards to protect the security or defense, or general effortsand confidentiality of personal data, providing notification of data breaches, and taking certain measures when engaging third-party processors. The GDPR increases our obligations with respect to reduceclinical trials conducted in the federal budget deficit could be viewedEEA by expanding the definition of personal data to include coded data and requiring changes to informed consent practices and more detailed notices for clinical trial subjects and investigators. In addition, the GDPR imposes strict rules on the transfer of personal data to countries outside the European Union, including the United States, governmentand, as a higher priority. These budgetary pressuresresult, increases the scrutiny that clinical trial sites located in the EEA should apply to transfers of personal data from such sites to countries that are considered to lack an adequate level of data protection, such as the United States. The GDPR also permits data protection authorities to require destruction of improperly gathered or used personal information and/or impose substantial fines for violations of the GDPR, which can be up to four percent of global revenues or 20 million Euros, whichever is greater, and it also confers a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the GDPR. In addition, the GDPR provides that European Union member states may make their own further laws and regulations limiting the processing of personal data, including genetic, biometric or health data.
Further, Brexit has led to, and could continue to lead to legislative and regulatory changes, which may increase our compliance costs. As of January 1, 2021 and the expiry of transitional arrangements agreed to between the United Kingdom and the European Union, data processing in the United Kingdom is governed by a United Kingdom version of the GDPR (combining the GDPR and the Data Protection Act 2018), exposing us to two parallel regimes, each of which authorizes similar fines and other potentially divergent enforcement actions for certain violations. On June 28, 2021, the European Commission adopted an Adequacy Decision for the United Kingdom, allowing for the relatively free exchange of personal information between the European Union and the United Kingdom, however, the European Commission may suspend the Adequacy Decision if it considers that the United Kingdom no longer provides for an adequate level of data protection. The UK has announced plans to reform the country’s data protection legal framework in its Data Reform Bill, which may introduce significant changes from the GDPR, which may lead to additional compliance costs. Other jurisdictions outside the European Union are similarly introducing or enhancing privacy and data security laws, rules and regulations.
Similar actions are either in place or under way in the United States. There are a broad variety of data protection laws that are applicable to our activities, and a wide range of enforcement agencies at both the state and federal levels that can review companies for privacy and data security concerns based on general consumer protection laws. The Federal Trade Commission and state Attorneys General all are aggressive in reviewing privacy and data security protections for consumers. New laws also are being considered at both the state and federal levels and several states have passed comprehensive privacy laws. For example, the California Consumer Privacy Act — which went into effect on January 1, 2020 — is creating similar risks and obligations as those created by the GDPR, though the California Consumer Privacy Act does exempt certain information collected as part of a clinical trial subject to the Federal Policy for the Protection of Human Subjects (the Common Rule). As of January 1, 2023, the California Consumer Privacy Act (as amended and expanded by the California Privacy Rights Act) is in full effect, with enforcement by California’s dedicated privacy enforcement agency expected to start later in 2023. While California was first among the states in adopting comprehensive data privacy legislation similar to the GDPR, many other states are following suit. Similar laws passed in Virginia, Colorado, Connecticut, and Utah took effect in 2023. Additionally, Delaware, Indiana, Iowa, Montana, Oregon, Tennessee and Texas have adopted
privacy laws, which take effect from July 1, 2024 through 2026. Many other states are considering similar legislation. Additionally, a broad range of legislative measures also have been introduced at the federal level. Accordingly, failure to comply with federal and state laws (both those currently in effect and future legislation) regarding privacy and security of personal information could expose us to fines and penalties under such laws. There also is the threat of consumer class actions related to these laws and the overall protection of personal data. This is particularly true with respect to data security incidents, and sensitive personal information, including health and biometric data. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our reputation and business.
Given the breadth and depth of changes in data protection obligations, preparing for and complying with these requirements is rigorous and time intensive and requires significant resources and a review of our technologies, systems and practices, as well as those of any third-party collaborators, service providers, contractors or consultants that process or transfer personal data collected in the European Union. The GDPR, new state privacy laws and other changes in laws or regulations associated with the enhanced protection of certain types of sensitive data, such as healthcare data or other personal information from our clinical trials, could require us to change our business practices and put in place additional compliance mechanisms, may interrupt or delay our development, regulatory and commercialization activities and increase our cost of doing business, and could lead to government enforcement actions, private litigation and significant fines and penalties against us and could have a material adverse effect on our business, financial condition and results of operations.
We and our partners may be subject to stringent privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security, and changes in such laws, regulations, policies or how they are interpreted or changes in contractual obligations could adversely affect our business.
There are numerous U.S. federal and state data privacy and protection laws and regulations that apply to the collection, transmission, processing, storage and use of personally-identifying information, which among other things, impose certain requirements relating to the privacy, security and transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve in jurisdictions worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Failure to comply with any of these laws and regulations could result in reduced allocationsenforcement action against us, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, damage to government agenciesour reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
If we are unable to properly protect the privacy and security of health-related information or other sensitive or confidential information in our possession, we could be found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face significant administrative, civil and criminal penalties. Enforcement activity can also result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that fund researchthreaten the privacy of state residents.
We may experience conflicts of interest with Viscient Biosciences, Inc. with respect to business opportunities and other matters.
Keith Murphy, our Executive Chairman, is the Chief Executive Officer, Chairman and principal stockholder of Viscient, a private company that he founded in 2017 that is focused on drug discovery and development activities. Past proposalsutilizing 3D tissue technology and multi-omics (genomics, transcriptomics, metabolomics). Jeffrey N. Miner, our former Chief Scientific Officer, is a co-founder, the Chief Scientific Officer and a significant stockholder of Viscient. In addition, Adam Stern, Douglas Jay Cohen and David Gobel (through the Methuselah Foundation and the Methuselah Fund), members of our Board, have invested funds through a convertible promissory note in Viscient, but do not serve as an employee, officer or director of Viscient. Additional members of our Research and Development organization also work at Viscient, and we expect that additional employees or consultants of ours will also be employees of or consultants to reduce budget deficitsViscient. We use certain Viscient-owned facilities and equipment and allow Viscient to use certain of our facilities and equipment. During fiscal 2024, we provided services to Viscient, and we expect to continue to provide services to Viscient and enter into additional agreements with Viscient in the future.
In addition, we license, as well as cross-license, certain intellectual property to and from Viscient and expect to continue to do so in the future. In particular, pursuant to an Asset Purchase and Non-Exclusive Patent License Agreement with Viscient, dated November 6, 2019, as amended, we have included reduced National Instituteprovided a paid up, worldwide, irrevocable, perpetual, non-exclusive license to Viscient under certain of Healthour patents and know-how to (a) make, have made, use, sell, offer to sell, import and otherwise exploit the inventions and subject matter covered by certain patents regarding certain bioprinter devices and bioprinting methods, engineered liver tissues, engineered renal tissues, engineered intestinal tissue and engineered tissue for in vitro research use, (b) to use and internally repair the bioprinters, and (c) to make additional bioprinters for internal use only in connection with drug discovery and development research, target identification and validation, compound screening, preclinical safety, absorption, distribution, metabolism, excretion and toxicology (ADMET) studies, and in vitro research to complement clinical development of a therapeutic compound. Although we have entered, and expect to enter, into agreements and arrangements that we believe appropriately govern the ownership of intellectual property created by joint employees or consultants of Viscient and/or using our or Viscient’s facilities or equipment, it is possible that we may disagree with Viscient as to the ownership of intellectual property created by shared employees or consultants, or using shared equipment or facilities.
On December 28, 2020, we entered into an intercompany agreement with Viscient and Organovo, Inc., our wholly-owned subsidiary (the “Intercompany Agreement”). Pursuant to the Intercompany Agreement, we agreed to provide Viscient certain services related to 3D bioprinting technology, which includes, but is not limited to, histology services, cell isolation, and proliferation of cells, and Viscient agreed to provide us certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, Viscient and we each agreed to share certain facilities and equipment and, subject to further agreement, to each make certain employees available for specified projects to the other party at prices to be determined in good faith by the parties. Under the Intercompany Agreement, each party will retain its own prior intellectual property and will obtain new intellectual property rights within their respectively defined fields of use.
Due to the interrelated nature of Viscient with us, conflicts of interest may arise with respect to transactions involving business dealings between us and Viscient, potential acquisitions of businesses or products, the development and ownership of technologies and products, the sale of products, markets and other researchmatters in which our best interests and development allocations. Any shift away from the funding of life sciences research and development or delays surrounding the approval of government budget proposals may cause our customers to delay or forego purchasesbest interests of our productsstockholders may
conflict with the best interests of the stockholders of Viscient. In addition, we and Viscient may disagree regarding the interpretation of certain terms of the arrangements we previously entered into with Viscient or may enter into in the future. We cannot guarantee that any conflict of interest will be resolved in our favor, or that, with respect to our transactions with Viscient, we will negotiate terms that are as favorable to us as if such transactions were with another third-party. In addition, executives that provide services which could seriously damageto us and Viscient may not dedicate all of their time to us and we may therefore compete with Viscient for the time commitments of our business.executive officers from time to time.
Risks Related to Our Common Stock and Liquidity Risks
Our securities are a “Penny Stock” and subjectWe could fail to specific rules governing their sale to investors.
The SEC has adopted Rule 15g-9 which establishesmaintain the definitionlisting of a “penny stock,” for the purposes relevant to our common stock as any equity securityon the Nasdaq Capital Market, which could seriously harm the liquidity of our stock and our ability to raise capital or complete a strategic transaction.
The Nasdaq Stock Market LLC (“Nasdaq”) has established continued listing requirements, including a requirement to maintain a minimum closing bid price of at least $1 per share. If a company trades for 30 consecutive business days below such minimum closing bid price, it will receive a deficiency notice from Nasdaq. Assuming it is in compliance with the other continued listing requirements, Nasdaq would provide such company a period of 180 calendar days in which to regain compliance by maintaining a closing bid price at least $1 per share for a minimum of ten consecutive business days. There can be no assurance that haswe will continue to maintain compliance with the minimum bid price requirement or other listing requirements necessary for us to maintain the listing of our common stock on the Nasdaq Capital Market.
A delisting from the Nasdaq Capital Market and commencement of trading on the Over-the-Counter Bulletin Board would likely result in a reduction in some or all of the following, each of which could have a material adverse effect on stockholders:
•the liquidity of our common stock;
•the market price of less than $5.00 per shareour common stock (and the accompanying valuation of our Company);
•our ability to obtain financing or with an exercise pricecomplete a strategic transaction;
•the number of
less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules requireinstitutional and other investors that
a broker or dealer approve a person’s account for transactionswill consider investing in
penny stocks; and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person; and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination; and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors sell shares of our common stock.
Disclosure also has to be made about stock;
•the risksnumber of investing in penny stocks in both public offeringsmarket markers or broker-dealers for our common stock; and in secondary
•the availability of information concerning the trading prices and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in casesvolume of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.shares of our common stock.
There is limited trading activityno assurance that an active market in our common stock andwill continue at present levels or increase in the future.
Our common stock is currently traded on the Nasdaq Capital Market, but there is no assurance that an active market in our common stock will developcontinue at present levels or increase in the future.
Recent trading activity in our Common Stock has been limited, averaging 100,000 shares traded per day since our Common Stock began trading February 14, 2012. Further, although our common stock is currently quoted on the OTCQB, trading of our common stock may be extremely sporadic. For example, several days may pass before any shares may be traded. As a result, an investor may find it difficult to dispose of or to obtain accurate quotations of the price of our common stock. There can be no assurance that a more active market for our common stock will develop, or if one should develop, there is no assurance that it will be sustained.on the timeline and at the volumes they desire. This severelyfactor limits the liquidity of our common stock and would likelymay have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
Because we became public by means of a reverse merger we may not be able to attract the attention of brokerage firms.
Additional risks may exist since we became public through a “reverse merger.” Securities analysts of brokerage firms may not provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on our behalf in the future.
Compliance with the reporting requirements of federal securities laws can be expensive.
We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, and the compliance obligations of the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports and other information with the SEC and furnishing audited reports to stockholders are substantial. In addition, we will incur substantial expenses in connection with the preparation of the Registration Statement and related documents with respect to the registration of resales of the common stock sold in the Offering.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of its business and its ability to obtain or retain listing of our common stock.
We may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. We may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business and our ability to obtain or retain listing of our shares of common stock on any stock exchange (assuming we elect to seek and are successful in obtaining such listing) could be adversely affected.
We may have undisclosed liabilities and any such liabilities could harm our revenues, business, prospects, financial condition and results of operations.
Even though our pre-merger assets and liabilities were transferred to the Split-Off Shareholders in the Split-Off, there can be no assurance that we will not be liable for any or all of such liabilities. Any such liabilities that survived the Merger could harm our revenues, business, prospects, financial condition and results of operations upon our acceptance of responsibility for such liabilities.
The transfer of the operating assets and liabilities to PSOS, coupled with the Split-Off of PSOS, will result in taxable income to us in an amount equal to the difference between the fair market value of the assets transferred and the pre-merger tax basis of the assets. Any gain recognized, to the extent not offset by our net operating loss carryforward, if any, will be subject to federal income tax at regular corporate income tax rates.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or detect fraud. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our stock.
We must maintain effective internal controls to provide reliable financial reports and detect fraud. We have been assessing our internal controls to identify areas that need improvement. We are in the process of implementing changes to internal controls, but have not yet completed implementing these changes. Failure to implement these changes to our internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm our operating results and cause investors to lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the trading price of our stock.
The price of our common stock may becomecontinue to be volatile, which could lead to losses by investors and costly securities litigation.
The trading price of our common stock is likely to be highly volatile and could fluctuate in response to factors such as:
actual or anticipated variations in our operating results;
announcements of developments by us or our competitors;
the timing of IDE and/or NDA approval, the completion and/or results of our clinical trials
regulatory actions regarding our products
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
•
our ability to execute on our new strategic plan;
•reduced government funding for research and development activities;
•actual or anticipated variations in our operating results;
•adoption of new accounting standards affecting the our industry;
•
additions or departures of key personnel;
introduction of new products by us or our competitors;
sales of the our common stock or other securities in the open market;
•degree of coverage of securities analysts and reports and recommendations issued by securities analysts regarding our business;
•
volume fluctuations in the trading of our common stock; and
•other events or factors, many of which are beyond our control.
The stock market is subject to significant price and volume fluctuations. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been initiated against such a company. Litigation initiated against us, whether or not successful, could result in substantial costs and diversion of our management’s attention and resources, which could harm our business and financial condition.
Investors may experience dilution of their ownership interests because of the future issuance of additional shares of our capital stock.
We are authorized to issue 200,000,000 shares of common stock and 25,000,000 shares of preferred stock. As of March 31, 2024, there were an aggregate of 12,585,625 shares of our common stock issued and outstanding and available for issuance on a fully diluted basis and no shares of preferred stock outstanding. That total for our common stock includes 2,462,899 shares of our common stock that may be issued upon the vesting of restricted stock units, the exercise of outstanding stock options, or is available for issuance under our equity incentive plans, and 45,000 shares of common stock that may be issued through our 2023 Employee Stock Purchase Plan (“ESPP”).
In the future, we may issue additional authorized but previously unissued equity securities resulting in the dilution of the ownership interests ofto raise funds to support our present stockholders.continued operations and to implement our business plan. We may also issue additional shares of our commoncapital stock or other securities that are convertible into or exercisable for our commoncapital stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. TheIf we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders may result. In addition, the future issuance of any such additional shares of commoncapital stock may create downward pressure on the trading price of our common stock. There can be no assurance that the we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the price at which shares of our common stock is currently quotedtraded on the OTCQB.Nasdaq Capital Market. Moreover, depending on market conditions, we cannot be sure that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or to our stockholders.
Our common stock is controlled by insiders.
Our officers and directors beneficially own approximately 22.9% of our outstanding shares of common stock. Such concentrated control may adversely affect the price of our common stock. Investors who acquire our common stock may have no effective voice in the management of our operations. Sales by our insiders or affiliates, along with any other market transactions, could affect the market price of our common stock.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our common stock to date and it is not anticipated that any dividends will be paid to holders of our common stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of our business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. As an investor, you should take note of the fact that a lack of a dividend can further affect the market value of our stock and could significantly affect the value of any investment.
USE OF PROCEEDS
We will not receive any proceeds from the sale of shares of our common stock by the selling security holders. A portion of the shares covered by this prospectus are issuable upon exercise of warrants to purchase our common stock. Upon any exercise of the warrants for cash, the selling security holders would pay us the exercise price of the warrants. Under certain conditions set forth in the warrants, the warrants are exercisable on a cashless basis. If the warrants are exercised on a cashless basis, we would not receive any cash payment from the selling security holders upon any exercise of the warrants. Instead, the selling security holders would satisfy their obligation to pay the exercise price through a formula-based transfer of warrant shares to us. The additional proceeds we could receive from the exercise of such warrants have not yet been earmarked for any specific use beyond working capital needs because there is no certainty that we will ever receive any proceeds from the exercise of such warrants.
The selling security holders will pay any underwriting discounts and commissions and expenses incurred by the selling security holders for brokerage, accounting, tax or legal services or any other expenses incurred by the selling security holders in disposing of the shares. We will bear all other costs, fees and expenses incurred in effecting the registration of the shares covered by this prospectus, including, without limitation, all registration and filing fees and fees and expenses of our counsel and our accountants.
DIVIDEND POLICY
We have never declared or paid dividends. We do not intend to pay cash dividends on our common stock for the foreseeable future, but currently intend to retain any future earnings to fund the development and growth of our business. The payment of dividends if any, on our common stock will rest solely within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements, financial condition, and other relevant factors.
MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND
ISSUER PURCHASES OF EQUITY SECURITIES
Prior to February 14, 2012, our Common Stock was available for trading in the over-the-counter market and was quoted on the OTCQB and the OTCBB under the symbol “RERR.” Effective February 14, 2012, our stock trades under the symbol “ONVO” and is quoted on the OTCQB. As of the December 31, 2011, there was no bid history for the “ONVO” Common Stock, because the Common Stock had never been traded.
The following table sets forth the high and low last-bid prices for our common stock for the periods indicated, as reported by the OTCQB. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
| | | | | | | | |
| | | 2012 | |
| | | High | | | Low | |
First quarter | | $ | 2.63 | | | $ | 1.24 | |
Second quarter (through June 12) | | $ | 6.70 | | | $ | 2.00 | |
Third quarter | | $ | — | | | $ | — | |
Fourth quarter | | $ | — | | | $ | — | |
Trades in our Common Stock may be subject to Rule 15g-9 of the Exchange Act, which imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction before the sale.
The SEC also has rules that regulate broker/dealer practices in connection with transactions in “penny stocks.” Penny stocks generally are equity securities with a price of less than $5.00 (other than securities listed on certain national exchanges, provided that the current price and volume information with respect to transactions in that security is provided by the applicable exchange or system). The penny stock rules require a broker/dealer, before effecting a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document prepared by the SEC that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker/dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker/dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker/dealer and salesperson compensation information, must be given to the customer orally or in writing before effecting the transaction, and must be given to the customer in writing before or with the customer’s confirmation. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for shares of our Common Stock. As a result of these rules, investors may find it difficult to sell their shares.
As of March 31, 2012, there were approximately 247 record holders (excluding an indeterminable number of stockholders whose shares are held in street or “nominee” name) of our common stock.
The following table summarizes our compensation plans under which our equity securities are authorized for issuance as of March 31, 2012:
EQUITY COMPENSATION PLAN INFORMATION
| | | | | | | | | | | | |
| | | Number of Shares to
be Issued Upon
Exercise of
Outstanding
Stock Options and
Restricted Stock
Units | | | Weighted-
Average
Exercise Price
of Outstanding
Stock Options | | | Number of Shares
Remaining Available
for Future Issuance
Under Equity
Compensation Plans | |
Equity compensation plans approved by security holders: | | | | | | | | | | | | |
2008 Equity Incentive Plan | | | 896,256 | | | $ | 0.08 | | | | — | |
2012 Equity Incentive Plan | | | — | | | | — | | | | 6,553,986 | |
Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Total | | | 896,256 | | | $ | 0.08 | | | | 6,553,986 | |
SELLING SECURITY HOLDERS
We are registering the following shares of common stock:
up to 15,247,987 shares of our common stock which were issued in our private placement (the “Offering”) of units consisting of (i) one share of our common stock and (ii) one warrant to purchase one share of our common stock at an exercise price of $1.00 per share (the “Units”), with closings of the Offering occurring on each of February 8, 2012 (the “Initial Closing”), February 29, 2012 and March 16, 2012 and shares of common stock issued to certain of the selling security holders on the date of the Initial Closing of the Offering in connection with the conversion of our $1,500,000 in principal amount of 6% convertible promissory notes due March 31, 2012 (the “Bridge Notes”) into 1,525,387 Units;
up to 15,247,987 shares of our common stock issuable upon the exercise of warrants issued to the selling security holders in our Offering of Units (excluding warrants issued to our placement agents in the Offering) and shares of common stock issuable upon the exercise of warrants issued to certain of the selling security holders on the date of the Initial Closing of the Offering in connection with the conversion of the Bridge Notes into 1,525,387 Units; and
up to 1,500,000 shares of our common stock issuable upon the exercise of warrants issued to certain selling security holders in connection with the original issuance of our Bridge Notes that where converted into 1,500,000 new warrants on the date of the Initial Closing, each exercisable at a price of $1.00 per share of our common stock.
The selling security holders may sell some, all or none of their shares. We do not know how long the selling security holders will hold the shares offered hereunder before selling them. We currently have no agreements, arrangements or understandings with the selling security holders regarding the sale of any of the shares by them other than the registration rights agreements referenced below in Description of Securities. The shares offered by this prospectus may be offered from time to time by the selling security holders. As used in this prospectus, the term “selling security holder” includes each of the selling security holders listed below, and any donee, pledgee, transferee or other successor in interest selling shares received after the date of this prospectus from a selling security holder as a gift, pledge, or other non-sale related transfer. The selling security holders may have sold or transferred, in transactions exempt from the registration requirements of the Securities Act, some or all of their shares since the date on which the information in the table is presented. Information about the selling security holders may change over time.
The following table sets forth the name of each selling security holder, the number of shares owned by such selling security holder as of June 1, 2012, the number of shares that may be offered under this prospectus by such selling security holder, and the number of shares of our common stock and the percentage (if one percent or more) of our common stock to be owned by such selling security holder after completion of this offering, assuming that all shares offered hereunder are sold as contemplated herein. The number of shares in the column “Shares of Common Stock Being Offered” represents all of the shares that a selling security holder may offer under this prospectus, which includes the shares issuable upon exercise of the warrants covered by this prospectus. Except as otherwise disclosed in this prospectus (or as disclosed in any document incorporated by reference) including information incorporated, none of the selling security holders has, or within the past three years has had, any position, office or other material relationship with us. The selling security holders have advised us that they may enter into short sales in the ordinary course of their business of investing and trading securities. The selling security holders participating in the Offering have also advised us that no short sales in our securities were entered into by them during the period beginning when the selling security holders obtained knowledge that we were contemplating a private placement and ending upon the public announcement of the Offering. Other than the costs of preparing and providing this prospectus and a registration fee to the SEC, we are not paying any costs relating to the sales by the selling security holders.
Ownership reflected in this table for each selling security holder is based upon information provided to us by the selling security holder and reflects holdings as of June 1, 2012. The percentages of common stock owned after the offering are based on 43,693,241 shares of our common stock outstanding as of June 1, 2012, including the shares of common stock issued in the PPO. Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Exchange Act. In computing the number of shares owned by and the percentage ownership of a selling security holder, shares of common stock that could be issued upon the exercise of outstanding options, warrants or other rights held by that selling security holder that are currently exercisable or exercisable within 60 days of June 1, 2012 are considered outstanding. However, such shares are not included in the shares outstanding as of June 1, 2012 when computing the percentage ownership of each other selling security holder.
Unless otherwise noted, each person or group identified possesses sole voting and investment power with respect to the shares, subject to community property laws where applicable.
| | | | | | | | | | | | | | | | | | | | | | | | |
Selling Security Holder | | Outstanding
Shares of
Common
Stock | | | Shares of
Common
Stock
Subject to
Warrants | | | Total Shares of
Common Stock
Beneficially
Owned | | | Shares of
Common
Stock Being
Offered in the
Offering (1) | | | Common
Stock
Beneficially
Owned After
Offering (1) | | | Percent
After
Offering | |
Aaron Lehmann | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
ABBA Properties Partnership | | | 70,000 | | | | 70,000 | | | | 140,000 | | | | 140,000 | | | | — | | | | * | |
ACP Partners Fund, LP | | | 125,000 | | | | 125,000 | | | | 250,000 | | | | 250,000 | | | | — | | | | * | |
ACP X, L.P. | | | 900,000 | | | | 900,000 | | | | 1,800,000 | | | | 1,800,000 | | | | — | | | | * | |
Allan Rothstein | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Andrew Fisher | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Andrew H. Kaufman | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Ann S. Totten | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Arun Virick | | | 5,000 | | | | 5,000 | | | | 10,000 | | | | 10,000 | | | | — | | | | * | |
Aspire Capital Fund, LLC | | | 250,000 | | | | 250,000 | | | | 500,000 | | | | 500,000 | | | | — | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Aubrey W. Gladstone & Marianne R. Gladstone | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Banque de Luxembourg—Client Account | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Barbara S. Dickler Trust | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Barry Michaels | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Bob Baltera | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Bradley Resources Company | | | 65,225 | | | | 80,225 | | | | 145,450 | | | | 145,450 | | | | — | | | | * | |
Bret Shupack | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Brian & Debbie Keller | | | 17,000 | | | | 17,000 | | | | 34,000 | | | | 34,000 | | | | — | | | | * | |
Brian Bauer | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Brian Joseph Murphy | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Brooks & Carmen McCartney JTWROS | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Bruce Levenbrook | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Byron C. Hughey | | | 12,500 | | | | 12,500 | | | | 25,000 | | | | 25,000 | | | | — | | | | * | |
Chenies Investor LLC | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Christine Hassuk | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Christopher J. Blum & Denise M. Blum JTWROS | | | 30,000 | | | | 30,000 | | | | 60,000 | | | | 60,000 | | | | — | | | | * | |
Christopher Travelle | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Cinema City Inc. | | | 152,826 | | | | 302,826 | | | | 455,652 | | | | 455,652 | | | | — | | | | * | |
Constance Hoidas | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
CRL Management LLC | | | 150,000 | | | | 150,000 | | | | 300,000 | | | | 300,000 | | | | — | | | | * | |
Cynergy Emerging Growth LLC | | | 101,500 | | | | 201,500 | | | | 303,000 | | | | 303,000 | | | | — | | | | * | |
Daniel W. Armstrong | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Daniel W. Hummell & Allaire D. Hummel JTWROS | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
David & Lillian Barry | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
David G. Rosen and Julie L. Rosen JTWROS | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
David Hochman | | | 12,688 | | | | 25,188 | | | | 37,876 | | | | 37,876 | | | | — | | | | * | |
David Kovacs | | | 75,000 | | | | 75,000 | | | | 150,000 | | | | 150,000 | | | | — | | | | * | |
Dawn E. Gunter | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
DCG&T Cust FBO John Dempsey IRA | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
DCG&T William C. Stone SEP IRA | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Deepak H. Aggarwal | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Delaware Charter Guarantee & Tr FBO Daniel K. Ho IRA | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Delaware Charter Guarantee & Tr FBO Raymond Coppede RO IRA | | | 75,000 | | | | 75,000 | | | | 150,000 | | | | 150,000 | | | | — | | | | * | |
Delaware Charter Guarantee & Trust Co FBO Bill L. Boad IRA | | | 35,000 | | | | 35,000 | | | | 70,000 | | | | 70,000 | | | | — | | | | * | |
Delaware Charter Guarantee & Trust Cust FBO Graham C. Short IRA | | | 73,000 | | | | 73,000 | | | | 146,000 | | | | 146,000 | | | | — | | | | * | |
Delaware Charter Guarantee & Trust Cust FBO Philip J. Benz IRA | | | 22,500 | | | | 22,500 | | | | 45,000 | | | | 45,000 | | | | — | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Derek J. Sroufe | | | 50,834 | | | | 100,834 | | | | 151,668 | | | | 151,668 | | | | — | | | | * | |
DIT Equity Holdings, LLC | | | 501,667 | | | | 601,667 | | | | 1,103,334 | | | | 1,103,334 | | | | — | | | | * | |
Douglas Jay Cohen | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Douglas P. Kaufman | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
ECPC Capital LLC | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Edward M. Dunn | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Edward N. and Carol Scott Robinson Revocable Trust April 6, 2005 | | | 50,750 | | | | 100,750 | | | | 151,500 | | | | 151,500 | | | | — | | | | * | |
Edward Rosenthal | | | 50,834 | | | | 100,834 | | | | 151,668 | | | | 151,668 | | | | — | | | | * | |
Elisabeth Stephens | | | 25,417 | | | | 50,417 | | | | 75,834 | | | | 75,834 | | | | — | | | | * | |
Eric Del Basso | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Fabrizio Balestri | | | 20,334 | | | | 40,334 | | | | 60,668 | | | | 60,668 | | | | — | | | | * | |
FEQ Realty, LLC | | | 201,667 | | | | 301,667 | | | | 503,334 | | | | 503,334 | | | | — | | | | * | |
Four Jr. Investments LTD. | | | 200,000 | | | | 200,000 | | | | 400,000 | | | | 400,000 | | | | — | | | | * | |
Gary H. Weitz | | | 35,000 | | | | 35,000 | | | | 70,000 | | | | 70,000 | | | | — | | | | * | |
George Karfunkel | | | 200,000 | | | | 200,000 | | | | 400,000 | | | | 400,000 | | | | — | | | | * | |
Gerald & Lynnette Hannahs JTWROS | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Great American Insurance Company | | | 500,000 | | | | 500,000 | | | | 1,000,000 | | | | 1,000,000 | | | | — | | | | * | |
Great American Life Insurance Company | | | 1,000,000 | | | | 1,000,000 | | | | 2,000,000 | | | | 2,000,000 | | | | — | | | | * | |
Greg Waisanen | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Harry L. Shufflebarger Revocable Trust | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Harvey Schilowitz & Linda Schilowitz JTWROS | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Henry Baumgart | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Henry Rothman | | | 50,225 | | | | 65,225 | | | | 115,450 | | | | 115,450 | | | | — | | | | * | |
Howard K. Fuguet | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Hyman Belzberg | | | 87,500 | | | | 87,500 | | | | 175,000 | | | | 175,000 | | | | — | | | | * | |
Ian Stern | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Immotrend Inc. | | | 250,000 | | | | 250,000 | | | | 500,000 | | | | 500,000 | | | | — | | | | * | |
Irwin Lampert | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
James Calvin MacKenzie LLC | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
James Lawler & Ali Rafie | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Jason Willis & Amanda Willis | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Jay Eisen | | | 12,688 | | | | 25,188 | | | | 37,876 | | | | 37,876 | | | | — | | | | * | |
Jeff and Pam Littrell | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Jeffrey Tarrand | | | 5,000 | | | | 5,000 | | | | 10,000 | | | | 10,000 | | | | — | | | | * | |
JKW Family LTD | | | 225,000 | | | | 225,000 | | | | 450,000 | | | | 450,000 | | | | — | | | | * | |
Joanne B. Schubert | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Joel Kovacs | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
John Berding | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
John C. Boyer | | | 40,000 | | | | 40,000 | | | | 80,000 | | | | 80,000 | | | | — | | | | * | |
John C. Ramsay | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
John Campo | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
John E. Dell | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
John F. Neary | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
John Menna | | | 40,000 | | | | 40,000 | | | | 80,000 | | | | 80,000 | | | | — | | | | * | |
John Smith | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
John T. Winebrenner Trust | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Jonathan Ardrey | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Kathleen S. McHugh | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Keith Eisenstark & Mary Beth Walsh | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Kenneth S. Goodwin | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Lamar A. Gwaltney | | | 50,834 | | | | 100,834 | | | | 151,668 | | | | 151,668 | | | | — | | | | * | |
Lance Siegall | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Larry W. Schwartz | | | 30,000 | | | | 30,000 | | | | 60,000 | | | | 60,000 | | | | — | | | | * | |
Lawrence Grossbard | | | 75,000 | | | | 75,000 | | | | 150,000 | | | | 150,000 | | | | — | | | | * | |
Lee K. Barba | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Lester Petracca | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Lewis B. Cullman | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Lincoln Trust FBO Thomas C. Stephens IRA | | | 176,667 | | | | 201,667 | | | | 378,334 | | | | 303,334 | | | | 75,000 | | | | * | |
Lon E. Bell | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Loren & Vivian Kramer | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Louis A. & Brenda K. Romeo | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Mark F. Adams | | | 5,000 | | | | 5,000 | | | | 10,000 | | | | 10,000 | | | | — | | | | * | |
Mark Volkov | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Marvin Boehm Family Trust | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Mary Divett | | | 20,700 | | | | 15,000 | | | | 35,700 | | | | 30,000 | | | | 5,700 | | | | * | |
Mary L. Marcus-West Declaration of Trust | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Michael & Sophie Mannarino | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Michael Cohen | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Michael J. Garnick | | | 175,000 | | | | 175,000 | | | | 350,000 | | | | 350,000 | | | | — | | | | * | |
Michael J. Pierce | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Michael L. and Ann J. Hetzner | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Michael Leiter | | | 70,000 | | | | 70,000 | | | | 140,000 | | | | 140,000 | | | | — | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Michael Lerner | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Michael Stephens | | | 25,375 | | | | 50,375 | | | | 75,750 | | | | 75,750 | | | | — | | | | * | |
Michael T. Dolen | | | 428,296 | | | | 603,296 | | | | 1,031,592 | | | | 1,031,592 | | | | — | | | | * | |
Michael Willis | | | 60,000 | | | | 60,000 | | | | 120,000 | | | | 120,000 | | | | — | | | | * | |
Michael Willis and Sharon Willis JTWROS | | | 220,000 | | | | 220,000 | | | | 440,000 | | | | 440,000 | | | | — | | | | * | |
Michael Zimmerman | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Micro Pipe Fund I, LLC | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Mitchell Lampert | | | 40,000 | | | | 40,000 | | | | 80,000 | | | | 80,000 | | | | — | | | | * | |
Mondas Investments Ltd. | | | 101,500 | | | | 201,500 | | | | 303,000 | | | | 303,000 | | | | — | | | | * | |
Montague Capital LP | | | 785,000 | | | | 785,000 | | | | 1,570,000 | | | | 1,570,000 | | | | — | | | | * | |
New Century Holdings LLP | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
NYPPEX Holdings, LLC 401K Retirement Plan | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Paul L. Schumacher | | | 20,600 | | | | 20,600 | | | | 41,200 | | | | 41,200 | | | | — | | | | * | |
Peter C. Gould | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Peter Einstein | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Peter P. Parthenis Trust | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Peter Sabo | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Philip Berman & Ingrid Berman JTWROS | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Philip M. Cannella | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Pierre Charpie | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
ProActive Capital Resources Group LLC | | | 25,375 | | | | 50,375 | | | | 75,750 | | | | 75,750 | | | | — | | | | * | |
QIP Holdings LLC | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Ralph L. Pawlick | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Ramos—Lujan Investment Group Corp. | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Raymond James Cust FBO Bruce Ferguson IRA | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Raymond Vollintine | | | 400,000 | | | | 400,000 | | | | 800,000 | | | | 800,000 | | | | — | | | | * | |
RBC Capital Markets Cust FBO Laurence G. Allen IRA | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Renald J. & Catherine C. Anelle JTWROS | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Richard Lieberman | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Richard M Spitalny | | | 5,000 | | | | 5,000 | | | | 10,000 | | | | 10,000 | | | | — | | | | * | |
Richard Neustadter | | | 200,000 | | | | 200,000 | | | | 400,000 | | | | 400,000 | | | | — | | | | * | |
Richard Todd Gross | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
RL Vollintine Construction, Inc. | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Robert D. Burke | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Robert D. deRose and Susan deRose Family Trust | | | 101,884 | | | | 201,884 | | | | 303,768 | | | | 303,768 | | | | — | | | | * | |
Robert G. Mulchrone Trust | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Robert Harris | | | 10,000 | | | | 10,000 | | | | 20,000 | | | | 20,000 | | | | — | | | | * | |
Robert L. Montgomery | | | 50,750 | | | | 100,750 | | | | 151,500 | | | | 151,500 | | | | — | | | | * | |
Robert M. Newsome | | | 250,000 | | | | 250,000 | | | | 500,000 | | | | 500,000 | | | | — | | | | * | |
Robyn Schreiber Irrevocable Trust, Warren Schreiber TTEE | | | 63,500 | | | | 63,500 | | | | 127,000 | | | | 127,000 | | | | — | | | | * | |
Ron Eller & Beth Eller JTWROS | | | 15,000 | | | | 15,000 | | | | 30,000 | | | | 30,000 | | | | — | | | | * | |
Royal Palm Investors, LLC | | | 101,884 | | | | 201,884 | | | | 303,768 | | | | 303,768 | | | | — | | | | * | |
RRC Bio Fund LP | | | 300,000 | | | | 300,000 | | | | 600,000 | | | | 600,000 | | | | — | | | | * | |
Ryan Modesto | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
S. Kent Adams | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Samuel Belzberg | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
San Diego Psychiatric Medical Group Inc. Combination Retirement Trust | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
Scott Anderson | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
ST Organovo LLC | | | 310,000 | | | | 310,000 | | | | 620,000 | | | | 620,000 | | | | — | | | | * | |
Stacy Paros Parthenis | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Stan Alex Miroshnik | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Stan Noah | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Stephen A. de Kanter | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Steve M. Payne | | | 200,000 | | | | 200,000 | | | | 400,000 | | | | 400,000 | | | | — | | | | * | |
Terence Oi | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
The Carnahan Trust | | | 200,000 | | | | 200,000 | | | | 400,000 | | | | 400,000 | | | | — | | | | * | |
Thomas G. Wales | | | 33,500 | | | | 33,500 | | | | 67,000 | | | | 67,000 | | | | — | | | | * | |
Tom Stephens | | | 50,750 | | | | 100,750 | | | | 151,500 | | | | 151,500 | | | | — | | | | * | |
UHURU Capital LLC | | | 100,000 | | | | 100,000 | | | | 200,000 | | | | 200,000 | | | | — | | | | * | |
Univest Management Inc. EPSP | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Vantage FBO Laurence E Lof Roth IRA | | | 50,000 | | | | 50,000 | | | | 100,000 | | | | 100,000 | | | | — | | | | * | |
Vekoe Partners, LLC | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
W. Ron Raecker | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
Wendy S. Flath Revocable Living Trust July 27, 2010 | | | 25,417 | | | | 50,417 | | | | 75,834 | | | | 75,834 | | | | — | | | | * | |
White Rock Capital Partners, LP | | | 500,000 | | | | 500,000 | | | | 1,000,000 | | | | 1,000,000 | | | | — | | | | * | |
William Belzberg Revocable Living Trust | | | 87,500 | | | | 87,500 | | | | 175,000 | | | | 175,000 | | | | — | | | | * | |
William C. Purdon & Debra B. Purdon JTWROS | | | 25,000 | | | | 25,000 | | | | 50,000 | | | | 50,000 | | | | — | | | | * | |
William N. Strawbridge | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
William Nowlin | | | 5,000 | | | | 5,000 | | | | 10,000 | | | | 10,000 | | | | — | | | | * | |
William P. Hogan | | | 20,000 | | | | 20,000 | | | | 40,000 | | | | 40,000 | | | | — | | | | * | |
William R. Lefever | | | 150,000 | | | | 150,000 | | | | 300,000 | | | | 300,000 | | | | — | | | | * | |
Total | | | 15,328,687 | | | | 16,747,987 | | | | 32,076,674 | | | | 31,995,974 | | | | 80,700 | | | | * | |
(1) | Includes shares of common stock issuable upon the exercise of warrants, and is adjusted to reflect the sale of shares pursuant to this offering. |
PLAN OF DISTRIBUTION
The selling security holders, which as used herein includes donees, pledgees, transferees, or other successors-in-interest selling shares of common stock or interests in shares of common stock received after the date of this prospectus from a selling security holder as a gift, pledge, partnership distribution, or other transfer, may, from time to time, sell, transfer, or otherwise dispose of any or all of their shares of common stock or interests in shares of common stock on any stock exchange, market, or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
If any of the selling security holders are deemed an “underwriter” within the meaning of Section 2(11) of the Securities Act in connection with the resale of our securities under this prospectus, any commissions received by such selling security holders and any profit on the resale of the shares of our common stock (including the shares of common stock issuable upon the exercise of the warrants) sold by such security holders while acting as principals will be deemed to be underwriting discounts or commissions. Because it will have been deemed to be an underwriter within the meaning of Section 2(11) of the Securities Act, such selling security holders will be subject to prospectus delivery requirements under the Securities Act.
The selling security holders may use any one or more of the following methods when disposing of shares or interests therein:
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
an exchange distribution in accordance with the rules of the applicable exchange;
privately negotiated transactions;
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
broker-dealers may agree with the selling security holders to sell a specified number of such shares at a stipulated price per share;
a combination of any such methods of sale; and
any other method permitted pursuant to applicable law.
The selling security holders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling security holders to include the pledgee, transferee or other successors in interest as selling security holders under this prospectus. The selling security holders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees, or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of our common stock or interests therein, the selling security holders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling security holders may also sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling security holders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities that require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the selling security holders from the sale of the common stock offered by them will be the purchase price of the common stock less discounts or commissions, if any. Each of the selling security holders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any of the proceeds from this offering. Upon any exercise of the warrants by payment of cash, however, we will receive the exercise price of the warrants.
The selling security holders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule.
To the extent required, the shares of our common stock to be sold, the names of the selling security holders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the selling security holders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the selling security holders and their affiliates. In addition, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling security holders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The selling security holders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the selling security holders against liabilities, including liabilities under the Securities Act and state securities laws, relating to the registration of the shares offered by this prospectus.
We have agreed to use reasonable efforts to maintain the effectiveness of this registration statement until the earlier of (i) the one year anniversary of the date the registration statement of which this prospectus forms a part is declared effective by the SEC or (ii) until Rule 144 of the Securities Act is available to the selling security holders with respect to all of their shares.
Penny Stock Regulations
You should note that our stock is a penny stock. The SEC has adopted Rule 15g-9, which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny-stock rules, which impose additional sales-practice requirements on broker-dealers that sell to persons other than established customers and “accredited investors.” The term “accredited investor” refers generally to institutions with assets in excess of $5,000,000 or individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly with their spouse. The penny-stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized-risk disclosure document in a form prepared by the SEC that provides information about penny stocks and the nature and level of risks in the penny-stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny-stock rules require that, prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny-stock rules. Consequently, these penny-stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny-stock rules discourage investor interest in and limit the marketability of our common stock.
Blue Sky Restrictions on Resale
If a selling security holder wants to sell shares of our common stock under this prospectus in the United States, the selling security holders will also need to comply with state securities laws, also known as “Blue Sky laws,” with regard to secondary sales. As a result, holders may not resell their shares of common stock in the United States without satisfying the applicable state securities law or qualifying for an exemption therefrom, including the exemptions provided under the U.S. National Securities Markets Improvement Act of 1996. The broker for a selling security holder will be able to advise a selling security holder as to which states our common stock is exempt from registration with that state for secondary sales.
Any person who purchases shares of our common stock from a selling security holder under this prospectus who then wants to sell such shares will also have to comply with Blue Sky laws regarding secondary sales. These restrictions and potential costs could be significant burdens to our stockholders seeking to effect resales of our common stock within the United States.
DESCRIPTION OF SECURITIES
Authorized Capital Stock
As of March 31, 2012, our authorized capital stock consisted of 150,000,000 shares of Common Stock, par value $0.001 per share, and 25,000,000 shares of preferred stock, par value $0.001 per share.
Issued and Outstanding Capital Stock
As of March 31, 2012, the following securities were issued and outstanding:
43,693,241 shares of common stock;
No shares of preferred stock;
Options to purchase 896,256 shares of common stock granted under our equity incentive plans; and
Warrants to purchase 24,256,932 shares of common stock exercisable at a price of $1.00 per share.
Description of Common Stock
The holders of Common Stock are entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of Common Stock that are present in person or represented by proxy. Except as otherwise provided by law, amendments to the certificate of incorporation generally must be approved by a majority of the votes entitled to be cast by all outstanding shares of Common Stock. The certificate of incorporation does not provide for cumulative voting in the election of directors. The Common Stock holders will be entitled to such cash dividends as may be declared from time to time by the Board from funds available. Upon our liquidation, dissolution or winding up, the Common Stock holders will be entitled to receive pro rata all assets available for distribution to such holders.
Description of Preferred Stock
Our Preferred Stock, par value $0.001 per share, may be issued from time to time in one or more series pursuant to a resolution or resolutions providing for such issue duly adopted by our Board of Directors (authority to do so being hereby expressly vested in the Board of Directors). The Board of Directors is further authorized, subject to limitations prescribed by law, to fix by resolution or resolutions the designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, of any wholly unissued series of Preferred Stock, including without limitation authority to fix by resolution or resolutions the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices, and liquidation preferences of any such series, and the number of shares constituting any such series and the designation thereof, or any of the foregoing.
Registration Rights Agreement
We are required to file within 90 days of the date of the final Closing of the Offering, a registration statement registering for resale all shares of common stock issued in the Offering, including common stock (i) included in the Units; and (ii) issuable upon exercise of the warrants included in the Units; consistent with the terms and provisions of the Registration Rights Agreement. A form of the Registration Rights Agreement is filed as Exhibit 10.5 to the registration statement of which this prospectus forms a part. The holders of any registrable securities removed from the registration statement a result of a Rule 415 or other comment from the SEC shall have “piggyback” registration rights for the shares of common stock or common stock underlying such warrants with respect to any registration statement filed by us following the effectiveness of the registration statement which would permit the inclusion of these shares. We have agreed to use reasonable efforts to have the registration statement of which this prospectus forms a part declared effective within 180 days of filing.
If the registration statement is not filed on or before the filing deadline or not declared effective on or before the effectiveness deadline, we shall pay to each holder of registrable securities an amount in cash equal to one-half of one percent (0.5%) of such holder’s investment in the Offering or in the Bridge Notes on every thirty (30) day anniversary of such filing deadline or effectiveness deadline failure until such failure is cured. The payment amount shall be prorated for partial thirty (30) day periods. The maximum aggregate amount of payments to be made by us as the result of such failures, whether by reason of a filing deadline failure, effectiveness deadline failure or any combination thereof, shall be an amount equal to 6% of each holder’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the holder’s registrable securities may be sold by such holder under Rule 144 or pursuant to another exemption from registration. Moreover, no such payments shall be due and payable with respect to any registrable securities we are unable to register due to limits imposed by the SEC’s interpretation of Rule 415 under the Securities Act.
We have agreed to use reasonable efforts to maintain the effectiveness of this registration statement until the earlier of (i) the one year anniversary of the date the registration statement of which this prospectus forms a part is declared effective by the SEC or (ii) until Rule 144 of the Securities Act is available to the selling security holders with respect to all of their shares.
Anti-Takeover Effects of Provisions of Delaware State Law
Anti-takeover provisions in our certificate of incorporationorganizational documents and Delaware law could make an acquisition more difficult and could prevent attempts by our stockholders to remove or replace current management.
Anti-takeover provisions of Delaware law and in our certificate of incorporation and our bylaws may discourage delay or prevent a change inof control, of our company, even if a change in controlan acquisition would be beneficial to our stockholders. In addition, these provisions may frustrate orstockholders, which could affect our stock price adversely and prevent any attempts by our stockholders to replace or remove our current managementmanagement.
Our Certificate of Incorporation, as amended (“Certificate of Incorporation”), and Amended and Restated Bylaws, as amended (“Bylaws”) contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions:
•authorize the issuance of preferred stock which can be created and issued by makingour board of directors without prior stockholder approval, with rights senior to those of the common stock;
•provide for a classified board of directors, with each director serving a staggered three-year term;
•provide that each director may be removed by the stockholders only for cause;
•prohibit our stockholders from filling board vacancies, calling special stockholder meetings, or taking action by written consent; and
•require advance written notice of stockholder proposals and director nominations.
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These and other provisions in our Certificate of Incorporation, Bylaws and Delaware law could make it more difficult for stockholders or potential acquirers to replace membersobtain control of our board of directors. In particular, underdirectors or initiate actions that are opposed by our certificatethen-current board of incorporationdirectors, including delaying or impeding a merger, tender offer, or proxy contest involving our company. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
Risks Related to Our Intellectual Property
If we are not able to adequately protect our proprietary rights, our business could be harmed.
Our success will depend to a significant extent on our ability to obtain patents and maintain adequate protection for our technologies, intellectual property and products and service offerings in the United States and other countries. If we do not protect our intellectual property adequately, competitors may be able to use our technologies and gain a competitive advantage.
To protect our products and technologies, we, and our collaborators and licensors, must prosecute and maintain existing patents, obtain new patents and pursue other intellectual property protection. Our existing patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies. Changes in either the patent laws or interpretations of patent laws in the United States and other countries may also affect the value of our licensed or owned intellectual property or create uncertainty. Moreover, the patent positions of many biotechnology and pharmaceutical companies are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, we cannot guarantee that:
•any patent applications filed by us will issue up to 25,000,000 shares of preferred stock withas patents;
•third parties will not challenge our proprietary rights, and privilegesif challenged that might be seniora court or an administrative board of a patent office will hold that our patents are valid and enforceable;
•third parties will not independently develop similar or alternative technologies or duplicate any of our technologies by inventing around our claims;
•any patents issued to us will cover our technology and products as ultimately developed;
•we will develop additional proprietary technologies that are patentable;
•the patents of others will not have an adverse effect on our business; or
•as issued patents expire, we will not lose some competitive advantage.
As previously disclosed, we have recommenced certain historical operations and are now focusing our future efforts on developing highly customized 3D human tissues as living, dynamic models for healthy and diseased human biology for drug development. Previously, we focused our efforts on developing our in vivo liver tissues to treat end-stage liver disease and a select group of life-threatening, orphan diseases, for which there were limited treatment options other than organ transplant. We also explored the development of other potential pipeline in vivo tissue constructs. As we focus our business on developing highly customized 3D human tissues, we may sell, discontinue, adjust or abandon certain patents and patent applications relating to our common stock,
historical operations. There can be no assurance that we will be successful at such efforts or sell or otherwise monetize such assets on acceptable terms, if at all. There is also no guarantee that our remaining patents will be sufficiently broad to prevent others from using our technologies or from developing competing products and technologies.We may not be able to protect our intellectual property rights throughout the world.
Certain foreign jurisdictions have an absolute requirement of novelty that renders any public disclosure of an invention immediately fatal to patentability in such jurisdictions. Therefore, there is a risk that we may not be able to protect some of our intellectual property in the United States or abroad due to disclosures, which we may not be aware of, by our collaborators or licensors. Some foreign jurisdictions prohibit certain types of patent claims, such as “method-of-treatment/use-type” claims; thus, the scope of protection available to us in such jurisdictions is limited.
Moreover, filing, prosecuting and defending patents on all of our potential products and technologies throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not sought or obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our future products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Patents covering our products could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. We may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office (the “USPTO”), or become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review (“IPR”), or interference proceedings or other similar proceedings challenging our patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we may have to participate in interference proceedings declared by the consentUSPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge our priority of invention or other features of patentability with respect to our patents and patent applications. Such challenges may result in loss of patent rights, in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the holderspatent protection of our technology or products. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
For example, our U.S. Patent Nos. 9,855,369 and 9,149,952, which relate to our bioprinter technology, were the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”), one of our competitors. Likewise, U.S. Patent Nos. 9,149,952, 9,855,369, 8,931,880, 9,227,339, 9,315,043 and 10,967,560 (all assigned to Organovo, Inc.) and U.S. Patent Nos. 7,051,654, 8,241,905, 8,852,932 and 9,752,116 (assigned to Clemson University and the University of Missouri, respectively) were implicated in a declaratory judgment complaint filed against Organovo, Inc., our wholly owned subsidiary, by BICO Group AB and certain of its subsidiaries in the United States District Court for the District of Delaware. All of these matters were eventually settled in February 2022.
Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the common stock. Moreover, without any further voteforegoing could have a material adverse effect on our business, financial condition or action onresults of operations. We may become involved in lawsuits to protect or enforce our inventions, patents or other intellectual property or the partpatents of the stockholders, the board of directors would have the authority to determine the price, rights, preferences, privileges,our licensors, which could be expensive and restrictions of the preferred stock. This preferred stock,time consuming.
In addition, if it is ever issued, may have preference over, and harm the rights of, the holders of common stock. Although the issuance of this preferred stock would provide us with flexibility in connection with possible acquisitions and other corporate purposes, this issuance may make it more difficult forwe initiate legal proceedings against a third party to acquireenforce a majoritypatent covering our products, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may also raise claims challenging the validity or enforceability of our outstanding voting stock. patents before administrative bodies in the United States or abroad, even outside the context of litigation, including through re-examination, post-grant review,IPR, interference proceedings, derivation proceedings and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of or amendment to our patents in such a way that they no longer cover our products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a third party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our products. Such a loss of patent protection would have a material adverse effect on our business, financial condition, and results of operations.
We may be involved in lawsuits or other proceedings to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our collaborators or licensors or our licensors may breach or otherwise prematurely terminate the provisions of our license agreements with them. To counter infringement or unauthorized use, we may be required to file infringement claims or lawsuits, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our collaborators or licensors is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our other patent applications at risk of not issuing. Additionally, our licensors may continue to retain certain rights to use technologies licensed by us for research purposes. Patent disputes can take years to resolve, can be very costly and can result in loss of rights, injunctions or substantial penalties. Moreover, patent disputes and related proceedings can distract management’s attention and interfere with running our business.
Furthermore, because of the potential for substantial discovery in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments which could harm our business.
As more companies file patents relating to bioprinters and bioprinted tissues, it is possible that patent claims relating to bioprinters or bioprinted human tissue may be asserted against us. In addition, the drug candidates we pursue may also be pursued by other companies, and it is possible that patent claims relating to such drug candidates may also be asserted against us. Any patent claims asserted against us could harm our business. Moreover, we may face claims from non-practicing entities, which have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation and diversion of resources, cause product shipment or delays or require us to enter into royalty or license agreements. These licenses may not be available on acceptable terms, or at all. Even if we are successful in defending such claims, infringement and other intellectual property litigation can be expensive and time-consuming to litigate and divert management’s attention from our core business. Any of these events could harm our business significantly.
Our current and future research, development and commercialization activities also must satisfy the obligations under our license agreements. Any disputes arising under our license agreements could be costly and distract our management from the conduct of our business. Moreover, premature termination of a license agreement could have an adverse impact on our business.
In addition to infringement claims against us, if third parties have prepared and filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the United States Patent and Trademark Office (“PTO”) to determine the priority of invention and opposition proceedings outside of the United States. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party.
Third parties may also attempt to initiate reexamination, post grant review or inter partes review of our patents or those of our collaborators or licensors in the PTO. We may also become involved in similar opposition proceedings in the European Patent Office or similar offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology.
*Changes in U.S. patent law or the patent law of other countries or jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity and is costly, time-consuming and inherently uncertain. For example, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act included a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and that may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application is typically entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO, and may become involved in post-grant proceedings, including opposition, derivation, reexamination, inter partes review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope or enforceability of, or invalidate, our patent rights, which could adversely affect our competitive position.
In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we might obtain in the future.
Similarly, changes in patent law and regulations in other countries or jurisdictions or changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our authorized but unissuedability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future. For example, the complexity and uncertainty of European patent laws have also increased in recent years. In Europe, in June 2023, a new unitary patent system was introduced, which will significantly impact European patents, including those granted before the introduction of the system. Under the unitary patent system, after a European patent is granted, the patent proprietor can request unitary effect, thereby getting a European patent with unitary Effect, or a Unitary Patent. Each Unitary Patent is subject to the jurisdiction of the Unitary Patent Court, or the UPC. As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty of any litigation. Patents granted before the implementation of the UPC will have the option of opting out of the jurisdiction of the UPC and remaining as national patents in the UPC countries. Patents that remain under the jurisdiction of the UPC may be potentially vulnerable to a single UPC-based revocation
challenge that, if successful, could invalidate the patent in all countries who are signatories to the UPC. We cannot predict with certainty the long-term effects of the new unitary patent system.
We depend on license agreements with University of Missouri, Clemson University and the Salk Institute for Biological Studies for rights to use certain patents, pending applications, and know how. Failure to comply with or maintain obligations under these agreements and any related or other termination of these agreements could materially harm our business and prevent us from developing or commercializing new product candidates.
We are party to license agreements with University of Missouri, Clemson University and the Salk Institute for Biological Studies under which we were granted exclusive rights to patents and patent applications that are important to our business and to our ability to develop and commercialize our 3D tissue products fabricated using our NovoGen Bioprinters and our FXR314 agonist in gastrointestinal disease. Our rights to use these patents and patent applications and employ the inventions claimed in these licensed patents are subject to the continuation of and our compliance with the terms of our license agreements. If we were to breach the terms of these license agreements and the agreements were terminated as a result, our ability to continue to develop and commercialize our NovoGen Bioprinters, 3D tissue products and the FXR314 agonist and to operate our business could be adversely impacted.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary and licensed technology and processes, we rely in part on confidentiality agreements with our corporate partners, employees, consultants, manufacturers, outside scientific collaborators and sponsored researchers and other advisors. These agreements may not effectively prevent disclosure of our confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We employ or engage individuals who were previously employed at other biopharmaceutical companies. Although we have no knowledge of any such claims against us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and even if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees. To date, none of our employees have been subject to such claims.
General Risk Factors
Compliance with the reporting requirements of federal securities laws can be expensive.
We are a public reporting company in the United States, and accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including the compliance obligations of the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley Act”). The costs of complying with the reporting requirements of the federal securities laws, including preparing and filing annual and quarterly reports and other information with the Securities and Exchange Commission (the “SEC”) and furnishing audited reports to stockholders, can be substantial.
If we fail to comply with the rules of Section 404 of the Sarbanes-Oxley Act related to accounting controls and procedures, or, if we discover material weaknesses and deficiencies in our internal control and accounting procedures, we may be subject to sanctions by regulatory authorities and our stock price could decline.
Section 404 of the Sarbanes-Oxley Act (“Section 404”) requires that we evaluate and determine the effectiveness of our internal control over financial reporting. We believe our system and process evaluation and testing comply with the management certification requirements of Section 404. We cannot be certain, however, that we will be able to satisfy the requirements in Section 404 in all future periods. If we are not able to continue to meet the requirements of Section 404 in a timely manner or with adequate compliance, we may be subject to sanctions or investigation by regulatory authorities, such as the SEC or Nasdaq. Any such action could adversely affect our financial results or investors’ confidence in us and could cause our stock price to fall. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we identify deficiencies in our internal controls that are deemed to be material weaknesses, we may be required to incur significant additional financial and management resources to achieve compliance.
Use of Proceeds
We estimate that we will receive net proceeds of approximately $[_________] million, after deducting the placement agent fees and estimated offering expenses payable by us (based on an assumed public offering price of $[___] per share, which was the last reported sales price of our common stock on the Nasdaq Capital Market on [________], 2024).
We intend to use the proceeds of this offering for working capital and general corporate purposes, which could include capital expenditures, research and development expenditures, regulatory affairs expenditures, clinical trial expenditures, legal expenditures, including intellectual property protection and maintenance expenditures, acquisitions of new technologies and investments, business combinations and the repurchase of capital stock. Pending these uses, we may invest the net proceeds in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the United States government.
However, because this is available for future issuance without stockholder approval.a best efforts offering and there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, the placement agent’s fees and net proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus.
Warrants
Set forth below is information concerning the various warrants issued by us toThis expected use of net proceeds from this offering represents our investors, placement agents, consultantsintentions based upon our current plans and other persons.
Warrants issued to investorsbusiness conditions, which could change in the Offering with closings on February 8, February 29future as our plans and March 16.
After the final closingbusiness conditions evolve. We cannot currently allocate specific percentages of the net proceeds to us from this offering that we may use for the purposes specified above. Our management will have broad discretion in the application of the net proceeds from this offering and could use them for purposes other than those contemplated at the time of this offering. Our stockholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not result in our being profitable or increase our market value. See “Risk Factors—Risks Related to this Offering there were warrants issuedand the Ownership of Our Common Shares—Our management has broad discretion as to purchase 15,247,987the use of the net proceeds from this offering.”
Capitalization
The following table sets forth our cash and cash equivalents and total capitalization as of December 31, 2023:
•a pro forma basis to reflect (i) the issuance of an aggregate of 237,721 shares of common stock held by investors purchasing Units inbetween January 1, 2024 and March 31, 2024 pursuant to the OfferingSales Agreement with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC (the “Investor Warrants”“Sales Agreement”). Each Investor Warrant entitles for net proceeds to us of approximately $0.3 million; and
•on an as adjusted basis, giving effect to the holder to purchase one shareissuance and sale of [__________] shares of common stock in this offering at the assumed public offering price of $[__________] per share, which is the last reported sale price of our common stock on the Nasdaq Capital Market on [__________], 2024, and after deducting placement agent fees and estimated offering expenses payable by us and assuming no sale of any pre-funded warrants in this offering.
The as adjusted information below is illustrative only, and our capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
You should read this information in conjunction with our financial statements and the related notes included elsewhere in this prospectus and in “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| | | | | | | | | |
| |
|
| | December 31, 2023 |
| | Actual (unaudited) | | Proforma | | Proforma as Adjusted |
| |
|
| | (In thousands, except share and per share data) |
Cash and cash equivalents | | $ | 5,295 | | $ | 5,571 | | $ | [ ] |
Stockholders' equity: | |
|
| |
|
| |
|
|
Common stock, par value $0.01 per share: 200,000,000 shares authorized, 9,838,755 shares issued and outstanding as of December 31, 2023, actual; 200,000,000 shares authorized,10,076,476 shares issued and outstanding, proforma; 200,000,000 shares authorized, [________] shares issued and outstanding, proforma as adjusted | | $ | 10 | | $ | 10 | | $ | [ ] |
Additional paid-in capital | |
| 342,796 | |
| 342,531 | |
| [ ] |
Accumulated deficit | |
| (336,624) | |
| (336,624) | |
| [ ] |
Accumulated other comprehensive loss | |
| 1 | |
| 1 | |
| [ ] |
Treasury stock, 46 shares at cost | |
| (1) | |
| (1) | |
| [ ] |
Total stockholders’ equity | |
| 6,182 | |
| 5,917 | |
| [ ] |
Total capitalization | | $ | 6,182 | | $ | 5,917 | | $ | [ ] |
The number of shares immediately outstanding following this offering is based on 9,838,755 shares of common stock outstanding as of December 31, 2023 and also gives effect to 237,712 shares of our common sold and issued through an “at the market offering”
pursuant to Sales Agreement and excludes:
•695,459 shares of common stock issuable upon the exercise of stock options outstanding at a purchaseweighted average exercise price of $1.00 duringapproximately $4.35 per share;
•123,892 shares of common stock issuable upon the five (5) year period commencing onvesting and settlement of outstanding restricted stock units;
•1,000 shares of common stock available for issuance pursuant to the 2021 Inducement Equity Plan;
•1,643,798 shares of common stock available for issuance pursuant to the 2022 Equity Incentive Plan; and
•45,000 shares of common stock available for issuance pursuant to the 2023 Employee Stock Purchase Plan.
Dilution
If you purchase our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share and the net tangible book value per share of our common stock after this offering.
As of December 31, 2023, we had net tangible book value of approximately $3.5 million, or $0.35 per share. Net tangible book value per share represents the amount of total tangible assets less total liabilities divided by the number of shares of our common stock outstanding.
Our pro forma net tangible book value as of December 31, 2023, before giving effect to this offering, was approximately $3.8 million, or $0.37 per share of our common stock. Pro forma net tangible book value, before the issuance and sale of shares of common stock in this offering, gives effect to the issuance of an aggregate of 237,721 shares of common stock between January 1, 2024 and March 31, 2024 pursuant to the Investor Warrants. We may callSales Agreement for net proceeds to us of approximately $0.3 million.
After giving effect to (i) the Investor Warrants at any timepro forma adjustments set forth above and (ii) the issuance and sale of the maximum of [______] shares of our common stock trades above $2.50 for twenty (20) consecutive days followingin this offering at an assumed public offering price of $[____] per share, the effectivenesslast reported sale price of our common stock on the registration statementNasdaq Capital Market on [_______], 2024, assuming no sale of whichpre-funded warrants in this prospectus forms a part covering the resale of the underlying Investor Warrant shares. The Investor Warrants can only be called if a registration statement registering the shares underlying the Investor Warrants is in effect at the time of the call.
The Investor Warrants, at the option of the holder, may be exercised by cash payment of the exercise price to us. The Investor Warrants may be exercised on a cashless basis commencing one yearoffering and after issuance if no registration statement registering the shares underlying the Investor Warrants is then in effect. Thededucting estimated placement agent fees and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of December 31, 2023 would have been approximately $[____] million, or approximately $[____] per share. This amount represents an immediate increase in pro forma net tangible book value of approximately $[____] per share to our existing stockholders and an immediate dilution in net tangible book value of approximately $[____] per share to new investors purchasing shares of common stock in this offering.
Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the Offering will receive a warrant solicitation fee equaloffering price per share paid by new investors. The following table illustrates this dilution:
| | | | | | | | |
|
|
|
|
|
|
|
|
|
Assumed public offering price per share |
|
|
|
|
| $ | [____] |
|
Net tangible book value per share as of December 31, 2023 |
| $ | 0.35 |
|
|
|
|
|
Increase in net tangible book value per share attributable to the issuance of 237,721 shares of common stock after December 31, 2023 pursuant to the Sales Agreement between January 1, 2024 and March 31, 2024 |
| $ | 0.02 |
|
|
|
|
|
Pro forma net tangible book value per share as of December 31, 2023 |
| $ | 0.37 |
|
|
|
|
|
Increase in pro forma net tangible book value per share attributable to this offering |
| $ | [____] |
|
|
|
|
|
Pro forma as adjusted net tangible book value per share, after giving effect to this offering |
|
|
|
|
| $ | [____] |
|
Dilution per share to investors purchasing shares in this offering |
|
|
|
|
| $ | [____] |
|
* Per share numbers may not add due to 5% ofrounding
The dilution information discussed above is illustrative only and may change based on the funds solicited byactual public offering price, the placement agent upon exercise of the Investor Warrants if we elect to call the Investor Warrants. The exercise price andactual number of shares of common stock issuable on exercisewe offer in this offering and other terms of the Investor Warrants may be adjusted in certain circumstances including a weighted average adjustmentthis offering determined at pricing.
A $0.50 increase or decrease in the eventassumed public offering price of future issuances$[___] per share of common stock, based on the last reported sale price for our common stock as reported on the Nasdaq Capital Market on [___], 2024, would decrease the number of shares of our equity securities at a price less thancommon stock offered in this offering by approximately [__] million shares or increase the exercise pricenumber of the Investor Warrant,shares of our common stock offered in the event of a stock dividend,this offering by approximately [__] million shares.
We may also increase or our recapitalization, reorganization, merger or consolidation.
No fractional shares will be issued upon exercise of the Investor Warrants. If, upon exercise of the Investor Warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round up to the nearest whole number,decrease the number of shares of common stock to be issued towe are offering. An increase of 100,000 in the Investor Warrant holder.
Simultaneous with the Initial Closingnumber of the Offering, former warrant holders and a former noteholder of Organovo were issued warrants to purchase an aggregate of 1,409,750 shares of common stock. These warrants are similar to the Investor Warrants, except that they do not have a call provisionstock offered by us would increase our as adjusted net tangible book value by approximately $[__] million, or registration rights.
Warrants issued in exchange for the warrants issued in connection with the Company’s bridge financing completed in October and November 2012 (the “Bridge Financing”).
There are 1,500,000 warrants outstanding (the “New Bridge Warrants”), all of which were issued at the Initial Closing of the Offering in exchange for the warrants issued in connection with the Bridge Financing (the “Bridge Warrants”). The New Bridge Warrants, which are exercisable at a price of $1.00$[__] per share, for a five year period, are substantially similarand decrease the dilution per share to investors participating in this offering by $[__] per share, assuming the Investor Warrants. Holders of the New Bridge Warrants receivedassumed offering price per share remains the same registration rights with respectand after deducting the estimated placement agent commissions and estimated offering expenses payable by us. Similarly, a decrease of 100,000 in the number of shares of common stock offered by us would decrease our as adjusted net tangible book value by approximately $[__] million or $[__] per share, and increase the dilution per share to investors participating in this offering by $[__] per share, assuming the assumed offering price per share remains the same and after deducting the estimated placement agent commissions and estimated offering expenses payable by us.
The number of shares immediately outstanding following this offering is based on 9,838,755 shares of common stock outstanding as of December 31, 2023 and also gives effect to 237,712 shares of our common sold and issued through an “at the market offering”
pursuant to Sales Agreement and excludes:
•695,459 shares of common stock issuable upon
the exercise of
the New Bridge Warrants as the investors in the Offering.Warrants issued to the placement agent in connection with the Bridge Financing and Offering.
The warrants issued to Spencer Trask Ventures, Inc., our placement agent in the Offering, permit the placement agent or its designees, to purchase forstock options outstanding at a five-year period, 5,489,040weighted average exercise price of approximately $4.35 per share;
•123,892 shares of common stock at an exercise priceissuable upon the vesting and settlement of $1.00 per share (the “Placement Agent Warrants”). Additionally, as compensation for the Bridge Financing, the placement agent was issued Organovo warrants that were subsequently exchanged for Placement Agent Warrants to purchase 610,155outstanding restricted stock units;
•1,000 shares of common stock at an exercise priceavailable for issuance pursuant to the 2021 Inducement Equity Plan;
•1,643,798 shares of $1.00 per share. The Placement Agent Warrantscommon stock available for issuance pursuant to the 2022 Equity Incentive Plan; and
•45,000 shares of common stock available for issuance pursuant to the 2023 Employee Stock Purchase Plan.
To the extent options, restricted units or warrants are additionally exercised or settled or other shares are issued, there may be further dilution to investors. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have no registration rightssufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
Business
Overview
Organovo Holdings, Inc. (“Organovo Holdings,” “we,” “us,” “our,” the “Company” and contain weighted average anti-dilution“our Company”) is a clinical stage biotechnology company thatis focused on developing FXR314 in inflammatory bowel disease (“IBD”), including ulcerative colitis (“UC”), based on demonstration of clinical promise in three-dimensional (“3D”) human tissues as well as strong preclinical data. FXR is a mediator of gastrointestinal and immediate cashless exercise provisions.
liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. FXR314 is the lead compound in our established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in UC.DESCRIPTION OF BUSINESS
OverviewOur current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). We plan to start a Phase 2a clinical trial in UC in the calendar year 2024. We are exploring the potential for combination therapies using FXR314 and currently approved mechanisms in preclinical animal studies and our IBD disease models.
Our second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. We have developed and are commercializing a platformuse our proprietary technology for the generation of three-dimensional (3D)to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. We believe these attributes can enable critical complex, multicellular disease models that can be employedused to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, we are initially focusing on the intestine and have ongoing 3D tissue development efforts in drug discoveryhuman tissue models of UC and development, biological research,CD. We use these models to identify new molecular targets responsible for driving the disease and as therapeutic implants forto explore the treatmentmechanism of damaged or degenerating tissuesaction of known drugs including FXR314 and organs.related molecules. We intend to introduce a paradigm shiftinitiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Our current understanding of intestinal tissue models and IBD disease models leads us to believe that we can create models that provide greater insight into the biology of these diseases than are generally currently available. We are creating high fidelity disease models, leveraging our prior work including the work found in our peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Our advances include cell type-specific compartments, prevalent intercellular tight junctions, and the approachformation of microvascular structures.
Using these disease models, we intend to identify and validate novel therapeutic targets. After finding therapeutic drug targets, we intend to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the generationdisease, and advance these novel drug candidates towards an Investigational New Drug (“IND”) filing and potential future clinical trials.
We expect to broaden our work into additional therapeutic areas over time and are currently exploring specific tissues for development. In our work to identify the areas of three-dimensional human tissues, by creation of constructs ininterest, we evaluate areas that might be better served with 3D that havedisease models than currently available models as well as the potential commercial opportunity. In line with these plans, we are building upon both our external and in house scientific expertise, which will be essential to replicate native human biology. We can improve on previous technologies by moving away from monolayer 2D cell cultures and by enabling all or partour drug development effort.
In February 2024, we formed our Mosaic Cell Sciences division (“Mosaic”) to serve as a key source of certain of the tissuesprimary human cells we create to be constructed solely of cells.utilize in our research and development efforts. We believe Mosaic can help us optimize our expertisesupply chain, reduce operating expenses related to cell sourcing and procurement and ensure that the cellular raw materials we use are of the highest quality and are derived from tissues that are ethically sourced in printing small-diameter, fully cellular human blood vessels in vitro provides a strong foundation upon which other tissues can be built to replicate human biologyfull compliance with state and human disease.federal guidelines. We believe that our broad and exclusive commercial rights to patented and patent-pending 3D bioprinting technology, combined with strengths in engineering and biology, put us in an ideal positionintend for Mosaic to provide a wide array of productsus with qualified human cells for use in our clinical research drug discovery and regenerative medicine therapies.development programs. In addition to supplying us with primary human cells, we intend for Mosaic to offer human cells for sale to life science customers, both directly and through distribution partners, which we expect to offset costs and over time become a profit center that offsets overall R&D spending by Organovo.
Our foundationalPlatform Technology
Our 3D human tissue platform is multifaceted. We approach each tissue agnostic to specific technologies, and intend to apply the best 3D technology to a given disease. We are developing novel disease models using high throughput systems, bioprinted and flow/stretch capable 3D systems as appropriate. Our proprietary technology derivesNovoGen Bioprinters® and related technologies for preparing bio-inks and bioprinting multicellular tissues with complex architecture are grounded in over a decade of peer-reviewed scientific publications, deriving originally from research led by Dr. Gabor Forgacs, one of our founders and a former George H. Vineyard Professor of BiophysicsBiological Physics at the University of Missouri.Missouri-Columbia (“MU”). We have a broad portfolio of intellectual property rights covering the principles, enabling instrumentation, applications, tissue constructs and methods of cell basedcell-based printing, including exclusive
licenses to certain patented and patent pending technologies from the University of Missouri-ColumbiaMU and Clemson University, and outright ownership of six pending patent applications (theUniversity. We own or exclusively license more than 100 patents and patent rights described in this paragraph are sometimes collectively referred to as the “Intellectual Property Rights”). See “—Intellectual Property “.pending applications worldwide covering specific tissue designs, uses, and methods of manufacture.
We believe that our portfolio of Intellectual Property Rights provides a strong and defensible market position for the commercialization of 3D bioprinting technology.
We believe we have the potential to build and maintain a sustainable business by leveraging our core technology platform across a variety of applications. As part of our business strategy we intend to pursue collaboration agreements with drug development companies that will allow us to further develop our 3D bioprinting technology and the potential uses of the cellular structures and tissues that can be produced with our technology. We also plan to develop research products with our 3D bioprinting technology that can be offered to third parties involved in drug discovery. We currently have collaborative research agreements currently in effect with Pfizer, Inc. (“ Pfizer “) and United Therapeutic Corporation (“ Unither “). We have also secured five federal grants in the aggregate amount of approximately $955,000 including Small Business Research Innovation grants and developed the NovoGen MMX Bioprinter™ (our first-generation 3D bioprinter) – within two and one half years of opening our first facilities. We believe these corporate achievements provide strong validation for the commercial viability of our technology.
As of March 31, 2012, we had devoted substantially all of our efforts to product development, raising capital and building infrastructure. We did not, as of that date, realize significant revenues from our planned principal operations. Accordingly, we are considered to be in the development stage.
The Technology
Our technology is centered around a core 3D bioprinting method, represented by our bioprinting instrument, the NovoGen MMX Bioprinter™. The 3D bioprinting technology enables a wide array of tissue compositions and architectures to be created, using combinations of cellular ‘bio-ink’ (building blocks comprised solely of cells), hydrogel (building blocks comprised of biocompatible gels), or hybrid ‘bio-ink’ (building blocks comprised of a mixture of cells and material such as hydrogel). A key distinguishing feature of our bioprinting platform is the ability to generate three-dimensional constructs that have all or some of their components comprised entirely of cells. The fully-cellular feature of our technology enables architecturally- and compositionally-defined 3D human tissues to be generated for in vitro use in drug discovery and development to potentially replicate the functional biology of a solid, fully cellular tissue. Furthermore, fully cellular constructs may offer specific advantages for regenerative medicine applications where bioactive cells are required and three-dimensional configuration is necessary, such as augmenting or replacing functional mass in tissues and organs that have sustained acute or chronic damage.
We plan to develop research products with our 3D bioprinting technology that can be offered to third parties involved in drug discovery. We intend to deliver the following products to the market:
Three-dimensional models of human tissue for utilization in traditional absorption, distribution, metabolism, excretion (ADME) / toxicology (TOX) / and drug metabolism and pharmacokinetics (DMPK) testing in drug development.
Specific models of human biology or pathophysiology, in the form of three-dimensional human tissues, and for use in drug discovery, development, and delivery.
Three-dimensional human tissues for use as therapeutic regenerative medicine products, such as blood vessels for bypass grafting, nerve grafts for nerve damage repair and cardiac patches for treatment of heart disease.
3D bioprinters for use in medical research.
A portfolio of consumables for use in 3D bioprinting.
As part of our business strategy we intend to pursue collaboration agreements with drug development companies that will allow us to further develop our 3D bioprinting technology and the potential uses of the cellular structures and tissues that can be produced with our technology. We currently have a collaborative research agreement with Pfizer to develop specific three-dimensional tissue models. We are engaged in the development of specific 3D human tissues to aid Pfizer in discovery of successful therapies in two areas of interest. In addition, in October 2011, we entered into a research agreement with Unither to establish and conduct a research program to discover treatments for pulmonary hypertension using our NovoGen MMX Bioprinter™ technology.
Market Opportunity
We believe that our bioprinting technology is uniquely positioned to provide three-dimensional human tissues for use in drug discovery and development as well as a broad array of tissues suitable for therapeutic use in regenerative medicine applications. While there are rapid-prototyping printers currently available that build three-dimensional structures out of polymers (often used for prototyping of plastic parts for tools or devices), these instruments are not specifically designed or intended for use with purely cellular inks in building biologic tissues and we do not believe that the firms working on these instruments have the required biology expertise to create tissues using these instruments at this time. There are multiple markets addressable by our technology platform:
1) | Specialized Models for Drug Discovery and Development: The NovoGen MMX Bioprinter™ can produce highly specialized three-dimensional human tissues that can be utilized to model a specific tissue physiology or pathophysiology. Our bioprinting technology has demonstrated the ability to create human blood vessel constructs, and to create fully human tissue containing capillary structures. These capabilities are anticipated to broaden the scope and scale of 3D tissues that can be generated, and to facilitate the development of disease models in such areas as cardiovascular disease, oncology, and fibrosis. |
2) | Biological Research Tools: Absorption, distribution, metabolism, excretion (ADME) testing is used to determine which factors enhance or inhibit how a potential drug compound reaches the blood stream. Distribution of a compound can be affected by binding to plasma proteins; age, genetics, and other factors can influence metabolism of a compound; and the presence of certain disease states can have effects on excretion of a compound. Many companies perform ADME studies utilizing various cell-based assays or automated bioanalytical techniques. Drug metabolism and pharmacokinetics (DMPK) testing is a subset of ADME. Determining the DMPK properties of a drug helps the drug developer to understand its safety and efficacy. Toxicology (TOX) testing is a further requirement to determine the detrimental effects of a particular drug on specific tissues. We believe that the NovoGen MMX Bioprinter™ is positioned to deliver highly differentiated products for use in traditional cell-based ADME / TOX / DMPK studies. Products in this arena may replace or complement traditional cell-based assays that typically employ primary hepatocytes, intestinal cell lines, renal epithelial cells and cell lines grown in a traditional two-dimensional format. Importantly, the combination of tissue-like three-dimensionality and human cellular components is believed to provide an advantage over non-human animal systems toward predicting in vivo human outcomes. |
3) | Regenerative Medicine: The field of regenerative medicine is advancing via multiple strategic approaches in development and practice, including cell therapies and scaffold-based products (+/- cells). The architectural precision and flexibility of our technology may facilitate the optimization, development, and clinical use of three-dimensional tissue constructs. Importantly, our technology offers a next-generation strategy whereby three-dimensional structures can be generated without the use of scaffolding or biomaterial components. The ultimate goal is to enable fully cellular constructs to be generated in a configuration compatible with surgical modes of delivery, thereby enabling restoration of significant functional mass to a damaged tissue or organ. |
We believe that our technology can capitalize, via strategic partnerships, on additional market opportunities in the provision of enabling tools for drug discovery and development as well as the discovery and development of therapeutic implants that augment or replace damaged tissues and organs. There are multiple short- and long-term revenue opportunities for us in these areas, including direct sales of 3D human tissue constructs for drug screening and development, licensing fees for commercial access to our technology, and royalties from product enablement, particularly in the area of therapeutic products for regenerative medicine.
Background on Bioprinting
The formation of ‘bio-ink’ — the cell-based building blocks that can be dispensed by our bioprinter — relies on the demonstrated principle that groups of individual cells will self-assemble to generate aggregates, through the actions of cell surface proteins that bind to each other and form junctions between cells. Furthermore, if two or more compatible self-assembled aggregates are placed in close proximity, under the proper conditions they will fuse to generate larger, more complex structures via physical properties analogous to those that drive fusion of liquid droplets. The concept of tissue liquidity originated in studies of developmental biology, where it was noted that developing tissues have liquid-like properties that enable individual cellular components to pattern each other, migrate, organize, and differentiate. As development progresses, tissues transition from a dynamic viscous liquid state to a more static semi-solid state, largely driven by the compartmentalized organization of cellular components and production within the organized tissue of extracellular matrix proteins that provide the mature tissue with the biomechanical properties required for tissue-specific function.
Figure 1 demonstrates self-assembly and tissue liquidity using cellular aggregates generated from developing chicken heart tissue, showing that two adjacent aggregates will fuse over time and generate a larger cellular structure. This basic behavior can be leveraged to form more complex structures whereby aggregates are arranged in a specific geometry that can recapitulate shapes and architectures commonly found in tissues and organs, including tubes and multi-layered structures.
Figure 2 shows that the phenomenon of aggregate fusion in embryonic tissue can be extended to adult-derived cultured mammalian cells, as demonstrated by the fusion of adult hamster ovary epithelial cell aggregates to form toroid (ring) structures when placed into that geometry and held for about 120 hours.
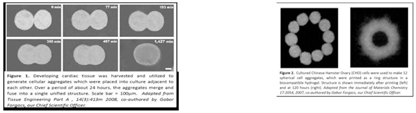
The NovoGen MMX Bioprinter™Bioprinter® Platform
Our NovoGen MMX Bioprinter™ is anBioprinters® are automated devicedevices that enablesenable the fabrication of three-dimensional (3D)3D living tissues comprised of mammalian cells. A custom graphic user interface (GUI)(“GUI”) facilitates the 3D design and execution of scripts that direct precision movement of themultiple dispensing heads to deposit defined cellular building blocks (‘bio-ink’)called bio-ink. Bio-ink can be formulated as a 100% cellular composition or supporting hydrogel. The unit fits easily intoas a standard biosafety cabinet, eliminatingmixture of cells and other matter (hydrogels, particles). Our NovoGen Bioprinters® can also dispense pure hydrogel formulations, provided the needphysical properties of the hydrogel are compatible with the dispensing parameters. Most typically, hydrogels are deployed to purchase ancillary equipmentcreate void spaces within specific locations in a 3D tissue or make facility modifications to maintain sterilityaid in the deposition of bioprinted tissues duringspecific cell types. We are able to employ a wide variety of proprietary cell- and hydrogel-based bio-inks in the printing process. The speedfabrication of tissues. Our NovoGen Bioprinters® also serve as important components of our tissue prototyping and precision of this instrument enables the production ofmanufacturing platform, as they are able to rapidly and precisely fabricate intricate small-scale tissue models for drug discoveryin vitro use as well as various drug absorption and toxicology assays. The NovoGen MMX Bioprinter™ (Figure 3) went from in-licensing and initial design to commercial production larger-scale tissues suitable for in less than two years.
vivo use.We are currently using a third party manufacturer, Invetech Pty.,Generation of Melbourne, Australia, to manufacture our NovoGen MMX Bioprinter. Under our manufacturing and supply agreement with Invetech, Invetech has agreed to manufacture our bioprinters for a certain budgeted cost, which cost decreases as we increasebio-ink comprising human cells is the number of bioprinters manufactured. Either party can terminate the manufacturing and supply agreement at any time. Although Invetech is currently a sole source manufacturer for our bioprinters, we believe we can locate a number of other third party manufacturers with the requisite expertise to manufacturer our bioprinters without significant delays or costs should Invetech elect to terminate their agreement with us.

The first step in bioprinting is preparation of the bio-ink aggregates, which are typically generated in spherical or cylindrical format. Bio-ink can be generated from aour standard bioprinting. A wide variety of cell types,cells and cell-laden hydrogels can be formulated into bio-ink and bioprinted tissues, including cell lines, primary cells, stromaland stem/progenitor cells. The majority of tissue designs employ two or more distinct varieties of bio-ink, usually comprised of cells epithelial cells, endothelialthat represent distinct compartments within a target tissue. For example, a 3D liver tissue might consist of two to three distinct bio-inks that are each made from a single cell type, a combination of cell types, and/or a combination of primary cells and progenitor cells. Bio-ink production begins with the creation of a thick ‘cell paste’ comprised of a slurry of cells and containing any other components required to be part of the final tissue composition. The cell paste is into spherical aggregates, cylindrical bio ink,one or another building block form. After a maturation period the bio-ink is loaded into the bioprinter, which then dispenses the building blocks in the geometry specified by the user, with amore bio-inert hydrogel servinghydrogels that serve as a physical supportsupports for the bioprinted tissue as well as occupying anyduring its maturation period, or to transiently occupy negative space includedspaces in thea tissue design.
TheResearch Collaborations
We continue to collaborate with several academic institutions by providing them with access to our NovoGen MMX Bioprinter™ has proved to be a powerful enabling tool for the design, optimization, and fabrication of viable 3D human tissues, based on our internal product discovery and development efforts as well as the experience of our corporate partners and customers. Continuing use of the NovoGen MMX Bioprinter™ in the pursuit of multiple drug discovery and therapeutic applications has provided key insights that will be utilized in the evolution of the bioprinter platform. We believe that purpose-driven improvements and added product features, combined with new capabilities enabled by additional in-licensed intellectual property, will enhance our ability to deliver commercially viable outputs for corporate partners in drug development and implantable therapeutics.
The NovoGen MMX Bioprinter has won the following awards and accolades:
2010 International Society for Biofabrication Meeting - Special Award
2010 TIME Magazine “50 Best Inventions of 2010”
2011 Australian Engineering Innovation Award, sponsored by the Australian government
Organovo was also celebrated as “Dealmaker of the Year 2011—Firm” by the Fermanian Business and Economic Institute and included in MIT Technology Review’s 2012 TR50 List of the World’s Most Innovative Companies.
In 2011 and early 2012 we provided, or will provide, NovoGen MMX Bioprinters™ for use by the following institutions, among others,Bioprinters® for research purposes: Harvard Medicalpurposes, including: Yale School Wake Forestof Medicine, Knight Cancer Institute at Oregon Health & Science University, and the Sanford Consortium for Regenerative Medicine (“SCRM”). The SCRM is a new institution which opened in November, 2011, comprised of faculty from the Salk Institute, The Scripps Research Institute, the University of California, San Diego, Sanford-Burnham Medical Research Institute, and La Jolla Allergy and Immunology Institute.Virginia. We believe that the use of our bioprinting platform by major research institutions will increasemay help to advance the valuecapabilities of the platform and create future opportunitiesgenerate new applications for intellectual property licensing.
Specific Applications for 3D Human Tissues
Our bioprinting technology and surrounding intellectual property and commercial rights serve as a platform for product generation across multiple markets that employ cell- and tissue-based products and services. The core capability ofbioprinted tissues. In prior instances, an academic institution or other third party provided funding to support the academic collaborator’s access to our technology is the productionplatform. This funding was typically reflected as collaboration revenues in our financial statements. Our academic research collaborations typically involve both parties contributing resources directly to projects. We are not currently generating any revenues from these collaborations.
Intellectual Property
We rely on a combination of human tissues with the potential to recapitulate human biology. Once generated, these in vivo- like human tissues may be suitable forpatents, trademarks, trade secrets, confidential know-how, copyrights and a variety of applicationscontractual mechanisms such as confidentiality, material transfer, licenses, research tools, specialized models of tissue pathobiology,collaboration, limited technology access, and implantable therapeuticsinvention assignment agreements, to protect our intellectual property. Our intellectual property portfolio for tissue engineering and regenerative medicine (Figure 4). Importantly, the basic fabrication and maturation protocols that generate functional micro-scale tissues for in vitro use will serve as a foundation for the design and manufacture of larger-scale tissues intended for therapeutic use to augment or replace damaged or degenerating organs.
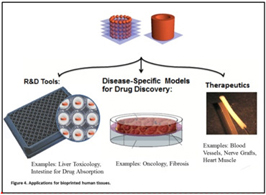
Collaborative Agreements
As part of our business strategy, we intend to pursue collaboration agreements with drug development companies that will allow us to further develop our bioprintercore technology was initially built through licenses from MU and the potential usesMedical University of the cellular structuresSouth Carolina. We subsequently expanded our intellectual property portfolio by filing our own patent and tissues that can be producedtrademark applications worldwide and negotiating additional licenses and purchases.
On an ongoing basis we review and analyze our full intellectual property portfolio to align it with our bioprinter technology. Under these collaboration agreements, wecurrent business needs, strategies and the drug development company will conduct research to pursue drug discovery utilizing the three dimensional cellular structures developed with our bioprinter technology. Currently, drug therapy researchobjectives. Based on that ongoing review, selected patents and testing generally involves testing drug candidates and therapies on monolayer two dimensional cell cultures that attempt to mimic damagedpatent applications in various countries are or degenerating tissues. We believe the use of our technology, which creates three dimensional cellular structures, will enhance and facilitate drug discovery.
Our collaboration agreements typically provide for the parties to mutually develop a research plan and timeline. Each collaboration partner is required to provide the other party reports describing the applicable party’s progress under such research plan. Our collaborative agreements generally have a term of the later of one to three years, or the completion by us of the applicable research plan. The agreements provide for certain upfront payments and milestone payments throughout the term related to our research and development obligations under the agreement. In addition, the collaboration agreements provide for a future licensing arrangement between the parties, with royalties payable to us, if the drug development company is successful in identifying a drug candidate or therapy utilizing our bioprinter technology. These agreements also provide customary mutual indemnities and contain standard representations and warranties.
Our first two collaboration agreements are with Pfizer, Inc. (“Pfizer”) and United Therapeutics Corporation (“Unither”). In December 2010, we entered into a collaborative research agreement with Pfizer to develop tissue based drug discovery assays in two therapeutic areas utilizing our NovoGen MMX Bioprinter ™ technology. To date, Pfizer has paid us all amounts due under the agreement and we anticipate completing the research plan by July 2012. We anticipate that the agreement will be extended past July 2012; although we can give no assurance that it will in fact be so extended. In October 2011, we entered into a research agreement with Unitherabandoned or allowed to establish and conduct a research program to discover treatments for pulmonary hypertension using our NovoGen MMX Bioprinter ™ technology, which remains in effect until the laterlapse. The numbers provided herein are reflective of 30 months from its commencementthose changes.
We solely own or our completion of the contracted research. Additionally, under the research agreement with Unither, we granted Unither an option to acquire from us a worldwide, royalty-bearing license in certain intellectual property created under the research agreement solely for use in the treatment or prevention of pulmonary hypertension and all other lung diseases. The license would provide for certain milestone payments and minimum annual royalties and sales-based royalties.
Federal Grants
We have received five federally funded grants to date. In August, 2009 and August, 2010 we received grants from National Heart, Lung, and Blood Institute, a division of the Department of Health and Human Services, to fund our research in connection with building and testing multi-layered fully biological blood vessel substitutes and bioprinting with specialized adult stem cells derived from adipose (fat) tissue. The total amount of these grants was $267,625. In October, 2010 we received two grants from the federal government relating to our projects titled “Biological 3D Bioprinted Blood Vessel” and “NovoGen 3D Bioprinter Development.” The total amount of these grants was $397,287. In March 2012, we received a $290,053 grant from the National Institutes of Health to support the development of functional human liver tissue utilizing our bioprinting technology.
Competition
We are subject to significant competition from pharmaceutical, biotechnology, and diagnostic companies; academic and research institutions; and government or other publicly-funded agencies that are pursuing the development of research tools and therapeutic products that otherwise address the needs of our potential customers.
We believe our future success will depend, in large part, on our ability to maintain a competitive position in our field. Biopharmaceutical technologies have undergone and are expected to continue to undergo rapid and significant change. We or our competitors may make rapid technological developments which may cause our research tools or therapeutic products to become obsolete before we recover the expenses incurred. The introduction of less expensive or more effective therapeutic discovery and development technologies, including technologies that may be unrelated to our field, may also make our technology less valuable or obsolete. We may not be able to make the necessary enhancements to our technologies or research tools to compete successfully with newly emerging technologies. The failure to maintain a competitive position in the biopharmaceutical field may result in decreased revenues.
We are a platform technology company dedicated to the development and production of 3D human tissues that service both the drug development and regenerative medicine industries. To our knowledge, there are no other companies with a similar platform technology or marketed products.
Set forth below is a discussion of competitive factors for each of the broad markets in which we intend to utilize our technology:
Highly Specialized Models for Drug Discovery: This aspect of our business is driven by leveraging our technology as a high-end partnered service that enables a customer to discover or optimally formulate a pharmacologic product that delivers a specific therapeutic effect, or avoids a particular side effect. In addition to revenue generated from the tissue production work, additional revenues are possible in the form of up-front license fees, milestone payments, know-how payments, and royalties. We can provide the customer access to tissues as a service or can produce and supply the tissues to customers; both options are designed to generate continuing revenue. Competition in this area arises mainly from two sources, traditional cell-based in vitro culture approaches and traditionalin vivo animal models and testing.
We believe that an important factor distinguishing our approach from that of our competitors is our ability to build models that are composed of human cells and have a 3D tissue-like configuration (i.e., able to generate results that are not subject to inherent limitations of 2D monolayer culture). We acknowledge, however, that there are some areas of research for which the existing methods (2D cell culture and/or animal studies) are adequate and 3D in vitro human tissues are not sufficiently advantageous.
Tools for Research and Drug Development: We intend to employ our technology to provide an array of broadly-applicable enabling tools and assays to the drug research markets. Examples of products in this segment of the business include future pipeline efforts in the development of 3D human tissue models that service the ADME/TOX/DMPK markets as alternatives or supplements to traditional cell-based assays and animal studies, and the NovoGen MMX Bioprinter™ instrument.
Competition in the bioprinter arena has been limited to date. We believe that we have a first mover advantage in being the first and only company to offer a purely cellular bioprinting system commercially, which does not rely on the presence of foreign, non-native polymer in the final tissue construct. Some academic groups have internally created inkjet bioprinting systems, but these systems have not been developed commercially to date and are unlikely to adapt as well to a commercial model.
Regenerative Medicine: This aspect of our business involves application of our 3D bioprinting technology to generate 3D human tissues suitable for implantation in vivo to augment or replace damaged or degenerating tissues. The majority of these efforts will be undertaken as partnered projects with leading therapeutic companies seeking to develop a tissue engineering / regenerative medicine product for a specific application. Near-term revenues would come from the funding of development work and, in some cases, licensing fees for access to our platform technologies. We expect longer-term revenues may arise from shared profits and royalties or other forms of income from successful clinical and commercial development of the tissue products. There are many companies pursuing the discovery, development, and commercialization of tissue-engineered products for a variety of applications, including but not limited to Organogenesis, Advanced BioHealing (recently acquired by Shire), Tengion, Genzyme (a subsidiary ofSanofi ), HumaCyte and Cytograft Tissue Engineering. These companies represent potential competition for us but can also be potential partners. For any tissue-engineered / regenerative medicine product where three-dimensionality is desired, our platform has a unique ability to enable generation of prototypes, optimization of prototypes and protocols, and production of the tissue.
Intellectual Property
Our success depends in large part on our ability to obtain and enforce patents, maintain protection of trade secrets and operate without infringing the proprietary rights of third parties. We hold exclusive licenses to one34 issued U.S. patent, threepatents and more than 45 issued international patents in foreign jurisdictions including Australia, Canada, China, Denmark, France, Great Britain, Germany, Ireland, Japan, Sweden, the Netherlands and Switzerland. We solely or jointly own or hold exclusive licenses to 17 pending U.S. patent applications and multiple correspondingmore than 5 pending international applications in foreign jurisdictions including Australia, Canada and China. These patent applications. We have filed six U.S. patent applications and corresponding international patent applications regardingfamilies relate to our bioprinting technology and itsour engineered tissue products and services, including our various uses in areas of tissue creation, andin vitro testing, utilization in drug discovery, and in vivo therapeutics.
In connection with the recent acquisition of the FXR program from Metacrine, we acquired the related patent portfolio by way of assignment. This includes filings on the lead candidate, FXR314, and selected filings on the prior candidate (no longer in development), FXR125. With respect to this FXR portfolio, we solely own 7 issued patents and 15 international patents in jurisdictions, including filings for specific tissue types.Australia, China, Eurasia, India, Israel, Mexico, Japan and South Africa. We solely own 8 pending U.S. patent applications and more than 50 pending international applications in foreign jurisdictions, including Argentina, Australia, Brazil, Chile, Canada, Eurasia, Europe, Israel, India, Japan, South Korea, Mexico, Philippines, Singapore, South Africa, Hong Kong and Taiwan.
These patent families relate to FXR125 and FXR314, including generic coverage, species coverage, methods of use, formulations and polymorph crystals.
In-Licensed Intellectual Property
In March, 2009 and 2010, we obtained a world-wide exclusive licenselicenses to a suite of intellectual property owned or licensed by MU and the Medical University of Missouri-Columbia (“MU”) covering the following two patent applications:
“Self-Assembling Cell Aggregates and Methods of Making Engineered Tissue Using the Same” (US 10/590,446); and
“Self-Assembling Multicellular Bodies and Methods of Producing a Three-Dimensional Biological Structure Using the Same” (PCT/US2009/48530) (the “MU 2009 License Agreement”).
The Company received official notification that theSouth Carolina, which now includes 7 issued U.S. Patent and Trademark Office (the “USPTO”) issued a patent (No. 8,143,055) for the above mentioned patent application. The patent provides the Company with intellectual property rights to create cellular aggregates, to use cellular aggregates to create engineered tissue, and to employ cellular aggregates to create engineered tissue with no scaffold present.
In addition, in March, 2010, we obtained a world-wide exclusive license to additional intellectual property from MU, including a patent application covering the composition and method of manufacture of a nerve conduit (the “MU 2010 License Agreement”, and together with the MU 2009 License Agreement, the “ MU License Agreements “). The patent application licensed to us under the MU 2009 License Agreement, entitled “Self-Assembling Multicellular Bodies and Methods of Producing a Three-Dimensional Biological Structure Using the Same” (Serial No. 12/491,228), of which an issue notification has been mailed by the U.S. Patent and Trademark Office assigning a projected U.S. Patent No. of 8,143,055, is expected to expire in June 2029. The remaining two patent applications licensed under the MU License Agreements are still under review at the U.S. Patent and Trademark Office.
Each of the MU License Agreements required us to make an upfront payment ranging from $5,000 to $25,000. They also require us to pay royalties ranging from 1% to 3% of net sales depending on the level of net sales reached and certain minimum annual royalties ranging from $5,000 to $25,000. Additionally, the MU 2010 License Agreement requires us to pay a minimum royalty of $12,500 if no net sales are achieved after five years from the effective date. Additionally, we are required to pay 20% of all revenue derived from any sublicense we grant under any of the MU License Agreements. The MU License Agreements terminate upon the last to expire licensed patents and may be terminated upon breach of either party, subject to standard cure provisions.
2 pending U.S. applications. Dr. Gabor Forgacs, one of our Foundersfounders and Scientific Advisors, is the common inventor of all of these works (the “Forgacs Intellectual Property “). The Forgacs Intellectual Property is the result of years of research by Dr. Gabor Forgacs, thea former George H. Vineyard Professor of Biophysics at MU, was one of the Universityco-inventors of Missouri-Columbia and his collaborators and research teams. Dr. Forgacs is a sought after expert in biofabrication with a long recordall of peer-reviewed publications. The Forgacsthese works (collectively, the “Forgacs Intellectual Property derives from work done in the labs of Dr. Forgacs and his collaborators, including the work done under a $5,000,000 Frontiers In Biological Research grant that Dr. Forgacs and his collaborators received from the National Science Foundation.
Property”). The Forgacs Intellectual Property provides us with intellectual property rights relating to create cellular aggregates, tothe use of cellular aggregates to create engineered tissue,tissues, and to employthe use of cellular aggregates to create engineered tissue with no scaffold present. The intellectual property rights derived from the Forgacs Intellectual Property also enables us to utilize our NovoGen MMX Bioprinter ™® to create engineered tissues, and provides us with rights to specific compositions with utility in the creation of nerve conduit.tissues.
In May, 2011, we obtained an exclusive license (the “CURF License Agreement”) to a U.S. patent entitled “Ink Jet Printing of Viable Cells” (US(U.S. Patent No. 7,051,654) fromowned by the Clemson University Research Foundation (“CURF Patent”). The Clemson University Research Foundation had been granted certains rights allowing it to offer exclusive rights to the CURF Patent. The CURF Patentthat provides us with the intellectual property rights relating to methods of using ink-jet printer technology to dispense cells and relating to createthe creation of matrices of bioprinted cells on gel materials. This patent is expected to expire in May 2024.
The CURF License Agreement requires us to make an upfront payment of $32,500, payable in four quarterly paymentsIn connection with the last payment dueacquisition of the FXR program from Metacrine in April 2012. Additionally,2023, we were assigned and assumed a license agreement with the CURF License Agreement requires us to pay royalties ranging from 1.5% to 3% of net sales dependingSalk Institute for Biological Studies requiring milestone and royalty payments based on the level of net sales reached and minimum annual royalties ranging from $20,000 to $40,000. Additionally, we are required to pay 40% of all revenue derived from any sublicense we grant under the CURF License Agreement. The CURF License Agreement terminates upon the last to expire licensed patents and may be terminated upon breach of either party, subject to standard cure provisions.
Under our license arrangements, we have full control and authority over the development and commercialization of any licensed products, including clinical trials, manufacturing, marketing,FXR314.
The patent rights we obtained through these exclusive licenses are not only foundational within the field of 3D bioprinting and regulatory filings. We were required to submit and have submitted plans for commercialization of all technologies and are required to make efforts to pursue commercial development of the technology.FXR agonist therapies but provide us with favorable priority dates. We are required to make ongoing royalty payments under these exclusive licenses based on an annual basis after commercializationnet sales of products and services that rely on the intellectual property we in-licensed. For additional information regarding our royalty obligations see “Note 5. Collaborative Research, Development, and License Agreements” in the Notes to maintain the license rights.Consolidated Financial Statements included elsewhere in this prospectus.
Company Owned Intellectual Property
In addition to the intellectual property we have in-licensed, we have historically innovated and grown our intellectual property portfolio.
With respect to our bioprinting platform, we have 11 issued U.S. patents and 13 issued foreign patents directed to our NovoGen Bioprinter® and methods of bioprinting: U.S. Patent Nos. 8,931,880; 9,149,952; 9,227,339; 9,315,043; 9,499,779; 9,855,369; 10,174,276, 10,967,560, 11,577,450, 11,577,451 and 11,413,805 ; Australia Patent Nos. 2015202836, 2013249569, and 2014296246; Canada Patent No. 2,812,766; China Patent Nos. ZL201180050831.4 and ZL201480054148.1; European Patent Nos. 2838985, 2629975, and 3028042; Japan Patent Nos. 6333231, 6566426 and 6842918, and Russian Patent No. 2560393. These issued patents and pending patent applications carry remaining patent terms ranging from over 20 years to over 7 years. We currently have additional U.S. continuation applications pending in these families as well foreign counterpart applications in multiple countries.
Our ExVive™ Human Liver Tissue is protected by U.S. Patent Nos. 9,222,932, 9,442,105, 10,400,219 and 11,127,774; Australia Patent Nos. 2014236780 and 2017200691; and Canada Patent No. 2,903,844. Our ExVive™ Human Kidney Tissue is protected by U.S. Patent Nos. 9,481,868, 10,094,821 and 10,962,526; Australian Patent No. 2015328173, Canadian Patent No. 2,962,778, European Patent No. 3204488 and Japan Patent No. 7021177. These issued patents and pending patent applications carry remaining patent terms ranging from over 11 years to over 9 years. We have additional U.S. patent applications pending to protect our proprietary methods and processes and have also filed, and intend to file, correspondingin these families, as well as foreign patentcounterpart applications. We believecurrently have pending numerous patent applications in the U.S. and globally that protectionare directed to additional features on bioprinters, additional tissue types, their methods of the proprietary nature of our productsfabrication, and technologies is essentialspecific applications.
Our U.S. Patent Nos. 9,855,369 and 9,149,952, which relate to our business. Accordingly, we have adopted and will continue a vigorous program to secure and maintain protection of our proprietary methods and processes. We file patent applications with respect to novel
bioprinter technology, and improvements thereof that are important to our business. We also rely upon trade secrets, unpatented know-how, continuing technological innovation and the pursuit of licensing opportunities to develop and maintain our competitive position. There can be no assurance that others will not independently develop substantially equivalent proprietary technology or that we can meaningfully protect our proprietary position.
Regulatory Considerations
We are not aware of any current FDA regulatory requirements for sales of research tools, such as bioprinters and bioprinted tissues, into a research setting. However, pharmaceutical industry corporate customers with whom we will enter into partnerships will face regulatory review of the research data they generate using our platform and research tools. Good Laboratory Practice (GLP) data is required in the development of any human therapeutic, and our platform has been designed to support compliance with GLP, although no independent testing has been performed to date to confirm this compliance. All product contact surfaces are sterilizable or disposable. GLP considerations around areas such as data integrity are the sole responsibility of the customer without regard to specifics of the research tool used.
Therapeutic tissues and other regenerative medicine products are subject to an extensive and uncertain regulatory approval process by the Food and Drug Administration (FDA) and comparable agencies in other countries. The regulation of new products is extensive, and the required process of laboratory testing and human studies is lengthy and expensive. The burden of these regulations will fall on our collaborating partners, or may be shared with us, to the extent that we are developing proprietary products that are the result of a collaboration effort. The burden of these regulations will fall on us to the extent we are developing proprietary products on our own. We may not be able to obtain FDA approvals for those products in a timely manner, or at all. We may encounter significant delays or excessive costs in our efforts to secure necessary approvals or licenses. Even if we obtain FDA regulatory approvals, the FDA extensively regulates manufacturing, labeling, distributing, marketing, promotion and advertising after product approval. Moreover, several of our product development areas may involve relatively new technology and have not beenwere the subject of extensive product testing in humans. The regulatory requirements governing these productsIPR proceedings filed by Cellink AB and related clinical procedures remain uncertainits subsidiaries (collectively, “BICO Group AB”), one of our competitors. Likewise, U.S. Patent Nos. 9,149,952, 9,855,369, 8,931,880, 9,227,339, 9,315,043 and 10,967,560 (all assigned to Organovo, Inc.) and U.S. Patent Nos. 7,051,654, 8,241,905, 8,852,932 and 9,752,116 (assigned to Clemson University and the products themselves may be subject to substantial reviewUniversity of Missouri, respectively) were implicated in a declaratory judgment complaint filed against Organovo, Inc., our wholly owned subsidiary, by foreign governmental regulatory authorities that could prevent or delay approvalBICO Group AB and certain of its subsidiaries in those countries. Regulatory requirements ultimately imposedthe United States District Court for the District of Delaware. All of these matters have since been settled in a favorable manner for the Company. Specifically, on our products could limit our ability to test, manufacture and, ultimately, commercialize our products and thereby could adversely affect our financial condition and results of operations.
As constructs move into clinical and commercial settings, useFebruary 23, 2022, we announced an agreement of a validatednon-exclusive license for BICO Group AB and Good Tissue Practices ( GTP ) Quality system will be required. Suitable designits affiliate companies to Organovo’s foundational patent portfolio in 3D bioprinting.
With respect to our FXR agonist program covering FXR314 and documentationFXR125, we have 6 issued U.S. patents and 14 issued foreign patents directed to composition of matter protection (generic and specific) for clinical useFXR314 and FXR125, as well claims directed to methods of
treatment of GI diseases, formulations of FXR314 and polymorphs of the bioprinter will beFXR314 molecule including United States Patent Nos.11,214,538, 10,705,712, 10,927,082, 10,961,198, 11,136,071 and 11,084,817, granted Australian Patent Nos. 2016323992 and 2018236275, Chinese Patent Nos. 201680066917 and 269065, Eurasian Patent Nos. 040003 and 040704, Israeli Patent Nos. 258011, 296068 and 296065, Indian Patent No. 380510, Japanese Patent Nos. 6905530 and 717709, Mexican Patent Nos. 386,752 and 397265 and South African Patent No. 2018/01750. In addition, we have 8 pending U.S. patent applications and over 50 pending foreign patent applications, including U.S. Patent Application Nos. 18/156,069, 17/532,618, 18/174,393, 17/349,757, 17/276,787, 17/906,580, 17/906,582 and 17/906,585 and over 50 pending international patent applications in a partnumber of future phases of printer design programs.countries including, Australia, Brazil, Canada, Chile, China, the Eurasian Patent Office, the European Patent Office, Israel, India, Japan, South Korea, Mexico, Singapore, Philippines and Hong Kong. These issued patents and pending patent applications carry remaining patent terms ranging from over 18 years to just over 15 years.
Employees and Human Capital
As of June 1, 2012,March 31, 2024, we have twenty-sevenhad 20 employees, of whom eighteenwhich 12 are employed full time.full-time. We have also engageretained some of our former employees as consultants, in addition to a number of expert consultants in specific scientific and operational areas. Our employees are not represented by labor unions or covered under any collective bargaining agreements. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and additional employees. The principal purposes of our equity incentive plans are to attract, retain and motivate selected employees, consultants and temporary employees fromdirectors through the granting of equity-based compensation awards.
Corporate Information
We are operating the business of our subsidiaries, including Organovo, Inc., our wholly-owned subsidiary, which we acquired in February 2012. Organovo, Inc. was incorporated in Delaware in April 2007. Our common stock has traded on The Nasdaq Stock Market LLC under the symbol “ONVO” since August 8, 2016 and our common stock currently trades on the Nasdaq Capital Market. Prior to that time, it traded on the NYSE MKT under the symbol “ONVO” and prior to timethat was quoted on the OTC Market.
Our principal executive offices are located at 11555 Sorrento Valley Rd, Suite 100, San Diego CA 92121 and our phone number is (858) 224-1000. Our Internet website can be found at http://www.organovo.com. The content of our website is not intended to provide servicesbe incorporated by reference into this prospectus or in any other report or document that relate to our bioprinting business and technology as well as for general administrative and accounting services.we file.
Available Information
Our investor relations website is located at http://ir.organovo.com. We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Reports filed with the SECSecurities and Exchange Commission (the “SEC”) pursuant to the Exchange Act, including annual and quarterly reports, and other reports we file, canare available free of charge, through our website. The content of our website is not intended to be inspected and copied at the publicincorporated by reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Investors may obtain informationinto this prospectus or in any other report or document that we file. We make them available on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. Investors can request copies of these documents upon payment of a duplicating fee by writing toour website as soon as reasonably possible after we file them with the SEC. The reports we file with the SEC are also available on the SEC’s website (http:(http://www.sec.gov)www.sec.gov).
DESCRIPTION OF PROPERTYLegal Proceedings
We lease officeIn addition to commitments and laboratory space in two locations in San Diego. Our primary office, including administrative and laboratory space, is located at the Oberlin Science Center, 5871 Oberlin Drive, San Diego, CA 92121. We also lease additional office space at 5897 Oberlin Drive, San Diego, CA 92121. Our current monthly base rent for our primary facility is $11,486 and our currently monthly base rent for our additional office space is $1,112.
We have entered into a lease agreement with our current landlord on a facility currently undergoing renovation, which we expect to occupy in July or August 2012. The new facility will house all of our operations under one roof, replacing the two facilities we now rent at a new base rate of $38,848. The new facility provides approximately three times our existing space and is expected to meet our business, research and operational needs for at least two years. The new facility will be delivered “turnkey”, thereby minimizing our need to utilize capital to fund tenant improvements to the laboratory or office spaces.
LEGAL PROCEEDINGS
From time to time we may be named in claims arisingobligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business. Currently, no
The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its financial statements. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not meaningful indicators of its potential liability.
The Company regularly reviews contingencies to determine the adequacy of its accruals and related disclosures. During the period presented, the Company has not recorded any accrual for loss contingencies associated with any claims or legal proceedings; determined that an unfavorable outcome is probable or reasonably possible; or determined that the amount or range of any possible loss is reasonably estimable. However, the outcome of legal proceedings government actions, administrative actions, investigationsand claims brought against the Company is subject to
significant uncertainty. Therefore, although management considers the likelihood of such an outcome to be remote, if one or claims are pendingmore legal matters were resolved against us or involve us that,the Company in a reporting period, the opinion of our management, could reasonably be expected to have a material adverse effect on our business and financial condition.
We anticipate that we will expend significant financial and managerial resources in the defense of our intellectual property rights in the future if we believe that our rights have been violated. We also anticipate that we will expend significant financial and managerial resources to defend against claims that our products and services infringe upon the intellectual property rights of third parties.
Organovo, Inc.
Financial Statements
CONTENTS
| | | | |
| | Page | |
| |
(a) Financial Statements of Organovo, Inc.
| | | | |
| |
Report of Independent Registered Public Accounting Firm
| | | 30 | |
| |
Balance Sheets as of December 31, 2011 and 2010
| | | 31 | |
| |
Statements of Operations as of December 31, 2011 and 2010, and from April 19, 2007 (Inception) through December 31, 2011
| | | 32 | |
| |
Statements of Stockholders’ Deficit from April 19, 2007 (Inception) through December 31, 2011
| | | 33 | |
| |
Statements of Cash Flows as of December 31, 2011 and 2010, and from April 19, 2007 (Inception) through December 31, 2011
| | | 34 | |
| |
Notes to Financial Statements
| | | 35 | |
| |
(b) Pro Forma Financial Information (unaudited)
| | | | |
| |
Unaudited Pro Forma Combined Financial Statements
| | | 52 | |
| |
Unaudited Pro Forma Combined Balance Sheets as of December 31, 2011
| | | 54 | |
| |
Unaudited Pro Forma Combined Statements of Operations for the Year Ended December 31, 2011
| | | 55 | |
| |
Notes to Unaudited Pro Forma Combined Financial Statements
| | | 56 | |
| |
(c) Unaudited Condensed Financial Statements of Organovo Holdings, Inc.
| | | | |
| |
Unaudited Balance Sheets as of March 31, 2012
| | | 59 | |
| |
Unaudited Statements of Operations as of March 31, 2012 and from April 19, 2007 (Inception) through March 31, 2012
| | | 60 | |
| |
Unaudited Statements of Stockholders’ Deficit from April 19, 2007 (Inception) through March 31, 2012
| | | 61 | |
| |
Unaudited Statements of Cash Flows as of March 31, 2012, and from April 19, 2007 (Inception) through March 31, 2012
| | | 62 | |
| |
Notes to Financial Statements
| | | 63 | |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Organovo, Inc.
San Diego, California
We have audited the accompanying balance sheets ofOrganovo, Inc. (the “Company”) as of December 31, 2011 and 2010, and the related statements of operations, stockholders’ deficit, and cash flows for the years then ended and for the period from April 19, 2007 (Inception) through December 31, 2011. TheseCompany’s consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.for that reporting period could be materially adversely affected.
We conducted our audits in accordance with the Public Company Accounting Oversight Board (United States). Those standards require thatProperties
In November 2020, we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position ofOrganovo, Inc. as of December 31, 2011 and 2010, and the results of its operations and its cash flows for the years then ended and for the period from April 19, 2007 (Inception) through December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
/s/ Mayer Hoffman McCann P.C.
San Diego, CA
March 30, 2012
Organovo, Inc.
(A development stage company)
Balance Sheets
| | | | | | | | |
| | | December 31, 2011 | | | December 31, 2010 | |
Assets | | | | | | | | |
| | |
Current Assets | | | | | | | | |
Cash and cash equivalents | | $ | 339,607 | | | $ | 285,308 | |
Grant receivable | | | — | | | | 59,744 | |
Inventory | | | 291,881 | | | | 68,022 | |
Deferred financing costs | | | 318,843 | | | | — | |
Prepaid expenses and other current assets | | | 79,874 | | | | 11,042 | |
| | | | | | | | |
Total current assets | | | 1,030,205 | | | | 424,116 | |
| | |
Fixed Assets - Net | | | 278,208 | | | | 295,539 | |
| | |
Other Assets - Net | | | 100,419 | | | | 40,743 | |
| | | | | | | | |
Total assets | | $ | 1,408,832 | | | $ | 760,398 | |
| | | | | | | | |
Liabilities and Stockholders’ Deficit | | | | | | | | |
| | |
Current Liabilities | | | | | | | | |
Accounts payable | | $ | 657,560 | | | $ | 284,217 | |
Accrued expenses | | | 437,837 | | | | 305,580 | |
Deferred revenue | | | 152,500 | | | | 106,925 | |
Related party note payable | | | — | | | | 25,000 | |
Accrued interest payable | | | 24,018 | | | | 251,536 | |
Convertible notes payable, current portion | | | 703,833 | | | | 200,000 | |
| | | | | | | | |
Total current liabilities | | | 1,975,748 | | | | 1,173,258 | |
| | |
Warrant liabilities | | | 1,266,869 | | | | — | |
Convertible notes payable, long-term portion | | | — | | | | 1,887,500 | |
| | | | | | | | |
Total liabilities | | $ | 3,242,617 | | | $ | 3,060,758 | |
| | |
Commitments and contingencies (Note 10) | | | | | | | | |
| | |
Stockholders’ Deficit | | | | | | | | |
Common stock, $0.0001 par value; 75,000,000 shares authorized, 22,445,254 and 14,707,020 shares issued and outstanding at December 31, 2011 and December 31, 2010, respectively | | | 2,245 | | | | 1,471 | |
Additional paid-in capital | | | 4,855,526 | | | | 6,463 | |
Deficit accumulated during the development stage | | | (6,691,556 | ) | | | (2,308,294 | ) |
| | | | | | | | |
Total stockholders’ deficit | | | (1,833,785 | ) | | | (2,300,360 | ) |
| | | | | | | | |
Total Liabilities and Stockholders’ Deficit | | $ | 1,408,832 | | | $ | 760,398 | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
Organovo, Inc.
(A development stage company)
Statements of Operations
| | | | | | | | | | | | |
| | | Year Ended
December 31, 2011 | | | Year Ended
December 31, 2010 | | | Period from
April 19, 1997
(Inception)
through
December 31, 2011 | |
Revenue | | | | | | | | | | | | |
Product | | $ | 223,500 | | | $ | — | | | $ | 223,500 | |
Collaborations | | | 688,088 | | | | 75,000 | | | | 763,088 | |
Grants | | | 56,925 | | | | 528,412 | | | | 664,112 | |
| | | | | | | | | | | | |
Total Revenue | | | 968,513 | | | | 603,412 | | | | 1,650,700 | |
| | | |
Cost of product revenue | | | 133,607 | | | | — | | | | 133,607 | |
Selling, general, and administrative expenses | | | 1,705,171 | | | | 577,914 | | | | 2,666,038 | |
Research and development expenses | | | 1,419,718 | | | | 1,203,716 | | | | 3,198,388 | |
| | | | | | | | | | | | |
Loss from Operations | | | (2,289,983 | ) | | | (1,178,218 | ) | | | (4,347,333 | ) |
| | | | | | | | | | | | |
Other Income (Expense) | | | | | | | | | | | | |
Interest expense | | | (2,066,889 | ) | | | (160,873 | ) | | | (2,318,442 | ) |
Interest income | | | 64 | | | | 81 | | | | 2,007 | |
Other expense | | | (26,454 | ) | | | 316 | | | | (27,788 | ) |
| | | | | | | | | | | | |
Total Other Income (Expense) | | | (2,093,279 | ) | | | (160,476 | ) | | | (2,344,223 | ) |
| | | | | | | | | | | | |
Net Loss | | $ | (4,383,262 | ) | | $ | (1,338,694 | ) | | $ | (6,691,556 | ) |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
Organovo, Inc.
(A development stage company)
Statements of Stockholders’ Deficit
Period from April 19, 2007 (Inception) through December 31, 2011
| | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional Paid-in | | | Deficit
Accumulated
During the
Development | | | Total | |
| | | Shares | | | Amount | | | Capital | | | Stage | | |
Balance at inception (April 19, 2007) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | |
Issuance of Common stock | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | — | |
Net Loss | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | |
Issuance of Common stock to founders | | | 1,729,532 | | | | 173 | | | | (173 | ) | | | — | | | | — | |
Issuance of restricted Common stock | | | 12,627,697 | | | | 1,263 | | | | (1,263 | ) | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 1,742 | | | | — | | | | 1,742 | |
Net Loss | | | — | | | | — | | | | — | | | | (97,559 | ) | | | (97,559 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 14,357,229 | | | $ | 1,436 | | | $ | 306 | | | $ | (97,559 | ) | | $ | (95,817 | ) |
| | | | | |
Issuance of restricted Common stock | | | 130,422 | | | | 13 | | | | (13 | ) | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 2,336 | | | | — | | | | 2,336 | |
Net Loss | | | — | | | | — | | | | — | | | | (872,041 | ) | | | (872,041 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 14,487,651 | | | $ | 1,449 | | | $ | 2,629 | | | $ | (969,600 | ) | | $ | (965,522 | ) |
| | | | | |
Issuance of restricted Common stock | | | 219,369 | | | | 22 | | | | (22 | ) | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 3,856 | | | | — | | | | 3,856 | |
Net Loss | | | — | | | | — | | | | — | | | | (1,338,694 | ) | | | (1,338,694 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 14,707,020 | | | $ | 1,471 | | | $ | 6,463 | | | $ | (2,308,294 | ) | | $ | (2,300,360 | ) |
| | | | | |
Issuance of Common stock through conversion of notes payable | | | 7,676,828 | | | | 768 | | | | 3,488,990 | | | | — | | | | 3,489,758 | |
Issuance of restricted Common stock | | | 61,406 | | | | 6 | | | | (6 | ) | | | — | | | | — | |
Warrants issued with convertible notes and upon conversion of notes payable | | | — | | | | — | | | | 1,111,364 | | | | — | | | | 1,111,364 | |
Beneficial conversion feature of convertible notes payable | | | — | | | | — | | | | 239,700 | | | | — | | | | 239,700 | |
Stock-based compensation expense | | | — | | | | — | | | | 9,015 | | | | — | | | | 9,015 | |
Net Loss | | | — | | | | — | | | | — | | | | (4,383,262 | ) | | | (4,383,262 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 22,445,254 | | | $ | 2,245 | | | $ | 4,855,526 | | | $ | (6,691,556 | ) | | $ | (1,833,785 | ) |
| | | | | | | | | | | | | | | | �� | | | | |
The accompanying notes are an integral part of these financial statements.
Organovo, Inc.
(A development stage company)
Statements of Cash Flows
| | | | | | | | | | | | |
| | | Year Ended
December 31, 2011 | | | Year Ended
December 31, 2010 | | | Period from
April 19, 2007
(Inception) through
December 31, 2011 | |
Cash Flows From Operating Activities | | | | | | | | | | | | |
Net loss | | $ | (4,383,262 | ) | | $ | (1,338,694 | ) | | $ | (6,691,556 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Amortization of debt discount | | | 1,187,569 | | | | — | | | | 1,187,569 | |
Depreciation and amortization | | | 68,064 | | | | 58,669 | | | | 156,328 | |
Amortization of deferred financing costs | | | 119,451 | | | | — | | | | 119,451 | |
Warrants issued in connection with exchange agreement | | | 527,629 | | | | — | | | | 527,629 | |
Stock-based compensation | | | 9,015 | | | | 3,856 | | | | 16,949 | |
Change in fair value of warrants | | | 6,569 | | | | — | | | | 6,569 | |
Increase (decrease) in cash resulting from changes in: | | | | | | | | | | | | |
Grants receivable | | | 59,744 | | | | (54,846 | ) | | | — | |
Inventory | | | (223,859 | ) | | | (68,022 | ) | | | (291,881 | ) |
Prepaid expenses and other current assets | | | (68,693 | ) | | | (2,409 | ) | | | (93,005 | ) |
Accounts payable | | | 373,343 | | | | 230,165 | | | | 657,560 | |
Accrued expenses | | | 132,257 | | | | 83,404 | | | | 437,837 | |
Deferred revenue | | | 45,575 | | | | 106,925 | | | | 152,500 | |
Accrued interest payable | | | 232,240 | | | | 160,856 | | | | 483,776 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (1,914,358 | ) | | | (820,096 | ) | | | (3,330,274 | ) |
| | | | | | | | | | | | |
Cash Flows From Investing Activities | | | | | | | | | | | | |
Purchases of fixed assets | | | (45,547 | ) | | | (48,072 | ) | | | (426,823 | ) |
Purchases of intangible assets | | | (65,000 | ) | | | (5,000 | ) | | | (95,000 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (110,547 | ) | | | (53,072 | ) | | | (521,823 | ) |
| | | | | | | | | | | | |
Cash Flows From Financing Activities | | | | | | | | | | | | |
Proceeds from issuance of convertible notes payable | | | 2,542,500 | | | | 992,500 | | | | 4,630,000 | |
Proceeds from issuance of related party notes payable | | | 225,000 | | | | 25,000 | | | | 250,000 | |
Repayment of related party notes payable | | | (250,000 | ) | | | — | | | | (250,000 | ) |
Deferred financing costs | | | (438,296 | ) | | | — | | | | (438,296 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 2,079,204 | | | | 1,017,500 | | | | 4,191,704 | |
| | | | | | | | | | | | |
Net Increase in Cash and Cash Equivalents | | | 54,299 | | | | 144,332 | | | | 339,607 | |
Cash and Cash Equivalents at Beginning of Period | | | 285,308 | | | | 140,976 | | | | — | |
| | | | | | | | | | | | |
Cash and Cash Equivalents at End of Period | | $ | 339,607 | | | $ | 285,308 | | | $ | 339,607 | |
| | | | | | | | | | | | |
|
| The accompanying notes are an integral part of these financial statements. | |
| | | |
| Supplemental Discloures of Cash Flow Information: | | | | | | | | | | | | |
| | | |
Interest | | $ | — | | | $ | — | | | $ | — | |
Income Taxes | | $ | 800 | | | $ | 1,600 | | | $ | 2,400 | |
Supplemental Disclosure of Noncash Investing and Financing Activities:
During 2008 the Company issued 1,729,532 shares of Common stock to the founders.
During 2011 and 2010 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued 61,406, 219,369 and 13,038,894, respectively, shares of restricted Common stock to certain employees, advisors and consultants of the Company.
During 2011 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued certain convertible notes payable that included warrants. The warrants and the related beneficial conversion feature, valued at $823,435 were classified as equity instruments and recorded as a discount to the carrying value of the related debt.
During 2011 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued warrants, valued at approximately $1,260,300, in connection with certain convertible notes payable. The warrants were recorded as a warrant liability and recorded as a discount to the carrying value related to debt.
During 2011, the Company issued 7,676,828 shares of Common stock to note holders for the conversion of Convertible Notes with a principal balance totaling $3,030,000 and accrued interest totaling $459,758.
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
1. Summary of Significant Accounting Policies | | A summary of the Company’s significant accounting policies consistently applied in the preparation of the accompanying financial statements follows. |
| |
Nature of operations | | Organovo, Inc. (“the Company”) was founded in Delaware in April 2007 and is a Delaware Corporation. Activities since the Company’s inception through 2011 were devoted primarily to developing a platform technology for the generation of three-dimensional (3D) human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs.
As of December 31, 2011, the Company has devoted substantially all of its efforts to product development, raising capital, and building infrastructure. The Company has not realized significant revenues from its planned principal operations. Accordingly, the Company is considered to be in the development stage.
On February 8, 2012, the Company merged with and into Organovo Acquisition Corp., a wholly-owned subsidiary of Organovo Holdings, Inc., a publicly traded Delaware corporation (“Organovo Holdings”), with the Company surviving the merger as a wholly-owned subsidiary of Organovo Holdings (the “Merger”). As a result of the Merger, Organovo Holdings acquired the business of the Company, and will continue the existing business operations of the Company.
|
| |
Liquidity | | As of December 31, 2011, the Company had an accumulated deficit of approximately $6,691,600. The Company also had negative cash flow from operations of $1,914,400 during the year ended December 31, 2011.
The Company expects to cover it’s anticipated 2012 operating expenses through cash on hand including the funds raised during the first quarter of 2012 through the Private Placement of its Securities and funds received through collaborative agreements, and other commercial arrangements.
On February 8, 2012, the Company received gross proceeds of approximately $6,500,000, including $1,500,000 previously received from the sale of convertible notes payable, in a private placement offering in conjunction with the Merger. The convertible notes automatically converted into equity at the time of the Merger. On February 29, 2012 and March 16, 2012, the Company completed two additional closings of its Private Placement Offering and received total gross proceeds of approximately $8,722,100. See Note 12.
While the likelihood of a liquidity crisis is considered remote, should one occur, there are no guarantees that the Company would be able to obtain sufficient cash from outside sources on a timely basis. Management does not believe the situation represents a significant risk to the Company as of the date of these financial statements.
The Company’s ability to continue its operations is dependent upon its ability to raise additional capital through equity or debt financing, and to generate capital through collaborative research agreements and other commercial arrangements. There can be no assurance that any additional financing will be available on acceptable terms or available at all. Any equity financing may result in dilution to existing stockholders and any debt financing may include restrictive covenants.
The accompanying financial statements do not include any adjustments to reflect the
possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of these uncertainties.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| |
Use of
estimates
| | The preparation of the financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. Significant estimates used in preparing the financial statements include those assumed in computing the valuation of warrants and conversion features, revenue recognized under the proportional performance model, the valuation of stock-based compensation expense, and the valuation allowance on deferred tax assets. |
| |
Cash and cash
equivalents
| | The Company considers all highly liquid investments with original maturities of 90 days or less to be cash equivalents. |
| |
Financial
instruments
| | For certain of the Company’s financial instruments, including cash and cash equivalents, grants receivable, inventory, prepaid expenses and other assets, accounts payable, accrued expenses, deferred revenue, notes payable to related parties and convertible notes payable, the carrying amounts are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. |
| |
Derivative financial
instruments
| | The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks.
The Company reviews the terms of convertible debt and equity instruments it issues to determine whether there are embedded derivative instruments, including an embedded conversion option that is required to be bifurcated and accounted for separately as a derivative financial instrument. In circumstances where the convertible instrument contains more than one embedded derivative instrument, including the conversion option, that is required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument. Also, in connection with the sale of convertible debt and equity instruments, the Company may issue freestanding warrants that may, depending on their terms, be accounted for as derivative instrument liabilities, rather than as equity.
Derivative instruments are initially recorded at fair value and are then revalued at each reporting date with changes in the fair value reported as non-operating income or expense. When the convertible debt or equity instruments contain embedded derivative instruments that are to be bifurcated and accounted for as liabilities, the total proceeds allocated to the convertible host instruments are first allocated to the fair value of all the bifurcated derivative instruments. The remaining proceeds, if any, are then allocated to the convertible instruments themselves, usually resulting in those instruments being recorded at a discount from their face value.
The discount from the face value of the convertible debt, together with the stated interest on the instrument, is amortized over the life of the instrument through periodic charges to interest expense, using the effective interest method.
|
| |
Grants
receivable
| | Grants receivable represent amounts due under: (i) two federal contracts with the National
Heart, Lung, and Blood Institute (NHLBI), a division of the National Institutes of Health (NIH), and (ii) two U.S. Department of Treasury grant awards. The Company considers the grants receivable to be fully collectible, and accordingly no allowance for doubtful amounts has been established. If amounts become uncollectible, they are charged to operations.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
Inventory | | Inventories are stated at the lower of the cost or market (first-in, first out). Inventory at December 31, 2011, consisted of approximately $235,000 in finished goods and approximately $56,900 in raw materials. Inventory at December 31, 2010 consisted of approximately $40,000 of work in process and approximately $28,000 in raw materials.
The Company provides inventory allowances based on excess or obsolete inventories determined based on anticipated use in the final product. There was no obsolete inventory reserve as of December 31, 2011 or 2010.
|
| |
Deferred financing costs | | As of December 31, 2011, deferred financing costs consisted of approximately $140,000 associated with the Merger transaction and approximately $179,000 associated with the private placement offering that was initiated in the fourth quarter of 2011. The deferred financing costs related to the private placement offering are being amortized over the life of the Convertible Notes. The deferred financing costs associated with the Merger transaction will be recorded to equity as an offset to the proceeds received as of the effective Merger date. See Note 5. |
| |
Other assets | | As of December 31, 2011, other assets consisted of approximately $13,100 in security deposits and $87,300 in net license fees related to a license obtained from Clemson University for bioprinting employing ink-jet technology, and a license obtained from the University of Missouri for 3D bioprinting. See Note 8.
|
| |
Fixed assets and
depreciation
| | Property and equipment are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the lease term. The estimated useful life of the fixed assets range between three and ten years. |
| |
Impairment of long- lived assets | | In accordance with authoritative guidance the Company reviews its long-lived assets, including property and equipment and other assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred in the period from inception through December 31, 2011. |
| |
Fair value measurement | | Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value: |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | ¨ Level 1 — Quoted prices in active markets for identical assets or liabilities.
¨ Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
¨ Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of December 31, 2011 and 2010, cash and cash equivalents were comprised of cash in checking accounts.
The Company used Level 3 inputs for its valuation methodology for the warrant derivative liabilities. The estimated fair values were determined using a Monte Carlo option pricing model based on various assumptions (see Note 4). The Company’s derivative liabilities are adjusted to reflect estimated fair value at each period end, with any decrease or increase in the estimated fair value being recorded in other income or expense accordingly, as adjustments to fair value of derivative liabilities.
At December 31, 2011, the estimated fair values of the liabilities measured on a recurring basis are as follows:
Fair Value Measurements at December 31, 2011
|
| | | | | | | | | | | | | | | | |
| | | Balance at
December, 31, 2011 | | | Quoted Prices in
Active Markets
(Level 1) | | | Significant Other
Observable Inputs
(Level 2) | | | Significant Other
Unobservable
Inputs (Level 3) | |
Warrant derivative liability | | $ | 1,266,869 | | | | — | | | | — | | | $ | 1,266,869 | |
| | |
| | The following table presents the activity for liabilities measured at estimated fair value using unobservable inputs for the year ended December 31, 2011:
Fair Value Measurements Using Significant Unobservable Inputs (Level 3)
|
| | | | |
| | | Warrant Derivative Liability | |
Beginning balance at December 31, 2010 | | $ | — | |
Issuances | | | 1,260,300 | |
Adjustments to estimated fair value | | | 6,569 | |
| | | | |
Ending balance at December 31, 2011 | | $ | 1,266,869 | |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
Revenue recognition | | The Company’s revenues are derived from the sale of bioprinter related products and services, NIH and U.S. Treasury Department Grants, collaboration agreements, and license agreements.
The Company recognizes revenue when the following criteria have been met: (i) persuasive evidence of an arrangement exists; (ii) services have been rendered or product has been delivered; (iii) price to the customer is fixed and determinable; and (iv) collection of the underlying receivable is reasonably assured.
Billings to customers or payments received from customers are included in deferred revenue on the balance sheet until all revenue recognition criteria are met. As of December 31, 2011 and 2010, the Company had approximately $152,500 and $107,000 in in deferred revenue related to its collaborative research programs.
Product Revenue
The Company recognizes product revenue at the time of shipment to the customer, provided all other revenue recognition criteria have been met. The Company recognizes product revenues upon shipment to distributors, provided that (i) the price is substantially fixed or determinable at the time of sale; (ii) the distributor’s obligation to pay the Company is not contingent upon resale of the products; (iii) title and risk of loss passes to the distributor at time of shipment; (iv) the distributor has economic substance apart from that provided by the Company; (v) the Company has no significant obligation to the distributor to bring about resale of the products; and (vi) future returns can be reasonably estimated. For any sales that do not meet all of the above criteria, revenue is deferred until all such criteria have been met.
Research and Development Revenue Under Collaborative Agreements.
The Company’s collaboration revenue consists of license and collaboration agreements that contain multiple elements, including non-refundable upfront fees, payments for reimbursement of third-party research costs, payments for ongoing research, payments associated with achieving specific development milestones and royalties based on specified percentages of net product sales, if any. The Company considers a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the elements are separable, whether there are determinable fair values and whether there is a unique earnings process associated with each element of a contract.
The Company recognizes revenue from research funding under collaboration agreements when earned on a “proportional performance” basis as research hours are incurred. The Company performs services as specified in each respective agreement on a best-efforts basis, and is reimbursed based on labor hours incurred on each contract. The Company initially defers revenue for any amounts billed or payments received in advance of the services being performed and recognizes revenue pursuant to the related pattern of performance, based on total labor hours incurred relative to total labor hours estimated under the contract.
In December 2010, the Company entered into a 12 month research contract agreement with a third party, whereby the Company was engaged to perform research and development services on a fixed-fee basis for approximately $600,000. Based on proportional performance criteria, the Company recognized approximately $450,000 in revenue related to the contract during 2011, and expects to recognize the remaining $150,000 in revenue during 2012.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | In October 2011, the Company entered into a research contract agreement with a third party, whereby the Company will perform research and development services on a fixed-fee basis for $1,365,000. The agreement includes an initial payment to the Company of approximately $239,000, with remaining payments expected to occur over a 21-month period. At December 31, 2011, the Company recorded approximately $239,000 in revenue related to the research contract in recognition of the proportional performance achieved by the Company during the fourth quarter of 2011.
Revenue Arrangements with Multiple Deliverables
The Company occasionally enters into revenue arrangements that contain multiple deliverables. Judgment is required to properly identify the accounting units of the multiple deliverable transactions and to determine the manner in which revenue should be allocated among the accounting units. Moreover, judgment is used in interpreting the commercial terms and determining when all criteria of revenue recognition have been met for each deliverable in order for revenue recognition to occur in the appropriate accounting period. For multiple deliverable agreements, consideration is allocated at the inception of the agreement to all deliverables based on their relative selling price. The relative selling price for each deliverable is determined using VSOE of selling price or third-party evidence of selling price if VSOE does not exist. If neither VSOE nor third-party evidence of selling price exists, the Company uses its best estimate of the selling price for the deliverable.
The Company recognizes revenue for delivered elements only when it determines there are no uncertainties regarding customer acceptance. While changes in the allocation of the arrangement consideration between the units of accounting will not affect the amount of total revenue recognized for a particular sales arrangement, any material changes in these allocations could impact the timing of revenue recognition, which could affect the Company’s results of operations.
The Company expects to periodically receive license fees for non-exclusive research licensing associated with funded research projects. License fees under these arrangements are recognized over the term of the contract or development period as it has been determined that such licenses do not have stand-alone value.
NIH and U.S. Treasury Grant Revenues
During 2010, the U.S. Treasury awarded the Company two one-time grants totaling approximately $397,300 for investments in qualifying therapeutic discovery projects under section 48D of the Internal Revenue Code. The grants cover reimbursement for qualifying expenses incurred by the Company in 2010 and 2009. The proceeds from these grants are classified in “Revenues – Grants” in the 2010 statement of operations.
During 2010 and 2009, the NHLBI, a division of the NIH, awarded the Company two research grants totaling approximately $267,600. Revenues from the NIH grants are based upon internal and subcontractor costs incurred that are specifically covered by the grant, and where applicable, an additional facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by subcontractors and as the Company incurs internal expenses that are related to the grant. Revenue recognized under these grants for the years ended December 31, 2011 and 2010 was approximately $56,900 and $131,100, respectively.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
Stock-based compensation | | The Company accounts for stock-based compensation in accordance with Financial Accounting Standards Board’s ASC Topic 718,Compensation – Stock Compensation, which establishes accounting for equity instruments exchanged for employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair value of the award, and is recognized as an expense, under the straight-line method, over the employee’s requisite service period (generally the vesting period of the equity grant).
The Company accounts for equity instruments, including restricted stock or stock options, issued to non-employees in accordance with authoritative guidance for equity based payments to non-employees. Stock options issued to non-employees are accounted for at their estimated fair value determined using the Black-Scholes option-pricing model. The fair value of options granted to non-employees is re-measured as they vest, and the resulting increase in value, if any, is recognized as expense during the period the related services are rendered. Restricted stock issued to non-employees is accounted for at their estimated fair value as they vest.
|
| |
Research and development | | Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Reseach and development costs are expensed as incurred. |
| |
Income taxes | | Deferred income taxes are recognized for the tax consequences in future years for differences between the tax basis of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the combination of the tax payable for the year and the change during the year in deferred tax assets and liabilities. |
| |
Comprehensive income (loss) | | Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at Comprehensive income (loss). For the years ended December 31, 2011 and 2010, and for the period April 19, 2007 (inception) through December 31, 2011, the comprehensive loss was equal to the net loss. |
| |
New accounting standards
| | In May 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2011-04, “Fair Value Measurement” to amend the accounting and disclosure requirements on fair value measurements. This ASU limits the highest-and-best-use measure to nonfinancial assets, permits certain financial assets and liabilities with offsetting positions in market or counterparty credit risks to be measured at a net basis, and provides guidance on the applicability of premiums and discounts. Additionally, this update expands the disclosure on Level 3 inputs by requiring quantitative disclosure of the unobservable inputs and assumptions, as well as description of the valuation processes and the sensitivity of the fair value to changes in unobservable inputs. ASU No. 2011-04 is to be applied prospectively and is effective during interim and annual periods beginning after December 15, 2011. The Company does not expect the adoption of this update to have a material effect on its financial statements. |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| |
| | In June 2011, FASB issued ASU No. 2011-05, “Presentation of Comprehensive Income.” This ASU presents an entity with the option to present the total of comprehensive income, the components of net income, and the component of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. This update eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity/deficit. The amendments in this update do not change the items that must be reported in other comprehensive income or when an item of other Comprehensive income must be reclassified to net income. ASU No. 2011-05 should be applied retrospectively and is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. As ASU No. 2011-05 relates only to the presentation of Comprehensive income, the Company does not expect the adoption of this update to have a material effect on its financial statements. |
| |
2. Fixed Assets | | Fixed assets consisted of the following: |
| | | | | | | | |
December 31, | | 2011 | | | 2010 | |
Laboratory equipment | | $ | 345,319 | | | $ | 309,057 | |
Leasehold improvements | | | 34,198 | | | | 34,198 | |
Computer software and equipment | | | 28,185 | | | | 28,185 | |
Furniture and fixtures | | | 19,123 | | | | 9,836 | |
| | | | | | | | |
| | | 426,825 | | | | 381,276 | |
Less accumulated depreciation and amortization | | | (148,617 | ) | | | (85,737 | ) |
| | | | | | | | |
| | $ | 278,208 | | | $ | 295,539 | |
| | | | | | | | |
| | |
| | Depreciation and amortization expense for the years ended December 31, 2011 and 2010 was approximately $62,900 and $57,100, respectively. Depreciation and amortization expense was approximately $148,600 for the period from April 19, 2007 (inception) through December 31, 2011. |
| |
3. Accrued Expenses | | Accrued expenses consisted of the following: |
| | | | | | | | |
December 31, | | 2011 | | | 2010 | |
Accrued compensation | | $ | 317,097 | | | $ | 129,234 | |
Other accrued expenses | | | 91,884 | | | | 116,424 | |
Deferred rent | | | 28,856 | | | | 59,922 | |
| | | | | | | | |
| | $ | 437,837 | | | $ | 305,580 | |
| | | | | | | | |
| | |
4. Derivative Liability | | As discussed in Note 5, the Company issued Convertible Notes in 2011 that provided for the issuance of five-year warrants to purchase the Company’s Common stock. The exercise price of the warrants is protected against down-round financing throughout the term of the warrant under certain conditions.
The protective provisions will be triggered if, prior to the expiration date of the warrants, the Company issues additional shares of common stock without consideration or for a consideration per share less than the exercise price of the warrants in effect immediately prior to such issue. In the event such an issuance occurs, the exercise price of the warrants will be reduced to a price (calculated to the nearest cent) determined by multiplying the exercise price by a fraction, (A) the numerator of which is (1) the number of shares of common stock outstanding immediately prior to such issue plus (2) the number of shares of common stock which the aggregate consideration received or to be received by the Company for the total number of additional shares of common stock so issued would purchase at the exercise price; and (B) the denominator of which is the number of shares of common stock outstanding immediately prior to such issue plus the number of such additional shares of common stock so issued.
For purposes of this calculation, (i) all shares of common stock issuable upon conversion or exchange of convertible securities outstanding immediately prior to such issue shall be deemed to be outstanding, and (ii) the number of shares of common stock deemed issuable upon conversion or exchange of such outstanding convertible securities shall be determined without giving effect to any adjustments to the conversion or exchange price or conversion or exchange rate of such convertible securities resulting from the issuance of additional shares of common stock that is the subject of this calculation.
For purposes of the foregoing calculations, the term “additional shares of common stock” means all shares of common stock issued by the Company after the issuance of the warrants (including any shares of common stock issuable upon conversion or exchange of any convertible securities or upon exercise of any option or warrant, on an as-converted basis), other than: (i) shares of common stock (and/or warrants for any class of equity securities of the Company) issued or issuable upon conversion or exchange of any convertible securities or exercise of any options or warrants outstanding on the date of issuance of the warrants; (ii) shares of common stock issued or issuable by reason of a dividend, stock split, split-up or other distribution on shares of common stock, including such events pursuant to a reorganization, reclassification, consolidation, merger or sale; (iii) shares of common stock (or options with respect thereto) issued or issuable to employees or directors of, or consultants to, the Company or any of its subsidiaries pursuant to a plan, agreement or arrangement approved by the Board of Directors of the Company; (iv) any securities issued or issuable by the Company pursuant to (A) the Securities Purchase Agreement pursuant to which the investors purchased the convertible promissory notes and the warrants, (B) the Selling Agreement with the Spencer Trask Ventures, Inc., the selling agent in the offering, (C) the reverse triangular merger of the Company into a publicly-held company, or (D) any private placement offering that closes (including subsequent closings) as part of the reverse triangular merger of the Company into a publicly-held company; and (v) securities issued pursuant to acquisitions or strategic transactions approved by a majority of disinterested directors of the Company, provided that any such issuance may only be to a person which is, itself or through its subsidiaries, an operating company in a business synergistic with the business of the Company and in which the Company receives benefits in addition to the investment of funds, and may not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
Upon each adjustment of the exercise price pursuant to the provisions stated above, the number of shares issuable upon exercise of the warrants shall be adjusted by multiplying a number equal to the exercise price in effect immediately prior to such adjustment by the number of shares issuable upon exercise of the warrants immediately prior to such adjustment and dividing the product so obtained by the adjusted exercise price.
Pursuant to ASC 815-15 and ASC 815-40, the fair value of the warrants of $1,260,300 was recorded as a derivative liability on the issuance date.
The fair value of the warrants was estimated at the issuance date and revalued at December 31, 2011, using a Monte Carlo simulation. At December 31, 2011, the Company has recorded a derivative liability of approximately $1,266,900. The change in fair value of the derivative liability of approximately $6,600 from the date of issuance to December 31, 2011 is included in other expense in the 2011 statement of operations.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
5. Convertible Notes Payable | | |
| |
Convertible notes | | From February 9, 2008 through December 31, 2011 the Company raised an aggregate of $2,390,000 in funds through loans consisting of convertible notes (“Convertible Notes”) to certain shareholders, management, vendors, and investors. The notes bore interest at rates ranging from 8% to 10% per annum and had maturity dates ranging from 2011 to 2018. The Convertible Notes were unsecured and subordinated to certain senior indebtedness of the Company, and for all Convertible Notes the principal plus accrued interest was convertible into the Company’s Common stock. During October 2011 the Convertible Notes and accrued interest converted into the Company’s Common stock, as discussed below. |
| |
Local Bridge | | During July and August 2011, $740,000 of Convertible Notes bearing interest at 20% per annum, and warrants to purchase shares of common stock were issued to investors. The Convertible Notes were due at the earlier of 1) one year from the issuance date or 2) one week after the consummation of the Merger (as discussed in Note 12). The number of warrants to be issued was equal to the note principal divided by the exercise price. The exercise price is the per share or per unit fair market value received in the Merger. The notes were convertible at a price per share equal to seventy-five percent (75%) of the per share fair market value of the total consideration received for a share of a public company’s Common stock to be determined to be identified upon consummation of a merger.
The Company determined that the beneficial conversion feature and the warrants did not represent embedded derivative instruments. Additionally, the Company did not record the discount for the beneficial conversion feature due to the contingencies surrounding conversion. The beneficial conversion feature was to be recorded when the contingencies are resolved. In accordance with ASC 470-20, Debt with Conversion and Other Options, the Company recorded a discount of approximately $583,700 for the warrants. The discount is being amortized to interest expense over the term of the Convertible Notes using the effective interest method.
The Company calculated the fair value of the warrants using the Black-Scholes Model using a volatility of 109.84%, an interest rate of 1.12% and a dividend yield of zero.
Certain of these Convertible Notes and accrued interest were converted into the Company’s Common stock in October 2011, as discussed below. Upon conversion the Company recognized the unamortized debt discount related to these notes to interest expense. The Company recognized approximately $583,700 of interest expense for the amortization of the note discount during the year ended December 31, 2011.
|
| |
Exchange agreement and release | | In October 2011, the Company’s Board of Directors and shareholders approved an Exchange Agreement and Release whereby the note holders could exchange their Convertible Notes and accrued interest for shares of the Company’s Common stock and warrants to purchase the Company’s Common stock. A total of $3,030,000 of principal and approximately $459,800 of accrued interest converted, at prices ranging from $0.27 to $0.75, into 7,676,828 shares of the Company’s Common stock, plus five-year warrants to purchase 1,309,750 Common shares at an exercise price of $1.00 per share. The Company calculated the fair value of the warrants using the Black-Scholes Model using a volatility of 110.13%, an interest rate of 1.11% and a dividend yield of zero. For the holders that elected to participate, the Exchange Agreement and Release resulted in the cancellation of the Convertible Notes and release from the note holders for any claims related to the Convertible Notes.
The Company determined that the warrants issued in connection with the Exchange Agreement and Release did not represent embedded derivative instruments. The warrants, valued at approximately $527,600, were classified as equity instruments and recorded as interest expense on the date of issuance.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | At December 31, 2011, a $100,000 Convertible Note remained outstanding, and was paid in cash at the close of the Merger. See Note 12. |
| |
Private placement | | On September 18, 2011, the Company’s Board of Directors authorized a private placement offering of up to 30 Units (the “Units”) of its securities at a price of $50,000 per Unit for an aggregate purchase price of $1,500,000. Each Unit consists of a convertible note in the principal amount of $50,000 accruing simple interest at the rate of 6% per annum, plus five-year warrants to purchase 50,000 shares of the next Qualified Round of Equity Securities, at an exercise price of $1.00 per share. The principal plus accrued interest was convertible into the common stock of a public shell company to be identified upon consummation of a merger transaction.
During October and November 2011, $1,500,000 of Convertible Notes bearing interest at 6% per annum with a maturity date of March 30, 2012, and five-year warrants to purchase 1,500,000 shares of the Company’s Common stock were issued to investors under the private placement. The Convertible Notes were outstanding at December 31, 2011, and were converted into common stock in connection with the Merger. See Note 12. The warrants are exercisable at $1.00 per share, expire in five years, and contain down-round price protection.
The Company determined that the warrants represent a derivative instrument due to the down-round price protection, and accordingly, the Company recorded a derivative liability related to the warrants of approximately $1,260,300. See Note 4. Additionally, the Company recorded the discount for the beneficial conversion feature of $239,700. The debt discount associated with the warrants and beneficial conversion feature are being amortized to interest expense over the life of the Convertible Notes. The Company recorded approximately $603,800 of interest expense for the amortization of the debt discount during the year ended December 31, 2011.
As consideration for locating investors to participate in this financing, the placement agent earned a cash payment of $195,000. Additionally, upon closing of a Merger transaction, the placement agent will earn five-year warrants to purchase 610,155 shares of the Company’s Common stock at $1.00 per share. These warrants contain down round protection and will be classified as derivative liabilities upon issuance.
As of December 31, 2011 and 2010, the outstanding principal balances on the Convertible Notes were $1,600,000 and $2,087,500, respectively. As of December 31, 2011 and 2010, the accrued interest balances on the outstanding Convertible Notes were approximately $24,000 and $252,000, respectively. As of December 31, 2011 and 2010, unamortized discounts relating to the outstanding principal balances were approximately $896,200 and $0, and the $896,200 is expected to be recognized as interest expense in 2012.
Interest expense, including amortization of the note discounts, for the years ended December 31, 2011 and 2010 was approximately $2,066,900 and $161,000, respectively. Interest expense, including amortization of the note discounts, for the period from April 19, 2007 (inception) through December 31, 2011 was approximately $2,318,000.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
6. Stockholders’ Equity | | |
| |
Common stock | | In September 2011, the Company amended its Certificate of Incorporation to increase its authorized Common stock from 100,000 shares to 75,000,000 shares. Each share of the Company’s Common stock is entitled to one vote and all shares rank equally as to voting and other matters.
On September 18, 2011, the Company approved a 362.282-for-1 forward stock split. The Company did not change the par value of the shares. The stockholders’ equity section of the accompanying financial statements and all share numbers disclosed throughout the financial statements have been retroactively adjusted to give effect to the forward stock split.
The Company issued 1,729,532 shares of Common stock to the founders in February 2008.
In October 2011, the Company issued 7,676,828 shares of Common stock to note holders for the conversion of Convertible Notes with a principal balance totaling $3,030,000 and accrued interest totaling approximately $459,800. See Note 5.
|
| |
Restricted stock awards | | In February 2008, four founders, including the Chief Executive Officer (“CEO”) and three directors of the Company received 11,779,960 shares of restricted Common stock, 25% vesting after the first year and the remaining 75% vesting in equal quarterly portions over the following three years.
On May 8, 2008, the Board of Directors of the Company approved the 2008 Equity Incentive Plan (the “2008 Plan”). The 2008 Plan authorized the issuance of up to 1,521,584 Common shares for awards of incentive stock options, non-statutory stock options, restricted stock awards, restricted stock unit awards, and stock appreciation rights. The 2008 Plan terminates on July 1, 2018.
From 2008 through 2011, the Company issued a total of 1,258,934 shares of restricted Common stock to various employees, advisors, and consultants of the Company. 1,086,662 of those shares were issued under the 2008 Plan and the remaining 172,272 shares were issued outside the plan.
|
| |
| | A summary of the Company’s restricted stock award activity is as follows: |
| | | | |
| | Number of Shares | |
Unvested at December 31, 2007
| | | — | |
Granted
| | | 12,627,697 | |
Vested
| | | (65,211 | ) |
Canceled / forfeited
| | | — | |
| | | | |
Unvested at December 31, 2008
| | | 12,562,486 | |
Granted
| | | 130,422 | |
Vested
| | | (5,373,004 | ) |
Canceled / forfeited
| | | — | |
| | | | |
Unvested at December 31, 2009
| | | 7,319,904 | |
Granted
| | | 219,369 | |
Vested
| | | (3,256,191 | ) |
Canceled / forfeited
| | | — | |
| | | | |
Unvested at December 31, 2010
| | | 4,283,082 | |
Granted
| | | 61,406 | |
Vested
| | | (3,233,193 | ) |
Canceled / forfeited
| | | — | |
| | | | |
Unvested at December 31, 2011
| | | 1,111,295 | |
| | | | |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | The fair value of each restricted Common stock award is recognized as stock-based expense over the vesting term of the award. The Company recorded restricted stock-based compensation expense in operating expenses for employees and non-employees of approximately $3,300 and $3,900 for the years ended December 31, 2011 and 2010, respectively. The Company recorded stock-based compensation expense of approximately $16,900 for the period from April 19, 2007 (inception) through December 31, 2011.
As of December 31, 2011 total unrecognized stock-based compensation expense was approximately $1,800, which will be recognized over a weighted average period of less than one year.
|
| |
Stock options | | Under the 2008 Plan, on October 12, 2011 the Company granted an officer of the Company incentive stock options to purchase 896,256 shares of the Company’s Common stock at an exercise price of $0.08 per share, vesting over a four-year period commencing in May, 2011. After this grant, no additional issuances are authorized under the 2008 plan.
The following table summarizes stock option activity as of December 31, 2011, and the changes for the year then ended:
|
| | | | | | | | | | | | |
| | | Options
Outstanding | | | Weighted-
Average
Exercise Price | | | Aggregate Intrinsic
Value | |
Outstanding at December 31, 2010 | | | — | | | | — | | | | — | |
Options Granted | | | 896,256 | | | $ | 0.08 | | | | — | |
Options Canceled | | | — | | | | — | | | | — | |
Options Exercised | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
Outstanding at December 31, 2011 | | | 896,256 | | | $ | 0.08 | | | $ | — | |
| | | | | | | | | | | | |
Vested and Exercisable at December 31, 2011 | | | — | | | $ | 0.08 | | | $ | — | |
| | | | | | | | | | | | |
| | |
| | The Company uses the Black-Scholes valuation model to calculate the fair value of stock options. The fair value of employee stock options was estimated at the grant date using the following assumptions: |
| | | | |
| | | December 31, 2011 | |
Weighted-average grant date fair value | | $ | 0.06 | |
Dividend yield | | | — | |
Volatility | | | 111% | |
Risk-free interest rate | | | 1.07% | |
Expected life of options | | | 5.0 years | |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. Due to the Company’s limited historical data, the estimated volatility incorporates the historical and implied volatility of comparable companies whose share prices are publicly available. The risk-free interest rate assumption was based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued. The weighted average expected life of options was estimated using the average of the contractual term and the weighted average vesting term of the options.
The total employee stock-based compensation recorded as operating expenses was approximately $5,800 for the year ended December 31, 2011 and for the period from April 19, 2007 (inception) through December 31, 2011.
The total unrecognized compensation cost related to unvested stock option grants as of December 31, 2011 was approximately $48,000, and the weighted average period over which these grants are expected to vest is 4 years
|
| |
Warrants | | During 2011, the Company issued warrants to purchase 2,909,750 shares of its Common stock. These warrants are immediately exercisable at $1.00 per share, and have remaining terms of approximately 4.8 years. None of the warrants were exercised as of December 31, 2011. See Notes 4 and 5. |
| |
Common stock reserved for future issuance | | Common stock reserved for future issuance consisted of the following at December 31, 2011: |
| | | | |
Common stock warrants outstanding
| | | 2,909,750 | |
Common stock options outstanding under the 2008 Plan
| | | 896,256 | |
Common stock warrants held for convertible debt issuance
| | | 1,500,000 | |
Authorized for future grant or issuance under the 2008 Plan
| | | — | |
| | | | |
Total
| | | 5,306,606 | |
| | | | |
| | |
7. Income Taxes | | Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows as of December 31, 2011 and 2010: |
| | | | | | | | |
December 31, | | 2011 | | | 2010 | |
Deferred tax asset: | | | | | | | | |
Net operating loss carryforwards | | $ | 1,620,000 | | | $ | 784,000 | |
Research & Development Credits | | | 190,000 | | | | 99,000 | |
Depreciation and amortization | | | 8,000 | | | | (2,000 | ) |
Accrued expenses and reserves | | | 107,000 | | | | 36,000 | |
| | | | | | | | |
Total deferred tax assets | | | 1,925,000 | | | | 917,000 | |
Valuation Allowance | | | (1,925,000 | ) | | | (917,000 | ) |
| | | | | | | | |
| | $ | — | | | $ | — | |
| | | | | | | | |
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| | A full valuation allowance has been established to offset the deferred tax assets as management cannot conclude that realization of such assets is more likely than not. The valuation allowance increased by approximately $1,008,000 in 2011.
At December 31, 2011, the Company had federal and state net operating loss carryforwards of approximately $4,067,000 and $4,063,000, respectively. The federal and state net operating loss carryforwards will begin expiring in 2029, unless previously utilized.
At December 31, 2011, the Company had federal and state research tax credit carryforwards of approximately $114,500 and $114,800, respectively. The federal research tax credit carryforwards begin expiring in 2029. The state research tax credit carryforwards do not expire.
The Company applies the authoritative guidance for uncertainty in income taxes pursuant to ASC 740-10. The adoption of this guidance did not have a material impact on the Company’s financial statements. The Company did not record any accruals for income tax accounting uncertainties for the years ended December 31, 2011 or 2010.
The Company’s policy is to recognize interest and penalties that would be assessed in relation to the settlement value of unrecognized tax benefits as a component of income tax expense. The Company did not accrue either interest or penalties as of December 31, 2011 or 2010.
The Company is subject to taxation in the United States, and the state of California. As of December 31, 2011, the Company’s tax years from inception are subject to examination by the tax authorities. The Company is not currently under examination by the United States federal or state jurisdictions.
|
| |
8. Licensing Agreements and Research Contracts | | |
| |
University of Missouri | | On March 24, 2009 the Company entered into a license agreement with the Curators of the University of Missouri to in-license certain technology and intellectual property relating to self-assembling cell aggregates and to intermediate cellular units. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company paid to the University of Missouri a nonrefundable license fee of $25,000 and has committed to reimburse the University of Missouri for certain prior and future patent costs. Each year the Company is required to pay the University of Missouri royalties ranging from 1% to 3% of net sales depending on the level of net sales achieved by the Company each year. A minimum annual royalty of $25,000 is due beginning 2 years after the calendar year of the first commercial sale and is credited to sales royalties. The license agreement terminates upon expiration of the patents licensed and is subject to certain conditions as defined in the license agreement, which are expected to expire after 2029. The $25,000 license fee is included in Other Assets in the accompanying balance sheets and is being amortized over the life of the related patent. |
| |
| | On March 12, 2010, the Company entered into a license agreement with the Curators of the University of Missouri to in-license certain technology and intellectual property relating to engineered biological nerve grafts. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company paid to University of Missouri a nonrefundable license fee of $5,000 and has committed to reimburse the University of Missouri for certain prior and future patent costs. In 2011 and 2010, the Company paid the University of Missouri $23,789 and $40,323, respectively, for prior patent costs relating to the license agreements with the University of Missouri. Each year the Company is required to pay the University of Missouri royalties ranging from 1% to 3% of net sales depending on the level of net sales achieved by the Company each year. A minimum annual royalty of $5,000 is due beginning 2 years after the calendar year of the first commercial sale and is credited to sales royalties. An additional royalty of $12,500 is due if there are no net sales within five years from the effective date of the license. The license agreement terminates upon expiration of the patents licensed and is subject to certain conditions as defined in the license agreement. The $5,000 license fee is included in Other Assets and is being amortized over the life of the related patent. |
| |
| | On May 2, 2011, the Company entered into a license agreement with Clemson University Research Foundation to in-license certain technology and intellectual property relating to ink-jet printing of viable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company agreed to pay Clemson University a nonrefundable license fee of $32,500, payable in four quarterly payments with the last payment due in April 2012. The Company has also committed to reimburse Clemson University for certain prior and future patent costs. In 2011 the Company paid Clemson University $23,793 for prior patent costs. Each year the Company is required to pay the University royalties ranging from 1.5% to 3% of net sales depending on the level of net sales reached each year and minimum annual fees ranging from $20,000 to $40,000. Specific terms of the royalty and license agreements are confidential. The license agreement terminates upon expiration of the patents licensed, which is expected to expire in May 2024, and is subject to certain conditions as defined in the license agreement.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
Clemson University 2011 licensing agreement | | On May 2, 2011 the Company entered into a license agreement with Clemson University Research Foundation to in-license certain technology and intellectual property relating to ink-jet printing of viable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company agreed to pay Clemson University a nonrefundable license fee in cash and in the form of a convertible promissory note. The Company has also committed to reimburse Clemson University for certain prior and future patent costs. Each year the Company is required to pay the University royalties. Specific terms of the royalty and license agreements are confidential. The license agreement terminates upon expiration of the patents licensed and is subject to certain conditions as defined in the license agreement.
No royalty fees have been incurred under the license agreements as of December 31, 2011.
|
| |
| | Capitalized license fees consisted of the following: |
| | | | | | | | |
December 31, | | 2011 | | | 2010 | |
License fees | | $ | 95,000 | | | $ | 30,000 | |
Less accumulated amortization | | | (7,700 | ) | | | (2,500 | ) |
| | | | | | | | |
License fees, net | | $ | 87,300 | | | $ | 27,500 | |
| | | | | | | | |
| | |
| | Amortization expense of licenses was approximately $5,200, $1,500 and 7,700 for 2011, 2010 and for the period from April 19, 2007 (inception) through December 31, 2011, respectively. At December 31, 2011, the weighted average remaining amortization period for all licenses was approximately 13 years. The annual amortization expense of licenses for the next five years is estimated to be approximately $6,000 per year. |
| |
9. Related Party Transactions | | |
| |
Note payable - related party | | In October 2010, the CEO loaned the Company $25,000 and was issued an interest-free note payable for the amount of the loan. At various points in 2011, the CEO made interest-free, short-term loans to the Company which in the aggregate totaled $225,000. All the notes were repaid in full during 2011. Imputed interest on the loans was minimal.
There was approximately $0 and $94,400 in amounts due to the CEO recorded in accounts payable as of December 31, 2011 and 2010, respectively.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | | | |
10. Commitments and Contingencies | | |
| |
Operating leases | | The Company leases office and laboratory space under non-cancelable operating leases. The Company records rent expense on a straight-line basis over the life of the lease and records the excess of expense over the amounts paid as deferred rent.
Rent expense was approximately $145,200 and $107,500 for the years ended December 31, 2011 and 2010, respectively. Rent expense was approximately $324,600 for the period from April 19, 2007 (inception) through December 31, 2011.
|
| |
| | Future minimum rental payments required under operating leases that have initial or remaining non-cancelable lease terms in excess of one year are as follows: |
| | | | |
Year Ending December 31, | |
2012 | | $ | 125,095 | |
Thereafter | | | — | |
| | | | |
Total | | $ | 125,095 | |
| | | | |
| | |
11. Concentrations | | |
| |
Credit risk | | Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions primarily located in San Diego. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. At times, balances may exceed federally insured limits. The Company has not experienced losses in such accounts, and management believes that the Company is not exposed to any significant credit risk with respect to its cash and cash equivalents. |
| |
12. Subsequent Events | | |
| |
Merger transaction | | On February 8, 2012, the Company merged with and into Organovo Acquisition Corp. (“Acquisition Corp.”), a wholly-owned subsidiary of Organovo Holdings, Inc., a publicly traded Delaware corporation (“Organovo Holdings”), with the Company surviving the merger as a wholly-owned subsidiary of Organovo Holdings (the “Merger”). As a result of the Merger, Organovo Holdings acquired the business of the Company, and will continue the existing business operations of the Company.
Simultaneously with the Merger, on February 8, 2012 (the “Closing Date”), all of the issued and outstanding shares of the Company’s common stock converted, on a 1 for 1 basis, into shares of Organovo Holding’s common stock, par value $0.001 per share (“Common Stock”). Also on the Closing Date, all of the issued and outstanding options to purchase shares of the Company’s common stock and other outstanding warrants to purchase the Company’s common stock, and all of the issued and outstanding Bridge Warrants (as defined below) to purchase shares of the Company’s Common Stock, converted, respectively, into options (the “New Options”), warrants (the “New Warrants”) and new bridge warrants (the “New Bridge Warrants”) to purchase shares of Organovo Holding’s Common Stock. The New Bridge Warrants, the New Warrants and the New Bridge Options were converted on a 1 for 1 basis. The New Options will be administered under the Company’s 2008 Equity Incentive Plan (the “2008 Plan”), which Organovo Holding’s assumed and adopted on the Closing Date in connection with the Merger.
Specifically, on the Closing Date, (i) 22,445,254 shares of Common Stock were issued to the Company’s former stockholders; (ii) New Options to purchase 896,256 shares of Common Stock granted under the 2008 Plan were issued to the Company’s optionees pursuant to the assumption of the 2008 Plan by Organovo Holdings; (iii) New Warrants to purchase 1,309,750 shares of Organovo Holdings’ Common Stock at $1.00 per share were issued to holders of the Company’s warrants; and (iv) New Bridge Warrants to purchase 1,500,000 shares of Organovo Holdings’ Common Stock at $1.00 per share were issued to the Company’s Bridge Investors.
In connection with three separate closings of a private placement transaction completed in connection with the Merger (the “Offering”), the Company received gross proceeds of approximately $6,500,000 (including $1,500,000 previously received from the conversion of outstanding convertible notes payable), $1,800,000 and $6,900,000 on February 8, 2012, February 29, 2012 and March 16, 2012, respectively.
For all three closings of the Offering, the Company raised total gross proceeds of $15,247,959 and total net proceeds of $11,593,065.91 (or $12,811,897.11, including the conversion of the Bridge Notes referred to above). The Company issued 15,247,987 shares of Organovo Holdings’ Common Stock and warrants to purchase 16,747,987 shares of Organovo Holdings’ Common Stock (including warrants to purchase 1,500,000 shares to former holders of the Bridge Notes) exercisable at $1.00 to investors in the Offering. The placement agent and its selected dealers were paid total cash commissions of $1,372,260 and the Placement Agent was paid an expense allowance of $411,678 and was issued Placement Agent warrants to purchase 6,099,195 shares of Organovo Holdings’ Common Stock at an exercise price of $1.00 per share (including warrants to purchase 610,155 shares issued in connection with issuance of the Bridge Notes and subsequently exchanged for new warrants in the Merger).
The Merger will be treated as a recapitalization of the Company for financial accounting.
On February 8, 2012, the Company merged with and into Organovo Acquisition Corp. (“Acquisition Corp.”), a wholly-owned subsidiary of Organovo Holdings, Inc., a publicly traded Delaware corporation (“Organovo Holdings”), with the Company surviving the merger as a wholly-owned subsidiary of Organovo Holdings (the “Merger”). As a result of the Merger, Organovo Holdings acquired the business of the Company, and will continue the existing business operations of the Company.
Simultaneously with the Merger, on February 8, 2012 (the “Closing Date”), all of the issued and outstanding shares of the Company’s common stock converted, on a 1 for 1 basis, into shares of Organovo Holding’s common stock, par value $0.001 per share (“Common Stock”). Also on the Closing Date, all of the issued and outstanding options to purchase shares of the Company’s common stock and other outstanding warrants to purchase the Company’s common stock, and all of the issued and outstanding Bridge Warrants (as defined below) to purchase shares of the Company’s Common Stock, converted, respectively, into options (the “New Options”), warrants (the “New Warrants”) and new bridge warrants (the “New Bridge Warrants”) to purchase shares of Organovo Holding’s Common Stock. The New Bridge Warrants, the New Warrants and the New Bridge Options were converted on a 1 for 1 basis. The New Options will be administered under the Company’s 2008 Equity Incentive Plan (the “2008 Plan”), which Organovo Holding’s assumed and adopted on the Closing Date in connection with the Merger.
Specifically, on the Closing Date, (i) 22,445,254 shares of Common Stock were issued to the Company’s former stockholders; (ii) New Options to purchase 896,256 shares of Common Stock granted under the 2008 Plan were issued to the Company’s optionees pursuant to the assumption of the 2008 Plan by Organovo Holdings; (iii) New Warrants to purchase 1,309,750 shares of Organovo Holdings’ Common Stock at $1.00 per share were issued to holders of the Company’s warrants; and (iv) New Bridge Warrants to purchase 1,500,000 shares of Organovo Holdings’ Common Stock at $1.00 per share were issued to the Company’s Bridge Investors.
In connection with three separate closings of a private placement transaction completed in connection with the Merger (the “Offering”), the Company received gross proceeds of approximately $6,500,000 (including $1,500,000 previously received from the conversion of outstanding convertible notes payable), $1,800,000 and $6,900,000 on February 8, 2012, February 29, 2012 and March 16, 2012, respectively.
For all three closings of the Offering, the Company raised total gross proceeds of $15,247,959 and total net proceeds of $11,593,065.91 (or $12,811,897.11, including the conversion of the Bridge Notes referred to above). The Company issued 15,247,987 shares of Organovo Holdings’ Common Stock and warrants to purchase 16,747,987 shares of Organovo Holdings’ Common Stock (including warrants to purchase 1,500,000 shares to former holders of the Bridge Notes) exercisable at $1.00 to investors in the Offering. The placement agent and its selected dealers were paid total cash commissions of $1,372,260 and the Placement Agent was paid an expense allowance of $411,678 and was issued Placement Agent warrants to purchase 6,099,195 shares of Organovo Holdings’ Common Stock at an exercise price of $1.00 per share (including warrants to purchase 610,155 shares issued in connection with issuance of the Bridge Notes and subsequently exchanged for new warrants in the Merger).
The Merger will be treated as a recapitalization of the Company for financial accounting purposes. The historical financial statements of Organovo Holdings before the Merger will be replaced with the historical financial statements of the Company before the Merger in all future filings with the Securities and Exchange Commission (the “SEC”).
Before the Merger, Organovo Holdings’ board of directors and stockholders adopted the 2012 Equity Incentive Plan (the “2012 Plan”). The 2012 Plan provides for the issuance of 6,553,9856 shares of Organovo Holdings’ Common Stock to executive officers, directors, advisory board members and employees. In addition, Organovo Holdings assumed and adopted the Company’s 2008 Plan, and as described above option holders under that plan were granted New Options to purchase Common Stock. No further options will be granted under the 2008 Plan. The parties have taken all actions necessary to ensure that the Merger is treated as a tax free exchange under Section 368(a) of the Internal Revenue Code of 1986, as amended.
|
Organovo, Inc.
(A development stage company)
Notes to Financial Statements
| | |
| |
New facilities lease | | The Company entered into a new facilities lease at 6275 Nancy Ridge Drive, San Diego, CA 92121. The lease was signed on February 27, 2012 with target occupancy of July 15, 2012. The base rent under the lease is approximately $38,800 per month with 3% annual escalators. The lease term is 48 months with an option for the Company to extend the lease at the end of the lease term. |
UNAUDITED PRO FORMA COMBINED FINANCIAL STATEMENTS
Organovo Holdings, Inc. (f/k/a Real Estate Restoration & Rental, Inc.), a Delaware corporation (the “Parent”), Organovo Acquisition Corp., a Delaware corporation (the “Acquisition Subsidiary”) and Organovo, Inc., a Delaware corporation (the “Company”), are collectively referred to as the “Parties.”
The Parties entered into a mergersixty-two month lease agreement on February 8, 2012 that providesfor our long term permanent premises, consisting of approximately 8,051 square feet of lab and office space. In November 2021, we amended the permanent lease agreement to add an additional 2,892 square of office space in the same building. In December 2021, we took occupancy of our permanent lab and office space, located at 11555 Sorrento Valley Road, San Diego, CA 92121. See “Note 7. Leases” of the Notes to the Consolidated Financial Statements contained elsewhere in this prospectus for a mergerfurther discussion of the Acquisition Subsidiary with and into the Company, with the Company remaining as the surviving entity after the merger and operating a wholly-owned subsidiary of Parent (the “Merger”). In the Merger, the stockholders of the Company received common stock of the Parent in exchange for their capital stock of the Company.
Simultaneously with the closing of the Merger, the Parent completed a Private Placement (the “Private Placement”) of 5,000,500 units at the purchase price of $1.00 per unit. Each unit consisted of one share of the Parent’s common stock, par value $0.001 per share, and one five year warrant to purchase one share of Parent common stock at an exercise price of $1.00 per share.
Also simultaneously with the closing of the Merger, the Company converted principal and interest of $1,525,387 related to its bridge financing (the “Bridge Conversion”) into 1,525,387 shares of common stock, and issued five year warrants to purchase 1,525,387 shares of common stock at $1.00 per share.
Immediately following the Merger, the Parent split-off its wholly owned subsidiary, Organovo Split Corp., a Delaware corporation (the “Split-Off Subsidiary”), through the sale of all of the outstanding capital stock of the Split-Off Subsidiary (the “Split-Off”) upon the terms and conditions of a split-off agreement.
The following unaudited pro forma combined balance sheet combines the historical balance sheet of the Parent as of December 31, 2011 and the historical balance sheet of the Company as of December 31, 2011, following the completion of the Merger, Private Placement, Bridge Conversion and Split-Off (collectively “the Transactions”). The Company remained as the surviving corporation of the Merger, becoming a wholly-owned subsidiary of the Parent. The pro forma combined balance sheet presented herein reflects the effects of the Transactions as if they had been consummated on December 31, 2011.
The following unaudited pro forma combined statements of operations combines the historical statements of operations of the Parent for the year ended December 31, 2011 and the Company for the year ended December 31, 2011, giving effect to the Transactions, as if they had occurred on January 1, 2011.
The following unaudited pro forma combined financial statements are presented to illustrate the estimated effects of the Transactions. The historical financial information has been adjusted to give effect to pro forma events that are directly attributable to the Transactions and factually supportable.
The following information should be read in conjunction with the pro forma combined financial statements.
properties.
Accompanying notes to the unaudited pro forma combined financial statements.40
Separate historical financial statements of the Parent for the year ended December 31, 2011 as filed in its Annual Report on Form 10-K with the Securities and Exchange Commission.
Separate historical financial statements of the Company for the year ended December 31, 2011 included it this Current Report on Form 8-K/A.
The unaudited pro forma combined financial statements are presented for informational purposes only. The pro forma information is not necessarily indicative of what the financial position or results of operations actually would have been had the Transactions been completed at the dates indicated. In addition, the unaudited pro forma combined financial statements do not purport to project the future financial position or operating results of the combined company.
The unaudited pro forma combined financial statements were prepared using the reverse acquisition application of the acquisition method of accounting as described in ASC 805-40-05-2, with the Company treated as the acquiror for U.S. GAAP accounting and financial reporting purposes. Accordingly, the unaudited pro forma combined financial statements are presented as a continuation of the Company’s financial statements with adjustments to reflect the Transactions.
Organovo Holdings, Inc.
Pro Forma Combined Balance Sheet at December 31, 2011
| | | | | | | | | | | | | | | | | | | | |
| | | Real Estate Restoration
& Rental, Inc. | | | Organovo, Inc. | | | Pro Forma
Adjustments | | | | | | Organovo
Holdings,
Inc. | |
| | | December 31, 2011 | | | December 31, 2011 | | | | | | | | | Pro Forma | |
Assets | | | | | | | | | | | | | | | | | | | | |
Current Assets | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 753 | | | $ | 339,607 | | | $ | (104,219 | ) | | | (1 | ) | | $ | 4,585,823 | |
| | | | | | | | | | | 5,000,500 | | | | (3 | ) | | | | |
| | | | | | | | | | | (650,065 | ) | | | (3 | ) | | | | |
| | | | | | | | | | | (753 | ) | | | (4 | ) | | | | |
Inventory | | | — | | | | 291,881 | | | | | | | | | | | | 291,881 | |
Deferred financing costs | | | — | | | | 318,843 | | | | (248,857 | ) | | | (2 | ) | | | 69,986 | |
Prepaid expenses and other current assets | | | 2,500 | | | | 79,874 | | | | (2,500 | ) | | | (4 | ) | | | 79,874 | |
| | | | | | | | | | | | | | | | | | | | |
Total current assets | | | 3,253 | | | | 1,030,205 | | | | 3,994,106 | | | | | | | | 5,027,564 | |
Fixed Assets - Net | | | — | | | | 278,208 | | | | | | | | | | | | 278,208 | |
Other Assets - Net | | | — | | | | 100,419 | | | | | | | | | | | | 100,419 | |
| | | | | | | | | | | | | | | | | | | | |
Total assets | | $ | 3,253 | | | $ | 1,408,832 | | | $ | 3,994,106 | | | | | | | $ | 5,406,191 | |
| | | | | | | | | | | | | | | | | | | | |
Liabilities and Stockholders’ Equity (Deficit) | | | | | | | | | | | | | | | | | | | | |
Current Liabilities | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 61,198 | | | $ | 657,560 | | | $ | (61,198 | ) | | | (4 | ) | | $ | 657,560 | |
Accrued expenses | | | — | | | | 437,837 | | | | | | | | | | | | 437,837 | |
Deferred revenue | | | 3,323 | | | | 152,500 | | | | (3,323 | ) | | | (4 | ) | | | 152,500 | |
Accrued interest payable | | | — | | | | 24,018 | | | | 796 | | | | (1 | ) | | | — | |
| | | | | | | | | | | (4,219 | ) | | | (1 | ) | | | | |
| | | | | | | | | | | 4,792 | | | | (2 | ) | | | | |
| | | | | | | | | | | (25,387 | ) | | | (2 | ) | | | | |
Convertible notes payable, net | | | 9,500 | | | | 703,833 | | | | (100,000 | ) | | | (1 | ) | | | — | |
| | | | | | | | | | | (1,500,000 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | 896,167 | | | | (2 | ) | | | | |
| | | | | | | | | | | (9,500 | ) | | | (4 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total current liabilities | | | 74,021 | | | | 1,975,748 | | | | (801,872 | ) | | | | | | | 1,247,897 | |
Derivative liabilities | | | | | | | 1,266,869 | | | | 52,876 | | | | (2 | ) | | | 1,573,169 | |
| | | | | | | | | | | 10,751 | | | | (2 | ) | | | | |
| | | | | | | | | | | 242,673 | | | | (3 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total liabilities | | | 74,021 | | | | 3,242,617 | | | | (495,572 | ) | | | | | | | 2,821,066 | |
| | | | | | | | | | | | | | | | | | | | |
Commitments and Contingencies | | | | | | | | | | | | | | | | | | | | |
| | | | | |
Stockholders’ Equity (Deficit) | | | | | | | | | | | | | | | | | | | | |
Common stock, $0.0001 par value; 75,000,000 shares authorized, 22,445,254 shares issued and outstanding prior to the merger; 28,971,141 shares issued and outstanding after the merger | | | 680 | | | | 2,245 | | | | 152 | | | | (2 | ) | | | 2,897 | |
| | | | | | | | | | | 500 | | | | (3 | ) | | | | |
| | | | | | | | | | | (680 | ) | | | (4 | ) | | | | |
Additional paid-in capital | | | 181,245 | | | | 4,855,526 | | | | 1,525,235 | | | | (2 | ) | | | 10,477,272 | |
| | | | | | | | | | | (10,751 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | 5,000,000 | | | | (3 | ) | | | | |
| | | | | | | | | | | (650,065 | ) | | | (3 | ) | | | | |
| | | | | | | | | | | (242,673 | ) | | | (3 | ) | | | | |
| | | | | | | | | | | (181,245 | ) | | | (4 | ) | | | | |
Deficit accumulated during the development stage | | | (252,693 | ) | | | (6,691,556 | ) | | | (796 | ) | | | (1 | ) | | | (7,895,044 | ) |
| | | | | | | | | | | (4,792 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | (896,167 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | (248,857 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | (52,876 | ) | | | (2 | ) | | | | |
| | | | | | | | | | | 252,693 | | | | (4 | ) | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total stockholders’ equity (deficit) | | | (70,768 | ) | | | (1,833,785 | ) | | | 4,489,678 | | | | | | | | 2,585,125 | |
| | | | | | | | | | | | | | | | | | | | |
Total Liabilities and Stockholders’ Equity (Deficit) | | $ | 3,253 | | | $ | 1,408,832 | | | $ | 3,994,106 | | | | | | | $ | 5,406,191 | |
| | | | | | | | | | | | | | | | | | | | |
See notes to unaudited pro forma combined financial information. | |
Organovo Holdings, Inc.
Pro Forma Combined Statement of Operations for the Year Ended December 31, 2011
| | | | | | | | | | | | | | | | | | | | |
| | | Real Estate
Restoration & Rental,
Inc. | | | Organovo, Inc. | | | Pro Forma
Adjustments | | | | | | Organovo
Holdings, Inc. | |
| | | 12 Months Ended
December 31, 2011 | | | Year Ended
December 31, 2011 | | | | | | | | | Pro
Forma | |
Revenues | | | | | | | | | | | | | | | | | | | | |
Product | | $ | 1,677 | | | $ | 223,500 | | | $ | (1,677 | ) | | | | | | $ | 223,500 | |
Collaborations | | | — | | | | 688,088 | | | | — | | | | | | | | 688,088 | |
Grants | | | — | | | | 56,925 | | | | — | | | | | | | | 56,925 | |
| | | | | | | | | | | | | | | | | | | | |
Total Revenues | | | 1,677 | | | | 968,513 | | | | (1,677 | ) | | | | | | | 745,013 | |
Costs of product revenue | | | | | | | 133,607 | | | | | | | | | | | | | |
Professional fees | | | 111,352 | | | | — | | | | (111,352 | ) | | | (4 | ) | | | — | |
General and administrative expenses | | | 21,824 | | | | 1,705,171 | | | | (21,824 | ) | | | (4 | ) | | | 1,705,171 | |
Research and development expenses | | | — | | | | 1,419,718 | | | | — | | | | | | | | 1,419,718 | |
Impairment of licensing rights | | | 27,723 | | | | — | | | | (27,723 | ) | | | (4 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Loss from Operations | | | (160,899 | ) | | | (2,289,983 | ) | | | 160,899 | | | | | | | | (2,379,876 | ) |
| | | | | | | | | | | | | | | | | | | | |
Other Income (Expense) | | | | | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | | (2,066,889 | ) | | | 2,066,889 | | | | (5 | ) | | | — | |
Interest income | | | — | | | | 64 | | | | — | | | | | | | | 64 | |
Miscellaneous income (expense) | | | — | | | | (26,454 | ) | | | — | | | | | | | | (26,454 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total Other Income (Expense) | | | — | | | | (2,093,279 | ) | | | 2,066,889 | | | | | | | | (26,390 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net Loss | | $ | (160,899 | ) | | $ | (4,383,262 | ) | | $ | 2,214,706 | | | | | | | $ | (2,406,266 | ) |
| | | | | | | | | | | | | | | | | | | | |
Basic and Diluted Net Loss Per Common Share | | $ | (0.02 | ) | | | | | | | | | | | | | | $ | (0.10 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted Average Common Shares - Basic and Diluted | | | 6,799,815 | | | | | | | | 16,125,879 | | | | (6 | ) | | | 22,925,694 | |
| | | | | | | | | | | | | | | | | | | | |
See notes to unaudited pro forma combined financial information.
NOTES TO UNAUDITED PRO FORMA
COMBINED FINANCIAL STATEMENTS
1. | Description of Transaction and Basis of Presentation |
Organovo Holdings, Inc. (f/k/a Real Estate Restoration & Rental, Inc.), a Delaware corporation (the “Parent”), Organovo Acquisition Corp., a Delaware corporation (the “Acquisition Subsidiary”) and Organovo, Inc., a Delaware corporation (the “Company”) are collectively referred to as the “Parties.”
The parties entered into a merger agreement on February 8, 2012 that provides for a merger of the Acquisition Subsidiary with and into the Company, with the Company remaining as the surviving entity after the merger and operating as a wholly-owned subsidiary of Parent (the “Merger”). In the Merger, the stockholders of the Company received common stock of the Parent in exchange for their capital stock of the Company.
Simultaneously with the closing of the Merger, the Parent completed a Private Placement (the “Private Placement”) of 5,000,500 units at the purchase price of $1.00 per unit. Each unit consisted of one share of the Parent’s common stock, par value $0.001 per share and one five year warrant to purchase one share of Parent common stock at an exercise price of $1.00 per share.
Also simultaneously with the closing of the Merger, the Company converted principal and interest of $1,525,387 related to its bridge financing (the “Bridge Conversion”) into 1,525,387 shares of common stock, and issued five year warrants to purchase 1,525,387 shares of common stock at $1.00 per share.
Immediately following the Merger, the Parent split-off its wholly owned subsidiary, Organovo Split Corp., a Delaware corporation (the “Split-Off Subsidiary”), through the sale of all of the outstanding capital stock of the Split-Off Subsidiary (the “Split-Off”) upon the terms and conditions of a split-off agreement.
The unaudited pro forma combined balance sheet combines the historical balance sheet of the Parent as of December 31, 2011 and the historical balance sheet of the Company as of December 31, 2011, following the completion of the Merger, Private Placement, Bridge Conversion and the Split-Off (collectively “the Transactions”). The Company remained as the surviving corporation of the Merger, becoming a wholly-owned subsidiary of the Parent. The pro forma combined balance sheet presented herein reflects the effects of the Transactions as if they had been consummated on December 31, 2011.
The unaudited pro forma combined statements of operations combines the historical statements of operations of the Parent for the year ended December 31, 2011 and the Company for year ended December 31, 2011, giving effect to the Transactions, as if they had occurred on January 1, 2011.
The unaudited pro forma combined financial statements are presented to illustrate the estimated effects of the Transactions. The historical financial information has been adjusted to give effect to pro forma events that are directly attributable to the Transactions and factually supportable.
There were no inter-company balances and transactions between the Parent and the Company as of the dates and for the periods of these pro forma condensed combined financial statements.
The pro forma adjustments included in the unaudited pro forma condensed combined financial statements are as follows:
| 1) | To record payment of a $100,000 convertible note and $4,219 of accrued interest at the Merger date. |
| 2) | To record the conversion of $1,500,000 in convertible notes payable and $25,387 in accrued interest into 1,525,387 shares of common stock issued in the Private Placement; and to record the discount of $896,167 as interest expense upon conversion; and to record interest expense of $179,177 for amortization of the deferred bridge financing costs upon conversion; and to record a reduction of equity of $139,667 to write-off merger related deferred financing costs; and to record interest expense of $52,600 related to the value of the 1,525,387 warrants issued in the Private Placement in connection with the conversion of the convertible notes; and to record offering costs of $21,040 related to the value of the 610,155 warrants issued to the placement agent. |
The exercise price of the warrants is protected against down-round financing throughout the term of the warrant. Pursuant to ASC 815-15 and ASC 815-40, the fair value of the warrants was recorded as a derivative liability on the issuance date. The Company calculated the fair value of the warrants using the Black-Scholes Model using a volatility of 109.84%, an interest rate of 0.83% and a dividend yield of zero. The use of a binomial valuation model might result in a different valuation.
| 3) | To record the issuance of 5,000,500 units in the Private Placement; and to record transaction expenses of $650,065 payable to the placement agent; and to record a derivative liability of $241,405 related to the value of the 5,000,500 warrants issued in the Private Placement and the 2,000,200 warrants issued to the placement agent. |
The exercise price of the warrants is protected against down-round financing throughout the term of the warrant. Pursuant to ASC 815-15 and ASC 815-40, the fair value of the warrants was recorded as a derivative liability on the issuance date. The Company calculated the fair value of the warrants using the Black-Scholes
Model using a volatility of 109.84%, an interest rate of 0.83% and a dividend yield of zero. The use of a binomial valuation model might result in a different valuation.
| 4) | To record the effect of the Split-Off. |
| 5) | To reverse interest expense of $2,066,889 related to convertible notes payable assumed to be converted as of January 1, 2011. |
| 6) | To reflect the shares issued in the Private Placement (5,000,500) and Bridge Conversion (1,525,387) as issued and outstanding as of January 1, 2011. |
3. | Pro Forma Net Loss Per Share |
The pro forma basic and diluted net loss per share are based on the number shares of common stock issued and outstanding of the Company after the Transactions, and assumes all common shares issued in the Transactions were issued and outstanding as of January 1, 2011.
Organovo Holdings Inc.
(A development stage company)
Balance Sheets
| | | | | | | | |
| | | March 31, 2012 | | | December 31, 2011 | |
| | | (Unaudited) | | | (Audited) | |
Assets | | | | | | | | |
Current Assets | | | | | | | | |
Cash and cash equivalents | | $ | 10,352,507 | | | $ | 339,607 | |
Inventory | | | 337,396 | | | | 291,881 | |
Deferred financing costs | | | — | | | | 318,843 | |
Prepaid expenses and other current assets | | | 144,800 | | | | 79,874 | |
| | | | | | | | |
Total current assets | | | 10,834,703 | | | | 1,030,205 | |
Fixed Assets - Net | | | 268,886 | | | | 278,208 | |
Restricted cash | | | 38,290 | | | | | |
Other Assets | | | 98,671 | | | | 100,419 | |
| | | | | | | | |
Total assets | | $ | 11,240,550 | | | $ | 1,408,832 | |
| | | | | | | | |
Liabilities and Stockholders’ Deficit | | | | | | | | |
Current Liabilities | | | | | | | | |
Accounts payable | | $ | 440,890 | | | $ | 657,560 | |
Accrued expenses | | | 401,183 | | | | 437,837 | |
Deferred revenue | | | 268,875 | | | | 152,500 | |
Accrued interest payable | | | — | | | | 24,018 | |
Convertible notes payable, current portion | | | — | | | | 703,833 | |
| | | | | | | | |
Total current liabilities | | | 1,110,948 | | | | 1,975,748 | |
Warrant Liabilities | | | 47,514,710 | | | | 1,266,869 | |
| | | | | | | | |
Total liabilities | | $ | 48,625,658 | | | $ | 3,242,617 | |
Commitments and contingencies (Note 5) | | | | | | | | |
Stockholders’ Deficit | | | | | | | | |
Common stock, $0.001 par value; 75,000,000 shares authorized, 43,693,241 and 22,445,254 issued and outstanding at March 31, 2012 and December 31, 2011, respectively | | | 43,693 | | | | 22,445 | |
Additional paid-in capital | | | 6,343,337 | | | | 4,835,326 | |
Deficit accumulated during the development stage | | | (43,772,138 | ) | | | (6,691,556 | ) |
| | | | | | | | |
Total stockholders’ deficit | | | (37,385,108 | ) | | | (1,833,785 | ) |
| | | | | | | | |
Total Liabilities and Stockholders’ Deficit | | $ | 11,240,550 | | | $ | 1,408,832 | |
| | | | | | | | |
The accompanying notes are an integral part of these condensed financial statements.
Organovo Holdings, Inc.
(A development stage company)
Unaudited Statements of Operations
| | | | | | | | | | | | |
| | | Three Months Ended
March 31, 2012 | | | Three Months Ended
March 31, 2011 | | | Period from
April 19, 2007
(Inception)
through
March 31, 2012 | |
Revenues | | | | | | | | | | | | |
Product | | $ | — | | | $ | 100,000 | | | $ | 223,500 | |
Collaborations | | | 120,000 | | | | 73,865 | | | | 883,088 | |
Grants | | | — | | | | 26,924 | | | | 664,112 | |
| | | | | | | | | | | | |
Total Revenues | | | 120,000 | | | | 200,789 | | | | 1,770,700 | |
Cost of product revenue | | | — | | | | 50,584 | | | | 133,607 | |
Selling, general, and administrative expenses | | | 901,843 | | | | 243,494 | | | | 3,567,881 | |
Research and development expenses | | | 547,287 | | | | 398,664 | | | | 3,745,675 | |
| | | | | | | | | | | | |
Loss from Operations | | | (1,329,130 | ) | | | (491,953 | ) | | | (5,676,463 | ) |
| | | | | | | | | | | | |
Other Income (Expense) | | | | | | | | | | | | |
Fair value of warrant liabilities in excess of proceeds received | | | (19,019,422 | ) | | | — | | | | (19,019,422 | ) |
Change in fair value of warrant liabilities | | | (13,505,819 | ) | | | — | | | | (13,512,388 | ) |
Financing transaction costs in excess of proceeds received | | | (2,129,500 | ) | | | — | | | | (2,129,500 | ) |
Interest expense | | | (1,087,453 | ) | | | (53,082 | ) | | | (3,405,895 | ) |
Interest income | | | 291 | | | | — | | | | 2,298 | |
Other expense | | | (9,549 | ) | | | (1,550 | ) | | | (30,768 | ) |
| | | | | | | | | | | | |
Total Other Income (Expense) | | | (35,751,452 | ) | | | (54,632 | ) | | | (38,095,675 | ) |
| | | | | | | | | | | | |
Net Loss | | $ | (37,080,582 | ) | | $ | (546,585 | ) | | $ | (43,772,138 | ) |
| | | | | | | | | | | | |
| | | |
Net loss per common share - basic and diluted | | $ | (1.17 | ) | | $ | (0.04 | ) | | | | |
Weighted average shares used in computing net loss per common share - basic and diluted | | | 31,591,663 | | | | 14,881,058 | | | | | |
The accompanying notes are an integral part of these condensed financial statements.
Organovo Holdings, Inc.
(A development stage company)
Unaudited Statements of Stockholders’ Deficit
Period from April 19, 2007 (Inception) through March 31, 2012
| | | | | | | | | | | | | | | | | | | | |
| | | | | | Additional | | | Deficit
Accumulated
During the
Development | | | | |
| | | Common Stock | | | | |
| | | Shares | | | Amount | | | Paid-in Capital | | | Stage | | | Total | |
Balance at inception (April 19, 2007) | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Issuance of Common stock | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | — | |
Net Loss | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Issuance of Common stock to founders | | | 1,729,532 | | | | 1,730 | | | | (1,730 | ) | | | — | | | | — | |
Issuance of restricted Common stock | | | 12,627,697 | | | | 12,628 | | | | (12,628 | ) | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 1,742 | | | | — | | | | 1,742 | |
Net Loss | | | — | | | | — | | | | — | | | | (97,559 | ) | | | (97,559 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 14,357,229 | | | $ | 14,358 | | | $ | (12,616 | ) | | $ | (97,559 | ) | | $ | (95,817 | ) |
Issuance of restricted Common stock | | | 130,422 | | | | 130 | | | | (130 | ) | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 2,336 | | | | — | | | | 2,336 | |
Net Loss | | | — | | | | — | | | | — | | | | (872,041 | ) | | | (872,041 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 14,487,651 | | | $ | 14,488 | | | $ | (10,410 | ) | | $ | (969,600 | ) | | $ | (965,522 | ) |
Issuance of restricted Common stock | | | 219,369 | | | | 219 | | | | (219 | ) | | | | | | | — | |
Stock-based compensation expense | | | | | | | | | | | 3,856 | | | | | | | | 3,856 | |
Net Loss | | | | | | | | | | | | | | | (1,338,694 | ) | | | (1,338,694 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 14,707,020 | | | $ | 14,707 | | | $ | (6,773 | ) | | $ | (2,308,294 | ) | | $ | (2,300,360 | ) |
Issuance of Common stock through conversion of notes payable | | | 7,676,828 | | | | 7,677 | | | | 3,482,081 | | | | | | | | 3,489,758 | |
Issuance of restricted Common stock | | | 61,406 | | | | 61 | | | | (61 | ) | | | — | | | | — | |
Warrants issued with convertible notes and conversion of notes | | | — | | | | — | | | | 1,111,364 | | | | — | | | | 1,111,364 | |
Beneficial conversion feature of convertible notes payable | | | — | | | | — | | | | 239,700 | | | | — | | | | 239,700 | |
Stock-based compensation expense | | | — | | | | — | | | | 9,015 | | | | — | | | | 9,015 | |
Net Loss | | | — | | | | — | | | | — | | | | (4,383,262 | ) | | | (4,383,262 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | | | 22,445,254 | | | $ | 22,445 | | | $ | 4,835,326 | | | $ | (6,691,556 | ) | | $ | (1,833,785 | ) |
Issuance of Common stock in connection with the merger | | | 6,000,000 | | | | 6,000 | | | | (6,000 | ) | | | — | | | | — | |
Issuance of Common stock through private placements in connection with the merger | | | 13,722,600 | | | | 13,723 | | | | 13,708,877 | | | | — | | | | 13,722,600 | |
Costs associated with the merger | | | — | | | | — | | | | (13,722,600 | ) | | | — | | | | (13,722,600 | ) |
Issuance of Common stock through conversion of notes payable and accrued interest in connection with the merger | | | 1,525,387 | | | | 1,525 | | | | 1,523,862 | | | | — | | | | 1,525,387 | |
Stock-based compensation expense | | | — | | | | — | | | | 3,872 | | | | — | | | | 3,872 | |
Net Loss | | | — | | | | — | | | | — | | | | (37,080,582 | ) | | | (37,080,582 | ) |
| | | | | | | | | | | | | | | | | | | | |
Balance at March 31, 2012 | | | 43,693,241 | | | $ | 43,693 | | | $ | 6,343,337 | | | $ | (43,772,138 | ) | | $ | (37,385,108 | ) |
| | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these condensed financial statements.
Organovo Holdings, Inc.
(A development stage company)
Unaudited Statements of Cash Flows
| | | | | | | | | | | | |
| | | Three Months Ended
March 31, 2012 | | | Three Months Ended
March 31, 2011 | | | Period from
April 19, 2007
(Inception)
through
March 31, 2012 | |
Cash Flows From Operating Activities | | | | | | | | | | | | |
Net loss | | $ | (37,080,582 | ) | | $ | (546,585 | ) | | $ | (43,772,138 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Amortization of deferred financing costs | | | 318,843 | | | | — | | | | 438,296 | |
Depreciation and amortization | | | 16,607 | | | | 15,596 | | | | 172,935 | |
Amortization of debt discount | | | 896,167 | | | | — | | | | 2,083,735 | |
Interest accrued on convertible notes payable | | | 11,616 | | | | 53,082 | | | | 495,392 | |
Fair value of warrant liabilities in excess of proceeds | | | 19,019,422 | | | | — | | | | 19,019,422 | |
Change in fair value of warrant liabilities | | | 13,505,819 | | | | — | | | | 13,512,388 | |
Stock-based compensation | | | 3,872 | | | | 1,187 | | | | 20,821 | |
Warrants issued in connection with exchange agreement | | | — | | | | — | | | | 527,629 | |
Increase (decrease) in cash resulting from changes in: | | | | | | | | | | | | |
Accounts receivable | | | — | | | | (199,213 | ) | | | — | |
Grants receivable | | | — | | | | 59,744 | | | | — | |
Inventory | | | (45,515 | ) | | | (10,634 | ) | | | (337,396 | ) |
Prepaid expenses and other assets | | | (64,926 | ) | | | (14,125 | ) | | | (157,932 | ) |
Accounts payable | | | (216,670 | ) | | | 61,630 | | | | 440,890 | |
Accrued expenses | | | (36,654 | ) | | | (872 | ) | | | 401,183 | |
Deferred revenue | | | 116,375 | | | | 268,423 | | | | 268,875 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (3,555,626 | ) | | | (311,767 | ) | | | (6,885,900 | ) |
| | | | | | | | | | | | |
Cash Flows From Investing Activities | | | | | | | | | | | | |
Restricted cash deposits | | | (38,290 | ) | | | — | | | | (38,290 | ) |
Purchases of fixed assets | | | (5,537 | ) | | | — | | | | (432,360 | ) |
Purchases of intangible assets | | | — | | | | — | | | | (95,000 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (43,827 | ) | | | — | | | | (565,650 | ) |
| | | | | | | | | | | | |
Cash Flows From Financing Activities | | | | | | | | | | | | |
Proceeds from issuance of convertible notes payable | | | — | | | | 160,000 | | | | 4,630,000 | |
Proceeds from issuance of common stock and warrants | | | 13,722,600 | | | | — | | | | 13,722,600 | |
Proceeds from issuance of related party notes payable | | | — | | | | — | | | | 250,000 | |
Repayment of related party notes payable | | | — | | | | (25,000 | ) | | | (250,000 | ) |
Repayment of convertible notes and interest payable | | | (110,247 | ) | | | — | | | | (110,247 | ) |
Deferred financing costs | | | — | | | | (901 | ) | | | (438,296 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 13,612,353 | | | | 134,099 | | | | 17,804,057 | |
| | | | | | | | | | | | |
Net Increase (Decrease) in Cash and Cash Equivalents | | | 10,012,900 | | | | (177,668 | ) | | | 10,352,507 | |
Cash and Cash Equivalents at Beginning of Period | | | 339,607 | | | | 285,308 | | | | — | |
| | | | | | | | | | | | |
Cash and Cash Equivalents at End of Period | | $ | 10,352,507 | | | $ | 107,640 | | | $ | 10,352,507 | |
| | | | | | | | | | | | |
| | | |
Supplemental Disclosure of Cash Flow Information: | | | | | | | | | | | | |
Interest | | $ | 10,247 | | | $ | — | | | | 10,247 | |
Income Taxes | | $ | 800 | | | $ | 2,400 | | | | 3,200 | |
Supplemental Disclosure of Noncash Investing and Financing Activities:
During 2008, the Company issued 1,729,532 shares of Common stock to its founders.
During 2011 and 2010 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued 61,406, 219,369 and 13,038,894, respectively, shares of restricted Common stock to certain employees, advisors and consultants of the Company.
During 2011 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued certain convertible notes payable that included warrants. The warrants and the related beneficial conversion feature, valued at $823,435 were classified as equity instruments and recorded as a discount to the carrying value of the related debt.
During 2011 and for the period from April 19, 2007 (Inception) through December 31, 2011, the Company issued warrants, valued at approximately $1,260,300, in connection with certain convertible notes payable. The warrants were recorded as a warrant liability and recorded as a discount to the carrying value related to debt.
During 2011, the Company issued 7,676,828 shares of Common stock to note holders for the conversion of Convertible Notes with a principal balance totaling $3,030,000 and accrued interest totaling $459,758.
During 2012, the Company issued 1,525,387 shares of Common stock to note holders for the conversion of Convertible Notes with a principal balance totaling $1,500,000 and accrued interest totaling $25,387.
The accompanying notes are an integral part of these condensed financial statements.
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
1. Summary of Significant Accounting Policies
|
Nature of operations and basis of presentation | | References in these notes to the unaudited condensed financial statements to “Organovo Holdings, Inc.,” “Organovo Holdings,” “we,” “us,” “our,” “the Company” and “our Company” refer to Organovo Holdings, Inc. and its consolidated subsidiary Organovo, Inc.
The Company has developed and is commercializing a platform technology for the generation of three-dimensional (3D) human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs.
As of March 31, 2012, the Company has devoted substantially all of its efforts to product development, raising capital, and building infrastructure. The Company has not realized significant revenues from its planned principal operations. Accordingly, the Company is considered to be in the development stage.
The accompanying interim condensed financial statements have been prepared by the Company, without audit, in accordance with the instructions to Form 10-Q and, therefore, do not necessarily include all information and footnotes necessary for a fair statement of its financial position, results of operations and cash flows in accordance with generally accepted accounting principles (“GAAP”). The balance sheet at December 31, 2011 is derived from the audited balance sheet at that date.
In the opinion of management, the unaudited financial information for the interim periods presented reflects all adjustments, which are only normal and recurring, necessary for a fair statement of financial position, results of operations and cash flows. These financial statements should be read in conjunction with the financial statements included in the Company’s Form 8-K/A for the fiscal year ended December 31, 2011 filed with the Securities and Exchange Commission (the “SEC”) on May 11, 2012. Operating results for interim periods are not necessarily indicative of operating results for the Company’s 2012 fiscal year.
|
| |
Merger transaction | | On February 8, 2012, Organovo, Inc., a privately held Delaware corporation, merged with and into Organovo Acquisition Corp., a wholly-owned subsidiary of the Company, a publicly traded Delaware corporation, with the Organovo, Inc. surviving the merger as a wholly-owned subsidiary of the Company (the “Merger”). As a result of the Merger, the Company acquired the business of the Organovo, Inc., and will continue the existing business operations of Organovo, Inc.
Simultaneously with the Merger, on February 8, 2012 (the “closing date”), all of the issued and outstanding shares of Organovo, Inc.’s common stock converted, on a 1 for 1 basis, into shares of the Company’s Common stock, par value $0.001 per share. Also on the closing date, all of the issued and outstanding options to purchase shares of Organovo, Inc.’s common stock and other outstanding warrants to purchase Organovo, Inc.’s common stock, and all of the issued and outstanding bridge warrants to purchase shares of Organovo, Inc.’s common stock, converted, respectively, on a 1 for 1 basis, into options, warrants and new bridge warrants to purchase shares of the Company’s common stock.
Immediately following the consummation of the Merger: (i) the former security holders of Organovo, Inc. common stock had an approximate 75% voting interest in the Company and the Company stockholders retained an approximate 25% voting interest, (ii) former executive management team of Organovo, Inc. remained as the only continuing executive management team for the Company, and (iii) the Company’s ongoing operations consist solely of the ongoing operations of Organovo, Inc. Based primarily on these factors, the Merger was accounted for as a reverse merger and a recapitalization in accordance with GAAP. As a result, these financial statements reflect the historical results of Organovo, Inc. prior to the Merger, and the combined results of the Company following the Merger. The par value of Organovo, Inc. common stock immediately prior to the Merger was $0.0001 per share. The par value subsequent to the Merger is $0.001 per share, and therefore the historical results of Organovo, Inc. prior to the Merger have been retroactively adjusted to affect the change in par value.
In connection with three separate closings of a private placement transaction completed in connection with the Merger (the “Private Placement”), the Company received gross proceeds of approximately $5,000,000, $1,800,000 and $6,900,000 on February 8, 2012, February 29, 2012 and March 16, 2012, respectively. The Company previously received $1,500,000 from the purchase of 6% convertible notes which were automatically converted into 1,500,000 shares of common stock, plus 25,387 shares for accrued interest of $25,387 on the principal, at February 8, 2012. See Note 3.
|
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
| | The cash transaction costs related to the Merger were approximately $2,129,500.
Before the Merger, Organovo Holdings’ board of directors and stockholders adopted the 2012 Equity Incentive Plan (the “2012 Plan”). The 2012 Plan provides for the issuance of 6,553,986 shares of the Company’s Common stock to executive officers, directors, advisory board members and employees. In addition, Organovo Holdings assumed and adopted Organovo, Inc.’s 2008 Equity Incentive Plan.
|
| |
Liquidity | | As of March 31, 2012, the Company had an accumulated deficit of approximately $43,772,000. The Company also had negative cash flow from operations of approximately $3,556,000 during the three months ended March 31, 2012.
On February 8, 2012, the Company received gross proceeds of approximately $5,000,000 in a private placement offering in conjunction with the Merger. On February 29, 2012 and March 16, 2012, the Company completed two additional closings of its Private Placement and received total gross proceeds of approximately $8,722,000.
The Company expects to cover its anticipated operating expenses over the next twelve months through cash on hand including the funds raised during the first quarter of 2012 through the Private Placement of its securities and funds received through collaborative agreements, and other commercial arrangements.
The Company’s ability to continue its operations is dependent upon its ability to raise additional capital through equity or debt financing, and to generate capital through collaborative research agreements and other commercial arrangements. There can be no assurance that any additional financing will be available on acceptable terms or available at all. Any equity financing may result in dilution to existing stockholders and any debt financing may include restrictive covenants.
The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of these uncertainties.
|
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
Use of estimates | | The preparation of the financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. Significant estimates used in preparing the financial statements include those assumed in computing the valuation of warrants and conversion features, revenue recognized under the proportional performance model, the valuation of stock-based compensation expense, and the valuation allowance on deferred tax assets. |
| |
Cash and cash equivalents | | The Company considers all highly liquid investments with original maturities of 90 days or less to be cash equivalents. |
| |
Restricted cash | | As of March 31, 2012, the Company had $38,290 of restricted cash deposited with a financial institution, held in certificates of deposit to support a letter of credit agreement related to the facility lease entered into during 2012. |
| |
Inventory | | Inventories are stated at the lower of the cost or market (first-in, first out). Inventory at March 31, 2012 consisted of approximately $279,000 in finished goods and approximately $58,000 in raw materials. Inventory at December 31, 2011 consisted of approximately $235,000 in finished goods and approximately $56,900 in raw materials.
The Company provides inventory allowances based on excess or obsolete inventories determined based on anticipated use in the final product. There was no obsolete inventory reserve as of March 31, 2012 or December 31, 2011.
|
| |
Deferred financing costs | | As of December 31, 2011, deferred financing costs consisted of approximately $140,000 associated with the Merger transaction and approximately $179,000 associated with convertible notes as part of the private placement offering that was initiated in the fourth quarter of 2011. The deferred financing costs related to the private placement offering were amortized over the life of the convertible notes and fully amortized to expense upon conversion of the convertible notes on February 8, 2012. The deferred financing costs associated with the Merger transaction in excess of the proceeds received were expensed at the effective Merger date. As of March 31, 2012, there were no deferred financing costs. |
| |
Fixed assets and depreciation | | Property and equipment are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the lease term. The estimated useful life of the fixed assets range between three and ten years. |
| |
Impairment of long-lived assets | | In accordance with authoritative guidance the Company reviews its long-lived assets, including property and equipment and other assets, for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred in the period from inception through March 31, 2012. |
| |
Fair value measurement | | Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value: |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
| | ¨ Level 1 — Quoted prices in active markets for identical assets or liabilities.
¨ Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
¨ Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of March 31, 2012 and December 31, 2011, cash and cash equivalents were comprised of cash in checking accounts.
The Company used Level 3 inputs for its valuation methodology for the warrant derivative liabilities. The estimated fair values were determined using a Monte Carlo option pricing model based on various assumptions (see Note 2). The Company’s derivative liabilities are adjusted to reflect estimated fair value at each period end, with any decrease or increase in the estimated fair value being recorded in other income or expense accordingly, as adjustments to fair value of derivative liabilities.
At March 31, 2012, the estimated fair values of the liabilities measured on a recurring basis are as follows:
Fair Value Measurements at March 31, 2012
|
| | | | | | | | | | | | | | | | |
| | | Balance at
March, 31, 2012 | | | Quoted Prices in
Active Markets
(Level 1) | | | Significant Other
Observable Inputs
(Level 2) | | | Significant Other
Unobservable
Inputs (Level 3) | |
| Warrant liability | | $ | 47,514,710 | | | | — | | | | — | | | $ | 47,514,710 | |
| | |
| | The following table presents the activity for liabilities measured at estimated fair value using unobservable inputs for the three months ended March 31, 2012:
Fair Value Measurements Using Significant Unobservable Inputs (Level 3)
|
| | | | |
| | | Warrant Derivative Liability | |
Beginning balance at December 31, 2011 | | $ | 1,266,869 | |
Issuances | | | 32,742,022 | |
Adjustments to estimated fair value | | | 13,505,819 | |
| | | | |
Ending balance at March 31, 2012 | | $ | 47,514,710 | |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
Revenue recognition | | Research and Development Revenue Under Collaborative Agreements.
In December 2010, the Company entered into a 12 month research contract agreement with a third party, whereby the Company was engaged to perform research and development services on a fixed-fee basis for approximately $600,000. Based on the proportional performance criteria, the Company recognized approximately $0 and $74,000 in revenue related to the contract during three months ended March 31, 2012 and 2011, respectively. Total revenue recognized on the contract from inception through March 31, 2012 was approximately $450,000.
In October 2011, the Company entered into a research contract agreement with a third party, whereby the Company will perform research and development services on a fixed-fee basis for $1,365,000. The agreement included an initial payment to the Company of approximately $239,000, with remaining payments expected to occur over a 21-month period. During the period ended March 31, 2012, the Company recorded approximately $120,000 in revenue related to the research contract in recognition of the proportional performance achieved by the Company during the first quarter of 2012. Total revenue recognized on the contract from inception through March 31, 2012 was approximately $359,000.
NIH and U.S. Treasury Grant Revenues
During 2010, the U.S. Treasury awarded the Company two one-time grants totaling approximately $397,300 for investments in qualifying therapeutic discovery projects under section 48D of the Internal Revenue Code. The grants cover reimbursement for qualifying expenses incurred by the Company in 2010 and 2009. The proceeds from these grants are classified in “Revenues – Grants” for the period from inception through March 31, 2012.
During 2010 and 2009, the NHLBI, a division of the NIH, awarded the Company two research grants totaling approximately $268,000. Revenues from the NIH grants are based upon internal and subcontractor costs incurred that are specifically covered by the grant, and where applicable, an additional facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by subcontractors and as the Company incurs internal expenses that are related to the grant. Revenue recognized under these grants for the three months ended March 31, 2012 and 2011 was approximately $0 and $27,000, respectively. Total revenue recorded under these grants from inception through March 31, 2012 was approximately $268,000.
Billings to customers or payments received from customers are included in deferred revenue on the balance sheet until all revenue recognition criteria are met. As of March 31, 2012 and December 31, 2011, the Company had approximately $268,900 and $152,500 in deferred revenue related to its collaborative research programs.
|
| |
Net loss per share | | Net loss per share is presented as both basic and diluted net loss per share. Basic net loss per share excludes any dilutive effects of options, shares subject to repurchase and warrants. Diluted net loss per share includes the impact of potentially dilutive securities. No dilutive effect was calculated for the three months ended March 31, 2012 and 2011 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive. The Company had outstanding common share equivalents of 25,817,738 and 2,243,350 at March 31, 2012 and 2011, respectively. |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
| |
2. Derivative Liability
| | During 2012, in relation to the reverse Merger and the three offerings under the Private Placement, the Company issued 21,347,182 five-year warrants to purchase the Company’s Common stock. The exercise price of the warrants is protected against down-round financing throughout the term of the warrant, as described below. The terms of the warrants issued in the first quarter of 2012 are the same as those issued in connection with the convertible notes in October and November 2011. Pursuant to ASC 815-15 and ASC 815-40, the fair value of the warrants of approximately $32,742,000 was recorded as a derivative liability on the issuance dates.
As of December 31, 2011, the Company had a warrant liability of $1,266,869 related to 1,500,000 warrants issued with Convertible Notes in the fourth quarter of 2011.
The Company revalued all of the warrants at the end of the period, and the estimated fair value of the warrant liabilities is $47,514,710 at March 31, 2012. The change in fair value of the derivative liabilities of approximately $13,505,800 is included in other expense in the 2012 statement of operations.
The derivative liabilities were valued at the closing dates of the Private Placement at March 31, 2012 using a Monte Carlo valuation model with the following assumptions:
|
| | | | | | | | |
| | | Closing dates | | | March 31, 2012 | |
| Closing price per share of common stock | | $ | N/A | | | $ | 2.47 | |
Exercise price per share | | $ | 1.00 | | | $ | 1.00 | |
Expected volatility | | | 105.8%-110.5% | | | | 103.5 | % |
Risk-free interest rate | | | 0.82%-1.07% | | | | 1.04 | % |
Dividend yield | | | — | | | | — | |
Remaining expected term of underlying securities (years) | | | 5 | | | | 4.90 | |
| | |
| | In addition, as of the valuation dates, management assessed the probabilities of future financings assumptions in the Monte Carlo valuation models. Management also applied a discount for lack of marketability to the valuation of the derivative liabilities based on such trading restrictions due to the shares not being registered.
If, prior to the expiration date of the warrants, the Company issues additional shares of Common Stock, as defined below, without consideration or for a consideration per share less than the exercise price of the warrants in effect immediately prior to such issue, then the exercise price shall be reduced, concurrently with such issue, to a price (calculated to the nearest cent) determined by multiplying such exercise price by a fraction, (A) the numerator of which shall be (1) the number of shares of Common stock outstanding immediately prior to such issue plus (2) the number of shares of Common stock which the aggregate consideration received or to be received by the Company for the total number of additional shares of Common stock so issued would purchase at such exercise price; and (B) the denominator of which shall be the number of shares of Common stock outstanding immediately prior to such issue plus the number of such additional shares of Common stock so issued; provided that (i) all shares of Common stock issuable upon conversion or exchange of convertible securities outstanding immediately prior to such issue shall be deemed to be outstanding, and (ii) the number of shares of Common stock deemed issuable upon conversion or exchange of such outstanding convertible securities shall be determined without giving effect to any adjustments to the conversion or exchange price or conversion or exchange rate of such convertible securities resulting from the issuance of additional shares of Common stock that is the subject of this calculation. For purposes of the warrants, “additional shares of common stock” shall mean all shares of Common stock issued by the Company after the effective date (including without limitation any shares of Common stock issuable upon conversion or exchange of any convertible securities or upon exercise of any option or warrant, on an as-converted basis), other than: (i) shares of Common stock (and/or warrants for any class of equity securities of the Company) issued or issuable upon conversion or exchange of any convertible securities or exercise of any options or warrants outstanding on the effective date; (ii) shares of Common stock issued or issuable by reason of a dividend, stock split, split-up or other distribution on shares of Common stock; (iii) shares of Common stock (or options with respect thereto) issued or issuable to employees or directors of, or consultants to, the Company or any of its subsidiaries pursuant to a plan, agreement or arrangement approved by the Board of Directors of the Company; (iv) any securities issued or issuable by the Company pursuant to (A) the Private Placement; or (B) the Merger; (v) securities issued pursuant to acquisitions or strategic transactions approved by a majority of disinterested directors of the Company, provided that any such issuance shall only be to a person which is, itself or through its subsidiaries, an operating company in a business synergistic with the business of the Company and in which the Company receives benefits in addition to the investment of funds, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities and (vi) securities issued to financial institutions, institutional investors or lessors in connection with credit arrangements, equipment financings or similar transactions approved by a majority of disinterested directors of the Company, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
Upon each adjustment of the exercise price pursuant to the provisions stated above, the number of warrant shares issuable upon exercise of the warrants shall be adjusted by multiplying a number equal to the exercise price in effect immediately prior to such adjustment by the number of warrant shares issuable upon exercise of the warrant immediately prior to such adjustment and dividing the product so obtained by the adjusted exercise price.
|
| |
3. Convertible Notes Payable | | |
| |
Convertible notes | | At December 31, 2011, an unsecured $100,000 Convertible Note, with interest at 10% and a maturity date of April 2014, remained outstanding. In February 2012, at the close of the Merger, the convertible note and accrued interest of approximately $110,740 were repaid. |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
Private placement | | On September 18, 2011, Organovo, Inc.’s Board of Directors authorized a private placement offering of up to 30 units of its securities at a price of $50,000 per unit for an aggregate purchase price of $1,500,000. Each unit consisted of a convertible note in the principal amount of $50,000 accruing simple interest at the rate of 6% per annum (the “Convertible Notes”), plus five-year warrants to purchase 50,000 shares of the next Qualified Round of Equity Securities, at an exercise price of $1.00 per share. The principal plus accrued interest was convertible into the Company’s common stock upon consummation of the Merger.
During October and November 2011, $1,500,000 of Convertible Notes bearing interest at 6% per annum with a maturity date of March 30, 2012, and five-year warrants to purchase 1,500,000 shares of the Company’s Common stock were issued to investors under the Private Placement. The warrants are exercisable at $1.00 per share, expire in five years, and contain down-round price protection. The Convertible Notes were outstanding at December 31, 2011, and were converted into 1,525,387 units during February 2012, in connection with the Merger.
The Company determined that the warrants represent a derivative instrument due to the down-round price protection, and accordingly, the Company recorded a derivative liability related to the warrants. See Note 4. Additionally, during 2011 at issuance of the notes, the Company recorded the discount for the beneficial conversion feature of $239,700. The debt discount associated with the warrants and beneficial conversion feature were amortized to interest expense over the life of the Convertible Notes, and fully amortized upon conversion of the Convertible Notes. The Company recorded approximately $896,200 of interest expense for the amortization of the debt discount during the three months ended March 31, 2012 and approximately $1,500,000 for the period from inception through March 31, 2012.
As consideration for locating investors to participate in the Private Placement, the placement agent earned a cash payment of $195,000. Additionally, upon closing of the Merger transaction, the placement agent earned five-year warrants to purchase 610,155 shares of the Company’s Common stock at $1.00 per share. These warrants contain down round protection and were classified as derivative liabilities upon issuance. See Note 2.
Interest expense, including amortization of the note discounts, for the three months ended March 31, 2012 and 2011 was approximately $1,087,453 and $53,100, respectively. Interest expense, including amortization of the note discounts, for the period from April 19, 2007 (inception) through December 31, 2011 was approximately $3,405,895.
During 2012, concurrently with the closing of the Merger and in contemplation of the Merger, the Company completed the initial closing of the Private Placement of up to 8,000,000 units of its securities, at a price of $1.00 per unit, with the ability to increase the offering to an aggregate of up to 16,000,000 units. Each unit consisted of one share of Common Stock and a warrant to purchase one share of Common Stock. The Company completed three closings under the Private Placement during the three months ended March 31, 2012, and raised total gross proceeds of $13,722,600 and total net proceeds of $11,593,066. The Company issued 13,722,600 shares of its Common Stock and warrants to purchase 15,247,987 shares of its Common Stock (including warrants to purchase 1,525,387 shares to former holders of the bridge notes) exercisable at $1.00 to investors in the Offering. The placement agent and its selected dealers were paid total cash commissions of $1,372,260 and the Placement Agent was paid an expense allowance of $411,678 and was issued Placement Agent warrants to purchase 6,099,195 shares of the Company’s Common Stock at an exercise price of $1.00 per share.
The warrants issued to the investors and the placement agent, as described above, contain down round protection, and accordingly, were classified as derivative liabilities upon issuance. On the closing date, the derivative liabilities were recorded at an estimated fair value of approximately $32,742,000. Given that the fair value of the derivative liabilities exceeded the total proceeds of the private placement of $13,722,600, no net amounts were allocated to the common stock. The amount by which the recorded liabilities exceeded the proceeds of approximately $19,019,400 was charged to other expense at the closing dates. The Company has revalued the derivative liability as of March 31, 2012, and will continue to do so on each subsequent balance sheet date until the securities to which to derivative liabilities relate are exercised or expire, with any changes in the fair value. See Note 2.
|
| |
Registration rights agreement | | The Company entered into a registration rights agreement (each, a “Registration Rights Agreement”) with the investors in the Offering. Under the terms of the Registration Rights Agreement, the Company agreed to file a registration statement covering the resale of the Common Stock underlying the Units and the Common Stock that is issuable on exercise of the Investor Warrants (but not the Common Stock that is issuable upon exercise of the warrants issued as compensation to the placement agent in connection with the Offering) within 90 days from the final closing date of the Offering (the “Filing Deadline”), and shall use commercially reasonable efforts to cause the registration statement to become effective no later than 180 days after it is filed (the “Effectiveness Deadline”).
The Company agreed to use reasonable efforts to maintain the effectiveness of the registration statement through the one year anniversary of the date the registration statement is declared effective by the Securities and Exchange Commission (the “SEC”), or until Rule 144 of the 1933 Act is available to investors in the Offering with respect to all of their shares, whichever is earlier. If the Company does not meet the Filing Deadline or Effectiveness Deadline, the Company will be liable for monetary penalties equal to one-half of one percent (0.5%) of each investor’s investment in the offering at the end of every 30 day period following such Filing Deadline or Effectiveness Deadline failure until such failure is cured. The payment amount shall be prorated for partial 30 day periods. The maximum aggregate amount of payments to be made by the Company as the result of such shall be an amount equal to 6% of each investor’s investment amount. Notwithstanding the foregoing, no payments shall be owed with respect to any period during which all of the investor’s registrable securities may be sold by such investor under Rule 144 or pursuant to another exemption from registration.
|
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | | | |
4. Stockholders’ Equity | | |
| |
Common stock | | During February and March 2012, the Company issued 21,247,987 shares of Common stock related to the Merger. See Notes 1 and 3. |
| |
Restricted stock awards | | In February 2008, four founders, including the Chief Executive Officer (“CEO”) and three directors of Organovo, Inc. received 11,779,960 shares of restricted Common stock, 25% vesting after the first year and the remaining 75% vesting in equal quarterly portions over the following three years.
From 2008 through December 31, 2011, Organovo, Inc. issued a total of 1,258,934 shares of restricted Common stock to various employees, advisors, and consultants of Organovo, Inc. 1,086,662 of those shares were issued under the 2008 Equity Incentive Plan and the remaining 172,272 shares were issued outside the plan. No restricted stock was issued during the three months ended March 31, 2012.
|
| |
| | A summary of the Company’s restricted stock award activity is as follows: |
| | | | | | |
| | Number of Shares | |
Unvested at December 31, 2007
| | | — | |
Granted
| | | 12,627,697 | |
Vested
| | | (65,211 | ) |
Canceled / forfeited
| | | — | |
| | | | | | |
Unvested at December 31, 2008
| | | 12,562,486 | |
Granted
| | | 130,422 | |
Vested
| | | (5,373,004 | ) |
Canceled / forfeited
| | | — | |
| | | | | | |
Unvested at December 31, 2009
| | | 7,319,904 | |
Granted
| | | 219,369 | |
Vested
| | | (3,256,191 | ) |
Canceled / forfeited
| | | — | |
| | | | | | |
Unvested at December 31, 2010
| | | 4,283,082 | |
Granted
| | | 61,406 | |
Vested
| | | (3,233,193 | ) |
Canceled / forfeited
| | | — | |
| | | | | | |
Unvested at December 31, 2011
| | | 1,111,295 | |
Granted
| | | — | |
Vested
| | | (446,745 | ) |
Canceled / forfeited
| | | — | |
| | | | | | |
Unvested at March 31, 2012
| | | 664,550 | |
| | | | | | |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
| | The fair value of each restricted Common stock award is recognized as stock-based expense over the vesting term of the award. The Company recorded restricted stock-based compensation expense in operating expenses for employees and non-employees of approximately $430 and $1,200 for the three months ended March 31, 2012 and 2011, respectively. The Company recorded stock-based compensation expense of approximately $14,400 for the period from April 19, 2007 (inception) through March 31, 2012.
As of March 31, 2012, total unrecognized stock-based compensation expense was approximately $1,370, which will be recognized over a weighted average period of less than one year.
|
| |
Stock options | | Under the 2008 Equity Incentive Plan, on October 12, 2011, Organovo, Inc. granted an officer incentive stock options to purchase 896,256 shares of Common stock at an exercise price of $0.08 per share, a quarter of which will vest on the one year anniversary of employment, in May 2012, and the remaining options will vest ratably over the remaining 36 month term. No other options have been issued under the Plan and after the October 2011 grant, no additional issuances are authorized under the 2008 plan.
The following table summarizes stock option activity for the three months ended March 31, 2012:
|
| | | | | | | | |
| | | Options
Outstanding | | | Weighted-
Average
Exercise Price | |
Outstanding at December 31, 2011 | | | 896,256 | | | $ | 0.08 | |
Options Granted | | | — | | | | — | |
Options Canceled | | | — | | | | — | |
Options Exercised | | | — | | | | — | |
| | | | | | | | |
Outstanding at March 31, 2012 | | | 896,256 | | | $ | 0.08 | |
| | | | | | | | |
Vested and Exercisable at March 31, 2012 | | | — | | | $ | 0.08 | |
| | | | | | | | |
| | |
| | No stock options were granted during the three months ended March 31, 2012 and 2011. |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
| | The total employee stock-based compensation recorded as operating expenses was approximately $3,400 for the three months ended March 31, 2012 and $6,400 for the period from April 19, 2007 (inception) through March 31, 2012.
The total unrecognized compensation cost related to unvested stock option grants as of March 31, 2012 was approximately $44,600, and the weighted average period over which these grants are expected to vest is 3.2 years
|
| |
Warrants | | During 2011, Organovo, Inc. issued warrants to purchase 2,909,750 shares of its Common stock. These warrants are immediately exercisable at $1.00 per share, and have remaining terms of approximately 4.8 years. None of the warrants were exercised as of December 31, 2011. See Notes 2 and 3.
During the three months ended March 31, 2012, the Company issued warrants to purchase 21,347,182 shares of its Common stock. These warrants are immediately exercisable at $1.00 per share, and have remaining terms of approximately 4.8 years. None of the warrants were exercised as of March 31, 2012. See Note 2.
|
| |
Common stock reserved for future issuance | | Common stock reserved for future issuance consisted of the following at March 31, 2012: |
| | | | |
Common stock warrants outstanding
| | | 24,256,932 | |
Common stock options outstanding under the 2008 Plan
| | | 896,256 | |
Common stock warrants held for convertible debt issuance
| | | — | |
| | | | |
Total
| | | 25,153,188 | |
| | | | |
Organovo Holdings, Inc.
(A development stage company)
Notes to Condensed Financial Statements
| | |
5. Commitments and Contingencies | | |
| |
Operating leases | | The Company leases office and laboratory space under non-cancelable operating leases. The Company records rent expense on a straight-line basis over the life of the lease and records the excess of expense over the amounts paid as deferred rent.
Rent expense was approximately $60,200 and $26,900 for the three months ended March 31, 2012 and 2011, respectively. Rent expense was approximately $384,800 for the period from April 19, 2007 (inception) through March 31, 2012.
The Company entered into a new facilities lease at 6275 Nancy Ridge Drive, San Diego, CA 92121. The lease was signed on February 27, 2012 with target occupancy of July 15, 2012. The base rent under the lease is approximately $38,800 per month with 3% annual escalators. The lease term is 48 months with an option for the Company to extend the lease at the end of the lease term.
Future minimum rental payments required under operating leases that have initial or remaining non-cancelable lease terms in excess of one year are as follows:
|
| | | | |
Year Ending December 31, | |
2012 | | $ | 303,229 | |
2013 | | | 466,170 | |
2014 | | | 466,170 | |
2015 | | | 466,170 | |
2016 | | | 252,500 | |
| | | | |
Total | | $ | 1,954,239 | |
| | | | |
| | |
6. Concentrations | | |
| |
Credit risk | | Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions primarily located in San Diego. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. At times, balances may exceed federally insured limits. The Company has not experienced losses in such accounts, and management believes that the Company is not exposed to any significant credit risk with respect to its cash and cash equivalents. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read theThe following management’s discussion and analysis of financial condition and results of operations should be read in conjunction with our historical consolidated financial statements and the related notes containedthereto for the fiscal year ended March 31, 2023 included elsewhere in this prospectus. This management’s discussion and analysis contains forward-looking statements, such as statements related to our plans, objectives, expectations and intentions. Any statements that involve risks, uncertaintiesare not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statementsthe like, and/or future tense or conditional constructions such as a result of a variety of factors, including those set forth under “Risk Factors” and elsewhere in this prospectus and those discussed in other documents we file with the SEC. In light“will,” “may,” “could,” “should,” or similar expressions, identify certain of these risks, uncertainties and assumptions, readers are cautioned not to place undue reliance on such forward-looking statements. These forward-looking statements represent beliefs and assumptionsspeak only as of the date of this prospectus. prospectus and are subject to risks and uncertainties, including those described discussed in the section titled “Risk Factors” in this prospectus, that could cause our actual results or events to differ materially from those expressed or implied by such forward-looking statements. Unless the context otherwise requires, the terms “Organovo,” the “Company”, “we”, “us” and “our” in this prospectus refer to Organovo Holdings, Inc. and its wholly owned subsidiaries, Organovo, Inc. and Opal Merger Sub, Inc.
Except asto the limited extent required by applicable law, we do not intendundertake any obligation to update or revise forward-looking statements contained in this prospectus to reflect future events or circumstances.
Basis of Presentation
On February 8, 2012, Organovo, Inc., a privately held Delaware corporation, merged with and into Organovo Acquisition Corp., a wholly-owned subsidiary of Organovo Holdings, Inc., with Organovo, Inc. surviving the merger as a wholly-owned subsidiary of Organovo Holdings, Inc. (the “Merger”). In the Merger, Organovo Holdings, Inc. acquired the business of Organovo, Inc., and will continue the existing business operations of Organovo, Inc. As the result of the change in Organovo Holdings, Inc.’s business and operations from a shell company to a biotechnology company, a discussion of the past financial results of the shell company is not pertinent, and the financial results of Organovo, Inc., the accounting acquirer, are considered the Company’s financial results on a historical and going-forward basis.
The discussion and analysis of our financial condition and results of operations are based on Organovo’s financial statements, which we have prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities atcircumstances occurring after the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate such estimates and judgments, including those described in greater detail below, on historical experience and on various other factors that we believe are reasonable under the circumstances, the resultsthis Quarterly Report.
Basis of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions.Presentation
The unaudited condensed consolidated financial statements included for the quarterly periods ended March 31, 2012 and 2011 in this prospectus have been prepared in accordance with SECthe Securities and Exchange Commission (the “SEC”) instructions to Quarterly Reports on Form 10-Q. Accordingly, the unaudited condensed consolidated financial statements as of and for the nine months ended December 31, 2023 presented elsewhere in this prospectus for the quarterly periods ended March 31, 2012 and 2011 and discussed below are unaudited and do not contain all the information required by U.S. GAAPgenerally accepted accounting principles (“GAAP”) to be included in a full set of financial statements. The audited financial statements for the year ended March 31, 2023 included elsewhere in this prospectus, include a summary of our significant accounting policies and should be read in conjunction with the unaudited condensed consolidated financial statements as of and for the nine months ended December 31, 2023. In the opinion of management, all material adjustments necessary to present fairly the results of operations for the quarterlysuch periods ended March 31, 2012 and 2011 have been included in this prospectus. All such adjustments are of a normal recurring nature. The results of operations for interim periods are not necessarily indicative of the results of operations for the entire year.
ReferencesOverview
We are a clinical stage biotechnology company that is focusing on clinical drug development of the farnesoid X receptor (“FXR”) agonist FXR314. FXR is a mediator of gastrointestinal and liver diseases. FXR agonism has been tested in this sectiona variety of preclinical models of inflammatory bowel disease ("IBD"). FXR314 is the lead compound in our established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in ulcerative colitis ("UC").
Our current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). We plan to “Organovo Holdings, Inc.,” “Organovo Holdings,” “we,” “us,” “our,” “the Company” and “our Company” referstart a Phase 2a clinical trial in UC in the calendar year 2024.
Our second focus is building high fidelity, 3D tissues that recapitulate key aspects of human disease. We use our proprietary technology to Organovo Holdings, Inc. and its consolidated subsidiary Organovo, Inc.
Overview
Organovo, Inc. was founded in Delaware in April 2007. Activities since Organovo, Inc.’s inception through 2011 were devoted primarily to developing a platform technology for the generation of three-dimensional (3D)build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. We believe these attributes can enable critical complex, multicellular disease models that can be employedused to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, we are initially focusing on the intestine and have ongoing 3D tissue development efforts in human tissue models of UC and CD. We use these models to identify new molecular targets responsible for driving the disease and to explore the mechanism of action of known drugs including FXR314 and related molecules. We intend to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
Our current understanding of intestinal tissue models and IBD disease models leads us to believe that we can create models that provide greater insight into the biology of these diseases than are generally currently available. We are creating high fidelity disease models, leveraging our prior work including the work found in our peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Our advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures.
Using these disease models, we intend to identify and validate novel therapeutic targets. After finding therapeutic drug targets, we intend to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug (“IND”) filing and potential future clinical trials.
We expect to broaden our work into additional therapeutic areas over time and are currently exploring specific tissues for development. In our work to identify the areas of interest, we evaluate areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, we are building upon both our external and in house scientific expertise, which will be essential to our drug development biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs.effort.
As of March 31, 2012, Organovo, Inc. had devoted substantially all of its efforts to product development, raising capital and building infrastructure. Organovo, Inc. did not, as of that date, realize significant revenues from its planned principal operations. Accordingly, Organovo, Inc. is considered to be in the development stage.
Critical Accounting Policies, Estimates, and Judgments
Our financial statements are prepared in accordance with GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We continually evaluate our estimates and judgments used in preparing our financial statements and related disclosures, none of which are considered critical. All estimates affect reported amounts of assets, liabilities, revenues and expenses, as well as disclosures of contingent assets and liabilities. These estimates and judgments are also based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known.
There have been no significant changes to our critical accounting policies since March 31, 2023. For a description of critical accounting policies that affect our significant judgments and estimates used in the preparation of our condensed consolidated financial statements, refer to Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Note 1. Description of Business and Summary of Significant Accounting Policies” in the Notes to Consolidated Financial Statements contained elsewhere in this prospectus.
Results of Operations
Comparison of the three months ended December 31, 2023 and 2022
The following table summarizes our results of operations for the three months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | | | | | |
| | December 31, | | | Increase (decrease) | |
| | 2023 | | | 2022 | | | $ | | | % | |
Revenues | | $ | 5 | | | $ | 131 | | | $ | (126 | ) | | | (96 | %) |
Research and development | | $ | 1,434 | | | $ | 1,185 | | | $ | 249 | | | | 21 | % |
Selling, general and administrative | | $ | 2,251 | | | $ | 2,305 | | | $ | (54 | ) | | | (2 | %) |
Other income | | $ | 76 | | | $ | 95 | | | $ | (19 | ) | | | (20 | %) |
Revenues
For the three months ended December 31, 2023 and 2022, total revenue was less than $0.1 million and $0.1 million, respectively. The revenue is related to sales-based royalties from licensing intellectual property. The decrease in royalty revenue year over year relates to a decrease in sales of royalty bearing products by the licensee. Given the trend of reduced sales during the fiscal year, we expect a decrease in sales of royalty bearing products by the licensee going forward.
Costs and Expenses
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months
Ended | | | | | | Three Months
Ended | | | | | | Increase (decrease) | |
| | December 31, 2023 | | | % of total | | | December 31, 2022 | | | % of total | | | $ | | | % | |
Research and development | | $ | 1,336 | | | | 93 | % | | $ | 1,023 | | | | 86 | % | | $ | 313 | | | | 31 | % |
Non-cash stock-based compensation | | | 40 | | | | 3 | % | | | 117 | | | | 10 | % | | | (77 | ) | | | (66 | %) |
Depreciation and amortization | | | 58 | | | | 4 | % | | | 45 | | | | 4 | % | | | 13 | | | | 29 | % |
Total research and development
expenses | | $ | 1,434 | | | | 100 | % | | $ | 1,185 | | | | 100 | % | | $ | 249 | | | | 21 | % |
For the three months ended December 31, 2023, total research and development expenses were $1.4 million, an increase of $0.2 million, or approximately 21%, from the prior year period. The increase year over year directly relates to the shift in strategic focus to advance the clinical drug development of FXR314 and start a Phase 2a clinical trial in the calendar year 2024. Our average full-time research and development staff decreased from an average of fifteen full-time employees for the three months ended December 31, 2022 to an average of fourteen full-time employees for the three months ended December 31, 2023. Our fiscal 2024 operations resulted in a $0.1 million increase in consulting expenses, and a $0.1 million increase in lab expenses. Going forward, we intend to continue to advance the clinical drug development of FXR314 and expect an associated increase in expenses.
Selling, General and Administrative Expenses
The following table summarizes our selling, general and administrative expenses for the three months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Three Months
Ended | | | | | | Three Months
Ended | | | | | | Increase (decrease) | |
| | December 31, 2023 | | | % of total | | | December 31, 2022 | | | % of total | | | $ | | | % | |
Selling, general and administrative | | $ | 2,120 | | | | 94 | % | | $ | 1,793 | | | | 77 | % | | $ | 327 | | | | 18 | % |
Non-cash stock-based compensation | | | 117 | | | | 5 | % | | | 427 | | | | 19 | % | | | (310 | ) | | | (73 | %) |
Depreciation and amortization | | | 14 | | | | 1 | % | | | 85 | | | | 4 | % | | | (71 | ) | | | (84 | %) |
Total selling, general and
administrative expenses | | $ | 2,251 | | | | 100 | % | | $ | 2,305 | | | | 100 | % | | $ | (54 | ) | | | (2 | %) |
For the three months ended December 31, 2023, total selling, general and administrative expenses were approximately $2.3 million, a decrease of less than $0.1 million, or approximately 2%, compared to the prior year period. The decrease year over year relates to a decrease in personnel. Our average full-time general and administrative staff decreased from an average of five full-time employees for the three months ended December 31, 2022 to an average of four full-time employees for the three months ended December 31, 2023. Our fiscal 2024 operations resulted in a $0.4 million decrease in personnel related costs, including stock based compensation, related to severance for the reduction in force in the second quarter of fiscal 2024. There was also a $0.1 decrease in other corporate expenses and a decrease of less than $0.1 million in depreciation and amortization expense in fiscal 2024. This was offset by a $0.2 million increase in legal expenses and a $0.3 million increase in investor relations expenses compared to the prior year period.
Other Income
Other income was $0.1 million for the three months ended December 31, 2023, which was related to interest income. Other income was $0.1 million for the three months ended December 31, 2022, which was related to interest income of approximately $0.1 million offset by a loss on investment in equity securities of less than $0.1 million.
Comparison of the nine months ended December 31, 2023 and 2022
The following table summarizes our results of operations for the nine months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | |
| | Nine Months Ended | | | | | | | |
| | December 31, | | | Increase (decrease) | |
| | 2023 | | | 2022 | | | $ | | | % | |
Revenues | | $ | 80 | | | $ | 208 | | | $ | (128 | ) | | | (62 | %) |
Research and development | | $ | 4,435 | | | $ | 3,436 | | | $ | 999 | | | | 29 | % |
Selling, general and administrative | | $ | 7,635 | | | $ | 6,724 | | | $ | 911 | | | | 14 | % |
Other income | | $ | 366 | | | $ | 154 | | | $ | 212 | | | | 138 | % |
Revenues
For the nine months ended December 31, 2023 and 2022, total revenue was less than $0.1 million and $0.2 million, respectively. The revenue is related to sales-based royalties from licensing intellectual property. The decrease in royalty revenue year over year relates to a decrease in sales of royalty bearing products by the licensee. Given the trend of reduced sales during the fiscal year, we expect a decrease in sales of royalty bearing products by the licensee going forward.
Costs and Expenses
Research and Development Expenses
The following table summarizes our research and development expenses for the nine months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months
Ended | | | | | | Nine Months
Ended | | | | | | Increase (decrease) | |
| | December 31, 2023 | | | % of total | | | December 31, 2022 | | | % of total | | | $ | | | % | |
Research and development | | $ | 4,179 | | | | 94 | % | | $ | 2,953 | | | | 86 | % | | $ | 1,226 | | | | 42 | % |
Non-cash stock-based compensation | | | 87 | | | | 2 | % | | | 363 | | | | 11 | % | | | (276 | ) | | | (76 | %) |
Depreciation and amortization | | | 169 | | | | 4 | % | | | 120 | | | | 3 | % | | | 49 | | | | 41 | % |
Total research and
development expenses | | $ | 4,435 | | | | 100 | % | | $ | 3,436 | | | | 100 | % | | $ | 999 | | | | 29 | % |
For the nine months ended December 31, 2023, total research and development expenses were $4.4 million, an increase of $1.0 million, or 29%, from the prior year period. Our average full-time research and development staff increased from an average of fourteen full-time employees for the nine months ended December 31, 2022 to an average of sixteen full-time employees for the nine months ended December 31, 2023. Our fiscal 2024 research and development activities resulted in a $0.3 million increase in personnel related costs, a $0.3 million increase in lab expenses, a $0.2 million increase in consulting expenses, a $0.1 million increase in depreciation and amortization expense, and a $0.1 million increase in facility costs. Going forward, we intend to continue to advance the clinical drug development of FXR314 with an associated increase in expenses.
Selling, General and Administrative Expenses
The following table summarizes our selling, general and administrative expenses for the nine months ended December 31, 2023 and 2022 (in thousands, except %):
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Nine Months
Ended | | | | | | Nine Months
Ended | | | | | | Increase (decrease) | |
| | December 31, 2023 | | | % of total | | | December 31, 2022 | | | % of total | | | $ | | | % | |
Selling, general and administrative | | $ | 6,374 | | | | 83 | % | | $ | 5,096 | | | | 75 | % | | $ | 1,278 | | | | 25 | % |
Non-cash stock-based compensation | | | 1,220 | | | | 16 | % | | | 1,514 | | | | 23 | % | | | (294 | ) | | | (19 | %) |
Depreciation and amortization | | | 41 | | | | 1 | % | | | 114 | | | | 2 | % | | | (73 | ) | | | (64 | %) |
Total selling, general and
administrative expenses | | $ | 7,635 | | | | 100 | % | | $ | 6,724 | | | | 100 | % | | $ | 911 | | | | 14 | % |
For the nine months ended December 31, 2023, total selling, general and administrative expenses were approximately $7.6 million, an increase of approximately $0.9 million, or approximately 14%, compared to the prior year period. The increase year over year relates to increases in legal and other corporate expenses. Our average full-time general and administrative staff remained consistent at an average of five full-time employees for both the nine months ended December 31, 2022 and December 31, 2023. However, there was a reduction in force event in the second quarter of fiscal 2024 that resulted in a decrease from five to four total employees for the quarter ended December 31, 2023. Our fiscal 2024 operations resulted in a $0.1 million decrease in personnel related costs, including stock based compensation, related to severance for the reduction in force event, a $0.1 million decrease in depreciation and amortization expense, offset by a $0.1 million increase in consulting costs, a $0.6 million increase in legal expenses, a $0.3 million increase in investor relations expenses, and a $0.1 million increase in other corporate expenses.
Other Income
Other income was $0.4 million for the nine months ended December 31, 2023, related to interest income. Other income was $0.2 million for the nine months ended December 31, 2022, which was related to interest income of approximately $0.3 million offset by a loss on investment in equity securities of approximately $0.1 million.
Financial Condition, Liquidity and Capital Resources
Going forward, we intend to focus on clinical drug development of FXR314, the lead compound in our established FXR program. Our current clinical focus is in advancing FXR314 in IBD, including UC and CD. We plan to start a Phase 2a clinical trial in UC in the calendar year 2024. Additionally, we plan to leverage our proprietary technology platform to develop therapeutic drugs, focusing on IBD, including CD and UC, with a goal of broadening our work into additional therapeutic areas over time.
The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. At December 31, 2023, we had cash and cash equivalents of approximately $5.3 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $336.6 million. The restricted cash was pledged as collateral for a letter of credit that the Company is required to maintain as a security deposit under the terms of the lease agreement for its facilities. We had negative cash flow from operations of approximately $12.0 million during the nine months ended December 31, 2023. At March 31, 2023, we had cash and cash equivalents of approximately $15.3 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $325.0 million.
At December 31, 2023, we had total current assets of approximately $6.2 million and current liabilities of approximately $1.7 million, resulting in working capital of $4.5 million. At March 31, 2023, we had total current assets of approximately $17.0 million and current liabilities of approximately $3.7 million, resulting in working capital of $13.3 million.
The following table summarizes the primary sources and uses of cash for the nine months ended December 31, 2023 and 2022 (in thousands):
| | | | | | | | |
| | Nine Months Ended | |
| | December 31, | |
| | 2023 | | | 2022 | |
Net cash (used in) provided by: | | | | | | |
Operating activities | | $ | (11,982 | ) | | $ | (7,675 | ) |
Investing activities | | | 803 | | | | (804 | ) |
Financing activities | | | 1,173 | | | | — | |
Net decrease in cash, cash equivalents, and restricted cash | | $ | (10,006 | ) | | $ | (8,479 | ) |
Operating activities
Net cash used in operating activities for the nine months ended December 31, 2023 was approximately $12.0 million as compared to $7.7 million used in operating activities for the nine months ended December 31, 2022. This $4.3 million increase in operating cash usage can be attributed primarily to the increase in research and development activities. Operating cash usage includes $2.0 million of cash outflows for acquired in-process research and development of Metacrine's FXR drug compound, related research data, and IP.
Investing activities
Net cash provided by investing activities was $0.8 million for the nine months ended December 31, 2023. Investing activities consisted of purchases of investments of $9.9 million, maturities of investments of $10.0 million, liquidation of equity securities of $0.7 million, and fixed asset purchases of less than $0.1 million. Net cash used in investing activities was $0.8 million for the nine months ended December 31, 2022, which consisted of purchases of equity securities of $1.1 million, purchases of investments of $9.9 million, maturities of investments of $10.0 million, sales of equity securities of $0.4 million, and fixed asset purchases of $0.2 million.
Financing activities
Net cash provided by financing activities was $1.2 million for the nine months ended December 31, 2023. Financing activities consisted of the sale of common stock through “at the market offerings” (“ATM offerings”). There were no financing activities in the nine months ended December 31, 2022.
Operations funding requirements
Through December 31, 2023, we have financed our operations primarily through the sale of common stock through public and ATM offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research-based services, grants, and collaborative research agreements, and from the sale of convertible notes.
Our ongoing cash requirements include research and development expenses, compensation for personnel, consulting fees, legal and accounting support, insurance premiums, facilities, maintenance of our intellectual property portfolio, license and collaboration agreements, listing on the Nasdaq Capital Market, and other miscellaneous fees to support our operations. We expect our total operating expense for the fiscal year ending March 31, 2024 to be between $13.0 million and $15.0 million. Based on our current operating plan and available cash resources, we will need substantial additional funding to support future operating activities. We have concluded that the prevailing conditions and ongoing liquidity risks faced by us raise substantial doubt about our ability to continue as a going concern for at least one year following the date these financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern.
We previously had an effective shelf registration statement on Form S-3 (File No. 333-222929) (the “2018 Shelf”) that registered $100.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing and expired on February 22, 2021. On January 19, 2021, we filed a shelf registration statement on Form S-3 (File No. 333-252224) to register $150.0 million of common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the “2021 Shelf”). The 2021 Shelf registration statement was declared effective by the SEC on January 29, 2021 and replaced the 2018 Shelf at that time. On January 26, 2024, we filed a new shelf registration statement on Form S-3 (File No. 333-276722) to register $150.0 million of the Company’s common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the “2024 Shelf”). The 2024 Shelf has not yet been declared effective by the SEC.
On March 16, 2018, we entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, we filed a prospectus supplement to the 2021 Shelf (the “2021 ATM Prospectus Supplement”), pursuant to which we could offer and sell, from time to time, through the
Agents, shares of our common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold will be issued pursuant to our 2021 Shelf until it is replaced by the 2024 Shelf.
During the nine months ended December 31, 2023, we sold 934,621 shares of common stock in ATM offerings pursuant to the 2021 ATM Prospectus Supplement. As of December 31, 2023, we have sold an aggregate of 2,515,483 shares of common stock in ATM offerings under the 2021 ATM Prospectus Supplement, for gross proceeds of approximately $22.9 million. As of December 31, 2023, there was approximately $100.0 million available in future offerings under the 2021 Shelf (excluding amounts available but not yet issued under the ATM Prospectus Supplement), and approximately $27.1 million available for future offerings through our ATM program under the 2021 ATM Prospectus Supplement.
On January 26, 2024, we also filed a prospectus to the 2024 Shelf (the “2024 ATM Prospectus”), pursuant to which we may offer and sell, from time to time, through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $2,605,728. Any shares offered and sold in these ATM sales transactions will be issued pursuant to the 2024 Shelf.
Having insufficient funds may require us to relinquish rights to our technology on less favorable terms than we would otherwise choose. Failure to obtain adequate financing could eventually adversely affect our ability to operate as a going concern. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business. We cannot be sure that additional financing will be available if and when needed, or that, if available, we can obtain financing on terms favorable to our stockholders. Any failure to obtain financing when required will have a material adverse effect on our business, operating results, financial condition and ability to continue as a going concern.
On October 12, 2022, our stockholders and the Board of Directors ("Board") approved the 2022 Equity Incentive Plan ("2022 Plan"), and it became effective on that date. The 2022 Plan replaced the 2012 Equity Incentive Plan ("2012 Plan") on the effective date. Upon the effective date, we ceased granting awards under the 2012 Plan and any shares remaining available for future issuance under the 2012 Plan were cancelled and are no longer available for future issuance. The 2012 Plan continues to govern awards previously granted under it. At the time the Board approved the 2022 Plan, an aggregate of 1,363,000 shares of our common stock was initially reserved for issuance under the 2022 Plan. We committed to reducing the new 2022 Plan share reserve by the number of shares that were granted under the 2012 Plan and the Inducement Plan between July 25, 2022 and October 12, 2022. From July 25, 2022 to October 12, 2022, we issued 126,262 shares of common stock under the 2012 Plan. As a result, the number of shares initially reserved for future issuance under the 2022 Plan was 1,236,738 shares of common stock as of October 12, 2022. We also committed to reducing the aggregate number of shares of common stock issuable pursuant to the 2021 Inducement Equity Incentive Plan ("Inducement Plan") from 750,000 shares to 51,000 shares (which includes 50,000 shares of its common stock issuable pursuant to an outstanding option to purchase common stock with an exercise price of $2.75 per share, leaving only 1,000 shares available for future issuance under the Inducement Plan) and the share reserve was reduced effective October 12, 2022.
As of December 31, 2023, we had 9,838,755 total issued and outstanding shares of common stock. Under the 2022 Plan, 1,643,798 shares remain available for issuance as of December 31, 2023, to executive officers, directors, advisory board members, employees and consultants. Additionally, 45,000 shares of common stock have been reserved for issuance under the 2023 Employee Stock Purchase Plan (“ESPP”), of which 45,000 shares remain available for future issuance as of December 31, 2023. Finally, 51,000 shares of common stock have been reserved for issuances under our Inducement Plan, of which 1,000 remain available for future issuance as of December 31, 2023. In aggregate, issued and outstanding common stock and shares issuable under outstanding equity awards or reserved for future issuance under the 2022 Plan, the 2012 Plan, the Inducement Plan, and the ESPP total 12,347,904 shares of common stock as of December 31, 2023.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources. We have certain options outstanding but we do not expect to receive sufficient proceeds from the exercise of these instruments unless and until the underlying securities are registered, and/or all restrictions on trading, if any, are removed, and in either case the trading price of our common stock is significantly greater than the applicable exercise prices of the options and warrants.
Comparison of the Years Ended March 31, 2023 and 2022
The following table summarizes our results of operations for the years ended March 31, 2023 and 2022 (in thousands, except percentages):
| | | | | | | | | | | | | | | |
| Year Ended March 31, | | | Increase (decrease) | |
| 2023 | | | 2022 | | | $ | | | % | |
Revenues | $ | 370 | | | $ | 1,500 | | | $ | (1,130 | ) | | | (75 | %) |
Research and development | $ | 8,885 | | | $ | 3,320 | | | $ | 5,565 | | | | 168 | % |
Selling, general and administrative | $ | 9,216 | | | $ | 9,659 | | | $ | (443 | ) | | | (5 | %) |
Other income | $ | 474 | | | $ | 33 | | | $ | 441 | | | | 1,336 | % |
Revenues
We had $0.4 million of royalty revenue for the year ended March 31, 2023, compared to $1.5 million revenue for the year ended March 31, 2022. The $1.5 million of royalty revenue for the year ended March 31, 2022 was an upfront payment related to the licensing of certain intellectual property (“IP”). The $0.4 million of royalty revenue for the year ended March 31, 2023, was related to the sales-based royalty revenue earned from the aforementioned licensing of IP.
Research and Development Expenses
The following table summarizes our research and development expenses for the years ended March 31, 2023 and 2022 (in thousands, except percentages):
| | | | | | | | | | | | | | | |
| Year Ended March 31, | | | Increase (decrease) | |
| 2023 | | | 2022 | | | $ | | | % | |
Research and development | $ | 8,247 | | | $ | 2,787 | | | $ | 5,460 | | | | 196 | % |
Non-cash stock-based compensation | | 473 | | | | 419 | | | | 54 | | | | 13 | % |
Depreciation and amortization | | 165 | | | | 114 | | | | 51 | | | | 45 | % |
Total research and development expenses | $ | 8,885 | | | $ | 3,320 | | | $ | 5,565 | | | | 168 | % |
Research and development expenses increased by $5.6 million, or 168%, from approximately $3.3 million for the year ended March 31, 2022 to approximately $8.9 million for the year ended March 31, 2023, as we significantly increased research and development activities. Our full-time research and development staff increased from an average of nine employees for the year ended March 31, 2022 to an average of fifteen employees for the year ended March 31, 2023. Research and development activities consisted of $2.4 million in personnel related costs, $5.2 million in lab and research expenses, $1.0 million in facility costs, and $0.3 million in consulting fees, depreciation, and other miscellaneous expenses. Of the $5.2 million in lab and research expenses, $4.0 million relates to acquired in-process research and development ("IPR&D") of Metacrine's FXR program, related research data, and IP.
Selling, General and Administrative Expenses
The following table summarizes our selling, general and administrative expenses for the years ended March 31, 2023 and 2022 (in thousands, except percentages):
| | | | | | | | | | | | | | | |
| Year Ended March 31, | | | Increase (decrease) | |
| 2023 | | | 2022 | | | $ | | | % | |
Selling, general and administrative | $ | 7,184 | | | $ | 7,794 | | | $ | (610 | ) | | | (8 | %) |
Non-cash stock-based compensation | | 1,904 | | | | 1,837 | | | | 67 | | | | 4 | % |
Depreciation and amortization | | 128 | | | | 28 | | | | 100 | | | | 357 | % |
Total selling, general and administrative
expenses | $ | 9,216 | | | $ | 9,659 | | | $ | (443 | ) | | | (5 | %) |
Selling, general and administrative expenses decreased approximately $0.4 million, or 5%, from $9.7 million for the year ended March 31, 2022 to approximately $9.2 million for the year ended March 31, 2023. Overall, the decrease year over year is due to a significant decrease in general corporate costs, most notably legal costs, as we were involved in litigation in fiscal 2022 which was resolved by the end of fiscal 2022. For the year ended March 31, 2022, we had an average of four full-time employees, which increased to an average of five full-time employees for the year ended March 31, 2023. Year over year, we had an increase in personnel related costs of approximately $0.4 million, an increase in consulting costs of approximately $0.3 million, an increase in depreciation and amortization of approximately $0.1 million. These increases were offset by a $1.2 million decrease in general
corporate costs, mostly attributable to a decrease in legal costs related to litigation regarding patent enforcement that occurred and ended in fiscal 2022.
Other Income (Expense)
Other income was $0.5 million and less than $0.1 million for the years ended March 31, 2023 and March 31, 2022, respectively. For the year ended March 31, 2023, interest income was approximately $0.5 million, due to higher interest rates compared to prior years. For the year ended March 31, 2022, other income consisted of a sale of a bioprinter asset to an academic research institution as well as interest income.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
On July 18, 2023, Mayer Hoffman McCann P.C. (“MHM”) informed the Company and the Audit Committee (the “Audit Committee”) of the Company’s Board of Directors that it would not stand for re-election as the Company’s independent registered public accounting firm for the audit of the Company’s financial statements for the fiscal year ending March 31, 2024. MHM ceased to serve as the Company’s independent registered public accounting firm on August 10, 2023, the date of the filing of the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2023.
The audit reports of MHM on the Company’s financial statements for the fiscal years ended March 31, 2023 and 2022 did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles, except for an explanatory paragraph regarding the existence of substantial doubt about the Company’s ability to continue as a going concern in the report for the fiscal year ended March 31, 2023.
During the Company’s two most recent fiscal years ended March 31, 2023 and 2022 and the subsequent interim period through July 18, 2023, there were no (a) disagreements, within the meaning of Item 304(a)(1)(iv) of Regulation S-K promulgated under the Securities Exchange Act of 1934, as amended (“Regulation S-K”), and the related instructions thereto, with MHM on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of MHM, would have caused it to make reference to the subject matter of the disagreements in connection with its reports, or (b) reportable events within the meaning of Item 304(a)(1)(v) of Regulation S-K and the related instructions thereto.
On August 31, 2023, the Audit Committee approved the appointment of Rosenberg Rich Baker Berman P.A. (“RRBB P.A.”) as the Company’s new independent registered public accounting firm, effective as of August 31, 2023. During the fiscal years ended March 31, 2023 and 2022 and the subsequent interim period through August 31, 2023, neither the Company, nor anyone on its behalf, consulted RRBB P.A. regarding either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the financial statements of the Company, and no written report or oral advice was provided to the Company by RRBB P.A. that RRBB P.A. concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was either the subject of a “disagreement” (as defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions) or a “reportable event” (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
Quantitative and Qualitative Disclosures About Market Risk
We invest our excess cash in short term, high quality interest bearing securities including US government and US government agency securities and high-grade corporate commercial paper. The primary objective of our investment activities is to preserve our capital for the purpose of funding our operations. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash, cash equivalents, and short-term investments in a variety of securities, including money market funds. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because the majority of our investments are comprised of cash and cash equivalents. We currently do not hedge interest rate exposure. Due to the nature of our short-term investments, we believe that we are not subject to any material market risk exposure. We have limited foreign currency risk exposure as our business operates primarily in U.S. dollars. We do not have significant foreign currency nor any other derivative financial instruments.
DESCRIPTION OF CAPITAL STOCK
We are offering up to [__________] shares of our common stock or pre-funded warrants to purchase up shares of our common stock. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. We are also registering the shares of common stock issuable from time to time upon exercise of the pre-funded warrants offered hereby.
General Matters
The following description summarizes the most important terms of our capital stock. Because it is only a summary of the provisions of our certificate of incorporation, as amended (the “Certificate of Incorporation”), and our amended and restated bylaws (the “Bylaws”), it does not contain all of the information that may be important to you. For a complete description of the matters set forth in this “Description of Capital Stock,” you should refer to our Certificate of Incorporation and Bylaws, each of which are included as exhibits to the registration statement of which this prospectus is a part, and to the applicable provisions of Delaware law.
Our authorized capital stock consists of 200,000,000 shares of common stock, $0.001 par value per share, and 25,000,000 shares of preferred stock, $0.001 par value per share. Our Board of Directors (the “Board”) may establish the rights and preferences of the preferred stock from time to time. As of March 31, 2024, there were 10,077,726 shares of our common stock issued and outstanding and no shares of preferred stock issued and outstanding.
Common Stock
Dividend Rights. Subject to limitations under Delaware law and preferences that may apply to any then-outstanding shares of preferred stock, holders of common stock are entitled to share ratably in dividends, if any, as may be declared from time to time by our Board in its discretion from funds legally available therefor. Dividends, if any, will be contingent upon our revenues and earnings, if any, and capital requirements and financial conditions. The payment of dividends, if any, will be within the discretion of our Board. We presently intend to retain all earnings, if any, and accordingly our Board does not anticipate declaring any dividends prior to a business combination.
Voting Rights. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of the stockholders. Our Certificate of Incorporation does not provide for cumulative voting for the election of directors. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes cast by all shares of common stock that are present in person or represented by proxy. The Certificate of Incorporation establishes a classified board of directors that is divided into three classes with staggered three-year terms. Only the directors in one class will be subject to election at each annual meeting of our stockholders, with the directors in the other classes continuing for the remainder of their respective three-year terms. The Certificate of Incorporation and the Bylaws also provide that the directors may be removed only for cause. In addition, Organovo has reserved the right to amend, alter, change or repeal any provision in the Certificate of Incorporation, subject to any approval of our stockholders as may be required under the DGCL, and our Board is authorized to adopt, amend or repeal the Bylaws.
No Preemptive or Similar Rights. Our common stock is not entitled to preemptive rights, and is not subject to conversion, redemption or sinking fund provisions.
Right to Receive Liquidation Distributions. In the event of a liquidation, dissolution or winding up, the holders of common stock are entitled to share pro rata all assets remaining after payment in full of all liabilities and after providing for each class of stock, if any, having preference over the common stock, subject to the liquidation preference of any then outstanding shares of preferred stock.
Fully Paid and Non-Assessable. All of the outstanding shares of our common stock are duly authorized, validly issued, fully paid and non-assessable.
Pre-Funded Warrants
The following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration and Exercise Price
Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.001. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
Each pre-funded warrant may be exercised, in cash or by a cashless exercise at the election of the holder at any time following the date of issuance and from time to time thereafter until the pre-funded warrants are exercised in full. The pre-funded warrants will be exercisable in whole or in part by delivering to the Company a completed instruction form for exercise and complying with the requirements for exercise set forth in the pre-funded warrant. Payment of the exercise price may be made in cash or pursuant to a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the pre-funded warrant.
Cashless Exercise
At the time a holder exercises its pre-funded warrants, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Exercise Limitation
In general, a holder will not have the right to exercise any portion of a pre-funded warrant if the holder (together with its Attribution Parties (as defined in the pre-funded warrant)) would beneficially own in excess of 4.99% or 9.99%, at the election of the holder, of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrant. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon notice to us, provided, that any increase in this limitation will not be effective until 61 days after such notice from the holder to us and such increase or decrease will apply only to the holder providing such notice.
Transferability
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, the number of shares of common stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder
Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Preferred Stock
As of the date of this prospectus, no shares of preferred stock are issued and outstanding. Our Board is authorized, subject to limitations prescribed by Delaware law, to issue up to 25,000,000 shares of preferred stock in one or more series, to determine and fix from time to time the number of shares to be included in such series, and to fix the designations, powers, preferences and rights, qualifications, limitations and restrictions thereof, including, without limitation, dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), redemption price or prices and liquidation
preferences of such series, in each case without further vote or action by the stockholders. Our Board can also increase or decrease the number of shares of any such series, but not above the total number of authorized shares and not below the number of shares of such series then outstanding, without any further vote or action by the stockholders.
Our Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock. We have no current plans to issue any shares of preferred stock.
Anti-Takeover Effects of Certain Provisions of our Certificate of Incorporation, Bylaws and the General Corporation Law of the State of Delaware
Certain provisions of Delaware law, along with the Certificate of Incorporation and the Bylaws, may have the effect of delaying, deferring or discouraging another person from acquiring control of us. These provisions are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to first negotiate with our Board. However, these provisions could have the effect of delaying, discouraging or preventing attempts to acquire us, which could deprive the stockholders of opportunities to sell their shares of our common stock at prices higher than prevailing market prices.
Delaware Law
We are subject to Section 203 of the General Corporation Law of the State of Delaware (“DGCL”), which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by our Board.
Choice of Forum
The Bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, another state or federal court located in the State of Delaware) will be the sole and exclusive forum for: (1) any derivative action or proceeding brought on our behalf; (2) any action asserting a claim of breach of a fiduciary duty by any of our directors, officers or stockholders to us or our stockholders; (3) any action arising pursuant to any provision of the DGCL or the Certificate of Incorporation or the Bylaws; or (4) any action asserting a claim governed by the internal affairs doctrine. The provision will not apply to suits brought to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the choice of forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. However, the Certificate of Incorporation does not relieve us of our duties to comply with federal securities laws and the rules and regulations thereunder, and our stockholders will not be deemed to have waived our compliance with these laws, rules and regulations. The Bylaws also provide that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock will be deemed to have notice of and to have consented to this choice of forum provision.
This choice of forum provision in the Bylaws may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, employees or agents, which may discourage such lawsuits against us and our directors, officers, employees or agents. In addition, stockholders who do bring a claim in the Court of Chancery in the State of Delaware could face additional litigation costs in pursuing any such claim, particularly if they do not reside in or near Delaware. Furthermore, the enforceability of similar choice of forum provisions in other companies’ governing documents has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable.
Board of Directors Vacancies. Any vacancy or newly created directorship in our Board, however occurring, shall be filled only by the vote of a majority of the directors then in office, although less than a quorum, and shall not be filled by the stockholders, unless our Board determines by resolution that any such vacancy or newly created directorship shall be filled by the stockholders. In addition, the number of directors constituting our Board shall be determined from time to time by a resolution adopted by our Board. These
provisions may prevent a stockholder from increasing the size of our Board and then gaining control of our Board by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of our Board and promotes continuity of management.
Classified Board. Our Board is divided into three classes. The directors in each class will serve for a three-year term, one class being elected each year by the stockholders. This system of electing and removing directors may tend to discourage a third party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors.
Stockholder Meetings. The Bylaws provide that a special meeting of stockholders may be called only by the chairperson of our Board, chief executive officer or president (in the absence of a chief executive officer) or a majority of the authorized number of directors, thus prohibiting a stockholder (in the capacity as a stockholder) from calling a special meeting. These provisions might delay the ability of the stockholders to force consideration of a proposal or for stockholders controlling a majority of the capital stock to take any action, including the removal of directors.
Elimination of Stockholder Action by Written Consent. The Certificate of Incorporation and the Bylaws eliminate the right of stockholders to act by written consent without a meeting unless the action to be effected by written consent and the taking of such action by written consent is approved in advance by resolution of our Board. As a result, a holder controlling a majority of the capital stock would not be able to amend the Bylaws or remove directors without holding a meeting of the stockholders called in accordance with the Bylaws.
Advance Notice Requirements for Stockholder Proposals and Director Nominations. The Bylaws establish advance notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors. The Bylaws also specify certain requirements regarding the form and content of a stockholder’s notice. These provisions might preclude the stockholders from bringing matters before an annual meeting of stockholders or from making nominations for directors at an annual meeting of stockholders if the proper procedures are not followed. We expect that these provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of us.
No Cumulative Voting. The Certificate of Incorporation does not permit stockholders to cumulate their votes in the election of directors. Accordingly, the holders of a majority of the outstanding shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they choose, other than any directors that holders of Preferred Stock may be entitled to elect.
Directors Removed Only for Cause. The Certificate of Incorporation and the Bylaws provide that no member of our Board may be removed from office by the stockholders except for cause.
Issuance of Undesignated Preferred Stock. The ability of our Board, without action by the stockholders, to issue up to 25,000,000 shares of undesignated preferred stock with voting or other rights or preferences as designated by our Board could impede the success of any attempt to change control of our company. This may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Amendment of Charter Provisions. Organovo has reserved the right to amend, alter, change or repeal any provision in the Certificate of Incorporation, subject to any approval of our stockholders as may be required under the DGCL, and our Board is authorized to adopt, amend or repeal the Bylaws. The provisions of the DGCL, the Certificate of Incorporation and the Bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition of our Board. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Transfer Agent and Registrar
The Transfer Agent and Registrar for our common stock is Continental Stock Transfer & Trust Company, One State Street, 30th Floor, New York, NY 10004.
Listing
Our common stock is listed on the Nasdaq Capital Market under the symbol “ONVO”.
MANAGEMENT
Board of Directors
Our Board of Directors is comprised of six directors. Our Board is divided into three classes, with one class standing for election each year for a three-year term. There are currently two Class I directors, two Class II directors, and two Class III directors.
In addition to the information set forth below regarding our directors and the skills that led our Board to conclude that these individuals should serve as directors, we also believe that all of our directors have a reputation for integrity, honesty and adherence to the highest ethical standards. We believe they each have demonstrated business acumen and an ability to exercise sound judgment, as well as a commitment of service to our Company and to their Board duties.
Information About Our Directors
The following sets forth information regarding the business experience of our current directors:
| | | | | | |
Name |
| Age(1) |
| Position(s) |
| Director Class |
Keith Murphy |
| 52 |
| Director and Executive Chairman |
| Class III |
Adam Stern |
| 60 |
| Director |
| Class III |
Douglas Jay Cohen |
| 53 |
| Lead Independent Director |
| Class II |
David Gobel |
| 71 |
| Director |
| Class II |
Alison Tjosvold Milhous |
| 44 |
| Director |
| Class I |
Vaidehi Joshi |
| 38 |
| Director and Director of Discovery Biology |
| Class I |
__________________
Class I Directors Continuing in Office until the 2024 Annual Meeting of Stockholders
Alison Tjosvold Milhous, Director, has served on our Board since September 2020. She has 20 years of audit and technical accounting experience and is a certified public accountant. She is currently the Vice President of Accounting at Erasca, Inc., a clinical-stage precision oncology company. Prior to joining Erasca, she was an independent consultant assisting public and private companies with accounting and reporting needs primarily within the life sciences and technology industries. Ms. Milhous was previously an audit partner at Grant Thornton LLP from August 2015 through September 2019 and held various positions with increasing responsibility at Grant Thornton from June 2002 as an audit associate through July 2015 as an audit senior manager. She began her career in June 2000 at Arthur Andersen LLP. Ms. Milhous served on the membership committee of Athena San Diego, a professional women’s leadership organization with a STEM focus, from August 2012 through September 2019 and was on the Pinnacle steering committee from September 2013 through April 2015. Ms. Milhous received a Bachelor of Science degree in Business Administration with a dual concentration in Accounting and Finance from California State Polytechnic University, San Luis Obispo.
We believe Ms. Milhous’ extensive financial and accounting experience and her experience providing audit and consulting services to life sciences companies qualify her to serve as a member of our Board.
Vaidehi Joshi, Director, has served on our Board since March 2022 and as our Director of Discovery Biology since April 2022. Ms. Joshi has over a decade of experience in early-stage biotech companies developing unique therapeutic solutions, cutting-edge research products, and cell-based therapies. Since November 2020, Ms. Joshi has served as Director of Discovery Biology at Viscient Biosciences, Inc., where she leads the MASH small molecule drug discovery program as well as 3D model development for other tissue programs. Prior to joining Viscient, Ms. Joshi worked her way up at Organovo, Inc., where she led the pre-clinical research program for the design, development, and manufacture of 3D bioprinted therapeutic human liver tissues targeted towards the treatment of inborn errors of metabolism and genetic diseases. She received her Bachelor of Engineering in Biotechnology at the Rashtreeya Vidyalaya College of Engineering (RVCE) in Bangalore, India and received a Master of Science in Biomedical Engineering from University of California, Los Angeles (UCLA), with a specialization in Tissue Engineering and Biomaterials. While at UCLA, her
focus was on primary intestinal epithelial stem cell isolations and 3D cultures for intestinal tissue regeneration for short gut syndrome. Ms. Joshi is an experienced speaker, panelist, and presenter at several biomedical conferences both in the U.S and internationally. Ms. Joshi has co-authored several published peer reviewed articles, and is an inventor on multiple Organovo patents and patent applications. We believe Ms. Joshi’s extensive scientific background, previous experience in the biotechnology field, and her educational experience qualify her to serve as a member of our Board.
We believe Ms. Joshi’s extensive scientific background, previous experience in the biotechnology field, and her educational experience qualify her to serve as a member of our Board.
Class II Directors Continuing in Office Until the 2025 Annual Meeting of Stockholders
Douglas Jay Cohen, Lead Independent Director, has served on our Board since September 2020 and has served as our Lead Independent Director since September 2022. He has served as president and Chief Executive Officer of IR Medtek LLC since January 2019, a medical device company developing a non-invasive probe for cancer detection by primary care physicians using a technology licensed from the Ohio State University. Prior to IR Medtek, Mr. Cohen served as President and Chief Executive Officer of Beacon Street Innovations, an advanced technology printing company from September 2016 to present. From January 1994 to September 2016, Mr. Cohen served as Vice President of Operations and Engineering at Screen Machine Industries, an industrial and construction heavy equipment manufacturer. As an active investor in startup companies, Mr. Cohen has invested in more than 20 biotech startups in the past 10 years, including investing in Organovo in 2013 and maintaining a position in the company ever since. Mr. Cohen received a B.S. from the Massachusetts Institute of Technology.
We believe Mr. Cohen’s experience in the life sciences industry, his experience in managing emerging growth companies and his experience in developing business strategies qualifies him to serve as a member of our Board.
David Gobel, Director, has served on our Board since September 2020. He has served as Chief Executive Officer of Methuselah Fund LLC since December 2016 and as Chief Executive Officer of Methuselah Foundation since September 2001, promoting increasing the healthy human lifespan by various means including: performance prizes, targeted grant making, education, and the creation/funding of biotech startups. Mr. Gobel became Chief Venture Strategist at Transportation Security Administration from January 2009 until March 2013, where he was responsible for strategic planning, innovation management and creation of a novel Venture Capital capability for TSA and then Department of Homeland Security by partnering with In-Q-Tel. Mr. Gobel was a member of the board of Volumetric Biotechnologies, a company that focuses on the development of bioholographic human tissue printing, from April 2018 to January 2020. Since July 2018, Mr. Gobel served as member of the board for Turn Bio, and since May 2020 as chairman of the board of Turn Bio. Mr. Gobel served as a board member of Leucadia Therapeutics from October 2015 to August 2022, and as an independent founding board member of Oisin Therapeutics since December 2014.
We believe Mr. Gobel’s previous services as chief executive officer for other biotechnology companies, his experience and expertise with human tissue printing companies and his extensive board experience qualify him to serve as a member of our Board.
Class III Directors Continuing in Office Until the 2026 Annual Meeting of Stockholders
Keith Murphy, Director and Executive Chairman, re-joined our Board in July 2020 and has served as our Executive Chairman since September 2020. Mr. Murphy is the Chief Executive Officer and Chairman of Viscient Biosciences, Inc. (“Viscient”), a private company that he founded in 2017 that is focused on drug discovery and development utilizing 3D tissue technology and multi-omics (genomics, transcriptomics, metabolomics). Mr. Murphy previously served as the President and Chief Executive Officer of Organovo from February 2012 through April 2017, and as Chairman from February 2012 through August 2017. Mr. Murphy also previously served as President, Chief Executive Officer, and Chairman of Organovo, Inc., Organovo’s primary operating company prior to its going-public transaction, from August 2007 to February 2012. Prior to founding Organovo, Mr. Murphy served in various roles at Amgen, Inc. from August 1997 to July 2007 including as Global Operations Leader for the osteoporosis/bone cancer drug Prolia/Xgeva (denosumab). Prior to joining Amgen, Mr. Murphy served at Alkermes, Inc., a biotechnology company, from July 1993 to July 1997, where he played a role on the development team for their first approved product, Nutropin (hGH) Depot. Mr. Murphy served as a member of the board of directors of Kintara Therapeutics, Inc. from August 2020 to February 2022, and served on its compensation committee and nominating and corporate governance committee. He holds a B.S. in Chemical Engineering from MIT and is an alumnus of the UCLA Anderson School of Management.
We believe Mr. Murphy’s previous experience in the biotechnology field, especially in developing novel products, his experience and expertise with our 3D bioprinting technology and product development opportunities and strategy, and his educational experience qualify him to be a member of our Board of Directors.
Adam Stern, Director, re-joined our Board in July 2020. Mr. Stern is currently the Chief Executive Officer of SternAegis Ventures, the private equity group at Aegis Capital Corp. responsible for venture capital and private equity financing, and has been the Head of Private Equity Banking at Aegis Capital Corp., a full-service investment banking firm, since December 2012. Prior to SternAegis, Mr. Stern served as Senior Managing Director at Spencer Trask Ventures, Inc., a private equity and venture firm, from 1997 to 2012, where he managed the structured finance group focusing primarily on technology and life sciences companies. From 1989 to 1997, Mr. Stern was at Josephthal & Co., Inc., Members of the New York Stock Exchange, where he served as Head of Private Equity and Managing Director. He has been a FINRA licensed securities broker since 1987 and a Registered General Securities Principal since 1991. Mr. Stern previously served as a director of Organovo from February 2012 to June 2013. Mr. Stern is a current director at DarioHealth Corp. (Nasdaq: DRIO), privately held Amplifica Holdings, Group, Inc., and Aerami Therapeutics Holdings Inc. Mr. Stern is a former director of Adgero Biopharmaceuticals Holdings, Matinas BioPharma Holdings, Inc. (NYSE: MTNB), Hydrofarm Holdings Group Inc. (Nasdaq: HYFM), InVivo Therapeutics, Inc. (Nasdaq: NVIV) and PROLOR Biotech prior to its sale in 2013 to Opko Health, Inc. (Nasdaq: OPK). Mr. Stern graduated with a Bachelor of Arts degree from the University of South Florida in 1987.
We believe Mr. Stern’s extensive experience in corporate finance, his expertise in the life sciences industries and his previous experience as a member of our Board qualify him to be a member of our Board of Directors.
No Family Relationships
There are no family relationships between any of our officers and directors.
Board Independence
Our shares of common stock are listed for trading on the Nasdaq Capital Market. As a result, our Board utilizes the definition of “independence” as that term is defined by the listing standards of the Nasdaq Capital Market and the rules and regulations of the SEC, including the additional independence requirements for members of our Audit Committee and the Compensation Committee. Our Board considers a director “independent” when the director is not an officer or employee of the Company or its subsidiaries, does not have any relationship which would, or could reasonably appear to, materially interfere with the independent judgment of such director, and the director otherwise meets the independence requirements under the listing standards of the Nasdaq Capital Market and the rules and regulations of the SEC. Our Board has reviewed the materiality of any relationship that each of our directors has with the Company, either directly or indirectly. Based on this review, our Board has affirmatively determined that the following four of our six current directors qualify as “independent” directors: Douglas Jay Cohen, David Gobel, Alison Tjosvold Milhous and Adam Stern. Keith Murphy and Vaidehi Joshi do not qualify as an independent director. Mr. Murphy currently serves as our Executive Chairman and as Chief Executive Officer of Viscient, which has made payments to the Company in sufficient amounts to qualify as related party transactions leading to director non-independence. Please see the section “Certain Relationships and Related Transactions” for an additional information. Ms. Joshi is currently our Director of Discovery Biology.
Executive Officers
The following persons are our executive officers as of the date of this prospectus and hold the positions set forth opposite their names as of April 12, 2024:
| | | | |
Name |
| Age |
| Position |
Keith Murphy |
| 52 |
| Executive Chairman |
Thomas Hess |
| 60 |
| President and Chief Financial Officer |
See the section entitled “Board of Directors Information”, above, for a description of the business experience and educational background of Mr. Murphy.
Thomas Hess, President and Chief Financial Officer, joined us in October 2021. Mr. Hess is currently employed by Danforth Advisors, LLC (“Danforth”), a professional financial consulting services firm. He has over twenty years of experience and has been with Danforth since September 2021. Mr. Hess recently served as Chief Financial Officer and Senior Vice President of Finance of Genomind, Inc., until his retirement in May 2021. From September 2011 until its sale in April 2014, Mr. Hess served as Chief Financial Officer and Executive Vice President of Finance of The Keane Organization. Mr. Hess also previously served in various other capacities including, but not limited to, Chief Financial Officer and Senior Vice President of Yaupon Therapeutics, Inc.; Chief Financial Officer and Vice President, Finance of Adolor Corporation; Corporate Controller of Vicuron Pharmaceuticals, Inc.; and Senior Manager, Accounting and Audit of KPMG. Mr. Hess received his B.S. in accounting from The Pennsylvania State University and his MBA from Katz Graduate School of Business, University of Pittsburgh and is a Certified Public Accountant in the State of Pennsylvania. He currently serves on the Alumni Council of Penn State.
Director Compensation
Our directors play a critical role in guiding our strategic direction and overseeing the management of our Company. Ongoing developments in corporate governance and financial reporting have resulted in an increased demand for such highly qualified and productive public company directors. The many responsibilities and risks and the substantial time commitment of being a director of a public company require that we provide adequate incentives for our directors’ continued performance by paying compensation commensurate with our directors’ workload. Our directors are compensated based upon their respective levels of Board participation and responsibilities, including service on Board committees.
Our director compensation is overseen by the Compensation Committee, which makes recommendations to our Board on the appropriate structure for our director compensation program and the appropriate amount of compensation. Our Board is responsible for final approval of our director compensation program and the compensation paid to our directors.
In connection with establishing our director compensation for the fiscal year ended March 31, 2024 (“Fiscal 2024”), the Compensation Committee retained Anderson Pay Advisors (“Anderson”) as its independent compensation consultant. With the assistance of Anderson, the Board and Compensation Committee conducted a formal review of our director compensation and incentive programs relative to the same peer group used in benchmarking the compensation for our executive officers.
Fiscal 2024 Director Compensation Framework
For Fiscal 2024, our Director Compensation Framework provided to both non-employee and employee directors annual cash retainers for Board service and for service as the chair or member of one of the standing Board committees. Our directors are not entitled to any Board meeting fees or Board committee meeting fees.
Annual Cash Retainers. For Fiscal 2024, each of our directors was eligible to receive an annual cash retainer of $66,300 for Board membership.
In addition, each of our directors are eligible to receive the applicable annual retainers set forth below for serving as committee chairs and for service as a member of a Board committee, with total cash compensation for each director not to exceed $105,000 per fiscal year:
| | | | | | | | | | | | | |
Position | | Audit Committee | | Compensation Committee |
| Nominating and Corporate Governance Committee |
| Science and Technology Committee |
|
Committee Chair | | $ | 25,500 | | $ | 25,500 |
| $ | 25,500 |
| $ | 25,500 |
|
Committee Member (excluding Chair) | | $ | 15,300 | | $ | 15,300 |
| $ | 15,300 |
| $ | 15,300 |
|
No additional meeting fees were paid to our directors for Fiscal 2024.
Equity Awards. In addition, in November 2023, each director received a restricted stock unit award with respect to 19,607 shares of common stock (a value of approximately $27,200), which will vest in full on the earlier of (i) November 17, 2024 or (ii) the date of our next annual meeting of the stockholders, subject to acceleration in the event of a change of control.
Reimbursement. Our directors are entitled to reimbursement for their reasonable travel and lodging expenses for attending Board and Board committee meetings.
Director Compensation Table
The following table sets forth the compensation earned and paid to each member of our Board for service as a director during Fiscal 2024:
| | | | | | | | | | | | | | | | | |
Name | | Fees Earned or Paid in Cash ($) |
| Stock Awards ($)(1) |
| Option Awards ($) |
|
| All Other Compensation ($) |
| Total ($) |
|
Douglas Jay Cohen | | $ | 105,000 |
| $ | 27,254 |
| $ | — |
|
| $ | — |
| $ | 132,254 |
|
David Gobel | | | 105,000 |
| | 27,254 |
| | — |
|
| | — |
| | 132,254 |
|
Alison Tjosvold Milhous | | | 105,000 |
| | 27,254 |
| | — |
|
| | — |
| | 132,254 |
|
Adam Stern | | | 96,900 |
| | 27,254 |
| | — |
|
| | — |
| | 121,154 |
|
Keith Murphy | | | 81,600 | | | 27,254 | | | — | | | | — | | | 108,854 | (2) |
Vaidehi Joshi | | | 91,800 |
| | 27,254 |
| | — |
|
| | — |
| | 119,054 |
|
______________________
(1)These amounts represent the grant date fair value of time-based restricted stock unit awards granted by the Board, determined in accordance with FASB ASC Topic 718. All awards are amortized over the vesting life of the award. For the assumptions used in our valuations, see “Note 5 – Stockholders’ Equity” of our notes to consolidated financial statements included elsewhere in this prospectus.
(2)Comprised solely of the compensation received by Mr. Murphy for his service as a member of the Board. Mr. Murphy’s additional compensation for Fiscal 2024 is included in the section entitled “Summary Compensation Table” on page 66 of this prospectus.
Executive Compensation
The following discussion is designed to provide our stockholders with an understanding of our compensation philosophy and objectives as well as an overview of the analysis that our Compensation Committee performed in setting the compensation of our executive officers for Fiscal 2024 (i.e., the period from April 1, 2023 to March 31, 2024).
This discussion summarizes the Compensation Committee’s determination of how and why, in addition to what, compensation actions were taken for our named executive officers, as follows:
•Keith Murphy, our Executive Chairman and Principal Executive Officer;
•Thomas Hess, our Chief Financial Officer;
•Thomas Jurgensen, our former General Counsel and Corporate Secretary(1); and
•Jeffrey Miner, our former Chief Scientific Officer(2).
(1)Mr. Jurgensen’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023.
(2)Dr. Miner’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023.
Other than Mr. Murphy, Mr. Hess was the only executive officer serving at the end of Fiscal 2024. These four individuals are collectively referred to in this prospectus as our “named executive officers”.
Recent “Say-on-Pay” Votes
Our recent stockholder advisory votes, commonly referred to as a “Say-on-Pay” vote, to approve the compensation of our named executive officers for Fiscal 2023 (i.e., the period from April 1, 2022 to March 31, 2023) was approved by our stockholders, with approximately 85% of stockholder votes cast in favor of the proposal.
During Fiscal 2024, our Executive Chairman, former General Counsel, and Chief Financial Officer maintained significant stockholder engagement efforts to monitor how our investors vote, obtain their views on key corporate governance and disclosure matters and determine how best to respond to feedback each year going forward. Specifically, we reached out to our stockholders representing over 95% of our institutional stock holdings multiple times. None of the stockholders indicated a need or desire to engage with the Company to discuss or express any concerns.
Going forward, we plan to continue to:
•at least annually, reach out to institutional stockholders representing a majority of the shares held by our institutional stockholders; and
•invite them to engage and participate in calls to discuss our executive compensation programs, their feedback and questions and how we may best address them.
One or more members of executive management are expected to be active participants on all such calls, as will one or more members of our Compensation Committee. All such feedback will be shared with our Board.
In evaluating potential changes to our executive compensation programs’ structure and disclosure, the Compensation Committee will closely examine, and aim to understand further, our stockholders’ feedback, including any common themes from our stockholders’ feedback. The Compensation Committee will also seek the advice of independent compensation consultants with respect to the design of our executive compensation program.
Compensation Philosophy and Objectives
Our executive compensation program focuses on creating alignment between our stockholders and executive officers by including both performance- and incentive-based compensation elements. Our compensation package also combines both short- and long-term components (cash and equity, respectively) at the levels the Compensation Committee determined to be appropriate to motivate, reward, and retain our executive officers. Our executive compensation program is designed to achieve the following key objectives:
•Attract, retain, and reward talented executives and motivate them to contribute to the Company’s success and to build long-term stockholder value;
•Establish financial incentives for executives to achieve our key financial, operational, and strategic goals;
•Enhance the relationship between executive pay and stockholder value by utilizing long-term equity incentives; and
•Recognize and reward executives for superior performance.
Use of Market Data and Benchmarking
The Compensation Committee endeavors to set compensation at competitive levels. In order to do this, the Compensation Committee compares our compensation packages with the packages offered by other peer companies that are similarly situated, and with which we compete for talent. Selection criteria includes:
•Industry, specifically biotechnology and medical research,
•Company focus, with an emphasis on technology platforms,
•Stage of leading drug candidate, with an emphasis on Phase II/III,
•Market capitalization, targeting less than $100 million,
•Number of employees, targeting less than 50 employees, and
•Location, specifically nationwide.
For Fiscal 2024, the Compensation Committee engaged Anderson, an independent compensation consultant, as the Compensation Committee’s advisor reporting directly to the chair of the Compensation Committee. The Compensation Committee determined that no conflict of interest exists that would preclude Anderson from serving as an independent consultant to the Compensation Committee.
The Compensation Committee requested Anderson conduct a review and analysis of our executive compensation programs as compared against competitive benchmarks. This included a benchmarking analysis against prevailing market practices of a peer group of comparable companies approved by the Compensation Committee and broader industry trends and benchmarks. The analysis included a review of the “Total Direct Compensation” (which includes salary, cash incentives, and equity awards) of our executive officers, and was based on an assessment of market trends covering available public information as well as proprietary information provided by Anderson.
For Fiscal 2024, based on recommendations from Anderson, our Compensation Committee determined that our peer group should be modified to better reflect our current market valuation as well as the growing importance of our therapeutics program to our overall business model. With input from Anderson, our Compensation Committee added a group of companies focused on technology platforms, with comparable size, revenues, market valuations, and stage of leading drug candidate. Our Compensation Committee also replaced some of the companies previously included in our peer group because their market valuations had grown too high for direct comparison to our Company, and/or their business focus had become less relevant for direct comparison to our Company. Our
Compensation Committee then used the compensation data from this revised peer group in setting executive compensation for Fiscal 2024.
The peer group for Fiscal 2024 included:
| | |
Aceragen* | Cohbar Inc.* | Onconova Therapeutics, Inc. |
Aligos Therapeutics, Inc.* | Fresh Tracks Therapeutics Inc.* | OncoSec Medical Incorporated |
Aprea Therapeutics, Inc. | Galectin Therapeutics Inc.* | Pulmatrix, Inc. |
aTyr Pharma, Inc. | Galmed Pharmaceuticals Ltd.* | Regulus Therapeutics Inc. |
Ayala Pharmaceuticals* | Hepion Pharmaceuticals, Inc.* | Seelos Therapeutics, Inc. |
Bellicum Pharmaceuticals, Inc. | Imunon, Inc.* | Soligenix, Inc. |
Capricor Therapeutics, Inc. | LadRx Corp* | Theriva Biologics, Inc.* |
* Indicates addition to peer group from Fiscal 2023.
Determination of Executive Compensation
In addition to peer group data, the Compensation Committee considered relevant publicly available market data and surveys and the compensation reports it received from Anderson. The Compensation Committee also reviewed and considered the compensation recommendations of our Executive Chairman, the Company’s overall performance during Fiscal 2024, the Company’s financial status and operating runway, each executive officer’s responsibilities and contribution to the Company’s achievement of the Fiscal 2024 corporate goals, and each executive officer’s individual performance during Fiscal 2024. With respect to new hires, our Compensation Committee considered the executive officer’s background and historical compensation in lieu of prior year performance in addition to benchmark data for the newly hired executive’s position.
Commitment to Good Compensation Governance Practices
In designing our executive compensation program, our Compensation Committee intends to create alignment between our stockholders and executive officers and to implement good compensation governance by:
•Annual Advisory Vote on the Compensation of our Named Executive Officers – We provide our stockholders with the ability to vote annually on the compensation of our named executive officers.
•Independent Compensation Consultant – The Compensation Committee engaged Anderson during Fiscal 2023 to serve as its independent compensation consultant. Anderson did not provide any other services to the Company during the periods it served as a consultant to the Compensation Committee.
•Performance and Incentive Based – Previously, approximately 40% of the Total Direct Compensation our executive officers could earn was performance and incentive based, thereby aligning the interests of our executive officers with our stockholders’ interests. As our current executive officers are retained through consulting firms, neither are eligible for performance based compensation.
•Compensation Risk Assessment – The Compensation Committee oversees and evaluates an annual risk assessment of the Company’s compensation program. The Compensation Committee believes that the performance goals established for incentives do not encourage excessive risk-taking or have the potential to encourage behavior that may have a material adverse effect on the Company.
•Prohibitions on Hedging, Pledging and Margin Activities – Our insider trading policy prohibits hedging transactions by Company employees. Under the policy, all short-term, speculative or hedging transactions in Organovo securities are prohibited by all employees. In addition, the policy specifically prohibits the use of Organovo securities for pledging and margin activities.
•No Single Trigger Change in Control Vesting- We do not provide for the acceleration of vesting solely upon the occurrence of a change in control in our equity awards for our directors and executive officers.
•No Excise Tax Gross Ups – We do not include excise tax gross ups for any change in control payments.
•Director and Executive Officer Stock Ownership Guidelines – We have adopted stock ownership guidelines that require each director and executive officer to accumulate and hold a specified value of our stock within five years of most recently starting employment with us or becoming a director.
The Compensation Committee believes that the program and policies described above demonstrate the Company’s commitment to, and consistent execution of, an effective performance-oriented executive compensation program.
Components of Executive Compensation
The framework established by the Compensation Committee, based on the data provided by Anderson, for our executive compensation program consists of a base salary, performance-based cash incentives and long-term equity-based incentives. The Compensation Committee endeavors to combine these compensation elements to develop a compensation package that provides competitive pay, rewards our executive officers for achieving our commercial, operational and strategic objectives and aligns the interests of our executive officers with those of our stockholders.
Salary. The Compensation Committee has provided, and will continue to provide, our executive officers with a base salary to compensate them for services provided during the fiscal year. In addition to benchmark data from our peer group, our Compensation Committee considers the Company’s overall performance during the prior fiscal year, cash burn, the Company’s financial status and operating profile, each executive officer’s responsibilities and contribution to the achievement of the prior year’s corporate goals, and each executive officer’s individual performance during the prior fiscal year. The evaluations and recommendations proposed by our Executive Chairman are also considered (other than with respect to determining his own compensation). With respect to new hires, the Compensation Committee considers an executive’s background and historical compensation in lieu of prior year performance as well as benchmark data for the new hire’s position. Our Compensation Committee evaluates and sets the base salaries for our executives following annual performance evaluations, as well as upon a promotion or other change in responsibility. Our Compensation Committee expects to continue to utilize these policies going forward.
For Fiscal 2024, the Compensation Committee determined that it was in the best interests of the Company and its stockholders to freeze Mr. Jurgensen’s and Dr. Miner’s salaries for Fiscal 2024 at Fiscal 2023 levels, or $381,600 for Mr. Jurgensen and $238,500 for Dr. Miner.
Pursuant to the terms of our consulting agreement with Multi Dimensional Bio Insight LLC (“MDBI”), a biotechnology consulting firm through which we retain Mr. Murphy, MDBI has the right, on an annual basis, to increase hourly consultant rates by up to 4%. From January 1, 2021 to May 1, 2022, the hourly rate for Mr. Murphy’s services was $375, which was increased to $413 effective May 1, 2022 and remained the same throughout the remainder of Fiscal 2023 and Fiscal 2024. The cash amount paid to MDBI increased from $543,781 for Fiscal 2023 to $657,984 for Fiscal 2024, with approximately a 21% increase in Mr. Murphy’s hours for Fiscal 2024 as compared to Fiscal 2023.
Pursuant to the terms of our consulting agreement with Danforth Advisors, LLC (“Danforth”), a financial consulting firm through which we retain Mr. Hess, Danforth has the right, on an annual basis, to increase hourly consultant rates by up to 4%. From April 1, 2022 to March 31, 2023, the hourly rate for Mr. Hess’ services was $433, which was increased to $450 effective January 1, 2024 and remained the same throughout the remainder of Fiscal 2024. The cash amount paid to Danforth decreased from $269,402 for Fiscal 2023 to $199,322 for Fiscal 2024, with approximately a 29% decrease in Mr. Hess’ hours for Fiscal 2024 as compared to Fiscal 2023.
The base salaries of our named executive officers for Fiscal 2024 as compared to Fiscal 2023 are set forth in the following table:
| | | | | | | | |
Name and Title |
| Fiscal 2024 Base Salary |
|
| Fiscal 2023 Base Salary |
|
Keith Murphy, Executive Chairman(1) |
| $ | 657,984 |
|
| $ | 543,781 |
|
Thomas Hess, Chief Financial Officer(2) |
|
| 199,322 |
|
|
| 269,402 |
|
Thomas Jurgensen, Former General Counsel and Corporate Secretary(3) |
|
| 381,262 |
|
|
| 381,262 |
|
Jeffrey Miner, Former Chief Scientific Officer(4) |
|
| 238,292 |
|
|
| 238,292 |
|
______________________
(1)Mr. Murphy was appointed our Executive Chairman on September 15, 2020. The Company retains Mr. Murphy through MDBI, a biotechnology consulting firm, pursuant to the terms of a consulting agreement, pursuant to which Company has agreed to pay MDBI $375-390 per hour of services provided by Mr. Murphy, with an annual increase in rates by up to 4%. The amounts reported under “Fiscal 2024 Base Salary” and “Fiscal 2023 Base Salary” are comprised of the actual amounts paid to MDBI for its consulting services. Mr. Murphy did not directly receive a base salary from the Company in Fiscal 2024 or Fiscal 2023. Mr. Murphy’s increase in base salary was primarily due to additional time commitment of Mr. Murphy in his consulting role as Executive Chairman of the Company.
(2)Mr. Hess was appointed our Chief Financial Officer on October 6, 2022. The Company retains Mr. Hess through Danforth, a financial consulting firm, pursuant to the terms of a consulting agreement, pursuant to which Company has agreed to pay Danforth $400 per hour of services provided by Mr. Hess, with an annual increase in rates by up to 4%. The amounts reported under “Fiscal 2024 Base Salary” and “Fiscal 2023 Base Salary” are comprised of the actual amounts paid to Danforth for its consulting services. Mr. Hess did not directly receive a base salary from the Company in Fiscal 2024 or Fiscal 2023. Mr. Hess’s decrease in base salary was primarily due to reduced time commitment of Mr. Hess in his consulting role as Chief Financial Officer of the Company.
(3)Mr. Jurgensen’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023.
(4)Dr. Miner’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023
Performance-Based Cash Incentive Awards. Our executive compensation program includes an annual performance-based cash incentive award, which provides our executive officers with an annual cash incentive opportunity as a percentage of their base salaries based upon the achievement of corporate and individual performance goals evaluated and approved by the Compensation Committee. For Fiscal 2024, the Compensation Committee determined that the annual target bonus opportunity expressed as a percentage of base salary for each of Mr. Jurgensen and Dr. Miner should be 40% of each of their respective base salaries. The Company continues to use an objectives and key results goal-setting framework (“OKRs”) used by individuals, teams, and the Company to define measurable goals and track their outcomes, originally developed and implemented by Andrew Grove at Intel. See: http://www.whatmatters.com. Each executive officer was eligible to receive an increase in his target bonus amount based on the achievement of individual and corporate OKRs. For Fiscal 2024, the Compensation Committee did not award bonuses to Mr. Jurgensen or Dr. Miner.
Mr. Murphy is retained through MDBI, a biotechnology consulting firm, pursuant to the terms of a consulting agreement. As such, Mr. Murphy is not eligible to receive a bonus for services provided during the fiscal year.
Mr. Hess is retained through Danforth, a financial consulting firm, pursuant to the terms of a consulting agreement. As such, Mr. Hess is not eligible to receive a bonus for services provided during the fiscal year.
Equity-Based Incentive Awards. In addition to base salaries and annual performance-based cash incentives, the Compensation Committee has provided long-term, equity-based incentive awards to our executive officers. In determining the size and terms of the awards, the Compensation Committee considered benchmark data from our peer group, publicly available market and survey data and the individual performance of the named executive officers. The Compensation Committee did not grant any awards for Fiscal 2024.
Other Benefits
In order to attract and retain qualified individuals and pay market levels of compensation, we have historically provided, and will continue to provide, our executives with the following benefits:
•Health Insurance – We provide each of our executives and their spouses and children the same health, dental, and vision insurance coverage we make available to our other eligible employees.
•Life and Disability Insurance – We provide each of our executives with the same life and disability insurance as we make available to our other eligible employees.
•Pension Benefits – We do not provide pension arrangements or post-retirement health coverage for our executives or employees. We implemented a 401(k) Plan effective January 1, 2014. We provide a company matching contribution up to 3.5% of compensation for all participants in the 401(k) plan, including our executive officers, to help attract and retain top talent.
•Nonqualified Deferred Compensation – We do not provide any nonqualified defined contribution or other deferred compensation plans to any of our employees.
•Perquisites – We limit the perquisites that we make available to our executive officers. In certain cases, we have reimbursed our executive officers for their relocation expenses on their initial hire.
Severance Arrangements
As of November 10, 2020, the Company implemented a change in control arrangement, which provides that, in the event an executive is terminated in connection with a Change in Control: the executive will receive (a) in the case of our Executive Chairman, 18 months of base salary or consulting fees, as applicable, and (b) in the case of our other executives, 12 months of base salary.
On September 7, 2023, in connection with Mr. Jurgensen’s termination, the Company entered into a Separation Agreement and General Release (the “Jurgensen Separation Agreement”) with Mr. Jurgensen, to be effective as of September 15, 2023. Pursuant to the Jurgensen Separation Agreement, Mr. Jurgensen released any claims against the Company and the Company paid Mr. Jurgensen an aggregate of $345,000 (less applicable federal, state, and local withholdings), in three separate installment payments of $115,000 on or before September 28, 2023, October 16, 2023 and January 5, 2024.
On September 19, 2023, in connection with Dr. Miner’s termination, the Company entered into a Separation Agreement and General Release (the “Miner Separation Agreement”) with Dr. Miner, to be effective as of September 27, 2023. Pursuant to the Miner
Separation Agreement, Dr. Miner released any claims against the Company and the Company (i) provided Dr. Miner a consulting contract for a period of six months, pursuant to which the Company paid Dr. Miner an aggregate of $169,250 for his consulting services, and (ii) granted Dr. Miner a stock option to purchase 40,000 shares of common stock of the Company (the “Option”). The Option will vest as follows: 13,000 shares vested immediately upon issuance, 13,500 shares will vest on the one year anniversary of the Miner Separation Agreement and 13,500 shares will vest on the two year anniversary of the Minder Separation Agreement. The exercise price is equal to the closing price of a share of Common Stock on the date the Option was approved by the Company’s Board of Directors.
Potential Payments upon Termination or Change in Control
The following table sets forth the amounts payable to each of our named executive officers based on an assumed termination as of March 31, 2024 based upon certain designated events.
| | | | | | | | | | | | | | | | |
Name |
| Cash Severance ($) |
| Health and Other Insurance Benefits ($) |
| Stock Options (Unvested and Accelerated) ($) |
| Restricted Stock Units (Unvested and Accelerated) ($) |
| Fiscal Year 2024 Total ($) |
|
Keith Murphy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination in connection with a Change in Control |
| $ | 1,288,560 |
| $ | — |
| $ | — |
| $ | — |
| $ | 1,288,560 |
|
Thomas Hess |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination in connection with a Change in Control |
| $ | 936,000 |
| $ | — |
| $ | — |
| $ | — |
| $ | 936,000 |
|
Thomas Jurgensen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination in connection with a Change in Control(1) |
| $ | 345,000 |
| $ | — |
| $ | — |
| $ | — |
| $ | 345,000 |
|
Jeffrey Miner |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Termination in connection with a Change in Control(2) |
| $ | 169,250 |
| $ | — |
| $ | — |
| $ | — |
| $ | 169,250 |
|
(1)Mr. Jurgensen’s employment was terminated as of August 25, 2023. Pursuant to the Jurgensen Separation Agreement, the Company paid Mr. Jurgensen an aggregate of $345,000.
(2)Dr. Miner’s employment was terminated as of August 25, 2023. Pursuant to the Miner Separation Agreement, for his consulting services, the Company paid Dr. Miner an aggregate of $169,250, and granted Dr. Miner a stock option to purchase 40,000 shares of common stock of the Company (the “Option”). The Option vests as follows: 13,000 shares vested immediately upon issuance, 13,500 shares will vest on the one year anniversary of the Miner Separation Agreement and 13,500 shares will vest on the two year anniversary of the Miner Separation Agreement.
Death or Disability Benefits
The outstanding equity awards held by our executive officers provide such executive officers with accelerated vesting if the executive officer terminates services with the Company as a result of death or disability. In order for an equity award to be eligible for accelerated vesting, the executive officer’s death or disability must occur more than 90 days after the date the equity award was granted. With respect to performance-based equity awards, an executive officer will vest at target levels upon the executive officer’s death or disability.
Summary Compensation Table
The following table summarizes the total compensation paid to or earned by each named executive officer for Fiscal 2024 and Fiscal 2023.
| | | | | | | | |
Name and Principal Position | Year or Period | Salary
($) | Bonus ($) | Stock Awards
($) | Option Awards
($)(1) | Non-Equity Incentive Plan Compensation
($)(2) | All Other Compensation
($) | Total
($) |
Keith Murphy(3) Executive Chairman | 2024 | 657,984 | — | — | — | — | 23,152(4) | 681,136 |
2023 | 543,781 | — | — | 73,921 | — | 3,746(4) | 621,448 |
Thomas Hess(5) Chief Financial Officer | 2024 | 199,322 | — | — | — | — | — | 199,322 |
Thomas Jurgensen Former General Counsel and
Corporate Secretary | 2024 | 161,446 | — | — | — | — | 348,454(6) | 509,900 |
2023 | 381,263 | — | — | 73,921 | — | 10,790(7) | 465,974 |
Jeffrey Miner Former Chief Scientific Officer | 2024 | 100,904 | — | — | 46,337(8) | — | 172,790(9) | 320,031 |
2023 | 238,292 | — | — | 73,921 | — | 3,540(7) | 315,753 |
______________________
(1)These amounts represent the grant date fair value of time-based stock option awards granted by the Company during the periods presented, determined in accordance with FASB ASC Topic 718. All awards are amortized over the vesting life of the award. For the assumptions used in our valuations, see “Note 5 – Stockholders’ Equity” of the Notes to Consolidated Financial Statements included elsewhere in this prospectus.
(2)Includes amounts paid under the Company’s Performance-Based Cash Incentive Award program based on the achievement of corporate and individual performance goals established and measured by the Compensation Committee.
(3)Mr. Murphy was appointed our Executive Chairman on September 15, 2020. The amounts reported under “Salary” are comprised of the amounts paid to MDBI for its consulting services. Mr. Murphy did not receive a base salary from the Company in Fiscal 2024 or Fiscal 2023. Mr. Murphy also received compensation for services as a member of the Board of Directors, which is included in the section entitled “Director Compensation Table” on page 60 of this prospectus.
(4)This amount includes $14,777 for expense reimbursements, $1,775 for administrative services and $6,600 for office rent in Fiscal 2024 and $1,996 for expense reimbursements, $1,200 for administrative services and $550 for office rent in Fiscal 2023.
(5)Mr. Hess was appointed our Chief Financial Officer on October 6, 2022. The amounts reported under “Salary” are comprised of the amounts paid to Danforth for its consulting services. Mr. Hess did not receive a base salary from the Company in Fiscal 2024. Mr. Hess’ compensation for Fiscal 2023 has been omitted from this table as Mr. Hess was not a named executive officer for Fiscal 2023.
(6)Pursuant to the Separation Agreement and General Release entered into on September 7, 2023 with Mr. Jurgensen, the Company agreed to pay Mr. Jurgensen an aggregate of $345,000. In addition, this amount includes $3,454 in matching contributions to the 401(k) Plan for Fiscal 2024. The formula for determining the matching contributions is the same for named executive officers as it is for all salaried employees (and are subject to the same statutory maximum). Excludes payments made for the reimbursement of medical insurance premiums and life insurance available for all salaried employees. For more information regarding these benefits, see above under “Other Benefits.”
(7)Consists of matching contributions to the 401(k) Plan. The formula for determining the matching contributions is the same for named executive officers as it is for all salaried employees (and are subject to the same statutory maximum). Excludes payments made for the reimbursement of medical insurance premiums and life insurance available for all salaried employees.
(8)Pursuant to the Separation Agreement entered into on September 10, 2023 with Dr. Miner, the Company granted Dr. Miner a stock option to purchase 40,000 shares of common stock, which will vest as follows: 13,000 shares will be vested immediately upon issuance, 13,500 shares will vest on the one year anniversary of the Separation Agreement and 13,500 shares will vest on the two year anniversary of the Separation Agreement.
(9)Pursuant to the Separation Agreement entered into on September 10, 2023 with Dr. Miner, the Company agreed to pay Dr. Miner an aggregate of $169,250 for his consulting services. In addition, this amount includes $3,540 in matching contributions to the 401(k) Plan for Fiscal 2024. The formula for determining the matching contributions is the same for named executive officers as it is for all salaried employees (and are subject to the same statutory maximum). Excludes payments made for the reimbursement of medical insurance premiums and life insurance available for all salaried employees. For more information regarding these benefits, see above under “Other Benefits.”
Outstanding Equity Awards at Fiscal Year End
The following table shows certain information regarding outstanding equity awards as of March 31, 2024 for our named executive officers:
| | | | | | | | | | | | | | | | | | | | |
|
| Option Awards |
|
| Stock Awards |
Name |
| No. of Securities Underlying Unexercised Options (#) Exercisable | |
| No. of Securities Underlying Unexercised Options (#) Unexercisable |
|
| Option Exercise Price ($) |
|
| Option Expiration Date |
|
| No. of Shares or Units of Stock That Have Not Vested (#) |
| Market Value of Shares or Units of Stock That Have Not Vested ($) |
|
Keith Murphy |
| 15,000 | (1) |
|
| 25,000 |
|
| $ | 2.36 |
|
| 8/30/2032 |
|
| 5,000 | (2) | $ | 5,150 |
|
Jeffrey Miner |
| 13,000 | (3) |
|
| 27,000 |
|
|
| 7.64 |
|
| 11/13/2033 |
|
|
|
|
|
|
|
______________________
(1)The option shares vest in 16 equal quarterly installments beginning August 31, 2022.
(2)25% of the shares subject to the restricted stock unit vested on March 8, 2022 and the remaining shares vest in 12 equal quarterly installments thereafter.
(3)Pursuant to the Separation Agreement entered into on September 10, 2023 with Dr. Miner, the Company granted Dr. Miner a stock option to purchase 40,000 shares of common stock, which will vest as follows: 13,000 shares will be vested immediately upon issuance beginning November 13, 2023, 13,500 shares will vest on the one year anniversary of the Separation Agreement and 13,500 shares will vest on the two year anniversary of the Separation Agreement.
As of March 31, 2024, neither Mr. Hess nor Mr. Jurgensen held any outstanding equity awards.
Compensation Committee Interlocks and Insider Participation
No member of our Compensation Committee has at any time been our employee. None of our executive officers serves, or has served during the last fiscal year, as a member of the board of directors or compensation committee of any other entity that has one or more executive officers serving as a member of our Board or our Compensation Committee.
Equity Compensation Plan Information
The following tables set forth certain information regarding the beneficial ownership of our common stock as of March 31, 2024 by (i) each of our directors and named executive officers (as disclosed in this prospectus); and (ii) all of our current executive officers and directors as a group. To our knowledge, based solely on our review of Schedules 13D and 13G filed with the SEC, no person beneficially owned more than 5% of our common stock as of March 31, 2024. Unless otherwise indicated in the table or the footnotes to the following table, each person named in the table has sole voting and investment power and such person’s address is c/o Organovo Holdings, Inc., 11555 Sorrento Valley Rd., Suite 100, San Diego, CA 92121.
We determined the number of shares of common stock beneficially owned by each person under rules promulgated by the SEC, based on information obtained from Company records and filings with the SEC on or before March 31, 2024. In cases of holders who are not directors or named executive officers, Schedules 13G or 13D filed with the SEC, as applicable (and, consequently, ownership reflected here), often reflect holdings as of a date prior to March 31, 2024. The information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power and also any shares which the individual or entity had the right to acquire within 60 days of March 31, 2024. These shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person or entity.
Applicable percentages are based on 10,077,726 shares of common stock outstanding as of March 31, 2024, as adjusted as required by the rules promulgated by the SEC. We have deemed shares of our common stock subject to stock options that are currently exercisable or exercisable within 60 days of March 31, 2024, or issuable pursuant to restricted stock units that are subject to vesting conditions expected to occur within 60 days of March 31, 2024, to be outstanding and to be beneficially owned by the person holding the stock option or restricted stock units for the purpose of computing the percentage ownership of that person. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person or entity.
| | | | | | | |
|
| Beneficial Ownership(1) |
|
Name of Beneficial Owner |
| Number of Common Shares |
|
|
| Percent of Common Shares |
|
Directors and Named Executive Officers |
|
|
|
|
|
|
|
Keith Murphy |
| 115,927 |
| (2) |
| 1.1% |
|
Douglas Jay Cohen |
| 84,107 |
| (3) |
| * |
|
Alison Tjosvold Milhous |
| 72,107 |
| (4) |
| * |
|
Adam Stern |
| 72,107 |
| (4) |
| * |
|
David Gobel |
| 52,500 |
| (5) |
| * |
|
Vaidehi Joshi |
| 42,941 |
| (6) |
| * |
|
Jeffrey Miner |
| 13,000 |
| (5) |
| * |
|
All current executive officers and directors as a group (7 persons) |
| 439,689 |
| (7) |
| 4.3% |
|
______________________
* Less than one percent.
(1)Beneficial ownership of shares and percentage ownership are determined in accordance with the rules of the SEC. Unless otherwise indicated and subject to community property laws where applicable, the individuals named in the table above have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
(2)Represents 100,927 shares of common stock held by Mr. Murphy and 15,000 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(3)Represents 29,607 shares of common stock held by Mr. Cohen, 2,000 shares held by Mr. Cohen’s children and 52,500 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(4)Represents 19,607 shares of common stock held and 52,500 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(5)Represents shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(6)Represents 19,607 shares of common stock held and 23,334 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(7)Comprised of shares included under “Directors and Named Executive Officers” other than Dr. Miner and Mr. Jurgensen, as neither is currently an executive officer.
Securities Authorized for Issuance Under Equity Compensation Plans.
The following table summarizes information about the Company’s equity compensation plans by type as of March 31, 2023:
| | | | | | | | | | |
| | (A) Number of securities to be issued upon exercise/vesting of outstanding options, warrants, units and rights | | | | | | (C) Number of securities available for future issuance under Equity Compensation Plans (excluding securities reflected in column (A)) | |
| | | | | (B) Weighted-average exercise price of outstanding options, warrants, units and rights | | | | |
| | | | |
| | | | |
| | | | |
| | | | |
Plan category | | | | |
Equity compensation plans approved by security holders (1) | | 1,528,934 | (2) | | $ | 6.62 | | | 1,129,897 | (3) |
Equity compensation plans not approved by security holders (4) | | 50,000 | (5) | | $ | 2.75 | | | 1,000 | (6) |
(1)Includes the 2008 Equity Incentive Plan, the Amended and Restated 2012 Equity Incentive Plan, the 2022 Equity Incentive Plan, and the Employee Stock Purchase Plan (the “ESPP”) of the Company.
(2)Includes stock options to purchase 1,401,217 shares of common stock with a per share weighted-average exercise price of $6.62. Also includes 127,717 restricted stock units with no exercise price.
(3)Includes 58,426 shares of common stock available for purchase under the ESPP as of March 31, 2023.
(4)Includes certain Inducement Award Stock Option Agreements and Inducement Award Performance-Based Restricted Stock Unit Agreements and the 2021 Inducement Equity Incentive Plan of the Company (the "Inducement Plan").
(5)Includes 50,000 stock options with a per share exercise price of $2.75 granted pursuant to the Inducement Plan.
(6)Includes 1,000 shares of common stock reserved for issuance pursuant to the Inducement Plan.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Since April 1, 2021, there have not been any transactions or series of similar transactions to which we were or are a party in which the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at fiscal year-end for the last two completed fiscal years, and in which any of our directors or executive officers, any holder of more than 5% of any class of our voting securities or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than (i) the transactions described below and (ii) the compensation arrangements with our executive officers and non-employee directors described in “Executive Compensation” and “Director Compensation,” respectively.
Intercompany Agreement with Viscient
Viscient is an entity for which Keith Murphy, our Executive Chairman and member of our Board, serves as the Chief Executive Officer and President. Dr. Jeffrey Miner, the Company’s former Chief Scientific Officer, is also the Chief Scientific Officer of Viscient, and Thomas Jurgensen, the Company’s former General Counsel, previously served as outside legal counsel to Viscient through his law firm, Optima Law Group, APC. In addition, Messrs. Stern, Cohen and Gobel (through the Methuselah Foundation and the Methuselah Fund) have invested funds through a convertible promissory note in Viscient, but do not serve as an employee, officer or director of Viscient.
On December 28, 2020, the Company entered into an intercompany agreement (the “Intercompany Agreement”) with Viscient and Organovo, Inc., the Company’s wholly-owned subsidiary, which included an asset purchase agreement for certain lab equipment. Pursuant to the Intercompany Agreement, the Company agreed to provide Viscient certain services related to 3D bioprinting technology which includes, but is not limited to, histology services, cell isolation, and proliferation of cells and Viscient agreed to provide the Company certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, the Company and Viscient each agreed to share certain facilities and equipment, and, subject to further agreement, to each make certain employees available for specified projects for the other party at prices to be determined in good faith by the parties. The Company evaluated the accounting for the Intercompany Agreement and concluded that any services provided by Viscient to the Company will be expensed as incurred, and any compensation for services provided by the Company to Viscient will be considered a reduction of personnel related expenses. Any services provided to Viscient do not fall under Financial Accounting Standards Board Topic 606 (“Revenue from Contracts with Customers”) as the Intercompany Agreement is not a contract with a customer. For the years ended March 31, 2024, 2023 and 2022, the Company provided approximately $14,000, $59,000 and $48,000 of histology services to Viscient, respectively. Additionally, for the year ended March 31, 2022, the Company incurred approximately $47,000 in consulting expenses from Viscient. No consulting services from Viscient were incurred for the years ended March 31, 2024 or 2023.
Related Party Transaction Policy and Procedures
Pursuant to our written Related Party Transaction Policy and Procedures, our executive officers, directors, and principal stockholders, including their immediate family members and affiliates, are prohibited from entering into a related party transaction with us without the prior consent of our Audit Committee or a committee of our independent directors. Any request for us to enter into a transaction with an executive officer, director, principal stockholder, or any of such persons’ immediate family members or affiliates, in which the amount involved exceeds $120,000, must first be presented to our Audit Committee for review, consideration and approval. In approving or rejecting the proposed agreement, our Audit Committee will consider the relevant facts and circumstances available and deemed relevant, including, but not limited to, the terms of the transaction, the nature of the related party’s interest in the transaction, the significance of the transaction to us and the related party, the nature of the related party’s relationship with us and whether the transaction would be likely to impair (or create an appearance of impairing) the judgement of a director or executive officer to act in our best interest. Our Audit Committee shall approve only those agreements that, in light of known circumstances, are in, or are not inconsistent with, our best interests, as our Audit Committee determines in the good faith exercise of its discretion.
Principal Stockholders
The following tables set forth certain information regarding the beneficial ownership of our common stock as of March 31, 2024 by (i) each of our directors and named executive officers (as disclosed in this prospectus); and (ii) all of our current executive officers and directors as a group. To our knowledge, based solely on our review of Schedules 13D and 13G filed with the SEC, no person beneficially owned more than 5% of our common stock as of March 31, 2024. Unless otherwise indicated in the table or the footnotes
to the following table, each person named in the table has sole voting and investment power and such person’s address is c/o Organovo Holdings, Inc., 11555 Sorrento Valley Rd., Suite 100, San Diego, CA 92121.
We determined the number of shares of common stock beneficially owned by each person under rules promulgated by the SEC, based on information obtained from Company records and filings with the SEC on or before March 31, 2024. In cases of holders who are not directors or named executive officers, Schedules 13G or 13D filed with the SEC, as applicable (and, consequently, ownership reflected here), often reflect holdings as of a date prior to March 31, 2024. The information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power and also any shares which the individual or entity had the right to acquire within 60 days of March 31, 2024. These shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person or entity.
Applicable percentages are based on 10,077,726 shares of common stock outstanding as of March 31, 2024, as adjusted as required by the rules promulgated by the SEC. We have deemed shares of our common stock subject to stock options that are currently exercisable or exercisable within 60 days of March 31, 2024, or issuable pursuant to restricted stock units that are subject to vesting conditions expected to occur within 60 days of March 31, 2024, to be outstanding and to be beneficially owned by the person holding the stock option or restricted stock units for the purpose of computing the percentage ownership of that person. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person or entity.
| | | | | | | |
|
| Beneficial Ownership(1) |
|
Name of Beneficial Owner |
| Number of Common Shares |
|
|
| Percent of Common Shares |
|
Directors and Named Executive Officers |
|
|
|
|
|
|
|
Keith Murphy |
| 115,927 |
| (2) |
| 1.1% |
|
Douglas Jay Cohen |
| 84,107 |
| (3) |
| * |
|
Alison Tjosvold Milhous |
| 72,107 |
| (4) |
| * |
|
Adam Stern |
| 72,107 |
| (4) |
| * |
|
David Gobel |
| 52,500 |
| (5) |
| * |
|
Vaidehi Joshi |
| 42,941 |
| (6) |
| * |
|
Jeffrey Miner (7) |
| 13,000 |
| (5) |
| * |
|
Thomas Jurgensen (8) | | - | | | | - | |
Thomas Hess | | - | | | | - | |
All current executive officers and directors as a group (7 persons) |
| 439,689 |
| (9) |
| 4.3% |
|
______________________
* Less than one percent.
(1)Beneficial ownership of shares and percentage ownership are determined in accordance with the rules of the SEC. Unless otherwise indicated and subject to community property laws where applicable, the individuals named in the table above have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
(2)Represents 100,927 shares of common stock held by Mr. Murphy and 15,000 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(3)Represents 29,607 shares of common stock held by Mr. Cohen, 2,000 shares held by Mr. Cohen’s children and 52,500 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(4)Represents 19,607 shares of common stock held and 52,500 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(5)Represents shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(6)Represents 19,607 shares of common stock held and 23,334 shares subject to options that are immediately exercisable or exercisable within 60 days of March 31, 2024.
(7)Dr. Miner’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023.
(8)Mr. Jurgensen’s employment with the Company was terminated as of August 25, 2023 in connection with Company’s reduction in force announced on August 18, 2023.
(9)Comprised of shares included under “Directors and Named Executive Officers” other than Dr. Miner and Mr. Jurgensen, as neither was an executive officer as of March 31, 2024.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our officers and directors, and persons who own more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. Officers, directors, and greater than 10% stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. Based solely upon a review of the copies of such forms and amendments thereto, we believe that, during Fiscal 2023, none of our officers, directors, and greater than 10% beneficial owners failed to file on a timely basis the reports required by Section 16(a).
Material U.S. Federal Income Tax Considerations for Non-U.S. Holders
The following is a summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our common stock and pre-funded warrants, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. Throughout this summary, all references to our common stock are meant to include our pre-funded warrants. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Code, Treasury Regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed or subject to differing interpretations, possibly with retroactive effect, with the resulting U.S. federal income tax consequences being different from those set forth below. We have not sought and will not seek any ruling from the Internal Revenue Service, or the IRS, with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS or a court will agree with such statements and conclusions.
This summary also does not address the tax considerations arising under the laws of any U.S. state or local or any non-U.S. jurisdiction, estate or gift tax, the 3.8% Medicare tax on net investment income or any alternative minimum tax consequences. In addition, this discussion does not address tax considerations applicable to a holder’s particular circumstances or to a holder that may be subject to special tax rules, including, without limitation:
•banks, insurance companies or other financial institutions;
•tax-exempt or government organizations;
•brokers or dealers in securities or currencies;
•traders in securities that elect to use a mark-to-market method of accounting for their securities holdings;
•persons that own, or are deemed to own, more than 5.0% of our capital stock;
•certain U.S. expatriates, citizens or former long-term residents of the United States;
•persons who hold our common stock as a position in a hedging transaction, “straddle,” “conversion transaction,” synthetic security, other integrated investment, or other risk reduction transaction;
•persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes);
•persons deemed to sell our common stock under the constructive sale provisions of the Code;
•partnerships, or other entities or arrangements treated as partnerships for U.S. federal income tax purposes, or investors in any such entities;
•persons for whom our stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code;
•integral parts or controlled entities of foreign sovereigns;
•passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; or
•persons that acquire our common stock as compensation for services.
In addition, if a partnership, including any entity or arrangement classified as a partnership for U.S. federal income tax purposes, holds our common stock, the tax treatment of a partner generally will depend on the status of the partner, the activities of the partnership, and certain determinations made at the partner level. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors regarding the U.S. federal income tax consequences to them of the purchase, ownership, and disposition of our common stock.
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock arising under the U.S. federal estate or gift tax rules or under the laws of any U.S. state or local or any non-U.S. or other taxing jurisdiction or under any applicable tax treaty.
Definition of a U.S. Holder
For purposes of this summary, a “U.S. Holder” is any beneficial owner of our common stock that is a “U.S. person,” and is not a partnership, or an entity treated as a partnership or disregarded from its owner, each for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
•an individual who is a citizen or resident of the United States;
•a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia;
•an estate, the income of which is subject to U.S. federal income tax regardless of its source; or
•a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a U.S. person for U.S. federal income tax purposes.
For purposes of this summary, a “Non-U.S. Holder” is any beneficial owner of our common stock that is not a U.S. Holder or a partnership, or other entity treated as a partnership or disregarded from its owner, each for U.S. federal income tax purposes.
Tax Consequences to U.S. Holders
Distributions on Common Stock
As discussed above under “Dividend Policy,” we do not currently expect to make distributions on our common stock. In the event that we do make distributions of cash or other property, distributions paid on common stock, other than certain pro rata distributions of common stock, will be treated as a dividend to the extent paid out of our current or accumulated earnings and profits, if any, and will be includible in income by the U.S. Holder and taxable as ordinary income when received. If a distribution exceeds our current and accumulated earnings and profits, the excess will be first treated as a tax-free return of the U.S. Holder’s investment, up to the U.S. Holder’s tax basis in the common stock. Any remaining excess will be treated as a capital gain. Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be eligible for taxation as “qualified dividend income” and therefore may be taxable at rates applicable to long-term capital gains. U.S. Holders should consult their tax advisers regarding the availability of the reduced tax rate on dividends in their particular circumstances. Dividends received by a corporate U.S. Holder will be eligible for the dividends-received deduction if the U.S. Holder meets certain holding period and other applicable requirements.
Sale or Other Disposition of Common Stock
For U.S. federal income tax purposes, gain or loss realized on the sale or other disposition of common stock will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the common stock for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the common stock disposed of and the amount realized on the disposition. Long-term capital gains recognized by non-corporate U.S. Holders will be subject to reduced tax rates. The deductibility of capital losses is subject to limitations.
Treatment of Pre-Funded Warrants
Although it is not entirely free from doubt, we believe a pre-funded warrant should be treated as common stock for U.S. federal income tax purposes and a holder of pre-funded warrants should generally be taxed in the same manner as a holder of our common stock, as described below. Accordingly, no gain or loss should be recognized upon the exercise of a pre-funded warrant and, upon exercise, the holding period of a pre-funded warrant should carry over to the common stock received. Similarly, the tax basis of the pre-funded warrant should carry over to the common stock received upon exercise, increased by the exercise price of $0.01 per share. However, our characterization of a pre-funded warrant is not binding on the IRS, and the IRS may treat our pre-funded warrants as warrants to acquire our common stock. If so, the amount and character of your gain with respect to an investment in our pre-funded warrants could change. Accordingly, each holder should consult his, her or its own tax advisor regarding the risks associated with the acquisition of pre-funded warrants pursuant to this offering (including potential alternative characterizations). The balance of this discussion generally assumes that our characterization described above is respected for U.S. federal income tax purposes.
Tax Consequences to Non-U.S. Holders
Distributions
As discussed in the section entitled “Dividend Policy,” we do not anticipate paying any dividends on our common stock in the foreseeable future. If we make distributions on our common, those payments will constitute dividends for U.S. federal income tax
purposes to the extent we have current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce a Non-U.S. Holder’s basis in our common stock, as applicable, but not below zero. Any excess will be treated as capital gain and will be treated as described below under the “—Gain on Sale or Other Disposition of Common Stock” section. Any such distributions would be subject to the discussions below regarding back-up withholding and the Foreign Account Tax Compliance Act, or FATCA.
Subject to the discussion below on effectively connected income, any dividend paid to a Non-U.S. Holder generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a Non-U.S. Holder must provide us or our agent with an IRS Form W-8BEN (generally including a U.S. taxpayer identification number), IRS Form W-8 BEN-E or another appropriate version of IRS Form W-8 (or a successor form), which must be updated periodically, and which, in each case, must certify qualification for the reduced treaty rate. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Dividends paid to a Non-U.S. Holder that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and that are not eligible for relief from U.S. (net basis) income tax under an applicable income tax treaty, generally are exempt from the (gross basis) withholding tax described above. To obtain this exemption from withholding tax, the Non-U.S. Holder must provide the applicable withholding agent with an IRS Form W-8ECI or successor form or other applicable IRS Form W-8 certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States. Such effectively connected dividends, if not eligible for relief under a tax treaty, would not be subject to a withholding tax, but would be taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits and if, in addition, the Non-U.S. Holder is a corporation, may also be subject to a branch profits tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty).
If you are eligible for a reduced rate of withholding tax pursuant to a tax treaty, you may be able to obtain a refund of any excess amounts withheld if you timely file an appropriate claim for refund with the IRS.
Gain on Sale or Other Disposition of Common Stock
Subject to the discussion below regarding backup withholding and FATCA, a Non-U.S. Holder generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
•the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States and not eligible for relief under an applicable income tax treaty, in which case the Non-U.S. Holder will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and for a Non-U.S. Holder that is a corporation, such Non-U.S. Holder may be subject to the branch profits tax at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items;
•the Non-U.S. Holder is an individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case the Non-U.S. Holder will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses (even though the Non-U.S. Holder is not considered a resident of the United States) (subject to applicable income tax or other treaties); or
•we are a “U.S. real property holding corporation” for U.S. federal income tax purposes, or a USRPHC, at any time within the shorter of the five-year period preceding the disposition or the Non-U.S. Holder’s holding period for our common stock. We believe we are not currently and do not anticipate becoming a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our United States real property interests relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to United States federal income tax if (a) shares of our common stock are “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, such as Nasdaq, and (b) the Non-U.S. Holder owns or owned, actually and constructively, 5% or less of the shares of our common stock throughout the five-year period ending on the date of the sale or exchange. If the foregoing exception does not apply, such Non-U.S. Holder’s proceeds received on the disposition of shares will generally be subject to withholding at a rate of 15% and such Non-U.S. Holder will generally be taxed on any gain in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax generally will not apply.
Information Reporting and Backup Withholding
Information returns may be filed with the IRS in connection with distributions on common, and the proceeds of a sale or other disposition of common stock. A non-exempt U.S. Holder may be subject to U.S. backup withholding on these payments if it fails to provide its taxpayer identification number to the withholding agent and comply with certification procedures or otherwise establish an exemption from backup withholding.
A Non-U.S. Holder may be subject to U.S. information reporting and backup withholding on these payments unless the Non-U.S. Holder complies with certification procedures to establish that it is not a U.S. person (within the meaning of the Code). The certification requirements generally will be satisfied if the Non-U.S. Holder provides the applicable withholding agent with a statement on the applicable IRS Form (or a suitable substitute or successor form), together with all appropriate attachments, signed under penalties of perjury, stating, among other things, that such Non-U.S. Holder is not a U.S. Person. Applicable Treasury Regulations provide alternative methods for satisfying this requirement. In addition, the amount of distributions on common stock paid to a Non-U.S. Holder, and the amount of any U.S. federal tax withheld therefrom, must be reported annually to the IRS and the holder. This information may be made available by the IRS under the provisions of an applicable tax treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides.
Payment of the proceeds of the sale or other disposition of common stock to or through a non-U.S. office of a U.S. broker or of a non-U.S. broker with certain specified U.S. connections generally will be subject to information reporting requirements, but not backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or an exemption otherwise applies. Payments of the proceeds of a sale or other disposition of common stock to or through a U.S. office of a broker generally will be subject to information reporting and backup withholding, unless the Non-U.S. Holder certifies under penalties of perjury that it is not a U.S. person or otherwise establishes an exemption.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment generally will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Accounts
The Code generally imposes a U.S. federal withholding tax of 30% on dividends and, subject to the discussion below regarding proposed regulations recently issued by the U.S. Treasury Department, the gross proceeds of a disposition of our securities paid to a “foreign financial institution” (as specifically defined for this purpose), unless such institution enters into an agreement with the U.S. government to, among other things, withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise qualifies for an exemption from these rules. A U.S. federal withholding tax of 30% also applies to dividends and, subject to the discussion below regarding proposed regulations recently issued by the U.S. Treasury Department, will apply to the gross proceeds of a disposition of our securities paid to a non-financial foreign entity (as defined in the Code), unless such entity provides the withholding agent with either a certification that it does not have any substantial direct or indirect. “United States owners” (as defined in the Code), provides information regarding each substantial United States owners of the entity, or otherwise qualifies for an exemption from these rules.
Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph.
The U.S. Treasury Department released proposed regulations which, if finalized in their present form, would eliminate the federal withholding tax of 30% applicable to the gross proceeds of a sale or other disposition of our common stock. In its preamble to such proposed regulations, the U.S. Treasury Department stated that taxpayers may generally rely on the proposed regulations until final regulations are issued. Prospective investors should consult their own tax advisors regarding the possible impact of these rules on their investment in our common stock, and the possible impact of these rules and the proposed regulations on the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of this 30% withholding tax.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR SECURITIES, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS. IN ADDITION, SIGNIFICANT CHANGES IN U.S. FEDERAL TAX LAWS WERE RECENTLY ENACTED. PROSPECTIVE INVESTORS SHOULD ALSO CONSULT WITH THEIR TAX ADVISORS WITH RESPECT TO SUCH CHANGES IN U.S. TAX LAW AS WELL AS POTENTIAL CONFORMING CHANGES IN STATE TAX LAWS.
Plan of Distribution
We have engaged JonesTrading Institutional Services LLC, or the placement agent, to act as our exclusive placement agent to solicit offers to purchase the shares of our common stock and pre-funded warrants offered by this prospectus. The placement agent is not purchasing or selling any such securities, nor is it required to arrange for the purchase and sale of any specific number or dollar amount of such securities, other than to use its “reasonable best efforts” to arrange for the sale of such securities by us. Therefore, we may not sell all of the shares of common stock and pre-funded warrants being offered. The terms of this offering were subject to market conditions and negotiations between us, the placement agent and prospective investors. The placement agent will have no authority to bind us by virtue of the engagement letter. This is a best efforts offering and there is no minimum offering amount required as a condition to the closing of this offering. The placement agent may retain sub-agents and selected dealers in connection with this offering. Investors purchasing securities offered hereby will have the option to execute a securities purchase agreement with us. In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers which enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract is material to larger purchasers in this offering as a means to enforce the following covenants uniquely available to them under the securities purchase agreement: (i) a covenant to not enter into variable rate financings for a period of [____] following the closing of the offering, subject to an exception; and (ii) a covenant to not enter into any equity financings for [_] days from closing of the offering, subject to certain exceptions.
The nature of the representations, warranties and covenants in the securities purchase agreements shall include:
•standard issuer representations and warranties on matters such as organization, qualification, authorization, no conflict, no governmental filings required, current in SEC filings, no litigation, labor or other compliance issues, environmental, intellectual property and title matters and compliance with various laws such as the Foreign Corrupt Practices Act;[ and
•covenants regarding matters such as registration of warrant shares, no integration with other offerings, filing of an 8-K to disclose entering into these securities purchase agreements, no shareholder rights plans, no material nonpublic information, use of proceeds, indemnification of purchasers, reservation and listing of common stock, and no subsequent equity sales for [_] days.
Delivery of the shares of common shares and pre-funded warrants offered hereby is expected to occur on or about [__________], 2024, subject to satisfaction of certain customary closing conditions.
We have agreed to pay the placement agent an aggregate fee equal to 6% of the gross proceeds received in the offering. In addition, we have agreed to reimburse the placement agent for its legal fees and expenses and other out-of-pocket expenses in an amount up to $75,000 and clearing expenses of $[______].
We estimate the total expenses of this offering paid or payable by us, exclusive of the placement agent's cash fee of 6% of the gross proceeds and expenses, will be approximately $[__] million. After deducting the fees due to the placement agent and our estimated expenses in connection with this offering, we expect the net proceeds from this offering will be approximately $[__] million, based on an assumed public offering price per share of $[___], which was the last reported sales price of our common stock on the Nasdaq Capital Market on [_________], 2024.
The following table shows the per share and total cash fees we will pay to the placement agent in connection with the sale of the common stock and shares of common stock underlying the pre-funded warrants pursuant to this prospectus.
| | | | | | | | | | | | |
|
| Per Share |
|
| Per Pre-Funded Warrant |
|
| Total |
|
Public offering price |
| $ |
|
|
| $ |
|
|
| $ |
|
|
Placement agent fees |
| $ |
|
|
| $ |
|
|
| $ |
|
|
Proceeds, before expenses, to us |
| $ |
|
|
| $ |
|
|
| $ |
|
|
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in our engagement letter with the placement agent. We have also agreed to contribute to payments the placement agent may be required to make in respect of such liabilities.
Lock-up Agreements
We and each of our executive officers and directors have agreed with the placement agent to be subject to a lock-up period of [___] days following the date of closing of the offering pursuant to this prospectus. This means that, during the applicable lock-up period, we and such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any of our shares of common stock or any securities convertible into, or exercisable or exchangeable for, shares of common stock, subject to customary exceptions. The placement agent may waive the terms of these lock-up agreements in its sole discretion and without notice. [In addition, we have agreed to not issue any securities that are subject to a price reset based on the trading prices of our common stock or upon a specified or contingent event in the future or enter into any agreement to issue securities at a future determined price for a period of [___] following the closing date of this offering, subject to an exception. The placement agent may waive this prohibition in its sole discretion and without notice.]
Other Relationships
From time to time, the placement agent may provide in the future various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. However, except with respect to a sales agreement that we entered into with H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC, the agents, on March 16, 2018, pursuant to which we may offer and sell through the agents shares of our common stock in ATM sales transactions, and as otherwise disclosed in this prospectus, we have no present arrangements with the placement agent for any further services.
Regulation M Compliance
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the sale of our securities offered hereby by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed their participation in the distribution.
Trading Market
Our common stock is listed on the Nasdaq Capital Market under the symbol “ONVO.” There is no established public trading market for the pre-funded warrants to be sold in this offering, and we do not expect a market to develop. In addition, we do not intend to apply for listing of the pre-funded warrants on any national securities exchange.
Legal Matters
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Paul Hastings LLP, Palo Alto, California. Certain legal matters will be passed upon for the placement agent by Duane Morris LLP, New York, New York.
Experts
The consolidated financial statements of Organovo Holdings, Inc. (“Company”) as of and for the years ended March 31, 2023 and 2022, appearing in this prospectus, have been audited by Mayer Hoffman McCann P.C., independent registered public accounting firm, as set forth in their report (which report includes an explanatory paragraph regarding the existence of substantial doubt about the Company’s ability to continue as a going concern), appearing elsewhere herein, and have been included in reliance upon such report given on the authority of such firm as experts in accounting and auditing, in giving said reports.
Where You Can Find More Information
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and persons controlling us pursuant to the provisions described in Item 14 of the registration statement of which this prospectus is a part or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our directors, officers, or controlling persons in the successful defense of any action, suit, or proceeding) is asserted by our directors, officers, or controlling persons in connection with the Common Stock being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of the issue.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and persons controlling us pursuant to the provisions described in Item 14 of the registration statement of which this prospectus is a part or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our directors, officers, or controlling persons in the successful defense of any action, suit, or proceeding) is asserted by our directors, officers, or controlling persons in connection with the Common Stock being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of the issue.
Organovo Holdings, Inc.
Index to Consolidated Financial Statements
Index to Unaudited Condensed Consolidated Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of:
Organovo Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Organovo Holdings, Inc. (“Company”) as of March 31, 2023 and 2022, and the related consolidated statements of operations and other comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended March 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended March 31, 2023, in conformity with accounting principles generally accepted in the United States which requireof America.
Going Concern Uncertainty
The accompanying financial statements have been prepared assuming that we make certain assumptions and estimates and, in connection therewith, adopt certain accounting policies. Our significant accounting policies are set forththe Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred recurring losses and negative cash flows from operations and is dependent on additional financing to fund operations. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Of those policies, weOur audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the policies discussed below may involvecurrent period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there were no critical audit matters.
/s/ Mayer Hoffman McCann P.C.
We served as the Company's auditor from 2011 to 2023.
San Diego, California
July 13, 2023
ORGANOVO HOLDINGS, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands except for share and per share data)
| | | | | | | | |
| | March 31, 2023 | | | March 31, 2022 | |
Assets | | | | | | |
Current Assets | | | | | | |
Cash and cash equivalents | | $ | 15,301 | | | $ | 28,675 | |
Accounts receivable | | | 152 | | | | — | |
Investment in equity securities | | | 706 | | | | — | |
Prepaid expenses and other current assets | | | 889 | | | | 858 | |
Total current assets | | | 17,048 | | | | 29,533 | |
Fixed assets, net | | | 902 | | | | 662 | |
Restricted cash | | | 143 | | | | 143 | |
Operating lease right-of-use assets | | | 1,705 | | | | 2,153 | |
Prepaid expenses and other assets, net | | | 515 | | | | 805 | |
Total assets | | $ | 20,313 | | | $ | 33,296 | |
Liabilities and Stockholders' Equity | | | | | | |
Current Liabilities | | | | | | |
Accounts payable | | $ | 331 | | | $ | 415 | |
Accrued expenses | | | 2,848 | | | | 489 | |
Operating lease liability, current portion | | | 492 | | | | 479 | |
Total current liabilities | | | 3,671 | | | | 1,383 | |
Operating lease liability, net of current portion | | | 1,313 | | | | 1,704 | |
Total liabilities | | | 4,984 | | | | 3,087 | |
Commitments and Contingencies | | | | | | |
Stockholders' Equity | | | | | | |
Common stock, $0.001 par value; 200,000,000 shares authorized,
8,716,906 and 8,710,627 shares issued and outstanding at
March 31, 2023 and 2022, respectively | | | 9 | | | | 9 | |
Additional paid-in capital | | | 340,317 | | | | 337,940 | |
Accumulated deficit | | | (324,998 | ) | | | (307,739 | ) |
Accumulated other comprehensive income | | | 2 | | | | — | |
Treasury stock, 46 shares at cost | | | (1 | ) | | | (1 | ) |
Total stockholders' equity | | | 15,329 | | | | 30,209 | |
Total Liabilities and Stockholders' Equity | | $ | 20,313 | | | $ | 33,296 | |
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE LOSS
(in thousands except for share and per share data)
| | | | | | | | |
| | Year Ended | | | Year Ended | |
| | March 31, 2023 | | | March 31, 2022 | |
Revenues | | | | | | |
Royalty revenue | | $ | 370 | | | $ | 1,500 | |
Total Revenues | | | 370 | | | | 1,500 | |
Research and development expenses | | | 8,885 | | | | 3,320 | |
Selling, general, and administrative expenses | | | 9,216 | | | | 9,659 | |
Total costs and expenses | | | 18,101 | |
| | 12,979 | |
Loss from Operations | | | (17,731 | ) | | | (11,479 | ) |
Other Income (Expense) | | | | | | |
Loss on fixed asset disposals | | | (9 | ) | | | — | |
Gain on investment in equity securities | | | 29 | | | | — | |
Interest income | | | 454 | | | | 8 | |
Other income | | | — | | | | 25 | |
Total Other Income | | | 474 | | | | 33 | |
Income Tax Expense | | | (2 | ) | | | (2 | ) |
Net Loss | | $ | (17,259 | ) | | $ | (11,448 | ) |
Other comprehensive income: | | | | | | |
Unrealized gain on available-for-sale debt securities | | | 2 | | | | — | |
Comprehensive loss | | $ | (17,257 | ) | | $ | (11,448 | ) |
Net loss per common share—basic and diluted | | $ | (1.98 | ) | | $ | (1.32 | ) |
Weighted average shares used in computing net loss per common share—basic
and diluted | | | 8,713,032 | | | | 8,703,596 | |
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | | | | | Treasury Stock | | | | | | | | | |
| | Shares | | | Amount | | | Additional Paid-in Capital | | | Shares | | Amount | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income | | Total | |
Balance at March 31, 2021 | | | 8,671 | | | $ | 9 | | | $ | 335,479 | | | | — | | $ | (1 | ) | | $ | (296,291 | ) | | | — | | $ | 39,196 | |
Issuance of common stock under employee and
director stock option, RSU and purchase plans | | | 13 | | | | — | | | | (46 | ) | | | — | | | — | | | | — | | | | — | | | (46 | ) |
Stock-based compensation expense | | | — | | | | — | | | | 2,256 | | | | — | | | — | | | | — | | | | — | | | 2,256 | |
Issuance of common stock from public offering,
net | | | 27 | | | | — | | | | 251 | | | | — | | | — | | | | — | | | | — | | | 251 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | — | | | | (11,448 | ) | | | — | | | (11,448 | ) |
Balance at March 31, 2022 | | | 8,711 | | | $ | 9 | | | $ | 337,940 | | | | — | | $ | (1 | ) | | $ | (307,739 | ) | | | — | | $ | 30,209 | |
Issuance of common stock under employee and
director stock option, RSU and purchase plans | | | 6 | | | | — | | | | — | | | | — | | | — | | | | — | | | | — | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 2,377 | | | | — | | | — | | | | — | | | | — | | | 2,377 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | — | | | | (17,259 | ) | | | — | | | (17,259 | ) |
Unrealized gain on available-for-sale debt securities | | | — | | | | — | | | | — | | | | — | | | — | | | | — | | | | 2 | | | 2 | |
Balance at March 31, 2023 | | | 8,717 | | | $ | 9 | | | $ | 340,317 | | | | — | | $ | (1 | ) | | $ | (324,998 | ) | | $ | 2 | | $ | 15,329 | |
The accompanying notes are an integral part of these consolidated financial statements.
ORGANOVO HOLDINGS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | |
| | Year Ended | | | Year Ended | |
| | March 31, 2023 | | | March 31, 2022 | |
Cash Flows From Operating Activities | | | | | | |
Net loss | | $ | (17,259 | ) | | $ | (11,448 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
Gain on investment in equity securities | | | (29 | ) | | | — | |
Loss on disposal of fixed assets | | | 9 | | | | — | |
Accretion on investments | | | (105 | ) | | | — | |
Depreciation and amortization | | | 293 | | | | 142 | |
Stock-based compensation | | | 2,377 | | | | 2,256 | |
Increase (decrease) in cash resulting from changes in: | |
| | |
| |
Accounts receivable | | | (152 | ) | | | — | |
Prepaid expenses and other assets | | | 177 | | | | 384 | |
Accounts payable | | | (148 | ) | | | 134 | |
Accrued expenses | | | 2,359 | | | | 49 | |
Operating right-of-use asset and lease liability, net | | | 70 | | | | 30 | |
Net cash used in operating activities | | | (12,408 | ) | | | (8,453 | ) |
Cash Flows From Investing Activities | | | | | | |
Purchases of fixed assets | | | (396 | ) | | | (409 | ) |
Purchases of investments | | | (9,893 | ) | | | — | |
Maturities of investments | | | 10,000 | | | | — | |
Purchases of equity securities | | | (1,061 | ) | | | — | |
Proceeds from sales of equity securities | | | 384 | | | | — | |
Net cash used in investing activities | | | (966 | ) | | | (409 | ) |
Cash Flows From Financing Activities | | | | | | |
Proceeds from issuance of common stock, net | | | — | | | | 251 | |
Employee taxes paid related to net share settlement of equity awards | | | — | | | | (46 | ) |
Net cash provided by financing activities | | | — | | | | 205 | |
Net Decrease in Cash, Cash Equivalents, and Restricted Cash | | | (13,374 | ) | | | (8,657 | ) |
Cash, cash equivalents, and restricted cash at beginning of period | | | 28,818 | | | | 37,475 | |
Cash, cash equivalents, and restricted cash at end of period | | $ | 15,444 | | | $ | 28,818 | |
Reconciliation of cash, cash equivalents, and restricted cash to the
consolidated balance sheets | | | | | | |
Cash and cash equivalents | | $ | 15,301 | | | $ | 28,675 | |
Restricted cash | | | 143 | | | | 143 | |
Total cash, cash equivalents and restricted cash | | $ | 15,444 | | | $ | 28,818 | |
Supplemental Disclosure of Cash Flow Information: | | | | | | |
Income taxes paid | | $ | 2 | | | $ | 2 | |
Operating lease liabilities arising from obtaining right-of-use assets | | $ | — | | | $ | 2,301 | |
Purchases of fixed assets in accounts payable | | $ | 64 | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements.
Organovo Holdings, Inc.
Notes to Consolidated Financial Statements
Note 1. Description of Business and Summary of Significant Accounting Policies
Nature of operations and basis of presentation
Organovo Holdings, Inc. (“Organovo Holdings,” “Organovo,” and the “Company”) is a higher degreebiotechnology company that focuses on building high fidelity, 3D tissues that recapitulate key aspects of judgmenthuman disease. The Company uses these models to identify gene targets responsible for driving the disease and mayintends to initiate drug discovery programs around these validated targets. The Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in ulcerative colitis (“UC”) and Crohn’s disease (“CD”). The Company intends to add additional tissues/diseases/targets to its portfolio over time. In line with these plans, the Company is building upon both its external and in house scientific expertise, which will be moreessential to its drug development effort.
The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. Organovo’s advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures. Management believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
The Company’s NovoGen Bioprinters® are automated devices that enable the fabrication of 3D living tissues comprised of mammalian cells. The Company believes that the use of its bioprinting platform as well as complementary 3D technologies will allow it to develop an accurate reflectionunderstanding of ourdisease biology that leads to validated novel drug targets and therapeutics to those targets to treat disease.
The majority of the Company’s current focus is in inflammatory bowel disease (“IBD”), including CD and UC. The Company is creating high fidelity disease models, leveraging its prior work including the work found in its peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) The Company’s current understanding of intestinal tissue models and IBD disease models leads it to believe that it can create models that provide greater insight into the biology of these diseases than are generally currently available. Using these disease models, the Company intends to identify and validate novel therapeutic targets. After finding therapeutic drug targets, the Company intends to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an Investigational New Drug (“IND”) filing and potential future clinical trials.
In March of 2023, the Company entered into and closed an asset purchase agreement with Metacrine, Inc to acquire their farnesoid X receptor ("FXR") program. FXR is a mediator of gastrointestinal ("GI") and liver diseases. FXR agonism has been tested in a variety of preclinical models of IBD. The acquired program contains two clinically tested compounds and over 2,000 discovery or preclinical compounds.
The Company expects to broaden its work into additional therapeutic areas over time and is currently exploring specific tissues for development. In the Company’s work to identify the areas of interest, it evaluates areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity.
Except where specifically noted or the context otherwise requires, references to “Organovo Holdings”, “the Company”, and “Organovo” in these notes to the consolidated financial conditionstatements refers to Organovo Holdings, Inc. and resultsits wholly owned subsidiaries, Organovo, Inc., and Opal Merger Sub, Inc.
Liquidity and Going Concern
The accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of operations.assets and satisfaction of liabilities in the normal course of business. As of March 31, 2023, the Company had cash and cash equivalents of approximately $15.3 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $325.0 million. The restricted cash was pledged as collateral for a letter of credit that the Company is required to maintain as a security deposit under the terms of the lease agreements for its facilities. The Company also had negative cash flows from operations of approximately $12.4 million during the year ended March 31, 2023.
Revenue RecognitionThrough March 31, 2023, the Company has financed its operations primarily through the sale of common stock through public and at-the-market (“ATM”) offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual
Our revenues are derivedF-7
property, products and research-based services, grants, and collaborative research agreements, and from the sale of bioprinter related productsconvertible notes. During the year ended March 31, 2023, the Company issued zero shares of its common stock through its ATM facility.
Based on our current operating plan and services, NIHavailable cash resources, we will need substantial additional funding to support future operating activities. We have concluded that the prevailing conditions and ongoing liquidity risks faced by us raise substantial doubt about our ability to continue as a going concern for at least one year following the date these financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern. As the Company continues its operations and is focusing its efforts on drug discovery and development, the Company will need to raise additional capital to implement this business plan. The Company cannot predict with certainty the exact amount or timing for any future capital raises. The Company will seek to raise additional capital through debt or equity financings, or through some other financing arrangement. However, the Company cannot be sure that additional financing will be available if and when needed, or that, if available, it can obtain financing on terms favorable to its stockholders. Any failure to obtain financing when required will have a material adverse effect on the Company’s business, operating results, and financial condition.
The preparation of the financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions.
Investments
Investments consist of investments in debt securities and investments in equity securities.
Investments in debt securities consist of investments in U.S. Treasury Department Grants, collaboration agreements, and license agreements.
We recognize revenue when the following criteria have been met: (i) persuasive evidence of an arrangement exists; (ii) services have been rendered or product has been delivered; (iii) the price to the customer is fixed and determinable; and (iv) collection of the underlying receivable is reasonably assured.
Billings to customers or payments received from customers are included in deferred revenue on the balance sheet until all revenue recognition criteria are met.bills. As of March 31, 20122023, all investments that have original maturities of three months or less are classified as cash equivalents on the Consolidated Balance Sheets. Prior to March 31, 2023, the Company classified certain investments as held-to-maturity. All investments previously classified as held-to-maturity matured prior to March 31, 2023. As of March 31, 2023, all investments are classified as available-for-sale, as the sale of such investments may be required prior to maturity to implement management strategies. Available-for-sale debt securities are recorded at fair value. Any unrealized gains and December 31, 2011losses are included in accumulated other comprehensive income as a component of stockholders' equity until realized. As U.S. Treasury bills are risk-free, any declines in fair value are considered temporary.
Investments in equity securities consist of investments in the common stock of entities traded in active markets. The Company does not have the ability to exercise significant influence over any entities. Therefore, initial investments are recorded at cost, and 2010, we had approximately $268,875, $152,500 and $107,000, respectively in deferred revenue related to our collaborative research programs.
Product Revenue
We recognize product revenueare remeasured at the time of shipment to the customer, provided all other revenue recognition criteria have been met. We recognize product revenues upon shipment to distributors, provided that (i) the price is substantially fixed or determinable at the time of sale; (ii) the distributor’s obligation to pay us is not contingent upon resalefair value as of the products; (iii) title and risk of loss passes to the distributor at time of shipment; (iv) the distributor has economic substance apart from that provided by us; (v) we have no significant obligation to the distributor to bring about resale of the products; and (vi) future returns can be reasonably estimated. For any sales that do not meet all of the above criteria, revenue is deferred until all such criteria have been met. Our collaboration revenue consists of license and collaboration agreements that contain multiple elements, including non-refundable upfront fees, payments for reimbursement of third-party research costs, payments for ongoing research, payments associated with achieving specific development milestones and royalties based on specified percentages of net product sales, if any. We consider a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the elements are separable, whether there are determinable fair values and whether there is a unique earnings process associated with each element of a contract.
Collaborative and License Revenue
We recognize revenue from research funding under collaboration agreements when earned on a “proportional performance” basis as research hours are incurred. We perform services as specified in each respective agreement on a best-efforts basis, and are reimbursed based on labor hours incurred on each contract. We initially defer revenue for any amounts billed,balance sheet date. Any gains or payments received, in advance of the services being performed and recognize revenue pursuant to the related pattern of performance, based on total labor hours incurred relative to total labor hours estimated under the contract.
In December 2010, we entered into a 12 month research contract agreement with a third party, whereby we were engaged to perform research and development services on a fixed-fee basis for approximately $600,000. Based on proportional performance criteria, we recognized approximately $450,000 in revenue related to the contract during 2011 and $0 in revenue related to the contract during the first quarter of 2012.
In October 2011, we entered into a research contract agreement with a third party, whereby we will perform research and development services on a fixed-fee basis for $1,365,000. The agreement included an initial payment of approximately $239,000, with remaining payments expected to occur over a 21-month period. At March 31, 2012, we recorded approximately $120,000 in revenue related to the research contract in recognition of the proportional performance achieved by us through the first quarter of 2012.
Revenue Arrangements with Multiple Deliverables
We occasionally enter into revenue arrangements that contain multiple deliverables. Judgment is required to properly identify the accounting units of the multiple deliverable transactions and to determine the manner in which revenue should be allocated among the accounting units. Moreover, judgment is used in interpreting the commercial terms and determining when all criteria of revenue recognition have been met for each deliverable in order for revenue recognition to occur in the appropriate accounting period. For multiple deliverable agreements, consideration is allocated at the inception of the agreement to all deliverables based on their relative selling price. The relative selling price for each deliverable is determined using VSOE of selling price or third-party evidence of selling price if VSOE does not exist. If neither VSOE nor third-party evidence of selling price exists, we use our best estimate of the selling price for the deliverable.
We recognize revenue for delivered elements only when we determine there are no uncertainties regarding customer acceptance. While changes in the allocation of the arrangement consideration between the units of accounting will not affect the amount of total revenue recognized for a particular sales arrangement, any material changes in these allocations could impact the timing of revenue recognition, which could affect our results of operations.
NIH and U.S. Treasury Grant Revenues
During 2010, the U.S. Treasury awarded us two one-time grants totaling approximately $397,300 for investments in qualifying therapeutic discovery projects under section 48D of the Internal Revenue Code. The grants cover reimbursement for qualifying expenses incurred by us in 2010 and 2009. The proceeds from these grants are classified in “Revenues – Grants” in the 2010 statement of operations.
During 2010 and 2009, the NHLBI, a division of the NIH, awarded us two research grants totaling approximately $267,600. Revenues from the NIH grants are based upon internal and subcontractor costs incurred that are specifically covered by the grant, and where applicable, an additional facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by subcontractors and as we incur internal expenses that are related to the grant. Revenue recognized under these grants for the years ended December 31, 2011 and 2010 was approximately $56,900 and $131,100, respectively. Revenue recognized under these grants for the three months ended March 31, 2012 was approximately 0.
Allowance for Doubtful Accounts
When needed we maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. The allowance for doubtful accounts is reviewed quarterly and is estimated based on the aging of account balances, collection history and known trends with current customers andchange in the economy in general. As a result of this review, the allowance is adjusted on a specific identification basis. An increase to the allowance for doubtful accounts results in a corresponding charge to sales, marketing and administrative expense. Historically our customer base is relatively concentrated and so we are subject to risk of concentration with any one particular customer. That risk is mitigated by the fact that payments from our collaborative agreements are typically prepaid, and our grant revenues are typically paid by units of the U.S. government. To-date we have fully collected all receivables. As a result our current and historic allowance is zero.
When we begin to sell commercial product we expect to establish a reserve for estimated sales returns thatfair value are recorded in net income. The investments in equity securities are classified as a reduction to revenue. That reserve will be maintained to account for future return of products sold in the current period. The reserve will be reviewed quarterly and will be estimated based on an analysis of our historical experience related to product returns.assets.
Fair value measurement
Derivative Financial Instruments
We do not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks.
We review the terms of convertible debt and equity instruments we issue to determine whether there are embedded derivative instruments, including an embedded conversion option that is required to be bifurcated and accounted for separately as a derivative financial instrument. In circumstances where the convertible instrument contains more than one embedded derivative instrument, including the conversion option, that is required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument. Also, in connection with the sale of convertible debt and equity instruments, we may issue freestanding warrants that may, depending on their terms, be accounted for as derivative instrument liabilities, rather than as equity.
Derivative instruments are initially recorded at fair value and are then revalued at each reporting date with changes in the fair value reported as non-operating income or expense. When the convertible debt or equity instruments contain embedded derivative instruments that are to be bifurcated and accounted for as liabilities, the total proceeds allocated to the convertible host instruments are first allocated to the fair value of all the bifurcated derivative instruments. The remaining proceeds, if any, are then allocated to the convertible instruments themselves, usually resulting in those instruments being recorded at a discount from their face value.
Fair Value Measurements
Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
•
Level 1 — Quoted prices in active markets for identical assets or liabilities.
•
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
We used Level 3 inputsFor certain of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other assets, accounts payable, accrued expenses, the carrying amounts are generally considered to be representative of their respective fair values because of the short-term nature of those instruments.
Cash and cash equivalents
The Company considers all highly liquid investments with original maturities of three months or less to be cash equivalents.
As of March 31, 2023 and 2022, the Company had approximately $0.1 million of restricted cash, respectively, deposited with a financial institution. The entire amount was held in certificates of deposit to support a letter of credit agreement related to the Company’s facility leases entered into in November 2020 and amended in November 2021.
Fixed assets and depreciation
Fixed assets are carried at cost. Expenditures that extend the life of the asset are capitalized and depreciated. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the remaining lease term. The estimated useful lives of the fixed assets range between one and seven years.
Impairment of long-lived assets
In accordance with authoritative guidance, the Company reviews its long-lived assets, including fixed assets and other assets, for our valuation methodologyimpairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may not be fully recoverable. To determine recoverability of its long-lived assets, the Company evaluates whether future undiscounted net cash flows will be less than the carrying amount of the assets and adjusts the carrying amount of its assets to fair value. Management has determined that no impairment of long-lived assets occurred as of March 31, 2023 and 2022.
Research and development expenses, including direct and allocated expenses, consist of independent research and development costs, as well as costs associated with sponsored research and development. Research and development costs are expensed as incurred.
Acquired in-process research and development
The Company has acquired drug candidates in development. The costs to acquire a drug candidate are immediately expensed as acquired in-process research and development, provided that the drug candidate has no alternative future use. Acquired in-process research and development expenses are included in total research and development expenses on the Consolidated Statements of Operations and Other Comprehensive Loss.
FXR Program
In March 2023, the Company acquired Metacrine's FXR program for $4.0 million. The FXR program was determined to have no alternative future use, and therefore was considered acquired in-process research and development and fully expensed. For the year ended March 31, 2023, the Company paid a $2.0 million upfront payment, and the remaining $2.0 million will be paid in the next fiscal year (see detail in "Note 4. Accrued Expenses") upon final transfer of the drug compounds, related data, and IP.
Income taxes
Deferred income taxes are recognized for the warrant derivativetax consequences in future years for differences between the tax basis of assets and liabilities and their financial reporting amounts at each year end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Income tax expense is the combination of the tax payable for the year and the change during the year in deferred tax assets and liabilities. The estimatedCompany’s policy regarding uncertainty in income taxes is pursuant to ASC 740-10. Interest and penalties that would be assessed in relation to the settlement value of unrecognized tax benefits is recognized as a component of income tax expense.
Revenue recognition
The Company has generated revenues from payments received from licensing intellectual property.
The Company has entered into a license agreement with a company that includes the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales, if any. At the initiation of the agreement, the Company has analyzed whether it results in a contract with a customer under Topic 606.
The Company has considered a variety of factors in determining the appropriate estimates and assumptions under these arrangements, such as whether the Company is a principal vs. agent, whether the elements are distinct performance obligations, whether there are determinable stand-alone prices, and whether any licenses are functional or symbolic. The Company has evaluated each performance obligation to determine if it can be satisfied and recognized as revenue at a point in time or over time. Typically, non-refundable upfront fees have been considered fixed, while sales-based royalty payments have been identified as variable consideration which must be evaluated to determine if it has been constrained and, therefore, excluded from the transaction price. Please refer to “Note 5: Collaborative Research, Development, and License Agreements” for further information.
Stock-based compensation
The Company accounts for stock-based compensation in accordance with the ASC Topic 718, Compensation — Stock Compensation, which establishes accounting for equity instruments exchanged for employee and non-employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair values were determinedvalue of the award (determined using aeither the Black-Scholes or Monte Carlo option pricing model basedoption-pricing models, depending on various assumptions (see the complexity of the equity grant), and is recognized as an expense, under the straight-line method, over the employee’s requisite service period (generally the vesting period of the equity grant).
Comprehensive income (loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at comprehensive income (loss).
Net loss per share
Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. The weighted-average number of shares used to compute diluted loss per share excludes any assumed exercise of stock options and warrants, shares reserved for purchase under the Company’s 2016 Employee Stock Purchase Plan (“ESPP”), the assumed release of restriction of restricted stock units (“RSUs”), and shares subject to repurchase as the effect would be anti-dilutive. No dilutive effect was calculated for the years ended March 31, 2023 and 2022 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive.
Common stock equivalents excluded from computing diluted net loss per share were approximately 1.6 million shares and 1.2 million shares for the years ended March 31, 2023 and 2022, respectively.
Note 42. Investments and fair value measurement
Investments in debt securities
As of March 31, 2023, the Company held $4.9 million of investments in debt securities (which are included in the $15.3 million of cash and cash equivalents). For the year ended March 31, 2023, there was $0.3 million of interest income related to the Financial Statementsinvestments in debt securities. There were less than $0.1 million of Organovo, Inc.unrealized gains recorded on investments in debt securities for the year ended DecemberMarch 31, 2011). Our derivative liabilities are adjusted to reflect estimated2023. As the investments in debt securities consist of U.S. Treasury bills from active markets, the fair value at each period end, with any decrease or increaseis measured using level 1 inputs.
The following table summarizes the Company's investments in the estimateddebt securities that are measured at fair value being recordedas of March 31, 2023 (in thousands):
| | | | | | | | | | | | | | | | |
| | Amortized costs basis | | | Gross unrealized gains | | | Gross unrealized losses | | | Fair value | |
As of March 31, 2023 | | | | | | | | | | | | |
Investment in debt securities | | $ | 4,943 | | | $ | 2 | | | $ | — | | | $ | 4,945 | |
Investments in other income or expense accordingly, as adjustments to fair valueequity securities
For the year ended March 31, 2023, there was $1.1 million of derivative liabilities.
Stock-Based Compensation
For purposesequity securities purchased, and $0.4 million of calculating stock-based compensation, we estimateequity securities sold. As of March 31, 2023, the fair value of investment in equity securities was $0.7 million, resulting in less than a $0.1 million unrealized gain on investment in equity securities. As the investments in equity securities consist of common stock optionsfrom active markets, the fair value is measured using level 1 inputs.
The following table presents the activity for investments in equity securities measured at fair value for the year ended March 31, 2023 (in thousands):
| | | | |
| | | |
| | Investment in Equity Securities
(in thousands) | |
Balance at March 31, 2022 | | $ | — | |
Purchases at cost | | | 1,061 | |
Sales | | | (384 | ) |
Gain on investment in equity securities | | | 29 | |
Balance at March 31, 2023 | | $ | 706 | |
Note 3. Fixed Assets
Fixed assets consisted of the following (in thousands):
| | | | | | | | |
| | March 31,
2023 | | | March 31,
2022 | |
Laboratory equipment | | $ | 1,575 | | | $ | 1,171 | |
Furniture and fixtures | | | 66 | | | | 38 | |
Computer software and equipment | | | 537 | | | | 524 | |
Fixed Assets, gross | | | 2,178 | | | | 1,733 | |
Less accumulated depreciation | | | (1,276 | ) | | | (1,071 | ) |
Fixed Assets, net | | $ | 902 | | | $ | 662 | |
As of March 31, 2023 and 2022, all of the Company’s fixed assets were active and in use. Depreciation expense for the years ended March 31, 2023 and 2022 was approximately $211,000 and $128,000, respectively.
Note 4. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| | | | | | | | |
| | March 31,
2023 | | | March 31,
2022 | |
Accrued compensation | | $ | 609 | | | $ | 434 | |
Accrued legal and professional fees | | | 193 | | | | 27 | |
Acquired in-process research and development | | | 2,000 | | | | — | |
Other accrued expenses | | | 46 | | | | 28 | |
| | $ | 2,848 | | | $ | 489 | |
Note 5. Collaborative Research, Development, and License Agreements
License Agreements
From June 2021 to February 2022, certain patents owned or sublicensed by the Company became the subject of IPR proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”). The Company and BICO Group AB were also engaged in litigation regarding patent infringement during the same time period. On February 22, 2022, the Company and BICO Group AB signed a Black-Scholes option-pricing model.settlement and patent license agreement (“License Agreement”) to close all matters noted above. In addition to closing all legal matters and patent disputes noted above, as part of the agreement, the Company agreed to grant a non-exclusive license to BICO
Group AB to use the Company’s aforementioned patents for its business operations of manufacturing and selling bioprinters as well as bioinks. The determinationCompany concluded that the nature of the license granted represents functional intellectual property.
As part of the License Agreement, BICO Group AB agreed to pay the Company a one time, nonrefundable upfront fee of $1,500,000. Based on Topic 606, the Company concluded that the performance obligation related to this upfront fee consisted of the Company filing stipulations of dismissal of all legal matters noted above, as well as the Company granting the non-exclusive license of the aforementioned patents within five days of receiving the upfront payment. The conditions of the performance obligation were satisfied, and therefore the Company recognized revenue of $1,500,000 on February 22, 2022, the executed date of the License Agreement.
Additionally, as part of the License Agreement, BICO Group AB agreed to pay the Company ongoing sales-based royalties (based on percentages of BICO Group AB’s net sales) for the use of the granted license. The sales-based royalties became effective beginning on February 22, 2022, the effective date of the License Agreement, and continue until the expiration of the last surviving licensed patent. As the sales-based royalties are required to be paid 45 days after the end of every quarter, there is variable consideration that must be estimated to determine royalty revenue within a given reporting period. Sales-based royalties that occurred prior to fiscal 2023 were recognized as revenue on a one quarter lag due to constraints on the estimates of variable consideration. During fiscal 2023, the Company began to estimate sales-based royalties earned each quarter. For the year ended March 31, 2023, the Company recorded $370,000 of royalty revenue based on sales-based royalties from the License Agreement. This recognized revenue is related to sales-based royalties earned from February 22, 2022 through March 31, 2023.
Also as part of the License Agreement, certain patents involved in the agreement are sublicensed by the Company from the University of Missouri and Clemson University. See below for further information.
University of Missouri
In March 2009, the Company entered into a license agreement with the Curators of the University of Missouri to in-license certain technology and intellectual property relating to self-assembling cell aggregates and to intermediate cellular units. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay the University of Missouri royalties ranging from 1% to 3% of net sales of covered tissue products, and of the fair market value of share-basedcovered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales achieved by the Company each year. The Company paid the minimum annual royalty of $25,000 in January 2022 for its respective calendar year, which is credited against royalties due during the subsequent twelve months. No payments have been made in excess of the minimum annual royalties in the years ended March 31, 2023 and 2022.
The license agreement with the University of Missouri also includes an additional sales royalty of 3% of all revenue received from a sublicensee, when such sublicense is entered pursuant to settlement of litigation. Such revenue shall include, but not be limited to, all option fees, license issue fees (upfront payments), license maintenance fees, equity, and all royalty payments. Such revenue shall not include research funding provided to licensee by sublicensee. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. As of March 31, 2023, the Company’s legal expenses exceeded royalties owed from the upfront payment awards utilizingand sales-based royalties related to the Black-Scholes model is affectedLicense Agreement. Therefore, no royalty expense to the University of Missouri was recorded for the year ended March 31, 2023. No royalty expense related to sales-based royalties has been recorded to date.
On December 5, 2022, the Company amended the license agreement with the University of Missouri, whereas the Company agreed to pay a single, upfront payment of $50,000 to the University of Missouri in exchange for the aforementioned licensed intellectual property to be fully paid up by our stock price andthe Company. As a number of assumptions, including expected volatility, expected life, risk-free interest rate and expected dividends. The expected volatility isresult, the Company will continue to have rights to the licensed intellectual property until its expiration, but will no longer owe minimum annual royalty payments, royalty payments based on net sales, or any other payments (other than patent annuities and any prosecution costs) in the historical volatilityfuture.
Clemson University
In May 2011, the Company entered into a license agreement with Clemson University Research Foundation to in-license certain technology and intellectual property relating to ink-jet printing of our common stockviable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay the university royalties ranging from 1.5% to 3% of net sales of covered tissue products and the fair market value of covered tissues transferred internally for use in the Company’s commercial service business, depending on the level of net sales reached each year. The license agreement terminates upon expiration of the patents licensed, which are expected to expire in May 2024, and is subject to certain conditions as defined in the license agreement. Minimum annual royalty payments of $20,000 were due for each of the two years beginning with
calendar 2014, and $40,000 per year beginning with calendar 2016. Royalty payments of $40,000 were made in each of the years ended March 31, 2023 and 2022. The annual minimum royalty is creditable against royalties owed during the same calendar year.
In addition to the annual royalties noted above, the University is owed 40% of all payments including but not limited to, upfront payments, license fees, issue fees, maintenance fees, and milestone payments received from third parties, including sublicensees, in consideration for sublicensing rights to licensed products. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. As of March 31, 2023, the Company’s legal expenses exceeded royalties owed from the upfront payment and sales-based royalties related to the License Agreement. Therefore, no royalty expense to Clemson University was recorded for the year ended March 31, 2023. No royalty expense related to sales-based royalties has been recorded to date.
Capitalized License Fees
Capitalized license fees consisted of the following (in thousands):
| | | | | | | | |
| | March 31,
2023 | | | March 31,
2022 | |
License fees | | $ | 114 | | | $ | 218 | |
Less accumulated amortization | | | (101 | ) | | | (124 | ) |
License fees, net | | $ | 13 | | | $ | 94 | |
The above license fees, net of accumulated amortization, are included in Other Assets in the accompanying consolidated balance sheets and are being amortized over the most recent period commensurate with the estimated expected term of the stock options. The expected life of the stock options is based on historicalrelated patents. Amortization expense of licenses was approximately $82,000 and other economic data trended into the future. The risk-free interest rate assumption is based on observed interest rates appropriate$14,000 for the expected terms of our stock options. The dividend yield assumption is based on our history and expectation of no dividend payouts. If factors change and we employ different assumptions, stock-based compensation expense may differ significantly from what we have recorded in the past. If there is a difference between the assumptions used in determining stock-based compensation expense and the actual factors which become known over time, specifically with respect to anticipated forfeitures, we may change the input factors used in determining stock-based compensation costs for future grants. These changes, if any, may materially impact our results of operations in the period such changes are made.
Results of Operations
Overview
Comparison of the three monthsyears ended March 31, 20122023 and 2011
Revenues
First quarter 2012 total revenues of $120,000 were $80,789, or 40%, below first quarter 2011 revenues of $200,789. Collaborative research revenues of $120,000 increased $46,135, or 62%, over the same period of prior year of $73,865. That growth was more than offset by no grant or product revenues in the first quarter of 2012, versus prior year, same period grant revenues of $26,924, and product revenues of $100,000. Revenue is expected to fluctuate between periods as the Company is in the development stage.
Operating Expenses
Overview.Operating expenses increased approximately $806,972, or 126%, in the first quarter of 2012 over the same period in 2011, from $642,158 in 2011 to $1,449,130 in 2012. Most significantly, relative to the same period in the prior year, the Company invested in infrastructure and outside services to support its transition from private ownership to a publicly owned and traded corporation. As expected in such transition, incremental initiatives were established in investor outreach, corporate governance, and SEC financial reporting including the need for audited financial statements. Non-payroll, non-audit related incremental public company expenses incurred in the first quarter of 2012 over the same period in 2011, were approximately $275,000, or approximately 34% of total incremental spending. Fees paid to our independent accounting firm to audit our financial statements were $139,000 in the first three months of 2012, representing approximately 17% of incremental spending over the first quarter 2011. Moreover, the Company invested in building its executive, research, and development staff, increasing first quarter 2012 payroll related expenses by $145,528, or 58%, over the same period 2011, from $250,399 to $395,927. Payroll related expenses accounted for approximately 18% of total year-to-year increase in operating expenses. The remaining incremental spending, period to period, were from numerous sources less significant than the foregoing. Executive search fees totaled $83,000, legal expenses related to licensing activity increased $43,000, newly established fees paid to our non-employee board members were approximately $35,000, and additional space was rented to accommodate our growing administrative and research staff at an incremental cost of $33,000 over the first quarter 2011.
Research and Development Expenses.First quarter 2012 research and development expenses increased by $148,623, or 37%, over the first quarter 2011 expenses of $398,664 to $547,287 as the Company increased its research staff to accommodate obligations under certain collaborative research agreements and to expand product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from four scientists, engineers and research associates at the three months ended March 31, 2011 to eleven in the same period 2012. Those staff additions yielded decreased consulting expenses by approximately $35,000 from the first quarter 2011 consulting expenses of $64,712. Those savings were offset by the Company incurring $61,457 in incremental executive search expenses during the first quarter 2012. In addition, laboratory supplies expenses increased from approximately $32,000 in the first quarter of 2011 to approximately $95,000 in the same period 2012, an increase of $63,000. That increase was related to purchases in support of our collaborative research conducted under our agreements.
General and Administrative Expenses.First quarter 2012 general and administrative expenses of $901,843 increased $658,349, or 270%, over same period 2011 expenses of $243,494. Expense increases were primarily driven by the Company’s transition from operating in a private environment to operating in a publicly traded environment. Expanded staff increased payroll and facilities expenses in 2012 over 2011 levels. Payroll related expenses increased from $76,917 in the first quarter 2011 to $236,266 for the same period 2012, an increase of $159,349 or 207%. The increase was primarily due to the addition of accounting staff to meet the expanded needs of operating in a publicly traded environment. In addition, the Board of Directors increased the base salary of our Chief Executive Officer from $220,000 per annum to $302,500.2022, respectively. At March 31, 20112023, the base salaryweighted average remaining amortization period for all licenses was approximately 2 years. The annual amortization expense of our CEO was $110,000. licenses for the next five years is estimated to be approximately $3,000 per year.
The 2012 adjustment, while large relativeSalk Institute for Biological Studies
In March 2023, the Company acquired the FXR Agonist program from Metacrine. All patent rights related to this program have been assigned to the first quarter 2011 base rate, isCompany in the lowest quartile of base salaries of peer companies as measured by an independent compensation expert retained by the board to access executive compensation. First quarter 2012 audit related expenses were approximately $139,000, whereas there were no audit related expenses during the same period 2011. Approximately $275,000 in other public company expenses were incurred in the first quarter of 2012 due to multiple reasons including increases to investor relations spending, financial printing, fees for non-employee Board members, rent and utilities, legal expenses, information technology investments in hardware, software and consulting services, and travel.
Other Income (Expense).The $35,696,820 increase in other expenses for the three month period ending March 31, 2012 over the same period of the prior year, was essentially due to the non-cash transaction costs associatedconnection with the first quarter 2012 Private Placement. During the first quarter of 2012 we incurred costs due to the placement agent for the first quarter Private Placement fees of $1,617,629 and reimbursed expenses and legal fees of $166,310. In addition, we issued warrants to purchase 6,099,195 shares of our common stock to the placement agent and warrants to purchase 15,247,987 of our common stock to investors in the Private Placement. The warrants issued to the placement agent and Private Placement investors were determined to be derivative liabilities as a result of the anti-dilution provisions in the warrant agreements that may result in an adjustment to the warrant exercise price. We will revalue the derivative liability on each subsequent balance sheet date until the securities to which the derivatives liabilities relate are exercised or expire. The fair value of warrant liabilities in excess of proceeds received was $19,019,422, while the change in fair value of warrant liabilities was $13,505,819. Financing transaction costs in excess of proceeds received was $2,129,500, and our interest expense was $1,087,453. The first quarter 2012 interest expense was primarily related to non-cash components including accretion of debt discounts and amortization of deferred financing costs.
Various factors are considered in the pricing models we use to value the warrants, including the company’s current stock price, the remaining life of the warrants, the volatility of the company’s stock price, and the risk free interest rate. Future changes in these factors will have a significant impact on the computed fair value of the warrant liability. As such, we expect future changes in the fair value of the warrants to continue to vary significantly from quarter to quarter.
Comparison of the twelve months ended December 31, 2011 and 2010
Revenues
2011 total revenues of $968,513 increased $365,101, or 61%, over 2010 revenues of $603,412. That increase was due to a $613,088 increase in collaborative agreement revenues, and a $223,500 increase in product revenues, partially offset by a $471,487 reduction in grant revenues. While grant revenues are expected to continue through 2012 they are expected to represent a declining portion of total revenues as we focus efforts on collaborative agreements and continued development of research tools.
Cost of Goods Sold, Gross Profit and Gross Profit Margin
Cost of goods sold (“COGS”) consists of purchased goods, and inventory-related costs. The Company did not have product revenues in 2010 and consequently did not have COGS. 2011 COGS of $133,607 were approximately 60% of product related revenues and 14% of total revenues.
Operating Expenses
Overview. Operating expenses increased approximately $1,343,259, or 75%, in 2011 over 2010, from $1,781,630 in 2010 to $3,124,889 in 2011. Most significantly, the Company invested in building its executive, research, and development staff, increasing payroll related expenses by $736,239 or 102% over 2010, from $720,759 to $1,456,998. Payroll related expenses accounted for approximately 55% of total year-to-year increase in operating expenses. General corporate expenses grew from $131,362 in 2010 to $421,063 in 2011, an increase of $289,700, or 221%, representing 22% of total operating expense growth. 85% of that expense increase was the result of increased legal activity, primarily focused on intellectual property (patent) protection.acquisition. In addition, the Company utilizedassumed and was assigned a license agreement with the services of outside consultantsSalk Institute for Biological Studies (hereafter “Salk”) that provides certain payments to Salk upon the successful development and research services to meet short-term spikes in scientific and professional service demands. Outsourcing those services to meet short-term demands increased Company expenses by $261,213, from $540,458 in 2010 to $801,671 in 2011, accounting for 19%commercialization of the total operating expense increases.lead compound, FXR314. The Company did not engage an independent accounting firm in 2010 but did so in 2011is required to audit the 2009 and 2010 financials. As a result overall operating expenses increased by $24,688 in 2011 over the prior year.
Research and Development Expenses.2011 research and development expenses increased by $216,002, or 18%, over 2010 expensespay Salk royalties ranging from 1% to 1.125% of $1,203,716 as the Company increased its research staff to accommodate its obligations under certain collaborative research agreements and to expand product development efforts in preparation for research-derived revenues. Full-time research and development staffing increased from four scientists and engineers at the 12 months ended December 31, 2010 to seven in in 2011.net sales of therapeutics based on FXR314. In addition, the Company outsourcedis required to make certain research related activitiesmilestone payments based on the successful initiation and/or completion of certain development milestones, including $500,000 within 45 days of the dosing of the first patient in responsea phase III clinical trial, $1,000,000 within 45 days of FDA approval of the first Licensed Product and $1,500,000 within 45 days of the first commercial sale of a Licensed Product in the Territory. There are also reduced milestone payments application to short-term demand spikes that increased expenses nearly $90,000 over prior year.a second or third licensed product, if any. Should the company sublicense the a licensed product to a third party, then it must pay a 3.5% of sublicensing revenue attributable to such a sublicense.
Note 6. Stockholders’ Equity
Selling, GeneralPreferred stock
The Company is authorized to issue 25,000,000 shares of preferred stock. There are no shares of preferred stock currently outstanding, and Administrative Expenses. Selling, general and administrative expenses grew from $577,914 in 2010 to $1,705,171 in 2011, an increase of $1,127,257 or 195%. Most notably the Company invested in its general and administrative staff, building needed infrastructurehas no present plans to meet the needs of operating in a publicly traded environment. Salaries, fringes and payroll related expenses increased by approximately $686,000, or 61% of the total increase. Legal expenses increased $244,861from $114,099 in 2010 to $358,960 in 2011. 78% of the legal expense increases were related to our patent related legal activities as we work diligently to secure additional patent protection in select markets. In 2011 we secured a short-term lease on office space near our main facility to accommodate our staff increases and need for additional meeting space. Rent expense grew from $107,481 in 2010 to $145, 218 for the year ended December 31, 2011, an increase of approximately $38,000. During 2011 we engaged an independent accounting firm to audit our 2009 and 2010 financial statements, adding approximately $25,000 in administrative expense that was not incurred in the prior year.
Interest Expense. Interest expense increased by $1,906,016 from $160,873 in 2010 to $2,066,889 in 2011. The 2011 interest expense was primarily related to non-cash components including:
| 1) | Accretion of debt discounts to interest expense of approximately $1.2 million |
| 2) | Amortization of deferred financing costs of approximately $119,500 |
| 3) | Fair value of warrants issued in connection with the exchange agreement of approximately $527.6K |
In the fourth quarter of 2011, the Company exchanged all outstanding convertible promissory notes for common stock equity, except for one $100,000 note, the principal and accrued but unpaid interest thereon to be paid at the close of a qualified equity financing. Following the exchange of earlier notes for equity, the Company completed a Bridge Financing, in which it sold $1,500,000 in principal amount of 6% promissory notes due March 31, 2012. Those notes will automatically convert to equity, including accrued but unpaid interest, upon the first close of a qualified equity financing.
Financial Condition, Liquidity and Capital Resources
Since our inception, we have primarily devoted our efforts to research and development, business planning, raising capital, recruiting management and technical staff, and acquiring operating assets. Accordingly, we are considered to be in the development stage.
Since inception, we incurred negative cash flows from operations. As of March 31, 2011, we had cash and cash equivalents of $339,607 and an accumulated deficit of $1,833,785. We also had negative cash flow from operations of $311,767 during the quarter ended March 31, 2011. At March 31, 2012, we had cash of $10,352,507 and an accumulated deficit of $37,385,108.
At March 31, 2012, we had total current assets of $10,834,703 and current liabilities of $1,110,948, resulting in working capital of $9,723,755. At March 31, 2011, we had total current assets of $1,030,205 and current liabilities of $1,975,748, resulting in a working capital deficit of $945,543.
Net cash used by operating activities for the quarter ended March 31, 2012 was $3,555,626. We raised approximately $13.7 million in gross proceeds from the sale of common stock and warrants, and $120,000 in revenue during the quarter.
Net cash used by operating activities for the quarter ended March 31, 2011 was $311,767. In the quarter ended March 31, 2011, we raised $200,789 in cash receipts from product sales, collaborative research agreements, and government grants.
We believe our cash and cash equivalents on hand as of March 31, 2012, together with amounts to be received from grants and our collaborate research agreements, should be sufficient to fund our ongoing operations as currently planned for at least the next 12 months. We have financed our operations primarily through the sale of common stock and convertible notes, and through revenue derived from grants or collaborative research agreements. We expect to cover our anticipated operating expenses through cash on hand, through additional financing from existing and prospective investors, and from revenue derived from collaborative research agreements.
We may need additional capital to further fund product development and commercialization of its human tissues that can be employed in drug discovery and development, biological research, and as therapeutic implants for the treatment of damaged or degenerating tissues and organs. We cannot be sure that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to us or to our stockholders. Having insufficient funds may require us to delay, scale back, or eliminate some or all of our development programs or relinquish some or even all of our licensed intellectual property. Failure to obtain adequate financing could eventually adversely affect our ability to operate as a going concern. If we raise additional funds from the issuance of equity securities, substantial dilution to our existing stockholders may result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
As of March 31, 2012, we had 43,693,241 total issued and outstandingissue shares of preferred stock.
Common Stock, and five year warrants forstock
In January 2012, the opportunity to purchase an additional 24,256,932 shares of Common Stock at $1.00 per share. If all warrants were exercised on a cash basis, we would realize an additional $24,256,932 in gross proceeds.
Board approved the 2012 Plan. The 2012 Equity Incentive Plan provides forauthorized the issuance of up to 6,553,986 shares, or approximately 15% of our outstanding Common Stock, to executive officers, directors, advisory board members and employees. In aggregate issued and outstanding common stock, shares underlying outstanding warrants, and shares reserved for the 2012 incentive plan total 74,504,159327,699 shares of common stock.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources. We have certain warrants and options outstanding but we do not expect to receive sufficient proceeds from the exercise of these instruments unless and until the underlying securities are registered, and/or all restrictions on trading, if any, are removed, and in either case the trading price of our Common Stock is significantly greater than the applicable exercise prices of the options and warrants.
Effect of Inflation and Changes in Prices
Management does not believe that inflation and changes in price will have a material effect on the Company’s operations.
DIRECTORS AND EXECUTIVE OFFICERS
The following persons are our executive officers, non-executive officers and directors and hold the positions set forth opposite their name as of June 1, 2012:
| | | | | | |
Name
| | Age | | | Position(s)
|
Keith Murphy
| | | 40 | | | Chairman of the Board, Chief Executive Officer, and President |
Sharon Collins Presnell, Ph.D.
| | | 43 | | | Executive Vice President of Research and Development |
Barry D. Michaels
| | | 62 | | | Chief Financial Officer |
Michael Renard
| | | 53 | | | Executive Vice President of Commercial Operations |
Eric Michael David, Ph.D.
| | | 40 | | | Chief Strategy Officer |
Robert Baltera, Jr.
| | | 46 | | | Director |
Andras Forgacs
| | | 35 | | | Director |
Adam K. Stern
| | | 48 | | | Director |
Keith Murphy, Chairman of the Board, Chief Executive Officer, and President, is one of our founders and joined us in July 2007. Mr. Murphy was formerly an employee of biotechnology company Alkermes, Inc., where he worked from July, 1993 to July, 1997 and played a role on the development teamstock for their first approved product, Nutropin (hGH) Depot. He moved to Amgen, Inc. in August, 1997 and developed several other novel formulation and device products. He has over 18 years of experience in biotechnology, including serving in Product Strategy and Director of Process Development roles at Amgen through July, 2007. He was previously Global Operations Leader for the largest development program in Amgen’s history, osteoporosis/bone cancer drug Prolia/Xgeva (denosumab). He holds a BS in Chemical Engineering from MIT, and is an alumnus of the UCLA Anderson School of Management.
Mr. Murphy’s previous experience in the biotechnology field and his educational experience qualify him to be a member of our Board of Directors.
Dr. Sharon Collins Presnell , Executive Vice President of Research and Development, joined us in May, 2011. Dr. Presnell has over 15 years of experience in the leadership of product-focused R&D. As an Assistant Professor at the University of North Carolina from 1998 to 2001 Dr. Presnell’s research in liver and prostate biology and carcinogenesis produced cell- and tissue-based technologies that were outlicensed for industrial applications. She joined Becton Dickinson & Co. (BD) in July, 2001 and played a key role in the early discovery and development of BD’s Discovery Platform and FACS CAP™ tools for the optimization of in vitro culture environments and flow cytometry-based characterization of cells. In her role at BD, she grew and led a large multi-disciplinary team to establish feasibility for the Discovery Platform and FACS CAP in multiple therapeutic areas, including diabetes, and stewarded both technologies through revenue-generating commercial partnerships. Dr. Presnell joined Tengion, Inc. in February, 2007 as the Senior Vice President of Regenerative Medicine Research, a position that she held until joining us in May 2011. At Tengion, Dr. Presnell was directly involved in the discovery and early development of Tengion’s Neo-Kidney Augment™ technology. Dr. Presnell holds a Ph.D. in Pathology from the Medical College of Virginia.
Barry D. Michaels, Chief Financial Officer, joined us in August, 2011. Mr. Michaels was the Chief Financial Officer of Cardima, Inc., a publicly-traded medical device company (NASDAQ: CRDM), from July, 2003 through June, 2005, and thereafter a consultant to the company through January, 2008. Mr. Michaels has been an independent consultant to medical device and technology companies since 1997, and has more than 30 years of combined industry experience. Since January, 2008 and prior to joining us, Mr. Michaels’s devoted his time to his consulting practice. In addition to his consulting practice, Mr. Michaels served as Chief Financial Officer of Lipid Sciences (NASDAQ: LIPD), a biotechnology company, from May, 2001 through January, 2003. Prior to joining Lipid Sciences, Mr. Michaels served as the Chief Financial Officer of IntraTherapeutics, Inc., an endovascular company, from March, 2000 until its acquisition by Sulzer Medica in May, 2001. Mr. Michaels received an MBA in finance from San Diego State University and is a graduate of the Executive Program at the University of California, Los Angeles.
Michael Renard, Executive Vice President of Commercial Operations, joined us in May, 2012. Mr. Renard has more than 29 years of experience in commercial operation, business development and sales and marketing for the life science industry. Since 1997, he has worked with Beckman Coulter holding various positions in program management, business operations and business development. He most recently was the vice president of marketing for North America commercial operations where he was responsible for achieving $2 billion in revenue across 11 major product lines. Before Beckman Coulter, he was vice president and general manager in a start-up development stage incubator division of Sanofi, Inc. and director of corporate accounts at Kallestad Diagnostics. He has a M.B.A from Rockhurst University and a B.A. in biology and chemistry from St. Olaf College.
Dr. Eric Michael David, Chief Strategy Officer,joined us in May, 2012. Dr. David was most recently associate partner at the consultancy McKinsey & Company, where he served private equity, pharmaceutical, biotech, diagnostic, and medical device clients to support pipeline and R&D strategy, as well as market entry strategy. Dr. David played a critical role in the commercial translation of 3D bioprinting as a founder and early director of Organovo, Inc. Prior to his time at McKinsey, Dr. David served as a freelance consultant to the Department of Health and Human Services in the use of genomic technologies for early detection of pathogens for public health preparedness. He completed his residency in Internal Medicine at New York Presbyterian Hospital, where he served as Assistant Chief Resident and received the Dick Bowman Award for scientific endeavor and dedication to patient care. He was also Assistant Professor at The Rogosin Institute and adjunct faculty at The Rockefeller University. He received his M.D. from Columbia University College of Physicians and Surgeons, his J.D. from Columbia University School of Law, and a B.A.in physics and fine arts from Amherst College. He is board certified in Internal Medicine and admitted to the Bar in New York State.
Robert Baltera, Jr., Director, joined us as a director in October, 2009. Most recently, Mr. Baltera was the Chief Executive Officer of Amira Pharmaceuticals, a position he held from July, 2007 through September, 2011. Amira was sold to Bristol-Myers Squibb in September, 2011 for $325 million in cash upfront, plus additional milestone payments of up to $150 million. Mr. Baltera is a seasoned pharmaceutical industry executive who has acquired a wealth of business and product management experience during his 17 years with biotech pioneer Amgen, beginning November, 1990. In his role leading Amira Pharmaceuticals, he was instrumental in focusing the company’s development efforts, strengthening and developing its pipeline and forging key collaborations with partners such as GlaxoSmithKline. Before becoming Amira’s CEO, he held a number of senior management positions at Amgen, the last being vice president of corporate and contract manufacturing. He served as Amgen’s team leader responsible for the approval of Kineret™ in rheumatoid arthritis. Mr. Baltera has an MBA from the Anderson School at UCLA and earned his bachelor’s degree in microbiology and a master’s degree in genetics from The Pennsylvania State University.
Mr. Baltera’s previous experience in the biotechnology field and his educational experience qualify him to be a member of our Board of Directors.
Andras Forgacs , Director, is one of our founders and joined us as a director in April, 2007. Mr. Forgacs has served as a Managing Director at Richmond Global, an international technology-focused venture fund, since July, 2008. In his role at Richmond, Mr. Forgacs focuses on the day-to-day management of the fund and the sourcing of new investment opportunities. Prior to joining Richmond, beginning in November, 2005, he was a consultant in the New York office of McKinsey & Company advising global financial institutions, healthcare/pharmaceutical companies and private equity/venture capital firms. Mr. Forgacs began his career with Citigroup as an investment banker in the Financial Strategy Group in July, 1999, and helped found the client-facing E-commerce Group. Mr. Forgacs is a Kauffman Fellow with the Center for Venture Education and a Term Member with the Council on Foreign Relations. He holds an MBA from the Wharton School of Business and
a Bachelor of Arts with honors from Harvard University. Mr. Forgacs is the son of Gabor Forgacs,Ph.D.,who developed Organovo’s breakthrough organ printing technology while leading a team of top regenerative medicine scientists from multiple universities, with the backing of a $5MM National Science Foundation Grant. Dr. Forgacs was one of the founders of the Company.
Mr. Forgacs’ previous experience with “start-up” companies in the equity/venture capital field and his educational experience qualify him to be a member of our Board of Directors.
Adam K. Stern, Director, joined us in February 2012 and is the Senior Managing Director of Spencer Trask Ventures. Mr. Stern has over 20 years of venture capital and investment banking experience focusing primarily on the technology and life science sectors of the capital markets. He currently manages the structured finance group of Spencer Trask Ventures, Inc. Mr. Stern joined Spencer Trask Ventures in September 1997 from Josephthal & Co., members of the New York Stock Exchange, where he served as Senior Vice President and Managing Director of Private Equity Marketing and held increasingly responsible positions from 1989 to 1997. He has been a licensed securities broker since 1987 and a General Securities Principal since 1991. Mr. Stern currently sits on the boards of various private companies and one other public company, InVivo Therapeutics Holdings Corp. (OTCBB:NVIV). Mr. Stern holds a Bachelor of Arts degree with honors from The University of South Florida in Tampa.
Mr. Stern’s experience as a board member of privately held and publicly traded companies qualifies him to be a member of our Board of Directors. Additionally, his 20 years of venture capital and investment banking focusing on technology and life science sectors will be an asset to the Board of the Directors if we attempt to raise capital in the future.
Family Relationships
Andras Forgacs is the son of Gabor Forgacs, who developed Organovo’s breakthrough organ printing technology while leading a team of top regenerative medicine scientists from multiple universities, with the backing of a $5MM National Science Foundation Grant. Dr. Forgacs was one of the founders of the Company.
Board of Directors and Corporate Governance
Our Board of Directors currently consists of four (4) members. On the Closing of the Merger, Deborah Lovig and James Coker, the members of the Board of Directors of Pubco, resigned, and simultaneously therewith, a new Board of Directors was appointed. Our Board consists of three (3) members who were former directors of Organovo and Adam K. Stern, who was appointed at the Closing of the Merger at the request of the Placement Agent.
Board Independence and Committees
We are not currently listed on any national securities exchange or in an inter-dealer quotation system that has a requirement that the Board of Directors be independent. However, in evaluating the independence of our members and the composition of the committees of our Board of Directors, our Board utilizes the definition of “independence” as that term is defined by applicable listing standards of the Nasdaq Stock Market and SEC rules, including the rules relating to the independence standards of an audit committee and the non-employee director definition of Rule 16b-3 promulgated under the Exchange Act.
Our Board of Directors expects to continue to evaluate its independence standards and whether and to what extent the composition of the Board and its committees meets those standards. We ultimately intend to appoint such persons to our Board and committees of our Board as are expected to be required to meet the corporate governance requirements imposed by a national securities exchange. Therefore, we intend that a majority of our directors will be independent directors of which at least one director will qualify as an “audit committee financial expert,” within the meaning of Item 407(d)(5) of Regulation S-K, as promulgated by the SEC.
Additionally, our Board of Directors is expected to appoint an audit committee, governance committee and compensation committee and to adopt charters relative to each such committee.
We believe that Robert Baltera is an “independent” director as that term is defined by SEC rules, including the rules relating to the independence standards of an audit committee and the non-employee director definition of Rule 16b-3 promulgated under the Exchange Act.
Code of Ethics
We have not adopted a written code of ethics. We intend to adopt a written code of ethics in the future.
Indemnification Agreements
Our Board has approved a form of indemnification agreement for our directors and executive officers (“ Indemnification Agreement “). Following Board approval, we entered into Indemnification Agreements with each of our current directors and executive officers.
The Indemnification Agreement provides for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreement also provides for the advancement of expenses in connection with a proceeding prior to a final, nonappealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreement.
The foregoing description is qualified in its entirety by reference to the form of Indemnification Agreement attached to this Report as Exhibit 10.17.
Classified Board
Our Board of Directors is divided into three classes (each, a “ Class “). The term of office of the initial Class I director (Mr. Murphy) shall expire at the first regularly-scheduled annual meeting of the stockholders following January 30, 2012, which was the date of our reincorporation in Delaware (the “ Effective Date “), the term of office of the initial Class II directors (Messrs. Forgacs and Stern) shall expire at the second annual meeting of the stockholders following the Effective Date and the term of office of the initial Class III director (Mr. Baltera) shall expire at the third annual meeting of the stockholders following the Effective Date. At each annual meeting of stockholders, commencing with the first regularly-scheduled annual meeting of stockholders following the Effective Date, each of the successors elected to replace the directors of a Class whose term expires at such annual meeting shall be elected to hold office for a three year term.
Scientific And Business Advisory Boards
Gabor Forgacs, Scientific Founder – PhD – University of Missouri and Clarkson University
Dr, Forgacs, is one of our founders. Dr. Forgacs is the Executive and Scientific Director of the Shipley Center for Innovation at Clarkson University and the George H. Vineyard Professor of Biological Physics at the University of Missouri. Dr. Forgacs has been with the University of Missouri since 1999 and has been with Clarkson University since 2011. He developed Organovo’s breakthrough bioprinting technology while leading a team of regenerative medicine scientists from multiple universities, with the backing of a $5 million National Science Foundation Grant. Dr. Forgacs is the author of more than 150 peer reviewed journal articles and the textbook Biological Physics of the Developing Embryo, (with Stuart Newman), published by Cambridge University Press. He holds a Ph.D. in theoretical physics from the Roland Eotvos University, Budapest, Hungary. He moved to the United States in the 1980’s from the Institute of Physics of the French Atomic Energy Agency in Saclay to accept a professorship at Clarkson University. Dr. Forgacs is the father of Andras Forgacs.
Gordana Vunjak-Novakovic, PhD—Columbia
Dr. Vunjak-Novakovic is the Mikati Foundation Professor of Biomedical Engineering and Medicine at Columbia University, where she directs the Laboratory for Stem Cells and Tissue Engineering, the Bioreactor Core of the NIH Tissue Engineering Center, the Stem Cell Imaging Core and the Craniofacial Regeneration Center. Prof. Vunjak-Novakovic has authored books as well as numerous book chapters, journal articles and issued, licensed and pending patents in the biomedical field. She is a Fellow of the American Institute for Medical and Biological Engineering.
Glenn Prestwich, PhD – University of Utah
Dr. Glenn D. Prestwich is Presidential Professor of Medicinal Chemistry and Special Presidential Assistant for Faculty Entrepreneurism at the University of Utah, where he leads the Entrepreneurial Faculty Scholars program. His university research includes the study of biomaterials for tissue repair and tissue engineering and biological reagents. He co-founded multiple companies, including Carbylan BioSurgery, Inc. (medical devices), Sentrx Animal Care, Inc. (veterinary wound care), and Glycosan BioSystems, Inc. (cell therapy and research tools). He received the Governor’s Medal for Science and Technology for 2006, the 1998 Paul Dawson Biotechnology Award and the 2008 Volwiler Research Award of the AACP, the 2010 University of Utah Distinguished Scholarly and Creative Research Award, and the 2010 “Rooster Prize” of the International Society for Hyaluronan Science.
David Mooney, PhD – Harvard University
Prof. David Mooney is a scientific author and a leader in the research of signaling mechanisms of tissue development. He studies the mechanisms by which chemical (for example, specific cell adhesion molecules) or mechanical signals (for example, cyclic strain) are sensed by cells and alter cells’ proliferation and specialization to either promote tissue growth or destruction. This work assists in the understanding of cell behavior post-processing by the organ printing technology. Dr. Mooney is the Pinkas Family Professor of Bioengineering at Harvard University, a member of the National Academy of Engineering, and holds a PhD from the Massachusetts Institute of Technology.
Dr. K. Craig Kent, MD – Columbia University/Weill Cornell Medical College
Dr. K. Craig Kent is the Chairman of the Department of Surgery at the University of Wisconsin School of Medicine and Public Health and previously served as Chief of the Division of Vascular Surgery at both Columbia University and Weill Cornell Medical College. Dr. Kent has authored or co-authored more than 300 manuscripts and chapters that have been published in peer-reviewed journals and textbooks on vascular disease. He is regularly invited to speak at local, national and international scientific meetings on a wide variety of vascular surgery topics. His National Institutes of Health (NIH)-funded basic science lab explores the mechanisms of failure for bypass grafts and angioplasty following vascular intervention. Dr. Kent served as the 2006-2007 president of the Society for Vascular Surgery. Dr. Kent was trained in general surgery at the University of California at San Francisco and completed his vascular surgery fellowship at Brigham and Women’s Hospital-Harvard Medical School, where he was awarded the prestigious annual E.J. Wylie Traveling Fellowship.
In March, 2008, we entered into consulting agreements with Dr. Glenn Prestwich, Prof. David Mooney, and Dr. K. Craig Kent, all of whom are members of our Scientific Advisory Board. In April, 2008, we entered into a consulting agreement with Prof. Gordana Vunjak-Novakovic, the fourth member of our Scientific Advisory Board. Per these agreements, we made restricted stock grants of 235,483 shares of our Common Stock to Dr. Prestwich and Prof. Vunjak-Novakovic and 117,741 shares of our Common Stock to Prof. Mooney and Dr. Kent. These grants vest in four annual equal installments with the first installment vesting on the one year anniversary of the member’s appointment to our Scientific Advisory Board. In addition, we agreed to pay Prof. Mooney $14,000 per year and Dr. Kent $7,000 per year. Each of the consulting agreements has a four year term which may be terminated by either us or the Scientific Advisory Board member on thirty days notice.
EXECUTIVE COMPENSATION
The following table sets forth information regarding each element of compensation that we paid or awarded to our named executive officers and for fiscal year ended December 31, 2011 and 2010.
Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position | | Year | | | Salary | | | Option
Awards
($) | | | Deferred
Compensation
($) | | | All Other
Compensation
($) | | | Total
Compensation
($) | |
Keith Murphy Chairman, Chief Executive Officer, and President | | | 2011 | | | $ | 217,711 | 1,2 | | | — | | | | — | | | | — 3 | | | $ | 217,711 | |
| | | 2010 | | | $ | 46,538 | | | | — | | | $ | 63,462 4 | | | | — 5 | | | $ | 110,000 | |
Barry D. Michaels Chief Financial Officer | | | 2011 | | | $ | 74,315 | | | | — | | | | — | | | | — 6 | | | $ | 74,315 | |
Sharon Presnell Executive Vice- President of Research and Development | | | 2011 | | | $ | 157,385 | | | $ | 3,163 | | | | — | | | | — 7 | | | $ | 160,548 | |
Employment Arrangements with Officers and Directors
Keith Murphy, one of our founders, has served as our President and Chief Executive Office since July, 2007. The terms of Mr. Murphy’s employment agreement, dated February 28, 2012, call for him to receive a base salary of $302,500 per year. The term of the employment agreement expires after one year from the effective date, and automatically renews thereafter, unless we provide Mr. Murphy advanced notice of nonrenewal. Mr. Murphy is also eligible to participate in our Annual Bonus Plan and other short-term incentive compensation plans established for our senior executives by our Board of Directors or the compensation committee. Mr. Murphy is also entitled to participate in our equity incentive awards plans.
Sharon Presnell became our Executive Vice President of Research and Development in May, 2011. The terms of Dr. Presnell’s employment arrangement call for her to receive a base salary of $248,014 per year. Dr. Presnell is also eligible to receive an annual bonus, which is targeted at 30% of her base salary but which may be adjusted based on her individual performance and our performance as a whole. In addition, on October 14, 2011 we issued to Dr. Presnell options to purchase 896,256 shares of Common Stock under the 2008 Plan, which will vest in equal installments over four years from May 2011. If we terminate Dr. Presnell’s employment without cause, we are required to pay her a severance of up to six months of her base salary (in effect immediately prior to the date of the termination of her employment) plus benefits.
1 | Effective August 16, 2011 Mr. Murphy’s annual base salary was increased to $220,000. |
2 | Mr. Murphy was paid an annual salary of $110,000 beginning March, 2009. |
3 | Excludes payments made for the reimbursement of medical insurance premiums and a personal computer used primarily for business in the aggregate of less than $10,000. |
4 | Base salary earned, but payment deferred to future periods. |
5 | Excludes payments made for the reimbursement of medical insurance premiums. |
6 | Excludes payments made for the reimbursement of medical insurance premiums in the aggregate of less than $10,000. |
7 | Excludes payments made for the reimbursement of medical insurance premiums in the aggregate of less than $10,000. Also excludes $24,681 in reimbursed relocation expenses that qualify under IRS guidelines as excludable from income. |
Barry Michaels became our Chief Financial Officer in August, 2011. The terms of Mr. Michaels’ employment arrangement call for him to receive a base salary of $230,022 per year. Mr. Michaels is also eligible to receive a bonus based on our and his attainment of certain goals and performance milestones. In addition, at the final closing of the Offering following the Closing Date of the Merger we intend to grant Mr. Michaels options to purchase up to 2% of our issued and outstanding Common Stock under the 2011 Plan, which will vest in equal installments over four years from August 2011. If we terminate Mr. Michaels’ employment without cause we are required to pay Mr. Michaels a severance of up to six months of his base salary (in effect immediately prior to the date of the termination of his employment) plus benefits.
Outstanding Equity Awards at Fiscal Year End
The following table summarizes the equity awards made to our named executive officers that were outstanding at December 31, 2011.
| | | | | | | | | | | | | | | | | | | | | | | | |
Name | | No. of Securities
Underlying
Unexercised
Options (#)
Exercisable | | | No. of Securities
Underlying
Unexercised
Options (#)
Unexercisable | | | Option Exercise
Price | | | Option Expiration
Date | | | Number of
shares or
Units of stock
that have not
vested(#) | | | Market Value of
shares or Units
of stock that
have not
vested($) | |
Keith Murphy (1) | | | — | | | | — | | | | — | | | | — | | | | 367,947 | | | $ | 57,422 | |
Sharon Presnell (2) | | | — | | | | 896,256 | | | $ | 0.08 | | | | 5/2021 | | | | — | | | | — | |
Barry Michaels | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
(1) | These shares vest in February 2012. |
(2) | The options were granted on October 14, 2011, and vest in equal installments over four years from May 2011. |
2012 Equity Incentive Plan
Our Board of Directors and stockholders adopted the 2012 Plan in January 2012. 6,553,986 shares of Common Stock are reserved for issuance under the 2012 Plan. If an incentive award granted under the 2012 Plan expires, terminates, is unexercised or is forfeited, or if any shares are surrendered to us in connection with an incentive award, the shares subject to such award and the surrendered shares will become available for further awards under the 2012 Plan. Additionally, shares used to pay the tax or exercise price of an award will become available for future grant or sale under the 2012 Plan. To the extent an award under the 2012 Plan is paid out in cash rather than shares, the cash payment will not result in reducing the number of shares available for issuance under the 2012 Plan. The maximum number of shares subject to awards that may be granted to any individual during any calendar year is 2,000,000 and the maximum aggregate amount of cash that may be paid in cash during any calendar year with respect to awards payable in cash is $2,000,000.
The number and class of shares of our Common Stock subject to the 2012 Plan, the number and class of shares subject to any numerical limit in the 2012 Plan, and the number, price and class of shares subject to awards will be adjusted in the event of any change in our outstanding Common Stock by reason of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
Administration
It is expected that the compensation committee of the Board, or the Board in the absence of such a committee, will administer the 2012 Plan. Subject to the terms of the 2012 Plan, the compensation committee would have complete authority and discretion to determine the terms of awards under the 2012 Plan.
Grants
The 2012 Plan authorizes the grant to 2012 Plan participants of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units,RSUs, performance units, performance shares, and other stock or cash awards intended to comply with Section 162(m)awards. The Board and stockholders of the Internal Revenue Code (as amended,Company approved an amendment to the “Code”) and stock appreciation rights, as described below:
Stock Options. Stock options entitle2012 Plan in August 2013 to increase the participant, upon exercise, to purchase a specified number of shares of common stock at a specified pricethat may be issued under the 2012 Plan by 250,000 shares. In August 2015, the Board and stockholders of the Company approved an amendment to the 2012 Plan to further increase the number of shares of common stock that may be issued under the 2012 Plan by 300,000 shares. In July 2018, the Board and stockholders of the Company approved an amendment to the 2012 Plan to further increase the number of shares of common stock that may be issued under the 2012 Plan by 550,000 shares. In October 2021, the Board and stockholders of the Company approved an amendment to the 2012 Plan to further increase the number of shares of common stock that may be issued under the 2012 Plan by 900,000, bringing the
aggregate shares issuable under the 2012 Plan to 2,327,699. The 2012 Plan as amended and restated became effective on July 26, 2018 and terminates ten years after such date.
In March 2021, the Board approved the Inducement Plan. The Inducement Plan authorized the issuance of up to 750,000 shares of common stock for a specified periodawards of time. The Administrator may grant incentive and/orstock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards. In February 2022, 50,000 incentive stock options were issued under the Inducement Plan.
On October 12, 2022, the Company's stockholders and the Board approved the 2022 Plan, and it became effective on that date. The 2022 Plan replaced the 2012 Plan on the effective date. Upon the effective date, the Company ceased granting awards under the 2012 Plan and any shares remaining available for future issuance under the 2012 Plan were cancelled and are no longer available for future issuance. The 2012 Plan continues to govern awards previously granted under it. At the time the Board approved the 2022 Plan, an aggregate of 1,363,000 shares of the Company’s common stock was initially reserved for issuance under the 2022 Plan. The Company committed to reducing the new 2022 Plan share reserve by the number of shares that were granted under the 2012 Plan and the Inducement Plan between July 25, 2022 and October 12, 2022. From July 25, 2022 to October 12, 2022, the Company issued 126,262 shares of its common stock under the 2012 Plan. As a result, the number of shares reserved for future issuance under the 2022 Plan is 1,236,738 shares of common stock. The Company also committed to reducing the aggregate number of shares of its common stock issuable pursuant to the Inducement Plan from 750,000 shares to 51,000 shares (which includes 50,000 shares of its common stock issuable pursuant to an outstanding option to purchase common stock with an exercise price of $2.75 per share, leaving only 1,000 shares available for eachfuture issuance under the Inducement Plan) and the share reserve was reduced effective October 12, 2022.
The Company previously had an effective shelf registration statement on Form S-3 (File No. 333-222929) and the related prospectus previously declared effective by the SEC on February 22, 2018 (the “2018 Shelf”), which registered $100.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing, that was set to expire on February 22, 2021. On January 19, 2021, the Company filed a shelf registration statement on Form S-3 (File No. 333-252224) to register $150.0 million of the Company’s common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the “2021 Shelf”) and a related prospectus. The 2021 Shelf was declared effective by the SEC on January 29, 2021 and replaced the 2018 Shelf at that time.
On March 16, 2018, the Company entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, the Company filed a prospectus supplement to the 2021 Shelf (the “ATM Prospectus Supplement”), pursuant to which the Company may offer and sell, from time to time through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold will be issued pursuant to the 2021 Shelf. During the year ended March 31, 2023, the Company issued zero shares of common stock in ATM offerings under the ATM Prospectus Supplement. As of March 31, 2023, the Company has sold an aggregate of 1,580,862 shares of common stock in ATM offerings under the ATM Prospectus Supplement, with gross proceeds of approximately $21.7 million. As of March 31, 2023, there was approximately $100.0 million available for future offerings under the 2021 Shelf (excluding amounts available but not yet issued under the ATM Prospectus Supplement), and approximately $28.3 million available for future offerings through the Company’s ATM program under the ATM Prospectus Supplement.
Restricted stock units
The following table summarizes the Company’s RSUs activity for the year ended March 31, 2023:
| | | | | | | | |
| | Number of
Shares | | | Weighted
Average Price | |
Unvested at March 31, 2022 | | | 15,500 | | | $ | 10.58 | |
Granted | | | 117,642 | | | $ | 1.53 | |
Vested | | | (5,425 | ) | | $ | 11.02 | |
Cancelled / forfeited | | | — | | | $ | — | |
Unvested at March 31, 2023 | | | 127,717 | | | $ | 2.22 | |
Stock options
During the year ended March 31, 2023 under both the 2022 Plan and 2012 Plan, 255,474 stock options were granted at various exercise prices, respectively.
On March 8, 2021, the Company granted 120,000 and 25,000 stock options, respectively, to its Executive Chairman and its Chief Scientific Officer under the 2012 Plan. On October 7, 2021, the Company granted an additional 120,000 and 25,000 stock options, respectively, to the aforementioned officers. These stock options have unique vesting criteria based on market conditions, more specifically the Company’s stock price. As these market condition based stock options require significant estimates and assumptions to calculate their fair value, the Company engaged with valuation specialists to calculate the fair value and requisite service periods using Monte Carlo simulations. The stock options will be expensed over their determined requisite service periods. As of March 31, 2023, half of the aforementioned stock options were fully expensed over their requisite service periods. However, to date, none of the stock options have vested.
On October 7, 2021, the Company granted 60,000 and 15,000 stock options, respectively, to its Executive Chairman and its Chief Scientific Officer under the 2012 Plan. These stock options have unique vesting criteria based on specific Company performance conditions. The vesting criteria for half of these options was relating to the Company recognizing $1.5 million of revenue per year based on three quarters of results, which was achieved on February 22, 2022 (refer to “Note 4. Collaborative Research, Development, and License Agreements” for more information). The remaining unvested options have vesting criteria relating to the Company closing a seven-figure cash up front deal with a major pharmaceutical company. As of March 31, 2023, management estimated there was a 0% probability of achievement, and therefore no expense has been recorded to date.
The following table summarizes stock option shall be determined byactivity for the Administrator but shall not be less than 100%year ended March 31, 2023:
| | | | | | | | | | | | |
| | Options
Outstanding | | | Weighted-
Average
Exercise Price | | | Aggregate
Intrinsic
Value | |
Outstanding at March 31, 2022 | | | 1,203,671 | | | $ | 7.36 | | | $ | 71,650 | |
Options granted | | | 255,474 | | | $ | 2.34 | | | $ | — | |
Options canceled | | | (7,928 | ) | | $ | 5.66 | | | $ | — | |
Options exercised | | | — | | | $ | — | | | $ | — | |
Outstanding at March 31, 2023 | | | 1,451,217 | | | $ | 6.49 | | | $ | 38,327 | |
Vested and Exercisable at March 31, 2023 | | | 559,685 | | | $ | 7.07 | | | $ | 472 | |
The weighted-average remaining contractual term of stock options exercisable and outstanding at March 31, 2023 was approximately 8.00 years.
During the years ended March 31, 2023 and 2022, the Company issued zero shares of common stock upon exercise of stock options.
Employee Stock Purchase Plan
In June 2016, the Board, and in August 2016, its stockholders subsequently approved, the ESPP. The Company reserved 75,000 shares of common stock for issuance thereunder. The ESPP permits employees after five months of service to purchase common stock through payroll deductions, limited to 15 percent of each employee’s compensation up to $25,000 per employee per year. Shares under the ESPP are purchased at 85 percent of the fair market value at the lower of (i) the closing price on the first trading day of the commonsix-month purchase period or (ii) the closing price on the last trading day of the six-month purchase period. The initial offering period commenced in September 2016. During the year ended March 31, 2023, 1,009 shares were issued under the ESPP. At March 31, 2023, there were 58,426 shares remaining available for the purchase under the ESPP.
Common stock reserved for future issuance
Common stock reserved for future issuance consisted of the following at March 31, 2023:
| | | | |
Common stock issuable pursuant to options outstanding and reserved under the 2012 Plan |
|
| 1,345,664 |
|
Common stock reserved under the 2012 Plan |
|
| — |
|
Common stock issuable pursuant to options outstanding and reserved under the 2022 Plan |
|
| 55,553 |
|
Common stock reserved under the 2022 Plan |
|
| 1,071,471 |
|
Common stock reserved under the ESPP |
|
| 58,426 |
|
Common stock reserved under the 2021 Inducement Equity Plan |
|
| 1,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2012 Plan |
|
| 10,075 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2022 Plan |
|
| 117,642 |
|
Common stock issuable pursuant to options outstanding and reserved under the Inducement Plan |
|
| 50,000 |
|
Total at March 31, 2023 |
|
| 2,709,831 |
|
Stock-based compensation expense and valuation information
Stock-based awards include stock options and RSUs under the Company's 2022 Equity Incentive Plan ("2022 Plan"), Amended and Restated 2012 Equity Incentive Plan (“2012 Plan”), inducement awards, performance-based RSUs under an Incentive Award Performance-Based Restricted Stock Unit Agreement, the 2021 Inducement Equity Incentive Plan (“Inducement Plan”), and rights to purchase stock under the ESPP. The Company calculates the grant date fair value of all stock-based awards in determining the stock-based compensation expense.
Stock-based compensation expense for all stock-based awards consists of the following (in thousands):
| | | | | | | | |
| | Year Ended
March 31, 2023 | | | Year Ended
March 31, 2022 | |
Research and development | | $ | 473 | | | $ | 419 | |
General and administrative | | | 1,904 | | | | 1,837 | |
Total | | $ | 2,377 | | | $ | 2,256 | |
The total unrecognized compensation cost related to unvested stock option grants as of March 31, 2023 was approximately $2,492,000 and the weighted average period over which these grants are expected to vest is 2.05 years.
The total unrecognized stock-based compensation cost related to unvested RSUs (not including performance-based RSUs) as of March 31, 2023 was approximately $210,000, which will be recognized over a weighted average period of 1.24 years.
As of March 31, 2023, there are no participants enrolled into the ESPP for the current purchase period, beginning March 1, 2023.
The Company uses either the Black-Scholes or Monte Carlo option-pricing models to calculate the fair value of stock options, depending on the complexity of the equity grants. Stock-based compensation expense is recognized over the vesting period using the straight-line method. The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses its Company-specific historical volatility rate. The risk-free interest rate assumption was based on U.S. Treasury rates. The weighted average expected life of options was estimated using the average of the contractual term and the weighted average vesting term of the options. The fair value of stock options was estimated at the grant date using the following weighted average assumptions:
| | | | | | | | |
| | Year Ended
March 31, 2023 | | | Year Ended
March 31, 2022 | |
Dividend yield | | | — | | | | — | |
Volatility | | | 95.53 | % | | | 95.65 | % |
Risk-free interest rate | | | 3.32 | % | | | 1.30 | % |
Expected life of options | | 6.00 years | | | 5.75 years | |
Weighted average grant date fair value | | $ | 1.83 | | | $ | 4.73 | |
The fair value of each RSU is recognized as stock-based compensation expense over the vesting term of the award. The fair value is based on the closing stock price on the date of the grant.
The “fair market value” means, ifCompany uses the stockBlack-Scholes valuation model to calculate the fair value of shares issued pursuant to the ESPP. Stock-based compensation expense is listedrecognized over the purchase period using the straight-line method. The fair value of ESPP shares was estimated at the purchase period commencement date using the following assumptions:
| | | | | | | | |
| | Year Ended
March 31, 2023 | | | Year Ended
March 31, 2022* | |
Dividend yield | | | — | | | | — | |
Volatility | | | 86.58 | % | | | 0.00 | % |
Risk-free interest rate | | | 3.34 | % | | | 0.00 | % |
Expected term | | 6 months | | | | — | |
Grant date fair value | | $ | 0.82 | | | $ | — | |
*There were no participants in the ESPP for the purchase periods March 1, 2021 – August 31, 2022 nor any participants in the ESPP for the current purchase period (beginning March 1, 2023).
The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses the Company-specific historical volatility rate as the indicator of expected volatility. The risk-free interest rate assumption was based on U.S. Treasury rates. The expected life is the 6-month purchase period.
Note 7. Leases
After the initial adoption of ASC 842, on an on-going basis, the Company evaluates all contracts upon inception and determines whether the contract contains a lease by assessing whether there is an identified asset and whether the contract conveys the right to control the use of identified asset in exchange for consideration over a period of time. If a lease is identified, the Company will apply the guidance from ASC 842 to properly account for the lease.
Operating Leases
From October 2019 to July 2021, the Company rented office space in Solana Beach, California. This agreement was a month-to-month contract and could be terminated at-will by either party at any established stock exchangetime. As such, the Company concluded that this agreement did not contain a lease and was expensed as incurred (referred to as “rent expense”). Monthly rental payments were approximately $4,000 per month.
On November 23, 2020, the Company entered into two lease agreements, pursuant to which the Company temporarily leased approximately 3,212 square feet of lab and office space (the “Temporary Lease”) in San Diego and permanently leased approximately 8,051 square feet of office space (the “Permanent Lease”) in San Diego once certain tenant improvements for the Company’s permanent premises were completed by the landlord and the premises were ready for occupancy. Additionally, on November 17, 2021, the Permanent Lease was amended to add an additional 2,892 square feet of office space in the same building. The Temporary Lease commenced on November 27, 2020 and served as temporary premises until the Permanent Lease was ready for occupancy. The Permanent Lease commenced on December 17, 2021 and is intended to serve as the Company’s permanent premises for approximately sixty-two months. Monthly rental payments are approximately $40,900 with 3% annual escalators.
The Company determined that the Temporary Lease is considered a short term lease under ASC 842 and therefore elected an accounting policy for short term leases to recognize lease payments as an expense on a straight-line basis over the lease term (referred to as “short term lease expense”). Variable lease expenses related to the short term lease, such as payments for additional monthly fees to cover the Company’s share of certain facility expenses (common area maintenance, or national market system,CAM) are expensed as incurred.
The Company determined that the closing sales pricePermanent Lease is considered an operating lease under ASC 842, and therefore upon the lease commencement date of December 17, 2021, recognized lease liabilities and corresponding right-of-use assets of $2.3 million. The Company aggregates all lease and non-lease components for each class of underlying assets into a single lease component. As the Permanent Lease did not have a discount rate implicit in the lease, the Company estimated its incremental borrowing rate to discount the lease payments based on information available at the lease commencement. The Company records operating lease expense on a straight-line basis over the life of the stock,lease (referred to as “operating lease expense”). Variable lease expenses associated with the Company’s leases, such as payments for additional monthly fees to cover the Company’s share of certain facility expenses (common area maintenance, or ifCAM) are expensed as incurred.
The table below summarizes the common stockCompany’s lease liabilities and corresponding right-of-use assets as of March 31, 2023 (in thousands):
| | | | |
| | March 31, 2023 | |
ASSETS | | | |
Operating lease right-of-use assets | | $ | 1,705 | |
Total lease right-of-use assets | | $ | 1,705 | |
| | | |
LIABILITIES | | | |
Current | | | |
Operating lease liability | | $ | 492 | |
Noncurrent | | | |
Operating lease liability, net of current portion | | $ | 1,313 | |
Total lease liabilities | | $ | 1,805 | |
| | | |
Weighted average remaining lease term: | | 3.83 years | |
Weighted average discount rate: | | | 6 | % |
The Company recorded rent expense of approximately zero and $18,000 for the years ended March 31, 2023 and 2022, respectively. Variable lease expense was approximately $146,000 and $59,000 for the years ended March 31, 2023 and 2022, respectively. Short term lease expense was approximately zero and $117,000 for the years ended March 31, 2023 and 2022, respectively. Lastly, operating lease expense was approximately $499,000 and $172,000 for the years ended March 31, 2023 and 2022, respectively.
Cash outflows associated with the Company’s operating lease for the years ended March 31, 2023 and 2022 were $430,000 and $183,000, respectively.
Future lease payments relating to the Company’s operating lease liabilities as of March, 31, 2023 are as follows (in thousands):
| | | | |
Fiscal year ending March 31, 2024 | | $ | 508 | |
Fiscal year ending March 31, 2025 | | | 523 | |
Fiscal year ending March 31, 2026 | | | 538 | |
Fiscal year ending March 31, 2027 | | | 460 | |
Thereafter | | | — | |
Total future lease payments | | | 2,029 | |
Less: Imputed Interest | | | (224 | ) |
Total lease obligations | | | 1,805 | |
Less: Current obligations | | | (492 | ) |
Noncurrent lease obligations | | $ | 1,313 | |
Note 8. Commitments and Contingencies
Legal matters
In addition to commitments and obligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business.
The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its financial statements. Because litigation is regularly quoted byinherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a recognized securities dealer, butmeaningful estimate due to a number of factors, including the selling pricesprocedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not reported,meaningful indicators of its potential liability.
The Company regularly reviews contingencies to determine the meanadequacy of its accruals and related disclosures. During the period presented, the Company has not recorded any accrual for loss contingencies associated with any claims or legal proceedings; determined that an unfavorable outcome is probable or reasonably possible; or determined that the amount or range of any possible
loss is reasonably estimable. However, the outcome of legal proceedings and claims brought against the Company is subject to significant uncertainty. Therefore, although management considers the likelihood of such an outcome to be remote, if one or more legal matters were resolved against the Company in a reporting period, the Company’s consolidated financial statements for that reporting period could be materially adversely affected.
Note 9. Income Taxes
A reconciliation of the statutory federal rate and the effective rate, for operations, is as follows for the years ended March 31, 2023 and 2022 (in thousands, except percentages):
| | | | | | | | | |
| March 31,
2023 | | | | March 31,
2022 | | |
Tax computed at federal statutory rate | $ | (3,624 | ) | 21% | | $ | (2,404 | ) | 21% |
State income tax, net of federal benefit | | (44 | ) | 0.2% | | | (6 | ) | 0% |
Stock-based compensation | | 167 | | -1% | | | 1,857 | | -16.2% |
Research credits | | 60 | | -0.4% | | | (249 | ) | 2.1% |
Change in tax rate | | 157 | | -0.9% | | | 454 | | -4.0% |
Removal of net operating losses and research development credits | | 1,410 | | -8.2% | | | 2,269 | | -19.8% |
Other | | 1 | | 0% | | | 20 | | -0.1% |
Valuation allowance | | 1,873 | | -10.7% | | | (1,941 | ) | 16.9% |
Provision (benefit) for income taxes | $ | — | | 0.0% | | $ | — | | 0.0% |
Deferred income taxes reflect the net tax effects of temporary differences between the high bidcarrying amounts of assets and low asked pricesliabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows as of March 31, 2023 and 2022 (in thousands, except percentages):
| | | | | | | |
| March 31,
2023 | | | March 31,
2022 | |
Deferred tax assets: | | | | | |
Amortization | $ | 598 | | | $ | — | |
Section 174 R&D capitalization | | 855 | | | | — | |
Accrued expenses and reserves | | 116 | | | | 110 | |
Operating lease liability | | 384 | | | | 611 | |
Stock-based compensation | | 755 | | | | 554 | |
Inventory | | 251 | | | | — | |
Other, net | | 3 | | | | 3 | |
Total deferred tax assets | | 2,962 | | | | 1,278 | |
Valuation allowance | | (2,458 | ) | | | (583 | ) |
Net deferred tax assets | $ | 504 | | | $ | 695 | |
Deferred tax liabilities: | | | | | |
Operating lease right-of-use assets | | (363 | ) | | | (603 | ) |
Depreciation | | (135 | ) | | | (92 | ) |
Investment in equity securities | | (6 | ) | | | — | |
Total deferred tax liabilities | $ | (504 | ) | | $ | (695 | ) |
| $ | — | | | $ | — | |
A full valuation allowance has been established to offset the deferred tax assets as management cannot conclude that realization of such assets is more likely than not. Under the Internal Revenue Code (“IRC”) Sections 382 and 383, annual use of the Company’s net operating loss and research tax credit carryforwards to offset taxable income may be limited based on cumulative changes in ownership. The Company has not completed an analysis to determine whether any such limitations have been triggered as of March 31, 2023. Until this analysis is completed, the Company has removed the deferred tax assets related to net operating losses from its deferred tax asset schedule. Further, until a study is completed and any limitation known, approximately $1.6 million and $1.5 million for the common stock onyears ended March 31, 2023 and 2022, respectively, would be considered as an uncertain tax position if netted against the daydeferred tax asset. Due to the existence of determination, orthe valuation allowance, future changes in the absenceCompany’s unrecognized tax benefits will not impact its effective tax rate. Any carryforwards that will expire prior to utilization as a result of an established marketsuch limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance. The valuation allowance increased by approximately $1,875,000 and decreased by approximately $1,941,000 for the stock,years ended March 31, 2023 and 2022, respectively.
The Company had federal and state net operating loss carryforwards of approximately $210.5 million and $40.9 million, respectively, as of March 31, 2023. Federal net operating loss carryforwards of approximately $66.8 million will carryforward indefinitely and be available to offset up to 80% of future taxable income each year subject to revisions made by the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”). The remaining federal net operating losses will begin to expire in 2028, unless previously utilized. The state net operating loss carryforwards (“NOLs”) will begin to expire in 2028, unless previously utilized.
The Company had federal and state research tax credit carryforwards of approximately $4.7 million and $4.3 million at March 31, 2023, respectively. The federal research tax credit carryforwards begin expiring in 2028. The state research tax credit carryforwards do not expire.
The Company did not record any accruals for income tax accounting uncertainties for the year ended March 31, 2023.
The Company did not accrue either interest or ifpenalties from inception through March 31, 2023.
The Company does not expect its unrecognized tax benefits to significantly increase or decrease within the stocknext 12 months.
The Company is subject to tax in the United States and in California. As of March 31, 2023, the Company’s tax years from inception are subject to examination by the tax authorities due to the generation of net operating losses. The Company is not regularly quotedcurrently under examination by any jurisdiction.
Note 10. Concentrations
Credit risk and significant customers
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions located within the United States. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. Balances may exceed federally insured limits. The Company is also potentially subject to concentrations of credit risk in its revenues and accounts receivable. However, the Company only receives royalty revenue from one licensee and has not historically experienced any accounts receivable write-downs.
Note 11. Related Parties
From time to time, the Company will enter into an agreement with a related party in the ordinary course of its business. These agreements are ratified by the Board or doesa committee thereof pursuant to its related party transaction policy.
Viscient Biosciences (“Viscient”) is an entity for which Keith Murphy, the Company’s Executive Chairman, serves as the Chief Executive Officer and President. Dr. Jeffrey Miner, the Company’s Chief Scientific Officer, is also the Chief Scientific Officer of Viscient, and Thomas Jurgensen, the Company’s General Counsel, previously served as outside legal counsel to Viscient through his law firm, Optima Law Group, APC.
On December 28, 2020, the Company entered into an intercompany agreement (the “Intercompany Agreement”) with Viscient and Organovo, Inc., the Company’s wholly-owned subsidiary, which included an asset purchase agreement for certain lab equipment. Pursuant to the Intercompany Agreement, the Company agreed to provide Viscient certain services related to 3D bioprinting technology, which includes, but is not have sufficient trades or bidlimited to, histology services, cell isolation, and proliferation of cells and Viscient agreed to provide the Company certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, the Company and Viscient each agreed to share certain facilities and equipment and, subject to further agreement, to each make certain employees available for specified projects for the other party at prices which would reflect the stock’s actual fair market value, the fair market value of the common stock willto be determined in good faith by the Administratorparties. The Company evaluated the accounting for the Intercompany Agreement and concluded that any services provided by Viscient to the Company will be expensed as incurred, and any compensation for services provided by the Company to Viscient will be considered a reduction of personnel related expenses. Any services provided to Viscient do not fall under Topic 606 as the Intercompany Agreement is not a contract with a customer. For the years ended March 31, 2023 and 2022, the Company incurred approximately zero and $47,000 in consulting expenses from Viscient, respectively. Additionally, for the years ended March 31, 2023 and 2022, the Company provided approximately $59,000 and $48,000 of histology services to Viscient, respectively.
Note 12. Defined Contribution Plan
The Company has a defined contribution 401(k) plan covering substantially all employees. During the year ended March 31, 2015, the 401(k) plan was amended (the “Amended Plan”) to include an employer matching provision. Under the terms of the Amended Plan, the Company will make matching contributions on up to the first 6% of compensation contributed by its employees. Amounts expensed under the Company’s 401(k) plan for the years ended March 31, 2023 and 2022 were approximately $10,000 and $25,000, respectively.
Note13. Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies. Unless otherwise stated, the Company believes that the impact of the recently issued accounting pronouncements that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption.
Organovo Holdings, Inc.
Condensed Consolidated Balance Sheets
(in thousands except for share and per share data)
| | | | | | | | |
| | December 31, 2023 | | | March 31, 2023 | |
| | (Unaudited) | | | | |
Assets | | | | | | |
Current Assets | | | | | | |
Cash and cash equivalents | | $ | 5,295 | | | $ | 15,301 | |
Accounts receivable | | | 33 | | | | 152 | |
Investment in equity securities | | | — | | | | 706 | |
Prepaid expenses and other current assets | | | 913 | | | | 889 | |
Total current assets | | | 6,241 | | | | 17,048 | |
Fixed assets, net | | | 739 | | | | 902 | |
Restricted cash | | | 143 | | | | 143 | |
Operating lease right-of-use assets | | | 1,403 | | | | 1,705 | |
Prepaid expenses and other assets, net | | | 355 | | | | 515 | |
Total assets | | $ | 8,881 | | | $ | 20,313 | |
Liabilities and Stockholders’ Equity | | | | | | |
Current Liabilities | | | | | | |
Accounts payable | | $ | 471 | | | $ | 331 | |
Accrued expenses | | | 727 | | | | 2,848 | |
Operating lease liability, current portion | | | 502 | | | | 492 | |
Total current liabilities | | | 1,700 | | | | 3,671 | |
Operating lease liability, net of current portion | | | 999 | | | | 1,313 | |
Total liabilities | | | 2,699 | | | | 4,984 | |
Commitments and Contingencies (Note 7) | | | | | | |
Stockholders’ Equity | | | | | | |
Common stock, $0.001 par value; 200,000,000 shares authorized, 9,838,755
and 8,716,906 shares issued and outstanding at December 31, 2023 and
March 31, 2023, respectively | | | 10 | | | | 9 | |
Additional paid-in capital | | | 342,796 | | | | 340,317 | |
Accumulated deficit | | | (336,624 | ) | | | (324,998 | ) |
Accumulated other comprehensive income | | | 1 | | | | 2 | |
Treasury stock, 46 shares at cost | | | (1 | ) | | | (1 | ) |
Total stockholders’ equity | | | 6,182 | | | | 15,329 | |
Total Liabilities and Stockholders’ Equity | | $ | 8,881 | | | $ | 20,313 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
Organovo Holdings, Inc.
Unaudited Condensed Consolidated Statements of Operations and Other Comprehensive Loss
(in thousands except share and per share data)
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | Three Months Ended | | | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023 | | | December 31, 2022 | | | December 31, 2023 | | | December 31, 2022 | |
Revenues | | | | | | | | | | | | |
Royalty revenue | | $ | 5 | | | $ | 131 | | | $ | 80 | | | $ | 208 | |
Total Revenues | | | 5 | | | | 131 | | | | 80 | | | | 208 | |
Research and development expenses | | | 1,434 | | | | 1,185 | | | | 4,435 | | | | 3,436 | |
Selling, general and administrative expenses | | | 2,251 | | | | 2,305 | | | | 7,635 | | | | 6,724 | |
Total costs and expenses | | | 3,685 | | | | 3,490 | | | | 12,070 | | | | 10,160 | |
Loss from Operations | | | (3,680 | ) | | | (3,359 | ) | | | (11,990 | ) | | | (9,952 | ) |
Other Income (Expense) | | | | | | | | | | | | |
(Loss) gain on investment in equity securities | | | — | | | | (48 | ) | | | 12 | | | | (123 | ) |
Interest income | | | 76 | | | | 143 | | | | 354 | | | | 277 | |
Total Other Income | | | 76 | | | | 95 | | | | 366 | | | | 154 | |
Income Tax Expense | | | — | | | | — | | | | (2 | ) | | | (2 | ) |
Net Loss | | $ | (3,604 | ) | | $ | (3,264 | ) | | $ | (11,626 | ) | | $ | (9,800 | ) |
Other Comprehensive Loss: | | | | | | | | | | | | |
Unrealized gain (loss) on available-for-sale debt
securities | | $ | — | | | $ | 3 | | | $ | (1 | ) | | $ | 3 | |
Comprehensive Loss | | $ | (3,604 | ) | | $ | (3,261 | ) | | $ | (11,627 | ) | | $ | (9,797 | ) |
Net loss per common share—basic and diluted | | $ | (0.40 | ) | | $ | (0.37 | ) | | $ | (1.31 | ) | | $ | (1.13 | ) |
Weighted average shares used in computing
net loss per common share—basic and
diluted | | | 9,115,455 | | | | 8,713,617 | | | | 8,850,881 | | | | 8,712,294 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
Organovo Holdings, Inc.
Unaudited Condensed Consolidated Statements of Stockholders’ Equity
(in thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Three and Nine Months Ended December 31, 2022 | |
| | Common Stock | | | | | | Treasury Stock | | | | | | | | | | |
| | Shares | | | Amount | | | Additional
Paid-in Capital | | | Shares | | | Amount | | | Accumulated
Deficit | | | Accumulated Other
Comprehensive
Income | | | Total | |
Balance at March 31, 2022 | | | 8,711 | | | $ | 9 | | | $ | 337,940 | | | | — | | | $ | (1 | ) | | $ | (307,739 | ) | | $ | — | | | $ | 30,209 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 660 | | | | — | | | | — | | | | — | | | | — | | | | 660 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,215 | ) | | | — | | | | (3,215 | ) |
Balance at June 30, 2022 (Unaudited) | | | 8,712 | | | $ | 9 | | | $ | 338,600 | | | | — | | | $ | (1 | ) | | $ | (310,954 | ) | | $ | — | | | $ | 27,654 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 673 | | | | — | | | | — | | | | — | | | | — | | | | 673 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,321 | ) | | | — | | | | (3,321 | ) |
Balance at September 30, 2022 (Unaudited) | | | 8,713 | | | $ | 9 | | | $ | 339,273 | | | | — | | | $ | (1 | ) | | $ | (314,275 | ) | | $ | — | | | $ | 25,006 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 544 | | | | — | | | | — | | | | — | | | | — | | | | 544 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,264 | ) | | | — | | | | (3,264 | ) |
Unrealized gain on available-for-sale debt securities | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3 | | | | 3 | |
Balance at December 31, 2022 (Unaudited) | | | 8,714 | | | $ | 9 | | | $ | 339,817 | | | | — | | | $ | (1 | ) | | $ | (317,539 | ) | | $ | 3 | | | $ | 22,289 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Three and Nine Months Ended December 31, 2023 | |
| | Common Stock | | | | | | Treasury Stock | | | | | | | | | | |
| | Shares | | | Amount | | | Additional
Paid-in Capital | | | Shares | | | Amount | | | Accumulated
Deficit | | | Accumulated Other
Comprehensive
Income | | | Total | |
Balance at March 31, 2023 | | | 8,717 | | | $ | 9 | | | $ | 340,317 | | | | — | | | $ | (1 | ) | | $ | (324,998 | ) | | $ | 2 | | | $ | 15,329 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 475 | | | | — | | | | — | | | | — | | | | — | | | | 475 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (4,028 | ) | | | — | | | | (4,028 | ) |
Balance at June 30, 2023 (Unaudited) | | | 8,718 | | | $ | 9 | | | $ | 340,792 | | | | — | | | $ | (1 | ) | | $ | (329,026 | ) | | $ | 2 | | | $ | 11,776 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 1 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation expense | | | — | | | | — | | | | 675 | | | | — | | | | — | | | | — | | | | — | | | | 675 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,994 | ) | | | — | | | | (3,994 | ) |
Unrealized loss on available-for-sale debt securities | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (1 | ) | | | (1 | ) |
Balance at September 30, 2023 (Unaudited) | | | 8,719 | | | $ | 9 | | | $ | 341,467 | | | | — | | | $ | (1 | ) | | $ | (333,020 | ) | | $ | 1 | | | $ | 8,456 | |
Issuance of common stock under employee and director stock
option, RSU, and purchase plans | | | 119 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock from public offering | | | 935 | | | | 1 | | | | 1,172 | | | | — | | | | — | | | | — | | | | — | | | | 1,173 | |
Stock-based compensation expense | | | 66 | | | | — | | | | 157 | | | | — | | | | — | | | | — | | | | — | | | | 157 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,604 | ) | | | — | | | | (3,604 | ) |
Balance at December 31, 2023 (Unaudited) | | | 9,839 | | | $ | 10 | | | $ | 342,796 | | | | — | | | $ | (1 | ) | | $ | (336,624 | ) | | $ | 1 | | | $ | 6,182 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
Organovo Holdings, Inc.
Unaudited Condensed Consolidated Statements of Cash Flows
(in thousands)
| | | | | | | | |
| | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023 | | | December 31, 2022 | |
Cash Flows From Operating Activities | | | | | | |
Net loss | | $ | (11,626 | ) | | $ | (9,800 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | |
(Gain) loss on investment in equity securities | | | (12 | ) | | | 123 | |
Accretion on investments | | | (128 | ) | | | (104 | ) |
Depreciation and amortization | | | 209 | | | | 234 | |
Stock-based compensation | | | 1,307 | | | | 1,877 | |
Increase (decrease) in cash resulting from changes in: | | | | | | |
Accounts receivable | | | 119 | | | | (76 | ) |
Prepaid expenses and other assets | | | 132 | | | | 18 | |
Accounts payable | | | 140 | | | | (173 | ) |
Accrued expenses | | | (2,121 | ) | | | 157 | |
Operating lease right-of-use assets and liabilities, net | | | (2 | ) | | | 69 | |
Net cash used in operating activities | | | (11,982 | ) | | | (7,675 | ) |
Cash Flows From Investing Activities | | | | | | |
Purchases of fixed assets | | | (42 | ) | | | (234 | ) |
Purchases of investments | | | (9,873 | ) | | | (9,893 | ) |
Maturities of investments | | | 10,000 | | | | 10,000 | |
Purchases of equity securities | | | — | | | | (1,061 | ) |
Sales of equity securities | | | — | | | | 384 | |
Liquidation of equity securities | | | 718 | | | | — | |
Net cash provided by (used in) investing activities | | | 803 | | | | (804 | ) |
Cash Flows From Financing Activities | | | | | | |
Proceeds from issuance of common stock, net | | | 1,173 | | | | — | |
Net cash provided by financing activities | | | 1,173 | | | | — | |
Net decrease in cash, cash equivalents, and restricted cash | | | (10,006 | ) | | | (8,479 | ) |
Cash, cash equivalents, and restricted cash at beginning of period | | | 15,444 | | | | 28,818 | |
Cash, cash equivalents, and restricted cash at end of period | | $ | 5,438 | | | $ | 20,339 | |
Reconciliation of cash, cash equivalents, and restricted cash to the condensed
consolidated balance sheets | | | | | | |
Cash and cash equivalents | | $ | 5,295 | | | $ | 20,196 | |
Restricted cash | | | 143 | | | | 143 | |
Total cash, cash equivalents, and restricted cash | | $ | 5,438 | | | $ | 20,339 | |
Supplemental Disclosure of Cash Flow Information: | | | | | | |
Income taxes paid | | $ | 2 | | | $ | 2 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
Organovo Holdings, Inc.
Notes to Unaudited Condensed Consolidated Financial Statements
Note1. Description of Business
Nature of Operations
Organovo Holdings, Inc. (“Organovo Holdings,” “Organovo,” and the advice“Company”) is a clinical stage biotechnology company that focuses on clinical drug development of the farnesoid X receptor (“FXR”) agonist FXR314. FXR is a qualified valuation expert.
Any stock options grantedmediator of gastrointestinal and liver diseases. FXR agonism has been tested in a variety of preclinical models of inflammatory bowel disease ("IBD"). FXR314 is the lead compound in the formCompany's established FXR program containing two clinically tested compounds (including FXR314) and over 2,000 discovery or preclinical compounds. FXR314 is a drug with safety and tolerability after daily oral dosing in Phase 1 and Phase 2 trials. Further, FXR314 has FDA clinical trial authorization for a Phase 2 trial in ulcerative colitis ("UC").
The Company’s current clinical focus is in advancing FXR314 in IBD, including UC and Crohn’s disease (“CD”). The Company plans to start a Phase 2a clinical trial in UC in the calendar year 2024.
A second focus of the Company is building high fidelity, 3D tissues that recapitulate key aspects of human disease. The Company uses its proprietary technology to build functional 3D human tissues that mimic key aspects of native human tissue composition, architecture, function and disease. Management believes these attributes can enable critical complex, multicellular disease models that can be used to develop clinically effective drugs across multiple therapeutic areas.
As with the clinical development program, the Company is initially focusing on the intestine and has ongoing 3D tissue development efforts in human tissue models of UC and CD. The Company uses these models to identify new molecular targets responsible for driving these diseases and to explore the mechanism of action of known drugs including FXR314 and related molecules. The Company intends to initiate drug discovery programs around these new validated targets to identify drug candidates for partnering and/or internal clinical development.
The Company’s current understanding of intestinal tissue models and IBD disease models leads it to believe that it can create models that provide greater insight into the biology of these diseases than are generally currently available. The Company is creating high fidelity disease models, leveraging its prior work including the work found in its peer-reviewed publication on bioprinted intestinal tissues (Madden et al. Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions. iScience. 2018 Apr 27;2:156-167. doi: 10.1016/j.isci.2018.03.015.) Organovo’s advances include cell type-specific compartments, prevalent intercellular tight junctions, and the formation of microvascular structures.
Using these disease models, the Company intends to identify and validate novel therapeutic targets. After finding therapeutic drug targets, the Company intends to focus on developing novel small molecule, antibody, or other therapeutic drug candidates to treat the disease, and advance these novel drug candidates towards an incentive stock optionInvestigational New Drug (“IND”) filing and potential future clinical trials.
The Company expects to broaden its work into additional therapeutic areas over time and is currently exploring specific tissues for development. In the Company’s work to identify the areas of interest, it evaluates areas that might be better served with 3D disease models than currently available models as well as the potential commercial opportunity. In line with these plans, the Company is building upon both its external and in house scientific expertise, which will be intendedessential to complyits drug development effort.
Except where specifically noted or the context otherwise requires, references to “Organovo Holdings”, “the Company”, and “Organovo” in these notes to the unaudited condensed consolidated financial statements refers to Organovo Holdings, Inc. and its wholly owned subsidiaries, Organovo, Inc., and Opal Merger Sub, Inc.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial information and the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not necessarily include all information and notes required by GAAP for complete financial statements. The condensed consolidated balance sheet at March 31, 2023 is derived from the Company’s audited consolidated balance sheet at that date.
The unaudited condensed consolidated financial statements include the accounts of Organovo and its wholly owned subsidiaries. All material intercompany accounts and transactions have been eliminated in consolidation. In the opinion of management, the unaudited financial information for the interim periods presented reflects all adjustments, which are only normal and recurring, necessary for a fair statement of the Company’s financial position, results of operations, stockholders’ equity and cash flows. These unaudited condensed consolidated financial statements should be read in conjunction with the requirements of Section 422audited financial statements and notes included in the Company’s Annual Report on Form 10-K for the year ended March 31, 2023, as filed with the Securities and Exchange Commission (“SEC”). Operating results for any interim period are not necessarily indicative of the Code. Only options granted to employees qualifyoperating results for incentive stock option treatment.any other interim period or the Company’s full fiscal year ending March 31, 2024 (see “Note 1. Description of Business”).
Liquidity and Going Concern
Each stock option shall expire at such timeThe accompanying consolidated financial statements have been prepared on the basis that we are a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. As of December 31, 2023, the Company had cash and cash equivalents of approximately $5.3 million, restricted cash of approximately $0.1 million and an accumulated deficit of approximately $336.6 million. The restricted cash was pledged as the Administrator shall determine at the time of grant. No stock option shall be exercisable later than the tenth anniversary of its grant. A stock option may be exercised in whole or in installments. A stock option may not be exercisablecollateral for a fractionletter of credit that the Company is required to maintain as a share. Sharessecurity deposit under the terms of the lease agreement for its facilities. The Company also had negative cash flows from operations of approximately $12.0 million during the nine months ended December 31, 2023.
Through December 31, 2023, the Company has financed its operations primarily through the sale of common stock purchased uponthrough public and at-the-market (“ATM”) offerings, the private placement of equity securities, from revenue derived from the licensing of intellectual property, products and research service-based services, grants, and collaborative research agreements, and from the sale of convertible notes. During the nine months ended December 31, 2023, the Company issued 934,621 shares of its common stock through its ATM facility.
Based on the Company's current operating plan and available cash resources, it will need substantial additional funding to support future operating activities. The Company has concluded that the prevailing conditions and ongoing liquidity risks faced by it raise substantial doubt about its ability to continue as a going concern for at least one year following the date these financial statements are issued. The accompanying consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern. As the Company continues its operations and is focusing its efforts on drug discovery and development, the Company will need to raise additional capital to implement this business plan. The Company cannot predict with certainty the exact amount or timing for any future capital raises. The Company will seek to raise additional capital through debt or equity financings, or through some other financing arrangement. However, the Company cannot be sure that additional financing will be available if and when needed, or that, if available, it can obtain financing on terms favorable to its stockholders. Any failure to obtain financing when required will have a material adverse effect on the Company’s business, operating results, and financial condition.
The preparation of the financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ from those estimates. On an ongoing basis, management reviews these estimates and assumptions.
Investments
Investments consist of investments in debt securities and investments in equity securities.
Investments in debt securities consist of investments in U.S. Treasury bills. All investments that have original maturities of three months or less are classified as cash equivalents on the Condensed Consolidated Balance Sheets. Prior to December 31, 2022, the Company classified certain investments as held-to-maturity. All investments previously classified as held-to-maturity matured prior to December 31, 2022. As of December 31, 2023 and March 31, 2023, all investments are classified as available-for-sale, as the sale of such investments may be required prior to maturity to implement management strategies. Available-for-sale debt securities are recorded at fair value. Any unrealized gains and losses are included in accumulated other comprehensive income as a component of stockholders' equity until realized. As U.S. Treasury bills have minimal risk, any declines in fair value are considered temporary.
Investments in equity securities consist of investments in the common stock of entities traded in active markets. The Company does not have the ability to exercise significant influence over any entities. Therefore, initial investments are recorded at cost, and are remeasured at fair value as of the balance sheet date. Any gains or losses resulting from the change in fair value are recorded in net income. The investments in equity securities are classified as current assets.
Fair value measurement
Financial assets and liabilities are measured at fair value, which is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The following is a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value:
•Level 1 — Quoted prices in active markets for identical assets or liabilities.
•Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Net Loss Per Share
Basic and diluted net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period. The weighted-average number of shares used to compute diluted loss per share excludes any assumed exercise of stock options, shares reserved for purchase under the Company’s 2016 Employee Stock Purchase Plan (“2016 ESPP”), the assumed vesting of restricted stock units (“RSUs”), and shares subject to repurchase as the effect would be anti-dilutive. No dilutive effect was calculated for each of the three and nine months ended December 31, 2023 and 2022 as the Company reported a net loss for each respective period and the effect would have been anti-dilutive.
Common stock equivalents excluded from computing diluted net loss per share due to their anti-dilutive effect were approximately 0.8 million at December 31, 2023 and 1.5 million at December 31, 2022.
Revenue recognition
The Company has generated revenues from payments received from licensing intellectual property.
The Company has entered into a license agreement with a company that includes the following: (i) non-refundable upfront fees and (ii) royalties based on specified percentages of net product sales, if any. At the initiation of the agreement, the Company has analyzed whether it results in a contract with a customer under Topic 606.
The Company has considered a variety of factors in determining the appropriate estimates and assumptions under these arrangements, such as whether the Company is a principal vs. agent, whether the elements are distinct performance obligations, whether there are determinable stand-alone prices, and whether any licenses are functional or symbolic. The Company has evaluated each performance obligation to determine if it can be satisfied and recognized as revenue at a point in time or over time. Typically, non-refundable upfront fees have been considered fixed, while sales-based royalty payments have been identified as variable consideration which must be evaluated to determine if it has been constrained and, therefore, excluded from the transaction price. Please refer to “Note 6: Collaborative Research, Development, and License Agreements” for further information.
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies. Unless otherwise stated, the Company believes that the impact of the recently issued accounting pronouncements that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption.
Note 3. Investments and Fair Value Measurement
Investments in debt securities
As of December 31, 2023, the Company held $2.0 million of investments in debt securities (which are included in the $5.3 million of cash and cash equivalents). For the three and nine months ended December 31, 2023, there was less than $0.1 million and $0.1 million, respectively, of interest income related to the investments in debt securities. As the investments in debt securities consist of U.S. Treasury bills from active markets, the fair value is measured using level 1 inputs.
The following table summarizes the Company's investments in debt securities that are measured at fair value as of December 31, 2023 (in thousands):
| | | | | | | | | | | | | | | | |
| | Amortized costs
basis | | | Gross unrealized gains | | | Gross unrealized losses | | | Fair value | |
As of March 31, 2023 | | | | | | | | | | | | |
Investment in debt securities | | $ | 4,943 | | | $ | 2 | | | $ | — | | | $ | 4,945 | |
As of December 31, 2023 | | | | | | | | | | | | |
Investment in debt securities | | $ | 1,995 | | | $ | — | | | $ | — | | | $ | 1,995 | |
Investments in equity securities
For the nine months ended December 31, 2023, there was $0.7 million of equity securities liquidated and less than a $0.1 million gain on the investment in equity securities. As of December 31, 2023, the fair value of investment in equity securities was zero, as a result of the liquidation of the shares by the underlying company on June 26, 2023. As the investment in equity securities consists of common stock from active markets, the fair value is measured using level 1 inputs.
The following table presents the activity for investments in equity securities measured at fair value for the nine months ended December 31, 2023 (in thousands):
| | | | |
| | Investment in Equity Securities
(in thousands) | |
Balance at March 31, 2023 | | $ | 706 | |
Liquidation of equity securities | | | (718 | ) |
Gain on investment in equity securities | | | 12 | |
Balance at December 31, 2023 | | $ | — | |
Note 4. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| | | | | | | | |
| | December 31,
2023 | | | March 31,
2023 | |
Accrued compensation | | $ | 439 | | | $ | 609 | |
Accrued legal and professional fees | | | 97 | | | | 193 | |
Acquired in-process research and development | | | — | | | | 2,000 | |
Other accrued expenses | | | 191 | | | | 46 | |
| | $ | 727 | | | $ | 2,848 | |
Note 5. Stockholders’ Equity
Preferred Stock
The Company is authorized to issue 25,000,000 shares of preferred stock. There are no shares of preferred stock currently outstanding, and the Company has no current plans to issue shares of preferred stock.
Common Stock
In March 2021, the Company's Board of Directors ("Board") approved the 2021 Inducement Equity Incentive Plan ("Inducement Plan"). The Inducement Plan authorized the issuance of up to 750,000 shares of common stock for awards of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, RSUs, performance units, performance shares, and other stock or cash awards. The only persons eligible to receive grants under the Inducement Plan are individuals who satisfy the standards for inducement grants under Nasdaq guidance. The Company also committed to reducing the aggregate number of shares of its common stock issuable pursuant to the Inducement Plan from 750,000 shares to 51,000 shares (which includes 50,000 shares of its common stock issuable pursuant to an outstanding option to purchase common stock with an exercise price of $2.75 per share, leaving only 1,000 shares available for future issuance under the Inducement Plan) and the share reserve was reduced accordingly effective October 12, 2022. As of December 31, 2023, there were 1,000 shares available for future grant under the Inducement Plan.
On October 12, 2022, the Company's stockholders and the Board approved the 2022 Equity Incentive Plan ("2022 Plan"), and it became effective on that date. The 2022 Plan replaced the Amended and Restated 2012 Equity Incentive Plan ("2012 Plan") on the effective date. Upon the effective date, the Company ceased granting awards under the 2012 Plan and any shares remaining available for future issuance under the 2012 Plan were cancelled and are no longer available for future issuance. The 2012 Plan continues to govern awards previously granted under it. At the time the Board approved the 2022 Plan, an aggregate of 1,363,000 shares of the Company’s common stock was initially reserved for issuance under the 2022 Plan. The Company committed to reducing the 2022 Plan share reserve by the number of shares that were granted under the 2012 Plan and the Inducement Plan between July 25, 2022 and October 12, 2022. From July 25, 2022 to October 12, 2022, the Company issued 126,262 shares of its common stock under the 2012 Plan. As a result, the number of shares initially reserved for future issuance under the 2022 Plan was 1,236,738 shares of common stock.
The Company previously had an effective shelf registration statement on Form S-3 (File No. 333-222929), declared effective by the SEC on February 22, 2018 (the “2018 Shelf”), which registered $100.0 million of common stock, preferred stock, warrants and units, or any combination of the foregoing, that expired on February 22, 2021. On January 19, 2021, the Company filed a shelf registration statement on Form S-3 (File No. 333-252224) to register $150.0 million of the Company’s common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the “2021 Shelf”). The 2021 Shelf was declared effective by the SEC on January 29, 2021 and replaced the 2018 Shelf at that time. On January 26, 2024, the Company filed a new shelf registration statement on Form S-3 (File No. 333-276722) to register $150.0 million of the Company’s common stock, preferred stock, debt securities, warrants and units, or any combination of the foregoing (the “2024 Shelf”). The 2024 Shelf has not yet been declared effective by the SEC.
On March 16, 2018, the Company entered into a Sales Agreement (“Sales Agreement”) with H.C. Wainwright & Co., LLC and Jones Trading Institutional Services LLC (each an “Agent” and together, the “Agents”). On January 29, 2021, the Company filed a prospectus supplement to the 2021 Shelf (the “2021 ATM Prospectus Supplement”), pursuant to which the Company may offer and sell, from time to time, through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $50.0 million. Any shares offered and sold will be issued pursuant to the 2021 Shelf until it is replaced by the 2024 Shelf. During each of the three and nine months ended December 31, 2023, the Company issued 934,621 shares of common stock in ATM offerings under the 2021 ATM Prospectus Supplement. As of December 31, 2023, the Company had sold an aggregate of 2,515,483 shares of common stock in ATM offerings, with gross proceeds of approximately $22.9 million. As of December 31, 2023, there was approximately $100.0 million available for future offerings under the 2021 Shelf (excluding amounts available but not yet issued under the ATM Prospectus Supplement), and approximately $27.1 million available for future offerings through the Company’s ATM program under the 2021 ATM Prospectus Supplement. On January 26, 2024, the Company filed a prospectus to the 2024 Shelf (the “2024 ATM Prospectus”), pursuant to which the Company may offer and sell, from time to time, through the Agents, shares of its common stock in ATM sales transactions having an aggregate offering price of up to $2,605,728. Any shares offered and sold in these ATM sales transactions will be issued pursuant to the 2024 Shelf.
Restricted Stock Units
The following table summarizes the Company’s RSUs activity for the nine months ended December 31, 2023:
| | | | | | | | |
| | Number of
Shares | | | Weighted
Average Price | |
Unvested at March 31, 2023 | | | 127,717 | | | $ | 2.22 | |
Granted | | | 117,642 | | | $ | 1.39 | |
Vested | | | (121,467 | ) | | $ | 1.81 | |
Cancelled / forfeited | | | — | | | $ | — | |
Unvested at December 31, 2023 | | | 123,892 | | | $ | 1.84 | |
Stock Options
During the three and nine months ended December 31, 2023, under the 2022 Plan, 40,000 and 171,257 stock options were granted at various exercise prices, respectively.
On August 28, 2023, the Company's Executive Chairman voluntarily forfeited 462,500 outstanding stock options, of which 312,918 were unvested and therefore cancelled, and 149,582 were vested and therefore expired. The forfeited stock option awards were not replaced by other awards or other compensation and there is no plan to replace the forfeited awards. Therefore, all previous unrecognized compensation expense associated with the forfeited awards, approximately $519,000, was recognized as a selling, general, and administrative expense on the date of forfeiture.
The following table summarizes the Company’s stock option activity from March 31, 2023 to December 31, 2023:
| | | | | | | | | | | | |
| | Options
Outstanding | | | Weighted
Average
Exercise Price | | | Aggregate
Intrinsic
Value | |
Outstanding at March 31, 2023 | | | 1,451,217 | | | $ | 6.49 | | | $ | 38,327 | |
Options granted | | | 171,257 | | | $ | 1.72 | | | $ | — | |
Options cancelled / forfeited | | | (593,384 | ) | | $ | 7.10 | | | $ | — | |
Options expired | | | (333,631 | ) | | $ | 7.42 | | | $ | — | |
Outstanding at December 31, 2023 | | | 695,459 | | | $ | 4.35 | | | $ | — | |
Vested and Exercisable at December 31, 2023 | | | 381,029 | | | $ | 5.83 | | | $ | — | |
The weighted average remaining contractual term of stock options exercisable and outstanding at December 31, 2023 was approximately 7.36 years.
Employee Stock Purchase Plan
In June 2016,the Board adopted, and in August 2016, the Company’s stockholders subsequently approved, the 2016 ESPP. The Company reserved 75,000 shares of common stock for issuance thereunder. As of October 31, 2023, the 2016 ESPP was replaced by the 2023 ESPP (as defined below).
In July 2023, the Board adopted, and on October 31, 2023, the Company's stockholders subsequently approved, the 2023 Employee Stock Purchase Plan (the "2023 ESPP"). The 2023 ESPP became effective on October 31, 2023 and replaced the 2016 ESPP on that date. The Company reserved 45,000 shares of common stock for issuance thereunder. The 2023 ESPP permits employees to purchase common stock through payroll deductions, limited to 15 percent of each employee’s compensation up to $25,000 per employee per year or 500 shares per employee per six-month purchase period. Shares under the 2023 ESPP are purchased at 85 percent of the fair market value at the lower of (i) the closing price on the first trading day of the six-month purchase period or (ii) the closing price on the last trading day of the six-month purchase period. The initial offering under the 2023 ESPP will commence in March 2024.
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consisted of the following at December 31, 2023:
| | | | |
Common stock issuable pursuant to options outstanding and reserved under the 2012 Plan |
|
| 440,591 |
|
Common stock reserved under the 2012 Plan |
|
| — |
|
Common stock issuable pursuant to options outstanding and reserved under the 2022 Plan |
|
| 204,868 |
|
Common stock reserved under the 2022 Plan |
|
| 1,643,798 |
|
Common stock reserved under the 2023 ESPP |
|
| 45,000 |
|
Common stock reserved under the 2021 Inducement Equity Plan |
|
| 1,000 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2012 Plan |
|
| 6,250 |
|
Common stock issuable pursuant to restricted stock units outstanding under the 2022 Plan |
|
| 117,642 |
|
Common stock issuable pursuant to options outstanding and reserved under the Inducement Plan |
|
| 50,000 |
|
Total at December 31, 2023 |
|
| 2,509,149 |
|
Stock-based Compensation Expense and Valuation Information
Stock-based awards include stock options and RSUs under the 2022 Plan, 2012 Plan, Inducement Plan, and rights to purchase stock under the 2023 ESPP. The Company calculates the grant date fair value of all stock-based awards in determining the stock-based compensation expense.
Stock-based compensation expense for all stock-based awards consists of the following (in thousands):
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | Three Months Ended | | | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023 | | | December 31, 2022 | | | December 31, 2023 | | | December 31, 2022 | |
Research and development | | $ | 40 | | | $ | 117 | | | $ | 87 | | | $ | 363 | |
General and administrative | | | 117 | | | | 427 | | | | 1,220 | | | | 1,514 | |
Total | | $ | 157 | | | $ | 544 | | | $ | 1,307 | | | $ | 1,877 | |
The total unrecognized compensation cost related to unvested stock option grants as of December 31, 2023 was approximately $0.6 million and the weighted average period over which these grants are expected to vest is 2.30 years.
The total unrecognized compensation cost related to unvested RSUs as of December 31, 2023 was $0.2 million, which will be recognized over a weighted average period of 0.97 years.
The Company uses either the Black-Scholes or Monte Carlo option-pricing models to calculate the fair value of stock options, depending on the complexity of the equity grants. Stock-based compensation expense is recognized over the vesting period using the straight-line method. The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses the Company-specific historical volatility rate as the indicator of expected volatility. The risk-free interest rate assumption is based on U.S. Treasury rates. The weighted average expected life of options was estimated using the average of the contractual term and the weighted average vesting term of the options. The measurement and classification of share-based payments to non-employees is consistent with the measurement and classification of share-based payments to employees. The fair value of stock options was estimated at the grant date using the following weighted average assumptions:
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | Three Months Ended | | | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023 | | | December 31, 2022 | | | December 31, 2023 | | | December 31, 2022 | |
Dividend yield | | | — | | | | — | | | | — | | | | — | |
Volatility | | | 100.31 | % | | | 95.93 | % | | | 98.93 | % | | | 95.43 | % |
Risk-free interest rate | | | 4.66 | % | | | 3.97 | % | | | 4.12 | % | | | 3.21 | % |
Expected life of options | | 6.00 years | | | 6.00 years | | | 6.00 years | | | 6.00 years | |
Weighted average grant
date fair value | | $ | 1.16 | | | $ | 1.23 | | | $ | 1.38 | | | $ | 1.96 | |
The fair value of each RSU is recognized as stock-based compensation expense over the vesting term of the award. The fair value is based on the closing stock price on the date of the grant.
The Company uses the Black-Scholes valuation model to calculate the fair value of shares issued pursuant to the 2016 ESPP and the 2023 ESPP. Stock-based compensation expense is recognized over the purchase period using the straight-line method. The fair value of ESPP shares was estimated at the purchase period commencement date using the following assumptions:
| | | | | | | | | | | | | | | | |
| | Three Months Ended | | | Three Months Ended | | | Nine Months Ended | | | Nine Months Ended | |
| | December 31, 2023* | | | December 31, 2022* | | | December 31, 2023* | | | December 31, 2022 | |
Dividend yield | | | — | | | | — | | | | — | | | | — | |
Volatility | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % | | | 86.58 | % |
Risk-free interest rate | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % | | | 3.34 | % |
Expected term | | | — | | | | — | | | | — | | | 6 months | |
Grant date fair value | | $ | — | | | $ | — | | | $ | — | | | $ | 0.82 | |
*There were no participants in the 2016 ESPP or the 2023 ESPP for the purchase periods beginning September 1, 2022, March 1, 2023 or September 1, 2023.
The assumed dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future. The Company uses the Company-specific historical volatility rate as the indicator of expected volatility. The risk-free interest rate assumption is based on U.S. Treasury rates. The expected life is the 6-month purchase period.
Note 6. Collaborative Research, Development, and License Agreements
License Agreements
From June 2021 to February 2022, certain patents owned or sublicensed by the Company became the subject of inter partes review proceedings filed by Cellink AB and its subsidiaries (collectively, “BICO Group AB”). The Company and BICO Group AB were also engaged in litigation regarding patent infringement during the same time period. On February 22, 2022, the Company and BICO Group AB signed a settlement and patent license agreement (“License Agreement”) to close all matters noted above. In addition to closing all legal matters and patent disputes noted above, as part of the agreement, the Company agreed to grant a non-exclusive license to BICO Group AB to use the Company’s aforementioned patents for its business operations of manufacturing and selling bioprinters as well as bioinks. The Company concluded that the nature of the license granted represents functional intellectual property.
As part of the License Agreement, BICO Group AB agreed to pay the Company ongoing sales-based royalties (based on percentages of BICO Group AB’s net sales) for the use of the granted license. The sales-based royalties became effective beginning on February 22, 2022, the effective date of the License Agreement, and continue until the expiration of the last surviving licensed patent. As the sales-based royalties are required to be paid 45 days after the end of every quarter, there is variable consideration that must be paidestimated to determine royalty revenue within a given reporting period. Once actual revenue earned is determined in the following fiscal quarter, an adjustment is made from the previously estimated amount. The Company estimated royalty revenue of $33,000 for the three months ended December 31, 2023. However, there was a decrease adjustment of approximately $28,000 relating to the prior quarter. For the three and nine months ended December 31, 2023, the Company recorded $5,000 and $80,000 of royalty revenue based on sales-based royalties from the License Agreement, respectively.
Also as part of the License Agreement, certain patents involved in full at the time of exercise in cash or such other consideration determinedagreement are sublicensed by the Administrator.Company from the University of Missouri and Clemson University. See below for further information.
Stock Appreciation Rights. A stock appreciation right (“SAR”University of Missouri
In March 2009, the Company entered into a license agreement with the Curators of the University of Missouri ("University of Missouri") to in-license certain technology and intellectual property relating to self-assembling cell aggregates and intermediate cellular units. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay the rightUniversity of Missouri royalties ranging from 1% to receive a payment equal to the excess3% of net sales of covered tissue products, and of the fair market value of a specified number of shares of common stockcovered tissues transferred internally for use in the Company’s commercial service business, depending on the datelevel of net sales achieved by the SARCompany each year.
The license agreement with the University of Missouri also includes an additional sales royalty of 3% of all revenue received from a sublicensee, when such sublicense is exercised over the exercise priceentered pursuant to settlement of the SAR. The exercise price for each SARlitigation. Such revenue shall include, but not be limited to, all option fees, license issue fees (up-front payments), license maintenance fees, equity, and all royalty payments. Such revenue shall not include research funding provided to licensee by sublicensee. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. No royalty expense related to sales-based royalties has been recorded to date.
On December 5, 2022, the Company amended the license agreement with the University of Missouri, where the Company agreed to pay a single, up-front payment of $50,000 to the University of Missouri in exchange for the aforementioned licensed intellectual property to be lessfully paid up by the Company. As a result, the Company will continue to have rights to the licensed intellectual property until its expiration, but will no longer owe minimum annual royalty payments, royalty payments based on net sales, or any other payments (other than 100%patent annuities and any prosecution costs) in the future.
Clemson University
In May 2011, the Company entered into a license agreement with Clemson University Research Foundation ("CURF") to in-license certain technology and intellectual property relating to ink-jet printing of viable cells. The Company received the exclusive worldwide rights to commercialize products comprising this technology for all fields of use. The Company is required to pay CURF royalties ranging from 1.5% to 3% of net sales of covered tissue products and the fair market value of covered tissues transferred internally for use in the common stockCompany’s commercial service business, depending on the level of net sales reached each year. The license agreement terminates upon expiration of the patents licensed, which are expected to expire in May 2024, and is subject to certain conditions as defined in the license agreement. Minimum annual royalty payments of $20,000 were due for two years beginning in calendar 2014, and $40,000 per year beginning in calendar 2016. Royalty payments of $40,000 were made in each of the years ended March 31, 2023 and 2022. The annual minimum royalty is creditable against royalties owed during the same calendar year.
In addition to the annual royalties noted above, CURF is owed 40% of all payments including but not limited to, upfront payments, license fees, issue fees, maintenance fees, and milestone payments received from third parties, including sublicensees, in consideration for sublicensing rights to licensed products. However, per the agreement, in the event that the Company defends the technology by litigation, it can offset any royalties due by legal expenses incurred. As of December 31, 2023, the Company’s legal expenses exceeded royalties owed from the upfront payment and sales-based royalties related to the license agreement. Therefore, no royalty expense to CURF was recorded for the nine months ended December 31, 2023. No royalty expense related to sales-based royalties has been recorded to date.
Note 7. Commitments and Contingencies
Legal Matters
In addition to commitments and obligations in the ordinary course of business, the Company may be subject, from time to time, to various claims and pending and potential legal actions arising out of the normal conduct of its business.
The Company assesses contingencies to determine the degree of probability and range of possible loss for potential accrual in its financial statements. Because litigation is inherently unpredictable and unfavorable resolutions could occur, assessing litigation contingencies is subjective and requires judgments about future events. When evaluating contingencies, the Company may be unable to provide a meaningful estimate due to a number of factors, including the procedural status of the matter in question, the presence of complex or novel legal theories, and/or the ongoing discovery and development of information important to the matters. In addition, damage amounts claimed in litigation against it may be unsupported, exaggerated or unrelated to possible outcomes, and as such are not meaningful indicators of its potential liability.
The Company regularly reviews contingencies to determine the adequacy of its accruals and related disclosures. During the period presented, the Company has not recorded any accrual for loss contingencies associated with any claims or legal proceedings; determined that an unfavorable outcome is probable or reasonably possible; or determined that the amount or range of any possible loss is reasonably estimable. However, the outcome of legal proceedings and claims brought against the Company is subject to significant uncertainty. Therefore, although management considers the likelihood of such an outcome to be remote, if one or more legal matters were resolved against the Company in a reporting period, the Company’s consolidated financial statements for that reporting period could be materially adversely affected.
Note 8. Leases
After the initial adoption of Accounting Standards Codification Topic 842 (“ASC 842”), on an on-going basis, the Company evaluates all contracts upon inception and determines whether the contract contains a lease by assessing whether there is an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration over a period of time. If a lease is identified, the Company will apply the guidance from ASC 842 to properly account for the lease.
Operating Leases
On November 23, 2020, the Company entered into a lease agreement, pursuant to which the Company permanently leased approximately 8,051 square feet of lab and office space (the “Permanent Lease”) in San Diego once certain tenant improvements were completed by the landlord and the premises were ready for occupancy. Additionally, on November 17, 2021, the Permanent Lease was amended to add an additional 2,892 square feet of office space in the same building. The Permanent Lease commenced on December 17, 2021 and is intended to serve as the Company’s permanent premises for approximately sixty-two months. Monthly rental payments will be approximately $40,800 with 3% annual escalators.
The Company determined that the Permanent Lease is considered an operating lease under ASC 842, and therefore upon the lease commencement date of December 17, 2021, recognized lease liabilities and corresponding right-of-use assets of $2.3 million. The Company records operating lease expense on a straight-line basis over the life of the lease (referred to as “operating lease expense”). Variable lease expenses associated with the Company’s leases, such as payments for additional monthly fees to cover the Company’s share of certain facility expenses (common area maintenance) are expensed as incurred.
The table below summarizes the Company’s lease liabilities and corresponding right-of-use assets as of December 31, 2023 (in thousands except the year and percentage):
| | | | |
| | December 31, 2023 | |
ASSETS | | | |
Operating lease right-of-use assets | | $ | 1,403 | |
Total lease right-of-use assets | | $ | 1,403 | |
| | | |
LIABILITIES | | | |
Current | | | |
Operating lease liability | | $ | 502 | |
Noncurrent | | | |
Operating lease liability, net of current portion | | $ | 999 | |
Total lease liabilities | | $ | 1,501 | |
| | | |
Weighted average remaining lease term: | | 3.08 | |
Weighted average discount rate: | | | 6 | % |
Variable lease expense was approximately $39,000 and $114,000 for the three and nine months ended December 31, 2023, respectively, and approximately $34,000 and $110,000 for the three and nine months ended December 31, 2022, respectively. Operating lease expense was approximately $125,000 and $377,000 for the three and nine months ended December 31, 2023, respectively, and approximately $114,000 and $373,000 for the three and nine months ended December 31, 2022, respectively.
Cash flows associated with the Company’s operating lease for the three and nine months ended December 31, 2023, were approximately $125,000 and $377,000, respectively, and approximately $59,000 and $304,000 for the three and nine months ended December 31, 2022, respectively.
Future lease payments relating to the Company’s operating lease liabilities as of December 31, 2023, are as follows (in thousands):
| | | | |
Fiscal year ending March 31, 2024 | | $ | 129 | |
Fiscal year ending March 31, 2025 | | | 523 | |
Fiscal year ending March 31, 2026 | | | 538 | |
Fiscal year ending March 31, 2027 | | | 460 | |
Total future lease payments | | | 1,650 | |
Less: Imputed interest | | | (149 | ) |
Total lease obligations | | | 1,501 | |
Less: Current obligations | | | (502 | ) |
Noncurrent lease obligations | | $ | 999 | |
Note 9. Concentrations
Credit risk and significant customers
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of temporary cash investments. The Company maintains cash balances at various financial institutions located within the United States. Accounts at these institutions are secured by the Federal Deposit Insurance Corporation. Balances may exceed federally insured limits. The Company is also potentially subject to concentrations of credit risk in its revenues and accounts receivable. However, the Company only receives royalty revenue from one licensee and has not historically experienced any accounts receivable write-downs.
Note 10. Related Parties
From time to time, the Company will enter into an agreement with a related party in the ordinary course of its business. These agreements are ratified by the Board or a committee thereof pursuant to its related party transaction policy.
Viscient Biosciences (“Viscient”) is an entity for which Keith Murphy, the Company’s Executive Chairman, serves as the Chief Executive Officer and President. Dr. Jeffrey Miner, the Company’s former Chief Scientific Officer, is also the Chief Scientific Officer of Viscient, and Thomas Jurgensen, the Company’s former General Counsel, previously served as outside legal counsel to Viscient through his law firm, Optima Law Group, APC.
On December 28, 2020, the Company entered into an intercompany agreement (the “Intercompany Agreement”) with Viscient and Organovo, Inc., the Company’s wholly-owned subsidiary, which included an asset purchase agreement for certain lab equipment. Pursuant to the Intercompany Agreement, the Company agreed to provide Viscient certain services related to 3D bioprinting technology, which include, but are not limited to, histology services, cell isolation, and proliferation of cells and Viscient agreed to provide the Company certain services related to 3D bioprinting technology, including bioprinter training, bioprinting services, and qPCR assays, in each case on payment terms specified in the Intercompany Agreement and as may be further determined by the parties. In addition, the Company and Viscient each agreed to share certain facilities and equipment and, subject to further agreement, to each make certain employees available for specified projects for the other party at prices to be determined in good faith by the parties. The Company evaluated the accounting for the Intercompany Agreement and concluded that any services provided by Viscient to the Company will be expensed as incurred, and any compensation for services provided by the Company to Viscient will be considered a reduction of personnel related expenses. Any services provided to Viscient do not fall under Topic 606 as the Intercompany Agreement is not a contract with a customer. For the three and nine months ended December 31, 2023 and 2022, the Company incurred no consulting expenses from Viscient. Additionally, for the three and nine months ended December 31, 2023, the Company provided approximately $3,000 and $13,000 of histology services to Viscient, respectively, and $17,000 and $44,000 for the three and nine months ended December 31, 2022, respectively.
Note 11. Restructuring
On August 18, 2023, the Company announced to its employees a plan to reduce the Company’s workforce, effective August 25, 2023, by approximately six employees, which represented approximately 24% of its employees as of August 18, 2023. The Company has refocused operations on FXR314, its clinical drug candidate. This decision to reduce the Company’s workforce was made in order to focus spending on the Company’s clinical program for FXR314, reduce ongoing operating expenses not related to clinical expenses, and extend the Company’s cash runway. The Company estimates that it will incur approximately $0.4 million of cash expenditures in connection with the reduction in force, which relate to severance pay, and are expected to be incurred through the quarter ending March 31, 2024. The Company anticipates annual cost savings of $1.5 million resulting from the reduction in force.
Approximately $0.4 million of restructuring charges were recorded during the nine months ended December 31, 2023, and no restructuring charges were recorded during the three months ended December 31, 2023.
| | | | | | | | |
| | Three Months Ended | | | Nine Months Ended | |
(in thousands) | | December 31, 2023 | | | December 31, 2023 | |
Severance for Involuntary Employee Terminations | | $ | — | | | $ | 380 | |
Total Restructuring Expense | | $ | — | | | $ | 380 | |
The following table summarizes the activity and balances of the restructuring reserve (in thousands):
| | | | |
| | Severance for Involuntary
Employee Terminations | |
Balance at March 31, 2023 | | $ | — | |
Increase to reserve | | | 380 | |
Utilization of reserve: | | | |
Payments | | | (263 | ) |
Balance at December 31, 2023 | | $ | 117 | |
Note 12. Subsequent Events
Between January 1, 2024 and the date of grant, and the termfiling of an SAR shall be no more than ten years fromthis Quarterly Report on Form 10-Q, the date of grant. At the discretion of the Administrator, the payment upon an SAR exercise may be in cash, in shares equivalent thereof, or in some combination thereof.
Upon exercise of an SAR, the participant shall be entitled to receive payment from Pubco in an amount determined by multiplying the excess of the fair market value of a share of common stock on the date of exercise over the exercise price of the SAR by the number of shares with respect to which the SAR is exercised.
Restricted Stock and Restricted Stock Units. Restricted stock and restricted stock units may be awarded or sold to participants under such terms and conditions as shall be established by the Administrator. Restricted stock and restricted stock units shall be subject to such restrictions as the Administrator determines, including a prohibition against sale, assignment, transfer, pledge or hypothecation, and a requirement that the participant forfeit such shares or units in the event of termination of employment. A restricted stock unit provides a participant the right to receive payment at a future date after the lapse of restrictions or achievement of performance criteria or other conditions determined by the Administrator.
Performance Stock. The Administrator shall designate the participants to whom long-term performance stock/units are to be awarded and determine the number of shares, the length of the performance period and the other vesting terms and conditions of each such award. Each award of performance stock/units shall entitle the participant to a payment in the form of shares/units of common stock upon the attainment of performance goals and other vesting terms and conditions specified by the Administrator. The Administrator may, in its discretion, make a cash payment equal to the fair market value ofCompany issued 201,319 shares of common stock otherwise required to be issued to a participant pursuant to a Performance Stock Award.
All awards madein ATM offerings under the 2012 Plan may be subject2021 ATM Prospectus Supplement for net proceeds of approximately $0.2 million.

Up to vesting[________] shares of Common Stock
Up to [________] Pre-Funded Warrants to Purchase up to [________] Shares of Common Stock
Up to [________] Shares of Common Stock Underlying the Pre-Funded Warrants
Common Stock
PROSPECTUS

[_________] , 2024
Part II
Information Not Required in the Prospectus
Item 13. Other Expenses of Issuance and other contingencies as determined by the Administrator and will be evidenced by agreements approved by the Administrator which set forth the terms and conditions of each award.Distribution.
Duration, Amendment, and Termination
Unless sooner terminated by the Board, the 2012 Plan will terminate ten years after its adoption. The Board may amend, alter, suspend or terminate the 2012 Plan at any time or from time to time without stockholder approval or ratification, unless necessary and desirable to comply with applicable law. However, before an amendment may be made that would adversely affect a participant who has already been granted an award, the participant’s consent must be obtained.
2011 Director Compensation
The following table sets forth compensation earnedan estimate of the fees and paid to each non-employee director for service as a director during 2011.
| | | | | | | | | | | | | | | | | | | | |
| Name | | Fees Earned or
Paid in Cash
($) | | | Stock
Awards
($) | | | Option
Awards
($) | | | All Other
Compensation
($) | | | Total
($) | |
Robert Baltera, Jr. (1) | | | — | | | $ | 2,898 | | | | — | | | | — | | | $ | 2,898 | |
Andras Forgacs (2) | | | — | | | | — | | | | — | | | | — | | | | — | |
Gabor Forgacs (3) | | | — | | | | — | | | | — | | | | — | | | | — | |
(1) | In October, 2009 we entered into a Memorandum of Understanding with Robert Baltera, Jr. in connection with his ongoing service as one of our directors. Pursuant to this arrangement we granted Mr. Baltera 36,228 shares of restricted Common Stock, which vest in four equal annual installments, commencing one year from the date of grant, provided Mr. Baltera remains a director on the applicable vesting date. In October 2011 we additionally granted Mr. Baltera 32,423 shares of restricted Common Stock, one quarter of which vested that month and the remainder of which will vest in three equal annual installments. Our arrangement with Mr. Baltera is terminable at will by either party. |
(2) | In February, 2008 we issued 60,365 shares of restricted Common Stock to Andras Forgacs as compensation for his services as a director. These shares vested to the extent of 25% of the original grant on the first anniversary of the grant date, and thereafter at the rate of 6.25% of the original grant on a quarterly basis, provided that Mr. Forgacs remains a director on the applicable vesting date. |
(3) | Gabor Forgacs resigned as a director effective February 8, 2012. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following tables set forth certain information regarding the beneficial ownership of our common stock as of June 1, 2012 by (i) each person who, to our knowledge, owns more than 5% of our common stock; (ii) each of our directors and named executive officers; and (iii) all of our executive officers and directors as a group. Unless otherwise indicated in the table or the footnotesexpenses relating to the following table, each person named in the table has sole votingissuance and investment power and that person’s address is c/o Organovo Holdings, Inc., 5871 Oberlin Drive, Suite 150, San Diego, CA 92121. Shares of Common Stock subject to options or warrants currently exercisable or exercisable within 60 days of June 1, 2012 are deemed outstanding for computing the share ownership and percentage of the person holding such options and warrants, but are not deemed outstanding for computing the percentage of any other person. Applicable percentages are based on 43,693,241 shares of common stock outstanding as of June 1, 2012.
| | | | | | | | |
| Name and address of Beneficial Owner | | No. Shares of Common Stock Beneficially Owned | | | Percent of Common Stock Outstanding | |
Keith Murphy (1) | | | 6,311,092 | (2) | | | 14.4 | % |
| | |
Gabor Forgacs | | | 6,057,741 | (3) | | | 13.9 | % |
| | |
Andras Forgacs (1) | | | 766,588 | | | | 1.8 | % |
| | |
Robert Baltera, Jr. (1) | | | 126,392 | (4) | | | * | |
| | |
Barry D. Michaels (1) | | | 20,000 | (8) | | | * | |
| | |
Sharon Collins Presnell (1) | | | 224,064 | (9) | | | * | |
| | |
Eric Michael David (1) | | | 814,306 | (10) | | | 1.9 | % |
| | |
Adam K. Stern (1)(5) c/o Spencer Trask Ventures 750 Third Avenue New York, NY 10017 | | | 1,604,484 | | | | 4.0 | % |
| | |
Kevin Kimberlin (6) 1700 East Putnam Avenue Suite 401 Greenwich, CT 06870 | | | 5,489,433 | | | | 11.4 | % |
| | |
All directors and executive officers as a group (7 persons) | | | 10,025,796 | (7) | | | 22.9 | % |
(1) | Executive officer and/or director. |
(2) | 255,255 of these shares are held by Equity Trust Co., Custodian FBO Keith Murphy IRA. Includes warrants to purchase 30,000 shares of Common Stock at an exercise price of $1.00 per share. |
(3) | Includes warrants to purchase 3,750 shares of Common Stock at an exercise price of $1.00 per share. |
(4) | 18,114 of these shares vested in or before October, 2011. Includes warrants to purchase 28,000 shares of Common Stock at an exercise price of $1.00 per share. |
(5) | Represents (i) 741,395 shares owned by Adam Stern, (ii) 360,000 shares underlying warrants owned by Adam Stern; (iii) 158,870 shares owned by ST Neuroscience Partners, LLC; (iv) 211,827 shares owned by Pavilion Capital Partners, LLC; and (v) 132,392 shares owned by Piper Venture Partners, LLC. Does not include shares underlying warrants held by the Placement Agent or its affiliates issued in connection with the Bridge Financing or the Offering. |
(6) | Represents (i) 1,082,489 shares held by Spencer Trask Investment Partners, LLP and (ii) 4,406,943 shares underlying warrants owned by Spencer Trask Ventures, Inc. issued in connection with the Bridge Financing or the Offering. |
(7) | Includes warrants to purchase 448,000 shares of Common Stock at an exercise price of $1.00 per share. Does not include shares underlying warrants issued to the Placement Agent in connection with the Bridge Financing or the Offering. |
(8) | Includes warrants to purchase 10,000 shares of Common Stock at an exercise price of $1.00 per share. |
(9) | Stock option shares that will vest within 60 days of June 1, 2012. 672,192 additional shares are subject to future vesting. 224,064 shares will vest on the first, second, third, and fourth anniversary of Dr. Presnell’s hire, May 4, 2011. |
(10) | Includes warrants to purchase 20,000 shares of Common Stock at an exercise price of $1.00 per share. |
Changes in Control
We are not aware of any or a party to arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in a change of control.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Pubco Shareholders
Forward Split, Split-Off and Share Cancellation
Real Estate Restoration and Rental, Inc.’s (“RERR”) common stock was forward-split on a 10.5913504 for 1 basis, with a record date of January 23, 2012 and an effective date of January 31, 2012. As a result of this stock split and the Reincorporation Merger, there were approximately 6,000,000 shares of Pubco’s common stock issued and outstanding before taking into account the issuance of shares of common stock to purchasers of Units in the Offering and in the Merger and after giving pro forma effect to the Split-Off, as discussed below.
Upon the closing of the Merger, Pubco transferred all of its operating assets and liabilities to Organovo Split Corp., a Delaware corporation (“PSOS”), and split-off PSOS (the “Split-Off”) through the sale of all of the outstanding capital stock of PSOS to its executive officers, directors and their affiliates (the” Split-Off Shareholders”). In connection with the Split-Off, 5,000,000 shares of common stock held by the Split-Off Shareholders were surrendered and cancelled without further consideration, other than the receipt of PSOS shares. An additional 1,236,000 shares of common stock were cancelled by other shareholders of Pubco for no or nominal consideration.
Transactions with the Placement Agent and its Related Parties
We retained Spencer Trask Ventures, Inc. to serve as our placement agent (the “Placement Agent”) in connection with the Bridge Financing (as defined below), the Merger and the Offering as described in this prospectus. Adam K. Stern, one of our directors, is a Senior Managing Director of the Placement Agent.
The Placement Agent acted as finder to Organovo in connection with our bridge financing, in which Organovo issued $1,500,000 in principal amount of its 6% convertible promissory notes due March 31, 2012 (the “Bridge Notes”) and warrants to purchase an aggregate of 1,500,000 shares of Organovo’s common stock at a price of $1.00 per share (the “Bridge Warrants”) to accredited investors (the “Bridge Financing”). The Placement Agent was issued warrants to purchase Organovo warrants that automatically converted into warrants to purchase 20% of the shares of Pubco Common Stock underlying the Units issued upon the conversion of the Bridge Notes in the Offering (as defined below) at a price of $1.00 per share per share as compensation for acting as a finder in the Bridge Financing. These warrants were exchanged at the initial close of the Offering for warrants (which are identical to the Placement Agent Warrants (as defined below) discussed below) to purchase 610,155 shares of common stock at an exercise price of $1.00 per share.
Prior to the initial closing of the Offering, several related parties to the Placement Agent purchased an aggregate of 219,705 shares of Pubco’s common stock (2,326,974 shares on a post stock split adjusted basis) from various shareholders of Pubco. The aggregate purchase price paid to such shareholders by the related parties for such shares was approximately $155,000. All of the foregoing shares of common stock are subject to a lock-up agreement. See “Lock-ups” below.
We engaged the Placement Agent as our exclusive placement agent in connection with a the Offering. For its services, we paid the Placement Agent (i) a cash fee equal to 10% of the gross proceeds raised in the Offering and (ii) a non-accountable expense allowance equal to 3% of the gross proceeds raised in the Offering. In addition, we granted to the Placement Agent or its designees, for nominal consideration, five-year warrants (“Placement Agent Warrants”) to purchase shares of common stock at an exercise price of $1.00 per share. The placement agent and its selected dealers were paid total cash commissions of $1,372,260 and the Placement Agent was paid an expense allowance of $411,678 and was issued Placement Agent Warrants to purchase 6,099,195 shares of common stock (including 610,155 warrants issued in connection with issuance of the bridge promissory notes and subsequently exchanged for new warrants in the Merger).
We have agreed to engage the Placement Agent as our warrant solicitation agent in the event the warrants issued to investors in the Offering (the “Investor Warrants”) are called for redemption and shall pay a warrant solicitation fee to the Placement Agent equal to five (5%) percent of the amount of funds solicited by the Placement Agent upon the exercise of the Investor Warrants following such redemption.
The Placement Agent was granted the right to designate one member to our Board of Directors and has designated Adam K. Stern to fill such Board seat.
The price of the Units was been determined following our discussions with the Placement Agent. Among the factors considered in the negotiations were our limited operating history, our history of losses, an assessment of our management and our proposed operations, our current financial condition, the prospects for the industry in which we operate, the prospects for the development of our business with the capital raised in the Offering and the general condition of the securities markets at the time of the Offering. The Offering price of the Units or the exercise price of the Investor Warrants did not necessarily bear any relationship to our assets, book value or results of operations or any other generally accepted criterion of value.
As a result of these transactions, as of April 13, 2012, Mr. Stern reported holding 741,395 shares of common stock and warrants to purchase 360,000 shares of common stock. He also reported indirect beneficial ownership of 158,870 shares owned by ST Neuroscience Partners, LLC, 211,827 shares owned by Pavilion Capital Partners, LLC; and 132,392 shares owned by Piper Venture Partners, LLC. As of April 27, 2012, Mr. Kimberlin reported indirect beneficial ownership of 1,082,489 shares held by Spencer Trask Investment Partners, LLP and warrants to purchase 4,406,943 shares owned by Spencer Trask Ventures, Inc. issued in connection with the Bridge Financing or the Offering.
We have agreed to indemnify the Placement Agent and other broker-dealers who are FINRA members selected by the Placement Agent to offer and sell Units, to the fullest extent permitted by law for a period of four (4) years from the closing of the Offering, against certain liabilities that may be incurred in connection with the Offering, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agent may be required to make in respect of such liabilities. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to the Placement Agent, pursuant to the foregoing provisions or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Lock-ups
Officers, directors and holders of 5% or more of our common stock have agreed to “lock-up” and not sell or otherwise transfer or hypothecate any of their shares for a term equal to the earlier of (i) twelve (12) months from the Closing Date of the Merger; or (ii) six (6) months following the effective date of the registration statement of which this prospectus forms a part registering the shares of common stock that were sold in the Offering.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the informational requirements of the Exchange Act. Accordingly, we file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any document that we file at the SEC’s public reference room at 100 F Street, NE, Washington, DC 20549. Information about the operation of the public reference room may be obtained by calling the SEC at 1-800-SEC-0330. Our SEC filings are also available to you free of charge at the SEC’s web site at http://www.sec.gov.
You can read and print press releases, financial statements, our most recent annual and quarterly reports and additional information about us, free of charge, at our web site at http://www.organovo.com.
This prospectus is a part of a registration statement on Form S-1 filed by us with the SEC under the Securities Act. This prospectus does not contain all of the information set forth in the registration statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC. For further information with respect to us and the shares of our common stock offered hereby, please refer to the registration statement. The registration statement may be inspected at the public reference facilities maintained by the SEC at the addresses set forth above. Statements in this prospectus about any document filed as an exhibit are not necessarily complete and, in each instance, you should refer to the copy of such document filed with the SEC. Each such statement is qualified in its entirety by such reference.
INTEREST OF NAMED EXPERTS AND COUNSEL
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validitydistribution of the securities being registered or uponhereby, other legal matters in connection with the registration or offeringthan underwriting discounts and commissions, all of the common stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriters, voting trustee, director, officer, or employee.
The consolidated financial statements of Organovo, Inc. as of December 31, 2011 and 2010 have been included in this prospectus in reliance upon the report of Mayer Hoffman McCann P.C., an independent registered public accounting firm, appearing elsewhere herein, given upon the authority of said firm as experts in accounting and auditing.
Certain legal matters in connection this registration statement are being passed upon by the law firm DLA Piper LLP (US), San Diego, California.
15,247,987 shares of Common Stock
16,747,987 shares of Common Stock Issuable Upon the exercise of Warrants
PRELIMINARY PROSPECTUS
, 2012
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. | Other Expenses of Issuance and Distribution. |
Expenses estimated towhich shall be incurredborne by Organovo Holdings, Inc. (the “Registrant”). All of such fees and expenses, except for the issuance and distribution of this prospectusSEC registration fee, are as follows:
| | | | |
SEC registration fee | | $ | 11,077 | |
Printing and reproduction costs | | | — | |
Legal and accounting fees and expenses | | | 25,000 | |
Total | | $ | 36,077 | |
estimates:
| Item 14. |
| Indemnification of Directors
|
SEC registration fee | $[___] |
FINRA filing fee | $[___] |
Legal fees and Officers.expenses | $[___] |
Printing fees and expenses | $[___] |
Accounting fees and expenses | $[___] |
Transfer agent fees and expenses | $[___] |
Miscellaneous fees and expenses | $[___] |
Total | $[___] |
Under
Item 14. Indemnification of Directors and Officers.
Section 145102 of the General Corporation Law of the State of Delaware we may indemnify our(the “DGCL”) permits a corporation to eliminate or limit the personal liability of directors and officers against liabilities they may incur in such capacities, including liabilities underof a corporation to the Securities Act. Our certificate of incorporation provides that, pursuant to Delaware law, our directors shall not be liablecorporation or its stockholders for monetary damages for a breach of the directors’ fiduciary duty of care to us and our stockholders. This provision does not eliminateas a director, except where the duty of care, and in appropriate circumstances equitable remedies such as injunctive or other forms of non-monetary relief will remain available under Delaware law. In addition, each director will continue to be subject to liability for breach of the director’sbreached his duty of loyalty to usthe corporation or ourits stockholders, for acts or omissions notfailed to act in good faith, or involvingengaged in intentional misconduct or knowing violationsknowingly violated a law, authorized the payment of a dividend or approved a stock repurchase or redemption in violation of the law, for actions leading toDGCL or derived an improper personal benefitbenefit. The Registrant’s certificate of incorporation, as amended, provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and for payment of dividends or approval of stock repurchases or redemptions that are unlawful under Delaware law. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities laws or state or federal environmental laws.
Our bylaws provide for the indemnification of its directors to the fullest extent permittedamounts paid in settlement actually and reasonably incurred by the Delaware General Corporation Law. Our bylaws further provide that our Board of Directors has discretion to indemnify our officers and other employees. We are required to advance, prior to the final disposition of any proceeding, promptly on request, all expenses incurred by any director or executive officerperson in connection with an action, suit or proceeding to which he or she was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, proceeding on receiptin the case of an undertakingactions brought by or on behalfin the right of the corporation, no indemnification shall be made with respect to judgments, fines and amounts paid in settlement in connection with such action, suit or proceeding or with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that directorthe Court of Chancery or executive officerother adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to repay those amounts if it shouldindemnity for such expenses which the Court of Chancery or such other court shall deem proper.
The Registrant’s amended and restated bylaws provide that the Registrant will indemnify each person who was or is a party or threatened to be determined ultimatelymade a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of the Registrant) by reason of the fact that he or she is not entitledor was a director or officer of the Registrant, or is or was serving, at the Registrant’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to be indemnified under our bylaws or otherwise. We are not, however, required to advance anyas an “Indemnitee”), against expenses (including, attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with anysuch action, suit or proceeding, if a determination is reasonably and promptly made by our Board of Directors by a majority vote of a quorum of disinterested Board members that (i) the party seeking an advancesuch Indemnitee acted in badgood faith and in a manner he or deliberately breachedshe reasonably believed to be in, or not opposed to, the Registrant’s best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her dutyconduct was unlawful. The Registrant’s bylaws, as amended, also provide that the Registrant will indemnify any Indemnitee who was or is a party to usan action or suit by or in the right of the Registrant to our stockholders and (ii)procure a judgment in the Registrant’s favor by reason of the fact that the Indemnitee is or was, a director or officer, or is or was serving, or has agreed to serve, at the Registrant’s request as a resultdirector, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including, attorneys’ fees) actually and reasonably
incurred by such Indemnitee in connection with the defense or settlement of such actions byaction or suit, if the party seeking an advance, itIndemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the Registrant’s best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the Registrant, unless, and only to the extent, that the Court of Chancery or the court in which such action or suit was brought determines, despite the adjudication of liability but in view of all the circumstances, he or she is more likely than not that it will ultimately be determined that such party is not entitled to indemnification of such expenses which the Court of Chancery or such other court shall deem proper. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
The Registrant has entered into indemnification agreements with each of its directors and executive officers that may be broader than the specific indemnification provisions contained in the DGCL. These indemnification agreements require the Registrant, among other things, to indemnify its directors and executive officers against liabilities that may arise by reason of their status or service. These indemnification agreements also require the Registrant to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit or proceeding, subject to certain exceptions.
The Registrant’s amended and restated bylaws provide that the Registrant may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the Registrant or is or was serving at the request of the Registrant as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against him or her in any such capacity, or arising out of his or her status as such, whether or not the Registrant would have the power to indemnify such person against such liability under the DGCL. The Registrant has obtained insurance under which, subject to the limitations of the insurance policies, coverage is provided to the Registrant’s directors and executive officers against loss arising from claims made by reason of breach of fiduciary duty or other wrongful acts as a director or executive officer, including claims relating to public securities matters, and to the Registrant with respect to payments that may be made by the Registrant to these directors and executive officers pursuant to the applicable sectionsRegistrant’s indemnification obligations or otherwise as a matter of our bylaws.law.
See also the undertakings set out in response to Item 17 herein.
Item 15. | Item 15. Recent Sales of Unregistered Securities. |
Sales by Organovoof Unregistered Securities.
From February 2008 through August 2011, Organovo sold unsecured convertible promissory notes in
Since January 1, 2021, the aggregate principal amount of $3,130,000 in private placements to a limited number of accredited investors. Under their original terms, these notes generallyRegistrant has issued the following securities that were to convert into shares of Organovo common stock upon the occurrence of certain events or, if not so converted, into shares of Organovo preferred stock at maturity. In addition, Organovo agreed to issue common stock purchase warrants to the noteholders upon conversion. The note sales were exempt from the registration requirements of Federal and State securities laws pursuant to Section 4(2) ofregistered under the Securities Act of 1933, as amended (the “Securities Act”) and Rule 506 of Regulation D under:
(1)On December 6, 2023, the
Securities Act. Prior to the closing of the Bridge Financing (as discussed below), $3,030,000 principal amount of these notes, plus accrued interest, were exchanged for an aggregate of 7,676,828 shares of Organovo common stock and 1,309,750 warrants to purchase Organovo common stock at an exercise price of $1.00 per share. One note, in the original principal amount of $100,000, plus accrued interest, was repaid from the proceeds of the Offering, at which time warrants to purchase 100,000 shares of Common Stock wereRegistrant issued
to the holder.In October and November 2011, Organovo completed its Bridge Financing wherein it sold $1,500,000 in principal amount of its 6% convertible promissory notes due March 31, 2012 (the “Bridge Notes”) and 1,500,000 common stock purchase warrants (the “Bridge Warrants”) to accredited investors (the “Bridge Financing”). Principal and accrued interest on the Bridge Notes were converted into Units in the Offering (as discussed below) and the Bridge Warrants were exchanged for 1,500,000 New Bridge Warrants to acquire 1,500,000 shares of our Common Stock at a price of $1.00 per share. The Placement Agent acted as a selling agent to Organovo in connection with the Bridge Financing and received as compensation for its services (i) a sales commission of 10% of the amount raised, or $150,000, (ii) a 3% non-accountable expense allowance, or $45,000 and (iii) Organovo warrants that converted upon the closing of the Merger into warrants to purchase 610,155 shares of our Common Stock at a price of $1.00 per share.
In February and March 2012, the Company received gross proceeds of $15,247,987 from the private placement (the “Offering”) of equity securities (the “Units”). On February 8, February 29, and March 16, 2012, the Company completed the first, second and final closings, respectively, of the private placement offering. In these three closings, 6,525,887 Units, 1,806,100 Units, and 6,916,000 Units, respectively, were sold to accredited investors at a price of $1.00 per Unit, including the conversion of $1,500,000 of principal and $25,379 of accrued interest under certain bridge promissory notes issued in 2011. The first closing was conducted simultaneously with the completion of the Company’s merger (the “Merger”) with Organovo, Inc. The notes automatically converted into equity securities on February 8, 2012, as part of the private placement offering. Each Unit consisted of one share of common stock of the Company, $0.001 par value per share, and a 5 year warrant to purchase one share of Common Stock at $1.00 per share. Total net proceeds were $11,593,065.91 (or $12,811,897.11, including the conversion of the bridge promissory notes referred to above). The Company issued 15,247,987 shares and 16,747,987 warrants (including 1,500,000 warrants to former holders of the bridge promissory notes).
The transactions described above were exempt from registration under Section 4(2) of the Securities Act and Rule 506 of Regulation D thereunder.
In April 2012, we issued stock options to our employees to purchase 305,658 shares of common stock under our 2012 Equity Incentive Plan at an exercise price of $2.25 per share. These issuances were exempt from registration under Rule 701 of the Securities Act.
Sales by Our Predecessor, RERR
On January 30, 2012, we issued common stock to stockholders of Organovo Holdings, Inc., a Nevada corporation (formerly known as Real Estate Restoration and Rental, Inc. or “RERR”) and our sole stockholder, in connection with our reincorporation in Delaware. Such transaction was not a “sale” within the meaning of Section 2(3) of the Securities Act because it came within the exemption under Rule 145(a)(2) of the Securities Act.
Deborah Lovig, RERR’s President, Chief Executive Officer, Chief Financial Officer and Director, purchased 5,000,000 (pre-split) shares of RERR common stock on December 19, 2009 for $100 in cash and $400 worth of services which she provided to RERR.
James Coker, RERR’s Secretary and Director, purchased 80,000 shares of RERR common stock on March 17, 2010 and an additional 15,000 shares of RERR common stock on April 2, 2010, for a total of 95,000 shares, for $9,500.
II-1
In June, 2010, RERR completed the sale of a total of 1,802,50065,789 unregistered shares of common stock to a numbermarketing firm as consideration for services pursuant to a marketing agreement.
The issuance of investors, at a pricethe shares of $0.10 per share, for aggregate offering proceeds of $180,250.
The transactions described above were exemptcommon stock was not registered under the Securities Act in reliance upon the exemption from registration underprovided by Section 4(2)4(a)(2) of the Securities Act and/promulgated by the SEC, and in reliance on similar exemptions under applicable state laws. The marketing firm represented that it was an accredited investor and was acquiring the shares of common stock for investment purposes only and not with a view to, or Rule 506for sale in connection with, any distribution thereof and that it could bear the risks of Regulation D thereunder.the investments and could hold the securities for an indefinite period of time.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
| Item 16. | |
Exhibit No. | Exhibits. |
(a) Exhibits required by Item 601 of Regulation S-K. The following exhibits are filed as a part of, or incorporated by reference into, this Registration Statement:
| | Description |
Exhibit No,
|
| Description
|
2.1 | |
2.1
| | Agreement and Plan of Merger and Reorganization, dated as of February 8, 2012,December 13, 2019, by and among Organovo Holdings,the Company, Opal Merger Sub, Inc. a Delaware corporation, Organovo Acquisition Corp., a Delaware corporation and Organovo,Tarveda Therapeutics, Inc., a Delaware corporation (incorporated by reference from Exhibit 2.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012)December 16, 2019). |
|
|
|
2.2 |
| CertificateFirst Amendment to Merger Agreement, dated as of January 26, 2020, by and among the Company, Opal Merger as filed with the Delaware Secretary of State effective February 8, 2012 (incorporated by reference from Exhibit 2.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
2.3 | | Articles of Merger as filed with the Nevada Secretary of State effective December 28, 2011Sub, Inc. and Tarveda Therapeutics, Inc. (incorporated by reference from Exhibit 2.1 to the Company’s Current Report on Form 8-K, as filed with the Securities and Exchange Commission (the “SEC”) on February 3, 2012 (the “February 2012 Form 8-K”) |
| |
2.4 | | Agreement and Plan of Merger, dated as of December 28, 2011, by and between Real Estate Restoration and Rental, Inc. and Organovo Holdings, Inc. (incorporated by reference from Exhibit 2.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on January 4, 2012)29, 2020). |
|
|
|
2.5 3.1 |
| Certificate of Merger as filed with the Delaware Secretary of State effective January 30, 2012 (incorporated by reference from Exhibit 2.3 to the February 2012 Form 8-K) |
| |
2.6 | | Agreement and Plan of Merger, dated as of January 30, 2012, by and between Organovo Holdings, Inc. (Nevada) and Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 2.2 to the February 2012 Form 8-K) |
| |
2.7 | | Articles of Merger as filed with the Nevada Secretary of State effective January 30, 2012 (incorporated by reference from Exhibit 2.4 to the February 2012 Form 8-K) |
| |
3.1(i) | | Articles of Incorporation of Real Estate Restoration and Rental, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s registration statement (SEC File No. 333-169928) on Form S-1, as filed with the SEC on October 13, 2010 |
| |
3.1(ii) | | Certificate of Incorporation, Certificate of Change of Registered Agent and/or Registered Office, Certificate of Correction, and Certificate of Amendment of Certificate of Incorporation, each of Organovo, Inc., as filed with the Secretary of State of the State of Delaware on April 19, 2007, January 30, 2009, July 29, 2010, and September 28, 2011 respectively (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K/A, as filed with the SEC on March 30, 2012) |
| |
3.1(iii) | | Certificate of Incorporation of Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 2012 Form 8-K)3, 2012). |
|
|
|
3.2 |
| BylawsCertificate of Amendment of Certificate of Incorporation of Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 3.2 to the February 2012 Form 8-K) |
| |
4.1 | | Form of Bridge Warrant of Organovo, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on July 27, 2018). |
|
|
|
3.3 |
| Certificate of Second Amendment of Certificate of Incorporation of Organovo Holdings, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K as filed with the SEC on August 17, 2020). |
|
|
|
3.4 |
| Amended and Restated Bylaws of Organovo Holdings, Inc., effective as of July 12, 2023 (incorporated by reference to Exhibit 3.4 to the Company's Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
4.1 |
| Description of Securities (incorporated by reference to Exhibit 4.1 to the Company's Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
4.2** |
| Form of Pre-Funded Warrant. |
|
|
|
5.1** |
| Opinion of Paul Hastings LLP. |
|
|
|
10.1+ |
| Organovo, Inc. 2008 Equity Incentive Plan (incorporated by reference from Exhibit 10.14 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012). |
|
|
|
4.210.2+ |
| Form of Bridge Promissory Note of Organovo, Inc. (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.3 | | Form of Warrant of Organovo, Inc. issued to former holders of Organovo, Inc. promissory notes (incorporated by reference from Exhibit 4.3 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.4 | | Form of Investor Warrant of Organovo Holdings, Inc. (incorporated by reference from Exhibit 4.4 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.5(i) | | Form of Warrant of Organovo Holdings, Inc. ($1.00 exercise price) issued to Placement Agent (incorporated by reference from Exhibit 4.2(i) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
4.5(ii) | | Form of Warrant of Organovo, Inc. ($1.00 exercise price) issued to Selling Agent (incorporated by reference from Exhibit 4.2(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16,Amended and Restated 2012 ) |
| |
4.5(iii) | | Form of Warrant of Organovo Holdings, Inc. ($1.00 exercise price) issued to Placement Agent in exchange for Organovo, Inc. warrant issued to Selling Agent (incorporated by reference from Exhibit 4.2(iii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
II-2
| | |
| |
4.5 | | Form of Warrant of Organovo Holdings, Inc. issued to former holders of Organovo, Inc. promissory notes (incorporated by reference from Exhibit 4.5 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.6 | | Form of New Bridge Warrant (incorporated by reference from Exhibit 4.6 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.7 | | Form of Lock-Up Agreement (incorporated by reference from Exhibit 4.7 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
5.1 | | Opinion of DLA Piper LLP (US) as to the validity of the shares of Organovo Holdings, Inc. common stock to be issued.* |
| |
10.1 | | Form of Securities Purchase Agreement between Organovo, Inc and the Bridge InvestorsEquity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012)October 6, 2021). |
|
|
|
10.210.3+ |
| EscrowForm of Stock Option Award Agreement by and among Organovo, Inc.,under the Selling Agent and Signature Bank2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.610.16 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16,February 13, 2012). |
|
|
|
10.310.4+ |
| Selling AgentForm of Indemnification Agreement (incorporated by reference from Exhibit 10.17 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012). |
|
|
|
10.5† |
| License Agreement dated as of March 24, 2009, by and between Organovo, Inc. and the Selling AgentCurators of the University of Missouri (incorporated by reference from Exhibit 10.23 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.6† |
| License Agreement dated as of March 12, 2010 by and between the Company and the Curators of the University of Missouri (incorporated by reference from Exhibit 10.24 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.7† |
| License Agreement dated as of May 2, 2011, by and between the Company and Clemson University Research Foundation (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 11, 2012). |
|
|
|
10.8+ |
| Form of Non-Employee Director Stock Option Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.35 to the Company’s Annual Report on Form 10-K, as filed with the SEC on June 9, 2015). |
|
|
|
10.9+ |
| Form of Executive Stock Option Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.36 to the Company’s Annual Report on Form 10-K, as filed with the SEC on June 9, 2015). |
|
|
|
10.10+ |
| Organovo Holdings, Inc. Severance and Change in Control Plan (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 9, 2015). |
|
|
|
10.11+ |
| Amendment to the Organovo Holdings, Inc. Severance and Change in Control Plan, dated May 19, 2020 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 20, 2020). |
|
|
|
10.12+ |
| Form of Organovo Holdings, Inc. Severance and Change in Control Plan Participation Agreement (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 9, 2015). |
| | |
Exhibit No. |
| Description |
|
|
|
10.13+ |
| Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement (Retention Form) under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.14+ |
| Form of Employee Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.15+ |
| Form of Non-Employee Director Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the 2012 Equity Incentive Plan (incorporated by reference from Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on August 4, 2016). |
|
|
|
10.16+ |
| Organovo Holdings, Inc. 2016 Employee Stock Purchase Plan (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on August 18, 2016). |
|
|
|
10.17+ |
| Organovo Holdings, Inc. Inducement Award Stock Option Agreement, dated April 24, 2017 (incorporated by reference from Exhibit 99.1 to the Company’s Registration Statement on Form S-8 (File No. 333-217437), as filed with the SEC on April 24, 2017). |
|
|
|
10.18+ |
| Organovo Holdings, Inc. Inducement Award Performance-Based Restricted Stock Unit Agreement, dated April 24, 2017 (incorporated by reference from Exhibit 99.2 to the Company’s Registration Statement on Form S-8 (File No. 333-217437), as filed with the SEC on April 24, 2017). |
|
|
|
10.19+ |
| Organovo Holdings, Inc. Inducement Award Stock Option Agreement, dated August 14, 2018 (incorporated by reference from Exhibit 99.1 to the Company’s Registration Statement on Form S-8 (File No. 333-226837), as filed with the SEC on August 14, 2018). |
|
|
|
10.20+ |
| Organovo Holdings, Inc. Inducement Award Restricted Stock Unit Agreement, dated August 14, 2018 (incorporated by reference from Exhibit 99.2 to the Company’s Registration Statement on Form S-8 (File No. 333-226837), as filed with the SEC on August 14, 2018). |
|
|
|
10.21+ |
| Consulting Agreement, dated September 15, 2020, by and between Organovo and Multi Dimensional Bio Insight LLC. (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.22+ |
| Consulting Agreement, dated August 25, 2020, by and between Organovo and Danforth Advisors (incorporated by reference from Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.23+ |
| Amendment No. 5 dated October 4, 2021 to Consulting Agreement dated August 25, 2021 by and between Company and Danforth Advisors LLC (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012)October 6, 2021). |
|
|
|
10.410.24+ |
| Offer Letter, dated September 15, 2020, between the Company and Jeffrey N. Miner (incorporated by reference from Exhibit 10.3 to the Company’s Quarterly Report on Form of Subscription10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.25+ |
| Engagement Agreement, dated July 23, 2020, by and between Organovo and Optima Law Group of San Diego (incorporated by reference from Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on November 5, 2020). |
|
|
|
10.26 |
| Lease Agreement dated November 23, 2020, between Organovo Holdings, Inc. and San Diego Inspire 1, LLC (Permanent Lease Agreement 176640186.8) (incorporated by reference from Exhibit 10.2 to the investors inCompany’s Current Report on Form 8-K, as filed with the offeringSEC on November 25, 2020). |
|
|
|
10.27 |
| Amended and Restated Lease Agreement dated November 23, 2020, between Organovo, Inc., as Tenant, and San Diego Inspire 2, LLC, as Landlord, as amended by First Amendment to Amended & Restated Lease, dated November 17, 2021, between San Diego Inspire 2, LLC, as Landlord, and Organovo, Inc., as Tenant (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on November 19, 2021). |
|
|
|
10.28# |
| Intercompany Agreement, dated December 28, 2020, by and among Organovo Holdings, Inc., Organovo, Inc. and Viscient Biosciences, Inc. (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on December 31, 2020). |
| | |
Exhibit No. |
| Description |
|
|
|
10.29+ |
| Offer Letter, dated December 28, 2020, between the Company and Tom Jurgensen (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q, as filed with the SEC on February 8, 2021). |
|
|
|
10.30 |
| Sales Agreement, dated March 16, 2018, by and among Organovo Holdings, Inc., H.C. Wainwright & Co., LLC and JonesTrading Institutional Services LLC. (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012 Form 8-K)2018). |
|
|
|
10.510.31+ |
| Form of Registration Rights Agreement, by and between Organovo Holdings, Inc. and the investors in the offering2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012)10, 2021). |
|
|
|
10.610.32+ |
| EscrowForm of Stock Option Agreement by and amongunder the Organovo Holdings, Inc., the Placement Agent and Signature Bank 2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 10.514.2 to the Company’s Registration Statement on Form S-8 (File No. 333-254714), as filed with the SEC on March 25, 2021). |
|
|
|
10.33+ |
| Form of Restricted Stock Unit Agreement under the Organovo Holdings, Inc. 2021 Inducement Equity Incentive Plan (incorporated by reference from Exhibit 4.3 to the Company’s Registration Statement on Form S-8 (File No. 333-254714), as filed with the SEC on March 25, 2021). |
|
|
|
10.34 |
| Settlement and Patent License Agreement, dated February 22, 2022, by and between Organovo Holdings, Inc. and BICO Group AB (incorporated by reference from Exhibit 10.34 to the Company's Annual Report on Form 10-K, as field with the SEC on June 10, 2022). |
|
|
|
10.35+ |
| Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012)October 14, 2022) |
|
|
|
10.6(i)10.36+ |
| Extension to EscrowForm of Global Stock Option Award Agreement under the Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 10.5(iii)4.2 to the Company's Registration Statement on Form S-8 (No. 333-268001), filed with the SEC on October 25, 2022). |
|
|
|
10.37+ |
| Form of Global Restricted Stock Unit Award Agreement under the Organovo Holdings, Inc. 2022 Equity Incentive Plan (incorporated by reference from Exhibit 4.3 to the Company's Registration Statement on Form S-8 (No. 333-268001), filed with the SEC on October 25, 2022). |
|
|
|
10.38* |
| Purchase Agreement, dated March 10, 2023, by and between Organovo Holdings, Inc. and Metacrine, Inc. (incorporated by reference from Exhibit 10.38 to the Company's Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
10.39 |
| Separation Agreement and General Release, effective as of September 15, 2023, between Organovo Holdings, Inc. and Tom Jurgensen (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012)September 12, 2023). |
|
|
|
10.7(i)10.40 |
| Joinder bySeparation Agreement and General Release, effective as of September 27, 2023, between Organovo Holdings, Inc. to Placement Agency Agreementand Jeffrey Miner (incorporated by reference from Exhibit 10.4(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.7(ii) | | Joinder by Organovo Holdings, Inc. to Escrow Agreement (incorporated by reference from Exhibit 10.5(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.8 | | Placement Agent Agreement between Organovo, Inc. and the Placement Agent (incorporated by reference from Exhibit 10.4(i) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.8(i) | | Extension to Placement Agent Agreement (incorporated by reference from Exhibit 10.4(iii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.9 | | Split-Off Agreement, by and among Organovo Holdings, Inc., Organovo Split Corp., Deborah Lovig and James Coker (incorporated by reference from Exhibit 10.9 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.10 | | General Release Agreement by and among Organovo Holdings, Inc., Organovo Split Corp., Deborah Lovig and James Coker (incorporated by reference from Exhibit 10.10 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.11 | | Form of Share Cancellation Agreement and Release (incorporated by reference from Exhibit 10.11 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.12 | | Offer Letter between Barry D. Michaels and Organovo, Inc. *** (incorporated by reference from Exhibit 10.12 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.13 | | Offer Letter between Sharon Collins Presnell and Organovo, Inc. *** (incorporated by reference from Exhibit 10.13 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.14 | | Organovo, Inc. 2008 Equity Incentive Plan *** (incorporated by reference from Exhibit 10.14 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.15 | | Organovo Holdings, Inc. 2012 Equity Incentive Plan*** (incorporated by reference from Exhibit 10.15 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.16 | | Form of Stock Option Award Agreement under the 2012 Equity Incentive Plan *** (incorporated by reference from Exhibit 10.16 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
II-3
| | |
| |
10.17 | | Form of Indemnification Agreement *** (incorporated by reference from Exhibit 10.17 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.18 | | Memorandum of Understanding between Organovo, Inc. and Robert Baltera, Jr. *** (incorporated by reference from Exhibit 10.18 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.19 | | Scientific Advisory Board Consulting Agreement, dated as of March 17, 2008, by and between Organovo, Inc. and Glenn Prestwich, Ph.D. (incorporated by reference from Exhibit 10.19 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.20 | | Scientific Advisory Board Consulting Agreement, dated as of March 17, 2008, by and between Organovo, Inc. and David Mooney, Ph.D. (incorporated by reference from Exhibit 10.20 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.21 | | Scientific Advisory Board Consulting Agreement, dated as of April 14, 2008, by and between Organovo, Inc. and Gordana Vunjak-Novakovic (incorporated by reference from Exhibit 10.21 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.22 | | Scientific Advisory Board Consulting Agreement, dated as of June 30, 2008, by and between Organovo, Inc. and K. Craig Kent, M.D. (incorporated by reference from Exhibit 10.22 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.23 | | License Agreement dated as of March 24, 2009, by and between Organovo, Inc. and the Curators of the University of Missouri, **** (incorporated by reference from Exhibit 10.23 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.24 | | License Agreement dated as of March 12, 2010 by and between the Company and the University of Missouri, **** (incorporated by reference from Exhibit 10.24 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.25 | | License Agreement dated as of May 2, 2011, by and between the Company and Clemson University Research Foundation, **** (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.26 | | 3D Bio-Printer Development Program Agreement, dated as of March 3, 2011, by and between Invetech Pty Ltd (“Invetech”) and Organovo Holdings, Inc. (incorporated by reference from Exhibit 10.2510.1 to the Company’s Current Report on Form 8-K/A, as filed with the SEC on March 30, 2012) ****September 22, 2023). |
|
|
|
21.110.41+ |
| Subsidiaries of Organovo Holdings, Inc. 2023 Employee Stock Purchase Plan (incorporated by reference from Exhibit 10.2510.1 to the Company’sCompany's Current Report on Form 8-K, as filed with the SEC on February 13, 2012)November 3, 2023). |
|
|
|
23.110.42** |
| Form of Securities Purchase Agreement. |
|
|
|
21.1 |
| Subsidiaries of Organovo Holdings, Inc. (incorporated by reference to Exhibit 21.1 to the Company's Annual Report on Form 10-K, as filed with the SEC on July 14, 2023). |
|
|
|
23.1* |
| Consent of Independent Registered Public Accounting Firm*Firm. |
|
|
|
23.224.1* |
| Consent of DLA Piper LLP (US) (included in Exhibit 5.1)* |
| |
24.1 | | Power of Attorney (included on signature page hereto)*. |
|
|
|
101†101.INS* |
| Inline XBRL Instance Document |
|
|
|
101.SCH* |
| Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents. |
|
|
|
104 |
| Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
107* |
| Filing Fee Table. |
II-5
** | To be filed by amendment. |
*** | Designates management contracts and compensation plans. |
**** |
* Filed herewith. ** To be filed by amendment. + Designates management contracts and compensation plans. † This Exhibit has been filed separately with the Secretary of the Securities and Exchange Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
† | XBRL (Extensible Business Reporting Language) information included herewith is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, and otherwise is not subject to liability under those sections. |
Certain Confidential Information contained in this Exhibit was omitted by means of redacting a portion of the textSecurities and replacing it with an asterisk.Exchange Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
(b) Financial Statements Schedules.
All financing statements schedules are# Certain identified information has been omitted pursuant to Item 601(b)(10) of Regulation S-K because they are not applicable or the requiredsuch information is shown inboth (i) not material and (ii) would likely cause competitive harm to the consolidated financial statements or other notes hereto.Registrant if publicly disclosed. The Registrant hereby undertakes to furnish supplemental copies of the unredacted exhibit upon request by the Securities and Exchange Commission.
Item 17. Undertakings
(a)
The undersigned registrantRegistrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) toTo include any prospectus required by sectionSection 10(a)(3) of the Securities Act of 1933 (the “Securities Act”);Act;
(ii) toTo reflect in the prospectus any facts or events arising after the effective date of thethis registration statement (or the most recent post-effective amendment
II-4
thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high endand of the estimated maximum offering range may be reflected in the form of prospectus filed with the SECSecurities and Exchange Commission (the “Commission”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) toTo include any material information with respect to the plan of distribution not previously disclosed in thethis registration statement or any material change to such information in this registration statement;
provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(b)
(4) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
The undersigned registrant hereby undertakes:
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrantRegistrant pursuant to the foregoing provisions, or otherwise, the registrantRegistrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrantRegistrant of expenses incurred or paid by a director, officer or controlling person of the registrantRegistrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrantRegistrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-5The undersigned registrant hereby undertakes that:
SIGNATURES
For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective.
For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,1933, as amended, the registrantRegistrant has duly caused this reportRegistration Statement to be signed on its behalf by the undersigned, hereuntothereunto duly authorized.authorized, in the City of San Diego, State of California, on April 12, 2024.
| | | | |
| | ORGANOVO HOLDINGS, INC. |
|
|
|
Date: June 13, 2012 | | By: |
| By: | /s/ Keith Murphy |
|
| | | Name: Keith Murphy |
|
| Executive Chairman
|
|
| Title: Chief Executive Officer
|
|
|
|
Power of Attorney
We, the undersigned officersPOWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS,that each person whose signature appears below constitutes and directors of Organovo Holdings, Inc., hereby severally constitute and appointappoints Keith Murphy and Barry D. Michaels,Thomas Hess, and botheach or any one of them, ouras his or her true and lawful attorneys-in-factattorney-in-fact and agents,agent, with full power of substitution and re-substitution in each of themresubstitution, for him or her and in his or her name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments)amendments and any related registration statements filed pursuant to Rule 462 and otherwise) to this registration statement,Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary or desirable to be done in and about the premises,connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorneys-in-fact and agents, or any of them, or their or his substitute or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statementRegistration Statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | |
Signature |
| Title |
| Date |
Signature
|
| Title
|
| Date |
| |
|
/s/ Keith Murphy |
| Executive Chairman |
| April 12, 2024 |
Keith Murphy |
| Chairman of the Board, Chief Executive Officer, and President
(Principal Executive Officer) |
| June 13, 2012 |
| |
|
|
|
|
|
|
/s/ Barry D. Michaels Barry D. MichaelsThomas Hess
|
| Chief Financial Officer |
| April 12, 2024 |
Thomas Hess |
| (Principal Financial and Principal Accounting Officer) |
| June 13, 2012 |
| |
|
/s/ Robert Baltera, Jr.
Robert Baltera, Jr.
|
| Director
|
| June 13, 2012 |
| |
|
/s/ Andras Forgacs Andras ForgacsAdam Stern
|
| Director |
| June 13, 2012 |
| | April 12, 2024 |
/s/ Adam K. Stern
Adam K. Stern
|
| Director
|
| June 13, 2012 |
EXHIBIT INDEX
| | |
Exhibit No,
|
| Description
|
|
|
/s/ Douglas Cohen |
| Director |
| April 12, 2024 |
2.1Douglas Cohen |
| Agreement and Plan of Merger and Reorganization, dated as of February 8, 2012, by and among Organovo Holdings, Inc. a Delaware corporation, Organovo Acquisition Corp., a Delaware corporation and Organovo, Inc., a Delaware corporation (incorporated by reference from Exhibit 2.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012)
|
|
|
|
|
|
|
|
2.2/s/ David Gobel |
| Certificate of Merger as filed with the Delaware Secretary of State effective February 8, 2012 (incorporated by reference from Exhibit 2.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012)Director |
| April 12, 2024 |
David Gobel |
|
|
|
|
2.3
|
| Articles of Merger as filed with the Nevada Secretary of State effective December 28, 2011 (incorporated by reference from Exhibit 2.1 to the Company’s Current Report on Form 8-K, as filed with the Securities and Exchange Commission (the “SEC”) on February 3, 2012 (the “February 2012 Form 8-K”)
|
|
|
/s/ Vaidehi Joshi |
| Director |
| April 12, 2024 |
2.4Vaidehi Joshi |
| Agreement and Plan of Merger, dated as of December 28, 2011, by and between Real Estate Restoration and Rental, Inc. and Organovo Holdings, Inc. (incorporated by reference from Exhibit 2.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on January 4, 2012)
|
|
|
|
|
|
|
|
2.5/s/ Alison Milhous |
| Certificate of Merger as filed with the Delaware Secretary of State effective January 30, 2012 (incorporated by reference from Exhibit 2.3 to the February 2012 Form 8-K)Director |
| April 12, 2024 |
Alison Milhous |
|
|
|
|
2.6
|
| Agreement and Plan of Merger, dated as of January 30, 2012, by and between Organovo Holdings, Inc. (Nevada) and Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 2.2 to the February 2012 Form 8-K) |
| |
2.7
| | Articles of Merger as filed with the Nevada Secretary of State effective January 30, 2012 (incorporated by reference from Exhibit 2.4 to the February 2012 Form 8-K) |
| |
3.1(i) | | Articles of Incorporation of Real Estate Restoration and Rental, Inc. (incorporated by reference from Exhibit 3.1 to the Company’s registration statement (SEC File No. 333-169928) on Form S-1, as filed with the SEC on October 13, 2010 |
| |
3.1(ii) | | Certificate of Incorporation, Certificate of Change of Registered Agent and/or Registered Office, Certificate of Correction, and Certificate of Amendment of Certificate of Incorporation, each of Organovo, Inc., as filed with the Secretary of State of the State of Delaware on April 19, 2007, January 30, 2009, July 29, 2010, and September 28, 2011 respectively (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K/A, as filed with the SEC on March 30, 2012) |
| |
3.1(iii) | | Certificate of Incorporation of Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 3.1 to the February 2012 Form 8-K) |
| |
3.2 | | Bylaws of Organovo Holdings, Inc. (Delaware) (incorporated by reference from Exhibit 3.2 to the February 2012 Form 8-K) |
| |
4.1 | | Form of Bridge Warrant of Organovo, Inc. (incorporated by reference from Exhibit 4.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.2 | | Form of Bridge Promissory Note of Organovo, Inc. (incorporated by reference from Exhibit 4.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.3 | | Form of Warrant of Organovo, Inc. issued to former holders of Organovo, Inc. promissory notes (incorporated by reference from Exhibit 4.3 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.4 | | Form of Investor Warrant of Organovo Holdings, Inc. (incorporated by reference from Exhibit 4.4 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.5(i) | | Form of Warrant of Organovo Holdings, Inc. ($1.00 exercise price) issued to Placement Agent (incorporated by reference from Exhibit 4.2(i) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
4.5(ii) | | Form of Warrant of Organovo, Inc. ($1.00 exercise price) issued to Selling Agent (incorporated by reference from Exhibit 4.2(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012 ) |
| |
4.5(iii) | | Form of Warrant of Organovo Holdings, Inc. ($1.00 exercise price) issued to Placement Agent in exchange for Organovo, Inc. warrant issued to Selling Agent (incorporated by reference from Exhibit 4.2(iii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
4.5 | | Form of Warrant of Organovo Holdings, Inc. issued to former holders of Organovo, Inc. promissory notes (incorporated by reference from Exhibit 4.5 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.6 | | Form of New Bridge Warrant (incorporated by reference from Exhibit 4.6 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
4.7 | | Form of Lock-Up Agreement (incorporated by reference from Exhibit 4.7 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| | |
| |
5.1 | | Opinion of DLA Piper LLP (US) as to the validity of the shares of Organovo Holdings, Inc. common stock to be issued.* |
| |
10.1 | | Form of Securities Purchase Agreement between Organovo, Inc and the Bridge Investors (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.2 | | Escrow Agreement, by and among Organovo, Inc., the Selling Agent and Signature Bank (incorporated by reference from Exhibit 10.6 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.3 | | Selling Agent Agreement between Organovo, Inc. and the Selling Agent (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.4 | | Form of Subscription Agreement, by and between Organovo Holdings, Inc. and the investors in the offering (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012 Form 8-K) |
| |
10.5 | | Form of Registration Rights Agreement, by and between Organovo Holdings, Inc. and the investors in the offering (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.6 | | Escrow Agreement, by and among Organovo, Inc., the Placement Agent and Signature Bank (incorporated by reference from Exhibit 10.51 to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.6(i) | | Extension to Escrow Agreement (incorporated by reference from Exhibit 10.5(iii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.7(i) | | Joinder by Organovo Holdings, Inc. to Placement Agency Agreement (incorporated by reference from Exhibit 10.4(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.7(ii) | | Joinder by Organovo Holdings, Inc. to Escrow Agreement (incorporated by reference from Exhibit 10.5(ii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.8 | | Placement Agent Agreement between Organovo, Inc. and the Placement Agent (incorporated by reference from Exhibit 10.4(i) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.8(i) | | Extension to Placement Agent Agreement (incorporated by reference from Exhibit 10.4(iii) to the Company’s Current Report on Form 8-K, as filed with the SEC on March 16, 2012) |
| |
10.9 | | Split-Off Agreement, by and among Organovo Holdings, Inc., Organovo Split Corp., Deborah Lovig and James Coker (incorporated by reference from Exhibit 10.9 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.10 | | General Release Agreement by and among Organovo Holdings, Inc., Organovo Split Corp., Deborah Lovig and James Coker (incorporated by reference from Exhibit 10.10 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.11 | | Form of Share Cancellation Agreement and Release (incorporated by reference from Exhibit 10.11 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.12 | | Offer Letter between Barry D. Michaels and Organovo, Inc. *** (incorporated by reference from Exhibit 10.12 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.13 | | Offer Letter between Sharon Collins Presnell and Organovo, Inc. *** (incorporated by reference from Exhibit 10.13 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.14 | | Organovo, Inc. 2008 Equity Incentive Plan *** (incorporated by reference from Exhibit 10.14 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.15 | | Organovo Holdings, Inc. 2012 Equity Incentive Plan*** (incorporated by reference from Exhibit 10.15 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.16 | | Form of Stock Option Award Agreement under the 2012 Equity Incentive Plan *** (incorporated by reference from Exhibit 10.16 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.17 | | Form of Indemnification Agreement *** (incorporated by reference from Exhibit 10.17 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.18 | | Memorandum of Understanding between Organovo, Inc. and Robert Baltera, Jr. *** (incorporated by reference from Exhibit 10.18 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.19 | | Scientific Advisory Board Consulting Agreement, dated as of March 17, 2008, by and between Organovo, Inc. and Glenn Prestwich, Ph.D. (incorporated by reference from Exhibit 10.19 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| | |
| |
10.20 | | Scientific Advisory Board Consulting Agreement, dated as of March 17, 2008, by and between Organovo, Inc. and David Mooney, Ph.D. (incorporated by reference from Exhibit 10.20 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.21 | | Scientific Advisory Board Consulting Agreement, dated as of April 14, 2008, by and between Organovo, Inc. and Gordana Vunjak-Novakovic (incorporated by reference from Exhibit 10.21 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.22 | | Scientific Advisory Board Consulting Agreement, dated as of June 30, 2008, by and between Organovo, Inc. and K. Craig Kent, M.D. (incorporated by reference from Exhibit 10.22 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
10.23 | | License Agreement dated as of March 24, 2009, by and between Organovo, Inc. and the Curators of the University of Missouri, **** (incorporated by reference from Exhibit 10.23 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.24 | | License Agreement dated as of March 12, 2010 by and between the Company and the University of Missouri, **** (incorporated by reference from Exhibit 10.24 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.25 | | License Agreement dated as of May 2, 2011, by and between the Company and Clemson University Research Foundation, **** (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K, as filed with the SEC on May 15, 2012) |
| |
10.26 | | 3D Bio-Printer Development Program Agreement, dated as of March 3, 2011, by and between Invetech Pty Ltd (“Invetech”) and Organovo Holdings, Inc. (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K/A, as filed with the SEC on March 30, 2012) **** |
| |
21.1 | | Subsidiaries of Organovo Holdings, Inc. (incorporated by reference from Exhibit 10.25 to the Company’s Current Report on Form 8-K, as filed with the SEC on February 13, 2012) |
| |
23.1 | | Consent of Independent Registered Public Accounting Firm* |
| |
23.2 | | Consent of DLA Piper LLP (US) (included in Exhibit 5.1)* |
| |
24.1 | | Power of Attorney (included on signature page hereto)* |
| |
101† | | Interactive Data File |
** | To be filed by amendment. |
*** | Designates management contracts and compensation plans. |
**** | This Exhibit has been filed separately with the Secretary of the Securities and Exchange Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
† | XBRL (Extensible Business Reporting Language) information included herewith is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, and otherwise is not subject to liability under those sections. |
Certain Confidential Information contained in this Exhibit was omitted by means of redacting a portion of the text and replacing it with an asterisk.II-8






