
As filed with the Securities and Exchange Commission on January 15, 2020.18, 2024
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Aditx Therapeutics, Inc.
ADITXT, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 82-3204328 | |||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial | |||
Classification Code Number) | (I.R.S. Employer Identification Number) |
Aditx Therapeutics, Inc.737 N. Fifth Street, Suite 200
11161 Anderson Street
Suite 105-10014
Loma Linda, CA 92354 Richmond, VA 23219
(909) 488-0844 (650) 870-1200
(Address and telephone number of registrant’s principal executive offices)
Amro Albanna
Aditx Therapeutics,Aditxt, Inc.
Chief Executive Officer
11161 Anderson737 N. Fifth Street, Suite 200
Suite 105-10014
Loma Linda, CA 92354 Richmond, VA 23219
(909) 488-0844 (650) 870-1200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| ||
| Sheppard, Mullin, Richter & Hampton LLP | ||
| 30 Rockefeller Plaza | ||
| New York, NY | ||
| Tel: (212) 653-8700
| ||
| Fax: (212) 653-8701 |
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ☐☒
If this Form is filed to register additional securities for a registration statementan offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same registration statement.offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same registration statement.offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same registration statement.offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |||||
| Non-accelerated filer ☒ | Smaller reporting company ☒ | |||||
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
Title of each Class of Securities to be Registered | Maximum Aggregate Offering Price(1) (2) | Amount of Registration | ||||||
| Common stock, par value $0.001 per share | $ | 12,017,500 | $ | 1,560 | ||||
| Warrants to purchase common stock, par value $0.001 per share(3) | ||||||||
| Shares of common stock issuable upon exercise of the Warrants | $ | 12,017,500 | $ | 1,560 | ||||
| Underwriter’s unit purchase option(4) | ||||||||
| Common Stock underlying underwriter’s unit purchase option(4) | $ | 721,050 | 94 | |||||
| Warrants underlying Underwriter’s unit purchase option(3) | ||||||||
| Common Stock underlying warrants included in Underwriter’s unit purchase option(4) | $ | 721,050 | $ | 94 | ||||
| Total | $ | 25,477,100 | $ | 3,308 | ||||
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant willRegistrant shall file a further amendment which specifically states that this registration statement willRegistration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement willRegistration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.determine.
The information in this preliminary prospectus is not complete and may be changed. These securitiesThe selling stockholders may not be soldsell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor doesthese securities and it seekis not soliciting an offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED JANUARY 18, 2024 |

SUBJECT TO COMPLETION,DATED JANUARY 15, 2020Aditxt, Inc.
PRELIMINARY PROSPECTUSUp to 3,785,569 Shares of Common Stock
1,900,000 Units
Pursuant to this prospectus, the selling stockholder identified herein (the “Selling Stockholder”) is offering on a resale basis an aggregate of 3,785,569 shares of common stock, par value $0.001 per share (the “Common Stock”) of Aditxt, Inc. (the “Company,” “we,” “us” or “our”) consisting of (i) 1,237,114 shares of Common Stock that are issuable upon exercise of pre-funded warrants (the “Pre-Funded Warrants”) issued pursuant to a securities purchase agreement entered into by and between us and the Selling Stockholder dated December 29, 2023 (the “Purchase Agreement”), (ii) up to 2,474,228 shares of Common Stock issuable upon exercise of warrants (the “Common Warrants”) issued pursuant to the Purchase Agreement, and (iii) up to 74,227 shares of Common Stock issuable upon exercise of warrants (the “Placement Agent Warrants”, together with the Pre-Funded Warrants and the Common Warrants, the “Warrants”) issued pursuant to the engagement letter dated as of December 3, 2023, as amended on December 29, 2023, by and between the Company and H.C. Wainwright & Co., LLC (the “Placement Agent”).

ADITX THERAPEUTICS, INC.The Selling Stockholders may sell or otherwise dispose of the Common Stock covered by this prospectus in a number of different ways and at varying prices. We provide more information about how the Selling Stockholders may sell or otherwise dispose of the Common Stock covered by this prospectus in the section entitled “Plan of Distribution” on page 86. Discounts, concessions, commissions and similar selling expenses attributable to the sale of Common Stock covered by this prospectus will be borne by the Selling Stockholders. We will pay all expenses (other than discounts, concessions, commissions and similar selling expenses) relating to the registration of the Common Stock with the Securities and Exchange Commission (the “SEC”).
ThisOur Common stock is an initial public offering of units of securities (the “Units”) of Aditx Therapeutics, Inc. Prior to this offering, there has been no public market for shares of our common stock. We have assumed a public offering price of $5.50 per Unit.
Each Unit consists of (a) one share of our common stock, and (b) one warrant to purchase one share of our common stock at an exercise price equal to $6.875 from the date that is six-months from the date of issuance until the fifth anniversary of the issuance date. The shares of our common stock and warrants are immediately separable and will be issued separately, but will be purchased together in this offering.
Prior to this offering, there has been no public market for our common stock. We have applied to list our common stocklisted on The Nasdaq Capital Market under the symbol “ADTX” upon our satisfaction of. On January 16, 2024, the exchange’s initial listing criteria. If our common stock is not approved for listingclosing price as reported on The Nasdaq Capital Market we will not consummate this offering. No assurance can be given that our application will be approved. We do not intend to applywas $4.59 per share. There is no established public trading market for any listing of the warrants on The Nasdaq Capital Market or any other securities exchange or nationally recognized trading system,Pre-Funded Warrants and the Common Warrants, and we do not expect a market to develop fordevelop. Without an active trading market, the warrants.liquidity of the Pre-Funded Warrants and Warrants will be limited. In addition, we do not intend to list the Pre-Funded Warrants or the Warrants on The Nasdaq Capital Market, any other national securities exchange or any other trading system.
We are an “emerging growth company” as that term is used inunder the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”)federal securities laws and, as such, have electedare subject to comply with certain reduced public company reporting requirements.
Investing in our securitiesCommon Stock involves risks.a high degree of risk. See “Risk Factors” beginning on page 7.13 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| The |
We have granted the underwriter an option, exercisable one or more times in whole or in part, to purchase up to 285,000 additional shares of common stock and/or warrants to purchase up to an aggregate of 285,000 shares of common stock, in any combinations thereof, from us at the public offering price per security, less the underwriting discounts and commissions, for 45 days after the date of this prospectus to cover over-allotments, if any.is , 2023
The underwriters are offering the Units as set forth under “Underwriting.” Delivery of the securities underlying the Units will be made on or about , 2019.
Sole Book-Running Manager
DAWSON JAMES SECURITIES, INC.
Prospectus dated , 2019
TABLE OF CONTENTS
-i-
This prospectus relates to the resale by the Selling Stockholders identified in this prospectus under the caption “Selling Stockholders,” from time to time, of up to an aggregate of 3,785,569 shares of Common Stock. We haveare not selling any shares of Common Stock under this prospectus, and we will not receive any proceeds from the underwriterssale of shares of Common Stock offered hereby by the Selling Stockholders, although we may receive cash from the exercise of the Warrants.
You should rely only on the information provided in this prospectus, including any information incorporated by reference. We have not authorized anyone to provide you with any other information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to whichand we have referred you. We take no responsibility for, and can provide no assuranceassurances as to the reliability of, any other information that others may give to you. The information contained in this prospectus is accuratespeaks only as of the date set forth on the cover page and may not reflect subsequent changes in our business, financial condition, results of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock.operations and prospects.
We are not, and the Selling Stockholders are not, making offers to sell these securities in any jurisdiction in which an offer or solicitation is not authorized or permitted or in which the person making such offer or solicitation is not qualified to do so or to any person to whom it is unlawful to make such an offer or solicitation. You should read this prospectus, including any information incorporated by reference, in its entirety before making an investment decision.
-ii-
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, which reflect the views of our management with respect to future events and financial performance. These forward-looking statements are subject to a number of uncertainties and other factors that could cause actual results to differ materially from such statements. Forward-looking statements are identified by words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “plans,” “projects,” “targets,” and similar expressions. Such forward-looking statements may be contained in the sections “Risk Factors,” and “Business,” among other places in this prospectus. Readers are cautioned not to place undue reliance on these forward-looking statements, which are based on the information available to management at this time and which speak only as of this date. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. For a discussion of some of the factors that may cause actual results to differ materially from those suggested by the forward-looking statements, please read carefully the information under “Risk Factors.”
The identification in this document of factors that may affect future performance and the accuracy of forward-looking statements is meant to be illustrative and by no means exhaustive. All forward-looking statements should be evaluated with the understanding of their inherent uncertainty. You may rely only on the information contained in this prospectus. No dealer, salesperson or other person
We have not authorized anyone to provide information different from that contained in this prospectus. Neither the delivery of this prospectus nor the sale of our Common Stock means that information contained in this prospectus is authorized to give information that is not contained incorrect after the date of this prospectus. This prospectus is not an offer to sell nor is it seekingor solicitation of an offer to buy these securities in any jurisdiction wherecircumstances under which the offer or salesolicitation is not permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of these securities.unlawful.
Through and including, , 2019 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
i-iii-
The followingThis summary highlights certain information about us, this offering and selected information contained elsewhere in this prospectus and in the documents incorporated by reference. This summary is qualified in its entirety by the more detailed informationnot complete and financial statements included elsewhere in this prospectus. It does not contain all the information that may be importantyou should consider before deciding whether to invest in our securities. For a more complete understanding of our company and this offering, we encourage you to read and your investment decision. You shouldconsider carefully readthe more detailed information contained in or incorporated by reference in this entire prospectus, including the matters set forthinformation contained under the heading “Risk Factors,” “Management’s Discussion and AnalysisFactors” beginning on page 13 of Financial Condition and Results of Operations,” and our financial statements and related notes included elsewhere in this prospectus. In this prospectus, unless context requires otherwise, references to “we,” “us,” “our,” “ADTX,” “Aditx Therapeutics,” or “the Company” refer to Aditx Therapeutics, Inc.
Overview and Mission
We believe the world needs—and deserves—a new approach to innovating that harnesses the power of large groups of stakeholders who work together to ensure that the most promising innovations make it into the hands of people who need them most.
We were incorporated in the State of Delaware on September 28, 2017, and our headquarters are located in Loma Linda, CA. We are a preclinical stage, life sciencesRichmond, Virginia. The company with the goal of developing nucleic acid (DNA)-based technologies to minimize rejection of transplanted organs by human recipients as well as address autoimmune diseases and allergies.
Our Business
We are a preclinical stage life sciences companywas founded with a mission of bringing stakeholders together, to prolongtransform promising innovations into products and services that could address some of the most challenging needs. The socialization of innovation through engaging stakeholders in every aspect of it, is key to transforming more innovations, more rapidly, and more efficiently.
At inception, the first innovation we took on was an immune modulation technology titled ADI/Adimune with a focus on prolonging life and enhanceenhancing life quality of patients that have undergone organ transplants. Since then, we expanded our portfolio of innovations, and we continue to evaluate a variety of promising health innovations.
Our Model
Aditxt is not about a single idea or a single molecule. It is about making sure the right innovation is made possible. Our business model has three main components as follows:
| (1) | Securing an Innovation: Our process begins with identifying and securing innovations through licensing or acquisition of an innovation asset. Assets come from a variety of sources including research institutions, government agencies, and private organizations. |
| (2) | Growing an Innovation: Once an innovation is secured, we surround it with activation resources that take a systemized approach to bringing that idea to life. Our activation resources include innovation, operations, commercialization, finance, content and engagement, personnel, and administration. |
| (3) | Monetizing an Innovation: Our goal is for each innovation to become commercial-stage and financially and operationally self-sustainable, to create shareholder value. |
We engage various stakeholders for each of our programs on every level. This includes identifying researchers and research institution partners, such as Stanford University; leading health institutions to get critical trials underway, such as Mayo Clinic; manufacturing partners who enable us to take innovations from preclinical to clinical; municipalities and governments, such as the city of Richmond and the state of Virginia and public health agencies who work with us to launch our program, Pearsanta’s laboratory; and thousands of shareholders around the globe. We seek to enable promising innovation to become purposeful products that have the power to change lives.
-1-
Our Value Proposition
We believe that far too often, promising treatment or technology does not reach commercialization due to lack of expertise, key resources, or efficiency. As a result, potentially life-changing and lifesaving treatments are not available to the individuals who so desperately need them.
Aditxt seeks to bring the holistic concept of an efficient, socialized ecosystem for advancing and accelerating innovations. Our process: We seek to license or acquire promising innovations. We will then form and build out a subsidiary around each innovation and support the subsidiaries through innovation, operation, commercialization, content and engagement, finance, personnel, and administration to thrive and grow as a successful, monetizable business.
Since our inception, we have built infrastructure consisting of innovation, operation, commercialization, content and engagement, finance, personnel, and administration, to support the rapid transformation of untapped innovations. Each of the main components of our infrastructure has established global access to partnerships with industry leaders, top-rated research and medical institutions, universities, manufacturing and distribution companies, and critical infrastructure such as CLIA-certified state-of-the art labs and GMP manufacturing.
The Shifting Landscape of Innovation
Innovation in general, and health innovations specifically, require significant resources. The convergence of biotech, high-tech, and media offers new possibilities of accelerating breakthrough innovations faster and more efficiently. This approach reflects our mission of “Making Promising Innovations Possible, Together”.
People deserve innovative solutions, which have never been more within reach. We believe the best idea, best product and the best solution will come from creating an ecosystem where all stakeholders, such as vendors, customers, municipalities, and shareholders contribute. When we disrupt the way we’re innovating, through our collaborative model, we believe we can move faster and more efficiently to activate viable solutions that have the potential to make a measurable impact.
Our Growth Strategy
We believe that the era of precision and personalized medicine is here and that people around the globe would benefit from health diagnostics and treatments that more accurately pinpoint the problems and more precisely treat the condition. In addition to our current programs, Adimune and Pearsanta, we look to bring in future health innovations in the areas of software and AI, medical devices, therapeutics, and other technologies that take a fundamentally different approach to health because they prioritize personalized precision medicine, timely disease root cause analysis, and targeted treatments.
Year over year, we plan to continue building our infrastructure and securing more personalized and precision health innovations that align with our mission. These opportunities may come in different forms such as IP, an early-stage company, or a late-stage company. We will continue to scale our systemized approach to the innovation process, making large-scale automation and enterprise systems available to our portfolio companies at every stage of their growth. Specifically, certain subsidiaries will need to grow through further M&A activities, operational infrastructure implementation, and development or acquisition of critical technologies.
Our Team
Aditxt is led by an entrepreneurial team with passion for transforming promising innovations into successful businesses. Our leadership come from a variety of different industries, with collective expertise in founding startup innovation companies, developing and marketing biopharmaceutical and diagnostic products, designing clinical trials, manufacturing, and management of private and public companies. We have deep experience in identifying and accessing promising health innovations and developing them into products and services with the ability to scale. We understand the capital markets, both public and private, as well as M&A and facilitating complex IPOs.
-2-
The following are profiles of three subsidiaries we have formed, including the terms of the intellectual property licenses that have been sublicensed from Aditxt to help build each of the businesses.
THE ADITXT PROGRAMS
ADIMUNE, INC.
Formed in January 2023, Adimune™, Inc. (“Adimune”) is focused on leading our immune modulation therapeutic programs. Adimune’s proprietary immune modulation product Apoptotic DNA Immunotherapy™, or ADI-100™, utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It includes two DNA molecules designed to deliver signals to induce tolerance. ADI-100 has been successfully tested in several preclinical models (e.g., skin grafting, psoriasis, type 1 diabetes, multiple sclerosis).
In May 2023, Adimune entered into a clinical trial agreement with Mayo Clinic to advance clinical studies targeting autoimmune diseases of the central nervous system (“CNS”) with the initial focus on the rare, but debilitating, autoimmune disease Stiff Person Syndrome (“SPS”). According to the National Organization of Rare Diseases, the exact incidence and prevalence of SPS is unknown; however, one estimate places the incidence at approximately one in one million individuals in the general population.
Pending approval by the International Review Board, a human trial for SPS is expected get underway in the second half of 2023 or the first half of 2024 with enrollment of 10-15 patients, some of whom may also have type 1 diabetes. ADI-100 will initially be tested for safety and efficacy. ADI-100 is designed to tolerize against an antigen known as glutamic acid decarboxylase (“GAD”), which is implicated in type-1 diabetes, psoriasis, and in many autoimmune diseases of the CNS.
Background
The discovery of immunosuppressive (anti-rejection)(anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation procedures. While these drugs prevent or delay organ rejection, transplanted organs often ultimately fail,procedures and about 40%blocking of transplanted organs survive no more than 5 years. Furthermore,unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable side effects, such as increased susceptibility to life-threatening infections and cancers, because it is not specifically targeted towards the transplanted organs; rather, it indiscriminately and broadly suppresses immune function throughout the body. While the use of these drugs has been justifiable because they prevent or delay organ rejection, their use for treatment of autoimmune diseases and allergies may not be acceptable because of the aforementioned side effects. Furthermore, often transplanted organs ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more than five years.
The opportunityThrough Aditxt, Adimune has the right of use to extend the life of a transplanted organ, even by a few years, may have substantial benefits to organ recipients. We have an exclusive worldwide license for commercializing aADI nucleic acid-based technology named Apoptotic DNA Immunotherapy™ (ADi™), which utilizes(which is currently at the pre-clinical stage) from Loma Linda University. ADI uses a novel approach that mimics the way the body naturally induces tolerance to our own tissues.tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. Thus, ADi™ADI may allow patients to live with transplanted organs with significantly reduced immune suppression. ADi™ADI is a technology platform which we believe can be engineered to address a wide variety of indications.
We plan to develop ADi™ products for organ transplantation, skin grafting, autoimmune diseases, allergies, and further indications, with the initial focus on skin allografts and psoriasis, asAdvantages
ADI™ is a nucleic acid-based technology (e.g., DNA-based), which we believe these indicationsselectively suppresses only those immune cells involved in attacking or rejecting self and transplanted tissues and organs. It does so by tapping into the body’s natural process of cell turnover (i.e., apoptosis) to retrain the immune system to stop unwanted attacks on self or transplanted tissues. Apoptosis is a natural process used by the body to clear dying cells and to allow recognition and tolerance to self-tissues. ADI triggers this process by enabling the cells of the immune system to recognize the targeted tissues as “self.” Conceptually, it is designed to retrain the immune system to accept the tissues, similar to how natural apoptosis reminds our immune system to be tolerant to our own “self” tissues.
-3-
While various groups have promoted tolerance through cell therapies and ex vivo manipulation of patient cells (i.e., takes place outside the body), to our knowledge, we will be most efficient in providing safety and efficacy data in clinical trials. To submit a BLA application for a biopharmaceutical product, clinical safety and efficacy must be demonstrated in a series of clinical studies conducted with human subjects. For productsunique in our classapproach of drugs, the first-in-human trialsusing in-body induction of apoptosis to promote tolerance to specific tissues. In addition, ADI treatment itself will be a combinationnot require additional hospitalization but only an injection of Phase I (safety/tolerability) and Phase II (efficacy) in affected subjects. To obtain approval to initiate the Phase I/IIa studies, an Investigational New Drug Application will be submitted to compile non-clinical efficacy data as well as manufacturing and pre-clinical safety/toxicology data. To date, we have conducted non-clinical studies in a stringent model of skin transplantation using genetically mismatched donor and recipient animals demonstrating a 3-fold increase in the survivalminute amounts of the skintherapeutic drug into the skin.
Moreover, preclinical studies have demonstrated that ADI treatment significantly and substantially prolongs graft survival, in animals that were tolerized withADi™ comparedaddition to animals that receive immune suppression alone. Prolongation of graft life was observed despite discontinuation of immune suppression after the first 5 weeks. Additionally, in an induced non-clinical model for psoriasis, ADi™ treatment resulted in a 68% reduction in skin thickness and a 38% decrease in skin flaking (two clinical parameters for assessment of psoriasis skin lesions). The Phase I/IIa studies in psoriasis will evaluate the safety/tolerability of ADi™ in patients diagnosed with psoriasis. In another Phase I/IIa study, patients requiring skin allografts will receive weekly intra-dermal injections of ADi™ in combination with standard immune suppression to assess safety/tolerability and possibility of reducing levels of immunosuppressive drugs as well as prolongation of graft life. Later phase trials are planned after successful completion of these studies in preparation for submission for a BLA to regulatory agencies.successfully “reversing” other established immune-mediated inflammatory processes.
License Agreement with Loma Linda University (“LLU”)
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris was party to a License Agreement with Loma Linda University (“LLU”), entered into and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,” and together with the Assignment Agreement, the “Sekris Agreements”). Pursuant to the Assignment Agreement, Sekris transferred and assigned all of its rights and obligations in and to and liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on March 8, 2018, we issued a warrant to Sekris to purchase up to 1,000,000 shares of our common stock (the “Sekris Warrant”). The warrant was immediately exercisable and has an exercise price of $2.00 per share. The expiration date of the warrant is March 8, 2023. On March 15, 2018, we entered into a Patent & Technology License Agreement directly with LLU, (the “LLU License Agreement”), which amends and restates the Sekris Agreements.
was subsequently amended on July 1, 2020. Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADi™Adi™ technology). See the section titled “Our Business—Intellectual Property—Patent Rights” for a summary of the patents and patent applications that we licensed from LLU pursuant to the LLU License Agreement. In consideration for the LLU License Agreement, we issued LLU 50,000625 shares of Common Stock to LLU.
PEARSANTA, INC.
Formed in January 2023, our subsidiary Pearsanta™, Inc. (“Pearsanta”) seeks to take personalized medicine to a whole new level by delivering “Health by the Numbers.” On November 22, 2023, Pearsanta entered into an assignment agreement with FirstVitals LLC, an entity controlled by Pearsanta’s CEO, Ernie Lee (“FirstVitals”), pursuant to which FirstVitals assigned its rights in certain intellectual property and website domain to Pearsanta in consideration of the issuance of 500,000 shares of Pearsanta common stock.stock to FirstVitals. On December 18, 2023, the board of directors of Pearsanta adopted the Pearsanta 2023 Omnibus Equity Incentive Plan (the “Pearsanta Omnibus Incentive Plan”), pursuant to which it reserved 15 million shares of common stock of Pearsanta for future issuance under the Pearsanta Omnibus Incentive Plan and the Pearsanta 2023 Parent Service Provider Equity Incentive Plan (the “Pearsanta Parent Service Provider Plan”) and approved the issuance of 9.32 million shares of Pearsanta common stock under the Pearsanta Parent Service Provider Plan.
Since its founding, Pearsanta has been building the platform for enabling our vision of lab quality testing, anytime, anywhere. Our plan for Pearsanta’s platform is for it to be the transactional backbone for sample collection, sample processing (on- and off-site), and reporting. This will require the development and convergence of multiple components developed by Pearsanta, or through transactions with third parties, including collection devices, “lab-on-a-chip” technologies, Lab Developed Test (LDT) assays, a data-driven analysis engine, and telemedicine. According to a comprehensive research report by Market Research Future, the clinical and consumer diagnostic market is estimated to hit $429.3 billion by 2030.
We believe that timely and personalized testing enables far more informed treatment decisions. Pearsanta’s platform is being developed as a seamless digital healthcare solution. This platform will integrate at-location sample collection, Point-of-Care (“POC”) and LDT assays, and an analytical reporting engine, with telemedicine-enabled visits with licensed physicians to review test results and, if necessary, order a prescription. Pearsanta’s goal of extending its platform to enable consumers to monitor their health more proactively as the goal is to provide a more complete picture about someone’s dynamic health status, factoring in genetic makeup and their response to medication. The POC component of Pearsanta would enable diagnostic testing at-home, at work, in pharmacies, and more to generate results quickly so that an individual can access necessary treatment faster. With certain infections, prescribing the most effective treatment according to one’s numbers can prevent hospital emergency room admissions and potentially life-threatening consequences.
Examples of indication-focused tests for the Test2Treat platform will include the evaluation for advanced urinary tract infections (“UTIs”), COVID-19/flu/respiratory syncytial virus, sexually transmitted infections, gut health, pharmacogenomics (i.e., how your genes affect the way your body responds to certain therapeutics), and sepsis. We believe that these offerings are novel and needed as the current standard of care using broad spectrum antibiotic treatment can be ineffective and potentially life-threatening. For example, improperly prescribed antibiotics may approach 50% of outpatient cases. Further, according to an article published in Physician’s Weekly, only 1% of board-certified critical care medicine physicians are trained in infectious disease.
Licensed Technologies – AditxtScoreTM
We intend to sublicense to Pearsanta an exclusive worldwide sub-license for commercializing the AditxtScore™ technology which provides a personalized comprehensive profile of the immune system. AditxtScore is intended to detect individual immune responses to viruses, bacteria, peptides, drugs, supplements, bone marrow and solid organ transplants, and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as emerging infectious agents.
1-4-
Pursuant
AditxtScore is being designed to enable individuals and their healthcare providers to understand, manage and monitor their immune profiles and to stay informed about attacks on or by their immune system. We believe AditxtScore can also assist the LLU License Agreement, we are requiredmedical community and individuals by being able to pay an annual license feeanticipate the immune system’s potential response to LLU. Additionally, upon completion of this offering, we willviruses, bacteria, allergens, and foreign tissues such as transplanted organs. This technology may be requiredable to pay $200,000 to LLUserve as a milestone paymentwarning signal, thereby allowing for more time to respond appropriately. Its advantages include the ability to provide simple, rapid, accurate, high throughput assays that was originally due within thirty (30) dayscan be multiplexed to determine the immune status with respect to several factors simultaneously, in approximately 3-16 hours. In addition, it can determine and differentiate between distinct types of July 31, 2018 upon the completioncellular and humoral immune responses (e.g., T and B cells and other cell types). It also provides for simultaneous monitoring of a “financing” round. Thereafter, we are required to pay to LLU milestone payments in connection with certain development milestones. As consideration for prior expenses incurred by LLU to prosecute, maintaincell activation and defend the LLU Patent and Technology Rights, we are currently obligated to pay LLU approximately $130,000. We are also required to defend the LLU Patent and Technology Rights during the termlevels of the LLU License Agreement. Additionally, we will owe royalty payments of (i) 1.5% of Net Product Sales and Net Service Sales on any Licensed Products (defined as any finished pharmaceutical products which utilizes the LLU Patent and Technology Rights in its development, manufacture or supply)cytokine release (i.e., and (ii) 0.75% of Net Product Sales and Net Service Sales for Licensed Products and Licensed Services not covered by a valid patent claim for technology rights and know-how for a three (3) year period beyond the expiration of all valid patent claims. We also are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf)cytokine storms).
The LLU License Agreement will terminate on the last day that a patent granted to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLU License Agreement may be terminated by mutual agreement or by us upon 90 days written notice to LLU. LLU may terminate the LLU License Agreement in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLU License Agreement by us in any 12-month period. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate a first-in-human clinical trials on or before March 31, 2020, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2022, (iii) the completion of Phase III clinical trials by March 31, 2024 and (iv) biologic licensing approval by the Food and Drug Administration (“FDA”) by March 31, 2025.
Our Team
We are actively involved in the regulatory approval process for AditxtScore assays for clinical use and securing manufacturing, marketing, and distribution partnerships for application in the various markets. To obtain regulatory approval to use AditxtScore as a clinical assay, we have assembled a teamconducted validation studies to evaluate its performance in detection of experts coming from a variety of different scientific fieldsantibodies and commercial backgrounds, with a collective experience that range from founding startup biotech companies,plan to developing and marketing biopharmaceutical products, to designing clinical trials, and management of private and public companies.continue conducting additional validation studies for new applications in autoimmune diseases.
Risks Related to Our BusinessAdvantages
Our business and our ability to execute our business strategy are subject to a numberThe sophistication of risks as more fully described in the section titled “Risk Factors” beginning on page 7. These risks include, among others:
AditxtScore technology includes the following:
| ● | ||
| ● |
| ● |
| ● |
| ● |
License Agreement with Leland Stanford Junior University (“Stanford”)
On February 3, 2020, we entered into an exclusive license agreement (the “February 2020 License Agreement”) with Stanford with regard to a patent concerning a method for detection and measurement of specific cellular responses. Pursuant to the February 2020 License Agreement, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and sale of Licensed Products (as defined in the agreement). The license to the patented technology is exclusive, including the right to sublicense, beginning on the effective date of the agreement, and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the “February 2020 License Agreement”). However, Stanford agreed not to grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory. On December 29, 2021, we entered into an amendment to the February 2020 License Agreement which extended our exclusive right to license the technology deployed in AditxtScoreTM and securing worldwide exclusivity in all fields of use of the licensed technology.
ADIVIR, INC.
Formed in April of 2023, Adivir™, Inc., is Aditxt’s most recently formed wholly owned subsidiary, dedicated to the clinical and commercial development efforts of innovative antiviral products. These products have the potential to address a wide range of infectious diseases, including those that currently lack viable treatment options.
-5-
Background
On April 18, 2023, we entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”), Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”), and the legal representative of Cellvera, pursuant to which, the Company will purchase Cellvera’s 50% ownership interest in G Response Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the Asset Purchase Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million, comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the antiviral medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”).
Additionally, upon the closing, the Share Exchange Agreement previously entered into as of December 28, 2021, between Cellvera Global Holdings, LLC f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements will be terminated.
The obligations of the Company to consummate the Closing are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following:
| (i) | Satisfactory completion of |
| (vii) | Execution of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the Company the rights to acquire the intellectual property from the third party under such agreement; |
| (viii) | Receipt of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and |
There can be no assurance that the conditions to closing will be satisfied or that the proposed acquisition will be completed as proposed or at all.
Our commitment to building our antiviral portfolio is strategic and timely. We believe that there has never has there been a more important time to address the growing global need to uncover new treatments or commercialize existing ones that treat life-threatening global viral infections.
-6-
Recent Developments
Merger Agreement with Evofem Biosciences, Inc.
As previously reported in a Current Report on Form 8-K, on December 11, 2023, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Adicure, Inc., a majority owned subsidiary of the Company (“Merger Sub”) and Evofem Biosciences, Inc. (“Evofem”), pursuant to which, Merger Sub will be merged into and with Evofem (the “Merger”), with Evofem surviving the Merger as a wholly owned subsidiary of the Company. Evofem is a commercial-stage women’s health company with a strong focus on innovation. Evofem is the creator of an FDA-approved hormone-fee contraceptive gel, Phexxi®.
At the effective time of the Merger (the “Effective Time”), (i) all issued and outstanding shares of common stock of Evofem (“Evofem Common Stock”), other than any shares of Evofem Common Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 610,000 shares of our Common Stock; and (ii) all issued and outstanding shares of Series E-1 Preferred Stock of Evofem (the “Evofem Unconverted Preferred Stock”), other than any shares of Evofem Unconverted Preferred Stock held by the Company or Merger Sub immediately prior to the Effective Time, will be converted into the right to receive an aggregate of 2,327 shares of our Series A-1 Convertible Preferred Stock, having such rights, powers, and preferences set forth in the form of Certificate of Designation of Series A-1 Convertible Preferred Stock, the form of which is attached as Exhibit C to the Merger Agreement. For additional information regarding the rights, powers and preferences of the Series A-1 Convertible Preferred Stock, see “Description of Capital Stock—Series A-1 Convertible Preferred Stock”.
The closing of the Merger is subject to the satisfaction or waiver of a number of conditions. Including but not limited to: (i) approval by our shareholders and the Evofem shareholders of the transactions contemplated by the Merger Agreement; (ii) the registration statement on Form S-4 pursuant to which the shares of our Common Stock issuable in the Merger having been declared effective by the SEC; (iii) all preferred stock of Evofem subject to certain exceptions shall have been converted to Evofem common stock; (iv) Evofem shall have received agreements from all holders of Evofem warrants which provide: (a) waivers with respect to any fundamental transaction, change in control or other similar rights that such warrant holder may have under any such Evofem warrants, and (b) an agreement to such Evofem warrants to exchange such warrants for not more than an aggregate (for all holders of Evofem warrants) of 551 shares of our preferred stock of Evofem; (v) the Company shall have obtained agreements from the holders of certain convertible notes and purchase rights of Evofem to exchange such convertible notes and purchase rights for not more than an aggregate (for all holders of Evofem convertible notes) of 86,153 shares of our preferred stock; and (vi) we shall have received waivers form the holders of certain of our securities which contain prohibitions on variable rate transactions.
The Merger Agreement may be terminated at any time prior to the consummation of the Closing by mutual written consent of us and Evofem. The Merger Agreement may also be terminated by us or Evofem if (i) the Merger shall not have been consummated on or before 5:00 p.m. Eastern Time on May 8, 2024; (ii) if any judgment, law or order prohibiting the Merger or the transactions contemplated in connection therewith has become final and non-appealable; (iii) the required vote of Evofem stockholders was not obtained; or (iv) in the event of any Terminable Breach (as defined in the Merger Agreement). We may also terminate the Merger Agreement if (i) prior to approval by the required vote of Evofem’s shareholders if the Evofem board of directors shall have effected a change in recommendation with respect to the Merger; or (ii) in the event that we determine, in our reasonable discretion, that the acquisition of Evofem could result in a material adverse amount of cancellation of indebtedness income to us. Evofem may terminate the Merger Agreement if (i) prior to approval by the required vote of Evofem’s shareholders if the Evofem board of directors determines to terminate the Merger Agreement in connection with a superior proposal in order to enter into a definitive agreement for such superior proposal provided that Evofem has paid the termination fee of $4 million; (ii) our Common Stock is no longer listed for trading on Nasdaq; or (iii) we have not made a loan to Evofem of no less than $3 million prior to January 31, 2024.
In connection with the Merger Agreement, we entered into an Assignment Agreement dated December 11, 2023 (the “Assignment Agreement”), with Evofem and the holders (the “Holders”) of certain senior indebtedness of Evofem (the “Notes”), pursuant to which the Holders assigned the Notes to us in consideration for the issuance by us of (i) an aggregate principal amount of $5 million in our secured notes due on January 2, 2024 (the “January 2024 Secured Notes”), (ii) an aggregate principal amount of $8 million in secured notes of the Company due on September 30, 2024 (the “September 2024 Secured Notes”), (iii) an aggregate principal amount of $5 million in ten-year unsecured notes (the “Unsecured Notes”), and (iv) payment of $154,480 in respect of net sales of Phexxi in respect of the calendar quarter ended September 30, 2023, which amount is due and payable on December 14, 2023. The January 2024 Secured Notes are secured by certain intellectual property assets of the Company and its subsidiaries pursuant to an Intellectual Property Security Agreement entered into in connection with the Assignment Agreement. The September 2024 Secured Notes are secured by the Notes and certain associated security documents pursuant to a Security Agreement entered into in connection with the Assignment Agreement.
On January 2, 2024, we entered into amendments to the January 2024 Secured Notes (“Amendment No. 1 to January 2024 Secured Notes”) with the Holders, pursuant to which the maturity date of the January 2024 Notes was extended to January 5, 2024. On January 5, 2024, we entered into amendments to the January 2024 Secured Notes (“Amendment No. 2 to January 2024 Secured Notes”) and amendments to the September 2024 Secured Notes (“Amendment No. 1 to September 2024 Secured Notes”) with the Holders, pursuant to which the Company and the Holders agreed that in consideration of a principal payment in the aggregate amount of $1 million on the January 2024 Secured Notes and in increase in the aggregate principal balance of $250,000 on the September 2024 Secured Notes, that the maturity date of the January 2024 Secured Notes would be further extended to January 31, 2024.
-7-
In connection with the Merger Agreement and the transactions contemplated thereby, on December 22, 2023, we entered into an Exchange Agreement (the “Exchange Agreement”) with the holders of an aggregate of 22,280 shares of Series F-1 Convertible Preferred Stock of Evofem, pursuant to which such holders agreed to exchange their respective shares of Evofem Series F-1 Preferred Stock for an aggregate of 22,280 shares of a new series of our convertible preferred stock of the Company designated as Series A-1 Convertible Preferred Stock. On December 26, 2023, in connection with the Exchange Agreement, we entered into a Registration Rights Agreement with the holders, pursuant to which we agreed to prepare and file with the SEC covering the resale of the shares of our Common Stock issuable upon conversion of the Series A-1 Convertible Preferred Stock (i) on the later of (x) the 15th calendar day after the closing date, or (y) the 2nd business day following the Stockholder Approval Date (as defined in the Exchange Agreement”), with respect to the initial registration statement and (ii) on the date on which the Company is required to file any additional Registration Statement pursuant to the terms of the Registration Rights Agreement with respect to any additional Registration Statements that may be required to be filed by the Company (the “Filing Deadline”). Pursuant to the Registration Rights Agreement, we are required (i) to have the initial Registration Statement declared effective by the SEC on the earlier of (x) the 60th calendar day after the Filing Deadline (or the 90th calendar day after the Filing Deadline if subject to a full review by the SEC), and (y) the 2nd business day after the date we are notified by the SEC that such Registration Statement will not be reviewed, and (ii) with respect to any additional Registration Statements that may be required to be filed, the earlier of (x) the 60th calendar day following the date on which we were required to file such additional Registration Statement (or the 90th calendar day if subject to a full review by the SEC), and (y) the 2nd business day after the date we are notified by the SEC that such Registration Statement will not be reviewed. In the event that we fail to file the Registration Statement by the Filing Deadline, have it declared effective by the Effectiveness Deadline, or the prospectus contained therein is not available for use or the investor is not otherwise able to sell its Warrant Shares pursuant to Rule 144, we will be required to pay the investor an amount equal to 2% of the stated value of such Holder’s Series A-1 Preferred Stock on the date of such failure and on every thirty date anniversary until such failure is cured. For additional information regarding the rights, powers and preferences of the Series A-1 Convertible Preferred Stock, see “Description of Capital Stock—Series A-1 Convertible Preferred Stock”.
Asset Purchase Agreement with MDNA Life Sciences, Inc.
As previously reported in a Current Report on Form 8-K, on December 17, 2023, the Company entered into an Asset Purchase Agreement (the “Purchase Agreement”) with Pearsanta, Inc., our majority owned subsidiary (“Pearsanta”) and MDNA Life Sciences, Inc. (“MDNA”), pursuant to which Pearsanta agreed to acquire certain intellectual property and other specified assets relating to MDNA’s early cancer detection platform (the “Acquired Assets”). MDNA’s Mitomic™ technology provides a tool for identifying biomarkers associated with various diseases that lead to mtDNA mutations. The Acquired Assets include, but are not limited to, the following:
| ● | The Mitomic Endometriosis Test (MET™) is in development as a blood-based assay for diagnosis of endometriosis. This test aims to provide early diagnostic insights, potentially reducing delays in diagnosing endometriosis. |
| ● | The Mitomic Prostate Test (MPT™) is currently under development as a blood-based assay for diagnosis of prostate cancer. We believe that this test holds the potential to provide more specific and clinically informative data especially in the prostate-specific antigen (PSA) grey zone. It aims to address the challenges of over-diagnosis and mitigate risks associated with low-grade cancers. |
-8-
Pursuant to the Purchase Agreement, the consideration for the transaction was to consist of: (i) an upfront working capital payment of $500,000 (the “Upfront Working Capital Payment”), which is payable upon the satisfaction of certain conditions set forth in the Purchase Agreement, (ii) a working capital payment at closing of $500,000, (iii) 50,000 shares of our Common Stock, (iv) a warrant to purchase 50,000 shares of our Common Stock exercisable for a term of 5 years at an exercise price equal to the opening price per share of our Common Stock as of the Closing Date (as defined below), and (v) 5,000 shares of Pearsanta Series A Preferred Stock, par value $0.001 per share (the “Pearsanta Preferred Stock”), provided, however, that if the value of such Pearsanta Preferred Stock, on an as-converted basis, at the time of the pricing of the Pearsanta common stock in connection with the sale of shares of Pearsanta common stock to the public in a firm-commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended does not equal $25,000,000, an additional amount of Pearsanta Preferred Stock (“Additional Pearsanta Preferred Stock”) so that the sum of the value of the Pearsanta Preferred Stock plus the Additional Pearsanta Preferred Stock (if any) shall equal $25,000,000. The Pearsanta Preferred Stock shall have such rights, powers, and preferences as set forth in the form of Certificate of Designation of Series A Preferred Stock, the form of which is attached as Exhibit D to the Purchase Agreement.
On January 4, 2024, we entered into a First Amendment to Asset Purchase Agreement with Pearsanta and MDNA, pursuant to which the parties agreed to: (i) the removal of the Upfront Working Capital Payment, (ii) the removal of the Closing Working Capital Payment (as defined in the Purchase Agreement”), and (iii) to increase the maximum amount of payments to be made by us under the Transition Services Agreement (as defined below) from $2.2 million to $3.2 million.
On January 4, 2024, Pearsanta and MDNA entered into a Transition Services Agreement (the “Transition Services Agreement”), pursuant to which MDNA agreed that it would perform, or cause certain of its affiliates or third parties to perform, certain services as described in the Transition Services Agreement for a term of three months in consideration for the payment by Pearsanta of certain fees as provided in the Transition Services Agreement, in an amount not to exceed $3.2 million.
On January 4, 2024, we completed its acquisition of the Acquired Assets and issued to MDNA 50,000 shares of our Common Stock, a warrant to purchase 50,000 shares of our Common Stock, and the Pearsanta Preferred Stock.
Set forth below is a summary of the rights, powers and preferences of the Pearsanta Series A Convertible Preferred Stock:
Series A Convertible Preferred Stock
On January 2, 2024, we filed a Certificate of Designations for the Pearsanta Series A Preferred Stock with the Secretary of State of Delaware (the “Series A Certificate of Designations”). The following is only a summary of the Series A Certificate of Designations, and is qualified in its entirety by reference to the full text of the Series A Certificate of Designations, a copy of which is filed as an exhibit to the Purchase Agreement.
Designation, Amount, and Par Value. The number of Series A Preferred Stock designated is 5,000 shares. The shares of Series A Preferred Stock have a par value of $0.001 per share and a stated value of $5,000 per share.
a)Conversion. each outstanding share of Series A Preferred Stock shall be mandatorily and automatically converted, with no further action on the part of the holders thereof, into 1,000 fully paid and nonassessable shares of Common Stock (1:1,000) (the “Conversion Ratio”) upon the consummation of a firm underwritten initial public offering of the Common Stock for cash effected pursuant to a registration statement or similar document filed by or on behalf of the Company under the Securities Act (a “Qualifying IPO”). If the product of (i) the number of shares of Common Stock issued upon conversion based on the Conversion Ratio and (ii) the initial trading price upon a Qualifying IPO (“IPO Price”), is less than $25,000,000 then the Conversion Ratio shall be adjusted to 1:X (such ratio, the “Adjusted Conversion Ratio”) where:
X = (25,000,000/ IPO Price) / 5000
and the number of shares of Common Stock issued or issuable upon conversion of the Series A Preferred Stock shall be determined based on such Adjusted Conversion Ratio.
-9-
Dividends. Holders of Series A Preferred Stock shall be entitled to receive, and the Corporation shall pay, dividends on shares of Series A Preferred Stock equal (on an as-if-converted-to-Common-Stock basis) to and in the same form as dividends actually paid on shares of the Common Stock
Liquidation. In the event of a liquidation, the holders the Series A Preferred Stock shall be entitled to receive in cash out of the assets of the Company, the same amount that a holder of Common Stock would receive if the Series A Preferred Stock were fully converted (disregarding for such purposes any conversion limitations hereunder) to Common Stock which amounts shall be paid pari passu with all holders of Common Stock.
Company Redemption. At any time prior to a Qualified IPO, the Company may redeem all, or any portion, of the Series A Preferred Stock for cash, at a price per share of Series A Preferred Stock equal to the Stated Value per share.
Voting Rights. The holders of the Series A Preferred Stock shall have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as expressly provided in the Series A Certificate of Designations and where required by the DGCL.
December 2023 PIPE
As previously reported in a Current Report on Form 8-K, on December 29, 2023, we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with the Selling Stockholder for the issuance and sale in a private placement of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,237,114 shares of our Common Stock at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 2,474,228 shares of our Common Stock, at a purchase price of $4.85 per share. The Common Warrants are exercisable immediately upon issuance at an exercise price of $4.60 per share and have a term of exercise equal to three years from the date of issuance. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until the Pre-Funded Warrants are exercised in full. A holder of Pre-Funded Warrants or Warrants (together with its affiliates) may not exercise any portion of a warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder 9.99%) of the Company’s outstanding common stock immediately after exercise.
Pursuant to the Purchase Agreement, we also agreed to reduce the exercise price of 106,594 outstanding warrants to purchase Common Stock of the Company (“Outstanding Warrants”) held by the Purchaser to $4.60 per share in consideration for the cash payment by the Purchaser of $0.125 per share of Common Stock underlying the Outstanding Warrants, effective immediately. As of the date of this prospectus, none of the repriced Outstanding Warrants have been exercised.
In connection with the Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”), dated as of December 29, 2023, with the Selling Stockholder, pursuant to which we agreed to prepare and file a registration statement with the Securities and Exchange Commission (the “SEC”) registering the resale of the shares of Common Stock underlying the Pre-Funded Warrants and the Common Warrants no later than 10 trading days after the date of the Registration Rights Agreement, and to use best efforts to have the registration statement declared effective as promptly as practical thereafter, and in any event no later than 45 days following the date of the Registration Rights Agreement (or 75 days following the date of the Registration Rights Agreement in the event of a “full review” by the SEC).
2-10-
Our financial statements have been prepared assuming
On January 3, 2024, we entered into a settlement agreement and general release with the Selling Stockholder, pursuant to which we agreed to settle an action filed in the United States District Court in the Southern District of New York by the Selling Stockholder against the Company (the “Action”) in consideration of the issuance by us of shares of our Common Stock (the “Settlement Shares”), which will continue asbe issued within two business days following court approval of a going concern, which contemplatesjoint motion to be filed by the realization of assetsSelling Stockholder and the satisfactionCompany. The number of liabilitiesSettlement Shares to be issued will be equal to $1.6 million divided by the closing price of our Common Stock on the day prior to court approval of the joint motion. On January 17, 2024, we issued 296,296 Settlement Shares. Following the issuance of the Settlement Shares, the Selling Stockholder will file a dismissal stipulation in the normal courseAction.
The private placement closed on January 4, 2024. The net proceeds to us from the private placement were approximately $5.5 million, after deducting placement agent fees and expenses and estimated offering expenses payable by us. The Company used a portion of business. Our future viability is largely dependent uponthe proceeds from the December 2023 PIPE to repay outstanding loans from our abilityChief Executive Officer, including accrued interest. In addition, the Company also used a portion of the proceeds to raisesatisfy outstanding obligations under the August Loan Agreement and the October MCA Agreement. As of the date of this prospectus, we are one payment in arrears on the August Loan Agreement.
Note Exchange
On December 29, 2023, we entered into an Exchange Agreement with the holder of our secured promissory note in the principal amount of $2.625 million (the “Note”), pursuant to which the holder agreed, subject to the terms and conditions set forth therein, to exchange the Note, including all accrued but unpaid interest thereon, for an aggregate of 2,625 shares of a new series of convertible preferred stock of the Company, designated as Series B-2 Convertible Preferred Stock. For additional capitalinformation regarding the rights, powers and preferences of the Series B-2 Convertible Preferred Stock, see “Description of Capital Stock—Series A-1 Convertible Preferred Stock”.
Nasdaq Compliance
As previously reported in a Current Report on Form 8-K, on September 29, 2023, we received written notice from The Nasdaq Capital Market (“Nasdaq”) that the Hearing Panel had granted us an exception through December 26, 2023 to finance our operations. Our management expects that future sources of funding may include sales of equity, obtaining loans, or other strategic transactions. Although our management continuesallow us to pursue these plans, there is no assurancecomplete its plan to demonstrate compliance with Nasdaq Listing Rule 5550(b)(1) (the “Stockholders’ Equity Rule”) and Nasdaq Listing Rule 5550(a)(4) (the “Public Float Rule”). On November 21, 2023, we received written notice from Nasdaq that we had regained compliance with the Public Float Rule. On December 29, 2023, we received written notice from Nasdaq that we had regained compliance with the Stockholders’ Equity Rule, but will be successful with this offering or in obtaining sufficient financing on terms acceptablesubject to us to continue to finance our operations, if at all. These circumstances raise substantial doubt on our ability to continue as a going concern, and our financial statements do not include any adjustments that might result from the outcomeMandatory Panel Monitor for a period of these uncertainties.one year.
Corporate Information
We were incorporated as a Delaware corporation on September 28, 2017. Our principal executive offices are located at 11161 Anderson737 N. Fifth Street, Suite 105-10014, Loma Linda, CA 92354,200 Richmond, VA 23219, and our telephone number is (909) 488-0844. Our website address is www.aditxt.com. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common shares.(650) 870-1200.
Implications of Being an Emerging Growth CompanyOur Common Stock trades on The Nasdaq Capital Market under the symbol “ADTX.”
As a company with less than $1.07 billion in revenues during our last fiscal year, we qualify as an emerging growth company as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in 2012. As an emerging growth company, we expect to take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
-11-
We may use these provisions until the last day of our fiscal year following the fifth anniversary of the completion of this offering. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.07 billion or we issue more than $1.07 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
As an emerging growth company, we intend to take advantage of an extended transition period for complying with new or revised accounting standards as permitted by The JOBS Act.
To the extent that we continue to qualify as a “smaller reporting company,” as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an emerging growth company may continue to be available to us as a smaller reporting company, including: (i) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act; (ii) scaled executive compensation disclosures; and (iii) the requirement to provide only two years of audited financial statements, instead of three years.
3
THE OFFERING
| Common stock outstanding prior to this offering | ||
| Common stock to be outstanding |
| |
| ||
| Use of proceeds | We will | |
| Risk | ||
|
(1) The number of shares of common stockour Common Stock to be outstanding after this offering as shown above is based on 7,780,1951,665,214 shares of common stock issued and outstanding as of January 15, 202017, 2023 and excludes the following:as of that date:
| ● | ||
| ● |
| |
Except as otherwise indicated herein, all information in this prospectus assumes:
| ● | ||
| ● |
4-12-
SUMMARY FINANCIAL DATA
We present below our summary historical financial and operating data. The historical financial data as of December 31, 2018 and for the period from September 28, 2017 (Inception) through December 31, 2017, and our unaudited financial data for the nine months ended September 30, 2019 and 2018 has been derived from our audited and unaudited financial statements and the related notes thereto, which are included elsewhere in this prospectus and which have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”).
Our historical results are not necessarily indicative of the financial results to be expected in any future periods. You should read this information in conjunction with our financial statements and related notes included elsewhere in this prospectus, as well as the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Balance Sheets
| September 30, | December 31, | |||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (unaudited) | ||||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS | ||||||||||||
| Cash | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
| TOTAL CURRENT ASSETS | 32,977 | 115,709 | 25,000 | |||||||||
| TOTAL ASSETS | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||
| CURRENT LIABILITIES | ||||||||||||
| Accounts payable and accrued expenses | $ | 1,735,016 | $ | 1,056,226 | $ | 8,566 | ||||||
| Accrued compensation to related parties | 833,651 | 432,615 | 51,334 | |||||||||
| Notes payable - related party | 10,000 | 42,502 | — | |||||||||
| Notes payable | 116,100 | 121,100 | — | |||||||||
| TOTAL CURRENT LIABILITIES | 2,694,767 | 1,652,443 | 59,900 | |||||||||
| Deposit on private placement | — | — | 25,000 | |||||||||
| TOTAL LIABILITIES | 2,694,767 | 1,652,443 | 84,900 | |||||||||
| COMMITMENTS AND CONTINGENCIES | — | — | — | |||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||||||
| Preferred stock, $0.001 par value, 3,000,000 shares authorized, 0 shares issued and outstanding | — | — | — | |||||||||
| Common stock, $0.001 par value, 27,000,000 shares authorized, 7,781,800, 7,527,850, and 7,100,000 shares issued and 7,580,195, 7,527,850, and 7,100,000 shares outstanding | 7,782 | 7,528 | 7,100 | |||||||||
| Treasury stock, 201,605, 0, and 0, shares | (201,605 | ) | — | — | ||||||||
| Additional paid-in capital | 8,270,900 | 4,357,961 | 145,986 | |||||||||
| Accumulated deficit | (10,738,867 | ) | (5,902,223 | ) | (212,986 | ) | ||||||
| TOTAL STOCKHOLDERS’ DEFICIT | (2,661,790 | ) | (1,536,734 | ) | (59,900 | ) | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
5
Statements of Operations
| September 30, | December 31, | |||||||||||||||
| 2019 | 2018 | 2018 | 2017 | |||||||||||||
| Statements of Operations Data: | (unaudited) | (unaudited) | ||||||||||||||
| REVENUE | — | — | — | — | ||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| General and administrative expenses | 4,771,567 | 4,551,814 | 5,044,634 | 212,986 | ||||||||||||
| Research and development | 108,449 | 525,587 | 525,000 | — | ||||||||||||
| Sales and marketing | 147 | 39,459 | 39,837 | — | ||||||||||||
| Total Operating Expenses | 4,880,163 | 5,116,860 | 5,609,471 | 212,986 | ||||||||||||
| NET LOSS FROM OPERATIONS | (4,880,163 | ) | (5,116,860 | ) | (5,609,471 | ) | (212,986 | ) | ||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||
| Interest expense | (1,481 | ) | (2,228 | ) | (3,009 | ) | — | |||||||||
| Gain on forgiveness of debt | 45,000 | — | — | — | ||||||||||||
| Amortization of debt discount | — | (69,237 | ) | (76,757 | ) | — | ||||||||||
| Total Other Income (Expense) | 43,519 | (71,465 | ) | (79,766 | ) | — | ||||||||||
| Net loss before provision for income taxes | (4,836,644 | ) | (5,188,325 | ) | (5,689,237 | ) | (212,986 | ) | ||||||||
| Provision for income taxes | — | — | — | — | ||||||||||||
| NET LOSS | (4,836,644 | ) | (5,188,325 | ) | (5,689,237 | ) | (212,986 | ) | ||||||||
| Net loss per share - basic and diluted | (0.63 | ) | (0.72 | ) | (0.78 | ) | (0.03 | ) | ||||||||
| Weighted average number of shares outstanding during the period - basic and diluted | 7,673,317 | 7,231,510 | 7,261,637 | 7,100,000 | ||||||||||||
An investment in our securities involves a high degree of risk. Before makingThis prospectus contains a discussion of the risks applicable to an investment decision,in our securities. Prior to deciding about investing in our securities, you should give careful considerationcarefully consider the specific factors discussed within this prospectus. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to the following risk factors, in addition to the other information included in this prospectus, includingus or that we currently deem immaterial may also affect our financial statements and related notes, before deciding whether to invest in our securities.operations. The occurrence of any of the adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operationsthese known or prospects. In that case, the trading price of our common stock could decline, andunknown risks might cause you mayto lose all or part of your investment.investment in the offered securities.
Risks Related to Our Financial Position and Need for Capital
We have generated no revenue from commercial sales to date and our future profitability is uncertain.Our financial situation creates doubt whether we will continue as a going concern.
We were incorporated in September 2017 and have a limited operating history and our business is subject to all of the risks inherent in the establishment of a new business enterprise. Our likelihood of success must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with development and expansion of a new business enterprise. Since inception, we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we commence our research and development efforts, conduct clinical trials, and develop manufacturing, sales, marketing, and distribution capabilities. Our net loss as ofattributable to common stockholders for the years ended December 31, 20182022 and for the period from September 28, 2017 (inception) through December 31, 20172021 was $5,689,237$27,612,199 and $212,986,$46,269,097, respectively, and our accumulated deficit as of December 31, 20182022 was $5,902,223.$95,040,362. Our net loss attributable to common stockholders for the nine months ended September 30, 20192023 and 20182022 was $4,836,644$21,579,795 and $5,188,325,$19,466,710, respectively, and our accumulated deficit as of September 30, 20192023 was $10,738,867.$116,620,157. There can be no assurance that the products under development by us will be approved for sale in the U.S. or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. If we are unable to achieve profitability, we may be unable to continue our operations. There can be no assurances that we will be able to achieve a level of revenues adequate to generate sufficient cash flow from operations or additional financing through private placements, public offerings and/or bank financing necessary to support our working capital requirements. To the extent that funds generated from any private placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial doubt about our ability to continue as a going concern. If adequate working capital is not available, we may be forced to discontinue operations, which would cause investors to lose their entire investment.
If we fail to obtain the capital necessary to fund our operations, we will be unable to continue or complete our product development and you will likely lose your entire investment.
We will need to continue to seek capital from time to time to continue development of our lead drug candidate beyond our initial combined Phase I/IIa clinical trial and to acquire and develop other product candidates. Once approved for commercialization, we cannot provide any assurances that any revenues it may generate in the future will be sufficient to fund our ongoing operations. We expect the net proceeds of this offering to be sufficient to satisfy our capital requirements for a period of eighteen (18) months from the date of this prospectus. Accordingly, we believe that we will need to raise substantial additional capital to fund our continuing operations and the development and commercialization of our product candidate in or before eighteen months from the date of this prospectus.
Our business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, develop new or enhance products, acquire complementary products, business or technologies, or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in preferred treatment modalities. In addition, we may need to accelerate the growth of our sales capabilities and distribution beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding when we need it or on favorable terms. We may not be able to raise sufficient funds to commercialize the product candidates we intend to develop.
If we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale back or eliminate our research and development activities, clinical studies, or future operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain technologies or products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. This could result in sharing revenues which we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition, and results of operations.
-13-
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our preclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
Our obligations to certain of our creditors are secured by security interests in our assets, so if we default on those obligations, our creditors could foreclose on some or all of our assets.
Our obligations to certain of our creditors are secured by security interests in our assets. As of December 31, 2023, approximately $24 million was owed to such secured creditors. Of this amount, $5 million has a maturity date of January 31, 2024. In addition, under certain other agreements, we are required to pay $248,000 on a weekly basis to such creditors. If we default on our obligations under these agreements, our secured creditors could foreclose on its security interests and liquidate some or all of these assets, which would harm our financial situation creates doubt whethercondition and results of operations and would require us to reduce or cease operations and possibly seek Bankruptcy Protection.
In the event we pursue Bankruptcy Protection, we will continuebe subject to the risks and uncertainties associated with such proceedings.
In the event we file for relief under the United States Bankruptcy Code, our operations, our ability to develop and execute our business plan and our continuation as a going concern.
The Company was incorporatedconcern will be subject to the risks and uncertainties associated with bankruptcy proceedings, including, among others: our ability to execute, confirm and consummate a plan of reorganization; the additional, significant costs of bankruptcy proceedings and related fees; our ability to obtain sufficient financing to allow us to emerge from bankruptcy and execute our business plan post-emergence, and our ability to comply with terms and conditions of that financing; our ability to continue our operations in the ordinary course; our ability to maintain our relationships with our consumers, business partners, counterparties, employees and other third parties; our ability to obtain, maintain or renew contracts that are critical to our operations on September 28, 2017reasonably acceptable terms and throughconditions; our ability to attract, motivate and retain key employees; the dateability of this prospectus has generated no revenues. Forthird parties to use certain limited safe harbor provisions of the year ended December 31, 2018,United States Bankruptcy Code to terminate contracts without first seeking Bankruptcy Court approval; the Company had a net lossability of $5,689,237. Forthird parties to force us to into Chapter 7 proceedings rather than Chapter 11 proceedings and the nine months ended September 30, 2019,actions and decisions of our stakeholders and other third parties who have interests in our bankruptcy proceedings that may be inconsistent with our operational and strategic plans. Any delays in our bankruptcy proceedings would increase the Company had a net lossrisks of $4,836,644.our being unable to reorganize our business and emerge from bankruptcy proceedings and may increase our costs associated with the bankruptcy process or result in prolonged operational disruption for us. Also, we would need the prior approval of the bankruptcy court for transactions outside the ordinary course of business during the course of any bankruptcy proceedings, which may limit our ability to respond timely to certain events or take advantage of certain opportunities. Because of the risks and uncertainties associated with any bankruptcy proceedings, we cannot accurately predict or quantify the ultimate impact of events that could occur during any such proceedings. There can be no assurancesguarantees that if we seek Bankruptcy Protection we will be able to achieve a level of revenues adequate to generate sufficient cash flowemerge from operations or obtain funding from this offering or additional financing through private placements, public offerings and/or bank financing necessary to support our working capital requirements. To the extent that funds generated from any private placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial doubt about our ability to continueBankruptcy Protection as a going concern. If adequate working capital is not available,concern or that holders of our Common Stock will receive any recovery from any bankruptcy proceedings.
In the event we are unable to pursue Bankruptcy Protection under Chapter 11 of the United States Bankruptcy Code, or, if pursued, successfully emerge from such proceedings, it may be forcednecessary to discontinue operations, whichpursue Bankruptcy Protection under Chapter 7 of the United States Bankruptcy Code for all or a part of our businesses.
In the event we are unable to pursue Bankruptcy Protection under Chapter 11 of the United States Bankruptcy Code, or, if pursued, successfully emerge from such proceedings, it may be necessary for us to pursue Bankruptcy Protection under Chapter 7 of the United States Bankruptcy Code for all or a part of our businesses. In such event, a Chapter 7 trustee would cause investorsbe appointed or elected to lose their entire investment. Our auditorsliquidate our assets for distribution in accordance with the priorities established by the United States Bankruptcy Code. We believe that liquidation under Chapter 7 would result in significantly smaller distributions being made to our stakeholders than those we might obtain under Chapter 11 primarily because of the likelihood that the assets would have indicated that these conditions raise substantial doubt about the Company’s ability to continuebe sold or otherwise disposed of in a distressed fashion over a short period of time rather than in a controlled manner and as a going concern.
-14-
We maywill need to raise substantial additional funding,capital, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or othercease operations.
We do not expect that the net proceeds of this offeringour current cash position will be sufficient to fund our current operations for at least the next 1812 months. However, ourOur operating plan may change as a resultbecause of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for, and to commercialize, our product candidates. Raising funds in the current economic environment may present additional challenges. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities may dilute our existing stockholders. The incurrence of indebtedness would result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay, or discontinue one or more of our research or development programs or the commercialization of any product candidate or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Even if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The capital markets have been unpredictable in the past for unprofitable companies such as ours. In addition, it is generally difficult for development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able tocan consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected.
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
The regulatory approval process is expensive, time-consuming, and uncertain and may prevent us from obtaining approvals for the commercialization of our future product candidates, if any.
We will not be permitted to market our product candidates in the United States until we receive approval from the FDA, or in any foreign countries until we receive the requisite approval from corresponding agencies in such countries. The testing, manufacturing, labeling, approval, selling, marketing and distribution of health-health and life science-related products are subject to extensive regulation, which regulations differ from country to country.
-15-
Successfully completing our clinical program and obtaining approval of a Biologics License Application (“BLA”) is a complex, lengthy, expensive and uncertain process, and the FDA or other applicable foreign regulator may delay, limit or deny approval of our product candidates for many reasons, including, among others, because:
| ● | we may not be able to demonstrate that our product candidates are safe and effective in treating patients to the satisfaction of the FDA or foreign regulator; |
| ● | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA or foreign regulator for marketing approval; |
| ● | the FDA or foreign regulator may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| ● | the FDA or foreign regulator may require that we conduct additional clinical trials; |
| ● | the FDA or foreign regulator may not approve the formulation, labeling or specifications of our product candidates; |
| ● | the contract research organizations (CROs) and other contractors that we may retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials; |
| ● | the FDA or foreign regulator may find the data from preclinical studies and clinical trials insufficient to demonstrate that our product candidate(s) are safe and effective for their proposed indications; |
| ● | the FDA or foreign regulator may disagree with our interpretation of data from our preclinical studies and clinical trials; |
| ● | the FDA or foreign regulator may not accept data generated at our clinical trial sites or may disagree with us over whether to accept efficacy results from clinical trial sites outside the United States or outside the EU, as applicable, where the standard of care is potentially different from that in the United States or in the EU, as applicable; |
| ● | if and when our BLAs or foreign equivalents are submitted to the applicable regulatory authorities, such agencies may have difficulties scheduling the necessary review meetings in a timely manner, may recommend against approval of our application or may recommend or require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| ● | the FDA or foreign regulator may require development of a Risk Evaluation and Mitigation Strategy (REMS), which would use risk minimization strategies to ensure that the benefits of certain prescription drugs outweigh their risks, as a condition of approval or post-approval; |
| ● | the FDA or other applicable foreign regulatory agencies may not approve the manufacturing processes or facilities of third-party manufacturers with which we contract; or |
| ● | the FDA or the other applicable foreign regulatory agencies may change their approval policies or adopt new regulations. |
-16-
We may encounter substantial delays in completing our clinical studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of applicable regulatory authorities.
It is difficult to predict if or when any of our product candidates, will prove safe or effective in humans or will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is expensive, time-consuming, and uncertain as to outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| ● | delays in reaching, or failing to reach, a consensus with regulatory agencies on study design; |
| ● | delays in reaching, or failing to reach, agreement on acceptable terms with a sufficient number of prospective contract research organizations (“CROs”) and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; | |
| ● | delays in obtaining required Institutional Review Board (“IRB”) or Ethics Committee (“EC”) approval at each clinical study site; | |
| ● | delays in recruiting a sufficient number of suitable patients to participate in our clinical | |
| ● | imposition of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites; |
| ● | failure by our CROs, other third parties or us to adhere to the clinical study, regulatory or legal requirements; | |
| ● | failure to perform in accordance with the FDA’s good clinical practices (“GCP”) or applicable regulatory guidelines in other countries; | |
| ● | delays in the testing, validation, manufacturing, and delivery of sufficient quantities of our product candidates to the clinical sites; | |
| ● | delays in having patients’ complete participation in a study or return for post-treatment follow-up; | |
| ● | clinical study sites or patients dropping out of a study; | |
| ● | delay or failure to address any patient safety concerns that arise during the course of a trial; | |
| ● | unanticipated costs or increases in costs of clinical trials of our product candidates; | |
| ● | occurrence of serious adverse events associated with the product candidates that are viewed to outweigh their potential benefits; or | |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
We could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs or ECs of the institutions in which such trials are being conducted, by an independent Safety Review Board (“SRB”) for such trial or by the FDA, European Medicines Agency (“EMA”), or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA, EMA, or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory and commercialization milestones, and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions.
-17-
Clinical study delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition, and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The outcome of preclinical studies and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Further, preclinical and clinical data are often susceptible to various interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have, nonetheless, failed to obtain marketing approval. If the results of our clinical studies are inconclusive or if there are safety concerns or adverse events associated with our other product candidates, we may:
| ● | be delayed in obtaining marketing approval for our product candidates, if approved at all; |
| ● | obtain approval for indications or patient populations that are not as broad as intended or desired; | |
| ● | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; | |
| ● | be required to change the way the product is administered; | |
| ● | be required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; | |
| ● | have regulatory authorities withdraw their approval of a product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy; | |
| ● | be sued; or | |
| ● | experience damage to our reputation. |
If we, ours collaborators, or our contract manufacturing organizations (“CMOs”) fail to comply with applicable regulatory requirements at any stage during the regulatory process, such noncompliance could result in, among other things delays in the approval of applications or supplements to approved applications; refusal of a regulatory authority, including the FDA, to review pending market approval applications or supplements to approved applications; warning letters; fines; import and/or export restrictions; product recalls or seizures; injunctions; total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
Additionally, our product candidates could potentially cause other adverse events that have not yet been predicted. The inclusion of ill patients in our clinical studies may result in deaths or other adverse medical events due to other therapies or medications that such patients may be using. As described above, any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our products.
We may not be able to meet requirements for the chemistry, manufacturing, and control of our drug product candidates.
To receive approval of our products by the FDA and comparable foreign regulatory authorities, we must show that we and our contract manufacturing partners are able to characterize, control and manufacture our drug products safely and in accordance with regulatory requirements. This includes synthesizing the active ingredient, developing an acceptable formulation, performing tests to adequately characterize the formulated product, documenting a repeatable manufacturing process, and demonstrating that our drug products meet stability requirements. Meeting these chemistry, manufacturing and control (“CMC”) requirements is a complex task that requires specialized expertise. If we are not able to meet the CMC requirements, we may not be successful in getting our products approved.
-18-
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
We may encounter delays or difficulties in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials on its current timelines, or at all, and even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Enrollment in our clinical trials may be slower than we anticipate, leading to delays in our development timelines. For example, we may face difficulty enrolling or maintaining a sufficient number of patients in our clinical trials due to the existing alternative treatments approved for any of our targeted indications as patients may decline to enroll or decide to withdraw from our clinical trials due to the risk of receiving placebo. Patient enrollment and retention in clinical trials depends on many factors, including the size of the patient population, the nature of the trial protocol, our ability to recruit clinical trial investigators with the appropriate competencies and experience, the existing body of safety and efficacy data with respect to the study drug, the number and nature of competing treatments and ongoing clinical trials of competing drugs for the same indication, the proximity of patients to clinical sites, the eligibility criteria for the trial and the proportion of patients screened that meets those criteria, our ability to obtain and maintain patient consents, and our ability to successfully complete prerequisite studies before enrolling certain patient populations.
Furthermore, any negative results or new safety signals we may report in clinical trials of our product candidates may make it difficult or impossible to recruit and retain patients in other clinical trials. Similarly, negative results reported by our competitors about their drug candidates may negatively affect patient recruitment in our clinical trials. Also, marketing authorization of competitors in this same class of drugs may impair our ability to enroll patients into our clinical trials, delaying or potentially preventing it from completing recruitment of one or more of our trials.
Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our product candidates or could render further development impossible. In addition, we expect to rely on CROs and clinical trial sites to ensure proper and timely conduct of our future clinical trials, and, while we intend to enter into agreements governing their services, we will be limited in our ability to compel their actual performance.
If our future pre-clinical development andor future clinical Phase I/II studies are unsuccessful, we may be unable to obtain regulatory approval of, or commercialize, our product candidates on a timely basis or at all.
The successful completion of pre-clinical development and multiple clinical trials is critical to the success of our future products. If the pre-clinical development and clinical trials are unsuccessful or produce inconsistent results or unanticipated adverse side effects, or if we are unable to collect reliable data, regulatory approval of our products could be delayed or not given and as a result we may be unable to commercialize our products. Generally, we expect to engage third parties such as consultants, universities or other collaboration partners to conduct clinical trials on our behalf. Incompatible practices or misapplication of our products by these third parties could impair the success of our clinical trials.
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from their sales, if any, may be limited.
If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community, including physicians, patients, and health care payors. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, dosing burden and ease of administration; | |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; |
-19-
| ● | efficacy of our product candidates compared to competing products; | |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; | |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; | |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our product candidates in applicable therapeutic and vaccine guidelines; | |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and patients, we may not generate sufficient revenues and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that does not include the labeling claims necessary or desirable for the successful commercialization for that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a Risk Evaluation and Mitigation Strategy (“REMS”) to assure the safe use of the drug. If the FDA or applicable foreign regulatory agency concludes a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the regulatory agencies will not approve the BLA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The regulatory agencies may also require a REMS for an approved product when new safety information emerges. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of our product candidates. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates.
-20-
Adverse events involving our products may lead the FDAor applicable foreign regulatory agencyto delay or deny clearance for our products or result in product recalls that could harm our reputation, business and financial results.
Once a product receives regulatory clearance or approval, the agency has the authority to require the recall of commercialized products in the event of adverse side effects, material deficiencies or defects in design or manufacture. The authority to require a recall must be based on a regulatory finding that there is a reasonable probability that the product would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency in a product is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a resultbecause of adverse side effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. The regulatory agencies require that certain classifications of recalls be reported to them within ten (10) working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the regulatory agency. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the regulatory agencies. If the regulatory agency disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the regulatory agency could take enforcement action for failing to report the recalls when they were conducted.
The in-licensing of technologies and the successful testing and early development of technologies in the laboratory may not be indicative of future results and may not result in commercially viable technologies or products. Further, our future products may have to be modified from their originally conceived versions in order to reach or be successful in the market.
Positive results from laboratory testing and early developmental successes, may not be predictive of future successful development, commercialization and sales results and should not be relied upon as evidence that products developed from our technologies will become commercially viable and successful. Further, the products we plan to develop in the future may have to be significantly modified from their originally conceived versions in order for us to control costs, compete with similar products, receive market acceptance, meet specific development and commercialization timeframes, avoid potential infringement of the proprietary rights of others, or otherwise succeed in developing our business and earning ongoing revenues. This can be a costly and resource draining activity. What appear to be promising technologies when we license them may not lead to viable technologies or products, or to commercial success.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
We are subject to the Clinical Laboratory Improvement Amendment of 1988, or CLIA, which is a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention, or treatment of disease. Our clinical laboratory is in Richmond, Virginia and must be certified under CLIA for us to perform testing on human specimens. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We currently hold a CLIA certificate to perform high-complexity testing. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing fewer complex tests. CLIA regulations require clinical laboratories like ours to comply with various operational, personnel, facilities administration, quality, and proficiency testing requirements intended to ensure that testing services are accurate, reliable, and timely. CLIA certification is a prerequisite for reimbursement eligibility for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make periodic inspections of our clinical laboratory outside of the renewal process. The failure to comply with CLIA requirements can result in enforcement actions, including the revocation, suspension, or limitation of our CLIA certificate of compliance, as well as a directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit and/or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for assays provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA program requirements and subjected to sanctions, our business and reputation could be harmed. Even if it were possible for us to bring our laboratory back into compliance, we could incur significant expenses and potentially lose revenue in doing so.
-21-
Additionally, certain states require laboratory licenses to test specimens from patients in those states or received from ordering physicians in those states. We may also be subject to regulation in foreign jurisdictions if we seek to expand international distribution of our assays outside the United States.
If we were to lose our CLIA certification or state laboratory licenses, whether because of a revocation, suspension or limitation, we would no longer be able to offer our assays (including our AditxtScore™ platform), which would limit our revenues and harm our business. If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to test specimens from those states.
Our AditxtScore™ tests are currently being offered as a LDTs. Should the FDA disagree that AditxtScore™ tests are LDTs, if our LDTs do not receive the required emergency use authorizations, or if the FDA’s regulatory approach to LDTs should change in the future, our commercialization strategy may be adversely affected, which would negatively affect our results of operations and financial condition.
The FDA has historically asserted its authority to regulate Laboratory Developed Tests (LDTs) as medical devices under the Federal Food, Drug, and Cosmetic Act (the “FDCA”), but it has generally exercised enforcement discretion regarding LDTs. This means that even though the FDA believes it can impose regulatory requirements on LDTs, such as requirements to obtain premarket approval, de novo classification, or clearance of LDTs, it has generally chosen not to enforce those requirements. The FDA has, on occasion, sent warning letters to laboratories offering LDTs that the agency believed were not eligible for enforcement discretion because of how they were developed, validated, performed or marketed and consequent risks to the public.
The FDA considers an LDT to be a test that is developed, validated, and performed within a single laboratory. We are providing AditxtScore™ as a service as a Laboratory Developed Test (LDT) to assess immunity status to COVID-19. Our AditxtScore™ tests are currently manufactured in our Mountain View, CA facility and performed in our Richmond, VA facility. If the FDA believes that the AditxtScore™ is not regulated as an LDT, we may be forced to stop performing AditxtScore™ while we worked to obtain the appropriate FDA authorizations which could negative affect our business, results of operations and financial condition.
On November 15, 2021, FDA revised its guidance document titled “Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised)” (“FDA COVID-19 Testing Guidance”) to require all COVID-19 diagnostic assays conducted as LDTs to apply for EUA authorization within a 60-day period from the revised guidance’s issuance date. The FDA COVID-19 Testing Guidance states that FDA does not intend to object to continued offering of LDTs that are the subject of submitted EUA requests while FDA reviews the EUA requests. The FDA COVID-19 Testing Guidance further states that if FDA declines to issue an EUA or otherwise decides not to authorize a test for any reason, including a determination that there is a lack of adequate data to support authorization, FDA generally expects developers to cease marketing and offering their test within 15 calendar days. Moreover, if FDA identifies a significant problem or concern with a test, based either on the provided information or external reports, FDA generally expects the developer to take appropriate steps to address such problems, which could include a recall of the test and/or notification concerning corrected test reports indicating prior test results may not be accurate. We have submitted EUA requests for our SARS-CoV-2 LDTs, and the applications are pending before FDA. There can be no assurance that the EUA requests that we submitted for our SARS-CoV-2 LDTs will be granted on a timely basis or at all. If FDA declines to issue a EUAs for our SARS-CoV-2 LDTs, we may be required to cease marketing the tests and our business, results of operations and financial condition could be negatively affected. Regardless of if our EUA applications are granted by FDA, we may recall, replace, or make corrections to our LDTs if we become aware of a product concern, which could negatively impact manufacturing, supply, and customer relationships, and may result in adverse regulatory action, including revision or revocation of an EUA.
In addition, there have been numerous legislative proposals to clarify the FDA’s regulatory authority over medical devices. These include two bills reintroduced in 2021: the VALID Act, which would expressly grant the FDA authority to regulate LDTs under a risk-based framework; and the VITAL Act, which would assign LDTs to regulation solely under CLIA and would direct CMS to update its CLIA regulations. We cannot predict if either of these bills will be enacted in their current (or any other) form and cannot quantify the effect of these bills on our business. In the meantime, the regulation by the FDA of LDTs remains uncertain. If FDA premarket review, classification or approval is required for AditxtScore™, our laboratory could be forced to stop performing AditxtScore™ while we worked to obtain the appropriate FDA authorizations which could negative affect our business, results of operations and financial condition.
-22-
We are subject to various governmental regulations relating to the labeling, marketing and sale of our products.
Both before and after a product is commercially released, we have ongoing responsibilities under regulations promulgated by the FDA, the Federal Trade Commission, and similar U.S. and foreign regulations governing product labeling and advertising, distribution, sale, and marketing of our products.
Manufacturers of medical devices are permitted to promote products solely for the uses and indications set forth in the device’s authorization. A number of enforcement actions have been taken against manufacturers that promote products for “off-label” uses (i.e., uses that are not described in the device’s authorization), including actions alleging that claims submitted to government healthcare programs for reimbursement of products that were promoted for “off-label” uses are fraudulent in violation of the Federal False Claims Act or other federal and state statutes and that the submission of those claims was caused by off-label promotion. The failure to comply with prohibitions on “off-label” promotion can result in significant monetary penalties, revocation or suspension of a company’s business license, suspension of sales of certain products, product recalls, civil or criminal sanctions, exclusion from participating in federal healthcare programs, or other enforcement actions. In the United States, allegations of such wrongful conduct could also result in a corporate integrity agreement with the U.S. government that imposes significant administrative obligations and costs.
We and our employees and contractors are subject, directly, or indirectly, to federal, state and foreign healthcare fraud and abuse laws, including false claims laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Our operations are subject to various federal, state, and foreign fraud and abuse laws. These laws may constrain our operations, including the financial arrangements and relationships through which we market, sell and distribute our products.
U.S. federal and state laws that affect our ability to operate include, but are not limited to:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind in return for, the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; |
| ● | federal physician self-referral law, which prohibits a physician from referring a patient to an entity with which the physician (or an immediate family member) has a financial relationship, for the furnishing of certain designated health services for which payment may be made by Medicare or Medicaid, unless an exception applies; |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals, or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other government payers that are false or fraudulent; |
| ● | Section 242 of HIPAA codified at 18 U.S.C. § 1347, which created new federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or from making false or fraudulent statements to defraud any healthcare benefit program (i.e., public or private); |
| ● | federal transparency laws, including the Physician Payments Sunshine Act which requires the tracking and disclosure to the federal government by pharmaceutical and medical device manufacturers of payments and other transfers of value to physicians and teaching hospitals as well as ownership and investment interests that are held by physicians and their immediate family members; and |
| ● | state law equivalents of each of these federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers; state laws that require pharmaceutical and medical device companies to comply with their industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government or otherwise restrict certain payments that may be made to healthcare providers and other potential referral sources; state laws that require drug and medical device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; state laws that prohibit giving gifts to licensed healthcare professionals; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts in certain circumstances, such as specific disease states. |
-23-
In particular, activities and arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, waste and other abusive practices. These laws and regulations may restrict or prohibit a wide range of activities or other arrangements related to the development, marketing or promotion of products, including pricing and discounting of products, provision of customer incentives, provision of reimbursement support, other customer support services, provision of sales commissions or other incentives to employees and independent contractors and other interactions with healthcare practitioners, other healthcare providers and patients.
Because of the breadth of these laws and the narrow scope of the statutory or regulatory exceptions and safe harbors available, our business activities could be challenged under one or more of these laws.
Government expectations and industry best practices for compliance continue to evolve and past activities may not always be consistent with current industry best practices. Further, there is a lack of government guidance as to whether various industry practices comply with these laws, and government interpretations of these laws continue to evolve, all of which create compliance uncertainties. Any non-compliance could result in regulatory sanctions, criminal or civil liability and serious harm to our reputation. It is not always possible to identify and deter misconduct concerning applicable laws, regulations, guidelines, policies and standards, and the precautions we take to detect and prevent this activity may not be effective in preventing such conduct, mitigating risks, or reducing the chance of governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations.
If a government entity opens an investigation into possible violations of any of these laws (which may include the issuance of subpoenas or civil investigative demands), we would have to expend significant resources to defend ourselves against the allegations. Allegations that we, our officers, or our employees violated any one of these laws can be made by individuals called “whistleblowers” who may be our employees, customers, competitors or other parties. Government policy is to encourage individuals to become whistleblowers and file a complaint in federal court alleging wrongful conduct. The government is required to investigate all of these complaints and decide whether to intervene. If the government intervenes and we are required to pay money back to the government, the whistleblower, as a reward, is awarded a percentage of the collection. If the government declines to intervene, the whistleblower may proceed on their own and, if they are successful, they will receive a percentage of any judgment or settlement amount the company is required to pay. The government may also initiate an investigation on its own. Such actions could have a significant impact on our business, including the imposition of significant fines, and other sanctions that may materially impair our ability to run a profitable business. In particular, if our operations are found to be in violation of any of the laws described above or if we agree to settle with the government without admitting to any wrongful conduct or if we are found to be in violation of any other governmental regulations that apply to us, we, our officers and employees may be subject to sanctions, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, the curtailment or restructuring of our operations and the imposition of a corporate integrity agreement, any of which could adversely affect our business, results of operations and financial condition.
Risks Related to theour Company and our Business
Our technology is subject to a licenselicenses from LLU and Stanford, each of which isare revocable in certain circumstances, including in the event we do not achieve certain Milestone Deadlines.payments and milestone deadlines. Without this license,these licenses, we may not be able to continue to develop our product candidates.
The LLU License Agreement may be terminated by LLU in the event of a breach by us of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU. Additional Milestone Deadlines include: (i) the requirement to have regulatory approval of an IND application to initiate first-in-human clinical trials on or before March 31, 2020,2022, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2022,2024, (iii) the completion of Phase III clinical trials by March 31, 20242026 and (iv) biologic licensing approval (BLA) by the FDA by March 31, 2025.2027. If the LLU License Agreement were to be terminated by LLU, we would lose our most significant asset and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations.
-24-
The February 2020 License Agreement with Stanford may be terminated by Stanford if we (i) are delinquent on any report or payments; (ii) are not diligently developing and commercializing Licensed Product (as defined in the February 2020 License Agreement); (iii) miss a milestone described in the agreement; (iv) are in breach of any other provision of the agreement; or (v) if we provide a false report to Stanford. The Termination discussed above will take effect only upon 30 days written notice by Stanford unless we remedy the breach within a 30-day cure period. If the February 2020 License Agreement were to be terminated by Stanford, we would lose a significant asset and may no longer be able to develop our product candidates, which would have a material adverse effect on our operations.
Our results of operations will be affected by the level of royalty and milestone payments that we are required to pay to third parties.
The LLU License Agreement requiresand February 2020 License Agreement with Stanford each require us to remit royalty payments and meet certain performance milestones related to in-licensed intellectual property. Any failure on our part to pay royalties owed or meet milestones could lead to us losing rights under our licenses and could thereby adversely affect our business. As our product sales increase, we may, from time-to-time, disagree with our third-party collaborators as to the appropriate royalties owed and the resolution of such disputes may be costly and may consume management’s time. Furthermore, we may enter into additional license agreements in the future, which may also include royalty payments.
Specifically, we were contractually obligated to pay LLU, within thirty (30) days of July 31, 2018, a milestone fee of $200,000 (the “Milestone Fee”) upon the completion of a “financing” round. As of the date of this prospectus, we have not received a written demand for payment from LLU. The Company intends to pay the Milestone Fee from the proceeds of this offering. There can be no assurance that the Company will raise sufficient capital to pay the Milestone Fee, or complete the offering at all, thus potentially causing a material adverse effect to the Company.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as products and processes being developed at universities and other research institutions. Our competitors have developed, are developing or will develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that may enter the market. We believe that a significant number of products are currently available, under development, and may become commercially available in the future, for the treatment of indications for which we may try to develop product candidates.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may have an advantage in marketing their approved products and may obtain regulatory approval of their product candidates before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less expensive than ours, and may also be more successful than us in manufacturing and marketing their products.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
-25-
Our technologies and products under development, and our business, may fail if we are not able to successfully commercialize them and ultimately generate significant revenues as a result.
Successful development of technologies and our product candidates will require significant additional investment, including costs associated with additional development, completing trials and obtaining regulatory approval, as well as the ability to manufacture or have others manufacture our products in sufficient quantities at acceptable costs while also preserving product quality. Difficulties often encountered in scaling up production include problems involving production yields, quality control and assurance, shortage of qualified personnel, production costs and process controls. In addition, we are subject to inherent risks associated with new technologies and products. These risks include the possibility that any of our technologies or future products may:
| ● | be found unsafe; |
| ● | be ineffective or less effective than anticipated; |
| ● | fail to receive necessary regulatory approvals; |
| ● | be difficult to competitively price relative to alternative solutions; |
| ● | be harmful to consumers or the environment; |
| ● | be difficult to manufacture on an economically viable scale; |
| ● | be subject to supply chain constraints for raw materials; |
| ● | fail to be developed and accepted by the market prior to the successful marketing of alternative products by competitors; |
| ● | be difficult to market because of infringement on the proprietary rights of third parties; or |
| ● | be too expensive for commercial use. |
Furthermore, we may be faced with lengthy market partner or distributor evaluation and approval processes. Consequently, we may incur substantial expenses and devote significant management effort in order to customize products for market partner or distributor acceptance, though there can be no assurance of such acceptance. As a result, we cannot accurately predict the volume or timing of any future sales.
Customers may not adopt our products quickly, or at all.
Customers in the sector in which we operate can be generally cautious in their adoption of new products and technologies. In addition, given the relative novelty of our future planned products (including our AditxtScore™ platform), customers of those products may require education regarding their utility and use, which may delay their adoption. There can be no assurance that customers will adopt our products quickly, or at all.
The significant level of competition in the markets for our products developed in the future may result in pricing pressure, reduced margins or the inability of our future products to achieve market acceptance.
The markets for our future products are intensely competitive and rapidly changing. We may be unable to compete successfully, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products.
-26-
Our competitors may have longer operating histories, significantly greater resources, greater brand recognition and large customer bases than we do. As a result, they may be able to devote greater resources to the manufacture, promotion or sale of their products, receive greater resources and support from market partners and independent distributors, initiate or withstand substantial price competition or more readily take advantage of acquisition or other opportunities.
We may rely on third parties for the productiondistribution of our current and future products, including our AditxtScore™ platform. If these parties do not distribute our products in a satisfactory or timely manner, in sufficient quantities or at an acceptable cost, our sales and development efforts could be delayed or otherwise negatively affected.
We rely on third parties for the distribution of our current and future products, including our AditxtScore™ platform. Our reliance on third parties to distribute products may present significant risks to us, including the risk that should any of these third parties fail to adequately distribute our products and services to end consumers and other market participants, our business may be materially harmed. Additionally, if we need to enter into agreements for the distribution of our future products with other third parties, there can be no assurance we will be able to do so on favorable terms, if at all.
We may rely on third parties to produce our future products. If these parties do not produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our sales and development efforts could be delayed or otherwise negatively affected.
We may rely on third parties for the manufacture of our future products. Our reliance on third parties to manufacture our future products may present significant risks to us, including the following:
| ● | reduced control over delivery schedules, yields and product reliability; |
| ● | price increases; |
| ● | manufacturing deviations from internal and regulatory specifications; |
| ● | the failure of a key manufacturer to perform as we require for technical, market or other reasons; |
| ● | difficulties in establishing additional manufacturer relationships if we are presented with the need to transfer our manufacturing process technologies to them; |
| ● | misappropriation of our intellectual property; and |
| ● | other risks in potentially meeting our product development schedule or satisfying the requirements of our market partners, distributors, direct customers and end users. |
If we need to enter into agreements for the manufacturing of our future products, there can be no assurance we will be able to do so on favorable terms, if at all.
If we are unable to establish successful relations with third-party market partners or distributors, or these market partners or distributors do not focus adequate resources on selling our products or are otherwise unsuccessful in selling them, sales of our products may not develop.
We anticipate relying on independent market partners and distributors to distribute and assist us with the marketing and sale of our products. Our future revenue generation and growth will depend in large part on our success in establishing and maintaining this sales and distribution channel. If our market partners and distributors are unable to sell our products, or receive negative feedback from end users, they may not continue to purchase or market our products. In addition, there can be no assurance that our market partners and distributors will focus adequate resources on selling our products to end users or will be successful in selling them. Many of our potential market partners and distributors are in the business of distributing and sometimes manufacturing other, possibly competing, products. As a result, these market partners and distributors may perceive our products as a threat to various product lines currently being distributed or manufactured by them. In addition, these market partners and distributors may earn higher margins by selling competing products or combinations of competing products. If we are unable to establish successful relationships with independent market partners and distributors, we will need to further develop our own sales and distribution capabilities, which would be expensive and time-consuming and might not be successful.
-27-
If we are not able to attract and retain highly skilled employees and contractors, we may not be able to implement our business model successfully.
We will rely upon employees and third-party consultant/contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled personnel. In order to do so, we may need to pay higher compensation, fees, and/or other incentives to our employees or consultants than we currently expect, and such higher compensation payments would have a negative effect on our operating results. Competition for experienced, high-quality employees, consultants and contractors is intense and we cannot assure that we will be able to recruit and retain such personnel. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel could impair our ability to develop new products and manage our business effectively.
The loss of our management team or other key personnel would have an adverse impact on our future development and impair our ability to succeed.
In the early stages of development, our business will be significantly dependent on the Company’s management team and other key personnel. Our success will be particularly dependent upon Mr. Amro Albanna and Dr. Shahrokh Shabahang. The loss of any one of these individuals or any other future key personnel could have a material adverse effect on the Company and our ability to further execute our intended business.
The use of our products may be limited by regulations, and we may be exposed to product liability and remediation claims.
The use of our planned products may be regulated by various local, state, federal and foreign regulators. Even if we are able to comply with all such regulations and obtain all necessary registrations, we cannot provide assurance that our future products will not cause injury to the environment, people, or animals and/or otherwise have unintended adverse consequences, under all circumstances. For example, our products may be improperly combined with other chemicals or, even when properly combined, our products may be blamed for damage caused by those other chemicals. The costs of remediation or products liability could materially adversely affect our results, financial condition and operations.
We may be held liable for, or incur costs to settle, liability and remediation claims if any products we develop, or any products that use or incorporate any of our technologies, cause injury or are found unsuitable during product testing, manufacturing, marketing, sale or use. These risks exist even with respect to products that have received, or may in the future receive, regulatory approval, registration or clearance for commercial use. We cannot guarantee that we will be able to avoid product liability exposure.
At the stage customary to do so, we expect to maintain product liability insurance at levels we believe are sufficient and consistent with industry standards for like companies and products. However, we cannot guarantee that our product liability insurance will be sufficient to help us avoid product liability-related losses. In the future, it is possible that meaningful insurance coverage may not be available on commercially reasonable terms or at all. In addition, a product liability claim could result in liability to us greater than our assets or insurance coverage. Moreover, even if we have adequate insurance coverage, product liability claims or recalls could result in negative publicity or force us to devote significant time and attention to these matters, which could harm our business.
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our Company.
We do not expect that internal control over financial accounting and disclosure, even if timely and well established, will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to prevent error or fraud could materially adversely affect our business.
-28-
We are currently in default of several outstanding promissory notes.COVID-19 may impact our operations.
Currently,On January 30, 2020, the World Health Organization declared the COVID-19 coronavirus outbreak a “Public Health Emergency of International Concern” and on March 10, 2020, declared it to be a pandemic. Actions taken around the world to help mitigate the spread of the coronavirus include restrictions on travel, and quarantines in certain areas, and forced closures for certain types of public places and businesses. The COVID-19 coronavirus and actions taken to mitigate it have had and are expected to continue to have an adverse impact on the economies and financial markets of many countries, including the geographical area in which the Company hasoperates. While it is unknown how long these conditions will last and what the complete financial effect will be to the Company, capital raise efforts and additional development of our technologies may be negatively affected.
Risks Related to Our Acquisition Strategy
Our acquisition strategy exposes us to substantial risk.
Our acquisition of companies is subject to substantial risk, including but not limited to the failure to identify material problems during due diligence (for which we may not be indemnified post-closing), the risk of over-paying for assets (or not making acquisitions on an accretive basis), the ability to obtain or retain customers and the risks of entering markets where we have limited experience. While we perform due diligence on prospective acquisitions, we may not be able to discover all potential operational deficiencies in such entities.
Our acquisition targets may not perform as expected or the returns from such businesses may not support the financing utilized to acquire them or maintain them. Furthermore, integration and consolidation of acquired businesses requires substantial human, financial and other resources and may divert management’s attention from our existing business concerns, disrupt our ongoing business or not be successfully integrated. Even if we consummate businesses that we believe will be accretive, those businesses may in fact result in a decrease in revenues as a result of incorrect assumptions in our evaluation of such businesses, unforeseen consequences, or other external events beyond our control. Furthermore, if we consummate any future acquisitions, our capitalization and results of operations may change significantly, and stockholders will generally not have the opportunity to evaluate the economic, financial, and other relevant information that we will consider in determining the application of these funds and other resources. As a result, the consummation of acquisitions may have a material adverse effect on our business, financial condition, results of operations and cash flows.
We may experience difficulty as we evaluate, acquire and integrate businesses that we may acquire, which could result in drains on our resources, including the attention of our management, and disruptions of our on-going business.
We acquire small to mid-sized businesses in various outstanding promissory notesindustry segments. Generally, because such businesses are privately held, we may experience difficulty in default. Thereevaluating potential target businesses as much of the information concerning these businesses is not publicly available. Therefore, our estimates and assumptions used to evaluate the operations, management and market risks with respect to potential target businesses may be subject to various risks and uncertainties. Further, the time and costs associated with identifying and evaluating potential target businesses may cause a substantial drain on our resources and may divert our management team’s attention away from the operations of our businesses for significant periods of time.
In addition, we may have difficulty effectively integrating and managing acquisitions. The management or improvement of businesses we acquire may be hindered by a number of factors, including limitations in the standards, controls, procedures and policies implemented in connection with such acquisitions. Further, the management of an acquired business may involve a substantial reorganization of the business’ operations resulting in the loss of employees and customers or the disruption of our ongoing businesses. We may experience greater than expected costs or difficulties relating to an acquisition, in which case, we might not achieve the anticipated returns from any particular acquisition.
-29-
We may not be able to effectively integrate the businesses that we acquire.
Our ability to realize the anticipated benefits of acquisitions will depend on our ability to integrate those businesses with our own. The combination of multiple independent businesses is a riskcomplex, costly and time-consuming process and there can be no assurance that we will be able to successfully integrate businesses into our business, or if such integration is successfully accomplished, that such integration will not be costlier or take longer than presently contemplated. Integration of future acquisitions may include various risks and uncertainties, including the continued defaultfactors discussed in the paragraph below. If we cannot successfully integrate and manage the businesses within a reasonable time, we may not be able to realize the potential and anticipated benefits of such notesacquisitions, which could preventhave a material adverse effect on our stock price, business, cash flows, results of operations and financial position.
We will consider acquisitions that we believe will complement, strengthen and enhance our growth. We evaluate opportunities on a preliminary basis from time to time, but these transactions may not advance beyond the preliminary stages or be completed. Such acquisitions are subject to various risks and uncertainties, including:
| ● | the inability to integrate effectively the operations, products, technologies and personnel of the acquired companies (some of which are in diverse geographic regions) and achieve expected synergies; |
| ● | the potential disruption of existing business and diversion of management’s attention from day-to-day operations; |
| ● | the inability to maintain uniform standards, controls, procedures and policies; |
| ● | the need or obligation to divest portions of the acquired companies; |
| ● | the potential failure to identify material problems and liabilities during due diligence review of acquisition targets; |
| ● | the potential failure to obtain sufficient indemnification rights to fully offset possible liabilities associated with acquired businesses; and |
| ● | the challenges associated with operating in new geographic regions. |
The integration of our acquisitions may result in significant accounting charges that adversely affect the announced results of our Company.
The financial results of our Company may be adversely affected by cash expenses and non-cash accounting charges incurred in connection with our recent acquisitions. In addition to the anticipated cash charges, costs associated with the amortization of intangible assets are expected. The price of our Common Stock could decline to the extent our financial results are materially affected by the foregoing charges or if the foregoing charges are larger than anticipated.
Our planned acquisitions may result in unexpected consequences to our business and results of operations.
Although we believe that our planned acquisitions will generally be subject to risks similar to those to which we are subject to in our existing operations, we may not have discovered all risks applicable to these businesses during the due diligence process. Some of these risks could produce unexpected and unwanted consequences for us. Undiscovered risks may result in us incurring financial liabilities, which could be material and have a negative impact on our business operations.
Failure to manage our growing and changing business could have a material adverse effect on our business, prospects, financial condition, and results of operations.
As we grow, we expect to encounter additional challenges to our internal processes, capital commitment process, and acquisition funding and financing capabilities. Our existing operations, personnel, systems, and internal control may not be adequate to support our growth and expansion and may require us to make additional unanticipated investments in our infrastructure. To manage the future growth of our operations, we will be required to improve our administrative, operational, and financial systems, procedures, and controls, and maintain, expand, train, and manage our growing employee base. If we are unable to manage our growth effectively, we may not be able to take advantage of market opportunities, execute our business strategies successfully or respond to competitive pressures. As a result, our business, prospects, financial condition, and results of operations could be materially and adversely affected.
-30-
We face competition for businesses that fit our acquisition strategy and, therefore, we may have to acquire targets at sub-optimal prices or, alternatively, forego certain acquisition opportunities.
Our acquisition strategy is focused on the acquisition of small to mid-sized businesses. In pursuing such acquisitions, we expect to face strong competition from a wide range of other potential purchasers. Although the pool of potential purchasers for such businesses is typically smaller than for larger businesses, those potential purchasers can be aggressive in their approach to acquiring such businesses. Furthermore, we expect that we will need to use third-party financing in order to fund some or all of these potential acquisitions, thereby increasing our acquisition costs. To the extent that other potential purchasers do not need to obtain third-party financing or are able to obtain such financing on more favorable terms, they may be in a position to be more aggressive with their acquisition proposals. As a result, in order to be competitive, our acquisition proposals may need to be aggressively priced, including at price levels that exceed what we originally determined to be fair or appropriate. Alternatively, we may determine that we cannot pursue on a cost-effective basis what would otherwise be an attractive acquisition opportunity.
We may not be able to successfully fund acquisitions due to the unavailability of equity or debt financing on acceptable terms, which could impede the implementation of our acquisition strategy.
We intend to finance acquisitions primarily through additional debt and equity financings. Because the timing and size of acquisitions cannot be readily predicted, we may need to be able to obtain funding on short notice to benefit fully from attractive acquisition opportunities. The sale of additional shares of any class of equity will be subject to market conditions and investor demand for such shares at prices that may not be in the best interest of our stockholders. The sale of additional equity securities could also result in dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if at all. These risks may materially adversely affect our ability to pursue our acquisition strategy.
We may change our management and acquisition strategies without the consent of our stockholders, which may result in a determination by us to pursue riskier business activities.
We may change our strategy at any time without the consent of our stockholders, which may result in our acquiring businesses or assets that are different from, raisingand possibly riskier than, the strategy described in this prospectus. A change in our strategy may increase our exposure to interest rate and currency fluctuations, subject us to regulation under the Investment Company Act or subject us to other risks and uncertainties that affect our operations and profitability.
In the future, we may seek to enter into credit facilities to help fund our acquisition capital and working capital needs. These credit facilities may expose us to additional capitalrisks associated with leverage and may inhibit our operating flexibility.
We may seek to enter into credit facilities with third-party lenders to help fund our acquisitions. Such credit facilities will likely require us to pay a commitment fee on the undrawn amount and will likely contain a number of affirmative and restrictive covenants. If we violate any such covenants, our lenders could accelerate the maturity of any debt outstanding. Such debt may be secured by our assets, including the stock we may own in businesses that we acquire. Our ability to meet our debt service obligations may be affected by events beyond our control and will depend primarily upon cash produced by businesses that we currently manage and may acquire in the future and distributed or paid to us. Any failure to comply with the terms of our indebtedness may have a material adverse effect on our financial condition.
In addition, we expect that such continued defaultcredit facilities will bear interest at floating rates which will generally change as interest rates change. We will bear the risk that the rates that we are charged by our lenders will increase faster than we can grow the cash flow from our businesses or businesses that we may acquire in the future, which could resultreduce profitability, materially adversely affect our ability to service our debt, cause us to breach covenants contained in our havingthird-party credit facilities and reduce cash flow available for distribution.
-31-
If, in the future, we cease to allocate somecontrol and operate our businesses or other businesses that we acquire in the future or engage in certain other activities, we may be deemed to be an investment company under the Investment Company Act.
We have the ability to make investments in businesses that we will not operate or control. If we make significant investments in businesses that we do not operate or control, or that we cease to operate or control, or if we commence certain investment-related activities, we may be deemed to be an investment company under the Investment Company Act. Our decision to sell a business will be based upon financial, operating and other considerations rather than a plan to complete a sale of a business within any specific time frame. If we were deemed to be an investment company, we would either have to register as an investment company under the proceedsInvestment Company Act, obtain exemptive relief from the Securities and Exchange Commission, or the SEC, or modify our investments or organizational structure or our contract rights to fall outside the definition of an investment company. Registering as an investment company could, among other things, materially adversely affect our financial condition, business and results of operations, materially limit our ability to borrow funds or engage in this offering away fromother transactions involving leverage and require us to add directors who are independent of us and otherwise will subject us to additional regulation that will be costly and time-consuming.
If intangible assets and goodwill that we recorded in connection with our acquisitions become impaired, we may have to take significant charges against earnings.
In connection with the accounting for our completed acquisitions, we may be required to record a significant amount of intangible assets, including developed technology, in-process research and development, budget.and customer relationships relating to the acquired product lines, and goodwill. Under generally accepted accounting principles in the United States, we must assess, at least annually and potentially more frequently, whether the value of indefinite-lived intangible assets and goodwill have been impaired. Intangible assets and goodwill are assessed for impairment in the event of an impairment indicator. Any reduction or impairment of the value of intangible assets and goodwill will result in a charge against earnings, which could materially adversely affect our results of operations and shareholders’ equity in future periods.
While we have entered into a Merger Agreement with Evofem, we cannot assure you that the transactions contemplated by the Merger Agreement will be consummated or, that if such transactions are consummated, they will be accretive to stockholder value.
The Merger Agreement may be terminated by us or Evofem if the merger has not been consummated by May 8, 2023. We can provide no assurance that the conditions to the closing of the merger, including but not limited to, the approval of the transaction by our stockholders, will be completed in the time frame or in the manner currently anticipated, or that we will recognize the anticipated benefits of the transaction.
In connection with the Merger Agreement, we entered into an Assignment Agreement with the holders of certain senior indebtedness of Evofem, pursuant to which we issued secured notes to such holders in the aggregate principal amount of $13 million, which we will remain obligated on whether or not the merger closes.
In connection with the Merger Agreement, we entered into an Assignment Agreement dated December 11, 2023 (the “Assignment Agreement”) with the holders of certain senior indebtedness of Evofem, pursuant to which such holders assigned their notes to the Company in consideration for the issuance by us of (i) an aggregate principal amount of $5 million in secured notes of the Company which are due on January 31, 2024, (ii) an aggregate principal amount of $8 million in secured notes of the Company which are due on September 30, 2024, (iii) an aggregate principal amount of $5 million in ten-year unsecured notes (the “Unsecured Notes”), and (iv) payment of $154,480 in respect of net sales of Phexxi in respect of the calendar quarter ended September 30, 2023, which was paid on December 14, 2023. We will remain obligated on such amounts whether or not the merger closes.
Risks Relating to Our Intellectual Property Rights
The failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively.
In order for our business to be viable and to compete effectively, we need to develop and maintain, and we will heavily rely on, a proprietary position with respect to our technologies and intellectual property. However, there are significant risks associated with our actual or proposed intellectual property. The risks and uncertainties that we face with respect to our rights principally include the following:
| ● | pending patent applications, we have filed or will file may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | we may be subject to interference proceedings; |
| ● | we may be subject to reexamination proceedings; |
| ● | we may be subject to post grant review proceedings; |
| ● | we may be subject tointer partesreview proceedings; |
-32-
| ● | we may be subject to derivation proceedings; |
| ● | we may be subject to opposition proceedings in the U.S. or in foreign countries; |
| ● | any patents that are issued to us may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge patents licensed or issued to us; |
| ● | other companies may have independently developed and patented (or may in the future independently develop and patent) similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design around technologies we have licensed or developed; |
| ● | enforcement of patents is complex, uncertain, and very expensive and we may not be able to secure, enforce and defend our patents; and |
| ● |
We cannot be certain that any patents will be issued as a resultbecause of any pending or future applications, or that any patents, once issued, will provide us with adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in scientific or patent literature often lags behind actual discoveries, we cannot be certain that we or our licensors were the first to invent or to file patent applications covering them.
It is also possible that others may have or may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. There is no guarantee that such licenses will be available based on commercially reasonable terms. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
If we are unable to obtain and maintain patent protection for our products, or if the scope of the patent protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products could be impaired.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our development output before it is too late to obtain patent protection.
The patent position of life science companies generally is highly uncertain, involves complex legal and factual questions and has in past years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States and we may fail to seek or obtain patent protection in all major markets. For example, unlike the U.S., European patent law restricts the patentability of methods of treatment of the human body. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection, even post-grant.
Recent patent reform legislation has increased the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office, or USPTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
-33-
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination,inter partesreview, post-grant review or interference proceedings challenging our patent rights (whether licensed or otherwise held) or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights (whether licensed or otherwise held), allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications (whether licensed or otherwise held) is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our patent applications (whether licensed or otherwise held) result in the issuance of patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our licensed or owned patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products. Given the amount of time required for the development, testing and regulatory review of new life science product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property rights portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our intellectual property rights, which could be expensive, time-consuming, and ultimately unsuccessful.
Competitors may infringe our intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property or that our intellectual property is invalid or unenforceable. In addition, in a patent infringement proceeding, a court may decide that a licensed or owned patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover that technology. Moreover, lawsuits to protect or enforce our intellectual property rights could be expensive, time-consuming, and ultimately unsuccessful.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the life sciences industry. We cannot guarantee that our product candidates will not infringe third-party patents or other proprietary rights. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, includinginter partesreview, interference, or derivation proceedings before the USPTO and similar bodies in other countries. Third parties may assert infringement claims against us based on existing intellectual property rights and intellectual property rights that may be granted in the future.
-34-
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our own patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees and annuities on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter our markets, which could have a material adverse effect on our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Certain of our employees and contractors were previously employed at universities or other companies, including potential competitors. Although we try to ensure that our employees and contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims, and any such litigation could have an unfavorable outcome.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and adverse results, and be a distraction to management.
-35-
Some intellectual property which we own or have licensed may have been discovered through government funded programs such as, for example, the government funded programs referenced in intellectual property licensed under the LLU License Agreement, and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for United States industry. Compliance with such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements and limit our ability to contract with non-U.S. manufacturers.
Some of the intellectual property rights we own or have licensed have been generated through the use ofusing United States government funding and may therefore be subject to certain federal regulations. As a result, the United States government may have certain rights to intellectual property embodied in our current or future products and product candidates pursuant to the Bayh-Dole Act of 1980. These United States government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the United States government has the right to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in rights”). The United States government also has the right to take title to these inventions if we fail to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the United States government may acquire title to these inventions in any country in which a patent application is not filed within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources. In addition, the United States government requires that any products embodying the subject invention or produced through the use ofusing the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for United States manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property. Any exercise by the government of any of the foregoing rights could harm our competitive position, business, financial condition, results of operations and prospects.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common stock.Common Stock. Such litigation or proceedings could increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
We may spend considerable resources developing and maintaining patents, licensing agreements and other intellectual property that may later be abandoned or may otherwise never result in products brought to market.
Not all technologies and candidate products that initially show potential as the basis for future products ultimately meet the rigors of our development process and as a result may be abandoned and/or never otherwise result in products brought to market. In some cases, prior to abandonment we may be required to incur significant costs developing and maintaining intellectual property and/or maintaining license agreements and our business could be harmed by such costs.
We rely on information technology, and if we are unable to protect against service interruptions, data corruption, cyber-based attacks or network security breaches, our operations could be disrupted, and our business could be negatively affected.
We rely on information technology networks and systems to process, transmit and store electronic and financial information; to coordinate our business; and to communicate within our Company and with customers, suppliers, partners and other third-parties.third parties. These information technology systems may be susceptible to damage, disruptions or shutdowns, hardware or software failures, power outages, computer viruses, cyber-attacks, telecommunication failures, user errors or catastrophic events. If our information technology systems suffer severe damage, disruption or shutdown, and our business continuity plans do not effectively resolve the issues in a timely manner, our operations could be disrupted, and our business could be negatively affected. In addition, cyber-attacks could lead to potential unauthorized access and disclosure of confidential information, and data loss and corruption. There is no assurance that we will not experience these service interruptions or cyber-attacks in the future.
-36-
Risks Related to the Offering and Our Common Stock
There is no assurance that an active and liquid trading market in our common stock will develop.
This offering will close only if our common stock is accepted to be listed on The Nasdaq Capital Market. There can be no assurance any broker will be interested in trading our common stock. Therefore, it may be difficult to sell any securities you purchase in this offering if you desire or need to sell them. Neither we nor the underwriters can provide any assurance that an active and liquid trading market in our common stock will develop or, if developed, that the market will continue.
There is no guarantee that we will successfully have our common stock listed on The Nasdaq Capital Market. Even if our common stock is accepted for listing on The Nasdaq Capital Market upon our satisfaction of the exchange’s initial listing criteria, the exchange may subsequently delist our common stock.
In the event we are able to list our common stock on The Nasdaq Capital Market upon our satisfaction of the exchange’s initial listing criteria, the exchange will require us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our common stock. If we fail to meet these continued listing requirements, our common stock may be subject to delisting. If our common stock is delisted and we are not able to list our common stock on another national securities exchange, we expect our securities would be quoted on an over-the-counter market. If this were to occur, our stockholders could face significant material adverse consequences, including limited availability of market quotations for our common stock and reduced liquidity for the trading of our securities. In addition, we could experience a decreased ability to issue additional securities and obtain additional financing in the future. Even if our common stock is listed on The Nasdaq Capital Market, there can be no assurance that an active trading market for our common stock will develop or be sustained after our initial listing.
The Nasdaq Capital Market may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
If we are approved to list our common stock on The Nasdaq Capital Market, we cannot assure you that our securities will be, or will continue to be, listed on such exchange in the future. In order to continue to have our securities listed on The Nasdaq Capital Market, we must maintain and comply with certain standards including, but not limited to, standards relating to corporate governance, stockholders’ equity and market value of listed securities. If we are unable to comply with the continued listing requirements of The Nasdaq Capital Market, our securities may be delisted from The Nasdaq Capital Market. If our securities are delisted from The Nasdaq Capital Market, we could face significant adverse consequences including, but not limited to:
The warrantsWe received a written notice from Nasdaq that we regained compliance with the filing requirement in Listing Rule 5550(b)(1) (the “Equity Rule”), however, we will remain subject to a Mandatory Panel Monitor for a period of one year ending on December 29, 2024. If, within that one-year monitoring period, we are unable to maintain compliance with the Equity Rule, it may not have value.result in our Common Stock being delisted from the Nasdaq Stock Market.
The warrantsOn September 29, 2023, we received written notice from Nasdaq that the Hearing Panel had granted the Company an exception through December 26, 2023 to allow the Company to complete its plan to demonstrate compliance with the Equity Rule and Nasdaq Listing Rule 5550(a)(4) (the “Public Float Rule”). On November 21, 2023, we received written notice from Nasdaq that we had regained compliance with the Public Float Rule. On December 29, 2023, we received written notice from Nasdaq that we had regained compliance with the Stockholders’ Equity Rule, but will be subject to a Mandatory Panel Monitor for a period of one year. If, within that one-year monitoring period, we are unable to maintain compliance with the Equity Rule, it may result in our Common Stock being offered by us in this offering are exercisable for the period commencing on the date that is six-monthsdelisted from the date of issuance of the warrant, have an exercise price of $6.875 per share, and expire three (3) years from the date of issuance. In the event that our common stock does not exceed the exercise price of the warrants during the period when the warrants are exercisable, the warrants may not have any value.
Holders of our warrants will have no rights as shareholders until they acquire shares of our common stock, if ever.
If you acquire the warrants to purchase shares of our common stock in this offering, you will have no rights with respect to our common stock until you acquire shares of such common stock upon exercise of your warrants. Upon exercise of your warrants, you will be entitled to exercise the rights of a holder of common stock only as to matters for which the record date occurs after the exercise date.
There is no public market for the warrants being offered by us in this offering and an active trading market for the warrants is not expected to develop.
There is no established public trading market for the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply for any listing of the warrants on The Nasdaq Capital Market or any other securities exchange or nationally recognized trading system. Without an active market, the liquidity of the warrants will be severely limited.Stock Market.
We do not expect to pay dividends in the foreseeable future.
We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any and all future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their securities, and holders may be unable to sell their securities on favorable terms or at all. We cannot assure you of a positive return on your investment or that you will not lose the entire amount of your investment.
Upon dissolution of our Company, you may not recoup all or any portion of your investment.
In the event of a liquidation, dissolution or winding-up of our Company, whether voluntary or involuntary, our assets would be used to pay all of our debts and liabilities, and only thereafter would any remaining assets be distributed to our stockholders, subject to rights of the holders of the Preferred Stock, if any, on apro ratabasis. There can be no assurance that we will have assets available from which to pay any amounts to our stockholders upon such a liquidation, dissolution or winding-up. In such an event, you would lose all of your investment.
Limitation of Liability and Indemnification of Management.
The Delaware General Corporation Law and the Company’s Amended and Restated Certificate of Incorporation providesprovide for the limitation of the liability of directors for monetary damages. Such provisions may discourage shareholders from bringing a lawsuit against directors for breaches of fiduciary duty and may also have the effect of reducing the likelihood of derivative litigation against directors and officers even though such action, if successful, might otherwise be a benefit to the Company’s shareholders. In addition, a shareholder’s investment in the Company may be adversely affected to the extent that costs of settlement and damage awards against the Company’s officers or directors are paid by the Company pursuant to such provisions. Additionally, in accordance with Delaware law and the Company’s Amended and Restated Certificate of Incorporation, the Company shall indemnify, hold harmless and provide advancement of expenses, to the fullest extent permitted by applicable law, directors, officers, employees, and agents that are made a party or threatened to be made a party to legal proceedings by reason of the fact that such parties were working at the request of the Company. We direct you to the Company’s Amended and Restated Certificate of Incorporation for more information.
Anti-takeover provisions under Delaware law could discourage, delay or prevent a change in control of our Company and could affect the trading price of our securities.
We are a Delaware corporation, and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders.
Upon completion of this offering, we will incur increased costs as a result of our public reporting obligations, and ourOur management team will beis required to devote substantial time to newpublic company compliance initiatives.
Upon the effectiveness of the Form S-1, of which this prospectus formsAs a part, particularly afterpublicly reporting company, we are no longer an “emerging growth company,” we will incur significant legal, accounting, and other expenses that we did not incur as a private company.expenses. Our management and other personnel would need to devote a substantial amount of time to comply with our reporting obligations. Moreover, these reporting obligations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
-37-
Failure to develop our internal controls over financial reporting as we grow could have an adverse impact on us.
As our Company matures, we will need to develop our current internal control systems and procedures to manage our growth. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish appropriate controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition, or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure of management’s assessment of our internal controls over financial reporting or disclosure of our public accounting firm’s attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
Our executive officers, directors, and their respective affiliates will continue to exercise significant control over our Company after this offering, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control.
Immediately following the completion of this offering, and disregarding any shares of common stock that they purchase in this offering, if any, the existing holdings of our executive officers, directors, and their affiliates, will represent beneficial ownership, in the aggregate, of approximately 35.97% of our outstanding common stock. Please see “Security Ownership of Beneficial Owners and Management” on page 51 for more information. As a result, these stockholders will be able to influence our management and affairs and control the outcome of matters submitted to our stockholders for approval, including the election of directors and any sale, merger, consolidation, or sale of all or substantially all of our assets. These stockholders acquired their shares of common stock for substantially less than the price of the shares of common stock being acquired in this offering, and these stockholders may have interests, with respect to their common stock, that are different from those of investors in this offering and the concentration of voting power among one or more of these stockholders may have an adverse effect on the price of our common stock. In addition, this concentration of ownership might adversely affect the market price of our common stock by:
If you purchase our Units in this offering, you will incur immediate and substantial dilution in the book value of your Shares.
You will suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. Assuming an initial offering price of $5.50 per unit, purchasers in this offering will experience immediate dilution of $4.57 per share of common stock in net tangible book value of the common stock. See “Dilution” on page 29 for a more detailed description of the dilution to new investors in the offering.
We have broad discretion in how we use the proceeds of this offering and may not use these proceeds effectively, which could affect our results of operations and cause our common stock and Warrant price to decline.
We will have considerable discretion in the application of the net proceeds of this offering. We intend to use the net proceeds from this offering to fund our business strategy, including without limitation, new and ongoing product development expenses, offering expenses, working capital and other general corporate purposes, which may include funding for the hiring of additional personnel. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
If our stock price fluctuates after the offering, you could lose a significant part of your investment.
The market price of our common stock could be subject to wide fluctuations in response to, among other things, the risk factors described in this section of this prospectus, and other factors beyond our control, such as fluctuations in the valuation of companies perceived by investors to be comparable to us. Furthermore, the stock markets have experienced price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions, such as recessions, interest rate changes or international currency fluctuations, may negatively affect the market price of our common stock. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
After the completion of this offering, we may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and pharmaceutical companies have experienced significant stock price volatility in past years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.Common Stock.
We could issue “blank check” preferred stock without stockholder approval with the effect of diluting interests of then-current stockholders and impairing their voting rights, and provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable.
Our Amended and Restated Certificate of Incorporation provides for the authorization to issue up to 3,000,000 shares of “blank check” preferred stock with designations, rights and preferences as may be determined from time to time by our board of directors. Our board of directors is empowered, without stockholder approval, to issue one or more series of preferred stock with dividend, liquidation, conversion, voting or other rights which could dilute the interest of, or impair the voting power of, our common stockholders. The issuance of a series of preferred stock could be used as a method of discouraging, delaying, or preventing a change in control. For example, it would be possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of our company. In addition, advanced notice is required prior to stockholder proposals, which might further delay a change of control.
Our Amended and Restated Certificate of Incorporation provides that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for substantially all disputes between the Company and its stockholders, which could limit stockholders’ ability to obtain a favorable judicial forum for disputes with the Company or its directors, officers or employees.
Our Amended and Restated Certificate of Incorporation provides that unless the Company consents in writing to the selection of an alternative forum, the State of Delaware is the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company, its directors, officers or employees arising pursuant to any provision of theDelaware General Corporation Law(the (the “DGCL”) or our Amended and Restated Certificate of Incorporation or the Company’s Amended and Restated Bylaws, or (iv) any action asserting a claim against the Company, its directors, officers, employees or agents governed by the internal affairs doctrine, except for, as to each of (i) through (iv) above, any claim as to which the Court of Chancery determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or for which the Court of Chancery does not have subject matter jurisdiction. This exclusive forum provision would not apply to suits brought to enforce any liability or duty created by the Securities Act or the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Furthermore,
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. However, our Amended and Restated Bylaws contain a federal forum provision which provides that unless the Company consents in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of the Corporation are deemed to have notice of and consented to this provision. The Supreme Court of Delaware has held that this type of exclusive federal forum provision is enforceable. There may be uncertainty, however, as to whether courts of other jurisdictions would enforce such a provision, if applicable.
This
-38-
These choice of forum provisionprovisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with the Company or its directors, officers or other employees, which may discourage such lawsuits against the Companyus and itsour directors, officers and other employees. Alternatively, if a court were to find theour choice of forum provisionprovisions contained in either our Amended and Restated Certificate of Incorporation or Amended and Restated Bylaws to be inapplicable or unenforceable in an action, the Companywe may incur additional costs associated with resolving such action in other jurisdictions, which could harm itsour business, results of operations, and financial condition.
We are an “emerging growth company” and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies, which could make our common stockCommon Stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, pursuant to Section 107 of the JOBS Act, as an “emerging growth company” we intend to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates for complying with new or revised accounting standards.
We cannot predict if investors will find our common stockCommon Stock less attractive because we may rely on these exemptions. If some investors find our common stockCommon Stock less attractive as a result, there may be a less active trading market for our common stockCommon Stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of thisour initial public offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties. You should not place undue reliance on these forward-looking statements. All statements other than statements of historical facts contained in this prospectus are forward-looking statements. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. In some cases, you can identify these forward-looking statements by terms such as “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expects,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms or other similar expressions, although not all forward-looking statements contain those words. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short-term and long-term business operations and objectives and financial needs. These forward-looking statements include, but are not limited to, statements concerning the following:
-39-
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
This prospectus contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. These data involve a number of assumptions and limitations and contain projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty, including those discussed in “Risk Factors.” We caution you not to give undue weight to such projections, assumptions and estimates. Further, industry and general publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that these publications, studies and surveys are reliable, we have not independently verified the data contained in them. In addition, while we believe that the results and estimates from our internal research are reliable, such results and estimates have not been verified by any independent source.
We estimate thatwill not receive any of the net proceeds from our issuance andthe sale by the Selling Stockholders of 1,900,000 Units in this offeringthe Common Stock. Upon any exercise of the Warrants by payment of cash, however, we will be approximately $9,404,000, assuming an initial public offeringreceive the exercise price of $5.50 per Unit, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their over-allotment optionWarrants, which, if exercised in full, we estimate that the net proceeds from this offering will be approximately $ , after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use approximately $5.3 million of the net proceeds from this offering to fund product development, including the commencement of Phase I/IIa clinical testing. We believe that such funds will be sufficient for the completion of Phase I/IIa clinical testing. The balance of the net proceeds is expected to be used for other general working capital purposes. We also intend to repay five outstanding promissory notes that have a total of $126,100 in principal outstanding. Two notes bear interest at four percent (4%) and the other three have an on-issuance discount totaling $44,100. The first note has $35,000 in principal outstanding and was due on November 12, 2018. The second note has $15,600 in principal outstanding and was due on October 8, 2018. The third note has $55,000 in principal outstanding and was due on October 16, 2018. The fourth note has $10,500 in principal outstanding and was due on October 16, 2018. The fifth note has $10,000 in principal outstanding and was due on September 21, 2019. Finally, we intend to pay a $200,000 Milestone licensing fee to LLU. Other than as described above, the Company intends to pay all currently due payments to LLU prior to the consummation of this offering.
We believe that the net proceeds from this offering and our existing cash cash equivalents and investments will be sufficient to fund our current operations for at least eighteen months from the date of this prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect.
Predicting the cost necessary to develop product candidates can be difficult and the amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization efforts, the status of and results from clinical trials, any collaborations that we may enter into with third parties for our product candidates and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering and our existing cash and cash equivalents.
In the ordinary course of our business, we expect to, from time to time, evaluate the acquisition of, investment in or in-license of complementary products, technologies or businesses, and we could use a portion of the net proceeds from this offering for such activities. We currently do not have any agreements, arrangements or commitments with respect to the 3,785,569 shares of Common Stock offered hereby, would result in gross proceeds to us of approximately $12.3 million. However, we cannot predict when and in what amounts or if the Warrants will be exercised by payments of cash and it is possible that the Warrants may expire and never be exercised, in which case we would not receive any potential acquisition, investment or license.cash proceeds.
We plan to retain any earnings for the foreseeable future for our operations. We have never paid anycash dividends on our common stockCommon Stock and we do not anticipate paying any cash dividends in the foreseeable future.future, but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends on our Common Stock will be at the sole discretion of our Boardboard of directors and will depend onbe dependent upon our financial condition, operating results of operations, capital requirements and such other factors as our Boardthe board of directors deems relevant.
CAPITALIZATIONDETERMINATION OF THE OFFERING PRICE
The following table sets forth our cash and cash equivalents and capitalization asprices at which the shares of September 30, 2019:
You should readCommon Stock covered by this capitalization table together with “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our financial statements andprospectus may actually be sold will be determined by the related notes appearing elsewhere in this prospectus.
| September 30, 2019 | ||||||||
| (in thousands, except share and per share data) | Actual | Pro Forma, As Adjusted (unaudited) | ||||||
| Cash | $ | 33 | 9,438 | |||||
| Common stock, par value $0.001 per share | 8 | 10 | ||||||
| Treasury stock | (202 | ) | (202 | ) | ||||
| Additional paid-in capital | 8,271 | 17,671 | ||||||
| Accumulated deficit | (10,739 | ) | (10,739 | ) | ||||
| Total stockholders’ equity/(deficit) | (2,662 | ) | 6,745 | |||||
| Total capitalization | $ | (2,662 | ) | 6,745 | ||||
The number ofprevailing public market price for shares of our common stock to be outstanding after this offering is based on 7,580,195 sharesCommon Stock or by negotiations between the Selling Stockholders and buyers of our common stock outstandingCommon Stock in private transactions or as otherwise described in “Plan of September 30, 2019, assumes (i) no exercise by the underwriter of its over-allotment option and (ii) no exercise of the unit purchase option to be issued to the underwriter in this offering, and excludes:
| ||
If you invest in our common stock, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
As of September 30, 2019 we had a historical net tangible book value (deficit) of $(2,661,790), or $(0.35) per share of common stock, based on shares of common stock outstanding at September 30, 2019. Our historical net tangible book value per share is the amount of our total tangible assets less our total liabilities at September 30, 2019, divided by the number of shares of common stock outstanding at September 30, 2019.
After giving effect to the sale of 1,900,000 Units (and the shares of common stock thereunder) in this offering at an assumed initial public offering price of $5.50 per Unit (assuming no exercise of the warrants included in the Units, no value is attributed to such warrants and such warrants are classified and accounted for as equity), and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value at September 30, 2019 would have been $8,834,210, or $0.71 per share of common stock. This represents an immediate increase in pro forma as adjusted net tangible book value of ($0.71) per share to existing shareholders and immediate dilution of $4.79 per share to new investors purchasing shares of common stock in this offering.
The following table illustrates this dilution on a per share basisDistribution.”
The number of shares of our common stock to be outstanding after this offering is based on 7,580,195 shares of our common stock outstanding as of September 30, 2019, assumes (i) no exercise by the underwriter of its over-allotment option and (ii) no exercise of the unit purchase option to be issued to the underwriter in this offering, and excludes:
| ||
The following tables set forth, assuming the sale of all of the shares offered for sale in this offering the total number of shares previously sold to existing stockholders as of September 30, 2019, the total consideration paid for the foregoing and the respective percentages applicable to such purchased shares and consideration paid based on an average price of $0.15 per share paid by our existing stockholders and $5.50 per share paid by investors in this Offering.
| Shares Purchased | Total Consideration | |||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||
| Assuming All Units Sold: | ||||||||||||||||
| Existing stockholders | 7,580,195 | 80 | % | $ | 1,125,600 | 10 | % | |||||||||
| New Investors | 1,900,000 | 20 | % | $ | 10,450,000 | 90 | % | |||||||||
| Total | 9,480,195 | 100 | % | $ | 11,575,600 | 100 | % | |||||||||
29-40-
SELECTED FINANCIAL DATABUSINESS
Our Mission
We believe the world needs—and deserves—a new approach to health innovation that is focused on harnessing the power of large groups of stakeholders who work together to ensure that the most promising treatments make it into the hands of people who need them most.
We were incorporated in the State of Delaware on September 28, 2017, and our headquarters are located in Richmond, Virginia. The following table sets forth our selected financial data ascompany was founded with a mission of bringing stakeholders together, to transform promising innovations into products and services that could address some of the datesmost challenging needs. The socialization of innovation through engaging stakeholders in every aspect of it, is key to transforming more innovations, more rapidly, and more efficiently.
At inception, the first innovation we took on was an immune modulation technology titled ADI/Adimune with a focus on prolonging life and enhancing life quality of patients that have undergone organ transplants. Since then, we expanded our portfolio of innovations, and we continue to evaluate a variety of promising health innovations.
Our Model
Our mission is to advance promising health innovations. Our business model begins with identifying and securing innovations through licensing or acquisition. Once an innovation is secured and becomes “An Aditxt Company,” we seek to accelerate its growth through supporting and scaling its innovation, operations, and commercialization.
Our model focuses on identifying promising health innovation and surrounding it with activation resources that take a systemized approach to bringing that idea to life. We seek to engage various stakeholders for each of our programs on every level: This includes identifying researchers and research institution partners, such as Stanford University; leading health institutions to get critical trials underway, such as the periods indicated. We have derivedMayo Clinic; manufacturing partners who enable us to take innovations from preclinical to clinical; municipalities and governments, such as the statementscity of operations data forRichmond and the year ended December 31, 2018state of Virginia and forpublic health agencies launch our programs, such as Pearsanta’s laboratory; and thousands of shareholders around the nine months ended September 30, 2019 from our audited and unaudited financial statements included elsewhere in this prospectus. The following summary financial data should be read with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes and other information included elsewhere in this prospectus. Our historical resultsglobe. What was once a promising innovation becomes a purposeful product that has the power to change lives.
We are not necessarily indicativeabout a single idea or a single innovation. It is about making sure the right innovation is made possible. With any type of innovation, there is power in numbers and power in people who believe in our mission of “Making Promising Innovations Possible, Together.”

Our Value Proposition
Far too often, promising treatment or technology does not reach commercialization due to lack of funds and critical infrastructure. As a result, potentially life-changing and lifesaving treatments are not available to the results to be expected in future periods.individuals who so desperately need them.
| September 30, 2019 | December 31, 2018 | |||||||
| Statements Of Operations Data: | (unaudited) | |||||||
| REVENUE | — | — | ||||||
| OPERATING EXPENSES | ||||||||
| General and administrative expenses, includes $3,533,016 and $3,417,526 in stock-based compensation | 4,771,567 | 5,044,634 | ||||||
| Research and development, includes $10,000 and $100,000 in stock-based compensation | 108,449 | 525,000 | ||||||
| Sales and marketing, includes $0 and $6,000 in stock-based compensation | 147 | 39,837 | ||||||
| Total Operating Expenses | 4,880,163 | 5,609,471 | ||||||
| NET LOSS FROM OPERATIONS | (4,880,163 | ) | (5,609,471 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense | (1,481 | ) | (3,009 | ) | ||||
| Gain on forgiveness of debt | 45,000 | — | ||||||
| Amortization of debt discount | — | (76,757 | ) | |||||
| Total Other Income (Expense) | 43,519 | (79,766 | ) | |||||
| Net loss before provision for income taxes | (4,836,644 | ) | (5,689,237 | ) | ||||
| Provision for income taxes | — | — | ||||||
| NET LOSS | (4,836,644 | ) | (5,689,237 | ) | ||||
| Net loss per share - basic and diluted | (0.63 | ) | (0.78 | ) | ||||
| Weighted average number of shares outstanding during the period - basic and diluted | 7,673,317 | 7,261,637 | ||||||
| Balance Sheet Data: | ||||||||
| Cash and cash equivalents | 32,977 | 115,709 | ||||||
| Working capital | (2,661,790 | ) | (1,536,734 | ) | ||||
| Total assets | 32,977 | 115,709 | ||||||
| Total liabilities | 2,694,767 | 1,652,443 | ||||||
| Total stockholders’ deficit | (2,661,790 | ) | (1,536,734 | ) | ||||
| Total liabilities and stockholders’ deficit | 32,977 | 115,709 | ||||||
30-41-
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND PLAN OF OPERATIONSOur mission is to make promising innovation a reality faster and more efficiently. Since inception, we have sought to provide the critical infrastructure consisting of a highly experienced team and stakeholders skilled in product development, operations, commercialization, engagement and content, finance and accounting, people, and legal. Our ecosystem has established access to industry leaders, top-rated research and medical institutions, universities, manufacturing and distribution companies, and critical infrastructure such as CLIA-certified state-of-the art labs and GMP manufacturing.
You should readWe bring the following discussionholistic concept of an efficient ecosystem for advancing and analysis of our financial conditionaccelerating innovations. Our process: We seek to discover, identify, and plan oflicense or acquire promising innovations. We then form and build out a subsidiary around each innovation and support the subsidiaries through innovation, operations, together with “Selected Financial Data”commercialization, and our financial statementscorporate functions that seek to thrive and the related notes appearing elsewhere in this prospectus. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this prospectus. All amounts in this report are in U.S. dollars, unless otherwise noted.
Overviewgrow as a successful, monetizable business.
The CompanyShifting Landscape of Health Innovation
Health innovation requires significant resources. The convergence of biotech and high-tech offers new possibilities of accelerating breakthrough innovations faster and more efficiently. This approach reflects our mission of “Making Promising Innovations Possible, Together”.
People deserve innovative solutions, which have never been more within reach. We believe the best idea, best product and the best solution will come from creating an ecosystem where all stakeholders, such as vendors, customers, municipalities, and shareholders contribute. When we disrupt the way we’re innovating, through our collaborative model, we believe we can move faster and more efficiently to activate viable solutions that have the potential to make a measurable impact.
Our Growth Strategy
The era of precision and personalized medicine is here. We believe that people around the globe would benefit from health diagnostics and treatments that more accurately pinpoint the problems and more precisely treat the condition.
In addition to our current programs, Adimune and Pearsanta, we look to bring in future health innovations in the areas of software and AI, medical devices, therapeutics, and other technologies that take a fundamentally different approach to health because they prioritize precision medicine, timely disease root cause analysis, and targeted treatments.
Year over year, we plan to continue building our infrastructure and adding more personalized and precision health innovations that align with our mission. These opportunities may come in different forms such as IP, an early-stage company, or a late-stage company. We will continue to scale our systemized approach to the innovation process, making large-scale automation and enterprise systems available to Aditxt portfolio companies at every stage of their growth. Specifically, certain subsidiaries will need to grow through further M&A activities, operational infrastructure implementation, and development or acquisition of critical technologies.
Our Team
Aditxt has assembled an entrepreneurial team of experts from a variety of different business, engineering, and scientific fields, and commercial backgrounds, with collective experience that ranges from founding startup innovation companies, to developing and marketing biopharmaceutical and diagnostic products, to designing clinical trials, and management of private and public companies. We have deep experience in identifying and accessing promising health innovations and developing them into products and services with the ability to scale. We understand the capital markets, both public and private, as well as M&A and facilitating complex IPOs.
-42-
THE ADITXT PROGRAMS
ADIMUNE, INC.
Formed in January 2023, Adimune™, Inc. (“Adimune”) is focused on leading Aditxt’s immune modulation therapeutic programs. Adimune’s proprietary immune modulation product Apoptotic DNA Immunotherapy, or ADI-100™, which utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It includes two DNA molecules designed to deliver signals to induce tolerance. ADI-100 has been successfully tested in several preclinical stage, life sciences companymodels (skin grafting, psoriasis, type 1 diabetes, multiple sclerosis).
In May 2023, Adimune and Mayo Clinic signed a clinical trial agreement to advance clinical studies targeting autoimmune diseases of the central nervous system (“CNS”) with the initial focus on the rare, but debilitating, autoimmune disease Stiff Person Syndrome (“SPS”). According to the National Organization of Rare Diseases, the exact incidence and prevalence of SPS is unknown; however, one estimate places the incidence at approximately 1 in 1 million individuals in the general population.
Pending approval by the International Review Board, a missionhuman trial for SPS will get underway in the second half of 2023 or the first half of 2024 with enrollment of 10-15 patients, some of whom also have type 1 diabetes. ADI-100 will be tested for safety and efficacy. ADI-100 is designed to prolong lifetolerize against an antigen known as glutamic acid decarboxylase (“GAD”), which is implicated in type-1 diabetes, psoriasis, and enhance life qualityin many autoimmune diseases of transplanted patients. the CNS.
Background
The discovery of immunosuppressive (anti-rejection)(anti-rejection and monoclonal) drugs over 40 years ago has made possible life-saving organ transplantation procedures. While these drugs prevent or delay organ rejection, transplanted organs often ultimately fail,procedures and about 40%blocking of transplanted organs survive no more than 5 years. Furthermore,unwanted immune responses in autoimmune diseases. However, immune suppression leads to significant undesirable side effects, such as increased susceptibility to life-threatening infections and cancers, because it is not specifically targeted towards the transplanted organs; rather, it indiscriminately and broadly suppresses immune function throughout the body. While the use of these drugs has been justifiable because they prevent or delay organ rejection, their use for treatment of autoimmune diseases and allergies may not be acceptable because of the aforementioned side effects. Furthermore, transplanted organs often ultimately fail despite the use of immune suppression, and about 40% of transplanted organs survive no more than 5 years.
The opportunityThrough Aditxt, Adimune has the right of use to extend the life of a transplanted organ, even by a few years, may have substantial benefits to organ recipients. We have an exclusive worldwide license for commercializing aADI nucleic acid-based technology named Apoptotic DNA Immunotherapy™ (ADi™) which utilizes(which is currently at the pre-clinical stage) from Loma Linda University. (See below). ADI uses a novel approach that mimics the way our bodiesthe body naturally induceinduces tolerance to our own tissues.tissues (“therapeutically induced immune tolerance”). While immune suppression requires continuous administration to prevent rejection of a transplanted organ, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. Thus, ADi™ADI may allow patients to live with transplanted organs with significantly reduced immune suppression. ADi™ADI is a technology platform which we believe can be engineered to address a wide variety of indications.
We plan to develop ADi™are developing ADI™ products for organ transplantation including skin grafting,allografting, autoimmune diseases, allergies, and further indications,allergies, with the initial focus on psoriasis, type 1 diabetes and skin allografting, indications for which we have compelling preclinical data. To submit a Biologics License Application (“BLA”) for a biopharmaceutical product, clinical safety and efficacy must be demonstrated in clinical studies conducted with human subjects. For products in our class of drugs, the first-in-human trials will be a combination of Phase I (safety/tolerability) and Phase II (efficacy) in affected subjects. To obtain approval to initiate the Phase I/IIa studies, an Investigational New Drug or Clinical Trial Application will be submitted that will include a compilation of non-clinical efficacy data as well as manufacturing and pre-clinical safety/toxicology data. To date, we have conducted non-clinical studies in a stringent model of skin transplantation using genetically mismatched donor and recipient animals demonstrating a 3-fold increase in the survival of the skin allograft in animals that were tolerized with ADI™ compared to animals that receive immune suppression alone. Prolongation of graft life was observed despite discontinuation of immune suppression after the first 5 weeks. In a non-obese diabetic mouse model of type 1 diabetes, we showed reversal of hyperglycemia with 80% of the animals showing durable glycemic control for the 40-week study period. Additionally, in an induced non-clinical model for psoriasis, ADI™ treatment resulted in a 69% reduction in skin thickness and a 38% decrease in skin flaking (two clinical parameters for assessment of psoriasis skin lesions). The Phase I/IIa studies in psoriasis will evaluate the safety/tolerability of ADI™ in patients diagnosed with psoriasis. Since the drug will be administered in subjects diagnosed with psoriasis, effectiveness of the drug to improve psoriatic lesions will also be evaluated. In the type 1 diabetes clinical studies, newly diagnosed subjects will receive ADI™ treatment to evaluate safety and efficacy. In another Phase I/IIa study, patients requiring skin allografts will receive weekly intra-dermal injections of ADI™ in combination with standard immune suppression to assess safety/tolerability and possibility of reducing levels of immunosuppressive drugs as well as prolongation of graft life.
-43-
New, focused therapeutic approaches are needed that modulate only the immune cells involved in rejection of the transplanted organ, as this approach can be safer for patients than indiscriminate immune suppression. Such approaches are referred to as immune tolerance, and when therapeutically induced, may be safer for patients and potentially allow long-termer survival of transplanted tissues and organs.
In the late 1990s, academic research on these approaches was conducted at the Transplant Center in Loma Linda University (“LLU”) in connection with a project that secured initial grant funding from the U.S. Department of Defense. The focus of that project was induction of tolerance for skin allografting for burn victims. Twenty years of research at LLU and an affiliated incubator led to a series of discoveries that have been translated into a large patent portfolio of therapeutic approaches that may be applied to the modulation of the immune system to induce tolerance to self and transplanted organs.
Advantages
ADI™ is a nucleic acid-based technology (e.g., DNA-based), which we believe selectively suppresses only those immune cells involved in attacking or rejecting self and transplanted tissues and organs. It does so by tapping into the body’s natural process of cell turnover (apoptosis) to retrain the immune system to stop unwanted attacks on self or transplanted tissues. Apoptosis is a natural process used by the body to clear dying cells and to allow recognition and tolerance to self-tissues. ADI™ triggers this process by enabling the cells of the immune system to recognize the targeted tissues as “self”. Conceptually, it is designed to retrain the immune system to accept the tissues, similar to how natural apoptosis reminds our immune system to be tolerant to our own “self” tissues.
While efforts have been made by various groups to promote tolerance through cell therapies and ex vivo manipulation of patient cells (takes place outside the body), we believe we will be unique in our approach of using in-body induction of apoptosis to promote tolerance to specific tissues. In addition, ADI treatment itself will not require additional hospitalization, only an injection of minute amounts of the therapeutic drug into the skin.
Reduce Chronic Rejection
Moreover, preclinical studies have demonstrated that ADI treatment significantly and substantially prolongs graft survival, in addition to successfully “reversing” other established immune-mediated inflammatory processes.
While immunosuppressants control acute rejection during the early time-period after receiving an organ, chronic rejection of the organ that occurs one or more years after the transplant procedure continues to pose a major challenge for organ recipients.
Chronic rejection has been likened to autoimmunity (a misdirected immune response that occurs when the immune system goes awry), where specific tissues in the transplanted organ become targets of immune attack. In other words, chronic rejection may not be caused just by differences between the donor and the recipient, but rather by an immune response by the recipient to specific tissues in the organ. Our pre-clinical studies suggest that ADI™ has the ability to tolerize to specific tissues in a transplanted organ, and conceivably, reduce incidences of chronic rejection.
Reduce immune suppression
Studies in animal models have shown that conditioning/desensitizing the animals to receive the transplant, prolongs the survival of the transplanted tissue or organ. These studies have used repeated exposure to low doses of protein components in specific organs to reduce immunologic recognition and attack on the transplanted organ.
Based on some of our data, we believe that with ADI™ treatment, recipients can be conditioned/desensitized, thereby retraining the immune system to more readily accept the organ and also reduce the levels of immunosuppressive drugs needed post-transplantation.
Preformed Antibodies
Studies have shown that presence of preformed antibodies prior to transplantation procedures increases the rate of organ rejection. Preformed antibodies can develop in previously transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. With more than 113,000 patients on transplant waiting lists in the U.S. alone, patients with pre-existing antibodies have much lower chances of qualifying to receive organs due to their increased risk of rejection – even with immune suppression.
-44-
Sadly, transplanted patients have a probability of needing re-transplantation at some point due to eventual chronic rejection of their transplanted organ, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Based on experimental data, we believe that ADI™ may have the potential to address this issue providing these individuals better opportunities for receiving an organ.
Technology Platform
ADI™ utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It is a technology platform, which we believe can be engineered to address a wide variety of indications. ADI™ includes two DNA molecules which are designed to deliver signals to induce tolerance. The first DNA molecule encodes a pro-apoptotic protein, which induces ‘programmed’ cell death (apoptosis). This is a core component of the technology because it is intended to greatly increase the recruitment of dendritic cells, which are implicated in regulating the immune system. The second DNA molecule encodes the protein of interest (guiding antigen), which is modified to promote a path of tolerance. The guiding antigen is intended to result in tolerance induction specific to the tissue where the protein is found.

ADI™ has shown efficacy in several preclinical models (skin grafting, psoriasis, type 1 diabetes, alopecia areata and multiple sclerosis) and its efficacy can be attributed to multiple factors:
| 1. | ADI™ does not rely on a single mechanistic approach. It has multiple components (interchangeable target antigen, apoptosis, methylated plasmid DNA) that affect different arms of the immune system, which can be manipulated. |
| 2. | ADI™ activates key immune cells known to maintain tolerance in test animals and humans. |
| 3. | ADI™ has been successfully applied to a stringent transplantation model. |
| 4. | ADI™ lends itself to repeat dosing, which may be required to achieve its full potential therapeutic effect. |
-45-
Proof of Concept: Skin Grafting
Results shown are 5 weeks post-transplantation
The proof-of-concept experiment performed in transplantation was a skin allograft transplantation procedure in which the donor skin was obtained from white BALB/c mice and transplanted to black C57BL/6 mice. The experiment was designed to address a more challenging scenario where the donor tissue was obtained from a donor which is genetically mismatched with the recipient. This is unlike clinical scenarios where the donor and recipient are genetically matched as much as possible. While these experiments were repeated in several separate experiments, the results shown here were obtained from a study conducted with 14 mice in the ADI™ treatment group and 7 mice in the control group. Prior to submission of an Investigational New Drug or Clinical Trial Application, additional non-clinical studies will be conducted to establish the precise protocol (e.g. timing of vaccine administration, dosing, and appropriate immunosuppressive agents that will be used in combination with ADI™) that will be used in the clinical trials. Pre-clinical safety/toxicology studies have already been conducted by a GLP lab to ensure product safety for clinical testing. These studies have shown no signs of toxicity to ADI™ treatment in mice.

-46-

Proof of Concept: Psoriasis

| ● | Psoriasis causes increased skin thickness and scaling in an established 10-day psoriasis model |
| ● | ADI™ treatment resulted in a 69% reduction in skin thickening and 38% reduction in scaling over the 10-day study period |
-47-
Proof of Concept: Type 1 Diabetes

Typically, 90% of female NOD mice developed spontaneous autoimmune diabetes. Disease progression may be different for individual animals.

-48-
ADI™ was administered once a week for 8 weeks after each animal developed hyperglycemia. All animals responded with 80% showing durable response for the entire 40-week study period.
| ● | Type 1 or autoimmune diabetes is a condition where the body’s immune system mistakenly attacks cells in the pancreas resulting in diminished production of insulin |
| ● | ADI™ incorporates an antigen (GAD) expressed in the pancreas |
| ● | Administration of ADI™ using GAD as the antigen over an 8-week period in animals with T1D restores insulin production and reverses hyperglycemia |
Pre-clinical and Clinical Plans
The resources and efforts used for the IND-enabling work summarized below supports both the psoriasis and TID clinical programs
High-level objectives for psoriasis clinical program:
| ● | Completion of IND-enabling work. Aditxt has initiated GMP manufacturing of clinical grade material that will be used for the first-in-human studies in subjects with psoriatic lesions. Included in the manufacturing program is stability studies; the regulatory agency requires one month of stability data for the GMP material for submission of the clinical trial application (CTA). Stability data will continue to be gathered while the clinical trials are ongoing and up to 24 months. Aditxt has also completed the in-life portion of the toxicology studies. Safety data have been recorded and Aditxt is now awaiting immunotoxicology data, which are forthcoming. | |
| ● | Upon completion of GMP manufacturing and toxicology studies, a CTA will be submitted in Q4 2022 to initiate the Phase I/II FIH clinical trials. |
The FIH clinical studies will combine Phase I (designed to test clinical safety) and Phase IIa (designed to obtain proof of effectiveness in human subjects), in subjects with psoriatic skin lesions. We have selected this indication for several reasons, including:
| 1. | Our existing preclinical data have shown promising results in reducing scaling and skin thickness in the mouse model; |
| 2. | The relative ease of visualization of healing of psoriatic lesions; and |
| 3. | The need for therapies that suitable and justifiable in individuals with mild to moderate psoriasis (current biologic therapies are primarily used in moderate to severe cases). |
We have identified a contract research organization with capabilities to conduct a multi-center study and ability to recruit the needed number of subjects to complete the clinical trials. Upon approval by the regulatory agency clinical trials will be initiated.
High-level objectives for type 1 diabetes (T1D) clinical program:
| ● | Completion of IND-enabling work. Aditxt has initiated GMP manufacturing of clinical grade material that will be used for the first-in-human studies in subjects with psoriatic lesions. Included in the manufacturing program is stability studies; the regulatory agency requires one month of stability data for the GMP material for submission of the clinical trial application (CTA). Stability data will continue to be gathered while the clinical trials are ongoing and up to 24 months. Aditxt has also completed the in-life portion of the toxicology studies. Safety data have been recorded and Aditxt is now awaiting immunotoxicology data, which are forthcoming. |
| ● | Clinical Phase I/II Study to demonstrate safety and clinical proof-of-concept in T1D |
-49-
Our clinical studies will combine Phase I (designed to test clinical safety) and Phase II (designed to obtain proof of effectiveness in human subjects), in T1D patients. We have selected this indication for several reasons, including:
| 1. | Our existing preclinical data have shown promising results using ADI™ to reverse hyperglycemia in the mouse model; and | |
| 2. | There is currently no treatment for T1D and the only option for patients suffering from T1D is insulin replacement therapy. |
We will be identifying clinical trial centers with adequate patients. Upon approval by the FDA and/or the applicable regulatory agency clinical trials will be initiated.
High-level objectives for skin allograft clinical program:
| ● | Completion of preclinical studies to identify the appropriate protocol for dosing and combination of ADI™ with immune suppression protocols. | |
| ● | Completion of IND-enabling work including GMP manufacturing and toxicology studies. | |
| ● | Clinical Phase I/II Study to demonstrate safety and clinical proof-of-concept in patients requiring skin allografts. |
Our clinical studies will combine Phase I (designed to test clinical safety) and Phase II (designed to obtain proof of effectiveness in human subjects), in patients requiring skin allografts. We have selected this indication for several reasons, including:
| 1. | Our existing preclinical data have shown promising results using ADI™ to prolong skin allograft survival in mismatched mouse model; and | |
| 2. | The relative ease of visualization of graft quality without the need for biopsies. |
We will be identifying clinical trial centers with adequate patients. Upon approval by the FDA and/or the applicable regulatory agency clinical trials will be initiated.
We are developing our immune monitoring platforms with the objective of utilizing them as clinical assays in pre-clinical and clinical studies. The multiplex technologies could potentially allow evaluation of more analytes with less tissue samples.
-50-
Drug Approval Process
In the United States, FDA approval is required before any new drugs can be introduced to the market. We currently have a product candidate for our first-in-human studies, but as of the date of report, we have not submitted an application to the regulatory agencies for approval.
We are working with a contract manufacturer who has the know-how, product ingredients including plasmid DNA molecules, and our patent-pending bacterial strain. Several batch runs have been successfully completed to demonstrate our ability to produce the DNA plasmids in a GMP facility. Based on validation studies, we are reasonably confident in our ability to produce clinical grade product candidates at larger scales. The contract manufacturer has provided a proposal for manufacturing of our clinical grade material, which will be signed and accepted once we are ready to initiate GMP manufacturing. We are not currently party to an agreement with this contract manufacturer.
The product candidate selected for clinical trials must be subjected to pre-clinical safety/toxicology studies by an independent GLP (Good Laboratory Practice) laboratory to demonstrate its suitability for clinical testing in human patients. Upon completion of manufacturing and safety/toxicology testing, an Investigational New Drug (IND) application will be prepared for submission to the regulatory agencies.
Upon receipt of clearance to initiate clinical testing, the ADI™ product can be tested in human patients. Our product will be tested in clinical trials, one in patients with psoriasis and one in patients who require skin allografting. Therefore, our first-in-human studies will be combined Phase I/Phase II studies in which safety and efficacy data will be obtained. We plan to start with in skin indications (psoriasis and skin allografting) because we believe these indications will be most efficient in providing safety and efficacy data in clinical trials. In parallel, we will continue to develop additional product formulations for other indications.
We are developing our immune monitoring platforms with the objective of utilizing them as clinical assays in pre-clinical and clinical studies. The multiplex technologies could potentially allow evaluation of more analytes with less tissue samples.
Target Market
Psoriasis affects close to 100 million people worldwide and presents a large market estimated at over $20 billion annually. Treatments range include topical and systemic therapeutics including vitamin D analogs, steroids, retinoids, immunosuppressants and biologics (i.e. monoclonal antibodies). While in more recent years, several classes of biologics have entered the market, most are primarily used for patients suffering from moderate to severe psoriasis because of their impairment of systemic immune responsiveness to infections and cancers. Aditxt believes that products based on the ADI™ platform will not be associated with similar side effects and can be targeted for use in mild to moderate cases.
T1D is one of the most common chronic disorders in children and affects nearly 2 million Americans, and has an incidence and prevalence increasing at alarming rates in industrialized countries. Current treatment consists of daily delivery of insulin as replacement therapy, but administration of the hormone can induce life-threatening hypoglycemia and does not completely prevent morbidity and mortality associated with the disease. Aditxt is leveraging the ADI™ technology to develop a new class of immunotherapy designed to arrest the autoimmune destruction of the insulin producing beta cells of the pancreas. This will be the first therapy to accomplish that long sought after goal, thus increasing life span and quality of life for up to 40,000 of US citizens and about 300,000 people around the world who develop T1D each year, with a 3-5% increase in yearly incidence.
In the U.S. alone, there are over 36,000 patients who receive organ transplantations each year, with more than 113,000 on transplant waiting lists.
The field of organ transplantation has been made possible and continues to rely on broad-acting immunosuppressive drugs, high levels of which can result in a compromised immune system that renders organ recipients susceptible to cancer and potentially life-threatening infections including re-activation of latent viruses.
-51-
In addition, immunosuppressants control acute rejection during the early time-period after receiving an organ but chronic rejection of the organ remains an unmet challenge for surgeons and transplant recipients.
While efforts have been made by various groups to promote tolerance through cell therapies and ex vivo manipulation of patient cells, these procedures take place outside the body and typically require hospitalization.
Moreover, transplanted patients will need re-transplantation at some point, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Preformed antibodies can develop in previously transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. These patients have much lower chances at qualifying to receive organs due to their increased risk of rejection - even with immune suppression. The potential to reduce formation of preformed antibodies in these patients will provide better opportunities for them to receive another transplanted organ.
There are gaps between current approaches and what the market needs. We believe that ADI™ addresses these gaps. ADI™ is easy to administer (does not require ex-vivo treatment of patient cells), it does not appear to suppress the immune system, it may allow patients to live with transplanted organs with significantly reduced immune suppression, it may provide for long-term survival of transplanted tissues and organs, may be more effective because it does not rely on a single immune pathway/mechanism, and potentially provides patients with pre-existing antibodies a chance to qualify to receive organs.
While these advantages present opportunities for unmet medical needs in the field of organ transplantation, the industry in which we operate is highly competitive. A small company such as us will meet significant challenges including regulatory requirements for approval of a new class of therapeutic agents, challenges in large scale manufacturing and marketing, cost of developing a novel therapeutic agent, which may require co-development partners who may or may not be willing to work with us, and the willingness of transplant surgeons to adopt our therapeutic vaccines in their existing immune suppression protocols. These challenges pose risks that we may not be able to overcome.
License Agreement with Loma Linda University -
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris was a party to a License Agreement with Loma Linda University (“LLU”), entered into and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,” and together with the Assignment Agreement, the “Sekris Agreements”). Pursuant to the Assignment Agreement, Sekris transferred and assigned all of its rights and obligations in and to and liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on March 8, 2018, we issued a warrant to Sekris to purchase up to 1,000,00010,000 shares of our common stockCommon Stock (the “Sekris Warrant”). The warrant was immediately exercisable and has an exercise price of $2.00$200.00 per share. The expiration date of the warrant is March 8, 2023. On March 15, 2018, as amended on July 1, 2020, we entered into a LLU License Agreement directly with Loma Linda University, which amends and restates the Sekris Agreements.
Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU and/or any of its affiliates (the “LLU Patent and Technology Rights”) and related to therapy for immune-mediated inflammatory diseases (the ADi™ADI™ technology). We refer you to the section titled “Our Business—Intellectual Property—Patent Rights” for a summary of the patents and patent applications that we licensed from LLU pursuant to the LLU License Agreement. In consideration for the LLU License Agreement, we issued 50,000500 shares of common stockCommon Stock to LLU.
Pursuant to the LLU License Agreement, we are required to pay an annual license fee to LLU. Also, upon completion of this offering, we will be required to pay $200,000 topaid LLU as a$455,000 in July 2020 for outstanding milestone payment within thirty (30) days of July 31, 2018.payments and license fees. We are also required to pay to LLU milestone payments in connection with certain development milestones. Specifically, we are required to make the following milestone payments: $175,000 on March 31, 2022; $100,000 on March 31, 2024; $500,000 on March 31, 2026; and $500,000 on March 31, 2027. Additionally, as consideration for prior expenses incurred by LLU to prosecute, maintain and defend the LLU Patent and Technology Rights, we are obligated to makemade the following payments to LLU:, $70,000 due at the end of December 2018, and a final payment of $60,000 due at the end of March 2019. We are required to defend the LLU Patent and Technology Rights during the term of the LLU License Agreement. Additionally, we will owe royalty payments of (i) 1.5% of Net Product Sales and Net Service Sales on any Licensed Products (defined as any finished pharmaceutical products which utilizes the LLU Patent and Technology Rights in its development, manufacture or supply), and (ii) 0.75% of Net Product Sales and Net Service Sales for Licensed Products and Licensed Services not covered by a valid patent claim for technology rights and know-how for a three (3) year period beyond the expiration of all valid patent claims. We also are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf).
-52-
The LLU License Agreement shall terminate on the last day that a patent granted in to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLU License Agreement may be terminated by mutual agreement or by us upon 90 days written notice to LLU. LLU may terminate the LLU License Agreement in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLU License Agreement by us in any 12-month period. Additional Milestone Deadlines include: (i) the requirement to have regulatory approvalsubmission of an INDIND/clinical trial application to initiate a first-in-human clinical trials on or before March 31, 2020,2022, (ii) the completion of first-in-human (phase I/II) clinical trials by March 31, 2022,2024, (iii) the completion of Phase III clinical trials by March 31, 20242026 and (iv) biologic licensing approval by the FDA by March 31, 2025.2027.
Going ConcernPEARSANTA, INC.
Formed in January 2023, our subsidiary PearsantaTM, Inc. (“Pearsanta”) is seeking to take personalized medicine to a whole new level by delivering “Health by the Numbers”. On November 22, 2023, Pearsanta entered into an assignment agreement with FirstVitals LLC, an entity controlled by Pearsanta’s CEO, Ernie Lee (“FirstVitals”), pursuant to which FirstVitals assigned its rights in certain intellectual property and website domain to Pearsanta in consideration of the issuance of 500,000 shares of Pearsanta common stock to FirstVitals. On December 18, 2023, the board of directors of Pearsanta adopted the Pearsanta 2023 Omnibus Equity Incentive Plan (the “Pearsanta Omnibus Incentive Plan”), pursuant to which it reserved 15 million shares of common stock of Pearsanta for future issuance under the Pearsanta Omnibus Incentive Plan and the Pearsanta 2023 Parent Service Provider Equity Incentive Plan (the “Pearsanta Parent Service Provider Plan”) and approved the issuance of 9.32 million shares of Pearsanta common stock under the Pearsanta Parent Service Provider Plan.
Since its foundation, we have been building the platform for enabling our vision of Any-test, Anytime, Anywhere. Our plan for Pearsanta’s platform is for it to be the transactional backbone for sample collection, sample processing (on- and off-site), and reporting. This requires the development and convergence of multiple components developed by Pearsanta or through transactions with third parties including collection devices, Lab on Chip technologies, Lab Developed Test (LDT) assays, data-driven analysis engine, and telemedicine. According to a comprehensive research report by Market Research Future, the clinical and consumer diagnostic market is estimated to hit $429.3 billion by 2030.
We believe that timely and personalized screening, enables for more informed decisions about treatments. Pearsanta’s platform is being developed to be a seamless digital healthcare solution. This platform will integrate at-location sample collection, Point-of-Care (POC) and LDT, and analytical reporting engine, with telemedicine enabled visits with licensed physicians to review test results and, if necessary, order a prescription. Pearsanta’s goal of extending its platform to provide a more complete picture about someone’s health status and how their dynamic status combined with their genetic makeup may affect their response to medication. The Company was incorporated on September 28, 2017POC component of Pearsanta would enable diagnostic testing at-home, at work, in pharmacies, and has not generated revenues to date. For the year and period ended December 31, 2018 and September 30, 2019, respectively, the Company had a net loss of $5,689,237 and $4,836,644 and will require significant additional capital in order to operate in the normal course of business and fund clinical studies. As a result, these conditions have raised substantial doubt regarding our ability to continue as a going concern beyond one year. While the Company believes in the viability of management’s strategymore to generate sufficient revenue, control costsresults quickly so that an individual can access necessary treatment faster. With certain infections, prescribing the most effective treatment according to one’s numbers can prevent hospital emergency room admissions and potentially life-threatening consequences.
Examples of potential indication-focused tests for the Test2Treat platform will include the evaluation for advanced urinary tract infections (“UTIs”), COVID-19/flu/respiratory syncytial virus, sexually transmitted infections, gut health, pharmacogenomics (i.e., how your genes affect the way your body responds to certain therapeutics), and sepsis. These offerings are novel and needed. We believe that the current standard of care using broad spectrum antibiotic treatment can be ineffective and life-threatening. For example, according to a recent CDC report, improperly prescribed antibiotics are used in 50% of outpatient cases. Further, according to a recent article published in Physicans Weekly, only 1% of board-certified critical care medicine physicians are trained in infectious disease.
Licensed Technologies - AditxtScoreTM
We intend to sublicense to Pearsanta an exclusive worldwide sub-license for commercializing the AditxtScore™ technology which provides a personalized comprehensive profile of the immune system. AditxtScore is intended to detect individual immune responses to viruses, bacteria, peptides, drugs, supplements, bone marrow and solid organ transplants and cancer. It has broad applicability to many other agents of clinical interest impacting the immune system, including those not yet identified such as emerging infectious agents.
-53-
AditxtScore will seek to enable individuals and their healthcare providers to understand, manage and monitor their immune profiles and to stay informed about attacks on or by their immune system. We believe AditxtScore can also assist the medical community and individuals by being able to anticipate the immune system’s potential response to viruses, bacteria, allergens, and foreign tissues such as transplanted organs. This technology may be able serve as warning signal thereby allowing for more time to respond appropriately. Its advantages include the ability to raise additional funds if necessary, thereprovide simple, rapid, accurate, high throughput assays that can be no assurancesmultiplexed to that effect. The Company’s abilitydetermine the immune status with respect to continue as a going concern is dependent uponseveral factors simultaneously, in 3-16 hours. In addition, it can determine and differentiate between distinct types of cellular and humoral immune responses (T and B cells and other cell types). It also provides for simultaneous monitoring of cell activation and levels of cytokine release (i.e., cytokine storms).
This capability may be possible by having the ability to complete clinical studiesdetermine the body’s potential response and implementfor developing a plan to deal with an undesirable reaction by the business plan, generate sufficient revenues and to control operating expenses.
Financial Results
We have a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light ofimmune system. Its advantages include the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. Our financial statements as of December 31, 2018, show a net loss of $5,689,237. Our financial statements as of September 30, 2019, show a net loss of $4,836,644 We expect to incur additional net expenses over the next several years as we continue to maintain and expand our existing operations. The amount of future losses and when, if ever, we will achieve profitability are uncertain.
Results of Operations
During the year ended December 31, 2018, we incurred a loss from operations of $5,609,471. This is due to general and administrative expenses of $5,044,634, which includes $3,417,526 in stock-based compensation, research and development of $525,000, and sales and marketing expenses of $39,837. The $525,000 in research and development is comprised of licensing fees.
During the period September 28, 2017 (“Inception”) through December 31, 2017, we incurred a loss from operations of $212,986. This is due to general and administrative expenses of $212,986.
During the nine months ended September 30, 2019, we incurred a loss from operations of $4,880,163. This is due to general and administrative expenses of $4,771,567, which includes $3,533,016 in stock-based compensation, research and development of $108,449, and sales and marketing expenses of $147. The $108,499 in research and development is comprised of $18,071 in licensing fees, $40,500 in product development, and $49,878 in other research and development expense.
During the nine months ended September 30, 2018, we incurred a loss from operations of $5,116,860. This is due to general and administrative expenses of $4,551,814, research and development of $525,587, and sales and marketing expenses of $39,459. The $525,587 in research and development is comprised of $525,000 in licensing fees and $587 in other research and development expense.
Liquidity
We have incurred substantial operating losses since inception and expect to continue to incur significant operating losses for the foreseeable future and may never become profitable. As of September 30, 2019, we had an accumulated deficit of approximately $10.7 million. The Company had a working capital deficit of $2,661,790 as of September 30, 2019.
Our financial statements have been prepared assuming that we will continue as a going concern. We will require additional capital to meet our long-term operating requirements.
The Company has funded its operations from proceeds from the sale of equity and debt securities. Currently, the Company has various promissory notes in default. There is a risk that the continued default of such notes could prevent management from raising additional capital in the future and such continued default could result in the Company having to allocate some of the proceeds away from the research and development budget. The Company will require significant additional capital to sustain its short-term operations and make the investments it needs to execute its longer-term business plan. The Company’s ability to successfully raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, even if it were successful, future equity issuances would result in dilution to its existing stockholders and any future debt or debt securities may contain covenantsprovide a simple, rapid, accurate, high throughput assays that limit the Company’s operations or ability to enter into certain transactions. The Company will need to raise additional funding through strategic relationships, public or private equity or debt financings, grants or other arrangements to develop and seek regulatory approvals for the Company’s existing and new product candidates. If such funding is not available or not available on terms acceptable to the Company, the Company’s current development plan and plans for expansion of its general and administrative infrastructure maycan be curtailed.
We will need to raise significant additional capital to continue to fund our operations and the clinical trials. We may seek to sell common stock, preferred stock or convertible debt securities, enter into a credit facility or another form of third-party funding or seek other debt financing. In addition, we may seek to raise cash through collaborative agreements or from government grants. The sale of equity and convertible debt securities may result in dilution to our stockholders and certain of those securities may have rights senior to those of our common shares. If we raise additional funds through the issuance of preferred stock, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict our operations. Any other third-party funding arrangement could require us to relinquish valuable rights.
The source, timing and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our clinical development program. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us to, among other things, delay, scale back or eliminate expenses including some or all of our planned development, including our clinical trials. There is substantial doubt about the Company’s ability to continue as a going concern (See page F-2 and F-7).
Critical accounting policies
Research and Development
We incur research and development costs during the process of researching and developing our technologies and future offerings. Our research and development costs consist primarily of licensing costs. We expense these costs as incurred unless such costs qualify for capitalization under applicable guidance.
Stock-Based Compensation
The Company accounts for stock-based compensation costs under the provisions of ASC 718, “Compensation—Stock Compensation,” which requires the measurement and recognition of compensation expense related to the fair value of stock-based compensation awards that are ultimately expected to vest. Stock-based compensation expense recognized includes the compensation cost for all stock-based payments granted to employees, officers, and directors based on the grant date fair value estimated in accordance with the provisions of ASC 718. ASC 718 is also applied to awards modified, repurchased, or canceled during the periods reported.
Fair value of Common Stock
In ordermultiplexed to determine the fair valueimmune status with respect to several factors simultaneously, in 3-16 hours. In addition, it can determine and differentiate between various types of sharescellular and humoral immune responses (T and B cells and other cell types). It also provides for simultaneous monitoring of our common stock, our boardcell activation and levels of directors considered, among other things, contemporaneous valuations of our common stock. Given the absence of a public trading market of our capital stock to date, our board of directors has exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of our common stock, including:
In estimating the fair market value of our common stock, our board of directors first determined the equity value of our business using accepted valuation methods.
Property
The Company does not own any real estate. The Company does not currently lease any office space, but intends to sign an office lease in Southern California upon the completion of this offering.
JOBS Act
On April 5, 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have chosen to take advantage of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards until those standards would otherwise apply to private companies provided under the JOBS Act. cytokine release (i.e., cytokine storms).
We are also evaluating plans to obtain regulatory approval for AditxtScore’s use as a clinical assay and seeking to secure manufacturing, marketing and distribution partnerships for application in the processvarious markets. To obtain regulatory approval to use AditxtScore™ as a clinical assay, we have conducted validation studies to evaluate its performance in detection of antibodies and plan to continue conducting additional validation studies for new applications in autoimmune diseases.
We plan to utilize AditxtScore™ in our upcoming pre-clinical and clinical studies to monitor subjects’ immune response before, during and after ADI™ drug administration. We are also evaluating plans to obtain regulatory approval for AditxtScore™’s use as a clinical assay and seeking to secure manufacturing, marketing and distribution partnerships for application in the benefitsvarious markets. To obtain regulatory approval to use AditxtScore™ as a clinical assay, we have conducted validation studies to evaluate its performance in detection of relying on other exemptionsantibodies and reduced reporting requirements providedplan to continue conducting additional validation studies for new applications in autoimmune diseases and transplantation.
(1) Organ Rejection
Typically, by the JOBS Act. Subject to certain conditions set forth intime a transplanted or a native organ shows signs of failure, the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b)damage is already done, and reversal of the Sarbanes-Oxley Acttissue injury becomes challenging. Access to early warning signs of damage would be invaluable to reverse or even prevent the damage. We believe that there are currently no practical, efficient assays available to measure cellular immune responses and (ii) complying with any requirement that mayavailable tools do not provide timely information for patients. AditxtScore™ can be adopted by the Public Company Accounting Oversight Board (“PCAOB”) regarding mandatory audit firm rotation orused to provide a supplementsensitive and rapid tool for pre-transplant monitoring and to the auditor’s reportdetermine T and B cell response and to differentiate between various types of cellular immune responses. It can be multiplexed providing additional information about the auditnumber of cells responding as well as quantifying the amounts of various cytokines released by the cells in the same assay. Determination of cellular response has valuable applications for prediction, monitoring, early detection, and treatment of disease, including organ failure/rejection, as well as treatment efficacy. It can also reveal dysfunction of the financial statements,immune system that can potentially contribute to more severe disease.
(2) Autoimmunity
Our immune system develops to differentiate self from non-self. In autoimmunity, the body’s ability to distinguish this difference is impaired. Detection of early signs of immune misrecognition may allow earlier intervention to reduce tissue destruction and to potential reverse the process more effectively. Better tools are needed to recognize immune responses to our own tissues earlier, and with more sensitivity and accuracy. We believe that AditxtScore™ harnesses the promise to develop such tools that can be used for early diagnosis, evaluation of treatment effectiveness and determination of the need for maintenance therapies when needed.
-54-
(3) Allergies
Our immune system protects us by acting as a barrier against foreign substances and by eliminating them when they penetrate our bodies. Once the initial exposure has occurred, memory cells develop to prepare the body against a future exposure. This process is called immunity. In certain situations, however, instead of immunity, the immune system develops memory cells that result in a more severe reaction during a future exposure to the same substance. This type of response is called a hypersensitivity response, commonly known as an allergic response. AditxtScore™ can be used to develop multiplex panels each designed to test and monitor immune response to allergens. Based on the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completionability of this offering; (iii) the date on which we have issuedtechnology to run multiple tests in a single assay, 100 or more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed tosubstances can potentially be a large accelerated filer under the rules of the Securities and Exchange Commission.
BUSINESStested simultaneously.
Overview(4) Drug/Vaccine Response
TransplantationWe believe that there are currently no effective assays to predict and easily assess responses to drugs or vaccines. To determine whether an individual has responded to a particular vaccine, antibody titers are measured. This process may take several days or even weeks. Furthermore, for vaccines that require a series of injections, titers are not measured between injections and may not be known for months. AditxtScore™ can be used to determine whether a patient is a responder or non-responder (e.g. individuals with a suppressed immune response may be non-responders). It can provide an effective and rapid tool for potentially determining beneficial responses to a vaccine and can be used to monitor levels of immune responsiveness post vaccination. It can allow evaluation of multiple vaccines in a single test (for memory B cell detection). We believe that this application can be useful for vaccines, cancer therapeutics anti-rejection drugs, anti-viral drugs, among others.
(5) Disease Susceptibility
Disease susceptibility can vary from one individual to another, and it can be a lifesaving treatment option, but the immune system continues to pose the greatest challenge to transplantation becoming routine medical treatment. This is due to the rejection that occurs when the recipient’s immune system recognizes the transplanted tissue or organ as foreign.
The discoveryfunction of immunosuppressants (anti-rejection drugs) over 40 years ago, such as cyclosporine, has allowed survival of transplanted organsvarious factors, including genetic variability and differences in human leukocyte antigens (HLA) encoded by preventing acute or early rejection. However, immunosuppressants fail to prevent the chronic or long-term rejection that occurs years after the initial transplant. About 40% of transplanted organs survivemajor histocompatibility complex (MHC) and responsible for no more than 5 years. Furthermore, immune suppression leads to significant undesirable side effects such as increased susceptibility to life-threatening infections and cancers because it is not specifically targeted towards the transplanted organs; rather, it indiscriminately and broadly suppresses immune function throughout the body.
New, focused therapeutic approaches are needed that modulate only the small portion of immune cells that are involved in rejection of the transplanted organ, as this approach can be safer for patients than indiscriminate immune suppression. Such approaches are referred to as immune tolerance, and when therapeutically induced, may be safer for patients and also potentially allow long-term survival of transplanted tissues and organs.
In the late 1990s, academic research on these approaches was conducted at the Transplant Center in Loma Linda University in connection with a project that secured initial grant funding from the U.S. Department of Defense (DoD). The focus of that project was for skin grafting for burn victims. Twenty years of research at LLU and an affiliated incubator led to a series of discoveries that have been translated into a large patent portfolio of therapeutic approaches that may be applied to the modulationregulation of the immune system in orderhumans. People with certain HLA types may have higher or lower susceptibility to induce tolerancediseases. AditxtScore™ can be used to selfdevelop assays to evaluate differences in HLA types in individuals to help elucidate the relationship between certain HLA types and transplanted organs.susceptibility to various diseases.
We have an exclusive worldwide license for commercializing this nucleic acid-based technology called Apoptotic DNA Immunotherapy™ (ADi™), which utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues (“therapeutically induced immune tolerance”). ADi™ is a technology platform, which we believe can be engineered to address a wide variety of indications.
We plan to develop ADi™ products for organ transplantation, skin grafting, autoimmune diseases, allergies, and other indications, with the initial focus on skin allografts and psoriasis, as we believe these indications will be most efficient in providing safety and efficacy data in clinical trials.
While immune suppression requires continuous administration to prevent acute or early rejection of transplanted organs, induction of tolerance has the potential to retrain the immune system to accept the organ for longer periods of time. Thus, ADi™ may allow patients to live with transplanted organs with significantly reduced dependence on immune suppression.
ADi™ Advantages
ADi™ is a nucleic acid-based technology (e.g., plasmid DNA-based) which we believe selectively suppresses only those immune cells involved in the rejection of tissue and organ transplants. It does so by tapping into the body’s natural process of cell death (apoptosis) to reprogram the immune system to stop unwanted attacks on self or transplanted tissues. Conceptually, it is designed to retrain the immune system to become accepting of the organ similar to how natural apoptosis reminds our immune system to be tolerant to our own “self” tissues.
While efforts have been made by various groups to promote tolerance through cell therapies andex vivo manipulation of patient cells (takes place outside the body typically requiring hospitalization), to our knowledge, we will be unique in our approach of using in-body induction of apoptosis to promote tolerance to specific tissues. In addition, ADi™ treatment itself will not require hospitalization, only an injection in minute amounts into the skin.
(6) Reduce Chronic RejectionInfectious Diseases
While immunosuppressants control acute rejection during the early time-period after receiving an organ, chronic rejection of the organ that occurs one or more years after the transplant procedure continues to pose a major challenge for organ recipients.
Chronic rejection has been likened to autoimmunity (a misdirected immune response that occurs when the immune system goes awry), where specific tissues in the transplanted organ are attacked by the immune system. In other words, chronic rejection may not be caused just by differences between the donor and the recipient, but rather by an immune response by the recipient to specific tissues in the organ. Our pre-clinical studies suggest that ADi™ has the ability to tolerize to specific tissues in a transplanted organ, and conceivably, reducing incidences of chronic rejection.
Moreover, preclinical studies have demonstrated that ADi™ treatment significantly and substantially prolongs graft survival, in addition to successfully “reversing” other established immune-mediated inflammatory processes.
Reduce immune suppression
Studies in animal models have shown that conditioning/desensitizing the animals to receive the transplant, prolongs the survival of the transplanted tissue or organ. These studies have used repeated exposure to low doses of protein components in specific organs to reduce immunologic recognition and attack on the transplanted organ.
Based on some of our data, we believe that with ADi™ treatment, recipients can be conditioned/desensitized ahead of transplantation, thereby retraining the immune system to more readily accept the organ and also reduce the levels of immunosuppressive drugs needed post-transplantation.
Preformed Antibodies
Studies have shown that presence of preformed antibodies prior to transplantation procedures increases the rate of organ rejection. Preformed antibodies can develop in previously-transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. With more than 113,000 patients on transplant waiting lists in the U.S. alone, patients with pre-existing antibodies have much lower chances at qualifying to receive organs due to their increased risk of rejection – even with immune suppression.
Sadly, transplanted patients have a probability of needing re-transplantation at some point due to eventual chronic rejection of their transplanted organ, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Based on experimental data, we believe that ADi™ may have the potential to address this issue providing these individuals better opportunities at receiving an organ transplantation.
ADi™ Key Differentiators
Ease of Delivery
Therapeutic products are typically administered systemically (i.e., by mouth in pill form or injected intramuscularly/intravenously). This requires repeated large doses of the drug to allow sufficient concentrations to reach the affected sites. ADi™ is a DNA-based product that can be injected directly into the skin where the target cells of the immune system reside, thereby significantly simplifying the delivery of the product and reducing the amount of product needed.
Repeat Dosing
DNA-based products are less likely to result in formation of neutralizing antibodies, which lend themselves to repeat dosing as may be required by ADi™ products.
Cost of Goods Advantage
ADi™ products are DNA-based and cost-effective to manufacture. Furthermore, DNA-based products are very stable and do not require adherence to cold chain (temperature-controlled) protocols for shipping. This also makes the product ideal for global distribution.
Simplified Therapy Delivery System
We believe that tolerance induction using ADi™infectious diseases can cause a major predicament for scientific and medical professionals, epidemiologists, and infectious disease specialists, who need to determine how to treat patients in real-time while efficacious therapies are still being developed. Proper decision making requires understanding why some affected individuals show minor or no symptoms, some recover, and others die. We feel that this is fundamental to creating effective targeted therapeutics which may potentially obviatediffer depending on the needunderlying profile of the individual at risk for, hospitalization because itor with, disease. The immune system plays a major role in how any given individual responds to the infectious agent. This response can simply injected intobe inadequate or too robust or appropriately effective. Regardless, the skin. This approach reduces kinetics of the response by the cellular and humoral (antibody) immune systems to the infectious agent are often unknown. A basic critical question, then, is what do the dynamics of the immune response look like from exposure to and through the disease period and during convalescence for those who survive and those who don’t; and how might vaccines and therapies alter these profiles such that predictions of vaccine/drug efficacy could be inferred prior to vaccination/treatment costsand/or disease severity or progression be prognosticated. We believe that AditxtScore™ can be used to help address these questions with multiplex assays each designed to test and complexities in treatment delivery. The anticipated administration of ADi™ will include an initial priming regimen that will require injections administered once a week for several weeks. Thereafter, booster or maintenance doses will be provided on an individual basis as determined bymonitor the immune and inflammation testing. ADi™ treatments will be significantly more convenient and comfortable for patients because they do not require removal of patient cells forex vivo manipulation.response to infectious agents.
ADi™ Technology Platform
ADi™ utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues. It is a technology platform which we believe can be engineered to address a wide variety of indications. ADi™ includes two DNA molecules which are designed to deliver signals to induce tolerance. The first DNA molecule encodes a pro-apoptotic protein, which induces ‘programmed’ cell death. This is a core component of the technology because it is intended to greatly increase the recruitment of dendritic cells, which are implicated in regulating the immune system. The second DNA molecule encodes the protein of interest (guiding antigen), which is modified to promote a path of tolerance. The guiding antigen is intended to result in tolerance induction specific to the tissue where the protein is found.

ADi™ has been successfully tested in several preclinical models and its efficacy can be attributed to multiple factors:
Proof of Concept: Skin Grafting
Results shown are 5 weeks post-transplantation
The proof of concept experiment performed in transplantation was a skin allograft transplantation procedure in which the donor skin was obtained from white BALB/c mice and transplanted to black C57BL/6 mice. The experiment was designed to address a more challenging scenario where the donor tissue was obtained from a donor which is genetically mismatched with the recipient. This is unlike clinical scenarios where the donor and recipient are genetically matched as much as possible. While these experiments were repeated in several separate experiments, the results shown here were obtained from a study conducted with 14 mice in the ADi™ treatment group and 7 mice in the control group. Prior to submission of an Investigational New Drug Application, additional non-clinical studies will be conducted in a pig model to establish the precise protocol (e.g. timing of vaccine administration, dosing, and appropriate immunosuppressive agents that will be used in combination with ADi™) that will be used in the clinical trials. In addition, IND-enabling safety/toxicology studies will be conducted by a GLP lab to ensure product safety for clinical testing.
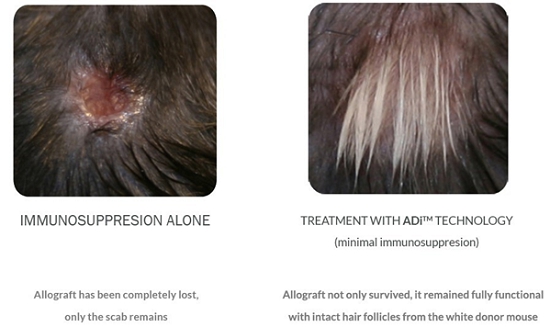
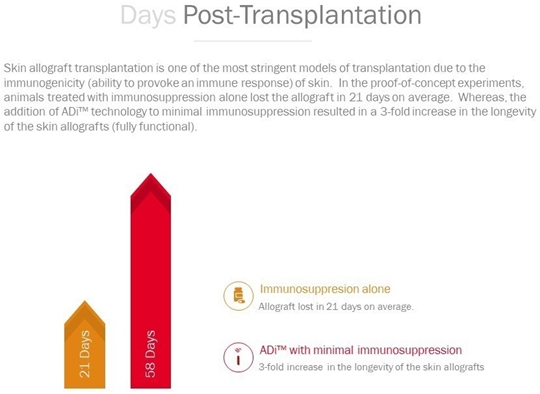
License Agreement with Loma LindaLeland Stanford Junior University (“Stanford”)
On March 8, 2018,February 3, 2020, we entered into an Assignment Agreementexclusive license agreement (the “Assignment“February 2020 License Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris was a partyStanford with regard to a License Agreement with Loma Linda University (“LLU”), entered intopatent concerning a method for detection and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,” and together with the Assignment Agreement, the “Sekris Agreements”).measurement of specific cellular responses. Pursuant to the AssignmentFebruary 2020 License Agreement, Sekris transferredother than as described below, we received an exclusive worldwide license to Stanford’s patent with regard to use, import, offer, and assigned allsale of its rights and obligationsLicensed Products (as defined in and to and liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on March 8, 2018, we issued a warrant to Sekris to purchase up to 1,000,000 shares of our common stock (the “Sekris Warrant”)agreement). The Sekris Warrant was immediately exercisable andlicense to the exercise pricepatented technology is $2.00 per share. The expirationexclusive, including the right to sublicense, beginning on the effective date of the warrant is March 8, 2023.agreement and ending when the patent expires. Under the exclusivity agreement, we acknowledged that Stanford had already granted a non-exclusive license in the Nonexclusive Field of Use, under the Licensed Patents in the Licensed Field of Use in the Licensed Territory (as those terms are defined in the February 2020 License Agreement”). However, Stanford agreed to not grant further licenses under the Licensed Patents in the Licensed Field of Use in the Licensed Territory. On March 15, 2018,December 29, 2021, we entered into an amendment to the LLUFebruary 2020 License Agreement directly with Loma Linda University, which amends extended our exclusive right to license the technology deployed in AditxtScoreTM and restatessecuring worldwide exclusivity in all fields of use of the Sekris Agreements (the “LLU License Agreement”).licensed technology.
Pursuant to the LLU License Agreement, we obtained the exclusive royalty-bearing worldwide license in and to all intellectual property, including patents, technical information, trade secrets, proprietary rights, technology, know-how, data, formulas, drawings, and specifications, owned or controlled by LLU (the “LLU Patent and Technology Rights”) and/or any of its affiliates and related to therapy for immune-mediated inflammatory diseases (the ADi™ technology). We refer you to the section titled “Our Business—Intellectual Property—Patent Rights” for a summary of the patents and patent applications that we licensed from LLU pursuant to the LLU License Agreement. In consideration for the LLU License Agreement, we issued 50,000 shares of common stock to LLU.
-55-
Pursuant to the LLU License Agreement, we are required
We were obligated to pay an annual licenseand paid a fee of $25,000 to LLU. Additionally,Stanford within 60 days of February 3, 2020. We also issued 375 shares of the Company’s Common Stock to Stanford. An annual licensing maintenance fee is payable by us on the first anniversary of the February 2020 License Agreement in the amount of $40,000 for 2021 through 2024 and $60,000 starting in 2025 until the license expires upon completionthe expiration of this offering, we will bethe patent. The Company is required to pay $200,000 to LLU as aand has paid $25,000 for the issuances of certain patents. The Company will pay milestone payment that was due within thirty (30) days of July 31, 2018. Additionally, the Company has agreed to pay license fees of $50,000 on the first commercial sales of a licensed product and $25,000 annually startingat the beginning of any clinical study for regulatory clearance of an in vitro diagnostic product developed and a potential licensed product. We are also required to: (i) provide a listing of the management team or a schedule for the recruitment of key management positions by March 31, 2020 milestone fees totaling up(which has been completed), (ii) provide a business plan covering projected product development, markets and sales forecasts, manufacturing and operations, and financial forecasts until at least $10,000,000 in revenue by June 30, 2020 (which has been completed), (iii) conduct validation studies by September 30, 2020 (which has been completed), (iv) hold a pre-submission meeting with the FDA by September 30, 2020 (which has been completed), (v) submit a 510(k) application to $1,725,000, andthe FDA, Emergency Use Authorization (“EUA”), or a past patent fee of $200,000 which is to be paid in quarterly installments between September 2018 andLaboratory Developed Test (“LDT”) by March 2019. As consideration31, 2021, (which has been completed), (vi) develop a prototype assay for prior expenses incurredhuman profiling by LLU to prosecute, maintain and defend the LLU Patent and Technology Rights, we are obligated to make the following payments to LLU: $70,000 that was dueDecember 31, 2021 (which has been completed), (vii) execute at the end of December 2018, and a final payment of $60,000 that was due at the end of March 2019. Asleast one partnership for use of the datetechnology for transplant, autoimmunity, or infectious disease purposes by March 31, 2022, and (viii) will provide further development and commercialization milestones for specific fields of this prospectus, we have not received any written demand for payment from LLU.use in writing by December 31, 2022.
We are requiredIn addition to defend the LLU Patent and Technology Rightsannual license maintenance fees outlined above, we will pay Stanford royalties on Net Sales (as such term is defined in the February 2020 License Agreement) during the of the term of the LLU License Agreement. Additionally, we will owe low single-digit royalty payments on any Licensed Products (definedagreement as any finished pharmaceutical product which utilizes the LLU Patent and Technology Rights in its development, manufacturefollows: 4% when Net Sales are below or supply). We alsoequal to $5 million annually or 6% when Net Sales are required to produce a written progress report to LLU, discussing our development and commercialization efforts, within 45 days following the end of each year. All intellectual property rights in and to LLU Patent and Technology Rights shall remain with LLU (other than improvements developed by or on our behalf).
above $5 million annually. The LLU License Agreement shall terminates on the last day that a patent granted in to us by LLU is valid and enforceable or the day that the last patent application licensed to us is abandoned. The LLUFebruary 2020 License Agreement may be terminated by mutual agreementupon our election on at least 30 days advance notice to Stanford, or by us upon 90 days written notice to LLU. LLU may terminate the LLU License AgreementStanford if we: (i) are delinquent on any report or payment; (ii) are not diligently developing and commercializing Licensed Product; (iii) miss certain performance milestones; (iv) are in the event of (i) non-payments or late payments of royalty, milestone and license maintenance fees not cured within 90 days after delivery of written notice by LLU, (ii) a breach of any non-payment provision (including the provision that requires us to meet certain deadlines for milestone events (each, a “Milestone Deadline”)) not cured within 90 days after delivery of written notice by LLU and (iii) LLU delivers notice to us of three or more actual breaches of the LLUFebruary 2020 License Agreement by usAgreement; or (v) provide any false report to Stanford. Should any events in any 12-month period. the preceding sentence occur, we have a thirty (30) day cure period to remedy such violation.
Plan of Operations
The next such Milestone Deadline isinitial application of the requirementplatform was AditxtScore™ for COVID-19, which was designed to provide a more complete a financing round by July 31, 2018. Additional Milestone Deadlines include: (i) the requirement to have regulatory approvalassessment of an IND applicationindividual’s infection and immunity status with respect to initiate a first-in-human clinical trials onthe SARS-CoV-2 virus. Infection status is determined by evaluating the presence or before March 31, 2020, (ii)absence of the completionvirus, and immunity status by measuring levels of first-in-human (phase I/II) clinical trials by March 31, 2022, (iii)antibodies against viral antigens and their ability to neutralize the completion of Phase III clinical trials by March 31, 2024 and (iv) biologic licensing approval by the FDA by March 31, 2025.
Plan of Operations
If we are successful in raising the $10,450,000 contemplated by this offering, we believe that the Company will have sufficient cash resources to fund its plan of operations for the next eighteen (18) months, which will be focused on Phase I/IIa Clinical Trial.
High-level Objectives:
Our first-in-human (FIH) clinical studies will combine Phase I (designed to test clinical safety) and Phase IIa (designed to obtain proof of effectiveness in human patients), in patients requiring skin and other organ and/or tissue allografts. We have selected this indication for several reasons, including:
We have already identified a clinical trial center with adequate patients, which we believe will simplify and reduce the time required for patient recruitment. Upon approval by the FDA and/or the applicable regulatory agency, and once the exact protocol has been determined in the preclinical studies, clinical trials will be initiated.
Drug Approval Processvirus.
In early 2021, we established our AditxtScore™ Immune Monitoring Center in Richmond, Virginia (the “Center”). The Center operates as a Clinical Laboratory Improvement Amendments (CLIA) certified facility for the United States, Foodprocessing of our AditxtScore™ for COVID-19 Lab Developed Test (LDT) for our prospective channel partners, including labs and Drug Administration (“FDA”) approval is required before any new drugs can be introducedhospitals.
In August 2020, we filed for an Emergency Use Authorization (EUA) with the FDA with the ultimate objective of filing a 510(K) application. On January 14, 2022, we submitted requests to obtain two EUAs for our antibody and neutralizing tests following an announcement on November 15, 2021 by the Department of Health and Human Services that COVID-19 related tests will require FDA review and FDA’s position that COVID-19 tests that have been in use prior to the market. We currently haveannouncement must submit applications for EUAs but can continue to operate unless informed otherwise. In the meantime, we are providing AditxtScore™ as a product candidate for our first-in-human studies, butservice as of the date of this prospectus, we have not submitted an applicationa Laboratory Developed Test (LDT) to the regulatory agencies for approval.assess immunity status to COVID-19.
WeThe public health emergency declaration for COVID-19 ended in May 2023. Thus, COVID-related assays are working with a contract manufacturer who has the know-how, product ingredients including plasmid DNA molecules,no longer considered as priority for review by FDA and our patent-pending bacterial strain. Several batch runs have been successfully completed to demonstrate our ability to produce the DNA plasmids in a GMP facility. Based on validation studies, we are reasonably confident in our ability to produce clinical grade product candidates at larger scales. The contract manufacturer has provided a proposalwill not be considered for manufacturing of our clinical grade material, which will be signed and accepted once this offering is completed and we are ready to initiate GMP manufacturing. WeEUAs. Assays that are not currently partydeveloped for evaluation of infection or immunity status to an agreement with this contract manufacturer. We intend to enter into such an agreement after the completion of this offering.
The product candidate selected for clinical trials must be subjected to pre-clinical safety/toxicology studies by an independent GLP (Good Laboratory Practice) laboratory to demonstrate its suitability for clinical testing in human patients. Upon completion of manufacturing and safety/toxicology testing, an Investigation New Drug (IND) application will be prepared for submission to the regulatory agencies.
Upon receipt of clearance to initiate clinical testing, the ADi™ product can be tested in human patients. Our product will be tested in clinical trials, one in patients with psoriasis and one in patients who require skin allografting. Therefore, our first-in-human studies will be combined Phase I/Phase IIa studies in which safety and efficacy data will be obtained. We plan to start with in skin indications (psoriasis and skin allografting) because we believe these indications will be most efficient in providing safety and efficacy data in clinical trials. In parallel, weSARS-CoV-2 will continue to develop additional product formulations for other indications.be offered as LDTs.
Target market
-56-
In the U.S. alone, there are over 36,000 patients who receive organ transplantations each year, with more than 113,000 on transplant waiting lists.
The field of organ transplantation has been made possible and continues to rely on broad-acting immunosuppressive drugs, high levels of which can result in a compromised immune system that renders organ recipients susceptible to cancer and potentially life-threatening infections including re-activation of latent viruses.
In addition, immunosuppressants control acute rejection during the early time-period after receiving an organ but chronic rejection of the organ remains an unmet challenge for surgeons and transplant recipients.
While efforts have been made by various groups to promote tolerance through cell therapies andex vivo manipulation of patient cells, these procedures take place outside the body and typically require hospitalization.
Moreover, transplanted patients will need re-transplantation at some point, with the possible exception of some newborn recipients. With increased incidence of preformed antibodies, these patients may never have the opportunity to receive another organ. Preformed antibodies can develop in previously-transplanted patients, patients who have given birth, and patients who have previously received blood transfusions. These patients have much lower chances at qualifying to receive organs due to their increased risk of rejection – even with immune suppression. The potential to reduce formation of preformed antibodies in these patients will provide better opportunities for them to receive another transplanted organ.
There are gaps between current approaches and what the market needs. We believe that ADi™ addresses these gaps. ADi™ is easy to administer (does not requireex-vivotreatment of patient cells), it does not appear to suppress the immune system, it may allow patients to live with transplanted organs with significantly reduced immune suppression, it may provide for long-term survival of transplanted tissues and organs, may be more effective because it does not rely on a single immune pathway/mechanism, and potentially provides patients with pre-existing antibodies a chance to qualify to receive organs.
While these advantages present opportunities for unmet medical needs in the field of organ transplantation, the industry in which we operate is highly competitive. A small company such as us will meet significant challenges including regulatory requirements for approval of a new class of therapeutic agents, challenges in large scale manufacturing and marketing, cost of developing a novel therapeutic agent, which may require co-development partners who may or may not be willing to work with us, and the willingness of transplant surgeons to adopt our therapeutic vaccines in their existing immune suppression protocols. These challenges pose risks that we may not be able to overcome.
Operational Advantages
Location
We intend to sign a lease in Southern California upon the completion of this offering. Assuming we do, we will be located near resources including Loma Linda University.
Strategic Partners
Our plan is to work with strategic partners to leverage common resources to accomplish milestones over the next 3 years. We hope that this strategy will reduce costs by obviating the need to duplicate resources.
Intellectual Property (IP)
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to our business, including seeking, maintaining and defending patent rights, whether developed internally or licensed from third parties. Our policy is to seek to protect our proprietary position by, among other methods, filing patent applications in the United States and in jurisdictions outside of the United States, to protect our proprietary technology, inventions, improvements and product candidates that are important to the development and implementation of our business. We also rely on trade secrets and know-how relating to our proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of immuno-therapy. We also plan to rely on data exclusivity, market exclusivity, and patent term extensions when available. Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions, and improvements; to preserve the confidentiality of our trade secrets; to obtain and maintain licenses to use intellectual property owned by third parties; to defend and enforce our proprietary rights, including any patents that we may own in the future; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third parties.
The ADi™ technologyOur innovation portfolio includes: (1) ADI™ immune modulation technologies, which are currently at the pre-clinical stage and its various componentsare designed to retrain the immune system to induce tolerance with an objective of addressing rejection of transplanted organs, autoimmune diseases, and allergies; and (2) AditxtScore™ immune monitoring technologies designed to provide a personalized comprehensive profile of the immune system. Both categories are protected by multiple families of patents and patent applications, including several issued U.S. and non-U.S. issuedpatents.
The projected expiration dates for the ADI™ patents and patents issuing from pending applications extend until 2043 for some patents. As of the date of this prospectus,report, our patent portfolio for ADI™ includes both patents and patent applications licensed from LLU includes seven U.S. patents, two U.S. pendingor Stanford and patent applications 56 foreignowned solely by Aditxt, including 120 granted patents, 2 allowed patent applications and 30 pending patient applications in U.S. and other regions. These patents and 15 foreign pending patent applications directed to ADi™ and related technologies. The ADi™ patents are divided intocover three families, one fordifferent technical aspects of ADI™, treatment of autoimmune diseases and type 1 diabetes, one fortreatment of organ transplantation, and a method of producing plasmid DNA that is mammalian-like to prevent immune activation. A fourth family provides patent protection for a composition of matter designed to serve as a general tolerance delivery system for antigens of interest that would be relevant for various given indications. This family is the basis for a platform allowing development of a new class of therapeutic vaccinesimmunotherapeutics for various indications. The patents and patent applications cover both methods of treatment for these indications as well as compositions of matter including plasmids that are able to induce tolerance to antigens or prevention of immune attack on antigens, depending on the indication, along with methods of producing such plasmids.
The AditxtScore™ technology is also protected by multiple families of patents and patent applications, including several issued U.S. and non-U.S. patents. The projected expiration dates for these AditxtScore™ patents and patents issuing from pending applications ranges from 20272037 to 2043. As of the date of this report, our patent portfolio for the earlierAditxtScore™ includes both patents for autoimmune diseases and diabetes to 2032 for the composition of matter patent applications licensed from Stanford and patent applications owned solely by Aditxt, including granted patents and 12 applications. These patents and patent applications encompass methods, systems and kits for the tolerance delivery system. detection and measurement of specific immune responses.
We also possess and/or in-license substantial know-how and trade secrets relating to the development and commercialization of our product candidates, including related manufacturing processes and technology. We plan to continue expanding and strengthening our IP portfolio with additional patent applications in the future.
In March 2021, we signed an agreement with a regulatory consultant based in Munich, Germany, which will play a central role in navigating the first ADI™ therapeutic program through the clinical trial and regulatory process. The firm has been working with the Aditxt’s ADI™ team to submit a clinical trial application to the regulatory agency in Germany. Psoriasis is the first indication being targeted for clinical trial in the ADI™ therapeutics pipeline. Other candidates that are advancing toward clinical trials include ADI™ for type 1 diabetes and skin allografting.
Advantages
The sophistication of the AditxtScore technology includes the following:
| ● | Greater sensitivity/specificity |
| ● | 20- fold higher dynamic range, greatly reducing signal to noise compared to conventional assays |
-57-
| ● | Ability to customize assays and multiplex a large number of analytes with speed and efficiency |
| ● | Ability to test for cellular immune responses, i.e. B & T cell, cytokines. |
| ● | Proprietary reporting algorithm. |
ADIVIR, INC.
Formed in April of 2023, Adivir™, Inc., is Aditxt’s most recently formed wholly owned subsidiary, dedicated to the clinical and commercial development efforts of innovative antiviral products. These products have the potential to address a wide range of infectious diseases, including those that currently lack viable treatment options.
Background
On April 18, 2023, we entered into an Asset Purchase Agreement (the “Asset Purchase Agreement”) with Cellvera Global Holdings LLC (“Cellvera Global”), Cellvera Holdings Ltd. (“BVI Holdco”), Cellvera, Ltd. (“Cellvera Ltd.”), Cellvera Development LLC (“Cellvera Development” and together with Cellvera Global, BVI Holdco, Cellvera Ltd. and Cellvera Development (the “Sellers”), AiPharma Group Ltd. (“Seller Owner” and collectively with the Sellers, “Cellvera”), and the legal representative of Cellvera, pursuant to which, the Company will purchase Cellvera’s 50% ownership interest in G Response Aid FZE (“GRA”), certain other intellectual property and all goodwill related thereto (the “Acquired Assets”). Unless expressly stated otherwise herein, capitalized terms used but not defined herein have the meanings ascribed to them in the Asset Purchase Agreement. Pursuant to the Asset Purchase Agreement, the consideration for the Acquired Assets consists of (A) $24.5 million, comprised of: (i) the forgiveness of the Company’s $14.5 million loan to Cellvera Global, and (ii) approximately $10 million in cash, and (B) future revenue sharing payments for a term of seven years. GRA holds an exclusive, worldwide license for the antiviral medication, Avigan® 200mg, excluding Japan, China and Russia. The other 50% interest in GRA is held by Agility, Inc. (“Agility”).
Additionally, upon the closing, the Share Exchange Agreement previously entered into as of December 28, 2021, between Cellvera Global Holdings, LLC f/k/a AiPharma Global Holdings, LLC (together with other affiliates and subsidiaries) and the Company, and all other related agreements will be terminated.
The obligations of the Company to consummate the Closing are subject to the satisfaction or waiver, at or prior to the Closing of certain conditions, including but not limited to, the following:
| (i) | Satisfactory completion of due diligence; |
| (ii) | Completion by the Company of financing sufficient to consummate the transactions contemplated by the Asset Purchase Agreement; |
| (iii) | Receipt by the Company of all required Consents from Governmental Bodies for the Acquisition, including but not limited to, any consents required to complete the transfer and assignment of Cellvera’s membership interests in GRA; |
| (iv) | Receipt of executed payoff letters reflecting the amount required to be fully pay all of each of Seller’s and Seller Owner’s Debt to be paid at Closing; |
| (v) | Receipt by the Company of a release from Agility; |
| (vi) | Execution of an agreement acceptable to the Company with respect to the acquisition by the Company of certain intellectual property presently held by a third party; |
-58-
| (vii) | Execution of an amendment to an asset purchase agreement previously entered into by Cellvera with a third party that effectively grants the Company the rights to acquire the intellectual property from the third party under such agreement; |
| (viii) | Receipt of a fairness opinion by the Company with respect to the transactions contemplated by the Asset Purchase Agreement; and |
| (ix) | Receipt by the Company from the Seller Owner of written consent, whether through its official liquidator or the Board of Directors of Seller Owner, to the sale and purchase of the Acquired Assets and Assumed Liabilities pursuant to the Assert Purchase Agreement. |
There can be no assurance that the conditions to closing will be satisfied or that the proposed acquisition will be completed as proposed or at all.
Our commitment to building our antiviral portfolio is strategic and timely. We believe that there has never has there been a more important time to address the growing global need to uncover new treatments or commercialize existing ones that treat life-threatening global viral infections.
MDNA
On December 17, 2023, we entered into an Asset Purchase Agreement (the “Purchase Agreement”) with Pearsanta, Inc., our majority owned subsidiary (“Pearsanta”) and MDNA Life Sciences, Inc. (“MDNA”), pursuant to which Pearsanta agreed to acquire certain intellectual property and other specified assets relating to MDNA’s early cancer detection platform (the “Acquired Assets”). MDNA’s Mitomic™ technology provides a tool for identifying biomarkers associated with various diseases that lead to mtDNA mutations. The Acquired Assets include, but are not limited to, the following:
| ● | The Mitomic Endometriosis Test (MET™) is in development as a blood-based assay for diagnosis of endometriosis. This test aims to provide early diagnostic insights, potentially reducing delays in diagnosing endometriosis. |
| ● | The Mitomic Prostate Test (MPT™) is currently under development as a blood-based assay for diagnosis of prostate cancer. We believe that this test holds the potential to provide more specific and clinically informative data especially in the prostate-specific antigen (PSA) grey zone. It aims to address the challenges of over-diagnosis and mitigate risks associated with low-grade cancers. |
Pursuant to the Purchase Agreement, the consideration for the transaction was to consist of: (i) an upfront working capital payment of $500,000 (the “Upfront Working Capital Payment”), which is payable upon the satisfaction of certain conditions set forth in the Purchase Agreement, (ii) a working capital payment at closing of $500,000, (iii) 50,000 shares of our Common Stock, (iv) a warrant to purchase 50,000 shares of our Common Stock exercisable for a term of 5 years at an exercise price equal to the opening price per share of our Common Stock as of the Closing Date (as defined below), and (v) 5,000 shares of Pearsanta Series A Preferred Stock, par value $0.001 per share (the “Pearsanta Preferred Stock”), provided, however, that if the value of such Pearsanta Preferred Stock, on an as-converted basis, at the time of the pricing of the Pearsanta common stock in connection with the sale of shares of Pearsanta common stock to the public in a firm-commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended does not equal $25,000,000, an additional amount of Pearsanta Preferred Stock (“Additional Pearsanta Preferred Stock”) so that the sum of the value of the Pearsanta Preferred Stock plus the Additional Pearsanta Preferred Stock (if any) shall equal $25,000,000. The Pearsanta Preferred Stock shall have such rights, powers, and preferences as set forth in the form of Certificate of Designation of Series A Preferred Stock, the form of which is attached as Exhibit D to the Purchase Agreement.
On January 4, 2024, we entered into a First Amendment to Asset Purchase Agreement with Pearsanta and MDNA, pursuant to which the parties agreed to: (i) the removal of the Upfront Working Capital Payment, (ii) the removal of the Closing Working Capital Payment (as defined in the Purchase Agreement”), and (iii) to increase the maximum amount of payments to be made by us under the Transition Services Agreement (as defined below) from $2.2 million to $3.2 million.
On January 4, 2024, Pearsanta and MDNA entered into a Transition Services Agreement (the “Transition Services Agreement”), pursuant to which MDNA agreed that it would perform, or cause certain of its affiliates or third parties to perform, certain services as described in the Transition Services Agreement for a term of three months in consideration for the payment by Pearsanta of certain fees as provided in the Transition Services Agreement, in an amount not to exceed $3.2 million.
On January 4, 2024, we completed its acquisition of the Acquired Assets and issued to MDNA 50,000 shares of our Common Stock, a warrant to purchase 50,000 shares of our Common Stock, and the Pearsanta Preferred Stock.
Employees
We have two (2) full-timethirty - nine (39) full time employees. We engage consultants on an as needed basis from time to time. Currently, we have engaged sixteen (16) consultants. We believe ourconsider the relations with our employees to be good.
-59-
Executive Officers and Directors
The following table setsSet forth below is certain information with respect to the name, ageindividuals who are our directors and position of each of our executive officers key employees and directors as of the date of this prospectus.
December 31, 2023:
| Name | Age | Positions | ||
| Amro Albanna | Chief Executive Officer, Director | |||
| Chief | ||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | Chief Innovation Officer, Director | |||
| Chief Operating Officer | ||||
| Thomas J. Farley | 50 | Chief Financial Officer | ||
| Charles Nelson | 70 | Director | ||
| Director | ||||
| Director |
Amro Albanna –- President, Chief Executive Officer and Chairman of the Board
Mr. Albanna has been our President, Chief Executive Officer and a Director since we were formed in 2017. He also served as our President from our inception through September 2021. In 2010, Mr. Albanna co-founded Innovation Economy Corporation (“IEC”), formed to license and commercialize innovations and create a group of life and health subsidiaries. From 2010 until 2017, Mr. Albanna was Chief Executive Officer and a Director of IEC and Olfactor Laboratories, Inc., a majority-owned subsidiary of IEC. From 2010 to August 2016, he was the Chief Executive Officer and a Director of Nano Engineered Applications, Inc., another majority-owned subsidiary of IEC. In 2003, Mr. Albanna founded Qmotions, Inc. (subsequently renamed Deal A Day Group Corp.). He served as its Chief Executive Officer and a Director until 2011. Qmotions used 3-D spatial tracking and pattern recognition technologies to develop motion-capturing video game controllers. In 2002, Mr. Albanna was a co-founder of Digital Angel Corporation –- a company formed via the merger of three private companies (one being TTC below) into a fourth publicly traded company (American Stock Exchange) and was placed in charge of commercializing its GPS/wireless technologies. Around that time, Mr. Albanna co-founded an incubator for startups at the University of California, Riverside Research Park which was acquired in 2007. In 1997, he founded Timely Technology Corporation (“TTC”), which designed and developed e-commerce software for education, retail and finance. TTC was acquired in 2000 by a Nasdaq-listed company. Mr. Albanna graduated from California State University San Bernardino in 1991 with a B.S. in Business Administration with concentration in Computer Information Systems. He completed graduate coursework in Computer Science and Engineering at California State University, Long Beach from 1992 to 1993. In 2019, Mr. Albanna completed coursework in Immunology and Genetics at Harvard Medical School HMX online learning platform. We believe that Mr. Albanna’s expertise leading technology companies across various sectors, leading private and public financing, and in positioning companies for mergers and acquisitions, qualifies him to serve as a director of our Company.
Corinne Pankovcin — Chief Commercialization Officer
Ms. Pankovcin has been our Chief Commercialization Officer since April 12, 2023. Ms. Pankovcin served as our President from September 2021 through April 2023. Ms. Pankovcin served as our Chief Financial Officer from July 2020 through August 2021. From December 2015 to July 2019, Ms. Pankovcin was the Chief Financial Officer and Managing Director and Treasurer of Business Development Corporation of America (“BDCA”), a business development company. Prior thereto, from January 2011 to August 2015, Ms. Pankovcin was the Chief Financial Officer and Treasurer of Blackrock Capital Investment Corporation (NASDAQ: BKCC), and a Managing Director of Finance at BlackRock Investment Management LLC. Prior to joining BlackRock, Ms. Pankovcin was a senior member of Finance & Accounting of Alternative Investments and served as Chief Financial Officer for the Global Emerging Markets products group at AIG Capital Partners. Ms. Pankovcin began her career with PricewaterhouseCoopers LLP, where she ultimately held the role of Senior Manager of Business Assurance for Consumer Products, Manufacturing, and Middle Market industries from 1991 to 2001. Ms. Pankovcin earned her B.S. in Accounting from Dowling College and her Master’s Degree in Business Administration from Hofstra University. She is a Certified Public Accountant.
-60-
Shahrokh Shabahang, –D.D.S., MS, Ph.D. - Chief Innovation Officer Director
Dr. Shabahang has been our Chief Innovation Officer and Director since our inception. In 2009, Dr. Shabahang co-founded Sekris Biomedical Inc. to incubate immunotherapy technologies. He served as its Chairman of the board and Chief Executive Officer since its inception. In 2004, Dr. Shabahang joined Genelux Corporation to lead its clinical development program and to serve as board secretary. Genelux developed an oncolytic virus technology for treatment of cancer, co-invented by Dr. Shabahang. During his tenure from 2004-2007, Genelux raised $20M+ and obtained regulatory approval to initiate First-In-Human clinical studies in Europe with patients who had not responded to chemotherapy. In 2001, Dr. Shabahang became the Director of the Microbiology and Molecular Biology Lab at Loma Linda University (“LLU”). He led the research and development of an antimicrobial therapeutic agent for treatment of dental infections, which was licensed and marketed by one of the largest dental distribution companies. Dr. Shabahang attended the University of California, Santa Barbara from 1982 to 1984 and later received his DDS from the University of Pacific in 1987. He earned his PhD in Microbiology and Molecular Genetics at LLU in 2001. During the same year, he established his laboratory at LLU to study infectious diseases and host immune responses. We believe that Dr. Shabahang’s experience leading biotech startups, leading clinical development programs, and his expertise in immunology and immune tolerance qualifies him to serve as a director of our Company.
David Briones –Rowena Albanna - Chief Operating Officer
Ms. Albanna has been our Chief Operating Officer since July 2020. From 2017 to immediately prior to her appointment as Chief Operating Officer, Ms. Albanna was an independent operations consultant for the Company. Prior thereto, from 2013 to 2017, Ms. Albanna was the Chief Operating Officer of Innovation Economy Corporation (“IEC”), formed to license and commercialize innovations and create a group of life and health subsidiaries. From 2010 to 2013, Ms. Albanna was Senior Vice President of IEC. From 2004 to 2009, Ms. Albanna was the founder and principal of Weezies, an online-based business focused on building and operating e-commerce stores and affiliate marketing sites. From 2003 to 2004, Ms. Albanna was the head of Product Development and Engineering of Qmotions Inc. Qmotions used 3-D spatial tracking and pattern recognition technologies to develop motion-capturing video game controllers. In 2002, Ms. Albanna was VP of Product Development at Digital Angel Systems where she led the development of devices which combined GPS, wireless, and biosensing. Prior to that, Ms. Albanna held multiple product development roles with increasing responsibilities for various technology companies in the areas of financial, medical, telecommunications, integrated circuit layout design, and defense. Ms. Albanna is a co-inventor of two patents related to systems for localizing, monitoring, and sensing objects. Ms. Albanna received a Bachelor of Science degree in Computer Science with a minor in Mathematics from California State University, San Bernardino in 1988. Ms. Albanna is the wife of Amro Albanna, our Chief Executive Officer.
Thomas J. Farley, CPA - Chief Financial Officer
Mr. BrionesFarley has been our Chief Financial Officer since January 2018. Mr. Briones is the founder and managing member of Brio Financial Group since its inception in October 2010, with over nineteen years of public accounting and executive level experience. He consults with various public companies in financial reporting, internal control development and evaluation, budgeting and forecasting. Mr. Briones has also been the Chief Financial Officer for Petro River Oil Corp., an independent energy company focused on the exploration and development of conventional oil and gas assets, since August 2013. From October 2017September 2021. Prior to May 2018this, Mr. BrionesFarley was the Chief FinancialPrincipal Accounting Officer and Controller from October of Bitzumi, Inc.2020 to September 2021. From December 2015 to June 2020, Mr. Farley was the Controller of Business Development Corporation of America (“BDCA”), a Bitcoin exchangepublicly listed business development company. Prior thereto, from January 2011 to August 2015, Mr. Farley was the Senior Controller of Blackrock Capital Investment Corporation (NASDAQ: BKCC). Prior to joining BlackRock Capital Investment Corporation, Mr. Farley was a Senior Controller for PineBridge Investments Emerging Markets practice. Mr. Farley was also an Accounting Manager for Bessemer Venture Partners prior to his tenure at PineBridge. Mr. Farley began his career with PricewaterhouseCoopers LLP, from 1996 to 2001. Mr. Farley earned his B.S. in Accounting from Long Island University and marketplace.is a Certified Public Accountant.
Brian Brady –- Director
Mr. Brian Brady has served as a Director since December 1, 2018. Mr. Brady has also been the Director of Investments at Prime Healthcarea large hospital system since March 2016, where he is responsible for the management of investment activity related to the organization and personal investments of the family that owns that company. From December 2011 to March 2016, Mr. Brady was the Vice President/Portfolio Manager at Northern Trust,a wealth advisory firm, where he served in an investment advisory role, including asset and portfolio management. Mr. Brady graduated in 2001 with a Bachelor’s degree in Finance from the University of Illinois at Chicago and in 2014 with a Master of Business Administration degree from the University of Chicago. We believe that Mr. Brady’s extensive experience with financial markets and management of investment activities qualifies him to serve as a director of our Company.
-61-
Namvar Kiaie –Charles Nelson - Director Nominee
Mr. Namvar Kiaie isNelson has served as a nominee fordirector since November 2023. Prior to his appointment as a director,member of the Board, Mr. Nelson was a consultant to the Company from September 2020 through September 2023. He began his financial career as a market representative with American International Group and such appointment will be effective upon completion of this offering.in 1979 joined Dean Witter Reynolds as a Financial Advisor, working with high net worth and institutional clients. In 1980, he joined Drexel Burnham and Lambert, and subsequently, at Ladenberg Thalmann and then at Auerbach Pollack and Richardson originating equity and investment banking transactions. Over the last 20 years, Mr. KiaieNelson has been associatedinvolved with Abbott Diabetes Care since December 2005 (Director of Engineering 2005-2007; R&D Director 2007-2010;financing companies in the fintech, healthcare and Senior Director of R&D 2010-present), where he is responsible forbio-pharma spaces through private equity and public financing including listings on the commercial launch of diabetes management related productsNasdaq and accessories, including blood glucose monitoring devices and data management software. Mr. Kiaie graduated in 1985 with a Bachelor of Science degree in Electrical Engineering and in 1986 with a Master of Science degree in Electrical Engineering, both from the University of California Santa Barbara.NYSE. We believe that Mr. Kiaie’sNelson’s extensive experience leading research and development efforts in the biotech industrycapital markets qualifies him to serve as a director of our Company.
Laura Anthony –Jeffrey W. Runge, M.D - Director Nominee
Ms. Laura Anthony is a nominee for appointmentDr. Runge has served as a director and such appointment will be effective upon completion of this offering. Ms. Anthony issince July 2020. From 2008 to the founding partner of Anthony L.G., PLLC, a corporate, securities and business transactions law firm andpresent, Dr. Runge has been practicing law since 1993. Ms. Anthonythe President and founder of Biologue, Inc., which provides corporate counselconsulting in biodefense, medical preparedness and injury control. From 2001 through August of 2008, Dr. Runge served in the Bush administration, first as the head of the National Highway Traffic Safety Administration, and, beginning in September 2005, as the Department of Homeland Security’s (DHS) first Chief Medical Officer. Dr. Runge founded the DHS Office of Health Affairs and was confirmed by the United States Senate as DHS’ first Assistant Secretary for Health Affairs in December of 2007. Dr. Runge also served as Acting DHS Undersecretary for Science and Technology from February through August 2006. In his role at DHS, Dr. Runge oversaw the operations of the department’s biodefense activities, medical preparedness and workforce health protection, as well as fulfilling DHS’ responsibilities in medical countermeasure development. Prior to small-caphis government service, Dr. Runge was Assistant Chairman and middle market privateDirector of Clinical Research in the Department of Emergency Medicine at Carolinas Medical Center in Charlotte, NC, from 1984 through 2001. Additionally, Dr. Runge is a Senior Advisor at The Chertoff Group, a firm providing advisory services in business risk management, security and public companies. For over twenty-five years, Ms. Anthonyhomeland defense. Since 2010, Dr. Runge has served clientson the boards of two public companies, including their Audit and Compensation committees, both of which underwent strategic acquisitions. He has also served as President and CEO of a SEC-regulated startup company in the areas including but not limited to compliance withhealth sector. Dr. Runge earned his medical degree from the Securities ActMedical University of 1933 offer saleSouth Carolina and registration requirements, including private and public offerings; initial public offerings; follow-on offerings and PIPE transactions; compliance withhis undergraduate degree from the NASDAQ and NYSE American initial and continued listing requirements; compliance withUniversity of the initial quotation and maintenance of standards for the OTCQB and OTCQX; working with foreign private issuers; Regulation A/A+ offerings; compliance with the registration and reporting requirements under the Securities Exchange Act of 1934; mergers and acquisitions; and general contract and business transactions. Ms. Anthony received a juris doctorate from Florida State University College of Law in 1993.South. We believe that Ms. Anthony’s extensiveDr. Runge’s experience asin medicine, medical research, public service, business and his prior service on public corporate counsel to private and public companiesboards qualifies herhim to serve as a director of our Company.
Board Leadership Structure and Risk Oversight
The Board oversees our business and considers the risks associated with our business strategy and decisions. The Board currently implements its risk oversight function as a whole. Each of the Board committees, when established, will also provide risk oversight in respect of its areas of concentration and reports material risks to the Board for further consideration.
Term of Office
Officers hold office until his or her successor is elected and qualified. Directors are appointed to serve for one year until the meeting of the Board following the annual meeting of stockholders and until their successors have been elected and qualified.
-62-
Director Independence
We use the definition of “independence” of The Nasdaq Stock Exchange LLC (“Nasdaq”) listing rules to make this determination. Nasdaq listing rules provide that an “independent director” is one who the board “affirmatively determines” has no “material relationship” with the company “either directly or as a partner, shareholder or officer of an organization that has a relationship with the Company. Nasdaq listing rules provide that a director cannot be considered independent if:
| ● | the director is, or has been within the last three (3) years, an employee of the Company or an immediate family member of director is, or has been within the last three (3) years, an executive officer of the Company; |
| ● | the director has received, or has an immediate family member who is an executive officer of the Company and has received, during any twelve-month period within the last three (3) years, more than $120,000 compensation directly from the Company (not including compensation received for director service, pension plan payments or deferred compensation for prior service not contingent on continued service); |
| ● | the director or an immediate family member is a current partner of the Company’s internal or external auditor; the director is a current employee of the auditor; an immediate family member is a current employee of the auditor and personally works on the Company’s audit; or the director or an immediate family member was within the last three (3) years a partner or employee of the auditor and personally worked on the Company’s audit within that time; |
| ● | the director or an immediate family member is, or has been within the last three (3) years, employed as an executive officer of another company where any of the Company’s present executive officers at the same time serves or served on that company’s compensation committee; or |
| ● | the director is a current employee, or an immediate family member is a current executive officer, of an organization that has made to or received from the Company payments for property or services in an amount which, in any of the last three fiscal (3) years, exceeds greater of 2% of such other company’s consolidated gross revenues or $1 million. Charitable contributions not considered “payments” for purposes of this prohibition but contributions meeting these thresholds must be disclosed on the Company’s website or in its annual proxy statement or its Annual Report on Form 10-K. |
Under such definitions, we consider Mr. Kiaie,Nelson, Mr. Brady, and Ms. AnthonyDr. Runge to be “independent.” Nasdaq listing rules permits a phase-in period of up to one year for an issuer registering securities in an initial public offering to comply with its requirement that a majority of the board of directors be made up of independent directors. However, our common stock is not currently quoted or listed on any national exchange or interdealer quotation system with a requirement that a majority of our Board be independent and, therefore, the Company is not subject to any director independence requirements. Upon listing on The Nasdaq Capital Market, however, we will beWe are subject to Nasdaq’s director independence requirements and we will beare required to structure our board of directors accordingly.
Committees of the Board
Upon the consummationOur board of this offering, the Company’s Board will establishdirectors has established three standing committees: Audit, Compensation, and Nominating and Corporate Governance. Each of thethese standing committees will operate pursuant to its respective charter. The committee charters will beare reviewed annually by the Nominating and Corporate Governance Committee. If appropriate, and in consultation with the chairs of the other committees, the Nominating and Corporate Governance Committee may propose revisions to the charters. The responsibilities of each committee are described in more detail below.
Nasdaq listing rules permits a phase-in period for an issuer registering securities in an initial public offering to meet the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee independence requirements. Under the initial public offering phase-in period, only one member of each committee is required to satisfy the heightened independence requirements at the time our registration statement becomes effective, a majority of the members of each committee must satisfy the heightened independence requirements within 90 days following the effectiveness of our registration statement, and all members of each committee must satisfy the heightened independence requirements within one year from the effectiveness of our registration statement.
-63-
The composition and functions of each committee are described below.
| Name | Independent | Audit | Nominating and Corporate Governance | Compensation | ||||||||||||
| Amro Albanna | ||||||||||||||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | ||||||||||||||||
| Brian Brady | X | X | * | X | X | |||||||||||
| Charles Nelson | X | X | X | X | * | |||||||||||
| Jeffrey Runge, M.D. | X | X | X | * | X | |||||||||||
| * | Chairman of the committee |
Audit Committee
The Audit Committee, among other things, will beis responsible for:
| ● | appointing; approving the compensation of; overseeing the work of; and assessing the independence, qualifications, and performance of the independent auditor; |
| ● | reviewing the internal audit function, including its independence, plans, and budget; |
| ● | approving, in advance, audit and any permissible non-audit services performed by our independent auditor; |
| ● | reviewing our internal controls with the independent auditor, the internal auditor, and management; |
| ● | reviewing the adequacy of our accounting and financial controls as reported by the independent auditor, the internal auditor, and management; |
| ● | overseeing our financial compliance system; and |
| ● | overseeing our major risk exposures regarding the Company’s accounting and financial reporting policies, the activities of our internal audit function, and information technology. |
The Board has affirmatively determined that each member of the Audit Committee meets the additional independence criteria applicable to audit committee members under SEC rules and Nasdaq listing rules. The Board will adopthas adopted a written charter setting forth the authority and responsibilities of the Audit Committee. The Board has affirmatively determined that each member of the Audit Committee is financially literate, and that Mr. Brady meets the qualifications of an Audit Committee financial expert.
The Audit Committee will consistconsists of Mr. Brady, Ms. Laura Anthony,Mr. Nelson, and Mr. Kiaie.Dr. Runge. Mr. Brady will chairchairs the Audit Committee. We believe that, after consummation of this offering, the functioning of the Audit Committee will comply with the applicable requirements of the rules and regulations of the Nasdaq listing rules and the SEC.
Compensation Committee
The Compensation Committee will beis responsible for:
| ● | reviewing and making recommendations to the Board with respect to the compensation of our officers and directors, including the CEO; |
| ● | overseeing and administering the Company’s executive compensation plans, including equity-based awards; |
| ● | negotiating and overseeing employment agreements with officers and directors; and |
| ● | overseeing how the Company’s compensation policies and practices may affect the Company’s risk management practices and/or risk-taking incentives. |
-64-
The Board will adopthas adopted a written charter setting forth the authority and responsibilities of the Compensation Committee.
The Compensation Committee will consistconsists of Ms. Laura Anthony, Mr. Brady, Mr. Nelson, and Dr. Runge. Mr. Kiaie. Mr. Kiaie will serveNelson serves as chairman of the Compensation Committee. The Board has affirmatively determined that each member of the Compensation Committee meets the independence criteria applicable to compensation committee members under SEC rules and Nasdaq listing rules. The Company believes that, after the consummation of the offering, the composition of the Compensation Committee will meet the requirements for independence under, and the functioning of such Compensation Committee will comply with, any applicable requirements of the rules and regulations of Nasdaq listing rules and the SEC.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee, among other things, will beis responsible for:
| ● | reviewing and assessing the development of the executive officers and considering and making recommendations to the Board regarding promotion and succession issues; |
| ● | evaluating and reporting to the Board on the performance and effectiveness of the directors, committees and the Board as a whole; |
| ● | working with the Board to determine the appropriate and desirable mix of characteristics, skills, expertise and experience, including diversity considerations, for the full Board and each committee; |
| ● | annually presenting to the Board a list of individuals recommended to be nominated for election to the Board; |
| ● | reviewing, evaluating, and recommending changes to the Company’s Corporate Governance Principles and Committee Charters; |
| ● | recommending to the Board individuals to be elected to fill vacancies and newly created directorships; |
| ● | overseeing the Company’s compliance program, including the Code of Conduct; and |
| ● | overseeing and evaluating how the Company’s corporate governance and legal and regulatory compliance policies and practices, including leadership, structure, and succession planning, may affect the Company’s major risk exposures. |
The Board of Directors will adopthas adopted a written charter setting forth the authority and responsibilities of the Nominating and Corporate Governance/NominatingGovernance Committee.
The Nominating and Corporate Governance Committee will consistconsists of Ms. Laura Anthony,Dr. Runge, Mr. Brady, and Mr. Kiaie. Ms. Laura Anthony will serveDr. Runge serves as chairman.chairman of the Nominating and Corporate Governance Committee. The Company’s Board of Directors has determined that each member of the Nominating and Corporate Governance Committee is independent within the meaning of the independent director guidelines of Nasdaq listing rules.
IndemnificationCompensation Committee Interlocks and Insider Participation
InNone of the Company’s executive officers serves, or in the past has served, as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any entity that has one or more executive officers who serve as members of the Company’s board of directors or its compensation committee. None of the members of the Company’s compensation committee is, or has ever been, an officer or employee of the Company.
Code of Business Conduct and Ethics
The Company’s board of directors adopted a code of business conduct and ethics applicable to its employees, directors and officers, in accordance with applicable U.S. federal securities laws and the corporate governance rules of the Nasdaq Capital Market. The code of business conduct and ethics is publicly available on the Company’s website. Any substantive amendments or waivers of the code of business conduct and ethics or code of ethics for senior financial officers may be made only by the Company’s board of directors and will be promptly disclosed as required by applicable U.S. federal securities laws and the corporate governance rules of the Nasdaq Capital Market.
-65-
Corporate Governance Guidelines
The Company’s board of directors has adopted corporate governance guidelines in accordance with the Delaware General Corporation Law and the Company’s Amended and Restated Certificate of Incorporation, the Company will indemnify, hold harmless and provide advancement of expenses, to the fullest extent permitted by applicable law, directors, officers, employees, and agents that are made a party or threatened to be made a party to legal proceedings by reasoncorporate governance rules of the fact that such parties were working at the request of the Company. For more information see the section of this prospectus titled “Risk Factors.”Nasdaq Capital Market.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is therefore unenforceable.
Involvement in Certain Legal Proceedings
To our knowledge, none of our current directors or executive officers has, during the past ten years:
| been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he or she was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Securities Exchange Act of 1934, as amended (the Exchange Act)), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
-66-
Except as set forth above and in our discussion below in “Certain Relationships and Related Transactions,” none of our directors or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Other than as set forth below, we are not currently a party to any legal proceedings, the adverse outcome of which, individually or in the aggregate, we believe will have a material adverse effect on our business, financial condition or operating results.
The Company, Amro Albanna, our President and Chief Executive Officer, and Dr. Shahrokh Shabahang, our Chief Innovation Officer, have been named as cross-defendants in a counterclaim filed by Christopher Sechrist in an action entitled Shahrokh Shabahang v. Christopher Sechrist, San Bernardino County Superior Court Case No. CIVDS1831323. In a cross-complaint, Mr. Sechrist contends that he was a partner in a dental practice with Dr. Shabahang, and that disputes arose as between those partners. Neither the Company nor Mr. Albanna were partners in, or otherwise have an interest in, the dental practice. Notwithstanding, and seemingly based solely on the fact that Dr. Shabahang became the Chief Innovation Officer for the Company, Mr. Sechrist has brought claims against the Company and Mr. Albanna. Both the Company and Mr. Albanna believe that the Counterclaims filed by Mr. Sechrist have no factual or legal merit, and they intend to vigorously defend themselves in the action and to seek a dismissal of the case as against them as soon as possible. On May 26, 2020, Mr. Sechrist filed a request for dismissal as to the Company and Mr. Albanna with the Superior Court of California, County of San Bernardino, San Bernardino District. The clerk of the court entered the dismissal with prejudice on May 26, 2020.
Our CEO,Chief Executive Officer, Amro Albanna, is a party to litigation matters unrelated to the Company or any of its properties. Such litigations relate to Innovation Economy Corporation (IEC), a company in which Mr. Albanna served as the CEO and a Director from 2010 until 2017, and its wholly-owned subsidiaries (Innovation Economy Corporation d/b/a ieCrowd). The first litigation (ieCrowd v. Kim, et. al, Superior Court, Riverside County) was originally commenced by IEC and its subsidiary after Mr. Albanna was no longer affiliated with IEC, against certain third-party defendants based upon claims related to their misconduct and mismanagement. Such defendants subsequently brought a countersuit against IEC and its subsidiary, in which they named Mr. Albanna and others as defendants, alleging that they were misled to invest in IEC and its subsidiary based upon misrepresentations by, among others, Mr. Albanna. The cases have now been consolidated. Mr. Albanna believes that the counteraction commenced by the third parties against him is without merit and intends to defend himself. The second matter (Calabria v. ieCrowd) was commenced by Calabria Ventures (the “Calabria Action”) more than 2 years after Mr. Albanna was no longer affiliated with IEC, related to uncollected rent. Mr. Albanna believes that the action commenced against him is without merit and intends to defend himself. IEC (either directly or through its Director and officer insurance policy) has covered all related legal costs to date. On August 5, 2020, the plaintiff in the Calabria Action filed a request for dismissal as to Mr. Albanna with the Superior Court of California, County of Riverside. The clerk of the court entered the dismissal without prejudice on August 5, 2020.
-67-
EXECUTIVE AND DIRECTOR COMPENSATION
The following table represents information regarding the total compensation for the named executive officers of the Company as of December 31, 20182023 and 2017:2022:
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||
| Amro Albanna | 2018 | 180,000 | -- | -- | -- | -- | 180,000 | (4) | ||||||||||||||||||
| Chief Executive Officer, President, and Director | 2017 | 51,000 | -- | -- | -- | 800 | (1) | 51,800 | ||||||||||||||||||
| Shahrokh Shabahang | 2018 | 180,000 | -- | -- | -- | -- | 180,000 | (5) | ||||||||||||||||||
| Chief Innovation Officer | 2017 | -- | -- | -- | -- | -- | -- | |||||||||||||||||||
| David Briones | 2018 | 36,484 | (2) | 36,484 | ||||||||||||||||||||||
| Chief Financial Officer | 2017 | 182,420 | (3) | 182,420 | ||||||||||||||||||||||
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Restricted Stock Units ($) | All Other Compensation ($)(4) | Total ($) | ||||||||||||||||||||||||
| Amro Albanna | 2023 | 432,119 | - | - | 47,114 | - | 40,000 | 519,233 | ||||||||||||||||||||||||
| Chief Executive Officer and Director | 2022 | 500,000 | - | - | - | - | 500,000 | |||||||||||||||||||||||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. | 2023 | 293,502 | - | - | 35,336 | - | 30,000 | 358,837 | ||||||||||||||||||||||||
| Chief Innovation Officer | 2022 | 325,000 | - | - | - | - | - | 325,000 | ||||||||||||||||||||||||
| Corinne Pankovcin | 2023 | 346,774 | - | - | 23,557 | - | 20,000 | 390,331 | ||||||||||||||||||||||||
| Chief Commercialization Officer, Former President(1), Former Chief Financial Officer(2) | 2022 | 385,000 | - | - | - | - | - | 385,000 | ||||||||||||||||||||||||
| Thomas J. Farley | 2023 | 337,894 | - | - | 23,557 | - | 20,000 | 381,451 | ||||||||||||||||||||||||
| Chief Financial Officer(4) | 2022 | 360,833 | - | - | - | - | 360,833 | |||||||||||||||||||||||||
| Matthew Shatzkes | 2023 | 198,670 | 890,893 | - | - | 34,076 | 1,123,639 | |||||||||||||||||||||||||
| Chief Legal Officer & General Counsel(5) | 2022 | 368,958 | 246,697 | - | - | 218,064 | - | 833,719 | ||||||||||||||||||||||||
Option awards represent granted options at the fair market value as of the date of grant. Restricted stock units represent granted restricted stock units at the fair market value as of the date of grant.
| (1) |
| (2) |
| (3) | |
| (4) |
Director Compensation
-68-
Employment Agreements
To date, we haveAmro Albanna, Chief Executive Officer
On November 14, 2021, the Company entered into an Amended and Restated Employment Agreement with Mr. Amro Albanna, the Chief Executive Officer of the Company (the “Amro Employment Agreement”). Pursuant to the Amro Employment Agreement, Mr. Albanna will receive (i) a base salary at the annual rate of $280,000 for the remainder of calendar year 2021, and effective January 1, 2022, $500,000 (prorated for any partial year) payable in bimonthly installments (ii) the opportunity to earn an annual bonus of 2% of the Company’s earnings before interest, taxes, depreciation, and amortization (EBITDA) with respect to an applicable year for which the bonus is payable, provided that such bonus will not compensated our directorsexceed two (2) times Mr. Albanna’s base salary, and (iii) eligible to earn an annual discretionary bonus as determined by the Board or its Compensation Committee in their sole discretion. In addition, for their servicecalendar year 2021, Mr. Albanna will be eligible to earn an additional discretionary bonus as determined by the Company.
The term of Mr. Albanna’s engagement under the Amro Employment AgreementsAgreement commences as of the Effective Date (as defined in the Amro Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Amro Employment Agreement. The term of Mr. Albanna’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Albanna or the Company.
We doUnder the Amro Employment Agreement, termination of Mr. Albanna by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Amro Employment Agreement), or resignation by Mr. Albanna without “Good Reason” (as defined in the Amro Employment Agreement), will not currently have employment agreements withrequire the Company to pay severance to Mr. Albanna. Upon any such termination, Mr. Albanna will be entitled to receive any Accrued Compensation (as defined in the Amro Employment Agreement), which in the case of our officerstermination by the Company for Cause or employees but intendresignation by Mr. Albanna for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Albanna by the Company without “Cause” or resignation by Mr. Albanna for “Good Reason,” then under the Amro Employment Agreement will require the Company to enter into employment agreementspay severance to Mr. Albanna. Upon any such termination, Mr. Albanna will be entitled to receive any Accrued Compensation and, subject to Mr. Albanna’s execution of an irrevocable release, receive (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Albanna’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the commencement of this offering.Effective Date (as defined in the Amro Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
Notwithstanding the foregoing, under the Amro Employment Agreement, termination of Mr. Albanna by the Company without Cause or resignation by Mr. Albanna for Good Reason and a Change of Control (as defined in the Amro Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Albanna in connection to such termination. Upon such termination, Mr. Albanna will be entitled to receive any Accrued Compensation, and subject to Mr. Albanna’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the product of three times Mr. Albanna’s salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Albanna’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
-69-
The Amro Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Albanna. To the extent any of the payments or benefits provided for under the Amro Employment Agreement or any other agreement or arrangement between Mr. Albanna and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Albanna the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Corinne Pankovcin, Chief Commercialization Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Pankovcin Employment Agreement”) with the Company’s President, Corinne Pankovcin, pursuant to which Ms. Pankovcin will continue to serve as the Company’s President and Secretary until the date upon which Ms. Pankovcin’s employment may be terminated in accordance with the terms of the Pankovcin Employment Agreement.
The term of Ms. Pankovcin’s engagement under the Pankovcin Employment Agreement commences as of the Effective Date (as defined in the Pankovcin Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Pankovcin Employment Agreement. The term of Ms. Pankovcin’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Ms. Pankovcin or the Company.
Pursuant to the Pankovcin Employment Agreement, Ms. Pankovcin will receive: (i) a base salary at the annual rate of $250,000 for the remainder of calendar year 2021, and effective January 1, 2022, $385,000 (prorated for any partial year) payable in bimonthly installments and (ii) eligible to earn an annual discretionary bonus with a target amount of 45% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Ms. Pankovcin shall be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Pankovcin Employment Agreement, termination of Ms. Pankovcin by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Pankovcin Employment Agreement), or resignation by Ms. Pankovcin for “Good Reason” (as defined in the Pankovcin Employment Agreement), will not require the Company to pay severance to Ms. Pankovcin. Upon any such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation (as defined in the Pankovcin Employment Agreement), which in the case of termination by the Company for Cause or resignation by Ms. Pankovcin for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Ms. Pankovcin by the Company without “Cause” or resignation by Ms. Pankovcin for “Good Reason,” then under the Pankovcin Employment Agreement will require the Company to pay severance to Ms. Pankovcin. Upon any such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation and, subject to Ms. Pankovcin’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Ms. Pankovcin’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Pankovcin Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
-70-
Notwithstanding the foregoing, under the Pankovcin Employment Agreement, termination of Ms. Pankovcin by the Company without Cause or resignation by Ms. Pankovcin for Good Reason and a Change of Control (as defined in the Pankovcin Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Ms. Pankovcin in connection to such termination. Upon such termination, Ms. Pankovcin will be entitled to receive any Accrued Compensation, and subject to Ms. Pankovcin’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the sum of (A) the product of two times Ms. Pankovcin’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Ms. Pankovcin’s Target Bonus; (ii) provide reimbursement to Ms. Pankovcin’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Pankovcin Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Ms. Pankovcin. To the extent any of the payments or benefits provided for under the Pankovcin Employment Agreement or any other agreement or arrangement between Ms. Pankovcin and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Ms. Pankovcin the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Thomas J. Farley, Chief Financial Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Farley Employment Agreement”) with the Company’s Chief Financial Officer, Thomas Farley, pursuant to which Mr. Farley will continue to serve as the Company’s Chief Financial Officer until the date upon which Mr. Farley’s employment may be terminated in accordance with the terms of the Farley Employment Agreement.
The term of Mr. Farley’s engagement under the Farley Employment Agreement commences as of the Effective Date (as defined in the Farley Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Farley Employment Agreement. The term of Mr. Farley’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Farley or the Company.
Pursuant to the Farley Employment Agreement, Mr. Farley will receive: (i) a base salary at the annual rate of $225,000 for the remainder of calendar year 2021, and effective January 1, 2022, $355,000 (prorated for any partial year) payable in bimonthly installments and, (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Mr. Farley will be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Farley Employment Agreement, termination of Mr. Farley by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Farley Employment Agreement), or resignation by Mr. Farley without “Good Reason” (as defined in the Farley Employment Agreement), will not require the Company to pay severance to Mr. Farley. Upon any such termination, Mr. Farley will be entitled to receive any Accrued Compensation (as defined in the Farley Employment Agreement which in the case of termination by the Company for Cause or resignation by Mr. Farley for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Farley by the Company without “Cause” or resignation by Mr. Farley for “Good Reason,” then under the Farley Employment Agreement will require the Company to pay severance to Mr. Farley. Upon any such termination, Mr. Farley will be entitled to receive any Accrued Compensation and, subject to Mr. Farley’s execution of an irrevocable release, receive (i) on the sixtieth day (60th) day following termination, a lump sum cash-payment equal to the sum of (A) the product of two times Mr. Farley’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Mr. Farley’s Target Bonus (as defined in the Farley Employment Agreement); (ii) provide reimbursement to Mr. Farley’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Farley Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
-71-
Notwithstanding the foregoing, under the Farley Employment Agreement, termination of Mr. Farley by the Company without Cause or resignation by Mr. Farley for Good Reason and a Change of Control (as defined in the Farley Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Farley in connection to such termination. Upon such termination, Mr. Farley will be entitled to receive any Accrued Compensation, and subject to Mr. Farley’s execution of an irrevocable release, receive (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the product of two times Mr. Farley’s salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Farley’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable (but not later than when the award would otherwise expire).
The Farley Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Farley. To the extent any of the payments or benefits provided for under the Farley Employment Agreement or any other agreement or arrangement between Mr. Farley and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Farley the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Shahrokh Shabahang, Chief Innovation Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Shabahang Employment Agreement”) with the Company’s Chief Innovation Officer, Shahrokh Shabahang, pursuant to which Mr. Shabahang will continue to serve as the Company’s Chief Innovation Officer until the date upon which Mr. Shabahang’s employment may be terminated in accordance with the terms of the Shabahang Employment Agreement.
The term of Mr. Shabahang’s engagement under the Shabahang Employment Agreement commences as of the Effective Date (as defined in the Shabahang Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Shabahang Employment Agreement. The term of Mr. Shabahang’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Mr. Shabahang or the Company.
Pursuant to the Shabahang Employment Agreement, Mr. Shabahang will receive: (i) a base salary at the annual rate of $210,000 for the remainder of calendar year 2021, and effective January 1, 2022, $325,000 (prorated for any partial year) payable in bimonthly installments, and (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Mr. Shabahang will be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Shabahang Employment Agreement, termination of Mr. Shabahang by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Shabahang Employment Agreement), or resignation by Mr. Shabahang without “Good Reason” (as defined in the Shabahang Employment Agreement), will not require the Company to pay severance to Mr. Shabahang. Upon any such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation (as defined in the Shabahang Employment Agreement), which in the case of termination by the Company for Cause or resignation by Mr. Shabahang for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Mr. Shabahang by the Company without “Cause” or resignation by Mr. Shabahang for “Good Reason,” then under the Shabahang Employment Agreement will require the Company to pay severance to Mr. Shabahang. Upon any such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation and, subject to Mr. Shabahang’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum cash-payment equal to the sum of (A) the product of two times Mr. Shabahangs’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Mr. Shabahang’s Target Bonus (as defined in the Shabahang Employment Agreement); (ii) provide reimbursement to Mr. Shabahang’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Shabahang Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
-72-
Notwithstanding the foregoing, under the Shabahang Employment Agreement, termination of Mr. Shabahang by the Company for without Cause or resignation by Mr. Shabahang for Good Reason and a Change of Control (as defined in the Shabahang Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Mr. Shabahang in connection to such termination. Upon such termination, Mr. Shabahang will be entitled to receive any Accrued Compensation, and subject to Mr. Shabahang’s execution of an irrevocable release, receive: (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the product of two times Mr. Shabahang’s salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Mr. Shabahang’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Shabahang Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Mr. Shabahang. To the extent any of the payments or benefits provided for under the Shabahang Employment Agreement or any other agreement or arrangement between Mr. Shabahang and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Shabahang the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Rowena Albanna, Chief Operating Officer
On November 14, 2021, Aditxt, Inc. (the “Company”) entered into a new employment agreement (the “Rowena Employment Agreement”) with the Company’s Chief Operating Officer, Rowena Albanna, pursuant to which Ms. Albanna will continue to serve as the Company’s Chief Operating Officer until the date upon which Ms. Albanna’s employment may be terminated in accordance with the terms of the Rowena Employment Agreement.
The term of Ms. Albanna’s engagement under the Rowena Employment Agreement commences as of the Effective Date (as defined in the Rowena Employment Agreement) and continues until November 14, 2023, unless earlier terminated in accordance with the terms of the Rowena Employment Agreement. The term of Ms. Albanna’s Employment Agreement is automatically renewed for successive one (1) year periods until terminated by Ms. Albanna or the Company.
Pursuant to the Rowena Employment Agreement, Ms. Albanna will receive: (i) a base salary at the annual rate of $210,000 for the remainder of calendar year 2021 and effective January 1, 2022, $325,000 (prorated for any partial year) payable in bimonthly installments, and (ii) eligible to earn an annual discretionary bonus with a target amount of 40% of Base Compensation, which is based on the achievement of performance objectives, which will be determined by the Board and Compensation Committee. In addition, for calendar year 2021, Ms. Albanna will be eligible to earn an additional discretionary bonus as determined by the Company.
Under the Rowena Employment Agreement, termination of Ms. Albanna by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Rowena Employment Agreement), or resignation by Ms. Albanna for “Good Reason” (as defined in the Rowena Employment Agreement), will not require the Company to pay severance to Ms. Albanna. Upon any such termination, Ms. Albanna will be entitled to receive any Accrued Compensation (as defined in the Rowena Employment Agreement), which in the case of termination by the Company for Cause or resignation by Ms. Albanna for Good Reason will not include payment of pro rata bonus; provided, however, if termination of Ms. Albanna by the Company without “Cause” or resignation by Ms. Albanna for “Good Reason” (as such terms are defined in the Rowena Employment Agreement), then under the Rowena Employment Agreement will require the Company to pay severance to Ms. Albanna. Upon any such termination, Ms. Albanna will be entitled to receive any Accrued Compensation and, subject to Ms. Albanna’s execution of an irrevocable release, receive: (i) on the sixtieth day (60th) day following termination, a lump sum amount equal to twelve (12) months base salary then in effect as of the date of termination, less applicable taxes and withholdings; (ii) provide reimbursement to Ms. Albanna’s medical insurance premiums for a period of twelve (12) months following the date of termination; and (iii) cause any equity awards granted prior to the Effective Date (as defined in the Rowena Employment Agreement), that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
-73-
Notwithstanding the foregoing, under the Rowena Employment Agreement, termination of Ms. Albanna by the Company without Cause or resignation by Ms. Albanna for Good Reason and a Change of Control (as defined in the Rowena Employment Agreement) of the Company occurs within six (6) months after such termination, or within twenty-four (24) months prior to such termination, the Company will pay severance to Ms. Albanna in connection to such termination. Upon such termination, Ms. Albanna will be entitled to receive any Accrued Compensation, and subject to Ms. Albanna’s execution of an irrevocable release, receive: (i) on the sixtieth (60th) day of termination, a lump sum cash-payment equal to the sum of (A) the product of two times Ms. Albanna’s salary then in effect as of the date of termination, less applicable taxes and withholdings, and (B) the product of two times Ms. Albanna’s Target Bonus; (ii) provide reimbursement to Ms. Albanna’s medical insurance premiums for a period of twenty-four (24) months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to the that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable for twenty-four (24) months (but not later than when the award would otherwise expire).
The Rowena Employment Agreement also contains customary non-solicitation and non-competition covenants, which covenants remain in effect for twelve (12) months following any cessation of employment with respect to Ms. Albanna. To the extent any of the payments or benefits provided for under the Rowena Employment Agreement or any other agreement or arrangement between Ms. Albanna and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Ms. Albanna the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
Matthew Shatzkes, Former Chief Legal Officer and General Counsel
On January 28, 2022, Aditxt, Inc. (the “Company”) entered into an employment agreement (the “Employment Agreement”) with Matthew Shatzkes, the Chief Legal Officer and General Counsel of the Company. Pursuant to the Employment Agreement, Mr. Shatzkes will (i) receive a base salary at the annual rate of $385,000 (the “Base Compensation”) payable in bimonthly installments, (ii) receive a one-time sign-on bonus (the “Sign-on Bonus”), (iii) a minimum 2022 quarterly bonus (the “Minimum 2022 Bonus”), and (iv) will be entitled to earn an annual discretionary bonus beginning in fiscal year 2022.
Following the first anniversary of the Employment Agreement (the “Anniversary Date”), in addition to Mr. Shatzkes’ Base Compensation, Mr. Shatzkes will be entitled to a minimum quarterly bonus (the “Subsequent Year Minimum Bonus”). Following the Anniversary Date, in addition to Mr. Shatzkes’ Base Compensation and Subsequent Year Minimum Bonus, Mr. Shatzkes will also be eligible to earn an annual discretionary bonus.
Under the Employment Agreement, Mr. Shatzkes will also receive (i) a restricted stock unit award that will entitle Mr. Shatzkes to receive 150,000 shares of the Company’s common stock which shall vest immediately, and (ii) a restricted stock unit award of an additional 330,000 shares of the Company’s common stock, which shall vest ratably over eight successive equal quarterly installments over a two-year period commencing on March 1, 2022 and ending on December 1, 2023.
The term of Mr. Shatzkes engagement under the Employment Agreement commences on the Effective Date (as defined in the Employment Agreement) and continues until January 16, 2024, unless earlier terminated in accordance with the terms of the Employment Agreement. The term of Mr. Shatzkes’ Employment Agreement is automatically renewed for successive one-year periods until terminated by Mr. Shatzkes or the Company.
-74-
Under the Employment Agreement, termination of Mr. Shatzkes by the Company for “Cause,” “Death,” or “Disability,” (as such terms are defined in the Employment Agreement), or resignation by Mr. Shatzkes without “Good Reason” (as defined in the Employment Agreement), will not require the Company to pay severance to Mr. Shatzkes. Upon any such termination, Mr. Shatzkes will be entitled to receive any Accrued Compensation (as defined in the Employment Agreement), which in the case of termination by the Company for Cause or resignation by Mr. Shatzkes for Good Reason will not include payment of pro rata bonus. If, however, termination of Mr. Shatzkes by the Company without “Cause”, resignation by Mr. Shatzkes for “Good Reason” or and a Change of Control (as defined in the Employment Agreement) event occurs, then the Employment Agreement will require the Company to pay severance to Mr. Shatzkes. Upon any such termination, Mr. Shatzkes will be entitled to receive any Accrued Compensation and, subject to Mr. Shatzkes’ execution of an irrevocable release, (i) on the sixtieth day following termination, a lump sum amount equal (a) twelve months of his Base Compensation, Sign-on Bonus and Minimum 2022 Bonus if his Employment Agreement is terminated prior to December 31, 2022, or (b) his Base Compensation and Subsequent Year Minimum Bonus if his Employment Agreement is terminated after December 31, 2022; (ii) provide reimbursement to Mr. Shatzkes’ medical insurance premiums for a period of twelve months following the date of termination; and (iii) notwithstanding any provision of any stock incentive plan, stock option agreement, realization bonus, restricted stock agreement or other agreement relating to capital stock of the Company, cause any equity awards granted prior to that termination that are then outstanding and unvested to immediately vest and, with respect to all options and stock appreciation rights, to become fully exercisable.
To the extent any of the payments or benefits provided for under the Employment Agreement or any other agreement or arrangement between Mr. Shatzkes and the Company (collectively, the “Payments”), (a) constitute an “excess parachute payment” within the meaning of Section 280G (“Section 280G”) of the Internal Revenue Code of 1986, as amended and restated (the “Code”), and (b) would otherwise be subject to the excise tax imposed by Section 4999 of the Code (“Section 4999”), then the Company will pay or provide the greater (whichever gives Mr. Shatzkes the highest net after-tax amount) of (i) all of the Payments or (ii) the portion of Payments not in excess of the greatest amount of Payments that can be paid that would not result in the imposition of the excise tax under Section 4999.
On July 21, 2023, Matthew Shatzkes tendered his resignation as Chief Legal Officer, General Counsel and Corporate Secretary of the Company. In connection with his resignation, the Company entered into a Separation Agreement and General Release (the “Separation Agreement”). Pursuant to the Separation Agreement, Mr. Shatzkes employment with the Company terminated on August 4, 2023 (the “Termination Date”). In addition, the Company agreed to pay Mr. Shatzkes within seven days after the Termination Date: (i) $122,292.32, representing all accrued salary and wages (inclusive of Base Compensation and earned Subsequent Quarterly Bonus amounts, as those terms are defined in Mr. Shatzkes employment agreement), and (ii) $32,575.84, representing Mr. Shatzkes accrued, but unused paid time off. The Company also agreed to pay Mr. Shatzkes: (i) $385,000, representing 12 months of Mr. Shatzkes Base Compensation (as that term is defined in Mr. Shatzkes employment agreement), and (ii) $290,000, representing Mr. Shatzkes Subsequent Year Minimum Bonus (as such term is defined in Mr. Shatzkes employment agreement), on the 60th day following the Termination Date. In addition, the Company shall reimburse Mr. Shatzkes COBRA premium for a period of 12 months and shall cause any restricted stock units granted to Mr. Shatzkes to immediately vest as of the Termination Date.
On August 15, 2023, the Company entered into an Amendment to Separation Agreement and General Release with Mr. Shatzkes (the “Separation Agreement Amendment”). Pursuant to the Separation Agreement Amendment, the Company was required to pay Mr. Shatzkes, upon the earlier of (i) September 1, 2023 or (ii) two business days following the closing of a capital raise by the Company, an amount equal to $91,060.16, which amount represents the balance of Mr. Shatzkes’ Accrued Salary and Wages and Accrued PTO plus an additional $1,000 to serve as consideration for entering into the Separation Agreement Amendment. In addition, under the Separation Agreement Amendment, the Company was required to pay Mr. Shatzkes the Severance Base Compensation and the Severance Bonus upon the earlier of (i) the 60th day following the Termination Date or (ii) two business days following the closing of a capital raise by the Company.
-75-
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
Transactions with Related Persons AND DIRECTOR INDEPENDENCE
Except as described below and except for employment arrangements which are described under “executive compensation,” since inception,January 1, 2018, there has not been, nor is there currently proposed, any transaction in which we are or were a participant, the amount involved exceeds the lesser of $120,000 or 1% of the average of the total assets at December 31, 2018,2022 and 2021, and any of our directors, executive officers, holders of more than 5% of our common stockCommon Stock or any immediate family member of any of the foregoing had or will have a direct or indirect material interest.
On December 6, 2023, Amro Albanna, the Chief Executive Officer of the Company loaned $200,000 to the Company. The loan was evidenced by an unsecured promissory note (the “December Note”). Pursuant to the terms of the December Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of June 6, 2024 or an event of default, as defined therein.
On November 30, 2023, Amro Albanna, the Chief Executive Officer of the Company loaned $10,000 to the Company. The loan was evidenced by an unsecured promissory note (the “November Note”). Pursuant to the terms of the November Note, it will accrue interest at the Prime rate of eight and one-half percent (8.5%) per annum and is due on the earlier of May 30, 2024 or an event of default, as defined therein.
On June 12, 2023, Amro Albanna, the Chief Executive Officer of the Company and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $200,000 and $100,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “June Notes”). Pursuant to the terms of the June Notes, each of the June Notes will accrue interest at the Prime rate of eight and one-quarter percent (8.25%) per annum and is due on the earlier of December 12, 2023 or an event of default, as defined therein.
On April 21, 2023, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $87,523 and $100,000, respectively, to the Company. The loans were each evidenced by an unsecured promissory note (the “April Note”). Pursuant to the terms each April Note, it will accrue interest at the Prime rate of eight percent (8.00%) per annum and is due on the earlier of October 21, 2023, or an event of default, as defined therein. As of September 30, 2023, the note was fully paid off.
On May 25, 2023, Amro Albanna, the Chief Executive Officer of the Company, loaned $200,000 to the Company. The loan was evidenced by an unsecured promissory note (the “May Note”). Pursuant to the terms of the May Note, it will accrue interest at a rate of eight and one-quarter percent (8.25%) per annum, the Prime rate on the date of signing, and is due on the earlier of November 25, 2023 or an event of default, as defined therein. As of September 30, 2023, the note was fully paid off.
On June 12, 2023, Amro Albanna, the Chief Executive Officer of the Company, and Shahrokh Shabahang, the Chief Innovation Officer of the Company, loaned $200,000 and $100,000, respectively, to the Company. The loans were evidenced by an unsecured promissory note (the “June Note”). Pursuant to the terms of the June Note, it will accrue interest at the Prime rate of eight and one-quarter percent (8.25%) per annum and is due on the earlier of December 12, 2023, or an event of default, as defined therein. As of September 30, 2023, the June Note was fully paid off.
On July 11, 2023, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, its Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series C Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $1,000.00 in cash. The sale closed on July 11, 2023.
On July 19, 2022, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, its Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which the Company agreed to issue and sell one (1) share of the Company’s Series B Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $20,000.00 in cash. The sale closed on July 19, 2022. The one share of Series B Preferred Stock was redeemed by the Company on Pctober 7, 2022 for $20,000 following the approval of the 2022 reverse stock split.
-76-
During the years ended December 31, 2019 and 2018, Rowena Albanna, the wife of Amro Albanna, our Chief Executive Officer, provided the Company with operations consulting services. In July 2020, Ms. Albanna joined the Company as its Chief Operating Officer. As of December 31, 2018, $112,000 was accrued as compensation. An additional $180,000 was expensed as compensation during the year ended December 31, 2019, and $17,000 was paid on the accrued balance. As of December 31, 2019, $275,000 remained accrued and outstanding.
On January 22, 2018, the Company issued an unsecured promissory note to Sekris for $40,000 that accrued interest of 4% annually. The note was due on the earlier of July 22, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of December 31, 2019.
On February 12, 2018, the Company issued an unsecured promissory note to Sekris for $50,000 that accrued interest of 4% annually. The note was due on the earlier of August 12, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of December 31, 2019.
On March 2, 2018, the Company issued an unsecured promissory note to Sekris for $10,000 that accrued interest of 4% annually. The note was due on the earlier of September 2, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of December 31, 2019.
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris. See “Summary—Overview—“Summary — Overview — License Agreement with Loma Linda University.” Dr. Shabahang, our Chief Innovative Officer, iswas the Chief Executive Officer of Sekris. Sekris was subsequently dissolved in 2019.
On March 8, 2018, we issued a warrant to purchase up to 1,000,00010,000 shares of our common stockCommon Stock to Sekris. On March 2, 2018, we issued a 4% unsecured promissory note to Sekris in the principal amount of $10,000. Principal and interest was due on September 2, 2018 or immediately upon an event of default. On February 12, 2018, we issued a 4% unsecured promissory note to Sekris in the principal amount of $50,000. Principal and interest was due on August 12, 2018 or immediately upon an event of default. On January 22, 2018, we issued a 4% unsecured promissory note to Sekris in the principal amount of $40,000. Principal and interest was due on July 22, 2018 or immediately upon an event of default.
On June 18, 2018, the Company issued an unsecured promissory note to Sekris for $17,502 that accrued interest of 4% annually. The note was due on the earlier of December 18, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of December 31, 2019.
On January 1, 2019, we entered into a consulting agreement with Rowena Albanna, the wife of Amro Albanna, our Chief Executive Officer, to perform operations consulting services. As part of this agreement, we pay Ms. Albanna $15,000 per month for her services. This agreement terminated on June 30, 2020. In July 2020, Ms. Albanna joined the Company as its Chief Operating Officer.
On March 21, 2019, the Companywe issued a promissory note to Dr. Shabahang, our Chief Innovative Officer. The note has a principal amount of $10,000, was due on September 21, 2019, and bears an interest rate of 4% per year. This note remains outstanding.
During the year ended December 31, 2019, we assumed an aggregate of $189,625 of liabilities from Sekris in exchange for the return of 94,813 shares of our Common Stock.
On January 20, 2020, we issued a promissory note to Brian Brady, a member of our board of directors. The note has a principal amount of $50,000, was due on the earlier of April 19, 2020 or within 10 days of the closing of our initial public offering. This note carried an original issue discount of $25,000. The note was amended on April 23, 2020 to extend the maturity date to the earlier of June 30, 2020 or within 10 days of the closing of our initial public offering. This note was repaid in July 2020.
Review, Approval and Ratification of Related Party Transactions
Given our small size and limited financial resources, we have not adopted formal policies and procedures for the review, approval or ratification of transactions, such as those described above, with our executive officer(s), Director(s) and significant stockholders. We intend to establish formal policies and procedures in the future, once we have sufficient resources and have appointed additional Directors, so that such transactions will be subject to the review, approval or ratification of our Board of Directors, or an appropriate committee thereof. On a moving forward basis, our Directors will continue to approve any related party transaction.
-77-
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND
MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding beneficial ownership of shares of our common stock as of the date of this prospectusJanuary 16, 2024 based on 1,665,214 shares issued and outstanding by (i) each person (or group of affiliated persons) who is known by us to beneficially own more than five percent (5%)5% of theour outstanding shares of our common stock, (ii) each director and executive officer, and (iii) all of our directors, (iii) our executive officers and director nominees(iv) all directors and executive officers as a group. AsShares are beneficially owned when an individual has voting and/or investment power over the shares or could obtain voting and/or investment power over the shares within 60 days of the date of this prospectus, there were 7,780,195 shares of our common stock issued and outstanding.
December 31, 2023. Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of our common stock owned by them, except to the extent that power may be shared with a spouse.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person currently owns or has the right to acquire within 60 days of the date of this prospectus. With respect to options and warrants, this would include options and warrants that are currently exercisable within 60 days. With respect to convertible securities, this would include securities that are currently convertible within 60 days.
Except as indicated in footnotes to this table, we believe that the stockholders named in thisthe table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned, by them, based on information providedsubject to us by such stockholders.community property laws, where applicable. Unless otherwise indicated, the address forof each director and executive officerbeneficial owner listed is:below is c/o Aditx Therapeutics,Aditxt, Inc., 11161 Anderson737 N. Fifth Street, Suite 105-10014, Loma Linda, CA 92354.200, Richmond, VA 23219.
| Number of shares of Common Stock Beneficially Owned | Percentage Before Completion of Offering | Percentage After Completion of Offering | ||||||||||
| Directors and Officers: | ||||||||||||
| Amro Albanna(1) | 2,000,000 | 22.27 | % | 18.38 | % | |||||||
| Shahrokh Shabahang(2) | 1,803,624 | 23.07 | % | 18.56 | % | |||||||
| David Briones(3) | 170,000 | 2.14 | % | 1.73 | % | |||||||
| Brian Brady | 15,000 | * | % | * | % | |||||||
| All directors and named executive officers as a group (3 persons) | 3,988,624 | 43.41 | % | 35.97 | % | |||||||
| Greater than 5% Beneficial Owners: | ||||||||||||
| Charles Crocker(4) | 511,875 | 6.55 | % | 5.27 | % | |||||||
| Bailey Family Trust | 1,053,925 | 13.55 | % | 10.89 | % | |||||||
| Leif Eldevik | 400,000 | 5.14 | % | 4.13 | % | |||||||
| Estate of Alan Escher | 703,174 | 9.04 | % | 7.26 | % | |||||||
| Number of shares of Common Stock Beneficially Owned | Percentage | |||||||
| Directors and Officers: | ||||||||
| Amro Albanna (1) | 10,103 | * | % | |||||
| Shahrokh Shabahang, D.D.S., MS, Ph.D. (2) | 7,780 | * | % | |||||
| Corinne Pankovcin (3) | 4,966 | * | % | |||||
| Rowena Albanna (4) | 4,956 | * | % | |||||
| Brian Brady (5) | 488 | * | % | |||||
| Jeffrey Runge, M.D. (6) | 483 | * | % | |||||
| Thomas J. Farley (7) | 4,812 | * | % | |||||
| Charles Nelson (8) | 731 | * | % | |||||
| All directors and executive officers as a group (9 persons) | 34,319 | 2.0 | % | |||||
| * |
| (1) |
| (2) |
| (3) | Includes |
| (4) | Includes |
| (5) | Includes (i) 13 shares held directly by Mr. Brady; and (ii) 475 shares issuable |
| (6) | Includes (i) 2 shares held by Biologue, Inc., over which Dr. Runge has voting and dispositive control; (ii) 6 shares held directly by Dr. Runge; and (iii) 475 shares issuable pursuant to options that are fully vested. |
| (7) | Includes (i) 80 shares held directly by Mr. Farley and (ii) 4,732 shares issuable pursuant to options that are fully vested. |
| (8) | Includes (i) 261 shares held by Siu Kim Athle International, LLC., over which Mr. Nelson has voting and dispositive control and (ii) 470 shares issuable pursuant to options that are fully vested. |
-78-
General
General
The following description of the Company’s capital stock and provisions of its certificateAmended and Restated Certificate of incorporationIncorporation and bylawsAmended and Restated Bylaws are summaries and are qualified by reference to the full text of the Company’s certificateAmended and Restated Certificate of incorporationIncorporation and bylaws.Amended and Restated Bylaws.
Description of Common Stock
The Companyfollowing description of our Common Stock is a summary and does not purport to be complete. It issubject to and qualified in its entirety by reference to our Amended and Restated Certificate of Incorporation, as amended (the “Certificate of Incorporation”) and our Amended and Restated Bylaws (the “Bylaws”), each of which are incorporated by reference as an exhibit to our Annual Report on Form 10-K. We encourage you toread our Certificate of Incorporation, Bylaws, and the applicable provisions of the Delaware General Corporation Law for additional information.
Authorized Capital Shares
Our authorized to issue 30,000,000capital shares consist of 100,000,000 shares of capital stock,Common Stock, $0.001 par value $0.001 per share, and 3,000,000 shares of preferred stock, $0.001 par value per share. As of December 31, 2023, there were 1,665,214 shares of Common Stock issued and 1,665,214 shares of Common Stock outstanding. As of December 31, 2023, there were 24,905 shares of preferred stock issued and outstanding, of which 27,000,000 are shares of common stock(i) 22,280 were designated as Series A-1 Convertible Preferred Stock, and 3,000,000 are shares of “blank check” preferred stock.(ii) 2,625 were designated as Series B-2 Convertible Preferred Stock.
As of January 15, 2020, the Company had outstanding 7,780,195 shares of common stock held by 100 shareholders of record.Voting Rights
Units Offered Hereby
We are offering 1,900,000 Units at a fixed priceHolders of $5.50 per Unit. Each Unit shall consist of (a) one share of our common stock, and (b) one warrant to purchase one share of our common stock, with an exercise price of $6.875 per share.
Common Stock
Voting
The holders of our common stock are entitled to one vote for eachper share held on all matters to be voted on by the Company’s stockholders. There shall be nostockholders, including the election of directors. Our Certificate of Incorporation and Bylaws do not provide for cumulative voting.voting in the election of directors.
DividendsDividend Rights
TheHolders of the Company’s Common Stock are entitled to receive dividends, if any, as may be declared from time to time by the board of directors in its discretion out of funds legally available for the payment of dividends.
Liquidation Rights
In the event of our liquidation, the holders of our Common Stock will be entitled to share ratably in any distribution of our assets after payment of all debts and other liabilities and the preferences payable to holders of shares of Preferred Stock then outstanding, if any.
Applicable Anti-Takeover Law
Set forth below is a summary of the provisions of the Certificate of Incorporation and the Bylaws that could have the effect of delaying or preventing a change in control of the Company. The following description is only a summary and it is qualified by refence to the Certificate of Incorporation, the Bylaws and relevant provisions of the Delaware General Corporation Law.
Blank Check Preferred Stock
The Certificate of Incorporation authorizes 3,000,000 undesignated shares of Preferred Stock and permits our common stockboard of directors to issue Preferred Stock with rights or preferences that could impede the success of any attempt to change control of the Company. For example, our board of directors, without stockholder approval, may create or issue Preferred Stock with conversion rights that could adversely affect the voting power of the holders of our Common Stock as well as rights to such Preferred Stock, in connection with implementing a stockholder rights plan. This provision may be deemed to have a potential anti-takeover effect, because the issuance of such Preferred Stock may delay or prevent a change of control of the Company. Furthermore, shares of Preferred Stock, if any are issued, may have other rights, including economic rights, senior to Common Stock, and as a result, the issuance thereof could depress the market price of our Common Stock.
Series A-1 Convertible Preferred Stock
On December 22, 2023, we filed a Certificate of Designation for its Series A-1 Preferred Stock with the Secretary of State of Delaware (the “Series A-1 Certificate of Designations”). The following is only a summary of the Series A-1 Certificate of Designations, and is qualified in its entirety by reference to the full text of the Series A-1 Certificate of Designations, a copy of which is filed as Exhibit 3.1 to our Current Report on Form 8-K filed on December 26, 2023 and is incorporated by reference herein.
Designation, Amount, and Par Value. The number of Series A-1 Preferred Stock designated is 22,280 shares. The shares of Series A-1 Preferred Stock have a par value of $0.001 per share and a stated value of $1,000 per share.
-79-
Conversion Price. The Series A-1 Preferred Stock will be convertible into shares of Common Stock at an initial conversion price of $4.44 (subject to adjustment pursuant to the Series A-1 Certificate of Designations) (the “Conversion Price”). The Certificate of Designations also provides that in the event of certain Triggering Events (as defined below) any holder may, at any time, convert any or all of such holder’s Series A-1 Preferred Stock at an alternate conversion rate equal to the product of (i) the Alternate Conversion Price (as defined below) and (ii) the quotient of (x) the 25% redemption premium multiplied by (y) the amount of Series A-1 Preferred Stock subject to such conversion. “Triggering Events” include, among others, (i) a suspension of trading or the failure to be traded or listed on an eligible market for five consecutive days or more, (ii) the failure to remove restrictive legends when required, (iii) the Company’s default in payment of indebtedness in an aggregate amount of $500,000 or more, (iv) proceedings for a bankruptcy, insolvency, reorganization or liquidation, which are not dismissed with 30 days, (v) commencement of a voluntary bankruptcy proceeding, and (viii) final judgments against the Company for the payment of money in excess of $100,000. “Alternate Conversion Price” means the lowest of (i) the applicable conversion price the in effect, (ii) the greater of (x) $0.888 (the “Floor Price”) and (y) 80% of the volume weighted average price (“VWAP”) of the Common Stock on the trading day immediately preceding the delivery of the applicable conversion notice. Further, the Series A-1 Certificate of Designations provides that if on any of the 90th and 180th day after each of the occurrence of any Stock Combination Event (as defined in the Series A-1 Certificate of Designations) and the Applicable Date (as defined in the Series A-1 Certificate of Designations), the conversion price then in effect is greater than the market price then in effect (the “Adjustment Price”), on such date then the conversion price shall automatically lower to the Adjustment Price.
Dividends. Holders of the Series A-1 Preferred Stock shall be entitled to receive dividends when and as declared by the Board, from time to time, in its sole discretion, which Dividends shall be paid by the Company out of funds legally available therefor, if,payable, subject to the conditions and other terms hereof, in cash, in securities of the Company or any other entity, or using assets as and when determined by the Board on the Stated Value of Directorssuch Preferred Share.
Liquidation. In the event of a Liquidation Event (as defined in the Series A-1 Certificate of Designation), the holders the Series A-1 Preferred Stock shall be entitled to receive in cash out of the assets of the Company, in their sole discretion, subjectbefore any amount shall be paid to provisionsthe holders of law, and any provisionother shares of capital stock of the Company’s Amended and RestatedCompany, equal to the greater of (A) 125% of the Conversion Amount (as defined in the Series A-1 Certificate of Incorporation,Designation) on the date of such payment and (B) the amount per share such holder of Series A-1 Preferred Stock would receive if they converted such share of Series A-1 Preferred Stock into Common Stock immediately prior to the date of such payment
Company Redemption. The Company may redeem all, or any portion, of the Series A-1 Preferred Stock for cash, at a price per share of Series A-1 Preferred Stock equal to 115% of the greater of (i) the Conversion Amount (as defined in the Series A-1 Certificate of Designation)being redeemed as amended from time to time. There are no preemptive, conversion or redemption privileges, nor sinking fund provisionsof the Company Optional Redemption Date (as defined in the Series A-1 Certificate of Designation) and (ii) the product of (1) the Conversion Rate (as defined in the Series A-1 Certificate of Designation) with respect to the common stock.
Liquidation
InConversion Amount being redeemed as of the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs,Company Optional Redemption Date multiplied by (2) the holders of our common stock will be entitled to share ratablygreatest Closing Sale Price (as defined in the net assets legally available for distribution to stockholders afterCertificate of Designation) of the payment of or provision for all of our debts and other liabilities.
Fully Paid and Non-assessable
All outstanding shares of common stock are, andCommon Stock on any Trading Day during the common stock to be outstanding upon completion of this offering will be, duly authorized, validly issued, fully paid and non-assessable.
Warrants Offered Hereby
The warrants entitle the registered holder to purchase one share of our common stock at a price equal to $6.875 per share, subject to adjustment as discussed below, at any timeperiod commencing on the date that is six-months fromimmediately preceding such Company Optional Redemption Notice Date (as defined in the consummationCertificate of this offeringDesignation) and terminating at 5:00 p.m., New York City time,ending on the fifth (5thTrading Day immediately prior to the date the Company makes the entire payment required to be made under the Certification of Designation.
Maximum Percentage. Holders of Series A-1 Preferred Stock are prohibited from converting shares of Series A-1 Preferred Stock into shares of Common Stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own in excess of 4.99% (the “Maximum Percentage”) anniversary of the datetotal number of issuance (the “Issuance Date”).shares of Common Stock issued and outstanding immediately after giving effect to such conversion.
Voting Rights. The holders of the Series A-1 Preferred Stock shall have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as expressly provided in the Certificate of Designations and where required by the DGCL.
-80-
Series B-2 Convertible Preferred Stock
The warrants will be issued in registered form underOn December 28, 2023, we filed a warrant agent agreementCertificate of Designations for its Series B-2 Preferred Stock with the Secretary of State of Delaware (the “Warrant Agent Agreement”) between us and our warrant agent, VStock Transfer, LLC (the “Warrant Agent”“Series B-2 Certificate of Designations”). The material provisionsfollowing is only a summary of the warrants are set forth hereinSeries B-2 Certificate of Designations, and is qualified in its entirety by reference to the full text of the Series B-2 Certificate of Designations, a copy of the Warrant Agent Agreement has beenwhich is filed as an exhibit to the Registration Statementour Current Report on Form S-1, of which this prospectus forms a part. The Company and8-K filed with the Warrant Agent may amend or supplement the Warrant Agent Agreement without the consent of any holder for the purpose of curing any ambiguity, or curing, correcting or supplementing any defective provision contained therein or adding or changing any other provisions with respect to matters or questions arising under the Warrant Agent Agreement as the parties thereto may deem necessary or desirable and that the parties determine, in good faith, shall not adversely affect the interest of the holders. All other amendments and supplements shall require the vote or written consent of holders of at least 50.1%
The exercise price and number of shares of common stock issuable upon exercise of the warrants may be adjusted in certain circumstances, including in the event of a stock dividend, extraordinary dividendSEC on or recapitalization, reorganization, merger or consolidation.January 2, 2024.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the Warrant Agent, with the exercise form attached to the warrant certificate completedDesignation, Amount, and executed as indicated, accompanied by full payment of the exercise price, by certified or official bank check payable to us, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No warrants will be exercisable unless at the time of the exercise a prospectus or prospectus relating to common stock issuable upon exercise of the warrants is current and the common stock has been registered or qualified or deemed to be exempt under the securities laws of the state of residence of the holder of the warrants. Under the terms of the Warrant Agent Agreement, we have agreed to use our best efforts to maintain a current prospectus or prospectus relating to common stock issuable upon exercise of the warrants until the expiration of the warrants. If we are unable to maintain the qualification or effectiveness of such registration statement until the expiration of the warrants, and therefore are unable to deliver registered shares of common stock, the warrants may become worthless. Such expiration would result in each holder paying the full Unit purchase price solely for the shares of common stock underlying the Units. Additionally, the market for the warrants may be limited if the prospectus or prospectus relating to the common stock issuable upon exercise of the warrants is not current or if the common stock is not qualified or exempt from qualification in the jurisdictions in which the holders of such warrants reside. In no event will the registered holders of a Warrant be entitled to receive a net-cash settlement, stock or other consideration in lieu of physical settlement in shares of our common stock.
No fractional shares of common stock will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round up to the nearest whole number the number of shares of common stock to be issued to the Warrant holder. If multiple warrants are exercised by the holder at the same time, we will aggregate the number of whole shares issuable upon exercise of all the warrants.
The price of the warrants has been arbitrarily established by us and the Underwriter after giving consideration to numerous factors, including but not limited to, the pricing of the Units in this offering. No particular weighting was given to any one aspect of those factors considered. We have not performed any method of valuation of the warrants.
Preferred StockPar Value.
We are authorized to issue up to 3,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock and the Notes. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Exclusive Forum
Our Amended and Restated Certificate of Incorporation provides that unless the Company consents in writing to the selection of an alternative forum, the State of Delaware is the sole and exclusive forum for: (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Company to the Company or the Company’s stockholders, (iii) any action asserting a claim against the Company, its directors, officers or employees arising pursuant to any provision of the DGCL or our Amended and Restated Certificate of Incorporation or the Company’s Bylaws, or (iv) any action asserting a claim against the Company, its directors, officers, employees or agents governed by the internal affairs doctrine, except for, as to each of (i) through (iv) above, any claim as to which the Court of Chancery determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or for which the Court of Chancery does not have subject matter jurisdiction.
Changes in Authorized Number
The number of authorizedSeries B-2 Preferred Stock designated is 2,625 shares. The shares of common stock may be increased or decreased subject to the Company’s legal commitments at any timeSeries B-2 Preferred Stock have a par value of $0.001 per share and from time to time to issue them, by the affirmative votea stated value of the holders of a majority of the stock of the Company entitled to vote. $1,000 per share.
Delaware Anti-Takeover Statute
We may become subject to Section 203 of the Delaware General Corporation Law, which prohibits persons deemed toConversion Price. The Series B-2 Preferred Stock will be “interested stockholders” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the Board of Directors. A Delaware corporation may “opt out” of these provisions with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or bylaws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have not opted out of these provisions. As a result, mergers or other takeover or change in control attempts of us may be discouraged or prevented.
Equity Incentive Plan
2017 Equity Incentive Plan
Our 2017 Equity Incentive Plan (the “2017 Plan”), was adopted by our board of directors and approved by our stockholders in October 2017. Our 2017 Plan provides for the grant of incentive stock options (within the meaning of Section 422 of the Code), or ISOs to employees, and non-statutory stock options, or NSOs, restricted stock, restricted stock units and stock appreciation rights, to our employees, directors, consultants and advisors.
Authorized Shares. A total of 1,200,000convertible into shares of our common stock have been reserved for issuanceCommon Stock at an initial conversion price of $4.71 (subject to adjustment pursuant to the 2017 Plan. On December 21, 2018, our boardSeries B-2 Certificate of directors amended the 2017 Plan to increase the numberDesignations) (the “Conversion Price”). The Series B-2 Certificate of authorized shares of common stock issuable thereunder to 5,000,000 shares, subject to shareholder approval. On December 20, 2019 our shareholders approved the increase.
Plan Administration. Currently, our board administers our 2017 Plan. Subject to the provisions of our 2017 Plan, the administrator has the power to determine the terms of the awards, including the exercise price, the number of shares subject to each such award, the exercisability of the awards, and the form of consideration, if any, payable upon exercise. The administratorDesignations also has the authority to amend existing awards to reduce their exercise price, to allow participants the opportunity to transfer outstanding awards to a financial institution or other person or entity selected by the administrator and to institute an exchange program by which outstanding awards may be surrendered in exchange for awards with a higher or lower exercise price.
Stock Options. The exercise price of options granted under our 2017 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed ten years, except that with respect to any participant who owns more than 10% of the voting power of all classes of our outstanding stock, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the grant date. Subject to the provisions of our 2017 Plan, the administrator will determine the term of all other options.
After the termination of service of an employee, director or consultant, he or she may exercise his or her option or stock appreciation right for the period of time stated in his or her award agreement. Generally, if termination is due to death or disability, the option or stock appreciation right will remain exercisable for six months. In no event may an option be exercised later than the expiration of its term.
Stock Appreciation Rights. Stock appreciation rights may be granted under our 2017 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Subject to the provisions of our 2017 Plan, the administrator determines the terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted Stock. Restricted stock may be granted under our 2017 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator determines the number of shares of restricted stock granted and may impose whatever conditions to vesting it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us). The administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
Restricted Stock Units. Restricted stock units may be granted under our 2017 Plan. Restricted stock units represent an amount equal to the fair market value of one share of our common stock. The administrator will determine the terms and conditions of restricted stock units, including the number of units granted, the vesting criteria (which may include accomplishing specified performance criteria or continued service to us), and the form and timing of payment. The administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Certain Adjustments. In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2017 Plan, the administrator will adjust the number and class of shares that may be delivered under the 2017 Plan or the number, class and price of shares covered by each outstanding award, and the numerical share limits set forth in the 2017 Plan.
Merger or Change in Control. Our 2017 Plan provides that in the event of certain Triggering Events (as defined below) any holder may, at any time, convert any or all of such holder’s Series B-2 Preferred Stock at an alternate conversion rate equal to the product of (i) the Alternate Conversion Price (as defined below) and (ii) the quotient of (x) the 125% redemption premium multiplied by (y) the amount of Series B-2 Preferred Stock subject to such conversion. “Triggering Events” include, among others, (i) a mergersuspension of trading or changethe failure to be traded or listed on an eligible market for five consecutive days or more, (ii) the failure to remove restrictive legends when required, (iii) the Company’s default in control, aspayment of indebtedness in an aggregate amount of $500,000 or more, (iv) proceedings for a bankruptcy, insolvency, reorganization or liquidation, which are not dismissed with 30 days, (v) commencement of a voluntary bankruptcy proceeding, and (viii) final judgments against the Company for the payment of money in excess of $500,000. “Alternate Conversion Price” means the lowest of (i) the applicable conversion price the in effect, (ii) the greater of (x) $0.9420 (the “Floor Price”) and (y) 80% of the lowest volume weighted average price (“VWAP”) of the Common Stock during the five consecutive trading day period ending and including the the trading day immediately preceding the delivery of the applicable conversion notice. Further, the Series B-2 Certificate of Designations provides that if on any of the 90th and 180th day after each of the occurrence of any Stock Combination Event (as defined in the 2017 Plan, each outstanding award will be treated as the administrator determines, including that the successor corporation or its parent or subsidiary will assume or substitute an equivalent award for each outstanding award. The administrator will not be required to treat all awards similarly. If there is no assumption or substitutionSeries B-2 Certificate of outstanding awards, the awards will fully vest, all restrictions will lapseDesignations) and the awards will become fully exercisable.Applicable Date (as defined in the Series B-2 Certificate of Designations), the conversion price then in effect is greater than the market price then in effect (the “Adjustment Price”), on such date then the conversion price shall automatically lower to the Adjustment Price.
PennyDividends. Holders of the Series B-2 Preferred Stock Regulation.
The SEC has adopted regulations which generally define “penny stock”shall be entitled to be any equity security that has a market price of less than $5.00 per share or an exercise price of less than $5.00 per share. Such securities are subject to rules that impose additional sales practice requirements on broker-dealers who sell them. For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchaser of such securitiesreceive dividends when and have received the purchaser’s written consent to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the rules require the delivery, prior to the transaction, of a disclosure schedule preparedas declared by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, among other requirements, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. As our common stock immediately following this offering may be subject to such penny stock rules, purchasers in this offering may find it more difficult to sell their common stock shares in the secondary market.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of substantial amounts of our common stock in the public market, or the anticipation of these sales, could materially and adversely affect market prices prevailingBoard, from time to time, in its sole discretion, which Dividends shall be paid by the Company out of funds legally available therefor, payable, subject to the conditions and could impair our ability to raise capital through salesother terms hereof, in cash, in securities of equitythe Company or equity-related securities.any other entity, or using assets as determined by the Board on the Stated Value of such Preferred Share.
OnlyLiquidation. In the event of a limitedLiquidation Event (as defined in the Series B-2 Certificate of Designations), the holders the Series B-2 Preferred Stock shall be entitled to receive in cash out of the assets of the Company, before any amount shall be paid to the holders of any other shares of capital stock of the Company, equal to the greater of (A) 125% of the Conversion Amount (as defined in the Series B-2 Certificate of Designation) on the date of such payment and (B) the amount per share such holder of Series B-2 Preferred Stock would receive if they converted such share of Series B-2 Preferred Stock into Common Stock immediately prior to the date of such payment.
Company Redemption. The Company may redeem all, or any portion, of the Series B-2 Preferred Stock for cash, at a price per share of Series B-2 Preferred Stock equal to 115% of the greater of (i) the Conversion Amount (as defined in the Series B-2 Certificate of Designations) being redeemed as of the Company Optional Redemption Date (as defined in the Series B-2 Certificate of Designations) and (ii) the product of (1) the Conversion Rate (as defined in the Series B-2 Certificate of Designations) with respect to the Conversion Amount being redeemed as of the Company Optional Redemption Date multiplied by (2) the greatest Closing Sale Price (as defined in the Series B-2 Certificate of Designations) of the Common Stock on any Trading Day during the period commencing on the date immediately preceding such Company Optional Redemption Notice Date (as defined in the Series B-2 Certificate of Designations) and ending on the Trading Day immediately prior to the date the Company makes the entire payment required to be made under the Certification of Designation.
Maximum Percentage. Holders of Series B-2 Preferred Stock are prohibited from converting shares of Series B-2 Preferred Stock into shares of Common Stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own in excess of 4.99% (the “Maximum Percentage”) of the total number of shares of Common Stock issued and outstanding immediately after giving effect to such conversion.
-81-
Voting Rights. The holders of the Series B-2 Preferred Stock shall have no voting power and no right to vote on any matter at any time, either as a separate series or class or together with any other series or class of share of capital stock, and shall not be entitled to call a meeting of such holders for any purpose nor shall they be entitled to participate in any meeting of the holders of Common Stock, except as expressly provided in the Series B-2 Certificate of Designations and where required by the DGCL.
No Cumulative Voting
The Certificate of Incorporation and the Bylaws do not provide holders of our commonCommon Stock cumulative voting rights in the election of directors. The absence of cumulative voting could have the effect of preventing stockholders holding a minority of our shares of Common Stock from obtaining representation on our board of directors. The absence of cumulative voting might also, under certain circumstances, render more difficult or discourage a merger, tender offer or proxy contest favored by a majority of our stockholders, the assumption of control by a holder of a large block of our stock or the removal of incumbent management.
Advance Notice Requirements for Stockholder Proposals and Director Nominees
The Bylaws require stockholders seeking to make nominations of candidates for election as directors or to bring other business before a meeting of our stockholders to provide timely notice of their intent in writing. To be timely, a stockholder’s notice must be delivered to the Secretary at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary of the immediately preceding annual meeting of the stockholders; provided, however, that in the event that the date of the annual meeting is more than 30 days before or after such anniversary date, notice by the stockholder to be timely must be so received not later than the close of business on the tenth day following the earlier of the date on which we first give notice or publicly announce the date of the meeting. A stockholder’s notice must include certain information about the stockholder and the nominee or proposal as specified in the Bylaws. These advance notice provisions may restrict the ability of the stockholders to make nominations for directors at or bring business before a meeting of the Company’s stockholders.
Listing
Our Common Stock is traded on Nasdaq Capital Market under the trading symbol “ADTX”.
Transfer Agent
The Company’s transfer agent is VStock Transfer, LLC.
Pre-Funded Warrants
General
The term “pre-funded” refers to the fact that the purchase price of the pre-funded warrants in this offering includes almost the entire exercise price that will be available for sale inpaid under the public marketpre-funded warrants, except for a periodnominal remaining exercise price of several months after completion$0.001. The purpose of the pre-funded warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding Common Stock following the consummation of this offering duethe opportunity to contractual and legalinvest capital into the Company without triggering their ownership restrictions, on resale described below. Nevertheless, sales of a substantial numberby receiving pre-funded warrants in lieu of shares of our common stockCommon Stock which would result in such ownership of more than 4.99% or 9.99%, as applicable, and receiving the public market after such restrictions lapse, or the perception that those sales may occur, could materially and adversely affect the prevailing market price of our common stock. Although we have been approved, subjectability to notice of issuance,exercise their option to list our common stock on The Nasdaq Capital Market, we cannot assure you that there will be an active market for our common stock.
Ofpurchase the shares to be outstanding immediately afterunderlying the completion of this offering, we expect that the shares to be sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act; these restricted securities may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act.
Rule 144
Affiliate Resales of Restricted Securities
Affiliates of ours must generally comply with Rule 144 if they wish to sell any shares of our common stock in the public market, whether or not those shares are “restricted securities.” “Restricted securities” are any securities acquired from us or one of our affiliates inpre-funded warrants at a transaction not involvingnominal price at a public offering. All shares of our common stock issued prior to the closing of the offering made hereby, are considered to be restricted securities. The shares of our common stock sold in this offering are not considered to be restricted securities.
Non-Affiliate Resales of Restricted Securities
Any person or entity who is not an affiliate of ours and who has not been an affiliate of ours at any time during the three months preceding a sale is only required to comply with Rule 144 in connection with sales of restricted shares of our common stock. Subject to the lock-up agreements described below, those persons may sell shares of our common stock that they have beneficially owned for at least one year without any restrictions under Rule 144 immediately following the effective date of the Registration Statement on Form S-1 of which this prospectus is a part.
Further, beginning 90 days after the effective date of the Registration Statement on Form S-1 of which this prospectus is a part, a person who is not an affiliate of ours at the time such person sells shares of our common stock, and has not been an affiliate of ours at any time during the three months preceding such sale, and who has beneficially owned such shares of our common stock, as applicable, for at least six months but less than a year, is entitled to sell such shares so long as there is adequate current public information, as defined in Rule 144, available about us.
Resales of restricted shares of our common stock by non-affiliates are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144, described above.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144.
Rule 701 also permits affiliates of ours to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701 and until expiration of the 180-day lock-up period described below.
Equity Incentive Awards
We intend to file a registration statement on Form S-8 under the Securities Act after the closing of this offering to register the shares of common stock that are issuable pursuant to our Plan. The registration statement is expected to be filed and become effective as soon as practicable after the completion of this offering. Accordingly, shares registered under the registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up arrangement described above, if applicable.
Lock-Up Agreements
We, our executive officers and directors, and holders of at 5% or more of our common stock have agreed to enter into lock-up agreements in connection with this offering. See “Underwriting” for additional information.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCKlater date.
The following is a brief summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the ownership and disposition of our common stock but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. No ruling on the U.S. federal, state, or local tax considerations relevant to our operations or to the purchase, ownership or disposition of our shares, has been requested from the IRS or other tax authority. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax consequences described below.
This summary also does not address the tax considerations arising under the laws of any non-U.S., state or local jurisdiction, or under U.S. federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock arising under the U.S. federal estate or gift tax rules or under the laws of any state, local, non-U.S., or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder (other than a partnership) if you are any holder other than:
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
Distributions
As described in “Dividend Policy,” we have never declared or paid cash dividends on our common stock and do not anticipate paying any dividends on our common stock in the foreseeable future. However, if we do make distributions on our common stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock as described below under “—Gain on Disposition of Common Stock.”
Subject to the discussion below on effectively connected income, backup withholding and foreign accounts, any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide us with an IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. A non-U.S. holder of shares of our common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by you in the United States) are generally exempt from such withholding tax. In order to obtain this exemption, you must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. You should consult your tax advisor regarding any applicable tax treaties that may provide for different rules.
Gain on Disposition of Common Stock
Subject to the discussion below regarding backup withholding and foreign accounts, you generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
We believe that we are not currently and will not become a USRPHC for U.S. federal income tax purposes, and the remainder of this discussion so assumes. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded common stock at any time during the shorter of the five-year period preceding your disposition of, or your holding period for, our common stock.
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. If you are an individual non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax (or such lower rate specified by an applicable income tax treaty) on the gain derived from the sale, which gain may be offset by U.S. source capital losses for the year (provided you have timely filed U.S. federal income tax returns with respect to such losses). You should consult any applicable income tax or other treaties that may provide for different rules.
Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for U.S. federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise. The test for whether an individual is a resident of the United States for U.S. federal estate tax purposes differs from the test used for U.S. federal income tax purposes. Some individuals, therefore, may be non-U.S. holders for U.S. federal income tax purposes, but not for U.S. federal estate tax purposes, and vice versa.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example, by properly certifying your non-U.S. status on an IRS Form W-8BEN, IRS Form W-8BEN-E or another appropriate version of IRS Form W-8.
Backup withholding is not an additional tax; rather, the U.S. federal income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance
The Foreign Account Tax Compliance Act, or FATCA, imposes withholding tax at a rate of 30% on dividends on and gross proceeds from the sale or other disposition of our common stock paid to “foreign financial institutions” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. FATCA also generally imposes a U.S. federal withholding tax of 30% on dividends on and gross proceeds from the sale or other disposition of our common stock paid to a “non-financial foreign entity” (as specially defined for purposes of these rules) unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. The withholding provisions under FATCA generally apply to dividends on our common stock, and under current transition rules, apply with respect to the gross proceeds from the sale or other disposition of our common stock. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock.
Each prospective investor should consult its tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed change in applicable laws.
Dawson James Securities, Inc. is acting as lead book-running manager of the offering and as representatives of the underwriters named below. Subject to the terms and conditions statedof the pre-funded warrants being offered by us. The following description is subject in all respects to the provisions contained in the underwriting agreement datedform of pre-funded warrant, the dateform of this prospectus, each underwriter named below has severally and not jointly agreed to purchase, and we have agreed to sell to that underwriter, the number of Units, consisting of shares of common stock and warrants set forth opposite the underwriter’s name.
The underwriting agreement provides that the obligations of the underwriters to purchase the shares of common stock and warrants included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriters are obligated to purchase all the shares of common stock and warrants (other than those covered by the over-allotment option described below) if they purchase any of the shares of common stock and warrants.
Shares of common stock and warrants sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares of common stock and warrants sold by the underwriters to securities dealers may be sold at a discount from the initial public offering price not to exceed $ per share and warrant. If all the shares of common stock and warrants are not sold at the initial offering price, the underwriters may change the offering price and the other selling terms.
We have granted to the underwriter an option, exercisable no later than 45 calendar days after the closing of this offering, to purchase up to an additional shares of common stock and/or additional warrants to purchase up to shares of common stock, in any combination thereof, from us to cover over-allotments, if any. If the underwriter exercises all or any part of this option, it will purchase shares and/or warrants covered by the option at the public offering price per share and the public offering price per warrant, respectively, less the underwriting discount. If this option is exercised in full, the total offering price to the public will be $ and the total net proceeds, before expenses, to us will be $ . We will pay the expenses associated with the exercise of the over-allotment option.
Underwriting discounts and commissions
The following table shows the underwriting discounts and commissions that we are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters’ over-allotment option.
We have also agreed to reimburse the underwriter for its expenses in connection with this offering, up to $120,000, and to reimburse the underwriter for certain “blue sky” expenses in an amount of $25,000. We estimate the total expenses of this offering which will be payable by us, excluding the underwriting discount and the underwriter’s expenses payable by us, will be approximately $ .
Indemnification
We have agreedfiled as an exhibit to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make because of any of those liabilities.
Right of First Refusal
Provided this offering is completed, for a period of twelve months from the closing date of this offering, Dawson James Securities, Inc. has a right of first refusal to act as our exclusive placement agent or lead underwriter and sole book runner, as applicable, in the event we decide to pursue an offering of our equity, equity-linked or debt securities during such period.
Underwriters’ Unit Purchase Option
We have also agreed to issue to the underwriters’ a unit purchase option to purchase a number of our securities equal to 6% of the securities and sold in this offering. The underwriters’ unit purchase option will have an exercise price equal to 125% of the public offering price of the combination of shares and warrants set forth on the cover of this prospectus (or $ per share and accompanying warrant) and may be exercised on a cashless basis. The underwriters’ unit purchase option is not redeemable by us. This prospectus also covers the sale of the underwriters’ unit purchase option and the shares of common stock and warrants (and shares of common stock underlying such warrants) issuable upon the exercise of the underwriters’ unit purchase option. The underwriters’ unit purchase option and the underlying securities have been deemed compensation by FINRA, and are therefore subject to FINRA Rule 5110(g)(1). In accordance with FINRA Rule 5110(g)(1), neither the underwriters’ unit purchase option nor any securities issued upon exercise of the underwriters’ unit purchase option may be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of such securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of the offering pursuant to which the underwriters’ unit purchase option is being issued, except the transfer of any security: (i) by operation of law or by reason of reorganization of our company; (ii) to any FINRA member firm participating in this offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction described above for the remainder of the time period; (iii) if the aggregate amount of our securities held by either an underwriter or a related person do not exceed 1% of the securities being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period.
Lock-up Agreements
We, our executive officers and directors, and holders of at 5% or more of our common stock have agreed to enter into lock-up agreements in connection with this offering. Under the lock-up agreements, subject to certain exceptions, we and each of these persons may not, without the prior written approval of the Underwriter, offer, sell, contract to sell, pledge, or otherwise dispose of, directly or indirectly, or hedge our common stock or securities convertible into or exchangeable or exercisable for our common stock. These restrictions remain in effect and will generally terminate on the six-month anniversary after the closing date.
In connection with this offering, we agreed that we will not: (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital stock; (ii) file or cause to be filed any registration statement with the SEC relating to the offering of any shares of our capital stock or any securities convertible into or exercisable or exchangeable for shares of our capital stock; or (iii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our capital stock, whether any such transaction described in clause (i), (ii) or (iii) above is to be settled by delivery of shares of our capital stock or such other securities, in cash or otherwise, in each case without the prior consent of the Underwriter for a period of six months after the date of the Underwriting Agreement, other than (A) the securities sold in this offering, or (B) the issuance by us of shares of our common stock upon the exercise of a stock option or warrant or the conversion of a security outstanding on the date of this prospectus, hereafter issued pursuant to our currently existing or hereafter adopted equity compensation plans or employment or consulting agreements or arrangements of which the Underwriter has been advised in writing or which have been filed with the SEC.
Offering Price Determination
Prior to this offering, there has been no public market for our common stock. The initial public offering price was negotiated between the representatives and us. In determining the initial public offering price of our common stock, the representative considered:
Stabilization, Short Positions and Penalty Bids
The representatives may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on The Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more of the underwriters and/or selling group members participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter or selling group member, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the representatives on the same basis as other allocations.
Other than the prospectus in electronic format, the information on any underwriter’s or selling group member’s web site and any information contained in any other web site maintained by an underwriter or selling group member is not part of the prospectus or the registration statement of which this prospectus forms a part.
-82-
Exercise price
Pre-funded warrants will have an exercise price of $0.001 per share. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Exercisability
The pre-funded warrants are exercisable at any time after their original issuance and until exercised in full. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part has not been approved and/or endorsed by delivering to us or any underwriter or selling group membera duly executed exercise notice and by payment in its capacity as underwriter or selling group member and shouldfull of the exercise price in immediately available funds for the number of shares of Common Stock purchased upon such exercise. As an alternative to payment in immediately available funds, the holder may elect to exercise the pre-funded warrant through a cashless exercise, in which the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the pre-funded warrant. No fractional shares of Common Stock will be issued in connection with the exercise of a pre-funded warrant.
Exercise limitations
The pre-funded warrants may not be reliedexercised by the holder to the extent that the holder, together with its affiliates, would beneficially own, after such exercise more than 4.99% of the shares of our Common Stock then outstanding (including for such purpose the shares of our Common Stock issuable upon by investors.such exercise). However, any holder may increase or decrease such beneficial ownership limitation upon notice to us, provided that such limitation cannot exceed 9.99%, and provided that any increase in the beneficial ownership limitation shall not be effective until 61 days after such notice is delivered. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding shares of Common Stock.
Listing on The Nasdaq Capital MarketTransferability
PriorSubject to this offering, there has beenapplicable laws, the pre-funded warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange listing
There is no publicestablished trading market for our common stock. We have applied to list our common stock on The Nasdaq Capital Market, and upon approval, we expect to list our common stock under the symbol “ADTX.” If our common stock is not approved for listing on The Nasdaq Capital Market, we will not consummate this offering.
We do not intend to apply for any listing of thepre-funded warrants The Nasdaq Capital Market or any other securities exchange or nationally recognized trading system, and we do not expect a market to developdevelop. In addition, we do not intend to apply for the listing of the pre-funded warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Fundamental transactions
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common Stock, upon consummation of such a fundamental transaction, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the pre-funded warrants.
Other RelationshipsNo rights as a stockholder
Except as otherwise provided in the pre-funded warrant or by virtue of such holder’s ownership of shares of our Common Stock, the holder of a pre-funded warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the pre-funded warrant. The pre-funded warrants will provide that holders have the right to participate in distributions or dividends paid on our Common Stock.
-83-
The underwritersCommon Stock being offered by the Selling Stockholders are those issuable to the Selling Stockholders, upon exercise of the Warrants. For additional information regarding the issuances of those securities, see “Prospectus Summary—Recent Developments—December 2023 PIPE” above. We are registering the shares of Common Stock in order to permit the Selling Stockholders to offer the shares for resale from time to time. Except for the ownership of the Warrants and certainas noted below, the Selling Stockholders have not had any material relationship with us within the past three years.
The table below lists the Selling Stockholders and other information regarding the beneficial ownership of the shares of Common Stock by each of the Selling Stockholders. The second column lists the number of shares of Common Stock beneficially owned by each Selling Stockholder, based on its ownership of the shares of Common Stock and Warrants, as of November 6, 2023, assuming exercise of the Warrants held by the Selling Stockholders on that date, without regard to any limitations on exercises.
The third column lists the shares of Common Stock being offered by this prospectus by the Selling Stockholders.
In accordance with the terms of a registration rights agreement with the Selling Stockholders, this prospectus generally covers the resale of the maximum number of shares of Common Stock issuable upon exercise of the Warrants, determined as if the outstanding Warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment as provided in the registration rights agreement, without regard to any limitations on the exercise of the Warrants. The fourth column assumes the sale of all of the shares offered by the Selling Stockholders pursuant to this prospectus.
Under the terms of the Warrants, a Selling Stockholder may not exercise the Warrants to the extent such exercise would cause such Selling Stockholder, together with its affiliates and attribution parties, to beneficially own a number of shares of Common Stock which would exceed 4.99% of our then outstanding Common Stock following such exercise, excluding for purposes of such determination shares of Common Stock issuable upon exercise of such Warrants which have not been exercised. The number of shares in the second and fourth columns do not reflect this limitation. The Selling Stockholders may sell all, some or none of their affiliates are full service financial institutions engagedshares in various activities, which may includethis offering. See “Plan of Distribution.”
-84-
| Name of Selling Stockholder | Number of Shares of Common Stock Beneficially Owned Prior to Offering(1) | Maximum Number of Shares of Common Stock to be Sold in this Offering | Number of Shares of Common Stock Beneficially Owned After Offering | Percentage of Shares Beneficially Owned after Offering(1) | ||||||||||||
| Sabby Volatility Warrant Master Fund, Ltd.(2) | 22,048 | 3,711,342 | 22,048 | 1.7 | % | |||||||||||
| Michael Vasinkevich(3) | 86,073 | 47,598 | 38,475 | 2.8 | % | |||||||||||
| Michael Mirsky(3) | 25,504 | 14,104 | 11,400 | * | ||||||||||||
| Noam Rubinstein(3) | 16,778 | 9,278 | 7,500 | * | ||||||||||||
| Craig Schwabe(3) | 4,530 | 2,505 | 2,025 | * | ||||||||||||
| Charles Worthman(3) | 1,342 | 742 | 600 | * | ||||||||||||
| * | less than 1% |
(1) | The ability to exercise the Warrants held by the Selling Stockholders is subject to a beneficial ownership limitation that, at the time of initial issuance of the Warrants was capped at 4.99% beneficial ownership of the Company’s issued and outstanding Common Stock (post-exercise). These beneficial ownership limitations may be adjusted up or down, subject to providing advanced notice to the Company. Beneficial ownership as reflected in the selling stockholder table reflects the total number of shares potentially issuable underlying the Warrants, and does not give effect to these beneficial ownership limitations. Accordingly, actual beneficial ownership, as calculated in accordance with Section 13(d) and Rule 13d-3 thereunder may be lower than as reflected in the table. |
| (2) | Sabby Management, LLC is the investment manager of Sabby Volatility Warrant Master Fund, Ltd. (“Sabby”) and shares voting and investment power with respect to these shares in this capacity. As manager of Sabby Management, LLC, Hal Mintz also shares voting and investment power on behalf of Sabby. Each of Sabby Management, LLC and Mr. Mintz disclaims beneficial ownership over the securities listed except to the extent of their pecuniary interest therein. Amount of shares beneficially owned by Sabby prior to the offering is based upon the Schedule 13G/A filed by Sabby on January 2, 2024. |
| (3) | The selling stockholder is affiliated with H.C. Wainwright & Co., LLC, a registered broker dealer with a registered address of H.C. Wainwright & Co., LLC, 430 Park Ave, 3rd Floor, New York, NY 10022, and has sole voting and dispositive power over the securities held. The number of shares beneficially owned prior to this offering consist of (a) shares of Common Stock issuable upon exercise of placement agent warrants, which were received as compensation in connection with the concurrent Registered Direct Offering and the Private Placements consummated by us in June 2023, and (b) shares of Common Stock issuable upon exercise of placement agent warrants, which were received as compensation in connection with the December 2023 PIPE. The selling stockholder acquired the placement agent warrants in the ordinary course of business and, at the time the placement agent warrants were acquired, the selling stockholder had no agreement or understanding, directly or indirectly, with any person to distribute such securities. |
-85-
Each Selling Stockholder of the securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certainany of their affiliates have,pledgees, assignees and successors-in-interest may, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for the issuer and its affiliates, for which they receivedsell any or may in the future receive customary fees and expenses.
In the ordinary courseall of their various business activities,securities covered hereby on the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer or its affiliates. If the underwriters or their affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the shares of common stock offered hereby. Any such credit default swaps or short positions could adversely affect future trading prices of the shares of common stock offered hereby. The underwriters and certain of their affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
This prospectus does not constitute an offer to sell to, or a solicitation of an offer to buy from, anyone in any country or jurisdiction (i) in which such an offer or solicitation is not authorized, (ii) in which any person making such offer or solicitation is not qualified to do so or (iii) in which any such offer or solicitation would otherwise be unlawful. No action has been taken that would, or is intended to, permit a public offer of the shares of common stock or possession or distribution of this prospectusprincipal Trading Market or any other offeringstock exchange, market or publicity material relating totrading facility on which the shares of common stocksecurities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any countryone or jurisdiction (other than the United States) where any such action for that purpose is required. Accordingly, each underwriter has undertaken that it will not, directly or indirectly, offer or sell any shares of common stock or have in its possession, distribute or publish any prospectus, form of application, advertisement or other document or information in any country or jurisdiction except under circumstances that will, to the best of its knowledge and belief, result in compliance with any applicable laws and regulations and all offers and sales of shares of common stock by it will be made on the same terms.
European Economic Area
In relation to each Member Statemore of the European Economic Area which has implemented the prospectus Directive (each, a “Relevant Member State”) an offer to the public of any common stock which are the subject of the offering contemplated herein may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any common stock may be made at any time under the following exemptions under the prospectus Directive, if they have been implemented in that Relevant Member State:methods when selling securities:
| ● |
| ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| ● | purchases by a broker-dealer as principal and resale by the |
| ● | an exchange distribution in accordance with the |
| ● | privately negotiated transactions; |
| ● | settlement of short sales; |
| ● | in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
| ● | through the writing or |
| ● | a combination of |
| ● |
Each personThe Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus. Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a Relevant Member State who receives any communication in respect of, or who acquires any common stock under, the offers contemplated here insupplement to this prospectus, willin the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
-86-
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to have represented, warranted and agreed to and with each underwriter and us that:
For the purposes of this representation and the provision above, the expression an “offer of common stock to the public” in relation to any common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any common stock to be offered so as to enable an investor to decide to purchase or subscribe for the common stock, as the same may be varied in that Relevant Member State by any measure implementing the prospectus Directive in that Relevant Member State, the expression “prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in each Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom
This prospectus has only been communicated or caused to have been communicated and will only be communicated or caused to be communicated as an invitation or inducement to engage in investment activity (within“underwriters” within the meaning of Section 21 of the Financial Services and MarketsSecurities Act of 2000 (the “FSMA”)) as received in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the issue or saleresale of the common stock in circumstances in which Section 21(1)securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
We are required to pay certain fees and expenses incurred by us incident to the registration of the FSMA doessecurities. We have agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not applybe sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to us. Allthe Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the FSMAExchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Common Stock by the Selling Stockholders or any other person. We will be complied with in respect to anything done in relationmake copies of this prospectus available to the common stock in, fromSelling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or otherwise involvingprior to the United Kingdom.time of the sale (including by compliance with Rule 172 under the Securities Act).
-87-
Notice to Residents of CanadaLEGAL MATTERS
The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
The validity of the issuance of the Units, and the common stock and warrants underlying the Units, offered by us in this will be passed upon for us by Sheppard, Mullin, Richter & Hampton LLP, New York, New York. Certain matters are being passed on forYork, will pass upon the underwriters by Schiff Hardin LLP, Washington, Districtvalidity of Columbia.the shares of our Common Stock offered hereby.
dbbmckennon, an independent registered public accounting firm, has audited our financial statements as of andincluded in our Annual Report on Form 10-K for the year ended December 31, 2018 and the period from September 28, 2017 (inception) to December 31, 2017,2022, as set forth in their report, which includes an unqualified opinion on the financial statements and an explanatory paragraph about the existence of substantial doubt concerning the Company’sas to our ability to continue as a going concern. Suchconcern, dated April 17, 2023, which is incorporated by reference in this prospectus and elsewhere in the registration statement. Our financial statements are includedincorporated by reference in reliance uponon dbbmckennon report, given on the reportauthority of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange CommissionSEC a Registration Statementregistration statement on Form S-1 under the Securities Act with respect to the common stocksecurities offered by this prospectus. This prospectus, which isconstitutes a part of the registration statement, omits certaindoes not contain all of the information exhibits, schedules and undertakings set forth in the registration statement.statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information pertainingwith respect to us and our common stock, reference is madesecurities, we refer you to the registration statement, andincluding the exhibits and schedules tofiled as a part of the registration statement. Statements contained in this prospectus as toconcerning the contents or provisions of any documents referred to in this prospectus arecontract or any other document is not necessarily complete, and in each instance wherecomplete. If a copy of thecontract or document has been filed as an exhibit to the registration statement, referenceplease see the copy of the contract or document that has been filed. Each statement is madethis prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. We are subject to the exhibit for a more complete descriptioninformational requirements of the matters involved.Exchange Act and in accordance therewith file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov. The registration statement and the documents referred to below under “Incorporation of Documents By Reference” are also available on our website, www.astrotechcorp.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
You may read and copy all or any portion
-88-
INCORPORATION OF DOCUMENTS BY REFERENCE
This prospectus is part of the registration statement without charge at the public reference room of the Securities and Exchange Commission at 100 F Street, N.E., Washington, D.C. 20549. Copies ofbut the registration statement mayincludes and incorporates by reference additional information and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be obtainedpart of this prospectus and you should read it with the same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from the Securitiesdate those documents are filed. We have filed with the SEC, and Exchange Commission at prescribed rates from the publicincorporate by reference room of the Securities and Exchange Commission at such address. You may obtain information regarding the operation of the public reference room by calling 1-800-SEC-0330. in this prospectus:
| ● | Annual Report on Form 10-K for the year ended December 31, 2022 originally filed with the SEC on April 17, 2023 and amended on April 28, 2023; |
| ● | Quarterly Report on Form 10-Q for the three months ended March 31, 2023 filed with the SEC on May 15, 2023; |
| ● | Quarterly Report on Form 10-Q for the six months ended June 30, 2023 filed with the SEC on August 14, 2023; |
| ● | Quarterly Report on Form 10-Q for the nine months ended September 30, 2023 filed with the SEC on November 14, 2023; |
| ● | Proxy Statement on Schedule 14A filed on July 20, 2023, as amended; and |
| ● | the description of our common stock and our preferred stock contained in our Registration Statement on Form 8-A12B/A filed with the Commission on June 17, 2020, and any amendments or reports filed updating such description. |
In addition, registration statements and certain other filings made with the Securities and Exchange Commission electronically are publicly available through the Securities and Exchange Commission’s website athttp://www.sec.gov. The registration statement, including all exhibits and amendmentsdocuments subsequently filed by us pursuant to the registration statement, has been filed electronically with the Securities and Exchange Commission.
Upon completion of this offering, we will become subject to the information and periodic reporting requirementsSections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, and, accordingly, willprior to the termination of the offering (excluding any information furnished rather than filed) shall be requireddeemed to file annual reports containing financialbe incorporated by reference into this prospectus.
Notwithstanding the statements audited by an independent public accounting firm, quarterly reports containing unaudited financial data, current reports, proxy statements andin the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information withthat we have “furnished” to the SEC pursuant to the Securities and Exchange Commission. You willAct of 1934, as amended shall be able to inspect and copy such periodic reports, proxy statements and other information at the Securities and Exchange Commission’s publicincorporated by reference room, and the website of the Securities and Exchange Commission referred to above.
Aditx Therapeutics, Inc.
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Aditx Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Aditx Therapeutics, Inc. (the “Company”) as of December 31, 2018 and 2017, the related statements of operations, stockholders’ deficit, and cash flows, for the year ended December 31, 2018 and the period from September 28, 2017 (“Inception”) through December 31, 2017, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for the period then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 of the financial statements, the Company has incurred losses since Inception and requires additional financing. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with respect to these matters are discussed in Note 2. The accompanying financial statements do not include any adjustments that might result from the outcome ofinto this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.prospectus.
We conductedwill furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to:
Aditxt, Inc.
737 N. Fifth Street, Suite 200
Richmond, VA 23219
Phone: (650) 870-1200
You also may access these filings on our audits in accordancewebsite at http://www.aditxt.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the standardsSEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement. Any statement contained herein or in any document incorporated or deemed to be incorporated by reference shall be deemed to be modified or superseded for purposes of the PCAOB andregistration statement of which this prospectus forms a part to the extent that a statement contained in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the auditany other subsequently filed document which also is or is deemed to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether duebe incorporated by reference modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Asconstitute a part of our audits, we are required to obtain an understandingthe registration statement of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to errorwhich this prospectus forms a part, except as so modified or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ dbbmckennon
We have served as the Company’s auditor since 2018.
Newport Beach, California
October 11, 2019
BALANCE SHEETS
| September 30, | December 31, | |||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (unaudited) | ||||||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS | ||||||||||||
| Cash | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
| TOTAL CURRENT ASSETS | 32,977 | 115,709 | 25,000 | |||||||||
| TOTAL ASSETS | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||||||
| CURRENT LIABILITIES | ||||||||||||
| Accounts payable and accrued expenses | $ | 1,735,016 | $ | 1,056,226 | $ | 8,566 | ||||||
| Accrued compensation to related parties | 833,651 | 432,615 | 51,334 | |||||||||
| Notes payable - related party | 10,000 | 42,502 | — | |||||||||
| Notes payable | 116,100 | 121,100 | — | |||||||||
| TOTAL CURRENT LIABILITIES | 2,694,767 | 1,652,443 | 59,900 | |||||||||
| Deposit on private placement | — | — | 25,000 | |||||||||
| TOTAL LIABILITIES | 2,694,767 | 1,652,443 | 84,900 | |||||||||
| COMMITMENTS AND CONTINGENCIES | — | — | — | |||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||||||
| Preferred stock, $0.001 par value, 3,000,000 shares authorized, 0 shares issued and outstanding | — | — | — | |||||||||
| Common stock, $0.001 par value, 27,000,000 shares authorized, 7,781,800, 7,527,850, and 7,100,000 shares issued and 7,580,195, 7,527,850, and 7,100,000 shares outstanding, respectively | 7,782 | 7,528 | 7,100 | |||||||||
| Treasury stock, 201,605, 0, and 0, shares, respectively | (201,605 | ) | — | — | ||||||||
| Additional paid-in capital | 8,270,900 | 4,357,961 | 145,986 | |||||||||
| Accumulated deficit | (10,738,867 | ) | (5,902,223 | ) | (212,986 | ) | ||||||
| TOTAL STOCKHOLDERS’ DEFICIT | (2,661,790 | ) | (1,536,734 | ) | (59,900 | ) | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 32,977 | $ | 115,709 | $ | 25,000 | ||||||
See accompanying notes to the financial statements.
STATEMENTS OF OPERATIONS
| For the Nine Months Ended September 30, | For the Year Ended December 31, | For the Period From September 28, (Inception) Through December 31, | ||||||||||||||
| 2019 | 2018 | 2018 | 2017 | |||||||||||||
| Statements of Operations Data: | (unaudited) | (unaudited) | ||||||||||||||
| REVENUE | — | — | — | — | ||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| General and administrative expenses, includes $3,533,016, $3,264,514, $3,417,526, and $153,086, in stock-based compensation | 4,771,567 | 4,551,814 | 5,044,634 | 212,986 | ||||||||||||
| Research and development, includes $10,000, $100,000, $100,000, and $0, in stock-based compensation | 108,449 | 525,587 | 525,000 | — | ||||||||||||
| Sales and marketing, includes $0 $6,000, $6,000, and $0, in stock-based compensation | 147 | 39,459 | 39,837 | — | ||||||||||||
| Total Operating Expenses | 4,880,163 | 5,116,860 | 5,609,471 | 212,986 | ||||||||||||
| NET LOSS FROM OPERATIONS | (4,880,163 | ) | (5,116,860 | ) | (5,609,471 | ) | (212,986 | ) | ||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||
| Interest expense | (1,481 | ) | (2,228 | ) | (3,009 | ) | — | |||||||||
| Gain on forgiveness of debt | 45,000 | — | — | — | ||||||||||||
| Amortization of debt discount | — | (69,237 | ) | (76,757 | ) | — | ||||||||||
| Total Other Income (Expense) | 43,519 | (71,465 | ) | (79,766 | ) | — | ||||||||||
| Net loss before provision for income taxes | (4,836,644 | ) | (5,188,325 | ) | (5,689,237 | ) | (212,986 | ) | ||||||||
| Provision for income taxes | — | — | — | — | ||||||||||||
| NET LOSS | (4,836,644 | ) | (5,188,325 | ) | (5,689,237 | ) | (212,986 | ) | ||||||||
| Net loss per share - basic and diluted | (0.63 | ) | (0.72 | ) | (0.78 | ) | (0.03 | ) | ||||||||
| Weighted average number of shares outstanding during the period - basic and diluted | 7,673,317 | 7,231,510 | 7,261,637 | 7,100,000 | ||||||||||||
See accompanying notes to the financial statements.
STATEMENTS OF STOCKHOLDERS’ DEFICIT
| Additional | Total | |||||||||||||||||||||||
| Common | Treasury | Paid-in | Accumulated | Stockholders’ | ||||||||||||||||||||
| Shares | Par | Shares | Capital | Deficit | Deficit | |||||||||||||||||||
| Balance September 28, 2017 (inception) | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||
| Issuance of founders’ shares | 7,100,000 | 7,100 | - | - | - | 7,100 | ||||||||||||||||||
| Stock option compensation | - | - | - | 82,086 | - | 82,086 | ||||||||||||||||||
| Fair value of services provided by founders | - | - | - | 63,900 | - | 63,900 | ||||||||||||||||||
| Net loss | - | - | - | - | (212,986 | ) | (212,986 | ) | ||||||||||||||||
| Balance December 31, 2017 | 7,100,000 | $ | 7,100 | $ | - | $ | 145,986 | $ | (212,986 | ) | $ | (59,900 | ) | |||||||||||
| Issuance of shares for cash, net of issuance costs | 349,850 | 350 | - | 655,870 | - | 656,220 | ||||||||||||||||||
| Issuance of shares for services and licenses | 78,000 | 78 | - | 155,922 | - | 156,000 | ||||||||||||||||||
| Stock option and warrant compensation | - | - | - | 3,367,526 | - | 3,367,526 | ||||||||||||||||||
| Warrants issued with notes | - | - | - | 32,657 | - | 32,657 | ||||||||||||||||||
| Net loss | - | - | - | - | (5,689,237 | ) | (5,689,237 | ) | ||||||||||||||||
| Balance December 31, 2018 | 7,527,850 | $ | 7,528 | $ | - | $ | 4,357,961 | $ | (5,902,223 | ) | $ | (1,536,734 | ) | |||||||||||
| Issuance of shares for cash, net of issuance costs | 212,950 | 213 | - | 369,964 | - | 370,177 | ||||||||||||||||||
| Issuance of shares for services and licenses | 41,000 | 41 | - | 81,959 | - | 82,000 | ||||||||||||||||||
| Stock option and warrant compensation | - | - | - | 3,461,016 | - | 3,461,016 | ||||||||||||||||||
| Treasury stock | (201,605 | ) | - | (201,605 | ) | - | - | (201,605 | ) | |||||||||||||||
| Net loss | - | - | - | - | (4,836,644 | ) | (4,836,644 | ) | ||||||||||||||||
| Balance September 30, 2019 (Unaudited) | 7,580,195 | $ | 7,782 | $ | (201,605 | ) | $ | 8,270,900 | $ | (10,738,867 | ) | $ | (2,661,790 | ) | ||||||||||
See accompanying notes to the financial statements.
STATEMENT OF CASH FLOWS
| For the Nine Months Ended September 30, | For the Year Ended December 31, | For the Period From September 28, 2017 (Inception) Through December 31, | ||||||||||||||
| 2019 | 2018 | 2018 | 2017 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||||||
| Net loss | $ | (4,836,644 | ) | $ | (5,188,325 | ) | $ | (5,689,237 | ) | $ | (212,986 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||||||
| Stock-based compensation | 3,543,016 | 3,370,514 | 3,523,526 | 153,086 | ||||||||||||
| Amortization of offering costs | - | - | 273,750 | - | ||||||||||||
| Amortization of debt discount | - | 69,237 | 76,757 | - | ||||||||||||
| Changes in operating assets and liabilities | ||||||||||||||||
| Accounts payable and accrued expenses | 477,185 | 1,016,130 | 1,047,660 | 8,566 | ||||||||||||
| Accrued compensation to related parties | 401,036 | 298,281 | 381,281 | 51,334 | ||||||||||||
| Net Cash Used In Operating Activities | (415,407 | ) | (434,163 | ) | (386,263 | ) | - | |||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||||||
| Proceeds from notes payable - related party | 10,000 | 117,502 | 117,502 | - | ||||||||||||
| Proceeds from notes payable | - | 182,000 | 182,000 | - | ||||||||||||
| Repayments of notes payable - related party | (42,502 | ) | (75,000 | ) | (75,000 | ) | - | |||||||||
| Repayments of notes payable | (5,000 | ) | - | (105,000 | ) | - | ||||||||||
| Common stock issued for cash, net of issuance costs | 370,177 | 237,500 | 631,220 | - | ||||||||||||
| Deferred offering costs | - | - | (273,750 | ) | - | |||||||||||
| Deposit on private placement | - | - | - | 25,000 | ||||||||||||
| Net Cash Provided By Financing Activities | 332,675 | 462,002 | 476,972 | 25,000 | ||||||||||||
| NET INCREASE (DECREASE) IN CASH | (82,732 | ) | 27,839 | 90,709 | 25,000 | |||||||||||
| CASH AT BEGINNING OF PERIOD | 115,709 | 25,000 | 25,000 | - | ||||||||||||
| CASH AT END OF PERIOD | $ | 32,977 | $ | 52,839 | $ | 115,709 | $ | 25,000 | ||||||||
| Supplemental cash flow information: | ||||||||||||||||
| Cash paid for income taxes | $ | - | $ | - | $ | - | $ | - | ||||||||
| Cash paid for interest expense | $ | - | $ | - | $ | - | $ | - | ||||||||
| NONCASH INVESTING AND FINANCING ACTIVITIES: | ||||||||||||||||
| Liabilities assumed for common stock repurchase | $ | 201,605 | $ | - | $ | - | $ | - | ||||||||
| Fair value of warrants issued with notes payable | $ | - | $ | 32,657 | $ | 32,657 | $ | - | ||||||||
| Common stock issued for deposit on private placement | $ | - | $ | 25,000 | $ | 25,000 | $ | - | ||||||||
See accompanying notes to the financial statements.
NOTES TO FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF BUSINESS
Company Background
Aditx Therapeutics, Inc. (“Aditx” or the “Company”) was incorporated on September 28, 2017, under the laws of the State of Delaware. The Company is a pre-clinical stage, life sciences company with a mission to prolong life and enhance life quality of transplanted patients.
Risks and Uncertainties
The Company has a limited operating history and has not generated revenue from intended operations. The Company’s business and operations are sensitive to general business and economic conditions in the U.S. and worldwide along with local, state, and federal governmental policy decisions. A host of factors beyond the Company’s control could cause fluctuations in these conditions. Adverse conditions may include: changes in biotechnology regulatory environment, technological advances that render our technologies obsolete, availability of resources for clinical trials, acceptance of technologies into the medical community, and competition from larger, more well-funded companies. These adverse conditions could affect the Company’s financial condition and the results of its operations.
NOTE 2 – GOING CONCERN ANALYSIS
Going Concern Analysis
The Company was incorporated on September 28, 2017 and has not generated revenues to date. For the year ended December 31, 2018, the Company had a net loss of $5,689,237 and will require significant additional capital in order to operate in the normal course of business and fund clinical studies. The Company will be conducting medical research and development, and the time at which the Company will begin generating revenue is unknown. As a result of these conditions, there is substantial doubt about the Company’s ability to continue as a going concern. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern.
The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the matters discussed herein. While we believe in the viability of our strategy to generate sufficient revenue, control costs and raise additional funds when necessary, there can be no assurances to that effect. The Company’s ability to continue as a going concern is dependent upon the ability to complete clinical studies and implement the business plan, generate sufficient revenues and to control operating expenses.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The unaudited interim financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information, within the rules and regulations of the United States Securities and Exchange Commission (the “SEC”). Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been condensed or omitted pursuant to such rules and regulations. The unaudited interim financial statements have been prepared on a basis consistent with the audited financial statements and in the opinion of management, reflect all adjustments, consisting of only normal recurring adjustments, necessary for the fair presentation of the results for the interim periods presented and of the financial condition as of the date of the interim balance sheet. The financial data and the other information disclosed in these notes to the interim financial statements related to the nine-month periods are unaudited. Unaudited interim results are not necessarily indicative of the results for the full fiscal year. These unaudited interim financial statements should be read in conjunction with the financial statements of the Company for the year ended December 31, 2018 and notes thereto that are included in the Company’s Registration Statement on Form S-1.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expense during the reporting period. Actual results could differ from those estimates. Significant estimates underlying the financial statements include the fair value of stock options and warrants.superseded.
F-7-89-
Fair Value Measurements and Fair Value of Financial Instruments
Aditxt, Inc.
The Company adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements. ASC Topic 820 clarifies the definition3,785,569 Shares of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:Common Stock
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The Company did not identify any assets or liabilities that are required to be presented on the balance sheets at fair value in accordance with ASC Topic 820.
Due to the short-term nature of all financial assets and liabilities, their carrying value approximates their fair value as of the balance sheet dates.
Concentrations of Credit Risk
The Company maintains its cash accounts at financial institutions which are insured by the Federal Deposit Insurance Corporation. At times, the Company may have deposits in excess of federally insured limits.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. As of September 30, 2019 and December 31, 2018 and 2017, the Company did not have any cash equivalents.
Offering Costs
The Company accounts for offering costs in accordance with ASC 340, Other Assets and Deferred Costs. Prior to the completion of an offering, offering costs were capitalized as deferred offering costs on the balance sheet. The deferred offering costs are netted against the proceeds of the offering in stockholders’ deficit or the related debt, as applicable. Costs related to unsuccessful offerings are expensed.
Debt Issued with Warrants
Debt issued with warrants is accounted for under the guidelines established by ASC 470-20, Accounting for Debt with Conversion or Other Options. We record the relative fair value of warrants related to the issuance of convertible debt as a debt discount or premium. The discount or premium is subsequently amortized to interest expense over the expected term of the convertible debt.
Stock-Based Compensation
The Company accounts for stock-based compensation costs under the provisions of ASC 718, Compensation—Stock Compensation, which requires the measurement and recognition of compensation expense related to the fair value of stock-based compensation awards that are ultimately expected to vest. Stock based compensation expense recognized includes the compensation cost for all stock-based payments granted to employees, officers, and directors based on the grant date fair value estimated in accordance with the provisions of ASC 718. ASC. 718 is also applied to awards modified, repurchased, or canceled during the periods reported.
Patents
The Company incurs fees from patent licenses. During the year and period ended December 31, 2018 and 2017, the Company had a licensing fee for the patents of $525,000 and $0, respectively. During the nine months ended September 30, 2019 and 2018, the Company had a licensing fee for the patents of $18,071 and $525,000, respectively.
Revenue Recognition
The adoption of ASC 606, Revenue From Contracts With Customers, represents a change in accounting principle that will more closely align revenue recognition with the delivery of the Company’s services and will provide financial statement readers with enhanced disclosures. In accordance with ASC 606, revenue is recognized when a customer obtains control of promised services. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to receive in exchange for these services. To achieve this core principle, the Company applies the following five steps: Identify the contract with a customer; Identify the performance obligations in the contract; Determine the transaction price; Allocate the transaction price to performance obligations in the contract; Recognize revenue when or as the Company satisfies a performance obligation. The Company has not recognized any revenue to date.
Research and Development
We incur research and development costs during the process of researching and developing our technologies and future offerings. Our research and development costs mainly consist of licensing costs. We expense these costs as incurred unless such costs qualify for capitalization under applicable guidance.
Advertising
The Company expenses the cost of advertising and promotions as incurred. Advertising expense was $39,837 and $0 for the year and period ended December 31, 2018 and 2017, respectively. Advertising expense was $147 and $39,459 for the nine months ended September 30, 2019 and 2018, respectively.
Basic and Diluted Net Loss per Common Share
Basic loss per common share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding for each period. Diluted loss per share is computed by dividing the net loss by the weighted average number of shares of common stock outstanding plus the dilutive effect of shares issuable through the common stock equivalents. The weighted-average number of common shares outstanding excludes common stock equivalents because their inclusion would be anti-dilutive. As of December 31, 2018, 1,005,000 stock options and 1,974,351 warrants were excluded from dilutive earnings per share as their effects were anti-dilutive. As of December 31, 2017, 240,000 stock options and no warrants were excluded from dilutive earnings per share as their effects were anti-dilutive. As of September 30, 2019, 2,205,000 stock options and 2,632,456 warrants were excluded from dilutive earnings per share as their effects were anti-dilutive. As of September 30, 2018, 1,005,000 stock options and 1,575,751 warrants were excluded from dilutive earnings per share as their effects were anti-dilutive.
Recent Accounting Pronouncements
In February 2016, FASB issued Accounting Standards Update (“ASU”) 2016-02: Leases (Topic 842). The new guidance generally requires an entity to recognize on its balance sheet operating and financing lease liabilities and corresponding right-of-use assets. The standard will be effective for the first interim period within annual reporting periods beginning after December 15, 2018 and early adoption is permitted. The new standard requires a modified retrospective transition for existing leases to each prior reporting period presented. The Company has elected to utilize the extended adoption period available to the Company as an emerging growth company and has not currently adopted this standard. This standard will be effective for the first interim period within annual reporting periods beginning after December 15, 2019. The Company is currently evaluating the impact of the adoption of ASU 2016-02 on its financial position, results of operations and cash flows once adopted.
In June 2018, the FASB issued ASU 2018-07, Compensation — Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, which expands the scope of Topic 718 to include all share-based payment transactions for acquiring goods and services from nonemployees. ASU 2018-07 specifies that Topic 718 applies to all share-based payment transactions in which the grantor acquires goods and services to be used or consumed in its own operations by issuing share-based payment awards. ASU 2018-07 also clarifies that Topic 718 does not apply to share-based payments used to effectively provide (1) financing to the issuer or (2) awards granted in conjunction with selling goods or services to customers as part of a contract accounted for under ASC 606. ASU 2018-07 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, with early adoption permitted, but no earlier than our adoption of ASC 606. The Company chose to early adopt ASU 2018-07. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480) and Derivatives and Hedging (Topic 815): I. Accounting for Certain Financial Instruments with Down Round Features; II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception. Part I of this update addresses the complexity of accounting for certain financial instruments with down round features. Down round features are features of certain equity-linked instruments (or embedded features) that result in the strike price being reduced based on the pricing of future equity offerings. Current accounting guidance creates cost and complexity for entities that issue financial instruments (such as warrants and convertible instruments) with down round features that require fair value measurement of the entire instrument or conversion option. Part II of this update addresses the difficulty of navigating Topic 480, Distinguishing Liabilities from Equity, because of the existence of extensive pending content in the FASB Accounting Standards Codification. This pending content is the result of the indefinite deferral of accounting requirements about mandatorily redeemable financial instruments of certain nonpublic entities and certain mandatorily redeemable noncontrolling interests. The amendments in Part II of this update do not have an accounting effect. This ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2018. The adoption of this standard did not have a material impact on the Company’s financial statements and related disclosures.
The FASB issues ASUs to amend the authoritative literature in ASC. There have been several ASUs to date, including those above, that amend the original text of ASC. Management believes that those issued to date either (i) provide supplemental guidance, (ii) are technical corrections, (iii) are not applicable to us or (iv) are not expected to have a significant impact our financial statements.
NOTE 4 – RELATED PARTY TRANSACTIONS
The Company’s Chief Executive Officer (“CEO”) has provided certain periods of service without payment. Accordingly, the Company has accrued $15,000 in compensation per month for services rendered during the year ended December 31, 2018. This amount was increased to $21,000 per month for the nine months ended September 30, 2019. As of September 30, 2019 and December 31, 2018, the CEO is owed $276,522 and $145,000, respectively, related to compensation.
During the period, a related party has provided the Company with operations consulting services. As of December 31, 2018, $112,000 was accrued as compensation. An additional $135,000 was expensed as compensation during the nine months ended September 30, 2019, and $17,000 was paid on the accrued balance. As of September 30, 2019, $230,000 remained accrued and outstanding.
On January 22, 2018, the Company entered into an unsecured promissory note with a related party for $40,000 that accrued interest of 4% annually. The note was due on the earlier of July 22, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of September 30, 2019.
On February 12, 2018, the Company entered into an unsecured promissory note with a related party for $50,000 that accrued interest of 4% annually. The note was due on the earlier of August 12, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of September 30, 2019.
On March 2, 2018, the Company entered into an unsecured promissory note with a related party for $10,000 that accrued interest of 4% annually. The note was due on the earlier of September 2, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of September 30, 2019.
On March 8, 2018, we entered into an Assignment Agreement (the “Assignment Agreement”) with Sekris Biomedical, Inc. (“Sekris”). Sekris is a related party due to Dr. Shabahang, our CTO, being the CEO of Sekris. Sekris was a party to a License Agreement with Loma Linda University (“LLU”), entered into and made effective on May 25, 2011, and amended on June 24, 2011, July 16, 2012 and December 27, 2012 (the “Original Agreement,” and together with the Assignment Agreement, the “Sekris Agreements”). Pursuant to the Assignment Agreement, Sekris transferred and assigned all of its rights and obligations in and to and liabilities under the Original Agreement, of whatever kind or nature, to us. In exchange, on March 8, 2018, we issued a warrant to Sekris to purchase up to 1,000,000 shares of our Common Stock (the “Sekris Warrant”). The warrant is immediately exercisable and the exercise price is $2.00 per share. The expiration date of the warrant is March 8, 2023. On March 15, 2018, we entered into a Patent & Technology License Agreement directly with LLU (the “New License Agreement”), which amends and restates the Sekris Agreements. Per this agreement, the Company agrees to pay license fees of $25,000 annually starting March 31, 2020, milestone fees totaling up to $1,725,000, and a past patent fee of $200,000 which is to be paid in quarterly installments between September 2018 and March 2019. The Company also issued 50,000 shares of its common stock as part of this agreement. These shares of common stock were valued at $2.00 per share based on the most recent sales price of the Company’s common stock during the period. The Company will also pay royalties of 1.5% of their net product sales and net service sales covered by a valid claim and 0.75% of their net product sales and net service sales not covered by a valid claim.
On June 18, 2018, the Company entered into an unsecured promissory note with a related party for $17,502 that accrued interest of 4% annually. The note was due on the earlier of December 18, 2018 or in the event of default, as defined in the agreement. This note has been repaid as of September 30, 2019.
On January 1, 2019, the Company entered into a consulting agreement with a related party to perform operating consulting services. As part of this agreement, the Company will pay the consultant $15,000 per month.
On March 21, 2019, the Company entered into a note with a related party. The note has a principle of $10,000, a maturity date of September 21, 2019, and an interest rate of 4% per year. There is $10,000 outstanding on this note as of September 30, 2019. This note is currently in default.
During the nine months ended September 30, 2019, the Company assumed $201,605 of liabilities from a related party in exchange for the return of 201,605 shares of the Company’s common stock.
NOTE 5 – STOCKHOLDERS’ DEFICIT
Preferred Stock
The Company has authorized the issuance of 3,000,000 shares of common stock, par value $0.001 per share.
Common Stock
The Company has authorized the issuance of 27,000,000 shares of common stock, par value $0.001 per share.
In November 2017, the Company commenced a private placement for the sale of 1,000,000 shares of common stock for gross proceeds of $2,000,000. The terms of the private placement were such that a minimum of $250,000 was to be raised before funds could be drawn on, and that the technology licensing agreement (Note 4) must be executed. During the first quarter of 2018, the Company received $237,500 in cash, and with the $25,000 received prior to December 31, 2017, the aggregate raised was $262,500 for which the Company issued 131,250 shares of common stock under the private placement at $2.00 per share. The Company also agreed to issue one warrant to purchase a share of common stock for each share purchased in the private placement. The warrant is exercisable at $2.00 per share and has a term of three years.
In October 2018, the Company commenced a private placement for the sale of 1,000,000 shares of common stock for gross proceeds of $2,000,000. During the year ended December 31, 2018, the Company received $437,200 in cash for which the Company issued 218,600 shares of common stock under the private placement at $2.00 per share. The Company also paid total issuance costs of $43,480, for net proceeds of $393,720. During the nine months ended September 30, 2019, the Company received $425,900 in cash for which the Company issued 212,950 shares of common stock under the private placement at $2.00 per share. The Company also paid total issuance costs of $55,723, for net proceeds of $370,177. The Company also agreed to issue one warrant to purchase a share of common stock for each share purchased in the private placement. The warrant is exercisable at $3.00 per share and has a term of three years.
During the year ended December 31, 2018, the Company issued 78,000 shares of common stock for services and recognized expense of $156,000 in stock compensation and license fees. These shares were valued based on the price which common shares were being sold in the above private placements.
During the nine months ended September 30, 2019, the Company issued 41,000 shares of common stock for services and recognized expense of $82,000 in stock compensation and license fees. During the nine months ended September 30, 2018, the Company issued 73,000 shares of common stock for services and recognized expense of $146,000 in stock compensation and licenses fees. These shares were valued based on the price which common shares were being sold in the above private placements.
Stock-Based Compensation
In October 2017, our Board of Directors adopted the Aditx Therapeutics, Inc. 2017 Equity Incentive Plan (the “2017 Plan”). The 2017 Plan provides for the grant of equity awards to employees, and consultants. Up to 1,200,000 shares of our common stock may be issued pursuant to awards granted under the 2017 Plan. The 2017 Plan is administered by our Board of Directors, and expires ten years after adoption, unless terminated earlier by the Board. On December 21, 2018, our board of directors amended the 2017 Plan to increase the number of authorized shares of common stock issuable thereunder to 5,000,000 shares, subject to shareholder approval.
During the year ended December 31, 2018, the Company granted 825,000 stock options with exercise prices of $2.00 per share vesting on issuance or vesting yearly. The total grant date fair value was determined to be $1,507,232. During the year ended December 31, 2017, the Company granted 240,000 stock options with exercise prices of $2.00 per share vesting monthly. The total grant date fair value was determined to be $437,793. During the nine months ended September 30, 2019, the Company granted 1,400,000 stock options with exercise prices of $2.00 per share vesting on issuance, of which 1,100,000 was to related parties. The total grant date fair value was determined to be $2,495,556. For all periods presented, the fair value of each stock option granted was estimated using the Black-Scholes assumption ranges and or factors as follows:
| Exercise price | $ | 2.00 | ||
| Expected dividend yield | 0 | % | ||
| Risk free interest rate | 2.47% - 2.65 | % | ||
| Expected life in years | 5 | |||
| Expected volatility | 141-151 | % |
The risk-free interest rate assumption for options granted is based upon observed interest rates on the United States government securities appropriate for the expected term of stock options.
The expected term of stock options is calculated using either the simplified method for employee options which takes into consideration the contractual life and vesting terms of the options, unless the options are expected to vest in which case the contractual term of the options.
The Company determined the expected volatility assumption for options granted using the historical volatility of comparable public companies’ common stock. The Company will continue to monitor peer companies and other relevant factors used to measure expected volatility for future stock option grants, until such time that the Company’s common stock has enough market history to use historical volatility.
The dividend yield assumption for options granted is based on the Company’s history and expectation of dividend payouts. The Company has never declared or paid any cash dividends on its common stock, and the Company does not anticipate paying any cash dividends in the foreseeable future.
Management estimated the fair value of common stock by looking at a market approach which takes into consideration past sales of stock to third parties and Company developments to date.
The Company recognizes stock option forfeitures as they occur as there is insufficient historical data to accurately determine future forfeitures rates.
The following is an analysis of the stock option grant activity under the Plan:
Weighted Average | Weighted Average | |||||||||||
| Number | Exercise Price | Remaining Life | ||||||||||
| Stock Options | ||||||||||||
| Outstanding September 28, 2017 (inception) | - | $ | - | - | ||||||||
| Granted | 240,000 | 2.00 | 5.00 | |||||||||
| Expired or forfeited | - | - | - | |||||||||
| Outstanding December 31, 2017 | 240,000 | 2.00 | 4.82 | |||||||||
| Granted | 825,000 | 2.00 | 5.00 | |||||||||
| Expired or forfeited | (60,000 | ) | 2.00 | - | ||||||||
| Outstanding December 31, 2018 | 1,005,000 | $ | 2.00 | 4.11 | ||||||||
| Nonvested Options | Shares | Weighted- Average Exercise Price | ||||||
| Nonvested at September 28, 2017 (inception) | - | $ | - | |||||
| Granted | 240,000 | 2.00 | ||||||
| Vested | (45,000 | ) | 2.00 | |||||
| Forfeited | - | - | ||||||
| Nonvested at December 31, 2017 | 195,000 | 2.00 | ||||||
| Granted | 825,000 | 2.00 | ||||||
| Vested | (760,000 | ) | 2.00 | |||||
| Forfeited | (60,000 | ) | 2.00 | |||||
| Nonvested at December 31, 2018 | 200,000 | $ | 2.00 | |||||
There are 805,000 options exercisable at December 31, 2018 with a weighted average exercise price of $2.00 and a weighted average life remaining of 4.09 years. There are 2,205,000 options exercisable at September 30, 2019 with a weighted average exercise price of $2.00 and a weighted average life remaining of 4.09 years.
The Company recognized compensation expense related to options issued and vesting of $1,369,824 during the year ended December 31, 2018, which is included in general and administrative expenses in the accompanying statements of operations. The remaining value to be expensed is $575,201 with a weighted average vesting life of 1.75 years as of December 31, 2018. The Company recognized compensation expense related to options issued and vesting of $82,086 during the period ended December 31, 2017, which is included in general and administrative expenses in the accompanying statements of operations.
The Company recognized compensation expense related to options issued and vesting of $2,513,826 during the nine months ended September 30, 2019, which is included in general and administrative expenses in the accompanying statements of operations. The remaining value to be expensed is $0 with a weighted average vesting life of 0 years as of September 30, 2019. The Company recognized compensation expense related to options issued and vesting of $1,296,772 during the period ended September 30, 2018, which is included in general and administrative expenses in the accompanying statements of operations.
Warrants
A summary of warrant issuances are as follows:
| Number | Weighted Average Exercise Price | Weighted Average Remaining Life | ||||||||||
| Warrants | ||||||||||||
| Outstanding December 31, 2017 | - | $ | - | - | ||||||||
| Granted | 1,974,351 | 2.16 | 4.53 | |||||||||
| Outstanding December 31, 2018 | 1,974,351 | $ | 2.16 | 3.89 | ||||||||
| Nonvested Warrants | Shares | Weighted- Average Exercise Price | ||||||
| Nonvested at December 31, 2017 | - | $ | - | |||||
| Granted | 1,974,351 | 2.16 | ||||||
| Vested | (1,374,351 | ) | 2.23 | |||||
| Forfeited | - | - | ||||||
| Nonvested at December 31, 2018 | 600,000 | $ | 2.00 | |||||
There are 1,374,351 warrants exercisable at December 31, 2018 with a weighted average exercise price of $2.23 and a weighted average life remaining of 3.77 years. There are 2,172,456 warrants exercisable at September 30, 2019 with a weighted average exercise price of $2.26 and a weighted average life remaining of 2.99 years.
The warrants are valued using similar inputs as noted in the stock options section above, with the exception of the expected life which is the contractual life.
The Company recognized compensation expense related to warrants issued and vesting of $2,030,359 during the year ended December 31, 2018, of which $1,997,702 is included in stock-based compensation and $32,657 is included in amortization of debt discount in the accompanying statements of operations. The remaining value to be expensed is $891,765 with a weighted average vesting term of 2.47 years as of December 31, 2018.
The Company recognized compensation expense related to warrants issued and vesting of $947,190 and $1,927,742 during the nine months ended September 30, 2019 and 2018, which is included in general and administrative in the accompanying statements of operations. The remaining value to be expensed is $643,851 with a weighted average vesting term of 1.78 years as of September 30, 2019.
On March 8, 2018, we entered into an Assignment Agreement with Sekris. This agreement called for the issuance of a warrant to Sekris to purchase up to 1,000,000 shares of our common stock (see Note 4).
In April 2018, the Company granted 420,000 warrants to purchase shares of common stock to two individuals for services. The warrants vest annually over three years.
During 2018, the Company issued 24,501 warrants to purchase shares of common stock in connection to three loans (see Note 7).
On December 1, 2018, the Company issued 180,000 warrants to purchase shares of common stock to a consultant. The warrants vest annually over three years.
During the nine months ended September 30, 2019, the Company issued 407,000 warrants to consultants for services.
On September 25, 2019, the Company issued a warrant to purchase 38,155 shares of its common stock to a placement agent as part of their agreement with the placement agent. (See Note 6).
The Company values warrants using inputs that are similar to those used in valuing the Company’s options.
NOTE 6 – AGREEMENTS
Effective March 1, 2018, the Company entered into a consulting agreement with a company for project management services for $13,000 per month plus any additional fees. The term of this agreement commenced on March 1, 2018 and remained in effect, as provided in the agreement, until May 31, 2018. The Company has entered into a new agreement with the consultant on November 16, 2018. This new agreement has the same compensation terms as the prior agreement.
On March 14, 2018, the Company entered into an agreement with a company to provide financial advisory and placement agent services. On March 14, 2018, an initial retainer fee of $55,000 was due for the Non-Accountable Expense Allowance with an additional $10,000 due upon filing of an offering statement as defined by the agreement. Based on the amended terms, the placement agent is entitled to a cash fee equal to seven percent (7%) of the aggregate gross proceeds received by the Company from the sale of the Units at each Closing of the Offering and a non-accountable expense allowance equal to one percent (1%) of the aggregate gross proceeds received by the Company from the sale of the Units at each Closing of the Offering. The placement agent will also receive a warrant to purchase an amount of shares, equal to an aggregate of ten percent (10%) of the Shares underlying the Units sold in the Offering, for an exercise price of $7.50. These warrants have not been issued as of the date of this filing. The agreement may be terminated after six months by either party.
On August 23, 2018, the Company entered into a consulting agreement with a company for investor relations for $7,500 per month. This agreement also issued 20,000 shares of the Company’s common stock to the consultant at the signing of the agreement. The service period for this agreement is from August 23, 2018 to February 22, 2019.
On August 27, 2018, the Company entered into a consulting agreement with a company for consulting and management services for $2,500 per month.
On October 23, 2018, the Company entered into an agreement with a placement agent. As part of this agreement the Company will pay the placement agent 10% of the purchase price of the securities sold by the placement agent. The Company will also pay a 3% fee based on the offering proceeds as non-accountable expense. The Company will issue to the consultant warrants to purchase the Company’s common stock at a rate of 10% of the shares sold at the offering. These warrants will have the same terms as the investor’s warrants.
On December 1, 2018, the Company entered into an independent director agreement. As part of this agreement the Company issued 5,000 shares of its common stock to the director and if the agreement remains in effect on June 30, 2019 the Company will issue an additional 10,000 shares of its common stock.
On December 1, 2018, the Company entered into a consulting agreement. As part of this agreement the Company will pay the consultant $3,000 per month and a 3% success fee for all payments received by the Company from qualified agreements. The Company has also issued 180,000 warrants to purchase shares of common stock to the consultant. These warrants have an exercise price of $2.00, an expiration date of December 1, 2022, and vest 60,000 options per year starting on December 1, 2019.
On January 1, 2019, the Company entered into a consulting agreement with a company for business advisory services. As part of this agreement, the Company will pay a monthly fee of $3,500. The Company will also issue 6,000 shares of its common stock and a warrant to purchase 12,000 shares of its common stock with an exercise price of $2.00 and an expiration date of January 1, 2022.
On January 1, 2019, the Company entered into a consulting agreement. As part of this agreement, the Company will pay the consultant $4,500 per month.
On January 1, 2019, the Company entered into a consulting agreement with a company for business advisory services. As part of this agreement, the Company will pay the consultant $14,700 per month. This agreement replaces the prior agreement with this consultant dated November 16, 2018. On July 1, 2019, the Company entered into another agreement with this consultant to extend the term of the agreement to December 31, 2019.
On January 21, 2019, the Company entered into a consulting agreement with a company for business advisory services. As part of this agreement, the Company will pay the consultant $6,500 for the first two months of service and $3,000 per month should the agreement be extended further.
On January 21, 2019, the Company entered into a consulting agreement with a company for advisory services. As part of this agreement, the Company will pay the consultant $5,000 per month.
On February 1, 2019, the Company entered into a consulting agreement with a company for investor relations for $7,500 per month. This agreement replaces the prior agreement with this consultant dated August 27, 2018.
On February 1, 2019, the Company entered into a consulting agreement. As part of this agreement, the Company will pay the consultant $3,000 per month and will issue the consultant 5,000 warrants to purchase shares of the Company’s common stock.
On February 1, 2019, the Company entered into a consulting agreement. As part of this agreement, the Company will pay the consultant $10,000 per month and an initial fee of $6,500. The monthly fee and initial fee will be due starting the month that the Company completes its initial public offering or July 30, 2019, whichever occurs first. The Company will also issue 20,000 shares of its common stock at a par value of $0.001 per share within business 10 days of the agreement being executed.
On March 11, 2019, the Company entered into a lease. As part of this lease, the Company will pay $3,900 per month. The lease ran until September 11, 2019, and was not renewed upon expiration.
On April 15, 2019, the Company entered into an option agreement to license a patent. The term of this option is until October 15, 2019. The Company will pay a fee of $5,000 on the signing of the agreement. The Company will also issue 5,000 shares of its common stock valued at $2.00 per share to the patent holder.
On April 15, 2019, the Company entered into a consulting agreement for pre-clinical guidance. As part of this agreement, the Company will pay the consultant $5,000 per month.
NOTE 7 – NOTES PAYABLE
On April 12, 2018, the Company entered into an unsecured promissory note for $35,000 that accrues interest of 4% annually. The note was due on the earlier of November 12, 2018 or in the event of default, as defined in the agreement. This note is currently in default.
On July 10, 2018, the Company entered into a bridge loan with a principle of $15,600 and an on-issuance discount of $3,600. The Company also issued 2,000 warrants to this individual. The Company calculated the relative fair value of the warrants using a Black-Scholes pricing model with similar inputs as noted in Note 5, resulting in an additional discount of $2,669. The total discount of $6,269 was fully amortized as of September 30, 2019. The note was due on the earlier of October 8, 2018 or in the event of default, as defined in the agreement. This note is currently in default.
On July 18, 2018, the Company entered into a bridge loan with a principle of $130,000 and an on-issuance discount of $30,000. The Company also issued 16,667 warrants to this individual. The Company calculated the relative fair value of the warrants using a Black-Scholes pricing model with similar inputs as noted in Note 5, resulting in an additional discount of $22,206. The total discount of $52,206 was fully amortized as of September 30, 2019. The note was due on the earlier of October 16, 2018 or in the event of default, as defined in the agreement. This note is currently in default.
On August 29, 2018, the Company entered into a bridge loan with a principle of $45,500 and an on-issuance discount of $10,500. The Company also issued 5,834 warrants to this individual. The Company calculated the relative fair value of the warrants using a Black-Scholes pricing model with similar inputs as noted in Note 5, resulting in an additional discount of $7,782. The total discount of $18,282 was fully amortized as of September 30, 2019. The note was due on the earlier of October 16, 2018 or in the event of default, as defined in the agreement. This note is currently in default.
NOTE 8 – INCOME TAX
The Tax Cuts and Jobs Act
At December 31, 2018, the Company has available for U.S. federal income tax purposes a net operating loss (“NOL”) carry-forwards of approximately $2,210,000 that may be used to offset future taxable income through the fiscal year ending December 31, 2038. These NOLs begin to expire in 2017. If not used, these NOLs may be subject to limitation under Internal Revenue Code Section 382 should there be a greater than 50% ownership change as determined under the regulations. The Company plans on undertaking a detailed analysis of any historical and/or current Section 382 ownership changes that may limit the utilization of the net operating loss carryovers. No tax benefit has been reported with respect to these net operating loss carry-forwards in the accompanying financial statements since the Company believes that the realization of its net deferred tax asset of approximately $659,000 was not considered more likely than not and accordingly, the potential tax benefits of the net loss carry-forwards are fully offset by a valuation allowance of $659,000.
Deferred tax assets consist primarily of the tax effect of NOL carry-forwards. The Company has provided a full valuation allowance on the deferred tax assets because of the uncertainty regarding its realizability. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon future generation for taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all the information available, Management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance. The valuation allowance increased by approximately $641,000 for the period from Inception through December 31, 2018.
For the year ended December 31, 2018, the Company has a current tax provision of $0 and deferred tax benefit of approximately $641,000 with a corresponding valuation allowance of approximately $641,000. For the period ended December 31, 2017, the Company has a current tax provision of $0 and deferred tax benefit of $18,000 with a corresponding valuation allowance of $18,000.
Components of deferred tax assets are as follows:
| December 31, 2018 | December 31, 2017 | |||||||
| Net deferred tax assets – Non-current: | ||||||||
| Expected income tax benefit from NOL carry-forwards | $ | 659,490 | $ | 18,000 | ||||
| Less valuation allowance | (659,490 | ) | (18,000 | ) | ||||
| Deferred tax assets, net of valuation allowance | $ | - | $ | - | ||||
A reconciliation of the federal statutory income tax rate and the effective income tax rate as a percentage of income before income taxes is as follows:
| For the year ended December 31, 2018 | For the Period from September 28, 2017 (inception) through December 31, 2017 | |||||||
| Federal and state statutory income tax rate | 29.84 | % | 34.0 | % | ||||
| Permanent difference | 62.93 | % | - | % | ||||
| Change in valuation allowance on net operating loss carry-forwards | (92.77 | )% | (34.0 | )% | ||||
| Effective income tax rate | 0.0 | % | 0.0 | % | ||||
NOTE 9 – SUBSEQUENT EVENTS
During October and November 2019, the Company received $100,000 in cash for shares of common stock under the private placement at $2.00 per share selling a total of 50,000 shares. The Company also agreed to issue one warrant to purchase a share of common stock for each share purchased in the private placement. The warrant is exercisable at $3.00 per share and has a term of three years.
On November 1, 2019, the Company entered into a bridge loan with a principle of $50,000. This loan does not accrue any interest. The note is due on the earlier of April 28, 2020 or in the event of default, as defined in the agreement. The note can convert into the same class of securities as those sold in the public offering with a conversion price of $2.00 per share.
On October 1, 2019, the Company entered into an agreement for accounting services. As part of this agreement the Company will issue 50,000 warrants to purchase share of the Company’s common stock to the consultant. The agreement expires on December 31, 2019.
On October 1, 2019, the Company entered into an agreement for consulting services. As part of this agreement the Company has issued 20,000 warrants to purchase share of the Company’s common stock to the consultant. The agreement expires on December 31, 2019.
On December 18, 2019, the Company extended the expiration date for 2,205,000 options to purchase shares of the Company’s common stock issued to ten individuals. These options have had their expiration date extended to October 5, 2027.
On December 18, 2019, the Company issued 10,000 warrants to purchase shares of the Company’s common stock. These warrants have a grant date of December 18, 2019, an expiration date of October 5, 2027, and an exercise price of $2.00.
On December 27, 2019, the Company entered into a consulting agreement for advisory services. As part of this agreement the Company will pay a monthly retainer of $2,000. The Company will also issue 20,000 shares of its common stock within ten business days of January 1, 2020. Additionally, the Company will issue 30,000 shares of its commons stock on within ten business days of July 1, 2020 and 30,000 shares of its common stock within ten business days of December 31, 2020.
On December 27, 2019, the Company entered into a consulting agreement for advisory services. As part of this agreement the Company will pay one-time retainer of $15,000. If the Company is successful in listing on an exchange the Company will be obligated to pay a success fee to the consultant. This success fee will consist of $7,5000 or an equivalent amount in the Company’s common stock. Additionally, if the listing is completed before February 14, 2020 the Company will also be obligated to pay an additional $10,000 and 10,000 shares of the Company’s common stock.
On January 5, 2020, the Company cancelled 38,155 warrants to purchase shares of the Company’s common stock that had been issued to a consultant and reissued 40,655 warrants to purchase shares of the Company’s common stock. These newly issued warrants have a grant date of January 6, 2020, and exercise price of $3.00 and expire on the third anniversary of the grant date.
On January 9, 2020, the Company entered into a consulting agreement for advisory services. As part of this agreement the Company will pay a monthly retainer of $4,000. The Company was obligated to issue 20,000 shares of its common stock within ten business days of January 1, 2020. Additionally, the Company will issue 30,000 shares of its commons stock on within ten business days of June 18, 2020 and 30,000 shares of its common stock within ten business days of December 17, 2020.
On January 10, 2020, the Company entered into a consulting agreement for advisory services. As part of this agreement the Company will issue 110,000 shares of its common stock within ten business days of January 10, 2020. Additionally, the Company will issue 110,000 shares of its common stock within ten business days of getting listed if the Company is successfully listed on Nasdaq Stock Market LLC or New York Stock Exchange prior to March 31, 2020.
On January 10, 2020, the Company entered into a bridge loan with a principle amount of $75,000. This loan does not accrue any interest. The note is due on the earlier of July 8, 2020 or in the event of default, as defined in the agreement. The note can convert into the same class of securities as those sold in the public offering with a conversion price of $2.00 per share. As of the date of this filing only $35,000 of the $75,000 principle has been received. The remaining $40,000 will be received upon the Company being approved for listing on the Nasdaq Stock Market LLC or the New York Stock Exchange.
PRELIMINARY PROSPECTUS
1,900,000 Units consisting of:
Common Stock
Warrants

Aditx Therapeutics, Inc.
PROSPECTUS
Sole Book-Running Manager
DAWSON JAMES SECURITIES, INC., 2024
Until , 2019 (25 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
, 2019
PART II—Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and DistributionDistribution.
The following table sets forth allWe estimate that expenses other than the underwriting discounts and commissions, payable by the registrant in connection with the distribution described in this registration statement (other than brokerage commissions, discounts or other expenses relating to the sale of the securities being registered. Allshares in this offering) will be as set forth below. We will pay all of the expenses with respect to the distribution, and such amounts, shown are estimates exceptwith the exception of the SEC registration fee and the FINRA filing fee.fee, are estimates.
| Amount to be paid | ||||
| SEC registration fee | $ | 3,308 | ||
| FINRA filing fee | $ | 2,000 | ||
| Nasdaq Capital Market initial listing fee | $ | 55,000 | ||
| Accounting fees and expenses | $ | 32,000 | ||
| Legal fees and expenses | $ | 250,000 | ||
| Printing and engraving expenses | $ | 10,000 | ||
| Miscellaneous | $ | 2,692 | ||
| Total | 355,000 | |||
| SEC expenses | $ | 2,693.17 | ||
| Legal fees and expenses | 25,000.00 | |||
| Accounting fees and expenses | 5,000.00 | |||
| Miscellaneous expenses | 5,000.00 | |||
| Total offering expenses (other than placement agent’s fees) | $ | 37,693.17 |
Item 14. Indemnification of Directors and Officers
Section 102 of theDelaware General Corporation Law of the State of Delaware (the “DGCL”) permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our amended and restated certificate of incorporation provides that no director of the CompanyAditxt shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Upon consummationOur Certificate of this offering, ourIncorporation as amended and restated certificate of incorporation and amended and restated bylaws willBylaws provide indemnification for our directors and officers to the fullest extent permitted by the DGCL. We will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our amended and restated certificate of incorporation and amended and restated bylaws will provide that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
Prior to the consummation of this offering, we intend to enter into separate indemnification agreements with each of our directors and executive officers. Each indemnification agreement will provide, among other things, for indemnification to the fullest extent permitted by law and our amended and restated certificate of incorporation and amended and restated bylaws against any and all expenses, judgments, fines, penalties and amounts paid in settlement of any claim. The indemnification agreements will provide for the advancement or payment of all expenses to the indemnitee and for the reimbursement to us if it is found that such indemnitee is not entitled to such indemnification under applicable law and our amended and restated certificate of incorporation and amended and restated bylaws.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act against certain liabilities.
Item 15. Recent Sales of Unregistered Securities
On January 31, 2022, the Company issued a consultant 2 shares of Common Stock for services rendered.
On February 28, 2022, the Company issued a consultant 2 shares of Common Stock for services rendered.
On June 27, 2022, the Company issued a consultant 768 shares of Common Stock for services rendered.
2017II-1
On December 7, 2022, the Company issued a consultant 3,729 shares of Common Stock for services rendered.
During 2017, weOn December 27, 2022, the Company issued a consultant 246 shares of Common Stock for services rendered.
On March 17, 2023, the securities below thatCompany issued a consultant 4,675 shares of Common Stock for services rendered.
The issuances above were not registered under the Securities Act. All of the securities discussed herein were issued in reliance onmade pursuant to Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder.Act.
On October 10, 2017,June 15, 2022, the Company issued 7,100,000 sharesentered into a letter agreement (the “Letter Agreement”) with a holder of certain of the Series C Warrants (the “Holder”). Pursuant to the Letter Agreement, the Holder has agreed to exercise in cash 4,486 of its common stock, including: (a) 3,500,000 sharesSeries C Warrants at a reduced exercise price of $300.00 per Share (reduced from $2,300.00 per Share), for gross proceeds to Sekris Biomedical, Inc. (“Sekris”), (b) 1,000,000 sharesthe Company of approximately $1.35 million. As an inducement to Dr. Shahrokh Shabahang, our co-founder and Chief Innovation Officer (c) 1,000,000 sharessuch exercise, the Company has agreed to Dr. Leonard Bailey,reduce the former Chairman of our board of directors and (d) 800,000 shares to Amro Albanna, our co-founder and Chief Executive Officer.
On March 8, 2018, we issued the Sekris Warrant to Sekris. The exercise price of the SekrisHolder’s remaining Series C Warrants to purchase up to 1,229 Shares from $2,300.00 to $495.80 per share (the “Amended Series C Warrant”). The Amended Series C Warrant is subject to adjustment in the eventwill be non-exercisable for a period of stock splits, stock dividends or similar events. Beginning in January 2018, we issued an aggregate principal amount of $100,000 of unsecured promissory notes to Sekris, which accrue interest at 4% and are due and payable six months from their respectivefollowing the closing date. In addition, the Company shall issue to the Holder a new warrant (the “New Warrant”) to purchase up to 10,200 shares of the Company’s common stock at an exercise price of $495.80 per share. The New Warrant will be non-exercisable for a period of six months following issuance dates or immediatelydate and have a term of five and one-half years. The Shares of common stock issuable upon an eventexercise of default.the Amended Series C Warrants are registered pursuant to the Company’s Registration Statement on Form S-3 (Registration No. 333-257645), which was initially filed with the Securities and Exchange Commission on July 2, 2021, and declared effective on July 13, 2021, and prospectus supplement thereto. The New Warrants were issued pursuant to Section 4(a)(2) of the Securities Act.
On March 15, 2018, we issued 50,000 shares of our common stock to LLU in considerationAugust 4, 2022, the Company entered into a Securities Purchase Agreement (the “SPA”) with certain accredited investors providing for the LLU License Agreement.
On March 17, 2018issuance and March 23, 2018, we entered into privatesale by the Company to the purchasers signatory thereto, of: (i) $1,477,777.78 in principal amount 10% Senior Secured Promissory Notes (the “August 2022 Notes”), resulting in gross proceeds to the Company of $1,330,000.00, exclusive of placement transactions with accredited investors pursuant to which we sold 125,000 sharesagent commission and 6,250fees and other offering expenses; (ii) 739 shares of common stock respectively, as commitment fees (the “August 2022 Commitment Shares”); and warrants (the “Warrants”) to purchase up to 3,138 shares (the “August 2022 Warrant Shares”) of the Company’s common stock (together with the August 2022 Notes, the August 2022 Commitment Shares and the August 2022 Warrant Shares, the “August 2022 Securities”).
The August 2022 Notes have a maturity date of twelve (12) months from the date of issuance and are convertible at the option of the Investor at any time prior to maturity in shares of Common Stock (the “Conversion Shares”) at an initial conversion price of $471.00 per share, subject to adjustment under certain circumstances. The holders of the aggregate, 131,250August 2022 Notes have the right, following any calendar day following the Commencement Date (as defined therein) to convert all or any portion of the then outstanding and unpaid principal amount and interest into fully paid and non-assessable shares of common stock for aggregate gross proceedsat the conversion price. The Company is prohibited from effecting a conversion of $262,500 (warrantsthe Note to purchase 125,000the extent that, as a result of such exercise, the Investor, together with the its affiliates, would beneficially own more than 4.99% of the number of shares of common stock were issued on March 17, 2018, and warrantsoutstanding immediately after giving effect to purchase 6,250the issuance of such shares. In addition, the sum of the aggregate number of shares of common stock werethat may be issued to all Investors under the August 2022 Securities is limited to 19.99% of the Company’s then outstanding shares of common stock as of the date of issuance unless Shareholder Approval (as defined in the SPA) is obtained to issue more than the 19.99%.
The August 2022 Warrants are exercisable for a period of five (5) years from period commencing on March 23, 2018). All such warrants havethe Commencement Date (as defined therein) and ending on 5:00 p.m. eastern standard time on the date that is five (5) years after the date of issuance, at an exercise price of $2.00 and expire three years$471.00, subject to adjustment provided therein (including cashless exercise). The Company is prohibited from their respectiveeffecting an exercise of the Warrants to the extent that, as a result of such exercise, the holder of the Warrant together with the holder’s affiliates, would beneficially own more than 4.99% of the number of shares of Common Stock of the Company outstanding immediately after giving effect to the issuance of the such shares. In addition, the sum of the aggregate number of shares of common stock that may be issued to all Investors under the August 2022 Securities is limited to 19.99% of the Company’s then outstanding shares of common stock as of the date of issuance (the “Private Placement Warrants”).
On June 8, 2018, we issued 3,000 shares of our common stockunless Shareholder Approval (as defined in the SPA) is obtained to a consultant ofissue more than the Company in compensation for videography services to be rendered to the Company.19.99%.
II-2
On October 10, 2017, we granted stock optionsIn connection with the Offering, the Company also entered into a registration rights agreement (the “Registration Rights Agreement”) with the Investors pursuant to Rod Turner, an advisor, to purchase 60,000which the Company shall prepare and file with the U.S. Securities and Exchange Commission (the “SEC”) a registration statement (the “Registration Statement”) covering the Note, the Conversion Shares, the Warrant, and the Warrant Shares and any additional shares of commonCommon Stock issued and issuable in connection with any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing (collectively, the “Registrable Securities”) on or prior to the 90th calendar day following the Closing Date (the “Filing Date”).
The Company shall use its best efforts to cause the registration statement covering the Registrable Securities to be declared effective (the “Effectiveness Date”) by the SEC within one hundred twenty (120) calendar days from the date hereof (or at an exercise price of $2.00 per share, with a five-year expiration date. Mr. Turner’s service was terminated in January 2018, and only 20,000 shares of common stock underlying such option vested. Such options were issued under our 2017 Equity Incentive Plan (our “2017 Plan”). On November 1, 2017, we granted stock optionsthe earliest possible date if prior to Gordon Winston, an advisor, and David Briones, our interim chief financial officer, to purchase an aggregate of 180,000 shares of common stock at an exercise price of $2.00 per share. Mr. Winston’s service was terminated on June 30, 2018 and such termination resulted inone hundred twenty (120) calendar days from the forfeiture of 20,000 of his stock options. Such options vest monthly over the term of one year and have a five-year expiration date. Such options were issued under our 2017 Plan. On February 9, 2018, we granted stock options to Rowena Albanna, a contractor, to purchase 100,000 shares of common stock at an exercise price of $2.00 per share. Such options were fully vested and immediately exercisable asdate of the grant date. On March 6, 2018, we granted stock options to David Alleva, an advisor, to purchase 300,000 shares of common stock at an exercise price of $2.00 per share. Such options vest one-third on each of September 30, 2018, September 30, 2019 and September 30, 2020 and have a five-year expiration date. Additionally, on March 6, 2018, we granted stock options to Amro Albanna, our CEO and Adrian Luchian, an advisor, to purchase an aggregate of 400,000 and 25,000 respectively shares of common stock at an exercise price of $2.00 per share. Such options were fully vested and immediately exercisable as of the grant date and have a five-year expiration date. All such options were issued under our 2017 Plan.
2018
During 2018, we issued the securities below that were not registered under the Securities Act. All of the securities discussed herein were issued in reliance on Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. Registration Rights Agreement.
In April 2018, we issued warrantsconnection with the Offering, the Company will issue 157 shares (the “Placement Agent Shares”) to purchase up to 420,000 sharesCrito Capital LLC.
The Company estimates that the net proceeds from the transaction will be approximately $1,244,000 after deducting estimated transaction fees and expenses. The net proceeds received by the Company from the transaction will be used for business development, working capital and other general corporate purposes.
The August 2022 Notes contain certain covenants, events of common stock to two (2) consultantsdefault and triggering events, which would require repayment of the Company.obligations outstanding pursuant to such instruments. The obligations of the Company pursuant to the August 2022 Notes are secured by certain assets of the Company pursuant to the Security Agreement dated August 4, 2022, by and among the Company and the holders of the August 2022 Notes.
In July 2018, weOn August 11, 2022 and September 12, 2022, the Company entered into private placement transactionsadditional SPAs with certain additional accredited investors (collectively, the “Subsequent Investors” and together with the August Investors, the “Investors”), pursuant to which wethe Company issued and sold an additional: (i) promissory notes which have$1,111,110 in principal amounts,amount of the August 2022 Notes, resulting in the aggregate of $145,600, and (ii) warrants to purchase, in the aggregate, 18,667 shares of common stock (the “July Private Placement Warrants”) for aggregate gross proceeds to the Company of $112,000. All such warrants have$1,000,000, exclusive of placement agent fees and other offering expenses; (ii) 556 August 2022 Commitment Shares; and (iii) August 2022 Warrants to purchase 2,384 shares of the Company’s common stock.
On August 31, 2022, the Company entered into a First Amendment and Waiver with each of the August Investors (the “August Amendment”). Pursuant to the August Amendment, the exercise price of the August 2022 Warrants was reduced to $300.00 per share and the Commencement Date (as defined in the SPA) was amended to mean the date on which the Company obtains shareholder approval for the issuance of any shares of common stock upon exercise of the August 2022 Warrants. The Warrant is exercisable for a period of five (5) years from period commencing on the Commencement Date (as defined therein) and ending on 5:00 p.m. eastern standard time on the date that is five (5) years after the date of issuance, at an exercise price of $6.00 per share and expire three (3) years$300.00, subject to adjustment provided therein (including cashless exercise). The Company is prohibited from their respectiveeffecting an exercise of the Warrant to the extent that, as a result of such exercise, the holder of the Warrant together with the holder’s affiliates, would beneficially own more than 4.99% of the number of shares of Common Stock of the Company outstanding immediately after giving effect to the issuance of such shares. In addition, the sum of the aggregate number of shares of common stock that may be issued under the Warrant is limited to 19.99% of the Company’s then outstanding shares of common stock as of the date of issuance.issuance unless Shareholder Approval (as defined in the Agreement for the Purchase and Sale of Future Receipts) is obtained to issue more than the 19.99%.
On August 23, 2018, we issued 20,000 shares of our common stock to a consultant in compensation for services to be rendered to31, 2022, the Company.
On August 29, 2018, weCompany entered into an Agreement for the Purchase and Sale of Future Receipts with a private placement transaction with an accredited investorcommercial funding source (the “Funder”) pursuant to which we sold (i) a promissory note which has principal amounts,the Company agreed to sell to the Funder certain future trade receipts in the aggregate of $45,500, and (ii) a warrant to purchase 5,834 shares of our common stockamount $288,000 (the “August Private Placement Warrants”“Purchased Amount”) for aggregate gross proceeds to the Company of $35,000.$200,000, less origination fees of $20,000. Pursuant to the Agreement, the Company granted the Funder a security interest in all of the Company’s present and future accounts receivable in an amount not to exceed the Purchased Amount. The Purchased Amount shall be repaid by the Company in 20 weekly installments of approximately $14,400. In connection with the Agreement, the Company also issued a warrant has an exercise price of $6.00 per share and expires three (3) years from its date of issuance.
In November 2018, we entered into private placement transactions with accredited investors pursuant to which we sold 54,900purchase 667 shares of the Company’s common stock and warrants to purchase, in the aggregate, 54,900 shares of common stock for aggregate gross proceeds of $109,800. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
In December 2018, we entered into private placement transactions with accredited investors pursuant to which we sold 163,700 shares of common stock and warrants to purchase, in the aggregate, 163,700 shares of common stock for aggregate gross proceeds of $327,400. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
On December 1, 2018, we issued 5,000 shares of common stock to a director of the Company.
2019
During 2019, we issued the securities below that were not registered under the Securities Act. All of the securities discussed herein were issued in reliance on Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder.
During 2019, we have issued a total of 515,155 warrants to purchase common stock to twelve (12) consultants of the Company.
In January 2019, we entered into private placement transactions with accredited investors pursuant to which we sold 40,000 shares of common stock and warrants to purchase, in the aggregate, 40,000 shares of common stock for aggregate gross proceeds of $80,000.
In February 2019, we issued 1,400,000 option to purchase shares of common stock to three related parties. These options are fully vested on issuance.
stock.
II-3
In February 2019, weOn April 20, 2023, the Company entered into private placement transactionsa securities purchase agreement (the “Purchase Agreement”) with accredited investorsan institutional investor, pursuant to which we sold 26,500the Company agreed to sell to such investor pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 39,634 shares of common stock and warrants toof the Company (the “Common Stock”) at a purchase in the aggregate, 26,500price of $48.76 per Pre-Funded Warrant. The Pre-Funded Warrants (and shares of common stock underlying the Pre-Funded Warrants) were offered by the Company pursuant to its shelf registration statement on Form S-3 (File No. 333-257645), which was declared effective by the Securities and Exchange Commission on July 13, 2021.
Concurrently with the sale of the Pre-Funded Warrants, pursuant to the Purchase Agreement in a concurrent private placement, for aggregate gross proceedseach Pre-Funded Warrant purchased by the investor, such investor received from the Company an unregistered warrant (the “Warrant”) to purchase two shares of $53,000. All suchCommon Stock. The warrants have an exercise price of $3.00$34.40 per share, and expireare exercisable for a three years from their respective dateyear period. The Warrant was issued were made pursuant to Section 4(a)(2) of issuance.the Securities Act.
On February 1, 2019, we issued 20,000 shares of common stock to a consultant of the Company.
In March 2019, we entered into private placement transactions with accredited investors pursuant to which we sold 45,000 shares of common stock and warrants to purchase, in the aggregate, 45,000 shares of common stock for aggregate gross proceeds of $90,000. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
In April 2019, we entered into private placement transactions with accredited investors pursuant to which we sold 9,000 shares of common stock and warrants to purchase, in the aggregate, 9,000 shares of common stock for aggregate gross proceeds of $18,000. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
On April 24, 2019, we issued 5,000 shares of common stock to a consultant of the Company.
On May 6, 2019,July 3, 2023, Aditxt, Inc. we entered into a private placement transactionSecurities Purchase Agreement (the “First Tranche Securities Purchase Agreement”) with an accredited investor pursuant to which we issued and sold 12,500a secured promissory note in the principal amount of $375,000 (the “First Tranche Note”) resulting in gross proceeds of $250,000. In connection with the issuance of the Note, we issued 3,907 shares of common stock (the “First Tranche Commitment Shares”) as a commitment fee to the investor. Pursuant to the Securities Purchase Agreement, we are obligated to obtain approval of our shareholders (“Shareholder Approval”) with respect to the issuance of any securities in connection with the Securities Purchase Agreement and warrantsthe Note in excess of 19.99% of our issued and outstanding shares on the closing date, which is equal to purchase,33,791 shares of our common stock. The First Tranche Note has a maturity date of December 31, 2023 and is convertible following Shareholder Approval and the occurrence of an Event of Default (as defined in the aggregate, 12,500 shares of common stock for aggregate gross proceeds of $25,000. All such warrants have an exerciseNote) at a conversion price of $3.00 and expire three years from their respective date of issuance.$18.00 per share.
On June 28, 2019,In connection with the Securities Purchase Agreement and the issuance of the First Tranche Note, we and certain of our subsidiaries also entered into a Security Agreement with the investor (the “First Tranche Security Agreement”) pursuant to which we granted the investor a security interest in certain Collateral (as defined in the First Tranche Security Agreement) to secure our obligations under the First Tranche Note. In addition, we entered into a private placement transactionRegistration Rights Agreement with the investor (the “First Tranche Registration Rights Agreement”) pursuant to which we agreed to prepare and file with the U.S. Securities and Exchange Commission a registration statement covering the resale of the First Tranche Commitment Shares and any shares of our Common Stock issuable upon conversion of the First Tranche Note within 120 days of the closing date and to have such registration statement declared effective within 150 days of the closing date.
On July 3, 2023, we entered into a Business Loan and Security Agreement (the “July Loan Agreement”) with a commercial funding source (the “July Lender”), pursuant to which we obtained a loan from the July Lender in the principal amount of $215,000, which includes origination fees of $10,750 (the “July Loan”). Pursuant to the July Loan Agreement, we granted the Lender a continuing secondary security interest in certain collateral (as defined in the Loan Agreement). The total amount of interest and fees payable by us to the July Lender under the July Loan will be (i) $322,285 and will be repaid in 13 weekly installments of $24,500 with a final payment of $3,785 in the fourteenth week.
On July 11, 2023, we entered into a Subscription and Investment Representation Agreement (the “Subscription Agreement”) with Amro Albanna, our Chief Executive Officer, who is an accredited investor (the “Purchaser”), pursuant to which we agreed to issue and sell one (1) share of the Company’s Series C Preferred Stock, par value $0.001 per share (the “Preferred Stock”), to the Purchaser for $1,000.00 in cash. The sale closed on July 11, 2023.
On July 11, 2023, we filed a certificate of designation (the “Certificate of Designation”) with the Secretary of State of Delaware, effective as of the time of filing, designating the rights, preferences, privileges and restrictions of the share of Preferred Stock. The Certificate of Designation provides that the share of Preferred Stock will have 250,000,000 votes and will vote together with the outstanding shares of our Common Stock as a single class exclusively with respect to any proposal to amend the Company’s Amended and Restated Certificate of Incorporation to effect a reverse stock split of our Common Stock. The Preferred Stock will be voted, without action by the holder, on any such proposal in the same proportion as shares of Common Stock are voted. The Preferred Stock otherwise has no voting rights except as otherwise required by the General Corporation Law of the State of Delaware.
II-4
The Preferred Stock is not convertible into, or exchangeable for, shares of any other class or series of stock or other securities of the Company. The Preferred Stock has no rights with respect to any distribution of assets of the Company, including upon a liquidation, bankruptcy, reorganization, merger, acquisition, sale, dissolution or winding up of the Company, whether voluntarily or involuntarily. The holder of the Preferred Stock will not be entitled to receive dividends of any kind.
The outstanding share of Preferred Stock shall be redeemed in whole, but not in part, at any time (i) if such redemption is ordered by the Board of Directors in its sole discretion or (ii) automatically upon the effectiveness of the amendment to the Certificate of Incorporation implementing a reverse stock split. Upon such redemption, the holder of the Preferred Stock will receive consideration of $1,000.00 in cash.
On July 24, 2023, we entered into a Securities Purchase Agreement (the “Second Tranche Securities Purchase Agreement”) with an accredited investor pursuant to which the Company issued and sold a secured promissory note in the principal amount of $2,625,000 (the “Second Tranche Note”) resulting in gross proceeds to the Company of $1,750,000. In connection with the issuance of the Note, we sold 10,000agreed to issue a total of 27,344 shares of common stockCommon Stock (the “Second Tranche Commitment Shares”) as a commitment fee to the investor. At the request of the investor, we issued 17,277 Second Tranche Commitment Shares and will issue the remaining 10,066 Second Tranche Commitment Shares within 120 days, subject to the investor’s discretion. Pursuant to the Second Tranche Securities Purchase Agreement, we are obligated to obtain approval of our shareholders with respect to the issuance of any securities in connection with the Second Tranche Securities Purchase Agreement and the Second Tranche Note in excess of 19.99% of our issued and outstanding shares on the closing date, which is equal to 38,026 shares of the Company’s Common Stock. The Second Tranche Note has a maturity date of December 31, 2023 and is convertible following shareholder approval and the occurrence of an Event of Default (as defined in the Note) at a conversion price of $15.60 per share.
In connection with the Second Tranche Securities Purchase Agreement and the issuance of the Second Tranche Note, we and certain of our subsidiaries also entered into a Security Agreement with the investor (the “Second Tranche Security Agreement”) pursuant to which we granted the investor a security interest in certain Collateral (as defined in the Second Tranche Security Agreement) to secure its obligations under the Second Tranche Note. In addition, we entered into a Registration Rights Agreement with the investor (the “Second Tranche Registration Rights Agreement”) pursuant to which we agreed to prepare and file with the U.S. Securities and Exchange Commission a registration statement covering the resale of the Second Tranche Commitment Shares and any shares of our Common Stock issuable upon conversion of the Second Tranche Note within 90 days of the closing date and to have such registration statement declared effective within 120 days of the closing date.
On August 23, 2023, we entered into a Business Loan and Security Agreement (the “August Loan Agreement”) with a commercial funding source (the “August Lender”) pursuant to which we obtained a loan from the August Lender in the principal amount of $1,400,000, which will satisfy the outstanding balances on loans that we originally obtained From the August Lender in April 2023 and July 2023, and includes origination fees of $70,000 (the “August Loan”). Pursuant to the August Loan Agreement, we granted the August Lender a continuing secondary security interest in certain collateral (as defined in the August Loan Agreement). The total amount of interest and fees payable by us to the August Lender under the August Loan will be $2,079,000, which will be repaid in 21 weekly installments of $99,000.
On August 31, 2023, we entered into a securities purchase agreement (the “Purchase Agreement”) with an institutional investor for the issuance and sale in a private placement (the “Private Placement”) of (i) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 1,000,000 shares of the Company’s Common Stock, par value $0.001 (the “Common Stock”), at an exercise price of $0.001 per share, and (ii) warrants (the “Common Warrants”) to purchase up to 1,000,000 shares of the Company’s Common Stock at an exercise price of $10.00 per share.
II-5
The Common Warrants are exercisable immediately upon issuance and have a term of exercise equal to five and one-half years from the date of issuance. The Pre-Funded Warrants are exercisable immediately and may be exercised at any time until the Pre-Funded Warrants are exercised in full. A holder of Pre-Funded Warrants or Warrants (together with its affiliates) may not exercise any portion of a warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder 9.99%) of the Company’s outstanding Common Stock immediately after exercise.
In connection with the Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”), dated as of August 31, 2023, with the investor, pursuant to which the Company agreed to prepare and file a registration statement with the Securities and Exchange Commission (the “SEC”) registering the resale of the shares of Common Stock underlying the Pre-Funded Warrants and the Common Warrants no later than 15 days after the date of the Registration Rights Agreement, and to use best efforts to have the registration statement declared effective as promptly as practical thereafter, and in any event no later than 45 days following the date of the Registration Rights Agreement (or 75 days following the date of the Registration Rights Agreement in the aggregate, 10,000 sharesevent of common stock fora “full review” by the SEC).
The Private Placement closed on September 6, 2023. We received net proceeds from the Private Placement of approximately $9 million, after deducting placement agent fees and expenses and estimated offering expenses payable by us.
H.C. Wainwright & Co., LLC (“Wainwright”) served as our exclusive placement agent in connection with the Private Placement, pursuant to those certain engagement letters, dated as of March 27, 2023 and April 25, 2023, as amended, between the Company and Wainwright (the “Engagement Letter”). Pursuant to the Engagement Letter, we paid Wainwright (i) a total cash fee equal to 7.75% of the aggregate gross proceeds of $20,000. All suchthe Private Placement, (ii) a management fee of 1.0% of the aggregate gross proceeds of the Private Placement, (iii) a non-accountable expense allowance of $50,000, and (iv) $100,000 for legal fees and other out-of-pocket expenses. In addition, we issued to Wainwright or its designees warrants have(the “Placement Agent Warrants”) to purchase up to an aggregate of 60,000 shares of Common Stock at an exercise price equal to $12.50 per share. The Placement Agent Warrants are exercisable immediately upon issuance and have a term of $3.00exercise equal to five and expire threeone-half years from their respectivethe date of issuance.
On June 30 2019, we issued 10,000 shares of common stock to a director of the Company for services rendered to the Company.
In July 2019, we entered into private placement transactions with accredited investors pursuant to which we sold 15,000 shares of common stock and warrants to purchase, in the aggregate, 15,000 shares of common stock for aggregate gross proceeds of $30,000. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
On August 2, 2019, we entered into a private placement transaction with an accredited investor pursuant to which we sold 4,950 shares of common stock and warrants to purchase, in the aggregate, 4,950 shares of common stock for aggregate gross proceeds of $9,900. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
On SeptemberDecember 19, 2019, we entered into a private placement transaction with an accredited investor pursuant to which we sold 50,000 shares of common stock and warrants to purchase, in the aggregate, 50,000 shares of common stock for aggregate gross proceeds of $100,000. All such warrants have an exercise price of $3.00 and expire three years from their respective date of issuance.
On October 1, 2019, we entered into an agreement for consulting services. As part of this agreement we have issued 20,000 warrants to purchase share of the Company’s common stock to the consultant.
During October and November 2019, we received $100,000 in cash for shares of common stock pursuant to a private placement at $2.00 per share, selling a total of 50,000 shares. The Company also agreed to issue one warrant to purchase a share of common stock for each share purchased in the private placement. The warrant is exercisable at $3.00 per share and has a term of three years.
On November 1, 2019, we issued a convertible promissory note with a principle amount of $50,000. This note does not accrue any interest. The note is due on the earlier of April 28, 2020 or in the event of default, as defined in the agreement. The note is convertible into the same class of securities as those sold in a qualified public offering with a conversion price of $2.00 per share.
On December 18, 2019, we issued 10,000 warrants to purchase shares of our common stock. These warrants have a grant date of December 18, 2019, an expiration date of October 5, 2027, and an exercise price of $2.00.
On December 27, 2019, we entered into an agreement for consulting services. As part of this agreement we issued 20,000 shares of our common stock to the consultant.
On January 9, 2020, we entered into an agreement for consulting services. As part of this agreement we issued 20,000 shares of our common stock to the consultant.
On January 5, 2020, we cancelled 38,155 warrants to purchase shares of the Company’s common stock that had been issued to a consultant and reissued 40,655 warrants to purchase shares of the Company’s common stock. These newly issued warrants have a grant date of January 6, 2020, and exercise price of $3.00 and expire on the third anniversary of the grant date.
On January 10, 2020,2023, we entered into a consulting agreement (the “Consulting Agreement”) with an independent consultant for a term of ninety days. Pursuant to the Consulting Agreement, the independent consultant agreed to provide the Company with business advisory services. As part of this agreement we issued 110,000services, guidance on growth strategies and networking with its clients on a non-exclusive basis for general business purposes (the “Services”). In consideration for the Services, the Company will issue to the independent consultant 70,000 shares of our common stockCommon Stock. The issuance of the Shares will not be registered under the Securities Act or the securities laws of any state, in reliance on the exemption from registration under the Securities Act, as provided by Section 4(a)(2) thereof.
On December 22, 2023, we entered into an Exchange Agreement with the holders of an aggregate of 22,280 shares of Series F-1 Convertible Preferred Stock of Evofem, pursuant to which such holders agreed to exchange their respective shares of Evofem Series F-1 Preferred Stock for an aggregate of 22,280 shares of our Series A-1 Convertible Preferred Stock, $0.001 par value. We issued the Series A-1 Convertible Preferred in reliance upon the exemption from registration provided by Section 4(a)(2) of the Securities Act and/or Rule 506(b) of Regulation D promulgated thereunder.
On December 29, 2023, we entered into an Exchange Agreement with the holder of our secured promissory note in the principal amount of $2.625 million (the “Note”), pursuant to which the holder agreed, subject to the consultant.terms and conditions set forth therein, to exchange the Note, including all accrued but unpaid interest thereon, for an aggregate of 2,625 shares of a new series of convertible preferred stock of the Company, designated as Series B-2 Convertible Preferred Stock. We issued the Series B-2 Convertible Preferred in reliance upon the exemption from registration provided by Section 4(a)(2) of the Securities Act and/or Rule 506(b) of Regulation D promulgated thereunder.
On January 10, 2020,4, 2024, we issued a convertible promissory note with a principle amount of $75,000. This note does not accrue any interest. The note is due on the earlier of July 8, 2020 or in the event of default, as defined in the agreement. The note can convert into the same class of securities as those sold in the public offering with a conversion price of $2.00 per share. Ascompleted our acquisition of the dateAcquired Assets of this filing only $35,000MDNA and issued to MDNA (i) 50,000 shares of our Common Stock, (ii) warrants to purchase 50,000 shares of our Common Stock, and (iii) 5,000 shares of Series A Preferred Stock, par value $0.001 per share of Pearsanta. The securities were issued in reliance upon the exemption from registration provided by Section 4(a)(2) of the $75,000 principle has been received. The remaining $40,000 will be received upon the Company being approved for listing on the Nasdaq Stock Market LLC or the New York Stock Exchange.
Securities Act
II-4II-6
Item 16. Exhibits and Financial Statement SchedulesSchedules.
EXHIBIT INDEX(a) Exhibits.
II-7
II-8
II-9
II-10
II-11
| * | Filed herewith |
* To be filed by amendment.
| ** | To be filed by amendment |
| † | Executive Compensation Plan or Agreement |
| # | Portions of this exhibit (indicated by asterisks) have been redacted in compliance with Regulation S-K Item 601(b)(10)(iv). |
II-12
Item 17. Undertakings.
Financial Statement SchedulesThe undersigned registrant hereby undertakes:
| 1. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to: |
Schedules have been omitted because
| i. | Include any prospectus required by Section 10(a)(3) of the Securities Act. |
| ii. | Reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| iii. | Include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that paragraphs (1)(i), (ii) and (iii) do not apply if the information required to be set forth thereinincluded in a post-effective amendment by those paragraphs is not applicablecontained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement, or is showncontained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
| 2. | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof; |
| 3. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; |
| 4. | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the financial statements or notes thereto.
Item 17. Undertakingsregistration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange CommissionSEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
II-5II-13
II-6
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Loma Linda,Richmond, State of California,Virginia, on the 1518thday of January, 2020.2024.
| By: | /s/ Amro Albanna | ||
| Name: | Amro Albanna | ||
| Title: | Chief Executive Officer | ||
We, the undersigned officers and directors of Aditx Therapeutics,Aditxt, Inc., hereby severally constitute and appoint Amro Albanna, our true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for us and in our stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement and all documents relating thereto, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing necessary or advisable to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or his substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
WITNESS our hands and common seal on the dates set forth below.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated below.
| Signature | Title | Date | ||
| /s/ Amro Albanna | Chief Executive Officer | January | ||
| Amro Albanna | (Principal Executive | |||
| /s/ | Chief Financial Officer | January | ||
| (Principal Financial and Accounting | ||||
| /s/ Brian Brady | Director | January 18, 2024 | ||
| Brian Brady | ||||
| /s/ Charles Athle Nelson | Director | January 18, 2024 | ||
| Charles Athle Nelson | ||||
| Director | January 18, 2024 | |||
| Jeffrey W. Runge, M.D. | ||||
| /s/ Shahrokh Shabahang | ||||
| Chief Innovation Officer and Director | January 18, 2024 | |||
| Shahrokh Shabahang | ||||
II-7II-14