
As filed with the Securities and Exchange Commission on OctoberSeptember 9, 2020.2021
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Virpax Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 82-1510982 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer | ||
Identification Number) |
1554 Paoli Pike, #2791055 Westlakes Drive, Suite 300
West Chester,Berwyn, PA 1938019312
(610) 727-4597
(484) 880-4588
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Anthony Mack
Chief Executive Officer
1055 Westlakes Drive, Suite 300
1554 Paoli Pike, #279
West Chester,Berwyn, PA 1938019312
(610) 727-4597
(484) 880-4588
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||
| Michael J. Lerner Steven M. Skolnick Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 (212) 262-6700 | Jeffrey J. Fessler | |
Sheppard, Mullin, Richter & Hampton LLP | ||
30 Rockefeller Plaza | ||
New York, NY 10112 | ||
(212) 634-3067 | ||
Approximate date of commencement of proposed sale to public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to Be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee(2) | ||||||
| Common Stock, $0.00001 par value per share (3) | $ | 17,250,000.00 | $ | 1,881.98 | ||||
| Underwriter’s warrant (4) | — | — | ||||||
| Common Stock underlying underwriter’s warrant (5) | $ | 1,078,125.00 | $ | 117.62 | ||||
| Total | $ | 18,328,125.00 | $ | 1,999.60 | ||||
| Title of Each Class of Securities to Be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee(2) | ||||||
| Common Stock, $0.00001 par value per share(3) | $ | 57,500,000 | $ | 6,273.25 | ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
| (3) | Includes shares of common stock which may be issued on exercise of a |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information contained in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED |
Shares
Common Stock

Virpax Pharmaceuticals, Inc
This is a firm commitment initial public3,401,360 Shares
Common Stock

| Virpax Pharmaceuticals, Inc. |
We are offering of3,401,360 shares of Virpax Pharmaceuticals, Inc. common stock. Prior to this offering, there has been no public market for our common stock. We anticipate that the initial public offering price of our common stock will be between $ and $ per share.on a firm commitment basis.
We have applied to list our shares ofOur common stock for tradingis listed on the Nasdaq Capital Market under the symbol “VRPX.” IfThe last reported sale price of our common stock is not approved for listing on the NASDAQNasdaq Capital Market we will not consummate this offering. No assurance can be given that our application will be approved.on September 8, 2021 was $14.70 per share.
We are an emerging growth company under the Jumpstart our Business Startups Act of 2012, or JOBS Act, and, as such, may elect to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 1114 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| $ | $ | |||||||
| Underwriting discounts and commissions | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
The underwriters may also exercise their option to purchase up to 510,204 additional shares from us at the public offering price, less the underwriting discounts and commissions, for 45 days after the date of this prospectus to cover over-allotments, if any.
The underwriters expect to deliver our shares in the offeringof common stock on or about , 2020.2021.
ThinkEquity
a division of Fordham Financial Management, Inc.
The date of this prospectus is , 20202021
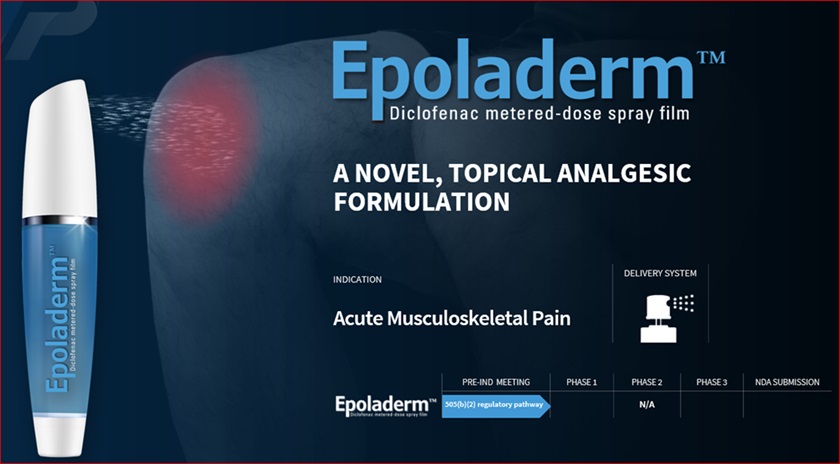




TABLE OF CONTENTS
We have not authorized anyone to provide you with different information, and we take no responsibility for any other information others may give you. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus.
No action is being taken in any jurisdiction outside the United States to permit a public offering of our common stock or possession or distribution of this prospectus in that jurisdiction. Persons who come into possession of this prospectus in jurisdictions outside the United States are required to inform themselves about and to observe any restrictions as to this offering and the distribution of this prospectus applicable to that jurisdiction.
We and the underwriters are offering to sell, and seeking offers to buy, our common shares only in jurisdictions where offers and sales are permitted. Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of our common shares and the distribution of this prospectus outside of the United States.
i
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider before making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our financial statements and the related notes thereto and the information set forth in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” If any of the risks materialize, our business, financial condition, operating results and prospects could be materially and adversely affected. In that event, the price of our common stock could decline, and you could lose part or all of your investment. Unless the context otherwise requires, we use the terms “Virpax,” “company,” “we,” “us” and “our” in this prospectus to refer to Virpax Pharmaceuticals, Inc.
Our Company
We are a preclinical stage biopharmaceuticalpreclinical-stage pharmaceutical company focused on becoming a global leader in pain management by developing and delivering innovative non-opioid and non-addictive pharmaceutical products using new drug delivery systems and technology. We are developing branded pharmaceutical product candidates forwith a focus on pain management by using advancedcutting-edge technology in an effort to enhance patients’ quality of life.
We have exclusive global rights to develop, sell and export (among other rights) a proprietary patented nonsteroidnonsteroidal anti-inflammatory Topical Spray Film Delivery Technology for acute musculoskeletal pain (“DSF100” or “EpoladermTM”) and for chronic osteoarthritis of the knee (“OSF200”). We also have exclusive global rights to a proprietary patented injectable, long-acting, “local anesthetic”local anesthetic Liposomal (Hydro) Gel Technology for postoperative pain management (“LBL100” or “ProbudurTM”“Probudur”). Additionally, we have exclusive global rights to a proprietary patented Molecular Envelope Technology (“MET”) that uses an intranasal device to deliver exogenous enkephalin for the management of acute and chronic pain, including pain associated with cancer (“NES100”Envelta”) and for the management of Post-Traumatic Stress Disorder (“PES200”). Enkephalins are pain-relieving pentapeptides produced in the body, and function to inhibit neurotransmitters in the pathway for pain perception, thereby reducing the physical impact of pain. While the Company is currently focused on the development of its non-opioid and non-addictive pain management pipeline of product candidates, the Company also plans on using its proprietary delivery technologies to develop anti-viral therapies (“AnQlar”) as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19).
We believe the Topical Spray Film Delivery Technology could provide a pathway for additional proprietary spray formulations and could potentially evolve into the preferred therapeutic treatment for topicals and transdermal deliveries due to itswith strong adhesion and accessibility properties, especially around joints and curved body surfaces. Pursuant to a Researchresearch and Option Agreement we have entered into with MedPharm Limitedoption agreement (the “MedPharm Research and Option Agreement”), with MedPharm Limited, (“MedPharm”), MedPharm will conduct certain research and development activities of proprietary formulations incorporating certain MedPharm technologies and certain of our proprietary molecules. These proprietary molecules relate to indications which include, but are not limited to, treatment of estrogen levels, Alzheimer’s disease, dementia, Parkinson’s disease, neuropathic issues, and acute and chronic pain. Under the agreement, we were granted an option to obtain an exclusive, world-wide, sub-licensable, royalty bearing, irrevocable license to research, develop, market, use, commercialize, and sell any product utilizing MedPharm’s spray formulation technology. See “Material Agreements” below for more information concerning this research and option agreement.
We believe NES100 and PES200 would support the current effort among prescribers, regulators, and patients to seek non-addictive treatment options to combat the opioid epidemic. We plan to utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and new chemical entity (“NCE”) product candidates for commercialization.
While we are currently focused on the development of our non-opioid and non-addictive pain management pipeline of product candidates, we also plan on using our proprietary delivery technologies to develop anti-viral therapies as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19). As of the date of this prospectus, activities related to our anti-viral therapies have not commenced. We plan to finance our anti-viral-related activities through the use of grants and do not plan on using any proceeds from this offering.
Our Portfolio
Our portfolio currently consists of sixmultiple preclinical stage product candidates: Epoladerm, OSF200, Probudur, NES100,Envelta, PES200 and MMS019.AnQlar. In the accompanying section we will describe each product candidate, its benefits, and our market strategy for each product candidate. The dates reflected in the below table are estimates only, and there can be no assurances that the events included in the table will be completed on the anticipated timeline presented, or at all.
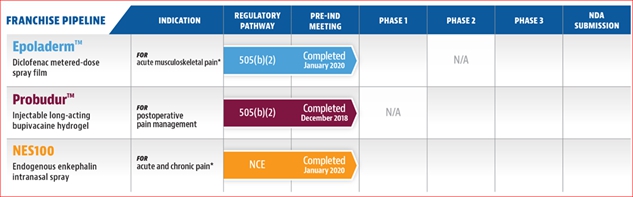

* We are also developing Epoladerm for a second indication, OSF200, which utilizes the same transdermal delivery system as Epoladerm, as a twice daily topical treatment for chronic osteoarthritis of the knee. OSF200 development plan is pending the approval of Epoladerm. In addition, we are also developing NES100 for a second indication, PES200, which utilizes the same delivery mechanism as NES100. PES200 enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain for post-traumatic stress disorder. PES200 development plan is pending a grant approval. Further, we recently entered into a Collaboration and License Agreement with Nanomerics Ltd. for the exclusive North American license to develop and commercialize a High-Density Molecular Masking Spray (“MMS019”) as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans. We plan to develop MMS019 primarily through grants and do not plan on using any proceeds from this offering.
1
| 1,2,3 | We are also developing Envelta for a second indication, PES200, which utilizes the same delivery mechanism as Envelta. PES200 enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain for the treatment of post-traumatic stress disorder. The Envelta IND enabling studies may be cross-referenced to the PES200 IND. We are also developing Epoladerm for a second indication, OSF200, which utilizes the same transdermal delivery system as Epoladerm, as a twice daily topical treatment for chronic osteoarthritis of the knee. OSF200’s development plan is pending the approval of Epoladerm. AnQlar has completed IND-enabling toxicology studies and ex-vivo studies demonstrated a reduction in infectivity from respiratory viruses like influenza and SARS-CoV-2. AnQlar also reduced nasal and viral load in its animal study. |
Diclofenac Epolamine Metered-Dose Spray Film (EpoladermTM)
We believe our Diclofenac Epolamine metered-dose spray film technology, which we refer to as Epoladerm, could provide a pathway for additional proprietary spray formulations with strong adhesion and accessibility properties upon application, especially around joints and curved body surfaces. We plan to develop and market Epoladerm as a topical nonsteroidal anti-inflammatory drug (“NSAID”) treatment for acute pain. We believe Epoladerm’s proprietary spray film technology may lead to adhesion capabilities superior to those of transdermal patches (e.g. Epoladerm does not require any tape reinforcement), while maintaining comparable skin absorption capabilities to transdermal patches currently on the market. Specifically, because the Epoladerm technology does not require a patch to deliver the drug through the skin, we believe Epoladerm may have better adhesion to the skin and may have better accessibility, particularly around joints and other curved body surfaces. Additionally, because Epoladerm is a spray, we believe it will be more aesthetically appealing than transdermal patches. As a spray, Epoladerm and OSF200 (as defined below) will bewere studied in non-clinical animal trials to demonstrateex-vivo skin studies (skin from abdominoplasty) and demonstrated drying times of between 60 and 90 seconds. Unlike other topical NSAIDs, Epoladerm does not require physical handling of the actual drug and enables metered dosing that provides an accurate amount of active ingredient per spray application.
When discussing nonopioid treatments for chronic pain, the Centers for Disease Control (“CDC”) notes clinicians should consider topical agents as alternative first-line analgesics, thought to be safer than systemic medications. In an August 18, 2020 article appearing in the Annals of Internal Medicine, the American College of Physicians and the American Academy of Family Physicians announced a joint clinical guideline, “Nonpharmacologic and Pharmacologic Management of Acute Pain from Non-Low Back, Musculoskeletal Injuries in Adults,” whereby they recommend topical NSAIDs as first-line therapy for patients experiencing pain from non-low back, musculoskeletal injuries. The clinical guideline also recommends that clinicians not prescribe opioids for these injuries except in cases of severe injury or if patients cannot tolerate first-line therapeutic options. We believe this creates a unique market opportunity for Epoladerm within the $3.3 billion (as of 2019) transdermal and topical non-opioid pain market. We plan to target our marketing and selling efforts to pain management clinics and high-prescribing healthcare practitioners, including orthopedic surgeons, rheumatologists, physical medicine and rehabilitation specialists and primary care physicians (“PCPs”).
The below image is a concept design for our metered-dose spray canister for the delivery of Epoladerm for the treatment of acute pain:


We believe Epoladerm represents a novel technology that administers a metered dose film based on our proprietary spray formulation. We are not aware of any other metered dose spray film product, on the market or in clinical development, that utilizes the same delivery mechanisms as Epoladerm. We believe that this transdermal delivery system could provide a pathway for additional proprietary spray formulations for us going forward. As a result of the Pre-Investigational New Drug (“IND”) review, the U.S. Food and Drug Administration (“FDA”) has indicated that it is reasonable for us to pursue a 505(b)(2) accelerated New Drug Application (“NDA”) for Epoladerm.Epoladerm to conduct a study to assess bioavailability and bioequivalence, and then move into Phase III clinical trials. A “505(b)(2)” NDA refers to an application for a new drug filed under Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act (“FDCA”). There can be no assurance that we will be successful in securing regulatory approval under the 505(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate.
The following is the planned development activity and status to bring Epoladerm to market:
2
We are also developing Epoladerm for a second indication utilizing the same transdermal delivery system as EpoladermEpoladerm. This second indication would be as a twice daily topical treatment for chronic osteoarthritis of the knee (“OSF200”). OSF200 will use the same formulation as Epoladerm; however, OSF200 would be applied twice daily. OSF200 is also covered under the same intellectual property as Epoladerm. As a result of the IND review, the FDA has indicated that it is reasonable for us to pursue a 505(b)(2) accelerated NDA for OSF200. There can be no assurance that we will be successful in securing regulatory approval under the 505(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate. OSF200 would be marketed as a topical NSAID treatment for chronic osteoarthritis of the knee. We believe OSF200’s adhesion capabilities and other attributes are the same as those listed for Epoladerm above. Our plan would beis to file a supplement for OSF200 to our potential NDA approval of Epoladerm for OSF200.Epoladerm. If we are able to obtain approval of the NDA for Epoladerm, we plan to conduct Phase III clinical trials for OSF200 with an anticipated NDA approval in approximately 18 months following a potential approval for Epoladerm. However, there can be no assurances that Epoladerm receives FDA approval.
Long-acting Bupivacaine Liposomal-gel 3.0% (LBL100 or ProbudurTM)Probudur)
Probudur is a drug product candidate based on a unique liposomal delivery system utilizing large multi-vesicular vesicles (“LMVVs”) encapsulating a high dose of the local anesthetic bupivacaine. These drug-loaded liposomes are composed of lecithin and cholesterol which are generally recognized as safe (“GRAS”) by the FDA. These LMVVs are embedded in hydrogel beads to form a Lipogel. The system delivers a local anesthetic/analgesic medicine from the Lipogel. Early non-clinical animal studies produced data which suggests that Probudur may be able to provide improved onset, duration and peak performance properties as compared to a similar product on the market. Early dataData from these animal studies indicatedemonstrated that after treatment with Probudur may provide pain(50 mg/kg), statistically significant analgesic activity (measured as threshold pressure at the withdrawal of the animal’s treated extremity) was observed in comparison to a control (vehicle), for up toas long as 96 hours.hours post-treatment (22.33±3.67g vs 5.00±0.58g; p<0.05), which is 24 hours longer than the leading product on the market. These animal studies were conducted by administering Probudur by local infiltration of the surgical site, which resulted in keeping the active ingredient localized at the surgical site for a longer period of time. Four trials were conducted using three animal models. The results of the animal studies show initial support for our belief that Probudur may potentially be safely administered to humans in a planned Phase IIA study.
Based on data from early animal studies, Probudur has indicated post-operative control for up to 96 hours, which is 24 hours longer than the leading product on the market. If we are able to demonstrate a successful Phase III clinical trial, we believe Probudur may represent the first long acting local anesthetic with an opioid sparing label. The slow release of the drug from the liposomal depot reduces the peak plasma levels. We believe this property may permit administration of higher bupivacaine doses (3% versus 1.3% in leading market product); however, there can be no assurances, based on these animal studies, that Probudur will be safe or effective. Further, there can be no assurance that Probudur will receive FDA approval.
We plan to market Probudur to general surgeons, anesthesiologists, and orthopedic surgeons within the $577 million (as of 2019) local anesthetic post-surgical market. If the product candidate is used appropriately, we believe this product candidate could potentially eliminate the need for opioids for post-operative pain relief. As a result of our IND review, the FDA has indicated that it is reasonable for us to pursue a 505(b)(2) accelerated NDA for Probudur. There can be no assurance that we will be successful in securing regulatory approval under the 502(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate.
Probudur has completed IND Enabling studies “in vitro,” “in vivo efficacy” and “in vivo toxicology.” Based upon discussions with the FDA, we intend to move Probudur directly into dose escalation studies in targeted patient populations. We believe the completed IND Enabling studies allow us to transition directly to a Phase II study. The following is the planned activity and status to bring Probudur to market:
| ||
| ||
3
Molecular Envelope Technology Enkephalin Intranasal Spray (NES100)(Envelta)
NES100Envelta is a nanotechnology-based intranasal spray drug product candidate which enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain. NES100It is manufactured using high pressure homogenization and spray drying. The MET nanoparticles are well tolerated via the nasal route at the dose administered. There is pharmacological evidence of activity of MET enabled enkephalin in morphine-tolerant animals. Preclinical studies were conducted in animals for between 6 and 28 days through intravenous, oral and intranasal dosing.
Twelve studies were conducted using three animal models whereby the animal studies were aimed at determining safety pharmacology and genetic toxicology. We believe theThe preliminary data from these early animal studies of NES100 support our beliefEnvelta have shown that NES100 may have comparable preclinical activity toEnvelta exhibited pain control in morphine in alltolerant animals, without the development of tolerance itself. These animal pain models tested without the drug seeking,anti-hyperalgesic effects in rats against evoked stimuli in a model of chronic inflammatory pain and against ongoing neuropathic pain in a conditioned placement preference model with spinal nerve ligation. Envelta and morphine were compared at the same dose level of 7.5 mg kg-1 in this model and Envelta was determined to have a similar analgesic effect. With respect to respiratory depression, and tolerance associated with opioids.delta opioid receptor agonists actually reverse the respiratory depression caused by morphine agonists, leading to our believe that Envelta will be unlikely to cause respiratory depression. However, there can be no assurances, based on these preclinical animal studies, that NES100Envelta will be safe and effective. Further, there can be no assurance that NES100Envelta will receive FDA approval.
We believe we have identified a large unmet need and market opportunity for current prescribers of opioids, including pain and hospice treatment centers. Currently, these prescribers may be using morphine-like opioids, which target three opioid receptors: mu, delta and kappa. Most analgesics used clinically target mu receptor, however, this receptor is also responsible for the majority of undesirable side effects associated with opioids. Currently, enkephalins are limited in their therapeutic potential by their pharmacokinetic profiles due to their inability to cross the blood-brain barrier to reach opioid receptors located in the central nervous system. However, we believe NES100’sEnvelta’s novel nasally delivered formulation, based on early animal studies, enhances enkephalin transport to the brain by protecting the drug in a molecular envelope (MET), facilitating its crossing of the blood-brain barrier. Enkephalins bind predominantly to the delta-receptor which is typically not associated with the dangers associated with opioids. We believe NES100Envelta may have analgesic potential without opioid tolerance, and Envelta has not exhibited any indications of withdrawal, respiratory depression, euphoria, or addiction in the early animal studies. A new study published in Proceedings of the National Academy of Sciences (PNAS)(“PNAS”) indicates, “Delta opioid receptors have a built-in mechanism for pain relief and can be precisely targeted with drug-delivering nanoparticles, making them a promising target for treating chronic inflammatory pain with fewer side effects.” There can be no assurances, based on these preclinical animal studies, that NES100Envelta will be safe and effective in human trials.
Additionally, we believe NES100Envelta may significantly reduce constipation and early animal clinical trials have not demonstrated any opioid dependence, drug seeking or respiratory depression. We plan to use the endogenous NCE regulatory pathway to bring this product candidate to market. We plan to target our marketing and selling efforts to pain specialists, anesthesiologists, orthopedics, surgeons, PCPs, Nurse Practitioners (“NPs”), oncologists, and neurologists within the $7 billion (as of 2019) analgesic narcotics market.
NES100Envelta is a neuroactive peptide drug product (enkephalin) with a proprietary composition formulated for administration by all routes, except the topical route. A preassembled device and cartridge would be used to propel the enkephalin formulation through the nose to the brain via the olfactory nerve/bulb route of transmission. A MET will encapsulate the drug product, protecting it from degradation, and help to carry the drug across the blood-brain barrier to promptly suppress pain.
NES100Envelta has completed IND enabling studies “in vitro,” “in vivo efficacy” and “in vivo toxicology.” The following is the planned activity and status to bring the product candidate to market:
We are also developing NES100Envelta for a second indication, Post-Traumatic Stress Disorder, utilizing the same delivery mechanism as NES100.Envelta. PES200 enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain and it is also covered under the same intellectual property listed elsewhere in this prospectus for NES100.Envelta. We believe PES200’s capabilities and attributes are the same as listed for NES100Envelta above. Our plan is to validate proof-of-concept for PES200 followed by IND-enabling studies for the development of a novel enkephalin-based formulation to treat Post-Traumatic Stress Disorder.
On August 25, 2020, we entered into a cooperative research and development agreement (the “CRADA”), with the National Center for Advancing Translational Sciences (NCATS) (the “CRADA”(“NCATS”), an institute/center of the National Institutes of Health (NIH)(“NIH”), U.S. Department of Health and Human Services. This collaboration is for the continued development of Virpax’sour product candidate, NES100,Envelta, an intranasal peptide, for the management of acute and chronic non-cancer pain. The term of the CRADA is for a period of four years from the effective date of the agreement and can be terminated by both parties at any time by mutual written consent. In addition, either party may unilaterally terminate the CRADA at any time by providing written notice of at least sixty (60) days before the desired termination date. The agreement provides for studies that are focused on the pre-clinical characterization of NES100Envelta as a novel analgesic for acute and chronic non-cancer pain, and for studies to further develop NES100Envelta through IND enabling studies.
4
High-Density Molecular Masking Spray Formulation for the Prevention of Respiratory Viruses (MMS019)(AnQlar)
MMS019AnQlar is high-density molecular masking spray we plan to develop as an anti-viral barrier to potentially prevent, or reduce the risk or the intensity of, viral infections in humans. We intend for this formulation to be delivered using a preassembled device and cartridge to propel the High-Density Molecular Sprayhigh-density molecular spray formulation into the nose. MMS019AnQlar will be used as a nasal powder spray to potentially prevent viral binding to epithelial cells in the nasal cavity and the upper respiratory tract, potentially reducing respiratory related infections.
WeAs an addition to standard personal protective equipment, we believe MMS019AnQlar may offer anotheran additional layer of protection, in the form of a Molecular Mask, to be added to standard personal protective equipmentmolecular mask, to protect healthcare workers and those at-riskat risk of serious disease from viral infections. MMS019AnQlar has completed IND-enabling toxicology studies and intends to demonstrate that pre-treatmentex-vivo studies which demonstrated a reduction in infectivity from respiratory viruses like influenza and SARS-CoV-2. During the ex-vivo study, in the presence of nasal mucosal cellsAnQlar, SARS-CoV-2 viral replication inhibition was observed in vitro prevents, or reduces, thea bronchial epithelial model reconstituted from healthy human donor cells. Virus replication was evaluated using RT-qPCR. The data was presented as a number of cells that get infected.viral copies per ml. A lower viral yield was detected in the cultures treated with AnQlar than in the control with phosphate buffered saline (“PBS”) after 72 hours of infection. We intendplan to finance activities relatedmarket AnQlar to MMS019 with grantsfirst responders, healthcare workers, clinics, military forces, transplant and not with any proceeds from this offering.other immune compromised or at risk patients within the $13 billion (as of 2019) anti-viral market.
We continue to seek opportunities to exploit our product portfolio through licensing and other strategic transactions to further develop our drug product candidates. This includes seeking potential partners in further developing our drug product candidates and responding to inquiries of interest we have received concerning our product portfolio.
Recent Developments
Initial Public Offering
On February 19, 2021, we closed our initial public offering (the “IPO”) of 1,800,000 shares of our common stock at a public offering price of $10.00 per share, for gross proceeds of $18.0 million, before deducting underwriting discounts and offering expenses.
Epoladerm
We have initiated a series of IND enabling toxicity studies for Epoladerm which are expected to take between eight months to one year to complete. If the completion of these studies are successful, we intend to submit an IND application to the FDA, including a trial design for a Phase I study.
Probudur
In March 2021, we engaged Charles River Laboratories to perform seven preclinical animal studies during the second half of 2021, including method, dosage, and toxicity as part of the required FDA enabling trials for an IND for Probudur. However, we elected to strategically delay these trials in order to enhance the formulation of Probudur to increase stability for manufacturing purposes and to possibly extend the lifetime of a relevant patent.
On June 29, 2021, we entered into an Agreement for Rendering of Research Services with LipocureRX, Ltd. (“Lipocure,” and the “June 2021 Lipocure Research Agreement”). Under the June 2021 Lipocure Research Agreement, we will provide funding for research and development related to the optimization of the Liposomal Bupivacaine formulation and eventual manufacture of pre-clinical batches including batches for stability testing, animal studies and toxicology work. This will also include work associated with the potential filing of additional provisional patent applications. We may terminate the agreement at any time upon 30 days written notice and will only be responsible to pay Lipocure for work performed through the date of such notice. In consideration for the research services, we agreed to pay research service fees of $200,000 upon execution as prepayment for research services, as well as $400,000 on July, 1 2021, and five quarterly payments of $270,000 initiating on September 1 2021. We also agreed to pay $250,000 to Lipocure upon the successful completion of a Chemistry, Manufacturing and Controls (“CMC”) filing with the FDA. All services to be provided under the June 2021 Lipocure Research Agreement initiated on July 1, 2021 and are anticipated to be completed towards the end 2022.
On June 30, 2021, we entered into an Agreement for Rendering of Research Services (the “June 2021 Yissum Research Agreement”) with Yissum Research Development Company of the Hebrew University of Jerusalem, Ltd. (“Yissum”). Under the June 2021 Yissum Research Agreement, we shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the optimization of the Liposomal Bupivacaine formulation and to increase stability for manufacturing purposes. We may terminate the agreement at any time upon 30 days written notice and shall be only responsible to pay Yissum for work performed through the date of such notice. In consideration for the research services, we agreed to pay research service fees of $337,500 in six equal quarterly installments. Each party will be entitled to terminate the agreement in the event of a breach by the other party of its obligations under the agreement, including, but not limited to, any payment failure, which is not remedied by the breaching party within 30 days of receipt of written notice from the non-breaching party. All services to be provided under the June 2021 Yissum Research Agreement initiated on July 1, 2021 and are anticipated to be completed towards the end of 2022.
Envelta
We plan to utilize the delivery technologies utilized for Envelta to selectively develop a portfolio of patented 505(b)(2) and NCE candidates for commercialization. The IND enabling studies for Envelta are being performed under a CRADA entered into by us and NCATS. To that end, we intend to use these studies as a source for INDs for two additional potential indications, cancer pain and post-traumatic stress disorder. To date, two of the four planned initial in vitro studies have been successfully completed. These pre-clinical studies under the CRADA are expected to be conducted through 2021 and 2022.
AnQlar
We have received a written pre-investigational new drug (“pre-IND”) response from the FDA for AnQlar, our patented and proprietary high-density molecular masking spray under development for use as an anti-viral barrier product. In its pre-IND response, the FDA provided guidance on our pathway to pursue prophylactic treatment against SARS-CoV-2 and influenza for daily use as an over-the-counter (“OTC”) product. We believe the results of the pre-IND response support further research on AnQlar as an intranasal protective that may limit transmission of the viruses to others. If we are able to successfully complete the required clinical trials for this product candidate, we intend to move forward and pursue an NDA for AnQlar as a once daily intranasal treatment. The FDA has indicated that, upon successful completion, we may pursue an NDA drug approval with the Office of Non-Prescription Drugs.
In August 2021, we engaged Syneos Health to assist with the optimal clinical trial design based on an efficient timeline. On August 25, 2021, we entered into a commercial manufacturing and supply agreement with Seqens, an integrated global leader in pharmaceutical solutions with 24 manufacturing sites worldwide and seven research and development facilities throughout the U.S. and Europe. The agreement with Seqens provides for both the supply material for our clinical studies as well as the long-term commercial supply of AnQlar. Seqens will conduct process development and validation of additional large scale commercial quantities of AnQlar at its facilities in Devens and Newburyport, Massachusetts.
Our Strengths
We are working to create and advance novel non-addictive treatments for the management of acute musculoskeletal pain, chronic pain, mononeuropathy, pain associated with cancer, and post-operative pain. Our goal is to develop and commercialize product candidates that improve the clinical outcomes of the treatments currently on the market. There can be no assurance that we will be able to execute on this strategy. With the support of the President, the U.S. Department of Health and Human Health Services (“HHS”), the National Institutes of Health (“NIH”) launched the initiative Helping to End Addiction Long-term (“HEAL”), to provide solutions to the national opioid crisis. Pursuant to this initiative, we have been awarded grantsin kind support related to NES100Envelta and have filed for additional grants that are currently pending. We believe HEAL and recent FDA guidance has made it more efficient to bring novel non-opioid medication to market.
We believe three of the below product candidates (Epoladerm, Probudur, and Envelta) have novel technologies that could improve pain management.management and one product candidate (AnQlar) could inhibit viral replication and decrease levels of virus in brain tissue. We believe these product candidates also have the following individual strengths and differentiators as compared to similar product candidates that we believe could make these product candidates more desirable if approved:
| ● | Epoladerm’s quick-drying spray film technology may permit consistent 12-hour dosing without the messy handling of much slower drying liquids, gels and spray foam formulations that must be rubbed into the skin on application. We believe this may allow for improved patient compliance, which may enhance therapeutic outcomes compared to the inconsistent adhesion of patch technology. |
| ● | Probudur may eliminate the need for opioids and catheters after surgery and may reduce the associated costs and length of stay. |
| ● |
| ● | AnQlar has demonstrated inhibited viral replication and |
Also, three of the sixour product candidates (Epoladerm, Probudur, and OSF200) could potentially be developed using the FDA’s 505(b)(2) regulatory pathway. This type of submission differs from the FDA’s standard 505(b)(1) NDA clinical development pathway typically used for most pharmaceutical new chemical entities (“NCEs”) in that the development candidate contains similar active ingredients to a previously approved drug. Consequently, the data included in the submission can rely, at least partially, on the FDA’s findings of safety and effectiveness related to the prior approved product, and as a result can mitigate many of the drug development risks faced by the drug developer. Companies utilizing the 505(b)(2) accelerated NDA regulatory pathway typically experience a shorter drug development program that requires less resources than the standard regulatory pathway. Under the 505(b)2(2) pathway, the FDA allows the use of data from a prior application that can be referenced by a new sponsor that can include part of the required preclinical or clinical studies for approval. Consequently, this alternative pathway can significantly lower the development cost and shorten the timeline to NDA approval.
We are focused on applying our spray film and liposomal gel technologies to already-approved pharmacological actives. We intend to seek to leverage the 505(b)(2) accelerated NDA pathway to accelerate our development timeline and potentially lower our clinical and regulatory risk for not only our Epoladerm drug candidate, but for the OSF200 and Probudur product candidates as well. However, there can be no assurance that the 505(b)(2) accelerated NDA pathway will lead to a faster development process or a faster regulatory review. While the 502(b)(2) pathway may potentially expedite development or the approval process, it does not change the FDA’s standards of approval or increase the likelihood that a product candidate will receive approval.
5
Our Team
We have assembled a highly experienced management team, board of directors (the “Board of Directors”) and scientific advisory board to execute on our mission to develop product candidates that effectively manage pain in all its complexities, while minimizing risks to patients and society. Our team has a proven track record in developing, launching and marketing multiple pain products.
Our Founder and Chief Executive Officer, Anthony Mack, is a business leader with over 25 years of experience in the pharmaceutical and finance industries. Our Chief Medical Officer, Jeffrey Gudin, M.D., is a Clinical Associate Professor in Anesthesiology at the Rutgers New Jersey Medical School and is Board Certified in Pain Medicine, Anesthesiology, Addiction Medicine and Hospice and Palliative Medicine. Our EVP, Commercial Operations Officer, Gerald W. Bruce, has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical and Medical Nutrition industry. Our Chief Financial Officer, Christopher M. Chipman, CPA, has more than 25 years of industry experience assisting public companies with financial reporting, forecasting, preparation of periodic reports required to be filed with the Securities and Exchange Commission and compliance with Section 404 of the Sarbanes Oxley Act of 2002 including pharmaceutical clients. Gerald W. Bruce EVP, Commercial Operations, has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical industry.
Our Strategy
We are focused on becoming a global leader in pain management by developing and delivering innovative pharmaceutical products to patients. We are developing branded pharmaceutical product candidates for pain management by using cutting-edge technology to enhance patients’ quality of life.
According to data from the CDC, in 2016, approximately 20% of adults in the United States had chronic pain (approximately 50 million people). Further, CDC data indicates that the prescribing of opioids by clinicians has increased threefold in the last 20 years, contributing to the problem of prescription opioid abuse. Accordingly, there is a push among prescribers, regulators, and patients to seek non-opioid and non-addictive treatment options to combat the opioid epidemic. We plan to utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and NCE product candidates for commercialization.
We have developed a marketing and sales strategy tailored to each individual product candidate within our portfolio which will be deployed if each product candidate is approved and brought to market. With a dedicated sales team, and niche non-opioid and non-addictive pain management product candidates, we believe once these strategies are put into place, they can produce products with greater pain management than opioid competitor products, potentially without any of the side effects caused by morphine/opioid related products.
6
Summary of Risks Associated with Our Business
Our business and an investment in our company is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this prospectus summary. Some of these risks include:
| ● | We are a pre-revenue company with a limited operating history; | |
| ● | We may not be able to successfully develop or commercialize new product candidates or do so on a timely or cost-effective basis; |
| ● | Our business may be negatively affected by the ongoing COVID-19 pandemic; | |
| ● | Our business may be negatively affected by ongoing litigation; |
| ● | We depend on a limited number of product candidates and our business could be materially adversely affected if one or more of our key product candidates do not perform as well as expected and do not receive regulatory approval; |
| ● | Our profitability depends on our major customers, and if our relationships with them do not continue as expected, our business, prospects and results of operations could materially suffer; |
| ● | We are, and will continue to be in the future, a party to legal proceedings that could result in adverse outcomes; |
| ● | Our competitors and other third parties may allege that we are infringing their intellectual property, forcing us to expend substantial resources in resulting litigation, and any unfavorable outcome of such litigation could have a material adverse effect on our business; |
| ● | We may experience failures of or delays in clinical trials which could jeopardize or delay our ability to obtain regulatory approval and commence product sales; |
| ● | We face intense competition from both brand and generic companies which could limit our growth and adversely affect our financial results; |
| ● | We are subject to extensive governmental regulation and we face significant uncertainties and potentially significant costs associated with our efforts to comply with applicable regulations; |
| ● | We may not be able to develop or maintain our sales capabilities or effectively market or sell our products; |
| ● | Manufacturing or quality control problems may damage our reputation, require costly remedial activities or otherwise negatively impact our business; |
| ● | Our profitability depends on coverage and reimbursement by third-party payors, and healthcare reform and other future legislation may lead to reductions in coverage or reimbursement levels; |
| ● | We face risks related to health epidemics and outbreaks, including the COVID-19 pandemic, which could significantly disrupt our preclinical studies and clinical trials, and therefore our receipt of necessary regulatory approvals could be delayed or prevented; |
| ● | We currently, and may in the future need to, license certain intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms; |
| ● | We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which might adversely affect our ability to develop, manufacture and market our products and product candidates; |
| ● | If we fail to comply with our obligations under any of our third-party agreements, we could lose license rights that are necessary to develop our product candidates; and |
| ● | After this offering, our directors, executive officers and certain stockholders (one of which is an affiliate of our Chief Executive Officer) will continue to own a significant percentage of our common stock and, if they choose to act together, will be able to exert significant control over matters subject to stockholder approval. |
Our Corporate Information
We were incorporated under the laws of the State of Delaware on May 12, 2017. Our principal executive offices are located at 1554 Paoli Pike, #279, West Chester,1055 Westlakes Drive, Suite 300, Berwyn, PA 19380.19312. Our telephone number is (484) 880-4588.(610) 727-4597.
Our website address is www.virpaxpharma.com. The information contained in, or accessible through, our website does not constitute a part of this prospectus. You should not rely on any such information in making your decision whether to purchase our common stock.
7
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, enacted in 2012. An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| ● | being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in this prospectus; |
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended; |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the completion of thisour initial public offering. However, if certain events occur prior to the end of such five-year period, including if we become a large accelerated filer, our annual gross revenue exceeds $1.07 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are choosing to “opt in” to this extended transition period, which will ensure us additional time to adopt these new accounting standard updates.
8
THE OFFERING
| Shares being offered | 3,401,360 shares of common stock based on an assumed public offering price of $14.70 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on September 8, 2021) | |
| Number of shares of common stock outstanding immediately before this offering | 5,045,181 shares | |
| Number of shares of common stock to be outstanding after this offering (1) | 8,446,541 shares (or 8,956,745 shares if the underwriters exercise the option to purchase additional shares in full) based on an assumed public offering price of $14.70 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on September 8, 2021). See “Description of Share Capital”. | |
| Use of proceeds | We expect to receive net proceeds, after deducting underwriting discounts and commissions and estimated expenses payable by us, of approximately | |
| We intend to use substantially all of the net proceeds from this offering to fund research and development of our Epoladerm, Probudur, Envelta and | ||
| Underwriters’ over-allotment option | We have granted the underwriters a | |
| Shares of our common stock are listed on the Nasdaq Capital Market under the symbol “VRPX.” | ||
| Risk factors | Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page |
| (1) | The number of shares of our common stock to be outstanding immediately after this offering is based on shares of our common stock outstanding as of |
| ● | 669,067 shares of common stock issuable upon exercise of stock options outstanding as of |
| ● |
| ● | ||
Unless otherwise indicated, this prospectus reflects and assumes the following:
| ● | no exercise of outstanding options or warrants described |
| ● | no exercise by the |
9
SUMMARY SELECTED FINANCIAL DATA
You should read the following summary selected financial data together with our financial statements and the related notes appearing at the end of this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus. We have derived the statement of operations data for the years ended December 31, 20192020 and December 31, 20182019 from our audited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the six months ended June 30, 20202021 and 20192020 and the balance sheet data as of June 30, 20202021 from our unaudited interim condensed financial statements appearing at the end of this prospectus. Our historical results are not necessarily indicative of results that should be expected in any future period.
| Year Ended December 31, | Six Months Ended June 30, | Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||||||||||||
| 2019 | 2018 | 2020 | 2019 | 2020 | 2019 | 2021 | 2020 | |||||||||||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||||||
| General and administrative | $ | 2,559,127 | $ | 1,483,786 | $ | 1,510,835 | $ | 1,364,326 | $ | 2,904,104 | $ | 2,559,127 | $ | 3,262,544 | $ | 1,510,835 | ||||||||||||||||
| Research and development | 622,741 | 1,142,176 | 335,553 | 154,559 | 1,291,615 | 622,741 | 1,391,565 | 335,553 | ||||||||||||||||||||||||
| Total operating expenses | 3,181,868 | 2,625,962 | 1,846,388 | 1,518,885 | 4,195,719 | 3,181,868 | 4,654,109 | 1,846,388 | ||||||||||||||||||||||||
| Loss from operations | (3,181,868 | ) | (2,625,962 | ) | (1,846,388 | ) | (1,518,885 | ) | (4,195,719 | ) | (3,181,868 | ) | (4,654,109 | ) | (1,846,388 | ) | ||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||||||
| Interest expense | (124,644 | ) | (14,976 | ) | (83,891 | ) | (55,637 | ) | (147,934 | ) | (124,644 | ) | (64,748 | ) | (83,891 | ) | ||||||||||||||||
| Other income | - | - | 4,000 | - | 4,000 | — | (3,833 | ) | 4,000 | |||||||||||||||||||||||
| Total other income (expense) | (143,934 | ) | (124,644 | ) | (68,581 | ) | (79,891 | ) | ||||||||||||||||||||||||
| Net loss | $ | (3,306,512 | ) | $ | (2,640,938 | ) | $ | (1,926,279 | ) | $ | (1,574,522 | ) | $ | (4,339,653 | ) | $ | (3,306,512 | ) | $ | (4,722,690 | ) | $ | (1,926,279 | ) | ||||||||
| Net loss per common share—basic and diluted(1) | $ | (0.23 | ) | $ | (0.19 | ) | $ | (0.13 | ) | $ | (0.11 | ) | ||||||||||||||||||||
| Weighted average common shares outstanding—basic and diluted(1) | 14,481,487 | 14,035,316 | 15,152,543 | 14,275,073 | ||||||||||||||||||||||||||||
| Net loss per common share – basic and diluted(1) | $ | (1.40 | ) | $ | (1.13 | ) | $ | (1.06 | ) | $ | (0.63 | ) | ||||||||||||||||||||
| Weighted average common shares outstanding – basic and diluted(1) | 3,107,502 | 2,929,005 | 4,454,877 | 3,065,636 | ||||||||||||||||||||||||||||
| (1) | See Note 2 to our audited and unaudited financial statements appearing at the end of this prospectus for further details on the calculation of basic and diluted net loss per common share. |
| June 30, 2021 | ||||||||||||||||
| Actual | Adjusted (1) | Actual | As Adjusted(1) | |||||||||||||
| (Unaudited) | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||||
| Balance Sheet Data: | ||||||||||||||||
| Cash | $ | 416,909 | $ | $ | 10,466,774 | $ | 56,690,274 | |||||||||
| Working capital | $ | (2,714,370 | ) | $ | $ | 8,557,320 | $ | 54,780,820 | ||||||||
| Total assets | $ | 898,152 | $ | $ | 11,387,696 | $ | 57,611,196 | |||||||||
| Notes payable and related party notes payable | $ | 1,463,663 | $ | $ | 1,072,100 | $ | 1,072,100 | |||||||||
| Accumulated deficit | $ | (8,234,470 | ) | $ | $ | (15,370,534 | ) | $ | (15,370,534 | ) | ||||||
| Stockholders’ (deficit) equity | $ | 7,535,730 | $ | 53,759,230 | ||||||||||||
| (1) |
10
RISK FACTORS
An investment in our common stock is speculative, illiquid and involves a high degree of risk including the risk of a loss of your entire investment. You should carefully consider the risks and uncertainties described below and the other information contained in this prospectus. The risks set forth below are not the only ones facing us. Additional unanticipated or unknown risks and uncertainties may exist that could also adversely affect our business, operations and financial condition in ways that are unknown to us or unpredictable. If any of the following risks actually materialize, our business, financial condition and/or operations could suffer. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock.
Risks Related to Our Financial Position and Need for Additional Capital
We are a preclinical stage biopharmaceutical company with a limited operating history.
We were established and began operations in 2017. Our operations to date have been limited to financing and staffing our company, licensing product candidates, conducting preclinical and clinical studies of Epoladerm for acute musculoskeletal pain, Probudur for postoperative hip and knee replacement pain management, and NES100Envelta for the management of acute and chronic pain.pain, including pain associated with cancer, and AnQlar as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans. We have not yet demonstrated the ability to successfully complete a large-scale, pivotal clinical trial, obtain marketing approval, manufacture a commercial scale product, arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially earlypreclinical stage clinical pharmaceutical companies such as ours. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history will face. In particular, potential investors should consider that we cannot assure you that we will be able to, among other things:
| ● | successfully implement or execute our current business plan, and we cannot assure you that our business plan is sound; |
| ● | successfully manufacture our clinical product candidates and establish commercial supply; |
| ● | successfully complete the clinical trials necessary to obtain regulatory approval for the marketing of our product candidates; |
| ● | secure market exclusivity and/or adequate intellectual property protection for our product candidates; |
| ● | attract and retain an experienced management and advisory team; |
| ● | secure acceptance of our product candidates in the medical community and with third-party payors and consumers; |
| ● | raise sufficient funds in the capital markets or otherwise to effectuate our business plan; and |
| ● | utilize the funds that we do have and/or raise in this offering or in the future to efficiently execute our business strategy. |
If we cannot successfully execute any one of the foregoing, our business may fail and your investment will be adversely affected.
We have incurred losses since inception and anticipate that we will continue to incur losses for the foreseeable future. We are not currently profitable, and we may never achieve or sustain profitability.
We are a preclinical stage biopharmaceutical company with a limited operating history and have incurred losses since our formation. We incurred net losses of approximately $3.3$4.3 million and approximately $2.6$3.3 million for the years ended December 31, 20192020 and 2018,2019, respectively, and incurred net losses of approximately $1.9$4.7 million and approximately $1.6$1.9 million for the six months ended June 30, 20202021 and 2019,2020, respectively. As of June 30, 2020,2021, we had an accumulated loss of approximately $8.2$15.4 million. We have not commercialized any product candidates and have never generated revenue from the commercialization of any product. To date, we have devoted most of our financial resources to research and development, including our preclinical and clinical work, and to intellectual property.
We expect to incur significant additional operating losses for the next several years, at least, as we advance Epoladerm, Probudur, Envelta and NES100AnQlar through clinical development, complete clinical trials, seek regulatory approval and commercialize Epoladerm, Probudur, Envelta and NES100,AnQlar, if approved. The costs of advancing product candidates into each clinical phase tend to increase substantially over the duration of the clinical development process. Therefore, the total costs to advance any of our product candidates to marketing approval in even a single jurisdiction will be substantial. Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to begin generating revenue from the commercialization of any products or achieve or maintain profitability. Our expenses will also increase substantially if and as we:
| ● | are required by the FDA, to complete Phase 1 trials in Epoladerm, Envelta and AnQlar and Phase 2 trials to support an NDA for |
| ● | are required by the FDA to complete Phase 3 trials to support NDAs for Epoladerm, Probudur, Envelta and |
| ● | establish a sales, marketing and distribution infrastructure to commercialize our drugs, if approved, and for any other product candidates for which we may obtain marketing approval; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| ● | hire additional clinical, scientific and commercial personnel; |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts, as well as to support our transition to a public reporting company; and |
| ● | acquire or in-license or invent other product candidates or technologies. |
Furthermore, our ability to successfully develop, commercialize and license any product candidates and generate product revenue is subject to substantial additional risks and uncertainties, as described under “Risks Related to Development, Clinical Testing, Manufacturing and Regulatory Approval” and “Risks Related to Commercialization.” As a result, we expect to continue to incur net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our stockholders’ equity and working capital. The amount of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If we are unable to develop and commercialize one or more product candidates, either alone or through collaborations, or if revenues from any product that receives marketing approval are insufficient, we will not achieve profitability. Even if we do achieve profitability, we may not be able to sustain profitability or meet outside expectations for our profitability. If we are unable to achieve or sustain profitability or to meet outside expectations for our profitability, the value of our common stock will be materially and adversely affected.
Even if this offering is successful, we will require additional capital to fund our operations, and if we fail to obtain necessary financing, we may not be able to complete the development and commercialization of our drugs.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to advance the clinical development of and launch and commercialize our product candidates if we receive regulatory approval. Following this offering, we will require additional capital for the further development and potential commercialization of Epoladerm, Probudur, Envelta and NES100AnQlar and may also need to raise additional funds sooner to pursue a more accelerated development of Epoladerm, Probudur, Envelta and NES100.AnQlar. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
We believe that the net proceeds from this offering will enable us to fund our operating expense requirements for at least 12 monthsthrough December 2024 following the closing of this offering. We have based this estimate on assumptions that may prove to be wrong, and we could deploy our available capital resources sooner than we currently expect. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to the:
| ● | initiation, progress, timing, costs and results of preclinical studies and clinical trials, including patient enrollment in such trials, for Epoladerm, Probudur, Envelta and |
| ● | clinical development plans we establish for Epoladerm, Probudur, Envelta and |
| ● | obligation to make royalty and non-royalty sublicense receipt payments to third-party licensors, if any, under our licensing agreements; |
| ● | number and characteristics of product candidates that we discover or in-license and develop; |
| ● | outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; |
| ● | costs of filing, prosecuting, defending and enforcing any patent claims and maintaining and enforcing other intellectual property rights; |
| ● | effects of competing technological and market developments; |
| ● | costs and timing of the implementation of commercial-scale manufacturing activities; |
| ● | costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval; and |
| ● | cost associated with being a public company. |
If we are unable to expand our operations or otherwise capitalize on our business opportunities due to a lack of capital, our ability to become profitable will be compromised.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements for the year ended December 31, 2019 with respect to this uncertainty. Our ability to continue as a going concern will require us to obtain additional funding.
Raising additional capital may cause dilution to our stockholders, including purchasers of common stock in this offering, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial revenue, we may finance our cash needs through a combination of equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or other sources. We do not currently have any committed external source of funds. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, intellectual property, future revenue streams or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate product candidate development or future commercialization efforts.
Changes in U.S. tax law may materially adversely affect our financial condition, results of operations and cash flows.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act, was signed into law to address the COVID-19 crisis. The CARES Act is an approximately $2 trillion emergency economic stimulus package that includes numerous U.S. federal income tax provisions, including the modification of: (i) net operating loss rules (as discussed below), (ii) the alternative minimum tax refund and (iii) business interest deduction limitations under Section 163(j) of the Internal Revenue Code of 1986, as amended, or the Code.
On December 22, 2017, President Trump signed into law federal tax legislation commonly referred to as the TCJA (defined below), which also significantly changed the U.S. federal income taxation of U.S. corporations. TCJA remains unclear in many respects and has been, and may continue to be, subject to amendments and technical corrections, as well as interpretations and implementing regulations by the Treasury and Internal Revenue Service, or the IRS, any of which could lessen or increase certain adverse impacts of TCJA. In addition, it is unclear how these U.S. federal income tax changes will affect state and local taxation, which often uses federal taxable income as a starting point for computing state and local tax liabilities.
While some of these U.S. federal income tax changes may adversely affect us in one or more reporting periods and prospectively, other changes may be beneficial on a going-forward basis. We continue to work with our tax advisors and auditors to determine the full impact TCJA and the CARES Act will have on us. We urge our investors to consult with their legal and tax advisors with respect to both TCJA and the CARES Act and the potential tax consequences of investing in our common stock.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
Our net operating loss carryforwards (“NOLs”), and certain other tax attributes could expire unused and be unavailable to offset future income tax liabilities because of their limited duration or because of restrictions under U.S. tax law. AsFrom the total of our federal NOL of $6,951,000 as of December 31, 2019, we had NOLs of approximately $4.3 million for federal and state income tax purposes. Our federal NOL of2020, $326,000 expireexpires in 2037, (withand the remaining federal NOLs havingNOL has an indefinite expiration date) and ourcarryover period. Our state NOL’s of $4,387,000 begin to$6,951,000 expire afterfrom 2037 through 2039.2040.
Under TCJA (defined below), federal NOLs generated in tax years ending after December 31, 2017 may be carried forward indefinitely. Under the CARES Act, NOL carryforwards arising in tax years beginning after December 31, 2017 and before January 1, 2021 may be carried back to each of the five tax years preceding the tax year of such loss. Due to our cumulative losses through June 30, 2020,2021, we do not anticipate that such provision of the CARES Act will be relevant to us. The deductibility of federal NOLs, particularly for tax years beginning after December 31, 2020, may be limited. It is uncertain if and to what extent various states will conform to TCJA or the CARES Act.
In addition, our NOLs are subject to review and possible adjustment by the U.S. Internal Revenue Service, or IRS, and state tax authorities. In general, under Sections 382 and 383 of the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to use its pre-change NOLs to offset future taxable income. Due to previous ownership changes, or if we undergo an ownership change in connection with or after this offering, our ability to use our NOLs could be limited by Section 382 of the Code. Future changes in our stock ownership, inclusive of a public offering and some of which are outside of our control, could result in an ownership change under Sections 382 and 383 of the Code. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to use a material portion of the NOLs, even if we attain profitability.
Our business, financial condition and results of operations may be adversely affected if we are unsuccessful in our current litigation with Sorrento Therapeutics, Inc. and Scilex Pharmaceuticals, Inc.
On March 12, 2021, we and our Chief Executive Officer, Anthony Mack were named as defendants in a complaint (the “Complaint”) filed by Sorrento Therapeutics, Inc. (“Sorrento”) and Scilex Pharmaceuticals Inc. (“Scilex” and together with Sorrento, the “Plaintiffs”) in the Court of Chancery of the State of Delaware. In the Complaint, the Plaintiffs allege (i) Mr. Mack breached a Restrictive Covenants Agreement, dated as of November 8, 2016, between himself and Sorrento (the “Restrictive Covenants Agreement”), (ii) we tortiously interfered with the Restrictive Covenants Agreement, and (iii) we tortiously interfered with Scilex’s relationship with Mr. Mack. On May 7, 2021, Plaintiffs filed an Amended Complaint (the “Amended Complaint”) asserting the same three causes of action. Simultaneously with filing the original Complaint, the Plaintiffs filed a motion to expedite, seeking a hearing in four weeks on their prospective motion for a preliminary injunction, prohibiting Mr. Mack’s alleged violations of the Restrictive Covenants Agreement and prohibiting the alleged tortious interference. On March 22, 2021, Vice Chancellor Paul A. Fioravanti Jr. issued a scheduling order, setting forth terms which had been agreed to by the parties, under which oral argument on Plaintiffs’ proposed motion for preliminary injunction was scheduled for July 15, 2021. On May 17, 2021, we and Mr. Mack filed a motion to dismiss the Amended Complaint. On May 18, 2021, the Vice Chancellor issued a revised scheduling order, also setting forth terms which had been agreed to by the parties, under which a briefing schedule was set with respect to Defendants’ motion to dismiss, plaintiffs agreed not to move for a preliminary injunction, and a trial in the action was set for February 9-11, 2022. As of July 12, 2021, the parties completed briefing on the motion to dismiss the Amended Complaint. The Court has scheduled December 9, 2021 for oral argument on the motion to dismiss. We believe that the allegations in the Amended Complaint are wholly without merit, and we intend to vigorously defend the action. However, we are unable to predict the ultimate outcome of the lawsuit at this time.
Defending this lawsuit may cause us to incur significant legal expenses. Furthermore, in the event that we are unsuccessful in the defense of this lawsuit, we could be required to pay damages which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Development, Clinical Testing, Manufacturing and Regulatory Approval
Clinical trials are expensive, time-consuming and difficult to design and implement, and involve an uncertain outcome.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Because the results of preclinical studies and early clinical trials are not necessarily predictive of future results, Epoladerm, Probudur, Envelta and NES100 AnQlar may not have favorable results in later preclinical and clinical studies or receive regulatory approval. We may experience delays in initiating and completing any clinical trials that we intend to conduct, and we do not know whether planned clinical trials will begin on time, need to be redesigned, enroll patients on time or be completed on schedule, or at all. Clinical trials can be delayed for a variety of reasons, including delays related to:
| ● | the FDA or comparable foreign regulatory authorities disagreeing as to the design or implementation of our clinical studies; |
| ● | obtaining regulatory approval to commence a trial; |
| ● | reaching an agreement on acceptable terms with prospective contract research organizations (“CROs”), and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | obtaining Institutional Review Board (“IRB”), approval at each site, or Independent Ethics Committee (“IEC”), approval at sites outside the United States; |
| ● | recruiting suitable patients to participate in a trial in a timely manner and in sufficient numbers; |
| ● | having patients complete a trial or return for post-treatment follow-up; |
| ● | imposition of a clinical hold by regulatory authorities, including as a result of unforeseen safety issues or side effects or failure of trial sites to adhere to regulatory requirements or follow trial protocols; |
| ● | clinical sites deviating from trial protocol or dropping out of a trial; |
| ● | addressing patient safety concerns that arise during the course of a trial; |
| ● | adding a sufficient number of clinical trial sites; or |
| ● | manufacturing sufficient quantities of product candidate for use in clinical trials. |
We could also encounter delays if a clinical trial is suspended or terminated by us, the IRBs or IECs of the institutions in which such trials are being conducted, the Data Safety Monitoring Board (“DSMB”) for such trial or the FDA or other regulatory authorities. Such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and, while we have agreements governing their committed activities, we have limited influence over their actual performance, as described in “Risks Related to Our Dependence on Third Parties”.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for Epoladerm, Probudur, Envelta and/or NES100AnQlar or any other product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain regulatory approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that we will never obtain regulatory approval for Epoladerm, Probudur, Envelta and/or NES100 AnQlaror any other product candidate. We are not permitted to market any of our product candidates in the United States until we receive regulatory approval of an NDA from the FDA. Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| ● | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| ● | serious and unexpected drug-related side effects experienced by participants in our clinical trials or by individuals using drugs similar to our product candidates, or other products containing the active ingredient in our product candidates; |
| ● | negative or ambiguous results from our clinical trials or results that may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| ● | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| ● | the data collected from clinical trials of our product candidates may not be acceptable or sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere, and we may be required to conduct additional clinical trials; |
| ● | the FDA or comparable foreign authorities may disagree regarding the formulation, labeling and/or the specifications of our product candidates; |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve or find deficiencies with the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, we must demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or foreign regulatory agencies, that such product candidates are safe and effective for their intended uses. Results from preclinical studies and clinical trials can be interpreted in different ways. Even if we believe the preclinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities.
The FDA or any foreign regulatory bodies can delay, limit or deny approval of our product candidates or require us to conduct additional preclinical or clinical testing or abandon a program for many reasons, including:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our safety interpretation of our product candidate; |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our efficacy interpretation of our product candidate; and |
| ● | the FDA or comparable foreign regulatory authorities may regard our CMC package as inadequate. |
Of the large number of drugs in development, only a small percentage successfully complete the regulatory approval processes and are commercialized. This lengthy approval process, as well as the unpredictability of future clinical trial results, may result in our failing to obtain regulatory approval to market Epoladerm, Probudur, Envelta and/or NES100AnQlar, or another product candidate, which would significantly harm our business, results of operations and prospects.
In addition, the FDA or the applicable foreign regulatory agency also may approve a product candidate for a more limited indication or patient population than we originally requested, and the FDA or applicable foreign regulatory agency may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
If we are unable to file for approval of Epoladerm and Probudur under Section 505(b)(2) of the FDCA or if we are required to generate additional data related to safety and efficacy in order to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines.
Our current plans for filing NDAs for Epoladerm and Probudur include efforts to minimize the data we will be required to generate in order to obtain marketing approval and therefore reduce the development time. We intend to file Section 505(b)(2) NDAs for Epoladerm and Probudur that might, if accepted by the FDA, save time and expense in the development and testing of these indications.
The timeline for filing and review of our NDAs for Epoladerm and Probudur is based on our plan to submit the NDAs under Section 505(b)(2) of the FDCA, which would enable us to rely in part on data in the public domain or elsewhere. We have not yet filed an NDA under Section 505(b)(2) for any of our product candidates. Depending on the data that may be required by the FDA for approval, some of the data may be related to products already approved by the FDA. If the data relied upon is related to products already approved by the FDA and covered by third-party patents, we would be required to certify that we do not infringe the listed patents or that such patents are invalid or unenforceable. As a result of the certification, the third-party would have 45 days from notification of our certification to initiate an action against us. In the event that an action is brought in response to such a certification, the approval of our NDA could be subject to a stay of up to 30 months or more while we defend against such a suit. Approval of our product candidates under Section 505(b)(2) may therefore be delayed until patent exclusivity expires or until we successfully challenge the applicability of those patents to our product candidates. Alternatively, we may elect to generate sufficient additional clinical data so that we no longer rely on data which triggers a potential stay of the approval of our product candidates. Even if no exclusivity periods apply to our applications under Section 505(b)(2), the FDA has broad discretion to require us to generate additional data on the safety and efficacy of our product candidates to supplement third-party data on which we may be permitted to rely. In either event, we could be required, before obtaining marketing approval for any of our product candidates, to conduct substantial new research and development activities beyond those we currently plan to engage in order to obtain approval of our product candidates. Such additional new research and development activities would be costly and time consuming.
We may not be able to realize a shortened development timeline for Epoladerm and Probudur, and the FDA may not approve either of our NDAs based on their review of the submitted data. Moreover, if products containing the reference drug are withdrawn from the market by the FDA for any safety reason, we may not be able to reference such products to support a 505(b)(2) NDA for our product candidates, and we may need to fulfill the more extensive requirements of Section 505(b)(1). If we are required to generate additional data to support approval, we may be unable to meet our anticipated development and commercialization timelines, may be unable to generate the additional data at a reasonable cost, or at all, and may be unable to obtain marketing approval of our lead product candidate.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. We may encounter delays in enrolling, or be unable to enroll, a sufficient number of patients to complete any of our clinical trials, and even once enrolled, we may be unable to retain a sufficient number of patients to complete any of our trials. Patient enrollment and retention in clinical trials depends on many factors, including:
| ● | the patient eligibility criteria defined in the protocol; |
| ● | the size of the patient population required for analysis of the trial’s primary endpoints; |
| ● | the nature of the trial protocol; |
| ● | the existing body of safety and efficacy data with respect to the product candidate; |
| ● | the proximity of patients to clinical sites; |
| ● | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| ● | clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating; |
| ● | competing clinical trials being conducted by other companies or institutions; |
| ● | our ability to maintain patient consents; and |
| ● | the risk that patients enrolled in clinical trials will drop out of the trials before completion. |
We face risks related to health epidemics and outbreaks, including the COVID-19 pandemic, which could significantly disrupt our preclinical studies and clinical trials, and therefore our receipt of necessary regulatory approvals could be delayed or prevented.
We face risks related to health epidemics or outbreaks of communicable diseases. For example, the recent outbreak around the world, including in the United States, the European Union (the “EU”) members, China and many other countries, of the highly transmissible and pathogenic COVID-19. The outbreak of such communicable diseases could result in a widespread health crisis that could adversely affect general commercial activity and the economies and financial markets of many countries, which in the case of COVID-19 has occurred. In addition, the COVID-19 pandemic is having a severe effect on the clinical trials of many drug candidates. Some trials have been merely delayed, while others have been cancelled. The extent to which the COVID-19 pandemic may impact our preclinical and clinical trial operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the duration and geographic reach of the outbreak, the severity of COVID-19, and the effectiveness of actions to contain and treat COVID-19. The continued spread of COVID-19 globally could adversely impact our clinical trial operations, including our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 if an outbreak occurs in their geography. Disruptions or restrictions on our ability to travel to monitor data from our clinical trials, or to conduct clinical trials, or the ability of patients enrolled in our studies to travel, or the ability of staff at study sites to travel, as well as temporary closures of our facilities or the facilities of our clinical trial partners and their contract manufacturers, would negatively impact our clinical trial activities. In addition, we rely on independent clinical investigators, CROs and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our preclinical studies and clinical trials, including the collection of data from our clinical trials, and the outbreak may affect their ability to devote sufficient time and resources to our programs or to travel to sites to perform work for us. Similarly, our preclinical trials could be delayed and/or disrupted by the COVID-19 pandemic. As a result, the expected timeline for data readouts of our preclinical studies and clinical trials and certain regulatory filings may be negatively impacted, which would adversely affect our ability to obtain regulatory approval for and to commercialize our product candidates, increase our operating expenses and have a material adverse effect on our financial results.
16
Results of preclinical studies, early clinical trials or analyses may not be indicative of results obtained in later trials.
The results of preclinical studies, early clinical trials or analyses of our product candidates may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. In addition, conclusions based on promising data from analyses of clinical results may be shown to be incorrect when implemented in prospective clinical trials. Even if our clinical trials for Epoladerm, Probudur, Envelta and/or NES100AnQlar are completed as planned, we cannot be certain that their results will support the safety and efficacy sufficient to obtain regulatory approval.
Interim “top-line” and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim “top-line” or preliminary data from our clinical studies. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available. Preliminary or “top-line” data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data we previously published. As a result, interim and preliminary data should be viewed with caution until the final data are available. Adverse differences between preliminary or interim data and final data could significantly harm our business prospects.
Our product candidates may cause serious adverse events or undesirable side effects, which may delay or prevent marketing approval, or, if approved, require them to be taken off the market, require them to include safety warnings or otherwise limit their sales.
Serious adverse events or undesirable side effects caused by Epoladerm, Probudur, Envelta and/or NES100AnQlar or any other product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. Results of any clinical trial we conduct could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. Any clinical trials for our drug product candidates which include Epoladerm, Probudur, and/or NES 100Envelta and AnQlar to date may fail to demonstrate acceptable levels of safety and efficacy which could prevent or significantly delay their regulatory approval or result in a more restrictive label by the FDA or other comparable foreign authorities.
If unacceptable side effects arise in the development of our product candidates, we, the FDA or the IRBs at the institutions in which our studies are conducted, or the DSMB, if constituted for our clinical trials, could recommend a suspension or termination of our clinical trials, or the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of a product candidate for any or all targeted indications. In addition, drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete a trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our product candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our product candidates. Inadequate training in recognizing or managing the potential side effects of our product candidates could result in patient injury or death. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw approvals of such product; |
| ● | regulatory authorities may require additional warnings on the label, such as a “black box” warning or contraindication; |
| ● | additional restrictions may be imposed on the marketing of the particular product or the manufacturing processes for the product or any component thereof; |
| ● | we may be required to implement a Risk Evaluation and Mitigation Strategy (“REMS”) or create a medication guide outlining the risks of such side effects for distribution to patients; |
| ● | we could be sued and held liable for harm caused to patients; |
| ● | the product may become less competitive; and |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of a product candidate, if approved, and could significantly harm our business, results of operations and prospects.
The market opportunities for Epoladerm, Probudur, Envelta and NES100,AnQlar, if approved, may be smaller than we anticipate.
We expect to initially seek approval for Epoladerm for acute musculoskeletal pain, Probudur for postoperative pain management, and NES100Envelta for the management for acute and chronic pain, including pain associated with cancer, and AnQlar as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, in the United States. Our estimates of market potential have been derived from a variety of sources, including scientific literature, patient foundations, and market research, and may prove to be incorrect. Even if we obtain significant market share for any product candidate, if approved, if the potential target populations are smaller than we anticipate, we may never achieve profitability without obtaining marketing approval for additional indications.
We have never obtained marketing approval for a product candidate and we may be unable to obtain, or may be delayed in obtaining, marketing approval for any of our product candidates.
We have never obtained marketing approval for a product candidate. It is possible that the FDA may refuse to accept for substantive review any NDAs that we submit for our product candidates or may conclude after review of our data that our application is insufficient to obtain marketing approval of our product candidates. If the FDA does not accept or approve our NDAs for our product candidates, it may require that we conduct additional clinical, preclinical, or manufacturing validation studies and submit that data before it will reconsider our applications. Depending on the extent of these or any other FDA-required studies, approval of any NDA that we submit may be delayed or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA to approve our NDAs.
Any delay in obtaining, or an inability to obtain, marketing approvals would prevent us from commercializing our product candidates, generating revenues, and achieving and sustaining profitability. If any of these outcomes occur, we may be forced to abandon our development efforts for our product candidates, which could significantly harm our business.
Even if we obtain FDA approval for Epoladerm, Probudur, Envelta and/or NES100AnQlar or any other product candidate in the United States, we may never obtain approval for or commercialize Epoladerm, Probudur, Envelta and/or NES100AnQlar or any other product candidate in any other jurisdiction, which would limit our ability to realize their full market potential.
In order to market any products in any particular jurisdiction, we must establish and comply with numerous and varying regulatory requirements on a country-by-country basis regarding safety and efficacy. Approval by the FDA in the United States does not ensure approval by regulatory authorities in other countries or jurisdictions. However, the failure to obtain approval in one jurisdiction may negatively impact our ability to obtain approval elsewhere. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country.
Approval processes vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking foreign regulatory approval could result in difficulties and increased costs for us and require additional preclinical studies or clinical trials which could be costly and time consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our products in those countries. We do not have any product candidates approved for sale in any jurisdiction, including in international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of any product we develop will be unrealized.
Even if we obtain regulatory approval for Epoladerm, Probudur, Envelta and/or NES100AnQlar or any product candidate, we will still face extensive and ongoing regulatory requirements and obligations and any product candidates, if approved, may face future development and regulatory difficulties.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, packaging, distribution, adverse event reporting, storage, recordkeeping, export, import, advertising and promotional activities for such product, among other things, will be subject to extensive and ongoing requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, establishment registration and drug listing requirements, continued compliance with current Good Manufacturing Practice (“cGMP”) requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping and Good Clinical Practice (“GCP”) requirements for any clinical trials that we conduct post-approval.
Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product candidate may be marketed or to the conditions of approval, including a requirement to implement a REMS. If any of our product candidates receive marketing approval, the accompanying label may limit the approved indicated use of the product candidate, which could limit sales of the product candidate. The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use, and if we market our products for uses beyond their approved indications, we may be subject to enforcement action for off-label marketing. Violations of the FDCA relating to the promotion of prescription drugs may lead to FDA enforcement actions and investigations alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes or failure to comply with regulatory requirements, may yield various results, including:
| ● | restrictions on manufacturing such products; |
| ● | restrictions on the labeling or marketing of products; |
| ● | restrictions on product distribution or use; |
| ● | requirements to conduct post-marketing studies or clinical trials; |
| ● | warning letters or untitled letters; |
| ● | withdrawal of the products from the market; |
| ● | refusal to approve pending applications or supplements to approved applications that we submit; |
| ● | recall of products; |
| ● | fines, restitution or disgorgement of profits or revenues; |
| ● | suspension or withdrawal of marketing approvals; |
| ● | refusal to permit the import or export of our products; |
| ● | product seizure; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
Further, the FDA’s policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, certain policies of the current presidential administration may impact our business and industry. Namely, the current presidential administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. It is difficult to predict how these executive actions will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these executive actions impose constraints on FDA’s ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted.
Potential product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
The use of Epoladerm,, Probudur,, Envelta and/or NES100AnQlar or any other product candidates we may develop in clinical trials and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated adverse effects. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | impairment of our business reputation and significant negative media attention; |
| ● | withdrawal of participants from our clinical trials; |
| ● | significant costs to defend the litigation; |
| ● | distraction of management’s attention from our primary business; |
| ● | substantial monetary awards to patients or other claimants; |
| ● | inability to commercialize Epoladerm, |
| ● | product recalls, withdrawals, or labeling, marketing, or promotional restrictions; |
| ● | decreased market demand for any product; and |
| ● | loss of revenue. |
Risks Related to Commercialization
We face significant competition from other biotechnology and pharmaceutical companies and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical and pharmaceutical industries are highly competitive and subject to significant and rapid technological change. Our success is highly dependent on our ability to acquire, develop, and obtain marketing approval for new products on a cost-effective basis and to market them successfully. If Epoladerm, Probudur, Envelta and/or NES100AnQlar is approved, we will face intense competition from a variety of businesses, including large, fully integrated pharmaceutical companies, specialty pharmaceutical companies and biopharmaceutical companies in the United States and other jurisdictions. These organizations may have significantly greater resources than we do and may conduct similar research; seek patent protection; and establish collaborative arrangements for research, development, manufacturing and marketing of products that may compete with us.
Our competitors may, among other things:
| ● | have significantly greater name recognition, financial, manufacturing, marketing, drug development, technical, and human resources than we do, and future mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors; |
| ● | develop and commercialize products that are safer, more effective, less expensive, more convenient, or easier to administer, or have fewer or less severe effects; |
| ● | obtain quicker regulatory approval; |
| ● | implement more effective approaches to sales and marketing; or |
| ● | form more advantageous strategic alliances. |
Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel; establishing clinical trial sites and patient registration; and in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are more effective, have fewer or less severe side effects, or are more convenient or are less expensive than Epoladerm, Probudur, Envelta and/or NES100.AnQlar. Our competitors may also obtain FDA or other regulatory approval for their product candidates more rapidly than we may obtain approval for Epoladerm, Probudur, Envelta and/or NES100,AnQlar, which could result in our competitors establishing or strengthening their market position before we are able to enter the market.
The successful commercialization of Epoladerm, Probudur, Envelta and/or NES100AnQlar and any other product candidate we develop will depend in part on the extent to which governmental authorities and health insurers establish adequate coverage, reimbursement levels, and pricing policies. Failure to obtain or maintain coverage and adequate reimbursement for our product candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue.
The availability and adequacy of coverage and reimbursement by governmental healthcare programs such as Medicare and Medicaid, private health insurers and other third-party payors are essential for most patients to be able to afford prescription medications such as Epoladerm, Probudur, Envelta and/or NES100,AnQlar, if approved. Our ability to achieve acceptable levels of coverage and reimbursement for products by governmental authorities, private health insurers and other organizations will have an effect on our ability to successfully commercialize our drug and any other product candidates we develop. Assuming we obtain coverage for our product candidates by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. We cannot be sure that coverage and reimbursement in the United States or elsewhere will be available for our product candidates or any product that we may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs or biologics when an equivalent generic drug, biosimilar, or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidates as substitutable and offer to reimburse patients only for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidates, pricing of existing drugs may limit the amount we will be able to charge for our product candidates. These payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in our product candidates. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on our product candidates.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs and biologics will be covered. The Medicare and Medicaid programs increasingly are used as models in the United States for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
No uniform policy for coverage and reimbursement for products exists among third-party payors in the United States. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely.
We may also be subject to extensive governmental price controls and other market regulations outside of the United States, and we believe the increasing emphasis on cost-containment initiatives in other countries have and will continue to put pressure on the pricing and usage of medical products. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits.
Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our product candidates may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of our product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and biologics and surgical procedures and other treatments, has become intense. As a result, increasingly high barriers are being erected to the entry of new products.
Even if Epoladerm, Probudur, Envelta and/or NES100AnQlar or any product candidate we develop receives marketing approval, it may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
If Epoladerm, Probudur, Envelta and/or NES100AnQlar or any product candidate we develop receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors, and others in the medical community. If it does not achieve an adequate level of acceptance, we may not generate significant product revenues or become profitable. The degree of market acceptance of our product candidates, if approved, will depend on a number of factors, including but not limited to:
| ● | the efficacy and potential advantages compared to alternative treatments; |
| ● | effectiveness of sales and marketing efforts; |
| ● | the cost of treatment in relation to alternative treatments, including any similar generic treatments; |
| ● | our ability to offer our products for sale at competitive prices; |
| ● | the convenience and ease of administration compared to alternative treatments; |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| ● | the strength of marketing and distribution support; |
| ● | the availability of third-party coverage and adequate reimbursement; |
| ● | the prevalence and severity of any side effects; and |
| ● | any restrictions on the use of our product together with other medications. |
Because we expect sales of our product candidates, if approved, to generate substantially all of our revenues for the foreseeable future, the failure of our product candidates to find market acceptance would harm our business and could require us to seek additional financing.
If we are unable to establish sales, marketing, and distribution capabilities either on our own or in collaboration with third parties, we may not be successful in commercializing Epoladerm, Probudur, Envelta and/or NES100,AnQlar, if approved.
We do not have any infrastructure for the sales, marketing, or distribution of Epoladerm, Probudur, Envelta and/or NES100,AnQlar, and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. In order to market and successfully commercialize our drug or any product candidate we develop, if approved, we must build our sales, distribution, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. We expect to build a focused sales, distribution and marketing infrastructure to market Epoladerm, Probudur, Envelta and/or NES100,AnQlar, if approved, in the United States and Europe. There are significant expenses and risks involved with establishing our own sales, marketing and distribution capabilities, including our ability to hire, retain and appropriately incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities could delay any product launch, which would adversely impact the commercialization of that product. For example, if the commercial launch of Epoladerm, Probudur, Envelta and/or NES100AnQlar for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| ● | our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to physicians or attain adequate numbers of physicians to prescribe our products; and |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
We do not anticipate having the resources in the foreseeable future to allocate to the sales and marketing of our product candidates, if approved, in certain markets overseas. Therefore, our future success will depend, in part, on our ability to enter into and maintain collaborative relationships for such capabilities, the collaborator’s strategic interest in a product and such collaborator’s ability to successfully market and sell the product. We intend to pursue collaborative arrangements regarding the sale and marketing of Epoladerm, Probudur, Envelta and/or NES100,AnQlar, if approved, for certain markets overseas; however, we cannot assure you that we will be able to establish or maintain such collaborative arrangements, or if able to do so, that they will have effective sales forces. To the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, and there can be no assurance that such efforts will be successful.
If we are unable to build our own sales force or negotiate a collaborative relationship for the commercialization of Epoladerm, Probudur, Envelta and/or NES100,AnQlar, we may be forced to delay the potential commercialization of the drug or reduce the scope of our sales or marketing activities. If we need to increase our expenditures to fund commercialization activities for Epoladerm, Probudur, Envelta and/or NES100AnQlar we will need to obtain additional capital, which may not be available to us on acceptable terms, or at all. We may also have to enter into collaborative arrangements for Epoladerm, Probudur, Envelta and/or NES100AnQlar at an earlier stage than otherwise would be ideal and we may be required to relinquish rights to it or otherwise agree to terms unfavorable to us. Any of these occurrences may have an adverse effect on our business, operating results, and prospects.
If we are unable to establish adequate sales, marketing, and distribution capabilities, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates and may never become profitable. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
A variety of risks associated with operating internationally could materially adversely affect our business.
We currently have no international operations, but our business strategy includes potentially expanding internationally if any of our product candidates receive regulatory approval. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations, such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
| ● | failure by us to obtain and maintain regulatory approvals for the use of our products in various countries; |
| ● | additional potentially relevant third-party patent rights; |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; |
| ● | difficulties in staffing and managing foreign operations; |
| ● | complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems; |
| ● | limits in our ability to penetrate international markets; |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| ● | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm any future international expansion and operations and, consequently, our results of operations.
22
Risks Related to Our Dependence on Third Parties
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors, and any third parties we may engage in connection with development and commercialization, may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
Our employees and independent contractors, including principal investigators, CROs, consultants, vendors and any third parties we may engage in connection with development and commercialization of our product candidates, could engage in misconduct, including intentional, reckless or negligent conduct or unauthorized activities that violate: the laws and regulations of the FDA or other similar regulatory requirements of other authorities, including those laws that require the reporting of true, complete and accurate information to such authorities; manufacturing standards; data privacy, security, fraud and abuse and other healthcare laws and regulations; or laws that require the reporting of true, complete and accurate financial information and data. Specifically, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Activities subject to these laws could also involve the improper use or misrepresentation of information obtained in the course of clinical trials, creation of fraudulent data in preclinical studies or clinical trials or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgements, possible exclusion from participation in Medicare, Medicaid, other U.S. federal healthcare programs or healthcare programs in other jurisdictions, individual imprisonment, other sanctions, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations.
We currently rely on third-party contract manufacturing organizations (“CMOs”) for the production of clinical supply of Epoladerm, Probudur, and/or NES100Envelta and AnQlar and intend to rely on CMOs for the production of commercial supply of Epoladerm, Probudur, and/or NES100,Envelta and AnQlar, if approved. Our dependence on CMOs may impair the development and commercialization of the drug, which would adversely impact our business and financial position.
We have limited personnel with experience in manufacturing, and we do not own facilities for manufacturing. Instead, we rely on and expect to continue to rely on CMOs for the supply of cGMP grade clinical trial materials and commercial quantities of Epoladerm, Probudur, and/or NES100Envelta and AnQlar and any product candidates we develop, if approved. Reliance on CMOs may expose us to more risk than if we were to manufacture our product candidates ourselves. We intend to have manufactured a sufficient clinical supply of Epoladerm, Probudur, and/or NES100Envelta and AnQlar drug substance to enable us to complete our clinical trials, and we have also engaged or intend to engage a CMO to provide clinical and commercial supplysupplies of the drug product.products.
The facilities used to manufacture our product candidates must be inspected by the FDA and comparable foreign authorities. While we provide oversight of manufacturing activities, we do not and will not control the execution of manufacturing activities by, and are or will be essentially dependent on, our CMOs for compliance with cGMP requirements for the manufacture of our product candidates. As a result, we are subject to the risk that our product candidates may have manufacturing defects that we have limited ability to prevent. If a CMO cannot successfully manufacture material that conforms to our specifications and the regulatory requirements, we will not be able to secure or maintain regulatory approval for the use of our product candidates in clinical trials, or for commercial distribution of our product candidates, if approved. In addition, we have limited control over the ability of our CMOs to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or comparable foreign regulatory authority finds deficiencies with or does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval or finds deficiencies in the future, we may need to find alternative manufacturing facilities, which would delay our development program and significantly impact our ability to develop, obtain regulatory approval for or commercialize our product candidates, if approved. In addition, any failure to achieve and maintain compliance with these laws, regulations and standards could subject us to the risk that we may have to suspend the manufacture of our product candidates or that obtained approvals could be revoked. Furthermore, CMOs may breach existing agreements they have with us because of factors beyond our control. They may also terminate or refuse to renew their agreement at a time that is costly or otherwise inconvenient for us. If we were unable to find an adequate CMO or another acceptable solution in time, our clinical trials could be delayed, or our commercial activities could be harmed.
We rely on and will continue to rely on CMOs to purchase from third-party suppliers the raw materials necessary to produce our product candidates. We do not and will not have control over the process or timing of the acquisition of these raw materials by our CMOs. Moreover, we currently do notonly have any agreementsone agreement for the production of these raw materials.materials (for AnQlar). Supplies of raw material could be interrupted from time to time and we cannot be certain that alternative supplies could be obtained within a reasonable timeframe, at an acceptable cost, or at all. In addition, a disruption in the supply of raw materials could delay the commercial launch of our product candidates, if approved, or result in a shortage in supply, which would impair our ability to generate revenues from the sale of our product candidates. Growth in the costs and expenses of raw materials may also impair our ability to cost effectively manufacture our product candidates. There are a limited number of suppliers for the raw materials that we may use to manufacture our product candidates and we may need to assess alternative suppliers to prevent a possible disruption of the manufacture of our product candidates.
Finding new CMOs or third-party suppliers involves additional cost and requires our management’s time and focus. In addition, there is typically a transition period when a new CMO commences work. Although we generally have not, and do not intend to, begin a clinical trial unless we believe we have on hand, or will be able to obtain, a sufficient supply of our product candidates to complete the clinical trial, any significant delay in the supply of our product candidates or the raw materials needed to produce our product candidates, could considerably delay conducting our clinical trials and potential regulatory approval of our product candidates.
As part of their manufacture of our product candidates, our CMOs and third-party suppliers are expected to comply with and respect the proprietary rights of others. If a CMO or third-party supplier fails to acquire the proper licenses or otherwise infringes the proprietary rights of others in the course of providing services to us, we may have to find alternative CMOs or third-party suppliers or defend against claims of infringement, either of which would significantly impact our ability to develop, obtain regulatory approval for or commercialize our product candidates, if approved.
We intend to rely on third parties to conduct, supervise and monitor our clinical trials. If those third parties do not successfully carry out their contractual duties, or if they perform in an unsatisfactory manner, it may harm our business.
We rely, and will continue to rely, on CROs, CRO-contracted vendors and clinical trial sites to ensure the proper and timely conduct of our clinical trials. Our reliance on CROs for clinical development activities limits our control over these activities, but we remain responsible for ensuring that each of our trials is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards.
We and our CROs will be required to comply with the Good Laboratory Practice (“GLP”) requirements for our preclinical studies and GCP requirements for our clinical trials, which are regulations and guidelines enforced by the FDA and are also required by comparable foreign regulatory authorities. Regulatory authorities enforce GCP requirements through periodic inspections of trial sponsors, principal investigators and clinical trial sites. If we or our CROs fail to comply with GCP requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. Accordingly, if our CROs fail to comply with these requirements, we may be required to repeat clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and we do not control whether or not they devote sufficient time and resources to our clinical trials. Our CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities, which could harm our competitive position. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationship with any CROs terminate, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management’s time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have an adverse impact on our business, financial condition and prospects.
| ● | the number and type of our collaborations could adversely affect our attractiveness to future collaborators or acquirers; and |
| ● | the loss of, or a disruption in our relationship with, any one or more collaborators could harm our business. |
If any collaborations do not result in the successful development and commercialization of products or if one of our collaborators terminates its agreement with us, we may not receive any future research and development funding or milestone or royalty payments under such collaborations. If we do not receive the funding we expect under these agreements, our continued development of our product candidates could be delayed, and we may need additional resources to develop additional product candidates. All of the risks relating to product development, regulatory approval and commercialization described in this prospectus also apply to the activities of any collaborators and there can be no assurance that our collaborations will produce positive results or successful products on a timely basis or at all.
In addition, subject to its contractual obligations to us, if one of our collaborators is involved in a business combination or otherwise changes its business priorities, the collaborator might deemphasize or terminate the development or commercialization of our product candidates. If a collaborator terminates its agreement with us, we may find it more difficult to attract new collaborators and the perception of our business and our stock price could be adversely affected.
We may in the future collaborate with additional pharmaceutical and biotechnology companies for development and potential commercialization of therapeutic products. We face significant competition in seeking appropriate collaborators. Our ability to reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our product candidates or bring them to market or continue to develop our programs, and our business may be materially and adversely affected.
Risks Related to Healthcare Laws and Other Legal Compliance Matters
Enacted and future healthcare legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates, if approved, and may affect the prices we may set.
In the United States and other jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively the “ACA”) was enacted, which substantially changed the way healthcare is financed by both governmental and private insurers. Among the provisions of the ACA, those of greatest importance to the pharmaceutical and biotechnology industries include the following:
| ● | an annual, non-deductible fee payable by any entity that manufactures or imports certain branded prescription drugs and biologic agents (other than those designated as orphan drugs), which is apportioned among these entities according to their market share in certain government healthcare programs; |
| ● | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| ● | new requirements to report certain financial arrangements with physicians and teaching hospitals, including reporting “transfers of value” made or distributed to prescribers and other healthcare providers and reporting investment interests held by physicians and their immediate family members; |
| ● | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| ● | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs and biologics that are inhaled, infused, instilled, implanted, or injected; |
| ● | extension of a manufacturer’s Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| ● | expansion of eligibility criteria for Medicaid programs thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
| ● | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| ● | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services, or CMS, to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; |
| ● | expansion of the entities eligible for discounts under the Public Health Service program; and |
| ● | a licensure framework for follow on biologic products. |
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. The current presidential administration and Congress will likely continue to seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the ACA. This includes enactment of the TCJA (as defined below), which, among other things, removes penalties for not complying with the ACA’s individual mandate to carry health insurance. It is uncertain the extent to which any such changes may impact our business or financial condition.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, the Budget Control Act of 2011, resulted in aggregate reductions of Medicare payments to providers of 2% per fiscal year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2027 unless additional action is taken by Congress. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Additionally, the orphan drug tax credit was reduced as part of a broader tax reform. These new laws or any other similar laws introduced in the future may result in additional reductions in Medicare and other health care funding, which could negatively affect our customers and accordingly, our financial operations.
In addition, there has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been Congressional inquiries and proposed federal and state legislation designed to bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drugs. Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Individual states in the United States have also become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our product candidates or put pressure on our product pricing.
In markets outside of the United States, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. We cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative action in the United States or any other jurisdiction. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our product candidates may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability.
Our business operations and current and future relationships with investigators, healthcare professionals, consultants, third-party payors, patient organizations, and customers will be subject to applicable healthcare regulatory laws, which could expose us to penalties.
Our business operations and current and future arrangements with investigators, healthcare professionals, consultants, third-party payors, patient organizations, and customers, may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell and distribute our product candidates, if approved. Such laws include:
| ● | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving, or providing any remuneration (including any kickback, bribe, or certain rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order, or recommendation of, any good, facility, item, or service, for which payment may be made, in whole or in part, under U.S. federal and state healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. The U.S. federal Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other hand; |
| ● | the U.S. federal false claims and civil monetary penalties laws, including the civil False Claims Act (the “FCA”) which, among other things, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. A claim includes “any request or demand” for money or property presented to the federal government. In addition, manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims; |
| ● | the U.S. federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), and their respective implementing regulations, which impose, among other things, specified requirements relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization by covered entities subject to the rule, such as health plans, healthcare clearinghouses and healthcare providers as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions; |
| ● | the FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices; |
| ● | the U.S. federal legislation commonly referred to as the Physician Payments Sunshine Act, enacted as part of the ACA, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics, and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to the government information related to certain payments and other transfers of value to physicians and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members; and |
| ● | analogous U.S. state laws and regulations, including: state anti-kickback and false claims laws, which may apply to our business practices, including but not limited to, research, distribution, sales, and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, which requires tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available under such laws, it is possible that some of our business activities, including our consulting agreements and other relationships with physicians and other healthcare providers, some of whom receive stock or stock options as compensation for their services, could be subject to challenge under one or more of such laws. Ensuring that our current and future internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations.
If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to the imposition of civil, criminal and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements or oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment, which could affect our ability to operate our business. Further, defending against any such actions can be costly, time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
Any clinical trial programs we conduct or research collaborations we enter into in the European Economic Area may subject us to the General Data Protection Regulation.
If we conduct clinical trial programs or enter into research collaborations in the European Economic Area, we may be subject to the General Data Protection regulation (“GDPR”). The GDPR applies extraterritorially and implements stringent operational requirements for processors and controllers of personal data, including, for example, high standards for obtaining consent from individuals to process their personal data, robust disclosures to individuals, a comprehensive individual data rights regime, data export restrictions governing transfers of data from the European Union (the “EU”) to other jurisdictions, short timelines for data breach notifications, limitations on retention of information, increased requirements pertaining to health data, other special categories of personal data and coded data and additional obligations if we contract third-party processors in connection with the processing of personal data. The GDPR provides that EU member states may establish their own laws and regulations limiting the processing of personal data, including genetic, biometric or health data, which could limit our ability to use and share personal data or could cause our costs to increase. If our or our partners’ or service providers’ privacy or data security measures fail to comply with the GDPR requirements, we may be subject to litigation, regulatory investigations, enforcement notices requiring us to change the way we use personal data and/or fines of up to 20 million Euros or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, as well as compensation claims by affected individuals, negative publicity, reputational harm and a potential loss of business and goodwill.
We are subject to environmental, health and safety laws and regulations, and we may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities.
Our operations, including our development, testing and manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions.
As with other companies engaged in activities similar to ours, we face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of or exposure to hazardous or biological materials. Environmental, health and safety laws and regulations are becoming more stringent. We may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, the production efforts of our third-party manufacturers or our development efforts may be interrupted or delayed.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under our existing intellectual property license, we risk losing the rights to our intellectual property.
Each of the material license agreements in which we have engaged, including the license agreements with MedPharm Limited, LipoCureRxLipoCureRX Ltd. and Nanomerics Ltd. has provisions by which each of those companies could terminate the license agreements thereby terminating our access to the intellectual property licensed under those agreements. Termination of these license agreements would prevent the commercialization of the productsproduct candidates we are developing.
If we are unable to obtain and maintain patent protection for our technology, products, and product candidates or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our drug development programs and product candidates, and products.candidates\. Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to Epoladerm, OSF200, Probudur, , NES100,Envelta, PES200 and MMS019AnQlar and any future products and product candidates. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our development programs and product candidates and products.candidates. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
If the patent applications we own or license with respect to our development programs and product candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for Epoladerm, OSF200, Probudur, NES100,Envelta, PES200 and MMS019AnQlar or any future product candidates, it could dissuade companies from collaborating with us to develop product candidates, and threaten our ability to commercialize future product candidates. Any such outcome could have a materially adverse effect on our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our patent rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than U.S. law does. However, in certain instances, the laws of the United States are more restrictive than those of foreign countries. For example, a recent series of Supreme Court Cases has narrowed the types of subject matter considered eligible for patenting.
Accordingly, certain diagnostic methods are considered ineligible for patenting in the U.S. because they are directed to a “law of nature”. Further, publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology, products, or product candidates, in whole or in part, or patents being issued which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated, held unenforceable, in whole or in part, or reduced patent term. Such a result could limit our ability to stop others from using or commercializing similar or identical technologies and products to ours. Moreover, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. While various extensions may be available, the life of a patent is limited. Without patent protection for our current or future products, we may be open to competition from generic versions of such products. Given the amount of time required for the development, testing and regulatory review of new products, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from using or commercializing technologies or products similar or identical to ours.
We may become subject to third parties’ claims alleging infringement of their patents and proprietary rights, or we may need to become involved in lawsuits to protect or enforce our patents, which could be costly, time consuming, delay or prevent the development and commercialization of our products and product candidates or put our patents and other proprietary rights at risk.
Our commercial success depends, in part, upon our ability to develop, manufacture, market and sell our products and product candidates without alleged or actual infringement, misappropriation or other violation of the patents and proprietary rights of third parties. Litigation relating to infringement or misappropriation of patent and other intellectual property rights in the pharmaceutical and biotechnology industries is common, including patent infringement lawsuits, interferences, oppositions, reexamination, derivation and post-grant proceedings before the U.S. Patent and Trademark Office (“USPTO”), and corresponding foreign patent offices. The various markets in which we plan to operate are subject to frequent and extensive litigation regarding patents and other intellectual property rights. In addition, many companies in intellectual property-dependent industries, including the biotechnology and pharmaceutical industries, have employed intellectual property litigation as a means to gain an advantage over their competitors. Numerous U.S., European and other foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing products and product candidates. Some claimants may have substantially greater resources than we do and may be able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time than we could. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our products and product candidates may be subject to claims of infringement of the intellectual property rights of third parties.
We may be subject to third-party claims including infringement, interference or derivation proceedings, post-grant review and inter partes review before the USPTO or similar adversarial proceedings or litigation in other jurisdictions. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, and the holders of any such patents may be able to block our ability to commercialize the applicable product candidate unless we obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable. These proceedings may also result in our patent claims being invalidated, held unenforceable or narrowed in scope. Similarly, if our patents or patent applications are challenged during interference or derivation proceedings, a court may hold that a third-party is entitled to certain patent ownership rights instead of us. Further, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our compositions, formulations, methods of manufacture, or methods of treatment, prevention or use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable products and product candidates unless we obtained a license or until such patents expire or are finally determined to be invalid or unenforceable. In addition, defending such claims would cause us to incur substantial expenses and, if successful, could cause us to pay substantial damages, if we are found to be infringing a third party’s patent rights. If we are found to have infringed such rights willfully, the damages may be enhanced and may include attorneys’ fees. Further, if a patent infringement suit is brought against us or our third-party service providers, our development, manufacturing or sales activities relating to the product or product candidate that is the subject of the suit may be delayed or terminated. As a result of patent infringement claims, or in order to avoid potential infringement claims, we may choose to seek, or be required to seek, a license from the third party, which may require us to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if a license can be obtained on acceptable terms, the rights may be nonexclusive, which could give our competitors access to the same intellectual property rights. If we are unable to enter into a license on acceptable terms, we could be prevented from commercializing one or more of our products and product candidates, forced to modify such products and product candidates, or to cease some aspect of our business operations, which could harm our business significantly. Modifying our products and product candidates to design around third-party intellectual property rights may result in significant cost or delay to us and could prove to be technically infeasible. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business. In addition, if the breadth or strength of protection provided the patents and patent applications we own or in-license is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future products and product candidates.
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products and product candidates, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the United States and in Europe, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of eligibility, lack of written description, lack of novelty, obviousness or non-enablement. Third parties might allege unenforceability of our patents because someone connected with prosecution of the patent withheld relevant information, or made a misleading statement, during patent prosecution. The outcome of proceedings involving assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity of patents, for example, we cannot be certain that there is no invalidating prior art of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our products and product candidates. Furthermore, our patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without infringing our patents or other intellectual property rights.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors view these announcements in a negative light, the price of common stock could be adversely affected.
Finally, even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors view these announcements in a negative light, the price of our common stock could be adversely affected. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have an adverse effect on our ability to compete in the marketplace.
We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which might adversely affect our ability to develop, manufacture and market our products and product candidates.
We cannot guarantee that any of our or our licensors’ patent searches or analyses, including but not limited to the identification of relevant patents, the scope of patent claims or the expiration of relevant patents, are complete or thorough, nor can we be certain that we have identified each and every third-party patent and pending application in the United States, Europe and elsewhere that is relevant to or necessary for the commercialization of our products and product candidates in any jurisdiction. For example, in the United States, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States, Europe and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our future products and product candidates, or their manufacture or use may currently be unpublished. Additionally, pending patent applications that have been published can, subject to certain limitations, be later amended in a manner that could cover our products and product candidates or the use thereof. The scope of a patent claim is determined by an interpretation of the law, the written disclosure in a patent and the patent’s prosecution history. Our interpretation of the relevance or the scope of a patent or a pending application may be incorrect, which may negatively impact our ability to market our products and product candidates. We may incorrectly determine that our products and product candidates are not covered by a third-party patent or may incorrectly predict whether a third party’s pending application will issue with claims of relevant scope. Our determination of the expiration date of any patent in the United States, Europe or elsewhere that we consider relevant may be incorrect, which may negatively impact our ability to develop and market our products and product candidates. Our failure to identify and correctly interpret relevant patents may negatively impact our ability to develop and market our products and product candidates.
From time to time we may identify patents or applications in the same general area as our products and product candidates. We may determine these third-party patents are irrelevant to our business based on various factors including our interpretation of the scope of the patent claims and our interpretation of when those patents expire. If the patents are asserted against us, however, a court may disagree with our determinations. Further, while we may determine that the scope of claims that will issue from a patent application does not present a risk, it is difficult to accurately predict the scope of claims that will issue from a patent application, our determination may be incorrect, and the issuing patent may be asserted against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we fail in any such dispute, in addition to being forced to pay monetary damages, we may be temporarily or permanently prohibited from commercializing our products and product candidates. We might, if possible, also be forced to redesign our products and product candidates so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products and product candidates.
As is the case with other biopharmaceutical and pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical and pharmaceutical industries involve both technological complexity and legal complexity. Therefore, obtaining and enforcing biopharmaceutical and pharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the America Invents Act, or the AIA, which was passed in September 2011, resulted in significant changes to the U.S. patent system.
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application, but circumstances could prevent us from promptly filing patent applications on our inventions.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent before the USPTO. This applies to all of our U.S. patents, even those effectively filed before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action.
Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. It is not clear what, if any, impact the AIA will have on the operation of our business. However, the AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned and in-licensed patent applications and the enforcement or defense of our owned and in-licensed patents.
Additionally, the U.S. Supreme Court has ruled on several patent cases in recent years either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. Similarly, the complexity and uncertainty of European patent laws has also increased in recent years. In addition, the European patent system is relatively stringent in the type of amendments that are allowed during prosecution. Complying with these laws and regulations could limit our ability to obtain new patents in the future that may be important for our business.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance and annuity fees on any issued patent are due to be paid to the USPTO, European Patent Office (“EPO”) and other foreign patent offices over the lifetime of a patent. In addition, the USPTO, EPO and other foreign patent offices require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent failure to make payment of such fees or to comply with such provisions can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which such noncompliance will result in the abandonment or lapse of the patent or patent application, and the partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents within prescribed time limits. If we or our licensors fail to maintain the patents and patent applications covering our products and product candidates or if we or our licensors otherwise allow our owned or licensed patents or patent applications to be abandoned or lapse, our competitors might be able to enter the market, which would hurt our competitive position and could impair our ability to successfully commercialize our products and product candidates in any indication for which they are approved.
We enjoy only limited geographical protection with respect to certain patents and we may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents covering our products and product candidates in all countries throughout the world would be prohibitively expensive. Competitors may use our owned and in-licensed technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we and our licensors have patent protection, but enforcement is not as strong as that in the United States or the Europe. These products may compete with our products and product candidates, and our owned or in-licensed patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
In addition, we may decide to abandon national and regional patent applications before grant. The grant proceeding of each national or regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant patent offices, while granted by others. For example, unlike other countries, China has a heightened requirement for patentability, and specifically requires a detailed description of medical uses of a claimed drug. Furthermore, generic drug manufacturers or other competitors may challenge the scope, validity or enforceability of our owned and in-licensed patents, requiring us or our licensors to engage in complex, lengthy and costly litigation or other proceedings. Generic drug manufacturers may develop, seek approval for and launch generic versions of our products. It is also quite common that depending on the country, the scope of patent protection may vary for the same product candidate or technology.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws or rules and regulations in the United States and Europe, and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in other jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Furthermore, while we intend to protect our intellectual property rights in our expected significant markets, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our product candidates. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate, which may have an adverse effect on our ability to successfully commercialize our product candidates in all of our expected significant foreign markets. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished, and we may face additional competition from others in those jurisdictions.
Some countries also have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, some countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired.
If we do not obtain patent term extension in the United States under the Hatch-Waxman Act and in foreign countries under similar legislation, thereby potentially extending the term of marketing exclusivity for our products, our business may be materially harmed.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our products are obtained, once the patent life has expired for a product, we may be open to competition from competitive medications, including generic medications. Given the amount of time required for the development, testing and regulatory review of new products, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from using or commercializing technologies or products similar or identical to ours.
Depending upon the timing, duration and conditions of FDA marketing approval of our product candidates, we may be able to extend the term of a patent covering each product candidate under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments and similar legislation in the EU. The Hatch-Waxman Amendments permit a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. Patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent that is applicable to and covers an approved drug may be extended. Similar provisions are available in Europe, such as supplementary protection certificates, and in certain other non-United States jurisdictions to extend the term of a patent that covers an approved drug. However, we may not receive an extension if we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of a patent term extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner. As a result, our revenue from applicable products could be reduced, possibly materially.
Further, under certain circumstances, the term of a patent covering our products may be extended for time spent during the pendency of the corresponding patent application in the USPTO (referred to as Patent Term Adjustment, or PTA). The laws and regulations underlying how the USPTO calculates the PTA is subject to change and any such PTA granted by the USPTO could be challenged by a third-party. If we do not prevail under such a challenge, the PTA may be reduced or eliminated, resulting in a shorter patent term, which may negatively impact our ability to exclude competitors.
Because PTA added to the term of patents covering pharmaceutical products has particular value, our business may be adversely affected if the PTA is successfully challenged by a third party and our ability to exclude competitors is reduced or eliminated.
Intellectual property rights do not address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| ● | others may be able to make products that are similar to Epoladerm, OSF200, Probudur, |
| ● | others may independently develop similar or alternative technologies or otherwise circumvent any of our technologies without infringing our intellectual property rights; |
| ● | we or any of our collaborators might not have been the first to conceive and reduce to practice the inventions covered by the patents or patent applications that we own, license or will own or license; |
| ● | we or any of our collaborators might not have been the first to file patent applications covering certain technologies we or they own or have obtained a license, or will own or obtain a license; |
| ● | it is possible that our owned and in-licensed pending patent applications will not lead to issued patents; |
| ● | issued patents that we own and in-licensed may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| ● | our competitors might conduct research and development activities in countries where we do not have patent rights, or in countries where research and development safe harbor laws exist, and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| ● | ownership and inventorship of our owned and in-licensed patents or patent applications may be challenged by third parties; and |
| ● | patents of third parties, or pending or future applications of third parties, if issued, may have an adverse effect on our business. |
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that our trade secrets will be misappropriated or disclosed, and confidentiality agreements with employees and third parties may not adequately prevent disclosure of trade secrets and protect other proprietary information.
We consider proprietary trade secrets or confidential know-how and unpatented know-how to be important to our business. We may rely on trade secrets or confidential know-how to protect our technology, especially where patent protection is believed by us to be of limited value. Because we expect to rely on third parties to manufacture Epoladerm, OSF200, Probudur, NES100,Envelta, PES200, and MMS019AnQlar and any future products and product candidates, and we expect to collaborate with third parties on the development of Epoladerm, OSF200, Probudur, NES100,Envelta, PES200, and MMS019AnQlar and any future products and product candidates, we must, at times, share trade secrets with them. We also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. However, trade secrets or confidential know-how can be difficult to maintain as confidential.
To protect this type of information against disclosure or appropriation by competitors, our policy is to require our employees, consultants, collaborators, contractors and advisors to enter into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with us prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. However, current or former employees, consultants, collaborators, contractors and advisors may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. The need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have an adverse effect on our business and results of operations. Enforcing a claim that a third party obtained illegally and is using trade secrets or confidential know-how is expensive, time consuming and unpredictable. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction.
In addition, these agreements typically restrict the ability of our employees, consultants, collaborators, contractors and advisors to publish data potentially relating to our trade secrets, although our agreements may contain certain limited publication rights. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any of our third-party collaborators. A competitor’s discovery of our trade secrets would impair our competitive position and have an adverse impact on our business.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our unregistered trademarks or trade names. Over the long term, if we are unable to successfully register our trademarks and trade names and establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
35
We may need to license certain intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary for the development or commercialization of Epoladerm, OSF200, Probudur, NES100,Envelta, PES200 and/or MMS019AnQlar or our future products or product candidates. It may be necessary for us to use the patented or proprietary technology of third parties to commercialize Epoladerm, OSF200, Probudur, NES100,Envelta, PES200, and/or MMS019AnQlar or our products or product candidates, in which case we would be required to obtain a license from these third parties. Such a license may not be available on commercially reasonable terms, or at all, which could materially harm our business. At this time, we are unaware of any intellectual property that interferes with ours or is complementary and needed to commercialize Epoladerm, OSF200, Probudur, NES100,Envelta, PES200, and/or MMS019.AnQlar.
We may be subject to claims that our employees, consultants, collaborators contractors or advisors have wrongfully used or disclosed confidential information of their former employers or other third parties.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. Although we seek to protect our ownership of intellectual property rights by ensuring that our agreements with our employees, consultants, collaborators, contractors, advisors and other third parties with whom we do business include provisions requiring such parties to assign rights in inventions to us, we may be subject to claims that we or our employees, consultants, collaborators, contractors and advisors have inadvertently or otherwise used or disclosed confidential information of their former employers or other third parties. We may also be subject to claims that the former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and if we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Even if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees.
Our proprietary information may be lost, or we may suffer security breaches.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, clinical trial data, proprietary business information, personal data and personally identifiable information of our clinical trial subjects and employees, in our data centers and on our networks. The secure processing, maintenance and transmission of this information is critical to our operations. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Although, to our knowledge, we have not experienced any such material security breach to date, any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, significant regulatory penalties, disrupt our operations, damage our reputation and cause a loss of confidence in us and our ability to conduct clinical trials, which could adversely affect our reputation and delay our clinical development of our product candidates.
Risks Related to Our Employees, Managing Our Growth and Our Operations
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
We are highly dependent on the development, regulatory, commercialization and business development expertise of Anthony Mack, our Chief Executive Officer, as well as the other principal members of our management, scientific and clinical teams. Although we have employment agreements, offer letters or consulting agreements with our executive officers, these agreements do not prevent them from terminating their services at any time.
If we lose one or more of our executive officers or key employees, our ability to implement our business strategy successfully could be seriously harmed. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop product candidates, gain regulatory approval, and commercialize new products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be engaged by entities other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize product candidates will be limited.
We expect to expand our development, regulatory, and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities or acquire new facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We may engage in acquisitions that could disrupt our business, cause dilution to our stockholders or reduce our financial resources.
In the future, we may enter into transactions to acquire other businesses, products or technologies. If we do identify suitable candidates, we may not be able to make such acquisitions on favorable terms, or at all. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt in connection with an acquisition or issue our common stock or other equity securities to the stockholders of the acquired company, which would reduce the percentage ownership of our existing stockholders. We could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by the indemnification we may obtain from the seller. In addition, we may not be able to successfully integrate the acquired personnel, technologies and operations into our existing business in an effective, timely and nondisruptive manner. Acquisitions may also divert management attention from day-to-day responsibilities, increase our expenses and reduce our cash available for operations and other uses. We cannot predict the number, timing or size of future acquisitions or the effect that any such transactions might have on our operating results.
Our business and operations would suffer in the event of system failures.
Our computer systems, as well as those of our CROs and other contractors and consultants, are vulnerable to damage from computer viruses, unauthorized access, natural disasters (including hurricanes), terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs. For example, the loss of preclinical or clinical trial data from completed, ongoing or planned trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of personal, confidential or proprietary information, we could incur liability and the further development of Epoladerm, Probudur and/or NES100Envelta or any other product candidate could be delayed.
37
We are increasingly dependent on information technology, and our systems and infrastructure face certain risks, including cybersecurity and data leakage risks.
Significant disruptions to our information technology systems or breaches of information security could adversely affect our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information, and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. The size and complexity of our information technology systems, and those of our third-party vendors with whom we contract, make such systems potentially vulnerable to service interruptions and security breaches from inadvertent or intentional actions by our employees, partners or vendors, from attacks by malicious third parties, or from intentional or accidental physical damage to our systems infrastructure maintained by us or by third parties. Maintaining the secrecy of this confidential, proprietary, or trade secret information is important to our competitive business position. While we have taken steps to protect such information and invested in information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches in our systems or the unauthorized or inadvertent wrongful use or disclosure of confidential information that could adversely affect our business operations or result in the loss, dissemination, or misuse of critical or sensitive information. A breach of our security measures or the accidental loss, inadvertent disclosure, unapproved dissemination, misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, fraud, trickery or other forms of deception, or for any other reason, could enable others to produce competing products, use our proprietary technology or information, or adversely affect our business or financial condition. Further, any such interruption, security breach, loss or disclosure of confidential information, could result in financial, legal, business, and reputational harm to us and could have a material adverse effect on our business, financial position, results of operations or cash flow.
Risks Related to this Offering and Our Common Stock
No active trading market for our common stock currently exists, and an active trading market may not develop.
Prior to this offering, there has not been an active trading market for our common stock. If an active trading market for our common stock does not develop following this offering, you may not be able to sell your shares quickly or at the market price. Our ability to raise capital to continue to fund operations by selling shares of our common stock and our ability to acquire other companies or technologies by using shares of our common stock as consideration may also be impaired. The initial public offering price of our common stock will be determined by negotiations between us and the underwriters and may not be indicative of the market prices of our common stock that will prevail in the trading market.
The market price of our common stock may behas been volatile and can fluctuate substantially, which could result in substantial losses for purchasers of our common stock in this offering.
The market price of our common stock is likely to be highly volatile and since our initial public offering in February 2021, the market price of our common stock has ranged from $3.70 to $36.00 per share. The recent fluctuations in our trading price and future trading in our common stock may be subject to wide fluctuations in response to a variety of factors, including the following:
| ● | any delay in the commencement, enrollment and ultimate completion of our clinical trials; |
| ● | any delay in submitting an NDA and any adverse development or perceived adverse development with respect to the FDA’s review of that NDA; |
| ● | failure to successfully develop and commercialize Epoladerm, Probudur |
| ● | results of ongoing litigation to which we are a party; |
| ● | inability to obtain additional funding; |
| ● | regulatory or legal developments in the United States and other countries applicable to Epoladerm, Probudur, |
| ● | adverse regulatory decisions; |
| ● | changes in the structure of healthcare payment systems; |
| ● | inability to obtain adequate product supply for Epoladerm, Probudur, |
| ● | introduction of new products, services or technologies by our competitors; |
| ● | failure to meet or exceed financial projections we provide to the public; |
| ● | failure to meet or exceed the estimates and projections of the investment community; |
| ● | changes in the market valuations of companies similar to ours; |
| ● | market conditions in the pharmaceutical and biotechnology sectors, and the issuance of new or changed securities analysts’ reports or recommendations; |
| ● | announcements of significant acquisitions, strategic collaborations, joint ventures or capital commitments by us or our competitors; |
| ● | significant lawsuits, including patent or stockholder litigation, and disputes or other developments relating to our proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| ● | additions or departures of key scientific or management personnel; |
| ● | sales of our common stock by us or our stockholders in the future; |
| ● | trading volume of our common stock; |
| ● | general economic, industry and market conditions; |
| ● | health epidemics and outbreaks, including the COVID-19 pandemic, which could significantly disrupt our preclinical studies and clinical trials, and therefore our receipt of necessary regulatory approvals could be delayed or prevented; and |
| ● | the other factors described in this “Risk Factors” section. |
In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors, as well as general economic, political, regulatory and market conditions, may negatively affect the market price of our common stock, regardless of our actual operating performance. The market price of our common stock may decline below the initial public offering price, and you may lose some or all of your investment. In particular, stock markets have experienced extreme volatility in 2020 and during the first six months of 2020ended June 30, 2021 due to the ongoing COVID-19 pandemic and investor concerns and uncertainty related to the impact of the pandemic on the economies of countries worldwide.
Our management has broad discretion in using the net proceeds from this offering.
We have stated, in only a general manner, how we intend to use the net proceeds from this offering. See “Use of Proceeds.” We cannot, with any assurance, be more specific at this time. We will have broad discretion in the timing of the expenditures and application of proceeds received in this offering. If we fail to apply the net proceeds effectively, we may not be successful in bringing our proposed products to market. You will not have the opportunity to evaluate all of the economic, financial or other information upon which we may base our decisions to use the net proceeds from this offering.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against companies following a decline in the market price of their securities. This risk is especially relevant for us because biotechnology companies have experienced significant share price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
After this offering, our directors, executive officers and certain stockholders (one of which is an affiliate of our Chief Executive Officer) will continue to own a significant percentage of our common stock and, if they choose to act together, will be able to exert significant control over matters subject to stockholder approval.
Upon the closing of this offering, our directors, executive officers, and stockholders affiliated with our directors and executive officers will beneficially own approximately %36.8% of the voting power of our outstanding common stock, or approximately %34.7% if the underwriters exercise their over-allotment option from us in full. In particular, Virpax Pharmaceuticals, LLC, which is controlled by Anthony Mack, our Chief Executive Officer, and of which Jeffrey Gudin, our Chief Medical Officer, is a member, will beneficially own shares representing approximately %32.3% of our outstanding capital stock immediately after this offering. As a result, such entities and individuals will have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those entities and individuals. These individuals also have significant control over our business, policies and affairs as officers and directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our common stock, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts may publish about us or our business. We do not have any control over these analysts. If our financial performance fails to meet analyst estimates or one or more of the analysts who cover us downgrade our common stock or change their opinion of our common stock, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. As a result, capital appreciation, if any, of our common stock would be your sole source of gain on an investment in our common stock for the foreseeable future. See “Dividend Policy” for additional information.
If you purchase shares of our common stock in this offering, you will incur immediate dilution in the book value of your shares.
The initial public offering price of our common stock will be substantially higher than the as adjusted net tangible book value per share of our common stock. Therefore, if you purchase our common stock in this offering, you will pay a price per share of our common stock that substantially exceeds the book value of our tangible assets after subtracting our liabilities. Based on anthe assumed initial public offering price of $$14.70 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on September 8, 2021), you will experience immediate dilution of $$8.27 per share, representing the difference between our net tangible book value per share, after giving effect to this offering, and the assumed initial public offering price. Further, the future exercise of any outstanding options and/or warrants to purchase shares of our common stock will cause you to experience additional dilution. See “Dilution.”
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a newly public company, and particularly after we no longer qualify as an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur previously. The Sarbanes-Oxley Act of 2002 (“SOX”), the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of Nasdaq, and other applicable securities rules and regulations impose various requirements on U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our boardBoard of directors.Directors. In addition, these rules and regulations are often subject to varying interpretations, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Pursuant to Section 404 of SOX (“Section 404”), we will be required to furnish a report by our senior management on our internal control over financial reporting.reporting in our next annual filing.
While we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To prepare for eventual compliance with Section 404, once we no longer qualify as an emerging growth company, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We are an “emerging growth company,” and the reduced reporting requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (“the JOBS Act”). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including exemption from compliance with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of this offering, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock held by non-affiliates exceeds $700 million as of the end of our prior second fiscal quarter, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
In addition, under the JOBS Act, emerging growth companies may delay adopting new or revised accounting standards until such time as those standards apply to private companies. WeAlthough we have not done so, we may elect not to avail ourselves of this exemption from new or revised accounting standards and, therefore, may be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Anti-takeover provisions contained in our certificate of incorporation and bylaws, to be adopted upon the closing of this offering, as well as provisions of Delaware law, could impair a takeover attempt.
Our certificate of incorporation, bylaws and Delaware law contain or will contain provisions which could have the effect of rendering more difficult, delaying or preventing an acquisition deemed undesirable by our boardBoard of directors.Directors. Our corporate governance documents include or will include provisions:
| ● | classifying our |
| ● | authorizing “blank check” preferred stock, which could be issued by our |
| ● | limiting the liability of, and providing indemnification to, our directors and officers; |
| ● | limiting the ability of our stockholders to call and bring business before special meetings; |
| ● | requiring advance notice of stockholder proposals for business to be conducted at meetings of our stockholders and for nominations of candidates for election to our |
| ● | controlling the procedures for the conduct and scheduling of |
| ● | providing our |
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management.
As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation law, which prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without approval of the holders of substantially all of our outstanding common stock.
Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our certificate of incorporation, as amended, designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our certificate of incorporation requires that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will, to the fullest extent permitted by law, be the sole and exclusive forum for each of the following:
| ● | any derivative action or proceeding brought on our behalf; |
| ● | any action asserting a claim for breach of any fiduciary duty owed by any director, officer or other employee of ours to the Company or our stockholders, creditors or other constituents; |
| ● | any action asserting a claim against us or any director or officer of ours arising pursuant to, or a claim against us or any of our directors or officers, with respect to the interpretation or application of any provision of, the DGCL, our certificate of incorporation or bylaws; or |
| ● | any action asserting a claim governed by the internal affairs doctrine; |
provided, that, if and only if the Court of Chancery of the State of Delaware dismisses any of the foregoing actions for lack of subject matter jurisdiction, any such action or actions may be brought in another state court sitting in the State of Delaware.
The exclusive forum provision is limited to the extent permitted by law, and it will not apply to claims arising under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or for any other federal securities laws which provide for exclusive federal jurisdiction.
Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our amended and restated certificate of incorporation provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring such a claim arising under the Securities Act against us, our directors, officers, or other employees in a venue other than in the federal district courts of the United States of America. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our amended and restated certificate of incorporation.
Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, this provision may limit or discourage a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
We note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging lawsuits against our directors and officers.
42
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This prospectus, including the sections entitled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Business,” contains forward-looking statements. The words “believe,” “may,” “will,” “potentially,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” and similar expressions that convey uncertainty of future events or outcomes are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements concerning the following:
| ● | our lack of operating history; |
| ● | wide fluctuations in the trading price of our common stock; |
| ● | the expectation that we will incur significant operating losses for the foreseeable future and will need significant additional capital following this offering; |
| ● | our current and future capital requirements to support our development and commercialization efforts for our product candidates and our ability to satisfy our capital needs; |
| ● | our dependence on our product candidates, which are still in preclinical or early stages of clinical development; |
| ● | our ongoing litigation; |
| ● | our, or that of our third-party manufacturers, ability to manufacture cGMP quantities of our product candidates as required for pre-clinical and clinical trials and, subsequently, our ability to manufacture commercial quantities of our product candidates; |
| ● | our ability to complete required clinical trials for our product candidates and obtain approval from the FDA or other regulatory agencies in different jurisdictions; |
| ● | our lack of a sales and marketing organization and our ability to commercialize our product candidates if we obtain regulatory approval; |
| ● | our dependence on third-parties to manufacture our product candidates; |
| ● | our reliance on third-party CROs to conduct our clinical trials; |
| ● | our ability to maintain or protect the validity of our intellectual property; |
| ● | our ability to internally develop new inventions and intellectual property; |
| ● | interpretations of current laws and the passages of future laws; |
| ● | acceptance of our business model by investors; |
| ● | the accuracy of our estimates regarding expenses and capital requirements; |
| ● | our ability to adequately support organizational and business growth; and |
| ● | the continued spread of COVID-19 and the resulting global pandemic and its impact on our preclinical studies and clinical studies. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors” and elsewhere in this prospectus. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in our forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations, except as required by law.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
43
INDUSTRY AND OTHER DATA
The following is a glossary of certain industry terms used throughout this prospectus:
| ● | “ANDA” refers to an Abbreviated New Drug Application, which is used to seek approval from the FDA of a generic drug. Generic drug applications are called “abbreviated” because they are generally not required to include preclinical and clinical data to establish safety and effectiveness. Instead, an applicant must scientifically demonstrate that its product is bioequivalent (i.e., performs in the same way as the innovator drug). Once approved, an applicant may manufacture and market the generic drug. |
| ● | “AB-rated drugs” refer to drugs that meet bioequivalence requirements and which the FDA considers to be therapeutically equivalent and, therefore, substitutable with a reference listed drug. |
| ● | “branded products” refer to products that are marketed under a proprietary, often trademark-protected name. |
| ● | “BX-rated drugs” refer to drugs for which the data that have been reviewed by the FDA are insufficient to determine therapeutic equivalence. In these situations, the FDA presumes these drugs are not therapeutically equivalent until the FDA has determined that there is adequate information to make a full evaluation of therapeutic equivalence. |
| ● | “generic products” refer to products that are comparable to a branded product in dosage form, strength, route of administration, quality and performance characteristics and intended use. Before approving a generic product, the FDA requires many rigorous tests and procedures to ensure that the generic product can be substituted for the branded product. The FDA bases evaluations of substitutability or therapeutic equivalence of generic products on scientific evaluations. By law, a generic product must contain the identical amounts of the same active ingredients as the branded product. Generic products evaluated as therapeutically equivalent can be expected to have the same clinical effect and safety profile as the branded, or reference, product when administered under the conditions specified in the labeling. |
| ● | “NDA” refers to a New Drug Application. When the sponsor of a new drug believes sufficient evidence of the drug’s safety and effectiveness has been obtained to meet the FDA’s requirements for marketing approval, the sponsor submits an NDA to the FDA. The application must contain certain data about the drug, including information about chemistry, pharmacology, medical, biopharmaceutics and statistics. If the NDA is approved, the product may be marketed in the United States. |
| ● | “non-promoted products” refer to our products that we do not actively market or do not intend to actively market upon receipt of regulatory approval. |
| ● | “promoted products” refer to our products that we actively market or intend to actively market upon receipt of regulatory approval. |
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research, surveys and studies conducted by third parties. We believe that each of these studies and publications is reliable. We also believe our internal company research as to such matters is reliable and the market definitions are appropriate. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors including those described in the section titled “Risk Factors”. These and other factors could cause results to differ materially from those expressed in the estimate made by the independent parties and by us.
44
TRADEMARKS, SERVICE MARKS AND TRADE NAMES
We own or have rights to use a number of registered and common law trademarks, service marks and/or trade names in connection with our business in the United States and/or in certain foreign jurisdictions.
Solely for convenience, the trademarks, service marks, logos and trade names referred to in this prospectus are without the ® and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. This prospectus contains additional trademarks, service marks and trade names of others, which are the property of their respective owners. All trademarks, service marks and trade names appearing in this prospectus are, to our knowledge, the property of their respective owners. We do not intend our use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Virpax® is a registered tradename for Virpax® Pharmaceuticals, Inc. It was registered under the United States Patent and Trademark Office under serial number 87897821 on December 11th, 2019. Our logo is a registered tradename for Virpax® Pharmaceuticals, Inc. It was registered under the United States Patent and Trademark Office under serial number 87897809 on January 1st, 2019. For the purpose of this prospectus, Virpax® will be referred to as Virpax. Additionally, “we”, “our”, “the company” will be synonymous with Virpax. We have obtained a notice of allowance for our trademarks PROBUDURTM and, EPOLADERMTM and EnveltaTM.
We have also filed for trademark protection with the USPTO on AnQlarTM (formerly known as “MMS019”) on March 2, 2021.
45
USE OF PROCEEDS
We estimate that the net proceeds we will receive from the sale of our common shares in this offering, after deducting underwriting discounts and commissions and estimated expenses payable by us, will be approximately $$46.2 million (or $$53.2 million if the underwriters exercise their option to purchase additional shares in full). This estimate assumes, based on an initialassumed public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forth on the cover of this prospectus.
The principal purposes of this offering are to increase our financial flexibility, create a public market for our common stock and to facilitate our access toon the public equity markets. Nasdaq Capital Market on September 8, 2021).
We currently expect to use the net proceeds from this offering for product development activities, including clinical and regulatory research and development for our product candidates, to repay outstanding promissory notes issued to our Chief Executive Officer in the aggregate amount of $1,301,975 (which promissory notes each bear interest at a rate of 11.9% per annum and mature on December 31, 2023) and to pay deferred compensation due to our Chief Executive Officer in the aggregate amount of $1,051,875, and the remainder for working capital and other general corporate purposes, including the associated costs of operating as a public company. We currently expect to use the net proceeds from this offering as follows:
Based on our current projections, we believe the net proceeds of this offering will fund our operations for at least 12 monthsthrough December 2024 after the closing of this offering. We expect the net proceeds from this offering to fund Epoladerm through the submission of an NDA, and Probudur and NES100 through the submission of an IND.
We may also use a portion of the net proceeds of this offering for the acquisition or licensing, as the case may be, of additional technologies, other assets or businesses, or for other strategic investments or opportunities, although we currently have no understandings, agreements or commitments to do so.
Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. The amounts and timing of our actual expenditures will depend upon numerous factors, including our sales and marketing and commercialization efforts, demand for our products, our operating costs and the other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
A $1.00 increase (decrease) in the assumed initial public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover of this prospectus,Nasdaq Capital Market on September 8, 2021), would increase (decrease) the net proceeds to us from this offering by approximately $$3.2 million (or approximately $$3.6 million if the underwriters exercise their option to purchase additional shares in full), assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and, after deducting underwriting discounts and commissions and estimated expenses payable by us. An increase (decrease) of 1,000,000100,000 in the number of shares offered by us in this offering, would increase (decrease) the net proceeds to us from this offering by approximately $$1.4 million (or approximately $$1.4 million if the underwriters exercise their option to purchase additional shares in full), assuming the initial public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover of this prospectus,Nasdaq Capital Market on September 8, 2021), remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. The information above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing.
The net proceeds from this offering, together with our cash and marketable securities, may not be sufficient for us to fund any of our product candidates through regulatory approval, and we may need to raise additional capital to complete the development and commercialization of our product candidates.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
46
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Investors should not purchase our common stock with the expectation of receiving cash dividends. Any future determination related to our dividend policy will be made at the discretion of our boardBoard of directorsDirectors after considering our financial condition, results of operations, capital requirements, business prospects and other factors our boardBoard of directorsDirectors deems relevant, and subject to the restrictions contained in any future financing instruments.
47
CAPITALIZATION
The following table sets forth our cash and our capitalization as of June 30, 2020:2021:
| ● | on an actual basis; and |
| ● | on an as adjusted basis, giving effect to our issuance and sale of 3,401,360 shares of our common stock in this offering |
The as adjusted information belowWe derived this table from, and it should be read in conjunction with and is illustrative only,qualified in its entirety by reference to, our historical financial statements and our capitalization following the completion ofaccompanying notes included elsewhere in this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing.prospectus. You should read the following table together with our financial statements and the related notes appearing at the end of this prospectus and the “Summary Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Description of Securities” sections of this prospectus.
| As of June 30, 2021 (in thousands) | ||||||||
| Actual | As Adjusted | |||||||
| Cash | $ | 10,467 | $ | 56,690 | ||||
| Liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 2,780 | $ | 2,780 | ||||
| Notes payable | 50 | 50 | ||||||
| Total current liabilities | 2,830 | 2,830 | ||||||
| Notes payable, net of current portion | 22 | 22 | ||||||
| Related party notes payable | 1,000 | 1,000 | ||||||
| Total non-current liabilities | 1,022 | 1,022 | ||||||
| $ | 3,852 | $ | 3,852 | |||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.00001 par value; 100,000,000 shares authorized, and 4,960,153 shares issued and outstanding, actual; 100,000,000 shares authorized, and 8,446,541 shares issued and outstanding, as adjusted | $ | - | $ | - | ||||
| Additional paid-in capital | 22,906 | 69,130 | ||||||
| Accumulated deficit | (15,370 | ) | (15,371 | ) | ||||
| Total stockholders’ equity | $ | 7,536 | $ | 53,759 | ||||
| Total capitalization | $ | 11,388 | $ | 57,611 | ||||
| As of June 30, 2020 | ||||||||
| Actual | As Adjusted | |||||||
| Cash | $ | 417 | $ | |||||
| Liabilities: | ||||||||
| Notes payable | $ | 463 | ||||||
| Related party notes payable | 1,000 | |||||||
| Total current liabilities | 1,463 | |||||||
| Notes payable, net of current portion | 43 | |||||||
| Total non-current liabilities | 43 | |||||||
| $ | 1,506 | $ | ||||||
| Stockholders’ deficit: | ||||||||
| Common stock, $0.00001 par value; 20,000,000 shares authorized, and 15,495,436 shares issued and outstanding, actual; shares authorized, as adjusted and shares issued and outstanding, as adjusted | $ | - | $ | |||||
| Additional paid-in capital | 5,550 | |||||||
| Accumulated deficit | (8,234 | ) | ||||||
| Total stockholders’ deficit | $ | (2,684 | ) | $ | ||||
| Total capitalization | $ | (1,178 | ) | $ | ||||
A $1.00 increase or decrease in the assumed initial public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover page of this prospectus,Nasdaq Capital Market on September 8, 2021), would increase or decrease the pro forma as adjusted amount of each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $$3.2 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. At an assumed initial public offering price of $ per share, the as adjusted amount of each of cash, additional paid-in capital, total stockholders’ equity and total capitalization wouldAn increase by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of the prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase in the initial public offering price above $ per share would increase the as adjusted amount of each of cash, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $ million, assuming the number of shares offered by us, as set forth on the cover of the prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Aor decrease of 1,000,000100,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase or decrease the pro forma as adjusted amount of each of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $$1.4 million, assuming no change in the assumed initial public offering price per share and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, to shares would increase the as adjusted amount of each of cash, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $ million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming the assumed initial public offering price stays the same. An increase in the number of shares offered by us, as set forth on the cover page of this prospectus, to shares would increase the as adjusted amount of each of cash, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $ million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming the assumed initial public offering price stays the same. An increase of 1,000,000 shares in the number of shares offered by us above would increase the as adjusted amount of each of cash, additional paid-in capital, total stockholders’ equity and total capitalization by approximately $ million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming the initial public offering price stays the same.
The table above is based on 4,960,153 shares of our common stock outstanding as of June 30, 2021 and does not include:include as of such date:
| ● |
| ● |
| ● |
DILUTION
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the initial public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after this offering.
Dilution results from the fact that the initial public offering price per share is substantially in excess of the book value per share attributable to the existing stockholders for the presently outstanding shares of common shares.stock. We calculate net tangible book value per share by dividing the net tangible book value (total tangible assets less total liabilities) by the number of outstanding shares of common shares.stock.
Our historical net tangible book value deficit as of June 30, 20202021 was $$7.5 million, or $$1.52 per share of our common stock. Our historical net tangible book value deficit is the amount of our total tangible assets less our total liabilities and our common stock. Historical net tangible book value deficit per share represents our historical net tangible book value deficit divided by the 4,960,153 shares of our common stock outstanding as of June 30, 2020.2021.
After giving effect to the receipt of the estimated net proceeds from our sale of shares of common stock in this offering, assumingbased on an initialassumed public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover of this prospectus,Nasdaq Capital Market on September 8, 2021), after deducting estimated underwriting discounts and the application of thecommissions and estimated net proceeds therefrom as described under “Use of Proceeds,”offering expenses payable by us, our as adjusted net tangible book value at June 30, 20202021 would have been approximately $$53.8 million, or $$6.43 per share. This represents an immediate increase in net tangible book value per share of $$4.91 to existing stockholders and an immediate decrease in net tangible book value per share of $$8.27 to you. The following table illustrates this dilution on a per share basis:basis to new investors:
| Assumed public offering price per share | $ | 14.70 | ||
| Historical net tangible book value deficit per share as of June 30, 2021 | $1.52 | |||
| Increase in net tangible book value per share attributable to this offering | $4.91 | |||
| As adjusted net tangible book value per share after this offering | $ | 6.43 | ||
| Dilution per share to new investors purchasing common stock in this offering | $ | 8.27 |
The dilution information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing. A $1.00$1.00 decrease in the assumed initial public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover page of this prospectus,Nasdaq Capital Market on September 8, 2021), would decrease our as adjusted net tangible book value per share after this offering by $$0.38 and dilution per share to new investors purchasing common stock in this offering by $ ,$0.62, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase in the assumed initial public offering price of $$14.70 per share which is the midpoint(the last reported sale price of the price range set forthour common stock on the cover page of this prospectusNasdaq Capital Market on September 8, 2021), would increase our as adjusted net tangible book value per share after this offering by $$0.38 and dilution per share to new investors purchasing common stock in this offering by $ ,$0.62, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. AAn increase or decrease of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease our as adjusted net tangible book value per share after this offering by $ and increase dilution per share to new investors purchasing common stock in this offering by $ , assuming no change in the assumed initial public offering price per share and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 1,000,000100,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase or decrease our pro forma as adjusted net tangible book value per share after this offering by $$0.09 and decrease dilution per share to new investors purchasing common stock in this offering by $ ,$0.09, assuming no change in the assumed initial public offering price per share and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters’ over-allotment option is exercised in full, our as adjusted net tangible book value per share after this offering would be $$6.85 and dilution per share to new investors purchasing common stock in this offering would be $ , assuming an initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.$7.85.
The following table summarizes, as of June 30, 2020, on an as adjusted basis, the total number of shares of common stock purchased from us on an as converted to common stock basis and the total consideration paid or to be paid and the average price per share paid or to be paid by existing stockholder and by new investors in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing our common stock in this offering will pay an average price per share substantially higher than our existing stockholder paid.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by new investors by $ million and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage points, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. An increase (decrease) of 1,000,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by new investors by $ and, in the case of an increase, would increase the percentage of total consideration paid by new investors by percentage points and, in the case of a decrease, would decrease the percentage of total consideration paid by new investors by percentage points, assuming no change in the assumed initial public offering price per share.
The table above assumes no exercise of the underwriters’ over-allotment option. If the underwriters’ over-allotment option is exercised in full, the number of shares of our common stock held by existing stockholder would be reduced to % of the total number of shares of our common stock outstanding after this offering, and the number of shares of common stock held by new investors purchasing common stock in this offering would be increased to % of the total number of shares outstanding after this offering.
The tablesdiscussion above do not include:
| ● |
| ● |
| ● |
To the extent any outstanding options or other equity awards are exercised or become vested or any additional options or other equity awards are granted and exercised or become vested or other issuances of our common shares are made, there may be further economic dilution to new investors.
50
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion summarizes the significant factors affecting the operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with “Prospectus Summary—Summary Financial Information,” “Selected Financial Information” and the financial statements and the related notes thereto included elsewhere in this prospectus. The statements in this discussion regarding industry outlook, our expectations regarding our future performance, liquidity and capital resources and all other non-historical statements in this discussion are forward-looking statements and are based on the beliefs of our management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this prospectus, particularly in the section entitled “Risk Factors.” This discussion and analysis are based upon the historical financial statements of Virpax Pharmaceuticals, Inc. included in this prospectus. All references to years, unless otherwise noted, refer to our fiscal years, which end on December 31.
Overview
Company Overview
We are a clinical-stagepreclinical-stage pharmaceutical company focused on becoming a global leader in pain management by developing and delivering innovative non-opioid and non-addictive pharmaceutical products using new drug delivery systems and technology. We developare developing branded pharmaceutical product candidates forwith a focus on pain management by using cutting-edge technology to enhance patients’ quality of life.
We have exclusive global rights to develop, sell and export (among other rights) a proprietary patented Topical Spray Film Delivery Technologynonsteroidal anti-inflammatory topical spray film delivery technology for acute musculoskeletal pain (“DSF100” or “EpoladermTM”Epoladerm”) and for chronic osteoarthritis of the knee (“OSF200”). We also have exclusive global rights to a proprietary patented injectable, “local anesthetic” Liposomal Gel Technologylong-acting, local anesthetic liposomal gel technology for postoperative pain management (“LBL100” or “ProbudurTM”“Probudur”). Additionally, we have exclusive global rights to a proprietary patented Nanomerics’ Molecular Envelope Technology (“MET”) that uses an intranasal device to deliver exogenous enkephalin for the management of acute and chronic pain, including pain associated with cancer (“NES100”Envelta”) and for the management of Post-Traumatic Stress Disorder (“PES200”). We believe NES100 would supportEnkephalins are pain-relieving pentapeptides produced in the current effort among prescribers, regulators,human body, and patientsfunction to seekinhibit neurotransmitters in the pathway for pain perception, thereby reducing the physical impact of pain. While we are currently focused on the development of our non-opioid and non-addictive treatment optionspain management pipeline of product candidates, we also plan on using our proprietary delivery technologies to combat the opioid epidemic. Further, we havedevelop a nanotechnology that enables the exclusive North American rights to develop and commercializedelivery of a High-Density Molecular Masking Spray (MS019)metabolically labile peptide drug into the brain via intranasal delivery (“AnQlar”) as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans. We planhumans, including, but not limited to, utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2)influenza and NCE candidates for commercialization.SARS-CoV-2 (COVID 19).
We believe the Topical Spray Film Delivery Technologyour topical spray film delivery technology could provide a pathway for additional proprietary spray formulations and could potentially evolve into the preferred therapeutic treatment for topicals and transdermal deliveries due to itswith strong adhesion and accessibility properties, especially around joints and curved body surfaces. Pursuant to the MedPharm Research and Option Agreement (as defined below in “Material Agreement”), MedPharm (as defined below in “Material Agreements”) will conduct certain research and development activities of proprietary formulations incorporating certain MedPharm technologies and certain of our proprietary molecules. These proprietary molecules relate to indications which include, but are not limited to, treatment of estrogen levels, Alzheimer’s disease, dementia, Parkinson’s disease, neuropathic issues, and acute and chronic pain. Under the agreement,MedPharm Research and Option Agreement, we were granted an option to obtain an exclusive, world-wide, sub-licensable, royalty bearing, irrevocable license to research, develop, market, use, commercialize, and sell any product utilizing MedPharm’s spray formulation technology. See “Material Agreements” below for more information concerning this research and option agreement.
We have never been profitable and have incurred net losses since inceptioninception. Our net losses were $3,306,512$4,339,653 and $2,640,938$3,306,512 for the years ended December 31, 20192020 and 2018,2019, respectively. Our net losses for the six months ended June 30, 2021 and 2020 were $4,722,690 and 2019 were $1,926,279, and $1,574,522, respectively, and our accumulated deficit at June 30, 20202021 was $8,234,470.$15,370,534. We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability.
Trends and Uncertainties—Uncertainties — COVID-19
We are subject to risks and uncertainties as a result of the COVID-19 pandemic. The extent of the impact of the COVID-19 pandemic on our business is highly uncertain and difficult to predict, as the responses that we, other businesses and governments are taking continue to evolve. Furthermore, capital markets and economies worldwide have also been negatively impacted by the COVID-19 pandemic, and it is possible that it could cause a local and/or global economic recession. Policymakers around the globe have responded with fiscal policy actions to support the healthcare industry and economy as a whole. The magnitude and overall effectiveness of these actions remain uncertain.
The severity of the impact of the COVID-19 pandemic on our business will depend on a number of factors, including, but not limited to, the duration and severity of the pandemic and the extent and severity of the impact on our service providers, suppliers, contract research organizations and our clinicalpreclinical trials, all of which are uncertain and cannot be predicted.
As of the date of this filing, the extent to which the COVID-19 pandemic may in the future materially impact our financial condition, liquidity or results of operations is uncertain.
Financial Operations Overview
The following discussion sets forth certain components of our statements of operations as well as factors that impact those items.
Research and Development Expenses
Costs for research and development are charged as incurred and include employee-related expenses (including salaries and benefits, travel and expenses incurred under agreements with CROs, CMOs and service providers that assist in conducting clinical and preclinical studies), costs associated with preclinical activities and development activities and costs associated with regulatory operations.
Costs for certain development activities, such as preclinical and clinical studies, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations or information provided to us by our vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the patterns of costs incurred, and are reflected in our financial statements as prepaid expenses or accrued expenses as applicable.
We expect that our research and development expenses for the next several years will be higher than in 20192020 as a result of increased expenditures for the work needed for our expected initiation of clinical trials for Epoladerm, Probudur and NES100.Envelta. These expenditures are subject to numerous uncertainties regarding timing and cost to completion. Completion of our clinical development and clinical trials may take several years or more and the length of time generally varies according to the type, complexity, novelty and intended use of our product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| ● | the number of sites included in the clinical trials; |
| ● | the length of time required to enroll suitable patients; |
| ● | the size of patient populations participating in the clinical trials; |
| ● | the duration of patient follow-ups; |
| ● | the development stage of the product candidates; and |
| ● | the efficacy and safety profile of the product candidates. |
Due to the early stage of our research and development, we are unable to determine the duration or completion costs of our development of Epoladerm, Probudur, Envelta and NES100.AnQlar. As a result of the difficulties of forecasting research and development costs of Epoladerm, Probudur, Envelta and NES100AnQlar as well as the other uncertainties discussed above, we are unable to determine when and to what extent we will generate revenues from the commercialization and sale of an approved product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and other related costs, including stock-based compensation, for personnel serving in our executive, finance, accounting, legal and human resource functions. Our general and administrative expenses also include professional fees for legal services, including patent-related expenses, consulting, tax and accounting services, insurance and general corporate expenses. We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates.
We expect that our general and administrative expenses for the next several years will be higher than in 2019 and 2020 as we increase our headcount. We also anticipate increased expenses relating to our operations as a public company, including increased costs for the hiring of additional personnel, and for payment to outside consultants, including lawyers and accountants, to comply with additional regulations, corporate governance, internal control and similar requirements applicable to public companies, as well as increased costs for insurance.
52
Interest Expense
Interest expense consists primarily of interest expense on our promissory notes and convertible debt.
Income Taxes
We account for income taxes using the asset-and-liability method in accordance with ASC 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and our respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rate is recognized in the period that includes the enactment date. A valuation allowance is recorded if it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized in future periods.
We follow the guidance in ASC Topic 740-10 in assessing uncertain tax positions. The standard applies to all tax positions and clarifies the recognition of tax benefits in the financial statements by providing for a two-step approach of recognition and measurement. The first step involves assessing whether the tax position is more-likely-than-not to be sustained upon examination based upon its technical merits. The second step involves measurement of the amount to be recognized. Tax positions that meet the more-likely than-not threshold are measured at the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate finalization with the taxing authority. We recognize the impact of an uncertain income tax position in the financial statements if we believe that the position is more likely than not to be sustained by the relevant taxing authority. We will recognize interest and penalties related to tax positions in income tax expense. As of June 30, 20202021 and December 31, 2019,2020, we had no uncertain income tax positions.
Critical Accounting Policies and Use of Estimates
We have based our management’s discussion and analysis of financial condition and results of operations on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to clinical development expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe to be appropriate under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully discussed in Note 2 to our audited financial statements appearing at the end of this prospectus, we believe that the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our financial statements.
Research and Development Expenses
We rely on third parties to conduct our preclinical studies and to provide services, including data management, statistical analysis and electronic compilation. Once our clinical trials begin, at the end of each reporting period, we will compare the payments made to each service provider to the estimated progress towards completion of the related project. Factors that we will consider in preparing these estimates include the number of patients enrolled in studies, milestones achieved and other criteria related to the efforts of our vendors. These estimates will be subject to change as additional information becomes available. Depending on the timing of payments to vendors and estimated services provided, we will record net prepaid or accrued expenses related to these costs.
Fair Value of Common Stock and Stock-Based Compensation
We account for grants of stock options to employees and non-employees, and warrants to consultants, based on their grant date fair value and recognize compensation expense over the vesting periods. We estimate the fair value of stock options as of the date of grant using the Black-Scholes option pricing model. Estimates in our stock-based compensation valuations are highly complex and subjective.
In the absence of a public trading market for our common stock, on each grant date, we develop an estimate of the fair value of our common stock underlying the option grants. We estimated the fair value of our common stock by referencing arms-length transactions inclusive of the common stock underlying which occurred on or near the valuation date(s). Once our common stock is publicly traded, we will no longer have to estimate the fair value of the common stock, rather we will determine the value based on quoted market prices. We determined the fair value of our common stock using methodologies, approaches and assumptions consistent with the AICPA Practice Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation and based in part on input from an independent third-party valuation firm.
During the year ended December 31, 2018,2019, we issued 675,000135,336 options with exercise prices of $2.00$9.89 per share. For purposes of recognizing compensation expense in our financial statements, we estimated the value of the common stock underlying these grants to be $2.00$9.89 per share. During the year ended December 31, 2019,2020, we issued 669,167269,868 options with exercise prices of $2.00 per share. During the six months ended June 30, 2019, we issued 419,167 options with exercise prices of $2.00$9.89 per share. During the six months ended June 30, 2020, we issued 1,334,336269,868 options with exercise prices of $2.00$9.89 per share. During the six months ended June 30, 2021, we issued 250,492 options and 90,000 warrants with exercise prices of $4.62 and $12.50 per share, respectively. For purposes of recognizing compensation expense in our financial statements, we estimated the value of the common stockoptions underlying these grants to be $2.00$3.09 per share.share for the six months ended June 30, 2021. We used the Black-Scholes option pricing model to value itsthe option and warrant awards. The assumptions used in calculating the fair value of share-based awards represents management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different assumptions, share-based compensation expense could be materially different for future awards.
Results of Operations
Six Months Ended June 30, 20202021 and 20192020
Operating expenses:
| Six Months Ended | ||||||||||||||||
| June 30, | Change | |||||||||||||||
| 2020 | 2019 | Dollars | Percentage | |||||||||||||
| Operating expenses: | ||||||||||||||||
| General and administrative | $ | 1,510,835 | $ | 1,364,326 | $ | 146,509 | 11 | % | ||||||||
| Research and development | 335,553 | 154,559 | 180,994 | 117 | % | |||||||||||
| Total operating expenses | $ | 1,846,388 | $ | 1,518,885 | $ | 327,503 | 22 | % | ||||||||
| Six Months Ended June 30, | Change | |||||||||||||||
| 2021 | 2020 | Dollars | Percentage | |||||||||||||
| Operating expenses: | ||||||||||||||||
| General and administrative | $ | 3,262,544 | $ | 1,510,835 | $ | 1,751,709 | 116 | % | ||||||||
| Research and development | 1,391,565 | 335,553 | 1,056,012 | 315 | % | |||||||||||
| Total operating expenses | $ | 4,654,109 | $ | 1,846,388 | $ | 2,807,721 | 152 | % | ||||||||
General and administrative expenses increased by $146,509,$1,751,709, or 11%116%, to $3,262,544 for the six months ended June 30, 2021 from $1,510,835 for the six months ended June 30, 2020 from $1,364,3262020. The primary reasons for the increase in general and administrative costs were (i) an increase in legal costs associated mainly with litigation efforts and legal costs associated with general corporate purposes of $1,257,665, (ii) an increase in insurance costs related to directors’ and officers’ insurance of $404,747, (iii) an increase in exchange listing fees of $85,333, and (iv) an increase in accounting consulting fees of $54,919. This was slightly offset by a decrease in investor relation costs of $76,339 as compared to the prior period.
Research and development expenses increased by $1,056,012, or 315%, to $1,391,565 for the six months ended June 30, 2019. The increase was primarily the result of an increase of $278,066 in share based compensation expense as well as an increase of $57,124 in employee compensation and benefits. This was offset by a reduction in accounting and professional fees of $168,9452021 from the prior period.
Research and development expenses increased by $180,994, or 117%, to $335,553 for the six months ended June 30, 2020 from $154,559 for the six months ended June 30, 2019.2020. The increase was primarily the result of pre-IND activitiesattributable to a $1,000,000 milestone payment made to Nanomerics associated with AnQlar, as well as increases in pre-clinical activity related to NES100 of $169,459 and pre-clinical work associated with Epoladerm of $55,947.$94,399 and Probudur of $22,407 as well as an increase in regulatory activities with AnQlar of $39,788. This was slightly offset by a decrease of $125,015 in research and development of Probudur during the period of $30,611.pre-clinical activities associated with Envelta.
As a result of the foregoing, our loss from operations for the six months ended June 30, 20202021 was $1,846,388,$4,654,109, compared to a loss from operations of $1,518,885$1,846,388 for the six months ended June 30, 2019.2020.
Other income (expense):
| Six Months Ended | ||||||||||||||||
| June 30, | Change | |||||||||||||||
| 2020 | 2019 | Dollars | Percentage | |||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | $ | (83,891 | ) | $ | (55,637 | ) | $ | (28,254 | ) | 51 | % | |||||
| Other income | 4,000 | - | 4,000 | 100 | % | |||||||||||
| Total other expense | $ | (79,891 | ) | $ | (55,637 | ) | $ | (24,254 | ) | 44 | % | |||||
| Six Months Ended June 30, | Change | |||||||||||||||
| 2021 | 2020 | Dollars | Percentage | |||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | $ | (64,748 | ) | $ | (83,891 | ) | $ | 19,143 | (23 | )% | ||||||
| Other income | (3,833 | ) | 4,000 | (7,833 | ) | (196 | )% | |||||||||
| Total other expense | $ | (68,581 | ) | $ | (79,891 | ) | $ | 11,310 | (14 | )% | ||||||
OtherInterest expense increaseddecreased by $24,254,$19,143, or 44%23%, to $79,891$64,748 for the six months ended June 30, 20202021 from $55,637$83,891 for the six months ended June 30, 2019.2020. The increasedecrease was primarily the result of an increasethe repayment of $28,254a convertible promissory note in interest expense related to our related party promissory notes and convertibles notes with RRD slightly offset by $4,000 of funds received pursuant to a grant under Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”).
February 2021.
54
Liquidity and Capital Resources
Six Months Ended June 30, 20202021 and 20192020
Capital Resources
| June 30, | December 31, | Change | ||||||||||||||||
2020 | 2019 | Dollars | Percentage | |||||||||||||||
| Current assets | $ | 825,152 | $ | 46,719 | $ | 778,433 | 1,666 | % | ||||||||||
| Current liabilities | $ | 3,539,522 | $ | 2,778,994 | $ | 760,528 | 27 | % | ||||||||||
| Working capital | $ | (2,714,370 | ) | $ | (2,732,275 | ) | $ | 17,905 | (1 | )% | ||||||||
| Change | ||||||||||||||||
| June 30, 2021 | December 31, 2020 | Dollars | Percentage | |||||||||||||
| Current assets | $ | 11,387,696 | $ | 73,069 | $ | 11,314,627 | 15,485 | % | ||||||||
| Current liabilities | $ | 2,830,376 | $ | 3,659,914 | $ | (829,538 | ) | (23 | )% | |||||||
| Working capital | $ | 8,557,320 | $ | (3,586,845 | ) | $ | 12,144,165 | 339 | % | |||||||
Since our inception in 2017, we have devoted most of our cash resources to research and development and general and administrative activities. We have financed our operations primarily with the proceeds from the sale of common stock, related party note payables and convertible promissory notes. To date, we have not generated any revenues from the sale of products, and we do not anticipate generating any revenues from the sales of products for the foreseeable future. We have incurred losses and generated negative cash flows from operations since inception. As of June 30, 2020,2021, our principal source of liquidity was our cash, which totaled $416,909.
Equity Financings
During the six months ended June 30, 2020, we issued 634,450 shares of common stock for gross proceeds totaling $893,900. As of June 30, 2020, we had an outstanding stock subscription receivable of $375,000 for the purchase of 187,500 shares of our common stock. We received the funds on July 13, 2020 related to this stock subscription receivable.
Debt
Refer to the “Description of Certain Indebtedness” section for further details regarding our indebtedness.
Cash Flows
The following table summarizes our cash flows from operating and financing activities:
| Six Months Ended | ||||||||
| June 30, | ||||||||
| 2020 | 2019 | |||||||
| Statement of cash flow data: | ||||||||
| Total net cash provided by (used in): | ||||||||
| Operating activities | $ | (590,627 | ) | $ | (587,702 | ) | ||
| Financing activities | 966,000 | 625,000 | ||||||
| Increase in cash | $ | 375,373 | $ | 37,298 | ||||
Operating Activities
For the six months ended June 30, 2020, cash used in operations was $590,627 compared to $587,702 for the six months ended June 30, 2019. The slight increase in cash used in operations was primarily the result of an increase in costs associated with our pre-IND activities related to NES100 offset by the timing of payment on accounts payable and accrued balances.
Financing Activities
Cash provided by financing activities was $966,000 during the six months ended June 30, 2020, mainly attributable to the issuance of 634,450 shares of common stock for proceeds totaling $893,900, net of stock subscription receivable of $375,000, as well as proceeds of $72,100 received from our under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) pursuant to the CARES Act.
Cash provided by financing activities was $625,000 during the six months ended June 30, 2019, attributable to the issuance of 62,500 shares of common stock for gross proceeds totaling $125,000 as well as gross proceeds of $500,000 in the form of a related party note payable.
$10,466,774. To continue to grow our business over the longer term, we plan to commit substantial resources to research and development, pre-clinical and clinical trials of our product candidates, and other operations and potential product acquisitions and in-licensing. We have evaluated and expect to continue to evaluate a wide array of strategic transactions as part of our plan to acquire or in-license and develop additional products and product candidates to augment our internal development pipeline. Strategic transaction opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur additional indebtedness, seek equity capital or both. In addition, we may pursue development, acquisition or in-licensing of approved or development products in new or existing therapeutic areas or continue the expansion of our existing operations. Accordingly, we expect to continue to opportunistically seek access to additional capital to license or acquire additional products, product candidates or companies to expand our operations, or for general corporate purposes. Strategic transactions may require us to raise additional capital through one or more public or private debt or equity financings or could be structured as a collaboration or partnering arrangement. Any equity financing would be dilutive to our stockholders. We have no arrangements, agreements, or understandings in place at the present time to enter into any acquisition, in-licensing or similar strategic business transaction.
Results To date, we have not generated any revenues from the sale of Operationsproducts, and we do not anticipate generating any revenues from the sales of products for the foreseeable future. We have incurred losses and generated negative cash flows from operations since inception.
Years Ended December 31, 2019 and 2018
Operating expenses:
| Year Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2019 | 2018 | Dollars | Percentage | |||||||||||||
| Operating expenses: | ||||||||||||||||
| General and administrative | $ | 2,559,127 | $ | 1,483,786 | $ | 1,075,341 | 72 | % | ||||||||
| Research and development | 622,741 | 1,142,176 | (519,435 | ) | (45 | %) | ||||||||||
| Total operating expenses | $ | 3,181,868 | $ | 2,625,962 | $ | 555,906 | 21 | % | ||||||||
General and administrative expenses increased by $1,075,341, or 72%, to $2,559,127 for the year ended December 31, 2019 from $1,483,786 for the year ended December 31, 2018. The increase was primarily the result of a $553,250 increase in professional fees, a $293,191 increase in share-based compensation expense and a $187,689 increase in employee compensation and benefits.
Research and development expenses decreased by $519,435, or 45%, to $622,741 for the year ended December 31, 2019 from $1,142,176 for the year ended December 31, 2018. The decrease was primarily the result of a decrease of approximately $589,739 related to IND enabling studies for Epoladerm in 2018.
As a result of the foregoing, our loss from operations for the year ended December 31, 2019 was $3,181,868, compared to a loss from operations of $2,625,962 for the year ended December 31, 2018.
56
Other expenses:
| Year Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2019 | 2018 | Dollars | Percentage | |||||||||||||
| Other expenses: | ||||||||||||||||
| Interest expense | $ | (124,644 | ) | $ | (14,976 | ) | $ | (109,668 | ) | 732 | % | |||||
| Total other expenses: | $ | (124,644 | ) | $ | (14,976 | ) | $ | (109,668 | ) | 732 | % | |||||
Interest expense increased by $109,668, or 732%, to $124,644 for the year ended December 31, 2019 from $14,976 for the year ended December 31, 2018. The increase was primarily the result of an increase in the principal amount of outstanding notes payable of $500,000 and $264,520 with Anthony Mack and RRD, respectively.
Liquidity and Capital Resources
Years Ended December 31, 2019 and 2018
Capital Resources
| Year Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2019 | 2018 | Dollars | Percentage | |||||||||||||
| Current assets | $ | 46,719 | 61,500 | $ | (14,781 | ) | (24 | %) | ||||||||
| Current liabilities | 2,778,994 | 1,254,224 | 1,524,770 | 122 | % | |||||||||||
| Working capital | (2,732,275 | ) | (1,192,724 | ) | (1,539,551 | ) | 129 | % | ||||||||
As of December 31, 2019, our principal source of liquidity was our cash, which totaled $41,536. To continue to grow our business over the longer term, we plan to commit substantial resources to research and development, clinical trials of product candidates, and other operations and potential product acquisitions and in-licensing. We have evaluated and expect to continue to evaluate a wide array of strategic transactions as part of our plan to acquire or in-license and develop additional products and product candidates to augment our internal development pipeline. Strategic transaction opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur additional indebtedness, seek equity capital or both. In addition, we may pursue development, acquisition or in-licensing of approved or development products in new or existing therapeutic areas or continue the expansion of our existing operations. Accordingly, we expect to continue to opportunistically seek access to additional capital to license or acquire additional products, product candidates or companies to expand our operations, or for general corporate purposes. Strategic transactions may require us to raise additional capital through one or more public or private debt or equity financings or could be structured as a collaboration or partnering arrangement. Any equity financing would be dilutive to our stockholders. We have no arrangements, agreements, or understandings in place at the present time to enter into any acquisition, in-licensing or similar strategic business transaction.
Equity Financings
During the yearsix months ended December 31, 2019,June 30, 2020, we issued 312,500128,303 shares of common stock for gross proceeds totaling $625,000. During$1,268,900, which included a stock subscription receivable of $375,000 as of June 30, 2020, for the year ended December 31, 2018,purchase of 37,917 shares of our common stock. We received the Company issued 560,000funds related to this issuance on July 13, 2020.
On February 16, 2021, we closed an initial public offering of 1,800,000 shares of our common stock at a public offering price of $10.00 per share, for net proceeds of $15.8 million, after deducting underwriting discounts and offering expenses. We intend to use substantially all of the net proceeds from the offering to fund research and development of our Epoladerm, Probudur, Envelta and AnQlar indications and other development programs, and for working capital and other general corporate purposes.
In conjunction with our initial public offering, we amended our Certificate of Incorporation to increase the number of shares of common stock for gross proceeds totaling $1,120,000,and preferred stock we are authorized to issue to 100,000,000 and 10,000,000, respectively.
Debt
Promissory Notes
On October 1, 2018, we issued an aggregate of $500,000 ofa promissory note, as amended (the “2018 Promissory Note”), pursuant to which we are obligated to pay Anthony Mack, our Chairman and Chief Executive Officer, the principal amount of promissory notes bearing an$500,000 that bears interest at a rate of 11.19%. per annum. The 2018 Promissory Note has a maturity date of the earlier of an Event of Default (as defined in the 2018 Promissory Note) and December 31, 2023. As of June 30, 2021, the balance on the 2018 Promissory note was $500,000, with accrued interest of $153,862.
On January 15, 2019, we issued an additional $500,000 ofa promissory note, as amended (the “2019 Promissory Note”), pursuant to which we are obligated to pay Mr. Mack the principal amount of promissory notes bearing an$500,000 that bears interest at a rate of 11.19%. per annum. The 2019 Promissory Note has a maturity date of the earlier of an Event of Default (as defined in the 2019 Promissory Note) and December 31, 2023. As of June 30, 2021, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $137,544.
We plan on using a portion of the net proceeds from this offering to repay the full amounts due under the 2018 Promissory Note and the 2019 Promissory Note, including all accrued but unpaid interest thereon.
In January 2021, we issued notes with an aggregate principal amount of $75,000 and $25,000, respectively. These notes were issued to Anthony Mack for $75,000 and Christopher Chipman, our Chief Financial Officer, for $25,000. These notes were subsequently repaid with proceeds from our initial public offering in February 2021.
RRD Note
On August 29, 2019, we issued thea service provider convertible note (the “RRD Note”) to RRD NoteInternational, LLC (“RRD”) with a maximum principal balance of $400,000 that bears interest at a rate of 10.00% per annum, which will automatically convert into equity or cash (at the holders’ option) upon a transaction where we sell our equity securities for at least $1.5 million (a “RRD Qualified Financing”). We anticipate that thisOur initial public offering will constituteconstituted an RRD Qualified Financing. The RRD Note was amended on March 20, 2020 to increase the maximum principal balance to $600,000. In October 2020, we amended the RRD Note to extend the maturity date from September 30, 2020 to November 30, 2020, increase the principal amount from $434,260 to $493,480, extend the RRD Qualified Financing deadline from September 30, 2020 to November 30, 2020, and provide for the payment of all interest accrued from April 1, 2020 through November 30, 2020, which was $30,431. On November 30, 2020, we amended the RRD Note to extend the conversion date to December 31, 2020 and on December 31, 2020, we amended the RRD Note to extend the conversion date to January 31, 2021. As of September 30,December 31, 2020, the principal balance on the RRD Note was $493,480, with accrued interest of $22,208.$34,544. In February 2021, we repaid in full the RRD Note, including accrued interest, utilizing net proceeds received from our initial public offering.
PPP Loan
On May 4, 2020, we entered into a promissory note (the “PPP Note”) with PNC Bank as the lender (the “Lender”), pursuant to which the Lender agreed to make a loan to us under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”).
The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to our full time headcount during the period ending October 31, 2020. On July 2, 2021, we were notified by the Small Business Administration that the forgiveness amount totaled $61,816. The remaining balance of $10,284 must be repaid on or before maturity.
Refer also to the “Description of Certain Indebtedness” section for further details regarding our indebtedness.
Cash Flows
The following table summarizes our cash flows from operating and financing activities:
| Six Months Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Statement of cash flow data: | ||||||||
| Total net cash provided by (used in): | ||||||||
| Operating activities | $ | (4,877,749 | ) | $ | (590,627 | ) | ||
| Financing activities | 15,289,727 | 966,000 | ||||||
| Increase in cash | $ | 10,411,978 | $ | 375,373 | ||||
Operating Activities
For the six months ended June 30, 2021, cash used in operations was $4,877,749 compared to $590,627 for the six months ended June 30, 2020. The increase in cash used in operations was primarily the result of the increase in net loss and an increase in prepaid insurance premiums.
Financing Activities
Cash provided by financing activities was $15,289,727 during the six months ended June 30, 2021, attributable primarily to net proceeds received from our initial public offering in February 2021 of $15,783,207, after deducting underwriting discounts and offering expenses. This was offset by repayment in full of our RRD Note of $493,480 in February 2021. Cash provided by financing activities was $966,000 during the six months ended June 30, 2020, attributable to $893,900 from the sale of 128,303 shares of our common stock. Of the shares of common stock issued, the Company recorded a stock subscription receivable of $375,000 as of June 30, 2020, for the purchase of 37,917 shares of our common stock. We received the funds on July 13, 2020 related to this stock subscription receivable.
Results of Operations
Years Ended December 31, 2020 and 2019
Operating expenses:
| Year Ended December 31, | Change | |||||||||||||||
| 2020 | 2019 | Dollars | Percentage | |||||||||||||
| Operating expenses: | ||||||||||||||||
| General and administrative | $ | 2,904,104 | $ | 2,559,127 | $ | 344,977 | 13 | % | ||||||||
| Research and development | 1,291,615 | 622,741 | 668,874 | 107 | % | |||||||||||
| Total operating expenses | $ | 4,195,719 | $ | 3,181,868 | $ | 1,013,851 | 32 | % | ||||||||
General and administrative expenses increased by $344,977, or 13%, to $2,904,104 for the year ended December 31, 2020 from $2,559,127 for the year ended December 31, 2019. The increase was primarily the result of an increase of $795,497 in share-based compensation expense, offset by a decrease of $343,014 in professional fees and a decrease of $107,506 in other general and administrative expenses.
Research and development expenses increased by $668,874, or 107%, to $1,291,615 for the year ended December 31, 2020 from $622,741 for the year ended December 31, 2019. The increase was primarily the result of: i) an increase of $617,236 related to pre-clinical studies, certain milestone payments, and pre-IND related work associated with Envelta during the year ended December 31, 2020, ii) an increase of $76,482 related mainly to pre-clinical work associated with Epoladerm, and iii) an increase of $86,525 in professional fees associated with all indications. The increase in research and development expenses was partially offset by a $105,122 decrease in pre-clinical work related to Probudur during the year ended December 31, 2020.
As a result of the foregoing, our loss from operations for the year ended December 31, 2020 was $4,195,719, compared to a loss from operations of $3,181,868 for the year ended December 31, 2019.
Other expenses:
| Year Ended December 31, | Change | |||||||||||||||
| 2020 | 2019 | Dollars | Percentage | |||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | $ | (147,934 | ) | $ | (124,644 | ) | $ | (23,290 | ) | 19 | % | |||||
| Other income (expense) | 4,000 | - | 4,000 | 100 | % | |||||||||||
| Total other expenses: | $ | (143,934 | ) | $ | (124,644 | ) | $ | (19,290 | ) | 15 | % | |||||
Interest expense increased by $23,290, or 19%, to $147,934 for the year ended December 31, 2020 from $124,644 for the year ended December 31, 2019. The increase was primarily the result of an increase in the principal amount of outstanding notes payable of $228,960 due to RRD in the current year.
Liquidity and Capital Resources
Years Ended December 31, 2020 and 2019
Capital Resources
| Year Ended December 31, | Change | |||||||||||||||
| 2020 | 2019 | Dollars | Percentage | |||||||||||||
| Current assets | $ | 73,069 | $ | 46,719 | $ | 26,350 | 56 | % | ||||||||
| Current liabilities | 3,659,914 | 2,778,994 | 880,920 | 32 | % | |||||||||||
| Working capital | (3,586,845 | ) | (2,732,275 | ) | (854,570 | ) | 31 | % | ||||||||
As of December 31, 2020, our principal source of liquidity was our cash, which totaled $54,796.
Equity Financings
During the year ended December 31, 2020, we issued 139,220 shares of common stock for gross proceeds totaling $1,376,900. During the year ended December 31, 2019, we issued 63,203 shares of common stock for gross proceeds totaling $625,000.
Debt
Promissory Notes
On October 1, 2018, we issued the 2018 Promissory Note, pursuant to which we are obligated to pay Anthony Mack, our Chairman and Chief Executive Officer, the principal amount of $500,000 that bears interest at a rate of 11.19% per annum. The 2018 Promissory Note has a maturity date of the earlier of an Event of Default (as defined below) and January 15, 2022. As of December 31, 2020, the balance on the 2018 Promissory Note was $500,000, with accrued but unpaid interest of $125,887. As of December 31, 2019, the balance on the 2018 Promissory Note was $500,000, with accrued but unpaid interest of $74,692.
On January 15, 2019, we issued the 2019 Promissory Note, pursuant to which we are obligated to pay Mr. Mack the principal amount of $500,000 that bears interest at a rate of 11.19% per annum. The 2019 Promissory Note has a maturity date of the earlier of an Event of Default (as defined below) and January 15, 2022. As of December 31, 2020, the balance on the 2019 Promissory Note was $500,000, with accrued but unpaid interest of $109,569. As of December 31, 2019, the balance on the 2019 Promissory Note was $500,000, with accrued but unpaid interest of $56,329.
On October 28, 2020, we amended our 2018 Promissory Note dated October 1, 2018 and 2019 Promissory Note dated January 15, 2019 with Anthony Mack, Chief Executive Officer and significant investor, to extend the maturity date to December 31, 2023 for both promissory notes. All other terms and conditions of these agreements remained unchanged.
PPP Loan
On May 4, 2020, we entered into the “PPP Note with PNC Bank, pursuant to which the Lender agreed to make the PPP Loan offered by the SBA in a principal amount of $72,100 pursuant to Title 1 of the CARES Act.
The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt.
Refer to the “Description of Certain Indebtedness” section for further details regarding our indebtedness.
Cash Flows
Years Ended December 31, 20192020 and 20182019
The following table summarizes our cash flows from operating and financing activities:
| Year Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Statement of cash flow data: | ||||||||
| Total net cash provided by (used in): | ||||||||
| Operating activities | $ | (1,131,834 | ) | $ | (1,799,636 | ) | ||
| Financing activities | 1,125,000 | 1,674,766 | ||||||
| Decrease in cash | $ | (6,834 | ) | $ | (124,870 | ) | ||
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Statement of cash flow data: | ||||||||
| Total net cash provided by (used in): | ||||||||
| Operating activities | $ | (1,384,860 | ) | $ | (1,131,834 | ) | ||
| Financing activities | 1,398,120 | 1,125,000 | ||||||
| Increase (decrease) in cash | $ | 13,260 | $ | (6,834 | ) | |||
Operating Activities
For the year ended December 31, 2019,2020, cash used in operations was $1,131,834$1,384,860 compared to $1,799,636$1,131,834 for the year ended December 31, 2018.2019. The decreaseincrease in cash used in operations was primarily the result of the increase in net loss, offset by an increase in interest and stock-based compensation expense from 2018,2019, and an increase in accounts payable and accrued expense balances.
Financing Activities
Cash provided by financing activities was $1,398,120 during the year ended December 31, 2020, attributable to $1,376,900 from the sale of 139,220 shares of our common stock and $72,100 in proceeds received from the issuance of the PPP Loan offset by payment of deferred costs related to our initial public offering of $50,880. Cash provided by financing activities was $1,125,000 during the year ended December 31, 2019, attributable to $625,000 from the sale of 312,50063,203 shares of our common stock and $500,000 from the issuance of debt. Cash provided by financing activities was $1,674,766 during the year ended December 31, 2018, attributable to $1,120,000 from the sale of 560,000 shares of our common stock and $554,766 from the issuance of debt.
Future Capital Requirements
We expect that the net proceeds from this offering and our existing cash will be sufficient to fund our operations, future research and development, and general working capital for at least 12 months followingthrough approximately the closingthird quarter of this offering.2022. However, it is difficult to predict our spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control.
Our expectations regarding future cash requirements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make in the future. We have no current understandings, agreements or commitments for any material acquisitions or licenses of any products, businesses or technologies. We may need to raise substantial additional capital in order to engage in any of these types of transactions.
We expect to continue to incur substantial additional operating losses for at least the next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval, the eventual commercialization of our product candidates. If we obtain marketing approval for our product candidates, we will incur significant sales, marketing and outsourced manufacturing expenses. In addition, we expect to incur additional expenses to add operational, financial and information systems and personnel, including personnel to support our planned product commercialization efforts. We also expect to continue to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to us as a public company following the closing of this offering.company.
Our future use of operating cash and capital requirements will depend on many forward-looking factors, including the following:
| ● | the initiation, progress, timing, costs and results of clinical trials for our product candidates; |
| ● | the clinical development plans we establish for each product candidate; |
| ● | the number and characteristics of product candidates that we develop or may in-license; |
| ● | the terms of any collaboration agreements we may choose to execute; |
| ● | the outcome, timing and cost of meeting regulatory requirements established by the DEA, the FDA, the EMA or other comparable foreign regulatory authorities; |
| ● | the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights; |
| ● | the cost of defending intellectual property disputes, including patent infringement actions brought by third parties against us; |
| ● | costs and timing of the implementation of commercial scale manufacturing activities; and |
| ● | the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own. |
To the extent that our capital resources are insufficient to meet our future operating and capital requirements, we must finance our cash needs through public or private equity offerings, debt financings, collaboration and licensing arrangements or other financing alternatives. We have no committed external sources of funds. Additional equity or debt financing or collaboration and licensing arrangements may not be available on acceptable terms, if at all.
If we raise additional funds by issuing equity securities, our stockholder will experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our stockholder. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
Liquidity and Going Concern
Since inception, we have been engaged in organizational activities, including raising capital and research and development activities. We have not generated revenues and have not yet achieved profitable operations, nor have we ever generated positive cash flow from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. We are subject to those risks associated with any pre-clinicalpreclinical stage pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that our research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, we operate in an environment of rapid technological change and isare largely dependent on the services of our employees and consultants. Further, our future operations are dependent on the success of the Company’sour efforts to raise additional capital. These uncertainties raise substantial doubt about our ability to continue as a going concern for 12 months after the issuance date of our financial statements. The accompanying financial statements have been prepared on a going concern basis which contemplates the continuation of operations, realization of assets and liquidation of liabilities in the ordinary course of business.
We incurred a net loss of $3,306,512$4,339,653 and $2,640,938$3,306,512 for the years ended December 31, 20192020 and 2018,2019, respectively, and had an accumulated deficit of $6,308,191$10,647,844 as of December 31, 2019.2020. We incurred a net loss of $4,722,690 and $1,926,279 for the six months ended June 30, 2021 and 2020, respectively, and had an accumulated deficit of $8,234,470$15,370,534 as of June 30, 2020.2021. We had cash and cash equivalents of $10,466,774 as of June 30, 2021. We anticipate incurring additional losses until such time, if ever, that it can generate significant revenue from our product candidates currently in development. Our primary source of capital has been the issuance of debt and equity securities. We believe that current cash is sufficient to fund operations and capital requirements through November 2020.the third quarter 2022. Additional financings will be needed by us to fund our operations, to complete clinical development of and to commercially develop our product candidates. There is no assurance that such financing will be available when needed or on acceptable terms. We also have the ability to curtail spending in research and development activities in order to conserve cash.
Global Pandemic Outbreak
In March 2020, the World Health Organization declared COVID-19 a global pandemic. The outbreak has become increasingly widespread in the United States, impacting the markets in which we operate. While the full impact of the pandemic continues to evolve, the financial markets have been subject to significant volatility that adversely impacts our ability to enter into, modify, and negotiate favorable terms and conditions relative to equity and debt financing initiatives. The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values also affect our ability to enter into collaborations, joint ventures, and license and royalty agreements. The outbreak and government measures taken in response to the pandemic have also had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, have spiked, while demand for other goods and services, such as travel, have fallen. The future progression of the pandemic and its effects on our business and operations are uncertain. We may face difficulties recruiting or retaining patients in our ongoing and planned preclinical and clinical trials if patients are affected by the virus or are fearful of traveling to our clinical trial sites because of the outbreak. We and our third-party contract manufacturers, CROs, and clinical sites may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak. While expected to be temporary, these disruptions may negatively impact our results of operations, financial condition, and liquidity in 2020,2021, and potentially beyond.
59
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Recently Issued Accounting Standards
For a discussion of recent accounting pronouncements, please see Note 2, Summary of Significant Accounting Policies to our financial statements included elsewhere in this prospectus.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”) was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an “emerging growth company”. As an “emerging growth company,” we are electing to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period is irrevocable.
Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we are not required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation. These exemptions will apply until the fifth anniversary of the completion of our initial public offering or until we no longer meet the requirements for being an “emerging growth company,” whichever occurs first.
BUSINESS
Our Company
We are a preclinical-stage pharmaceutical company focused on becoming a global leader in pain management by developing and delivering innovative non-opioid and non-addictive pharmaceutical products using new drug delivery systems and technology. We are developing branded pharmaceutical product candidates with a focus on pain management by using cutting-edge technology to enhance patients’ quality of life.
We have exclusive global rights to develop, sell and export (among other rights) a proprietary patented nonsteroidal anti-inflammatory Topical Spray Film Delivery Technology for acute musculoskeletal pain (“DSF100” or “Epoladerm(EpoladermTM”) and for chronic osteoarthritis of the knee (“OSF200”)(OSF200). We also have exclusive global rights to a proprietary patented injectable, long-acting, local anesthetic Liposomal Gel Technology for postoperative pain management (“LBL100”(LBL100 or “ProbudurProbudurTM”). Additionally, we have exclusive global rights to a proprietary patented METMolecular Envelope Technology (MET) that uses an intranasal device to deliver exogenous enkephalin for the management of acute and chronic pain, (“NES100”including pain associated with cancer (EnveltaTM) and for the management of Post-Traumatic Stress Disorder (“PES200”)(PES200). Enkephalins are pain-relieving pentapeptides produced in the body, and function to inhibit neurotransmitters in the pathway for pain perception, thereby reducing the physical impact of pain. While the Company is currently focused on the development of its non-opioid and non-addictive pain management pipeline of product candidates, the Company also plans on using its proprietary delivery technologies to develop anti-viral therapies (AnQlar) as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19).
We believe the Topical Spray Film Delivery Technologytopical spray film delivery technology could provide a pathway for additional proprietary spray formulations and could potentially evolve into the preferred therapeutic treatment for topicals and transdermal deliveries due to itswith strong adhesion and accessibility properties, especially around joints and curved body surfaces. Pursuant to the MedPharm Research and Option Agreement, MedPharm will conduct certain research and development activities of proprietary formulations incorporating certain MedPharm technologies and certain of our proprietary molecules. These proprietary molecules relate to indications which include, but are not limited to, treatment of estrogen levels, Alzheimer’s disease, dementia, Parkinson’s disease, neuropathic issues, and acute and chronic pain. Under the agreement, we were granted an option to obtain an exclusive, world-wide, sub-licensable, royalty bearing, irrevocable license to research, develop, make, market, use, commercialize, and sell any product utilizing MedPharm’s spray formulation technology. See “Material Agreements” below for more information concerning this research and option agreement.
We believe NES100Envelta and PES200 could support the current effort among prescribers, regulators, and patients to seek non-addictive treatment options. We plan to utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and NCE candidates for commercialization.
While we are currently focused on the development of our non-opioid and non-addictive pain management pipeline of product candidates, we also plan on using our proprietary delivery technologies to develop anti-viral therapies as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19). As of the date of this prospectus, activities related to our anti-viral therapies have not commenced. We plan to finance our anti-viral-related activities through the use of grants and do not plan on using any proceeds from this offering.
Our Portfolio
Our portfolio currently consists of sixmultiple preclinical-stage product candidates: Epoladerm, OSF200, Probudur, NES100,Envelta, PES200 and MMS019.AnQlar. In the accompanying section we will describe each product candidate, its benefits, and our market strategy for each product candidate. The dates reflected in the below table are estimates only, and there can be no assurances that the events included in the below table will be completed on the anticipated timeline presented, or at all.

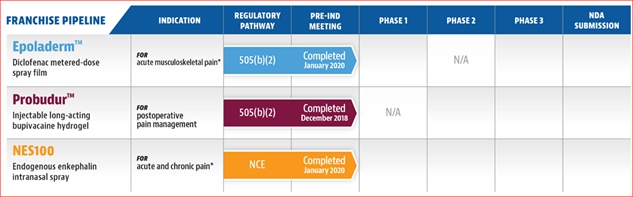
| 1,2,3 | We are also developing Envelta for a second indication, PES200, which utilizes the same delivery mechanism as Envelta. PES200 enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain for the treatment of post-traumatic stress disorder. The Envelta IND enabling studies may be cross-referenced to the PES200 IND. We are also developing Epoladerm for a second indication, OSF200, which utilizes the same transdermal delivery system as Epoladerm, as a twice daily topical treatment for chronic osteoarthritis of the knee. OSF200’s development plan is pending the approval of Epoladerm. AnQlar has completed IND-enabling toxicology studies and ex-vivo studies demonstrated a reduction in infectivity from respiratory viruses like influenza and SARS-CoV-2. AnQlar also reduced nasal and viral load in its animal study. |
61
* We are also developing Epoladerm for a second indication, OSF200, which utilizes the same transdermal delivery system as Epoladerm, as a twice daily topical treatment for chronic osteoarthritis of the knee. OSF200 development plan is pending the approval of Epoladerm. In addition, we are also developing NES100 for a second indication, PES200, which utilizes the same delivery mechanism as NES100. PES200 enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain for post-traumatic stress disorder. PES200 development plan is pending a grant approval. Further, we recently entered into a Collaboration and License Agreement with Nanomerics Ltd. for the exclusive North American license to develop and commercialize a High-Density Molecular Masking Spray (MMS019) as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans. We plan to develop MMS019 primarily through grants and do not plan on using any proceeds from this offering.
Diclofenac Epolamine Metered-Dose Spray Film (Epoladerm)
We believe our Diclofenac Epolamine metered-dose spray film technology, which we refer to as Epoladerm, could provide a pathway for additional proprietary spray formulations with strong adhesion and accessibility properties upon application, especially around joints and curved body surfaces. We plan to develop and market Epoladerm as a topical NSAID treatment for acute pain. We believe Epoladerm’s proprietary spray film technology may lead to adhesion capabilities superior to those of transdermal patches (e.g. Epoladerm does not require any tape reinforcement), while maintaining comparable skin absorption capabilities to transdermal patches currently on the market. Specifically, because the Epoladerm technology does not require a patch to deliver the drug through the skin, we believe Epoladerm may have better adhesion to the skin and may have better accessibility, particularly around joints and other curved body surfaces. Additionally, because Epoladerm is a spray, we believe it will be more aesthetically appealing than transdermal patches. As a spray, Epoladerm and OSF200 will bewere studied in non-clinical animal trials to demonstrateex-vivo skin studies (skin from abdominoplasty) and demonstrated drying times of between 60 and 90 seconds. Unlike other topical NSAIDs, Epoladerm does not require physical handling of the actual drug and enables metered dosing that provides an accurate amount of active ingredient per spray application. We plan to target our marketing and selling efforts on pain management clinics and high-prescribing healthcare practitioners including orthopedic surgeons, rheumatologists, physical medicine and rehabilitation specialists and primary care within the $3.3 billion (as of 2019) transdermal and topical non-opioid pain market.
Image 1, below, displays the expected delivery system of Epoladerm for the treatment of acute pain:
Image 1
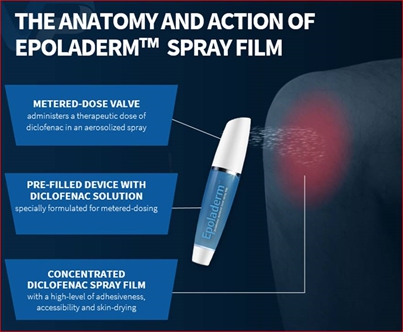
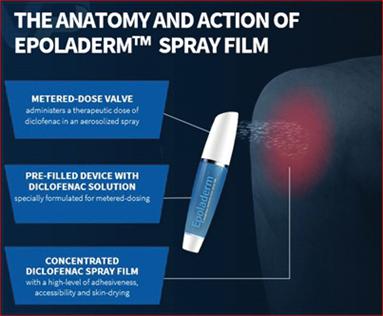
Image 2, below, illustrates the results of aMedPharm’s skin flux study which was conductedperformed a standard In Vitro Permeation Test (“IVPT”) experiment comparing the amount of diclofenac epolamine permeating through skin from both Epoladerm and Flector patch and noted that Epoladerm demonstrated similar absorption over the 24 hour period observed, despite a lower amount of drug product being applied to compare the skin absorption rates of Epoladerm and a leading commercially-available product:than contained in the Flector patch:

Image 2
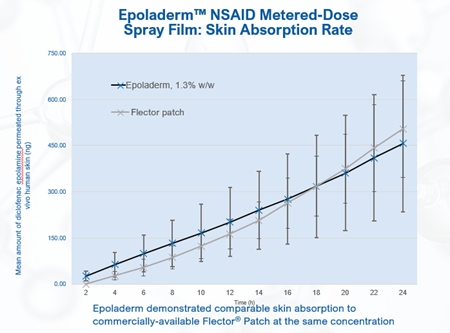
When discussing nonopioid treatments for chronic pain, the CDC notes clinicians should consider topical agents as alternative first-line analgesics, thought to be safer than systemic medications. In an August 18, 2020 article appearing in the Annals of Internal Medicine, the American College of Physicians and the American Academy of Family Physicians announced a joint clinical guideline, “Nonpharmacologic and Pharmacologic Management of Acute Pain from Non-Low Back, Musculoskeletal Injuries in Adults,” whereby they recommend topical NSAIDs as first-line therapy for patients experiencing pain from non-low back, musculoskeletal injuries. The clinical guideline also recommends that clinicians not prescribe opioids for these injuries except in cases of severe injury or if patients cannot tolerate first-line therapeutic options. We believe this creates a unique market opportunity for Epoladerm within the $3.3 billion (as of 2019) transdermal and topical non-opioid pain market. We plan to target our marketing and selling efforts to pain management clinics and high-prescribing healthcare practitioners, including orthopedic surgeons, rheumatologists, physical medicine and rehabilitation specialists and PCPs.
We believe Epoladerm represents a novel technology that administers a metered dose spray based on our proprietary spray. We are not aware of any other metered dose spray film product, on the market or in clinical development, that utilizes the same delivery mechanisms as Epoladerm. As a result of the Pre-IND review, the FDA has indicated that it is reasonable for us to pursue a 505(b)(2) accelerated NDA for Epoladerm. There can be no assurance that we will be successful in securing regulatory approval under the 505(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate.
The following is the planned development activity and status to bring Epoladerm to market:
We are also developing a second indication utilizing the same transdermal delivery system as Epoladerm as a twice daily topical treatment for chronic osteoarthritis of the knee (OSF200). OSF200 will use the same formulation as Epoladerm; however, OSF200 would be applied twice daily. OSF200 is also covered under the same intellectual property listed above for Epoladerm. As a result of the IND review, the FDA has indicated that it is reasonable for us to pursue a 505(b)(2) NDA for OSF200. There can be no assurance that we will be successful in securing regulatory approval under the 505(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate. OSF200 would be marketed as a topical NSAID treatment of chronic osteoarthritis for the knee. We believe OSF200’s adhesion capabilities and attributes are the same as listed for Epoladerm. Our plan would be to file a supplement to our potential NDA approval of Epoladerm. If we are able to obtain approval of the NDA for Epoladerm, we plan to conduct Phase III clinical trials for OSF200 with an anticipated NDA approval in approximately 18 months following a potential approval for Epoladerm. However, there can be no assurance that Epoladerm is approved by the FDA.
We have initiated a series of IND enabling toxicity studies for Epoladerm which are expected to take from eight months to one year to complete. If the completion of these studies are successful, we intend to submit an IND application to the FDA, including a trial design for a Phase I study.
Long-acting Bupivacaine Liposomal-gel 3.0% (LBL100 or ProbudurTM)
Probudur is a drug product candidate based on a unique liposomal delivery system LMVVs encapsulating a high dose of the local anesthetic bupivacaine. These drug-loaded liposomes are composed of lecithin and cholesterol which are GRAS by the FDA. These LMVVs are embedded in hydrogel beads to form a Lipogel. The system delivers a local analgesic medicine from the Lipogel. Early non-clinical animal studies produced data which suggests that Probudur may be able to provide improved onset, duration and peak performance properties as compared to a similar product on the market. Early data from these animal studies indicate that Probudur may provide pain control for up to 96 hours. TheseThe animal studies were conducted by administering Probudur by local infiltration of the surgical site which resulted in keeping the active ingredient localized at the surgical site for a longer period of time. Four trials were conducted using three animal models. The resultsData from these animal studies showed that after treatment with Probudur (50 mg/kg), statistically significant analgesic activity (measured as threshold pressure at animal’s withdrawal of the animal studies show initial supporttreated extremity) was observed in comparison to control (vehicle), for our belief that Probudur may potentially be safely administered to humans in a planned Phase IIA study.
Based on data from early animal studies, Probudur has indicated post-operative control for up toas long as 96 hours post-treatment (22.33±3.67g vs 5.00±0.58g; p<0.05), which is 24 hours longer than the leading product on the market.
If we are able to demonstrate a successful Phase III clinical trial, we believe Probudur may represent the first long acting local anesthetic with an opioid sparing label. The slow release of the drug from the liposomal depot reduces the peak plasma levels, reducing toxicity while also potentially providing longer-lasting post-operative pain control. We believe this property may permit administration of higher bupivacaine doses (3% versus 1.3% in leading market product); however, there can be no assurances, based on these animal studies, that Probudur will be safe and effective as these determinations are solely within the authority of the FDA. Further, there can be no assurance that Probudur will receive FDA approval.
Image 43 below illustrates the results of the early animal studies of Probudur:
Image 4 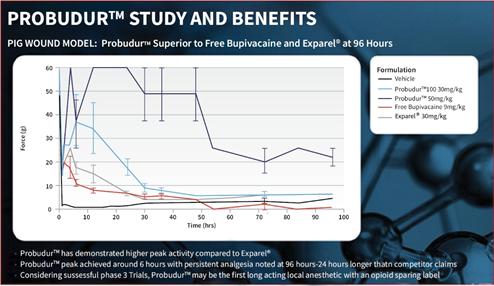
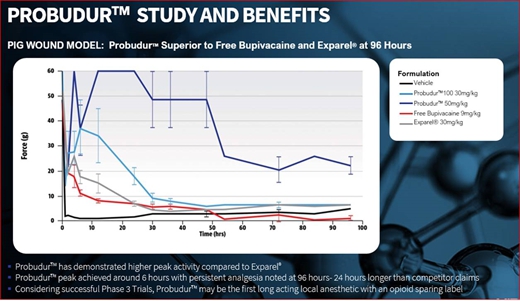
We plan to market Probudur to general surgeons, anesthesiologists, and orthopedic surgeons within the $577 million (as of 2019) local anesthetic post-surgical market. If the product candidate is used appropriately, we believe this product candidate could potentially eliminate the need for opioids for post-operative pain relief. As a result of our IND review, the FDA has indicated that it is reasonable for us to pursue a 505(b)(2) accelerated NDA for Probudur. There can be no assurance that we will be successful in securing regulatory approval under the 505(b)(2) pathway or that we will be successful in mitigating risks associated with the clinical development of this product candidate.
Image 5,4, below, displays the planned delivery of Probudur at the wound site:
Image 5 
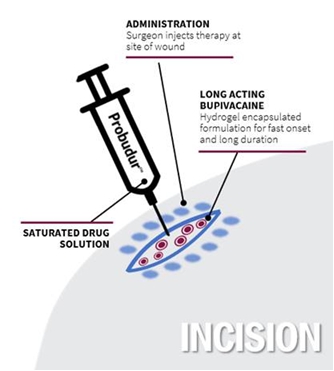
Probudur has completed IND Enabling studies “in vitro,” “in vivo efficacy” and “in vivo toxicology.” Based upon discussions with the FDA, we intend to move Probudur directly into dose escalation studies in targeted patient populations. We believe the completed IND Enabling studies allow us to transition directly to a Phase II study. The following is the planned development activity and status to bring Probudur to market:
| ||||
| ||||
Molecular Envelope Technology Enkephalin Intranasal Spray (NES100)(EnveltaTM)
NES100EnveltaTM is a nanotechnology-based intranasal spray drug product candidate which enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain. NES100Envelta is manufactured using high pressure homogenization and spray drying. The MET nanoparticles are well tolerated via the nasal route at the dose administered. Preclinical studies were conducted in animals for between 6 and 28 days through intravenous, oral and intranasal dosing. Twelve studies were conducted using three animal models whereby the animal studies were aimed at determining safety pharmacology and genetic toxicology. We believe theThe preliminary data from these early animal studies of NES100 support our beliefEnvelta have shown that NES100 may have comparable preclinical activity toEnvelta exhibited pain control in morphine in alltolerant animals, without the development of tolerance itself. These animal pain models tested without the drug seeking,anti-hyperalgesic effects in rats against evoked stimuli in a model of chronic inflammatory pain and against ongoing neuropathic pain in a conditioned placement preference model with spinal nerve ligation. Envelta and morphine were compared at the same dose level of 7.5 mg kg-1 in this model and Envelta was determined to have a similar analgesic effect. This analgesic effect was measured using the Complete Freund’s Adjuvant model (“CFA”) to induce inflammation in animals and determined Envelta produced relief of ongoing neuropathic pain similar to morphine. The baseline paw withdrawal threshold in the CFA model, using von Frey filaments, ranged from 22.3 ± 5.7 to 26.0 ± 0 g, reducing to a range of 2.9 ± 1.2 to 3.7 ± 1.5 g twenty-four hours after the intraplantar injection of CFA (0.1 mL). Five days after CFA dosing, the baseline paw withdrawal threshold ranged from 4.3 ± 0.3 to 5.7 ± 0.3 g. The baseline paw withdrawal thresholds, using von Frey filaments, for the sham and spinal ligation animals were 13.68 ± 0.72 g and 6.00 ± 0.82 g respectively. With respect to respiratory depression, and tolerance associated with opioids.delta opioid receptor agonists actually reverse the respiratory depression caused by morphine agonists, meaning that we believe Envelta will be unlikely to cause respiratory depression. However, there can be no assurances, based on these preclinical studies, that NES100Envelta will be safe and effective. Further, there can be no assurance that NES100Envelta will receive FDA approval.
We believe we have identified a large unmet need and market opportunity for current prescribers of opioids, including pain and hospice treatment centers. Currently, these prescribers may be using morphine-like opioids, which target three opioid receptors: mu, delta and kappa. Most analgesics used clinically target mu receptor, however, this receptor is also responsible for the majority of undesirable side effects associated with opioids. Currently, enkephalins are limited in their therapeutic potential by their pharmacokinetic profiles due to their inability to cross the blood-brain barrier to reach opioid receptors located in the central nervous system. However, we believe NES100’sEnvelta’s novel nasally delivered formulation, based on early animal studies, may enhance enkephalin transport to the brain by protecting the drug in a molecular envelope (MET), facilitating its crossing of the blood-brain barrier. Enkephalins bind predominantly to the delta-receptor which is typically not associated with the dangers associated with opioids. We believe NES100Envelta may have analgesic potential without opioid tolerance and has not exhibited any indications of withdrawal, respiratory depression euphoria, or addiction in the early animal studies. We plan to use the endogenous NCE regulatory pathway to bring this product candidate to market. We plan to target our marketing and selling efforts to pain specialists, anesthesiologists, orthopedic surgeons, PCPs, NPs, oncologists, and neurologists within the $7 billion (as of 2019) analgesic narcotics pain market. There can be no assurances, based on these preclinical animal studies, that NES100Envelta will be safe and effective in human trials.
NES100Envelta is a neuroactive peptide drug product (enkephalin) with a proprietary composition formulated for administration by all routes except the topical route. A preassembled device and cartridge would be used to propel the enkephalin formulation through the nose to the brain via the olfactory nerve/bulb route of transmission. A MET will encapsulate the drug product, protecting it from degradation and help to carry the drug across the blood-brain barrier to promptly suppress pain.
Image 65 and Image 7,6, below, display the planned delivery of enkephalin peptide nanoparticles to the brain via the olfactory route:
Image 6



Image 7,
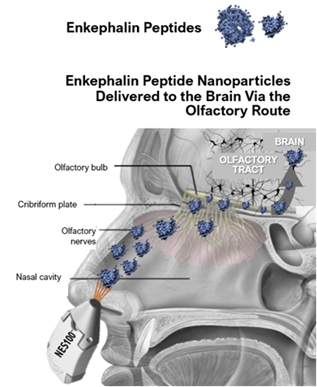
Image 8 below, illustrates the concept NES100Envelta delivery device:
Image 8 

NES100Envelta has completed IND enabling studies “in vitro,” “in vivo efficacy” and “in vivo toxicology.” The following is the planned activity and development status to bring the product candidate to market:
We are also developing NES100Envelta for a second indication utilizing the same delivery mechanism as NES100Envelta (“PES200”) which enables the delivery of a metabolically labile peptide drug (Enkephalin) into the brain. PES200 is also covered under the same intellectual property listed elsewhere in this prospectus for NES100.Envelta. We believe PES200’s capabilities and attributes are the same as NES100.Envelta. Our plan is to validate proof-of-concept followed by IND-enabling studies for the development of a novel enkephalin-based formulation to treat Post-Traumatic Stress Disorder. To date, two of the four planned initial in vitro studies have been successfully completed. These pre-clinical studies under the CRADA will be conducted through 2021 and 2022.
On August 25, 2020, we entered into a CRADA with NCATS. This collaboration is for the continued development of Virpax’s product candidate, NES100,Envelta, an intranasal peptide, for the management of acute and chronic non-cancer pain. The term of the CRADA is for a period of four years from the effective date of the agreement and can be terminated by both parties at any time by mutual written consent. In addition, either party may unilaterally terminate the CRADA at any time by providing written notice of at least sixty (60) days before the desired termination date. The agreement provides for studies that are focused on the pre-clinical characterization of NES100Envelta as a novel analgesic for acute and chronic non-cancer pain, and for studies to further develop NES100Envelta through IND enabling studies. There are certain development “Go/No Go” provisions within the agreement whereby, if certain events occur, or do not occur, NCATS may terminate the CRADA. These “No GO” provisions include: i) lack of efficacy in all animal pain models, ii) no reliable and sensitive bioanalytical method can be developed, iii) manufacturing failure due to inherent process scalability issues, iv) unacceptable toxicity or safety profile to enable clinical dosing, and v) inability to manufacture the NES100Envelta dosage form.
High-Density Molecular Masking Spray Formulation for the Prevention of Respiratory Viruses (MMS019)(AnQlar)
MMS019AnQlar is high-density molecular masking spray we plan to develop as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans. We intend for this formulation to be delivered using a
preassembled device and cartridge to propel the High-Density Molecular Sprayhigh-density molecular spray formulation into the nose. MMS019AnQlar will be used as a nasal powder spray to potentially prevent viral binding to epithelial cells in the nasal cavity and the upper respiratory tract, potentially reducing respiratory related infections.
WeIn addition to the standard personal protective equipment, we believe MMS019AnQlar may offer another layer of protection, in the form of a Molecular Mask, to be added to standard personal protective equipmentmolecular mask to protect healthcare workers and those at-risk of serious disease from viral infections. MMS019 has completed
Our results from an animal study of AnQlar demonstrated inhibited viral replication and decreased levels of virus in animal brain tissue. The animal study model included transgenic mice expressing the human ACE2 protein under the human cytokeratin 18 promoter (a type of protein found on epithelial cells, inside and outside of the body). Each experimental group consisted of 10 animals with 14 animals in the control group. AnQlar was administered once daily intranasally in the treatment group, and the control group received Remdesivir intramuscularly. Initially, the animals were infected intranasally with the SARS-CoV-2 virus. The virus was amplified and titrated in commonly used cell cultures [Vero cells]. Mice received AnQlar or Remdesivir every 24 hours from Day 1 until Day 6 post-infection. On day 6 post-infection, the animals were euthanized, and selected tissues were collected for analysis; viral RNA was isolated, and the viral infection was quantified by means of the RT-qPCR system that detects genetic material of the virus using a lab technique called polymerase chain reaction (PCR). The initial viral titer was comparatively higher than would be contained in an infected human droplet. However, after treatment with AnQlar, there was a marked inhibition of viral replication in the mouse nasal passages, and decreased levels of the virus were also recorded for the brain tissue, indicating the limited systemic infection. No adverse effects were observed during the experiment. We have submitted a pre-IND briefing package to the FDA and plan to finance our clinical development activities mainly through the use of grants.
Image 8 below illustrates the results of the AnQlar IND-enabling toxicology studies ex-vivo:
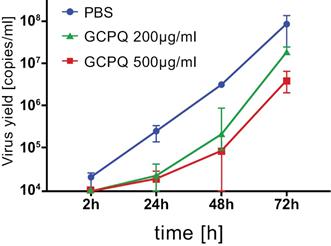
A lower viral yield was detected in the cultures treated with AnQlar than in the control with PBS after 72 hours of infection. We plan to market AnQlar to first responders, healthcare workers, clinics, military forces, transplant and intendsother immune compromised or at risk patients in the $13 billion (as of 2019) anti-viral market.
On August 17, 2021, we announced that we had received a written pre-investigational new drug response from the FDA for AnQlar providing guidance on our pathway to demonstratepursue prophylactic treatment Against SARS-CoV-2 and influenza for daily use as an over-the-counter (“OTC”) Product. We believe the results of the pre-IND response support further research on AnQlar as an intranasal protective that pre-treatmentmay limit transmission of nasal mucosal cells in vitro prevents, or reduces,influenza and SARS-CoV-2 to others. If we are able to successfully complete the number of cells that get infected. Werequired clinical trials for this product candidate, we intend to finance activities relatedmove forward and pursue an NDA for AnQlar as a once daily intranasal treatment. The FDA has indicated that, upon successful completion, we may pursue an NDA drug approval with the Office of Non-Prescription Drugs. We have engaged Syneos Health to MMS019assist with grants and not with any proceeds from this offering.the optimal clinical trial design based on an efficient timeline.
We continue to seek to exploit our product candidate portfolio through licensing and other strategic transactions to further develop our drug product candidates. This includes seeking potential partners in further developing our drug product candidates and responding to inquiries of interest we have received concerning our product portfolio.
Our Strengths
We are working within this larger context to create and advance novel non-addictive treatments for the management of acute musculoskeletal pain, chronic pain, mononeuropathy and post-operative pain. Our strategy is to develop product candidates that may provide a clinical advantage over current treatments. However, there can be no assurance that we will be able to execute on this strategy. With the support of the President, the U.S. Department of Health and Human Health Services (“HHS”), the National Institutes of Health (“NIH”) launched the initiative Helping to End Addiction Long-term (“HEAL”), to provide solutions to the national opioid crisis. Pursuant to this initiative, we have been awarded grantsin kind support related to NES100Envelta and have filed for additional grants that are currently pending. We believe HEAL and recent FDA guidance has made it more efficient to bring novel non-opioid medication to market.
We believe three of the below product candidates (Epoladerm, Probudur, and Envelta) have novel technologies that could improve pain management.management and one product candidate (AnQlar) could inhibit viral replication and decrease levels of virus in brain tissue. We believe these product candidates also have the following individual strengths and differentiators as compared to similar product candidates that we believe could make these product candidates more desirable if we obtain FDA approval:approved:
| ● |
| ● |
| ● |
| ● | AnQlarhas demonstrated inhibited viral replication and |
Also, three of the sixour product candidates (Epoladerm, Probudur, and OSF200) could potentially be developed using the FDA’s 505(b)(2) regulatory pathway. This type of submission differs from the FDA’s standard 505(b)(1) NDA clinical development pathway typically used for most pharmaceutical NCEs in that the development candidate (e.g., Diclofenac Epolamine Metered-Dose spray) in question contains similar active ingredients to a previously approved drug. Consequently, the data included in the submission can rely, at least partially, on the FDA’s findings of safety and effectiveness related to the other product, and as a result can mitigate many of the drug development risks faced by the drug developer. Companies utilizing the 505(b)(2) regulatory pathway typically experience a shorter drug development program that requires less resources than the standard regulatory pathway. Under the 505(b)2 pathway, the FDA allows the use of data from a prior application that can be referenced by a new sponsor that can include part of the required preclinical or clinical studies for approval. Consequently, this alternative pathway can significantly lower the development cost and shorten the timeline to NDA approval.
We are focused on applying our spray film and liposomal gel technologies to already-approved pharmacological actives. We intend to seek to leverage the 505(b)(2) accelerated regulatory pathway to accelerate our development timeline and lower our clinical and regulatory risk for not only our Epoladerm drug candidate, but for the OSF200 and Probudur product candidates as well.
There can be no assurance that the 505(b)(2) accelerated NDA pathway will lead to a faster development process or regulatory review. Further, while the 502(b)(2) pathway may expedite development or the approval process, it does not change the FDA’s standards of approval or increase the likelihood that a product candidate will receive approval.
Our Team
We have assembled a highly experienced management team, boardBoard of directorsDirectors and scientific advisory board to execute on our mission to develop product candidates that effectively manage pain in all its complexities, while minimizing risks to patients and society. Our team has a proven track record in developing, launching and marketing multiple pain products.
Our Founder and Chief Executive Officer, Anthony Mack, is a business leader with over 25 years of experience in the pharmaceutical and finance industries. Our Chief Medical Officer, Jeffrey Gudin, M.D., is a Clinical Associate Professor in Anesthesiology at the Rutgers New Jersey Medical School and is Board Certified in Pain Medicine, Anesthesiology, Addiction Medicine and Hospice and Palliative Medicine. Our EVP, Commercial Operations Officer, Gerald W. Bruce, has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical and Medical Nutrition industry. Our Chief Financial Officer, Christopher M. Chipman, CPA, has more than 25 years of industry experience assisting public companies with financial reporting, forecasting, preparation of periodic reports required to be filed with the Securities and Exchange Commission and compliance with Section 404 of the Sarbanes Oxley Act of 2002 including pharmaceutical clients. Gerald W. Bruce EVP, Commercial Operations, has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical industry.
Our Strategy
We are focused on becoming a global leader in pain management by developing and delivering innovative pharmaceutical products to patients. We are developing branded pharmaceutical product candidates for pain management by using cutting-edge technology to enhance patients’ quality of life. While we are currently focused on developing our non-opioid and non-addictive pain management pipeline of product candidates, we are also using our proprietary delivery technologies to develop anti-viral therapies as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19).
According to data from the CDC, in 2016, approximately 20% of adults in the United States had chronic pain (approximately 50 million people). Further, CDC data also indicates that the prescribing of opioids by clinicians has increased threefold in the last 20 years, contributing to the problem of prescription opioid abuse. Accordingly, there is a push among prescribers, regulators, and patients to seek non-opioid and non-addictive treatment options to combat the opioid epidemic. We plan to utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and NCE candidates for commercialization.
We plan to enter the prescription analgesic/pain market with our fivemultiple proprietary non-opioid and non-addictive products. If approved, we believe Epoladerm will be the only transdermal Diclofenac Epolamine Spray film with an indication in sprains, strains, and bruises. In addition, based on early data from our animal studies, we believe Probudur, our proprietary single injectable liposomal gel local anesthetic for postoperative pain management, could be the only liposomal gel bupivacaine with 96 hours of postoperative pain control (96 hours is 24 hours longer than any other liposomal bupivacaine). Further, we believe NES100Envelta may significantly reduce moderate to severe pain with a fast onset of activity, improved mood, and the elimination of opioid-induced constipation. We also believe MMS019AnQlar may offer another layer of protection, in the form of a Molecular Mask, to be added to standard personal protective equipment to protect healthcare workers, and those at-risk of serious disease, from viral infections.
Additionally, we have developed a marketing and sales strategy tailored to each individual product candidate within our portfolio which will be deployed if each product candidate is approved and brought to market. With a dedicated sales team, and niche non-opioid and non-addictive pain management product candidates, we believe if the product candidates receive approval and these strategies are put into place, they can produce products with greater pain management than opioid competitor products, but potentially without any of the side effects caused by morphine/opioid related products.
Epoladerm
We believe we may have a significant advantage over our competitors due to the potential superior adhesion and faster onset of action for which Epoladerm is currently being studied.
Probudur
We believe we have a significant advantage over our competitors due to early data which seems to support a longer efficacy period of 96 hours as compared to 72 hours for the leading market indicator.
NES100
Envelta
If NES100Envelta obtains FDA approval, we intend for it to be sold as an innovative, non-addictive analgesic. We believe it has significant potential to participate in the moderate – severe pain markets. We plan to target specialists of rheumatology, orthopedic, neurology, anesthesiology, pain management, LTC, hospice care, surgery, oncology, PCPs, and geriatrics. Our sales team plans to target 32,000 physicians and market to 200,000 health care providers.
Intellectual Property
We strive to protect and enhance the proprietary technologies, inventions and improvements that we believe are important to our business, including seeking, maintaining and defending patent rights, whether developed internally or licensed from third parties. Our policy is to seek to protect our proprietary position by, among other methods, pursuing and obtaining patent protection in the United States and in jurisdictions outside of the United States related to our proprietary technology, inventions, improvements, platforms and our product candidates that are important to the development and implementation of our business.
As of October 7, 2020September 8, 2021, our portfolio of owned and licensed patents and pending patent applications consisting of 8 issued U.S. patents, 1 pending U.S. patent application, 1 pending Patent Cooperation Treaty (“PCT”) application, 25 issued patents and 2 pending foreign applications. These include US Patent Nos. 7,741,474, 8,470,371, 10,213,474, 8,278,277, 8,920,819, 9,713,591, 8,349,297 and 8,695,592 as well as patents in Europe, Canada, Japan, China, Australia, New Zealand, Russia and South Korea. Below is a breakdown of patents by indication:
Epoladerm
The product candidate is covered by US Patent No. 8,349,297 (expires December 4, 2028) as well as issued patents in South Africa, Russian Federation, New Zealand, Norway, Mexico, Republic of Korea, Japan, China, Canada, Australia, Turkey, Slovakia, Slovenia, Sweden, Portugal, Poland, Netherlands, Latvia, Luxembourg, Lithuania, Italy, Ireland, Hungary, Greece, United Kingdom, France, Finland, Spain, Denmark, Germany, Czech Republic, Switzerland, Belgium and Austria (all of which expire September 14, 2026). The patents contain broad composition claims to a platform of pharmaceutical formulations which form a film on spray administration where the active agent is present at least 80% saturation and there is no undissolved active agent in the formulation. The claims also include a method of treatment and an aerosol dispenser containing the formulation.
Probudur
The product candidate is covered by US Patent No. 9,713,591 (expires July 24, 2030) as well as a European patent (expires October 11, 2029) and a Chinese patent (expires October 11, 2029). There is also a pending US application which is a continuation of US Patent No. 9,713,591 and for which a notice of allowance has been received and the issue fee paid. The patent contains composition claims to pharmaceutical compositions having an external storage solution containing an active pharmaceutical ingredient and particles of liposomes embedded in a polymeric matrix contained within the storage solution.
NES100
Envelta
The product candidate is covered by the following patent families which protect the chemistry of the MET polymer and its use in pharmaceutical products: Patent Family 1 includes US Patent No. 7,741,474 (expires March 18, 2026), and a Japanese Patent, Canadian Patent and European Patent (all which expire September 22,2023)22, 2023). This patent family covers carbohydrate polymers with hydrophobic and hydrophilic side-groups suitable for solubilizing, for example, hydrophobic drugs.
Patent Family 2 includes US Patent No. 8,470,371 (expires July 29, 2029) and a Japanese Patent (expires August 8, 2027) and a European Patent (which expire August 8, 2027). This family covers both composition and method claims for amphiphilic carbohydrate polymers which are capable of self-assembling to form micellar clusters in which the carbohydrate amphiphiles aggregate into hierarchically organized micellar clusters of individual aggregates. These micellar clusters formed by the aggregation of individual micelles may be transformed into stable nanoparticles with drugs, especially hydrophobic drugs that have poor aqueous solubility. This provides molar polymer/drug ratios that are greater than the ratios observed with block copolymers and improve the transfer of hydrophobic drugs across biological barriers.
Patent Family 3 includes US Patent Nos. 8,278,277 (expires August 16, 2030) and 8,920,819 (expires April 29, 2029), a Canadian patent (expires March 1, 2030) and a pending European application (which would expire March 1, 2030). This family covers lipophilic derivatives of hydrophilic drugs comprising a hydrophilic drug and a cleavable linker as well as methods of treatment using these compositions. In particular, the patents relate to compositions of a lipophilic derivative of the hydrophilic neuropeptide Leucine [5]-Enkephalin and an amphiphile compound, where the derivative includes a lipophilic linker attached to the side chain oxygen of the tyrosine in the Leucine [5]-Enkephalin, and where the amphiphile compound is quaternary palmitoyl glycol chitosan (GCPQ).
Patent Family 4 includes US Patent No. 10,213,474 (expires November 3, 2034), a Japanese patent (expires November 3, 2034), a European patent (expires November 3, 2034) and a pending Canadian patent application (which would expire November 3, 2034). The patents cover methods for treating pain, comprising intranasally administering to a human or animal a composition comprising a therapeutically effective amount of a hydrophilic neuroactive peptide and an amphiphilic quaternary ammonium palmitoyl glycol chitosan (GCPQ); wherein the amphiphilic GCPQ is capable of self-assembly in aqueous media into particles having a mean particle size between 20-500 nm; where intranasally administering the composition delivers the hydrophilic neuroactive peptide to the brain of the human or animal.
In addition to the patent families which protect the chemistry of the MET polymer and its use in pharmaceutical products there is a patent family which covers the delivery device that can be used to administer the pharmaceutical compositions including US Patent No. 8,695,592 (expires October 11, 2029), and a European patent (expires October 11, 2029). This family covers a capsule for use in dispensing a drug which has a pressurized container for a fluid, a chamber for containing a particulate, at least one channel running between the container and the chamber to provide fluidic communication and at least two distinct concave surfaces which impart rotational motion to a fluid flow so that within the chamber a rotationally turbulent flow of fluid is produced in order to engage with the particulate and to produce a mobile fluid comprising the particulate.
AnQlar
This product candidate is specifically covered by a recently filed PCT application (August 24, 2021) which describes a molecular masking spray for use as an anti-viral barrier to potentially prevent, or reduce the risk or the intensity of, viral infections in humans. Depending on the formulation and mode of administration of AnQlar, the product candidate may also be covered by many of the patents and patent application which cover Envelta.
Individual patents extend for varying periods depending on the date of filing of the patent application and the legal term of patents in the countries in which they are obtained. Generally, patents issued for regularly filed applications in the United States are granted a term of 20 years from the earliest effective non-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office, or USPTO, delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. However, the actual protection afforded by a patent varies on a product by product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Furthermore, we rely upon trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our collaborators, employees, contractors, consultants and advisors and invention assignment agreements with our employees. We also have confidentiality agreements or invention assignment agreements with our collaborators, contractors and selected consultants. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our collaborators, employees, contractors and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our drugs or processes, or to obtain licenses or cease certain activities. Our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our future drugs may have an adverse impact on us. If third parties have prepared and filed patent applications prior to the date of our earliest filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the USPTO, to determine priority of invention. For more information, please see “Risk Factors—Factors — Risks Related to Our Intellectual Property.”
Material Agreements
MedPharm Limited
Research and Option Agreement
On April 11, 2017, we entered into a research and option agreement, as amended on May 30, 2018 (the “MedPharm Research and Option Agreement”), with MedPharm Limited, a company organized and existing under the laws of the United Kingdom (“MedPharm”), pursuant to which MedPharm granted us an option to obtain an exclusive, world-wide, royalty bearing license to use certain technology developed by MedPharm. Pursuant to the agreement, MedPharm will conduct certain research and development of proprietary formulations incorporating certain MedPharm technologies and certain of our proprietary molecules.
Under the MedPharm Research and Option Agreement, MedPharm granted us an option (the “MedPharm Option”) to obtain an exclusive (even to MedPharm), worldwide, sub-licensable (through multiple tiers), royalty bearing, irrevocable license to research, develop, market, commercialize, and sell any product utilizing MedPharm’s spray formulation technology which is the result of the activities performed under the MedPharm Research and Option Agreement, subject to our entry into a definitive license agreement with MedPharm. In order to exercise the MedPharm Option, we must provide MedPharm with written notice of such exercise before the end of the Option Period (as defined in the MedPharm Research and Option Agreement). The Option Period is subject to extension upon our mutual agreement with MedPharm.
Pursuant to the MedPharm Research and Option Agreement, we have a right of first refusal with respect to any license or commercial arrangement involving any Licensed Intellectual Property (as defined in the MedPharm Research and Option Agreement) in combination with any Virpax Molecule (as defined in the MedPharm Research and Option Agreement). In the event that MedPharm reaches an agreement with respect to a license or other commercial arrangement that involves technology or molecules covered by the right of first refusal, we have ten business days from the date of notice to notify MedPharm of our intention to exercise the right of first refusal and our intention to match the financial terms of the other license or commercial arrangement.
License Agreement
On June 6, 2017, as a result of our exercise of the MedPharm Option under the MedPharm Research and Option Agreement, we entered into a license agreement, as amended on September 2, 2017 and October 31,9, 2017 (the “MedPharm License Agreement”), with MedPharm for the exclusive global rights to discover, develop, make, sell, market, and otherwise commercialize any pharmaceutical composition or preparation (in any and all dosage forms) in final form containing one or more compounds, including Epoladerm and OSF200, that was developed, manufactured or commercialized utilizing MedPharm’s spray formulation technology (“MedPharm Product”), to be used for any and all uses in humans (including all diagnostic, therapeutic and preventative uses). Under the MedPharm License Agreement, we are required to make future milestone and royalty payments to MedPharm. We are obligated to make aggregate milestone payments to MedPharm of up to $1.125£1.15 million upon the achievement of specified development milestones (payable in Great British Pounds). Royalty payments must be paid to MedPharm in an amount equal to a single-digit percentage of net sales of all MedPharm Product sold by us during the royalty term in the territory. Royalties shall be payable, on a country-by-country basis, during the period of time commencing on the first commercial sale and ending upon the expiration of the last-to-expire patent claim on the licensed product.product, which is set to expire on December 4, 2028. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
LipoCureRx,LipoCureRX, Ltd.
On March 19, 2018, we entered into a license and sublicense agreement (the “LipoCure Agreement”) with LipoCureRx,LipoCureRX, Ltd., a company organized and existing under the laws of Israel (“LipoCure”), for the sole and exclusive global license and sub-license rights to discover, develop, make, sell, market, and otherwise commercialize bupivacaine liposome, in injectable gel or suspension (“Licensed Compound”) or any pharmaceutical composition or preparation (in any and all dosage forms) in final form, including any combination product, containing a Licensed Compound (“Licensed Product”), including Probudur. Under the LipoCure Agreement, we were required to pay an upfront fee upon signing of $150,000 and are required to make future milestone and royalty payments to LipoCure. We are obligated to make aggregate milestone payments of up to $19.8 million upon the achievement of specified development and commercial milestones. Royalty payments must be paid in an amount equal to a single digit to low double-digit percentage of annual net sales of royalty qualifying products, subject to certain adjustments. Royalties shall be payable during the period of time, on a country-by-country basis, commencing on the first commercial sale and ending upon the expiration of the last-to-expire patent claim on the licensed product.product, which is set to expire on July 24, 2030. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
On June 29, 2021, we entered into an Agreement for Rendering of Research Services (the “June 2021 Lipocure Research Agreement”) with Lipocure. Under the June 2021 Lipocure Research Agreement, we shall provide funding for research and development related to the optimization of the Liposomal Bupivacaine formulation and eventual manufacture of pre-clinical batches including batches for stability testing, animal studies and toxicology work. This will also include work associated with the potential filing of additional provisional patent applications. We may terminate the agreement at any time upon 30 days written notice and shall be only responsible to pay Lipocure for work performed through the date of such notice. In consideration for the research services, we agreed to pay research service fees of $200,000 upon execution as prepayment for research services included in Prepaid Expenses and Other Current Assets on the accompanying balance sheet as of June 30, 2021, as well as $400,000 on July, 1 2021, and five quarterly payments of $270,000 initiating on September 1 2021. We also agreed to pay $250,000 to Lipocure upon successful completion of a Chemistry, Manufacturing and Controls “CMC” filing with the U.S. Food and Drug Administration (the (“FDA”). All services to be provided under the June 2021 Lipocure Research Agreement initiated on July 1, 2021 and are anticipated to be completed towards the end 2022.
Nanomerics Ltd.
Nanomerics Collaboration Agreement
On April 11, 2019, we entered into an exclusive collaboration and license agreement, as amended (the “Nanomerics Collaboration Agreement”), with Nanomerics Ltd. (“Nanomerics”), a company organized and existing under the laws of United Kingdom, for the exclusive world-wide license to develop and commercialize products, including NES100,Envelta, which contain hydrophilic neuropeptide Leucin5-Enkephalin and an amphiphile compound which is quaternary ammonium palmitoyl glycol chitosan, and to engage in a collaborative program utilizing Nanomerics’ knowledge, skills and expertise in the clinical development of products and in attracting external funding for such development. The Nanomerics Collaboration Agreement was also amended to include a program for the pre-clinical development of a product for post-traumatic stress disorder.
Under the Nanomerics Collaboration Agreement, we are required to make royalty payments equal to a single digit percentage of annual net sales of royalty qualifying products. We are also required to make aggregate milestone payments of up to $103 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships we enter into subsequent to the Nanomerics Collaboration Agreement. Our obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of our licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim (patent is set to expire on November 3, 2034); and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. We have the right to terminate the agreement upon 180 days’ prior written notice to Nanomerics. Upon termination, we shall assign to Nanomerics all its right title and interest in all results other than results specific to (a) the Device (as defined in the Nanomerics Collaboration Agreement), its manufacture or use; and (b) the Technology, but excluding any clinical Results relating to the Compound or Licensed Products (all terms as defined in the Nanomerics Collaboration Agreement).
Nanomerics License Agreement
On August 7, 2020, we entered into a collaboration and license agreement with Nanomerics (the “Nanomerics License Agreement”) for the exclusive North American license to develop and commercialize a High-Density Molecular Masking Spray (MMS019)(AnQlar) as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans. Under the Nanomerics License Agreement, we are required to make royalty payments within a range of 5% to 15% of annual net sales of royalty qualifying products. Our obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of our licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th)(10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim; and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. We are also required to make aggregate milestone payments of up to $50 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships we enter into subsequent to the Nanomerics License Agreement.Agreement (any patent that issues from the currently filed provisional patent application would expire on August 24, 2041). We have the right to terminate the Nanomerics License Agreement upon 60 days’ prior written notice to Nanomerics. Upon termination, we shall assign to Nanomerics all its rights, title and interest in all of our results. Nanomerics has the right to terminate the agreement upon 60 days’ prior written notice if we have not secured funding by the Funding Expiry Date (as defined in the Nanomerics License Agreement). On December 31, 2020, we amended the Nanomerics License Agreement to extend the Funding Expiry Date to March 31, 2021. Upon the closing of our initial public offering on February 19, 2021, the Funding Expiry Date provision was satisfied.
Yissum
License Agreement
On August 11, 2019, we entered into an exclusive research and license agreement, as amended (the “Yissum License Agreement”), with Yissum Research Development Company of the Hebrew University of Jerusalem, Ltd., a company organized and existing under the laws of Israel (“Yissum”). Under the Yissum Agreement, we shall provide funding for research and development studies to be performed by researchers at Hebrew University related to technology enabling the creation of quick-onset and long-acting formulations of opioid antagonists. Under the Yissum License Agreement, we have the exclusive right to license the commercial technology resulting from the activities of the researches at the Hebrew University. We have agreed to use commercially reasonable efforts to carry out the development, regulatory, manufacturing and marketing work necessary to develop and commercialize any of the products which result from such research.
The Yissum License Agreement requires us to pay an annual license maintenance fee of $50,000, a royalty fee equal to a single digit percentage of annual net sales of any product, aggregate milestone payments of up to $1.19 million upon the achievement of various development and commercial milestones, and a percentage of sublicense fees based on the timing of execution of a sublicense agreement. The license agreement expires on a country-by-country, product-by-product basis, upon the later of: (i) the date of expiration in such country of the last to expire licensed patent included in the licensed technology; (ii) the date of expiration of any exclusivity on the product granted by a regulatory or government body in such country; or (iii) the end of a period of twenty (20) years from the date of the first commercial sale in such country. We have the right to terminate the Yissum License Agreement by written notice immediately if the licensor passes a resolution for voluntary winding up or a winding up application is made against it and not set aside within 60 days. Additionally, we may terminate the Yissum License Agreement if either a receiver or liquidator is appointed for us, or we enter into a winding up or insolvency or bankruptcy proceeding.
Research Agreement
On May 12, 2019,June 30, 2021, we entered into an Agreement for Rendering of Research Services as amended (the “Yissum“June 2021 Yissum Research Agreement”), with Yissum.Yissum Research Development Company of the Hebrew University of Jerusalem, Ltd. (“Yissum”). Under the June 2021 Yissum Research Agreement, we shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the formulation, preparation and characterizationoptimization of the Liposomal Bupivacaine formulation and to increase stability for size zeta potential, drug loadingmanufacturing purposes. We may terminate the agreement at any time upon 30 days written notice and rateshall be only responsible to pay Yissum for work performed through the date of drug release.such notice. In consideration for the research services, we agreed to pay research service fees of $81,000$337,500 in six equal monthlyquarterly installments. We retain ownership in all our intellectual property rights and any intellectual property belonging to either us or Yissum prior to the execution of the Yissum Research Agreement will remain the sole property of either us or Yissum, respectively. All data generated from the provision of the Yissum Research Agreement, including any reports, which are specifically required and contemplated under the Yissum Research Agreement, shall be owned by us upon full payment of the research services fees. Each party will be entitled to terminate the agreement in the event of a breach by the other party of its obligations under the agreement, including, but not limited to, any payment failure, which is not remedied by the breaching party within 30 days of receipt of written notice from the non-breaching party. All services to be provided under the June 2021 Yissum Research Agreement willinitiated on July 1, 2021 and are anticipated to be completed by September 14, 2021.towards the end of 2022.
NCATS-NIH Cooperative Research and Development Agreement
On August 25, 2020, we entered into athe CRADA with NCATS. This collaboration is for the continued development of our product candidate, NES100,Envelta, an intranasal peptide, for the management of acute and chronic non-cancer pain. The term of the CRADA is for a period of four years from the effective date of the agreement and can be terminated by both parties at any time by mutual written consent. In addition, either party may unilaterally terminate the CRADA at any time by providing written notice of at least sixty (60) days before the desired termination date. The agreement provides for studies that are focused on the pre-clinical characterization of NES100Envelta as a novel analgesic for acute and chronic non-cancer pain, and for studies to further develop NES100Envelta through IND enabling studies. There are certain development “Go/No Go” provisions within the agreement whereby, if certain events occur, or do not occur, NCATS may terminate the CRADA. These “No GO” provisions include: i) lack of efficacy in all animal pain models, ii) no reliable and sensitive bioanalytical method can be developed, iii) manufacturing failure due to inherent process scalability issues, iv) unacceptable toxicity or safety profile to enable clinical dosing, and v) inability to manufacture the NES100Envelta dosage form.
With respect to NCATS rights to any invention made solely by an NCATS employee(s) or made jointly by an NCATS employee(s) and our employee(s), the CRADA grants to us an exclusive option to elect an exclusive or nonexclusive commercialization license. For inventions owned solely by NCATS or jointly by NCATS and us, and licensed pursuant to our option, we must grant to NCATS a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government. For inventions made solely by an employee of ours, we grant to the United States government a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government for research or other government purposes.
Sales and Marketing
If Epoladerm, OSF200, Probudur, NES100,Envelta, PES200 and/or MMS019AnQlar are approved, we plan to enter into sales and marketing agreements with one or several pharmaceutical companies to sell to pain management clinics and specialists, general and orthopedic surgeons, anesthesiologists, PCPs, NPs, oncologists, and neurologists.
On August 30, 2018, we entered into a Master Service Agreement (the “MSA”) with INC Research, LLC, a Syneos Health™ group company (“Syneos Health”) to operate as our Contract Sales Organization (“CSO”). Services provided by Syneos Health include clinical research services, bioanalytical analysis, statistics, validations, pharmacokinetics, and/or consulting, advertising, and public relations (communications), field team sales and education recruiting and deployment, and patient adherence services.
Manufacturing
NES100Envelta is an intranasal spray and we rely on third-party contractors for manufacturing clinical supplies and plan to do so for commercial amounts also.
On August 25, 2021, we entered into a commercial manufacturing and supply agreement with Seqens, an integrated global leader in pharmaceutical solutions with 24 manufacturing sites worldwide and seven research and development facilities throughout the U.S. and Europe. The agreement with Seqens provides for both the supply material for our clinical studies as well as the long-term commercial supply of AnQlar. Seqens will conduct process development and validation of additional large scale commercial quantities of AnQlar at its facilities in Devens and Newburyport, Massachusetts.
We continue to explore manufacturing sources, in order to ensure that we have access to sufficient manufacturing capacity in order to meet potential demand for any of our product candidates in a cost-efficient manner. We plan to secure supply sources and contract with these or other parties to manufacture commercial quantities of any products we successfully develop. Among the conditions for FDA approval of a pharmaceutical product is the requirement that the manufacturer’s quality control and manufacturing procedures conform to cGMP,Current Good Manufacturing Practice (“cGMP”), which must be followed at all times. The FDA typically inspects manufacturing facilities on an ongoing basis. In complying with Current Good Manufacturing Practice (“cGMP”)cGMP regulations, pharmaceutical manufacturers must expend resources and time to ensure compliance with product specifications as well as production, record keeping, quality control, reporting, and other requirements.
Competition
Competition
The pharmaceutical industry is intensely competitive and subject to rapid and significant technological change. We will continue to face competition from various global pharmaceutical, biotechnology, specialty pharmaceutical and generic drug companies that engage in drug development activities. Our key competitors include Pacira Biosciences, Inc. (which is developing EXPAREL®) and Pfizer Inc. (which is developing the Flector Patch). Many of our competitors have similar products that focus on the same diseases and conditions that our current and future pipeline product candidates address. Many of our competitors have greater financial flexibility to deploy capital in certain areas as well as more commercial and other resources, marketing and manufacturing organizations, and larger research and development staff. As a result, these companies may be able to pursue strategies or approvals that we are not able to finance or otherwise pursue and may receive FDA, or other applicable regulatory approvals more efficiently or rapidly than us. Also, our competitors may have more experience in marketing and selling their products post-approval and gaining market acceptance more quickly. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Our product candidates could become less competitive if our competitors are able to license or acquire technology that is more effective or less costly and thereby offer an improved or a cheaper alternative to our product candidates.
We expect any product candidates that we develop and commercialize will compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price and the availability of reimbursement from government and other third-party payors. We also expect to face competition in our efforts to identify appropriate collaborators or partners to help commercialize our product candidate portfolio in our target commercial markets.
Government Regulation and Approval Process
Government authorities in the United States at the federal, state and local level, including the FDA, the FTC and the DEA, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, recordkeeping, promotion, advertising, distribution, marketing and export and import of products such as those we market. For both currently marketed and future products, failure to comply with applicable regulatory requirements can, among other things, result in suspension of regulatory approval and possible civil and criminal sanctions. Regulations, enforcement positions, statutes and legal interpretations applicable to the pharmaceutical industry are constantly evolving and are not always clear. Significant changes in regulations, enforcement positions, statutes and legal interpretations could have a material adverse effect on our financial condition and results of operations.
Additionally, future healthcare legislation or other legislative proposals at the federal and state levels could bring about major changes in the affected health care systems, including statutory restrictions on the means that can be employed by brand and generic pharmaceutical companies to settle Paragraph IV patent litigations. We cannot predict the outcome of such initiatives, but such initiatives, if passed, could result in significant costs to us in terms of costs of compliance and penalties associated with failure to comply.
With the support of the President and the HHS, the NIH launched the initiative Helping to End Addiction Long-term (“HEAL”), to provide solutions to the national opioid crisis. With the focus on ending the opioid crisis in America, HEAL, and new FDA guidance has made it more efficient to bring novel non-opioid medication to market.
The Office of National Drug Control Policy (“ONDCP”) is a component of the Executive Office of the President which works to reduce drug use and its consequences by leading and coordinating the development, implementation, and assessment of U.S. drug policy. In addition to its vital ongoing work, ONDCP also provided administrative and financial support to the President’s Commission on Combating Drug Addiction and the Opioid Crisis, established by Executive Order on March 29, 2017 by President Donald J. Trump. This commission was created to make recommendations to the President on how to best combat opioid addiction and abuse. In August 2017, the commission issued a preliminary report calling on President Trump to officially declare the crisis of opioid abuse a national emergency. On October 26, 2017, President Trump declared the opioid crisis a “national public health emergency.” The commission’s final report was released in early November 2017. In July 2017, the Pharmaceutical Care Management Association, a trade association representing pharmacy benefit managers, wrote a letter to the commissioner of FDA in which it expressed support for, among other things, the CDC guidelines and a seven-day limit on the supply of opioids for acute pain. In September 2018, CVS Pharmacy announced that it would only fill first time opioid prescriptions for acute pain for a seven-day supply.
Law enforcement and regulatory agencies may apply policies that seek to limit the availability of opioids. This creates the potential for aggressive enforcement, unfavorable publicity regarding the use or misuse of opioid drugs or the limitations of abuse-deterrent formulations, litigation, public inquiries or investigations related to the abuse, sales, marketing, distribution or storage of opioid products.
In addition, efforts by the FDA and other regulatory bodies to combat the abuse of opioids will positively impact the market for our novel non-opioid and non-addictive product candidates. We expect that the FDA will continue to evaluate the impact of abuse-deterrent opioids in the future, and this could impose further restrictions to opioid products currently on the market, which may include changing labeling, imposing additional prescribing restrictions, or seeking a product’s removal from the market.
CoincidingIn harmony with HEAL, there isFDA has issued and plans to issue several new FDA guidance that streamlines and broadens the range of novel non-opioid medications. The first guidance will address medications that can reduce the use of opioids in the treatment of acute pain, including how sponsors can demonstrate a clinically meaningful reduction in the use of opioid pain medications in the acute setting. The second guidance will focus on assessing the benefits and risks of developing new opioid pain drugs, including drafting an updated framework for evaluating the risks associated with intentional or illicit misuse or abuse of these substances. The third guidance will “outline a path for developing extended-release local anesthetics,” including clinical pharmacology, proper evaluation of safety and efficacy, and the types of studies that may support approval of these product candidates. Finally, FDA will issue guidanceguidances on the development and clinical usage of “newopioid and non-opioid pain medications for acute and chronic pain that can provide therapeutic alternatives to the use of opioids.”medications.
Pharmaceutical Regulation in the United States
In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, Warning Letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved drug or a generic version of a previously approved drug, can be marketed in the United States.
The process required by the FDA before a new drug may be marketed in the United States generally involves:
| ● | Completion of preclinical laboratory and animal testing and formulation studies in compliance with the FDA’s current GLP regulations; |
| ● | Submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin in the United States; |
| ● | Approval by an IRB at each clinical site before each trial may be initiated; |
| ● | Performance of adequate and well-controlled human clinical trials in accordance with the FDA to establish the safety and efficacy of the proposed drug product for each intended use; |
| ● | Satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s cGMP regulations to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; |
| ● | Submission to the FDA of an NDA; |
| ● | Satisfactory completion of a potential review by an FDA advisory committee, if applicable; and |
| ● | FDA review and approval of the NDA. |
Preclinical Studies
When developing a branded product and bringing it to market, the first step in proceeding to clinical studies is preclinical testing. Preclinical tests are intended to provide a laboratory or animal study evaluation of the product to determine its chemistry, formulation and stability. Toxicology studies are also performed to assess the potential safety of the product. The conduct of the preclinical tests must comply with federal regulations and requirements, including GLPs. The results of these studies are submitted to the FDA as part of an IND application along with other information, including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long-term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND application is submitted.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it initiates at that institution. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on their website, www.ClinicalTrials.gov.www.ClinicalTrials.gov.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
| ● | Phase I: The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. |
| ● | Phase II: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| ● | Phase III: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2, and Phase 3 trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Disclosure of Clinical Trial Information
Sponsors of certain clinical trials of FDA-regulated products, including drugs, are required to register and disclose certain clinical trial information on www.ClinicalTrials.gov.www.ClinicalTrials.gov. Information related to the product, subject population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss certain results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Marketing Approval
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the United States. The NDA must include, among other things, the results of all preclinical, clinical and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture and controls. Under federal law, the submission of most NDAs is subject to a substantial application user fee, and the manufacturer or sponsor under an approved NDA is also subject to annual program fees. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information and is subject to payment of additional user fees. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. Under the Prescription Drug User Fee Act, as amended, the FDA has agreed to certain performance goals in the review of NDAs through a two-tiered classification system, Standard Review and Priority Review. Priority Review designation is given to drugs that are intended to treat a serious condition and, if approved, would provide a significant improvement in safety or effectiveness over existing therapies. The FDA endeavors to review most applications subject to Standard Review within ten to twelve months whereas the FDA’s goal is to review most Priority Review applications within six to eight months, depending on whether the drug is a new molecular entity.
The FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. Before approving an NDA, the FDA may inspect one or more clinical sites to assure compliance with GCP requirements. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the NDA unless it determines that the manufacturing process and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications and the NDA contains data that provide substantial evidence that the drug is safe and effective for the labeled indication.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter to indicate that the application is not ready for approval. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. Even with submission of this additional information, the FDA may ultimately decide that an application does not satisfy the regulatory criteria for approval. If, or when, the deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
As a condition of NDA approval, the FDA may require a REMS to help ensure that the benefits of the drug outweigh the potential risks. If the FDA determines a REMS is necessary during review of the application, the drug sponsor must agree to the REMS plan at the time of approval. A REMS may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate healthcare providers of the drug’s risks, limitations on who may prescribe or dispense the drug, or other elements to assure safe use, such as special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. In addition, the REMS must include a timetable to periodically assess the strategy. The requirement for a REMS can materially affect the potential market and profitability of a drug.
Moreover, as a condition of product approval, the FDA may require substantial post-approval testing, known as Phase IV testing, and/or surveillance to monitor the drug’s safety or efficacy, and the FDA has the authority to prevent or limit further marketing of a product based on the results of these post-marketing programs. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or certain problems are identified following initial marketing. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling, and, even if the FDA approves a product, it may limit the approved indications for use for the product or impose other conditions, including labeling or distribution restrictions or other risk-management mechanisms.
Further changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented, which may require us to develop additional data or conduct additional preclinical studies and clinical trials. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the similar procedures in reviewing NDA supplements as it does in reviewing NDAs.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to drug listing and registration, recordkeeping, periodic safety reporting, product sampling and distribution, adverse event reporting and advertising, marketing and promotion, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. Drugs may be marketed only for the approved indications and in a manner consistent with the provisions of the approved labeling. While physicians may prescribe for off-label uses, manufacturers may only promote for the approved indications and in accordance with the provisions of the approved labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. There also are extensive DEA regulations applicable to controlled substances.
Adverse event reporting and submission of periodic reports is also required following FDA approval of an NDA. Additionally, the FDA may place conditions on an approval, in addition to REMS programs of Phase IV testing, that could restrict the distribution or use of the product. Drug manufacturers and certain of their subcontractors are required to register their establishments and list their marketed products with the FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality-control to maintain compliance with cGMPs, including quality control and manufacturing processes. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing or if previously unrecognized problems are subsequently discovered. The FDA may also impose a REMS requirement on a drug already on the market if the FDA determines, based on new safety information, that a REMS is necessary to ensure that the drug’s benefits outweigh its risks. In addition, regulatory authorities may take other enforcement action, including, among other things, Warning Letters, the seizure of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations, refusal to approve pending applications or supplements to approved applications, civil penalties and criminal prosecution.
78
The Hatch-Waxman Amendments
505(b)(2) NDAs
The FDA is authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference from the data owner. The applicant may rely upon the FDA’s findings of safety and efficacy for an approved product that acts as the “listed drug.” The FDA may also require 505(b)(2) applicants to perform additional studies or measurements to support the change from the listed drug. The FDA may then approve the new product candidate for all, or some, of the conditions of use for which the branded reference drug has been approved, or for a new condition of use sought by the 505(b)(2) applicant.
Abbreviated New Drug Applications (“ANDAs”)
The Hatch-Waxman amendments to the FDCA established a statutory procedure for submission and FDA review and approval of ANDAs for generic versions of listed drugs. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient (“API\I”), drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. Premarket applications for generic drugs are termed abbreviated because they generally do not include clinical data to demonstrate safety and effectiveness. However, a generic manufacturer is typically required to conduct bioequivalence studies of its test product against the listed drug. Bioequivalence is established when there is an absence of a significant difference in the rate and extent for absorption of the generic product and the reference listed drug. For some drugs, other means of demonstrating bioequivalence may be required by the FDA, especially where rate or extent of absorption are difficult or impossible to measure. The FDA will approve an ANDA application if it finds that the generic product does not raise new questions of safety and effectiveness as compared to the reference listed drug. A product is not eligible for ANDA approval if the FDA determines that it is not bioequivalent to the reference listed drug if it is intended for a different use or if it is not subject to, and requires, an approved Suitability Petition.
Patent Exclusivity and Orange Book Listing
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the Orange Book. Any applicant who files an ANDA seeking approval of a generic equivalent version of a drug listed in the Orange Book or a 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA (i) that there is no patent listed with the FDA as covering the relevant branded product, (ii) that any patent listed as covering the branded product has expired, (iii) that the patent listed as covering the branded product will expire prior to the marketing of the generic product, in which case the ANDA will not be finally approved by the FDA until the expiration of such patent or (iv) that any patent listed as covering the branded drug is invalid or will not be infringed by the manufacture, sale or use of the generic product for which the ANDA is submitted. A notice of the Paragraph IV certification must be provided to each owner of the patent that is the subject of the certification and to the holder of the approved NDA to which the ANDA or 505(b)(2) application refers. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
If the reference NDA holder and patent owners assert a patent challenge directed to one of the Orange Book listed patents within 45 days of the receipt of the Paragraph IV certification notice, the FDA is prohibited from approving the application until the earlier of 30 months from the receipt of the Paragraph IV certification, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the applicant. The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the branded reference drug has expired as described in further detail below.
Non-Patent Exclusivity
In addition to patent exclusivity, the holder of the NDA for the listed drug may be entitled to a period of non-patent exclusivity, during which the FDA cannot approve an ANDA or 505(b)(2) application that relies on the listed drug.
For example, a drug that is considered new chemical entity (NCE) at the time of approval may be awarded a five-year period of marketing exclusivity, starting at the time of product approval. An ANDA or 505(b)(2) application referencing that drug may not be approved until the five-year period expires. Also, an ANDA or 505(b)(2) application referencing that drug may not be filed with the FDA until the expiration of five years, unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval.
A drug, including one approved under Section 505(b)(2), may obtain a three-year period of exclusivity for a particular condition of approval, or change to a marketed product, such as a new formulation for a previously approved product, if one or more new clinical studies (other than bioavailability or bioequivalence studies) was essential to the approval of the application and was conducted/sponsored by the applicant.
DEA Regulation
Our product candidates may be regulated as “controlled substances” as defined in the Controlled Substances Act of 1970, as amended, which establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered by the U.S. Drug Enforcement Agency (the “DEA”). The DEA is concerned with, among other things, the control of handlers of controlled substances and with the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce.
The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. The manufacture, shipment, storage, sale and use of Schedule II drugs are subject to a high degree of regulation. For example, Schedule X drug prescriptions generally must be signed by a physician and may not be refilled without a new prescription. Substances in Schedule IV are considered to have a lower potential for abuse relative to substances in Schedule II. A prescription for controlled substances in Schedule IV may be issued by a practitioner through oral communication, in writing or by facsimile to the pharmacist and may be refilled if so, authorized on the prescription or by call-in. In the future, our other potential products may also be listed by the DEA as controlled substances.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which schedules of controlled substances are authorized.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Required security measures include background checks on employees and physical control of inventory through measures such as cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances and periodic reports must be made to the DEA, including, for example, distribution reports for Schedule II controlled substances, Schedule III substances that are narcotics and other designated substances. Reports must also be made for thefts or losses of any controlled substance and authorization must be obtained to destroy any controlled substance. In addition, special authorization and notification requirements apply to imports and exports.
In addition, a DEA quota system controls and limits the availability and production of controlled substances in Schedule II. Distributions of any Schedule II controlled substance must also be accompanied by special order forms, with copies provided to the DEA. The DEA establishes annually an aggregate quota for how much of a Schedule II substance may be produced in total in the United States based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. This limited aggregate amount of any particular Schedule II substance that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual production and procurement quotas. We and our contract manufacturers must receive an annual quota from the DEA in order to produce or procure any Schedule II substance for use in manufacturing. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. Our and our contract manufacturers’ quota of an active ingredient may not be sufficient to meet commercial demand or complete clinical trials. Any delay or refusal by the DEA in establishing our and our contract manufacturers’ quota for controlled substances could delay or stop our clinical trials or product launches, which could have a material adverse effect on our business, financial position and results of operations.
To meet its responsibilities, the DEA conducts periodic inspections of registered establishments that handle controlled substances. Failure to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to revoke those registrations. In certain circumstances, violations could result in criminal proceedings.
Individual states also regulate controlled substances, and we and our contract manufacturers will be subject to state regulation on distribution of these products.
Pricing and Reimbursement
Successful commercialization of our products depends, in part, on the availability of governmental and third-party payor reimbursement for the cost of our products. Government authorities and third-party payors increasingly are challenging the price of medical products and services. On the government side, there is a heightened focus, at both the federal and state levels, on decreasing costs and reimbursement rates for Medicaid, Medicare and other government insurance programs. This has led to an increase in federal and state legislative initiatives related to drug prices, which could significantly influence the purchase of pharmaceutical products, resulting in lower prices and changes in product demand. If enacted, these changes could lead to reduced payments to pharmaceutical manufacturers. Many states have also created preferred drug lists and include drugs on those lists only when the manufacturers agree to pay a supplemental rebate. If our current products or future drug candidates are not included on these preferred drug lists, physicians may not be inclined to prescribe them to their Medicaid patients, thereby diminishing the potential market for our products.
In addition, third-party payors have been imposing additional requirements and restrictions on coverage and limiting reimbursement levels for pharmaceutical products. Third-party payors may require manufacturers to provide them with predetermined discounts from list prices and limit coverage to specific pharmaceutical products on an approved list, or formulary, which might not include all of the FDA-approved pharmaceutical products for particular indications. Third-party payors may challenge the price and examine the medical necessity and cost-effectiveness of pharmaceutical products in addition to their safety and efficacy. Manufacturers may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of pharmaceutical products in addition to the costs required to obtain the FDA approvals. Adequate third-party reimbursement may not be available to enable manufacturers to maintain price levels sufficient to realize an appropriate return on their investment in drug development.
Healthcare Reform
In the United States, there have been a number of federal and state proposals during the last several years regarding the pricing of pharmaceutical products, government control and other changes to the healthcare system of the United States. It is uncertain what other legislative proposals may be adopted or what actions federal, state, or private payors may take in response to any healthcare reform proposals or legislation. We cannot predict the effect such reforms may have on our business, and no assurance can be given that any such reforms will not have a material adverse effect.
By way of example, in March 2010, the ACA was signed into law, which, among other things, includes changes to the coverage and payment for drug products under government health care programs. The law includes measures that (i) significantly increase Medicaid rebates through both the expansion of the program and significant increases in rebates, (ii) substantially expand the Public Health System (340B) program to allow other entities to purchase prescription drugs at substantial discounts, (iii) extend the Medicaid rebate rate to a significant portion of Managed Medicaid enrollees, (iv) assess a rebate on Medicaid Part D spending in the coverage gap for branded and authorized generic prescription drugs, and (v) levy a significant excise tax on the industry to fund the healthcare reform.
In addition to the changes brought about by the ACA, other legislative changes have been proposed and adopted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs and reform government program reimbursement methodologies for drug products. At the federal level, the Trump Administration’s budget proposal for fiscal year 2019 contains furtherBiden Administration is expected to release major proposals on drug price control measures that could be enacted duringpricing in the 2019 budget processlate summer or in other future legislation, including, for example, measures tofall of 2021, which may, permit Medicare Part D plans to negotiate the price of all or certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid and to eliminate cost sharing for generic drugs for low-income patients. While any proposed measures will require authorization through additional legislation to become effective, Congress and the Trump Administration have each indicated an intent to continue to seek new legislative or administrative measures to control drug costs.that those plans cover. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Healthcare Regulations
Pharmaceutical companies are subject to various federal and state laws that are intended to combat health care fraud and abuse and that govern certain of our business practices, especially our interactions with third-party payors, healthcare providers, patients, customers and potential customers through sales and marketing or research and development activities. These include anti-kickback laws, false claims laws, sunshine laws, privacy laws and FDA regulation of advertising and promotion of pharmaceutical products.
Anti-kickback laws, including the federal Anti-Kickback Statute, make it a criminal offense knowingly and willfully to offer, pay, solicit, or receive any remuneration to induce or reward referral of an individual for, or the purchase, order or recommendation of, any good or service reimbursable by, a federal health care program (including our products). The federal Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act. The penalties for violating the federal Anti-Kickback Statute include administrative civil money penalties, imprisonment for up to five years, fines of up to $25,000 per violation and possible exclusion from federal healthcare programs such as Medicare and Medicaid.
The federal civil and criminal false claims laws, including the civil False Claims Act, prohibit knowingly presenting, or causing to be presented, claims for payment to the federal government (including Medicare and Medicaid) that are false or fraudulent (and, under the Federal False Claims Act, a claim is deemed false or fraudulent if it is made pursuant to an illegal kickback). Manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the False Claims Act can result in significant monetary penalties, including fines ranging from $11,181 to $22,363 for each false claim, and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other improper sales and marketing practices. The government has obtained multi-million and multi-billion dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. In addition, companies have been forced to implement extensive corrective action plans and have often become subject to consent decrees or corporate integrity agreements, severely restricting the manner in which they conduct their business. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
The Federal Civil Monetary Penalties Law prohibits, among other things, the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. Noncompliance can result in civil money penalties of up to $15,270 for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the federal healthcare programs.
Federal criminal statutes prohibit, among other actions, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, the ACA amended the intent standard for certain healthcare fraud statutes under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Analogous state and foreign laws and regulations, including state anti-kickback and false claims laws, may apply to products and services reimbursed by non-governmental third-party payors, including commercial payors. Additionally, there are state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or that otherwise restrict payments that may be made to healthcare providers as well as state and foreign laws that require drug manufacturers to report marketing expenditures or pricing information.
Sunshine laws, including the Federal Open Payments law enacted as part of the ACA, require pharmaceutical manufacturers to disclose payments and other transfers of value to physicians and certain other health care providers or professionals, and in the case of some state sunshine laws, restrict or prohibit certain such payments. Pharmaceutical manufacturers are required to submit reports to the government by the 90th day of each calendar year. Failure to submit the required information may result in civil monetary penalties of up to an aggregate of $165,786 per year (or up to an aggregate of $1.105 million per year for “knowing failures”) for all payments, transfers of value or ownership or investment interests not reported in an annual submission, and may result in liability under other federal laws or regulations. Certain states and foreign governments require the tracking and reporting of gifts, compensation and other remuneration to physicians.
Privacy laws, such as the privacy regulations implemented under HIPAA, restrict covered entities from using or disclosing protected health information. Covered entities commonly include physicians, hospitals and health insurers from which we may seek to acquire data to aid in our research, development, sales and marketing activities. Although pharmaceutical manufacturers are not covered entities under HIPAA, our ability to acquire or use protected health information from covered entities may be affected by privacy laws. Specifically, HIPAA, as amended by HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information.
Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, thus complicating compliance efforts.
The FDA regulates the sale and marketing of prescription drug products and, among other things, prohibits pharmaceutical manufacturers from making false or misleading statements and from promoting products for unapproved uses. There has been an increase in government enforcement efforts at both the federal and state level. Numerous cases have been brought against pharmaceutical manufacturers under the Federal False Claims Act, alleging, among other things, that certain sales or marketing-related practices violate the Anti-Kickback Statute or the FDA’s regulations, and many of these cases have resulted in settlement agreements under which the companies were required to change certain practices, pay substantial fines and operate under the supervision of a federally appointed monitor for a period of years. Due to the breadth of these laws and their implementing regulations and the absence of guidance in some cases, it is possible that our practices might be challenged by government authorities. Violations of fraud and abuse laws may be punishable by civil and criminal sanctions including fines, civil monetary penalties, as well as the possibility of exclusion of our products from payment by federal health care programs.
Government Price Reporting
Government regulations regarding reporting and payment obligations are complex, and we are continually evaluating the methods we use to calculate and report the amounts owed with respect to Medicaid and other government pricing programs. Our calculations are subject to review and challenge by various government agencies and authorities, and it is possible that any such review could result either in material changes to the method used for calculating the amounts owed to such agency or the amounts themselves. Because the process for making these calculations, and our judgments supporting these calculations, involve subjective decisions, these calculations are subject to audit. In the event that a government authority challenges or finds ambiguity with regard to our report of payments, such authority may impose civil and criminal sanctions, which could have a material adverse effect on our business. From time to time we conduct routine reviews of our government pricing calculations. These reviews may have an impact on government price reporting and rebate calculations used to comply with various government regulations regarding reporting and payment obligations.
Many governments and third-party payors reimburse the purchase of certain prescription drugs based on a drug’s AWP. In the past several years, state and federal government agencies have conducted ongoing investigations of manufacturers’ reporting practices with respect to AWP, which they have suggested have led to excessive payments by state and federal government agencies for prescription drugs. We and numerous other pharmaceutical companies have been named as defendants in various state and federal court actions alleging improper or fraudulent practices related to the reporting of AWP.
Drug Pedigree Laws
State and federal governments have proposed or passed various drug pedigree laws which can require the tracking of all transactions involving prescription drugs from the manufacturer to the pharmacy (or other dispensing) level. Companies are required to maintain records documenting the chain of custody of prescription drug products beginning with the purchase of such products from the manufacturer. Compliance with these pedigree laws requires implementation of extensive tracking systems as well as heightened documentation and coordination with customers and manufacturers. While we fully intend to comply with these laws, there is uncertainty about future changes in legislation and government enforcement of these laws. Failure to comply could result in fines or penalties, as well as loss of business that could have a material adverse effect on our financial results.
Federal Regulation of Patent Litigation Settlements and Authorized Generic Arrangements
As part of the Medicare Prescription Drug Improvement and Modernization Act of 2003, companies are required to file with the U.S. Federal Trade Commission (“FTC”) and the U.S. Department of Justice (the “DOJ”) certain types of agreements entered into between brand and generic pharmaceutical companies related to the settlement of patent litigation or manufacture, marketing and sale of generic versions of branded drugs. This requirement could affect the manner in which generic drug manufacturers resolve intellectual property litigation and other disputes with brand pharmaceutical companies and could result generally in an increase in private-party litigation against pharmaceutical companies or additional investigations or proceedings by the FTC or other governmental authorities.
Other
The U.S. federal government, various states and localities have laws regulating the manufacture and distribution of pharmaceuticals, as well as regulations dealing with the substitution of generic drugs for branded drugs. Our operations are also subject to regulation, licensing requirements and inspection by the states and localities in which our operations are located or in which we conduct business.
Certain of our activities are also subject to FTC enforcement actions. The FTC also enforces a variety of antitrust and consumer protection laws designed to ensure that the nation’s markets function competitively, are vigorous, efficient and free of undue restrictions. Federal, state, local and foreign laws of general applicability, such as laws regulating working conditions, also govern us.
In addition, we are subject to numerous and increasingly stringent federal, state and local environmental laws and regulations concerning, among other things, the generation, handling, storage, transportation, treatment and disposal of toxic and hazardous substances, the discharge of pollutants into the air and water and the cleanup of contamination. We are required to maintain and comply with environmental permits and controls for some of our operations, and these permits are subject to modification, renewal and revocation by the issuing authorities. Our environmental capital expenditures and costs for environmental compliance may increase in the future as a result of changes in environmental laws and regulations or increased manufacturing activities at any of our facilities. We could incur significant costs or liabilities as a result of any failure to comply with environmental laws, including fines, penalties, third-party claims and the costs of undertaking a clean-up at a current or former site or at a site to which our wastes were transported. In addition, we have grown in part by acquisition, and our diligence may not have identified environmental impacts from historical operations at sites we have acquired in the past or may acquire in the future.
Employees and Labor Relations
As of the date of this prospectus, we have a total of 26 full time employees and 2 employees working as contractors.1 part time employee. We have no collective bargaining agreements with our employees, and none are represented by labor unions. We consider our current relations with our employees to be good.
Facilities
Our principal address is 1554 Paoli Pike, #279, West Chester,1055 Westlakes Drive, Suite 300, Berwyn, PA 19380.19312. We believe our facilities are adequate to meet our current needs, although we may seek to negotiate new leases or evaluate additional or alternate space for our operations. We believe appropriate alternative space would be readily available on commercially reasonable terms.
Legal Proceedings
From time to time we may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we do not currently have any pending litigation to which we are a party other than what is detailed below or to which our property is subject that we believe to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting our overall operations.
84On March 12, 2021, we and our Chief Executive Officer, Anthony Mack were named as defendants in a complaint (the “Complaint”) filed by Sorrento Therapeutics, Inc. (“Sorrento”) and Scilex Pharmaceuticals Inc. (“Scilex” and together with Sorrento, the “Plaintiffs”) in the Court of Chancery of the State of Delaware. In the Complaint, the Plaintiffs allege (i) Mr. Mack breached a Restrictive Covenants Agreement, dated as of November 8, 2016, between himself and Sorrento (the “Restrictive Covenants Agreement”), (ii) we tortiously interfered with the Restrictive Covenants Agreement, and (iii) we tortiously interfered with Scilex’s relationship with Mr. Mack. On May 7, 2021, Plaintiffs filed an Amended Complaint (the “Amended Complaint”) asserting the same three causes of action. Simultaneously with filing the original Complaint, the Plaintiffs filed a motion to expedite, seeking a hearing in four weeks on their prospective motion for a preliminary injunction, prohibiting Mr. Mack’s alleged violations of the Restrictive Covenants Agreement and prohibiting the alleged tortious interference. On March 22, 2021, Vice Chancellor Paul A. Fioravanti Jr. issued a scheduling order, setting forth terms which had been agreed to by the parties, under which oral argument on Plaintiffs’ proposed motion for preliminary injunction was scheduled for July 15, 2021. On May 17, 2021, we and Mr. Mack filed a motion to dismiss the Amended Complaint. On May 18, 2021, the Vice Chancellor issued a revised scheduling order, also setting forth terms which had been agreed to by the parties, under which a briefing schedule was set with respect to Defendants’ motion to dismiss, plaintiffs agreed not to move for a preliminary injunction, and a trial in the action was set for February 9-11, 2022. As of July 12, 2021, the parties completed briefing on the motion to dismiss the Amended Complaint. The Court has scheduled December 9, 2021 for oral argument on the motion to dismiss. We believe that the allegations in the Amended Complaint are wholly without merit, and we intend to vigorously defend the action. However, we are unable to predict the ultimate outcome of the lawsuit at this time.
MANAGEMENT
Executive Officers and Directors
Below is a list of the names, ages as of September 30, 2020,8, 2021, and positions of the individuals who serve as our executive officers and directors. Our Certificate of Incorporation will provide that each of our directors will, subject to any earlier resignation or removal in accordance with the terms of our Certificate of Incorporation, serve until the first annual meeting of stockholder following the completion of this offering.
| Name | Age | Position | ||
| Executive Officers | ||||
| Anthony Mack, MBA | 59 | |||
| Chief Executive Officer (Principal Executive Officer) and Chairman of the Board of Directors, Class III Director | ||||
| Jeffrey Gudin, MD | Executive Vice President, Chief Medical Officer, and Class III Director | |||
| Gerald Bruce | Executive Vice President, Commercial Operations, and Class I Director | |||
| Christopher M. Chipman, CPA | Chief Financial Officer (Principal Accounting Officer) and Corporate Secretary | |||
| Directors | ||||
| Eric Floyd, PhD | Independent Class III Director and Compensation Committee Chair | |||
| Jerrold Sendrow, CFP | Independent Class II Director and Audit Committee Chair | |||
| Thani Jambulingam, PhD | Independent Class II Director and Corporate Governance Committee Chair | |||
| Gary Jacob, PhD | Independent Class I Director | |||
| Vanila M. Singh, MD | Independent Class I Director | |||
| Michael F. Dubin, CPA | 66 | Independent Class II Director |
Executive Officers
Anthony Mack, MBA became a director and our Chairman of the Board of Directors and Chief Executive Officer in May 2017. Mr. Mack has more than 25 years of experience in the pharmaceutical and finance industries. Prior to founding Virpax in 2016, Mr. Mack founded SCILEX Pharmaceuticals in September 2012 and served as its President and CEO until ZTlido was approved in February 2018. Mr. Mack founded his first pharmaceutical company, ProSolus Pharmaceuticals, in 2009, where he served as President before selling the company to Mission Pharmacal in June 2015. Mr. Mack has held high-level management positions with Purdue Pharma, Endo Pharmaceuticals Inc., Novartis AG, and EKR Therapeutics Inc. (acquired by Cornerstone Therapeutics Inc.). He has led training, marketing, and commercial distribution for billion-dollar pain management products and has forged key strategic alliances. Mr. Mack also founded and servesserved as a Director of IACTA Pharmaceuticals Inc. By serving on executive boards, Mr. Mack has utilized his skills and experience to implement policies relating to corporate governance and overall business strategy. Mr. Mack holds an Executive MBA in Pharmaceutical and Healthcare Marketing from Saint Joseph’s University. Mr. Mack was selected as a director due to his leadership experience at other companies and his history of founding and operating specialty pharmaceutical companies.
Jeffrey Gudin, MD became an Executive Vice President, and our Chief Medical Officer in January 2017. Prior to joining us, Dr. Gudin, was Director of Pain Management and Palliative Care at Englewood Hospital and Medical Center in New Jersey for almost 20 years. He is a Clinical Associate Professor in Anesthesiology at the Rutgers New Jersey Medical School. Dr. Gudin is Board Certified in Pain Medicine, Anesthesiology, Addiction Medicine and Hospice and Palliative Medicine. He is an active speaker in the field of pain management. His clinical and research focus includes pain management, opioid abuse and potential solutions, and increasing clinician awareness of pain assessment and risk management. Dr. Gudin completed a residency in anesthesiology at Yale University School of Medicine, in New Haven, Connecticut. He continued his training with an extended postdoctoral fellowship in pain medicine at the Yale Center for Pain Management, where he was actively involved in research and teaching.
Gerald W. Bruce became an Executive Vice President, and our Commercial Operations Officer in August 2017. Mr. Bruce has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical and Medical Nutrition industry. He started his career in May 1983 at Johnson & Johnson Inc. (NYSE: JNJ) where he was an award-winning sales representative and held leadership positions of increasing responsibility in sales and marketing ending with his role as Group Product Director of Analgesics in September 1998.From September 1998 to November 2000, he served as Vice President of Sales at Bristol-Myers Squibb Co. (NYSE: BMY) where he led the Cardiovascular and Metabolic sales force. From November 2000 to January 2006 he served as Vice President of Managed Markets where he led the team responsible for the development and implementation of the reimbursement strategy for Bristol-Myers Squibb’s US portfolio. From January 2006 to June 2008, Mr. Bruce was the Senior Vice President of Commercial Operations at NitroMed, Inc. where he was responsible for building the commercial strategy and led the team responsible for the development and implementation of the commercial plan for the start-up company’s first product for the treatment of Heart Failure. From April 2009 to November 2018, Mr. Bruce served as Vice President of Sales for Nutricia North America, Danone Medical Nutrition Division. Mr. Bruce currently serves on the Board of Trustees for Lincoln University and is a Board member for the National Sales Network. He received his bachelor’s degree in Business Administration from Lincoln University and a master’s degree in Leadership from the McDonough School of Business at Georgetown University.. See below under “Directors.”
85
Christopher M. Chipman, CPA became our Chief Financial Officer in May 2020. Mr. Chipman has more than 20 years of public and private accounting experience. Since November 2000, Mr. Chipman has been a managing member of Chipman & Chipman, LLC, a consulting firm that assists public companies with the preparation of periodic reports required to be filed with the Securities and Exchange Commission and compliance with Section 404 of the Sarbanes Oxley Act of 2002. Mr. Chipman is a CPA and was Chief Financial Officer and Secretary of Capital Gold Corporation from March 2006 to June 2011. Capital Gold Corporation was a publicly-held gold production and exploration company, until its acquisition by AuRico Gold, Inc. (formerly, Gammon Gold). From July 1996 to August 1998, Mr. Chipman was a senior accountant with the accounting firm of Grant Thornton LLP and from August 1998 to March 2000 he was a Senior Financial Analyst for GlaxoSmithKline. Prior to that, from July 1994 to July 1996, he was an Audit Examiner for Wells Fargo Corporation. Mr. Chipman received a B.A. in Economics from Ursinus College in 1994.
Directors
Eric Floyd, PhD became a director in January 2017. Dr. Floyd currently serves as Chief Regulatory Officer at Neurogene Inc. He has nearly 21 years of regulatory experience within the pharmaceutical industry. Most recently, from November 2018 to December 2019 he was Senior Vice President, Regulatory Affairs, for Axovant Sciences. Prior to that, he served as President of Compliance Services and Chief Scientific Officer at Dohmen Life Science Services, Inc. from June 2015, Senior Vice President, U.S. Regulatory Affairs and Clinical Quality Compliance at Lundbeck Inc. December 2011, Global Vice President of Regulatory Affairs at Hospira Inc. (later acquired by Pfizer Inc.) from January 2010, Vice President of Worldwide Regulatory Affairs and Quality Assurance at Cephalon Inc. (later acquired by Teva Pharmaceuticals Industries Ltd.) from January 2007 and VP and Global Head of Respiratory, Dermatology, and Tropical Medicines Drug Regulatory Affairs at Novartis AG from February 2005. Dr. Floyd has also held senior leadership roles at Bristol Myers Squibb Co., Aventis Pharma and Merck Research Laboratories (a division of Merck & Co.). Dr. Floyd received a Ph.D. in Neurophysiology from Meharry Medical College, Nashville, an executive MBA from St. Joseph’s University, Philadelphia, an MS from Tennessee State University, a BS from the University of Illinois and has served as an Assistant Professor at Harvard University School of Medicine. Dr. Floyd served as an outside director on the Boardboard of Directorsdirectors of SCILEX Pharmaceuticals Inc. from April 2014 to November 2016. Dr. Floyd was selected as a director due to his extensive experience at pharmaceutical companies and knowledge of the pharmaceutical industry.
Jerrold Sendrow, CFP became a director in January 2017. Mr. Sendrow has been a Certified Financial Planner since 1986 and continues to maintain his practice. Mr. Sendrow also served as an outside Director on the Boardboard of Directorsdirectors of SCILEX Pharmaceuticals Inc. from April 2014 to November 2016. Prior to that, Mr. Sendrow was an accountant in the audit departments of Touche Ross & Co. and Peat Marwick Mitchell & Co after returning from military service of two tours in the Vietnam conflict. Mr. Sendrow holds business degrees from Bernard Baruch College of the City University of New York and Adelphi University. Mr. Sendrow was selected as a director due to his leadership experience at other growth-stage companies and his financial accounting experience.
Gerald W. Bruce became a director in July 2021 and an Executive Vice President and our Commercial Operations Officer in August 2017. Mr. Bruce has spent over 30 years, including 20 years in senior leadership roles, in the Pharmaceutical and Medical Nutrition industry. He started his career in May 1983 at Johnson & Johnson Inc. (NYSE: JNJ) where he was an award-winning sales representative and held leadership positions of increasing responsibility in sales and marketing ending with his role as Group Product Director of Analgesics in September 1998.From September 1998 to November 2000, he served as Vice President of Sales at Bristol-Myers Squibb Co. (NYSE: BMY) where he led the Cardiovascular and Metabolic sales force. From November 2000 to January 2006 he served as Vice President of Managed Markets where he led the team responsible for the development and implementation of the reimbursement strategy for Bristol-Myers Squibb’s US portfolio. From January 2006 to June 2008, Mr. Bruce was the Senior Vice President of Commercial Operations at NitroMed, Inc. where he was responsible for building the commercial strategy and led the team responsible for the development and implementation of the commercial plan for the start-up company’s first product for the treatment of heart failure. From April 2009 to November 2018, Mr. Bruce served as Vice President of Sales for Nutricia North America, Danone Medical Nutrition Division. Mr. Bruce currently serves on the Board of Trustees for Lincoln University and is a Board member for the National Sales Network. He received his bachelor’s degree in Business Administration from Lincoln University and a master’s degree in Leadership from the McDonough School of Business at Georgetown University. Mr. Bruce was selected as a director due to his extensive experience at pharmaceutical companies and knowledge of the pharmaceutical industry.
Thani Jambulingam, PhD became a director in January 2017. Dr. Jambulingam is a Pfizer Fellow and Professor in the Department of Pharmaceutical and Healthcare Marketing at St Joseph’s University, Erivan K. Haub School of Business, in Philadelphia, Pennsylvania. He teaches in the executive MBA program for biopharmaceutical, medical device and physician executives. Dr. Jambulingam served as the chair of the department for eight years, from June 2003 to June 2010. Dr. Jambulingam’s research is focused on pharmaceutical and healthcare strategy and innovation. His research is regularly published in marketing and management journals. Dr. Jambulingam has also served as a consultant and facilitated training sessions in innovation and strategy for senior leadership and/or brand teams within several small, mid and large pharma and healthcare firms including Alkermes Plc, Abbott Industries, AstraZeneca plc, Cardinal Health, FMC, IQVIA, Lancaster General Hospital, Leo Pharma, Merck & Co., Novo Nordisk, Pfizer Inc., Sanofi, Solvay and Procter & Gamble Inc. During his sabbatical from Saint Joseph’s University, from July 2011 to August 2012, he joined Pfizer Inc. (NYSE: PFE) with the Prevenar Global Commercial Team contributing to development of Prevenar franchise positioning, healthy aging platform development, vaccine business strategy for emerging markets, pediatric expanded age strategy (life cycle management) and conducted strategy sessions for executive leadership within the specialty care division of Pfizer. Dr. Jambulingam is a pharmacist and obtained his Ph.D. from the University of Wisconsin-Madison. Dr. Jambulingam completed the case method of teaching at Harvard. He has been inducted to the Rho Chi, the honor society in pharmacy and Beta Gamma Sigma, the honor society for business. For the past seven years, Dr. Jambulingam has been a faculty member conducting Bio-Entrepreneurship Bootcamp at the annual meeting at the Biotechnology Industry Organization (BIO). Dr. Jambulingam is also a visiting professor in the Wharton MBA Global program and teaches healthcare courses in India. Dr. Jambulingam was selected as a director due to his leadership experience at other companies and his extensive knowledge of the pharmaceutical industry.
Gary S. Jacob, PhD became a director in April 2020. Dr. Jacob has over 35 years of extensive experience in the pharmaceutical and biotechnology industries across multiple disciplines, including research and development, operations, business development, capital financing activities, and senior management expertise. He is the Co-Founder and former CEO and Chairman of Synergy Pharmaceuticals and has developed broad and influential contacts throughout the biopharmaceutical, financial, banking, and investor communities. He served as Chairman of the Board, President and Chief Executive Officer of Synergy Pharmaceuticals, Inc. where he held various positions from July 2008 until October 2018 and is the co-inventor of the FDA-approved drug Trulance® which is currently marketed in the U.S. by Bausch Health Companies, Inc. (NYSE: BHC) to treat functional GI disorders. From November 2018 to March 2020 Dr. Jacob served as the CEO and Managing Director of Immuron, Ltd., an Australian biotechnology company dual-listed on the Australian ASX exchange and on NASDAQ. Since March 2014, Dr. Jacob has served as Chairman of the Board of Hepion Pharmaceuticals, Inc., a public company with a drug in clinical development to treat nonalcoholic steatohepatitis, and since February 2009 has served on the Boardboard of Directorsdirectors of Cardiff Oncology Inc., a public oncology company. He served as Chief Executive Officer and Director of Callisto Pharmaceuticals, Inc. from May 2003 until January 2013. Prior to his involvement with Callisto and Synergy, Dr. Jacob spent a number of years at Monsanto/G.D. Searle, where he was Director of Glycobiology and a Monsanto Science Fellow, specializing in the field of Glycobiology and drug discovery. Dr. Jacob holds over 30 patents and is the co-inventor of one FDA-approved pharmaceutical drug. Dr. Jacob earned a B.S. in Chemistry cum laude from the University of Missouri –— St. Louis and holds a Ph.D. in Biochemistry from the University of Wisconsin-Madison. Dr. Jacob was selected as a director due to his leadership experience at other companies and his extensive knowledge of the pharmaceutical industry.
Vanila M. Singh, MD became a director in June 2020. From June 2017 to July 2019, Dr. Singh is the former Chief Medical Officer of the U.S. Department of Health and Human Services, where she served as the Chairperson of the highly regarded HHS Pain and Opioid Task Force in conjunction with the Department of Defense and the Veterans Administration. Since November 2019, Dr. Singh has been a director of Biodelivery Sciences International, Inc. (NASDAQ: BDSI), and since June 2004, Dr. Singh has been a clinical associate professor of Anesthesiology, Pain and Peri-operative Medicine at Stanford University and is a teaching mentor at Walter Reed National Military Medical Center. For over ten years, Dr. Singh served on medical ethics as well as on scientific editorial boards, committees for the American Society of Regional Anesthesia, American Society of Interventional Pain Physicians, California Medical Association, and the Santa Clara County Medical Association. Dr. Singh, who is double board-certified in pain and anesthesiology, focuses her practice on regional anesthesia and peri-operative, subacute, and the development of chronic pain, with an appreciation for complimentary and traditional medicine approaches that emphasize an individualized patient-centered approach. Dr. Singh received her medical degree from George Washington University Medical School and her B.A. from U.C. Berkeley in Molecular and Cell Biology and Economics. Dr. Singh was selected as a director due to her leadership experience and her extensive knowledge of the pharmaceutical industry and the regulatory environment.
Corporate Governance
Michael F. Dubin, CPA became a director in July 2021. From 2001 to 2016, Mr. Dubin held the title of Managing Partner, PA/SNJ Offices, with RSMUS LLP (RSM), a $2.8 billion professional services company with over 12,000 employees. He was presented with RSM’s “National Achievement Award” in 2010, and was a finalist for the company’s “National Integrity Award.” Mr. Dubin obtained a BS in Economics (magna cum laude) from the Wharton School of Business, University of Pennsylvania. He served as a Board Member for RSM for four years. Mr. Dubin also is currently a board member and the Audit Committee Chairman for a privately held business in Philadelphia engaged in supplying energy efficiency services and facilities, and a board member and the Risk Management Committee Chairman for a commercial bank in Pennsylvania. Mr. Dubin is professionally affiliated with the PICPA and AICPA. He was also an adjunct faculty member and course teacher for the Wharton School of Business for two years and has also been a guest lecturer at the Wharton School of the University of Pennsylvania, Temple University, University of Scranton and Lehigh University. He also served as an expert witness/consultant for the Federal Deposit Insurance Corporation and the Resolution Trust Corporation. Mr. Dubin was selected as a director due to his long history of success in manufacturing, distribution, financial services, business and professional services, pharma, technology, retail and various other industries.
Corporate Governance
The bylaws to be adopted upon the closing of this offering will delegate the authority over our management and officers to our boardBoard of directors (the “Board of Directors”).Directors. The Board of Directors may then delegate management of the Company to committees of the Board of Directors, or such other persons based on its reasonable discretion.
Regardless of any delegation, the Board of Directors will remain responsible for the proper management of our affairs. The Board of Directors may create new committees or change the responsibilities of existing committees from time to time.
Board Structure and Committee Composition
Our business and affairs are managed under the direction of our Board of Directors. Our Board of Directors currently consists of sevennine directors. The Certificate of Incorporation that will be in effect upon closing of this offering provides that our Board of Directors shall consist of at least one director but not more than nine directors and that the number of directors may be fixed from time to time by resolution of our Board of Directors.
In accordance with the terms of the Certificate of Incorporation and bylaws, that will become effective upon the completionour Board of this offering, our board of directors will beDirectors is divided into three classes, Class I, Class II and Class III, with each class serving staggered three-year terms. Upon the expiration of the term of a class of directors, directors in that class will beare eligible to be elected for a new three-year term at the annual meeting of stockholderstockholders in the year in which their term expires. Our directors will beare divided among the three classes as follows:
| ● | The Class I directors |
| ● | The Class II directors |
| ● | The Class III directors |
We expect that any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control.
Under the Certificate of Incorporation, that will become effective upon the closing of this offering, directors have the authority to appoint one or more directors to our Board of Directors, subject to the maximum number of directors allowed for in our Certificate of Incorporation. A vacancy on our Board of Directors may be filled by the remaining directors and any director so appointed will hold office until our next annual general meeting. During any vacancy on our Board of Directors, the remaining directors will have full power to act as the board.
Upon consummation of this offering, we willWe have an Audit Committee, a Compensation Committee, a Nominating and Corporate Governance Committee and a Science and Technology Committee with the composition and responsibilities described below. Each committee will operateoperates under a charter that will behas been approved by our Board of Directors. The members of each committee are appointed by the Board of Directors and serve until their successor is elected and qualified unless they are earlier removed or resign. In addition, from time to time, special committees may be established under the direction of the board of directors when necessary to address specific issues.
Audit Committee
Effective upon completion of this offering, ourOur Audit Committee will beis comprised of Mr. Sendrow, Dr. Floyd, and Dr. Jambulingam, with Mr. Sendrow serving as Chairman of the audit committee. Our Board of Directors has determined that each member of the Audit Committee meets the independence requirements of Rule 10A-3 under the Exchange Act and the applicable rules of the Nasdaq Capital Market. Our Board of Directors has determined that Mr. Sendrow is an “audit committee financial expert” within the meaning of SEC regulations and the applicable rules of the Nasdaq Capital Market. The Audit Committee’s responsibilities upon completion of this offering will include:
| ● | appointing, approving the compensation of, and assessing the qualifications, performance and independence of our independent registered public accounting firm, and in particular the provision of additional services to each entity covered by the committee; |
| ● | pre-approving audit and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| ● | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures as well as critical accounting policies and practices used by us; |
| ● | monitoring the audit of our financial statements; |
| ● | setting policies for our hiring of employees or former employees of our independent registered public accounting firm; |
| ● | reviewing our significant risks or exposures and assessing the steps that management has taken or should take to monitor and minimize such risks or exposures; |
| ● | reviewing the adequacy of our internal control over financial reporting, including information system controls and security; |
| ● | monitoring the effectiveness of our systems of internal control, internal audit and risk management for each entity covered by the committee; |
| ● | establishing policies and procedures for the receipt and retention of accounting-related complaints and concerns; |
| ● | recommending, based upon the audit committee’s review and discussions with management and the independent registered public accounting firm, whether our audited financial statements shall be included in our Annual Report on Form 10-K; |
| ● | monitoring our compliance with legal and regulatory requirements as they relate to our financial statements and accounting matters; |
| ● | preparing the audit committee report required by the rules of the SEC to be included in our annual proxy statement; |
| ● | reviewing all related party transactions for potential conflict of interest situations and approving all such transactions; and |
| ● | reviewing and discussing with management and our independent registered public accounting firm our earnings releases and scripts. |
88
Compensation Committee
Effective upon completion of this offering, ourOur Compensation Committee will beis composed of Dr. Floyd, Mr. Sendrow, and Dr. Jambulingam, with Dr. Floyd serving as Chairman of the committee. Our Board of Directors has determined that each director serving on the Compensation Committee is “independent” as defined under the applicable listing standards of the Nasdaq Capital Market. Further, the Board of Directors has determined that the directors serving on the Compensation Committee are “non-employee directors” as defined in rule 16b-3 promulgated under the Exchange Act and are “outside directors” as that term is defined in Section 162(m) of the Internal Revenue Code of 1986, as amended. The compensation committee’s responsibilities upon completion of this offering will include:
| ● | reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, the officers who report directly to the chief executive officer and all officers who are “insiders” subject to Section 16 of the Exchange Act; |
| ● | evaluating the performance of our chief executive officer and such other officers in light of such corporate goals and objectives and determining and approving, or recommending to our board of directors for approval, the compensation of our chief executive officer and such other officers; |
| ● | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| ● | conducting the independence assessment outlined in the listing standards of the Nasdaq Capital Market with respect to any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| ● | annually reviewing and reassessing the adequacy of the committee charter; |
| ● | reviewing and establishing our overall management compensation and our compensation philosophy and policy; |
| ● | overseeing and administering our equity compensation and other compensatory plans; |
| ● | reviewing and approving our equity and incentive policies and procedures for the grant of equity-based awards and approving the grant of such equity-based awards; |
| ● | reviewing and making recommendations to our board of directors with respect to non-employee director compensation; and |
| ● | producing a report, if required, on executive compensation to be included in our annual proxy statement or Annual Report on Form 10-K. |
Nominating and Corporate Governance Committee
Effective upon completion of this offering, ourOur nominating and corporate governance committee will be composed of Dr. Jambulingam, Mr. Sendrow, and Dr. Floyd, with Mr.Dr. Jambulingam serving as chairmanChairman of the committee. Our board of directors has determined that Dr. Jambulingam is “independent” as defined in the applicable rules of the Nasdaq Capital Market. The nominating and corporate governance committee’s responsibilities upon completion of this offering will include:
| ● | establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholder; |
| ● | identifying individuals qualified to become members of our board of directors; |
| ● | recommending to our board of directors the persons to be nominated for election as directors and to each of our board’s committees; |
| ● | developing and recommending to our board of directors a set of corporate governance principles; |
| ● | articulating to each director what is expected, including reference to the corporate governance principles and directors’ duties and responsibilities; |
| ● | reviewing and recommending to our board of directors’ practices and policies with respect to directors; |
| ● | reviewing and recommending to our board of directors the functions, duties and compositions of the committees of our board of directors; |
| ● | reviewing and assessing the adequacy of the committee charter and submitting any changes to our board of directors for approval; |
| ● | considering and reporting to our board of directors any questions of possible conflicts of interest of board of directors’ members; |
| ● | providing for new director orientation and continuing education for existing directors on a periodic basis; |
| ● | performing an evaluation of the performance of the committee; and |
| ● | overseeing the evaluation of our board of directors. |
Science and Technology Committee
Our Science and Technology Committee is comprised of Dr. Floyd and Dr. Jambulingam, with Dr. Floyd serving as the chairmanChairman of the committee. Our Science and Technology Committee is responsible for, among other things:
| ● | periodically |
| ● |
| ● |
| ● | periodically |
Code of Business Conduct and Ethics
We have adopted written code of business conduct and ethics (“Code of Ethics”) that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. The Code of Ethics is available on our website at www.virpaxpharma.com/code-of- ethics. In addition, we intend to post on our website all disclosures that are required by law or the Nasdaq Capital Market rules concerning any amendments to, or waivers from, any provision of the Code of Ethics. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
Legal Proceedings
We are not aware of any of our directors or officers being involved in any legal proceedings in the past 10 years relating to bankruptcy, insolvency or criminal proceedings (other than traffic and other minor offenses) or being subject to any of the items set forth under Item 401(f) of Regulation S-K, except for Dr. Gary S. Jacob. From 2008 to 2017, Dr. Jacob served as President and Chief Executive Officer of Synergy Pharmaceuticals, Inc. and as Chairman from 2013 to October 2018. In December of 2018, Synergy Pharmaceuticals, Inc. filed a petition for relief under Chapter 11 of the U.S. Bankruptcy Code.
90
EXECUTIVE AND DIRECTOR COMPENSATION
As an emerging growth company under the JOBS Act we have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies,” which require compensation disclosure for our principal executive officer and the two most highly compensated executive officers (other than our principal executive officer) serving as executive officers at the end of our most recently completed fiscal year (collectively, our “Named Executive Officers”). This section describes the executive compensation program in place for our Named Executive Officers during the year ended December 31, 2019,2020, who are the individuals who served as our principal executive officer and two most highly compensated executive officers.
This section discusses the material components of the executive compensation program for our executive officers who are named in the “Summary Compensation Table” below and the non-employee members of our board of directors. In 2019,2020, our “Named Executive Officers” and their positions were:
| ● | Anthony Mack, our Chief Executive Officer and Chairman of the Board of Directors; |
| ● | Jeffrey Gudin, MD, our Executive Vice President, Chief Medical Officer; |
| ● | Christopher M. Chipman, our Chief Financial Officer: and |
| ● | Michele Linde, our former Executive Vice President, Chief Legal Officer. |
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt following the completion of this offering may differ materially from the currently planned programs summarized in this discussion.
2019 Summary Compensation Table
The following table sets forth information concerning the compensation of our Named Executive Officers for the year ended December 31, 20192020 and 2018:2019:
| Name & | Salary/ | Stock | Option | Non-Equity Incentive Plan | Change in Pension Valued and NQDC | All Other | |||||||||||||||||||||||||||||
| Principal Position | Year | Fees | Bonus | Awards | Awards | Compensation | Earnings ($) | Compensation | Total | ||||||||||||||||||||||||||
| Anthony Mack | 2019 | $ | 375,000 | $ | - | $ | - | $ | 102,000 | (2) | $ | - | $ | - | $ | - | $ | 477,000 | |||||||||||||||||
| Chief Executive Officer, Chairman (1) | 2018 | $ | 296,875 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 296,875 | ||||||||||||||||||
| Jeffrey Gudin, MD | 2019 | $ | - | $ | - | $ | - | $ | 77,000 | (2) | $ | - | $ | - | $ | - | $ | 77,000 | |||||||||||||||||
| Executive VP, Chief Medical Officer | 2018 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||||
| Michele Linde (3) | 2019 | $ | 126,000 | $ | - | $ | 288,000 | (4) | $ | 153,000 | (2) | $ | - | $ | - | $ | - | $ | 567,000 | ||||||||||||||||
| Executive VP, Chief Legal Officer | 2018 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||||
| Name & Principal Position | Year | Salary | Salary/ Bonus | Stock Awards | Option Awards | Total | ||||||||||||||||
| Anthony Mack | 2020 | $ | 375,000 | $ | — | $ | — | $ | 230,000 | (2) | $ | 605,000 | ||||||||||
| Chief Executive Officer, Chairman(1) | 2019 | $ | 375,000 | $ | — | $ | — | $ | 102,000 | (2) | $ | 477,000 | ||||||||||
| Jeffrey Gudin, MD | 2020 | $ | — | $ | — | $ | — | $ | 173,000 | (2) | $ | 173,000 | ||||||||||
| Executive VP, Chief Medical Officer | 2019 | $ | — | $ | — | $ | — | $ | 77,000 | (2) | $ | 77,000 | ||||||||||
| Christopher Chipman | 2020 | $ | 48,000 | $ | — | $ | — | $ | 228,000 | (2) | $ | 276,000 | ||||||||||
| Chief Financial Officer | 2019 | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||
| Michele Linde(3) | 2020 | $ | 128,000 | $ | — | $ | 69,000 | (4) | $ | 228,000 | (2) | $ | 425,000 | |||||||||
| Executive VP, Chief Legal Officer | 2019 | $ | 126,000 | $ | — | $ | 288,000 | (4) | $ | 153,000 | (2) | $ | 567,000 | |||||||||
| (1) | This represents deferred compensation due to Mr. Mack as of December 31, |
| (2) | Amounts reflect the full grant date fair value of stock options granted during the year ended December 31, |
| (3) | Michele Linde was engaged as a consultant during the year ended December 31, 2017 and was hired as a full-time employee on May 1, 2019. We terminated Ms. Linde as our Executive Vice President, Chief Legal Officer as of May 15, 2020. |
| (4) | Represents the full grant date fair value of restricted shares granted during the year ended December 31, 2020 and 2019 pursuant to the employment agreement entered into with Ms. Linde on May 1, 2019. Upon the termination of Ms. Linde’s employment on May 15, 2020, $200,000 of the fair value of the restricted shares was forfeited. |
Employment arrangementsCompensation Arrangements with our Named Executive Officers
Mr. Mack.
On September 18, 2018, we entered into an employment agreement with Mr. Mack. The term of the agreement initiated upon the commencement of the agreement and terminates upon either death, disability, for cause, for good reason, or for other reasons by the Company or Mr. Mack. Under Mr. Mack’s employment agreement, Mr. Mack is paid an annual base salary of $375,000 with reviews by the board of directors for annual increases commencing in 2020 and an annual performance bonus in an amount of up to 50% of his base salary based on the achievement of our corporate objectives and Mr. Mack’s individual performance metrics, in each case as established by the board of directors in consultation with Mr. Mack. For the fiscal year 2019, no bonus was paid to Mr. Mack under his employment agreement. Mr. Mack’s employment agreement may be terminated by us immediately upon written notice to Mr. Mack, or by Mr. Mack upon 30 days‘days’ notice provided to us. Concurrent with the execution of his employment agreement, we and Mr. Mack agreed to an executive confidentiality agreement (the “Executive Confidentiality Agreement”) that contains standard non-disclosure and non-competition provisions. In the event we terminate Mr. Mack’s employment agreement other than for cause, or Mr. Mack terminates the employment agreement for good reason, we will pay him the then effective base salary for a period of twelve months following the effective date of the termination. However, payment of the effective base salary is subject to the execution of a release of claims and the compliance by Mr. Mack with such release and all terms and provisions of the employment agreement and Executive Confidentiality Agreement that survive the termination of Mr. Mack’s employment.
Mr. Mack has elected to forgo his salary and defer his compensation.compensation through March 2021. As of JulyDecember 31, 2020, Mr. Mack is due approximately $840,000$1,005,000 in deferred compensation payable pursuant to Mr. Mack’s employment agreement. We plan to pay such deferred compensation to Mr. Mack with a portion of the net proceeds from this offering.
Dr. Gudin
Mr. Bruce
Mr. Bruce was appointed our Executive VP of Commercial Operations in March 2020 and is currently working as a consultant for us. On March 21, 2020,April 15, 2021, we entered into a consultingan employment agreement with Mr. BruceDr. Gudin (the “Gudin Employment Agreement”). The Gudin Employment Agreement provides for Dr. Gudin to continue to serve as our Executive Vice President and Chief Medical Officer reporting to our Chief Executive Officer, and provides for an annual base salary of $150,000. Under the Gudin Employment Agreement, Dr. Gudin is eligible for an annual bonus with a target amount of 30% of his base salary, which will be awarded by the Company’s board of directors (the “Board”) in its sole discretion based on the achievement of the Company and Dr. Gudin of corporate and personal performance metrics established by the Board on an annual basis. To receive any bonus, Dr. Gudin must be employed by the Company at the time of payment. Dr. Gudin may also receive, in the discretion of the Board, equity awards under the 2017 Plan or any other equity incentive plan that the Company may adopt in the future. Dr. Gudin will also be eligible to receive other customary benefits described in the Gudin Employment Agreement.
We may terminate the Gudin Employment Agreement upon written notice to Dr. Gudin in the event of Disability (as defined in the Gudin Employment Agreement), in which event we would have no further obligations under the Gudin Employment Agreement, except for any Accrued Obligations (as defined in the Gudin Employment Agreement) and any portion of an earned annual bonus which remains unpaid at the time of termination. We may also terminate the Gudin Employment Agreement for Cause (as defined in the Gudin Employment Agreement) immediately upon providing written notice of such termination to Dr. Gudin. If we terminate the Gudin Employment Agreement for Cause, we would have no further obligation under the Gudin Employment Agreement, except for any Accrued Obligations due. We may terminate the Gudin Employment Agreement without Cause immediately upon written notice of termination to Dr. Gudin. If we terminate the Gudin Employment Agreement without Cause, in addition to any Accrued Obligations due, Dr. Gudin is entitled to receive (i) severance payments in an amount equal to Dr. Gudin’s base salary for a 1 year term, under which Mr. Bruce will perform consulting services which include, but are not limited to, reviewing business development opportunitiesperiod of six months after the effective date of the termination and business analytics and modeling, establishing market segmentation guidelines with business analytics, assisting with commercial infrastructure, advising(ii) reimbursement of medical insurance premiums until the Board, President and CEO, overseeing our CSO, and advising commercial strategy for the IPO. Pursuant to the consulting agreement, we granted Mr. Bruce a stock option equivalent to 75,000 sharesearlier of our common stock at an exercise price of $2.00 per share. The stock options vest upon one year from(1) six months or (2) the date Dr. Gudin becomes eligible for medical benefits through another employer, subject to certain conditions.
Dr. Gudin may terminate his agreement for Good Reason (as defined in the Gudin Employment Agreement) upon providing written notice of grant. This represented the sole compensation under the consulting agreement. The stock optionsuch termination to us. If Dr. Gudin terminates his employment for Good Reason, Dr. Gudin will be entitled to receive the same payments and benefits on the same terms and conditions as would be applicable upon termination by us without Cause.
If the Gudin Employment Agreement is terminated by Dr. Gudin for Good Reason or by us without Cause (other than on account of Dr. Gudin’s death or Disability), in each case within twelve months following a Change in Control (as defined in the Gudin Employment Agreement), Dr. Gudin will be entitled to receive the Accrued Obligations and, subject to Dr. Gudin’s compliance with the terms of our Amendedthe Gudin Employment Agreement, Dr. Gudin will be entitled to receive the following: (i) a lump sum payment equal to two times the sum of Dr. Gudin’s base salary for the year in which the termination date occurs (or if greater, the year immediately preceding the year in which the Change in Control occurs), (ii) a lump sum payment equal to two times the sum of Dr. Gudin’s cash bonus for the calendar year in which the termination date occurs (or if greater, the year in which the Change in Control occurs), and Restated(iii) accelerated vesting of any award granted to Dr. Gudin under the 2017 Equity IncentivePlan.
The Gudin Employment Agreement has a term of three years from the effective date. In connection with his entry into the Gudin Employment Agreement, Dr. Gudin entered into a customary Confidential Disclosure Invention Assignment Agreements with us.
Mr. Chipman
On April 7, 2021, we entered into an employment agreement with Christopher Chipman (the “Chipman Employment Agreement”). The Chipman Employment Agreement provides for Mr. Chipman to continue to serve as the Company’s Chief Financial Officer reporting to the Company’s Chief Executive Officer, and provides for an annual base salary of $250,000. Under the Chipman Employment Agreement, Mr. Chipman is eligible for an annual bonus equal to 30% of his base salary, which will be awarded by the Company’s board of directors (the “Board”) in its sole discretion based on the achievement of the Company and Mr. Chipman of corporate and personal performance metrics established by the Board on an annual basis. To receive any bonus, Mr. Chipman must be employed by the Company at the time of payment. Mr. Chipman may also receive, in the discretion of the Board, equity awards under the 2017 Plan, and a stock option agreement.or any other equity incentive plan that the Company may adopt in the future. Mr. BruceChipman will also be reimbursedeligible to receive other customary benefits described in the Chipman Employment Agreement.
We may terminate the Chipman Employment Agreement upon written notice to Mr. Chipman in the event of Disability (as defined in the Chipman Employment Agreement), in which event the Company would have no further obligations under the Chipman Employment Agreement, except for all reasonable, documented travelany Accrued Obligations (as defined in the Chipman Employment Agreement) and direct out-of-pocket expenses relatedany portion of an earned annual bonus which remains unpaid at the time of termination. We may also terminate the Chipman Employment Agreement for Cause (as defined in the Chipman Employment Agreement) immediately upon providing written notice of such termination to Mr. Chipman. If we terminate the Chipman Employment Agreement for Cause, we would have no further obligation under the Chipman Employment Agreement, except for any Accrued Obligations due. We may terminate the Chipman Employment Agreement without Cause immediately upon written notice of termination to Mr. Chipman. If we terminate the Chipman Employment Agreement without Cause, in addition to any Accrued Obligations due, Mr. Chipman is entitled to receive (i) severance payments in an amount equal to Mr. Chipman’s base salary for a period of six months after the effective date of the termination and (ii) reimbursement of medical insurance premiums until the earlier of (1) six months or (2) the date Mr. Chipman becomes eligible for medical benefits through another employer, subject to certain conditions.
Mr. Chipman may terminate his consulting services, provided we have approvedagreement for Good Reason (as defined in the Chipman Employment Agreement) upon providing written notice of such expenditures advance. The consulting agreement maytermination to us. If Mr. Chipman terminates his employment for Good Reason, Mr. Chipman will be entitled to receive the same payments and benefits on the same terms and conditions as would be applicable upon termination by us without Cause.
If the Chipman Employment Agreement is terminated by either party at any time upon 15 days advance written notice. However, if Mr. Bruce remains engaged underChipman for Good Reason or by us without Cause (other than on account of Mr. Chipman’s death or Disability), in each case within twelve months following a Change in Control (as defined in the Chipman Employment Agreement), Mr. Chipman will be entitled to receive the Accrued Obligations and, subject to Mr. Chipman’s compliance with the terms of his consulting agreement at the time we complete an IPO or commercially launches its first product, he isChipman Employment Agreement, Mr. Chipman will be entitled to be offeredreceive the following: (i) a full time position.lump sum payment equal to two times the sum of Mr. Bruce’s employment agreement contains standard non-solicitationChipman’s base salary for the year in which the termination date occurs (or if greater, the year immediately preceding the year in which the Change in Control occurs), (ii) a lump sum payment equal to two times the sum of Mr. Chipman’s cash bonus for the calendar year in which the termination date occurs (or if greater, the year in which the Change in Control occurs), and non-compete provisions.(iii) accelerated vesting of any award granted to Mr. Chipman under the 2017 Plan.
The Chipman Employment Agreement has a term of three years from the effective date. In connection with his entry into the Chipman Employment Agreement, Mr. Chipman entered into a customary Confidential Disclosure Invention Assignment Agreements with the Company.
Severance subject to release of claims. Our obligation to provide an executive with severance payments and other benefits under each executive’s employment or consulting agreement, as applicable, is conditioned on the executive signing (and not subsequently revoking) an effective release of claims in favor of us.
Director compensation
The following table sets forth information concerning the compensation paid to certain of our non-employee directors during the year ended December 31, 2019.2020.
| Name | Option Awards ($)(1) | Total ($) | ||||||
| Eric Floyd, PhD (2) | $ | 54,000 | $ | 54,000 | ||||
| Jerrold Sendrow, CFP (3) | $ | 35,000 | $ | 35,000 | ||||
| Thani Jambulingam, PhD (4) | $ | 37,000 | $ | 37,000 | ||||
| Name | Option Awards ($)(1) | Total ($) | ||||||
| Eric Floyd, PhD(2) | $ | 54,000 | $ | 54,000 | ||||
| Jerrold Sendrow, CFP(3) | $ | 38,000 | $ | 38,000 | ||||
| Thani Jambulingam, PhD(4) | $ | 37,000 | $ | 37,000 | ||||
| Vanila M. Singh, MD(5) | $ | 115,000 | $ | 115,000 | ||||
| Gary S. Jacob, PhD(6) | $ | 114,000 | $ | 114,000 | ||||
| (1) | Amounts reflect the full grant date fair value of stock options granted during |
| (2) | On January 1, |
| (3) | On January 1, |
| (4) | On January 1, |
| (5) | On June 15, 2020, Dr. Singh was granted an option to purchase 20,225 shares of common stock with an exercise price of $9.89 per share. This option will fully vest on the first anniversary of the original grant date and has a term of ten years. |
| (6) | On April 26, 2020, Mr. Jacob was granted an option to purchase 20,225 shares of common stock with an exercise price of $9.89 per share. This option will fully vest on the first anniversary of the original grant date and has a term of ten years. |
On May 21, 2018,Michael F. Dubin was appointed to our boardBoard of directorsDirectors on July 16, 2021. Upon his appointment, Mr. Dubin was granted an option to purchase 20,225 shares of common stock with an exercise price of $4.32 per share. This option will fully vest on the first anniversary of the original grant date and has a term of ten years.
Our Board of Directors has approved a director compensation policy applicable to our Non-Employee Directors. This policy provides for:
| ● | On January 1 of each year, each then serving Non-Employee Director of the Board shall be automatically granted that number of stock options having a value of $25,000 calculated on the grant date in accordance with the Black-Scholes option pricing model and shall be exercisable as to 100% of stock option on the first anniversary of the grant date. These stock options have a term of ten years and shall have an exercise price equal to 100% of the fair market value of a share of common stock on the date of grant. |
| ● | On January 1 of each year, each then serving member of the Science and Technology Committee shall be automatically granted stock options to purchase |
| ● | On January 1 of each year, the Chair of the Science and Technology Committee shall be granted stock options to purchase an additional |
All options granted under this policy are granted pursuant to the 2017 Plan. Further, the 2017 Plan provides for the granting of an option to purchase 20,225 shares of our common stock upon the initial appoint to our Board of Directors for each non-employee director.
93
Equity compensation
Outstanding equity awards at fiscal year-end table
The following table sets forth information concerning the outstanding equity awards held by each of our Named Executive Officers as of December 31, 2019:2020:
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights that Have Not Vested (#) | |||||||||||||||||||||||||
| Anthony Mack | 100,000 | — | 2.00 | 5/18/2029 | — | — | — | — | ||||||||||||||||||||||||||
| Chief Executive Officer | ||||||||||||||||||||||||||||||||||
| Jeffrey Gudin, MD | 75,000 | — | 2.00 | 5/18/2029 | — | — | — | — | ||||||||||||||||||||||||||
| Chief Medical Officer | ||||||||||||||||||||||||||||||||||
| Michele Linde | 25,000 | — | — | 2.00 | 7/20/2028 | 100,000 | $ | 200,000 | 100,000 | — | ||||||||||||||||||||||||
| Executive VP, Chief Legal Officer | — | 50,000 | — | 2.00 | 5/18/2029 | 43,750 | $ | 106,250 | — | — | ||||||||||||||||||||||||
| — | 100,0000 | — | 2.00 | 10/31/2029 | — | — | — | — | ||||||||||||||||||||||||||
| Option Awards | ||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | ||||||||||
| Anthony Mack | — | 40,450 | 9.89 | 5/22/2030 | ||||||||||
| Chief Executive Officer | 20,225 | — | 9.89 | 5/18/2029 | ||||||||||
| Jeffrey Gudin, MD | — | 30,338 | 9.89 | 5/22/2030 | ||||||||||
| Chief Medical Officer | 15,169 | — | 9.89 | 5/18/2029 | ||||||||||
| Christopher Chipman | 10,112 | 30,338 | 9.89 | 5/1/2030 | ||||||||||
| Chief Financial Officer | ||||||||||||||
| Michele Linde | 40,450 | — | 9.89 | 5/15/2030 | ||||||||||
| Executive VP, | 10,112 | — | 9.89 | 5/18/2029 | ||||||||||
| Chief Legal Officer | 5,056 | — | 9.89 | 10/31/2029 | ||||||||||
| 5,056 | — | 9.89 | 7/20/2028 | |||||||||||
Employee benefits plans
We currently provide broad-based health and welfare benefits that are available to all of our employees, including our Named Executive Officers, including medical, dental, vision, life and disability insurance.
Limitation of Directors Liability and Indemnification
The Delaware General Corporation Law authorizes corporations to limit or eliminate, subject to certain conditions, the personal liability of directors to corporations and their stockholder for monetary damages for breach of their fiduciary duties. The Certificate of Incorporation to be adopted upon the closing of this offering limits the liability of our directors to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with all of our directors and named executive officers whereby we have agreed to indemnify those directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of ours, provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, our best interests.
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act. The Certificate of Incorporation and bylaws that will be adopted upon closing of this offering will also provide that we will indemnify our directors and officers who, by reason of the fact that he or she is or was one of our officers or directors of our Company, is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative related to their board role with us.
There is no pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
94
Indemnification Agreements
We have entered into Indemnification Agreements with each of our current directors and executive officers. The Indemnification Agreements provide for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreements also provide for the advancement of expenses in connection with a proceeding prior to a final, nonappealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreements.
Incentive plan
2017 Incentive Plan
On May 20, 2017, our Board of Directors adopted the 2017 Plan, and on May 21, 2018, our Board of Directors adoptedapproved the Amended and Restated 2017 Plan.Plan, which was further amended on April 25, 2020. The following summary describes the material terms of the 2017 Plan. This summary is not a complete description of all provisions of the 2017 Plan and is qualified in its entirety by reference to the 2017 Plan, which will be filed as an exhibit to the registration statement of which this prospectus is a part.
The purpose of the 2017 Plan is to encourage the participants to contribute materially to our growth as a company, thereby benefitting our stockholder, and will align the economic interests of the participants with those of the stockholder.
Administration. The plan is administered by the Compensation Committee, with the caveat that the entire Board of Directors may act in lieu of the Compensation Committee on any matter of the plan. The Compensation Committee shall have authority in its discretion to determine the eligible persons to whom, and the time or times at which, awards may be granted, the number of shares, units or other rights subject to each award, the exercise, base or purchase price of an award (if any), the time or times at which an award will become vested, exercisable or payable, the performance criteria, performance goals and other conditions of an award, the duration of the award, and all other terms of the award.
Available shares. The maximum aggregate number of shares of common stock which may be issued under all awards granted to participants under the plan initially shall be 1,500,000303,382 shares. All 1,500,000303,382 of such authorized shares initially available may be issued in respect of incentive stock options. The number of authorized shares available for issuance under the plan shall automatically increase on January 1st of each year, in an amount equal to 6% of the total number of shares of common stock outstanding on December 31st of the preceding calendar year. However, the Board of Directors may act prior to the first day of any calendar year, to provide that there shall be no increase in the share reserve for such calendar year or that the increase in the share reserve for such calendar year shall be a lesser number of shares of common stock than would otherwise occur. As of January 1, 2021, the maximum aggregate number of shares of common stock which may be issued under the plan is 1,009,505 shares.
Eligibility for participation. An incentive stock option may only be granted to an employee, officer, director, or consultant who is considered an employee of ours or any subsidiary of ours.
Individual limits. The maximum number of shares of common stock with respect to any award may be granted to any one eligible person under the plan during a calendar year shall be 300,00060,676 shares.
Types of awards. The 2017 Plan provides for the grant of nonqualified stock options, incentive stock options (“ISOs”), stock appreciation rights (“SARs”), restricted stock, stock units, performance shares, performance units, incentive bonus awards, and other cash and stock-based awards.
| ● | Stock options and SARs. The Compensation Committee may grant stock options, including ISOs, and SARs. A stock option is a right entitling the holder to acquire our common shares upon payment of the applicable exercise price. A SAR is a right entitling the holder upon exercise to receive an amount (payable in cash or shares of equivalent value) equal to the excess of the fair market value of the shares subject to the right over the base value from which appreciation is measured. The exercise price of each stock option, and the base value of each SAR, granted under the 2017 Plan shall be no less than 100% of the fair market value of a share of common stock on the date of grant (110% in the case of certain ISOs). Each stock option and SAR will have a maximum term of not more than ten years from the date of grant. |
| ● | Restricted and unrestricted stock and stock units. The administrator of the 2017 Plan may grant awards of shares, stock units, restricted stock and restricted stock units. A stock unit is an unfunded and unsecured promise, denominated in shares, to deliver shares or cash measured by the value of shares in the future, and a restricted stock unit is a stock unit that is subject to the satisfaction of specified performance or other vesting conditions. Restricted stock are shares subject to restrictions requiring that they be redelivered or forfeited to the company if specified conditions are not satisfied. |
| ● | Performance awards. The administrator of the 2017 Plan may grant performance awards, which are awards subject to performance criteria. Each performance share shall have an initial value equal to the fair market value of a share on the date of grant. The Compensation Committee shall set performance goals in its discretion that, depending on the extent to which they are met over a specified time period, shall determine the number of performance shares that shall be paid to a participant. |
| ● | Other stock-based awards. The administrator of the 2017 Plan may grant other awards that are convertible into or otherwise based on our common shares, subject to such terms and conditions as it determines. |
| ● | Substitute awards. The administrator of the 2017 Plan may grant substitute awards in connection with certain corporate transactions, which may have terms and conditions that are inconsistent with the terms and conditions of the 2017 Plan. |
Change in control. The Compensation Committee may, at the time of the grant of an award and as set forth in the applicable award agreement, provide for the effect of a “Change in Control” on an award. Such provisions may include any one or more of the following: (i) the acceleration or extension of time periods for purposes of exercising, vesting in, or realizing gain from any award; (ii) the elimination or modification of performance or other conditions related to the payment or other rights under an award; (iii) provision for the cash settlement of an award for an equivalent cash value, as determined by the Committee, or (iv) such other modification or adjustment to an award as the Committee deems appropriate to maintain and protect the rights and interests of participants upon or following a Change in Control. To the extent necessary for compliance with Section 409A of the Code, an award agreement shall provide that an award subject to the requirements of Section 409A that would otherwise become payable upon a Change in Control shall only become payable to the extent that the requirements for a “change in control” for purposes of Section 409A have been satisfied.
“Change in Control” means, unless otherwise provided in an award agreement, the occurrence of any one of the following events:
| (i) | any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Exchange Act, but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular Participant, the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (A) the combined voting power of the Company’s then outstanding securities; or (B) the then outstanding shares of Common Stock (in either such case other than as a result of an acquisition of securities directly from the Company); or |
| (ii) | any consolidation or merger of the Company where our stockholders, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Exchange Act), directly or indirectly, shares representing in the aggregate 50% or more of the combined voting power of the securities of the corporation issuing cash or securities in the consolidation or merger (or of its ultimate parent corporation, if any); or |
| (iii) | there shall occur (A) any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of our assets, other than a sale or disposition by us of all or substantially all of our assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership in us immediately prior to such sale; or (B) the approval by our stockholders of any plan or proposal for our liquidation or dissolution. |
Notwithstanding the foregoing, no event or condition shall constitute a Change in Control to the extent that, if it were, a 20% tax would be imposed under Section 409A of the Code; provided that, in such a case, the event or condition shall continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such 20% tax.
Stockholder rightsrights.. Except as otherwise provided in the applicable award agreement, and with respect to an award of restricted stock, a participant will have no rights as a stockholder with respect to shares of our common stock covered by any award until the participant becomes the record holder of such shares.
Amendments and termination. The Board of Directors may suspend or terminate the 2017 Plan (or any portion thereof) at any time and may amend the 2017 Plan at any time and from time to time in such respects as the Board of Directors may deem advisable or in our best interests; provided, however, that stockholder approval is required for any amendment to the 2017 Plan that (i) increases the number of shares of common stock available for issuance under the 2017 Plan, or (ii) changes the persons or class of persons eligible to receive awards under the 2017 Plan.
Transferability of awards. Except as the Administrator may otherwise determine, awards may not be transferred other than by will or by the laws of descent and distribution.
Claw back. The administrator of the 2017 Plan may provide that any outstanding award or the proceeds of any award or share acquired thereunder will be subject to reduction, cancellation, forfeiture, or recoupment upon the occurrence of certain specified events, in addition to any otherwise applicable vesting or performance conditions of an award. Such events shall include, but not limited to, termination of continuous service for cause, violation of material company policies, breach of noncompetition, confidentiality or other restrictive covenants that may apply to the participant, or other conduct by the participant that is detrimental to our business or reputation. The Compensation Committee may also specify in an award agreement that the participant’s rights, payments and benefits with respect to an award shall be conditioned upon the participant making a representation regarding compliance with noncompetition, confidentiality or other restrictive covenants. The participant’s rights, payments and benefits with respect to an award shall be subject to reduction, cancellation, forfeiture or recoupment on account of a breach of such representation.
Potential Limitation on Company Deductions. Section 162(m) of the Tax Code generally disallows a tax deduction for compensation in excess of $1 million paid in a taxable year by a publicly held corporation to its chief executive officer and certain other “covered employees”. The Board of Directors and the Compensation Committee intend to consider the potential impact of Section 162(m) on grants made under the 2017 Plan but reserve the right to approve grants of options and other awards for an executive officer that exceed the deduction limit of Section 162(m).
Tax Withholding. As and when appropriate, we shall have the right to require each optionee purchasing shares of common stock and each grantee receiving an award of shares of common stock under the 2017 Plan to pay any federal, state or local taxes required by law to be withheld.
97
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 20182019 to which we have been a party in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets as of December 31, 20192020 and 2018,2019, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive and Director Compensation.” We also describe below certain other transactions with our directors, executive officers, and stockholder.
Initial Capitalization
In May 2017, we issued 13,500,000 shares of our common stock to Virpax Pharmaceuticals, LLC. Anthony Mack, our Chief Executive Officer, and Jeffrey Gudin, our Executive Vice President and Chief Medical Officer, are the members of Virpax Pharmaceuticals, LLC. Virpax Pharmaceuticals, LLC currently owns approximately 87% of our outstanding common stock prior to the closing of this offering and will own approximately % of our outstanding common stock immediately after the closing of this offering. These shares were issued to Virpax Pharmaceutical, LLC as consideration for founding our company.
Independent Contractor Agreement with Chipman & Chipman
On May 1, 2020, we entered into an Independent Contractor Agreement (“Contractor Agreement”) with Chipman & Chipman, LLC (“C&C”), whereby C&C will provideprovided us with consulting and advisory services typically provided by a Chief Financial Officer of a company of our size and stage of development. The engagement term iswas for 1one year, for a fee equal to $6,000 per month. Our Chief Financial Officer, Christopher Chipman, is the managing director of C&C, and was appointed as our CFO pursuant to the Contractor Agreement. Pursuant to the Contractor Agreement, upon the closing of this offering,our IPO, we will be obligated to paypaid C&C an amount equal to $6,000 perfor each month C&C has provided services under the agreement, at the time of the closing.which was $57,000. Upon the signing of the Contractor Agreement, pursuant to the 2017 Plan we granted to C&C an option exercisable for 200,00040,450 shares of our common stock, with 50,00010,112 shares vesting upon the grant date, 100,00020,225 shares vesting upon closing of this offeringour IPO and the remaining 50,00010,113 shares vesting on the first anniversary of the closing of this offering.our IPO. On April 7, 2021, we entered into the Chipman Employment Agreement with Mr. Chipman, pursuant to which Mr. Chipman serves as our Chief Financial Officer.
Promissory Notes with Anthony MackNotes-Executive Officers
We have entered into twothree promissory notes with our Chief Executive Officer, Anthony Mack.Mack, and one promissory note with our Chief Financial Officer, Christopher Chipman. Please refer to the “Description of Certain Indebtedness” section below for further details.
Policies and Procedures for Related Person Transactions
Our Board of Directors intends to adopthas adopted a written related person transaction policy to be effective upon the closing of this offering, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover,covers, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets as of December 31, 20192020 and 20182019 and a related person had, has or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
98
PRINCIPAL STOCKHOLDERS
The following table sets forth information with respect to the beneficial ownership of our common stock, as of October 8, 2020the date of this prospectus by:
| ● | each person or group of affiliated persons known by us to beneficially own more than 5% of our common stock; |
| ● | each of our executive officers; |
| ● | each of our directors; and |
| ● | all of our executive officers and directors as a group. |
The number of shares beneficially owned by each stockholder is determined under rules issued by the SEC. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. Applicable percentage ownership is based on 15,550,6275,045,181 shares of common stock outstanding as of OctoberSeptember 8, 2020.2021. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to the exercise of options, warrants or other rights held by such person that are currently exercisable or will become exercisable within 60 days of OctoberSeptember 8, 20202021 are counted as outstanding. Unless noted otherwise, the address of all listed stockholder is 1554 Paoli Pike, #279, West Chester,1055 Westlakes Drive, Suite 300, Berwyn, PA 19380.19312. Each of the stockholder listed has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
| Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | |||||||||||
| Name of Beneficial Owner | Prior to Offering | Prior to Offering | After Offering | |||||||||
| 5% or Greater Stockholders | ||||||||||||
| Virpax Pharmaceuticals, LLC | 13,500,000 | (1)(2)(3) | 86.8 | % | % | |||||||
| Executive Officers and Directors Other Than 5% or Greater Stockholders | ||||||||||||
| Anthony Mack | 850,000 | (1)(2) | 5.4 | % | % | |||||||
| Jeffrey Gudin, MD | 112,500 | (3) | % | % | ||||||||
| Gerald Bruce | 20,000 | (4) | % | % | ||||||||
| Christopher Chipman | 50,000 | (5)(6) | % | % | ||||||||
| Eric Floyd, PhD | 163,889 | (7) | % | % | ||||||||
| Jerrold Sendrow, CFP | 134,389 | (8) | % | % | ||||||||
| Thani Jambulingam, PhD | 131,389 | (9) | % | % | ||||||||
| Gary Jacob, PhD | - | (10) | % | % | ||||||||
| Vanila Singh, MD, MACM | - | (11) | % | % | ||||||||
| Directors and Officers as a Group (9 persons) | 14,962,167 | 92.4 | % | |||||||||
| Number of Shares Beneficially | Percentage of Shares Beneficially Owned | |||||||||||
| Name of Beneficial Owner | Owned Prior to Offering | Prior to Offering | After Offering | |||||||||
| 5% or Greater Stockholders | ||||||||||||
| Virpax Pharmaceuticals, LLC | 2,730,438 | (1) | 54.1 | % | 32.3 | % | ||||||
| Executive Officers and Directors Other Than 5% or Greater Stockholders | ||||||||||||
| Anthony Mack | 212,362 | (1)(2) | 4.2 | % | 2.5 | % | ||||||
| Jeffrey Gudin, MD | 53,091 | (3) | 1.0 | % | * | |||||||
| Gerald Bruce | 49,214 | (4) | 1.0 | % | * | |||||||
| Christopher Chipman | 30,337 | (5)(6) | * | * | ||||||||
| Eric Floyd, PhD | 42,876 | (7) | * | * | ||||||||
| Jerrold Sendrow, CFP | 33,875 | (8) | * | * | ||||||||
| Thani Jambulingam, PhD | 33,370 | (9) | * | * | ||||||||
| Gary Jacob, PhD | 23,725 | (10) | * | * | ||||||||
| Vanila Singh, MD, MACM | 20,225 | (11) | * | * | ||||||||
| Michael F. Dubin, CPA | — | (12) | * | * | ||||||||
| Directors and Officers as a Group (10 persons) | 3,229,513 | 60.1 | % | 36.8 | % | |||||||
| * | Less than 1%. |
| (1) | Anthony Mack, our Chief Executive Officer, and Jeffrey Gudin, our Executive Vice President and Chief Medical Officer, are the members of Virpax Pharmaceuticals, LLC. Due to Mr. Mack’s ownership of 88.8888% of the outstanding member units of Virpax Pharmaceuticals, LLC, he may be deemed to have sole voting and dispositive control over the shares of our common stock held by Virpax Pharmaceuticals, LLC. As a result, Mr. Mack may be deemed to beneficially own the shares of our common stock held by Virpax Pharmaceuticals, LLC. |
| (2) | Includes |
| (3) | Includes |
| (4) | Includes |
| (5) | The shares of common stock reported herein are held by Chipman & Chipman LLC, a consulting firm of which Mr. Chipman is the Managing Member. As a result, Mr. Chipman has the sole voting and dispositive control over the shares of our common stock held by Chipman & Chipman LLC. As a result, Mr. Chipman may be deemed to beneficially own the shares of our common stock held by Chipman & Chipman LLC. |
| (6) | Includes |
| (7) | Includes |
| (8) | Includes |
| (9) | Includes |
| (10) |
| 2021. Does not include |
| (11) | Includes 20,225 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days of September 8, 2021. Does not include 8,183 shares of common stock issuable upon exercise of stock options that are not exercisable within 60 days of September 8, 2021. |
| (12) | Does not include 20,225 shares of common stock issuable upon exercise of stock options that are not exercisable within 60 days of September 8, 2021. |
100
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
The Company’s common stock trades on Nasdaq under the symbol “VRPX.”
As of September 8, 2021, there were approximately 49 holders of record of the Company’s common stock. This number does not include beneficial owners whose shares were held in street name. The actual number of holders of our common stock is greater than this number of record holders and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers or held by other nominees.
DESCRIPTION OF CERTAIN INDEBTEDNESS
The following is a summary of certain of our indebtedness that is currently outstanding. The following descriptions do not purport to be complete and are qualified in their entirety by reference to the agreements and related documents referred to herein, copies of which have been filed as exhibits to the registration statement of which this prospectus forms a part, and may be obtained as described under “Where You Can Find More Information” in this prospectus.
Promissory Notes
On October 1, 2018, we issued a promissory note, as amended (the “2018the 2018 Promissory Note”),Note, pursuant to which we are obligated to pay Anthony Mack the principal amount of $500,000. The 2018 Promissory Note has a maturity date of the earlier of an Event of Default (as defined below) and January 15, 2022. As of June 30, 2021, the balance on the 2018 Promissory note was $500,000, with accrued interest of $153,862. As of December 31, 2020, the balance on the 2018 Promissory Note was $500,000, with accrued but unpaid interest of $107,606.$125,887. As of December 31, 2019, the balance on the 2018 Promissory Note was $500,000, with accrued but unpaid interest of $74,692. As of December 31, 2018, the balance on the 2018 Promissory note was $500,000, with accrued interest of $14,118.
On January 15, 2019, we issued a promissory note, as amended (the “20192019 Promissory Note”),Note, pursuant to which we are obligated to pay Mr. Mack the principal amount of $500,000. The 2019 Promissory Note has a maturity date of the earlier of an Event of Default (as defined below) and January 15, 2022. As of June 30, 2021, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $137,544. As of December 31, 2020, the balance on the 2019 Promissory Note was $500,000, with accrued but unpaid interest of $88,190.$109,569. As of December 31, 2019, the balance on the 2019 Promissory Note was $500,000, with accrued but unpaid interest of $56,329.
On August 29, 2019, we agreed to pay certain compensation due to RRD International, LLC (“RRD”) in the form of a service provider convertible note purchase agreement, as amended (the “RRD Note”). The RRD Note states that a maximum principal balance of $400,000 can be applied for services provided to us by RRD, which principal amount can be converted, at RRD’s option, into equity or cash (all or in part) upon the earlier of a RRD Qualified Financing (which we anticipate this offering to be) or November 30, 2020. At December 31, 2019, the principal balance on the RRD Note was $264,520, with accrued interest of $7,741. On March 20,October 28, 2020, we amended the RRDour 2018 Promissory Note dated October 1, 2018 and 2019 Promissory Note dated January 15, 2019 with Anthony Mack, Chief Executive Officer and significant investor, to extend the maturity date to December 31, 2023 for both promissory notes. All other terms and conditions of these agreements remained unchanged.
We intend to use a portion of the net proceeds from March 31, 2020this offering to September 30, 2020, increaserepay the maximum2018 Promissory Note and 2019 Promissory Note, including any accrued but unpaid interest thereon.
In January 2021, the Company issued notes with an aggregate principal amount of $75,000 and $25,000, respectively. These notes were issued to Anthony Mack, Chief Executive Officer, for $75,000 and Christopher Chipman, Chief Financial Officer, for $25,000. These notes bore interest as a rate of 1.35% per annum and were subsequently repaid with proceeds from $400,000 to $600,000, extend the RRD Qualified Financing deadline from March 31, 2020 to September 30, 2020, and provide for the payment of all interest accrued up to March 31, 2020, which was $16,435. At June 30, 2020, the principal balance on the RRD Note was $434,260, withour IPO, including accrued interest of $10,363. In October 2020, we amended the RRD Note to extend the maturity date from September 30, 2020 to November 30, 2020, increase the principal amount from $434,260 to $493,480, extend the RRD Qualified Financing deadline from September 30, 2020 to November 30, 2020,$122 and provide for the payment of all interest from April 1, 2020 through November 30, 2020, which was $30,431.$41, respectively.
Interest Rate and Fees
Borrowings under the 2018 Promissory Note and 2019 Promissory Note bear interest at a rate of 11.19% per annum. Any overdue and unpaid principal amounts will bear interest at a rate of 15% for each day that such amounts are overdue.
Borrowings under the RRD Note bear simple interest on the outstanding principal amount of the RRD Note until paid in full at the fixed rate of 10% per annum.
Conversion
Borrowings under the RRD Note are subject to a qualified conversion feature. If on or prior to November 30, 2020 (the “Conversion Date”), an RRD Qualified Financing has not occurred, then all outstanding principal shall be automatically converted into shares of our common stock at a conversion price of $2.00. A “RRD Qualified Financing” means a transaction prior to November 30, 2020 in which we issue and sell shares of our equity securities to one or more third parties in the amount of at least $1.5 million.
Amortization and Final Maturity
Under the 2018 Promissory Note, principal and interest shall be due upon the earlier of (i) January 15, 2022 and (ii) an event of default.
Under the 2019 Promissory Note, principal and interest shall be due upon the earlier of (i) January 15, 2022 and (ii) an event of default.
Under the RRD Note, the payment of all amounts is due at the earlier of (i) the date of an RRD Qualified Financing, (ii) an event of default, and (iii) a change in control. Upon one of these events, RRD shall have the option to receive cash or convert any or all of the principal and accrued interest into shares of our common stock. RRD has indicated to us that it plans to elect to receive cash for all amounts due under the RRD Note. A portion of the net proceeds from this offering will be used to pay the total amounts due to RRD under the RRD Note, which, as of September 30, 2020, was $523,911 (which amount includes interest through November 30, 2020).
Certain Covenants and Events of Default
For the borrowings under the 2018 Promissory Note and 2019 Promissory Note, an Event of Default consists of one or more of the following:
| ● |
| ● |
| ● |
| ● |
For the borrowings under the RRD Note, an Event of Default consists of one or more of the following:
Paycheck Protection Program Loan (“PPP Loan”)Loan
On May 4, 2020, we entered into a Promissorythe PPP Note (the “PPP Note”) with PNC Bank as the lender, (the “Lender”), pursuant to which the Lender agreed to make a loan to us under the Paycheck Protection Program (the “PPP Loan”)PPP Loan offered by the U.S. Small Business Administration (the “SBA”)SBA in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”).CARES Act.
The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to our full time headcount during the period ending October 31, 2020.
The interest rate on the PPP Note is a fixed rate of 1% per annum. To the extent that the amounts owed under the PPP Loan, or a portion of them, are not forgiven, we will be required to make principal and interest payments in monthly installments beginning seven months from April 2020. The PPP Note matures in two years.on May 4, 2022.
The PPP Note includes customary events of default including, among others, (i) the nonpayment of any principal, interest or other indebtedness under the PPP Note when due; (ii) the occurrence of any event of default or any default and the lapse of any notice or cure period, or our failure to observe or perform any covenant or other agreement, under or contained in any loan document; (iii) the filing by or against us of any proceeding in bankruptcy, receivership, insolvency, reorganization, liquidation, conservatorship or similar proceeding; and (iv) the breach of any representation or warranty on our part. Upon the occurrence of an event of default, the Lender will have the right to exercise remedies against us, including the right to require immediate payment of all amounts due under the PPP Note.
On July 2, 2021, the SBA notified the Company that the forgiveness amount totaled $61,816. The remaining balance of $10,284 must be repaid by the Company on or before maturity.
102
DESCRIPTION OF SECURITIES
The following description summarizes some of the terms of our Amended and Restated Certificate of Incorporation and amendedAmended and restated bylaws that will become effective upon the closing of this offering.Restated Bylaws. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description, you should refer to our restated Certificate of Incorporation and amendedAmended and restated bylaws,Restated Bylaws, copies of which have been or will be filed as exhibits to the registration statement of which this prospectus is a part. The description of our common stock reflects changes to our capital structure that will occur immediately prior to the closing of this offering.
Following the closing of this offering, our authorized capital stock will consist of million shares of common stock, par value $0.00001 per share.
Authorized Capitalization
Upon completion of this offering, we willWe have 110,000,000 shares of capital stock authorized under our Amended and Restated Certificate of Incorporation, consisting of 100,000,000 shares of common stock with a par value of $0.00001 per share and no10,000,000 shares of preferred stock with a par value of $0.00001 per share. As of October 7, 2020,September 8, 2021, we had 15,550,6275,045,181 shares of common stock outstanding and no shares of preferred stock outstanding. In addition, as of October 7, 2020,September 8, 2021, we had outstanding options to purchase an aggregate of 2,403,503669,067 shares of our common stock under the 2017 Plan, at a weighted average exercise price of $2.00$7.75 per share. Our authorized but unissued shares of common stock and preferred stock are available for issuance without further action by our stockholder, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded in the future.
Common Stock
Holders of our common stock are entitled to such dividends as may be declared by our boardBoard of directorsDirectors out of funds legally available for such purpose. The shares of common stock are neither redeemable nor convertible. Holders of common stock have no preemptive or subscription rights to purchase any of our securities.
Each holder of our common stock is entitled to one vote for each such share outstanding in the holder’s name. No holder of common stock is entitled to cumulate votes in voting for directors.
In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to receive pro rata our assets, which are legally available for distribution, after payments of all debts and other liabilities. All of the outstanding shares of our common stock are fully paid and non-assessable. The shares of common stock offered by this prospectus will also be fully paid and non-assessable.
Preferred Stock
Upon completion of this offering, ourOur Board of Directors will havehas the authority, without further action by our stockholder,stockholders, to issue up to 10,000,000 shares of preferred stock in one or more classes or series and to fix the designations, rights, preferences, privileges and restrictions thereof, without further vote or action by the stockholder. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such class or series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our company or other corporate action. Immediately after completion of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Stock Options
As of October 7, 2020,September 8, 2021, we had reserved the following shares of common stock for issuance pursuant to stock options under the 2017 Plan described below:
| ● |
| ● |
Underwriter Warrants
We have agreed to sell toIn conjunction with our initial public offering, we granted the underwriters for nominal consideration, warrants to purchase 90,000 shares of our common stock as additional consideration to the underwriters in this offering. The underwriter warrants will haveat an exercise price equalof $12.50 per share, of which warrants to 125%purchase a total of the public offering price in this offering13,380 shares of our common stock remain outstanding. The warrants have a five-year term and shall bebecame exercisable for a period of four years and six months and provide for cashless exercise. See “Underwriting.”on August 16, 2021.
Anti-Takeover Effects of Delaware law and Our Certificate of Incorporation and Bylaws
The provisions of Delaware law, our Amended and Restated Certificate of Incorporation and our Amended and Restated Bylaws, to be adopted upon the closing of this offering, described below may have the effect of delaying, deferring or discouraging another party from acquiring control of us.
Section 203 of the Delaware General Corporation Law
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| ● | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| ● | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholder, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines business combination to include the following:
| ● | any merger or consolidation involving the corporation and the interested stockholder; |
| ● | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| ● | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| ● | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| ● | the receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Certificate of Incorporation and Bylaws
Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws to be adopted upon the closing of the offering providesprovide for:
| ● | authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| ● | limiting the removal of directors by the stockholders; |
| ● | requiring a supermajority vote of stockholders to amend our bylaws or certain provisions our certificate of incorporation; |
| ● | prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
| ● | eliminating the ability of stockholders to call a special meeting of stockholders; |
| ● | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings; |
| ● | establishing Delaware as the exclusive jurisdiction for certain stockholder litigation against us; and |
| ● | a classified board of directors. |
Potential Effects of Authorized but Unissued Stock
We have shares of common stock and preferred stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital stock.
The existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board of directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent permissible under the Delaware General Corporation Law and subject to any limitations set forth in our certificate of incorporation. The purpose of authorizing the board of directors to issue preferred stock and to determine the rights and preferences applicable to such preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the effect of making it more difficult for a third-party to acquire, or could discourage a third-party from acquiring, a majority of our outstanding voting stock.
Choice of Forum
Unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any stockholder to bring (i) any derivative action or proceeding brought on behalf of the Company, (ii) any action asserting a claim of breach of fiduciary duty owed by any director, officer or other employee of the Company or the Company’s stockholders, creditors or constituents, (iii) any action asserting a claim against the Company or any director or officer of the Company arising pursuant to, or a claim against the Company or any director or officer of the Company, with respect to the interpretation or application of any provision of, the DGCL, our certificate of incorporation or bylaws, or (iv) any action asserting a claim governed by the internal affairs doctrine, except for, in each of the aforementioned actions, any claims to which the Court of Chancery of the State of Delaware determines it lacks jurisdiction. This provision will not apply to claims arising under the Exchange Act, the Securities Act or for any other federal securities laws which provide for exclusive federal jurisdiction. However, the exclusive forum provision provides that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Therefore, this provision could apply to a suit that falls within one or more of the categories enumerated in the exclusive forum provision and that asserts claims under the Securities Act, inasmuch as Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. There is uncertainty as to whether a court would enforce such an exclusive forum provision with respect to claims under the Securities Act.
We note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging lawsuits against our directors and officers.
Transfer Agent and Registrar
The transfer agent and registrar for our shares of common sharesstock is V-Stock Transfer LLC.
National Securities Exchange Listing
We intend to apply to list ourOur shares of common sharesstock are listed on the Nasdaq Capital Market under the symbol “VRPX,“VRPX.” which listing we expect to occur upon consummation of this offering.
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was no public market for our common shares, and we cannot predict what effect, if any, market sales of our common shares or the availability of our common shares for sale will have on the market price of our common shares prevailing from time to time. Nevertheless, future sales of substantial amounts of our common shares in the public market, or the perception that such sales could occur, could materially and adversely affect the market price of our common shares and could impair our future ability to raise capital through the sale of our equity or equity-related securities at a time and price that we deem appropriate.
Upon the consummation of this offering, we will have outstanding an aggregate of approximately common shares. Of the outstanding shares, the shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, may be sold only in compliance with the limitations described below.
The remaining outstanding common shares will be deemed restricted securities, as defined under Rule 144. Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which we summarize below. Approximately of these shares will be subject to lock-up agreements described below.
Taking into account the lock-up agreements described below, and assuming we do not release stockholders from these agreements, the following shares will be eligible for sale in the public market at the following times, subject to the provisions of Rule 144 and Rule 701:
Lock-Up Agreements
Pursuant to certain “lock-up” agreements, we and our executive officers and directors and our stockholders, have agreed not to, without the prior written consent of the representative of the underwriters, offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, for a period of nine months, in the case of our officers and directors, and six months90 days from the date of this prospectus, in the case of us and all other stockholders. See “Underwriting — Lock-Up Agreements” for additional information.
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, a person who is not our affiliate and has not been our affiliate at any time during the preceding three months will be entitled to sell any of our common shares that such person has beneficially owned for at least six months, including the holding period of any prior owner other than one of our affiliates, without regard to volume limitations. Sales of our common shares by any such person would be subject to the availability of current public information about us if the shares to be sold were beneficially owned by such person for less than one year.
Approximately shares of our common stock that are not subject to the lock-up agreements described below will be eligible for sale under Rule 144 immediately upon the consummation of this offering.
Beginning 90 days after the date of this prospectus, our affiliates who have beneficially owned our common shares for at least six months, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
prospectus.
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, any of an issuer’s employees, directors, officers, consultants or advisors who purchases shares from an issuer in connection with a compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Act is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The Securities and Exchange Commission has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Registration Statements on Form S-8
Immediately after the completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act to register all of our common shares issued or reserved for future issuance under our equity incentive plans. This registration statement would cover approximately shares. Shares registered under the registration statement will generally be available for sale in the open market after the 180-day lock-up period immediately following the date of this prospectus.
Registration Rights
Beginning six months after the date of this prospectus, subject to certain exceptions, holders of shares of our common shares will be entitled to the registration rights described under “Certain Relationships and Related Party Transactions — Stockholders’ Agreement.” Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon effectiveness of the registration.
107
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the purchase, ownership and disposition of the shares of common stock issued pursuant to this offering but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or foreign tax laws are not discussed. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U IRS, in effect as of the date of this offering. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a non-U.S. holder of our common stock. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position regarding the tax consequences of the purchase, ownership and disposition of our common stock.
This discussion is limited to non-U.S. holders that hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a non-U.S. holder’s particular circumstances, including the impact of the alternative minimum tax or the unearned income Medicare contribution tax. In addition, it does not address consequences relevant to holders subject to particular rules, including, without limitation:
| ● | U.S. expatriates and certain former citizens or long-term residents of the United States; |
| ● | persons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| ● | banks, insurance companies, and other financial institutions; |
| ● | regulated investment companies or real estate investment trusts; |
| ● | brokers, dealers or traders in securities or currencies; |
| ● | controlled foreign corporations, “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| ● | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| ● | tax-exempt organizations or governmental organizations; |
| ● | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| ● | persons for whom our common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or as “Section 1244 stock” for purposes of Section 1244 of the Code; |
| ● | persons subject to special tax accounting rules as a result of any item of gross income with respect to our common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
| ● | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; |
| ● | tax-qualified retirement plans; and |
If a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) holds our common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT INTENDED AS LEGAL OR TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
108
Definition of a Non-U.S. Holder
For purposes of this discussion, a “non-U.S. holder” is any beneficial owner of our common stock that is not a “U.S. person,” a partnership or an entity disregarded as separate from its owner, each for United States federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is:
| ● | an individual who is a citizen or resident of the United States; |
| ● | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| ● | a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more United States persons (within the meaning of Section 7701(a)(30) of the Code), or (2) has made a valid election under applicable Treasury Regulations to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our common stock in the foreseeable future. However, if we do make distributions on our common stock, such distributions of cash or property on our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a non-U.S. holder’s adjusted tax basis in its common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below in the section relating to the sale or disposition of our common stock. Because we may not know the extent to which a distribution is a dividend for U.S. federal income tax purposes at the time it is made, for purposes of the withholding rules discussed below we or the applicable withholding agent may treat the entire distribution as a dividend.
Subject to the discussion below on backup withholding and foreign accounts, dividends paid to a non-U.S. holder of our common stock that are not effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty).
Non-U.S. holders will be entitled to a reduction in or an exemption from withholding on dividends as a result of either (a) an applicable income tax treaty or (b) the non-U.S. holder holding our common stock in connection with the conduct of a trade or business within the United States and dividends being effectively connected with that trade or business. To claim such a reduction in or exemption from withholding, the non-U.S. holder must provide the applicable withholding agent with a properly executed (a) IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) claiming an exemption from or reduction of the withholding tax under the benefit of an income tax treaty between the United States and the country in which the non-U.S. holder resides or is established, or (b) IRS Form W-8ECI stating that the dividends are not subject to withholding tax because they are effectively connected with the conduct by the non-U.S. holder of a trade or business within the United States, as may be applicable. These certifications must be provided to the applicable withholding agent prior to the payment of dividends and must be updated periodically. Non-U.S. holders that do not timely provide the applicable withholding agent with the required certification, but that qualify for a reduced rate under an applicable income tax treaty, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
If dividends paid to a non-U.S. holder are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such dividends are attributable), then, although exempt from U.S. federal withholding tax (provided the non-U.S. holder provides appropriate certification, as described above), the non-U.S. holder will be subject to U.S. federal income tax on such dividends on a net income basis at the regular graduated rates. In addition, a non-U.S. holder that is a corporation may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits for the taxable year that are attributable to such dividends, as adjusted for certain items. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty and regarding any applicable treaties that may provide for different rules.
109
Sale or Other Taxable Disposition of Common Stock
Subject to the discussions below on backup withholding and foreign accounts, a non-U.S. holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our common stock unless:
| ● | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment fixed base in the United States to which such gain is attributable); |
| ● | the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| ● | our common stock constitutes U.S. real property interests, or USRPIs, by reason of our status as a U.S. real property holding corporation, or USRPHC, for U.S. federal income tax purposes. |
Gain described in the first bullet point above will generally be subject to U.S. federal income tax on a net income basis at the regular rates. A non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
A non-U.S. holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder (even though the individual non-U.S. holder is not considered a resident of the United States) provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we are not currently and do not anticipate becoming a USRPHC. Because the determination of whether we are a USRPHC depends on the fair market value of our USRPIs relative to the fair market value of our other business assets and our non-U.S. real property interests and our other business assets, however, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a non-U.S. holder of our common stock will not be subject to U.S. federal income tax if our common stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such non-U.S. holder owned, actually and constructively, 5% or less of our common stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition or the non-U.S. holder’s holding period. If we are a USRPHC and either our common stock is not regularly traded on an established securities market or a non-U.S. holder holds, or is treated as holding, more than 5% of our outstanding common stock, directly or indirectly, during the applicable testing period, such non-U.S. holder’s gain on the disposition of shares of our common stock generally will be taxed in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax generally will not apply. If we are a USRPHC and our common stock is not regularly traded on an established securities market, a non-U.S. holder’s proceeds received on the disposition of shares will also generally be subject to withholding at a rate of 15%. Prospective investors are encouraged to consult their tax advisors regarding the possible consequences to them if we are, or were to become, a USRPHC.
Non-U.S. holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Subject to the discussion below on foreign accounts, a non-U.S. holder will not be subject to backup withholding (currently at a rate of 24%) with respect to distributions on our common stock we make to the non-U.S. holder, provided the applicable withholding agent does not have actual knowledge or reason to know such holder is a United States person and the holder certifies its non-U.S. status, such as by providing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns generally will be filed with the IRS in connection with any distributions (including deemed distributions) made on our common stock to the non-U.S. holder, regardless of whether any tax was actually withheld.
In addition, proceeds of a sale or other taxable disposition of our common stock within the United States or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting, if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that the beneficial owner is a U.S. person, or such holder otherwise establishes an exemption. Proceeds of a disposition of our common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under Sections 1471 through 1474 of the Code (such Sections are commonly referred to as the Foreign Account Tax Compliance Act (“FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends (including deemed dividends) paid on our common stock, or, subject to the proposed Treasury Regulations discussed below, gross proceeds from the sale or other disposition of our common stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends (including deemed dividends) paid on our common stock. While withholding under FATCA would also have applied to payments of gross proceeds from the sale or other disposition of stock on or after January 1, 2019, proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued. Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock.
UNDERWRITING
ThinkEquity a division of Fordham Financial Management, Inc., is acting as representative of the underwriters ofsole book-running manager for this offering. We have entered into an underwriting agreement dated , 2020,2021, with the representative.ThinkEquity. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below,ThinkEquity, and each underwriter named belowThinkEquity has severally agreed to purchase from us, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common sharesstock listed next to its name in the following table:
| Underwriters | Number of Shares | ||||
| ThinkEquity LLC | |||||
The underwriting agreement provides that the underwriters areunderwriter is committed to purchase all shares offered by us other than those covered by the over-allotment option described below, if any are purchased. The obligations of the underwritersunderwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’underwriter’s obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwritersunderwriter of officers’ certificates and legal opinions.
The underwriters areunderwriter is offering the shares subject to prior sale, when, as and if issued to and accepted by them,it, subject to approval of legal matters by theirits counsel, and other conditions. The underwriters reserveunderwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The underwriters proposeunderwriter proposes to offer the shares offered by us to the public at the public offering price set forth on the cover of the prospectus. After the shares are released for sale to the public, the underwritersunderwriter may change the offering price and other selling terms at various times
Over-Allotment Option
We have granted the underwritersunderwriter an over-allotment option. This option, which is exercisable for up to 3045 days after the date of this prospectus, permits the representative to purchase a maximum of 510,204 additional shares of common stock (15% of the shares sold in this offering) from us to cover over-allotments, if any. If the representativeunderwriter exercises all or part of this option, it will purchase shares covered by the option at the public offering price per share that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total offering price to the public will be approximately $ million and the total net proceeds, before expenses, to us will be approximately $ .million.
Discount
The following table shows the public offering price, underwriting discounts, and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | ||||||||||||
| Total Without Over-Allotment Option | Total with Over-Allotment Option | |||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting | $ | $ | $ | |||||||||
| Proceeds, before expense, to us | $ | $ | $ | |||||||||
| (1) | We have agreed to pay ThinkEquity an underwriting discount or spread of |
We have agreed to pay a non-accountable expense allowance to the underwriters equal to 1% of the gross proceeds received in this offering (excluding proceeds received from exercise of the underwriters’ over-allotment option).
We have paid a $20,000 advance to ThinkEquity upon execution of our engagement letter with ThinkEquity, and will pay an additional $15,000 upon filing of this Registration Statement, which shall be applied against actual out-of-pocket-accountable expenses, which will be returned to us to the extent such out-of-pocket accountable expenses are not actually incurred in accordance with FINRA Rule 5110(f)(2)(C).
In addition, we have agreed to pay the following expenses of the underwriters relating to the offering: (a) all fees, expenses and disbursements relating to background checks of our officers, directors and entities in an amount not to exceed $1,000 per individual or entity or $15,000$1,500 in the aggregate; and (b) the fees and expenses of the Underwriter’s legal counsel not to exceed $90,000; (c) the $29,500 cost associated with the use of Ipreo’s book building, prospectus tracking and compliance software for the Offering and (d) up to $20,000 of ThinkEquity’s actual accountable “road show” expenses for the offering.
$75,000.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, and non-accountable expense allowance, will be approximately $ .$276,500.
Representative’s Warrants
We have agreed to issue to the representative warrants to purchase up to 5% of the aggregate number of shares of common stock sold in this offering, excluding shares of common stock sold upon exercise of the underwriters’ over-allotment option (the “Representative’s Warrants”), for an aggregate purchase price of $ . The Representative’s Warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share of the shares of common stock sold in this offering. The Representative’s Warrants are exercisable at any time, from time to time, in whole or in part, during the four and one half year period commencing six months from the effective date of the registration statement related to this offering.
The Representative’s Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to FINRA Rule 5110(g)(1). Except as permitted by Rule 5110(g)(2), the underwriters (or permitted assignees under the Rule) will not sell, transfer, assign, pledge, or hypothecate the warrants or the securities underlying the warrants, nor will any of them engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the option or the underlying securities for a period of 180 days from the date of effectiveness of the registration statement of which this prospectus forms a part or the commencement of sales under this prospectus. We will bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants, other than underwriting commissions incurred and payable by the holders.
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreement
Pursuant to certain “lock-up” agreements, we, and our executive officers and directors and our stockholders, have agreed not to, without the prior written consent of the representative, offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, for a period of nine months, in the case of our officers and directors, and six months90 days from the date of this prospectus, in the case of us and all other stockholders.prospectus.
Right of First RefusalIndemnification
Subject to certain limited exceptions, until eighteen months after the closing of this offering, ThinkEquity has a right of first refusal to act as sole investment banker, sole book-runner and/or sole placement agent, at ThinkEquity’s sole discretion, for each and every future public and private equity and debt offering, including all equity-linked offerings, by us or any of our successors during such eighteen-month period on terms customary to the representative.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
113
Electronic Offer, Sale and Distribution of Shares
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum and are engaged in for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress.
Over-allotment transactions involve sales by the underwriters of securities in excess of the number of securities that underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriters is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing securities in the open market.
Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared with the price at which they may purchase securities through exercise of the over-allotment option. If the underwriters sell more securities than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the securities in the open market that could adversely affect investors who purchase in the offering.
Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the securities originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As a result, the price of our securities in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our securities. These transactions may be effected on The Nasdaq Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive Market Making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common stock on The Nasdaq Capital Market or on the OTCQB in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the securities and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
114
Other Relationships
Certain of the underwriters and their affiliates may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they may receive customary fees and commissions. However, we have not yet had, and have no present arrangements with any of the underwriters for any further services.
Listing
We intend to apply to have our common stock approved for listing on The Nasdaq Capital Market under the trading symbol “VRPX”.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Area — Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| ● | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); |
| ● | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2°and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2°and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo(Comissao do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
117
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company. In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
LEGAL MATTERS
Lowenstein Sandler LLP, New York, New York, will pass upon the validity of the shares of common stock offered by this prospectus and certain other legal matters. Sheppard, Mullin, Richter & Hampton LLP, New York, New York, is acting as legal counsel to the underwriters.
EXPERTS
The balance sheets of Virpax Pharmaceuticals, Inc. as of December 31, 20192020 and 20182019 and the related statements of operations, stockholders’ deficit, and cash flows for each of the years then ended have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report which is incorporated herein. Such financial statements have been incorporated herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
No expert named in the registration statement of which this prospectus forms a part as having prepared or certified any part thereof (or named as having prepared or certified a report or valuation for use in connection with such registration statement) or counsel named in this prospectus as having given an opinion upon the validity of the securities being offered pursuant to this prospectus or upon other legal matters in connection with the registration or offering of such securities was employed for such purpose on a contingency basis. At the time of such preparation, certification or opinion or at any time thereafter, through the date of effectiveness of such registration statement or that part of such registration statement to which such preparation, certification or opinion relates, no such person had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in our Company. Nor was any such person connected with our Company as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
120
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed thereto. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. Upon completion of this offering, we will be required to file periodic reports, proxy statements, and other information with the Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934. Securities and Exchange Commission also maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the Securities and Exchange Commission. The address of that site is www.sec.gov.
INDEX TO FINANCIAL STATEMENTS
VIRPAX PHARMACEUTICALS, INC
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Virpax Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Virpax Pharmaceuticals, Inc. (the “Company”) as of December 31, 20192020 and 2018,2019, and the related statements of operations, stockholders’ deficit, and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20192020 and 2018,2019, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has not generated revenues and has not yet achieved profitable operations nor generated positive cash flows from operations that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ EisnerAmper LLP
We have served as the Company’s auditor since 2020.
EISNERAMPER LLP
Philadelphia, Pennsylvania
August 10, 2020
F-2March 31, 2021
VIRPAX Pharmaceuticals, Inc.
BALANCE SHEETS
| December 31, 2020 | December 31, 2019 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 54,796 | $ | 41,536 | ||||
| Prepaid expenses and other current assets | 18,273 | 5,183 | ||||||
| Total current assets | 73,069 | 46,719 | ||||||
| Deferred financing costs | 392,337 | — | ||||||
| Total assets | $ | 465,406 | $ | 46,719 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Accounts payable and accrued expenses | $ | 3,115,924 | $ | 1,514,474 | ||||
| Notes payable | 543,990 | 264,520 | ||||||
| Related party notes payable | — | 1,000,000 | ||||||
| Total current liabilities | 3,659,914 | 2,778,994 | ||||||
| Notes payable, net of current portion | 21,590 | — | ||||||
| Related party notes payable, net of current portion | 1,000,000 | — | ||||||
| Total long-term liabilities | 1,021,590 | — | ||||||
| Total liabilities | 4,681,504 | 2,778,994 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred stock, par value $0.00001, 1,000,000 shares authorized, 0 shares issued and outstanding | — | — | ||||||
| Common stock, $0.00001 par value; 20,000,000 shares authorized, 3,145,153 shares issued and outstanding as of December 31, 2020; 3,018,673 shares issued and outstanding as of December 31, 2019 | 31 | 30 | ||||||
| Additional paid-in capital | 6,431,715 | 3,575,886 | ||||||
| Accumulated deficit | (10,647,844 | ) | (6,308,191 | ) | ||||
| Total stockholders’ deficit | (4,216,098 | ) | (2,732,275 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 465,406 | $ | 46,719 | ||||
BALANCE SHEETS
| December 31, 2019 | December 31, 2018 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 41,536 | $ | 48,370 | ||||
| Prepaid expenses and other current assets | 5,183 | 13,130 | ||||||
| Total current assets | 46,719 | 61,500 | ||||||
| Total assets | $ | 46,719 | $ | 61,500 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Accounts payable and accrued expenses | $ | 1,514,474 | $ | 754,224 | ||||
| Convertible notes payable | 264,520 | - | ||||||
| Related party notes payable | 1,000,000 | 500,000 | ||||||
| Total current liabilities | 2,778,994 | 1,254,224 | ||||||
| Total liabilities | 2,778,994 | 1,254,224 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred stock, par value $0.00001, 1,000,000 shares authorized, no shares issued and outstanding | - | - | ||||||
| Common stock, $0.00001 par value; 20,000,000 shares authorized, 14,925,158 shares issued and outstanding as of December 31, 2019; 14,212,000 shares issued and outstanding as of December 31, 2018 | 149 | 142 | ||||||
| Additional paid-in capital | 3,575,767 | 1,808,813 | ||||||
| Accumulated deficit | (6,308,191 | ) | (3,001,679 | ) | ||||
| Total stockholders’ deficit | (2,732,275 | ) | (1,192,724 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 46,719 | $ | 61,500 | ||||
See Notes to the Financial Statements
VIRPAX Pharmaceuticals, Inc.
STATEMENTS OF OPERATIONS
| For the Year Ended December 31, 2020 | For the Year Ended December 31, 2019 | |||||||
| OPERATING EXPENSES | ||||||||
| General and administrative | $ | 2,904,104 | $ | 2,559,127 | ||||
| Research and development | 1,291,615 | 622,741 | ||||||
| Total operating expenses | 4,195,719 | 3,181,868 | ||||||
| Loss from operations | (4,195,719 | ) | (3,181,868 | ) | ||||
| OTHER (EXPENSE) INCOME | ||||||||
| Interest expense | (147,934 | ) | (124,644 | ) | ||||
| Other income | 4,000 | — | ||||||
| (143,934 | ) | (124,644 | ) | |||||
| Loss before tax provision | (4,339,653 | ) | (3,306,512 | ) | ||||
| Benefit from income taxes | — | — | ||||||
| Net loss | $ | (4,339,653 | ) | $ | (3,306,512 | ) | ||
| Basic and diluted net loss per share | $ | (1.40 | ) | $ | (1.13 | ) | ||
| Basic and diluted weighted average common stock outstanding | 3,107,502 | 2,929,005 | ||||||
| For the Year Ended December 31, 2019 | For the Year Ended December 31, 2018 | |||||||
| OPERATING EXPENSES | ||||||||
| General and administrative | $ | 2,559,127 | $ | 1,483,786 | ||||
| Research and development | 622,741 | 1,142,176 | ||||||
| Total operating expenses | 3,181,868 | 2,625,962 | ||||||
| Loss from operations | (3,181,868 | ) | (2,625,962 | ) | ||||
| OTHER EXPENSE | ||||||||
| Interest expense | (124,644 | ) | (14,976 | ) | ||||
| Other expense | (124,644 | ) | (14,976 | ) | ||||
| Loss before tax provision | (3,306,512 | ) | (2,640,938 | ) | ||||
| Benefit from income taxes | - | - | ||||||
| Net loss | $ | (3,306,512 | ) | $ | (2,640,938 | ) | ||
| Basic and diluted net loss per share | $ | (0.23 | ) | $ | (0.19 | ) | ||
| Basic and diluted weighted average common stock outstanding | 14,481,487 | 14,035,316 | ||||||
See Notes to the Financial Statements
F-4
VIRPAX Pharmaceuticals, Inc.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
| Preferred stock | Common stock | Additional paid-in | Accumulated | Total stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | deficit | ||||||||||||||||||||||
| Balance at January 1, 2019 | — | $ | — | 2,874,437 | $ | 29 | $ | 1,808,926 | $ | (3,001,679 | ) | $ | (1,192,724 | ) | ||||||||||||||
| Common stock issued pursuant to subscription agreements | — | — | 63,203 | 1 | 624,999 | — | 625,000 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | — | — | 46,903 | — | 463,815 | — | 463,815 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 678,146 | — | 678,146 | |||||||||||||||||||||
| Restricted stock awards granted | — | — | 34,130 | — | — | — | — | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (3,306,512 | ) | (3,306,512 | ) | |||||||||||||||||||
| Balance at December 31, 2019 | — | — | 3,018,673 | 30 | 3,575,886 | (6,308,191 | ) | (2,732,275 | ) | |||||||||||||||||||
| Common stock issued pursuant to subscription agreements | — | — | 139,220 | 1 | 1,376,899 | — | 1,376,900 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | — | — | 533 | — | 5,288 | — | 5,288 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 1,473,642 | — | 1,473,642 | |||||||||||||||||||||
| Restricted stock awards granted | — | — | 6,952 | — | — | — | — | |||||||||||||||||||||
| Restricted stock awards forfeited | — | — | (20,225 | ) | — | — | — | — | ||||||||||||||||||||
| Net loss | — | — | — | — | — | (4,339,653 | ) | (4,339,653 | ) | |||||||||||||||||||
| Balance at December 31, 2020 | — | $ | — | 3,145,153 | $ | 31 | $ | 6,431,715 | $ | (10,647,844 | ) | $ | (4,216,098 | ) | ||||||||||||||
| Preferred stock | Common stock | Additional paid-in | Accumulated | Total stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | deficit | ||||||||||||||||||||||
| Balance at January 1, 2018 | - | $ | - | 13,577,000 | $ | 136 | $ | 153,864 | $ | (360,741 | ) | $ | (206,741 | ) | ||||||||||||||
| Common stock issued pursuant to subscription agreements | - | - | 560,000 | 5 | 1,119,995 | - | 1,120,000 | |||||||||||||||||||||
| Issuance of common stock upon conversion of convertible note | - | - | 75,000 | 1 | 149,999 | - | 150,000 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 384,955 | - | 384,955 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (2,640,938 | ) | (2,640,938 | ) | |||||||||||||||||||
| Balance at December 31, 2018 | - | $ | - | 14,212,000 | $ | 142 | $ | 1,808,813 | $ | (3,001,679 | ) | $ | (1,192,724 | ) | ||||||||||||||
| Common stock issued pursuant to subscription agreements | - | - | 312,500 | 3 | 624,997 | - | 625,000 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | - | - | 231,908 | 2 | 463,813 | - | 463,815 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 678,146 | - | 678,146 | |||||||||||||||||||||
| Restricted stock awards granted | - | - | 168,750 | 2 | (2 | ) | - | - | ||||||||||||||||||||
| Net loss | - | - | - | - | - | (3,306,512 | ) | (3,306,512 | ) | |||||||||||||||||||
| Balance at December 31, 2019 | - | $ | - | 14,925,158 | $ | 149 | $ | 3,575,767 | $ | (6,308,191 | ) | $ | (2,732,275 | ) | ||||||||||||||
See Notes to the Financial Statements
F-5
VIRPAX Pharmaceuticals, Inc.
STATEMENTS OF CASH FLOWS
| For the Year Ended December 31, 2020 | For the Year Ended December 31, 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (4,339,653 | ) | $ | (3,306,512 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Non-cash interest expense | 147,934 | 124,644 | ||||||
| Stock-based compensation | 1,473,642 | 678,146 | ||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | 5,288 | 439,999 | ||||||
| Change in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (13,090 | ) | 7,947 | |||||
| Accounts payable and accrued expenses | 1,341,019 | 923,942 | ||||||
| Net cash used in operating activities | (1,384,860 | ) | (1,131,834 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of debt | 72,100 | 500,000 | ||||||
| Deferred financing costs | (50,880 | ) | - | |||||
| Proceeds from the issuance of stock | 1,376,900 | 625,000 | ||||||
| Net cash provided by financing activities | 1,398,120 | 1,125,000 | ||||||
| Net change in cash | 13,260 | (6,834 | ) | |||||
| Cash, beginning of year | 41,536 | 48,370 | ||||||
| Cash, end of year | $ | 54,796 | $ | 41,536 | ||||
| Supplemental disclosure of cash and non-cash financing activities | ||||||||
| Cash paid for interest | $ | — | $ | — | ||||
| Cash paid for taxes | $ | — | $ | — | ||||
| Debt issued in payment of consulting services and settlement of accounts payable | $ | 228,960 | $ | 264,520 | ||||
| Common stock issued in payment of consulting services and settlement of accounts payable | $ | — | $ | 23,816 | ||||
| Deferred financing costs, included in accounts payable and accrued expenses | $ | 341,457 | $ | — | ||||
For the Year Ended December 31, | For the Year Ended December 31, | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (3,306,512 | ) | $ | (2,640,938 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Non-cash interest expense | 124,644 | 14,976 | ||||||
| Stock-based compensation | 678,146 | 384,955 | ||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | 439,999 | - | ||||||
| Change in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | 7,947 | (13,130 | ) | |||||
| Accounts payable and accrued expenses | 923,942 | 454,501 | ||||||
| Net cash used in operating activities | (1,131,834 | ) | (1,799,636 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of debt | 500,000 | 554,766 | ||||||
| Proceeds from the issuance of stock | 625,000 | 1,120,000 | ||||||
| Net cash provided by financing activities | 1,125,000 | 1,674,766 | ||||||
| Net change in cash | (6,834 | ) | (124,870 | ) | ||||
| Cash, beginning of year | 48,370 | 173,240 | ||||||
| Cash, end of year | $ | 41,536 | $ | 48,370 | ||||
| Supplemental disclosure of cash and non-cash financing activities | ||||||||
| Cash paid for interest | $ | - | $ | - | ||||
| Cash paid for taxes | $ | - | $ | - | ||||
| Issuance of common stock upon conversion of convertible note | $ | - | $ | 150,000 | ||||
| Debt issued in payment of consulting services and settlement of accounts payable | $ | 264,520 | $ | - | ||||
| Common stock issued in payment of consulting services and settlement of accounts payable | $ | 23,816 | $ | - | ||||
See Notes to the Financial Statements
VIRPAX Pharmaceuticals, Inc.
NOTES TO FINANCIAL STATEMENTS
Note 1. Business and Liquidity
Business
Business
Virpax Pharmaceuticals, Inc. (“Virpax” or “Company”) was incorporated on May 12, 2017 in the state of Delaware. Virpax is a company specializing in developing pharmaceutical products for pain management by using new drug delivery systems. Virpax has exclusive global rights to a proprietary patented Topical Spray Film Delivery Technology for acute musculoskeletal pain (“Epoladerm”). Virpax also has exclusive global rights to a proprietary patented injectable “local anesthetic” Liposomal Gel Technology for postoperative pain management (“Probudur”). Additionally, Virpax has exclusive global rights to a proprietary patented Nanomerics’ Molecular Envelope Technology (“MET”) that uses an intranasal device to deliver enkephalin for the management of acute and chronic pain, including pain associated with cancer (“NES100”). NES100 would support the current effort among prescribers, regulators, and patients to seek non-opioid and non-addictive treatment options to combat the opioid epidemic. Virpax will utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and new chemical entity (“NCE”) candidates for commercialization.
Liquidity and Going Concern
The Company, since inception, has been engaged in organizational activities, including raising capital and research and development activities. The Company has not generated revenues and has not yet achieved profitable operations, nor has it ever generated positive cash flow from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. The Company is subject to those risks associated with any preclinical stage pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company’s research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants. Further, the Company’s future operations are dependent on the success of the Company’s efforts to raise additional capital.
These uncertainties raise substantial doubt about the Company’s ability to continue as a going concern for 12 months after the issuance date of these financial statements. The accompanying financial statements have been prepared on a going concern basis which contemplates the continuation of operations, realization of assets and liquidation of liabilities in the ordinary course of business. The Company incurred a net loss of $3,306,512$4,339,653 and $2,640,938$3,306,512 for the years ended December 31, 20192020 and 2018,2019, respectively, and had an accumulated deficit of $6,308,191$10,647,844 as of December 31, 2019.2020. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenue from its product candidates currently in development. The Company’s primary source of capital has been the issuance of debt and equity securities. The Company closed its initial public offering in February 2021 raising net proceeds of approximately $16,000,000 from the issuance of 1,800,000 shares of its common stock.
In addition, with respect to the ongoing and evolving coronavirus (“COVID-19”) outbreak, which was designated as a pandemic by the World Health Organization on March 11, 2020, the outbreak has caused substantial disruption in international and U.S. economies and markets and if repercussions of the outbreak are prolonged, could have a significant adverse impact on the Company’s business.
Management believes that current cash is sufficient to fund operations and capital requirements through October 2020.approximately November 2022. Additional financings will be needed by the Company to fund its operations, to complete clinical development of and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation — The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and reflect the operations of the Company. Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Use of Estimates — The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, including disclosure of contingent assets and liabilities, at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Due to the uncertainty of factors surrounding the estimates or judgments used in the preparation of the financial statements, actual results may materially vary from these estimates.
Significant items subject to such estimates and assumptions include research and development accruals and the valuation of stock-based compensation. Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
Basic and Diluted Loss per Share — Basic net loss per share is determined using the weighted average number of shares of common stock outstanding during each period. Diluted net income per share includes the effect, if any, from the potential exercise or conversion of securities, such as stock options and warrants, which would result in the issuance of incremental shares of common stock. The computation of diluted net loss per shares does not include the conversion of securities that would have an antidilutive effect. Equivalent common shares consisting of 1,169,167 and 675,000 stock options, and 132,260 and 0 common shares related to the conversion of the RRD Note, are excluded from the calculation of diluted net loss per share for the years ended December 31, 2019 and 2018, respectively, since their effect is antidilutive due to the net loss of the Company.Company consisted of the following:
| Year Ended December 31, 2020 | Year Ended December 31, 2019 | |||||||
| Equivalent common shares | ||||||||
| Stock options | 486,101 | 236,458 | ||||||
| Warrants | 5,056 | — | ||||||
| RRD note conversion | 49,897 | 26,746 | ||||||
Cash — At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation (“FDIC”). There were no accounts that exceeded federally insured limits at December 31, 20192020 or December 31, 2018.2019.
Fair Value of Financial Instruments — The carrying amounts of the Company’s financial instruments, including cash and accounts payable approximate fair value due to the short-term nature of those instruments.
Research and Development — Research and development costs are expensed as incurred. These expenses include the costs of proprietary efforts, as well as costs incurred in connection with certain licensing arrangements and external research and development expenses incurred under arrangements with third parties, such as contract research organizations (“CROs”) and consultants. At the end of each reporting period, the Company compares the payments made to each service provider to the estimated progress towards completion of the related project. Factors that the Company considers in preparing these estimates include the number of patients enrolled in studies, milestones achieved, and other criteria related to the efforts of its vendors. These estimates will be subject to change as additional information becomes available.
Stock-based Compensation — Stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which is generally the vesting period. The Company early adopted ASU No. 2018-07 on January 1, 2018 whichCompany’s policy permits the valuation of stock-based awards granted to non-employees to be measured at fair value at the grant date rather than on an accelerated attribution basis over the vesting period.
Determining the appropriate fair value of share-based awards requires the use of subjective assumptions, including the fair value of the Company’s common shares, and for options, the excepted life of the option and expected share price volatility. The Company usinguses the Black-Scholes option pricing model to value its option awards. The assumptions used in calculating the fair value of share-based awards represents management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different assumptions, share-based compensation expense could be materially different for future awards.
The expected life of options was estimated using the simplified method, as the Company has historical information to develop reasonable expectations about future exercise patterns and post-vesting employment.
Income Taxes — The Company accounts for income taxes using the asset-and-liability method in accordance with ASC 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rate is recognized in the period that includes the enactment date. A valuation allowance is recorded if it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized in future periods.
The Company follows the guidance in ASC Topic 740-10 in assessing uncertain tax positions. The standard applies to all tax positions and clarifies the recognition of tax benefits in the financial statements by providing for a two-step approach of recognition and measurement. The first step involves assessing whether the tax position is more-likely-than-not to be sustained upon examination based upon its technical merits. The second step involves measurement of the amount to be recognized. Tax positions that meet the more-likely than-not threshold are measured at the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate finalization with the taxing authority. The Company recognizes the impact of an uncertain income tax position in the financial statements if it believes that the position is more likely than not to be sustained by the relevant taxing authority. The Company will recognize interest and penalties related to tax positions in income tax expense. As of December 31, 2019,2020, the Company had no uncertain income tax positions.
Recent Accounting Pronouncements
In February 2016, the FASB issued its final standard on lease accounting, ASU No. 2016-02, as amended Leases (Topic 842), which superseded Topic 840. The new pronouncement requires the recognition on the balance sheet of right-of-use assets and lease liabilities for all long-term leases, including operating leases, on the balance sheet. The pronouncement requires that lease arrangements longer than 12 months result in an entity classifying leases as finance or operating leases. However, unlike current U.S. GAAP, which requires only capital leases to be recognized on the balance sheet, Topic 842 will require both types of leases to be recognized on the balance sheet. Topic 842 also requires disclosures about the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative requirements, providing additional information about the amounts recorded in the financial statements. The effective date of Topic 842 for non-public business entities, including smaller reporting companies, to fiscal years beginning after December 15, 2021. The Company intends to adopt the new guidance as of January 1, 2022. The adoption of this standard is not expected to have any material impact on the financial statements and related disclosures.
Note 3. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consistedconsists of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Prepaid insurance | $ | 8,257 | $ | 3,328 | ||||
| Legal retainer | 3,643 | — | ||||||
| Consulting fees | 5,838 | — | ||||||
| Accounts receivable, related party | - | 1,388 | ||||||
| Other prepaid expenses and current assets | 534 | 1,855 | ||||||
| $ | 18,273 | $ | 5,183 | |||||
| December 31, 2019 | December 31, 2018 | |||||||
| Prepaid insurance | $ | 3,328 | $ | 2,630 | ||||
| Governmental affairs consulting | - | 10,500 | ||||||
| Accounts receivable, related party | 1,388 | - | ||||||
| Other prepaid expenses and current assets | 467 | - | ||||||
| $ | 5,183 | $ | 13,130 | |||||
Note 4. Accounts Payable and Accrued Liabilities
Accounts payable and accrued liabilities consistedconsists of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Deferred CEO compensation | $ | 1,028,110 | $ | 680,400 | ||||
| Research and development expenses | 1,045,505 | 275,429 | ||||||
| Legal expenses | 437,471 | 200,062 | ||||||
| Professional fees | 253,462 | 177,096 | ||||||
| Interest payable/accrued | 270,000 | 138,762 | ||||||
| Accounting consulting fees | 45,904 | 24,765 | ||||||
| Taxes | 13,365 | 14,465 | ||||||
| Other | 22,107 | 3,495 | ||||||
| $ | 3,115,924 | $ | 1,514,474 | |||||
| December 31, 2019 | December 31, 2018 | |||||||
| Accrued payroll | $ | 680,400 | $ | 291,600 | ||||
| Research and development expenses | 275,429 | 180,168 | ||||||
| Legal expenses | 200,062 | 184,580 | ||||||
| Professional fees | 177,096 | 55,911 | ||||||
| Interest payable/accrued | 138,762 | 14,118 | ||||||
| Accounting consulting fees | 24,765 | 13,829 | ||||||
| Tax expenses | 14,465 | 7,465 | ||||||
| Other | 3,495 | 6,553 | ||||||
| $ | 1,514,474 | $ | 754,224 | |||||
Note 5. Notes Payable
On May 26, 2017 and October 4, 2017, Virpax entered into convertible promissory notes (the “Convertible Notes”), which promised to pay Anthony Mack, Chief Executive Officer and significant investor, the principal amounts of $100,000 and $20,000, respectively. On December 31, 2017, the Company entered into an Amended and Restated Unsecured Convertible Promissory Note (“Amended Convertible Note”) with Anthony Mack. The Amended Convertible Note consolidated the Convertible Notes (including accrued interest of $22,917) with an additional loan from Anthony Mack to the Company of $27,083. The Amended Convertible Note had an initial principal balance of $150,000. The Convertible Notes were not fully funded upon execution and the amount of debt outstanding as of January 1, 2018 was $95,234. Under this agreement, the maturity date was extended to December 31, 2018. If a qualified equity financing occurs before the maturity date, the Amended Convertible Note would be automatically converted into common shares of Virpax equal to the outstanding principal and accrued interest of the note at the time of qualified equity financing. On February 8, 2018, Anthony Mack exercised the conversion of the Amended Convertible Note payable upon the closing of a qualified equity financing. The Amended Convertible Note balance of $150,000 was converted into 75,000 shares of common stock at $2.00 per share. As of December 31, 2018, there was no outstanding balance for the Amended Convertible Note.
On October 1, 2018, Virpaxthe Company entered into a promissory note (the “2018 Promissory Note”), which promises to pay Anthony Mack, Chief Executive Officer and significant investor, the principal amount of $500,000, and bear interest at a rate of 11.19% per annum. The 2018 Promissory Note states that the principal shall be paid at the earlier of an event of default and the first anniversary of the date of the note. As of December 31, 2019,2020, the balance on the 2018 Promissory Note was $500,000, with accrued interest of $74,692.$125,887. As of December 31, 2018,2019, the balance on the 2018 Promissory note was $500,000, with accrued interest of $14,118. Please refer to Note 12. Subsequent Events for further detail regarding an amendment to the 2018 Promissory Note.$74,692.
On January 15, 2019, Virpaxthe Company entered into a promissory note (the “2019 Promissory Note”), which promises to pay Anthony Mack the principal amount of $500,000, and bear interest at a rate of 11.19% per annum. The 2019 Promissory Note states that the principal shall be paid at the earlier of an event of default and the first anniversary of the date of the note. On April 6, 2020, VIRPAX and Anthony Mack entered into an amendment to the 2019 Promissory Note which extended the maturity date from the first anniversary of the date of the 2019 Promissory Note to January 15, 2021 with all other terms remaining consistent. As of December 31, 2020, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $109,569. As of December 31, 2019, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $56,329. Please refer to
On October 28, 2020, the Company amended its 2018 Promissory Note 12. Subsequent Events for further detail regarding an amendment to thedated October 1, 2018 and its 2019 Promissory Note.Note dated January 15, 2019 with Anthony Mack, Chief Executive Officer and significant investor, to extend the maturity date to December 31, 2023 for both promissory notes. All other terms and conditions of these promissory notes remained unchanged.
On August 29, 2019, Virpaxthe Company entered into a service provider convertible note purchase agreement (the “RRD Note”) with RRD International, LLC (“RRD”). Under this agreement, Virpax and RRD agreed to make certain compensation payable in the form of a convertible promissory note. The convertible promissory note states that a maximum principal balance of $400,000 can be applied for services provided by RRD to Virpax, which can be converted into equity or cash (all or in part) upon a Qualified Financing or the Conversion Date of March 31, 2020. Borrowings under the RRD Note bear simple interest on the outstanding principal amount of the RRD Note until paid in full at the fixed rate of 10% per annum. On March 20, 2020, VIRPAX and RRD entered into an amendment which extended the maturity date from March 31, 2020 to September 30, 2020, increased the amount of principal from $400,000 to $600,000, extended the Qualified Financing deadline from March 31, 2020 to September 30, 2020, and provided for the payment of all interest accrued up to March 31, 2020 which totaled $16,435. As of December 31, 2020, the balance on the RRD Note was $493,480, with accrued interest of $34,544. As of December 31, 2019, the balance on the RRD Note was $264,520, with accrued interest of $7,741. Please refer
In October 2020, the Company amended the RRD Note to extend the maturity date from September 30, 2020 to November 30, 2020, and to extend the RRD Qualified Financing deadline from September 30, 2020 to November 30, 2020, and provide for the payment of all interest accrued from April 1, 2020 through November 30, 2020, which was $30,431. On November 30, 2020, the Company amended the RRD Note 12.to extend the conversion date to December 31, 2020. On December 31, 2020, we amended the RRD Note to extend the conversion date to January 31, 2021 (See also Note 12 – Subsequent Events for further detail regarding an amendmentinformation on the RRD Note).
On May 4, 2020, the Company entered into a Promissory Note (the “PPP Note”) with PNC Bank as the lender (the “Lender”), pursuant to which the Lender agreed to make a loan to us under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”). The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to the RRDCompany’s full time headcount during the period ending October 31, 2020. The interest rate on the PPP Note is a fixed rate of 1% per annum. To the extent that the amounts owed under the PPP Loan, or a portion of them, are not forgiven, the note shall convert to an amortizing term loan and the Company will be required to make principal and interest payments in monthly installments beginning seven months from April 2020. The PPP Note matures in two years. The PPP Note includes events of default. Upon the occurrence of an event of default, the Lender will have the right to exercise remedies against us, including the right to require immediate payment of all amounts due under the PPP Note.
The following table summarizes the Company’s notes payable:payables:
| December 31, 2019 | ||||||||||||||||
| Balance as of January 1, 2019 | Notes issued | Conversions of notes to equity | Outstanding principal | |||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | - | $ | - | $ | 500,000 | ||||||||
| Anthony Mack 2019 Promissory Note | - | 500,000 | - | 500,000 | ||||||||||||
| Total related party notes payable | 500,000 | 500,000 | - | 1,000,000 | ||||||||||||
| RRD Note | - | 264,520 | 264,520 | |||||||||||||
| Total notes payable | $ | 500,000 | $ | 764,520 | $ | - | $ | 1,264,520 | ||||||||
| December 31, 2018 | ||||||||||||||||
| Balance as of January 1, 2018 | Notes issued | Conversions of notes to equity | Outstanding principal | |||||||||||||
| Anthony Mack Convertible Note | $ | 95,234 | $ | 54,766 | $ | (150,000 | ) | $ | - | |||||||
| Anthony Mack 2018 Promissory Note | - | 500,000 | - | 500,000 | ||||||||||||
| Total related party notes payable | $ | 95,234 | $ | 554,766 | $ | (150,000 | ) | $ | 500,000 | |||||||
The interest expense associated with the notes payable was $124,644 and $14,976 for the years, ended December 31, 2019 and 2018, respectively.
| December 31, 2020 | ||||||||||||
| Balance as of January 1, 2020 | Notes issued | Balance as of December 31, 2020 | ||||||||||
| Related party notes payable | ||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | — | $ | 500,000 | ||||||
| Anthony Mack 2019 Promissory Note | 500,000 | — | 500,000 | |||||||||
| Total related party notes payable | 1,000,000 | — | 1,000,000 | |||||||||
| RRD Note | 264,520 | 228,960 | 493,480 | |||||||||
| SBA PPP Loan | — | 72,100 | 72,100 | |||||||||
| Total notes payable | $ | 1,264,520 | $ | 301,060 | $ | 1,565,580 | ||||||
| Less: Current portion of notes payable | 1,264,520 | 279,470 | 543,990 | |||||||||
| Total non-current portion of notes payable | $ | — | $ | 21,590 | $ | 1,021,590 | ||||||
F-10
| December 31, 2019 | ||||||||||||
| Balance as of January 1, 2019 | Notes Issued | Balance as of December 31, 2019 | ||||||||||
| Related party notes payable | ||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | — | $ | 500,000 | ||||||
| Anthony Mack 2019 Promissory Note | — | 500,000 | 500,000 | |||||||||
| Total related party notes payable | 500,000 | 500,000 | 1,000,000 | |||||||||
| RRD Note | — | 264,520 | 264,520 | |||||||||
| Total notes payable | 500,000 | 764,520 | 1,264,520 | |||||||||
| Less: Current portion of notes payable | 500,000 | 764,520 | 1,264,520 | |||||||||
| Total non-current portion of notes payable | $ | — | $ | — | $ | — | ||||||
Note 6. Commitments and Contingencies
Leases
The Company leases its Malvern, PA office under a non-cancelable operating lease that expires in 2020. Total rentalInterest expense was $42,468$147,934 and $38,963$124,644 for the year ended December 31, 2020 and 2019, and 2018, respectively.
Principal payments on note payables are due as follows:
| Years ending December 31, | ||||
| 2021 | $ | 543,990 | ||
| 2022 | 21,590 | |||
| 2023 | 1,000,000 | |||
| 2024 | — | |||
| Total payments | $ | 1,565,580 | ||
Note 6. Commitments and Contingencies
Employment Agreements
The Company has an employment agreement with the Chief Executive Officer, effective September 18, 2018. The agreement may be terminated by either party at any time upon written notice provided to the other party. Concurrent with the employment agreement, the CEO and the Company agreed to an Executive Confidentiality Agreement that contains standard non-closure and non-competition provisions. In the event we terminate the employment agreement other than for cause, or the CEO terminates the agreement for good reason, we will pay the CEO the then effective base salary for a period of twelve months following the effective date of the termination. However, payment of the effective base salary is subject to the execution of a release form and the compliance by the CEO with the release and all terms and provisions of the employment agreement and Executive Confidentiality Agreement that survive the termination of employment. The Company’s Chief Executive Officer has also elected to forego a salary temporarily and defer compensation. Deferred compensation due to the Company’s Chief Executive Officer amounted to $630,000$1,005,000 and $270,000$630,000 as of December 31, 20192020 and 2018,2019, respectively, which is included in accounts payable and accrued expenses on the accompanying balance sheet.
The Company has an employment agreement with the Executive VP of Global Corporate Governance, effective May 1, 2019. The term of the agreement will continue in effect until notice is provided 10 days prior to the termination by either party. Upon termination of the agreement by the Company for any reason other than for cause, death or disability, or by the Executive VP of Global Corporate Governance for good reason, the Company shall pay any accrued salary, bonuses, or benefits that have not yet been paid to the Executive VP of Global Corporate Governance. Please refer to Note 12. Subsequent Events for further detail regarding the subsequent departure of the Executive VP of Global Corporate Governance from the Company.
Litigation
The Company is subject, from time to time, to claims by third parties under various legal disputes. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows. AsSee also Note 12 – Subsequent Events.
Global Pandemic Outbreak
In March 2020, the World Health Organization declared the outbreak of December 31, 2019,a novel coronavirus (“COVID-19”) a global pandemic. The outbreak has become increasingly widespread in the United States, impacting the markets in which the Company did notoperates. While the full impact of the pandemic continues to evolve, the financial markets have any pending legal actions.been subject to significant volatility that adversely impacts the Company’s ability to enter into, modify, and negotiate favorable terms and conditions relative to equity and debt financing initiatives. The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values also affect the Company’s ability to enter into collaborations, joint ventures, and license and royalty agreements. The outbreak and government measures taken in response to the pandemic have also had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, have spiked, while demand for other goods and services, such as travel, have fallen. The future progression of the outbreak and its effects on our business and operations are uncertain. We may face difficulties recruiting or retaining patients in our ongoing and planned clinical trials if patients are affected by the virus or are fearful of traveling to our clinical trial sites because of the outbreak. We and our third-party contract manufacturers, contract research organizations, and clinical sites may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak.
While expected to be temporary, these disruptions may negatively impact the Company’s results of operations, financial condition, and liquidity in 2021 and potentially beyond.
Note 7. Stockholders’ Equity
Overview
Preferred Stock
The Company’s Certificate of Incorporation, filed on May 12, 2017, authorizes the issuance of preferred stock. The total number of shares which the Company is authorized to issue is 1,000,000, each with a par value of $0.00001 per share.
Common Stock
The Company’s Certificate of Incorporation, filed on May 12, 2017, authorizes the issuance of common stock. The total number of shares which the Company is authorized to issue is 20,000,000, each with a par value of $0.00001 per share.
SinceFrom inception through December 31, 2020, the Company has raised funds through private placements at $2.00$9.89 per share utilizing subscriptions agreements.share.
During the year ended December 31, 2020, the Company issued 139,220 shares of common stock for gross proceeds totaling $1,376,900. The Company’s CEO and an immediate family member purchased 40,450 and 8,999 of these shares of common stock for gross proceeds totaling $400,000 and $89,000, respectively.
During the year ended December 31, 2019, the Company issued 312,50063,203 shares of common stock for gross proceeds totaling $625,000. The Company’s CEO purchased 300,00060,674 of these shares of common stock for gross proceeds totaling $600,000.
During the year ended December 31, 2018, the Company issued 560,000 shares of common stock for gross proceeds totaling $1,120,000, and the Company issued 75,000 shares of common stock in conversion of the Amended Convertible Note balance of $150,000. The Company’s CEO and CMO purchased 250,000 and 37,500 of these shares of common stock for gross proceeds totaling $500,000 and $75,000, respectively.
In addition, during the year ended December 31, 2019,2020, the Company issued 231,908533 shares of common stock in payment of consulting services and settlement of accounts payable for gross proceeds totaling $463,815. No$5,288. The Company issued 46,903 shares were issuedof common stock in payment of consulting services and settlement of accounts payable for gross proceeds totaling $463,815 during the year ended December 31, 2018.2019.
Reverse Stock Split
F-11
On November 19, 2020, the Company filed an amendment to its Articles of Incorporation and effected 1-for-4.944260256 reverse stock split of its issued and outstanding shares of common stock, $0.00001 par value, whereby 15,550,627 outstanding shares of the Company’s common stock were exchanged for 3,145,153 newly issued shares of the Company’s common stock. Under the terms of the reverse stock split, fractional shares issuable to stockholders were rounded to the nearest whole share, with cash paid in lieu of any fractional shares, resulting in a reverse split of 1-for-4.944260256. All per share amounts and number of shares (other than authorized shares) in the financial statements and related notes have been retroactively restated to reflect the reverse stock split resulting in the transfer of $119 and $113 from common stock to additional paid in capital at January 1, 2020 and 2019, respectively.
Note 8. Stock-Based Compensation
Restricted Stock Awards
On May 20, 2017, the Company established the Virpax Pharmaceuticals, Inc. Amended and Restated 2017 Equity Incentive Plan (the “Plan”). The Company’s Board of Directors, acting through its Equity Incentive Plan Committee, has determined that it would be to the advantage and best interest of the Company and its stockholders to grant restricted stock awards to certain individuals as compensation to serve as an employee of the Company and as an incentive for increased efforts during such service.
As of December 31, 2020, there were 5,056 unvested restricted stock awards issued totaling $50,000 of unearned deferred stock based compensation, based on a fair value of stock of $9.89 per share. In addition, during the year ended December 31, 2020, there were 6,952 restricted stock awards granted and 20,225 of restricted stock awards forfeited during the period. Also, during the year ended December 31, 2020, there were 15,800 restricted stock awards that vested resulting in $156,250 in stock-based compensation expense during the year. As of December 31, 2019, there were 168,75034,130 restricted stock awards issued and unvested totaling $337,500 of unearned deferred stock based compensation, based on a fair value of stock of $2.00$9.89 per share as determined by recent sales of stock to unrelated third parties. There were no restricted stock awards issued as of December 31, 2018.share.
Note 8. Stock-Based Compensation
Stock Options
The Company’s Plan provides a means whereby eligible employees, officers, non-employee directors and other individual service providers develop a sense of proprietorship and personal involvement in the development and financial success of the Company and to encourage them to devote their best efforts to the business of the Company, thereby advancing the interests of the Company and its stockholders. The Company, by means of the Plan, seeks to retain the services of such eligible persons and to provide incentives for such persons to exert maximum efforts for the success of the Company. The Plan commenced on the Effective Date and the Plan is administered by the Compensation Committee (“Committee”); provided that the entire Board may act in lieu of the Committee on any matter. The maximum aggregate number of shares of common stock which may be issued under all Awards granted to Participants under the Plan initially shall be 1,500,000303,382 shares. The number of authorized shares available for issuance under the Plan shall automatically increase on January 1st1st of each year commencing with the January 1 following the Effective Date and on each January 1 thereafter until the expiration date, in an amount equal to six percent (6%) of the total number of shares of common stock outstanding on December 31st31st of the preceding calendar year. The Plan shall remain in effect, subject to the right of the board of directors of the Company to amend or terminate the Plan at any time until the earlier of the tenth (10th)(10th) anniversary of the Effective Date. In the event of a termination of continuous service (other than as a result of a change of control, as defined in the Plan), unvested stock options generally shall terminate and, with regard to vested stock options, the exercise period shall be the lesser of the original expiration date or three months from the date continuous service terminates.
On May 21, 2018, the Company amended the Plan to grant stock options to Non-Employee Directors. Stock options to purchase 25,0005,056 shares of common stock shall automatically be granted under the Plan to each Non-Employee Director who is first appointed or elected to the Board. In addition, On January 1 of each year, each then serving Non-Employee Director of the Company shall automatically be granted under the Plan (i) that number of options having a value of $25,000 calculated on the grant date in accordance with the Black-Scholes option pricing model and shall be exercisable as to 100% of the number of shares of Stock covered thereby on the twelve-month anniversary of the grant date, and shall have an exercise price equal to 100% of the Fair Market Value of a share of Common Stock on the Date of Grant. Also, on January 1, of each year, each then serving member of the Science and Technology Committee shall automatically be granted stock options to purchase 10,0002,022 shares of Common Stock under the Plan, and the Chair of the Science and Technology Committee shall be granted stock options to purchase an additional 15,0003,033 shares of Common Stock under the Plan. These options have the same terms and conditions as the Non-Employee Directors noted above.
Stock-based compensation expense for the year ended December 31, 2020 and 2019 was $1,473,642 and 2018 was $678,146, and $384,955, respectively, which is included in general and administrative expense on the accompanying statement of operations.
The fair value of option awards is estimated using the Black-Scholes option-pricing model. Exercise price of each award is generally not less than the per share fair value in effect as of that award date. The determination of fair value using the Black-Scholes model is affected by the Company’s share fair value as well as assumptions regarding a number of complex and subjective variables, including expected price volatility, risk-free interest rate and projected employee share option exercise behaviors. Options granted or modified under the Plan during the years ended December 31, 20192020 and 20182019 were valued using the Black-Scholes option-pricing model with the following weighted-average assumptions:
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Expected term (years) | 5.24 | 5.54 | ||||||
| Risk-free interest rate | 0.48 | % | 2.03 | % | ||||
| Expected volatility | 68.50 | % | 57.23 | % | ||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
| For the Years Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Expected term (years) | 5.54 | 5.50 | ||||||
| Risk-free interest rate | 2.03 | % | 2.81 | % | ||||
| Expected volatility | 57.23 | % | 65.00 | % | ||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
In the absence of a public trading market for our common stock, on each grant date, the Company develops an estimate of the fair value of our common stock underlying the option grants. The Company estimated the fair value of our common stock by referencing arms-length transactions inclusive of the common stock underlying which occurred on or near the valuation date(s). Once the Company’s common stock is publicly traded, the Company will no longer have to estimate the fair value of the common stock, rather the value will be determined based on quoted market prices. The Company determined the fair value of common stock using methodologies, approaches and assumptions consistent with the AICPA Practice Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation and based in part on input from an independent third-party valuation firm.
The Company estimates its expected volatility by using a combination of historical share price volatilities of similar companies within our industry. The risk-free interest rate assumption is based on observed interest rates for the appropriate term of the Company’s options on a grant date. The expected option term assumption is estimated using the simplified method and is based on the mid-point between vest date and the remaining contractual term of the option, since the Company does not have sufficient exercise history to estimate expected term of its historical option awards.
The following is a summary of stock option activity under the stock option plans for the years ended December 31, 20192020 and 2018:2019:
| Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | ||||||||||||||
| Options outstanding at January 1, 2019 | 136,515 | $ | 9.89 | 9.51 | $ | — | |||||||||||
| Forfeited | (30,337 | ) | 9.89 | ||||||||||||||
| Cancelled | (5,056 | ) | 9.89 | ||||||||||||||
| Granted | 135,336 | 9.89 | |||||||||||||||
| Options outstanding at December 31, 2019 | 236,458 | 9.89 | 8.96 | — | |||||||||||||
| Forfeited | (20,225 | ) | 9.89 | ||||||||||||||
| Granted | 269,868 | 9.89 | |||||||||||||||
| Options outstanding at December 31, 2020 | 486,101 | $ | 9.89 | 8.68 | $ | — | |||||||||||
| Options exercisable at December 31, 2020 | 266,164 | $ | 9.89 | 8.15 | $ | — | |||||||||||
| Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Options outstanding at January 1, 2018 | - | $ | - | $ | - | |||||||||||
| Forfeited | - | - | ||||||||||||||
| Cancelled | - | - | ||||||||||||||
| Exercised | - | - | ||||||||||||||
| Granted | 675,000 | 2.00 | ||||||||||||||
| Options outstanding at December 31, 2018 | 675,000 | 2.00 | 9.51 | - | ||||||||||||
| Forfeited | (150,000 | ) | 2.00 | |||||||||||||
| Cancelled | (25,000 | ) | 2.00 | |||||||||||||
| Exercised | - | - | ||||||||||||||
| Granted | 669,167 | 2.00 | ||||||||||||||
| Options outstanding at December 31, 2019 | 1,169,167 | $ | 2.00 | 8.96 | $ | - | ||||||||||
| Options exercisable at December 31, 2019 | 600,000 | $ | 2.00 | 8.51 | $ | - | ||||||||||
The weighted-average grant-date fair value of stock options granted during the yearyears ended December 31, 2020 and 2019 was $5.66 and 2018 was $1.05 and $1.18,$5.21, respectively.
As of December 31, 2019,2020, there was $268,393$547,693 of total time-based unrecognized compensation costs related to unvested stock options stock. These costs are expected to be recognized over a weighted average period of 1.510.50 years.
On June 15, 2020, the Company and Michele Linde entered into a consulting agreement in conjunction with Ms. Linde separating from the Company as Executive Vice President, General Counsel and Corporate Secretary of the Company with an effective date of May 15, 2020. As part of the consulting agreement, Ms. Linde agreed to perform consulting and advisory services in exchange for 40,450 nonqualified stock options in the Company pursuant to a nonqualified stock option grant. The options shall vest evenly over a six-month term beginning on the effective date. The options from the consulting agreement were granted in exchange for the forfeiture of the 20,225 unvested restricted stock award and 15,169 unvested nonqualified stock options that were originally granted on October 30, 2019. These modifications are treated as an option modification and the Company accounted for the option modification under ASC Topic 718, Compensation — Stock Compensation. The fair value of the forfeited options and restricted stock award was determined to be in excess of the fair value of the options granted from the consulting agreement. As a result, the fair value of the forfeited options and restricted stock award were recognized over the six-month term of the consulting award, beginning with the effective date.
On June 15, 2020, the Company and Ms. Linde also executed an amendment with an effective date of May 15, 2020 to modify three nonqualified stock option grant agreements (the “NQSO Amendment”) that were entered into on July 20, 2018, May 18, 2019, and October 30, 2019, respectively. The nonqualified stock option grants were for 5,056, 10,112, and 5,056 options, respectively. The NQSO Amendment extended the post-termination exercisability period of the vested nonqualified stock options held by Ms. Linde from 90 days following termination of employment to ten years after the initial option grant. The NQSO Amendment also amended the May 19, 2019 grant agreement to vest all 10,112 options on May 18, 2020, regardless if Ms. Linde was employed by the Company at that date. These modifications are treated as an option modification and the Company accounted for the option modification under ASC Topic 718, Compensation — Stock Compensation. As a result of the modification, the Company recognized $90,050 in incremental compensation expense during year ended December 31, 2020.
Note 9. Income Taxes
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s deferred tax assets relate primarily to its net operating loss carryforwards and other balance sheet basis differences. In accordance with ASC 740, the Company recorded a valuation allowance to fully offset the gross deferred tax asset, because it is not more likely than not that the Company will realize future benefits associated with these deferred tax assets at December 31, 2020 and 2019. During the years ended December 31, 20192020 and 2018,2019, there was no income tax expense.
At December 31, 2019, the Company had deferred tax assets of $1,826,000, against which a full valuation allowance had been recorded. The change in the valuation allowance for the year ended December 31, 2019 was an increase of $954,000.
At December 31, 2018, the Company had deferred tax assets of $872,000, against which a full valuation allowance had been recorded. The change in the valuation allowance for the year ended December 31, 2018 was an increase of $768,000.
Significant components of the Company’s deferred tax assets at December 31, 20192020 and 2018:2019:
December 31, 2020 | December 31, 2019 | |||||||
| Deferred tax assets: | ||||||||
| Net-operating loss carryforwards | $ | 2,008,000 | $ | 1,267,000 | ||||
| Stock-based compensation | 688,000 | 307,000 | ||||||
| Accrued payroll | 297,000 | 197,000 | ||||||
| R&D credit | 10,000 | 10,000 | ||||||
| Other | 77,000 | 45,000 | ||||||
| Total deferred tax assets | 3,080,000 | 1,826,000 | ||||||
| Valuation allowance | (3,080,000 | ) | (1,826,000 | ) | ||||
| Net deferred tax asset | $ | — | $ | — | ||||
| December 31, 2019 | December 31, 2018 | |||||||
| Deferred tax assets: | ||||||||
| Net-operating loss carryforwards | $ | 1,267,000 | $ | 655,000 | ||||
| Stock-based compensation | 307,000 | 111,000 | ||||||
| Accrued payroll | 197,000 | 84,000 | ||||||
| R&D credit | 10,000 | 10,000 | ||||||
| Other | 45,000 | 12,000 | ||||||
| Total deferred tax assets | 1,826,000 | 872,000 | ||||||
| Valuation allowance | (1,826,000 | ) | (872,000 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
The change in the valuation allowance for the years ended December 31, 2020 and 2019 was an increase of $1,254,000 and 954,000, respectively.
A
The Company’s reconciliation of the federal statutory tax rate and the effective tax rates for the years ended December 31, 20192020 and 20182019 is as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Federal statutory rate | 21.0 | % | 21.0 | % | ||||
| Increase (decrease) in tax expense at federal statutory rate | ||||||||
| Change in valuation allowance | -21.0 | % | -20.9 | % | ||||
| Other | - | -0.1 | % | |||||
| Effective tax rate | 0.0 | % | 0.0 | % | ||||
| December 31, 2019 | December 31, 2018 | |||||||
| Federal statutory rate | 21.0 | % | 21.0 | % | ||||
| Increase (decrease) in tax expense at federal statutory rate | ||||||||
| Change in valuation allowance | -20.9 | % | -20.5 | % | ||||
| Other | -0.1 | % | -0.5 | % | ||||
| Effective tax rate | 0.0 | % | 0.0 | % | ||||
The Company had approximately $4,387,000 and $4,387,000$6,951,000 of gross net operating loss (“NOL”) carryforwards, (federalfor both federal and state, respectively) as of December 31, 2019.2020.
Sections 382 and 383 of the Internal Revenue Code, and similar state regulations, contain provisions that may limit the NOL carryforwards available to be used to offset income in any given year upon the occurrence of certain events, including changes in the ownership interests of significant stockholders. In the event of a cumulative change in ownership in excess of 50% over a three-year period, the amount of the NOL carryforwards that the Company may utilize in any one year may be limited.
TheFrom the total of the Company’s federal NOL of $6,951,000, $326,000 expires in 2037, and the remaining federal NOL’s haveNOL has an indefinite carryover period. The Company’s state NOL’s of $4,387,000 begin to$6,951,000 expire afterfrom 2037 through 2039.2040.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act, or the CARES Act, was signed into law to address the COVID-19 crisis. The CARES Act is an approximately $2 trillion emergency economic stimulus package that includes numerous U.S. federal income tax provisions, including the modification of: (i) net operating loss rules, (ii) the alternative minimum tax refund and (iii) business interest deduction limitations under Section 163(j) of the Internal Revenue Code of 1986, as amended, or the Code. The effects of this legislation will be taken into consideration in the period of enactment in 2020. The changes in federal tax will not have a material effect on the tax liability of the Company.
Note 10. Related-Party Transactions
As discussed in Note 5, in October 2018 and January 2019 the Company issued notes with an aggregate principal amount of $1,000,000. These notes were issued to Anthony Mack, Chief Executive Officer and significant investor of the Company.
As discussed in Note 3, Thethe Company maintained an account receivable, related party balance of $1,388 as of December 31, 2019. This amount relatesrelated to the reimbursement due back to the Company for healthcare insurance on behalf of Jeffrey Gudin, the Company’s Chief Medical Officer.
Note 11. Research and Development and License Agreements
MedPharm Limited
Research and Option Agreement
On April 11, 2017, the Company entered into a research and option agreement, as amended on May 30, 2018 (the “MedPharm Research and Option Agreement”), with MedPharm Limited, a company organized and existing under the laws of the United Kingdom (“MedPharm”), pursuant to which MedPharm granted the Company an option to obtain an exclusive, world-wide, royalty bearing license to use certain technology developed by MedPharm. Pursuant to the agreement, MedPharm will conduct certain research and development of proprietary formulations incorporating certain MedPharm technologies and certain of the Company’s proprietary molecules.
Under the MedPharm Research and Option Agreement, MedPharm granted the Company an option (the “MedPharm Option”) to obtain an exclusive (even to MedPharm), worldwide, sub-licensable (through multiple tiers), royalty bearing, irrevocable license to research, develop, market, commercialize, and sell any product utilizing MedPharm’s spray formulation technology which is the result of the activities performed under the MedPharm Research and Option Agreement, subject to the Company’s entry into a definitive license agreement with MedPharm. In order to exercise the MedPharm Option, the Company must provide MedPharm with written notice of such exercise before the end of the Option Period (as defined in the MedPharm Research and Option Agreement). The Option Period is subject to extension upon mutual agreement with MedPharm.
Pursuant to the MedPharm Research and Option Agreement, the Company has a right of first refusal with respect to any license or commercial arrangement involving any Licensed Intellectual Property (as defined in the MedPharm Research and Option Agreement) in combination with any Virpax Molecule (as defined in the MedPharm Research and Option Agreement). In the event that MedPharm reaches an agreement with respect to a license or other commercial arrangement that involves technology or molecules covered by the right of first refusal, the Company has ten business days from the date of notice to notify MedPharm of its intention to exercise the right of first refusal and the Company’s intention to match the financial terms of the other license or commercial arrangement.
License Agreement
On June 6, 2017, as a result of the Company’s exercise of the MedPharm Option under the MedPharm Research and Option Agreement, the Company entered into a license agreement, as amended on September 2, 2017 and October 31, 2017 (the “MedPharm License Agreement”), with MedPharm for the exclusive global rights to discover, develop, make, sell, market, and otherwise commercialize any pharmaceutical composition or preparation (in any and all dosage forms) in final form containing one or more compounds, including Epoladerm and OSF200, that was developed, manufactured or commercialized utilizing MedPharm’s spray formulation technology (“MedPharm Product”), to be used for any and all uses in humans (including all diagnostic, therapeutic and preventative uses). Under the MedPharm License Agreement, the Company is required to make future milestone and royalty payments to MedPharm. The Company isWe are obligated to make aggregate milestone payments to MedPharm of up to 1GBP 1.150 million Great British pounds upon the achievement of specified development milestones (payable in Great British Pounds). Additional milestone payments are due upon the achievement of certain development and an additional 100,000 GBP percommercial milestones achieved outside the first marketing approval ofUnited States, payable on a product in each country in the European market for any indication.by country basis. Royalty payments must be paid to MedPharm in an amount equal to a single digitsingle-digit percentage (5% or lower) of net sales of all MedPharm Product sold by the Companyus during the royalty term in the territory. Royalties shall be payable, on a country-by-country basis, during the period of time commencing on the first commercial sale and ending upon the expiration of the last-to-expire patent claim on the licensed product, which is set to expire on December 4, 2028. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
LipoCureRx, Ltd.
On March 19, 2018, the Company entered into a license and sublicense agreement (the “LipoCure Agreement”) with LipoCureRx, Ltd., a company organized and existing under the laws of Israel (“LipoCure”), for the sole and exclusive global license and sub-license rights to discover, develop, make, sell, market, and otherwise commercialize bupivacaine liposome, in injectable gel or suspension (“Licensed Compound”) or any pharmaceutical composition or preparation (in any and all dosage forms) in final form, including any combination product, containing a Licensed Compound (“Licensed Product”)., including Probudur. Under the LipoCure Agreement, the Company iswas required to pay an upfront fee upon signing of $150,000 and isare required to make future milestone and royalty payments to LipoCure. The upfront fee of $150,000 was recorded as research and development expense in April 2018. The Company is obligated to make aggregate milestone payments of up to $19.8 million upon the achievement of specified development and commercial milestones. Royalty payments must be paid in an amount equal to a single digit to low double digitdouble-digit percentage of annual net sales of royalty qualifying products, subject to certain adjustments. These royalty payments are basedRoyalties shall be payable during the period of time, on a country-by-country basis, commencing on the first commercial sale and ending upon aggregate net salesthe expiration of certain qualifying products by Virpax and related parties in each calendar year of such royalty term, at 8% for the annual portion of sales less than or equallast-to-expire patent claim on the licensed product, which is set to $250 million, at 9% for the portion of annual net sales between $250-$500 million, and 11% for the portion that is greater than $500 million.expire on July 24, 2030. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
Nanomerics Ltd.
Nanomerics Collaboration Agreement
On April 11, 2019, the Company entered into an exclusive collaboration and license agreement, as amended (the “Nanomerics Collaboration Agreement”), with Nanomerics Ltd. (“Nanomerics”), a company organized and existing under the laws of United Kingdom, for the exclusive world-wide license to develop and commercialize products, including NES100, which contain hydrophilic neuropeptide Leucin5-Enkephalin and an amphiphile compound which is quaternary ammonium palmitoyl glycol chitosan, and to engage in a collaborative program utilizing Nanomerics’ knowledge, skills and expertise in the clinical development of products and in attracting external funding for such development. The Nanomerics Collaboration Agreement was also amended to include a program for the pre-clinical development of a product for post-traumatic stress disorder.
Under the Nanomerics Collaboration Agreement, the Company are required to make royalty payments equal to a single digit percentage (7.5%) of annual net sales of the licensed product and a certain percentage of annual net sales of combination products containing the compound together with one or more other active ingredients.royalty qualifying products. The Company areis also required to make aggregate milestone payments of up to $103 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships the Company enterswe enter into subsequent to the Nanomerics Collaboration Agreement. The Company’s obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of its licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim (patent is set to expire on November 3, 2034); and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. The Company has the right to terminate the agreement upon 180 days’ prior written notice to Nanomerics. Upon termination, the Company shall assign to Nanomerics all its right title and interest in all results other than results specific to (a) the Virpax ResultsDevice (as defined in the Nanomerics Collaboration Agreement). Through December 31, 2019, none of, its manufacture or use; and (b) the development and commercial milestones were met.
Yissum
On August 11, 2019, the Company entered into an exclusive research and license agreement, as amended (the “Yissum Agreement”), with Yissum Research Development Company of the Hebrew University of Jerusalem, Ltd., a company organized and existing under the laws of Israel (“Yissum”). Under the Yissum Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to technology enabling the creation of quick-onset and long-acting formulations of opioid antagonists. Under the Yissum Agreement, the Company has the exclusive right to license the commercial technology resulting from the activities of the researches at the Hebrew University. The Company has agreed to use commercially reasonable efforts to carry out the development, regulatory, manufacturing and marketing work necessary to develop and commercializeTechnology, but excluding any of the products which result from such research.
The Yissum Agreement requires us to pay an annual license maintenance fee, a royalty fee equal to a single digit percentage of annual net sales of any product, aggregate milestone payments of up to $1.19 million upon the achievement of various development and commercial milestones, and a percentage of sublicense fees based on the timing of execution of a sublicense agreement. The Company has the right to terminate the Yissum Agreement by written notice immediately if the licensor passes a resolution for voluntary winding up or a winding up application is made against it and not set aside within 60 days. Additionally, we may terminate the Yissum Agreement if either a receiver or liquidator is appointed for the licensor, or the licensor enters into a winding up or insolvency or bankruptcy proceeding.
Research and development expense was $622,741 and $1,142,176 for the year ended December 31, 2019 and 2018, respectively.
Note 12. Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through August 10, 2020. The following are material subsequent events:
Subsequentclinical Results relating to the year ended December 31, 2019 and throughCompound or Licensed Products (all terms as defined in the date these financial statements have been issued, the Company has raised gross proceeds of approximately $1,348,682 from the issuance of 674,403 shares of its common stock.Nanomerics Collaboration Agreement).
Subsequent to the year ended December 31, 2019 and through the date these financial statements have been issued, the Company granted a total of 1,334,336 stock options to its officers, directors, employees and consultants under the Company’s Plan as incentive compensation. The stock options have a term of ten years and shall vest and become exercisable either over a period of one year or upon reaching certain milestones. The exercise price of the stock options was $2.00 per share. In addition, on January 1, 2020, the number of authorized shares available for issuance under the 2017 Plan automatically increased by 895,509 shares.Nanomerics License Agreement
On August 7, 2020, the Company entered into a collaboration and license agreement with Nanomerics Ltd. (the “Nanomerics License Agreement”), a company organized and existing under the laws of United Kingdom, for the exclusive North American license to develop and commercialize a High-Density Molecular Masking Spray (MMS019) as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans. Under the Nanomerics License Agreement, the Company is required to make royalty payments equalwithin a range of 5% to a low double digit percentage15% of annual net sales of royalty qualifying products. The Company’s obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of its licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim; and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. The Company is also is required to make aggregate milestone payments of up to $50 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships the Company enterswe enter into subsequent to the Nanomerics License Agreement.Agreement (any patent that issues from the currently filed provisional patent application would expire on August 24, 2041). The Company has the right to terminate the agreementNanomerics License Agreement upon 60 days’ prior written notice to Nanomerics. Upon termination, the Companywe shall assign to Nanomerics all its rights, title and interest in all results of the Company.its results. Nanomerics has the right to terminate the agreement upon 60 days’ prior written notice upon if the Company has not secured funding by the Funding Expiry Date (as defined in the Nanomerics License Agreement). On December 31, 2020, the Company amended the Nanomerics License Agreement to extend the Funding Expiry Date to March 31, 2021.
Yissum
Research Agreements
On May 12, 2019, the Company entered into an Agreement for Rendering of Research Services (the “May 2019 Yissum Research Agreement”), with Yissum. Under the May 2019 Yissum Research Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the formulation, preparation and characterization of Liposomal Bupivacaine for size zeta potential, drug loading and rate of drug release. In consideration for the research services, The Company agreed to pay research service fees of $81,000 in equal monthly installments. The Company retains ownership in all our intellectual property rights and any intellectual property belonging to either the Company or Yissum prior to the execution of the May 2019 Yissum Research Agreement will remain the sole property of either the Company or Yissum, respectively. All data generated from the provision of the May 2019 Yissum Research Agreement, including any reports, which are specifically required and contemplated under the May 2019 Yissum Research Agreement, shall be owned by the Company upon full payment of the research services fees. Each party will be entitled to terminate the agreement in the event of a breach by the other party of its obligations under the agreement, including, but not limited to, any payment failure, which is not remedied by the breaching party within 30 days of receipt of written notice from the non-breaching party. All services to be provided under the May 2019 Yissum Research Agreement will be completed by March 31, 2020.
On October 11, 2020, the Company entered into an Agreement for Rendering of Research Services with Yissum (the “October 2020 Yissum Research Agreement”) on substantially similar terms and conditions as detailed above in the May 2019 Yissum Research Agreement. Under the October 2020 Yissum Research Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the formulation of Liposomal Bupivacaine as well as efficacy and PK studies in animals. In consideration for the research services, the Company agreed to pay research service fees of $81,000 in six equal monthly installments. In connection with the completion of our initial public offering, we paid Yissum $40,500 towards the total consideration of $81,000. All services to be provided under the October 2020 Yissum Research Agreement will be completed by June 30, 2021.
NCATS-NIH Cooperative Research and Development Agreement
On August 25, 2020, the Company entered into a CRADA with NCATS. This collaboration is for the continued development of its product candidate, NES100, an intranasal peptide, for the management of acute and chronic non-cancer pain. The term of the CRADA is for a period of four years from the effective date of the agreement and can be terminated by both parties at any time by mutual written consent. In addition, either party may unilaterally terminate the CRADA at any time by providing written notice of at least sixty (60) days before the desired termination date. The agreement provides for studies that are focused on the pre-clinical characterization of NES100 as a novel analgesic for acute and chronic non-cancer pain, and for studies to further develop NES100 through IND enabling studies. There are certain development “Go/No Go” provisions within the agreement whereby, if certain events occur, or do not occur, NCATS may terminate the CRADA. These “No GO” provisions include: i) lack of efficacy in all animal pain models, ii) no reliable and sensitive bioanalytical method can be developed, iii) manufacturing failure due to inherent process scalability issues, iv) unacceptable toxicity or safety profile to enable clinical dosing, and v) inability to manufacture the NES100 dosage form.
With respect to NCATS rights to any invention made solely by an NCATS employee(s) or made jointly by an NCATS employee(s) and our employee(s), the CRADA grants to the Company an exclusive option to elect an exclusive or nonexclusive commercialization license. For inventions owned solely by NCATS or jointly by NCATS and the Company, and licensed pursuant to its option, the Company must grant to NCATS a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government. For inventions made solely by an employee of ours, we grant to the United States government a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government for research or other government purposes.
Note 12. Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through March 31, 2021. The following are material subsequent events:
Initial Public Offering
On February 16, 2021, the Company closed its initial public offering of 1,800,000 shares of its common stock at a public offering price of $10.00 per share, for gross proceeds of $18.0 million, before deducting underwriting discounts and offering expenses, and net proceeds of $16 million. The Company intends to use substantially all of the net proceeds from the offering, after the repayment of notes payable – see below, to fund research and development of its Epoladerm™, Probudur™, Envelta™ and MMS019 indications and other development programs, and for working capital and other general corporate purposes.
In conjunction with the initial public offering, the Company amended its Certificate of Incorporation to increase the number of shares of common stock and preferred stock it is authorized to issue to 100,000,000 and 10,000,000, respectively.
On February 16, 2021, upon closing the initial public offering, 5,056 restricted stock awards vested totaling $50,000 of stock based compensation.
Notes Payable
In January 2021, the Company issued notes with an aggregate principal amount of $75,000 and $25,000, respectively. These notes were issued to Anthony Mack, Chief Executive Officer and significant investor of the Company for $75,000 and Christopher Chipman, Chief Financial Officer, for $25,000. These notes were subsequently repaid with proceeds from the initial public offering in February 2021.
In February 2021, the Company paid the balance on its RRD Note of $493,480, including accrued interest, with proceeds from the Company’s initial public offering.
Legal Proceedings
On April 6, 2020 VirpaxMarch 12, 2021, we and Anthony Mack, our President and Chief Executive Officer, were named as defendants in a complaint (the “Complaint”) filed by Sorrento Therapeutics, Inc. (“Sorrento”) and Scilex Pharmaceuticals Inc. (“Scilex” and together with Sorrento, the “Plaintiffs”) in the Court of Chancery of the State of Delaware (the “Court”). In the Complaint the Plaintiffs allege (i) Mr. Mack breached a Restrictive Covenants Agreement, dated as of November 8, 2016, between himself and Sorrento (the “Restrictive Covenants Agreement”), (ii) we tortiously interfered with the Restrictive Covenants Agreement, and (iii) we tortiously interfered with Scilex’s relationship with Mr. Mack.
Simultaneously with filing the Complaint, the Plaintiffs filed a motion to expedite, seeking a hearing in four weeks on their prospective motion for a preliminary injunction, prohibiting Mr. Mack’s alleged violations of the Restrictive Covenants Agreement and prohibiting our alleged tortious interference. On March 19, 2021, the parties reached an agreement to schedule the hearing for a date in July 2021, which the Court so ordered. We believe that the allegations in the Complaint and the motion for a preliminary injunction are wholly without merit and we intend to vigorously defend the action. However, we are unable to predict the ultimate outcome of the lawsuit at this time.
VIRPAX Pharmaceuticals, Inc.
CONDENSED BALANCE SHEETS
| June 30, 2021 | December 31, 2020* | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 10,466,774 | $ | 54,796 | ||||
| Prepaid expenses and other current assets | 920,922 | 18,273 | ||||||
| Total current assets | 11,387,696 | 73,069 | ||||||
| Deferred financing costs | — | 392,337 | ||||||
| Total assets | $ | 11,387,696 | $ | 465,406 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Accounts payable and accrued expenses | $ | 2,779,866 | $ | 3,115,924 | ||||
| Notes payable | 50,510 | 543,990 | ||||||
| Total current liabilities | 2,830,376 | 3,659,914 | ||||||
| Notes payable, net of current portion | 21,590 | 21,590 | ||||||
| Related party notes payable | 1,000,000 | 1,000,000 | ||||||
| Total long-term liabilities | 1,021,590 | 1,021,590 | ||||||
| Total liabilities | 3,851,966 | 4,681,504 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity (deficit) | ||||||||
| Preferred stock, par value $0.00001, 10,000,000 shares authorized, no shares issued and outstanding | — | — | ||||||
| Common stock, $0.00001 par value; 100,000,000 shares authorized, 4,960,153 shares issued and outstanding as of June 30, 2021; 3,145,153 shares issued and outstanding as of December 31, 2020 | 50 | 31 | ||||||
| Additional paid-in capital | 22,906,214 | 6,431,715 | ||||||
| Accumulated deficit | (15,370,534 | ) | (10,647,844 | ) | ||||
| Total stockholders’ equity (deficit) | 7,535,730 | (4,216,098 | ) | |||||
| Total liabilities and stockholders’ equity (deficit) | $ | 11,387,696 | $ | 465,406 | ||||
| * | Derived from audited financial statements |
See Notes to the Condensed Financial Statements
VIRPAX Pharmaceuticals, Inc.
CONDENSED STATEMENTS OF OPERATIONS
(UNAUDITED)
For the Three June 30, | For the Three Months Ended June 30, | For the Six Months Ended June 30, 2021 | For the Six June 30, | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| General and administrative | $ | 1,988,972 | $ | 1,061,850 | $ | 3,262,544 | $ | 1,510,835 | ||||||||
| Research and development | 316,565 | 168,673 | 1,391,565 | 335,553 | ||||||||||||
| Total operating expenses | 2,305,537 | 1,230,523 | 4,654,109 | 1,846,388 | ||||||||||||
| Loss from operations | (2,305,537 | ) | (1,230,523 | ) | (4,654,109 | ) | (1,846,388 | ) | ||||||||
| OTHER (EXPENSE) INCOME | ||||||||||||||||
| Interest expense | (34,049 | ) | (43,261 | ) | (64,748 | ) | (83,891 | ) | ||||||||
| Other income (expense), net | (3,833 | ) | 4,000 | (3,833 | ) | 4,000 | ||||||||||
| Loss before tax provision | (2,343,419 | ) | (1,269,784 | ) | (4,722,690 | ) | (1,926,279 | ) | ||||||||
| Benefit from income taxes | — | — | — | — | ||||||||||||
| Net loss | $ | (2,343,419 | ) | $ | (1,269,784 | ) | $ | (4,722,690 | ) | $ | (1,926,279 | ) | ||||
| Basic and diluted net loss per share | (0.47 | ) | (0.41 | ) | $ | (1.06 | ) | $ | (0.63 | ) | ||||||
| Basic and diluted weighted average common stock outstanding | 4,958,999 | 3,086,384 | 4,454,877 | 3,065,636 | ||||||||||||
See Notes to the Condensed Financial Statements
VIRPAX Pharmaceuticals, Inc.
CONDENSED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(UNAUDITED)
| Additional | Total | |||||||||||||||||||||||||||
| Preferred stock | Common stock | paid-in | Accumulated | stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | equity/(deficit) | ||||||||||||||||||||||
| Balance at December 31, 2019 | — | $ | — | 3,018,673 | $ | 30 | $ | 3,575,886 | $ | (6,308,191 | ) | $ | (2,732,275 | ) | ||||||||||||||
| Common stock issued pursuant to subscription agreements | — | — | 42,978 | — | 425,000 | — | 425,000 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | — | — | 180 | — | 1,781 | — | 1,781 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 130,990 | — | 130,990 | |||||||||||||||||||||
| Restricted stock awards granted | — | — | 3,792 | — | — | — | — | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (656,495 | ) | (656,495 | ) | |||||||||||||||||||
| Balance at March 31, 2020 | — | — | 3,065,623 | 30 | 4,133,657 | (6,964,686 | ) | (2,830,999 | ) | |||||||||||||||||||
| Common stock issued pursuant to subscription agreements | — | — | 85,325 | 1 | 843,899 | — | 843,900 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | — | — | 113 | — | 1,125 | — | 1,125 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 571,691 | — | 571,691 | |||||||||||||||||||||
| Restricted stock awards granted | — | — | 3,160 | — | — | — | — | |||||||||||||||||||||
| Restricted stock awards forfeited | — | — | (20,225 | ) | — | — | — | — | ||||||||||||||||||||
| Net loss | — | — | — | — | — | (1,269,784 | ) | (1,269,784 | ) | |||||||||||||||||||
| Balance at June 30, 2020 | — | $ | — | 3,133,996 | $ | 31 | $ | 5,550,372 | $ | (8,234,470 | ) | $ | (2,684,067 | ) | ||||||||||||||
| Balance at December 31, 2020 | — | — | 3,145,153 | 31 | 6,431,715 | (10,647,844 | ) | (4,216,098 | ) | |||||||||||||||||||
| Common stock issued pursuant to initial public offering, net of offering costs of $2,216,793 | — | — | 1,800,000 | 18 | 15,783,189 | — | 15,783,207 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 369,884 | — | 369,884 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (2,379,271 | ) | (2,379,271 | ) | |||||||||||||||||||
| Balance at March 31, 2021 | — | — | 4,945,153 | 49 | 22,584,788 | (13,027,115 | ) | 9,557,722 | ||||||||||||||||||||
| Restricted stock awards granted | — | — | 15,000 | 1 | (1 | ) | — | — | ||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 321,427 | — | 321,427 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (2,343,419 | ) | (2,343,419 | ) | |||||||||||||||||||
| Balance at June 30, 2021 | — | $ | — | 4,960,153 | $ | 50 | $ | 22,906,214 | $ | (15,370,534 | ) | $ | 7,535,730 | |||||||||||||||
See Notes to the Condensed Financial Statements
VIRPAX Pharmaceuticals, Inc.
CONDENSED STATEMENTS OF CASH FLOWS
(UNAUDITED)
For the Six June 30, | For the Six Months Ended June 30, 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (4,722,690 | ) | $ | (1,926,279 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Non-cash interest expense | 64,748 | 83,891 | ||||||
| Stock-based compensation | 691,311 | 702,681 | ||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | - | 2,906 | ||||||
| Change in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (902,649 | ) | 346,940 | |||||
| Deferred financing costs | - | (73,000 | ) | |||||
| Subscription receivable | - | (375,000 | ) | |||||
| Accounts payable and accrued expenses | (8,469 | ) | 647,234 | |||||
| Net cash used in operating activities | (4,877,749 | ) | (590,627 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayment of notes payable | (493,480 | ) | - | |||||
| Proceeds from the issuance of debt | - | 72,100 | ||||||
| Proceeds from related party notes payable | 100,000 | - | ||||||
| Repayment of related party notes payable | (100,000 | ) | ||||||
| Proceeds from the issuance of stock | - | 893,900 | ||||||
| Offering costs related to initial public offering | (2,216,793 | ) | - | |||||
| Proceeds from initial public offering of common stock | 18,000,000 | - | ||||||
| Net cash provided by financing activities | 15,289,727 | 966,000 | ||||||
| Net change in cash | 10,411,978 | 375,373 | ||||||
| Cash, beginning of period | 54,796 | 41,536 | ||||||
| Cash, end of period | $ | 10,466,774 | $ | 416,909 | ||||
| Supplemental disclosure of cash and non-cash financing activities | ||||||||
| Cash paid for interest | $ | 34,707 | $ | — | ||||
| Cash paid for taxes | $ | — | $ | — | ||||
| Debt issued in payment of consulting services and settlement of accounts payable | $ | — | $ | 169,740 | ||||
See Notes to the Condensed Financial Statements
VIRPAX Pharmaceuticals, Inc.
NOTES TO CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 1. Business and Liquidity
Business
Virpax Pharmaceuticals, Inc. (“Virpax” or the “Company”) was incorporated on May 12, 2017 in the state of Delaware. Virpax is a company specializing in developing pharmaceutical products for pain management by using new drug delivery systems. Virpax has exclusive global rights to a proprietary patented Topical Spray Film Delivery Technology for acute musculoskeletal pain (“Epoladerm”). Virpax also has exclusive global rights to a proprietary patented injectable “local anesthetic” Liposomal Gel Technology for postoperative pain management (“Probudur”). Additionally, Virpax has exclusive global rights to a proprietary patented Nanomerics’ Molecular Envelope Technology (“MET”) that uses an intranasal device to deliver enkephalin for the management of acute and chronic pain, including pain associated with cancer (“NES100”). NES100 would support the current effort among prescribers, regulators, and patients to seek non-opioid and non-addictive treatment options to combat the opioid epidemic. Virpax will utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and new chemical entity (“NCE”) candidates for commercialization. While the Company is currently focused on the development of its non-opioid and non-addictive pain management pipeline of product candidates, the Company also plans on using its proprietary delivery technologies to develop anti-viral therapies (“MMS019”) as an anti-viral barrier to potentially prevent or reduce the risk or the intensity of viral infections in humans, including, but not limited to, influenza and SARS-CoV-2 (COVID 19).
The Company, since inception, has been engaged in organizational activities, including raising capital and research and development activities. The Company has not generated revenues and has not yet achieved profitable operations, nor has it ever generated positive cash flow from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. The Company is subject to those risks associated with any preclinical stage pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company’s research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants. Further, the Company’s future operations are dependent on the success of the Company’s efforts to raise additional capital.
The Company incurred a net loss of $4,722,690 and $1,926,279 for the six months ended June 30, 2021 and 2020, respectively, and had an accumulated deficit of $15,370,534 as of June 30, 2021. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenue from its product candidates currently in development. The Company’s primary source of capital has been the issuance of debt and equity securities.
On February 16, 2021, the Company announced the pricing of its initial public offering (the “IPO”) of 1,800,000 shares of its common stock at an initial offering price of $10.00 per share. The Company’s common stock commenced trading on the NASDAQ on February 17, 2021 under the ticker symbol “VRPX”. The IPO closed on February 19, 2021. The gross proceeds from the IPO were $18.0 million. The net proceeds of the IPO were approximately $15.8 million after deducting underwriting discounts, commissions and offering expenses payable by the Company, including offering costs paid and offering costs accrued and unpaid as of December 31, 2020. In conjunction with the IPO, the Company granted the underwriters warrants to purchase 90,000 shares of Company common stock at an exercise price of $12.50 per share, which was 125% of the initial public offering price.
In addition, with respect to the ongoing and evolving coronavirus (“COVID-19”) outbreak, which was designated as a pandemic by the World Health Organization on March 11, 2020, the outbreak has caused substantial disruption in international and U.S. economies and markets and if repercussions of the outbreak are prolonged, could have a significant adverse impact on the Company’s business.
Management believes that current cash is sufficient to fund operations and capital requirements through the third quarter 2022. Additional financings will be needed by the Company to fund its operations, to complete clinical development of and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms. The Company also has the ability to curtail spending in research and development activities in order to conserve cash.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation — The interim condensed financial statements included herein are unaudited. In the opinion of management, these statements include all adjustments, consisting only of normal, recurring adjustments, necessary for a fair presentation of the financial position of Virpax at June 30, 2021, and its results of operations and its cash flows for the three and six months ended June 30, 2021 and 2020. The interim results of operations are not necessarily indicative of the results to be expected for a full year. These interim unaudited financial statements should be read in conjunction with the audited financial statements for the years ended December 31, 2020 and 2019 and notes thereto. The accompanying financial statements have been prepared in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification (“ASC”) of the Financial Accounting Standards Board (“FASB”). Certain information and note disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been omitted pursuant to such rules and regulations of the Securities and Exchange Commission (“SEC”) relating to interim financial statements. The December 31, 2020 balance sheet information was derived from the audited financial statements as of that date.
Use of Estimates — The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, including disclosure of contingent assets and liabilities, at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Due to the uncertainty of factors surrounding the estimates or judgments used in the preparation of the financial statements, actual results may materially vary from these estimates.
Significant items subject to such estimates and assumptions include research and development accruals and the valuation of stock-based compensation. Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
Basic and Diluted Loss per Share — Basic net loss per share is determined using the weighted average number of shares of common stock outstanding during each period. Diluted net income per share includes the effect, if any, from the potential exercise or conversion of securities, such as stock options and warrants, which would result in the issuance of incremental shares of common stock. The computation of diluted net loss per shares does not include the conversion of securities that would have an antidilutive effect. Equivalent common shares excluded from the calculation of diluted net loss per share since their effect is antidilutive due to the net loss of the Company consisted of the following:
Three Months Ended June 30, 2021 | Three Months Ended June 30, 2020 | Six Months | Six Months Ended June 30, 2020 | |||||||||||||
| Equivalent common shares | ||||||||||||||||
| Stock options | 736,593 | 486,101 | 736,593 | 486,101 | ||||||||||||
| Warrants | 95,056 | — | 95,056 | — | ||||||||||||
| RRD note conversion | — | 43,909 | — | 43,909 | ||||||||||||
Cash — At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation. Total cash was $10,466,774 and $54,796 as of June 30, 2021 and December 31, 2020, respectively.
Fair Value of Financial Instruments — The carrying amounts of the Company’s financial instruments, including cash and accounts payable approximate fair value due to the short-term nature of those instruments.
Research and Development — Research and development costs are expensed as incurred. These expenses include the costs of proprietary efforts, as well as costs incurred in connection with certain licensing arrangements and external research and development expenses incurred under arrangements with third parties, such as contract research organizations (“CROs”) and consultants. At the end of each reporting period, the Company compares the payments made to each service provider to the estimated progress towards completion of the related project. Factors that the Company considers in preparing these estimates include the number of patients enrolled in studies, milestones achieved, and other criteria related to the efforts of its vendors. These estimates will be subject to change as additional information becomes available.
Stock-based Compensation — Stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which is generally the vesting period. The Company’s policy permits the valuation of stock-based awards granted to non-employees to be measured at fair value at the grant date rather than on an accelerated attribution basis over the vesting period.
Determining the appropriate fair value of share-based awards requires the use of subjective assumptions, including the fair value of the Company’s common shares, and for options, the expected life of the option and expected share price volatility. The Company uses the Black-Scholes option pricing model to value its option awards. The assumptions used in calculating the fair value of share-based awards represents management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different assumptions, share-based compensation expense could be materially different for future awards.
The expected life of options was estimated using the simplified method, as the Company has historical information to develop reasonable expectations about future exercise patterns and post-vesting employment.
Income Taxes — The Company accounts for income taxes using the asset-and-liability method in accordance with ASC 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rate is recognized in the period that includes the enactment date. A valuation allowance is recorded if it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized in future periods.
The Company follows the guidance in ASC Topic 740-10 in assessing uncertain tax positions. The standard applies to all tax positions and clarifies the recognition of tax benefits in the financial statements by providing for a two-step approach of recognition and measurement. The first step involves assessing whether the tax position is more-likely-than-not to be sustained upon examination based upon its technical merits. The second step involves measurement of the amount to be recognized. Tax positions that meet the more-likely than-not threshold are measured at the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate finalization with the taxing authority. The Company recognizes the impact of an uncertain income tax position in the financial statements if it believes that the position is more likely than not to be sustained by the relevant taxing authority. The Company will recognize interest and penalties related to tax positions in income tax expense. As of June 30, 2021, the Company had no uncertain income tax positions.
Note 3. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consists of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Prepaid insurance | $ | 703,316 | $ | 8,257 | ||||
| Prepaid research and development | 200,000 | - | ||||||
| Legal retainer | 3,050 | 3,643 | ||||||
| Consulting fees | 8,825 | 5,838 | ||||||
| Other prepaid expenses and current assets | 5,731 | 534 | ||||||
| $ | 920,922 | $ | 18,273 | |||||
Note 4. Accounts Payable and Accrued Liabilities
Accounts payable and accrued liabilities consists of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Deferred CEO compensation | $ | 1,073,075 | $ | 1,028,110 | ||||
| Research and development expenses | 69,605 | 1,045,503 | ||||||
| Insurance premiums | 448,789 | 2,874 | ||||||
| Legal expenses | 768,811 | 437,471 | ||||||
| Professional fees | 47,748 | 253,462 | ||||||
| Interest payable/accrued | 291,406 | 270,000 | ||||||
| Accounting consulting fees | 6,235 | 45,904 | ||||||
| Tax expenses | 10,000 | 13,365 | ||||||
| Other | 64,197 | 19,235 | ||||||
| $ | 2,779,866 | $ | 3,115,924 | |||||
Note 5. Notes Payable
On October 1, 2018, the Company entered into a promissory note (the “2018 Promissory Note”), which promises to pay Anthony Mack, Chief Executive Officer and significant investor, the principal amount of $500,000, and bears interest at a rate of 11.19% per annum. The 2018 Promissory Note states that the principal shall be paid at the earlier of an amendment toevent of default (as defined in the 2018 Promissory Note which extended the maturity date fromNote) and the first anniversary of the date of the 2018 Promissory Note. As of June 30, 2021, the balance on the 2018 Promissory note was $500,000, with accrued interest of $153,862. As of December 31, 2020, the balance on the 2018 Promissory Note was $500,000, with accrued interest of $125,887.
On January 15, 2019, the Company entered into a promissory note (the “2019 Promissory Note”), which promises to October 1, 2020 with all other terms remaining consistent.
pay Anthony Mack the principal amount of $500,000, and bears interest at a rate of 11.19% per annum. The 2019 Promissory Note states that the principal shall be paid at the earlier of an event of default (as defined in the 2019 Promissory Note) and the first anniversary of the date of the 2019 Promissory Note. On April 6, 2020, Virpaxthe Company and Anthony Mack entered into an amendment to the 2019 Promissory Note which extended the maturity date from the first anniversary of the date of the 2019 Promissory Note to January 15, 2021, with all other terms remaining consistent. As of June 30, 2021, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $137,544. As of December 31, 2020, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $109,569.
On October 28, 2020, the Company amended its 2018 Promissory Note and its 2019 Promissory Note with Anthony Mack to extend the maturity date to December 31, 2023 for both promissory notes. All other terms and conditions of these promissory notes remained unchanged.
In January 2021, the Company issued notes with an aggregate principal amount of $75,000 and $25,000, respectively. These notes were issued to Anthony Mack for $75,000 and Christopher Chipman, Chief Financial Officer, for $25,000. These notes bore interest as a rate of 1.35% per annum and were subsequently repaid with proceeds from the IPO, including accrued interest of $122 and $41, respectively.
On March 20, 2020, Virpax and RRDAugust 29, 2019, the Company entered into an amendment to thea service provider convertible note purchase agreement (the “RRD Note”) with RRD International, LLC (“RRD”). Under the RRD Note, the Company and RRD agreed to make certain compensation due to RRD payable in the form of a convertible promissory note. The RRD Note stated that a maximum principal balance of $400,000 could be applied for services provided by RRD to the Company, which extendedcould be converted into equity or cash (all or in part) upon a Qualified Financing (as defined in the RRD Note) or the Conversion Date of March 31, 2020. Borrowings under the RRD Note bore simple interest on the outstanding principal amount of the RRD Note until paid in full at the fixed rate of 10% per annum. During 2020, the RRD Note was amended to increase the maximum principal to $600,000 and to extend the maturity date from Marchand conversion dates through to January 31, 2021. As of December 31, 2020, the balance on the RRD Note was $493,480, with accrued interest of $34,544.
In February 2021, the Company paid the balance on its RRD Note of $528,024, including $34,544 of accrued interest, with proceeds from the Company’s IPO.
On May 4, 2020, the Company entered into a Promissory Note (the “PPP Note”) with PNC Bank as the lender (the “Lender”), pursuant to September 30, 2020, increasedwhich the Lender agreed to make a loan to the Company under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”). The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to the Company’s full time headcount during the period ending October 31, 2020. The interest rate on the PPP Note is a fixed rate of 1% per annum. To the extent that the amounts owed under the PPP Loan, or a portion of them, are not forgiven, the note shall convert to an amortizing term loan and the Company will be required to make principal and interest payments in monthly installments beginning seven months from $400,000April 2020. The PPP Note matures in two years. The PPP Note includes events of default. Upon the occurrence of an event of default, the Lender will have the right to $600,000, extendedexercise remedies against us, including the Qualified Financing deadline from March 31, 2020right to September 30, 2020, and provided for therequire immediate payment of all interest accrued upamounts due under the PPP Note. On July 2, 2021, the SBA notified the Company that the forgiveness amount totaled $61,816. The remaining balance of $10,284 must be repaid by the Company on or before maturity.
The following table summarizes the Company’s notes payables:
| June 30, 2021 | ||||||||||||||||
| Balance as of January 1, 2021 | Notes Issued | Note Payments | Balance as of June 30, 2021 | |||||||||||||
| Related party notes payable | ||||||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | — | $ | — | $ | 500,000 | ||||||||
| Anthony Mack 2019 Promissory Note | 500,000 | — | — | 500,000 | ||||||||||||
| Related party notes payable | — | 100,000 | (100,000 | ) | — | |||||||||||
| Total related party notes payable | 1,000,000 | 100,000 | (100,000 | ) | 1,000,000 | |||||||||||
| RRD Note | 493,480 | — | (493,480 | ) | — | |||||||||||
| SBA PPP Loan | 72,100 | — | — | 72,100 | ||||||||||||
| Total notes payable | 1,565,580 | 100,000 | (593,480 | ) | 1,072,100 | |||||||||||
| Less: Current portion of notes payable | 543,990 | 100,000 | (593,480 | ) | 50,510 | |||||||||||
| Total non-current portion of notes payable | $ | 1,021,590 | $ | — | $ | — | $ | 1,021,590 | ||||||||
Interest expense was $64,748 and $83,891 for the six months ended June 30, 2021 and 2020, respectively. Interest expense was $34,049 and $43,261 for the three months ended June 30, 2021 and 2020, respectively.
Principal payments on note payables are due as follows:
| Years ending December 31, | ||||
| 2021 | $ | 50,510 | ||
| 2022 | 21,590 | |||
| 2023 | 1,000,000 | |||
| Total payments | $ | 1,072,100 | ||
Note 6. Commitments and Contingencies
Employment Agreements
The Company has an employment agreement with Anthony Mack, the Company’s Chief Executive Officer (“CEO”), effective September 18, 2018. The agreement may be terminated by either party at any time upon written notice provided to Marchthe other party. Concurrent with the employment agreement, the CEO and the Company agreed to an Executive Confidentiality Agreement that contains standard non-closure and non-competition provisions. In the event we terminate the employment agreement other than for cause, or the CEO terminates the agreement for good reason, we will pay the CEO the then effective base salary for a period of twelve months following the effective date of the termination. However, payment of the effective base salary is subject to the execution of a release form and the compliance by the CEO with the release and all terms and provisions of the employment agreement and Executive Confidentiality Agreement that survive the termination of employment. The Company’s Chief Executive Officer also elected to defer salary temporarily. Deferred compensation due to the Company’s Chief Executive Officer amounted to $1,052,000 and $1,005,000 as of June 30, 2021 and December 31, 2020, respectively, which totaled $16,435.is included in accounts payable and accrued expenses on the accompanying condensed balance sheets. In March 2021, the Company’s Chief Executive Officer ceased deferring compensation.
Litigation
From time to time the Company is subject to claims by third parties under various legal disputes. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows.
On March 12, 2021, the Company and Mr. Mack (the “defendants”) were named as defendants in a complaint (the “Complaint”) filed by Sorrento Therapeutics, Inc. (“Sorrento”) and Scilex Pharmaceuticals Inc. (“Scilex” and together with Sorrento, the “Plaintiffs”) in the Court of Chancery of the State of Delaware. In the Complaint the Plaintiffs allege (i) Mr. Mack breached a Restrictive Covenants Agreement, dated as of November 8, 2016, between himself and Sorrento (the “Restrictive Covenants Agreement”), (ii) the Company tortiously interfered with the Restrictive Covenants Agreement, and (iii) the Company tortiously interfered with Scilex’s relationship with Mr. Mack. On May 7, 2021 Plaintiffs filed an Amended Complaint (the “Amended Complaint”) asserting the same three causes of action. Simultaneously with filing the original Complaint, the Plaintiffs filed a motion to expedite, seeking a hearing in four weeks on their prospective motion for a preliminary injunction, prohibiting Mr. Mack’s alleged violations of the Restrictive Covenants Agreement and prohibiting the alleged tortious interference. On March 22, 2021, Vice Chancellor Paul A. Fioravanti Jr. issued a scheduling order, setting forth terms which had been agreed to by the parties, under which oral argument on Plaintiffs’ proposed motion for preliminary injunction was scheduled for July 15, 2021. On May 17, 2021, Mr. Mack and the Company filed a motion to dismiss the Amended Complaint. On May 18, 2021, the Vice Chancellor issued a revised scheduling order, also setting forth terms which had been agreed to by the parties, under which a briefing schedule was set with respect to Defendants’ motion to dismiss, Plaintiffs agreed not to move for a preliminary injunction, and a trial in the action was set for February 9-11, 2022. As of July 12, 2021, the parties completed briefing on the motion to dismiss the Amended Complaint. The Court has not yet scheduled a date for oral argument on the motion to dismiss. The Company believes that the allegations in the Amended Complaint are wholly without merit, and the Company intends to vigorously defend the action. However, the Company is unable to predict the ultimate outcome of the lawsuit at this time.
Global Pandemic Outbreak
In March 2020, the World Health Organization declared the outbreak of a novel coronavirus (“COVID-19”) a global pandemic. The outbreak has become increasingly widespread in the United States, impacting the markets in which the Company operates. While the full impact of the pandemic continues to evolve, the financial markets have been subject to significant volatility that adversely impacts the Company’s ability to enter into, modify, and negotiate favorable terms and conditions relative to equity and debt financing initiatives. The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values also affect the Company’s ability to enter into collaborations, joint ventures, and license and royalty agreements. The outbreak and government measures taken in response to the pandemic have also had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, have spiked, while demand for other goods and services, such as travel, have fallen. The future progression of the outbreak and its effects on our business and operations are uncertain. We may face difficulties recruiting or retaining patients in our ongoing and planned clinical trials if patients are affected by the virus or are fearful of traveling to our clinical trial sites because of the outbreak. We and our third-party contract manufacturers, contract research organizations, and clinical sites may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak.
While expected to be temporary, these disruptions may negatively impact the Company’s sales, its results of operations, financial condition, and liquidity in 2020.
Paycheck Protection Program Loan (“PPP Loan”)
On May 4, 2020, the Company entered into a Promissory Note (the “PPP Note”) with PNC Bank as the lender (the “Lender”), pursuant to which the Lender agreed to make a loan to us under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”).
The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to the Company’s full time headcount during the period ending October 31, 2020.
The interest rate on the PPP Note is a fixed rate of 1% per annum. To the extent that the amounts owed under the PPP Loan, or a portion of them, are not forgiven, we will be required to make principal and interest payments in monthly installments beginning seven months from April 2020. The PPP Note matures in two years.
The PPP Note includes events of default. Upon the occurrence of an event of default, the Lender will have the right to exercise remedies against us, including the right to require immediate payment of all amounts due under the PPP Note.
F-18
CONDENSED BALANCE SHEETS
(UNAUDITED)
| June 30, 2020 | December 31, 2019 | |||||||
| (Unaudited) | * | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 416,909 | $ | 41,536 | ||||
| Subscription receivable | 375,000 | - | ||||||
| Prepaid expenses and other current assets | 33,243 | 5,183 | ||||||
| Total current assets | 825,152 | 46,719 | ||||||
| Deferred financing costs | 73,000 | - | ||||||
| Total assets | $ | 898,152 | $ | 46,719 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 2,075,859 | $ | 1,514,474 | ||||
| Notes payable | 463,663 | 264,520 | ||||||
| Related party notes payable | 1,000,000 | 1,000,000 | ||||||
| Total current liabilities | 3,539,522 | 2,778,994 | ||||||
| Notes payable, net of current portion | 42,697 | - | ||||||
| Total liabilities | 3,582,219 | 2,778,994 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred stock, par value $0.00001, 1,000,000 shares authorized, -0- shares issued and outstanding as of both June 30, 2020 and December 31, 2019 | - | - | ||||||
| Common stock, $0.00001 par value; 20,000,000 shares authorized, 15,495,436 shares issued and outstanding as of June 30, 2020; 20,000,000 shares authorized, 14,925,158 shares issued and outstanding as of December 31, 2019 | 155 | 149 | ||||||
| Additional paid-in capital | 5,550,248 | 3,575,767 | ||||||
| Accumulated deficit | (8,234,470 | ) | (6,308,191 | ) | ||||
| Total stockholders’ deficit | (2,684,067 | ) | (2,732,275 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 898,152 | $ | 46,719 | ||||
See Notes to the Condensed Financial Statements
CONDENSED STATEMENTS OF OPERATIONS
(UNAUDITED)
| For the Six Months Ended June 30, 2020 | For the Six Months Ended June 30, 2019 | |||||||
| OPERATING EXPENSES | ||||||||
| General and administrative | $ | 1,510,835 | $ | 1,364,326 | ||||
| Research and development | 335,553 | 154,559 | ||||||
| Total operating expenses | 1,846,388 | 1,518,885 | ||||||
| Loss from operations | (1,846,388 | ) | (1,518,885 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense | (83,891 | ) | (55,637 | ) | ||||
| Other income | 4,000 | - | ||||||
| Other expense | (79,891 | ) | (55,637 | ) | ||||
| Loss before tax provision | (1,926,279 | ) | (1,574,522 | ) | ||||
| Benefit from income taxes | - | - | ||||||
| Net loss | $ | (1,926,279 | ) | $ | (1,574,522 | ) | ||
| Basic and diluted net loss per share | $ | (0.13 | ) | $ | (0.11 | ) | ||
| Basic and diluted weighted average common stock outstanding | 15,152,543 | 14,275,073 | ||||||
See Notes to the Condensed Financial Statements
STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
(UNAUDITED)
| Preferred stock | Common stock | Additional paid-in | Accumulated | Total stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | deficit | deficit | ||||||||||||||||||||||
| Balance at January 1, 2020 | - | $ | - | 14,925,158 | $ | 149 | $ | 3,575,767 | $ | (6,308,191 | ) | $ | (2,732,275 | ) | ||||||||||||||
| Common stock issued pursuant to subscription agreements | - | - | 634,450 | 6 | 1,268,894 | - | 1,268,900 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | - | - | 1,453 | - | 2,906 | - | 2,906 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 702,681 | - | 702,681 | |||||||||||||||||||||
| Restricted stock awards granted | - | - | 34,375 | - | - | - | - | |||||||||||||||||||||
| Restricted stock awards forfeited | - | - | (100,000 | ) | - | - | - | - | ||||||||||||||||||||
| Net loss | - | - | - | - | - | (1,926,279 | ) | (1,926,279 | ) | |||||||||||||||||||
| Balance at June 30, 2020 | - | $ | - | 15,495,436 | $ | 155 | $ | 5,550,248 | $ | (8,234,470 | ) | $ | (2,684,067 | ) | ||||||||||||||
| Balance at January 1, 2019 | - | $ | - | 14,212,000 | 142 | 1,808,813 | (3,001,679 | ) | (1,192,724 | ) | ||||||||||||||||||
| Common stock issued pursuant to subscription agreements | - | - | 62,500 | - | 125,000 | - | 125,000 | |||||||||||||||||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | - | - | 120,000 | 1 | 239,999 | - | 240,000 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 424,615 | - | 424,615 | |||||||||||||||||||||
| Restricted stock awards granted | - | - | 6,250 | - | - | - | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | (1,574,522 | ) | (1,574,522 | ) | |||||||||||||||||||
| Balance at June 30, 2019 | - | $ | - | 14,400,750 | $ | 143 | $ | 2,598,427 | $ | (4,576,201 | ) | $ | (1,977,631 | ) | ||||||||||||||
See Notes to the Condensed Financial Statements
CONDENSED STATEMENTS OF CASH FLOWS
(UNAUDITED)
For the Six Months Ended June 30, | For the Six Months Ended June 30, | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (1,926,279 | ) | $ | (1,574,522 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Non-cash interest expense | 83,891 | 55,637 | ||||||
| Stock-based compensation | 702,681 | 424,615 | ||||||
| Common stock issued in payment of consulting services and settlement of accounts payable | - | 216,184 | ||||||
| Change in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | 346,940 | (12,878 | ) | |||||
| Subscription receivable | (375,000 | ) | - | |||||
| Deferred financing costs | (73,000 | ) | - | |||||
| Accounts payable and accrued expenses | 650,140 | 303,770 | ||||||
| Due from related party, net | - | (508 | ) | |||||
| Net cash used in operating activities | (590,627 | ) | (587,702 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of debt | 72,100 | 500,000 | ||||||
| Proceeds from the issuance of stock | 893,900 | 125,000 | ||||||
| Net cash provided by financing activities | 966,000 | 625,000 | ||||||
| Net increase in cash | 375,373 | 37,298 | ||||||
| Cash, beginning of period | 41,536 | 48,370 | ||||||
| Cash, end of period | $ | 416,909 | $ | 85,668 | ||||
| Supplemental disclosure of cash and non-cash financing activities | ||||||||
| Cash paid for interest | $ | - | $ | - | ||||
| Cash paid for taxes | $ | - | $ | - | ||||
| Debt issued in payment of consulting services and settlement of accounts payable | $ | 169,740 | $ | - | ||||
| Common stock issued in payment of consulting services and settlement of accounts payable | $ | 2,906 | $ | 23,816 | ||||
| Common stock subscription receivable | $ | 375,000 | $ | - | ||||
See Notes to the Condensed Financial Statements
NOTES TO CONDENSED FINANCIAL STATEMENTS
(UNAUDITED)
Note 1. Business and Liquidity
Business
Virpax Pharmaceuticals, Inc. (“Virpax” or “Company”) was incorporated on May 12, 2017 in the state of Delaware. Virpax is a company specializing in developing pharmaceutical products for pain management by using new drug delivery systems. Virpax has exclusive global rights to a proprietary patented Topical Spray Film Delivery Technology for acute musculoskeletal pain (“Epoladerm”). Virpax also has exclusive global rights to a proprietary patented injectable “local anesthetic” Liposomal Gel Technology for postoperative pain management (“Probudur”). Additionally, Virpax has exclusive global rights to a proprietary patented Nanomerics’ Molecular Envelope Technology (“MET”) that uses an intranasal device to deliver enkephalin for the management of acute and chronic pain (“NES100”). NES100 would support the current effort among prescribers, regulators, and patients to seek non-opioid and non-addictive treatment options to combat the opioid epidemic. Virpax will utilize these delivery technologies to selectively develop a portfolio of patented 505(b)(2) and new chemical entity (“NCE”) candidates for commercialization.
Liquidity and Going Concern
The Company, since inception, has been engaged in organizational activities, including raising capital and research and development activities. The Company has not generated revenues and has not yet achieved profitable operations, nor has it ever generated positive cash flow from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. The Company is subject to those risks associated with any preclinical stage pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company’s research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants. Further, the Company’s future operations are dependent on the success of the Company’s efforts to raise additional capital.
These uncertainties raise substantial doubt about the Company’s ability to continue as a going concern for 12 months after the issuance date of these financial statements. The accompanying financial statements have been prepared on a going concern basis which contemplates the continuation of operations, realization of assets and liquidation of liabilities in the ordinary course of business. The Company incurred a net loss of $1,926,279 and $1,574,522 for the six months ended June 30, 2020 and 2019, respectively, and had an accumulated deficit of $8,234,470 as of June 30, 2020. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenue from its product candidates currently in development. The Company’s primary source of capital has been the issuance of debt and equity securities. In addition, with respect to the ongoing and evolving coronavirus (“COVID-19”) outbreak, which was designated as a pandemic by the World Health Organization on March 11, 2020, the outbreak has caused substantial disruption in international and U.S. economies and markets and if repercussions of the outbreak are prolonged, could have a significant adverse impact on the Company’s business. Management believes that current cash is sufficient to fund operations and capital requirements through November 2020. Additional financings will be needed by the Company to fund its operations, to complete clinical development of and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation of Interim Unaudited Condensed Financial Statements — The interim condensed financial statements included herein are unaudited. In the opinion of management, these statements include all adjustments, consisting only of normal, recurring adjustments, necessary for a fair presentation of the financial position of Virpax at June 30, 2020, and its results of operations and its cash flows for the six months ended June 30, 2020 and 2019. The interim results of operations are not necessarily indicative of the results to be expected for a full year. These interim unaudited financial statements should be read in conjunction with the audited financial statements for the years ended December 31, 2019 and 2018 and notes thereto. The accompanying financial statements have been prepared in conformity with U.S. generally accepted accounting principles (U.S. GAAP). Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification (ASC) of the Financial Accounting Standards Board (FASB). Certain information and note disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been omitted pursuant to such rules and regulations of the SEC relating to interim financial statements. The December 31, 2019 balance sheet information was derived from the audited financial statements as of that date.
Use of Estimates — The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, including disclosure of contingent assets and liabilities, at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Due to the uncertainty of factors surrounding the estimates or judgments used in the preparation of the financial statements, actual results may materially vary from these estimates.
Significant items subject to such estimates and assumptions include research and development accruals and the valuation of stock-based compensation. Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
Basic and Diluted Loss per Share — Basic net loss per share is determined using the weighted average number of shares of common stock outstanding during each period. Diluted net income per share includes the effect, if any, from the potential exercise or conversion of securities, such as stock options, which would result in the issuance of incremental shares of common stock. The computation of diluted net loss per shares does not include the conversion of securities that would have an antidilutive effect. Equivalent common shares, consisting of 2,403,503 and 1,044,167 stock options, and 217,130 and no common shares related to the conversion of the RRD Note, are excluded from the calculation of diluted net loss per share for the six months ended June 30, 2020 and 2019, respectively, since their effect is antidilutive due to the net loss of the Company.
Cash — At times, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation (“FDIC”). There were no accounts that exceeded federally insured limits as of June 30, 2020 or December 31, 2019.
Fair Value of Financial Instruments — The carrying amounts of the Company’s financial instruments, including cash, subscription receivable and prepaids and other current assets, notes payable, and accounts payable approximate fair value due to the short-term nature of those instruments.
Research and Development — Research and development costs are expensed as incurred. These expenses include the costs of proprietary efforts, as well as costs incurred in connection with certain licensing arrangements and external research and development expenses incurred under arrangements with third parties, such as contract research organizations (“CROs”) and consultants. At the end of each reporting period, the Company compares the payments made to each service provider to the estimated progress towards completion of the related project. Factors that the Company considers in preparing these estimates include the number of patients enrolled in studies, milestones achieved, and other criteria related to the efforts of its vendors. These estimates will be subject to change as additional information becomes available.
Stock-based Compensation — Stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which is generally the vesting period.
Determining the appropriate fair value of share-based awards requires the use of subjective assumptions, including the fair value of the Company’s common shares, and for options, the excepted life of the option and expected share price volatility. The Company using the Black-Scholes option pricing model to value its option awards. The assumptions used in calculating the fair value of share-based awards represents management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different assumptions, share-based compensation expense could be materially different for future awards.
The expected life of options was estimated using the simplified method, as the Company does not have historical information to develop reasonable expectations about future exercise patterns and post-vesting employment.
Income Taxes — The Company accounts for income taxes using the asset-and-liability method in accordance with ASC 740, Income Taxes (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rate is recognized in the period that includes the enactment date. A valuation allowance is recorded if it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized in future periods.
The Company follows the guidance in ASC Topic 740-10 in assessing uncertain tax positions. The standard applies to all tax positions and clarifies the recognition of tax benefits in the financial statements by providing for a two-step approach of recognition and measurement. The first step involves assessing whether the tax position is more-likely-than-not to be sustained upon examination based upon its technical merits. The second step involves measurement of the amount to be recognized. Tax positions that meet the more-likely than-not threshold are measured at the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate finalization with the taxing authority. The Company recognizes the impact of an uncertain income tax position in the financial statements if it believes that the position is more likely than not to be sustained by the relevant taxing authority. The Company will recognize interest and penalties related to tax positions in income tax expense. As of June 30, 2020, the Company had no uncertain income tax positions.
Recent Accounting Pronouncements
In February 2016, the FASB issued its final standard on lease accounting, ASU No. 2016-02, as amended Leases (Topic 842), which superseded Topic 840. The new pronouncement requires the recognition on the balance sheet of right-of-use assets and lease liabilities for all long-term leases, including operating leases, on the balance sheet. The pronouncement requires that lease arrangements longer than 12 months result in an entity classifying leases as finance or operating leases. However, unlike current U.S. GAAP, which requires only capital leases to be recognized on the balance sheet, Topic 842 will require both types of leases to be recognized on the balance sheet. Topic 842 also requires disclosures about the amount, timing, and uncertainty of cash flows arising from leases. These disclosures include qualitative and quantitative requirements, providing additional information about the amounts recorded in the financial statements. The effective date of Topic 842 for non-public business entities, including smaller reporting companies, to fiscal years beginning after December 15, 2021. The Company intends to adopt the new guidance as of January 1, 2022. The adoption of this standard is not expected to have any material impact on the financial statements and related disclosures.
In August 2020, the FASB issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. This ASU amends the guidance on convertible instruments and the derivatives scope exception for contracts in an entity’s own equity, and also improves and amends the related EPS guidance for both Subtopics. The ASU will be effective for annual reporting periods after December 15, 2021 and interim periods within those annual periods and early adoption is permitted. We are assessing the impact of ASU 2020-06 on our financial statements.
Note 3. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following:
| June 30, 2020 | December 31, 2019 | |||||||
| Prepaid insurance | $ | 10,587 | $ | 3,328 | ||||
| Governmental affairs consulting | 7,500 | - | ||||||
| Consulting fees | 13,040 | - | ||||||
| Accounts receivable, related party | - | 1,388 | ||||||
| Other prepaid expenses and current assets | 2,116 | 467 | ||||||
| $ | 33,243 | $ | 5,183 | |||||
potentially beyond.
F-25
Note 4. Accounts Payable and Accrued Liabilities
Accounts payable and accrued liabilities consisted of the following:
| June 30, 2020 | December 31, 2019 | |||||||
| Accrued payroll | $ | 6,073 | $ | - | ||||
| Deferred compensation | 849,304 | 680,400 | ||||||
| Accrued severance | 77,007 | - | ||||||
| Research and development expenses | 309,541 | 275,429 | ||||||
| Legal expenses | 116,258 | 200,062 | ||||||
| Professional fees | 336,732 | 177,096 | ||||||
| Interest payable | 206,159 | 138,762 | ||||||
| Accounting consulting fees | 94,860 | 24,765 | ||||||
| Audit fees | 26,000 | - | ||||||
| Tax preparation fees | 21,110 | 14,465 | ||||||
| Rent expense | 15,000 | - | ||||||
| Other | 17,815 | 3,495 | ||||||
| $ | 2,075,859 | $ | 1,514,474 | |||||
Note 5. Notes Payable
On October 1, 2018, the Company entered into a promissory note (the “2018 Promissory Note”), which promises to pay Anthony Mack, Chief Executive Officer and significant investor, the principal amount of $500,000, and bear interest at a rate of 11.19% per annum. The 2018 Promissory Note states that the principal shall be paid at the earlier of an event of default and the first anniversary of the date of the note. As of June 30, 2020, the balance on the 2018 Promissory Note was $500,000, with accrued interest of $107,606. As of December 31, 2019, the balance on the 2018 Promissory note was $500,000, with accrued interest of $74,692.
On January 15, 2019, the Company entered into a promissory note (the “2019 Promissory Note”), which promises to pay Anthony Mack the principal amount of $500,000, and bear interest at a rate of 11.19% per annum. The 2019 Promissory Note states that the principal shall be paid at the earlier of an event of default and the first anniversary of the date of the note. On April 6, 2020, VIRPAX and Anthony Mack entered into an amendment to the 2019 Promissory Note which extended the maturity date from the first anniversary of the date of the 2019 Promissory Note to January 15, 2021 with all other terms remaining consistent. As of June 30, 2020, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $88,190. As of December 31, 2019, the balance on the 2019 Promissory Note was $500,000, with accrued interest of $56,329.
On August 29, 2019, the Company entered into a service provider convertible note purchase agreement (the “RRD Note”) with RRD International, LLC (“RRD”). Under this agreement, Virpax and RRD agreed to make certain compensation payable in the form of a convertible promissory note. The convertible promissory note states that a maximum principal balance of $400,000 can be applied for services provided by RRD to Virpax, which can be converted into equity or cash (all or in part) upon a Qualified Financing or the Conversion Date of March 31, 2020. Borrowings under the RRD Note bear simple interest on the outstanding principal amount of the RRD Note until paid in full at the fixed rate of 10% per annum. On March 20, 2020, VIRPAX and RRD entered into an amendment which extended the maturity date from March 31, 2020 to September 30, 2020, increased the amount of principal from $400,000 to $600,000, extended the Qualified Financing deadline from March 31, 2020 to September 30, 2020, and provided for the payment of all interest accrued up to March 31, 2020 which totaled $16,435. As of June 30, 2020, the balance on the RRD Note was $434,260, with accrued interest of $10,363. As of December 31, 2019, the balance on the RRD Note was $264,520, with accrued interest of $7,741.
On May 4, 2020, the Company entered into a Promissory Note (the “PPP Note”) with PNC Bank as the lender (the “Lender”), pursuant to which the Lender agreed to make a loan to us under the Paycheck Protection Program (the “PPP Loan”) offered by the U.S. Small Business Administration (the “SBA”) in a principal amount of $72,100 pursuant to Title 1 of the Coronavirus Aid, Relief and Economic Security Act (the “CARES Act”). The PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. The amount that will be forgiven will be calculated in part with reference to the Company’s full time headcount during the period ending October 31, 2020. The interest rate on the PPP Note is a fixed rate of 1% per annum. To the extent that the amounts owed under the PPP Loan, or a portion of them, are not forgiven, the note shall convert to an amortizing term loan and the Company will be required to make principal and interest payments in monthly installments beginning seven months from April 2020. The PPP Note matures in two years. The PPP Note includes events of default. Upon the occurrence of an event of default, the Lender will have the right to exercise remedies against us, including the right to require immediate payment of all amounts due under the PPP Note.
F-26
The following table summarizes the Company’s notes payables:
| June 30, 2020 | ||||||||||||
Balance as of | Notes issued | Balance as of June 30, 2020 | ||||||||||
| Related party notes payable | ||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | - | $ | 500,000 | ||||||
| Anthony Mack 2019 Promissory Note | 500,000 | - | 500,000 | |||||||||
| Total related party notes payable | 1,000,000 | - | 1,000,000 | |||||||||
| RRD Note | 264,520 | 169,740 | 434,260 | |||||||||
| SBA PPP Loan | - | 72,100 | 72,100 | |||||||||
| Total notes payable | $ | 1,264,520 | $ | 241,840 | $ | 1,506,360 | ||||||
| Less: Current portion of notes payable | 1,264,520 | 199,143 | 1,463,663 | |||||||||
| Total non-current portion of notes payable | $ | - | $ | 42,697 | $ | 42,697 | ||||||
| December 31, 2019 | ||||||||||||
| Balance as of January 1, 2019 | Notes Issued | Balance as of Dec 31, 2019 | ||||||||||
| Related party notes payable | ||||||||||||
| Anthony Mack 2018 Promissory Note | $ | 500,000 | $ | - | $ | 500,000 | ||||||
| Anthony Mack 2019 Promissory Note | - | 500,000 | 500,000 | |||||||||
| Total related party notes payable | 500,000 | 500,000 | 1,000,000 | |||||||||
| RRD Note | - | 264,520 | 264,520 | |||||||||
| Total notes payable | 500,000 | 764,520 | 1,264,520 | |||||||||
| Less: Current portion of notes payable | 500,000 | 764,520 | 1,264,520 | |||||||||
| Total non-current portion of notes payable | $ | - | $ | - | $ | - | ||||||
Interest expense was $83,891 and $55,637 for the six months ended June 30, 2020 and 2019, respectively.
Principal payments on note payables are due as follows:
Years ending December 31,
| 2020 (Remaining six months) | $ | 438,450 | ||
| 2021 | 1,050,552 | |||
| 2022 | 17,358 | |||
| Total payments | $ | 1,506,360 |
Note 6. Commitments and Contingencies
Employment Agreements
The Company has an employment agreement with the Chief Executive Officer, effective September 18, 2018. The agreement may be terminated by either party at any time upon written notice provided to the other party. Concurrent with the employment agreement, the CEO and the Company agreed to an Executive Confidentiality Agreement that contains standard non-closure and non-competition provisions. In the event we terminate the employment agreement other than for cause, or the CEO terminates the agreement for good reason, we will pay the CEO the then effective base salary for a period of twelve months following the effective date of the termination. However, payment of the effective base salary is subject to the execution of a release form and the compliance by the CEO with the release and all terms and provisions of the employment agreement and Executive Confidentiality Agreement that survive the termination of employment. The Company’s Chief Executive Officer has also elected to forego a salary temporarily and defer compensation. Deferred compensation due to the Company’s CEO amounted to $817,500 and $630,000 as of June 30, 2020 and December 31, 2019, respectively, which is included in accounts payable and accrued expenses on the accompanying balance sheet.
F-27
Litigation
The Company is subject, from time to time, to claims by third parties under various legal disputes. The defense of such claims, or any adverse outcome relating to any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows. As of June 30, 2020, the Company did not have any pending legal actions.
Global Pandemic Outbreak
In March 2020, the World Health Organization declared the outbreak of a novel coronavirus (“COVID-19”) a global pandemic. The outbreak has become increasingly widespread in the United States, impacting the markets in which the Company operates. While the full impact of the pandemic continues to evolve, the financial markets have been subject to significant volatility that adversely impacts the Company’s ability to enter into, modify, and negotiate favorable terms and conditions relative to equity and debt financing initiatives. The uncertain financial markets, disruptions in supply chains, mobility restraints, and changing priorities as well as volatile asset values also affect the Company’s ability to enter into collaborations, joint ventures, and license and royalty agreements. The outbreak and government measures taken in response to the pandemic have also had a significant impact, both direct and indirect, on businesses and commerce, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, have spiked, while demand for other goods and services, such as travel, have fallen. The future progression of the outbreak and its effects on our business and operations are uncertain. We may face difficulties recruiting or retaining patients in our ongoing and planned clinical trials if patients are affected by the virus or are fearful of traveling to our clinical trial sites because of the outbreak. We and our third-party contract manufacturers, contract research organizations, and clinical sites may also face disruptions in procuring items that are essential to our research and development activities, including, for example, medical and laboratory supplies used in our clinical trials or preclinical studies, in each case, that are sourced from abroad or for which there are shortages because of ongoing efforts to address the outbreak.
While expected to be temporary, these disruptions may negatively impact the Company’s sales, its results of operations, financial condition, and liquidity in 2020.
Note 7. Stockholders’ Equity
Overview
Preferred Stock
The Company’s Certificate of Incorporation, filed on May 12, 2017, and amended and restated on February 16, 2021, authorizes the issuance of preferred stock. The total number of shares which the Company is authorized to issue is 1,000,000, each10,000,000, with a par value of $0.00001 per share.
Common Stock
The Company’s Certificate of Incorporation, filed on May 12, 2017, and amended and restated on February 16, 2021, authorizes the issuance of common stock. The total number of shares which the Company is authorized to issue is 20,000,000, each100,000,000, with a par value of $0.00001 per share.
During the six monthmonths ended June 30, 2021, the Company issued 1,800,000 shares of common stock related to the Company’s IPO in February 2021, for net proceeds totaling $15,783,207, after deducting underwriting discounts and offering expenses.
During the three and six months ended June 30, 2020, the Company issued 634,45085,325 and 128,303 shares of common stock for gross proceeds totaling $1,268,894. The$425,000 and $1,268,900, respectively. Anthony Mack, the Company’s CEO, and an immediate family member of Mr. Mack purchased 200,00040,450 and 8,393 of these shares of common stock for gross proceeds totaling $400,000.
As of June 30, 2020, we had an outstanding stock subscription receivable of $375,000$400,000 and $83,000, respectively, for the purchase of 187,500 shares of our common stock. We received the funds on July 13, 2020 related to this stock subscription receivable.
During the six months ended June 30, 2019, the Company issued 62,500 shares of common stock for gross proceeds totaling $125,000. The Company’s CEO purchased 50,000 of these shares of common stock for gross proceeds totaling $100,000.2020.
In addition, during the three and six months ended June 30, 2020, the Company issued 1,453113 and 293 shares of common stock were issued in payment of consulting services and settlement of accounts payable totaling $1,125 and $2,906, respectively.
Warrants
In conjunction with the IPO, the Company granted the underwriters warrants to purchase 90,000 shares of the Company’s common stock at an exercise price of $12.50 per share, which is 125% of initial public offering price. The warrants have a five-year term and are not exercisable prior to August 16, 2021. All of the warrants were outstanding at June 30, 2021 and the fair value allocated to the warrants of $639,000 was accounted for gross proceeds totaling $2,906.as a component of stockholders’ equity. The fair value of the warrants was estimated using the Black-Scholes option-pricing model and is affected by the Company’s share fair value as well as assumptions regarding a number of complex and subjective variables, including a term of 5 years, expected price volatility of 100%, and a risk-free interest rate of 0.6%.
Reverse Stock Split
On November 19, 2020, the Company filed an amendment to its Articles of Incorporation and effected 1-for-4.944260256 reverse stock split of its issued 120,000and outstanding shares of common stock, $0.00001 par value, whereby 15,550,627 outstanding shares of the Company’s common stock were exchanged for 3,145,153 newly issued shares of the Company’s common stock. Under the terms of the reverse stock split, fractional shares issuable to stockholders were rounded to the nearest whole share, with cash paid in paymentlieu of consulting servicesany fractional shares, resulting in a reverse split of 1-for-4.944260256. All per share amounts and settlementnumber of accounts payable for gross proceeds totaling $240,000 duringshares (other than authorized shares) in the six months ended June 30, 2019.financial statements and related notes have been retroactively restated to reflect the reverse stock split resulting in the transfer of $119 from common stock to additional paid in capital at January 1, 2020.
Note 8. Stock-Based Compensation
Restricted Stock Awards
On May 20, 2017, the Company established the Virpax Pharmaceuticals, Inc. Amended and Restated 2017 Equity Incentive Plan (the “Plan”). The Company’s Board of Directors (the “Board”), acting through its Equity Incentive Plan Committee, has determined that it would be to the advantage and best interest of the Company and its stockholders to grant restricted stock awards to certain individuals as compensation to serve as an employee of the Company and as an incentive for increased efforts during such service.
As of June 30, 2020, thereThere were 25,00014,723 and 5,506 unvested restricted stock awards issued totaling $83,888 and $50,000 of unearned deferred stock based compensation, based on a fair value of the Company’s common stock on the date of $2.00 per sharegrant, as determined by recent sales of stock to unrelated third parties. As ofJune 30, 2021 and December 31, 2019,2020, respectively. During the three months ended June 30, 2021 and 2020, there were 168,750 unvested15,000 and 3,160 restricted stock awards issued totaling $337,500granted during the period, respectively, and 20,225 of unearned deferredrestricted stock awards forfeited during the three months ended June 30, 2020. The Company recognized $4,688 and $156,250 of stock based compensation.compensation for vested restricted shares during the three months ended June 30, 2021 and June 30, 2020, respectively. In addition, during the six months ended June 30, 2021 and 2020, there were 34,37515,000 and 6,952 restricted stock awards granted during the period, respectively, and 100,00020,225 of restricted stock awards forfeited during the period. During the six months ended June 30, 2019, there were 6,2502020. The Company recognized $34,629 and $156,250 of stock based compensation for vested restricted stock awards granted during the period. Also,shares during the six months ended June 30, 2021 and June 30, 2020, there were 78,125 restricted stock awards that vested resulting in $156,250 in stock-based compensation expense during the period.respectively.
Stock Options
Note 8. Stock-Based Compensation
The Company’s Plan provides a means wherebyfor eligible employees, officers, non-employee directors and other individual service providers (collectively, “eligible persons”) to develop a sense of proprietorship and personal involvement in the development and financial success of the Company and to encourage them to devote their best efforts to the business of the Company, thereby advancing the interests of the Company and its stockholders. The Company, by means of the Plan, seeks to retain the services of such eligible persons and to provide incentives for such eligible persons to exert maximum efforts for the success of the Company. The Plan commenced on the Effective DateMay 20, 2017 (the “Effective Date”) and the Plan is administered by the Compensation Committee (“of the Board (the “Compensation Committee”); provided that the entire Board may act in lieu of the Compensation Committee on any matter. The maximum aggregate number of shares of common stock which may be issued under all Awardsawards granted to Participantsparticipants under the Plan initially shall be 1,500,000303,382 shares. The number of authorized shares available for issuance under the Plan shall automatically increase on January 1st1st of each year commencing with theon January 1 following the Effective Date and on each January 1 thereafter until the expiration date, in an amount equal to six percent (6%) of the total number of shares of common stock outstanding on December 31st31st of the preceding calendar year. The Plan shall remain in effect, subject to the right of the board of directors of the CompanyBoard to amend or terminate the Plan at any time until the earlier of the tenth (10th)(10th) anniversary of the Effective Date. In the event of a termination of continuous service (other than as a result of a change of control, as defined in the Plan), unvested stock options generally shall terminate and, with regard to vested stock options, the exercise period shall be the lesser of the original expiration date or three months from the date continuous service terminates.
On May 21, 2018,April 25, 2020, the Company amended and restated the Plan to grant stock options to Non-Employee Directors.non-employee directors. Stock options to purchase 25,00020,225 shares of common stock shall automatically be granted under the Plan to each Non-Employee Directornon-employee director who is first appointed or elected to the Board. In addition, On January 1 of each year, each then serving Non-Employee Directornon-employee director of the Company shall automatically be granted under the Plan (i) that number of options having a value of $25,000 calculated on the grant date in accordance with the Black-Scholes option pricing model and shall be exercisable as to 100% of the number of shares of Stockcommon stock covered thereby on the twelve-month anniversary of the grant date, and shall have an exercise price equal to 100% of the Fair Market Value (as defined in the Plan) of a share of Common Stock on the Datedate of Grant.grant. Also, on January 1 of each year, each then serving member of the Science and Technology Committee of the Board (the “Science and Technology Committee”) shall automatically be granted stock options to purchase 10,0002,022 shares of Common Stock under the Plan, and the Chair of the Science and Technology Committee shall be granted stock options to purchase an additional 15,0003,033 shares of Common Stock under the Plan. These options have the same terms and conditions as the Non-Employee Directorsoptions granted to the non-employee directors noted above. The options due on January 1, 2021 pursuant to the Plan were granted by the Board on April 7, 2021.
On April 7, 2021, the Board, upon recommendation of the Company’s Compensation Committee, approved equity compensation awards for the Company’s executive officers, employees and consultants. The Board approved awards of options to purchase 197,500 shares of Common Stock to officers of the Company pursuant to the 2017 Plan. The options have an exercise price of $4.62 per share, the fair market value of the Common Stock on the date of grant of April 7, 2021. The options granted to the officers will vest in three equal annual installments, commencing on the one-year anniversary of the grant date, and have a ten-year expiration date. The grant date fair value of these options using the Black-Scholes option pricing model was $612,050.
The Board also approved awards of 52,922 options to the Company’s non-employee directors pursuant to the 2017 Plan. These options have exercise prices of $4.62, the fair market value of the common stock on the date of grant as of April 7, 2021, and will fully vest upon the one-year anniversary of the grant date, and have a ten-year expiration date. The grant date fair value of these options using the Black-Scholes option pricing model was $162,156.
Stock-based compensation expense for the three months ended June 30, 2021 and 2020 was $321,427 and $571,691, respectively. The Company recorded $302,993 and $571,691 of this stock-based compensation within general and administrative expense and $18,433 and $0 within research and development expense on the accompanying statement of operations for the three months ended June 30, 2021 and 2020, respectively. Stock-based compensation expense for the six months ended June 30, 2021 and 2020 was $691,310 and 2019 was $702,681, respectively. The Company recorded $672,877 and $424,615, respectively, which is included in$702,681 of this stock-based compensation within general and administrative expense and $18,433 and $0 within research and development expense on the accompanying statement of operations.operations for the six months ended June 30, 2021and 2020, respectively.
The fair value of option awards is estimated using the Black-Scholes option-pricing model. Exercise price of each award is generally not less than the per share fair value in effect as of that award date. The determination of fair value using the Black-Scholes model is affected by the Company’s share fair value as well as assumptions regarding a number of complex and subjective variables, including expected price volatility, risk-free interest rate and projected employee share option exercise behaviors. Options granted or modified under the Plan during the yearssix months ended June 30, 20202021 and December 31, 20192020 were valued using the Black-Scholes option-pricing model with the following weighted-average assumptions:
For the Six Months Ended | For the Year Ended | |||||||
| June 30, 2020 | December 31, 2019 | |||||||
| Expected term (years) | 5.24 | 5.54 | ||||||
| Risk-free interest rate | 0.49 | % | 2.03 | % | ||||
| Expected volatility | 68.47 | % | 57.23 | % | ||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
| For the Six Months Ended June 30, | ||||||||
| 2021 | 2020 | |||||||
| Expected term (years) | 5.77 | 5.24 | ||||||
| Risk-free interest rate | 1.05 | % | 0.49 | % | ||||
| Expected volatility | 79.40 | % | 68.47 | % | ||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
In the absence of a public trading market for our common stock, on each grant date, the Company develops an estimate of the fair value of our common stock underlying the option grants. The Company estimated the fair value of our common stock by referencing arms-length transactions inclusive of the common stock underlying which occurred on or near the valuation date(s). Once the Company’s common stock is publicly traded, the Company will no longer have to estimate the fair value of the common stock, rather the value will be determined based on quoted market prices. The Company determined the fair value of common stock using methodologies, approaches and assumptions consistent with the AICPA Practice Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation and based in part on input from a valuation firm.
The Company estimates its expected volatility by using a combination of historical share price volatilities of similar companies within our industry. The risk-free interest rate assumption is based on observed interest rates for the appropriate term of the Company’s options on a grant date. The expected option term assumption is estimated using the simplified method and is based on the mid-point between vest date and the remaining contractual term of the option, since the Company does not have sufficient exercise history to estimate expected term of its historical option awards.
The following is a summary of stock option activity under the stock option plansplan for the six months ended June 30, 2021 and for the year ended December 31, 2020:
| Number of Shares | Weighted- | Weighted- Average Remaining Contractual Term (Years) | Aggregate | |||||||||||||
| Options outstanding at January 1, 2020 | 1,169,167 | 2.00 | 8.96 | - | ||||||||||||
| Forfeited | (100,000 | ) | 2.00 | |||||||||||||
| Cancelled | - | - | ||||||||||||||
| Exercised | - | - | ||||||||||||||
| Granted | 1,334,336 | 2.00 | ||||||||||||||
| Options outstanding at June 30, 2020 | 2,403,503 | $ | 2.00 | 9.28 | $ | - | ||||||||||
| Options exercisable at June 30, 2020 | 1,140,000 | $ | 2.00 | 8.47 | $ | - | ||||||||||
| Number of Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
| Options outstanding at January 1, 2020 | 236,458 | $ | 9.89 | 8.96 | $ | - | ||||||||||
| Forfeited | (20,225 | ) | 9.89 | |||||||||||||
| Cancelled | - | - | ||||||||||||||
| Granted | 269,868 | 9.89 | ||||||||||||||
| Options outstanding at December 31, 2020 | 486,101 | 9.89 | 8.68 | - | ||||||||||||
| Forfeited | - | - | ||||||||||||||
| Cancelled | - | - | ||||||||||||||
| Granted | 250,492 | 4.62 | - | - | ||||||||||||
| Options outstanding at June 30, 2021 | 736,593 | $ | 8.10 | 8.73 | $ | - | ||||||||||
| Options exercisable at June 30, 2021 | 502,197 | $ | 9.58 | 8.27 | $ | - | ||||||||||
The weighted-average grant-date fair value of stock options granted during the yearsix months ended June 30, 20202021 and year ended December 31, 20192020 was $1.14$3.09 and $1.05,$5.66, respectively.
As of June 30, 2020,2021, there was $1,239,510$665,560 of total time-based unrecognized compensation costs related to unvested stock options stock. These costs are expected to be recognized over a weighted average period of 1.122.24 years.
On June 15, 2020, the Company and Michele Linde entered into a consulting agreement in conjunction with Ms. Linde separating from the Company as Executive Vice President, General Counsel and Corporate Secretary of the Company with an effective date of May 15, 2020. As part of the consulting agreement, Ms. Linde agreed to perform consulting and advisory services in exchange for 200,000 nonqualified stock options in the Company pursuant to a nonqualified stock option grant. The options shall vest evenly over a six-month term beginning on the effective date. The options from the consulting agreement were granted in exchange for the forfeiture of the 100,000 unvested restricted stock award and 75,000 unvested nonqualified stock options that were originally granted on October 30, 2019. These modifications are treated as an option modification and the Company accounted for the option modification under ASC Topic 718, Compensation – Stock Compensation. The fair value of the forfeited options and restricted stock award was determined to be in excess of the fair value of the options granted from the consulting agreement. As a result, the fair value of the forfeited options and restricted stock award were recognized over the six-month term of the consulting award, beginning with the effective date.
On June 15, 2020, the Company and Ms. Linde also executed an amendment with an effective date of May 15, 2020 to modify three nonqualified stock option grant agreements (the “NQSO Amendment”) that were entered into on July 20, 2018, May 18, 2019, and October 30, 2019, respectively. The nonqualified stock option grants were for 25,000, 50,000, and 25,000 options, respectively. The NQSO Amendment extended the post-termination exercisability period of the vested nonqualified stock options held by Ms. Linde from 90 days following termination of employment to ten years after the initial option grant. The NQSO Amendment also amended the May 19, 2019 grant agreement to vest all 50,000 options on May 18, 2020, regardless if Ms. Linde was employed by the Company at that date. These modifications are treated as an option modification and the Company accounted for the option modification under ASC Topic 718, Compensation – Stock Compensation. As a result of the modification, the Company recognized $90,050 in incremental compensation expense during the six months ended June 30, 2020.
Note 9. Related-Party Transactions
As discussed in Note 5, in October 2018 and January 2019 the Company issued notes with an aggregate principal amount of $1,000,000. These notes were issued to Anthony Mack, Chief Executive Officer and significant investor of the Company. In addition, in January 2021, the Company issued notes with an aggregate principal amount of $75,000 and $25,000, respectively. These notes were issued to Anthony Mack, Chief Executive Officer and significant investor of the Company for $75,000 and Christopher Chipman, Chief Financial Officer, for $25,000, and were paid off with the proceeds from the initial public offering.
As discussed in Note 3, The Company maintained an account receivable, related party balance of $06, the Company’s Chief Executive Officer elected to temporarily defer his salary. Deferred compensation due to the Company’s Chief Executive Officer amounted to $1,052,000 and $1,388$1,005,000 as of June 30, 20202021 and December 31, 2019, respectively. This amount related to2020, respectively, which is included in accounts payable and accrued expenses on the reimbursement due back to the Company for healthcare insurance on behalf of Jeffrey Gudin,accompanying condensed balance sheets. In March 2021, the Company’s Chief Medical Officer.Executive Officer ceased deferring compensation.
Note 10. Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through October 9, 2020. The following are material subsequent events:
Subsequent to the six months ended June 30, 2020 and through the date these financial statements have been issued, the Company has raised gross proceeds of approximately $108,000 from the issuance of 54,000 shares of its common stock inclusive of $6,000 from the issuance of 3,000 shares to a related party. In addition, the Company issued 1,191 shares its common stock for services provided amounting to $2,382.
In October 2020, the Company amended the RRD Note to extend the maturity date from September 30, 2020 to November 30, 2020, increase the principal amount from $434,260 to $493,480, extend the RRD Qualified Financing deadline from September 30, 2020 to November 30, 2020, and provide for the payment of all interest accrued from April 1, 2020 through November 30, 2020, which was $30,431. As of September 30, 2020, the principal balance on the RRD Note was $493,480, with accrued interest of $22,208.
Research and Development and License Agreements
MedPharm Limited
Research and Option Agreement
On April 11, 2017, the Company entered into a research and option agreement, as amended on May 30, 2018 (the “MedPharm Research and Option Agreement”), with MedPharm Limited, a company organized and existing under the laws of the United Kingdom (“MedPharm”), pursuant to which MedPharm granted the Company an option to obtain an exclusive, world-wide, royalty bearing license to use certain technology developed by MedPharm. Pursuant to the agreement, MedPharm will conduct certain research and development of proprietary formulations incorporating certain MedPharm technologies and certain of the Company’s proprietary molecules.
Under the MedPharm Research and Option Agreement, MedPharm granted the Company an option (the “MedPharm Option”) to obtain an exclusive (even to MedPharm), worldwide, sub-licensable (through multiple tiers), royalty bearing, irrevocable license to research, develop, market, commercialize, and sell any product utilizing MedPharm’s spray formulation technology which is the result of the activities performed under the MedPharm Research and Option Agreement, subject to the Company’s entry into a definitive license agreement with MedPharm. In order to exercise the MedPharm Option, the Company must provide MedPharm with written notice of such exercise before the end of the Option Period (as defined in the MedPharm Research and Option Agreement). The Option Period is subject to extension upon mutual agreement with MedPharm.
Pursuant to the MedPharm Research and Option Agreement, the Company has a right of first refusal with respect to any license or commercial arrangement involving any Licensed Intellectual Property (as defined in the MedPharm Research and Option Agreement) in combination with any Virpax Molecule (as defined in the MedPharm Research and Option Agreement). In the event that MedPharm reaches an agreement with respect to a license or other commercial arrangement that involves technology or molecules covered by the right of first refusal, the Company has ten business days from the date of notice to notify MedPharm of its intention to exercise the right of first refusal and the Company’s intention to match the financial terms of the other license or commercial arrangement.
License Agreement
On June 6, 2017, as a result of the Company’s exercise of the MedPharm Option under the MedPharm Research and Option Agreement, the Company entered into a license agreement, as amended on September 2, 2017 and October 31, 2017 (the “MedPharm License Agreement”), with MedPharm for the exclusive global rights to discover, develop, make, sell, market, and otherwise commercialize any pharmaceutical composition or preparation (in any and all dosage forms) in final form containing one or more compounds, including Epoladerm and OSF200, that was developed, manufactured or commercialized utilizing MedPharm’s spray formulation technology (“MedPharm Product”), to be used for any and all uses in humans (including all diagnostic, therapeutic and preventative uses). Under the MedPharm License Agreement, the Company is required to make future milestone and royalty payments to MedPharm. We are obligated to make aggregate milestone payments to MedPharm of up to GBP 1.150 million upon the achievement of specified development milestones (payable in Great British Pounds). Additional milestone payments are due upon the achievement of certain development and commercial milestones achieved outside the United States, payable on a country by country basis. Royalty payments must be paid to MedPharm in an amount equal to a single-digit percentage of net sales of all MedPharm Product sold by us during the royalty term in the territory. Royalties shall be payable, on a country-by-country basis, during the period of time commencing on the first commercial sale and ending upon the expiration of the last-to-expire patent claim on the licensed product, which is set to expire on December 4, 2028. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
LipoCureRx, Ltd.
On March 19, 2018, the Company entered into a license and sublicense agreement (the “LipoCure Agreement”) with LipoCureRx, Ltd., a company organized and existing under the laws of Israel (“LipoCure”), for the sole and exclusive global license and sub-license rights to discover, develop, make, sell, market, and otherwise commercialize bupivacaine liposome, in injectable gel or suspension (“Licensed Compound”) or any pharmaceutical composition or preparation (in any and all dosage forms) in final form, including any combination product, containing a Licensed Compound (“Licensed Product”), including Probudur. Under the LipoCure Agreement, the Company was required to pay an upfront fee upon signing of $150,000 and is required to make future milestone and royalty payments to LipoCure. The Company is obligated to make aggregate milestone payments of up to $19.8 million upon the achievement of specified development and commercial milestones. Royalty payments must be paid in an amount equal to a single digit to low double-digit percentage of annual net sales of royalty qualifying products, subject to certain adjustments. Royalties shall be payable during the period of time, on a country-by-country basis, commencing on the first commercial sale and ending upon the expiration of the last-to-expire patent claim on the licensed product, which is set to expire on July 24, 2030. Each party has the right to terminate the agreement in its entirety upon written notice to the other party if such other party is in material breach of the agreement and has not cured such breach within ninety (90) days after notice from the terminating party indicating the nature of such breach.
Nanomerics Ltd.
Nanomerics Collaboration Agreement
On April 11, 2019, the Company entered into an exclusive collaboration and license agreement, as amended (the “Nanomerics Collaboration Agreement”), with Nanomerics Ltd., a company organized and existing under the laws of United Kingdom (“Nanomerics”), for the exclusive world-wide license to develop and commercialize products, including NES100, which contain hydrophilic neuropeptide Leucin5-Enkephalin and an amphiphile compound which is quaternary ammonium palmitoyl glycol chitosan, to engage in a collaborative program utilizing Nanomerics’ knowledge, skills and expertise in the clinical development of products and to attract external funding for such development. The Nanomerics Collaboration Agreement was also amended to include a program for the pre-clinical development of a product for post-traumatic stress disorder.
Under the Nanomerics Collaboration Agreement, the Company is required to make royalty payments equal to a single digit percentage of annual net sales of royalty qualifying products. The Company is also required to make aggregate milestone payments of up to $103 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships we enter into subsequent to the Nanomerics Collaboration Agreement. The Company’s obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of its licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim (patent is set to expire on November 3, 2034); and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. The Company has the right to terminate the agreement upon 180 days’ prior written notice to Nanomerics. Upon termination, the Company shall assign to Nanomerics all its right title and interest in all results other than results specific to (a) the Device (as defined in the Nanomerics Collaboration Agreement), including its manufacture or use; and (b) the Technology, but excluding any clinical Results relating to the Compound or Licensed Products (all terms as defined in the Nanomerics Collaboration Agreement).
Nanomerics License Agreement
On August 7, 2020, the Company entered into a collaboration and license agreement with Nanomerics Ltd. (the “Nanomerics License Agreement”), a company organized and existing under the laws of United Kingdom, for the exclusive North American license to develop and commercialize a High-Density Molecular Masking Spray (MMS019) as an anti-viral barrier to prevent or reduce the risk or the intensity of viral infections in humans. Under the Nanomerics License Agreement, the Company is required to make royalty payments equalwithin a range of 5% to a low double digit percentage15% of annual net sales of royalty qualifying products. The Company’s obligation to pay royalties, on a country-by-country basis, shall commence on the date of first commercial sale of its licensed products and shall expire with respect to each separate licensed product, on the latest to occur of (a) the tenth (10th) anniversary of the first commercial sale of the first licensed product; (b) the expiration date of the last to expire of any valid claim; and, (c) the date upon which a generic product has been on the market for a period of no fewer than ninety (90) days. The Company is also is required to make aggregate milestone payments of up to $50 million upon the achievement of specified development and commercial milestones, and sublicense fees for any sublicense relationships the Company enters into subsequent to the Nanomerics License Agreement.Agreement (any patent that issues from the currently filed provisional patent application would expire on August 24, 2041). The Company has the right to terminate the agreementNanomerics License Agreement upon 60sixty (60) days’ prior written notice to Nanomerics. Upon termination, the Company shall assign to Nanomerics all its rights, title and interest in all results of the Company.its results. Nanomerics has the right to terminate the agreement upon 60sixty (60) days’ prior written notice upon if the Company has not secured funding by the Funding Expiry Date (as defined in the Nanomerics License Agreement). On December 31, 2020, the Company amended the Nanomerics License Agreement to extend the Funding Expiry Date to March 31, 2021. Upon the closing of the Company’s IPO, the Funding Expiry Date provision was satisfied on February 19, 2021.
Research Agreements
Yissum
On May 12, 2019, the Company entered into an Agreement for Rendering of Research Services (the “May 2019 Yissum Research Agreement”), with Yissum Research Development Company of the Hebrew University of Jerusalem, Ltd. (“Yissum”). Under the May 2019 Yissum Research Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the formulation, preparation and characterization of Liposomal Bupivacaine for size zeta potential, drug loading and rate of drug release. In consideration for the research services, the Company agreed to pay research service fees of $81,000 in equal monthly installments. The Company retains ownership in all its intellectual property rights and any intellectual property belonging to either the Company or Yissum prior to the execution of the May 2019 Yissum Research Agreement will remain the sole property of either the Company or Yissum, respectively. All data generated from the provision of the May 2019 Yissum Research Agreement, including any reports, which are specifically required and contemplated under the May 2019 Yissum Research Agreement, shall be owned by the Company upon full payment of the research services fees. Each party will be entitled to terminate the agreement in the event of a breach by the other party of its obligations under the agreement, including, but not limited to, any payment failure, which is not remedied by the breaching party within thirty (30) days of receipt of written notice from the non-breaching party. All services to be provided under the May 2019 Yissum Research Agreement were completed by March 31, 2020.
On October 11, 2020, the Company entered into an Agreement for Rendering of Research Services with Yissum (the “October 2020 Yissum Research Agreement”) on substantially similar terms and conditions as detailed above under the May 2019 Yissum Research Agreement. Under the October 2020 Yissum Research Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the formulation of Liposomal Bupivacaine as well as efficacy and PK studies in animals. In consideration for the research services, the Company agreed to pay research service fees of $81,000 in six equal monthly installments. In connection with the completion of the Company’s IPO, the Company paid Yissum $40,500 towards the total consideration of $81,000. All services to be provided under the October 2020 Yissum Research Agreement were completed by June 30, 2021.
On June 30, 2021, the Company entered into an Agreement for Rendering of Research Services with Yissum (the “June 2021 Yissum Research Agreement”) on substantially similar terms and conditions as detailed above under the October 2020 Yissum Research Agreement. Under the June 2021 Yissum Research Agreement, the Company shall provide funding for research and development studies to be performed by researchers at Hebrew University related to the optimization of the Liposomal Bupivacaine formulation and to increase stability for manufacturing purposes. The Company may terminate the agreement at any time and shall be only responsible to pay Yissum for work performed through the date of termination. In consideration for the research services, the Company agreed to pay research service fees of $337,500 in six equal quarterly installments. All services to be provided under the June 2021 Yissum Research Agreement initiated on July 1, 2021 and are anticipated to be completed towards the end 2022.
Lipocure
On June 29, 2021, the Company entered into an Agreement for Rendering of Research Services (the “June 2021 Lipocure Research Agreement”) with Lipocure RX, Ltd. (“Lipocure”). Under the June 2021 Lipocure Research Agreement, the Company shall provide funding for research and development related to the optimization of the Liposomal Bupivacaine formulation and eventual manufacture of pre-clinical batches including batches for stability testing, animal studies and toxicology work. This will also include work associated with the potential filing of additional provisional patent applications. The Company may terminate the agreement at any time upon 30 days written notice and shall be only responsible to pay Lipocure for work performed through the date of such notice. In consideration for the research services, the Company agreed to pay research service fees of $200,000 upon execution as prepayment for research services included in Prepaid Expenses and Other Current Assets on the accompanying balance sheet as of June 30, 2021, as well as $400,000 on July, 1 2021, and five quarterly payments of $270,000 initiating on September 1 2021. The Company also agreed to pay $250,000 to Lipocure upon successful completion of a Chemistry, Manufacturing and Controls “CMC” filing with the U.S. Food and Drug Administration (the (“FDA”). All services to be provided under the June 2021 Lipocure Research Agreement initiated on July 1, 2021 and are anticipated to be completed towards the end 2022.
NCATS-NIH Cooperative Research and Development Agreement
On August 25, 2020, the Company entered into a cooperative researchCooperative Research and development agreementDevelopment Agreement (“CRADA”) with the National Center for Advancing Translational Sciences (NCATS), an institute/center of the National Institutes of Health (NIH), U.S. Department of Health and Human Services.Science (“NCATS”). This collaboration is for the continued development of Virpax’sthe Company’s product candidate, NES100, an intranasal peptide, for the management of acute and chronic non-cancer pain. The term of the agreementCRADA is for a period of four years from theMay 6, 2020 (the effective date of the agreementagreement) and can be terminated by both parties at any time by mutual written consent. In addition, either party may unilaterally terminate this agreementthe CRADA at any time by providing written notice of at least sixty (60) days before the desired termination date. The agreement provides for studies that are focused on the further developmentpre-clinical characterization of NES100 as a novel analgesic for acute and chronic non-cancer pain, and for studies to further develop NES100 through INDinvestigative new drug (“IND”) enabling studies. There are certain development “Go/No Go” provisions within the agreement whereby, if certain events occur, or do not occur, NCATS may terminate the CRADA. These “No GO” provisions include: i) lack of efficacy in all animal pain models, ii) no reliable and sensitive bioanalytical method can be developed, iii) manufacturing failure due to inherent process scalability issues, iv) unacceptable toxicity or safety profile to enable clinical dosing, and v) inability to manufacture the NES100 dosage form.
With respect to NCATS rights to any invention made solely by an NCATS employee(s) or made jointly by an NCATS employee(s) and our employee(s), the CRADA grants to the Company an exclusive option to elect an exclusive or nonexclusive commercialization license. For inventions owned solely by NCATS or jointly by NCATS and the Company, and licensed pursuant to the Company’s option, the Company must grant to NCATS a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government. For inventions made solely by an employee of the Company, we grant to the United States government a nonexclusive, nontransferable, irrevocable, paid-up license to practice the invention or have the invention practiced throughout the world by or on behalf of the United States government for research or other government purposes.
Note 11. Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through August 10, 2021. The following are material subsequent events:
On July 26, 2021, the Company, based on the recommendation of the Corporate Governance and Nominating Committee, appointed Mr. Gerald Bruce and Mr. Michael Dubin as directors of the Company, effective July 26, 2021, and determined Mr. Dubin qualifies as an “independent director” for purposes of the NASDAQ Capital Market listing standards.
On July 26, 2021, the Company’s Board approved an equity compensation award for the Company’s new independent director. The Board approved this award of options (the “Options”) to purchase 20,225 shares of Common Stock to the new director of the Company pursuant to the 2017 Plan. The Options have an exercise price of $4.32 per share, the fair market value of the Common Stock on the date of grant of July 26, 2021. The Options granted to the directors will vest upon the one-year anniversary of the grant date, and have a ten-year expiration date.
3,401,360 Shares of Common Stock


Virpax Pharmaceuticals, Inc.
____________________________
VIRPAX PHARMACEUTICALS, INC
PRELIMINARY PROSPECTUS
____________________________
ThinkEquity
, 2021
PROSPECTUS
ThinkEquity
a division of Fordham Financial Management, Inc.
, 2020
Through and including , 2020 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and the Nasdaq Capital Market listing fee.
| Amount | ||||
| Securities and Exchange Commission registration fee | $ | 1,999.60 | ||
| FINRA filing fee | $ | 3,250.00 | ||
| Underwriter Legal Fees and Expenses | $ | 90,000.00 | ||
| Nasdaq Capital Market listing fees | $ | 50,000.00 | ||
| Accountants’ fees and expenses | ||||
| Legal fees and expenses | ||||
| Transfer Agent’s fees and expenses | ||||
| Printing and engraving expenses | ||||
| Non-accountable expenses to underwriters | ||||
| Miscellaneous | ||||
| Total expenses | $ | |||
| Amount | ||||
| Securities and Exchange Commission registration fee | $ | 6,273.25 | ||
| FINRA Filing Fee | $ | 8,000.00 | ||
| Accountants’ fees and expenses | $ | 50,000.00 | ||
| Legal fees and expenses | $ | 126,500.00 | ||
| Underwriters’ legal fees and expenses | $ | 76,500.00 | ||
| Printing and engraving expenses | $ | 8,000.00 | ||
| Miscellaneous | $ | 1,226.75 | ||
| Total expenses | $ | 276,500.00 | ||
Item 14. Indemnification of Directors and Officers.
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our restated certificate of incorporation provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the General Corporation Law of the State of Delaware prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Our restated certificate of incorporation provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our restated certificate of incorporation provides that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
II-1
We have entered into indemnification agreements with each of our directors and officers. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any other company or enterprise to which the person provides services at our request.
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act of 1933, as amended, or the Securities Act, against certain liabilities.
Item 15. Recent Sales of Unregistered Securities.
Initial Capitalization
In May 2017, we issued 13,500,000 shares of our common stock to Virpax Pharmaceuticals, LLC. Anthony Mack, our Chief Executive Officer, and Jeffrey Gudin, our Executive Vice President and Chief Medical Officer, are the members of Virpax Pharmaceuticals, LLC. These shares were issued to Virpax Pharmaceutical, LLC as consideration for founding the Company.
Issuances of Capital Stock
Between June 2017 and October 7, 2020,January 8, 2021, we issued a total of 1,744,858346,303 shares of our common stock in private placements to certain investors at a purchase price of $2.00$9.89 per share.
Stock Options
Between June 2017 and October 7, 2020,July 26, 2021, we granted stock options to purchase an aggregate of 2,678,503812,436 shares of our common stock with ana weighted average exercise price of $2.00$8.13 per share to our employees, consultants and directors pursuant to the 2017 Plan.
Service Provider Warrant
On April 2, 2020, as compensation under a Contractor Agreement between us and a service provider, we granted a stock option to purchase 25,0005,056 shares of our common stock with an exercise price of $2.00$9.89 per share to the service provider. The Contractor Agreement terminated pursuant to its terms in March 2020. Pursuant to the terms of the stock option agreement, the stock option was forfeited 90 days after grant if it had not been exercised earlier. Upon discussions with the service provider and in satisfaction of all compensation due under the Contractor Agreement, we issued to the service provider a warrant exercisable for 25,0005,056 shares of our common stock with an exercise price of $2.00$9.89 per share.
Underwriter Warrants
In conjunction with the IPO, we granted the underwriters warrants to purchase 90,000 shares of the Company’s common stock at an exercise price of $12.50 per share, which is 125% of initial public offering price. The warrants have a five-year term and became exercisable on August 16, 2021.
II-2
Securities Act Exemptions
We deemed the offers, sales and issuances of the securities described above under “Initial Capitalization,” “Issuances of Stock” and, “Service Provider Warrant” and “Underwriter Warrants” to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances of common stock upon exercise of such options described above under “Stock Options” to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
II-2
Item 16. Exhibits and Financial Statement Schedules.
II-3
II-3
| * |
| To be filed by |
| Filed herewith. |
| † | Denotes management compensation plan or contract. |
| # |
II-4
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (a) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| (b) | The undersigned registrant hereby undertakes that: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. | |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of West Chester,Berwyn, Pennsylvania, on the 9th day of October, 2020.September, 2021.
| Virpax Pharmaceuticals, Inc. | |||
| By: | /s/ Anthony Mack | ||
| Name: | Anthony Mack | ||
| Title: | Chief Executive Officer | ||
II-6
We, the undersigned officers and directors of Virpax Pharmaceuticals, Inc., hereby severally constitute and appoint Anthony Mack, Christopher Chipman, and each of them singly (with full power to each of them to act alone), to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated:
| Signature | Title | Date | ||
| Chief Executive Officer (Principal Executive Officer) | September 9, 2021 | |||
| Anthony Mack | and Chairman of the Board of Directors | |||
| Chief Financial Officer (Principal Financial Officer) | September 9, 2021 | |||
| Christopher Chipman | ||||
| Director | September 9, 2021 | |||
| Eric Floyd, Ph.D. | ||||
| Chief Medical Officer, Director | September 9, 2021 | |||
| Jeffrey Gudin, MD | ||||
| Director | September 9, 2021 | |||
| Jerrold Sendrow, CFP | ||||
| Director | September 9, 2021 | |||
| Thani Jambulingam, Ph.D. | ||||
| Director | September 9, 2021 | |||
| Gary Jacob, Ph.D. | ||||
| /s/ Vanila M. Singh, MD | Director | September 9, 2021 | ||
| Vanila M. Singh, MD | ||||
| /s/ Gerald Bruce | EVP, Chief Commercial Officer, Director | September 9, | ||
| Gerald Bruce | ||||
| /s/ Michael F. Dubin | Director | September 9, 2021 | ||
| Michael F. Dubin |
II-7II-6