

As filed with the Securities and Exchange Commission on July 24, 2019.October 2, 2023.
Registration No. 333-333-272623
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON,Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Qualigen Therapeutics, Inc.
RITTER PHARMACEUTICALS, INC.
(Exact nameName of Registrant as specifiedSpecified in its charter)Its Charter)
| Delaware | 2834 | 26-3474527 | ||
| (State or | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
Qualigen Therapeutics, Inc.
1880 Century Park East #10005857 Owens Avenue, Suite 300
Los Angeles, CA 90067Carlsbad, California 92008
(310) 203-1000(760) 452-8111
(Address, including zip code,Including Zip Code, and telephone number, including area code,Telephone Number, Including Area Code, of Registrant’s principal executive offices)Principal Executive Offices)
Andrew J. RitterMichael S. Poirier
Chief Executive Officer
Ritter Pharmaceuticals,Qualigen Therapeutics, Inc.
1880 Century Park East #10005857 Owens Avenue, Suite 300
Los Angeles, CA 90067Carlsbad, California 92008
(310) 203-1000(760) 452-8111
(Name, address, including zip code,Address, Including Zip Code, and telephone number, including area code,Telephone Number, Including Area Code, of agent for service)Agent For Service)
Copies to:
Michael Sanders, Esq.
Ashok W. Mukhey, Esq. William D. Davis II, Esq. Reed Smith LLP 1901 Avenue of the Stars, Suite 700 Los Angeles, California 90067
| Leslie Marlow, Esq. Patrick J. Egan, Esq. Blank Rome LLP 1271 Avenue of the Americas New York, New York 10020 (212) 885-5000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.Registration Statement.
If any of the securities being registered on this Formform are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. [X]☒
If this Formform is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]☐
If this Formform is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]☐
If this Formform is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.Act:
| Large accelerated filer | Accelerated filer | |||
| Non-accelerated filer | Smaller reporting company | |||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. [ ]☐
| Title of each class of securities to be registered | Amount to be registered (1) | Proposed maximum offering price per share (2) | Proposed maximum aggregate offering price (2) | Amount of registration fee (2) | ||||||||||||
| Common Stock, $0.001 par value per share | 7,600,000 | $ | 0.98 | $ | 7,448,000 | $ | 903 | |||||||||
The Registrant hereby amends this registration statementRegistration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statementRegistration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statementRegistration Statement shall become effective on such date as the Commission, acting pursuant to suchsaid Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. The selling stockholderThese securities may not sell these securitiesbe sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it iswe are not soliciting an offeroffers to buy these securities in any jurisdiction where the offer or sale is not permitted.


7,600,000Up to Shares
of Common Stock
Up to Pre-Funded Warrants to Purchase up to Shares of Common Stock
This prospectus relatesUp to Shares of Common Stock Underlying the Pre-Funded Warrants
Up to Common Stock Warrants to Purchase up to Shares of Common Stock
Up to Shares of Common Stock Underlying the Common Stock Warrants
We are offering on a “reasonable best efforts” basis up to $ million of shares of common stock together with common stock warrants to purchase shares of common stock. Each share of our common stock is being sold together with a common stock warrant to purchase shares of our common stock. The shares of common stock and common stock warrants are immediately separable and will be issued separately in this offering but must be purchased together as a unit in this offering. The common stock warrants will have an initial exercise price of $ per share and will have a five-year term.
We are also offering pre-funded warrants to purchase up to an aggregate of shares of common stock to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, in lieu of shares of common stock that would result in beneficial ownership in excess of 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant is exercisable for one share of our common stock, has an exercise price of $0.001 per share and an indefinite term. Each pre-funded warrant is being offered together with a common stock warrant. The pre-funded warrants and common stock warrants are immediately separable and will be issued separately in this offering but must be purchased together as a unit in this offering. For each pre-funded warrant that we sell, the number of shares of common stock we are offering will be reduced on a one-for-one basis.
There is no established trading market for the pre-funded warrants or common stock warrants, and we do not expect a market to develop. In addition, we do not intend to list the pre-funded warrants or common stock warrants on Nasdaq, any other national securities exchange or any other trading system. Without an active trading market, the liquidity of the pre-funded warrants and common stock warrants may be limited.
We have engaged A.G.P./Alliance Global Partners (whom we refer to herein as the “Placement Agent”) to act as our exclusive placement agent in connection with the securities offered by this prospectus. The Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities but has agreed to use its reasonable best efforts to arrange for the sale of upthe securities offered by this prospectus. We have agreed to 7,600,000pay the Placement Agent a fee based upon the aggregate gross proceeds raised in this offering as set forth in the table below.
The actual public offering price of the securities described in this prospectus will be determined by us, the Placement Agent and the investors in the offering, and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price.
Pursuant to this prospectus, we are also offering the shares of common stock issuable upon the exercise of pre-funded warrants and common stock warrants offered hereby.
The shares of our common stock, pre-funded warrants or common warrants being offered will be sold in a single closing. We will deliver all securities to be issued in connection with this offering delivery versus payment (DVP)/receipt versus payment (RVP) upon receipt of investor funds received by Aspire Capital Fund, LLC,us. Accordingly, neither we nor the Placement Agent have made any arrangements to place investor funds in an escrow account or Aspire Capital. Aspire Capitaltrust account since the Placement Agent will not receive investor funds in connection with the sale of the securities offered hereunder. Because there is also referredno minimum number of securities or minimum aggregate amount of proceeds for this offering to close, we may sell fewer than all of the securities offered hereby, and investors in this prospectus as the selling stockholder. The prices at which the selling stockholder may sell the shares will be determined by the prevailing market price for the shares or in negotiated transactions. Weoffering will not receive a refund in the event that we do not sell an amount of securities sufficient to pursue the business goals outlined in this prospectus. Because there is no escrow account and there is no minimum offering amount, investors could be in a position where they have invested in our company, but we are unable to fulfill our objectives due to a lack of interest in this offering. Also, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan. The offering of the shares by the selling stockholder. However, we may receive proceeds of up to $6.5 million from the sale of our common stock, pre-funded warrants or common warrants will terminate no later than , 2023 unless the offering is fully subscribed before that date or we decide to terminate the selling stockholder,offering (which we may do at any time in our discretion) prior to that date; however, the shares of our common stock underlying the pre-funded warrants and the common warrants will be offered on a continuous basis pursuant to a common stock purchase agreement entered into with the selling stockholder on July 23, 2019, or the Purchase Agreement, once the registration statement, of which this prospectus is a part, is declared effective.
The selling stockholder is an “underwriter” within the meaning ofRule 415 under the Securities Act of 1933, as amended. We will pay the expenses of registering these shares, but all selling and other expenses incurred by the selling stockholder will be paid by the selling stockholder.amended (the “Securities Act”).
Our common stock is listed on theThe Nasdaq Capital Market under the ticker symbol “RTTR.“QLGN.” On July 23, 2019, theThe last reported salesales price per share of our common stock on The Nasdaq Capital Market on September 29, 2023 was $1.01 per share.
You should read this prospectus and We do not intend to list the pre-funded warrants or the common stock warrants on any prospectus supplement, together with additional information described under the headings “Incorporation of Certain Documents by Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
We are an “emerging growth company” as defined by the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. Please see “Prospectus Summary – Implications of Being an Emerging Growth Company.”national securities exchange or other nationally recognized trading system.
InvestingAn investment in our securities involves a high degree of risk. See “Risk Factors”significant risks. You should carefully consider the risk factors beginning on page 58 of this prospectus.prospectus before you make your decision to invest in our securities.
Neither the Securities and Exchange Commission nor any state securities commissionother regulatory body has approved or disapproved of these securities or passed upon the adequacyaccuracy or accuracyadequacy of this prospectus. Any representation to the contrary is a criminal offense.
Per Share Common Stock | Per Pre-Funded Warrant and Accompanying Common Stock Warrant | Total | ||||||||||
| Public offering price | $ | $ | ||||||||||
| Placement Agent fees(1) | $ | $ | ||||||||||
| Proceeds to us, before expenses(2) | $ | $ | ||||||||||
| (1) | Does not include certain expenses of the Placement Agent. See “Plan of Distribution” beginning on page 23 of this prospectus for additional information regarding compensation to be received by the Placement Agent. |
| (2) | The amount of proceeds, before expenses, to us does not give effect to any exercise of the pre-funded warrants or common warrants. |
Delivery of the shares of our common stock, pre-funded warrants or common warrants is expected to be made on or about , 2023.
Sole Placement Agent
A.G.P.
The date of this prospectus is , 2019.2023
TABLE OF CONTENTS
We incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Incorporation of Certain Information by Reference,” before deciding to invest in our common stock.
Neither we nor the selling stockholder hasPlacement Agent have authorized anyone to providegive any information or to make any representations other than asthose contained or incorporated by reference in this prospectus or in any free writing prospectuses we have prepared. We and the selling stockholder take no responsibility for, and provide no assurance as to the reliability of,prospectus. You must not rely on any information that others may give you.or representations not contained or incorporated by reference in this prospectus. This prospectus is an offer to sell only the sharessecurities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
For investors outside of the United States: Neither we nor the selling stockholder have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about, and to observe any restrictions relating to, this offering and the distribution of this prospectus outside of the United States.
This summary highlights certain information about us, this offering and selected information contained elsewhere in theor incorporated by reference into this prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our common stock. For a more complete understanding of our companysecurities. You should carefully read this entire prospectus and the documents and reports incorporated by reference into this offering, we encourage you to read prospectus before making an investment decision, including the information presented under the headings “Risk Factors” and consider“Cautionary Note Regarding Forward-Looking Statements and Industry Data and Market Information” in this prospectus and the more detailed information in the prospectus, including ourhistorical financial statements and the related notes and other documentsthereto incorporated by reference into this prospectus. You should pay special attention to the information contained under the caption titled “Risk Factors” in this prospectus, as well asin our most recent Annual Report on Form 10-K, in any subsequent Quarterly Reports on Form 10-Q and in our other reports filed from time to time with the information under the caption “Risk Factors” hereinSecurities and under similar headings in the other documents thatExchange Commission, which are incorporated by reference into this prospectus.. Unless we specify otherwise, all references inprospectus, before deciding to buy our securities. In this prospectus, to “Ritter Pharmaceuticals,the terms “Qualigen,” “we,“Qualigen Therapeutics, Inc.,” “our,the “Company,” “us” “we,” “our,” “ours” and “our company” “us” refer to Ritter Pharmaceuticals,Qualigen Therapeutics. Inc. and its subsidiaries.
Company Overview
Ritter Pharmaceuticals, Inc. develops novel therapeutic products that modulate the gut microbiome to treat digestive disordersWe are a clinical stage therapeutics company focused on developing treatments for adult and gastrointestinal diseases. pediatric cancers of high unmet medical need with potential for Orphan Drug designation.
Our cancer therapeutics pipeline includes QN-302, our Pan-RAS Inhibitor platform (formerly RAS-F) and QN-247.
Our lead product candidate, RP-G28,oncology therapeutics program, QN-302, is an orally administered,a high purity galacto-oligosaccharide, currentlypotency small molecule selective transcription inhibitor with strong binding affinity to G-Quadruplexes (G4s) prevalent in Phase 3 clinical development for the treatment of lactose intolerance (“LI”), a condition that affects millions worldwide. RP-G28 is designedcancer cells. By binding to, selectively stimulate the growth of lactose-metabolizing bacteria in the colon, thereby effectively adapting the gut microbiome to assist in digesting lactose (the sugar found in milk) that reaches the large intestine. RP-G28and stabilizing G4s against “unwinding,” QN-302 could potentially help inhibit cancer cell proliferation and induces cancer cell death. QN-302 has the potential to become the first drug approved byreceived Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA) to proceed with its Phase 1 clinical trial. Earlier this year, QN-302 also received Orphan Drug Designation (ODD) from FDA for the indication of pancreatic cancer.
Our Pan-RAS portfolio consists of a family of Pan-RAS inhibitor small molecules believed to inhibit or block mutated RAS genes’ proteins from binding to their effector proteins. Preventing this binding could stop tumor growth, especially in RAS-driven tumors such as pancreatic, colorectal and lung cancers.
Our investigational oligonucleotide-based drug candidate QN-247 binds nucleolin, a key multi-functional regulatory phosphoprotein that is overexpressed in cancer cells. Such binding could inhibit the cancer cells’ proliferation in nucleolin-expressing malignancies.
| 1 |
Therapeutics Pipeline
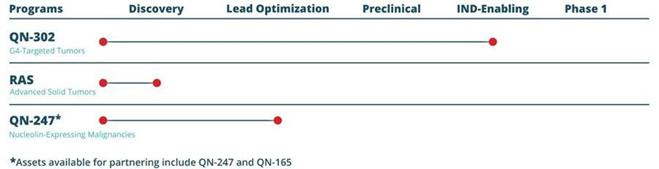
Our lead drug compound QN-302 (formerly SOP1812) is being developed to target regulatory regions of cancer genes that down-regulate gene expression in multiple cancer pathways for potential treatment of G4-targeted tumors (e.g., pancreatic cancer).
The investigational compounds within our Pan-RAS Inhibitor portfolio are designed to suppress the interaction of endogenous RAS with c-RAF, upstream of the KRAS, HRAS and NRAS effector pathways, and is being developed for the potential treatment of RAS-driven tumors.
Our anticancer drug candidate, QN-247 (formerly referred to as ALAN or AS1411-GNP) is a DNA aptamer conjugated to a gold nanoparticle, which potentially gives it dramatically increased potency and versatility. On May 2, 2023, we announced that we have de-prioritized deploying our internal resources to our QN-247 program and are seeking a partner to further its development.
On May 26, 2022, we acquired a 52.8% interest in NanoSynex, Ltd. (“FDA”NanoSynex”). NanoSynex is a nanotechnology diagnostics company domiciled in Israel. NanoSynex’s technology is an Antimicrobial Susceptibility Testing (AST) that aims to enable better targeting of antibiotics for their most suitable uses to ultimately result in faster and more efficacious treatment, hence reducing hospitals mortality and morbidity rates. As described below, we have recently reduced our ownership interest in NanoSynex to below 50%.
QN-302 (formerly referred to as SOP1812)
We exclusively in-licensed the global rights to the G4 selective transcription inhibitor platform from University College London (“UCL”) in January 2022. The licensed technology comprises lead compound QN-302 (formerly SOP1812) and back-up compounds that target regulatory regions of cancer genes that down-regulate gene expression in multiple cancer pathways. The license agreement requires (if and when applicable) tiered royalty payments in the low to mid-single digits, clinical/regulatory/sales milestone payments, and a percentage of any non-royalty sublicensing consideration paid to Qualigen.
Developed by Dr. Stephen Neidle and his group at UCL, the G-Quadruplex (G4) binding concept is derived from over 30 years in nucleic acid research, including research on G4s, which are higher order DNA and RNA structures formed by sequences containing guanine-rich repeats. G4s are overrepresented in telomeres (a region of repetitive DNA sequences at the end of a chromosome) as well as promoter sequences and untranslated regions of many oncogenes. Their prevalence is therefore significantly greater in cancer cells compared to normal human cells.
G4-selective small molecules such as QN-302 and backup compounds target the regulatory regions of cancer genes, which have a high prevalence of enriched G4s. Stable G4-QN-302 complexes can be impediments to replication, transcription or translation of those cancer genes containing G4s, and the drugs’ binding to G4s are believed to stabilize the G4s against possible “unwinding.” G4 binders like QN-302 could potentially be efficacious in a variety of cancer types with a high prevalence of G4s.
Pancreatic cancer is the tenth most common cancer and third deadliest cancer in the United States and has one of the lowest rates of survival of all cancer types, with 91% of those diagnosed dying from the disease and one in four dying within the first month of diagnosis. The chemotherapy drug Gemcitabine has been standard of care for patients with metastatic pancreatic cancer for more than 15 years. Numerous clinical trials have tested new drugs, either alone or in combination, with Gemcitabine. Based upon our pre-clinical in-vitro and in-vivo studies, we believe that QN-302 has the potential to demonstrate positive activity against pancreatic ductal adenocarcinoma (“PDAC”).
Our pre-clinical in-vitro and in-vivo studies have shown that G4 stabilization by QN-302 resulted in inhibition of target gene expression and cessation of cell growth in various cancers, including PDAC, which represents 98% of pancreatic cancers. In in-vitro studies, QN-302 was potent in inhibiting the growth of several PDAC cell lines at low nanomolar concentrations. Similarly, in in-vivo studies, QN-302 showed a longer survival duration in a KPC genetic mouse model for PDAC than Gemcitabine has historically shown. Additional preclinical in-vivo studies suggest activity in gemcitabine-resistant PDAC. Data further demonstrated that QN-302 had significant anti-tumor activity in three patient-derived PDAC xenograft models. Early safety indicators suggest no significant adverse toxic effects at proposed therapeutic doses in pancreatic cancer mouse in-vivo models.
| 2 |
On January 9, 2023, the FDA granted Orphan Drug Designation (“ODD”) to QN-302 for the indication of pancreatic cancer. ODD provides advantages to pharmaceutical companies that are developing investigational drugs or biological products that show promise in treating rare diseases or conditions that affect fewer than 200,000 people in the United States, including seven-year marketing exclusivity if the product receives the first FDA approval for the disease for which it has such designation, and eligibility to receive regulatory support and guidance from the FDA in the design of an overall drug development plan.
There are also economic advantages to receiving ODD, including a 25% federal tax credit for expenses incurred in conducting clinical research on the orphan designated product within the United States. Tax credits may be applied to the prior year or applied to up to 20 years of future taxes. ODD recipients may also have their Prescription Drug User Fee Act (PDUFA) application fees waived, a potential savings of around $3.2 million (as of fiscal year 2023) for applications requiring covered clinical data and may qualify to compete for research grants from the Office of Orphan Products Development that support clinical studies.
Pan-RAS Inhibitor Platform (formerly RAS-F)
We entered into a sponsored research agreement with University of Louisville (“UofL”) on March 4, 2019, pursuant to which UofL agreed to conduct ongoing discovery and preclinical efforts for the Pan-RAS platform on our behalf. Under the terms of the agreement, as amended, the collaboration extends until the fourth quarter of 2023.
In July 2020, we entered into an exclusive worldwide license agreement with UofL for the intellectual property covering the “RAS” family of pan RAS inhibitor small molecule drug candidates, which was subsequently amended on March 17, 2021 and June 15, 2023. The Pan-RAS inhibitor compounds are believed to work by blocking RAS mutations directly, thereby inhibiting tumor formation (especially in pancreatic, colorectal and lung cancers). Pursuant to the license agreement, we in-licensed the Pan-RAS compound family of drug candidates and will seek to identify and develop a lead drug candidate from the compound family. The license agreement requires (if and when applicable) tiered royalty payments in the low to mid-single digits, clinical/regulatory/sales milestone payments, and a percentage of any non-royalty sublicensing consideration paid to Qualigen.
RAS is the most common oncogene in human cancer. Activating mutations in one of the three human RAS gene isoforms (KRAS, HRAS or NRAS) are present in about one-fourth to one-third of all cancers. For example, mutant KRAS is found in 98% of pancreatic ductal adenocarcinomas, 52% of colon cancers, and 32% of lung adenocarcinomas. For these three cancer types, cancers with mutant KRAS are diagnosed in more than 170,000 people each year in the United States and cause more than 120,000 deaths. Drugs that target signaling downstream of RAS are available; however, such drugs have shown disappointing clinical durability because RAS is a “hub” that activates multiple effectors, so drugs that block a single pathway downstream may not account for the many other activated pathways.
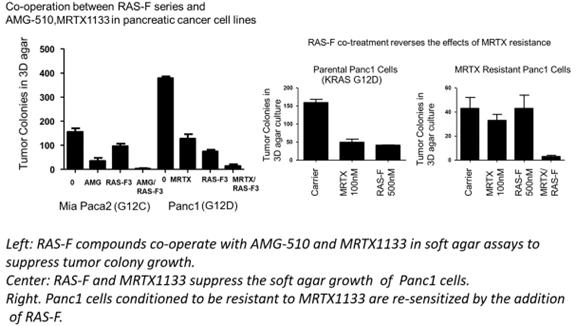
On June 5, 2023, we announced a poster featuring our pan-RAS inhibitor program in pancreatic cancer, which was presented as part of the Scientific Program at the American Society of Clinical Oncology’s (ASCO) 2023 Annual Meeting held June 2 - 6, 2023 in Chicago.
The poster outlines results of concurrent administration of one of our pan-RAS inhibitor molecules (referred to as RAS-F in the figure below) with commonly used therapeutics against pancreatic cancer. In-vivo activity was confirmed in xenograft experiments with cell lines and PDX models, and the molecules were shown to co-operate with AMG-510 (sotorasib) (G12C specific inhibitor) and MRTX1133 (G12D specific inhibitor) against pancreatic cancer cell lines. This agent was also shown to suppress resistance to MRTX1133 in-vitro, which led authors to believe these pan-RAS compounds may have the potential to overcome resistance to AMG-510 and MRTX1133 in-vivo.
| 3 |
QN-247
In June 2018, we entered into an exclusive, worldwide license agreement with UofL for the intellectual property covering QN-247, which was subsequently amended on January 17, 2023 and March 16, 2023.
We also entered into a sponsored research agreement with UofL in August 2018, which was subsequently amended in October 2020, pursuant to which UofL performed various animal studies on our behalf to assess antitumor efficacy and safety of different QN-247 compositions. The sponsored research agreement with UofL for QN-247 expired on August 31, 2022.
QN-247 is an oligonucleotide-based drug candidate that is designed to treat different types of nucleolin-expressing cancers, including liquid and solid tumors. QN-247 inhibits nucleolin, a key multi-functional regulatory phosphoprotein that is overexpressed in cancer cells, and may thereby be able to inhibit the cells’ proliferation. QN-247 has shown promise in preclinical studies for the treatment of LI. We are further exploring the functionality and discovering the therapeutic potential that gut microbiome changesacute myeloid leukemia (“AML”). This novel technology may have several other potential applications, including enhancement of radiation therapy, enhancement of tumor imaging, and delivery of other anti-cancer compounds directly to tumor cells.
QN-247 is an enhanced version of QN-165 (formerly referred to as AS1411), where the DNA oligonucleotide aptamer is conjugated. A key component of QN-247, DNA oligonucleotide aptamer QN-165, has been shown, primarily on treating/preventing a varietypreclinical basis, to have the potential to target and destroy cancer cells. This component has been administered in Phase 1 and Phase 2 clinical trials to over 100 AML or renal cell carcinoma cancer patients and appears to be well tolerated with no evidence of conditions including gastrointestinal diseases,severe adverse events in such trials, with at least seven patients appearing to have clinical responses.
An in-vivo efficacy study with a triple negative breast cancer metabolic,(TNBC) MDA-MB-231 xenograft mouse model was performed with 12 daily doses (1 mg/kg) of QN-247. This study showed statistically significant reductions in mean tumor volumes for all QN-247 formulations compared to baseline and liver diseases.to vehicle control. QN-247 formulations with higher oligonucleotide loading appeared to reduce tumor volumes more than lower oligonucleotide loading. No evidence of adverse toxicity was observed.
| 4 |
We do not have in-house manufacturing capability for our therapeutics product candidates.
Research and Development
For research and development of our drug candidates, we are leveraging the scientific and technical resources and laboratory facilities of UofL and UCL, through technology licensing, sponsored research, and other consulting agreements, which are focused on aptamer technology and applications. We would engage contract research organizations (“CROs”) for any clinical trials of our drug candidates. We intend to expandfocus our product pipelineinternal research and create added value in the future by evaluating RP-G28 in other indications, including orphan indications, developing additional products baseddevelopment on our underlying microbiome-modulating technology, and/or in-licensing complementary products to treatoversight of these or other, conditions.organizations.
In July 2019,Recent Developments
FDA IND Clearance to Initiate Phase 1 Clinical Trial of QN-302
On August 1, 2023, we announced that the last patient had completed their final visitFDA has cleared our IND application for QN-302. Based on this clearance, we plan to initiate the Phase 1 clinical trial in the Company’s first pivotal Phase 3 clinical trial of RP-G28, that trial finalization leading to data lock and top-line data readout had begun, and that data is expected to be publicly released in early fourth quarter of 2019.2023 and will enroll patients with advanced or metastatic solid tumors. The proposed Phase 1 clinical trial is a multicenter, open-label, dose escalation, safety, pharmacokinetic, and pharmacodynamic study with dose expansion to evaluate safety, tolerability, and antitumor activity of QN-302 in patients with advanced solid tumors that have not responded to or that have recurred following treatment with available therapies. We expect ouranticipate the dosing of at least 24 patients in Phase 3 clinical program will include two confirmatory clinical trials1a can be completed by the end of similar trial design.2024, funded in part by proceeds received by the divestiture of the Company’s diagnostics business in July 2023 as described immediately below.
We have devoted substantiallySale of Diagnostics Business
On July 20, 2023, we sold all of the issued and outstanding shares of common stock of Qualigen, Inc., a wholly-owned subsidiary and the legal entity operating our resourcesFastPack™ diagnostic business, to development efforts relatingChembio Diagnostics, Inc. (“Chembio”), a subsidiary of Biosynex, S.A. As consideration for the shares of Qualigen, Inc., we received a cash payment of approximately $4.7 million, which payment is subject to RP-G28, including conducting clinical trialspost-closing adjustments. An additional $450,000 was delivered by Chembio to an escrow account to satisfy our indemnification obligations. Any amounts remaining in the escrow account that have not been offset or reserved for claims will be released to us within five business days following the date that is 18 months after the closing of RP-G28, providing generalthe transaction. Following the consummation of the transaction, Qualigen, Inc. became a wholly-owned subsidiary of Chembio.
Amendment and administrative supportSettlement Agreement with NanoSynex Ltd.
On July 20, 2023, we entered into an Amendment and Settlement Agreement with NanoSynex (the “NanoSynex Amendment”), which amended the Master Funding Agreement for these operationsthe Operational and protectingTechnology Funding of Nanosynex Ltd., dated May 26, 2022, by and between us and NanoSynex (the “Original NanoSynex Agreement”), to, among other things, provide for the further funding of NanoSynex, as contemplated by the Original NanoSynex Agreement.
Pursuant to the terms of the NanoSynex Amendment, we agreed to advance to NanoSynex an aggregate amount of $1,610,000 as follows: (i) $380,000 within five business days of the execution of the NanoSynex Amendment; (ii) $560,000 on or before November 30, 2023, against which NanoSynex will issue a promissory note to us with a face value in the amount of such funding; and (iii) $670,000 on or before March 31, 2024, against which NanoSynex will issue a promissory note to us with a face value in the amount of such funding. The NanoSynex Amendment further provides that the initial payment of $380,000 would be satisfied by our intellectual property. We currentlysurrender of the 281,000 Preferred B Shares of NanoSynex then held by us, and such share surrender has resulted in our ownership in NanoSynex being reduced from approximately 52.8% to approximately 49.97% of the issued and outstanding voting equity of NanoSynex. In the event that we do not satisfy these payment obligations, there would be an additional surrender of our shares in NanoSynex in such number as provided in the NanoSynex Amendment. The NanoSynex Amendment supersedes any payments contemplated by the Original NanoSynex Agreement, such that except as described in the NanoSynex Amendment, we will have any products approvedno further payment obligations to NanoSynex under the Original NanoSynex Agreement or otherwise (including by way of equity investment, loan financing or credit lines).
Reverse Stock Split
Effective as of November 23, 2022, we completed a 1-for-10 reverse stock split of our common stock in order to regain compliance with Nasdaq Listing Rule 5550(a)(2), which requires a minimum bid price of $1.00 per share. As a result of the reverse stock split, each 10 shares of our common stock issued and outstanding as of 12:01 a.m. Eastern Time on November 23, 2022 were combined and converted into one share of common stock. On December 9, 2022, we received written notice from the Listing Qualifications Department of The Nasdaq Stock Market LLC stating that because our shares of common stock had a closing bid price at or above $1.00 per share for salea minimum of 10 consecutive business days, the Company has regained compliance with the minimum bid price requirement of $1.00 per share for continued listing on The Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(a)(2), and wethat the matter is now closed. All references to numbers of shares of Common Stock and per-share information in this prospectus have not generated any revenue from product sales since our inception.been adjusted retroactively, as appropriate, to reflect the 1-for-10 reverse stock split.
Corporate Information
We wereRitter Pharmaceuticals, Inc. (our predecessor) was formed as a Nevada limited liability company on March 29, 2004 under the name Ritter Natural Sciences, LLC. In September 2008, wethis company converted into a Delaware corporation under the name Ritter Pharmaceuticals, Inc. On May 22, 2020, upon completing a “reverse recapitalization” transaction with Qualigen, Inc., Ritter Pharmaceuticals, Inc. was renamed Qualigen Therapeutics, Inc. Qualisys Diagnostics, Inc. was formed as a Minnesota corporation in 1996, reincorporated to become a Delaware corporation in 1999, and then changed its name to Qualigen, Inc. in 2000. Qualigen, Inc. was a wholly-owned subsidiary of the Company. On July 20, 2023, we sold all of the issued and outstanding shares of common stock of Qualigen, Inc. to Chembio, a wholly-owned subsidiary of Biosynex, S.A. Following the consummation of this transaction, Qualigen, Inc. became a wholly-owned subsidiary of Chembio.
Our principal executive offices are located at 1880 Century Park East,5857 Owens Avenue, Suite 1000, Los Angeles, California 90067, and our300, Carlsbad, CA 92008. Our telephone number is (310) 203-1000.(760) 452-8111. Our corporate website address is www.qlgntx.com. Our website address iswww.ritterpharmaceuticals.comand we regularly post copies of our press releases as well as additionalthe information about uscontained on, our website. Information contained in, or that can be accessed through, our website iswill not be deemed to be incorporated by reference intoin, and are not considered part of, this prospectus, and youprospectus. You should not consider informationrely on our website or any such information in making your decision whether to be part of this prospectus.
This prospectus may contain references topurchase our trademark and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
Implications of Being an Emerging Growth Companysecurities.
We are an emerging growth company,a “smaller reporting company” as defined in the Jumpstart Our Business Startups ActItem 10(f)(1) of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, weRegulation S-K. Smaller reporting companies may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act,certain reduced disclosure obligations, regarding executive compensation in this prospectus and our periodic reports and proxy statements and exemptions from the requirementsincluding, among other things, providing only two years of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved.audited financial statements. We canwill remain an emerging growtha smaller reporting company until the earlier of (1) the last day of the fiscal year (a) ending December 31, 2020, which is the end of the fiscal year following the fifth anniversary of the closing of our initial public offering, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means(1) the market value of our common stock that isheld by non-affiliates exceeds $250 million as of the prior June 30, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our common stock held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. Additionally, even if we no longer qualify as an emerging growth company, as long as we are neither a “large accelerated filer” nor an “accelerated filer,” we would not be required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act.30.
We cannot predict if investors will find our securities less attractive because we may rely on these exemptions, which could result in a less active trading market for our securities and increased volatility in the price of our securities.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. We have elected to use this extended transition period. As a result of this election, our timeline to comply with these standards will in many cases be delayed as compared to other public companies that are not eligible to take advantage of this election or have not made this election. Therefore, our financial statements may not be comparable to those of companies that comply with the public company effective dates for these standards.
In addition, if we cease to be an emerging growth company, we will no longer be able to use the extended transition period for complying with new or revised accounting standards. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
THE OFFERINGThe Offering
Securities we are offering | Up to shares of common stock together with common stock warrants to purchase up to shares of our common stock, and pre-funded warrants to purchase up to shares of common stock together with common stock warrants to purchase up to shares of common stock. The shares of common stock being | ||
| We are offering to those purchasers whose purchase of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the closing of this offering, in lieu of purchasing common stock, pre-funded warrants to purchase up to an aggregate of shares of our common stock. Each pre-funded warrant is exercisable for one share of our common stock. The purchase price of each pre-funded warrant is equal to the price at which a share of common stock is being sold to the public in this offering, minus $0.001. We are also registering the issuance of up to shares of our common stock issuable upon exercise of the pre-funded warrants. For each pre-funded warrant that we sell, the number of shares of common stock that we are offering will be reduced on a one-for-one basis. | |||
Common immediately before this offering | |||
Common stock outstanding immediately after this offering | Up to shares, assuming no exercise of the pre-funded warrants and common stock warrants issued in this offering. | ||
| Use of | We estimate that the net proceeds from this offering will We intend to use the net proceeds from the sale of the | ||
| Risk Factors | |||
| Lock-up | We have agreed, subject to certain exceptions and without the approval of the Placement Agent and purchasers of our securities in this offering, not to (1) issue, enter into any agreement to issue or announce the issuance or proposed issuance of, any shares of common stock (or securities convertible into or exercisable for common stock) or file any registration statement, including any amendments or supplements for a period of days following the closing of the offering of the shares and (2) enter into a variable rate transaction for a period of days following the closing of this offering. Our directors and officers have agreed not to offer, sell, pledge or otherwise transfer or dispose of any of our |
The Nasdaq Capital Market listing symbol | “QLGN.” There is no established trading market for the pre-funded warrants or the common stock warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the pre-funded warrants or the common stock warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the pre-funded warrants and common stock warrants will be limited. |
| 6 |
The number of shares of our common stock to be outstanding followingafter this offering is based on an aggregate5,052,463 shares of 9,350,026common stock outstanding on September 27, 2023, does not give effect to the shares outstanding as of July 23, 2019, butcommon stock issuable upon exercise of the pre-funded warrants and common warrants issued in this offering and excludes:
| ● | ||
| ● | ||
| ● | Approximately 1,762,396 shares of common stock issuable | |
| ● | ||
| ● |
Common Stock Issued and IssuableUnless otherwise indicated, all information in this prospectus gives effect to the Selling Stockholder1-for-10 reverse stock split effectuated on November 23, 2022.
The Purchase Agreement provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $6.5 million of shares of our common stock, or the Purchase Shares, over the term of the Purchase Agreement, which matures on March 31, 2021.
Concurrently with entering into the Purchase Agreement, we also entered into an amended and restated registration rights agreement with Aspire Capital, or the Registration Rights Agreement, in which we agreed to file one or more registration statements, including the registration statement of which this prospectus is a part, as permissible and necessary to register under the Securities Act of 1933, as amended, or the Securities Act, the sale of the shares of our common stock that may be issued to Aspire Capital under the Purchase Agreement.
As of July 23, 2019 there were 9,350,026 shares of our common stock outstanding, excluding the 7,600,000 shares of common stock that we may issue to Aspire Capital after this registration statement is declared effective under the Securities Act. If all of such 7,600,000 shares of our common stock offered hereby were issued and outstanding as of the date hereof, such shares would represent 44.8% of the total common stock outstanding as of the date hereof. The number of shares of our common stock ultimately offered for sale by Aspire Capital is dependent upon the number of shares purchased by Aspire Capital under the Purchase Agreement.
Pursuant to the terms of the Purchase Agreement, the aggregate number of shares that we may issue to Aspire Capital under the Purchase Agreement may in no case exceed 1,807,562 shares of our common stock, unless (i) stockholder approval is obtained to issue more, in which case this 1,807,562 share limitation will not apply, or (ii) stockholder approval has not been obtained and at any time the 1,807,562 share limitation is reached and at all times thereafter the average price paid for all shares issued under the Purchase Agreement is equal to or greater than $0.86, or the Minimum Price; provided that at no point in time shall Aspire Capital (together with its affiliates) beneficially own more than 19.99% of our common stock.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we are registering 7,600,000 shares of our common stock under the Securities Act that we may issue to Aspire Capital after this registration statement is declared effective under the Securities Act. All 7,600,000 shares of common stock are being offered pursuant to this prospectus.
After the SEC has declared effective the registration statement of which this prospectus is a part, on any trading day on which the closing sale price of our common stock exceeds $0.25, or the Floor Price, we have the right, in our sole discretion, to present Aspire Capital with a purchase notice, or each a Purchase Notice, directing Aspire Capital (as principal) to purchase up to 100,000 shares of our common stock per trading day, up to $6.5 million of our common stock in the aggregate at a per share price, or the Purchase Price, calculated by reference to the prevailing market price of our common stock (as more specifically described below in the section titled “The Aspire Capital Transaction”); however, in no event shall the shares purchased exceed Five Hundred Thousand Dollars ($500,000) per business day.
In addition, on any date on which we submit a Purchase Notice for 100,000 shares to Aspire Capital, we also have the right, in our sole discretion, to present Aspire Capital with a volume-weighted average price purchase notice, or each a VWAP Purchase Notice, directing Aspire Capital to purchase an amount of stock equal to up to 30% of the aggregate shares of the Company’s common stock traded on the Nasdaq Capital Market on the next trading day, or the VWAP Purchase Date, subject to a maximum number of shares we may determine, or the VWAP Purchase Share Volume Maximum, and a minimum trading price, or the VWAP Minimum Price Threshold (as more specifically described below). The purchase price per Purchase Share pursuant to such VWAP Purchase Notice, or the VWAP Purchase Price, is calculated by reference to the prevailing market price of our common stock (as more specifically described below).
The Purchase Agreement provides that the Company and Aspire Capital will not effect any sales under the Purchase Agreement on any purchase date where the closing sale price of our common stock is less than the Floor Price. This Floor Price and the respective prices and share numbers in the preceding paragraphs will be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction. There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement.
Aspire Capital may not assign its rights or obligations under the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
Investing in our common stock, pre-funded warrants and common stock warrants involves a high degree of risk. YouBefore investing in our common stock, pre-funded warrants and common stock warrants, you should consider carefully the following risks and uncertainties as well as the risks and uncertainties described in the section entitled “Risk Factors” contained discussed under “Risk Factors” in our Annual Reportlatest annual report on Form 10-K forand subsequent quarterly reports on Form 10-Q and current reports on Form 8-K, which are incorporated by reference herein in their entirety. You should carefully consider each of the year ended December 31, 2018, as filedfollowing risks, together with the Securities and Exchange Commission, or SEC, on April 1, 2019, as well as in our subsequent Quarterly and Annual Reports filed with the SEC, which descriptions are incorporatedall other information set forth in this prospectus and incorporated by reference in their entirety, as well as in any prospectus supplement hereto. These risksherein, including our consolidated financial statements and uncertainties are not the only risksrelated notes, before deciding to buy our common stock, pre-funded warrants and uncertainties we face. Additional risks and uncertainties not currently known to us, or that we currently view as immaterial, may also impair our business.common stock warrants. If any of the following risks or uncertainties described in our SEC filings or any additional risks and uncertainties actually occur,occurs, our business financial condition, results of operations and cash flow could be materially and adversely affected.harmed. In that case, the trading price of our common stock could decline, and you mightmay lose all or part of your investment. You should carefully consider the following information about risks, together with the other information contained in this prospectus, before making an investment in our common stock.
We will need to raise substantial additional capital in the future to fund our operations and we may be unable to raise such funds when needed and on acceptable terms.
We will need to raise substantial additional capital in the future to fund our operations. The extent to which we utilize the Purchase Agreement with Aspire Capital as a source of funding will depend on a number of factors, including the prevailing market price of our common stock, the volume of trading in our common stock and the extent to which we are able to secure funds from other sources. The number of shares that we may sell to Aspire Capital under the Purchase Agreement on any given day and during the term of the Purchase Agreement is limited. See “The Aspire Capital Transaction” for additional information. Additionally, we and Aspire Capital may not effect any sales of shares of our common stock under the Purchase Agreement during the continuance of an event of default or on any trading day that the closing sale price of our common stock is less than $0.25 per share. Even if we are able to access the full $6.5 million under the Purchase Agreement, we will still need additional capital to fully implement our business, operating and development plans.
The sale of our common stock to Aspire Capital may cause substantial dilution to our existing stockholders and the sale of the shares of common stock acquired by Aspire Capital could cause the price of our common stock to decline.
We are registering for sale 7,600,000 shares that we may sell to Aspire Capital from time to time under the Purchase Agreement. It is anticipated that shares registered in this offering will be sold until the term of the Purchase Agreement matures on March 31, 2021. The number of shares ultimately offered for sale by Aspire Capital under this prospectus is dependent upon the number of shares we elect to sell to Aspire Capital under the Purchase Agreement. Depending on a variety of factors, including market liquidity of our common stock, the sale of shares under the Purchase Agreement may cause the trading price of our common stock to decline.
Aspire Capital may ultimately purchase all, some or none of the $6.5 million of common stock that is the subject of this prospectus. Aspire Capital may sell all, some or none of our shares that it holds or comes to hold under the Purchase Agreement. Sales by us to Aspire Capital of shares pursuant to the Purchase Agreement may result in dilution to the interests of other holders of our common stock. The sale of a substantial number of shares of our common stock by Aspire Capital in this offering, or anticipation of such sales, could cause the trading price of our common stock to decline or make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise desire.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This prospectus and the documents incorporated by reference herein also contain forward-looking statements that reflectinvolve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks faced by us described below and elsewhere in this prospectus. See “Cautionary Note Regarding Forward-Looking Statements” for information relating to these forward-looking statements.
Risks Related to this Offering
The price of our management’s beliefscommon stock may be highly volatile.
The market price of our securities, like that of many other research and viewsdevelopment public pharmaceutical and biotechnology companies, has been highly volatile and the price of our common stock may be volatile in the future due to a wide variety of factors, including:
| ● | announcements by us or others of results of pre-clinical testing and clinical trials; | |
| ● | our quarterly operating results and performance; | |
| ● | developments or disputes concerning patents or other proprietary rights; | |
| ● | mergers or acquisitions or disposition; | |
| ● | litigation and government proceedings; | |
| ● | adverse legislation or regulatory matters; | |
| ● | changes in government regulations; | |
| ● | our available working capital; | |
| ● | failure of our common stock to continue to be listed or quoted on a national exchange or market system, such as Nasdaq or the New York Stock Exchange | |
| ● | economic and other external factors; and | |
| ● | general market conditions. |
Since January 1, 2023, the closing stock price of our common stock has fluctuated between a high of $1.51 per share to a low of $0.87 per share. On September 27, 2023, the last reported sales prices of our common stock on The Nasdaq Capital Market was $1.03 per share. The fluctuation in the price of our common stock has sometimes been unrelated or disproportionate to our operating performance. In addition, potential dilutive effects of future sales of shares of common stock, options and warrants by us, as well as the potential sale of common stock by the holders of options, warrants and the Debenture could have an adverse effect on the market price of our shares.
Our failure to meet the continued listing requirements of Nasdaq could result in a delisting of our common stock, which would limit the ability of broker-dealers to sell our securities and the ability of shareholders to sell their securities in the secondary market and negatively impact our ability to raise capital.
If we fail to satisfy the continued listing requirements of Nasdaq, Nasdaq may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so.
We have in the past been in noncompliance with other Nasdaq’s continued listing rules. For example, on November 23, 2022, we effected a 1-for-10 reverse stock split of our outstanding common stock to cure our noncompliance, for a period of more than 30 consecutive business days, with Nasdaq Listing Rule 5550(a)(2), which requires listed securities to maintain a minimum bid price of $1.00 per share.
In addition, on April 20, 2023, we received a notification letter from the Listing Qualifications Department of Nasdaq indicating that, as a result of our delay in filing our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, we were not in compliance with the timely filing requirements for continued listing under Nasdaq Listing Rule 5250(c)(1). We regained compliance with this listing rule by filing our Annual Report on Form 10-K on May 2, 2023.
| 8 |
If we are unable to maintain compliance with Nasdaq’s continued listing requirements, our stock could be delisted. In the event of a delisting, we would take action to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s other listing requirements.
If our common stock were to be delisted from Nasdaq, it would have a material negative impact on the actual and potential liquidity of our securities, as well as a material negative impact on our ability to raise future capital. If, for any reason, Nasdaq were to delist our common stock from trading on its exchange and we were unable to obtain listing on another national securities exchange or take action to restore our compliance with the Nasdaq continued listing requirements, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our stockholders:
| ● | the liquidity of our common stock; | |
| ● | the market price of our common stock; | |
| ● | our ability to obtain financing for the continuation of our operations; | |
| ● | the number of institutional and general investors that will consider investing in our securities; | |
| ● | the number of market makers in our common stock; | |
| ● | the availability of information concerning the trading prices and volume of our common stock; and | |
| ● | the number of broker-dealers willing to execute trades in shares of our common stock. |
Further, we would likely become a “penny stock”, which would make trading of our common stock much more difficult.
Investors will experience immediate and substantial dilution as a result of this offering and may suffer substantial dilution related to issued stock warrants and options.
Investors will incur immediate and substantial dilution as a result of this offering. After giving effect to this offering for aggregate gross proceeds of $ million, based on a public offering price of $ per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on , 2023, assuming no sale of pre-funded warrants and no exercise of any common stock warrants issued in this offering, and after deducting estimated offering expenses payable by us, investors in this offering can expect immediate dilution of $ per share of common stock. See “Dilution.”
As of September 27, 2023, we had outstanding options to purchase 416,215 shares of common stock, at a weighted average exercise price of $35.50, and warrants to purchase 4,059,934 shares of common stock, at a weighted average exercise price of $2.19. In addition, the Debenture is convertible, at any time, and from time to time, at the holder’s option, into shares of our common stock, subject to the terms and conditions described in the Debenture, and, subject to the terms and conditions described in the Debenture, we may elect to pay all or a portion of the $110,000 Monthly Redemption Amount (as defined in the Debenture) and/or interest required by the Debenture in shares of our common stock. On July 13, 2023, we obtained stockholder approval, for purposes of complying with Nasdaq Listing Rule 5635(d), the issuance to Alpha Capital Anstalt (“Alpha”) of more than 20% of our issued and outstanding common stock pursuant to the terms and conditions of (a) the Debenture, and (b) our common stock purchase warrant dated December 22, 2022 issued to Alpha. After the first two monthly redemptions, we may elect to pay all or a portion of a Monthly Redemption Amount in shares of our common stock, based on a conversion price equal to the lesser of (i) the then conversion price of the Debenture and (ii) 85% of the average of the VWAPs (as defined in the Debenture) for the five consecutive trading days ending on the trading day that is immediately prior to the applicable Monthly Redemption Date (such average, the “VWAP Price”), subject to the Equity Conditions (as defined in the Debenture) having been satisfied. Accordingly, to the extent that the VWAP Price of our common stock is less than approximately $1.55 per share immediately prior to the applicable Monthly Redemption Date for which we have elected to make a payment of the Monthly Redemption Amount in shares instead of cash, the number of shares we issue to satisfy the Monthly Redemption Amount will increase. As of September 27, 2023, approximately 1,762,396 shares of common stock were issuable under the Debenture, based on the closing sale price of the Company’s common stock as reported on Nasdaq on September 27, 2023.
We also have an incentive compensation plan for our management, employees and consultants and an employee stock purchase plan, which has been temporarily suspended. We have granted, and expect to grant in the future, options to purchase shares of our common stock to our directors, employees and consultants. To the extent that options are exercised, our stockholders will experience dilution and our stock price may decrease.
The sale, or even the possibility of a sale, of the shares of common stock underlying these options, warrants and the Debenture could have an adverse effect on the market price for our securities or on our ability to obtain future financing.
If the offering price of the common stock or the exercise price of the common stock warrants in this offering is lower than the current exercise price of certain of our outstanding warrants with anti-dilution price protection provisions, then, as a result of this offering, such outstanding warrants will have their exercise prices reduced to the offering price.
If the offering price of the common stock or the exercise price of the common stock warrants in this offering is lower than $1.32 per share, which is the current lowest exercise price among our outstanding warrants with anti-dilution price protection provisions, then, as a result of this offering, such warrants, which, prior to this offering, are exercisable for up to 3,856,619 shares of our common stock, will have their exercise prices reduced to at least the offering price per share in this offering. These warrants include: (i) certain Series C preferred stock warrants originally issued in 2004 (as subsequently extended and exchanged for our common stock purchase warrants) which, prior to this offering, are currently exercisable for up to 1,349,571 shares of our common stock, (ii) warrants issued to Alpha in May 2020 which, prior to this offering, are currently exercisable for up to 7,048 shares of our common stock, and (iii) a common stock purchase warrant issued to Alpha in December 2022 which, prior to this offering, is currently exercisable for up to 2,500,000 shares of our common stock.
If the offering price of the common stock or the exercise price of the common stock warrants in this offering is lower than the current conversion price of the Debenture issued to Alpha, then, as a result of this offering, such conversion price will be reduced to the offering price and therefore the number of shares of common stock issuable upon full conversion of the Debenture will increase.
If the offering price of the common stock or the exercise price of the common stock warrants in this offering is lower than $1.32 per share, which is the current conversion price of the Debenture, then this offering could be considered a “Dilutive Issuance” (as defined below) and the conversion price of the Debenture shall be reduced to equal the offering price per share in this offering. As a result, the number of shares of common stock issuable upon full conversion of the Debenture will increase. As an example, if the offering price of the common stock and the exercise price of the common stock warrants in this offering equals the assumed offering price of $ , then the Debenture would be convertible into approximately shares instead of the shares of common stock the Debenture is convertible into prior to this offering.
Our shares of common stock are thinly traded, so stockholders may be unable to sell at or near ask prices or at all if they need to sell shares to raise money or otherwise desire to liquidate their shares.
Our common stock has from time to time been “thinly-traded,” meaning that the number of persons interested in purchasing our common stock at or near ask prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we become more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give stockholders any assurance that a broader or more active public trading market for our common shares will develop or be sustained, or that current trading levels will be sustained.
| 9 |
We do not currently intend to pay dividends on our common stock in the foreseeable future, and consequently, our stockholders’ ability to achieve a return on their investment will depend on appreciation in the price of our common stock.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any cash dividends to holders of our common stock in the foreseeable future. Consequently, our stockholders must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Upon our dissolution, our stockholders may not recoup all or any portion of their investment.
In the event of our liquidation, dissolution or winding-up, whether voluntary or involuntary, the proceeds and/or our assets remaining after giving effect to such transaction, and the payment of all of our debts and liabilities, including the Debenture, will be distributed to the holders of common stock on a pro rata basis. There can be no assurance that we will have available assets to pay to the holders of common stock, or any amounts, upon such a liquidation, dissolution or winding-up. In this event, our stockholders could lose some or all of their investment.
Our board of directors can, without stockholder approval, cause preferred stock to be issued on terms that adversely affect holders of our common stock.
Under our Amended and Restated Certificate of Incorporation (as amended, the “Certificate of Incorporation”), our board of directors is authorized to issue up to 15,000,000 shares of preferred stock, of which none are issued and outstanding as of the date of this prospectus. Also, our board of directors, without stockholder approval, may determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares. If our board of directors causes shares of preferred stock to be issued, the rights of the holders of our common stock would likely be subordinate to those of preferred holders and therefore could be adversely affected. Our board of directors’ ability to determine the terms of preferred stock and to cause its issuance, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire a majority of our outstanding common stock. Preferred shares issued by our board of directors could include voting rights or super voting rights, which could shift the ability to control the Company to the holders of the preferred stock. Preferred stock could also have conversion rights into shares of our common stock at a discount to the market price of our common stock, which could negatively affect the market for our common stock. In addition, preferred stock would have preference in the event of liquidation of the Company, which means that the holders of preferred stock would be entitled to receive the net assets of the Company distributed in liquidation before the holders of our common stock receive any distribution of the liquidated assets.
Our management will have broad discretion over the use of the net proceeds from this offering and we may use the net proceeds in ways with which you disagree, or which do not produce beneficial results.
We currently intend to use the net proceeds from this offering for our operations and for other general corporate purposes, including, but not limited to, our internal research and development programs and the development of new programs, general working capital and possible future acquisitions (see “Use of Proceeds”). We have not allocated specific amounts of the net proceeds from this offering for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us or our stockholders. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, and results of operation.
This is a best efforts offering; no minimum amount of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business.
The Placement Agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, Placement Agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth in this prospectus. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to pursue the business goals outlined in this prospectus. Thus, we may not raise the amount of capital we believe is required for our business and may need to raise additional funds, which may not be available or available on terms acceptable to us. Despite this, any proceeds from the sale of securities offered by us will be available for our immediate use, and because there is no escrow account and no minimum offering amount in this offering, investors could be in a position where they have invested in us, but we are unable to fulfill our objectives due to a lack of interest in this offering.
| 10 |
There is no public market for the pre-funded warrants and common stock warrants being offered in this offering.
There is no established public trading market for the pre-funded warrants or common stock warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants or common stock warrants on any securities exchange or nationally recognized trading system. Without an active market, the liquidity of the pre-funded warrants and common stock warrants will be extremely limited.
The warrants offered by this prospectus may not have any value.
The common stock warrants have an exercise price of $ per share and will have a five-year term. In the event our common stock price does not exceed the exercise price of the common warrants during the period when the warrants are exercisable, the common stock warrants may not have any value.
Holders of the pre-funded warrants or common stock warrants will not have rights of holders of our shares of common stock until such pre-funded warrants or common stock warrants are exercised.
The pre-funded warrants or common stock warrants in this offering do not confer any rights of share ownership on their holders, but rather merely represent the right to acquire shares of our common stock at a fixed price. Until holders of pre-funded warrants or common stock warrants acquire shares of our common stock upon exercise of the pre-funded warrants or common stock warrants, as applicable, holders of pre-funded warrants or common stock warrants will have no rights with respect to future eventsour shares of common stock underlying such pre-funded warrants or common stock warrants.
If we do not maintain a current and are subjecteffective registration statement relating to substantial risksthe common stock issuable upon exercise of the pre-funded warrants and uncertaintiescommon stock warrants being offered in this offering, holders will be able to exercise such warrants on a “cashless” basis and we may not receive any additional funds upon the exercise of such warrants.
If we do not maintain a current and effective registration statement relating to the common stock issuable upon exercise of the pre-funded warrants and common stock warrants being offered in this offering, such warrants may be exercised by way of a “cashless” exercise, meaning that the holder would not pay a cash purchase price upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the warrant. Accordingly, we may not receive any additional funds upon the exercise of such warrants.
Purchasers who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase agreement.
In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement.
Additionally, in connection with this offering, we may agree to amend the terms of certain of our outstanding warrants held by certain significant purchasers in this offering who will enter into the securities purchase agreement. Any such amendments may, among other things, decrease the exercise prices to be the same as the exercise prices of the common stock warrants offered in this offering, or increase the term of exercise of those warrants.
| 11 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the information incorporated herein by reference contain forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934,Act. These forward-looking statements are often identified by words such as amended“may,” “should,” “would,” “expect,” “intend,” “anticipate,” “believe,” “estimate,” “continue,” “plan,” “potential” and the safe harbor provisionssimilar expressions. These statements involve estimates, assumptions and uncertainties that could cause actual results to differ materially from those expressed for the U.S. Private Securities Litigation Reform Act of 1955. All statements, other than statements of historical fact, containedreasons described in this prospectus and in the documents incorporated herein by reference herein, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, arereference. You should not place undue reliance on these forward-looking statements. In some cases, forward-looking statements can be identified by the use of forward-looking words such as “aim,” “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “pro forma,” “project,” “seek,” “should,” “target,” “will,” “would” or other similar words or expressions (including their use in the negative), or by discussions of future matters such as the development of products, technology enhancements, possible changes in legislation, and other statements that are not historical.
We have based theseYou should be aware that our actual results could differ materially from those contained in the forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, resultsdue to a number of operations, business strategy and financial needs. These forward-looking statements are subject to known and unknown risks, uncertainties and assumptions, including risks described in “Risk Factors” and elsewhere in this prospectus and the documents incorporated by reference herein, regarding, among other things:factors, including:
| ● | ||
| there can be no assurance that we will successfully develop any drugs or therapeutic devices; | ||
| ● | ||
| candidate drugs or therapeutic devices will be successful; | ||
| ● | ||
| trials will be approved to begin by or will actually begin by or will proceed as contemplated by any projected timeline; | ||
| ● | ||
| projected timeline; | ||
| ● | ||
| products or lack negative impacts; | ||
| ● | ||
| there can be no assurance that any drugs or therapeutic devices will receive required regulatory approvals or that they will be commercially successful; | ||
| ● | there can be no assurance that we will be able to procure or earn sufficient working capital to complete the development, testing and launch of our | |
| prospective therapeutic products; | ||
| ● | there can be no assurance that patents will issue on our | |
| in-licensed patent applications; | ||
| ● | ||
| in-licensed patents would prevent competition; | ||
| ● | ||
| diagnostic products generally, including as a result of FastPack reimbursement pricing challenges; | ||
| ● | ||
| business strategy; | ||
| ● | we are and will continue to face competition, including the | |
| products superior to our products; and | ||
| ● | other factors, including those set forth under the | |
We may not actually achieve
You should also consider carefully the plans, intentions or expectations disclosedstatements under the section titled “Risk Factors” in this prospectus, and documents incorporated herein by reference including the sections titled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” incorporated by reference from our most recent Annual Report on Form 10-K and in our Quarterly Reports on Form 10-Q, as well as any amendments thereto, filed with the SEC, which address additional factors that could cause our actual results to differ from those set forth in the forward-looking statements. We operatestatements and could materially and adversely affect our business, operating results and financial condition. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in a very competitivetheir entirety by the applicable cautionary statements.
The forward-looking statements speak only as of the date on which they are made, and, rapidly changing environment. Itexcept to the extent required by federal securities laws, we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is not possible for our managementmade or to predict all risks, nor canreflect the occurrence of unanticipated events. In addition, we cannot assess the impact of all factorseach factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make, and accordingly you should not place undue reliance on our forward-looking statements. We have included important factors in the cautionary statements included in this prospectus, particularly in the “Risk Factors” section in this prospectus and the documents incorporated by reference herein, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus, the documents incorporated by reference herein and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus and the documents incorporated by reference herein by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
THE ASPIRE CAPITAL TRANSACTIONUSE OF PROCEEDS
GeneralWe estimate that net proceeds from this offering will be approximately $ after deducting estimated Placement Agent fees and estimated offering expenses payable by us, and assuming no sale of any pre-funded warrants in this offering.
The Purchase Agreement provides that, uponWe intend to use the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $6.5 million of shares of our common stock, or the Purchase Shares, until the term of the Purchase Agreement matures on March 31, 2021.
Concurrently with entering into the Purchase Agreement, we also entered into a registration rights agreement with Aspire Capital, or the Registration Rights Agreement, in which we agreed to file one or more registration statements, including the registration statement of which this prospectus is a part, as permissible and necessary to register under the Securities Act of 1933, as amended, or the Securities Act,net proceeds from the sale of the sharessecurities offered by us pursuant to this prospectus, if any, for our operations and for other general corporate purposes, including, but not limited to, our internal research and development programs and the development of new programs, general working capital and possible future acquisitions. We have not determined the amount of net proceeds to be used specifically for such purposes and, as a result, management will retain broad discretion over the allocation of net proceeds. The occurrence of unforeseen events or changed business conditions could result in the application of the net proceeds from this offering in a manner other than as described in this prospectus. Pending their uses, we intend to invest the net proceeds of this offering in interest-bearing bank accounts or in short-term, interest-bearing, investment-grade securities.
| 13 |
We have not paid cash dividends on our common stock, that may be issued to Aspire Capital under the Purchase Agreement.
As of July 23, 2019 there were 9,350,026 shares of our common stock outstanding, excluding the 7,600,000 shares of common stock that we may issue to Aspire Capital after this registration statement is declared effective under the Securities Act. If all of such 7,600,000 shares of our common stock offered hereby were issued and outstanding as of the date hereof, such shares would represent 44.8% of the total common stock outstanding as of the date hereof. The number of shares of our common stock ultimately offered for sale by Aspire Capital is dependent upon the number of shares purchased by Aspire Capital under the Purchase Agreement.
Pursuant to the terms of the Purchase Agreement, the aggregate number of shares that we may issue to Aspire Capital under the Purchase Agreement may in no case exceed 1,807,562 shares of our common stock, unless (i) stockholder approval is obtained to issue more, in which case this 1,807,562 share limitation will not apply, or (ii) stockholder approval has not been obtained and at any time the 1,807,562 share limitation is reached and at all times thereafter the average price paid for all shares issued under the Purchase Agreement is equal to or greater than $0.86, or the Minimum Price; provided that at no point in time shall Aspire Capital (together with its affiliates) beneficially own more than 19.99% of our common stock.
Pursuant to the Purchase Agreement and the Registration Rights Agreement, we are registering 7,600,000 shares of our common stock under the Securities Act that we may issue to Aspire Capital after this registration statement is declared effective under the Securities Act. All 7,600,000 shares of common stock are being offered pursuant to this prospectus. Under the Purchase Agreement, we have the right but not the obligation to issue more than the 7,600,000 shares of common stock included in this prospectus to Aspire Capital. As of the date hereof, we do not have any plansanticipate that we will declare or intent to issue to Aspire Capital any shares of common stock in addition to the 7,600,000 shares of common stock offered hereby.
After the SEC has declared effective the registration statement of which this prospectus is a part,pay dividends on any trading day on which the closing sale price of our common stock exceeds $0.25, we have the right, in our sole discretion, to present Aspire Capital with a purchase notice, or each a Purchase Notice, directing Aspire Capital (as principal) to purchase up to 100,000 shares of our common stock per trading day, up to $6.5 million of our common stock in the aggregate at a per share price, orforeseeable future. Payment of dividends, if any, is within the Purchase Price, calculated by reference to the prevailing market pricesole discretion of our common stock (as more specifically described below); however, in no event shallboard of directors and will depend, among other factors, upon our earnings, capital requirements and our operating and financial condition. To the shares purchased exceed Five Hundred Thousand Dollars ($500,000) per business day.extent we have any earnings, we likely will retain earnings to pay down debt, or expand corporate operations and not use such earnings to pay dividends.
In addition, on any date on which we submit a Purchase Notice to Aspire Capital for 100,000 shares, we also have the right, in our sole discretion, to present Aspire Capital with a VWAP Purchase Notice directing Aspire Capital to purchase an amount of stock equal to up to 30% of the aggregate shares of our common stock traded on the Nasdaq Capital Market on the next trading day, subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold. The VWAP Purchase Price is calculated by reference to the prevailing market price of our common stock (as more specifically described below).
The Purchase Agreement provides that neither we nor Aspire Capital will effect any sales under the Purchase Agreement on any purchase date where the closing sale price of our common stock is less than the Floor Price. There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement.
Aspire Capital may not assign its rights or obligations under the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
Purchase Of Shares Under The Common Stock Purchase AgreementCAPITALIZATION
Under the termsThe following table sets forth our cash, total long-term liabilities and capitalization as of the Purchase Agreement, on any trading day selected by us on which the closing sale price of our common stock exceeds $0.25 per share, we may direct Aspire Capital to purchase up to 100,000 shares of our common stock per trading day. The Purchase Price of such shares is equal to the lesser of:June 30, 2023 on:
| ● | an actual basis; and | |
| ● | on an as adjusted basis, to give effect to this offering for aggregate gross proceeds of up to $ , based on a public offering price of $ per share of common stock, which was the |
You should read this capitalization table together with the section titled “Use of Proceeds” in this prospectus, and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included in our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2023, which are incorporated by reference in this prospectus.
| At June 30, 2023 | ||||||||
| Actual | As Adjusted (unaudited) | |||||||
| Cash | $ | 1,341,659 | $ | |||||
| Total Liabilities | 9,841,528 | |||||||
| Stockholders’ equity: | ||||||||
| Qualigen Therapeutics, Inc. stockholders’ equity: | ||||||||
| Common stock, $0.001 par value; 225,000,000 shares authorized; 5,052,463 shares issued and outstanding as of June 30, 2023 | 42,952 | |||||||
| Additional paid-in capital | 112,554,830 | |||||||
| Accumulated other comprehensive income | 131,891 | |||||||
| Accumulated deficit | (110,695,598 | ) | ||||||
| Total Qualigen Therapeutics, Inc. stockholders’ equity | 2,034,075 | |||||||
| Noncontrolling interest | 1,273,969 | |||||||
| Total stockholders’ equity | 3,308,044 | |||||||
| Total capitalization | $ | 13,149,572 | ||||||
The number of shares of common stock to be outstanding after this offering set forth in the table above is based on 5,052,463 shares of common stock outstanding on June 30, 2023, does not give effect to the shares of common stock issuable upon exercise of the pre-funded warrants and common stock warrants issued in this offering and excludes:
| ● | 445,163 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2023, at a weighted average exercise price of $34.68 per share; | |
| ● |
In addition, on any date on which we submit a Purchase Notice to Aspire Capital for purchase of 100,000 shares, we also have the right to direct Aspire Capital to purchase an amount of stock equal to up to 30% of the aggregate shares of our common stock traded on the Nasdaq Capital Market on the next trading day, subject to the VWAP Purchase Share Volume Maximum and the VWAP Minimum Price Threshold, which is equal to the greater of (a) 80% of the closing price of the Company’s common stock on the business day immediately preceding the VWAP Purchase Date or (b) such higher price as set forth by the Company in the VWAP Purchase Notice. The VWAP Purchase Price of such shares is the lower of:
| ● |
| ● | ||
| ● |
The Purchase Price will be adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the trading day(s) used to compute the Purchase Price. We may deliver multiple Purchase Notices and VWAP Purchase Notices to Aspire Capital from time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed.
| 15 |
UnderPurchasers of common stock, or pre-funded warrants, and accompanying common stock warrants in this offering will experience immediate dilution to the Purchase Agreement, weextent of the difference between the public offering price per share of common stock in this offering and Aspire Capital may not effect any salesthe net tangible book value per share of common stock immediately after this offering.
Our net tangible book value as of June 30, 2023 was approximately $(3.2) million, or $(0.62) per share of common stock. Net tangible book value per share is determined by dividing the net of total tangible assets less total liabilities, by the aggregate number of shares of our common stock underoutstanding as of June 30, 2023.
After giving further effect to this offering for aggregate gross proceeds of up to $ , based on a public offering price of $ per share, which was the Purchase Agreement on any trading day that the closinglast reported sale price of our common stock is less thanon the Floor Price.
EventsNasdaq Capital Market on , 2023, assuming no sale of Default
No sales are permitted to be made under the Purchase Agreement upon the occurrencepre-funded warrants and no exercise of any of the following, among other, events of default:
Our Termination Rights
The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
No Short-Selling or Hedging by Aspire Capital
Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of our common stock during any time prior to the termination of the Purchase Agreement.
Effect of Performance of the Purchase Agreement on Our Stockholders
The Purchase Agreement does not limit the ability of Aspire Capital to sell any or all of the 7,600,000 shares registered in this offering. It is anticipated that shares registered in this offering will be sold until the term of the Purchase Agreement matures on March 31, 2021. The sale by Aspire Capital of a significant amount of shares registered in this offering at any given time could cause the market price of our common stock to decline and/or to be highly volatile. Aspire Capital may ultimately purchase all, some or none of the 7,600,000 shares of common stock not yetwarrants issued but registered in this offering, and it may sell all, some or none of the shares issued to it by us. Therefore, sales to Aspire Capital by us pursuant to the Purchase Agreement also may result in substantial dilution to the interests of other holders of our common stock. However, we have the right to control the timing and amount of any sales of our shares to Aspire Capital and the Purchase Agreement may be terminated by us at any time at our discretion without any penalty or cost to us.
Percentage of Outstanding Shares After Giving Effect to the Purchased Shares Issued to Aspire Capital
In connection with entering into the Purchase Agreement, we authorized the sale to Aspire Capital of up to $6.5 million of our shares of common stock, which may be sold by us to Aspire Capital until the term of the Purchase Agreement matures on March 31, 2021. We will sell no more than 7,600,000 shares to Aspire Capital under the Purchase Agreement, all of which are included in this offering. Subject to any required approval by our board of directors, we have the right but not the obligation to issue more than the 7,600,000 shares included in this prospectus to Aspire Capital under the Purchase Agreement. In the event we elect to issue more than 7,600,000 shares under the Purchase Agreement, we will be required to file a new registration statement and have it declared effective by the SEC. The number of shares ultimately offered for sale by Aspire Capital in this offering is dependent upon the number of shares purchased by Aspire Capital under the Purchase Agreement. The following table sets forth the number and percentage of outstanding shares to be held by Aspire Capital after giving effect to the sale of shares of common stock issued to Aspire Capital at varying purchase prices:
| Assumed Average Purchase Price | Estimated Proceeds from the Sale of Purchase Shares to Aspire Capital Under the Purchase Agreement Registered in this Offering | Estimated Number of Purchase Shares to be Issued in this Offering at the Assumed Average Purchase Price | Estimated Percentage of Outstanding Shares After Giving Effect to the Purchased Shares Issued to Aspire Capital (1) | |||||||||||
| $ | .50 | $ | 3,800,000 | 7,600,000 | 44.8 | % | ||||||||
| $ | 1.00 | $ | 6,500,000 | 6,500,000 | 41.0 | % | ||||||||
| $ | 1.50 | $ | 6,500,000 | 4,333,333 | 32.7 | % | ||||||||
| $ | 2.00 | $ | 6,500,000 | 3,250,000 | 25.8 | % | ||||||||
| $ | 3.00 | $ | 6,500,000 | 2,166,666 | 18.8 | % | ||||||||
| $ | 3.50 | $ | 6,500,000 | 1,857,142 | 16.6 | % | ||||||||
| $ | 4.00 | $ | 6,500,000 | 1,625,000 | 14.8 | % | ||||||||
This prospectus relates to shares of our common stock that may be offered and sold from time to time by Aspire Capital. We will not receive any proceeds upon the sale of shares by Aspire Capital. However, we may receive proceeds up to $6.5 million from the sale of shares to Aspire Capital under the Purchase Agreement. The proceeds received from the sale of the shares under the Purchase Agreement will be used for working capital and general corporate purposes, including research and development activities. This anticipated use of net proceeds from the sale of our common stock to Aspire Capital under the Purchase Agreement represents our intentions based upon our current plans and business conditions.
The sale of our common stock to Aspire Capital pursuant to the Purchase Agreement will have a dilutive impact on our stockholders. As a result, our net income/(loss) per share would decrease/increase in future periods and the market price of our common stock could decline. In addition, the lower our stock price is at the time we exercise our right to sell shares to Aspire Capital, the more shares of our common stock we will have to issue to Aspire Capital pursuant to the Purchase Agreement and our existing stockholders would experience greater dilution.
After giving effect to the sale of 7,600,000 shares of common stock (the number of shares registered in this offering) at an assumed offering price of $1.01 per share (the closing price of our common stock on July 23, 2019), and after deducting the estimated offering expenses of $60,000$ payable by us, our pro formaas adjusted net tangible book value as of March 31, 2019June 30, 2023 would have been $12.3 million,$ , or $0.74$ per share of common stock. This represents an immediate increase in pro formathe net tangible book value of $0.23$ per share to our existing shareholdersstockholders and an immediate dilution in pro forma net tangible book value of $0.27 per share to investors participating in this offering.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share | $ | 1.01 | ||||||
| Net tangible book value per share as of March 31, 2019 | $ | 0.51 | ||||||
| Increase in net tangible book value per share attributable to this offering | $ | 0.23 | ||||||
| Pro forma net tangible book value per share after this offering | $ | 0.74 | ||||||
| Dilution per share to investors participating in this offering | $ | 0.27 |
The shares sold in this offering, if any, may be sold from time to time at various prices.
Each $.50 increase in the per share price at which we sell shares to Aspire Capital under the Purchase Agreement from the assumed offering price of $1.01 per share would increase our pro forma net tangible book value by $3.8 million, our pro forma net tangible book value per share by $0.23 and dilution$ per share to new investors purchasing shares of common stocksecurities in this offering by $0.27, assuming that the number of shares of common stock offered, as set forth on the cover page of this prospectus, remains the same and after deducting estimated aggregate offering expenses payable by us. This information is supplied for illustrative purposes only.offering.
The table and calculations set forth above are basedbelow illustrates this dilution on the numbera per-share basis, assuming no sale of shares of common stock outstanding as of March 31, 2019 and assumes no exercise of any outstanding options or warrants. To the extent that options orpre-funded warrants, are exercised, there will be further dilution to new investors.
The selling stockholder may from time to time offer and sell any or all of the shares of our common stock set forth below pursuant to this prospectus. When we refer to the “selling stockholder” in this prospectus, we mean the entity listed in the table below, and its respective pledgees, donees, permitted transferees, assignees, successors and others who later come to hold any of the selling stockholder’s interests in shares of our common stock other than through a public sale.
The following table sets forth, as of the date of this prospectus, the name of the selling stockholder for whom we are registering shares for sale to the public, the number of shares of common stock beneficially owned by the selling stockholder prior to this offering, the total number of shares of common stock that the selling stockholder may offer pursuant to this prospectus andwhich, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis, and excludes the selling stockholder will beneficially own after thisproceeds, if any, from the exercise of any pre-funded warrants or common stock warrants issued in the offering. Except as noted below, the selling stockholder does not have, or within the past three years has not had, any material relationship with us or any of our predecessors or affiliates and the selling stockholder is not or was not affiliated with registered broker-dealers.
Based on the information provided to us by the selling stockholder, assuming that the selling stockholder sells all of the shares of our common stock beneficially owned by it that have been registered by us and does not acquire any additional shares during the offering, the selling stockholder will not own any shares other than those appearing in the column entitled “Beneficial Ownership After This Offering.” We cannot advise you as to whether the selling stockholder will in fact sell any or all of such shares of common stock. In addition, the selling stockholder may have sold, transferred or otherwise disposed of, or may sell, transfer or otherwise dispose of, at any time and from time to time, the shares of our common stock in transactions exempt from the registration requirements of the Securities Act of 1933 after the date on which it provided the information set forth in the table below.
| Assumed public offering price per share of common stock | $ | |||||||
| Net tangible book value per share as of June 30, 2023 | $ | |||||||
| Increase in net tangible book value per share attributable to new investors in this offering | $ | |||||||
| As adjusted net tangible book value per share as of June 30, 2023, after giving effect to this offering (1) | $ | |||||||
| Dilution per share to new investors purchasing securities in this offering | $ | |||||||
| Beneficial Ownership After this Offering(1) | ||||||||||||||||||||
| Name | Shares of Beneficially Owned Prior to this Offering | Percentage of Outstanding Shares Beneficially Owned Prior to this Offering | Shares of Common to be Sold in the Offering | Number of Shares | %(2) | |||||||||||||||
| Aspire Capital Fund, LLC(3) | 0 | (4) | — | % | 7,600,000 | 0 | — | % | ||||||||||||
| (1) | A $0.50 increase or decrease in the assumed public offering price per share of common stock and accompanying common stock warrant of $ , which was the last reported sale price of Similarly, an increase of 500,000 in the shares of common stock |
The common stock offered by this prospectus is being offered by Aspire Capital, the selling stockholder. The common stock may be sold or distributed from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the common stock offered by this prospectus may be effected in one or more of the following methods:above table excludes:
| ● | ||
| ● | ||
| ● | ||
| ● | ||
| ● | ||
In order to comply withTo the securities laws of certain states, if applicable,extent that options or warrants are exercised, new options are issued under our 2020 Plan, shares are issued under the shares may be sold only through registeredDebenture, or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the registration or qualification requirement is available and complied with.
The selling stockholder may transfer thewe issue additional shares of common stock by other means not describedin the future, there may be further dilution to investors participating in this prospectus.offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
Brokers, dealers, underwriters, or agents participatingBecause there is no minimum offering amount required as a condition to the closing of this offering, the dilution per share to purchasers in the distributionoffering may be more than that indicated above in the event that the actual number of shares sold, if any, is less than the maximum number of shares of our common stock we are offering.
| 16 |
The following description of the terms of our securities is not complete and is qualified in its entirety by reference to our Certificate of Incorporation, and our amended and restated bylaws (the “Bylaws”), both of which are filed as exhibits to our Annual Report on Form 10-K.
Under our Certificate of Incorporation and Bylaws, we are authorized to issue 240,000,000 shares of capital stock, consisting of 225,000,000 shares of common stock, par value $0.001 per share, and 15,000,000 shares of preferred stock, $0.001 par value per share, including 7,000 shares that have been designated as agentsSeries Alpha Preferred Stock. As of September 27, 2023, there were 5,052,463 shares of our common stock outstanding and no shares of our Series Alpha Preferred Stock outstanding.
Common Stock
Pursuant to the terms of our Certificate of Incorporation, the holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders, except on matters relating solely to terms of preferred stock. Subject to preferences that may be applicable to any outstanding preferred stock, the holders of common stock will be entitled to receive compensationratably such dividends, if any, as may be declared from time to time by our Board of Directors out of funds legally available therefor. In the event of liquidation, dissolution or winding up, the stockholders will be entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The holders of our common stock will have no preemptive or conversion rights or other subscription rights. There will be no redemption or sinking fund provisions applicable to our common stock.
Preferred Stock
Pursuant to the formterms of commissions, discounts,our Certificate of Incorporation, our Board of Directors has the authority to issue preferred stock in one or concessions frommore classes or series and to fix the selling stockholder and/designations, powers, preferences and rights, and the qualifications, limitations or purchasersrestrictions thereof, including dividend rights, conversion right, voting rights, terms of redemption, liquidation preferences and the number of shares constituting any class or series, without further vote or action by the stockholders.
The issuance of shares of preferred stock, or the issuance of rights to purchase such shares, may decrease the amount of earnings and assets available for distribution to the holders of common stock, could adversely affect the rights and powers, including voting rights, of the common stock, for whomand could have the broker-dealers may act as agent. Aspire Capital has informedeffect of delaying, deterring or preventing a change of control of us that each such broker-dealer will receive commissions from Aspire Capital which will not exceed customary brokerage commissions.or an unsolicited acquisition proposal.
Aspire Capital isStock Options
As of September 27, 2023, we had outstanding options to acquire 416,215 shares of our common stock, having a weighted-average exercise price of $35.50 per share.
Warrants
As of September 27, 2023, we had outstanding warrants to purchase an “underwriter” withinaggregate of 4,059,934 shares of our common stock, having a weighted-average exercise price of $2.19 per share.
If the meaningoffering price of the Securities Act.common stock or the exercise price of the common stock warrants in this offering is lower than $1.32 per share, which is the current lowest exercise price among our outstanding warrants with anti-dilution price protection provisions, as a result of this offering, such warrants, which are exercisable for up to 3,856,619 shares of our common stock, will have their exercise prices reduced to at least the offering price per share in this offering. These warrants include: (i) certain Series C preferred stock warrants originally issued in 2004 (as subsequently extended and exchanged for our common stock purchase warrants) which, prior to this offering, are exercisable for up to 1,349,571 shares of our common stock, (ii) warrants issued to Alpha in May 2020 which, prior to this offering, are exercisable for up to 7,048 shares of our common stock, and (iii) a common stock purchase warrant issued to Alpha in December 2022 which, prior to this offering, is currently exercisable for up to 2,500,000 shares of our common stock.
NeitherDebenture
On December 22, 2022, we nor Aspire Capital can presently estimateissued to Alpha an 8% Senior Convertible Debenture in the aggregate principal amount of compensation that any agent will receive. We know$3,300,000 for a purchase price of no existing arrangements between Aspire Capital, any other stockholder, broker, dealer, underwriter, or agent relating$3,000,000 pursuant to the sale or distributionterms of a Securities Purchase Agreement, dated December 21, 2022 (the “2022 Securities Purchase Agreement”). The Debenture has a maturity date of December 22, 2025 and is convertible, at any time, and from time to time, until the Debenture is no longer outstanding, at Alpha’s option, into shares offered by this prospectus. Atof our common stock (the “Conversion Shares”), at a price equal to $1.32 per share, subject to adjustment as described in the time a particular offerDebenture and other terms and conditions described in the Debenture. On July 13, 2023, we obtained stockholder approval, for purposes of shares is made, a prospectus supplement, if required, will be distributed that will set forthcomplying with Nasdaq Listing Rule 5635(d), for the namesissuance to Alpha of any agents, underwriters, or dealersmore than 20% of our issued and any compensation from the selling stockholder, and any other required information.
We will pay all of the expenses incident to the registration, offering, and sale of the shares to the public other than commissions or discounts of underwriters, broker-dealers, or agents. We have agreed to indemnify Aspire Capital and certain other persons against certain liabilities in connection with the offering ofoutstanding shares of common stock offered hereby, including liabilities arisingpursuant to the terms and conditions of (a) the Debenture, and (b) the common stock purchase warrant dated December 22, 2022 issued by us to Alpha. Between January 9 and 12, 2023, we issued 841,726 shares of common stock upon Alpha’s partial conversion of the Debenture at $1.32 per share for a total of $1,111,078 principal. As of September 27, 2023, we paid an aggregate of $440,000 as the Monthly Redemption Amounts (as defined below) under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paidDebenture in respectcash for June, July, August and September. As of such liabilities. Aspire Capital has agreed to indemnify us against liabilities underSeptember 27, 2023, the Securities Act that may arise from certain written information furnished to us by Aspire Capital specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
Aspire Capital and its affiliates have agreed not to engage in any direct or indirect short selling or hedgingDebenture was convertible into approximately 1,762,396 shares of our common stock, duringbased on the termclosing sale price of the Purchase Agreement.Company’s common stock as reported on Nasdaq on September 27, 2023. The Debenture includes a beneficial ownership blocker of 9.99%, which may only be waived by Alpha upon 61 days’ notice to us.
We have advised Aspire Capital that
| 17 |
Except in respect of an Exempt Issuance (as defined in the 2022 Securities Purchase Agreement), if, at any time while itthe Debenture is engagedoutstanding, we or any of our subsidiaries as listed in a distributionthe 2022 Securities Purchase Agreement, as applicable, sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of the shares included in this prospectus it is requiredor issues (or announces any sale, grant or any option to comply with Regulation M promulgated under the Securities Exchange Act of 1934, as amended. With certain exceptions, Regulation M precludes the selling stockholder, any affiliated purchasers, and any broker-dealerpurchase or other person who participates in the distribution from bidding fordisposition), any shares of our common stock or purchasing, or attempting to inducecommon stock equivalents entitling any person to bidacquire shares of our common stock at an effective price per share that is lower than the then Conversion Price (such lower price, the “Base Conversion Price” and such issuances, collectively, a “Dilutive Issuance”), then simultaneously with the consummation (or, if earlier, the announcement) of each Dilutive Issuance, the Conversion Price will be reduced to equal the Base Conversion Price, provided that the Base Conversion Price will not be less than $0.260 (subject to adjustment for or purchase any security which isreverse and forward stock splits, recapitalizations and similar transactions following the subjectdate of the distribution until2022 Securities Purchase Agreement). Accordingly, if the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize theoffering price of a security in connection with the distribution of that security. Allcommon stock or the exercise price of the foregoing may affectcommon stock warrants in this offering is lower than the marketabilitycurrent conversion price, $1.32 per share, then this offering could be considered a “Dilutive Issuance” and the conversion price of the shares offered herebyDebenture shall be reduced to equal the offering price per share in this prospectus.
We may suspend the sale of shares by Aspire Capital pursuant to this prospectus for certain periods of time for certain reasons, includingoffering.As an example, if the prospectus is required tooffering price of the common stock and the exercise price of the common stock warrants in this offering equals the assumed offering price of $ , then the Debenture would be supplemented or amended to include additional material information.
This offering will terminate on the date that allconvertible into approximately shares offered by this prospectus have been sold by Aspire Capital.
Reed Smith LLP, Los Angeles, California will pass upon the validityinstead of the shares of common stock offered hereby.the Debenture is convertible into prior to this offering.
Commencing June 1, 2023 (the “Initial Monthly Redemption Date”) and continuing on the first day of each month thereafter until the earlier of (i) December 22, 2025 and (ii) the full redemption of the Debenture (each such date, a “Monthly Redemption Date”), we must redeem $110,000 plus accrued but unpaid interest, liquidated damages and any amounts then owing under the Debenture (the “Monthly Redemption Amount”). The Monthly Redemption Amount must be paid in cash; provided that after the first two monthly redemptions, we may elect to pay all or a portion of a Monthly Redemption Amount in shares of our common stock, based on a conversion price equal to the lesser of (i) the then conversion price of the Debenture and (ii) 85% of the average of the VWAPs (as defined in the Debenture) for the five consecutive trading days ending on the trading day that is immediately prior to the applicable Monthly Redemption Date, subject to the Equity Conditions (as defined in the Debenture) having been satisfied, including our receipt of the necessary stockholder approvals. We intend to pay the Monthly Redemption Amount for October 2023 in shares of our common stock, pursuant to a wavier by Alpha of the Equity Conditions. The conversion price to be used for such redemption in shares will be determined pursuant to the formula referenced above. We may also redeem some or all of the then outstanding principal amount of the Debenture at any time for cash in an amount equal to 105% of the then outstanding principal amount of the Debenture being redeemed plus accrued but unpaid interest, liquidated damages and any amounts then owing under the Debenture, subject to the Equity Conditions having been satisfied.
The Debenture accrues interest at the rate of 8% per annum, which does not begin accruing until December 1, 2023, and will be payable on a quarterly basis. Interest may be paid in cash or shares of our common stock or a combination thereof at our option; provided that interest may only be paid in shares if the Equity Conditions have been satisfied, including our receipt of the necessary stockholder approvals.
Except as otherwise set forth in this Debenture, we may not prepay any portion of the principal amount of the Debenture without Alpha’s prior written consent.
Registration Rights
In December 2022, pursuant to the terms of the Securities Purchase Agreement, we entered into a registration rights agreement with Alpha (the “Registration Rights Agreement”), pursuant to which we agreed to file one or more registration statements, as necessary, and to the extent permissible, to register under the Securities Act the resale of the remaining Underlying Shares not otherwise registered under the Company’s registration statement on Form S-3 (File No. 333-266430). The Registration Rights Agreement requires that the Company file, within 30 days after signing, a resale registration statement and use commercially reasonable efforts to cause the resale registration statement to be declared effective by the SEC on or before the 60th calendar day following the date of signing of the Registration Rights Agreement (or 120 days if such registration statement is subject to full review by the SEC). We filed a resale registration statement on Form S-3 pursuant to the requirements of the Registration Rights Agreement on December 2022 (File Number 333-269088), which registration statement was declared effective by the SEC on January 5, 2023. On September 1, 2023, we filed a Post-Effective Amendment No. 1 to Form S-3 on Form S-1 (File No. 333-269088) in order to maintain the registration of the resale by Alpha, which Post-Effective Amendment No. 1 was declared effective by the SEC on September 7, 2023. On September 29, 2023, we filed the final resale prospectus on Form 424(b)(3).
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
The provisions of Delaware law and our Certificate of Incorporation and Bylaws could discourage or make it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or in our best interests. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change of our control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. Such provisions also may have the effect of preventing changes in our management.
Delaware Statutory Business Combinations Provision. We are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law (the “DGCL”). Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. For purposes of Section 203, a “business combination” is defined broadly to include a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and, subject to certain exceptions, an “interested stockholder” is a person who, together with his or her affiliates and associates, owns, or within three years prior, did own, 15% or more of the corporation’s voting stock.
| 18 |
Election and Removal of Directors. Except as may otherwise be provided by the DGCL, any director or the entire board of directors may be removed, with or without cause, at an annual meeting or a special meeting called for that purpose, by the holders of a majority of the shares then entitled to vote at an election of directors, provided a quorum is present. Vacancies on our board of directors resulting from the removal of directors and newly created directorships resulting from any increase in the number of directors may be filled solely by the affirmative vote of a majority of the remaining directors then in office (although less than a quorum) or by the sole remaining director. This system of electing and removing directors may discourage a third party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of our directors. Our Certificate of Incorporation and Bylaws do not provide for cumulative voting in the election of directors.
Advance Notice Provisions for Stockholder Proposals and Stockholder Nominations of Directors. Our Bylaws provide that, for nominations to the board of directors or for other business to be properly brought by a stockholder before a meeting of stockholders, the stockholder must first have given timely notice of the proposal in writing to our Secretary. For an annual meeting, a stockholder’s notice generally must be delivered not less than 90 days or more than 120 days before the anniversary of the previous year’s annual meeting.
Special Meetings of Stockholders. Special meetings of the stockholders may be called at any time only by the board of directors, the Chairman of the board of directors, the Chief Executive Officer or the President, subject to the rights of the holders of any series of preferred stock then outstanding.
Blank-Check Preferred Stock. Our board of directors is authorized to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve.
Transfer Agent
The transfer agent and registrar for our common stock is Equiniti Trust Company. Its address is P.O. Box 64945, Saint Paul MN 55164-0945 and its telephone number is (800) 468-9716.
Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “QLGN.”
| 19 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
Common Stock
The material terms and provisions of our common stock are described under the section titled “Description of Capital Stock” on page 17.
Pre-Funded Warrants
The following summary of certain terms and conditions of the pre-funded warrants is not complete and is subject to, and qualified in its entirety by, the provisions of pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
General
The term “pre-funded” refers to the fact that the purchase price of the pre-funded warrants in this offering includes almost the entire exercise price that will be paid under the pre-funded warrants, except for a nominal remaining exercise price of $0.001. The purpose of the pre-funded warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, at the election of the holder, 9.99%) of our outstanding common stock following the consummation of this offering the opportunity to invest capital into the Company without triggering their ownership restrictions, by receiving pre-funded warrants in lieu of shares of our common stock which would result in such ownership of more than 4.99% (or, at the election of the holder, 9.99%), and receiving the ability to exercise their option to purchase the shares underlying the pre-funded warrants at a nominal price at a later date.
Form
The pre-funded warrants will be issued as individual warrant agreements to the investors. You should review the form of pre-funded warrant, filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the pre-funded warrants.
Exercisability
The pre-funded warrants are exercisable at any time after their original issuance and will be exercisable until exercised in full. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full by wire transfer or cashier’s check drawn on a United States bank for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as described below). A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase or decrease such beneficial ownership limitation, provided that the limitation in no event exceeds 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share of common stock.
Duration and Exercise Price
The exercise price per whole share of our common stock purchasable upon the exercise of the pre-funded warrants is $0.001 per share of common stock. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price of the pre-funded warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock.
Cashless Exercise
If, at any time after the issuance of the pre-funded warrants, the holder exercises its pre-funded warrants and a registration statement registering the issuance of the shares of common stock underlying the pre-funded warrants under the Securities Act is not then effective (or the prospectus contained therein is not available for the issuance of shares of common stock underlying the pre-funded warrants), then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder shall instead receive upon such exercise (either in whole or in part) only the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants. Notwithstanding anything to the contrary, in the event we do not have or maintain an effective registration statement, there are no circumstances that would require us to make any cash payments or net cash settle the pre-funded warrants to the holders.
| 20 |
Transferability
Subject to applicable laws, the pre-funded warrants and all right thereunder are transferable, in whole or in part, at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon the making of such transfer.
Exchange Listing
There is no established trading market for the pre-funded warrants, and we do not plan on applying to list the pre-funded warrants on The Nasdaq Capital Market any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of 50% or more of our outstanding voting power of the common equity, or any person or group becoming the beneficial owner of 50% or more of the voting power represented by our outstanding common equity, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the pre-funded warrants.
Rights as a Stockholder
Except by virtue of such holder’s ownership of shares of our common stock, the holder of a pre-funded warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the pre-funded warrant.
Common Stock Warrants
The following summary of certain terms and provisions of the common stock warrants is not complete and is subject to, and qualified in its entirety by, the provisions of the common stock warrants, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of common stock warrant for a complete description of the terms and conditions of the common stock warrants.
Form
The common stock warrants will be issued as individual warrant agreements to the investors. You should review the form of common stock warrant, filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the common stock warrants.
Exercisability
The common stock warrants are exercisable upon issuance and have a five-year term. The common stock warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full by wire transfer or cashier’s check drawn on a United States bank for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as described below). A holder (together with its affiliates) may not exercise any portion of the common stock warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase or decrease such beneficial ownership limitation, provided that the limitation in no event exceeds 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the common stock warrants. No fractional shares of common stock will be issued in connection with the exercise of common stock warrants. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share of common stock.
Duration and Exercise Price
The exercise price per whole share of our common stock purchasable upon the exercise of the common stock warrants is $ per share of common stock. The common stock warrants have a five-year term. The exercise price of the common stock warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock.
| 21 |
Cashless Exercise
If the holder exercises its common stock warrants and a registration statement registering the issuance of the shares of common stock underlying the common warrants under the Securities Act is not then effective (or the prospectus contained therein is not available for the issuance of shares of common stock underlying the common stock warrants), then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder shall instead receive upon such exercise (either in whole or in part) only the net number of shares of common stock determined according to a formula set forth in the common stock warrants. Notwithstanding anything to the contrary, in the event we do not have or maintain an effective registration statement, there are no circumstances that would require us to make any cash payments or net cash settle the common stock warrants to the holders.
Transferability
Subject to applicable laws, the common stock warrants and all right thereunder are transferable, in whole or in part, at the option of the holder upon surrender of the common stock warrant to us together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon the making of such transfer.
Exchange Listing
There is no established trading market for the common stock warrants, and we do not plan on applying to list the common stock warrants on any national securities exchange or nationally recognized trading system.
Fundamental Transactions
In the event of a fundamental transaction, as described in the common stock warrants, and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of 50% or more of our outstanding voting power of the common equity, or any person or group becoming the beneficial owner of 50% or more of the voting power represented by our outstanding common equity, the holders of the common stock warrants will be entitled to receive, upon exercise of the common stock warrants, the kind and amount of securities, cash or other property that the holders would have received had they exercised the common stock warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the common stock warrants.
Rights as a Stockholder
Except by virtue of such holder’s ownership of shares of our common stock, the holder of a common stock warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the common stock warrant.
| 22 |
A.G.P. has agreed to act as our exclusive placement agent in connection with this offering subject to the terms and conditions of the placement agent agreement dated , 2023. The Placement Agent is not purchasing or selling any of the securities offered by this prospectus, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities but has agreed to use its reasonable best efforts to arrange for the sale of all of the securities offered hereby. Therefore, we may not sell the entire amount of securities offered pursuant to this prospectus. We will enter into a securities purchase agreement directly with certain investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities in this offering.
We will deliver the securities being issued to the investors upon receipt of such investor’s funds for the purchase of the securities offered pursuant to this prospectus. We will deliver the securities being offered pursuant to this prospectus upon closing. We expect this offering to be completed not later than two (2) business days following the commencement of this offering and we will deliver all securities to be issued in connection with this offering delivery versus payment (DVP)/receipt versus payment (RVP) upon receipt of investor funds received by us. We expect to deliver the securities being offered pursuant to this prospectus on or about , 2023.
We have agreed to indemnify the Placement Agent and specified other persons against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the Placement Agent may be required to make in respect thereof.
Fees and Expenses
We have engaged A.G.P. as our exclusive placement agent in connection with this offering. This offering is being conducted on a “best efforts” basis and the Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay the Placement Agent a fee based on the aggregate proceeds as set forth in the table below:
Per Share of Common Stock and Accompanying Common Stock Warrant | Per Pre-Funded Warrant and Accompanying Common Stock Warrant | Total | ||||||||||
| Public offering price | $ | $ | ||||||||||
| Placement Agent fees(1) | $ | $ | ||||||||||
| Proceeds to us, before expenses(2) | $ | $ | ||||||||||
| (1) | We have agreed to pay the Placement Agent a cash placement commission equal to 7% of the aggregate proceeds from the sale of the shares of common stock, the common stock warrants and pre-funded warrants sold in this offering. We have also agreed to reimburse the Placement Agent for certain expenses incurred in connection with this offering. |
| (2) | The amount of the offering proceeds to us presented in this table does not give effect to any exercise of the pre-funded warrants or common stock warrants being issued in this offering. |
We have also agreed to reimburse the Placement Agent at closing (i) for legal and other expenses incurred by them in connection with the offering in an aggregate amount up to $100,000, and (ii) non-accountable expenses payable to the Placement Agent of up to $50,000. We estimate the total expenses payable by us for this offering, excluding the Placement Agent fees and expenses, will be approximately $ .
The Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the shares sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the Placement Agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of shares by the Placement Agent acting as principal. Under these rules and regulations, the Placement Agent:
| ● | may not engage in any stabilization activity in connection with our securities; and | |
| ● | may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution. |
Listing
Our common stock is listed on The Nasdaq Capital Market under the trading symbol “QLGN.” We do not plan to list the pre-funded warrants or the common stock warrants on the Nasdaq Capital Market or any other securities exchange or trading market.
| 23 |
Lock-Up Agreements
Our directors and officers have entered into lock-up agreements. Under these agreements, these individuals agreed, subject to specified exceptions, not to sell or transfer any shares of common stock or securities convertible into, or exchangeable or exercisable for, common stock during a period ending 90 days after the completion of this offering, without first obtaining the written consent of the Placement Agent. Specifically, these individuals agreed, in part, subject to certain exceptions, not to:
| ● | offer for sale, sell, pledge, or otherwise transfer or dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) any shares of common stock or securities convertible into or exercisable or exchangeable for common stock; | |
| ● | enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of common stock; or | |
| ● | make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any of our securities. |
No Sales of Similar Securities
We have agreed, subject to certain exceptions, not to issue, enter into any agreement to issue or announce the issuance or proposed issuance of, any shares of common stock (or securities convertible into or exercisable for common stock) or, subject to certain exceptions, file any registration statement, including any amendments or supplements thereto (other than the prospectus supplement, registration statement or amendment to the registration statement relating to the securities offered hereunder and a registration statement on Form S-8), until days after the completion of this offering. We have also agreed not to enter into a variable rate transaction (as defined in the securities purchase agreement) for days after the completion of this offering.
Discretionary Accounts
The Placement Agent does not intend to confirm sales of the securities offered hereby to any accounts over which it has discretionary authority.
Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “QLGN.”
Transfer Agent and Registrar
We have appointed Equiniti Trust Company as the transfer agent and registrar for our common stock.
Other Activities and Relationships
The Placement Agent and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The Placement Agent and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses, including placement agent fees of $617,400, $420,000 and $350,000 received for acting as placement agent in registered direct offerings that closed on December 1, 2021, December 18, 2020 and July 10, 2020.
In the ordinary course of their various business activities, the Placement Agent and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the Placement Agent or its affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The Placement Agent and its affiliates may hedge such exposure by entering into transactions that consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The Placement Agent and certain of its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
The foregoing does not purport to be a complete statement of the terms and conditions of the placement agent agreement or the securities purchase agreement, copies of which are attached to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
| 24 |
The validity of the securities being offered will be passed upon for us by Reed Smith LLP, Los Angeles, California. The Placement Agent is being represented by Blank Rome LLP, New York, New York in connection with this offering.
Mayer Hoffman McCann P.C., our independent registered public accounting firm, has audited our balance sheets as
The consolidated financial statements of December 31, 2018 and 2017, andQualigen Therapeutics, Inc. for the related Statements of Operations and Comprehensive Loss, Statement of Changes in Stockholders’ Equity and Statements of Cash Flows for each of the two years in the periodyear ended December 31, 2018, as set forth2022 incorporated in theirthis Registration Statement and Prospectus and have been so incorporated in reliance on the report whichof Baker Tilly US, LLP. (which report expresses an unqualified opinion and includes an explanatory paragraph relating to ourthe Company’s ability to continue as a going concern.concern), an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC, which means that we can disclose important information to you by referring you to those documents. Any information referenced this way is considered to be part of this prospectus, and any information that we file later with the SEC will automatically update and, where applicable, supersede this information. We incorporate by reference the following documents that we have included our financialfiled with the SEC (other than, in each case, documents or information deemed to have been furnished and not filed in accordance with the SEC’s rules):
| (1) | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, as filed with the SEC on May 2, 2023, as amended on Form 10-K/A filed with the SEC on July 7, 2023; | |
| (2) | Our Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2023 and June 30, 2023, as filed with the SEC on May 15, 2023 and August 14, 2023, respectively; | |
| (3) | Our Current Reports on Form 8-K filed with the SEC on January 10, 2023, January 20, 2023, April 24, 2023, May 5, 2023, May 19, 2023, June 26, 2023, July 13, 2023, July 26, 2023, August 1, 2023, August 4, 2023, and September 28, 2023; and | |
| (4) | the description of our common stock, which is registered under Section 12 of the Exchange Act, in our registration statement on Form 8-A, filed with the SEC on June 15, 2015, as updated by Exhibit 4.9 to Amendment No. 1 to our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed on July 7, 2023. |
Additionally, all documents filed by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after (i) the date of the initial filing of the registration statement and prior to effectiveness of the registration statement, and (ii) the date of this prospectus and before the termination or completion of any offering hereunder, shall be deemed to be incorporated by reference into this prospectus from the respective dates of filing of such documents, except that we do not incorporate any document or portion of a document that is “furnished” to the SEC, but not deemed “filed.”
We undertake to provide without charge to each person (including any beneficial owner) who receives a copy of this prospectus, upon written or oral request, a copy of all of the preceding documents that are incorporated by reference (other than exhibits, unless the exhibits are specifically incorporated by reference into these documents). We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the reports or documents that we incorporate by reference in this prospectus contained in the registration statement (except exhibits to the documents that are not specifically incorporated by reference) at no cost to you, by writing or calling us at: Qualigen Therapeutics. Inc., Attn: Corporate Secretary, 5857 Owens Avenue, Suite 300, Carlsbad, California 92008, telephone number: (760) 452-8111.
Any statements contained in a document incorporated by reference in this prospectus shall be deemed to be modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus (or in any other subsequently filed document which also is incorporated by reference in this prospectus) modifies, supersedes or replaces such statement. Any statement so modified, superseded or replaced shall not be deemed, except as so modified, superseded or replaced, to constitute a part of this prospectus. Statements contained in this prospectus and any document incorporated by reference as to the contents of any contract, agreement or other document referred to are not necessarily complete, and in thiseach instance, reference is made to the copy of the contract, agreement or other document filed as an exhibit to the registration statement in reliance on the report of Mayer Hoffman McCann P.C. given on their authority as experts in accounting and auditing.or any incorporated document, each statement being so qualified by this reference.
| 25 |
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect tofor the securitiesshares of common stock, pre-funded warrants and accompanying common stock warrants, and the shares of common stock issuable upon exercise of the pre-funded warrants and the common stock warrants being offered by this prospectus. This prospectus, which is part of the registration statement, does not contain all of the information included in the registration statement and itsthe exhibits. For further information with respect toabout us and the securitiescommon stock, pre-funded warrants and accompanying common warrants offered by this prospectus, weyou should refer you to the registration statement and its exhibits. Statements containedReferences in this prospectus as to the contentsany of any contractour contracts or any other document referred todocuments are not necessarily complete, and in each instance, weyou should refer you to the copy of the contract or other document filed as an exhibitexhibits attached to the registration statement. Each of these statements is qualified in all respects by this reference.
We are subject to the information and periodic reporting requirementsstatement for copies of the Exchange Act, andactual contract or document. Additionally, we file periodicannual, quarterly and current reports, proxy statements and other information with the SEC. You can read our
The SEC filings,maintains an internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may also request a copy of these filings, at no cost, by writing us, at 1880 Century Park East, Suite 1000, Los Angeles, California 90067 or telephoning us at (310) 203-1000.http://www.sec.gov. We also maintain a website atwww.ritterpharmaceuticals.com, at which you may access these materialsmake available, free of charge, on our website at www.qlgntx.com, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports and statements as soon as reasonably practicable after they are electronically filed with or furnished to, the SEC. The information contained in, or that can be accessed through,contents of our website is not incorporated by reference in, and isthe SEC’s websites are not part of this prospectus.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows usprospectus, and the reference to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus.
We incorporateour and the SEC’s websites do not constitute incorporation by reference into this prospectus and the registration statement of which this prospectus form a part the information orcontained at those sites, other than documents listed below that we have filedfile with the SEC and any future filings we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, and until the termination of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form8-K):
Any statement contained in this prospectus or in a document incorporated or deemed to bethat are specifically incorporated by reference into this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus modifies or supersedes the statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.

7,600,000Up to Shares
of Common Stock

Up to Shares of Common Stock Underlying the Pre-Funded Warrants
Up to Common Warrants to Purchase up to Shares of Common Stock
Up to Shares of Common Stock Underlying the Common Stock Warrants
PROSPECTUS
________, 2019
Sole Placement Agent
A.G.P.
, 2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and DistributionDistribution.
The following table sets forth the estimated costs and expenses to be incurredpaid or payable by the registrant in connection with the issuance and distribution of the securities being registered under this Registration Statement.other than the Placement Agent fees. All the amounts shown are estimates except the SEC registration fee and the Financial Industry Regulatory Authority, Inc. (“FINRA”) filing fee.
| Total | Amount to be Paid | |||||||
| SEC registration fee | $ | 903 | $ | 2,645 | ||||
| FINRA filing fee | 2,300 | |||||||
| Printing and engraving expenses | * | |||||||
| Legal fees and expenses | $ | 35,000 | * | |||||
| Accounting fees and expenses | $ | 20,000 | * | |||||
| Transfer agent’s fees | * | |||||||
| Miscellaneous fees and expenses | $ | 4,097 | * | |||||
| Total | $ | 60,000 | $ | * | ||||
| * | To be filed by amendment. |
Item 14. Indemnification of Directors and OfficersOfficers.
Our amended and restated certificate of incorporation (as amended, the “Certificate of Incorporation”) provides that we shall indemnify, to the fullest extent authorized by the Delaware General Corporation Law (“DGCL”), each person who is involved in any litigation or other proceeding because such person is or was a director or officer of Ritter Pharmaceuticals,Qualigen Therapeutics, Inc. or is or was serving as an officer or director of another entity at our request, against all expense, loss or liability reasonably incurred or suffered in connection therewith. Our amended and restated certificateCertificate of incorporationIncorporation provides that the right to indemnification includes the right to be paid expenses incurred in defending any proceeding in advance of its final disposition provided, however, that such advance payment will only be made upon delivery to us of an undertaking,the fullest extent authorized by or on behalf of the director or officer, to repay all amounts so advanced if it is ultimately determined that such director is not entitled to indemnification. If we do not pay a proper claim for indemnification in full within 30 days after we receive a written claim for such indemnification, our certificate of incorporation and our bylaws authorize the claimant to bring an action against us and prescribe what constitutes a defense to such action.Delaware General Corporation Law.
Section 145 of the Delaware General Corporation Law permits a corporation to indemnify any director or officer of the corporation against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with any action, suit or proceeding brought by reason of the fact that such person is or was a director or officer of the corporation, if such person acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, if he or she had no reason to believe his or her conduct was unlawful. In a derivative action, (i.e.(i.e., one brought by or on behalf of the corporation), indemnification may be provided only for expenses actually and reasonably incurred by any director or officer in connection with the defense or settlement of such an action or suit if such person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation, except that no indemnification shall be provided if such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action or suit was brought shall determine that the defendant is fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability.
Pursuant to Section 102(b)(7) of the Delaware General Corporation Law, our certificateCertificate of incorporationIncorporation eliminates the liability of a director to us or our stockholders for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
| ● | from any breach of the director’s duty of loyalty to us or our stockholders; | |
| ● | from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; | |
| ● | under Section 174 of the | |
| ● | from any transaction from which the director derived an improper personal benefit. |
We carry insurance policies insuring our directors and officers against certain liabilities that they may incur in their capacity as directors and officers.
In addition, we have entered into indemnification agreements with each of our current directors and executive officers. These agreements require us to indemnify these individuals toprovide for the fullest extent permitted under Delaware lawindemnification of such persons for all reasonable expenses and liabilities incurred in connection with any action or proceeding brought against liabilities that may arisethem by reason of the fact that they are or were serving in such capacity. We believe that these indemnification agreements are necessary to attract and retain qualified persons as directors and officers. Furthermore, we have obtained director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their serviceservices to us and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. us.
We also intend to enter into indemnification agreements withmaintain general liability insurance which covers certain liabilities of our future directors and executive officers.officers arising out of claims based on acts or omissions in their capacities as directors or officers, including liabilities under the Securities Act of 1933, as amended.
The above discussion is qualified in its entirety by reference to the Company’s Certificate of Incorporation and bylaws.
| II-1 |
Item 15. Recent Sales of Unregistered SecuritiesSecurities.
InThe following is a summary of transactions during the preceding three years preceding the filing(since September 27, 2020) involving sales of this registration statement, we have issued the followingour securities that were not registered under the Securities Act. The following issuancesAll share and per share data have been adjusted retrospectively to reflect the 1-for-10 reverse stock split that was effective March 23, 2018.
As consideration for Ricerche Sperimentali Montale SpA, or RSM, entering into Amendment No. 2 to the Clinical Supply and Cooperation Agreement, on November 30, 2015, we issued 10,000 shares of common stock to RSM pursuant to a stock purchase agreement, dated as of November 30, 2015.Reverse Stock Split.
On December 18, 2015,February 10 and 11, 2021, we issued to Aspire Capital Fund, LLC, or Aspire Capital, 18,886an aggregate of 2,500 shares of our common stock asto Atlanta Capital Partners, LLC, and Investor Awareness, Inc. in exchange for services valued at $101,750. No underwriter was involved. These issuances were undertaken in reliance upon the exemption from registration described in Section 4(a)(2) of the Securities Act.
On December 3, 2021, we issued a commitment feecommon stock warrant to a consultant, entitling the consultant to purchase up to 60,000 of our shares of common stock at an exercise price of $13.20 per share. This warrant expired on September 14, 2023. No underwriter was involved. The issuance was undertaken in reliance upon the exemption from registration described in Section 4(a)(2) of the Securities Act.
On May 26, 2022, we issued 350,000 shares of our common stock and solda pre-funded common stock purchase warrant to Aspirepurchase 331,464 shares of our common stock to Alpha Capital 50,000Anstalt (“Alpha”) in exchange for 2,232,861 preferred shares of NanoSynex Ltd. No underwriter was involved. The issuance to Alpha was undertaken in reliance upon the exemption from registration described in Section 4(a)(2) of the Securities Act.
On December 22, 2022, we issued to Alpha an 8% Senior Convertible Debenture (the “Debenture”) in the aggregate principal amount of $3,300,000 for a purchase price of $3,000,000. The Debenture is convertible, at any time, and from time to time, at Alpha’s option, into shares of our common stock, at $2.00a price equal to $1.32 per share, for gross proceeds of $100,000. Subsequently,subject to adjustment as described in the Debenture. Additionally, we soldissued a common stock purchase warrant (the “Alpha Warrant”) to Aspire Capital 157,769Alpha to purchase up to 2,500,000 shares of our common stock for total proceedsat a price of approximately $3.0 million.$1.65 per share, subject to adjustment as described in the Warrant. No underwriter was involved. The issuance to Alpha was undertaken in reliance upon the exemption from registration described in Section 4(a)(2) of the Securities Act.
On May 4, 2017,
Between January 9 and 12, 2023, we issued to Aspire Capital 13,732841,726 shares of our common stock (adjustedupon Alpha’s partial conversion of the Debenture at $1.32 per share for the reverse stock split that was effected March 23, 2018) as a commitment fee.total of $1,111,078 principal.
On October 30, 2018, we entered into a Securities Purchase Agreement with the purchasers named therein, or the Purchasers, pursuant to which sold 6,000 shares of Series B convertible preferred stock, with a stated value of $1,000 per share, or the Series B Shares, together with common stock purchase warrants, or the Warrants, to purchase 2,307,692 shares of our common stock (representing 50% of the aggregate number of shares of common stock into which the Series B Shares were convertible) to the Purchasers for aggregate gross proceeds of $6 million to us. Pursuant to the terms of the Securities Purchase Agreement, certain Purchasers who owned shares of the Company’s Series A convertible preferred stock were also permitted to exchange, on a 1-for-1 share basis, their shares of Series A convertible preferred stock for shares of the Company’s newly designated Series C convertible preferred stock, with a stated value of $1,000 per share and convertible into shares of the Company’s common stock at an initial conversion price per share of $1.64, or the Series C Shares. The offering closed on November 5, 2018. A.G.P./Alliance Global Partners, or the Placement Agent, served as the exclusive placement agent and Roth Capital Partners acted as a financial advisor for the offering. Pursuant to the terms and conditions of the Placement Agency Agreement, entered into by us and the Placement Agent on October 30, 2018, we paid the Placement Agent an aggregate cash fee equal to 7% of the aggregate gross proceeds raised in the Offering (less $750,000 paid by one of the Purchasers) and reimbursed the Placement Agent for approximately $35,000 of certain of its expenses with respect to the Offering.
Except with respect to the offerings described in the paragraphs immediately above, no underwriters were used in the foregoing transactions. The securities described above were issued and sold in reliance on the exemptions from registration provided by Section 4(a)(2) of the Securities Act and/or Rule 506 of Regulation D promulgated under the Securities Act. Each of the purchasers in these transactions represented to us in connection with its purchase that it was acquiring the securities for investment and not for distribution and that it could bear the risks of the investment. Each purchaser received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from registration. All of the foregoing securities were deemed restricted securities for the purposes of the Securities Act, except for the securities that were issued to Aspire Capital and the Purchasers, which were registered for sale by Aspire Capital and the Purchasers, respectively, in previously filed registration statements.
| II-2 |
Item 16. Exhibits and Financial Statement SchedulesSchedules.
| (a) | Exhibits: |
| 10.1+ | Executive Employment Agreement, by and between Qualigen, Inc. and Michael Poirier, dated as of February 1, 2017 and as amended on January 9, 2018 | 8-K | 001-37428 | 10.1 | 5/29/2020 | |||||
| 10.2+ | Executive Employment Agreement, by and between Qualigen, Inc. and Christopher Lotz, dated as of February 1, 2017 and as amended on January 9, 2018 | 8-K | 001-37428 | 10.2 | 5/29/2020 | |||||
| 10.3+ | Executive Employment Agreement dated December 10, 2021 with Amy Broidrick | 10-K | 001-37428 | 10.53 | 3/31/2022 |
| II-3 |
| II-4 |
| * | Filed herewith. |
| ** | Previously filed. |
| *** | To be filed by amendment. |
| + | Indicates management contract or compensatory plan or arrangement. |
| (b) | Consolidated Financial Statement Schedules: All schedules are omitted because the required information is inapplicable or the information is presented in the consolidated financial statements and the related notes. |
| II-5 |
* Filed herewith.
+ Indicates management contract or compensatory plan or arrangement.
Item 17. Undertakings
| (a) | The undersigned registrant hereby |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (i), (ii) and (iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Exchange Act that are incorporated by reference in this Registration Statement or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the Registration Statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this Registration Statement, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(i) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-6 |
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrantregistrant has duly caused this Registration Statement on Form S-1registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Los Angeles,Carlsbad, State of California, on the 24thday of July, 2019.October 2, 2023.
| By: | /s/ | |
| Chairman of the Board, Chief Executive Officer | ||
We the undersigned officers and directors of Ritter Pharmaceutics, Inc., hereby severally constitute and appoint Andrew J. Ritter and John W. Beck, and each of them singly, our true and lawful attorneys with full power to any of them, and to each of them singly, to sign for us and in our names in the capacities indicated below the registration statement on Form S-1 filed herewith and any and all pre-effective and post-effective amendments to said registration statement and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same with all exhibits thereto, and the other documents in connection therewith, with the Securities and Exchange Commission, and generally to do all such things in our name and behalf in our capacities as officers and directors to enable Ritter Pharmaceuticals, Inc. to comply with the provisions of the Securities Act of 1933, as amended, and all requirements of the Securities and Exchange Commission, hereby ratifying and confirming our signatures as they may be signed by our said attorneys, or any of them, to said registration statement and any and all amendments thereto.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statementregistration statement has been signed below by the following persons in the capacities and on the dates indicated below .indicated:
| Signature | Title | Date | ||
| /s/ | Chairman of the Board, Chief Executive Officer | October 2, 2023 | ||
| Michael S. Poirier | ||||
| (Principal Executive Officer) | ||||
| /s/ | Vice President of Finance, Chief Financial Officer | |||
| Director | ||||
| Director | ||||
| Matthew | ||||
| Director | ||||
| Director | ||||
| By: | /s/ Michael S. Poirier | |
Michael S. Poirier Attorney-in-fact |
| II-7 |