
As filed with the Securities and Exchange Commission on December 17, 2018.11, 2023
Registration No. 333- 333-275121
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
UNITED STATESFORM S-1
SECURITIES AND EXCHANGE COMMISSION(Amendment No. 2)
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
Motus
MOTUS GI Holdings, Inc.HOLDINGS, INC.
(Exact Namename of Registrantregistrant as Specifiedspecified in its Charter)charter)
| Delaware | 3841 | 81-4042793 | ||||
(State or other jurisdiction of
| (Primary Standard Industrial Classification Code Number) | (
|
Identification |
1301 East Broward Boulevard, 3rd Floor
Ft. Lauderdale, FL 33301
Telephone: (954) 541-8000
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Timothy P. Moran
Mark Pomeranz
Chief Executive Officer
Motus GI Holdings, Inc.
1301 East Broward Boulevard, 3rd3rd Floor
Ft. Lauderdale, FL 33301
Telephone: (954) 541-8000
(Address,Name, address, including zip code, and telephone number,
including area code, of agent for service)
CopiesPlease send copies of all communications to:
Steven M. Skolnick, Esq.
Lowenstein Sandler LLP 1251 Avenue of the Americas New York, NY 10020 Telephone: (212) 262-6700 | Faith L. Charles Thompson Hine LLP 300 Madison Avenue, 27th Floor New York, New York Telephone: (212) |
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable on or after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.¨box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨ ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company”,company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | Accelerated filer |
| Non-accelerated filer | Smaller reporting company |
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.x ☒
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to Be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee | ||||||
| Common Stock, par value $0.0001 per share | $ | 18,572,500 | $ | 2,250.99 | ||||
The Registrantregistrant hereby amends this Registration Statementregistration statement on such date or dates as may be necessary to delay its effective date until the Registrantregistrant shall file a further amendment whichthat specifically states that this Registration Statementregistration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 as amended, or until this Registration Statementthe registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to suchsaid Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it iswe are not soliciting an offer to buy these securities in any statejurisdiction where the offer or sale is not permitted.
Motus GI Holdings, Inc.SUBJECT TO COMPLETION, DATED DECEMBER 11, 2023

5,000,000 Shares
Common StockPRELIMINARY PROSPECTUS

Up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Pre-Funded Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Series A Common Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Series B Common Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Shares of Common Stock issuable upon exercise of the Pre-Funded Warrants
Up to 3,022,670 Shares of Common Stock issuable upon exercise of the Common Warrants
We are offering 5,000,000on a “reasonable best efforts” basis up to $6.0 million of shares of our common stock and Series A Common Warrants and Series B Common Warrants (collectively, the “Common Warrants”) to purchase shares of our common stock (and the shares of common stock that are issuable from time to time upon exercise of the Common Warrants), at an assumed public offering price of $3.97 per share and accompanying Series A Common Warrant to purchase one share of common stock and Series B Common Warrant to purchase one share of common stock, which was the closing price of our common stock on The Nasdaq Capital Market on December 8, 2023. We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants to purchase shares of our common stock, in lieu of shares of common stock that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock. The purchase price of each pre-funded warrant and accompanying Common Warrants will be equal to the price at which a share of common stock and accompanying Common Warrants are sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. The pre-funded warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. Each share of common stock and pre-funded warrant is being sold together with a Series A Common Warrant to purchase one share of our common stock and a Series B Common Warrant to purchase one share of our common stock, each with an exercise price of $ per share (representing 100% of the price at which a share of common stock and accompanying Common Warrants are sold to the public in this offering). The Common Warrants will be exercisable immediately and will expire as follows: the Series A Common Warrant will expire on the fifth anniversary of the date of issuance and the Series B Common Warrant will expire on the eighteen month anniversary of the date of issuance. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue two Common Warrants for each share of our common stock and two Common Warrants for each pre-funded warrant to purchase one share of our common stock sold in this offering, the number of Common Warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. The shares of common stock and pre-funded warrants, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. This offering also relates to the shares of common stock issuable upon exercise of any Common Warrants and pre-funded warrants sold in this offering.
Immediately following the closing of this offering, pursuant to the 2021 Loan Agreement (as defined in this prospectus), an aggregate of $4.0 million of the principal amount under such loan will be automatically converted into an assumed 1,007,556 shares of common stock (and/or pre-funded warrants in lieu thereof), at a conversion price equal to the public offering price per share of common stock and accompanying Common Warrants in this offering, based on the assumed combined public offering price of $3.97 per share of common stock and accompanying Common Warrants, which is the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023. The Lender (as defined in this prospectus) will also be issued Conversion Common Warrants (as defined in this prospectus) to purchase an assumed 2,015,112 shares of common stock, representing warrant coverage on the shares being issued to the Lender in the same form and terms and at the same proportion as investors in this offering, and will have executed a customary lock-up agreement with respect to such shares and warrants for a 90-day period following the closing. We will also prepay $1.5 million of the principal amount of such loan from the net proceeds of this offering. The securities issued to the Lender in the conversion will be issued in reliance on the exemption from registration set forth in Section 3(a)(9) of the Securities Act, and this offering does not relate to the issuance of such securities.
Our common stock is listed on theThe Nasdaq Capital Market under the symbol “MOTS.” On December 13, 2018,8, 2023 the last reported sale price of our common stock as reported on theThe Nasdaq Capital Market was $3.23$3.97 per share. There is no established public trading market for the pre-funded warrants or the Common Warrants, and we do not expect a market to develop. In addition, we do not intend to apply for a listing of the pre-funded warrants or the Common Warrants on any national securities exchange. Without an active trading market, the liquidity of the pre-funded warrants and the Common Warrants will be limited.
The public offering price per share of common stock and accompanying Common Warrants and any pre-funded warrant and accompanying Common Warrants, as the case may be, will be determined by us at the time of pricing, may be at a discount to the current market price, and the recent market price used throughout this prospectus may not be indicative of the final offering price.
You should read this prospectus, together with additional information described under the headings “Information Incorporated by Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
We are an “emerging growth company” as defined under the federal securities laws.laws and, as such, have elected to comply with certain reduced public company reporting requirements.
Investing in our common stocksecurities involves risks that are described in the “Risk Factors” sectiona high degree of risk. See “Risk Factors” beginning on page 8 of this prospectus.prospectus and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered in connection with an investment in our securities.
The underwriters may also exercise their option to purchase up to an additional 750,000 shares from us at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined ifpassed upon the adequacy or accuracy of this prospectus is truthful or complete.prospectus. Any representation to the contrary is a criminal offense.
We may sell fewer than all of the securities offered hereby, provided that we will only consummate an offering of $5.0 million or more in gross proceeds. This offering may be closed without further notice to you. We expect to close the offering on , 2023, but the offering will be terminated on or before December 31, 2023, provided that the closing of the offering has not occurred by such date, and may not be extended. Investors will deposit their investment in us with the placement agent, which will settle the offering in a single closing on a Delivery Versus Payment (“DVP”) settlement basis (i.e., on the closing date, we will issue the securities registered in the investors’ names and addresses and released by the transfer agent (with respect to common stock) or us (with respect to warrants) directly to the account(s) at the placement agent identified by each investor; upon receipt of such securities, the placement agent will promptly electronically deliver such securities to the applicable investor, and payment therefor shall be made by the placement agent (or its clearing firm) by wire transfer to us). Also, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan.
We have engaged A.G.P./Alliance Global Partners (“A.G.P.”) as our placement agent in connection with this offering. We refer to A.G.P. as the “placement agent” in this prospectus. The placement agent is not purchasing or selling the securities offered by us, and is not required to arrange for the purchase or sale of any specific number or dollar amount of our securities, but will use its reasonable best efforts to solicit offers to purchase the securities offered by this prospectus. The securities will be offered at a fixed price and are expected to be issued in a single closing. Although we will only consummate an offering of $5.0 million or more in gross proceeds, the actual public offering amount, placement agent’s fees, and proceeds to us, if any, are not presently determinable and may be less than the total maximum offering amounts set forth above, subject to the $5.0 million minimum.
| Per Share and Accompanying Common Warrants | Per Pre-Funded Accompanying Common Warrants | Total | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Placement agent fees (1) | $ | $ | $ | |||||||||
| Proceeds to us, before expenses | $ | $ | $ | |||||||||
(1) We have agreed to pay the placement agent a cash fee equal to 7% (or 5% with respect to proceeds from certain investors agreed upon between us and the placement agent). We have also agreed to reimburse the placement agent for certain of their offering-related expenses. See “Plan of Distribution” for a description of the compensation to be received by the placement agent.
The underwriters expectdelivery of the securities offered hereby to deliver the shares against payment thereforpurchasers is expected to be made on or about , 2018.2023.
Piper JaffraySole Placement Agent
A.G.P.
The date of this prospectus is, 2018.2023.
TABLE OF CONTENTS
TABLE OF CONTENTSABOUT THIS PROSPECTUS
We incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Information Incorporated by Reference,” before deciding to invest in our securities.
You should rely only onNeither we nor the placement agent have authorized anyone to provide you with additional information or information different from that contained or incorporated by reference in this prospectus filed with the Securities and any related free writing prospectus that we mayExchange Commission (the “SEC”). We take no responsibility for, and can provide no assurance as to you in connection with this offering. We have not, and the underwriters have not, authorizedreliability of, any other personinformation that others may give you. The placement agent is seeking offers to provide you with different information. If anyone provides you with differentbuy our securities only in jurisdictions where offers and sales are permitted. The information contained in this prospectus, or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing ordocument incorporated by reference in this prospectus, is accurate only as of the date onof those respective documents, regardless of the front covertime of delivery of this prospectus or the dateany sale of the applicable document incorporated by reference.our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
The information incorporated by reference or provided in this prospectus contains statistical data and estimates, including those relating to market size and competitive position of the markets in which we participate, that we obtained from our own internal estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable. While we believe our internal company research is reliable and the definitions of our market and industry are appropriate, neither this research nor these definitions have been verified by any independent source.
For investors outside the United States (“U.S.”): neither we nor any ofWe and the underwriters hasplacement agent have not done anything that would permit this offering or the possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purposethose purposes is required, other than in the United States.U.S. Persons outside the United StatesU.S. who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stocksecurities and the distribution of this prospectus and any such free writing prospectus outside of the United States.U.S.
CAUTIONARY NOTE CONCERNING FORWARD-LOOKING STATEMENTS
InThis prospectus contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements contained in this prospectus we rely on and refer to information and statisticsother than statements of historical fact, including statements regarding our industry. We obtained this statistical, marketstrategy, future operations, future financial position, liquidity, future revenue, projected expenses, results of operations, expectations concerning the timing and other industryour ability to commence and subsequently report data from planned non-clinical studies and forecastsclinical trials, prospects, plans and objectives of management are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “plan,” “expect,” “predict,” “potential,” “opportunity,” “goals,” or “should,” and similar expressions are intended to identify forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from publicly available information.those projected in the forward-looking statements as a result of many factors.
We based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in “Risk Factors” in this prospectus, and under a similar heading in any other annual, periodic or current report incorporated by reference into this prospectus or that we may file with the SEC in the future. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge quickly and from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this prospectus, may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement.
You should also read carefully the factors described in the “Risk Factors” section of this prospectus, and under a similar heading in any other annual, periodic or current report incorporated by reference into this prospectus, to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. You are advised to consult any further disclosures we make on related subjects in our future public filings.
This summary highlights information about our company, this offering and information contained in greater detail in other parts of this prospectus andor incorporated by reference into this prospectus from our filings with the SEC listed in the documents incorporatedsection entitled “Information Incorporated by reference.Reference.” Because it is only a summary, it does not contain all of the information that you should consider before purchasing our securities in making your investment decision. Before investingthis offering and it is qualified in our common stock, youits entirety by, and should carefullybe read in conjunction with, the more detailed information appearing elsewhere or incorporated by reference into this prospectus. You should read the entire prospectus, the registration statement of which this prospectus is a part, and the documentsinformation incorporated by reference into this prospectus in their entirety, including the “Risk Factors” included in this prospectus at page 8 and incorporated by reference and “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and theour financial statements and the related notes to those financial statements incorporated by reference into this prospectus, before purchasing our securities in this offering.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus before investing in our common stock.
When used herein, unless the context requires otherwise, references to the “Company,” “Holdings,“the Company,” “we,” “our”“us” and “us”“our” refer to Motus GI Holdings, Inc., a Delaware corporation, collectively with and our direct wholly-owned subsidiaries, Motus GI Medical Technologies, Ltd., an Israeli corporation, and Motus GI, Inc., a Delaware corporation.subsidiaries.
Corporate Overview
All trademarksMotus GI Holdings, Inc. (“we,” “us,” “our” or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Our Company
General
We have“Company”), has developed the Pure-Vu System, (the “Pure-Vu System”), a medical device that has received 510(k) clearance frombeen cleared by the U.S. Food and Drug Administration (the “FDA”) to help facilitate the cleansing of a poorly prepared gastrointestinal tract during colonoscopy and to help facilitate upper gastrointestinal (“GI”) endoscopy procedures. The Pure-Vu System is also CE mark approvalmarked in the European Economic Area.Area (EEA) for use in colonoscopy. The Pure-Vu System is indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes, as well as gastroscopes, to enable safeimprove visualization during colonoscopy and rapid cleansing during the procedureupper GI procedures while preserving established procedural workflow and techniquestechniques. Through irrigation and evacuation of debris, the Pure-Vu System is designed to provide better-quality exams. Challenges exist for inpatient colonoscopy and endoscopy, particularly for patients who are elderly, with comorbidities, or active bleeds, where the ability to visualize, diagnose and treat is often compromised due to debris, including fecal matter, blood, or blood clots. We believe this is especially true in high acuity patients, like GI bleeding where the existence of blood and blood clots can impair a physician’s view and removing them can be critical in allowing a physician the ability to identify and treat the source of bleeding on a timely basis. We believe use of the Pure-Vu System may lead to positive outcomes and lower costs for hospitals by irrigatingsafely and quickly improving visualization of the colon and evacuatingupper GI tract, potentially enabling effective diagnosis and treatment without delay. In multiple clinical studies to date, involving the irrigation fluid (water)treatment of challenging inpatient and outpatient cases, the Pure-Vu System has consistently helped achieve adequate bowel cleanliness rates greater than 95% following a reduced prep regimen. We also believe that the technology may be useful in the future as a tool to help reduce user dependency on conventional pre-procedural bowel prep regimens. Based on our review and analysis of 2019 market data and 2021 projections for the U.S. and Europe, as obtained from iData Research Inc., feces and other bodily fluids and matter. Challenges with bowel preparation for inpatient colonoscopy represent a significant area of unmet needwe believe that directly affects clinical outcomes and increases the cost of care for a hospital in a market segment where most of the reimbursement is under a bundle payment based on a Diagnostic Related Group (a “DRG”), comprising ofduring 2022 approximately 1.5 million annualinpatient colonoscopy procedures were performed in the U.S. and approximately 3.84.8 million annual procedures worldwide. Upper GI bleeds occurred in the U.S. at a rate of approximately 400,000 cases per year in 2019, according to iData Research Inc. The Pure-Vu System has been assigned an ICD-10 code in the US. The system does not currently have a unique reimbursement codecodes with any private or governmental third-party payors in any country. To date, as part ofother country or for any other use; however, we may pursue reimbursement activities in the limited market development launch, we have focused on collecting additional clinical and health economic data, as exemplified byfuture, particularly in the recently initiated Reliable Endoscopic Diagnosis Utilizing Cleansing Enhancement Study (the “REDUCE Study”), along with garnering valuable experienceoutpatient colonoscopy market. We received 510(k) clearance in key hospitals onOctober 2023 from the use ofFDA for the Pure-Vu EVS System to support a planned full launchfor use in the United States inpatient colonoscopyUpper GI tract as well as an enhanced version for the colon. We expect to begin market introduction of these products in 2019. We dothe coming months. The Company does not expect to generate significant revenue from product sales unless and until we expand ourit further expands its commercialization efforts.
efforts, which is subject to significant uncertainty.
Recent Developments
Clinical Data & Safety2021 Loan Amendment
On July 16, 2021 (the “Effective Date”), we entered into a loan facility (the “2021 Loan Agreement”) with a private institutional lender (the “Lender”). Under the 2021 Loan Agreement, the Lender agreed to provide us with access to term loans in an aggregate principal amount of up to $12.0 million (the “Loan”) in three tranches as follows: (a) on the Effective Date, a loan in the aggregate principal amount of $4.0 million (the “Convertible Note”, or “Tranche A”), (b) on the effective date of the Loan, a loan in the aggregate principal amount of $5.0 million (“Tranche B”), and (c) available until December 31, 2021, a loan in the aggregate principal amount of $3.0 million (“Tranche C” and, together with Tranche B, the “Term Loan”). The 2021 Loan Agreement contains customary representations and warranties, indemnification provisions in favor of the Lender, events of default and affirmative and negative covenants, including, among others, covenants that limit or restrict our ability to, among other things, incur additional indebtedness, merge or consolidate, make acquisitions, pay dividends or other distributions or repurchase equity, make investments, dispose of assets and enter into certain transactions with affiliates, in each case subject to certain exceptions. Outstanding borrowings under the Loan are secured by a first priority security interest on substantially all of our personal property assets, including our material intellectual property and equity interests in its subsidiaries. There are no liquidity or financial covenants.
InThe Convertible Note and Tranche B were funded on the effective date. As of December 31, 2021, we drew down the full $3 million aggregate principal amount of Tranche C.
The Convertible Note requires forty-eight monthly interest only payments at 7.75% per annum commencing after the Effective Date and thereafter full payment of the then outstanding principal balance of the Convertible Note on July 1, 2025. The Tranche B loan requires interest only monthly payments commencing on the Effective Date until September 30, 2022 and, thereafter, thirty-three (33) monthly payments of principal and interest accrued thereon until June 1, 2025. The Tranche C loan, to the extent drawn on or prior to December 31, 2021, requires monthly payments of interest only commencing on the date drawn until September 30, 2022 and, thereafter, thirty-three (33) monthly payments of principal and interest accrued thereon until June 1, 2025. Interest on the Tranche B and Tranche C loans accrues at 9.5% per annum.
The 2021 Loan Agreement contains features that would permit the Lender to convert all or any portion of the outstanding principal balance of the Convertible Note at any time, pursuant to which the converted part of the Convertible Note will be converted into that number of shares of our common stock to be issued to the Lender at a recent clinical study performedprice per share equal to the conversion price, of $420.00 per share. Following the conversion of any portion of the outstanding principal balance of the Convertible Note, the principal balance of the Convertible Note remaining outstanding shall continue to bear interest at 7.75% per annum.
On November 28, 2023, we and the Lender entered into a First Amendment to the 2021 Loan Agreement (the “Amendment”), pursuant to which, among other things, (a)(i) on the effective date of the Amendment, we paid to the Lender a sum of $750,000 in cash via wire transfer in immediately available funds (the “Amendment Execution Date Payment”), and (ii) upon consummation of a First Amendment Capital Raise (as defined below) and immediately following the Convertible Note Securities Exchange (as defined below), we will prepay to the Lender a sum of $1,500,000 in cash via wire transfer in immediately available funds (the “Closing Payment”), which sums set forth in (i) and (ii) shall be applied towards partial prepayment of the outstanding principal balance of the Term Loan; and (b) subject to the satisfaction (or waiver by Lender) of certain Exchange Conditions (as defined in the United States,Amendment), immediately following the consummation of a First Amendment Capital Raise, which we assume this offering will be, $4.0 million (the “Conversion Amount”) of the outstanding aggregate principal balance of the Convertible Note will automatically convert into such number of shares of our common stock (the “Convertible Note Securities Exchange”) at a price per share equal to the public offering price per share in the First Amendment Capital Raise representing the Conversion Amount; provided, that, (A) the Lender shall have executed a customary lock-up agreement for a 90-day period following the Convertible Note Securities Exchange, (B) the Lender shall receive the same warrant coverage (the “Conversion Common Warrants”) per share of common stock, if any, as investors purchasing securities in the First Amendment Capital Raise and (C) the Lender shall receive a pre-funded warrant (the “Conversion Pre-Funded Warrant”) in lieu of shares of common stock otherwise issuable upon the Convertible Note Securities Exchange for such number of shares that would represent more than 4.5% of the pre-exercise outstanding shares of common stock, providing that the Lender will not own (x) more than 4.99% of the post-exercise outstanding shares of common stock at any time and (y) to the extent required under the rules of The Nasdaq Capital Market, more than 19.99% of the shares of common stock outstanding immediately prior to the Convertible Note Securities Exchange (but after the consummation of the First Amendment Capital Raise) unless applicable shareholder approval is obtained. “First Amendment Capital Raise” means the Company raising additional cash through one equity financing registered under the Securities Act (to be consummated no later than December 29, 2023) with gross proceeds of at least $5.0 million. The securities issued to Lender in the Convertible Note Securities Exchange will be issued in reliance on the exemption from registration set forth in Section 3(a)(9) of the Securities Act, and this offering does not relate to the issuance of such securities. We also agreed to file a resale registration statement to register the securities being issued to Lender in the Convertible Note Securities Exchange as promptly as practicable (and in no event later than 91 calendar days after the closing of the Convertible Note Securities Exchange). Assuming this offering will satisfy the definition of the First Amendment Capital Raise, we will issue to the Lender an aggregate of 1,007,556 shares of our common stock (or Conversion Pre-Funded Warrants in lieu thereof) and Conversion Common Warrants to purchase 2,015,112 shares of our common stock at an exercise price equal to the exercise price and a term of each of the classes of the Common Warrants sold in this offering, based on the assumed combined public offering price of $3.97 per share of common stock and accompanying Common Warrants, which is the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023. We do not intend to price this offering for less than $5.0 million in gross proceeds.
Nasdaq Deficiencies and Reverse Stock Split
As previously disclosed, we received a letter from the Nasdaq Stock Market, LLC (“Nasdaq”) indicating that we are not in compliance with the minimum stockholders’ equity requirement for continued listing on Nasdaq under Rule 5550(b)(1) (the “Equity Rule”). In addition, as previously disclosed on the Current Report on Form 8-K filed April 5, 2023, we received a letter from Nasdaq indicating that the bid price of the Company’s common stock had failed to close above the minimum $1 requirement for the past 30 trading days in violation of Listing Rule 5550(a)(2) (the “Bid Price Rule”). The Company was provided 180 calendar days, or until September 27, 2023, to regain compliance with the Bid Price Rule. On September 27, 2023, we received notice that the Nasdaq Hearings Panel (the “Hearings Panel”) granted us an extension to regain compliance with the Equity Rule and the Bid Price Rule until January 2, 2024.
At our annual meeting of stockholders held on September 21, 2023, our stockholders approved a proposed amendment to our Certificate of Incorporation to effect a reverse stock split of our outstanding common stock at a ratio of not less than two-for-one (2:1) and not greater than twenty-for-one (20:1), at any time prior to the one year anniversary date of our annual meeting of stockholders, with the exact ratio to be determined by our Board of Directors. On November 2, 2023, we effected a reverse stock split of our issued and outstanding common stock at a ratio of 1-for-15 (the “2023 Reverse Stock Split”), and on November 21, 2023, we received a letter from Nasdaq confirming that we had regained compliance with the Bid Price Rule. All historical information in this prospectus (other than information incorporated by reference herein dated prior to November 2, 2023) has been retroactively adjusted for the 2023 Reverse Stock Split. We intend to regain compliance with the Equity Rule through the consummation of this offering and the Convertible Note Securities Exchange. Even if we regain compliance with the Equity Rule prior to the January 2, 2024 deadline, we expect that we will need to raise additional capital to remain in compliance with the Equity Rule for future reporting periods, which capital raises may result in additional dilution to investors in our securities.
Operations in Israel
We have research and development capabilities in electrical and mechanical engineering with laboratories in our facility in Israel for development and prototyping, and electronics design and testing. Currently, the workstation and loading fixture component of our Pure-Vu System demonstrated safe and effective colonic cleaningis manufactured by Sanmina Corporation at their facilities in 46 patients receiving a reduced prep regimen.Israel. The study was initially designed to compare two different minimal bowel preparation regimens. Initially patients were randomized to receive one of two minimal bowel preparations: three doses of 17 gr. MiraLAX each mixed in 8.5 oz. of clear liquids or two doses of 7.5 oz. magnesium citrate (MgC) each taken with 19.5 oz. of clear liquid. A study amendment early on replaced the MiraLAX arm, due to obvious inferior Boston Bowel Preparation Scale (“BBPS”), a validated assessment instrument, scoring from the outset. The replacement arm consisted of two doses of 5 oz. MgC taken with 16 oz. of clear liquid. All patients were allowed to eat a low residue diet on the day prior and were asked to avoid seeds and nuts for five days prior to their procedure. Study objectives evaluated for each study arm included: (1) improvement of colon cleansing from presentation baseline to completion of the procedure (as assessed by the BBPS) through the usedisposable portion of the Pure-Vu System, (2) time required to reach the cecum, (3) total procedure time, and (4) safety. No significant differences were found between the three groups with regard to demographics or indication for colonoscopy. No serious adverse events related to the device were reported.EVS is manufactured by Sterling Industries in their Michigan, U.S. facility. The usedisposable portion of the Pure-Vu System enabled successful intraprocedural cleansing of the colon and ensured successful completion of all colonoscopies performed (100% success rate). Although there were only 46 patients in the study, there was a highly significant difference in the study population (p value <0.0001) between the baseline preparation and that seen post cleansing with the Pure-Vu System. The use of the Pure-Vu System added some time to the procedure, but the total procedure time was approximately 25 minutes in this study.
The clinical data showing performance of the Pure-Vu System in this study using the BBPS is shown below. The clinical results from the study were presented at the 2018 American College of Gastroenterology (“ACG”) Annual Meeting in October 2018.
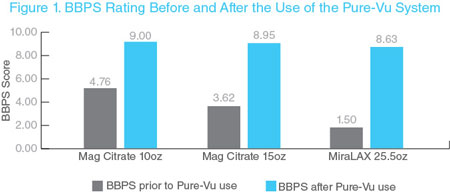
REDUCE Study
Published studies have found that the inpatient population experiences rates of insufficiently prepped colons at the time of colonoscopy as high as 55%. This has been shown to lead directly to significantly longer hospital stays and other additional costs due to the need for repeated preps, repeated colonoscopies and additional diagnostic procedures. This is exemplified in a recently published study from Northwestern University Hospital System which showed an average hospital stay extension of two days and cost increase of as much as $8,000 per patient as a result of challenges associated with bowel preparation. We believe the Pure-Vu System may improve outcomes and lower costs for hospitals by reducing the time to a successful colonoscopy, minimizing delayed and incomplete procedures, and improving the quality of an exam.
On May 23, 2018, we announced the initiation of the Reliable Endoscopic Diagnosis Utilizing Cleansing Enhancement Study (the “REDUCE Study”). The REDUCE Study is a multi-center inpatient prospective trial designed to evaluate the Pure-Vu System’s ability to consistently and reliably cleanse the colon to facilitate a successful colonoscopy in a timely manner in patients who are indicated for a diagnostic colonoscopy. The study will enroll approximately 100 subjects in at least five hospital centers in the United States and Europe.
We expect to announce a top-line readout of the REDUCE Study data in the first quarter of 2019. The primary endpoint of the study is to determine the Pure-Vu System’s rate of improved bowel cleansing level using the BBPS index. Other key data being collected in the study includes the proportion of patients who receive a successful colonoscopy for the intended indication in the first attempt, which impacts the quality of the exam as well as hospital length of stay and costs required for the episode of care. The savings to the hospital can be meaningful as National Inpatient Sample (“NIS”) and other literature sources note that the cost for a standard hospital bed averages $2,298 and the cost for an intensive care unit (“ICU”) bed averages $6,546 per day in the U.S.
FDA Clearance to Market Pure-Vu Slim Sleeve
On September 13, 2018, we announced that we received special 510(k) clearance from FDA for the Pure-Vu Slim Sleeve (the “Pure-Vu Slim Sleeve”), a compatible extension to the Pure-Vu System for slim colonoscopes. The Pure-Vu Slim Sleeve design allows the Pure-Vu System access to the full range of procedures in the colonoscopy market as we estimate, through consultation with colonoscope manufacturing companies, approximately 30% of procedures are performed with a slim colonoscope. The Pure-Vu Slim Sleeve has the same cleansing performance as the standard Pure-Vu System sleeve, and both versions work with the same Pure-Vu workstation control system. The Pure-Vu Slim Sleeve has been designed to be compatible with smaller diameter and more flexible slim colonoscopes with additional enhancements to our low friction lubricious coating technology to aid in navigation through the colon. The first successful clinical cases using the Pure-Vu Slim Sleeve were completed in October 2018.
Second Generation of Pure-Vu
We expect to submit a Special 510(k) Notice to FDA in the first quarter of 2019 for the Second-Generation (“Gen 2”) of the Pure-Vu System. This premarket notification is a premarket submission used to review with FDA modifications to our devices that can be validated within our Quality Systems and which do not affect the device’s intended use or alter the device’s fundamental scientific technology.
The Gen 2 Pure-Vu System has been designed to improveis manufactured by Polyzen, Inc., at their facilities in North Carolina, U.S. Both Sterling Industries and Polyzen use Medacys in Shenzhen, China as key sub-supplier for the mobility and logisticsinjection molded parts in the setupPure Vu disposables. On October 7, 2023, the “Swords of Iron” war stroke between Israel and the system and will retain allterrorist organizations in the same functionality asGaza Strip, following a surprise attack on Israel led by certain armed groups in the current generation ofGaza Strip. To date, our operations in Israel have not been significantly impacted by the Pure-Vu System in terms of how it cleansesongoing war or the colon. The Gen 2 Pure-Vu System Workstation will have a reduced footprint and be mounted on a roll stand to allow nursing staff to easily move the Gen 2 Pure-Vu System to different procedure rooms or to the ICU as needed. The Gen 2 Pure-Vu System also has improvements that reduce the number of steps to set up the system and simplifies the loading process onto the colonoscope.
Additional Clinical Studies and Market Expansion OpportunitiesOctober 7, 2023 terrorist attack.
We expectFor additional information, see “Risk Factors—Risks Related to initiate the EXPEDITE Study (the “EXPEDITE Study”)Our Operations in the first quarter of 2019. The EXPEDITE Study is a planned feasibility study in hospitalized patients which will be designed to analyze the Pure-Vu System’s ability to minimize the preparation in order to shorten the time to a successful colonoscopy in the inpatient population. We are also working with key centers to generate clinical data on outpatient populations that have difficulty with the pre-procedural preparation to study the Pure-Vu System’s ability to allow these patients to have a successful exam. This data is expected to lay the ground work for future expansion into high need outpatient populations.Israel.”
Our resources are currently focused on the planned full launch of the Pure-Vu System in the United States inpatient colonoscopy market in 2019. However, we have identified two follow-on market expansion opportunities we may explore in the future. These include the inpatient upper gastrointestinal bleed (“Upper GI”) endoscopy market and the outpatient high medical need colonoscopy market. In the Upper GI bleed endoscopy market, we believe the Pure-Vu System has the potential to be used during endoscopy procedures to remove clots and debris to provide a clear field of view for the endoscopist. Separately, the outpatient high medical need colonoscopy market presents a large potential commercial market opportunity for the Pure-Vu System, as close to 26 million outpatient colonoscopy procedures are performed worldwide. Based on published literature in several peer reviewed journals from 2010 to 2015 and surveys of physicians conducted in 2015, approximately 23% of such colonoscopy patients can have an inadequate preparation, which may lead to repeat procedures earlier than the medical guidelines suggest. We believe use of the Pure-Vu System has the potential to reduce the need for such repeat procedures if used in the outpatient high medical need colonoscopy market. Additionally, if we choose to explore either market, we may be able to leverage our existing hospital and doctor relationships developed through our inpatient colonoscopy sales force to facilitate such expansion.
Intellectual Property Update
Our intellectual property position comprises a highly innovative portfolio covering technologies rooted in systems and methods for cleaning body cavities with or without the use of an endoscope. Currently we have six granted or allowed patents in the U.S., six patents in Asia, four patents in the EU and 27 (11 in the U.S.) pending patent applications in various regions of the world with a focus on the U.S., EU and Japan.
On March 20, 2018, we announced that the European Patent Office (“EPO”) issued Patent No. 3079556 to us titled, “Apparatus and method for coupling between a colonoscope and add-on tubes.” The patent provides intellectual property protection for our use of the Pure-Vu System in the European market through June 2035.
On March 27, 2018, we announced that the U.S. Patent and Trademark Office (“USPTO”) issued U.S. Patent No. 9,895,483 to us titled, “Systems and methods for cleaning body cavities.” The patent provides intellectual property protection for our use of the Pure-Vu System in the United States through January 2031.
On May 21, 2018, we announced that the Chinese Patent Office (“SIPO”) issued Patent No. ZL201580037467.6 to us titled, “Apparatus and method for coupling between a colonoscope and add on tubes.” The patent provides intellectual property protection for our use of the Pure-Vu System in China through June 2035.
On July 30, 2018, we announced that the Japanese Patent Office (“JPO”) issued Patent No. 6,362,640 to us titled, “System and method for cleaning body cavities.” The patent provides intellectual property protection for our use of the Pure-Vu System in Japan through June 2031.
Our Risks
Investing in our common stock involves a high degree of risk. You should carefully consider all of the information in this prospectus and in the documents incorporated by reference prior to investing in our common stock. These risks are discussed more fully in the section titled “Risk Factors” beginning on page 8 herein and in our Annual Report on Form 10-K for the year ended December 31, 2017, as updated by our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018, which are incorporated by reference in this prospectus. These risks and uncertainties include, but are not limited to, the following:
Implications of Being an Emerging Growth Company
We are an “emerging growth company,”company” as defined in Section 2(a)(19) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”),. As such, we are eligible for and for as long as we continue to be an “emerging growth company,” we may chooseintend to take advantage of certain exemptions from various reporting requirements applicable to other public companies butthat are not to “emergingemerging growth companies” for as long as we continue to be an emerging growth company, including, but not limited to, not being required to comply with(i) the exemption from the auditor attestation requirements ofwith respect to internal control over financial reporting under Section 404404(b) of the Sarbanes-Oxley Act of 2002, as amended, (the “Sarbanes-Oxley“Sarbanes Oxley Act”), (ii) the exemptions from say-on-pay, say-on-frequency and say-on-golden parachute voting requirements and (iii) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions fromstatements.
Our eligibility to qualify as an emerging growth company will end on December 31, 2023 (which is the requirementslast day of holding a nonbinding advisory vote on executive compensation and stockholder approvalthe fiscal year following the fifth anniversary of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years from the dateclosing of our initial public offering, in February 2018, or untilwhich occurred during 2018). In addition, the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.07 billion, (ii) the dateJOBS Act provides that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three-year period. We intend toan emerging growth company can take advantage of these reporting exemptions described above until we are no longer an “emerging growth company.” Under the JOBS Act, “emerging growth companies” can also delay adoptingextended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until such time as those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption fromextended transition period and, as a result, we will adopt new or revised accounting standards and, therefore, we are subject toon the same new or revised accountingrelevant dates on which adoption of such standards asis required for non-public companies instead of the dates required for other public companies that are not “emerging growth companies.”
Corporate History and Information
We are a Delaware corporation formed in September 2016 under the name Eight-Ten Merger Corp. In November 2016, we changed our name to Motus GI Holdings, Inc. We are the parent company of Motus GI Medical Technologies Ltd., an Israeli corporation, and Motus GI, LLC (formerly Motus GI, Inc.), a Delaware corporation.
On February 16, 2018, we closed our initial public offering (the “IPO”) in which we sold 3,500,000 shares of our common stock atlimited liability company. Motus GI, Inc. was converted from a public offering price of $5.00 per share. In connection with the closing of the IPO, we received net proceeds of approximately $15 million after deducting underwriting discounts and commissions of approximately $1.4 million and other offering expenses of approximately $1.1 million. In addition, on March 12, 2018, we closed the sale of an additional 56,000 shares of our common stock pursuant toCorporation into a partial exercise of the underwriters’ 30-day option to purchase up to an additional 525,000 shares of our common stock in connection with the IPO, resulting in net proceeds to us of approximately $258,000 after deducting underwriting discounts and commissions and other offering expenses. Shares of our common stock commenced trading on the Nasdaq Capital Market under the symbol “MOTS” on February 14, 2018.Limited Liability Company effective January 1, 2021.
Our principal executive offices are located at 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL 33301. Our phone number is (954) 541-8000 and our web address is http://www.motusgi.com. InformationOur website and the information contained inon, or accessiblethat can be accessed through, our web site is not, and shouldwebsite will not be deemed to be incorporated by reference in, orand are not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase our common stock.
“Motus GI,” “Pure-Vu,” and our other registered or common law trademarks, service marks or trade names appearing herein are the property of Motus GI Holdings, Inc. Some trademarks referred to in this prospectusreport are referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
The following summary contains basic information about this offering. The summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus and in the documents incorporated by reference.
Common Stock | Up to 1,511,335 shares, based on the sale of our common stock at an assumed combined public offering price of $3.97 per share of common stock and accompanying Common Warrants, which is the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023. | |
| Pre-funded Warrants to be Offered | ||
Common Warrants to be Offered | Up to 3,022,670 Common Warrants to purchase an aggregate of up to 3,022,670 shares of our common stock, based on the sale of our common stock at an assumed combined public offering price of $3.97 per share of common stock and accompanying Common Warrants, which is the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023. Each share of our common stock and each pre-funded warrant to purchase one share of our common stock is being sold together with a Series A Common Warrant to purchase one share of our common stock and a Series B Common Warrant to purchase one share of our common stock. Each Common Warrant will have an exercise price of $ per share (representing 100% of the price at which a share of common stock and accompanying Common Warrants are sold to the public in this offering), will be immediately exercisable and will expire (i) in the case of the Series A Common Warrant, on the fifth anniversary of the original issuance date and (ii) in the case of the Series B Common Warrant, on the eighteen month anniversary of the original issuance date. The shares of common stock and pre-funded warrants, and the accompanying Common Warrants, as the case may be, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the Common Warrants. |
| Debt Conversion | Immediately following the closing of this offering, pursuant to the 2021 Loan Agreement, an aggregate of $4.0 million of the principal amount under such loan will be automatically converted into an assumed 1,007,556 shares of common stock (and/or pre-funded warrants in lieu thereof), at a conversion price equal to the public offering price per share of common stock and accompanying Common Warrants in this offering, based on the assumed combined public offering price of $3.97 per share of common stock and accompanying Common Warrant, which is the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023. The Lender will also be issued Conversion Common Warrants to purchase an assumed 2,015,112 shares of common stock, representing warrant coverage on the shares being issued to the Lender in the same form and terms and at the same proportion as investors in this offering, and will have executed a customary lock-up agreement with respect to such shares and warrants for a 90-day period following the closing. We will also prepay $1.5 million of the principal amount of such loan from the net proceeds of this offering. The securities issued to the Lender in the conversion will be issued in reliance on the exemption from registration set forth in Section 3(a)(9) of the Securities Act, and this offering does not relate to the issuance of such securities The Nasdaq Capital Market. | |||
| Common Stock to be | ||||
| Use of Proceeds | We estimate that
We currently intend to use the net proceeds from this offering to | |||
| Risk Factors | ||||
| National Securities Exchange Listing | Our common stock is listed on The Nasdaq Capital Market under the symbol |
(1) The number of shares of our common stock tothat will be outstanding upon completion ofimmediately after this offering is based on 15,690,151690,247 shares of our common stock outstanding as of September 30, 2018November 15, 2023, and assumes the sale and issuance by us of 1,511,335 shares of common stock (and no sale of any pre-funded warrants) in this offering and excludes:
| ● | ||
| ● | 61,975 shares of common stock issuable upon the exercise of | |
| ● | 312,107 shares of |
| ● | ||
| ● | ||
| ● |
Unless otherwise indicated, all information in this prospectus reflects and assumes no issuances or assumes the following:
exercises of any other outstanding shares, options or warrants after November 15, 2023.
An investmentInvesting in our common stock is speculative and illiquid andsecurities involves a high degree of risk includingrisk. We urge you to carefully consider all of the information contained in this prospectus and other information which may be incorporated by reference in this prospectus as provided under “Information Incorporated by Reference.” In particular, you should consider the risk of a loss of your entire investment. You should carefully consider the following risk factors as well asbelow, together with those set forth under the heading “Risk Factors” in our most recent Annual Report on Form 10-K, for the year ended December 31, 2017, as updated by our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018, which areis incorporated by reference ininto this prospectus. Theseprospectus, as those risk factors contain, in addition to historical information, forward looking statements that involveare amended or supplemented by our subsequent filings with the SEC. These risks and uncertainties. Our actual results could differ significantly from the results discussed in the forward looking statements. The order in which the following risks are presented is not intended to reflect the magnitude of the risks described. The occurrence of any of the following adverse developments described in the following risk factors and in the documents incorporated by reference could materially and adversely harm our business, financial condition, results of operations or prospects. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of the money that you pay for our common stock. In addition, the risks and uncertainties discussed below and in the documents incorporated by reference are not the only onesrisks and uncertainties we face. Our business, financial condition, results of operations or prospects could also be harmed byAdditional risks and uncertainties not currently known to us, or that we currently do not believe are material, and theseview as immaterial, may also impair our business. If any of the risks or uncertainties described below or in our SEC filings or any additional risks and uncertainties actually occur, our business, financial condition, results of operations and cash flow could results inbe materially and adversely affected. As a complete lossresult, you could lose all or part of your investment. In assessing the risks and uncertainties described below, you should also refer to the other information contained in this prospectus (as supplemented or amended) and the documents incorporated by reference in this prospectus.
Risks RelatedRISKS RELATED TO OUR OPERATIONS IN ISRAEL
Our research and development facilities and some of our suppliers are located in Israel and, therefore, our business, financial condition and results of operation may be adversely affected by political, economic and military instability in Israel.
Our research and development facilities are located in northern Israel. In addition, most of our employees are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the State of Israel was established in 1948, the State of Israel and its economy has experienced significant growth and expansion, coupled with an increase in the standard of living, and has developed one of the most advanced high-tech industries in the world. However, it continues to face many geo-political and other challenges that may affect companies located in Israel, such as ours. For example, a number of armed conflicts have occurred between Israel and its Arab neighbors. Although Israel has entered into peace agreements with Egypt and Jordan, comprehensive agreements with the Palestinian Authority, and other agreements with neighboring Arab countries regarding public normalization of relations, there continues to be unrest and terrorist activity in Israel with varying levels of severity, as well as ongoing hostilities and armed conflicts between Israel and the Palestinian Authority, and other groups in the West Bank and Gaza Strip, recent unrest was due to the United States’ relocation of its embassy from Tel Aviv to Jerusalem. The effects of these hostilities and violence on the Israeli economy and our operations are unclear, and we cannot predict the effect on us of a further increase in these hostilities or any future armed conflict, political instability or violence in the region. We could be harmed by any major hostilities involving Israel, the interruption or curtailment of trade between Israel and its trading partners, boycotts or a significant downturn in the economic or financial condition of Israel. The impact of Israel’s relations with its Arab neighbors in general, or on our operations in the region in particular, remains uncertain. The establishment of new fundamentalist Islamic regimes or governments more hostile to Israel could have serious consequences for the stability in the region, place additional political, economic and military confines upon Israel, materially adversely affect our operations and limit our ability to sell our products to countries in the region.
In particular, on October 7, 2023, the “Swords of Iron” war stroke between Israel and the terrorist organizations in the Gaza Strip, following a surprise attack on Israel led by certain armed groups in the Gaza Strip. To date, our operations in Israel have not been significantly impacted by the ongoing war or the October 7, 2023 terrorist attack, however we will disclose via Current Report on Form 8-K any material changes to our operations resulting from the conflict.
Additionally, several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries and groups have imposed or may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. These restrictions may limit our ability to sell our products to companies in these countries. Furthermore, the Boycott, Divestment and Sanctions Movement, a global campaign attempting to increase economic and political pressure on Israel to comply with the stated goals of the movement, may gain increased traction and result in a boycott of Israeli products and services. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturn in the economic or financial condition of Israel, could adversely affect our business, results of operations and financial condition.
Our Financial Positioncommercial insurance policy does not cover losses associated with armed conflicts and Needterrorist attacks. Although the Israeli government in the past covered the reinstatement value of certain damages that were caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for Capitaldamages incurred. Any losses or damages incurred by us could have a material adverse effect on our business.
Our operations could also be disrupted by the obligations of some of our employees to perform military service. Some of our employees in Israel may be called upon to perform up to 54 days in each three year period (and in the case of military officers, up to 84 days in each three year period) of military reserve duty until they reach the age of 40 (and in some cases, depending on their specific military profession and rank up to 45 or even 49 years of age) and, in certain emergency circumstances, may be called to immediate and unlimited active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists and it is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of employees related to military service, which could materially adversely affect our business and results of operations. To date, none of our employees have been called upon to perform reserve duty as a result of the October 7, 2023 attack and ongoing war.
RISKS RELATED TO THIS OFFERING
There is substantial doubt about our ability to continue as a going concern, which will affect our ability to obtain future financing and may require us to curtail our operations.
Our financial statements as of December 31, 2017 were prepared under the assumption that we will continue as a going concern. The independent registered public accounting firm that audited our 2017 financial statements, in their report, included an explanatory paragraph referring to our recurring losses since inception and expressing substantial doubt in our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our ability to continue as a going concern depends on our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. We cannot assure you, however, that we will be able to achieve any of the foregoing.
Risks Related to this Offering and Ownership of our Common Stock
After this offering, our officers, directors, and principal stockholders will continue to exercise significant control over our Company, and will control our company for the foreseeable future, including the outcome of matters requiring stockholder approval.
When this offering is completed our officers, directors, entities controlled by our officers and directors, and principal stockholders who beneficially own more than 5% of our common stock before this offering will in the aggregate, beneficially own shares representing approximately 47.76% of our outstanding capital stock immediately after this offering (based upon an assumed public offering price of $3.23 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018). As a result, such entities and individuals have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as: (i) a merger or a sale of our company, (ii) a sale of all or substantially all of our assets, and (iii) amendments to our certificate of incorporation and bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those entities and individuals. These individuals also have significant control over our business, policies and affairs as officers and directors of our company. Therefore, you should not invest in reliance on your ability to have any control over our company.
Investors in this offering will pay a higher price than the book value of our common stock.
If you purchase shares of common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. You will incurexperience immediate and substantial dilution of $2.07in your investment. You will experience further dilution if we issue additional equity or equity-linked securities in the future.
Because the price per share representingof our common stock being offered is substantially higher than the difference between ourpro forma as adjusted net tangible book value per share after giving effectof our common stock, you will suffer immediate and substantial dilution with respect to the net tangible book value of the common stock you purchase in this offering andoffering. Based on an assumed combined public offering price of $3.23$3.97 per share the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018. To the extent any outstanding options or warrants are ultimately converted or exercised,and accompanying Common Warrants being sold in this offering, and our pro forma as adjusted net tangible book value as of September 30, 2023 of $2.08 per share, if you purchase shares of common stock in this offering, you will sustain further dilution. For further information, seesuffer immediate and substantial dilution of $1.89 per share with respect to the pro forma as adjusted net tangible book value of the common stock. See the section entitled “Dilution.”
If we sell shares“Dilution” for a more detailed discussion of ourthe dilution you will incur if you purchase common stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.this offering.
We may from time to timeIf we issue additional shares of common stock, or securities convertible into or exchangeable or exercisable for shares of common stock, our stockholders, including investors who purchase shares of common stock and/or pre-funded warrants and accompanying Common Warrants in this offering, will experience additional dilution, and any such issuances may result in downward pressure on the price of our common stock. We also cannot assure you that we will be able to sell shares or other securities in any other offering at a discountprice per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders.
This is a reasonable best efforts offering, which we will only consummate for $5.0 million or more in gross proceeds.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. Although we will only consummate an offering of $5.0 million or more in gross proceeds, the actual public offering amount, placement agent’s fees, and proceeds to us, if any, are not presently determinable and may be less than the total maximum offering amounts set forth above, subject to the $5.0 million minimum, and there can be no assurance that the offering contemplated hereby will ultimately be consummated.
If we fail to regain compliance with the requirements for continued listing on Nasdaq, our common stock could be delisted from trading, which would adversely affect the liquidity of our common stock and our ability to raise additional capital.
The Nasdaq Capital Market’s rules for listed companies requires us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our common stock. In order to maintain our listing on Nasdaq, we must satisfy the continued listing requirements of Nasdaq for inclusion in The Nasdaq Capital Market, including among other things, a minimum stockholders’ equity of $2.5 million and a minimum bid price for our common stock of $1.00 per share.
As previously disclosed, we received a letter from the currentNasdaq Stock Market, LLC (“Nasdaq”) indicating that we are not in compliance with the minimum stockholders’ equity requirement for continued listing on Nasdaq under Rule 5550(b)(1) (the “Equity Rule”). In addition, as previously disclosed on the Current Report on Form 8-K filed April 5, 2023, we received a letter from Nasdaq indicating that the bid price of the Company’s common stock had failed to close above the minimum $1 requirement for the past 30 trading days in violation of Listing Rule 5550(a)(2) (the “Bid Price Rule”). The Company was provided 180 calendar days, or until September 27, 2023, to regain compliance with the Bid Price Rule. On September 27, 2023, we received notice that the Nasdaq Hearings Panel (the “Hearings Panel”) granted us an extension to regain compliance with the Equity Rule and the Bid Price Rule until January 2, 2024.
At our annual meeting of stockholders held on September 21, 2023, our stockholders approved a proposed amendment to our Certificate of Incorporation to effect a reverse stock split of our outstanding common stock at a ratio of not less than two-for-one (2:1) and not greater than twenty-for-one (20:1), at any time prior to the one year anniversary date of our annual meeting of stockholders, with the exact ratio to be determined by our Board of Directors. On November 2, 2023, we effected a reverse stock split of our issued and outstanding common stock at a ratio of 1-for-15.
We intend to regain compliance with the applicable continued listing requirements of The Nasdaq Capital Market prior to the end of the compliance period set forth in the abovementioned letter. On November 21, 2023, we received a letter from Nasdaq confirming that we had regained compliance with the Bid Price Rule. We intend to regain compliance with the Equity Rule through the consummation of this offering and the Convertible Note Securities Exchange. However, until Nasdaq has reached a final determination that we have regained compliance with all of the applicable continued listing requirements, there can be no assurances regarding the continued listing of our common stock on Nasdaq. The delisting of our common stock from Nasdaq would have a material adverse effect on our access to capital markets, and any limitation on market liquidity or reduction in the price of our common stock as a result of that delisting would adversely affect our ability to raise capital on terms acceptable to us, if at all. Even if we regain compliance with the Equity Rule prior to the January 2, 2024 deadline, we expect that we will need to raise additional capital to remain in compliance with the Equity Rule for future reporting periods, which capital raises may result in additional dilution to investors in our securities.
Future sales of substantial amounts of our common stock or securities convertible into or exchangeable or exercisable for shares of common stock, either by us or by our existing stockholders, or the possibility that such sales could occur, could adversely affect the market price of our common stock. As a result, our stockholders would experience immediate dilution upon
Future sales in the purchasepublic market of any shares of our common stock sold at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. If we issue common stock or securities convertible into or exchangeable or exercisable for shares of common stock, including shares referred to in the foregoing risk factor, shares held by our existing stockholders or shares issued upon exercise of our outstanding stock options or warrants, or the perception by the market that these sales could occur, could lower the market price of our common stockholders would experiencestock or make it difficult for us to raise additional dilution and, as a result, our stock price may decline.capital.
There is no public market for the pre-funded warrants or Common Warrants being offered in this offering.
There is no established public trading market for the pre-funded warrants or Common Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants or Common Warrants on any securities exchange or nationally recognized trading system, including The Nasdaq Capital Market. Without an active market, the liquidity of the pre-funded warrants and Common Warrants will be limited.
Holders of pre-funded warrants and Common Warrants purchased in this offering will have no rights as common stockholders until such holders exercise such warrants and acquire our common stock.
Until holders of pre-funded warrants or Common Warrants acquire shares of our common stock upon exercise of such warrants, holders of pre-funded warrants or Common Warrants will have no rights with respect to the shares of our common stock underlying such warrants. Upon exercise of the pre-funded warrants or Common Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
We will have broad discretion in how wethe use of our existing cash and cash equivalents, including the net proceeds offrom this offering. Weoffering, and may invest or spend our cash in ways with which you do not agree and in ways that may not use these proceeds effectively, which could affect our resultsincrease the value of operations and cause our stock price to decline.your investment.
We will have considerablebroad discretion inover the applicationuse of our cash and cash equivalents, including the net proceeds from this offering. You may not agree with our decisions, and our use of this offering, including forcash may not yield any of the purposes described in the section entitled “Use of Proceeds.”return on your investment. We intend to use the net proceeds from this offering for commercialization activities,to support the market introduction of our Upper GI and next generation system, research and development, activities, including clinical and regulatory development and the continued development and enhancement of our Pure-Vu System, and fortrials, working capital and other general corporate purposes. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions forpurposes, including the useprepayment of $1.5 million of principal under the balance of2021 Loan Agreement and future debt payments under the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest2021 Loan Agreement. Our failure to apply the net proceeds from this offering in a manner that does not produce income or that loses value.
An active trading market for our common stock may not be sustained.
Prior to the closing of our IPO, there had been no public market for our common stock. Although our common stock is listed on the Nasdaq Capital Market, the market for our shares has demonstrated varying levels of trading activity. Furthermore, the current level of trading may not be sustained in the future. The lack of an active market for our common stock may impair investors’ ability to sell their shares at the time they wish to sell them or at a price that they consider reasonable, may reduce the fair market value of their shares and may impaireffectively could compromise our ability to raise capital to continue to fund operations by selling sharespursue our growth strategy and may impair our ability to acquire additional intellectual property assets by using our shares as consideration.
Our share price maywe might not be volatile, which could subject us to securities class action litigation and prevent you from being able to sell your shares at or above the offering price.
You may be unable to sell your shares of common stock at or above the offering price. The market price foryield a significant return, if any, on our common stock has been and may continue to be volatile and subject to wide fluctuations in response to factors including the following:
In particular, the market prices of early commercial-stage companies like ours have been highly volatile due to factors, including, but not limited to:
These and other market and industry factors may cause the market price and demand for our common stock to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, the stock market in general, and the Nasdaq Capital Market and emerging growth companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Innet proceeds. You will not have the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any ofopportunity to influence our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention ofdecisions on how to use our management.
Shareholders will experience dilution by exercises of outstanding warrants and options.
As of September 30, 2018, there were 2,586,551 shares of our common stock issuable upon the exercise of outstanding warrants, with a weighted average exercise price of $5.20 per share, and options to purchase an aggregate of up to 1,964,454 shares of our common stock, with a weighted average exercise price of $4.48 per share. Since September 30, 2018, we have issued (i) an additional 560,000 options to purchase shares of our common stock pursuant to our equity incentive plan, with a weighted average exercise price of $3.78 per share, and (ii) an additional42,917 warrants to purchase shares of our common stock with a weighted average exercise price of $7.48 per share.
The exercise of such warrants and options will result in dilution of your investment. As a result ofnet proceeds from this dilution, you may receive significantly less than the full purchase price you paid for our securities in the event of liquidation.
We do not currently intend to pay dividends on our common stock in the foreseeable future, and consequently, any gains from an investment in our common stock will likely depend on appreciation in the price of our common stock.
We have never declared or paid cash dividends on our common stock and do not anticipate paying any cash dividends to holders of our common stock in the foreseeable future. Consequently, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference in this prospectus contain, and our officers and representatives may from time to time make, “forward-looking statements,” which include information relating to future events, future financial performance, financial projections, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “goal,” “seek,” “project,” “strategy,” “likely,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements are neither historical facts, nor should they be read as a guarantee of future performance or results and may not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
The foregoing does not represent an exhaustive list of matters that may be covered by the forward-looking statements contained herein and in the documents incorporated by reference herein or risk factors that we are faced with that may cause our actual results to differ from those anticipate in our forward-looking statements. Please see “Risk Factors” beginning on page 8 for additional risks which could adversely impact our business and financial performance.
Moreover, new risks regularly emerge and it is not possible for our management to predict or articulate all risks we face, nor can we assess the impact of all risks on our business or the extent to which any risk, or combination of risks, may cause actual results to differ from those contained in any forward-looking statements. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended, do not protect any forward-looking statements that we make in connection with this offering. All forward-looking statements included in this prospectus and in the documents incorporated by reference in this prospectus are based on information available to us on the date of this prospectus or the date of the applicable document incorporated by reference. Except to the extent required by applicable laws or rules, we undertake no obligation to publicly update or revise any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future events or otherwise. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained above and throughout this prospectus and in the documents incorporated by reference in this prospectus. We qualify all of our forward-looking statements by these cautionary statements.
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY OUR MANAGEMENT. IN REVIEWING THIS PROSPECTUS AND THE DOCUMENTS INCORPORATED BY REFERENCE IN THIS PROSPECTUS, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT THERE MAY BE OTHER POSSIBLE RISKS THAT COULD BE IMPORTANT.
We estimate that we will receive net proceeds of approximately $14.57$5.2 million from the sale of the shares of common stocksecurities offered by us in this offering, or approximately $16.82 million if the underwriters exercise their over-allotment option in full, based on an assumed combined public offering price of $3.23$3.97 per share (theand accompanying Common Warrants, which was the last reported salesales price of our common stock on theThe Nasdaq Capital Market on December 13, 2018) and8, 2023, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the assumed public offering price of $3.23 per share (last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and commissionsplacement agent fees and estimated offering expenses payable by us, by approximately $4.65excluding the proceeds, if any, from the exercise of the Common Warrants issued in this offering.
The foregoing discussion assumes no sale of pre-funded warrants, which if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis.
We currently intend to use the net proceeds from this offering to support the market introduction of our Upper GI and next generation system, research and development, including clinical trials, working capital and general corporate purposes, including the prepayment of $1.5 million assumingof principal under the 2021 Loan Agreement and future debt payments under the 2021 Loan Agreement. See “Risk Factors” for a discussion of certain risks that may affect our intended use of the net proceeds from this offering.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot currently allocate specific percentages of the net proceeds that we may use for the purposes specified above, and we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors, including our ability to obtain additional financing, the progress, cost and results of our preclinical and clinical development programs, and whether we are able to enter into future licensing or collaboration arrangements.
Pending the use of the net proceeds from this offering, we intend to invest the net proceeds in investment-grade, interest-bearing instruments, certificates of deposit or direct or guaranteed obligations of the U.S.
A 250,000 increase or decrease in the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
We may alsowould increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from thisby approximately $0.9 million, based on the assumed public offering price of $3.97 per share remaining the same, and after deducting the underwriting discounts and commissionsestimated placement agent fees and estimated offering expenses payable by us, by approximately $3.00 million, assuming the public offering price stays the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018), would increase the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $8.58 million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018), would decrease the net proceeds to us from this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $6.72 million. We do not expect that a change in the offering price or the number of shares by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although it may impact the amount of time prior to which we may need to seek additional capital.us.
We expect to use the net proceeds from this offering for commercialization activities, research and development activities, including clinical and regulatory development and the continued development and enhancement of our Pure-Vu System, and for working capital and other general corporate purposes. Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. The amounts and timing of our actual expenditures will depend upon numerous factors, including our sales and marketing and commercialization efforts, demand for our products, our operating costs and the other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
Although we may use a portion of the net proceeds of this offering for the acquisition or licensing, as the case may be, of additional technologies, other assets or businesses, or for other strategic investments or opportunities, we have no current understandings, agreements or commitments to do so.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities.
We have never paid any cash dividends on our common stock. We anticipate that we will retain funds and future earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future following this offering. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements and other factors that our board of directors deems relevant. In addition, the terms of any future debt or credit financings may preclude us from paying dividends.
In addition, the ability of Motus GI Medical Technologies Ltd., an Israeli Company (“Opco”), our direct wholly-owned operating subsidiary, to distribute dividends may be limited by Israeli law. The Israeli Companies Law, 1999, or the Israeli Companies Law, restricts Opco’s ability to declare dividends. Unless otherwise approved by a court, Opco can distribute dividends only from “profits” (as defined by the Israeli Companies Law). Dividends may be paid with the approval of a court, at a company’s request, provided that there is no reasonable concern that payment of the dividend will prevent the company from satisfying its current and foreseeable obligations, as they become due.
The payment of dividends by Opco to Holdings may be subject to Israeli withholding taxes.
The following table sets forth our cash and cash equivalents and short-term investments balance and capitalization as of September 30, 2018:2023:
| ● | on an actual basis; |
| ● | on a pro forma basis to give effect to (i) the payment of the $750,000 Amendment Execution Date Payment under the amended 2021 Loan Agreement, to be applied towards partial prepayment of the outstanding principal balance of the Term Loan, on November 29, 2023, (ii) the issuance of an aggregate of 161,268 shares of common stock upon exercises of previously issued pre-funded warrants, for nominal cash payment, between October 1, 2023 and November 15, 2023 and (iii) the cancellation of 1,470 fractional shares in connection with the 2023 Reverse Stock Split (collectively, the “pro forma events”); and |
| ● | on a pro forma, as adjusted basis, to give further effect to (i) the sale of 1,511,335 (or 1,202,784 in the fourth column) shares of common stock and the accompanying Common Warrants in this offering at an assumed combined public offering price of $3.97 per share and accompanying Common Warrants, which was the last reported sale price of our common stock on The Nasdaq Capital Market on December 8, 2023, (ii) the payment of the $1,500,000 Closing Payment under the 2021 Loan Agreement, to occur immediately following the closing of this offering, and (iii) the issuance, under the 2021 Loan Agreement, of an assumed 1,007,556 shares of common stock upon the conversion of $4.0 million of principal amount of the Convertible Note (net of unamortized debt discount of $64,000 solely for accounting purposes), to occur immediately following the closing of this offering at a conversion price equal to the assumed combined public offering price per share and accompanying Common Warrants in this offering (which assumes no issuance of any Conversion Pre-Funded Warrants), and after deducting estimated placement agent fees and estimated offering expenses payable by us, excluding the proceeds, if any, from the exercise of the Common Warrants issued in this offering, and assuming no sale of pre-funded warrants in this offering (collectively, the “closing events”). |
You should read this table together with “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited and unaudited financial statements and related notes thereto incorporated by reference in this prospectus.
| As of September 30, 2023 | Pro Forma As Adjusted ($6 million | Pro Forma As Adjusted ($5 million | ||||||||||||||
| Actual | Pro Forma | offering) | offering) | |||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | |||||||||||||
| Cash | $ | 5,724,000 | $ | 4,974,000 | $ | 8,704,000 | $ | 7,774,000 | ||||||||
| Total liabilities | 10,969,000 | 10,219,000 | 4,783,000 | 4,783,000 | ||||||||||||
| Stockholders’ deficit: | ||||||||||||||||
| Common stock, $0.0001 par value, 115,000,000 shares authorized as of September 30, 2023, and 530,449 shares, actual, 690,247 shares, pro forma, 3,209,138 shares, pro forma as adjusted ($6 million offering) and 2,861,554 shares, pro forma as adjusted ($5 million offering), issued as of September 30, 2023 | 53 | 69 | 321 | 296 | ||||||||||||
| Additional paid-in capital | $ | 148,935,000 | $ | 148,935,000 | $ | 158,164,748 | $ | 157,234,773 | ||||||||
| Accumulated deficit | $ | (151,413,000 | ) | $ | (151,413,000 | ) | $ | (151,477,000 | ) | $ | (151,477,000 | ) | ||||
| Total stockholders’ (deficit) equity | $ | (2,477,947 | ) | $ | (2,477,931 | ) | $ | 6,688,069 | $ | 5,758,069 | ||||||
| Total capitalization | $ | 8,491,053 | $ | 7,741,069 | $ | 11,471,069 | $ | 10,541,069 | ||||||||
The table and discussion above is based on 530,449 shares of common stock outstanding as of September 30, 2023 and excludes:
| ● | ||
| ● | 5,134 shares of common stock reserved for future issuance under 2016 Equity Incentive Plan, as amended, as of September 30, 2023; | |
| ● | 473,408 shares of common stock issuable upon the exercise of other warrants outstanding as of September 30, 2023, with a weighted average exercise price of $9.16 per share; | |
| ● | the shares of common stock issuable upon the exercise of the pre-funded warrants issued in this offering; | |
| ● | up to 3,022,670 shares of common stock issuable upon the exercise of the Common Warrants issued in this offering; and | |
| ● |
You should read the selected data in the table below together with the sections titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2017 and our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2018, June 30, 2018 and September 30, 2018 which are incorporated by reference in this prospectus, and our consolidated financial statements and related notes incorporated by reference in this prospectus.
| As of September 30, 2018 | ||||||||
| Actual | As Adjusted | |||||||
| (unaudited) | ||||||||
| (in thousands, except per share data) | ||||||||
| Cash and cash equivalents and short-term investments | $ | 11,631 | $ | 26,201 | ||||
| Long-term liabilities | $ | 1,990 | $ | 1,990 | ||||
| Stockholders’ equity: | ||||||||
Common stock, $0.0001 par value; 50,000,000 shares authorized, 15,690,151 shares issued and outstanding (actual); 20,690,151 shares issued and outstanding (as adjusted) | 2 | 2 | ||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized, zero shares issued and outstanding (actual), 10,000,000 shares authorized, zero shares issued and outstanding (as adjusted) | — | — | ||||||
| Additional paid-in capital | 65,251 | 79,821 | ||||||
| Accumulated deficit | (55,811 | ) | (55,811 | ) | ||||
| Total shareholders’ equity | 9,442 | 24,012 | ||||||
| Total capitalization | $ | 11,432 | $ | 26,002 | ||||
Each $1.00 increase (decrease) in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the amount of cash and cash equivalents and short-term investments, additional paid-in capital, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $4.65 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) cash and cash equivalents and short-term investments, total stockholders’ equity and total capitalization on an as adjusted basis by approximately $3.00 million, assuming the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) remains the same, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Each one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase each of cash and cash equivalents and short-term investments and total stockholders’ equity by approximately $8.58 million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, each one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would decrease each of cash and cash equivalents and short-term investments and total stockholders’ equity by approximately $6.72 million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will be adjustedadjust based on the actual public offering price, the actual number of shares and Common Warrants that we offer in this offering, and other terms of this offering determined at pricing.
The Except as indicated otherwise, the discussion and table above assume (i) no sale of pre-funded warrants, which, if sold, would reduce the number of shares of our common stock to be outstanding upon completionthat we are offering on a one-for-one basis and (ii) no exercise of this offering is based on 15,690,151Common Warrants accompanying the shares of our common stock outstanding as of September 30, 2018 and excludes:sold in this offering.
If you invest in our common stock in this offering,securities, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after the closing of this offering.
Our historical net tangible book value (deficit) as of September 30, 2023 was $(2.5) million, or $(4.67) per share of common stock. Our historical net tangible book value is the amount of our total tangible assets less our total liabilities. Net historicalHistorical net tangible book value per share of common stock is our historical net tangible book value divided by the number of shares of common stock outstanding as of September 30, 2018. 2023.
Our historicalpro forma net tangible book value (deficit) as of September 30, 20182023 was $9.4$(2.5) million, or $0.60$(3.59) per share of common stock.
As adjusted net book value is our The pro forma net tangible book value plusgives effect to (i) the payment of the $750,000 Amendment Execution Date Payment under the amended 2021 Loan Agreement, to be applied towards partial prepayment of the outstanding principal balance of the Term Loan, on November 29, 2023, (ii) the issuance of an aggregate of 161,268 shares of common stock upon exercises of previously issued pre-funded warrants, for nominal cash payment, between October 1, 2023 and November 15, 2023 and (iii) the cancellation of 1,470 fractional shares in connection with the 2023 Reverse Stock Split (collectively, the “pro forma events”).
After giving further effect ofto (i) the sale of 1,511,335 shares of our common stock and the accompanying Common Warrants in this offering at thean assumed combined public offering price of $3.23$3.97 per share (theand accompanying Common Warrants, which was the last reported sale price of our common stock on theThe Nasdaq Capital Market on December 13, 2018)8, 2023, (ii) the payment of the $1,500,000 Closing Payment under the 2021 Loan Agreement, to occur immediately following the closing of this offering, and (iii) the issuance, under the 2021 Loan Agreement, of an assumed 1,007,556 shares of common stock upon the conversion of $4.0 million of principal amount of the Convertible Note (net of unamortized debt discount of $64,000 solely for accounting purposes), to occur immediately following the closing of this offering at a conversion price equal to the assumed combined public offering price per share and accompanying Common Warrants in this offering (which assumes no issuance of any Conversion Pre-Funded Warrants), and after deducting the underwriting discounts and commissionsestimated placement agent fees and estimated offering expenses payable by us. Ourus, excluding the proceeds, if any, from the exercise of the Common Warrants issued in this offering, and assuming no sale of pre-funded warrants in this offering (collectively, the “closing events”), our pro forma as adjusted net tangible book value as of September 30, 20182023 would have been approximately $24.01be $6.7 million, or $1.16$2.08 per share.share of common stock. This amount represents an immediate increase in as adjustedpro forma net tangible book value of $0.56$5.67 per share to our existing stockholders and an immediate dilution of $2.07$1.89 per share to new investors participating in this offering. DilutionWe determine dilution per share to new investors is determinedparticipating in this offering by subtracting pro forma as adjusted net tangible book value per share after this offering from the assumed combined public offering price per share paid by new investors.investors participating in this offering.
The following table illustrates this dilution on a per share basis:basis to new investors:
| Assumed public offering price per share | $ | 3.23 | ||||||
| Historical net tangible book value per share as of September 30, 2018 | $ | 0.60 | ||||||
| Increase in net tangible book value per share as of September 30, 2018, attributable to new investors | $ | 0.56 | ||||||
| As adjusted net tangible book value per share, after giving effect to this offering | 1.16 | |||||||
| Dilution of as adjusted net tangible book value per share to new investors | $ | 2.07 |
| Assumed combined public offering price per share and accompanying Common Warrants | $ | 3.97 | ||||||
| Historical net tangible book value (deficit) per share as of September 30, 2023 | $ | (4.67 | ) | |||||
| Increase in historical net tangible book value per share attributable to the pro forma events | 1.08 | |||||||
| Pro forma net tangible book value (deficit) per share as of September 30, 2023 | (3.59 | ) | ||||||
| Increase in net tangible book value per share attributable to the closing events | 5.67 | |||||||
| Net tangible book value per share after giving effect to this offering | $ | 2.08 | ||||||
| Dilution per share to new investors in this offering | $ | 1.89 |
Each $1.00We may increase (decrease)or decrease the number of shares we are offering. An increase of 250,000 in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase (decrease) the as adjusted net tangible book value, by $0.22 per share and would decrease (increase) the dilution to new investors by $0.22 per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated expenses payable by us. Each increase of one million shares offered by us would increase the pro forma as adjusted net tangible book value by $0.08 per share by approximately $0.12 and decrease the dilution per share to new investors participating in this offering by $0.08 per share, assuming theapproximately $0.12, based on an assumed combined public offering price of $3.23$3.97 per share (theand accompanying Common Warrants, which was the last reported sale price of our common stock on theThe Nasdaq Capital Market on December 13, 2018) remains8, 2023, remaining the same and after deducting underwriting discountsestimated placement agent fees and commissions and estimated offering expenses payable by us. Similarly, each decreaseA reduction of one million250,000 in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease the pro forma as adjusted net tangible book value by $0.09 per share after this offering by approximately $0.14 and increase the dilution per share to new investors participating in this offering by $0.09 per share, assuming theapproximately $0.14, based on an assumed combined public offering price of $3.23$3.97 per share (theand accompanying Common Warrants, which was the last reported sale price of our common stock on theThe Nasdaq Capital Market on December 13, 2018) remains8, 2023, remaining the same and after deducting underwriting discountsestimated placement agent fees and commissions and estimated expenses payable by us. Each one million share increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would increase the as adjusted net tangible book value by $0.34 per share and decrease the dilution to new investors by $0.34 per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, each one million share decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed public offering price of $3.23 per share (the last reported sale price of our common stock on the Nasdaq Capital Market on December 13, 2018) would decrease the as adjusted net tangible book value by $0.28 per share and increase the dilution to new investors by $0.28 per share, after deducting underwriting discounts and commissions and any estimated offering expenses payable by us.
If the underwriters exercise their over-allotment option in full, the as adjusted net tangible book value per share after giving effect to this offering would be $1.23 per share, which amount represents an immediate increase in the as adjusted net tangible book value of $0.62 per share of our common stock to existing stockholdersThe table and an immediate dilution in net tangible book value of $2.00 per share of our common stock to new investors purchasingdiscussion above is based on 530,449 shares of common stock in this offering.
If any shares are issued upon the exercise of outstanding options or warrants, you will experience further dilution. The above discussion and table are based on 15,690,151 shares of our common stock outstanding as of September 30, 20182023 and excludes:
| ● |
| ● | ||
| ● | 473,408 shares of common stock issuable upon the exercise of other warrants outstanding | |
| ● | ||
| ● | up to | |
| ● |
The following table sets forth information regarding the beneficial ownership of our common stock as of September 30, 2018 by:
Beneficial ownership is determined based on the rules and regulations of the SEC. A person has beneficial ownership of shares if such individual has the power to vote and/or dispose of shares. This power may be sole or shared and direct or indirect. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of our common stock that are subject to options or warrants held by that person and exercisable as of, or within 60 days of, September 30, 2018 are counted as outstanding. These shares, however, are not counted as outstanding for the purposes of computing the percentage ownership of any other person(s). Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of our common stock set forth opposite that person’s name. Unless indicated below, the address of each individual listed below is c/o Motus GI Holdings, Inc., 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL 33301.
Applicable percentage ownership in the following table is based on 15,690,151 shares of our common stock outstanding as of September 30, 2018 and also lists applicable percentage ownership based on shares of our common stock assumed to be outstanding after completion of the offering, assuming no exercise by the underwriters of their option to purchase additional shares of our common stock.
Beneficial ownership representing less than 1% is denoted with an asterisk (*).
| Name of Beneficial Owner | Number of Shares Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||||||||||
| Before Offering | After Offering | |||||||||||||||
| Officers and Directors | ||||||||||||||||
| Timothy P. Moran (1) | 0 | * | * | |||||||||||||
| Mark Pomeranz (2) | 448,491 | 2.78% | 2.12 | % | ||||||||||||
| David Hochman (3)(4)(6)(7)(8)(9) | 3,976,361 | 24.58% | 18.78 | % | ||||||||||||
| Darren Sherman (4)(5)(6)(7)(8)(9) | 3,864,861 | 23.94% | 18.28 | % | ||||||||||||
| Gary Jacobs (10)(11) | 925,498 | 5.83% | 4.43 | % | ||||||||||||
| Samuel Nussbaum (12) | 35,000 | * | * | |||||||||||||
| Shervin Korangy (13) | 52,500 | * | * | |||||||||||||
| Andrew Taylor (14) | 81,999 | * | * | |||||||||||||
| Gary Pruden (15) | 50,000 | * | * | |||||||||||||
| Directors and Officers as a Group (9 persons) | 5,629,149 | 33.12% | 25.59 | % | ||||||||||||
| 5% Stockholders | ||||||||||||||||
| Ascent Biomedical Ventures II, L.P. (16) | 907,364 | 5.67% | 4.32 | % | ||||||||||||
| ABV, LLC (16)(17) | 1,607,163 | 9.99% | 7.62 | % | ||||||||||||
| Geoffrey W. Smith (8) (16)(17) | 3,607,163 | 22.41% | 17.10 | % | ||||||||||||
| Orchestra MOTUS Co-Investment Partners, LLC (7) | 1,345,101 | 8.47% | 6.44 | % | ||||||||||||
| Orchestra Medical Ventures, LLC (7) | 1,345,101 | 8.47% | 6.44 | % | ||||||||||||
| Orchestra BioMed, Inc. (8) | 2,000,000 | 12.75% | 9.67 | % | ||||||||||||
| Jacobs Investment Company LLC (11) | 870,148 | 5.50% | 4.18 | % | ||||||||||||
| Perceptive Life Sciences Master Fund Ltd. (18) | 3,046,596 | 19.12% | 14.55 | % | ||||||||||||
| Perceptive Advisors LLC (17) | 3,046,596 | 19.12% | 14.55 | % | ||||||||||||
The information discussed above is illustrative only and will adjust based on the actual public offering price, the actual number of shares and Common Warrants that we offer in this offering, and other terms of this offering determined at pricing. Except as indicated otherwise, the discussion and table above assume (i) no sale of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis and (ii) no exercise of Common Warrants accompanying the shares of common stock sold in this offering.
In addition, we may choose to raise additional capital in the future. To the extent that capital is raised through equity or convertible securities, the issuance of those securities may result in further dilution to the holders of common stock.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONSDESCRIPTION OF CAPITAL STOCK
Other than compensation arrangements for our named executive officers and directors, we describe below each transaction or seriesDescription of similar transactions, since January 1, 2015, to which we were a party or will be a party, in which:Common Stock
Our authorized capital stock consists of:
| ● | ||
Compensation arrangements for our named executive officers and directors are described in the section titled “Executive Compensation” in our Annual Report on Form 10-K for the year ended December 31, 2017, which is incorporated by reference in this prospectus.
Voting Agreement
Effective on December 1, 2016, Opco, and the holders of all issued and outstanding shares of capital stock of Opco (the “Opco Stockholders”), entered into a share exchange agreement (the “Share Exchange Agreement”) with us. Pursuant to the terms of the Share Exchange Agreement, as a condition of and contemporaneously with the initial closing (the “Initial Closing”) of the private placement offering of units we conducted from December 2016 to February 2017 (the “2017 Private Placement”), the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco (the “Share Exchange Transaction”) and Opco became our direct wholly-owned subsidiary. In connection with the Initial Closing of the 2017 Private Placement, the Opco Stockholders and our stockholders prior to the Share Exchange Transaction and the 2017 Private Placement (the “Formation Stockholders”), including Jacobs Investments Company LLC, an entity in which our director Gary Jacobs is the beneficial owner of the shares held by such entity, and Accelerated Technologies, Inc., Orchestra Medical Ventures II, L.P., Orchestra MOTUS Co-Investment Partners, LLC, and Orchestra Medical Ventures II GP, LLC, entities in which our directors David Hochman and Darren Sherman share beneficial ownership of the shares held by such entities, entered into a Voting Agreement (the “Voting Agreement”). Pursuant to the terms of the Voting Agreement, (i) the Opco Stockholders held the right to nominate four (4) members to our board of directors (the “Opco Stockholders’ Nominees”), (ii) the Formation Stockholders were to vote in favor of the election of the Opco Stockholders’ Nominees, (iii) the Formation Stockholders were to vote in favor of the election of the Placement Agent Nominee (defined below) to our board of directors, (iv) the Opco Stockholders were to vote in favor of the election of the Placement Agent Nominee and (v) the Opco Stockholders and the Formation Stockholders were permitted to vote in favor of up to two additional independent candidates to the board of directors acceptable to the Placement Agent Nominee and the Opco Stockholders’ Nominees. The Voting Agreement expired upon the closing of our IPO and is of no further force or effect.
Board of Directors Composition
Pursuant to the Voting Agreement described above, the placement agent for the 2017 Private Placement (the “Placement Agent”) held a right to appoint one member of our board of directors for a two-year term from the Initial Closing of the 2017 Private Placement (the “Placement Agent Nominee”). However, the Voting Agreement expired upon the closing of our IPO and is of no further force or effect.
Convertible Note, Convertible Warrant, and 2017 Private Placement - Related Party Participation
Pursuant to the terms of a convertible note agreement (the “CNA”), as amended, Opco issued convertible notes (the “Convertible Notes”) to certain investors (the “Convertible Holders”) in a series of closings from June 2015 through November 2016. From August 2015 through November 2016, Orchestra Medical Ventures II, L.P., an affiliate of Orchestra Medical Ventures II GP, LLC (an entity in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Members) purchased Convertible Notes in an aggregate principal amount of $1,649,062. On December 22, 2016, Orchestra Medical Ventures II, L.P. exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 299,244 shares of our common stock and (ii) 99,748 shares of our Series A Convertible Preferred Stock. In addition, Orchestra Medical Ventures II, L.P. received five (5) year warrants to purchase an aggregate of 108,838 shares of our common stock at an exercise price of $5.00 per share in an amount equal to thirty-three percent (33%) of the principal amount of such Convertible Note divided by $5.00 (the “Exchange Warrants,” see “Description of Securities – Warrants – Exchange Warrants” for a description of the Exchange Warrants.). Orchestra Medical Ventures II, L.P. and Orchestra Medical Ventures II GP, LLC are no longer beneficial owners of more than five percent of our common stock.
In addition, from August 2015 through November 2016, Orchestra MOTUS Co-Investment Partners, LLC, an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) purchased Convertible Notes in an aggregate principal amount of $1,047,511. On December 22, 2016, Orchestra MOTUS Co-Investment Partners, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 195,114 shares of our common stock and (ii) 65,038 shares of our Series A Convertible Preferred Stock. In addition, Orchestra MOTUS Co-Investment Partners, LLC received Exchange Warrants to purchase an aggregate of 69,136 shares of our common stock.
From June 2015 through November 2016, Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, purchased Convertible Notes in an aggregate principal amount of $1,044,032. On December 22, 2016, Jacobs Investment Company, LLC exchanged its Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 189,865 shares of our common stock and (ii) 63,289 shares of our Series A Convertible Preferred Stock. In addition, Jacobs Investment Company, LLC received Exchange Warrants to purchase an aggregate of 68,906 shares of our common stock.
From June 2015 through August 2016, Ascent Biomedical Ventures II, L.P., and from July 2015 through October 2015, Ascent Biomedical Ventures Synecor, L.P. (collectively, the “Ascent Entities”) purchased Convertible Notes in an aggregate principal amount of $2,790,412 (the “Ascent Convertible Notes”). ABV, LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Ascent Entities. On December 22, 2016, the Ascent Entities exchanged the Ascent Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 511,776 shares of our common stock and (ii) 170,593 shares of our Series A Convertible Preferred Stock. In addition, the Ascent Entities received Exchange Warrants to purchase an aggregate of 184,167 shares of our common stock.
On October 27, 2016, Perceptive Life Sciences Master Fund Ltd., and on October 28, 2016, Titan Perc, Ltd. (collectively, the “Perceptive Entities”) purchased Convertible Notes in an aggregate principal amount of $1,000,000 (the “Perceptive Convertible Notes”). Perceptive Advisors LLC, a beneficial owner of more than five percent of our common stock, is the beneficial owner of the securities held by the Perceptive Entities. On December 22, 2016, the Perceptive Entities exchanged the Perceptive Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 169,155 shares of our common stock and (ii) 56,386 shares of our Series A Convertible Preferred Stock. In addition, the Perceptive Entities received Exchange Warrants to purchase an aggregate of 66,000 shares of our common stock. Additionally, the Perceptive entities purchased an aggregate of 800,000 units in our 2017 Private Placement, at a purchase price of $5.00 per unit, comprised of an aggregate of (i) 600,000 shares of our common stock and (ii) 200,000 shares of our Series A Convertible Preferred Stock.
From January 2016 through November 2016 The Peierls Bypass Trust, UD E.F. Peierls for Brian E. Peierls, UD E.F. Peierls for E. Jeffrey Peierls, UD E.S. Peierls for E.F. Peierls et al, UD J.N. Peierls for Brian Eliot Peierls, UD J.N. Peierls for E. Jeffrey Peierls, UW E.S. Peierls for Brian E. Peierls - Accumulation, UW E.S. Peierls for E. Jeffrey Peierls - Accumulation, UW J.N. Peierls for Brian E. Peierls, UW J.N. Peierls for E. Jeffrey Peierls (collectively, the “Peierls Trusts”) and The Peierls Foundation, Inc. and UD Ethel F. Peierls Charitable Lead Trust (collectively, the “Peierls Entities”) purchased Convertible Notes in an aggregate principal amount of $1,448,000 (the “Peierls Convertible Notes”). E. Jeffrey Peierls, a beneficial owner of more than five percent of our common stock prior to the IPO, is the beneficial owner of the securities held by the Peierls Trusts and the Peierls Entities. On December 22, 2016, the Peierls Trusts and the Peierls Entities exchanged the Peierls Convertible Notes, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 257,385 shares of our common stock and (ii) 85,801 shares of our Series A Convertible Preferred Stock. In addition, the Peierls Trusts and the Peierls Entities received Exchange Warrants to purchase an aggregate of 95,568 shares of our common stock. Additionally, the Peierls Trusts and the Peierls Entities purchased an aggregate of 100,000 units in our 2017 Private Placement, at a purchase price of $5.00 per unit, comprised of an aggregate of (i) 75,000 shares of our common stock and (ii) 25,000 shares of our Series A Convertible Preferred Stock. As a result of the IPO, E. Jeffrey Peierls is no longer a beneficial owner of more than five percent of our common stock.
On October 27, 2016, AKS Family Partners, LP, a beneficial owner of more than five percent of our common stock prior to the Initial Closing, purchased a Convertible Note in an aggregate principal amount of $250,000. On December 22, 2016, AKS Family Partners, LP exchanged its Convertible Note, together with accrued and unpaid interest thereon calculated through December 22, 2016 at a rate of 10% per annum, for units sold in the 2017 Private Placement at a price of $4.50 per unit resulting in the receipt of (i) 42,290 shares of our common stock and (ii) 14,097 shares of our Series A Convertible Preferred Stock. In addition, AKS Family Partners, LP received Exchange Warrants to purchase an aggregate of 16,500 shares of our common stock. As a result of the Initial Closing, AKS Family Partners, LP is no longer a beneficial owner of more than five percent of our common stock.
Share Exchange Transaction
Effective on December 1, 2016, Opco, and the Opco Stockholders, entered into the Share Exchange Agreement with us. Pursuant to the terms of the Share Exchange Agreement, the Opco Stockholders sold to us, and we acquired, all of the issued and outstanding shares of capital stock of Opco and Opco became our wholly owned subsidiary.
At the closing of the Share Exchange Transaction, on December 22, 2016, (i) Orchestra Medical Ventures II, L.P., an affiliate of Orchestra Medical Ventures II GP, LLC (an entity in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Members) and Orchestra MOTUS Co-Investment Partners, LLC, an affiliate of Orchestra Medical Ventures, LLC (an investment firm in which David Hochman, Chairman of the Board, and Darren Sherman, one of our Directors, serve as Managing Partners) sold to us, and we acquired, all of the capital stock of Opco held by Orchestra Medical Ventures II, L.P. and Orchestra MOTUS Co-Investment Partners, LLC in exchange for 670,800 and 899,816 shares of our common stock, respectively, (ii) Jacobs Investment Company, LLC, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, sold to us, and we acquired, all of the capital stock of Opco held by Jacobs Investment Company, LLC in exchange for 474,802 shares of our common stock, and (iii) ABV, LLC, a beneficial owner of more than five percent of our common stock, through the Ascent Entities, sold to us, and we acquired, all of the capital stock of Opco held by the Ascent Entities in exchange for an aggregate of 1,520,353 shares of our common stock.
Ten Percent Warrants - Related Party Participation
In connection with the 2017 Private Placement, we entered into a registration rights agreement (the “Registration Rights Agreement”) with the 2017 Private Placement investors. Upon the completion of our IPO in February 2018, we issued warrants to certain of our Series A Convertible Preferred Stock holders, pursuant to an amendment to the Registration Rights Agreement and an amendment to the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock, to purchase an aggregate of 1,095,682 shares of our common stock (the “Ten Percent Warrants”), including (i) Ten Percent Warrants to purchase 300 shares of our common stock to David Hochman, the Chairman of our Board, (ii) Ten Percent Warrants to purchase 300 shares of our common stock to Darren Sherman, one of our directors, (iii) Ten Percent Warrants to purchase an aggregate of 220,274 shares of our common stock to the Ascent Entities, beneficial owners of more than five percent of our common stock, (iv) Ten Percent Warrants to purchase 106,980 shares of our common stock to Orchestra Medical Ventures II, L.P., a former beneficial owner of more than five percent of our common stock, (v) Ten Percent Warrants to purchase 115,997 shares of our common stock to Orchestra MOTUS Co-Investment Partners, LLC, a beneficial owner of more than five percent of our common stock, (vi) Ten Percent Warrants to purchase 72,386 shares of our common stock to Jacobs Investment Company, an investment firm in which Gary Jacobs, one of our Directors, serves as Founder and Managing Director, (vii) Ten Percent Warrants to purchase 180,055 shares of our common stock to Perceptive Life Sciences Master Fund Ltd., a beneficial owner of more than five percent of our common stock, (viii) Ten Percent Warrants to purchase an aggregate of 57,035 shares of our common stock to E. Jeffrey Peierls, including the Peierls Trusts and the Peierls Entities, a former beneficial owner of more than five percent of our common stock. See “Description of Securities – Warrants – Ten Percent Warrants” for a description of the Ten Percent Warrants.
Sales and Marketing Services Arrangement with FreeHold Surgical, Inc.
In August, 2017, we began paying a monthly fee to FreeHold Surgical, Inc., or FreeHold, an entity in which David Hochman, the Chairman of our Board, serves as a Director, and Darren Sherman, one of our Directors, serves as a Director and President. Pursuant to the fee arrangement, we paid FreeHold a monthly amount of approximately $25,000 as all-in compensation for sales and marketing services performed for us, on a part time basis, by two FreeHold sales representatives (the “FreeHold Services”), through June 2018. Effective July 2018, pursuant to an amendment to the fee arrangement, we pay FreeHold a monthly amount of approximately $8,333 as all-in compensation for the FreeHold Services. We have agreed with FreeHold to terminate the fee arrangement effective as of November 30, 2018. As of November 30, 2018 our payment obligations to FreeHold pursuant to the fee arrangement have terminated and all FreeHold Services obligations by FreeHold have ceased.
Participation in the IPO
In addition to the shares issued pursuant to the directed share program described below, all of our directors and executive officers, and certain of our existing stockholders who held greater than 5% of our common stock, including stockholders affiliated with certain of our directors, purchased an aggregate of 1,435,000 shares of our common stock in our IPO, completed February 2018, at the public offering price of $5.00 per share, including (i) Perceptive Life Sciences Master Fund Ltd., a greater than 5% shareholder, which purchased 1,000,000 shares, (ii) Orchestra Medical Ventures II, L.P., a greater than 5% shareholder, which purchased 40,000 shares, (iii) Gary Pruden, one of our directors, who purchased 50,000 shares, (iv) David Hochman, the chairman of our board, who purchased 75,000 shares, (v) Shervin Korangy, one of our directors, who purchased 20,000 shares, (vi) Mark Pomeranz, our president, who purchased 8,000 shares, (vii) Samuel Nussbaum, one of our directors, who purchased 10,000 shares, (viii) Darren Sherman, one of our directors, who purchased 5,000 shares and (ix) Andrew Taylor, our chief financial officer, who purchased 2,000 shares.
Directed Share Program
At our request, the underwriters sold at the IPO price 175,000 shares of our common stock, or five percent (5%) of the shares offered in our IPO, to our employees and other persons associated with us, including Gary Jacobs, one of our Directors, who purchased 5,000 shares of our common stock at the IPO price. The directed share program was arranged through the representative of the underwriters in the IPO.
Indemnification Agreements
We have entered into indemnification agreements with all of our directors and named executive officers. These agreements require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. We also intend to enter into indemnification agreements with our future directors and executive officers.
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest (collectively “related parties”), are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the Audit Committee or, in certain circumstances, the chairman of the Audit Committee. Any request for us to enter into a transaction with a related party, in which the amount involved exceeds $100,000 and such related party would have a direct or indirect interest must first be presented to our Audit Committee, or in certain circumstances the chairman of our Audit Committee, for review, consideration and approval. In approving or rejecting any such proposal, our Audit Committee, or the chairman of our Audit Committee, is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related party’s interest in the transaction.
Director Independence
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that Mr. Hochman, Mr. Sherman, Mr. Jacobs, Dr. Nussbaum, Mr. Korangy and Mr. Pruden do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the Rules of the Nasdaq Market and the SEC.
Our current certificate of incorporation, as amended, authorizes us to issue:
| ● | 10,000,000 shares of preferred stock, par value $0.0001 per share. |
As of September 30, 2018 there were 15,690,151The additional shares of our common stock outstanding, held of record by approximately 207 stockholders of record, and no shares of preferred stock outstanding.
The following statements are summaries only of provisions of our authorized capital stock available for issuance may be issued at times and are qualifiedunder circumstances so as to have a dilutive effect on earnings per share and on the equity ownership of the holders of our common stock. The ability of our board of directors to issue additional shares of stock could enhance the board’s ability to negotiate on behalf of the stockholders in a takeover situation but could also be used by the board to make a change-in-control more difficult, thereby denying stockholders the potential to sell their entirety byshares at a premium and entrenching current management. The following description is a summary of the material provisions of our certificate of incorporation, as amended.common stock. You should review these documents for a description of the rights, restrictions and obligations relatingrefer to our capital stock. CopiesCertificate of our certificateIncorporation and Bylaws, both of incorporation may be obtained fromwhich are on file with the Company upon written request.SEC as exhibits to previous SEC filings, for additional information. The summary below is qualified by provisions of applicable law.
Common Stock
Voting.Voting. The holders of our common stock are entitled to one vote for each share held of record on all matters on which the holders are entitled to vote (or consent to). When a quorum is present at any meeting of stockholders, any matter before any such meeting (other than an election of a director or directors) shall be decided by a majority of the votes properly cast on such matter, except where a different vote is required by our Certificate of Incorporation, by our Bylaws, by law, by the rules or regulations of any stock exchange applicable to us, or pursuant to any regulation applicable to us or our securities, in which case, such different vote shall apply. A majority in voting power of the shares entitled to vote at the meeting, present in person or represented by proxy, shall constitute a quorum at any meeting of stockholders.
Dividends.Dividends. The holders of our common stock are entitled to receive, ratably, dividends only if, when and as declared by our board of directors out of funds legally available therefor and after provision is made for each class of capital stock having preference over our common stock.
Liquidation Rights.Rights. In the event of our liquidation, dissolution or winding-up, the holders of our common stock are entitled to share, ratably, in all assets remaining available for distribution after payment of all liabilities and after provision is made for each class of capital stock having preference over our common stock.
Conversion Rights.Right. The holders of our common stock have no conversion rights.
Preemptive and Similar Rights.Rights. The holders of our common stock have no preemptive or similar rights.
Redemption/Put Rights.Rights. There are no redemption or sinking fund provisions applicable to our common stock. All of the outstanding shares of our common stock are fully-paid and non-assessable.
Transfer Restrictions.SharesAnti-Takeover Effects of our common stock are subject to transfer restrictions. HoldersDelaware Law and Our Certificate of our common stock may not transfer their securities unless (a) a registration statement is in effect under the Securities Act covering the proposed transferIncorporation and such transfer is made in accordance with such registration statement or (b) the securities are transferred in a transaction exempt from the registration requirements of the Securities Act and any related requirements imposed by applicable state securities laws. In the case of any transfer permitted under clause (b), the holder must notify us in writing of the proposed transfer and furnish us with an opinion of counsel, reasonably satisfactory to us, that the transfer will not require registration under the Securities Act or any applicable state securities laws. Each certificate representing a security contains a legend referring to this restriction on transfer and any legends required by state securities laws.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of “blank check” preferred stock, par value $0.0001 per share, with such designations, rights, and preferences as may be determined from time to time by our board of directors. Accordingly, our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock could have the effect of restricting dividends on our common stock, diluting the voting power of our common stock, impairing the liquidation rights of our common stock, or delaying or preventing a change in control of our company. We currently have no plans to issue any shares of preferred stock.
Royalty Payment Rights Certificates
We have issued Royalty Payment Rights Certificates (the “Royalty Payment Rights Certificates”) entitling the holders of such certificates to the following royalty payment rights (the “Royalty Payment Rights”):
Royalties. If and when we generate sales of the current and potential future versions of the Pure-Vu System, including disposables, parts, and services, or if we receive any proceeds from the licensing of the current and potential future versions of the Pure-Vu System, then we will pay to the holders of our Royalty Payment Rights Certificates (the “Holders”) with the allocation of such Royalty Payment Rights between Holders determined as set forth below under “Allocation of Royalty Payments”, a royalty (the “Royalty Amount”) equal to, in the aggregate, in royalty payments in any calendar year for all products:Bylaws
| ||
* Notwithstanding the foregoing, with respect to Net Sales based Royalty Amounts, (a) no Net Sales based Royalty Amount shall begin to accrue or become payable until we have first generated, in the aggregate, since inception, Net Sales equal to $20 million (the “Initial Net Sales Milestone”), and royalties shall only be computed on, and due with respect to, Net Sales generated in excess of the Initial Net Sales Milestone, and (b) the total Net Sales based Royalty Amount due and payable in any calendar year shall be subject to a cap per calendar year of $30 million. “Net Sales” is defined in the Royalty Payment Rights Certificates.
** Notwithstanding the foregoing, with respect to Licensing Proceeds based Royalty Amounts, (a) no Licensing Proceeds based Royalty Amount shall begin to accrue or become payable until we have first generated, in the aggregate, since inception, Licensing Proceeds equal to $3.5 million (the “Initial Licensing Proceeds Milestone”), and royalties shall only be computed on, and due with respect to, Licensing Proceeds in excess of the Initial Licensing Proceeds Milestone and (b) the total Licensing Proceeds based Royalty Amount due and payable in any calendar year shall be subject to a cap per calendar year of $30 million. “Licensing Proceeds” is defined in the Royalty Payment Rights Certificates.
We currently have not licensed any of our products to any third-party and are not in negotiations with respect to any such license. There is no guarantee that we will ever generate sales of, or Licensing Proceeds from, our products. The Holders may never receive any royalty payments and these Royalty Payment Rights may expire worthless.
Timing of Royalty Payments. With respect to products that we commercialize directly, royalty payments, if any, will be paid annually 15 business days after the issuance of our audited financial statements for the prior year in which such Net Sales were generated; for the avoidance of doubt, such payments shall begin only upon achievement of the Initial Net Sales Milestone without regard to whether the Initial Licensing Proceeds Milestone has been met. With respect to products that we sublicense or otherwise dispose of to a third-party, royalty payments, if any, will be paid 10 business days after the end of the applicable quarter in which such Licensing Proceeds are received by us; for the avoidance of doubt, such payments shall begin only upon achievement of the Initial Licensing Proceeds Milestone without regard to whether the Initial Net Sales Milestone has been met.
The royalty will be payable up to the later of (i) the latest expiration date of our patents issued as of December 22, 2016, or (ii) the latest expiration date of any pending patents as of December 22, 2016 that have since been issued or may be issued in the future (which is currently November 2034). Following the expiration of all such patents, the Holders of the Royalty Payment Rights will no longer be entitled to any further royalties for any period following the latest to occur of such patent expiration.
Allocation of Royalty Payment. Once the aggregate Royalty Payment Amount is calculated based on the criteria set forth above under “Royalties,” that amount will be allocated to the holders of the Royalty Payment Rights Certificates by multiplying the aggregate Royalty Payment Amount by the percentage set forth in each Holder’s Royalty Payment Rights Certificate.
Separability. The Royalty Payment Rights Certificates may be transferred, subject to the availability of an exemption from registration under applicable state and federal securities laws.
Unsecured Obligations. The Royalty Payment Rights are unsecured obligations of ours.
Placement Agent Royalty Payment Rights
In connection with completion of the 2017 Private Placement, we issued the Placement Agent royalty payment rights certificates (the “Placement Agent Royalty Payment Rights Certificates”) to receive, in the aggregate, 10% of the amount of payments paid to the holders of the Royalty Payment Rights Certificates. The Placement Agent Royalty Payment Rights Certificates are on substantially similar terms as the Royalty Payment Rights Certificates.
Warrants
Exchange Warrants.In connection with the Share Exchange Transaction and the CNA, on December 22, 2016, we issued warrants to each former Convertible Holder to purchase an aggregate 907,237 shares of our common stock (the “Exchange Warrants”). The Exchange Warrants are exercisable for our common stock at an exercise price equal to $5.00 per share (the “Exercise Price”). The Exchange Warrants are exercisable immediately upon issuance and have a five year term, and provide for cashless exercise. The Exchange Warrants may be exercised at any time in whole or in part at the applicable exercise price until expiration of the Exchange Warrants. No fractional shares will be issued upon the exercise of the Exchange Warrants.
Placement Agent Warrants.In connection with completion of the 2017 Private Placement, on February 24, 2017, we issued the placement agent, and its designees, warrants to purchase 403,632 shares of our common stock at an exercise price of $5.00 as partial compensation (the “Placement Agent Warrants”). These warrants have a five year term and provide cashless exercise.
Ten Percent Warrants. In connection with our initial public offering, on February 16, 2018, we issued warrants to purchase 1,095,682 shares of our common stock (the “Ten Percent Warrants”). The Ten Percent Warrants are exercisable for our common stock at an exercise price of $5.00 per share. The Ten Percent Warrants have a five year term, and provide for cashless exercise. No fractional shares will be issued upon the exercise of the Ten Percent Warrants.
Service Provider Warrants. We have additional warrants outstanding, or which are issuable by us or pursuant to contractual obligations, to purchase an aggregate of 257,917 shares of our common stock with a weighted average exercise price of $8.03 per share. We have an additional warrant which is issuable by us pursuant to a contractual obligation to purchase an estimated 6,380 shares of our common stock, subject to adjustment, with an estimated weighted average exercise price of $3.23 per share, subject to adjustment, expected to be issued October 2019.
Registration Rights
Service Provider Warrants. Certain of our other warrants are entitled to piggyback registration rights with respect to the shares issuable upon exercise of such warrants. If we register any of our securities either for our own account or for the account of other security holders, such holders are entitled to include their shares in the registration. In the event we register securities in connection with an underwritten offering, the underwriters will have the right to limit the number of shares included in such offering. The holder of all of our outstanding warrants entitled to piggyback registration rights has executed a notice and waiver of piggyback registration rights, waiving any such piggyback registration rights in connection with this offering.
Lock-Up Agreements
Each of our directors and officers and the holders of substantially all of five percent (5%) or more of our common stock as of the time of the Initial Closing of the 2017 Private Placement have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, which includes any shares of our common stock acquired upon the exercise of any warrants acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending February 13, 2019, without our prior written consent and the prior written consent of the Placement Agent.
In connection with the formation of Motus GI Holdings, Inc. in September 2016, certain affiliates of the Placement Agent and certain other parties not affiliated with us or the Placement Agent subscribed for an aggregate of 1,650,000 shares of our common stock (the “Formation Shares”), for which they paid an aggregate of $82,500 ($0.05 per share). Each of the holders of the Formation Shares have agreed that they will not (a) offer, sell, contract to sell, grant any option to purchase, hypothecate, pledge or otherwise dispose of or (b) transfer title to any shares of our common stock acquired prior to the 2017 Private Placement, for a period beginning on December 22, 2016 and ending February 13, 2019, without our prior written consent and the prior written consent of the Placement Agent.
In March 2017, we signed a consulting service agreement by which we granted the service provider 90,000 shares of our common stock for past services provided. The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to 45,000 of the subject shares, for a period beginning the effective date of the consulting agreement and ending February 13, 2019, without our prior written consent.
In March 2018, we granted a service provider 15,000 shares of our common stock for services pursuant to a consulting agreement. The consultant has agreed that they will not (a) offer, pledge, sell, contract to sell, grant any option or contract to purchase, purchase any option or contract to sell, or otherwise dispose of, directly or indirectly, or (b) transfer title to the 15,000 shares for a period beginning the effective date of the consulting agreement and ending February 13, 2019, without our prior written consent.
Our officersCertificate of Incorporation and directors have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of their common stock or securities convertible into or exchangeable for shares of common stock for a period through the date 90 days after the date of this prospectus, except with the prior written consent of Piper Jaffray & Co., as the representative of the underwriters. The representative may in their sole discretion and at any time without notice release some or all of the shares subject to lock-up agreements prior to the expiration of the 90-day period. When determining whether or not to release shares from the lock-up agreements, the representative may consider, among other factors, the stockholder’s reasons for requesting the release, the number of shares for which the release is being requested and market conditions at the time.
Transfer Agent and Registrar
Continental Stock Transfer and Trust, located at 1 State Street 30th Floor, New York, NY 10004, is the transfer agent and registrar for our common stock and preferred stock.
Quotation of Securities
Shares of our common stock are listed on the Nasdaq Capital Market under the trading symbol“MOTS”.
Anti-Takeover Effect of Delaware Law, Certain Charter and Bylaw Provisions
Our certificate of incorporation and bylawsBylaws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company.control. These provisions are as follows:
| ● | they provide that special meetings of stockholders may be called by the board of directors or at the request in writing by stockholders of record owning at least twenty (20%) percent of the issued and outstanding voting shares of our common stock; | |
| ● | they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and | |
| ● | they allow us to issue, without stockholder approval, up to 10,000,000 shares of preferred stock that could adversely affect the rights and powers of the holders of our common stock. |
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware,DGCL, an anti-takeover law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in the following prescribed manner:
| ● | prior to the time of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; | |
| ● | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the stockholder owned at least eighty-five percent (85%) of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding; (1) shares owned by persons who are directors and also officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; and | |
| ● | on or subsequent to the time of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least sixty-six and two-thirds percent (66 2/3%) of the outstanding voting stock which is not owned by the interested stockholder. |
Generally, for purposes of Section 203, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns or, within three (3) years prior to the determination of interested stockholder status, owned fifteen percent (15%) or more of a corporation’s outstanding voting securities.
ChoiceRequirements for Advance Notification of ForumStockholder Nominations and Proposals
Our certificateBylaws establish advance notice procedures with respect to stockholder proposals and the nomination of incorporation,candidates for election as amended,directors, other than nominations made by or at the direction of our board of directors or a committee of our board of directors. These provisions may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
Choice of Forum
Our Certificate of Incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us, or any of our officers or Directors, arising pursuant to the Delaware General Corporation Law,DGCL, our certificateCertificate of incorporation, as amended,Incorporation or our bylaws;Bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. This exclusive forum provision may limit the ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for the disputes listed above, which may discourage such lawsuits against us, or any of our officers or directors.
Potential Effects of Authorized but Unissued Stock
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF OUR COMMON STOCKWe have shares of common stock and preferred stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital stock.
The followingexistence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board of directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent permissible under the DGCL and subject to any limitations set forth in our Certificate of Incorporation. The purpose of authorizing the board of directors to issue preferred stock and to determine the rights and preferences applicable to such preferred stock is to eliminate delays associated with a summarystockholder vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the material United States federal income tax consequenceseffect of making it more difficult for a third-party to non-U.S. holders (as defined below)acquire, or could discourage a third-party from acquiring, a majority of their ownershipour outstanding voting stock.
Transfer Agent and dispositionRegistrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company. The transfer agent address is Continental Stock Transfer & Trust Company, 1 State Street, 30th Floor, New York, NY 10004, (212) 509-4000.
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering shares of our common stock but doesor pre-funded warrants to purchase shares of our common stock and Common Warrants to purchase shares of our common stock. Each share of common stock or pre-funded warrant is being sold together with a Series A Common Warrant to purchase one share of our common stock and a Series B Common Warrant to purchase one share of our common stock. The shares of common stock or pre-funded warrants and accompanying Common Warrants will be issued separately. We are also registering the shares of common stock issuable from time to time upon exercise of the pre-funded warrants and Common Warrants offered hereby.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Pre-Funded Warrants
The following summary of certain terms and provisions of pre-funded warrants that are being offered hereby is not purportcomplete and is subject to, be a complete analysis of alland qualified in its entirety by, the potential tax considerations relating thereto. This summary is based upon current provisions of the Internal Revenue Code, or Code, existingpre-funded warrant, the form of which will be filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and proposed United States Treasury Regulations promulgated thereunder, current administrative rulings, and judicial decisions, all as in effect asprovisions of the date hereof. These authoritiesform of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration and Exercise Price. Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.0001. The pre-funded warrants will be immediately exercisable and may be changed, possibly retroactively, so asexercised at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to resultappropriate adjustment in United States federal tax consequences different from those set forth below. We have not obtained,the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and do not intend to obtain, any opinion of counsel or rulingthe exercise price. The pre-funded warrants will be issued separately from the Internal Revenue Service, or the IRS, with respect to the statements madeaccompanying Common Warrants and the conclusions reached in the following summary, and there canmay be no assurance that the IRS will agree with such statements and conclusions.transferred separately immediately thereafter.
This summary also does not addressExercisability. The pre-funded warrants will be exercisable, at the tax considerations arising under the lawsoption of any non-United States, state or local jurisdiction or under any non-income tax laws, including United States federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address the potential application of the tax on net investment income or the alternative minimum tax. This discussion may not apply,each holder, in whole or in part, by delivering to particular non-U.S. holdersus a duly executed exercise notice accompanied by payment in lightfull for the number of their individual circumstances or to holders subject to special treatment under the United States federal income tax laws, including, without limitation:
In addition, this discussion does not address the tax treatment of partnerships, including any entity or arrangement treated as a partnership for United States federal income tax purposes. Generally, the tax treatment of a person treated as a partner in such an entity will depend on the status of the partner, the activities of the partner and the partnership, and certain determinations made at the partner level. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
THIS SUMMARY IS NOT INTENDED TO BE CONSTRUED AS LEGAL ADVICE. WE RECOMMEND THAT PROSPECTIVE INVESTORS CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES TO THEM OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS, ANY APPLICABLE INCOME TAX TREATIES, OR ANY OTHER U.S. FEDERAL TAX LAWS (INCLUDING ESTATE AND GIFT TAX LAWS).
Definition of Non-U.S. Holder
For purposes of this discussion, you are a non-U.S. holder if you are a beneficial ownershares of our common stock that is not, for United States federal income tax purposes:
Distributions to non-U.S. Holders
As describedpurchased upon such exercise (except in the section titled “Dividend Policy,” we do not anticipate paying any cash dividends or making distributionscase of other property on oura cashless exercise as discussed below). Purchasers of the pre-funded warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the pre-funded warrants at closing to have their pre-funded warrants exercised immediately upon issuance and receive shares of common stock inunderlying the foreseeable future. However, if we do make distributionspre-funded warrants upon closing of cash or property on our common stock, those payments will constitute dividends for United States federal income tax purposesthis offering. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Tothat the extent those distributions exceed both our current and our accumulated earnings and profits,holder would own more than 4.99% of the excess will constitute a return of capital and will first reduce a non-U.S. holder’s tax basis in ouroutstanding common stock but not below zero, and then will be treated by a non-U.S. holder as gainimmediately after exercise, except that upon at least 61 days’ prior notice from the saleholder to us, the holder may increase the amount of ownership of outstanding stock as described below under “Gain on Dispositions of Our Common Stock by Non-U.S. Holders.”
Subjectafter exercising the holder’s pre-funded warrants up to the discussion below on effectively connected income, any dividend paid to a non-U.S. holder generally will be subject to United States withholding tax either at a rate of 30%9.99% of the gross amountnumber of the dividend or such lower rate as may be specified by an applicable income tax treaty. To receive a reduced treaty rate, a non-U.S. holder must provide us with an IRS Form W-8BEN or W-8BEN-E (or applicable successor form) and certify qualification for the reduced rate. If a non-U.S. holder is eligible for a reduced rate of United States withholding tax pursuant to an income tax treaty, such non-U.S. holder may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS. If a non-U.S. holder holds our common stock through a financial institution or other agent acting on such non-U.S. holder’s behalf, appropriate documentation will need to be provided to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by a non-U.S. holder that are effectively connected with such non-U.S. holder’s conduct of a trade or business in the United States (and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States), are generally exempt from the 30% withholding tax if certain certification and disclosure requirements are satisfied. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI (or applicable successor form) properly certifying such exemption. However, such effectively connected dividends, although not subject to withholding tax, generally are taxed at the same United States federal income tax rates applicable to United States persons, net of certain deductions and credits. In addition, dividends received by a corporate non-U.S. holder that are effectively connected with the conduct of a trade or business in the United States may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. Non-U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Any documentation provided to an applicable withholding agent may need to be updated in certain circumstances. The certification requirements described above also may require a non-U.S. holder to provide a United States taxpayer identification number.
For additional withholding rules that may apply to dividends, including dividends paid to foreign financial institutions (as specifically defined by the applicable rules) or to certain other foreign entities that have substantial direct or indirect United States owners, see the discussion below under the headings “Information Reporting and Backup Withholding” and “Withholdable Payments to Foreign Financial Institutions and Other Foreign Entities.”
Gain on Disposition of Our Common Stock by Non-U.S. Holders
Subject to the discussion below under the headings “Information Reporting and Backup Withholding” and “Withholdable Payments to Foreign Financial Institutions and Other Foreign Entities,” a non-U.S. holder generally will not be required to pay United States federal income tax or withholding tax on any gain recognized upon the sale, exchange or other taxable dispositionshares of our common stock unless:
We believe that we are not currently and will not become a USRPHC and the remainderexercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this discussion so assumes. However, becauseoffering may also elect prior to the determinationissuance of whether we are a USRPHC depends on the fair market valuepre-funded warrants to have the initial exercise limitation set at 9.99% of our United States real property relative to the fair market valueoutstanding common stock. No fractional shares of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as United States real property interests only ifissued in connection with the exercise of a non-U.S.pre-funded warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder actually or constructively holds more than 5%exercises its pre-funded warrants, a registration statement registering the issuance of such regularly tradedthe shares of common stock at any time duringunderlying the shorterpre-funded warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the five-year period precedingaggregate exercise price, the holder may elect instead to receive upon such non-U.S. holder’s disposition of,exercise (either in whole or holding period for, our common stock. Non- U.S. holders should consult with tax advisors regarding any applicable income tax or other treaties that may provide for different rules.
Information Reporting and Backup Withholding
We (orin part) the applicable paying agent) must report annually to the IRS the amount of dividends on our common stock paid to non-U.S. holders and the amount of tax withheld, if any. A similar report will be sent to each non-U.S. holder. Copies of this information reporting may also be made available under the provisions of a specific income tax treaty or agreement with the tax authorities in a non-U.S. holder’s country of residence.
Non-U.S. holders will generally be subject to backup withholding (at a current rate of 24%) for dividends on our common stock paid to such non-U.S. holders unless an exemption is established such as by, for example, properly certifying non-United States status on an IRS Form W-8BEN or W-8BEN-E (or applicable successor form). Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that a holder of our common stock is a United States person.
Information reporting and backup withholding generally are not required with respect to the amount of any proceeds from the sale or other disposition of our common stock by a non-U.S. holder outside the United States through a foreign office of a foreign broker that does not have certain specified connections to the United States. However, if a non-U.S. holder sells or otherwise disposesnet number of shares of common stock throughdetermined according to a United States brokerformula set forth in the pre-funded warrants.
Transferability. Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
| 17 |
Exchange Listing. There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system. We do not intend to list the United States officespre-funded warrants on any securities exchange or nationally recognized trading system.
Right as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Fundamental Transaction. In the event of a foreign broker,fundamental transaction, as described in the brokerpre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the pre-funded warrants will generally be requiredentitled to reportreceive upon exercise of the pre-funded warrants the kind and amount of proceeds paidsecurities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such non-U.S. holderfundamental transaction.
Common Warrants
The following summary of certain terms and provisions of Common Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Common Warrants, the form of which will be filed as exhibits to the IRSregistration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and alsoprovisions of the form of Common Warrants for a complete description of the terms and conditions of the Common Warrants.
Duration and Exercise Price. Each Common Warrant offered hereby will have an initial exercise price per share equal to backup withhold$ . The Common Warrants will be immediately exercisable and will expire as follows: in the case of the Series A Common Warrant, on that amount unless the broker is provided appropriate certificationfifth anniversary of status as a non-United States person or an exemption is otherwise established. Information reporting will also apply if a non-U.S. holder sellsthe original issuance date and in the case of the Series B Common Warrant, on the eighteen month anniversary of the original issuance date. The exercise price and number of shares of common stock throughissuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The Common Warrants will be issued separately from the common stock (or pre-funded warrants) and may be transferred separately immediately thereafter. Two Common Warrants to purchase one share of our common stock each will be issued for every share of common stock (or pre-funded warrant to purchase a foreign broker derivingshare of common stock) purchased in this offering.
Exercisability. The Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of any Common Warrant to the extent that the holder would own more than a specified percentage4.99% of its incomethe outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from United States sources or having certain other connectionsthe holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s Common Warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the United States, unlessexercise, as such broker has documentary evidencepercentage ownership is determined in accordance with the terms of the Common Warrants. No fractional shares of common stock will be issued in connection with the exercise of a Common Warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its records that such non-U.S. holderCommon Warrants, a registration statement registering the issuance of the shares of common stock underlying the Common Warrants under the Securities Act is a non-United States personnot then effective or available and certain other conditions are met, or an exemption from registration under the Securities Act is not available for the issuance of such shares, then in lieu of making the cash payment otherwise established.contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Common Warrants.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules fromTransferability. Subject to applicable laws, a paymentCommon Warrant may be refunded or credited against a non-U.S. holder’s United States federal income tax liability, if any, provided that an appropriate claim is timely filedtransferred at the option of the holder upon surrender of the Common Warrant to us together with the IRS. Non-U.S. holders should consult with tax advisors regardingappropriate instruments of transfer.
Exchange Listing. There is no established public trading market for the applicationCommon Warrants, and we do not expect a market to develop. In addition, we do not intend to list the Common Warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the information reporting and backup withholding rules to investmentCommon Warrants will be limited.
Right as a Stockholder. Except as otherwise provided in the Common Warrants or by virtue of such holder’s ownership of shares of our common stock.stock, the holders of the Common Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Common Warrants.
Withholdable Payments to Foreign Financial InstitutionsFundamental Transaction. In the event of a fundamental transaction, as described in the form of Common Warrant, and other Foreign Entities
The Foreign Account Tax Compliance Act,generally including any reorganization, recapitalization or FATCA, imposes a United States federal withholding taxreclassification of 30% on certain payments to “foreign financial institutions” (as specifically defined under these rules) and certain other non-United States persons that fail to comply with certain information reporting and certification requirements pertaining to their direct and indirect United States security holders and/or United States account holders. Such payments include dividends on and, on or after January 1, 2019, gross proceeds fromour common stock, the sale, transfer or other disposition of all or substantially all of our common stock. Under certain circumstances, a non-U.S. holder may be eligible for refundsproperties or creditsassets, our consolidation or merger with or into another person, the acquisition of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult with tax advisors regarding the possible implicationsmore than 50% of this legislation and any applicable intergovernmental agreements on investment in our common stock.
U.S. Federal Estate Tax
Ouroutstanding common stock, beneficially ownedor any person or group becoming the beneficial owner of 50% of the voting power represented by an individual whoour outstanding common stock, the holders of the Common Warrants will be entitled to receive upon exercise of the Common Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Common Warrants immediately prior to such fundamental transaction. In the event of a fundamental transaction approved by our Board of Directors, the holders of the Common Warrants have the right to require us or a successor entity to redeem the Common Warrants for cash in the amount of the Black Scholes Value (as defined in each Common Warrant) of the unexercised portion of the Common Warrants as of the date of the consummation of the fundamental transaction. In the event of a fundamental transaction which is not a citizen or residentapproved by our Board of Directors, the holders of the United States (as definedCommon Warrants have the right to require us or a successor entity to redeem the Common Warrants for United States federal estate tax purposes) at the time of their death will generally be includableconsideration paid in the decedent’s gross estate for United States federal estate tax purposes and, therefore, may be subject to United States federal estate tax unless an applicable estate tax treaty or other treaty provides otherwise. Investors are urged to consult their own tax advisors regardingfundamental transaction in the United States federal estate tax consequencesamount of the ownership or dispositionBlack Scholes Value of our common stock.
THIS SUMMARY IS NOT INTENDED TO BE CONSTRUED AS LEGAL ADVICE. NON-U.S. HOLDERS ARE URGED TO CONSULT WITH TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE UNITED STATES FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATION, AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE UNITED STATES FEDERAL ESTATE OR GIFT TAX RULES OR UNDER THE LAWS OF ANY STATE, LOCAL, NON-UNITED STATES OR OTHER TAXING JURISDICTION OR UNDER ANY APPLICABLE TAX TREATY, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
the unexercised portion of the Common Warrants as of the date of the consummation of the fundamental transaction.
UNDERWRITINGPLAN OF DISTRIBUTION
SubjectA.G.P./Alliance Global Partners (“A.G.P.”), has agreed to act as our sole placement agent on a reasonable best efforts basis in connection with this offering subject to the terms and conditions of a placement agency agreement, dated , 2023 between A.G.P. and us. The placement agent is not purchasing or selling any shares in this offering but has arranged for the sale of the securities offered hereby. The public offering price of the securities in this offering has been determined based upon arm’s-length negotiations between the purchasers and us. The placement agent agreement will provide certain representations, warranties and covenants, including indemnifications, from us. A.G.P. will have no authority to bind us. We will enter into a securities purchase agreement directly with the investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase agreement entered into with Piper Jaffray & Co., as representativeof our securities in this offering. All of the several underwritersshares will be sold at the offering price specified in this prospectus and, we expect, at a single closing. Investors will deposit their investment in us with the placement agent, which will settle the offering in a single closing on a Delivery Versus Payment (“DVP”) settlement basis (i.e., on the closing date, we will issue the securities registered in the investors’ names and addresses and released by the transfer agent (with respect to common stock) or us (with respect to warrants) directly to the account(s) at the placement agent identified by each investor; upon receipt of thissuch securities, the placement agent will promptly electronically deliver such securities to the applicable investor, and payment therefor shall be made by the placement agent (or its clearing firm) by wire transfer to us).
We may sell fewer than all of the securities offered hereby, provided that we will only consummate an offering of $5.0 million or more in gross proceeds. This offering may be closed without further notice to you. We expect to close the offering on , 2023 but the offering will be terminated on or before December 31, 2023, provided that the closing of the offering has not occurred by such date, and may not be extended. Also, any proceeds from the sale of securities offered by us will be available for our immediate use, despite uncertainty about whether we would be able to use such funds to effectively implement our business plan.
Commissions and Expenses
We have agreed to sellpay the placement agent an aggregate cash placement fee equal to seven percent (7.0%) (or 5.0% with respect to proceeds from certain investors agreed upon between us and the placement agent) of the gross proceeds in this offering from sales arranged for by the placement agent.
Subject to certain conditions, we have also agreed to pay the following expenses relating to this offering: (a) all filing fees and expenses relating to the underwriters,registration with the SEC of the securities sold in this offering; (b) all FINRA public offering filing fees; (c) all fees and expenses relating to the listing of the Company’s equity or equity-linked securities on the Nasdaq Stock Exchange; (d) all fees, expenses and disbursements relating to the registration or qualification of the securities under the “blue sky” securities laws of such states and other jurisdictions as A.G.P. may reasonably designate (including, without limitation, all filing and registration fees, and the underwriters have severally agreed to purchase from usreasonable fees and disbursements of the number of shares of common stock indicated in the table below:
The underwriters have advised us that they propose to offer the shares of common stock to the public at $ per share of common stock to certain dealers at that price less a concessionCompany’s “blue sky” counsel, which will be A.G.P.’s counsel) unless such filings are not in excess of $ per share of common stock. After the offering, this figure may be changed by the underwriters. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The following table shows the per share of common stock and total underwriting discounts to be paid to the underwritersrequired in connection with this offering assuming both no exercisethe Company’s proposed listing with Nasdaq; (e) all fees, expenses and full exercisedisbursements relating to the registration, qualification or exemption of the underwriters’ optionsecurities under the securities laws of such foreign jurisdictions as A.G.P. may reasonably designate; (f) the costs of all mailing and printing of the offering documents; (g) transfer and/or stamp taxes, if any, payable upon the transfer of securities from the Company to purchase additional sharesA.G.P.; (h) the fees and expenses of common stock:the Company’s accountants; (i) $100,000 for reasonable legal fees and disbursements for A.G.P.’s counsel; (j) up to $25,000 for reimbursement of non-accountable expenses and (k) up to $10,000 for clearing costs.
We estimate that the total fees andoffering expenses of this offering that will be payable by us, excluding underwriting discounts and commissionsthe placement agent fees, will be approximately $450,000, which includes up$325,000. After deducting the placement agent fees and our estimated offering expenses, we expect the net proceeds from this offering to $125,000 that we have agreed to reimburse the underwriters for fees incurred by them in connection with this offering.be approximately $5.2 million.
Lock-Up Agreements.
In connection with this offering, each of our IPO we grantedexecutive officers and directors and certain shareholders have agreed, subject to certain exceptions set forth in the representativelock-up agreements, not to sell, offer, agree to sell, contract to sell, hypothecate, pledge, grant any option to purchase, make any short sale of, or otherwise dispose of, directly or indirectly, any common stock, or any securities convertible into or exercisable or exchangeable for common stock, for ninety (90) days following the closing of the underwritersoffering.
Securities Issuance Standstill
In addition, we have agreed, subject to certain exceptions, that for a rightperiod of first refusal duringninety (90) days from the thirty month period following February 13, 2019, the effectiveclosing date of our IPO prospectus, to act as our exclusive financial advisor, sole book-running manager, or exclusivethe offering, without the prior written consent of the placement agent, aswe will not (a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of the case may be, in connection with any restructuring transaction, any acquisition or disposition transaction, any public offering, any Rule 144A offeringCompany or any private placementsecurities convertible into or exercisable or exchangeable for equity of the Company; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any equity of the Company or any securities untilconvertible into or exercisable or exchangeable for equity of the earlierCompany; or (c) enter into any agreement or announce the intention to effect any of (i)the actions described in subsections (a) or (b) hereof (all of such matters, the “Standstill”). So long as none of such equity securities shall be saleable in the public market until the expiration of the thirty monthninety (90) day period described above, the following February 13, 2019,matters shall not be prohibited by the effective dateStandstill: (i) the adoption of an equity incentive plan and the grant of awards or equity pursuant to any equity incentive plan and the issuance of shares of our IPO prospectus, orcommon stock pursuant to any outstanding convertible securities, and the filing of a registration statement on Form S-8; and (ii) the aggregate gross proceedsissuance of equity securities in connection with an acquisition or a strategic relationship, which may include the sale of equity securities. We have also agreed not to enter into a variable rate transaction (as defined in the securities purchase agreement) for 120 days after the completion of this offering.
Determination of Offering Price
The public offering price of the securities we are offering was negotiated between us and the investors, in such transactions exceeds $50,000,000, subject to certain specified exceptions. Any such engagement will beconsultation with the placement agent based on terms and conditions customarythe trading of our common stock prior to the representativeoffering, among other things. Other factors considered in determining the public offering price of the underwriterssecurities we are offering include our history and prospects, the stage of development of our business, our business plans for similarthe future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Passive Market Making
In connection with this offering, the placement agent may engage in passive market making transactions in our common stock on the Nasdaq Stock Market in accordance with Rule 103 of Regulation M promulgated under the Exchange Act during a period before the commencement of offers or sales of shares of our common stock and will be governed by separate agreement.extending through the completion of the distribution.
Indemnification
We have agreed to indemnify the underwritersplacement agent against certain liabilities, including civil liabilities under the Securities Act, and liabilities arising from breaches of representations and warranties contained in the placement agency agreement, or to contribute to payments that the underwritersplacement agent may be required to make in respect of those liabilities.
We, eachPotential Conflicts of Interest
The placement agent and its affiliates have in the past and may in the future, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which it may receive customary fees and reimbursement of expenses. In the ordinary course of its various business activities, the placement agent and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for its own accounts and for the accounts of its customers and such investment and securities activities may involve securities and/or instruments of our directorsCompany. The placement agent and officersis affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and certainmay at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Electronic Distribution
This prospectus may be made available in electronic format on websites or through other online services maintained by the placement agent or by an affiliate. Other than this prospectus, the information on the placement agent’s website and any information contained in any other website maintained by the placement agent is not part of our shareholders have entered into lock-up agreements withthis prospectus or the underwriters. Under these agreements, weregistration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent, and each of these persons mayshould not without the prior written approval of the underwriter, subject to limited exceptions, offer, pledge, sell, offer to sell, contract to sell or lend, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwisebe relied upon by investors.
Transfer Agent and Registrar
The transfer or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeableagent and registrar for our common stock or publicly disclose the intention to make any offer, sale, pledge or disposition, or enter into any swap, hedge or similar arrangements, or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock or such other securities. In addition, subject to limited exceptions, we have agreed not to file any registration statement under the Securities Act for any such transaction or which registers, or offers for sale, our common stock or any securities convertible into or exercisable or exchangeable for our common stock,is Continental Stock Transfer and each of our directors and officers has agreed not to make any demand for, or exercise any right with respect to, the registration under the Securities Act of any shares of our common stock or any security convertible into or exercisable or exchangeable for our common stock, in each case without the prior written approval of Piper Jaffray & Co. These restrictions will be in effect for a period of 90 days after the date of this prospectus supplement.Trust.
The Nasdaq Capital Market Listing
Our shares of common stock areis currently listed on The Nasdaq Capital Market under the symbol “MOTS.”
Price Stabilization and Short Positions
To facilitate the offering being made pursuant to this prospectus supplement or the concurrent preferred stock offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock during and after the offering. Specifically, the underwriters may over-allot or otherwise create a short position in the common stock for its own account by selling more shares of common stock than we have sold to the underwriters. Short sales involve the sale by the underwriters of a greater number of shares than the underwriters is required to purchase in the offering. The underwriters may close out a short position by exercising the over-allotment option or by purchasing shares in the open market.
In addition, the underwriters may attempt to stabilize or maintain the price of the common stock by bidding for or purchasing shares of common stock in the open market and may impose penalty bids. If penalty bids are imposed, selling concessions allowed to other broker-dealers participating in the offering are reclaimed if shares of common stock previously distributed in the offering are repurchased, whether in connection with stabilization transactions or otherwise. The effect of these transactions may be to stabilize or maintain the market price of the common stock at a level above that which might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of the common stock to the extent that it discourages resales of the common stock. The magnitude or effect of any stabilization or other transactions is uncertain. These transactions may be effected on The Nasdaq Stock Market or otherwise and, if commenced, may be discontinued at any time. The underwriters may also engage in passive market making transactions in our common stock. Passive market making consists of displaying bids on The Nasdaq Stock Market limited by the prices of independent market makers and effecting purchases limited by those prices in response to order flow. Rule 103 of Regulation M promulgated by the SEC limits the amount of net purchases that each passive market maker may make and the displayed size of each bid. Passive market making may stabilize the market price of the common stock at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of shares of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
This prospectus may be made available on a website maintained by the underwriters and the underwriters may distribute this prospectus electronically.
Selling Restrictions
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), each underwriter represents and agrees that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, it has not made and will not make an offer of securities which are the subject of the offering contemplated by this prospectus supplement to the public in that Relevant Member State other than:
For the purposes of this provision, the expression an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression Prospectus Directive means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
Each of the underwriters severally represents, warrants and agrees as follows:
Notice to Prospective Investors in Israel
In the State of Israel this prospectus supplement shall not be regarded as an offer to the public to purchase securities under the Israeli Securities Law, 5728 — 1968, which requires a prospectus to be published and authorized by the Israel Securities Authority, if it complies with certain provisions of Section 15 of the Israeli Securities Law, 5728 — 1968, including, inter alia, if: (i) the offer is made distributed or directed to not more than 35 investors, subject to certain conditions, or the Addressed Investors; or (ii) the offer is made, distributed or directed to certain qualified investors defined in the First Addendum of the Israeli Securities Law, 5728 — 1968, subject to certain conditions, or the Qualified Investors. The Qualified Investors shall not be taken into account in the count of the Addressed Investors and may be offered to purchase securities in addition to the 35 Addressed Investors. Our company has not and will not take any action that would require it to publish a prospectus in accordance with and subject to the Israeli Securities Law, 5728 — 1968. We have not and will not distribute this prospectus supplement or make, distribute or direct an offer to subscribe for our securities to any person within the State of Israel, other than to Qualified Investors and up to 35 Addressed Investors.
Qualified Investors may have to submit written evidence that they meet the definitions set out in of the First Addendum to the Israeli Securities Law, 5728 — 1968. In particular, we may request, as a condition to be offered securities, that Qualified Investors will each represent, warrant and certify to us or to anyone acting on our behalf: (i) that it is an investor falling within one of the categories listed in the First Addendum to the Israeli Securities Law, 5728 — 1968; (ii) which of the categories listed in the First Addendum to the Israeli Securities Law, 5728 — 1968 regarding Qualified Investors is applicable to it; (iii) that it will abide by all provisions set forth in the Israeli Securities Law, 5728 — 1968 and the regulations promulgated thereunder in connection with the offer to be issued securities; (iv) that the securities that it will be issued are, subject to exemptions available under the Israeli Securities Law, 5728 — 1968: (a) for its own account; (b) for investment purposes only; and (c) not issued with a view to resale within the State of Israel, other than in accordance with the provisions of the Israeli Securities Law, 5728 — 1968; and (v) that it is willing to provide further evidence of its Qualified Investor status. Addressed Investors may have to submit written evidence in respect of their identity and may have to sign and submit a declaration containing, inter alia, the Addressed Investor’s name, address and passport number or Israeli identification number.
Notice to Prospective Investors in Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions, and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption form, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in Hong Kong
The securities may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the securities may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”).
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (2) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (3) where no consideration is or will be given for the transfer, (4) where the transfer is by operation of law, (5) as specified in Section 276(7) of the SFA, or (6) as specified in Regulation 32.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), or the FIEA. The securities may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This offering document does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons (the “Exempt Investors”) who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This offering document contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this offering document is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Dubai International Financial Centre
This offering document relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This offering document is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth in this prospectus and has no responsibility for the offering document. The securities to which this offering document relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this offering document you should consult an authorized financial advisor.
Notice to Prospective Investors in Switzerland
We have not and will not register with the Swiss Financial Market Supervisory Authority (“FINMA”) as a foreign collective investment scheme pursuant to Article 119 of the Federal Act on Collective Investment Scheme of 23 June 2006, as amended (“CISA”), and accordingly the securities being offered pursuant to this prospectus have not and will not be approved, and may not be licenseable, with FINMA. Therefore, the securities have not been authorized for distribution by FINMA as a foreign collective investment scheme pursuant to Article 119 CISA and the securities offered hereby may not be offered to the public (as this term is defined in Article 3 CISA) in or from Switzerland. The securities may solely be offered to “qualified investors,” as this term is defined in Article 10 CISA, and in the circumstances set out in Article 3 of the Ordinance on Collective Investment Scheme of 22 November 2006, as amended (“CISO”), such that there is no public offer. Investors, however, do not benefit from protection under CISA or CISO or supervision by FINMA. This prospectus and any other materials relating to the securities are strictly personal and confidential to each offeree and do not constitute an offer to any other person. This prospectus may only be used by those qualified investors to whom it has been handed out in connection with the offer described in this prospectus and may neither directly or indirectly be distributed or made available to any person or entity other than its recipients. It may not be used in connection with any other offer and shall in particular not be copied and/or distributed to the public in Switzerland or from Switzerland. This prospectus does not constitute an issue prospectus as that term is understood pursuant to Article 652a and/or 1156 of the Swiss Federal Code of Obligations. We have not applied for a listing of the securities on the SIX Swiss Exchange or any other regulated securities market in Switzerland, and consequently, the information presented in this prospectus does not necessarily comply with the information standards set out in the listing rules of the SIX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SIX Swiss Exchange.
LEGAL MATTERSINFORMATION INCORPORATED BY REFERENCE
The validitySEC allows us to “incorporate by reference” information into this document, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede this information.
We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act made subsequent to the date of this prospectus until the termination of the offering of the securities offereddescribed in this prospectus (other than information in such filings that was “furnished,” under applicable SEC rules, rather than “filed”). We incorporate by reference the following documents or information that we have filed with the SEC:
| ● | our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023; | |
| ● | our Definitive Proxy Statement on Schedule 14A, filed with the SEC on August 7, 2023 (other than the portions thereof that are furnished and not filed); | |
| ● | our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2023, June 30, 2023 and September 30, 2023, filed with the SEC on May 10, 2023, August 14, 2023 and November 13, 2023 respectively; and | |
| ● | our Current Reports on Form 8-K filed with the SEC on April 5, 2023, April 13, 2023, May 11, 2023, May 17, 2023, May 22, 2023, June 5, 2023, July 7, 2023, July 14, 2023, July 28, 2023, September 6, 2023, September 14, 2023, September 21, 2023,October 2, 2023, November 2, 2023, November 27, 2023 and November 28, 2023 (other than any portions thereof deemed furnished and not filed). |
Any statement contained in this prospectus or contained in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed to be modified or superseded to the extent that a statement contained in this prospectus or any subsequently filed supplement to this prospectus, or document deemed to be incorporated by reference into this prospectus, modifies or supersedes such statement. Any statements so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may request a copy of these filings at no cost, by writing or telephoning us at the following address:
Motus GI Holdings, Inc.
Attn: Mark Pomeranz, Chief Executive Officer
1301 East Broward Boulevard, 3rd Floor
Ft. Lauderdale, FL 33301
You may also access these filings on our website at www.motusgi.com. You should rely only on the information incorporated by reference or provided in this prospectus. We have not authorized anyone else to provide different or additional information on our behalf. An offer of these securities is not being made in any jurisdiction where the offer or sale is not permitted. You should not assume that the information in this prospectus is being passed upon for us by Lowenstein Sandler LLP, New York, New York. Goodwin Procter LLP, New York, New York has actedaccurate as counsel forof any date other than the underwriters in connection with certain legal matters related to this offering.date of those respective documents.
The financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2017, have been audited by Brightman Almagor Zohar & Co., a member firm of Deloitte Touche Tohmatsu Limited and an independent registered public accounting firm, as stated in their report, which is incorporated by reference in this prospectus and elsewhere in this registration statement (which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph referring to the Company’s ability to continue as a going concern). Such financial statements have been incorporated by reference in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
| 21 |
WHERE YOU CAN FIND ADDITIONALMORE INFORMATION
We haveThis prospectus is part of a registration statement we filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus.SEC. This prospectus which is partdoes not contain all of the information set forth in the registration statement omits certain information,and the exhibits schedules and undertakings set forth into the registration statement. For further information pertainingwith respect to us and our common stock, reference is madethe securities we are offering under this prospectus, we refer you to our SEC filings and the registration statement and the exhibits and schedules tofiled as a part of the registration statement. StatementsYou should rely only on the information contained in this prospectus asor incorporated by reference into this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any jurisdiction where the contents or provisions of any documents referred tooffer is not permitted. You should assume that the information contained in this prospectus, are not necessarily complete, andor any document incorporated by reference in each instance where a copythis prospectus, is accurate only as of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete descriptiondate of those respective documents, regardless of the matters involved.
In addition, registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the SEC.time of delivery of this prospectus or any sale of our securities.
We are subject to the informationfile annual, quarterly and periodic reporting requirements of the Securities Exchange Act of 1934, as amended, and, in accordance with such requirements, will file periodiccurrent reports, proxy statements and other information with the SEC. These periodic reports, proxy statements,Our SEC filings are available to the public from commercial document retrieval services and other information will be available for inspection and copyingover the Internet at the web site of the SEC referred to above. SEC’s website at http://www.sec.gov.
We also maintain a website at http://www.motusgi.com, at which youwww.motusgi.com. You may access these materialsour annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after they aresuch material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of,incorporated by reference into, and is not incorporated into,part of, this prospectus. We
The validity of the common stock and certain other legal matters will be passed upon for us by Lowenstein Sandler LLP, New York, New York. Thompson Hine LLP, New York, New York, has acted as counsel to the placement agent in connection with this offering.
The consolidated balance sheets of Motus GI Holdings, Inc. and Subsidiaries (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of comprehensive loss, changes in shareholders’ equity, and cash flows for each of the years then ended, have included our website addressbeen audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report which is incorporated herein by reference, which report includes an explanatory paragraph about the existence of substantial doubt concerning the Company’s ability to continue as a going concern. Such financial statements have been incorporated herein by reference in reliance on the report of such firm given upon their authority as experts in accounting and auditing.

Up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Pre-Funded Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Series A Common Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Series B Common Warrants to Purchase up to 1,511,335 Shares of Common Stock
Up to 1,511,335 Shares of Common Stock issuable upon exercise of the Pre-Funded Warrants
Up to 3,022,670 Shares of Common Stock issuable upon exercise of the Common Warrants
PROSPECTUS
Sole Placement Agent
A.G.P.
The date of this prospectus solely as an inactive textual reference.is , 2023.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCEPART II
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (File No. 001-38389):
In addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, prior to the termination of the offering (excluding any information furnished rather than filed) shall be deemed to be incorporated by reference into this prospectus.
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Securities Exchange Act of 1934, as amended shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Motus GI Holdings, Inc., Attn: Chief Financial Officer, 1301 East Broward Boulevard, 3rd Floor, Ft. Lauderdale, FL, 33301. You may also direct any requests for documents to us by telephone at (954) 541-8000 or e-mail at IR@MotusGI.com.
You also may access these filings on our website at http://www.motusgi.com. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
MOTUS GI HOLDINGS, INC.
5,000,000 Shares
Common Stock
Piper Jaffray
PROSPECTUS
, 2018
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all feesthe costs and expenses, to be paidother than placement agent fees, payable by us other than estimated underwriting discounts and commissions, in connection with this offering.the sale and distribution of the securities being registered. All of the amounts shown are estimates, except for the SEC registration fee and the FINRA filing fee and the Nasdaq Market initial listing fee.fee:
| Amount | ||||
| SEC Registration Fee | $ | 2,250.99 | ||
| FINRA filing fee | $ | 3,285.88 | ||
| Printing and engraving expenses | $ | 10,000.00 | ||
| Accounting Fees and Expenses | $ | 120,000.00 | ||
| Transfer agent and registrar fees and expenses | $ | 5,000.00 | ||
| Legal Fees and Expenses | $ | 300,000.00 | ||
| Miscellaneous Fees and Expenses | $ | 9,463.13 | ||
| Total Expenses | $ | 450,000.00 | ||
| Amount to be paid | ||||
| SEC registration fee | $ | 2,657 | ||
| FINRA filing fee | 2,300 | |||
| Legal fees and expenses | 250,000 | |||
| Accounting fees and expenses | 50,000 | |||
| Miscellaneous | 20,043 | |||
| Total expenses | $ | 325,000 | ||
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our certificate of incorporation and bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the DGCL, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any amendment by stockholders or directors resolution.
Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification.modification
We have director and officer liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters arising under the Securities Act.
We have entered into indemnification agreements with all of our directors and named executive officers whereby we have agreed to indemnify those directors and officers to the fullest extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer, employee or agent of Motus GI Holdings, Inc. (the “Company”),the Company provided that such director or officer acted in good faith and in a manner that the director or officer reasonably believed to be in, or not opposed to, the best interests of the Company.
The Underwriting Agreement filed as Exhibit 1.1 to this registration statement provides for indemnification by the underwriters of us, our executive officers and directors, and by us of the underwriters for certain liabilities, including liabilities arising under the Securities Act and otherwise.
| II-1 |
Since September 2016 (dateItem 15. Recent Sales of inception),Unregistered Securities.
In the three years preceding the filing of this registration statement, the Company made sales of the following unregistered securities:
Original Issuances of Stock, Warrants andRoyalty Exchange
As previously reported, the Company issued certain (i) Royalty Payment Rights Certificates,
Formation of Holdings
In connection with our formation in September 2016, we sold an aggregate of 1,650,000 shares of our common stock for an aggregate of $82,500 ($0.05 per share), which includes 450,000 shares of our common stock owned by an affiliate as amended (“Royalty Payment Rights Certificates”) to the former holders of the Placement Agent for the 2017 Private Placement.
2017 Private Placement
From December 2016 through February of 2017, we sold an aggregate of 4,743,311 shares of our common stock and 1,581,128Company’s shares of Series A Convertible Preferred Stock, at a price of $5.00par value $0.0001 per unit, inclusive of 2,432,808 shares of our common stock and 810,960 shares of Seriesshare (the “Series A Convertible Preferred Stock issued pursuant toStock” and such holders, the Exchange of Convertible Notes, to 229 accredited investors.
In connection“Certificate Holders”), with the 2017 Private Placement, we issued (i)right to receive certain single digit royalties for the Placement Agent Warrants to the Placement Agent to purchase 403,632 sharesachievement of our common stock with an exercise price of $5.00 per sharecertain commercialization milestones (the “Royalty Amount”), and (ii) the Placement Agent Royalty Payment Rights Certificates dated December 22, 2016 (the “Placement Agent Payment Rights Certificates”) to Aegis Capital Corp., a New York corporation (the “Placement Agent”) or its designees, with the right to receive ina payment equal to a percentage of the aggregate 10% of the amount of paymentsRoyalty Amount paid to the Certificate Holders (the “Certificate Payment”).
On September 12, 2023 (the “Effective Date”), the Company, entered into an Amendment Agreement (the “Amendment Agreement”) with the holders of the Series A Convertible Preferred Stock, or the holdersa majority of the Royalty Payment Rights Certificates uponto cancel the conversionrights of all Certificate Holders to receive the Royalty Amounts in exchange for an aggregate of 88,221 shares of the Series A Convertible Preferred StockCompany’s common stock (the “Certificate Holder Securities”). As a result, the right of the holders of the Placement Agent Payment Rights Certificates to receive the Certificate Payment was also cancelled, in exchange for an aggregate of 8,821 shares (such shares, together with the Certificate Holder Securities, the “Exchange Securities”). The Company issued the applicable number of Exchange Securities in exchange for cancellation of all Royalty Payment Right Certificates held by the Certificate Holders (as well as the Placement Agent Payment Rights Certificates held by the Placement Agent or its designees) as of September 12, 2023. As part of the amendment, the Exchange Securities are also subject to a 180-day lock-up from the Effective Date. The Exchange Securities are being issued in a cashless exchange, exempt from registration pursuant to Section 3(a)(9) of the Securities Act of 1933, as amended.
Private Placement
On May 17, 2023, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with an institutional and accredited investor (the “Purchaser”), pursuant to which the Company issued and sold to the Purchaser in a private placement (the “Private Placement”) an aggregate of (i) 35,000 shares (the “Shares”) of the Company’s common stock, $0.0001 par value per share (“Common Stock”), (ii) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to an aggregate of 241,134 shares of ourCommon Stock and (iii) common stock.warrants (the “May Common Warrants”) to purchase up to an aggregate of 276,134 shares of Common Stock, in each case, in accordance with the terms and conditions of the Securities Purchase Agreement, at a combined offering price of $12.675 per Share and accompanying May Common Warrant to purchase one share of Common Stock and $0.12.6735 per Pre-Funded Warrant to purchase one share of Common Stock and accompanying May Common Warrant to purchase one share of Common Stock, for gross proceeds of approximately $3.5 million.
Share Exchange Transaction
PursuantThe May Common Warrants have an exercise price of $10.80 per share. The May Common Warrants are immediately exercisable and may be exercised at any time after their original issuance until November 20, 2028. The Pre-Funded Warrants have an exercise price of $0.0015 per share. The Pre-Funded Warrants are immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. A holder of May Common Warrants or Pre-Funded Warrants may not exercise any portion of such holder’s May Common Warrants or Pre-Funded Warrants to the termsextent that the holder, together with its affiliates, would beneficially own more than 4.99% (or, at the election of the Share Exchange Agreement, as a condition of and contemporaneously with the Initial Closingholder, 9.99%) of the 2017 Private Placement, the Opco Stockholders sold to us, and we acquired, all of the issued andCompany’s outstanding shares of capital stock of Opco and Opco became our wholly-owned subsidiary. PursuantCommon Stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to the Share Exchange Transaction (i)Company, the Opco Stockholders received an aggregateholder may increase the beneficial ownership limitation to up to 9.99% of 4,000,000the number of shares of our common stock in exchange for all of the issued andCommon Stock outstanding shares of capital stock of Opco, (ii) the Convertible Notes and the Convertible Note Warrants (as defined below) were exchanged for our securities, as described below, and (iii) all of the issued and outstanding options to purchase or otherwise acquire shares of Opco capital stock that were outstanding and unexercised as of immediately priorafter giving effect to the Initial Closing were substituted for 125,730 options to acquire shares of our common stock pursuant to our 2016 Equity Incentive Plan.exercise.
| II-2 |
The net proceeds to the Company from the Private Placement were approximately $3.0 million, after deducting placement agent fees and expenses and estimated offering expenses payable by the Company. The Company used the net proceeds from the Private Placement for general corporate purposes. The Private Placement closed on May 19, 2023.
In connection with the Private Placement, the Company entered into a registration rights agreement, dated May 17, 2023 (the “Registration Rights Agreement”), with the Purchaser, pursuant to which, among other things, the Company agreed to prepare and file with the Securities and Exchange Commission a registration statement on Form S-3 (the “Registration Statement”) to register for resale the Shares and the shares of Convertible NotesCommon Stock issuable upon the exercise of the Common Warrants and Pre-Funded Warrants by June 1, 2023.
In connection with the Private Placement, the Company entered into a warrant amendment (the “Warrant Amendment”), dated May 17, 2023 with the holder named therein, pursuant to which the Company agreed to amend certain existing warrants to purchase up to an aggregate of 19,999 shares of Common Stock that were previously issued in January 2021 through February 2021 at an exercise price of $636.00 per share, such that effective upon the closing of the Private Placement the amended warrants have a reduced exercise price of $10.80 per share, at an additional offering price of $0.1875 per amended warrant.
Pursuant to the termsSecurities Purchase Agreement, the Company agreed that for a period of the CNA, as amended, Opco issued the Convertible Notes in a series of closings from June 2015 through November 2016, in an aggregate amount of $14,596,683 (inclusive of accrued interest through December 22, 2016,60 days following the date that the Registration Statement is declared effective (the “Effective Date”), the Company shall not issue, enter into an agreement to issue or announce the issuance or proposed issuance of the Initial Closing). Certain related parties purchased Convertible NotesCommon Stock or any other securities convertible into, or exercisable or exchangeable for, Common Stock or file any registration statement or any amendment or supplement thereto, in each case other than as contemplated pursuant to the CNA, see “Certain Relationships and Related Party Transactions - Convertible Note, Convertible Warrant, and 2017Registration Rights Agreement. The Company also agreed not to enter into any Variable Rate Transaction (as defined in the Securities Purchase Agreement) for a period of one year following the Effective Date, subject to certain exceptions.
H.C. Wainwright & Co., LLC (“Wainwright”) acted as the exclusive placement agent for the Private Placement. The Company paid Wainwright a cash fee equal to 7.0% of the aggregate gross proceeds of the Private Placement - Related Party Participation.” At the Initial Closing, Convertible Holders exchanged their Convertible Notes, together with accrued and unpaid interest thereon calculated through the datea management fee equal to 1.0% of the Initial Closing at a rate of 10% per annum, for unitsgross proceeds of the 2017 Private Placement, atand paid Wainwright a pricenon-accountable expense allowance of $4.50 per unit. As a result, upon consummation$75,000. Additionally, the Company issued to Wainwright, or its designees, warrants to purchase up to an aggregate of 13,806 shares of Common Stock, equal to 5.0% of the Initial Closing, the Convertible Notes were exchanged for units representing (i) 2,432,808aggregate number of Shares and shares of our common stock (inclusive of shares of our common stock resulting fromCommon Stock underlying the accruedPre-Funded Warrants placed in the Private Placement (the “Placement Agent Warrants”). The Placement Agent Warrants are exercisable immediately, expire on November 20, 2028 and unpaid interest of the Convertible Notes through the date of the Initial Closing) and (ii) 810,960 shares of Series A Convertible Preferred Stock (inclusive of shares of Series A Convertible Preferred Stock resulting from the accrued and unpaid interest of the Convertible Notes through the date of the Initial Closing).
Exchange of Convertible Note Warrants
In connection with, and pursuant to the terms of, the CNA, each Convertible Holder also received a seven (7) year warrant (the “Convertible Note Warrants”) to purchase Preferred A Shares of Opco, nominal value in Israeli New Shekel (“NIS”) 0.01 per share, with an exercise price per share of $1.00 (the “Convertible Note Warrant Exercise Price”). Each Convertible Note Warrant entitled the holder to purchase that number of shares of Preferred A Shares of Opco equal to thirty-three percent (33%) of the principal amount of the Convertible Note in connection with which it was issued. Convertible Note Warrants to purchase an aggregate 4,536,188 shares of Preferred A Shares of Opco withhave an exercise price of $1.00$15.8445 per share (equal to 125% of the combined offering price per Share and accompanying Common Warrant).
The Shares, Pre-Funded Warrants, May Common Warrants, Placement Agent Warrants and the shares of Common Stock issuable upon the exercise of May Common Warrants, Pre-Funded Warrants and Placement Agent Warrants have not been registered under the Securities Act of 1933, as amended, and were issued in connection with the CNA. Certain related parties held Convertible Note Warrantsoffered pursuant to the CNA, see “Certain Relationshipsexemption from registration provided in Section 4(a)(2) under the Securities Act of 1933, as amended, and Related Party Transactions - Convertible Note, Convertible Warrant,Rule 506(b) promulgated thereunder.
2021 Loan
On July 16, 2021 (the “Effective Date”), the Company and 2017 Private Placement - Related Party Participation.” Atits wholly owned subsidiaries, Motus GI, LLC (the “US Subsidiary”) and Motus GI Medical Technologies, LTD (the “IL Subsidiary” together with the Initial Closing,Company and the holdersUS Subsidiary, the “Borrower”), entered into that certain Agreement for the Provision of a Loan Facility (the “Loan Agreement”) with a private institutional lender (“Lender”), a limited partnership incorporated in Jersey. Under the Convertible Note Warrants exchanged their Convertible Note Warrants for five (5) year warrants (the “Exchange Warrants”)Loan Agreement, Lender will provide the Company with access to purchaseterm loans in an aggregate 907,237 shares of our common stock at an exercise price of $5.00 per share, such amount being equal to thirty-three percent (33%) of the principal amount of up to $12.0 million in three tranches as follows: (a) on the Effective Date, a loan in the aggregate principal amount of $4.0 million (the “Convertible Bullet Loan” or the “Convertible Note”), (b) on the Effective Date, a loan in the aggregate principal amount of $5.0 million (“Tranche B”), and (c) available until December 31, 2021, a loan in the aggregate principal amount of $3.0 million (“Tranche C”, together with the Convertible Notes divided by $5.00.
Service Provider StockBullet Loan and Warrants
In March 2017, we issued a service provider (a) 90,000 shares of our common stock, subject to a lock-up agreement,Tranche B, the “Loan” or “Loans”). The Convertible Bullet Loan and (b) five (5) year warrants to purchase 30,000 shares of our common stock with an exercise price of $8.00 per share (the “May 2017 Consultant Warrant”), as partial payment for services pursuant to a consulting agreement betweenTranche B were funded on the service provider and us. In July 2018, we entered into an amendment to this consulting agreement and issued the service provider 30,000 shares of our common stock and a warrant to purchase 90,000 shares of our common stock. The warrants (the “May 2017 Additional Consultant Warrant”) are fully vested, are exercisable at $8.50 per share and expire five years from the date of issuance.
During August, September, and October of 2017, we issued a service provider an aggregate of 4,167 shares, pursuant to the terms of the agreement with such service provider.
On March 27, 2018, we issued a service provider 15,000 shares of our common stock, subject to a lock-up agreement, as partial payment for services pursuant to a consulting agreement between the service provider and us.
In June 2018, we entered into a consultant agreement pursuant to which we (a) issued a warrant, on June 6, 2018, to purchase 10,000 shares of our common stock, with an exercise price of $5.25 per share, (b) issued a warrant, on October 6, 2018, to purchase 10,000 shares of our common stock, with an exercise price of $6.25 per share, and (c) agreed to issue a warrant to purchase 10,000 shares of our common stock, with an exercise price of $7.25 per share, issuable on February 6, 2019, provided the consultant is still engaged at that time, as payment for services pursuant to the consulting agreement. Each warrant (each a “June 2018 Consultant Warrant”) issued or issuable under the consultant agreement has or will have a five year term and a cashless exercise provision.Effective Date.
| II-3 |
In July 2018, we entered into a consulting agreement pursuantThe Company intends to which we (a) issued a warrantuse the proceeds of the Loans to refinance the Company’s existing indebtedness in the amount of approximately $8.2 million, and to enhance the Company’s product development and commercial growth plans, and for general corporate purposes.
The Convertible Bullet Loan requires forty-eight (48) monthly interest only payments commencing after the Effective Date and thereafter full payment of the then outstanding principal balance of the Bullet Loan on July 2, 20181, 2025. The Tranche B loan requires interest only monthly payments commencing on the Effective Date until September 30, 2022 and, thereafter, thirty-three (33) monthly payments of principal and interest accrued thereon until June 1, 2025. The Tranche C loan, to purchase 25,000 sharesthe extent drawn on or prior to December 31, 2021, requires monthly payments of our common stockinterest only commencing on the date drawn until September 30, 2022 and, thereafter, thirty-three (33) monthly payments of principal and interest accrued thereon until June 1, 2025. Notwithstanding the foregoing, in the event the Borrower completes a capital raise of a minimum of $20.0 million prior to September 30, 2022, the repayment terms of the Tranche B and Tranche C loans shall automatically be amended so that the interest only period will be extended to June 30, 2023, and, thereafter, the Borrower shall pay twenty-four (24) monthly payments of principal and interest accrued thereon until June 1, 2025.
Interest on the Convertible Bullet Loan accrues at 7.75% per annum. Interest on the Tranche B and Tranche C loans accrues at 9.5% per annum.
The Borrower may prepay all, but not less than all, of the outstanding principal balance of any of the Loans. In case of prepayment within 6 months of the Effective Date, the Borrower will pay a sum equal to (i) the principal balance then outstanding, plus (ii) an aggregate of all remaining interest payments discounted to present value at the then-applicable Wall Street Journal Prime Rate less 3%, with a floor of 0%. In case of prepayment within 7-24 months of the Effective Date, the Borrower will pay a sum equal to 102% of principal balance then outstanding. In case of prepayment within 25-36 months of the Effective Date, the Borrower will pay a sum equal to 101% of the principal balance then outstanding. If prepayment is made after 36 months of the Effective Date, the Borrower will pay a sum equal to the principal balance then outstanding. In connection with any prepayment, the Borrower will also pay the End of Loan Payment (as defined below) and any other unpaid fees or costs, if any.
The Loans are subject to mandatory accelerated repayment provisions that require repayment of the outstanding principal amount of the Loan, and all accrued and unpaid interest thereon, upon the occurrence of an exercise priceevent of $7.39 per share, which warrant hasdefault, subject to certain limitations and cure rights. In addition, in the event of acceleration upon an exerciseevent of default (a) the Borrower will be required to pay the aggregate of the monthly interest payments scheduled to be paid by the Borrower for the period of 12 months from the date of agreement, (b) issued a warrant on July 2, 2018acceleration to purchase 25,000 sharesthe expiry of our common stock with an exercise price of 7.39 per share, which warrant has an exercise period of 18 monthsthe applicable Loan, in each case discounted from the applicable monthly repayment date to the date of prepayment at the agreement, (c) issued a warrant on October 2, 2018rate of 2% per annum and (b) the other prepayment penalty obligations described above will not apply.
The Lender may elect to purchase 25,000convert all or part of the Convertible Bullet Loan into shares of ourthe Company’s common stock, with an exercise price of 8.75par value $0.0001 per share which warrant has an exercise period(the “Common Stock”) at the Conversion Price (as defined below) at any time while the Convertible Bullet Loan remains outstanding. The “Conversion Price” is set at $420.00, subject to certain customary adjustments for stock splits, combinations, stock dividends or similar events as specified in the Loan Agreement. Should the Lender elect to convert the Convertible Bullet Loan, the End of 18 months fromLoan Fee for the dateportion of loan converted would not be payable by the Borrower.
The Company may elect to convert all or part of the Convertible Bullet Loan into shares of Common Stock at the Conversion Price at any time while the Convertible Bullet Loan remains outstanding, if the average closing price per share of Common Stock for twenty (20) consecutive trading days, including the day of the actual conversion, is greater than 200% of the Conversion Price.
In connection with entering into the Loan Agreement, the Company will pay Lender a fee of up to $50,000 for legal and other ancillary fees. Pursuant to the Loan Agreement, upon the execution of the agreement, the Company paid Lender (a) a transaction fee equal to $150,000 and (d) agreed(b) an advance payment in the amount of $171,406.21 which reflects the last month’s payment of principal and interest for Tranche B. Additionally, Borrower will be required to issuepay Lender an end of loan payment equal to 1.75% of the amount of each tranche drawn down upon the six month anniversaryexpiration of each such tranche (each and “End of Loan Payment”). If the Lender elects to convert any part of the dateConvertible Bullet Loan, then the aforementioned End of Loan Payment shall not apply with respect to such converted part of the agreement, provided the consultant is still engaged at the respective time of issuance, a warrant to purchase 25,000 shares of our common stock with an exercise price of $10.00 per share, which warrant has an exercise period of 24 months from the date of the agreement. The warrants (each a “July 2018 Consultant Warrant”) issued under this agreement are callable by us.
In July 2018, we entered into a consulting agreement with an executive search firm pursuant to which we issued a warrant on November 8, 2018 to purchase 7,917 shares of our common stock, with an exercise price of $5.00 per share. The warrant (the “November 2018 Consultant Warrant”) issued under the consultant agreement has a three (3) year term. An additional warrant is issuable by us pursuant to the consulting agreement to purchase an estimated 6,380 shares of our common stock, subject to adjustment, with an estimated exercise price of $3.23 per share, subject to adjustment. The warrant (the “Contingent Consultant Warrant”) to be issued under the consulting agreement is expected to have a three (3) year term and is expected to be issued October 2019.
Ten Percent Warrants
Simultaneously with the closing of our IPO, we issued warrants to purchase an aggregate of 1,095,682 shares of our common stock (the “Ten Percent Warrants”) to certain of the holders of our formerly outstanding Series A Convertible Preferred Stock, pursuant to an amendment to the Registration Rights Agreement entered into with the investors in our 2017 Private Placement offering and an amendment to the Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock. The Ten Percent Warrants are exercisable for our common stock at an exercise price of $5.00 per share. The Ten Percent Warrants are exercisable any time on or after the 180 day anniversary of the completion of our IPO, have a five year term, and provide for cashless exercise. No fractional shares will be issued upon the exercise of the Ten Percent Warrants.
Conversion of Series A Convertible Preferred Stock
Simultaneously with the closing of our IPO, all 1,581,128 previously outstanding shares of our Series A Convertible Preferred Stock were converted, on a one-to-one basis, into shares of our common stock. At such time we issued (i) 1,581,128 shares of our common stock and (ii) the Royalty Payment Rights Certificates to each former holder of our Series A Convertible Preferred Stock, entitling the holders of such Royalty Payment Rights Certificates to the same Royalty Payment Rights as were included in the Series A Convertible Preferred Stock.
Issuance Pursuant to Exercise of Stock Option
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003), we issued 1,148 shares of our common stock pursuant the exercise of stock options.
Stock Options
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003) we granted stock options under our 2016 Equity Incentive Plan to purchase an aggregate of 2,114,769 shares of our common stock, with a weighted average exercise price of $4.51 per share.
Unrestricted Stock Awards
From September 2016 (date of inception) to March 29, 2018, the date of the filing of our registration statement on Form S-8 (File No. 333-224003), we granted unrestricted stock awards under our 2016 Equity Incentive Plan for an aggregate of 5,000 shares of our common stock.Bullet Loan.
| II-4 |
Securities Act ExemptionsOutstanding borrowings under the Loan Agreement are secured by a first priority security interest on substantially all of the personal property assets of the Borrower, including Borrower’s material intellectual property and equity interests in its subsidiaries. In conjunction with the security interests granted under the Loan Agreement, the Borrower’s obligations are further secured, pursuant to the terms of (A) a Security Agreement (the “Company Security Agreement”), between the Company and the Lender, (B) a Security Agreement, between the US Subsidiary and the Lender (the “US Subsidiary Security Agreement”), (C) a Debenture – Fixed Charge, between the IL Subsidiary and the Lender (the “IL Subsidiary Debenture – Fixed Charge”), (D) a Debenture – Floating Charge, between the IL Subsidiary and the Lender (the “IL Subsidiary Debenture – Floating Charge”), and (E) a US Intellectual Property Security Agreement, between the IL Subsidiary and the Lender (the “IL Subsidiary US IP Security Agreement”, together with the Company Security Agreement, the US Subsidiary Security Agreement, the IL Subsidiary Debenture – Fixed Charge, and the IL Subsidiary Debenture – Floating Charge, the “Initial Security Documents”), each dated as of the Effective Date.
We deemedThe Loan Agreement contains customary representations and warranties, indemnification provisions in favor of Lender, events of default and affirmative and negative covenants, including, among others, covenants that limit or restrict the offers, salesCompany’s ability to, among other things, incur additional indebtedness, merge or consolidate, make acquisitions, pay dividends or other distributions or repurchase equity, make investments, dispose of assets and issuancesenter into certain transactions with affiliates, in each case subject to certain exceptions. There are no liquidity or financial covenants. Borrower has also granted Lender certain information rights.
In connection with the Loan Agreement, the Company also issued to Lender a warrant (“Warrant”), dated July 16, 2021, to purchase up to 636 shares of the Company’s common stock, at an exercise price of $314.22 per share, payable in cash or on a cashless basis according to the formula set forth in the Warrant. The exercise price of the Warrant and the number of shares issuable upon exercise of the Warrant are subject to adjustments for stock splits, combinations, stock dividends or similar events. The Warrant is exercisable until the date that is ten (10) years after the date of issuance.
On November 28, 2023, we and the Lender entered into a First Amendment to the 2021 Loan Agreement (the “Amendment”), pursuant to which, among other things, (a)(i) on the effective date of the Amendment, we paid to the Lender a sum of $750,000 in cash via wire transfer in immediately available funds (the “Amendment Execution Date Payment”), and (ii) upon consummation of a First Amendment Capital Raise (as defined below) and immediately following the Convertible Note Securities Exchange (as defined below), we will prepay to the Lender a sum of $1,500,000 in cash via wire transfer in immediately available funds (the “Closing Payment”), which sums set forth in (i) and (ii) shall be applied towards partial prepayment of the outstanding principal balance of the Term Loan; and (b) subject to the satisfaction (or waiver by Lender) of certain Exchange Conditions (as defined in the Amendment), immediately following the consummation of a First Amendment Capital Raise, which we assume this offering will be, $4.0 million (the “Conversion Amount”) of the outstanding aggregate principal balance of the Convertible Note will automatically convert into such number of shares of our common stock (the “Convertible Note Securities Exchange”) at a price per share equal to the public offering price per share in the First Amendment Capital Raise representing the Conversion Amount; provided, that, (A) the Lender shall have executed a customary lock-up agreement for a 90-day period following the Convertible Note Securities Exchange, (B) the Lender shall receive the same warrant coverage (the “Conversion Common Warrants”) per share of common stock, if any, as investors purchasing securities described abovein the First Amendment Capital Raise and (C) the Lender shall receive a pre-funded warrant (the “Conversion Pre-Funded Warrant”) in lieu of shares of common stock otherwise issuable upon the Convertible Note Securities Exchange for such number of shares that would represent more than 4.5% of the pre-exercise outstanding shares of common stock, providing that the Lender will not own (x) more than 4.99% of the post-exercise outstanding shares of common stock at any time and (y) to the extent required under “Original Issuancesthe rules of Stock, Warrants and Payment Rights Certificates”The Nasdaq Capital Market, more than 19.99% of the shares of common stock outstanding immediately prior to be exempt from registrationthe Convertible Note Securities Exchange (but after the consummation of the First Amendment Capital Raise) unless applicable shareholder approval is obtained. “First Amendment Capital Raise” means the Company raising additional cash through one equity financing registered under the Securities Act (to be consummated no later than December 29, 2023) with gross proceeds of 1933, as amended (the “Securities Act”),at least $5.0 million. The securities issued to Lender in the Convertible Note Securities Exchange will be issued in reliance on the exemption from registration set forth in Section 3(a)(9) of the Securities Act. We also agreed to file a resale registration statement to register the securities being issued to Lender in the Convertible Note Securities Exchange as promptly as practicable (and in no event later than 91 calendar days after the closing of the Convertible Note Securities Exchange).
The Company will issue the Convertible Bullet Loan, the Warrant and the shares of Common Stock underlying the Convertible Bullet Loan and the Warrant to Lender in reliance on the exemption from registration provided for under Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions byAct.
Exercise Agreement
As previously reported, on August 28, 2020, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with an issuer not involving a public offering. All purchasers of securities in transactions exempt from registrationinstitutional investor (the “Holder”), pursuant to Regulation D representedwhich the Company issued to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risksInvestor, securities of the investmentCompany, including warrants (the “Existing Warrants”) to purchase up to 29,112 shares of common stock, par value $0.0001 per share (the “Common Stock”), of the Company (the “Warrant Shares”). The Existing Warrants were immediately exercisable at an exercise price of $390.00 per share and could holdexpired on the securitiesfifth anniversary of the date of issuance.
On January 27, 2021, the Company entered into a Warrant Exercise Agreement (the “Exercise Agreement”) with the Holder. Pursuant to the Exercise Agreement, in order to induce the Holder to exercise all of the remaining 26,666 outstanding Existing Warrants for cash, pursuant to the terms of and subject to beneficial ownership limitations contained in the Existing Warrants, the Company agreed to issue to the Holder, new warrants (the “New Warrants”) to purchase 0.75 shares of Common Stock for each share of Common Stock issued upon such exercise of the remaining 26,666 outstanding Existing Warrants pursuant to the Exercise Agreement or an indefinite periodaggregate of time.20,000 New Warrants. The purchasers received written disclosuresterms of the New Warrants will be substantially similar to those of the Existing Warrants, except that the securities hadNew Warrants will have an exercise price of $636.00 (a 20% premium to the closing price of the Company’s Common Stock on The Nasdaq Capital Market on January 26, 2021), will be immediately exercisable and will expire five years from the date of the Exercise Agreement. The Holder will pay an aggregate of $600,000 to the Company for the purchase of the New Warrants. The Company expects to receive aggregate gross proceeds before expenses of approximately $11.0 million from the exercise of all of the remaining 400,000 outstanding Existing Warrants held by the Holder and the payment of the purchase price for the New Warrants.
Pursuant to the Exercise Agreement, the Holder has agreed, until the date that no Existing Warrants are held by such Holder (i) not been registeredto purchase any shares of Common Stock, other than pursuant to exercises of the Existing Warrants and (ii) not to transfer any Existing Warrants other than to transferees who assume the obligations under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options and issuances of our common stock upon exercise of such options, and the restricted share awards, described above to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder and Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
Exercise Agreement.
| II-5 |
The Company will issue the New Warrants and the shares of Common Stock underlying the New Warrants to the Holder in reliance on the exemption from registration provided for under Section 4(a)(2) of the Securities Act. The Company relied on this exemption from registration for private placements based in part on the representations made by the Holder, including the representations with respect to the Holder’s status as an “accredited investor,” as such term is defined in Rule 501(a) of the Securities Act, and the Holder’s investment intent.
Item 16. Exhibits.
The list of exhibits following the signature page of this registration statement is incorporated by reference herein.
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakesTo file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(b) That, for purposesthe purpose of determining any liability under the Securities Act, of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statementsuch post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(b) The(c) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant hereby undertakes to deliver or cause to be delivered withthat in a primary offering of securities of the prospectus, to each person to whom the prospectus is sent or given, the latest annual report to security holders that is incorporated by reference in the prospectus and furnishedundersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and meetingwill be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the requirements of Rule 14a-3 or Rule 14c-3 underundersigned registrant relating to the Securities Exchange Act of 1934; and where interim financial informationoffering required to be presentedfiled pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by Article 3or on behalf of Regulation S-X are not set forththe undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the prospectus, to deliver, or cause to be delivered to each person to whomoffering made by the prospectus is sent or given, the latest quarterly report that is specifically incorporated by reference in the prospectus to provide such interim financial information.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuantundersigned registrant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.purchaser.
(d) The undersigned Registrant hereby undertakes that:
| (2) | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | The undersigned registrant hereby undertakes that: |
(1)(a) For purposes of determining any liability under the Securities Act, of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance uponon Rule 430A and contained in a form of prospectus filed by the Registrantundersigned registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.effective; and
(2)(b) For the purpose of determining any liability under the Securities Act, of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| (4) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Ft. Lauderdale, State of Florida, on December 17, 2018.11, 2023.
| MOTUS GI HOLDINGS, INC. | |||
| By: | /s/ | ||
| Chief Executive Officer | |||
KNOW ALL MEN BY THESE PRESENTS, that the undersigned officers and directors of Motus GI Holdings, Inc., a Delaware corporation, do hereby constitute and appoint each of Mark Pomeranz and Andrew Taylor as his or her true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto and other documents in connection therewith) to this Registration Statement and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended, which relates to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.December 11, 2023.
| /s/ | Chief Executive Officer and Director | |||
| Mark Pomeranz | (Principal Executive Officer) | |||
| /s/ Ravit Ram | Chief Financial Officer | |||
| Ravit Ram | (Principal Financial Officer) | |||
| /s/ Elad Amor | Chief Accounting Officer | |||
| Elad Amor | (Principal Accounting Officer) | |||
| * | Director and | |||
| Timothy P. Moran | ||||
| ||||
| Chairman of the Board | ||||
| Director | ||||
| Sonja Nelson | ||||
| Director | ||||
| Gary J. Pruden | ||||
| Director | ||||
| Scott Durbin |
| /s/ Mark Pomeranz | |||||
| Mark Pomeranz | |||||
| Attorney-in-fact |
EXHIBIT INDEX
| II-9 |
| II-10 |
| II-11 |
| II-12 |
| * | To be filed by amendment. |
| ** | Previously filed. |
| # | The schedules and exhibits to this agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the Securities and Exchange Commission upon request. |
| II-13 |