As filed with the Securities and Exchange Commission on September 1, 2016
April 9, 2024Registration No. ___________
333‑ 278326 UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C.D. C. 20549
Amendment No. 1
to
FORM S-1
S‑1REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Commission File Number: 001-37479
VISUALANT, INCORPORATED
(Exact name of registrant as specified in charter)
| 90-0273142 |
(State(Exact name of registrant as specified in its charter) |
Nevada | | 3920 | | 90‑0273142 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
| |
| 3920 |
| (Primary Standard Industrial Classification Code Number) |
| |
500 Union Street, Suite 420, Seattle, Washington USA
| 98101
|
(Address of principal executive offices) | (Zip Code)(IRS Employer Identification No.) |
| 206-903-1351 | |
| (Registrant's telephone number, including area code) | |
| | |
| | |
| N/A | |
| (Former name, address, and fiscal year, if changed since last report) | |
| | |
| Ronald P. Erickson, Chief Executive Officer
Visualant, Incorporated
500 Union Street, Suite 420
Seattle, WA 9810
| |
| (Name, address, including zip code, and telephone number, including area code, of agent for service) | |
| | |
| Copies to:
Lawrence W. Horwitz, Esq.
James A. Murphy, Esq.
Horwitz + Armstrong, A Professional Law Corporation
14 Orchard, Suite 200
Lake Forest, California 92630
(949) 540-6540
| |
500 Union Street, Suite 810
Seattle, Washington 98101
206‑903‑1351
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Ronald P. Erickson
Chief Executive Officer
500 Union Street, Suite 810
Seattle, Washington 98101
206‑903‑1351
(Names, address, including zip code and telephone number, including area code, of agent for service)
Copies to:
Joshua E. Little
Dentons Durham Jones Pinegar P.C.
192 East 200 North, 3rd Floor
St. George, UT 84770
(435) 674-0400
joshua.e.little@dentons.com
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statementregistration statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effectivepost‑effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effectivepost‑effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-acceleratednon‑accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of "large“large accelerated filer," "accelerated filer"” “accelerated filer,” “smaller reporting company,” and "smaller reporting company"“emerging growth company” in Rule 12b-212b‑2 of the Exchange Act.
Large accelerated filer | | | Accelerated filer | ☐
|
Non-acceleratedNon‑accelerated filer | ☐
☒ | | Smaller reporting company | ☒ |
CALCULATION OF REGISTRATION FEE
| Title of Each Class | | | | |
| of Securities to | | | | |
| be Registered | | | | |
| Common Stock, $0.001 par value per share related to the potential | | | | |
| conversion of up to $1,250,000 of Series C Convertible Preferred Stock | | | | |
| offered by selling stockholder (3) | 1,785,714 | $0.70 | $1,250,000 | $125.87 |
| | | | | |
| Common Stock, $0.001 par value per share, issuable upon exercise of | | | | |
| Series E Warrants (3) | 1,785,714 | 0.70 | 1,250,000 | 125.87 |
| Total | 3,571,428 | $0.70 | $2,500,000 | $251.75 |
(1)
| Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act.
|
| | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission acting pursuant to such Section 8(a), may determine.
EXPLANATORY NOTE
This Amendment No. 1 to the Registration Statement on Form S-1 of Know Labs, Inc. (the “Company”) is being filed solely to (i) update the Incorporation by Reference section hereto, and (ii) to revise Item 16. “Exhibits”.
Except as described above, no changes have been made to the Registration Statement.
|
(2)
| Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
| |
(3)
|
There is being registered hereunder an indeterminate number of shares of common stock issuable upon conversion of Series C Convertible Preferred Stock. The Series C Convertible Preferred Stock is convertible at any time at an initial conversion price of $0.70 per share of our common stock subject to adjustment for certain events. Pursuant to Rule 416 under the Securities Act of 1933, as amended, the shares of common stock registered hereby also include an indeterminate number of additional shares of common stock that may be issued in connection with a stock split, stock dividend, recapitalization or similar event or adjustment in the number of shares of common stock issuable as provided in the Series C Convertible Redeemable Preferred Stock Designation. |
|
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARYThe information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT OF 1933 OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SAID SECTION 8(A), MAY DETERMINE.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED. COMPLETION, DATED APRIL 9, 2024
Subject to completion, dated September 1, 2016
Visualant, Incorporated
500 Union Street, Suite 420
Seattle, WA 98101
206-903-1351

KNOW LABS, INC.
10,800,000 Shares of Common Stock
This prospectus covers the resale by the selling stockholder (the “Selling Stockholder”): (i) up to
1,785,714 shares of our common stock that we may issuerelates to the Selling Stockholder upon conversion of Series C Redeemable Convertible Preferred Stock at a conversion price of $0.70 per share, subject to certain adjustments, and (ii) up to 1,785,714 shares (the “Warrant Shares”) of common stock issuable upon the exercise of outstanding Series E Warrants (“Series E Warrant Shares”) at an exercise price of $0.70 per share, subject to certain adjustments. The common stock covered by this prospectus may be offered for resale, from time to time, by the Selling Stockholderselling stockholder identified in this prospectus under the caption “Selling Stockholder,” of up to 4,800,000 shares of Common Stock of Know Labs, Inc., a Nevada corporation (the “Company”), $0.001 par value (the “Common Stock”), which are issuable upon conversion of a secured, convertible note in accordance with the termsprincipal amount of $4,800,000 (the “Note”), at an initial conversion price of $1.00 per share, subject to adjustment, and up to 6,000,000 shares of Common Stock underlying a common stock purchase warrant (the “Warrant”) at an initial exercise price of $0.80 per share.For the details about the selling stockholder, please see “Selling Stockholder.” The selling stockholder may sell these shares from time to time in the principal market on which our Common Stock is traded at the prevailing market price, in negotiated transactions, or through any other means described in the section entitledtitled “Plan of Distribution.”
We are not The selling
anystockholder may be deemed an underwriter within the meaning of the Securities Act of 1933, as amended, of the shares of
our common stock in this offering and, as a result, weCommon Stock that they are offering. We will pay the expenses of registering these shares. We will not receive
any proceeds from the sale of the common stock covered by this prospectus. All of the net proceeds from the sale of our
common stock will goshares by the selling stockholder that are covered by this prospectus.The shares are being registered to permit the Selling Stockholder. Upon exercise ofselling stockholder, or its pledgees, donees, transferees or other successors-in-interest, to sell the Series E Warrants, however, we will receive up to $0.70 per share or such lower price as may result from the anti-dilution protection features of such warrants. Any proceeds received from the exercise of such warrants will be used for general working capital and other corporate purposes.
The Selling Stockholder may sell common stockshares from time to time at prices established onin the Overpublic market. We do not know when or in what amount the Counter Bulletin Board ("OTCBB")selling stockholder may offer the securities for sale. The selling stockholder may sell some, all or as negotiated in private transactions, or as otherwise described under the heading "Plan of Distribution." The common stock may be sold directly or through agents or broker-dealers acting as agents on behalfnone of the Selling Stockholder. The Selling Stockholder may engage brokers, dealers or agents who may receive commissions or discounts from the Selling Stockholder. We will pay all the expenses incident to the registration of the shares; however, we will not pay for sales commissions or other expenses applicable to the sale of our common stock registered hereunder.
securities offered by this prospectus.Our common stock is quoted on the OTCQB Marketplace, operated by OTC Markets Group,NYSE American under the symbol "VSUL".“KNW.” On August 30, 2016,March 25, 2024, the last reported saleclosing price forof our common stock on the OTCQB MarketplaceNYSE American was $1.10$0.65 per share.
On June 17, 2015, we completed a 1-for-150 reverse stock splitThe selling stockholder may sell its shares of our common stock. All warrant, option, share and per share informationCommon Stock described in this prospectus gives retroactive effectin a number of different ways, at prevailing market prices or privately negotiated prices and there is no termination date of the selling stockholder’s offering.
You should read this prospectus, together with additional information described under the headings “Incorporation of Certain Information by Reference” and “Where You Can Find More Information”, carefully before you invest in any of our securities.
Investing in our securities involves a high degree of risk. See “Risk Factors” starting on page 8 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the 1-for-150 split with all numbers rounded up to the nearest whole share.
INVESTING IN OUR SECURITIES INVOLVES A HIGH DEGREE OF RISK. SEE THE SECTION ENTITLED "RISK FACTORS" BEGINNING ON PAGE 11 IN THIS PROSPECTUS. YOU SHOULD CAREFULLY CONSIDER THESE RISK FACTORS, AS WELL AS THE INFORMATION CONTAINED IN THIS PROSPECTUS, BEFORE YOU INVEST.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
No dealer, salesperson or any other personcontrary is authorized to give any information or make any representations in connection with this offering other than those contained in this prospectus and, if given or made, the information or representations must not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any security other than the securities offered by this prospectus, or an offer to sell or a solicitation of an offer to buy any securities by anyone in any jurisdiction in which the offer or solicitation is not authorized or is unlawful.
criminal offense.The date of this prospectus is September 1, 2016
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus andor in any applicablefree writing prospectus supplement.that we may specifically authorize to be delivered or made available to you. We have not authorized anyone to provide you with differentany information other than that contained in this prospectus or additional information. If anyone provides you with differentin any free writing prospectus we may authorize to be delivered or inconsistentmade available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information you should not rely on it.that others may give you. This prospectus may only be used where it is legal to offer and sell our securities. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities described in this prospectus. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information we have previously filed with the Securities and Exchange Commission, is accurate as of the date on the front of those documents only.our securities. Our business, financial condition, results of operations and prospects may have changed since those dates.
For investors outside the United States: neither we nor the underwriters have done anything that
would permit this offering or possession or distributiondate. We are not making an offer of
this prospectus or any free writing prospectus we may provide to you in connection with this offeringthese securities in any jurisdiction where
action for that purposethe offer is
required, other than innot permitted.Unless the United States. You are required to inform yourselves aboutcontext otherwise requires, the terms “Know Labs,” the “Company”, “we,” “us” and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
Unless otherwise indicated, information contained“our” in this prospectus
concerning ourrefer to Know Labs, Inc., and its subsidiaries and “this offering” refers to the offering contemplated in this prospectus.INDUSTRY AND MARKET DATA
This prospectus includes information with respect to market and industry and the markets in which we operate, including our general expectations and market position, market opportunityconditions and market share isfrom third-party sources or based on information from our own managementupon estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based onusing such sources when available. We believe that such information and knowledge, whichestimates are reasonable and reliable. We also believe the information extracted from publications of third-party sources has been accurately reproduced. However, we believe to be reasonable. Our management estimates have not independently verified any of the data from third-party sources. Similarly, our internal research is based upon our understanding of industry conditions, and such information has not been verified by any independent source,sources.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
We own or have rights to trademarks, service marks and trade names that we have not independently verified any third-party information. In addition, assumptions and estimatesuse in connection with the operation of our business, including our corporate name, logos and our industry's future performance are necessarily subject to a high degree of uncertaintywebsite names. Other trademarks, service marks and risk due to a variety of factors, including those described in "Risk Factors". These and other factors could cause our future performance to differ materially from our assumptions and estimates. See "Special Note Regarding Forward-Looking Statements".
Our trademarks Visualant™ and ChromaID™ are used throughout this prospectus. This prospectus also includes trademarks, trade names and service marks thatappearing in this report are the property of other organizations.their respective owners. Solely for convenience, some of the trademarks, service marks and trade names referred to in this prospectus appearreport are listed without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to theseour trademarks, service marks and trade names.
This report may include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included in this prospectus are the property of their respective owners.PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that you should consider before investingdeciding whether to invest in our common stock.securities. You should carefully read thisthe entire prospectus, carefully, especiallyincluding the "Risk Factors"risks associated with an investment in our company discussed in the “Risk Factors” section of this prospectus and our financial statements and the related notes appearing at the end of this prospectus, before making an investment decision. As usedSome of the statements in this prospectus unlessare forward‑looking statements. See the context otherwise requires, references to "we," "us," "our," "our company," “Visualant, Inc.section titled “Cautionary Statement Regarding Forward‑Looking Statements.” and "Visualant" refer to Visualant, Incorporated and our wholly-owned subsidiary TransTech Systems, Inc., unless the context otherwise requires.
On June 17, 2015, we effected a 1-for-150 reverse stock split of our common stock. All warrant, option, share and per share informationOUR COMPANY
Overview
Know Labs is an emerging leader in this prospectus gives retroactive effect to the 1-for-150 split with all numbers rounded up to the nearest whole share
Overview
Our Company
non-invasive medical diagnostics. We are focused primarily on the development and commercialization of aour proprietary sensor technology whichutilizing radio and microwave spectroscopy. When paired with our machine learning platform, our technology is capable of uniquely identifying and authenticatingmeasuring almost any substancematerial or analyte using lightelectromagnetic energy to create,detect, record, identify, and detectmeasure the unique digital “signature” of said materials or analytes.The first application of our sensor technology is in a product to non-invasively monitor blood glucose levels. Our device will provide the substance.user with real-time information on their blood glucose levels. We call thisrecently announced our “ChromaID™” technology.
Our ChromaID™ Technology
Generation 1 working prototype device. This device embodies the sensor which has been used in internal clinical testing. We have
also announced the work our R&D team is doing on the Generation 2 of our device, which is a wearable format and could be a final form factor, ready for commercial application. We are expanding our testing, both internally and externally, and will refine the device over time, which will require FDA clearance before entering the market.Following FDA clearance of our non-invasive blood glucose monitoring device, Know Labs plans to expand its sensor technology to other non-invasive medical diagnostic applications. As a platform technology, it can identify numerous other analytes in the human body that are important in medical diagnostics and human health and wellness.
While medical diagnostics applications, with blood glucose monitoring paramount, are the focus of Know Labs, the Company’s proprietary radio frequency and microwave spectroscopy platform have broad applicability outside of the medical diagnostic realm. Over time, as resources allow, the Company will explore those opportunities.
The Know Labs Technology
We have internally and under contract with third parties developed a proprietary platform technology to uniquely identify and authenticatemeasure almost any substance. Thisorganic and inorganic material or analyte. Our patented technology utilizes light atdirects electromagnetic energy in the photon (elementary particle of light) levelradio wave and microwave frequencies through a series of emitters and detectorssubstance or material to generatecapture a unique signature or “fingerprint” from a scan of almost any solid, liquid or gaseous material. This signature of reflected or transmitted light is digitized, creating a unique ChromaIDmolecular signature. Each ChromaID signature is comprised of from hundredsWe then perform analytics which will allow the Company to thousands of specific data points.
The ChromaID technology looks beyond visible light frequencies to areas of near infra-redaccurately identify and ultraviolet light that are outside the humanly visible light spectrum. The data obtained allows us to create a very specificmeasure materials and unique ChromaID signature of the substance for a myriad of authentication and verification applications.
Traditional light-based identification technology, called spectrophotometry, has relied upon a complex system of prisms, mirrors and visible light. Spectrophotometers typically have a higher cost and utilize a form factor more suited to a laboratory setting and require trained laboratory personnel to interpret the information. The ChromaID technology uses lower cost LEDs and photodiodes and specific frequencies of light resulting in a more accurate, portable and easy-to-use solution for a wide variety of applications. The ChromaID technology not only has significant cost advantages as compared to spectrophotometry, it is also completely flexible is size, shape and configuration. The ChromaID scan head can range in size from endoscopic to a scale that could be the size of a large ceiling-mounted florescent light fixture.
In normal operation, a ChromaID master or reference scan is generated and stored in a database. The Visualant scan head can then scan similar materials to identify, authenticate or diagnose them by comparing the new ChromaID digital signature scan to that of the original or reference ChromaID signature or scan result.
ChromaID was invented by scientists from the University of Washington under contract with Visualant. We have pursued an aggressive intellectual property strategy and have been granted ten patents. We also have 20 patents pending. We possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, Intellectual Ventures through its subsidiary IDMC.
In 2010, we acquired TransTech Systems, Inc. (“TransTech”) as an adjunct to our business. TransTech is a distributor of products for employee and personnel identification. TransTech currently provides substantially all of our revenues. We intend, however, to further develop and market our ChromaID technology.
The following summarizes our plans for our proprietary ChromaID technology. Based on our anticipated expenditures on this technology, the expected efforts of our management and our relationship with Intellectual Ventures and its subsidiary, IDMC, and our other strategic partner, Sumitomo Precision Products, Ltd., we expect our ChromaID technology to provide an increasing portion of our revenues in future years from product sales, licenses, royalties and other revenue streams., as discussed further below.
ChromaID: A Foundational Platform Technology
analytes. Our ChromaID technology provides a unique platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. The ChromaIDOur radio frequency spectroscopy technology is an enabling“enabling” technology that brings the science of light and photonicselectromagnetic energy to low cost, real worldlow-cost, real-world commercialization opportunities across multiple industries. The technology is foundational and, as such, the basis upon which we believe a significant businessbusinesses can be built.
As While we are pursuing our core focus on commercializing our non-invasive glucose monitor, we believe non-core clinical, non-clinical and medical research applications represent a multitude of opportunities for strategic collaboration, joint development, and licensing agreements with
other foundational technologies, a single application may reach across multipleleading companies in their respective industries.
The ChromaIDWe believe an important competitive differentiator for our sensor technology can, for example effectively differentiate and identify different brands of clear vodkas that appear identical to the human eye. By extension this same technology can identify pure water from water with contaminants present. It can provide real time detection of liquid medicines such as morphine that have been adulterated or compromised. It can detect if jet fuel has water contamination present. It could determine when it is time to change oil in a deep fat fryer. These are but a few of the potential applications of the ChromaID technology based upon extensions ofbe its ability to not only identify different clear liquids.
a wide range of organic and inorganic materials and analytes, but to do so non-invasively, and in real-time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring.Competitive Advantages
We believe our key competitive strengths include:
| · | Through first principles, our sensor technology’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, accurately, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. |
| | |
| · | Our sensor technology is non-invasive, using radio waves to identify and measure what is going on inside the body. |
| | |
| · | Our sensor technology platform can be integrated into a variety of wearable, mobile, or counter-top form factors, and we believe eventual interoperability with existing products from current market leaders. |
| | |
| · | No needles nor invasive transmitters in your body, making our sensor convenient and pain-free. |
| | |
| · | No expensive supplies, such as test strips and lancets, are required to operate our device. |
| | |
| · | A core focus on accessibility and affordability for the populations we will serve around the globe. |
| | |
| · | The current prototype sensor collects approximately 1.5 million data points per hour, which allows us to potentially build a deep understanding of health and wellness that other sensors may not be able to. |
| | |
| · | Know Labs is the world intellectual property leader in non-invasive blood glucose monitoring, according to ipCG Capital and PatSnap. |
Growth Strategy
The cornerstone of a company with a foundational platform technology is its intellectual property. ChromaID was invented by scientists from the University of Washington under contract with Visualant. We have pursued an aggressive intellectual property strategy and have been granted ten patents. We currently have 20 patents pending. We possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, the IDMC subsidiary of Intellectual Ventures.
At the Photonics West trade show held in San Francisco in February 2013, we were honored to receive a PRISM award from the Society of Photo-Optical Instrumentation Engineers International, better known as SPIE. The PRISM awards recognizes photonic products that break with conventional ideas, solve problems, and improve life through the application of light-based technologies.
IDMC Relationship
In November 2013, we entered into a strategic relationship with Invention Development Management Company, a subsidiary of Intellectual Ventures, a private intellectual property fund with over $5 billion under management. Intellectual Ventures owns over 40,000 IP assets and has broad global relationships for the invention of technology, the filing of patents and the licensing of intellectual property. IDMC has worked to expand the reach and the potential application of the ChromaID technology and has filed ten patents base on the ChromaID technology, which it has licensed to us. In connection with IDMC’s work to expand our intellectual property portfolio, we agreed to curtail outbound marketing activitieskey elements of our technology through the fourth calendar quarter of 2014.
Initial testing in our laboratories and the work of the IDMC inventors have shown that the ChromaID technology has a number of broad and useful applications a few of which include:
●
Milk identification for quality, protein and fat content and impurities
●
Identification of liquids for counterfeits or contaminants
●
Detecting adulterants in food and food products compromising its quality
●
Color grading of diamonds
●
Identifying real cosmetics versus counterfeit cosmetics
●
Identifying counterfeit medications versus real medications
●
Identifying regular flour versus gluten free flour
●
Authenticating secure identification cards
Products
Our first delivered product, the ChromaID Lab Kit, scans and identifies solid surfaces. We are marketing this productstrategy to customers who are considering licensing the technology. Target markets include, but are not limited to, commercial paint manufacturers, pharmaceutical equipment manufacturers, process control companies, currency paper and ink manufacturers, security cards, cosmetic companies, scanner manufactures and food processing companies.
Our second product, the ChromaID Liquid Lab Kit, scans and identifies liquids. This product is currently in prototype form. Similar to our first product, it will be marketed to customers who are considering licensing the technology. Rather than use an LED emitter to reflect light off of a surface that is captured by a photodiode to generate a ChromaID signature the liquid analysis product shines light through the liquid (transmissive) with the LEDs positioned on one side of the liquid sample and the photo detectors on the opposite side. This device is in a functional state in our laboratory and we anticipate having a Liquid ChromaID Lab Kit available for customers by the Company during the fall of 2015. Target markets include, but are not limited to, water companies, petrochemical companies, pharmaceutical companies, and numerous consumer applications.
The ChromaID Lab Kits allows potential licensors of our technology to work with our technology and develop solutions for their particular application. Our contractual arrangements with IDMC are described in greater detail below.
Our next planned product should be an exemplar product is a prototype that will be produced to address several markets. The primary purpose of this prototype will be to demonstrate the technology to prospective business partners, and will consist of a small, hand held, battery powered, Bluetooth enabled scanning device. The scanner should wirelessly connect to a smart phone or tablet to transfer the scanned data. The smart phone application will include two or three industry specific but generic applications that allow for the demonstration of the scanning and matching of the ChromaID signatures. The applications will focus on drug identification, food safety and liquid detection. The prototype device will lend itself to consumer applications and can be a consumer product as well.
Our Commercialization Plans for the ChromaID Technology
We shipped our first ChromaID product, the ChromaID Lab Kits, to our strategic partner IDMC during the last calendar quarter of 2013 and first calendar quarter of 2014, after we completed final assembly and testing. As part of our agreement with IDMC, we curtailed our ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, we began to actively market the ChromaID Lab Kits to interested and qualified customers. Some ChromaID Lab Kits are provided free of charge to potential customers. Others are sold for a modest price. To date, we have achieved limited revenue from the sale of our ChromaID Lab Kits.
The Lab Kit includes the following:
ChromaID Scanner. A small device made with electronic and optical components and firmware which pulses light onto a flat material and records and digitizes the light that is reflected back from that material. The device is the size of a typical flashlight (5.5” long and 1.25” diameter). However, the technology can be incorporated into almost any size, shape and configuration.
ChromaID Lab Software. A software application that runs on a Windows PC. The software allows for configuration of the scanner, controls the behavior of the ChromaID Scanner, displays a graph of the captured ChromaID signature profile, stores the ChromaID signature in a database and uses algorithms to compare the accuracy of the match of the unknown scan to the known ChromaID signature profile. This software is intended for lab and experimental use only and is not required for commercialized product applications.
Software Development Toolkit. A collection of software applications, API (an abbreviation of application program interface – a set of routines, protocols, and tools for building software applications) definitions and file descriptions that allow a customer to extract the raw data from the ChromaID signatures and run their own software routines against that raw data.
The ChromaID Lab Kit allows customers to experiment with and evaluate the ChromaID technology and determine if it is appropriate for their specific applications. The primary electronic and optical parts of the ChromaID scanner, called the “scan head,” could be supplied to customers to integrate into their own products. A set of ChromaID Developer Tools are also available. These allow customers to develop their own applications and products based on the ChromaID technology.
ChromaID signatures must be stored, managed, and readily accessible for comparison, matching and authentication purposes. The database can be owned and operated by the end customer, but in the case of thousands of ChromaID signatures, database management may be outsourced to us or a third party provider. These database services could be made available on a per-access transaction basis or on a monthly or annual subscription basis. The actual storage location of the database can be cloud-based, on a stand-alone scanning device or on a mobile device via a Bluetooth connection depending on the requirements of access, size of the database and security as defined by the customer. As a result, large databases can be accessed by cell phone or other mobile technologies using either local storage or cloud based storage.
Based on the commercialization plans outlined above,grow our business model anticipates deriving revenue from several sources:
●
Sales of the ChromaID Lab Kit and ChromaID Liquid Lab Kit
●
Non Recurring Engineering (NRE) fees to assist customers with scan integration into their products
●
Licensing of the ChromaID technology
●
Royalties per unit generated from the sales of scan heads
●
Multi-unit sales of the above referenced exemplar product for as yet to be determined consumer product applications
●
Per click transaction revenue from accessing the unique ChromaID signatures
●
Developing custom product applications for customers
●
ChromaID database administration and management services
Our Acceleration of Business Development in the United States and Around the World
We are coordinating our internal business development, sales and marketing efforts with those of our strategic partners IDMC, and Sumitomo Precision Products to leverage market data and information in order to focus on specific target vertical markets which have the greatest potential for early adoption. The ChromaID Lab Kit provides a means for us to demonstrate the technology to customers in these markets. It also allows customers to experiment with developing unique applications for their particular use. Our Business Development team is pursuing license opportunities with customers in our target markets. As an example, in March 2016 we entered into a Collaboration Agreement and License with Intellicheck Mobilisa. The agreement provides Intellicheck with exclusive rights to our ChromaID technology in the areas of homeland security, law enforcement and crime prevention.
There is no requirement for FDA or other government approval for the current applications of our ChromaID technology. Over time, as we explore the application of our ChromaID technology for medical diagnostics and other applications, we expect that there will be requirements for FDA and other government approvals before applications using the technology in medical and other regulated fields can enter the marketplace.
Research and Development
Our research and development efforts are primarily focused improving the core foundational ChromaID technology and developing new and unique applications for the technology. As part of this effort, we typically conduct testing to ensure that ChromaID application methods are compatible with the customer’s requirements, and that they can be implemented in a cost effective manner. We are also actively involved in identifying new application methods. Our team has considerable experience working with the application of light-based technologies and their application to various industries. We believe that its continued development of new and enhanced technologies relating to our core business is essential to our future success. We spent $243,114 during the nine months ended June 30, 2016 and $362,661 and $670,742 for the years ended September 30, 2015 and 2014, respectively, on development activities. Our research and development efforts are supported internally, through its relationship with IDMC and through contractors led by Dr. Tom Furness and his team at RATLab LLC.
Our Patents
We believe that our ten patents, 20 patent applications, and two registered trademarks, and our trade secrets, copyrights and other intellectual property rights are important assets for us. Our patents will expire at various times between 2027 and 2033. The duration of our trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The patents that have been granted to Visualant include:
On August 9, 2011, we were issued US Patent No. 7,996,173 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy,” by the United States Office of Patents and Trademarks. The patent expires August 24, 2029.
On December 13, 2011, we were issued US Patent No. 8,076,630 B2 entitled “System and Method of Evaluating an Object Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires November 7, 2028.
On December 20, 2011, we were issued US Patent No. 8,081,304 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 28, 2030.
On October 9, 2012, we were issued US Patent No. 8,285,510 B2 entitled “Method, Apparatus, and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On February 5, 2013, we were issued US Patent No. 8,368,878 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 12, 2013, we were issued US Patent No. 8,583,394 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 21, 2014, we were issued US Patent No. 8,888,207 B2 entitled “Systems, Methods, and Articles Related to Machine-Readable Indicia and Symbols” by the United States Office of Patents and Trademarks. The patent expires February 7, 2033.
On March 23, 2015, we were issued US Patent No. 8,988,666 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On May 26, 2015, we were issued patent US Patent No. 9,041,920 B2 entitled “Device for Evaluation of Fluids using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
On April 19, 2016, we were issued patent US Patent No. 9,316,581 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Substances Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
We pursue an aggressive patent strategy to expand our unique intellectual property in the United States and other countries.
Services and License Agreement Invention Development Management Company, L.L.C.
In November 2013, we entered into a Services and License Agreement with Invention Development Management Company. IDMC is a subsidiary of Intellectual Ventures, which collaborates with inventors and partners with pioneering companies and invests both expertise and capital in the process of invention. On November 19, 2014, we amended the Services and License Agreement with IDMC. This amendment exclusively licenses 10 filed patents to us
The agreement requires IDMC to identify and engage inventors to develop new applications of our ChromaID™ technology, present the developments to us for approval, and file at least 10 patent applications to protect the developments. IDMC is responsible for the development and patent costs. We provided the Chroma ID Lab Kits to IDMC at no cost and are providing ongoing technical support. In addition, to provide time for this accelerated expansion of its intellectual property we delayed the selling of the ChromaID Lab Kits for 140 days except for certain select accounts. We have continued our business development efforts during this period and have worked with IDMC and their global business development resources to secure potential customers and licensees for the ChromaID technology. We shipped 20 ChromaID Lab Kits to inventors in the IDMC network during December 2013 and January 2014. As part of our agreement with IDMC, we curtailed our ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, we began to actively market the ChromaID Lab Kits to interested and qualified customers.
We have received a worldwide, nontransferable, exclusive license to the intellectual property developed under the IDMC agreement during the term of the agreement, and solely within the identification, authentication and diagnostics field of use, to (a) make, have made, use, import, sell and offer for sale products and services; (b) make improvements; and (c) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to the useful intellectual property held by IDMC within the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer to sell products and services and (b) grant sublicenses to any and all of the foregoing rights. The option to acquire this license may be exercised for up to two years from the effective date of the Agreement.
IDMC is providing global business development services to us for geographies not being pursued by Visualant. Also, IDMC has introduced us to potential customers, licensees and distributors for the purpose of identifying and pursuing a license, sale or distribution arrangement or other monetization event.
We granted to IDMC a nonexclusive, worldwide, fully paid, nontransferable, sublicenseable, perpetual license to our intellectual property solely outside the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer for sale products and services and (b) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We granted to IDMC a nonexclusive, worldwide, fully paid up, royalty-free, nontransferable, non-sublicenseable, perpetual license to access and use our technology solely for the purpose of marketing the aforementioned sublicenses of our intellectual property to third parties outside the designated fields of use.
In connection with the original license agreement, we issued a warrant to purchase 97,169 shares of common stock to IDMC as consideration for the exclusive intellectual property license and application development services. The warrant has a current exercise price of $2.50 per share and expires November 10, 2018. The per share price is subject to adjustment based on any issuances below $2.50 per share except as described in the warrant.
We agreed to pay IDMC a percentage of license revenue for the global development business services and a percentage of revenue received from any company introduce to us by IDMC. We also have also agreed to pay IDMC a royalty when we receive royalty product revenue from an IDMC-introduced company. IDMC has agreed to pay us a license fee for the nonexclusive license of our intellectual property.
The term of both the exclusive intellectual property license and the nonexclusive intellectual property license commences on the effective date of November 11, 2013, and terminates when all claims of the patents expire or are held in valid or unenforceable by a court of competent jurisdiction from which no appeal can be taken.
The term of the Agreement commences on the effective date until either party terminates the Agreement at any time following the fifth anniversary of the effective date by providing at least ninety days’ prior written notice to the other party.
TransTech Systems, Inc.
Our wholly owned subsidiary, TransTech Systems, Inc., is a distributor of products, including systems solutions, components and consumables, for employee and personnel identification in government and the private sector, document authentication, access control, and radio frequency identification. TransTech provides these products and services, along with marketing and business development assistance to value-added resellers and system integrators throughout North America.
We expect our ownership of TransTech to accelerate our market entry and penetration through well-operated and positioned dealers of security and authentication systems, thus creating a natural distribution channel for products featuring our proprietary ChromaID technology. TransTech currently provides substantially all of our revenues. Its management team functions independently from Visualant’s and its operations require a minimal commitment of our management time and other resources. Our acquisition of TransTech in June 2010 and its operations are described in greater detail below.
Agreements with Sumitomo Precision Products Co., Ltd.
In May 2012, we entered into a Joint Research and Product Development Agreement with SumitomoPrecision Products Co., Ltd., a publicly-traded Japanese corporation, for the commercialization of our ChromaID technology. In March 2013, we entered into an amendment to this agreement, which extended the Joint Development Agreement from March 31, 2013 to December 31, 2013. The extension provided for continuing work between Sumitomo and Visualant focused upon advancing the ChromaID technology and market research aimed at identifying the most significant markets for the ChromaID technology. This collaborative work supported the development of the ChromaID Lab Kit. This agreement expired December 31, 2013. The current version of the technology was introduced to the marketplace as a part of our ChromaID Lab Kit during the fourth quarter of 2013. Sumitomo invested $2,250,000 in exchange for 115,385 shares of restricted shares of common stock priced at $19.50 per share that was funded on June 21, 2012.
We also entered into a License Agreement with Sumitomo in May 2012, under which Sumitomo paid the Company an initial payment of $1 million. The License Agreement granted Sumitomo an exclusive license for the then extant ChromaID technology. The territories covered by this license include Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines). The Sumitomo License fee was recorded as revenue over the life the Joint Research and Product Development Agreement and was fully recorded as of May 31, 2013. On May 21, 2015, we entered into an amendment to the License Agreement, which, effective as of June 18, 2014, eliminated the Sumitomo exclusivity and provides that if we sell products in certain territories – Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines) – the Company will pay Sumitomo a royalty rate of 2% of net sales (excluding non-recurring engineering revenues) over the remaining term of the five-year License Agreement (through May 2017).
Risks That We Face
Our business is subject to a number of risks of which you should be aware before making an investment decision. We are exposed to various risks related to our business and financial position (specifically our need for additional financing), this offering, our common stock and our recent reverse stock split. These risks are discussed more fully in the "Risk Factors" section of this prospectus beginning on page 11.
| · | Initially, entering the diabetes glucose monitoring market with our non-invasive glucose monitoring device. |
| | |
| · | Following our entry into the glucose monitoring market, entering other clinical monitoring markets for continuous, non-invasive hormone, medication metabolites, endocrinology components, and biomolecular monitoring. |
| · | Applying our platform technology to lifestyle analysis, clinical trials, and chronic illnesses. We believe that potential use cases include real-time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency. |
| | |
| · | With a potential ever-growing body of non-invasively determined analytes available from individuals utilizing our technology we believe, over time, with longitudinal data we will be able to engage in so-called “predictive health” and provide early warnings of the onset of disease. |
| | |
| · | Significantly, every new application will likely function utilizing the same sensor. We expect that hardware changes will not be required to target new analytes, so you will not need a new device, but an updated software algorithm will be required. |
| | |
| · | Each new application provides potential new opportunities for monetization of the platform technology. Each additional analyte we identify over time may require its own subsequent FDA clearance. |
Corporate Information
We were incorporated under the laws of the State of Nevada on October 8, 1998. Our executive offices areoffice is located at 500 Union Street, Suite 420,810, Seattle, WA 98101. Our telephone number is (206) 903-1351 and our principal website address is located at www.visualant.net.
www.knowlabs.co. The information contained on or that can be accessed through, our website is not incorporated intoby reference in and is not deemed a part of this prospectus. You should not rely on our website or any such information in making your decision whether to purchase our common stock.
Securities offered:Common Stock being offered by Selling Stockholder | | 3,571,428Up to 4,800,000 shares of Common Stock, issuable upon full conversion of a Note in the principal amount of $4,800,000, and up to 6,000,000 shares of Common Stock underlying the Warrant. The selling stockholder may sell its shares of Common Stock at prevailing market prices or privately negotiated prices. We will not receive any proceeds from the sales by the selling stockholder. |
| |
Use of Proceeds | We will not receive any proceeds from the sale of shares by the selling stockholder. |
| |
Common stock outstanding immediately after the offering | 87,312,146 shares |
| |
Trading Symbol | Our common stock is listed on the NYSE American stock exchange under the symbol “KNW.” |
| |
Risk Factors | The securities offered by this prospectus are speculative and involve a high degree of risk and investors purchasing securities should not purchase the securities unless they can afford the loss of their entire investment. You should read “Risk Factors,” beginning on page 8 as well as those risk factors in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023, subsequent Quarterly Reports on Form 10-Q for the period ended December 31, 2023, and our other filings with the SEC, all of which includes (i) upare incorporated by reference herein, before deciding to 1,785,714 invest in our common stock. |
| |
Transfer Agent | Equiniti Trust Company located at 6201 15th Avenue, Brooklyn, New York 11219, telephone number (800) 937-5449, as the transfer agent for our common stock. |
The number of shares of common stock outstanding immediately following this offering is based on 82,512,146 shares outstanding as of March 25, 2024 and excludes:
| · | 29,347,106 shares of common stock that we may issue to the Selling Stockholder upon conversion of Series C Redeemable Convertible Preferred Stock, and (ii) up to 1,785,714 shares ofour common stock issuable upon the exercise of options outstanding Series E Warrants Shares. Our Common Stock is described in further detail in the sectionas of the prospectus titled “DESCRIPTION OF SECURITIES”March 25, 2024 under our 2021 Equity Incentive Plan (the “2021 Plan”), at a weighted average exercise price of $0.853 per share (including unearned stock option grants totaling 4,179,825 shares related to performance milestones); |
| | |
Common stock outstanding before the offering (1): | | 2,356,152 shares2 |
| | |
Common stock to be outstanding after this offering (2):Table of Contents |
| · | 5,950,914 shares
|
| | |
Use of Proceeds:
| | We will not receive any of the proceeds from the sale of7,111,706 additional shares of common stock by the Selling Stockholder. Upon exercise of the Series E Warrants, however, we will receive up to $0.70 per share or such lower price as may result from the anti-dilution protection features of such warrants. Any proceeds received from the exercise of such warrants will be used for general working capital and other corporate purposes. |
| | |
Terms of Warrants: | | Each Series E Warrant entitles the holder thereof to purchase one common share at an exercise price or $0.70 per full share, for a five year period ending August 5, 2021. The price per Warrant Share shall be subject to adjustment for stock splits, combinations, and similar recapitalization events and anti-dilution protection features. |
| | |
Risk Factors:
| | An investment in our common stock involves a high degree of risk. You should carefully consider the risk factors set forththat are reserved for issuance under the "Risk Factors" section hereunder and the other information contained in this prospectus before making an investment decision regarding our common stock. Our common stock should not be purchased by investors who cannot afford the loss of their entire investment.2021 Plan; |
| | |
OTCQB Symbol: | · | Our common stock is currently quoted on the OTCQB (the “OTCQB”) under the symbol “VSUL”. |
| | |
Reverse Split:
| | On June 17, 2015, we effected a 1-for-150 reverse stock split of our common stock. All warrant, option, share and per share information in this prospectus gives retroactive effect to the 1-for-150 split with all numbers rounded up to the nearest whole share. |
(1)
The number of shares of our common stock outstanding before this offering is based on 2,356,152 shares of our common stock outstanding as of September 1, 2016, and excludes, as of that date:
● 50,942 shares of our common stock issuable upon the exercise of outstanding stock options outstanding at a weighted-average exercise price of $18.04 per share;
● 23,334 shares of our common stock issuable upon the conversion of Series A Convertible Preferred Stock;
● 3,188,734 shares of our common stock issuable upon the exercise of outstanding warrants at an average exercise price of $0.89 per share. 1,785,714 shares of our common stock issuable upon the exercise of outstanding warrants, at an exercise price of $0.70, are being registered in this offering. The warrants will expire on or before August 2021;
● Up to 277,106 shares of our common stock issuable upon the exercise of placement agent warrants exercisable at $1.12 per share.
● An unknown number of shares of our common stock issuable upon the conversion of $810,000 of Convertible Notes Payable and an unknown number of our common shares issuable upon the exercise of $710,000 of warrants related to Convertible Notes Payable;
● 27,391 additional shares of our common stock available for future issuance under our 2011 Stock Incentive Plan;
● 1,785,714 shares of our common stock issuable upon the conversion of Series C Convertible Preferred Stock, at an exercise price of $0.70, subject to certain adjustments. These shares of common stock are being registered in this offering.
(2) | This total includes 23,3348,108,356 shares of our common stock issuable upon the conversion of Series AC Convertible Preferred Stock, 1,785,714 and Series D Convertible Preferred Stock as of March 25, 2024, and approximately 3,201,534 shares of common stock issuable upon conversionshares reserved to pay dividends on the outstanding shares of Series C Convertible Preferred Stock and 1,785,714Series D Convertible Preferred Stock, through December 31, 2023; |
| | |
| · | 9,020,264 shares of our common stock issuable upon the conversion of convertible debentures outstanding as of March 25, 2024; and |
| | |
| · | 25,984,961 shares of our common stock issuable upon exercise of all Series E Warrants.warrants outstanding as of March 25, 2024 at a weighted average exercise price of $1.028 per share. |
SUMMARY FINANCIAL INFORMATION
The following tables set forth a summary ofCAUTIONARY STATEMENT REGARDING FORWARD‑LOOKING STATEMENTS
This prospectus contains forward‑looking statements that are based on our historical financial data as of,management’s beliefs and for the period endedassumptions and on the dates indicated. We have derived theinformation currently available to us. All statements other than statements of operations data forhistorical facts are forward‑looking statements. The forward‑looking statements are contained principally in, but not limited to, the years ended September 30, 2015 and 2014 from our audited financial statements included in this prospectus. Historical results for any prior period are not necessarily indicative of results to be expected in any future period. You should read the following summary financial data together with our financial statements and the related notes appearing at the end of this prospectus and the "Capitalization” and "Management'ssections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations" sectionsOperations” and “Business.” These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward‑looking statements. Forward‑looking statements include, but are not limited to, statements about:
| · | our goals and strategies; |
| | |
| · | our future business development, financial condition and results of operations; |
| | |
| · | expected product development outcomes, including obtaining regulatory clearance; |
| | |
| · | expected changes in our revenue, costs or expenditures; |
| | |
| · | growth of and competition trends in our industry; |
| | |
| · | our expectations regarding demand for, and market acceptance of, our products; |
| | |
| · | our expectations regarding our relationships with investors, institutional funding partners and other parties with whom we collaborate; |
| | |
| · | our expectation regarding the use of proceeds from this offering; |
| | |
| · | fluctuations in general economic and business conditions in the markets in which we operate; and |
| | |
| · | relevant government policies and regulations relating to our industry. |
In some cases, you can identify forward‑looking statements by terms such as “may,” “could,” “will,” “should,” “would,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward‑looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the heading “Risk Factors” in this prospectus and in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023, subsequent Quarterly Report on Form 10-Q for the period ended December 31, 2023, and our other filings with the SEC, all of which are incorporated by reference. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward‑looking statements. No forward‑looking statement is a guarantee of future performance.
The forward‑looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus.
StatementsAlthough we will become a public company after this offering and have ongoing disclosure obligations under United States federal securities laws, we do not intend to update or otherwise revise the forward‑looking statements in this prospectus, whether as a result of Operations Data:
(in thousands, except for share and per share data)
| | | | | | | |
| | | Years Ended September 30, |
| | | | | | | |
| | | | | | | |
| STATEMENT OF OPERATIONS DATA: | | | | | | |
| Net revenue | $4,586 | $6,291 | $7,983 | $8,573 | $7,924 | $9,136 |
| Cost of goods sold | 3,835 | 5,274 | 6,694 | 6,717 | 6,344 | 7,570 |
| Gross profit | 751 | 1,017 | 1,289 | 1,856 | 1,580 | 1,566 |
| Research and development expenses | 243 | 363 | 670 | 1,169 | 177 | 134 |
| General and administrative expenses | 2,297 | 2,984 | 3,180 | 4,581 | 3,625 | 3,691 |
| Operating (loss) | (1,789) | (2,330) | (2,561) | (3,894) | (2,222) | (2,259) |
| Other income (expense) | 1,194 | (271) | 1,538 | (2,741) | (533) | (146) |
| Net (loss) | (595) | (2,601) | (1,023) | $(6,635) | $(2,755) | $(2,405) |
| Income taxes expense (current benefit) | - | 30 | (6) | $(30) | $(29) | $(9) |
| Net (loss) | (595) | (2,631) | (1,017) | (6,605) | (2,726) | (2,396) |
| Noncontrolling interest | - | - | - | $17 | $6 | $14 |
| Net (loss) attributable to Visualant, Inc. and Subsidiaries common shareholders | $(595) | $(2,631) | $(1,017) | $(6,622) | $(2,732) | $(2,410) |
| Net (loss) per share | $(0.48) | $(2.33) | $(0.92) | $(8.06) | $(6.24) | $(8.42) |
| Weighted average number of shares | 1,236,721 | 1,131,622 | 1,108,964 | 819,563 | 437,049 | 284,552 |
Balance Sheet Data:
(in thousands)
| |
| |
BALANCE SHEET DATA: | |
Total current assets | $1,706
|
Total assets | 3,055
|
Total current liabilities | 7,230
|
Total current liabilities without derivative liabilities | 6,491
|
Total liabilities | 7,230
|
Stockholders' (deficiency) | (4,174)
|
Stockholders' (deficiency) without derivative liabilities | (3,435)
|
new information, future events or otherwise.
RISK FACTORS
InvestingAn investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, including the shares of common stock offered by this prospectus, you should carefully consider the risks and uncertainties described under “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023, subsequent Quarterly Reports on Form 10-Q for the period ended December 31, 2023, and our other filings with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial condition and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value of our securities could decline and you could lose some or all of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. If any of these risks occur, our business, results of operations or financial condition and prospects could be harmed. In that event, the market price of our common stock, and you could lose all or part of your investment. Some statements in this prospectus, including statements in the following risk factors, constitute forward-looking statements. Please refer to the section titled “Cautionary Statement Regarding Forward-Looking Statements.”
Summary of Risk Factors
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the “Risk Factors” section immediately following this summary. These risks include, but are not limited to, the following:
Risks Related to Our Business and uncertaintiesIndustry
| · | We might not be able to continue as a going concern. We believe that our cash on hand will be sufficient to fund our operations at least through August 31, 2024. |
| | |
| · | We are still in the early stages of commercialization, refining our technology. Our success depends on our ability to conclude development and market devices that are recognized as accurate, safe, and cost-effective as other options currently available in the market and cleared by FDA. |
| | |
| · | We are subject to extensive regulation by FDA, which could restrict the sales and marketing of our products and could cause us to incur significant costs; |
Risks Related to Ownership of Our Common Stock
| · | The market price of our common stock may fluctuate, and you could lose all or part of your investment. |
| | |
| · | We may not be able to maintain a listing of our common stock on the NYSE American. |
| | |
| · | We do not expect to declare or pay dividends in the foreseeable future. |
| | |
| · | Future issuances of our common stock or securities convertible into, or exercisable or exchangeable for, our common stock, or the expiration of lock-up agreements that restrict the issuance of new common stock or the trading of outstanding common stock, could cause the market price of our securities to decline and would result in the dilution of your holdings. |
| | |
| · | Future issuances of debt securities, which would rank senior to our common stock upon our bankruptcy or liquidation, and future issuances of preferred stock, which could rank senior to our common stock for the purposes of dividends and liquidating distributions, may adversely affect the level of return you may be able to achieve from an investment in our common stock. |
RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully read and consider all of the risks described below, together with all of the other information contained or referred to in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus,report, before decidingmaking an investment decision with respect to invest in our common stock. If any of the following risks actuallyevents occur, our business, prospects, operating results and financial condition, could sufferbusiness and results of operations (including cash flows) may be materially adversely affected. In that event, the tradingmarket price of our common stock could decline, and you could lose all or part of your investment.
Risks RelatingRelated to the Commercialization of Our Products
We may not be able to generate sufficient revenue from the commercialization of our ChromaID technology and related products to achieve or sustain profitability.
We are in the process of commercializing our ChromaID™ technology. To date, we have entered into one License Agreement with Sumitomo Precision Products Co., Ltd. and have a strategic relationship with IDMC. Failure to sell our ChromaID products, grant additional licenses and obtain royalties or develop other revenue streams will have a material adverse effect on our business, financial condition and results of operations.
We believe that our commercialization success is dependent upon our ability to significantly increase the number of customers that are using our products. To date, we have generated minimal revenue from sales of our ChromaID products. In addition, demand for our ChromaID products may not increase as quickly as planned and we may be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in introducing the ChromaID technology and related products to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability.
We are in the early stages of commercialization and our ChromaID technology and related products may never achieve significant commercial market acceptance.
Our success depends on our ability to develop and market products that are recognized as accurate and cost-effective. Many of our potential customers may be reluctant to use our new technology. Market acceptance will depend on many factors, including our ability to convince potential customers that our ChromaID technology and related products are an attractive alternative to existing light-based technologies. We will need to demonstrate that our products provide accurate and cost-effective alternatives to existing light-based authentication technologies. Compared to most competing technologies, our technology is relatively new, and most potential customers have limited knowledge of, or experience with, our products. Prior to implementing our ChromaID technology and related products, potential customers are required to devote significant time and effort to testing and validating our products. In addition, during the implementation phase, customers may be required to devote significant time and effort to training their personnel on appropriate practices to ensure accurate results from our technology and products. Any failure of our ChromaID technology or related products to meet customer expectations could result in customers choosing to retain their existing testing methods or to adopt systems other than ours.
Many factors influence the perception of a system including its use by leaders in the industry. If we are unable to induce industry leaders in our target markets to implement and use our ChromaID technology and related products, acceptance and adoption of our products could be slowed. In addition, if our products fail to gain significant acceptance in the marketplace and we are unable to expand our customer base, we may never generate sufficient revenue to achieve or sustain profitability.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We commenced our formal commercial launch in the fourth fiscal quarter of 2014 and anticipate growth in our business operations. Since our inception in 1998, we have increased our number of employees to 16 as of June 30, 2016 and we expect to increase our number of employees further as our business grows. This future growth could create strain on our organizational, administrative and operational infrastructure, including quality control, customer service and sales and marketing. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and management controls, as well as our reporting systems and procedures. If our current infrastructure is unable to handle our growth, we may need to expand our infrastructure and staff and implement new reporting systems. The time and resources required to implement such expansion and systems could adversely affect our operations. Our expected future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend, in part, on our ability to manage this potential future growth effectively, without compromising quality.
Risks Relating to our Business and Financial Condition
We have a history of operating losses and there can be no assurance that we can achieve or maintain profitability.
We have experienced net losses since inception. As of June 30, 2016, we had an accumulated deficit of $24.8 million and net losses in the amount of $595,000, $2,631,000 and $1,017,000 for the nine months ended June 30, 2016 and the years ended September 30, 2015 and 2014, respectively. There can be no assurance that we will achieve or maintain profitability.If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair our ability to sustain operations and adversely affect the price of our common stock and our ability to raise capital. Our operating expenses may increase as we spend resources on growing our business, and if our revenue does not correspondingly increase, our operating results and financial condition will suffer. Our ChromaID business has produced limited revenues, and may not produce significant revenues in the near term, or at all, which would harm our ability to continue our operations or obtain additional financing and require us to reduce or discontinue our operations. You must consider our business and prospects in light of the risks and difficulties we will encounter as business with an early-stage technology in a new and rapidly evolving industry. We may not be able to successfully address these risks and difficulties, which could significantly harm our business, operating results and financial condition.
IndustryWe need additional financing to support our technology development and ongoing operations, pay our debts and maintain ownership of our intellectual properties; we will not receive any of the proceeds from the sale of the common stock by the selling stockholder.
property.We are currently operating at a loss.loss and using substantial cash to fund our operation. We believe that our cash on hand will be sufficient to fund our operations through September 30, 2016.
August 31, 2024. We may need additional financing to implement our business plan and to service our ongoing operations, pay our current debts (described below) and maintain ownership of our intellectual property. There can be no assurance that we will be able to secure any needed funding, or that if such funding is available, the terms or conditions would be acceptable to us. If we are unable to obtain additional financing when necessary,it is needed, we will need to restructure our operations and/or divest all or a portion of our business. We may seekare seeking additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, and could increase our expenses and require that our assets secure such debt. Equity financing, if obtained, could result in dilution to our then-existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back, or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. Our services and license agreement with Invention Development Management Company, LLC is important There can be no assurance that we will be able to our business strategy and operations.
In November 2013, we entered into a Services and License Agreement with Invention Development Management Company. IDMC is a subsidiary of Intellectual Ventures, which collaborates with inventors, partners with companies and invests both expertise and capital in the process of invention. This agreement was amended in November 2014 to license ten patents filed by IDMC related to the ChromaID technology to us.
The amended agreement with IDMC covers asell that number of areas that are important to our operations, including the following:
●
The agreement requires IDMC to identify and engage inventors to develop new applications of our ChromaID technology, present the developments to us for approval, and file at least ten patent applications to protect the developments;
●
We received a worldwide, nontransferable, exclusive license to the licensed intellectual property developed under this agreement within the identification, authentication and diagnostics field of use;
●
We received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to intellectual property held by IDMC within that same field of use; and
●
We granted to IDMC certain licenses to our intellectual property outside the identification, authentication and diagnostics field of use.
Failure to operate in accordance with the IDMC agreement, or an early termination or cancellation of this agreement for any reason,would have a material adverse effect on ability to execute our business strategy and on our results of operations and business.
shares, if any. We need to continue as a going concern if our business is to succeed.
Because we have generated limited revenues and currently operate at a loss, we are completely dependent on the continued availability of financing in order to continue our recurringbusiness. There can be no assurance that financing sufficient to enable us to continue our operations will be available to us in the future.
We have cash and cash equivalents of $4,821,000 and net working capital of approximately $3,796,000 (exclusive of convertible notes payable) as of December 31, 2023. We anticipate that we will record losses and negative cash flows from operations the audit report of our independent registered public accountants on our consolidated financial statements for the year ended September 30, 2015 contains an explanatory paragraph statingforeseeable future. We believe that there iswe have enough available cash to operate until August 31, 2024. As of December 31, 2023, our accumulated deficit was $125,351,000. We intend to seek additional cash via equity and debt offerings. As a result of not having at least twelve months of cash available and not having any firm commitment for debt or equity financing, substantial doubt about ourthe Company’s ability to continue as a going concern. Factors identified in the report include our historical net losses, negative working capital, and the need for additional financing to implement our business plan and service our debt repayments. If we are not able to attain profitability in the near future our financial condition could deteriorate further, which would have a material adverse impact on our business and prospects and result in a significant or complete loss of your investment. Further, we may be unable to pay our debt obligations as they become due, which include obligations to secured creditors.
If we are unable to continue as a going concern we mightexists. We have to liquidatefinanced our assetscorporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the valuesexercise of warrants. During the remainder of 2024, we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements. Additionally, we are subject to customary operational covenants, including limitations on our ability to incur liens or additional debt, pay dividends, redeem stock, make specified investments and engage in merger, consolidation or asset sale transactions, among other restrictions. In addition, the inclusion of an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern and our lack of cash resources may materially adversely affect our share price and our abilityexpect to raise new capitaladditional funds through the issuance of preferred stock, convertible debentures or equity.
The proceeds of warrants currently outstanding, to enter into critical contractual relations with third parties.
the extent not exercised on a cashless basis, may generate potential proceeds. We
have obligations to repaycannot provide assurance that any of these warrants will be exercised.As of December 31, 2023, we owed approximately $2,696,435 in various loans in the near future,$2,958,000 and if we do not satisfy these obligations, the lenders may have the right to demand payment in full or exercise other remedies.
We have a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which currently matures onowe $2,762,000 under various convertible promissory notes as of December 31, 20162023, including $1,301,000 to Clayton Struve who owns 100% of outstanding Series C preferred stock and provides for interest at 3.25% per year.
RelatedD preferred stock, and $1,461,000 owed to the Umpqua Loan, we entered into a demand promissory note for $200,000 on January 10, 2014 with an entity with whichentities controlled by Ronald P. Erickson, our Chief Executive Officer, is affiliated. This demand promissory note will be effective in case of a default by us under the Umpqua Loan.We also have two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between JanuaryCEO and July 2014, provide for interest of 3% per year and now mature on September 30, 2016. They also provide for a second lien on our assets if not repaid by September 30, 2016 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson.Chairman. Mr. Erickson and/or entities with which he is affiliated also have advanced $668,500accounts payable and have unreimbursed expenses and compensation of approximately $398,000. We owe Mr. Erickson, or entities with which he is affiliated, $1,686,500accrued liabilities $196,000 as of September 1, 2016.
December 31, 2023 related to accrued interest. We also have convertible notes payable to investors totaling $710,000 to fund short-term working capital. These notes accrue interest at a rate of 8% per annum, become due during September 2016 and February 2017 and are convertible into common stock as part of our next financing, at a conversion price equal to the price of the common stock sold in our next public financing. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing.
We entered into a Convertible Note Payable with JSJ Investments, Inc. on August 1, 2016 for $100,000 to fund short-term working capital. The JSJ Note accrued interest at a rate of 12% per annum and becomes due on May 1, 2017 and is convertible into common stock on January 1, 2017. The JSJ Note is convertible at the lower of $1.35 per share or 65% of the average of the lowest twenty day trading price prior to the date of the conversion notice. We received $88,000 net of all fees. On August 30, 2016, we paid $110,000 to JSJ to repay the Note Payable in full.
We requiremay need additional financing, to service and/or repay these debt obligations. If we raise additional capital through borrowing or other debt financing, we may incur substantial interest expense. If and when we raise more equity capital in the future, it will result in substantial dilution to our current stockholders.
Our management has concludedWe have a history of operating losses and there can be no assurance that we can achieve or maintain profitability.
We have material weaknessesexperienced net losses since inception. As of December 31, 2023, we had an accumulated deficit of $125,351,000 and net losses in our internal controls over financial reportingthe amount of $3,447,000, $15,289,000 and that our disclosure controls$20,071,000 during the three months ended December 31, 2023 and procedures are not effective.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company's annual or interim financial statements will not be prevented or detected on a timely basis. During the audit of our financial statements for the yearyears ended September 30, 2015, our management identified material weaknesses in our internal control over financial reporting.2023 and 2022, respectively. There can be no assurance that we will achieve or maintain profitability. If these weaknesses continue, investors could lose confidencewe achieve profitability in the accuracyfuture, we may not be able to sustain profitability in subsequent periods. Failure to become and completeness ofremain profitable would impair our financial reportsability to sustain operations and other disclosures.
In addition, our management has concluded that our disclosure controls and procedures were not effective due toadversely affect the lack of an audit committee “financial expert.” These material weaknesses, if not remediated, create an increased risk of misstatement of the Company’s financial results, which, if material, may require future restatement thereof. A failure to implement improved internal controls, or difficulties encountered in their implementation or execution, could cause future delays in our reporting obligations and could have a negative effect on us and the trading price of our common stock.
Ifstock and our ability to raise capital. Our operating expenses may increase as we spend resources on growing our business, and if our revenue does not correspondingly increase, our operating results and financial condition will suffer. Our businesses have produced minimal revenues and may not produce significant revenues in the
company werenear term, or at all, which would harm our ability to
dissolvecontinue our operations or
wind-up, holdersobtain additional financing and require us to reduce or discontinue our operations. You must consider our business and prospects in light of the risks and difficulties we will encounter as business with an early-stage technology in a new and rapidly evolving industry. We may not be able to successfully address these risks and difficulties, which could significantly harm our business, operating results, financial condition and common stock price per share.We may not be able to generate sufficient revenue from the commercialization of our common stock would not receive a liquidation preference.
If we weretechnology and related products to wind-upachieve or dissolve our company and liquidate and distribute our assets, our common stockholders would share in our assets only after we satisfy any amounts we owe to our creditors and preferred equity holders. If our liquidation or dissolution were attributable to our inability to profitably operate our business, then it is likely that we would have material liabilities at the time of liquidation or dissolution. Accordingly, it is very unlikely that sufficient assets will remain available after the payment of our creditors and preferred equity holders to enable you to receive any liquidation distribution with respect to any common stock you hold.
If components used in our finished products become unavailable, or third-party manufacturers otherwise experience delays, we may incur delays in shipment to our customers, which would damage our business.
sustain profitability.We depend on third-party suppliers for substantially all of our components and products. We purchase these products and components from third-party suppliers that serve the advanced lighting systems market and we believe that alternative sources of supply are readily available for most products and components. However, consolidation could result in one or more current suppliers being acquired by a competitor, rendering us unable to continue purchasing necessary amounts of key components at competitive prices. In addition, for certain of our customized components, arrangements for additional or replacement suppliers will take time and result in delays. We purchase products and components pursuant to purchase orders placed from time to time in the ordinary courseearly stages of business. This means we are vulnerablecommercializing our technology. Failure to unanticipated price increasesdevelop and product shortages. Any interruption or delay in the supply of components andsell products orbased upon our inability to obtain components and products from alternate sources at acceptable prices intechnology could have a timely manner, could harmmaterial adverse effect on our business, financial condition and results of operations.
While To date, we
have not generated revenue from sales of our technology or products. We believe
alternative manufacturersthat our commercialization success is dependent upon our ability to significantly increase the number of customers that will use our products. In addition, demand for
theseour products may not materialize, or increase as quickly as planned, and we may therefore be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in introducing our technology and related products to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability.We are subject to extensive regulation by the U.S. Food and Drug Administration, which could require us to take significant time and could cause us to incur significant costs.
Our KnowU and UBand glucose monitoring products are available,subject to extensive regulation by FDA. These regulations relate to manufacturing, labeling, sale, promotion, distribution and shipping. Before a new medical device, or a new intended use of a legally marketed device, can be marketed in the United States, it must be cleared or approved by FDA through the applicable premarket review process (510(k), PMA, or de novo classification), unless an exemption applies.
The KnowU and UBand glucose monitoring products and substantially equivalent devices of this type that may later receive marketing authorization are similar to products referred to as integrated continuous glucose monitoring (CGM) systems. Integrated continuous glucose monitoring systems are generally classified by FDA as Class II devices and have established special controls outlining requirements for assuring CGM accuracy, reliability, and clinical relevance. FDA also has descriptions of the types of studies and data required to demonstrate acceptable CGM performance. Though it is our current belief that our initial product, the KnowU and UBand glucose monitoring products, are appropriate for a de novo classification request (i.e., a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device that is described in more detail below), we have selected these particular manufacturers basedexpect similar classification, special controls, and testing.
If we receive 510(k) clearance for our KnowU and UBand glucose monitoring products, we may be required to obtain new 510(k) clearances for significant post-market modifications. Each premarket submission and review process can be expensive and lengthy, and entail significant user fees, unless exempt. The classification and special controls for all other products using the Company’s proprietary radio frequency and microwave spectroscopy platform will be dependent on their abilityproduct type and explored as applicable.
In addition, regulatory clearance or approval by FDA does not ensure registration, clearance, approval, or certification by regulatory authorities or notified bodies internationally. While the regulatory requirements for marketing in international markets may require that we obtain clearance, approval, or certification by an international specified regulatory body or notified body. Complying with foreign regulatory requirements, including obtaining registrations, clearances, approvals, or certifications, can be expensive and time consuming, and we may not receive regulatory clearances, approvals, or certifications in each country or region in which we plan to consistently produce thesemarket our products peror we may be unable to do so on a timely basis. In turn, this could limit our specifications ensuring the best quality product at the most cost effective price. We depend on our third-party manufacturers to satisfy performanceexpected international growth and quality specifications and to dedicate sufficient production capacity within scheduled delivery times. Accordingly, the loss of all or one of these manufacturers or delays in obtaining shipmentsprofitability, which could have a material adverse effect on our business, financial condition, and results of operations.
The clinical trial process is lengthy and expensive with uncertain outcomes. Results of earlier studies may not be predictive of future clinical trial results, or the safety or efficacy profile for such products.
Clinical trials are generally required to support an application for clearance of a new device type such as our KnowU and UBand glucose monitoring products. All clinical trials must be conducted in accordance with FDA’s Investigational Device Exemption (IDE) regulations, which govern investigational device labeling, prohibit promotion, and specify an array of Good Clinical Practice requirements, which include among other things, recordkeeping, reporting, and monitoring responsibilities of study sponsors and study investigators. Clinical trials must further comply with FDA’s regulations for institutional review board approval and for informed consent and other human subject protections. Required records and reports are subject to inspection by FDA.
Results of clinical testing may be unfavorable or, even if the intended safety and efficacy success criteria are achieved, may not be considered sufficient for FDA to grant approval or clearance of a product. In additional, the commencement or completion of any of our clinical trials may be delayed or halted for numerous reasons, including, but not limited to, the following:
| · | we may be required to submit an investigational device exemption application, or IDE, to FDA, which must become effective prior to commencing certain human clinical trials of medical devices, and FDA may reject our IDE and notify us that we may not begin clinical trials; |
| | |
| · | the cost of clinical trials may be greater than we anticipate; |
| | |
| · | FDA or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold; |
| | |
| · | patients do not enroll in clinical trials at the rate we expect; |
| | |
| · | patients do not comply with trial protocols; |
| | |
| · | patient follow-up is not at the rate we expect; |
| | |
| · | patients experience adverse side effects; |
| | |
| · | patients die during a clinical trial, even though their death may not be related to our products; |
| | |
| · | we may not reach agreement on acceptable terms with prospective contract research organizations (CROs), and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| | |
| · | institutional review boards and third-party clinical investigators may delay or reject our trial protocol; |
| | |
| · | third-party clinical investigators decline to participate in a trial or do not perform a trial on our anticipated schedule or consistent with the clinical trial protocol, good clinical practices, or other FDA requirements; |
| · | data collection, monitoring, and analysis is not performed in a timely or accurate manner or consistent with the clinical trial protocol or investigational or statistical plans; |
| | |
| · | regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials; |
| | |
| · | changes in governmental regulations or administrative actions applicable to our trial protocols, including, for example, recent legislation passed by Congress requiring clinical trial sponsors to increase engagement with FDA on matters related to appropriate representation of racial and ethnic minorities in clinical trial data for pivotal studies; |
| | |
| · | the interim or final results of the clinical trial are inconclusive or unfavorable as to safety or effectiveness; and |
| | |
| · | FDA concludes that the results from our trial and/or trial design are inadequate to demonstrate safety and effectiveness of the product. |
Additionally, the ability of FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel, the availability of industry-paid user fees, and statutory, regulatory, and policy changes. Average review times for product approvals at FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies on which our operations untilmay rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at FDA and other agencies, including those resulting from global concerns (e.g., the ongoing COVID-19 global pandemic), may also slow the time necessary for new products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, if a prolonged government shutdown and/or government employee furloughs were to occur, or if FDA’s response to a global issue diverts FDA resources and attention to other regulatory efforts, then the ability of FDA to timely review and process our regulatory submissions could be significantly impacted, which could have a material adverse effect on our business, financial condition, and results of operations. Further, in our operations as a public company, future government shutdowns, furloughs, or public health emergencies could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Any of these occurrences may significantly harm our business, financial condition, and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Moreover, even if our products are cleared in the U.S., commercialization of our products in foreign countries would require clearance or approval by regulatory authorities in those countries. Clearance or approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials.
The safety and efficacy of our products is not yet supported by long-term clinical data, which could limit sales, and our products might therefore prove to be less safe or effective than initially thought.
Given the regulatory environment in which we operate, we lack the breadth of published long-term clinical data supporting the safety and efficacy of The KnowU and UBand glucose monitoring products and the benefits it offers that might have been generated in connection with other marketing authorization pathways. For these reasons, clinicians may be slow to adopt our products, we may not have comparative data that our competitors have or are generating, and we may be subject to greater regulatory and product liability risks. Further, future patient studies or clinical experience may indicate that treatment with our product does not improve patient outcomes. Such results would slow the adoption of our product by physicians, would significantly reduce our ability to achieve expected sales, and could prevent us from achieving and maintaining profitability.
In addition, because the KnowU and UBand glucose monitoring products have never been marketed, we have limited complaints or patient success rate data with respect to using these products. If future patient studies or clinical testing do not support our belief that our products offer a more advantageous blood glucose monitoring, then market acceptance of our products could fail to increase or could decrease, and our business could be harmed. Moreover, if future results and experience indicate that our product has potentially recurring malfunctions or causes unexpected or serious complications or other unforeseen negative effects, then we could be subject to mandatory or voluntary product recalls, suspension or withdrawal of FDA clearance, as well as significant legal liability or harm to our business reputation and financial results.
If we choose to, or are required to, conduct additional clinical studies and the outcome of such timestudies are not positive, then this could reduce the rate of coverage and reimbursement for the KnowU and UBand glucose monitoring products. This may slow the market adoption of our product by physicians, significantly reduce our ability to achieve expected revenues and prevent us from becoming profitable.
We believe that publications of scientific and medical results in peer-reviewed journals and presentations at leading conferences are critical to the broad adoption of our products. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving our products sufficiently novel or worthy of publication. The failure to be listed in physician guidelines or to be published in peer-reviewed journals could limit the adoption of our products. Unless specifically stated to be “peer-reviewed,” the studies referred to in this filing are not peer reviewed.
We are subject to extensive regulation which could restrict the sales and marketing of our products and could cause us to incur significant costs.
Medical devices may be marketed only for the indications for which they are approved or cleared. Further, clearances can be revoked if safety or effectiveness problems develop once the device is on the market.
The current regulatory requirements to which we are subject may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by FDA, which may include any of the following sanctions:
| · | modification to our training and promotional materials; |
| | |
| · | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| | |
| · | customer notification, or orders for repair, replacement or refunds; |
| | |
| · | voluntary or mandatory recall or seizure of our current or future products; |
| | |
| · | administrative detention by FDA of medical devices believed to be adulterated or misbranded; |
| | |
| · | imposing operating restrictions, suspension or shutdown of production; |
| | |
| · | refusing our requests for clearance, PMA or de novo classification of any new products, new intended uses or modifications to our products; |
| | |
| · | FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; |
| | |
| · | withdraws or suspension of 510(k) clearance that has already been granted, resulting in prohibitions on sales of our products; and |
| | |
| · | criminal prosecution. |
The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in stockholders losing their entire investment.
Additionally, any relationships we may have with healthcare professionals, clinical investigators, and payors in connection with our current and future business activities may be subject to federal and state healthcare fraud and abuse laws, false claims laws, transparency laws, and health information privacy and security laws, which could expose us to, among other things, criminal sanctions, civil penalties, contractual damages, exclusion from governmental healthcare programs, reputational harm, administrative burdens, and diminished profits and future earnings.
Healthcare providers and payors play a primary role in the recommendation and/or prescription of any product candidates for which we obtain future marketing approval. Our current and future arrangements with healthcare professionals, clinical investigators, payors, and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
| · | the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| | |
| · | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which can be enforced by private citizens through civil whistleblower or qui tam actions, prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, prohibits, among other things, executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| | |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information; |
| | |
| · | the federal Physician Payments Sunshine Act requires applicable manufacturers of covered drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to annually report to Centers for Medicare & Medicaid Services (CMS) starting in 2022 information regarding payments and other transfers of value to physicians, certain other healthcare providers, and teaching hospitals, as well as information regarding ownership and investment interests held by physicians and their immediate family members. The information reported will be publicly available on a searchable website, with disclosure required annually; and |
| | |
| · | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. For instance, the collection and use of health data in the European Union is governed by the General Data Protection Regulation, or the GDPR, which extends the geographical scope of European Union data protection law to non-European Union entities under certain conditions, tightens existing European Union data protection principles, creates new obligations for companies and new rights for individuals. Failure to comply with the GDPR may result in substantial fines and other administrative penalties. In addition, on June 28, 2018, the State of California enacted the California Consumer Privacy Act, or CCPA, which took effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and similar laws have been proposed at the federal level and in other states.
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve on-going substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, then we may be subject to significant penalties, including civil, criminal, and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, temporary or permanent debarment, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. Defending against any such actions can be costly, time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, then they may be subject to criminal, civil, or administrative sanctions, including exclusions from government funded healthcare programs.
A variety of risks associated with marketing our product candidates internationally could materially adversely affect our business.
We may seek regulatory approval of our product candidates outside of the U.S., and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
| · | differing regulatory requirements and reimbursement regimes in foreign countries; |
| | |
| · | unexpected changes in tariffs, trade barriers, price and exchange controls, and other regulatory requirements; |
| | |
| · | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| | |
| · | compliance with tax, employment, immigration, and labor laws for employees living or traveling abroad; |
| | |
| · | foreign taxes, including withholding of payroll taxes; |
| | |
| · | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| | |
| · | difficulties staffing and managing foreign operations; |
| | |
| · | workforce uncertainty in countries where labor unrest is more common than in the U.S.; |
| | |
| · | potential liability under the Foreign Corrupt Practices Act (FCPA) or comparable foreign regulations; |
| | |
| · | challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the U.S.; |
| | |
| · | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| | |
| · | business interruptions resulting from geo-political actions, including war and terrorism. |
These and other risks associated with our international operations may materially adversely affect our ability to attain or maintain profitable operations.
We may face difficulties with respect to coverage and reimbursement by various payors.
Sales of any medical device depend often, in part, on the extent to which the product will be covered and reimbursed by government payors (e.g., federal and state healthcare programs), third-party payors (e.g., commercial insurance and managed healthcare organizations), and other payors (e.g., foreign government healthcare programs). In the United States, various glucose monitoring products are covered for individuals with both Type 1 and Type 2 diabetes by Medicare and Medicaid in the majority of states and by commercial insurers, subject to satisfaction of certain eligibility and coverage criteria.
But significant uncertainty exists as to the coverage and reimbursement status of any newly approved product. For example, there is no assurance that a product will be considered medically reasonable and necessary for a specific indication, will be considered cost-effective by payors, that an adequate level of reimbursement will be established even if coverage is available, or that the payors’ reimbursement policies will not adversely affect the ability for manufacturers to sell products profitably.
Decisions regarding the extent of coverage and reimbursement amount are generally made on a plan-by-plan basis meaning one payors’ decision to cover a particular product does not ensure that other payors will also provide similar coverage. As a result, the coverage determination process can require manufactures to provide scientific and clinical support for the use of a product, and require providers to show medical necessity for use, to each payor separately. This process can be time-consuming, with no assurance that coverage and adequate reimbursement will be applied consistently or even obtained.
Payors are also increasingly reducing reimbursements for devices through continued implementation of cost-containment programs, including price controls and restrictions on coverage and reimbursement, which could further limit sales of any product. In addition, payors continue to question safety and efficacy while also challenging the prices charged, examining medical necessity and reviewing the cost effectiveness of devices in an effort to avoid coverage and reimbursement. But decreases of this nature surrounding the reimbursement for any product or a decision by a government and third-party payor not to cover a product could result in reduced physician usage and patient demand for the product.
Moreover, in international markets, reimbursement and healthcare payment systems vary significantly by country, with many countries have instituted price ceilings on specific products and therapies.
The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of FDA or another governmental authority, could have a negative impact on us.
We are subject to FDA’s medical device reporting regulations, which require us to report to FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event.
We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the initial use of the device. If we fail to comply with our reporting obligations, FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, seizure of our products, or, if premarket review is required in the future, delay in clearance of future products.
FDA and foreign regulatory bodies have the authority to require the recall of commercialized medical device products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects, or other deficiencies or failures to comply with applicable regulations. We cannot assure you that product defects or other errors will not occur in the future. Recalls involving our products could have a material adverse effect on our business, financial condition, and results of operations.
Moreover, medical device manufacturers are required to maintain certain records of recalls and corrections, even if they are not reportable to FDA. We may initiate voluntary withdrawals or corrections for our devices in the future that we determine do not require notification of FDA. If FDA disagrees with our determinations, then it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability and malpractice claims against us and negatively affect our sales.
We may face difficulties from changes to current regulations and future legislation, both in the U.S. as well as in other foreign jurisdictions where we may be operating.
Existing regulations and regulatory policies may change, and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of our product candidates. Legislative changes may impact our future business and operations, including those that may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our product candidates, if approved, and accordingly, our business, financial condition, and results of operations.
Both before and after a product is commercially released, we have ongoing responsibilities under various laws and regulations. If a regulatory authority were to conclude that we are not in compliance with applicable laws or regulations, or that any of our products are ineffective or pose an unreasonable risk for the end-user, then the authority may ban such devices, detain or seize adulterated or misbranded devices, order a recall, repair, replacement, or refund of such instruments, and require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health. A regulatory authority may also impose operating restrictions, enjoin and restrain certain violations of applicable law pertaining to medical devices, and assess civil or criminal penalties against our officers, employees, or us. The regulatory authority may also recommend prosecution by law enforcement agencies. Any governmental law or regulation, existing or imposed in the future, or enforcement action taken may have a material adverse effect on our business, financial condition, and results of operations.
We cannot predict the likelihood, nature, or extent of any legislative changes will be enacted or government regulation that may arise from future legislation or administrative action, either in the U.S. or abroad. Similarly, we cannot predict whether FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, then we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
Our industry is highly competitive and subject to significant or rapid technological change.
Our fields of therapeutic interest is highly competitive and subject to significant and rapid technological change. Accordingly, our success may depend, in part, on our ability to respond quickly to such change through the development and introduction of new products.
If our product candidates are approved by FDA, then potential competitors who seek to introduce similar product candidates may seek to take advantage of a shorter and less costly development program for a product that competes with our products. Our ability to compete successfully against currently existing and future alternatives to our product candidates and systems and competitors who compete directly with us may depend, in part, on our ability to attract and retain skilled scientific and research personnel, develop technologically superior products, develop competitively priced products, obtain patent or other required regulatory approvals for our products, be an early entrant to the market and manufacture, market, and sell our products, independently or through collaborations.
We currently rely upon external resources for many engineering and product development services. If we are unable to secure engineering or product development partners or establish satisfactory engineering and product development capabilities, we may not be able to successfully commercialize our technology.
Our success depends upon our ability to develop products that are accurate and provide solutions for our customers. Achieving the desired results for our customers requires solving engineering issues in concert with them. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing methods or to adopt systems other than ours.
Historically, we have not had sufficient internal resources to work on all necessary engineering and product development matters. We have used third parties in the past and will continue to do so. These resources are not always readily available, and the absence of their availability could inhibit our research and development efforts and our responsiveness to our customers. Our inability to secure those resources could impact our ability to provide engineering and product development services and could have an impact on our customers’ willingness to use our technology. Moreover, third parties have their own internal demands on time and resources which may not always align with ours. Hence, our own expectations for development and product timelines may not be shared by third parties upon whom we rely.
We are in the early stages of commercialization and our technology and related products may never achieve significant commercial market acceptance.
Our success depends on our ability to develop and market devices that are recognized as accurate, safe and cost-effective. They must be safe and deliver the required level of accuracy under any condition, regardless of the user, as determined by their intended use. This will be achieved through continued refinement of our technology. Before presenting it to the FDA, additional development is needed to increase its generalizability.
Many of our potential customers may be reluctant to use our new technology. Market acceptance will depend on many factors, including our ability to convince potential customers that our technology and related products are an attractive alternative manufacturerto existing technologies. We will need to demonstrate that our products provide accurate and cost-effective alternatives to existing technologies. Compared to most competing technologies, our technology is new, and most potential customers will have limited knowledge of, or experience with, our products. Prior to implementing our technology and related products, some potential customers may be required to devote significant time and effort to testing and validating our products. Any failure of our technology or related products to meet customer expectations could result in customers choosing to retain their existing methods or to adopt systems other than ours.
Many factors influence the perception of a new technology including its use by leaders in the industry. If we are unable to induce industry leaders in our target markets to implement and use our technology and related products, acceptance and adoption of our products could be found.
slowed. In addition, if our products fail to gain significant acceptance in the marketplace and we are unable to expand our customer base, we may never generate sufficient revenue to achieve or sustain profitability.Additionally, we may not be able to penetrate or successfully operate in international markets or encounter difficulty expanding into international markets because of limited brand recognition in certain parts of the world, which may lead to delayed acceptance of our products by consumers in these international markets. If we are unable to expand internationally and manage the complexity of international operations successfully, then it could have a material adverse effect on our business, financial condition, and results of operations. If our efforts to introduce our products into foreign markets are not successful, then we may have expended significant resources without realizing the expected benefit. Ultimately, the investment required for expansion into foreign markets could exceed the results of operations generated from this expansion.
We are dependent on key personnel.
Our success depends to a significant degree upon the continued contributions of key management and other personnel, some of whom could be difficult to replace, including Ronald P. Erickson,replace. While our Chief Executive Officer. We currently docontinued operation and ultimate success is not employ a Chief Financial Officer. We do not maintain key person life insurance covering any ofdependent upon one individual, our officers. Our success willdoes depend on the performance of our officers, our ability to retain and motivate our officers, our ability to integrate new officers into our operations, and the ability of all personnel to work together effectively as a team. Our officers do not currently have employment agreements. Our failure to retain and recruit officers and other key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success also depends on our continued ability to identify, attract, hire, train, retain and motivate highly skilled technical, managerial, manufacturing, administrative and sales and marketing personnel. Competition for these individuals is intense, and we may not be able to successfully recruit, assimilate or retain sufficiently qualified personnel. In particular, we may encounter difficulties in recruiting and retaining a sufficient number of qualified technical personnel, which could harm our ability to develop new products and adversely impact our relationships with existing and future customers. The inability to attract and retain necessary technical, managerial, manufacturing, administrative and sales and marketing personnel could harm our ability to obtain new customers and develop new products and could adversely affect our business and operating results.We rely on the timely supply of components and parts and could suffer if suppliers fail to meet their delivery obligations, raise prices or cease to supply us with components or parts.
The manufacture of our products is complex and requires the integration of a number of components from several sources of supply. We rely on numerous critical suppliers for various key components that are used in the manufacturing of our products. We can make no assurance that we will be able to maintain such supply arrangements. If we are unable to maintain supply arrangements, our access to key components could be reduced, which could harm our business.
Additionally, if demand for our products decreases, we may have excess inventory and inventory that may expire, which could result in inventory write-offs that would have a material adverse effect on our business, financial condition, and results of operations. We may also encounter defects in materials and/or workmanship, which could lead to a failure to adhere to regulatory requirements. Any defects could delay operations at our contract manufacturers’ facilities, lead to regulatory fines, or halt or discontinue manufacturing indefinitely. Any of these outcomes could have a material adverse effect on our business, financial condition, and results of operations.
This reliance also adds additional risks to the manufacturing process that are beyond our control. For example, the occurrence of epidemics or pandemics may cause one or more of our suppliers to close or reduce the scope of their operations either temporarily or permanently. In addition, these suppliers may provide components and products to our competitors. The medical device industry’s reliance on a limited number of key components and product suppliers subjects us to the risk that in the event of an increase in demand, our suppliers may fail to provide supplies to us in a timely manner while they continue to supply our competitors, many of which have greater purchasing power than us, or seek to supply components to us at a higher cost.
The failure of our suppliers to deliver components or products in a timely fashion could have disruptive effects on our ability to produce our products in a timely manner, or we may be required to find new suppliers at an increased cost.
Moreover, our reputation and the quality of our products are in part dependent on the quality of the components that we source from third-party suppliers. If we are unable to control the quality of the components supplied to us or to address known quality problems in a timely manner, then our reputation in the market may be damaged and sales of our products may suffer. As a result, we may experience a material adverse effect on our business, financial condition, and results of operations.
We have limited insurance which may not cover claims by third parties against us or our officers and directors.
We have limited directors’ and officers’ liability insurance and commercial liability insurance policies. Claims, however, by third parties against us may exceed policy amounts and we may not have amounts to cover these claims. Any significant claims would have a material adverse effect on our business, financial condition and results of operations. In addition, our limited directors’ and officers’ liability insurance may affect our ability to attract and retain directors and officers.
Our inability to effectively protect our intellectual property would adversely affect our ability to compete effectively, our revenue, our financial condition and our results of operations.
We rely on a combination of patent, trademark, and trade secret laws, and confidentiality procedures and licensing arrangements to protect our intellectual property rights.
ObtainingCreating and maintaining a strong patent positionportfolio is important to our business. Patent law relating to the scope of claims in the technology fields in which we operate is complex and uncertain, so we cannot be assured that we will be able to obtain or maintain patent rights, or that the patent rights we may obtain will be valuable, provide an effective barrier to competitors or otherwise provide competitive advantages. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions or demonstrate that we did not derive our invention from another, we may have to participate in interference or derivation proceedings in the USPTOUnited States Patent and Trademark Office or in court that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will prevail over those filed by others. Also, our intellectual property rights may be subject to other challenges by third parties. Patents we obtain could be challenged in litigation or in administrative proceedings such as ex parte reexam, inter partesparties review, or post grant review in the United States or opposition proceedings in Europe or other jurisdictions.There can be no assurance that:
● | · | any of our existing patents will continue to be held valid, if challenged; |
● | | |
| · | patents will be issued for any of our pending applications; |
● | | |
| · | any claims allowed from existing or pending patents will have sufficient scope or strength to protect us; |
● | | |
| · | our patents will be issued in the primary countries where our products are sold in order to protect our rights |
| | |
| · | and potential commercial advantage; or |
● | | |
| · | any of our products or technologies will not infringe on the patents of other companies. |
If we are enjoinedprevented from selling our products, or if we are required to develop new technologies or pay significant monetary damages or are required to make substantial royalty payments, our business and results of operations would be harmed.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may or may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could have a material adverse effect on our results of operations and business.
Claims by others that our products infringe their patents or other intellectual property rights could prevent us from manufacturing and selling some of our products or require us to pay royalties or incur substantial costs from litigation or development of non-infringing technology.
In recent years, there has been significant litigation in the United States involving patents and other intellectual property rights. We may receive notices that claim we have infringed upon the intellectual property of others. Even if these claims are not valid, they could subject us to significant costs. Any such claims, with or without merit, could be time-consuming to defend, result in costly litigation, divert our attention and resources, cause product shipment delays or require us to enter into royalty or licensing agreements. Such royalty or licensing agreements, if required, may not be available on terms acceptable to us or at all. We have not been engaged in litigation andbut litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the proprietary rights of others. Litigation may also be necessary to defend against claims of infringement or invalidity by others. A successful claim of intellectual property infringement against us and our failure or inability to license the infringed technology or develop or license technology with comparable functionality could have a material adverse effect on our business, financial condition and operating results.
Our TransTech vendor baseThe analysis of our patent portfolio by PatSnap Research and ipCapital Group is concentrated.
Evolis, Fargo, Ultra Electronics - Magicard Divisionnot a legal analysis and NiSCA, are major vendors of TransTech whose products account for approximately 73% of TransTech’s revenue. TransTech buys, packages and distributes products from these vendors after issuing purchase orders. Any lossdoes not predict the outcome of any legal challenges we or others might make in regard to patents, nor does it constitute a view on the overall legal strength of these vendors would have a material adverse effect on our business, financial condition and results of operations.
We currently have a very small sales and marketing organization. patents.If we are unable to secure a sales and marketing partner or establish satisfactory sales and marketing capabilities at our company, we may not be able to successfully commercialize our ChromaID technology.
We currently have one full-time sales and business development manager for the ChromaID technology. This individual oversees sales of our products and IP licensing and manages critical customer and partner relationships. In addition, he manages and coordinates the business development resources at our strategic partners IDMC and Sumitomo Precision Products as they relate to our ChromaID technology. We also work with third party entities that are focused in specific market verticals where they have business relationships that can be leveraged. Our subsidiary, TransTech Systems, has six sales and marketing employees on staff to support the ongoing sales efforts of that business. In order to commercialize products that are approved for commercial sales, we sell directly to our customers, collaborate with third parties that have such commercial infrastructure and work with our strategic business partners to generate sales. If we are not successful entering into appropriate collaboration arrangements or recruiting sales and marketing personnel or in building a sales and marketing infrastructure, we will have difficulty successfully commercializing our ChromaID technology, which would adversely affect our business, operating results and financial condition.
We may not be able to enter into collaboration agreements on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize a positive return on this investment. In addition, we will have tomust compete with established and well-funded pharmaceutical and biotechnology companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize ChromaIDtechnology without strategic partners or licensees include:
● | · | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
● | | |
| · | the lack of complementary products to be offered by sales personnel, which may put us at a competitive |
| | |
| · | disadvantage relative to companies with more extensive product lines; and |
● | | |
| · | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Government regulatory approval may be necessary before some of our products can be sold and there is no assurance such approval will be granted.
Although we do not need regulatory approval for our current applications, our ChromaID technology may have a number of potential applications in fields of use which will require prior governmental regulatory approval before the technology can be introduced to the marketplace. For example, we are exploring the use of our ChromaID technology for certain medical diagnostic applications. There is no assurance that we will be successful in developing medical applications for our ChromaID technology. If we were to be successful in developing medical applications of our technology, prior approval by the FDA and other governmental regulatory bodies may be required before the technology could be introduced into the marketplace. There is no assurance that such regulatory approval would be obtained for a medical diagnostic or other applications requiring such approval.
We may engage in acquisitions, mergers, strategic alliances, joint ventures and divestures that could result in final results that are different than expected
.In the normal course of business, we engage in discussions relating to possible acquisitions, equity investments, mergers, strategic alliances, joint ventures and divestitures. Such transactions are accompanied by a number of risks, including the use of significant amounts of cash, potentially dilutive issuances of equity securities,
incurrence of debt on potentially unfavorable terms as well as impairment expenses related to goodwill and amortization expenses related to other intangible assets, the possibility that we may pay too much cash or issue too many of our shares as the purchase price for an acquisition relative to the economic benefits that we ultimately derive from such acquisition, and various potential difficulties involved in integrating acquired businesses into our operations.From time to time, we have also engaged in discussions with candidates regarding the potential acquisitions of our product lines, technologies and businesses. If a divestiture such as this does occur, we cannot be certain that our business, operating results and financial condition will not be materially and adversely affected. A successful divestiture depends on various factors, including our ability to effectively transfer liabilities, contracts, facilities and employees to any purchaser; identify and separate the intellectual property to be divested from the intellectual property that we wish to retain; reduce fixed costs previously associated with the divested assets or business; and collect the proceeds from any divestitures.
If we do not realize the expected benefits of any acquisition or divestiture transaction, our financial position, results of operations, cash flows and stock price could be negatively impacted.
Our growth strategy depends in part on our ability to execute successfulWe may make strategic acquisitions. We have made strategic acquisitions in the past and may do so in the future, and if the acquired companies do not perform as expected, this could adversely affect our operating results, financial condition and existing business.
We may continue to expand our business through strategic acquisitions. The success of any acquisition will depend on, among other things:
| ● | · | the availability of suitable candidates; |
| ● | |
| · | higher than anticipated acquisition costs and expenses; |
| ● | |
| · | competition from other companies for the purchase of available candidates; |
| ● | |
| · | our ability to value those candidates accurately and negotiate favorable terms for those acquisitions; |
| ● | |
| · | | the availability of funds to finance acquisitions and obtaining any consents necessary under our credit facility; |
| · | the ability to establish new informational, operational and financial systems to meet the needs of our business; |
| ● | |
| · | the ability to achieve anticipated synergies, including with respect to complementary products or services; and |
| ● | |
| · | the availability of management resources to oversee the integration and operation of the acquired businesses. |
We may not be successful in effectively integrating acquired businesses and completing acquisitions in the future. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions. Acquired businesses may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition as we do. If these risks materialize, our stock price could be materially adversely affected.
Government regulatory approval may be necessary before some of our products can be sold and there is no assurance such approval will be granted.
Our technology will have a number of potential applications in fields of use that will require prior governmental regulatory approval before the technology can be introduced to the marketplace. For example, we are exploring the use of our technology for certain medical diagnostic applications, with an initial focus on the monitoring of blood glucose. There is no assurance that we will be successful in developing glucose monitoring medical applications for our technology. If we were to be successful in developing glucose monitoring medical applications of our technology, prior clearance by FDA and other governmental regulatory bodies will be required before the technology could be introduced into the marketplace. Our devices leverage Machine Learning (ML) and Artificial Intelligence (AI) to process the massive data collected through the Bio-RFID sensor. ML/AI also controls the sensor operation, enabling the device to emit and capture data, and, ultimately, to identify and measure blood glucose levels. Machine learning-enabled device software functions (ML-DSF) continue to be evaluated by FDA, which recently released new guidance proposing a science-based approach for AI/ML-enabled medical devices to be modified and improved more quickly. There is no assurance that such regulatory approval would be obtained for a glucose monitoring medical diagnostic device or other applications requiring such approval. FDA can refuse to grant, delay, and limit or deny approval of an application for clearance of marketing a glucose monitoring device for many reasons. We may not obtain the necessary regulatory approvals or clearances to market these glucose monitoring systems in the United States or outside of the United States. Any delay in, or failure to receive or maintain, approval or clearance for our products could prevent us from generating revenue from these products or achieving profitability.
We or our manufacturers may be unable to obtain or maintain international regulatory clearances or approvals for our current or future products, or our distributors may be unable to obtain necessary qualifications, which could harm our business thus limited sales to the U.S.
Sales of our products internationally are subject to foreign regulatory requirements that vary widely from country to country. In addition, FDA regulates exports of medical devices from the U.S. Complying with international regulatory requirements can be an expensive and time-consuming process, and marketing approval or clearance is not certain. The time required to obtain clearances or approvals, if required by other countries, may be longer than that required for FDA clearance or approvals, and requirements for such clearances or approvals may significantly differ from FDA requirements. We may rely on third-party distributors to obtain regulatory clearances and approvals required in other countries, and these distributors may be unable to obtain or maintain such clearances or approvals. Our distributors may also incur significant costs in attempting to obtain and in maintaining foreign regulatory approvals or clearances, which could increase the difficulty of attracting and retaining qualified distributors. If our distributors experience delays in receiving necessary qualifications, clearances or approvals to market our products outside the U.S., or if they fail to receive those qualifications, clearances or approvals, then we may be unable to market our products or enhancements in international markets effectively, or at all.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we market and sell our products outside of the U.S., we may be subject to rigorous international regulation in the future. In these circumstances, we would be required to rely on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our product in foreign countries.
Cybersecurity risks and cyber incidents could result in the compromise of confidential data or critical data systems and give rise to potential harm to customers, remediation and other expenses, expose us to liability under consumer protection laws, or other common law theories, subject us to litigation and federal and state governmental inquiries, damage our reputation, and otherwise be disruptive to our business and operations.
Cyber incidents can result from deliberate attacks or unintentional events. We collect and store on our networks sensitive information, including intellectual property, proprietary business information and personally identifiable information of our customers. The secure maintenance of this information and technology is critical to our business operations. We have implemented multiple layers of security measures to protect the confidentiality, integrity and availability of this data and the systems and devices that store and transmit such data. We utilize current security technologies, and our defenses are monitored and routinely tested internally and by external parties. Despite these efforts, threats from malicious persons and groups, new vulnerabilities and advanced new attacks against information systems create risk of cybersecurity incidents. These incidents can include, but are not limited to, gaining unauthorized access to digital systems for purposes of misappropriating assets or sensitive information, corrupting data, or causing operational disruption. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and may not immediately produce signs of intrusion, we may be unable to anticipate these incidents or techniques, timely discover them, or implement adequate preventative measures.
These threats can come from a variety of sources, ranging in sophistication from an individual hacker to malfeasance by employees, consultants or other service providers to state-sponsored attacks. Cyber threats may be generic, or they may be custom crafted against our information systems. Over the past several years, cyber-attacks have become more prevalent and much harder to detect and defend against. Our network and storage applications may be vulnerable to cyber-attack, malicious intrusion, malfeasance, loss of data privacy or other significant disruption and may be subject to unauthorized access by hackers, employees, consultants or other service providers. In addition, hardware, software or applications we develop or procure from third parties may contain defects in design or manufacture or other problems that could unexpectedly compromise information security. Unauthorized parties may also attempt to gain access to our systems or facilities through fraud, trickery or other forms of deceiving our employees, contractors and temporary staff.
There can be no assurance that we will not be subject to cybersecurity incidents that bypass our security measures, impact the integrity, availability or privacy of personal health information or other data subject to privacy laws or disrupt our information systems, devices or business, including our ability to deliver services to our customers. As a result, cybersecurity, physical security and the continued development and enhancement of our controls, processes and practices designed to protect our enterprise, information systems and data from attack, damage or unauthorized access remain a priority for us. As cyber threats continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any cybersecurity vulnerabilities.
Additionally, the U.S. may institute additional cybersecurity requirements especially for medical devices. For example, the data security requirements in the Food and Drug Omnibus Reform Act (“FDORA”), enacted in December 2022, that among other provisions, requires developers of certain “cyber devices” to design and implement plans to monitor, identify and address cybersecurity vulnerabilities of those devices and to submit those plans to FDA as part of every new 510(k) or PMA for a cyber device. “Cyber devices” are defined as devices that include software, connect to the internet, and contain any technological features that could be vulnerable to cybersecurity threats. This provision entered into effect on March 29, 2023, and FDA has indicated that it expects sponsors of cyber devices to begin to comply with these requirements as of October 1, 2023. FDA has stated that failure to comply with these requirements will result in FDA denying approval of the cyber device application.
We are subject to corporate governance and internal control requirements, and our costs related to compliance with, or our failure to comply with existing and future requirements could adversely affect our business.
We must comply with corporate governance requirements under the Sarbanes-Oxley Act of 2002 and the Dodd–Frank Wall Street Reform and Consumer Protection Act of 2010, as well as additional rules and regulations currently in place and that may be subsequently adopted by the Securities and Exchange Commission, or the SEC, and the Public Company Accounting Oversight Board. These laws, rules, and regulations continue to evolve and may become increasingly stringent in the future. The financial cost of compliance with these laws, rules, and regulations is expected to remain substantial.
Our management has concluded that our disclosure controls and procedures were not effective due to the lack of an audit committee “financial expert.” We expect to appoint an additional independent director to serve as Audit Committee Chairman. This director will be an “audit committee financial expert” as defined by the SEC. However, we cannot assure you that we will be able to fully comply with these laws, rules, and regulations that address corporate governance, internal control reporting, and similar matters in the future. Failure to comply with these laws, rules and regulations could materially adversely affect our reputation, financial condition, and the value of our securities.
The Capital Source credit facility contains covenants that may limit our flexibility in operating our business and failureRisks Related to comply with anyOwnership of these covenants could have a material adverse effect on our business
.
In December 8, 2009, we entered into the Capital Source credit facility. The Capital Source credit facility contains covenants that limit our ability to engage in specified types of transactions. These covenants limit our ability to, among other things:
| ● | | sell, transfer, lease or dispose of certain assets; |
| ● | | engage in certain mergers and consolidations; |
| ● | | incur debt or encumber or permit liens on certain assets, except in the limited circumstances permitted under the loan and security agreements; |
| ● | | make certain restricted payments, including paying dividends on, or repurchasing or making distributions with respect to, our common stock; and |
| ● | | enter into certain transactions with affiliates. |
A breach of any of the covenants under the Capital Source credit facility could result in a default under the Capital Source credit facility. Upon the occurrence of an event of default under the Capital Source credit facility, the lenders could elect to declare all amounts outstanding to be immediately due and payable and terminate all commitments to extend further credit. Our Common StockIf we are unable to repay those amounts,comply with the lenders could proceed against the collateral granted to them to secure such indebtedness.
The exercise pricescontinued listing requirements of the
IDMC warrant, Series A, B, CNYSE American, then our common stock would be delisted from the NYSE American, which would limit investors’ ability to effect transactions in our common stock and
Dsubject us to additional trading restrictions.Our common stock is currently listed on the NYSE American and placement agent warrants may require further adjustment.
In connectionthe continued listing of our common stock on the NYSE American is contingent on our continued compliance with a number of listing requirements. If we are unable to comply with the
June 2013 Special Situations financing described below under “Liquidity and Capital Resources”, ascontinued listing requirements of
September 1, 2016 we have outstanding Series A Warrants to purchase a total of 252,060 shares ofthe NYSE American, our common stock
with a current exercise price of $0.70 per share, and Series B Warrantswould be delisted from the NYSE American, which would limit investors’ ability to
purchase a total of 252,060 shares ofeffect transactions in our common stock
and subject us to additional trading restrictions. In order to maintain our listing, we must maintain certain share prices, financial and share distribution targets, including maintaining a minimum amount of stockholders’ equity and a minimum number of public stockholders, as well as satisfy other listing requirements of the NYSE American. In addition to these objective standards, NYSE American may delist the securities of any issuer for other reasons involving the judgment of NYSE American. We have been informally advised by the staff of NYSE American that, given our current stockholders equity and history of net losses, we may be subject to the equity standards set forth in Section 1003(a)(ii) and (iii) of the NYSE American Company Guide, and that we may not satisfy these standards or the exemption criteria for these standards. There is no assurance that we will be able to maintain compliance with a current exercise price of $0.70 per share, the IDMC warrant to purchase 97,169 shares ofNYSE American continued listing rules and/or continue its listing on the NYSE American in the future.
If the NYSE American delists our common stock with a current exercise price of $0.70 per share, Series Cfrom trading on its exchange and D Warrantswe are not able to purchase 23,334 shares oflist our securities on another national securities exchange, we expect the common stock with a current exercise price of $0.70 per share and placement agent warrantswould qualify to purchase 20,439 shares of common stock atbe quoted on an exercise price of current exercise price of $0.70 per share (collectively, the “Special Situations Warrants”). The Special Situations Warrants contain an adjustment provision that would require an adjustment in the exercise price of the Special Situations Warrants ifover-the-counter market. If this were to occur, we issue common stock, warrants or equity below the price that is reflected in the Special Situations Warrants (currently $0.70 per share). If we issue any additional shares of common stock, warrants or other equity securities at a price below the exercise prices of the Special Situations Warrants, it would result in a reduction in the exercise price of the Special Situations Warrants. A downward adjustment in the exercise price of the Special Situations Warrants could also affect the market price of the common stock.
Risks Relating to Our Stock
face significant material adverse consequences, including: | · | a limited availability of market quotations for our securities; |
| | |
| · | reduced liquidity for our securities; |
| | |
| · | substantially impair our ability to raise additional funds; |
| | |
| · | result in a loss of institutional investor interest and a decreased ability to issue additional securities or obtain additional financing in the future; |
| | |
| · | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| | |
| · | a limited amount of news and analyst coverage; and |
| | |
| · | potential breaches of representations or covenants of our agreements pursuant to which we made representations or covenants relating to our compliance with applicable listing requirements, which, regardless of merit, could result in costly litigation, significant liabilities and diversion of our management’s time and attention and could have a material adverse effect on our financial condition, business and results of operations. |
The price of our common stock is volatile, which may cause investment losses for our stockholders
. stockholders. The market price of our common stock has been and is likely in the future to be volatile. Our common stock price may fluctuate in response to factors such as:
| ●· | Announcements by us regarding liquidity, significant acquisitions, equity investments and divestitures, strategic relationships, addition or loss of significant customers and contracts, capital expenditure commitments and litigation; |
| ● | |
| · | Issuance of convertible or equity securities and related warrants for general or merger and acquisition purposes; |
| ● | |
| · | Issuance or repayment of debt, accounts payable or convertible debt for general or merger and acquisition purposes; |
| ● | |
| · | Sale of a significant number of shares of our common stock by stockholders; |
| ● | |
| · | General market and economic conditions; |
| ● | |
| · | Quarterly variations in our operating results; |
| ● | |
| · | Investor and public relation activities; |
| ● | |
| · | Announcements of technological innovations; |
| ● | |
| · | New product introductions by us or our competitors; |
| ● | |
| · | Competitive activities; |
| | |
| · | Low liquidity; and |
| ● | |
| · | Additions or departures of key personnel. |
These broad market and industry factors may have a material adverse effect on the market price of our common stock, regardless of our actual operating performance. These factors could have a material adverse effect on our business, financial condition, and results of operations.
Clayton A. Struve and Dale Broadrick could have significant influence over matters submitted to stockholders for approval.
As of September 1, 2016, Clayton A. Struve and Dale Broadrick in the aggregate, assuming the exercise of all warrants to purchase common stock, hold shares representing approximately 90.1% of our common stock on a fully-converted basis and could be considered a control group for purposes of SEC rules. However, the agreement with the accredited investor limits their ownership to 4.99% individually. Beneficial ownership includes shares over which an individual or entity has investment or voting power and includes shares that could be issued upon the exercise of options and warrants within 60 days after the date of determination. If these persons were to choose to act together, they would be able to significantly influence all matters submitted to our stockholders for approval, as well as our officers, directors, management and affairs. For example, these persons, if they choose to act together, could significantly influence the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of us on terms that other stockholders may desire.
We were in dispute with an Institutional Investor related to our Series B Redeemable Convertible Preferred Stock
On March 8, 2016, we closed a Stock Purchase Agreement with an institutional investor pursuant to which we issued 255 Shares of Series B Redeemable Preferred Shares of the Company at $10,000 per share with a 5.0% original issue discount for the sum of $2,500,000.
At closing, we sold 51 Series B Preferred Shares in exchange for payment to the Company of $500,000 in cash and issued an additional 204 Series B Preferred Shares in exchange for delivery of a full recourse 1% Promissory Note for $1,995,000 and payment to the Company of $5,000 in cash (paid). The Note was collateralized by the Series B Preferred Shares. Under the terms of the Note, we were to receive an additional $500,000 for each $5 million, or in certain cases a lower amount, in aggregate trading volume of the common stock, so long as it meets certain other requirements. Any remaining balance under the Note was payable at its maturity in seven years. The Series B Preferred Shares were convertible into common stock at $7.50 per share; provided that the institutional investor may not convert any Series B Preferred Shares into common stock until that portion of the Note underlying the purchase of the converted portion of Series B Preferred Shares was paid in cash us.
During the nine months ended June 30, 2016, we issued 74,064 shares of common stock through the conversion of 34 shares of Series B Redeemable Preferred Stock valued at $339,998, or $4.591 per share. On August 5, 2016, we closed the First Amendment to Stock Purchase Agreement by and between the Company and this institutional investor. As a result of this amendment agreement, we paid the institutional investor $505,000, issued 52,000 shares of common stock valued at $169,000, cancelled the remaining 221 shares of Series B Redeemable Preferred Stock, and terminated the relationship and all aspects of the Stock Purchase Agreement described above in its entirety. We expect to expense $674,000 related to this amendment agreement during the three months ended September 30, 2016 and are currently cancelling the Certificate of Designations of Preferences, Rights and Limitations of Series B Redeemable Preferred Stock.
Transfers of our securities may be restricted by virtue of state securities “blue sky” laws, which prohibit trading absent compliance with individual state laws. These restrictions may make it difficult or impossible to sell shares in those states.
Transfers of our common stock may be restricted under the securities or securities regulations laws promulgated by various states and foreign jurisdictions, commonly referred to as "blue sky" laws. Absent compliance with such individual state laws, our common stock may not be traded in such jurisdictions. Because the securities held by many of our stockholders have not been registered for resale under the blue sky laws of any state, the holders of such shares and persons who desire to purchase them should be aware that there may be significant state blue sky law restrictions upon the ability of investors to sell the securities and of purchasers to purchase the securities. These restrictions may prohibit the secondary trading of our common stock. Investors should consider the secondary market for our securities to be a limited one.
The sale of a significant number of our shares of common stock could depress the price of our common stock.
Sales or issuances of a large number of shares of common stock in the public market or the perception that sales may occur could cause the market price of our common stock to decline. As of September 1, 2016,December 31, 2023, we had
2,356,15281,346,524 shares of common stock issued and outstanding,outstanding. As of September 1, 2016,December 31, 2023, there were options outstanding for the purchase of 50,94228,220,473 shares of our common stock and(including unearned stock option grants totaling 4,179,825 shares related to performance targets), warrants for the purchase of 3,188,734 shares of common stock. In addition, 23,33420,984,961 shares of our common stock, are8,108,356 shares of the Company’s common stock issuable, collectively, upon the conversion of our Series AC Convertible Preferred Stock and 1,785,714Series D Convertible Preferred Stock, and approximately 3,201,534 shares of our common stock, issuable upon the conversion ofcollectively, reserved to pay accrued dividends on our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock. Finally, up to 277,106In addition, the Company currently has 9,020,264 shares of ourits common stock are issuable uponat the exercisecurrent price of placement agent warrants $0.25 per share reserved and an unknown number of shares are issuable upon conversion of $810,000 in convertible promissory notes and an unknown numberdebentures of $2,761,931. Further, under the current terms of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock, and assuming no changes in the ownership thereof, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock, which are issuable if such dividends become payable as additional shares issuable uponof preferred stock, and such preferred stock is then converted into common stock. All of the exerciseforegoing shares could potentially dilute future earnings per share but are excluded from the December 31, 2023, calculation of $710,000 of warrants related to Convertible Notes Payable.net loss per share because their impact is antidilutive.Significant shares of common stock are held by our principal stockholders, other company insiders and other large stockholders. As “affiliates” of Visualant,“affiliates,” as defined under Securities and Exchange Commission Rule 144 under the Securities Act, of 1933, our principal stockholders, other of our insiders and other large stockholders may only sell their shares of common stock in the public market pursuant to an effective registration statement or in compliance with Rule 144.
These options, warrants, convertible notes payable and convertible preferred stock could result in further dilution to common stock holdersstockholders and may affect the market price of the common stock.
Future issuancecapital raises or other issuances of
additionalequity or debt securities may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.Pursuant to our articles of incorporation, we are authorized to issue 200,000,000 shares of common stock and/or preferred stock could dilute existing stockholders.
We have and may issue preferred stock that could have rights that are preferential to the rights of common stock that could discourage potentially beneficially transactions to our common stockholders.Pursuant to our certificate of incorporation, we currently have authorized 100,000,000 shares of common stock and 5,000,000 shares of preferred stock. To the extent that common shares arestock is available for issuance, subject to compliance with applicable stock exchange listing rules, our board of directors has the ability to issue additional shares of common stock in the future for such consideration as the board of directors may consider sufficient. The issuance of any additional securitiesshares could, among other things, result in substantial dilution of the percentage ownership of our stockholders at the time of issuance, result in substantial dilution of our earnings per share and adversely affect the prevailing market price for our common stock.
An issuancePursuant to our articles of incorporation, we are also authorized to issue 5,000,000 shares of blank check preferred stock of which 30,000 shares have been designated as our Series C Convertible Preferred Stock and 20,000 shares have been designated as our Series D Convertible Preferred Stock. Such preferred stock is senior to our common stock in terms of dividend priority and liquidation preference. Any preferred stock that we issue in the future may rank ahead of our common stock in terms of dividend priority or liquidation preference and may have greater voting rights than our common stock. In addition, such preferred stock may contain provisions allowing those shares to be converted into shares of common stock, which could dilute the value of our common stock to current stockholders and could adversely affect the market price, if any, of our common stock. In addition, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company. Although we have no present intention to designate or issue any shares of our authorized blank check preferred stock, there can be no assurance that we will not do so in the future.
As a result of the modifications of our Series C Convertible Preferred Stock and Series D Convertible Preferred Stock (see Description of Securities—Preferred Stock), assuming no changes in the amount of outstanding Series C preferred stock or Series D preferred stock ownership, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock. Future accreted dividends will be settled by issuing additional shares of preferred stock could result in a class of outstanding securities that would have preferences with respectwhich can then be converted to voting rights and dividends and in liquidation over our common stock and could, upon conversion or otherwise, have all of the rights of our common stock. Our Board of Directors' authority to issue preferred stock could discourage potential takeover attempts or could delay or prevent a change in control through merger, tender offer, proxy contest or otherwise by making these attempts more difficult or costly to achieve. The issuance of preferred stock could impair
In the voting, dividend and liquidation rights of common stockholders without their approval.
Future capital raises may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.
Iffuture, we
raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced and these stockholders may experience substantial dilution. We may also
issue equity securities that provide for rights, preferences and privileges seniorattempt to
those ofincrease our
common stock. If we raise additional fundscapital resources by
issuingoffering debt
securities, thesesecurities. These debt securities would have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets.
Because our decision to issue securities or incur debt in our future offerings will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings and debt financing. Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future. Thus, you will bear the risk of our future offerings reducing the value of your shares and diluting your interest in us.
The exercise prices of certain warrants, and the conversion prices of our outstanding convertible notes payable and our preferred stock may require further adjustment.
If in the future, the Company sells its common stock at a price below $0.25 per share, the conversion price of our outstanding shares of series C convertible preferred stock and series D convertible preferred stock would adjust below $0.25 per share pursuant to the documents governing such instruments. In addition, the conversion price of the convertible promissory notes referred to above and the exercise price of certain outstanding warrants to purchase 7,684,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments.
If our company were to dissolve or wind-up operations, holders of our common stock would not receive a liquidation preference.
If we raise additional funds through collaborationswere to wind up or dissolve our company and licensing arrangements,liquidate and distribute our assets, our common stockholders would share in our assets only after we may be required to relinquish some rightssatisfy any amounts we owe to our technologiescreditors and preferred equity holders. If our liquidation or candidate products,dissolution were attributable to our inability to profitably operate our business, then it is likely that we would have material liabilities at the time of liquidation or dissolution. Accordingly, it is very unlikely that sufficient assets will remain available after the payment of our creditors and preferred equity holders to grant licenses on terms that are not favorableenable common stockholders to us.
receive any liquidation distribution with respect to any common stock.We do not anticipate paying any cash dividends on our capital stock in the foreseeable future.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business, and we do not anticipate paying any cash dividends on our capital stock in the foreseeable future. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Anti-takeover provisions may limit the ability of another party to acquire our company, which could cause our stock price to decline.
Our certificate of incorporation, as amended, our bylaws and Nevada law contain provisions that could discourage, delay or prevent a third party from acquiring our company, even if doing so may be beneficial to our stockholders. In addition, these provisions could limit the price investors would be willing to pay in the future for shares of our common stock.
Our articles of incorporation allow for our board to create new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock; our Series A Preferred Stock contains provisions that restrict our ability to take certain actions without the consent of at least 66% of the Series A Preferred Stock then outstanding.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our Board of Directors also has the authority to issue preferred stock without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our Board of Directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders.
In addition, our articles of incorporation restrict our ability to take certain actions without the approval of at least 66% of the Series A Preferred Stock then outstanding. These actions include, among other things;
●
authorizing, creating, designating, establishing or issuing an increased number of shares of Series A Preferred Stock or any other class or series of capital stock ranking senior to or on a parity with the Series A Preferred Stock;
●
adopting a plan for the liquidation, dissolution or winding up the affairs of our company or any recapitalization plan (whether by merger, consolidation or otherwise);
●
amending, altering or repealing, whether by merger, consolidation or otherwise, our articles of incorporation or bylaws in a manner that would adversely affect any right, preference, privilege or voting power of the Series A Preferred Stock; and
●
declaring or paying any dividend (with certain exceptions) or directly or indirectly purchase, redeem, repurchase or otherwise acquire any shares of our capital stock, stock options or convertible securities (with certain exceptions).
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes statements that are, or may be deemed, "forward-looking statements." In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms "believes", "estimates", "anticipates", "expects", "plans", "intends", "may", "could", "might", "will", "should", "approximately" or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this prospectus and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned exploration activities, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and rare earth element market developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this prospectus. In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this prospectus, they may not be predictive of results or developments in future periods.
Any forward-looking statements that we make in this prospectus speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this prospectus.
You should also read carefully the factors described in the "Risk Factors" section of this prospectus to better understand the risks and uncertainties inherent in our business and underlying any forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. We disclaim any obligation to update or revise any forward-looking statement as a result of new information, future events or for any other reason.
We are not selling any shares of our common stock in this offering and, as a result, we will not receive any proceeds from the sale of the common stock covered by this prospectus. All of the net proceeds from the sale of our common stock will go to the Selling Stockholder. Upon exercise of the Series E Warrants, however, we will receive up to $0.70 per share or such lower price as may result from the anti-dilution protection features of such warrants. Any proceeds received from the exercise of such warrants will be used for general working capital and other corporate purposes. See “Selling Security Holders” and “Plan of Distribution.”
To the extent the Selling Stockholder exercises all of the Series E Warrants covering the 1,785,714 shares of common stock issuable upon exercise of all of the Warrants held by such Selling Stockholder, we would receive up to $0.70 per share from the exercise of the Series E Warrants, or such lower price as may result from the anti-dilution protection features of such Warrants. The Warrants may expire without having been exercised. Even if some or all of these Warrants are exercised, we cannot predict when they will be exercised and when we would receive the proceeds. We intend to use any proceeds we receive upon exercise of the warrants for general working capital and other corporate purposes.
With the exception of any brokerage fees and commissions which are the respective obligations of the Selling Stockholder, we are responsible for the fees, costs and expenses of this Registration Statement, which includes our legal and accounting fees, printing costs, and filing and other miscellaneous fees and expenses.
PRICE RANGE OF OUR COMMON STOCK
Our common stock is currently quoted on the OTCQB under the symbol "VSUL". The following table sets forth the range of the high and low sale prices of the common stock for the periods indicated. The quotations reflect inter-dealer prices, without retail markup, markdown or commission, and may not represent actual transactions. Consequently, the information provided below may not be indicative of our common stock price under different conditions.
Trades in our common stock may be subject to Rule 15g-9 of the Exchange Act, which imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction before the sale.
| Period Ended | | |
| Year Ending September 30, 2016 | | |
| July 1, 2016 to August 30, 2016 | $3.50 | $0.95 |
| June 30, 2016 | $9.35 | $2.25 |
| March 31, 2016 | $8.04 | $5.00 |
| December 31, 2015 | $9.00 | $4.30 |
| | | |
| Year Ending September 30, 2015 | | |
| September 30, 2015 | $8.00 | $2.80 |
| June 30, 2015 | $13.50 | $6.00 |
| March 31, 2015 | $13.50 | $7.50 |
| December 31, 2014 | $18.00 | $7.50 |
| | | |
| Year Ending September 30, 2014 | | |
| September 30, 2014 | $13.50 | $9.00 |
| June 30, 2014 | $15.00 | $9.00 |
| March 31, 2014 | $16.50 | $10.50 |
| December 31, 2013 | $19.50 | $9.00 |
As of August 30, 2016, the high and low sales price of our common stock was $1.10 per share and $1.15 per share, respectively. As of September 1, 2016, there were 2,356,152 shares of common stock outstanding held by approximately 50 stockholders of record. This number does not include approximately 2,300 beneficial owners whose shares are held in the names of various security brokers, dealers and registered clearing agencies.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock and intend, for the foreseeable future, to retain any future earnings to finance the growth and development of our business. Our future dividend policy will be determined by our Board of Directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2016 and on a pro forma basis to give effect to this offering and other equity transactions since June 30, 2016.
In thousands, except for share and per share data
| | | |
| | | |
| | | |
| Cash and cash equivalents | $277 | $1,808 |
| | | |
| Convertible notes payable | 957 | 810 |
| | | |
| STOCKHOLDERS' DEFICIT | | |
| Series A Convertible Preferred stock | - | - |
| Series B Redeemable Convertible Preferred stock | - | - |
| Series C Convertible Preferred Stock | - | 2 |
| Common stock | 1 | 4 |
| Additional paid in capital | 20,586 | 24,205 |
| Accumulated deficit | (24,761) | (25,803) |
| Total stockholders' (deficit) | (4,174) | (1,592) |
| Total capitalization | $(3,217) | $(782) |
(1) Pro Forma balances include certain activities occurring after June 30,2016, including:
●
Proceeds of $1,250,000 from the assumed exercise of Series E warrants at $0.70 per share.
●
Proceeds of $1,250,000 from the issuance of 350,000 shares Series C Convertible Preferred Stock.
●
Payoff of convertible debentures of $265,000 in August 2016.
●
Payment of $505,000 in cancellation of the Series B Redeemable Preferred Stock.
●
Proceeds from the issuance of $118,000 in convertible notes that closed in August 2016.
●
Payoff of other short term liabilities totaling $400,000 in August 2016.
●
Proceeds from an equity investment for $500,000 that closed in August 2016.
●
Payment of $310,000 in banker fees.
●
Issuance of 945,524 shares of common stock through the exercise of warrants, debt conversions, or in satisfaction of other liabilities totaling $1,124,000.
You should read this table together with the sections entitled "Summary Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and the related notes included elsewhere in this prospectus.
The number of shares of our common stock outstanding before this offering is based on 2,356,152 shares of our common stock outstanding as of September 1, 2016, and excludes, as of that date:
●
50,942 shares of our common stock issuable upon the exercise of outstanding stock options outstanding at a weighted-average exercise price of $18.04 per share;
●
23,334 shares of our common stock issuable upon the conversion of Series A Convertible Preferred Stock;
●
3,188,734 shares of our common stock issuable upon the exercise of outstanding warrants at an average exercise price of $0.89 per share. 1,785,714 shares of our common stock issuable upon the exercise of outstanding warrants, at an exercise price of $0.70, are being registered in this offering. The warrants will expire on or before August 2021;
●
Up to 277,106 shares of our common stock issuable upon the exercise of placement agent warrants exercisable at $1.12 per share.
●
An unknown number of shares of our common stock issuable upon the conversion of $810,000 of Convertible Notes Payable and an unknown number of our common shares issuable upon the exercise of $710,000 of warrants related to Convertible Notes Payable;
●
27,391 additional shares of our common stock available for future issuance under our 2011Common Stock Incentive Plan;
●
1,785,714 shares of our common stock issuable upon the conversion of Series C Convertible Preferred Stock, at an exercise price of $0.70, subject to certain adjustments. These shares of common stock are being registered in this offering.
The pro forma information discussed above is to illustrate only and will change based on the actual public offering price, number of shares and other terms of this offering determined in pricing.
DILUTION
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
Our historical net tangible book value deficit of ($3,842,000) is the amount of our total tangible assets less our total liabilities as of June 30, 2016 and other equity transactions since June 30, 2016 (1). Net historical tangible book value (deficit) per share of ($1.615) is our historical net tangible book value deficit divided by 1,410,628 shares of common stock outstanding as of June 30, 2016, plus 23,334 shares of common stock issuable upon the conversion of Series A Convertible Preferred Stock and 945,524 shares of common stock that have been issued since June 30, 2016.
Pro forma as adjusted net book value is our pro forma net tangible book value (deficit), after giving effect to the sale of shares of our common stock by the selling stockholder in this offering at a public offering price of $0.70. Our pro forma as adjusted net book value as of June 30, 2016, after giving effectpursuant to this offering and other equity transactions since June 30, 2016, would have been approximately ($1,592,000), or ($0.268) per share. This amount represents an immediate increase in pro forma as adjusted net tangible book value of $1.347 per share to our existing stockholders, and an immediate dilution of $0.968 per share to new investors participating in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the public offering price per share paid by new investors.
The following table illustrates this dilution on a per share basis:
Assumed public offering price per share | | $0.700
|
Pro forma net tangible book value per share as of June 30, 2016 | $(1.615)
| |
Increase in net tangible book value per share attributable to this offering | $1.347
| |
Pro forma as adjusted net tangible book value per share after this offering | | $(0.268)
|
Amount of dilution in net tangible book value per share to new investors in this offering | | $0.968
|
Notes to Dilution Summary Table:
(1)
Historical net tangible book value (deficit) represents the ($4,174,406) balance at June 30, 2016 adjusted for the following activities occurring after June 30,2016:
●
Payoff of convertible debentures of $265,000 in August 2016.
●
Payment of $505,000 in cancellation of the Series B Redeemable Preferred Stock.
●
Proceeds from the issuance of 118,000 in convertible notes that closed in August 2016.
●
Payoff of other short term liabilities totaling $400,000 in August 2016.
●
Proceeds from an equity investment for $500,000 that closed in August 2016.
●
Payment of $310,000 in banker fees.
●
Issuance of 945,524 shares of common stock through the exercise of warrants, debt conversions, or in satisfaction of other liabilities totaling $1,124,000.
The number of shares of our common stock outstanding before this offering is based on 2,356,152 shares of our common stock outstanding as of September 1, 2016, and excludes, as of that date:
●
50,942 shares of our common stock issuable upon the exercise of outstanding stock options outstanding at a weighted-average exercise price of $18.04 per share;
●
23,334 shares of our common stock issuable upon the conversion of Series A Convertible Preferred Stock;
●
3,188,734 shares of our common stock issuable upon the exercise of outstanding warrants at an average exercise price of $0.89 per share. 1,785,714 shares of our common stock issuable upon the exercise of outstanding warrants, at an exercise price of $0.70, are being registered in this offering. The warrants will expire on or before August 2021;
●
Up to 277,106 shares of our common stock issuable upon the exercise of placement agent warrants exercisable at $1.12 per share.
●
An unknown number of shares of our common stock issuable upon the conversion of $810,000 of Convertible Notes Payable and an unknown number of our common shares issuable upon the exercise of $710,000 of warrants related to Convertible Notes Payable;
●
27,391 additional shares of our common stock available for future issuance under our 2011 Stock Incentive Plan;
●
1,785,714 shares of our common stock issuable upon the conversion of Series C Convertible Preferred Stock, at an exercise price of $0.70, subject to certain adjustments. These shares of common stock are being registered in this offering.
We may choose to raise additional capital through the sale of equity or convertible debt securities due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that any of these options or warrants are exercised, new options are issued under our equity incentive plans or we issue additional shares of common stock or other equity securities in the future, there may be further dilution to new investors participating in this offering.
SELLING SECURITY HOLDERS
This prospectus covers the resale by our Selling Stockholder of 3,571,428 shares of common stock, including: (i) up to 1,785,714. shares of common stock issuable upon the conversion of the Company’s Series C Preferred Stock which were sold to Clayton A. Struve (the “Selling Stockholder”), on August 4, 2016 in a private placement, and (ii) 1,785,714 common stock shares issuable upon the exercise of the Selling Stockholder’s Series E Warrants at an exercise price of $0.70 previously issued in connection with the private placement with the Selling Stockholder on August 4, 2016.
We are registering these securities in order to permit the selling stockholder to dispose of its shares of common stock from time to time. The Selling stockholder may decide to sell all, some, or none of the securities listed below. See the section entitled “Plan of Distribution.” We cannot provide an estimate of the number of our securities that the selling stockholder will hold in the future. For purposes of this table, beneficial ownership is determined in accordance with the rules of the SEC, and includes voting power and investment power with respect to such securities.
The Selling Stockholder has had no material relationship with us or our affiliates during the last three years, other than as a purchaser of the Series C Shares from us in the private placement. To our knowledge, the Selling Stockholder is not a registered broker-dealer or an affiliate of a broker-dealer
The table below lists the selling stockholder and other information regarding the beneficial ownership of the shares of common stock by the selling stockholder. Column B lists the number of shares of common stock beneficially owned by the selling stockholder prior to this offering. Column C lists the shares of common stock covered by this prospectus that may be disposed of by the selling stockholder. Column D lists the number of shares of common stock that will be beneficially owned by the selling stockholder assuming all of the shares covered by this prospectus are sold. Column E lists the percentage of shares of common stock that will be beneficially owned by the selling stockholder assuming all of the shares covered by this prospectus are sold. Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act.
| | | | |
| | | | |
| | | | |
| | | | |
Name of Selling Shareholder (A) | | | | |
Clayton A. Struve | -
| -
| 3,571,428
| *
|
| | | | |
| | | | |
| -
| -
| 3,571,428
| *
|
*Less than 1% ownership.
DESCRIPTION OF SERIES C PREFERRED STOCK AND WARRANT PURCHASE AGREEMENT
We currently have 5,000,000 shares of Preferred Stock, par value of $0.001 authorized. On August 4, 2016, we authorized the designation 1,784,715 shares of Series C Convertible Preferred Stock (“Series C Preferred”). On August 11, 2016, we applied with the State of Nevada for approval of the Certificate of Designations, Preferences, and Rights of Series C Convertible Preferred Stock. The Series C Preferred Stock is convertible into shares of common stock at a price of $0.70 per share or by multiplying the number of Series C Preferred Stock shares by the stated value and dividing by the conversion price then in effect, subject to certain diluted events, and has the right to vote the number of shares of common stock the Series C Preferred Stock would be issuable on conversion, subject to a 4.99% blocker.
On August 5, 2016, we closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The Preferred Series C has an annual yield of 8% and an ownership blocker of 4.99%. In addition, the investor received 100% warrant coverage with five year warrants having a strike price of $0.70. The underlying common stock upon the conversion of the Series C Preferred and Series E Warrants issued were required to be included in a registration statement as filed by the Company.
Garden State Securities, Inc., a FINRA member, acted as our exclusive placement agent. They received a 10% cash fee and 250,000 5 year warrants at an exercise price of $1.00.
PLAN OF DISTRIBUTION
We are registering under this prospectus 1,785,714 shares of common stock issuable upon the conversion of Series C Preferred Stock and 1,785,714 shares of common stock issuable upon exercise of the Series E Warrants to purchase shares of our common stock, that may be issued by us to the Selling Stockholder in order to permit the resale of these shares of common stock. The Series C Convertible Preferred Stock was sold to the Selling Stockholder on August 4, 2016, and we are required under the terms of the Preferred Stock and Warrant Purchase Agreement between the Company and the investor to register the common stock issuable upon conversion of the Series C Convertible Preferred Stock and Series E Warrants. We are not selling any shares of our common stock in this offering and, as a result, we will not receive anyprospectus. All proceeds from the sale of the common stock covered by this prospectus. Allshares will be for the account of the net proceeds from the sale of our common stock will go to the Selling Stockholder. Upon exercise of the Series E Warrants, however, we will receive up to $0.70 per share or such lower price asselling stockholder. The selling stockholder may result from the anti-dilution protection features of such warrants. Any proceeds received from the exercise of such warrants will be used for general working capital and other corporate purposes. Under the terms of the Preferred Stock and Warrant Purchase Agreement, we have agreed to pay all fees and expenses incident to our obligation to registersell these shares of common stock.
The Selling Stockholder may decide not to sell any of its shares of common stock, or may sell all or a portion of its shares of common stock. The Selling Stockholder will act independently of us in making decisions with respect to the timing, manner and size of any sale of shares, and may sell the shares directly or through one or more broker-dealers or agents. To the extent that the Selling Stockholder employs broker-dealers or other agents in connection with the sale of its stock, the Selling Stockholder will pay any commissions, discounts or other amounts due to such broker-dealers or agents. To our knowledge, the selling stockholder has not entered into any agreement, arrangement or understanding with any particular broker-dealer oropen market maker with respect to the sale or distribution of the shares of common stock offered hereby.
The Selling Stockholder, which as used herein includes its donees, pledgees, transferees or other successors-in-interest selling shares of common stock or interests in shares of common stock received after the date of this prospectus from a Selling Stockholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise, dispose of any or all of its shares of common stock or interests in shares of common stock on any stock exchange,at market or trading facility on which the shares are traded, or in private transactions. These dispositions may be at fixed prices at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The Selling Stockholder may useselling stockholder will pay any one or more of the following methods when disposing of shares or interests therein:
-
ordinary brokerage transactionsunderwriting discounts and transactions in which the broker-dealer solicits purchasers;
-
block trades in which the broker-dealer will attempt to sell the shares as agent, but may positioncommissions and resell a portion of the block as principal to facilitate the transaction;
-
purchases by a broker-dealer as principal and resaleexpenses incurred by the broker-dealerselling stockholder for its account;
-
an exchange distribution in accordance with the rules of the applicable exchange;
-
privately negotiated transactions;
-
short sales effected after the date the Registration Statement of which this prospectus is a part is declared effectivebrokerage or legal services or any other expenses incurred by the SEC;
-
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
-
broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share;
-
a combination of any such methods of sale; and
-
any other method permitted by applicable law.
The Selling Stockholder may, from time to time, pledge or grant a security intereststockholder in some or alldisposing of the shares of common stock owned by it and, if it defaultsincluded in the performance of its secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of Selling Stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The Selling Stockholder also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling stockholders for purposes of this prospectus.
In connection with the sale of our common stock or interests therein, the Selling Stockholder may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The Selling Stockholder may also sell shares of our common stock short and deliver these securities to close out its short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The Selling Stockholder may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus as supplemented or amended to reflect such transaction.
The aggregate proceeds to the Selling Stockholder from the sale of the common stock will be the purchase price of the common stock less discounts or commissions, if any. The Selling Stockholder reserves the right to accept and, together with its agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any ofbear all other costs, fees and expenses incurred in effecting the proceeds from these stock sales by the Selling Stockholder.
The Selling Stockholder also may resell all or a portion of its shares of common stock in open market transactions in reliance upon Rule 144 under the Securities Act of 1933, provided that it meets the criteria and conform to the requirements of that rule.
To the extent required, the shares of our common stock to be sold, the names of the Selling Stockholder(s), the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the Registration Statement that includes this prospectus.
In order to comply with the securities laws of some states, if applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the Selling Stockholder that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the Selling Stockholder and its affiliates. In addition, to the extent applicable we will make copies of this prospectus as it may be supplemented or amended from time to time available to the Selling Stockholder for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Stockholder may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the Selling Stockholder against liabilities, including liabilities under the Securities Act and state securities laws, arising out of or based upon any untrue statement or alleged untrue statement of a material fact contained in the Registration Statement, prospectus, prospectus supplement, or any information incorporated by reference therein, or arising out of or based upon any omission or alleged omission to state a material fact necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading; provided, however, that we will not be liable for any liabilities finally adjudicated to be caused solely by a false statement of material fact contained within written information provided by such the Selling Stockholder expressly for the purpose of including it in this Registration Statement or the prospectus that is part of this Registration Statement.
We also have agreed with the Selling Stockholder to keep the Registration Statement of which this prospectus constitutes a part effective until the earlier of (1) the date on which all of the shares covered by this prospectus,
have been sold,including all registration and filing fees and fees and expenses of our counsel and accountants.DETERMINATION OF OFFERING PRICE
The selling stockholder may sell these shares in the over-the-counter market or (2)otherwise, at market prices prevailing at the date on which alltime of sale, at prices related to the prevailing market price, or at negotiated prices. We will not receive any proceeds from the sale of shares may be sold without restriction pursuant to Rule 144 ofby the Securities Act.
You should read theThe following discussion and analysis summarizes the significant factors affecting our operating results, financial condition, liquidity and cash flows of our financial conditioncompany as of and results of operations togetherfor the periods presented below. The following discussion and analysis should be read in conjunction with our financial statements and the related notes appearing at the end ofthereto included elsewhere in this prospectus. SomeThe discussion contains forward-looking statements that are based on the beliefs of themanagement, as well as assumptions made by, and information containedcurrently available to, our management. Actual results could differ materially from those discussed in this discussionor implied by forward-looking statements as a result of various factors, including those discussed below and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should read the "Risk Factors" section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements containedparticularly in the following discussionsections titled “Risk Factors” and analysis.
“Cautionary Statement Regarding Forward-Looking Statements.”BUSINESS
Overview
Visualant was incorporatedKnow Labs is an emerging leader in 1998. Since 2007 we have beennon-invasive medical diagnostics. We are focused primarily on the development and commercialization of aour proprietary sensor technology whichutilizing radio and microwave spectroscopy. When paired with our machine learning platform, our technology is capable of uniquely identifying and authenticatingmeasuring almost any material or analyte using electromagnetic energy to detect, record, identify, and measure the unique “signature” of said materials or analytes.
The first application of our sensor technology is in a product to non-invasively monitor blood glucose levels. Our device will provide the user with real-time information on their blood glucose levels. We recently announced our Generation 1 working prototype device. This device embodies the sensor which has been used in internal clinical testing. We have also announced the work our R&D team is doing on the Generation 2 of our device, which is a wearable format and could be a final form factor, ready for commercial application. We are expanding our testing, both internally and externally, and will refine the device over time, which will require FDA clearance before entering the market.
Following FDA clearance of our non-invasive blood glucose monitoring device, Know Labs plans to expand its sensor technology to other non-invasive medical diagnostic applications. As a platform technology, it can identify numerous other analytes in the human body that are important in medical diagnostics and human health and wellness.
While medical diagnostics applications, with blood glucose monitoring paramount, are the focus of Know Labs, the Company’s proprietary radio frequency and microwave spectroscopy platform have broad applicability outside of the medical diagnostic realm. Over time, as resources allow, the Company will explore those opportunities.
Corporate History and Structure
Know Labs, Inc. was incorporated under the laws of the State of Nevada in 1998. Since 2007, our company has been focused primarily on research and development of proprietary spectroscopic technologies spanning the electromagnetic spectrum.
Know Labs has one wholly owned subsidiary, Particle, Inc. incorporated on April 30, 2020. AI Mind, Inc., Know Labs’ former wholly owned subsidiary, was incorporated on September 17, 2021 and dissolved in early 2023. At this time there is no material activity in the Particle subsidiary while the Company gives all of its attention to its focus on its sensor technology and glucose monitoring device development.
The Know Labs Technology
We have internally and under contract with third parties developed proprietary platform technology to uniquely identify and measure almost any organic and inorganic material or analyte. Our patented technology directs electromagnetic energy in the radio wave and microwave frequencies through a substance using lightor material to capture a unique molecular signature. We then perform analytics which will allow the Company to accurately identify and measure materials and analytes.
Our technology provides a unique platform upon which a myriad of applications can be developed. Our radio frequency spectroscopy technology is an “enabling” technology that brings the science of electromagnetic energy to low-cost, real-world commercialization opportunities across multiple industries. The technology is foundational and, as such, the basis upon which we believe significant businesses can be built. While we are pursuing our core focus on commercializing our non-invasive glucose monitor, we believe non-core clinical, non-clinical and medical research applications represent a multitude of opportunities for strategic collaboration, joint development, and licensing agreements with leading companies in their respective industries.
We believe an important competitive differentiator for our sensor technology to be its ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, and in real-time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring.
Know Labs Sensor Technology: Hardware and Software
Our sensor technology embodies two key components: hardware and software. The key hardware component includes a sensor which both sends and receives a radio frequency signal. The data obtained by the receiving aspect of the sensor is analyzed by software. Today, the sensor portion of our hardware development is complete. This sensor is currently being used in our internal tests, and has been for the past several months, gathering millions of data points to further refine our algorithm. It is the core component in our Generation 1 working prototype device and the Generation 2 device under development. This sensor technology will be the core component of future versions of our devices.
As a consequence, a significant amount of our focus has shifted to algorithm development. This involves sophisticated development of algorithms which derive meaningful information from the raw data obtained by our sensor. These algorithms are developed through the utilization of machine learning (ML) by means of training various models. We will continue data collection to further refine the accuracy of the algorithm until we feel confident that we can be successful in FDA clinical trials and bring to the market the first non-invasive blood glucose monitor.
Early Results
We previously announced the results of an internal exploratory study comparing tests between our sensor technology and the leading continuous glucose monitors from Abbott Labs (Freestyle Libre®) and DexCom (G6®). These results provided evidence of a high degree of correlation between our technology and the current industry leaders and their continuous glucose monitors. Our patented technology is fundamentally differentiated from these industry leaders as our technology completely non-invasively monitors blood glucose levels. We also believe our technology successfully addresses the limiting qualities of non-invasive optical technologies whose diagnostic capacities may be inhibited by skin tones and other factors.
We are currently underway with an internal study comparing data from our sensor technology and lab-based reference devices, the Nova Primary and Nova Stat Strip. We have also expanded our study to include participants with diabetes, an important step in clinical development. These studies will allow us to further refine our algorithm and obtain results compared to lab-based reference devices, which will be required for future FDA clearance.
We continue to build the internal and external development team necessary to commercialize our technology. Our ability to obtain exacting results from the data collected through our sensor technology is enabled by our trade secret algorithms built through our machine learning platform. We have been and continue to refine these algorithms so they can accurately determine blood glucose levels across a broad population. We believe our algorithms can also provide accurate measurements for blood alcohol and blood oxygen levels, which we have identified in preliminary tests. We expect them to provide the analytics for the long list of other potential analytes in the human body many of which are set forth in our issued patent USPTO 11,033,208 B1.
Validation and FDA Clearance
We are also focused on building strong external validation of the technology. This on-going initiative should provide additional evidence and support as we look to approach FDA approval. Over the past year, we have announced several significant validating studies. They include:
The results of a proof-of-principle study titled, “Detecting Unique Analyte-Specific Radio Frequency Spectral Responses in Liquid Solutions, Implications for Non-Invasive Physiologic Monitoring.” This study was conducted in collaboration with Mayo Clinic, sponsored by the Company, and its results were presented at the “photon” level to detect2023 American Physiological Society (APS) Summit. The study demonstrated the unique digital “signature”accuracy of the substance. sensor in quantifying three different analytes in vitro. In the peer-reviewed publication, it was found Bio-RFID achieved 100% accuracy in quantifying these three different analytes in vitro. The study was peer-reviewed by Sensors Journal and American Physiology Society.
The results of our technical feasibility study titled, “Technical Feasibility of a Novel Sensor for Non-Invasive Blood Glucose Monitoring Compared to Dexcom G6®.” These results were presented at the American Association of Clinical Endocrinology (AACE) Annual Meeting in Seattle, WA on May 5, 2023. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle. The purpose of this technical feasibility study was to demonstrate hardware and software infrastructure stability, and to collect additional data to determine the accuracy of the sensor at quantifying BGCin vivo non-invasively using radio frequency by means of training a neural network (NN) model to predict readings of the Dexcom G6® as a proxy for BGC. The study was peer-reviewed by the American Association of Clinical Endocrinology.
The results of a new study titled, "Algorithm Refinement in the Non-Invasive Detection of Blood Glucose Using Know Labs’ Bio-RFID Technology." The study demonstrates that algorithm optimization using a light gradient-boosting machine (lightGBM) machine learning model improved the accuracy of Know Labs’ Bio-RFID™ sensor technology at quantifying blood glucose using predicted readings of the Dexcom G6® as a proxy for BGC, demonstrating an overall Mean Absolute Relative Difference (MARD) of 12.9% – which is within the range of independently reported values for certain FDA-cleared blood glucose monitoring devices. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle, and reviewed by members of Know Labs' Scientific Advisory Board.
The results from a 2023 study titled, "Novel data preprocessing techniques in an expanded dataset improve machine learning model accuracy for a non-invasive blood glucose monitor." The study demonstrates that continued algorithm refinement and more high-quality data improved the accuracy of Know Labs’ proprietary Bio-RFID sensor technology, resulting in an overall Mean Absolute Relative Difference (MARD) of 11.3%. As with all Know Labs’ previous research, this study was designed to assess the ability of the Bio-RFID sensor to non-invasively and continuously quantify blood glucose, using the Dexcom G6® continuous glucose monitor (CGM) as a reference device and proxy for BGC. In this new study where data collection was completed in May of 2023, Know Labs applied novel data preprocessing techniques and trained a light gradient-boosting machine (lightGBM) model to predict blood glucose values of Dexcom G6® CGM using 3,311 observations – or reference device values – from over 330 hours of data collected from 13 healthy participants. With this method, Know Labs was able to predict blood glucose in the test set – the dataset that provides a blind evaluation of model performance – with a MARD of 11.3%. The study was performed by the Know Labs Clinical Development Team at Know Labs Research Laboratory in Seattle and reviewed by members of Know Labs' Scientific Advisory Board.
The interim results from a 2024 study titled, “Non-Invasive Blood Glucose Monitoring in People with Diabetes Using an RF Sensor and Venous Blood Comparator” presented at the 17th Annual ATTD Conference in Florence, Italy on March 6, 2024. The study assessed the accuracy of Know Labs’ proprietary radiofrequency (RF) dielectric sensor in non-invasively measuring blood glucose in participants with prediabetes and Type 2 diabetes using venous blood as a comparative reference – resulting in an overall MARD of 11.1%. As with all Know Labs’ previous research, this study was designed to assess the ability of the Bio-RFID sensor to non-invasively and continuously quantify blood glucose, using the Dexcom G6® CGM as a reference device and proxy for BGC. These interim results are from a preliminary portion of a larger study where data collection is ongoing. Know Labs applied novel data preprocessing techniques and trained a lightGBM model to predict blood glucose values of Dexcom G6® CGM. With this method, Know Labs was able to predict blood glucose in the test set – the dataset that provides a blind evaluation of model performance – with a MARD of 11.1%.
As the Company successfully completed our foundational studies, created a stable sensor that delivers repeatable results, and developed a software infrastructure to manage and interpret large, novel datasets, it will continue to expand its testing and data gathering with larger and more diverse populations in order to continue increasing the accuracy of our algorithms across diverse populations.
We callhave also begun the internal and external process to pursue FDA clearance for our non-invasive blood glucose monitor. Our Chief Medical Officer, medical and regulatory advisory board, our entire executive team along with external advisors guide us in this process. Additionally, our “ChromaID™” technology.
third-party quality assurance and documentation consultants help ensure that the rigorous requirements of FDA are met. We are unable to estimate the time necessary for FDA approval or the likelihood of success in that endeavor.Product Strategy
We have announced the development of our non-invasive glucose monitor and our desire to obtain FDA clearance for the marketing of this product. We are currently undertaking internal development work of this product for the commercial marketplace. We have also announced the engagement of several strategic partners and advisors focused on sensor technology, product design, data science, machine learning, manufacturing and regulatory affairs, who we will work with to bring this product to market. The announcement of our Generation 1 working prototype device was a significant milestone for the Company. It has been used in internal testing and sensor characterization work to inform the development of our Generation 2 prototype device. We have also announced the work being done on the Generation 2 prototype device, which is a wearable format and may be a potential final format that would be presented to the FDA for market clearance. We will make further announcements regarding the product as development, testing, manufacturing, and regulatory approval work progresses.
Our efforts are entirely focused on productizing our sensor technology and collecting high quality data for validation purposes, including third-party studies, and appropriate and required clinical trials. At this point in our development cycle, the hardware continues to be miniaturized and optimized, the product form factor is moving in the direction of a final product that will be used for FDA clinical trials and the algorithms which provide results from the data collected by our sensor are being refined to improve accuracy.
Sales and Marketing
While we continue with our internal development efforts and the move toward clinical trials for FDA clearance of our non-invasive blood glucose monitor, we will explore the several potential avenues for moving our first product and potential follow-on products into the marketplace. The avenues being explored include direct to consumer, initial launch partners, broad distribution partners, licensing partners and private label approaches to the market, among others. As part of our growth strategy, we have begun discussions around joint development agreements with potential biopharma, medical device, and consumer electronics partners. These would be strategic collaborations that could help us accelerate development and commercial launch. In 2010,parallel, we acquired TransTech Systems, Inc.have begun to build our internal commercial and marketing team in preparation for detailed strategic thinking about the optimal approach to the marketplace. We attend and engage in conferences focused on diabetes management and technology, which are valuable for building Know Labs’ reputation and network in the space.
Competition
The technology industry, generally, and blood glucose monitoring and other medical diagnostic markets in particular, are intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities by industry participants. To compete successfully, we will need to demonstrate the advantages of our products and technologies over well-established alternative solutions, products, and technologies, including legacy providers of blood glucose monitoring technology, as an adjunctwell as newer ones that are working to achieve a non-invasive solution or more acceptable blood glucose monitoring solutions which may or may not be similar to our business. TransTech istechnology, and convince consumers and enterprises of the advantages of our products and technologies.
We group our competition into three large categories. Those are (i) large global technology companies who may enter the blood glucose monitoring and other medical diagnostic markets, (ii) legacy providers of blood glucose monitoring technology, and (iii) new entrants working to achieve a distributornon-invasive solution or more acceptable blood glucose monitoring solutions which may or may not be similar to our technology. With regard to companies in each category, we perform due diligence from all publicly available sources of information on their relevant technologies and their product plans. This information informs and refines our activities and underscores our sense of urgency as we work to bring our own technology to the marketplace. As it relates to all competitors, we continue to focus on building the world’s most robust patent portfolio in this space. PatSnap Research and ipCapital Group, two leading patent analytic firms, have ranked Know Labs #1 for global patent leadership in non-invasive glucose monitoring patents. We have retained both organizations to perform patent related work. We continue to build out our patent portfolio and grow our trade secret AI and ML driven algorithms. Patents issued, pending, and in-process increased from 107 to 264 year over year (+147% vs. market +35%) reflecting our high rate of innovation.
With respect to our planned non-invasive glucose monitor, we will face direct and indirect competition from a number of competitors who have developed or are developing products for employeecontinuous monitoring of glucose levels. These competitors include DexCom, Inc., Abbott Laboratories, Medtronic plc, Roche Diagnostics, LifeScan, Inc., Ascensia Diabetes Care Holdings AG, Senseonics Holdings, Inc., Integrity Applications, Inc., Nemaura Medical, Biolinq Inc., and personnel identification. TransTech currently provides substantially allProfusa, Inc. Our planned solution will also compete with traditional glucometers, which remain an inexpensive alternative. We also compete with companies who are seeking to create non-invasive glucose monitors, such as Movano, Inc., Hagar, and DiaMonTech AG. Because of the large size of the potential market for our products, it is possible that new or existing competitors may develop competing products, procedures, or clinical solutions that could prove to be more effective, safer, or less costly than our solution. The introduction of new products, procedures, or clinical solutions by competitors may result in price reductions, reduced margins, or loss of market share, or may render our products obsolete. Many of the companies we will compete with enjoy significantly greater name recognition and have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and sales and marketing of approved products than we have.
Mergers and acquisitions in the medical device, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our revenues. competitors. Other small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. There are also several academic and other institutions involved in various phases of technology development regarding blood glucose monitoring devices.
Competitive Advantages
We intend, however,believe our key competitive strengths include:
| · | Through first principles, our sensor technology’s ability to not only identify a wide range of organic and inorganic materials and analytes, but to do so non-invasively, accurately, and in real time, which potentially enables new multivariate models of clinical diagnostics, and health and wellness monitoring. |
| | |
| · | Our sensor technology is non-invasive, using radio waves to identify and measure what is going on inside the body. |
| | |
| · | Our sensor technology platform can be integrated into a variety of wearable, mobile, or counter-top form factors, and we believe eventual interoperability with existing products from current market leaders. |
| | |
| · | No needles nor invasive transmitters in your body, making our sensor convenient and pain-free. |
| | |
| · | No expensive supplies, such as test strips and lancets, are required to operate our device. |
| | |
| · | A core focus on accessibility and affordability for the populations we will serve around the globe. |
| | |
| · | The current prototype sensor collects approximately 1.5 million data points per hour, which allows us to potentially build a deep understanding of health and wellness that other sensors may not be able to. |
| | |
| · | Know Labs is the world intellectual property leader in non-invasive blood glucose monitoring, according to ipCG Capital and PatSnap. |
Growth Strategy
The key elements of our strategy to use a majoritygrow our business include:
| · | Initially, entering the diabetes glucose monitoring market with our non-invasive glucose monitoring device. |
| | |
| · | Following our entry into the glucose monitoring market, entering other clinical monitoring markets for continuous, non-invasive hormone, medication metabolites, endocrinology components, and biomolecular monitoring. |
| | |
| · | Applying our platform technology to lifestyle analysis, clinical trials, and chronic illnesses. We believe that potential use cases include real-time wearable medication monitoring and detection of, for example, ovulation and hormone deficiency. |
| | |
| · | With a potential ever-growing body of non-invasively determined analytes available from individuals utilizing our technology we believe, over time, with longitudinal data we will be able to engage in so-called “predictive health” and provide early warnings of the onset of disease. |
| | |
| · | Significantly, every new application will likely function utilizing the same sensor. We expect that hardware changes will not be required to target new analytes, so you will not need a new device, but an updated software algorithm will be required. |
| | |
| · | Each new application provides potential new opportunities for monetization of the platform technology. Each additional analyte we identify over time may require its own subsequent FDA clearance. |
Research and Development
Our current research and development efforts are primarily focused on improving our radio frequency spectroscopy technology for the monitoring of the proceedsblood glucose. As part of this offeringeffort, we continuously perform clinical testing of our devices following IRB-approved protocols, and we conduct on-going laboratory testing to further developensure that application methods are compatible with the end-user and marketregulatory requirements, and that they can be implemented in a cost-effective manner. As resources permit, we plan to focus on extending the capacity of our ChromaID technology.
sensor technology to identify new analytes and applications. Our current internal team along with outside consultants have considerable experience working with the application of our technologies. We
are inengage third party experts as required to supplement our internal team. We incurred expenses of $1,486,000 and $1,743,000 for the
processthree months ended December 31, 2023 and 2022, respectively, on development activities.The cornerstone of commercializing our ChromaID™ technology.foundational platform technology is our intellectual property portfolio. We have pursued an active intellectual property strategy which includes focus on patents where appropriate and a diligent protection of trade secrets. To date, we have entered into one License Agreement with Sumitomo Precision Products Co., Ltd.been granted 33 patents and 26 design patents. These include 13 patents on our early work on the visible and near visible portions of the electromagnetic spectrum, which were a point of creative departure as we explored and invented our current radio frequency sensor technology. These also include 9 patents related to our Particle subsidiary. We currently have a strategic relationshipnumber of patents pending and continue, on a regular basis, with IDMC. Failurethe filing of new patents. If we include pending patents, our IP portfolio reaches 261 patents issued and pending, which positions the company as the top worldwide IP holder in non-invasive blood glucose monitoring, according to sellipCapital Group, a leading IP and innovation consulting firm. We possess all rights, title and interest to the issued patents.
Our issued patents will expire at various times between 2027 and 2047. Pending patents, if and when issued, may have expiration dates that extend further in time. The duration of our ChromaID products, granttrademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The issued patents cover the fundamental aspects of our radio frequency spectroscopy technology and a number of unique applications. We have filed patents, which are pending, on the additional licensesfundamental aspects of our technology and obtain royaltiesgrowing number of unique applications. We will continue, over time, to expand our patent portfolio.
Additionally, significant aspects of our technology are maintained as trade secrets which may not be disclosed through the patent filing process. We are diligent in maintaining and securing our trade secrets, in particular as they involve our AI and ML driven algorithms.
We shall also have an exclusive, perpetual and royalty free right to any patent(s) or other intellectual property which Phillip Bosua, someone working under direction of Phillip Bosua, or any successor or assignee develops, relating to Know Labs’ technology within a period of five years after January 23, 2023.
Related Patent Assets
Inherent in a platform technology is the ability to develop or license technology in diverse fields of use apart from our core focus. We focus on human health and wellness with a first focus on the non-invasive monitoring of blood glucose. We plan to pursue the identification of a multitude of analytes in the human body that are important to diagnostics over time. We also plan to identify, over time, opportunities for our intellectual property to be deployed in areas outside of human health and wellness.
We may, although we cannot guarantee that we will, create other revenue streamssuch subsidiaries over time. Additionally, we may license our intellectual property to third parties so that they may pursue activities that are not a part of our core focus.
Employees
As of December 31, 2023, we had 12 full-time and part-time employees. Our senior management and other personnel are co-located in our Seattle, Washington offices and remote. The Company expanded its utilization of consulting firms and individual contractors to supplement our reduced workforce in an effort to reduce fixed expenses and extend operating resources.
RESULTS OF OPERATIONS
Overview
We are focused on the development and commercialization of our proprietary sensor technology utilizing radio and microwave spectroscopy. When paired with our machine learning platform, our technology is capable of uniquely identifying and measuring almost any material or analyte using electromagnetic energy to detect, record, identify, and measure the unique “signature” of said materials or analytes. The first application of our sensor technology is in a product to non-invasively monitor blood glucose levels. This device will require US Food and Drug Administration (FDA) clearance before entering the market.
On April 30, 2020, we incorporated our wholly owned subsidiary, Particle, Inc. Particle was focused on the development and commercialization of our extensive intellectual property relating to electromagnetic energy outside of the medical diagnostic arena, which remains our company’s singular focus. Since incorporation, Particle was engaged in research and development activities on threaded light bulbs that have a material adverse effectwarm white light and can inactivate germs, including bacteria and viruses. Particle is now looking for partners to take this product to market.
On September 17, 2021, we incorporated our wholly owned subsidiary, AI Mind, Inc., for the purpose of identifying and capitalizing on market opportunities for our business, financial conditionAI deep learning platform (discussed below). The first activity undertaken by AI Mind was the creation of graphical images expressed as non-fungible tokens, or NFTs, utilizing the AI deep learning platform. During the year ended September 30, 2022,AI Mind, operating our AI deep learning platform, began generating revenue from digital asset sales of NFT’s and resultshad sales of operations.
$4,360,000. AI Mind was dissolved on July 25, 2023.Recent Developments
On September 15, 2023, we signed amendments to the convertible promissory or OID notes, held by Clayton A. Struve and Ron Erickson, who also serves as our Chief Executive Officer, to extend the due dates to September 30, 2024.
On September 29, 2023, we closed an offering of our common stock pursuant to which we sold 28,000,000 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $5,472,791. As part of the offering, we issued common stock purchase warrants to the underwriter representatives to purchase an aggregate of 1,960,000 shares of Common Stock at an exercise price of $0.25 per share, subject to adjustments. The warrants are immediately exercisable and may be exercised at any time and from time to time, in whole or in part, until September 26, 2028 and may be exercised on a cashless basis.
On October 26, 2023, we closed an offering of our common stock pursuant to which we sold 883,061 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $203,105. We believeissued common stock purchase warrants to Boustead Securities, LLC and The Benchmark Company, LLC to purchase an aggregate of 123,648 shares of Common Stock at an exercise price of $0.25 per share, subject to adjustments.
Based upon the modified terms and conditions of our Series C preferred stock and Series D preferred stock certificates of designations, it was determined that Series C preferred stock and Series D preferred stock dividends need to be accreted going forward. As of December 31, 2023, cumulative unpaid Series C preferred stock and Series D preferred stock dividends totaled approximately $800,000, which on a converted-to-common-stock basis represents approximately 3,202,000 shares of common stock. We have recorded $3,590,283 in cumulative deemed dividends related to Series C preferred stock and D preferred stock which have not been paid, net of the approximately $351,000 of accumulated dividends with respect to the Series D preferred stock that were settled for 1,402,784 shares of common stock. Mr. Struve is subject to an ownership blocker limiting his ownership to 4.99% of our commercialization success is dependent upon our ability to significantly increaseoutstanding shares of common stock and thus the number of customerscommon shares he can receive for dividends. Unpaid accreted stock dividends will be issued to Mr. Struve if he converts preferred stock or if the Board declares a dividend thereon, limited to his 4.99% ownership blocker. Assuming no changes in the amount of outstanding Series C preferred stock or Series D preferred stock ownership, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock, which are issuable if such dividends become payable as additional shares of preferred stock, and such preferred stock is then converted into common stock.
On February 27, 2024, we (a) entered into a securities purchase agreement with the selling stockholder pursuant to which we may issue to the selling stockholder one or more senior convertible notes in the aggregate principal amount of up $14,400,000 for an aggregate purchase price equal to up to $12,000,000 and common stock purchase warrants and (b) issued the Note and the Warrant to the selling stockholder in exchange for a purchase price of $4,000,000.
On March 20, 2024, we entered into an At the Market Offering Agreement with The Benchmark Company, LLC pursuant to which we may, from time to time, offer and sell shares of our common stock through or to The Benchmark Company, LLC as our sales agent or manager in an aggregate amount of up to $5,000,000.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by the following factors:
| · | the ability of our research and development team to produce an FDA clearance quality technology; |
| | |
| · | our ability to recruit and maintain quality personnel with the talent to bring our technology to the market; |
| | |
| · | the production of market ready products that can sustain FDA clearance quality results; |
| | |
| · | the clearance by FDA after their rigorous clinical trial process of our products for the marketplace; |
| | |
| · | the receptivity of the marketplace and the addressable diabetes community to our new non-invasive glucose monitoring technology; and |
| | |
| · | access to sufficient capital to support us until our products achieve FDA clearance and are accepted in the marketplace. |
Segment Reporting
The Financial Accounting Standards Board, or FASB, Accounting Standard Codification, or ASC, Topic 280, Segment Reporting, requires that are purchasingan enterprise report selected information about reportable segments in its financial reports issued to its stockholders. The Company considers the business to currently have one operating segment: the development of its radio frequency spectroscopy technology with a first focus on non-invasively ascertaining blood glucose levels. Previous segments included (i) Particle, Inc. technology; and using our products. To date we have generated minimal revenue from(ii) AI Mind, Inc. sales of our ChromaIDNFT products. In addition, demandParticle commenced operations in the year ended September 30, 2020. It is now looking for our ChromaID products may not increase as quickly as planned and we may be unablepartners to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in introducingtake the ChromaID technology and related productsproduct to our target markets, we may not be able to generate sufficient revenue to achieve or sustain profitability.
market. AI Mind commenced operations during the year ended September 30, 2022. AI Mind was dissolved on July 25, 2023.Results of Operations
Nine During the Three Months Ended June 30, 2016 Compared to Nine Months Ended June 30, 2015
December 31, 2023 and 2022The following table presents certain consolidated statementsets forth key components of our results of operations information and presentation of that data as a percentage of change from year-to-year.
(dollars in thousands)
| | Nine Months Ended June 30, |
| | | | | |
| | | | | |
| Revenue | $4,586 | $4,825 | $(239) | -5.0% |
| Cost of sales | 3,835 | 4,041 | (206) | 5.1% |
| Gross profit | 751 | 784 | (33) | -4.2% |
| Research and development expenses | 243 | 286 | (43) | 15.0% |
| Selling, general and administrative expenses | 2,297 | 2,349 | (52) | 2.2% |
| Operating loss | (1,789) | (1,851) | 62 | 3.3% |
| Other income (expense): | | | | |
| Interest expense | (255) | (138) | (117) | -84.8% |
| Other (expense) income | (10) | 24 | (34) | -141.7% |
| Gain on change - derivative liability | 1,966 | 1,809 | 157 | 8.7% |
| (Loss) on conversion of debt | (507) | - | (507) | -100.0% |
| Total other income | 1,194 | 1,695 | (501) | -29.6% |
| Loss before income taxes | (595) | (156) | (439) | -281.4% |
| Income taxes - current (benefit) | - | (8) | 8 | -100.0% |
| Net loss | $(595) | $(148) | $(447) | -302.0% |
Sales
Net revenue forduring the ninethree months ended June 30, 2016 decreased $239,000 to $4,586,000 as compared to $4,825,000 for the nine months ended June 30, 2015. The decrease was due to lower sales at TransTech of $4,580,000. TransTech has experienced reduced sales from one printer product line related to a vendor issue.
Cost of Sales
Cost of sales for the nine months ended June 30, 2016 decreased $206,000 to $2,835,000 as compared to $4,041,000 for the nine months ended June 30, 2015. The decrease was due to lower sales at TransTech.
Gross profit was $751,000 for the nine months ended June 30, 2016 as compared to $784,000 for the nine months ended June 30, 2015. Gross profit was 16.4% for the nine months ended June 30, 2016 as compared to 16.3% for the nine months ended June 30, 2015.
December 31, 2023 and 2022 (dollars in thousands).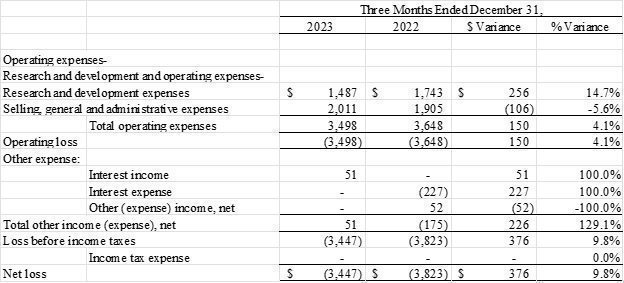
Research and Development Expenses
.Research and development expenses for the ninethree months ended June 30, 2016December 31, 2023 decreased $43,000$256,000 to $243,000$1,487,000 as compared to $286,000$1,743,000 for the ninethree months ended June 30, 2015.December 31, 2022. The decrease was due to decreased personnel, use of consultant, expenditures for suppliers related to the commercializationdevelopment of our ChromaIDradio frequency spectroscopy Bio-RFID™ technology.
During the year ended September 30, 2023, we reduced our headcount by nine and operating expenses and used external consultants to reduce the future cost of the development of our Bio-RFID™ technology.Selling, General and Administrative Expenses
.Selling, general and administrative expenses for the ninethree months ended June 30, 2016 decreased $52,000December 31, 2023 increased $106,000 to $2,297,000$2,011,000 as compared to $2,349,000$1,905,000 for the ninethree months ended June 30, 2015.December 31, 2022. The decreaseincrease primarily was due to (i) an increase of $280,000 in salaries; (ii) a decrease in legal expensesinsurance of $64,000, offset by$101,000; and (iii) a decrease in other expenses of $12,000.$73,000. As part of the selling, general and administrative expenses for the ninethree months ended June 30, 2016,December 31, 2023 and 2022, we incurredrecorded $69,000 and $52,000, respectively, of investor relation expenses of $104,000relationship and business development expenses of $284,000.
expenses.Other Income/Expense
Income (Expense), Net.Other income (expense), net for the ninethree months ended June 30, 2016December 31, 2023 was $1,194,000$51,000 as compared to $1,695,000other expense net of $175,000 for the ninethree months ended June 30, 2015.December 31, 2022. The other income, net for the ninethree months ended June 30, 2016December 31, 2023 included a gain on change - derivative liabilityinterest income of $1,966,000, offset by$51,000.The other expense, net for the three months ended December 31, 2022 included (i) interest expense of $255,000, other expense of $10,000 and loss on settlement of debt of $507,000. The gain on change derivative liability related to derivative instruments included in the June 2013 private placement, the November 2013 IDMC Services and License Agreement, our convertible notes payable and the issuance of Series A Convertible Preferred Stock
and Series B Redeemable Preferred Stock. The other income for the nine months ended June 30, 2015 included other$227,000, offset by (ii) interest income of $24,000 and gain on change - derivative liability of $1,809,000, offset by interest expense of $138,000. The gain on change derivative liability warrants related to derivative instruments included in the June 2013 private placement, the November 2013 IDMC Services and License Agreement, our convertible notes payable and the issuance of Series A Convertible Preferred Stock.
$52,000.Net (Loss) Income
Loss.Net loss for the ninethree months ended June 30, 2016December 31, 2023 was $595,000$3,447,000 as compared to net loss of $148,000$3,823,000 for the ninethree months ended June 30, 2015. NetDecember 31, 2022. The net loss for the ninethree months ended June 30, 2016December 31, 2023 included non-cash other incomeexpenses of $823,000 and included change – derivative liability of $1,966,000, offset by$792,000. The non-cash items include (i) depreciation and amortization of $136,000;$19,000; (ii) issuance of capital stock for services and expenses of $222,000; (iii) stock based compensationcompensation- stock options of $36,000; (iv) expenses related to the issuance of convertible notes payable of $149,000; (v)$699,000; (iii) issuance of common stock related to convertible notes payablefor services of $26,000; and Series B Redeemable Convertible Preferred Stock(iv) amortization of $577,000; and (vi) loss on saleoperating lease right-of-use asset of assets of $23,000. TransTech’s net loss from operations was $97,000 for the nine months ended June 30, 2016 as compared net loss of $98,000 for the nine months ended June 30, 2015.
$48,000. The net loss for the ninethree months ended
June 30, 2015, December 31, 2022 included non-cash incomeexpenses of $1,327,000, including$1,097,000. The non-cash items include (i) gain on change- derivative liability warrants of $1,809,000, (ii) other of $8,000, offset by (iii) depreciation and amortization of $299,000; (iv)$103,000; (ii) stock based compensationcompensation- stock options of $53,000;$744,000; (iii) expenses for extension of notes and warrants of $207,000; (iv) amortization of operating lease right-of-use asset of $44,000 and offset by (v) share and warrant issuancesother of $138,000. We expect losses to continue as we commercialize our ChromaID technology.
Year$1,000.Results of Operations During the Years Ended September 30, 2015 Compared to Year Ended September 30, 2014
2023 and 2022The following table presents certain consolidated statementsets forth key components of our results of operations information and presentation of that data as a percentage of change from year-to-year:
(dollars in thousands).
| | Years Ended September 30, |
| | | | | |
| | | | | |
| Revenue | $6,291 | $7,983 | $(1,692) | -21.2% |
| Cost of sales | 5,274 | 6,694 | (1,420) | 21.2% |
| Gross profit | 1,017 | 1,289 | (272) | -21.1% |
| Research and development expenses | 363 | 670 | (307) | 45.8% |
| Selling, general and administrative expenses | 2,984 | 3,180 | (196) | 6.2% |
| Operating loss | (2,330) | (2,561) | 231 | 9.0% |
| Other income (expense): | | | | |
| Interest expense | (170) | (105) | (65) | -61.9% |
| Other income | 41 | 38 | 3 | 7.9% |
| Loss on conversion of debt | (34) | - | (34) | -100.0% |
| (Loss) gain on change- derivative liability warrants | (108) | 1,605 | (1,713) | -100.0% |
| Total other (expense) income | (271) | 1,538 | (1,809) | -117.6% |
| Loss before income taxes | (2,601) | (1,023) | (1,578) | -154.3% |
| Income taxes - current (provision) benefit | 30 | (6) | 36 | -600.0% |
| Net loss | (2,631) | (1,017) | (1,614) | -158.7% |
| Noncontrolling interest | - | - | - | 0.0% |
| Net loss attributable to Visualant, Inc. common shareholders | $(2,631) | $(1,017) | $(1,614) | -158.7% |
Sales
Net revenue forduring the yearyears ended September 30, 2015 decreased $1,692,000 to $6,291,000 as compared to $7,983,000 for the year ended September 30, 2014. The decrease was due to lower sales at TransTech of $1,692,000 resulting from a reduction2023 and 2022 (dollars in sales from the products of one large vendor.
Cost of Sales
Costthousands).
Revenues. Revenue- digital asset sales for the year ended September 30, 2015 decreased $1,420,000 to $5,274,0002023 was $0 as compared to $6,694,000$4,360,000 for the year ended September 30, 2014. The decrease was due to lower2022. We do not expect future activity or revenue from that source. Our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $4,360,000 from Non-Fungible Token (NFT) sales.
Gross profit was $1,017,000 for the year ended September 30, 2015 as compared to $1,289,000 for the year ended September 30, 2014. Gross profit was 16.2% for the year ended September 30, 2015 as compared to 16.1% for the year ended September 30, 2014.
Research and Development Expenses
.Research and development expenses for the year ended September 30, 2015 decreased $307,0002023 increased $2,341,000 to $363,000$7,727,000 as compared to $670,000$5,386,000 for the year ended September 30, 2014.2022. The decreaseincrease was due to reducedincreased personnel, use of consultants, expenditures for suppliers related to the commercializationdevelopment of our ChromaID technology andradio frequency spectroscopy Bio-RFID™ technology. During the effectend of the Servicesthree months ended June 30, 2023, we reduced our headcount by nine and License Agreement with IDMC, which reduced cash expenditures.
operating expenses and used external consultants to reduce the future cost of the development of our Bio-RFID™ technology. Selling, General and Administrative Expenses
.Selling, general and administrative expenses for the year ended September 30, 20152023 decreased $196,000$1,547,000 to $2,984,000$6,571,000 as compared to $3,180,000$8,118,000 for the year ended September 30, 2014.
2022. The decrease primarily was due to lower expenses at TransTech ($127,000), reduced corporate development ($65,000), reduced amortization ($66,000), and(i) a decrease of $1,466,000 in stock based compensation; (ii) a decrease in other general expenses ($72,000),compensation expense of $451,000 related to warrants issued for services; (iii) a decrease in common stock issued for services of $183,000; offset by increased stock based compensation(iv) an increase in insurance of $420,000; and (v) an increase in other expenses ($134,000).of $133,000. As part of the selling, general and administrative expenses for the year ended September 30, 2015,2023 and 2022, we incurredrecorded $305,000 and $380,000, respectively, of investor relation expenses of $39,000relationship and business development expenses of $105,000.
Other Income (Expense)
Other expenseexpenses. Selling and Transactional Costs for Digital Asset Sales.Selling and transactional for digital asset sales were ($274,000) as compared $3,430,000 for the year ended September 30, 2015 was $271,000 as compared to other income2022. During 2022, our Artificial Intelligence (AI) deep learning platform has generated revenue- digital asset sales of $1,538,000$4,360,000 from Non-Fungible Token (NFT) sales. The benefit for the year ended September 30, 2014. The other expense2023 included an adjustment of our estimate for sales and use tax related to the sale of digital assets. Such costs for the year ended September 30, 20152022 included digital asset conversion loss, consulting, bonus compensation transaction fees, taxes, royalties and other income of $41,000, offset by loss on change - derivative liability of $108,000, interestcosts.
Other (Expense), Net.Other expense, of $170,000. The loss on change derivative liability warrants related to derivative instruments included in the June 2013 private placement, the November 2013 IDMC Services and License Agreement, our convertible notes payable and the issuance of Series A Convertible Preferred Stock.
The incomenet for the year ended September 30,
20142023 was $1,265,000 as compared to other expense, net of $7,497,000 for the year ended September 30, 2022. The other expense, net for the year ended September 30, 2023 included
gain(i) interest income of $127,000; offset by (ii) interest expense of $390,000 related to convertible notes payable and the modification and extension of terms; (iii) loss on
change - derivative liabilitydebt extinguishment of
$1,605,000$507,000 related to the extension of convertible notes payable; and
(iv) other expense of $495,000 related to the write-off of certain equipment.The other expense, net for the year ended September 30, 2022 included (i) interest expense of $8,034,000 related to convertible notes payable and the amortization of the beneficial conversion feature and value of warrants issued; and offset by (ii) other income of $38,000, offset by$522,000 primarily related to the forgiveness of notes payable- PPP loans and other debt and (iii) interest expenseincome of $104,000. The gain on change- derivative liability warrants relates to derivative instruments included in the June 2013 private placement and the November 2013 IDMC Services and License Agreement.
.Net loss for the year ended September 30, 20152023 was $2,631,000$15,289,000 as compared to a net loss of $1,017,000$20,071,000 for the year ended September 30, 2015. The increase was primarily due to an increase in loss on change - derivative liability of $1,713,000. The net loss for the year ended September 30, 2015, included non-cash expenses of $706,000, including (i) loss on change- derivative liability warrants of $108,000, (ii) other of $42,000, offset by (iii) depreciation and amortization of $353,000; (iv) stock based compensation of $65,000; and (v) share and warrant issuances of $138,000. TransTech’s net loss from operations was $194,000 for the year ended September 30, 2015 as compared to a net loss of $64,000 for the year ended September 30, 2014.
2022. The net loss for the year ended September 30, 20142023 included non-cash incomeexpenses of $819,000, including$4,768,000. The non-cash items include (i) depreciation and amortization of $418,000;$313,000; (ii) loss on sale of assets of $550,000; (iii) loss on debt extinguishment of $507,000; (iv) modification of notes and warrants- interest expense of $350,000; (v) stock based compensationcompensation- stock options of $88,000;$2,956,000; and (iii) share and warrant issuances(vi) amortization of $308,000, offset by (i) gain on change- derivative liability warrantsoperating lease right-of-use asset of $1,605,000; and (ii) other of $28,000. TransTech$142,000. The net loss from operations was $64,000 for the year ended
September 30, 2014 2022 included non-cash expenses of $12,164,000. The non-cash items include (i) depreciation and amortization of $321,000; (ii) issuance of common stock for services of $183,000; (iii) issuance of common stock warrants for services of $452,000; (iv) stock based compensation- stock options of $4,422,000; (v) amortization of debt discount as compared to a net lossinterest expense of $406,000 for the year ended September 30, 2013. We expect losses to continue as we commercialize our ChromaID™ technology.
$7,273,000; (vi) other of $35,000; offset by (vii) gain on debt settlement of $269,000; and (viii) gain on forgiveness of note payable- PPP loans of $253,000.Liquidity and Capital Resources
During the Three Months Ended December 31, 2023 and 2022Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures.
We hadhave cash and cash equivalents of $276,000$4,821,000 and net working capital deficit of approximately $4,785,000 (excluding the derivative liability- warrants$3,796,000 (exclusive of $739,000convertible notes payable) as of June 30, 2016.December 31, 2023. We expectanticipate that we will record losses to continue as we commercialize our ChromaID™ technology. Our cash used infrom operations for the nineforeseeable future. As of December 31, 2023, our accumulated deficit was $125,351,000 and net losses in the amount of $3,447,000, $15,289,000 and $20,071,000 during the three months ended JuneDecember 31, 2023 and years ended September 30, 20162023 and 2022, respectively. We incurred non-cash expenses of $792,000, $4,768,000, and $12,164,000 during the three months ended December 31, 2023 and the years ended September 30, 20152023 and 2014 was $1,857,000, $240,000 and $1,379,000,2022, respectively.
We believe that our cash on hand will be sufficient to fund our operations through September 30, 2016.
The opinion of our independent registered public accounting firm on our audited financial statements as of and for the year ended September 30, 2015 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon raising capital from financing transactions.
We need additional financing to implement our business plan and to service our ongoing operations and pay our current debts. There can be no assurance that we will be able to secure any needed funding, or that if such funding is available, the terms or conditions would be acceptable to us. If we are unable to obtain additional financing when it is needed, we will need to restructure our operations, and divest all or a portion of our business. We may seek additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, and could increase our expenses and require that our assets secure such debt. Equity financing, if obtained, could result in dilution to our then-existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected.
We received commitments from debtors to convert $1,000,000 into our common stock as part of our proposed listing on The NASDAQ Capital Market. These conversions are expected to increase stockholder’s equity by $1,000,000.
In connection with the June 2013 Special Situations financing described below under “Liquidity and Capital Resources”, as of September 1, 2016 we have outstanding Series A Warrants to purchase a total of 252,060 shares of common stock with a current exercise price of $0.70 per share, and Series B Warrants to purchase a total of 252,060 shares of common stock with a current exercise price of $0.70 per share, the IDMC warrant to purchase 97,169 shares of common stock with a current exercise price of $0.70 per share, Series C and D Warrants to purchase 23,334 shares of common stock with a current exercise price of $0.70 per share and placement agent warrants to purchase 20,439 shares of common stock at an exercise price of current exercise price of $0.70 per share (collectively, the “Special Situations Warrants”). The Special Situations Warrants contain an adjustment provision that would require an adjustment in the exercise price of the Special Situations Warrants if we issue common stock, warrants or equity below the price that is reflected in the Special Situations Warrants (currently $0.70 per share). If we issue any additional shares of common stock, warrants or other equity securities at a price below the exercise prices of the Special Situations Warrants, it would result in a reduction in the exercise price of the Special Situations Warrants. A downward adjustment in the exercise price of the Special Situations Warrants could also affect the market price of the common stock.
We issued convertible notes payable to investors totaling $710,000 to fund short-term working capital. These notes accrue interest at a rate of 8% per annum, become due during September 2016 to February 2017 and are convertible into common stock as part of our next public financing, at a conversion price equal to the price of the common stock sold in our next public financing. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing.
On August 4, 2016, we closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve for purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred has a yield of 8% and a ownership blocker of 4.99%. In addition, the investor received 100% warrant coverage with five year warrants having a strike price of $0.70. Both the Series C and warrants will be included in this registration statement.
On August 10, 2016, we closed a Stock Purchase Agreement with Dale Broadrick and affiliate of the Company for the purchase of $500,000 of our common stock at $0.70 per share. In addition, the investor received 100% warrant coverage with a five year warrant having a strike price of $0.70. These common shares and warrants are not subject to a registration statement.
We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale common stock, issuance of common stock in conjunction with an equity line of credit, loans by our Chief Executive Officer and the exercise of warrants.
We finance TransTech from operations During the remainder of 2024, we expect to raise additional funds through the issuance of preferred stock, convertible debentures or equity.On September 29, 2023, we closed an offering of our common stock pursuant to which we sold 28,000,000 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and a Secured Credit Facility with Capital Source Business Finance Group. On December 9, 2008, TransTech entered into a $1,000,000 secured credit facility with Capital Source to fund its operations. On June 9, 2016,other offering expenses, we received net proceeds of $5,472,791.
During the secured credit facility was renewed for an additional six months, with a floor for prime interestend of 4.5% (currently 4.5%) plus 2.5%. The eligible borrowing is based on 80% of eligible trade accounts receivable, not to exceed $1,000,000. The secured credit facility is collateralized by the assets of TransTech, with a guarantee by Visualant, including a security interest in all assets of Visualant. Availability under this Secured Credit ranges from $0 to $175,000 ($20,000 as ofquarter ended June 30, 2016)2023, the Company made some adjustments to its staffing level, and the impact of those adjustments, plus the departure of our chief technology and executive officer, has significantly reduced our monthly burn rate. The Company will further adjust its cost structure if new debt or equity capital is not received. We believe that we have enough available cash to operate until August 31, 2024.
The proceeds of warrants currently outstanding, to the extent not exercised on a daily basis. The remaining balance on the accounts receivable linecashless basis, may generate potential proceeds. We cannot provide assurance that any of $747,652 as of June 30, 2016 mustthese warrants will be repaid by the time the secured credit facility expires on December 12, 2016, or we renew by automatic extension for the next successive six-month term.
Nine Months Ended June 30, 2016
exercised.Operating Activities
Net cash used in operating activities for the ninethree months ended June 30, 2016December 31, 2023 and 2022 was $1,857,000. This amount$3,393,000 and $2,917,000, respectively. The net cash used in operating activities for the three months ended December 31, 2023 was primarily related to (i) a net loss of $595,000$3,447,000; (ii) working capital changes of $738,000; and an increase in accounts receivable of $483,000, an increase in inventory of $89,000 and non-cash other income of $823,000 and other of $6,000, offset by an increase in accounts payable(iii) non-cash expenses of $131,000 and other of $8,000.$792,000. The non-cash other income of $823,000 includes change – derivative liability of $1,966,000, offset by (i)items include (iv) depreciation and amortization of $136,000; (ii) issuance of capital stock for services and expenses of $222,000; (iii)$19,000; (v) stock based compensationcompensation- stock options of $36,000; (iv) expenses related to the issuance of convertible notes payable of $149,000; (v)$699,000; (vi) issuance of common stock for services of $26,000; and (vii) amortization of operating lease right-of-use asset of $48,000.
The net cash used in operating activities for the three months ended December 31, 2022 was primarily related to convertible notes payable(i) a net loss of 3,823,000; (ii) working capital changes of $194,000; and Series B Redeemable Convertible Preferred Stock(iii) non-cash expenses of $577,000;$1,097,000.
Investing Activities
Net cash used in investing activities for the three months ended December 31, 2023 and (vi) loss on sale of assets of $23,000.
2022 was $13,000 and $11,000, respectively. There amounts were primarily related to the investment in equipment for research and development.Financing Activities
Net cash provided by financing activities for the ninethree months ended June 30, 2016December 31, 2023 and 2022 was $2,047,000. This amount$203,000 and $15,000, respectively. The net cash provided by financing activities for the three months ended December 31, 2023 was primarily related to issuance of common stock for a common stock offering, net of expenses of $203,000. On October 26, 2023, we closed an offering of our common stock pursuant to which we sold 883,061 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $203,105.
The net cash provided by financing activities for the three months ended December 31, 2022 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $349,000;$13,000; and (ii) proceeds from convertible notes of $925,000; (iii) proceeds from line of credit of $383,000; and (iv) proceeds from the issuance of
Series B Redeemable Preferred Stockcommon stock for the exercise of $505,000, offsetstock option grants of $2,000.Our contractual cash obligations as of December 31, 2023 are summarized in the table below:
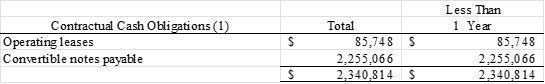
(1) Convertible notes payable includes $2,255,066 (excluding $506,865 adjustment for debt extinguishment accounting) that can be converted into common stock upon demand. We expect to incur capital expenditures related to the development of the “Bio-RFID™” and “ChromaID” technologies. None of the expenditures are contractual obligations as of December 31, 2023.
Liquidity and Capital Resources During the Years Ended September 30, 2023 and 2022
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by the repaymentoperations, levels of accounts receivable and accounts payable and capital expenditures.
We have cash and cash equivalents of $8,024,000 and net working capital of approximately $6,264,000 (exclusive of convertible notes payable) as of $115,000.
Year Ended September 30, 2015
2023. We anticipate that we will record losses from operations for the foreseeable future. As of September 30, 2023, our accumulated deficit was $121,841,000 and net losses in the amount of $15,289,000 and $20,071,000 during the years ended September 30, 2023 and 2022, respectively. We incurred non-cash expenses of $4,768,000, and $12,164,000 during the years ended September 30, 2023 and 2022, respectively.We have financed our corporate operations and our technology development through the issuance of convertible debentures, the issuance of preferred stock, the sale of common stock and the exercise of warrants. During the remainder of 2024, we expect to raise additional funds through the issuance of preferred stock, convertible debentures or equity.
On September 29, 2023, we closed an offering of our common stock pursuant to which we sold 28,000,000 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $5,472,791.
During the end of the quarter ended June 30, 2023, the Company made some adjustments to its staffing level, and the impact of those adjustments, plus the departure of our chief technology and executive officer, has significantly reduced our monthly burn rate. The Company will further adjust its cost structure if new debt or equity capital is not received. We believe that we have enough available cash to operate until August 31, 2024.
The proceeds of warrants currently outstanding, to the extent not exercised on a cashless basis, may generate potential proceeds. We cannot provide assurance that any of these warrants will be exercised.
Operating Activities
Net cash used in operating activities for the year ended September 30, 20152023 and 2022 was $240,000. This amount$10,354,000 and $6,920,000, respectively. The net cash used in operating activities for the year ended September 30, 2023 was primarily related to (i) a net loss of $2,631,000,$15,289,000; offset by (ii) working capital changes of $167,000; and (iii) non-cash otherexpenses of $4,768,000. The non-cash items include (iv) depreciation and amortization of $313,000; (v) loss on disposal assets of $550,000; (vi) loss on debt extinguishment of $507,000; (vii) modification of notes and warrants- interest expense of $706,000, decrease$350,000; (viii) stock based compensation- stock options of $2,956,000; and (ix) amortization of operating lease right-of-use asset of $142,000.
The net cash used in accounts receivableoperating activities for the year ended September 30, 2022 was primarily related to (i) a net loss of $167,000, a decrease in inventory$20,071,000; offset by (ii) working capital changes of $195,000$987,000 related to Our Artificial Intelligence (AI) Deep Learning Platform has generated initial revenue from Non-Fungible Token (NFT) sales and an increase accounts payableincurred certain expenses; and accrued(iii) non-cash expenses of $1,290,000.
$12,164,000. The non-cash items include (iv) depreciation and amortization of $321,000; (v) issuance of common stock for services of $183,000; (vi) issuance of common stock warrants for services of $452,000; (vii) stock based compensation- stock options of $4,422,000; (viii) amortization of debt discount as interest expense of $7,273,000; (ix) other of $13,000; offset by (x) gain on debt settlement of $269,000; and (xi) gain on forgiveness of note payable- PPP loans of $253,000. Investing Activities
Net cash provided byused in investing activities for the year ended September 30, 20152023 and 2022 was $21,000. This amount was$81,000 and $855,000, respectively. There amounts were primarily related to the investment of proceeds from the sale ofin equipment of $21,000.
for research and development. Financing Activities
Net cash provided by financing activities for the year ended September 30, 20152023 and 2022 was $230,000. This amount$5,865,000 and $8,111,000, respectively. The net cash provided by financing activities for the year ended September 30, 2023 was primarily related to (i) proceeds from the saleissuance of preferredcommon stock for the exercise of $350,000 andwarrants of $387,000; (ii) proceeds from convertible notethe issuance of $173,000,common stock for the exercise of stock option grants of $5,000; issuance of common stock for a common stock offering, net of expenses of $5,472,791. On September 29, 2023, we closed an offering of our common stock pursuant to which we sold 28,000,000 shares of common stock, at a purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $5,472,791.
The net cash provided by financing activities for the year ended September 30, 2022 was primarily related to (i) proceeds from the issuance of common stock for the exercise of warrants of $838,000; (ii) proceeds from the issuance of common stock for the exercise of stock option grants of $27,000; issuance of common stock for NYSE uplisting, net of expenses of $7,425,000; and offset by the repayment of linenotes payable- PPP loans of credit$179,000. On September 20, 2022, we completed a public offering of $124,000, repaymentour common stock pursuant to which we sold 4,140,000 shares of convertible notecommon stock, at a purchase price of $167,000$2.00. After deducting underwriting commissions and repaymentother offering expenses, we received net proceeds of $7,425,000.

(1) Convertible notes payable includes $2,255,066 (excluding $506,865 adjustment for debt extinguishment accounting) that can be converted into common stock upon demand. We expect to incur capital leasesexpenditures related to the development of $3,000.
Contractual Obligations
Ourthe “Bio-RFID™” and “ChromaID” technologies. None of the expenditures are contractual cash obligations as of JuneSeptember 30, 2016 are summarized in the table below:
| | | | | | |
| Contractual Cash Obligations | | | | | |
| Operating leases | $23,401 | $23,401 | $- | $- | $- |
| Convertible notes payable | 957,310 | 957,310 | - | - | - |
| Notes payable | 1,547,587 | 1,547,587 | - | - | - |
| Capital expenditures | 100,000 | 20,000 | 40,000 | 40,000 | - |
| | $2,628,298 | $2,548,298 | $40,000 | $40,000 | $- |
2023.Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements (as that term is defined in Item 303 of Regulation S-K) that are reasonably likely to have a current or future material effect on our financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies andInvolving Significant Estimates
The applicationfollowing discussion relates to critical accounting policies for our Company which involve significant estimates. The preparation of financial statements in conformity with United States generally accepted accounting principles, or GAAP, involves the exercise of varying degrees of judgment. On an ongoing basis, we evaluaterequires our management to make assumptions, estimates and judgments based on historical experiencethat affect the amounts reported, including the notes thereto, and various other factorsrelated disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are believedsignificant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Revenue Recognition. We determine revenue recognition from contracts with customers through the following steps:
| · | identification of the contract, or contracts, with the customer; |
| | |
| · | identification of the performance obligations in the contract; |
| | |
| · | determination of the transaction price; |
| | |
| · | allocation of the transaction price to the performance obligations in the contract; and |
| | |
| · | recognition of the revenue when, or as our company satisfies a performance obligation. |
Revenue is recognized when control of the promised goods or services is transferred to the customers, in an amount that reflects the consideration we expect to be reasonable underentitled to in exchange for those goods or services.
Research and Development Expenses. Research and development expenses consist of the circumstances.
Actual results may differ from these estimates under different assumptions or conditions. We believe thatcost of our significant accounting policies (see summary of significant accounting policies more fully describedofficers, employees, consultants and contractors who design, engineer and develop new products and processes as well as materials, supplies and facilities used in Note 2 to the financial statements set forth in this report), the following policies involve a higher degree of judgment and/or complexity:
Inventories – Inventories consist primarily of printers and consumable supplies, including ribbons and cards, badge accessories, capture devices, and access control components held for resale and are stated at the lower of cost or market on the first-in, first-out (“FIFO”) method. Inventories are considered available for resale when drop shipped and invoiced directly to a customer from a vendor, or when physically received by TransTech at a warehouse location. We record a provision for excess and obsolete inventory whenever an impairment has been identified. There is a $20,000 reserve for impaired inventory as of June 30, 2016 and September 30, 2015, respectively.
producing prototypes.Fair Value Measurements and Financial Instruments
–. ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). Revenue Recognition – Visualant and TransTech revenue are derived from other products and services. Revenue is considered realized when the services have been provided to the customer, the work has been accepted by the customer and collectability is reasonably assured. Furthermore, if an actual measurement of revenue cannot be determined, we defer all revenue recognition until such time that an actual measurement can be determined. If during the course of a contract management determines that losses are expected to be incurred, such costs are charged to operations in the period such losses are determined. Revenues are deferred when cash has been received from the customer but the revenue has not been earned.
Stock Based Compensation – We have share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options to purchase shares of our common stock at the fair market value at the time of grant. Stock-based compensation cost is measured by us at the grant date, based on the fair value of the award, over the requisite service period. For options issued to employees, we recognize stock compensation costs utilizing the fair value methodology over the related period of benefit. Grants of stock options and stock to non-employees and other parties are accounted for in accordance with the ASC 505.
Revenue Recognition
Visualant and TransTech revenue are derived from the sale of products and services. Service revenue is considered realized when the services have been provided to the customer, the work has been accepted by the customer and collectability is reasonably assured. Furthermore, if an actual measurement of revenue cannot be determined, we defer all revenue recognition until such time that an actual measurement can be determined. If during the course of a contract management determines that losses are expected to be incurred, such costs are charged to operations in the period such losses are determined. Revenues are deferred when cash has been received from the customer but the revenue has not been earned. The Sumitomo License fee was recorded as revenue over the life the Joint Development Agreement and was fully recorded as of May 31, 2013.
Stock Based Compensation
We have share-based compensation plans under which employees, consultants, suppliers and directors may be granted shares of our restricted common stock, as well as options to purchase shares of our common stock at the fair market value at the time of grant. Stock-based compensation cost is measured by us at the grant date, based on the fair value of the award, over the requisite service period. For options issued to employees, we recognize stock compensation costs utilizing the fair value methodology over the related period of benefit. Grants of stock options and shares of restricted common stock to non-employees and other parties are accounted for in accordance with the ASC 505.
Quantitative and Qualitative Disclosure about Market Risk
We have no investments in any market risk sensitive instruments either held for trading purposes or entered into for other than trading purposes.
DESCRIPTION OF OUR BUSINESS
The Company is focused primarily on the development of a proprietary technology which is capable of uniquely identifying and authenticating almost any substance using light to create, record and detect the unique digital “signature” of the substance. We call this our “ChromaID™” technology.
Our ChromaID™ Technology
We have developed a proprietary technology to uniquely identify and authenticate almost any substance. This patented technology utilizes light at the photon (elementary particle of light) level through a series of emitters and detectors to generate a unique signature or “fingerprint” from a scan of almost any solid, liquid or gaseous material. This signature of reflected or transmitted light is digitized, creating a unique ChromaID signature. Each ChromaID signature is comprised of from hundreds to thousands of specific data points.
The ChromaID technology looks beyond visible light frequencies to areas of near infra-red and ultraviolet light that are outside the humanly visible light spectrum. The data obtained allows us to create a very specific and unique ChromaID signature of the substance for a myriad of authentication and verification applications.
Traditional light-based identification technology, called spectrophotometry, has relied upon a complex system of prisms, mirrors and visible light. Spectrophotometers typically have a higher cost and utilize a form factor more suited to a laboratory setting and require trained laboratory personnel to interpret the information. The ChromaID technology uses lower cost LEDs and photodiodes and specific frequencies of light resulting in a more accurate, portable and easy-to-use solution for a wide variety of applications. The ChromaID technology not only has significant cost advantages as compared to spectrophotometry, it is also completely flexible is size, shape and configuration. The ChromaID scan head can range in size from endoscopic to a scale that could be the size of a large ceiling-mounted florescent light fixture.
In normal operation, a ChromaID master or reference scan is generated and stored in a database. The Visualant scan head can then scan similar materials to identify, authenticate or diagnose them by comparing the new ChromaID digital signature scan to that of the original or reference ChromaID signature or scan result.
ChromaID was invented by scientists from the University of Washington under contract with Visualant. We have pursued an aggressive intellectual property strategy and have been granted ten patents. We also have 20 patents pending. We possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, Intellectual Ventures through its subsidiary IDMC.
In 2010, we acquired TransTech Systems, Inc. (“TransTech”) as an adjunct to our business. TransTech is a distributor of products for employee and personnel identification. TransTech currently provides substantially all of our revenues. We intend, however, to further develop and market our ChromaID technology.
The following summarizes our plans for our proprietary ChromaID technology. Based on our anticipated expenditures on this technology, the expected efforts of our management and our relationship with Intellectual Ventures and its subsidiary, IDMC, and our other strategic partner, Sumitomo Precision Products, Ltd., we expect our ChromaID technology to provide an increasing portion of our revenues in future years from product sales, licenses, royalties and other revenue streams., as discussed further below.
ChromaID: A Foundational Platform Technology
Our ChromaID technology provides a platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. The ChromaID technology is an enabling technology that brings the science of light and photonics to low cost, real world commercialization opportunities across multiple industries. The technology is foundational and as such, the basis upon which we believe a significant business can be built.
As with other foundational technologies, a single application may reach across multiple industries. The ChromaID technology can, for example effectively differentiate and identify different brands of clear vodkas that appear identical to the human eye. By extension this same technology can identify pure water from water with contaminants present. It can provide real time detection of liquid medicines such as morphine that have been adulterated or compromised. It can detect if jet fuel has water contamination present. It could determine when it is time to change oil in a deep fat fryer. These are but a few of the potential applications of the ChromaID technology based upon extensions of its ability to identify different clear liquids.
The cornerstone of a company with a foundational platform technology is its intellectual property. ChromaID was invented by scientists from the University of Washington under contract with Visualant. We have pursued an aggressive intellectual property strategy and have been granted ten patents. We currently have 20 patents pending. We possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, the IDMC subsidiary of Intellectual Ventures.
At the Photonics West trade show held in San Francisco in February 2013, we were honored to receive a PRISM award from the Society of Photo-Optical Instrumentation Engineers International, better known as SPIE. The PRISM awards recognizes photonic products that break with conventional ideas, solve problems, and improve life through the application of light-based technologies.
IDMC Relationship
In November 2013, we entered into a strategic relationship with Invention Development Management Company, a subsidiary of Intellectual Ventures, a private intellectual property fund with over $5 billion under management. Intellectual Ventures owns over 40,000 IP assets and has broad global relationships for the invention of technology, the filing of patents and the licensing of intellectual property. IDMC has worked to expand the reach and the potential application of the ChromaID technology and has filed ten patents base on the ChromaID technology, which it has licensed to us. In connection with IDMC’s work to expand our intellectual property portfolio, we agreed to curtail outbound marketing activities of our technology through the fourth calendar quarter of 2014.
Initial testing in our laboratories and the work of the IDMC inventors have shown that the ChromaID technology has a number of broad and useful applications a few of which include:
●
Milk identification for quality, protein and fat content and impurities
●
Identification of liquids for counterfeits or contaminants
●
Detecting adulterants in food and food products compromising its quality
●
Color grading of diamonds
●
Identifying real cosmetics versus counterfeit cosmetics
●
Identifying counterfeit medications versus real medications
●
Identifying regular flour versus gluten free flour
●
Authenticating secure identification cards
Products
Our first delivered product, the ChromaID Lab Kit, scans and identifies solid surfaces. We are marketing this product to customers who are considering licensing the technology. Target markets include, but are not limited to, commercial paint manufacturers, pharmaceutical equipment manufacturers, process control companies, currency paper and ink manufacturers, security cards, cosmetic companies, scanner manufactures and food processing companies.
Our second product, the ChromaID Liquid Lab Kit, scans and identifies liquids. This product is currently in prototype form. Similar to our first product, it will be marketed to customers who are considering licensing the technology. Rather than use an LED emitter to reflect light off of a surface that is captured by a photodiode to generate a ChromaID signature the liquid analysis product shines light through the liquid (transmissive) with the LEDs positioned on one side of the liquid sample and the photo detectors on the opposite side. This device is in a functional state in our laboratory and we anticipate having a Liquid ChromaID Lab Kit available for customers by the Company during the fall of 2015. Target markets include, but are not limited to, water companies, petrochemical companies, pharmaceutical companies, and numerous consumer applications.
The ChromaID Lab Kits allows potential licensors of our technology to work with our technology and develop solutions for their particular application. Our contractual arrangements with IDMC are described in greater detail below.
Our next planned product should be an exemplar product is a prototype that will be produced to address several markets. The primary purpose of this prototype will be to demonstrate the technology to prospective business partners, and will consist of a small, hand held, battery powered, Bluetooth enabled scanning device. The scanner should wirelessly connect to a smart phone or tablet to transfer the scanned data. The smart phone application will include two or three industry specific but generic applications that allow for the demonstration of the scanning and matching of the ChromaID signatures. The applications will focus on drug identification, food safety and liquid detection. The prototype device will lend itself to consumer applications and can be a consumer product as well.
Our Commercialization Plans for the ChromaID Technology
We shipped our first ChromaID product, the ChromaID Lab Kits, to our strategic partner IDMC during the last calendar quarter of 2013 and first calendar quarter of 2014, after we completed final assembly and testing. As part of our agreement with IDMC, we curtailed our ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, we began to actively market the ChromaID Lab Kits to interested and qualified customers. Some ChromaID Lab Kits are provided free of charge to potential customers. Others are sold for a modest price. To date, we have achieved limited revenue from the sale of our ChromaID Lab Kits.
The Lab Kit includes the following:
ChromaID Scanner. A small device made with electronic and optical components and firmware which pulses light onto a flat material and records and digitizes the light that is reflected back from that material. The device is the size of a typical flashlight (5.5” long and 1.25” diameter). However, the technology can be incorporated into almost any size, shape and configuration.
ChromaID Lab Software. A software application that runs on a Windows PC. The software allows for configuration of the scanner, controls the behavior of the ChromaID Scanner, displays a graph of the captured ChromaID signature profile, stores the ChromaID signature in a database and uses algorithms to compare the accuracy of the match of the unknown scan to the known ChromaID signature profile. This software is intended for lab and experimental use only and is not required for commercialized product applications.
Software Development Toolkit. A collection of software applications, API (an abbreviation of application program interface – a set of routines, protocols, and tools for building software applications) definitions and file descriptions that allow a customer to extract the raw data from the ChromaID signatures and run their own software routines against that raw data.
The ChromaID Lab Kit allows customers to experiment with and evaluate the ChromaID technology and determine if it is appropriate for their specific applications. The primary electronic and optical parts of the ChromaID scanner, called the “scan head,” could be supplied to customers to integrate into their own products. A set of ChromaID Developer Tools are also available. These allow customers to develop their own applications and products based on the ChromaID technology.
ChromaID signatures must be stored, managed, and readily accessible for comparison, matching and authentication purposes. The database can be owned and operated by the end customer, but in the case of thousands of ChromaID signatures, database management may be outsourced to us or a third party provider. These database services could be made available on a per-access transaction basis or on a monthly or annual subscription basis. The actual storage location of the database can be cloud-based, on a stand-alone scanning device or on a mobile device via a Bluetooth connection depending on the requirements of access, size of the database and security as defined by the customer. As a result, large databases can be accessed by cell phone or other mobile technologies using either local storage or cloud based storage.
Based on the commercialization plans outlined above, our business model anticipates deriving revenue from several sources:
●
Sales of the ChromaID Lab Kit and ChromaID Liquid Lab Kit
●
Non Recurring Engineering (NRE) fees to assist customers with scan integration into their products
●
Licensing of the ChromaID technology
●
Royalties per unit generated from the sales of scan heads
●
Multi-unit sales of the above referenced exemplar product for as yet to be determined consumer product applications
●
Per click transaction revenue from accessing the unique ChromaID signatures
●
Developing custom product applications for customers
●
ChromaID database administration and management services
Our Acceleration of Business Development in the United States and Around the World
We are coordinating our internal business development, sales and marketing efforts with those of our strategic partners IDMC, and Sumitomo Precision Products to leverage market data and information in order to focus on specific target vertical markets which have the greatest potential for early adoption. The ChromaID Lab Kit provides a means for us to demonstrate the technology to customers in these markets. It also allows customers to experiment with developing unique applications for their particular use. Our Business Development team is pursuing license opportunities with customers in our target markets. As an example, in March 2016 we entered into a Collaboration Agreement and License with Intellicheck Mobilisa. The agreement provides Intellicheck with exclusive rights to our ChromaID technology in the areas of homeland security, law enforcement and crime prevention.
There is no requirement for FDA or other government approval for the current applications of our ChromaID technology. Over time, as we explore the application of our ChromaID technology for medical diagnostics and other applications, we expect that there will be requirements for FDA and other government approvals before applications using the technology in medical and other regulated fields can enter the marketplace.
Research and Development
Our research and development efforts are primarily focused improving the core foundational ChromaID technology and developing new and unique applications for the technology. As part of this effort, we typically conduct testing to ensure that ChromaID application methods are compatible with the customer’s requirements, and that they can be implemented in a cost effective manner. We are also actively involved in identifying new application methods. Our team has considerable experience working with the application of light-based technologies and their application to various industries. We believe that its continued development of new and enhanced technologies relating to our core business is essential to our future success. We spent $243,114 during the nine months ended June 30, 2016 and $362,661 and $670,742 for the years ended September 30, 2015 and 2014, respectively, on development activities. Our research and development efforts are supported internally, through its relationship with IDMC and through contractors led by Dr. Tom Furness and his team at RATLab LLC.
Our Patents
We believe that our ten patents, 20 patent applications, and two registered trademarks, and our trade secrets, copyrights and other intellectual property rights are important assets for us. Our patents will expire at various times between 2027 and 2033. The duration of our trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The patents that have been granted to Visualant include:
On August 9, 2011, we were issued US Patent No. 7,996,173 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy,” by the United States Office of Patents and Trademarks. The patent expires August 24, 2029.
On December 13, 2011, we were issued US Patent No. 8,076,630 B2 entitled “System and Method of Evaluating an Object Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires November 7, 2028.
On December 20, 2011, we were issued US Patent No. 8,081,304 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 28, 2030.
On October 9, 2012, we were issued US Patent No. 8,285,510 B2 entitled “Method, Apparatus, and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On February 5, 2013, we were issued US Patent No. 8,368,878 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 12, 2013, we were issued US Patent No. 8,583,394 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 21, 2014, we were issued US Patent No. 8,888,207 B2 entitled “Systems, Methods, and Articles Related to Machine-Readable Indicia and Symbols” by the United States Office of Patents and Trademarks. The patent expires February 7, 2033.
On March 23, 2015, we were issued US Patent No. 8,988,666 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On May 26, 2015, we were patent US Patent No. 9,041,920 B2 entitled “Device for Evaluation of Fluids using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
On April 19, 2016, we were issued patent US Patent No. 9,316,581 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Substances Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
We pursue an aggressive patent strategy to expand our unique intellectual property in the United States and other countries.
Services and License Agreement Invention Development Management Company, L.L.C.
In November 2013, we entered into a Services and License Agreement with Invention Development Management Company. IDMC is a subsidiary of Intellectual Ventures, which collaborates with inventors and partners with pioneering companies and invests both expertise and capital in the process of invention. On November 19, 2014, we amended the Services and License Agreement with IDMC. This amendment exclusively licenses 10 filed patents to us.
The agreement requires IDMC to identify and engage inventors to develop new applications of our ChromaID™ technology, present the developments to us for approval, and file at least 10 patent applications to protect the developments. IDMC is responsible for the development and patent costs. We provided the Chroma ID Lab Kits to IDMC at no cost and are providing ongoing technical support. In addition, to provide time for this accelerated expansion of its intellectual property we delayed the selling of the ChromaID Lab Kits for 140 days except for certain select accounts. We have continued our business development efforts during this period and have worked with IDMC and their global business development resources to secure potential customers and licensees for the ChromaID technology. We shipped 20 ChromaID Lab Kits to inventors in the IDMC network during December 2013 and January 2014. As part of our agreement with IDMC, we curtailed our ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, we began to actively market the ChromaID Lab Kits to interested and qualified customers.
We have received a worldwide, nontransferable, exclusive license to the intellectual property developed under the IDMC agreement during the term of the agreement, and solely within the identification, authentication and diagnostics field of use, to (a) make, have made, use, import, sell and offer for sale products and services; (b) make improvements; and (c) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to the useful intellectual property held by IDMC within the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer to sell products and services and (b) grant sublicenses to any and all of the foregoing rights. The option to acquire this license may be exercised for up to two years from the effective date of the Agreement.
IDMC is providing global business development services to us for geographies not being pursued by Visualant. Also, IDMC has introduced us to potential customers, licensees and distributors for the purpose of identifying and pursuing a license, sale or distribution arrangement or other monetization event.
We granted to IDMC a nonexclusive, worldwide, fully paid, nontransferable, sublicenseable, perpetual license to our intellectual property solely outside the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer for sale products and services and (b) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We granted to IDMC a nonexclusive, worldwide, fully paid up, royalty-free, nontransferable, non-sublicenseable, perpetual license to access and use our technology solely for the purpose of marketing the aforementioned sublicenses of our intellectual property to third parties outside the designated fields of use.
In connection with the original license agreement, we issued a warrant to purchase 97,169 shares of common stock to IDMC as consideration for the exclusive intellectual property license and application development services. The warrant has a current exercise price of $2.50 per share and expires November 10, 2018. The per share price is subject to adjustment based on any issuances below $2.50 per share except as described in the warrant.
We agreed to pay IDMC a percentage of license revenue for the global development business services and a percentage of revenue received from any company introduce to us by IDMC. We also have also agreed to pay IDMC a royalty when we receive royalty product revenue from an IDMC-introduced company. IDMC has agreed to pay us a license fee for the nonexclusive license of our intellectual property.
The term of both the exclusive intellectual property license and the nonexclusive intellectual property license commences on the effective date of November 11, 2013, and terminates when all claims of the patents expire or are held in valid or unenforceable by a court of competent jurisdiction from which no appeal can be taken.
The term of the Agreement commences on the effective date until either party terminates the Agreement at any time following the fifth anniversary of the effective date by providing at least ninety days’ prior written notice to the other party.
TransTech Systems, Inc.
Our wholly owned subsidiary, TransTech Systems, Inc., is a distributor of products, including systems solutions, components and consumables, for employee and personnel identification in government and the private sector, document authentication, access control, and radio frequency identification. TransTech provides these products and services, along with marketing and business development assistance to value-added resellers and system integrators throughout North America.
We expect our ownership of TransTech to accelerate our market entry and penetration through well-operated and positioned dealers of security and authentication systems, thus creating a natural distribution channel for products featuring our proprietary ChromaID technology. TransTech currently provides substantially all of our revenues. Its management team functions independently from Visualant’s and its operations require a minimal commitment of our management time and other resources. Our acquisition of TransTech in June 2010 and its operations are described in greater detail below.
Agreements with Sumitomo Precision Products Co., Ltd.
In May 2012, we entered into a Joint Research and Product Development Agreement with SumitomoPrecision Products Co., Ltd., a publicly-traded Japanese corporation, for the commercialization of our ChromaID technology. In March 2013, we entered into an amendment to this agreement, which extended the Joint Development Agreement from March 31, 2013 to December 31, 2013. The extension provided for continuing work between Sumitomo and Visualant focused upon advancing the ChromaID technology and market research aimed at identifying the most significant markets for the ChromaID technology. This collaborative work supported the development of the ChromaID Lab Kit. This agreement expired December 31, 2013. The current version of the technology was introduced to the marketplace as a part of our ChromaID Lab Kit during the fourth quarter of 2013. Sumitomo invested $2,250,000 in exchange for 115,385 shares of restricted shares of common stock priced at $19.50 per share that was funded on June 21, 2012.
We also entered into a License Agreement with Sumitomo in May 2012, under which Sumitomo paid the Company an initial payment of $1 million. The License Agreement granted Sumitomo an exclusive license for the then extant ChromaID technology. The territories covered by this license include Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines). The Sumitomo License fee was recorded as revenue over the life the Joint Research and Product Development Agreement and was fully recorded as of May 31, 2013. On May 21, 2015, we entered into an amendment to the License Agreement, which, effective as of June 18, 2014, eliminated the Sumitomo exclusivity and provides that if we sell products in certain territories – Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines) – the Company will pay Sumitomo a royalty rate of 2% of net sales (excluding non-recurring engineering revenues) over the remaining term of the five-year License Agreement (through May 2017).
Potential Markets and Customers
Our plan is to develop markets and customers who have a need to authenticate, detect, identify, verify or diagnose materials or substances which may include, but are not limited to, commercial paint manufacturers, pharmaceutical equipment manufacturers, process control companies, water purification and quality companies, currency paper and ink manufacturers, security card manufacturers, cosmetic companies and food processing companies.
Market opportunities include identification, detection, or diagnosis of:
●
Pharmaceuticals – pill counting and verification
●
Food safety – testing for contaminants and quality
●
Gemstones – diamond color grading
●
Liquid analysis – water purity
●
Law enforcement - illicit drug identification for law enforcement applications
●
ID badges – counterfeit ID detection
●
Secure packaging - Container seals and packaging materials with invisible markings
●
Cosmetics – matching skin tones to correct products
●
Documents and Currency– detect counterfeit paper and inks
●
Medical - Noninvasive skin analysis for discovery of diseases or medical conditions
Our Strategy
To date, the substantial portion of our non-TransTech revenue has been generated from the development license with Sumitomo Precision Products and sales of our ChromaID Lab Kits. We expect to continue to grow revenues from sales of our Lab Kits, non-recurring engineering fees, licenses, per unit royalties and subscriptions, as well as “per click” revenues. Key aspects of our strategy include:
Customize and Refine our Solutions to Meet Potential Customers’ Needs
We are continuously improving and expanding our potential product offerings by testing the incorporation of our technologies into different media, such as the new ChromaID Liquid Lab Kit that is in the prototype stage. Each vertical market has specific requirements for their potential product application that will involve determining the range of LEDs and photodiodes that will provide optimum performance and the associated form factor required for their product. Our goal is to develop a cost-effective scanning system for each potential industry and customer that can be incorporated into that potential customer’s products that they will then take to market.
Continue to Expand Applications for ChromaID Technology
While we have basic proof of concepts for applications in several large markets to date, we plan to continue our ongoing effort to expand proof-of-concept testing in other vertical markets that have yet to be tested. We have also identified and are further examining opportunities to collaborate with companies and universities to develop new applications for the ChromaID technology. We believe the strength of our solutions is based on the unique and proprietary ChromaID signature that is created from every scan.
Target Potential High-Volume Markets
We will continue to focus our efforts on target vertical markets that are characterized by a high level of vulnerability to counterfeiting, product tampering, piracy, fraud, identity theft, contamination and adulteration. We believe the ChromaID technology can be a lower cost, real time, flexible form factor solution in the following areas: access control, quality and process control, food safety, water quality, law enforcement support, standardization and medical diagnostics. Our current target markets include pharmaceuticals, food quality and safety, gemstone grading, water purity, law enforcement, paint color matching, identity cards, chemical identification, cosmetics, currency, process control and healthcare. If and when we have significantly penetrated these markets, we intend to expand into additional related high volume markets.
Pursue Strategic Acquisitions and Alliances
We intend to pursue strategic acquisitions of companies and technologies that strengthen and complement our core technologies, improve our competitive positioning, allow us to penetrate new markets, and grow our customer base. We also intend to work in collaboration with potential strategic partners in order to continue to market and sell new product lines derived from, but not limited to, ChromaID technology.
Target Additional Markets
In fourth fiscal quarter of 2014, we began introducing our technology and services in Europe, the United States and Asia. Several potential customers are currently analyzing our technology. At the present time, we are focusing our efforts on the pharmaceutical industry, the food safety industry, law enforcement and homeland security. In the future, we plan to expand our focus to include identification cards and other secure documents, industrial materials, agrochemicals, pharmaceuticals, consumer products, cosmetics, currency and medical diagnostics.
Industry Background
Visualant’s ChromaID is a part of the broad industry built upon photonics or light-based technology. Photonics science includes the generation, emission, transmission, modulation, signal processing, switching, amplification, and detection/sensing of light. Though covering all light's technical applications over the whole spectrum, most photonic applications are in the range of visible and near-infrared light. The term photonics developed as an outgrowth of the first practical semiconductor light emitters invented in the early 1960s and optical fibers developed in the 1970s.
Photonics came into common use in the 1980s as fiber-optic data transmission was adopted by telecommunications network operators. At that time, the term was used widely at Bell Laboratories. Its use was confirmed when the IEEE Lasers and Electro-Optics Society established an archival journal named Photonics Technology Letters at the end of the 1980s.
Photonics covers a huge range of science and technology applications, including laser manufacturing, biological and chemical sensing, medical diagnostics and therapy, display technology, and optical computing.
Applications of photonics includes all areas from everyday life to the most advanced science, e.g. light detection, telecommunications, information processing, lighting, metrology, spectroscopy, holography, medicine (surgery, vision correction, endoscopy, health monitoring), military technology, laser material processing, visual art, biophotonics, agriculture and robotics.
The world photonics market, according to the World Photonics Report of 2013 was a $350 billion market and will grow to a $650 billion market by 2020.
Our business model is focused on the use of structured light - a disruptive conceptual breakthrough in photonics. Light-emitting diodes (LEDs) shine a single wavelength of pulsed light in increasing steps of intensity onto a subject. Photodiodes capture the light that is returned via reflection or re emission of that light. The photodiode produces an analog signal that is then converted into a 24 bit digital data point for each pulse of light. A typical scan is comprised of hundreds of pulses of light across a number of specific frequency LED’s creating a unique ChromaID signature for the subject being scanned. In a typical application a “reference” or “master” ChromaID signature is captured and stored in a database for that specific subject. When an unknown substance is scanned to produce its own ChromaID signature, (the “discovery scan”), the unknown substance’s ChromaID signature is compared to that of the known (or “reference”) ChromaID signature. Algorithms are used to compare the two sets of data and determine if the “discovery” signature is the same as the “reference” ChromaID signature. This accuracy threshold can be adjusted from 51 % to 99.995 % accuracy based on the requirements for each specific application of the ChromaID technology. Historically, a number of the applications for ChromaID technology were performed by spectrophotometers. The sales of spectrophotometers by companies such as Ocean Optics, Perkin Elmer, Fisher Thermo Scientific and Agilent are multibillion dollar businesses. Spectrophotometers combine broad-spectrum light; a diffraction grating to split it; and a linear array for graphical presentation in software. They tend to be bulky, fragile, and expensive; scanning and analysis are complex. We believe our ChromaID technology uses lower cost components, provides more accurate data, has a very flexible form factor and the information it provides can be easily understood. The use of structured light by our ChromaID technology provides a platform for the development of a myriad of applications in the categories of identification, authentication and diagnostics.
We believe that the ChromaID technology is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. The ChromaID technology may be considered an enabling technology that brings the science of light and photonics to low cost, real world commercialization opportunities across multiple industries. ChromaID is a sensor technology which, with its low cost, small form factor, and ease of connectivity can be an enabling technology for the broad Internet of Things and integrated into many aspects of everyday life providing useful information relating health, life and safety. The technology is foundational and as such, the basis upon which we believe a significant business can be built.
A number of our current and potential markets are set forth below.
Current and Potential Markets
PHARMACEUTICALS
The ChromaID technology has many potential applications in the pharmaceutical industry. These may include counterfeit drug detection, raw materials verification, clean room environment verification, process and quality control applications, medical waste disposal and packaging verification. There are many issues in the pharmaceutical industry that have health safety, litigation and financial loss implications resulting in billions of dollars globally.
Internal tests of the ChromaID technology to cost effectively address a number of the issues that are of concern for the pharmaceutical industry. The ChromaID technology has been proven that it is able to distinguish authentic pills from counterfeit pills and one manufacturer’s aspirin from another manufacturer’s aspirin by simply scanning and comparing the scan results. We have worked with potential partners to use the ChromaID technology to identify controlled substances prior to their safe and secure disposal in hospital environments. We are currently working with a partner who is exploring using the ChromaID technology in high speed pill sorting and counting equipment to authenticate and verify that the correct pill is going into the package or bottle.
We have worked with a company to verify raw materials prior to them being processed into nutraceutical products.
With the ChromaID Liquid Development Kit we have been able to detect pure liquids from those with contaminants in them with potential applications in production control as well as real-time monitoring of patient liquid medicine delivery systems for potential dilution and verifiability issues.
The pharmaceutical industry faces major problems relative to counterfeit, diluted or falsely labeled drugs that make their way through healthcare systems worldwide, posing a health threat to patients and a financial threat to drug manufacturers and distributors. According to the Center for Medicine in the Public Interest in the United States of America, worldwide sales of counterfeit medicines could top $75 billion this year, a 90% rise in five years. In some countries, counterfeit prescription drugs comprise as much as 70% of the drug supply and have been responsible for thousands of deaths in some of the world’s most impoverished nations, according to the WHO. Counterfeit pharmaceuticals are estimated to be a billion-dollar industry, though some estimate it to be much larger. In 2010, the WHO reported that in over 50% of cases, medicines purchased over the Internet from illegal sites that conceal their physical address have been found to be counterfeit. According to the WHO, counterfeiting can apply to both branded and generic products and counterfeit pharmaceuticals may include products with the correct ingredients but false packaging, with the wrong ingredients, without active ingredients or with insufficient active ingredients.
Throughout the channel of distribution, from raw material sourcing to end consumer purchasing, the ChromaID technology can be used to detect erroneous or counterfeit products and help provide safety and security throughout the supply chain from manufacturers to consumers.
FOOD SAFETY AND QUALITY
Counterfeit food threats are becoming more common as supply chains become more global and as imaging and manufacturing technology become more accessible. There are numerous alarming examples of counterfeit foods that have been reported. For instance, long-grain rice is being labelled and sold as basmati rice, Spanish olive oil is being bottled and sold as Italian olive oil, and mixtures of industrial solvents and alcohol are being sold as vodka. In addition, herbal teas have been found to contain no herbs or tea and juices have been found to contain vegetable oil, which is used as a flame retardant, and labeled tuna turns out to be an unidentifiable concoction of random meats. Although many of these stories have emerged from the UK and Europe, the fake-food problem is also relevant in the United States.
Around the world, food fraud is an epidemic and we believe the ChromaID technology can make a significant contribution in the area of food authentication, food safety and food quality. We are currently in discussions with potential customers about a consumer-based food safety testing device. We are in discussions with another potential customer who has expressed interest in using the technology to perform milk quality analysis including protein and fat content.
In our labs we have been able to identify and differentiate whole milk from 2%, 1% and fat free products. We have also been able to differentiate the same milk product, 2% milk for example, from different manufacturers. We intend to work with research laboratories and universities to perform validation studies that the technology can detect bacteria such as Listeria and E. coli on the surfaces of meat, fish and poultry.
We have performed initial testing on nutraceutical products and are able to differentiate pure products such as green tea, dried blueberry and dried barberry from those containing small amounts of Maltodextrin, which is a product “extender”.
ChromaID technology also may have applicability in the field both in aiding in pest and disease management and in using our ability to determine the best time for crop harvest. Light reflectivity can also indicate the maturity of a plant’s fruit. The timing of a harvest has a direct impact upon a crop’s value.
GEMSTONE COLOR AND QUALITY
Visualant is currently working with parties who are directly involved with the assessment and grading of diamonds and gemstones. In certain cases the acknowledged global standard for gemstone certification, the GIA (Gemological Institute of America), uses subjective evaluation for elements of diamond quality such as color. These evaluations are subject to the inconsistencies of how one individual perceives one color as compared to another individual. The difference in evaluation can impact the value of the gem being graded.
Laboratory testing that has been performed to date indicates that the ChromaID technology can uniquely and accurately differentiate diamond colors between stones and grades of stones. This would provide for a uniform and consistent evaluation of investment grade diamonds in different markets so, for example, there would be consistency between New York, Tel Aviv and Antwerp for the same stone.
FLUID ANALYSIS
Laboratory experimentation with Visualant’s ChromaID technology has shown that the technology can identify and differentiate between identical looking clear fluids. Laboratory tests on water samples were able to detect and identify the pure water sample and clear samples that had various concentrations of salt or sugar dissolved into the solution.
In another laboratory test the ChromaID technology was able to effectively differentiate between different clear vodkas and accurately identify Smirnoff, Stolichnaya and Grey Goose. All were in a clear plastic containers yet the ChromaID technology could quickly and accurately identify them. These tests were all performed with the flat surface scanner. Visualant’s newest product, the ChromaID Liquid Lab Kit, has been designed specifically for liquid analysis. This technology shines light through the liquid using a transmissive derivative of the ChromaID technology which will provide even more accurate results with fluids.
Testing to determine adherence to environmental standards of safety and quality in the US is done primarily by small private commercial labs. These labs are required by municipal and government dictates to use mass spectrometry and gas chromatography for their tests. Globally, the water testing market totals $3.5 billion annually, according to Global Water Intelligence. In addition, there is an approaching boom in this market as China as their awareness of their environmental impact and costs continues to grow.
Further, there is a potential application for the ChromaID technology to be inserted in stacks and effluent pipes to monitor quality of discharges. Currently, mass spectrometer bulbs and sensors are inserted, but they quickly foul due to precipitates and heat reaction of chemicals in the flow.
Among the applications that potential customers have shown an interest in are as follows:
●
Low cost water quality testing device for use in third world environments.
●
Field-deployable water testing tool for fracking water analysis at the well site.
●
Fuel and Oil Analysis. This area includes all analysis done to detect and identify contaminants in fuel and oil inventories. This analysis may be done in the field or in controlled settings. An example of this would be testing for the presence of water in aviation fuel either in line in the aircraft system, or in the hanger as part of routine sampling.
●
Real time monitoring of almost any liquid in a production environment such as water desalination, petrochemical production, bottling plants etc. Today samples must be pulled from the production lines and sent to a laboratory for analysis taking hours or days to obtain results.
●
Inline intravenous drip monitoring of liquid medications such as morphine for dilution, contaminants or adulterants.
The promise of a flow-through liquid quality detection device that provides real time results at a lower cost has many applications.
LAW ENFORCEMENT
In fiscal year 2011, the US government provided $3.9 billion for drug interdiction. Currently there is no reusable, reliable and easily portable field-based detection system available to law enforcement agencies. There are many chemical-based tests, but these require a careful adherence to process.
Law enforcement organizations are always seeking a system they can use which will provide absolute proof of authentication. Laboratory experimentation with the ChromaID technology has successfully shown that it can differentiate salt from sugar, baking powder from flour, one manufacturer’s baking powder from another manufacturer’s baking powder, regular flour from gluten free flour, and aspirin from two different producers.
We have worked with a potential customer in the controlled substance disposal area and, using the ChromaID technology, they were able to successfully identify several similar looking controlled substances from one another. Putting a controlled substance detection device that provides results in real time in the hands of law enforcement for less than $1,000 would be very valuable tool.
The ChromaID technology could be applied in the area of forensics by identifying automobile paint and soil samples from a crime scene. A database of all automobile paints by manufacturer and model exists and can be scanned and stored in a ChromaID database. This would allow for the paint chips at the scene of a crime to be scanned and identify not just the color of the car but also the year, make and model of the suspect vehicle. Law enforcement would know exactly what make and year of car they were looking for, not just a “yellow” car.
PAINT AND COLOR MATCHING
Laboratory testing of the ChromaID technology has demonstrated that the technology is capable of differentiating minute differences in paint colors and finishes. Our experimentation with Pantone color chips has demonstrated that the ChromaID technology can detect more subtle color differences than a traditional hand held spectrophotometer. In experimenting with a Pantone skin color collection the ChromaID technology could detect a difference in every skin tone sample. A spectrophotometer could only detect a difference in every tenth skin tone sample.
The ChromaID technology could be incorporated into a consumer device that could accurately capture a paint color in the home from a wall, a piece of furniture or even a plant and take that information to paint retailer for correct matching. Industrial applications could include replacement of the expensive and inaccurate spectrophotometers installed in most paint retailers, or the ability to correctly color metal roofing or siding materials prior to them being manufactured or ordered.
Printing color verification is another application of the technology for accurately verifying the color on a particular printing job before thousands of prints are run. The ChromaID can also differentiate between flat, eggshell, semi-gloss and gloss finishes, something impossible to do with a spectrophotometer.
SECURITY BADGING, IDENTIFICATION CARDS, DOCUMENTS AND CURRENCY
Governments are increasingly vulnerable to counterfeiting, terrorism and other security threats at least in part because currencies, identity and security cards and other official documents can be counterfeited, often with relative ease. Havocscope, a company that collects black market intelligence and identifies security threats, reports that they believe there are over 1 million fake ID’s in use in the United States.
The physical security and access control market is experiencing a major shift toward intellectual property-based solutions. Until now most of the security solutions were analog, but intellectual property-based solutions have started to garner more confidence in the market. This shift is influencing equipment purchases, upgrades, processes and training. The US government’s decision to deploy an integrated, agency-wide, common smart card platform may continue to raise the awareness of smart card technology and hence increase the demand for contactless smart card proximity readers in the public and private sectors alike.
Visualant, with its digital platform including software, is ideally positioned for this trend.
According to the U.S. Immigration and Customs Enforcement, document and benefit fraud poses a severe threat to national security and public safety because it creates a vulnerability that may enable terrorists, criminals and illegal aliens to gain entry to and remain in the United States. As of 2013, almost 40 million "travel" documents were reported as lost or stolen since 2002, according to Interpol.
Maintaining the integrity of the U.S. passport is essential to the State Department’s efforts to protect U.S. citizens from terrorists, criminals and others. The State Department issued approximately 15.5 million passports in fiscal year 2015. In 2004, the States’ Bureau of Diplomatic Security arrested about 500 individuals for passport fraud, and about 300 persons were convicted. Passport fraud is often intended to facilitate other crimes, including illegal immigration, drug trafficking, and alien smuggling.
The low initial and maintenance costs of the Visualant ChromaID technology, together with the robustness and accuracy of the system, could create opportunities for other innovative applications. This innovation may involve, among other things, the mode and location of the sensor head, or the form factor of the unit.
Internal testing performed by Visualant on various currency papers, document papers and currency inks have indicated that the technology can accurately detect minute differences in the properties of the papers and inks allowing the counterfeit currency or document to be easily identified using a low cost Chroma ID scanner in either a hand held form factor or incorporated into an ATM type device. The ChromaID scan is very fast. It could be incorporated in a currency counter, for example, as each complete scan may take as little as 0.006 seconds.
The ChromaID solution can be used for all types of identification and official documents, such as passports; lawful permanent resident, or “green” cards; visas; drivers’ licenses; Social Security cards; military identification cards; national transportation cards; security cards for access to sensitive physical locations; and other important identity cards, official documents and security-related cards.
TAGGANTS AND CHEMICAL IDENTIFIERS
The idea of using an invisible chemical marker either as a coating or incorporated into a part or product has been successfully tested in our laboratory using the ChromaID technology. The project involved using a unique chemical compound, often referred to as a taggant, in a batch of PVC security badges. The chemical was acquired from a taggant manufacturer and added to a batch of PVC that was then pressed and cut into standard security badge sized cards.
There was no visible difference in the off white color of a standard PVC card vs. the treated taggant card. Once the ChromaID signature for the taggant treated card was recorded, the two batches of cards were mixed together and underwent a series of ChromaID discovery scans. The ChromaID technology was able to identify the “secure” taggant treated security badge even though both cards looked identical to the naked eye.
Adding holograms, RFID chips, overlays and other secure identification methods drives the cost up substantially from a few cents to a few dollars. Adding a small amount of a chemical compound is very inexpensive yet provides very secure results. In order to produce a counterfeit card the counterfeiter would need to know (a) that there was an invisible marker, (b) what the chemical was, (c) what the concentration of the chemical was and (d) what the proprietary ChromaID signature was, making it very difficult process for the counterfeiter.
These taggant agents could be incorporated into the material that the part was made of or applied as a low cost coating over parts, in packing tape, in plastic packaging, in credit cards, etc.
There are two distinct advantages of the ChromaID technology over other methods such as DNA certification. The cost would be lower for the chemical taggant to be added to the product and there would be virtually no waiting for results. The ChromaID reader would provide immediate feedback as to whether or not the part is authentic. In conjunction with a DNA test, a two-tier authentication methodology could be developed using the Chroma ID technology as a primary test and a DNA test as a secondary test.
MICROCIRCUITS AND OTHER ELECTRONICS
According to the research firm IHS Inc., counterfeit semiconductors have proliferated through corporations and the military and are a $169 billion risk to the electronics supply chain.
The global trade in recycled electronics parts is enormous and growing rapidly, driven by a confluence of cost pressures, increasingly complex supply chains and the huge growth in the amount of electronic waste disposed around the world, especially Asia. Recycled parts, relabeled and sold as new, threaten not only military systems but also commercial transportation systems, medical devices and systems, and the computers and networks that run financial markets and communications systems. The vast majority of counterfeits discovered in military equipment are semiconductors, the stamp-sized silicon wafers that act as the “brains” of nearly every type of modern electronic system. According to an article in Defense One, the U.S. military is an important consumer of these products; a single F-35 Joint Strike Fighter jet is controlled by more than 2,500 semiconductors.
The United States military and the federal government’s national security agencies have faced significant counterfeiting of electronic chips, chipsets and components. In a government-mandated survey of companies involved in the avionics electronics supply chain, the Commerce Department’s Bureau of Industry and Security found 7,383 electronics counterfeit incidents during 2008. This was an increase from the 5,747 incidents reported in 2007.
The ChromaID technology could be utilized as counterfeit detection system through a coating and scanning methodology. If the surface of the microchip is not unique enough on its own, a clear coating containing a unique chemical identifier could be applied to the product. When scanned with the ChromaID device it would return the correct ChromaID signature in real time. Some manufacturers are currently using DNA as an authentication methodology for microchips; however, this requires laboratory testing and verification which can take 24 hours or more.
PRINTING AND PACKAGING
The scourge of counterfeiting in packaging has greatly intensified in recent years. Counterfeiting has spiked, causing detrimental health concerns for consumers, safety concerns for law enforcement agencies, and financial concerns for businesses worldwide. As a result, the global anti-counterfeit packaging market is estimated to reach approximately $128.6 billion by the year 2019, according to the August 2014 issue of the publication Markets and Markets.
Billions of dollars per year are at stake for companies as they seek ways to ensure that the products sold with their logos and branding are authorized and authentic. The proliferation of counterfeiting requires brand owners and their converter/printer partners to work together to create a multi-layered protection plan so that their packaging and labels protect their brands and deter those trying to profit at their (and their reputation’s) expense.
Counterfeiters have become so good at their unlawful activity that spotting the difference between legitimate and counterfeit products can be daunting. These criminals have many ways to subvert legitimate brands and they are capable of using legitimate packaging with certain knock off products.
As we say at Visualant, a counterfeiter cannot counterfeit what they cannot see. Our ability to see colors outside the humanly visible portion of the color spectrum could significantly reduce counterfeiting. That, combined with our ability to randomize what we are referring to in our proprietary database, could further frustrate counterfeiters and make our approach virtually “unhackable.”
There are several areas where the ChromaID technology is applicable to protecting products in packaging. As mentioned above, unique chemicals or taggants that are invisible to the naked eye can be added to packaging material for minimal cost. Some current technology uses a fluorescent light that causes the packaging material to glow red or green. This technology has already been compromised by counterfeiters around the world. Using the same methods, but using a ChromaID scanner for verification could result in a very accurate outcome that is extremely difficult to counterfeit. We have been working on an informal basis with taggant manufacturers on developing these solutions that could be added to packaging material, plastic wrap or plastic sealing tape, all with no visible indication that they have unique properties that a counterfeiter might detect.
Visualant was recently granted a patent for a technology called “invisible bar coding.” Because the ChromaID technology looks at light outside the humanly visible spectrum the technology, can “see” information that is invisible to the naked eye. Most bar code printers can produce color information outside the visible spectrum. This information can then be used to not only authenticate a barcode label on a product package but also enhance the amount information that can be stored on that label.
CONSUMER PRODUCTS
ChromaID is a platform technology. A ChromaID scanner connected to a smartphone via a Bluetooth connection or embedded in a smartphone or tablet would provide the opportunity for the development of numerous applications. Several ideas for consumer applications of the ChromaID technology have been suggested. One example is a hand-held paint color checker for use by homeowners, interior designers and paint manufacturers. The form factor could be a small scanner that could connect to a cell phone via Bluetooth connectivity. An application on the phone could then provide color matching and identification on that mobile device. Another application might be for food safety, food quality or the protein and fat content of some foods. Another could be used to test the ripeness of a selected fruit or vegetable at the grocery store or in the field. Cosmetic skin color matching is yet another potential application of the ChromaID technology in the consumer market space.
Counterfeit items are a significant and growing problem with all kinds of consumer packaged goods, especially in the retail and apparel industries. According to Havoscope, the counterfeit clothing and shoes market were worth $24 billion in 2013. We have developed and are currently marketing a number of solutions aimed at brand protection and authentication for the retail and apparel industries, including the clothing, accessories, fragrances and cosmetics segments.
HOMELAND SECURITY
There are several possible applications for the ChromaID technology in the Homeland Security market. Currently spectrophotometry is used for detection and identification of explosives or toxins. We believe a ChromaID scanner would be a low cost, field-deployable method for doing the same work in real time.
As mentioned under Security Badging, the ChromaID scanner cold be used to create invisible markers in security badges or driver’s licenses, and on equipment and other applications that require high security. In many cases just adding a simple but invisible chemical to a security badge provides a significantly more secure badge for a minimal increase in cost.
In 2011, a U.S. Commerce Department report indicated separate cases of counterfeiting rose to 9,356 in 2010 from 3,868 in 2005. In 2008, Robert P. Ernst, who led research into counterfeit parts for the U.S. Navy’s Aging Aircraft Program, claimed that as much as 15 percent of all of the replacements the Pentagon was purchasing could be considered counterfeit.
On September 9, 2010, Homeland Security Newswire published an article “Fake chips from China threaten U.S. military systems” in which a U.S. Chamber of Commerce estimate finds that the global market for counterfeit electronics may be as large as $100 billion. While these references include daunting statistics, the underlying problem has not changed because there was no satisfactory technological solution. Senate hearings in November 2011 revealed the discovery of over 1,800 incidents, totaling over 1 million parts, of counterfeit electronic parts in the defense supply chain According to the semiconductor industry association anti-counterfeiting task force, counterfeiting results in a $7.5 billion loss in revenue annually as well as a loss of 11,000 U.S. jobs.
PROCESS CONTROL
Spectrophotometers have been used in production and manufacturing process control applications for many years. These devices are generally quite large and expensive, limiting where they can be used in a production environment and how many can be cost justified. In internal laboratory testing, using a potential customer’s coating samples, the ChromaID technology was very accurate in determining the “cure level” of a coating applied over a substrate in terms of whether the coating was properly cured, under-cured or over-cured.
The ChromaID scanner was applied to all five samples and each sample had a unique ChromaID signature. This meant that each sample was uniquely identifiable with the ChromaID technology even though all of the samples were the same "color. The samples had been provided to Visualant for testing with anonymous markings A, B, C, D and E.
When we informed the customer of the test results and that each sample had a unique ChromaID signature or identity, they informed us that samples A and B were under-cured, C and D were properly cured and F was over- cured. These results meant that they could compare the ChromaID signature profiles to determine if the coating had been properly cured. This example is one instance where the technology can be applied. The applications in food processing, drug manufacture, parts production and other applications can deploy a lower cost and highly accurate sensing technology to improve the production process by detecting problems in the production line on a real time basis.
MEDICAL DIAGNOSTICS
The American Diabetes Association has determined that the cost of diabetes in the US was $245 billion in 2012, as compared to a total cost of $174 billion in 2007.
The development of a non-invasive, lower cost, easy to use blood glucose testing device is an area that Visualant is pursuing. It is known that UV frequencies of light are able to penetrate beneath the skin for blood analysis applications. Additionally, the ChromaID scanner / sensor could potentially access a developed library of skin conditions and scan a person’s skin to determine the likelihood of a mole being cancerous or not.
Other medical detection applications of the technology may be to scan a patient as they enter the emergency room to detect if they are sick or have other medical conditions that they are unaware of prior to entering the hospital.
Sales and Marketing
We currently have one employee directly engaged in sales and marketing. This employee also manages the activities of several independent business development contractors with relationships in specific vertical markets and fields of use. We also collaborate with our business development partners at IDMC and Sumitomo. We expect to hire additional sales directors and/or consultants to assist us with sales and marketing efforts with respect to our target vertical markets in the areas of pharmaceuticals, printing and packaging and consumer asset marking. Our TransTech subsidiary has five people involved in sales and marketing.
Development of License, Royalty and Other Opportunities
Our plan is to develop license and royalty producing opportunities and partners, including major companies in the US, Europe and Asia. We expect to expand our patent portfolio by continually extending the reach and application of our intellectual property.
Our first major license was signed May 31, 2012 with Sumitomo. Our Business Development team is pursuing other license opportunities with customers in our target markets.
Our Acquisition of Visualant Related Assets of the RATLab LLC
On June 7, 2011, we closed the acquisition of all Visualant related assets of the RATLab, namely the rights to the medical field of use of the Chroma ID technology. The RATLab is a Seattle based research and development laboratory created by Dr. Tom Furness, founder and Director of the HITLab International, with labs at Seattle, University of Canterbury in New Zealand, and the University of Tasmania in Australia. With this acquisition, we consolidated all intellectual property relating to the ChromaID technology, except for environmental field of use which was held by Javelin LLC and which was acquired separately (see below). We acquired these assets of the RATLab for (I) 6,667 shares of our common stock at closing valued at $30.00 per share, the price during the negotiation of this agreement; (ii) payment of $250,000; and (iii) payment of the outstanding promissory note owing to Mr. Furness in the amount of $65,000 with accrued interest of $24,675.
On October 23, 2008, the Company and RATLab entered into definitive agreements which provide for a non-commercial non-exclusive license of the Company’s technology to RATLab for the purpose of continuing research and development with a license back to the Company for enhancements that are developed. Further, an exclusive license was entered into between the Company and RATLab for selected fields of use.
Our Acquisition of Environmental Field of Use Rights from Javelin LLC
On July 31, 2012, we closed the acquisition of all rights to the ChromaID technology in the environmental field of use from Javelin LLC. We acquired these assets of Javelin for (i) 8,334 shares of our common stock valued at $19.50 per share, the price during the negotiation of the acquisition agreement; and (ii) $100,000 in cash, with $20,000 payable at closing and $80,000 to be paid in four equal installments over a period of eight months, all of which have now been paid.
Our Acquisition of TransTech Systems, Inc.
In June 2010 we acquired TransTech Systems, Inc., based in Aurora, Oregon. TransTech, which is now a wholly owned subsidiary, is a distributor of products, including systems solutions, components and consumables, for employee and personnel identification in government and the private sector, document authentication, access control, and radio frequency identification. TransTech provides these products and services, along with marketing and business development assistance to a growing channel of value-added resellers and system integrators throughout North America.
TransTech provides its channel partners pre-and post-sales support in the industry. Technical Services covers training and installation support, in-warranty repair, out of warranty repair, and spares programs. Our Customer Service team, provides full sales, configuration and design, and logistics services. An increasing number of manufacturers are turning to TransTech Systems for channel development and introduction of their products to our market space.
We closed the acquisition of TransTech on June 8, 2010. We acquired our 100% interest in TransTech by issuing a Promissory Note to James Gingo, the President and sole stockholder of TransTech, in the amount of $2,300,000, plus interest at the rate of three and one-half percent per annum from the date of the Note. The Note was secured by a security interest in the stock and assets of TransTech, and was payable over a period of three years. The final balance of $1,000,000 on the Note and accrued interest of $30,397 were paid to Mr. Gingo on June 12, 2013, to complete payment of the purchase price for the TransTech stock.
On June 8, 2010 in connection with the acquisition of TransTech, we issued a total of 25,334 shares of restricted common stock of the Company to James Gingo, Jeff Kruse and Steve Waddle, executives of TransTech, and Paul Bonderson, a TransTech investor. The parties valued the shares in this transaction at $76,000 or $3.00 per share, the closing bid price during negotiations.
This acquisition is expected to accelerate market entry and penetration through well-operated and positioned dealers of security and authentication systems, thus creating a natural distribution channel for products featuring the company’s proprietary ChromaID technology.
TransTech Products
TransTech products include:
●
ID Systems & Components: Provision of ID personalization systems to the security industry. These systems include components such as ID cards, printers, software, supplies, data collection devices, document scanners, photo capture products, document authentication devices, and signature capture products.
●
Logical and Physical Access Control: Logical access readers used for logging onto computer networks and VPNs, physical access control readers used to gain access into buildings or secure areas, software such as visitor management & temporary card solutions, and additional applications outside of security.
●
Radio Frequency Identification and Tracking: These products include RF antennas, readers, cards, tags, labels, tracking software, systems integration software and even video surveillance cameras to tie video clips of the asset or article movement to the personnel using them or to record other events surrounding asset and article movement.
●
Kiosk printers for the self service industry – The self service industry is expanding from ATM’s and grocery store check-out lines to fully integrated systems for paying bills, depositing cash or checks, and using financial services. TransTech provides Kiosk card printers. The mechanical functions of the printers are the same as a standard desktop card printer but typically do not have the protective housing and may come with much higher volume feeder capacities.
TransTech Markets
TransTech’s markets include:
●
Regions: Revenues are derived from over 400 resellers and national accounts in the United States.
●
Route to Market: TransTech’s focus is on its reseller channel. Approximately 90% of sales are through the reseller channel and government prime vendors. The remaining approximately 10% is direct to end users.
●
Distribution Network Development: TransTech is exploring a closer position with its direct channel for tighter market feedback, insurance against manufacturer’s policies, and for financial benefits. This exploration includes partnering, LLCs, Joint Ventures, and potential acquisitions.
●
Applications and Verticals: The primary use of TransTech products is for security applications. These fit within many verticals, including but not limited to, commercial industries, manufacturing, distribution, transportation, government, health care, education, entertainment. In recent years there has been growth into several non-security applications such as gaming/player’s cards, loyalty cards, gift cards, direct marketing, certifications, amusement, payment, and guest cards.
Key TransTech Partners
●
Customers: We currently do not have any customer concentrations where one customer exceeds 10% of net revenues on an annual basis.
●
Suppliers: Evolis, Fargo, Ultra Electronics - Magicard Division and NiSCA are major vendors whose products account for approximately 70% of TransTech’s revenue. TransTech buys, packages and distributes products from these vendors after issuing purchase orders. Our products do not have any limit on availability, subject to proper payment of outstanding invoices.
TransTech Distribution Methods
Distribution is fragmented in the security and authentication marketplace. There are large companies who increasingly sell directly to customers via the Internet and smaller regional and national distributors who sell to these same customers and provide value added services and support. Often called value added resellers or VARs, distributors such as TransTech work hard to maintain their customer relationships through the provision of outstanding service and support.
The Visualant technology will be primarily sold as INTELLECTUAL PROPERTY, licensing and component parts of third party solutions and products. The sales and business development efforts are therefore focused on developing business relationships with those potential customers who have a need for faster, more accurate and lower cost discovery, authentication and verification of surfaces or substances via the spectral pattern creation, recording and storage capabilities provided by the Visualant ChromaID technology. These applications may be in the industrial, commercial or government security sector, but the end user products most likely will be produced by a third party incorporating the Visualant scan head component as a part of the overall product.
We should be able to leverage our TransTech channel of distribution and obtain a speed to market advantage. At the same time, where appropriate, Visualant expects to utilize broad global channels of distribution for the ChromaID technology. We also expect to enter into joint ventures with co-development partners who may have their own channels of distribution.
Our Competition
While we have not seen any direct competition to the patented ChromaID technology and are not aware of any direct competitors using technology with the same or similar capabilities as the Visualant Spectral Pattern Matching technology in the security and authentication marketplace, there are several indirect competitors in the form of other methods for determining the authenticity of products and people. These competitive products include the use of RFID chips, holograms, iris scans, fingerprints, taggants and other means of determining whether a person or product is authentic.
Competitors to the ChromaID technology include major corporations focused on the spectrophotometer industry such as Perkin Elmer, Ocean Optics and Fisher Thermo Scientific. The use of light for analysis and testing is a multi-billion-dollar industry driven by these and other corporations. New entrants, such as SCiO, use light to perform certain specific tasks. We are not aware of any legacy company or new entrant that possesses the breakthrough foundational technology embodied in the patents which cover ChromaID and its many applications.
There are competitors who do use spectroscopy and IR light to sense and validate various substances. While these methods are not identical to Chroma ID technology, they are functional, but at a relatively higher price. The FDA recently developed an internal product for checking on illegal drugs, and companies like Thermo Scientific and Centice are using Ramen light scattering technologies to analyze various substances confirming that the market is interested the light identification solutions. The previously mentioned products, however, are large and expensive, costing over $10,000 for each product. Many companies compete in the security and authentication marketplace with various solutions, many of which perform well. We believe that we can provide an accurate, cost effective component which will add value to customers looking for additional inexpensive redundancies to solve their security and authentication problems.
TransTech faces direct competition from OEMs and manufacturers selling directly to end users/customers and from other distributors of both the same products as TransTech distributes and competing products.
Intellectual Property and Proprietary Rights
We rely on a combination of patent, trademark, and trade secret laws, confidentiality procedures and licensing arrangements to protect our intellectual property rights.
A discussion of the patents held and licensed to the Company can be found in the section of this prospectus titled “Description of Our Business”, subsection “Our Patents”.
Government Regulation
Our ChromaID technology may have a number of potential applications in fields of use which require prior governmental regulatory approval before the technology can be introduced to the marketplace. For example, the Company is exploring the use of its ChromaID technology for certain medical diagnostic applications. If it were to be successful in developing medical applications of its technology, prior approval by the FDA and other governmental regulatory bodies may be required before the technology could be introduced into the marketplace.
Properties and Operating Leases
We are obligated under various non-cancelable operating leases for our various facilities and certain equipment.
Corporate Offices
Our executive office is located at 500 Union Street, Suite 420, Seattle, Washington, USA, 98101. We lease 1,180 square feet and its net monthly payment is $3,048. We lease this office on a month to month basis.
TransTech Facilities
TransTech is located at 12142 NE Sky Lane, Suite 130, Aurora, OR 97002. TransTech leases a total of approximately 9,750 square feet of office and warehouse space for its administrative offices, product inventory and shipping operations. We leased this office until June 30, 2016 for $5,486 per month. Effective July 1, 2016, we lease this office on a month to month basis at $6,120 per month.
Employees
As of the date of this prospectus we had 18 full-time employees, 14 of whom were employed by TransTech. Our senior management is located in the Seattle, Washington office. None of our employees are subject to a collective bargaining agreement or represented by a trade or labor union. We believe that we have a good relationship with our employees.
Legal Proceedings
We may from time to time become a party to various legal proceedings arising in the ordinary course of our business. We are currently not a party to any pending legal proceeding that is not ordinary routine litigation incidental to our business.
MANAGEMENT
Identification of Directors and Executive Officers
The following table sets forth certain information about our current directors and executive officers:
Name | Age | Director/ Executive Officer |
Directors- | | |
Ronald P. Erickson | 72 | Chairman of the Board, Chief Executive Officer and President (1) |
Jon Pepper | 65 | Director (2) |
Ichiro Takesako | 57 | Director |
| | |
| | |
Executive Officers- | | |
Mark E. Scott | 63 | Former Chief Financial Officer and Secretary (3) |
Todd Martin Sames | 62 | Executive Vice President of Business Development |
(1) Chairman of the Nominations and Governance Committee.
(2) Chairman of the Audit and Compensation Committees.
(3) On August 12, 2016, Mark E. Scott resigned as chief Financial Officer and Secretary effective August 31, 2016. Mr. Scott had no disagreement with the Company on any matter relating to the registrant’s operations, policies or practices.
Term of Office
Each of our officers is elected by the Company's Board of Directors to serve until the next annual meeting of Directors or until their successors are duly elected and qualified. Each of our directors is elected by the Company's Board of Directors and shall hold office until the next annual meeting of stockholders and until his/her successor shall have been duly elected and qualified.
Background and Business Experience
Ronald P. Erickson has been a director and officer of Visualant since April 2003. He was appointed as our CEO and President in November 2009 and as Chairman of the Board in February 2015. Previously, Mr. Erickson was our President and Chief Executive Officer from September 2003 through August 2004, and was Chairman of the Board from August 2004 until May 2011.
A senior executive with more than 30 years of experience in the high technology, telecommunications, micro-computer, and digital media industries, Mr. Erickson was the founder of Visualant. He is formerly Chairman, CEO and Co-Founder of Blue Frog Media, a mobile media and entertainment company; Chairman and CEO of eCharge Corporation, an Internet-based transaction procession company, Chairman, CEO and Co-founder of GlobalTel Resources, a provider of telecommunications services; Chairman, Interim President and CEO of Egghead Software, Inc. a software reseller where he was an original investor; Chairman and CEO of NBI, Inc.; and Co-founder of MicroRim, Inc. the database software developer. Earlier, Mr. Erickson practiced law in Seattle and worked in public policy in Washington, DC and New York, NY. Additionally, Mr. Erickson has been an angel investor and board member of a number of public and private technology companies. In addition to his business activities, Mr. Erickson serves on the Board of Trustees of Central Washington University where he received his BA degree. He also holds a MA from the University of Wyoming and a JD from the University of California, Davis. He is licensed to practice law in the State of Washington.
Mr. Erickson is our founder and was appointed as a director because of his extensive experience in developing technology companies.
Ichiro Takesako has served as a director since December 28, 2012. Mr. Takesako has held executive positions with Sumitomo Precision Products Co., Ltd or Sumitomo since 1983. Mr. Takesako graduated from Waseda University, Tokyo, Japan where he majored in Social Science and graduated with a Degree of Bachelor of Social Science.
In the past few years, Mr. Takesako has held the following executive position in Sumitomo and its affiliates:
June 2008: | appointed as General Manager of Sales and Marketing Department of Micro Technology Division |
April 2009: | appointed as General Manager of Overseas Business Department of Micro Technology Division, in charge of M&A activity of certain business segment and assets of Aviza Technology, Inc. |
July 2010: | appointed as Executive Director of SPP Process Technology Systems, 100% owned subsidiary of Sumitomo Precision Products then, stationed in Newport, Wales |
August 2011: | appointed as General Manager, Corporate Strategic Planning Group |
January 2013:
| appointed as Chief Executive Officer of M2M Technologies, Inc., a company invested by Sumitomo Precision products
|
April 2013: | appointed as General Manager of Business Development Department, in parallel of CEO of M2M Technologies, Inc.
|
April 2014:
| relieved from General Manager of Business Development Department and is responsible for M2M Technologies Inc. as its CEO
|
Mr. Takesako was appointed as a Director based on his position with Sumitomo and Sumitomo's significant partnership with the Company.Jon Pepper has served as an independent director since April 2006. Mr. Pepper founded Pepcom in 1980, and continues as the founding partner of Pepcom, an industry leader at producing press-only technology showcase events around the country. Prior to that, Mr. Pepper started the DigitalFocus newsletter, a ground-breaking newsletter on digital imaging that was distributed to leading influencers worldwide. Mr. Pepper has been closely involved with the high technology revolution since the beginning of the personal computer era. He was formerly a well-regarded journalist and columnist; his work on technology subjects appeared in The New York Times, Fortune, PC Magazine, Men's Journal, Working Woman, PC Week, Popular Science and many other well-known publications. Pepper was educated at Union College in Schenectady, New York and the Royal Academy of Fine Arts in Copenhagen.
Mr. Pepper was appointed as a director because of his marketing skills with technology companies.
Other Executive Officers
Mark E. Scott was our Chief Financial Officer, Secretary and Treasurer from May 2010 to August 31, 2016. Mr. Scott has significant financial, capital market and relations experience in public microcap companies. He currently serves as Consulting Chief Financial Officer of GrowLife, Inc., a publicly traded cultivation services provider, since July 2014. Mr. Scott served as the Chairman of its Audit Committee and a Director of GrowLife from June 2014 until October 2015. Mr. Scott also provides consulting services to other entities from time to time.
Mr. Scott was Chief Financial Officer of U.S. Rare Earths, Inc., a consulting position he held from December 2011 to April 2014, and Chief Financial Officer of Sonora Resources Corporation, a consulting position he held from June 2011 to August 2014. Mr. Scott was Chief Financial Officer, Secretary and Treasurer of WestMountain Gold from February 2011 to December 2013 and was a consultant to that company from December 2010 to February 2011. Mr. Scott previously served as Chief Financial Officer and Secretary of IA Global, Inc. (NASDAQ: IAQ) from October 2003 to June 2011. Previously, he held executive financial positions with Digital Lightwave (NASDAQ: DIGL); Network Access Solutions; and Teltronics, Inc. He has also held senior financial positions at Protel, Inc., Crystals International, Inc., Ranks Hovis McDougall, LLP and Brittania Sportswear, and worked at Arthur Andersen. Mr. Scott is also a certified public accountant and received a Bachelor of Arts in Accounting from the University of Washington.
Todd Martin Sames joined the Company as Vice President, Business Development in September 2012. Mr. Sames was appointed Executive Vice President, Business Development in March 2015. Mr. Sames is responsible for global business development and sales of the ChromaID technology, customer relations and creating new licensing agreements resulting in the commercialization of Visualant’s technology across a wide range of applications with device and equipment manufacturers in several business verticals.
Mr. Sames brings over 25 years of successful emerging technology sales and sales management experience in the areas of enterprise software, audio and video conferencing and networking solutions to corporate clients. From 2010 to 2012, Mr. Sames held a Business Unit Director position at INX, focused on unified communications and collaboration solutions for Fortune 1000 clients. From 2007 to 2010, Mr. Sames held a Regional Management position at BT Conferencing, Video. Prior to that, Mr. Sames was the original corporate sales resource for then start-up Portable Software, now Concur Technologies,
During his tenure at Egghead Software, Mr. Sames was the Midwest Regional Manager for Corporate Sales based in Chicago and ultimately Director of Corporate Relationships overseeing corporate purchasing contracts, special projects and innovative new corporate service programs. Mr. Sames has a Bachelor of Arts Degree from the University of Puget Sound and additional certifications in communications technology from Cisco Systems, Polycom, TANDBERG and other technology systems providers.
Board of Directors Composition
Our business is managed under the direction of our Board of Directors. Our Board of Directors currently consists of three members. Our Board of Directors conducts its business through meetings of our Board of Directors and our committees. During fiscal 2015, our current Board of Directors held two meetings and acted by unanimous written consent six times. All members of our current Board of Directors attended 75% of the meetings of our board during 2015.
There are no family relationships among any of our directors or executive officers.
Communication with our Board of Directors
Our stockholders and other interested parties may communicate with our Board of Directors by sending written communication in an envelope addressed to "Board of Directors" in care of the Secretary, 500 Union Street, Suite 420, Seattle, Washington 98101.
Director Independence
Our Board of Directors has determined that Jon Pepper satisfies the independence requirements of the SEC and the NASDAQ Capital Market and is considered an independent director. In making such determinations, our Board of Directors considered the relationships that each such non-employee director has with us and all other facts and circumstances that our Board of Directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Code of Ethics
We have adopted conduct and ethics standards titled the code of ethics, which is available at www.visualant.net. These standards were adopted by our Board of Directors to promote transparency and integrity. The standards apply to our Board of Directors, executives and employees. Waivers of the requirements of our code of ethics or associated polices with respect to members of our Board of Directors or executive officers are subject to approval of the full board.
Board Committees
The Board has three standing committees to facilitate and assist the Board in the execution of its responsibilities. The committees are currently the Audit Committee, the Nominations and Governance Committee, and the Compensation Committee. The Committees were formed in July 2010. The Audit and Compensation Committees are comprised solely of non-employee, independent directors. The Nominations and Governance Committee has one management director, Ronald Erickson, as Chairman. Charters for each committee are available on our website at www.visualant.net. The discussion below describes current membership for each of the standing Board committees.
Audit Committee
Our Board of Directors established an audit committee in July 2010. Our audit committee provides assistance to the Board in fulfilling its responsibilities to our stockholders relating to: (1) maintaining the integrity of our financial reports, including our compliance with legal and regulatory requirements, (2) the independent auditor's qualifications and independence, (3) the performance of our internal audit function in cooperation with the independent auditors, and (4) the preparation of the report required by the rules of the SEC to be included in our annual proxy statement. Our audit committee is directly responsible for the appointment, compensation and oversight of the independent auditors (including the resolution of any disagreements between management and the independent auditors regarding financial reporting), approving in advance all auditing services, and approving in advance all non-audit services provided by the independent auditors. The independent auditors report directly to the committee. In addition, our audit committee is to review our annual and quarterly financial reports in conjunction with the independent auditors and financial management.
Our Board of Directors has adopted a written charter for the audit committee, a copy of which is available on our website at www.visualant.net.
Compensation Committee
Our Board of Directors established a compensation committee in July 2010. Our compensation committee is responsible for: (1) reviewing and approving goals and objectives underlying the compensation of our Chief Executive Officer, evaluating the CEO's performance in accordance with those goals and objectives, and determining and approving the CEO's compensation; (2) recommending to the board the compensation of executive officers other than the CEO, subject to board approval; (3) administering any incentive compensation and equity-based plans, subject to board approval; (4) preparing the compensation report required by the rules and regulations of the SEC for inclusion in our annual proxy statement; and (5) periodically reviewing the results of our executive compensation and perquisite programs and making recommendations to the board with respect to annual compensation (salaries, fees and equity) for our executive officers and non-employee directors.
Our Board of Directors has adopted a written charter for the compensation committee, a copy of which is available on our website at www.visualant.net.
Nominations and Governance Committee
Our Board of Directors established the nominations and governance committee in July 2010 for the purpose of: (1) assisting the board in identifying individuals qualified to become board members and recommending to the board the nominees for election as directors at the next annual meeting of stockholders; (2) assist the board in determining the size and composition of the board committees; (3) develop and recommend to the board the corporate governance principles applicable to us; and (4) serve in an advisory capacity to the board and the Chairman of the Board on matters of organization, management succession planning, major changes in our organizational and the conduct of board activities.
Our Board of Directors has adopted a written charter for the nominations and governance committee, a copy of which is available on our website at www.visualant.net.
Involvement in Certain Legal Proceedings
None of our current directors or executive officers has, to the best of our knowledge, during the past ten years:
● Had any petition under the federal bankruptcy laws or any state insolvency law filed by or against, or had a receiver, fiscal agent, or similar officer appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time hereof, or any corporation or business association of which he was an executive officer at or within two years before the time hereof;
● Been convicted in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
● Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
● Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
● Engaging in any type of business practice; or
● Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of federal or state securities laws or federal commodities laws;
● Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any federal or state authority barring, suspending, or otherwise limiting for more than 60 days the right of such person to engage in any activity described in (i) above, or to be associated with persons engaged in any such activity;
● Been found by a court of competent jurisdiction in a civil action or by the SEC to have violated any federal or state securities law, where the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated; or
● Been found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any federal commodities law, where the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended, or vacated.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934 requires our directors and executive officers and persons who beneficially own more than ten percent of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of change in ownership of our common stock and other equity securities. Officers, directors and greater than ten percent stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. Based solely upon a review of Forms 3 and 4 and amendments thereto furnished to us under Rule 16a-3(e) during the year ended September 30, 2015, Forms 5 and any amendments thereto furnished to us with respect to the year ended September 30, 2016, and the representations made by the reporting persons to us, we believe that our executive officers and directors and all persons who own more than ten percent of a registered class of our equity securities complied with all Section 16(a) filing requirements.
EXECUTIVE AND DIRECTOR COMPENSATION
The following table provides information concerning remuneration of the chief executive officer, the chief financial officer and another named executive officer for the fiscal years ended September, 2015 and 2014:
Summary Compensation Table
| | | | | | | | All | |
| | | | | | Stock | Option | Other | |
| | | | Salary | Bonus | Awards | Awards | Compensation | Total |
| Name | Principal Position | | ($) | ($) | ($) (6) | ($) (6) | ($) | ($) |
| Salary- | | | | | | | | |
| Ronald P. Erickson (1) | Chief Executive Officer | 9/30/2015 | $ 180,000 | $ - | $ - | $ - | $ - | $ 180,000 |
| | | 9/30/2014 | $ 180,000 | $ - | $ - | $ - | $ - | $ 180,000 |
| | | | | | | | | |
| Mark E. Scott (2) | Chief Financial Officer | 9/30/2015 | $ 120,000 | $ - | $ 20,010 | $ - | $ - | $ 140,010 |
| | Secretary | 9/30/2014 | $ 120,000 | $ - | $ - | $ - | $ - | $ 120,000 |
| | | | | | | | | |
| Richard Mander, Ph.D. (3) | Chief Technology Officer | 9/30/2015 | $ 20,834 | $ - | $ - | $ - | $ 1,250 | $ 22,084 |
| | | 9/30/2014 | $ 187,500 | $ - | $ - | $ - | $ 12,000 | $ 199,500 |
| | | | | | | | | |
| Todd Martin Sames (4) | Vice President of Business Development | 9/30/2015 | $ 120,000 | $ - | $ 15,000 | $ - | $ - | $ 135,000 |
| | | 9/30/2014 | $ 120,000 | $ - | $ - | $ 13,500 | $ - | $ 133,500 |
| | | | | | | | | |
| Jeffrey Kruse (5) | President of TransTech Systems, Inc. | 9/30/2015 | $ 162,000 | $ 3,000 | $ 15,000 | $ - | $ 5,760 | $ 185,760 |
| | | 9/30/2014 | $ 162,000 | $ 4,500 | $ - | $ - | $ 6,780 | $ 173,280 |
(1) During the years ended September 30, 2015 and 2014, Mr. Erickson was paid a monthly salary of $15,000. As of September 30, 2015 and 2014, Mr. Erickson had accrued but unpaid salary of $180,000 and $105,000, respectively. This accrual was based on the tight cash flow of the Company and agreed to by Mr. Erickson, but there was no formal deferral agreement. There was no accrued interest paid on the unpaid salary.
(2) During the year ended September 30, 2014 and 2013, Mr. Scott was paid a monthly salary of $10,000. As of September 30, 2015, Mr. Scott had accrued but unpaid salary of $40,000. This accrual was based on the tight cash flow of the Company and agreed to by Mr. Scott, but there was no formal deferral agreement. The 2015 stock award amount for Mr. Scott reflects 1,334 shares of restricted common stock issued by us on January 23, 2015. The restricted common stock was issued at the grant date market value of $15.00 per share. Mr. Scott resigned August 12, 2016, effective August 31, 2016. Mr. Scott cancelled his stock options grants effective August 12, 2016.
(3) Mr. Mander was paid a monthly salary of $12,500 from October 1, 2013 to December 31, 2013. From January 1, 2014 to September 30, 2014, Mr. Mander was paid a monthly salary of $16,667. Mr. Mander was paid $1,000 per month for medical expenses. On November 7, 2014, the Company accepted the resignation of Mr. Mander as Chief Technology Officer. Mr. Mander’s stock option grants expired March 31, 2015 in connection with his resignation.
(4) During the year ended September 30, 2015 and 2014, Mr. Sames was paid a monthly salary of $10,000. As of September 30, 2015, Mr. Sames had accrued but unpaid salary of $40,000. This accrual was based on the tight cash flow of the Company and agreed to by Mr. Sames, but there was no formal deferral agreement. The 2015 stock award amount for Mr. Sames reflects 1,000 shares of restricted common stock issued by us on January 23, 2015. The restricted common stock was issued at the grant date market value of $15.00 per share. The 2014 stock option grant amount for Mr. Sames reflects 2,000 shares issued by us on April 2, 2014. The grant was issued at the grant date market value of $15.00 per share and vested by April 1, 2017.
(5) Mr. Kruse was appointed as President of TransTech in July 2013. As President, Mr. Kruse was paid at the monthly rate of $13,500 from July 2013 to September 30, 2015. As of September 30, 2015, Mr. Kruse had accrued but unpaid salary of $21,000. This accrual was based on the tight cash flow of the Company and agreed to by Mr. Kruse, but there was no formal deferral agreement. Mr. Kruse was paid bonuses of $3,000 and $4,500 for achieving profitability at TransTech during quarters in the years ended September 30, 2015 and 2014, respectively. The 2015 stock award amount for Mr. Kruse reflects 1,000 shares of restricted common stock issued by us on January 23, 2015. The restricted common stock was issued at the grant date market value of $15.00 per share. Mr. Kruse also was eligible to participate in the TransTech 401k plan. Mr. Kruse has assumed the part time position of General Manager of TransTech effective September 30, 2015. He is no longer considered an officer of Visualant for SEC reporting purposes.
(6) These amounts reflect the grant date market value as required by Regulation S-K Item 402(n)(2), computed in accordance with FASB ASC Topic 718.
Outstanding Equity Awards as of Fiscal Year End
The table below summarizes the outstanding equity awards to our executive officers as of September 30, 2015.
| | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | Option |
| | | | | Expiration |
| Name | (#) | (#) | | Date |
| | | | | |
| Ronald P. Erickson (1) | 13,334 | - | $22.50 | 5/9/2020 |
| | 6,667 | - | $19.50 | 6/5/2022 |
| | | | | |
| Mark E. Scott (2) | 5,668 | - | $19.50 | 6/5/2022 |
| | | | | |
| Richard Mander, Ph.D. (3) | - | - | $- | |
| | - | - | $- | |
| | | | | |
| Todd Martin Sames (4) | 6,667 | - | $19.50 | 9/4/2017 |
| | 1,111 | 889 | $15.00 | 4/1/2019 |
| | - | 6,668 | $15.00 | 1/22/2020 |
| | | | | |
| Jeffrey Kruse (5) | 2,000 | - | $13.50 | 6/7/2020 |
| | 4,001 | 1,334 | $15.00 | 8/26/2018 |
| | - | 3,334 | $15.00 | 1/22/2020 |
(1)
Mr. Erickson’s stock option grants consist of (i) 20,000 shares which vested quarterly over two years from May 10, 2010; and (ii) 6,667 shares which vested quarterly over one year from June 5, 2012.
(2)
Mr. Scott’s stock option grants consist of (i) 3,334 shares which vested March 21, 2013; and (ii) 3,334 shares which vested quarterly over one year from June 5, 2012. Mr. Scott resigned August 12, 2016, effective August 31, 2016. Mr. Scott cancelled his stock options grants effective August 12, 2016.
(3)
Mr. Mander’s stock option grants consist of (i) 6,667 shares which vest quarterly over three years from June 26, 2012; and (ii) 3,334 shares which vest quarterly over three years from August 27, 2013. Mr. Mander’s stock option grants expired March 31, 2015.
(4)
Mr. Sames’ stock options grants consist of (i) 6,667 shares which vest quarterly over three years from September 5, 2012; and (ii) 2,000 shares which vest quarterly over three years from April 1, 2014. The 2015 stock option grant for Mr. Sames reflects 6,668 shares at an exercise price of $15.00 per share. The grant vests quarterly over three years after being earned and expires January 22, 2020. As of September 30, 2015, the stock option grant was not earned.
(5)
Mr. Kruse’s stock options grants consist of (i) 2,000 shares which vested 25% at six months and 25% annually thereafter from June 8, 2010; (ii) 667 shares which vested quarterly over three years from November 29, 2011; (ii) 2,000 shares which vest quarterly over three years from April 1, 2014; and (iii) 5,334 shares which vest quarterly over three years from August 27, 2013. The 2015 stock option grant for Mr. Kruse reflects 3,334 shares at an exercise price of $15.00 per share. The grant vests quarterly over three years after being earned and expires January 22, 2020. As of September 30, 2015, the stock option grant was not earned.
(6)
These amounts reflect the grant date market value as required by Regulation S-K Item 402(n)(2), computed in accordance with FASB ASC Topic 718.
Option Exercises and Stock Vested
Our Named Executive Officers did not exercise any stock options during the years ended September 30, 2015 or 2014.
Pension Benefits
We do not provide any pension benefits.
Nonqualified Deferred Compensation
We do not have a nonqualified deferral program.
Employment Agreements
We do not have employment agreements with our Named Executive Officers.
Potential Payments upon Termination or Change in Control
We do not have any potential payments upon termination or change in control with our Named Executive Officers.
Compensation of Directors
We primarily use stock options grants to incentive compensation to attract and retain qualified candidates to serve on the Board. This compensation reflected the financial condition of the Company. In setting director compensation, we consider the significant amount of time that Directors expend in fulfilling their duties to the Company as well as the skill-level required by our members of the Board. During the year ended September 30, 2015, Ronald Erickson did not receive any compensation for his service as a director. The compensation disclosed in the Summary Compensation Table represents the total compensation.
Director Summary Compensation Table
The table below summarizes the compensation paid by us to non-employee directors during the year ended September 30, 2015.
| | | | | |
| Name | | | | |
| Marco Hegyi (1) | $40,005 | $- | $- | $40,005 |
| Jon Pepper | 30,000 | - | - | 30,000 |
| Ichiro Takesako (2) | - | - | - | - |
| | | | | |
| Total | $70,005 | $- | $- | $70,005 |
(1)
Mr. Hegyi’s stock award amount reflects 2,667 restricted shares issued at the grant date market value of $15.00 per share. Mr. Hegyi resigned as a director in February 2015.
(2)
Mr. Pepper’s stock award amount reflects 2,000 restricted shares issued at the grant date market value of $15.00 per share.
(3)
These amounts reflect the grant date market value as required by Regulation S-K Item 402(r)(2), computed in accordance with FASB ASC Topic 718.
Our independent non-employee directors are not compensated in cash. The only compensation has been in the form of stock awards (see Director Compensation Table) and during the fiscal year ended September 30, 2015, we did not make any grants of stock options to our independent non-employee directors. There is no formal stock compensation plan for independent non-employee directors.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Since September 2013, we have engaged in the following reportable transactions with our directors, executive officers, holders of more than 5% of our voting securities and affiliates, or immediately family members of our directors, executive officers and holders of more than 5% of our voting securities.
Review and Approval of Related Person Transactions
We have operated under a Code of Conduct for many years. Our Code of Conduct requires all employees, officers and directors, without exception, to avoid the engagement in activities or relationships that conflict, or would be perceived to conflict, with the Company’s interests or adversely affect its reputation. It is understood, however, that certain relationships or transactions may arise that would be deemed acceptable and appropriate upon full disclosure of the transaction, following review and approval to ensure there is a legitimate business reason for the transaction and that the terms of the transaction are no less favorable to the Company than could be obtained from an unrelated person.
The Audit Committee is responsible for reviewing and approving all transactions with related persons. The Company has not adopted a written policy for reviewing related person transactions. The Company reviews all relationships and transactions in which the Company and our directors and executive officers or their immediate family members are participants to determine whether such persons have a direct or indirect material interest. As required under SEC rules, transactions that are determined to be directly or indirectly material to the Company or a related person are disclosed.
Relationship with Invention Development Management Company, L.L.C.
In November 2013, we entered into a Services and License Agreement with IDMC. IDMC is a subsidiary of Intellectual Ventures, which collaborates with inventors and partners with pioneering companies and invests both expertise and capital in the process of invention. On November 19, 2014, we amended the Services and License Agreement with IDMC. This amendment exclusively licenses 10 filed patents to us.
The agreement requires IDMC to identify and engage inventors to develop new applications of our ChromaID™ technology, present the developments to us for approval, and file at least 10 patent applications to protect the developments. IDMC is responsible for the development and patent costs. We provided the Chroma ID Lab Kits to IDMC at no cost and are providing ongoing technical support. In addition, to provide time for this accelerated expansion of its intellectual property we delayed the selling of the ChromaID Lab Kits for 140 days except for certain select accounts. We have continued our business development efforts during this period and have worked with IDMC and their global business development resources to secure potential customers and licensees for the ChromaID technology. We shipped 20 ChromaID Lab Kits to inventors in the IDMC network during December 2013 and January 2014. As part of our agreement with IDMC, we curtailed our ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, we began to actively market the ChromaID Lab Kits to interested and qualified customers.
We have received a worldwide, nontransferable, exclusive license to the intellectual property developed under the IDMC agreement during the term of the agreement, and solely within the identification, authentication and diagnostics field of use, to (a) make, have made, use, import, sell and offer for sale products and services; (b) make improvements; and (c) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to the useful intellectual property held by IDMC within the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer to sell products and services and (b) grant sublicenses to any and all of the foregoing rights. The option to acquire this license may be exercised for up to two years from the effective date of the Agreement.
IDMC is providing global business development services to us for geographies not being pursued by Visualant. Also, IDMC has introduced us to potential customers, licensees and distributors for the purpose of identifying and pursuing a license, sale or distribution arrangement or other monetization event.
We granted to IDMC a nonexclusive, worldwide, fully paid, nontransferable, sublicenseable, perpetual license to our intellectual property solely outside the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer for sale products and services and (b) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
We granted to IDMC a nonexclusive, worldwide, fully paid up, royalty-free, nontransferable, non-sublicensable, perpetual license to access and use our technology solely for the purpose of marketing the aforementioned sublicenses of our intellectual property to third parties outside the designated fields of use.
In connection with the original license agreement, we issued a warrant to purchase 97,169 shares of common stock to IDMC as consideration for the exclusive intellectual property license and application development services. The warrant has a current exercise price of $2.50 per share and expires November 10, 2018. The per share price is subject to adjustment based on any issuances below $2.50 per share except as described in the warrant.
We agreed to pay IDMC a percentage of license revenue for the global development business services and a percentage of revenue received from any company introduce to us by IDMC. We also have also agreed to pay IDMC a royalty when we receive royalty product revenue from an IDMC-introduced company. IDMC has agreed to pay us a license fee for the nonexclusive license of our intellectual property.
The term of both the exclusive intellectual property license and the nonexclusive intellectual property license commences on the effective date of November 11, 2013, and terminates when all claims of the patents expire or are held in valid or unenforceable by a court of competent jurisdiction from which no appeal can be taken.
The term of the Agreement commences on the effective date until either party terminates the Agreement at any time following the fifth anniversary of the effective date by providing at least ninety days’ prior written notice to the other party.
Purchase Agreement with Special Situations and 40 other Accredited Investors
On June 14, 2013, we entered into a Purchase Agreement, Warrants, and Stock Purchase Agreement with Special Situations Technology Funds and forty other accredited investors, pursuant to which we issued 348,685 shares of common stock at $15.00 per share for a total of $5,230,000, which amount includes the conversion of $500,000 in outstanding debt of the Company owed to one of its officers. As part of the transaction, which closed on June 14, 2013, we issued to the investors (i) five-year Series A Warrants to purchase a total of 348,685 shares of common stock at $22.50 per share; and (ii) five year Series B Warrants to purchase a total of 348,685 shares of common stock at $30.00 per share. We also issued 34,871 placement agent warrants exercisable at $15.00 per share to GVC Capital, with an obligation to issue up to 34,871 additional placement agent warrants exercisable at $22.50 per share. The placement agent warrants shall issue only upon the exercise of the Series A Warrants by the investors, and are issuable ratably based upon the number of Warrants exercised by the investors. The placement agent warrants have a term of five years from the date of closing of the transaction. The transaction was entered into to strengthen our balance sheet, complete the purchase of our TransTech subsidiary, and provide working capital to support the rapid movement of our ChromaID technology into the marketplace. On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due the issuance of common stock at this price.
Agreements with Sumitomo Precision Products Co., Ltd.
In May 2012, we entered into a Joint Research and Product Development Agreement with SumitomoPrecision Products Co., Ltd., a publicly-traded Japanese corporation, for the commercialization of our ChromaID technology. In March 2013, we entered into an amendment to this agreement, which extended the Joint Development Agreement from March 31, 2013 to December 31, 2013. The extension provided for continuing work between Sumitomo and Visualant focused upon advancing the ChromaID technology and market research aimed at identifying the most significant markets for the ChromaID technology. This collaborative work supported the development of the ChromaID Lab Kit. This agreement expired December 31, 2013. The current version of the technology was introduced to the marketplace as a part of our ChromaID Lab Kit during the fourth quarter of 2013. Sumitomo invested $2,250,000 in exchange for 115,385 shares of restricted shares of common stock priced at $19.50 per share that was funded on June 21, 2012.
We also entered into a License Agreement with Sumitomo in May 2012, under which Sumitomo paid the Company an initial payment of $1 million. The License Agreement granted Sumitomo an exclusive license for the then extant ChromaID technology. The territories covered by this license include Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines). The Sumitomo License fee was recorded as revenue over the life the Joint Research and Product Development Agreement and was fully recorded as of May 31, 2013. On May 21, 2015, we entered into an amendment to the License Agreement, which, effective as of June 18, 2014, eliminated the Sumitomo exclusivity and provides that if we sell products in certain territories – Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines) – the Company will pay Sumitomo a royalty rate of 2% of net sales (excluding non-recurring engineering revenues) over the remaining term of the five-year License Agreement (through May 2017).
Related Party Transactions with Ronald P. Erickson
Entities affiliated with Mr. Erickson have made advances and loans to us in the total principal amount of $960,000 on or before the date hereof at an average annual interest rate of 4.2%. In addition, Mr. Erickson and/or entities with which Mr. Erickson is affiliated also have unreimbursed 2013 expenses and unpaid salary and interest from 2013 on the outstanding principal amount of the Loans totaling approximately $65,000 as of June 14, 2013. Mr. Erickson and related entities converted $500,000 of the advances and loans as part of the private placement which closed June 14, 2013. The remaining amounts were paid to Mr. Erickson and related entities prior to June 30, 2013.
We have a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which currently matures on December 31, 2016 and provides for interest at 3.25% per year. Related to the Umpqua Loan, we entered into a demand promissory note for $200,000 on January 10, 2014 with an entity with which Ronald P. Erickson, our Chief Executive Officer, is affiliated. This demand promissory note will be effective in case of a default by us under the Umpqua Loan.
We also have two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between January and July 2014, provide for interest of 3% per year and now mature on September 30, 2016. They also provide for a second lien on our assets if not repaid by September 30, 2016 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson. Mr. Erickson and/or entities with which he is affiliated also have advanced $668,500 and have unreimbursed expenses and compensation of approximately $398,000. We owe Mr. Erickson, or entities with which he is affiliated, $1,686,500 as of September 1, 2016.
On January 26, 2015, Mr. Erickson cancelled 6,667 in previous issued stock option at $22.50 per share.
Related Party Transaction with Mark E. Scott
Mr. Mark E. Scott, our former Chief Financial Offer, invested $10,000 in the private placement which closed June 14, 2013.
On January 26, 2015, Mr. Scott cancelled 1,000 in previously issued stock options at $19.50 per share. Mr. Scott cancelled 5,668 in previously issued stock options grants effective August 12, 2016.
Indemnification
Our articles of incorporation provide that we will indemnify our directors and officers to the fullest extent permitted by Nevada law. In addition, we have an Indemnification Agreements with the current Board of Directors.
Policies and Procedures for Related Person Transactions
We have operated under a Code of Conduct and Ethics since December 28, 2012. Our Code of Conduct and Ethics requires all employees, officers and directors, without exception, to avoid the engagement in activities or relationships that conflict, or would be perceived to conflict, with our interests.
Prior to the adoption of our related person transaction policy, there was a legitimate business reason for all the related person transactions described above and we believe that, where applicable, the terms of the transactions are no less favorable to us than could be obtained from an unrelated person.
Our Audit Committee reviews all relationships and transactions in which we and our directors and executive officers or their immediate family members are participants to determine whether such persons have a direct or indirect material interest.
As required under SEC rules, transactions that are determined to be directly or indirectly material to us or a related person are disclosed.
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information regarding the ownership of our common stock as of September 1, 2016 by:
| ● | each director and nominee for director; |
| | |
| ● | each person known by us to own beneficially 5% or more of our common stock; |
| | |
| ● | each executive officer named in the summary compensation table elsewhere in this
report; and
|
| | |
| ● | all of our current directors and executive officers as a group. |
The amounts and percentages of common stock beneficially owned are reported on the basis of regulations of the SEC governing the determination of beneficial ownership of securities. Under the rules of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares voting power,” which includes the power to vote or to direct the voting of such security, or has or shares “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has the right to acquire beneficial ownership within 60 days. Under these rules more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Unless otherwise indicated below, each beneficial owner named in the table has sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable. The address for each person shown in the table is c/o Visualant, Inc. 500 Union Street, Suite 420, Seattle Washington, unless otherwise indicated.
| | Shares Beneficially Owned | Shares Beneficially Owned |
| | | |
| | | | | |
| Directors and Officers- | | | | |
| Ronald P. Erickson (1) | 175,087 | 7.4% | 175,087 | 2.9% |
| Mark E. Scott (2) | 11,791 | * | 11,791 | * |
| Jon Pepper | 13,000 | * | 13,000 | * |
| Richard Mander (3) | - | * | - | * |
| Todd Martin Sames (4) | 9,834 | * | 9,834 | * |
| Jeffrey Kruse (5) | - | * | - | * |
| Sumitomo Precision Products Co., Ltd./ Ichiro Takesako | 115,385 | 4.9% | 115,385 | 1.9% |
| Total Directors and Officers (7 in total) | 325,097 | 13.8% | 325,097 | 5.5% |
* Less than 1%.
(1) Includes 86,668 shares of shares of common stock beneficially owned and stock option grants to purchase 20,001 shares of our common stock that are exercisable within 60 days, and also includes 66,667 Series A and B Warrants to purchase shares of our common stock that are exercisable within 60 days.
(2) Includes 10,457 shares of shares of common stock beneficially owned and stock option grants totaling 5,668 shares that Mr. Scott has the right to acquire in 60 days, and also includes 1,334 Series A and B Warrants to purchase shares of our common stock that are exercisable within 60 days.
(3) Includes stock option grants for 10,000 shares which Mr. Mander forfeited on March 31, 2015 in connection with his resignation.
(4) Includes 1,667 shares of shares of common stock beneficially owned and stock option grants totaling 8,000 shares that Mr. Sames has the right to acquire in 60 days.
(5) Mr. Kruse was not a named executive officer as of September 30, 2015.
| | Shares Beneficially Owned | | Shares Beneficially Owned |
| | Prior to this Offering | | After this Offering |
| | Number | Percentage | | Number | Percentage |
| Greater Than 5% Ownership | | | | | |
| | | | | | |
| Sumitomo Precision Products Co., Ltd./ Ichiro Takesako (1) | 115,385 | 4.9% | | 115,385 | 1.9% |
| | | | | | |
| Special Situations Technology Funds, L.P./ Adam Stettner (2) | 318,000 | 12.4% | | 318,000 | 5.2% |
| | Blocker at 9.99% | | Blocker at 9.99% |
| | | | | | |
| Invention Development Management Company, L.L.C. (3) | 97,169 | 4.0% | | 97,169 | 1,6% |
| | | | | | |
| Clayton A. Struve (4) | 3,571,428 | 60.3% | | 3,571,428 | 60.3% |
| | Blocker at 4.99% | | Blocker at 4.99% |
| | | | | | |
| Dale Broadrick (5) | 1,768,087 | 57.6% | | 1,768,087 | 29.8% |
(1) Reflects the shares beneficially owned by Sumitomo Precision Products Co., Ltd as stated in a Schedule 13D filed with the SEC on June 23, 2012, and which has subsequently confirmed the ownership related to the private placement which closed June 14, 2013. Their address is 1-10 Fuso-cho, Amagasaki, Hyogo, Japan.
(2) Reflects the shares beneficially owned by Special Situations Technology Funds, L.P. This total includes 106,000 shares and a total of 212,000 Series A and B Warrants to purchase shares of our common stock. The address of Special Situations Technology Funds, L.P. is 527 Madison Avenue, Suite 2600, New York City, New York.
(3) Reflects a warrant to purchase 97,169 shares of our common stock that are exercisable within 60 days. The address for Invention Development Management Company, L.L.C. is 3150 139th Avenue SE, Building 4, Bellevue, Washington.
(4) Reflects the shares beneficially owned by Clayton A. Struve. This total includes 1,785,714 shares of Series C Preferred Stock and 1,785,714 of Series E Warrants to purchase shares of our common stock. The address of the accredited investor is 175 West Jackson Blvd., Suite 440, Chicago, IL 60604.
(5) Reflects the shares beneficially owned by Dale Broadrick as stated in a Schedule 13D filed with the SEC on August 31, 2016, and which has subsequently confirmed the ownership. Mr. Broadrick’s address 3003 Brick Church Pike, Nashville, TN 37207.
DESCRIPTION OF CAPITAL STOCK
General
The following description of our capital stock and provisions of our articles of incorporation and bylaws are summaries and are qualified by reference to our articles of incorporation, as amended and restated, and our bylaws, as amended and restated. We have filed copies of these documents with the SEC as exhibits to our Registration Statement, of which this prospectus forms a part.
Authorized Capital Stock
We have authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares of voting preferred stock, par value $0.001 per share.
Capital Stock Issued and Outstanding
As of September 1, 2016, we had 2,356,152 shares of common stock issued and outstanding, which excludes the following shares, as of that date:
● 50,942 shares of our common stock issuable upon the exercise of outstanding stock options outstanding at a weighted-average exercise price of $18.04 per share;
● 23,334 shares of our common stock issuable upon the conversion of Series A Convertible Preferred Stock;
● 3,188,734 shares of our common stock issuable upon the exercise of outstanding warrants at an average exercise price of $0.89 per share. 1,785,714 shares of our common stock issuable upon the exercise of outstanding warrants, at an exercise price of $0.70, are being registered in this offering. The warrants will expire on or before August 2021;
● Up to 277,106 shares of our common stock issuable upon the exercise of placement agent warrants exercisable at $1.12 per share.
● An unknown number of shares of our common stock issuable upon the conversion of $810,000 of Convertible Notes Payable and an unknown number of our common shares issuable upon the exercise of $710,000 of warrants related to Convertible Notes Payable;
● 27,391 additional shares of our common stock available for future issuance under our 2011 Stock Incentive Plan;
● 1,785,714 shares of our common stock issuable upon the conversion of Series C Convertible Preferred Stock, at an exercise price of $0.70, subject to certain adjustments. These shares of common stock are being registered in this offering.
Voting Common Stock
We are authorized to issue up to 100,000,000 shares of common stock with a par value of $0.001. As of September 1, 2016, we had 2,356,152 shares of common stock issued and outstanding, held by 50 stockholders of record. The number of stockholders, including beneficial owners holding shares through nominee names, is approximately 2,300. Each share of common stock entitles its holder to one vote on each matter submitted to the stockholders for a vote, and no cumulative voting for directors is permitted. Stockholders do not have any preemptive rights to acquire additional securities issued by us. As of September 1, 2016, there were options outstanding for the purchase of 50,942 shares of common stock and warrants for the purchase of 3,188,734 shares of common stock. In addition, 23,334 shares of our common stock are issuable upon the conversion of Series A Convertible Preferred Stock and 1,785,714 shares of our common stock issuable upon the conversion of Series C Convertible Preferred Stock. Finally, up to 277,106 shares of our common stock are issuable upon the exercise of placement agent warrants and an unknown number of shares are issuable upon conversion of $810,000 in convertible promissory notes and an unknown number of our common shares issuable upon the exercise of $710,000 of warrants related to Convertible Notes Payable, all of which could potentially dilute future earnings per share.
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights for the election of directors. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. On all other matters, the affirmative vote of the holders of a majority of the stock present in person or represented by proxy and entitled to vote is required for approval, unless otherwise provided in our articles of incorporation, bylaws or applicable law. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our Board of Directors, subject to any preferential dividend rights of outstanding preferred stock.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Voting Preferred Stock
As of September 1, 2016, we are authorized to issue up to 5,000,000 shares of preferred stock with a par value of $0.001.
On July 21, 2015, we filed with the Nevada Secretary of State an Amended and Restated Certificate of Designations, Preferences and Rights for our Series A Convertible Preferred Stock. Among other things, the Amended and Restated Certificate changed the conversion price and the stated value of the Series A Preferred from $0.10 (pre reverse stock split) to $30.00 (post-reverse stock split), and added a provision adjusting the conversion price upon the occurrence of certain events.
Under the Amended and Restated Certificate, we had 11,667 shares of Series A Preferred authorized, all of which are outstanding. Each holder of outstanding shares of Series A Preferred is entitled to the number of votes equal to the number of whole shares of common stock into which the shares of Series A Preferred held by such holder are then convertible as of the applicable record date. We cannot amend, alter or repeal any preferences, rights, or other terms of the Series A Preferred so as to adversely affect the Series A Preferred, without the written consent or affirmative vote of the holders of at least 66% of the then outstanding shares of Series A Preferred, voting as a separate voting group, given by written consent or by vote at a meeting called for such purpose for which notice shall have been duly given to the holders of the Series A Preferred.
During the year ended September 30, 2015, we sold 11,667 Series A Preferred Stock to two investors totaling $350,000. These shares are expected to be convertible into 11,667 shares of common stock at $30.00 per share, subject to adjustment, for a period of five years. The Series A Preferred Stock has voting rights and may not be redeemed without the consent of the holder. We also issued (i) a Series C five-year Warrant for 23,334 shares of common stock at an exercise price of $30.00 per share, which is callable at $60.00 per share; and (ii) a Series D five-year Warrant for 23,334 shares of common stock at an exercise price of $45.00 per share, which is callable at $90.00 per share. The Series A Preferred Stock and Series C and D Warrants had registration rights.
On July 20, 2015, the two investors entered into an Amendment to Series A Preferred Stock Terms whereby they agreed to the terms of the Amended and Restated Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock and waived all registration rights.
On March 8, 2016, we received approval from the State of Nevada for the Correction to the Company’s Amended and Restated Certificate of Designations, Preferences and Rights of its Series A Convertible Preferred Stock. The Amended and Restated Certificate filed July 21, 2015 changed the conversion price and the stated value from $0.10 (pre reverse stock split) to $30.00 (post-reverse stock split), and adding a provision adjusting the conversion price upon the occurrence of certain events. On February 19, 2016, the holders of Series A Convertible Preferred Stock entered into Amendment 2 of Series A Preferred Stock Terms and increased the number of Preferred Stock Shares to properly account for the reverse stock split. We have 23,334 Series A Preferred Stock issued and outstanding.
On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due to the issuance of common stock at that price. On August 4, 2016, the warrant exercise price was adjusted to $0.70 per share due to the issuance of common stock at that price.
Series B Redeemable Convertible Preferred Stock
On March 8, 2016, we received approval from the State of Nevada for the Certificate of Designations of Preferences, Powers, Rights and Limitations of Series B Redeemable Convertible Preferred Stock. The Certificate authorized 5,000 shares of Series B Preferred Stock at a par value of $.001 per share that is convertible into common stock at $7.50 per share, subject to certain adjustments as set forth in the Certificate.
We entered into a Stock Purchase Agreement with an institutional investor pursuant to which the Company issued 255 Shares of Series B Redeemable Preferred Shares (“Series B Preferred Shares”) of the Company at $10,000.00 per share with a 5.0% original issue discount for the sum of $2,500,000. We are currently cancelling the Certificate of Designations of Preferences, Rights and Limitations of Series B Redeemable Preferred Stock.
Series C Convertible Preferred Stock
On August 11, 2016, we have applied with the State of Nevada for the approval of the Certificate of Designations, Preferences, and Rights of Series C Convertible Preferred Stock. The Certificate designated 1,785,715 shares as Series C Convertible Preferred Stock at a par value of $.001 per share that is convertible into common stock at $0.70 per share, with certain adjustments as set forth in the Certificate.
Warrants to Purchase Common Stock
In connection with the June 2013 Special Situations financing described below under “Liquidity and Capital Resources”, we have outstanding Series A Warrants to purchase a total of 252,060 shares of common stock with a current exercise price of $0.70 per share, and Series B Warrants to purchase a total of 252,060 shares of common stock with a current exercise price of $0.70 per share, the IDMC warrant to purchase 97,169 shares of common stock with a current exercise price of $0.70 per share, Series C and D Warrants to purchase 23,334 shares of common stock with a current exercise price of $0.70 per share and placement agent warrants to purchase 20,439 shares of common stock at an exercise price of current exercise price of $0.70 per share (collectively, the “Special Situations Warrants”). The Special Situations Warrants contain an adjustment provision that would require an adjustment in the exercise price of the Special Situations Warrants if we issue common stock, warrants or equity below the price that is reflected in the Special Situations Warrants (currently $0.70 per share). If we issue any additional shares of common stock, warrants or other equity securities at a price below the exercise prices of the Special Situations Warrants, it would result in a reduction in the exercise price of the Special Situations Warrants. A downward adjustment in the exercise price of the Special Situations Warrants could also affect the market price of the common stock. In addition, up to 1,785,714 shares (the “Warrant Shares”) of common stock issuable upon the exercise of outstanding Series E Warrants at an exercise price of $0.70 per share, subject to certain adjustments.
DESCRIPTION OF SECURITIES BEING REGISTERED
This prospectus covers the resale by the selling stockholder named herein of up to 3,571,428 shares of our common stock. The common stock covered by this prospectus will be offered for resale from time to time by the selling stockholder identified in this prospectus in accordance with the terms described in the section entitled “Plan of Distribution.” We will not receive any of the proceeds from the resale of the common stock by the selling stockholder.
Common Stock
The material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common stock are described under the caption “Description of Capital Stock” in this prospectus.
Options to Purchase Common Stock
Our 2011 Stock Incentive Plan was approved by our stockholders in April 2011. We were authorized to issue options to purchase up to 46,668 shares of common stock under the 2011 Stock Incentive Plan. In March 2013, our stockholders approved an increase in the number of shares under the 2011 Stock Incentive Plan to 93,333. We have currently reserved 50,942 shares of our common stock for issuance under the 2011 Stock Incentive Plan.
Dividend Policy
We have not previously declared or paid any cash dividends on our common stock and do not anticipate or contemplate paying dividends on our common stock in the foreseeable future. We currently intend to use all of our available funds to finance the growth and development of our business. We can give no assurances that we will ever have excess funds available to pay dividends. In addition, our articles of incorporation restrict our ability to pay any dividends on our common stock without the approval of 66% of our then outstanding Series A Preferred Stock.
Anti-Takeover Provisions
Nevada Revised Statutes
Acquisition of Controlling Interest Statutes. Nevada's "acquisition of controlling interest" statutes contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These "control share" laws provide generally that any person who acquires a "controlling interest" in certain Nevada corporations may be denied certain voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These statutes provide that a person acquires a "controlling interest" whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the Nevada Revised Statutes, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become "control shares" to which the voting restrictions described above apply. Our articles of incorporation and bylaws currently contain no provisions relating to these statutes, and unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest were to provide otherwise, these laws would apply to us if we were to (i) have 200 or more stockholders of record (at least 100 of which have addresses in the State of Nevada appearing on our stock ledger) and (ii) do business in the State of Nevada directly or through an affiliated corporation. As of June 30, 2016 we have less than 200 record stockholders. If these laws were to apply to us, they might discourage companies or persons interested in acquiring a significant interest in or control of the company, regardless of whether such acquisition may be in the interest of our stockholders.
Combinations with Interested Stockholders Statutes. Nevada's "combinations with interested stockholders" statutes prohibit certain business "combinations" between certain Nevada corporations and any person deemed to be an "interested stockholder" for two years after the such person first becomes an "interested stockholder" unless (i) the corporation's board of directors approves the combination (or the transaction by which such person becomes an "interested stockholder") in advance, or (ii) the combination is approved by the board of directors and sixty percent of the corporation's voting power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an "interested stockholder" is any person who is (x) the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the outstanding voting shares of the corporation, or (y) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the then outstanding shares of the corporation. The definition of the term "combination" is sufficiently broad to cover most significant transactions between the corporation and an "interested stockholder". Subject to certain timing requirements set forth in the statutes, a corporation may elect not to be governed by these statutes. We have not included any such provision in our articles of incorporation.
The effect of these statutes may be to potentially discourage parties interested in taking control of us from doing so if it cannot obtain the approval of our Board of Directors.
Articles of Incorporation and Bylaws Provisions
Our articles of incorporation, as amended and restated, and our bylaws, as amended and restated, contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change in control, including changes a stockholder might consider favorable. In particular, our articles of incorporation and bylaws, among other things:
● permit our Board of Directors to alter our bylaws without stockholder approval;
● provide that vacancies on our Board of Directors may be filled by a majority of directors in office, although less than a quorum;
● authorize the issuance of preferred stock, which can be created and issued by our Board of Directors without prior stockholder approval, with rights senior to our common stock, which may render more difficult or discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise; and
● establish advance notice procedures with respect to stockholder proposals relating to the nomination of candidates for election as directors and other business to be brought before stockholder meetings, which notice must contain information specified in our bylaws.
In addition, our articles of incorporation restrict our ability to take certain actions without the approval of at least 66% of the Series A Preferred Stock then outstanding. These actions include, among other things;
● authorizing, creating, designating, establishing or issuing an increased number of shares of Series A Preferred Stock or any other class or series of capital stock ranking senior to or on a parity with the Series A Preferred Stock;
● adopting a plan for the liquidation, dissolution or winding up the affairs of our company or any recapitalization plan (whether by merger, consolidation or otherwise);
● amending, altering or repealing, whether by merger, consolidation or otherwise, our articles of incorporation or bylaws in a manner that would adversely affect any right, preference, privilege or voting power of the Series A Preferred Stock; and
● declaring or paying any dividend (with certain exceptions) or directly or indirectly purchase, redeem, repurchase or otherwise acquire any shares of our capital stock, stock options or convertible securities (with certain exceptions).
Such provisions may have the effect of discouraging a third-party from acquiring us, even if doing so would be beneficial to our stockholders. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our Board of Directors and in the policies formulated by them, and to discourage some types of transactions that may involve an actual or threatened change in control of our company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage some tactics that may be used in proxy fights. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company outweigh the disadvantages of discouraging such proposals because, among other things, negotiation of such proposals could result in an improvement of their terms.
However, these provisions could have the effect of discouraging others from making tender offers for our shares that could result from actual or rumored takeover attempts. These provisions also may have the effect of preventing changes in our management.
Transfer Agent
Our transfer agent is American Stock Transfer & Trust Company located at 6201 15th Avenue, Brooklyn, New York 11219, and their telephone number is (800) 937-5449.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
LEGAL MATTERS
Unless otherwise indicated in the applicable prospectus supplement, Horwitz + Armstrong, A Professional Law Corporation, Lake Forest, California, will provide opinions regarding the validity of the shares of our Common Stock. Horwitz + Armstrong, A Professional Law Corporation may also provide opinions regarding certain other matters.
EXPERTS
PMB Helin Donovan, LLP, independent registered public accounting firm, has audited our financial statements at September 30, 2015 and 2014, and for each of the two years in the period ended September 30, 2015, as set forth in their report which includes an explanatory paragraph relating to our ability to continue as a going concern, included elsewhere in this prospectus. We have included our financial statements in this prospectus and elsewhere in this Registration Statement in reliance on PMB Helin Donovan, LLP's report, given on their authority as experts in accounting and auditing.
Except as noted below, no expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the shares and warrants and its underlying securities was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a Registration Statement on Form S-1 under the Securities Act of 1933 with respect to the shares of common stock we are offering to sell. This prospectus, which constitutes part of the Registration Statement, does not include all of the information contained in the Registration Statement and the exhibits, schedules and amendments to the Registration Statement. For further information with respect to us and our common stock, we refer you to the Registration Statement and to the exhibits and schedules to the Registration Statement. Statements contained in this prospectus about the contents of any contract, agreement or other document are not necessarily complete, and, in each instance, we refer you to the copy of the contract, agreement or other document filed as an exhibit to the Registration Statement. Each of these statements is qualified in all respects by this reference.
You may read and copy the Registration Statement of which this prospectus is a part at the SEC's public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. You can request copies of the Registration Statement by writing to the Securities and Exchange Commission and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC's public reference room. In addition, the SEC maintains a website, which is located at www.sec.gov, that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the Registration Statement of which this prospectus is a part at the SEC's website.
We are subject to the information reporting requirements of the Securities Exchange Act of 1934 and are required to file reports, proxy statements and other information with the SEC. All documents filed with the SEC are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at www.growlifeinc.com. You may access our reports, proxy statements and other information free of charge at this website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information on such website is not incorporated by reference and is not a part of this prospectus.
INDEX TO FINANCIAL STATEMENTS |
Title of Document | | Page |
| | |
Consolidated Balance Sheets as of June 30, 2016 and September 30, 2015 (audited) | | F-2 |
| | |
Consolidated Statements of Operations for the three and nine months ended June 30, 2016 and 2015 | | F-3 |
| | |
Consolidated Statements of Cash Flows for the nine months ended June 30, 2016 and 2015 | | F-4 |
| | |
Notes to the Financial Statements | | F-5 |
| | |
| | |
Report of PMB Helin Donovan, LLP | | F-22 |
| | |
Consolidated Balance Sheets as of September 30, 2015 and 2014 | | F-23 |
| | |
Consolidated Statements of Operations for the years ended September 30, 2015 and 2014 | | F-24 |
| | |
Consolidated Statements of Changes in Stockholders' Equity (Deficit) for the years ended September 30, 2015 and 2014 | | F-25 |
| | |
Consolidated Statements of Cash Flows for the years ended September 30, 2015 and 2014 | | F-26 |
| | |
Notes to the Financial Statements | | F-27 |
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED BALANCE SHEETS |
| | | |
| ASSETS | | |
| | | |
| CURRENT ASSETS: | | |
| Cash and cash equivalents | $276,879 | $82,266 |
| Accounts receivable, net of allowance of $40,000 and $40,000, respectively | 1,102,821 | 619,849 |
| Prepaid expenses | 19,742 | 27,774 |
| Inventories, net | 306,529 | 217,824 |
| Total current assets | 1,705,971 | 947,713 |
| | | |
| EQUIPMENT, NET | 303,584 | 366,250 |
| | | |
| OTHER ASSETS | | |
| Intangible assets, net | 56,875 | 158,000 |
| Goodwill | 983,645 | 983,645 |
| Other assets | 5,070 | 5,070 |
| | | |
| TOTAL ASSETS | $3,055,145 | $2,460,678 |
| | | |
| LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | |
| | | |
| CURRENT LIABILITIES: | | |
| Accounts payable - trade | $2,655,741 | $2,520,223 |
| Accounts payable - related parties | 3,996 | 73,455 |
| Accrued expenses | 37,190 | 4,068 |
| Accrued expenses - related parties | 1,288,743 | 1,256,861 |
| Derivative liability | 738,984 | 2,704,840 |
| Convertible notes payable | 957,310 | 109,000 |
| Notes payable - current portion of long term debt | 1,547,587 | 1,164,692 |
| Deferred revenue | - | 5,833 |
| Total current liabilities | 7,229,551 | 7,838,972 |
| | | |
| COMMITMENTS AND CONTINGENCIES | - | - |
| | | |
| STOCKHOLDERS' DEFICIT | | |
| Preferred stock - $0.001 par value, 5,000,000 shares authorized, 0 shares issued and | | |
| outstanding at 6/30/2016 and 9/30/2015, respectively | - | - |
| Series A Convertible Preferred stock - $0.001 par value, 23,334 shares authorized, 23,334 | | |
| and 11,667 issued and outstanding at 6/30/2016 and 9/30/2015, respectively | 23 | 12 |
| Series B Redeemable Convertible Preferred stock - $0.001 par value, 5,000 shares authorized, | | |
| 221 and 0 shares issued and outstanding at 6/30/2016 and 9/30/2015, respectively | 5 | - |
| Common stock - $0.001 par value, 100,000,000 shares authorized, 1,410,628 | | |
| and 1,155,991 shares issued and outstanding at 6/30/2016 and 9/30/2015, respectively | 1,411 | 1,156 |
| Additional paid in capital | 20,585,633 | 18,786,694 |
| Accumulated deficit | (24,761,478) | (24,166,156) |
| Total stockholders' deficit | (4,174,406) | (5,378,294) |
| | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $3,055,145 | $2,460,678 |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS OF OPERATIONS |
| | | |
| | | | | |
| | | | | |
| REVENUE | $1,833,283 | $1,546,931 | $4,585,548 | $4,825,099 |
| COST OF SALES | 1,495,568 | 1,291,046 | 3,834,521 | 4,041,058 |
| GROSS PROFIT | 337,715 | 255,885 | 751,027 | 784,041 |
| RESEARCH AND DEVELOPMENT EXPENSES | 69,387 | 89,097 | 243,114 | 285,627 |
| SELLING, GENERAL AND ADMINISTRATIVE EXPENSES | 743,016 | 821,101 | 2,297,001 | 2,349,326 |
| OPERATING LOSS | (474,688) | (654,313) | (1,789,088) | (1,850,912) |
| | | | | |
| OTHER INCOME (EXPENSE): | | | | |
| Interest expense | (118,486) | (17,124) | (254,557) | (138,144) |
| Other (expense) income | (14,199) | 5,336 | (10,934) | 23,784 |
| Gain on change - derivative liability | 2,835,362 | 1,428,093 | 1,965,856 | 1,809,116 |
| (Loss) on conversion of debt | (506,599) | - | (506,599) | - |
| Total other income | 2,196,078 | 1,416,305 | 1,193,766 | 1,694,756 |
| | | | | |
| INCOME (LOSS) BEFORE INCOME TAXES | 1,721,390 | 761,992 | (595,322) | (156,156) |
| | | | | |
| Income taxes - current provision | - | (5,288) | - | (8,377) |
| | | . | | . |
| NET INCOME (LOSS) | $1,721,390 | $767,280 | $(595,322) | $(147,779) |
| | | | | |
| Basic income (loss) per common share attributable to Visualant, | | | | |
| Inc. and subsidiaries common shareholders- | | | | |
| Basic income (loss) per share | $1.29 | $0.68 | $(0.48) | $(0.13) |
| | | | | |
| Weighted average shares of common stock outstanding- basic | 1,339,484 | 1,131,960 | 1,236,721 | 1,127,252 |
| | | | | |
| Diluted income (loss) per common share attributable to Visualant, | | | | |
| Inc. and subsidiaries common shareholders- | | | | |
| Diluted income (loss) per share | $0.97 | $0.68 | $(0.48) | $(0.13) |
| | | | | |
| Weighted average shares of common stock outstanding- diluted | 1,779,650 | 1,131,960 | 1,236,721 | 1,127,252 |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS OF CASH FLOWS |
| | |
| | | |
| | | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | |
| Net loss | $(595,322) | $(147,779) |
| Adjustments to reconcile net loss to net cash (used in) | | |
| operating activities | | |
| Depreciation and amortization | 136,129 | 299,493 |
| Issuance of capital stock for services and expenses | 222,061 | 137,500 |
| Stock based compensation | 35,511 | 53,222 |
| Loss (gain) on sale of assets | 22,367 | (20,042) |
| Gain on change - derivative liability | (1,965,856) | (1,809,116) |
| Provision for losses on accounts receivable | 211 | 12,035 |
| Non-cash related to issuance of convertible notes payable | 148,790 | - |
| Issuance of common stock related to convertible notes payable | | |
| and Series B Redeemable Convertible Preferred stock | 577,478 | - |
| Changes in operating assets and liabilities: | | |
| Accounts receivable | (483,183) | 131,146 |
| Prepaid expenses | 8,032 | 9,741 |
| Inventory | (88,705) | 143,527 |
| Accounts payable - trade and accrued expenses | 131,063 | 1,040,396 |
| Income tax receivable | - | (8,377) |
| Deferred revenue | (5,833) | 8,333 |
| CASH (USED IN) OPERATING ACTIVITIES | (1,857,257) | (149,921) |
| | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | |
| Capital expenditures | (1,290) | - |
| Proceeds from sale of equipment | 6,585 | 21,452 |
| NET CASH PROVIDED BY INVESTING ACTIVITIES: | 5,295 | 21,452 |
| | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | |
| Proceeds (repayments) from line of credit | 382,895 | (139,558) |
| Proceeds from sale of preferred stock | 505,000 | 350,000 |
| Proeeds from warrant exercises | 349,159 | - |
| Proceeds from convertible notes payable | 924,500 | 64,000 |
| Repayments of convertible notes | (114,979) | (166,500) |
| Repayments of capital leases | - | (2,290) |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,046,575 | 105,652 |
| | | |
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 194,613 | (22,817) |
| | | |
| CASH AND CASH EQUIVALENTS, beginning of period | 82,266 | 70,386 |
| | | |
| CASH AND CASH EQUIVALENTS, end of period | $276,879 | $47,569 |
| | | |
| Supplemental disclosures of cash flow information: | | |
| Interest paid | $33,646 | $97,290 |
| Taxes paid | $- | $- |
| | | |
| Gain on change - derivative liability warrants | $- | $17,727 |
| Issuance of common stock for debt conversion | $- | $25,499 |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Visualant, Incorporated (the “Company,” “Visualant, Inc.” or “Visualant”) was incorporated under the laws of the State of Nevada in 1998. The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares preferred stock, par value $0.001 per share. On July 21, 2015, the Company filed with the Secretary of State of Nevada an Amended and Restated Certificate of Designations, Preferences and Rights for our Series A Convertible Preferred Stock. On March 8, 2016, the Company received approval from the State of Nevada for the Certificate of Designations of Preferences, Powers, Rights and Limitations of Series B Redeemable Convertible Preferred Stock. The Certificate authorized 5,000 shares of Series B Preferred Stock at a par value of $.001 per share that is convertible into common stock at $7.50 per share, subject to certain adjustments as set forth in the Certificate.
Since 2007 the Company has been focused primarily on the development of a proprietary technology which is capable of uniquely identifying and authenticating almost any substance using light at the “photon” level to detect the unique digital “signature” of the substance. The Company calls this its “ChromaID™” technology.
In 2010, the Company acquired TransTech Systems, Inc. as an adjunct to its business. TransTech is a distributor of products for employee and personnel identification. TransTech currently provides substantially all of the Company’s revenues.
The Company is in the process of commercializing its ChromaID™ technology. To date, the Company has entered into one License Agreement with Sumitomo Precision Products Co., Ltd. and has a strategic relationship with Invention Development Management Company, L.L.C. (“IDMC”).
The Company believes that its commercialization success is dependent upon its ability to significantly increase the number of customers that are purchasing and using its products. To date the Company has generated minimal revenue from sales of its ChromaID products. The Company is currently not profitable. Even if the Company succeeds in introducing the ChromaID technology and related products to its target markets, the Company may not be able to generate sufficient revenue to achieve or sustain profitability.
ChromaID was invented by scientists from the University of Washington under contract with Visualant. The Company has pursued an aggressive intellectual property strategy and have been granted ten patents. The Company also has 20 patents pending. The Company possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to the Company in perpetuity by the Company’s strategic partner, Intellectual Ventures through its subsidiary IDMC.
On May 6, 2015, the Company’s stockholders approved a reverse split of our common stock, in a ratio to be determined by the Company’s Board of Directors, of not less than 1-for-50 nor more than 1-for-150. On June 9, 2015, the Company’s Board of Directors determined that the ratio of the reverse split would be 1-for-150. All warrant, option, share and per share information in this Form 10-Q gives retroactive effect for a 1-for-150 split with all numbers rounded up to the nearest whole share.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company incurred net losses of $595,322, $2,631,037 and $1,017,291 for the nine months ended June 30, 2016 and the years ended September 30, 2015 and 2014, respectively. Net cash used in operating activities was $1,857,257, $239,877 and $1,379,397 for the for the nine months ended June 30, 2016 and the years ended September 30, 2015 and 2014, respectively.
The Company anticipates that it will record losses from operations for the foreseeable future. As of June 30, 2016, the Company’s accumulated deficit was $24,761,478. The Company has limited capital resources, and operations to date have been funded with the proceeds from private equity and debt financings and loans from Ronald P. Erickson, our Chief Executive Officer, or entities with which he is affiliated. These conditions raise substantial doubt about our ability to continue as a going concern. The audit report prepared by the Company’s independent registered public accounting firm relating to our financial statements for the year ended September 30, 2015 includes an explanatory paragraph expressing the substantial doubt about the Company’s ability to continue as a going concern.
Continuation of the Company as a going concern is dependent upon obtaining additional working capital. The financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
3. | SIGNIFICANT ACCOUNTING POLICIES: ADOPTION OF ACCOUNTING STANDARDS |
Basis of Presentation– The accompanying unaudited consolidated financial statements include the accounts of the Company. Intercompany accounts and transactions have been eliminated. The preparation of these unaudited condensed consolidated financial statements were prepared in conformity with U.S. generally accepted accounting principles (“GAAP”).
The unaudited consolidated financial statements of the Company and the accompanying notes included in this Quarterly Report on Form 10-Q are unaudited. In the opinion of management, all adjustments necessary for a fair presentation of the Consolidated Financial Statements have been included. Such adjustments are of a normal, recurring nature. The Consolidated Financial Statements, and the accompanying notes, are prepared in accordance with generally accepted accounting principles in the United States ("GAAP") and do not contain certain information included in the Company's Annual Report on Form 10-K for the fiscal year ended September 30, 2015. The interim Condensed Consolidated Financial Statements should be read in conjunction with that Annual Report on Form 10-K.
Principles of Consolidation – The consolidated financial statements include the accounts of the Company and its wholly owned and majority-owned subsidiaries, TransTech Systems, Inc. Inter-Company items and transactions have been eliminated in consolidation.
Cash and Cash Equivalents – The Company classifies highly liquid temporary investments with an original maturity of three months or less when purchased as cash equivalents. The Company maintains cash balances at various financial institutions. Balances at US banks are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk for cash on deposit.
Accounts Receivable and Allowance for Doubtful Accounts – Accounts receivable consist primarily of amounts due to the Company from normal business activities. The Company maintains an allowance for doubtful accounts to reflect the expected non-collection of accounts receivable based on past collection history and specific risks identified within the portfolio. If the financial condition of the customers were to deteriorate resulting in an impairment of their ability to make payments, or if payments from customers are significantly delayed, additional allowances might be required.
Inventories – Inventories consist primarily of printers and consumable supplies, including ribbons and cards, badge accessories, capture devices, and access control components held for resale and are stated at the lower of cost or market on the first-in, first-out (“FIFO”) method. Inventories are considered available for resale when drop shipped and invoiced directly to a customer from a vendor, or when physically received by TransTech at a warehouse location. The Company records a provision for excess and obsolete inventory whenever an impairment has been identified. There is a $20,000 for impaired inventory as of June 30, 2016 and September 30, 2015, respectively.
Equipment – Equipment consists of machinery, leasehold improvements, furniture and fixtures and software, which are stated at cost less accumulated depreciation and amortization. Depreciation is computed by the straight-line method over the estimated useful lives or lease period of the relevant asset, generally 2-10 years, except for leasehold improvements which are depreciated over 5-20 years.
Intangible Assets/ Intellectual Property – The Company amortizes the intangible assets and intellectual property acquired in connection with the acquisition of TransTech, over sixty months on a straight - line basis, which was the time frame that the management of the Company was able to project forward for future revenue, either under agreement or through expected continued business activities. Intangible assets and intellectual property acquired from RATLab LLC and Javelin are recorded likewise. The Company performs annual assessments and has determined that no impairment is necessary. On June 7, 2011, the Company closed the acquisition of all Visualant related assets of the RATLab LLC, namely the rights to the medical field of use of the Chroma ID technology. On July 31, 2012, the Company closed the acquisition of all rights to the ChromaID technology in the environmental field of use from Javelin LLC.
Goodwill – Goodwill is the excess of cost of an acquired entity over the fair value of amounts assigned to assets acquired and liabilities assumed in a business combination. With the adoption of ASC 350, goodwill is not amortized, rather it is tested for impairment annually, and will be tested for impairment between annual tests if an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is done at a reporting unit level. Reporting units are one level below the business segment level, but are combined when reporting units within the same segment have similar economic characteristics. Under the criteria set forth by ASC 350, the Company has one reporting unit based on the current structure. An impairment loss generally would be recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit. The Company performs annual assessments and has determined that no impairment is necessary.
Long-Lived Assets – The Company reviews its long-lived assets for impairment annually or when changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Long-lived assets under certain circumstances are reported at the lower of carrying amount or fair value. Assets to be disposed of and assets not expected to provide any future service potential to the Company are recorded at the lower of carrying amount or fair value (less the projected cost associated with selling the asset). To the extent carrying values exceed fair values, an impairment loss is recognized in operating results.
Fair Value Measurements and Financial Instruments– ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
Level 1 – Quoted prices in active markets for identical assets and liabilities; |
Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and.
Level 3 - Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
|
Level 1 – Quoted prices in active markets for identical assets and liabilities;
Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and
Level 3 – Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, and accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities at June 30, 2016as of December 31, 2023 and September 30, 20152023 are based upon the short-term nature of the assets and liabilities.
We have a money market account which is considered a Level 1 asset. The balance as of December 31, 2023 and September 30, 2023 was $4,787,378, and $7,836,393, respectively. No other assets or liabilities are required to be recorded at fair value on a recurring nature.
Derivative financial instruments -
The Company evaluatesFinancial Instruments. Pursuant to ASC 815 “Derivatives and Hedging”, we evaluate all of itsour financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. We then determine if embedded derivative must be bifurcated and separately accounted for. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statements of operations. For stock-based derivative financial instruments, the Company useswe use a weighted average Black-Scholes-Merton option pricing model to value the derivative instruments at inception and on subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative instrument liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within twelve months of the balance sheet date.Revenue Recognition – Visualant We determined that the conversion features for purposes of bifurcation within convertible notes payable issued during 2020 and TransTech revenue are derived from products2021 were immaterial and services. Revenue is considered realized when the products or servicesas of December 31, 2023 all such convertible notes have been providedconverted to the customer, the work has been accepted by the customer and collectability is reasonably assured. Furthermore, if an actual measurement of revenue cannot be determined, the Company defers all revenue recognition until such time that an actual measurement can be determined. If during the course of a contract management determines that losses are expected to be incurred, such costs are charged to operations in the period such losses are determined. Revenues are deferred when cash has been received from the customer but the revenue has not been earned.
common stock. Stock Based Compensation
– The Company has. We have share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options and warrants to purchase shares of Company common stock at the fair market value at the time of grant. Stock-based compensation cost to employees is measured by the Companyus at the grant date, based on the fair value of the award, over the requisite service period.period under ASC 718. For options issued to employees, the Company recognizeswe recognize stock compensation costs utilizing the fair value methodology over the related period of benefit. Grants of stock options and stock to non-employees and other parties are accounted for in accordance with the ASC 505. Convertible Securities
– .Based upon ASC 815-15, we have adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities issued subsequent to September 30, 2015.determine if an instrument should be accounted for as equity or a liability. We will evaluate our contracts based upon the earliest issuance date. DIRECTORS AND EXECUTIVE OFFICERS
The following sets forth, as of March 25, 2024, the name, age, position and certain information of each executive officer and director and the tenure in office of each director of the Company.
Identification of Directors and Executive Officers
The following table sets forth certain information about our current directors and executive officers:
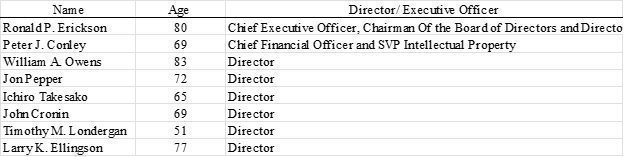
Set forth below is information regarding our directors and executive officers.
Ronald P. Erickson. Mr. Erickson was appointed as Chief Executive Officer in January 2023. Mr. Erickson previously served as our Chief Executive Officer from November 2009 to April 2018. He has served as Chairman of the Board from 2004 to 2011 and from 2015 to the present. A senior executive with more than 30 years of experience in the technology, telecommunications, software, and digital media industries, Mr. Erickson was the founder of our company. He is formerly Chairman, CEO and Co-Founder of Blue Frog Media, a mobile media and entertainment company; Chairman and CEO of eCharge Corporation, an Internet-based transaction procession company; Chairman, CEO and Co-founder of GlobalTel Resources, a provider of telecommunications services; Chairman, Interim President and CEO of Egghead Software, Inc., a software reseller where he was an original investor; Chairman and CEO of NBI, Inc.; and Co-founder of MicroRim, Inc., the database software developer. Earlier, Mr. Erickson practiced law in Seattle and worked in public policy in Washington, DC and New York, NY. Additionally, Mr. Erickson has been an angel investor and board member of a number of public and private technology companies. In addition to his business activities, Mr. Erickson was Chairman and a member of the Board of Trustees from 2010 to 2021 of Central Washington University where he received his BA degree. He also holds an MA from the University of Wyoming and a JD from the University of California, Davis. He is licensed to practice law in the State of Washington. Mr. Erickson is our founder and was appointed as a director because of his extensive experience in developing technology companies.
Peter J. Conley.Mr. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022.In addition, Mr. Conley currently serves as Senior Managing Director and Head of Intellectual Property Banking at Boustead Securities, LLC, a position he has held since October 2014, where he provides equity financing and M&A advisory services to small-cap public companies. Prior to that, from 2012 to 2016, Mr. Conley was a cofounder and Chief Operating Officer of ipCreate, a global IP development and innovation services company serving large multinational companies. He also served as managing director of ipCapital Venture Group, where he provided IP strategy and venture advisory services. During his career spanning more than 35 years, Mr. Conley has held leadership roles at MDB Capital Group, The Analytiq Group / RDEX Research, Roth Capital Partners, and Lehman Brothers. He was on the founding team and Head of Equity Capital Markets at E*Offering, the investment bank of E*Trade. Mr. Conley attended the University of Hawaii at Manoa and the University of London, Center for Financial & Management Studies, SOAS.
William A. Owens. Admiral Owens has served as an independent director since May 2018. William A. Owens is the co-founder and executive chairman of Red Bison Technology Group, a company which installs and operates high speed telecoms networks and technology in large office buildings. He is the Chairman of Visionary Vehicles which is building a series of automobiles focused on electric and hydrogen powered cars, Kyrrex which is a successful and growing Crypto Currency Exchange operating in Europe, and Massif, an electric bicycle company. Owens serves on the board of directors of the public companies, Siply, Know Labs, and Compass, and is a director of the private companies: TruU, Tethr, ViruSight, Prism, Steel Grove, JennyCo, Axxess Capital, Versium, and Viome. Owens was the chairman of the board of CenturyLink Telecom (now Lumen), the third largest telecommunications company in the United States and SAP USA. Owens is on the board of trustees of Seattle University, and the Fiscal Responsibility Amendment (CFFRA) Association which aims to establish a balanced budget amendment to the US Constitution. He is a member of the Council of Foreign Relations. He is the Founder and senior General on a China US forum to bring 4 star generals together for China US cooperation. He is a Senior Fellow at Stimson Institute. From 2007 to 2015, Owens was the Chairman and Senior Partner of AEA Investors Asia, a private equity firm located in Hong Kong, and Vice Chairman of the NYSE for Asia. Owens also served as the Chairman of Eastern Airlines. He has served on over 25 public boards including Daimler, British American Tobacco, Telstra, Nortel Networks, and Polycom.
Owens was the CEO of Nortel, a fortune 500 company, the CEO/Chairman of Teledesic, a Bill Gates/Craig McCaw company bringing worldwide broadband through an extensive satellite network and was the President of Science Applications International Corporation (SAIC). He also served on the boards of the not-for-profit organizations; Fred Hutchinson Cancer Research Center, Carnegie Corporation of New York, Brookings Institution, East West Institute, and RAND Corporation.
Owens is a retired four-star US Navy Admiral. He was Vice Chairman of the Joint Chiefs of Staff, the second-ranking United States military officer in the US, with responsibility for reorganizing and restructuring the armed forces in the post- Cold War era. He is widely recognized for bringing commercial high-grade technology into the Department of Defense for military applications. Owens was the architect of the Revolution in Military Affairs (RMA), an advanced systems technology approach to military operations, the most significant change in the system of requirements, budgets and technology for the four armed forces since World War II. Owens was Commander of the U.S. Sixth Fleet from 1990 to 1992, which included Operation Desert Storm. Owens also served as the deputy Chief of Naval Operations for Resources and Requirements. Owens was the Senior Military Assistant to two Secretaries of Defense (Cheney and Carlucci) and served in the Office of Program Appraisal for the Secretary of the Navy. He began his military career as a nuclear submariner. He served on four strategic nuclear-powered submarines and three nuclear attack submarines, including tours as Commanding Officer of the USS Sam Houston, USS Michigan, and USS City of Corpus Christi.
Owens is a 1962 honor graduate of the United States Naval Academy in mathematics, holds bachelors and masters degrees in politics, philosophy and economics from Oxford University, and a master’s degree in management from George Washington University. He has written more than 50 articles on national security and authored the book “High Seas.” His book, “Lifting the Fog of War,” was published in April 2000 with a revision published in Mandarin in 2009. And his book “China-US 2039: The Endgame” was published in 2019 in both English and Mandarin.
Owens has received numerous recognitions and awards: the “Légion d’Honneur” by France, and the highest awards given to foreigners by the countries of Indonesia and Sweden. He was named as one of The 50 Most Powerful People in Networking by Network World, one of the 100 Best Board Members in the United States for 2011 and again in 2016 awarded by NACD, and the Intrepid Salute Award in recognition of his business achievements and support of important philanthropic activities. Owens is active in philanthropy to foster Chinese – American relations including dialogues between the most senior retired officers in the United States and Chinese militaries. He is a North Dakota’s Roughriders recipients, the award given annually to the most prominent North Dakotans. Admiral Owens was appointed as a director of Know Labs because of his financials and governance skills.
Jon Pepper.Mr. Pepperhas served as an independent director since April 2006. Mr. Pepper founded Pepcom, a company that become the industry leader at producing press-only technology showcase events around the country and internationally, in 1980. He sold his stake in the corporation and retired as a partner at the end of 2018. Prior to that, Mr. Pepper started the DigitalFocus newsletter, a ground-breaking newsletter on digital imaging that was distributed to leading influencers worldwide. Mr. Pepper has been closely involved with the high technology revolution since the beginning of the personal computer era. He was formerly a well-regarded journalist and columnist. His work on technology subjects appeared in The New York Times, Fortune, PC Magazine, Men’s Journal, Working Woman, PC Week, Popular Science and many other well-known publications. Mr. Pepper was educated at Union College in Schenectady, New York and the Royal Academy of Fine Arts in Copenhagen. He continues to be active in non-profit work and private company boards and in 2017 founded Mulberry Tree Films, a non-profit that supports independent high-quality documentary films and other publishing and creative projects that are oriented toward increasing the understanding of human potential and creativity. Mulberry Tree funded and produced the acclaimed documentary, “The Gates of Shinto” and is currently at work on additional projects. Mr. Pepper was appointed as a director because of his marketing skills with technology companies. Mr. Pepper was appointed as a director because of his marketing skills with technology companies.
Ichiro Takesako. Mr. Takesako has served as a director since December 2012. Mr. Takesako has held executive positions with Sumitomo Precision Products Co., Ltd, or Sumitomo, and its affiliates since 1983. In the past few years, Mr. Takesako has held the following executive position in Sumitomo and its affiliates: in June 2008, he was appointed as General Manager of Sales and Marketing Department of Micro Technology Division; in April 2009, he was appointed as General Manager of Overseas Business Department of Micro Technology Division, in charge of M&A activity of certain business segment and assets of Aviza Technology, Inc.; in July 2010, he was appointed as Executive Director of SPP Process Technology Systems, a 100% owned subsidiary of Sumitomo Precision Products at the time; in August 2011, he was appointed as General Manager, Corporate Strategic Planning Group; in January 2013, he was appointed as Chief Executive Officer of M2M Technologies, Inc., a company invested by Sumitomo Precision products; in April 2013, has was appointed as General Manager of Business Development Department, in parallel of CEO of M2M Technologies, Inc.; in April 2014, he was relieved from General Manager of Business Development Department and is responsible for M2M Technologies Inc. as its CEO; in March 2017, he established At Signal, Inc. which took over the entire business operation from M2M Technologies, Inc.; and in April 2017, he was appointed as Chief Executive Officer of At Signal, Inc. Mr. Takesako graduated from Waseda University, Tokyo, Japan where he majored in Social Science and graduated with a Degree of Bachelor of Social Science. Mr. Takesako was appointed as a director based on his previous position with Sumitomo and Sumitomo’s previous significant partnership with our company. Mr. Takesako was appointed as a director based on his previous position with Sumitomo and Sumitomo’s previous significant partnership with our company.
John Cronin. Mr. Cronin has served as an independent director since November 2023. Mr. Cronin is an experienced inventor and intellectual property strategist. Mr. Cronin is Chairman and CEO of ipCapital Group, Inc. (“ipCG”), a globally recognized IP strategy consulting firm founded in 1998, offering more than 45 different services. Mr. Cronin has authored greater than 1,600 patents and applications across hundreds of technology spaces, leveraging the ipCapital Methodology. Before forming ipCG, Mr. Cronin spent over 17 years at IBM and became its top inventor with over 100 patents and 150 patent publications. He created and ran the IBM Patent Factory, which was essential in helping IBM become number one in US patents and led the team that contributed to the startup and success of IBM’s licensing program. Mr. Cronin is also the Chair of the Board of Directors of AdrenalineIP, Chairman of IX-Innovations, and is the Founder of HarvestWeb, a 501(c)3 charitable organization that provides an easy online way to make donations to food pantries. Mr. Cronin previously served on the board of directors of HopTo Inc. (OTC: HPTO) from 2014 to September 2018, and ImageWare® Systems, Inc. (OTCQB: IWSY) from 2012 until April 2020. Mr. Cronin has a B.S. (E.E.), an M.S. (E.E), and a B.A. degree in Psychology from the University of Vermont. As of the year ended September 30, 2023, we have paid ipCapital Group approximately $713,000 in professional fees. Mr. Cronin was appointed as a director based on his domain expertise and extensive experience in patent strategy, development and monetization.
Timothy M. Londergan, Ph.D.Mr. Londerganhas served as an independent director since November 2023. Mr. Londergan is a creative and results oriented business executive with 20 years of experience with early-stage technology companies. In May 2020, Mr. Londergan founded Tangibly, Inc., a company focused on helping companies manage their most valuable assets, trade secrets, through an innovative AI platform that can analyze patents and predict related trade secrets. Mr. Londergan continues to act as CEO of Tangibly, Inc. From May 2017-2021, Mr. Londergan founded and was CEO of WaveFront Venture Labs Pte Ltd, focused on helping leading Fortune 100 corporations incubate new companies based on highly promising, yet currently dormant technologies and was developing a platform to systematically evaluate and extract the most promising technologies trapped inside these companies and create paths to market for them via new company formation. WaveFront was acquired by Boustead Securities in 2021. From January 2018 to June 2020, Mr. Londergan was co-founder and CEO of Operem, Inc., a company that created blockchain based tools for managing intangible assets and was acquired by Abaxx. Mr. Londergan was recently named IAM 300 World’s Leading IP Strategist 2023. Mr. Londergan has 30+ issued patents, 20+ applications along with over 20 publications in peer reviewed journals and conference proceedings. Mr. Londergan holds a Ph.D. in Organic Chemistry, 1998, from the University of Southern California and a B.S. in Chemistry, 1995, from St. Bonaventure University. Mr. Londergan was appointed as a director based on his extensive industry experience working with early-stage technology companies and significant expertise in patent monetization and development of trade secrets programs.
Larry K. Ellingson.Mr. Ellingsonhas served as an independent director since November 2023. Mr. Ellingson holds a BS, Pharmacy, from North Dakota State University (NDSU), Fargo, North Dakota and an Executive MBA from Babson College, Babson Park, Massachusetts. From April 23, 2019 to present, Mr. Ellingson has served as a member of Know Labs advisory board. From 2013 to present, Mr. Ellingson is Co-Founder of the Diabetes Leadership Council and Vice Chair Global initiatives. Since 2006 to present, Mr. Ellingson has been President of Global Diabetes Consulting LTD.
Mr. Ellingson retired from Eli Lilly and Company in May 2001 after having been involved as a leader of global diabetes for Lilly for more than half of his career. He has held several other positions at Lilly including Director of Pharmaceutical New Product Planning for gastrointestinal, skeletal, endocrine and infectious diseases, along with responsibility for marketed products in those areas in the late 1980s.
Mr. Ellingson continues to remain active with committee work and board positions for a multitude of organizations, among them are NDSU, Research Park, International Diabetes Federation, Academy of Nutrition and Dietetics, Nurse Practitioners Healthcare Foundation and the American Diabetes Association®. His contributions to the Association have been abundant and far-reaching and have spanned over 20 years. He has held numerous positions within the Association such as member of the Industry Advisory, Strategic Marketing Task Force, Strategic Planning Task Force, Big Ticket Task Force, Pinnacle Society and the Income Development Committee. He has been Chair or Vice Chair for an equally extensive list of bodies within the Association including the Board of Directors, Fundraising Committee, Executive Committee and Nominating Committee. He has unquestionably been a positive force and an integral part of mission delivery.
Mr. Ellingson has been honored several times for his achievements in his field. He was honored by being the first and only non–scientist to receive Eli Lilly’s President’s Award and the Lilly Research Award for contributions to diabetes research. In 2001, Eli Lilly created the Ellingson Legacy Award to honor those who provide outstanding service to the customer. Ellingson was the first recipient of the award. The ADA, Indiana affiliate awarded Mr. Ellingson the J.K. Lilly Award in 2004 for his contributions & service to the field of diabetes. NDSU awarded him the highest honor in 2007, naming him An Outstanding Alumni of the Year for his contributions to the field and to the University. The American Diabetes Association® recognized Mr. Ellingson in 2006 with the Charles H. Best Medal for Outstanding Service for his exceptional contributions as Chair of the Board. The ADA recognized Mr. Ellingson with the prestigious Wendell Mayes Jr. Award in 2013 for his long-term service in diabetes. Mr. Ellingson received an Honorary Membership in 2020 to the Academy of Nutrition and Dietetics for his contributions to the Academy. He continues to be engaged in diabetes programs and projects through the Diabetes Leadership Council which he cofounded in 2013. He continues to be engaged in diabetes programs and projects through the Diabetes Leadership Council which he cofounded in 2013. Mr. Ellingson was appointed as a director based on his substantial experience in the diabetes industry and his global thought leadership in the field of diabetes.
Term of Office
Our directors currently have terms which will end at our next annual meeting of stockholders or until their successors are elected and qualify, subject to their prior death, resignation or removal. Officers serve at the discretion of the Board.
Family Relationships
There are no family relationships among any of our officers or directors.
Involvement in Certain Legal Proceedings
To the best of our knowledge, except as described below, none of our directors or executive officers has, during the past ten years:
| · | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| | |
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| | |
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| | |
| · | been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| | |
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | |
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
The Board’s Role in Risk Oversight
Our Board oversees that the assets of our company are properly safeguarded, that the appropriate financial and other controls are maintained, and that our business is conducted wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the Board’s oversight of the various risks facing our company. In this regard, our Board seeks to understand and oversee critical business risks. Our Board does not view risk in isolation. Risks are considered in virtually every business decision and as part of our business strategy. Our Board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is essential for our company to be competitive on a global basis and to achieve our objectives.
While the Board oversees risk management, company management is charged with managing risk. Management communicates routinely with the Board and individual directors on the significant risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management.
Our Board administers its risk oversight function as a whole by making risk oversight a matter of collective consideration; however, much of the work is delegated to committees, which will meet regularly and report back to the full Board. The audit committee oversees risks related to our financial statements, the financial reporting process, accounting and legal matters, the compensation committee evaluates the risks and rewards associated with our compensation philosophy and programs, and the nominating and corporate governance committee evaluates risks associated with management decisions and strategic direction.
Attendance at Annual Meetings of Stockholders
We expect that all of our Board members will attend our annual meetings of stockholders in the absence of a showing of good cause for failure to do so and all attended our 2023 annual meeting of stockholders in person or by telephone.
Board Meetings and Committees
During our last fiscal year, each of our directors attended at least 75% of the aggregate of (i) the total number of Board meetings and (ii) the total number of meetings of the committees on which the director served.
Independent Directors
NYSE American’s rules generally require that a majority of an issuer’s board of directors must consist of independent directors. Our board of directors currently consists of seven (7) directors, six (6) of whom, Messrs. Owens, Pepper, Takesako, Cronin, Londergan, and Ellingson are independent within the meaning of NYSE American rules.
Committees of the Board of Directors
Our Board has established an audit committee, a compensation committee and a nominating and corporate governance committee, each with its own charter approved by the Board. Each committee’s charter is available on our website at www.knowlabs.co. In addition, our Board may, from time to time, designate one or more additional committees, which shall have the duties and powers granted to it by our Board.
Audit Committee
William A. Owens, Jon Pepper and Tim Londergan, each of whom satisfies the “independence” requirements of Rule 10A-3 under the Exchange Act and NYSE American’s rules, serve on our audit committee, with Mr. Pepper serving as the chairman. Our Board has determined that Mr. Owens qualifies as an “audit committee financial expert” as defined by applicable SEC rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of the Company.
The audit committee is responsible for, among other things: (i) retaining and overseeing our independent accountants; (ii) assisting the Board in its oversight of the integrity of our financial statements, the qualifications, independence and performance of our independent auditors and our compliance with legal and regulatory requirements; (iii) reviewing and approving the plan and scope of the internal and external audit; (iv) pre-approving any audit and non-audit services provided by our independent auditors; (v) approving the fees to be paid to our independent auditors; (vi) reviewing with our chief executive officer and principal financial officer and independent auditors the adequacy and effectiveness of our internal controls; (vii) reviewing hedging transactions; and (viii) reviewing and assessing annually the audit committee’s performance and the adequacy of its charter. The audit committee is also responsible for preparing a report to be included with this Proxy Statement. Our audit committee met 4 times during the last fiscal year.
Compensation Committee
William A. Owens, John Cronin and Jon Pepper, each of whom satisfies the “independence” requirements of Rule 10C-1 under the Exchange Act and NYSE American’s rules, serve on our compensation committee, with Mr. Owens serving as the chairman. The members of the compensation committee are also “non-employee directors” within the meaning of Section 16 of the Exchange Act. The compensation committee assists the Board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers.
The compensation committee is responsible for, among other things: (i) reviewing and approving the remuneration of our executive officers; (ii) making recommendations to the Board regarding the compensation of our independent directors; (iii) making recommendations to the Board regarding equity-based and incentive compensation plans, policies and programs; and (iv) reviewing and assessing annually the compensation committee’s performance and the adequacy of its charter. Our compensation committee met 4 times during the last fiscal year.
No member of our compensation committee is or has been our current or former officer or employee. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director or member of our compensation committee during the fiscal year ended September 30, 2023.
Nominating and Corporate Governance Committee
Larry K. Ellingson, Jon Pepper and Ichiro Takesako, each of whom satisfies the “independence” requirements of NYSE American’s rules, serve on our nominating and corporate governance committee, with Mr. Ellingson serving as the chairman. The nominating and corporate governance committee assists the Board in selecting individuals qualified to become our directors and in determining the composition of the Board and its committees.
The nominating and corporate governance committee is responsible for, among other things: (i) identifying and evaluating individuals qualified to become members of the Board by reviewing nominees for election to the Board submitted by stockholders and recommending to the Board director nominees for each annual meeting of stockholders and for election to fill any vacancies on the Board; (ii) advising the Board with respect to Board organization, desired qualifications of Board members, the membership, function, operation, structure and composition of committees (including any committee authority to delegate to subcommittees), and self-evaluation and policies; (iii) advising on matters relating to corporate governance and monitoring developments in the law and practice of corporate governance; (iv) overseeing compliance with our code of ethics; and (v) approving any related party transactions.
The nominating and corporate governance committee’s methods for identifying candidates for election to our Board (other than those proposed by our stockholders, as discussed below) include the solicitation of ideas for possible candidates from a number of sources – members of our Board, our executives, individuals personally known to the members of our Board, and other research. The nominating and corporate governance committee may also, from time-to-time, retain one or more third-party search firms to identify suitable candidates.
In making director recommendations, the nominating and corporate governance committee may consider some or all of the following factors: (i) the candidate’s judgment, skill, and experience with other organizations of comparable purpose, complexity and size, and subject to similar legal restrictions and oversight; (ii) the interplay of the candidate’s experience with the experience of other Board members; (iii) the extent to which the candidate would be a desirable addition to the Board and any committee thereof; (iv) whether or not the person has any relationships that might impair his or her independence; and (v) the candidate’s ability to contribute to the effective management of the Company, taking into account the needs of the Company and such factors as the individual’s experience, perspective, skills and knowledge of the industry in which we operate.
A stockholder may nominate one or more persons for election as a director at an annual meeting of stockholders if the stockholder complies with the notice and information provisions contained in our Bylaws. Such notice must be received in writing to our Company not later than the close of business fourteen (14) days nor earlier than the close of business eighty (80) days prior to the first anniversary of the preceding year’s annual meeting; provided, however, that if less than twenty-one (21) days’ notice of the meeting is given to stockholders, such writing shall be received by the Secretary of the Corporation not later than the close of the seventh (7th) day following the day on which notice of the meeting was mailed to stockholders. In addition, stockholders furnishing such notice must be a holder of record on both (i) the date of delivering such notice and (ii) the record date for the determination of stockholders entitled to vote at such meeting.
Code of Ethics
We have adopted a code of ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. Such code of ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, and reporting of violations of the code.
A copy of the code of ethics has been filed as an exhibit to our registration statement on Form S-1, as amended, July 29, 2022, and is also available on our website as www.knowlabs.io. We are required to disclose any amendment to, or waiver from, a provision of our code of ethics applicable to our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar functions. We intend to use our website as a method of disseminating this disclosure as well as by SEC filings, as permitted or required by applicable SEC rules. Any such disclosure will be posted to our website within four (4) business days following the date of any such amendment to, or waiver from, a provision of our code of ethics.
Communication with our Board of Directors
Our stockholders and other interested parties may communicate with our Board of Directors by sending written communication in an envelope addressed to “Board of Directors” in care of the Secretary, 500 Union Street, Suite 810, Seattle, Washington 98101.
Section 16(a) Beneficial Ownership Reporting Compliance
Our executive officers, directors and 10% stockholders are required under Section 16(a) of the Exchange Act to file reports of ownership and changes in ownership with the SEC. Copies of these reports must also be furnished to us.
Based solely on a review of copies of reports furnished to us, as of September 30, 2023 our executive officers, directors and 10% holders complied with all filing requirements except as follows:
Todd Baszucki filed a Form 4 on October 25, 2023 that was required to be filed on October 3, 2023.
Phillip A. Bosua filed a Form 4 on February 14, 2023 that was required to be filed on January 25, 2023.
William A. Owens filed a Form 4 on May 22, 2023 that was required to be filed on May 18, 2023.
Ichiro Takesako filed a Form 4 on April 10, 2023 that was required to be filed on February 17, 2023.
Ichiro Takesako filed a Form 4 on April 20, 2023 that was required to be filed on March 27, 2023.
Jon Pepper filed a Form 4 on April 10, 2023 that was required to be filed on February 17, 2023.
EXECUTIVE COMPENSATION
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons (our “named executive officers”) for services rendered in all capacities during the years ended September 30, 2023 and September 30, 2022, respectively. The Company meets the requirements of a “smaller reporting company” and has utilized the scaled reporting requirements available to qualifying companies. No other executive officers received total annual salary and bonus compensation in excess of $100,000.
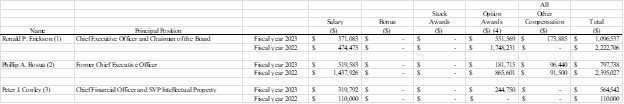
(1) During the years ended September 30, 2023 and 2022, the Compensation Committee and the Board compensated Ronald P. Erickson with a salary of $300,000 from April 1, 2021 to March 15, 2022 and $325,000 from March 15, 2022 to September 30, 2022. From December 14, 2022, Mr. Erickson has been compensated with an annual salary of $375,000. An entity affiliated with and controlled by Mr. Erickson, J3E2A2Z LP, was paid interest of $173,855 during the year ended September 30, 2023. Mr. Erickson was paid deferred compensation of $165,000 during the year ended September 30, 2022, respectively. See “Outstanding Equity Awards at Year-End” for a discussion of option award compensation.
(2) Mr. Bosua resigned from the Board of Directors and from his position as Chief Executive Officer on January 23, 2023. Mr. Bosua is party to a Separation and Release Agreement with the Company, pursuant to which he was entitled to receive severance payments. Such payments are described in greater detail below under “Employment and Separation Agreements.” During the years ended September 30, 2023 and 2022, the Compensation Committee and the Board compensated Phillip A. Bosua at an annual salary of $350,000 from April 1, 2021 to January 23, 2023. Mr. Bosua was also paid $400,000 in severance and $96,440 in rent and other costs during the year ended September 30, 2023. Mr. Bosua was paid $1,097,928 in compensation and $91,500 in rent expenses for services provided to AI Mind, a wholly owned subsidiary of the Company, in connection with the development of NFT sales for the year ended September 30, 2022. See the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Digital Asset Sales” and Note 4 to the Notes to our Consolidated Financial Statements, See “Outstanding Equity Awards at Year-End” for a discussion of option award compensation.
(3) Mr. Peter J. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022.During the year ended September 30, 2022, the Compensation Committee and the Board compensated Mr. Conley with an annual salary of $300,000 from May 20, 2022 to September 30, 2022. From December 14, 2022 to September 30, 2023, Mr. Conley has been compensated with an annual salary of $325,000. See “Outstanding Equity Awards at Year-End” for a discussion of option award compensation.
(4) These amounts reflect the aggregate grant date fair value of awards granted in the fiscal year ended September 30, 2022, as required by Regulation S-K Item 402(n)(2), computed in accordance with the FASB Accounting Standards Codification Topic 718 (“FASB ASC Topic 718”). All assumptions made in the valuations are contained and described in footnote 8 to the Company’s financial statements for Fiscal 2023 contained in this Annual Report on Form 10-K for the fiscal year ended September 30, 2023. The amounts shown in the table reflect the total fair value on the date of the grant.
Employment Agreements
On April 10, 2018, we entered into an amended employment agreement for Ronald P. Erickson which amends our employment agreement with him dated July 1, 2017. The employment agreement provides for a base salary of $180,000 per year, which was increased to $215,000 from May 1, 2020 to March 31, 2021, to $300,000 from April 1, 2021 to March 15, 2022 and to $325,000 from March 15, 2022 to September 30, 2022. From December 14, 2022, Mr. Erickson has been compensated with an annual salary of $375,000. Mr. Erickson will be entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. The employment agreement is for an initial term of 12 months (subject to earlier termination) and will be automatically extended for additional 12-month terms unless either party notifies the other party of its intention to terminate the employment agreement at least ninety (90) days prior to the end of the initial term or renewal term. If we terminate Mr. Erickson’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Erickson terminates his employment at any time for “good reason” or due to a “disability,” Mr. Erickson will be entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months. On January 23, 2023, the Board appointed Mr. Erickson our Chief Executive Officer. Mr. Erickson was appointed to serve until his successor is duly elected.
On April 10, 2018, we entered into an employment agreement with Phillip A. Bosua reflecting his appointment as Chief Executive Officer. The employment agreement provided for a base salary of $225,000 per year, which was increased to $260,000 from May 1, 2020 to March 31, 2021 and to $350,000 from April 1, 2021 to January 23, 2023. Mr. Bosua also received 500,000 shares of common stock valued at $0.33 per share and was entitled to bonuses and equity awards at the discretion of the Board or a committee of the Board. Mr. Bosua was entitled to participate in all group employment benefits that are offered by us to our senior executives and management employees from time to time, subject to the terms and conditions of such benefit plans, including any eligibility requirements. The employment agreement was for an initial term of 12 months (subject to earlier termination) and was automatically extended for additional 12-month terms unless either party notified the other party of its intention to terminate the employment agreement at least ninety (90) days prior to the end of the initial term or renewal term. If we terminated Mr. Bosua’s employment at any time prior to the expiration of the term without cause, as defined in the employment agreement, or if Mr. Bosua terminated his employment at any time for “good reason” or due to a “disability,” Mr. Bosua was entitled to receive (i) his base salary amount for one year; and (ii) medical benefits for eighteen months.
On January 23, 2023, Mr. Bosua resigned from the Board and from his position as our Chief Executive Officer. In connection with his resignation, we entered into a Separation and Release Agreement (the “Separation Agreement”) with Mr. Bosua containing customary terms and mutual releases, pursuant to which Mr. Bosua is entitled receive a $400,000 severance payment and benefits pursuant to his prior employment agreement. Pursuant to the Separation Agreement, Mr. Bosua’s outstanding stock options ceased vesting as of January 23, 2023, and all vested stock options remain exercisable through January 23, 2024. Mr. Bosua has been engaged as a consultant to the Company for a period of one year at a rate of $10,000 per month. Mr. Bosua also entered into a lock up and leak out agreement with respect to 3,005,000 common shares owned by Mr. Bosua and shares issuable upon exercise of his vested option awards. During the period commencing March 17, 2023 through March 17, 2024, Mr. Bosua may sell no more than 1,500,000 shares. During the period commencing April 1, 2024 through June 30, 2026, Mr. Bosua may sell no more than 375,000 shares per quarter (or 1,500,000 shares per year), unless the stock price of our common stock exceeds $5.00 per share on the NYSE American (the “Stock Price Threshold”), then Mr. Bosua may sell a maximum of 750,000 shares during any such quarter that the Stock Price Threshold is met. Notwithstanding the foregoing, any lock-up or leak-out restrictions are waived for any sales of shares from Mr. Bosua to Todd Baszucki.
On May 13, 2022, we entered into an employment agreement with Peter J. Conley reflecting his appointment as our Chief Financial Officer and Senior Vice President, Intellectual Property. The employment agreement provides for a base salary of $300,000. From December 14, 2022 to September 30, 2023, Mr. Conley has been compensated with an annual salary of $325,000, Mr. Conley may also be entitled to bonuses from time to time as determined by our Board or our compensation committee in their sole discretion. Mr. Conley is eligible to participate in all our employee benefit plans, policies and arrangements that are applicable to other executive officers, as such plans, policies and arrangements may exist or change from time to time at our discretion. We will reimburse Mr. Conley for reasonable travel, entertainment and other expenses he incurs in the furtherance of his duties under the employment agreement. The employment agreement is at will, meaning either we or Mr. Conley may terminate the employment relationship at any time, with or without cause, upon written notice to the other party. The employment agreement provides for severance pay equal to 12 months of then-in-effect base salary if Mr. Conley is terminated without “cause” or voluntarily terminates his employment for “good reason,” as defined in the employment agreement.
Outstanding Equity Awards at Fiscal Year-End
The following table includes certain information with respect to the value of all unexercised options and unvested shares of restricted stock previously awarded to the executive officers named above at the fiscal year ended September 30, 2023.
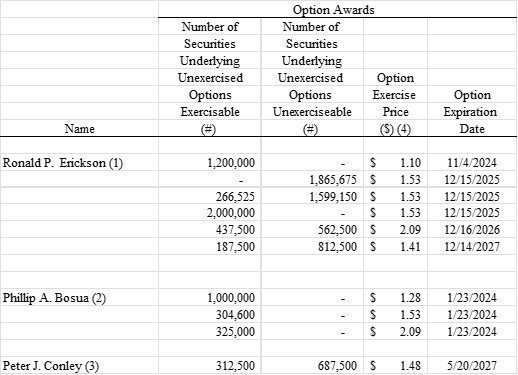
(1) On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vested upon uplisting to the NASDAQ or NYSE exchanges. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $1,207,200 during the year ended September 30, 2022. On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vests in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. We estimated at grant date the fair value of these options at approximately $520,869 which is being amortized over 5 years. As of September 30, 2023 and 2022, we recorded an expense of $104,172 and a cumulative expense of $186,657, respectively. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2023 and 2022. On December 15, 2020, we issued an additional stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $263,593 during the year ended September 30, 2022. The stock option grants vest when earned based on certain performance criteria. On December 15, 2020, we issued a fully vested warrant to Ronald P. Erickson for 2,000,000 shares of common stock. The five year warrant is exercisable for cash or non-cash at $1.53 per share and was valued using a Black-Scholes model at $1,811,691. On December 16, 2021, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years. On December 14, 2022, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $1.41 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
(2) On July 30, 2018, Mr. Bosua was awarded a stock option grant for 1,000,000 shares of our common stock that was awarded at $1.28 per share. The stock option grant vests quarterly over four years. The performance grant was not earned as of September 30, 2022. On November 4, 2019, we granted a stock option grant to Philip A. Bosua for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon FDA approval of the UBAND blood glucose monitor. On December 15, 2020, we issued a stock option grant to Phillip A. Bosua for 2,132,200 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vested in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. We estimated at grant date the fair value of these options at approximately $595,277 which is being amortized over 5 years. As of September 30, 2023 and 2022, we recorded an expense of $37,370 and a cumulative expense of $231,321, respectively. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2023 and 2022. On December 15, 2020, we issued another stock option grant to Phillip A. Bosua for 2,132,195 shares at an exercise price of $1.53 per share. The stock option grants expire in five years. The stock option grants vest when earned based on certain performance criteria. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $301,249 during the year ended September 30, 2022. On December 16, 2021, we issued a stock option grant to Phillip A. Bosua for 1,300,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years. On December 14, 2022, we issued a stock option grant to Philip A. Bosua for 1,250,000 shares at an exercise price of $1.41 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
Mr. Bosua resigned from the Board of Directors and from his position as Chief Executive Officer on January 23, 2023. Pursuant to the Separation Agreement, as of January 23, 2023, all of Mr. Bosua’s outstanding stock options listed above ceased vesting as of January 23, 2023, and his vested stock options will remain exercisable until January 23, 2024. Mr. Bosua forfeited stock option grants for 7,384,795 shares of common stock.
(3) Mr. Peter J. Conley has served as our Chief Financial Officer and SVP Intellectual Property since May 2022.On May 20, 2022, we issued a stock option grant to Mr. Conley for 1,000,000 shares at an exercise price of $1.48 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years, with no vesting during the first six months.
(4) These amounts reflect the grant date market value as required by Regulation S-K Item 402(n)(2), computed in accordance with FASB ASC Topic 718.
Additional Narrative Disclosure
Retirement Benefits
We have not maintained, and do not currently maintain, a defined benefit pension plan, nonqualified deferred compensation plan or other retirement benefits.
We maintain a 401(k) plan and/or other health and welfare benefit plans in which our NEOs are eligible to participate.
Potential Payments upon Termination or Change in Control
We have the following potential payments upon termination or change in control with Ronald P. Erickson:

(1) | Reflects a salary for twelve months. |
(2) | Reflects the vesting of stock option grants-non cash. |
(3) | Reflects the cost of medical benefits for eighteen months. |
We have the following potential payments upon termination or change in control with Peter J. Conley:

(1) | Reflects a salary for twelve months. |
(2) | Reflects the vesting of stock option grants- non cash, |
Director Compensation
Our independent non-employee directors are primarily compensated with stock option grants and stock grants to attract and retain qualified candidates to serve on the Board, in addition to a $12,500 cash retainer in consideration of board services. In setting director compensation, we consider the significant amount of time that directors expend in fulfilling their duties to us as well as the skill-level required by our members of the Board.
The table below sets forth the compensation paid to our non-employee directors during the fiscal year ended September 30, 2023. Ronald P. Erickson and Phillip A. Bosua did not receive any compensation for their services as directors. The compensation disclosed in the Summary Compensation Table above represents the total compensation for Mr. Erickson and Mr. Bosua.
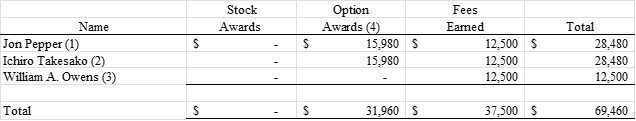
(1) | The stock option grant for 20,000 shares of common stock was issued on February 15, 2023 to Mr. Pepper and was valued at the black scholes value of $0.799 per share. Mr. Pepper was paid $12,500 for board services. As of September 30, 2023, Mr. Pepper has stock option grants for 77,500 shares of common stock and warrants to purchase common stock of 40,000 shares. |
| |
(2) | The stock option grant for 20,000 shares of common stock was issued on February 15, 2023 to Mr. Takesako and was valued at the black scholes value of $0.799 per share. Mr. Takesako was paid $12,500 for board services. As of September 30, 2023, Mr. Takesako has stock option grants for 77,500 shares of common stock and warrants to purchase common stock of 40,000 shares. |
(3) | Mr. Owens was paid $12,500 for board services. As of September 30, 2023, Mr. Owens has stock option grants for 0 shares of common stock and warrants to purchase common stock of 40,000 shares. |
| |
(4) | These amounts reflect the grant date market value as required by Regulation S-K Item 402(n)(2), computed in accordance with FASB ASC Topic 718. All assumptions made in the valuations are contained and described in footnote 8 to the Company’s financial statements for Fiscal 2023 contained in this Annual Report on Form 10-K for the fiscal year ended September 30, 2023. The amounts shown in the table reflect the total fair value on the date of grant and do not necessarily reflect the actual value, if any, that may be realized by the listed executives. |
Mr. Cronin, Mr. Londergan, and Mr. Ellingson were each appointed as directors in November 2023, as such they received no compensation as directors in the year ended September 30, 2023.
2021 Equity Incentive Plan
On August 12, 2021, we established the Know Labs, Inc. 2021 Equity Incentive Plan (the “2021 Plan”), which was adopted by our stockholders on October 15, 2021. The following summary briefly describes the principal features of the 2021 Plan and is qualified in its entirety by reference to the full text of the 2021 Plan, which is filed as an exhibit to this report.
Awards that may be granted include: (a) incentive stock options, (b) non-qualified stock options, (c) stock appreciation rights, (d) restricted awards, (e) performance share awards, and (f) performance compensation awards. These awards offer our officers, employees, consultants and directors the possibility of future value, depending on the long-term price appreciation of our common stock and the award holder’s continuing service with our company. All of the permissible types of awards under the 2021 Plan are described in more detail below.
Purposes of 2021 Plan: The purposes of the 2021 Plan are to attract and retain officers, employees and directors for our company and our subsidiaries; motivate them by means of appropriate incentives to achieve long-range goals; provide incentive compensation opportunities; and further align their interests with those of our stockholders through compensation that is based on our common stock.
Administration of the 2021 Plan: The 2021 Plan is administered by our compensation committee (which we refer to as the plan administrator). Among other things, the plan administrator has the authority to select persons who will receive awards, determine the types of awards and the number of shares to be covered by awards, and to establish the terms, conditions, performance criteria, restrictions and other provisions of awards. The plan administrator has authority to establish, amend and rescind rules and regulations relating to the 2021 Plan.
Eligible Recipients: Persons eligible to receive awards under the 2021 Plan will be those officers, employees, consultants, and directors of our company and our subsidiaries who are selected by the plan administrator.
Shares Available Under the 2021 Plan: 20,000,000 shares of our common stock were originally authorized as the maximum number of shares of our common stock that may be delivered to participants under the 2021 Plan, subject to adjustment for certain corporate changes affecting the shares, such as stock splits. This number was increased to 22,000,000 shares of common stock as of January 1, 2022 as a result of the automatic share reserve increase discussed below. Shares subject to an award under the 2021 Plan for which the award is canceled, forfeited or expires again become available for grants under the 2021 Plan. Shares subject to an award that is settled in cash will not again be made available for grants under the 2021 Plan. As of the date of this report, all shares remain available for issuance under the 2021 Plan. The 2021 Plan also authorizes for issuance the sum of (A) any shares of our common stock that, as of the date of stockholder approval of the 2021 Plan, have been reserved but not issued pursuant to any awards granted under our 2011 Stock Incentive Plan and (B) any shares of our common stock subject to stock options or similar awards granted under our 2011 Stock Incentive Plan that, after the date of stockholder approval of the 2021 Plan, expire or otherwise terminate without having been exercised in full and shares of our common stock issued pursuant to awards granted under our 2011 Stock Incentive Plan that are forfeited to or repurchased by us, with the maximum number of shares of our common stock to be added to the 2021 Plan pursuant to clause (B) equal to 7,592,825.
Automatic Share Reserve Increase: Subject to the provisions of Section 14 of the 2021 Plan, the number of shares available for issuance under the 2021 Plan will be increased on the first day of each calendar year beginning January 1, 2022 and ending on and including January 1, 2030 in an amount equal to the least of (i) 2,000,000 shares of our common stock, (ii) four percent (4%) of the outstanding shares of our common stock on the last day of the immediately preceding fiscal year or (iii) such number of shares of our common stock determined by our board of directors; provided, that such determination under clause (iii) will be made no later than the last day of the immediately preceding fiscal year.
Stock Options:Stock options give the option holder the right to acquire from us a designated number of shares of common stock at a purchase price that is fixed upon the grant of the option. The exercise price will not be less than the market price of the common stock on the date of grant. Stock options granted may be either tax-qualified stock options (so-called “incentive stock options”) or non-qualified stock options.
General. Subject to the provisions of the 2021 Plan, the plan administrator has the authority to determine all grants of stock options. That determination will include: (i) the number of shares subject to any option; (ii) the exercise price per share; (iii) the expiration date of the option; (iv) the manner, time and date of permitted exercise; (v) other restrictions, if any, on the option or the shares underlying the option; and (vi) any other terms and conditions as the plan administrator may determine.
Option Price. The exercise price for stock options will be determined at the time of grant. Normally, the exercise price will not be less than the fair market value on the date of grant. As a matter of tax law, the exercise price for any incentive stock option awarded may not be less than the fair market value of the shares on the date of grant. However, incentive stock option grants to any person owning more than 10% of our voting stock must have an exercise price of not less than 110% of the fair market value on the grant date.
Exercise of Options. An option may be exercised only in accordance with the terms and conditions for the option agreement as established by the plan administrator at the time of the grant. The option must be exercised by notice to us, accompanied by payment of the exercise price. Payments may be made in cash or, at the option of the plan administrator, by actual or constructive delivery of shares of common stock to the holder of the option based upon the fair market value of the shares on the date of exercise.
Expiration or Termination. Options, if not previously exercised, will expire on the expiration date established by the plan administrator at the time of grant. In the case of incentive stock options, such term cannot exceed ten years provided that in the case of holders of more than 10% of our voting stock, such term cannot exceed five years. Options will terminate before their expiration date if the holder’s service with our company or a subsidiary terminates before the expiration date. The option may remain exercisable for specified periods after certain terminations of employment, including terminations as a result of death, disability or retirement, with the precise period during which the option may be exercised to be established by the plan administrator and reflected in the grant evidencing the award.
Incentive and Non-Qualified Options. As described elsewhere in this summary, an incentive stock option is an option that is intended to qualify under certain provisions of the Internal Revenue Code of 1986, as amended, or the Code, for more favorable tax treatment than applies to non-qualified stock options. Any option that does not qualify as an incentive stock option will be a non-qualified stock option. Under the Code, certain restrictions apply to incentive stock options. For example, the exercise price for incentive stock options may not be less than the fair market value of the shares on the grant date and the term of the option may not exceed ten years. In addition, an incentive stock option may not be transferred, other than by will or the laws of descent and distribution, and is exercisable during the holder’s lifetime only by the holder. In addition, no incentive stock options may be granted to a holder that is first exercisable in a single year if that option, together with all incentive stock options previously granted to the holder that also first become exercisable in that year, relate to shares having an aggregate market value in excess of $100,000, measured at the grant date.
Stock Appreciation Rights: Stock appreciation rights, or SARs, which may be granted alone or in tandem with options, have an economic value similar to that of options. When a SAR for a particular number of shares is exercised, the holder receives a payment equal to the difference between the market price of the shares on the date of exercise and the exercise price of the shares under the SAR. The exercise price for SARs normally is the market price of the shares on the date the SAR is granted. Under the 2021 Plan, holders of SARs may receive this payment — the appreciation value — either in cash or shares of common stock valued at the fair market value on the date of exercise. The form of payment will be determined by us.
Stock Awards: Restricted shares are shares of common stock awarded to participants at no cost. Restricted shares can take the form of awards of restricted stock, which represent issued and outstanding shares of our common stock subject to vesting criteria, or restricted stock units, which represent the right to receive shares of our common stock, subject to satisfaction of the vesting criteria. Those may include requirements for continuous service and/or the achievement of specified performance goals. Restricted shares are forfeitable and non-transferable until the shares vest. The vesting date or dates and other conditions for vesting are established when the shares are awarded.
Cash Awards: A cash award is an award that may be in the form of cash or shares of common stock or a combination, based on the attainment of pre-established performance goals and other conditions, restrictions and contingencies identified by the plan administrator.
Section 162(m) of the Code: Section 162(m) of the Code limits publicly-held companies to an annual deduction for U.S. federal income tax purposes of $1.0 million for compensation paid to each of their principal executive officer or principal financial officer and their three highest compensated executive officers (other than the principal executive officer or principal financial officer) determined at the end of each year, referred to as covered employees.
Performance Criteria: Under the 2021 Plan, one or more performance criteria will be used by the plan administrator in establishing performance goals. Any one or more of the performance criteria may be used on an absolute or relative basis to measure the performance of our company, as the plan administrator may deem appropriate, or as compared to the performance of a group of comparable companies or published or special index that the plan administrator deems appropriate. In determining the actual size of an individual performance compensation award, the plan administrator may reduce or eliminate the amount of the award through the use of negative discretion if, in its sole judgment, such reduction or elimination is appropriate. The plan administrator shall not have the discretion to (i) grant or provide payment in respect of performance compensation awards if the performance goals have not been attained or (ii) increase a performance compensation award above the maximum amount payable under the 2021 Plan.
Other Material Provisions: Awards will be evidenced by a written agreement, in such form as may be approved by the plan administrator. In the event partial reclassification of contracts subjectvarious changes to ASC 815-40-25 is necessary, due tothe capitalization of our inability to demonstrate we have sufficient shares authorizedcompany, such as stock splits, stock dividends and unissued, sharessimilar re-capitalizations, an appropriate adjustment will be allocated onmade by the basisplan administrator to the number of issuanceshares covered by outstanding awards or to the exercise price of such awards. The plan administrator is also permitted to include in the written agreement provisions that provide for certain changes in the award in the event of a change of control of our company, including acceleration of vesting. Except as otherwise determined by the plan administrator at the date withof grant, awards will not be transferable, other than by will or the earliest issuance date receiving first allocationlaws of shares. If a reclassificationdescent and distribution. Prior to any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any employee withholding tax requirements. Our board also has the authority, at any time, to discontinue the granting of awards. The board also has the authority to alter or amend the 2021 Plan or any outstanding award or may terminate the 2021 Plan as to further grants, provided that no amendment will, without the approval of our stockholders, to the extent that such approval is required by law or the rules of an instrument were required, it would result inapplicable exchange, increase the instrument issued latest being reclassified first
Income Taxes – Income taxes are calculated based uponnumber of shares available under the asset and liability method of accounting. Deferred income taxes are recorded to reflect2021 Plan, change the tax consequences in future years of differences betweenpersons eligible for awards under the tax basis of assets and liabilities and their financial reporting amounts at each year-end. A valuation allowance is recorded against deferred tax assets if management does not believe2021 Plan, extend the Company has met the “more likely than not” standard to allow for recognition of such an asset. In addition, realization of an uncertain income tax position musttime within which awards may be estimated as “more likely than not” (i.e., greater than 50% likelihood of receiving a benefit) before it can be recognized in the financial statements. Further, the recognition of tax benefits recorded in the financial statements, if any, is based on the amount most likely to be realized assuming a review by tax authorities having all relevant information.
Net Loss per Share – Undermade, or amend the provisions of ASC 260, “Earnings Per Share,the 2021 Plan related to amendments. No amendment that would adversely affect any outstanding award made under the 2021 Plan can be made without the consent of the holder of such award. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of December 31, 2023 for (i) each of our named executive officers and directors; (ii) all of our named executive officers and directors as a group; and (iii) each other stockholder known by us to be the beneficial owner of more than 5% of our outstanding common stock. Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o our company, 500 Union Street, Suite 810, Seattle, WA 98101.
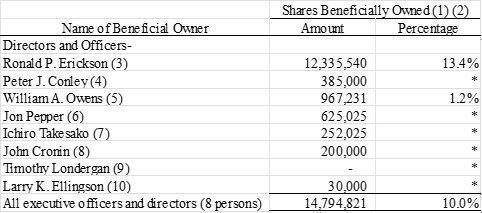
* Less than 1%
(1) | Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares that such person or any member of such group has the right to acquire within sixty (60) days. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within sixty (60) days of December 31, 2023 are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership by any person. |
| |
(2) | Based on 81,346,524 shares of common stock issued and outstanding as of December 31, 2023. |
| |
(3) | Consists of (i) 1,488,085 shares of shares of our common stock beneficially owned by Ronald P. Erickson or entities controlled by Mr. Erickson, (ii) 2,216,325 shares of our common stock issuable upon the exercise of options exercisable within 60 days, (iii) 3,894,666 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days, and (iv) 4,736,264 shares of our common stock issuable upon the conversion of convertible notes that are convertible within 60 days. |
| |
(4) | Consists of (i) 10,000 shares of our common stock held directly by Peter Conley and (ii) 375,000 shares of our common stock issuable upon the exercise of options exercisable within 60 days. |
| |
(5) | Consists of (i) 847,703 shares of our common stock held directly by William A Owens, (ii) 79,528 shares of our common stock issuable upon the exercise of options exercisable within 60 days, and (iii) 40,000 shares of our common stock issuable upon the exercise of warrants that are exercisable within 60 days. |
| |
(6) | Consists of (i) 428,000 shares of our common stock held directly by Jon Pepper, (ii) 157,025 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 40,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
| |
(7) | Consists of (i) 55,000 shares of our common stock held directly by Ichiro Takesako, (ii) 157,025 shares of our common stock issuable upon the exercise of options exercisable within 60 days and (iii) 40,000 shares of our common stock issuable upon the exercise of warrants exercisable within 60 days. |
| |
(8) | Consists of 200,000 shares of our common stock issuable to John Cronin upon the exercise of options exercisable within 60 days. |
| |
(9) | Timothy Londergan was appointed to the board of directors in November 2023. |
| |
(10) | Consists of 30,000 shares of our common stock issuable to Larry K. Ellingson upon the exercise of options exercisable within 60 days. |

(1) | Consists of (i) 1,402,784 shares of our common stock, (ii) 6,269,715 shares of our common stock issuable upon the exercise of warrants, (iii) 5,000,000 shares of our common stock issuable upon the conversion of our series C convertible preferred stock, (iv) 3,108,356 shares of our common stock issuable upon the conversion of our series D convertible preferred stock and (v) 4,284,000 shares of our common stock issuable upon the conversion of convertible notes; and excludes additional shares of preferred stock issuable as accreted preferred dividends pursuant to terms of the series C convertible preferred stock and series D convertible preferred stock. All of the warrants, series C convertible preferred stock, series D convertible preferred stock and convertible notes held by Mr. Struve are subject to a 4.99% blocker pursuant to which shares of our common stock may not be issued to the extent that such issuance would cause Mr. Struve to beneficially own more than 4.99% of our common stock. The address of Mr. Struve is 175 West Jackson Blvd., Suite 440, Chicago, IL 60604. |
| |
(2) | Includes (i) 17,200,000 shares of our common stock held directly by Todd Baszucki and (ii) 1,000,000 shares of our common stock issuable upon the exercise of warrants. The address for Mr. Baszucki is 395 Del Monte Center, Unit 306, Monterey, CA 93940. |
| |
(3) | See above for Ronald P. Erickson or entities controlled by Mr. Erickson.The address for Mr. Erickson is 500 Union Street, Suite 810, Seattle, WA 98101. |
RELATED PARTY TRANSACTIONS OF DIRECTORS AND EXECUTIVE OFFICERS
Transactions with Related Persons
The following includes a summary of transactions since the beginning of our 2021 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last three completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation”
basic earningsabove). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.Transactions with Clayton Struve
On December 7, 2022, we signed an Extension of Warrant Agreement with Clayton Struve, extending the exercise dates as follows:
Warrant No./Class | | Issue Date | | No. Warrant Shares | | | Exercise Price | | | Current Expiration Date | | Amended Expiration Date | |
Clayton A. Struve Warrant | | 08-14-2017 | | | 1,440,000 | | | $ | 0.25 | | | 08-13-2024 | | 08-13-2025 | |
Clayton A. Struve Warrant | | 12-12-2017 | | | 1,200,000 | | | $ | 0.25 | | | 12-11-2024 | | 12-11-2025 | |
Clayton A. Struve Warrant | | 08-04-2016 | | | 1,785,715 | | | $ | 0.25 | | | 08-04-2024 | | 08-04-2025 | |
Clayton A. Struve Warrant | | 02-28-2018 | | | 1,344,000 | | | $ | 0.25 | | | 02-28-2024 | | 02-28-2025 | |
We recorded interest expense of $194,019 during the year ended September 30, 2023 related to the extension of the warrants. We recorded the original value of warrants in equity and as such, we recorded the extension value as an expense with an offset to additional paid in capital.
On December 7, 2022, we signed an Extension of Warrant Agreement with Clayton Struve, extending the exercise dates. We recorded interest expense of $194,019 during the year ended September 30, 2023 related to the extension of the warrants. We recorded the original value of warrants in equity and as such, we recorded the extension value as an expense with an offset to additional paid in capital.
Convertible Promissory Notes with Clayton A. Struve
We owe Clayton A. Struve, a significant stockholder, $1,301,005 under convertible promissory or OID notes. We recorded accrued interest of $95,952 and $94,062 as of December 31, 2023 and September 30, 2023, respectively. On December 7, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2023. On September 15, 2023, the due dates on the notes was further extended to September 30, 2024. We expensed $230,005 as loss on debt extinguishment during the year ended September 30, 2023 related to the extension of the notes. We recorded in convertible note payable the incremental value related to the conversion feature and as such, we recorded the extension value as an expense with an offset to convertible note payable. The extension value will be reclassified to equity upon conversion.
Series C and D Preferred Stock, Warrants and Dividends
See “Description of Securities” for the terms of our Series C preferred stock and D preferred stock, warrants and dividends.
On June 27, 2023, at Mr. Struve’s request, we settled all cash dividends with respect to the Series D preferred stock accrued and accumulated through December 31, 2022 in exchange for the issuance to Mr. Struve of 1,402,784 shares of our common stock in reliance on the exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended. In connection with this transaction, we recorded $1,627,230 in dividends, representing the fair market value of the 1,402,784 shares issued.
Transactions with Ronald P. Erickson
We owe Ronald P. Erickson and J3E2A2Z, an entity affiliated controlled by Ronald P. Erickson $1,460,926 under convertible promissory notes. On March 16, 2018, we entered into a promissory note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z promissory notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of our for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The Company recorded accrued interest of $196,241 and $218,334 as of December 31, 2023 and September 30, 2023, respectively. On December 7, 2022, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to January 30, 2023. On January 25, 2023, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2023. On September 15, 2023, the due dates on the notes was further extended to September 30, 2024. We expensed $276,860 as interest during the year ended September 30, 2023 related to the extension of the notes. We recorded in convertible note payable the incremental value related to the conversion feature and as such, we recorded the extension value as an expense with an offset to convertible note payable. The extension value will be amortized to equity upon conversion.
On November 4, 2019, we granted a stock option grant to Ronald P. Erickson for 1,200,000 shares with an exercise price of $1.10 per share. The performance grant expires November 4, 2024 and vests upon uplisting to the NASDAQ or NYSE exchanges. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $1,207,200 during the year ended September 30, 2022.
On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. The grant vests in increments if the market capitalization of our commons stock exceeds for 20 consecutive trading days starting at $100 million to $1 billion. We estimated at grant date the fair value of these options at approximately $520,869 which is calculatedbeing amortized over 5 years. As of September 30, 2023 and 2022, we recorded an expense of $104,172 and a cumulative expense of $186,657, respectively. We are valuing this stock option using the Monte Carlo pricing model which included key assumptions of 100% stock volatility, five year life and no forfeitures. The stock option grant was not vested as of September 30, 2022.
On December 15, 2020, we issued a stock option grant to Ronald P. Erickson for 1,865,675 shares at an exercise price of $1.53 per share. The stock option grant expires in five years. Our common stock began trading on NYSE American under the symbol “KNW” on September 16, 2022 and we expensed $263,593 during the year ended September 30, 2022. The stock option grants vest when earned based on certain performance criteria.
On December 16, 2021, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $2.09 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On December 14, 2022, we issued a stock option grant to Ronald P. Erickson for 1,000,000 shares at an exercise price of $1.41 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
On January 19, 2023, we signed an Extension of Warrant Agreements with Ronald P. Erickson and an entity controlled by dividing net income Mr. Erickson, extending the exercise dates from January 30, 2023 to January 30, 2024.
Mr. Erickson and/or loss availableentities with which he is affiliated also have expenses and interest of approximately $196,000 as of December 31, 2023, respectively.
On October 10, 2023, we issued a stock option grant to Ronald P. Erickson for 4,640,844 shares at an exercise price of $0.25 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
Transactions with Peter J. Conley
On May 20, 2022, we issued a stock option grant to Mr. Conley for 1,000,000 shares at an exercise price of $1.48 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years, with no vesting during the first six months.
On October 10, 2023, we issued a stock option grant to Peter J. Conley for 3,001,000 shares at an exercise price of $0.25 per share. The stock option grant expires in five years. The stock option grant vests quarterly over four years.
Transactions with Directors
On January 5, 2022, we issued 30,000 shares each to three directors for services rendered during 2021.
On January 5, 2022, we issued 20,000 warrants to purchase common stock each to three directors at an exercise price of $1.70 per share. The warrants expire on January 5, 2027.
On February 15, 2023, we issued stock option grants to two directors for a total of 50,000 shares at an exercise price of $1.24 per share. The stock option grant expires in five years. The stock option grants vested at issuance.
Mr. Cronin has served as an independent director since November 2023. Mr. Cronin is an experienced inventor and intellectual property strategist. Mr. Cronin is Chairman and CEO of ipCapital Group, Inc. As of the year ended September 30, 2023, we have paid ipCapital Group approximately $713,000 in professional fees.
On October 10, 2023, we issued 105,000 fully vested stock awards total to three directors at an exercise price of $0.25 per share for director services.
On October 10, 2023, we issued stock option grants to three directors for a total of 238,584 shares at an exercise price of $0.25 per share. The stock option grant expires in five years. The stock option grants vested at issuance.
Indemnification
Our articles of incorporation provide that we will indemnify our directors and officers to the fullest extent permitted by Nevada law. In addition, we have Indemnification Agreements with the current Board of Directors.
DESCRIPTION OF SECURITIES
The following description summarizes certain terms of our capital stock and the securities being sold in this offering. Because this is a summary description, it does not contain all of the information that may be important to you. This summary does not purport to be complete and is qualified in its entirety by the provisions of our articles of incorporation as amended, restated and supplemented to date, or our articles of incorporation, and our second amended and restated bylaws, or our bylaws, which have been filed as exhibits to the registration statement of which this prospectus is a part, as well as the applicable provisions of the Nevada Revised Statutes.
The following description summarizes important terms of the classes of our capital stock as of December 31, 2023.
Authorized Capital Stock. The Company’s authorized capital stock currently consists of: 200,000,000 shares of common stock, par value $0.001 per share and 5,000,000 shares of “blank check” preferred stock, par value $0.001 per share, of which 30,000 shares have been designated as our Series C Convertible Preferred Stock, $0.001 par value per share and 20,000 shares have been designated as our Series D Convertible Preferred Stock, $0.001 par value per share.
Outstanding Shares of Capital Stock. The Company’s common stock is the only security of the Company registered pursuant to Section 12 of the Securities Exchange Act of 1934, as amended. All outstanding shares of the Company’s capital stock are fully paid and nonassessable. As of December 31, 2023, there were 81,346,524 shares of common stock issued and outstanding, held by holders of record; 17,858 shares of Series C Convertible Preferred Stock issued and outstanding, held by one holder of record; and 10,161 shares of Series D Convertible Preferred Stock issued and outstanding, held by one holder of record.
Securities Subject to Price Adjustments
If in the future, the Company sells its common stock at a price below $0.25 per share, the conversion price of our outstanding shares of series C convertible preferred stock and series D convertible preferred stock would adjust below $0.25 per share pursuant to the documents governing such instruments. In addition, the conversion price of the convertible promissory notes referred to above and the exercise price of certain outstanding warrants to purchase 7,684,381 shares of common stock would adjust below $0.25 per share pursuant to the documents governing such instruments.
Series C and D Preferred Stock, Warrants and Dividends
On August 5, 2016, the Company closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve, an accredited investor for the purchase of $1,250,000 of preferred stock with a conversion price of $0.70 per share. The preferred stock has a cumulative dividend of 8% and an ownership blocker of 4.99%. Dividends are due and payable in cash when declared by the Company or when the stock is converted. Series C Preferred stock is senior to Series D preferred stock and is entitled to receive equal dividends paid to Series D. In addition, Mr. Struve received a five-year warrant to acquire 1,785,714 shares of common stock at $0.70 per share. On August 14, 2017, the price of the Series C preferred stock and warrant and its conversion price, were adjusted to $0.25 per share pursuant to the documents governing such instruments. As of December 31, 2023, Mr. Struve owns all of the 17,858 issued and outstanding shares of Series C preferred stock. Each holder of Series C preferred stock is allowed to vote as a common shareholder as if the shares were converted to common stockholdersstock up to the ownership blocker of 4.99%.
In 2017 the Company closed a $750,000 Series D preferred stock and Warrant offering with Mr. Struve. As of December 31, 2023, Mr. Struve owns all of the 10,161 issued and outstanding shares of Series D Preferred Stock. Each outstanding share of series D preferred stock will accrue cumulative cash dividends at a rate equal to 8.0% per annum, subject to adjustment as provided in the series D preferred stock certificate of designations. Dividends are due and payable in cash when declared by the Company or when the stock is converted. In addition, On August 14, 2017, the price of the Series D Preferred Stock were adjusted to $0.25 per share pursuant to the documents governing such instruments. Each holder of Series D preferred stock is allowed to vote as a common shareholder as if the shares were converted to common stock up to the ownership blocker of 4.99%.
In August, 2023, as part of a modification of the Series C preferred stock and Series D preferred stock certificates of designation, such preferred stock does not accrue or pay cash dividends. All future dividends will be accrued and paid in Series C preferred stock or Series D preferred stock, as applicable. As was the case prior to the modifications of the Series C preferred stock and Series D preferred stock, although accrual of dividends is required as described below, no dividends are actually paid, and no shares actually issued, until a conversion of such stock or declaration of the dividend by the Board of Directors. Additionally, the Series D preferred stock will no longer be required to automatically convert to common stock based on listing of the Company’s common stock on the NYSE American, except if the volume weighted average price of the common stock is at least $2.50 per share for 20 trading days and certain other requirements are satisfied. The cumulative dividends accrued and paid in preferred stock will be determined based upon a $.70 stated value. The conversion from preferred stock into common stock is determined based dividing the $0.70 stated value by the $0.25 conversion price. In June, 2023, as part of the anticipated modification of the certificates of designation of the Series C preferred stock and Series D preferred stock, at Mr. Struve’s request, the Company settled all cash dividends with respect to the Series D preferred stock accrued and accumulated through December 31, 2022 in exchange for the issuance to Mr. Struve of 1,402,784 shares of the Company’s common stock in reliance on the exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended. In connection with this transaction, the Company recorded $1,627,230 in dividends, representing the fair market value of the 1,402,784 shares issued.
Based upon the modified terms and conditions of Series C preferred stock and D preferred stock certificates of designations, it was determined that Series C preferred stock and D preferred stock dividends need to be accreted going forward. As of December 31, 2023, cumulative unpaid Series C preferred stock and D preferred stock dividends totaled approximately $800,000, which on a converted-to-common-stock basis represents approximately 3,202,000 shares of common stock. Company has recorded $3,590,283 in cumulative deemed dividends related to Series C preferred stock and D preferred stock which have not been paid, net of the approximately $351,000 of accumulated dividends with respect to the Series D preferred stock that were settled for 1,402,784 shares of common stock as noted above. Mr. Struve is subject to an ownership blocker limiting his ownership to 4.99% and thus the number of common shares he can receive for dividends. Unpaid accreted stock dividends will be issued to Mr. Struve if he converts preferred stock or if the Board declares a dividend thereon, limited to his 4.99% ownership blocker. Assuming no changes in the amount of outstanding Series C preferred stock or Series D preferred stock ownership, going forward on a quarterly basis the Company will accrete as a preferred dividend the value of approximately 160,000 shares of common stock, which are issuable if such dividends become payable as additional shares of preferred stock, and such preferred stock is then converted into common stock.
Equity Incentive Plan
There are 28,220,473 (including unearned stock option grants totaling 4,179,825 shares related to performance milestones) options to purchase common stock at an average exercise price of $0.907 per share outstanding as of December 31, 2023 under the 2021 Plan. The expiration dates of these stock options range from now to November 11, 2028.
Warrants to Purchase Common Stock
As of December 31, 2023, we have issued warrants for the purchase of 20,984,961 shares of common stock at a weighted average exercise price of $1.059. The expiration dates of these warrants range from February 25, 2024 to October 26, 2028.
Clayton A. Struve has warrants to purchase 6,269,715 shares of common stock that have a beneficial ownership blocker at 4.99%.
The proceeds of warrants currently outstanding, to the extent not exercised on a cashless basis, may generate potential proceeds. We cannot provide assurance that any of these warrants will be exercised.
Convertible Promissory Notes with Clayton A. Struve
We owe Clayton A. Struve, a significant stockholder, $1,301,005 under convertible promissory or OID notes. We recorded accrued interest of $95,952 and $94,062 as of December 31, 2023 and September 30, 2023, respectively. On December 7, 2022, the Company signed Amendments to the convertible promissory or OID notes, extending the due dates to September 30, 2023. On September 15, 2023, the due dates on the notes was further extended to September 30, 2024. We expensed $230,005 as loss on debt extinguishment during the year ended September 30, 2023 related to the extension of the notes. We recorded in convertible note payable the incremental value related to the conversion feature and as such, we recorded the extension value as an expense with an offset to convertible note payable. The extension value will be reclassified to equity upon conversion.
Convertible Redeemable Promissory Notes with J3E2A2Z
We owe Ronald P. Erickson and J3E2A2Z, an entity affiliated controlled by Ronald P. Erickson $1,460,926 under convertible promissory notes. On March 16, 2018, we entered into a promissory note and Account Payable Conversion Agreement pursuant to which (a) all $664,233 currently owing under the J3E2A2Z Notes was converted to a Convertible Redeemable Promissory Note in the principal amount of $664,233, and (b) all $519,833 of the J3E2A2Z Account Payable was converted into a Convertible Redeemable Promissory Note in the principal amount of $519,833 together with a warrant to purchase up to 1,039,666 shares of common stock of our for a period of five years. The initial exercise price of the warrants described above is $0.50 per share, also subject to certain adjustments. The Company recorded accrued interest of $196,241 and $218,334 as of December 31, 2023 and September 30, 2023, respectively. On December 7, 2022, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to January 30, 2023. On January 25, 2023, we approved Amendments to the convertible redeemable promissory notes with Ronald P. Erickson and J3E2A2Z, extending the due dates to September 30, 2023. On September 15, 2023, the due dates on the notes was further extended to September 30, 2024. We expensed $276,860 as interest during the year ended September 30, 2023 related to the extension of the notes. We recorded in convertible note payable the incremental value related to the conversion feature and as such, we recorded the extension value as an expense with an offset to convertible note payable. The extension value will be amortized to equity upon conversion.
Anti-takeover Provisions
Anti-Takeover Effects of Certain Provisions of Nevada Law and our Governing Documents
Provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws could have the effect of delaying or preventing a third-party from acquiring us, even if the acquisition could benefit our stockholders. Such provisions of the Nevada Revised Statutes, our articles of incorporation and our bylaws can have the effect of enhancing continuity and stability in the composition of our board of directors and the policies formulated by the board of directors, and can also have the effect of discouraging certain types of transactions that may involve an actual or threatened change of control of our company. These provisions also may have the effect of reducing our vulnerability to an unsolicited proposal for a takeover that does not contemplate the acquisition of all of our outstanding shares, or an unsolicited proposal for the restructuring or sale of all or part of our company.
Nevada Anti-Takeover Statutes
The Nevada Revised Statutes, or NRS, contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. Nevada’s “acquisition of controlling interest” statutes (NRS 78.378 through 78.3793, inclusive) contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These laws will apply to us as of a particular date if we were to have 200 or more stockholders of record (at least 100 of whom have addresses in Nevada appearing on our stock ledger at all times during the 90 days immediately preceding that date) and do business in the State of Nevada directly or through an affiliated corporation, unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest provide otherwise. These laws provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the NRS, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become ���control shares” to which the voting restrictions described above apply. These laws may have a chilling effect on certain transactions if our articles of incorporation or bylaws are not amended to provide that these provisions do not apply to us or to an acquisition of a controlling interest, or if our disinterested stockholders do not confer voting rights in the control shares.
Nevada’s “combinations with interested stockholders” statutes (NRS 78.411 through 78.444, inclusive) provide that specified types of business “combinations” between certain Nevada corporations and any person deemed to be an “interested stockholder” of the corporation are prohibited for two years after such person first becomes an “interested stockholder” unless the corporation’s board of directors approves the combination (or the transaction by which such person becomes an “interested stockholder”) in advance, or unless the combination is approved by the board of directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an “interested stockholder” is any person who is (1) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (2) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to cover most significant transactions between a corporation and an “interested stockholder”. These laws generally apply to Nevada corporations with 200 or more stockholders of record. However, a Nevada corporation may elect in its articles of incorporation not to be governed by these particular laws, but if such election is not made in the corporation’s original articles of incorporation, the amendment (1) must be approved by the affirmative vote of the holders of stock representing a majority of the outstanding voting power of the corporation not beneficially owned by interested stockholders or their affiliates and associates, and (2) is not effective until 18 months after the vote approving the amendment and does not apply to any combination with a person who first became an interested stockholder on or before the effective date of the amendment. Neither our original articles of incorporation nor our current articles of incorporation include such an election.
NRS 78.139 also provides that directors may resist a change or potential change in control of the impactcorporation if the board of directors determines that the change or potential change is opposed to or not in the best interest of the corporation upon consideration of any potentially dilutiverelevant facts, circumstances, contingencies or constituencies pursuant to NRS 78.138(4). The Nevada Revised Statutes also provide that any director may be removed from our board of directors by the vote or written consent of stockholders representing not less than two-thirds of the voting power of the issued and outstanding shares entitled to vote, and this standard is also reflected in our bylaws.
Bylaws
Our bylaws contain limitations as to who may call special meetings and also establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock equivalents. Diluted earningsare available for our board of directors to issue without stockholder approval. We may use these additional shares for a variety of corporate purposes, including future public or private offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of our authorized but unissued shares of common stock could render it more difficult or discourage an attempt to obtain control of our company by means of a proxy contest, tender offer, merger or other transaction. Our authorized but unissued shares may be used to delay, defer or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by our stockholders.
Market Price of and Dividends on Common Equity and Related Stockholder Matters
Our common stock trades on the NYSE American under the symbol “KNW”. Trades in our common stock may be subject to Rule 15g-9 of the Exchange Act, which imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, broker/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser’s written agreement to the transaction before the sale.
As of March 25, 2024, we had 82,512,146 shares of common stock issued and outstanding, held by 195 stockholders of record. This number does not include approximately 5,690 beneficial owners whose shares are held in the names of various security brokers, dealers and registered clearing agencies.
Transfer Agent and Registrar
We have appointed Equiniti Trust Company located at 6201 15th Avenue, Brooklyn, New York 11219, telephone number (800) 937-5449, as the transfer agent for our common stock.
SELLING STOCKHOLDER
This prospectus relates to the offering and sale, from time to time, of up to 4,800,000 shares of our Common Stock issuable upon conversion of the Note and up to 6,000,000 shares of Common Stock underlying the Warrant held by the selling stockholders named in the table below. We are registering the shares to permit the selling stockholder and its pledgees, donees, transferees and other successors-in-interest that receive their shares from the selling stockholder as a gift, partnership distribution or other non-sale related transfer after the date of this prospectus to resell the shares when and as they deem appropriate in the manner described in the “Plan of Distribution.” As of March 25, 2024, there were 82,512,146 shares of Common Stock issued and outstanding.
The following table sets forth:
| · | · the name of the selling stockholder, |
| | |
| · | · the number of shares of our Common Stock that the selling stockholder beneficially owned prior to the offering for resale of the shares under this prospectus, |
| | |
| · | · the maximum number of shares of our Common Stock that may be offered for resale for the account of the selling stockholder under this prospectus, and |
| | |
| · | · the number and percentage of shares of our Common Stock beneficially owned by the selling stockholder after the offering of the shares (assuming all of the offered shares are sold by the selling stockholder). |
Unless set forth below, the selling stockholder received its securities in a private transaction with the Company.
The selling stockholder may offer for sale all or part of the shares of Common Stock from time to time. The table below assumes that the selling stockholder will sell all of the shares of Common Stock offered for sale. The selling stockholder is under no obligation, however, to sell any shares of Common Stock pursuant to this prospectus.
Name of selling stockholder | | Shares of Common Stock Beneficially Owned Prior To offering | | | Maximum Number of Shares of Common Stock To Be Sold | | | Number of Shares of Common Stock Owned After offering (1) | | | Percentage Ownership After offering (2) | |
Lind Global Fund II LP (3) | | | 10,800,000 | | | | 10,800,000 | | | | - | | | | 0.00 | % |
(1) | Since we do not have the ability to control how many, if any, of the shares of Common Stock the selling stockholder will sell, we have assumed that the selling stockholder will sell all of the shares offered herein for purposes of determining how many Shares it will own after the offering and its percentage of ownership following the offering. |
| |
(2) | All percentages have been rounded up to the nearest one hundredth of one percent. |
| |
(3) | Consists of 4,800,000 shares of Common Stock issuable upon the conversion of the Note, which is calculated based on the initial conversion price of $1.00 per share, and 6,000,000 shares of Common Stock underlying the Warrant. Both the Note and the Warrant contain blocker provisions such that they cannot be exercised to the extent such exercise would cause the holder, together with its affiliates, to beneficially own in excess of 4.99% of the outstanding Equity Interests (as defined in the Note and Warrant) of such class. The number of shares of Common Stock set forth in second and fourth columns do not give effect to such blocker provisions, but the percentages set forth in fifth column do give effect to such blocker provisions. The address for Lind Global Fund II LP is c/o The Lind Partners LLC, 444 Madison Avenue, Floor 41, New York, NY 10022. Lind Global Partners II LLC, the general partner of Lind Global Fund II LP, may be deemed to have sole voting and dispositive power with respect to the shares held by Lind Global Fund II LP. Jeff Easton, the managing member of Lind Global Partners II LLC, may be deemed to have sole voting and dispositive power with respect to the shares held by Lind Global Fund II LP. |
PLAN OF DISTRIBUTION
The selling stockholder and any of its pledgees, donees, assignees and other successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. The selling stockholder may use any one or more of the following methods when selling shares:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits the purchaser; |
| | |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal; |
| | |
| · | facilitate the transaction; |
| | |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| | |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| | |
| · | privately-negotiated transactions; |
| | |
| · | broker-dealers may agree with the selling stockholder to sell a specified number of such shares at a stipulated price per share; |
| | |
| · | through the writing of options on the shares; |
| | |
| · | a combination of any such methods of sale; and |
| | |
| · | any other method permitted pursuant to applicable law. |
The selling stockholder may also sell shares under Rule 144 of the Securities Act of 1933, as amended (the “Securities Act”), if available, rather than under this prospectus. The selling stockholder shall have the sole and absolute discretion not to accept any purchase offer or make any sale of shares if it deems the purchase price to be unsatisfactory at any particular time.
The selling stockholder or its pledgees, donees, transferees or other successors in interest, may also sell the shares directly to market makers acting as principals and/or broker-dealers acting as agents for themselves or their customers. Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from the selling stockholder and/or the purchasers of shares for whom such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing the shares will do so for their own account and at their own risk. It is possible that the selling stockholder will attempt to sell shares of common stock in block transactions to market makers or other purchasers at a price per share reflectwhich may be below the potential dilutionthen existing market price. We cannot assure that all or any of the shares offered in this prospectus will be issued to, or sold by, the selling stockholder. The selling stockholder and any brokers, dealers or agents, upon effecting the sale of any of the shares offered in this prospectus, may be deemed to be “underwriters” as that term is defined under the Securities Act, the Exchange Act and the rules and regulations of such acts. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act.
We are required to pay all fees and expenses incident to the registration of the shares, including fees and disbursements of counsel to the selling stockholder, but excluding brokerage commissions or underwriter discounts.
The selling stockholder, alternatively, may sell all or any part of the shares offered in this prospectus through an underwriter. The selling stockholder have not entered into any agreement with a prospective underwriter and there is no assurance that any such agreement will be entered into.
The selling stockholder may pledge its shares to its brokers under the margin provisions of customer agreements. If the selling stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares. The selling stockholder and any other persons participating in the sale or distribution of the shares will be subject to applicable provisions of the Exchange Act, and the rules and regulations under such act, including, without limitation, Regulation M. These provisions may restrict certain activities of, and limit the timing of purchases and sales of any of the shares by, the selling stockholder or any other such person. In the event that the selling stockholder is deemed an affiliated purchaser or distribution participant within the meaning of Regulation M, then the selling stockholder will not be permitted to engage in short sales of common stock. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and certain other activities with respect to such securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. In addition, if a short sale is deemed to be a stabilizing activity, then the selling stockholder will not be permitted to engage in a short sale of our common stock. All of these limitations may affect the marketability of the shares.
If the selling stockholder notifies us that could share in our earnings throughit has a material arrangement with a broker-dealer for the conversionresale of the common shares issuable via outstanding stock, options and stock warrants, except where their inclusionthen we would be anti-dilutive. Total outstandingrequired to amend the registration statement of which this prospectus is a part, and file a prospectus supplement to describe the agreements between the selling stockholder and the broker-dealer.
In compliance with the guidelines of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum consideration or discount to be received by any member of the FINRA may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus.
MARKET FOR OUR COMMON STOCK, DIVIDENDS AND RELATED STOCKHOLDER INFORMATION
Market Information. As of March 25, 2024, our common stock, equivalents at June 30, 2016par value $.001 per share (the “Common Stock”), is currently quoted on the NYSE American under the symbol “KNW.”
Holders. As of March 25, 2024, we had approximately 195 shareholders of record of our common stock. This number does not include approximately 5,690 beneficial owners whose shares are held in the names of various security brokers, dealers and 2015 were 772,833 and 969,225 respectively. Dilutedregistered clearing agencies.
Dividends. Holders of our common shares outstanding were calculated using the Treasury Stock Method for the three months ended June 30, 2016 and 2015 werestock are entitled to receive such dividends as follows:
| | | |
| Weighted average number of common shares used in basic net income per common share | 1,339,484 | 1,131,960 |
| Dilutive effects of outstanding stock options and warrants | 440,166 | - |
| Weighted average number of common shares used in diluted net income per common share | 1,779,650 | 1,131,960 |
Dividend Policy –may be declared by our board of directors. The Company has never paid any cash dividends and intends, for the foreseeable future, to retain any future earnings for the development of ourits business. OurThe Company’s future dividend policy will be determined by the board of directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
Patent Policy—Securities Authorized for Issuance Under Equity Compensation Plans
The Company expensesfollowing table sets forth certain information about the securities authorized for issuance under our incentive plans as of December 31, 2023.

On August 12, 2021, we established the 2021 Plan, which was adopted by our stockholders on October 15, 2021. The maximum number of shares of common stock that may be issued pursuant to awards granted under the 2021 Plan is 22,000,000 shares and all costs incurredof these shares remained available for patents applications and registered trademarks.
Useissuance as of Estimates – December 31, 2023. See Item 11 “Executive Compensation—2021 Equity Incentive Plan” for a complete description of the 2021 Plan.LEGAL MATTERS
The preparationvalidity of our common stock offered hereby will be passed upon for us by Dentons Durham Jones & Pinegar P.C., St. George, Utah.
EXPERTS
The consolidated financial statements in conformity with accounting principles generally acceptedof Know Labs, Incorporated and subsidiaries as of September 30, 2023 and 2022 and for the each of the two years in the United States requires managementperiod ended September 30, 2023, have been incorporated in this prospectus by reference to make estimatesthe Annual Report on Form 10-K for the year ended September 30, 2023, in reliance upon the report (which contains an explanatory paragraph relating to the Company’s ability to continue as a going concern as described in Note 2 to the consolidated financial statements) of BPM LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and assumptionsaccounting.
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and special reports, and other information with the SEC. All such reports and other information are available to the public through the SEC’s website at http://www.sec.gov.
We also maintain a website where you can obtain information about us at www.knowlabs.co. Our website includes our annual, quarterly and current reports, together with any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. The information contained on our website is not part of this prospectus, and you should not consider it to be part of this document.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that affectin the reported amountsopinion of assetsthe SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by one of our directors, officers or controlling persons in the successful defense of any action, suit or proceeding) is asserted by that director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether that indemnification by us is against public policy as expressed in the Securities Act and disclosurewill be governed by the final adjudication of contingent assetsthat issue.
INCORPORATION BY REFERENCE
The following documents filed with the SEC are incorporated by reference into this prospectus:
| · | our Annual Report on Form 10-K for the year ended September 30, 2023, filed with the SEC on December 19, 2023; |
| | |
| · | our Quarterly Reports on Form 10-Q for the period ended December 31, 2023, filed with the SEC on February 14, 2024; |
| | |
| · | our Current Reports on Form 8-K filed with the SEC on October 30, 2023, October 31, 2023, November 8, 2023, December 1, 2023, December 14, 2023, February 14, 2024, February 27, 2024, as amended, February 29, 2024, March 6, 2024 and March 20, 2024 (other than any portions thereof deemed furnished and not filed); |
| | |
| · | our Definitive Proxy Statement on Schedule 14A filed with the SEC on August 4, 2023; and |
| | |
| · | the description of our common stock contained in our Registration Statement on Form 8-A, filed with the SEC on September 15, 2022, including any amendments thereto or reports filed for the purposes of updating this description, including Exhibit 4.5 to our Annual Report on Form 10-K for the year ended September 30, 2022, filed with the SEC on December 20, 2022. |
We also incorporate by reference all documents we file pursuant to Section 13(a), 13(c), 14 or 15 of the Exchange Act (other than any portions of filings that are furnished rather than filed pursuant to Items 2.02 and liabilities at7.01 of a Current Report on Form 8-K) after the date of the financial statementsinitial registration statement of which this prospectus is a part and prior to effectiveness of such registration statement. All documents we file in the reported amountsfuture pursuant to Section 13(a), 13(c), 14 or 15(d) of revenuesthe Exchange Act after the date of this prospectus and prior to the termination of the offering are also incorporated by reference and are an important part of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of this registration statement to the extent that a statement contained herein or in any other subsequently filed document which also is or deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this registration statement.
You may request and obtain a copy of any of the filings incorporated herein by reference, at no cost, by writing or telephoning us at the following address or phone number:
Know Labs Inc.
500 Union Street, Suite 810
Seattle, WA 98101
(206) 903-1351
ask@knowlabs.co
www.knowlabs.co
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, duringother than underwriting discounts and commissions, payable by us in connection with the reporting period. Actual results could differ from thosesale of common shares being registered. All amounts, other than the SEC registration fee and FINRA filing fee, are estimates.
Recent Accounting Pronouncements
We will pay all these expenses.
Item 14. Indemnification of Directors and Officers
We are a Nevada corporation. The Nevada Revised Statutes, or NRS, and certain provisions of our articles of incorporation and bylaws under certain circumstances provide for indemnification of our directors, officers, employees and agents against certain liabilities which they may incur in such capacities. A varietysummary of proposed or otherwise potential accounting standards are currently under studythe circumstances in which such indemnification is set forth below, but this description is qualified in its entirety by standard setting organizationsreference to our articles of incorporation and various regulatory agencies. Duebylaws and to the tentativerelevant statutory provisions, including NRS 78.7502, 78.751 and preliminary nature78.752.
In general, our articles of those proposed standards, management hasincorporation provide that any officer, director, employee or agent may be indemnified against expenses, fines, settlements or judgments arising in connection with a legal proceeding to which such person is a party, if that person acted in good faith and in a manner which he or she reasonably believed to be in or not determined whether implementation of such proposed standards would be materialopposed to our consolidated financial statements.
4. | DEVELOPMENT OF OUR CHROMAID™ TECHNOLOGY |
The Company is focused primarily on the development of a proprietary technology which is capable of uniquely identifying and authenticating almost any substance using light to create, record and detect the unique digital “signature”best interests of the substance. The Company, calls this its “ChromaID™” technology.
The Company’s ChromaID™ Technology
Theand, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. Our bylaws further provide that each person who was or is made a party or is threatened to be made a party to any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact such person is or was a director or officer of the Company has developed a proprietary technology to uniquely identify and authenticate almost any substance. This patented technology utilizes lightor is or was serving at the photon (elementary particlerequest of light) level throughthe Company as a seriesdirector or officer of emittersanother enterprise, shall be indemnified and detectors to generate a unique signature or “fingerprint” from a scan of almost any solid, liquid or gaseous material. This signature of reflected or transmitted light is digitized, creating a unique ChromaID signature. Each ChromaID signature is comprised of from hundreds or thousands of specific data points.
The ChromaID technology looks beyond visible light frequencies to areas of near infra-red and ultraviolet light that are outside the humanly visible light spectrum. The data obtained allowsheld harmless by the Company to create a very specific and unique ChromaID signature of the substance for a myriad of authentication and verification applications.
Traditional light-based identification technology, called spectrophotometry, has relied upon a complex system of prisms, mirrors and visible light. Spectrophotometers typically have a higher cost and utilize a form factor more suited to a laboratory setting and require trained laboratory personnel to interpret the information. The ChromaID technology uses lower cost LEDs and photodiodes and specific frequencies of light resulting in a more accurate, portable and easy-to-use solution for a wide variety of applications. The ChromaID technology not only has significant cost advantages as compared to spectrophotometry, it is also completely flexible is size, shape and configuration. The ChromaID scan head can range in size from endoscopic to a scale that could be the size of a large ceiling-mounted florescent light fixture.
In normal operation, a ChromaID master or reference scan is generated and stored in a database. The Visualant scan head can then scan similar materials to identify, authenticate or diagnose them by comparing the new ChromaID digital signature scan to that of the original or reference ChromaID signature or scan result.
The following summarizes the Company’s plans for its Company’s proprietary ChromaID technology. Based on the Company’s anticipated expenditures on this technology, the expected efforts of its management and its relationship with Intellectual Ventures and its subsidiary, IDMC, and the Company’s other strategic partner, Sumitomo Precision Products, Ltd., the Company expects its ChromaID technology to provide an increasing portion of its revenues in future years from product sales, licenses, royalties and other revenue streams., as discussed further below.
ChromaID: A Foundational Platform Technology
The Company’s ChromaID technology provides a platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. The ChromaID technology is an enabling technology that brings the science of light and photonics to low cost, real world commercialization opportunities across multiple industries. The technology is foundational and as such, the basis upon which the Company believes a significant business can be built.
As with other foundational technologies, a single application may reach across multiple industries. The ChromaID technology can, for example effectively differentiate and identify different brands of clear vodkas that appear identical to the human eye. By extension this same technology can identify pure water from water with contaminants present. It can provide real time detection of liquid medicines such as morphine that have been adulterated or compromised. It can detect if jet fuel has water contamination present. It could determine when it is time to change oil in a deep fat fryer. These are but a few of the potential applications of the ChromaID technology based upon extensions of its ability to identify different clear liquids.
The cornerstone of a company with a foundational platform technology is its intellectual property. ChromaID was invented by scientists from the University of Washington under contract with Visualant. The Company has pursued an aggressive intellectual property strategy and has been granted nine patents. The Company currently has 20 patents pending. The Company possesses all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, the IDMC subsidiary of Intellectual Ventures.
IDMC Relationship
In November 2013, the Company entered into a strategic relationship with IDMC, a subsidiary of Intellectual Ventures, a private intellectual property fund. IDMC has worked to expand the reach and the potential application of the ChromaID technology and has filed ten patents base on the ChromaID technology, which it has licensed to the Company. In connection with IDMC’s work to expand the Company’s intellectual property portfolio, the Company agreed to curtail outbound marketing activities of its technology through the fourth fiscal quarter of 2014.
Products
The Company first delivered product, the ChromaID Lab Kit, scans and identifies solid surfaces. The Company is marketing this product to customers who are considering licensing the technology. Target markets include, but are not limited to, commercial paint manufacturers, pharmaceutical equipment manufacturers, process control companies, currency paper and ink manufacturers, security cards, cosmetic companies, scanner manufactures and food processing companies.
The Company’s second product, the ChromaID Liquid Lab Kit, scans and identifies liquids. This product is currently in prototype form. Similar to the Company’s first product, it will be marketed to customers who are considering licensing the technology. Rather than use an LED emitter to reflect light off of a surface that is captured by a photodiode to generate a ChromaID signature the liquid analysis product shines light through the liquid (transmissive) with the LEDs positioned on one side of the liquid sample and the photo detectors on the opposite side. This device is in a functional state in our laboratory and the Company anticipates having a Liquid ChromaID Lab Kit available for customersfullest extent permitted by the
Company during the fall of 2015. Target markets include, but areNRS against all expense, liability and loss (including attorneys’ fees, judgments, fines or penalties and amounts paid in settlement) reasonably incurred or suffered by such person in connection therewith.NRS 78.751 provides that indemnification may not limitedbe made to water companies, petrochemical companies, pharmaceutical companies, and numerous consumer applications.
The ChromaID Lab Kits allows potential licensors of our technology to work with our technology and develop solutions for their particular application. Our contractual arrangements with IDMC are described in greater detail below.
The Company’s next planned product should be an exemplar product is a prototype that will be produced to address several markets. The primary purpose of this prototype will be to demonstrate the technology to prospective business partners, and will consist of a small, hand held, battery powered, Bluetooth enabled scanning device. The scanner should wirelessly connect to a smart phone or tablet to transfer the scanned data. The smart phone application will include two or three industry specific but generic applications that allow for the demonstration of the scanning and matching of the ChromaID signatures. The applications will focus on drug identification, food safety and liquid detection. The prototype device will lend itself to consumer applications and can be a consumer product as well.
Research and Development
The Company’s research and development efforts are primarily focused improving the core foundational ChromaID technology and developing new and unique applications for the technology. As part of this effort, the Company typically conduct testing to ensure that ChromaID application methods are compatible with the customer’s requirements, and that they can be implemented in a cost effective manner. The Company is also actively involved in identifying new application methods. Visualant’s team has considerable experience working with the application of light-based technologies and their application to various industries. The Company believes that its continued development of new and enhanced technologies relating to our core business is essential to its future success. The Company spent $243,114 during the nine months ended June 30, 2016 and $362,661 and $670,742 during the years ended September 30, 2015 and 2014, respectively, on research and development activities. The Company’s research and development efforts are supported internally, through its relationship with IDMC and through contractors led by Dr. Tom Furness and his team at RATLab LLC.
The Company’s Patents
The Company believes that it’s ten patents, 20 patent applications, and two registered trademarks, and our trade secrets, copyrights and other intellectual property rights are important assets. The Company’s patents will expire at various times between 2027 and 2033. The duration of the Company’s trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The patents that have been granted to Visualant include:
On August 9, 2011, the Company was issued US Patent No. 7,996,173 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy,” by the United States Office of Patents and Trademarks. The patent expires August 24, 2029.
On December 13, 2011, the Company was issued US Patent No. 8,076,630 B2 entitled “System and Method of Evaluating an Object Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires November 7, 2028.
On December 20, 2011, the Company was issued US Patent No. 8,081,304 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 28, 2030.
On October 9, 2012, the Company was issued US Patent No. 8,285,510 B2 entitled “Method, Apparatus, and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On February 5, 2013, the Company was issued US Patent No. 8,368,878 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 12, 2013, the Company was issued US Patent No. 8,583,394 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 21, 2014, the Company was issued US Patent No. 8,888,207 B2 entitled “Systems, Methods, and Articles Related to Machine-Readable Indicia and Symbols” by the United States Office of Patents and Trademarks. The patent expires February 7, 2033.
On March 23, 2015, the Company was issued US Patent No. 8,988,666 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On May 26, 2015, the Company was issued patent US Patent No. 9,041,920 B2 entitled “Device for Evaluation of Fluids using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
On April 19, 2016, the Company was issued patent US Patent No. 9,316,581 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Substances Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
The Company pursues an aggressive patent strategy to expand its unique intellectual property in the United States and other countries.
Services and License Agreement Invention Development Management Company, L.L.C.
In November 2013, the Company entered into a Services and License Agreement with IDMC. IDMC is affiliated with Intellectual Ventures, which collaborates with inventors and partners with pioneering companies and invests both expertise and capital in the process of invention. On November 19, 2014, the Company amended the Services and License Agreement with IDMC. This amendment exclusively licenses 10 filed patents to us.
The agreement requires IDMC to identify and engage inventors to develop new applications of Visualant’s ChromaID™ technology, present the developments to us for approval, and file at least 10 patent applications to protect the developments. IDMC is responsible for the development and patent costs. The Company provided the Chroma ID Lab Kits to IDMC at no cost and are providing ongoing technical support. In addition, to provide time for this accelerated expansion of its intellectual property the Company delayed the selling of the ChromaID Lab Kits for 140 days except for certain select accounts. The Company continued its business development efforts during this period and have worked with IDMC and their global business development resources to secure potential customers and licensees for the ChromaID technology. The Company shipped 20 ChromaID Lab Kits to inventors in the IDMC network during December 2013 and January 2014. As part of the agreement with IDMC, the Company curtailed its ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, the Company began to actively market the ChromaID Lab Kits to interested and qualified customers.
The Company has received a worldwide, nontransferable, exclusive license to the intellectual property developed under the IDMC agreement during the term of the agreement, and solely within the identification, authentication and diagnostics field of use, to (a) make, have made, use, import, sell and offer for sale products and services; (b) make improvements; and (c) grant sublicensesbehalf of any and all of the foregoing rights (including the right to grant further sublicenses).
The Company received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to the useful intellectual property held by IDMC within the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer to sell products and services and (b) grant sublicenses to any and all of the foregoing rights. The option to acquire this license may be exercised for up to two years from the effective date of the Agreement.
IDMC is providing global business development services to us for geographies not being pursued by Visualant. Also, IDMC has introduced the Company to potential customers, licensees and distributors for the purpose of identifying and pursuing a license, saledirector or distribution arrangement or other monetization event.
The Company granted to IDMC a nonexclusive, worldwide, fully paid, nontransferable, sublicenseable, perpetual license to our intellectual property solely outside the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer for sale products and services and (b) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
The Company granted to IDMC a nonexclusive, worldwide, fully paid up, royalty-free, nontransferable, non-sublicenseable, perpetual license to access and use the Company’s technology solely for the purpose of marketing the aforementioned sublicenses of our intellectual property to third parties outside the designated fields of use.
In connection with the original license agreement, the Company issued a warrant to purchase 97,169 shares of common stock to IDMC as consideration for the exclusive intellectual property license and application development services. The warrant has a current exercise price of $2.50 per share and expires November 10, 2018. The per share price is subject to adjustment based on any issuances below $2.50 per share except as described in the warrant.
The Company agreed to pay IDMC a percentage of license revenue for the global development business services and a percentage of revenue received from any company introduced to us by IDMC. The Company also have also agreed to pay IDMC a royalty when the Company receives royalty product revenue from an IDMC-introduced company. IDMC has agreed to pay the Company a license fee for the nonexclusive license of the Company’s intellectual property.
The term of both the exclusive intellectual property license and the nonexclusive intellectual property license commences on the effective date of November 11, 2013, and terminates when all claims of the patents expire or are held in valid or unenforceableofficer finally adjudged by a court of competent jurisdiction, from which no appeal canafter exhaustion of any appeals taken therefrom, to be taken.
The termliable for intentional misconduct, fraud or a knowing violation of the Agreement commences on the effective date until either party terminates the Agreement at any time following the fifth anniversarylaw if such intentional misconduct, fraud or a knowing violation of the effective date by providing at least ninety days’ prior written noticelaw was material to the other party.
cause of action. However, NRS 78.752 permits us to purchase and maintain insurance on behalf of our directors, officers, employees or agents against any liability asserted against or incurred by such person in any such capacity or arising out of such person’s status as such, whether or not we would have the power to indemnify such person against such liabilities.To the maximum extent permitted by law, our articles of incorporation eliminate or limit the liability of our directors to us or our stockholders for monetary damages for breach of a director’s fiduciary duty as a director. NRS 78.138(7) further provides that, subject to limited statutory exceptions and unless the articles of incorporation or an amendment thereto (in each case filed on or after October 1, 2003) provide for greater individual liability, a director or officer is not individually liable to a Nevada corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his or her capacity as a director or officer unless the presumption established by NRS 78.138(3) has been rebutted and it is proven that (i) his or her act or failure to act constituted a breach of his or her fiduciary duties as a director or officer, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
In May 2012, the CompanyWe have entered into a Joint Researchseparate indemnification agreements with our directors and Product Development Agreement (the “Joint Development Agreement”) with Sumitomo Precision Products Co., Ltd., a publicly-traded Japanese corporation,executive officers. Each indemnification agreement provides, among other things, for indemnification to the fullest extent permitted by law and our articles of incorporation and bylaws against any and all expenses, judgments, fines, penalties and amounts paid in settlement of any claim. The indemnification agreements will provide for the commercializationadvancement or payment of all expenses to the indemnitee and for reimbursement to us if it is found that such indemnitee is not entitled to such indemnification under applicable law and our ChromaID technology. In March 2013,articles of incorporation and bylaws.
We have a directors’ and officers’ liability insurance policy in place pursuant to which its directors and officers are insured against certain liabilities, including certain liabilities under the Company entered into an amendmentSecurities Act and the Exchange Act of 1934, as amended.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to this agreement, which extendeddirectors, officers or persons controlling us pursuant to the Joint Development Agreement from March 31, 2013 to December 31, 2013. The extension provided for continuing work between Sumitomo and Visualant focused on advancingforegoing provisions, we have been informed that, in the ChromaID technology and market research aimed at identifying the most significant markets for the ChromaID technology. This agreement expired December 31, 2013. This collaborative work supported the developmentopinion of the ChromaID Lab Kit. The current version of the technology was introduced to the marketplaceSEC, such indemnification is against public policy as a part of our ChromaID Lab Kit during the fourth quarter of 2013.
The Company also entered into a License Agreement with Sumitomo in May 2012 which provides for an exclusive license for the then-extant ChromaID technology. The territories covered by this license include Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines). On May 21, 2015, the Company entered into an amendment to the License Agreement, which, effective as of June 18, 2014, which eliminated the Sumitomo exclusivity and provides that if the Company sells products in certain territories – Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines) – the Company will pay Sumitomo a royalty rate of 2% of net sales (excluding non-recurring engineering revenues) over the remaining term of the five-year License Agreement (through May 2017).
6.
| ACCOUNTS RECEIVABLE/CUSTOMER CONCENTRATION
|
Accounts receivable were $1,102,821 and $619,849, net of allowance, as of June 30, 2016 and September 30, 2015, respectively. The Company had one customer (14.8%) in excess of 10% of our consolidated revenues for the nine months ended June 30, 2016. The Company had three customers (28.1%, 14.5% and 11.2%) with accounts receivable in excess of 10% as of June 30, 2016. The Company does expect to have customers with consolidated revenues or accounts receivable balances of 10% of total accounts receivableexpressed in the foreseeable future.
Inventories were $306,529Securities Act and $217,824 as of June 30, 2016 and September 30, 2015, respectively. Inventories consist primarily of printers and consumable supplies, including ribbons and cards, badge accessories, capture devices, and access control components held for resale. There is a $20,000 reserve for impaired inventory as of June 30, 2016 and September 30, 2015, respectively.
Fixed assets, net of accumulated depreciation, was $303,584 and $366,250 as of June 30, 2016 and September 30, 2015, respectively. Accumulated depreciation was $778,313 and $803,705 as of June 30, 2016 and September 30, 2015, respectively. Total depreciation expense, was 55,100 and $61,727 for the nine months ended June 30, 2016 and 2015, respectively. All equipment is used for selling, general and administrative purposes and accordingly all depreciation is classified in selling, general and administrative expenses.
Property and equipment as of June 30, 2016 was comprised of the following:
| | Estimated | |
| | Useful Lives | | | |
| Machinery and equipment | 2-10 years | $252,636 | $69,581 | $322,217 |
| Leasehold improvements | 5-20 years | 548,612 | - | 548,612 |
| Furniture and fixtures | 3-10 years | 73,977 | 101,260 | 175,237 |
| Software and websites | 3- 7 years | 35,831 | - | 35,831 |
| Less: accumulated depreciation | | (607,472) | (170,841) | (778,313) |
| | $303,584 | $- | $303,584 |
therefore unenforceable. In April 2008, the FASB issued a pronouncement that provides guidance on determining what types of instruments or embedded features in an instrument held by a reporting entity can be considered indexed to its own stock for the purpose of evaluating the first criteria of the scope exception in the pronouncement on accounting for derivatives. This pronouncement was effective for financial statements issued for fiscal years beginning after December 15, 2008. The adoption of these requirements can affect the accounting for warrants and many convertible instruments with provisions that protect holders from a decline in the stock price (or “down-round” provisions). For example, warrants or conversion features with such provisions are no longer recorded in equity. Down-round provisions reduce the exercise price of a warrant or convertible instrument if a company either issues equity shares for a price that is lower than the exercise price of those instruments or issues new warrants or convertible instruments that have a lower exercise price.
Derivative liability as of June 30, 2016 is as follows:
| | | | | |
| | Fair Value Measurements Using Inputs | |
| Financial Instruments | | | | |
| | | | | |
| Liabilities: | | | | |
| Derivative Instruments | $- | $738,984 | $- | $738,984 |
| | | | | |
| Total | $- | $738,984 | $- | $738,984 |
Derivative liability as of September 30, 2015 is as follows:
| | | | | |
| | Fair Value Measurements Using Inputs | |
| Financial Instruments | | | | |
| | | | | |
| Liabilities: | | | | |
| Derivative Instruments | $- | $2,704,840 | $- | $2,704,840 |
| | | | | |
| Total | $- | $2,704,840 | $- | $2,704,840 |
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the warrants.
Derivative Instruments – Warrants with the June 2013 Private Placement
|
The Company issued warrants to purchase 697,370 shares of common stock in connection with our June 2013 private placement of 348,685 shares of common stock. The exercise price of these warrants is $2.50 per share. These warrants were not issued with the intent of effectively hedging any future cash flow, fair value of any asset, liability or any net investment in a foreign operation. These warrants were issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional shares of our common stock or securities exercisable, convertible or exchangeablea claim for the Company’s common stock were issued at a price lessindemnification against such liabilities (other than the exercise price. Therefore,payment by the fair valueregistrant of these warrants were recorded asexpenses incurred or paid by a liabilitydirector, officer or controlling person of the registrant in the consolidated balance sheet and are marked to market each reporting period until they are exercisedsuccessful defense of any action, suit or expireproceeding) is asserted by such director, officer or otherwise extinguished.
The proceeds from the private placement were allocated between the shares of common stock and the warrants issuedcontrolling person in connection with the
private placement based upon their estimated fair values as of the closing date at June 14, 2013, resultingsecurities being registered, we will, unless in the
aggregate amountopinion of
$2,494,710 allocatedour counsel the matter has been settled by controlling precedent, submit to
stockholders’ equity and $2,735,290 allocated toa court of appropriate jurisdiction the
warrant derivative. The Company recognized $1,448,710 of other expense resulting from the increasequestion whether such indemnification by us is against public policy as expressed hereby in the
fair valueSecurities Act and we will be governed by the final adjudication of
the warrant liability at September 30, 2013. such issue.Item 15. Recent Sales of Unregistered Securities
During the year ended September 30, 2014,past three years, we issued the Company recognized $2,092,000 of other income resulting from the decrease in the fair value of the warrant liability at September 30, 2014. During the year ended September 30, 2015, the Company recognized $104,716 of other expense resulting from the decrease in the fair value of the warrant liability at September 30, 2015. During the nine months ended June 30, 2016, the Company recognized $1,650,339 of other income resulting from the increase in the fair value of the warrant liability at June 30, 2016.
Derivative Instruments – Warrant with the November 2013 IDMC Services and License Agreement
|
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the warrants.
The Company issued a warrant to purchase 97,169 shares of common stock in connection with the November 2013 IDMC Services and License Agreement. The warrant price of $30.00 per share expires November 10, 2018 and the per share price is subject to adjustment. In August 2015 the exercise price was reset to $2.50 per shares. This warrant wasfollowing securities, which were not issued with the intent of effectively hedging any future cash flow, fair value of any asset, liability or any net investment in a foreign operation. This warrant was issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional shares of our common stock or securities exercisable, convertible or exchangeable for our common stock were issued at a price less than the exercise price. Therefore, the fair value of these warrants was recorded as a liability in the consolidated balance sheet and are marked to market each reporting period until they are exercised or expire or otherwise extinguished. During the year ended September 30, 2014, the Company recognized $320,657 of other expense related to the IDMC warrant. During the year ended September 30, 2015, the Company recognized $14,574 of other income related to the IDMC warrant. During the nine months ended June 30, 2016, the Company recognized $213,286 of other income from the increase in the fair value of the warrant liability at June 30, 2016.
Derivative Instrument – Series A Convertible Preferred Stock
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the Series A Convertible Preferred Stock.
The Company issued 11,667 shares of Series A Convertible Preferred Stock with attached warrants during the year ended September 30, 2015. The Company allocated $233,322 to stockholder’s equity and $116,678 to the derivative warrant liability. The warrants were issued with a down round provision. The warrants have a term of five years, 23,334 are exercisable at $30 per common share and 23,334 are exercisable at $45 per common share. On August 14, 2015 the exercise price was adjusted to $2.50 per share. During the year ended September 30, 2015, the Company recognized $30,338 of other expense related to the warrant liability. During the nine months ended June 30, 2016, the Company recognized $102,436 of other income resulting from the increase in the fair value of the warrant liability at June 30, 2016.
Derivative Instrument – Convertible Note Payable Vis Vires Group, Inc.
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on February 19, 2016 for $100,000 to fund short-term working capital. The Vis Vires Note accrues interest at a rate of 8% per annum and becomes due on November 22, 2016 and is convertible into common stock on August 19, 2016. The Vis Vires Note is convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. The Company recorded accrued interest of $2,915 as of June 30, 2016. During the nine months ended June 30, 2016, the Company recognized a $65,278 debt discount resulting from the fair value of the conversion option and other income resulting from the increase in the fair value of the warrant liability at June 30, 2016 of $55,243. The Company also recorded other financing expense in the amount of $14,000 which was expensed to interest immediately due to the short term nature of the note.
Liabilities measured at fair value on a recurring basis for the Vis Vires Convertible Note Payable are summarized as follows:
| |
Market price and estimated fair value of common stock: | $3.25
|
Exercise price | 2.50
|
Expected term (years) | 0.25
|
Dividend yield | -
|
Expected volatility | 86.8%
|
Risk-free interest rate | 0.01%
|
Derivative Instrument – Convertible Note Payable Vis Vires Group, Inc.
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on August 10, 2015 for $84,000 to fund short-term working capital. The Vis Vires Note accrued interest at a rate of 8% per annum and was due on May 12, 2016 and was convertible into common stock on February 5, 2016. The Vis Vires Note was convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. The Company recorded accrued interest of $405 as of September 30, 2015. During the year ended September 30, 2015, the Company recognized $55,038 of other expense related to the Vis Vires Note. On February 6, 2016, the Company paid $114,979 to Vis Vires to repay the Note Payable in full. The Company recognized other income of $47,028 during the nine months ended June 30, 2016.
Intangible assets as of June 30, 2016 and September 30, 2015 consisted of the following:
| | Estimated | | |
| | Useful Lives | | |
| | | | |
| Customer contracts | 5 years | $983,645 | $983,645 |
| Technology | 5 years | 712,500 | 712,500 |
| Less: accumulated amortization | | (1,639,270) | (1,538,145) |
| Intangible assets, net | | $56,875 | $158,000 |
Total amortization expense was $101,125 and $238,028 for the nine months ended June 30, 2016 and 2015, respectively.
The fair value of the TransTech intellectual property acquired was $983,645, estimated by using a discounted cash flow approach based on future economic benefits associated with agreements with customers, or through expected continued business activities with its customers. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
The fair value of the RATLab intellectual property associated with the assets acquired was $450,000 estimated by using a discounted cash flow approach based on future economic benefits. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
The fair value of the Javelin intellectual property acquired was $262,500 estimated by using a discounted cash flow approach based on future economic benefits associated with the assets acquired. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
Accounts payable were $2,655,741 and $2,520,233 as of June 30, 2016 and September 30, 2015, respectively. Such liabilities consisted of amounts due to vendors for inventory purchases and technology development, external audit, legal and other expenses incurred by the Company.
The Company had two vendors (12.0% and 10.9%) with accounts payable in excess of 10% of its accounts payable as of June 30, 2016. The Company does expect to have vendors with accounts payable balances of 10% of total accounts payable in the foreseeable future.
12. | CONVERTIBLE NOTES PAYABLE |
Convertible notes payable as of June 30, 2016 consisted of the following:
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on August 10, 2015 for $84,000 to fund short-term working capital. The Vis Vires Note accrued interest at a rate of 8% per annum and becomes due on May 12, 2016 and was convertible into common stock on February 5, 2016. The Vis Vires Note was convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. On February 6, 2015, the Company paid $114,979 to Vis Vires to repay the Note Payable in full.
The Company entered into Convertible Promissory Notes totaling $710,000 with accredited investors during September 2015 to February 2016 to fund short-term working capital. The Notes accrue interest at a rate of 8% per annum and become due September 2016 to February 2017 and are convertible into common stock at the same price of our next financing. The Company recorded accrued interest of $31,275 as of June 30, 2016. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing.
The Company entered into 8%-10% Convertible Promissory Notes and Securities Purchase Agreements with three accredited investors on February 4, 2016, totaling $165,000 with an original issue discount of $15,000 to fund short-term working capital. The Notes become due on February 3, 2017 and are convertible into common stock after six months from issuance. The Notes are convertible at 60% of the average of the lowest trading price in the 25 days prior to conversion but not less than $0.001 per share. The Company issued a total of 10,500 shares of restricted common stock to the investors valued at $70,875 and paid $7,500 in legal fees. The Company received $128,500 net of all fees. Due to the short term of the note the Company expensed all fees and interest immediately.
On February 5, 2016, the Company valued the beneficial conversion feature of this senior secured convertible redeemable debenture at $110,000 and recorded additional paid in capital of $110,000. During the nine months June 30, 2016, the Company recorded interest expense of $142,086, related to the amortization of the debt discount associated with the Convertible Promissory Notes. As of June 30, 2016, the Company has $33,087 to be amortized to expense related to debt discount associated with the Convertible Promissory Notes.
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on February 19, 2016 for $100,000 to fund short-term working capital. The Vis Vires Note accrues interest at a rate of 8% per annum and becomes due on November 22, 2016 and is convertible into common stock on August 19, 2016. The Vis Vires Note is convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. The Company recorded accrued interest of $2,915 as of June 30, 2016. During the nine months ended June 30, 2016, the Company recognized a $65,278 debt discount resulting from the fair value of the conversion option and other income resulting from the increase in the fair value of the warrant liability at June 30, 2016 of $55,243. The Company also recorded other financing expense in the amount of $14,000 which was expensed to interest immediately due to the short term nature of the note.
13. | NOTES PAYABLE, CAPITALIZED LEASES AND LONG TERM DEBT |
Notes payable, capitalized leases and long term debt as of June 30, 2016 and September 30, 2015 consisted of the following:
| | | |
| | | |
| | | |
| Capital Source Business Finance Group | $747,652 | $364,757 |
| Note payable to Umpqua Bank | 199,935 | 199,935 |
| Secured note payable to J3E2A2Z LP - related party | 600,000 | 600,000 |
| Total debt | 1,547,587 | 1,164,692 |
| Less current portion of long term debt | (1,547,587) | (1,164,692) |
| Long term debt | $- | $- |
Capital Source Business Finance Group Secured Credit Facility
The Company finances its TransTech operations from operations and a Secured Credit Facility with Capital Source Business Finance Group. On December 9, 2008, TransTech entered into a $1,000,000 secured credit facility with Capital Source to fund its operations. On June 9, 2016, the secured credit facility was renewed for an additional six months, with a floor for prime interest of 4.5% (currently 4.5%) plus 2.5%. The eligible borrowing is based on 80% of eligible trade accounts receivable, not to exceed $1,000,000. The secured credit facility is collateralized by the assets of TransTech, with a guarantee by Visualant, including a security interest in all assets of Visualant. Availability under this Secured Credit ranges from $0 to $175,000 ($20,000 as of June 30, 2016) on a daily basis. The remaining balance on the accounts receivable line of $747,652 as of June 30, 2016 must be repaid by the time the secured credit facility expires on December 12, 2016, or we renew by automatic extension for the next successive six-month term.
Note Payable to Umpqua Bank
The Company has a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which matures on June 30, 2016 and provides for interest at 3.25% per year. On December 19, 2015, the Umpqua Loan maturity was extended to December 31, 2016 and provides for interest at 3.50% per year related to this Umpqua Loan, the Company entered into a demand promissory note for $200,000 on January 10, 2014 with an entity affiliated with Ronald P. Erickson, our Chief Executive Officer. This demand promissory note will be effective in case of a default by the Companyregistered under the Umpqua Loan. The Company recorded accrued interest of $14,844 as of June 30, 2016.
Note Payables to Ronald P. Erickson or J3E2A2Z LP
The Company also has two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between January and July 2014, provide for interest of 3% per year and now mature on September 30, 2016. They also provide for a second lien on our assets if not repaid by September 30, 2016 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson. The Company recorded accrued interest of $35,679 as of June 30, 2016.
Aggregate maturities totaling $1,547,587 are all due within twelve months.
Authorized Capital Stock
The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares of voting preferred stock, par value $0.001 per share.
Voting Preferred Stock
As of June 30, 2016, the Company is authorized to issue up to 5,000,000 shares of preferred stock with a par value of $0.001.
On July 21, 2015, the Company filed with the Nevada Secretary of State an Amended and Restated Certificate of Designations, Preferences and Rights for our Series A Convertible Preferred Stock. Among other things, the Amended and Restated Certificate changed the conversion price and the stated value of the Series A Preferred from $0.10 (pre reverse stock split) to $30.00 (post-reverse stock split), and added a provision adjusting the conversion price upon the occurrence of certain events.
Under the Amended and Restated Certificate, the Company had 11,667 shares of Series A Preferred authorized, all of which are outstanding. Each holder of outstanding shares of Series A Preferred is entitled to the number of votes equal to the number of whole shares of common stock into which the shares of Series A Preferred held by such holder are then convertible as of the applicable record date. The Company cannot amend, alter or repeal any preferences, rights, or other terms of the Series A Preferred so as to adversely affect the Series A Preferred, without the written consent or affirmative vote of the holders of at least 66% of the then outstanding shares of Series A Preferred, voting as a separate voting group, given by written consent or by vote at a meeting called for such purpose for which notice shall have been duly given to the holders of the Series A Preferred.
During the year ended September 30, 2015, the Company sold 11,667 Series A Preferred Stock to two investors totaling $350,000. These shares are expected to be convertible into 11,667 shares of common stock at $30.00 per share, subject to adjustment, for a period of five years. The Series A Preferred Stock has voting rights and may not be redeemed without the consent of the holder.
The Company also issued (i) a Series C five-year Warrant for 23,334 shares of common stock at an exercise price of $30.00 per share, which is callable at $60.00 per share; and (ii) a Series D five-year Warrant for 23,334 shares of common stock at an exercise price of $45.00 per share, which is callable at $90.00 per share. The Series A Preferred Stock and Series C and D Warrants had registration rights.
On July 20, 2015, the two investors entered into an Amendment to Series A Preferred Stock Terms whereby they agreed to the terms of the Amended and Restated Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock and waived all registration rights.
On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due to the issuance of common stock at that price.
On March 8, 2016, the Company received approval from the State of Nevada for the Correction to the Company’s Amended and Restated Certificate of Designations, Preferences and Rights of its Series A Convertible Preferred Stock. The Amended and Restated Certificate filed July 21, 2015 changed the conversion price and the stated value from $0.10 (pre reverse stock split) to $30.00 (post-reverse stock split), and adding a provision adjusting the conversion price upon the occurrence of certain events. On February 19, 2016, the holders of Series A Convertible Preferred Stock entered into Amendment 2 of Series A Preferred Stock Terms and increased the number of Preferred Stock Shares to properly account for the reverse stock split. We have 23,334 Series A Preferred Stock issued and outstanding.
Series B Redeemable Convertible Preferred Stock
On March 8, 2016, the Company received approval from the State of Nevada for the Certificate of Designations of Preferences, Powers, Rights and Limitations of Series B Redeemable Convertible Preferred Stock. The Certificate authorized 5,000 shares of Series B Preferred Stock at a par value of $.001 per share that is convertible into common stock at $7.50 per share, subject to certain adjustments as set forth in the Certificate.
The Company entered into a Stock Purchase Agreement with an institutional investor pursuant to which the Company issued 255 Shares of Series B Redeemable Preferred Shares (“Series B Preferred Shares”) of the Company at $10,000 per share with a 5.0% original issue discount for the sum of $2,500,000.
At closing, the Company sold 51 Series B Preferred Shares in exchange for payment to the Company of $500,000 in cash and issued an additional 204 Series B Preferred Shares in exchange for delivery of a full recourse 1% Promissory Note (“Note”) for $1,995,000 and payment to the Company of $5,000 in cash (paid). The Note is collateralized by the Series B Preferred Shares. Under the terms of the Note, the Company is to receive an additional $500,000 for each $5 million, or in certain cases a lower amount, in aggregate trading volume of the common stock, so long as it meets certain other requirements. Any remaining balance under the Note is payable at its maturity in seven years. Due to the uncertainty on the receipt of achieving future funding, the Company has not booked the full recourse 1% Promissory Note.
The Series B Preferred Shares are convertible into common stock at $7.50 per share; provided that the institutional investor may not convert any Series B Preferred Shares into common stock until that portion of the Note underlying the purchase of the converted portion of Series B Preferred Shares is paid in cash to Company.
The Company may issue, at our sole discretion in lieu of cash, as a conversion premium or in payment of dividends on such shares of Series B Preferred Shares. The number of additional common shares that we may issue as a conversion premium or in payment of dividends, is dependent on the dividend rate which can vary depending on our underlying stock price at the time of conversion and assuming no triggering event has occurred.
The Company filed a Registration Statement on Form S-1, which was declared effective May 6, 2016, to register $2,675,000 for the resale of all shares of common stock issuable upon conversion of the Series B Shares.
During the three months ended June 30, 2016, the Company issued 74,084 shares of common stock to this institutional investor at $4.591 or $339,998 and expensed $506,599 related to the conversion of 34 Series B Preferred Shares. The institutional investor has presented conversion notices for an additional 29,166 to 161,428 shares of common stock related to the conversion of 34 Series B Preferred Shares. The Company has denied these requests and expects to negotiate with the institutional investor.
Common Stock
Securities Act.All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non-accreditednon‑accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non-accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities.
The following equity issuances occurred during the nine months ended June 30, 2016:
Eleven investors exercised warrants at $2.50 per share and were issued 139,668 shares of common stock, for a total of $349,165 in proceeds to the Company.
On October 21, 2015, the Company entered into a Public Relations Agreement with Financial Genetics LLC for public relation services. Under the Agreement, Financial Genetics was issued 26,696 shares of our common stock. The Company expensed $195,171 during the nine months ended June 30, 2016.
On October 6, 2015, the Company entered into a Consulting Agreement with Joshua Conroy for business development services. Under the Agreement, Mr. Conroy was issued 1,711 shares of our common stock. The Company expensed $11,977 during the nine months ended June 30, 2016.
The Company entered into Convertible Promissory Notes totaling $710,000 with non‑accredited investors during September 2015 to February 2016 to fund short-term working capital. The Notes accrue interest at a rate of 8% per annum and become due September 2016 to February 2017 and are convertible into common stock as part of our next financing. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing.
The Company entered into 8%-10% Convertible Promissory Notes and Securities Purchase Agreements with three accredited investors on February 4, 2016, totaling $165,000 with an original issue discount of $15,000 to fund short-term working capital. The Notes become due on February 3, 2017 and are convertible into common stock after six months from issuance. The Notes are convertible at 60% of the average of the lowest trading price in the 25 days prior to conversion but not less than $0.001 per share. The Company issued a total of 10,500 shares of restricted common stock to the investors valued at $70,875 and paid $7,500 in legal fees and recorded a total debt discount of $165,000 during the nine months ended June 30, 2016. The Company received $128,500 net of all fees.
On February 23, 2016, the Company entered into a Consulting Agreement with David Markowski for business development services. On February 29, 2016, Mr. Markowski was issued 2,000 shares of our common stock. The Company expensed $14,600 during the nine months ended June 30, 2016.
During the three months ended June 30, 2016, the Company issued 74,084 shares of common stock to this institutional investor at $5.591 or $339,998 and expensed $506,599 related to the conversion of 34 Series B Preferred Shares.
On May 6, 2015, the Company’s stockholders approved a reverse split of our common stock, in a ratio to be determined by the Company’s Board of Directors, of not less than 1-for-50 nor more than 1-for-150. On June 9, 2015, the Company’s Board of Directors determined that the ratio of the reverse split would be 1-for-150. All warrant, option, share and per share information in this Form 10-Q gives retroactive effect for a 1-for-150 split with all numbers rounded up to the nearest whole share.
Warrants to Purchase Common Stock
The following warrant exercises occurred during the nine months ended June 30, 2016:
Eleven investors exercised warrants at $2.50 per share and were issued 139,668 shares of common stock, for a total of $349,165 in proceeds to the Company.
A warrant to acquire 1,679 shares of common stock at $19.36 per share expired.
The Company entered into Convertible Promissory Notes totaling $710,000 with accredited investors during September 2015 to February 2016 to fund short-term working capital. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing. The number of warrants convertible into shares of common stock is not known at this time as the number of shares is determined by the price of the next up-lift offering by an investment banker.
A summary of the warrants issued as of June 30, 2016 were as follows:
| | |
| | | |
| | | |
| | | |
| | | |
| Outstanding at beginning of period | 899,750 | $3.18 |
| Issued | - | - |
| Exercised | (139,668) | (2.50) |
| Forfeited | - | - |
| Expired | (1,679) | 19.36 |
| Outstanding at end of period | 758,403 | $3.27 |
| Exerciseable at end of period | 758,403 | |
A summary of the status of the warrants outstanding as of June 30, 2016 is presented below:
| |
| | | | |
| | | | |
| | | | |
| | | | |
736,398 | 1.71 | $2.50 | 736,398 | $2.50 |
1,667 | 0.38 | 19.50-22.50 | 1,667 | 19.50-22.50 |
20,338 | 1.09 | 30.00 | 20,338 | 30.00 |
758,403 | 1.54 | $3.27 | 758,403 | $3.27 |
The significant weighted average assumptions relating to the valuation of the Company’s warrants for the period ended June 30, 2016 were as follows:
Dividend yield | 0% |
Expected life | 3 |
Expected volatility | 90% |
Risk free interest rate | 0.7% |
There were vested warrants of 736,398 as of June 30, 2016 with an aggregate intrinsic value of $414,224.
Description of Stock Option Plan
On April 29, 2011, the Company’s 2011 Stock Incentive Plan was approved at the Annual Stockholder Meeting. The Company was authorized to issue options for, and has reserved for issuance, up to 46,667 shares of common stock under the 2011 Stock Incentive Plan. On March 21, 2013, an amendment to the Stock Option Plan was approved by the stockholders of the Company, increasing the number of shares reserved for issuance under the Plan to 93,333 shares.
Determining Fair Value under ASC 505
The Company records compensation expense associated with stock options and other equity-based compensation using the Black-Scholes-Merton option valuation model for estimating fair value of stock options granted under our plan. The Company amortizes the fair value of stock options on a ratable basis over the requisite service periods, which are generally the vesting periods. The expected life of awards granted represents the period of time that they are expected to be outstanding. The Company estimates the volatility of our common stock based on the historical volatility of its own common stock over the most recent period corresponding with the estimated expected life of the award. The Company bases the risk-free interest rate used in the Black Scholes-Merton option valuation model on the implied yield currently available on U.S. Treasury zero-coupon issues with an equivalent remaining term equal to the expected life of the award. The Company has not paid any cash dividends on our common stock and does not anticipate paying any cash dividends in the foreseeable future. Consequently, the Company uses an expected dividend yield of zero in the Black-Scholes-Merton option valuation model and adjusts share-based compensation for changes to the estimate of expected equity award forfeitures based on actual forfeiture experience. The effect of adjusting the forfeiture rate is recognized in the period the forfeiture estimate is changed.
Stock Option Activity
The Company had the following stock option transactions during the nine months ended June 30, 2016:
During the nine months ended June 30, 2016, employees forfeited stock option grants for 797 shares of common stock at $36.21 per share.
There are currently 56,610 options to purchase common stock at an average exercise price of $18.19 per share outstanding as of June 30, 2016 under the 2011 Stock Incentive Plan. The Company recorded $35,511 and $53,222 of compensation expense, net of related tax effects, relative to stock options for the nine months ended June 30, 2016 and 2015 in accordance with ASC 505. Net loss per share (basic and diluted) associated with this expense was approximately ($0.03) and ($0.05) per share, respectively. At June 30, 2016, there is approximately $149,700 of total unrecognized costs related to employee granted stock options that are not vested. These costs are expected to be recognized over a period of approximately 3.58 years.
Stock option activity for the nine months ended June 30, 2016 and the years ended September 30, 2015 and 2014 was as follows:
| | |
| | | | |
| Outstanding as of September 30, 2013 | 84,900 | $18.954 | $1,609,200 |
| Granted | 2,633 | 15.002 | 39,500 |
| Exercised | - | - | - |
| Forfeitures | (200) | (32.500) | (6,500) |
| Outstanding as of September 30, 2014 | 87,333 | 18.804 | 1,642,200 |
| Granted | 11,335 | 15.000 | 170,025 |
| Exercised | - | - | - |
| Forfeitures | (41,261) | (18.286) | (754,500) |
| Outstanding as of September 30, 2015 | 57,407 | 18.43 | 1,057,725 |
| Granted | - | - | - |
| Exercised | - | - | - |
| Forfeitures | (797) | (35.210) | (28,062) |
| Outstanding as of June 30, 2016 | 56,610 | $18.189 | $1,029,663 |
The following table summarizes information about stock options outstanding and exercisable as of June 30, 2016:
| | | | | |
| | | | | |
| | | | | |
| | | | | |
13.500 | 3,334 | 2.00 | $13.50 | 3,334 | $13.50 |
15.000 | 20,939 | 3.08 | 15.00 | 9,103 | 15.00 |
19.500 | 19,003 | 3.84 | 19.50 | 19,002 | 19.50 |
22.500 | 13,334 | 3.88 | 22.50 | 13,334 | 22.50 |
| 56,610 | 3.58 | $18.19 | 44,773 | $19.58 |
There were exercisable options of 56,610 as of June 30, 2016 with an aggregate intrinsic value of $0.
16. | OTHER SIGNIFICANT TRANSACTIONS WITH RELATED PARTIES |
Related Party Transactions with Ronald P. Erickson
See Note 13 for Notes Payable to Ronald P. Erickson, our Chief Executive Officer Chief and/or entities in which Mr. Erickson has a beneficial interest.
Note Payable to Umpqua Bank
The Company has a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which matures on June 30, 2016 and provides for interest at 3.25% per year. On December 19, 2015, the Umpqua Loan maturity was extended to December 31, 2016 and provides for interest at 3.50% per year related to this Umpqua Loan, the Company entered into a demand promissory note for $200,000 on January 10, 2014 with an entity affiliated with Ronald P. Erickson, our Chief Executive Officer. This demand promissory note will be effective in case of a default by the Company under the Umpqua Loan. The Company recorded accrued interest of $14,844 as of June 30, 2016.
Note Payables to Ronald P. Erickson or J3E2A2Z LP
The Company also has two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between January and July 2014, provide for interest of 3% per year and now mature on September 30, 2016. They also provide for a second lien on our assets if not repaid by September 30, 2016 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson. The Company recorded accrued interest of $35,679 as of June 30, 2016.
Other Amounts Due to Mr. Erickson
Mr. Erickson and/or entities with which he is affiliated also have advanced $668,500 and have unreimbursed expenses and compensation of approximately $398,004. We owe Mr. Erickson, or entities with which he is affiliated, $1,686,503 as of June 30, 2016.
17. | COMMITMENTS, CONTINGENCIES AND LEGAL PROCEEDINGS |
Legal Proceedings
The Company may from time to time become a party to various legal proceedings arising in the ordinary course of our business. The Company is currently not a party to any pending legal proceeding that is not ordinary routine litigation incidental to our business.
During the three months ended June 30, 2016, the Company issued 74,084 shares of common stock to this institutional investor at $5.591 or $339,998 and expensed $506,599 related to the conversion of 34 Series B Preferred Shares. The institutional investor has presented conversion notices for an additional 29,166 to 161,428 shares of common stock related to the conversion of 34 Series B Preferred Shares. The Company has denied these requests and expects to negotiate with the institutional investor.
Properties and Operating Leases
The Company is obligated under various non-cancelable operating leases for its various facilities and certain equipment.
Corporate Offices
The Company’s executive office is located at 500 Union Street, Suite 420, Seattle, Washington, USA, 98101. The Company leases 1,014 square feet and its net monthly payment is $2,535. The Company leases this office on a month to month basis.
TransTech Facilities
TransTech is located at 12142 NE Sky Lane, Suite 130, Aurora, OR 97002. TransTech leases a total of approximately 9,750 square feet of office and warehouse space for its administrative offices, product inventory and shipping operations. We leased this office until June 30, 2016 for $5,486 per month. Effective July 1, 2016, the Company leases this office on a month to month basis at $6,120 per month.
The aggregate future minimum lease payments under operating leases as of June 30, 2016 were $20,826.
The Company evaluates subsequent events, for the purpose of adjustment or disclosure, up through the date the financial statements are available. Subsequent to June 30, 2016, there were no material transactions that require disclosure:
Subsequent to June 30, 2016, executives of the Company exercised warrants at $2.50 per share and were issued 68,001 shares of common stock.
On July 12, 2016, a supplier converted accounts payable totaling $232,966 into 77,665 shares of common stock valued at $3.00 per share.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
Visualant, Inc.:
We have audited the accompanying consolidated balance sheets of Visualant, Inc. (the “Company”) as of September 30, 2015 and 2014 and the related consolidated statements of operations, stockholders’ (deficit) equity, and cash flows for the years ended September 30, 2015 and 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Visualant, Inc. as of September 30, 2015 and 2014, and the results of its operations and its cash flows for the years ended September 30, 2015 and 2014 in conformity with generally accepted accounting principles in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has sustained a net loss from operations and has an accumulated deficit since inception. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in this regard are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
PMB Helin Donovan, LLP
/s/ PMB Helin Donovan, LLP
November 4, 2015
Seattle, Washington
|
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED BALANCE SHEETS |
| | | September 30, 2015 | | | September 30, 2014 | |
| ASSETS | | | | | | |
| | | | | | | |
| CURRENT ASSETS: | | | | | | |
| Cash and cash equivalents | | $ | 82,266 | | | $ | 70,386 | |
| Accounts receivable, net of allowance of $40,000 and $39,000, respectively | | | 619,849 | | | | 815,460 | |
| Prepaid expenses | | | 27,774 | | | | 25,067 | |
| Inventories, net | | | 217,824 | | | | 412,831 | |
| Refundable tax assets | | | - | | | | 29,590 | |
| Total current assets | | | 947,713 | | | | 1,353,334 | |
| | | | | | | | | |
| EQUIPMENT, NET | | | 366,250 | | | | 447,236 | |
| | | | | | | | | |
| OTHER ASSETS | | | | | | | | |
| Intangible assets, net | | | 158,000 | | | | 431,653 | |
| Goodwill | | | 983,645 | | | | 983,645 | |
| Other assets | | | 5,070 | | | | 5,070 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 2,460,678 | | | $ | 3,220,938 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| CURRENT LIABILITIES: | | | | | | | | |
| Accounts payable - trade | | $ | 2,520,223 | | | $ | 2,234,123 | |
| Accounts payable - related parties | | | 73,455 | | | | 66,729 | |
| Accrued expenses | | | 4,068 | | | | 31,369 | |
| Accrued expenses - related parties | | | 1,256,861 | | | | 260,687 | |
| Derivative liability - warrants | | | 2,704,840 | | | | 2,579,157 | |
| Convertible notes payable | | | 109,000 | | | | 166,500 | |
| Notes payable - current portion of long term debt | | | 1,164,692 | | | | 1,290,960 | |
| Deferred revenue | | | 5,833 | | | | - | |
| Total current liabilities | | | 7,838,972 | | | | 6,629,525 | |
| | | | | | | | | |
| COMMITMENTS AND CONTINGENCIES | | | - | | | | - | |
| | | | | | | | | |
| STOCKHOLDERS' DEFICIT | | | | | | | | |
| Series A Convertible Preferred stock - $0.001 par value, 5,000,000 shares authorized, | | | | | | | | |
| 11,667 and 0 shares issued and outstanding at 9/30/2015 and 9/30/2014, respectively | | | 12 | | | | - | |
| Common stock - $0.001 par value, 100,000,000 shares authorized, 1,155,991 | | | | | | | | |
| and 1,121,150 shares issued and outstanding at 9/30/2015 and 9/30/2014, respectively | | | 1,156 | | | | 1,121 | |
| Additional paid in capital | | | 18,786,694 | | | | 18,125,411 | |
| Accumulated deficit | | | (24,166,156 | ) | | | (21,535,119 | ) |
| Total stockholders' deficit | | | (5,378,294 | ) | | | (3,408,587 | ) |
| | | | | | | | | |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | | $ | 2,460,678 | | | $ | 3,220,938 | |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS OF OPERATIONS |
| | | Years Ended, | |
| | | September 30, 2015 | | | September 30, 2014 | |
| | | | | | | |
| REVENUE | | $ | 6,290,794 | | | $ | 7,983,352 | |
| COST OF SALES | | | 5,274,334 | | | | 6,694,274 | |
| GROSS PROFIT | | | 1,016,460 | | | | 1,289,078 | |
| RESEARCH AND DEVELOPMENT EXPENSES | | | 362,661 | | | | 670,742 | |
| SELLING, GENERAL AND ADMINISTRATIVE EXPENSES | | | 2,983,751 | | | | 3,179,699 | |
| OPERATING LOSS | | | (2,329,952 | ) | | | (2,561,363 | ) |
| | | | | | | | | |
| OTHER INCOME (EXPENSE): | | | | | | | | |
| Interest expense | | | (170,157 | ) | | | (104,818 | ) |
| Other income | | | 40,653 | | | | 38,534 | |
| Loss on conversion of debt | | | (34,035 | ) | | | - | |
| (Loss) gain on change - derivative liability warrants | | | (107,956 | ) | | | 1,604,843 | |
| Total other (expense) income | | | (271,495 | ) | | | 1,538,559 | |
| | | | | | | | | |
| LOSS BEFORE INCOME TAXES | | | (2,601,447 | ) | | | (1,022,804 | ) |
| | | | | | | | | |
| Income taxes - current provision (benefit) | | | 29,590 | | | | (5,513 | ) |
| | | | | | | | . | |
| NET LOSS | | | (2,631,037 | ) | | | (1,017,291 | ) |
| | | | | | | | | |
| NONCONTROLLING INTEREST | | | - | | | | - | |
| | | | | | | | | |
| NET LOSS ATTRIBUTABLE TO VISUALANT, INC. AND SUBSIDIARIES | | | | | | | | |
| COMMON SHAREHOLDERS | | $ | (2,631,037 | ) | | $ | (1,017,291 | ) |
| Basic and diluted loss per common share attributable to Visualant, | | | | | | | | |
| Inc. and subsidiaries common shareholders- | | | | | | | | |
| Basic and diluted loss per share | | $ | (2.33 | ) | | $ | (0.92 | ) |
| | | | | | | | | |
| Weighted average shares of common stock outstanding- basic and diluted | | | 1,131,622 | | | | 1,108,964 | |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' (DEFICIT) EQUITY |
| | | | | | | | | | | | | | | Additional | | | | | | Total | |
| | | Preferred Stock | | | Common Stock | | | Paid in | | | Accumulated | | | Stockholders' | |
| | | Amount | | | Amount | | | Amount | | | Amount | | | Capital | | | Deficit | | | (Deficit) | |
| Balance as of September 30, 2013 | | | - | | | | - | | | | 1,101,817 | | | | 1,101 | | | | 17,729,731 | | | | (20,537,825 | ) | | | (2,806,993 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock compensation expense - employee options | | | - | | | | - | | | | - | | | | - | | | | 87,550 | | | | - | | | | 87,550 | |
| Issuance of common stock for services | | | - | | | | - | | | | 8,667 | | | | 9 | | | | 90,991 | | | | - | | | | 91,000 | |
| Issuance of common stock for debt conversion | | | - | | | | - | | | | 10,667 | | | | 11 | | | | 159,989 | | | | - | | | | 160,000 | |
| Issuance of warrants for services | | | - | | | | - | | | | - | | | | - | | | | 57,150 | | | | - | | | | 57,150 | |
| Sale of noncontrolling interest | | | - | | | | - | | | | - | | | | - | | | | - | | | | 19,987 | | | | 19,987 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (1,017,281 | ) | | | (1,017,281 | ) |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (1,017,281 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of September 30, 2014 | | | - | | | | - | | | | 1,121,150 | | | | 1,121 | | | | 18,125,411 | | | | (21,535,119 | ) | | | (3,408,587 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock compensation expense - employee options | | | - | | | | - | | | | - | | | | - | | | | 65,463 | | | | - | | | | 65,463 | |
| Issuance of preferred stock | | | 11,667 | | | | 12 | | | | - | | | | - | | | | 233,310 | | | | - | | | | 233,322 | |
| Issuance of common stock for services | | | - | | | | - | | | | 9,169 | | | | 9 | | | | 137,491 | | | | - | | | | 137,500 | |
| Issuance of common stock for debt conversion | | | - | | | | - | | | | 24,710 | | | | 25 | | | | 91,781 | | | | - | | | | 91,806 | |
| Reversal of derivative liability for debt repayment | | | - | | | | - | | | | - | | | | - | | | | 98,940 | | | | - | | | | 98,940 | |
| Effect of reverse stock split | | | - | | | | - | | | | 962 | | | | 1 | | | | 263 | | | | - | | | | 264 | |
| Loss on conversion of debt | | | - | | | | - | | | | - | | | | - | | | | 34,035 | | | | - | | | | 34,035 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,631,037 | ) | | | (2,631,037 | ) |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (2,631,037 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance as of September 30, 2015 | | | 11,667 | | | | 12 | | | | 1,155,991 | | | $ | 1,156 | | | $ | 18,786,694 | | | $ | (24,166,156 | ) | | $ | (5,378,294 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES |
CONSOLIDATED STATEMENTS OF CASH FLOWS |
| | | | | | | |
| | | Years Months Ended, | |
| | | September 30, 2015 | | | September 30, 2014 | |
| | | | | | | |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | |
| Net loss | | $ | (2,631,037 | ) | | $ | (1,017,281 | ) |
| Adjustments to reconcile net loss to net cash (used in) | | | | | | | | |
| operating activities | | | | | | | | |
| Depreciation and amortization | | | 353,229 | | | | 418,271 | |
| Issuance of capital stock for services and expenses | | | 137,500 | | | | 91,000 | |
| Issuance of warrants for services and expenses | | | - | | | | 57,150 | |
| Issuance of capital stock for accrued liabilities | | | - | | | | 160,000 | |
| Stock based compensation | | | 65,463 | | | | 87,550 | |
| (Gain) on sale of assets | | | (20,042 | ) | | | (28,363 | ) |
| Loss (gain) on change - derivative liability warrants | | | 107,956 | | | | (1,604,843 | ) |
| Provision for losses on accounts receivable | | | 28,266 | | | | 36 | |
| Loss on conversion of debt | | | 34,035 | | | | - | |
| Changes in operating assets and liabilities: | | | - | | | | | |
| Accounts receivable | | | 167,345 | | | | 191,578 | |
| Prepaid expenses | | | (2,707 | ) | | | 31,464 | |
| Inventory | | | 195,007 | | | | 87,959 | |
| Other assets | | | - | | | | 1,091 | |
| Accounts payable - trade and accrued expenses | | | 1,289,685 | | | | 144,808 | |
| Income tax receivable | | | 29,590 | | | | 183 | |
| Deferred revenue | | | 5,833 | | | | - | |
| CASH (USED IN) OPERATING ACTIVITIES | | | (239,877 | ) | | | (1,379,397 | ) |
| | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | |
| Proceeds from sale of equipment | | | 21,452 | | | | 29,300 | |
| NET CASH PROVIDED BY INVESTING ACTIVITIES: | | | 21,452 | | | | 29,300 | |
| | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | |
| Repayments from line of credit | | | (123,633 | ) | | | (260,925 | ) |
| Proceeds from sale of preferred stock | | | 350,000 | | | | - | |
| Proceeds from notes payable | | | - | | | | 200,000 | |
| Proceeds from notes payable- related party | | | - | | | | 600,000 | |
| Repayment of convertible notes | | | (166,500 | ) | | | - | |
| Proceeds from convertible notes payable | | | 173,000 | | | | 166,500 | |
| Repayments of capital leases | | | (2,562 | ) | | | (3,138 | ) |
| Change in controlling interest | | | - | | | | (29,083 | ) |
| NET CASH PROVIDED BY FINANCING ACTIVITIES | | | 230,305 | | | | 673,354 | |
| | | | | | | | | |
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | | 11,880 | | | | (676,743 | ) |
| | | | | | | | | |
| CASH AND CASH EQUIVALENTS, beginning of period | | | 70,386 | | | | 747,129 | |
| | | | | | | | | |
| CASH AND CASH EQUIVALENTS, end of period | | $ | 82,266 | | | $ | 70,386 | |
| | | | | | | | | |
| Supplemental disclosures of cash flow information: | | | | | | | | |
| Interest paid | | $ | 114,907 | | | $ | 52,755 | |
| Taxes paid | | $ | - | | | $ | - | |
| | | | | | | | | |
| Non-cash investing and financing activities: | | | | | | | | |
| Loss (gain) on change - derivative liability warrants | | $ | 17,727 | | | $ | - | |
| Issuance of common stock for debt conversion | | $ | 91,806 | | | $ | - | |
The accompanying notes are an integral part of these consolidated financial statements.
VISUALANT, INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Visualant, Incorporated (the “Company,” “Visualant, Inc.” or “Visualant”) was incorporated under the laws of the State of Nevada in 1998. The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares preferred stock, par value $0.001 per share. On July 21, 2015, the Company filed with the Secretary of State of Nevada an Amended and Restated Certificate of Designations, Preferences and Rights for our Series A Convertible Preferred Stock.
Since 2007 the Company has been focused primarily on the development of a proprietary technology which is capable of uniquely identifying and authenticating almost any substance using light at the “photon” level to detect the unique digital “signature” of the substance. The Company calls this its “ChromaID™” technology.
In 2010, the Company acquired TransTech Systems, Inc. as an adjunct to its business. TransTech is a distributor of products for employee and personnel identification. TransTech currently provides substantially all of the Company’s revenues.
The Company is in the process of commercializing its ChromaID™ technology. To date, the Company has entered into one License Agreement with Sumitomo Precision Products Co., Ltd. and has a strategic relationship with Invention Development Management Company, L.L.C. (“IDMC”).
The Company believes that its commercialization success is dependent upon its ability to significantly increase the number of customers that are purchasing and using its products. To date the Company has generated minimal revenue from sales of its ChromaID products. The Company is currently not profitable. Even if the Company succeeds in introducing the ChromaID technology and related products to its target markets, the Company may not be able to generate sufficient revenue to achieve or sustain profitability.
ChromaID was invented by scientists from the University of Washington under contract with Visualant. The Company has pursued an aggressive intellectual property strategy and have been granted eight patents. The Company also has 22 patents pending. The Company possess all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to the Company in perpetuity by the Company’s strategic partner, Intellectual Ventures through its subsidiary IDMC.
On May 6, 2015, the Company’s stockholders approved a reverse split of our common stock, in a ratio to be determined by the Company’s Board of Directors, of not less than 1-for-50 nor more than 1-for-150. On June 9, 2015, the Company’s Board of Directors determined that the ratio of the reverse split would be 1-for-150. All warrant, option, share and per share information in this Form 10-K gives retroactive effect for a 1-for-150 split with all numbers rounded up to the nearest whole share.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company incurred net losses of $2,631,037 and $1,017,291 for the years ended September 30, 2015 and 2014, respectively. Net cash used in operating activities was $(239,877) and $(1,379,397) for the years ended September 30, 2015 and 2014, respectively.
The Company anticipates that it will record losses from operations for the foreseeable future. As of September 30, 2015, the Company’s accumulated deficit was $24,166,156. The Company has limited capital resources, and operations to date have been funded with the proceeds from private equity and debt financings and loans from Ronald P. Erickson, our Chief Executive Officer, or entities with which he is affiliated. These conditions raise substantial doubt about our ability to continue as a going concern. The audit report prepared by the Company’s independent registered public accounting firm relating to our financial statements for the year ended September 30, 2015 includes an explanatory paragraph expressing the substantial doubt about the Company’s ability to continue as a going concern.
Continuation of the Company as a going concern is dependent upon obtaining additional working capital. The financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
3. | SIGNIFICANT ACCOUNTING POLICIES: ADOPTION OF ACCOUNTING STANDARDS |
Basis of Presentation – The accompanying unaudited consolidated financial statements include the accounts of the Company. Intercompany accounts and transactions have been eliminated. The preparation of these unaudited condensed consolidated financial statements were prepared in conformity with U.S. generally accepted accounting principles (“GAAP”).
Principles of Consolidation – The consolidated financial statements include the accounts of the Company and its wholly owned and majority-owned subsidiaries, TransTech Systems, Inc. Inter-Company items and transactions have been eliminated in consolidation.
Cash and Cash Equivalents – The Company classifies highly liquid temporary investments with an original maturity of three months or less when purchased as cash equivalents. The Company maintains cash balances at various financial institutions. Balances at US banks are insured by the Federal Deposit Insurance Corporation up to $250,000. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant risk for cash on deposit.
Accounts Receivable and Allowance for Doubtful Accounts – Accounts receivable consist primarily of amounts due to the Company from normal business activities. The Company maintains an allowance for doubtful accounts to reflect the expected non-collection of accounts receivable based on past collection history and specific risks identified within the portfolio. If the financial condition of the customers were to deteriorate resulting in an impairment of their ability to make payments, or if payments from customers are significantly delayed, additional allowances might be required.
Inventories – Inventories consist primarily of printers and consumable supplies, including ribbons and cards, badge accessories, capture devices, and access control components held for resale and are stated at the lower of cost or market on the first-in, first-out (“FIFO”) method. Inventories are considered available for resale when drop shipped and invoiced directly to a customer from a vendor, or when physically received by TransTech at a warehouse location. The Company records a provision for excess and obsolete inventory whenever an impairment has been identified. There is a $20,000 and $10,000 reserve for impaired inventory as of September 30, 2015 and 2014, respectively.
Equipment – Equipment consists of machinery, leasehold improvements, furniture and fixtures and software, which are stated at cost less accumulated depreciation and amortization. Depreciation is computed by the straight-line method over the estimated useful lives or lease period of the relevant asset, generally 2-10 years, except for leasehold improvements which are depreciated over 5-20 years.
Intangible Assets/ Intellectual Property – The Company amortized the intangible assets and intellectual property acquired in connection with the acquisition of TransTech, over sixty months on a straight - line basis, which was the time frame that the management of the Company was able to project forward for future revenue, either under agreement or through expected continued business activities. Intangible assets and intellectual property acquired from RATLab LLC and Javelin are recorded likewise. The Company performs annual assessments and has determined that no impairment is necessary. On June 7, 2011, the Company closed the acquisition of all Visualant related assets of the RATLab LLC, namely the rights to the medical field of use of the Chroma ID technology. On July 31, 2012, the Company closed the acquisition of all rights to the ChromaID technology in the environmental field of use from Javelin LLC.
Goodwill – Goodwill is the excess of cost of an acquired entity over the fair value of amounts assigned to assets acquired and liabilities assumed in a business combination. With the adoption of ASC 350, goodwill is not amortized, rather it is tested for impairment annually, and will be tested for impairment between annual tests if an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is done at a reporting unit level. Reporting units are one level below the business segment level, but are combined when reporting units within the same segment have similar economic characteristics. Under the criteria set forth by ASC 350, the Company has one reporting unit based on the current structure. An impairment loss generally would be recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit. The Company performs annual assessments and has determined that no impairment is necessary.
Long-Lived Assets – The Company reviews its long-lived assets for impairment annually or when changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Long-lived assets under certain circumstances are reported at the lower of carrying amount or fair value. Assets to be disposed of and assets not expected to provide any future service potential to the Company are recorded at the lower of carrying amount or fair value (less the projected cost associated with selling the asset). To the extent carrying values exceed fair values, an impairment loss is recognized in operating results.
Fair Value Measurements and Financial Instruments – ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy, which requires classification based on observable and unobservable inputs when measuring fair value. The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
Level 1 – Quoted prices in active markets for identical assets and liabilities; |
Level 2 – Inputs other than level one inputs that are either directly or indirectly observable; and |
Level 3 – Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
Derivative Instruments – Warrants with the June 2013 Private Placement
Liabilities measured at fair value on a recurring basis are summarized as follows:
| | | | | | | | | | | | Carrying | |
| | | Fair Value Measurements Using Inputs | | | Amount at | |
| Financial Instruments | | Level 1 | | | Level 2 | | | Level 3 | | | September 30, 2015 | |
| | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | |
| Derivative Instruments - Warrants | | $ | - | | | $ | 2,196,716 | | | $ | - | | | $ | 2,196,716 | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | - | | | $ | 2,196,716 | | | $ | - | | | $ | 2,196,716 | |
| | | September 30, 2015 | |
| Market price and estimated fair value of common stock: | | $ | 5.600 | |
| Exercise price | | | 2.50 | |
| Expected term (years) | | | 0.25 | |
| Dividend yield | | | - | |
| Expected volatility | | | 105.1 | % |
| Risk-free interest rate | | | 0.001 | % |
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the warrants.
The Company issued warrants to purchase 697,370 shares of common stock in connection with our June 2013 private placement of 348,685 shares of common stock. The exercise price of these warrants is $2.50 per share. These warrants were not issued with the intent of effectively hedging any future cash flow, fair value of any asset, liability or any net investment in a foreign operation. These warrants were issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional shares of our common stock or securities exercisable, convertible or exchangeable for the Company’s common stock were issued at a price less than the exercise price. Therefore, the fair value of these warrants were recorded as a liability in the consolidated balance sheet and are marked to market each reporting period until they are exercised or expire or otherwise extinguished.
The proceeds from the private placement were allocated between the shares of common stock and the warrants issued in connection with the private placement based upon their estimated fair values as of the closing date at June 14, 2013, resulting in the aggregate amount of $2,494,710 allocated to stockholders’ equity and $2,735,290 allocated to the warrant derivative. The Company recognized $1,448,710 of other expense resulting from the increase in the fair value of the warrant liability at September 30, 2013. During the year ended September 30, 2014, the Company recognized $2,092,000 of other income resulting from the decrease in the fair value of the warrant liability at September 30, 2014. During the year ended September 30, 2015, the Company recognized $104,716 of other expense resulting from the decrease in the fair value of the warrant liability at September 30, 2015.
Liabilities measured at fair value on a recurring basis are summarized as follows:
| | | | | | | | | | | | Carrying | |
| | | Fair Value Measurements Using Inputs | | | Amount at | |
| Financial Instruments | | Level 1 | | | Level 2 | | | Level 3 | | | September 30, 2015 | |
| | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | |
| Derivative Instruments - Warrants | | $ | - | | | $ | 306,082 | | | $ | - | | | $ | 306,082 | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | - | | | $ | 306,082 | | | $ | - | | | $ | 306,082 | |
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the warrants.
| | | September 30, 2015 | |
| Market price and estimated fair value of common stock: | | $ | 5.60 | |
| Exercise price | | | 2.50 | |
| Expected term (years) | | | 0.25 | |
| Dividend yield | | | - | |
| Expected volatility | | | 105.1 | % |
| Risk-free interest rate | | | 0.001 | % |
The Company issued a warrant to purchase 97,169 shares of common stock in connection with the November 2013 IDMC Services and License Agreement. The warrant price of $30.00 per share expires November 10, 2018 and the per share price is subject to adjustment. This warrant was not issued with the intent of effectively hedging any future cash flow, fair value of any asset, liability or any net investment in a foreign operation. This warrant was issued with a down-round provision whereby the exercise price would be adjusted downward in the event that additional shares of our common stock or securities exercisable, convertible or exchangeable for our common stock were issued at a price less than the exercise price. Therefore, the fair value of these warrants was recorded as a liability in the consolidated balance sheet and are marked to market each reporting period until they are exercised or expire or otherwise extinguished. During the year ended September 30, 2014, the Company recognized $320,657 of other expense related to the IDMC warrant. During the year ended September 30, 2015, the Company recognized $14,574 of other income related to the IDMC warrant.
Derivative Instrument – Convertible Note Payable KBM Worldwide, Inc.
The Company entered into a Convertible Note Payable with KBM Worldwide, Inc. on August 25, 2014 for $103,500. The Note was paid off on March 2, 2015. The Company entered into a Convertible Note Payable with KBM on September 24, 2014 for $63,000. The Note was repaid March 27, 2015. The Company entered into a Convertible Note Payable with KBM on January 27, 2015 for $64,000. The KBM Note accrued interest at a rate of 8% per annum and becomes due on October 27, 2015 and was convertible into common stock on July 26, 2015. On August 3, 10, 13 and 14, 2015, the Company issued a total of 23,010 shares of common stock to KBM Worldwide, Inc. related to the conversion of $64,000 of debt and interest of $2,560 pursuant to a Securities Purchase Agreement dated January 27, 2015. The shares were issued at an average of $2.785 per share, with a low price of $2.50 per share.
During the year ended September 30, 2014, the Company recognized $166,500 of other expense related to the KBM Note. During the year ended September 30, 2015, the Company recognized $29,529 of other income and allocated $98,940 to stockholder’s equity related to the KBM Note. The Company recorded accrued interest of $898 as of June 30, 2015. The Company recorded on a loss on conversion of $34,035 and allocated $34,035 to stockholder’s equity.
Derivative Instrument – Series A Convertible Preferred Stock
| | | | | | | | | | | | Carrying | |
| | | Fair Value Measurements Using Inputs | | | Amount at | |
| Financial Instruments | | Level 1 | | | Level 2 | | | Level 3 | | | September 30, 2015 | |
| | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | |
| Derivative Instruments - Warrants | | $ | - | | | $ | 147,004 | | | $ | - | | | $ | 147,004 | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | - | | | $ | 147,004 | | | $ | - | | | $ | 147,004 | |
Liabilities measured at fair value on a recurring basis are summarized as follows:
| | | September 30, 2015 | |
| Market price and estimated fair value of common stock: | | $ | 5.60 | |
| Exercise price | | | 2.50 | |
| Expected term (years) | | | 0.25 | |
| Dividend yield | | | - | |
| Expected volatility | | | 105.1 | % |
| Risk-free interest rate | | | 0.001 | % |
The risk-free rate of return reflects the interest rate for the United States Treasury Note with similar time-to-maturity to that of the Series A Convertible Preferred Stock.
The Company issued 11,667 shares of Series A Convertible Preferred Stock with attached warrants during the year ended September 30, 2015. The Company allocated $233,322 to stockholders equity and $116,678 to the derivative warrant liability. The warrants were issued with a down round provision. During the year ended September 30, 2015, the Company recognized $30,338 of other expense related to the warrant liability.
Derivative Instrument – Convertible Note Payable Vis Vires Group, Inc.
| | | | | | | | | | | | Carrying | |
| | | Fair Value Measurements Using Inputs | | | Amount at | |
| Financial Instruments | | Level 1 | | | Level 2 | | | Level 3 | | | September 30, 2015 | |
| | | | | | | | | | | | | |
| Liabilities: | | | | | | | | | | | | |
| Derivative Instruments - Convertible Promissory Note | | $ | - | | | $ | 55,038 | | | $ | - | | | $ | 55,038 | |
| | | | | | | | | | | | | | | | | |
| Total | | $ | - | | | $ | 55,038 | | | $ | - | | | $ | 55,038 | |
Liabilities measured at fair value on a recurring basis are summarized as follows:
| | | September 30, 2015 | |
| Market price and estimated fair value of common stock: | | $ | 5.60 | |
| Exercise price | | | 3.64 | |
| Expected term (years) | | | 0.375 | |
| Dividend yield | | | - | |
| Expected volatility | | | 105.1 | % |
| Risk-free interest rate | | | 0.75 | % |
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on August 10, 2015 for $84,000 to fund short-term working capital. The Vis Vires Note accrues interest at a rate of 8% per annum and becomes due on May 12, 2016 and is convertible into common stock on February 5, 2016. The Vis Vires Note is convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. The Company recorded accrued interest of $405 as of September 30, 2015.
During the year ended September 30, 2015, the Company recognized $55,038 of other expense related to the Vis Vires Note.
The recorded value of other financial assets and liabilities, which consist primarily of cash and cash equivalents, accounts receivable, other current assets, and accounts payable and accrued expenses approximate the fair value of the respective assets and liabilities at September 30, 2015 and 2014 based upon the short-term nature of the assets and liabilities.
Revenue Recognition – Visualant and TransTech revenue are derived from products and services. Revenue is considered realized when the products or services have been provided to the customer, the work has been accepted by the customer and collectability is reasonably assured. Furthermore, if an actual measurement of revenue cannot be determined, the Company defers all revenue recognition until such time that an actual measurement can be determined. If during the course of a contract management determines that losses are expected to be incurred, such costs are charged to operations in the period such losses are determined. Revenues are deferred when cash has been received from the customer but the revenue has not been earned.
Stock Based Compensation – The Company has share-based compensation plans under which employees, consultants, suppliers and directors may be granted restricted stock, as well as options to purchase shares of Company common stock at the fair market value at the time of grant. Stock-based compensation cost is measured by the Company at the grant date, based on the fair value of the award, over the requisite service period. For options issued to employees, the Company recognizes stock compensation costs utilizing the fair value methodology over the related period of benefit. Grants of stock options and stock to non-employees and other parties are accounted for in accordance with the ASC 505.
Convertible Securities – Based upon ASC 815-15, we have adopted a sequencing approach regarding the application of ASC 815-40 to convertible securities issued subsequent to September 30, 2015. We will evaluate our contracts based upon the earliest issuance date. In the event partial reclassification of contracts subject to ASC 815-40-25 is necessary, due to our inability to demonstrate we have sufficient shares authorized and unissued, shares will be allocated on the basis of issuance date, with the earliest issuance date receiving first allocation of shares. If a reclassification of an instrument were required, it would result in the instrument issued latest being reclassified first.
Income Taxes – Income taxes are calculated based upon the asset and liability method of accounting. Deferred income taxes are recorded to reflect the tax consequences in future years of differences between the tax basis of assets and liabilities and their financial reporting amounts at each year-end. A valuation allowance is recorded against deferred tax assets if management does not believe the Company has met the “more likely than not” standard to allow for recognition of such an asset. In addition, realization of an uncertain income tax position must be estimated as “more likely than not” (i.e., greater than 50% likelihood of receiving a benefit) before it can be recognized in the financial statements. Further, the recognition of tax benefits recorded in the financial statements, if any, is based on the amount most likely to be realized assuming a review by tax authorities having all relevant information.
The Company recognizes refundable and deferred assets to the extent that management has determined their realization. As of September 30, 2015 and 2014, the Company had refundable tax assets related to TransTech of $0 and $29,590, respectively.
Net Loss per Share – Under the provisions of ASC 260, “Earnings Per Share,” basic loss per common share is computed by dividing net loss available to common shareholders by the weighted average number of shares of common stock outstanding for the periods presented. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that would then share in the income of the Company, subject to anti-dilution limitations. The common stock equivalents have not been included as they are anti-dilutive. As of September 30, 2015, there were options outstanding for the purchase of 57,407 common shares, warrants for the purchase of 899,750 common shares, 11,667 shares of our common stock issuable upon the conversion of Series A Convertible Preferred Stock, up to 34,871 shares of our common stock issuable upon the exercise of placement agent warrants and an unknown number of shares related to the conversion of $109,000 in convertible promissory notes which could potentially dilute future earnings per share. As of September 30, 2014, there were options outstanding for the purchase of 87,333 common shares, warrants for the purchase of 857,083 common shares and up to 34,871 shares of our common stock issuable upon the exercise of placement agent warrants and an unknown number of shares related to the conversion of $166,500 in convertible promissory notes which could potentially dilute future earnings per share.
Dividend Policy – The Company has never paid any cash dividends and intends, for the foreseeable future, to retain any future earnings for the development of our business. Our future dividend policy will be determined by the board of directors on the basis of various factors, including our results of operations, financial condition, capital requirements and investment opportunities.
Use of Estimates – The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recent Accounting Pronouncements
A variety of proposed or otherwise potential accounting standards are currently under study by standard setting organizations and various regulatory agencies. Due to the tentative and preliminary nature of those proposed standards, management has not determined whether implementation of such proposed standards would be material to our consolidated financial statements.
In August 2014, FASB issued ASU 2014-15—Presentation of Financial Statements—Going Concern (ASC Subtopic 205-40): “Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern”. The update requires management to assess a company’s ability to continue as a going concern and to provide related footnote disclosures in certain circumstances. All entities are required to apply the new requirements in annual periods ending after December 15, 2016, and interim periods thereafter. Early application is permitted. As such, GrowLife, Inc. is required to adopt these provisions for the annual period ending December 31, 2016. The Company is currently evaluating the impact of FASB ASU 2014-15 but does not expect the adoption thereof to have a material effect on the Company’s financial statements.
In May 2014, FASB issued ASU 2014-09—Revenue from Contracts with Customers (Topic 606): “Section A—Summary and Amendments That Create Revenue from Contracts with Customers, (Topic 606) and Other Assets and Deferred Costs—Contracts with Customers (Subtopic 340-40), Section B—Conforming Amendments to Other Topics and Subtopics in the Codification and Status Tables, Section C—Background Information and Basis for Conclusions”. The guidance in this update affects any entity that enters into contracts with customers to transfer goods or services and supersedes the revenue recognition requirements in Topic 605, Revenue Recognition. The update is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. Early application is not permitted. As such, GrowLife, Inc. is required to adopt these provisions as of December 31, 2016. The Company is currently evaluating the impact of FASB ASU 2014-09 but does not expect the adoption thereof to have a material effect on the Company’s financial statements.
In July 2013, FASB issued ASU 2013-11—Income Taxes (ASC Topic 740): “Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists (a consensus of the FASB Emerging Issues Task Force)”. The amendments in this update provide explicit guidance on the financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists at the reporting date. The update is effective for fiscal years, and interim periods within those years, beginning after December 15, 2013. The adoption of FASB ASU 2013-11 did not have a material effect on the Company’s financial statements.
New Accounting Standards Issued But Not Yet Adopted
In July 2015, the FASB issued ASU 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory ("ASU 2015-11"). This ASU requires inventories measured under any methods other than last-in, first-out ("LIFO") or the retail inventory method to be subsequently measured at the lower of cost or net realizable value, rather than at the lower of cost or market. Subsequent measurement of inventory using LIFO or the retail inventory method is unchanged by this ASU. ASU 2015-11 is effective for public companies for interim and annual periods beginning after December 15, 2016. The Company is currently evaluating the impact that this standard will have on the consolidated financial statements and does not anticipate a significant impact to the Company's financial position as a result of this change.
In February 2015, the FASB issued ASU No. 2015-02, Consolidation (Topic 810): Amendments to the Consolidation Analysis (“ASU 2015-02”). ASU 2015-02 affects reporting entities that are required to evaluate whether they should consolidate certain legal entities. ASU 2015-02 is effective for us on January 1, 2016, with early adoption permitted. The Company does not believe that this pronouncement will have an impact on the Company’s consolidated financial statements.
In April 2015, the FASB issued ASU No. 2015-03, Interest - Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”). The amendments in ASU 2015-03 require that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amendments in ASU 2015-03. ASU 2015-03 is effective for the Company on January 1, 2016, with early adoption permitted. The Company is currently evaluating the potential changes from this ASU to the Company’s future financial reporting and disclosures.
4. | DEVELOPMENT OF OUR CHROMAID™ TECHNOLOGY |
The Company is focused primarily on the development of a proprietary technology which is capable of uniquely identifying and authenticating almost any substance using light to create, record and detect the unique digital “signature” of the substance. The Company calls this its “ChromaID™” technology.
The Company’s ChromaID™ Technology
The Company has developed a proprietary technology to uniquely identify and authenticate almost any substance. This patented technology utilizes light at the photon (elementary particle of light) level through a series of emitters and detectors to generate a unique signature or “fingerprint” from a scan of almost any solid, liquid or gaseous material. This signature of reflected or transmitted light is digitized, creating a unique ChromaID signature. Each ChromaID signature is comprised of from hundreds or thousands of specific data points.
The ChromaID technology looks beyond visible light frequencies to areas of near infra-red and ultraviolet light that are outside the humanly visible light spectrum. The data obtained allows the Company to create a very specific and unique ChromaID signature of the substance for a myriad of authentication and verification applications.
Traditional light-based identification technology, called spectrophotometry, has relied upon a complex system of prisms, mirrors and visible light. Spectrophotometers typically have a higher cost and utilize a form factor more suited to a laboratory setting and require trained laboratory personnel to interpret the information. The ChromaID technology uses lower cost LEDs and photodiodes and specific frequencies of light resulting in a more accurate, portable and easy-to-use solution for a wide variety of applications. The ChromaID technology not only has significant cost advantages as compared to spectrophotometry, it is also completely flexible is size, shape and configuration. The ChromaID scan head can range in size from endoscopic to a scale that could be the size of a large ceiling-mounted florescent light fixture.
In normal operation, a ChromaID master or reference scan is generated and stored in a database. The Visualant scan head can then scan similar materials to identify, authenticate or diagnose them by comparing the new ChromaID digital signature scan to that of the original or reference ChromaID signature or scan result.
The following summarizes the Company’s plans for its Company’s proprietary ChromaID technology. Based on the Company’s anticipated expenditures on this technology, the expected efforts of its management and its relationship with Intellectual Ventures and its subsidiary, IDMC, and the Company’s other strategic partner, Sumitomo Precision Products, Ltd., the Company expects its ChromaID technology to provide an increasing portion of its revenues in future years from product sales, licenses, royalties and other revenue streams., as discussed further below.
ChromaID: A Foundational Platform Technology
The Company’s ChromaID technology provides a platform upon which a myriad of applications can be developed. As a platform technology, it is analogous to a smartphone, upon which an enormous number of previously unforeseen applications have been developed. The ChromaID technology is an enabling technology that brings the science of light and photonics to low cost, real world commercialization opportunities across multiple industries. The technology is foundational and as such, the basis upon which the Company believes a significant business can be built.
As with other foundational technologies, a single application may reach across multiple industries. The ChromaID technology can, for example effectively differentiate and identify different brands of clear vodkas that appear identical to the human eye. By extension this same technology can identify pure water from water with contaminants present. It can provide real time detection of liquid medicines such as morphine that have been adulterated or compromised. It can detect if jet fuel has water contamination present. It could determine when it is time to change oil in a deep fat fryer. These are but a few of the potential applications of the ChromaID technology based upon extensions of its ability to identify different clear liquids.
The cornerstone of a company with a foundational platform technology is its intellectual property. ChromaID was invented by scientists from the University of Washington under contract with Visualant. The Company has pursued an aggressive intellectual property strategy and has been granted nine patents. The Company currently have 21 patents pending. The Company possesses all right, title and interest to the issued patents. Ten of the pending patents are licensed exclusively to us in perpetuity by our strategic partner, the IDMC subsidiary of Intellectual Ventures.
At the Photonics West trade show held in San Francisco in February 2013, we were honored to receive a PRISM award from the Society of Photo-Optical Instrumentation Engineers International, better known as SPIE. The PRISM awards recognizes photonic products that break with conventional ideas, solve problems, and improve life through the application of light-based technologies.
IDMC Relationship
In November 2013, the Company entered into a strategic relationship with IDMC, a subsidiary of Intellectual Ventures, a private intellectual property fund with over $5 billion under management. Intellectual Ventures owns over 40,000 IP assets and has broad global relationships for the invention of technology, the filing of patents and the licensing of intellectual property. IDMC has worked to expand the reach and the potential application of the ChromaID technology and has filed ten patents base on the ChromaID technology, which it has licensed to the Company. In connection with IDMC’s work to expand the Company’s intellectual property portfolio, the Company agreed to curtail outbound marketing activities of its technology through the fourth fiscal quarter of 2014.
Initial testing in the Company’s laboratories and the work of the IDMC inventors have shown that the ChromaID technology has a number of broad and useful applications a few of which include:
●
Milk identification for quality, protein and fat content and impurities
●
Identification of liquids for counterfeits or contaminants
●
Detecting adulterants in food and food products compromising its quality
●
Color grading of diamonds
●
Identifying real cosmetics versus counterfeit cosmetics
●
Identifying counterfeit medications versus real medications
●
Identifying regular flour versus gluten free flour
●
Authenticating secure identification cards
Products
The Company first delivered product, the ChromaID Lab Kit, scans and identifies solid surfaces. The Company is marketing this product to customers who are considering licensing the technology. Target markets include, but are not limited to, commercial paint manufacturers, pharmaceutical equipment manufacturers, process control companies, currency paper and ink manufacturers, security cards, cosmetic companies, scanner manufactures and food processing companies.
The Company’s second product, the ChromaID Liquid Lab Kit, scans and identifies liquids. This product is currently in prototype form. Similar to the Company’s first product, it will be marketed to customers who are considering licensing the technology. Rather than use an LED emitter to reflect light off of a surface that is captured by a photodiode to generate a ChromaID signature the liquid analysis product shines light through the liquid (transmissive) with the LEDs positioned on one side of the liquid sample and the photo detectors on the opposite side. This device is in a functional state in our laboratory and the Company anticipates having a Liquid ChromaID Lab Kit available for customers by the Company during the fall of 2015. Target markets include, but are not limited to, water companies, petrochemical companies, pharmaceutical companies, and numerous consumer applications.
The ChromaID Lab Kits allows potential licensors of our technology to work with our technology and develop solutions for their particular application. Our contractual arrangements with IDMC are described in greater detail below.
Our Commercialization Plans for the ChromaID Technology.
The Company shipped its first ChromaID product, the ChromaID Lab Kits, to our strategic partner IDMC during the last calendar quarter of 2013 and first calendar quarter of 2014, after we completed final assembly and testing. As part of the Company’s agreement with IDMC, the Company curtailed its ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, the Company began to actively market the ChromaID Lab Kits to interested and qualified customers. Some ChromaID Lab Kits are provided free of charge to potential customers. Others are sold for a modest price. To date, the Company has achieved limited revenue from the sale of our ChromaID Lab Kits.
The Lab Kit includes the following:
ChromaID Scanner. A small device made with electronic and optical components and firmware which pulses light onto a flat material and records and digitizes the light that is reflected back from that material. The device is the size of a typical flashlight (5.5” long and 1.25” diameter). However, the technology can be incorporated into almost any size, shape and configuration.
ChromaID Lab Software. A software application that runs on a Windows PC. The software allows for configuration of the scanner, controls the behavior of the ChromaID Scanner, displays a graph of the captured ChromaID signature profile, stores the ChromaID signature in a database and uses algorithms to compare the accuracy of the match of the unknown scan to the known ChromaID signature profile. This software is intended for lab and experimental use only and is not required for commercialized product applications.
Software Development Toolkit. A collection of software applications, API (an abbreviation of application program interface – a set of routines, protocols, and tools for building software applications) definitions and file descriptions that allow a customer to extract the raw data from the ChromaID signatures and run their own software routines against that raw data.
The ChromaID Lab Kit allows customers to experiment with and evaluate the ChromaID technology and determine if it is appropriate for their specific applications. The primary electronic and optical parts of the ChromaID scanner, called the “scan head,” could be supplied to customers to integrate into their own products. A set of ChromaID Developer Tools are also available. These allow customers to develop their own applications and products based on the ChromaID technology.
ChromaID signatures must be stored, managed, and readily accessible for comparison, matching and authentication purposes. The database can be owned and operated by the end customer, but in the case of thousands of ChromaID signatures, database management may be outsourced to us or a third party provider. These database services could be made available on a per-access transaction basis or on a monthly or annual subscription basis. The actual storage location of the database can be cloud-based, on a stand-alone scanning device or on a mobile device via a Bluetooth connection depending on the requirements of access, size of the database and security as defined by the customer. As a result, large databases can be accessed by cell phone or other mobile technologies using either local storage or cloud based storage.
Based on the commercialization plans outlined above, the Company’s business model anticipates deriving revenue from several sources:
●
Sales of the ChromaID Lab Kit and ChromaID Liquid Lab Kit
●
Non Recurring Engineering (NRE) fees to assist customers with scan integration into their products
●
Licensing of the ChromaID technology
●
Royalties per unit generated from the sales of scan heads
●
Per click transaction revenue from accessing the unique ChromaID signatures
●
Developing custom product applications for customers
●
ChromaID database administration and management services
The Company’s Acceleration of Business Development in the United States and Around the World
The Company is coordinating its internal business development, sales and marketing efforts with those of its strategic partners IDMC, and Sumitomo Precision Products to leverage market data and information in order to focus on specific target vertical markets which have the greatest potential for early adoption. The ChromaID Lab Kit provides a means for us to demonstrate the technology to customers in these markets. It also allows customers to experiment with developing unique applications for their particular use. Visualant’s Business Development team is pursuing license opportunities with customers in our target markets.
There is no requirement for FDA or other government approval for the current applications of our ChromaID technology. Over time, as the Company explores the application of its ChromaID technology for medical diagnostics and other applications, the Company expects that there will be requirements for FDA and other government approvals before applications using the technology in medical and other regulated fields can enter the marketplace.
Research and Development
The Company’s research and development efforts are primarily focused improving the core foundational ChromaID technology and developing new and unique applications for the technology. As part of this effort, the Company typically conduct testing to ensure that ChromaID application methods are compatible with the customer’s requirements, and that they can be implemented in a cost effective manner. The Company is also actively involved in identifying new application methods. Visualant’s team has considerable experience working with the application of light-based technologies and their application to various industries. The Company believes that its continued development of new and enhanced technologies relating to our core business is essential to its future success. The Company spent $362,661 and $670,742 during the years ended September 30, 2015 and 2014, respectively, on research and development activities. The Company’s research and development efforts are supported internally, through its relationship with IDMC and through contractors led by Dr. Tom Furness and his team at RATLab LLC.
The Company’s Patents
The Company believes that it’s nine patents, 21 patent applications, and two registered trademarks, and our trade secrets, copyrights and other intellectual property rights are important assets. The Company’s patents will expire at various times between 2027 and 2033. The duration of the Company’s trademark registrations varies from country to country. However, trademarks are generally valid and may be renewed indefinitely as long as they are in use and/or their registrations are properly maintained.
The patents that have been granted to Visualant include:
On August 9, 2011, the Company was issued US Patent No. 7,996,173 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy,” by the United States Office of Patents and Trademarks. The patent expires August 24, 2029.
On December 13, 2011, the Company was issued US Patent No. 8,076,630 B2 entitled “System and Method of Evaluating an Object Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires November 7, 2028.
On December 20, 2011, the Company was issued US Patent No. 8,081,304 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 28, 2030.
On October 9, 2012, the Company was issued US Patent No. 8,285,510 B2 entitled “Method, Apparatus, and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On February 5, 2013, the Company was issued US Patent No. 8,368,878 B2 entitled “Method, Apparatus and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 12, 2013, the Company was issued US Patent No. 8,583,394 B2 entitled “Method, Apparatus and Article to Facilitate Distributed Evaluation of Objects Using Electromagnetic Energy by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On November 21, 2014, the Company was issued US Patent No. 8,888,207 B2 entitled “Systems, Methods, and Articles Related to Machine-Readable Indicia and Symbols” by the United States Office of Patents and Trademarks. The patent expires February 7, 2033.
On March 23, 2015, the Company was issued US Patent No. 8,988,666 B2 entitled “Method, Apparatus, and Article to Facilitate Evaluation of Objects Using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires July 31, 2027.
On May 26, 2015, the Company was issued patent US Patent No. 9,041,920 B2 entitled “Device for Evaluation of Fluids using Electromagnetic Energy” by the United States Office of Patents and Trademarks. The patent expires March 12, 2033.
The Company pursues an aggressive patent strategy to expand its unique intellectual property in the United States and other countries.
Services and License Agreement Invention Development Management Company, L.L.C.
In November 2013, the Company entered into a Services and License Agreement with IDMC. IDMC is affiliated with Intellectual Ventures, which collaborates with inventors and partners with pioneering companies and invests both expertise and capital in the process of invention. On November 19, 2014, the Company amended the Services and License Agreement with IDMC. This amendment exclusively licenses 10 filed patents to us.
The agreement requires IDMC to identify and engage inventors to develop new applications of Visualant’s ChromaID™ technology, present the developments to us for approval, and file at least 10 patent applications to protect the developments. IDMC is responsible for the development and patent costs. The Company provided the Chroma ID Lab Kits to IDMC at no cost and are providing ongoing technical support. In addition, to provide time for this accelerated expansion of its intellectual property the Company delayed the selling of the ChromaID Lab Kits for 140 days except for certain select accounts. The Company continued its business development efforts during this period and have worked with IDMC and their global business development resources to secure potential customers and licensees for the ChromaID technology. The Company shipped 20 ChromaID Lab Kits to inventors in the IDMC network during December 2013 and January 2014. As part of the agreement with IDMC, the Company curtailed its ChromaID marketing efforts through the fourth calendar quarter of 2014 while IDMC worked to expand our intellectual property portfolio. Thereafter, the Company began to actively market the ChromaID Lab Kits to interested and qualified customers.
The Company has received a worldwide, nontransferable, exclusive license to the intellectual property developed under the IDMC agreement during the term of the agreement, and solely within the identification, authentication and diagnostics field of use, to (a) make, have made, use, import, sell and offer for sale products and services; (b) make improvements; and (c) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
The Company received a nonexclusive and nontransferable option to acquire a worldwide, nontransferable, nonexclusive license to the useful intellectual property held by IDMC within the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer to sell products and services and (b) grant sublicenses to any and all of the foregoing rights. The option to acquire this license may be exercised for up to two years from the effective date of the Agreement.
IDMC is providing global business development services to us for geographies not being pursued by Visualant. Also, IDMC has introduced the Company to potential customers, licensees and distributors for the purpose of identifying and pursuing a license, sale or distribution arrangement or other monetization event.
The Company granted to IDMC a nonexclusive, worldwide, fully paid, nontransferable, sublicenseable, perpetual license to our intellectual property solely outside the identification, authentication and diagnostics field of use to (a) make, have made, use, import, sell and offer for sale products and services and (b) grant sublicenses of any and all of the foregoing rights (including the right to grant further sublicenses).
The Company granted to IDMC a nonexclusive, worldwide, fully paid up, royalty-free, nontransferable, non-sublicenseable, perpetual license to access and use the Company’s technology solely for the purpose of marketing the aforementioned sublicenses of our intellectual property to third parties outside the designated fields of use.
In connection with the original license agreement, the Company issued a warrant to purchase 97,169 shares of common stock to IDMC as consideration for the exclusive intellectual property license and application development services. The warrant has a current exercise price of $2.50 per share and expires November 10, 2018. The per share price is subject to adjustment based on any issuances below $2.50 per share except as described in the warrant.
The Company agreed to pay IDMC a percentage of license revenue for the global development business services and a percentage of revenue received from any company introduced to us by IDMC. The Company also have also agreed to pay IDMC a royalty when the Company receives royalty product revenue from an IDMC-introduced company. IDMC has agreed to pay the Company a license fee for the nonexclusive license of the Company’s intellectual property.
The term of both the exclusive intellectual property license and the nonexclusive intellectual property license commences on the effective date of November 11, 2013, and terminates when all claims of the patents expire or are held in valid or unenforceable by a court of competent jurisdiction from which no appeal can be taken.
The term of the Agreement commences on the effective date until either party terminates the Agreement at any time following the fifth anniversary of the effective date by providing at least ninety days’ prior written notice to the other party.
5.
AGREEMENTS WITH SUMITOMO PRECISION PRODUCTS CO., LTD.
In May 2012, the Company entered into a Joint Research and Product Development Agreement (the “Joint Development Agreement”) with Sumitomo Precision Products Co., Ltd., a publicly-traded Japanese corporation, for the commercialization of our ChromaID technology. In March 2013, the Company entered into an amendment to this agreement, which extended the Joint Development Agreement from March 31, 2013 to December 31, 2013. The extension provided for continuing work between Sumitomo and Visualant focused on advancing the ChromaID technology and market research aimed at identifying the most significant markets for the ChromaID technology. This agreement expired December 31, 2013. This collaborative work supported the development of the ChromaID Lab Kit. The current version of the technology was introduced to the marketplace as a part of our ChromaID Lab Kit during the fourth quarter of 2013.
The Company also entered into a License Agreement with Sumitomo in May 2012 which provides for an exclusive license for the then-extant ChromaID technology. The territories covered by this license include Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines). On May 21, 2015, the Company entered into an amendment to the License Agreement, which, effective as of June 18, 2014, which eliminated the Sumitomo exclusivity and provides that if the Company sells products in certain territories – Japan, China, Taiwan, Korea and the entirety of Southeast Asia (Burma, Indonesia, Thailand, Cambodia, Laos, Vietnam, Singapore and the Philippines) – the Company will pay Sumitomo a royalty rate of 2% of net sales (excluding non-recurring engineering revenues) over the remaining term of the five-year License Agreement (through May 2017).
6.
ACQUISITION OF TRANSTECH
The Company’s wholly owned subsidiary, TransTech Systems, Inc., is a distributor of products, including systems solutions, components and consumables, for employee and personnel identification in government and the private sector, document authentication, access control, and radio frequency identification. TransTech provides these products and services, along with marketing and business development assistance to value-added resellers and system integrators throughout North America.
The Company expects its ownership of TransTech to accelerate our market entry and penetration through well-operated and positioned dealers of security and authentication systems, thus creating a natural distribution channel for products featuring its proprietary ChromaID technology. TransTech currently provides substantially all of our revenues. TransTech’s management team functions independently from Visualant’s and its operations require a minimal commitment of our management time and other resources. The Company’s acquisition of TransTech in June 2010.
7.
ACCOUNTS RECEIVABLE/CUSTOMER CONCENTRATION
Accounts receivable were $619,845 and $815,460, net of allowance, as of September 30, 2015 and 2014, respectively. The Company had one customer (11.1%) in excess of 10% of the Company’s consolidated revenues for the year ended September 30, 2015. The Company had two customers (13.5% and 11.1%) with accounts receivable in excess of 10% as of September 30, 2015. The Company does expect to have customers with consolidated revenues or accounts receivable balances of 10% of total accounts receivable in the foreseeable future.
Inventories were $217,824 and $412,831 as of September 30, 2015 and 2014, respectively. Inventories consist primarily of printers and consumable supplies, including ribbons and cards, badge accessories, capture devices, and access control components held for resale. There is a $20,000 and $10,000 reserve for impaired inventory as of September 30, 2015 and 2014, respectively.
Fixed assets, net of accumulated depreciation, was $366,250 and $447,236 as of September 30, 2015 and 2014, respectively. Accumulated depreciation was $803,705 and $742,676 as of September 30, 2015 and 2014, respectively. Total depreciation expense, was $79,576 and $64,357 for the years ended September 30, 2015 and 2014, respectively. All equipment is used for selling, general and administrative purposes and accordingly all depreciation is classified in selling, general and administrative expenses.
Property and equipment as of September 30, 2015 was comprised of the following:
| | Estimated | | June 30, 2015 | |
| | Useful Lives | | Purchased | | | Capital Leases | | | Total | |
| Machinery and equipment | 2-10 years | | $ | 192,374 | | | $ | 87,038 | | | $ | 279,412 | |
| Leasehold improvements | 5-20 years | | | 603,612 | | | | - | | | | 603,612 | |
| Furniture and fixtures | 3-10 years | | | 77,039 | | | | 101,260 | | | | 178,299 | |
| Software and websites | 3- 7 years | | | 63,783 | | | | 44,849 | | | | 108,632 | |
| Less: accumulated depreciation | | | | (570,558 | ) | | | (233,147 | ) | | | (803,705 | ) |
| | | | $ | 366,250 | | | $ | - | | | $ | 366,250 | |
Intangible assets as of September 30, 2015 and 2014 consisted of the following:
| | Estimated | | |
| | Useful Lives | | |
| | | | |
| Customer contracts | 5 years | $983,645 | $983,645 |
| Technology | 5 years | 712,500 | 712,500 |
| Less: accumulated amortization | | (1,538,145) | (1,264,492) |
| Intangible assets, net | | $158,000 | $431,653 |
Total amortization expense was $273,653 and $339,229 for the years ended September 30, 2015 and 2014, respectively.
The fair value of the TransTech intellectual property acquired was $983,645, estimated by using a discounted cash flow approach based on future economic benefits associated with agreements with customers, or through expected continued business activities with its customers. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results. The TransTech intellectual property was fully amortized as of September 30, 2015.
The fair value of the RATLab intellectual property associated with the assets acquired was $450,000 estimated by using a discounted cash flow approach based on future economic benefits. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
The fair value of the Javelin intellectual property acquired was $262,500 estimated by using a discounted cash flow approach based on future economic benefits associated with the assets acquired. In summary, the estimate was based on a projected income approach and related discounted cash flows over five years, with applicable risk factors assigned to assumptions in the forecasted results.
Accounts payable were $2,520,223 and $2,234,123 as of September 30, 2015 and 2014, respectively. Such liabilities consisted of amounts due to vendors for inventory purchases and technology development, external audit, legal and other expenses incurred by the Company. The Company had one vendor (12.5%) with accounts payable in excess of 10% of its accounts payable as of September 30, 2015. The Company does expect to have vendors with accounts payable balances of 10% of total accounts payable in the foreseeable future.
12.
CONVERTIBLE NOTES PAYABLE
The Company entered into a Convertible Note Payable with KBM Worldwide, Inc. on August 25, 2014 for $103,500. The Note was paid off on March 2, 2015. The Company entered into a Convertible Note Payable with KBM on September 24, 2014 for $63,000. The Note was repaid March 27, 2015. The Company entered into a Convertible Note Payable with KBM on January 27, 2015 for $64,000. The KBM Note accrued interest at a rate of 8% per annum and becomes due on October 27, 2015 and was convertible into common stock on July 26, 2015. On August 3, 10, 13 and 14, 2015, the Company issued a total of 23,010 shares of common stock to KBM Worldwide, Inc. related to the conversion of $64,000 of debt and interest of $2,560 pursuant to a Securities Purchase Agreement dated January 27, 2015. The shares were issued at an average of $2.785 per share, with a low price of $2.50 per share.
During the year ended September 30, 2014, the Company recognized $166,500 of other expense related to the KBM Note. During the year ended September 30, 2015, the Company recognized $29,529 of other income and allocated $98,940 to stockholder’s equity related to the KBM Note. The Company recorded accrued interest of $898 as of June 30, 2015. The Company recorded on a loss on conversion of $34,035 and allocated $34,035 to stockholder’s equity.
The Company entered into a Convertible Note Payable with Vis Vires Group, Inc. on August 10, 2015 for $84,000 to fund short-term working capital. The Vis Vires Note accrues interest at a rate of 8% per annum and becomes due on May 12, 2016 and is convertible into common stock on February 5, 2016. The Vis Vires Note is convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. The Company recorded accrued interest of $405 as of September 30, 2015.
The Company entered into a Convertible Promissory Note with Planning Partners, Inc. on September 24, 2015 for $25,000 to fund short-term working capital. The Planning Partners Note accrues interest at a rate of 8% per annum and becomes due on September 23, 2016 and is convertible into common stock as part of our next financing. The Company recorded accrued interest of $38 as of September 30, 2015.
13. | NOTES PAYABLE, CAPITALIZED LEASES AND LONG TERM DEBT |
Notes payable, capitalized leases and long term debt as of September 30, 2015 and 2014 consisted of the following:
| | | September 30, | | | September 30, | |
| | | 2015 | | | 2014 | |
| | | | | | | |
| Capital Source Business Finance Group | | $ | 364,757 | | | $ | 488,398 | |
| Note payable to Umpqua Bank | | | 199,935 | | | | 200,000 | |
| Secured note payable to J3E2A2Z LP - related party | | | 600,000 | | | | 600,000 | |
| TransTech capitalized leases, net of capitalized interest | | | 0 | | | | 2,562 | |
| Total debt | | | 1,164,692 | | | | 1,290,960 | |
| Less current portion of long term debt | | | (1,164,692 | ) | | | (1,290,960 | ) |
| Long term debt | | $ | - | | | $ | - | |
Capital Source Business Finance Group Secured Credit Facility
The Company finances its TransTech operations from operations and a Secured Credit Facility with Capital Source Business Finance Group. On December 9, 2008, TransTech entered into a $1,000,000 secured credit facility with Capital Source to fund its operations. On June 12, 2015, the secured credit facility was renewed for an additional six months, with a floor for prime interest of 4.5% (currently 4.5%) plus 2.5%. The eligible borrowing is based on 80% of eligible trade accounts receivable, not to exceed $1,000,000. The secured credit facility is collateralized by the assets of TransTech, with a guarantee by Visualant, including a security interest in all assets of Visualant. Availability under this Secured Credit ranges from $0 to $175,000 ($24,000 as of September 30, 2015) on a daily basis. The remaining balance on the accounts receivable line of $364,757 as of September 30, 2015 must be repaid by the time the secured credit facility expires on December 12, 2015, or we renew by automatic extension for the next successive six-month term.
Note Payable to Umpqua Bank
The Company has a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which currently matures on December 31, 2015 and provides for interest at 3.25% per year. Related to this Umpqua Loan, the Company entered into a demand promissory note for $200,000 on January 10, 2014 with an entity affiliated with Ronald P. Erickson, our Chief Executive Officer. This demand promissory note will be effective in case of a default by the Company under the Umpqua Loan. The Company recorded accrued interest of $10,340 as of September 30, 2015.
Note Payables to Ronald P. Erickson or J3E2A2Z LP
The Company also has two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between January and July 2014, provide for interest of 3% per year and now mature on December 31, 2015. They also provide for a second lien on our assets if not repaid by December 31, 2015 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson. The Company recorded accrued interest of $22,167 as of September 30, 2015.
Aggregate maturities totaling $1,164,692 are all due within twelve months.
Authorized Capital Stock
The Company has authorized 105,000,000 shares of capital stock, of which 100,000,000 are shares of voting common stock, par value $0.001 per share, and 5,000,000 are shares of voting preferred stock, par value $0.001 per share.
Voting Preferred Stock
The Company is authorized to issue up to 5,000,000 shares of preferred stock with a par value of $0.001.
On July 21, 2015, the Company filed with the Nevada Secretary of State an Amended and Restated Certificate of Designations, Preferences and Rights for our Series A Convertible Preferred Stock. Among other things, the Amended and Restated Certificate changed the conversion price and the stated value of the Series A Preferred from $0.10 (pre reverse stock split) to $30.00 (post-reverse stock split), and added a provision adjusting the conversion price upon the occurrence of certain events.
Under the Amended and Restated Certificate, the Company has 11,667 shares of Series A Preferred authorized, all of which are outstanding. Each holder of outstanding shares of Series A Preferred is entitled to the number of votes equal to the number of whole shares of common stock into which the shares of Series A Preferred held by such holder are then convertible as of the applicable record date. The Company cannot amend, alter or repeal any preferences, rights, or other terms of the Series A Preferred so as to adversely affect the Series A Preferred, without the written consent or affirmative vote of the holders of at least 66% of the then outstanding shares of Series A Preferred, voting as a separate voting group, given by written consent or by vote at a meeting called for such purpose for which notice shall have been duly given to the holders of the Series A Preferred.
During the year ended September 30, 2015, the Company sold 11,667 Series A Preferred Stock to two investors totaling $350,000. These shares are expected to be convertible into 11,667 shares of common stock at $30.00 per share, subject to adjustment, for a period of five years. The Series A Preferred Stock has voting rights and may not be redeemed without the consent of the holder. The Company also issued (i) a Series C five-year Warrant for 23,334 shares of common stock at an exercise price of $30.00 per share, which is callable at $60.00 per share; and (ii) a Series D five-year Warrant for 23,334 shares of common stock at an exercise price of $45.00 per share, which is callable at $90.00 per share. The Series A Preferred Stock and Series C and D Warrants had registration rights.
On July 20, 2015, the two investors entered into an Amendment to Series A Preferred Stock Terms whereby they agreed to the terms of the Amended and Restated Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock and waived all registration rights.
On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due to the issuance of common stock at that price.
Common Stock
All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non-accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non-accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities.
The following equity issuances occurred during the year ended September 30, 2015:
On December 14, 2014, the Company entered into an Advisory Agreement with Lester Garfinkel for financial consulting services. Under the Advisory Agreement, Mr. Garfinkel was awarded 167 shares of our common stock. The Company expensed $2,500 during the year ended September 30, 2015.
On January 23, 2015, the Company issued 9,002 shares of restricted common stock to seven employees and directors for services during 2014. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $15.00 per share, the market price of our common stock. The Company expensed $135,000 during the year ended September 30, 2015.
On February 23, 2015, the Company issued 1,700 shares of common stock to NVPR LLC related to a conversion of $25,499 under a 7% Convertible Debenture.
On April 24, 2015, the Company filed a registration statement on Form S-1 to register $10 million of Company securities in a proposed public offering. The Company has applied for listing of the Company’s common stock and the warrants on The NASDAQ Capital Market.
On May 6, 2015, the Company’s stockholders approved a reverse split of our common stock, in a ratio to be determined by the Company’s Board of Directors, of not less than 1-for-50 nor more than 1-for-150. On June 9, 2015, the Company’s Board of Directors determined that the ratio of the reverse split would be 1-for-150 , and the reverse split became effective on June 17, 2015. All warrant, option, share and per share information in this Form 10-Q gives retroactive effect for a 1-for-150 split with all numbers rounded up to the nearest whole share. The Company issued 962 fractional shares related to the reverse split.
On August 3, 10, 13 and 14, 2015, the Company issued a total of 23,010 shares of common stock to KBM Worldwide, Inc. related to the conversion of $64,000 of debt and interest of $2,560 pursuant to a Securities Purchase Agreement dated January 27, 2015. The shares were issued at an average of $2.785 per share, with a low price of $2.50 per share. The Company recorded on a loss on conversion of $34,035 and allocated $34,035 to stockholder’s equity.
The Company had the following equity transactions during the year ended September 30, 2014:
On May 15, 2014, the Company issued 10,667 shares of common stock to White Oak Capital LLC related to a conversion under a 7% Convertible Debenture. The shares were valued at $160,000 or $15.00 per share.
On June 12, 2014, the Company issued 2,000 shares of common stock to Dynasty Wealth, Inc. related to Financial Public Relations Group dated June 9, 2014. The shares were valued at $60,000 or $30.00 per share.
On August 27, 2014, the Company entered into an Addendum to a Financial Consultant Agreement or Agreement with D. Weckstein and Co, Inc. for financial consulting and investment banking services. Under the Addendum, Weckstein was awarded 6,667 shares of the Company’s common stock on August 27, 2014. The shares were valued at $30.00 per share by the parties. The Company expensed $70,000 during the year ended September 30, 2014 or $10.50, the closing price on August 27, 2014.
Warrants to Purchase Common Stock
The following warrant issuances occurred during the year ended September 30, 2015:
On June 14, 2013, the Company entered into a Purchase Agreement, Warrants, and Registration Rights Agreement with Special Situations Technology Funds and forty other accredited investors, pursuant to which the Company issued 348,685 shares of common stock at $15.00 per share for a total of $5,230,000, which amount includes the conversion of $500,000 in outstanding debt of the Company owed to one of its officers. As part of the transaction, which closed on June 14, 2013, the Company issued to the investors (i) five year Series A Warrants to purchase a total of 348,685 shares of common stock at $22.50 per share; and (ii) five year Series B Warrants to purchase a total of 348,685 shares of common stock at $30.00 per share. The Company also issued 34,871 placement agent warrants exercisable at $15.00 per share to GVC Capital, with an obligation to issue up to 34,871 additional placement agent warrants exercisable at $22.50 per share. The placement agent warrants shall issue only upon the exercise of the Series A Warrants by the investors, and are issuable ratably based upon the number of Warrants exercised by the investors. The placement agent warrants have a term of five years from the date of closing of the transaction. On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due the issuance of common stock at this price.
Warrants to purchase 4,000 shares of common stock at $15.00 per share were forfeited.
The following warrant issuances occurred during the year ended September 30, 2014:
The Company issued a warrant to purchase 97,169 shares of common stock as consideration for the exclusive IP license and application development services to IDMC signed on November 11, 2013. The warrant price of $30.00 per share expires November 10, 2018 and the per share price is subject to adjustment. On August 14, 2015, the warrant exercise price was adjusted to $2.50 per share due the issuance of common stock at this price.
On April 2, 2014, the Company issued a warrant to purchase 6,667 shares of common stock to Thomas Furness, a supplier, at an exercise price of $30.00 per share. The Warrant expires on April 1, 2019.
On April 2, 2014, the Company issued a warrant to purchase 1,334 shares of common stock to Delacore LLC, a supplier, at an exercise price of $30.00 per share. The Warrant expires on April 1, 2017.
On June 11, 2014, the Company issued a warrant to purchase 3,334 shares of common stock to Designsense Ltd, a supplier, at an exercise price of $30.00 per share. The Warrant expires on June 10, 2017.
On June 11, 2014, the Company issued a warrant to purchase 1,667 shares of common stock to Alan Tompkins, a supplier, at an exercise price of $30.00 per share. The Warrant expires on June 10, 2017.
On June 12, 2014, the Company issued a warrant for 1,334 shares of common stock to Dynasty Wealth, Inc. The warrants vested on June 12, 2014, are exercisable at $30.00 per share expire on September 3, 2016.
Warrants to purchase 11,180 shares of common stock at $46.35 per share were forfeited.
A summary of the warrants issued as of September 30, 2015 were as follows:
| | | September 30, 2015 | |
| | | | | | Weighted | |
| | | | | | Average | |
| | | | | | Exercise | |
| | | Shares | | | Price | |
| Outstanding at beginning of period | | | 857,083 | | | $ | 26.28 | |
| Issued | | | 46,667 | | | | 37.50 | |
| Exercised | | | - | | | | - | |
| Forfeited | | | - | | | | - | |
| Expired | | | (4,000 | ) | | | 15.00 | |
| Outstanding at end of period | | | 899,750 | | | $ | 3.18 | |
| Exerciseable at end of period | | | 899,750 | | | | | |
A summary of the status of the warrants outstanding as of September 30, 2015 is presented below:
| | | | September 30, 2015 | |
| | | | Weighted | | | Weighted | | | | | | Weighted | |
| | | | Average | | | Average | | | | | | Average | |
| Number of | | | Remaining | | | Exercise | | | Shares | | | Exercise | |
| Warrants | | | Life ( In Years) | | | Price | | | Exerciseable | | | Price | |
| | 876,078 | | | | 3.31 | | | $ | 2.50 | | | | 876,078 | | | $ | 2.50 | |
| | 3,334 | | | | 1.13 | | | | 19.50-22.50 | | | | 3,334 | | | | 19.50-22.50 | |
| | 20,338 | | | | 1.83 | | | | 30.00 | | | | 20,338 | | | | 30.00 | |
| | 899,750 | | | | 2.94 | | | $ | 3.18 | | | | 899,750 | | | $ | 3.18 | |
The significant weighted average assumptions relating to the valuation of the Company’s warrants for the year ended September 30, 2015 were as follows:
Dividend yield | 0% |
Expected life | 3 |
Expected volatility | 90% |
Risk free interest rate | 0.7% |
At September 30, 2015, vested warrants totaling 876,078 shares had an aggregate intrinsic value of $2,715,842.
Description of Stock Option Plan
On April 29, 2011, the Company’s 2011 Stock Incentive Plan was approved at the Annual Stockholder Meeting. The Company was authorized to issue options for, and has reserved for issuance, up to 46,667 shares of common stock under the 2011 Stock Incentive Plan. On March 21, 2013, an amendment to the Stock Option Plan was approved by the stockholders of the Company, increasing the number of shares reserved for issuance under the Plan to 93,333 shares.
Determining Fair Value under ASC 505
The Company records compensation expense associated with stock options and other equity-based compensation using the Black-Scholes-Merton option valuation model for estimating fair value of stock options granted under our plan. The Company amortizes the fair value of stock options on a ratable basis over the requisite service periods, which are generally the vesting periods. The expected life of awards granted represents the period of time that they are expected to be outstanding. The Company estimates the volatility of our common stock based on the historical volatility of its own common stock over the most recent period corresponding with the estimated expected life of the award. The Company bases the risk-free interest rate used in the Black Scholes-Merton option valuation model on the implied yield currently available on U.S. Treasury zero-coupon issues with an equivalent remaining term equal to the expected life of the award. The Company has not paid any cash dividends on our common stock and does not anticipate paying any cash dividends in the foreseeable future. Consequently, the Company uses an expected dividend yield of zero in the Black-Scholes-Merton option valuation model and adjusts share-based compensation for changes to the estimate of expected equity award forfeitures based on actual forfeiture experience. The effect of adjusting the forfeiture rate is recognized in the period the forfeiture estimate is changed.
Stock Option Activity
The Company had the following stock option transactions during the year ended September 30, 2015:
During the year ended September 30, 2015, twelve employees and directors, forfeited stock option grants for 41,621 shares of common stock at $18.29 per share.
On January 23, 2015, three employees were issued performance grants for 11,335 shares of common stock at $15.00 per share. The grants were issued in accordance with the 2011 Stock Incentive Plan, vest quarterly over three years after being earned and expire January 22, 2020. As of September 30, 2015, none of the stock option grants were earned.
The Company had the following stock option transactions during the year ended September 30, 2014:
During the year ended September 30, 2015, two employees of TransTech, forfeited stock option grants for 200 shares of common stock at $31.50 per share.
On April 2, 2014, the Company issued stock option grants to two employees totaling 2,633 shares at $15.00 per share. The grants vest quarterly over three years and expire on April 1, 2019.
There are currently 57,407 options to purchase common stock at an average exercise price of $18.435 per share outstanding as of September 30, 2015 under the 2011 Stock Incentive Plan. The Company recorded $65,463 and $87,550 of compensation expense, net of related tax effects, relative to stock options for the years ended September 30, 2015 and 2014 in accordance with ASC 505. Net loss per share (basic and diluted) associated with this expense was approximately ($0.058) and ($.079) per share, respectively. At September 30, 2015, there is approximately $185,211 of total unrecognized costs related to employee granted stock options that are not vested. These costs are expected to be recognized over a period of approximately 4.24 years.
Stock option activity for the year ended September 30, 2015 and 2014 was as follows:
| | | Weighted Average | |
| | | Options | | | Exercise Price | | | $ | |
| Outstanding as of September 30, 2013 | | | 84,900 | | | | 18.954 | | | | 1,609,200 | |
| Granted | | | 2,633 | | | | 15.000 | | | | 39,500 | |
| Exercised | | | - | | | | - | | | | - | |
| Forfeitures | | | (200 | ) | | | (32.500 | ) | | | (6,500 | ) |
| Outstanding as of September 30, 2014 | | | 87,333 | | | | 18.804 | | | | 1,642,200 | |
| Granted | | | 11,335 | | | | 15.000 | | | | 170,025 | |
| Exercised | | | - | | | | - | | | | - | |
| Forfeitures | | | (41,261 | ) | | | (18.286 | ) | | | (754,500 | ) |
| Outstanding as of September 30, 2015 | | | 57,407 | | | $ | 18,425 | | | $ | 1,057,725 | |
The following table summarizes information about stock options outstanding and exercisable at September 30, 2015:
| | | | | | | Weighted | | | Weighted | | | | | | Weighted | |
| | | | | | | Average | | | Average | | | | | | Average | |
| Range of | | | Number | | | Remaining Life | | | Exercise Price | | | Number | | | Exercise Price | |
| Exercise Prices | | | Outstanding | | | In Years | | | Exerciseable | | | Exerciseable | | | Exerciseable | |
| | 13.500 | | | | 3,334 | | | | 2.38 | | | $ | 13.50 | | | | 3,334 | | | $ | 13.50 | |
| | 15.000 | | | | 20,970 | | | | 3.83 | | | | 15.00 | | | | 6,837 | | | | 15.00 | |
| | 19.500 | | | | 19,002 | | | | 4.50 | | | | 19.50 | | | | 19,002 | | | | 19.50 | |
| | 22.500 | | | | 13,334 | | | | 4.63 | | | | 22.50 | | | | 13,334 | | | | 22.50 | |
| | 36.000 | | | | 767 | | | | - | | | | 36.00 | | | | 934 | | | | 36.00 | |
| | | | | | 57,407 | | | | 4.24 | | | $ | 18.43 | | | | 43,441 | | | $ | 20.35 | |
There is no aggregate intrinsic value of the exercisable options as of September 30, 2015.
16. | OTHER SIGNIFICANT TRANSACTIONS WITH RELATED PARTIES |
Related Party Transactions with Ronald P. Erickson
See Note 13 for Notes Payable to Ronald P. Erickson, our Chief Executive Officer Chief and/or entities in which Mr. Erickson has a beneficial interest.
The Company a $199,935 Business Loan Agreement with Umpqua Bank (the “Umpqua Loan”), which currently matures on December 31, 2015 and provides for interest at 3.25% per year. Related to the Umpqua Loan, the Company entered into a demand promissory note for $200,000 on January 10, 2014 with an entity with which Ronald P. Erickson, our Chief Executive Officer, is affiliated. This demand promissory note will be effective in case of a default by us under the Umpqua Loan.
The Company have two other demand promissory notes payable to entities affiliated with Mr. Erickson, totaling $600,000. Each of these notes were issued between January and July 2014, provide for interest of 3% per year and now mature on December 31, 2015. They also provide for a second lien on our assets if not repaid by December 31, 2015 or converted into convertible debentures or equity on terms acceptable to the Mr. Erickson. Mr. Erickson and/or entities with which he is affiliated also have advanced $708,500 and have unreimbursed expenses and compensation of approximately $344,221. The Company Mr. Erickson, or entities with which he is affiliated, $1,652,721 as of September 30, 2015.
17.
COMMITMENTS, CONTINGENCIES AND LEGAL PROCEEDINGS
Legal Proceedings
The Company may from time to time become a party to various legal proceedings arising in the ordinary course of our business. The Company is currently not a party to any pending legal proceeding that is not ordinary routine litigation incidental to our business.
Properties and Operating Leases
The Company is obligated under various non-cancelable operating leases for its various facilities and certain equipment.
Corporate Offices
The Company’s executive office is located at 500 Union Street, Suite 420, Seattle, Washington, USA, 98101. The Company leases 2,244 square feet and its net monthly payment is $2,535. The Company leases this office on a month to month basis.
TransTech Facilities
TransTech is located at 12142 NE Sky Lane, Suite 130, Aurora, OR 97002. TransTech leases a total of approximately 9,750 square feet of office and warehouse space for its administrative offices, product inventory and shipping operations. In March 2011, the lease was extended for a five year term at a monthly rental of $4,751. There are two additional five year renewals available with a set accelerating increase of 10% per 5 year term.
The aggregate future minimum lease payments under operating leases as of June 30, 2015 were $26,330.
The Company has incurred losses since inception, which have generated net operating loss carryforwards. The net operating loss carryforwards arise from United States sources.
Pretax losses arising from United States operations were approximately $773,000 for the year ended September 30, 2015.
Pretax losses arising from United States operations were approximately $835,000 for the year ended September 30, 2014.
The Company has net operating loss carryforwards of approximately $18,901,000, which expire in 2020-2033. Because it is not more likely than not that sufficient tax earnings will be generated to utilize the net operating loss carryforwards, a corresponding valuation allowance of approximately $6,426,000 was established as of September 30, 2015. Additionally, under the Tax Reform Act of 1986, the amounts of, and benefits from, net operating losses may be limited in certain circumstances, including a change in control. The Company is subject to possible tax examination for the years 2011 through 2015.
Section 382 of the Internal Revenue Code generally imposes an annual limitation on the amount of net operating loss carryforwards that may be used to offset taxable income when a corporation has undergone significant changes in its stock ownership. There can be no assurance that the Company will be able to utilize any net operating loss carryforwards in the future.
For the year ended September 30, 2015, the Company’s effective tax rate differs from the federal statutory rate principally due to net operating losses and warrants issued for services.
The principal components of the Company’s deferred tax assets at September 30, 2015 are as follows:
| | | 2015 | | | 2014 | |
| U.S. operations loss carry forward at statutory rate of 34% | | $ | (6,426,360 | ) | | $ | (6,163,645 | ) |
| Non-U.S. operations loss carry forward at statutory rate of 20.5% | | | 0 | | | | 0 | |
| Total | | | (6,426,360 | ) | | | (6,163,645 | ) |
| Less Valuation Allowance | | | 6,426,360 | | | | 6,163,645 | |
| Net Deferred Tax Assets | | | - | | | | - | |
| Change in Valuation allowance | | $ | 6,426,360 | | | $ | 6,163,645 | |
A reconciliation of the United States Federal Statutory rate to the Company’s effective tax rate for the period ended September 30, 2015 and 2014 is as follows:
| | | 2015 | | | 2014 | |
| Federal Statutory Rate | | | -34.0 | % | | | -34.0 | % |
| Increase in Income Taxes Resulting from: | | | | | | | | |
| Change in Valuation allowance | | | 34.0 | % | | | 34.0 | % |
| Effective Tax Rate | | | 0.0 | % | | | 0.0 | % |
The Company evaluated subsequent events, for the purpose of adjustment or disclosure, up through the date the financial statements were issued.
As of November 3, 2015, the Company received commitments from debtors to convert $1,000,000 into common stock of the Company as part of the Company’s proposed listing on The NASDAQ Capital Market. These conversions are expected to increase stockholder’s equity by $1,000,000.
The Company entered into convertible notes payable with accredited investors during September 2015 and October 2015 totaling $255,000 to fund short-term working capital. Notes payable accrue interest at a rate of 8% per annum and becomes due during September and October 2016 and are convertible into common stock as part of the Company’s next financing.
PROSPECTUS
VISUALANT, INCORPORATED
500 Union Street, Suite 420
Seattle, WA 98101
1,785,714 shares of common stock issuable upon conversion of Series C Preferred Stock and
1,785,714 shares of common stock issuable upon exercise of Series E Warrants
DEALER PROSPECTUS DELIVERY OBLIGATION
Until _______________, 2016, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
____________________, 2016
PART II—INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The expenses payable by us in connection with the issuance and distribution of the securities being registered other than underwriting discounts and commissions, if any are set forth below. Each item listed is estimated as follows:
Securities and Exchange Commission registration fee | $500
|
Accountant's fees and expenses | 15,000
|
Legal fees and expenses | 25,000
|
Blue Sky fees and expenses | 5,000
|
Transfer agent's fees and expenses | 1,000
|
Miscellaneous | 3,500
|
| |
Total expenses | $50,000
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Nevada Revised Statutes, or NRS, Sections 78.7502 and 78.751 provide us with the power to indemnify any of our directors and officers. The director or officer must have conducted himself/herself in good faith and reasonably believe that his/her conduct was in, or not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have had reasonable cause to believe his/her conduct was unlawful.
Under NRS Section 78.751, advances for expenses may be made by agreement if the director or officer affirms in writing that he/she believes he/she has met the standards and will personally repay the expenses if it is determined such officer or director did not meet the standards.
Our articles of incorporation include an indemnification provision under which we have the power to indemnify our directors, officers, employees and other agents of the company to the fullest extent permitted by applicable law.
We have a directors’ and officers’ liability insurance policy in place pursuant to which its directors and officers are insured against certain liabilities, including certain liabilities under the Securities Act of 1933, as amended and the Securities and Exchange Act of 1934, as amended.
The underwriting agreement we will enter into in connection with the offering of common stock and warrants being registered hereby provides that the underwriters will indemnify, under certain conditions, our directors and officers (as well as certain other persons) against certain liabilities arising in connection with such offering.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
In the three years preceding the filing of this Registration Statement, we have issued the following securities that were not registered under the Securities Act.
All of the offerings and sales described below were deemed to be exempt under Rule 506 of Regulation D and/or Section 4(a)(2) of the Securities Act. No advertising or general solicitation was employed in offering the securities, the offerings and sales were made to a limited number of persons, all of whom were accredited investors and transfer was restricted by the company in accordance with the requirements of Regulation D and the Securities Act. All issuances to accredited and non-accredited investors were structured to comply with the requirements of the safe harbor afforded by Rule 506 of Regulation D, including limiting the number of non-accredited investors to no more than 35 investors who have sufficient knowledge and experience in financial and business matters to make them capable of evaluating the merits and risks of an investment in our securities. We have not employed any underwriters in connection with any of the below transactions, and, except as otherwise noted below, the individuals and entities to whom we issued securities are not affiliated with us. Except as noted below, none of the holders of the securities have any contractual rights to have such securities registered with the Securities and Exchange Commission.
September 2013 to the Year Ended September 30, 2013
On September 4, 2013, we issued 2,000 shares to the Liolios Group related to public relation services. The shares have “piggyback” registration rights. In addition, we issued a warrant for 1,334 shares of common stock to Liolios related to public relation services. The warrants vested on September 4, 2013, are exercisable at $30.00 per share expire on September 3, 2016. The warrant has “piggyback” registration rights.
2021We issued a warrant to Genesis Select Corporation related to a Strategic Consulting Services Agreement dated September 15, 2013 for 1,334 shares of common stock. The warrants vested on September 15, 2013, are exercisable at $30.00 per share and expire on September 14, 2016. The warrant does not have piggyback registration rights.
We issued a warrant to Jason Eichenholz on September 18, 2013 related to a Technical Advisor Agreement dated July 18, 2013 for 3,334 shares of common stock. The warrants vested on September 18, 2013, are exercisable at $30.00 per share and expire on September 17, 2016. The warrant does not have piggyback registration rights.
Year Ended September 30, 2014
We issued a warrant to purchase 97,169 shares of common stock as consideration for the exclusive IP license and application development services to IDMC signed on November 11, 2013. The warrant price of $30.00 per share expires November 10, 2018 and the per share price is subject to adjustment.
On April 2, 2014, we issued a warrant to purchase 6,667 shares of common stock to Thomas Furness, a supplier, at an exercise price of $30.00 per share. The Warrant expires on April 1, 2019.
On April 2, 2014, we issued a warrant to purchase 1,334 shares of common stock to Delacore LLC, a supplier, at an exercise price of $30.00 per share. The Warrant expires on April 1, 2017.
On May 15, 2014, we issued 10,667 shares of common stock to White Oak Capital LLC related to a conversion under a 7% Convertible Debenture. The shares were valued at $160,000, or $15.00 per share.
On June 11, 2014, we issued a warrant to purchase 3,334 shares of common stock to Designsense Ltd, a supplier, at an exercise price of $30.00 per share. The Warrant expires on June 10, 2017.
On June 11, 2014, we issued a warrant to purchase 1,667 shares of common stock to Alan Tompkins, a supplier, at an exercise price of $30.00 per share. The Warrant expires on June 10, 2017.
On June 12, 2014, we issued 2,000 shares of common stock to Dynasty Wealth, Inc. related to Financial Public Relations Group dated June 11, 2017. In addition, we issued a warrant for 1,334 shares of common stock to Dynasty. The warrants vested on June 12, 2014, are exercisable at $30.00 per share expire on September 3, 2016.
On August 27, 2014, we entered into an Addendum to a Financial Consultant Agreement or Agreement with D. Weckstein and Co, Inc. for financial consulting and investment banking services. Under the Addendum, Weckstein was awarded 6,667 shares of our common stock on August 27, 2014. The shares were valued at $30.00 per share by the parties.
We issued a Convertible Note to KBM Worldwide, Inc. on August 25, 2014 for $103,500. The Note was paid off on March 2, 2015. We issued a Convertible Note to KBM on September 24, 2014 for $63,000. The Note was repaid March 27, 2015.
Year Ended September 30, 2015
On December 14, 2014, we entered into an Advisory Agreement with Lester Garfinkel for financial consulting services. Under the Advisory Agreement, Mr. Garfinkel was awarded 167 shares of our common stock.
Subsequent to September 30, 2014, we sold 11,667 Series A Preferred Stock to two investors totaling $350,000. These shares are currently convertible into 11,667 shares of common stock at $30.00 per share, subject to adjustment, for a period of five years. The Series A Preferred Stock has voting rights and may not be redeemed without the consent of the holder. The Company also issued (i) a Series C five-year Warrant for 23,3343,676,542 shares of common stock at an
exerciseaverage price of
$30.00$0.582 per share
which is callablerelated to the exercise of warrants.We issued 97,000 shares related to services. The shares were valued at $60.00the fair market value of $202,820.
We issued 16,875 shares related to the exercise of stock option grants at $1.38 per share; and (ii) a Series D five-year Warrant for 23,334share.
We issued 480,600 shares of common stock at an exerciseaverage price of $45.00$2.00 per share which is callable at $90.00 per share. The Series A Preferred Stock and Series C and D Warrants have registration rights upon the closing of this offering.
On January 23, 2015, we issued 9,002 shares of restricted common stock to seven employees and directors for services during 2014. The shares were issued in accordance with the 2011 Stock Incentive Plan and were valued at $15.00 per share, the market price of our common stock.
We issued a Convertible Note to KBM Worldwide, Inc. on January 27, 2015 for $64,000. On August 3, 10, 13 and 14, 2015, we issued a total of 23,010 shares of common stock to KBM Worldwide, Inc.or $961,200 related to the conversion of $64,000Particle Simple Agreements for Future Equity into shares of debt and interest of $2,560 pursuant to a Securities Purchase Agreement dated January 27, 2015. The shares werethe Company’s common stock.Year Ended September 30, 2022
We issued at an average of $2.785 per share, with a low price of $2.50 per share.
On February 23, 2015, we issued 1,7001,045,724 shares of common stock to NVPR LLC related to a conversion under a 7% Convertible Debenture.
The exercise prices of certain of the warrants described in this Item 15 will be adjustedwarrant exercises and received $838,487.We issued 26,293 shares related to the exercise of stock option grants and received $26,887.
The Company issued 104,634 shares each to three directors and three consultants at $1.749 per share price in this offering. The exercise prices of certain of our warrants are subject to further adjustment if we sell shares in the future at less than the per share price of this offering.
We entered into a Convertible Note Payable with Vis Vires Group, Inc. on August 10, 2015 for $84,000 to fund short-term working capital. The Vis Vires Note accrued interest at a rate of 8% per annum and became due on May 12, 2016 and was convertible into common stock on February 5, 2016. The Vis Vires Note was convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. On February 6, 2016, we paid $114,979 to Vis Vires to repay the Note Payable in full.
Subsequent to the share.Year Ended September 30, 2015
Fourteen investors exercised warrants at $2.50 per share and were2023We issued 207,66750,000 shares of common stock for a total of $519,168 in proceedsrelated to the Company.
On October 6, 2015, we entered into a Consulting Agreement with Joshua Conroy for business development services. Under the Agreement, Mr. Conroy wasexercise of warrants and received $12,500.We issued 1,711 shares of our common stock. We expensed $11,977 during the during the period subsequent to September 30, 2015.
On October 21, 2015, we entered into a Public Relations Agreement with Financial Genetics LLC for public relation services. Under the Agreement, Financial Genetics was issued 35,268 shares of our common stock. We expensed $218653 during the period subsequent to September 30, 2015.
We entered into Convertible Promissory Notes totaling $710,000 with accredited investors during September 2015 to February 2016 to fund short-term working capital. The Notes accrue interest at a rate of 8% per annum and become due September 2016 to February 2017 and are convertible into common stock as part of our next financing. The investors received $710,000 in warrants that are exercisable into common stock at the price equal to the price of the common stock sold in our next public financing.
We entered into 8%-10% Convertible Promissory Notes and Securities Purchase Agreements with three accredited investors on February 4, 2016, totaling $165,000 with an original issue discount of $15,000. The Notes become due on February 3, 2017 and are convertible into common stock after six months from issuance. The Notes were convertible at 60% of the average of the lowest trading price in the 25 days prior to conversion but not less than $0.001 per share. The Company issued a total of 10,500 shares of restricted common stock to the investors valued at $70,875 and paid $7,500 in legal fees. The Company received $128,500 net of all fees. On August 5, 2016, we paid $217,366 to the three accredited investors to repay the Notes Payable in full.
We entered into a Convertible Note Payable with Vis Vires Group, Inc. on February 19, 2016 for $100,000. The Vis Vires Note accrued interest at a rate of 8% per annum and becomes due on November 22, 2016 and is convertible into common stock on August 19, 2016. The Vis Vires Note is convertible at 65% of the average of the lowest three day trading price in the 10 days prior to conversion. On August 11, 2016, we paid $136,769.86 to Vis Vires Group, Inc. to repay the Note Payable in full.
On February 23, 2016, we entered into a Consulting Agreement with David Markowski for business development services. On February 29, 2016, Mr. Markowski was issued 2,000 shares of our common stock. We expensed $14,600 during the nine months ended June 30, 2016.
During the period subsequent to September 30, 2015, we issued 74,0642,632,727 shares of common stock
throughrelated to warrant exercises and received $374,835.On June 27, 2023, Mr. Struve converted dividends of $350,696 into 1,402,784 shares of its common stock related to the conversion of 34 shares of Series B Redeemable Preferred Stock valued at $339,998, or $4.591 per share. D preferred stock.
On August 5, 2016,September 29, 2023, we closed the First Amendmentan offering of our common stock pursuant to Stock Purchase Agreement by and between the Company and this institutional investor. As a result of this amendment agreement,which we paid the institutional investor $505,000, issued 52,000sold 28,000,000 shares of common stock, valued at $169,000, cancelleda purchase price of $0.25 per share. After deducting underwriting commissions and other offering expenses, we received net proceeds of $5,472,791.
During the remaining 221 sharesThree Months Ended December 31, 2023
On October 10, 2023, the Company issued 105,000 fully vested stock awards total to three directors at an exercise price of Series B Redeemable Preferred Stock, and terminated$0.25 per share for director services.
On October 26, 2023, the relationship and all aspectsCompany closed an offering of the Stock Purchase Agreement described above in its entirety. We expectour common stock pursuant to expense $674,000 related to this amendment agreement during the three months ended September 30, 2016 and are currently cancelling the Certificate of Designations of Preferences, Rights and Limitations of Series B Redeemable Preferred Stock.
On July 11, 2016,which we issued 77,665sold 883,061 shares of common stock, to White Oak Capital LLC related toat a conversion of accounts payable. The shares were valued at $232,995, or $3.00 per share.
On August 1, 2016, we entered into an Engagement Letter with Axiom Financial, Inc. for financial and marketing services. Under the Letter, Axiom was issued 25,000 shares of our common stock. We expensed $29,000 during the period subsequent to September 30, 2015.
On August 4, 2016, we closed a Series C Preferred Stock and Warrant Purchase Agreement with Clayton A. Struve for purchase of $1,250,000 of preferred stock with a conversion price of $0.70$0.25 per share. The preferred has a yield of 8%After deducting underwriting commissions and an ownership blocker of 4.99%. In addition, the investor received 100% warrant coverage with five year warrants having a strike price of $0.70. Both the Series C and warrants will be included in this registration statement.
On August 10, 2016, we closed a Stock Purchase Agreement with Dale Broadrick and affiliate ofother offering expenses, the Company
received net proceeds of $203,105.Item 16. Exhibits.
(a) Exhibits.
Exhibit No. | | Description |
| | |
3.1 | | Restatement of the Articles of Incorporation, dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K, field August 14, 2023) |
3.2 | | Second Amended and Restated Bylaws, dated October 15, 2021 (incorporated by reference to the Company’s Current Report on Form 8‑K, filed December 7, 2021) |
3.3 | | Amended and Restate Series C Certificate of Designation, dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K filed August 14, 2023) |
3.4 | | Third Amended and Restated Series D Certificate of Designation, dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K filed August 14, 2023) |
3.5 | | Series D Certificate of Correction of Know Labs, Inc., dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K filed August 14, 2023) |
3.6 | | Series C Certificate of Correction of Know Labs, Inc., dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K filed August 14, 2023) |
3.7 | | Certificate of Withdrawal of Series F Preferred Stock, dated August 11, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K filed August 14, 2023) |
3.8 | | Certificate of Designation of Series F Preferred Stock (incorporated by reference to the Company’s Current Report on Form 8‑K, filed August 3, 2018) |
3.9 | | Certificate of Amendment to Articles of Incorporation dated December 6, 2021 (incorporated by reference to the Company’s Current Report on Form 8‑K, filed December 7, 2021) |
4.1† | | Know Labs, Inc. 2021 Equity Incentive Plan (incorporated by reference to the Company’s Form S‑8 Filed December 10, 2021) |
5.1** | | Opinion of Dentons Durham Jones Pinegar P.C. |
10.1 | | Form of Preferred Stock and Warrant Purchase Agreement, Form of Amended and Restated Registration Rights Agreement. and Form of Series F Warrant to Purchase common stock by and between Visualant, Incorporated and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed May 5, 2017) |
10.2 | | Securities Purchase Agreement dated August 14, 2017 by and between Visualant, Incorporated and accredited investor (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 18, 2017) |
10.3 | | Senior Secured Convertible Redeemable Debenture dated December 12, 2017 by and between Visualant, Incorporated and accredited investor. (incorporated by reference to the Company’s Current Report on Form 8-K, filed December 22, 2017) |
10.4 | | Senior Secured Convertible Redeemable Debenture dated February 28, 2018 by and between Visualant, Incorporated and accredited investor. (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 7, 2018) |
10.5 | | Note and Account Payable Conversion Agreement and related notes and warrants dated January 31, 2018 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 21, 2018) |
10.8† | | Amended Employment Agreement dated April 10, 2018 by and between Visualant, Incorporated and Ronald P. Erickson. (incorporated by reference to the Company’s Annual Report on Form 10-K, filed December 21, 2018) |
10.9† | | Employment Agreement dated May 13, 2022 by and between Know Labs, Inc. and Peter Conley. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed August 12, 2022) |
10.11 | | Common Stock Purchase Warrant issued by Know Labs, Inc. to Boustead Securities, LLC on September 20, 2022 (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 21, 2022) |
10.12 | | Extension of Warrant Agreement dated December 7, 2022 by and between Know Labs, Inc. and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed December 9, 2022) |
10.13 | | Extension of Warrant Agreement dated January 19, 2023 by and between Know Labs, Inc. and Ronald P. Erickson (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 23, 2023). |
10.14 | | Extension of Warrant Agreement dated January 19, 2023 by and between Know Labs, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 23, 2023). |
10.15 | | Amendment 10 dated September 15, 2023 to Convertible Redeemable Promissory Note dated January 31, 2018, by and between Know Labs, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.16 | | Amendment 10 dated September 15, 2023 to Convertible Redeemable Promissory Note dated January 31, 2018, by and between Know Labs, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.17 | | Amendment 9 dated September 15, 2023 to Senior Secured Convertible Redeemable Note dated September 30, 2016 by and between Know Labs, Inc. and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.18 | | Amendment 9 dated September 15, 2023 to Senior Secured Convertible Redeemable Note dated August 14, 2017 by and between Know Labs, Inc. and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.19 | | Amendment 9 dated September 15, 2023 to Senior Secured Convertible Redeemable Note dated December 12, 2017 by and between Know Labs, Inc. and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.20 | | Amendment 8 dated September 15, 2023 to Senior Secured Convertible Redeemable Note dated February 28, 2018 by and between Know Labs, Inc. and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 19, 2023). |
10.21 | | Underwriting Agreement, dated September 26, 2023, between Know Labs, Inc., Boustead Securities, LLC and The Benchmark Company, LLC (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 29, 2023). |
10.22 | | Common Stock Purchase Warrant issued by Know Labs, Inc. to Boustead Securities, LLC on September 29, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 29, 2023). |
10.23 | | Common Stock Purchase Warrant issued by Know Labs, Inc. to The Benchmark Company, LLC on September 29, 2023 (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 29, 2023). |
10.24 | | Securities Purchase Agreement, dated February 27, 2024, between Know Labs, Inc. and Lind Global II, LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 29, 2024). |
10.25 | | Form of Convertible Secured Promissory Note issued by Know Labs, Inc. to Lind Global II, LP on February 27, 2024 (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 29, 2024). |
10.26 | | Form of Warrant to Purchase Common Stock issued by Know Labs, Inc. to Lind Global II, LP on February 27, 2024 (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 29, 2024). |
10.27 | | Security Agreement, dated February 27, 2024, between Know Labs, Inc. and Lind Global II, LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 29, 2024). |
10.28 | | Guaranty, dated February 27, 2024, between Know Labs, Inc. and Lind Global II, LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 29, 2024). |
14.1 | | Code of Ethics dated November 2018 (incorporated by reference to the Company’s Current Report on Form 8‑K, filed November 27, 2018) |
21.1 | | Subsidiaries of the Registrant. (incorporated by reference to the Company’s Annual Report on Form 10-K, filed December 19, 2023) |
23.1** | | Consent of BPM LLP, Independent Registered Public Accounting Firm |
23.2** | | Consent of Dentons Durham Jones Pinegar P.C. (included in Exhibit 5.1) |
24.1** | | Power of Attorney (included in the signature page of this Form S-1 filed March 28, 2024) |
99.1 | | Audit Committee Charter dated November 2018 (incorporated by reference to the Company’s Current Report on Form 8-K, filed November 27, 2018) |
99.2 | | Compensation Committee Charter dated November 2018 (incorporated by reference to the Company’s Current Report on Form 8-K, filed November 27, 2018) |
99.3 | | Nominations and Corporate Governance Committee Charter dated November 2018 (incorporated by reference to the Company’s Current Report on Form 8-K, filed November 27, 2018) |
107* | | Exhibit Filing Fees |
______________
† | Executive compensation plan or arrangement. |
* | Filed herewith. |
** | Previously Filed. |
(b) Financial Statement Schedules.
All financial statement schedules are omitted because the information called for the purchase of $500,000 of our common stock at $0.70 per share. In addition, the investor received 100% warrant coverage with a five year warrant having a strike price of $0.70. These common shares and warrants areis not subject to a registration statement.
We entered into a Convertible Note Payable with JSJ Investments, Inc. on August 1, 2016 for $100,000 to fund short-term working capital. The JSJ Note accrued interest at a rate of 12% per annum and becomes due on May 1, 2017 andrequired or is convertible into common stock on January 1, 2017. The JSJ Note is convertible at the lower of $1.35 per share or 65% of the average of the lowest twenty day trading price prior to the date of the conversion notice. We received $88,000 net of all fees. On August 30, 2016, we paid $110,000 to JSJ to repay the Note Payable in full.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
The exhibits to the Registration Statement are listedshown either in the Exhibit Index attached hereto and incorporated by reference herein.
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individuallyfinancial statements or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: notes thereto.Item 17. Undertakings
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that, in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
(5) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(6) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
hereby undertakes:(1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| | |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| | |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
(2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
(3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| |
(4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| A. | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| | |
| B. | Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
(5) | That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| i. | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| | |
| ii. | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| iii. | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| | |
| iv. | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
(6) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| |
(7) | The undersigned registrant hereby undertakes that: |
| i. | For purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
| | |
| ii. | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and |
| | |
| iii. | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this Registration Statementregistration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Seattle, State of Washington, on September 1, 2016.
April 9, 2024. | VISUALANT, INCORPORATEDKnow Labs, Inc. | |
| | |
| By: | /s/ Ronald P. Erickson | |
| | Chairman of the Board, Chief Executive Officer and President |
| Director | | |
| By: | /s/ Ronald P. Erickson |
| | Interim Chief Financial Officer and Secretary |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
SIGNATURESSignature | TITLE | DATETitle | | Date |
| | | | |
/s/ Ronald P. Erickson | | Chief Executive Officer | | April 9, 2024 |
Ronald P. Erickson | | (Principal Executive Officer), Director | | |
| | | | |
/s/ Peter Conley | | Chief Financial Officer | | April 9, 2024 |
Peter Conley | | (Principal Financial and Accounting Officer) | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
Jon Pepper | | | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
William A. Owens | | | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
Ichiro Takesako | | | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
John Cronin | | | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
Timothy Londergan | | | | |
| | | | |
/s/ *** | | Director | | April 9, 2024 |
Larry K. Ellingson | | | | |
***By: | /s/ Ronald P. Erickson | |
| Ronald P. Erickson Attorney-in-fact | |
| |
/s/ Ronald P. Erickson | Chairman of the Board, Chief Executive Officer, President, Interim Chief Financial Officer and Director | September 1, 2016 |
Ronald P. Erickson | | |
| | |
/s/ Jon Pepper | Director | September 1, 2016 |
Jon Pepper | | |
| | |
/s/ Ichiro Takesako | Director | September 1, 2016 |
Ichiro Takesako | | 75 |
Exhibit Index
3.1
Restatement of the Articles of Incorporation dated September 13, 2013 (incorporated by reference to the Company’s Current Report on Form 8-K/A2, filed September 17, 2013)
3.2
Certificate of Designation, Preferences and Rights for the Company’s Series A Convertible Preferred Stock (incorporated by reference to the Company’s Current Report on Form 8-K, filed February 27, 2015)
3.3
Amended and Restated Bylaws (incorporated by reference to the Company’s Form 8-K, filed August 17, 2012)
3.4
Certificate of Amendment to the Restatement of the Articles of Incorporation dated June 11, 2015 (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 17, 2015)
3.5
Amended and Restated Certificate of Designations, Preferences and Rights of the Company’s Series A Convertible Preferred Stock dated July 21, 2015 (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 29, 2015)
3.6
Correction to Amended and Restated Certificate of Designations, Preferences and Rights of its Series A Convertible Preferred Stock dated March 8, 2016 (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 15, 2016)
3.7
Amendment 2 of Series A Preferred Stock Terms dated February 19, 2016 (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 15, 2016)
3.8
Certificate of Designations of Preferences, Powers, Rights and Limitations of Series B Redeemable Convertible Preferred Stock dated March 8, 2016 (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 15, 2016)
3.9
Certificate of Designations, Preferences and Rights of Series C Convertible Preferred Stock (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
4.1
2011 Stock Incentive Plan (incorporated by reference to the Company’s Definitive Proxy Statement on Schedule 14A, filed January 11, 2013)
4.2
Form of Warrant Agreement for Offering (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed June 17, 2015)
10.1
Financial Consulting Agreement effective October 5, 2011 by and between Visualant, Incorporated and D. Weckstein & Co. Inc. (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed August 16, 2013)
10.2
License Agreement dated May 31, 2012 by and between Visualant, Incorporated and Sumitomo Precision Products Co., Ltd. (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 4, 2012)
10.3
Joint Research and Product Development Agreement dated May 31, 2012 by and between Visualant, Incorporated and Sumitomo Precision Products Co. (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 7, 2013)
10.4
Lease dated July 11, 2012 by and between Visualant, Inc. and Harbor Properties Inc. (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed September 16, 2013)
10.5
Job Offer Letter dated August 9, 2012 by and between Visualant, Inc. and Todd Sames (incorporated by reference to the Company’s Registration Statement on Form S-1, filed April 24, 2015)
10.6
Settlement and Release Agreement dated September 6, 2012 by and between Visualant, Incorporated and Bradley E. Sparks (incorporated by reference to the Company’s Current Report on Form 8-K, filed September 11, 2012)
10.7
Amendment to Joint Research and Product Development Agreement dated March 29, 2013 by and between Visualant, Incorporated and Sumitomo Precision Products Co. (incorporated by reference to the Company’s Registration Statement on Form S1/A, filed September 16, 2013)
10.8
Stock Purchase Agreement dated May 31, 2012 by and between Visualant, Incorporated and Sumitomo Precision Products Co., Ltd. (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 4, 2012)
10.9
Form of Purchase Agreement by and between Visualant, Incorporated and investors (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 18, 2013)
10.10
Form of Series A and Series B Warrant by and between Visualant, Incorporated and investors (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 18, 2013)
10.11
Form of Stock Purchase Agreement by and between Visualant, Incorporated and investors (incorporated by reference to the Company’s Current Report on Form 8-K, filed June 18, 2013)
10.12
Security Agreement dated June 12, 2013 by and between Visualant, Incorporated and BFI Business Finance (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed August 15, 2013)
10.13
General Continuing Guaranty dated June 12, 2013 by and between TransTech Systems Inc., Visualant, Incorporated and BFI Business Finance (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 7, 2013)
10.14
Third Modification to Loan and Security Agreement dated June 12, 2013 by and between TransTech Systems Inc. and BFI Business Finance (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 7, 2013)
10.15
[Intentionally Omitted]
10.16
Amendment No. 1 to Lease dated June 14, 2013 by and between Visualant, Inc. and Logan Building (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed August 16, 2013)
10.17
Form of Placement Agent Warrant by and between Visualant, Incorporated and placement agents (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed October 7, 2013)
10.18
Warrant to Purchase Common Stock dated November 11, 2013 by and between Visualant, Incorporated and Invention Development Management Company, L.L.C. (incorporated by reference to the Company’s Current Report on Form 8-K, filed November 21, 2013)
10.19
Services and License Agreement dated November 11, 2013 by and between Visualant, Incorporated and Invention Development Management Company, L.L.C (incorporated by reference to the Company’s Registration Statement on Form S-1/A, filed January 24, 2014)
10.20
Demand Promissory Note dated January 10, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 15, 2014)
10.21
Secured Promissory Note dated March 19, 2014 by and between TransTech System, Inc. and BFI Finance (incorporated by reference to the Company’s Quarterly Report Form 10-Q, filed May 14, 2014)
10.22
Letter Agreement dated March 19, 2014 by and between TransTech System, Inc. and BFI Finance (incorporated by reference to the Company’s Quarterly Report Form 10-Q, filed May 14, 2014)
10.23
Demand Promissory Note dated March 31, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed April 3, 2014)
10.24
Amendment to Demand Promissory Note dated March 31, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed April 3, 2014)
10.25
Financial Public Relations Agreement dated June 9, 2014 by and between Visualant, Incorporated and Dynasty Wealth, Inc. (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed August 14, 2014)
10.26
Second Amendment to Office Lease dated June 18, 2014 by and between Visualant, Incorporated and Logan Building LLC (incorporated by reference to the Company’s Annual Report on Form 10-K, filed January 13, 2015)
10.27
Demand Promissory Note dated July 17, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 18, 2014, and incorporated by reference.
10.28
Amendment to Demand Promissory Note dated July 17, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 18, 2014)
10.29
Amendment 2 to Demand Promissory Note dated July 17, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 18, 2014)
10.30
Addendum to Letter dated August 27, 2014 by and between Visualant, Incorporated and D. Weckstein Co., Inc. (incorporated by reference to the Company’s Annual Report on Form 10-K, filed January 13, 2015)
10.31
Amendment to Services and License Agreement dated November 18, 2014 by and between Visualant, Inc. and Invention Development Management Company, LLC (incorporated by reference to the Company’s Current Report on Form 8-K, filed November 25, 2014)
10.32
Third Amendment to Office Lease dated December 18, 2014 by and between Visualant, Incorporated and Logan Building LLC (incorporated by reference to the Company’s Annual Report on Form 10-K, filed January 13, 2015)
10.33
Amendment to Demand Promissory Note dated December 31, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 2, 2015)
10.34
Amendment 2 to Demand Promissory Note dated December 31, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 2, 2015)
10.35
Amendment 3 to Demand Promissory Note dated December 31, 2014 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 2, 2015)
10.36
Form of Purchase Agreement related to Series A Preferred Stock offering by and between Visualant, Incorporated and investors (incorporated by reference to the Company’s Registration Statement on Form S-1, filed April 24, 2015)
10.37
Form of Series C Warrant between Visualant, Incorporated and investors (incorporated by reference to the Company’s Registration Statement on Form S-1, filed April 24, 2015)
10.38
Form of Series D Warrant between Visualant, Incorporated and investors (incorporated by reference to the Company’s Registration Statement on Form S-1, filed April 24, 2015)
10.39
Form of Stock Purchase Agreement related to preferred stock by and between Visualant, Incorporated and investors (incorporated by reference to the Company’s Registration Statement on Form S-1, filed April 24, 2015)
10.40
Amendment 2 to Demand Promissory Note dated March 31, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed April 3, 2015)
10.41
Amendment 3 to Demand Promissory Note dated March 31, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed April 3, 2015)
10.42
Amendment 4 to Demand Promissory Note dated March 31, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed April 3, 2015)
10.43
Amendment to License Agreement received May 21, 2015, effective June 18, 2014 by and between Visualant, Incorporated and Sumitomo Precision Products Co., Ltd. (incorporated by reference to the Company’s Current Report on Form 8-K, filed May 27, 2015)
10.44
Amendment 3 to Demand Promissory Note dated July 15, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 17, 2015)
10.45
Amendment 4 to Demand Promissory Note dated July 15, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 17, 2015)
10.46
Amendment 5 to Demand Promissory Note dated July 15, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 17, 2015)
10.47
Form of Amendment to Series A Preferred Stock Terms (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 29, 2015)
10.48
Amendment 5 to Demand Promissory Note dated September 30, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed October 2, 2015)
10.49
Amendment 5 to Demand Promissory Note dated September 30, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed October 2, 2015)
10.50
Amendment 6 to Demand Promissory Note dated September 30, 2015 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed October 2, 2015)
10.51
Amendment 5 to Demand Promissory Note dated December 31, 2015 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 4, 2016)
10.52
Amendment 6 to Demand Promissory Note dated December 31, 2015 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 4, 2016)
10.53
Amendment 7 to Demand Promissory Note dated December 31, 2015 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 4, 2016)
10.54
Form of Note and Warrant Purchase Agreement (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed February 16, 2016)
10.55
Form of Subordinated Convertible Promissory Note Agreement (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed February 16, 2016)
10.56
Form of Warrant to Purchase Shares Agreement (incorporated by reference to the Company’s Quarterly Report on Form 10-Q, filed February 16, 2016)
10.57
Stock Purchase Agreement dated March 8, 2016 by and between Visualant, Incorporated and institutional investor (incorporated by reference to the Company’s Current Report on Form 8-K, filed March 10, 2016)
10.58
Amendment 6 to Demand Promissory Note dated April 29, 2016 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed May 2, 2016)
10.59
Amendment 7 to Demand Promissory Note dated April 29, 2016 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed May 2, 2016)
10.60
Amendment 8 to Demand Promissory Note dated April 29, 2016 by and between Visualant, Inc. and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed May 2, 2016)
10.61
Amendment 7 to Demand Promissory Note dated June 30, 2016 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 6, 2016).
10.62
Amendment 8 to Demand Promissory Note dated June 30, 2016 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 6, 2016).
10.63
Amendment 9 to Demand Promissory Note dated June 30, 2016 by and between Visualant, Incorporated and J3E2A2Z LP (incorporated by reference to the Company’s Current Report on Form 8-K, filed July 6, 2016).
10.64
Form of Preferred Stock and Warrant Purchase Agreement by and between Visualant, Incorporated and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
10.65
Form of Warrant to Purchase Shares by and between Visualant, Incorporated and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
10.66
Form of Registration Rights Agreement by and between Visualant, Incorporated and Clayton A. Struve (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
10.67
Form of Stock Purchase Agreement by and between Visualant, Incorporated and Dale Broadrick (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
10.68
Form of Warrant for the Purchase of Common Stock by and between Visualant, Incorporated and Dale Broadrick (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
10.69
First Amendment to Stock Purchase Agreement by and between Visualant, Incorporated and institutional investor (incorporated by reference to the Company’s Current Report on Form 8-K, filed August 11, 2016)
14.1
Code of Conduct and Ethics dated November 30, 2012 (incorporated by reference to the Company’s Current Report on Form 8-K, filed January 3, 2013)
21.1
Subsidiaries of the Registrant (incorporated by reference to the Company’s Annual Report on Form 10-K, filed November 4, 2015)
23.2
Consent of Horwitz + Armstrong, A Professional Law Corporation (included in Exhibit 5.1) (filed herewith)
















