risks emerge from time to time. It is not possible for
may cause actual results to differ materially from those contained in any forward-looking
forward-looking statements.
capital. We
have not set an offering price for the shares registered hereunder, as the only shares being registered are those sold pursuant to the GHS Financing Agreement. GHS may
sell all oralso use a portion of the
shares being offered pursuantnet proceeds to
acquire or invest in businesses, technologies, and products that are complementary to our own, although we have no current binding agreements with respect to any acquisitions as of the date of this
Prospectus at fixed pricesprospectus.This expected use of the net proceeds from this offering represents our intentions based upon our current plans and prevailing market prices atbusiness conditions. Pending our use of the timenet proceeds from this offering, we intend to invest the net proceeds in a variety of sale, at varying prices or at negotiated prices.capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
DILUTION
Not applicable. The shares registeredMARKET FOR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock is listed on the Nasdaq Capital Market under this Registration Statement are not being offered for purchase. The shares are being registered on behalfthe symbol “SBFM.”
As of February 6, 2024, there were approximately 149 holders of record of our selling stockholders pursuant to the GHS Financing Agreement.
SELLING STOCKHOLDERS
The Selling Stockholder identified in this Prospectus may offer and sell up to 266,417,879 shares of our Common Stock, which consists of shares of Common Stock to be sold by GHS pursuant to the Financing Agreement. If issued presently, the shares of Common Stock registered for resale by GHS would represent approximately 16.8% of our issued and outstanding shares of Common Stock as of September 20, 2018.
We may require the Selling Stockholder to suspend the sales of the shares of our Common Stock being offered pursuant to this Prospectus upon the occurrence of any event that makes any statement in this Prospectus or the related Registration Statement untrue in any material respect or that requires the changing of statements in those documents in order to make statements in those documents not misleading.
The Selling Stockholder identified in the table below may from time to time offer and sell under this Prospectus any or all of the shares of Common Stock described under the column “Shares of Common Stock Being Offered” in the table below.
GHS will be deemed to be an underwriter within the meaning of the Securities Act. Any profits realized by such Selling Stockholder may be deemed to be underwriting commissions.
common stock.Equity Compensation Plan Information concerning the Selling Stockholder may change from time to time and, if necessary, we will amend or supplement this Prospectus accordingly. We cannot give an estimate as to the number of shares of Common Stock that will actually be held by the Selling Stockholder upon termination of this offering, because the Selling Stockholder may offer some or all of the Common Stock under the offering contemplated by this Prospectus or acquire additional shares of Common Stock. The total number of shares that may be sold, hereunder, will not exceed the number of shares offered, hereby. Please read the section entitled “PLAN OF DISTRIBUTION” in this Prospectus.
The manner in which the Selling Stockholder acquired or will acquire shares of our Common Stock is discussed below under “THE OFFERING.”
The following table sets forth the name of each Selling Stockholder, the number of shares ofinformation regarding our Common Stock beneficially owned by such stockholder before this offering, the number of shares to be offered for such stockholder’s account and the number and (if one percent or more) the percentage of the class to be beneficially owned by such stockholder after completion of the offering. The number of shares owned are those beneficially owned, as determined under the rules of the SEC, and such information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares of our Common Stock as to which a person has sole or shared voting power or investment power and any shares of Common Stock which the person has the right to acquire within 60 days, through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement, and such shares are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. Beneficial ownership percentages are calculated based on 1,585,628,494 shares of our Common Stock outstandingequity compensation plans as of September 20, 2018.
Unless otherwise set forth below, (a) the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the Selling Stockholder’s name, subject to community property laws, where applicable, and (b) no Selling Stockholder had any position, office or other material relationship within the past three years, with us or with anyDecember 31, 2023.| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans |
| Equity compensation plans approved by security holders(1) | -- | -- | 3,320,988 |
| Equity compensation plans not approved by security holders | -- | -- | -- |
(1) Represents our predecessors or affiliates. The number of shares of Common Stock shown as beneficially owned before the offering is based on information furnished to us or otherwise based on information available to us at the timing of the filing of the Registration Statement of which this Prospectus forms a part.
| | | Shares Owned by the Selling Stockholders | | Number of Shares to be Owned by Selling Stockholder After the Offering and Percent of Total Issued and Outstanding Shares | |
| Name of Selling Stockholder | | | | | |
| GHS Investments LLC (3) | | 0 | 266,417,879(4) | 0 | 0% |
Notes:
(1) Beneficial ownership is determined in accordance with Securities and Exchange Commission rules and generally includes voting or investment power with respect to shares of Common Stock. Shares of Common Stock subject to options, warrants and convertible debentures currently exercisable or convertible, or exercisable or convertible within 60 days, are counted as outstanding. The actual number of shares of Common Stock issuable upon the conversion of the convertible debentures is subject to adjustment depending on, among other factors, the future market price of our Common Stock, and could be materially less or more than the number estimated in the table.
(2) Because the Selling Stockholder may offer and sell all or only some portion of the 266,417,879 shares of our Common Stock being offered pursuant to this Prospectus and may acquire additional shares of our Common Stock in the future, we can only estimate the number and percentage of shares of our Common Stock that any of the Selling Stockholder will hold upon termination of the offering.
(3) Mark Grober exercises voting and dispositive power with respect to the shares of our Common Stock that are beneficially owned by GHS Investments LLC.
(4) Consists of up to 266,417,879 shares of Common Stock to be sold by GHS pursuant to the Financing Agreement.
THE OFFERING
On September 10, 2018, we entered into an2023 Equity Financing Agreement (the “Financing Agreement”) with GHS Investments LLC (“GHS”). Although we are not mandated to sell shares under the Financing Agreement, the Financing Agreement gives us the option to sell to GHS, up to $10,000,000 worth of our Common Stock over the period ending thirty six (36) months after the date this Registration Statement is deemed effective. The $10,000,000 was stated as the total amount of available funding in the Financing Agreement because this was the maximum amount that GHS agreed to offer us in funding. In connection with the Financing Agreement, the Company executed a promissory note dated September 10, 2018, in the principal amount of $20,000 (the “Note”) as payment of the commitment fee for the Financing Agreement. The Note bears interest at the rate of 8% per annum and has a maturity date of June 30, 2019. There is no assurance the market price of our Common Stock will increase in the future. The number of common shares that remain issuable may not be sufficient, dependent upon the share price, to allow us to access the full amount contemplated under the Financing Agreement. If the bid/ask spread remains the same we will not be able to place a put for the full commitment under the Financing Agreement. Based on the average of the three lowest VWAP’s of our Common Stock during the ten (10) consecutive trading day period preceding the filing date of this Registration Statement was approximately $0.00148, the Registration Statement covers the offer and possible sale of $319,382 worth of our shares.
The purchase price of the Common Stock will be set at eighty one percent (81%) of the average of the three lowest VWAPs of the Company’s Common Stock during the ten (10) consecutive trading day period immediately preceding the date on which the Company delivers a put notice to GHS. In addition, there is a maximum ownership limit for GHS of 9.99% of the issued and outstanding Common Stock of the Company.
GHS is not permitted to engage in short sales involving our Common Stock during the term of the commitment period. In accordance with Regulation SHO, however, sales of our Common Stock by GHS after delivery of a put notice of such number of shares reasonably expected to be purchased by GHS under a put will not be deemed a short sale.
In addition, we must deliver the other required documents, instruments and writings required. GHS is not required to purchase the put shares unless:
●
Our Registration Statement with respect to the resale of the shares of Common Stock delivered in connection with the applicable put shall have been declared effective;
●
We shall have obtained all material permits and qualifications required by any applicable state for the offer and sale of the registrable securities; and
●
We shall have filed all requisite reports, notices, and other documents with the SEC in a timely manner.
As we issue puts under the Financing Agreement, shares of our Common Stock will be sold into the market by GHS. The sale of these shares could cause our stock price to decline. In turn, if our stock price declines and we issue more puts, more shares will come into the market, which could cause a further drop in our stock price. You should be aware that there is an inverse relationship between the market price of our Common Stock and the number of shares to be issued under the Financing Agreement. If our stock price declines, we will be required to issue a greater number of shares. We have no obligation to utilize any or the full amount available under the Financing Agreement.
Neither the Financing Agreement nor any of our rights or GHS’s rights thereunder may be assigned to any other person.
PLAN OF DISTRIBUTION
The Selling Stockholder named above and any of its pledgees and successors-in-interest may, from time to time, sell any or all of their shares of Common Stock on OTC Markets or any other stock exchange, market or trading facility on which the shares of our Common Stock are traded or in private transactions. These sales may be at fixed prices and prevailing market prices at the time of sale, at varying prices or at negotiated prices. The Selling Stockholder may use any one or more of the following methods when selling shares:
●
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
●
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
●
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
●
privately negotiated transactions;
●
broker-dealers may agree with the Selling Stockholder to sell a specified number of such shares at a stipulated price per share; or
●
a combination of any such methods of sale.
Broker-dealers engaged by the Selling Stockholder may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholder (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
GHS is an underwriter within the meaning of the Securities Act of 1933 and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act of 1933 in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act of 1933. GHS has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the Common Stock of our company. Pursuant to a requirement by FINRA, the maximum commission or discount to be received by any FINRA member or independent broker-dealer may not be greater than 8% of the gross proceeds received by us for the sale of any securities being registered pursuant to Rule 415 promulgated under the Securities Act of 1933.
Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by the Selling Stockholder. The Selling Stockholder may agree to indemnify any agent, dealer, or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that person under the Securities Act of 1933.
We are required to pay certain fees and expenses incurred by us incident to the registration of the shares covered by this Prospectus. We have agreed to indemnify the Selling Stockholder against certain losses, claims, damages and liabilities, including liabilities under the Securities Act of 1933. We will not receive any proceeds from the resale of any of the shares of our Common Stock by the Selling Stockholder. We may, however, receive proceeds from the sale of our Common Stock under the Financing Agreement with GHS. Neither the Financing Agreement with GHS nor any rights of the parties under the Financing Agreement with GHS may be assigned or delegated to any other person.
Pursuant to the terms of the Financing Agreement, we have agreed with GHS to keep this Prospectus effective until GHS has sold all of the common shares purchased by it under the Financing Agreement.
The resale shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Securities Exchange Act of 1934, any person engaged in the distribution of the resale shares may not simultaneously engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholder will be subject to applicable provisions of the Securities Exchange Act of 1934 and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of the Common Stock by the Selling Stockholder or any other person. We will make copies of this Prospectus available to the Selling Stockholder.
DESCRIPTION OF SECURITIES TO BE REGISTERED
Capital Stock
The Company’s authorized capital is comprised of 3,000,000,000 shares of $0.001 par value Common Stock and 30,000,000 shares of $0.10 par value Preferred Stock, to have such rights and preferences as the Directors of the Company have or may assign from time to time. Out of the authorized Preferred Stock, the Board has designated 850,000 shares as Series “A” Preferred Stock (“Series A”) and 500,000 shares as Series B Preferred Stock (“Series B”). The Company currently has 1,585,628,494 shares of Common Stock, 0 shares of Series A Preferred Stock and 500,000 shares of Series B Preferred Stock issued and outstanding. The 500,000 shares of Series B Preferred Stock are held by Dr. Steve N. Slilaty, the CEO of the Company. The Series B Preferred Stock are non-convertible, non-redeemable and non-contractible. They give the holder the right to 1,000 votes per share and may vote together with the Common Stock.
Each share of Common Stock shall have one (1) vote per share. Our Common Stock does not provide a preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights. Our Common Stock holders are not entitled to cumulative voting for election of Board of Directors.
Dividends
Incentive Plan.Dividend Policy
We have not paid any dividends on our Common Stock or Preferred Stock since our inceptionincorporation and do not intend to payanticipate paying any dividends in the foreseeable future.
The declaration of any future cash dividends At present, our policy is
at the discretion of our board of directors and depends upon ourto retain earnings, if any,
to develop and market our
products. Our payment of dividends in the future will depend upon, among other factors, our earnings, capital requirements, and
operating financial
position,conditions.CAPITALIZATION
The following table sets forth our general economic conditions,cash and other pertinent conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Warrants
As of the date hereof, there are no outstanding warrants of any kind issued and outstanding.
Options
As of the date hereof, there are no outstanding options of any kind issued and outstanding. .
Securities Authorized for Issuance Under Equity Compensation Plans
Anti-Takeover EffectsFinancial Condition and Results of Various Provisions of Colorado Law
Provisions of the Colorado Revised Statutes, our articles of incorporation, as amended,Operations,” and our bylaws could make it more difficult to acquire us by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions, summarized below, would be expected to discourage certain types of takeover practices and takeover bids our Board may consider inadequate and to encourage persons seeking to acquire control of us to first negotiate with us. We believe that the benefits of increased protection of our ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us will outweigh the disadvantages of discouraging takeover or acquisition proposals because, among other things, negotiation of these proposals could result in an improvement of their terms.
Blank Check Preferred Stock
Our articles of incorporation permit our Board to issue Preferred Stock with voting, conversion and exchange rights that could negatively affect the voting power or other rights of our Common Stockholders. The issuance of our Preferred Stock could delay or prevent a change of control of our Company.
Limitations on Liability and Indemnification of Officers and Directors
The Colorado Revised Statutes and the Colorado Business Corporation Act (the “CBCA”) limits or eliminates the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties as directors.
Section 7-109-102(1) of the Colorado Business Corporation Act (the “CBCA”) permits indemnification of a director of a Colorado corporation, in the case of a third party action, if the director (a) conducted himself or herself in good faith, (b) reasonably believed that (i) in the case of conduct in his or her official capacity, his or her conduct was in the corporation’s best interest, or (ii) in all other cases, his or her conduct was not opposed to the corporation’s best interest, and (c) in the case of any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful. Section 7-109-103 further provides for mandatory indemnification of directors and officers who are successful on the merits or otherwise in litigation.
Section 7-109-102(4) of the CBCA limits the indemnification that a corporation may provide to its directors in two key respects. A corporation may not indemnify a director in a derivative action in which the director is held liable to the corporation, or in any proceeding in which the director is held liable on the basis of his improper receipt of a personal benefit. Sections 7-109-104 of the CBCA permits a corporation to advance expenses to a director, and Section 7-109-107(1)(c) of the CBCA permits a corporation to indemnify and advance litigation expenses to officers, employees and agents who are not directors to a greater extent than directors if consistent with law and provided for by the bylaws, a resolution of directors or shareholders, or a contract between the corporation and the officer, employee or agent.
Authorized but Unissued Shares
Our authorized but unissued shares of Common Stock and Preferred Stock will be available for future issuance without stockholder approval, except as may be required under the listing rules of any stock exchange on which our Common Stock is then listed. We may use additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued shares of Common Stock and Preferred Stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Penny Stock Considerations
Our shares will be “penny stocks” as that term is generally defined in the Securities Exchange Act of 1934 to mean equity securities with a price of less than $5.00 per share. Thus, our shares will be subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in certain transactions involving a penny stock. Under the penny stock regulations, a broker-dealer selling a penny stock to anyone other than an established customer must make a special suitability determination regarding the purchaser and must receive the purchaser’s written consent to the transaction prior to the sale, unless the broker-dealer is otherwise exempt.
In addition, under the penny stock regulations, the broker-dealer is required to:
●
Deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the Securities and Exchange Commission relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt;
●
Disclose commissions payable to the broker-dealer and our registered representatives and current bid and offer quotations for the securities;
●
Send monthly statements disclosing recent price information pertaining to the penny stock held in a customer’s account, the account’s value, and information regarding the limited market in penny stocks; and
●
Make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction, prior to conducting any penny stock transaction in the customer’s account.
Because of these regulations, broker-dealers may encounter difficulties in their attempt to sell shares of our Common Stock, which may affect the ability of selling shareholders or other holders to sell their shares in the secondary market and have the effect of reducing the level of trading activity in the secondary market. These additional sales practice and disclosure requirements could impede the sale of our securities, if our securities become publicly traded. In addition, the liquidity for our securities may be decreased, with a corresponding decrease in the price of our securities. Our shares in all probability will be subject to such penny stock rules and our shareholders will, in all likelihood, find it difficult to sell their securities.
INTERESTS OF NAMED EXPERTS AND COUNSEL
The consolidated financial statements for the Company as of December 31, 2017period ended September 30, 2023, and 2016 and for the years then endedrelated notes thereto, included in this Prospectus have been audited by BF Borgers CPA PC, respectively, an independent registered public accounting firm, to the extentprospectus.| | | As of September 30, 2023 | |
| | | Actual | | | As adjusted | |
| Cash and cash equivalents | | $ | 18,846,140 | | | $ | 27,296,140 | |
| Total liabilities | | | 5,686,801 | | | | 5,686,801 | |
| Stockholders’ equity: | | | | | | | | |
| Series B Preferred Stock, $0.10 par value: 1,000,000 shares authorized; 10,000 shares issued and outstanding | | | 1,000 | | | | 1,000 | |
| Common Stock, $0.001 par value: 3,000,000,000 shares authorized; 25,678,290 shares issued and outstanding, actual; 64,893,977 shares issued and outstanding, as adjusted | | | 25,678 | | | | 64,894 | |
| Capital paid in excess of par value | | | 84,387,890 | | | | 92,798,674 | |
| Accumulated comprehensive income | | | 204,549 | | | | 204,549 | |
| Accumulated (deficit) | | | (62,655,634 | ) | | | (62,655,634 | ) |
| Total stockholders’ equity | | | 21,963,483 | | | | 30,413,483 | |
The above table is based on 25,678,290 shares of common stock outstanding as of September 30, 2023, and for the periods set forth in our report and are incorporated herein in relianceexcludes 23,395,046 shares issuable upon such report given upon the authorityexercise of said firm as experts in auditing and accounting.
The legality of the shares offered under this Registration Statement will be passed upon by Lucosky Brookman LLP.
INFORMATION WITH RESPECT TO THE REGISTRANT
History
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” Until October 2009, our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies.
In October 2009, we acquired Sunshine Biopharma, Inc., a Colorado corporation holding an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a. As a result of this transaction we changed our name to “Sunshine Biopharma, Inc. and our officers and directors resigned their positions with us and were replaced by Sunshine’s management at the time, including our current CEO, Dr. Steve N. Slilaty, and our current CFO, Camille Sebaaly each of whom remain part of our current management. Our principal business became that of a pharmaceutical company focusing on the development of our licensed Adva-27a anticancer compound. In December 2015 we acquired all issued and pending patents pertaining to our Adva-27a technology and terminated the license.
In July 2014, we formed a wholly owned Canadian subsidiary, Sunshine Biopharma Canada Inc. (“Sunshine Canada”), for the purposes of offering generic pharmaceutical products in Canada and elsewhere around the world. Sunshine Canada has recently signed licensing agreements for four (4) generic prescription drugs for the treatment of cancer and BPH (Benign Prostatic Hyperplasia).
In January 2018, we acquired Atlas Pharma Inc., a certified company dedicated to chemical analysis of pharmaceutical and other industrial samples whose operations are authorized by a Drug Establishment License issued by Health Canada.
In March 2018, we formed NOX Pharmaceuticals, Inc., a Colorado corporation and assigned all of our interest in our Adva-27a anticancer compound to that company.
Our principal place of business is located at 6500 Trans-Canada Highway, 4th Floor, Pointe-Claire, Quebec, Canada H9R 0A5. . Our phone number is (514) 426-6161and our website address is www.sunshinebiopharma.com.
Business Operations
As of the date of this Registration Statement on Form S-1 we are operating through the following wholly owned subsidiaries:
●
NOX Pharmaceuticals, Inc., a recently formed Colorado company focused on the research, development and commercialization of proprietary drugs for the treatment of cancer including Adva-27a, a multi-purpose anti-tumor compound targeted for the treatment of multidrug resistant cancer;
●
Sunshine Biopharma Canada Inc., a Canadian company, which offers generic prescription drugs for the treatment of cancer and other acute and chronic indications; and
●
Atlas Pharma Inc., a Canadian company acquired in January 2018, offering certified chemical analysis of pharmaceutical and other industrial samples.
Proprietary Drug Development Operations
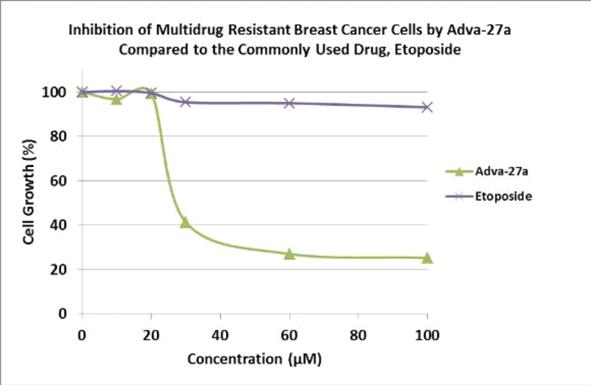
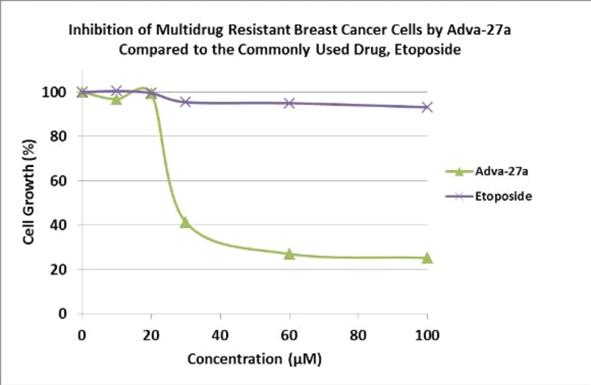
Since inception, our proprietary drug development activities have been focused on the development of a small molecule called Adva-27a for the treatment of aggressive forms of cancer. A Topoisomerase II inhibitor, Adva-27a has been shown to be effective at destroying Multidrug Resistant Cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma cells (Published in ANTICANCER RESEARCH, Volume 32, Pages 4423-4432, October 2012). Sunshine Biopharma is direct owner of all issued and pending worldwide patents pertaining to Adva-27a including U.S. Patent Number 8,236,935. See “Intellectual Property.”

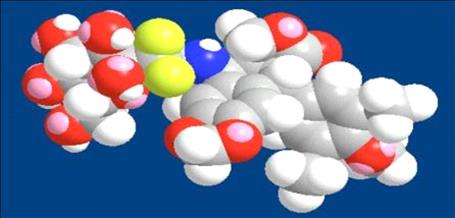
Adva-27a is a GEM-difluorinated C-glycoside derivative of Podophyllotoxin. Another derivative of Podophyllotoxin called Etoposide is currently on the market and is used to treat various types of cancer including leukemia, lymphoma, testicular cancer, lung cancer, brain cancer, prostate cancer, bladder cancer, colon cancer, ovarian cancer, liver cancer and several other forms of cancer. Etoposide is one of the most widely used anticancer drugs. Adva-27a and Etoposide are similar in that they both attack the same target in cancer cells, namely the DNA unwinding enzyme, Topoisomerase II. Unlike Etoposide, and other anti-tumor drugs currently in use, Adva-27a is able to destroy Multidrug Resistant Cancer cells. Adva-27a is the only compound known today that is capable of destroying Multidrug Resistant Cancer. In addition, Adva-27a has been shown to have distinct and more desirable biological and pharmacological properties compared to Etoposide. In side-by-side studies using Multidrug Resistant Breast Cancer cells and Etoposide as a reference, Adva-27a showed markedly improved cell killing activity (see Figure below). Our preclinical studies to date have shown that:
●
Adva-27a is effective at killing different types of Multidrug Resistant cancer cells, including Pancreatic Cancer Cells (Panc-1), Breast Cancer Cells (MCF-7/MDR), Small-Cell Lung Cancer Cells (H69AR), and Uterine Sarcoma Cells (MES-SA/Dx5).
●
Adva-27a is unaffected by P-Glycoprotein, the enzyme responsible for making cancer cells resistant to anti-tumor drugs.
●
Adva-27a has excellent clearance time (half-life = 54 minutes) as indicated by human microsomes stability studies and pharmacokinetics data in rats.
●
Adva-27a clearance is independent of Cytochrome P450, a mechanism that is less likely to produce toxic intermediates.
●
Adva-27a is an excellent inhibitor of Topoisomerase II with an IC50 of only 13.7 micromolar (this number has recently been reduce to 1.44 micromolar as a result of resolving the two isomeric forms of Adva-27a).
●
Adva-27a has shown excellent pharmacokinetics profile as indicated by studies done in rats.
●
Adva-27a does not inhibit tubulin assembly.
These and other preclinical data have been published in ANTICANCER RESEARCH, a peer-reviewed International Journal of Cancer Research and Treatment. The publication which is entitled “Adva-27a, a Novel Podophyllotoxin Derivative Found to Be Effective Against Multidrug Resistant Human Cancer Cells” [ANTICANCER RESEARCH 32: 4423-4432 (2012)] is available on our website atwww.sunshinebiopharma.com.
We have been delayed in our clinical development program due to lack of funding. Our fund raising efforts are continuing and as soon as adequate financing is in place we will continue our clinical development program of Adva-27a by conducting the following next sequence of steps:
●
GMP Manufacturing of 2 kilogram for use in IND-Enabling Studies and Phase I Clinical Trials
●
Regulatory Filing (Fast-Track Status Anticipated)
●
Phase I Clinical Trials (Pancreatic Cancer Indication)
On November 14, 2014, we entered into a Manufacturing Services Agreement with Lonza Ltd. and Lonza Sales Ltd. (hereinafter jointly referred to as “Lonza”), whereby we engaged Lonza to be the manufacturer of our Adva-27a anticancer drug. In June 2015 we received a sample of the pilot manufacturing run for evaluation. Our laboratory analyses showed that, while the sample meets all of the required chemical, physical and biological specifications, the amount of material generated (the “Yield”) by the pilot run was found to be significantly lower than anticipated. We are currently working towards finding possible solutions to increase the Yield and define a path forward. During the course of our discussions concerning the problem of the low Yield, Lonza informed us that they required us to pay them $687,818 prior to moving forward with any activity pertaining to the manufacturing agreement we have with them. We have repeatedly indicated to Lonza that a clear path defining exactly how the extremely low Yield issue would be addressed is imperative prior to us making any payments. As of the date of this Registration Statement on Form S-1, neither party has changed its position.
Adva-27a’s initial indication will be pancreatic cancer for which there are currently little or no treatment options available. We are planning to conduct our clinical trials at McGill University’s Jewish General Hospital in Montreal, Canada. All aspects of the clinical trials in Canada will employ FDA standards at all levels. We estimate that Phase I clinical trials will take 18 months to complete.
According to the American Cancer Society, nearly 1.5 million new cases of cancer are diagnosed in the U.S. each year. We believe that upon successful completion of Phase I Clinical Trials we may receive one or more offers from large pharmaceutical companies to buyout or license our drug. However, there are no assurances that our Phase I Trials will be successful, or if successful, that any pharmaceutical companies will make an acceptable offer to us. In the event we do not consummate such a transaction, we will require significant capital in order to conduct additional clinical trials, manufacture and market our new drug.
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Generic Pharmaceuticals Operations
In 2016, our Canadian wholly owned subsidiary, Sunshine Biopharma Canada Inc. (“Sunshine Canada”), signed Cross Referencing Agreementsoutstanding warrants with a major pharmaceutical company for four prescription generic drugs for the treatment of Breast Cancer, Prostate Cancer and Enlarged Prostate. Following this acquisition we have been working towards commencement of marketing of these pharmaceutical products under our own Sunshine Biopharma label. These four generic products are as follows:
●
Anastrozole (brand name Arimidex® by AstraZeneca) for treatment of Breast Cancer;
●
Letrozole (brand name Femara® by Novartis) for treatment of Breast Cancer;
●
Bicalutamide (brand name Casodex® by AstraZeneca) for treatment of Prostate Cancer;
●
Finasteride (brand name Propecia® by Merck) for treatment of BPH (Benign Prostatic Hyperplasia)
Worldwide sales of the brand name version of these products as reported by the respective pharmaceutical company, owner of the registered trademark are as follows:
Sunshine Canada is currently in the process of securing a Drug Identification Number (“DIN”) for each of these products from Health Canada. We are planning to use part of the already approved Atlas Pharma Inc. space as a drug warehouse to facilitate the process of obtaining a Drug Establishment License (“DEL”) from Health Canada. Upon receipt of the DEL and DIN’s, we will be able to accept orders for our own label SBI-Anastrozole, SBI-Letrozole, SBI-Bicalutamide and SBI-Finasteride. We cannot estimate the timing in our obtaining either the DIN’s or the DEL due to variables involved that are out of our control. The figure below shows our 30-Pill blister pack of Anastrozole.

We currently have twenty three (23) additional Generic Pharmaceuticals under review for in-licensing. While no assurances can be provided that we will acquire the rights to all or any of these drugs, we are confident we will acquire most, if not all of these rights. We believe that a larger product portfolio will provide us with more opportunities and a greater reach into the marketplace. We hope to further build our generics portfolio of “SBI” label Generic Pharmaceuticals over time. There are no assurances this will occur.
Various publicly available sources indicate that the worldwide sales of generic pharmaceuticals are approximately $200 billion per year. In the United States and Canada, the sales of generic pharmaceuticals are approximately $50 billion and $5 billion, respectively. The generic pharmaceuticals business is fairly competitive and there are several multinational players in the field including Teva (Israel), Novartis - Sandoz (Switzerland), Hospira (USA), Mylan (Netherlands), Sanofi (France), Fresenius Kabi (Germany) and Apotex (Canada). While no assurances can be provided, with our offering of Canadian approved products we believe that we will be able to access at least a small percentage of the generic pharmaceuticals marketplace.
As part of a subscription agreement entered into in 2016, we have an obligation to pay a royalty of 5% of net sales on one of our generic products (Anastrozole) for a period of three (3) years from the date of the first sale of that product. As of the date of this Registration Statement on Form S-1 we have not yet commenced marketing efforts and no sales or royalty payments have been made. On May 28, 2018 we issued 1,000,000 shares of our Common Stock valued at $5,900 in exchange for cancellation of this royalty obligation.
While no assurances can be provided and subject to the availability of adequate financing, of which there is no assurance, we anticipate that profits from the sales of Generic Products will be used to finance our proprietary drug development program, including Adva-27a, our flagship anticancer compound. In addition to near-term revenue generation, building the generics business infrastructure and securing the proper permits will render us appropriately positioned for the marketing and distribution of our proprietary Adva-27a drug candidate, provided that Adva-27a is approved for such marketing and distribution, of which there can be no assurance.
Analytical Chemistry Services Operations
On January 1, 2018, we entered into an agreement (the “Atlas Agreement”) to acquire Atlas Pharma Inc. (“Atlas”). The purchase price was $848,000 Canadian ($684,697 US). Payment of the purchase price was comprised of (i) a cash payment of $100,500 Canadian ($80,289 US), (ii) the issuance of 20,000,000 shares of our Common Stock valued at $246,000, and (iii) a promissory note in the principal amount of $450,000 Canadian ($358,407 US), with interest payable at the rate of 3% per annum. We are required to make payments of $10,000 Canadian (approximately $8,000 US) per calendar quarter, due and payable on or before the end of each such calendar quarter through December 31, 2023.
Atlas is a certified company dedicated to chemical analysis of pharmaceutical and other industrial samples. Atlas has 9 full-time employees and generated revenues of approximately $500,000 Canadian (approximately $400,000 US) in 2017. Housed in a 5,250 square foot facility, Atlas’s operations are authorized by a Drug Establishment License (DEL) issued by Health Canada and are fully compliant with the requirements of Good Manufacturing Practices (GMP). Atlas is also registered with the FDA.
Atlas is the owner of a relatively large portfolio of analytical chemistry methodology and Standard Operating Procedure. This intellectual property is protected as company secrets and controlled through employee and management confidentiality agreements.
On June 18, 2018, we purchased laboratory equipment at a total cost of $235,870 Canadian (approximately $181,580 US) for Microbiology Testing as part of our plan to expand the operations services offering of Atlas. Presently, Atlas offers Analytical Chemistry Testing and intends to offer Microbiology Testing soon.
Government Regulations
All of our business operations, including the Generic Pharmaceutical Operations, the Proprietary Drug Development Operations, and our newly acquired Analytical Chemistry Services Operations are subject to extensive and frequently changing federal, state, provincial and local laws and regulations.
In the U.S, the Federal Government agency responsible for regulating drugs is the U.S. Food and Drug Administration (“FDA”). The Canadian counterpart to the FDA is the Health Products and Food Branch (“HPFB”) of Health Canada. Both the FDA and HPFB have similar requirements for a drug to be approved for marketing. In addition, the quality standards for brand name drugs and generic drugs are the same. The ingredients, manufacturing processes and facilities for all drugs must meet the guidelines for Good Manufacturing Practices (“GMP”). Moreover, all drug manufacturers must perform a series of tests, both during and after production, to show that every drug batch made meets the regulatory agency’s requirements for that product.
In connection with our development of the new chemical entity, Adva-27a, we will be subject to significant regulations in the U.S. in order to obtain the approval of the FDA to offer our product on the market. The approximate procedure for obtaining FDA approval involves an initial filing of an IND application following which the FDA would review the application and if all the data are in order and acceptable would give the go ahead for the drug sponsor to proceed with Phase I clinical (human) trials. Following completion of Phase I, the results are filed with the FDA and a request is made to proceed to Phase II. Similarly, following completion of Phase II the data are filed with the FDA and a request is made to proceed to Phase III. Following completion of Phase III, a request is made for marketing approval. Depending on various issues and considerations, the FDA could provide limited marketing approval on a humanitarian basis if the drug treats terminally ill patients with limited treatment options available. As of the date of this Registration Statement on Form S-1 we have not made any filings with the FDA or other regulatory bodies in other jurisdictions. We have however had extensive discussions with clinicians at the McGill University’s Jewish General Hospital in Montreal where we plan to undertake our Phase I study for pancreatic cancer and multidrug resistant breast cancer they believe that Health Canada is likely to grant us a so-called fast-track process on the basis of the terminal nature of the cancer types which we will be treating. There are no assurances this will occur.
Employees
As of the date of this Registration Statement on Form S-1 we have a total of twelve (12) employees. In addition to our management team which is comprised of our three (3) officers and directors, new wholly owned subsidiary acquired on January 1, 2018, Atlas Pharma Inc., has 9 full-time employees. We anticipate that if we receive financing we will need additional employees in both our generic pharmaceutical and proprietary drug development operations including accounting, regulatory affairs, marketing, sales and laboratory personnel.
Competition
In the area of proprietary anticancer drug development, we will be competing with large publicly and privately held companies engaged in developing new cancer therapies. There are numerous other entities engaged in this business that have greater resources, both financial and otherwise, than the resources presently available to us. Nearly all major pharmaceutical companies including Amgen, Roche, Pfizer, Bristol-Myers Squibb and Novartis, to name just a few, have on-going anti-cancer drug development programs and some of the drug they may develop could be in direct competition with our drug. Also, a number of small companies are also working in the area of cancer and could develop drugs that may be in competition with ours. However, none of these competitor companies can use molecules similar to ours as they would be infringing our patents.
The generic pharmaceuticals business is fairly competitive and there are many players in the field including several multinationals such as Teva (Israel), Novartis - Sandoz (Switzerland), Hospira (USA), Mylan (Netherlands), Sanofi (France), Fresenius Kabi (Germany) and Apotex (Canada)with annual sales in the range of approximately $2 billion to over $10. With our offering of Canadian approved generic products, we believe that we will be able to access at least a small percentage of the generic pharmaceuticals market.
Intellectual Property
Effective October 8, 2015, we executed a Patent Purchase Agreement (the “October Purchase Agreement”), with Advanomics, a related party, pursuant to which we acquired all of the right, title and interest in and to U.S. Patent Number 8,236,935 (the “US Patent”) for our anticancer compound, Adva-27a. On December 28, 2015, we executed a second Patent Purchase Agreement (the “December Purchase Agreement”), with Advanomics, pursuant to which we acquired all of the right, title and interest in and to all of the remaining worldwide rights covered by issued and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 (the “Worldwide Patents”) for our anticancer compound, Adva-27a.
Effective December 28, 2015, we entered into amendments (the “Amendments”) of these Purchase Agreements pursuant to which the total purchase price was reduced from $17,142,499 to $618,810, the book value of this intellectual property on the financial statements of Advanomics. Further, the Amendments provided for automatic conversion of the promissory notes representing the new purchase price into an aggregate of 321,305,415 shares of our Common Stock once we increase our authorized capital such that these shares can be issued. In July 2016 we increased our authorized capital and issued the 321,305,415 Common shares to Advanomics thereby completing all aspects of the patent purchase arrangements and securing direct ownership of all worldwide patents and rights pertaining to Adva-27a.
In addition, in 2016 we signed Cross Referencing Agreements with a major pharmaceutical company for four (4) prescription generic drugs for the treatment of Breast Cancer, Prostate Cancer and Enlarged Prostate. These agreements give us the right to register the four (4) generic products, Anastrozole, Letrozole, Bicalutamide and Finasteride in Canada under our own label and obtain a DIN for each in order to be able to place them on the market.
Our new wholly owned subsidiary, Atlas Pharma Inc., which we acquired on January 1, 2018 holds a Drug Establishment License from Health Canada and is registered with the FDA. Atlas Pharma Inc. is the owner of a relatively large portfolio of analytical chemistry methodology and Standard Operating Procedure. This intellectual property is protected as company secrets and controlled through employee and management confidentiality agreements.
Equity Financing Agreement and Registration Rights Agreement
On September 10, 2018, Sunshine Biopharma, Inc., a Colorado corporation (the “Company”), entered into an Equity Financing Agreement (“Equity Financing Agreement”) and Registration Rights Agreement (“Registration Rights Agreement”) with GHS Investments LLC, a Nevada limited liability company (“GHS”). Under the terms of the Equity Financing Agreement, GHS agreed to provide the Company with up to $10,000,000 upon effectiveness of a Registration Statement on Form S-1 (the “Registration Statement”) filed with the U.S. Securities and Exchange Commission (the “Commission”).
Following effectiveness of the Registration Statement, the Company shall have the discretion to deliver puts to GHS and GHS will be obligated to purchase shares of the Company’s Common Stock, par value $0.001 per share (the “Common Stock”) based on the investment amount specified in each put notice. The maximum amount that the Company shall be entitled to put to GHS in each put notice shall not exceed two hundred fifty percent (250%) of theweighted average
daily trading dollar volume of the Company’s Common Stock during the ten (10) trading days preceding the put date, so long as such amount does not exceed $300,000. Pursuant to the Equity Financing Agreement, GHS and its affiliates will not be permitted to purchase and the Company may not put shares of the Company’s Common Stock to GHS that would result in GHS’s beneficial ownership equaling more than 9.99% of the Company’s outstanding Common Stock. Theexercise price of
each put share shall be equal to eighty one percent (81%) of the Market Price (as defined in the Equity Financing Agreement). Puts may be delivered by the Company to GHS until the earlier of thirty-six (36) months after the effectiveness of the Registration Statement or the date on which GHS has purchased an aggregate of $10,000,000 worth of Common Stock under the terms of the Equity Financing Agreement. Additionally, in accordance with the Equity Financing Agreement, the Company shall issue GHS a promissory note in the principal amount of $20,000 to offset transaction costs (the “Note”). The Note bears interest at the rate of 8% per annum, is not convertible and is due on June 30, 2019.$1.94.The Registration Rights Agreement provides that the Company shall (i) use its best efforts to file with the Commission the Registration Statement within 30 days of the date of the Registration Rights Agreement; and (ii) have the Registration Statement declared effective by the Commission within 30 days after the date the Registration Statement is filed with the Commission, but in no event more than 90 days after the Registration Statement is filed.
MARKET PRICE OF THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
Trading of our Common Stock commenced on the OTC MARKETS in September 2007 under the symbol “MWBN.” Effective November 30, 2009, the trading symbol for our Common Stock was changed to “SBFM” as a result of our name change discussed above.
The table below sets forth the reported high and low bid prices for the periods indicated. The bid prices shown reflect quotations between dealers, without adjustment for markups, markdowns or commissions, and may not represent actual transactions in our Common Stock.
| Quarter Ended | | |
| | | |
| March 31, 2016 | $0.0088 | $0.0052 |
| June 30, 2016 | $0.0110 | $0.0061 |
| September 30, 2016 | $0.0039 | $0.0030 |
| December 31, 2016 | $0.0040 | $0.0032 |
| | | |
| March 31, 2017 | $0.0025 | $0.0025 |
| June 30, 2017 | $0.0134 | $0.0110 |
| September 30, 2017 | $0.0155 | $0.0141 |
| December 31, 2017 | $0.0130 | $0.0100 |
| | | |
| March 31, 2018 | $$0.0175 | $0.0079 |
| June 30, 2018 | $0.0087 | $0.0041 |
| September 30, 2018 (through September 26, 2018) | $$0.0078 | $0.0013 |
Trading volume in our Common Stock varies between a few hundred thousand shares to several million shares per day. As a result, the trading price of our Common Stock is subject to significant fluctuations.
Holders of Common Equity
As of the date hereof, there were approximately 146 stockholders of record. An additional number of stockholders are beneficial holders of our Common Stock in “street name” through banks, brokers and other financial institutions that are the record holders.
Dividend Information
We have not paid any cash dividends to our holders of Common Stock or Preferred Stock. The declaration of any future cash dividends is at the discretion of our board of directors and depends upon our earnings, if any, our capital requirements and financial position, our general economic conditions, and other pertinent conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATION
You should read theOPERATIONSThe following discussion ofhighlights the principal factors that have affected our financial condition and results of operations as well as our liquidity and capital resources for the periods described. This discussion should be read in conjunction with our financial statements and the related notes thereto included elsewhere in this Prospectus. The followingprospectus. This discussion contains forward-looking statements that reflect our plans, estimatesstatements. Please see “Cautionary Note Regarding Forward-Looking Statements” for a discussion of the uncertainties, risks and beliefs. Our actual results could differ materially from those discussed in theassumptions associated with these forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Prospectus, particularly in the section labeled “Risk Factors.”
This sectionResults of the Prospectus includes a numberOperations
Comparison of forward-looking statements that reflect our current views with respect to future events and financial performance. Forward-looking statements are often identified by words like “believe,” “expect,” “estimate,” “anticipate,” “intend,” “project,” and similar expressions, or words that, by their nature, refer to future events. You should not place undue certainty on these forward-looking statements, which apply only asresults of the date of this Prospectus. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from historical results or our predictions.
Overview And History
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” Until October 2009, our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies.
In October 2009, we acquired Sunshine Biopharma, Inc., a Colorado corporation holding an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a. As a result of this transaction we changed our name to “Sunshine Biopharma, Inc.” and our officers and directors resigned their positions with us and were replaced by Sunshine Biopharma, Inc.’s management at the time, including our current CEO, Dr. Steve N. Slilaty, and our current CFO, Camille Sebaaly each of whom remain part of our current management. Our principal business became that of a pharmaceutical company focusing on the development of our licensed Adva-27a anticancer compound. In December 2015 we acquired all issued and pending patents pertaining to our Adva-27a technology and terminated the license.
In July 2014, we formed a wholly owned Canadian subsidiary, Sunshine Biopharma Canada Inc. (“Sunshine Canada”),operations for the
purposes of offering generic pharmaceutical productsthree months ended September 30, 2023 and 2022During the three months ended September 30, 2023, we generated $5,957,668 in Canada and elsewhere around the world. Sunshine Canada has signed licensing agreements for four (4) generic prescription drugssales, compared to $132,808 for the treatmentthree months ended September 30, 2022, an increase of breast cancer, prostate cancer and BPH (Benign Prostatic Hyperplasia). We have applied for and are currently awaiting the issuance$5,824,860. The increase is attributable to sales generated by Health Canada of a Drug Establishment License and a Drug Identification Number for each of our four (4) generic products in order to begin marketing of the same.
In January 2018, we acquired Atlas Pharma Inc. (“Atlas”), a certified company dedicated to chemical analysis of pharmaceutical and other industrial samples whose operations are authorized by a Drug Establishment License issued by Health Canada. Atlas has been generating revenues since its inception in September 2013. The revenues reported in our consolidated financial statements for the first calendar quarter of 2018 are a result of the Atlas operations.
In March 2018, we formed NOX Pharmaceuticals, Inc., a Colorado corporation, and assigned all of our interest in our Adva-27a anticancer compound to that company. NOX Pharmaceuticals Inc.’s mission is to research, develop and commercialize proprietary drugs including Adva-27a.
As a result, we are now a holding company operating through these three wholly owned subsidiaries.
Our principal place of business is located at 6500 Trans-Canada Highway, 4th Floor, Pointe-Claire, Quebec, Canada H9R 0A5. Our phone number is (514) 426-6161and our website address is www.sunshinebiopharma.com.
We have not been subject to any bankruptcy, receivership or similar proceeding.
Operations
Proprietary Drug Development Operations
Since inception, our proprietary drug development activities have been focused on the development of a small molecule called Adva-27a for the treatment of aggressive forms of cancer. A Topoisomerase II inhibitor, Adva-27a has been shown to be effective at destroying Multidrug Resistant Cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma cells (Published in ANTICANCER RESEARCH, Volume 32, Pages 4423-4432, October 2012). Sunshine Biopharma Inc. owns all of the rights, as well as, all of the issued and pending worldwide patents pertaining to Adva-27a, including U.S. Patent Number 8,236,935.
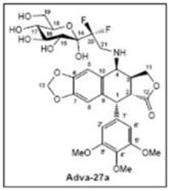
Adva-27a is a GEM-difluorinated C-glycoside derivative of Podophyllotoxin. Another derivative of Podophyllotoxin called Etoposide is currently on the market and is used to treat various types of cancer including leukemia, lymphoma, testicular cancer, lung cancer, brain cancer, prostate cancer, bladder cancer, colon cancer, ovarian cancer, liver cancer and several other forms of cancer. Etoposide is one of the most widely used anticancer drugs. Adva-27a and Etoposide are similar in that they both attack the same target in cancer cells, namely the DNA unwinding enzyme, Topoisomerase II. Unlike Etoposide, and other anti-tumor drugs currently in use, Adva-27a is able to destroy Multidrug Resistant Cancer cells. Adva-27a is the only compound known today that is capable of destroying Multidrug Resistant Cancer. In addition, Adva-27a has been shown to have distinct and more desirable biological and pharmacological properties compared to Etoposide. In side-by-side studies using Multidrug Resistant Breast Cancer cells and Etoposide as a reference, Adva-27a showed markedly improved cell killing activity (see Figure below). Our preclinical studies to date have shown that:
●
Adva-27a is effective at killing different types of Multidrug Resistant cancer cells, including Pancreatic Cancer Cells (Panc-1), Breast Cancer Cells (MCF-7/MDR), Small-Cell Lung Cancer Cells (H69AR), and Uterine Sarcoma Cells (MES-SA/Dx5).
●
Adva-27a is unaffected by P-Glycoprotein, the enzyme responsible for making cancer cells resistant to anti-tumor drugs.
●
Adva-27a has excellent clearance time (half-life = 54 minutes) as indicated by human microsomes stability studies and pharmacokinetics data in rats.
●
Adva-27a clearance is independent of Cytochrome P450, a mechanism that is less likely to produce toxic intermediates.
●
Adva-27a is an excellent inhibitor of Topoisomerase II with an IC50 of only 13.7 micromolar (this number has recently been reduce to 1.44 micromolar as a result of resolving the two isomeric forms of Adva-27a).
●
Adva-27a has shown excellent pharmacokinetics profile as indicated by studies done in rats.
●
Adva-27a does not inhibit tubulin assembly.
These and other preclinical data have been published in ANTICANCER RESEARCH, a peer-reviewed International Journal of Cancer Research and Treatment. The publication which is entitled “Adva-27a, a Novel Podophyllotoxin Derivative Found to Be Effective Against Multidrug Resistant Human Cancer Cells” [ANTICANCER RESEARCH 32: 4423-4432 (2012)] is available on our website atwww.sunshinebiopharma.com.
We have been delayed in our clinical development program due to lack of funding. Our fund raising efforts are continuing and as soon as adequate financing is in place we will continue our clinical development program of Adva-27a by conducting the following next sequence of steps:
●
GMP Manufacturing of 2 kilogram for use in IND-Enabling Studies and Phase I Clinical Trials
●
Regulatory Filing (Fast-Track Status Anticipated)
●
Phase I Clinical Trials (Pancreatic Cancer Indication)
Adva-27a’s initial indication will be Pancreatic Cancer for which there are currently little or no treatment options available. We are planning to conduct our clinical trials at McGill University’s Jewish General Hospital in Montreal, Canada. All aspects of the clinical trials in Canada will employ FDA standards at all levels. We estimate that the Pancreatic Cancer clinical trials will take approximately 18 months from start to finish.
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Generic Pharmaceuticals Operations
In 2016, our Canadian wholly owned subsidiary, Sunshine Biopharma Canada Inc. (“Sunshine Canada”), signed Cross Referencing Agreements with a major pharmaceutical company for four prescription generic drugs for the treatment of Breast Cancer, Prostate Cancer and Enlarged Prostate. Following this acquisition we have been working towards commencement of marketing of these pharmaceutical products under our own Sunshine Biopharma label. These four generic products are as follows:
●
Anastrozole (brand name Arimidex® by AstraZeneca) for treatment of Breast Cancer;
●
Letrozole (brand name Femara® by Novartis) for treatment of Breast Cancer;
●
Bicalutamide (brand name Casodex® by AstraZeneca) for treatment of Prostate Cancer;
●
Finasteride (brand name Propecia® by Merck) for treatment of BPH (Benign Prostatic Hyperplasia)
Worldwide sales of the brand name version of these products as reported by the respective pharmaceutical company, owner of the registered trademark are as follows:
In June 2017, Sunshine Canada submitted an application to Health Canada for the procurement of a Drug Establishment License (“DEL”), a requirement for the Company’s drug handling and pharmaceutical operations. Health Canada has assigned the Company DEL Application No. 3002475 and File No. 17938. We are currently awaiting Health Canada to set a date for physical inspection of our warehouse and drug management operations. In addition, we are currently in the process of filing applications for a Drug Identification Number (“DIN”) for each of its four (4) generic products, Anastrozole, Letrozole, Bicalutamide and Finasteride. The Figure below shows our 30-Pill blister pack of Anastrozole.

We currently have twenty three (23) additional Generic Pharmaceuticals under review for in-licensing. We believe that a larger product portfolio will provide us with more opportunities and a greater reach into the marketplace. Our plan is to fund our Proprietary Drug Development Program, including Adva-27a, through the sales of Generic Drugs. In addition to near-term revenue generation, building the generics business infrastructure and securing the proper permits will render us appropriately positioned for the marketing and distribution of our own proprietary drugs as they become available.
Analytical Chemistry Services Operations
On January 1, 2018, we entered into an agreement (the “Atlas Agreement”) to acquire AtlasNora Pharma, Inc. (“Atlas”). The purchase price was $848,000 Canadian ($684,697 US). Payment of the purchase price was comprised of (i) a cash payment of $100,500 Canadian ($80,289 US), (ii) the issuance of 20,000,000 shares of our Common Stock valued at $246,000, and (iii) a promissory note in the principal amount of $450,000 Canadian ($358,407 US), with interest payable at the rate of 3% per annum. We are required to make payments of $10,000 Canadian (approximately $8,000 US) per calendar quarter, due and payable on or before the end of each such calendar quarter through December 31, 2023.
Atlas is a certified company dedicated to chemical analysis of pharmaceutical and other industrial samples. Atlas has 9 full-time employees and generated revenues of approximately $500,000 Canadian (approximately $400,000 US) in 2017. Housed in a 5,250 square foot facility, Atlas’s operations are authorized by a Drug Establishment License (DEL) issued by Health Canada and are fully compliant with the requirements of Good Manufacturing Practices (GMP). Atlas is also registered with the FDA.
Atlas is the owner of a relatively large portfolio of analytical chemistry methodology and Standard Operating Procedure. This intellectual property is protected as company secrets and controlled through employee and management confidentiality agreements.
On June 18, 2018, we purchased laboratory equipment at a total cost of $235,870 Canadian (approximately $181,580 US) for Microbiology Testing as part of our plan to expand the operations services offering of Atlas. Presently, Atlas offers Analytical Chemistry Testing and intends to offer Microbiology Testing soon.
Results Of Operations
Comparison of Results of Operations for the six months ended June 30, 2018 and 2017
During the six months ended June 30, 2018, we generated revenues of $198,418 from the operations of our new wholly owned subsidiary, Atlas Pharma Inc. (“Atlas”), which we acquired on January 1, 2018.in October 2022. The direct cost for generating these revenuessales was $190,913, which$3,967,412 (66.6%) for the three months ended September 30, 2023, compared to $65,783 (49.5%) for the three months ended September 30, 2022. The increase in the cost of goods sold in 2023 is compriseddue to increased cost of salaries ($113,495), laboratory supplies ($24,264), rent ($37,960) and depreciation ($15,194). We did not generate any revenues duringmanufacturing of the comparable period in 2017.
generic prescription drugs sold by Nora Pharma. Our gross profit grew to $1,990,256 for the three months ended September 30, 2023, compared to $67,025 for the three months ended September 30, 2022.General and administrative expenseexpenses during the six monthsthree-month period ended JuneSeptember 30, 2018 was $745,153,2023, were $2,769,730, compared to $581,208$1,785,005 during the six monthsthree-month period ended JuneSeptember 30, 2017,2022, an increase of $163,945. The principal reason for this$984,725. This increase was an increase of $133,051 in executive compensation, as well as increases in accounting, legal and office expenses. These increases were athe result of increased overhead associated with being a Nasdaq listed company and expenses related to Nora Pharma operations. Specifically, we incurred increased costs in consulting ($58,929), office ($467,397), salaries ($549,377) and taxes ($52,586). Overall, we incurred a loss of $779,474 from our increased business activities relatingoperations for the three months ended September 30, 2023, compared to Atlas, as well asa loss of $1,717,980 from our continuing efforts to raise additional funding. The only category that saw a decrease was consulting fees by $32,067, as efforts were made to complete more work in-house.
We incurred $93,338operations in
losses arising from debt conversionthe three-month period ended September 30, 2022.In addition, we had net interest income of $168,904 during the sixthree months ended JuneSeptember 30, 2018,2023, compared to $76,929 in losses from debt conversiona net interest income of approximately $260,936 during the similar period in 2017three months ended September 30, 2022, as a result of some convertible notes having been paid off or reduced prior to maturity.
interest earned on cash on hand.As a result, we incurred a net loss of $909,944$651,482 ($0.000.04 per share) for the six month periodthree months ended JuneSeptember 30, 2018,2023, compared to a net loss of $681,146$1,457,019 ($0.000.08 per share) duringfor the six monththree-month period ended JuneSeptember 30, 2017.
2022.Comparison of Resultsresults of Operationsoperations for the threenine months ended JuneSeptember 30, 20182023 and 2017
For2022During the threenine months ended JuneSeptember 30, 2018,2023, we generated $107,250 in revenues of $16,412,586, compared to no revenues of $405,760 for the same threenine months ended September 30, 2022, an increase of 2017. All of these revenues were$16,006,826. The increase is attributable to sales generated from the operations ofby our newrecently acquired wholly owned subsidiary, Atlas Pharma Inc. (“Atlas”), which we acquired on January 1, 2018.Nora Pharma. The direct cost for generating these revenues was $91,631, which$10,641,461 (64.8%) for the nine months ended September 30, 2023, compared to $200,311 (49.4%) for the nine months ended September 30, 2022. The increase in the cost of goods sold in 2023 is compriseddue to increased cost of salaries ($55,937), laboratory supplies ($10,194), rent ($16,438) and depreciation ($9,062). We did not generate any revenues duringmanufacturing of the comparablegeneric prescription drugs sold by Nora Pharma. Our gross profit increased to $5,771,125 for the nine months ended September 30, 2023, compared to a gross profit of $205,449 for the same period in 2017.
2022.General and administrative expenses during the three monthnine-month period ended JuneSeptember 30, 20182023, were $586,570,$9,369,203 compared to general and administrative expense of $472,218 incurred$3,842,589 during the three monthnine-month period ended JuneSeptember 30, 2017,2022, an increase of $114,352.$5,526,614. This increase is attributable to an increase in executive compensationwas the result of $98,229, as well as increases accounting fees, legal fees, and office expenses due to costsincreased overhead associated with being a Nasdaq listed company and expenses related to Nora Pharma operations. Specifically, we incurred increased costs in accounting ($63,608), consulting ($475,817), office costs ($972,328), research and development ($269,407), salaries ($3,239,801) and taxes ($212,953). Overall, we incurred a loss of $3,598,078 from our operations in the acquisition of Atlas. The only category that saw a decrease was consulting fees by $10,248, as efforts were made to complete more work in-house.
We also incurred $28,375 in interest expense during the three monthsnine-month period ended
JuneSeptember 30,
2018,2023, compared to
$9,598a loss from operations of $3,637,140 in
interest expense during the similar period
in 2017of 2022.In addition, we had net interest income of $517,163 during the nine months ended September 30, 2023, compared to a net interest income of $394,118 during the nine months ended September 30, 2022, as a result of increased borrowings. However, we incurred $54,998 in losses arising from debt conversion during the three months ended June 30, 2018, compared to $0 in losses from debt conversion during the similar period in 2017 as a result of some convertible notes having been paid off prior to maturity in the same period of 2017.
interest earned on cash on hand.As a result, we incurred a net loss of $644,308$3,256,020 ($0.000.12 per share) for the three monthnine-month period ended JuneSeptember 30, 2018,2023, compared to a net loss of $485,444$3,232,125 ($0.000.26 per share) duringfor the three monthnine-month period ended JuneSeptember 30, 2017.
2022.Comparison of Results of Operations for the fiscal years ended December 31, 2022 and 2021
During our fiscal year ended December 31, 2022, we generated revenues of $4,345,603, compared to revenues of $228,426, in 2021. The increase was the result of our acquisition of Nora Pharma in October 2022, which accounted for $3,803,106 of these revenues. The cost of sales in 2022 and 2021 for generating these revenues was $2,649,028 and $117,830, respectively.
General and administrative expenses for our fiscal year ended December 31, 2022, were $28,697,325, compared to $2,550,730 during our fiscal year ended December 31, 2021, an increase of $26,146,595. The increase was largely a result of goodwill impairment of $18,326,719 and costs and expenses relating to the Nora Pharma acquisition.
We also incurred $39,412 in interest expense and $0 in losses from debt conversion in 2022, compared to $328,818 in interest expense and $9,726,485 in losses from debt conversion in 2021. The decrease in interest expense and losses from debt conversion in 2022 was due to our repayment of all outstanding debt in 2022.
As a result, we incurred a net loss of $26,511,136 for the year ended December 31, 2022, compared to a net loss of $12,436,447 for the year ended December 31, 2021.
Liquidity and Capital Resources
As of JuneSeptember 30, 2018,2023, we had cash or cash equivalents of $36,395.
$18,846,140.Net cash used in operating activities was $292,898$6,085,435 during the six monthnine months ended September 30, 2023, compared to $3,001,746 during the nine-month period ended JuneSeptember 30, 2018,2022. The increase was a result of the addition of Nora Pharma’s operations.
Cash flows used in investing activities were $386,920 for the nine months ended September 30, 2023, compared to $185,850$0 for the six month periodnine months ended JuneSeptember 30, 2017. We anticipate that overhead costs and other expenses will2022. The increase was the result of cash invested in the future as we move forward with our proprietary drug development activities and expansion of our generic pharmaceuticals operations as well as our newly acquired analytical chemistry services operations as discussed above.
Nora Pharma.Cash flows fromprovided by financing activities were $249,975 for$3,456,106 during the six month periodsnine months ended JuneSeptember 30, 2018,2023, compared to $275,665$41,561,363 during the sixnine months ended JuneSeptember 30, 2017. Cash flows used by investing activities were $22,428 for2022. The decrease was primarily as a result of one offering made during the six month periodnine months ended JuneSeptember 30, 20182023, compared to $22,295 duringthree offerings completed in February, March, and April 2022, and to a lesser extent due to our repurchase of a total of $540,629 in common stock in the same six month period in 2017.first and third quarter of 2023.
As of December 31, 2022, we had cash and cash equivalents of $21,826,437.
On February 17, 2022, we completed an underwritten public offering of common stock and warrants for gross proceeds of $8 million. We received net proceeds of approximately $6.8 million from the offering.
On March 14, 2022, we completed a private placement of common stock and warrants for gross proceeds of $8 million. We received net proceeds of approximately $6.8 million from the private placement.
On April 28, 2022, we completed a private placement of common stock and warrants for gross proceeds of approximately $19.5 million. We received net proceeds of approximately $16.8 million from the private placement.
During the three months periodfiscal year ended June 30, 2018,December 31, 2022, we received aggregate proceeds of $13,193,177 in connection with warrant exercises.
During the year ended December 31, 2021, we issued a total of 185,369,308559,144 shares of our Common Stock. Of these, 42,584,566 sharescommon stock valued at $290,039 were issued upon$12,705,214 for the conversion of outstanding notes payable, reducing the debt by $188,568$2,867,243 and interest payable by $8,133$127,986 and generating a loss on conversion of $93,585. In addition,$9,726,485.
During the year ended December 31, 2021, we issued 20,000,000 sharesdid not sell any of our Common Stock valued at $246,000 or $0.0123 per share as partcapital stock for cash; however, we entered into the following new debt arrangements:
| · | On January 12, 2021, we issued a note in the principal amount of $150,000 with interest accruing at 5% per year, due January 12, 2023. The note was convertible after 180 days from issuance into common stock at a price of $0.30 per share. This note was converted to common stock on December 20, 2021. |
| | |
| · | On January 27, 2021, we issued a note in the principal amount of $300,000 with interest accruing at 5% per year, due January 27, 2023. The note was convertible after 180 days from issuance into common stock at a price equal to $0.50 per share. This note was converted to common stock on December 20, 2021. |
| | |
| · | On February 12, 2021, we issued a note in the principal amount of $700,000 with interest accruing at 5% per year, due February 12, 2023. The note was convertible after 180 days from issuance into common stock at a price of $0.60 per share. This note was converted to common stock on December 20, 2021. |
| | |
| · | On April 5, 2021, we issued a note in the principal amount of $330,000 with interest accruing at 10% per year, due January 5, 2022. The note was convertible after 180 days from issuance into common stock at a price 35% below market value. On October 13, 2021, the noteholder converted $330,000 in principal and $16,500 in accrued interest into 26,250 shares of common stock leaving a principal balance of $0. We repaid this note. |
| | |
| · | On April 20, 2021, we issued a note in the principal amount of $500,000 with interest accruing at 5% per year, due April 20, 2023. The note was convertible after 180 days from issuance into common stock at a price of $0.30 per share. We repaid this note following the closing of our public offering in February 2022. |
| | |
| · | On July 6, 2021, we issued a note in the principal amount of $900,000 with interest accruing at 5% per year, due July 6, 2023. The note was convertible after 180 days from issuance into common stock at a price of $0.30 per share. We repaid this note following the closing of our public offering in February 2022. In connection with this debt financing, we agreed to allow the lender, who is also the holder of a note dated November 25, 2020, to convert a total of $240,000 in principal into 120,000 shares of common stock leaving a principal balance of $10,000 and accrued interest of $7,750. On July 6, 2021, we paid off the remaining principal balance of this note and received forgiveness of the accrued interest. |
| | |
| · | On August 18, 2021, we issued a note in the principal amount of $500,000 with interest accruing at 5% per year, due August 18, 2023. The note is convertible after 180 days from issuance into common stock at a price equal to $0.30 per share. We repaid this note following the closing of our public offering in February 2022. |
Cash flows used in investing activities were $14,619,390 during the year ended December 31, 2022, compared to $0 during our fiscal year ended December 31, 2021. The reason for the increase was due to the acquisition of Atlas Pharma Inc.
On January 12, 2018, we received net proceedsNora Pharma. Net cash flows provided by financing activities were $39,465,107 in 2022 compared to $2,904,675 in 2021. The increase was primarily a result of $100,000 in exchange for a note payable having a face value of $102,000 and accruing interest at the rate of 8% per annum. The note, due on October 30, 2018, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. We estimate that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On February 7, 2018, we received net proceeds of $142,500 in exchange for a note payable having a face value of $150,000 and accruing interest at the rate of 8% per annum. The note, due on February 7, 2019, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. We estimate that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On February 22, 2018, we received net proceeds of $83,000 in exchange for a note payable having a face value of $85,000 and accruing interest at the rate of 8% per annum. The note, due on November 30, 2018, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. We estimate that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On May 29, 2018, we received net proceeds of $25,000 in exchange for a note payable having a face value of $26,750 and accruing interest at the rate of 8% per annum. The note, due on February 28, 2019 is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. We estimate that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On June 27, 2018, we issued a note payable having a face value of $53,000 and accruing interest at the rate of 8% per annum. The net proceeds of $51,000 from this note were received by us on July 2, 2018. The note, due on April 15, 2019 is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. We estimate that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
During the six month period ended June 30, 2018, the holders of certain notes payable converted principal and interest of $290,039 into 42,584,566 shares of Common Stock.
As part of a subscription agreement entered into in 2016, we had an obligation to pay a royalty of 5% of net sales on one of one of our generic products (Anastrozole) for a period of three (3) years fromrounds of financing which took place in February, March, and April 2022. Net cash used in operations was $5,248,358 in 2022, compared to $1,829,128 in 2021. The reason for the dateincrease was the acquisition of the first sale of that product. On May 28, 2018 we issued 1,000,000 shares of our Common Stock valued at $5,900 in exchange for cancellation of this royalty obligation.
Nora Pharma.We are not generating adequate revenues from our operations to fully implement our business plan as set forth herein. AsOn February 17, 2022, we received net proceeds of approximately $6.8 million from the sale of common stock and warrants in an underwritten public offering. On March 14, 2022, we received net proceeds of approximately $6.8 million from the sale of common stock and warrants in a result,private placement. On April 28, 2022, we received net proceeds of approximately $16.8 million from the sale of common stock and warrants in a private placement. On May 16, 2023, we received net proceeds of approximately $4.1 million from the sale of common stock and warrants in a private placement. We believe our future success will depend on the future availability of financing, among other things. Such financingexisting cash will be requiredsufficient to enable usfund our operations, including general and administrative expenses, research and development activities, and the generic pharmaceuticals sales business, for the next 18 to expand24 months. There is no assurance our Analytical Chemistry Services business and further develop our Generic Pharmaceuticals operations and Proprietary Drug Development program. We intend to raise funds through private placements of our Common Stock and/or debt financing. We estimateestimates will be accurate.
Management estimates that we will requireneed additional capital in the amount of approximately $7 million ($2$30 million for the Analytical Chemistryexpansion of our drug development activities and Generic Pharmaceuticalsgeneric pharmaceuticals operations, including possibly a Phase I clinical trial. Additional capital may not be available on terms acceptable to us, or at all. Currently, we do not have any committed arrangements for financing and $5 million for the Proprietary Drug Development program) to fully implement our business plan in the future and there arecan provide no assurancesassurance that we will be able to raise this capital.obtain financing when required. No assurance can be given that we will obtain access to capital markets in the future or that financing, adequate to satisfy the cash requirements of implementing our business will be available on acceptable terms. Our inability to obtain sufficient funds from external sources when needed willacceptable financing could have a materialan adverse effect on our plan of operation,upon the results of our operations and financial condition. Our plan is to fund our Proprietary Drug Development Program, including Adva-27a, through the sales of Generic Drugs if we are unable to find any additional financing. There are also no assurances that we will generate sufficient revenues and profits from our Proprietary Drug Development Program to accomplish these objectives.
Our cost of operations is expected to increase as we move forward with implementation of our business plan. We do not have sufficient funds to cover the anticipated increase in the relevant expenses. We need to raise additional capital in order to continue our existing operations and finance our expansion plans for the next year. If we are successful in raising additional funds, we expect our operations and business efforts to continue and expand. There are no assurances this will occur.
In August 2017 we signed an agreement with Jitney Trade Inc. (“Jitney”), a Canadian broker-dealer, to raise up to $10 million Canadian (approximately $8 million US) in a private offering being undertaken only in Canada (the “Offering”) in order to provide the funding we have estimated we need to implement our business plan. The Offering expired on February 28, 2018 without any funds having been raised. On May 3, 2018, we signed an agreement with Jitney Trade Inc. whereby the parties agreed to extend the proposed equity financing that was previously announced of up to $10,000,000 Canadian (approximately $8,000,000 US), until August 31, 2018. The terms and conditions of the financing remained unchanged. We intend to offer at up to 400,000,000 shares of our Common Stock at a price of $0.025 Canadian (approximately $0.02 US) per share. As of the date of this report no funds have been raised. There are no assurances that Jitney will sell any shares of our Common Stock in this proposed offering.
On July 5, 2018, the holder of a note payable dated November 14, 2017 elected to convert $20,000 in principal into 6,505,122 shares of Common Stock leaving a principal balance of $93,000.
On July 11, and August 2, 2018, the holder of a note payable dated October 26, 2017 elected to convert a total of $44,000 in principal and $2,531 in accrued interest into 20,821,004 shares of Common Stock leaving a principal balance of $23,000.
On July 17, 23, 26, and August 2, 7, and 10, 2018, the holder of a note payable dated January 12, 2018 elected to convert a total of $81,000 in principal into an aggregate of 47,805,452 shares of Common Stock leaving a principal balance of $21,000.
On September 10, 2018, Sunshine Biopharma, Inc., a Colorado corporation (the “Company”), entered into an Equity Financing Agreement (“Equity Financing Agreement”) and Registration Rights Agreement (“Registration Rights Agreement”) with GHS Investments LLC, a Nevada limited liability company (“GHS”). Under the terms of the Equity Financing Agreement, GHS agreed to provide the Company with up to $10,000,000 upon effectiveness of a Registration Statement on Form S-1 (the “Registration Statement”) filed with the U.S. Securities and Exchange Commission (the “Commission”).
Following effectiveness of the Registration Statement, the Company shall have the discretion to deliver puts to GHS and GHS will be obligated to purchase shares of the Company’s Common Stock, par value $0.001 per share (the “Common Stock”) based on the investment amount specified in each put notice. The maximum amount that the Company shall be entitled to put to GHS in each put notice shall not exceed two hundred fifty percent (250%) of the average daily trading dollar volume of the Company’s Common Stock during the ten (10) trading days preceding the put date, so long as such amount does not exceed $300,000. Pursuant to the Equity Financing Agreement, GHS and its affiliates will not be permitted to purchase and the Company may not put shares of the Company’s Common Stock to GHS that would result in GHS’s beneficial ownership equaling more than 9.99% of the Company’s outstanding Common Stock. The price of each put share shall be equal to eighty one percent (81%) of the Market Price (as defined in the Equity Financing Agreement). Puts may be delivered by the Company to GHS until the earlier of thirty-six (36) months after the effectiveness of the Registration Statement or the date on which GHS has purchased an aggregate of $10,000,000 worth of Common Stock under the terms of the Equity Financing Agreement. Additionally, in accordance with the Equity Financing Agreement, the Company shall issue GHS a promissory note in the principal amount of $20,000 to offset transaction costs (the “Note”). The Note bears interest at the rate of 8% per annum, is not convertible and is due on June 30, 2019.
The Registration Rights Agreement provides that the Company shall (i) use its best efforts to file with the Commission the Registration Statement within 30 days of the date of the Registration Rights Agreement; and (ii) have the Registration Statement declared effective by the Commission within 30 days after the date the Registration Statement is filed with the Commission, but in no event more than 90 days after the Registration Statement is filed.
Off Balance Sheet Arrangements
None
Fiscal Years Ended 2017 And 2016
Results of Operations
Comparison of Results of Operations for the fiscal years ended December 31, 2017 and 2016
During our fiscal years ended December 31, 2017 and 2016, we did not generate any revenues.
Total expenses for our fiscal year ended December 31, 2017 were $857,190, compared to $993,108 during our fiscal year ended December 31, 2016, a decrease of $135,918. The expense categories that saw a decrease were consulting fees by $80,388, amortization and depreciation by $54,102, research and development by $32,793, and licenses by $19,203. The decreases in these categories of expenses were offset to some extent by relatively modest increases in legal fees, accounting fees and officer and director compensation. The decrease in consulting fees in 2017 was due to the fact that a substantial amount of the work required for setting up the generic pharmaceuticals operations had been completed. Similarly, we incurred no licensing fees in 2017 as we acquired the Adva-27a rights and as a result, terminated the License Agreement we had for the same with Advanomics Corporation. The license expense of $19,203 we paid in 2016 was incurred in order to obtain the rights for our four (4) generic products.
We also incurred $104,829 in interest expense and $76,929 in losses from debt conversion during the year ended December 31, 2017, compared to $34,732 in interest expense and $1,945,898 in losses from debt conversion during the similar period in 2016. In addition, we incurred a loss of $556,120 in 2016 as a result of impairment of the patents we purchased in 2015.
As discussed elsewhere in this Registration Statement on Form S-1, on October 8, 2015, we acquired U.S. Patent Number 8,236,935 (the “US Patent”) for the anticancer compound, Adva-27a from a related entity (Advanomics Corporation), which includes all rights to this intellectual property within the United States, in exchange for an interest-free note payable for $4,320,000 (the “October Patent Purchase Agreement”). On December 28, 2015, we acquired the remaining worldwide issued and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 (the “Worldwide Patents”) for the Adva-27a anticancer compound from the same related entity (Advanomics Corporation) in exchange for a note payable for $12,822,499 (the “December Patent Purchase Agreement”).
We believe that purchase of the US Patent and the Worldwide Patents (the “Patents”) would facilitate our ability to obtain the funding necessary to complete the development and FDA approval process for Adva-27a. As a related party transaction, purchased patents are required to be recorded at the purchase price or the book value on the seller’s financial statements, whichever is lower. Effective December 28, 2015, the parties agreed to amend the October Patent Purchase Agreement and the December Patent Purchase Agreement. Pursuant to the amendment agreements (the “Amendments”), the Patents were purchased from a related party, Advanomics Corporation, at Advanomics’ cost less the amortization through December 28, 2015, the effective date of the transfer. The Amendments amended the purchase price of the Patents to $835,394, eliminated all cash payments obligations and replaced the non-convertible notes totaling $17,142,499 with two (2) convertible notes totaling $835,394 that automatically convert into an aggregate of 321,305,415 shares of our Common Stock. We needed to amend our Articles of Incorporation to establish additional authorized common shares in order to issue this stock. In July 2016, having completed the increase of our authorized capital to 3 billion shares of Common Stock, we issued the 321,305,415 Common Shares to Advanomics thereby completing all aspects of the patent purchase arrangements and securing direct ownership of all worldwide patents and rights pertaining to Adva-27a.
In 2016, following a review of the status of our intellectual property, the remaining value of the Patents ($556,120) on our Balance Sheet was impaired as required under applicable accounting rules.
As a result, we incurred a net loss of $3,496,687 (approximately $0.01 per share) for the year ended December 31, 2016, compared to a net loss of $1,040,236 (approximately $0.00 per share) during the year ended December 31, 2017.
Because we did not generate revenue in the last two years, following is our Plan of Operation.
Plan of Operation
As of the date of this report we are operating through the following wholly owned subsidiaries:
●
NOX Pharmaceuticals, Inc., a recently formed Colorado company focused on the research, development and commercialization of proprietary drugs for the treatment of cancer including Adva-27a, a multi-purpose anti-tumor compound targeted for the treatment of multidrug resistant cancer;
●
Sunshine Biopharma Canada Inc., a Canadian company formed in July 2014, which offers generic prescription drugs for the treatment of cancer and other acute and chronic indications; and
●
Atlas Pharma Inc., a Canadian company acquired in January 2018, offers certified chemical analysis of pharmaceutical and other industrial samples.
NOX Pharmaceuticals, Inc. and Atlas Pharma Inc. are not included in the 2017 and 2016 financials.
See Business, above, for a more detailed description of these businesses.
Liquidity and Capital Resources
As of December 31, 2017, we had cash or cash equivalents of $107,532.
Net cash used in operating activities was $543,520 during our fiscal year ended December 31, 2017, compared to $314,182 during our fiscal year ended December 31, 2016. We anticipate that our cash requirements for our operations will increase in the future before we reach profitability levels.
Cash flows used in investing activities were $84,008 during our fiscal year ended December 31, 2017. For the fiscal year ended December 31, 2016, cash flows used in investing activities were $3,439 arising primarily out of the purchase of laboratory and generic drugs warehouse equipment in 2017. Net cash flows provided by financing activities totaled $670,705 in 2017, compared to $324,622 during our fiscal year ended December 31, 2016.
We have issued convertible and non-convertible notes to both related and unaffiliated parties in order to fund our operations.
In December 2016, we received monies from our CEO in exchange for a note payable having a principal amount of $90,000 Canadian ($67,032 US) with interest at 12% due March 31, 2017. The note was convertible any time after the date of issuance into shares of our Common Stock at a price 35% below market value. At the time, this note was collateralized by all of our assets. In the event of default, the interest rate will increased to 18% per annum and a penalty of $1,000 Canadian ($752 US) per day will accrue. On March 31, 2017, the note, together with accrued interest of $3,021 Canadian ($2,271 US) and an additional principal amount of $3,000 Canadian ($2,247 US) was renewed for a 90-day period under the same terms and conditions as the original note. The new note then having a face value of $96,021 Canadian ($72,198 US) was due on June 30, 2017. On June 30, 2017, the note, together with accrued interest of $2,873 Canadian ($2,005 US), was renewed for a 90-day period under the same terms and conditions as the original note except that the new note was not- convertible. The new note then having a face value of $98,894 Canadian ($76,072US) was due on September 30, 2017. On September 30, 2017, the note, together with accrued interest of $2,991 Canadian ($2,397 US) was renewed for a 90-day period under the same terms and conditions as the June 2017 note. The note, then having a principal balance of $101,885 Canadian ($81,640 US) matured December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as the September 2017 note except that this new note is unsecured and nonconvertible. The new note has a face value of $104,942 Canadian ($83,649 US) and matures on December 31, 2018.
A note payable held by a private individual who subsequently became a principal shareholder of our Company, having a face value of $100,000 at December 31, 2016 and a maturity date of March 31, 2017, accrues interest at 12%. The Note is convertible any time from the date of issuance into shares of our Common Stock at a 35% discount from market price. On March 31, 2017, the note’s principal balance of $100,000 plus accrued interest of $11,715 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note then having a face value of $111,715 matured on June 30, 2017. On June 30, 2017, the note’s principal balance of $111,715, plus accrued interest of $3,342 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note then had a face value of $115,057 and matured on September 30, 2017. On September 30, 2017, the note’s principal balance of $115.057 plus accrued interest of $3,480 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note then had a principal balance of $118,537, which matured on December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as before. The new note has a face value of $122,093 and matures on December 31, 2018.
A Note Payable having a Face Value of $21,439 at December 31, 2016, and accruing interest at 12% was due December 31, 2017. On December 31, 2017, we renewed the note, together with accrued interest of $2,573, for a 12-month period. The new note has a Face Value of $24,012 and accrues interest at 12%. This note is convertible anytime from the date of issuance into shares of our Common Stock at a 35% discount from market price and is due December 31, 2018.
On April 1, 2017, we received monies in exchange for a note payable having a Face Value of $100,000 Canadian ($79,710 US) with interest payable quarterly at 9%, which is due April 1, 2019. The note is convertible any time after issuance into shares of our Common Stock at a price of $0.015 Canadian (approximately $0.012 US) per share.
On September 22, 2017, we received monies in exchange for a note having a Face Value of $62,000, with interest accruing at 8%, which is due June 30, 2018. The note is convertible after 180 days from issuance into shares of our Common Stock at a price 35% below market value.
On October 26, 2017, we received monies in exchange for a note payable having a Face Value of $115,000 with interest accruing at 8%, which is due October 26, 2018. The note is convertible after 180 days from issuance into shares of our Common Stock at a price 35% below market value.
On November 14, 2017, we received monies in exchange for a note payable having a Face Value of $113,000 with interest accruing at 8%, which is due November 14, 2018. The note is convertible after 180 days from issuance into shares of our Common Stock at a price 35% below market value.
On December 1, 2017, we received monies in exchange for a note payable having a Face Value of $50,000 Canadian ($39,855 US) with interest accruing at 8%, due November 30, 2018. The note is convertible after 180 days from issuance into shares of our Common Stock at a price 35% below market value.
On February 10, 2017, we received monies in exchange for a note payable having a Face Value of $50,000 with interest accruing at 8%, which is due November 20, 2017. The note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. In August 2017, the note was paid off with an additional $17,422 as a prepayment penalty.
On April 26, 2017, we received monies in exchange for a note payable having a Face Value of $ 65,000 with interest accruing at 8%, which is due April 26, 2018. The note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. In October 2017, we issued payment in the amount of $85,107 to pay off the note including a prepayment penalty of $20,107.
On August 3, 2017, we received monies in exchange for a note payable having a Face Value of $80,000 with interest accruing at 8%, which is due August 3, 2018. The note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value.
On August 21, 2017, we received monies in exchange for a note payable having a Face Value of $83,000 with interest accruing at 8%, which is due May 30, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. In February 2018, the note was paid off with an additional $32, 370 as a prepayment penalty.
On July 1, 2016, we received monies in exchange for a note payable having a Face Value of $55,000 with interest accruing at 10%, which is due April 1, 2017. The note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 40% below market value. In December 2016 and January 2017, the note, together with $3,022 in accrued interest, was fully converted into 47,528,125 shares of our Common Stock.
During the fiscal year ended December 31, 2017, we issued an aggregate of 149,336,640 shares of our Common Stock as follows:
●
40,000,000 shares for cash in the amount of $100,000 Canadian or $78,312 US
●
11,004,167 shares for the purchase of laboratory and generic drugs warehouse equipment valued at $56,700
●
42,000,000 shares valued at $336,000 as compensation to the Company’s Directors and Officers
●
13,804,348 shares for services rendered to the Company by third parties valued at $77,000
●
42,528,125 shares valued at $128,451 in connection with the conversion of $48,500 in debt and interest of $3,022 resulting in a $76,929 loss on conversion
Except as indicated, we relied upon the exemption from registration provided by Regulation D and Section 4(a)(1) of the Securities Act of 1933, as amended, to issue the respective shares.
We are not generating revenue from our operations, and our ability to implement our business plan as set forth herein will depend on the future availability of financing. Such financing will be required to enable us to further develop our generic pharmaceuticals business, proprietary drug development program, and analytical chemistry operations acquired in January 2018. We intend to raise funds through private placements of our Common Stock and/or debt financing. We estimate that we will require approximately $8 million ($1 million for the generic pharmaceutical operations, $1 million for expansion of the analytical chemistry operations, and $6 million for the proprietary drug development program) to fully implement our business plan in the future and there are no assurances that we will be able to raise this capital.
In late 2017 we signed an agreement with Jitney Trade Inc. (“Jitney”), a Canadian broker-dealer, to raise up to $10 million Canadian (approximately $8 million US) in a private offering being undertaken only in Canada (the “Offering”) in order to provide the funding we have estimated we need to implement our business plan. The Offering expired on February 28, 2018 without any funds having been raised. As of the date of this Registration Statement on Form S-1, we are engaged in negotiations with Jitney concerning the terms for extending the Offering. There are no assurances that any funds will be raised for us in this situation.
Our cost to continue operations as they are now conducted is nominal, but these are expected to increase as we move forward with implementation of our enhanced business plan. We do not have sufficient funds to cover the anticipated increase in the relevant expenses. We need to raise additional funds in order to continue our existing operations and planned expansions.
Going Concern
Our financial statements accompanying this Registration Statement on Form S-1 have been prepared assuming that we will continue as a going concern, which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The financial statements do not include any adjustment that might result from the outcome of this uncertainty. We have a minimal operating history and minimal revenues or earnings from operations. We have no significant assets or financial resources. We will, in all likelihood, sustain operating expenses without corresponding revenues for the immediate future. See “Financial Statements and Notes.”
Inflation
Although our operations are influenced by general economic conditions, we do not believe that inflation had a material effect on our results of operations during our fiscal year ended December 31, 2017.
Critical Accounting Policies and Estimates
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We follow the guidance in SFAS No. 13 “
AccountingASC 842 “Accounting for Leases,,” as amended, which requires us to evaluate the lease agreements we enter into to determine whether they represent operating or capital leases at the inception of the lease.Our wholly owned subsidiary, Nora Pharma, currently occupies a 23,500 square foot facility located at 1565 Boulevard Lionel-Boulet, Varennes, Quebec, Canada, J3X 1P7 pursuant to a lease agreement that expires in January 2030, with an option to extend for 5 years. This site is composed of 18,500 square feet of warehouse space and 5,000 square feet of executive office space. The facility houses all administrative, marketing, quality control, regulatory affairs, and other operations personal, as well as a Health Canada licensed warehouse space. We pay a monthly rent of $27,250 CAD (approximately $19,900 USD), including taxes.
Recently Adopted Accounting Standards
In November 2016,February 2020, the FASB issued ASU 2020-02, Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842) - Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 2016-17, Income Taxes119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic 740): Balance Sheet Classification of Deferred Taxes. The standard requires that deferred tax assets and liabilities be classified as noncurrent on842) which amends the balance sheet rather than being separated into current and noncurrent. ASU 2016-17 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2017. Early adoption is permitted and the standard may be applied either retrospectively or on a prospective basis to all deferred tax assets and liabilities. We adopted ASU 2016-17 during our first quarterdate of the year ended December 31, 2017, on a retrospective basis. The adoption of 2016-17 had no impact on our financial statements.
In February 2017, the FASB issuedoriginal pronouncement for smaller reporting companies. ASU No. 2017-02, Leases (Topic 842), to provide guidance on recognizing lease assets2016-13 and lease liabilities on the balance sheet and disclosing key information about leasing arrangements, specifically differentiating between different types of leases. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from all leases. The recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee have not significantly changed from previous GAAP. There continues to be a differentiation between finance leases and operating leases. However, the principal difference from previous guidance is that the lease assets and lease liabilities arising from operating leases should be recognized in the balance sheet. The accounting applied by a lessor is largely unchanged from that applied under previous GAAP. Theits amendments will be effective for the Company for interim and annual periods in fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, and early2022. The Company believes the adoption will modify the way the Company analyzes financial instruments, but it does not anticipate a material impact on results of operations. The Company is permitted. In transition, lessees and lessors are required to recognize and measure leases atin the beginningprocess of determining the earliest period presented using a modified retrospective approach.
The modified retrospective approach includes a number of optional practical expedients that entities may elect to apply. These practical expedients relate to the identification and classification of leases that commenced before the effective date, initial direct costs for leases that commenced before the effective date, and the ability to use hindsight in evaluating lessee options to extend or terminate a lease or to purchase the underlying asset.
An entity that elects to apply the practical expedientseffects adoption will in effect, continue to account for leases that commence before the effective date in accordance with previous GAAP unless the lease is modified, except that lessees are required to recognize a right-of-use asset and a lease liability for all operating leases at each reporting date basedhave on the present value of the remaining minimum rental payments that were tracked and disclosed under previous GAAP. We are currently evaluating the impact of these amendments on ourits consolidated financial statements.
In March 2017,August 2020, the FASB issued ASU No. 2017-08, Revenue from2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts with Customers (Topic 606): Principal versus Agent Considerations, to clarify the implementation guidance on principal versus agent considerations and address how an entity should assess whether it is the principal or the agent in contracts that include three or more parties. The effective date and transition requirements for these amendments are the same as the effective date and transition requirements of Entity’s Own Equity (Subtopic 815 – 40), (“ASU 2014-09 (discussed above)2020-06”). We are currently evaluating the impact of these amendments on our financial statements.
In March 2017, the FASB issued ASU No. 2017-09, Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting, to reduce complexity in accounting standards involving several aspects of2020-06 simplifies the accounting for employee share-based payment transactions, including (1) the income tax consequences, (2) classificationcertain financial instruments with characteristics of awards as either equity or liabilities and (3) classificationequity, including convertible instruments and contracts on the statement of cash flows.an entity’s own equity. The ASU2020-06 amendments will be effective for financial statements issued for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, and early adoption is permitted. Amendments related to the timing of when excess tax benefits are recognized, minimum statutory withholding requirements, forfeitures, and intrinsic value should be applied using a modified retrospective transition method, amendments related to the presentation of employee taxes paid on the statement of cash flows when an employer withholds shares to meet the minimum statutory withholding requirement should be applied retrospectively, amendments requiring recognition of excess tax benefits and tax deficiencies in the income statement and the practical expedient for estimating expected term should be applied prospectively, and amendments related to the presentation of excess tax benefits on the statement of cash flows can be applied using either a prospective transition method or a retrospective transition method. An entity that elects early adoption must adopt all of the amendments in the same period. We are currently evaluating the impact of these amendments on our financial statements.
In April 2017, the FASB issued ASU No. 2017-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing, to clarify the following two aspects of Topic 606: 1) identifying performance obligations, and 2) the licensing implementation guidance. The effective date and transition requirements for these amendments are the same as the effective date and transition requirements of ASU 2014-09 (discussed above). We are currently evaluating the impact of these amendments on our financial statements.
In May 2017, the FASB issued ASU No. 2017-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing, to clarify certain core recognition principles including collectability, sales tax presentation, noncash consideration, contract modifications and completed contracts at transition and disclosures no longer required if the full retrospective transition method is adopted.
The effective date and transition requirements for these amendments are the same as the effective date and transition requirements of ASU 2014-09 (discussed above). We are currently evaluating the impact of these amendments on our financial statements.
In August 2017, the FASB issued ASU No. 2017-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, to clarify how certain cash receipts and cash payments are presented and classified in the statement of cash flows. The amendments should be applied using a retrospective transition method, and are effective for fiscal years beginning after December 15, 2017,2023, and interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. We are currentlyThe Company is evaluating the impact of these amendments on our financial statements.
In November 2017, the FASB issued ASU No. 2017-18, Statement of Cash Flows (Topic 230): Restricted Cash (a consensus of the FASB Emerging Issues Task Force), to providethis guidance on the presentation of restricted cash or restricted cash equivalents in the statement of cash flow. The amendments should be applied using a retrospective transition method, and are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. We are currently evaluating the impact of these amendments on ourits unaudited consolidated financial statements.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources and would be considered material to investors.
BUSINESS
History
We were incorporated in the State of Colorado on August 31, 2006, and on October 15, 2009, we acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition.
Sunshine Biopharma, Inc. held an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a (the “License Agreement”). Upon completion of the reverse acquisition transaction, we changed our name to Sunshine Biopharma, Inc. and began operating as a pharmaceutical company.
In December 2015, we acquired all worldwide issued (US Patent Number 8,236,935, and 10,272,065) and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 for the Adva-27a anticancer compound and terminated the License Agreement.
In early 2020, we initiated a new R&D project focused on the development of a treatment for COVID-19 and on May 22, 2020, we filed a provisional patent application in the United States for the new coronavirus treatment. The patent application covers composition subject matter pertaining to small molecules for inhibition of the main Coronavirus protease, Mpro. On April 30, 2021, we filed a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like protease, PLpro.
In June 2021, we initiated another R&D project in which we set out to determine if certain mRNA molecules can be used as anticancer agents. The data obtained for mRNA molecules bearing the laboratory name K1.1 became the subject of a new patent application filed in April 2022.
In October 2022, we acquired Nora Pharma, a Canadian generic pharmaceuticals company based in the greater Montreal area. Nora Pharma has 41 employees and operates in a 23,500 square foot facility certified by Health Canada. Nora Pharma currently sells 51 generic prescription drugs in Canada. The consolidated financial statements contained in this prospectus include the results of operations of Nora Pharma and Sunshine Canada.
Generic Prescription Drugs on the Market
As a result of the acquisition of Nora Pharma we now have the following generic prescription drugs on the market in Canada:
| Drug | Action/Indication | Reference Brand |
| Alendronate | Osteoporosis | Fosamax® |
| Amlodipine | Cardiovascular | Norvasc® |
| Apixaban | Cardiovascular | Eliquis® |
| Atorvastatin | Cardiovascular | Lipitor® |
| Azithromycin | Antibacterial | Zithromax® |
| Candesartan | Hypertension | Atacand® |
| Candesartan HCTZ | Hypertension | Atacand Plus® |
| Celecoxib | Anti-inflammatory | Celebrex® |
| Cetirizine | Allergy | Reactine® |
| Ciprofloxacin | Antibiotic | Cipro® |
| Citalopram | Central nervous system | Celexa® |
| Clindamycin | Antibiotic | Dalacin® |
| Clopidogrel | Cardiovascular | Plavix® |
| Dapagliflozin | Diabetes | Forxiga® |
| Donepezil | Central nervous system | Aricept® |
| Duloxetine | Central nervous system | Cymbalta® |
| Dutasteride | Urology | Avodart® |
| Escitalopram | Central nervous system | Cipralex® |
| Ezetimibe | Cardiovascular | Ezetrol® |
| Finasteride | Urology | Proscar® |
| Flecainide | Cardiovascular | Tambocor® |
| Fluconazole | Antifungal | Diflucan® |
| Fluoxetine | Central nervous system | Prozac® |
| Hydroxychloroquine | Antimalarial | Plaquenil® |
| Lacosamide | Central nervous system | Vimpat® |
| Letrozole | Oncology | Femara® |
| Levetiracetam | Central nervous system | Keppra® |
| Mirtazapine | Central nervous system | Remeron® |
| Metformin | Diabetes | Glucophage® |
| Montelukast | Allergy | Singulair® |
| Olmesartan | Cardiovascular | Olmetec® |
| Olmesartan HCTZ | Cardiovascular | Olmetec Plus® |
| Pantoprazole | Gastroenterology | Pantoloc® |
| Paroxetine | Central nervous system | Paxil® |
| Perindopril | Cardiovascular | Coversyl® |
| Pravastatin | Cardiovascular | Pravachol® |
| Pregabalin | Central nervous system | Lyrica® |
| Quetiapine | Central nervous system | Seroquel® |
| Quetiapine XR | Central nervous system | Seroquel XR® |
| Ramipril | Cardiovascular | Altace® |
| Rizatriptan ODT | Central nervous system | Maxalt® ODT |
| Rosuvastatin | Cardiovascular | Crestor® |
| Sertraline | Central nervous system | Zoloft® |
| Sildenafil | Urology | Viagra® |
| Tadalafil | Urology | Cialis® |
| Telmisartan | Cardiovascular | Micardis® |
| Telmisartan HCTZ | Cardiovascular | Micardis Plus® |
| Topiramate | Anticonvulsant | Topamax® |
| Tramadol Acetaminophen | Central nervous system | Tramacet® |
| Zolmitriptan | Central nervous system | Zomig® |
| Zopiclone | Central nervous system | Imovane® |
Generic Prescription Drugs Pipeline
In addition to the 51 drugs on the market, we currently have the following roster of generic prescription drugs scheduled to be launched in 2024 and 2025:
| Generic Drugs | Therapeutic Area(s) | Development Stage | Launch Date |
| Group A (2 Products) | Cardiovascular, CNS* | Under manufacturing | 2024Q1 |
| Group B (6 Products) | Oncology, Gastroenterology, CNS* | Under regulatory review | 2024Q2 |
| Group C (3 Products) | Central Nervous System, Diabetes, CNS* | Under regulatory review | 2024Q3 |
| Group D (5 Products) | Cardiovascular, Urology, Endocrinology | Under regulatory review | 2024Q4 |
| Group E (16 Products) | Cardiovascular, Oncology, Anti-infectives, Anti- inflammatory, Diabetes, Gastroenterology, CNS* | Soon to be under regulatory review | 2025 |
* Central Nervous System
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
None.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS, AND CONTROL PERSONS
FollowingWe believe the addition of these products to our existing portfolio will strengthen our presence in the Canadian generic drugs marketplace and provide us with greater access to pharmacies as we become more of a go-to supplier for every-day and specialty medicines.
Proprietary Drugs in Development
We are currently developing the following drug candidates:
| Proprietary Drugs | Therapeutic Area | Development Stage | Launch Date |
| Adva-27a (Small Molecule) | Oncology (Pancreatic Cancer) | Paused (See below) | TBD* |
| K1.1 (mRNA LNP) | Oncology (Liver Cancer) | Preclinical | TBD* |
| SBFM-PL4 (Small Molecule) | Antiviral (COVID-19) | Preclinical | TBD* |
* To be determined
Adva-27a Anticancer Drug
Adva-27a is a listsmall molecule designed for the treatment of aggressive forms of cancer. A Topoisomerase II inhibitor, Adva-27a has been shown to be effective at destroying Multidrug Resistant Cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma cells (Published in ANTICANCER RESEARCH, Volume 32, Pages 4423-4432, October 2012). We are the direct owner of all patents pertaining to Adva-27a including U.S. Patents Number 8,236,935 and 10,272,065.
In December 2022, we entered into a research agreement with the Jewish General Hospital (“JGH”), to conduct the IND-enabling studies of Adva-27a (the “Research Agreement”). In August 2023, we were informed by the JGH that the lab results on testing of the Adva-27a molecule were not favorable. After conclusion of an internal review of the lab results on November 2, 2023, we provided notice to JGH of termination of the Research Agreement. We have now paused the IND-enabling studies of Adva-27a pending a review of the possibility of chemical modification of the compound to address the suboptimal performance of the molecule in certain studies.
K1.1 Anticancer mRNA
In June 2021, we initiated a new research project in which we set out to determine if certain mRNA molecules can be used as anti-cancer agents. The data collected to date have shown that a selected group of mRNA molecules are capable of destroying cancer cells in vitro including multidrug resistant breast cancer cells (MCF-7/MDR), ovarian adenocarcinoma cells (OVCAR-3), and pancreatic cancer cells (SUIT-2). Studies using non-transformed (normal) human cells (HMEC cells) showed that these mRNA molecules had little cytotoxic effects. These new mRNA molecules, bearing the laboratory name K1.1, are readily adaptable for delivery into patients using the mRNA vaccine technology. In April 2022, we filed a provisional patent application in the United States covering the subject mRNA molecules.
We recently concluded an agreement with a specialized partner for the purposes of formulating our K1.1 mRNA molecules into lipid nanoparticles, ready for use to conduct studies in xenograft mice. Such xenograft, and other, studies are currently underway.
SBFM-PL4 Coronavirus Treatment
The initial genome expression products following infection by Betacoronavirus, the causative agent of COVID-19, are two large polyproteins, referred to as pp1a and pp1ab. These two polyproteins are cleaved at 15 specific sites by two virus encoded proteases, called Mpro and PLpro, to generate 16 different non-structural proteins essential for viral replication. Mpro and PLpro represent attractive anti-viral drug development targets as they play a central role in the early stages of viral replication. PLpro is of particular interest as a therapeutic target in that, in addition to processing essential viral proteins, it is also responsible for suppression of the human immune system making the virus more life-threatening. PLpro is present only in Betacoronaviruses, the subgroup of Coronaviruses represented by the highly pathogenic SARS-CoV, MERS-CoV, and SARS-CoV-2.
Our Anti-Coronavirus research effort has been focused on developing an inhibitor of PLpro and, on May 22, 2020, we filed a patent application in the United States covering composition subject matter pertaining to small molecules for inhibition of the Coronavirus PLpro as well as Mpro.
In February 2022, we expanded our PLpro inhibitors research effort by entering into a research agreement with the University of Arizona for the purposes of conducting research focused on determining the in vivo safety, pharmacokinetics, and dose selection properties of three University of Arizona owned PLpro inhibitors, to be followed by efficacy testing in mice infected with SARS-CoV-2 (the “Research Project”). Under the agreement, the University of Arizona granted the Company a first option to negotiate a commercial, royalty-bearing license for all intellectual property developed by University of Arizona under the Research Project. In addition, the Company and the University of Arizona entered into an option agreement (the “Option Agreement”) whereby the Company was granted a first option to negotiate a royalty-bearing commercial license for the underlying technology of the Research Project. On September 13, 2022, we exercised our options, and on February 24, 2023, we entered into an exclusive worldwide license agreement with the University of Arizona for all of the technology related to the Research Project.
We have recently expanded our objective to include the development of an injectable candidate of first-in-class PLpro inhibitor to treat SARS-CoV2 and potentially SARS-CoV and MERS-CoV infection in patients who could not use Paxlovid, Molnupiravir, or Remdesivir, due to concerns about drug interaction and possible ‘rebound’ infections and other side effects.
Intellectual Property
We are the sole owner of all worldwide rights pertaining to Adva-27a. These patent rights are covered by PCT/FR2007/000697 and PCT/CA2014/000029. The patent applications filed under these two PCT's have been issued in the United States (US Patent Number 8,236,935 and 10,272,065), Europe, and Canada.
On May 22, 2020, we filed a provisional patent application in the United States for a new treatment for Coronavirus infections. Our patent application covers composition subject matter pertaining to small molecules for inhibition of the main Coronavirus protease, Mpro, an enzyme that is essential for viral replication. The patent application has a priority date of May 22, 2020. On April 30, 2021, we filed a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like protease, PLpro. The priority date of May 22, 2020, has been maintained in the newly filed PCT application.
On April 20, 2022, we filed a provisional patent application in the United States covering mRNA molecules capable of destroying cancer cells in vitro. The patent application contains composition and utility subject matter pertaining to the structure and sequence of the relevant mRNA molecules.
Our wholly owned subsidiary, Nora Pharma, owns 180 Drug Identification Numbers (“DIN’s”) issued by Health Canada for prescription drugs currently on the market in Canada. These DIN’s were secured through in-licenses or cross-licenses from international manufacturers of generic pharmaceutical products.
In addition, we are the owner of two Natural Product Numbers (“NPN’s”) issued by Health Canada: NPN 80089663 authorizes us to manufacture and sell our in-house developed OTC product, Essential 9™, and NPN 80093432 authorizes us to manufacture and sell the OTC product, Calcium-Vitamin D under the brand name Essential Calcium-Vitamin D™.
Manufacturing
Our generic drugs are manufactured by several different international partners under long-term contracts.
We currently do not have any proprietary drugs on the market. Research quantities of our officersproprietary drug candidates are currently manufactured at the University of Arizona located in Tucson, Arizona (Anti-Coronavirus compounds), WuXi App Tech located in Hong Kong, China (Adva-27a compound), and directors:
Our OTC products are manufactured under contract by INOV Pharma Inc. located in Montreal, Canada.
Marketing and Sales
Our generic drugs are currently being sold across Canada. All of our generic drug sales are conducted by Nora Pharma’s sales representatives based in key Provinces across Canada. In addition, a segment of our marketing team offers human resources and commercial assistance to pharmacies and pharmacy owners by providing experienced pharmacists and technical assistant recruitment services as well as training and education support.
Our OTC products are currently sold in the U.S. and Canada through Amazon.com and Amazon.ca, respectively. Our personnel together with outside consultants develop and place ads on Google, YouTube, Amazon, and other media outlets. The same team manages our accounts with Amazon.
Government Regulations
All of our business operations, including our generic drugs, proprietary drugs, and OTC products operations, are subject to extensive and frequently changing federal, state, provincial and local laws and regulations.
In the United States, the Federal Government agency responsible for regulating prescription drugs and nonprescription OTC supplements is the U.S. Food and Drug Administration (“FDA”). The Canadian counterpart to the FDA is Health Canada. Though the FDA and Health Canada have generally similar requirements for drugs and OTC supplements to be approved or allowed to be marketed, approval in one jurisdiction does not automatically result in approval in the other. In Canada, prescription drugs and nonprescription OTC supplements are authorized through the issuance by Health Canada of a Drug Identification Number (DIN) for the former and a Natural Product Number (NPN) for the latter. In the United States, the marketing of OTC supplements does not require prior approval from the FDA, provided that the ingredients are known to the FDA. In both the U.S. and Canada, the ingredients, manufacturing processes and facilities for all drugs and OTC supplements must meet the guidelines for Good Manufacturing Practices (“GMP”). Moreover, all drug manufacturers must perform a series of tests, both during and after production, to show that every drug or supplement batch made meets the regulatory requirements for that product.
Our generic prescription medicines are produced following the same Good Manufacturing Practices (GMP) guidelines as for brand-name drugs. Prescription drugs dossiers are filed with Health Canada in order to obtain a manufacturing Notice of Compliance (NOC) and a Drug Identification Number (DIN). The same grant the applicant marketing authorization in Canada. In the case of Nora Pharma’s products, Nora Pharma secures cross-licenses from supply partners holding NOC’s and in turn applies to Health Canada to obtain DIN’s issued in Nora Pharma’s name in order to commercialize in Canada. In Canada, the pan-Canadian Pharmaceutical Alliance (pCPA), an alliance of the provincial, territorial and federal governments that collaborates on a range of public drug plan initiatives to increase and manage access to clinically effective and affordable drug treatments, determines generic drugs pricing based on a percentage of the brand-name reference products.
In the area of proprietary drug development where our Anti-Coronavirus and Anti-Cancer compounds fall, we will be subject to significant regulations in the U.S. in order to obtain approval of the FDA to offer our products for sale. The approximate procedure for obtaining FDA approval involves an initial filing of an IND application following which the FDA would review and allow for the drug developer to proceed with Phase I clinical trials. Following completion of Phase I, the results are filed with the FDA and a request is made to proceed to Phase II. Similarly, following completion of Phase II the data are filed with the FDA and a request is made to proceed to Phase III. Following completion of Phase III, a new drug application, or NDA is submitted and a request is made for marketing approval. Depending on various issues and considerations, the FDA could provide “emergency use authorization” or limited approval for “compassionate-use” if the drug treats terminally ill patients with limited other treatment options available. As of the date of the filing of this prospectus, we have not made any filings with the FDA or other regulatory bodies in other jurisdictions. We anticipate filing an initial IND application for an anti-Covid-19 compound within approximately one year and filing an initial IND for our anti-cancer compound within approximately two years. We have however had discussions with clinicians and as a result we believe that the FDA and Health Canada are likely to grant us a so-called “fast-track” process on the basis of the ongoing Covid-19 pandemic and the terminal nature of the cancer type we are planning to treat. There are no assurances this will occur.
In connection with OTC supplements, the FDA regulates the formulation, manufacturing, packaging, storage, labeling, promotion, distribution, and sale of such products, while the Federal Trade Commission (“FTC”) regulates marketing and advertising claims. In August 2007, a rule issued by the FDA went into effect requiring companies that manufacture, package, label, distribute or hold OTC supplements to meet certain GMP requirements to ensure such products are of the quality specified and are properly packaged and labeled. We are committed to meeting or exceeding the standards set by the FDA and the FTC and we believe we are currently operating within both the FDA and FTC mandates.
Employees
As of the date of this prospectus we have a total of 46 employees, composed of our management team (5) and employees of Nora Pharma (41).
Presently, our proprietary drug development activities are subcontracted out to specialized service providers in the U.S. and Canada. We also use consultants for various other activities including legal, marketing, accounting, and IT.
Labors laws in Quebec provide for certain guaranteed minimum entitlements, including minimum wages, maternity leave, medical leave, employee termination conditions, etc. Moreover, the Province of Quebec has various language laws governing language use. These laws require corporate operations carried out in the Province of Quebec to be conducted to a large extent, and some cases entirely, in French. We and our Canadian subsidiaries operating in the Province of Quebec are fully compliant with these laws.
Competition
The Canadian generic pharmaceuticals market is valued at approximately $7.2 billion CAD (approximately $5.3 billion USD). Generic pharmaceutical companies produce and deliver more than 70% of the prescribed medicines with high quality at affordable prices. There are more than 35 active generic players in the market, of which, the top 3 hold approximately 50% share of the market. Nora Pharma is relatively new in this space but has demonstrated one of the fastest year-over-year sales increase amongst its peers.
Our Anti-Coronavirus drug development project is in direct competition with several companies in the U.S. that have developed effective vaccines or treatment options for Covid-19. The companies focused on treatments include Pfizer, Merck, Gilead, Eli Lilly, and Regeneron. Today two leading vaccines (Pfizer’s, and Moderna’s) and two antibody treatments (Regeneron’s, and Eli Lilly’s) are in use. Gilead’s Remdesivir, an antiviral injectable, was approved by the FDA for treatment of Covid-19 in October 2020. In addition, in December 2021, Pfizer received Emergency Use Authorization (“EUA”), for its antiviral pill, Paxlovid, and, in the same month, the FDA granted Merck EUA for its antiviral pill, Molnupiravir. While the approved vaccines, pills and injectable treatments are effective, we believe that additional treatment options such as the one we are developing which targets a different part of the virus could potentially form an important component of the range of anti-coronavirus treatment options available to attending physicians.
In the area of anticancer drug development, we compete with large publicly and privately held companies engaged in developing new cancer therapies. There are numerous other entities engaged in oncology therapeutics development that have greater resources than the resources presently available to us. Nearly all major pharmaceutical companies including Merck, Amgen, Roche, Pfizer, Bristol-Myers Squibb and Novartis, to name a few, have on-going anticancer drug development programs and some of the drugs they may develop could be in direct competition with our own. In addition, a number of smaller companies are working in the area of cancer therapy and could develop drugs that may be in competition with ours.
Similarly, our OTC products fall directly within a very crowded and highly competitive product sector. As of the date of this prospectus, we believe Essential 9™ is the only Essential Amino Acid product that comprises all 9 essential amino acids in capsule form. We believe this may provide us with a competitive advantage, at least for the near future but there are no assurances that this will occur.
Properties
Our principal place of business is located at 1177 Avenue of the Americas, 5th Floor, New York, NY 10036, pursuant to a month-to-month arrangement and a pay-per-use plan. Our minimum monthly rent is $289.00.
Our wholly owned subsidiary, Nora Pharma, currently occupies a 23,500 square foot facility located at 1565 Boulevard Lionel-Boulet, Varennes, Quebec, Canada, J3X 1P7 pursuant to a lease agreement that expires in January 2030, with an option to extend for 5 years. This site is composed of 18,500 square feet of warehouse space and 5,000 square feet of executive office space. The facility houses all administrative, marketing, quality control, regulatory affairs, and other operations personal, as well as a Health Canada licensed warehouse space. We pay a monthly rent of $27,250 CAD (approximately $19,900 USD), including taxes.
Legal Proceedings
We are not party to, and our property is not the subject of, any material legal proceedings.
MANAGEMENT
Directors and Executive Officers
The following table and biographical summaries set forth information, including principal occupation and business experience about our directors and executive officers:
| Name | | Age | | Position(s) |
| | | | | |
| Dr. Steve N. Slilaty | | 6671 | | President, Chief Executive Officer and Chairman |
| | | | | |
| Dr. Abderrazzak Merzouki | | 5559 | | Chief OperatingScience Officer and Director |
| | | | | |
| Camille Sebaaly | | 5962 | | Chief Financial Officer Secretary and Secretary |
| | | | |
| Dr. Rabi Kiderchah | | 51 | | Director |
| | | | |
| David Natan | | 70 | | Director |
| | | | |
| Dr. Andrew Keller | | 70 | | Director |
| | | | |
| Malek Chamoun | | 39 | | Chief Development Officer and President of Nora Pharma Inc. |
| | | | |
| Marc Beaudoin | | 57 | | Chief Operating Officer |
Our directors serve as directors until our next Annual Meeting of Stockholders and the election and qualification of the director’s respective successor or until the director’s earlier death, removal or resignation.
Following is biographical information of our current management:
Dr. Steve N. Slilaty
was appointed as our CEO, Presidentchief executive officer and Chairmanchairman of our Boardboard of Directorsdirectors on October 15, 2009. Dr. Slilaty is an accomplished scientist and business executive. His scientific publications are widely cited including university textbooks.cited. Sunshine Biopharma is the third in a line of biotechnology companies that Dr. Slilaty founded and managed through their early and mid-stages of development.managed. The first,Quantum Biotechnologies Inc.later known asQbiogene Inc., was founded in 1991 and grew to over $60 million in annual sales. Today,Qbiogeneis now a member of a family of companies owned byMP Biomedicals, one of the largest international suppliers of biotechnology reagents and other research products. The second company which Dr. Slilaty founded,Genomics One Corporation, later known asAlert B&C Corporation, conducted an initial public offering (IPO) of its capital stock in 1999 and, on the basis of its ownership of Dr. Slilaty’s patented TrueBlue®TrueBlue Technology,Genomics Onebecame one of the key participants in the Human Genome Project.Project and reached a market capitalization of $1 billion in 2000. Formerly, Dr. Slilaty was a research team leader ofat theBiotechnology Research Institute (Montreal), a division of theNational Research Council of Canada, Dr. Slilaty also served as a consultant in a management and advisory capacity for a major Canadian biotechnology company between 1995 and 1997 during which time the company completed one of the largest biotechnology IPO‘s in Canada.. Dr. Slilaty is one of the pioneers of Gene Therapy having developed the first gene delivery system applicable to humans in 1983 [Science [Science 220:725-727 (1983)]. Dr. Slilaty's other distinguished scientific career accomplishments includeaccomplishment was the discovery of a new class of enzymes, the S24 Family of Proteases (IUBMB Enzyme Nomenclature:Enzyme: EC 3.4.21.88), development of [Proc. Natl. Acad. Sci. U.S.A. 84: 3987-3991 (1987)]. In addition, Dr. Slilaty (i) developed the first site-directed mutagenesis system applicable to double-stranded DNA cloning[Analyt. Biochem. 185: 194-200 (1990)], (ii) cloned the gene for the first yeast-lytic enzyme (lytic b-1,3-glucanase) [J. Biol. Chem. 266: 1058-1063 (1991)], developing(iii) developed a new molecular strategy for increasing the rate of enzyme reactions inventing[Protein Engineering 4: 919-922 (1991)], and (iv) constructed a powerful new cloning system for genomic cloning and gene discoverysequencing (TrueBlue® Technology) [Gene 213: 83-91 (1998)]. Most recently, Dr. Slilaty, in collaboration with Institut National des Sciences Appliquée (France), State University of New York at Binghamton (USA) and developing a new transcriptomics technology for generating entire RNA profiles.École Polytechnique, Université de Montréal (Canada), designed, patented, and advanced the development the first, and currently the only known anticancer compound (Adva-27a) capable of destroying multidrug resistant cancer cells [Anticancer Res. 32: 4423 (2011) and US Patent Numbers: 8,236,935 and 10,272,065]. These and other works of Dr. Slilaty are cited in research papers, editorials, review articles and textbooks. Dr. Slilaty is the author of 18 original research papers and 10 issued and pending. These and other works of Dr. Slilaty are cited in research papers, editorials, review articles and textbooks. Dr. Slilaty received his Ph.D. degree in Molecular Biology from the University of Arizona in 1983 and Bachelor of Science degree in Genetics and Biochemistry from Cornell University in 1976. In addition, Dr. Slilaty holds a position as Adjunct Professor at Université du Québechas received research grants from the NIH and NSF and he is the recipient of the 1981 University of Arizona Foundation award for Meritorious Performance in the DepartmentTeaching. Dr. Slilaty’s scientific knowledge and experience qualifies him to serve on our board of Microbiology and Biotechnology.directors.
Dr. Abderrazzak Merzouki
was appointed has served as a Directordirector since February 2016 and our Chief Operating Officer inas chief science officer since January 2024. He served as chief operating officer from February 2016.2016 to January 2024. In addition to his new positions with our Company since January 2016 he has been self-employed as a consultant in the fields of biotechnology and pharmacology. From July 2007 through December 2016, Dr. Merzouki worked at the Institute of Biomedical Engineering in the Department of Chemical Engineering at Ecole Polytechnique de Montreal, where he taught and acted as a senior scientist involved in the research and development of plasmid and siRNA-based therapies. Dr. Merzouki is a molecular biologist and an immunologist with extensive experience in the area of gene therapy where he performed several preclinical studies for pharmaceutical companies involving the use of adenoviral vectors for cancer therapy and plasmid vectors for the treatment of peripheral arterial occlusions. Dr. Merzouki also has extensive expertise in the design of expression vectors, and production and purification of recombinant proteins. He developed technologies for production of biogeneric therapeutic proteins for the treatment of various diseases including cancer, diabetes, hepatitis and multiple sclerosis. Dr. Merzouki obtained his Ph.D. in Virology and Immunology from Institut Armand-Frappier in Quebec and received his post-doctoral training at the University of British Columbia and the BC Center for Excellence in HIV/AIDS research. Dr. Merzouki has over 30 publications and 70 communications in various, highly respected scientific journals in the field of cellular and molecular biology. Dr. Merzouki’s scientific knowledge and experience qualifies him to serve on our board of directors.Camille Sebaaly
was appointed as our Chief Financial Officer, Secretarychief financial officer, secretary and a Directordirector of our Company on October 15, 2009. He resigned as a director of the Company in October 2021. Since 2001, Mr. Sebaaly has been self-employed as a business consultant, primarily in the biotechnology and biopharmaceutical sectors. He held a number of senior executive positions in various areas including financial management, business development, project management and finance. As an executive and an entrepreneur, he combines expertise in strategic planning and finance with strong skills in business development and deal structure and negotiations. In addition, Mr. Sebaaly worked in operations, general management, investor relations, marketing and business development with emphasis on international business and marketing of advanced technologies including hydrogen generation and energy saving. In the area of marketing, Mr. Sebaaly has evaluated market demands and opportunities, created strategic marketing and business development plans, designed marketing communications and launched market penetration programs. Mr. Sebaaly was a cofounder of Advanomics Corporation with Dr. Slilaty. Mr. Sebaaly graduated from State University of New York at Buffalo with an Electrical and Computer Engineering Degree in 1987. There are no family relationships between any of our former or current officers and directors.
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a)Dr. Rabi Kiderchah has served as a director of the Securities Exchange ActCompany since October 2021. Dr. Kiderchah is a licensed physician in Canada. From 2000 until August 2021, he was working at Argenteuil Hospital, Lachute, Quebec, Canada, as an emergency room physician. He has also worked as what is referred to in Canada as a “medecins depanneurs”, working in rural areas where there are not enough ER doctors. Since August 2011 he has worked at Rabi Kiderchah Medecin Inc. as a freelance physician in the Quebec, Canada area. He received a Bachelor of 1934 (the “34 Act”) requiresScience degree in 1994 and an MD degree in 1998 from the University of Montreal. Dr. Kiderchah’s medical and scientific knowledge and experience qualifies him to serve on our officers and directors and persons owning more than ten percentboard of directors.
David Natan has served as a director of the Common Stock,Company since February 2022. He currently serves as CEO of Natan & Associates, LLC, a consulting firm offering CFO services to file initial reportspublic and private companies since 2007. From February 2010 to May 2020, Mr. Natan served as CEO of ownershipForceField Energy, Inc. (OTCMKTS: FNRG), a company focused on LED lighting products. From February 2002 to November 2007, Mr. Natan served as CFO of PharmaNet Development Group, Inc., a drug development company, and, changesfrom June 1995 to February 2002, as CFO and VP of Global Technovations, Inc., a manufacturer and marketer of speaker components. Prior to that, Mr. Natan served in ownershipvarious roles with the SecuritiesDeloitte & Touche LLP. From April 2020 through June 2023, Mr. Natan was Executive Vice President and Exchange Commission (“SEC”). Additionally, Item 405 of Regulation S-K under the 34 Act requires us to identify in our Form 10-K and proxy statement those individualsChief Financial Officer for whom one of the above referenced reports was not filed onAirborne Motorworks, Inc., Spokane, WA, a timely basis during the most recent year or prior years. To our best knowledge, all reports that were required to be filed were filed, but were filed late.
CODE OF ETHICS
Our board of directors has not adoptedprivately-held aerospace transportation company. Mr. Natan currently serves as a code of ethics but plans to do so in the near future.
COMMITTEES OF OUR BOARD OF DIRECTORS
There are no committeesmember of the Board of Directors
but itand Chair of the Audit Committee of NetBrands, Inc. (OTC: NBND), a distributor of snack products, since February 2021; and serves as a member of the Board of Directors and Chair of the Audit Committee of Titan Pharmaceuticals Inc. (NASDAQ: TTNP) a pharmaceutical company, since August 2022. Additionally, in November 2023, Mr. Natan was appointed to the board of Directors and Audit Committee Chair of Minim Inc. (NASDAQ: MINM). Mr. Natan holds a B.A. in Economics from Boston University. Mr. Natan’s experience as a business executive and as a director and chairperson of audit committees for public companies qualifies him to serve on our board of directors.Dr. Andrew M. Keller has served as a director of the Company since February 2022. From 2016 through November 2019, Dr. Keller was the Chief Medical Officer at the Western Connecticut Medical Group, Bethel CT, a multispecialty organization. He was employed by this group beginning in 1989, and in 2003 became Chief – Section of Cardiovascular Diseases. In 2014 he was appointed Chief Medical Informatics Officer. Previously, Dr. Keller was an Assistant Professor of Medicine/Radiology at Columbia University, The College of Physicians and Surgeons, NY, NY. Dr. Keller retired as a practicing physician in 2019. Upon his retirement as a practicing physician Dr. Keller enrolled as a full time student at Quinnipiac University College of Law, where he graduated with a Juris Doctor degree in 2023. In July 2023, Dr. Keller passed the Bar exam and was admitted to practice law in the State of Connecticut in November 2023. Since November 2023 he has been employed at the Law Office of Robin P. Keller LLC, Norwalk, CT advocating for the educational needs of disabled children with medically complex diagnoses. Dr. Keller received a Doctor of Medicine degree in 1979 from The Ohio State University and a Bachelor of Arts degree in Physics, Magna Cum Laude from Ithaca College in 1975. Dr. Keller’s medical, scientific and legal knowledge and experience qualify him to serve on our board of directors.
Malek Chamoun was appointed as our Chief Development Officer in January 2024. In addition, he is anticipatedPresident of Nora Pharma, Inc., our wholly owned subsidiary that we will establishacquired in October 2022. In 2017 he founded Nora Pharma, where he has been the President and CEO since inception. Mr. Chamoun received a bachelor’s degree in business administration from Hautes Études Commerciales, Montreal, Quebec, Canada in 2008 and became a licensed CPA in Canada in 2012. He devotes all of his business time to Nora Pharma’s affairs.
Marc Beaudoin was appointed as our Chief Operating Officer in January 2024. Mr. Beaudoin was the sole owner of M.A. Beaudoin Consulting Group Inc., a privately held business strategy consulting company in the Canadian pharmaceutical and biopharmaceutical sectors since 2016. From January 2018 through February 2019, he was employed by the KDA Group, Inc., a publicly held Canadian healthcare company, as the COO of KDA Group and CEO of its Canadian generic pharmaceutical division, Pharapar. From 2006 to 2016, he held several executive positions at Sandoz Canada in various areas including Marketing and Communications, Strategic Planning, Business Development & Portfolio Management. As an executive and an entrepreneur, he combines expertise in strategic planning with operational and commercial execution. Mr. Beaudoin obtained his MBA from Sherbrooke University in 2018. He also holds multiple certifications (including a fellowship) from the Association for Supply Chain Management.
Board of Directors Term of Office
Directors are elected at our annual meeting of shareholders and serve for one year until the next annual meeting of shareholders or until their successors are elected and qualified.
Director Independence
Our independent directors consist of Dr. Kidershah, Mr. Natan and Dr. Keller.
Committees of our Board of Directors
The Company has established an audit committee, a compensation committee, and a corporate governance and nominating committee and governance committee once independentof our board of directors, are appointed,each of which is expected to occur in the near future.
INVOLVEMENT IN CERTAIN LEGAL PROCEEDINGS
On November 14, 2014, we entered into a Manufacturing Services Agreement with Lonza Ltd. and Lonza Sales Ltd. (hereinafter jointly referred to as “Lonza”), whereby we engaged Lonza to be the manufacturercomprised of each of our
Adva-27a anticancer drug. In June 2016 we received a sampleindependent directors.No Family Relationships
There is no family relationship between any director and executive officer or among any directors or executive officers.
Involvement in Certain Legal Proceedings
Our directors and executive officers have not been involved in any of the pilot manufacturing run for evaluation. Our laboratory analyses showed that, whilefollowing events during the sample meets allpast ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| | |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| | |
| 4. | being found by a court of competent jurisdiction in a civil action, the SEC or the CFTC to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| | |
| 5. | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| | |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Code of the required chemical, physical and biological specifications, the amount of material generated (the “Yield”) by the pilot run was found to be significantly lower than anticipated. We are currently working towards finding possible solutions to increase the Yield and define a path forward. During the course of our discussions concerning the problem of the low Yield, Lonza informed us that they required us to pay them $687,818 prior to moving forward with any activity pertaining to the manufacturing agreement we have with them. Ethics
We have repeatedly indicated to Lonzaadopted a Code of Ethics that a clear path defining exactly how the extremely low Yield issue would be addressed is imperative prior to us making any payments. We issued a letter to them in June 2017 advising of our position. As of the date of this Report we have not received a responseapplies to our letterprincipal executive officer, principal financial officer, and no further action has been taken by either party.
In June 2018 we filed an action in the Superior Courtprincipal accounting officer. Our Code of the Province of Quebec in the District of Montreal (Canada) against one ofEthics is available on our existing shareholders residing in Quebec City (Canada) arising out of a possible equity investment intended to be completed in the near future. The complaint alleges among other things, claims of misrepresentations and misleading conduct resulting in damages to the Company. As of the date of this report we are awaiting a court date for the hearings to commence.
Other than the foregoing, there are no known pending legal proceedings to which the Company is a party or in which any director, officer or affiliate of the Company, any owner of record or beneficially of more than 5% of any class of voting securities of the Company, or security holder is a party adverse to the Company or has a material interest adverse to the Company. The Company’s property is not the subject of any other pending legal proceedings
EXECUTIVE COMPENSATION
website at www.sunshinebiopharma.com.Executive Compensation
The following table sets forth compensation information concerningfor services rendered by our executive officers in all cashcapacities during the last two completed fiscal years.
| Name and Principal Position | | Year | | Salary ($) | | Bonus ($) | | Stock Awards ($) | | All Other Compensation ($) | | Total ($) |
| | | | | | | | | | | | | |
| Dr. Steve N. Slilaty | | 2022 | | 360,000 | | 10,000 | | – | | – | | 370,000 | |
| Chief Executive Officer and Director | | 2023 | | 378,000 | | 182,000 | | – | | – | | 560,000 | |
| | | | | | | | | | | | | | |
| Camille Sebaaly | | 2022 | | 300,000 | | 630,000 | | – | | – | | 930,000 | |
| Chief Financial Officer | | 2023 | | 300,000 | | 420,000 | | – | | – | | 720,000 | |
| | | | | | | | | | | | | | |
| Dr. Abderrazzak Merzouki | | 2022 | | 240,000 | | 245,000 | | – | | – | | 485,000 | |
| Chief Operating Officer and Director | | 2023 | | 240,000 | | – | | – | | – | | 240,000 | |
Employment Agreements
On April 8, 2022, we entered into an employment agreement with Dr. Steve N. Slilaty, our Chief Executive Officer. Pursuant to the employment agreement, Dr. Slilaty will continue to serve as our CEO and non-cashwill be paid a base annual salary of $360,000 (which will increase annually at the rate of the Consumer Price Index or 5%, whichever is higher). The employment agreement has a term of four years and will renew automatically for a term of an additional three years. In the event the employment agreement is terminated by the Company without cause, the Company will pay Dr. Slilaty $10 million. Upon expiration of the employment agreement, the Company will pay Dr. Slilaty $2 million.
Outstanding Equity Awards at 2023 Fiscal Year-End
We did not have any outstanding equity awards as of December 31, 2023.
Director Compensation
The following table sets forth compensation awarded to, earned by orwe paid to our directors during the year ended December 31, 2023.
| Name | | Fees Earned or Paid in Cash ($) | | Stock Awards | | Option Awards | | All Other Compensation | | Total ($) |
| Dr. Rabi Kiderchah | | 80,000 | | – | | – | | – | | 80,000 |
| Mr. David Natan | | 80,000 | | – | | – | | – | | 80,000 |
| Dr. Abderrazzak Merzouki | | 80,000 | | – | | – | | – | | 80,000 |
| Dr. Andrew Keller | | 80,000 | | – | | – | | – | | 80,000 |
| Dr. Steve N. Slilaty | | 80,000 | | – | | – | | – | | 80,000 |
TRANSACTIONS WITH RELATED PERSONS
A note payable dated December 31, 2019, held by our chief executive officers. We do not currently have an established policy to provide compensation to members of our Board of Directors for their services in that capacity, although we may choose to adoptofficer, having a policy in the future.
| | | Bonus | | Option Awards | | |
Name and Principal Position | | | ($) | | ($) | | |
| | | | | | | |
Dr. Steve N. Slilaty, Chief Executive Officer and Director | | -0- | | | | 50,000(1) | 50,000 |
| | 1,000 | | | | 164,600(2) | 165,600 |
| | 155,641(4) | | | | 112,000(3) | 267,641 |
| | | | | | | |
Camille Sebaaly, Chief Financial Officer and Director | 2015 | -0- | | | | -0- | -0- |
| 2016 | 4,597 | | | | 164,600(2) | 169,197 |
| 2017 | 16,099 | | | | 112,000(3) | 128,099 |
| | | | | | | | |
Abderrazzak Merzouki, Chief Operating Officer and Director | 2015 | -0- | | | | -0- | -0- |
| 2016 | -0- | | | | 164,600(2) | 164,600 |
| 2017 | 12,531 | | | | 112,000(3) | 124,531 |
1)
In consideration for services valued at $50,000, Dr. Slilaty was issued 500,000 shares of Series “B” Preferred Stock having 1,000 votes per share. The Series “B” Preferred Stock is non-convertible, non-redeemable, non-retractable and has a statedface value of
$0.10$128,269 and accruing interest at 12% was due December 31, 2020. On December 31, 2020, we renewed the note together with accrued interest of $15,392 for a 12-month period. The new note had a face value of $143,661, accrued interest at 12% per
share. Theseyear, and had a maturity date of December 31, 2021. On August 24, 2021, we paid off the entire principal balance of this note, together with accrued interest of $12,929 by making a cash payment of $156,590.On February 22, 2022, we redeemed 990,000 shares of Series B Preferred Stock are restrictedheld by Dr. Steve Slilaty, our CEO, at a redemption price equal to the stated value of $0.10 per share.
On February 8, 2024, the Company issued and may not be sold or transferred without prior written consent of the Board of Directors of the Company.
2)
In 2016, each member of our Board of Directors was issued 26,000,000 and 12,000,00020,000 shares of our CommonSeries B Preferred Stock valued at $80,600 and 84,000, respectively. These Officers and Directors shares are restricted and may not be sold without prior written consentto Dr. Steve Slilaty for a purchase price equal to the stated value of the Board of Directors of the Company.
In 2017, each member of our Board of Directors was issued 14,000,000 shares of our Common Stock valued at $112,000. These Officers and Directors shares are restricted and may not be sold without prior written consent of the Board of Directors of the Company.
4)
Includes $147,695 paid to Advanomics Corporation, a company controlled by our CEO.
Salaries are established by our Board of Directors. We currently do not have a Compensation Committee but expect to have one in place in the future once we have independent directors. We have not and do not expect to pay any other compensation to our current executive officers or directors until such time as we are able to secure adequate funding for our operations.
EMPLOYMENT AGREEMENTS
None of our executive officers is party to an employment agreement with us.
STOCK PLAN
We have not adopted any stock option or other employee plans as of the date of this Registration Statement on Form S-1. We may adopt such plans in the future.
DIRECTOR COMPENSATION
We have not established standard compensation arrangements for our directors and the compensation, if any payable to each individual for their service on our Board will be determined from time to time by our Board of Directors based upon the amount of time expended by each of the directors on our behalf. No member of our Board of Directors received compensation for their services for the fiscal year ended December 31, 2017.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the ownership of Common Stock and Preferred Stock voting with the Common Stockcommon stock as of the date of this Registration Statement on Form S-1February 8, 2024, by (i) each person known to us to own more than 5% of our outstanding Common Stock as of the date of this Registration Statement on Form S-1, (ii) each of our directors, (iii)(ii) each of our executive officers, and (iv)(iii) all of our directors and executive officers as a group.group, and (iv) any person or group as those terms are used in Section 13(d)(3) of the Exchange Act, believed by us to beneficially own more than 5% of our common stock. Unless otherwise indicated, all shares are owned directly and the indicated person has sole voting and investment power. The information provided ispercentages listed are based upon 1,585,628,494 Common Shares28,024,290 common shares issued and 500,000 shares ofoutstanding and 30,000 Series B Preferred Stock issued andshares outstanding as of February 8, 2024. Unless otherwise indicated, the dateaddress of this Registration Statement on Form S-1.each holder is c/o Sunshine Biopharma, Inc., 1177 Avenue of the Americas, 5th Floor, New York, NY 10036.
| Title of Class | | Name and Address of Beneficial Owner | | Amount and Nature of Beneficial Ownership | | | Percent of Class | |
| | | | | | | | | |
| Common | | Dr. Steve N. Slilaty(1) | | | 3,821,024 | (3) | | | 13.7% | |
| Preferred | | | | | 30,000 | (2) | | | 100% | |
| | | | | | | | | | | |
| Common | | Camille Sebaaly(1) | | | 174,465 | | | | * | |
| | | | | | | | | | | |
| Common | | Dr. Abderrazzak Merzouki(1) | | | 116,720 | | | | * | |
| | | | | | | | | | | |
| Common | Dr. Andrew Keller(1) | | | 0 | | | | * | |
| | | | | | | | | | |
| Common | David Natan(1) | | | 0 | | | | * | |
| | | | | | | | | | |
| Common | Dr. Rabi Kiderchah(1) | | | 1,625 | | | | * | |
| | | | | | | | | | |
| Common | | Malek Chamoun(1) | | | 3,700,000 | (3) | | | 13.2% | |
| | | | | | | | | | | |
| Common | | Marc Beaudoin(1) | | | 0 | | | | * | |
| | | | | | | | | | | |
Common | | All Officers and Directors as Group (8 persons): | | | 4,113,834 | (2)(3) | | | 14.7% | |
___________________
* Less than 1%.
| (1) | Officer and/or director of our Company. |
| (2) | Includes 30,000 shares of the Company’s Series B Preferred Stock. Each share of Series B Preferred Stock is entitled to 1,000 votes. |
| (3) | Includes 3,700,000 common shares owned by Malek Chamoun, the President of Nora Pharma Inc., a company acquired by the Company in October 2022. Dr. Slilaty controls the voting of Mr. Chamoun’s shares through a voting agreement between Mr. Chamoun and Dr. Slilaty dated October 20, 2022. |
| Title of Class | | Name and Address of Beneficial Owner | | Amount and Nature of Beneficial Ownership | | Percent of Common Class(5)(6) | | Percent of Voting Shares |
| | | | | | | | | |
Common Series B Preferred | | Dr. Steve N. Slilaty(1) 579 rue Lajeunesse Laval, Quebec Canada H7X 3K4 | | 332,398,597(2) 500,000,000(3) | | 21.0% 0% | | 15.9% 24.0% |
| | | | | | | | | |
| Common | | Dr. Abderrazzak Merzouki(1) 731 Place de l’Eeau Vive Laval, Quebec Canada H7Y 2E1 | | 118,467,000 | | 7.5% | | 5.7% |
| | | | | | | | | |
| Common | | Camille Sebaaly(1) 14464 Gouin West, #B Montreal, Quebec Canada H9H 1B1 | | 246,703,300(4) | | 15.6% | | 11.8% |
| | | | | | | | | |
| Common | | All Officers and Directors As Group (3 persons) | | 697,568,897 | | 44.0% | | 57.4% |
(1) Officer and Director.
(2) Includes 215,014,224 shares held in the nameDESCRIPTION OF CAPITAL STOCK
General
Our authorized capital stock consists of Advanomics Corporation. Dr. Slilaty is an officer, director and principal shareholder of Advanomics Corporation and, as a result, controls the disposition of these shares.
(3) Comprised of 500,0003,000,000,000 shares of
$0.10common stock, par value
Series “B” Preferred Stock having 1,000 votesof $0.001 per share, and 30,000,000 shares of preferred stock, par value $0.10 per share.
The1,000,000 shares of our preferred stock are designated as Series
“B”B Preferred
Stock is non-convertible, non-redeemable, non-retractable and has a superior liquidation valueStock.As of $0.10 per share.
(4) Includes 129,488,927 shares held in the name of 4019318 Canada, Inc. Mr. Sebaaly is the sole officer and director of this company and, as a result, controls the disposition of these shares.
(5) Beneficial ownership is determined in accordance with Rule 13D-3(a) of the Exchange Act and generally includes voting or investment power with respect to securities.
(6) The percentages in the table have been calculated on the basis of treating as outstanding for a particular person, allFebruary 8, 2024, there were 28,024,290 shares of our common stock, outstanding, on that date and all shares of our common stock issuable to that holder in the event of exercise of outstanding options, warrants, rights or conversion privileges owned by that person at that date which are exercisable within 60 days of that date. Based on 1,585,628,494 shares of common stock as of September 27, 2018
TRANSACTIONS WITH RELATED PERSONS
On November 27, 2014, we issued a note payable in the principal amount of $128,000 to an individual who subsequently became a principal shareholder. The note accrues interest at 10% per annum and was convertible into shares of our Common Stock at a price of $0.20 per share. On June 30, 2015, we renewed this note with the addition of accrued interest of $7,540 and an origination fee of $25,600. The new Note had a face value of $161,140 and accrued interest at 12% per annum. The new note was due December 31, 2015, and was convertible any time from the date of issuance into shares of our Common Stock at a 35% discount from market price. On December 31, 2015, we again renewed this note with the addition of accrued interest amounting to $9,668 and an origination fee of $32,228. The new note now has a face value of $203,036 and accrues interest at 12% per annum. The new note was due June 30, 2016 and was convertible anytime from the date of issuance into shares of our Common Stock at a 35% discount from market price. In January 2016, $38,036 of the principal was converted, leaving a principal balance of $165,000. In connection therewith, 7,705,186 shares of our Common Stock, valued $231,156 were issued generating a loss on conversion of $193,120. On June 30, 2016, we renewed this note again with the addition of accrued interest amounting to $9,852. The renewed note had a face value of $174,852 and accrues interest at 12% per annum. It was due on March 31, 2017. In October 2016, $74,852 of the principal amount was converted, leaving a principal balance of $100,000. On March 31, 2017, the note’s principal balance of $100,000 plus accrued interest of $11,715 was renewed for a period of 90 days under the same terms and conditions as the previous note. The new note now had a face value of $111,715 and matured on June 30, 2017. On June 30, 2017, the note’s principal balance of $111,715 plus accrued interest of $3,342 was renewed for a period of 90 days under the same terms and conditions as its predecessor. The new note had a face value of $115,057 and matured on September 30, 2017. On September 30, 2017, the note’s principal balance of $115.057 plus accrued interest of $3,480 was renewed for a period of 90 days under the same terms and conditions as the previous note. The new note had a principal balance of $118,537 and matured on December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as the prior note. The new note has a face value of $122,093 and matures on December 31, 2018.
In December 2016, we received monies from our CEO in exchange for a note payable having a principal amount of $90,000 Canadian ($67,032 US) with interest at 12%. The note was convertible any time after the date of issuance into shares of our Common Stock at a price 35% below market value. In addition, the note was collateralized by all our assets. On March 31, 2017, the note, together with all accrued interest thereon and an additional principal amount of $3,000 Canadian paid to us in March 2017, was renewed for a 90-day period under the same terms and conditions as the original note. The new note now having a face value of $96,021 Canadian ($72,198 US) was due on June 30, 2017. On June 30, 2017, the note, together with accrued interest of $2,873 Canadian ($2,005 US), was renewed for a 90-day period under the same terms and conditions as the original note except that the new note is nonconvertible. The new note now having a face value of $98,894 Canadian ($76,072US) was due on September 30, 2017. On September 30, 2017, the note, together with accrued interest of $2,991 Canadian ($2,397 US), was renewed for a 90-day period under the same terms and conditions as its predecessor. The new note now having a principal balance of $101,885 Canadian ($81,640 US) matured on December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as before except that this new note is unsecured and now non-convertible. The new note has a face value of $104,942 Canadian ($83,649 US) and matures on December 31, 2018.
On October 8, 2015, we acquired U.S. Patent Number 8,236,935 (the “US Patent”) for the anticancer compound, Adva-27a from a related entity (Advanomics Corporation), which includes all rights to this intellectual property within the United States, in exchange for an interest-free note payable for $4,320,000 (the “October Patent Purchase Agreement”). On December 28, 2015, we acquired the remaining worldwide issued and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 (the “Worldwide Patents”) for the Adva-27a anticancer compound from the same related entity (Advanomics Corporation) in exchange for a note payable for $12,822,499 (the “December Patent Purchase Agreement”). We believe that purchase of the US Patent and the Worldwide Patents (the “Patents”) would facilitate our ability to obtain the funding necessary to complete the development and FDA approval process for Adva-27a. In related party transactions, purchased patents are required to be recorded at the purchase price or the book value on the seller’s financial statements, whichever is lower. Effective December 28, 2015, the parties agreed to amend the October Patent Purchase Agreement and the December Patent Purchase Agreement. Pursuant to the amendment agreements (the “Amendments”), the Patents were purchased from the related party, Advanomics Corporation, at Advanomics’ cost less the amortization through December 28, 2015, the effective date of the transfer. The Amendments amended the purchase price of the Patents to $835,394, eliminated all cash payments obligations and replaced the non-convertible notes totaling $17,142,499 with two (2) convertible notes totaling $835,394 that automatically convert into an aggregate of 321,305,415 shares of our Common Stock when we successfully amend our Articles of Incorporation to increase our authorized capital of Common Stock to 3 billion. In July 2016, having completed the increase of our authorized capital to 3 billion shares of Common Stock, we issued the 321,305,415 Common Shares to Advanomics thereby completing all aspects of the patent purchase arrangements and securing direct ownership of all worldwide patents and rights pertaining to Adva-27a.
In 2016 and 2017 our principal place of business was located at 469 Jean-Talon West, 3rd Floor, Montreal, Quebec, Canada, H3N 1R4. This was also the location of our former licensor, Advanomics Corporation, who provided this space to us on a rent free basis.. Dr. Steve N. Slilaty, our Chief Executive Officer and a Director, is an Officer, Director and principal shareholder of Advanomics. Starting January 1, 2017 we took over the lease from Advanomics until we moved to our current location on June 1, 2017.
In February and April 2016, we paid $30,000 and $50,487 to Advanomics for the balance of 2015 licensing fees.
During the fiscal year ended December 31, 2016, Advanomics Corporation paid on our behalf $13,725 Canadian in patenting fees. Advanomics was fully reimbursed by us in January 2017.
During the fiscal ended December 31, 2017, we issued to our Board of Directors 42,000,000 shares of par value $0.001 Common Stock valued at $336,000 or $0.008 per share. During the same period, our Directors and Officers were paid $184,271 in cash. Of this amount, $147,695 was paid to Advanomics Corporation, a company controlled by our CEO.
During the period ended December 31, 2016, we issued 78,000,000 shares of par value $0.001 Common Stock to our Directors and Officers valued at $241,800 or $0.0031 per share. We also issued to the Board of Directors 36,000,000 shares of $0.001Common Stock valued at $252,000 or $0.0078 per share. In addition, we paid or Directors and Officers $5,597 in cash.
Certain members of our management, including Dr. Steve N. Slilaty, our President, CEO and a Director and Camille Sebaaly, our Secretary, CFO and a Director, hold similar positions with Advanomics.
There are no other related party transactions that are required to be disclosed pursuant to Regulation S-K promulgated under the Securities Act of 1933, as amended.
On January 1, 2018 as part of the acquisition of Atlas Pharma Inc., the Company issued a note payable in the amount of $450,000 Canadian ($358,407 US) and accruing interest at the rate of 3% per annum. The note is due on December 31, 2023. Payments on this note are $10,000 Canadian (approximately $8,000 US) per quarter. The outstanding principal balance at June 30, 2018 is $331,668. The note is secured by the Atlas Pharma Inc. shares held by the Company.
The Company paid its Officers and Directors cash compensation totaling $17,344 and $12,415 and $95,131 and $55,380 for the three and six month periods ended June 30, 2018 and 2017, respectively. The Company also paid its Officers and Directors non-cash compensation in the form of shares of Common Stock valued at $429,300 and $336,000 and $429,300 and $336,000 during the three and six month periods ended June 30, 2018 and 2017 respectively. In June 2018 the Company expensed an additional $429,300 in compensation to the directors in exchange for 81,000,000 shares of $0.001 par value Common Stock issued in June 2018 valued at $0.0053 per share. Stock issued for executive compensation is valued at the closing price on the date of issuance.
DIRECTOR INDEPENDENCE
None of our current directors are deemed “independent” pursuant to SEC rules. We anticipate appointing independent directors in the foreseeable future.
does not include: | PART I – FINANCIAL INFORMATION· | 23,395,046 shares issuable upon exercise of outstanding warrants with a weighted average exercise price of $1.94; and |
| | | |
| · | 30,000 outstanding shares of Series B Preferred Stock, which are not convertible into common stock. |
Common Stock
Holders of our common stock are entitled to one vote for each share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting rights. Therefore, holders of a majority of the voting power of our stockholders for the election of directors can elect all of the directors. Holders of one-third of the voting power of the Company’s stockholders, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of stockholders. A vote by the holders of a majority of the voting power of the Company’s stockholders is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to the Company’s certificate of incorporation.
Holders of our common stock are entitled to share in all dividends that the board of directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock. The Company’s common stock has no pre-emptive rights, no conversion rights and there are no withdrawal provisions applicable to the Company’s common stock.
Pre-Funded Warrants to be issued in this offering
The following summary of certain terms and provisions of the Pre-Funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of, the Pre-Funded Warrant. Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant for a complete description of the terms and conditions of the Pre-Funded Warrants.
The term “pre-funded” refers to the fact that the purchase price of our common stock in this offering includes almost the entire exercise price that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.001. The purpose of the Pre-Funded Warrants is to enable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, upon election of the holder, 9.99%) of our outstanding shares of common stock following the consummation of this offering the opportunity to make an investment in the Company without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of our common stock which would result in such ownership of more than 4.99% (or 9.99%), and receive the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at such nominal price at a later date.
Duration. The Pre-Funded Warrants offered hereby will entitle the holders thereof to purchase our shares of common stock at a nominal exercise price of $0.001 per share, commencing immediately on the date of issuance. There is no expiration date for the Pre-Funded Warrants.
Exercise Limitation. A holder will not have the right to exercise any portion of the Pre-Funded Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, upon election of the holder, 9.99%) of the number of our shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. However, any holder may increase or decrease such percentage (up to 9.99%), provided that any increase will not be effective until the 61st day after such election. It is the responsibility of the holder to determine whether any exercise would exceed the exercise limitation.
Exercise Price. The Pre-Funded Warrants will have an exercise price of $0.001 per share. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our shareholders.
Transferability. Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned without our consent.
Absence of Trading Market. There is no established trading market for the Pre-Funded Warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Pre-Funded Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Pre-Funded Warrants will be limited.
Fundamental Transactions. In the event of a fundamental transaction, generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation, merger, amalgamation or arrangement with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holder will have the right to receive, for each share of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of the successor or acquiring corporation or of us if we are the surviving corporation, and any additional consideration receivable as a result of such fundamental transaction by a holder of the number of shares for which the Pre-Funded Warrant was exercisable immediately prior to such fundamental transaction. The holders of the Pre-Funded Warrants may also require us to purchase the Pre-Funded Warrants from the holders by paying to each holder an amount equal to the Black Scholes value of the remaining unexercised portion of the Pre-Funded Warrants on the date of the fundamental transaction.
No Rights as a Shareholder. Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of our shares of common stock, the holder of Pre-Funded Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Pre-Funded Warrant.
Warrant Stockholder Approval
Under Nasdaq listing rules, the alternative cashless exercise option (described below) in the Series A Warrants, certain anti-dilution provisions in the Series B Warrants (described below), and the reverse stock split provision in both Series A Warrants and Series B Warrants (each described below) will not be effective until, and unless, we obtain the approval of our stockholders. While we intend to promptly seek stockholder approval, there is no guarantee that the Warrant Stockholder Approval will ever be obtained. If we are unable to obtain the Warrant Stockholder Approval, the foregoing provisions will not become effective and the Series A Warrants and Series B Warrants will have substantially less value. In addition, we will incur substantial cost, and management will devote substantial time and attention, in attempting to obtain the Warrant Stockholder Approval.
Series A Warrants and Series B Warrants to be issued in this offering
The following summary of certain terms and provisions of the Series A Warrants and Series B Warrants included in the Units and Pre-offered hereby is not complete and is subject to, and qualified in its entirety by the provisions of the forms of Series A Warrant and Series B Warrant, which are filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the forms of Series A Warrant and Series B Warrant.
Exercisability. The Series A Warrants and Series B Warrants are exercisable immediately and at any time up to the date that is two-and-a-half years (with respect to the Series A Warrants) or five years (with respect to the Series B Warrants) after their original issuance. The Series A Warrants and Series B Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares of common stock underlying the Series A Warrants and Series B Warrants under the Securities Act is effective and available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise. If a registration statement registering the issuance of the shares of common stock underlying the Series A Warrants or Series B Warrants under the Securities Act is not effective, the holder may elect to exercise the Series A Warrants or Series B Warrants through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the warrant. No fractional shares of common stock will be issued in connection with the exercise of Series A Warrants or Series B Warrants. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
On or after receipt of the Warrant Stockholder Approval, a holder may also effect an “alternative cashless exercise” at any time while the Series A Warrants are outstanding. In such event, the aggregate number of shares issuable in such alternative cashless exercise will be equal to the number of Series A Warrants being exercised multiplied by two.
Exercise Limitation. A holder will not have the right to exercise any portion of the Series A Warrants or Series B Warrants if the holder (together with its affiliates) would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Series A Warrants and Series B Warrants. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days following notice from the holder to us.
Exercise Price. The exercise price per whole share of common stock purchasable upon exercise of the Series A Warrants is $3.825, and the exercise price per whole share of common stock purchasable upon exercise of the Series B Warrants is $4.335. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Subsequent Financing. In addition, conditioned upon the receipt of the Warrant Stockholder Approval, and subject to certain exemptions, if we sell, enter into an agreement to sell, or grant any option to purchase, or sell, enter into an agreement to sell, or grant any right to reprice, or otherwise dispose of or issue (or announce any offer, sale, grant or any option to purchase or other disposition) any shares of common stock, at an effective price per share less than the exercise price of the Series B Warrants then in effect, the exercise price of the Series B Warrants will be reduced to such price (subject to a floor of $[*] prior to the Warrant Stockholder Approval), and the number of shares issuable upon exercise will be proportionately adjusted such that the aggregate exercise price will remain unchanged.
Reverse Stock Split. Conditioned upon the receipt of the Warrant Stockholder Approval, if at any time on or after the date of issuance there occurs any share split, share dividend, share combination recapitalization or other similar transaction involving our common stock and the lowest daily volume weighted average price during the period commencing five consecutive trading days immediately preceding and the five consecutive trading days immediately following such event is less than the exercise price of the Series A Warrants or Series B Warrants then in effect, then the exercise price of the Series A Warrants and Series B Warrants will be reduced to the lowest daily volume weighted average price during such period and the number of shares issuable upon exercise will be proportionately adjusted such that the aggregate price will remain unchanged.
Transferability. Subject to applicable laws, the Series A Warrants and Series B Warrants may be offered for sale, sold, transferred or assigned without our consent.
Warrant Agent. The Series A Warrants and Series B Warrants will be issued in registered form under a warrant agency agreement between Equiniti Trust Company, as warrant agent, and us. The Series A Warrants and Series B Warrants will initially be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Series A Warrants and Series B Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Series A Warrants and Series B Warrants will be entitled to receive upon exercise of the Series A Warrants and Series B Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Series A Warrants and Series B Warrants immediately prior to such fundamental transaction. The holders of the Series A Warrants and Series B Warrants may also require us to purchase the Series A Warrants and Series B Warrants from the holders by paying to each holder an amount equal to the Black Scholes value of the remaining unexercised portion of the Series A Warrants and Series B Warrants on the date of the fundamental transaction.
Rights as a Stockholder. Except as otherwise provided in the Series A Warrants or Series B Warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a Series A Warrants or Series B Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Series A Warrant or Series B Warrants.
Governing Law. The Series A Warrants, Series B Warrants, and the warrant agency agreement are governed by New York law.
Reverse Stock Split. The Company shall effect a reverse stock split within seven (7) business days after the date that is the earlier of the date on which (x) the first meeting of stockholders to obtain Warrant Stockholder Approval is held or (y) the items to be approved under the Warrant Stockholder Approval have been approved in accordance with the applicable laws and corporate governing documents of the Company (the “First Reverse Split Date”). No reverse stock split shall be effectuated before the First Reverse Split Date, except if the consent has been obtained from a purchasers of the majority of the Units.
Blank Check Preferred Stock
Our articles of incorporation authorize the issuance of 30,000,000 shares of preferred stock, par value $0.10 per share, in one or more series, subject to any limitations prescribed by law, without further vote or action by the stockholders. Each such series of preferred stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by our board of directors, which may include, among others, dividend rights, voting rights, liquidation preferences, conversion rights and preemptive rights.
Series B Preferred Stock
1,000,000 shares of our authorized preferred stock have been designated Series B Preferred Stock. 30,000 shares of Series B Preferred Stock are outstanding and held by our chief executive officer, Dr. Steve N. Slilaty.
The Series B Preferred Stock votes together with the common stock on all matters submitted to a vote of the Company’s stockholders. Each share of Series B Preferred Stock entitles the holder to 1,000 votes.
Upon any liquidation or dissolution of the Company, the Series B Preferred Stock will be entitled to a payment equal to the stated value of $0.10 per share, prior to any payments being made with respect to the common stock. The Series B Preferred Stock is not redeemable by the Company and is entitled to dividends when, as and if declared by the board of directors in its sole discretion.
UNDERWRITING
Aegis Capital Corp., or Aegis, is acting as the underwriter of the offering. We have entered into an underwriting agreement dated , 2024 with the underwriter. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of Units including a share of common stock, and the number of Units including a Pre-Funded Warrants, listed next to its name in the following table:
| Underwriter | | Page No.Number of Units including Common Stock | | Number of
Units including Pre-Funded Warrant |
| Aegis Capital Corp. | | | | - |
| Total | | | | |
The underwriter is committed to purchase all the Units offered by us, other than those covered by the over-allotment option described below, if they purchase any Units. The obligations of the underwriter may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriter’s obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriter of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriter against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriter may be required to make in respect thereof.
The underwriter is offering the Units, shares of common stock, Pre-Funded Warrants, Series A Warrants and Series B Warrants subject to prior sale, when, as and if issued to and accepted by it, subject to approval of legal matters by its counsel and other conditions specified in the underwriting agreement. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Over-allotment Option
We have granted to the underwriter an Over-Allotment Option exercisable not later than 45 days after the closing date of this offering to purchase up to a number of additional shares of Common Stock and/or Pre-Funded Warrants and/or Series A Warrants and/or Series B Warrants equal to 15% of the number of securities sold in this offering at the applicable public offering price listed on the cover of this prospectus, less the underwriting discounts and commissions. The underwriter may exercise its Over-Allotment Option, if any, made in connection with this offering. If any additional shares of Common Stock and/or Pre-Funded Warrants and/or Series A Warrants and/or Series B Warrants are purchased, the underwriter will offer these securities on the same terms as those on which the other securities are being offered.
Discounts, Commissions and Reimbursement
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriter of its over-allotment option.
Item 1. | Unaudited Consolidated Financial Statements | F-1Per Unit including Common Stock | | | Per Unit including Pre-Funded Warrant | | | Total with no Over-Allotment | | | Total with Over-Allotment | |
| Public offering price | | $ | | | | $ | | | | $ | | | | $ | | |
| Underwriting discounts and commissions (8.0%) | | $ | | | | $ | | | | $ | | | | $ | | |
| Non-accountable expense allowance (1.0%)(1) | | $ | | | | $ | | | | $ | | | | $ | | |
| Proceeds, before expenses, to us | | $ | | | | $ | | | | $ | | | | $ | | |
(1) We have agreed to pay a non-accountable expense allowance to the underwriter equal to 1.0% of the gross proceeds received in this offering.
The underwriter proposes to offer the Units to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriter may offer some of the Units to other securities dealers at such price less a concession not in excess of $ per Unit. If all of the Units offered by us are not sold at the public offering price, the underwriter may change the offering price and other selling terms by means of a supplement to this prospectus.
We have also agreed to pay $150,000 of the underwriter’s legal expenses relating to the offering.
We estimate that the total expenses of the offering payable by us, excluding the discount and non-accountable expense allowance, will be approximately $650,000.
Discretionary Accounts
The underwriter does not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to “lock-up” agreements, our executive officers and directors and shareholders holding at least five percent (5%) of the outstanding shares of common stock have agreed, subject to limited exceptions, without the prior written consent of the underwriter not to directly or indirectly offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities or publicly disclose the intention to do any of the foregoing, for a period of 90 days from the closing date of this offering.
Company Standstill
We have agreed that without the prior written consent of the underwriter, we will not, for a period of ninety (90) days after the closing of this offering, subject to certain exceptions, (a) offer, sell, issue, or otherwise transfer or dispose of, directly or indirectly, any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; (b) file or caused to be filed any registration statement with the Commission relating to the offering of any equity of the Company or any securities convertible into or exercisable or exchangeable for equity of the Company; or (c) enter into any agreement or announce the intention to effect any of the actions described in subsections (a) or (b) hereof.
Right of First Refusal
We have granted the underwriter a right of first refusal, for a period of three years from the consummation of this offering, to act as sole book-runner, sole manager, sole placement agent, sole agent, sole book-runner, sole book-running manger and/or sole underwriter, at the underwriter’s sole discretion, for each and every future public and private equity or debt offering or debt refinancing, including all equity linked financings (each, a “Subject Transaction”), during such three year period, of the Company, or any successor to or subsidiary of the Company, on terms and conditions customary to the underwriter for such Subject Transactions.
Tail Financing
In addition, we have agreed to pay the above cash compensation to the extent that any fund which the underwriter contacted or introduced to us during the term of our engagement agreement with the underwriter dated January 23, 2024, provides financing or capital in any public or private offering or capital raising transaction during the three-month period following the closing of this offering or expiration or termination of our engagement letter with the underwriter dated January 23, 2024.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriter or selling group members. The underwriter may agree to allocate a number of securities to underwriter and selling group members for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriter and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
Stabilization
In connection with this offering, the underwriter may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress.
Over-allotment transactions involve sales by the underwriter of shares in excess of the number of shares the underwriter is obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriter may close out any short position by exercising its over-allotment option and/or purchasing shares in the open market.
Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the over-allotment option. If the underwriter sells more shares than could be covered by exercise of the over-allotment option and, therefore, has a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be affected in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making
In connection with this offering, the underwriter and selling group members may engage in passive market making transactions in our common stock on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships
The underwriter and its affiliates have in the past and may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they have in the past and may in the future receive customary fees. In February 2022, Aegis served as the underwriter in connection with a public offering of units, with each unit consisting of one share of common stock and two warrants, each warrant exercisable for one share of common stock, pursuant to an underwriting agreement between Aegis and us containing standard terms. Aegis received an underwriting discount of 8%, and a non-accountable expense allowance equal to 1% of the gross proceeds of the public offering. In March 2022, Aegis acted as the placement agent in connection with a private placement for the Company’s common stock or pre-funded warrants and warrants exercisable for common stock. Aegis was paid a commission equal to 10% of the gross proceeds received by the Company in the private placement and 2% of the gross proceeds as a non-accountable expense allowance. The Company paid Aegis certain fees and expenses including attorney fees. In April 2022, Aegis acted as the placement agent in connection with the private placement for the Company’s common stock or pre-funded warrants and warrants exercisable for common stock. Aegis was paid a commission equal to 10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 5% of the proceeds from any exercise of warrants, payable on exercise. In May 2023, Aegis acted as the placement agent in connection with the private placement for the Company’s common stock, pre-funded warrants, each exercisable to purchase one share of common stock, and warrants, each exercisable to purchase one share of common stock. Aegis was paid a commission equal to 10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 10% of the proceeds from any exercise of warrants, payable on exercise.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
LEGAL MATTERS
We are being represented by Sichenzia Ross Ference Carmel LLP, New York, New York, with respect to certain legal matters as to United States federal securities and New York State law. The enforceability of the pre-funded warrants, Series A Warrants, and Series B Warrants will be passed upon for us by Sichenzia Ross Ference Carmel LLP, New York, New York. The validity of the securities being offered by this prospectus, including the shares, Units, Pre-Funded Warrants, Series A Warrants, Series B Warrants, and shares underlying the Pre-Funded Warrants, Series A Warrants, and Series B Warrants, will be passed upon for us by Andrew I. Telsey, P.C., Englewood, Colorado. Certain legal matters in connection with this offering have been passed upon for the underwriter by Kaufman & Canoles, P.C., Richmond, Virginia.
EXPERTS
The consolidated financial statements of Sunshine Biopharma, Inc. at December 31, 2022 and 2021, and for each of the two years in the period ended December 31, 2022, included in this prospectus have been audited by B F Borgers CPA PC, independent registered public accounting firm, as set forth in their report thereon, appearing therein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus, which constitutes a part of the registration statement on Form S-1 that we have filed with the SEC under the Securities Act, does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the securities offered by this prospectus, you should refer to the registration statement and the exhibits filed as part of that document. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We are subject to the reporting requirements of the Exchange Act, and file annual, quarterly and current reports, and other information with the SEC. The SEC maintains an Internet site that contains these reports and other information filed electronically by us with the SEC, which are available on the SEC’s website at http://www.sec.gov. We also maintain a website at https://sunshinebiopharma.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
Sunshine Biopharma, Inc.
CONSOLIDATED FINANCIAL STATEMENTS
At September 30, 2023 and December 31, 2022
TABLE OF CONTENTS
CONSOLIDATED FINANCIAL STATEMENTS
With Independent Accountant’s Audit Report
At December 31, 2022 and 2021
Sunshine Biopharma, Inc.
Consolidated Balance Sheets
| | | | | | | |
| | | September 30, | | | December 31, | |
| | | 2023 | | | 2022 | |
| | | (Unaudited) | | | | |
| ASSETS | | | | | | | | |
| Current Assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 18,846,140 | | | $ | 21,826,437 | |
| Accounts receivable | | | 2,034,119 | | | | 1,912,153 | |
| Inventory | | | 4,517,044 | | | | 3,289,945 | |
| Prepaid expenses | | | 37,556 | | | | 283,799 | |
| Total Current Assets | | | 25,434,859 | | | | 27,312,334 | |
| | | | | | | | | |
| Property and equipment | | | 334,922 | | | | 394,249 | |
| Intangible assets | | | 1,216,207 | | | | 776,856 | |
| Right-of-use-asset | | | 664,296 | | | | 760,409 | |
| TOTAL ASSETS | | $ | 27,650,284 | | | $ | 29,243,848 | |
| | | | | | | | | |
| LIABILITIES | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable and accrued expenses | | $ | 2,220,870 | | | $ | 2,802,797 | |
| Earnout payable | | | 2,547,831 | | | | 3,632,000 | |
| Income tax payable | | | 201,541 | | | | 373,191 | |
| Right-of-use-liability | | | 117,840 | | | | 123,026 | |
| Total Current Liabilities | | | 5,088,082 | | | | 6,931,014 | |
| | | | | | | | | |
| Long-Term Liabilities: | | | | | | | | |
| Deferred tax liability | | | 43,032 | | | | 43,032 | |
| Right-of-use-liability | | | 555,687 | | | | 642,232 | |
| Total Long-Term Liabilities | | | 598,719 | | | | 685,264 | |
| TOTAL LIABILITIES | | | 5,686,801 | | | | 7,616,278 | |
| | | | | | | | | |
| SHAREHOLDERS' EQUITY | | | | | | | | |
| Preferred Stock, Series B $0.10 par value per share; 1,000,000 shares authorized; 10,000 Shares issued and outstanding | | | 1,000 | | | | 1,000 | |
| Common Stock, $0.001 par value per share; 3,000,000,000 shares authorized; 25,678,290 and 22,585,632 shares issued and outstanding as of September 30, 2023 and December 31, 2022, respectively | | | 25,678 | | | | 22,585 | |
| Capital paid in excess of par value | | | 84,387,890 | | | | 80,841,752 | |
| Accumulated comprehensive income | | | 204,549 | | | | 161,847 | |
| Accumulated (Deficit) | | | (62,655,634 | ) | | | (59,399,614 | ) |
| TOTAL SHAREHOLDERS' EQUITY | | | 21,963,483 | | | | 21,627,570 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | | $ | 27,650,284 | | | $ | 29,243,848 | |
The accompanying notes are an integral part of these unaudited financial statements
Sunshine Biopharma, Inc.
Consolidated Statements of Operations and Comprehensive Loss (Unaudited)
| | | | | | | | | | | | | | | | | |
| | | 3 Months Ended September 30, | | | 9 Months Ended September 30, | |
| | | 2023 | | | 2022 | | | 2023 | | | 2022 | |
| | | | | | | | | | | | | |
| Sales | | $ | 5,957,668 | | | $ | 132,808 | | | $ | 16,412,586 | | | $ | 405,760 | |
| Cost of sales | | | 3,967,412 | | | | 65,783 | | | | 10,641,461 | | | | 200,311 | |
| Gross profit | | | 1,990,256 | | | | 67,025 | | | | 5,771,125 | | | | 205,449 | |
| | | | | | | | | | | | | | | | | |
| General and Administrative Expenses: | | | | | | | | | | | | | | | | |
| Accounting | | | 56,350 | | | | 122,913 | | | | 301,381 | | | | 237,773 | |
| Consulting | | | 221,781 | | | | 162,852 | | | | 745,850 | | | | 270,033 | |
| Director fees | | | 100,000 | | | | 100,000 | | | | 300,000 | | | | 200,000 | |
| Legal | | | 133,302 | | | | 146,467 | | | | 392,874 | | | | 403,386 | |
| Marketing | | | 241,897 | | | | 217,666 | | | | 502,987 | | | | 400,386 | |
| Office | | | 544,215 | | | | 76,818 | | | | 1,422,058 | | | | 449,730 | |
| R&D | | | 238,012 | | | | 362,500 | | | | 1,039,502 | | | | 770,095 | |
| Salaries | | | 1,144,377 | | | | 595,000 | | | | 4,344,801 | | | | 1,105,000 | |
| Taxes | | | 52,586 | | | | – | | | | 212,953 | | | | – | |
| Depreciation | | | 37,210 | | | | 789 | | | | 106,797 | | | | 6,186 | |
| Total General and Administrative Expenses: | | | 2,769,730 | | | | 1,785,005 | | | | 9,369,203 | | | | 3,842,589 | |
| | | | | | | | | | | | | | | | | |
| (Loss) from operations | | | (779,474 | ) | | | (1,717,980 | ) | | | (3,598,078 | ) | | | (3,637,140 | ) |
| | | | | | | | | | | | | | | | | |
| Other Income (Expense): | | | | | | | | | | | | | | | | |
| Foreign exchange | | | 40 | | | | 25 | | | | (206 | ) | | | 45 | |
| Interest income | | | 207,431 | | | | 260,938 | | | | 624,361 | | | | 406,984 | |
| Debt release | | | – | | | | – | | | | – | | | | 10,852 | |
| Interest expense | | | (38,527 | ) | | | (2 | ) | | | (107,198 | ) | | | (12,866 | ) |
| Total Other Income (Expense) | | | 168,944 | | | | 260,961 | | | | 516,957 | | | | 405,015 | |
| | | | | | | | | | | | | | | | | |
| Provision for income taxes | | | (40,952 | ) | | | – | | | | (174,899 | ) | | | – | |
| Net (Loss) | | $ | (651,482 | ) | | $ | (1,457,019 | ) | | $ | (3,256,020 | ) | | $ | (3,232,125 | ) |
| | | | | | | | | | | | | | | | | |
| Gain (Loss) from foreign exchange translation | | | (460,507 | ) | | | (45,126 | ) | | | 42,702 | | | | (56,764 | ) |
| Comprehensive (Loss) | | $ | (1,111,989 | ) | | $ | (1,502,145 | ) | | $ | (3,213,318 | ) | | $ | (3,288,889 | ) |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Basic (Loss) per common share | | $ | (0.04 | ) | | $ | (0.08 | ) | | $ | (0.133 | ) | | $ | (0.26 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted Average Common Shares Outstanding (Basic and Diluted) | | | 25,690,449 | | | | 18,885,632 | | | | 24,507,122 | | | | 12,789,733 | |
The accompanying notes are an integral part of these unaudited financial statements
Sunshine Biopharma, Inc.
Consolidated Statements of Cash Flows (Unaudited)
| | | | | | | |
| | | September 30, | | | September 30, | |
| | | 2023 | | | 2022 | |
| | | | | | | |
| Cash Flows From Operating Activities: | | | | | | | | |
| Net (Loss) | | $ | (3,256,020 | ) | | $ | (3,232,125 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 106,794 | | | | 6,186 | |
| Foreign exchange | | | (374 | ) | | | 45 | |
| Debt release | | | – | | | | (10,852 | ) |
| Accounts receivable | | | (118,482 | ) | | | 7,776 | |
| Inventory | | | (1,221,112 | ) | | | (163,991 | ) |
| Prepaid expenses | | | 247,977 | | | | 2,235 | |
| Accounts payable and accrued expenses | | | (587,973 | ) | | | 437,267 | |
| Income tax payable | | | (172,076 | ) | | | – | |
| Interest payable | | | (1,084,169 | ) | | | (48,287 | ) |
| Net Cash Flows (Used) in Operations | | | (6,085,435 | ) | | | (3,001,746 | ) |
| | | | | | | | | |
| Cash Flows From Investing Activities: | | | | | | | | |
| Reduction in Right-of-use asset | | | 97,498 | | | | – | |
| Purchase of intangible assets | | | (19,804 | ) | | | – | |
| Purchase of equipment | | | (464,614 | ) | | | – | |
| Net Cash Flows (Used) in Investing Activities | | | (386,920 | ) | | | – | |
| | | | | | | | | |
| Cash Flows From Financing Activities: | | | | | | | | |
| Common stock issued | | | 4,089,218 | | | | 43,560,363 | |
| Exercise of warrants | | | 1,156 | | | | – | |
| Purchase of treasury stock | | | (541,143 | ) | | | (99,000 | ) |
| Lease liability | | | (93,125 | ) | | | – | |
| Payments of notes payable | | | – | | | | (1,900,000 | ) |
| Net Cash Flows Provided by Financing Activities | | | 3,456,106 | | | | 41,561,363 | |
| | | | | | | | | |
| Cash and Cash Equivalents at Beginning of Period | | | 21,826,437 | | | | 2,045,167 | |
| Net increase (decrease) in cash and cash equivalents | | | (3,016,249 | ) | | | 38,559,617 | |
| Effect of exchange rate changes on cash | | | – | | | | (105,617 | ) |
| Foreign currency translation adjustment | | | 35,952 | | | | 56,764 | |
| Cash and Cash Equivalents at End of Period | | $ | 18,846,140 | | | $ | 40,555,931 | |
| | | | | | | | | |
| Supplementary Disclosure of Cash Flow Information: | | | – | | | | – | |
| Cash paid for interest | | $ | – | | | $ | 61,151 | |
| Cash paid for income taxes | | $ | – | | | $ | – | |
The accompanying notes are an integral part of these unaudited financial statements
Sunshine Biopharma, Inc.
Consolidated Statement of Shareholders' Equity (Unaudited)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Number Of Common Shares | | | Common | | | Capital Paid in Excess of Par | | | Number Of Preferred Shares | | | Preferred | | | Comprehensive | | | Accumulated | | | | |
| | | Issued | | | Stock | | | Value | | | Issued | | | Stock | | | Income | | | Deficit | | | Total | |
| Three Month Period Ended September 30, 2023 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at June 30, 2023 | | | 25,746,302 | | | $ | 25,746 | | | $ | 84,422,143 | | | | 10,000 | | | $ | 1,000 | | | $ | 665,056 | | | $ | (62,004,152 | ) | | $ | 23,109,793 | |
| Repurchase stock | | | (68,012 | ) | | | (68 | ) | | | (34,253 | ) | | | – | | | | – | | | | – | | | | – | | | | – | |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | (460,507 | ) | | | (651,482 | ) | | | (1,111,989 | ) |
| Balance at September 30, 2023 | | | 25,678,290 | | | $ | 25,678 | | | $ | 84,387,890 | | | | 10,000 | | | $ | 1,000 | | | $ | 204,549 | | | $ | (62,655,634 | ) | | $ | 21,963,483 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Nine Month Period Ended September 30, 2023 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance December 31, 2022 | | | 22,585,632 | | | $ | 22,585 | | | $ | 80,841,752 | | | | 10,000 | | | $ | 1,000 | | | $ | 161,847 | | | $ | (59,399,614 | ) | | $ | 21,627,570 | |
| Repurchase of common stock | | | (513,723 | ) | | | (514 | ) | | | (540,629 | ) | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock and pre-funded warrants issued in a private offering | | | 2,450,000 | | | | 2,451 | | | | 4,086,767 | | | | – | | | | – | | | | – | | | | – | | | | 4,089,218 | |
| Exercise of warrants | | | 1,156,381 | | | | 1,156 | | | | – | | | | – | | | | – | | | | – | | | | – | | | | 1,156 | |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | 42,702 | | | | (3,256,020 | ) | | | (3,213,318 | ) |
| Balance at September 30, 2023 | | | 25,678,290 | | | $ | 25,678 | | | $ | 84,387,890 | | | | 10,000 | | | $ | 1,000 | | | $ | 204,549 | | | $ | (62,655,634 | ) | | $ | 21,963,483 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Three Month Period Ended September 30, 2022 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at June 30, 2022 | | | 18,885,632 | | | $ | 18,886 | | | $ | 76,331,451 | | | | 10,000 | | | $ | 1,000 | | | $ | (34,777 | ) | | $ | (34,430,280 | ) | | $ | 41,886,280 | |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | (45,126 | ) | | | (1,457,019 | ) | | | (1,502,145 | ) |
| Balance at September 30, 2022 | | | 18,885,632 | | | $ | 18,886 | | | $ | 76,331,451 | | | | 10,000 | | | $ | 1,000 | | | $ | (79,903 | ) | | $ | (35,887,299 | ) | | $ | 40,384,135 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Nine Month Period Ended September 30, 2022 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance December 31, 2021 | | | 2,595,620 | | | $ | 2,596 | | | $ | 32,787,379 | | | | 1,000,000 | | | $ | 100,000 | | | $ | (23,139 | ) | | $ | (32,655,174 | ) | | $ | 211,662 | |
| Common stock and pre-funded warrants issued in public offering | | | 6,656,526 | | | | 6,657 | | | | 30,360,528 | | | | – | | | | – | | | | – | | | | – | | | | 30,367,185 | |
| Exercise of warrants | | | 9,633,486 | | | | 9,633 | | | | 13,183,544 | | | | – | | | | – | | | | – | | | | – | | | | 13,193,177 | |
| Preferred stock purchased from related party | | | – | | | | – | | | | – | | | | (990,000 | ) | | | (99,000 | ) | | | – | | | | – | | | | (99,000 | ) |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | (56,764 | ) | | | (3,232,125 | ) | | | (3,288,889 | ) |
| Balance at September 30, 2022 | | | 18,885,632 | | | $ | 18,886 | | | $ | 76,331,451 | | | | 10,000 | | | $ | 1,000 | | | $ | (79,903 | ) | | $ | (35,887,299 | ) | | $ | 40,384,135 | |
The accompanying notes are an integral part of these unaudited financial statements
Sunshine Biopharma, Inc.
Notes to Unaudited Consolidated Financial Statements
|
Consolidated Condensed Balance Sheet |
|
| | | |
| | | |
| | | |
| ASSETS | | |
| Current Assets: | | |
| Cash and cash equivalents | $36,395 | $107,532 |
| Accounts receivable | 108,230 | - |
| Other receivable | 51,000 | - |
| Prepaid expenses | 1,089 | 9,667 |
| | | |
| Total Current Assets | 196,714 | 117,199 |
| | | |
| Equipment (net of $25,864 and $9,132 depreciation resepctively) | 299,946 | 59,996 |
Patents (net of $58,918 amortization and $556,120 impairment) | - | -
|
| Deposits | - | 80,290 |
| Goodwill | 673,646 | - |
| | | |
| TOTAL ASSETS | $1,170,306 | $257,485 |
| | | |
| LIABILITIES AND SHAREHOLDERS' EQUITY | | |
| | | |
| Current Liabilities: | | |
| Notes payable | 719,730 | 516,867 |
| Notes payable - related party | 242,672 | 205,742 |
| Bank overdraft | 1,012 | - |
| Accounts payable & accrued expenses | 139,020 | 19,314 |
| Interest payable | 34,334 | 9,215 |
| | | |
| Total Current Liabilities | 1,136,768 | 751,138 |
| | | |
| Long-term Liabilities: | | |
| Note payable | - | 79,710 |
| Related party note payable | 309,668 | - |
| | | |
| TOTAL LIABILITIES | 1,446,436 | 830,848 |
| | | |
| COMMITMENTS AND CONTINGENCIES | | |
| | | |
| SHAREHOLDERS' EQUITY (DEFICIT) | | |
| | | |
| Preferred stock, Series A $0.10 par value per share; | | |
Authorized 850,000 Shares; Issued and outstanding -0- shares. | -
| -
|
| | | |
| Preferred stock, Series B $0.10 par value per share; | | |
Authorized 500,000 Shares; Issued and outstanding 500,000 shares. | 50,000
| 50,000
|
| | | |
| Common Stock, $0.001 par value per share; | | |
| Authorized 3,000,000,000 Shares; Issued | | |
| and outstanding 1,104,105,806 and 918,736,498 at | | |
| June 30, 2018 and December 31, 2017 respectively | 1,104,106 | 918,737 |
Reserved for issuance 572,727,700 shares at June 30, 2018
| | |
| | |
| Capital paid in excess of par value | 13,106,164 | 12,075,586 |
| | | |
| Accumulated comprehensive income | (8,266) | 504 |
| | | |
| Accumulated (Deficit) | (14,528,134) | (13,618,190) |
| | | |
| TOTAL SHAREHOLDERS' EQUITY (DEFICIT) | (276,130) | (573,363) |
| | | |
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT) | $1,170,306 | $257,485 |
See Accompanying Notes To These Financial Statements.
|
Unaudited Consolidated Condensed Statement Of Operations and Comprehensive Loss |
|
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| | | | | |
| Revenue: | $107,250 | $- | $198,418 | $- |
Cost of Revenue
| 91,631 | - | 190,913 | - |
|
| |
| |
| 15,619
| - | 7,505
| - |
| | | | | |
| General & Administrative Expenses | | | | |
| Accounting | 61,939 | 48,415 | 89,939 | 64,015 |
| Consulting | 23,682
| 33,930 | 27,800
| 59,867 |
| Legal | 31,600 | 27,920 | 59,085 | 42,824 |
| Office | 20,068 | 13,032 | 39,116 | 22,098 |
| Officer & director renumeration | 446,644 | 348,415 | 524,431 | 391,380 |
| Rent | 2,035
| | 3,572
| |
| Depreciation | 602
| 506 | 1,210
| 1,024 |
| | | | | |
| Total G & A | 586,570
| 472,218 | 745,153
| 581,208 |
| | | | | |
| (Loss) from operations | (570,951) | (472,218) | (737,648) | (581,208) |
| | | | | |
| Other Income (expense): | | | | |
| Foreign exchange gain (loss) | 10,016 | (3,628) | 24,884 | (4,267) |
| Interest expense | (28,375) | (9,598) | (103,842) | (18,742) |
| Loss on debt conversions | (54,998) | - | (93,338) | (76,929) |
| | | | | |
| Total Other (Expense) | (73,357) | (13,226) | (172,296) | (99,938) |
| | | | | |
| Net (loss) | $(644,308) | $(485,444) | $(909,944) | $(681,146) |
| | | | | |
| Basic (Loss) per common share | $0.00 | $0.00 | $0.00 | $0.00 |
| | | | | |
| Weighted Average Common Shares Outstanding | 1,000,371,607 | 857,473,771 | 971,151,423 | 811,800,080 |
| | | | | |
| Net Income (Loss) | $(644,308) | $(485,444) | $(909,944) | $(681,146) |
| Other comprehensive income: | | | | |
| Gain (Loss) from foreign exchange translation | (4,056) | 2,680 | (5,786) | 3,795 |
| Comprehensive (Loss) | (648,364) | (482,764) | (915,730) | (677,351) |
| | | | | |
| Basic (Loss) per Common Share | $0.00 | $0.00 | $0.00 | $0.00 |
| | | | | |
| Weighted Average Common Shares Outstanding | 1,000,371,607 | 857,473,771 | 971,151,423 | 811,800,080 |
See Accompanying Notes To These Financial Statements.
|
Unaudited Consolidated Condensed Statement Of Cash Flows |
|
| | | |
| | | |
| | | |
| | | |
| | | |
Cash Flows From Operating Activities: | | |
| Net Loss | $(909,944) | $(681,146) |
| Depreciation and amortization | 16,404 | 1,024 |
| Foreign exchange loss | (24,884) | 4,267 |
| Stock issued for licenses, services, and other assets | 505,100
| 64,000 |
| Stock issued for payment interest | 7,886 | 3,022 |
| Loss on debt conversion | 93,585 | 76,929 |
| Interest forgiven | (247) | |
| Stock issued for payment of expenses | - | 14,400 |
| (Increase) decrease in accounts receivable | (28,722) | - |
| (Increase) decrease in prepaid expenses | 8,578 | (7,530) |
| Increase (decrease) in Accounts Payable & accrued expenses | 14,227
| 342,773 |
| Increase (decrease) in interest payable | 25,119 | (3,589) |
| | | |
| Net Cash Flows Used in Operations | (292,898) | (185,850) |
| | | |
Cash Flows From Investing Activities: | | |
| Cash received from acquisition of subsidiary | 4,942 | |
| Purchase of equipment | (27,370) | (22,295) |
| | | |
| Net Cash Flows Used in Investing Activities | (22,428) | (22,295) |
| | | |
Cash Flows From Financing Activities: | | |
| Proceed from notes payables | 381,885 | 188,444 |
| Sale of common stock | | 63,912 |
| Payment of notes payable | (146,184) | - |
| Advances from related parties | 12,240 | |
| Payments to related parties | (13,216) | |
| Note payable used to pay expenses | | 13,962 |
| Note payable used to pay origination fees & interest | 15,250 | 9,347 |
| | | |
| Net Cash Flows Provided by Financing Activities | 249,975
| 275,665 |
| | | |
| | | |
| Net Increase (Decrease) In Cash and cash equivalents | (65,351) | 67,520 |
| Foreign currency translation adjustment | (5,786) | 3,795 |
| Cash and cash equivalents at beginning of period | 107,532 | 57,453 |
| | | |
| Cash and cash equivalents at end of period | $36,395
| $128,768 |
| | - | |
Supplementary Disclosure Of Cash Flow Information: | | |
| Stock issued for services, licenses and other assets | $679,908
| $78,400 |
| Stock issued for note conversions including interest | $290,039 | $128,451 |
| Stock issued for acqusition of subsidiary | $246,000 | $- |
Note payable issued for acquisition of subsidiary
| $358,407
| $-
|
| Cash paid for interest | $13,622 | $- |
| Cash paid for income taxes | $- | $- |
See Accompanying Notes To These Financial Statements.
Notes To Unaudited Consolidated Financial Statements
For The Threethe Nine Months Ended September 30, 2023 and Six Month Interim Period Ended June 30, 2018
2022
Note 1 – NatureDescription of Business and Basis of Presentation
The Company was originally incorporated under the name Mountain West Business Solutions, Inc. (“MBS”) on August 31, 2006, in the State of Colorado. Until October 2009, the Company was operating as a business consultancy firm. Effective October 15, 2009, the Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. Sunshine Biopharma, Inc. was holding an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a. Upon completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and began operating as a pharmaceutical company focusingcompany.
In addition to conducting its own drug development activities, Sunshine Biopharma operates two wholly owned subsidiaries: (i) Nora Pharma Inc. (“Nora Pharma”), a Canadian corporation with a portfolio of pharmaceutical products consisting of 51 generic prescription drugs on the development the licensed Adva-27a anticancer drug.
In July 2014, the Company formed a wholly owned Canadian subsidiary,market in Canada, and (ii) Sunshine Biopharma Canada Inc. (“Sunshine Canada”) for, a Canadian corporation which develops and sells nonprescription over-the-counter (“OTC”) products. In addition to the purposes of offering generic pharmaceutical products in Canada and elsewhere around the world. Sunshine Canada has signed licensing agreements for four (4)51 generic prescription drugs for treatment of breast cancer, prostate cancer and BPH (Benign Prostatic Hyperplasia). Sunshine Canada is currently evaluating several other generic products for in-licensing and is working on securing a Drug Establishment License (“DEL”) and a Drug Identification Number (“DIN”) per product from Health Canada. Once the DEL and the DIN’s are secured, Sunshine Canada will begin its generic pharmaceuticals marketing and sales programmarket in Canada, and overseas.
On January 1, 2018 the Company acquired all of the issuedhas 32 additional generic prescription drugs scheduled to be launched in 2024 and outstanding shares of Atlas Pharma Inc. (“Atlas”), a Canadian privately held company. The purchase price for the shares was Eight Hundred Forty Eight Thousand Dollars $848,000 Canadian ($684,697 US). The purchase price included a cash payment of $100,500 Canadian ($80,289 US), plus the issuance of 20,000,000 shares of the Company’s Common Stock valued at $246,000 or $0.0123 per share, and a promissory note2025 in the principal amount of $450,000 Canadian ($358,407 US), with interest payable at the rate of 3% per annum. Atlas is a certified company dedicated to chemical analysis of pharmaceutical and other industrial samples. Atlas’ operations are authorized by a Drug Establishment License issued by Health Canada. Atlas is also registered with the FDA.
The Company has performed analysisdetermined that it has two reportable segments:
| · | Prescription Generic Pharmaceuticals (“Generic Pharmaceuticals”) |
| · | Nonprescription Over-The-Counter Products (“OTC Products) |
Through December 31, 2022 and as of September 30, 2023, sales from the Generic Pharmaceuticals segment represented approximately 97% of total revenues of the fair market valueCompany while the remaining approximately 3% was generated from the sale of Atlas Pharma Inc. assetsOTC Products. Based on these results, the Company deems segmentation reporting to be immaterial at September 30, 2023.
The Company is not subject to material customer concentration risks as it sells its products directly to pharmacies in several Canadian Provinces. Provincial governments in Canada reimburse patients for their prescription drugs expenditures to various degrees under drug reimbursement programs, making generic drugs prices highly dependent on government regulations which may change over time. The most recent negotiations between the pan-Canadian Pharmaceutical Alliance and liabilities.the Canadian Generic Pharmaceutical Association have resulted in updated generic pricing for certain products which took effect on October 1, 2023. The following table summarizes the allocation of the purchase price as of the acquisition date:
Cash | $4,942
|
Accounts receivable
| 79,508
|
Prepaids | 1,428
|
Propertyupdated prices are valid for three years and equipment | 62,990
|
Goodwill | 673,646
|
| |
Less: Liabilities assumed ($172,899 Canadian) | ( 137,817)
|
| |
Total consideration | $684,697
|
While the agreement
contains an option to
acquire Atlas Pharma Inc. was signed effective January 1, 2018, there are several matters which are yet to be completed. extend for an additional two years.
In addition, as of the date of this report, the audit of Atlas Pharma Inc. has not been completed. As a result, various disclosures in this report may have to be updated. The updated information may differ and the difference may be material.
In March 2018, the Company formed NOX Pharmaceuticals, Inc., a wholly owned Colorado corporation and assigned all of the Company’s interest in the Adva27a anticancer drug to that company. NOX Pharmaceuticals Inc.’s mission is to research, develop and commercialize proprietary drugs including Adva-27a.
The financial statements represent the consolidated activity of Sunshine Biopharma, Inc., Sunshine Biopharma Canada Inc., Atlas Pharma Inc. and NOX Pharmaceuticals, Inc. (herein collectively referred to as the "Company").
Sunshine Biopharma, Inc.
Notes To Unaudited Consolidated Financial Statements
For The Three and Six Month Interim Period Ended June 30, 2018
The Company has been and continues to work on the development of its proprietary anticancer drug, Adva-27a. The next series of stepsengaged in the development of
Adva-27a include (i) GMP-manufacturing of a 2-kilogram quantity of the
drug, (ii) completing the requisite IND-enabling studies, and (iii) conducting Phase I clinical trials. In the preclinical studies, Adva-27a was shown to be effective at destroying multidrug resistant cancer cells including Pancreatic Cancer cells, Breast Cancer cells, Small-Cell Lung Cancer cells, and Uterine Sarcoma cells.following proprietary drugs:
| · | Adva-27a, a small chemotherapy molecule for treatment of pancreatic cancer (IND-enabling studies were paused on November 2, 2023 due to unfavorable results. See Note 13 – Subsequent Events) |
| · | K1.1 mRNA, a lipid nano-particle (LNP) targeted for liver cancer |
| · | SBFM-PL4, a protease inhibitor for treatment of Coronavirus infections |
During the last three month period the Company has continued to raise money through stock sales and borrowings.
The Company’s activities are subject to significant risks and uncertainties, including failing to secure additional funding to operationalize the Company’s generics business and proprietary drug development program.
Note 2 – Basis of Presentation of Unaudited Condensed Financial Information
The unaudited condensed financial statements of the Company for the three and six monthnine months periods ended JuneSeptember 30, 20182023 and 20172022 have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information and pursuant to the requirements for reporting on Form 10-Q and Regulation S-K.S-X. Accordingly, they do not include all the information and footnotes required by accounting principles generally accepted in the United States of America for complete financial statements. However, such information reflects all adjustments (consisting solely of normal recurring adjustments), which are, in the opinion of management, necessary for the fair presentation of the financial position and the results of operations. Results shown for interim periods are not necessarily indicative of the results to be obtained for a full fiscal year. The balance sheet information as of June 30, 2018December 31, 2022, was derived from the audited financial statements included in the Company's financial statements as of and for the year ended December 31, 20172022, included in the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”) on April 2, 2018.4, 2023. These financial statements should be read in conjunction with that report.
Recently Issued Accounting Pronouncements
Recently issued amendments by the FASB are effective for fiscal years beginning after December 15, 2017, and should be applied prospectively on or after the adoption date. Early adoption is permitted, including adoption in an interim period. The Company does not expect these amendments to have a material impact on its financial statements.
In March 2017, the FASB issued ASU No. 2017-08, Receivables—Nonrefundable Fees and Other Costs (Subtopic 310-20): Premium Amortization on Purchased Callable Debt Securities, to amend the amortization period for certain purchased callable debt securities held at a premium. The ASU shortens the amortization period for the premium to the earliest call date. Under current Generally Accepted Accounting Principles (“GAAP”), entities generally amortize the premium as an adjustment of yield over the contractual life of the instrument. The amendments should be applied on a modified retrospective basis, and are effective for fiscal years beginning after December 15, 2018. Early adoption is permitted, including adoption in an interim period. The Company is currently evaluating the impact of this amendment on its financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases, effective for annual reporting periods beginning on or after December 15, 2018, and interim periods within those annual periods. Earlier application is permitted as of the beginning of an interim or annual period. This update requires organizations to recognize lease assets and lease liabilities on the balance sheet with lease terms of more than 12 months and also disclose certain qualitative and quantitative information about leasing arrangements. The Company does not expect adoption of this amendment to have a material impact on the financial statements.
Sunshine Biopharma, Inc.
Notes To Unaudited Consolidated Financial Statements
For The Three and Six Month Interim Period Ended June 30, 2018
Note 2 – Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Since inception, the Company has had recurring operating losses and negative operating cash flows. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
The Company’s continuation as a going concern is dependent on its ability to obtain additional financing to fund operations, implement its business, and ultimately, attain profitability. The Company will need to secure additional funds through various means, including equity and debt financing. There can be no assurance that the Company will be able to obtain additional equity or debt financing, if and when needed, on terms acceptable to the Company, or at all. Any additional equity or debt financing may involve substantial dilution to the Company’s stockholders, restrictive covenants or high interest costs. The Company’s long-term liquidity also depends upon its ability to generate revenues and achieve profitability.
Private Placement
On January 12, 2018,May 16, 2023, the Company received netcompleted a private placement pursuant to a securities purchase agreement with a single institutional investor for gross proceeds of $100,000 in exchange for a note payable having a face value of $102,000 and accruing interest at the rate of 8% per annum. The note, due on October 30, 2018, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assignedapproximately $5 million, before deducting fees to the beneficial conversion feature.
On February 7, 2018,placement agent and other offering expenses payable by the Company received net proceeds of $142,500 in exchange for a note payable having a face value of $150,000 and accruing interest at the rate of 8% per annum. The note, due on February 7, 2019, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On February 20, 2018, the Company received net proceeds of $83,000 in exchange for a note payable having a face value of $85,000 and accruing interest at the rate of 8% per annum. The note, due on November 30, 2018, is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On May 29, 2018, the Company received net proceeds of $25,000 in exchange for a note payable having a face value of $26,750 and accruing interest at the rate of 8% per annum. The note, due on February 28, 2019 is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature.
On June 27, 2018, the Company issued a note payable having a face value of $53,000 and accruing interest at the rate of 8% per annum.Company. The net proceeds
of $51,000 from this note were received by the Company
on July 2, 2018. The note, due on April 15, 2019 is convertible after 180 days from issuance into $0.001 par value Common Stockwere $4,089,218.
In connection with the private placement, the Company issued (i) 2,450,000 shares of common stock, (ii) 3,502,381 pre-funded warrants (the “May Pre-Funded Warrants”), and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at $0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price 35% below market value.of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of $0.839. The Company estimates thatMay Pre-Funded Warrants are immediately exercisable at a nominal exercise price of $0.001, and may be exercised at any time until all of the fair valueMay Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of this convertible debt approximates$0.59 per share (subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the face value, sodate of issuance. As of September 30, 2023, a total of 1,156,381 May Pre-Funded Warrants and no value hasMay Investor Warrants have been assigned toexercised. The net proceeds received from the beneficial conversion feature.
At June 30, 2018 and December 31, 2017, accrued interest on Notes Payable was $34,334 and $9,481, respectively.
Sunshine Biopharma, Inc.
Notes To Unaudited Consolidated Financial Statements
For The Three and Six Month Interim Period Ended June 30, 2018
exercise of May Pre-Funded Warrants were $1,156.
Note 4 – Notes Payable - Related Party
On January 1, 2018 as partAcquisition of the acquisition of AtlasNora Pharma Inc., the Company issued a note payable in the amount of $450,000 Canadian ($358,407 US) and accruing interest at the rate of 3% per annum. The note is due on December 31, 2023. Payments on this note are $10,000 Canadian (approximately $8,000 US) per quarter. The outstanding principal balance at June 30, 2018 is $331,668. The note is secured by the Atlas Pharma Inc. shares held by the Company.
In addition to the above, on June 30, 2018 the Company had notes payable from related parties amounting to $201,786 and accrued interest of $12,125.
Note 5 – Issuance of Common Stock
During the six months ended June 30, 2018, the Company issued a total of 185,369,308 shares of $0.001 par value Common Stock. Of these, 42,584,566 shares valued at $290,286 were issued upon conversion of outstanding notes payable, reducing the debt by $188,568 and interest payable by $8,133 and generating a loss on conversion of $93,585. The Company also issued 92,650,000 shares valued at $499,200 for services, and 29,134,742 shares valued at $174,808 in exchange for equipment.
In addition, 20,000,000 shares valued at $246,000 or $0.0123 per share were issued as part of the acquisition of Atlas Pharma Inc. The Company also issued 1,000,000 shares valued at $5,900 in exchange for cancellation of a royalty obligation.
The Company declared no dividends through June 30, 2018.
Note 6 – Commitments
The Company’s subsidiary, Atlas Pharma Inc., has entered into long-term lease agreements for the rental of buildings which call for minimum lease payments of $228,113 and additional lease payments based on operating expenses. The lease expires on May 21, 2021. Minimum lease payments for the next four years are $62,213 in 2018, $62,213 in 2019, $62,213 in 2020, and $41,474 in 2021.
Note 7 – Earnings (Loss) Per Share
Earnings (loss) per share is computed using the weighted average number of Common Shares outstanding during the period. The Company has adopted ASC 260 (formerly SFAS128), “Earnings per Share”.
Note 8 – Goodwill
On January 1, 2018,October 20, 2022, the Company acquired all of the issued and outstanding shares of AtlasNora Pharma Inc. ("Atlas"), a Canadian privately held company. The purchase price for the shares was Eight Hundred Forty Eight Thousand Dollars ($848,000) Canadian ($684,697 US). The book value$18,860,637 (USD), $14,346,637 of which was paid in cash and the fixed assets acquiredremainder was $11,051. The remainderpaid through the issuance of the purchase price ($673,646) was applied to Goodwill.
Sunshine Biopharma, Inc.
Notes To Unaudited Consolidated Financial Statements
For The Three and Six Month Interim Period Ended June 30, 2018
Note 9 – Income Taxes
Deferred income taxes arise from the temporary differences between financial statement and income tax recognition of net operating losses and other items. Loss carryovers are limited under the Internal Revenue Code should a significant change in ownership occur.
A deferred tax asset at each date has been offset by a 100% valuation allowance.
Note 10 – Royalties Payable
As part of a subscription agreement entered into in 2016, the Company has an obligation to pay a royalty of 5% of net sales on one of its generic products (Anastrozole) for a period of three (3) years from the date of the first sale of that product. During the period ended June 30, 2018 1,000,0003,700,000 shares of the Company’s common stock valued at
$5,900$4,514,000 or $1.22 per share. Nora Pharma sells generic pharmaceutical products in Canada. Nora Pharma’s operations are authorized by a Drug Establishment License issued by Health Canada.
The following table summarizes the allocation of the purchase price as of October 20, 2022, the acquisition date using Nora Pharma’s balance sheet assets and liabilities:
| Schedule of allocation of purchase price | | | |
| Accounts receivable | | $ | 1,358,121 | |
| Inventory | | | 3,181,916 | |
| Intangible assets | | | 659,571 | |
| Equipment & furniture | | | 210,503 | |
| Other assets | | | 1,105,093 | |
| Total assets | | | 6,515,204 | |
| Liabilities assumed | | | (5,981,286 | ) |
| Net assets | | | 533,918 | |
| Goodwill | | | 18,326,719 | |
| Total Consideration | | $ | 18,860,637 | |
The value of the 3,700,000 common shares issued as part of the consideration paid for Nora Pharma was issueddetermined based on the closing market price of the Company’s common shares on the acquisition date, October 20, 2022 ($1.22 per share).
The Company impaired 100% of the goodwill amount in exchange for cancellation of this royalty obligation.
Note 11 – Related Party Transactions
In addition2022 and plans to
depreciate the
related party transactionsintangible assets as detailed in Note
45 below.
As part of the consideration paid for Nora Pharma, the Company agreed to a $5,000,000 CAD ($3,632,000 USD) earnout amount payable to Mr. Malek Chamoun, the Seller of Nora Pharma. The earnout is payable in the form of twenty (20) payments of $250,000 CAD for every $1,000,000 CAD increase in gross sales (as defined in the Purchase Agreement) above Nora Pharma’s June 30, 2022 gross sales, provided that his employment with the Company is not terminated pursuant to the Company’s employment agreement with him. The total earnout amount of $3,632,000 has been recorded as a salary payable. During the nine-month period ended September 30, 2023, the Company paid its Officersan earn-out amount of $1,084,169 leaving a balance earn-out to be paid of $2,547,831 at September 30, 2023.
The unaudited financial information in the table below summarizes the combined results of operations of the Company and Directors cash compensation totaling $17,344Nora Pharma for the years ended December 31, 2022 and $12,415 and $95,131 and $55,3802021, on a pro forma basis, as though the two companies had been combined as of January 1, 2021. The unaudited pro forma financial information does not purport to be indicative of the Company's combined results of operations which would have been obtained had the acquisition taken place on January 1, 2021, nor should it be taken as indicative of future consolidated results of operations:
| Schedule of Pro Forma results from acquisition | | | | | | |
| Pro Forma Results From Acquisition | | December 31,
2022 | | | December 31,
2021 | |
| Total revenues | | $ | 14,758,115 | | | $ | 7,927,165 | |
| Net (loss) from operations | | $ | (26,192,503 | ) | | $ | (2,224,253 | ) |
| Net (loss) | | $ | (26,164,764 | ) | | $ | (12,289,655 | ) |
| Basic and fully diluted (loss) per share | | $ | (1.74 | ) | | $ | (4.70 | ) |
| Weighted average number of shares outstanding | | | 15,056,097 | | | | 2,612,061 | |
Note 5 – Intangible Assets
Intangible assets, net, consisted of the following at September 30, 2023:
| Schedule of intangible assets | | | | |
| Balance June 30, 2023 | | $ | 1,233,570 | |
| Dossier fee additions | | | 13,905 | |
| Balance at September 30, 2023 | | | 1,247,475 | |
| Less accumulated amortization | | | (31,268 | ) |
| Finite-lived intangible assets, net, at September 30, 2023 | | $ | 1,216,207 | |
| | | | | |
| Balance December 31, 2022 | | $ | 776,856 | |
| Dossier fee additions | | | 470,619 | |
| Balance at September 30, 2023 | | | 1,247,475 | |
| Less accumulated amortization | | | (31,268 | ) |
| Finite-lived intangible assets, net, at September 30, 2023 | | $ | 1,216,207 | |
Amortization expense for the three months period ended September 30, 2023, and the nine months period ended September 30, 2023, amounted to $10,797 and $26,746, respectively.
As of September 30, 2023, estimated amortization expense of the Company’s intangible assets for each of the next five years is as follows:
| Schedule of estimated amortization expense | | | | |
| 2024 | | $ | 55,418 | |
| 2025 | | | 55,418 | |
| 2026 | | | 54,240 | |
| 2027 | | | 15,599 | |
| 2028 | | | 7,370 | |
Note 6 – Reverse Stock Splits
Effective February 9, 2022, the Company completed a 1 for 200 reverse split of its common stock. The Company had previously completed two 20 to 1 reverse stock splits, one in 2019 and the other in 2020. The Company’s financial statements reflect all three reverse stock splits on a retroactive basis for all periods presented and for all references to common stock, unless specifically stated otherwise.
Note 7 – Capital Stock
The Company’s authorized capital is comprised of 3,000,000,000 shares of common stock, par value $0.001, and 30,000,000 shares of preferred stock, $0.10 par value. As of December 31, 2022 and September 30, 2023, the Company had authorized 1,000,000 shares of Series B Preferred Stock. The Series B Preferred Stock is non-convertible, non-redeemable and non-retractable. It has superior liquidation rights to the common stock at $0.10 per share and gives the holder the right to 1,000 votes per share. As of September 30, 2023 and December 31, 2022, 10,000 shares of Series B Preferred Stock are outstanding and held by the Company’s chief executive officer.
On February 17, 2022, the Company completed a public offering and received net proceeds of $6,833,071 from the offering. Pursuant to the public offering, the Company issued and sold an aggregate of 1,882,353 shares of common stock and 4,102,200 warrants to purchase shares of common stock (the “Tradeable Warrants”).
On February 22, 2022, the Company redeemed 990,000 shares of Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $0.10 per share. The remaining 10,000 shares of Series B Preferred Stock could not be voted pursuant to a warrant agent agreement relating to the Tradeable Warrants (the “Warrant Agent Agreement”). On October 12, 2023, the Company held a special meeting of the holders of the outstanding Tradeable Warrants in which the holders of the majority of the outstanding Tradeable Warrants approved an amendment to the Warrant Agent Agreement to eliminate the provision that prohibited the Company’s CEO from exercising his voting rights under the Series B Preferred Stock, as well as to lower the exercise price of the Tradeable Warrants to $0.11. The Company entered into the amendment to the Warrant Agent Agreement on October 18, 2023.
On March 14, 2022, the Company completed a private placement and received net proceeds of $6,781,199. In connection with this private placement, the Company issued (i) 2,301,353 shares of its common stock together with investor warrants (“Investor Warrants”) to purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of common stock, together with Investor Warrants to purchase up to 1,302,251 shares of common stock. Each share of common stock and accompanying Investor Warrant was sold together at a combined offering price of $2.22 and each Pre-Funded Warrant and accompanying Investor Warrant were sold together at a combined offering price of $2.219. The Pre-Funded Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The Investor Warrants have an exercise price of $2.22 per share (subject to adjustment as set forth in the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On April 28, 2022, the Company completed another private placement and received net proceeds of $16,752,915. In connection with this private placement, the Company issued (i) 2,472,820 shares of its common stock together with warrants (“April Warrants”) to purchase up to 4,945,640 shares of common stock, and (ii) 2,390,025 pre-funded warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of common stock, together with April Warrants to purchase up to 4,780,050 shares of common stock. Each share of common stock and accompanying two April Warrants were sold together at a combined offering price of $4.01 and each Pre-Funded Warrant and accompanying two April Warrants were sold together at a combined offering price of $4.009. The Pre-Funded Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The April Warrants have an exercise price of $3.76 per share (subject to adjustment as set forth in the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On October 20, 2022, the Company issued 3,700,000 shares of common stock as part of the acquisition of Nora Pharma. These shares were valued at $4,514,000, or $1.22 per share.
On January 19, 2023, the Company announced a stock repurchase program of up to $2 million (“Stock Repurchase Program”). During the six month periodsmonths ended June 30, 20182023, the Company repurchased a total of 445,711 shares of common stock at an average price of $1.1371 per share for a total cost of $506,822. The 445,711 repurchased common shares were cancelled and 2017,returned to treasury reducing the number of issued and outstanding shares from 22,585,632 to 22,139,921.
On May 16, 2023, the Company completed a private placement pursuant to a securities purchase agreement with a single institutional investor for gross proceeds of approximately $5 million, before deducting fees to the placement agent and other offering expenses payable by the Company. The net proceeds received by the Company were $4,089,218. In connection with the private placement, the Company issued (i) 2,450,000 shares of common stock, (ii) 3,502,381 pre-funded warrants (the “May Pre-Funded Warrants”), and (iii) investor warrants (the “May Investor Warrants”) to purchase up to 11,904,762 shares of common stock at $0.59 per share. Each share of common stock and accompanying two May Investor Warrants were sold together at a combined offering price of $0.84 and each May Pre-Funded Warrant and accompanying two May Investor Warrants were sold together at a combined offering price of $0.839. The May Pre-Funded Warrants are immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the May Pre-Funded Warrants are exercised in full. The May Investor Warrants which have an exercise price of $0.59 per share (subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
In 2022 and the first six months of 2023, the Company issued a total of 10,789,867 shares of common stock in connection with warrant exercises for aggregate net proceeds of $13,194,335.
In July 2023, the Company repurchased a total of 68,012 shares of common stock on the open market under the Stock Repurchase Program announced on January 19, 2023, at an average price of $0.5046 per share for a total cost of $34,321. In October 2023, the 68,012 repurchased common shares were cancelled and returned to treasury reducing the number of issued and outstanding shares from 25,746,302 to 25,678,290.
As of September 30, 2023 and December 31, 2022, the Company has a total of 25,678,290 and 22,585,632 shares of common stock issued and outstanding, respectively.
The Company also paid its Officershas declared no dividends since inception.
Note 8 – Warrants
The Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants are considered a liability if they are mandatorily redeemable and Directors non-cash compensationthey require settlement in cash, other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the formfair value of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
In 2022 and during the first nine months of 2023, the Company completed four financing events, and in connection therewith, it issued warrants as follows:
| Schedule of warrants issued with financing | | | | | | |
| Type | | Number | | Exercise Price | | Expiry Date |
| Pre-Funded Warrants | | 3,692,276 | | $0.001 | | Unlimited |
| Tradeable Warrants | | 4,102,200 | | $2.22* | | February 2027 |
| Investor Warrants | | 3,603,604 | | $2.22 | | March 2027 |
| April Warrants | | 9,725,690 | | $3.76 | | April 2027 |
| May Pre-Funded Warrants | | 3,502,381 | | $0.001 | | Unlimited |
| May Investor Warrants | | 11,904,762 | | $0.59 | | November 2028 |
| * | The Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company's private placement on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof. |
As of September 30, 2023, all of the Pre-Funded Warrants and a total of 3,138,507 Tradeable Warrants, 2,802,703 Investor Warrants, and 1,156,381 May Pre-Funded Warrants were exercised resulting in aggregate proceeds of $13,194,335 received by the Company.
The Company’s outstanding warrants at September 30, 2023 consisted of the following:
| Schedule of outstanding warrants | | | | | | |
| Type | | Number | | Exercise Price | | Expiry Date |
| Pre-Funded Warrants | | None | | $0.001 | | Unlimited |
| Tradeable Warrants | | 963,693 | | $2.22* | | February 2027 |
| Investor Warrants | | 800,901 | | $2.22 | | March 2027 |
| April Warrants | | 9,725,690 | | $3.76 | | April 2027 |
| May Pre-Funded Warrants | | 2,346,000 | | $0.001 | | Unlimited |
| May Investor Warrants | | 11,904,762 | | $0.59 | | November 2028 |
| * | On October 12, 2023, the Company held a special meeting of the holders of its outstanding Tradeable Warrants in which a majority of the holders approved an amendment to the Warrant Agent Agreement to reduce the exercise price of the Tradeable Warrants to $0.11 per warrant. The amendment was executed on October 18, 2023. |
Note 9 – Net Loss Per Common Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock valuedoutstanding during the period, without consideration for common stock equivalents.
Diluted net loss per share is calculated by dividing the net loss by the weighted-average number of shares of common stock outstanding during the period, taking into consideration common stock equivalents.
In February 2022, the Company issued 4,102,200 Tradeable Warrants pursuant to the Company’s Public Offering. In March and April 2022, the Company issued 3,603,604 Investor Warrants and 9,725,690 April Warrants pursuant to two private placements. In May 2023, the Company issued 11,904,762 May Investor Warrants pursuant to two private placements. As of September 30, 2023, 3,138,507 Tradeable Warrants and 2,802,703 Investor Warrants were exercised, leaving 963,693 Tradeable Warrants, 800,901 Investor Warrants, 9,725,690 April Warrants, and 11,904,762 May Investor Warrants outstanding. These warrants are dilutive and were included in the diluted earnings per share.
In March and April 2022, the Company issued and sold Pre-Funded Warrants to purchase an aggregate of 3,692,276 shares of common stock at $429,300a nominal exercise price of $0.001 per share. During the nine months ended September 30, 2023, all of these warrants were exercised and $336,000therefore had no remaining dilutive effect.
In May 2023, the Company issued and $429,300sold May Pre-Funded Warrants to purchase an aggregate of 3,502,381 shares of common stock at a nominal exercise price of $0.001 per share. During the nine months ended September 30, 2023, 1,156,381 of these warrants were exercised leaving 2,346,000outstanding. These warrants were not included in the calculation of weighted average outstanding shares as they would be ant-dilutive.
Note 10 – Lease
The Company has obligations as a lessee for office space with initial non-cancellable terms in excess of one year. The Company classified the lease as an operating lease. The lease contains a renewal option for a period of five years. Because the Company is certain to exercise the renewal option, the optional period is included in determining the lease term, and $336,000 duringassociated payments under the renewal option are included in the lease payments. The Company’s lease does not include termination options for either party to the lease or restrictive financial or other covenants. Payments due under the lease contract include fixed payments plus a variable Payment. The Company’s office space lease requires it to make variable payments for the Company’s proportionate share of building’s property taxes, insurance, and common area maintenance. These variable lease payments are not included in lease payments used to determine lease liability and are recognized as variable costs when incurred.
Amounts reported on the balance sheet as of September 30, 2023 were as follows:
| Schedule of lease information | |
| Operating lease ROU asset | $664,296 |
| Operating Lease liability - Short-term | $117,840 |
| Operating lease liability - Long-term | $555,687 |
| Remaining lease term | 6 years 3 months |
| Discount rate | 6% |
Amounts disclosed for ROU assets obtained in exchange for lease obligations and reductions of ROU assets resulting from reductions of lease obligations include amounts reduced from the carrying amount of ROU assets resulting from deferred rent.
Maturities of lease liabilities under non-cancellable operating leases at September 30, 2023 are as follows:
| Schedule of maturities of lease liabilities | | |
| 2023 | $ | 30,124 |
| 2024 | | 116,090 |
| 2025 | | 116,277 |
| 2026 | | 110,134 |
| 2027 | | 103,736 |
| Thereafter | | 197,166 |
Note 11 – Management and Director Compensation
The Company paid its officers cash compensation totaling $245,000 and $362,500 and $1,290,000 and $770,095 for the three and six monthnine-month periods ended JuneSeptember 30, 20182023 and 20172022, respectively. In June 2018
The Company paid its directors cash compensation totaling $100,000 and $300,000 and $100,000 and $200,000 for the Company expensed an additional $429,300 in compensation to the directors in exchange for 81,000,000 shares of $0.001 par value common stock issued in June 2018 valued at $0.0053 per share. Stock issued for executive compensation is valued at the closing price on the date of issuance.
three and nine-month periods ended September 30, 2023 and 2022, respectively.
Note 12 – Revenue Recognition
As of January 1, 2018,Income Taxes
In calculating the provision for income taxes on an interim basis, the Company adopted ASU No. 201409, “Revenue from Contracts with Customers” (ASU 201409). Under the new guidance,uses an entity will recognize revenue to depict the transfer of promised goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services. A five-step model has been introduced for an entity to apply when recognizing revenue. The new guidance also includes enhanced disclosure requirements. The guidance was effective January 1, 2018 and was applied on a modified basis. The adoption did not have an impact on the Company's financial statements. A
llestimate of the revenues ofannual effective tax rate based upon currently known facts and circumstances and applies that rate to its year-to-date earnings or losses. The Company’s effective tax rate is based on expected income and statutory tax rates and takes into consideration permanent differences between financial statement and tax return income applicable to the Company are generated by Atlas Pharma Inc.,in the Company's wholly owned Canadian subsidiaryvarious jurisdictions in which provides laboratory testing services. Local governmental regulations require that companies recognize revenues upon completionthe Company operates. The effect of discrete items, such as changes in estimates, changes in rates or tax status, and unusual or infrequently occurring events, is recognized in the work by issuing an invoiceinterim period in which the discrete item occurs. The accounting estimates used to compute the provision for income taxes may change as new events occur, additional information is obtained or as the result of new judicial interpretations or regulatory or tax law changes.
The Company’s interim effective tax rate, inclusive of discrete items, for the nine-month periods ended September 30, 2023 and remitting the applicable sales taxes (GST and QST) to the appropriate government agency. Atlas Pharma Inc.'s revenue recognition policy is in compliance with these local regulations.
2022 was 26.83%.
Note 13 – Accounts Receivable
Accounts receivable consistSubsequent Events
On October 12, 2023, the Company held a special meeting of trade accounts arisingthe holders of its outstanding Tradeable Warrants in which the normal courseholders of businessthe majority of the outstanding Tradeable Warrants approved an amendment to the Warrant Agent Agreement to (i) reduce the exercise price of the Tradeable Warrants to $0.11, subject to further adjustment as provided therein, and are classified as current assets and carried at original invoice amounts less(ii) eliminate the provision that prohibits the Company’s CEO from exercising his voting rights under his Series B Preferred Stock.
In December 2022, the Company had entered into a research agreement with the Jewish General Hospital (“JGH”), Montreal, Canada to conduct IND-enabling studies of the Company’s anticancer drug candidate, Adva-27a (the “Research Agreement”). In August 2023, the Company was advised by JGH that the lab results on testing of the Adva-27a molecule were not favorable. After conclusion of an estimate for doubtful receivables basedinternal review of the lab results on November 2, 2023, the Company provided notice of termination of the Research Agreement, which will become effective on December 2, 2023, pursuant to the terms of the Research Agreement. The Company has now paused the IND-enabling studies of Adva-27a pending a review of outstanding balances on a monthly basis. The estimatethe possibility of allowance for doubtful accounts is based on the Company's bad debt experience, market conditions, and aging of accounts receivable, among other factors. If the financial conditionchemical modification of the Company's customers deteriorates resultingcompound to address the suboptimal performance of the molecule in the customer's inability to pay the Company's receivables as they come due, additional allowances for doubtful accounts will be required.
Sunshine Biopharma, Inc.
Notes To Unaudited Consolidated Financial Statements
For The Three and Six Month Interim Period Ended June 30, 2018
Note 14 – Subsequent Events
On July 5, 2018, the holder of a note payable dated November 14, 2017 elected to convert $20,000 in principal into 6,505,122 shares of Common Stock leaving a principal balance of $93,000.
On July 11, and August 2, 2018, the holder of a note payable dated October 26, 2017 elected to convert a total of $44,000 in principal and $2,531 in accrued interest into 20,821,004 shares of Common Stock leaving a principal balance of $23,000.
On July 17, 23, 26, and August 2, 7, and 10, 2018, the holder of a note payable dated January 12, 2018 elected to convert a total of $81,000 in principal into an aggregate of 47,805,452 shares of Common Stock leaving a principal balance of $21,000.
certain studies.
| | Page No.
|
| F-14 | |
Report of Independent Registered Public Accounting Firm | F-11 |
| |
Consolidated Balance Sheets as of December 31, 2017 and 2016 | F-12 |
| |
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2017 and 2016
| F-13 |
| |
Consolidated Statements of Cash Flows for the years ended December 31, 2017 and 2016 | F-14 |
| |
Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2017 and 2016
| F-15 |
| |
Notes to Consolidated Financial Statements | F-16 |
Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of Sunshine Biopharma, Inc.
:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Sunshine Biopharma, Inc. (the "Company") as of December 31, 20172022 and 2016,2021, the related consolidated statements of operations stockholders'and comprehensive income (loss), shareholders' equity, (deficit), and cash flows for each of the two years thenin the period ended December 31, 2022, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20172022 and 2016,2021, and the results of its operations and its cash flows for each of the two years thenin the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the Company has suffered recurring losses from operations and has a significant accumulated deficit. In addition, the Company continues to experience negative cash flows from operations. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits.audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our auditsaudit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engageengaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our auditsaudit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our auditsaudit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provideaudit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or are required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved especially challenging, subjective, or complex judgments.
We determined that there are no critical audit matters.
/s/ BF Borgers CPA PC
(PCAOB ID 5041)BF Borgers CPA PC
We have served as the Company's auditor since 2013.
April 2, 2018
March 31, 2023
PCAOB ID 5041
Sunshine Biopharma, Inc. |
Consolidated Balance SheetF-15 | |
| | | |
| | | |
| ASSETS | | |
| | | |
| Current Assets: | | |
| | | |
| Cash and cash equivalents | $107,532 | $57,453 |
| Prepaid expenses | 9,667 | 1,007 |
| | | |
| Total Current Assets | 117,199 | 58,460 |
| | | |
| Equipment (net of $9,132 and $2,228 depreciation) | 59,996 | 5,944 |
| | | |
Patents (net of $58,918 amortization and $556,120 impairment) | - | - |
| | | |
| Non-current asset - Deposits | 80,290 | - |
| | | |
| TOTAL ASSETS | $257,485 | $64,404 |
| | | |
| LIABILITIES AND SHAREHOLDERS' EQUITY | | |
| | | |
| Current Liabilities: | | |
| | | |
| Current portion of notes payable | 516,867 | 69,939 |
| Current portion of notes payable - related entity | 205,742 | 167,032 |
| Accounts payable | 19,314 | 28,122 |
| Interest payable | 9,215 | 9,011 |
| | | |
| Total Curent Liabilities | 751,138 | 274,104 |
| | | |
| Long-term liabilities - Notes payable | 79,710 | - |
| | | |
| | | |
| TOTAL LIABILITIES | 830,848 | 274,104 |
| | | |
| | | |
| SHAREHOLDERS' EQUITY | | |
| | | |
Preferred Stock, Series A, $0.10 par value per share; Authorized 5,000,000 Shares; Issued and outstanding -0- shares at December 31, 2017 and 2016, respectively. | - | - |
| | | |
Preferred Stock, Series B $0.10 par value per share; Authorized 500,000 Shares; Issued and outstanding 500,000 and 500,000 shares at December 31, 2017 and 2016, respectively. | 50,000 | 50,000 |
| | | |
Common Stock, $0.001 par value per share; Authorized 3,000,000,000 Shares; Issued and outstanding 918,736,498 and 769,399,858 at December 31, 2017 and 2016, respectively | 918,736 | 769,400 |
| | |
Reserved for issuance 394,808,684 at December 31, 2017
| | |
| | |
| Capital paid in excess of par value | 12,075,586 | 11,548,460 |
| | | |
| Accumulated other comprehesive income | 504 | 394 |
| | | |
| Accumulated (Deficit) | (13,618,190) | (12,577,954) |
| | | |
| TOTAL SHAREHOLDERS' DEFICIT | (573,363) | (209,700) |
| | | |
| TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIT | $257,485 | $64,404 |
| | | |
See Accompanying Notes To These Financial Statements. |
Sunshine Biopharma, Inc.
Consolidated Balance Sheets
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2022 | | | 2021 | |
| ASSETS | | | | | | | | |
| | | | | | | | | |
| Current Assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 21,826,437 | | | $ | 2,045,167 | |
| Accounts receivable | | | 1,912,153 | | | | 7,798 | |
| Inventory | | | 3,289,945 | | | | 105,650 | |
| Prepaid expenses | | | 283,799 | | | | 29,625 | |
| Deposits | | | – | | | | 7,590 | |
| Total Current Assets | | | 27,312,334 | | | | 2,195,830 | |
| | | | | | | | | |
| Property and equipment | | | 394,249 | | | | 7,061 | |
| Intangible assets | | | 776,856 | | | | – | |
| Right-of-use-asset | | | 760,409 | | | | – | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 29,243,848 | | | $ | 2,202,891 | |
| | | | | | | | | |
| LIABILITIES | | | | | | | | |
| | | | | | | | | |
| Current Liabilities: | | | | | | | | |
| Accounts payable & accrued expenses | | $ | 2,802,796 | | | $ | 42,942 | |
| Earn-out payable | | | 3,632,000 | | | | – | |
| Interest payable | | | – | | | | 48,287 | |
| Income tax payable | | | 373,191 | | | | – | |
| Current portion - Right-of-use-liability | | | 123,026 | | | | – | |
| Total Current Liabilities | | | 6,931,014 | | | | 91,229 | |
| | | | | | | | | |
| Long-Term Liabilities: | | | | | | | | |
| Notes payable | | | – | | | | 1,900,000 | |
| Right-of-use-liability | | | 642,232 | | | | – | |
| Deferred tax liability | | | 43,032 | | | | – | |
| Total Long-Term Liabilities | | | 685,264 | | | | 1,900,000 | |
| | | | | | | | | |
| TOTAL LIABILITIES | | | 7,616,278 | | | | 1,991,229 | |
| | | | | | | | | |
| SHAREHOLDERS' EQUITY | | | | | | | | |
| | | | | | | | | |
| Preferred Stock, Series B $0.10 par value per share; 1,000,000 shares authorized; 10,000 and 1,000,000 shares issued and outstanding as of December 31, 2022 and December 31, 2021, respectively | | | 1,000 | | | | 100,000 | |
| Common Stock, $0.001 par value per share; 3,000,000,000 shares authorized; 22,585,632 and 2,591,240 shares issued and outstanding as of December 31, 2022 and December 31, 2021, respectively | | | 22,585 | | | | 2,591 | |
| Capital paid in excess of par value | | | 80,841,752 | | | | 32,787,384 | |
| Accumulated comprehensive income | | | 161,847 | | | | (23,139 | ) |
| Accumulated (Deficit) | | | (59,399,614 | ) | | | (32,655,174 | ) |
| | | | | | | | | |
| TOTAL SHAREHOLDERS' EQUITY | | | 21,627,570 | | | | 211,662 | |
| | | | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY | | $ | 29,243,848 | | | $ | 2,202,891 | |
See Accompanying Notes to These Consolidated Financial Statements.
Sunshine Biopharma, Inc. |
Consolidated Statement Of Operations and comprehensive lossF-16 | |
| | | |
| | | |
| Revenue: | $- | $- |
| | | |
| General & Administrative Expenses | | |
| | | |
| Accounting | 81,643 | 70,413 |
| Legal | 75,908 | 57,955 |
| Consulting | 127,013
| 207,401 |
| Office | 45,726 | 45,215 |
| Licenses | - | 19,203 |
| Officer & director renumeration | 520,271
| 499,397 |
| Research & development | - | 32,793 |
| Amortization & depreciation | 6,629 | 60,731 |
| | | |
| Total G & A | 857,190 | 993,108 |
| | | |
| (Loss) from operations | (857,190) | (993,108) |
| | | |
| Other (expense): | | |
| Interest expense | (104,829) | (34,732) |
| Loss on conversion of notes payable | (76,929) | (1,945,898) |
| Loss on impairment of patents | - | (556,120) |
| Litigation settlement proceeds | - | 25,000 |
| (Loss) from foreign exchange transactions | (1,288) | - |
| Gain on interest forgiveness | - | 381 |
| Debt release | - | 7,790 |
| | | |
| Total Other (Expense) | (183,046) | (2,503,579) |
| | | |
| Net (loss) | $(1,040,236) | $(3,496,687) |
| | | |
| Basic (Loss) per common share | $0.00 | $(0.01) |
| | | |
| Weighted Average Common Shares Outstanding | 872,685,608 | 424,874,458 |
| | | |
| Net Income (Loss) | $(1,040,236) | $(3,496,687) |
| Other comprehensive income: | | |
Unrealized foreign currency Gain (Loss)
| 110
| (346) |
| Comprehensive (Loss) | (1,040,126) | (3,497,033) |
| | | |
| Basic (Loss) per common share | (0.00) | (0.01) |
| | | |
| Weighted Average Common Shares Outstanding | 872,685,608 | 424,874,458 |
Sunshine Biopharma, Inc.
Consolidated Statements of Operations and Comprehensive Loss
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Revenue | | $ | 4,345,603 | | | $ | 228,426 | |
| Cost of sales | | | 2,649,028 | | | | 117,830 | |
| Gross profit | | | 1,696,575 | | | | 110,596 | |
| | | | | | | | | |
| General and Administrative Expenses: | | | | | | | | |
| Accounting | | | 341,139 | | | | 118,423 | |
| Consulting | | | 842,894 | | | | 50,873 | |
| Directors Fees | | | 300,000 | | | | – | |
| Legal | | | 565,265 | | | | 232,616 | |
| Marketing | | | 578,085 | | | | – | |
| Office | | | 796,007 | | | | 248,561 | |
| R&D | | | 811,858 | | | | 672,209 | |
| Salaries | | | 6,054,962 | | | | 1,215,307 | |
| Taxes | | | 55,233 | | | | – | |
| Depreciation and amortization | | | 25,163 | | | | 12,741 | |
| Goodwill impairment | | | 18,326,719 | | | | – | |
| Total General and Administrative Expenses | | | 28,697,325 | | | | 2,550,730 | |
| | | | | | | | | |
| (Loss) from operations | | | (27,000,750 | ) | | | (2,440,134 | ) |
| | | | | | | | | |
| Other Income (Expenses): | | | | | | | | |
| Loss on debt conversions | | | – | | | | (9,726,485 | ) |
| Foreign exchange | | | (476 | ) | | | 50 | |
| Interest income | | | 518,650 | | | | – | |
| Interest expense | | | (39,412 | ) | | | (328,818 | ) |
| Debt forgiveness | | | 10,852 | | | | 51,031 | |
| Interest forgiveness | | | – | | | | 7,909 | |
| Total Other Income (Expenses) | | | 489,614 | | | | (9,996,313 | ) |
| | | | | | | | | |
| Net (loss) before income taxes | | | (26,511,136 | ) | | | (12,436,447 | ) |
| Provision for income taxes | | | 233,304 | | | | – | |
| Net (Loss) | | | (26,744,440 | ) | | | (12,436,447 | ) |
| Comprehensive income (loss): | | | | | | | | |
| Gain (Loss) from foreign exchange translation | | | 184,986 | | | | (20,268 | ) |
| Comprehensive (Loss) | | $ | (26,559,454 | ) | | $ | (12,456,715 | ) |
| | | | | | | | | |
| Basic and diluted (Loss) per common share | | $ | (1.76 | ) | | $ | (4.76 | ) |
| | | | | | | | | |
| Weighted average common shares outstanding (Basic & Diluted) | | | 15,180,868 | | | | 2,612,061 | |
See Accompanying Notes Toto These Consolidated Financial Statements.
| Sunshine Biopharma, Inc. | | |
| Consolidated Statement Of Cash Flows | |
| | | |
| | | |
| | | |
Cash Flows From Operating Activities: | | |
| | | |
| Net (Loss) | $(1,040,236) | $(3,496,687) |
Amortization and Depreciation
| 6,629 | 60,731 |
| Stock issued for services | 427,400 | 702,300 |
| Loss on impairment of patents | - | 556,120 |
| Loss on conversion of notes payable | 76,929 | 1,945,898 |
| Stock issued for payment of interest | 3,022 | 9,270 |
| Debt forgiveness | - | (1,313) |
| (Increase) decrease in prepaid expenses | (8,660) | 2,104 |
| Increase (decrease) in Accounts Payable | (8,808) | (18,960) |
| Increase in Accounts Payable - related entity | - | (80,000) |
| Increase(decrease) in interest payable | 204 | 6,355 |
| | | |
| Net Cash Flows (used) in Operations | (543,520) | (314,182) |
| | | |
Cash Flows From Investing Activities: | |
| | | |
| Purchase equipment | (3,718) | (3,439) |
| Deposits on business acquisition | (80,290) | - |
| | | |
| Net Cash Flows (used) in Investing Activities | (84,008) | (3,439) |
| | | |
Cash Flows From Financing Activities: | |
| | | |
| Proceed from note payable | 660,565 | 131,150 |
Notes Payable - Interest expense
| 33,977 | |
| Payment of notes payable | (115,000) | - |
| Origination fees | 25,000 | 22,312 |
Notes payable - related party
| 2,251 | 67,032 |
| Note payable related entity for patent purchase | - | - |
| Sale of common stock | 63,912 | 104,128 |
| | | |
| Net Cash Flows Provided by Financing Activities | 670,705 | 324,622 |
| | | |
| | | |
| Net Increase (Decrease) In Cash and cash equivalents | 43,177 | 7,001 |
| Foreign currency translation adjustment | 6,902 | (346) |
| Cash and cash equivalents at beginning of period | $57,453 | $50,798 |
| | | |
| | $107,532 | $57,453 |
Supplementary Disclosure Of Cash Flow Information: |
| Cash paid for interest | $21,900 | $5,264 |
| Cash paid for income taxes | $- | $- |
| | | |
| Stock issued for services | $427,400 | $702,300 |
| Stock issued for note conversions | $128,451 | $3,077,950 |
| Stock issued to buy equipment | $56,700 | $- |
| Loan issued for interest | $58,977 | $- |
| Stock issued for payment of interest | $3,022 | $- |
| | | |
Sunshine Biopharma, Inc.
Consolidated Statements of Cash Flows
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2022 | | | 2021 | |
| Cash Flows From Operating Activities: | | | | | | |
| Net (Loss) | | $ | (26,744,440 | ) | | $ | (12,436,447 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 25,163 | | | | 12,741 | |
| Goodwilll impairment | | | 18,326,719 | | | | – | |
| Foreign exchange (gain) | | | 548 | | | | (50 | ) |
| Stock issued for services | | | – | | | | 918,000 | |
| Debt release | | | (10,852 | ) | | | – | |
| Loss on debt conversion | | | – | | | | 9,726,485 | |
| Gain on interest and debt forgiveness | | | – | | | | 58,940 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (524,486 | ) | | | (5,882 | ) |
| Inventory | | | 42,983 | | | | (81,879 | ) |
| Prepaid expenses | | | 82,846 | | | | (26,847 | ) |
| Accounts payable & accrued expenses | | | 3,359,141 | | | | (18,156 | ) |
| Deferred tax liability | | | 3,628 | | | | – | |
| Income tax payable | | | 238,679 | | | | – | |
| Interest payable | | | (48,287 | ) | | | 23,967 | |
| Net Cash Flows (Used) in Operations | | | (5,248,358 | ) | | | (1,829,128 | ) |
| | | | | | | | | |
| Cash Flows From Investing Activities: | | | | | | | | |
| Reduction in Right of use asset | | | 33,379 | | | | – | |
| Nora Pharma Inc. acquisition | | | (14,346,637 | ) | | | – | |
| Cash from Nora Pharma Inc. acquisition | | | (1,135 | ) | | | – | |
| Purchase of intangible assets | | | (111,015 | ) | | | – | |
| Purchase of equipment | | | (193,982 | ) | | | – | |
| Net Cash Flows (Used) in Investing Activities | | | (14,619,390 | ) | | | – | |
| | | | | | | | | |
| Cash Flows From Financing Activities: | | | | | | | | |
| Proceeds public and private offerings of common stock, net | | | 30,367,185 | | | | – | |
| Warrant exercises | | | 13,193,177 | | | | – | |
| Purchase of preferred shares | | | (99,000 | ) | | | – | |
| Reduction in lease liability | | | (31,924 | ) | | | – | |
| Payoff of Nora Pharma Inc.’s debt | | | (2,064,331 | ) | | | – | |
| Proceeds from notes payable | | | – | | | | 3,318,500 | |
| Note payable used to pay fees | | | – | | | | 61,500 | |
| Payments of notes payable | | | (1,900,000 | ) | | | (475,325 | ) |
| Net Cash Flows Provided by Financing Activities | | | 39,465,107 | | | | 2,904,675 | |
| | | | | | | | | |
| Cash and Cash Equivalents at Beginning of Period | | | 2,045,167 | | | | 989,888 | |
| Net Increase (Decrease) in cash and cash equivalents | | | 19,597,359 | | | | 1,075,547 | |
| Effect of exchange rate changes on cash | | | (1,075 | ) | | | – | |
| Foreign currency translation adjustment | | | 184,986 | | | | (20,268 | ) |
| Cash and Cash Equivalents at End of Period | | $ | 21,826,437 | | | $ | 2,045,167 | |
| | | | | | | | | |
| Supplementary Disclosure of Cash Flow Information: | | | – | | | | – | |
| Cash paid for interest | | $ | 48,287 | | | $ | 38,117 | |
| Cash paid for income taxes | | $ | – | | | $ | – | |
| Stock issued for note conversions | | $ | – | | | $ | 12,705,214 | |
| Stock issued for acquisition of Nora Pharma, Inc. | | $ | 4,514,000 | | | $ | – | |
See Accompanying Notes Toto These Consolidated Financial Statements.
Sunshine Biopharma, Inc.
|
Statement of Shareholders' Equity
F-18 | |
| | | | | | | |
| |
| | | | | | | | | |
| | | | | | | | | |
| Balance at December 31, 2015 | 198,265,118 | $198,265 | $8,235,217 | 500,000 | $50,000 | $740 | $(9,081,267) | $(597,045) |
| | | | | | | | | |
| Common stock issued for cash | 12,555,556 | 12,556 | 91,572 | | | | | 104,128 |
| | | | | | | | | |
| Common stock issued for services | 146,750,000 | 146,750 | 555,550 | | | | | 702,300 |
| | | | | | | | | |
| Common stock issued for the reduction of note payable | | | | | | | | |
| and payment of interest | 411,829,184 | 411,829 | 2,666,121 | | | | | 3,077,950 |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| Net Income (Loss) | - | - | - | | | (346) | (3,496,687) | (3,497,033) |
| Balance at December 31, 2016 | 769,399,858 | $769,400 | $11,548,460 | 500,000 | $50,000 | $394 | $(12,577,954) | $(209,700) |
| | | | | | | | | |
| Common stock issued for cash | 34,000,000 | 34,000 | 29,912 | | | | | 63,912 |
| | | | | | | | | |
| Common stock issued for services | 61,804,348 | 61,804 | 365,596 | | | | | 427,400 |
| | | | | | | | | |
| Common stock issued for equipment | 11,004,167 | 11,004 | 45,696 | | | | | 56,700 |
| | | | | | | | | |
| Common stock issued for the reduction of note payable | | | | | | | | |
| and payment of interest | 42,528,125 | 42,528 | 85,923 | | | | | 128,451 |
| | | | | | | | | |
| | | | | | | | | |
| Net Income (Loss) | - | - | - | | | 110 | (1,040,236) | (1,040,126) |
| Balance at December 31, 2017 | 918,736,498 | $918,736 | $12,075,586 | 500,000 | $50,000 | $504 | $(13,618,190) | $(573,363) |
Sunshine Biopharma, Inc.
Consolidated Statement of Shareholders' Equity
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Number Of Common Shares | | | Common | | | Capital Paid in Excess of Par | | | Number Of Preferred Shares | | | Preferred | | | Compre- hensive | | | Accumulated | | | | |
| | | Issued | | | Stock | | | Value | | | Issued | | | Stock | | | Income | | | Deficit | | | Total | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance December 31, 2020 | | | 1,732,096 | | | $ | 1,732 | | | $ | 19,165,029 | | | | 1,000,000 | | | $ | 100,000 | | | $ | (2,871 | ) | | $ | (20,218,727 | ) | | $ | (954,837 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common stock issued for the reduction of debt and payment of interest | | | 559,144 | | | | 559 | | | | 12,704,655 | | | | – | | | | – | | | | – | | | | – | | | | 12,705,214 | |
| Common stock issued for services | | | 300,000 | | | | 300 | | | | 917,700 | | | | – | | | | – | | | | – | | | | – | | | | 918,000 | |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | (20,268 | ) | | | (12,436,447 | ) | | | (12,456,715 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2021 | | | 2,591,240 | | | $ | 2,591 | | | $ | 32,787,384 | | | | 1,000,000 | | | $ | 100,000 | | | $ | (23,139 | ) | | $ | (32,655,174 | ) | | | 211,662 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Fractional shares issued for reverse stock split | | | 4,380 | | | | 4 | | | | (4 | ) | | | – | | | | – | | | | – | | | | – | | | | – | |
| Common stock and warrants issued in offerings | | | 6,656,526 | | | | 6,657 | | | | 30,360,528 | | | | – | | | | – | | | | – | | | | – | | | | 30,367,185 | |
| Exercise of warrants | | | 9,633,486 | | | | 9,633 | | | | 13,183,544 | | | | – | | | | – | | | | – | | | | – | | | | 13,193,177 | |
| Preferred stock purchased from related party | | | – | | | | – | | | | – | | | | (990,000 | ) | | | (99,000 | ) | | | – | | | | – | | | | (99,000 | ) |
| Common stock issued as part of Nora Pharma Inc. acquisition | | | 3,700,000 | | | | 3,700 | | | | 4,510,300 | | | | – | | | | – | | | | – | | | | – | | | | 4,514,000 | |
| Net (loss) | | | – | | | | – | | | | – | | | | – | | | | – | | | | 184,986 | | | | (26,744,440 | ) | | | (26,559,454 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at December 31, 2022 | | | 22,585,632 | | | $ | 22,585 | | | $ | 80,841,752 | | | | 10,000 | | | $ | 1,000 | | | $ | 161,847 | | | $ | (59,399,614 | ) | | $ | 21,627,570 | |
See Accompanying Notes Toto These Consolidated Financial Statements.
Notes to Consolidated Financial Statements
December 31, 20172022 and 2016
2021
Note 1 – Description of Business
The Company
Sunshine Biopharma, Inc. (the “Company”) was originally incorporated under the name Mountain West Business Solutions, Inc. (“MWBS”) on August 31, 2006, in the State of Colorado.
Effective October 15, 2009, MWBSthe Company acquired Sunshine Biopharma, Inc. in a transaction classified as a reverse acquisition. MWBS concurrentlySunshine Biopharma, Inc. held an exclusive license to a new anticancer drug bearing the laboratory name, Adva-27a (the “License Agreement”). Upon completion of the reverse acquisition transaction, the Company changed its name to Sunshine Biopharma, Inc. and Sunshine Biopharma, Inc. changed its name to Sunshine Etopo, Inc.began operating as a pharmaceutical company focusing on the development of the licensed Adva-27a anticancer drug. In December 2015, Sunshine Etopo, Inc. became inactive and was recently dissolved.
On January 1, 2018, the Company acquired
Atlas Pharma Inc.,all rights to Adva-27a by purchasing PCT/FR2007/000697 and PCT/CA2014/000029 and terminated the License Agreement.
On May 22, 2020, the Company filed a fully certified Canadian company offering chemical analysisprovisional patent application in the United States for a new treatment for Coronavirus infections. The Company’s patent application covers composition subject matter pertaining to small molecules for inhibition of pharmaceuticalthe main Coronavirus protease, Mpro, an enzyme that is essential for viral replication. The patent application has a priority date of May 22, 2020. On April 30, 2021, the Company filed a PCT application containing new research results and other industrial samples. Asextending coverage to include the Coronavirus Papain-Like protease, PLpro. The priority date of May 22, 2020 has been maintained in the newly filed PCT application. The Company’s lead Anti-Coronavirus compound arising from these patents bears the laboratory name SBFM-PL4.
On April 20, 2022, the Company filed a resultprovisional patent application in the United States covering mRNA molecules capable of thisdestroying cancer cells in vitro. The patent application contains composition and utility subject matter pertaining to the recent formationstructure and sequence of NOX Pharmaceuticals, Inc., Sunshine Biopharma, Inc. is now operating through three wholly owned subsidiaries, including:
●
NOX Pharmaceuticals, Inc.,such mRNA molecules.
On February 18, 2022, the Company entered into a recently formed Colorado company focused onresearch agreement (the “SRA”) with the research, development and commercializationUniversity of proprietary drugs for the treatment of cancer including Adva-27a, a multi-purpose anti-tumor compound targeted for the treatment of multidrug resistant cancer;
●
Sunshine Biopharma Canada Inc., a Canadian company formed in July 2014, which offers generic prescription drugs for the treatment of cancer and other acute and chronic indications; and
●
Atlas Pharma Inc., a Canadian company acquired in January 2018, offering certified chemical analysis of pharmaceutical and other industrial samples.
The financial statements represent the consolidated activity of Sunshine Biopharma, Inc. and its subsidiaries (hereinafter collectively referred to as the "Company"). The Company was originally formedArizona for the purposes of conducting research
developmentfocused on determining the in vivo safety, pharmacokinetics, and
commercializationdose selection properties of
drugsthree University of Arizona owned PLpro inhibitors, to be followed by efficacy testing in mice infected with SARS-CoV-2 (the “Research Project”). Under the SRA, the University of Arizona granted the Company a first option to negotiate a commercial, royalty-bearing license for all intellectual property developed by University of Arizona personnel under the Research Project. In addition, the Company and the University of Arizona entered into an Option Agreement whereby the Company was granted a first option to negotiate a royalty-bearing commercial license for the
treatmentunderlying technology of
various forms of cancer. The Company may also engage in any other business that is permitted by law, as designatedthe Research Project. Encouraged by the
Boardresults to date, the Company submitted a Notice of
DirectorsOption Exercise to the University of
Arizona on September 13, 2022.
On October 20, 2022, the Company. NOX Pharmaceuticals, Inc. and AtlasCompany acquired Nora Pharma Inc. are not included(“Nora Pharma”), a Canadian generic pharmaceuticals company. Based in the Company's 2017 financials.
greater Montreal area, Nora Pharma has 37 employees and operates in a 15,000 square foot facility certified by Health Canada. Nora Pharma currently offers 60 products, including 49 generic prescription drugs, and 11 OTC products. Nora Pharma sales were $10.7 million USD during its fiscal year ended June 30, 2022. The consolidated financial statements contained in this report include the results of operations of Nora Pharma from October 20, 2022 through December 31, 2022.
During the last year the Company has continued to raise money through stock sales and borrowings.
The Company’s activities are subject to significant risks and uncertainties, including failing to secure additional funding to operationalize the Company’s generic pharmaceuticals business and proprietary drug development program.
Note 2 – Summary of Significant Accounting Policies
This summary of significant accounting policies is presented to assist the reader in understanding the Company's financial statements. The consolidated financial statements and notes are representations of the Company's management, which is responsible for their integrity and objectivity. These accounting policies conform to generally accepted accounting principlesGenerally Accepted Accounting Principles and have been consistently applied in the preparation of the financial statements.
IMPACT OF CORONAVIRUS (COVID-19) PANDEMIC
In March 2020, the World Health Organization declared Coronavirus and its associated disease, COVID-19, a global pandemic. Conditions surrounding the Coronavirus outbreak are continuing to evolve and government authorities around the world have and continue to implement various measures to mitigate the spread of the virus. The outbreak and related mitigation measures have had and will continue to have a material adverse impact on the world economies and the Company's business activities. It is not possible for the Company to predict the duration or magnitude of the adverse conditions of the outbreak and their effects on the Company’s business or ability to raise funds. No adjustments have been made to the amounts reported in the Company's financial statements as a result of this matter.
PRINCIPLES OF CONSOLIDATION
The accompanying consolidated financial statements include the accounts of the Company and its subsidiaries, all wholly owned subsidiary.owned. All intercompany accounts and transactions have been eliminated in consolidation.
The preparation of financial statements in conformity with U.S. generally accepted accounting principlesUS Generally Accepted Accounting Principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The more significant estimates and assumptions made by management are valuation of equity instruments, depreciation of property and equipment, and deferred tax asset valuation. Actual results could differ from those estimates as the current economic environment has increased the degree of uncertainty inherent in these estimates and assumptions.
TRADE ACCOUNTS RECEIVABLE AND ALLOWANCE FOR DOUBTFUL ACCOUNTS
Trade accounts receivable are stated at net realizable value. The majority of customers are not extended credit and therefore time to maturity for receivables is short. On a periodic basis, management evaluates its trade accounts receivable and determines whether to record an allowance for doubtful accounts or if any accounts should be written off based on a past history of write-offs, collections and current credit conditions. A receivable is considered past due if the Company has not received payments based on agreed-upon terms. The Company generally does not require any security or collateral to support its receivables.
INVENTORY VALUATION
Inventory is valued at the lower of cost and net realizable value. Cost is determined using the first in, first out method. Net realizable value is the estimated selling price in the ordinary course of business, less the costs of completion and costs necessary to make the sale. The cost of inventory includes the purchase price and other costs directly attributable to the acquisition of finished goods.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
CASH AND CASH EQUIVALENTS
For the Balance Sheets and Statements of Cash Flows, all highly liquid investments with maturity of 90 days or less are considered to be cash equivalents. The Company had a cash balance of $107,532$21,826,437 and $57,453$2,045,167 as of December 31, 20172022 and December 31, 2016,2021, respectively. At times such cash balances may be in excess of the FDIC limit of $250,000 in the U.S. or the equivalent in Canada.
Property and equipment isare reviewed for recoverability when events or changes in circumstances indicate that its carrying value may exceed future undiscounted cash inflows. As of December 31, 20172022 and 2016,2021, the Company had not identified any such impairment. Repairs and maintenance are charged to operations when incurred and improvements and renewals are capitalized.
Property and equipment are stated at cost. Depreciation is calculated usingaccording to the straight-line methodfollowing methods at the following annual rates and period for financial reporting purposes and accelerated methods for tax purposes. Their estimated useful lives are as follows:
| Schedule of estimated useful lives | Office Equipment: | 5-7 Years |
| Office Equipment: | Laboratory EquipmentStraight-line and Declining balance method | 55-7 Years / 20% |
| Computer Equipment: | VehiclesDeclining balance method | 55% |
| Laboratory Equipment: | Straight-line method | 5 Years |
| Vehicles: | Straight-line and Declining balance method | 5 Years / 30% |
Sunshine Biopharma, Inc.
Notes
INTANGIBLE ASSETS
Intangible assets are amortized over their estimated useful lives according to Consolidated Financial Statements
December 31, 2017the following methods at the following annual rates and 2016
period:
| Licenses: | Straight-line method | 5 Years |
| Website: | Declining balance method | 55% |
Intangible assets are tested for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. The carrying amount of a long-lived asset is not recoverable when it exceeds the sum of the undiscounted cash flows expected to result from its use and eventual disposal. In such a case, an impairment loss must be recognized and is equivalent to the excess of the carrying amount of a long-lived asset over its fair value.
INTELLECTUAL PROPERTY RIGHTS - PATENTS
The cost of patents acquired is capitalized and will beis amortized over the shorter of the term of the patent life (20 years) or the remaining life of the underlying patents.
The Company evaluates recoverability of identifiable intangible assets whenever events or changes in circumstances indicate that intangible assets carrying amount may not be recoverable. Such circumstances include but are not limited to: (1) a significant decrease in the market value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used, or (3) an accumulation of cost significantly in excess of the amount originally expected for the acquisition of an asset. The Company measures the carrying amount of thesuch assets against the estimated undiscounted future cash flows associated with it.
There was an impairment
BASIC AND DILUTED NET GAIN (LOSS) PER SHARE
The Company computes loss per share in accordance with ASC 260, Earnings per Share. ASC 260 requires presentation of $556,120both basic and diluted earnings per share (“EPS”) on the face of the income statement.
Basic net income (loss) per share is calculated by dividing net (loss) by the weighted-average common shares outstanding. Diluted net income per share is calculated by dividing net income by the weighted-average common shares outstanding during the period using the treasury stock method or the two-class method, whichever is more dilutive. As the Company incurred net losses for the year ended December 31, 2016.
The Company’s management determined that the expected cash flows would be less than the carrying amount of assets being evaluated; therefore an impairment loss was recognized. The impairment loss was calculated as the amount by which the carrying amount of the assets, exceed fair value.
EARNINGS PER SHARE
The Company has adopted the Financial Accounting Standards Board (FASB) ASC Topic 260 regarding earnings / loss per share, which provides for calculation of “basic” and “diluted” earnings / loss per share. Basic earnings / loss per share includes no dilution and is computed by dividing net income / loss available to common shareholders by the weighted average common shares outstanding for the period. Diluted earnings / loss per share reflect the potential dilution of securities that could share in the earnings of an entity similar to fully diluted earnings / loss per share.
There were2022, no potentially dilutive
instruments outstanding duringsecurities were included in the
period ended December 31, 2017 orcalculation of diluted earnings per share as the
year ended December 31, 2016.impact would have been anti-dilutive.
In accordance with ASC 740 -– Income Taxes, the provision for income taxes is computed using the asset and liability method. The liability method measures deferred income taxes by applying enacted statutory rates in effect at the balance sheet date to the differences between the tax basis of assets and liabilities and their reported amounts on the financial statements. The resulting deferred tax assets or liabilities have been adjusted to reflect changes in tax laws as they occur. A valuation allowance is provided when it is more likely than not that a deferred tax asset will not be realized.
The Company expects to recognize the financial statement benefit of an uncertain tax position only after considering the probability that a tax authority would sustain the position in an examination. For tax positions meeting a "more-likely-than-not" threshold, the amount to be recognized in the financial statements will be the benefit expected to be realized upon settlement with the tax authority. For tax positions not meeting the threshold, no financial statement benefit is recognized. As of December 31, 2017,2022 the Company had no uncertain tax positions. The Company recognizes interest and penalties, if any, related to uncertain tax positions as general and administrative expenses. The Company currently has no federal or state tax examinations nor has it had any federal or state examinations since its inception. To date, the Company has not incurred any interest or tax penalties.
For Canadian and US tax purposes, the Company’s 20142019 through 20162021 tax years remain open for examination by the tax authorities under the normal three-year statute of limitations.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
The U.S. dollar is the functional currency of the Company which is operating in the United States. The functional currency for the Company's Canadian subsidiarysubsidiaries is the Canadian dollar.
The Company translates its Canadian subsidiary'ssubsidiaries' financial statements into U.S. dollars as follows:
●
Assets and liabilities are translated at the exchange rate in effect as of the financial statement date.
●
Income statement accounts are translated using the weighted average exchange rate for the period.
| · | Assets and liabilities are translated at the exchange rate in effect as of the financial statement date. |
| · | Income statement accounts are translated using the weighted average exchange rate for the period. |
The Company includes translation adjustments from currency exchange and the effect of exchange rate changes on intercompany transactions of a long-term investment nature as a separate component of shareholders’ equity. There are currently no transactions of a long-term investment nature, nor any gains or losses from non U.S. currency transactions.
CONCENTRATION OF CREDIT RISKS
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash equivalents notes receivables, deposits, and trade receivables. The Company places its cash equivalents with high credit quality financial institutions. As of December 31, 2017 and 2016 there were no trade receivables.
FINANCIAL INSTRUMENTS AND FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company applies the provisions of accounting guidance, FASB Topic ASC 825, Financial Instruments.Instruments. ASC 825 requires all entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for which it is practicable to estimate fair value, and defines fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties. As of December 31, 20172022 and 2016,2021, the fair value of cash, accounts receivable and notes receivable, accounts payable, accrued expenses, and other payables approximated carrying value due to the short maturity of the instruments, quoted market prices or interest rates which fluctuate with market rates.
The Company defines fair value as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements).
Level 1 — Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date.
Level 2 — Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. If the asset or liability has a specified (contractual) term, a Level 2 input must be observable for substantially the full term of the asset or liability.
Level 3 — Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
| · | Level 1 – Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. |
| | |
| · | Level 2 – Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly. If the asset or liability has a specified (contractual) term, a Level 2 input must be observable for substantially the full term of the asset or liability. |
| | |
| · | Level 3 – Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
The carrying value of financial assets and liabilities recorded at fair value is measured on a recurring or nonrecurring basis. Financial assets and liabilities measured on a non-recurring basis are those that are adjusted to fair value when a significant event occurs. The Company had no financial assets or liabilities carried and measured on a nonrecurring basis during the reporting periods. Financial assets and liabilities measured on a recurring basis are those that are adjusted to fair value each time a financial statement is prepared.
Borrowings are recognized initially at fair value, net of transaction costs incurred. Borrowings are subsequently carried at amortized cost; any difference between the proceeds (net of transaction costs) and the redemption value is recognized in the income statement over the period of the borrowings using the effective interest method.
ACCOUNTING FOR DERIVATIVES LIABILITIES
The Company evaluates stock options, stock warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for under the relevant sections of ASC Topic 815-40, Derivative Instruments and Hedging: Contracts in Entity’s Own Equity.Equity. The result of this accounting treatment could be that the fair value of a financial instrument is classified as a derivative instrument and is marked-to-market at each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income or other expense.
Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity. Financial instruments that are initially classified as equity that become subject to reclassification under ASC Topic 815-40 are reclassified to a liability account at the fair value of the instrument on the reclassification date. The Company determined that none of the Company’s financial instruments meet the criteria for derivative accounting as of December 31, 20172022 and 2016.
2021.
EQUITY INSTRUMENTS ISSUED TO EMPLOYEES OR NON-EMPLOYEES FOR AQUIRINGACQUIRING GOODS OR SERVICES
Issuances
The stock-based compensation expense for both employee and non-employee awards is generally recognized on a straight-line basis over the requisite service period of the Company’s commonaward. The Company accounts for stock-based compensation to employees in conformity with the provisions of ASC Topic 718, Stock Based Compensation. Stock-based compensation to employees consisting of stock or warrants for acquiring goods or servicesoption grants and restricted shares are measuredrecognized in the statement of operations based on their fair values at the fair valuedate of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable.grant. The measurement dateCompany accounts for the fair value of the equity instruments issued to consultantsnon-employees in accordance with the provisions of ASC Topic 718, based upon the fair-value of the underlying instrument.
REVENUE RECOGNITION
The Company generates sales from three revenue streams: (1) Generic Drugs, (2) OTC Supplements, and (3) Commissions Income.
In Canada, governmental regulations require that companies recognize revenues upon completion of the work by issuing an invoice and remitting the applicable sales taxes (GST and QST) to the appropriate government agency. The Company’s wholly owned Canadian subsidiaries' revenue recognition policy is in compliance with these local regulations.
The Company recognizes revenues for product sales and commissions when title and risk of loss has passed to the customer, which is typically upon delivery to the customer, when estimated rebates are reasonably determinable, and when collectability is reasonably assured.
Trade sales and commissions are accounted for when persuasive evidence of an arrangement exists, the goods have been received by the client, the price is fixed or vendorsdeterminable and collection is determinedreasonably assured.
LEASES
The Company recognizes and measures its leases in accordance with FASB ASC 842, Leases. The Company is a lessee in a non-cancellable operating lease for office space. The Company determines if an arrangement is a lease, or contains a lease, at inception of a contract and when the terms of an existing contract are changed. The Company recognizes a lease liability and a right-of-use (ROU) asset at the earliercommencement date. The lease liability is initially and subsequently recognized based on the present value of (i)its future lease payments. Variable payments are included in the date at whichfuture lease payments when those variable payments depend on an index or a commitment for performance to earnrate. The discount rate is the equity instruments is reached (a “performance commitment” which would include a penalty considered to be of a magnitude that is a sufficiently large disincentive for nonperformance) or (ii) the date at which performance is complete. Whenimplicit rate if it is appropriate forreadily determinable or otherwise the Company uses its incremental borrowing rate. The implicit rates of the Company's lease are not readily determinable and accordingly, the Company uses its incremental borrowing rate based on the information available at the commencement date for all leases. The Company’s incremental borrowing rate for a lease is the 6% interest it would have to recognize the cost ofpay on a transaction during financial reporting periods priorcollateralized basis to borrow an amount equal to the measurement date, for purposes of recognition of costs during those periods,lease payments under similar terms and in a similar economic environment. The ROU asset is subsequently measured throughout the equity instrument is measuredlease term at the then-current fair values at each of those interim financial reporting dates.
NONCASH EQUITY TRANSACTIONS
Shares of equity instruments issued for noncash consideration are recorded at the estimated fair marketremaining amount (i.e. present value of the
consideration granted basedremaining lease payments), plus unamortized initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of lease incentives received, and any impairment recognized. Lease cost for lease payments is recognized on
a straight-line basis over the
estimated market valuelease term.
The Company has elected, for all underlying classes of assets, not to recognize ROU assets and lease liabilities for short-term leases that have a lease term of 12 months or less at lease commencement, and do not include an option to purchase the equity instrument, or at the estimated value of the goods or services received whichever is more readily determinable.
RELATED PARTIES
A party is considered to be related tounderlying asset that the Company if the party directly or indirectly or through one or more intermediaries, controls, is controlled by, or is under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the otherreasonably certain to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. A party which can significantly influence the management or operating policies of the transacting parties or if it has an ownership interest in one of the transacting parties and can significantly influence the other to an extent that one or more of the transacting parties might be prevented from fully pursuing its own separate interests is also a related party.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
GENERAL AND ADMINISTRATIVE EXPENSES
General and administrative expenses consisted of professional service fees, rent and utility expenses, meals, travel and entertainment expenses, and other general and administrative overhead costs. Expenses are recognized when incurred.
BASIC AND DILUTED NET GAIN (LOSS) PER SHARE
exercise. The Company computes loss per share in accordancerecognizes the lease cost associated with ASC 260,Earnings per Share.ASC 260 requires presentation of both basic and diluted earnings per share (“EPS”)its short-term leases on a straight-line basis over the face oflease term.
Under the income statement.
Basic net income (loss) per share is calculated by dividing net (loss) by the weighted-average common shares outstanding. Diluted net income per share is calculated by dividing net income by the weighted-average common shares outstanding during the period using the treasury stock method or the two-class method, whichever is more dilutive. As the Company incurred net lossesavailable practical expedient, we account for the year ended December 31, 2017 no potentially dilutive securities were included inlease and non-lease components as a single lease component for all classes of underlying assets as both a lessee and lessor. Further, we elected a short-term lease exception policy on all classes of underlying assets, permitting us to not apply the calculation of diluted earnings per share as the impact would have been anti-dilutive.
Therefore, basic and dilutive net (loss) per share were the same as of December 31, 2017 and 2016.
REVENUE RECOGNITION
The Company is focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. The Company does not expect to generate revenues until clinical trials of its proposed products are completed. Once completed, revenues would be recognized as its technology is licensed or sold or its products become marketable.
IMPACT OF NEW ACCOUNTING STANDARDS
In March 2017, the FASB issued ASU No. 2017-08, Receivables — Nonrefundable Fees and Other Costs (Subtopic 310-20): Premium Amortization on Purchased Callable Debt Securities, to amend the amortization period for certain purchased callable debt securities held at a premium. The ASU shortens the amortization period for the premium to the earliest call date. Under current Generally Accepted Accounting Principles (“GAAP”), entities generally amortize the premium as an adjustment of yield over the contractual life of the instrument. The amendments should be applied on a modified retrospective basis, and are effective for fiscal years beginning after December 15, 2018. Early adoption is permitted, including adoption in an interim period. The Company is currently evaluating the impactrecognition requirements of this
amendment on its financial statements.standard to short-term leases (i.e. leases with terms of 12 months or less).
In February 2017, the FASB issued ASU No. 2017-05, Other Income—Gains and Losses from the Derecognition of Nonfinancial Assets (Subtopic 610-20): Clarifying the Scope of Asset Derecognition Guidance and Accounting for Partial Sales of Nonfinancial Assets, to clarify the scope of Subtopic 610-20, Other Income—Gains and Losses from the Derecognition of Nonfinancial Assets, and to add guidance for partial sales of nonfinancial assets. Subtopic 610-20, which was issued in May 2014 as a part of ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), provides guidance for recognizing gains and losses from the transfer of nonfinancial assets in contracts with noncustomers. The amendments are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years, which is the same time as the amendments in ASU No. 2014-09, and early adoption is permitted. The Company is currently evaluating the impact of this amendment on its financial statements.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
In January 2017, the FASB issued ASU No. 2017-03, Accounting Changes and Error Corrections (Topic 250). The ASU adds SEC disclosure requirements for both the quantitative and qualitative impacts that certain recently issued accounting standards will have on the financial statements of a registrant when such standards are adopted in a future period. Specially, these disclosure requirements apply to the adoption of ASU No. 2014- 09, Revenue from Contracts with Customers (Topic 606); ASU No. 2016-02, Leases (Topic 842); and ASU No. 2016-13, Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The Company is currently evaluating the impact of these amendments on its financial statements.
Between May 2014 and December 2016, the FASB issued several ASU’s on Revenue from Contracts with Customers (Topic 606). These updates will supersede nearly all existing revenue recognition guidance under current U.S. generally accepted accounting principles (GAAP). The core principle is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services.
A five-step process has been defined to achieve this core principle, and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing U.S. GAAP. The standards are effective for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standards in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting the standards recognized at the date of adoption (which includes additional footnote disclosures). The Company is currently evaluating the impact of its pending adoption of these standards on its financial statements and has not yet determined the method by which it will adopt the standard in 2018.
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash (a consensus of the FASB Emerging Issues Task Force), to provide guidance on the presentation of restricted cash or restricted cash equivalents in the statement of cash flow. The amendments should be applied using a retrospective transition method, and are effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The Company is currently evaluating the impact of these amendments on its financial statements.
DIRECTOR AND OFFICER COMPENSATION
For the period ended December 31, 2017, the Company issued to the Board of Directors 42,000,000 shares of par value $0.001 Common Stock valued at $336,000 or $0.008 per share. During the year ended December 31, 2017, the Directors and Officers were paid $184,271 in cash. Of this amount, $147,695 was paid to Advanomics Corporation, a company controlled by the CEO of the Company.
For the period ended December 31, 2016, the Company issued 78,000,000 shares of par value $0.001 Common Stock to the three Company officers/directors valued at $241,800 or $0.0031 per share. The Company also issued to the Board of Directors 36,000,000 shares of $0.001 Common Stock valued at $252,000 or $0.0078 per share. In addition, the Company paid its officers $5,597 in cash.
During the years ended December 31, 20172022 and 2016,2021, the legal fees incurred were incurred largely as a result ofrelated to services provided to the Company to assist with its regulatory requirementsin connection with the Securities and Exchange Commission requirements and a litigationother regulatory and contracts matters.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
The Company has implemented all new accounting pronouncements that are in which it was involvedeffect and since been resolved.
DATE OF MANAGEMENT’S REVIEW
Subsequent eventsthat may impact its financial statements and does not believe that there are any other new pronouncements that have been evaluated through March 29, 2018, which is the date the Financial Statements were available to be issued.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
issued that might have a material impact on its financial position or results of operations.
Note 3 – Going Concern
The accompanying financial statements have been prepared assumingAcquisition of Nora Pharma Inc.
On October 20, 2022 the Company will continueacquired all of the issued and outstanding shares of Nora Pharma Inc. (“Nora” Pharma), a Canadian privately held company. The purchase price for the shares was $18,860,637 which was paid in cash ($14,346,637) and by the issuance of 3,700,000 shares of the Company’s common stock valued at $4,514,000 or $1.22 per share. Nora Pharma is a certified company offering generic pharmaceutical products in Canada. Nora Pharma’s operations are authorized by a Drug Establishment License issued by Health Canada. Nora Pharma is also registered with the FDA.
The following table summarizes the allocation of the purchase price as a going concern, which contemplates, among other things,of October 20, 2022, the realization ofacquisition date using Nora Pharma’s balance sheet assets and satisfaction of liabilitiesliabilities:
| Schedule of allocation of the purchase price | | | | |
| Accounts receivable | | $ | 1,358,121 | |
| Inventory | | | 3,181,916 | |
| Intangible assets | | | 659,571 | |
| Equipment & furniture | | | 210,503 | |
| Other assets | | | 1,105,093 | |
| Total assets | | | 6,515,204 | |
| | | | | |
| Liabilities assumed | | | (5,981,286 | ) |
| | | | | |
| Net assets | | | 533,918 | |
| | | | | |
| Goodwill | | | 18,326,719 | |
| | | | | |
| Total Consideration | | $ | 18,860,637 | |
Management has determined that going forward it is in the normal coursebest interest of business.the Company to impair 100% of the goodwill in the current, 2022 fiscal year. The Company hadwill review the value of the intangible and other assets on an accumulated deficitannual basis and make adjustments to the carrying amounts as necessary.
The fair value of approximately $13,618,190the 3,700,000 common shares issued as part of the consideration paid for Nora Pharma was determined on the basis of the closing market price of the Company’s common shares on the acquisition date, October 20, 2022 ($1.22 per share).
The fair value of the financial assets acquired includes receivables, Inventory, furniture, fixtures, and $12,577,954 at December 31, 2017processing equipment, and 2016, respectively, had a net lossright to use assets was $5,858,369.
The unaudited financial information in the table below summarizes the combined results of approximately $1,040,236operations of the Company (Sunshine Biopharma and Nora Pharma) for the yearyears ended December 31, 20172022 and 2021, on a net losspro forma basis, as though the companies had been combined as of $3,496,687January 1, 2021. The unaudited pro forma financial information does not purport to be indicative of the Company's combined results of operations which would actually have been obtained had the acquisition taken place on January 1, 2021, nor should it be taken as indicative of future consolidated results of operations.
| Pro Forma results from acquisition | | December 31,
2022 | | | December 31,
2021 | |
| Total revenues | | $ | 14,758,115 | | | $ | 7,927,165 | |
| Net (loss) from operations | | $ | (26,192,503 | ) | | $ | (2,224,253 | ) |
| Net (loss) | | $ | (26,164,764 | ) | | $ | (12,289,655 | ) |
| | | | | | | | | |
| Basic and fully (loss) per share | | $ | (1.74 | ) | | $ | (4.70 | ) |
| Weighted average shares outstanding | | | 15,056,097 | | | | 2,612,061 | |
In addition, the Company paid off Nora Pharma’s debt by making cash payments totaling $2,064,331 directly to Nora Pharma creditors at or before closing in order to secure creditor consent for the fiscal year ended December 31, 2016, and Shareholders’ Deficitacquisition transaction.
Note 4 – Earnout
As part of approximately $573,363 and $209,700 at December 31, 2017 and 2016, respectively.
These matters, among others, raise substantial doubt about the Company’s abilityNora Pharma acquisition the Company agreed to continue as a going concern.an earnout of $5,000,000 CAD ($3,632,000 USD) payable to Mr. Chamoun, the Seller. The Company believes it can raise capital through equityearnout is payable in the form of twenty (20) payments of $250,000 CAD for every $1,000,000 CAD increase in gross sales and borrowing to fund its operations. Management believes this will contribute toward its subsequent profitability. The accompanying Financial Statements do not include any adjustments(as defined in the Purchase Agreement) above Nora Pharma’s June 30, 2022 gross sales, provided that might be necessary ifhis employment with the Company is unablenot terminated pursuant to continuethe Company’s Employment Agreement with him. The total earnout amount of $3,632,000 has been recorded as a going concern.
salary payable.Note 45 – Goodwill and Intangible Assets
As result of the Nora Pharma acquisition the Company now has goodwill of $18,226,881 and intangible assets of $659,571 on its balance sheet. Management has determined that it is in the best interest of the Company to (i) impair 100% of the goodwill in the current, 2022 fiscal year, and (ii) review the intangible assets for amortization or possible partial of full impairment on an annual basis.
Note 6 – Patents
and Other Intellectual Property
The following is a summarylist of the Patentspatents and other intellectual property held by the Company at December 31, 2017 and 2016:
On October 8,2022:
In December 2015, the Company acquired U.S.all worldwide issued (US Patent Number 8,236,935, (the “US Patent”) for the Adva-27a anticancer compound from Advanomics Corporation (“Advanomics”), a related party, in exchange for an interest-free note payable for $4,320,000. Effective December 28, 2015, the parties executed an amendment pursuant to which this note payable for $4,320,000 was cancelled and replaced with a new interest-free convertible note having a face value of $210,519, comprised of $155,940 in principal amount which is the Advanomics book value of the US Patent plus $54,579 as an adjustment for the currency exchange difference. The new note is automatically convertible into 80,968,965 shares of the Company’s Common Stock upon the Company increasing its authorized capital to a level that would permit the issuance of such shares.
On December 28, 2015, the Company acquired the remaining worldwide issuedNumber 10,272,065) and pending patents under PCT/FR2007/000697 and PCT/CA2014/000029
(the “Worldwide Patents”) for the Adva-27a anticancer
compound from Advanomics,compound.
On May 22, 2020, the Company filed a related party,provisional patent application in exchangethe United States for a note payablenew treatment for $12,822,499. Effective December 28, 2015, the parties executed an amendment pursuantCoronavirus infections. The Company’s patent application covers composition subject matter pertaining to which this note payablesmall molecules for $12,822,499 was cancelled and replaced with a new interest-free convertible note having a face value of $624,875, comprised of $462,870 in principal amount, which is the Advanomics book valueinhibition of the Worldwide Patents, plus $162,005 asmain Coronavirus protease, Mpro, an adjustmentenzyme that is essential for the currency exchange difference.viral replication. The new note is automatically convertible into 240,336,451 sharespatent application has a priority date of the Company’s Common Stock uponMay 22, 2020. On April 30, 2021, the Company increasing its authorized capitalfiled a PCT application containing new research results and extending coverage to include the Coronavirus Papain-Like protease, PLpro. The priority date of May 22, 2020 has been maintained in the newly filed PCT application.
On April 20, 2022, the Company filed a level that would permitprovisional patent application in the issuanceUnited States covering mRNA molecules capable of destroying cancer cells in vitro. The patent application contains composition and utility subject matter pertaining to the structure and sequence of such shares. mRNA molecules.
In addition, the Company owns 152 DIN’s issued by Health Canada for prescription drugs currently on the market in Canada. These DIN’s were secured through in-licenses or cross-licenses from international manufacturers of generic pharmaceutical products.
The US PatentCompany also owns two NPN’s issued by Health Canada: (i) NPN 80089663 authorizes us to manufacture and sell our in-house developed OTC supplement, Essential 9™, and (ii) NPN 80093432 authorizes us to manufacture and sell the OTC supplement, Calcium-Vitamin D under the brand name Essential Calcium-Vitamin D™.
Note 7 – Reverse Stock Splits
Effective February 9, 2022, the Company completed a 1 for 200 reverse split of its common stock. The Company had previously completed two 20 to 1 reverse stock splits, one in 2019 and the Worldwide Patents are herein referredother in 2020.
The Company’s financial statements reflects all three reverse stock splits on a retroactive basis for all periods presented and for all references to as the “Patents.”
The Patents were therefore acquired from the related party (Advanomics) for a total of $835,394, including a total of $216,584 in adjustments for the currency exchange difference ($618,810 net). Patents expire 20 years from the priority date and are therefore amortized over 20 years. The oldest of the Patents expires on April 25, 2026 and therefore the Company has deemed that the Patents have approximately 10 years remaining on their useful life.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
In July 2016, the Company issued 321,305,416 shares of $0.001 par value Common Stock in exchange for notes payable totaling $835,394.
| | | |
| | | |
| Adva-27a US Patent | $ | $155,940 |
| Adva-27a Worldwide Patents | $ | $462,870 |
| | | |
| Total | $ | $618,810 |
| Less: accumulated amortization | | (62,690) |
| Loss on impairment | | (556,120) |
| Total | | |
| | $-0- | $-0- |
common stock, unless specifically stated otherwise.
Note 58 – Capital Stock
The Company’s authorized capital is comprised of 3,000,000,000 shares of $0.001 par value Common Stockcommon stock and 30,000,000 shares of $0.10 par value Preferred Stock,preferred stock, to have such rights and preferences as the Directors of the Company have or may assign from time to time. Out of the authorized Preferred Stock, the Company hashad previously designated 850,000 shares as Series “A” Preferred Stock (“Series A”). TheAt December 31, 2019, the Company had no issued and outstanding shares of Series A. On June 17, 2020, the Company filed an amendment to its Articles of Incorporation (the “Amendment”) eliminating the Series A is convertible at any time after issuance into 20shares and the designation thereof, which shares were returned to the status of undesignated shares of Preferred Stock. In addition, the Company's Common Stock with no further consideration, has full voting rights at 20 votes per share, and has superior liquidation rightsAmendment increased the number of authorized Series B Preferred Shares from five hundred thousand (500,000) to the Common Stock. During the year ended December 31, 2015, the Company authorized 500,000 shares of $0.10 par value Series “B” Preferred Stock (“Series B”).one million (1,000,000) shares. The Series B Preferred Stock is non-convertible, non-redeemable and non-retractable. It has superior liquidation rights to the Common Stockcommon stock at $0.10 per share and gives the holder the right to 1,000 votes per share. AllAs of December 31, 2021, there were 1,000,000 shares of the Series B Preferred Stock are held by the CEO of the Company.
On February 17, 2022, the Company’s public offering closed and the Company received net proceeds of $6,833,071 from the offering. Pursuant to the public offering, the Company issued and sold an aggregate of 1,882,353 shares of common stock and 4,102,200 warrants to purchase shares of common stock (the “Tradeable Warrants”) (including 337,494 Tradeable Warrants resulting from partial exercise of the overallotment option granted to the underwriter).
On February 22, 2022, the Company redeemed 990,000 shares of Series B Preferred Stock from the CEO of the Company at a redemption price equal to the stated value of $0.10 per share.
On March 14, 2022, the Company completed a private placement and received net proceeds of $6,781,199. In connection with this private placement, the Company issued (i) 2,301,353 shares of its common stock together with investor warrants (“Investor Warrants”) to purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of common stock, together with Investor Warrants to purchase up to 1,302,251 shares of common stock. Each share of common stock and accompanying Investor Warrant was sold together at a combined offering price of $2.22 and each Pre-Funded Warrant and accompanying Investor Warrant were sold together at a combined offering price of $2.219. The Pre-Funded Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The Investor Warrants have an exercise price of $2.22 per share (subject to adjustment as set forth in the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On April 28, 2022, the Company completed another private placement and received net proceeds of $16,752,915. In connection with this private placement, the Company issued (i) 2,472,820 shares of its common stock together with warrants (“April Warrants”) to purchase up to 4,945,640 shares of common stock, and (ii) 2,390,025 pre-funded warrants (“Pre-Funded Warrants”) with each Pre-Funded Warrant exercisable for one share of common stock, together with April Warrants to purchase up to 4,780,050 shares of common stock. Each share of common stock and accompanying two April Warrants were sold together at a combined offering price of $4.01 and each Pre-Funded Warrant and accompanying two April Warrants were sold together at a combined offering price of $4.009. The Pre-Funded Warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. The April Warrants have an exercise price of $3.76 per share (subject to adjustment as set forth in the warrant), are exercisable upon issuance and will expire five years from the date of issuance.
On October 20, 2022, the Company issued 3,700,000 shares of Common Stock as part of the acquisition of Nora Pharma. These shares were valued at $4,514,000, or $1.22 per share.
During the fiscal year ended December 31, 2021, the Company issued an aggregate of 559,144 shares of its Common Stock valued at $12,705,214 in connection with the conversion of $2,867,243 in debt and interest of $127,986 resulting in a loss of $9,726,485 on conversion. In addition, the Company issued 300,000 shares of its Common Stock valued at $918,000 as compensation to its directors. In total, 859,114 shares of Common Stock were issued during the fiscal year ended December 31, 2021.
Through December 31, 20172022 and December 31, 2016,2021, the Company has issued and outstanding a total of 918,736,49822,585,632 and 769,399,8582,591,240 shares of Common Stock, respectively. Through the same periods, the Company has issued and outstanding a total of -0- and -0- shares of Series A Preferred Stock and 500,000 and 500,000 shares of Series B Preferred Stock, respectively.
During the fiscal year ended December 31, 2017, the Company issued an aggregate of 149,336,640 shares of its Common Stock as follows:
●
40,000,000 shares for cash in the amount of $100,000 Canadian or $78,312 US
●
11,004,167 shares for the purchase of laboratory and generic drugs warehouse equipment valued at $56,700
●
42,000,000 shares valued at $336,000 as compensation to the Company’s Directors and Officers
●
13,804,348 shares for services rendered to the Company by third parties valued at $77,000
●
42,528,125 valued at $128,451 shares in connection with the conversion of $48,500 in debt and interest of $3,022 resulting in a $76,929 loss on conversion
During the fiscal year ended December 31, 2016, the Company issued 411,829,184 shares of Common Stock for the conversion of $1,122,782 in debt and interest of $9,270 generating a loss of $1,945,898 on conversion. The Company sold 12,555,556 shares of Common Stock for cash of $104,128 and issued 146,750,000 shares of Common Stock in exchange for services valued at $702,300. In 2016, 114,000,000 shares valued at $493,800 were issued to the Directors and Officers of the Company. The Officers and Directors shares are restricted and may not be sold without prior written consent of the Board of Directors of the Company.
The Company has declared no dividends since inception.
Note 9 – Warrants
The Company accounts for issued warrants either as a liability or equity in accordance with ASC 480-10 or ASC 815-40. Under ASC 480-10, warrants are considered a liability if they are mandatorily redeemable and they require settlement in cash, other assets, or a variable number of shares. If warrants do not meet liability classification under ASC 480-10, the Company considers the requirements of ASC 815-40 to determine whether the warrants should be classified as a liability or as equity. Under ASC 815-40, contracts that may require settlement for cash are liabilities, regardless of the probability of the occurrence of the triggering event. Liability-classified warrants are measured at fair value on the issuance date and at the end of each reporting period. Any change in the fair value of the warrants after the issuance date is recorded in the consolidated statements of operations as a gain or loss. If warrants do not require liability classification under ASC 815-40, in order to conclude warrants should be classified as equity, the Company assesses whether the warrants are indexed to its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP standard. Equity-classified warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
During the fiscal year ended December 31, 20172022, the Company completed three financing events, and 2016
in connection therewith, it issued warrants as follows:| Warrants issued with financing | | | | | | |
| Type | | Number | | Exercise Price | | Expiry Date |
| Pre-Funded Warrants | | 3,692,276 | | $0.001 | | Unlimited |
| Tradeable Warrants | | 4,102,200 | | $2.22* | | February 2027 |
| Investor Warrants | | 3,603,604 | | $2.22 | | March 2027 |
| April Warrants | | 9,725,690 | | $3.76 | | April 2027 |
| * | The Tradeable Warrants had an initial exercise price of $4.25, subject to adjustment. Upon the closing of the Company’s private placement on March 14, 2022, the exercise price of the Tradeable Warrants was reduced to $2.22, in accordance with the terms thereof. |
During the fiscal year ended December 31, 2022, all of the Pre-Funded Warrants and a total of 3,138,507 Tradeable Warrants were exercised resulting in aggregate proceeds of $6,971,178 received by the Company. In addition, during the fiscal year ended December 31, 2022, a total of 2,802,703 Investor Warrants were exercised resulting in aggregate proceeds of $6,222,001 received by the Company.
The Company’s outstanding warrants at December 31, 2022 consisted of the following:
| Schedule of outstanding warrants | | | | | | |
| Type | | Number | | Exercise Price | | Expiry Date |
| Pre-Funded Warrants | | None | | $0.001 | | Unlimited |
| Tradeable Warrants | | 963,693 | | $2.22 | | February 2027 |
| Investor Warrants | | 800,901 | | $2.22 | | March 2027 |
| April Warrants | | 9,725,690 | | $3.76 | | April 2027 |
At December 30, 2022, the final trading day of the year, the closing price of the Company’s common stock was $0.64 per share, a value well below the exercise price of these warrants.
Note 610 – Earnings perPer Share
The following table sets forth the computation of basic and diluted net income per share for the years ended December 31:
| Schedule of earnings per share computation | | | | | | | | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Net gain (loss) attributable to common stock | | $ | (26,744,440 | ) | | $ | (12,436,447 | ) |
| Basic weighted average outstanding shares of common stock | | | 15,180,868 | | | | 2,612,061 | |
| Dilutive common share equivalents | | | 0 | | | | 0 | |
| Dilutive weighted average outstanding shares of common stock | | | 15,180,868 | | | | 2,612,061 | |
| Net gain (loss) per share attributable to common stock | | $ | (1.76 | ) | | $ | (4.76 | ) |
| | | |
| Net (loss) attributable to Common Stock | $(1,040,236) | $(3,496,687) |
| Basic weighted average outstanding shares of Common Stock | 872,685,608 | 424,874,458 |
| Dilutive effects of common share equivalents | -0- | -0- |
| Dilutive weighted average outstanding shares of common stock | 872,685,608 | 424,874,458 |
| Net loss per share of Common Stock | | |
| Basic and Diluted | $(0.00) | $(0.01) |
The Company files a United States federal income tax return and a Canadian branch return on a calendar year basis. The Company and its wholly-owned subsidiary, Sunshine Biopharma Canada Inc., have not generated taxable income since inception.
Deferred income taxes arise from the temporary differences between financial statement and income tax recognition of net operating losses. These loss carryovers are limited under the Internal Revenue Code should a significant change in ownership occur. The Company accounts for income taxes pursuant to ASC 740.
Deferred income taxes arise from the temporary differences between financial statement and income tax recognition of net operating losses and other items. Loss carryovers are limited under the Internal Revenue Code should a significant change in ownership occur.
The Company follows FASB Statement Accounting Standards Codification No. 740, “Accounting for Income Taxes”, which requires, among other things, an asset and liability approach to calculating deferred income taxes.
The components of the provision for income taxes were as follows:
| Provision for income taxes | | | |
| Current: | | | |
| Federal | | $ | – | |
| State | | | – | |
| Foreign | | | 139,856 | |
| Deferred: | | | | |
| Federal | | | – | |
| State | | | – | |
| Foreign | | | 3,628 | |
| Total | | $ | 143,484 | |
The components of the net deferred income tax assets and liabilities arising under ASC No. 740 were as follows:
| Components of net deferred tax assets | | | |
| Deferred Tax Assets: | | | |
| Net Operating Loss, Credits and Carryforwards | | $ | 4,323,025 | |
| Fixed Assets | | | 98,957 | |
| Intangibles | | | 1,021,230 | |
| Research and Development | | | 90,000 | |
| Other DTA | | | 161,719 | |
| Lease Liability | | | 202,793 | |
| Valuation Allowance | | | (5,596,431 | ) |
| Total Deferred Tax Assets | | | 301,293 | |
| | | | | |
| Deferred Tax Liabilities: | | | | |
| Fixed Assets | | | – | |
| Intangibles | | | (142,817 | ) |
| Right-of-Use Asset | | | (201,508 | ) |
| Total Deferred Tax Liabilities | | | (344,325 | ) |
| | | | | |
| Net Deferred Tax Liability | | $ | (43,032 | ) |
There were no deferred income taxes
Note 12 – Notes Payable
As of December 31, 2022 and December 31, 2021, the Company had $0 and $1,900,000, respectively in notes payable outstanding. At December 31, 2022 and December 31, 2021, total accrued interest on Notes Payable was $0 and $48,287, respectively.
The Company’s Notes Payable at December 31, 2017 and 2016.
The types of temporary differences between the tax basis of assets and their financial reporting amounts that give rise to a significant portion2021 consisted of the deferred assets and liabilities are as follows:
| | | |
| | | | | |
| Deferred tax assets: | | | | |
| Net operating loss US | $10,611,921 | $3,932,778 | $9,609,340 | $3,561,221 |
| Net operating loss Canada | 266,498 | 71,421 | 202,188 | 46,099 |
| Total | 10,878,419 | 4,004,199 | 9,811,528 | 3,607,320 |
| | | | | |
| Valuation allowance | (10,878,419) | (4,004,199) | (9,811,528) | (3,607,320) |
| | | | | |
| Total deferred tax asset | -0- | -0- | -0- | -0- |
| | | | | |
| Net deferred tax asset | $-0- | $-0- | $-0- | $-0- |
Sunshine Biopharma, Inc.
Notesfollowing:
On April 20, 2021, the Company received monies in exchange for a Note Payable having a Face Value of $500,000 with interest accruing at 5% due April 20, 2023. The Note was convertible after 180 days from issuance into common stock at a price equal to Consolidated Financial Statements
December 31, 2017 and 2016
Deferred income taxes arise$0.30 per share. On February 17, 2022, the Company paid off the entire principal balance of this Note, together with accrued interest of $20,753 by making cash payment of $520,753.
On July 6, 2021, the Company received monies in exchange for a Note Payable having a Face Value of $900,000 with interest accruing at 5%, due July 6, 2023. The Note was convertible after 180 days from issuance into common stock at a price equal to $0.30 per share. On February 17, 2022, the temporary differences between financial statement and income tax recognitionCompany paid off the entire principal balance of net operating losses. These loss carryovers are limited underthis Note, together with accrued interest of $27,863 by making cash payment of $927,863.
On August 18, 2021, the Internal Revenue Code shouldCompany received monies in exchange for a significant change in ownership occur.
Note Payable having a Face Value of $500,000 with interest accruing at 5%, due August 18, 2023. The Note was convertible after 180 days from issuance into common stock at a price equal to $0.30 per share. On February 17, 2022, the Company paid off the entire principal balance of this Note, together with accrued of $12,534 by making cash payment of $512,534.
At December 31, 20172022 and December 31, 2016,2021, total accrued interest on Notes Payable was $-0- and $48,287, respectively.
Note 13 – Notes Payable - Related Party
A Note Payable dated December 31, 2019 held by the CEO of the Company had approximately $10,611,921having a Face Value of $128,269 and $9,609,340, respectivelyaccruing interest at 12% was due December 31, 2020. On December 31, 2020, the Company renewed the Note together with accrued interest of $15,392 for a 12-month period. The new Note has a face Value of $143,661, accrues interest at 12% per annum, and has a maturity date of December 31, 2021. On August 24, 2021, the Company paid off the entire principal balance of this Note, together with accrued interest of $12,929 by issuing cash payment of $156,590.
Note 14 – Lease
The Company has obligations as a lessee for office space with initial non-cancellable terms in unused federal netexcess of one year. The Company classified the lease as an operating loss carryforwards, which beginlease. The lease contains a renewal option for a period of five years. Because the Company is certain to expire principallyexercise the renewal option, the optional period is included in determining the lease term, and associated payments under the renewal option are included in the year 2029. A deferred tax asset at each datelease payments. The Company’s lease does not include termination options for either party to the lease or restrictive financial or other covenants. Payments due under the lease contract include fixed payments plus a variable Payment. The Company’s office space lease requires it to make variable payments for the Company’s proportionate share of approximately $3,950,013building’s property taxes, insurance, and $3,607,320common area maintenance. These variable lease payments are not included in lease payments used to determine lease liability and are recognized as variable costs when incurred.
Amounts reported on the balance sheet as of December 31, 2022 were as follows:
| Lease information | |
| Operating lease ROU asset | $760,409 |
| Operating Lease liability - Short-term | $123,026 |
| Operating lease liability - Long-term | $642,232 |
| Remaining lease term | 7 years |
| Discount rate | 6% |
Amounts disclosed for ROU assets obtained in exchange for lease obligations and reductions of ROU assets resulting from reductions of lease obligations include amounts reduced from the loss carryforwards has been offset by a 100% valuation allowance. The change in the valuation allowance for the period endedcarrying amount of ROU assets resulting from deferred rent.
Maturities of lease liabilities under non-cancellable operating leases at December 31, 20172022 are as follows:
| Maturities of lease liabilities | |
| 2023 | $123,026 |
| 2024 | $115,879 |
| 2025 | $116,066 |
| 2026 | $109,934 |
| 2027 | $103,547 |
| Thereafter | $196,807 |
Note 15 – Management and December 31, 2016 was approximately $342,693and $521,180, respectively.
A reconciliation of the U.S. statutory federal income tax rate to the effective tax rate is as follows:
| | |
| | | |
| U.S. Federal statutory graduated rate | 34.00% | 34.00% |
State income tax rate, net of federal benefit | 3.06% | 3.06% |
| Net rate | 37.06% | 37.06% |
| | | |
| Net operating loss used | 0.00% | 0.00% |
Net operating loss for which no tax benefit is currently available | -37.06% | -37.06% |
| | 0.00% | 0.00% |
Director Compensation
The Company’s income tax filings are subject to audit by various taxing authorities. The Company’s open audit periods are 2014, 2015,Company paid its officers cash compensation totaling $1,785,000 and 2016, although, the statute of limitations for the 2014 tax year will expire effective March 15, 2018. In evaluating the Company’s provisions and accruals, future taxable income, and reversal of temporary differences, interpretations and tax planning strategies are considered.
Note 8 – Notes Payable
Notes payable consist of the following: | | |
| | | |
| A Note Payable having a Face Value of $21,439 at December 31, 2016 and accruing interest at 12% was due December 31, 2017. On December 31, 2017, the Company renewed the note, together with accrued interest of $2,573, for a 12-month period. The new note has a Face Value of $24,012 and is due December 31, 2018. The new note accrues interest at 12% and is convertible anytime from the date of issuance into $0.001 par value Common Stock at a 35% discount from market price. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $24,012 | $21,439 |
| | | |
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
On July 1, 2016, the Company received monies in exchange for a note payable having a Face Value of $55,000 with interest accruing at 10% is due April 1, 2017. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 40% below market value. In December 2016, $6,500 of the principal was converted into 5,000,000 shares of $0.001 par value Common Stock valued at $20,000 and generating a loss of $13,500 on conversion. In January 2017, the remaining principal amount of $48,500 together with accrued interest of $3,022 was converted into 42,528,125 shares of $0.001 par value Common Stock valued at $128,451 and generating a loss of $76,929 on conversion. | $-0- | $48,500 |
| | | |
| On February 10, 2017, the Company received $48,000 cash in exchange for a note payable having a Face Value of $50,000 with interest accruing at 8%, which is due November 20, 2017. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. In August 2017, the note was paid off with additional $1,863 in accrued interest and $15,559 as prepayment penalty. | $-0- | $-0- |
| | | |
On April 1, 2017, the Company received monies in exchange for a note payable having a Face Value of $100,000 Canadian ($79,710 US) with interest payable quarterly at 9%, which is due April 1, 2019. The Note is convertible any time after issuance into $0.001 par value Common Stock at a price of $0.015 Canadian (approximately $0.012 US) per share. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $79,710 | $-0- |
| | | |
On April 26, 2017, the Company received $63,000 cash in exchange for a note having a Face Value of $ 65,000 with interest accruing at 8%, which is due April 26, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. In August 2017 the note was paid off with additional $2,607 in accrued interest and $19,500 as prepayment penalty. | $-0- | $-0- |
| | | |
On August 3, 2017, the Company received $76,000 in exchange for a note payable having a Face Value of $ 80,000 with interest accruing at 8%, which is due August 3, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $80,000 | $-0- |
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
On August 21, 2017, the Company received $80,000 cash in exchange for a note payable having a Face Value of $ 83,000 with interest accruing at 8% , which is due May 30, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $83,000 | $-0- |
| | | |
On September 22, 2017, the Company received $60,000 cash in exchange for a note having a Face Value of $ 62,000 with interest accruing at 8%, which is due June 30, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $62,000 | $-0- |
| | | |
On October 26, 2017, the Company received $110,000 cash in exchange for a note payable having a Face Value of $ 115,000 with interest accruing at 8%, which is due October 26, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $115,000 | $-0- |
| | | |
On November 14, 2017, the Company received $106,000 cash in exchange for a note payable having a Face Value of $ 113,000 with interest accruing at 8%, which is due November 14, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $113,000 | $-0- |
| | | |
On December 1, 2017 the Company received monies in exchange for a note having a Face Value of $ 50,000 Canadian ($39,855 US) with interest accruing at 8%, due November 30, 2018. The Note is convertible after 180 days from issuance into $0.001 par value Common Stock at a price 35% below market value. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $39,855 | $-0- |
| | | |
| Total Current Debt | $596,577 | $69,939 |
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
Interest expense$297,307 for the years ended December 31, 20172022 and 2016 was $79,833 and $34,732,2021, respectively. The balance of interest payable at December 31, 2017 and 2016 was $9,215 and $9,011, respectively. Loss on conversion of notes payable for the years ended December 31, 2017 and 2016 was $76,929 and $1,945,898, respectively.
Note 9 – Notes Payable Related Party
| Notes payable to related parties consist of the following: | | |
| | | |
| A note payable held by a private individual who subsequently became a principal shareholder of the Company having a face value of $100,000 at December 31, 2016 and a maturity date of March 31, 2017, accrues interest at 12%. The Note is convertible any time from the date of issuance into $0.001 par value Common Stock at a 35% discount from market price. On March 31, 2017, the note’s principal balance of $100,000 plus accrued interest of $11,715 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a face value of $111,715 matures on June 30, 2017. On June 30, 2017, the note’s principal balance of $111,715 plus accrued interest of $3,342 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a face value of $115,057 matures on September 30, 2017. On September 30, 2017, the note’s principal balance of $115.057 plus accrued interest of $3,480 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a principal balance of $118,537 matures on December 31, 2017. On December 31, 2017 the note plus accrued interest of $3,556 was renewed for a 12-month period under the same terms and conditions as before. The new note has a face value of $122,093 and matures on December 31, 2018. The Company estimates that the fair value of this convertible debt approximates the face value, so no value has been assigned to the beneficial conversion feature. Any gain or loss will be recognized at conversion. | $122,093 | $100,000
|
| | | |
| In December 2016, the Company received monies from its CEO in exchange for a note payable having a principal amount of $90,000 Canadian ($67,032 US) with interest at 12% due March 31, 2017. The note was convertible any time after the date of issuance into $0.001 par value Common Stock at a price 35% below market value. This note was collateralized by all of the assets of the Company. In the event of default, the interest rate will increased to 18% per annum and a penalty of $1,000 Canadian ($752 US) per day will accrue. On March 31, 2017, the note, together with accrued interest of $3,021 Canadian ($2,271 US) and an additional principal amount of $3,000 Canadian ($2,247 US) paid to the Company on March 28, 2017, was renewed for a 90-day period under the same terms and conditions as the original note. The new note now having a face value of $96,021 Canadian ($72,198 US) was due on June 30, 2017. On June 30, 2017, the note, together with accrued interest of $2,873 Canadian ($2,005 US), was renewed for a 90-day period under the same terms and conditions as the original note except that the new note is non-convertible. The new note now having a face value of $98,894 Canadian ($76,072US) is due on September 30, 2017. On September 30, 2017, the note, together with accrued interest of $2,991 Canadian ($2,397 US) was renewed for a 90-day period under the same terms and conditions as the original note except that the new note is nonconvertible. The new note now having a principal balance of $101,885 Canadian ($81,640 US) matures December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as before except that this new note is unsecured and nonconvertible. The new note has a face value of $104,942 Canadian ($83,649 US) and matures on December 31, 2018. | $83,649
| $67,032 |
| | | |
| Total Current Related Party Debt | $205,742 | $167,032 |
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
December 31, 2017 and 2016
Note 10 – Related Party Transactions
In December 2016, the Company received monies from our CEO in exchange for a note payable having a principal of $90,000 Canadian ($67,032 US) with interest at 12% due March 31, 2017. The Note is convertible any time after the date of issuance into shares of our Common Stock at a price 35% below market value. We estimated that the fair value of the convertible debt approximates the face value, so no value has been assignedOf these amounts attributable to the beneficial conversion feature. This Note is collateralized by all of the assets of the Company. On March 31, 2017, the note, together with accrued interest of $3,021 Canadian ($2,271 US)Company’s CEO, $60,000 and an additional principal amount of $3,000 Canadian ($2,247 US) paid to the Company on March 28, 2017, was renewed for a 90-day period under the same terms and conditions as the original note. The new note now having a face value of $96,021 Canadian ($72,198 US) was due on June 30, 2017. On June 30, 2017, the note, together with accrued interest of $2,873 Canadian ($2,005 US), was renewed for a 90-day period under the same terms and conditions as the original note except that the new note is nonconvertible. The new note now having a face value of $98,894 Canadian ($76,072US) is due on September 30, 2017. On September 30, 2017, the note, together with accrued interest of $2,991 Canadian ($2,397 US), was renewed for a 90-day period under the same terms and conditions as the original note except that the new note is nonconvertible. The new note now having a principal balance of $101,885 Canadian ($81,640 US) matures December 31, 2017. On December 31, 2017, the note was renewed for a 12-month period under the same terms and conditions as the original note except that this note is unsecured and non-convertible. The new note has a face value of $104,942 Canadian ($84,649 US) and matures on December 31, 2018.
A note payable held by a private individual who subsequently became a principal shareholder of the Company having a face value of $100,000 at December 31, 2016 and a maturity date of March 31, 2017, accrues interest at 12%. The Note is convertible any time from the date of issuance into $0.001 par value Common Stock at a 35% discount from market price. On March 31, 2017, the note’s principal balance of $100,000 plus accrued interest of $11,715 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a face value of $111,715 matures on June 30, 2017. On June 30, 2017, the note’s principal balance of $111,715 plus accrued interest of $3,342 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a face value of $115,057 matures on September 30, 2017. On September 30, 2017, the note’s principal balance of $115.057 plus accrued interest of $3,480 was renewed for a period of 90 days under the same terms and conditions as the original note. The new note now having a principal balance of $118,537 matures on December 31, 2017. On December 31, 2017 the note was renewed for a 12-month period under the same terms and conditions as before. The new note has a face value of $122,093 and matures on December 31, 2018.
Until June 1, 2017, the Company’s principal place of business was located at 469 Jean-Talon West, 3rd Floor, Montreal, Quebec, Canada H3N R4. This was also the location of the Company’s former licensor, Advanomics Corporation (“Advanomics”), who provided this space to the Company on a rent free basis in 2015 and 2016. Starting January 1, 2017, the Company took over the lease from Advanomics for this space until it moved to its current location in June 2017.
In February and April 2016, the Company paid $30,000 and $50,487 to Advanomics for the balance of 2015 licensing fees.
In 2016, Advanomics Corporation paid on behalf of the Company $13,725 Canadian in patenting fees. Advanomics was fully reimbursed by the Company in January 2017.
Dr. Steve N. Slilaty, the Company’s Chief Executive Officer and a Director, is an Officer, Director and principal shareholder of Advanomics.
During the period ended December 31, 2017, the Company issued to its Directors and Officers 42,000,000 shares of $0.001 par value Common Stock valued at $336,000 or $0.008 per share. In addition, the Directors and Officers were paid an aggregate of $184,271 for their services in 2017. Of this amount, $147,695$110,000, respectively was paid to Advanomics Corporation, a company controlled by the CEO of the Company.
During the period ended December 31, 2016, the Company issued 78,000,000 shares of $0.001 par value Common Stock to the three Company officers valued at $241,800 or $0.0031 per share. During the same period, the Company also issued to the Board of Directors 36,000,000 shares of $0.001 par value Common Stock valued at $252,000 or $0.0078 per share. In addition, the Company
issued 300,000 shares of common stock valued at $918,000 to its officers during year ended December 31, 2021. The value of these shares was based upon the closing price of the Company’s common stock of $3.06 on the issuance date.
The Company paid its officers $5,597 in cash.
Sunshine Biopharma, Inc.
Notes to Consolidated Financial Statements
directors cash compensation totaling $300,000 and $0 for the years ended December 31, 20172022 and 2016
2021, respectively.
Note 1116 – Royalties Payable
As part of a subscription agreement entered into in February 2016,Subsequent Events
On January 19, 2023, the Company has an obligationannounced a stock repurchase program of up to pay a royalty of 5% of net sales on one of its generic products (Anastrozole) for a period of three (3) years from the date of the first sale of that product.$2 million. As of the date hereof,of this report, the Company has not received revenues from the sale of this product.
Note 12 – Acquisition of Atlas Pharma Inc.
In December 2017, the Company issued a payment of $100,500 Canadian ($80,290 US) to Mr. Mohamed Belhai as a deposit towards the acquisition of Atlas Pharma Inc. On January 1, 2018, the Company entered into a Share Purchase Agreement with Mr. Mohamed Belhaj and Atlas Pharma Inc. (the “Atlas Agreement”), wherein the Company acquired all of the issued and outstanding shares (the “Shares”) of Atlas Pharma Inc., (“Atlas”) from Mr. Belhaj. The purchase price for the Shares was $848,000 Canadian (approximately $678,400 US). The purchase price included a cash payment of $100,500 Canadian ($80,290 US), plus issuance of 20,000,000 shares of the Company’s Common Stock, plus a promissory note in the principal amount of $450,000 Canadian (approximately $360,000 US), with interest payable at the rate of 3% per annum. The Company is required to make payments of $10,000 per calendar quarter, due and payable on or before the end of each such calendar quarter through December 31, 2023.
Note 13 – Subsequent Events
On January 1, 2018, the Company acquired Atlas Pharma Inc., a Montreal-based, fully certified analytical chemistry company dedicated to chemical analysis of pharmaceutical and other industrial samples. More information about Atlas Pharma is available at www.atlaspharmainc.ca.
On January 12, 2018, the Company received monies in exchange for a convertible note payable having a face value of $102,000.
On February 6, 2018, the Company issued payment in the amount of $51,613 to pay off approximately half of a note payable dated August 3, 2017, and on February 12 and 21 the remainder was converted intorepurchased a total of 6,555,761445,711 shares of the Company's Common Stock.
On February 7, 2018, the Company received monies in exchangeStock at an average price of $1.1371 per share for a
convertible note payable having a face valuetotal cost of
$150,000.$506,822. As of the date of this report, the repurchased shares have not been returned to treasury.
On February 16, 2018, the Company issued payment in the amount
39,215,687 Units, Each Unit Consisting of $115,370One Share of Common Stock or One Pre-Funded Warrant to pay off a note payable dated August 21, 2017.
On February 20, 2018, the Company received monies in exchange for a convertible note payable having a face valuePurchase One Share of $85,000.
On March 27, 2018, the holderCommon Stock, one-tenth of a convertible note having a face valueSeries A Warrant to Purchase one Share of $62,000 elected to convert $15,000 of the outstanding principal amount into 2,727,273 shares of the Company's Common Stock leavingand two-tenths of a principal balanceSeries B Warrant to Purchase one Share of $47,000.
Common Stock11,764,706 Shares of Common Stock Underlying the Series A and Series B Warrants

PROSPECTUS
Aegis Capital Corp.
, 2024
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
Item 13. Other Expenses of Issuance and Distribution.
The following table presents fees for professional audit services renderedsets forth all costs and expenses paid or payable by B F Borgers CPA PC, our independent auditors, during our fiscal year ended December 31, 2017us in connection with the sale of the securities being registered, other than underwriting discounts and 2016:
| | | |
| Audit Fees | $21.600 | $21,600 |
| | | |
| Tax Fees | | |
| All Other Fees | | |
| Total | $21,600 | $21,600 |
Audit Fees. Consist ofcommissions. All amounts billed for professional services renderedshown are estimates except for the auditSecurities and Exchange Commission, or SEC, registration fee, the Nasdaq listing fee, and the FINRA filing fee.| Expense | | Amount
Paid or
to be Paid | |
| SEC registration fee | | $ | 10,015 | |
| FINRA filing fee | | | 10,678 | |
| Legal fees and expenses | | | 450,000 | |
| Accounting fees and expenses | | | 20,000 | |
| Miscellaneous expenses | | | 10,000 | |
| Expense reimbursement to underwriter | | | 150,000 | |
| Total | | $ | 650,693 | |
Item 14. Indemnification of our annual financial statements included in our Annual Reports on Forms 10-K for our fiscal years ended December 31, 2017Directors and 2014 and reviews of our interim financial statements included in our Quarterly Reports on Form 10-Q.
Tax Fees. Consists of amounts billed for professional services rendered for tax return preparation, tax planning and tax advice.
All Other Fees. Consists of amounts billed for services other than those noted above.
We do not have an audit committee and as a result our entire board of directors performs the duties of an audit committee. Our board of directors evaluates the scope and cost of the engagement of an auditor before the auditor renders audit and non-audit services.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Officers.Section 7-108-402 of the Colorado Business Corporation Act (the “CBCA”) provides, generally, that the articles of incorporation may contain a provision eliminating or limiting the personal liability of a director to the corporation or its shareholders for monetary damages for breach of fiduciary duty as a director, except that any such provision shall not eliminate or limit the liability of a director for (i) for any breach of the director’s duty of loyalty to the corporation or its shareholders, (ii) acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) acts specified in Section 7-108-403 of the CBCA,
or (iv) any transaction from which the director directly or indirectly derived an improper personal benefit.Section 7-109-102(1) of the CBCA permits indemnification of a director of a Colorado corporation, in the case of a third party action, if the director (a) conducted himself or herself in good faith, (b) reasonably believed that (i) in the case of conduct in his or her official capacity, his or her conduct was in the corporation’s best interest, or (ii) in all other cases, his or her conduct was not opposed to the corporation’s best interest, and (c) in the case of any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful. Section 7-109-103 further provides for mandatory indemnification of directors and officers who are successful on the merits or otherwise in litigation.
Section 7-109-102(4) of the CBCA limits the indemnification that a corporation may provide to its directors in two key respects. A corporation may not indemnify a director in a derivative action in which the director is held liable to the corporation, or in any proceeding in which the director is held liable on the basis of his improper receipt of a personal benefit. Sections 7-109-104 of the CBCA permits a corporation to advance expenses to a director, and Section 7-109-107(1)(c) of the CBCA permits a corporation to indemnify and advance litigation expenses to officers, employees and agents who are not directors to a greater extent than directors if consistent with law and provided for by the bylaws, a resolution of directors or shareholders, or a contract between the corporation and the officer, employee or agent.
Our bylaws include provisions that require the company to indemnify our directors or officers against monetary damages for actions taken as a director or officer of our Company. We are also expressly authorized to carry directors’ and officers’ insurance to protect our directors, officers, employees and agents for certain liabilities. Our articles of incorporation do not contain any limiting language regarding director immunity from liability.
The limitation of liability and indemnification provisions under the CBCA and our bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. However, these provisions do not limit or eliminate our rights, or those of any stockholder, to seek non-monetary relief such as injunction or rescission in the event of a breach of a director’s fiduciary duties. Moreover, the provisions do not alter the liability of directors under the federal securities laws. In addition, your investment may be adversely affected to the extent that, in a class action or direct suit, we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
RECENT SALES OF UNREGISTERED SECURITIES
The following sets forth information regarding all unregistered securities sold by us in transactions that were exempt from the requirements of the Securities Act in the last three years. Except where noted, all of the securities discussed in this Item 15 were all issued in reliance on the exemption under Section 4(a)(2) of the Securities Act. Unless otherwise indicated, all of the share issuances described below were made in reliance on the exemption from registration provided by Section 4(a)(2) of the Securities Act.
During the six months ended June 30, 2018, we issued a total of 185,369,308 shares of our Common Stock. Of these, 42,584,566 shares valued at $290,286 were issued upon conversion of outstanding notes payable, reducing outstanding debt by $188,568 and interest payable by $8,133 and generating a loss on conversion of $93,585. We also issued 93,650,000 shares valued at $558,200 for services, and 29,134,742 shares valued at $174,808 in exchange for equipment.
During the fiscal year ended December 31, 2017, we issued an aggregate of 149,336,640 shares of our Common Stock as follows:
●
40,000,000 shares for cash in the amount of $100,000 Canadian or $78,312 US
●
11,004,167 shares for the purchase of laboratory and generic drugs warehouse equipment valued at $56,700
●
42,000,000 shares valued at $336,000 as compensation to the Company’s Directors and Officers
●
13,804,348 shares for services rendered to the Company by third parties valued at $77,000
●
42,528,125 shares valued at $128,451 in connection with the conversion of $48,500 in debt and interest of $3,022 resulting in a $76,929 loss on conversion.
During the fiscal year ended December 31, 2016, the Company issued 411,829,184 shares of Common Stock for the conversion of $1,122,782 in debt and interest of $9,270 generating a loss of $1,945,898 on conversion. The Company sold 12,555,556 shares of Common Stock for cash of $104,128 and issued 146,750,000 shares of Common Stock in exchange for services valued at $702,300. In 2016, 114,000,000 shares valued at $493,800 were issued to the Directors and Officers of the Company. The Officers and Directors shares are restricted and may not be sold without prior written consent of the Board of Directors of the Company.
During the fiscal year ended December 31, 2015, the Company issued 124,714,077 shares of Common Stock. Of these, 102,914,077 were issued for the conversion of $501,624 in debt and $12,886 in interest, generating a loss of $575,144 on conversion. In addition, the Company sold 20,000,000 shares of Common Stock for cash of $236,550 and issued 1,800,000 shares of Common Stock in exchange for services valued at $66,500. In 2015, the Company also issued 500,000 shares of Series “B” Preferred Stock to the CEO of the Company in exchange for services valued at $50,000.
The preceding securities were not registered under the Securities Act of 1933, as amended (the “Securities Act”), but qualified for exemption under Section 4(a)(2) of the Securities Act. The securities were exempt from registration under Section 4(a)(2) of the Securities Act because the issuance of such securities by the Company did not involve a “public offering,” as defined in Section 4(a)(2) of the Securities Act, due to the insubstantial number of persons involved in the transaction, size of the offering, and manner of the offering and number of securities offered. The Company did not undertake an offering in which it sold a high number of securities to a high number of investors. In addition, the Investor had the necessary investment intent as required by Section 4(a)(2) of the Securities Act since they agreed to, and received, the securities bearing a legend stating that such securities are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these securities would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, the Company has met the requirements to qualify for exemption under Section 4(a)(2) of the Securities Act.
EXHIBITS
The following exhibits are included herewith:
Exhibit No. | | Description |
| | Opinion of Lucosky Brookman LLP |
| | Consent of BF Borgers CPA PC |
23.2 | | Opinion of Lucosky Brookman LLP (see Exhibit 5.1 herein) |
Following are a list of exhibits which we previously filed in other reports which we filed with the SEC, including the Exhibit No., description of the exhibit and the identity of the filing where the exhibit was filed.
No. | | Description | | Filed With | | Date |
| | Articles of Incorporation | | Form SB-2 Registration Statement | | October 19, 2007 |
| | Bylaws | | Form SB-2 Registration Statement | | October 19, 2007 |
| | Articles of Amendment (Name Change) | | Form 8-K Dated November 2, 2009 | | November 6, 2009 |
| | Statement of Share and Equity Capital Exchange | | Form 10-Q For Quarter Ended June 30, 2010 | | August 4, 2010 |
| | Articles of Amendment (Add Preferred and Series A Preferred to Authorized) | | Form 10-Q For Quarter Ended June 30, 2010 | | August 4, 2010 |
4.1 | | Promissory Note | | Form 8-K dated September 14, 2018 | | September 14, 2018 |
| | Share Exchange Agreement with Sunshine Biopharma, Inc. | | Form 8-K dated October 15, 2009 | | October 20, 2009 |
| | License Agreement with Advanomics, Inc. | | Form 8-K/A dated October 15, 2009 | | January 19, 2010 |
| | Amendment No. 1 to License Agreement with Advanomics, Inc. | | Form 8-K/A dated October 15, 2009 | | January 19, 2010 |
| | Research Agreement with The Research Foundation of the State University of New York | | Form 8-K dated January 17, 2011 | | January 19, 2011 |
| | Research Agreement with Jewish General Hospital | | Form 8-K dated June 14, 2011 | | June 17, 2011 |
| | Amendment No. 2 to License Agreement with Advanomics | | Form 8-K dated December 21, 2011 | | December 27, 2011 |
| | Investment Agreement with Dutchess Investment Group II | | Form 8-K dated April 28, 2014 | | April 28, 2014 |
| | Registration Rights Agreement with Dutchess Investment Group II | | Form 8-K dated April 28, 2014 | | April 28, 2014 |
| | Patent Purchase Agreement with Advanomics Corporation | | Form 8-K dated October 8, 2016 | | October 9, 2016 |
| | Second Patent Purchase Agreement with Advanomics Corporation | | Form 8-K dated December 28, 2015 | | December 28, 2015 |
| | Amendment No. 1 to Patent Purchase Agreement with Advanomics Corporation dated October 8, 2016, including Secured Convertible Promissory Note. | | Form 8-K dated March 14, 2016 | | March 14, 2016 |
| | Amendment No. 1 to Patent Purchase Agreement with Advanomics Corporation dated December 28, 2016, including Secured Convertible Promissory Note | | Form 8-K dated March 14, 2016 | | March 14, 2016 |
10.13 | | Share Purchase Agreement with Mohamed Belhaj and Atlas Pharma, Inc. | | Form 8-K dated January 4, 2018 | | January 4, 2018 |
10.14 | | Equity Financing Agreement | | Form 8-K dated September 14, 2018 | | September 14, 2018 |
10.15 | | Registration Rights Agreement | | Form 8-K dated September 14, 2018 | | September 14, 2018 |
21.1 | | List of Subsidiaries | | Form 10-K dated April 2, 2018 | | April 2, 2018 |
*Filed herewith
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes
1.
To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
i.
To include any Prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii.
To reflect in the Prospectus any facts or events arising after the effective date of the Registration Statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the Registration Statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of Prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement.
iii.
To include any material information with respect to the plan of distribution not previously disclosed in the Registration Statement or any material change to such information in the Registration Statement;
2.
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3.
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
4.
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
i.
Any Preliminary Prospectus or Prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
ii.
Any free writing Prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
iii.
The portion of any other free writing Prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
iv.
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
5.
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: Each Prospectus filed pursuant to Rule 424(b) as part of a Registration Statement relating to an offering, other than Registration Statements relying on Rule 430B or other than Prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the Registration Statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a Registration Statement or Prospectus that is part of the Registration Statement or made in a document incorporated or deemed incorporated by reference into the Registration Statement or Prospectus that is part of the Registration Statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the Registration Statement or Prospectus that was part of the Registration Statement or made in any such document immediately prior to such date of first use.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers andor persons controlling persons,us pursuant to the foregoing provisions, we have been advisedinformed that, in the opinion of the Securities and Exchange Commission,SEC, such indemnification is against public policy as expressed in the Securities Act of 1933 and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by usthe registrant of expenses incurred or paid by a director, officer or controlling person of the corporationregistrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by a controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by us is against public policy as expressed hereby in the Securities Act of 1933, as amended, and we will be governed by the final adjudication of such case.issue.
Item 15. Recent Sales of Unregistered Securities.
On February 12, 2021, we issued a note in the principal amount of $700,000, convertible after 180 days from issuance into common stock at a price equal to $0.60 per share. This note was converted to common stock on December 20, 2021.
On April 5, 2021, we issued a note in the principal amount of $330,000. The note was convertible after 180 days from issuance into common stock at a price equal to the lower of $60.00 or 35% below market. On October 13, 2021, the noteholder converted $330,000 in principal and $16,500 in accrued interest into 26,250 shares of common stock leaving a principal balance of $0.
On April 20, 2021, we issued a note in the principal amount of $500,000. The note is convertible after 180 days from issuance into common stock at a price equal to $0.30 per share.
On April 22, 2021, a note holder converted a total of $11,028 in principal and $4,472 in accrued interest into 77,500 shares of common stock.
On July 6, 2021, the Company issued a note in the principal amount of $900,000, convertible after 180 days from issuance into common stock at a price of $0.30 per share. In connection with this debt financing, the Company agreed to allow the lender, who is also the holder of a note dated November 25, 2020 (the “November Note”), to convert a total of $240,000 in principal amount of the November Note into 120,000 shares of common stock leaving a principal balance of $10,000 and accrued interest of $7,750.
On August 18, 2021, the Company issued a issued a note in the principal amount of $500,000, convertible after 180 days from issuance into common stock at a price of $0.30 per share.
During the nine months ended September 30, 2021, the Company issued a total of 518,370 shares of common stock valued at $11,981,072 for the conversion of outstanding notes, reducing the debt by $1,233,028 and interest payable by $38,201 and generating a loss on conversion of $10,709,843.
During the nine months ended September 30, 2021, the Company issued 300,000 shares of common stock to its officers and directors as compensation for their services to the Company.
On December 20, 2021, the Company issued 14,524 shares of common stock upon conversion of $1,361,000 in convertible debt.
On March 10, 2022, the Company entered into a securities purchase agreement with certain accredited investors for a private placement for the Company’s common stock or pre-funded warrants and warrants exercisable for common stock. Pursuant to the purchase agreement, the Company sold (i) 2,301,353 shares of its common stock together with warrants to purchase up to 2,301,353 shares of common stock, and (ii) 1,302,251 pre-funded warrants with each pre-funded warrant exercisable for one share of common stock, together with warrants to purchase up to 1,302,251 shares of common stock. Each share of common stock and accompanying warrant were sold together at a combined offering price of $2.22, and each pre-funded warrant and accompanying warrant were sold together at a combined offering price of $2.219. The pre-funded warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrants had an initial exercise price of $2.22 per share, were exercisable upon issuance and will expire five years from the date of issuance. Aegis Capital Corp. acted as the placement agent in connection with the private placement. Aegis was paid a commission equal to 10% of the gross proceeds received by the Company in the private placement and 2% of the gross proceeds as a non-accountable expense allowance. The Company paid Aegis $100,000 for fees and expenses including attorney fees.
On April 25, 2022, the Company entered into a securities purchase agreement with certain accredited investors for a private placement for the Company’s common stock or pre-funded warrants, and warrants exercisable for common stock. Pursuant to the purchase agreement, the Company sold (i) 2,472,820 shares of its common stock, (ii) warrants to purchase up to 9,725,690 shares of common stock, and (iii) 2,390,025 pre-Funded warrants with each pre-funded warrant exercisable for one share of common stock. Each share of common stock and accompanying warrants were sold together at a combined offering price of $4.01, and each pre-funded warrant and accompanying two warrants were sold together at a combined offering price of $4.009. The pre-funded warrants were immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrants had an initial exercise price of $3.76 per share (subject to adjustment as set forth therein), were exercisable upon issuance and will expire five years from the date of issuance.
Aegis acted as the placement agent in connection with the private placement and was paid a commission equal to 10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 5% of the proceeds from any exercise of warrants, payable on exercise.
During the three months ended June 30, 2022, the Company issued 2,802,703 shares of common stock upon exercise of warrants with an exercise price of $2.22, and 3,692,276 shares of common stock upon exercise of pre-funded warrants with an exercise price of $0.001.
Effective October 20, 2022, the Company entered into a share purchase agreement with Malek Chamoun and Nora Pharma, wherein the Company acquired all of the issued and outstanding shares of Nora Pharma in consideration of a purchase price of $30,000,000 CAD (approximately $21,900,000 USD), which was paid as follows: (a) $5,000,000 CAD (approximately $3,650,000 USD) by issuance of 3,700,000 shares of the Company’s common stock; (b) $20,000,000 CAD (approximately $14,600,000 USD) in cash, which was subject to customary adjustments in accordance with the Purchase Agreement; and (c) 5,000,000 CAD (approximately $3,650,000 USD) in the form of earn-out payable to Mr. Chamoun once earned in a maximum of twenty (20) payments of $250,000 CAD for every $1,000,000 CAD increase in gross sales (as defined in the Purchase Agreement) above Nora Pharma’s June 30, 2022 gross sales, provided that his employment with the Company is not terminated pursuant to the Company’s employment agreement with him.
On May 12, 2023, the Company entered into a securities purchase agreement with a certain accredited investor for a private placement for the Company’s common stock, pre-funded warrants, each exercisable to purchase one share of common stock, and warrants, each exercisable to purchase one share of common stock. Pursuant to the purchase agreement, the Company sold (i) 2,450,000 shares of its common stock, (ii) warrants to purchase up to 11,904,762 shares of common stock, and (iii) 3,502,381 pre-funded warrants. Each share of common stock and accompanying two warrants were sold together at a combined offering price of $0.84, and each pre-funded warrant and accompanying two warrants were sold together at a combined offering price of $0.839. The pre-funded warrants are immediately exercisable, at a nominal exercise price of $0.001, and may be exercised at any time until all of the pre-funded warrants are exercised in full. The warrants have an initial exercise price of $0.59 per share (subject to adjustment as set forth therein), are exercisable upon issuance and will expire five and a half years from the date of issuance.
Aegis acted as the placement agent in connection with the private placement and was paid a commission equal to 10% of the gross proceeds received by the Company, and a non-accountable expense allowance equal to 2% of the gross proceeds, and will receive 10% of the proceeds from any exercise of warrants, payable on exercise.
On February 8, 2024, the Company issued and sold 20,000 shares of Series B Preferred Stock to Dr. Steve Slilaty for a purchase price equal to the stated value of $0.10 per share.
In connection with the foregoing, we relied upon the exemption from registration provided by Section 4(a)(2) under the Securities Act of 1933, as amended, for transactions not involving a public offering.
Item 16. Exhibits and Financial Statement Schedules.
| 1.1 | | Form of Underwriting Agreement (filed herewith) |
| 3.1 | | Articles of Incorporation(2) |
| 3.2 | | Certificate of Amendment to Articles of Incorporation filed November 2, 2009(3) |
| 3.3 | | Statement of Share and Equity Capital Exchange(4) |
| 3.4 | | Articles of Amendment to Articles of Incorporation filed July 13, 2010(4) |
| 3.5 | | Articles of Amendment to Articles of Incorporation filed May 27, 2015(5) |
| 3.6 | | Articles of Amendment to Articles of Incorporation(6) |
| 3.7 | | Articles of Amendment to Articles of Incorporation(7) |
| 3.8 | | Bylaws(14) |
| 4.1 | | Description of Registrant’s Securities (16) |
| 5.1 | | Opinion of Andrew I. Telsey, P.C. (filed herewith) |
| 5.2 | | Opinion of Sichenzia Ross Ference Carmel LLP (filed herewith) |
| 10.1 | | Patent Purchase Agreement with Advanomics Corporation(8) |
| 10.2 | | Second Patent Purchase Agreement with Advanomics Corporation(9) |
| 10.3 | | Amendment No. 1 to Patent Purchase Agreement with Advanomics Corporation dated October 8, 2016, including Secured Convertible Promissory Note(10) |
| 10.4 | | Amendment No. 1 to Patent Purchase Agreement with Advanomics Corporation dated December 28, 2016, including Secured Convertible Promissory Note(10) |
| 10.5 | | Form of Warrant, dated February 17, 2024(1) |
| 10.6 | | Warrant Agent Agreement between the Company and Equiniti , dated February 17, 2022(1) |
| 10.7 | | Sponsored Research Agreement, dated October 6, 2020, between the Company and the University of Georgia Research Foundation, Inc.(11) * |
| 10.8 | | Research Agreement between the Company and Arizona Board of Regents on behalf of the University of Arizona(12) |
| 10.9 | | Form of Warrant, dated March 14, 2022 (15) |
| 10.10 | | Form of Amendment to Warrant, dated March 24, 2022 (17) |
| 10.11 | | Employment Agreement between Sunshine Biopharma, Inc. and Dr. Steve Slilaty (18) |
| 10.12 | | Form of Warrant, dated April 28, 2022 (19) |
| 10.13 | | Share Purchase Agreement between Sunshine Biopharma, Inc., Malek Chamoun and Nora Pharma Inc. (20) |
| 10.14 | | Employment Agreement between Sunshine Biopharma, Inc., Nora Pharma Inc. and Malek Chamoun (20) |
| 10.15 | | License Agreement between the Company and the University of Arizona (22) ** |
| 10.16 | | Form of Warrant, dated May 16, 2023 (22) |
| 10.17 | | Amendment No. 1 to Warrant Agent Agreement, dated October 18, 2023 (23) |
_______________________
| * | Portions of the exhibit have been omitted. |
| (1) | Incorporated by reference to 8-K filed with the SEC on February 17, 2022 |
| (2) | Incorporated by reference to SB-2 filed with the SEC on October 19, 2007. |
| (3) | Incorporated by reference to 8-K filed with the SEC on November 6, 2009. |
| (4) | Incorporated by reference to 10-Q filed with the SEC on August 4, 2010. |
| (5) | Incorporated by reference to 8-K filed with the SEC on June 1, 2015. |
| (6) | Incorporated by reference to 8-K filed with the SEC on June 24, 2020. |
| (7) | Incorporated by reference to 8-K filed February 9, 2022. |
| (8) | Incorporated by reference to 8-K filed with the SEC on October 9, 2015. |
| (9) | Incorporated by reference to 8-K filed with the SEC on December 28, 2015. |
| (10) | Incorporated by reference to 8-K filed with the SEC on March 14, 2016. |
| (11) | Incorporated by reference to S-1/A filed with the SEC on January 24, 2022. |
| (12) | Incorporated by reference to 8-K filed with the SEC on February 25, 2022. |
| (13) | Incorporated by reference to 10-K filed with the SEC on May 1, 2020. |
| (14) | Incorporated by reference to 8-K filed with the SEC on April 19, 2023. |
| (15) | Incorporated by reference to 8-K filed with the SEC on March 15, 2022. |
| (16) | Incorporated by reference to 10-K filed with the SEC on March 21, 2022. |
| (17) | Incorporated by reference to 8-K filed with the SEC on March 24, 2022. |
| (18) | Incorporated by reference to 8-K filed with the SEC on April 8, 2022. |
| (19) | Incorporated by reference to 8-K filed with the SEC on April 28, 2022. |
| (20) | Incorporated by reference to 8-K filed with the SEC on October 20, 2022. |
| (21) | Incorporated by reference to 8-K filed with the SEC on February 28, 2023. |
| (22) | Incorporated by reference to 8-K filed with the SEC on May 16, 2023. |
| (23) | Incorporated by reference to 8-K filed with the SEC on October 20, 2023. |
| (24) | Incorporated by reference to S-8 filed with the SEC on January 8, 2024. |
| (25) | Incorporated by reference to 10-K filed with the SEC on April 4, 2023. |
(b) Financial statement schedule.
None.
Item 17. Undertakings.
| (a) | The undersigned registrant hereby undertakes: |
| |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement: |
| |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: |
| |
| (i) | If the registrant is relying on Rule 430B (§230.430B of this chapter): |
| |
| (A) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) (§230.424(b)(3) of this chapter) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| |
| (B) | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) (§230.424(b)(2), (b)(5), or (b)(7) of this chapter) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) (§230.415(a)(1)(i), (vii), or (x) of this chapter) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or |
| (ii) | If the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
| |
| The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter); |
| |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue |
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statementregistration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of New York, State of New York, on October 8, 2018.
February 9, 2024.DATE | | SIGNATURE | | TITLESUNSHINE BIOPHARMA, INC. |
| | | | | |
October 8, 2018 | | /s/Dr. Steve N. Slilaty | | President, Chief Executive Officer |
| | Dr. Steve N. Slilaty | | and Chairman |
| | | |
| | (PrincipalBy: | /s/ Dr. Steve N. Slilaty |
| | Dr. Steve N. Slilaty |
| | Chief Executive Officer)Officer |
In accordance withPursuant to the requirements of the Securities Act of 1933, this Registration Statement wasregistration statement has been signed by the following persons in the capacities and on the dates stated:
indicated.DATESignature | | SIGNATURETitle | | TITLEDate |
| | | | | |
October 8, 2018 | | /s/Dr. Steve N. Slilaty | | President, Chief Executive Officer and Chairman |
| Director | | February 9, 2024 |
| Dr. Steve N. Slilaty | | Officer (Principal(Principal Executive Officer) | | |
| | | | | |
October 8, 2018 | | /s/ Camille Sebaaly * | | Chief Financial Officer , Secretary | | February 9, 2024 |
| Camille Sebaaly | | (Principal Financial and DirectorAccounting Officer) |
| | | Camille Sebaaly | | (Principal Financial Officer) (Principal Accounting Officer) |
| | | | | |
October 8, 2018 | | /s/ Dr. Abderrazzak Merzouki* | | Chief Operating Officer and Director | | February 9, 2024 |
| Dr. Abderrazzak Merzouki | | | | |
| | | | | |
| /s/ David Natan * | | Director | | February 9, 2024 |
| David Natan | | | | |
| | | | |
/s/ Dr. Abderrazzak MerzoukiAndrew Keller * | | Director | | February 9, 2024 |
| Dr. Andrew Keller | | | | |
| | | | |
| /s/ Dr. Rabi Kiderchah * | | Director | | February 9, 2024 |
| Dr. Rabi Kiderchah | | | | |
* By /s/ Dr. Steve N. Slilaty
Attorney-in-fact