
UNITED STATES
Amendment No. 45
Adeza Biomedical Corporation
| Delaware | 2835 | 77-0054952 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
1240 Elko Drive
Emory V. Anderson
Copies to:
| Sarah A. O’Dowd Heller Ehrman White & McAuliffe LLP 275 Middlefield Road Menlo Park, California 94025 (650) 324-7000 | Frederick W. Kanner Dewey Ballantine LLP 1301 Avenue of the Americas New York, NY 10019 (212) 259-8000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, check the following box. o
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to Section 8(a), may determine.
3,750,000 Shares

This is the initial public offering of our common stock. No public market currently exists for our common stock. We are offering all of the shares of common stock offered by this prospectus. We expect the public offering price to be between $14.00 and $16.00 per share.
Our common stock has been approved for quotation on The Nasdaq National Market under the symbol “ADZA.”
Investing in our common stock involves a high degree of risk. Before buying any shares, you should carefully read the discussion of material risks of investing in our common stock in “Risk factors” beginning on page 7 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters may also purchase up to an additional 562,500 shares of our common stock at the public offering price, less the underwriting discounts and commissions payable by us, to cover over-allotments, if any, within 30 days from the date of this prospectus. If the underwriters exercise this option in full, the total underwriting discounts and commissions will be $ and our total proceeds, before expenses, will be $ .
The underwriters are offering the common stock as set forth under “Underwriting.” Delivery of the shares will be made on or about , 2004.
UBS Investment Bank
SG Cowen & Co.
Through and including , 2004 (the 25th day after the date of this prospectus), federal securities laws may require all dealers that effect transactions in our common stock, whether or not participating in this offering, to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
TABLE OF CONTENTS
Adeza Biomedical Corporation Trademarks and Registered Trademarks are trademarks of Adeza. Our trademarks and trade names include the stylized A, Adeza®, E-tegrity® Test, Full Term and TLiIQ® System. Other service marks, trademarks and trade names referred to in this prospectus are the property of their respective owners.
Prospectus summary
This summary highlights selected information appearing elsewhere in this prospectus. While this summary highlights what we consider to be the most important information about us, you should carefully read this prospectus and the registration statement of which this prospectus is a part in their entirety before investing in our common stock, especially the risks of investing in our common stock, which we discuss later in “Risk factors,” and our financial statements and related notes beginning on page F-1. Unless the context requires otherwise, the words “Adeza,” “we,” “Company,” “us” and “our” refer to Adeza Biomedical Corporation.
OUR BUSINESS
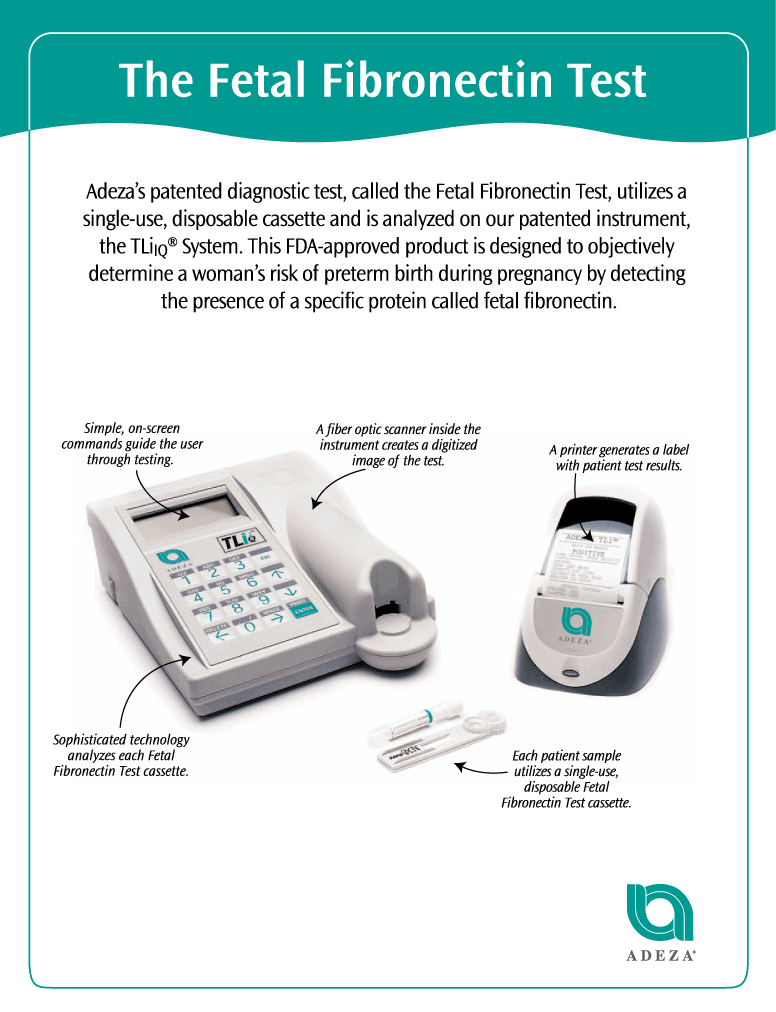
We design, develop, manufacture and market innovative products for women’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the Fetal Fibronectin Test, that utilizes a single-use, disposable cassette and is analyzed on our patented instrument, the TLiIQ System. This product is approved by the Food and Drug Administration, or FDA, for broad use in assessing the risk of preterm birth.
Our Fetal Fibronectin Test is designed to objectively determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. Testing for fetal fibronectin during pregnancy provides a more accurate assessment of the likelihood of a preterm birth than traditional methods. According to the New England Journal of Medicine, preterm births have historically accounted for up to 85% of all pregnancy-related complications and deaths in the United States. The March of Dimes estimated that over $13 billion in costs were associated with the care of preterm or low birth weight infants in 2001. By correctly identifying women at risk for preterm birth, we believe our Fetal Fibronectin Test leads to improved patient care and significant cost savings and has the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women.
The patient population for which our Fetal Fibronectin Test is approved can be divided into three patient categories. The first category consists of women who present with signs and symptoms of preterm labor and are typically directed to the hospital. The second and third categories include women designated as either “high-risk” or “low-risk” for preterm birth by their healthcare providers, and who currently exhibit no signs and symptoms of preterm labor. We believe that by using the Fetal Fibronectin Test periodically during a pregnancy, healthcare providers can more accurately assess the likelihood that women in all three categories will not deliver preterm.
As of September 30, 2004, we have shipped more than 1,270 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes for use in hospital and clinical laboratories. As of September 30, 2004, our direct sales force consisted of 67 representatives who sell to hospital and clinical laboratories, health plans and healthcare providers. Our Fetal Fibronectin Test has been assigned a reimbursement code used for insurance processing of claims for the Fetal Fibronectin Test, and we believe that reimbursement for our Fetal Fibronectin Test has been regularly available through health plan organizations and most state Medicaid programs.
We also market and sell our E-tegrity Test, an infertility-related test based on a proprietary analyte specific reagent, to assess receptivity of the uterus to embryo implantation in women with unexplained infertility. The E-tegrity Test can be particularly useful for women who are considering assisted reproductive technologies, including in vitro fertilization, or IVF. We are also seeking to expand the indications for use of our Fetal Fibronectin Test.
We were profitable for both the year ended December 31, 2003 and for the nine months ended September 30, 2004. For the year ended December 31, 2001, we had product sales of approximately $6.7 million and a net loss of approximately $5.7 million. For the year ended December 31, 2002, we had product sales of approximately $14.3 million and a net loss of approximately $0.3 million. For the year ended December 31, 2003, we had product sales of approximately $26.5 million and net income of $3.2 million. For the nine months ended September 30, 2004, we had product sales of approximately $24.4 million and net income of approximately $7.3 million, including a one-time reduction in cost of product sales for accrued royalties of $2.7 million. Our accumulated deficit incurred from inception through September 30, 2004 is approximately $46.4 million.
OUR PRIMARY MARKET
Preterm birth
There are approximately four million births in the United States annually. Births occurring before 37 weeks of pregnancy are defined as preterm, and in recent years, according to a 2003 publication by the Centers for Disease Control and Prevention, preterm births represent approximately 12% of all births. On average, this equates to over 1,300 preterm births per day, or 480,000 preterm births per year. According to the Centers for Disease Control and Prevention, the percentage of preterm births in the United States grew to 12% of all births in 2002, an increase of 29% since 1981. This increase in the preterm birth rate is a growing public health concern. In January 2003, the March of Dimes launched a five-year, $75 million campaign to reduce the number of preterm births.
Historically, healthcare providers have had difficulty accurately predicting the likelihood of preterm birth, which has contributed to increased costs and complications. According to a 1994 publication from the National Conference of State Legislators, the costs of newborn intensive care in the United States ranged between $20,000 and $400,000 per infant. According to a 2001 study from the March of Dimes, the average hospital charge for preterm/low birth weight infants was $75,000, compared to $1,300 for an uncomplicated newborn stay.
OUR SOLUTION
We believe that our proprietary, FDA-approved diagnostic test and instrument, the single-use, disposable Fetal Fibronectin Test and the TLiIQ System, have the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women and to become a standard of care for use in pregnancy. The clinical efficacy of our Fetal Fibronectin Test for preterm birth has been demonstrated in numerous peer-reviewed clinical publications, including two multi-center clinical trial publications.
The Fetal Fibronectin Test and the TLiIQ System have the following key characteristics:
We believe women who present with signs and symptoms of preterm labor are candidates for one Fetal Fibronectin Test per episode, and women designated as “high-risk” may be tested multiple times
OUR STRATEGY
Our goal is to be a leader in the development and commercialization of proprietary diagnostic products for reproductive healthcare. We believe that our products can assist healthcare providers in making more timely and accurate diagnoses, leading to improved quality of care and significant cost savings for the healthcare system. In order to effectively execute this strategy, we plan to:
RISKS ASSOCIATED WITH OUR BUSINESS
Our business is subject to numerous risks and uncertainties, including those that we highlight under “Risk factors” beginning on page 7. For example, substantially all of our revenue has resulted from sales of our principal product line, consisting of our Fetal Fibronectin Test, the TLiIQ System and related consumables. Our commercial success depends on our ability to expand sales of our principal product line in hospitals and clinical laboratories. Our accumulated deficit incurred from inception through September 30, 2004 is approximately $46.4 million. Our business strategy includes expanding international sales, which will require that we obtain and maintain foreign regulatory approval to market our products and rely on international distributors to sell our products in those markets. We also intend to develop and market our Fetal Fibronectin Test in new pregnancy-related applications and for applications in oncology, which will require that we expand our research and development efforts and seek regulatory approvals in domestic and international markets.
OUR CORPORATE INFORMATION
Our business was incorporated in California in 1985 as Aspen Diagnostics Corporation. We changed our name to Adeza Biomedical Corporation in 1989, and in 1996 we re-incorporated in the State of Delaware. Our principal executive offices are located at 1240 Elko Drive, Sunnyvale, CA 94089, and our telephone number is (408) 745-0975. Our websites are located athttp://www.adeza.comandhttp://www.ffntest.com. We do not intend for the information contained on our websites to be incorporated by reference into, or to form any part of, this prospectus.
The offering
The number of shares of our common stock to be outstanding immediately after this offering is based on 12,139,841 shares of common stock outstanding as of September 30, 2004 after giving effect to the conversion of all 15,409,062 shares of preferred stock outstanding as of September 30, 2004 into 11,957,322 shares of common stock, which will become effective at the closing of this offering.
The number of shares of our common stock outstanding immediately after this offering excludes:
Unless otherwise indicated, all information in this prospectus:
Summary financial data
The following summary financial data for the years ended December 31, 2001, 2002 and 2003 have been derived from our financial statements audited by Ernst & Young LLP, which appear elsewhere in this prospectus. The summary financial data for the nine months ended September 30, 2003 and 2004 have been derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited summary financial data include, in the opinion of management, all adjustments, consisting of only normal recurring adjustments, that are necessary for a fair presentation of the financial position and results for the interim unaudited periods. Operating results for the nine months ended September 30, 2004 are not necessarily indicative of the results that may be expected for the year ending December 31, 2004. The summary financial data set forth below should be read together with the financial statements and the related notes to those statements, as well as “Management’s discussion and analysis of financial condition and results of operations,” appearing elsewhere in this prospectus.
| Nine months ended | |||||||||||||||||||||
| Years ended December 31, | September 30, | ||||||||||||||||||||
| Statement of | |||||||||||||||||||||
| operations data: | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||||||||
| Product sales | $6,742 | $14,277 | $26,499 | $19,185 | $24,405 | ||||||||||||||||
| Cost of product sales | 2,521 | 3,715 | 6,087 | 4,621 | 895 | (1) | |||||||||||||||
| Gross profit | 4,221 | 10,562 | 20,412 | 14,564 | 23,510 | ||||||||||||||||
| Contract revenues | 811 | 1,059 | — | — | — | ||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||||||
| Selling and marketing | 6,437 | 7,819 | 12,259 | 9,023 | 11,638 | ||||||||||||||||
| General and administrative | 2,033 | 2,069 | 2,730 | 1,791 | 2,631 | ||||||||||||||||
| Research and development | 2,145 | 2,047 | 2,001 | 1,519 | 1,755 | ||||||||||||||||
| Total operating costs and expenses | 10,615 | 11,935 | 16,990 | 12,333 | 16,024 | ||||||||||||||||
| Income (loss) from operations | (5,583 | ) | (314 | ) | 3,422 | 2,231 | 7,486 | ||||||||||||||
| Other expenses, net | (21 | ) | (8 | ) | (33 | ) | (10 | ) | — | ||||||||||||
| Interest income (expense), net | (85 | ) | (7 | ) | (19 | ) | (23 | ) | 104 | ||||||||||||
| Income (loss) before income taxes | (5,689 | ) | (329 | ) | 3,370 | 2,198 | 7,590 | ||||||||||||||
| Provision for income taxes | — | — | 135 | 113 | 307 | ||||||||||||||||
| Net income (loss) | $(5,689 | ) | $(329 | ) | $3,235 | $2,085 | $7,283 | ||||||||||||||
| Net income (loss) per share: | |||||||||||||||||||||
| Basic | $(36.27 | ) | $(1.82 | ) | $17.78 | $11.46 | $39.97 | ||||||||||||||
| Diluted | $(36.27 | ) | $(1.82 | ) | $0.26 | $0.17 | $0.55 | ||||||||||||||
| Pro forma basic (unaudited) | $0.27 | $0.60 | |||||||||||||||||||
| Pro forma diluted (unaudited) | $0.26 | $0.55 | |||||||||||||||||||
| Shares used in computing net income (loss) per share: | |||||||||||||||||||||
| Basic | 156,857 | 181,188 | 181,965 | 181,900 | 182,223 | ||||||||||||||||
| Diluted | 156,857 | 181,188 | 12,515,063 | 12,429,763 | 13,364,494 | ||||||||||||||||
| Pro forma basic (unaudited) | 11,986,026 | 12,139,545 | |||||||||||||||||||
| Pro forma diluted (unaudited) | 12,515,063 | 13,364,494 | |||||||||||||||||||
The following table contains a summary of our balance sheet as of September 30, 2004:
| As of September 30, 2004 | ||||||||||||
| Pro forma | ||||||||||||
| Balance sheet data: | Actual | Pro forma | as adjusted | |||||||||
| (unaudited, in thousands) | ||||||||||||
| Cash and cash equivalents | $14,638 | $14,638 | $65,151 | |||||||||
| Working capital | 17,454 | 17,454 | 67,967 | |||||||||
| Total assets | 23,813 | 23,813 | 74,326 | |||||||||
| Convertible preferred stock | 61,484 | — | — | |||||||||
| Accumulated deficit | (46,354 | ) | (46,354 | ) | (46,354 | ) | ||||||
| Total stockholders’ equity (deficit) | (43,541 | ) | 17,943 | 68,456 | ||||||||
Risk factors
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below with all of the other information included in this prospectus before deciding to invest in our common stock. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In this event, the market price of our common stock could decline and you could lose part or all of your investment.
RISKS RELATING TO OUR BUSINESS
Because our revenues and financial results depend significantly on a limited product line, if we are unable to manufacture or sell our products in sufficient quantities and in a timely manner, our business will suffer.
To date, substantially all of our revenue has resulted from sales of our principal product line, our Fetal Fibronectin Test, the TLiIQ System (and its predecessor, the TLi System) and related consumables. Although we have introduced new products such as the E-tegrity Test, and intend to introduce additional products, we expect sales of the Fetal Fibronectin Test to account for substantially all of our near-term revenue. Because our business is highly dependent on our Fetal Fibronectin Tests, the TLiIQ System and the related consumables, factors adversely affecting the pricing of or demand for these products could have a material and adverse effect on our business and cause the value of our securities to decline substantially. We will lose revenue if alternative diagnostic products or technologies gain commercial acceptance or if reimbursement is limited. We cannot assure you that we will be able to continue to manufacture these products in commercial quantities at acceptable costs. Our inability to do so would adversely affect our operating results and cause our business to suffer.
If our products do not achieve and sustain market acceptance, we may fail to generate sufficient revenue to maintain our business.
Our commercial success depends in large part on our ability to achieve and sustain market acceptance of our principal product line, the Fetal Fibronectin Test and the TLiIQ System. A key element of our business plan calls for us to expand sales of our TLiIQ System in hospitals and clinical laboratories and increase the related sales of the Fetal Fibronectin Test and other consumables used in conjunction with the TLiIQ System. To accomplish this, we will need to convince healthcare providers of the benefits of our products through various means, including through published papers, presentations at scientific conferences and additional clinical trials. If existing users of our products determine that these products do not satisfy their requirements, or if our competitors develop a product perceived to better satisfy their requirements, our sales of Fetal Fibronectin Tests and other consumables may decline, and our revenues may correspondingly decline.
In addition, our commercial success may depend on our ability to gain market acceptance for our other products and product candidates. Market acceptance of our product portfolio will depend on our ability to develop additional applications of our existing products and to introduce new products to additional markets, including the oncology diagnostic market, the reproductive endocrinology and infertility markets and other women’s health markets.
Other factors that might influence market acceptance of our products include the following:
In addition, our marketing and development efforts could require us to expend significant time and resources, and we cannot assure you that we will succeed in these efforts. If our products are unable to achieve or maintain broad market acceptance, our revenues and operating results may be negatively impacted and our business would suffer.
Our quarterly revenues and operating results are subject to significant fluctuations, and our stock price may decline if we do not meet the expectations of investors and analysts.
As of September 30, 2004, we had an accumulated deficit of $46.4 million. For the nine months ended September 30, 2004, we had net income of $7.3 million, including a one-time reduction in cost of product sales for accrued royalties of $2.7 million. However, we cannot assure you that we will sustain profitability or that losses will not occur in the future. Our quarterly revenues and operating results are difficult to predict and have in the past and may in the future fluctuate significantly from quarter to quarter due to a number of factors, many of which are outside our control. These factors include, but are not limited to:
These and other factors make it difficult for us to predict sales for subsequent periods and future performance. If our quarterly operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly. We believe quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
In addition, we expect to incur additional expenses to execute our business plan, and these expenses will increase as we expand our marketing efforts, research and development activities, clinical testing and manufacturing capacity. These expenses, among other things, may cause our net income and working capital to decrease. If sales do not continue to grow, we may not be able to maintain profitability. Our expansion efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues sufficiently to offset these higher expenses. If we fail to do so, the market price for our common stock will likely decline.
If third-party payors do not adequately reimburse our customers, market acceptance of our products may be impaired, which may adversely affect our revenues and our operating results.
Market acceptance of our products and the majority of our sales depend, in large part, on the availability of adequate reimbursement for the use of our products from government insurance plans, including Medicare and Medicaid, managed care organizations, private insurance plans and other third-party payors in the United States and abroad. Third-party payors are often reluctant to reimburse healthcare providers for the use of medical diagnostic products incorporating new technology.
Because each third-party payor individually approves reimbursement, obtaining these approvals can be a time-consuming and costly process that requires us to provide scientific and clinical support for the use of each of these products to each third-party payor separately with no assurance that approval will be obtained. For example, while the policies of some third-party payors limit reimbursement for the use of our Fetal Fibronectin Test to women with signs and symptoms of preterm labor, other third-party payors provide reimbursement for broader use of our Fetal Fibronectin Test. This individualized process can delay the market acceptance of new products and may have a negative effect on our revenues and operating results.
Market acceptance of our products internationally may depend in part upon the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country and include both government sponsored healthcare and private insurance. We may not obtain international reimbursement approvals in a timely manner, if at all. Our failure to receive international reimbursement approvals may negatively impact market acceptance of our products in the international markets in which those approvals are sought.
We believe third-party payors are increasingly limiting coverage for medical diagnostic products in the United States and internationally, and in many instances are exerting pressure on medical products suppliers to reduce their prices. Consequently, third-party reimbursement may not be consistently available or adequate to cover the cost of our products. Additionally, third-party payors who have previously approved a specific level of reimbursement may reduce that level. We cannot assure you that under prospective payment systems, in which healthcare providers may be reimbursed a set amount based on the type of diagnostic procedure performed, such as those utilized by Medicare and in many privately managed care systems, the cost of our products will be justified and reimbursed. This could limit our ability to commercialize and sell new products and continue to sell our existing products, or may cause the prices of our existing products to be reduced, which may adversely affect our revenues and operating results.
If we fail to properly manage our anticipated growth in the United States or abroad, we may incur significant additional costs and expenses and our operating results may suffer.
Rapid growth of our business is likely to place a significant strain on our managerial, operational and financial resources and systems. In the United States, while we anticipate hiring additional personnel to assist in the planned expansion of sales efforts for our current products and the development of future products, we cannot assure you that we will be able to successfully increase sales of current products or introduce new products and meet our growth goals. To manage our anticipated growth, we must attract and retain qualified personnel and manage and train them effectively. We will depend on our personnel and third parties to effectively market our products to an increasing number of hospitals, physicians and other healthcare providers. We will also depend on our personnel to develop next generation technologies. Further, our anticipated growth will place additional strain on our suppliers and manufacturers, as well as our own internal manufacturing processes, resulting in an increased need for us to carefully monitor for quality assurance. In addition, we may choose or be required to relocate or expand our manufacturing facility to accommodate potential growth in our business. Any
Our plans to significantly expand our presence in international markets will cause us to incur various costs and expenses and may strain our operating and financial systems and resources in a manner that could materially and adversely affect our operating results. We will be subject to the regulatory oversight of additional authorities as we expand internationally. These authorities may impose regulations and restrictions on the sales and marketing of our products that are different and potentially more restrictive than those placed on us by regulators in the United States. We may be required to expend considerable resources to comply with these requirements. We cannot assure you that we will ultimately be able to comply with such regulations in a timely manner, if at all. If we are unable to satisfy these requirements on commercially reasonable terms, our ability to commercialize our products would be hampered and our revenues may be adversely affected.
We will need to devote considerable resources to comply with federal, state and foreign regulations and, if we are unable to fully comply, we could face substantial penalties.
We are directly or indirectly through our customers subject to extensive regulation by both the federal government and the states and foreign countries in which we conduct our business. Companies such as ours are required to expend considerable resources complying, in particular, with laws such as the following:
Companies such as ours are also required to comply with laws and regulations regarding the practice of medicine by non-physicians, consumer protection and Medicare and Medicaid payments. If our past or present operations are found to be in violation of any of the laws described above or the other governmental regulations to which we or our customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. If we are required to obtain permits or licenses under these laws that we do not already possess, we may become subject to substantial additional regulation or incur significant expense. Any penalties, damages, fines, curtailment or restructuring of our operations may adversely affect our ability to operate our business and our financial results. Because many of these laws have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations and additional legal or regulatory change, we may be at a heightened risk of being found to be in violation of these laws. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
If we are unable to maintain our existing regulatory approvals and clearances for our existing products, or obtain new regulatory approvals and clearances for our product candidates, our ability to commercially distribute our products and our business may be significantly harmed.
The US Food and Drug Administration, or FDA, and comparable agencies of other countries generally regulate our products as medical devices. In the United States, FDA regulations govern, among other things, the activities that we perform, including product development, product testing, product labeling, product storage, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution. Most of the new products that we plan to develop and commercialize in the United States will require either pre-market notification, also known as 510(k) clearance, or pre-market approval, from the FDA prior to marketing. The 510(k) clearance process requires us to notify the FDA of our intent to market a medical device. The overall 510(k) clearance process usually takes from three to twelve months from the time of submission to being able to sell a product in the market, but can take significantly longer. The pre-market approval process, often referred to as the PMA process, is much more costly, lengthy, uncertain and generally takes between one and three years from submission to PMA approval, but may take significantly longer and such clearance or approval may never be obtained.
All of the products that we have submitted and may submit in the future for FDA clearance or approval are or will be subject to substantial restrictions, including, among other things, restrictions on the indications for which we may market our products, which could result in reductions in or an inability to grow our revenues. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or certain requirements for costly post-marketing testing and surveillance to monitor the performance and clinical utility of the product. For example, any of our products that have received FDA approval, such as our Fetal Fibronectin Test or TLiIQ System, remain subject to ongoing post-marketing regulation and oversight by the FDA. The marketing claims that we are permitted to make in labeling our diagnostic products, if cleared or approved by the FDA, are limited to those specified in any clearance or approval. Our intention to expand the use of our products into new areas such as the prediction of successful induction of labor and oncology will require us to make new submissions to the FDA.
In addition, we are subject to review, periodic inspection and marketing surveillance by the FDA to determine our compliance with regulatory requirements for any product for which we obtain marketing approval. Following approval, our manufacturing processes, subsequent clinical data and promotional activities are subject to ongoing regulatory obligations. If the FDA finds that we have failed to comply with these requirements or later discovers previously unknown problems with our products, including unanticipated adverse events of unanticipated severity or frequency, manufacturer or manufacturing processes or failure to comply with regulatory requirements, it can institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions, including:
Any of these enforcement actions could affect our ability to commercially distribute our products in the United States and may also harm our ability to conduct the clinical trials necessary to support the marketing, clearance or approval of these products and could materially and adversely affect our business.
Our PMA supplement seeking approval for use of our Fetal Fibronectin Test in predicting successful induction of labor has been submitted to the FDA. The FDA has placed its review of the application on hold while a third party we have engaged conducts an audit of all of the clinical study sites because of the number of protocol deviations, in order to confirm the accuracy of the data. Upon completion of the third-party audit, we will need to submit new analyses of the data and a corrective action plan to the FDA before it will resume its review of the application. We cannot assure you that the new analyses of the data or the corrective action plan will be acceptable to us or to the FDA or that we will continue to pursue or obtain FDA approval for this application.
We rely on our CLIA-certified laboratory located at our facility in Sunnyvale, California to process E-tegrity Tests. The Centers for Medicare and Medicaid Services, or the CMS, requires that operators of CLIA-certified laboratories submit to surveillance and follow-up inspections. If we are unable to meet the CMS’s requirements for continued operation pursuant to CLIA, our laboratory may lose its CLIA certification, and we may be unable to continue to process E-tegrity Tests. As a result, our business may be harmed.
If we modify our marketed products, we may be required to obtain new 510(k) clearances or PMAs, or we may be required to cease marketing or recall the modified products until clearances are obtained.
Any modification to a 510(k)-cleared or pre-market approved diagnostic device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or PMA, such as the development of our Fetal Fibronectin Test as a diagnostic test for the induction of labor. The FDA requires every manufacturer to make the determination of whether new clearance or approval is required for 510(k)-cleared devices. The FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or PMA for any modification to a previously cleared or approved product, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Any recall or FDA requirement that we seek additional approvals or clearances could result in delays, fines, costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
If we or any of our third-party manufacturers do not operate in accordance with Quality System Regulations, we could be subject to FDA enforcement actions, including the seizure of our products and the halt of our production.
We and any third-party manufacturers that we currently rely on or will rely on in the future, including those we rely on to produce components of our products, must continuously adhere to the current good manufacturing practices, or cGMP, set forth in the FDA’s Quality System Regulations, or QSR, and enforced by the FDA through its facilities inspection program. In complying with QSR, we and our third-party manufacturers must expend significant time, money and effort in design and development, testing, production, record keeping and quality control to assure that our products meet applicable specifications and other regulatory requirements. The failure to comply with these specifications and other requirements could result in an FDA enforcement action, including the seizure of products and shutting down of production. We or any of these third-party manufacturers may also be subject to comparable or more stringent regulations of foreign regulatory authorities. In any of these circumstances, our ability to develop, produce and sell our products could be impaired.
We have received regulatory approvals for some of the operations located at our Sunnyvale, California headquarters, including our CLIA-certified laboratory. Should we choose to relocate, or if for some reason we are required to relocate some or all of our facilities from this location, we may be required to apply for regulatory approvals for the new location. It may be difficult or impossible for us to obtain the necessary approvals to continue our business in its present form at any such new location, and our business may be harmed as a result.
If we experience delays in the development of new products or delays in planned improvements to our products, our commercial opportunities will be reduced and our future competitive position may be adversely affected.
To improve our competitive position, we believe that we will need to develop new products as well as improve our existing instruments, reagents and ancillary products. Improvements in automation and the number of tests that can be performed in a specified period of time will be important to the competitive position of our products as we market to a broader, perhaps less technically proficient, group of customers. Our ability to develop new products and make improvements in our products may face difficult technological challenges leading to delays in development. If we are unable to successfully complete development of new products or if we are unable to successfully complete the planned enhancements to our products, in each case without significant delays, our future competitive position may be adversely affected.
If other companies develop and market technologies or diagnostic products faster than we do, or if those products are more cost effective or useful than our products, our commercial opportunities will be reduced or eliminated.
The extent to which any of our technologies and products achieve and sustain market acceptance will depend on numerous competitive factors, many of which are beyond our control. Competition in the medical devices and diagnostic products industries is intense and has been accentuated by the rapid pace of technological development. While no company directly competes with us in our core markets, there are other diagnostic techniques currently in use to diagnose the likelihood of preterm birth, such as ultrasound. In addition, other companies may develop new diagnostic products or technologies that could compete with or entirely displace our products and technologies. For example, other biomarkers, including cytokines and other proteins indicative of infection, and proteomics are the subject of research that may yield new products or technologies. The effectiveness of these alternative techniques may improve with time and additional research by clinicians or manufacturers. The medical devices and diagnostic products industries include large diagnostics and life sciences companies. Most of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing, sales and service resources than we do. Some of them also have more experience than we do in research and development, clinical trials, regulatory matters, manufacturing, marketing and sales. These organizations also compete with us to:
If we cannot successfully compete with new products or technologies, sales of our products and our competitive position will suffer, and our stock price might be adversely affected. Because of their greater experience with commercializing technologies and larger research and development capabilities, other companies might succeed in developing and commercializing technologies or products earlier and obtaining regulatory approvals and clearances from the FDA more rapidly than we do. Other companies also might develop more effective technologies or products that are more predictive, more
We rely on a limited number of suppliers, and if these suppliers fail or are unable to perform in a timely and satisfactory manner, we may be unable to manufacture our products or satisfy product demand in a timely manner, which could delay the production or sale of these products.
We rely on a limited number of suppliers for both raw materials and components necessary for the manufacture of our products, including our Fetal Fibronectin Test and TLiIQ System. We acquire all of these components, assemblies and raw materials on a purchase-order basis, which means that the supplier is not required to supply us with specified quantities over a certain period of time or to set aside part of its inventory for our forecasted requirements. If we need alternative sources for key components, assemblies or raw materials for any reason, such components, assemblies or raw materials may not be immediately available. If alternative suppliers are not immediately available, we will have to identify and qualify alternative suppliers, and delivery of such components, assemblies or raw materials may be delayed. Consequently, if we do not forecast properly, or if our suppliers are unable or unwilling to supply us in sufficient quantities or on commercially acceptable terms, we may not have access to sufficient quantities of these components, assemblies and raw materials on a timely basis and may not be able to satisfy product demand. We also rely upon a fulfillment provider to process orders for our products, coordinate invoicing and collections, as well as ship our products to customers in the United States. We may not be able to find an adequate alternative supplier or fulfillment provider in a reasonable time period, or on commercially acceptable terms, if at all. Our inability to obtain a supplier for the manufacture of our products may force us to curtail or cease operations, which would have a material adverse effect on our product sales and profitability.
In addition, if any of these components, assemblies or raw materials are no longer available in the marketplace, we will be forced to further develop our technologies to incorporate alternate components, assemblies and raw materials and to do so in compliance with QSR. If we incorporate new components, assemblies or raw materials into our products, we may need to seek and obtain additional approvals or clearances from the FDA or foreign regulatory agencies, which could delay the commercialization of these products.
We depend on distributors to market and sell our products in overseas markets, and if our foreign distributors fail in their efforts or are unwilling or unable to devote sufficient resources to market and sell our products, our ability to effectively market our products and our business will be harmed.
Our international sales currently depend upon the marketing efforts of and sales by certain distributors in Europe, Australia, the Pacific Rim region and South America. In most instances, our distribution arrangements are governed by short-term purchase orders. We also rely upon certain of these distributors to assist in obtaining product registration and reimbursement approvals in certain international markets, and we may not be able to engage qualified distributors in our targeted markets. The distributors that we are able to obtain may not perform their obligations. If a distributor fails to invest adequate resources and support in promoting our products and training physicians, hospitals and other healthcare providers in the proper techniques for using our products or in awareness of our products, or if a distributor ceases operations, we would likely be unable to achieve significant sales in the territory represented by the distributor. If we decide to market new products abroad, we will likely need to educate our existing or new distributors about these new products and convince them to distribute the new products. If these distributors are unwilling or unable to market and sell our products, we may experience delayed or reduced market acceptance and sales of our products outside the United States. Our failure to engage adequate distributors, or the failure of the distributors to perform their obligations as expected, may harm our ability to effectively market our products and our business.
The regulatory approval process outside the United States varies depending on foreign regulatory requirements and may limit our ability to develop, manufacture and sell our products internationally.
To market any of our products outside of the United States, we and our collaborative partners, including certain of our distributors, are subject to numerous and varying foreign regulatory requirements, implemented by foreign health authorities, governing the design and conduct of human clinical trials and marketing approval for diagnostic products. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval. The foreign regulatory approval process includes all of the risks associated with obtaining FDA approval set forth above, and approval by the FDA does not ensure approval by the health authorities of any other country, nor does the approval by foreign health authorities ensure approval by the FDA.
If our products do not perform as expected, we may experience reduced revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high quality medical diagnostic devices. Our customers are particularly sensitive to product defects and errors because of the use of our products in medical practice. Our reputation and the public image of our products may be impaired for any of the following reasons:
Even after any underlying problems are resolved, any manufacturing defects or performance errors in our products could result in lost revenue, delay in market acceptance, damage to our reputation, increased service and warranty costs and claims against us.
If product liability suits or other claims and product field actions are initiated against us, we may be required to engage in expensive and time-consuming litigation, pay substantial damages, face increased insurance rates and sustain damage to our reputation, which would significantly impair our financial condition.
Our business exposes us to potential product liability claims and field action risks that are inherent in the testing, manufacturing, marketing and sale of diagnostic products. We may be unable to avoid product liability claims or field actions, including those based on claims that the use or failure of our products resulted in a misdiagnosis or harm to a patient. Although we believe that our liability coverage is adequate for our current needs, and while we intend to expand our product liability insurance coverage to any products for which we obtain marketing approval, insurance may be unavailable, prohibitively expensive or may not fully cover our potential liabilities. If we are unable to maintain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims or field actions, we may be unable to continue to market our products and develop new markets. Defending a lawsuit could be costly and significantly divert management’s attention from conducting our business. A successful product liability claim brought against us in excess of any insurance coverage we have at that time could cause us to incur substantial liabilities, potentially in excess of our total assets, and our business to fail. In addition, we are a specialty company focused on women’s health. We have a narrow customer base that is subject to significant malpractice litigation that may place us at risk of the same. In addition, product liability claims or product field action or other regulatory proceedings may damage our reputation by raising questions about our products’ safety and efficacy, could significantly harm our reputation, interfere with our
We depend on the services of key personnel to implement our strategy, and if we lose key management or scientific personnel, scientific collaborators or other advisors or are unable to attract and retain other qualified personnel, we may be unable to execute our business plan and our operations and business would suffer.
Our success depends, in large part, on the efforts and abilities of Emory Anderson, who is our President and Chief Executive Officer, Dr. Durlin Hickok, who is our Vice President, Medical Affairs, Dr. Robert Hussa, our Vice President, Research and Development, Mark Fischer-Colbrie, who is our Vice President of Finance and Administration and Chief Financial Officer, and Marian Sacco, our Vice President, Sales and Marketing, as well as the other members of our senior management and our scientific and technical personnel. While we have executed management continuity agreements, we do not currently have employment agreements with any of these individuals. We do not currently carry key person insurance on the lives of any of these executives. Many of these people have been members of our executive team for several years, and their knowledge of our business would be difficult or time-consuming to replace. We also depend on our scientific collaborators and other advisors, particularly with respect to our research and development efforts. If we lose the services of one or more of our key officers, employees or consultants, or are unable to retain or attract the services of existing or new scientific collaborators and other advisors, our research and development and product development efforts could be delayed or curtailed, our ability to execute our business strategy would be impaired, and our stock price might be adversely affected.
Most of our operations are currently conducted at a single location that may be at risk from earthquakes and other natural or unforeseen disasters.
We currently conduct all of our manufacturing, development and management activities at a single location in Sunnyvale, California near known fault zones. In addition, our E-tegrity Tests are currently processed solely through our CLIA-certified laboratory located at our Sunnyvale facility. Despite precautions taken by us, any future natural or man-made disaster, such as a fire, earthquake or terrorist activity, could cause substantial delays in our operations, damage or destroy our equipment or inventory, and reduce our sales or cause us to incur additional expenses. In addition, the facility and some pieces of manufacturing equipment would be difficult to replace and could require substantial replacement lead-time. A disaster could seriously harm our business and results of operations. While we carry insurance for certain business interruptions, some natural and man-made disasters are excluded from our insurance policies, including those caused by terrorist acts or earthquakes. We believe that our insurance coverage is generally adequate for our current needs in the event of losses not caused by excluded events, but we may be subject to interruptions caused by excluded events or extraordinary events resulting in losses in excess of our insurance coverage or for which we have no coverage. This could impair our operating results and financial condition.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our research and development activities sometimes involve the controlled use of potentially harmful biological materials, hazardous materials and chemicals that are dangerous to human health and safety or the environment. We are subject on an ongoing basis to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations might be significant and could negatively affect our profitability. We believe our safety procedures for handling and disposing of these materials comply in all material aspects with federal, state and local laws and regulations and to date, we have not been required to take any action to correct any noncompliance. However, we cannot completely eliminate the risk of accidental contamination or injury to third parties from the use, storage, handling or
Potential business combinations could require significant management attention and prove difficult to integrate with our business, which could distract our management, disrupt our business, dilute stockholder value and adversely affect our operating results.
If we become aware of potential business combination candidates that are complementary to our business, we may decide to combine with such businesses or acquire their assets in the future. We have acquired businesses or product lines in the past. For example, we acquired exclusive rights to the SalEst Test in 2003. While we have not encountered such difficulties following our prior acquisitions, business combinations generally involve a number of additional difficulties and risks to our business, including:
In addition, we may not realize benefits from any business combination we may undertake in the future. If we fail to successfully integrate such businesses, or the technologies associated with such business combinations into our company, the revenue and operating results of the combined company could be adversely affected. Any integration process would require significant time and resources, and we may not be able to manage the process successfully. If our customers are uncertain about our ability to operate on a combined basis, they could delay or cancel orders for our products. We may not successfully evaluate or utilize the acquired technology or accurately forecast the financial impact of a combination, including accounting charges or volatility in the stock price of the combined entity. If we fail to successfully integrate other companies with which we may combine in the future, our business and your investment could be harmed.
If we fail to obtain necessary funds for our operations, we will be unable to continue to develop and commercialize new products and technologies and we would need to downsize or halt our operations.
We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, commercialization, manufacturing, clinical trials and research and development activities. We believe the net proceeds of this offering, together with our cash and cash equivalents, will be sufficient to meet our operating and capital requirements for at least the next two years. However, our present and future funding requirements will depend on many factors, including, among other things:
As a result of these factors, we may need to raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms when needed, if at all. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our future products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
If we are unable to protect our proprietary rights, we may not be able to compete effectively.
Our success depends significantly on our ability to protect our proprietary rights to the technologies used in our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions, to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our pending US and foreign patent applications may not issue as patents at all, or if they do, they may not issue as patents in a form that will be advantageous to us or may issue and be subsequently successfully challenged by others and invalidated. Additionally, our family of issued patents and patent applications, if and when issued, relating to our Fetal Fibronectin Test and TLiIQ System, have a range of expiration dates from 2007 to 2025. We cannot assure you that, upon the expiration of one or more patents relating to our Fetal Fibronectin Test and TLiIQ System, we will be able to protect our proprietary rights relating to the technologies used in these products. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. Both the patent application process and the process of managing patent disputes can be time-consuming and expensive. Competitors may be able to design around our patents or develop products that provide outcomes comparable to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States.
Although we may initiate litigation to stop the infringement of our patent claims or to attempt to force an unauthorized user of our patented inventions or trade secrets to compensate us for the infringement or unauthorized use, patent and trade secret litigation is complex and often difficult and expensive, and would consume the time of our management and other significant resources. If the outcome of litigation is adverse to us, third parties may be able to use our technologies without payments to us. Moreover, other companies against whom we might initiate litigation may be better able to sustain the costs of litigation because they have substantially greater resources. Because of these factors relating to litigation, we may be effectively unable to prevent misappropriation of our patent and other proprietary rights.
Our rights to use technologies and patents licensed to us by third parties are not within our control, and we may not be able to commercialize our products without these technologies.
We have licensed a number of patents, including patents related to our Fetal Fibronectin Test and our E-tegrity Test from third parties, including the Fred Hutchinson Cancer Research Center, Inverness Medical and the University of Pennsylvania. Our business may significantly suffer if one or more of these licenses terminate, if we or our licensors fail to abide by the terms of the licenses or fail to prevent infringement by third parties or if the licensed patents are found to be invalid.
If we violate the terms of our licenses, or otherwise lose our rights to these patents, we may be unable to continue developing and selling our products. Our licensors or others may dispute the scope of our rights under any of these licenses. The licensors under these licenses may breach the terms of their respective agreements or fail to prevent infringement of the licensed patents by third parties. Loss of any of these licenses for any reason could materially harm our financial condition and operating results.
In addition, if we determine that our products do not incorporate the patented technology that we have licensed from third parties, or that one or more of the patents that we have licensed is not valid, we may dispute our obligation to pay royalties to our licensors.
Any dispute with a licensor could be complex, expensive and time-consuming and an outcome adverse to us could materially harm our business and impair our ability to commercialize our products, including our Fetal Fibronectin Test. As a result, our stock price might be adversely affected.
If the use of our technologies conflicts with the intellectual property rights of third parties, we may incur substantial liabilities, and we may be unable to commercialize products based on these technologies in a profitable manner, if at all.
Other companies may have or acquire patent rights that they could enforce against us. If they do so, we may be required to alter our technologies, pay licensing fees or cease activities. If our technologies conflict with patent rights of others, third parties could bring legal action against us or our licensees, suppliers, customers or collaborators, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we might have to obtain a license in order to continue to manufacture or market the affected products. A required license under the related patent may not be available on acceptable terms, if at all.
Because patent applications can take many years to issue, there may be currently pending applications unknown to us or reissuance applications that may later result in issued patents upon which our technologies may infringe. There could also be existing patents of which we are unaware that our technologies may infringe. In addition, if third parties file patent applications or obtain patents claiming technology also claimed by us in pending applications, we may have to participate in interference proceedings in the US Patent and Trademark Office to determine priority of invention. If third parties file oppositions in foreign countries, we may also have to participate in opposition proceedings in foreign tribunals to defend the patentability of the filed foreign patent applications. We may have to participate in interference proceedings involving our issued patents or our pending applications.
If a third party claims that we infringe upon its proprietary rights, it could cause our business to suffer in a number of ways, including:
If any of these events occur, our business will suffer and the market price of our common stock may decline.
If we are involved in intellectual property claims and litigation, the proceedings may divert our resources and subject us to significant liability for damages, substantial litigation expense and the loss of our proprietary rights.
In order to protect or enforce our patent rights, we may initiate patent litigation. In addition, others may initiate patent litigation against us. We may become subject to interference proceedings conducted in patent and trademark offices to determine the priority of inventions. There are numerous issued and pending patents in the medical device field. The validity and breadth of medical technology patents may involve complex legal and factual questions for which important legal principles may remain unresolved.
Litigation may be necessary to assert or defend against infringement claims, enforce our issued and licensed patents, protect our trade secrets or know-how or determine the enforceability, scope and validity of the proprietary rights of others. Our involvement in intellectual property claims and litigation could:
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other diagnostic companies, including our potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business.
If we cannot obtain additional licenses to intellectual property owned by third parties that we desire to incorporate into new products we plan to develop, we may not be able to develop or commercialize these future products.
We are developing diagnostic products designed to expand the utility of fetal fibronectin in multiple applications. The technology that we ultimately may use in the development and commercialization of
RISKS RELATING TO THIS OFFERING
You will suffer immediate and substantial dilution.
We expect the initial public offering price of our shares to be substantially higher than the book value per share of our outstanding common stock. Accordingly, investors purchasing shares of common stock in this offering will:
To the extent outstanding stock options, warrants or the underwriters’ over-allotment option are exercised after this offering, there will be further dilution to new investors. See “Dilution.”
If our principal stockholders, executive officers and directors choose to act together, they may be able to control our management and operations, which may prevent us from taking actions that may be favorable to you.
Our executive officers, directors and principal stockholders, and entities affiliated with them, will beneficially own in the aggregate approximately 64.88% of our common stock following this offering. This significant concentration of share ownership may adversely affect the trading price of our common stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders. These stockholders, acting together, will have the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors and any proposed merger, consolidation or sale of all or substantially all of our assets. In addition, they could dictate the management of our business and affairs. This concentration of ownership could have the effect of delaying, deferring or preventing a change in control of us or impeding a merger or consolidation, takeover or other business combination that could be favorable to you.
The future sale of our securities could dilute your investment and negatively affect our stock price.
After this offering, we will have approximately 15,889,841 shares of common stock outstanding, or 16,452,341 shares if the underwriters exercise their over-allotment option in full. The 3,750,000 shares sold in this offering, or 4,312,500 shares if the underwriters exercise their over-allotment option in full, will be freely tradable without restriction under the federal securities laws unless purchased by our affiliates. The remaining shares of common stock outstanding after this offering will be available for public sale subject in some cases to volume and other limitations. See “Shares eligible for future sale.” We, our executive officers and directors and our existing stockholders holding an aggregate of over 97% of our shares outstanding (including securities convertible into or exchangeable or exercisable for our common stock) have entered into lock-up agreements with the underwriters that generally expire 180 days after the completion of the offering as described under “Underwriting.”
If our common stockholders sell substantial amounts of common stock in the public market, or the market perceives that such sales may occur, the market price of our common stock could fall. After this offering, the holders of approximately 12,139,841 shares of our common stock and the holders of warrants to purchase 196,915 shares of our common stock will have rights, subject to some
If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. Furthermore, we may enter into financing transactions at prices that represent a substantial discount to market price. Raising funds through the issuance of equity securities will dilute the ownership of our existing stockholders. A negative reaction by investors and securities analysts to any sale of our equity securities could result in a decline in the trading price of our common stock.
If an active, liquid trading market for our common stock does not develop, you may be unable to sell your shares quickly or at the market price.
Prior to this offering, there was no public market for our common stock. An active trading market for our common stock may not develop following this offering. You may not be able to sell your shares quickly or at the market price if trading in our stock is not active. The initial public offering price may not be indicative of prices that will prevail in the trading market. See “Underwriting” for more information regarding the factors considered in determining the initial public offering price.
If the price and volume of our common stock experience extreme fluctuations, this could lead to costly litigation for us.
Because we operate within the medical devices and diagnostic products industries, our stock price is likely to be volatile. The market price of our common stock may fluctuate substantially due to a variety of factors, including:
The market prices of the securities of medical devices and diagnostic products companies, particularly companies like ours without consistent product sales and earnings, have been highly volatile and are likely to remain highly volatile in the future. This volatility has often been unrelated to the operating performance of particular companies. Moreover, market prices for stocks of biotechnology-related companies, particularly following an initial public offering, frequently reach levels that bear no relationship to the operating performance of these companies. These market prices generally are not sustainable and are highly volatile. In the past, companies that experience volatility in the market price of their securities have often faced securities class action litigation. Whether or not meritorious, litigation brought against us could result in substantial costs, divert our management’s attention and resources and harm our ability to grow our business.
We have reserved discretion in how we allocate our use of the net proceeds of this offering and if we do not use these proceeds effectively, we may fail to achieve our objectives and our stock price could decline.
We will have flexibility in applying the net proceeds of this offering among the categories of identified uses described in the “Use of proceeds” section of this prospectus. Although we expect to use the net proceeds in the approximate allocations described elsewhere in this prospectus, if we use the net proceeds for corporate purposes that do not yield a significant return or any return at all for our stockholders, our stock price could decline, and you may also not agree with how we allocate the net proceeds of this offering.
Anti-takeover provisions in our certificate of incorporation and bylaws and under Delaware law may inhibit a change in control or a change in management that you consider favorable.
Provisions in our certificate of incorporation and bylaws could delay or prevent a change of control or change in management that would provide you with a premium to the market price of your common stock. These provisions include those:
In addition, Section 203 of the Delaware General Corporation Law limits business combination transactions with 15% stockholders that have not been approved by our board of directors. These provisions and others could make it difficult for a third party to acquire us, or for members of our board of directors to be replaced, even if doing so would be beneficial to our stockholders. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt to replace the current management team. If a change of control or change in management is delayed or prevented, you may lose an opportunity to realize a premium on your shares of common stock or the market price of our common stock could decline.
We do not expect to pay dividends in the foreseeable future. As a result, you must rely on stock appreciation for any return on your investment.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. Any payment of cash dividends will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. Accordingly, you will have to rely on capital appreciation, if any, to earn a return on your investment in our common stock. Furthermore, we may in the future become subject to contractual restrictions on, or prohibitions against, the payment of dividends.
Special note regarding forward-looking statementsEXPLANATORY NOTE
This prospectus, including the sections titled “Prospectus summary,” “Risk factors,” “Management’s discussion and analysis of financial condition and results of operations” and “Business,” contains forward-looking statements. Forward-looking statements convey our current expectations or forecasts of future events. All statements contained in this prospectus other than statements of historical fact are forward-looking statements. Forward-looking statements include statements regarding our future financial position, business strategy, budgets, projected costs, plans and objectives of management for future operations. The words “may,” “continue,” “estimate,” “intend,” “plan,” “will,” “believe,” “project,” “expect,” “could,” “would,” “anticipate” and similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. These forward-looking statements include, among other things, statements about:
Any or all of our forward-looking statements in this prospectus may turn out to be inaccurate. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. They may be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including the risks, uncertainties and assumptions described in “Risk factors.” In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur as contemplated, and actual results could differ materially from those anticipated or implied by the forward-looking statements.
These forward-looking statements speak only as of the date of this prospectus. Unless required by law, we undertake no obligation to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this prospectus. See “Where you can find additional information.”
Use of proceeds
We estimate that the net proceeds to us from the sale of the shares of common stock we are offering will be approximately $50.5 million, assuming an initial public offering price of $15.00 per share, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, we estimate the net proceeds to us from this offering will be approximately $58.4 million.
The primary purposes of this offering are to create a public market for our common stock, raise additional equity capital and facilitate future access to public markets. We intend to use the net proceeds of this offering as follows:
We may also use a portion of the net proceeds to acquire or in-license complementary businesses, products or technologies. Although we have no specific arrangements with respect to acquisitions, we evaluate acquisition opportunities and engage in related discussions from time to time. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds from this offering. We reserve the right to change the allocation of the net proceeds based on the changing needs of our business, including the progress and results of our research and development activities, the results of our sales and marketing efforts, acquisition opportunities and competitive developments. Accordingly, our management will have broad discretion in the application of the net proceeds and investors will be relying on the judgment of our management regarding the application of the proceeds of this offering.
Pending use of the proceeds from this offering, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments.
Dividend policy
We have never declared or paid any cash dividends on our capital stock and we do not currently anticipate declaring or paying cash dividends on our capital stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance operations. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and covenants and other factors that our board of directors may deem relevant.
Capitalization
The following table summarizes our capitalization as of September 30, 2004:
You should read the following table in conjunction with “Management’s discussion and analysis of financial condition and results of operations” and our financial statements and related notes appearing elsewhere in this prospectus.
| As of September 30, 2004 | ||||||||||||||
| Pro forma | ||||||||||||||
| Actual | Pro forma | as adjusted | ||||||||||||
| (in thousands, except share and | ||||||||||||||
| per share data) | ||||||||||||||
| Cash and cash equivalents | $ | 14,638 | $ | 14,638 | $ | 65,151 | ||||||||
| Convertible preferred stock, $0.001 par value, 16,516,335 shares authorized, actual; 5,000,000 shares authorized, pro forma and pro forma as adjusted; 15,409,062 shares issued and outstanding, actual; no shares issued and outstanding, pro forma and pro forma as adjusted | $ | 61,484 | $ | — | $ | — | ||||||||
| Stockholders’ equity (deficit): | ||||||||||||||
| Common stock, $0.001 par value, 25,000,000 shares | ||||||||||||||
| authorized, actual; 100,000,000 shares authorized, pro forma and pro forma as adjusted; 182,519 shares issued and outstanding, actual; 12,139,841 shares issued and outstanding, pro forma; 15,889,841 shares issued and outstanding, pro forma as adjusted | — | 12 | 16 | |||||||||||
| Additional paid-in capital | 6,272 | 67,744 | 118,253 | |||||||||||
| Deferred compensation | (3,459 | ) | (3,459 | ) | (3,459 | ) | ||||||||
| Accumulated deficit | (46,354 | ) | (46,354 | ) | (46,354 | ) | ||||||||
| Total stockholders’ equity (deficit) | (43,541 | ) | 17,943 | 68,456 | ||||||||||
| Total capitalization | $ | 17,943 | $ | 17,943 | $ | 68,456 | ||||||||
The number of shares of our common stock to be outstanding immediately after this offering is based on 12,139,841 shares of common stock outstanding as of September 30, 2004 after giving effect to the conversion of all 15,409,062 shares of preferred stock outstanding as of September 30, 2004 into 11,957,322 shares of common stock, which will become effective at the closing of this offering.
The number of shares of our common stock outstanding immediately after this offering excludes:
We effected a three-for-four reverse split of our common stock on December 6, 2004. All common stock share prices and amounts set forth in the table above have been adjusted to give effect to this stock split.
Dilution
If you invest in our common stock, your interest will be diluted immediately to the extent of the difference between the public offering price per share you will pay in this offering and the pro forma net tangible book value per share of our common stock immediately after this offering.
Our net tangible book value as of September 30, 2004 was approximately $(43.7) million, or $(239.59) per share of common stock. Net tangible book value per share is equal to our total tangible assets minus total liabilities, all divided by 182,519 shares of common stock outstanding as of September 30, 2004.
Our pro forma net tangible book value per share as of September 30, 2004 was approximately $1.46 per share. Pro forma net tangible book value per share gives effect to the conversion of all outstanding shares of our preferred stock as of September 30, 2004 into 11,957,322 shares of our common stock, which will become effective at the closing of this offering.
After giving effect to the sale of the 3,750,000 shares of common stock we are offering at an assumed initial public offering price of $15.00 per share, and after deducting underwriting discounts and commissions and our estimated offering expenses, our pro forma as adjusted net tangible book value as of September 30, 2004 would have been approximately $68.3 million, or $4.30 per share.
This represents an immediate increase in pro forma net tangible book value of $2.84 per share and an immediate dilution of $10.70 per share to new investors. The following table illustrates this calculation on a per share basis:
| Assumed initial public offering price per share | $ | 15.00 | ||||||
| Net tangible book value per share as of September 30, 2004 | $ | (239.59 | ) | |||||
| Pro forma increase in net tangible book value per share attributable to conversion of preferred stock outstanding at September 30, 2004 | 241.05 | |||||||
| Pro forma net tangible book value per share of common stock as of September 30, 2004 | 1.46 | |||||||
| Pro forma increase per share attributable to the offering | 2.84 | |||||||
| Pro forma as adjusted net tangible book value per share of common stock after this offering | 4.30 | |||||||
| Pro forma dilution per share to new investors | $ | 10.70 | ||||||
If the underwriters exercise their over-allotment option in full, our pro forma as adjusted book value will increase to $4.63 per share, representing an increase to existing holders of $3.17 per share, and there will be an immediate dilution of $10.37 per share to new investors.
The following table summarizes, on a pro forma as adjusted basis as of September 30, 2004, after giving effect to this offering, and the pro forma adjustments referred to above, the total number of shares of our common stock purchased from us and the total consideration and average price per share by existing stockholders and by new investors:
| Total shares | Total consideration | ||||||||||||||||||||
| Average price | |||||||||||||||||||||
| Number | Percent | Amount | Percent | per share | |||||||||||||||||
| Existing stockholders | 12,139,841 | 76.4 | % | $ | 64,917,000 | 53.6 | % | $ | 5.35 | ||||||||||||
| New investors | 3,750,000 | 23.6 | 56,250,000 | 46.4 | 15.00 | ||||||||||||||||
| Total | 15,889,841 | 100.0 | % | $ | 121,167,000 | $ | 100.0 | % | |||||||||||||
If the underwriters exercise their over-allotment option in full, the following will occur:
The tables and calculations above are based on 182,519 shares of our common stock outstanding as of September 30, 2004 and excludes:
If all of our outstanding options and warrants as of September 30, 2004 were exercised, the pro forma as adjusted net tangible book value per share after this offering would be $4.34 per share, representing an increase to existing holders of $2.88 per share, and there will be an immediate dilution of $10.66 per share to new investors.
Selected financial data
We have derived the following statements of operations data for each of the three fiscal years ended December 31, 2001, 2002 and 2003 and the balance sheet data at December 31, 2002 and 2003 from our financial statements which have been audited by Ernst & Young LLP, independent registered public accounting firm, and which financial statements and the report thereon we include elsewhere in this prospectus. We have derived our statements of operations data for the fiscal years ended December 31, 1999 and 2000 and the balance sheet data at December 31, 1999, 2000 and 2001 from our audited financial statements that we do not include in this prospectus. The selected financial data at September 30, 2004 and for the nine months ended September 30, 2003 and 2004 have been derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited selected financial data include, in the opinion of management, all adjustments, consisting of only normal recurring adjustments, that are necessary for a fair presentation of the financial position and the results of operations for the interim unaudited periods. Operating results for the nine months ended September 30, 2004 are not necessarily indicative of the results that may be expected for the year ending December 31, 2004. You should read the selected financial data in conjunction with our financial statements and the related notes and “Management’s discussion and analysis of financial condition and results of operations” appearing elsewhere in this prospectus.
| Nine months ended | |||||||||||||||||||||||||||||
| Years ended December 31, | September 30, | ||||||||||||||||||||||||||||
| Statement of | |||||||||||||||||||||||||||||
| operations data: | 1999 | 2000 | 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||||||
| (in thousands, except share and per share data) | |||||||||||||||||||||||||||||
| (unaudited) | |||||||||||||||||||||||||||||
| Product sales | $3,776 | $3,404 | $6,742 | $14,277 | $26,499 | $19,185 | $24,405 | ||||||||||||||||||||||
| Cost of product sales | 1,880 | 2,023 | 2,521 | 3,715 | 6,087 | 4,621 | 895 | (1) | |||||||||||||||||||||
| Gross profit | 1,896 | 1,381 | 4,221 | 10,562 | 20,412 | 14,564 | 23,510 | ||||||||||||||||||||||
| Contract revenues | 444 | 515 | 811 | 1,059 | — | — | — | ||||||||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||||||||||||||
| Selling and marketing | 3,630 | 5,337 | 6,437 | 7,819 | 12,259 | 9,023 | 11,638 | ||||||||||||||||||||||
| General and administrative | 1,481 | 1,641 | 2,033 | 2,069 | 2,730 | 1,791 | 2,631 | ||||||||||||||||||||||
| Research and development | 2,014 | 1,893 | 2,145 | 2,047 | 2,001 | 1,519 | 1,755 | ||||||||||||||||||||||
| Total operating costs and expenses | 7,125 | 8,871 | 10,615 | 11,935 | 16,990 | 12,333 | 16,024 | ||||||||||||||||||||||
| Income (loss) from operations | (4,785 | ) | (6,975 | ) | (5,583 | ) | (314 | ) | 3,422 | 2,231 | 7,486 | ||||||||||||||||||
| Other expenses, net | (11 | ) | (17 | ) | (21 | ) | (8 | ) | (33 | ) | (10 | ) | — | ||||||||||||||||
| Interest income (expense), net | (779 | ) | (646 | ) | (85 | ) | (7 | ) | (19 | ) | (23 | ) | 104 | ||||||||||||||||
| Income (loss) before income taxes | (5,575 | ) | (7,638 | ) | (5,689 | ) | (329 | ) | 3,370 | 2,198 | 7,590 | ||||||||||||||||||
| Provision for income taxes | — | — | �� | — | 135 | 113 | 307 | ||||||||||||||||||||||
| Net income (loss) | $(5,575 | ) | $(7,638 | ) | $(5,689 | ) | $(329 | ) | $3,235 | $2,085 | $7,283 | ||||||||||||||||||
| Net income (loss) per share: | |||||||||||||||||||||||||||||
| Basic | $(28.11 | ) | $(45.75 | ) | $(36.27 | ) | $(1.82 | ) | $17.78 | $11.46 | $39.97 | ||||||||||||||||||
| Diluted | $(28.11 | ) | $(45.75 | ) | $(36.27 | ) | $(1.82 | ) | $0.26 | $0.17 | $0.55 | ||||||||||||||||||
| Pro forma basic (unaudited) | $0.27 | $0.60 | |||||||||||||||||||||||||||
| Pro forma diluted (unaudited) | $0.26 | $0.55 | |||||||||||||||||||||||||||
| Shares used in computing net income (loss) per share: | |||||||||||||||||||||||||||||
| Basic | 198,358 | 166,942 | 156,857 | 181,188 | 181,965 | 181,900 | 182,223 | ||||||||||||||||||||||
| Diluted | 198,358 | 166,942 | 156,857 | 181,188 | 12,515,063 | 12,429,763 | 13,364,494 | ||||||||||||||||||||||
| Pro forma basic (unaudited) | 11,986,026 | 12,139,545 | |||||||||||||||||||||||||||
| Pro forma diluted (unaudited) | 12,515,063 | 13,364,494 | |||||||||||||||||||||||||||
| As of December 31, | ||||||||||||||||||||||||
| As of September 30, | ||||||||||||||||||||||||
| Balance sheet data: | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | ||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Cash and cash equivalents | $3,071 | $6,515 | $16,674 | $10,751 | $12,092 | $ | 14,638 | |||||||||||||||||
| Working capital | (2,586 | ) | (1,888 | ) | 7,094 | 6,195 | 9,653 | 17,454 | ||||||||||||||||
| Total assets | 6,137 | 8,234 | 17,714 | 15,731 | 18,716 | 23,813 | ||||||||||||||||||
| Convertible preferred stock | 36,577 | 45,637 | 60,984 | 60,984 | 61,484 | 61,484 | ||||||||||||||||||
| Accumulated deficit | (43,216 | ) | (50,854 | ) | (56,543 | ) | (56,872 | ) | (53,637 | ) | (46,354 | ) | ||||||||||||
| Total stockholders’ equity (deficit) | (42,188 | ) | (48,540 | ) | (54,217 | ) | (54,537 | ) | (51,279 | ) | (43,541 | ) | ||||||||||||
Management’s discussion and analysis of financial condition and results of operations
The following discussion of our financial conditions and results of operations should be read in conjunction with our financial statements and the notes to those financial statements appearing elsewhere in this prospectus. This discussion contains forward-looking statements that involve significant risks and uncertainties. As a result of many factors, such as those set forth under “Risk factors” and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
OVERVIEW
We design, develop, manufacture and market innovative products for women’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the Fetal Fibronectin Test, that utilizes a single-use, disposable cassette and is analyzed on our patented instrument, the TLiIQ System. This FDA-approved product is designed to objectively determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. We began selling our single-use, disposable Fetal Fibronectin Test in 1999 and launched our second-generation system, the TLiIQ System, in 2001. Sales of TLiIQ Systems to hospital and clinical laboratories allow healthcare providers access to the Fetal Fibronectin Test, resulting in the potential for better patient care and for significant cost savings by avoiding unnecessary medical treatment. To date, we have shipped over 1,270 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes.
We believe the key factors underlying our growth since 1999 include greater healthcare provider acceptance, demonstrated cost savings, expanded reimbursement coverage by insurance companies, expansion of our sales force and increased marketing efforts. Continued growth in test volume and revenue will depend on a number of factors, including placing additional TLiIQ Systems in hospitals and clinical laboratories, increasing utilization of existing TLiIQ Systems and developing additional applications or products.
Product sales
Our product sales are derived primarily from the sale of our disposable Fetal Fibronectin Test cassettes. In addition, we derive a small portion of our revenues from the sale of TLiIQ Systems and other consumables. Sales in the United States accounted for 97% of our product sales in both the nine months ended September 30, 2004 and the year ended December 31, 2003. International sales accounted for 3% of our product sales in both the nine months ended September 30, 2004 and the year ended December 31, 2003. We currently use distributors for sales outside of the United States and Canada. Our business has been in the past and may continue to be seasonal and is affected by customer ordering patterns, which may involve quarterly or semi-annual orders, as well as other factors which may cause quarterly variances in our revenue. As such, revenue may not increase in sequential quarters and our net income may fluctuate significantly.
Cost of product sales
Our cost of product sales represents the cost of materials, overhead associated with the manufacture of our products, direct labor, delivery charges, lab services, royalties and product warranty obligations.
Royalty costs were $1.4 million or 5.9% of revenue for the nine months ended September 30, 2004 as compared to $2.4 million or 12.8% of revenue for the nine months ended September 30, 2003, excluding the effect of a one-time benefit of $2.7 million recorded in the 2004 period. The ongoing reduction in royalty costs and the one-time reduction of $2.7 million in the nine months ended
Selling and marketing expenses
Selling and marketing expenses consist primarily of sales and marketing personnel compensation, sales force incentive compensation, travel, trade-shows, promotional materials and programs, advertising and healthcare provider education materials and events.
General and administrative expenses
Our general and administrative expenses consist primarily of personnel expenses for accounting, human resources, information technology and corporate administration functions. Other costs include facility costs and professional fees for legal and accounting services.
Research and development expenses
Our research and development expenses consist of costs incurred for company-sponsored and collaborative research and development activities. These expenses consist primarily of direct and research-related allocated overhead expenses such as facilities costs, salaries and benefits, and material and supply costs.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
We prepare our financial statements in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. Our critical accounting policies are as follows:
Revenue recognition
Our revenue from product sales is recognized when there is persuasive evidence an arrangement exists, the price is fixed or determinable, delivery to the customer has occurred and collectibility is reasonably assured. We use contracts and customer purchase orders to determine the existence of an arrangement. We assess whether the fee is fixed or determinable based on the terms of the agreement associated with the transaction. We use shipping documents and, if necessary, third-party proof of delivery to verify delivery. In order to determine whether collection is probable, we assess a number of factors, including past transaction history with the customer and the credit-worthiness of the customer. Revenue from our laboratory services is recognized as tests are performed. Contract revenues are recorded over the life of the contract or as performance occurs and the related earnings process is completed based on the performance requirements of the contract.
With respect to sales to distributors, revenue is generally recognized upon shipment, as the title, risks and rewards of ownership of the products pass to the distributors and the selling price of our product is fixed and determinable at that point. The selling prices on sales to a certain distributor through June 30, 2002 were not fixed and determinable until the distributor shipped the products to the end user. Consequently, for this distributor, we recognized revenue only after the shipment of product to the end user. Additionally, on July 1, 2002, we entered into a services agreement with a laboratory and fulfillment company that performs diagnostic tests. Under the terms of the agreement, this company provides certain domestic product distribution and testing services for us. We recognize revenue upon the shipment of products from this company to the end user as the title, risks and rewards of ownership of the products pass from us to the end user at that time. Any advance payments received in excess of revenue recognized are classified as deferred revenue.
Valuation of inventory
Inventories are stated at the lower of standard cost (which approximates actual cost) or market. We calculate an inventory reserve for estimated obsolescence or excess inventory based upon historical demand and assumptions about future demand for our products and market conditions. If our current assumptions about future demand change and if actual market conditions are less favorable than anticipated, additional inventory reserves may be required which would negatively impact our gross profit.
Allowance for doubtful accounts
We maintain an allowance for doubtful accounts related to the estimated losses that may result from the inability of our customers to make required payments. This allowance is determined based upon historical experience and any specific customer collection issues that have been identified. Historically, we have not experienced significant credit losses related to an individual customer or groups of customers in any particular industry or geographic area. However, deterioration in our ability to collect our receivables could result in an increase in our allowance for doubtful accounts and increase our general and administrative expenses.
Stock-based compensation expense
In connection with the grant of stock options to employees and directors, we record deferred stock compensation as a component of stockholders’ equity. Deferred stock compensation for options granted to employees and directors is recorded if the estimated fair value of our common stock on the date the options are granted is greater than their exercise prices. Deferred stock compensation is amortized as a charge to operations over the vesting periods of the options using the straight-line method. Additionally, for options granted to non-employees, the fair value of the options, estimated using the Black-Scholes valuation model, is periodically re-measured with the resulting value charged to expense over the period of the related services being rendered. We recorded stock-based compensation expense related to all of our options of $0, $5,000, and $23,000 for the years ended December 31, 2001, 2002 and 2003, respectively, and $454,000 for the nine months ended September 30, 2004. As of September 30, 2004, we had $3.5 million of deferred stock compensation that will be expensed over the next four years. The amount of stock-based compensation expense to be recorded in future periods may decrease if unvested options, for which deferred stock compensation has been recorded, are subsequently canceled.
Cost of product sales
Royalty costs are included as a cost of product sales. The royalty costs are determined by applying the royalty rate in each license agreement to the specific product offerings included in that particular agreement, including any deductions to sales or royalty cost allowed under the royalty terms. The determination of royalty costs can be affected by various factors including changes in the terms of the
RESULTS OF OPERATIONS
Nine months ended September 30, 2004 as compared to the nine months ended September 30, 2003
Product sales
Product sales for the nine months ended September 30, 2004 were $24.4 million, an increase of 27.2%, or $5.2 million, from $19.2 million for the nine months ended September 30, 2003. The growth in sales was primarily due to an approximate $5.4 million increase in sales of Fetal Fibronectin Test cassettes, partially offset by slight decreases in other revenue of approximately $0.2 million.
Cost of product sales
Cost of product sales for the nine months ended September 30, 2004 was $0.9 million, a decrease of $3.7 million from $4.6 million for the nine months ended September 30, 2003. The decrease in cost of product sales was primarily the result of reduced royalty costs of $1.0 million and a one-time reduction in accrued royalties of $2.7 million that we determined were in excess of amounts actually due. The decrease and one-time reduction of $2.7 million were primarily related to significant new information received in October 2004 which allowed us to conclude that no royalties were due or are required under a license agreement. In addition, a decrease in other product costs of $0.6 million was offset by $0.6 million of costs related to increased unit volumes for the nine months ended September 2004 as compared to the nine months ended September 2003. Non-royalty related costs were unchanged for the period ended September 30, 2004 from the period ended September 30, 2003 as costs related to increased unit sales were offset by lower unit manufacturing costs. As a percent of revenue, cost of product sales was 14.9% of revenue for the nine months ended September 30, 2004, excluding the effect of the one-time reduction in accrued royalties, as compared to 24.1% for the same period in the prior year due to the reduction in royalty costs.
Gross profit
Our gross profit for the nine months ended September 30, 2004 was $23.5 million, an increase of $8.9 million from $14.6 million in the nine months ended September 30, 2003. The gross profit increase was primarily due to increased sales adding $4.6 million, a one-time reduction of accrued royalties of $2.7 million, and $1.6 million in reduced product costs. Gross margin was 85.1%,
Selling and marketing expenses
Selling and marketing expenses for the nine months ended September 30, 2004 were $11.6 million, an increase of approximately 29% from $9.0 million for the nine months ended September 30, 2003. Selling and marketing expenses as a percentage of product sales increased slightly to 47.7% in the nine months ended September 30, 2004 from 47.0% in the nine months ended September 30, 2003. The increase was largely attributable to $1.4 million related to the expansion of our direct sales force, $0.6 million in increased marketing expenses to support the growth in product sales, and $0.3 million in stock-based compensation expense.
General and administrative expenses
General and administrative expenses for the nine months ended September 30, 2004 were $2.6 million, an increase of $0.8 million or 46.9% from $1.8 million for the nine months ended September 30, 2003. General and administrative expenses as a percentage of product sales were 10.8% and 9.3% for the nine months ended September 30, 2004 and 2003, respectively. The increase was primarily attributable to patent and legal expense increases of $0.5 million and increased personnel related expenses of $0.3 million.
Research and development expenses
Research and development expenses for the nine months ended September 30, 2004 were $1.8 million, an increase of 15.5% from $1.5 million for the same period in 2003. The increase was attributable to increased clinical trial expenses related to the induction of labor indication for the Fetal Fibronectin Test and personnel and related costs. Research and development expenses as a percentage of revenue decreased to 7.2% for the nine months ended September 30, 2004 from 7.9% for the same period in 2003.
Interest income (expense)
We recognized interest income of $104,000 for the nine months ended September 30, 2004, an increase of $18,000 from the same period in 2003, primarily due to higher cash balances earning interest. There was no interest expense for the nine months ended September 30, 2004 as compared to $109,000 of interest expense in the same period of 2003. The interest expense in 2003 was primarily related to notes payable in conjunction with the conclusion of a co-promotion and distribution agreement with a major US distributor. The note was repaid by June 30, 2003.
Provision for income taxes
We recorded a provision for income taxes of $307,000 for the nine months ended September 30, 2004 related to federal alternative minimum taxes and state taxes compared to approximately $113,000 for the same period in 2003.
Our effective tax rate for the nine months ended September 30, 2004 was 4.0% compared to 5.1% for the nine months ended September 30, 2003. The effective tax rate for both periods is lower than the statutory rate due primarily to tax benefits arising from the utilization of net operating losses to the extent allowable under current law.
Year ended December 31, 2003 as compared to year ended December 31, 2002
Product sales
Product sales for the year ended December 31, 2003 were $26.5 million, an increase of 85.6%, or $12.2 million from $14.3 million in 2002. The increase was primarily attributable to growth in unit sales of $6.0 million. The increase was also attributable to the reduction in revenue sharing of
Cost of product sales
Cost of product sales for the year ended December 31, 2003 was $6.1 million, an increase of 63.8% from $3.7 million in 2002. The increase was related primarily to the conclusion of the co-promotion and distribution agreement and the resulting increase in our product sales of Fetal Fibronectin Test cassettes. As a percentage of product sales, cost of product sales in 2003 decreased to 23.0% in the year ended December 31, 2003 from 26.0% in the year ended December 31, 2002.
Gross profit
Gross profit was $20.4 million in the year ended December 31, 2003 and $10.6 million in the year ended December 31, 2002. Gross margin for the year ended December 31, 2003 was 77.0%, as compared to 74.0% in 2002. The increase in gross profit was primarily due to the conclusion of the co-promotion and distribution agreement and the resulting increase in our product sales.
Contract revenue
We had no contract revenue in the year ended December 31, 2003. Contract revenue of $1.1 million was recognized in the year ended December 31, 2002. In 1999, we entered into a co-promotion and distribution agreement with a major US distributor. Under the agreement, the distributor paid us a nonrefundable one-time payment of $2.0 million in 1999. This one-time payment was being recognized as revenue over the estimated five-year life of the agreement. At the termination of the agreement in 2002, we recognized the balance of this deferred contract revenue. The distributor was also to pay us a total of up to $2.0 million during 2001 and 2002 to fund certain portions of the research and development efforts related to the products and services covered by this agreement. During the year ended December 31, 2002, we recognized $176,000 of such research and development revenue.
Selling and marketing expenses
Selling and marketing expenses for the year ended December 31, 2003 were $12.3 million, an increase of 56.8% from $7.8 million for the same period in 2002. The increase was primarily due to $3.0 million in costs associated with the expansion of our direct sales force and $0.9 million related to marketing expenditures.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2003 were $2.7 million, an increase of 31.9% from $2.1 million for the same period in 2002. The increase was primarily attributable to increased patent and other legal fees of approximately $0.4 million and increased insurance expense of $0.2 million.
Research and development expenses
Research and development expenses for the year ended December 31, 2003 were $2.0 million, unchanged from the same period in 2002. Lower clinical trial expenses in 2003 were offset by higher usage of supplies and service charges relating to FDA filing and user fees.
Interest income (expense)
We recognized interest income of $112,000 in 2003, a decrease of 47.2% from $212,000 in 2002. The decrease was primarily due to a higher average cash balance in 2002 and lower interest rates in 2003. We recognized interest expense of $131,000 in 2003, a decrease of 40.2% from $219,000 in 2002. The decrease is attributable to interest on capital lease obligations and notes payable which were fully paid in 2002, and interest from the note payable related to the terminated co-promotion and distribution agreement issued in June 2002.
Provision for income taxes
We recorded an income tax provision of $135,000 for the year ended December 31, 2003 related to federal alternative minimum taxes and state taxes. There was no tax provision recorded for the year ended December 31, 2002 due to an operating loss.
Because of our lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance.
As of December 31, 2003, we had federal and state net operating loss carryforwards of approximately $40.3 million and $22.8 million, respectively. We also had federal and state research and development tax credit carryforwards of approximately $1.1 million and $0.9 million, respectively. The federal net operating loss and tax credit carryforwards will expire at various dates beginning in 2004 through 2022, if not utilized. The state net operating loss carryforwards will expire at various dates beginning in 2004 through 2013, if not utilized. The state research and development tax credits carry forward indefinitely.
Utilization of our net operating losses may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses before utilization.
Year ended December 31, 2002 as compared to year ended December 31, 2001
Product sales
Product sales for the year ended December 31, 2002 were $14.3 million, an increase of 111.8% from $6.7 million for 2001. The increase was mainly attributable to an increase in unit sales of Fetal Fibronectin Test cassettes of $3.3 million and the favorable impact of $3.0 million from the conclusion of the co-promotion and distribution agreement with a major US distributor on June 30, 2002 with the addition of $1.2 million due to an increase in average selling price.
Cost of product sales
Cost of product sales for the year ended December 31, 2002 was $3.7 million, an increase of 47.4% from $2.5 million in 2001. The absolute dollar increase was primarily due to the favorable impact of the conclusion of the co-promotion and distribution agreement with a major US distributor. As a percentage of product revenue, cost of product sales in 2002 was 26.0%, significantly lower than the 37.4% in 2001.
Gross profit
Gross profit was $10.6 million in the year ended December 31, 2002 and $4.2 million in the year ended December 31, 2001. Gross margin was 74.0% in 2002 and 62.6% in 2001. The increase was primarily attributable to the conclusion of the co-promotion and distribution agreement with a major US distributor and the resulting increase in our sales.
Contract revenue
Contract revenue for the year ended December 31, 2002 was $1.1 million, an increase of 30.6% from $0.8 million for 2001. In the years ended December 31, 2002 and 2001, respectively, we recognized $176,000 and $244,000 of research and development revenue from a co-promotion and distribution agreement with a major US distributor. Additionally, the Company received a nonrefundable, one-time $2.0 million payment from the distributor in 1999 that was being recognized as revenue over the estimated five-year life of the agreement. The amount of revenue recognized for the years ended December 31, 2002 and 2001 from this one-time payment was $574,000 and $400,000, respectively. Also included in contract revenue are reimbursements for research and development expenses from National Institutes of Health, or NIH, grants. Reimbursements recognized as revenue in 2002 and 2001 were $35,000 and $167,000, respectively.
Selling and marketing expenses
Selling and marketing expenses for the year ended December 31, 2002 were $7.8 million, an increase of 21.5% from $6.4 million in 2001. The increase was primarily due to $0.9 million of expenses related to the expansion of our direct sales force.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2002 were $2.1 million, relatively consistent with $2.0 million in 2001. Increases in personnel related expenses of $171,000, and legal fees of $47,000 were partially offset primarily by reduced bad debt expenses of $73,000.
Research and development expenses
Research and development expenses for the year ended December 31, 2002 were $2.0 million, a decrease of 4.6% from $2.1 million in 2001. The decrease was primarily due to lower clinical trial expenses partially offset by increased personnel expenses.
Interest income (expense)
We recognized interest income of $212,000 in the year ended December 31, 2002, a decrease of 22.1% from $272,000 in 2001. The decrease was primarily due to lower interest rates.
We recognized interest expense of $219,000 in the year ended December 31, 2002, a decrease of 38.7% from $357,000 in 2001. The decrease was attributable to declining interest expense related to capital lease obligations and notes payable.
Provision for income taxes
We recorded no income tax provision for 2002 or 2001 due to operating losses.
Because of our lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance.
LIQUIDITY AND CAPITAL RESOURCES
Since our inception, our operations have been primarily financed through private equity investments, with the addition of capital leases, and research and development contracts. As of September 30, 2004 our cash and cash equivalents were $14.6 million. All of our cash equivalents have original maturities of three months or less.
During the nine months ended September 30, 2004, our operating activities provided cash of approximately $2.7 million, compared to approximately $3.7 million during the nine months ended September 30, 2003. The decrease was due primarily to an increase in net income more than offset by
Our investing activities used cash of approximately $138,000 during the nine months ended September 30, 2004 compared to $357,000 for the nine months ended September 30, 2003. Investing activities in 2004 were related to investments in equipment, while the investing activity in 2003 was primarily due to our acquisition of the SalEst Test.
No cash was used for financing activities during the nine months ended September 30, 2004 compared to $4.3 million used during the nine months ended September 30, 2003. The $4.3 million used during the nine months ended September 30, 2003 was primarily related to repayments of notes payable.
For the year ended December 31, 2003 our operating activities provided cash of approximately $5.5 million. This was an increase of $9.4 million from the cash used in operating activities of $3.9 million for the year ended December 31, 2002. This change was primarily due to a loss of $0.3 million in the year ended December 31, 2002 in comparison to net income of $3.2 million in 2003. Additionally, accounts receivable increased by $1.3 million in the year ended December 31, 2003, in comparison to an increase of $3.9 million in 2002, and deferred revenue increased by $0.2 million in 2003, in comparison to a decrease of $1.9 million in 2002. These changes in accounts receivable and deferred revenue were due primarily to the conclusion of the co-promotion and distribution agreement with a major US distributor.
For the year ended December 31, 2003 our investing activities used cash of approximately $0.4 million. This was an increase of $0.2 million from cash used in investing activities of $0.1 million for the year ended December 31, 2002. The increase was due to the purchase of intangible assets and equipment.
For the year ended December 31, 2003 our financing activities used $3.8 million. This was an increase of $1.9 million from cash used in financing activities of approximately $1.9 million for the year ended December 31, 2002. The increase was primarily attributable to payments on notes payable.
As of September 30, 2004, we had no long-term debt, capital lease obligations or long-term purchase agreements or commitments other than a facility lease which we have for a one-year term with two one-year renewal options and an operating lease for a new telephone system. Future operating lease payments under both of these leases are included in the table below. The table also reflects our expectations regarding reductions in accrued royalties.
| Payments due in | ||||||||||||||||||||||||||||
| Contractual obligations | Total | 2004 | 2005 | 2006 | 2007 | 2008 | After 2008 | |||||||||||||||||||||
| Operating leases | $ | 254,000 | $ | 77,000 | $ | 148,000 | $ | 13,000 | $ | 13,000 | $ | 3,000 | — | |||||||||||||||
| Accrued royalties | $ | 547,000 | — | $ | 547,000 | — | — | — | — | |||||||||||||||||||
| Total | $ | 801,000 | $ | 77,000 | $ | 695,000 | $ | 13,000 | $ | 13,000 | $ | 3,000 | — | |||||||||||||||
In addition to cash generated from product sales, we believe our existing cash and cash equivalents, together with the estimated net proceeds from this offering, will be sufficient to meet our anticipated cash requirements for at least the next two years. However, future research and development, clinical trials and sales and marketing expenses, as well as administration support, may require additional capital resources. We may raise additional funds through public or private equity offerings, debt financings, capital lease transactions, corporate collaborations or other means. Due to the uncertainty of financial markets, financing may not be available to us on acceptable terms or at all. Therefore, we may raise additional capital from time to time due to favorable market conditions or strategic considerations even if we have sufficient funds for planned operations.
Our future capital requirements are difficult to forecast and will depend on many factors, including:
If at any time sufficient capital is not available, either through existing capital resources or through raising additional funds, we may be required to delay, reduce the scope of, eliminate or divest one or more of our research, clinical, sales and marketing programs or our entire business.
RECENT ACCOUNTING PRONOUNCEMENTS
In January 2003, the Financial Accounting Standards Board, or FASB, issued InterpretationAmendment No. 46,Consolidation of Variable Interest Entities(FIN 46). FIN 46 requires a variable interest entity to be consolidated by a company if that company is subject to a majority of the risk of loss from the variable interest entity’s activities or entitled to receive a majority of the entity’s residual returns or both. A variable interest entity is a corporation, partnership, trust or any other legal structure used for business purposes that either (a) does not have equity investors with voting rights or (b) has equity investors that do not provide sufficient financial resources for the entity to support its activities. Our adoption of the requirements of FIN 46 did not have an impact on our financial position or results of operations.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
To date, all of our sales have been denominated in US dollars. Accordingly, we believe that there is no material exposure to risk from changes in foreign currency exchange rates.
We hold no derivative financial instruments and do not currently engage in hedging activities.
Our exposure to interest rate risk at December 31, 2003 and September 30, 2004 is related to the investment of our excess cash into highly liquid financial investments with original maturities of three months or less. We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments. Due to the short term nature of our investments, we have assessed that there is no material exposure to interest rate risk arising from them.
Business
OVERVIEW
We design, develop, manufacture and market innovative products for women’s health. Our initial focus is on reproductive healthcare, using our proprietary technologies to predict preterm birth and assess infertility. Our principal product is a patented diagnostic test, the Fetal Fibronectin Test, that utilizes a single-use, disposable cassette, and is analyzed on our patented instrument, the TLiIQ System. This test is approved by the FDA for broad use in assessing the risk of preterm birth.
Our Fetal Fibronectin Test is designed to objectively determine a woman’s risk of preterm birth by detecting the presence of a specific protein, fetal fibronectin, in vaginal secretions during pregnancy. Testing for fetal fibronectin during pregnancy provides a more accurate assessment of the likelihood of a preterm birth than traditional methods. According to the New England Journal of Medicine, preterm births have historically accounted for up to 85% of all pregnancy-related complications and deaths in the United States. The March of Dimes estimated that over $13 billion in costs were associated with the care of preterm or low birth weight infants in 2001. By correctly identifying women at risk for preterm birth, we believe our Fetal Fibronectin Test leads to improved patient care and significant cost savings and has the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women.
Healthcare providers have historically had difficulty with accurately predicting when a woman is likely to give birth. Data from numerous clinical studies have demonstrated that our Fetal Fibronectin Test has a greater predictive value than traditional risk assessment methods for identifying women at risk of preterm birth. For example, a negative Fetal Fibronectin Test for a woman presenting with signs and symptoms of preterm labor indicates a 99.5% probability that she will not deliver in the next 7 days. A negative test result enables the healthcare provider to avoid unnecessary and costly hospitalization and drug treatment. Although a positive Fetal Fibronectin Test result does not have the same predictive value as a negative test result, if the Fetal Fibronectin Test result is positive, the healthcare provider may proactively prescribe various treatments to delay or manage preterm labor and birth.
The patient population for which our Fetal Fibronectin Test is approved can be divided into three patient categories. The first category consists of women who present with signs and symptoms of preterm labor and are typically directed to the hospital. The second and third categories include women designated as either “high-risk” or “low-risk” for preterm birth by their healthcare providers, and who currently exhibit no signs and symptoms of preterm labor. We believe that by using the Fetal Fibronectin Test periodically during a pregnancy, healthcare providers can more accurately assess the likelihood that women in all three categories will not deliver preterm.
As of September 30, 2004, we have shipped more than 1,270 TLiIQ Systems and over 1.1 million Fetal Fibronectin Test cassettes for use in hospital and clinical laboratories. As of September 30, 2004, our direct sales force consisted of 67 representatives who sell to hospital and clinical laboratories, health plans and healthcare providers. Our Fetal Fibronectin Test has been assigned a reimbursement code used for insurance processing of claims for the Fetal Fibronectin Test, and we believe that reimbursement for our Fetal Fibronectin Test has been regularly available through health plan organizations and most state Medicaid programs.
We also market and sell the E-tegrity Test, an infertility-related test based on a proprietary analyte specific reagent, to assess receptivity of the uterus to embryo implantation in women with unexplained infertility. The E-tegrity Test can be particularly useful for women who are considering assisted reproductive technologies, including in vitro fertilization, or IVF. We are also seeking to expand the indications for use of our Fetal Fibronectin Test for predicting successful induction of labor, for predicting delivery at term and for diagnostic applications in oncology, including bladder cancer.
OVERVIEW OF TARGETED MARKETS
Preterm birth
There are approximately four million births in the United States annually. Births occurring before 37 weeks of pregnancy are defined as preterm, and in recent years, according to a 2003 publication by the Center for Disease Control and Prevention, or CDC, preterm births represent approximately 12% of all births. On average, this equates to over 1,300 preterm births per day, or 480,000 preterm births per year. According to the Centers for Disease Control and Prevention, the percentage of preterm births in the United States grew to 12% of all births in 2002, an increase of 29% since 1981. This increase in the preterm birth rate is a growing public health concern. In January 2003, the March of Dimes launched a five-year, $75 million campaign to reduce the number of preterm births.
According to a 1994 publication from the National Conference of State Legislators, the costs of newborn intensive care in the United States ranged between $20,000 and $400,000 per infant. According to a 2001 study from the March of Dimes, the average hospital charge for preterm/low birth weight infants was $75,000, compared to $1,300 for an uncomplicated newborn stay. Infants born preterm often receive specialized care in a neonatal intensive care unit, or NICU, with charges ranging from approximately $800 to $2,700 per day in 1998. In addition, medical costs following discharge for preterm births can be substantial as a result of ongoing physical and mental developmental complications. We believe medical costs can be reduced if women at risk of preterm birth could be identified earlier and appropriately treated.
Preterm birth market segments
Women that are evaluated and potentially treated for preterm birth fall into three categories:
Current treatments and interventions for preterm birth
Identification of women at risk for preterm birth can be critical because the use of certain interventions to delay preterm birth may improve infant survival rates and reduce the severity of complications. Interventions may include hospitalization, consultation with a highly-skilled specialist, transport to a more advanced NICU facility, drug treatments such as tocolytics, which are used to delay the onset of birth by reducing contractions, corticosteroids for acceleration of fetal lung development, administration of progesterone to reduce the likelihood of preterm birth, antibiotics, and lifestyle modifications, including bed rest and discontinuing work.
There is ongoing research into the development of medications to further slow or prevent preterm births. For example, a multi-center, randomized, controlled, clinical trial was conducted by the National Institutes of Health and published in the New England Journal of Medicine in 2003 that used a progesterone formulation to prevent preterm birth in a group of “high-risk” women with a prior preterm birth. The study showed that in women with a prior preterm birth, there was a reduction in preterm birth by more than 33% in the treatment group as compared to a group treated with a placebo.
Infertility
According to the CDC National Center for Health Statistics, approximately 6.1 million women in the United States are affected by some form of infertility. It was estimated that approximately 1.2 million women had an infertility appointment in 1995 in the United States. Based upon a 1998 publication by the American Society for Reproductive Medicine, we believe approximately 10% to 15% of infertile women are classified as suffering from unexplained infertility where extensive tests for known factors have failed to reveal a cause. Infertile women are candidates for assisted reproductive technologies, including IVF.
According to the American Society of Reproductive Medicine, the average cost of an IVF cycle is $12,400. Receptivity of the uterus to undergo implantation varies by patient, and we are unaware of any current methods by which to evaluate women on this basis. The ability to predict good candidates for IVF procedures would help prevent unnecessary and costly IVF cycles.
Induction of labor
Induction of labor is the process by which medications and other treatments are used to initiate labor and delivery. According to a 2003 publication by the CDC, in 2002, 820,000 of the estimated four million births in the United States were induced. The same publication indicated that the percentage of induced labor more than doubled from approximately 9% in 1989 to approximately 21% in 2002. Induction of labor may be required for certain maternal or fetal conditions. In addition, we believe a number of inductions are elective in nature and performed for the convenience of the patient or healthcare provider. Healthcare providers have traditionally assessed women as candidates for successful induction of labor through the presence of certain clinical characteristics such as softness, dilation and thickness of the woman’s cervix. These clinical characteristics are not always reliable
We believe the current use of subjective evaluation techniques to predict the successful induction of labor contributes to the annual cesarean section rate in the United States. Based upon a 2003 publication by the CDC, cesarean sections represented 26% of all births in the United States in 2002. This is the highest rate ever reported in the United States and has risen 26% since 1996. Delivery by cesarean section typically results in costs of approximately $2,000 more than vaginal delivery based on a 2002 article published by the American Journal of Obstetrics and Gynecology. In addition, unsuccessful induction of labor may be associated with medical complications for both the mother and infant, as well as increased hospital stays and neonatal costs. In addition, women who undergo a cesarean section are often encouraged to continue to have cesarean sections in subsequent pregnancies. An objective diagnostic test to assist healthcare providers in predicting the successful induction of labor would help improve labor success rates and reduce unnecessary cesarean sections.
Oncology — bladder cancer
According to a 2003 publication in the Journal of Clinical Ligand Assay, bladder cancer is the fifth most common cancer in the United States. The American Cancer Society estimates that there will be more than 60,000 new cases diagnosed in the United States in 2004. The National Cancer Institute found in a 1995 study that the existing patient population for bladder cancer is approximately 500,000 cases. According to a 2004 article published in Clinical Chemistry, following treatment, even patients initially diagnosed with superficial tumors must be monitored closely as two-thirds of these patients will experience a recurrence within five years and almost 90% will have a recurrence within 15 years. Current diagnostic tools and techniques include visual observation through cystoscopy, evaluation of potential cancer cells through cytology and assessment of tissue biopsies. There are several FDA-cleared tests for monitoring patients and a limited number of tests that have been approved by the FDA for use in screening for bladder cancer. However, these tests detect bladder cancer with varying degrees of success and are generally more successful in detecting more advanced cancers. We believe there is a market opportunity for a more accurate and reliable test to monitor and screen for bladder cancer at an early stage.
FETAL FIBRONECTIN OVERVIEW
Fetal fibronectin is a protein expressed in the fetal membranes and placenta at the interface between the mother and fetus. Fetal fibronectin is believed to play a role in the adhesion of the fetal membranes to the wall of the uterus. In a normal pregnancy, the level of fetal fibronectin in vaginal secretions is typically elevated through weeks 16 to 20 of gestation as the fetal membranes adhere to the uterine wall. From week 20 through week 35 of gestation, fetal fibronectin levels are typically low. As the pregnancy reaches term, the fetal fibronectin level rises significantly. Therefore, low levels of fetal fibronectin in vaginal secretions between week 20 and week 35 of gestation are highly correlated with a low risk of preterm birth, while high levels of fetal fibronectin during this time period indicate a greater risk of preterm birth. Testing for the presence or absence of fetal fibronectin enables healthcare providers to identify women at risk for preterm birth, and may also be useful in predicting the successful induction of labor.
Fetal fibronectin has also been shown to be present in certain forms of cancer. In oncology, fetal fibronectin is referred to as oncofetal fibronectin. The protein is expressed in several forms of cancer where its adhesive properties may help the cancer to attach to tissue and grow. Oncofetal fibronectin can potentially be detected in cancerous tissues or in fluids that come into contact with those tissues.
THE ADEZA SOLUTION
We believe that our proprietary, FDA-approved diagnostic test and instrument, the single-use, disposable Fetal Fibronectin Test and the TLiIQ System have the potential to fundamentally change how healthcare providers select the appropriate course of treatment for pregnant women and to become a standard of care for use in pregnancy. The clinical efficacy of our fetal fibronectin test for preterm birth has been demonstrated in numerous peer-reviewed clinical publications, including the Peaceman et al. trial, published in the American Journal of Obstetrics and Gynecology, and the Goldenberg et al. trial, published in the American Journal of Public Health and Obstetrics & Gynecology, two multi-center clinical trials. Our Fetal Fibronectin Test was used to obtain the results described in each of these publications. In addition, cost savings resulting from the use of our test have also been confirmed in peer-reviewed publications such as articles published by Joffe et al. and Giles et al. in the American Journal of Obstetrics and Gynecology.
The Fetal Fibronectin Test and the TLiIQ System have the following key characteristics:
We market the Fetal Fibronectin Test to healthcare providers for women who are seeking urgent medical care for signs and symptoms of preterm labor in the hospital. While a woman5 is being evaluated, the Fetal Fibronectin Test is run in the hospital laboratory with the result generated in less than 25 minutes. A negative Fetal Fibronectin Test for women with signs and symptoms of preterm labor effectively rules out the chance of preterm birth in the next seven days, with a 99.5% probability, as reported by Peaceman et al. in the American Journal of Obstetrics and Gynecology and incorporated in our FDA labeling. We believe this avoids unnecessary hospitalization, medications, hospital transport, or bed rest. A positive Fetal Fibronectin Test provides the healthcare provider with a more accurate assessment of who will deliver preterm in the next seven days than traditional risk factors. The probability of a preterm birth within seven days of a positive Fetal Fibronectin Test is 12.7%, according to data collected by Peaceman et al. and incorporated in our FDA labeling. By comparison, the Peaceman et al. data indicates that traditional risk factors such as genital tract infection, uterine activity, vaginal bleeding and cervical dilation have positive predictive values of only 1.7%, 6.3%, 7.6% and 8.5%, respectively. If the Fetal Fibronectin Test is positive, the healthcare provider may proactively prescribe various treatments to delay or manage preterm labor and birth.
We also market the Fetal Fibronectin Test to healthcare providers for women who have been designated as “high-risk.” These women should be carefully monitored throughout their pregnancy, and a Fetal Fibronectin Test can be used multiple times in their pregnancy during their frequent visits to their healthcare provider’s office. In those cases, fetal fibronectin samples are collected from women in healthcare providers’ offices and picked up for testing by a clinical laboratory. Results are typically returned to the healthcare provider within 24 hours. The use of the Fetal Fibronectin Test for this patient population helps to identify women who are not at risk of delivering preterm.
In addition, we believe that our product has the potential to be used in routine patient visits for women who have been designated as “low-risk” by their healthcare providers. According to a 2001 article published in the American Journal of Obstetrics and Gynecology, over 50% of all preterm births occur when no major risk factors are present. Our Fetal Fibronectin Test may provide an
In these “high-risk” and “low-risk” patient categories, a large, multi-center, peer reviewed clinical study by Goldenberg, et al. in the American Journal of Obstetrics and Gynecology comparing the most common predictors of preterm birth demonstrated that the Fetal Fibronectin Test was the single strongest predictor of preterm birth at less than 35 weeks of pregnancy.
A peer-reviewed, cost-benefit study published in 1999 by Joffe, et al. in the American Journal of Obstetrics and Gynecology addressed the potential impact of the Fetal Fibronectin Test in a hospital system. Data obtained from a year when the Fetal Fibronectin Test was utilized was compared to the prior year when the test was not available. Use of the Fetal Fibronectin Test resulted in a significant cost savings for the hospital system by reducing preterm labor hospital admissions, length of stay, and prescriptions for tocolytic agents. Preterm labor hospital admissions alone were decreased by approximately 40%.
We believe our potential aggregate market size is defined by the number of pregnant women who are potential candidates for the Fetal Fibronectin Test and the number of tests they may receive during their pregnancies. We believe that every year approximately 800,000 women with signs and symptoms of preterm labor are candidates for one Fetal Fibronectin Test per episode during their hospital visit, and that there are approximately 1.2 million women each year who are designated as “high-risk” and who are candidates to be tested three times, on average, during their regularly scheduled office visits. We estimate that these two patient populations represent a total market opportunity of approximately 4.4 million tests, or over $400 million, annually. If we are able to expand the use of the Fetal Fibronectin Test with the approximately 2.8 million women per year who we believe are designated as “low-risk” and who are candidates to be tested three times, on average, during their regularly scheduled office visits, this would represent an additional potential market opportunity of 8.4 million tests annually. We estimate that the potential aggregate annual market size for all Fetal Fibronectin Tests, including preterm and other uses, can be greater than $1 billion.
PRODUCTS
The following table summarizes information related to our principal products and certain products under development.
We also sell certain consumables related to our principal products and a fetal fibronectin test intended for certain international markets. None of these consumable or international specific products represent a material portion of our revenues.
Marketed products
Preterm birth products
Our Fetal Fibronectin Test has been approved by the FDA for assessing the risk of preterm birth. We manufacture and market the patented single-use, disposable Fetal Fibronectin Test, which is performed on our patented instrument, the TLiIQ System. The Fetal Fibronectin Test cassette is sold directly to hospital and clinical laboratories that perform the test and provide results to healthcare providers.
TLiIQ System.The TLiIQ System consists of the TLiIQ instrument and printer. The TLiIQ instrument is small and compact, approximately eight inches long by seven inches wide by three inches high, and is composed of:
The printer for the TLiIQ System generates a label with the patient test result.
Fetal Fibronectin Test cassette.The Fetal Fibronectin Test cassette is a single-use, dry chemistry cassette. All the necessary reagents for one test are contained within the cassette.
Test procedure.A healthcare provider collects a sample of the patient’s vaginal secretions using our Fetal Fibronectin Specimen Collection Kit. The Fetal Fibronectin Specimen Collection Kit contains a sterile polyester swab and specimen transport tube. The swab is placed into the transport tube, which contains a proprietary buffer solution that extracts and stabilizes the fetal fibronectin sample during transport. The transport tube containing the patient sample is sent to the hospital or clinical laboratory for analysis on the TLiIQ instrument. At the laboratory, the Fetal Fibronectin Test cassette is inserted into the chamber of the TLiIQ instrument, and then the patient sample is dispensed into the sample well to begin the Fetal Fibronectin Test. The TLiIQ instrument produces a positive or negative test result in less than 25 minutes and prints the test result on a label that can be affixed to the patient’s record. The Fetal Fibronectin Test can be easily run with minimal training of laboratory personnel required.
Infertility products
E-tegrity Test.The E-tegrity Test is a patented diagnostic test designed to determine receptivity of the uterus to embryo implantation. The E-tegrity Test identifies the presence or absence of a unique protein, alpha-v beta-3 integrin, important for implantation to occur. This unique protein is the basis of our proprietary analyte specific reagent, or ASR, on which the E-tegrity Test is based. If a woman is missing the alpha-v beta-3 integrin protein between days 20 to 24 of her menstrual cycle, the fertilized egg may not attach properly to the epithelial lining of the uterus. As a result, a woman’s chances of a successful pregnancy are significantly decreased.
The E-tegrity Test provides healthcare providers with a new method for potentially explaining the cause of a woman’s infertility. Women who may be helped by the E-tegrity Test include women that are having difficulty getting pregnant by natural means, women who are considering assistive reproductive technologies, and women who have already tried IVF without success. A negative test provides a potential reason for the woman’s infertility, and the healthcare provider can then initiate appropriate treatments to potentially increase the chance for successful embryo implantation. We believe the E-tegrity Test can provide significant cost savings by potentially reducing the number of failed IVF cycles. Peer-reviewed publications have shown that women missing alpha-v beta-3 integrin can benefit from drug therapy that improves uterine receptivity for embryo implantation. We perform the E-tegrity Test exclusively in our CLIA-certified laboratory, Adeza Diagnostic Services.
Products under development
Pregnancy products
Induction of labor
Preterm birth
Delivery date
Oncology products
We are exploring applications for oncofetal fibronectin in oncology and have initially focused our efforts in the area of bladder cancer. In addition, we are developing and evaluating protocols, and plan to perform preliminary feasibility studies for the use of oncofetal fibronectin for the detection of cervical and ovarian cancer.
Bladder cancer
We are developing an oncofetal fibronectin test for the detection of bladder cancer using urine samples. A third-party preliminary feasibility study conducted in Germany, published in 2001 in the peer-reviewed journal Oncology Reports, on 40 bladder cancer patients and 20 non-cancer control patients evaluated whether oncofetal fibronectin could be detected in the urine of bladder cancer patients using our test. The results of this study showed that 38 of the 40 bladder cancer patients tested positive for oncofetal fibronectin using our test (a 95% detection rate) while all 20 of the control patients tested negative. In this study, the relationship between a positive oncofetal fibronectin test and the presence of bladder cancer was statistically significant (p < .001). This preliminary feasibility study included a small number of patients, and later studies may not confirm the results from this study. We recently initiated another study in Germany to expand on the earlier study and to obtain further clinical data.
OUR STRATEGY
Our goal is to be a leader in the development and commercialization of proprietary diagnostic products for reproductive healthcare. In addition, we intend to commercialize products for diagnostic applications in oncology. We believe that our products can assist healthcare providers in making more timely and accurate diagnoses, leading to improved quality of care and significant cost savings for the healthcare system. The key elements of our strategy are to maximize the value of our commercialized products and build for future growth by continuing to develop our product pipeline. In order to effectively execute this strategy we plan to:
Leverage existing sales and marketing infrastructure to increase market penetration
As of September 30, 2004, our existing sales force consisted of 67 direct sales representatives. We intend to leverage these representatives to increase market penetration of our TLiIQ System in hospital and clinical laboratories as well as to increase the number of Fetal Fibronectin Tests run on each TLiIQ System within our current customer base. Our direct sales representatives call on healthcare providers in the hospital and office setting, as well as on clinical laboratories and health plans, to broaden the overall awareness of our Fetal Fibronectin Test and its associated benefits. Our sales representatives use peer-reviewed publications, cost-benefit data, case studies and other marketing materials to promote the benefits of our Fetal Fibronectin Test and drive increased utilization. In addition, our marketing organization has implemented a national speakers program where healthcare providers, such as
Increase marketing for women designated as “high-risk”
We have expanded our target market for the Fetal Fibronectin Test to include women designated as “high-risk” for preterm birth by their healthcare provider. Of our 67 direct sales representatives, 47 are primarily focused on healthcare providers in the office setting to drive utilization of our Fetal Fibronectin Test for “high-risk” women to address a need to more accurately assess risk at multiple times during pregnancy. As we further penetrate the “high-risk” market, we plan to increase the number of direct sales representatives focused on healthcare providers serving this patient population. In addition to targeting healthcare providers, we are increasing our direct marketing efforts to women and health plans for this “high-risk” patient population. We provide specific patient-focused material for placement in healthcare provider offices. We also conduct presentations to medical directors and case managers of health plan organizations, exhibiting the benefits of utilizing our Fetal Fibronectin Test in this “high-risk” patient population.
Expand marketing to “low-risk” patient population
As we further penetrate the market for women with signs and symptoms of preterm birth and women designated as “high-risk,” we plan to expand our focus to market our Fetal Fibronectin Test for routine patient visits in “low-risk” women. By increasing market penetration and continuing to educate healthcare providers on the benefits of our Fetal Fibronectin Test, we believe we have the potential to penetrate the “low-risk” patient population for the assessment of the risk of preterm birth. Because over 50% of all preterm births occur when no major risk factors are present, there is a need to more accurately assess risk at multiple times during pregnancy. Our Fetal Fibronectin Test could be used to predict the likelihood of preterm birth during a woman’s routine visits to her healthcare provider. Our sales and marketing efforts will ultimately be focused on penetrating this “low-risk” patient population for routine patient visits.
Expand international sales
We are currently focused on the US market, but we intend to expand into additional geographic regions. We currently sell directly into Canada and have relationships with approximately 15 international distributors in areas such as Mexico, Europe, the Pacific Rim and South America. Although we have not yet targeted specific foreign countries, we intend to hire additional sales representatives to focus on international markets and to increase our international distribution network through strategic partners and distributors that have significant presence and experience in their local markets. We believe that populous countries with high birth rates may have markets sufficiently large to justify investment in developing distribution channels there. We intend to begin the expansion of our international sales and distribution efforts within 12 months.
Seek additional indications for our Fetal Fibronectin Test
In addition to the current indications for our Fetal Fibronectin Test, we have submitted a PMA supplement for FDA review and approval to expand the labeling of the Fetal Fibronectin Test to be used as a predictor of the successful induction of labor. Of the more than 820,000 births in the United States annually that are induced, we believe a number are elective in nature due to patient or healthcare provider convenience. Many women endure prolonged dysfunctional induced labor, increased hospital stays, elevated risk of cesarean section and increased neonatal costs. Our Fetal Fibronectin Test may provide an objective diagnostic test to assist healthcare providers in predicting the successful induction of labor. In addition, we are developing a product to more accurately predict delivery date in full-term pregnancies.
Develop products for applications in oncology
We are developing an oncofetal fibronectin test for the detection of bladder cancer. The results from a preliminary feasibility clinical study in Germany suggests that bladder cancer can be detected in the urine of bladder cancer patients with our oncofetal fibronectin test. We recently initiated another study in Germany to expand on the earlier study and to obtain further clinical data from a larger patient population. In addition, we are developing and evaluating protocols for cervical and ovarian cancer detection studies.
Strategically acquire or in-license complementary businesses, products or technologies
In addition to our own product development efforts, we intend to expand our product portfolio by selectively acquiring or in-licensing complementary businesses, products or technologies that could be sold by our existing direct sales force. For example, we acquired the exclusive rights to the SalEst Test in late 2003. We are continually identifying and evaluating new opportunities that would meet these criteria and further strengthen or expand our current programs.
SALES AND MARKETING
We focus our sales and marketing efforts on increasing awareness of our products and services among healthcare providers, hospitals, laboratories, health plans, and patients, where we have initiated branding of our test as Full Term, The Fetal Fibronectin Test. Our strategy is to sell and market our products through our direct sales force in the United States and Canada, and distributors worldwide. We choose our partners and distributors on a country-by-country basis. As of September 30, 2004, we had 67 direct sales representatives in North America, including 16 who have a primary focus on hospital and laboratory sales, 47 who have a primary focus on healthcare providers in the office setting, three health plan sales representatives and one sales representative covering Canada. Our direct sales force includes some full-time sales representatives provided to us by a third party, who are directly managed by us, sell our products exclusively and are an integral part of our sales team.
US sales and marketing
Our hospital and clinical laboratory sales representatives focus primarily on selling the TLiIQ System and Fetal Fibronectin Test to hospital and clinical laboratories, including some of the leading national laboratories in the United States. Our healthcare provider sales representatives focus on the estimated 1,300 maternal-fetal medicine specialists, who focus on high-risk pregnancies, 25,000 Ob/ Gyn physicians, as well as nurses and midwives. Our health plan sales representatives focus on chief medical officers, medical directors, and case managers of health plans.
We are focused on increasing healthcare provider, hospital, laboratory, health plan, and patient awareness of the Fetal Fibronectin Test and its associated benefits through our direct sales and marketing efforts. In our selling process, we use peer-reviewed publications, cost-benefit data and case studies. Peer-to-peer selling is also a critical element of our strategy. Our marketing organization has implemented a national speakers program where healthcare providers such as maternal-fetal medicine specialists, obstetricians, nurses, and midwives make educational presentations at hospitals, professional meetings, and conferences to increase awareness of our Fetal Fibronectin Test. We also conduct presentations at health plan organizations to medical directors and case managers. Our marketing professionals support sales of our Fetal Fibronectin Test with product literature and training materials for healthcare providers, laboratories, and health plans.
An additional element of our educational efforts is our relationship with the March of Dimes. We are a national corporate supporter of the March of Dimes Prematurity Campaign. The goals of our relationship are to educate families on the problems associated with preterm birth, as well as to highlight the immediate and long term costs of preterm birth for health plans, through co-branded educational brochures and medical seminars.
International sales and marketing
Our international sales and marketing efforts address the particular healthcare systems of individual countries through a network of approximately 15 international distributors with expertise in their markets. Our internal marketing professionals support these distributors. We intend to expand our international marketing efforts by increasing our international direct sales force, as well as broadening our international distribution network through strategic partners and distributors that have significant presence and experience in their local markets. In Japan and South Korea, we have a strategic partnership with Daiichi Pure Chemicals Co. Ltd.
We also manufacture and market other fetal fibronectin test formats that are sold in international markets and are designed to meet certain criteria specific to these markets.
MANUFACTURING
We conduct a majority of the manufacturing for our Fetal Fibronectin Test cassettes and TLiIQ System at our 17,600 square foot manufacturing facility, located in Sunnyvale, California. This facility is subject to the current good manufacturing practices, or cGMP, enforced by the FDA. The manufacturing process for our products includes assembly, testing, packaging, labeling, component inspection and final inspection of products that have been manufactured by us or to our specifications by outside contractors. Our quality assurance group independently inspects our products to verify that all components and finished products comply with our specifications and applicable regulatory requirements.
We are licensed by the State of California, Department of Health Services Food and Drug Branch and are also registered with the FDA as a device manufacturer.
We purchase components for our Fetal Fibronectin Test and TLiIQ System products from various suppliers. The components we purchase are generally available from more than one supplier. For those components for which there are relatively few alternate sources of supply, we believe that even in the event of a disruption of supply of any required materials, we could establish additional or replacement sources of supply without materially interrupting the availability of our products.
Our quality assurance systems are required to be in conformance with the Quality System Regulations, or QSR, as mandated by the FDA. We have ISO 13485 certification for our quality system which is required in Canada. Our products are CE marked in accordance with the European In Vitro Diagnostic Directive.
RESEARCH AND DEVELOPMENT
Our research and development efforts are conducted internally and through collaborations with academic investigators and clinicians. Our research and development is focused on enhancements to existing products and developing additional products within women’s health, including pregnancy and infertility, and in oncology. As of September 30, 2004, we had 11 employees conducting research and development.
GOVERNMENT REGULATION
United States
The research, development, manufacture, labeling, distribution and marketing of our products are subject to extensive regulation by the Food and Drug Administration and other regulatory bodies. The FDA regulates our currently marketed products as medical devices and we are required to obtain review and clearance or approval from the FDA prior to commercializing our devices.
FDA’s premarket clearance and approval requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval, or PMA, from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risk are placed in either Class I or II, which requires the manufacturer to submit to the FDA a premarket notification requesting permission for commercial distribution of a device that is substantially equivalent to a predicate device that has already received 510(k) clearance or was commercialized prior to May 28, 1976. This process is known as 510(k) clearance and was used for authorization of our Fetal Fibronectin Specimen Collection Kit. Some low-risk Class I devices are exempt from the 510(k) requirement altogether. Devices deemed by the FDA to pose greater risk, or devices deemed not substantially equivalent to a previously cleared 510(k) device are placed in Class III, most of which require premarket approval, such as our Fetal Fibronectin Test and TLiIQ System. Both premarket clearance and premarket approval applications are subject to the payment of user fees, paid at the time of submission for FDA review.
510(k) clearance pathway
To obtain 510(k) clearance, a premarket notification must be submitted, demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications. The FDA’s 510(k) clearance pathway usually takes from three to six months from the date the application is submitted, but it can take significantly longer.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new 510(k) clearance or could require premarket approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If the FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
Premarket approval pathway
A PMA application must be submitted if the device cannot be cleared through the 510(k) process, and is usually utilized for Class III medical devices, or devices that pose a significant safety risk, including unknown risks related to the novelty of the device. A PMA application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. Technical performance data required forin vitro diagnostic device PMA applications may include validation of the performance of hardware and software under repeat testing, calibration of mechanical components and stability of reagents and other products used in specimen collection, storage and testing. Preclinical trials may include tests to determine product stability and biocompatibility, among other features. Preclinical studies must be conducted in accordance with Good Laboratory Practices. PMA clinical trials are conducted under an Investigational Device Exemption and are designed to demonstrate the performance characteristics of the device relating to safety and effectiveness for the commercially intended use.
After a PMA approval application is complete, the FDA begins an in-depth review of the submitted information, which generally takes between one and three years, but may take significantly longer. During this review period, the FDA may request additional information or clarification of information already provided. For example, the FDA has placed its review of our PMA supplement seeking
Also during the PMA review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. New PMA applications or PMA supplements are required for significant modifications to the manufacturing process, labeling or design of an approved device. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA, and may not require as extensive clinical data or the convening of an advisory panel, which makes recommendations to the FDA concerning the approval of a PMA.
In vitro diagnostic medical devices
In addition to our FDA-approved and cleared medical devices in distribution, we provide the E-tegrity Test as a test run in our own CLIA-certified laboratory, Adeza Diagnostic Services, for the detection of defects in uterine receptivity. This in-house test is permitted to utilize an analyte specific reagent, an ASR or active ingredient, the use of which has been validated in our clinical laboratory. While we receive specimens in our lab for the E-tegrity Test, the active ingredient of the test itself has not been validated outside of, and is not marketed outside of, our lab. Under the FDA’s requirements, E-tegrity Test results are provided with a disclaimer concerning the absence of an FDA requirement for clearance or approval of ASRs. We do not intend to seek FDA clearance or approval for broader use of the E-tegrity Test.
Continuing FDA regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
We are also subject to unannounced inspections by the FDA and the Food and Drug Branch of the California Department of Health Services.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA approval, and the requirements may differ.
INTELLECTUAL PROPERTY
Protection of our intellectual property is a strategic priority for our business, and we rely on a combination of patent, trademark, copyright and trade secret laws to protect our interests. Our ability to protect and use our intellectual property rights in the continued development and commercialization of our technologies and products, operate without infringing the proprietary rights of others, and prevent others from infringing our proprietary rights, is crucial to our continued success. We will be able to protect our products and technologies from unauthorized use by third parties only to the extent that they are covered by valid and enforceable patents, trademarks or copyrights, or are effectively maintained as trade secrets, know-how or other proprietary information.
We seek US and international patent protection for our reagents, collection kits, diagnostic systems and other components of our products, and all other commercially important technologies we develop.
We devote significant resources to obtaining, enforcing and defending patents and other intellectual property and protecting our other proprietary information. We have already obtained patents or filed patent applications on a number of our technologies, including important patents and patent applications relating to fetal fibronectin, our TLiIQ System, and the Fetal Fibronectin Test. If valid and enforceable, these patents may give us a means of blocking competitors from using similar or alternative technology to compete directly with our products. We also have certain proprietary trade secrets that are not patentable or for which we have chosen to maintain secrecy rather than file for patent protection. With respect to proprietary know-how that is not patentable, we have chosen to rely on trade secret protection and confidentiality agreements to protect our interests.
We believe that our portfolio of issued patents and patent applications, together with our exclusively licensed patents described under “License and Other Agreements”, provides patent coverage for our proprietary technologies and products. As of September 30, 2004, our intellectual property estate consisted of 157 issued patents or patent applications, as follows:
Each of the foreign filings corresponds in subject matter to a US patent filing. We solely own or have exclusively licensed all of the patents and patent applications set forth above.
Our patent portfolio as of September 30, 2004 is summarized in the following table:
| Foreign patents | Foreign applications | |||||||||||||||
| Category | US patents granted | US applications filed | granted | filed | ||||||||||||
| Methods of Use | 14 | 7 | 54 | 7 | ||||||||||||
| Detection Systems | 8 | 3 | 46 | 3 | ||||||||||||
| Platforms/Other Devices | 6 | 3 | 2 | 4 | ||||||||||||
| Total | 28 | 13 | 102 | 14 | ||||||||||||
We believe that our portfolio of issued patents and patent applications provides patent coverage for our proprietary technologies and products. We have patents and patent applications relating to our Fetal Fibronectin Test and TLiIQ System in the categories of methods of use, detection systems, platforms and other devices. Our family of issued patents and patent applications, if and when issued, relating to our Fetal Fibronectin Test and TLiIQ System have a range of expiration dates from 2007 to 2025. Although patents to a reagent used in the Fetal Fibronectin Test expire in 2007, we have additional issued patents that relate to the Fetal Fibronectin Test and the TLiIQ System. Thus, we believe that our currently issued patents provide protection for our Fetal Fibronectin Test to 2009 by protecting the method upon which the test is based and to 2011 by protecting various reagents and kit embodiments employed in the test. In addition, we have issued patents relating to our TLiIQ System and the associated Fetal Fibronectin Test that expire in 2018. We have filed patent applications that, if and when issued, could provide protection for measurement and detection of fetal fibronectin for current and additional indications. These patents, if and when issued, could expire as late as 2025. With respect to our E-tegrity Test, we have exclusively licensed patents and pending patent applications involving detection of beta-3 integrin subunit as an infertility/ fertility indicator. The issued patents that involve detection of beta-3 integrin subunit have an expiration of 2012, and any patents that issue based upon the pending applications should have an expiration date of 2012.
LICENSE AND OTHER AGREEMENTS
While we own much of our intellectual property, including patents, patent applications, trademarks, copyrights, trade secrets, know-how and proprietary information, we also license related technology of importance to commercialization of our products. To continue developing and commercializing our current and future products, we may license intellectual property from commercial or academic entities to obtain the rights to technology that may be complementary to, or required for, our research, development and commercialization activities.
In August 1992, we entered into an amended and restated license agreement with the Fred Hutchinson Cancer Research Center under which we were granted a worldwide, exclusive license to a US patent and corresponding foreign patents related to fetal fibronectin and antibodies made against fetal fibronectin. These licensed patents are used in our Fetal Fibronectin Test, and may be used in other fetal fibronectin or oncofetal fibronectin products that we develop. We are obligated to pay royalties to the Hutchinson Center on net product sales in the United States by us during the remainder of the term of a licensed patent, subject to an annual minimum royalty of $10,000, and to date, we have paid approximately $1.1 million. We are also obligated to indemnify the Hutchinson Center for certain claims related to the license agreement. The agreement terminates upon the expiration of the licensed patents in 2007, subject to earlier termination by either party in the event that the other party materially breaches the agreement and fails to correct the breach in a timely manner.
In December 1998, we entered into a license agreement with Unilever plc, which was subsequently assigned to Inverness Medical Inc., under which we were granted a non-exclusive license to five US patents and corresponding foreign patents, relating to the chemistry of our Fetal Fibronectin Test, which expire in 2014. We are obligated to pay royalties on net sales of our Fetal Fibronectin Test until the expiration of the last to expire of the licensed patents, provided that our Fetal Fibronectin Test
We were previously party to a marketing agreement with Matria Healthcare, which was terminated as of March 1998 pursuant to the terms of an Agreement and Release. Under the terms of the Agreement and Release, we agreed to pay Matria royalties on sales of certain of our products. To date, we have paid approximately $0.4 million. This agreement has no expiration.
In July 1997, we entered into a license agreement with the University of Pennsylvania under which we were granted a worldwide, sublicensable, exclusive license to three US patents and corresponding foreign patents relating to the use of a specific protein found in the lining of the uterus as a predictor of endometriosis and for the determination of uterine receptivity toward embryo implantation. Our E-tegrity Test incorporates the technology covered by these patents, which expire in 2012 and 2013. We are obligated to pay royalties to the university on net sales of our E-tegrity Test during the remainder of the term to the last to expire of the licensed patents, subject to an annual minimum royalty equal to the greater of $10,000 or 20% of the royalties paid to the university in the prior year, and to date, we have paid approximately $0.2 million. The agreement terminates upon the expiration of the last to expire of the licensed patents, subject to earlier termination by the university in the event that we materially breach the agreement and fail to correct the breach in a timely manner, fail to pay royalties when due or file for bankruptcy.
We also have agreements with other third parties pursuant to which we have royalty-bearing, non-exclusive licenses to patents held by those third parties.
COMPETITION
Rapid product development, technological advances, intense competition and a strong emphasis on proprietary products characterize the medical devices and diagnostic products industries. Our products could be rendered obsolete or uneconomical by the introduction and market acceptance of competing products, by technological advances of potential competitors or by other approaches.
We are currently the only provider of a fetal fibronectin test for predicting preterm birth. However, we could experience competition for our preterm birth diagnostic products from companies that manufacture and market pregnancy-related diagnostic products and services. These companies may be significantly larger and have access to substantially more capital for new product development and sales and marketing. These companies may develop new diagnostic products or technologies that could compete with or entirely displace our products and technologies. For example, other biomarkers, including cytokines and other proteins indicative of infection, and proteomics are the subject of research that may yield new products or technologies.
In addition, healthcare providers use diagnostic techniques such as clinical examination and ultrasound to diagnose the likelihood of preterm birth. Healthcare providers may choose to continue using these techniques to assess their patients, rather than use our Fetal Fibronectin Test. They may also choose to use these techniques in conjunction with our Fetal Fibronectin Test to predict preterm birth.
We believe that in light of the increased rate of preterm birth, cesarean section delivery and assisted reproductive procedures, the market for our products is growing. As a result, we expect additional competition from companies with greater financial, managerial, and technical resources than we have.
EMPLOYEES
As of September 30, 2004, we had 83 employees, including 44 in sales, marketing and business development, 13 in manufacturing and laboratory services, 11 in research and development, 10 in administration and 5 in quality assurance. In addition, our direct sales force includes some full-time sales representatives provided to us by a third party, who are directly managed by us, sell our products exclusively, and are an integral part of our sales team. The agreement with such third party is currently scheduled to expire on May 14, 2006. None of our employees is represented by a labor union or is covered by a collective bargaining agreement. We have never experienced any employment-related work stoppages and consider our employee relations to be good. We also employ independent contractors to support our development, regulatory, sales, marketing and administrative activities.
FACILITIES
We maintain our headquarters in Sunnyvale, California in one leased facility of approximately 17,600 square feet, which contains laboratory, research and development, manufacturing, sales and marketing, and general administration. We believe that our existing facilities are adequate to meet our immediate needs and that suitable additional space will be available in the future on commercially reasonably terms as needed.
LEGAL PROCEEDINGS
We are not currently party to any material legal proceedings.
Management
EXECUTIVE OFFICERS, SIGNIFICANT EMPLOYEES, AND DIRECTORS
Set forth below is the name, age, position and a brief account of the business experience of each of our executive officers and directors as of December 3, 2004.
Emory V. Andersonhas been our President and Chief Executive Officer since February 1997. From October 1992 to February 1997, Mr. Anderson was our Vice President and Chief Financial Officer. Prior to joining us, Mr. Anderson served as Executive Vice President and Chief Operating Officer of Indesys, Inc., which he co-founded in 1984. Previously, he held the position of Director of Finance for Atari, Inc.
Mark D. Fischer-Colbriehas been our Vice President of Finance and Administration and Chief Financial Officer since February 2001. From March 1992 to January 2001, Mr. Fischer-Colbrie served as Vice President, Finance and Administration and Chief Financial Officer for KeraVision, Inc., a company that filed for bankruptcy under federal bankruptcy laws in March 2001. He also held several financial positions at Maxtor Corporation from April 1986 through February 1992, including Vice President of Finance and Corporate Controller.
Durlin E. Hickok, MD, MPH,has been our Vice President of Medical Affairs since November 1998. From 1996 to 1998, Dr. Hickok was Vice President and Medical Director of Omnia, Inc., a women’s healthcare management company. He was also Chief of Obstetrics and Gynecology at the Virginia Mason Medical Center from 1993 to 1996, and Associate Director of Perinatal Medicine at Swedish Hospital Medical Center in Seattle, Washington from 1982 to 1993. Previously, he was an Assistant Professor at the University of Washington.
Robert O. Hussa, PhD,has been our Vice President of Research and Development since May 1993. From January 1990 to May 1993, Dr. Hussa was Vice President of Imaging and Therapeutics Research and Development at Hybritech, Inc. From June 1986 to December 1989, he was Director of Assay Development at Hybritech. Prior to joining Hybritech, Dr. Hussa was a Professor of Gynecology & Obstetrics and of Biochemistry at the Medical College of Wisconsin for 18 years.
Marian E. Saccohas been our Vice President of Sales and Marketing since September 1997. From 1996 to 1997, Ms. Sacco was the Vice President of Marketing at Behring Diagnostics. Previously, Ms. Sacco was the Director of Worldwide Oncology Business and Worldwide Marketing Manager for CBA Corning/ Chiron Diagnostics from 1991 to 1996, and was the US Sales and Marketing Manager for Centocor Diagnostics from 1987 to 1991.
Andrew E. Senyei, MD, Chairman of the Board, has served as one of our directors since 1987. Dr. Senyei has been a Managing Director and a General Partner of Enterprise Partners, a venture capital firm, since 1987. Dr. Senyei was a founder of Molecular Biosystems and, prior to joining Enterprise Partners, was a practicing clinician and Adjunct Associate Professor of Obstetrics, Gynecology and Pediatrics at the University of California at Irvine. He serves on the boards of directors of numerous private healthcare companies.
Nancy D. Burrushas served as one of our directors since December of 1994. Ms. Burrus has been a general partner of Suez Ventures, a venture capital firm, since 1990. Suez Ventures manages STF II, L.P., one of our institutional shareholders. Prior to joining Suez Ventures, Ms. Burrus was a Vice President with Morgan Stanley Ventures. She serves on the boards of directors of several private companies.
Craig C. Taylorhas served as one of our directors since 1986. Mr. Taylor heads the life science investments at Alloy Ventures and has been active in venture capital since 1977 when he joined Asset Management Company. Alloy Ventures is the institutional fund management successor to Asset Management Company, a venture management firm founded in 1965. He serves on the boards of directors of Pharmacyclics and Lynx Pharmaceuticals, both publicly held companies, and several private companies.
Kathleen D. LaPortehas served as one of our directors since April of 2002. Ms. LaPorte has been a general partner of Sprout Group, a venture capital firm, since 1994. Prior to joining the Sprout Group, Ms. LaPorte was a principal at Asset Management Company, a venture capital firm focused on early stage investments. She has also worked as a financial analyst with The First Boston Corporation. She is the past President, and a member of the Board of the Western Association of Venture Capitalists. She serves on the board of directors of VNUS Medical Technologies, Inc. and ISTA Pharmaceuticals, Inc., both publicly held companies, and several private companies.
BOARD COMPOSITION
Our board of directors has five members. Upon the completion of this offering and in accordance with the terms of our amended and restated certificate of incorporation, our board of directors will be divided into three classes:
At each annual meeting of stockholders, or special meeting in lieu thereof, after the initial classification of the board of directors, the successors to directors whose terms will then expire will be elected to serve from the time of election and qualification until the third annual meeting following the election or special meeting in lieu thereof. The authorized number of directors may be changed only by resolution adopted by a majority of the board of directors. This classification of the board of directors may have the effect of delaying or preventing changes of control or management.
BOARD COMMITTEES
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Pursuant to our bylaws, our board of directors may from time to time establish other committees to facilitate the management of our business and operations.
Audit committee
Our audit committee currently consists of Ms. Burrus, Mr. Taylor and Dr. Senyei. Our audit committee is responsible for assuring the integrity of our financial control, audit and reporting functions and reviews with our management and our independent auditors the effectiveness of our financial controls and accounting and reporting practices and procedures. In addition, this committee reviews the qualifications of our independent auditors, is responsible for their appointment, compensation, retention and oversight and reviews the scope, fees and results of activities related to audit and non-audit services. The composition of the audit committee will satisfy the independence requirements of The Nasdaq National Market and the SEC.
Compensation committee
Our compensation committee consists of Ms. Burrus and Dr. Senyei. The compensation committee’s principal responsibility is to administer our stock plans and to set the salary and incentive compensation, including stock option grants, for our Chief Executive Officer and senior staff members. The composition of the compensation committee will satisfy the independence requirements of The Nasdaq National Market.
Nominating and corporate governance committee
Our nominating and governance committee consists of Ms. LaPorte and Mr. Taylor. The nominating and governance committee is responsible for reviewing and making recommendations on the composition of our board and selection of directors, periodically assessing the functioning of our board of directors and its committees, and making recommendations to our board of directors regarding corporate governance matters and practices. The composition of the nominating and governance committee will satisfy the independence requirements of The Nasdaq National Market.
We strive to operate within a comprehensive plan of corporate governance for the purpose of defining responsibilities, setting high standards of professional and personal conduct and assuring compliance with these responsibilities and standards. We have implemented changes to our corporate governance structure and procedures in response to the Sarbanes-Oxley Act of 2002 and the adopted changes in The Nasdaq National Market’s listing standards regarding corporate governance. We believe that our current corporate governance structure and procedures comply with existing corporate governance requirements. We will strive to maintain our board of directors and committees in full compliance with these corporate governance requirements on an ongoing basis. We will also continue to regularly monitor developments in the area of corporate governance.
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
No interlocking relationship is expected to exist between our board of directors or compensation committee and the board of directors or compensation committee of any other entity, nor has any interlocking relationship existed in the past.
DIRECTOR COMPENSATION
Our 2004 Equity Incentive Plan, or the 2004 Plan, provides for automatic grants of options to purchase shares of our common stock to our non-employee directors. The 2004 Plan provides for the automatic grant to a non-employee director who is on our board of directors upon the completion of this offering, or who is appointed or elected to our board of directors at a later date, of an option to purchase 30,000 shares of our common stock for our chairman, and 22,500 shares of our common stock to each remaining non-employee director. Such grant is referred to as the Initial Option. The 2004 Plan provides for an automatic grant of an option to purchase 11,250 shares to our chairman and 7,500 shares to each remaining non-employee director on the date of each annual meeting of the stockholders that occurs on or after the date that the 2004 Plan first becomes effective, provided that
Each Initial Option and Annual Option will have an exercise price equal to the fair market value of a share of our common stock on the date of grant and will have a term of ten years. Each Initial Option will vest and become exercisable in 48 equal installments on each monthly anniversary of the date of grant so long as the non-employee director continuously remains a director or consultant to our company. Each Annual Option will vest and become exercisable in 12 equal installments on each monthly anniversary of the date of grant of the option for so long as the non-employee director remains a director or a consultant to, our company. All automatic non-employee director options granted under the 2004 Plan will be non-statutory stock options. Options must be exercised, if at all, within three months after a non-employee director’s termination of service, except in the case of death, in which event the director’s estate shall have one year from the date of death to exercise the option. In no event, however, shall any option granted to a director be exercisable later than the expiration date of the option’s term. In the event of our merger with another corporation or another change of control, the non-employee director options will become fully vested and exercisable. For a description of the 2004 Plan see “Equity Compensation Plan Information.”
We currently do not pay cash compensation to our chairman or other non-employee directors for their service as directors.
LIMITATION ON LIABILITY AND INDEMNIFICATION OF OFFICERS AND DIRECTORS
Our amended and restated certificate of incorporation limits the liability of our directors to the maximum extent permitted by Delaware law. Delaware law provides that a corporation may eliminate the personal liability of its directors for monetary damages for breach of their fiduciary duties as directors, except liability for any of the following acts:
Our bylaws provide that we will indemnify our directors, officers, employees and other agents to the fullest extent permitted by the Delaware General Corporation Law. Our bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent of ours for any liability arising out of his or her actions in such capacity, regardless of whether the Delaware General Corporation Law would permit a corporation to indemnify for such liability.
We have obtained directors’ and officers’ insurance providing indemnification for all of our directors and officers for certain liabilities. We believe that these provisions and this insurance are necessary to attract and retain qualified directors and officers. At present, there is no pending litigation or proceeding involving any of our directors, officers, employees or agents where indemnification would be required or permitted. We are not aware of any pending or threatened litigation or proceeding that might result in a claim for such indemnification.
EXECUTIVE COMPENSATION
Summary compensation table
The following table summarizes the compensation paid to, awarded to or earned during the fiscal year ended December 31, 2003 by our Chief Executive Officer and each of the four most highly compensated executive officers whose total salary and bonus exceed $100,000 for services rendered to us in all capacities during 2003. The executive officers listed in the table below are referred to in this prospectus as our named executed officers.
| Long-term | |||||||||||||||||||||
| compensation | |||||||||||||||||||||
| Annual compensation | |||||||||||||||||||||
| Securities | |||||||||||||||||||||
| Other annual | underlying | All other | |||||||||||||||||||
| Name and principal position(s) | Salary | Bonus | compensation(1) | options | compensation | ||||||||||||||||
Emory V. Anderson(2) | $310,277 | $108,597 | — | — | — | ||||||||||||||||
| President and Chief Executive Officer | |||||||||||||||||||||
Mark D. Fischer-Colbrie(3) | 216,293 | 43,259 | $33,500(4) | — | — | ||||||||||||||||
| Vice President, Finance and Administration and Chief Financial Officer | |||||||||||||||||||||
Durlin E. Hickok(5) | 218,720 | 43,744 | — | — | — | ||||||||||||||||
| Vice President, Medical Affairs | |||||||||||||||||||||
Robert O. Hussa(6) | 199,346 | 19,935 | — | — | — | ||||||||||||||||
| Vice President, Research and Development | |||||||||||||||||||||
Marian E. Sacco(7) | 209,388 | 41,878 | — | — | — | ||||||||||||||||
| Vice President, Sales and Marketing | |||||||||||||||||||||
Option grants in fiscal year 2003
We did not grant any options to any of our named executive officers during our fiscal year ended December 31, 2003.
Aggregated option exercises in last fiscal year and fiscal year-end option values
There were no option exercises by the named executive officers during our fiscal year ended December 31, 2003. The following table summarizes the value of options held by them as of December 31, 2003. There was no public trading market for our common stock as of December 31, 2003. Accordingly, the value of unexercised in-the-money options listed below has been calculated on
| Number of securities | Value of unexercised | |||||||||||||||||||||||
| underlying unexercised options | in-the-money options | |||||||||||||||||||||||
| at December 31, 2003 | at December 31, 2003 | |||||||||||||||||||||||
| Shares acquired | Value | |||||||||||||||||||||||
| Name | upon exercise | realized | Exercisable(1) | Unexercisable | Exercisable | Unexercisable | ||||||||||||||||||
| Emory V. Anderson | — | $ | — | 609,540 | — | $ | 7,732,038.87 | $ | — | |||||||||||||||
| Mark D. Fischer-Colbrie | — | — | 131,947 | — | 1,539,381.67 | — | ||||||||||||||||||
| Durlin E. Hickok | — | — | 131,962 | — | 1,688,787.25 | — | ||||||||||||||||||
| Robert O. Hussa | — | — | 54,467 | — | 790,451.76 | — | ||||||||||||||||||
| Marian E. Sacco | — | — | 136,187 | — | 1,812,899.65 | — | ||||||||||||||||||
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
In December 2000, in connection with his offer of employment, we agreed to loan Mark Fischer-Colbrie up to $183,000 in connection with his offer letter. On February 1, 2003, we entered into a promissory note with Mr. Fischer-Colbrie in the amount of $109,800. The terms of the note provided that the principal amount would be due on January 31, 2006, and interest on the unpaid principal balance would accrue at an annual rate of 1.65%. The note also provides that the loan amount is forgiven by $36,600 in principal along with accumulated interest at the end of each 12 months of employment. The full amount of the note, including interest, would be due and payable within 30 days of Mr. Fischer-Colbrie’s resignation from our company or his termination by us for cause. In the event of a change of control transaction, a merger with another company or his termination without cause by us, the loan and any accumulated interest would be forgiven. In August 2004, the $76,000 unpaid balance and interest of Mr. Fischer-Colbrie’s loan was forgiven.
Management continuity agreements
We have entered into a management continuity agreement with each of Mr. Anderson, Mr. Fischer-Colbrie, Dr. Hickok, Dr. Hussa and Ms. Sacco. Mr. Anderson’s agreement provides that if we experience a “change of control” (as defined in the agreement), the vesting of each equity award granted to Mr. Anderson shall accelerate such that 75% of the aggregate number of unvested awards shall become vested immediately prior to the effective date of the transaction. In the event that Mr. Anderson’s employment is terminated other than for “cause” or if he resigns for “good reason” (each as defined in the agreement) at any time within 12 months following a change of control, each equity award shall become 100% vested as of the termination date. In addition, Mr. Anderson will receive severance benefits equal to 18 months of his salary, a lump sum payment equal to 75% of the bonus payment made to Mr. Anderson for the prior fiscal year and continuation of his health insurance benefits at our expense under COBRA for up to 18 months. In the event that Mr. Anderson’s termination or resignation occurs for one of the reasons specified above, at any time prior to, or more than 12 months following, a change of control, he will receive severance benefits equal to 12 months of his base salary, 50% of the prior year’s bonus, continuation of his health insurance benefits at our expense under COBRA for up to 12 months, and 12 months acceleration of his unvested equity awards. In both cases, equity awards granted on or after July 2004 shall remain exercisable for 18 months following his termination date.
Mr. Fischer-Colbrie’s agreement provides that if we experience a “change of control” (as defined in the agreement), the vesting of each equity award granted to Mr. Fischer-Colbrie shall accelerate such that 50% of the aggregate number of unvested awards shall become vested immediately prior to the
Under the agreements with Dr. Hickok, Dr. Hussa and Ms. Sacco, if we experience a “change of control” (as defined in the agreement), the vesting of each equity award granted to the officer shall accelerate such that 50% of the aggregate number of unvested awards shall become immediately vested immediately prior to the effective date of the transaction. In the event that the officer’s employment is terminated other than for “cause” or if he or she resigns for “good reason” (each as defined in the agreement) at any time within 12 months following a change of control, the officer will receive severance benefits equal to 9 months of his or her salary, a lump sum payment equal to 37.5% of the bonus payment made to the officer for the prior fiscal year, continuation of his or her health insurance benefits at our expense under COBRA for up to 9 months and 12 months acceleration of his or her unvested equity awards. In addition, equity awards granted on or after July 2004 shall remain exercisable for 18 months following his or her termination date. In the event that the officer’s termination or resignation occurs for one of the reasons specified above, at any time prior to, or more than 12 months following, a change of control, he or she will receive severance benefits equal to 6 months of his or her base salary, 25% of the prior year’s bonus and continuation of his or her health insurance benefits at our expense under COBRA for up to 6 months.
Each of these agreements further provides that, to the extent the severance payments and benefits payable under the agreements would cause the officer to be liable for “parachute payment” excise taxes applicable by reason of Section 4999 of the Internal Revenue Code, the officer will receive an additional “gross up” payment to indemnify the officer for the effect of the excise taxes.
As a condition of receipt of the severance payments and benefits under the agreements, each officer must execute a release of claims agreement in the form we provide and confirm his or her obligations to us under our standard form of proprietary information agreement.
Other agreements
All of our current employees and consultants have entered into agreements with us relating to the protection of our confidential information and the assignment of inventions.
None of our employees are employed for a specified term and each employee’s employment with us is subject to termination at any time by either party for any reason, with or without cause, without further liability or obligation.
EQUITY COMPENSATION PLAN INFORMATION
1995 Stock Option and Restricted Stock Plan
In 1995, our board of directors and stockholders approved the Adeza Biomedical Corporation 1995 Stock Option and Restricted Stock Plan, or the 1995 Plan. The 1995 Plan was adopted as a successor to our 1988 Employee Stock Plan and 1992 Key Executive Stock Plan. The 1995 Plan is designed to provide employees, non-employee members of the board of directors or non-employee members of the board of directors of any parent or subsidiary and consultants who provide services to us or any
Under the 1995 Plan, we are authorized to grant shares and stock options for the purchase of up to a maximum of 2,282,613 shares of our common stock. As of September 30, 2004:
Upon the effectiveness of this offering, we will no longer issue any additional options under the 1995 Plan. However, upon the effectiveness of this offering, any shares that are available for future grant under the 1995 Plan, and any shares issuable upon exercise of outstanding options that are subsequently forfeited, expire or are canceled, will be allocated to the 2004 Equity Incentive Plan.
Administration of the 1995 Plan
The 1995 Plan is administered by our board of directors, or a committee thereof, (the plan administrator), which determines all terms of grants under the 1995 Plan. The plan administrator also determines who will receive grants under the 1995 Plan and the number of shares of common stock subject to the grant.
Eligibility
All employees, non-employee members of the board of directors or non-employee members of the board of directors of any parent or subsidiary, and consultants who provide services to us are eligible to participate in the 1995 Plan.
Options
The 1995 Plan authorizes the plan administrator to grant options in an amount and at an exercise price to be determined by it. The exercise price of an incentive stock option cannot be less than 100% of the fair market value of our common stock on the option grant date. The exercise of non-statutory stock options must generally be at least 85% of the fair market value of the common stock on the option grant date. With respect to options granted to a 10% shareholder, the exercise price shall not be less than 110% of the fair market value of common stock on the option grant date. The exercise price shall be immediately due upon the exercise of the option, and is generally payable:
The 1995 Plan also authorizes the plan administrator to determine the vesting schedule (if any) applicable to the option shares. Generally, options granted under the 1995 plan vest in 48 successive equal monthly installments over four years. The plan administrator also determines the maximum term for which the option is to remain outstanding. The term of an option cannot exceed ten years from the option grant date, and the term of an incentive option granted to a 10% stockholder cannot exceed
Upon the termination of an optionee’s employment or other relationship with us, such optionee will have a limited time within which to exercise any outstanding options.
The 1995 Plan also authorizes the plan administrator to grant options that are exercisable for unvested shares of common stock. To the extent that an optionee holds unvested shares of common stock upon termination, we will have the right to repurchase any or all of such unvested shares at the exercise price paid by the optionee for such shares. The plan administrator will determine the terms of such options, but the vesting schedule may not be more restrictive than 20% per year vesting, beginning one year after the grant of the option. Such minimum vesting requirement shall not apply to options granted to a highly compensated employee.
Until our common stock is first registered under Section 12(g) of the Securities Exchange Act, we retain a right of first refusal with respect to any disposition by an optionee of any shares of common stock issued under the 1995 Plan. Options issued under the 1995 Plan may not be assigned or transferred other than by will or the laws of descent and distribution following the optionee’s death, except that non-statutory stock options may be assigned in accordance with the terms of a qualified domestic relations order.
The aggregate fair market value of the shares of common stock for which one or more incentive stock options granted to any employee under the 1995 Plan may for the first time become exercisable during any one calendar year shall not exceed the sum of $100,000.
Change in control
Prior to its amendment in August 2004, the 1995 Plan provided that in the event of a corporate transaction where the acquiror does not assume or replace options granted under the 1995 Plan, each outstanding option will terminate on the effective date of the corporate transaction. Options assumed or replaced in connection with a corporate transaction shall be appropriately adjusted.
Following the amendment of the 1995 Plan, the 1995 Plan provides with regard to options granted after that date, that in the event of a corporate transaction where the acquiror does not assume or replace options granted under the 1995 Plan, such outstanding options will become fully vested and exercisable immediately prior to the consummation of the corporate transaction. Additionally the amendment 1995 Plan provides that in the event of a corporate transaction where the acquiror assumes or substitutes options granted under the 1995 Plan, options issued under the 1995 Plan will become fully vested only if the optionee is involuntarily terminated without cause within 12 months of the corporate transaction or if the optionee voluntarily resigns following a relocation of the optionee’s worksite to a location more than 50 miles from the original location within 12 months of the corporate transaction. Options assumed or replaced by an acquiror in connection with a corporate transaction shall be appropriately adjusted.
A “corporate transaction” is defined as either of the following shareholder approved transactions to which we are a party: (i) a merger or consolidation in which securities possessing more than 50% of the total combined voting power of our outstanding securities are transferred to a person or persons different from the persons holding those securities immediately prior to such transaction, or (ii) a sale, transfer or other disposition of all or substantially all of our assets in complete liquidation or dissolution of our company.
All outstanding repurchase rights shall automatically terminate upon a corporate transaction, except to the extent the repurchase rights are assigned to the successor corporation in connection with such corporate transaction. As of August 1, 2004, the 1995 Plan provides that for options granted after that date in the event of a corporate transaction where outstanding repurchase rights are assigned to the successor corporation, all outstanding repurchase rights shall automatically terminate if the
Stock issuances
The plan administrator may grant direct and immediate issuances of shares of common stock pursuant to the 1995 Plan’s stock issuance program. The purchase price of common stock issued under the stock issuance program must be at least 85% of the fair market value of common stock on the stock issuance date. With respect to issuances to a 10% stockholder, the exercise price shall not be less than 110% of the fair market value of common stock on the stock issuance date. Payment for the exercise price may be made in cash, check or past services rendered to us (or any parent or subsidiary).
Shares of common stock issued under the stock issuance program may, in the discretion of the plan administrator, be fully and immediately vested upon issuance or may vest in one or more installments over the term of the recipient’s service or upon attainment of specified performance objectives. The plan administrator may not impose a vesting schedule that is more restrictive than 20% per year vesting, beginning one year after the stock issuance date. Such minimum vesting requirement shall not apply to stock issued to a highly compensated employee. An individual who is issued shares of common stock under the stock issuance program will, upon issuance, have full stockholder rights with respect to such shares whether or not such shares are vested as of the date of issuance. To the extent that an individual who was issued common stock pursuant to the stock issuance program either terminates his or her employment (or other relationship) with us while holding unvested shares of common stock or fails to attain the specified performance objectives upon which such vesting of such common stock was contingent, such shares shall be immediately surrendered to us for cancellation in exchange for a refund of any cash or cash-equivalent consideration paid for such unvested shares. The plan administrator may waive the surrender and cancellation of unvested shares of common stock.
Until our common stock is first registered under Section 12(g) of the Securities Exchange Act, we retain a right of first refusal with respect to any disposition by the participant of any shares of common stock issued under the stock issuance program.
Prior to amendment in August 2004, the 1995 Plan provided that in the event of a corporate transaction where the outstanding repurchase rights under the stock issuance program are not assigned to the successor corporation in connection with such corporate transaction, all of the outstanding repurchase rights under the stock issuance program shall terminate automatically upon a corporate transaction. Following amendment of the 1995 Plan, the 1995 Plan provides that, with regard to shares of common stock issued under the stock issuance program after the date of amendment in the event of a corporate transaction where the repurchase rights are assigned to the successor corporation, the outstanding purchase rights will terminate if the stockholder is involuntarily terminated without cause within 12 months of the corporate transaction or if the stockholder voluntarily resigns following a relocation of the stockholder’s worksite to a location more than 50 miles from the original location within 12 months of the corporate transaction.
Amendment and termination
The board of directors has the complete and exclusive power and authority to amend or modify the 1995 Plan in any or all respects. No such amendment or modification shall adversely affect the rights and obligations with respect to options or unvested stock issuances at the time outstanding under the 1995 Plan, unless the optionee or participant consents to such amendment or modification. To increase the maximum number of shares issuable under the 1995 Plan, to materially modify the eligibility requirements for participation, or to materially increase the benefits accruing to plan participants, stockholder approval is required.
The 1995 Plan shall terminate upon the earliest of the expiration of the ten-year period of the 1995 Plan, the date on which all shares available for issuance have been issued, or the termination of all outstanding options in connection with a corporate transaction. Upon termination of the 1995 Plan, all outstanding options and unvested stock issuances shall continue to have full force and effect.
2004 Equity Incentive Plan
In August 2004, our board of directors and stockholders approved the 2004 Equity Incentive Plan, or the 2004 Plan, which will become effective upon the completion of this offering.
Stock options, stock appreciation rights, or SARs, stock awards and cash awards may be granted under the 2004 Plan. Each is referred to as an award in the 2004 Plan. Options granted under the 2004 Plan may be either “incentive stock options,” as defined under Section 422 of the Internal Revenue Code of 1986, as amended, or nonstatutory stock options.
Share reserve
We have reserved a total of 1,875,000 shares of our common stock, subject to adjustment, for issuance under the 2004 Plan, all of which are available for future grant. The number of shares initially reserved under the 2004 Plan will be increased by up to 17,056 shares, represented by shares available for grant under the 1995 Plan as of the completion of the offering. In addition, options outstanding under the 1995 Plan that are forfeited, expire or are cancelled after the date of this prospectus will be available for future grant under the 2004 Plan after the completion of this offering.
Automatic annual increase of share reserve
The 2004 Plan provides that the share reserve will be cumulatively increased on January 1 of each year, beginning January 1, 2005 and for nine years thereafter, by a number of shares that is equal to the lesser of (a) 3% of the number of our company’s shares issued and outstanding as of the preceding December 31, (b) 525,000 shares and (c) a number of shares set by our board.
Automatic grants to non-employee directors
The 2004 Plan provides that non-employee directors will be automatically granted options under the 2004 Plan in the following amounts: (a) an option to purchase 22,500 shares of our common stock to non-employee directors upon the completion of this offering or upon their initial appointment or election, as applicable, to our board, and (b) commencing in 2005 and provided that such individual has served as a non-employee director for at least six months, an option to purchase 7,500 shares annually thereafter on the date of each stockholder meeting following which the non-employee director continues to serve on our board. In the case of a non-employee director who serves as the chairman of the board, such director will receive (a) an option to purchase 30,000 shares of our common stock upon the completion of this offering, or upon initial appointment or election as the chairman, as applicable, and (b) an option to purchase 11,250 shares annually thereafter on the date of each stockholder meeting following which the non-employee director continues to serve as chairman of our board.
Administration
Our board of directors or a committee of the board will administer the 2004 Plan. The board or its committee is referred to in the 2004 Plan as the administrator.
Eligibility
Awards under the 2004 Plan may be granted to our employees, directors and consultants. Incentive stock options may be granted only to our employees. The administrator, in its discretion, approves awards granted under the 2004 Plan.
Change in control
In the event of a corporate transaction, any or all outstanding awards may be assumed, converted or replaced. Awards assumed, converted or replaced in connection with a corporate transaction shall be appropriately adjusted. In the event of a corporate transaction where the acquiror does not assume or substitute awards granted under the 2004 Plan, the vesting with respect to such awards shall fully and immediately accelerate or the repurchase rights shall fully and immediately terminate, as the case may be, immediately prior to the consummation of the corporate transaction. In the event of a corporate transaction where the acquiror assumes or substitutes awards granted under the 2004 Plan, the vesting with respect to such awards shall fully and immediately accelerate or the repurchase rights shall fully and immediately terminate, as the case may be, if an awardee voluntarily resigns following relocation of the awardee’s workplace to a location more than 50 miles from the original location within 12 months following the corporate transaction.
Termination of awards
Generally, if an awardee’s service to us terminates other than by reason of death or disability, vested options and SARs will remain exercisable for a period of three months following the termination of the awardee’s service, or if earlier, the expiration of the term of such award. Unless otherwise provided for by the administrator in the award agreement, if an awardee’s termination is due to death or disability, the awardee’s vested options and SARs will be exercisable for one year following the awardee’s death or disability, or if earlier, the expiration of the term of such award.
Nontransferability of awards
Unless otherwise determined by the administrator, awards granted under the 2004 Plan are not transferable other than by will, a domestic relations order, or the laws of descent and distribution and may be exercised during the awardee’s lifetime only by the awardee.
Exercise price of stock options
The administrator determines the exercise price of options at the time the options are granted. The exercise price of an incentive stock option may not be less than 100% of the fair market value of our common stock on the date of grant. With respect to incentive stock options granted to a 10% stockholder, the exercise price shall not be less than 110% of the fair market value of our common stock on the date of grant. Unless otherwise determined by the administrator, the exercise price of a nonstatutory stock option will generally not be less than 85% of the fair market value of our common stock on the date of grant. The fair market value of our common stock will generally be the closing sales price as quoted on The Nasdaq National Market on the date of the grant.
Exercise of option; form of payment
The administrator determines when options become exercisable. The means of payment for shares issued on exercise of an option are specified in each award agreement. The 2004 Plan permits payment to be made by cash, check, wire transfer, other shares of our common stock that meet certain holding period requirements (valued at the fair market value of our common stock on the exercise date), a broker-assisted same-day sale transaction or cancellation of any debt owed by us or any of our affiliates to the optionholder.
Term of option
The term of an option may be no more than ten years from the date of grant. No option may be exercised after the expiration of its term. Any incentive stock option granted to a 10% stockholder may not have a term of more than five years.
Stock appreciation rights
The administrator may grant SARs alone, in addition to, or in tandem with, any other awards under this plan. A SAR entitles the participant to receive the amount by which the fair market value of a specified number of shares on the exercise date exceeds an exercise price established by the administrator. The excess amount will be payable in ordinary shares, in cash or in a combination thereof, as determined by the administrator. The terms and conditions of a SAR will be contained in an award agreement. The grant of a SAR may be made contingent upon the achievement of objective performance conditions.
Stock awards
The administrator may grant stock awards of restricted shares as payment of a bonus, as payment of any other compensation obligation, upon the occurrence of a special event or as otherwise determined by the administrator. The terms and conditions of a stock award will be found in an award agreement. Vesting and restrictions on the ability to exercise such stock awards may be conditioned upon the achievement of one or more goals, as determined by the administrator in its discretion. Recipients of restricted shares may have voting rights and may receive dividends on the granted shares prior to the time the restrictions lapse.
Cash awards
The administrator may grant cash awards, which entitle the recipient to a cash payment on the satisfaction of performance goals described in the award. The administrator determines the terms, conditions and restrictions related to cash awards.
Amendment and termination of the 2004 Plan
Our board of directors may amend, alter, suspend or terminate the 2004 Plan, or any part thereof, at any time and for any reason. However, we will solicit stockholder approval for any amendment to the 2004 Plan to the extent necessary and desirable to comply with applicable laws. Generally, no such action by our board of directors or stockholders may alter or impair any award previously granted under the 2004 Plan without the written consent of the awardee. The 2004 Plan has a term of 10 years, but it may be terminated by our board of directors at any time.
401(k) PLAN
We have established and maintain a retirement savings plan under section 401(k) of the Internal Revenue Code, or the Code, to cover our eligible employees. The Code allows eligible employees to defer a portion of their compensation, within prescribed limits, on a tax-deferred basis through contributions to the 401(k) plan. Our 401(k) plan is qualified under Section 401(a) of the Code and its associated trust is exempt from federal income taxation under Section 501(a) of the Code. Our 401(k) permits us to make matching contributions on behalf of eligible employees; however, we currently do not make any matching contributions.
Certain relationships and related party transactions
From January 1, 2001 until the date of this prospectus, there has not been any transaction or series of similar transactions, nor is there currently proposed any transaction or series of similar transactions, to which we were, are, or would be a party, and in which the amount involved exceeded or would exceed $60,000 and in which any of our directors or executive officers, any holder of more than 5% of any class of our voting securities or any member of the immediate family of any of these persons had or will have a direct or indirect material interest, other than the compensation arrangements (including with respect to equity compensation) described in “Management,” and the transactions described below.
We believe that we have executed all of the transactions described below on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors and principal stockholders and their affiliates are approved by a majority of our board of directors, including a majority of the independent and disinterested members of our board of directors, and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
ISSUANCES OF PREFERRED STOCK AND WARRANTS
We have engaged in transactions regarding sales of our preferred stock with one of our directors and with the beneficial owners of at least 5% of our voting securities. On September 19, 2001, we sold an aggregate of 3,247,408 shares of our Series 5 Preferred Stock at a purchase price of $4.63 per share. On September 29, 2000, we sold an aggregate of 2,203,108 shares of our Series 4 Preferred Stock at a purchase price of $4.63 per share. On November 18, 1996, we sold an aggregate of 4,463,100 shares of our Series 3 Preferred Stock at a purchase price of $2.92 per share and an additional 19,361 shares and 134,357 shares pursuant to the exercise of warrants on March 27, 2001 and April 26, 2001, respectively, for $2.92 per share. On December 19, 2003, we sold 190,259 shares of our Series 3 Preferred Stock pursuant to the exercise of warrants at a purchase price of $2.63 per share. On December 21, 1994, we sold 3,322,893 shares of Series 2 Preferred Stock and an additional 183,872 shares and 1,642 shares from warrants exercised on December 23, 1999, and January 10, 2000, respectively, for $2.40 per share. In December 1994, our stockholders approved an amendment to the Articles of Incorporation to change our capital structure. This amendment effected a conversion of the outstanding Series A through F Preferred Stock into a new Series 1 Preferred Stock and a reverse stock split resulting in 1,654,719 shares of Series 1 Preferred Stock. The Series A through F Preferred Stock were converted and reconstituted in the following manner:
The following table summarizes the shares of our preferred stock purchased in these transactions by our directors, by our 5% stockholders and by the persons and entities associated with them in these private placement transactions. We effected a three-for-four reverse split of our common stock on December 6, 2004. Each share of Series 1 Preferred Stock, Series 2 Preferred Stock, Series 4 Preferred Stock and Series 5 Preferred Stock will convert into 0.75 share of common stock upon the closing of this offering, after giving effect to the reverse split of common stock into which the preferred stock will be converted. Each share of Series 3 Preferred Stock will convert into 0.83333 shares of common stock, upon the closing of this offering, after giving effect to the reverse split of the common stock into which the preferred stock will be converted.
| Series 1 | Series 2 | Series 3 | Series 4 | Series 5 | ||||||||||||||||
| Preferred | Preferred | Preferred | Preferred | Preferred | ||||||||||||||||
| Investor | Stock | Stock | Stock | Stock | Stock | |||||||||||||||
Directors | ||||||||||||||||||||
Craig Taylor(1) | — | — | — | — | 21,598 | |||||||||||||||
5% Stockholders(2) | ||||||||||||||||||||
Entities affiliated with Sprout Capital(3) | — | — | 3,424,658 | — | — | |||||||||||||||
Enterprise Partners V, L.P.(4) | — | — | — | 1,079,914 | 1,295,896 | |||||||||||||||
Entities affiliated with Charter Venture Capital(5) | 180,305 | 590,904 | 265,939 | 204,242 | 291,577 | |||||||||||||||
| Aeneas Venture Corporation | 338,775 | 773,890 | 87,106 | — | — | |||||||||||||||
Entities affiliated with Alliance Technology Ventures(6) | 130,851 | 239,230 | 41,294 | 647,949 | 55,076 | |||||||||||||||
Entities affiliated with Asset Management(7) | 136,735 | 472,360 | 207,298 | 162,399 | 86,393 | |||||||||||||||
| Pantheon Global PCC Limited acting in respect and on behalf of Pantheon Secondary Interests Cell | 136,735 | 422,802 | 207,292 | 54,349 | 197,300 | |||||||||||||||
STF II, L.P.(8) | — | 416,666 | 314,836 | 54,255 | — | |||||||||||||||
INDEMNIFICATION AGREEMENTS
We have entered into indemnification agreements with our directors and executive officers for the indemnification of and advancement of expenses to these persons to the fullest extent permitted by law. We also intend to enter into these agreements with our future directors and executive officers.
EMPLOYMENT, SEVERANCE AND CHANGE OF CONTROL AGREEMENTS
See “Management—Employment, Severance and Change of Control Agreements.”
LOANS TO EXECUTIVE OFFICERS
We loaned Mark Fischer-Colbrie $109,800 in February 2003. In August 2004, the $76,000 unpaid balance and interest of Mr. Fischer-Colbrie’s loan was forgiven. See “Management—Employment, Severance and Change of Control Agreements.”
Principal stockholders
The following table shows information with respect to the beneficial ownership of our common stock as of September 30, 2004 and as adjusted to reflect the sale of the common stock being offered in this offering by:
Beneficial ownership and percentage ownership are determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock underlying options and warrants that are exercisable within 60 days of September 30, 2004 are considered to be outstanding. To our knowledge, except as indicated in the footnotes to the following table and subject to community property laws where applicable, the persons named in this table have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
The following table reflects the conversion of all shares of our preferred stock outstanding as of September 30, 2004 into an aggregate of 11,957,322 shares of our common stock that will become effective at the closing of this offering (after giving effect to a three-for-four reverse split of our outstanding common stock). This table is based on 12,139,841 shares of our common stock outstanding as of September 30, 2004 and 15,889,841 shares outstanding immediately after this offering.
Unless otherwise indicated, the address of each stockholder is c/o Adeza Biomedical Corporation, 1240 Elko Drive, Sunnyvale, California 94089.
| Number of securities | Percent of shares | |||||||||||||||
| beneficially owned | beneficially owned | |||||||||||||||
| prior to offering | ||||||||||||||||
| Before | After | |||||||||||||||
| Name and address of beneficial owner | Stock | Options | this offering | this offering | ||||||||||||
Directors and executive officers | ||||||||||||||||
Kathleen D. LaPorte(1) | 2,853,875 | — | 23.51% | 17.96% | ||||||||||||
Andrew E. Senyei(2)(3) | 1,793,804 | 8,729 | 14.84% | 11.34% | ||||||||||||
Craig C. Taylor(4) | 839,183 | 1,880 | 6.93% | 5.29% | ||||||||||||
Nancy D. Burrus(5) | 615,551 | — | 5.07% | 3.87% | ||||||||||||
Emory V. Anderson(6) | — | 778,290 | 6.02% | 4.67% | ||||||||||||
Mark D. Fischer-Colbrie(7) | — | 169,447 | 1.38% | 1.06% | ||||||||||||
Robert O. Hussa(8) | 3,453 | 73,217 | * | * | ||||||||||||
Marian E. Sacco(9) | — | 173,687 | 1.41% | 1.08% | ||||||||||||
Durlin E. Hickok(10) | — | 169,462 | 1.38% | 1.06% | ||||||||||||
All directors and executive officers as a group (9 persons)(15)(16) | 6,105,866 | 1,374,712 | 55.35% | 43.33% | ||||||||||||
Five percent stockholders | ||||||||||||||||
Entities affiliated with Sprout Capital(1) | 2,853,875 | — | 23.51% | 17.96% | ||||||||||||
Enterprise Partners V, L.P.(2) | 1,781,857 | — | 14.68% | 11.21% | ||||||||||||
Entities affiliated with Charter Venture Capital(11) | 1,179,981 | — | 9.72% | 7.43% | ||||||||||||
Aeneas Venture Corporation(12) | 907,011 | — | 7.47% | 5.71% | ||||||||||||
Entities affiliated with Alliance Technology Ventures(13) | 845,291 | — | 6.96% | 5.32% | ||||||||||||
Entities affiliated with Asset Management(4) | 822,985 | 1,880 | 6.79% | 5.19% | ||||||||||||
Pantheon Global PCC Limited acting in respect and on behalf of Pantheon Secondary Interests Cell(14) | 787,958 | — | 6.49% | 4.96% | ||||||||||||
STF II, L.P.(5) | 615,551 | — | 5.07% | 3.87% | ||||||||||||
Description of capital stock
The description below of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws are summaries and are qualified by reference to the amended and restated certificate of incorporation and the amended and restated bylaws that will become effective upon closing of this offering. These documents will be filed as exhibits to the registration statement of which this prospectus is a part. The descriptions of the common stock and preferred stock reflect changes to our capital structure that will occur upon the closing of this offering.
GENERAL
Our amended and restated certificate of incorporation, to become effective upon the closing of this offering, authorizes the issuance of up to 100,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share. The rights and preferences of the preferred stock may be established from time to time by our board of directors. The following description of our capital stock gives effect to a three-for-four reverse stock split of our common stock that was effected on December 6, 2004.
COMMON STOCK
Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, except matters that relate only to one or more of the series of preferred stock and each holder does not have cumulative voting rights. Accordingly, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they so choose.
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the board of directors out of legally available funds.
Holders of common stock have no preemptive or conversion rights or other subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are, and the shares of common stock offered by us in this offering, when issued and paid for, will be fully paid and nonassessable. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock, which we may designate in the future.
PREFERRED STOCK
Upon the closing of this offering, our board of directors will be authorized, subject to any limitations prescribed by law, without stockholder approval, to issue up to an aggregate of 5,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions
WARRANTS
In March 1999, we issued two warrants to purchase a total of 219,178 shares of Series 3 Preferred Stock at a pre-conversion exercise price of $2.92 per share to lenders pursuant to a credit facility. In March 2000, we issued a warrant to purchase 17,123 shares of Series 3 Preferred Stock at a pre-conversion exercise price of $2.92 per share to a lender in connection with a credit facility. After this offering, the warrants will be exercisable for 196,915 shares of common stock at an exercise price of $3.50 per share or expire on the later of ten years from the date of grant or five years after the closing of this public offering.
STOCK OPTIONS
We intend to file a registration statement under the Securities Act covering up to 4,157,613 shares of common stock reserved for issuance under our stock plans as of September 30, 2004. That registration statement is expected to become effective upon filing with the SEC. Accordingly, common stock registered under that registration statement will, subject to vesting provisions and limitations as to the volume of shares that may be held by our affiliates under Rule 144 described below, be available for sale in the open market unless the holder is subject to the 180-day lock-up period.
As of September 30, 2004, options to purchase 2,163,582 shares of common stock were issued and outstanding at a weighted average exercise price of $4.70 per share. Upon the expiration of the lock-up period described above, at least 1,549,083 shares of common stock will be subject to vested options.
REGISTRATION RIGHTS
We and the holders of our preferred stock entered into an amended and restated investor rights agreement, dated September 19, 2001. This agreement provides these holders with customary demand and piggyback registration rights with respect to the shares of common stock to be issued upon conversion of their preferred stock or exercise of their warrants.
Demand registration
According to the terms of the amended and restated investor rights agreement, holders of 40% of common stock issued or issuable upon conversion of the outstanding preferred stock and upon the exercise of stock purchase warrants of the company have the right to require us to register their shares with the SEC for resale to the public. To demand such a registration, holders who hold together an aggregate of at least 40% of the shares having registration rights must request a registration that is reasonably expected to have an aggregate offering price which equals or exceeds $7,500,000 including underwriting discounts and commissions. We are not required to effect more than two demand registrations. We have currently not effected, or received a request for, any demand registrations.
Piggyback registration
If we file a registration statement for a public offering of any of our securities (other than a registration relating solely to employee stock option or purchase plans, or a registration relating solely to an SEC Rule 145 transaction, or a registration on any form other than Form S-1, S-2 or S-3, or any
Form S-3 registration
At any time after we become eligible to file a registration statement on Form S-3, the holders of common stock issued or issuable upon conversion of the outstanding preferred stock may require us to file a Form S-3 registration statement, provided that the aggregate disposition price of such registration must be at least $500,000. Such holders have the right to request an unlimited number of registrations on Form S-3, but we are obligated to file only one Form S-3 registration statement in any 12-month period.
These registration rights are subject to certain conditions and limitations, including the right of the underwriters of an offering to limit the number of shares of common stock to be included in the registration. We are generally required to bear the expenses of all registrations, except underwriting discounts and commissions. However, we will not pay for any expenses of any demand or S-3 registration if the request is subsequently withdrawn by the holders who requested such registration unless the withdrawal is based on material adverse information about the company not available at the time of the registration request or the right to demand one registration is forfeited by all holders of the right. The investors’ rights agreement also contains our commitment to indemnify the holders of registration rights for losses attributable to statements or omissions by us incurred with registrations under the agreement.
ANTI-TAKEOVER EFFECTS OF PROVISIONS OF OUR AMENDED AND RESTATED CERTIFICATE OF INCORPORATION AND BYLAWS AND DELAWARE LAW
Amended and restated certificate of incorporation and bylaws
Some provisions of Delaware law and our amended and restated certificate of incorporation and bylaws contain provisions that could make the following transactions more difficult:
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids and to promote stability in our management.
These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors.
Delaware anti-takeover statute
We are subject to Section 203 of the Delaware General Corporation Law. This law prohibits a publicly held Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless:
Section 203 defines “business combination” to include:
In general, Section 203 defines an “interested stockholder” as an entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
LIMITATION OF LIABILITY
Our amended and restated certificate of incorporation provides that no director shall be personally liable to us or to our stockholders for monetary damages for breach of fiduciary duty as a director, except that the limitation shall not eliminate or limit liability to the extent that the elimination or limitation of such liability is not permitted by the Delaware General Corporation Law as the same exists or may hereafter be amended.
THE NASDAQ NATIONAL MARKET
Our common stock has been approved for quotation on The Nasdaq National Market under the symbol “ADZA.”
TRANSFER AGENT AND REGISTRAR
Upon the closing of this offering, the transfer agent and registrar for our common stock will be Wells Fargo Bank, N.A.
Shares eligible for future sale
Prior to this offering, there was no public market for our common stock. Future sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur, could adversely affect the price of our common stock.
Based on the number of shares outstanding as of September 30, 2004, we will have approximately 15,889,841 shares of our common stock outstanding after the completion of this offering (approximately 16,452,341 shares if the underwriters exercise their over-allotment option in full). Of those shares, the 3,750,000 shares of common stock sold in this offering (4,312,500 shares if the underwriters exercise their over-allotment option in full) will be freely transferable without restriction, unless purchased by our affiliates. The remaining 12,139,841 shares of common stock to be outstanding immediately following the completion of this offering, which are “restricted securities” under Rule 144 of the Securities Act of 1933, or Rule 144, as well as any other shares held by our affiliates, may not be resold except pursuant to an effective registration statement or an applicable exemption from registration, including an exemption under Rule 144.
The holders of approximately 11,898,058 shares of outstanding common stock as of the closing of this offering, and the holders of approximately 2,253,009 shares of common stock underlying options and warrants as of the closing of this offering, including all of our officers and directors, have entered into lock-up agreements pursuant to which they have generally agreed, subject to certain exceptions, not to, without the prior written approval of UBS Securities LLC, offer, sell, contract to sell or otherwise dispose of, directly or indirectly, or hedge our common stock or securities convertible into or exchangeable or exercisable for our common stock. These restrictions will be in effect for a period of 180 days after the date of this prospectus. The 180-day lock-up period may be extended under certain circumstances where we release, or pre-announce a release of, our earnings or material news or a material event shortly before or after the termination of the 180-day period. At any time and without public notice, UBS Securities LLC may in its sole discretion release all or some of the securities from these lock-up agreements. See “Underwriting.”
After the offering, the holders of 11,957,322 shares of our common stock, and the holders of warrants to purchase 196,915 shares of our common stock will be entitled to certain registration rights. For more information on these registration rights, see “Description of capital stock— Registration Rights.”
In general, under Rule 144, as currently in effect, an affiliate of ours who beneficially owns shares of our common stock that are not restricted securities, or a person who beneficially owns for more than one year shares of our common stock that are restricted securities, may generally sell, within any three-month period, a number of shares that does not exceed the greater of:
Sales under Rule 144 are also subject to requirements with respect to manner of sale, notice and the availability of current public information about us. Generally, a person who was not our affiliate at any time during the three months before the sale, and who has beneficially owned shares of our common stock that are restricted securities for at least two years, may sell those shares without regard to the volume limitations, manner of sale provisions, notice requirements or the requirements with respect to availability of current public information about us.
Generally, an employee, officer, director or consultant who purchased shares of our common stock before the effective date of the registration statement of which this prospectus is a part, or who holds
Neither Rule 144 nor Rule 701 supersedes the contractual obligations of our security holders set forth in the lock-up agreements described above.
The 12,139,841 pro forma shares of our common stock that were outstanding on September 30, 2004 will, assuming conversion of our preferred stock in connection with this initial public offering, and assuming no shares are released from the lock-up agreements described above prior to 180 days after the date of this prospectus, become eligible for sale pursuant to Rule 144 or Rule 701 without registration approximately as follows:
Additionally, of the 2,360,497 shares issuable upon exercise of options and warrants to purchase our common stock outstanding as of September 30, 2004, approximately 1,745,998 shares will be vested and eligible for sale 180 days after the date of this prospectus.
EQUITY COMPENSATION
We have reserved an aggregate of 4,157,613 shares of our common stock for issuance under our stock plans as of the completion of this offering. We intend to register the shares reserved for issuance under our 1995 Plan and 2004 Plan on a registration statement under the Securities Act of 1933 on Form S-8 following this offering. Subject to the lock-up agreements and the restrictions imposed under our 1995 Plan and 2004 Plan, shares of common stock issued under our 1995 Plan and 2004 Plan after the effective date of any registration statement on Form S-8 will be available for sale in the public market without restriction to the extent that they are held by persons who are not our affiliates.
Underwriting
We are offering the shares of our common stock described in this prospectus through the underwriters named below. UBS Securities LLC, SG Cowen & Co., LLC, Thomas Weisel Partners LLC, and William Blair & Company, L.L.C. are the representatives of the underwriters. UBS Securities LLC is the sole book-running manager of this offering. We have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of common stock listed next to its name in the following table:
The underwriting agreement provides that the underwriters must buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Our common stock is offered subject to a number of conditions, including:
The representatives have advised us that the underwriters intend to make a market in our common stock but that they are not obligated to do so and may discontinue making a market at any time without notice.
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
Sales of shares made outside of the United States may be made by affiliates of the underwriters.
OVER-ALLOTMENT OPTION
We have granted the underwriters an option to buy up to 562,500 additional shares of our common stock. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above.
COMMISSIONS AND DISCOUNTS
Shares sold by the underwriters to the public will initially be offered at the initial offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from the initial public offering price. If all the shares are not sold at the initial public offering price, the representatives may change the offering pricefiling Exhibits 3.5, 10.3 and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to
The following table shows the per share and total underwriting discounts and commissions we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional 562,500 shares.
We estimate that the total expenses of the offering payable by us, excluding underwriting discounts and commissions, will be approximately $1,800,000.
NO SALES OF SIMILAR SECURITIES
We, our executive officers and directors and our existing stockholders holding an aggregate of over 97% of our shares outstanding (including securities convertible into or exchangeable or exercisable for our common stock) have entered into lock-up agreements with the underwriters. Under these agreements, subject to certain exceptions, we and each of these persons may not, without the prior written approval of UBS Securities LLC, offer, sell, contract to sell or otherwise dispose of, directly or indirectly, or hedge our common stock or securities convertible into or exchangeable or exercisable for our common stock. These restrictions will be in effect for a period of 180 days after the date of this prospectus. The 180-day lock-up period may be extended under certain circumstances where we release, or pre-announce a release of, our earnings or material news or a material event shortly before or after the termination of the 180-day period. At any time and without public notice, UBS Securities LLC may in its sole discretion release all or some of the securities from these lock-up agreements.
INDEMNIFICATION
We have agreed to indemnify the underwriters against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities.
NASDAQ NATIONAL MARKET QUOTATION
Our common stock has been approved for quotation on The Nasdaq National Market under the trading symbol “ADZA.”
PRICE STABILIZATION, SHORT POSITIONS
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including:
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. These transactions
The underwriters may close out any covered short position either by exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option.
Naked short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on The Nasdaq National Market, in the over-the-counter market or otherwise.
DETERMINATION OF OFFERING PRICE
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiation by us and the representatives of the underwriters. The principal factors to be considered in determining the initial public offering price include:
AFFILIATIONS
Certain of the underwriters and their affiliates have in the past provided and may from time to time provide certain commercial banking, financial advisory, investment banking and other services for us for which they were and will be entitled to receive separate fees.
We retained UBS Securities LLC, one of the underwriters, to act as our exclusive financial advisor and capital markets advisor in connection with strategic alternatives and, subject to the execution of a satisfactory underwriting agreement, as bookrunner and lead manager of the initial public offering of our common stock. UBS Securities LLC would be entitled to receive additional compensation if certain strategic transactions were to be consummated.
Legal matters
The validity of the common stock offered hereby will be passed upon for us by Heller Ehrman White & McAuliffe LLP, Menlo Park, California. Dewey Ballantine LLP, New York, New York, is counsel for the underwriters in connection with this offering.
Experts
Ernst & Young LLP, independent registered public accounting firm, have audited our financial statements at December 31, 2002 and 2003, and for each of the three years in the period ended December 31, 2003, as set forth in their report. We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
Where you can find more information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933 with respect to the common stock we are offering. This prospectus, which constitutes a part of the registration statement, does not contain all of the information in the registration statement and the exhibits of the registration statement. For further information with respect to us and our common stock, we refer you to the registration statement and to the exhibits to the registration statement.
You may read and copy the registration statement of which this prospectus is a part at the SEC’s Public Reference Room, which is located at 450 Fifth Street, N.W., Washington, D.C. 20549. You can request copies of the registration statement by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s Public Reference Room. In addition, the SEC maintains an Internet web site, which is located at www.sec.gov, which contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC’s Internet web site. Upon completion of this offering, we will be subject to the information reporting requirements of the Securities Exchange Act of 1934, and we will file reports, proxy statements and other information with the SEC.
We maintain Internet websites athttp://www.adeza.comandhttp://www.ffntest.com. We have not incorporated by reference into this prospectus the information on our web sites, and you should not consider them to be a part of this prospectus.
This prospectus includes statistical data obtained from industry publications. These industry publications generally indicate that the authors of these publications have obtained information from sources believed to be reliable but do not guarantee the accuracy and completeness of their information. While we believe these industry publications to be reliable, we have not independently verified their data.
Notice to investors
You should rely only on the information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional information or information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock.
Report of Ernst & Young LLP, independent registered public accounting firm
The Board of Directors and Stockholders
We have audited the accompanying balance sheets of Adeza Biomedical Corporation as of December 31, 2003 and 2002, and the related statements of operations, convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2003. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Adeza Biomedical Corporation at December 31, 2003 and 2002, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2003 in conformity with U.S. generally accepted accounting principles.
Palo Alto, California
| December 31, | Pro forma at | ||||||||||||||||
| September 30, | September 30, | ||||||||||||||||
| 2002 | 2003 | 2004 | 2004 | ||||||||||||||
| (unaudited) | (unaudited) | ||||||||||||||||
| Assets | |||||||||||||||||
| Current assets: | |||||||||||||||||
| Cash and cash equivalents | $ | 10,751 | $ | 12,092 | $ | 14,638 | |||||||||||
Accounts receivable, net of allowance of $335, $264 and $249 at December 31, 2002 and 2003 and September 30, 2004(unaudited), respectively | 4,042 | 5,294 | 6,541 | ||||||||||||||
| Inventories | 513 | 590 | 597 | ||||||||||||||
| Prepaid and other current assets | 173 | 188 | 1,548 | ||||||||||||||
| Total current assets | 15,479 | 18,164 | 23,324 | ||||||||||||||
| Property and equipment, net | 252 | 252 | 301 | ||||||||||||||
| Note receivable-related party | — | 76 | — | ||||||||||||||
| Intangible assets, net | — | 224 | 188 | ||||||||||||||
| Total assets | $ | 15,731 | $ | 18,716 | $ | 23,813 | |||||||||||
| Liabilities and stockholders’ equity (deficit) | |||||||||||||||||
| Current liabilities: | |||||||||||||||||
| Accounts payable | $ | 1,716 | $ | 2,428 | $ | 3,287 | |||||||||||
| Accrued compensation | 1,421 | 1,675 | 1,228 | ||||||||||||||
| Accrued royalties | 1,483 | 3,449 | 547 | ||||||||||||||
| Other accrued liabilities | 221 | 545 | 751 | ||||||||||||||
| Notes payable | 4,222 | — | — | ||||||||||||||
| Deferred revenue | 218 | 414 | 57 | ||||||||||||||
| Capital lease obligations | 3 | — | — | ||||||||||||||
| Total current liabilities | 9,284 | 8,511 | 5,870 | ||||||||||||||
Convertible preferred stock, $0.001 par value; issuable in series; 16,516,335 shares authorized, actual; 5,000,000 shares authorized pro forma; 15,218,803, 15,409,062 and 15,409,062 shares issued and outstanding, actual at December 31, 2002 and 2003, and September 30, 2004(unaudited), respectively; no shares issued and outstanding pro forma(unaudited); aggregate liquidation preference of $76,872 at December 31, 2003 and September 30, 2004(unaudited) | 60,984 | 61,484 | 61,484 | $ | — | ||||||||||||
| Commitments and contingencies | |||||||||||||||||
| Stockholders’ equity (deficit): | |||||||||||||||||
Common stock, $0.001 par value; 25,000,000 shares authorized, actual; 100,000,000 shares authorized, pro forma; 181,380, 182,160 and 182,519 shares issued and outstanding, actual at December 31, 2002 and 2003, and September 30, 2004(unaudited), respectively; 12,139,841 shares issued and outstanding pro forma(unaudited) | — | — | — | 12 | |||||||||||||
| Additional paid-in capital | 2,335 | 2,358 | 6,272 | 67,744 | |||||||||||||
| Deferred compensation | — | — | (3,459 | ) | (3,459 | ) | |||||||||||
| Accumulated deficit | (56,872 | ) | (53,637 | ) | (46,354 | ) | (46,354 | ) | |||||||||
| Total stockholders’ equity (deficit) | (54,537 | ) | (51,279 | ) | (43,541 | ) | 17,943 | ||||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 15,731 | $ | 18,716 | $ | 23,813 | |||||||||||
See accompanying notes.
| Nine months ended | |||||||||||||||||||||
| Years ended December 31, | September 30, | ||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| Product sales | $ | 6,742 | $ | 14,277 | $ | 26,499 | $ | 19,185 | $ | 24,405 | |||||||||||
| Cost of product sales | 2,521 | 3,715 | 6,087 | 4,621 | 895 | ||||||||||||||||
| Gross profit | 4,221 | 10,562 | 20,412 | 14,564 | 23,510 | ||||||||||||||||
| Contract revenues | 811 | 1,059 | — | — | — | ||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||||||
| Selling and marketing | 6,437 | 7,819 | 12,259 | 9,023 | 11,638 | ||||||||||||||||
| General and administrative | 2,033 | 2,069 | 2,730 | 1,791 | 2,631 | ||||||||||||||||
| Research and development | 2,145 | 2,047 | 2,001 | 1,519 | 1,755 | ||||||||||||||||
| Total operating costs and expenses | 10,615 | 11,935 | 16,990 | 12,333 | 16,024 | ||||||||||||||||
| Income (loss) from operations | (5,583 | ) | (314 | ) | 3,422 | 2,231 | 7,486 | ||||||||||||||
| Interest income | 272 | 212 | 112 | 86 | 104 | ||||||||||||||||
| Interest expense | (357 | ) | (219 | ) | (131 | ) | (109 | ) | — | ||||||||||||
| Other expenses, net | (21 | ) | (8 | ) | (33 | ) | (10 | ) | — | ||||||||||||
| Income (loss) before income taxes | (5,689 | ) | (329 | ) | 3,370 | 2,198 | 7,590 | ||||||||||||||
| Provision for income taxes | — | — | 135 | 113 | 307 | ||||||||||||||||
| Net income (loss) | $ | (5,689 | ) | $ | (329 | ) | $ | 3,235 | $ | 2,085 | $ | 7,283 | |||||||||
| Basic net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 17.78 | $ | 11.46 | $ | 39.97 | |||||||||
| Diluted net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 0.26 | $ | 0.17 | $ | 0.55 | |||||||||
| Pro forma basic net income per share (unaudited) | $ | 0.27 | $ | 0.60 | |||||||||||||||||
| Pro forma diluted net income per share (unaudited) | $ | 0.26 | $ | 0.55 | |||||||||||||||||
| Shares used to compute basic net income (loss) per share | 156,857 | 181,188 | 181,965 | 181,900 | 182,223 | ||||||||||||||||
| Shares used to compute diluted net income (loss) per share | 156,857 | 181,188 | 12,515,063 | 12,429,763 | 13,364,494 | ||||||||||||||||
| Shares used to compute pro forma basic net income per share (unaudited) | 11,986,026 | 12,139,545 | |||||||||||||||||||
| Shares used to compute pro forma diluted net income per share (unaudited) | 12,515,063 | 13,364,494 | |||||||||||||||||||
See accompanying notes.
| Convertible | Total | |||||||||||||||||||||||||||||
| preferred stock | Common stock | Additional | stockholders’ | |||||||||||||||||||||||||||
| paid-in | Deferred | Accumulated | equity | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | capital | Compensation | deficit | (deficit) | |||||||||||||||||||||||
| Balances at December 31, 2000 | 11,817,677 | $ | 45,637 | 151,789 | $ | — | $ | 2,314 | $ | — | $ | (50,854 | ) | $ | (48,540 | ) | ||||||||||||||
| Exercise of Series 3 warrants for cash of $2.92 per share | 134,357 | 392 | — | — | — | — | — | — | ||||||||||||||||||||||
| Exercise of Series 3 warrants from net exercise | 19,361 | — | — | — | — | — | — | — | ||||||||||||||||||||||
| Issuance of Series 5 convertible preferred stock at $4.63 per share for cash, net of issuance costs of $80 | 3,247,408 | 14,955 | — | — | — | — | — | — | ||||||||||||||||||||||
| Exercise of stock options at $0.32 to $3.33 per share for cash | — | — | 8,003 | — | 12 | — | — | 12 | ||||||||||||||||||||||
| Exercise of common stock warrant for cash of $0.01 per share | — | — | 18,750 | — | — | — | — | — | ||||||||||||||||||||||
| Net and comprehensive loss | — | — | — | — | — | — | (5,689 | ) | (5,689 | ) | ||||||||||||||||||||
| Balances at December 31, 2001 | 15,218,803 | 60,984 | 178,542 | — | 2,326 | — | (56,543 | ) | (54,217 | ) | ||||||||||||||||||||
| Exercise of stock options at $0.97 to $3.33 per share for cash | — | — | 2,838 | — | 4 | — | — | 4 | ||||||||||||||||||||||
| Stock-based compensation related to stock options issued to nonemployees | — | — | — | — | 5 | — | — | 5 | ||||||||||||||||||||||
| Net and comprehensive loss | — | — | — | — | — | — | (329 | ) | (329 | ) | ||||||||||||||||||||
| Balances at December 31, 2002 | 15,218,803 | 60,984 | 181,380 | — | 2,335 | — | (56,872 | ) | (54,537 | ) | ||||||||||||||||||||
| Exercise of Series 3 preferred stock warrants for cash at $2.63 per share | 190,259 | 500 | — | — | — | — | — | — | ||||||||||||||||||||||
| Stock-based compensation related to stock options issued to nonemployees | — | — | — | — | 23 | — | — | 23 | ||||||||||||||||||||||
| Exercise of stock options at $0.32 per share for cash | — | — | 780 | — | — | — | — | — | ||||||||||||||||||||||
| Net and comprehensive income | — | — | — | — | — | — | 3,235 | 3,235 | ||||||||||||||||||||||
| Balances at December 31, 2003 | 15,409,062 | 61,484 | 182,160 | — | 2,358 | — | (53,637 | ) | (51,279 | ) | ||||||||||||||||||||
| Deferred compensation related to stock options issued to employees | — | — | — | — | 3,625 | (3,625 | ) | — | — | |||||||||||||||||||||
| Stock-based compensation related to stock options issued to nonemployees | — | — | — | — | 288 | — | — | 288 | ||||||||||||||||||||||
| Amortization of deferred compensation related to stock options issued to employees | — | — | — | — | — | 166 | — | 166 | ||||||||||||||||||||||
| Exercise of stock options at $3.33 per share for cash | — | — | 359 | — | 1 | — | — | 1 | ||||||||||||||||||||||
| Net and comprehensive income | — | — | — | — | — | — | 7,283 | 7,283 | ||||||||||||||||||||||
| Balance at September 30, 2004 | 15,409,062 | 61,484 | 182,519 | — | 6,272 | (3,459 | ) | (46,354 | ) | (43,541 | ) | |||||||||||||||||||
See accompanying notes.
| Nine months ended | ||||||||||||||||||||||
| Years ended December 31, | September 30, | |||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||
Operating activities | ||||||||||||||||||||||
| Net income (loss) | $ | (5,689 | ) | $ | (329 | ) | $ | 3,235 | $ | 2,085 | $ | 7,283 | ||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | ||||||||||||||||||||||
| Depreciation and amortization | 147 | 129 | 142 | 100 | 126 | |||||||||||||||||
| Stock based compensation expense | — | 5 | 23 | 16 | 454 | |||||||||||||||||
| Other non-cash charges | 174 | — | (25 | ) | — | — | ||||||||||||||||
| Non-cash interest expense | — | 204 | 131 | 81 | — | |||||||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||||||||
| Accounts receivable | 665 | (3,919 | ) | (1,252 | ) | (450 | ) | (1,247 | ) | |||||||||||||
| Inventories | (47 | ) | 22 | (77 | ) | (126 | ) | (7 | ) | |||||||||||||
| Prepaid and other assets | (28 | ) | (34 | ) | (116 | ) | (145 | ) | (1,285 | ) | ||||||||||||
| Accounts payable | 325 | 206 | 712 | 581 | 859 | |||||||||||||||||
| Accrued royalties | — | 1,483 | 1,966 | 1,239 | (2,902 | ) | ||||||||||||||||
| Accrued compensation | 379 | 491 | 253 | 310 | (447 | ) | ||||||||||||||||
| Other accrued liabilities | 340 | (312 | ) | 324 | 191 | 206 | ||||||||||||||||
| Deferred revenue | 293 | (1,854 | ) | 196 | (195 | ) | (357 | ) | ||||||||||||||
| Net cash provided by (used in) operating activities | (3,441 | ) | (3,908 | ) | 5,512 | 3,687 | 2,683 | |||||||||||||||
Investing activities | ||||||||||||||||||||||
| Purchase of intangible assets | — | — | (240 | ) | (240 | ) | — | |||||||||||||||
| Purchases of property and equipment | (64 | ) | (140 | ) | (125 | ) | (117 | ) | (138 | ) | ||||||||||||
| Proceeds from sales of property and equipment | 6 | 2 | — | — | — | |||||||||||||||||
| Net cash used in investing activities | (58 | ) | (138 | ) | (365 | ) | (357 | ) | (138 | ) | ||||||||||||
Financing activities | ||||||||||||||||||||||
| Payments on capital lease obligations | (101 | ) | (38 | ) | (3 | ) | (3 | ) | — | |||||||||||||
| Payment of notes payable | (1,600 | ) | (1,843 | ) | (4,303 | ) | (4,303 | ) | — | |||||||||||||
| Net proceeds from issuance of common stock | 12 | 4 | — | — | 1 | |||||||||||||||||
| Net proceeds from the issuance of convertible preferred stock | 15,347 | — | 500 | — | — | |||||||||||||||||
| Net cash provided by (used in) financing activities | 13,658 | (1,877 | ) | (3,806 | ) | (4,306 | ) | 1 | ||||||||||||||
| Net increase (decrease) in cash and cash equivalents | 10,159 | (5,923 | ) | 1,341 | (976 | ) | 2,546 | |||||||||||||||
| Cash and cash equivalents at beginning of period | 6,515 | 16,674 | 10,751 | 10,751 | 12,092 | |||||||||||||||||
| Cash and cash equivalents at end of period | $ | 16,674 | $ | 10,751 | $ | 12,092 | $ | 9,775 | $ | 14,638 | ||||||||||||
Supplemental cash flow information | ||||||||||||||||||||||
| Cash paid for interest | $ | 206 | $ | 48 | $ | — | $ | — | $ | — | ||||||||||||
| Cash paid for income taxes | $ | — | $ | — | $ | 15 | $ | 15 | $ | 165 | ||||||||||||
Supplemental schedule of noncash investing and financing activities | ||||||||||||||||||||||
| Conversion of deferred revenue to notes payable upon termination of Distributor contract | $ | — | $ | 5,498 | $ | — | $ | — | $ | — | ||||||||||||
See accompanying notes.
Organization and business
Basis of Presentation
Use of estimates
Unaudited interim results
Unaudited pro forma information
Reverse stock split
Revenue recognition
In June 1999, Adeza entered into a Co-Promotion and Exclusive Distribution Agreement with a major US distributor (the “Distributor”). The Co-Promotion and Exclusive Distribution Agreement with the Distributor was terminated as of June 30, 2002. Under the terms of the agreement through June 30, 2002, the revenue earned for the sale of products to the Distributor was not fixed and determinable until the time the products were sold by the Distributor to the end user. Consequently, the Company recognized revenue only after the shipment by the Distributor of the products to the end users. Any payments received prior to the point at which the selling price was fixed and determinable were recorded as deferred revenue. Revenue from laboratory tests performed by the Distributor’s contracted laboratory service provider was recognized as tests were performed.
Effective July 1, 2002, Adeza entered into a services agreement with a national laboratory that performs diagnostic tests. Under the terms of the agreement, the laboratory provides certain domestic product distribution and testing services for Adeza. The Company recognizes revenue upon the shipment of products to the end user as the title, risks and rewards of ownership of the products pass from the Company to the end user at that time. Revenue from the Company’s laboratory services is recognized as tests are performed.
Revenue on all other product sales is recognized upon shipment to distributors as the title, risks, and rewards of ownership of the products pass to the distributors and the selling price of Adeza products is fixed and determinable at that point. Any advance payments received in excess of revenue recognized are classified as deferred revenue on the accompanying balance sheets. Customers have the right to return products that are defective. There are no other return rights.
During the years ended December 31, 2001, 2002 and 2003, 87%, 94%, and 97% and the periods ended September 30, 2003 and 2004, 96% and 97%, respectively, of the Company’s product revenues were derived from customers located in the United States.
Warranty policy
and are reasonably estimable. The Company’s product warranty obligations are included in other accrued liabilities as follows:
| Nine months | |||||||||||||||||||||
| Years ended | ended | ||||||||||||||||||||
| December 31, | September 30, | ||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||
| Balance at beginning of period | $ | 97 | $56 | $20 | $ | 20 | $16 | ||||||||||||||
| Charges to cost of product sales | 25 | 4 | 8 | 6 | 14 | ||||||||||||||||
| Warranty costs incurred | (10 | ) | (22 | ) | (12 | ) | (10 | ) | (21 | ) | |||||||||||
| Change in estimate related to accrued warranty costs | (56 | ) | (18 | ) | — | — | — | ||||||||||||||
| Balance at end of period | $ | 56 | $20 | $16 | $ | 16 | $9 | ||||||||||||||
Research and development
Concentrations of risk
Cash and cash equivalents
Derivative financial instruments
Property and equipment
Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
Intangible assets
Long-lived assets
Income taxes
Stock-based compensation
Pro forma information regarding net loss is required by SFAS 123, as if Adeza had accounted for its employee and director stock options granted under the fair value method of SFAS 123. The fair value
for these options was estimated at the date of grant using the Black-Scholes option pricing model and the following weighted-average assumptions:
| Nine months ended | ||||||||||||||||||||
| Years ended December 31, | September 30, | |||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Volatility factor | 50% | 85% | 85% | 85% | 85% | |||||||||||||||
| Risk-free interest rate | 4.3% | 3.9% | 3.4% | 3.4% | 3.1% | |||||||||||||||
| Dividend yield | 0% | 0% | 0% | 0% | 0% | |||||||||||||||
| Expected life of options | 4.9 years | 4.9 years | 4.0 years | 4.0 years | 4.0 years | |||||||||||||||
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. In addition, the model requires the input of highly subjective assumptions, including the expected life of the option. Because Adeza’s employee and director stock options have characteristics significantly different from those of traded options and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee and director stock options.
For purposes of pro forma disclosures, the estimated fair value of the options is amortized to expense over the options’ vesting period. The pro forma information is as follows:
| Nine months ended | |||||||||||||||||||||
| Years ended December 31, | September 30, | ||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands, except per share information) | |||||||||||||||||||||
| Net income (loss): | |||||||||||||||||||||
| As reported | $ | (5,689 | ) | $ | (329 | ) | $ | 3,235 | $ | 2,085 | $ | 7,283 | |||||||||
| Add: Total stock based employee and director compensation expense determined under intrinsic value method for all awards | — | — | — | — | 166 | ||||||||||||||||
| Less: Total stock based employee and director compensation expense determined under fair value method for all awards | (37 | ) | (916 | ) | (670 | ) | (544 | ) | (1,309 | ) | |||||||||||
| Pro forma | $ | (5,726 | ) | $ | (1,245 | ) | $ | 2,565 | $ | 1,541 | $ | 6,140 | |||||||||
| Reported basic net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 17.78 | $ | 11.46 | $ | 39.97 | |||||||||
| Reported diluted net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 0.26 | $ | 0.17 | $ | 0.55 | |||||||||
| Pro forma basic net income (loss) per share | $ | (36.51 | ) | $ | (6.87 | ) | $ | 14.10 | $ | 8.47 | $ | 33.69 | |||||||||
| Pro forma diluted net income (loss) per share | $ | (36.51 | ) | $ | (6.87 | ) | $ | 0.21 | $ | 0.12 | $ | 0.46 | |||||||||
Allowance for doubtful accounts
Historically, the Company has not experienced significant credit losses related to an individual customer or groups of customers in any particular industry or geographic area.
The Company’s allowance for bad debt is included in accounts receivable as follows:
| Nine months | |||||||||||||||||||||
| Years ended | ended | ||||||||||||||||||||
| December 31, | September 30, | ||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||
| Balance at beginning of period | $ | 27 | $ | 5 | $ | 95 | $ | 95 | $ | 164 | |||||||||||
| Charges to bad debt expense | 22 | — | 112 | 29 | 5 | ||||||||||||||||
| Bad debt costs incurred | (11 | ) | (4 | ) | (43 | ) | (43 | ) | — | ||||||||||||
| Change in estimate | (33 | ) | 94 | — | — | — | |||||||||||||||
| Balance at end of period | $ | 5 | $ | 95 | $ | 164 | $ | 81 | $ | 169 | |||||||||||
In addition to the bad debt reserve, the Company’s allowance for sales returns is included in accounts receivable as follows:
| Nine months | |||||||||||||||||||||
| Years ended | ended | ||||||||||||||||||||
| December 31, | September 30, | ||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | |||||||||||||||||
| (unaudited) | |||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||
| Balance at beginning of period | $ | — | $ | — | $ | 240 | $ | 240 | $ | 100 | |||||||||||
| Charges to product sales returns | — | 159 | 9 | 48 | — | ||||||||||||||||
| Sales returns incurred | — | (2 | ) | (4 | ) | — | — | ||||||||||||||
| Change in estimate | — | 83 | (145 | ) | (109 | ) | (20 | ) | |||||||||||||
| Balance at end of period | $ | — | $ | 240 | $ | 100 | $ | 179 | $ | 80 | |||||||||||
Inventories
Advertising expense
Shipping and handling costs
Reclassifications
Recent accounting pronouncements
Net income (loss) per share
| Nine months ended | ||||||||||||||||||||||
| Years ended December 31, | September 30, | |||||||||||||||||||||
| 2001 | 2002 | 2003 | 2003 | 2004 | ||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||
| (in thousands, except share and per share information) | ||||||||||||||||||||||
| Numerator: | ||||||||||||||||||||||
| Net income (loss) | $ | (5,689 | ) | $ | (329 | ) | $ | 3,235 | $ | 2,085 | $ | 7,283 | ||||||||||
| Denominator: | ||||||||||||||||||||||
| Denominator for basic earnings per share-weighted-average common shares outstanding | 156,857 | 181,188 | 181,965 | 181,900 | 182,223 | |||||||||||||||||
| Effect of dilutive securities: | ||||||||||||||||||||||
| Stock options | — | — | 500,988 | 440,654 | 1,092,208 | |||||||||||||||||
| Warrants | — | — | 28,049 | 8,433 | 132,741 | |||||||||||||||||
| Convertible preferred stock | — | — | 11,804,061 | 11,798,776 | 11,957,322 | |||||||||||||||||
| Dilutive potential common shares | — | — | 12,333,098 | 12,247,863 | 13,182,271 | |||||||||||||||||
| Denominator for diluted earnings per share-weighted-average common shares outstanding and dilutive potential common shares | 156,857 | 181,188 | 12,515,063 | 12,429,763 | 13,364,494 | |||||||||||||||||
| Denominator for basic earnings per share | 181,965 | 182,223 | ||||||||||||||||||||
| Assumed conversion of preferred stock (unaudited) | 11,804,061 | 11,957,322 | ||||||||||||||||||||
| Denominator for pro forma basic earnings per share (unaudited) | 11,986,026 | 12,139,545 | ||||||||||||||||||||
| Effect of other dilutive securities: | ||||||||||||||||||||||
| Stock options (unaudited) | 500,988 | 1,092,200 | ||||||||||||||||||||
| Warrants (unaudited) | 28,049 | 132,741 | ||||||||||||||||||||
| Denominator for pro forma dilutive earnings per share (unaudited) | 12,515,063 | 13,364,494 | ||||||||||||||||||||
| Basic net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 17.78 | $ | 11.46 | $ | 39.97 | ||||||||||
| Diluted net income (loss) per share | $ | (36.27 | ) | $ | (1.82 | ) | $ | 0.26 | $ | 0.17 | $ | 0.55 | ||||||||||
| Pro forma basic net income per share (unaudited) | $ | 0.27 | $ | 0.60 | ||||||||||||||||||
| Pro forma diluted net income per share (unaudited) | $ | 0.26 | $ | 0.55 | ||||||||||||||||||
| Weighted average common shares related to securities not included above as anti-dilutive: | ||||||||||||||||||||||
| Stock options | 701,478 | 1,145,449 | — | — | — | |||||||||||||||||
| Warrants | 371,748 | 355,465 | — | 196,915 | — | |||||||||||||||||
| Convertible preferred stock | 11,756,719 | 11,798,776 | — | — | — | |||||||||||||||||
| Total | 12,829,945 | 13,299,690 | — | 196,915 | — | |||||||||||||||||
On September 9, 2003, the Company completed the acquisition of substantially all of the assets of Biex, Inc. to expand its diagnostic product pipeline. Biex’s assets primarily consisted of several patents relating to FDA-approved preterm labor diagnostic testing products. The Biex acquisition has been accounted for as an acquisition of assets rather than as a business combination in accordance with the criteria outlined in Emerging Issues Task Force 98-3, because, at the date of acquisition, Biex lacked all of the elements of a business because it did not have any employees or processes.
The aggregate purchase price of the Biex assets was $250,000 in cash. The following table summarizes the purchase price allocation (in thousands):
| Fixed assets | $ | 10 | ||
| Patents | 240 | |||
| Total purchase price | $ | 250 | ||
The purchased patents relate to methods of predicting premature labor. The Company believes that the patents may allow for complementary products to its existing product lines.
The Company allocated the purchase price to the tangible and intangible assets acquired based on their estimated fair values. Such valuations require management to make significant estimates and assumptions, especially with respect to intangible assets. Management’s estimates of fair value are based upon assumptions believed to be reasonable, but which are inherently uncertain and unpredictable.
Adeza has entered into license, clinical trial, supply, and sponsored research and development agreements with universities, research organizations, and commercial companies. Certain of these agreements require payments of royalties on future sales of products resulting from such agreements and may subject Adeza to minimum annual royalty payments to such contract partners. During the years ended December 31, 2001, 2002, and 2003, the total of such royalty costs recorded were approximately $368,000, $1,399,000 and $3,187,000, respectively, and $2,449,000 and $1,439,000 for the nine months ended September 30, 2003 and 2004, respectively, excluding the effect of a one-time reduction in accrued royalties of $2.7 million that was recorded in the nine-month period ended September 30, 2004. Royalty costs are included in cost of product sales. The one-time reduction of $2.7 million was primarily related to significant new information that Adeza received in October 2004 which allowed Adeza to conclude that no royalties were due or are required under a license agreement.
In June 1999, Adeza entered into a Co-Promotion and Exclusive Distribution Agreement with the Distributor. This agreement was mutually terminated by both parties as of June 30, 2002. Under the terms of this agreement, the Distributor had the right to co-market and distribute, within the United States of America and its territories and possessions excluding the Commonwealth of Puerto Rico, certain existing products and next-generation improvements to those existing products related to their use for certain clinical indications owned by Adeza.
In consideration of the co-promotion and exclusive distribution rights, and other terms and conditions, the Distributor paid Adeza a nonrefundable one-time payment of $2,000,000 in 1999. The Distributor was also to pay Adeza a total of up to $2,000,000 during 2001 and 2002 to fund certain portions of
the research and development efforts related to the products and services covered by this agreement. During the years ended December 31, 2001, 2002 and 2003, the Company received $244,000, $176,000, and $0, respectively, of such research and development funding. No amounts were received in the nine months ended September 30, 2003 and 2004. This funding is included within contract revenue in the statements of operations.
The 1999 nonrefundable, one-time $2,000,000 payment received from the Distributor was deferred and was being recognized as revenue over the estimated five-year life of the agreement. At the termination of the agreement, Adeza recognized the balance of the deferred contract revenue in 2002, net of costs related to the contract termination. The amount of revenue recognized for the years ended December 31, 2001, 2002 and 2003 was $400,000, $574,000, and $0, respectively.
As a result of the mutual termination of the contract, Adeza agreed to pay the Distributor $5,771,000. This amount, primarily related to deferred product revenue received by Adeza from sales to the Distributor, was reclassified from deferred revenue to notes payable excluding $273,000 of imputed interest to be recorded over the repayment period, in 2002 (see Note 7).
Inventories consist of the following (in thousands):
| December 31, | ||||||||||||
| September 30, | ||||||||||||
| 2002 | 2003 | 2004 | ||||||||||
| (unaudited) | ||||||||||||
| Raw materials | $ | 252 | $ | 320 | $ | 308 | ||||||
| Work in process | 158 | 136 | 145 | |||||||||
| Finished goods | 103 | 134 | 144 | |||||||||
| $ | 513 | $ | 590 | $ | 597 | |||||||
Property and equipment consists of the following (in thousands):
| December 31, | ||||||||||||
| September 30, | ||||||||||||
| 2002 | 2003 | 2004 | ||||||||||
| (unaudited) | ||||||||||||
| Laboratory and other equipment | $ | 2,033 | $ | 2,120 | $ | 2,217 | ||||||
| Furniture and fixtures | 150 | 152 | 156 | |||||||||
| Leasehold improvements | 130 | 130 | 130 | |||||||||
| 2,313 | 2,402 | 2,503 | ||||||||||
| Less accumulated depreciation and amortization | (2,061 | ) | (2,150 | ) | (2,203 | ) | ||||||
| Total net property and equipment | $ | 252 | $ | 252 | $ | 301 | ||||||
Included in laboratory and other equipment at December 31, 2002 are assets with a cost of $70,000 acquired pursuant to capital lease obligations. Related accumulated amortization for these leased assets was $51,000 as of December 31, 2002. During 2003, the capital lease reached its term. As such, the assets underlying this lease were purchased and are no longer carried under capital lease. The assets continue to be included within laboratory and other equipment.
Leases
| Year ending December 31, 2004 | $ | 59 |
In October 2004, the Company entered into a facility lease for a one-year term with two one-year renewal options. Future minimum obligations under this lease at September 30, 2004 are payments totaling $200,000 through October 2005.
Rent expense was approximately $300,000, $297,000, $195,000, $146,000, and $153,000 for the years ended December 31, 2001, 2002, 2003, and the nine-month periods ended September 30, 2003 and 2004 (unaudited), respectively.
Notes payable
In June 2002, Adeza entered into an agreement to terminate the Co-Promotion and Distribution Agreement between Adeza and the Distributor (see Note 4). Under the terms of the agreement, Adeza agreed to pay the Distributor $5,771,000 under a note payable due through June 30, 2003. In December 2002, $1,443,000 was paid to the Distributor. The remaining payments were paid in March and June 2003. The note was secured by Adeza’s assets. In the years ended December 31, 2002 and 2003, Adeza recorded $167,000 and $106,000, respectively, of interest expense related to this note. $106,000 was recorded in the nine-month period ended September 30, 2003. No interest expense was recorded in the nine-month period ended September 30, 2004.
Convertible preferred stock
| December 31, 2003 and September 30, 2004 | ||||||||||||
| Shares | ||||||||||||
| Shares | issued and | Liquidation | ||||||||||
| authorized | outstanding | preference | ||||||||||
| Series 1 | 1,880,572 | 1,654,719 | $ | 3,971 | ||||||||
| Series 2 | 3,591,087 | 3,496,750 | 8,392 | |||||||||
| Series 3 | 5,084,676 | 4,807,077 | 14,037 | |||||||||
| Series 4 | 2,700,000 | 2,203,108 | 20,401 | |||||||||
| Series 5 | 3,260,000 | 3,247,408 | 30,071 | |||||||||
| Total | 16,516,335 | 15,409,062 | $ | 76,872 | ||||||||
Upon completion of, and subordinate to, the distribution of any Series 5 convertible preferred stock dividends, each share of Series 4 convertible preferred stock entitles the holder to receive, if and as declared, cumulative dividends of $0.463. Upon completion of and subordinate to the distribution to holders of Series 4 convertible preferred stock dividends, each share of Series 1, 2, and 3 convertible preferred stock entitles the holder to receive, if and as declared, noncumulative dividends of $0.24, $0.24, and $0.292 per share, respectively. If dividends are declared on Series 1, 2, 3, or 4 convertible preferred stock, all such series of convertible preferred stock must receive dividends. Series 5 convertible preferred stock entitles the holder to receive, if and as declared, cumulative dividends of $0.463 per share. No dividends have been declared to date.
Each share of Series 1, 2, 4, and 5 convertible preferred stock is convertible, at the option of the holder, into 0.75 of a share of common stock. Each share of Series 3 convertible preferred stock is convertible, at the option of the holder, into 0.83333 of a share of common stock. The Series 1, 2, 3, 4, and 5 convertible preferred stock is convertible at the option of the holder, automatically upon a public offering with a price per share of at least $9.27 and aggregate proceeds greater than $15,000,000 or on the affirmative vote or written consent of the holders of at least 66 2/3% of Series 1, 2, 3, 4, or 5 convertible preferred stockholders consenting as a single class, respectively.
Upon liquidation of Adeza, the holders of Series 5 convertible preferred stock shall have a liquidation preference, prior and in preference to any distribution to the holders of Series 1, 2, 3, and 4 convertible preferred stock of $9.26 per share plus any declared but unpaid dividends. Following the liquidation payments to the holders of Series 5 preferred stock, the holders of Series 4 convertible preferred stock shall have a liquidation preference, prior and in preference to any distribution to the holders of Series 1, 2, or 3 convertible preferred stock of $9.26 per share plus any declared but unpaid dividends. Following the liquidation payments to the holders of Series 4 and 5 preferred stock, the holders of Series 1, 2, and 3 convertible preferred stock shall be entitled to $2.40, $2.40, and $2.92 per share, respectively, plus any declared but unpaid dividends. A merger or consolidation of the Company in which the Company receives a distribution in cash or securities of another company shall be treated as a liquidation unless the stockholders of the Company will hold at least 50% of the voting equity securities of the surviving company. This change-in-control provision requires the convertible preferred stock to be classified outside of stockholders’ equity as a purchaser of the Company could acquire the required percentage of the voting power of the outstanding stock without company approval. Therefore, such a payment to the preferred stockholders is not solely within the Company’s control. The carrying value of the convertible preferred stock has not been adjusted to the liquidation value of such shares as it is not probable that such a payment will occur due to the potential for conversion of the preferred stock prior to any such change-in-control.
After the payments to holders of preferred stock as described above, the holders of common stock are entitled to receive $0.3867 per share. Any remaining assets of Adeza available for distribution shall be distributed ratably among the holders of common stock and preferred stock on an as-if-converted basis.
The Series 1, 2, 3, 4, and 5 preferred stockholders have voting rights equal to the common stockholders on an as-if-converted basis.
The Company has reserved 236,301 shares of convertible preferred stock for issuance upon the exercise of warrants at December 31, 2003 and September 30, 2004.
Warrants
In conjunction with the loan and security agreement with MMC/ GATX Partnership No. 1 and Transamerica Business Credit Corporation, Adeza issued warrants to purchase 236,301 shares of Series 3 convertible preferred stock at an exercise price of $2.92 per share. The warrants are scheduled to expire on the later of ten years from the date of the grant or five years after the closing of a public offering. The fair value assigned to these warrants, as determined using the Black-Scholes valuation model, was approximately $475,000. In determining the fair value of the warrants the following assumptions were used: expected volatility of 50%; expected life of 10 years; expected dividend yield of 0%; risk-free interest rate of 6%; stock price at date of grant and exercise price of $2.92 per share. The fair value of these warrants was netted against the related debt and was amortized to interest expense over the terms of the various notes. In the years ended December 31, 2001, 2002 and 2003, and the nine month periods ended September 30, 2003 and 2004, Adeza amortized as interest expense $150,000, $33,000, $24,000, $2,600 and $0, respectively, related to these warrants. The warrants are outstanding and exercisable at September 30, 2004.
All preferred stock underlying the warrants is convertible into common stock at a conversion ratio of 1:0.83333.
Common stock
| December 31, | ||||||||||||
| September 30, | ||||||||||||
| 2002 | 2003 | 2004 | ||||||||||
| (unaudited) | ||||||||||||
| Options outstanding | 1,446,429 | 1,471,404 | 2,163,582 | |||||||||
| Shares reserved for future option grants | 160,119 | 140,715 | 17,056 | |||||||||
| Convertible preferred stock issued and outstanding | 11,798,776 | 11,957,322 | 11,957,322 | |||||||||
| Warrants outstanding— convertible preferred stock | 339,612 | 196,915 | 196,915 | |||||||||
| 13,744,936 | 13,766,356 | 14,334,875 | ||||||||||
Stock option plan
Option activity under the 1995 Plan is as follows:
| Options | Options | Weighted- | |||||||||||
| available | outstanding | average | |||||||||||
| for grant | and exercisable | exercise price | |||||||||||
| Balance at December 31, 2001 | 890,458 | 712,677 | $ | 2.29 | |||||||||
| Shares authorized | 6,251 | — | |||||||||||
| Options granted | (745,390 | ) | 745,390 | $ | 3.33 | ||||||||
| Options exercised | — | (2,838 | ) | $ | 1.44 | ||||||||
| Options canceled | 8,800 | (8,800 | ) | $ | 2.92 | ||||||||
| Balance at December 31, 2002 | 160,119 | 1,446,429 | $ | 2.29 | |||||||||
| Shares authorized | 6,351 | — | — | ||||||||||
| Options granted | (34,622 | ) | 34,622 | $ | 3.33 | ||||||||
| Options exercised | — | (780 | ) | 0.32 | |||||||||
| Options canceled | 8,867 | (8,867 | ) | $ | 3.05 | ||||||||
| Balance at December 31, 2003 | 140,715 | 1,471,404 | $ | 2.32 | |||||||||
Shares authorized(unaudited) | 568,878 | — | — | ||||||||||
Options granted(unaudited) | (703,276 | ) | (703,276 | ) | $ | 9.65 | |||||||
Options exercised(unaudited) | — | (359 | ) | — | |||||||||
Options canceled(unaudited) | 10,739 | (10,739 | ) | $ | 2.99 | ||||||||
Balance at September 30, 2004(unaudited) | 17,056 | 2,163,582 | $ | 4.70 | |||||||||
The following summarizes options outstanding and exercisable as of December 31, 2003:
| Options outstanding and exercisable | ||||||||||||
| Weighted- | ||||||||||||
| average | ||||||||||||
| remaining | ||||||||||||
| contractual | Weighted- | |||||||||||
| Number | life | average | ||||||||||
| Exercise prices | outstanding | (in years) | exercise price | |||||||||
| $0.32 | 141,726 | 1.42 | $ | 0.32 | ||||||||
| $0.97 | 452,948 | 3.91 | $ | 0.97 | ||||||||
| $3.33 | 872,980 | 8.29 | $ | 3.33 | ||||||||
| $6.17 | 3,750 | 9.58 | $ | 6.17 | ||||||||
| 1,471,404 | 6.28 | $ | 2.32 | |||||||||
The following summarizes options outstanding and exercisable as of September 30, 2004:
| Options outstanding and exercisable | ||||||||||||
| Weighted- | ||||||||||||
| average | ||||||||||||
| remaining | ||||||||||||
| contractual | Weighted- | |||||||||||
| Number | life | average | ||||||||||
| Exercise price | outstanding | (in years) | exercise price | |||||||||
| $ 0.32 | 141,463 | 0.68 | $ | 0.32 | ||||||||
| $ 0.97 | 451,705 | 3.16 | $ | 0.97 | ||||||||
| $ 3.33 | 883,414 | 7.58 | $ | 3.33 | ||||||||
| $ 6.17 | 3,750 | 8.83 | $ | 6.17 | ||||||||
| $ 8.51 | 76,125 | 13.84 | $ | 8.51 | ||||||||
| $10.00 | 607,125 | 9.84 | $ | 10.00 | ||||||||
| 2,163,582 | 7.06 | $ | 4.70 | |||||||||
During the nine months ended September 30, 2004, the Company recorded deferred stock compensation for the excess of the estimated fair value of its common stock over option exercise prices at the date of grant of approximately $3,625,000 related to options granted to employees and directors. Stock-based compensation expense is being recognized over the option vesting period of four years using the straight-line method.
For options granted to nonemployees, the Company determined the estimated fair value of the options using the Black-Scholes option pricing model. Compensation expense is generally being recognized over the option vesting period. For the years ended December 31, 2002 and 2003 and the nine months ended September 30, 2004, the Company recorded stock-based compensation expense of approximately $5,000, $23,000 and $288,000, respectively, in connection with options granted to nonemployees. No stock-based compensation expense was recorded for the years ended December 31, 2001 and the nine months ended September 30, 2003.
The Company’s accumulated deficit differs from the federal net operating loss carryforwards due to the losses of Adeza’s former foreign subsidiary and temporary differences, which consist primarily of certain expenses not currently deductible for tax reporting purposes.
The provision for income taxes consists of the following:
| Years ended | |||||||||||||
| December 31, | |||||||||||||
| 2001 | 2002 | 2003 | |||||||||||
| (in thousands) | |||||||||||||
| Current: | |||||||||||||
| Federal | $ | — | $ | — | $ | 65 | |||||||
| State | — | — | 70 | ||||||||||
| Total current | $ | — | $ | — | $ | 135 | |||||||
The Company’s income tax provision differs from the amounts computed by applying the federal statutory income tax rate of 34% to pretax income (loss) as follows:
| Years ended December 31, | |||||||||||||
| 2001 | 2002 | 2003 | |||||||||||
| (in thousands) | |||||||||||||
| U.S. federal taxes (benefit) | |||||||||||||
| Expected provision at federal statutory rate | $ | (1,934 | ) | $ | (112 | ) | $ | 1,146 | |||||
| State taxes, net of federal benefit | — | — | 46 | ||||||||||
| Net operating losses not benefitted (benefitted) | 1,899 | 61 | (1,132 | ) | |||||||||
| Other individually immaterial items | 35 | 51 | 75 | ||||||||||
| Total | $ | — | $ | — | $ | 135 | |||||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows:
| December 31, | |||||||||||||
| 2001 | 2002 | 2003 | |||||||||||
| (in thousands) | |||||||||||||
| Deferred tax assets: | |||||||||||||
| Net operating losses | $ | 15,500 | $ | 15,900 | $ | 15,040 | |||||||
| Research credits | 1,400 | 1,500 | 1,740 | ||||||||||
| Capitalized research and development | 600 | 900 | 180 | ||||||||||
| Deferred revenue | 3,100 | 100 | — | ||||||||||
| Other, net | 400 | 800 | 380 | ||||||||||
| Total deferred tax assets | 21,000 | 19,200 | 17,340 | ||||||||||
| Valuation allowance | (21,000 | ) | (19,200 | ) | (17,340 | ) | |||||||
| Net deferred tax assets | $ | — | $ | — | $ | — | |||||||
Because of Adeza’s lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance (increased) decreased by $(3,200,000), $1,800,000 and $1,860,000 during the years ended December 31, 2001, 2002 and 2003, respectively.
As of December 31, 2003, the Company had federal and state net operating loss carryforwards of approximately $40,300,000 and $22,800,000, respectively. The Company also had federal and state research and development tax credit carryforwards of approximately $1,100,000 and $900,000, respectively. The federal net operating loss and tax credit carryforwards will expire at various dates beginning in 2004 through 2022, if not utilized. The state net operating loss carryforwards will expire at various dates beginning in 2004 through 2013, if not utilized. The state research and development tax credits carry forward indefinitely.
Utilization of the Company’s net operating losses and research and development tax credits may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation could result in the expiration of the net operating losses and research and development tax credits before utilization.
The Company’s 401(k) Plan allows eligible employees to make contributions of their qualified compensation subject to IRS limits. The Company has the discretion to make matching contributions each year. The Company has not made any matching contributions to date.
In 2000, a loan offer was made to an officer of the Company. The agreement to the loan was ratified by the Board of Directors on April 21, 2001, for an amount of $183,000, with the minimum interest rate allowed by the internal revenue service. According to the terms agreed upon, 20% of the loan would be forgiven in principal and accrued interest at the end of each twelve months of employment. The loan would be due and payable within 30 days following termination by Adeza for cause. In the event of a change of control or merger with another company or of termination without cause, the loan and accumulated interest would be forgiven. The loan contemplated was executed on February 28, 2003 for $109,800 which was paid to the officer at that time. All other terms were in accordance with the original loan offer. Subject to the officer’s continued employment, the loan and related interest would have been forgiven in 2003 to 2006. On August 4, 2004, the Company, upon approval of its Board of Directors, forgave the remaining balance of the loan. In the year ended December 31, 2003 and the nine months ended to September 30, 2004, $33,550 and $76,000, respectively, of the principal was forgiven and recorded to general and administrative expenses.
Employee option plans
2004 Equity Incentive Plan
1995 Stock Option and Restricted Stock Plan

Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, to be paid by the Registrant in connection with the sale of the common stock being registered. All amounts other than the SEC registration fee, the NASD filing fees and the Nasdaq National Market listing fee are estimates.
| Amount | |||||
| to be paid | |||||
| SEC registration fee | $ | 8,742.30 | |||
| NASD filing fee | 7,400.00 | ||||
| Nasdaq National Market application fee | 5,000.00 | ||||
| Nasdaq National Market entry fee | 95,000.00 | ||||
| Nasdaq National Market annual fee (prorated for 2004) | 4,416.67 | ||||
| Legal fees and expenses | 900,000.00 | ||||
| Accounting fees and expenses | 500,000.00 | ||||
| Printing and engraving | 200,000.00 | ||||
| Blue Sky fees and expenses (including legal fees) | 20,000.00 | ||||
| Transfer agent and registrar fees | 5,000.00 | ||||
| Miscellaneous | 54,441.03 | ||||
| Total | $ | 1,800,000.00 | |||
Item 14. Indemnification of directors and officers
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act of 1933, as amended (the “Securities Act”).
As permitted by the Delaware General Corporation Law, the Registrant’s amended restated certificate of incorporation includes a provision that eliminates the personal liability of its directors for monetary damages for breach of fiduciary duty as a director.
As permitted by the Delaware General Corporation Law, the bylaws of the Registrant provide that (1) the Registrant is required to indemnify its directors and officers to the fullest extent permitted by the Delaware General Corporation Law, subject to certain very limited exceptions, (2) the Registrant is required to advance expenses, as incurred, to its directors and executive officers in connection with a legal proceeding to the fullest extent permitted by the Delaware General Corporation Law, subject to certain very limited exceptions and (3) the rights conferred in the restated bylaws are not exclusive.
The Registrant has entered into indemnification agreements with each of its directors and executive officers to give such directors and officers additional contractual assurances regarding the scope of the indemnification set forth in the Registrant’s restated certificate of incorporation and to provide additional procedural protections. The Registrant also intends to enter into indemnification agreements with any new directors and executive officers in the future. At present, there is no pending litigation or proceeding involving any of our directors, officers, employees, or agents, where indemnification by us will be required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for such indemnification.
The Underwriting Agreement provides for indemnification by the underwriters of the officers, directors and controlling persons of the Registrant against certain liabilities, including liabilities arising under the Securities Act. Reference is made to the form of underwriting agreement filed as Exhibit 1.1 hereto.
The indemnification provisions in the Registrant’s restated certificate of incorporation, restated bylaws and the indemnification agreements entered into between the Registrant and each of its directors and executive officers may be sufficiently broad to permit indemnification of the Registrant’s directors and executive officers for liabilities arising under the Securities Act.
The Registrant has obtained liability insurance for its officers and directors.
Reference is made to the following documents filed as exhibits to this Registration Statement regarding relevant indemnification provisions described above and elsewhere in this prospectus:
| Exhibit | ||||
| Document | number | |||
| Form of Underwriting Agreement | 1.1 | |||
| Form of Restated Certificate of Incorporation of Registrant | 3.2 | |||
| Form of Restated Bylaws of Registrant | 3.4 | |||
| Form of Indemnification Agreement for Directors and Officers | 10.12 | |||
Item 15. Recent sales of unregistered securities
In the preceding three years, beginning October 1, 2001 and ending December 3, 2004, the filing of this registration statement, we have issued the following securities that were not registered under the Securities Act of 1933, as amended (the “Securities Act”). The offers, sales and issuances of these securities were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, and/or Regulation D and the other rules and regulations promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions not involving a public offering or transactions under compensatory benefit plans and contracts relating to compensation as provided under such Rule 701. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and warrants issued in such transactions. All common share amounts give effect to a three-for-four reverse stock split of our common stock effected on December 6, 2004.
1. We have issued options to purchase an aggregate of 1,483,438 shares of common stock to our directors, employees and consultants, of which options to purchase 796,438 shares were granted at an exercise price of $3.33 per share, options to purchase 607,125 shares of common stock were granted at an exercise price of $10.00 per share, options to purchase 3,750 shares of common stock were granted at an exercise price of $6.17, options to purchase 76,125 shares of common stock were granted at an exercise price of $8.51 and 9,266 shares of common stock have been issued upon exercise of options. The sales of the above securities were deemed to be exempt from registration pursuant to either Section 4(2) of the Securities Act (in the case of our officers) or Rule 701 promulgated under the Securities Act (in the case of optionees other than officers).
2. In September 2001, we issued 3,247,408 shares of our Series 5 preferred stock to accredited investors for an aggregate cash consideration of approximately $15,035,500. The issuance of these securities was exempt from registration under the Securities Act pursuant to Rule 506 under Regulation D promulgated under the Securities Act, and a Form D was filed with respect to this issuance.
3. In November 2001, we issued 18,750 shares of our common stock to Fred Hutchinson Cancer Research Center, Inc. for an aggregate cash consideration of $250. The sale of the shares was exempt from registration pursuant to Section 4(2) of the Securities Act.
4. In December 2003, we issued 190,259 shares of our Series 3 preferred stock upon exercise of warrants to STF II L.P., Asset Management Associates 1984, L.P., Asset Management Associates 1989,
Item 16. Exhibits and Financial Statement Schedules
| Exhibit | ||||
| number | Description | |||
| 1.1* | * | Form of Underwriting Agreement. | ||
| 3.1* | * | Amended and Restated Certificate of Incorporation. | ||
| 3.2* | * | Form of Amended and Restated Certificate of Incorporation to be effective upon the completion of the offering. | ||
| 3.3* | * | Bylaws. | ||
| 3.4* | * | Form of Amended and Restated Bylaws to be effective upon the completion of the offering. | ||
| 3.5 | Certificate of Amendment of Amended and Restated Certificate of Incorporation. | |||
| 4.1* | * | Specimen Stock Certificate. | ||
| 5.1* | * | Opinion of Heller Ehrman White & McAuliffe LLP. | ||
| 10.1* | * | 1995 Stock Option and Restricted Stock Plan. | ||
| 10.2* | * | 2004 Equity Incentive Plan. | ||
| Exclusive License Agreement, dated August 12, 1992, between Adeza and the Fred Hutchinson Cancer Research Center, together with the First Amendment to Exclusive License Agreement and Consent dated May 9, 1996 and Amendment No. 1 to Exclusive License Agreement dated April 30, 1998.† | ||||
| 10.4* | * | Investors’ Rights Agreement, dated September 19, 2001, between Adeza and certain Stockholders of Adeza. | ||
| 10.5* | * | License Agreement, dated July 25, 1997, between Adeza and the Trustees of the University of Pennsylvania.† | ||
| Agreement and Release, dated March 3, 1998, between Adeza and Matria Healthcare, Inc.† | ||||
| * | Net Industrial Space Lease, dated July 7, 1999, between Adeza and Tasman V, LLC. | |||
| 10.8* | * | Service Agreement, dated as of March 31, 1999, between Adeza and Ventiv Health U.S. Sales LLC (formerly known as Snyder Healthcare Sales Inc.), together with First Amendment to Service Agreement dated March 8, 2002, Second Amendment to Service Agreement dated July 22, 2002, and Third Amendment to Service Agreement dated May 15, 2004.† | ||
| 10.9* | * | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 23, 1999, between Adeza and Transamerica Business Credit Corporation and its assignees. | ||
| 10.10 | ** | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 31, 2000, between Adeza and TBCC Funding Trust II and its assignees. | ||
| 10.11 | ** | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 23, 1999, between Adeza and MMC/GATX Partnership No. I and its assignees. | ||
| 10.12 | ** | Form of Indemnification Agreement for Directors and Officers. | ||
| 10.13 | ** | Agreement, dated December 24, 1998, between Adeza and Unilever PLC.† | ||
| 10.14 | ** | Second Amendment to Lease, dated October 12, 2004, between Adeza and Tasman V, LLC. | ||
| 10.15 | ** | Management Continuity Agreement, dated October 21, 2004, between Adeza and Emory Anderson. | ||
| 10.16 | ** | Management Continuity Agreement, dated October 21, 2004, between Adeza and Mark Fischer-Colbrie. | ||
| 10.17 | ** | Form of Management Continuity Agreement, dated October 21, 2004, between Adeza and Durlin Hickok, Robert Hussa and Marian Sacco. | ||
| * | Consent of Ernst & Young LLP, independent registered public accounting firm. | |||
| 23.2* | * | Consent of Heller Ehrman White & McAuliffe LLP (included in Exhibit 5.1). | ||
| 24.1* | * | Powers of Attorney (included on signature page). | ||
| * | To be filed by amendment. |
| ** | Previously filed. |
| † | Application has been made to the Securities and Exchange Commission to seek confidential treatment of certain provisions of this exhibit under Rule 406 of the Securities Act of 1933. Omitted material for which confidential treatment has been requested has been filed separately with the Securities and Exchange Commission. |
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424 (b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and | |
| (2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fideoffering thereof. |
Signatures
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 45 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Menlo Park, California on this 6th7th day of December, 2004.
| ADEZA BIOMEDICAL CORPORATION |
| By: | /s/ EMORY V. ANDERSON |
| Emory V. Anderson | |
| President and Chief Executive Officer |
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 45 to the Registration Statement has been signed by the following persons in the capacities indicated on December 6,7, 2004:
| Signature | Title(s) | |||
| /s/ EMORY V. ANDERSON Emory V. Anderson | President, Chief Executive Officer and Director (principal executive officer) | |||
| /s/ MARK D. FISCHER-COLBRIE Mark D. Fischer-Colbrie | Vice President, Finance and Administration and Chief Financial Officer (principal financial and accounting officer) | |||
| /s/ ANDREW E. SENYEI, MD* Andrew E. Senyei, MD | Chairman of the Board | |||
| /s/ NANCY D. BURRUS* Nancy D. Burrus | Director | |||
| /s/ CRAIG C. TAYLOR* Craig C. Taylor | Director | |||
| /s/ KATHLEEN D. LAPORTE* Kathleen D. LaPorte | Director | |||
| *By: | ||||
| /s/ EMORY V. ANDERSON Emory V. Anderson Attorney-in-Fact | ||||
Exhibit Index
| Exhibit | ||||
| number | Description | |||
| 1.1** | Form of Underwriting Agreement. | |||
| 3.1** | Amended and Restated Certificate of Incorporation. | |||
| 3.2** | Form of Amended and Restated Certificate of Incorporation to be effective upon the completion of the offering. | |||
| 3.3** | Bylaws. | |||
| 3.4** | Form of Amended and Restated Bylaws to be effective upon the completion of the offering. | |||
| 3.5 | Certificate of Amendment of Amended and Restates Certificate of Incorporation. | |||
| 4.1** | Specimen Stock Certificate. | |||
| 5.1** | Opinion of Heller Ehrman White & McAuliffe LLP. | |||
| 10.1** | 1995 Stock Option and Restricted Stock Plan. | |||
| 10.2** | 2004 Equity Incentive Plan. | |||
| Exclusive License Agreement, dated August 12, 1992, between Adeza and the Fred Hutchinson Cancer Research Center, together with the First Amendment to Exclusive License Agreement and Consent dated May 9, 1996 and Amendment No. 1 to Exclusive License Agreement dated April 30, 1998.† | ||||
| 10.4** | Investors’ Rights Agreement, dated September 19, 2001, between Adeza and certain Stockholders of Adeza. | |||
| 10.5** | License Agreement, dated July 25, 1997, between Adeza and the Trustees of the University of Pennsylvania.† | |||
| Agreement and Release, dated March 3, 1998, between Adeza and Matria Healthcare, Inc.† | ||||
| Net Industrial Space Lease, dated July 7, 1999, between Adeza and Tasman V, LLC. | ||||
| 10.8** | Service Agreement, dated as of March 31, 1999, between Adeza and Ventiv Health U.S. Sales LLC (formerly known as Snyder Healthcare Sales Inc.), together with First Amendment to Service Agreement dated March 8, 2002, Second Amendment to Service Agreement dated July 22, 2002, and Third Amendment to Service Agreement dated May 15, 2004.† | |||
| 10.9** | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 23, 1999, between Adeza and Transamerica Business Credit Corporation and its assignees. | |||
| 10.10* | * | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 31, 2000, between Adeza and TBCC Funding Trust II and its assignees. | ||
| 10.11* | * | Warrant to Purchase Shares of Series 3 Preferred Stock, dated March 23, 1999, between Adeza and MMC/GATX Partnership No. I and its assignees. | ||
| 10.12* | * | Form of Indemnification Agreement for Directors and Officers. | ||
| 10.13* | * | Agreement, dated December 24, 1998, between Adeza and Unilever PLC.† | ||
| 10.14* | * | Second Amendment to Lease, dated October 12, 2004, between Adeza and Tasman V, LLC. | ||
| 10.15* | * | Management Continuity Agreement, dated October 21, 2004, between Adeza and Emory Anderson. | ||
| 10.16* | * | Management Continuity Agreement, dated October 21, 2004, between Adeza and Mark Fischer-Colbrie. | ||
| 10.17* | * | Form of Management Continuity Agreement, dated October 21, 2004, between Adeza and Durlin Hickok, Robert Hussa and Marian Sacco. | ||
| Consent of Ernst & Young LLP, independent registered public accounting firm. | ||||
| 23.2** | Consent of Heller Ehrman White & McAuliffe LLP (included in Exhibit 5.1). | |||
| 24.1** | Powers of Attorney (included on signature page). | |||
| * | To be filed by amendment. |
| ** | Previously filed. |
| † | Application has been made to the Securities and Exchange Commission to seek confidential treatment of certain provisions of this exhibit under Rule 406 of the Securities Act of 1933. Omitted material for which confidential treatment has been requested has been filed separately with the Securities and Exchange Commission. |