As filed with the Securities and Exchange Commission on May 17, 2010January 31, 2011
RegistrationNo. 333-166146333-__________
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
Amendment No. 2
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
REGENERX BIOPHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
|
| Delaware | | 2834 | | 52-1253406 |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
15245 Shady Grove Road, Suite 470
Rockville, MD 20850
(301) 208-9191
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
J.J. Finkelstein
President and Chief Executive Officer
15245 Shady Grove Road, Suite 470
Rockville, MD 20850
(301) 208-9191
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| | |
Darren K. DeStefano, Esq.
Brian F. Leaf, Esq.
Cooley LLP
One Freedom Square, Reston Town Center
11951 Freedom Drive
Reston, VA 20190-5656
Brian F. Leaf, Esq.
Cooley LLP
One Freedom Square, Reston Town Center
11951 Freedom Drive
Reston, VA20190-5656
(703) 456-8000 | | Steven M. Skolnick, Esq.
Lowenstein Sandler PC
65 Livingston Avenue
Roseland, NJ 07068-1791
(973) 597-2382 |
Approximate date of commencement of proposed sale to the public:As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration number of the earlier effective registration statement for the same offering.o
CALCULATION OF REGISTRATION FEE
| | | | | | | | | | | | | | | | | | | |
| | | | | | | Proposed Maximum
| | | Proposed Maximum
| | | Amount of
|
Title of Each Class of
| | | Amount to be
| | | Offering
| | | Aggregate
| | | Registration
|
| Securities Being Registered | | | Registered(1) | | | Price per Security(2) | | | Offering Price(2) | | | Fee |
| Units, each consisting of one share of common stock, $0.001 par value per share, and 0.4 warrants to purchase common stock(1) | | | 13,225,000
units | | | $ | 0.56 | | | | $ | 7,406,000 | | | | $ | 528.05 | |
| Common Stock, $0.001 par value per share, included in the Units(1) | | | 13,225,000
shares | | | | — | | | | | — | | | | | —(3 | ) |
| Warrants included in the Units(1) | | | 5,290,000
warrants | | | | — | | | | | — | | | | | —(3 | ) |
| Shares of common stock underlying the warrants included in the Units | | | 5,290,000
shares | | | $ | 0.62 | | | | $ | 3,279,800 | | | | $ | 233.85 | |
| Representative’s Warrant | | | 1 warrant | | | $ | 100.00 | | | | $ | 100 | | | | $ | 0.01 | |
| Shares of common stock underlying the Representative’s Warrant | | | 805,000
shares | | | $ | 0.62 | | | | $ | 499,100 | | | | $ | 35.59 | |
| Total | | | | | | $ | | | | | $ | 11,185,000 | | | | $ | 797.50(5 | ) |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| |
| | | | | | | | Proposed Maximum | | | Proposed Maximum | | | Amount of | |
| | Title of Each Class of | | | Amount to be | | | Offering | | | Aggregate | | | Registration | |
| | Securities Being Registered | | | Registered(1) | | | Price per Share(2) | | | Offering Price(2) | | | Fee | |
| | Common Stock, $0.001 par value per share | | | 15,000,000 shares | | | $ | 0.22 | | | | $ | 3,300,000 | | | | $ | 383.13 | | |
| |
| | |
(1) | | Includes 1,725,000 units, consisting of 1,725,000 shares of common stock and 690,000 warrants to purchase common stock, which may be issued upon exercise of a45-day option granted to the underwriters to cover over-allotments, if any. |
(1) Pursuant to Rule 416 under the Securities Act, the shares being registered hereunder include such indeterminate number of shares of common stock as may be issuable with respect to the shares being registered hereunder as a result of stock splits, stock dividends or similar transactions.
| | |
(2) | | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(c) under the Securities Act. |
|
(3) | | No fee pursuant to Rule 457(g) under the Securities Act. |
|
(4) | | Represents 7% of the shares underlying the units to be sold in the offering. |
(2) Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(c) under the Securities Act. The price per share and aggregate offering price are based on the average of the high and low sales prices of the registrant’s common stock on January 28, 2011, as reported on the Over-the-Counter Bulletin Board.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule12b-2 of the Exchange Act. (Check one):
| | | | | | | |
|
Large accelerated filero | | Accelerated filero | | Non-accelerated filero
(Do not check if a smaller reporting company) | | Smaller reporting companyþ |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance withSection 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
|
SUBJECT TO COMPLETION, DATED MAY 17, 2010Subject To Completion, Dated January 31, 2011
PROSPECTUS
15,000,000 Shares
Common Stock
11,500,000 Units
Common Stock
Warrants
We are offering 11,500,000 units, each unit consistingThis prospectus relates to the sale of one shareup to 15,000,000 shares of our common stock and 0.4 warrantswhich may be offered by the selling stockholder, Lincoln Park Capital Fund, LLC, or Lincoln Park, from time to purchasetime. The shares of common stock at an exercise price per whole share equalbeing offered by the selling stockholder are issuable pursuant to 110%the Lincoln Park Purchase Agreement, which we refer to in this prospectus as the Purchase Agreement. Please refer to the section of this prospectus entitled “The Lincoln Park Transaction” for a description of the closing bid pricePurchase Agreement and the section entitled “Selling Stockholder” for additional information. Such registration does not mean that Lincoln Park will actually offer or sell any of these shares. We will not receive any proceeds from the sales of shares of our common stock onby the dateselling stockholder; however, we may receive proceeds of this prospectus. No fractional warrants will be issued. The units will separate immediately andup to $11,000,000 under the common stock and warrants will be issued separately. There will be no market for the units. Each unit will be sold at a purchase price of $ .
Purchase Agreement.
Our common stock is currently listedquoted on the NYSE Amex stock exchangeOver-the-Counter Bulletin Board under the symbol “RGN.“RGRX.” On May 14, 2010,January 28, 2011, the last reported sale price of our common stock on the NYSE Amex was $0.56$0.22 per share. Currently, no public market exists for the warrants offered by this prospectus. It is anticipated that the warrants will be quoted on the OTC Bulletin Board under the symbol “ ” promptly after the date of this prospectus. We cannot assure you that our common stock will continue to be listed on the NYSE Amex or that our warrants will continue to be quoted on the OTC Bulletin Board.
Entities and individuals affiliated with Sigma-Tau, our largest stockholder, have expressed an interest in purchasing units in this offering.
Investing in our securities involves a high degree of risk. See “Risk Factors”
beginning on page 78 of this prospectus for a discussion of information that should be
considered in connection with an investment in our securities.
| | | | | | | | |
| | Per Unit | | Total |
|
Public offering price | | $ | | | | $ | | |
Underwriting discounts and commissions(1) | | $ | | | | $ | | |
Proceeds, before expenses, to us | | $ | | | | $ | | |
| | |
(1) | | Does not include a corporate finance fee in the amount of 1% of the gross proceeds of the offering or warrants to be issued to the representative of the underwriters. No underwriting discount will be paid on units purchased by Sigma-Tau. See “Underwriting” beginning on page 70 of this prospectus. |
We have grantedThe selling stockholder is an “underwriter” within the underwritersmeaning of the Securities Act of 1933, as amended. The selling stockholder is offering these shares of common stock and may sell all or a45-day option portion of these shares from time to purchase up to antime in market transactions, in negotiated transactions or otherwise, and at prices and on terms that will be determined by the then prevailing market price or at negotiated prices directly or through a broker or brokers, who may act as agent or as principal or by a combination of such methods of sale. For additional 1,725,000 units from usinformation on the same terms and conditions as set forth above.
methods of sale, you should refer to the section entitled “Plan of Distribution.”
Neither the Securities and Exchange Commission nor any state securities regulators have approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the units to purchasers on or about , 2010.
| |
Maxim Group LLC
| Boenning & Scattergood, Inc. |
The date of this prospectus is , 2010.___, 2011.
TABLE OF CONTENTS
| | | | | |
| | | Page |
| |
| | | 1 | |
| | | 78 | |
| | | 22 | |
| | | 2423 | |
| | | 2524 | |
| | | 2625 | |
| | | 2726 | |
| | | 2827 | |
| | | 2928 | |
| | | 3836 | |
| | | 4845 | |
| | | 54 | |
| | | 6162 | |
| | | 6365 | |
| | | 67 | |
| | | 6571 | |
| | | 7074 | |
| | | 7675 | |
| | | 7675 | |
| | | 7675 | |
| | | 7775 | |
| | | F-1 | |
| EX-5.1 |
| EX-23.1 |
You should rely only on the information contained in this prospectus and any related free writing prospectus we may authorize to be delivered to you.prospectus. We have not, and the underwriters haveselling stockholder has not, authorized any person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. Neither thisThis prospectus nor any related free writing prospectus is not an offer to sell, nor are theyis the selling stockholder seeking an offer to buy, these securities in any state where the offer or solicitation is not permitted. The information contained in this prospectus is complete and accurate as of the date on the front cover of this prospectus, but information may have changed since that date. We are responsible for updating this prospectus to ensure that all material information is included and will update this prospectus to the extent required by law.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that these industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data and we do not make any representation as to the accuracy of the information.
-i-
PROSPECTUS SUMMARY
The items in the following summary are described in more detail later in this prospectus. This summary does not contain all of the information you should consider. Before investing in our securities, you should read the entire prospectus carefully, including the “Risk Factors” beginning on page 78 and the financial statements and related notes beginning onpage F-1. Unless the context indicates otherwise (i) as used in this prospectus, the terms “RegeneRx,” “our company,” “we,” “us” and “our” refer to RegeneRx Biopharmaceuticals, Inc. and (ii) the information in this prospectus assumes no exercise of the underwriters’ over-allotment option.
Overview
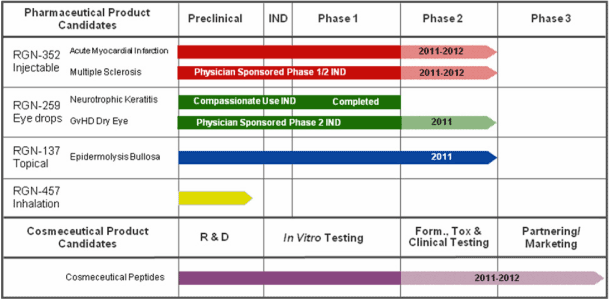
We are a biopharmaceutical company focused on the development of a novel therapeutic peptide, Thymosin beta 4, or Tß4, for tissue and organ protection, repair, and regeneration. We have formulated Tß4 into three distinct product candidates currently in clinical development:
| | |
| | • | | RGN-352, an injectable product candidate to treat cardiovascular diseases, central nervous system diseases, and other medical indications that may be treated by systemic administration, for which we intend to initiate a Phase 2 clinical trial in the second half of 2010;administration; |
| |
| | • | | RGN-259, a topical eye drop for ophthalmic indications that was evaluated in a small Phase 2 clinical trial and is currently being supported in compassionate use studies;regeneration of corneal tissues damaged by injury, disease or other pathology; and |
| |
| | • | | RGN-137, a topically applied gel for chronic dermal wounds and reduction of scar tissue that is currently in a Phase 2 clinical trial for the treatment of the skin defect epidermolysis bullosa, or EB.tissue. |
We have a fourth product candidate, RGN-457, in preclinical development. RGN-457 is an inhaled formulation of Tß4 targeting cystic fibrosis and other pulmonary diseases.
We are continuing strategic partnership discussions with biotechnology and pharmaceutical companies regarding the further clinical development of all of our product candidates.
In addition to our four pharmaceutical product candidates, we are also pursuing the commercial development of peptide fragments and derivatives of Tß4 for potential cosmeceutical use. CosmeceuticalsThese fragments are cosmetic products with biologically active ingredients.amino acid sequences, and variations thereof, within the Tß4 molecule that have demonstrated activity in several in vitro preclinical research studies that we have sponsored. We believe the biological activities of these fragments may be useful, for example, in developing novel cosmeceutical products for the anti-aging market. Our strategy is to enter into a collaboration with another company to develop cosmeceutical formulations based on these peptides.
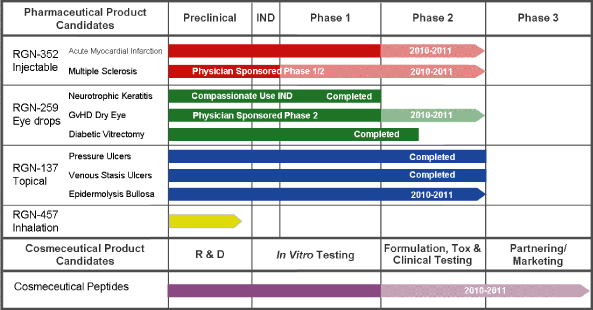
The following chart provides an overview of our product candidates and their development status:
1
We are engaged in research collaborations with at least 25 research institutions throughout the world, which we believe indicates significant independent research interest in the clinical potential of Tß4. Most of these
1
institutions, including the U.S. military, are conducting research on Tß4 at their own expense. We have also entered into a license agreement with the U.S. National Institutes of Health, or NIH, under which we received an exclusive worldwide license for Tß4 for several clinical indications. We have similarly in-licensed other rights related to Tß4 that we believe support our current or expected future clinical development. We have applied for or hold over 60 worldwide patents onand patent applications covering peptide compositions, uses and formulations related to cardiac, central nervous system, ophthalmic and dermal indications, among others, as well as for cosmetic and consumer products.
Our Tß4-Based Product Candidates
Tß4 is a naturally occurring43-amino acid peptide that was originally isolated from bovine thymus glands. Preclinical animal research has identified several important biological activities of Tß4 that we believe make it potentially useful as a wound healing, repair and tissue regenerating agent, including:
| | |
| | • | | signaling adult epicardial progenitor cells, or EPCs, to differentiate into coronary blood vessels; |
| |
| | • | | forming cardiomyocytes that repair damaged heart tissue; |
| |
| | • | | triggering the maturation of stem cells into cells that produce myelin, the outer covering of nerve cells in the central nervous system; |
| |
| | • | | improving neurologic functional recovery; |
| |
| | • | | regulating actin, which is critical to cell structure and mobility; |
| |
| | • | | stimulating angiogenesis, or blood vessel development; |
| |
| | • | | reducing inflammation, which is implicated in many medical indications; |
| |
| | • | | stimulating the formation of collagen and up-regulation of laminin-5 to accelerate tissue repair; and |
| |
| | • | | preventing apoptosis, or programmed cell death. |
We have developed a synthetic version of Tß4 and have formulated it for various routes of administration, targeting medical indications with significant unmet needs and market potential, as well as orphan indications that we believe could also provide substantial commercial value. Our product candidates are intended to provide solutions to these medical indications and to offer improvements to current standards of care.
RGN-352
Our product candidate RGN-352 is an injectable formulation of Tß4 for systemic administration. We have initially targeted RGN-352 for patients who have suffered an acute myocardial infarction, or AMI, commonly known as a heart attack. Preclinical research published in the scientific journalNaturehas indicated that Tß4 can guide specific types of stem cells from the outer layer of the heart to generate new myocardial blood vessels and tissue at injured sites.
During 2009, we completed a Phase 1 clinical trial evaluating the safety of RGN-352 in 60 healthy subjects. The product candidate was well-tolerated, and there were no reported drug-related adverse events.
Based on the results of this Phase 1 trial and subject to available funding,extensive preclinical efficacy data published in peer-reviewed journals, we intend to initiateinitiated a Phase 2 clinical trial in the second half of 2010 to evaluate RGN-352’s ability to salvage and regenerate damaged cardiac tissue and improve cardiac function after a heart attack. Wean AMI.
Additionally, recent preclinical research published in the scientific journalsNeuroscience and theJournal of Neurosurgeryindicate that RGN-352 may prove useful for patients with multiple sclerosis, or MS, as well as stroke and traumatic brain injury. In these studies, the administration of Tß4 resulted in regeneration of neuronal tissue and improvement of neurological function. Based on this preclinical research, we intend to usesupport a portion of the proceeds of this offering to initiate and conduct thisproposed Phase 1/2 clinical trial although depending onto be conducted at a major U.S. medical center under a physician-sponsored investigational new drug application, or
2
IND, in order to evaluate the amounttherapeutic potential of proceeds we may not be ableRGN-352 in patients with MS. We are planning to complete the trial without additional capital. Depending on our capital resources, we may conduct the trial while also continuing strategic partnership discussions with biotechnologysupply RGN-352 and pharmaceutical companiesprovide clinical and regulatory guidance for the further development of RGN-352.trial.
In May 2010, we were awarded a $3 million grant from the National Heart, Lung and Blood Institute, one of the institutes of the NIH, to support the further development of RGN-352.
Recent preclinical research published in the scientific journalNeurosciencealso indicates that RGN-352 may prove useful for patients with multiple sclerosis, or MS, and stroke. In research involving mice, the administration of Tß4 resulted in statistically significant improvement in neurological functional recovery.
2
Based on this research, we intend to support a proposed Phase 1/2 clinical trial to be conducted at a major U.S. medical center under a physician-sponsored investigational new drug application, or IND, in order to evaluate the therapeutic potential of RGN-352 in patients with MS. We are planning to supply RGN-352 and provide clinical and regulatory guidance for the trial. We believe that we can support this trial from our existing capital resources, although we intend to use a portion of the proceeds from this offering to provide additional support.
RGN-259
Our product candidate RGN-259 is a sterile topical eye drop formulation of Tß4 for ophthalmic indications. Emerging human clinical data from two compassionate use studies have demonstrated the ability of RGN-259 to repair and regenerate corneal tissue in patients with non-healing corneal lesions in the eye. This data has been reported at medical conferences and published in scientific journals and provided theproof-of-concept data that we initially sought for RGN-259. Based on these data and slower than expected patient accrual in a Phase 2 ophthalmic wound healing trial that we had initiated with RGN-259, in 2009, we closed the Phase 2 trial after enrolling the initial low-dose cohort. The results from evaluating this initial cohort indicated increased corneal epithelial thickening and reduced cell and flare inflammation in the Tß4-treated patients, as compared to patients who were administered a placebo. We believe these results are indicative of Tß4’s activities in corneal re-epithelialization and healing.
We are continuing to support the development of RGN-259 in ophthalmic indications under compassionate use INDs and expect to report final patient data from these trials in the third quarter of 2010. We are also planning to supportsupporting a physician-sponsored clinical trial in patients with dry eye secondary to graft versus host disease, or GvHD, in order to gain further insight intoevaluate RGN-259’s ability to repair and regenerate damaged ophthalmic tissues. Our support includes manufacturing and supplying RGN-259 for the trial and providing regulatory and clinical guidance. We are continuing to collaborate with the U.S. military to evaluate the potential of RGN-259 to prevent or reduce eye damage caused by chemical warfare agents. We are also engaged in discussions with potential partners regarding the clinical development of this product candidate. Once enough human data is generated, we intend to seek strategic partnerships with one or more ophthalmic specialty companies.
RGN-137
Our product candidate RGN-137 is a topical gel formulation of Tß4 intended to promote dermal wound healing and tissue regeneration. Preclinical research has demonstrated that Tß4 can accelerate dermal regeneration after a wound, while more recent research indicates that Tß4 can reduce scarring after injury in the skin and heart. In 2005, based on research conducted at the NIH, we initiated a series of Phase 2 clinical trials to evaluate RGN-137 for the treatment of three different types of skin wounds.
The first trial evaluatedWe are evaluating the use of RGN-137 in the treatment of patients with epidermolysis bullosa, or EB, which is a genetic defect that results in fragile skin and other epidermalepithelial tissues that can blister at the slightest trauma or friction, creating a wound that at times does not heal or heals poorly. A portion of this trial was funded by a grant from the FDA due to the orphan nature of the indication.U.S. Food and Drug Administration, or FDA. Despite the small patient population with this disease,EB, we are continuingcontinue to enroll patients in this Phase 2 trial and expect to complete itthe trial in late 2010 or early 2011. Once we complete our Phase 2 EB trial, we will analyze the data in conjunction with our two other completed Phase 2 trials of RGN-137, along with the preclinical data indicating Tß4’s ability to reduce scarring, at which time we will further evaluate our strategy for the clinical development of RGN-137.
Additionally, we intend to discuss with the FDA the possibility of converting the Phase 2 trial to a pivotal trial for marketing approval, should the data from the Phase 2 trial warrant. We believe that there is precedent for this process, particularly in cases of product candidates under development for ultra-orphan indications, where patient accrual is limited but the clinical data confirms both safety and efficacy.
Relationship with Sigma-Tau
Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. is an international pharmaceutical company and an affiliate of Sigma-Tau Finanziaria S.p.A., which together with its affiliates comprise our largest stockholder group and are referred to in this prospectus as Sigma-Tau. Sigma-Tau has licensed certain rights to our product candidates for marketing in Europe and other surrounding countries, for which we would be the exclusive supplier of Tß4 and would receive royalties on commercial sales, if any. Sigma-Tau conducted and funded our
3
completed Phase 2 trial in Italy and Poland to evaluate RGN-137 for the treatment of patients with venous stasis ulcers.
Commercialization Strategy
Our strategy is to seek strategic partners and to out-license rights for each drug candidate and our peptide fragments, with
3
certain exceptions. For example, we believe we can commercially develop and marketRGN-137 for EB, since the patient population is small and well-defined, as is the population of pediatric dermatologists who specialize in treating this disease. We continue to hold strategic discussions with pharmaceutical and biotechnology companies at each development milestone for each product candidate.
Corporate Information
We were incorporated in Delaware in 1982 under the name Alpha 1 Biomedicals, Inc. In 2000, we changed our corporate name to RegeneRx Biopharmaceuticals, Inc. at which time we began hiring our current management team.Inc.. Our principal executive office is located at 15245 Shady Grove Road, Suite 470, Rockville, Maryland 20850. Our telephone number is(301) 208-9191. Our website address iswww.regenerx.com.Information contained in, or accessible through, our website does not constitute a part of, and is not incorporated into, this prospectus.
We use RegeneRxtmTM and the RegeneRx logo as trademarks and service marks in the United States.States and other countries. All other trademarks or trade names referred to in this prospectus are the property of their respective owners.
Recent Developments
NYSE Amex Delisting
On October 26, 2010, we received a notice from the staff of the NYSE Amex, or the Exchange, stating that we had failed to regain compliance with the Exchange’s continued listing standards and that, accordingly, our common stock was subject to a delisting proceeding. The Exchange advised us that we were not in compliance with Section 1003(a)(iii) of the Exchange’s Company Guide because our stockholders’ equity was less than $6,000,000. In accordance with Sections 1009(d) and 1203 of the Exchange’s Company Guide, we appealed the determination of the Exchange’s staff and initially requested a hearing before the Exchange’s Listing Qualifications Panel. The hearing was scheduled to occur on December 17, 2010.
On December 15, 2010, we notified the Exchange of our intent to withdraw the request for a hearing, and our common stock was suspended from the Exchange as of the commencement of trading on December 23, 2010. Our common stock began to be quoted on the OTC Bulletin Board as of December 23, 2010.
Purchase Agreements with Lincoln Park
We have entered into two purchase agreements and a registration rights agreement with Lincoln Park. Under the terms of a Securities Purchase Agreement, on January 7, 2011 Lincoln Park purchased 1,851,852 shares of our common stock, together with a warrant to purchase an additional 740,741 shares of our common stock at an exercise price of $0.38 per share, for gross proceeds of $500,000. The warrant will be exercisable on July 7, 2011 and thereafter until July 7, 2016. This offering was made pursuant to a shelf registration statement and related prospectus, as supplemented by a prospectus supplement filed with the Securities and Exchange Commission, or SEC, on January 7, 2011.
On January 4, 2011, we also entered into a Purchase Agreement and a Registration Rights Agreement, under which Lincoln Park is irrevocably committed to purchase from us up to $11,000,000 of our common stock, from time to time, over a 30-month period. We have filed a registration statement, of which this prospectus is a part, covering the resale of the shares that may be issued to Lincoln Park under the Purchase Agreement. Under the Purchase Agreement, we have also agreed to issue to Lincoln Park up to an aggregate of 1,916,666 shares of common stock as a fee for Lincoln Park’s commitment to purchase our shares. Of these commitment shares, we issued one-half, or 958,333 shares, upon entering into the agreement with Lincoln Park. The remaining commitment shares are issuable to Lincoln Park on a pro rata basis as additional purchases are made under the Purchase Agreement. For additional information about the terms of the Purchase Agreement and the Registration Rights Agreement, please refer to the section of this prospectus entitled “The Lincoln Park Transaction.”
Purchase Agreements with Sigma-Tau
In addition to the agreements entered into with Lincoln Park, on January 7, 2011, we consummated a private placement of our securities to affiliates of Sigma-Tau. In this private placement, we issued an additional 3,518,519 shares of our common stock at a price per share of $0.27, for gross proceeds of $950,000. In connection with the private placement, we issued to the purchasers warrants to purchase an additional 1,407,407 shares of common stock in the aggregate at an exercise price of $0.38 per share. The warrants will be exercisable on July 7, 2011 and thereafter until July 7, 2016. In connection with this private placement, we also agreed to amend the terms of certain outstanding warrants held by Sigma-Tau in order to reduce their exercise prices to $0.38 per share and to extend their expiration dates to December 31, 2011.
4
The Offering
| | |
SecuritiesCommon stock outstanding prior to the offering | | 79,860,282 shares, including the 958,333 initial commitment shares already issued to Lincoln Park under the Purchase Agreement and the 1,851,852 shares already issued to Lincoln Park outside of the Purchase Agreement |
| | |
Common Stock offered by usthe selling stockholder | | 11,500,000 units, each unit15,000,000 shares, consisting of one share of common stock, par value $0.001 per share, and 0.4 tradeable warrantsup to purchase common stock (each, a “unit”). Each whole warrant will represent the right958,333 shares to purchase a whole share of our common stock. No fractional warrants will be issued. The units will separate immediatelyissued to Lincoln Park as additional commitment shares and the common stock and warrants willremaining shares to be issued separately. There will be no market forpurchased from time to time under the units.Purchase Agreement |
| | |
Common stock to be outstanding after
this offering giving effect to the issuance of 15,000,000 shares to Lincoln Park under the Purchase Agreement | | 71,906,82894,860,282 shares |
| | |
TermsUse of the warrants offered by usproceeds | | Each warrantWe will be exercisable during the period commencing 30 days after original issuance and ending five yearsnot receive any proceeds from the original date of issuance at an exercise price of $ per whole share of common stock, which is equal to 110% of the closing bid price of our common stock on the date of this prospectus. See “Description of Securities.” This prospectus also relates to the offeringsale of the shares of common stock issuable upon exerciseby Lincoln Park. However, we may receive up to $11,000,000 from sales of shares under the warrants. |
|
Redemption ofPurchase Agreement. Any proceeds that we receive from sales to Lincoln Park under the warrants issued as
part of the units | | In the event the closing sale pricePurchase Agreement will be used to further development of our common stock is at least $ per share, which is equal to 350% of the closing bid price of our common stock on the date of this prospectus, for any 20 trading days within a 30 consecutive trading day period, we may call the warrants for redemption, at a redemption price of $0.01 per warrant, by providing at least 30 days notice to each warrant holder. Holders of the warrants will be entitled to exercise the warrants prior to the date scheduled for redemption, but there can be no assurance that the price of our common stock will exceed the call price or the warrant exercise price after the redemption call is made. |
| | |
Over-allotment option | | 1,725,000 units |
| | |
Use of proceeds | | We intend to use the net proceeds from this offering, and our existing cash and cash equivalents, to fund ongoing research and development activities, including contemplated clinical trials,drug candidates and for general corporate purposes, including working capital.purposes. See “Use of Proceeds.” |
| | |
Market for our securities | | Our common stock is currently listed on the NYSE Amex under the symbol “RGN.” It is anticipated that the warrants underlying the units will be quoted on the OTC Bulletin Board under the symbol “ ” promptly after the date of this prospectus. There will be no market for the units. | | RGRX |
| | |
| Risk factors | | This investment involves a high degree of risk. See “Risk Factors” for a discussion of factors you should consider carefully before making an investment decision. |
The numbernumbers of shares of our common stock that will be outstanding immediately after this offering isare based on 60,406,82879,860,282 shares of common stock outstanding as of March 31, 2010,January 7, 2011, and excludes:
| | |
| | • | 4,914,112 | 5,348,863 shares of our common stock issuable upon the exercise of outstanding stock options, outstanding under our 2000 stock option plan as of March 31, 2010, atwith a weighted average exercise price of $1.53$1.37 per share; |
| |
| | • | 1,550,888 | 4,327,500 shares of our common stock available for future issuance under our 2000 stock option plan;2010 Equity Incentive Plan; |
| |
| | • | 7,933,851 | 16,136,900 shares of our common stock issuable upon the exercise of outstanding warrants, as of March 31, 2010, atwith a weighted-average exercise price of $2.01$0.89 per share; and |
| |
| | • | | additional shares of our common stock that are potentially issuable upon exercise of warrants to be issued in connection withLincoln Park under the Purchase Agreement beyond the 15,000,000 shares being offered by this offering.prospectus. |
On January 4, 2011, we executed a Purchase Agreement and a Registration Rights Agreement with the selling stockholder, Lincoln Park Capital Fund, LLC, or Lincoln Park. Under the Purchase Agreement, we have the right to sell to Lincoln Park up to $11,000,000 of our common stock at our option as described below.
Pursuant to the Registration Rights Agreement, we are filing this registration statement and prospectus with the SEC covering the shares that may be issued to Lincoln Park under the Purchase Agreement. We do not have the right to commence any sales of our shares to Lincoln Park until the SEC has declared effective the registration statement. Thereafter, over approximately 30 months, and subject to certain terms and conditions, we have the right to direct Lincoln Park to purchase up to $11,000,000 of our common stock in periodic amounts of up to 400,000 shares and as often as every two business days. The purchase price of the shares will be based on the market prices of our shares immediately prior to the time of sale as computed under the Purchase Agreement without any fixed discount. In no event, however, will Lincoln Park be obligated to purchase shares of our common stock under the Purchase Agreement at a price of less than $0.15 per share. We may, at any time, and in our sole discretion, terminate the Purchase Agreement without fee, penalty or cost upon notice to Lincoln Park.
5
Upon signing the Purchase Agreement, we issued 958,333 shares of our common stock to Lincoln Park as a commitment fee for entering into the Purchase Agreement (which commitment shares are not part of this offering), and we may issue up to an additional 958,333 shares pro rata (which shares are included in this offering) if and when we sell additional shares to Lincoln Park under the Purchase Agreement. In addition to the purchases contemplated by the Purchase Agreement, Lincoln Park invested $500,000 in our company on January 7, 2011 and received 1,851,852 shares of our common stock, together with a warrant to purchase 740,741 shares of our common stock at an exercise price of $0.38 per share. These securities were offered and sold pursuant to an effective shelf registration statement and are not included in this offering.
Under the Purchase Agreement and the Registration Rights Agreement, we are required to register the $11,000,000 of shares which we may sell to Lincoln Park and the 958,333 shares which we are required to issue pro rata in the future as a commitment fee if and when we sell shares to Lincoln Park under the Purchase Agreement. Although the Purchase Agreement provides that we may sell up to $11,000,000 of our common stock to Lincoln Park, we are only registering 15,000,000 shares to be purchased thereunder, which may or may not cover all such shares purchased by Lincoln Park under the Purchase Agreement, depending on the purchase price per share.
Of the 15,000,000 shares offered under this prospectus:
| • | | 958,333 shares represent shares that we are required to issue proportionally in the future, as a commitment fee, if and when we sell additional shares to Lincoln Park under the Purchase Agreement; and |
|
| • | | The remainder represent shares we may sell to Lincoln Park under the Purchase Agreement. |
Except as otherwise indicated herein, all information in this prospectus, including the number of shares that will be outstanding after this offering, assumes or gives effect to:to no exercise of options or warrants outstanding on the date of this prospectus or in the future, except as specifically set forth herein.
| | |
| • | no exercise of options or warrants outstanding on the date of this prospectus, except as specifically set forth herein; and |
|
| • | no exercise of the underwriters’ over-allotment option. |
As of January 7, 2011, there were 79,860,282 shares outstanding, of which 45,743,676 shares were held by non-affiliates (including the 958,333 shares already issued to Lincoln Park under the Purchase Agreement and 1,851,852 shares issued to Lincoln Park outside of the Purchase Agreement). If all of the 15,000,000 shares offered by Lincoln Park were issued and outstanding as of the date hereof, such shares would represent 15.8% of the total common stock outstanding, or 24.8% of the non-affiliates’ shares outstanding. The number of shares ultimately offered for sale by Lincoln Park is dependent upon the number of shares that we sell to Lincoln Park under the Purchase Agreement. If we elect to issue more than the 15,000,000 shares offered under this prospectus, which we have the right but not the obligation to do, we must first register under the Securities Act the resale by Lincoln Park of any additional shares we may elect to sell to Lincoln Park before we can sell such additional shares.
56
Summary Financial Data
The following tables summarize our financial data. We have derived the following summary of our statement of operations data for the years ended December 31, 2009 and 2008 from our audited financial statements appearing later in this prospectus. We have derived the following summary of our statement of operations data for the threenine months ended March 31,September 30, 2010 and 2009 and balance sheet data as of March 31,September 30, 2010 from our unaudited financial statements appearing later in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. You should read the summary of our financial data set forth below together with our financial statements and the related notes to those statements, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing later in this prospectus.
| | | | | | | | | | | | | | | | | |
| | | Year Ended | | | Nine Months Ended | |
| | | December 31, | | | September 30, | |
| | | 2009 | | | 2008 | | | 2010 | | | 2009 | |
Statement of Operations Data: | | | | | | | | | | | | | | | | |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | | | $ | 53,819 | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | | | | 1,819,036 | | | | 3,064,248 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | | | | 2,345,619 | | | | 2,161,539 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | | | | 4,164,655 | | | | 5,225,787 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) | | | (4,110,836 | ) | | | (5,225,787 | ) |
| | | | | | | | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | | | | 6,840 | | | | 10,304 | |
| | | | | | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | | (10,636,965 | ) | | | (4,103,996 | ) | | | (5,215,483 | ) |
| | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.12 | ) | | $ | (0.21 | ) | | $ | (0.06 | ) | | $ | (0.10 | ) |
| | | | | | | | | | | | | |
| Shares used to compute basic and diluted net loss per share | | | 55,680,525 | | | | 50,967,617 | | | | 66,729,519 | | | | 54,216,430 | |
| | | | | | | | | | | | | |
We have presented the summary balance sheet data:
| | |
| • | on an actual basis as of March 31, 2010; and |
| | |
| • | on an as adjusted basis to give effect to our sale of 11,500,000 units in this offering at an assumed public offering price of $0.56 per unit (based on the last reported sale price of our common stock on May 14, 2010), after deducting estimated underwriting discounts and commissions, the 1% corporate finance fee payable to the representative of the underwriters and estimated offering expenses payable by us. |
| | | | | | | | | | | | | | | | | |
| | | Year Ended
| | | Three Months Ended
| |
| | | December 31, | | | March 31, | |
| | | 2009 | | | 2008 | | | 2010 | | | 2009 | |
| |
Statement of Operations Data: | | | | | | | | | | | | | | | | |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | | | | 470,434 | | | | 1,661,600 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | | | | 678,068 | | | | 859,568 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | | | | 1,148,502 | | | | 2,521,168 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) | | | (1,148,502 | ) | | | (2,521,168 | ) |
| | | | | | | | | | | | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | | | | 2,793 | | | | 6,518 | |
| | | | | | | | | | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) | | $ | (1,145,709 | ) | | $ | (2,514,650 | ) |
| | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.12 | ) | | $ | (0.21 | ) | | $ | (0.02 | ) | | $ | (0.05 | ) |
| | | | | | | | | | | | | | | | | |
| Shares used to compute basic and diluted net loss per share | | | 55,680,525 | | | | 50,967,617 | | | | 60,406,828 | | | | 53,622,491 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| | | As of March 31, 2010 |
| | | Actual | | As Adjusted |
| |
Balance Sheet Data: | | | | | | | | |
| Cash and cash equivalents | | $ | 3,189,990 | | | $ | 8,637,974 | |
| Working capital | | | 2,689,485 | | | | 8,096,771 | |
| Total assets | | | 3,455,854 | | | | 8,903,838 | |
| Common stock | | | 60,407 | | | | 71,907 | |
Additionalpaid-in capital | | | 88,276,191 | | | | 93,712,675 | |
| Accumulated deficit | | | (85,647,113 | ) | | | (85,647,113 | ) |
| Total stockholders’ equity | | | 2,689,485 | | | | 8,137,469 | |
| | | | | |
| | | As of September 30, 2010 | |
Balance Sheet Data: | | | | |
| Cash and cash equivalents | | $ | 4,975,947 | |
| Working capital | | | 4,423,905 | |
| Total assets | | | 5,615,670 | |
| Common stock | | | 73,531 | |
| Additional paid-in capital | | | 92,997,669 | |
| Accumulated deficit | | | (88,605,400 | ) |
| Total stockholders’ equity | | | 4,465,800 | |
67
RISK FACTORS
InvestingBefore you make a decision to invest in our securities, involves a high degree of risk. Youyou should consider carefully consider the risks described below, as well as thetogether with other information included in this prospectus, before you decide to purchase our securities.prospectus. If any of the following risksevents actually occurs, they may harmoccur, our business, operating results, prospects or financial condition could be materially and operating results. As a result,adversely affected. This could cause the trading price of our securities couldcommon stock to decline and you may lose all or part of your investment. The risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also significantly impair our business operations and could lose part or allresult in a complete loss of your investment.
Risks Related to Our Liquidity and Need for Financing
Even afterBefore giving effect to the proceeds of this offering,any future sales to Lincoln Park, we estimate that our existing capital resources will only be sufficient to fund our operations into the second quarterhalf of 2011.
We intend to use the proceeds from this offeringour recent investment transactions and proceeds of a grant we were recently awarded from the National Institutes of Healthour other existing capital resources to fund our ongoing research and development activities; however, we may not be able to complete all of theour active trials and those we intend to initiate in 2010 or beyond 20102011 without additional funding. We expect that the proceeds of this offeringthe recent investment transactions, together with our other capital resources, will provide us the ability to fund our operations into the second quarterhalf of 2011. We intend2011, without giving effect to use a portion ofany other financing activities, including any purchases under the proceeds of this offering, togetherPurchase Agreement with our existing capital resources, to fund our existingLincoln Park. Our research initiatives in supportinginclude support for a Phase 1/2 clinical trial ofRGN-352 in patients with multiple sclerosis supporting ongoing compassionate use studies of RGN-259 in patients with corneal defects, supportingand a physician-sponsored clinical trial in patients with dry eye secondary to graft versus host diseaseGvHD using RGN-259, and completing our ongoing Phase 2 trial of RGN-137 in patients with EB. We also intend to use a portion of the proceeds of this offering to initiate and conduct at least a portion of a Phase 2 clinical trial of RGN-352 in patients who have suffered an acute myocardial infarction. We expect that the Phase 2 trial design will allow for an interim review of patient data, fromwhich, if positive, we believe will facilitate discussions with potential strategic partners.
In January 2011, we entered into the Purchase Agreement with Lincoln Park, under which we may direct Lincoln Park to purchase up to an initial groupadditional $11,000,000 worth of evaluated patients, andshares of our common stock over a 30-month period, once the registration statement of which this prospectus forms a part has been declared effective by the SEC. If we currently expect thatmake sales of our common stock under the proceeds of this offering, together with our cash resources and the $3 million grant from the NIH, will be sufficient to reach this point in the trial. We will notPurchase Agreement, we would be able to completefund our operations for a longer period of time. However, the contemplated Phase 2 clinical trialextent to which we will rely on the Purchase Agreement with Lincoln Park as a source ofRGN-352 without additional capital.
funding will depend on a number of factors, including the prevailing market price of our common stock and volume of trading and the extent to which we are able to secure working capital from other sources. Specifically, Lincoln Park does not have the obligation to purchase any shares of our common stock on any business day that the price of our common stock is less than $0.15 per share.
We are registering the resale of 15,000,000 shares by Lincoln Park pursuant to this prospectus. In the event we elect to issue more than the 15,000,000 shares offered hereby, we would be required to file a new registration statement and have it declared effective by the SEC. If obtaining sufficient funding from Lincoln Park does not occur or is prohibitively dilutive, we will need to secure another source of funding in order to satisfy our working capital needs. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our business, operating results, financial condition and prospects.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in these risk factors.this prospectus. We have based this estimate on assumptions that may prove to be wrong, and we could use our available capital resources and the proceeds from this offering sooner than we currently expect.
In addition to our current development objectives, we will need substantial additional capital for the continued development of product candidates through marketing approval and for our longer-term future operations.
Beyond our current liquidity needs, we anticipate that substantial new capital resources will be required to continue our longer-term independent product development efforts, including any and all follow-on trials that will result from our current clinical programs beyond those currently contemplated, and to scale up manufacturing processes for our product candidates. TheWe may be able to obtain funding under the Purchase Agreement with Lincoln Park in order to further some of these efforts. However, the actual amount of funds that we will need will be determined by many factors, some of which are beyond our control. These factors include, without limitation:
8
| | |
| | • | | the scope of our clinical trials, which is significantly influenced by the quality of clinical data achieved as trials are completed and the requirements established by regulatory authorities; |
| |
| | • | | the speed with which we complete our clinical trials, which depends on our ability to attract and enroll qualifying patients and the quality of the work performed by our clinical investigators; |
| |
| | • | | the time required to prosecute, enforce and defend our intellectual property rights, which depends on evolving legal regimes and infringement claims that may arise between us and third parties; |
| |
| | • | | the ability to manufacture at scales sufficient to supply commercial quantities of any of our product candidates that receive regulatory approval, which may require levels of effort not currently anticipated; and |
7
| | |
| | • | | the successful commercialization of our product candidates, which will depend on our ability to either create or partner with an effective commercialization organization and which could be delayed or prevented by the emergence of equal or more effective therapies. |
Emerging biotechnology companies like us may raise capital through corporate collaborations and by licensing intellectual property rights to other biotechnology or pharmaceutical enterprises. We intend to pursue this strategy, but there can be no assurance that we will be able to license our intellectual property or product development programs on commercially reasonable terms, if at all. There are substantial challenges and risks that will make it difficult to successfully implement any of these alternatives. If we are successful in raising additional capital through such a license or collaboration, we may have to give up valuable rights to our intellectual property. In addition, the business priorities of a strategic partner may change over time, which creates the possibility that the interests of the strategic partner in developing our technology may diminish whichand could have a potentially material negative impact on the value of our interest in the licensed intellectual property or product candidates.
We also intend to apply for federal cash grants and tax credits that have been set aside for small biotechnology companies under recently enacted healthcare reform legislation. However, there can be no assurance that we will qualify for or otherwise be able to obtain any such grants or credits.
Further, if we raise additional funds by selling shares of our common stock or securities convertible into our common stock, including to Lincoln Park under the Purchase Agreement, the ownership interest of our existing stockholders may be significantly diluted. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and may involve significant fees, interest expense, restrictive covenants or the granting of security interests in our assets.
Our failure to successfully address long-term liquidity requirements would have a material negative impact on our business, including the possibility of surrendering our rights to some technologies or product opportunities, delaying our clinical trials or ceasing our operations.
We have incurred losses since inception and expect to incur significant losses in the foreseeable future and may never become profitable.
We have not commercialized any product candidates to date and incurred net operating losses every year since our inception in 1982. We believe these losses will continue for the foreseeable future, and may increase, as we pursue our product development efforts related to Tß4. As of March 31,September 30, 2010, our accumulated deficit totaled approximately $85.6$88.6 million.
As we expand our research and development efforts and seek to obtain regulatory approval of our product candidates to make them commercially viable, we anticipate substantial and increasing operating losses. Our ability to generate additional revenues and to become profitable will depend largely on our ability, alone or through the efforts of third-party licensees and collaborators, to efficiently and successfully complete the development of our product candidates, obtain necessary regulatory approvals for commercialization,scale-up commercial quantity manufacturing capabilities either internally or through third-party suppliers, and market our product candidates. There can be no assurance that we will achieve any of these objectives or that we will ever become profitable or be able to maintain profitability. Even if we do achieve profitability, we cannot predict the level of such profitability. If we sustain losses over an extended period of time and are not otherwise able to raise necessary funds to continue our development efforts and maintain our operations, we may be forced to cease operations.
We are currently not in compliance with NYSE Amex rules regarding the minimum shareholders’ equity requirement and are at risk of beingOur common stock has been delisted from the NYSE Amex stock exchange, which may subjectsubjects us to the SEC’s penny stock rules and will further decrease the liquidity of our common stock.
Because of our historical losses from operations, NYSE Amex rules require that we maintain minimum stockholders’ equity of $6 million, unless our market capitalization exceeds $50 million. We are not currently in compliance with either of these continued listing standards. In the second quarter of 2009, we submittedwere previously operating under a compliance plan intended to the NYSE Amex that forecasted our abilityallow us to regain compliance with the listing standardsExchange’s stockholders’ equity requirement by October 25, 2010. On October 26, 2010, we were notified by the Exchange that we had
89
by October 2010. NYSE Amex has accepted our compliance plan, which is subject to periodic review by NYSE Amex to determine whether we are making progress consistent with the plan. Our compliance plan contemplates the raise of additional equity capital, such as through this offering. While the proceeds of this offering may enable us to achievenot timely regained compliance with the $6 million stockholders’ equity requirement, we will likely require additional capital in the future to maintain compliance with thisExchange’s continued listing standard. Additionally,standards. As a result, the notice indicated that our securities were subject to delisting from the Exchange. We were initially granted a hearing before the Exchange’s Listing Qualifications Panel that was scheduled for December 17, 2010. On December 15, 2010, we cannot assure you that we will meet or maintain compliance with the $50 million market capitalization alternative standard after completion of this offering. Even if we raise net proceeds equal to or greater than the amount required to satisfy the $6 million stockholders’ equity requirement, there can be no assurance that, because ofwithdrew our significant operating cash requirements, we will be able to maintain compliance with that requirement or remain eligiblerequest for continued listing on the NYSE Amex. Further, even if we raise net proceeds equal to or greater than the amount necessary to regain compliance, there can be no assurance that NYSE Amex will agree that we have satisfied the compliance plan.
If we do not achieve or maintain compliance with NYSE Amex listing rules, we expect thata hearing, and our common stock wouldwas suspended from trading on the Exchange as of the commencement of trading on December 23, 2010 and will be delisted from the NYSE Amex exchange. Following any such delisting,delisted.
As of December 23, 2010, our common stock may beis tradedover-the-counter on the OTC Bulletin Board or in the “pink sheets.” These alternativeBoard. Over-the-counter markets however, are generally considered to be less efficient than, and not as broad as, the NYSE Amexa stock exchange. If our common stock is delisted from NYSE Amex, thereThere may be a limited market for our stock now that it is quoted on the OTC Bulletin Board, trading in our stock may become more difficult and our share price could decrease even further.decrease. Specifically, you may not be able to resell your shares of common stock at or above the price you paid for such shares or at all.
In addition, if our common stock is delisted, our ability to raise additional capital may be impaired because of the less liquid nature of the OTC Bulletin Board and the pink sheets.over-the-counter markets. While we cannot guarantee that we would be able to complete an equity financing on acceptable terms, or at all, we believe that dilution from any equity financing while our shares are quoted on the OTC Bulletin Board or the pink sheetsan over-the-counter market would likely be substantially greater than if we were to complete thea financing while our common stock is traded on a national securities exchange. Further, now that our stock is not traded on an exchange, upon the NYSE Amex exchange.filing of our annual report for the year ended December 31, 2010, we will no longer be eligible to use short-form registration statements on Form S-3 for the registration of our securities, which could impair our ability to raise additional capital as needed.
In the event ourOur common stock is delisted, it may also become subject to penny stock rules.rules, which impose additional sales practice requirements on broker-dealers who sell our common stock. The SEC generally defines “penny stock” as an equity security that has a market price of less than $5.00 per share, subject to certain exceptions. We are not currently subject to the penny stock rules because our common stock qualifies for an exception to the SEC’s penny stock rules for companies that have an equity security that is quoted on an exchange. However, if we were delisted, our common stock would become subject to the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell our common stock. If our common stock were considered penny stock, theThe ability of broker-dealers to sell our common stock and the ability of our stockholders to sell their shares in the secondary market wouldwill be limited and, as a result, the market liquidity for our common stock wouldwill likely be adversely affected. We cannot assure you that trading in our securities will not be subject to these or other regulations in the future.
The report of our independent registered public accounting firm contains explanatory language that substantial doubt exists about our ability to continue as a going concern.
The report of our independent registered public accounting firm on our financial statements for the year ended December 31, 2009 contains explanatory language that substantial doubt exists about our ability to continue as a going concern, without raising additional capital. AlthoughWe estimate that our existing capital resources, without giving effect to any proceeds that we expect thatmay receive from sales of our shares to Lincoln Park under the proceeds of this offeringPurchase Agreement, will provide us the abilityonly be sufficient to fund our operations into the second quarterhalf of 2011, we will continue to have a need for additional financing after this offering, which we may not be able to complete either on favorable terms or at all.2011. As a result, unless we cannot assure you that, after taking into accountsell shares under the proceeds of this offering and our anticipated expenditures for the remainder of 2010,Purchase Agreement or obtain significant additional financing, the report of our independent registered public accounting firm for the year endingended December 31, 2010 will notcontinue to express substantial doubt about our ability to continue as a going concern. If this were to occur, we would once again face the need to obtain sufficient financing in the short term, and the failure to do so would, in all likelihood, create severe liquidity problems and cause us to have to curtail our operations. If we were to curtail our operations, we could be placed into bankruptcy or undergo liquidation, the result of which would adversely affect the value of our common stock.
9
Risks Related to Our Business and Operations
All of our drug candidates are based on a single compound that has yet to be proven effective in human subjects.
Our current primary business focus is the development of Tß4, and its analogues, derivatives and fragments, for the improvement of cardiac function, the acceleration of corneal healing, the treatment of non-healing wounds and other conditions. Unlike many pharmaceutical companies that have a number of unique chemical entities in development, we are dependent on a single molecule, formulated for different routes of administration and different clinical indications, for our potential commercial success. As a result, any common safety or efficacy concerns for Tß4-based products that cross formulations would have a much greater impact on our business prospects than if our product pipeline were more diversified.
We may never be able to commercialize our product candidates.
Although Tß4 has shown biological activity inin vitroand animal models, we cannot assure you that our product candidates will exhibit activity or importance in humans. Our drug candidates are still in research and development, and we do not expect them to be commercially available for the foreseeable future, if at all. Only a small number of research and development programs ultimately result in commercially successful drugs. Potential products that appear to be promising at early stages of development may not reach the market for a number of reasons. These include the possibility that the potential products may:
10
| | |
| | • | | be found ineffective or cause harmful side effects during preclinical studies or clinical trials; |
| |
| | • | | fail to receive necessary regulatory approvals; |
| |
| | • | | be precluded from commercialization by proprietary rights of third parties; |
| |
| | • | | be difficult to manufacture on a large scale; or |
| |
| | • | | be uneconomical or otherwise fail to achieve market acceptance. |
If any of these potential problems occurs, we may never successfully market Tß4-based products.
We are subject to intense government regulation, and we may not receive regulatory approvals for our drug candidates.
Our product candidates will require regulatory approvals prior to sale. In particular, therapeutic agents are subject to stringent approval processes, prior to commercial marketing, by the FDA and by comparable agencies in most foreign countries. The process of obtaining FDA and corresponding foreign approvals is costly and time-consuming, and we cannot assure you that such approvals will be granted. Also, the regulations we are subject to change frequently and such changes could cause delays in the development of our product candidates.candidates
Three of our drug candidates are currently in the clinical stage, and we cannot be certain that we or our collaborators will successfully complete the clinical trials necessary to receive regulatory product approvals. The regulatory approval process is lengthy, unpredictable and expensive. To obtain regulatory approvals in the United States, we or a collaborator must ultimately demonstrate to the satisfaction of the U.S. Food and Drug Administration, or FDA, that our product candidates are sufficiently safe and effective for their proposed administration to humans. Many factors, known and unknown, can adversely impact clinical trials and the ability to evaluate a product candidate’s safety and efficacy, including:
| | |
| | • | | the FDA or other health regulatory authorities, or institutional review boards, or IRBs, do not approve a clinical trial protocol or place a clinical trial on hold; |
| |
| | • | | suitable patients do not enroll in a clinical trial in sufficient numbers or at the expected rate, for reasons such as the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the perceptions of investigators and patients regarding safety, and the availability of other treatment options; |
10
| | |
| | • | | clinical trial data is adversely affected by trial conduct or patient withdrawal prior to completion of the trial; |
| |
| | • | | there may be competition with ongoing clinical trials and scheduling conflicts with participating clinicians; |
| |
| | • | | patients experience serious adverse events, including adverse side effects of our drug candidates, for a variety of reasons that may or may not be related to our product candidates, including the advanced stage of their disease and other medical problems; |
| |
| | • | | patients in the placebo or untreated control group exhibit greater than expected improvements or fewer than expected adverse events; |
| |
| | • | | third-party clinical investigators do not perform the clinical trials on the anticipated schedule or consistent with the clinical trial protocol and good clinical practices, or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| |
| | • | | service providers, collaborators or co-sponsors do not adequately perform their obligations in relation to the clinical trial or cause the trial to be delayed or terminated; |
| |
| | • | | we are unable to obtain a sufficient supply of manufactured clinical trial materials; |
| |
| | • | | regulatory inspections of manufacturing facilities, which may, among other things, require us or aco-sponsor to undertake corrective action or suspend the clinical trials; |
| |
| | • | | the interim results of the clinical trial are inconclusive or negative; |
|
11
| | • | | the clinical trial, although approved and completed, generates data that is not considered by the FDA or others to be sufficient to demonstrate safety and efficacy; and |
| |
| | • | | changes in governmental regulations or administrative actions affect the conduct of the clinical trial or the interpretation of its results. |
There can be no assurance that our clinical trials will in fact demonstrate, to the satisfaction of the FDA and others, that our product candidates are sufficiently safe or effective. The FDA or we may also restrict or suspend our clinical trials at any time if either believes that we are exposing the subjects participating in the trials to unacceptable health risks.
Clinical trials for product candidates such as ours are often conducted with patients who have more advanced forms of a particular condition or other unrelated conditions. For example, in clinical trials for our product candidate RGN-137, we have studied patients who are not only suffering from chronic epidermal wounds but who are also older and much more likely to have other serious adverse conditions. During the course of treatment with our product candidates, patients could die or suffer other adverse events for reasons that may or may not be related to the drug candidate being tested. Further, and as a consequence ofthat all of our drug candidates beingare based on Tß4, crossover risk exists such that a patient in one trial may be adversely impacted by one drug candidate, and that adverse event may have implications for our other trials and other drug candidates. However, even if unrelated to our product candidates, such adverse events can nevertheless negatively impact our clinical trials, and our business prospects would suffer.
These factors, many of which may be outside of our control, may have a negative impact on our business by making it difficult to advance product candidates or by reducing or eliminating their potential or perceived value. As a consequence, we may need to perform more or larger clinical trials than planned. Further, if we are forced to contribute greater financial and clinical resources to a study, valuable resources will be diverted from other areas of our business. If we fail to complete or if we experience material delays in completing our clinical trials as currently planned, or we otherwise fail to commence or complete, or experience delays in, any of our other present or planned clinical trials, including as a result of the actions of third parties upon which we rely for these functions, our ability to conduct our business as currently planned could materially suffer.
11
We may not successfully establish and maintain development and testing relationships with third-party service providers and collaborators, which could adversely affect our ability to develop our product candidates.
We have only limited resources, experience with and capacity to conduct requisite testing and clinical trials of our drug candidates. As a result, we rely and expect to continue to rely on third-party service providers and collaborators, including corporate partners, licensors and contract research organizations, or CROs, to perform a number of activities relating to the development of our drug candidates, including the design and conduct of clinical trials, and potentially the obtaining of regulatory approvals. For example, we currently rely on several third-party contractors to manufacture and formulate Tß4 into the product candidates used in our clinical trials, develop assays to assess Tß4’s effectiveness in complex biological systems, recruit clinical investigators and sites to participate in our trials, manage the clinical trial process and collect, evaluate and report clinical results.
We may not be able to maintain or expand our current arrangements with these third parties or maintain such relationships on favorable terms. Our agreements with these third parties may also contain provisions that restrict our ability to develop and test our product candidates or that give third parties rights to control aspects of our product development and clinical programs. In addition, conflicts may arise with our collaborators, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any conflicts arise with our existing or future collaborators, they may act in their self-interest, which may be adverse to our best interests. Any failure to maintain our collaborative agreements and any conflicts with our collaborators could delay or prevent us from developing our product candidates. We and our collaborators may fail to develop products covered by our present and future collaborations if, among other things:
| | |
| | • | | we do not achieve our objectives under our collaboration agreements; |
| |
| | • | | we or our collaborators are unable to obtain patent protection for the products or proprietary technologies we develop in our collaborations; |
| |
| | • | | we are unable to manage multiple simultaneous product development collaborations; |
| |
| | • | | our collaborators become competitors of ours or enter into agreements with our competitors; |
|
12
| | • | | we or our collaborators encounter regulatory hurdles that prevent commercialization of our product candidates; or |
| |
| | • | | we develop products and processes or enter into additional collaborations that conflict with the business objectives of our other collaborators. |
We also have less control over the timing and other aspects of our clinical trials than if we conducted the monitoring and supervision entirely on our own. Third parties may not perform their responsibilities for our clinical trials on our anticipated schedule or consistent with a clinical trial protocol or applicable regulations. We also rely on clinical research organizations to perform much of our data management and analysis. They may not provide these services as required or in a timely manner. If any of these parties do not meet deadlines or follow proper procedures, including procedures required by law, the preclinical studies and clinical trials may take longer than expected, may be delayed or may be terminated, which would have a materially negative impact on our product development efforts. If we were forced to find a replacement entity to perform any of our preclinical studies or clinical trials, we may not be able to find a suitable entity on favorable terms or at all. Even if we were able to find a replacement, resulting delays in the tests or trials may result in significant additional expenditures and delays in obtaining regulatory approval for drug candidates, which could have a material adverse impact on our results of operations and business prospects.
We are subject to intense competition from companies with greater resources and more mature products, which may result in our competitors developing or commercializing products before or more successfully than we do.
We are engaged in a business that is highly competitive. Research and development activities for the development of drugs to treat indications within our focus are being sponsored or conducted by private and
12
public research institutions and by major pharmaceutical companies located in the United States and a number of foreign countries. Most of these companies and institutions have financial and human resources that are substantially greater than our own and they have extensive experience in conducting research and development activities and clinical trials and in obtaining the regulatory approvals necessary to market pharmaceutical products that we do not have. As a result, they may develop competing products more rapidly that are safer, more effective, or have fewer side effects, or are less expensive, or they may develop and commercialize products that render our product candidates non-competitive or obsolete.
We have initially targeted our product candidate RGN-352 for cardiovascular indications. Most large pharmaceutical companies and many smaller biomedical companies are vigorously pursuing the development of therapeutics to treat patients after heart attacks and other cardiovascular indications. With respect to our product candidate RGN-259 for corneal defects, there are also numerous ophthalmic companies developing drugs for corneal wound healing and otheroutside-of-the-eye diseases and injuries. Amniotic membranes have been successfully used to treat corneal wounds in certain cases, as have topical steroids and antibacterial agents. With respect to our product candidate RGN-137 for wound healing, Johnson & Johnson has previously marketed RegranextmRegranex™ for this purpose in patients with diabetic foot ulcers. Other companies, such as Novartis, are developing and marketing artificial skins, which we believe could also compete with RGN-137. Moreover, wound healing is a large and highly fragmented marketplace attracting many companies, large and small, to develop products for treating acute and chronic wounds, including, for example, honey-based ointments, hyperbaric oxygen therapy, and low frequency cavitational ultrasound.
We are also developing potential cosmeceutical products, which are loosely defined as products that bridge the gap between cosmetics and pharmaceuticals, for example, by improving skin texture and reducing the appearance of aging. This industry is intensely competitive, with potential competitors ranging from large multinational companies to very small specialty companies. New cosmeceutical products often have a short product life and are frequently replaced with newer products developed to address the latest trends in appearance and fashion. We may not be able to adapt to changes in the industry as quickly as larger and more experienced cosmeceutical companies. Further, larger cosmetics companies have the financial and marketing resources to effectively compete with smaller companies like us in order to sell products aimed at larger markets.
Even if approved for marketing, our technologies and product candidates are unproven and they may fail to gain market acceptance.
Our product candidates, all of which are based on the molecule Tß4, are new and unproven and there is no guarantee that health care providers or patients will be interested in our product candidates, even if they are approved for use. If any of our product candidates are approved by the FDA, our success will depend in part on our ability to demonstrate sufficient clinical benefits, reliability, safety, and cost effectiveness of our product candidates relative to other approaches, as well as on our ability to continue to develop our product candidates to respond to competitive and technological changes. If the market does
13
not accept our product candidates, when and if we are able to commercialize them, then we may never become profitable. Factors that could delay, inhibit or prevent market acceptance of our product candidates may include:
| | |
| | • | | the timing and receipt of marketing approvals; |
| |
| | • | | the safety and efficacy of the products; |
| |
| | • | | the emergence of equivalent or superior products; |
| |
| | • | | the cost-effectiveness of the products; and |
| |
| | • | | ineffective marketing. |
It is difficult to predict the future growth of our business, if any, and the size of the market for our product candidates because the markets are continually evolving. There can be no assurance that our product candidates will prove superior to products that may currently be available or may become available in the future or that our research and development activities will result in any commercially profitable products.
13
We have no marketing experience, sales force or distribution capabilities. If our product candidates are approved, and we are unable to recruit key personnel to perform these functions, we may not be able to commercialize them successfully.
Although we do not currently have any marketable products, our ability to produce revenues ultimately depends on our ability to sell our product candidates if and when they are approved by the FDA and other regulatory authorities. We currently have no experience in marketing or selling pharmaceutical products, and we do not have a marketing and sales staff or distribution capabilities. Developing a marketing and sales force is also time-consuming and could delay the launch of new products or expansion of existing product sales. In addition, we will compete with many companies that currently have extensive and well-funded marketing and sales operations. If we fail to establish successful marketing and sales capabilities or fail to enter into successful marketing arrangements with third parties, our ability to generate revenues will suffer.
If we enter markets outside the United States our business will be subject to political, economic, legal and social risks in those markets, which could adversely affect our business.
There are significant regulatory and legal barriers to entering markets outside the United States that we must overcome if we seek regulatory approval to market our product candidates in countries other than the United States. We would be subject to the burden of complying with a wide variety of national and local laws, including multiple and possibly overlapping and conflicting laws. We also may experience difficulties adapting to new cultures, business customs and legal systems. Any sales and operations outside the United States would be subject to political, economic and social uncertainties including, among others:
| | |
| | • | | changes and limits in import and export controls; |
| |
| | • | | increases in custom duties and tariffs; |
| |
| | • | | changes in currency exchange rates; |
| |
| | • | | economic and political instability; |
| |
| | • | | changes in government regulations and laws; |
| |
| | • | | absence in some jurisdictions of effective laws to protect our intellectual property rights; and |
| |
| | • | | currency transfer and other restrictions and regulations that may limit our ability to sell certain product candidates or repatriate profits to the United States. |
Any changes related to these and other factors could adversely affect our business if and to the extent we enter markets outside the United States.
Governmental and third-party payors may subject any product candidates we develop to sales and pharmaceutical pricing controls that could limit our product revenues and delay profitability.
The successful commercialization of our product candidates, if they are approved by the FDA, will likely depend on our
14
ability to obtain reimbursement for the cost of the product and treatment. Government authorities, private health insurers and other organizations, such as health maintenance organizations, are increasingly seeking to lower the prices charged for medical products and services. Also, the trend toward managed health care in the United States, the growth of healthcare maintenance organizations, and recently enacted legislation reforming healthcare and proposals to reform government insurance programs could have a significant influence on the purchase of healthcare services and products, resulting in lower prices and reducing demand for our product candidates. The cost containment measures that healthcare providers are instituting and any healthcare reform could reduce our ability to sell our product candidates and may have a material adverse effect on our operations. We cannot assure you that reimbursement in the United States or foreign countries will be available for any of our product candidates, and that any reimbursement granted will be maintained, or that limits on reimbursement available from third-party payors will not reduce the demand for, or the price of, our product candidates. The lack or inadequacy of third-party reimbursements for our product candidates would decrease the potential profitability of our operations. We cannot forecast what
14
additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect the legislation or regulation would have on our business.
We have no manufacturing or formulation capabilities and are dependent upon third-party suppliers to provide us with our product candidates. If these suppliers do not manufacture our product candidates in sufficient quantities, at acceptable quality levels and at acceptable cost, or if we are unable to identify suitable replacement suppliers if needed, our clinical development efforts could be delayed, prevented or impaired.
We do not own or operate manufacturing facilities and have little experience in manufacturing pharmaceutical products. We currently rely, and expect to continue to rely, primarily on peptide manufacturers to supply us with Tß4 for further formulation into our product candidates. We have engaged three separate smaller drug formulation contractors for the formulation of clinical grade product candidates, one for each of our three product candidates in clinical development. We currently do not have an alternative source of supply for either Tß4 or the individual drug candidates. If these suppliers, together or individually, are not able to supply us with either Tß4 or individual product candidates on a timely basis, in sufficient quantities, at acceptable levels of quality and at a competitive price, or if we are unable to identify a replacement manufacturer to perform these functions on acceptable terms as needed, our development programs could be seriously jeopardized.
The risks of relying solely on single suppliers for each of our product candidates include:
| | |
| | • | Their | their respective abilities to ensure quality and compliance with regulations relating to the manufacture of pharmaceuticals; |
| |
| | • | Their | their manufacturing capacity may not be sufficient or available to produce the required quantities of our product candidates based on our planned clinical development schedule, if at all; |
| |
| | • | They | they may not have access to the capital necessary to expand their manufacturing facilities in response to our needs; |
| |
| | • | Commissioning | commissioning replacement suppliers would be difficult and time-consuming; |
| |
| | • | Individual | individual suppliers may have used substantial proprietary know-how relating to the manufacture of our product candidates and, in the event we must find a replacement or supplemental supplier, our ability to transfer this know-how to the new supplier could be an expensiveand/or time-consuming process; |
| |
| | • | An | an individual supplier may experience events, such as a fire or natural disaster, that force it to stop or curtail production for an extended period; |
| |
| | • | An | an individual supplier could encounter significant increases in labor, capital or other costs that would make it difficult for them to produce our products cost-effectively; or |
| |
| | • | An | an individual supplier may not be able to obtain the raw materials or validated drug containers in sufficient quantities, at acceptable costs or in sufficient time to complete the manufacture, formulation and delivery of our product candidates. |
Our suppliers may use hazardous and biological materials in their businesses. Any claims relating to improper handling, storage or disposal of these materials could be time-consuming and costly to us, and we are not insured against such claims.
15
Our product candidates and processes involve the controlled storage, use and disposal by our suppliers of certain hazardous and biological materials and waste products. We and our suppliers and other collaborators are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. Even if we and these suppliers and collaborators comply with the standards prescribed by law and regulation, the risk of accidental contamination or injury from hazardous materials cannot be completely eliminated. In the event of an accident, we could be held liable for any damages that result, and we do not carry insurance for this type of claim. We may also incur significant costs to comply with current or future environmental laws and regulations.
15
We face the risk of product liability claims, which could adversely affect our business and financial condition.
We may be subject to product liability claims as a result of our testing, manufacturing, and marketing of drugs. In addition, the use of our product candidates, when and if developed and sold, will expose us to the risk of product liability claims. Product liability may result from harm to patients using our product candidates, such as a complication that was either not communicated as a potential side effect or was more extreme than anticipated. We require all patients enrolled in our clinical trials to sign consents, which explain various risks involved with participating in the trial. However, patient consents provide only a limited level of protection, and it may be alleged that the consent did not address or did not adequately address a risk that the patient suffered. Additionally, we will generally be required to indemnify our clinical product manufacturers, clinical trial centers, medical professionals and other parties conducting related activities in connection with losses they may incur through their involvement in the clinical trials.
Our ability to reduce our liability exposure for human clinical trials and commercial sales, if any, of Tß4 is dependent in part on our ability to obtain sufficient product liability insurance or to collaborate with third parties that have adequate insurance. Although we intend to obtain and maintain product liability insurance coverage if we gain approval to market any of our product candidates, we cannot guarantee that product liability insurance will continue to be available to us on acceptable terms, or at all, or that its coverage will be sufficient to cover all claims against us. A product liability claim, even one without merit or for which we have substantial coverage, could result in significant legal defense costs, thereby potentially exposing us to expenses significantly in excess of our revenues, as well as harm to our reputation and distraction of our management.
If any of our key employees discontinue their services with us, our efforts to develop our business may be delayed.
We are highly dependent on the principal members of our management team. The loss of our chairman and chief scientific advisor, Allan Goldstein, or our chief executive officer, J.J. Finkelstein, could prevent or significantly delay the achievement of our goals. We have employment agreements with Dr. Goldstein and Mr. Finkelstein. For part of 2009, we effected salary reductions for certain of our employees, including Dr. Goldstein and Mr. Finkelstein. Although their salaries were restored effective as of October 1, 2009, weWe cannot assure you that they, or other key employees, maywill not elect to terminate their employment as a result of the salary reductions or for other reasons.employment. In addition, we do not maintain a key man life insurance policy with respect to Dr. Goldstein or Mr. Finkelstein. In the future, we anticipate that we may need to add additional management and other personnel. Competition for qualified personnel in our industry is intense, and our success will depend in part on our ability to attract and retain highly skilled personnel. We cannot assure you that our efforts to attract or retain such personnel will be successful.
Mauro Bove, a member of our Board, is also a director and officer of entities affiliated with Sigma-Tau, a relationship which could give rise to a conflict of interest involving Mr. Bove.
Mauro Bove, a member of our Board of Directors, is also a director and officer of entities affiliated with Sigma-Tau, which collectively make up our largest stockholder group. Sigma-Tau has provided us with significant funding, may continue doing so in the future, and is also our strategic partner in Europe with respect to the development of certain of our drug candidates. During 2008 and 2009, weWe have issued shares of common stock and common stock warrants to Sigma-Tau in four separateseveral private placement financing transactions, including as recently as January 2011, but we retained the right to repurchase some of these shares under certain circumstances.
We have licensed certain rights to our product candidates generally for the treatment of dermal and internal wounds to Sigma-Tau. Under the license agreement, upon the completion of a Phase 2 clinical trial of either of these product candidates that yields positive results in terms of clinical efficacy and safety, Sigma-Tau is obligated to either make a $5 million milestone payment to us or to initiate and fund a pivotal Phase 3 clinical trial of the product candidate. In 2009, we completed two Phase 2 clinical trials of RGN-137 in the treatment of pressure ulcers and venous stasis ulcers. However, due to the lack of statistical significance of the
16
reported efficacy results, these trials arewere not sufficient to trigger the milestone obligation described above. There can be no assurance that we will ever receive this payment or be able to initiate a pivotal Phase 3 clinical trial of RGN-137 that would be funded by Sigma-Tau. As a result of Mr. Bove’s relationship with Sigma-Tau, there could be a conflict of interest between Sigma-Tau and our other stockholders with respect to these and other agreements and circumstances that may require the exercise of the Board’s discretion with respect to Sigma-Tau. Any decision in the best
16
interests of Sigma-Tau may not be in the best interest of our other stockholders.
Risks Related To Our Intellectual Property
We are heavily reliant on our license from the National Institutes of Health for the rights to Tß4, and any loss of these rights would adversely affect our business.
We have received an exclusive worldwide license to intellectual property discovered at the National Institutes of Health, or NIH, pertaining to the use of Tß4 in wound healing and tissue repair. The intellectual property rights from this license form the basis for our current commercial development focus with Tß4. This license terminates upon the last to expire of the patent applications that are filed, or any patents that may issue from such applications, in connection with the license. This license requires us to pay a minimum annual royalty to the NIH, regardless of the success of our product development efforts, plus certain other royalties upon the sale of products created by the intellectual property granted under the license. This license may be terminated for a number of reasons, including our non-payment of the royalty or lack of continued product development, among others. While to date we believe that we have complied with all requirements to maintain the license, the loss of this license would have a material adverse effect on our business and business prospects and may require us to cease development of our current line of Tß4-based product candidates.
If we are not able to maintain adequate patent protection for our product candidates, we may be unable to prevent our competitors from using our technology or technology that we license.
Our success will depend in substantial part on our ability to obtain, defend and enforce patents, maintain trade secrets and operate without infringing upon the proprietary rights of others, both in the United States and abroad. Pursuant to an exclusive worldwide license from the NIH, we have exclusive rights to use Tß4 in the treatment of non-healing wounds. While patents covering our use of Tß4 have issued in some countries, we cannot guarantee whether or when corresponding patents will be issued, or the scope of any patents that may be issued, in other countries. We have attempted to create a substantial intellectual property portfolio, submitting patent applications for various compositions of matter, methods of use and fragments and derivatives of Tß4. We have also in-licensed other intellectual property rights from third parties that could be subject to the same risks as our own patents. If any of these patent applications do not issue, or do not issue in certain countries, or are not enforceable, the ability to commercialize Tß4 in various medical indications could be substantially limited or eliminated.
In addition, the patent positions of the products being developed by us and our collaborators involve complex legal and factual uncertainties. As a result, we cannot assure you that any patent applications filed by us, or by others under which we have rights, will result in patents being issued in the United States or foreign countries. In addition, there can be no assurance that any patents will be issued from any pending or future patent applications of ours or our collaborators, that the scope of any patent protection will be sufficient to provide us with competitive advantages, that any patents obtained by us or our collaborators will be held valid if subsequently challenged or that others will not claim rights in or ownership of the patents and other proprietary rights we or our collaborators may hold. Unauthorized parties may try to copy aspects of our product candidates and technologies or obtain and use information we consider proprietary. Policing the unauthorized use of our proprietary rights is difficult. We cannot guarantee that no harm or threat will be made to our or our collaborators’ intellectual property. In addition, changes in, or different interpretations of, patent laws in the United States and other countries may also adversely affect the scope of our patent protection and our competitive situation.
17
Due to the significant time lag between the filing of patent applications and the publication of such patents, we cannot be certain that our licensors were the first to file the patent applications we license or, even if they were the first to file, also were the first to invent, particularly with regards to patent rights in the United States. In addition, a number of pharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to our product candidates. Some of these technologies, applications or patents may conflict with our or our licensors’ technologies or patent applications. A conflict could limit the scope of the patents, if any, that we or our licensors may be able to obtain or result in denial of our or our licensors’ patent applications. If patents that cover our activities are issued to other companies, we may not be able to develop or obtain alternative technology.
Additionally, there is certain subject matter that is patentable in the United States but not generally patentable outside of the United States. Differences in what constitutes patentable subject matter in various countries may limit the protection we can obtain outside of the United States. For example, methods of treating humans are not patentable in many countries outside of the United States. These and other issues may prevent us from obtaining patent protection outside of the United
17
States, which would have a material adverse effect on our business, financial condition and results of operations.
Changes to U.S. patent laws could materially reduce any value our patent portfolio may have.
The value of our patents depends in part on their duration. A shorter period of patent protection could lessen the value of our rights under any patents that may be obtained and may decrease revenues derived from its patents. For example, the U.S. patent laws were previously amended to change the term of patent protection from 17 years following patent issuance to 20 years from the earliest effective filing date of the application. Because the time from filing to issuance of biotechnology applications may be more than three years depending on the subject matter, a20-year patent term from the filing date may result in substantially shorter patent protection. Future changes to patent laws could shorten our period of patent exclusivity and may decrease the revenues that we might derive from the patents and the value of our patent portfolio.
We may not have adequate protection for our unpatented proprietary information, which could adversely affect our competitive position.
In addition to our patents, we also rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. However, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. To protect our trade secrets, we may enter into confidentiality agreements with employees, consultants and potential collaborators. However, we may not have such agreements in place with all such parties and, where we do, these agreements may not provide meaningful protection of our trade secrets or adequate remedies in the event of unauthorized use or disclosure of such information. Also, our trade secrets or know-how may become known through other means or be independently discovered by our competitors. Any of these events could prevent us from developing or commercializing our product candidates.
We may be subject to claims that we or our employees have wrongfully used or disclosed alleged trade secrets of former employers.
As is commonplace in the biotechnology industry, we employ now, and may hire in the future, individuals who were previously employed at other biotechnology or pharmaceutical companies, including competitors or potential competitors. Although there are no claims currently pending against us, we may be subject to claims that we or certain employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and would be a significant distraction to management.
18
Risks Related To Our Securities and This Offering
Our common stock price is volatile, and has had limited trading volume,our stock is highly illiquid, and any investment in our securities could decline substantially in value.
For the period from January 1, 2009 through December 31, 2010, the dateclosing price of this prospectus, our closingcommon stock price has fluctuated between prices of $0.42ranged from $0.21 to $1.75, per share, with an average daily trading volume of approximately 80,000117,000 shares. We expect the trading volume of our common stock to decline further in light of our recent delisting from the NYSE Amex exchange. In light of our small size and limited resources, as well as the uncertainties and risks that can affect our business and industry, our stock price is expected to continue to be highly volatile and can be subject to substantial drops, with or even in the absence of news affecting our business. The following factors, in addition to the other risk factors described in this prospectus, and the potentially low volume of trades in our common stock, may have a significant impact on the market price of our common stock, some of which are beyond our control:
| • | | the recent delisting of our common stock from the NYSE Amex exchange; |
|
| | • | | results of preclinicalpre-clinical studies and clinical trials; |
| |
| | • | | commercial success of approved products; |
| |
| | • | | corporate partnerships; |
| |
| | • | | technological innovations by us or competitors; |
|
18
| | • | | changes in laws and government regulations both in the U.S. and overseas; |
| |
| | • | | changes in key personnel at our company; |
| |
| | • | | developments concerning proprietary rights, including patents and litigation matters; |
| |
| | • | | public perception relating to the commercial value or safety of any of our product candidates; |
| |
| | • | | future sales of our common stock;stock, including to Lincoln Park under the Purchase Agreement; |
| |
| | • | future issuance | other issuances of our common stock causing dilution; |
| |
| | • | | anticipated or unanticipated changes in our financial performance; |
| |
| | • | | general trends related to the biopharmaceutical and biotechnological industries; and |
| |
| | • | | general conditions in the stock market. |
The stock market in general has recently experienced relatively large price and volume fluctuations. In particular, the market prices of securities of smaller biotechnology companies have experienced dramatic fluctuations that often have been unrelated or disproportionate to the operating results of these companies. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in its value. You should also be aware that price volatility may be worse if the trading volume of the common stock remains limited or declines.
Our principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
Following this offering, we estimatethe investment transactions that were consummated on January 7, 2011, our officers, directors and principal stockholders together will controlcontrolled approximately 40%43% of our outstanding common stock, not including the exercise of options or warrants.stock. Included in this group is Sigma-Tau and its affiliates, which will holdtogether held outstanding shares representing approximately 33%38% of our outstanding common stock following the offering, excluding the effect of any options and warrants, and not giving effect to any potential participation by Sigma-Tau in this offering. Sigma-Tau has expressed an interest in purchasing units in this offering.stock. A portion of the shares of common stock currently held by Sigma-Tau representing approximately 18% of our outstanding common stock, isand its affiliates are subject to voting agreements under which we controlour Board controls the voting power of these shares.such stock. We cannot assure you that thesesuch voting agreements would prevent Sigma-Tau and its affiliates from taking actions not in your best interests and effectively exercising control over us. These voting agreements are currently scheduled to expire between June 2010 andperiodically through September 2012. After their expiration, we will have no control over the voting of these shares controlled by Sigma-Tau, including with respect to the
19
election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock, and therefore may not be in the best interest of our other stockholders.
An active trading market for the warrants being sold in this offering may not develop.
Prior to this offering, there has been no public market for the warrants that are part of the units. It is anticipated that the warrants will be quoted on the OTC Bulletin Board promptly after the date of this prospectus. However, an active trading market for our warrants may never develop, and an active market for our common stock may not be sustained. If an active market for our securities does not develop, it may be difficult for you to sell the securities you purchase in this offering without depressing the market price for such securities.
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price of our common stock and other securities and their trading volume could decline.
The trading market for our common stock and other securities will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If securities or industry analysts do not commence or maintain coverage of us, the trading price for our common stock and other securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our common stock and other securities and their trading volume to decline.
Our rights to repurchase certain shares of stock held by Sigma-Tau expire over time, and we may never be able or elect to exercise these rights.
Until June 2010, we have the right to repurchase at a price of $5.00 per share a number of shares of common stock issued to Sigma-Tau equal to the lesser of the shares sold to Sigma-Tau in connection with our private placement of securities in June 2005, or the number of shares necessary to reduce Sigma-Tau’s ownership of our outstanding capital stock to an aggregate of approximately 30% at the time of such repurchase. In addition, we have the right to repurchase at any time until December 31, 2010, for $2.50 per share, up to 5,000,000 shares of common stock issued to Sigma-Tau in connection with a private placement of securities in February 2008. After December 31, 2010, our rights to repurchase common stock held by Sigma-Tau will expire. These provisions could, under certain circumstances, allow us to reduce dilution by repurchasing these shares at prices lower than the then-prevailing market price of our common stock. However, we cannot assure you that our share price will increase sufficiently to make such repurchases economically feasible or that we would avail ourselves of the opportunity to make such repurchases even if our share price had risen to such a level.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future. This could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. Upon completionAfter giving effect to the issuance of this offering,15,000,000 shares to Lincoln Park, we will have outstanding 71,906,82894,860,282 shares of common stock, assuming no exercise of outstanding options or warrants. OfSubstantially all of these shares, the 11,500,000 shares underlying the units sold in this offering and 28,851,031 additional outstanding shares and any shares issued upon exercise of warrants issued in this offering, will be freely tradable, and 31,555,797 additional shares of common stock will be available for sale in the public market beginning 90 days after the date of this prospectus following the expiration oflock-up agreements betweentradable. If our officers, directors and certain stockholders and the representative of the underwriters, subject to restrictions under federal securities laws. The representative of the underwriters may release these officers, directors and stockholders from theirlock-up agreements with the underwriters at any time and without notice, which would allow for earlier sales of shares in the public market. If our
20
stockholders sell, or the market perceives that our stockholders intend to sell, substantial amounts of our common stock in the public market following this offering, the market price of our common stock could decline significantly.
19
If you purchase securities in this offering, you will suffer immediate dilution of your investment.
We expect the public offering price of the units to be substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase units in this offering, you will effectively pay a price per share that substantially exceeds our net tangible book value per share after this offering. Based on an assumed public offering price of $0.56 per unit (based on the last reported sale price of our common stock on May 14, 2010), you will experience immediate dilution of $0.45 per share underlying each unit, representing the difference between our net tangible book value per share after giving effect to this offering and the effective public offering price per share of our common stock.
The exercise of options and warrants and other issuances of shares of common stock or securities convertible into common stock will dilute your interest.
As of March 31, 2010,the date of this prospectus, there wereare outstanding options to purchase an aggregate of 4,914,1125,348,863 shares of our common stock at exercise prices ranging from $0.28$0.27 per share to $3.82 per share of which optionsand outstanding warrants to purchase 3,613,069 shares were exercisable as of such date. As of March 31, 2010, there were warrants outstanding to purchase 7,933,85116,136,900 shares of our common stock at a weighted average exercise price of $2.01 per share. We will issue additional warrants as part of the units being sold in this offering, with an exercise price equal to $$0.89 per share. The exercise of options and warrants at prices below the market price of our common stock could adversely affect the price of shares of our common stock. Additional dilution may result from the issuance of shares of our capital stock in connection with collaborations or manufacturing arrangements or in connection with other financing efforts.efforts, including pursuant to the Purchase Agreement with Lincoln Park.
Any issuance of our common stock that is not made solely to then-existing stockholders proportionate to their interests, such as in the case of a stock dividend or stock split, will result in dilution to each stockholder by reducing his, her or its percentage ownership of the total outstanding shares. Moreover, if we issue options or warrants to purchase our common stock in the future and those options or warrants are exercised or we issue restricted stock, stockholders may experience further dilution. Holders of shares of our common stock have no preemptive rights that entitle them to purchase their pro rata share of any offering of shares of any class or series.
In addition, certainmost of the outstanding warrants to purchase shares of our common stock currently containhave an exercise price above the current market price for theour common stock, or above-market warrants, as will the warrants issued in connection with this offering.stock. As a result, these warrants may not be exercised prior to their expiration, andin which case we maywould not realize any proceeds from their exercise.
The sale of shares of our common stock to Lincoln Park under the Purchase Agreement may cause substantial dilution to our existing stockholders and could cause the price of our common stock to decline.
Under the Purchase Agreement, we may sell to Lincoln Park, from time to time and under certain circumstances, up to $11,000,000 of our common stock. We do not have the right to commence any sales of our shares to Lincoln Park under the Purchase Agreement until the SEC has declared effective the resale registration statement of which this prospectus forms a part. After the SEC has declared effective the resale registration statement, over approximately 30 months, generally, we have the right, but no obligation, to direct Lincoln Park to periodically purchase up to $11,000,000 of our common stock in specific amounts under certain conditions, which periodic purchase amounts can be increased under specified circumstances.
We have also agreed to issue to Lincoln Park up to an aggregate of 1,916,666 shares of common stock as a fee for Lincoln Park’s commitment to purchase our shares. Of these commitment shares, we issued one-half, or 958,333 shares, upon entering into the agreement with Lincoln Park. The remaining commitment shares are issuable to Lincoln Park on a pro rata basis as purchases are made under the Purchase Agreement.
Depending upon market liquidity at the time, sales of shares of our common stock to Lincoln Park may cause the trading price of our common stock to decline. Lincoln Park may ultimately purchase all, some or none of the $11,000,000 of common stock, and after it has acquired shares, Lincoln Park may sell all, some or none of those shares. Therefore, sales to Lincoln Park by us could result in substantial dilution to the interests of other holders of our common stock. The sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales. However, we have the right to control the timing and amount of any sales of our shares to Lincoln Park, and the Purchase Agreement may be terminated by us at any time at our discretion without any cost to us.
Our certificate of incorporation, our stockholder rights plan and Delaware law contain provisions that could discourage or prevent a takeover or other change in control, even if such a transaction would be beneficial to our stockholders, which could affect our stock price adversely and prevent attempts by our stockholders to replace or remove our current management.
Our certificate of incorporation provides our Board with the power to issue shares of preferred stock without stockholder approval. In addition, under our stockholder rights plan, our Board has the discretion to issue certain rights to purchase our capital stock to our stockholders when a person acquires in excess of 25% of our outstanding common shares. These provisionsOur Board has exempted purchases by Sigma-Tau to date and purchases that may be made by Lincoln Park under the Purchase Agreement from the operation of our stockholder rights plan. The stockholder rights plan may make it more difficult for stockholders to take corporate actions and may have the effect of delaying or preventing a change in control, even if such actions or change in control would be in your best interests. In addition, we are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law. Subject to specified exceptions, this section provides that a corporation may not engage in any business combination with any interested stockholder, as defined in that statute, during the three-year period following
20
the time that such stockholder becomes an interested stockholder. This provision could also have the effect of delaying or preventing a change of control of our company. The foregoing factors could reduce the price that investors or an acquirer might be willing to pay in the future for shares of our common stock.
21
We may become involved in securities class action litigation that could divert management’s attention and harm our business and our insurance coverage may not be sufficient to cover all costs and damages.
The stock market has from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of pharmaceutical and biotechnology companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against that company. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could hurt our business, operating results and financial condition.
WeManagement will have broad discretion inas to the use of the proceeds from this offeringsales under the Purchase Agreement, and we may invest ornot use the proceeds effectively.
We have not designated any portion of the proceeds from sales to Lincoln Park under the Purchase Agreement to be used for any particular purpose. Accordingly, our management will have broad discretion as to the application of the proceeds from any such sales and could spend the proceeds in ways with which youthat do not agree andnecessarily improve our operating results or enhance the value of our common stock.
You will experience immediate dilution in ways that may not yield a return.the book value per share of the common stock you purchase.
WeBecause the price per share of our common stock being offered is likely to be substantially higher than the book value per share of our common stock, you will have broad discretion oversuffer substantial dilution in the usenet tangible book value of proceeds fromthe common stock you purchase in this offering. You may not agree withBased on the assumed minimum allowed offering price of $0.15 per share in this offering and a pro forma net tangible book value per share of our decisions,common stock of $0.09 as of September 30, 2010, if you purchase securities in this offering, you will suffer immediate and our usesubstantial dilution of $0.06 per share in the net tangible book value of the proceeds may not yield any returncommon stock purchased. See “Dilution” on your investment in us. Our failure to apply the net proceeds of this offering effectively could havepage 26 for a material adverse effect on our business, financial condition and results of operations.
As a public company, we continue to be subject to the requirements of Section 404more detailed discussion of the Sarbanes-Oxley Act. If we are unable to complydilution you will incur in connection with Section 404 in a timely manner it may affect the reliability of our internal control over financial reporting.
Assessing our staffing and training procedures to improve our internal control over financial reporting is an ongoing process. We are currently required to comply with Section 404 of the Sarbanes-Oxley Act of 2002 and to make an assessment of the effectiveness of our internal control over financial reporting. However, our independent registered public accounting firm has not been engaged to express, nor have they expressed, an opinion on the effectiveness of our internal control over financial reporting.
We plan to continue to assess our internal controls and procedures and intend to take further action as necessary or appropriate to address any other matters we identify. Under current SEC rules, our independent registered public accounting firm will also be required to deliver an attestation report on the operating effectiveness of our internal control over financial reporting beginning with the year ending December 31, 2010.
We cannot be certain at this time that we will be able to successfully complete the attestation requirements of Section 404 or that we or our independent registered public accounting firm will not identify material weaknesses in our internal control over financial reporting. If we fail to comply with the requirements of Section 404 or if we or our independent registered public accounting firm identify and report a material weakness, it may affect the reliability of our internal control over financial reporting, which could adversely affect our stock price.offering.
21
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” but are also contained elsewhere in this prospectus. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a
22
combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain. Forward-looking statements include, but are not limited to, statements about:
| | |
| | • | | our anticipated cash needs and our estimates regarding our capital requirements and our needs for additional financing; |
| |
| | • | | the progress, outcome, timing or success of preclinical studies and clinical trials; |
| |
| | • | | the expected timing of clinical trials and availability of data from those trials; |
| |
| | • | | our ability to obtain and maintain regulatory approval for our product candidates from the FDA or foreign regulatory authorities; |
| |
| | • | | future demand for our product candidates and our ability to sustain such demand; |
| |
| | • | | the size of the potential market for our product candidates; |
| |
| | • | | our plans to seek collaborative relationships and the success of those relationships; |
| |
| | • | | the success of competing therapies that are or become available; |
| |
| | • | | our compliance with federal, state and foreign regulatory requirements, and regulatory developments that impact those requirements; |
| |
| | • | | our estimates and assumptions with respect to disease incidence; |
| |
| | • | | our intellectual property and our strategies regarding filing additional patent applications to attempt to strengthen our intellectual property rights; |
| |
| | • | | our ability to retain key management and scientific personnel; |
| |
| | • | | estimates of our future financial performance; |
| |
| | • | | our ability to implement financial controls and procedures on a timely basis; and |
| |
| | • | | anticipated trends and challenges in our business. |
In addition, you should refer to the “Risk Factors” section of this prospectus for a discussion of other important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate or that we will achieve the plans, intentions or expectations expressed or implied in our forward-looking statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Any forward-looking statements we make in this prospectus speak only as of its date, and we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the
22
registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
23
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of 11,500,000 units in this offering will be approximately $5.4 million, or approximately $6.3 million if the underwriters exercise their over-allotment option in full, based upon an assumed public offering price of $0.56 per unit (based on the last reported sale price This prospectus relates to shares of our common stock on May 14, 2010), after deducting estimated underwriting discountsthat may be offered and commissions,sold from time to time by Lincoln Park. We will not receive any proceeds upon the 1% corporate finance feesale of shares by Lincoln Park. However, we may receive proceeds of up to $11,000,000 under the Purchase Agreement with Lincoln Park, subject to the terms and estimated offering expenses payable by us.
conditions of the agreement.
We currently expectintend to use the net proceeds from this offering,sales under the Purchase Agreement for preclinical and our existing cash and cash equivalents, to fund research andclinical development activities, including our anticipated Phase 2 clinical trial of RGN-352 in AMI patients, as well as the completion of our ongoing Phase 2 clinical trial of RGN-137 in patients with EBdrug candidates and our support of compassionate use studies using RGN-259 and a potential Phase 1/2 clinical trial of RGN-352 in patients with multiple sclerosis. We also expect to use a portion of the net proceeds for general corporate purposes, including working capital.
In addition, we may use a portion of the proceeds to acquire drugs or drug candidates, technologies, businesses or other assets. The expected usetiming and amount of net proceeds from this offering represents our intentionsactual expenditures will be based uponon many factors, including the progress, timing and success of our present plans and business conditions. We will not be able to complete the contemplated Phase 2 clinical trial of RGN-352 without additional capital. Further, we expect that the net proceeds will not be sufficient to complete clinical trials to obtain regulatory approval for the marketing ofand other development efforts, whether we partner any of our current product candidates. As described elsewhere in this prospectus, the completion of these trials may be delayed for a number of reasons. As of the date of this prospectus,development programs, and whether we cannot predict with certainty all of the particular uses for the proceeds of this offering or the amounts that we will actually spend on the uses set forth above. The amount and timing of actual expenditures may vary significantly depending upon a number of factors, such as the progresschoose to curtail some of our development efforts, regulatory requirements, commercialization efforts in the event that we obtain regulatory approval, the amount of cash, if any, we generate from strategic collaborations that we may enter into or from other sources, andresearch activities, as well as the amount of cash used byin our operations. Accordingly,We will retain broad discretion in determining how we will have significant flexibility in applyingallocate the netproceeds from any sales to Lincoln Park.
Until we use the proceeds of this offering.
Pending their use,any such sales, we intend to invest the net proceeds of this offeringfunds in a variety of capital-preservation investments, including short- and intermediate-term,short-term, investment grade, interest-bearing investment-grade securities.
2423
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2010:September 30, 2010.
| | |
| • | on an actual basis; and |
| | |
| • | on an as adjusted basis to give effect to our sale of 11,500,000 units in this offering at an assumed public offering price of $0.56 per unit (based on the last reported sale price of our common stock on May 14, 2010), after deducting estimated underwriting discounts and commissions, the 1% corporate finance fee payable to the representative of the underwriters and estimated offering expenses payable by us. |
| | | | | | | | | |
| | | Actual | | | As Adjusted | |
| | | (In thousands) | |
| |
| Cash and cash equivalents | | $ | 3,190 | | | $ | 8,638 | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Preferred stock, $0.001 per share, 1,000,000 shares authorized, no shares issued or outstanding, actual or as adjusted | | | — | | | | — | |
| Common stock, $0.001 par value, 100,000,000 shares authorized, 60,406,828 shares issued and outstanding, actual; 100,000,000 shares authorized, 79,406,828 shares issued and outstanding, as adjusted; | | | 60 | | | | 72 | |
Additionalpaid-in-capital | | | 88,276 | | | | 93,713 | |
| Accumulated deficit | | | (85,647 | ) | | | (85,647 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 2,689 | | | | 8,137 | |
| | | | | | | | | |
| Total capitalization | | $ | 2,689 | | | $ | 8,137 | |
| | | | | | | | | |
| | | | | |
|
| Cash and cash equivalents | | $ | 4,975,947 | |
| | | | |
| Stockholders’ equity: | | | | |
| Preferred stock, $0.001 per share, 1,000,000 shares authorized, no shares issued or outstanding | | | — | |
| Common stock, $0.001 par value, 200,000,000 shares authorized, 73,531,578 shares issued and outstanding | | | 73,531 | |
| Additional paid-in-capital | | | 92,997,669 | |
| Accumulated deficit | | | (88,605,400 | ) |
| | | | |
| |
| Total stockholders’ equity | | | 4,465,800 | |
| | | | |
| |
| Total capitalization | | $ | 4.465,800 | |
| | | | |
The number of shares of common stock outstanding in the table above does not include:excludes, as of September 30, 2010:
| • | | |
| | • | 4,914,1125,348,863 shares of our common stock issuable upon the exercise of outstanding stock options, outstanding under our 2000 stock option plan as of March 31, 2010, atwith a weighted average exercise price of $1.53$1.37 per share; |
| | |
| • | • | 1,550,8884,327,500 shares of our common stock available for future issuance under our 2000 stock option plan; |
| | 2010 Equity Incentive Plan; |
| |
| • | 7,933,851 | 13,988,751 shares of our common stock issuable upon the exercise of outstanding warrants, as of March 31, 2010, atwith a weighted-average exercise price of $2.01$1.38 per share; and |
| | |
| • | • | shares issuable upon exercise of warrantsour common stock issued to be issuedLincoln Park and Sigma-Tau in connection with this offering.January 2011. |
2524
PRICE RANGE OF COMMON STOCK AND DIVIDEND POLICY
Our common stock tradesis quoted on the OTC Bulletin Board under the symbol “RGRX.” Our common stock last traded at $0.22 on January 28, 2011. Prior to December 23, 2010, our stock traded on the NYSE Amex previously known as the American Stock Exchange,stock exchange under the symbol “RGN.” The prices, as presented below, representfollowing table provides the highesthigh and lowest bid or salelow closing prices for our common stock by quarterfor each quarterly period within the two most recent fiscal years as quoted on the NYSE Amex. On May 14, 2010,Amex or reported by the last sale price of our common stockOTC Bulletin Board, as appropriate. The quotation reported onby the NYSE Amex was $0.56 per share.OTC Bulletin Board reflects inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
| | | | | | | | | |
| 2009 | | High | | | Low | |
| First Quarter | | $ | 1.75 | | | $ | 0.42 | |
| Second Quarter | | $ | 0.85 | | | $ | 0.45 | |
| Third Quarter | | $ | 1.12 | | | $ | 0.52 | |
| Fourth Quarter | | $ | 0.83 | | | $ | 0.55 | |
| | | | | | | | | |
| 2010 | | High | | | Low | |
| First Quarter | | $ | 0.65 | | | $ | 0.53 | |
| Second Quarter | | $ | 0.68 | | | $ | 0.26 | |
| Third Quarter | | $ | 0.35 | | | $ | 0.24 | |
| Fourth Quarter | | $ | 0.30 | | | $ | 0.21 | |
| | | | | | | | | |
| 2011 | | High | | | Low | |
| First Quarter (through January 28, 2011) | | $ | 0.27 | | | $ | 0.20 | |
| | | | | | | | | |
2008 | | High | | Low |
| |
| First Quarter | | $ | 1.10 | | | $ | 0.80 | |
| Second Quarter | | $ | 1.92 | | | $ | 0.83 | |
| Third Quarter | | $ | 1.43 | | | $ | 1.02 | |
| Fourth Quarter | | $ | 1.66 | | | $ | 0.85 | |
| | | | | | | | | |
2009 | | High | | Low |
| |
| First Quarter | | $ | 1.75 | | | $ | 0.42 | |
| Second Quarter | | $ | 0.85 | | | $ | 0.45 | |
| Third Quarter | | $ | 1.12 | | | $ | 0.52 | |
| Fourth Quarter | | $ | 0.83 | | | $ | 0.55 | |
| | | | | | | | | |
2010 | | High | | Low |
| |
| First Quarter | | $ | 0.65 | | | $ | 0.53 | |
| Second Quarter (through May 14, 2010) | | $ | 0.68 | | | $ | 0.46 | |
As of April 14,December 31, 2010, we had 851832 holders of record of our common stock.
Each unit to be issued in this offering consists of sharesstock and over 4,100 beneficial holders of our common stock and a tradeable warrant to purchase additional shares of our common stock. The units will separate immediately and the common stock and the warrants will be issued separately. There will be no market for the units. Currently, no public market exists for the warrants. We intend to apply for listing of the warrants on the NYSE Amex, and we expect that the warrants will begin trading on or promptly after the date of this prospectus, subject to listing.
We have never declared or paid any dividends on our common stock or any other securities. We anticipate that we will retain all of our future earnings, if any, for use in the operation of our business and do not anticipate paying cash dividends in the foreseeable future.
2625
DILUTION
If you invest in this offering, your interest Investors who purchase our common stock will be diluted to the extent of the difference between the public offering price per share of our common stock assuming no value is attributed to the warrants included in each unit, and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering. Net tangible book value per share is determined by dividing our total tangible assets less total liabilities by the number of outstanding shares of our common stock. As of March 31,September 30, 2010, we had a net tangible book value of $2.7$4.5 million, or approximately $0.04$0.06 per share of common stock.
Investors participating in this offering will incur immediate and substantial dilution. After giving effect to the issuance of an aggregate of 6,328,704 shares in connection with sales to Lincoln Park and saleSigma-Tau on January 4, 2011 and January 7, 2011 that generated net proceeds of 11,500,000 units$1.45 million, our as adjusted net tangible book value would have been $5.9 million, or approximately $0.07 per share, as of September 30, 2010.
Dilution in net tangible book value per share represents the difference between the amount per share paid by purchasers of common stock in this offering, at an assumed public offeringassuming a purchase price of $0.56$0.15 per unit (based onshare, which is the last reportedminimum purchase price at which shares can be sold under the Purchase Agreement, and the pro forma as adjusted net tangible book value per share of common stock immediately after the completion of this offering. After giving effect to our assumed receipt of $2.2 million in estimated proceeds from the sale of 14,806,506 shares of common stock issuable under the Purchase Agreement and registered in this offering (assuming a purchase price of our common stock$0.15 per share and the issuance of 193,404 additional commitment shares for no additional cash consideration, and assuming all such sales and issuances were made on May 14,September 30, 2010), and after deducting estimated underwriting discounts and commissions, the 1% corporate finance fee payable to the representative of the underwriters and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of March 31,September 30, 2010 would have been approximately $8.1 million, or approximately $0.11$0.09 per share of common stock.share. This representswould represent an immediate increase in the net tangible book value of $0.07$0.02 per share to existing stockholders and an immediate dilution in the net tangible book value of $0.45 per shareattributable to investors purchasing units in this offering. The following table illustrates this per share dilution:
| | | | | | | | | |
| Assumed public offering price per share of common stock (minimum allowed price) | | | | | | $ | 0.15 | |
| As adjusted net tangible book value per share as of September 30, 2010 | | $ | 0.07 | | | | | |
| Increase in as adjusted net tangible book value per share attributable to this offering | | | 0.02 | | | | | |
| | | | | | | | |
| Pro forma net tangible book value per share after this offering | | | | | | | 0.09 | |
| | | | | | | | |
| Dilution per share to new investors | | | | | | $ | 0.06 | |
| | | | | | | | |
| | | | | | | | | |
| Assumed public offering price per share of common stock underlying each unit | | | | | | $ | 0.56 | |
| Actual net tangible book value per share as of March 31, 2010 | | $ | 0.04 | | | | | |
| Increase in net tangible book value per share attributable to new investors participating in this offering | | | 0.07 | | | | | |
| | | | | | | | | |
| Net tangible book value per share after this offering | | | | | | | 0.11 | |
| | | | | | | | | |
| Dilution per share to investors participating in this offering | | | | | | $ | 0.45 | |
| | | | | | | | | |
IfTo the underwriters exercise their optionextent that we sell more or less than $2.2 million worth of shares under the Purchase Agreement, or to the extent that some or all sales are made at prices in full toexcess of the minimum allowable purchase 1,725,000 additional units in this offering, the pro forma net tangible book valueprice of $0.15 per share, afterthen the offering would be $0.12 per share, the increasedilution reflected in the pro forma net tangible book value per sharetable above will differ. The above table is based on 73,531,578 shares of our common stock outstanding as of September 30, 2010, adjusted for the issuance of 6,328,704 shares issued to existing stockholders would be $0.08 per shareLincoln Park and Sigma-Tau on January 4, 2011 and January 7, 2011 for aggregate gross proceeds of $1.45 million, and the dilutionassumed sale of $2.2 million in shares to new investors purchasing unitsLincoln Park under the Purchase Agreement at the assumed minimum purchase price described above. In addition, the calculations in this offering would be $0.44 per share.the foregoing table do not take into account, as of September 30, 2010:
The table above excludes:
| | |
| | • | 4,914,112 | 5,348,863 shares of our common stock issuable upon the exercise of outstanding stock options, outstanding under our 2000 stock option plan as of March 31, 2010, atwith a weighted average exercise price of $1.53$1.37 per share; |
| | |
| | • | 1,550,888 | 4,327,500 shares of our common stock available for future issuance under our 2000 stock option plan;2010 Equity Incentive Plan; and |
| | |
| | • | 7,933,851 | 13,988,751 shares of our common stock issuable upon the exercise of outstanding warrants, as of March 31, 2010, atwith a weighted-average exercise price of $2.01$1.38 per share; andshare. |
| | |
| • | shares issuable upon exercise of warrants to be issued in connection with this offering. |
To the extent that options or warrants are exercised, new options are issued under our equity benefit plans, or we issue additional shares of common stock in the future, there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
2726
SELECTED FINANCIAL DATA
You should read the following selected financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and accompanying notes included later in this prospectus. The selected financial data in this section is not intended to replace our financial statements and the accompanying notes.
We have derived the selected balance sheet data as of December 31, 2009 and 2008 and the selected statement of operations data for the years ended December 31, 2009 and 2008 from our audited financial statements that are included in this prospectus. We have derived the selected balance sheet data as of December 31, 2007, 2006 and 2005 and the selected statement of operations data for the years ended December 31, 2007, 2006 and 2005 from our audited financial statements that are not included in this prospectus. We have derived the selected statement of operations data for threethe nine months ended March 31,September 30, 2010 and 2009 and the selected balance sheet data as of March 31,September 30, 2010 from our unaudited financial statements that are included in this prospectus.
Our historical results are not necessarily indicative of the results to be expected in any future period.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | Nine Months Ended | |
| | | Year Ended December 31, | | | September 30, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2010 | | | 2009 | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | | | $ | 240,324 | | | $ | 272,491 | | | $ | — | | | $ | 53,819 | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | | | | 8,887,255 | | | | 6,396,524 | | | | 3,155,735 | | | | 1,819,036 | | | | 3,064,248 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | | | | 3,197,685 | | | | 2,665,652 | | | | 2,513,792 | | | | 2,345,619 | | | | 2,161,539 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | | | | 12,084,940 | | | | 9,062,176 | | | | 5,669,527 | | | | 4,164,655 | | | | 5,225,787 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) | | | (11,844,616 | ) | | | (8,789,685 | ) | | | (5,669,527 | ) | | | (4,110,836 | ) | | | (5,225,787 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | | | | 666,458 | | | | 522,704 | | | | 214,676 | | | | 6,840 | | | | 10,304 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) | | $ | (11,178,158 | ) | | $ | (8,266,981 | ) | | $ | (5,454,851 | ) | | $ | (4,103,996 | ) | | | (5,215,483 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.12 | ) | | $ | (0.21 | ) | | $ | (0.24 | ) | | $ | (0.21 | ) | | $ | (0.15 | ) | | $ | (0.06 | ) | | | (0.10 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares used to compute basic and diluted net loss per share | | | 55,680,525 | | | | 50,967,617 | | | | 46,465,982 | | | | 40,116,367 | | | | 36,843,609 | | | | 66,729,519 | | | | 54,216,430 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Three Months Ended
| |
| | | Year Ended December 31, | | | March 31, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2010 | | | 2009 | |
| |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | | | $ | 240,324 | | | $ | 272,491 | | | $ | — | | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | | | | 8,887,255 | | | | 6,396,524 | | | | 3,155,735 | | | | 470,434 | | | | 1,661,600 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | | | | 3,197,685 | | | | 2,665,652 | | | | 2,513,792 | | | | 678,068 | | | | 859,568 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | | | | 12,084,940 | | | | 9,062,176 | | | | 5,669,527 | | | | 1,148,502 | | | | 2,521,168 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) | | | (11,844,616 | ) | | | (8,789,685 | ) | | | (5,669,527 | ) | | | (1,148,502 | ) | | | (2,521,168 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | | | | 666,458 | | | | 522,704 | | | | 214,676 | | | | 2,793 | | | | 6,518 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) | | $ | (11,178,158 | ) | | $ | (8,266,981 | ) | | $ | (5,454,851 | ) | | $ | (1,145,709 | ) | | $ | (2,514,650 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.12 | ) | | $ | (0.21 | ) | | $ | (0.24 | ) | | $ | (0.21 | ) | | $ | (0.15 | ) | | $ | (0.02 | ) | | $ | (0.05 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Shares used to compute basic and diluted net loss per share | | | 55,680,525 | | | | 50,967,617 | | | | 46,465,982 | | | | 40,116,367 | | | | 36,843,609 | | | | 60,406,828 | | | | 53,622,491 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | As of
| | | | |
| | | As of December 31, | | | March 31,
| | | | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2010 | | | | |
| |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 4,355,768 | | | $ | 5,655,367 | | | $ | 3,696,878 | | | $ | 13,052,308 | | | $ | 4,896,143 | | | $ | 3,189,990 | | | | | |
| Short-term investments | | | — | | | | — | | | | 4,579,592 | | | | 4,000,000 | | | | 2,679,693 | | | | — | | | | | |
| Working capital | | | 3,671,910 | | | | 4,565,932 | | | | 6,102,596 | | | | 16,187,188 | | | | 6,939,195 | | | | 2,648,787 | | | | | |
| Total assets | | | 4,583,754 | | | | 5,922,576 | | | | 8,621,793 | | | | 17,501,625 | | | | 7,724,634 | | | | 3,455,854 | | | | | |
| Total liabilities | | | 880,404 | | | | 1,325,912 | | | | 2,469,069 | | | | 1,249,290 | | | | 714,127 | | | | 766,369 | | | | | |
| Accumulated deficit | | | (84,501,404 | ) | | | (78,007,544 | ) | | | (67,405,579 | ) | | | (56,227,421 | ) | | | (47,960,440 | ) | | | (85,647,113 | ) | | | | |
| Stockholders’ equity | | | 3,703,350 | | | | 4,596,664 | | | | 6,152,724 | | | | 16,252,335 | | | | 7,010,507 | | | | 2,689,485 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | As of | |
| | | As of December 31, | | | September 30, | |
| | | 2009 | | | 2008 | | | 2007 | | | 2006 | | | 2005 | | | 2010 | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 4,355,768 | | | $ | 5,655,367 | | | $ | 3,696,878 | | | $ | 13,052,308 | | | $ | 4,896,143 | | | $ | 4,975,947 | |
| Short-term investments | | | — | | | | — | | | | 4,579,592 | | | | 4,000,000 | | | | 2,679,693 | | | | — | |
| Working capital | | | 3,671,910 | | | | 4,565,932 | | | | 6,102,596 | | | | 16,187,188 | | | | 6,939,195 | | | | 4,423,905 | |
| Total assets | | | 4,583,754 | | | | 5,922,576 | | | | 8,621,793 | | | | 17,501,625 | | | | 7,724,634 | | | | 5,615,670 | |
| Total liabilities | | | 880,404 | | | | 1,325,912 | | | | 2,469,069 | | | | 1,249,290 | | | | 714,127 | | | | 1,149,870 | |
| Accumulated deficit | | | (84,501,404 | ) | | | (78,007,544 | ) | | | (67,405,579 | ) | | | (56,227,421 | ) | | | (47,960,440 | ) | | | (88,605,400 | ) |
| Stockholders’ equity | | | 3,703,350 | | | | 4,596,664 | | | | 6,152,724 | | | | 16,252,335 | | | | 7,010,507 | | | | 4,465,800 | |
2827
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of our operations together with our financial statements and the related notes to those statements included later in this prospectus. In addition to historical financial information, this discussion contains forward-looking statements reflecting our current plans, estimates, beliefs and expectations that involve risks and uncertainties. As a result of many important factors, particularly those set forth under “Special Note Regarding Forward-Looking Statements” and “Risk Factors,” our actual results and the timing of events may differ materially from those anticipated in these forward-looking statements.
Overview
We are a biopharmaceutical company focused on the development of a novel therapeutic peptide, Thymosin beta 4, or Tß4, for tissue and organ protection, repair, and regeneration. We have formulated Tß4 into three distinct product candidates currently in clinical development:
| | |
| | • | | RGN-352, an injectable product candidate to treat cardiovascular diseases, central nervous system diseases, and other medical indications that may be treated by systemic administration, for which we intend to initiate a Phase 2 clinical trial in the second half of 2010;administration; |
| |
| | • | | RGN-259, a topical eye drop for ophthalmic indications that was evaluated in a small Phase 2 clinical trial and is currently being supported in compassionate use studies;regeneration of corneal tissues damaged by injury, disease or other pathology; and |
| |
| | • | | RGN-137, a topically applied gel for chronic dermal wounds and reduction of scar tissue that is currently in a Phase 2 clinical trial for the treatment of the skin defect epidermolysis bullosa.tissue. |
We have a fourth product candidate, RGN-457, in preclinical development. RGN-457 is an inhaled formulation of Tß4 targeting cystic fibrosis and other pulmonary diseases.
In addition to our fourWe are continuing strategic partnership discussions with biotechnology and pharmaceutical product candidates, we are also pursuingcompanies regarding the commercialfurther clinical development of peptide fragments and derivativesall of Tß4 for cosmeceutical use. We believe the biological activities of these fragments may be useful, for example, in developing novel cosmeceutical products for the anti-aging market.
our product candidates.
During 2009, we completed a Phase 1 clinical trial evaluating the safety of RGN-352 in 60 healthy subjects. Based on the results of this Phase 1 trial and subject to available funding,extensive preclinical efficacy data published in peer-reviewed journals, we intend to initiateinitiated a Phase 2 clinical trial in the second half of 2010 to evaluate RGN-352’s ability to salvage and regenerate damaged cardiac tissue and improve cardiac function after an acute myocardial infarction, or AMI, commonly known as a heart attack. We
Additionally, recent preclinical research published in the scientific journalsNeuroscience and theJournal of Neurosurgeryindicate that RGN-352 may prove useful for patients with multiple sclerosis, or MS, as well as stroke and traumatic brain injury. In these studies, the administration of Tß4 resulted in regeneration of neuronal tissue and improvement of neurological function. Based on this preclinical research, we intend to usesupport a portion of the proceeds of this offering in support of this Phase 2 clinical trial, although we will not be able to complete the trial without additional capital. In May 2010, we were awarded a $3 million grant from the National Heart, Lung and Blood Institute, one of the institutes of the NIH, to support the further development of RGN-352. We also intend to supply RGN-352 and may provide other assistance, depending on our available financial resources, in support of aproposed Phase 1/2 clinical trial proposed to be conducted at a major U.S. medical center under a physician-sponsored investigational new drug application, or IND, in order to evaluate the therapeutic potential of this product candidateRGN-352 in patients with multiple sclerosis.
MS. We are continuingplanning to supportsupply RGN-352 and provide clinical and regulatory guidance for the development of RGN-259 in ophthalmic indications under compassionate use INDs.trial.
We are also planning to supportsupporting a physician-sponsored clinical trial in patients with dry eye secondary to graft versus host disease, or GvHD, in order to gain further insight intoevaluate RGN-259’s ability to repair and regenerate damaged ophthalmic tissues. Our support includes manufacturing and supplying RGN-259 for the trial and providing regulatory and clinical guidance. We are also collaboratingcontinuing to collaborate with the U.S. military to evaluate the potential of RGN-259 to prevent or reduce eye damage caused by chemical warfare agents.
We are currently conducting a Phase 2 clinical trial evaluating the use of RGN-137 forin the treatment of patients with epidermolysis bullosa, or EB, which is a genetic defect that results in fragile skin and other epithelial tissues that can blister at the slightest trauma or friction, creating a wound that at times does not heal or heals poorly. A portion of this trial was funded by a grant from the U.S. Food and Drug Administration, or FDA. Despite the small patient population with EB, we continue to enroll patients in this Phase 2 trial and expect to complete the trial in late 2010 or early 2011. Once we complete our Phase 2 EB trial, we will analyze the data in conjunction with our two other completed Phase 2 trials of RGN-137, along with the preclinical data indicating Tß4’s ability to reduce scarring, at which time we will further evaluate our strategy for the clinical development of RGN-137. Additionally, we intend to discuss with the FDA the possibility of converting the Phase 2 trial to a pivotal trial for marketing approval, should the data from the Phase 2 trial warrant. We believe that there is precedent for this process, particularly in cases of product candidates under development for ultra-orphan indications, where patient accrual is limited but the clinical data confirms both safety and efficacy.
2928
In addition to our four pharmaceutical product candidates, we are also pursuing the commercial development of peptide fragments and derivatives of Tß4 for potential cosmeceutical use. These fragments are amino acid sequences, and variations thereof, within the Tß4 molecule that have demonstrated activity in severalin vitropreclinical research studies that we have sponsored. We believe the biological activities of these fragments may be useful, for example, in developing novel cosmeceutical products for the anti-aging market. Our strategy is to enter into a collaboration with other companiesanother company to develop cosmeceutical formulations based on these peptides.
As of the date of this prospectus, we believe we have sufficient liquidity and capital resources to fund our operations, including our ongoing clinical trials and other research initiatives, into the third quartersecond half of 2010,2011, without consideringgiving effect to any proceeds from this offeringpotential sales of our common stock under the Purchase Agreement or any other sources of funding. With the proceeds of this offering,capital. During 2011 we believe that we will be able to initiate and conduct at least a portion of our contemplated Phase 2 AMI trial of RGN-352 as well as to complete our ongoing Phase 2 trial of RGN-137 in EB patients and also expect to be able to conduct a portion of our Phase 2 AMI trial for RGN-352 and to support portions of the compassionate use studies ofphysician-sponsored Phase 2 GvHD trial for RGN-259 and the proposed Phase 1/2 trial of RGN-352 infor multiple sclerosis patients. However, we will need substantial additional funds beyond the proceeds of this offering in order to initiate and complete further clinical trials beyond those currently contemplated and to continue to fund our operations.
We incurred net losses of $1.1 million, $6.5 million and $10.6 million for the three months ended March 31, 2010 and the years ended December 31, 2009 and 2008, respectively. As of March 31, 2010, we had an accumulated deficit of $85.6 million. During 2009, we issued shares of common stock and warrants to purchase additional shares of our common stock to Sigma-Tau for gross proceeds of $1.6 million. In October 2009, we also issued shares of common stock and warrants to purchase additional shares of our common stock to new institutional investors for gross proceeds of approximately $3.7 million. From April to September 2009, we also reduced our ongoing monthly cash outflows through salary reductions and reductions in director fees in exchange for the issuance of stock options to our non-employee directors and certain of our executives and employees, which reduced our cash outflows by approximately $300,000 during this period. We restored salaries and directors fees to their prior levels in October 2009 and have continued our research efforts through the date of this prospectus. We intend to maintain tight cost controls and continue to operate under a closely monitored budget approved by the Board of Directors until sufficient funding is obtained to enable expanded research activities.
Financial Operations Overview
Historically, we received only immaterial amounts of revenue from non-refundable government grants. As described elsewhere in this prospectus, we were recently awarded a grant from the NIH to support the further clinical development of RGN-352. Our receipt of the full award will be subject to a number of terms and conditions, and we may never receive future grants. We have never generated product revenues, and we do not expect to generate product revenues until the FDA approves one of our product candidates, if ever, and we begin marketing it. Subject to the availability of financing, we expect to invest increasingly significant amounts in the furtherance of our current clinical programs and may add additional preclinicalnonclinical studies and new clinical trials as we explore the potential of our current product candidates in other indications and explore new formulations of Tß4-based product candidates. As we expand our clinical development initiatives, we expect to incur substantial and increasing losses. Accordingly, we will need to generate significant product revenues in order to ultimately achieve and then maintain profitability. Also, we expect that we will need to raise substantial additional capital in addition to the proceeds of this offering in order to meet product development requirements. We cannot assure investors that such capital will be available when needed, on acceptable terms, or at all.
Most of our expenditures to date have been for research and development, or R&D, activities and general and administrative, or G&A, activities. R&D costs include all of the wholly-allocable costs associated with our various clinical programs passed through to us by our outsourced vendors. Those costs include manufacturing Tß4 and peptide fragments, formulation of Tß4 into our product candidates, stability studies for both Tß4, and the various formulations, preclinical toxicology, safety and pharmacokinetic studies, clinical trial management, medical oversight, laboratory evaluations, statistical data analysis, regulatory compliance, quality assurance
30
and other related activities. R&D includes cash and non-cash compensation, employee benefits, travel and other miscellaneous costs of our internal R&D personnel, seven persons in total, who are wholly dedicated either on a full or part-time basis to R&D efforts. R&D also includes a proration of our common infrastructure costs for office space and communications. We expense our R&D costs as they are incurred.
R&D expenditures are subject to the risks and uncertainties associated with clinical trials and the FDA review and approval process. As a result, these expenses could exceed our expectations, possibly materially. We are uncertain as to what we will incur in future research and development costs for our clinical studies, as these amounts are subject to the outcome of current studies, management’s continuing assessment of the economics of each individual research and development project and the internal competition for project funding. As described below under “Sources of Liquidity,” in May 2010 we were awarded a grant from the National Institutes of Health, or NIH, to support the development of RGN-352. Subject to our compliance with the terms and conditions of the grant, we are eligible to receive up to $3.0 million over a three-year period in cost reimbursements related to the purposes set forth in the grant.
G&A costs include outside professional fees for legal, business development, audit and accounting services, including the costs to maintain our intellectual property portfolio.services. G&A also includes cash and non-cash compensation, employee benefits, travel and other miscellaneous costs of our internal G&A personnel, three in total, who are wholly dedicated to G&A efforts. G&A also includes a proration of our common infrastructure costs for office space, and communications.
Our G&A expenses also include costs to maintain our intellectual property portfolio. We have expanded our patent prosecution activities and have been reviewing our pending patent applications in the United States, Europe and other countries with the advice of outside legal counsel. In some cases, we have filed patent applications for non-critical strategic purposes intended to prevent others from filing similar patent claims. We continue to closely monitor our patent applications to determine if they will continue to provide strategic benefits. In cases where we believe the benefit has been realized or it becomes unnecessary due to the issuance of other patents, or for other reasons that will not affect the strength of our intellectual property portfolio, we will abandon these patent applications in order to reduce our costs of prosecution.
29
Critical Accounting Policies
We prepare our financial statements in conformity with accounting principles generally accepted in the United States. Such accounting principles require that our management make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Our actual results could differ materially from those estimates. The items in our financial statements that have required us to make significant estimates and judgments are as follows:
Share-Based Payment
We account for share-based compensation based on the estimated grant date fair value of the award using the Black-Scholes option-pricing model. The estimated grant date fair value is recognized over the requisite service period.
Determining the appropriate fair value model and calculating the fair value of share-based payment awards require the input of highly subjective assumptions, including the expected life of the share-based payment awards and stock price volatility. Since our historical data is limited, the expected life was determined in accordance with SEC Staff Accounting Bulletin No. 107 guidance for “plain vanilla” options. Since our historical trading volume is relatively low, we estimated the expected volatility based on monthly closing prices for a period consistent with the expected life of the option.
The assumptions used in calculating the fair value of share-based payment awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future. In addition, we are required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. If our actual forfeiture rate is materially different from our estimate, the stock-based compensation expense could be significantly different from what we have recorded in the current period. See Note 2 to our financial statements included in this prospectus for a further discussion on stock-based compensation and the relative ranges of our historical underlying assumptions.
Costs of Preclinical Studies and Clinical Trials
We accrue estimated costs for preclinical studies and clinical trials conducted by contract research organizations and participating hospitals. These costs are a significant component of research and development expenses. We accrue costs for preclinical studies and clinical trials performed by contract research organizations based on estimates of work performed under the contracts. Costs of setting up hospital sites for participation in trials are accrued immediately. Hospital costs related to patient enrollment are accrued as patients are entered in the trial.
31
Recent Accounting Pronouncements
In FebruaryApril 2010, the Financial Accounting Standards Board or FASB, issued Accounting Standards Update, (ASU 2010 — 09)or ASU, No. 2010-17, “Revenue Recognition—Milestone Method,” which provides guidance on defining a milestone and determining when it may be appropriate to address potential practice issues associated with FASB ASC 855 (formerly SFAS 165), “Subsequent Events.”apply the milestone method of revenue recognition for research or development transactions. Research or development arrangements frequently include payment provisions whereby all or a portion of the consideration is contingent upon milestone events such as successful completion of phases in a study or achieving a specific result from the research or development efforts. The amendments in this ASU provide guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. The ASU was effective upon issuance and eliminated the requirement for entities that file or furnish financial statements with the SEC to disclose the date through which subsequent events have been evaluated in originally issued and reissued financial statements. Other entities would continue to be required to disclose the date through which subsequent events have been evaluated; however, disclosures about the date would be required only in financial statements revised because of an error correction or retrospective application of U.S. GAAP. Our adoption of this standard changed our presentation of subsequent events when preparing our financial statements.
In September 2009, the FASB ratified ASU2009-13 (formerlyEITF 08-1), “Revenue Recognition” (ASC 605): Multiple-Deliverable Revenue Arrangements, the final consensus reached by the Emerging Issues Task Force that revised the authoritative guidance for revenue arrangements with multiple deliverables. The guidance addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how the arrangement consideration should be allocated among the separate units of accounting. The guidance will beis effective for our fiscal yearyears and interim periods within those years beginning January 1, 2011on or after June 15, 2010, with early adoption permitted. The guidance may be applied retrospectively or prospectively for new or materially modified arrangements. We currently do not have any multiple-deliverable revenue arrangements, accordingly, the adoption of the guidance will not have an impact on our financial statements.
In August 2009, the FASB issued ASUNo. 2009-05, “Fair Value Measurements and Disclosures (ASC 820) — Measuring Liabilities at Fair Value” (ASU2009-05). ASU2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset or the quoted prices for similar liabilities or similar liabilities when traded as assets. The guidance provided is effective for the first reporting period (including interim periods) beginning after issuance. Our adoption of ASU2009-05 did No. 2010-17 is not impact our financial position or results of operations.
In June 2009, the FASB issued ASC 105 (formerly SFAS 168), “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (ASC 105). ASC 105 is now the source of authoritative U.S. GAAP recognized by the FASBexpected to be applied by nongovernment entities. It also modifies the GAAP hierarchy to include only two levels of GAAP: authoritative and non-authoritative. ASC 105 is effective for financial statements issued for interim and annual periods ending after September 15, 2009. The adoption of this standard in 2009 changed how we reference various elements of U.S. GAAP when preparing our financial statement disclosures, but did not have ana material impact on our financial position, or results of operations.
operations or cash flows.
OtherIn July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act was signed into law. This legislation includes an exemption for companies with less than $75 million in market capitalization from the requirement set forth in Section 404(b) of the Sarbanes-Oxley Act of 2002 to include an external auditor’s report on the effectiveness of a registrant’s internal control over financial reporting. As a result of the new pronouncements issued butlegislation, our independent registered public accounting firm will not effective until after March 31, 2010 are not expectedbe required to have a significant effect onissue an attestation report with respect to our internal control over financial position or resultsreporting. However, we will continue to be subject to the requirement of operations.
Section 404 of the Sarbanes-Oxley Act of 2002 for our management to make an annual assessment of the effectiveness of our internal control over financial reporting.
Results of Operations
Comparison of the threenine months ended March 31,September 30, 2010 and 2009
Revenue. We recognized approximately $54,000 in revenue for the nine months ended September 30, 2010 from the NIH
30
grant awarded in May 2010. The revenue recognized was based on the costs incurred during the period related to this grant. There were no revenue-generating grants or other sources of revenue during 2009.
Research and Development Expense.R&D Expenses. For the threenine months ended March 31,September 30, 2010, our R&D expenses decreased by approximately $1.2$1.3 million, or 72%41%, to $470,000,approximately $1.8 million, from approximately $1.7$3.1 million for the same period in 2009. The decrease was primarily the result of reduced clinical activity in 2010 as compared to 2009. During the threenine months ended March 31,September 30, 2009, we concluded our Phase 2 clinical trials evaluating RGN-137 in patients with pressure ulcers and venous stasis ulcers, as well as the clinical portion of our Phase 1 trial evaluating the safety of RGN-352 in healthy subjects. In January 2009, weWe also terminated the clinical portion of our Phase 2 clinical trial evaluating the safety and efficacy of RGN-259 to treat diabetic patients whose corneal epithelium had been scraped during vitrectomy surgery.
Offsetting these cost reductions we initiated several research activities, including drug formulation and other preparatory work for our Phase 2 clinical trial with AMI patients, our Phase 2 clinical trial with GvHD patients and two pre-clinical dry eye studies.
General and Administrative Expense.G&A Expenses.For the threenine months ended March 31,September 30, 2010, our G&A expenses decreasedincreased by $181,000,approximately $200,000, or 21%9%, to approximately $678,000,$2.4 million from approximately $859,000$2.2 million for the same period
32
in 2009. The decrease was largely the result of lower legal, accounting and business development expenses during the three months ended March 31, 2010. In addition, during the second quarter of 2009 we changed our assumed forfeiture rate for stock-based awards, which had the effect of reducing non-cash stock-based compensation expense by $85,000 during the three months ended March 31, 2010 as compared to the same period in 2009.
This net change included increases of approximately $310,000 in support of our intellectual property portfolio, approximately $55,000 primarily due to the engagement of an outside investor relations firm and other investor relations activities, approximately $10,000 in additional compensation costs, and approximately $8,000 in additional insurance premiums. These increases were offset by a reduction in legal and other outside consulting costs of approximately $183,000.
Comparison of years ended December 31, 2009 and 2008
Revenues.Revenue. For the year ended December 31, 2009, we did not recognize any grant revenue aswas $0 compared to approximately $168,000 for the year ended December 31, 2008. Our2008 as our grant from the FDA’s Office of Orphan Products DevelopmentNIH for the trial of RGN-137 trial forto treat the treatment oforphan indication EB was exhausted during the year ended December 31, 2008, and weexhausted. We do not expect to receive any additional grant funding for this trial.
Research and Development Expense.R&D Expenses. For the year ended December 31, 2009, our R&D expenditures decreased by approximately $3.4 million, or 48%, to approximately $3.7 million, from approximately $7.1 million in 2008. Our outsourced R&D costs, which are costs paid directly to contract research organizations and outside consultants, decreased by approximately $2.9 million, or 58%, to approximately $2.1 million, from approximately $5.0 million. This net decrease is directly related to the conclusion of several clinical trials in late 2008 and early 2009.
For RGN-352, we completed the majority of work associated with a Phase 1 safety trial in 2008, including the initiation and completion of a Phase 1A portion followed by the initiation of the Phase 1B portion. During 2009, only a relatively minor portion of clinical activity on Phase 1B occurred for the remaining subjects in the study, along with that phase’s data evaluation and wrap up. Consequently, our R&D expenditures for RGN-352 decreased by approximately $1.2 million, or 65%, to approximately $0.6 million in 2009, from approximately $1.8 million in 2008.
For RGN-259, during 2008, we were actively enrolling our Phase 2 trial to treat diabetic patients whose corneal epithelium was scraped during vitrectomy surgery. In January 2009, we completed enrollment of the first cohort of our Phase 2 diabetic vitrectomy study and terminated the trial. Consequently, our R&D expenditures for RGN-259 decreased by approximately $0.1 million, or 15%, to approximately $0.8 million in 2009, from approximately $0.9 million in 2008.
Throughout 2008 we were actively enrolling our Phase 2 trials of RGN-137 to treat patients with pressure ulcers as well as EB. Having completed enrollment of our Phase 2 pressure ulcer trial at the end of 2008, we incurred relatively less cost in early 2009 to evaluate the trial’s data and report the information, while our Phase 2 EB trial continued enrollment throughout both periods. Consequently, our R&D expenditures for RGN-137 decreased by approximately $1.3 million, or 89%, to approximately $0.2 million, from approximately $1.5 million in 2008.
Regarding RGN-352, we completed the majority of work associated with a Phase 1 safety trial in 2008 including the initiation and completion of a Phase 1A portion with 40 healthy volunteers followed by the initiation and treatment of approximately 30 healthy volunteers in Phase 1B. During 2009, only a relatively minor portion of clinical activity on Phase 1B occurred for the remaining 10 healthy volunteers, along with that phase’s data evaluation and wrap up. Consequently, our R&D expenditures for RGN-352 decreased by approximately $1.2 million, or 65%, to approximately $0.6 million, from approximately $1.8 million in 2008.
Regarding RGN-259, during 2008 we were actively enrolling our Phase 2 trial to treat diabetic patients whose corneal epithelium was scraped during vitrectomy surgery. In January 2009 we completed enrollment of the first cohort of our Phase 2 diabetic vitrectomy study and terminated the trial. Consequently, our R&D expenditures for RGN-259 decreased by approximately $0.1 million, or 15%, to approximately $0.8 million, from approximately $0.9 million in 2008.
Some of our outsourced R&D costs are for various miscellaneous development efforts or are for certain services that span several formulations or trials. These include certain stability, pharmacokinetic, and medical monitoring services. Given the overall decrease in clinical activity between years, these costs decreased by approximately $0.3 million, or 35%, to approximately $0.5 million, in 2009, from approximately $0.8 million in 2008.
Our internal R&D costs decreased by approximately $0.5 million, or 25%, to approximately $1.6 million, in 2009, from approximately $2.1 million in 2008. As described elsewhere in this prospectus, wemillion. We implemented a salary reduction program for six months of 2009. Additionally, we reduced our R&D headcount by one person during the year and some of our R&D personnel only worked part-timepart time during a portion of 2009. Finally, as described in Note 7 to our financial statements included in this prospectus, weWe also increased our forfeiture assumption for stock options, based on historical experience, which reduced the employee-related non-cash stock-based compensation expense associated with the grant of stock options. In combination, these variances yielded a decrease in
31
our cost of employment for our R&D personnel of approximately $0.4 million, or 23%, to approximately $1.4 million, in 2009, from approximately $1.8 million in 2008. Our other cost-cuttingcost cutting measures as well as a reduction in travel associated with less clinical activity resulted inyielded a decrease in
33
our other internal R&D costs of approximately $0.1 million, or 35%, to approximately $0.2 million, in 2009, from approximately $0.3 million in 2008.
General and Administrative Expense.G&A Expenses. For the year ended December 31, 2009, our G&A expenses decreased by approximately $1.0 million, or 27%, to approximately $2.8 million, from approximately $3.8 million in 2008. The combination of our 2009 salary reduction program and reductions in stock-based compensation expense resulting from changes in forfeiture assumptions yielded a decrease in our G&A personnel expenses of approximately $0.5 million, or 32%, to approximately $0.9 million, in 2009, from approximately $1.4 million in 2008. We also reduced our outside accounting, legal and business development personnel costs by $0.5 million, or 28%, to approximately $1.5 million, in 2009, from approximately $2.0 million in 2008. Our other G&A costs for facilities, investor relations, insurance, and travel remained consistent between years at approximately $0.4 million.
Interest Income.Income. For the year ended December 31, 2009, our interest income decreased by $137,000, or 92%, to approximately $12,000, from approximately $150,000 in 2008. The decrease was due to lower average interest-bearing cash balances during 2009.
Liquidity and Capital Resources
Overview
We have not commercialized any of our product candidates to date and have incurred significant losses since inception. We have primarily financed our operations through the issuance of common stock and common stock warrants in private and public financings, although as discussed below we have recently been awarded a government grant and intend to apply for additional federal cash grants and tax credits.will continue to pursue other governmental funding sources. The report of our independent registered public accounting firm regarding our financial statements for the year ended December 31, 2009 contains an explanatory paragraph regarding our ability to continue as a going concern based upon our history of net losses and dependence on future financing in order to meet our planned operating activities.
We incurred net losses of $6.5 million and $1.1 million for the year ended December 31, 2009 and $4.1 million for the threenine months ended March 31, 2010, respectively.September 30, 2010. We had cash and cash equivalents totaling $3.2$5.0 million and $4.4 million and $5.7 million at March 31,September 30, 2010 and December 31, 2009, and 2008, respectively. The decreaseincrease during the threenine months ended March 31,September 30, 2010 was largelyresulted from the resultsale of shares of our common stock and warrants to purchase shares of our common stock, partially offset by our net loss during thisthe period. The $1.3 million decrease during 2009 was the result of $6.2 million used in operating activities, offset by $4.9 million in cash raised through the private placement of common stock and warrants. As of the dateSeptember 30, 2010, we had an accumulated deficit of this prospectus, we have approximately $2.7 million of cash and cash equivalents.$88.6 million.
Based on our current operations, we believe our existing cash resources along with the expected net proceeds of this offering, will be adequate to fund our operations into the second quarterhalf of 2011.
2011, without considering any potential sales to Lincoln Park under the Purchase Agreement or any other sources of capital. Accordingly, we will continue to have a need for financing, which we may not be able to complete either on favorable terms or at all. If we raise additional funds by selling shares of our common stock or securities convertible into our common stock, such as in this offering,
Cash Flows for the ownership interest of our existing stockholders may be significantly diluted. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferencesNine Months Ended September 30, 2010 and privileges senior to our common stock and may involve significant fees, interest expense, restrictive covenants or the granting of security interests in our assets. We are also in the process of exploring other alternatives, including corporate collaborations and licensing arrangements, and the sale of certain of our intellectual property rights. There are substantial challenges and risks that will make it difficult to successfully implement any of these opportunities.
Cash Flows2009
Net Cash Used in Operating Activities. Net Our net cash used in operating activities was approximately $1.2$3.9 million and $1.8$5.1 million for the threenine months ended March 31,September 30, 2010 and 2009, respectively. In both periods, the net cash used in operating activities was primarily the result of our net losses during the periods. Included in these net losses were non-cash expenses related to employee stock compensation and depreciation
34
of $135,000approximately $369,000 and $271,000$620,000 for the threenine months ended March 31,September 30, 2010 and 2009, respectively. Also included in the net loss for the threenine months ended March 31,September 30, 2010 was a $141,000 non-cash gain upon the settlement of accrued liabilities. Finally, changes in working capital resulted in net cash inflows of approximately $4,000$15,000 during the threenine months ended March 31,September 30, 2010, as opposed to net cash inflowsoutflows of $492,000$525,000 during the threenine months ended MarchSeptember 30, 2009. During the nine months ended September 30, 2010, we spent approximately $24,000 for the purchase of furniture and equipment, which was our only investing activity during the period, and there were no investing activities during the same period of 2009. During the nine months ended September 30, 2010, we raised net cash proceeds of approximately $4.5 million from the sale of units in a public offering. During the same period of 2009, we raised approximately $574,000 in net proceeds from a private placement of our common stock and warrants.
Cash Flows for the Years Ended December 31, 2009.
2009 and 2008
NetOur net cash used in operating activities was approximately $6.2 million and $10.6 million for the years ended December 31, 2009 and 2008, respectively. While our reported net loss for the year ended December 31, 2009 was approximately $6.5 million, it included approximately $0.8 million in non-cash expenses, primarily non-cash share-based compensation, which
32
was offset by approximately $0.5 million of cash used to retire current liabilities as compared to the liabilities reported as of December 31, 2008. Our net loss in 2008 of $10.6 million approximated the net cash used in operating activities in the same period as the non-cash share based compensation expenses of approximately $1.1 million were fully offset by a similar reduction in liabilities as compared to those reported as of December 31, 2007.
Net Cash Provided by Investing Activities. Net Our net cash provided by investing activities was approximately $0 and approximately $4.6 million for the yearyears ended December 31, 2009 and 2008, and there were no investing activities during 2009. During the three months ended March 31, 2010, we spent approximately $18,000 for the purchase of furniture and equipment, which was our only investing activity during the period. There were no investing activities during the three months ended March 31, 2009.respectively. In early 2008 we sold all of our short-term, highly-liquid, investment-grade financial instruments that had more than a90-day maturity from the date of purchase and invested the proceeds in cash equivalents.
Net Cash Provided by Financing Activities. There were no financing activities during either of the three months ended March 31, 2010 or 2009. Netcash-equivalents. Our net cash provided by financing activities totaled approximately $4.9 million and $7.9 million for the years ended December 31, 2009 and 2008, respectively. In both periods, these net proceeds result from the issuance of common stock and warrants to purchase common stock.
Future Funding Requirements
The expenditures that will be necessary to execute our business plan are subject to numerous uncertainties that may adversely affect our liquidity and capital resources. As described elsewhere in this prospectus, during 2009 we completed two Phase 2 clinical trials, closed one additional Phase 2 clinical trial and completed a Phase 1 clinical trial. Currently, we are actively enrolling patients in onea Phase 2 trial for RGN-137 in EB patients while we are supporting small compassionate use studies of RGN-259. Subject to available funding, we intend to commenceand a Phase 2 clinical trial of RGN-352 for AMI patients in the second half of 2010 and support a Phase 1/2 clinical trial of RGN-352 for MS patients, as well aspatients. We are also supporting a physician-sponsored Phase 2 clinical trial of RGN-259 in patients with dry eye secondary to graft versus host disease.GvHD, and a planned Phase 1/2 clinical trial of RGN-352 for MS patients. We currently do not have sufficient capital resources to continue clinical development beyondinto the third quartersecond half of 2010,2011, without giving effect any sales to this offering. As described elsewhere in this prospectus, we were recently awarded a grant fromLincoln Park under the NIH, whichPurchase Agreement, but will allow usrequire substantial capital resources to continue the development of RGN-352 without exhausting our current capital resources.
operations beyond that time.
In addition, theThe length of time required for clinical trials varies substantially according to the type, complexity, novelty and intended use of a product candidate. Some of the factors that could impact our liquidity and capital needs include, but are not limited to:
| | |
| | • | | the progress of our clinical trials; |
| |
| | • | | the progress of our research activities; |
| |
| | • | | the number and scope of our research programs; |
| |
| | • | | the progress of our preclinical development activities; |
| |
| | • | | the costs involved in preparing, filing, prosecuting, maintaining, enforcing and defending patent and other intellectual property claims; |
35
| | |
| | • | | the costs related to development and manufacture of preclinical, clinical and validation lots for regulatory purposes and commercialization of drug supply associated with our product candidates; |
| |
| | • | | our ability to enter into corporate collaborations and the terms and success of these collaborations; |
| |
| | • | | the costs and timing of regulatory approvals; and |
| |
| | • | | the costs of establishing manufacturing, sales and distribution capabilities. |
In addition, the duration and the cost of clinical trials may vary significantly over the life of a project as a result of differences arising during the clinical trial protocol, including, among others, the following:
| | |
| | • | | the number of patients that ultimately participate in the trial; |
| |
| | • | | the duration of patientfollow-up that seems appropriate in view of the results; |
| |
| | • | | the number of clinical sites included in the trials; and |
| |
| | • | | the length of time required to enroll suitable patient subjects. |
Also, we test our potential product candidates in numerous preclinical studies to identify indications for which they may be product candidates. We may conduct multiple clinical trials to cover a variety of indications for each product candidate. As we obtain results from trials, we may elect to discontinue clinical trials for certain product candidates or for certain indications in order to focus our resources on more promising product candidates or indications.
Our proprietary product candidates also have not yet achieved FDA regulatory approval, which is required before we can
33
market them as therapeutic products. In order to proceed to subsequent clinical trial stages and to ultimately achieve regulatory approval, the FDA must conclude that our clinical data establish safety and efficacy. Historically, the results from preclinical studies and early clinical trials have often not been predictive of results obtained in later clinical trials. A number of new drugs and biologics have shown promising results in clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals.
In addition to our obligations under clinical trials, we are committed under an office space lease through January 2013 that requires average base rental payments of approximately $7,300 per month.
Sources of Liquidity
We have not commercialized any of our product candidates to date and have primarily financed our operations through the issuance of common stock and common stock warrants in private and public financings. Sigma-Tau has historically provided significant equity capital to us. In 2009, Sigma-Tau provided approximatelyus, including through private placements of $950,000 in January 2011 and $1.6 million in gross proceeds outOctober 2009. In January 2011, we also raised $500,000 from a registered direct offering of our securities to Lincoln Park. During the first half of 2010, we raised approximately $4.5 million from an underwritten public offering of our securities, and during 2009, we raised approximately $3.7 million from a registered direct offering of our securities.
In January 2011, we also entered into the Purchase Agreement with Lincoln Park. We have filed a registration statement, of which this prospectus is a part, with regard to the resale by Lincoln Park of the common stock issuable under the Purchase Agreement. We do not have the right to commence any sales of our shares to Lincoln Park until the SEC has declared the registration statement effective. Thereafter, over approximately $5.3 million30 months, we have the right but not the obligation to direct Lincoln Park to purchase up to 200,000 shares of common stock every third business day at a purchase price calculated by reference to the prevailing market price of our common stock without any fixed discount, subject to the floor price of $0.15 per share. We may sell up to $11,000,000 worth of shares under the Purchase Agreement. There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Lincoln Park. Lincoln Park has no right to require any sales by us, but is obligated to make purchases from us as we direct in total grossaccordance with the Purchase Agreement. We can also accelerate the amount of common stock to be purchased under certain circumstances. There are no limitations on use of proceeds, raised duringfinancial or business covenants, restrictions on future funding, rights of first refusal, participation rights, penalties or liquidated damages in the year, with new investors providing the remaining $3.7 million. Sigma-Tau provided all of the $8.0 million in gross proceeds raised during 2008.
Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
As described below under “Business — Material Agreements,” weWe are also party to a license agreement with a subsidiary of Sigma-Tau that provides the opportunity for us to receive milestone payments upon specified events and royalty payments uponin connection with commercial sales of Tß4 in Europe. However, we have not received any milestone payments to date, and there can be no assurance that we will be able to attain such milestones and generate any such payments under the agreement.
As a result of recent government initiatives, we have engaged a consulting firm to assist us in identifying sources of Federal government funding. We are also aggressively pursuing federally-sourcedgovernment funding and in May 2010 were recently awarded a $3 million grant from the NIH, as describedNIH’s National Heart, Lung and Blood Institute to support the requisite nonclinical development of RGN-352 for patients who have suffered a heart attack. These nonclinical activities are being conducted in this prospectus. Recentlyparallel with our ongoing Phase 2 clinical trial of RGN-352. Subject to our compliance with the terms and conditions of the grant, we are eligible to receive up to $3.0 million over a three-year period in cost reimbursements for our associated costs incurred for the purposes set forth in the grant. Revenue from the grant will be recorded during the same periods when we incur eligible expenses.
The Patient Protection and Affordable Care Act enacted healthcare reform legislation also provides forin 2010 included a qualifying therapeutic discovery project credit, or Therapeutic Credit, as annew incentive for small biotechnology companies like us. Theours, known as the Qualifying Therapeutic Credit will allowDiscovery Project grant program. Under this program, small businesses were able to apply for a federal grant in an amount equal to 50% of their eligible investment in qualifying therapeutic discovery projects for 2009 and 2010. Qualifying therapeutic discovery projects includeincluded those designed to treat or
36
prevent diseases or conditions by conducting preclinicalpre-clinical or clinical activities for the purpose of securing FDA approval of a product. We believe that our entire Tß4 development program may qualify for the Therapeutic Credit, and we currently estimate that 50%submitted three applications, covering each of our qualifying costs for 2009clinical-stage product candidates, and in October 2010 could approximate up to $4.0 million. We expect that the Therapeutic Credit program will be highly competitive, and there can be no assurance that we will be able to secure any fundingwere awarded an aggregate of $733,438 under this program. We are also collaborating with
Additionally, the FederalU.S. government inis evaluating RGN-259, our sterile eye drop product candidate,formulation, in animals exposed to caustic agents, and wechemical warfare agents. We believe that our other formulations may also be of interest in healing damaged tissues for indications that result from battlefield or homeland security situations.
As such, we have engaged a consulting firm to help us identify other sources of funding from U.S. government agencies. There can be no assurance, however, that we will be able to secure additional funds from the U.S. government or other governmental sources.
Other potential sources of outside capital include entering into strategic business relationships, publicadditional issuances of
34
equity securities or private sales of shares of our capital stock, or debt financing or other similar financial instruments. While we sold common stock and warrants to purchase common stock to Sigma-Tau and new investors in the fourth quarter of 2009, we do not have any committed sources of outside capital at this time. Consequently, there can be no assurance that we will be able to obtain additional capital in sufficient amounts, on acceptable terms, or at all.
If we raise additional capital through such a strategic business relationship, we may have to give up valuable rights to our intellectual property. If we raise funds by selling additional shares of our common stock or securities convertible into our common stock, such as in this offering, the ownership interest of our existing stockholders may be significantly diluted. In addition, if additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and may involve significant fees, interest expense, restrictive covenants and the granting of security interests in our assets.
Our failure to successfully address ongoing liquidity requirements would have a materially negative impact on our business, including the possibility of surrendering our rights to some technologies or product opportunities, delaying our clinical trials, or ceasing operations.
There can be no assurance that we will be able to obtain additional capital in sufficient amounts, on acceptable terms, or at all.
Off Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as such term is defined in Item 303(a)(4) ofRegulation S-K.
Quantitative and Qualitative Disclosures about Market Risk
Our cash equivalents, which are generally comprised of Federally-insured bank deposits and short-term U.S. government debt securities, are subject to default, changes in credit rating and changes in market value. These investments are also subject to interest rate risk and will decrease in value if market interest rates increase. As of March 31,September 30, 2010, these cash equivalents were $3.2 million. Due to the short-term nature of these investments, if market interest rates differed by 10% from their levels as of March 31,September 30, 2010, the change in fair value of our financial instruments would not have been material.
3735
BUSINESS
General
General
We are a biopharmaceutical company focused on the development of a novel therapeutic peptide, Tß4, for tissue and organ protection, repair, and regeneration. We have formulated Tß4 into three distinct product candidates currently in clinical development:
| | |
| | • | | RGN-352, an injectable product candidate to treat cardiovascular diseases, central nervous system diseases, and other medical indications that may be treated by systemic administration, for which we intend to initiateinitiated a Phase 2 clinical trial in the second half of 2010; |
| | |
| | • | | RGN-259, a topical eye drop for ophthalmic indications that was evaluated infor which we are supporting a small Phase 2physician-sponsored clinical trial and is currently being supported in compassionate use studies;patients with dry eye secondary to graft versus host disease, or GvHD; and |
| | |
| | • | | RGN-137, a topically applied gel for chronic dermal wounds and reduction of scar tissue that is currently in a Phase 2 clinical trial for the treatment of the skin defect epidermolysis bullosa, or EB. |
We have a fourth product candidate, RGN-457, in preclinical development. RGN-457 is an inhaled formulation of Tß4 targeting cystic fibrosis and other pulmonary diseases.
In addition to our four pharmaceutical product candidates, we are also pursuing the commercial development of peptide fragments and derivatives of Tß4 for cosmeceutical use. Cosmeceuticals are cosmetic products with biologically active ingredients. We believe the biological activities of these fragments may be useful, for example, in developing novel cosmeceutical products for the anti-aging market.
Overview of Tß4
Tß4 is a naturally occurring43-amino acid peptide that was originally isolated from bovine thymus glands. It plays a vital role in cell structure and motility and in the protection, regeneration, remodeling and healing of tissues.
Although it is recognized that wound healing is a complex process, most companies working to develop new drugs in this area have focused primarily on the development of growth factors to stimulate healing and have, to date, failed to demonstrate dramatic improvements in the healing process. Unlike growth factors, numerous preclinical animal studies, published by independent researchers, have identified several important biological activities involving Tß4 that we believe make it potentially useful as a wound healing, repair and tissue regenerating agent. These activities include:
| | |
| | • | | Progenitor (Stem) Cell Differentiation.Research published in the journalNaturein November 2006 featured the discovery that Tß4 is the key signaling molecule that triggers adult epicardial progenitor cells, or EPCs, to differentiate into coronary blood vessels. EPCs are partially differentiated stem cells that can further differentiate into specific cell types when needed. Confirmatory research published in 2009 in theJournal of Molecular and Cellular Cardiologyconcluded that Tß4 is responsible for the initiation of the embryonic coronary developmental program and EPC differentiation in adult mice. These publications confirm that Tß4’s interaction with EPCs is necessary for the maintenance of a healthy adult animal heart, as well as normal fetal animal heart development. |
The 2006Naturepublication also concluded that Tß4’s interaction with EPCs resulted in the formation of cardiomyocytes that repaired damaged myocardium, or heart tissue, in mice after an induced AMI.acute myocardial infarction, or AMI, commonly known as a heart attack. Research published in the journalCirculationin April 2008 showed Tß4’s cardioprotective effects in a pig ischemic-reperfusion model. This pig model is accepted as an important model upon which to base human clinical research, as pigs are larger mammals, the anatomy of the pig heart is similar to the human heart, and vascular response processes are completed five to six times faster in pigs than in humans, so that long-term results can be obtained in a relatively short period of time. This research also identified Tß4’s interaction with EPCs as the underlying basis of cardioprotection through the differentiation of EPCs into cardiomyocytes, yielding statistically significant cardiac functional recovery results when compared to the administration of placebo.
38
Similar research in the area of brain tissue was published in the journalNeurosciencein September 2009. This publication concluded that Tß4 triggered the differentiation of oligodendrocyte progenitor cells to form myelin-producing oligodendrocytes, which led to the remyelination of axons in the brain of mice with experimental autoimmune
36
encephalomyelitis, or EAE. This mouse model is an accepted small animal model for the study of multiple sclerosis.
| | |
| | • | | Actin Regulation.Tß4 regulates actin, which comprises up to 10% of the protein of non-muscle cells in the body and plays a central role in cell structure and in the movement of cells. Research studies have indicated that Tß4 stimulates the migration of human keratinocytes, or skin cells, human endothelial cells, and progenitor cells. Endothelial cells are the major cell type responsible for the formation of new blood vessels, a process known as angiogenesis. Certain of these studies conducted at the NIH were the first to suggest the role of Tß4 in wound healing. The data from these studies encouraged us to license the rights to Tß4 from the NIH in 2001 and to launch an initial clinical development program that targeted the use Tß4 for chronic dermal wounds. |
| | |
| | • | | Reduction of Inflammation.Uncontrolled inflammation is the underlying basis of many pathologies and injuries. Research has shown that Tß4 is a potent anti-inflammatory agent in skin cells and in corneal epithelial cells in the eye. Tß4 has also been shown to decrease the levels of inflammatory mediators and to significantly reduce the influx of inflammatory cells in the reperfused heart of animals. More recent preclinical research suggests that Tß4 blocks activation of the NFκNFкB pathway, which is involved in DNA activation of inflammatory mediators, thereby modulating inflammation in the body. This anti-inflammatory activity may explain, in part, the mechanism by which Tß4 appeared to improve functional outcome in the mouse multiple sclerosis model described above, as well as promoting repair in the heart and skin. Identifying a factor such as Tß4 that blocks activation of NFκNFкB suggests that Tß4 could have additional important therapeutic applications for inflammation-related diseases, such as cancer, osteoarthritis, rheumatic diseases, autoimmune diseases, inflammatory pulmonary disease and pancreatitis. |
| | |
| | • | | Collagen and Laminin-5 Stimulation.Tß4 has a number of additional biological activities shown to reduce inflammation, stimulate the formation of collagen, and up-regulate the expression of laminin-5, a subepithelial basement membrane protein. Both collagen and laminin-5 are central to healthy tissue and the prevention of disease. |
| |
| | • | | Apoptosis.Tß4 has been shown to prevent apoptosis, or programmed cell death, in two animal models and in two tissue types. In the rodent model, corneal apoptosis, or loss of corneal epithelial cells leading to corneal epithelial thinning, was prevented through topical administration of Tß4, and in the heart muscle of ischemic animal models, such as in mice and pigs, cell death was prevented by the systemic administration of Tß4. |
In combination, we believe that these various biological activities work together to play a vital role in the healing and repair of injured or damaged tissue and suggest that Tß4 is an essential component of the tissue protection and regeneration process that may lead to many potential medical applications. All of our product candidates are based on Tß4, manufactured as a synthetic copy of the naturally occurring peptide and formulated for various routes of administration and applications.
Our Product Candidates
RGN-352
Our product candidate RGN-352 is an injectable formulation of Tß4 for systemic administration. We have initially targeted RGN-352 for patients who have suffered an acute myocardial infarction, or AMI, commonly known as a heart attack.AMI. Preclinical research published in the scientific journalNaturehas indicated that Tß4 can guide specific types of stem cells from the outer layer of the heart to generate new myocardial blood vessels and tissue at injured sites.
Clinical Development.In 2009, we completed a Phase 1 clinical trial evaluating the safety, tolerability and the pharmacokinetics of the intravenous administration of RGN-352. We also designed this trial to explore the use of RGN-352 in other indications in which acute administration of Tß4 may be warranted. We conducted the Phase
39
1 trial in two consecutive parts, referred to as Phase 1A and Phase 1B, both of which were double-blind, placebo-controlled, and dose-escalating over four doses. We enrolled a total of 60 healthy subjects in the trial, consisting of 40 subjects in each phase, of which 20 subjects participated in both phases. In Phase 1A, we evaluated a single administration of RGN-352, and in Phase 1B we evaluated once daily administration for 14 consecutive days.
In September 2008, we reported the results of Phase 1A. The single intravenous injection of RGN-352 was well-tolerated at all four dose levels. In December 2009, we reported the results of Phase 1B. A daily intravenous injection of RGN-352 for 14 consecutive days was also observed to be well-tolerated at all four dose levels. There were no reported dose-limiting adverse events in either Phase 1A or Phase 1B.
37
Future Plans.Based on the results of the Phase 1 trial, and subject to available funding, we intend to initiateinitiated a Phase 2 clinical trial in the second half of 2010 to evaluate RGN-352 in patients who have suffered an AMI. We are currently designinghave designed this trial to observe RGN-352’s cardioprotective effects and its ability to salvage and regenerate damaged cardiac tissue and improve cardiac function after a heart attack. We intend to use a portion of the proceeds of this offering to initiate and conduct at least a portion of this Phase 2 clinical trial, although we will not be able to complete the trial without additional capital. In May 2010, we were awarded a $3 million grant from the NIH’s Blood, Heart and Lung Institute to support the further development of RGN-352. We expect that the Phase 2 trial design will allow for an interim review of patient data from an initial group of evaluated patients, and we currently expect that the proceeds of this offering will be sufficient to reach this point in the trial.patients. Depending on our capital resources, we may conduct the trial while continuing strategic partnership discussions with biotechnology and pharmaceutical companies for the further clinical development of RGN-352.
Recent preclinical research published in the scientific journaljournalsNeuroscienceandJournal of Neurosurgeryalso indicates that RGN-352 may prove useful for patients with multiple sclerosis, or MS, as well as stroke and stroke.traumatic brain injury. In research involving mice,these studies, the administration of Tß4 resulted in statistically significantregeneration of neuronal tissue and improvement inof neurological functional recovery.function. Based on this research, we intend to support a proposed Phase 1/2 clinical trial to be conducted at a major U.S. medical center under a physician-sponsored IND in order to evaluate the therapeutic potential of RGN-352 in patients with MS. We are planning to supply RGN-352 and provide clinical and regulatory guidance for the trial. We believe that we can support this trial from our existing capital resources, although we intend to use a portion of the proceeds from this offering to provide additional support.
RGN-259
Our product candidate RGN-259 is a sterile topical eye drop formulation of Tß4 for ophthalmic indications.
Clinical Development.Emerging human clinical data from two compassionate use studies have demonstrated the ability of RGN-259 to repair and regenerate corneal tissue. AIn the first compassionate study, a middle-aged diabetic woman had undergone corneal epithelial debridement during surgery. The resultant corneal defect had not healed for 23 days prior to treatment with RGN-259. Typically, these wounds heal within a few days after surgery. Following treatment with RGN-259, the patient experienced reduced ocular irritation and the wound fully healed within 11 days.
In the second compassionate use study, a corneal specialist has received approval from the FDA to treat up to ten patients with neurotrophic keratitis, or NK, with RGN-259. NK is a rare degenerative corneal disease induced by a nerve impairment. The most common causes of NK include the herpes zoster virus. To date,treated nine patients have been treated in an open label protocol for periods of 28 or 49 days. The NK patients being evaluated have non-healing defects that have lasted at least six weeks and up to greater than ten years.
Patients in the study were divided into two groups. The first group consisted of six patients with a single non-healing measurable eye ulcer.ulcer resulting from neurotrophic keratitis, or NK, a rare degenerative corneal disease commonly caused by the herpes zoster virus and induced by a nerve impairment resulting in painful corneal lesions that can lead to blindness. The NK patients evaluated had defects that had not healed for at least six weeks and in some cases for several years. The second group consisted of three patients with diffuse punctate erosions, a corneal defectdefects that appearsappear as numerous small pinhole-sized lesions.
All nine patients were treated with RGN-259 for periods of up to 49 days. The six NK patients with single lesionsnon-healing ulcers showed clinically significant improvement during the treatment with RGN-259 and thefollow-up period, with four of the six patients healing completely. The completely healed ulcers remained healed during thefollow-up period, and those that had demonstrated significant improvement continued to improve after completion of treatment with RGN-259. The three patients with diffuse punctate erosions demonstrated no significant improvement, although they did report reduced ocular irritation.
In all nine patients treated, RGN-259 has been well-tolerated, and there have been no drug-related adverse events. A tenth patient with a single lesion has recently been enrolled in the study, and we expect to report final results of the compassionate use study later in 2010. Based on the preliminary findings, we believe that RGN-259 may provide a novel approach to the treatment of patients with non-healing neurotrophic corneal ulcers.
40
We had previously initiated a Phase 2 clinical trial to evaluate RGN-259 in diabetic patients undergoing corneal epithelial debridement, or removal of the outer transparent tissue layer of the front part of the eye, during vitrectomy surgery. In this randomized, double-blind, placebo-controlled, dose-response trial conducted at several U.S. clinical sites, we originally intended to evaluate the safety, tolerability, and healing efficacy of three different concentrations of RGN-259 compared to placebo, applied as eye drops, four times daily for up to 14 consecutive days.
While we did not view this particular ophthalmic indication as a significant commercial opportunity, we believed that it represented a“proof-of-concept” “proof-of-concept” clinical model to evaluate the safety and efficacy ofRGN-259 for the treatment of corneal indications. We intended to obtain initial data that could be used to address other ophthalmic indications with larger market potential. Patient enrollment in the trial was significantly slower than anticipated due to newer surgical techniques and equipment that reduced the need for corneal epithelial debridement required for the trial. We closed the trial in January 2009, after completion of the first low-dose cohort of 12 patients, in order to focus our research on other commercial opportunities. The encouraging compassionate use data described above, which we received during the course of the trial, also influenced our decision to close the trial earlier than originally intended.
In the 12 patients evaluated in the trial, there were no reported drug-related adverse events associated with RGN-259. We observed increased corneal epithelial thickening and reduced cell flare and flare inflammation in the low-dose patients treated with RGN-259 as compared to patients receiving placebo, which we believe to be indicative of corneal re-epithelialization and healing. None of the results from the trial are considered to be statistically significant. We expect
38
In all patients treated to report final results later in 2010 following the submission of the clinical study reportdate, RGN-259 has been well-tolerated, and there have been no drug-related adverse events. Based on these preliminary findings, we believe that RGN-259 may provide a novel approach to the FDA.treatment of patients with corneal defects.
Future Plans.We are continuing to support the evaluation of RGN-259 in NK patients under a compassionate use IND and expect to report final patient data from these trials in the third quarter of 2010. We are also planning to supportsupporting a physician-sponsored clinical trial in patients with dry eye secondary to GvHD in order to gain further insight into RGN-259’s ability to repair and regenerate ophthalmic tissues. Our support includes manufacturing and supplying RGN-259 for the trial and providing regulatory and clinical guidance. We are continuing to collaborate with the U.S. military to evaluate the potential of RGN-259 to prevent or reduce eye damage caused by chemical warfare agents. We are also engaged in discussions with potential partners regarding the clinical development of this product candidate. Once enough human data is generated, we intend to seek strategic partnerships with one or more ophthalmic specialty companies.
RGN-137
Our product candidate RGN-137 is a topical gel formulation of Tß4 intended to promote dermal wound healing and tissue regeneration. Preclinical research has demonstrated that Tß4 can accelerate dermal regeneration after a wound, while more recent research indicates that Tß4 can reduce scarring after injury in the skin and heart. Based on research conducted at the NIH, we initiated a series of Phase 2 clinical trials to evaluate RGN-137 for the treatment of three different types of skin wounds.
Clinical Development — Epidermolysis Bullosa.In 2005, we began enrolling patients in a Phase 2 trial designed to assess the safety and effectiveness of RGN-137 for the treatment of patients with EB. EB is a genetic defect that results in fragile skin and other epidermal tissues that can blister at the slightest trauma or friction, creating a wound that at times does not heal or heals poorly. In this randomized, double-blind, placebo-controlled, dose-response trial, nine U.S. clinical sites are enrolling a total of 36 patients to evaluate the safety, tolerability, and wound healing effectiveness of three different concentrations of RGN-137 compared to placebo. RGN-137 is being applied topically to the skin, once daily for up to 56 consecutive days.
EB has been designated as an “orphan” indication by the FDA. We estimate the prevalence of EB in the United States to be between 20,000 and 30,000 patients, with a subpopulation of approximately 5,000 patients in the group eligible for inclusion in our Phase 2 clinical trial. We received a grant of $681,000 from the FDA’s Office of Orphan Products Development to partially fund this trial. While enrollment has been difficult due to the small addressable patient population, we currently expect to complete this trial by late 2010 or earlyin 2011.
41
Clinical Development — Pressure Ulcers.In late 2005, we began enrolling patients in a Phase 2 clinical trial designed to assess the safety and effectiveness of RGN-137 for the treatment of patients with chronic pressure ulcers, commonly known as bedsores. In this randomized, double-blind, placebo-controlled, dose-response trial, 15 clinical sites in the United States enrolled a total of 72 patients to evaluate the safety, tolerability, and wound healing effectiveness of three different concentrations of RGN-137 compared to placebo. RGN-137 was applied topically to the ulcers, once daily for up to 84 consecutive days. Patients in the trial were between 19 and 85 years old and had at least one stable Stage III or IV pressure ulcer with a surface area between 5 and 70 cm2. Stage III and IV pressure ulcers are full thickness wounds that penetrate through the skin and muscle, sometimes completely to the bone.
In January 2009, we reported final data from this trial. RGN-137 was well-tolerated at all three dose levels studied, with no dose-limiting adverse events, which achieved the primary objective of the study. As for efficacy, all Tß4 doses performed similarly compared to placebo, with no statistically significant efficacy results. Patients treated with the middle dose showed a 17% rate of wound healing, which was the highest rate among the three active doses evaluated. The improvement in ulcer healing in this middle dose group following nine weeks of treatment was equal to the improvement in patients treated with placebo after 12 weeks of treatment.
Clinical Development — Venous Stasis Ulcers.In 2006, we began enrolling patients in a Phase 2 trial designed to assess the safety and effectiveness of RGN-137 for the treatment of patients with venous stasis ulcers. In this randomized, double-blind dose-response trial, eight clinical sites in Italy and Poland enrolled a total of 73 patients to evaluate the safety, tolerability, and wound healing effectiveness of three different concentrations of RGN-137 compared to placebo. RGN-137 was applied topically to the ulcers, once daily for up to 84 consecutive days. Patients in the trial were between 18 to 79 years old and had at least one venous stasis ulcer with a surface area between 3 and 30 cm2. We were the sponsor of the trial, and it was conducted and funded by Sigma-Tau.
In March 2009, we reported final data from the trial. RGN-137 was well-tolerated at all three dose levels, with no dose-limiting adverse events, which achieved the primary objective of the study. Thirty-three percent (33%) of the patients who
39
received the middle dose of RGN-137 had their ulcers heal completely after the 12 weeks of treatment, compared to 24% of patients receiving the placebo, 16% of the patients receiving the lowest drug dose and 17% of patients receiving the highest drug dose. Of the patients receiving the middle dose whose ulcers healed completely, the median time to complete healing decreased by approximately 45%, as compared to a 37% decrease in the time to healing for patients in the placebo-treated group. None of the differences observed between RGN-137 and placebo were statistically significant.
Future Plans.Once we complete our Phase 2 EB trial, and evaluate the results, we will analyze the data in conjunction with our two other completed Phase 2 trials of RGN-137, along with preclinical data indicating Tß4’s ability to reduce scarring, at which time we will further evaluate its potential valueour strategy for acceleration of dermal wound healing and whether to continuethe clinical development of this product candidate.RGN-137. Additionally, we intend to discuss with the FDA the possibility of converting the Phase 2 trial to a pivotal trial for marketing approval, should the data from the Phase 2 trial warrant. We believe that preclinical research indicating the ability of Tß4 to reduce scarring in rats, complimented by reduced scarring in the hearts of mice and pigs after an induced heart attack, may also be relevant in suggesting that Tß4 may be effective in reducing dermal scar tissue. Subject to available funding, we plan to continue research and development of RGN-137there is precedent for this potential application.
process, particularly in cases of product candidates under development for ultra-orphan indications, where patient accrual is limited but the clinical data confirms both safety and efficacy.
RGN-457
Our preclinical product candidate RGN-457 is based on Tß4 formulated as an inhaled therapeutic agent. We have completed a substantial amount of preclinical work necessary for an IND application, and we are currently seeking a strategic partner to assist in the development of RGN-457 for the treatment of cystic fibrosis, or CF. CF is a life-threatening, hereditary disease that impairs the patient’s ability to breathe due to the accumulation of mucus secretions in the airways of the lungs. The predicted median age of survival for patients with cystic fibrosis is 37 years. There are estimated to be approximately 30,000 CF patients in the United States and approximately 40,000 CF patients in Europe. It is therefore considered to be an orphan disease in both territories. While we believe RGN-457 may prove beneficial in the treatment of CF, we remain focused primarily on development of our other product candidates while we continue strategic partnership discussions with respect to RGN-457.
42
Peptide Fragments for Cosmeceutical Applications
We are also seeking to identify and evaluate Tß4 peptide fragments and derivatives that may be useful as novel components in cosmeceutical and consumer products. We have identified several amino acid sequences, and variations thereof, within the Tß4 molecule that have demonstratedin vitro activity in preclinical research studies that we have sponsored, and we have filed a number of patent applications related to this research. We believe the biological activities of these fragments may be useful, for example, in developing novel cosmeceutical products for the anti-aging market. To date, research has suggested that these fragments suppress inflammation, accelerate the deposition of certain types of collagen, promote the production of elastin, and inhibit programmed cell death, among other activities. Our development and commercialization strategy is to identify suitable commercial partners to license these novel fragments for various cosmeceutical applications. We are currently holding discussions with several multinational cosmetics and consumer products companies for potential collaborations to further develop and commercialize these fragments.
Our Strategy
We seek to maximize the value of our product candidates by advancing their clinical development and then identifying suitable partners for further development, regulatory approval, and marketing. We intend to engage in strategic partnerships with companies with clinical development and commercialization strengths in desired pharmaceutical therapeutic fields. We are actively seeking partners with suitable infrastructure, expertise and a long-term initiative in our medical fields of interest.
For example, in 2004, we entered into a strategic partnership with Defiante Farmaceutica S.A., or Defiante, a subsidiary of Sigma-Tau, for development and marketing of RGN-137 and RGN-352 for specified indications in Europe and other contiguous countries. Sigma-Tau also funded and co-managed our Phase 2 clinical trial of RGN-137 in Europe for the treatment of venous stasis ulcers.
Manufacturing
We use a contract manufacturer to produce bulk Tß4 by an established and proven manufacturing process known as solid-phase peptide synthesis, and we are in the early stages of qualifying backup manufacturers. While we do not currently have long-term supply agreements in place, we intend to establish a long-term supply arrangement with at least one manufacturer once practicable. No assurance can be given, however, that such agreements will be negotiated on favorable terms, or at all. Contractors are selected on the basis of their supply capability, ability to produce a drug substance in accordance with current Good Manufacturing Practice requirements of the FDA, and ability to meet our established specifications.
40
We also use a number of outside contract manufacturers to formulate bulk Tß4 into our product candidates. All of these formulations may require modifications along with additional studies as we move through our clinical development programs.
Competition
We are engaged in a business that is highly competitive, and our target medical indications are ones with significant unmet needs. Moreover, the cosmetic and cosmeceutical industries are rapidly developing new products based on new scientific research. Consequently, there are many enterprises, both domestic and foreign, pursuing therapies and products that could compete with ours. Most of these entities have financial and human resources that are substantially greater than ours, specifically with regard to the conduct of clinical research and development activities, clinical testing and in obtaining the regulatory approvals necessary to market pharmaceutical products. Brief descriptions of some of these competitive products follow:
| | |
| | • | | RGN-352.Currently, there are no approved pharmaceutical products for regenerating cardiac tissue following a heart attack, nor are there approved pharmaceutical products for the remyelination of axons for patients with multiple sclerosis. However, many pharmaceutical companies and research organizations are developing products and technologies that are intended to prevent cardiac damage, improve cardiac function, and regenerate cardiac muscle after a heart attack. There are also companies |
43
| | |
| | developing products that remyelinate neurons and provide functional improvement for multiple sclerosis patients. If we were to successfully develop RGN-352 for other cardiovascular indications, such as acute or chronic heart failure, such a product would have to compete with other drugs or therapies currently marketed by large pharmaceutical companies for similar indications, as would products for the treatment of multiple sclerosis. |
| | |
| | • | | RGN-259.Most specialty ophthalmic companies have a number of products on the market that could compete with RGN-259. There are numerous antibiotics to treat eye infections that cause corneal wounds and many eye lubrication products to help eye healing and function, many of which are sold without prescriptions. Companies also market steroids to treat certain severe conditions within our area of interest. Allergan, Inc. has marketed Restasistm TM, a relatively new approved eye drop to treat dry eye. Dry eye is a condition related to a number of diseases and one that we believe could benefit from the use of RGN-259. |
| |
| | • | | RGN-137.Johnson & Johnson has marketed RegranextmTM for patients with diabetic foot ulcers. Companies such as Novartis are developing and marketing artificial skins, which would compete with RGN-137 in the treatment of dermal wound healing. There are other companies developing new pharmaceutical products for wound healing. Products and therapies such as antibiotics, honey-based ointments and low frequency cavitational ultrasound are also used to treat certain types of dermal wounds. Moreover, dermal wound healing is a large and highly fragmented marketplace that includes numerous therapeutic products and medical devices for treating acute and chronic dermal wounds. |
| |
| | • | | RGN-457.CF is a genetic defect for which there is no cure. There are mucolytic agents and antibiotic drugs on the market, such as Genentech’s pulmozyme and Novartis’ TOBI®, an inhaled version of tobramycin, that relieve the symptoms posed by CF and could potentially compete with RGN-457. |
| |
| | • | | Cosmeceuticals.The cosmetics industry is highly competitive and dependent on effective marketing and distribution. There are multiple products currently launched by major international cosmetic enterprises that claim the same or similar benefits that may be claimed with our product candidates. |
Government Regulation
In the United States, the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing, manufacturing, labeling, storing, recordkeeping, distribution, advertising and promotion of our product candidates. Regulation by governmental authorities in the United States and foreign countries will be a significant factor in the manufacturing and marketing of our product candidates and in our ongoing research and product development activities. Any product candidate we develop will require regulatory approval by governmental agencies prior to commercialization. In particular, human therapeutic products are subject to rigorous preclinical studies, clinical trials and other approval procedures by the FDA and similar health authorities in foreign countries. The process of obtaining these approvals and subsequent compliance with appropriate federal and state statutes and regulations requires the expenditure of substantial resources.
41
Preclinical studies must ordinarily be conducted to evaluate an investigational new drug’s potential safety by toxicology studies and potential efficacy by pharmacology studies. The results of these studies, among other things, are submitted to the FDA as part of an Investigational New Drug Application, or IND, which must be reviewed by the FDA before clinical trials can begin. Typically, clinical evaluation involves a three-stage process. Phase 1 clinical trials are conducted with a small number of healthy volunteers to determine the safety profile and the pattern of drug absorption, distribution, metabolism and excretion, and to assess the drug’s effect on the patient. Phase 2, or therapeutic exploratory, trials are conducted with somewhat larger groups of patients, who are selected by relatively narrow criteria yielding a more homogenous population that is afflicted with the target disease, in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. Phase 2 trials should allow for the determination of the dose to be used in Phase 3 clinical trials. Phase 3, or therapeutic confirmatory, large scale, multi-center, comparative trials are conducted with patients afflicted with a target disease in order to provide enough data for the statistical proof of safety and efficacy required by the FDA and other regulatory authorities. The primary objective of Phase 3 clinical trials
44
is to show that the drug confers therapeutic benefit that outweighs any safety risks. All clinical trials must be registered with a central public database, such as www.clinicaltrials.gov, and once completed, results of the clinical trials must be entered in the database.
The results of all of these preclinical studies and clinical trials, along with detailed information on manufacturing, are submitted to the FDA in the form of a New Drug Application, or NDA, for approval to commence commercial sales. The FDA’s review of an NDA requires the payment of a user fee currently in excess of $1 million, which may be waived for the first NDA submitted by a qualifying small business. In responding to an NDA, the FDA may refuse to file the application if the FDA determines that the application does not satisfy its regulatory approval criteria, request additional information or grant marketing approval. Therefore, even if we complete Phase 3 clinical trials for our product candidates and submit an NDA to the FDA, there can be no assurance that the FDA will grant marketing approval, or if granted, that it will be granted on a timely basis. If the FDA does approve a product candidate, it may require, among other things, post-marketing testing, including potentially expensive Phase 4 trials, which monitor the safety of the drug. In addition, the FDA may in some circumstances impose risk evaluation and mitigation strategies that may be difficult and expensive to administer. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market.
Among the conditions for NDA approval is the requirement that the applicable clinical, pharmacovigilance, quality control and manufacturing procedures conform on an ongoing basis with current Good Clinical Practices, Good Laboratory Practices, current Good Manufacturing Practices, and computer information system validation standards. During the review of an NDA, the FDA will perform a pre-licensing inspection of select clinical sites, manufacturing facilities and the related quality control records to determine the applicant’s compliance with these requirements. To assure compliance, applicants must continue to expend time, money and effort in the area of training, production and quality control. After approval of any product, manufacturers are subject to periodic inspections by the FDA. If a company fails to comply with FDA regulatory requirements, FDA may pursue a wide range of remedial actions, including seizure of products, corrective actions, warning letters and fines.
In June 2004, we received orphan drug designation from the FDA for Tß4 for the treatment of EB. The FDA may designate a product or products as having orphan drug status to treat a disease or condition that affects less than 200,000 individuals in the United States, or, if patients of a disease number more than 200,000, the sponsor can establish that it does not realistically anticipate its product sales will be sufficient to recover its costs. If a product candidate is designated as an orphan drug, then the sponsor may receive incentives to undertake the development and marketing of the product, including grants for clinical trials, as well as a waiver of the user fees for submission of an NDA application. For example, as described above, we received a grant of approximately $681,000 in the aggregate for our ongoing Phase 2 clinical trial of RGN-137 to treat patients with EB.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to marketing exclusivity for a period of seven years in the United States. There may be multiple designations of orphan drug status for a given drug and for different indications. Orphan drug designation does not guarantee that a product candidate will be approved by the FDA for marketing for the designation, and even if a sponsor of a product candidate for an indication for use with an orphan drug designation is the first to obtain FDA approval of an NDA for that designation and obtains marketing exclusivity, another sponsor’s application for the same drug product may be approved by the FDA during the period of exclusivity if the FDA concludes that the competing product is clinically superior. In this instance, the orphan designation and marketing exclusivity originally granted would be lost in favor of the clinically superior product.
Intellectual Property
42
We have applied for or hold over 60 worldwide patents onand patent applications covering peptide compositions, uses and formulations related to dermal and ophthalmic indications and other organ and tissue repair activities, as well as for cosmetic and consumer product applications. In 2001, we entered into a license agreement with the NIH under
45
which we received an exclusive worldwide license from the NIH for all claims within the scope of the NIH’s patent application, and any issued patents, covering the use of Tß4 as a tissue repair and regeneration factor. During 2007, a patent was issued in Europe and the U.S. related to the original NIH patent application, which patent expires in July 2019. Corresponding patents have been granted in Hong Kong, Australia and China and certain other territories. The issued European patent was opposed by a third party at the European Patent Office and in December 2009, we argued the case before the Opposition Division of the European Patent Office in Munich, Germany and prevailed.prevailed with certain amendments to the claims. In exchange for the exclusive license, we agreed to make certain minimum royalty and milestone payments to the NIH. Through December 31, 2009,2010, we have complied with all minimum royalty requirements, and no milestone payments have been required under the agreement.
We hold a U.S. patent relating to the use of Tß4 for treatment of alopecia, an autoimmune skin disease that results in hair loss, which expires in 2017, with corresponding patents in Europe and Singapore that expire in 2018. In 2006, we were issued a patent in China for the use of Tß4 to treat EB, which expires in 2022.
Under a research agreement with The George Washington University, or GWU, we funded Tß4 research at GWU and received a sole and exclusive worldwide license to any resulting patents. While we no longer fund any research under this agreement, we remain obligated to pay GWU a royalty of 4% of the net sales, if any, of specified products covered by patents issued in connection with the agreement. Pursuant to the research agreement, we have exclusive rights to patent applications filed in the United States and in Europe disclosing the use of Tß4 for the treatment of septic shock and associated syndromes, including Adult Respiratory Distress Syndrome. Two U.S. patents covered by this agreement have been issued, which expire in 2013 and 2014.
We have also filed numerous additional U.S. and international patent applications covering various compositions, uses, formulations and other components of Tß4, as well as for novel peptides resulting from our research efforts.efforts, the latest of which were filed during 2010. There can be no assurance that these, or any other future patent applications under which we have rights, will result in the issuance of a patent or that any patent issued will not be subject to challenge or opposition. In the case of a claim of patent infringement by or against us, there can be no assurance that we will be able to afford the expense of any litigation that may be necessary to enforce our proprietary rights.
Material Agreements
National Institutes of Health
We have entered into a license agreement with NIH under which we are obligated to pay an annual minimum royalty of $25,000. Additionally, we are obligated to pay the NIH a percentage of sales of qualifying product candidates, if any. There have been no such sales to date.
Defiante/Sigma-Tau
We have exclusively licensed certain internal and external wound healing European rights to Tß4 to Defiante Farmaceutica, S.A., or Defiante, a Portuguese company that is a wholly owned subsidiary of Sigma-Tau.Defiante. These licensed rights to Tß4 include its use to treat indications that are the subject of all of our current dermal clinical trials as well as the treatment of heart attacks. The license excludes the use of Tß4 in ophthalmic indications and other indications that are disease-based and not the result of a wound. Under the agreement, Sigma-Tau will develop Tß4 for the treatment of internal and external wounds in Europe and certain other contiguous and geographically relevant countries. The license agreement expires on acountry-by-country basis upon the later of the expiration of the last to expire of any granted patent in the territory having at least one valid claim covering the products then on the market, the expiration of any other exclusive or proprietary marketing rights, or January 2016.
Under the license agreement, Sigma-Tau is obligated to pay us a royalty on commercial sales, if any, and we will supply all required Tß4 for development. Upon the completion of a Phase 2 clinical trial for the covered indications that yields positive results in terms of efficacy and safety, Sigma-Tau must either pay us a $5 million milestone payment or initiate and fund a pivotal Phase 3 clinical trial for the applicable product candidate in order to maintain the license. As described elsewhere in this prospectus, in 2009, we completed
46
two Phase 2 clinical trials of RGN-137 for the treatment of pressure ulcers and venous stasis ulcers, which, due to the lack of statistical significance of the reported efficacy results, have not triggered the milestone obligation described above.
The license agreement with Defiante also contains future clinical and regulatory milestones in the licensed territory. If those milestones are attained, certain performance criteria regarding commercial registration and minimum annual royalties
43
will be payable to us in each licensed country. The agreement does not prevent us from sublicensing the technology in countries outside the licensed territory, and has no impact on any U.S. rights.
Development Agreements
We have entered into agreements with outside service providers for the manufacture and development of Tß4, the formulation of Tß4 into our product candidates, the conduct of nonclinical safety, toxicology and efficacy studies in animal models, and the management and execution of clinical trials in humans. Terms of these agreements vary in that they can last from a few months to more than a year in duration. Certain of these agreements require initial up front payments ranging from 25% to 50% of the total estimated cost. For additional information regarding our research and development expenses over the past two years, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Results of Operations” in this prospectus.
Employees
To balance costs and optimize control, we utilize an outsourcing business strategy, whereby our management oversees the outsourced activities for many of our research and development and administrative functions. We currently have nine full-time employees and one part-time employee, and we retain several independent contractors on an as-needed basis. We believe that we have good relations with our employees.
Facilities
Our corporate headquarters are located in Rockville, Maryland where we lease approximately 3,500 square feet of office space with an average base rent of $7,300 per month and a term through January 2013. We believe that our facilities are generally suitable to meet our needs for the foreseeable future, although we will continue to seek alternate or additional space as needed.
Corporate Information
We were incorporated in Delaware in 1982 under the name Alpha 1 Biomedicals, Inc. In 2000, we changed our corporate name to RegeneRx Biopharmaceuticals, Inc. Our principal executive office is located at 15245 Shady Grove Road, Suite 470, Rockville, Maryland 20850. Our telephone number is(301) 208-9191.
Legal Proceedings
We are not currently a party to or engaged in any material legal proceedings. However, we may be subject to various claims and legal actions arising in the ordinary course of business from time to time.
4744
MANAGEMENT
Executive Officers and Directors
The following table sets forth as of the date of this prospectus the name, age and position of each person who serves as an executive officer or director of our company. There are no family relationships among any of our executive officers or directors, with the exception that Mr. Finkelstein is the first cousin of Dr. Goldstein’s wife.
We seek to assemble a board that, as a whole, possesses the appropriate balance of professional and industry knowledge. financial expertise and high-level management experience necessary to oversee and direct our business. To that end, our board intends to maintain membership of directors who complement and strengthen the skills of other members and who also exhibit integrity, collegiality, sound business judgment and other qualities that we view as critical to effective functioning of the board. The brief biographies below include information, as of the date of this prospectus, regarding the specific and particular experience, qualifications, attributes or skills of each director or nominee that led the board to believe that the director should serve on the board.
| | | | | | | |
Name | | Age | | Position |
|
| J.J. Finkelstein | | | 58 | | | President, Chief Executive Officer and Director |
| C. Neil Lyons | | | 53 | | | Chief Financial Officer |
| David R. Crockford | | | 6465 | | | Vice President, Clinical and Regulatory Affairs |
| Allan L. Goldstein, Ph.D. | | | 7273 | | | Director, Chairman of the Board and Chief Scientific Advisor |
Richard J. HindinR. Don Elsey | | | 6757 | | | Director |
| Joseph C. McNay | | | 7677 | | | Director |
| Mauro Bove | | | 5556 | | | Director |
| L. Thompson Bowles, M.D., Ph.D. | | | 7879 | | | Director |
Mr. Finkelsteinhas served as our President and Chief Executive Officer and a member of our Board of Directors since 2002. Mr. Finkelstein also served as our Chief Executive Officer from 1984 to 1989 and as the Vice Chairman of our Board of Directors from 1989 to 1991. Mr. Finkelstein has worked as an executive officer and consultant in the bioscience industry for the past 28 years, including serving from 1989 to 1996 as chief executive officer of Cryomedical Sciences, Inc., a publicly-traded medical device company. Mr. Finkelstein has significant experience in developing early-stage companies. He has been responsible for the regulatory approval and marketing of several medical devices in the U.S. and abroad. Mr. Finkelstein has served on the executive committee of the Board of Directors of the Technology Council of Maryland since 2006, MdBio, Inc. since 1998 and currently chairs the MdBio Foundation, all of which are non-profit entities that support bioscience development and education in the State of Maryland. Mr. Finkelstein received a business degree in finance from the University of Texas. The Board believes that Mr. Finkelstein’s history and long tenure as our Chief Executive Officer positions him to contribute to the Board his extensive knowledge of our company and to provide Board continuity. In addition, the Board believes that his experience at prior companies has provided him with operational and industry expertise, as well as leadership skills that are important to the Board.
Mr. Lyonshas served as our Chief Financial Officer and Treasurer since 2005. With more than 25 years of experience, Mr. Lyons has developed expertise related to operations, finance, SEC compliance, complex transactions, strategy, information systems and corporate governance. From 1979 to 1990, Mr. Lyons practiced with Deloitte, providing assurance and advisory services to several public companies in the Washington, D.C. metro area. Following that, Mr. Lyons served as a senior financial executive with HFS, Inc., a major Department of Defense contractor, from 1990 to 1996, with Bell Atlantic from 1996 to 1998, with SkyBridge LP, an international satellite broadbandstart-up affiliated with Alcatel, from 1998 to 2003, and consulted with area businesses regarding financial management, including the initial implementations of the Sarbanes-Oxley
48
Act from 2003 to 2005. Mr. Lyons is a certified public accountant and received a Bachelor of Science degree
45
in accounting, magna cum laude, from Florida Southern College.
Mr. Crockfordhas served as our Vice President of Clinical and Regulatory Affairs since March 2005 and was a consultant to the Company from 2000 until his appointment as Vice President. He has more than 25 years of experience in the biotechnology and pharmaceutical industries. During his career as a clinical and regulatory affairs professional, Mr. Crockford has established strategic plans, implemented and obtained marketing approval for 18 drug products, including one of the first human growth hormone preparations sold in the U.S., 17 in vitro diagnostic tests, and an intraoperative medical device to detect and treat cancer. Mr. Crockford’s other clinical and regulatory achievements include the cost-effective and timely development of a number of innovative investigational drugs. Mr. Crockford is the author of a number of publications, includingDevelopment of Thymosin ß4 for Treatment of Patients with Ischemic Heart Disease, and is an inventor or co-inventor on approximately two dozen patents related to drug development. Mr. Crockford has a B.A. degree in biology and chemistry from Boston University. He also completed biochemistry and clinical chemistry course studies in Princeton, New Jersey, and seminars in reproductive medicine at medical schools at Wayne State University and UCLA.
Dr. Goldsteinhas served as the Chairman of our Board of Directors and our Chief Scientific Advisor since he founded our company in 1982. Dr. Goldstein has been a Professor of Biochemistry since 1978 and served as Chairman of the Department of Biochemistry and Molecular Biology at the George Washington University School of Medicine and Health Sciences until 2009. Dr. Goldstein is a recognized expert in the field of immunology and protein chemistry, having authored over 430 scientific articles in professional journals. He is also the inventor on over 25 issuedand/or pending patents in biochemistry, immunology, cardiology, cancer and wound healing. Dr. Goldstein discovered several important compounds, including Tα1, which is marketed worldwide, and Tß4, which is the basis for RegeneRx’s clinical program. Dr. Goldstein has served on the Board of Trustees of the Sabin Vaccine Institute since 2000 and on the Board of Directors of the Richard B. and Lynne V. Cheney Cardiovascular Institute since 2006. Dr. Goldstein has also done pioneering work in the area of medical education, developing distance learning programs offered through “Frontiers in Medicine,” a medical education series that Dr. Goldstein developed. The Board believes that Dr. Goldstein’s scientific expertise, industry background and prior experience as our founder all position him to make an effective contribution to the medical and scientific understanding of the Board, which the Board believes to be particularly important as we continue our Tß4 development efforts.
Mr. HindinElseyhas served as a member of our Board of Directors since 2002. Mr. Hindin has been an entrepreneur during his more than 40 year career and is currently the principal stockholder of Chicken Out Rotisserie, Inc., which operates 15 restaurants in three states and the District of Columbia. Mr. Hindin has served since 1987 as a member and since 1989 as the chairman of the board of directors of The Institute for Advanced Studies in Aging & Geriatric Medicine, or IASIA, a non-profit corporation that disseminates medical information to the public as well as providing the pharmaceutical industry with an independent source for testing vaccines and drugs for the elderly. Mr. Hindin’s entrepreneurial background includes several companies and commenced with Britches of Georgetown, Inc., a clothing retailer specializing in the sale of upscale men’s and women’s apparel and accessories which he co-founded. Mr. Hindin has also served as Chairman of the Board of Hinsilblon Laboratories Ltd., a company based in Fort Myers, Florida which sells odor neutralization products and delivery systems, since 1990. Finally, Mr. HindinSeptember 2010. He has served as Presidentsenior vice president, finance and administration of Adworks Inc,Emergent BioSolutions Inc., a Washington D.C.-based advertisingpublicly held biopharmaceutical company, since May 2007, and marketing consulting agency,as its chief financial officer since 1987. During 2009,March 2006 and Treasurer since June 2005. Mr. Hindin filed for personal bankruptcy underElsey previously served as vice president, finance of Emergent BioSolutions from June 2005 to May 2007. He served as the U.S. Bankruptcy Codedirector of finance and administration at IGEN International, Inc., a publicly held biotechnology company, and its successor BioVeris Corporation, from April 2000 to June 2005. Prior to joining IGEN, Mr. Elsey served as director of finance at Applera, a genomics and sequencing company, and in several finance positions at International Business Machines, Inc. He received an M.B.A. in finance and a B.A. in economics from Michigan State University. Mr. Elsey is currently involved in proceedings related to the matter.a certified management accountant. The Board believes that Mr. Hindin’s extensiveElsey’s experience as an entrepreneur will be increasinglychief financial officer of a public company is particularly valuable to the Board as we seekour business in that it positions him to expandcontribute to our Board’s and finance our operations.Audit Committee’s understanding of financial matters.
Mr. McNayhas served as a member of our Board of Directors since 2002. He is currently Chairman, Chief Investment Officer and Managing Principal of Essex Investment Management Company, LLC, positions he has held since 1976 when he founded Essex. He has direct portfolio management responsibilities for a variety of funds and on behalf of private clients. He is also a member of the firm’s Management Board. Prior to founding Essex, Mr. McNay was Executive Vice President and Director of Endowment Management & Research Corp. from 1967. Prior to that, Mr. McNay was Vice President and Senior Portfolio Manager at the
49
Massachusetts Company. Currently he is serving as Trustee of National Public Radio, Trustee of the Dana Farber Cancer Institute, and is a Trustee and member of the Children’s Hospital Investment Committee. He received his A.B. degree from Yale University and his M.B.A. degree in finance from the Wharton School of the University of Pennsylvania. The Board believes that Mr. McNay’s extensive financial experience is valuable to our business and also positions him to contribute to the Audit Committee’s understanding of financial matters.
Mr. Bovehas served as a member of our Board of Directors since 2004 and has more than 25 years of business and management experience within the pharmaceutical industry. Mr. Bove is currently the Head of Corporate & Business Development and serves on the board of Sigma-Tau Finanziaria S.p.A., the holding company of Sigma-Tau Group, a leading international pharmaceutical company, and certain Sigma-Tau affiliates, positions he has held since 1993. Sigma-Tau Finanziaria S.p.A. and its affiliates are collectively our largest stockholder. Mr. Bove has also held a number of senior positions in business, licensing and corporate development within Sigma-Tau Group, which has subsidiaries in most European countries and the United States. Mr. Bove obtained his law degree at the University of Parma, Italy, in 1980. In 1985, he attended the Academy of American and International Laws at the International and Comparative Law Center, Dallas, Texas. The Board believes that Mr. Bove’s extensive business and management experience within the pharmaceutical
46
industry allows him to recognize and advise the Board with respect to recent industry developments.
Dr. Bowleshas served as a member of our Board of Directors since 2006. He retired from his career as a thoracic surgeon in 1988. Dr. Bowles served as Dean of Medicine and Professor of Surgery at The George Washington University, or GWU, School of Medicine and Health Sciences from 1976 to 1988 and as Vice President for Medical Affairs and Executive Dean of the GWU Medical Center from 1988 to 1992. Dr. Bowles previously served as President of the National Board of Medical Examiners, a medical accrediting organization, from 1992 to 2000. He has also been a member of the National Academy of Sciences Institute of Medicine since 1988 and currently serves as a member of several other national medical societies including: The American College of Surgeons, The American Association for Thoracic Surgery, The Society of Thoracic Surgeons, The American College of Chest Physicians, The American Gerontological Society, The Society of Medical Administrators, The College of Physicians of Philadelphia, and The Washington Academy of Surgeons. Dr. Bowles has served on the editorial board of a number of medical journals, including the Journal of Medical Education and continued on as chairman of its newly revised updated version, Academic Medicine. Dr. Bowles has been President of the District of Columbia’s medical licensing board called the Healing Arts Commission(1977-1979), and was a member of the National Library of Medicine’s Board of Regents(1982-1986), chairman(1984-1986), member of the Special Medical Advisory Group of Veterans Administration (now Dept. of Veterans Affairs)1984-1992, chairman1992-1994. Dr. Bowles was also chairman of the National Committee on Foreign Medical Education and Accreditation,1994-1996. Dr. Bowles received his medical degree from Duke University and his Ph.D. in higher education from New York University. The Board believes that Dr. Bowles’ distinguished medical career positions him to bring extensive medical and clinical trial experience to the Board. The Board expects that this experience will permit Dr. Bowles to provide leadership and insight as we translate our laboratory discoveries into human clinical trials and advance our product candidates through clinical development toward commercialization.
Independence of the Board of Directors
As required underUnder NYSE Amex listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the Board. Although our common stock is no longer listed on the NYSE Amex exchange, we have determined the independence of our directors using the NYSE Amex definitions of independence. The Board consults with counsel to ensure that the Board’s determinations are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of the NYSE Amex, as in effect from time to time.
Consistent with these considerations, after review of all relevant identified transactions or relationships between each director, or any of his family members, and us, our senior management and our independent auditors, the Board has affirmatively determined that the following four directors are independent directors within the meaning of the applicable NYSE Amex listing standards: Mr. Hindin,Elsey, Mr. Bove, Mr. McNay and Dr. Bowles. In making this determination, the Board found that none of the these directors had a material or
50
other disqualifying relationship with us. Mr. Finkelstein, our President and Chief Executive Officer, and Dr. Goldstein, our Chief Scientific Advisor, are not independent directors by virtue of their employment with us.
In determining the independence of Mr. Bove, the board of directors took into account the significant ownership of our common stock by Sigma-Tau and its affiliates. The board of directors does not believe that any of the transactions with Sigma-Tau and its affiliates described would interfere with Mr. Bove’s exercise of independent judgment in carrying out his responsibilities as a director of our company.
Information Regarding Committees of the Board of Directors
The Board has two standing committees: an Audit Committee and a Compensation Committee. The Board does not have a separate nominating and corporate governance committee. Rather, the independent members of the full Board perform the functions of a nominating and corporate governance committee.
Below is a description of each committee of the Board. Each of the committees has authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. The Board has determined that each member of each committee meets the applicable NYSE Amex rules and regulations regarding “independence” and that each member is free of any relationship that would impair his individual exercise of independent judgment with regard to our company.
Audit Committee
The Audit Committee of the Board consists of Messrs. Hindin andMr. McNay, and Dr. Bowles and Mr. Elsey, with Mr. HindinMcNay acting as the
47
Chairman of the committee. The Audit Committee meets no less than quarterly with management and our independent registered public accounting firm, both jointly and separately, has sole authority to engage and terminate our independent registered public accounting firm, and reviews our financial reporting process on behalf of the Board. The Audit Committee operates under a formal written charter available on our website at www.regenerx.com.
Each member of the Audit Committee is an independent director in accordance with both Section 121A of the NYSE Amex listing standards andRule 10A-3 of the Exchange Act. Furthermore, the Board has determined that Messrs. HindinMr. McNay and McNayMr. Elsey qualify as “audit committee financial experts” as defined under SEC rules.
The Audit Committee pre-approves all audit and non-audit engagement fees, and terms and services. On an ongoing basis, management communicates specific projects and categories of services for which advance approval of the Audit Committee is required. The Audit Committee reviews these requests and advises management and the independent auditors if the Audit Committee pre-approves the engagement of the independent auditors for such projects and services. On a periodic basis, the independent auditors report to the Audit Committee the actual spending for such projects and services compared to the approved amounts.
Compensation Committee
The Compensation Committee is composed of four directors: Messrs. Hindin,Dr. Bowles, Mr. McNay, andMr. Bove and Mr. Elsey, with Dr. Bowles. All members of our Compensation Committee are independent,Bowles acting as independence is currently defined in Section 803AChairman of the NYSE Amex listing standards.committee. The Compensation Committee has adopted a written charter that is available to stockholders on our website at www.regenerx.com.
The Compensation Committee of the Board acts on behalf of the Board to review, adopt and oversee our compensation strategy, policies, plans and programs, including:
| | |
| | • | | establishment of corporate and individual performance objectives relevant to the compensation of our chief executive officer, other executive officers and Board members; |
| |
| | • | | evaluation of performance in light of these stated objectives; |
51
| | |
| | • | | review and approval of the compensation and other terms of employment or service, including severance andchange-in-control arrangements, of our Chief Executive Officer and the other executive officers; and |
| |
| | • | | administration of our equity compensation plans and other similar plan and programs. |
Nominating and Corporate Governance
The Board does not have a standing nominating and corporate governance committee. Instead, pursuant to Section 804 of the NYSE Amex listing standards, the independent members of the Board, consisting of Messrs. Hindin,Elsey, McNay and Bove and Dr. Bowles, are responsible for performing key nominating and corporate governance activities on behalf of the Board, including identifying, reviewing and evaluating candidates to serve as our directors, reviewing and evaluating incumbent directors, selecting candidates for election to the Board, making recommendations to the Board regarding the membership of the committees of the Board, assessing the performance of management and developing and maintaining a set of corporate governance principles for us. All members of the Board performing the role of a nominating and corporate governance committee are “independent” as defined in Section 803A of the NYSE Amex listing standards.
Director Compensation
The following table set forth certain information for the fiscal year ended December 31, 20092010 with respect to the compensation of our directors. Mr. Finkelstein’s compensation is disclosed in the Summary Compensation Table below, and he does not receive any additional compensation for his service as a director. Dr. Goldstein is an employee of our company and his compensation as an employee is set forth in the table below. He does not receive any additional compensation for his service as a director.
Each non-employee director is eligible to receive an annual cash retainer of $13,905. The chairman of each of our audit committee and compensation committee is eligible to receive a supplemental annual cash retainer of $10,300. Mr. HindinMcNay currently serves as the chairmanChairman of eachthe Audit Committee and Dr. Bowles currently serves as the Chairman of these committees.the Compensation Committee.
Directors also receive $1,288 for each board meeting attended in person and $412 for each Board meeting attended by telephone. Additionally, members of each committee of the board of directors are eligible to receive $515 for each committee meeting attended, whether in person or by telephone.
48
Additionally, non-employee directors receive a nonqualified stock option under our stock optionequity incentive plan to purchase 15,00020,000 shares of common stock upon their re-election as a director at each annual meeting of stockholders. Newly elected or appointed non-employee directors receive a nonqualified stock option under our stock optionequity incentive plan to purchase 35,00040,000 shares of common stock. All options granted to directors under this policy vest over four years, with 25% of the shares underlying the option vesting on the first through fourth anniversaries of the date of grant.
We also reimburse directors for expenses incurred in attending meetings of the board and other events attended on our behalf and at our request.
Of note, our annual rates of director compensation in effect at December 31, 2008 and 2009 remain the same. However, given our limited cash resources during most of 2009, the Board elected to reduce the cash fees payable to the Board for their services by 35% for the period from April 1 to September 30, 2009. Consequently, the following charts of actual compensation may differ from the disclosed annual rates of compensation currently in effect.
52
In return for the 35% director fee reduction, each director received options to purchase shares of our common stock at an exercise price of $0.57 per share. We granted options to purchase an aggregate of 64,157 shares of our common stock pursuant to this arrangement. Effective October 1, 2009, director fees were restored to the levels in effect at December 31, 2008 and, therefore, the options ceased vesting as of September 30, 2009 but remain exercisable to the extent vested as of September 30, 2009 in accordance with the terms of our stock option plan. The number of shares vested and outstanding from these option grants are included within the amounts set forth in Footnote 1 to the table below.
Director Compensation for Fiscal 20092010
| | | | | | | | | | | | | | | | | |
| | | Fees Earned | | | | | | | | | | |
| | | or Paid | | | Option | | | All Other | | | | |
| | | in Cash | | | Awards | | | Compensation | | | Total | |
| Name | | ($) | | | ($)(1) | | | ($) | | | ($) | |
| Allan Goldstein, Ph.D. | | | — | | | | 15,284 | | | | 187,460 | (2) | | | 202,744 | |
| R. Don Elsey | | | 7,726 | | | | 6,403 | | | | — | | | | 14,129 | |
| L. Thompson Bowles M.D., Ph.D. | | | 28,017 | | | | 3,103 | | | | — | | | | 31,120 | |
| Joseph McNay | | | 25,028 | | | | 3,103 | | | | — | | | | 28,131 | |
| Mauro Bove | | | 17,303 | | | | 3,103 | | | | — | | | | 20,406 | |
| Richard Hindin (3) | | | 21,836 | | | | — | | | | — | | | | 21,836 | |
| | | | | | | | | | | | | | | | | |
| | | Fees Earned
| | | | | | |
| | | or Paid
| | Option
| | All Other
| | |
| | | in Cash
| | Awards
| | Compensation
| | Total
|
Name | | ($) | | ($)(1) | | ($) | | ($) |
| |
| Allan Goldstein, Ph.D. | | | — | | | | 71,348 | | | | 162,466 | (2) | | | 233,814 | |
| Richard Hindin | | | 37,877 | | | | 16,039 | | | | — | | | | 53,916 | |
| L. Thompson Bowles M.D., Ph.D. | | | 21,105 | | | | 11,875 | | | | — | | | | 32,980 | |
| Joseph McNay | | | 18,028 | | | | 10,917 | | | | — | | | | 28,945 | |
| Mauro Bove | | | 16,292 | | | | 10,459 | | | | — | | | | 26,751 | |
| | |
| (1) | | These amounts reflect the aggregate full grant date fair values (computed in accordance with FASB ASC Topic 718) of options granted to directors during 2009, a portion of which vested during 2009 as described above.2010. Options held by each Board member as of December 31, 2009,2010, are as follows: |
| | | | | |
|
| Allan Goldstein, Ph.D. | | | 696,942795,442 | |
Richard Hindin | | | 237,749 | |
| R. Don Elsey | | | 40,000 | |
| | | | |
| L. Thompson Bowles M.D., Ph.D. | | | 154,843174,843 | |
| | | | |
| Joseph McNay | | | 228,024248,024 | |
| | | | |
| Mauro Bove | | | 227,155247,155 | |
| | |
| (2) | | In addition to being Chairman of our Board of Directors, Dr. Goldstein also serves as our Chief Scientific Advisor. In this capacity, Dr. Goldstein received a base salary of $153,093$187,460 for 2009, and a discretionary cash bonus of $9,373.2010. Under Dr. Goldstein’s employment agreement, in the event that his employment is terminated by us without “cause,” as defined in his employment agreement, or if he voluntarily terminates his employment within 12 months following a “change in control,” as defined in his employment agreement, then in each case, subject to Dr. Goldstein’s entering into and not revoking a release of claims in a form acceptable to us, Dr. Goldstein will be entitled to receive a lump sum severance payment equal to his annual base salary then in effect, plus any earned bonus as of the date of termination, in each case less applicable taxes and withholdings. Dr. Goldstein is not entitled to receive any continuing health and welfare benefits as part of our severance obligation to him. If Dr. Goldstein’s employment had been terminated for any of the reasons described in this paragraph as of December 31, 2009,2010, he would have been entitled to receive a lump sum payment of $187,460, less taxes and withholdings. Dr. Goldstein is eligible to receive options to purchase common stock under the 2000 Plan.our equity incentive plans. The decision to grant any such options and the terms of such options are within the discretion of our board of directors or the compensation committee. In addition, if Dr. Goldstein’s employment is terminated without “cause,” or if there is a “change in control” event, in each case as defined in either the 2000 Planapplicable benefit plan or in Dr. Goldstein’s employment agreement, then the unvested portion of Dr. Goldstein’s options would accelerate in full. All vested options are exercisable for a period of time following any termination of Dr. Goldstein’s employment as may be set forth in the 2000 Planapplicable benefit plan or in any option agreement between Dr. Goldstein and us. |
|
| (3) | | Mr. Hindin’s term as a director ended in July 2010. |
5349
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table shows, for the fiscal years ended December 31, 20092010 and 2008,2009, compensation awarded to or paid to, or earned by, our chief executive officer and our two other most highly compensated executive officers during 2009 who were serving as executive officers at December 31, 2009.2010. For purposes of this prospectus, we refer to these officers as the named executive officers.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Non-Equity | | | | | | | |
| | | | | | | | | | | | | | | | | | | Incentive | | | | | | | |
| | | | | | | | | | | | | | | Option | | | Plan | | | All Other | | | | |
| | | | | | | Salary(1) | | | Bonus(2) | | | Awards(3) | | | Compensation(4) | | | Compensation(5) | | | Total | |
| Name and Principal Position | | Year | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | | | ($) | |
| J.J. Finkelstein, President and | | | 2010 | | | | 299,520 | | | | — | | | | 19,396 | | | | — | | | | 18,425 | | | | 337,341 | |
| Chief Executive Officer | | | 2009 | | | | 244,608 | | | | 18,720 | | | | 116,198 | | | | — | | | | 13,005 | | | | 392,531 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| C. Neil Lyons, | | | 2010 | | | | 202,537 | | | | 2,000 | | | | 15,284 | | | | — | | | | 6,886 | | | | 226,707 | |
| Chief Financial Officer | | | 2009 | | | | 167,093 | | | | 11,140 | | | | 74,395 | | | | — | | | | 4,999 | | | | 257,627 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| David R. Crockford, | | | 2010 | | | | 210,223 | | | | 2,000 | | | | 15,284 | | | | — | | | | 10,321 | | | | 237,828 | |
| Vice President, Clinical and Regulatory Affairs | | | 2009 | | | | 210,223 | | | | 5,781 | | | | — | | | | — | | | | 6,818 | | | | 222,822 | |
Of note, our annual rates of compensation for our named executive officers in effect at December 31, 2008 and 2009 remain the same. However, given our limited cash resources during 2009, the named executive officers other than Mr. Crockford had their annual base salaries reduced by 35% for the period from April 1 to September 30, 2009. Consequently, the salary amounts set forth in the following table may differ from the disclosed annual base salaries currently in effect.
In return for the 35% salary reduction, Mr. Finkelstein and Mr. Lyons received options to purchase 172,122 and 116,592 shares, respectively, of our common stock at an exercise price of $0.57 per share. Effective October 1, 2009, their salaries were restored to the levels in effect at December 31, 2008 and, therefore, the options ceased vesting as of September 30, 2009 but remain exercisable in accordance with the terms of our stock option plan. The number of shares vested and outstanding from these option grants are set forth in the table within the “Outstanding Equity Awards at December 31, 2009” section below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Non-Equity
| | | | |
| | | | | | | | | | | Incentive
| | | | |
| | | | | | | | | Option
| | Plan
| | All Other
| | |
| | | | | Salary(1)
| | Bonus(2)
| | Awards(3)
| | Compensation(4)
| | Compensation(5)
| | Total
|
Name and Principal Position | | Year | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) |
| |
| J.J. Finkelstein, President and | | | 2009 | | | | 244,608 | | | | 18,720 | | | | 116,198 | | | | — | | | | 13,005 | | | | 392,531 | |
| Chief Executive Officer | | | 2008 | | | | 299,520 | | | | 22,464 | | | | 86,137 | | | | 14,976 | | | | 17,690 | | | | 440,787 | |
| C. Neil Lyons, | | | 2009 | | | | 167,093 | | | | 11,140 | | | | 74,395 | | | | — | | | | 4,999 | | | | 257,627 | |
| Chief Financial Officer | | | 2008 | | | | 200,817 | | | | 12,152 | | | | 68,848 | | | | 10,127 | | | | 8,508 | | | | 300,452 | |
| David R. Crockford, | | | 2009 | | | | 210,223 | | | | 5,781 | | | | — | | | | — | | | | 6,818 | | | | 222,822 | |
| Vice President, Clinical and Regulatory Affairs | | | 2008 | | | | 209,203 | | | | 12,613 | | | | 51,682 | | | | 10,511 | | | | 11,681 | | | | 295,690 | |
| | |
| (1) | | Reflects base salary before pretax contributions and therefore includes compensation deferred under our 401(k) plan. |
| |
| (2) | | Reflects the discretionary portion of our bonus plan. |
| |
| (3) | | These amounts reflect the aggregate full grant date fair values (computed in accordance with FASB ASC Topic 718) of options granted to executives during the respective fiscal years. |
| |
| (4) | | Reflects amounts earned under our bonus plan subject to the achievement of corporate performance goals. |
| |
| (5) | | Primarily reflects our match of executive compensation deferrals into our 401(k) plan, along with supplemental life and disability insurance premiums. None of the individual items exceeded $10,000. |
Employment Agreements; Potential Payments Upon Termination or Change in Control
We are party to written employment agreements with our named executive officers. These employment agreements contain severance and other provisions that may provide for payments to the named executive officers following termination of employment with us in specified circumstances. The following is a summary of the material terms of these employment agreements with our named executive officers.
J.J. Finkelstein.We entered into an employment agreement with Mr. Finkelstein in January 2002 for him to serve as our president and chief executive officer. Mr. Finkelstein’s employment agreement had an initial three-year term, which is automatically renewed for additional one-year periods unless either we or Mr. Finkelstein elect not to renew it. This agreement was amended and restated during 2008 and again in 2009. Mr. Finkelstein’s annual base salary is $299,520. Mr. Finkelstein’s salary may not be adjusted downward
54
without his written consent, except in a circumstance which is part of a general reduction or other concessionary arrangement affecting all employees or affecting senior executive officers. Mr. Finkelstein is also eligible to receive an annual bonus in an amount established by the board of directors and is entitled to participate in and receive all standard employee benefits and to participate in all of our applicable incentive plans, including stock option, stock, bonus, savings and retirement plans. We also provide him with $5 million in life and disability insurance.
Mr. Finkelstein is eligible to receive options to purchase common stock under our Amended and Restated 2000 Stock Option and Incentive Plan, which we refer to in this prospectus as the 2000 Plan.equity incentive plans. The decision to grant any such options and the terms of such options are within the discretion of our board of directors or the compensation committee thereof. All vested options are exercisable for a period of time following any termination of Mr. Finkelstein’s employment as may be set forth in the 2000 Planapplicable benefit plan or in any option agreement between Mr. Finkelstein and us.
50
In the event that Mr. Finkelstein’s employment is terminated by us without “cause” or by Mr. Finkelstein for “good reason,” each as defined in his employment agreement, or if Mr. Finkelstein voluntarily terminates his employment within 12 months following a “change in control,” as defined in his employment agreement, then in each case, subject to Mr. Finkelstein’s entering into and not revoking a release of claims in a form acceptable to us, Mr. Finkelstein will be entitled to receive (i) a lump sum severance payment equal to his annual base salary then in effect (or if his base salary is less than the amount in effect as of March 31, 2009, the base salary in effect as of March 31, 2009), plus (ii) any earned bonus, and (iii) if he timely elects and remains eligible for continuation of healthcare benefits, that portion of the continued healthcare premiums that we were paying prior to the date of termination for a period of 12 months, in each case less applicable taxes and withholdings. If Mr. Finkelstein’s employment had been terminated for any of the reasons described in this paragraph as of December 31, 2009,2010, he would have been entitled to receive a lump sum payment of $299,520, less taxes and withholdings, plus continuation of healthcare benefits with a value of $8,772.$9,204.
In addition, if Mr. Finkelstein’s employment is terminated without “cause,” or if there is a “change in control” event, in each case as defined in either the 2000 Planapplicable benefit plan or in Mr. Finkelstein’s employment agreement, then the unvested portion of Mr. Finkelstein’s options outstanding as of December 31, 20092010 would accelerate in full.
C. Neil Lyons.We entered into an employment agreement with Mr. Lyons in April 2007 for him to serve as our chief financial officer. Mr. Lyons’ employment agreement had an initial one-year term, which is automatically renewed for additional one-year periods unless either we or Mr. Lyons elect not to renew it. The agreement was amended and restated during 2008 and again in 2009. Under the employment agreement, as amended to date, Mr. Lyons’ base salary is $202,537. Mr. Lyons is also eligible to receive an annual bonus in an amount established by the board of directors and chief executive officer and is entitled to participate in and receive all standard employee benefits and to participate in all of our applicable incentive plans, including stock option, stock, bonus, savings and retirement plans. We also reimburse Mr. Lyons for two-thirds of his annual term life insurance premium, for term life insurance coverage not to exceed two times his annual base salary.
Mr. Lyons is eligible to receive options to purchase common stock under the 2000 Plan.our equity incentive plans. The decision to grant any such options and the terms of such options are within the discretion of our board of directors or the compensation committee thereof. All vested options are exercisable for a period of time following any termination of Mr. Lyons’ employment as may be set forth in the 2000 Planapplicable benefit plan or in any option agreement between Mr. Lyons and us.
In the event that Mr. Lyons’ employment is terminated by us without “cause” as defined in his employment agreement, or if Mr. Lyons voluntarily terminates his employment within 12 months following a “change in control,” as defined in his employment agreement, then in each case, subject to Mr. Lyons’ entering into and not revoking a release of claims in a form acceptable to us, Mr. Lyons will be entitled to receive (i) severance payments equal to his annual base salary then in effect, plus (ii) any earned bonus, and (iii) if he timely elects and remains eligible for continuation of healthcare benefits, that portion of the continued healthcare premiums that we were paying prior to the date of termination for a period of 12 months, in each
55
case less applicable taxes and withholdings. If Mr. Lyons’s employment had been terminated for any of the reasons described in this paragraph as of December 31, 2009,2010, he would have been entitled to receive severance payments of $202,537, less taxes and withholdings, plus continuation of healthcare benefits with a value of $17,208.
$17,844.
In addition, if Mr. Lyons’ employment is terminated without “cause,” or if there is a “change in control” event, in each case as defined in either the 2000 Planapplicable benefit plan or in Mr. Lyons’ employment agreement, then the unvested portion of Mr. Lyons’ options outstanding as of December 31, 20092010 would accelerate in full.
David R. Crockford.We entered into an employment agreement with Mr. Crockford in March 2005 for him to serve as our vice president of clinical and regulatory affairs. Mr. Crockford’s employment agreement had an initial one-year term, which is automatically renewed for additional one-year periods unless either we or Mr. Crockford elect not to renew it. The agreement was amended and restated during 2008 and again in 2009. Under the employment agreement, as amended to date, Mr. Crockford’s base salary is $210,223. Mr. Crockford is also eligible to receive an annual bonus in an amount established by the board of directors and chief executive officer and is entitled to participate in and receive all standard employee benefits and to participate in all of our applicable incentive plans, including stock option, stock, bonus, savings and retirement plans. We also reimburse Mr. Crockford for two-thirds of his annual term life insurance premium, for term life insurance coverage not to exceed two times his annual base salary.
Mr. Crockford is eligible to receive options to purchase common stock under the 2000 Plan.our equity incentive plans. The decision to grant any such options and the terms of such options are within the discretion of our board of directors or the compensation committee thereof. All vested options are exercisable for a period of time following any termination of Mr. Crockford’s employment as may be set forth in the 2000 Planapplicable benefit plan or in any option agreement between Mr. Crockford and us.
51
In the event that Mr. Crockford’s employment is terminated by us without “cause” as defined in his employment agreement, or if Mr. Crockford voluntarily terminates his employment within 12 months following a “change in control,” as defined in his employment agreement, then in each case, subject to Mr. Crockford’s entering into and not revoking a release of claims in a form acceptable to us, Mr. Crockford will be entitled to receive (i) severance payments equal to his annual base salary then in effect, plus (ii) any earned bonus, and (iii) if he timely elects and remains eligible for continuation of healthcare benefits, that portion of the continued healthcare premiums that we were paying prior to the date of termination for a period of 12 months, in each case less applicable taxes and withholdings. If Mr. Crockford’s employment had been terminated for any of the reasons described in this paragraph as of December 31, 2009,2010, he would have been entitled to receive severance payments of $210,223, less taxes and withholdings, plus continuation of healthcare benefits with a value of $14,664.$15,324. In addition, upon a “change in control,” all of Mr. Crockford’s unvested options will accelerate in full, but there is no such acceleration upon a termination without cause.
56
Outstanding Equity Awards at December 31, 20092010
The following table shows certain information regarding outstanding equity awards at December 31, 20092010 for the named executive officers, all of which were stock options.
| | | | | | | | | | | | | | | | | | | | | |
| | | Number of | | | | | | | | | | | | | |
| | | Shares | | | Number of Shares | | | | | | | | | | |
| | | Underlying | | | Underlying | | | | | | | | | | |
| | | Unexercised | | | Unexercised | | | Option | | | | | | | |
| | | Options (#) | | | Options (#) | | | Exercise Price | | | Option | | | | |
| Name | | Exercisable | | | Unexercisable | | | ($) | | | Expiration Date | | | Note | |
| Mr. Finkelstein | | | 500,000 | | | | — | | | | 0.33 | | | | 1/1/2012 | | | | | |
| | | | 100,000 | | | | — | | | | 3.21 | | | | 4/1/2015 | | | | | |
| | | | 93,750 | | | | 31,250 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 62,500 | | | | 62,500 | | | | 1.15 | | | | 4/15/2015 | | | | (1 | ) |
| | | | 114,748 | | | | — | | | | 0.57 | | | | 4/10/2019 | | | | | |
| | | | 31,250 | | | | 93,750 | | | | 0.76 | | | | 10/11/2016 | | | | (1 | ) |
| | | | — | | | | 125,000 | | | | 0.27 | | | | 07/14/2017 | | | | (1 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Mr. Lyons | | | 166,667 | | | | 33,333 | | | | 3.10 | | | | 4/7/2015 | | | | (2 | ) |
| | | | 56,250 | | | | 18,750 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 37,500 | | | | 37,500 | | | | 1.50 | | | | 6/15/2015 | | | | (1 | ) |
| | | | 77,728 | | | | — | | | | 0.57 | | | | 4/10/2019 | | | | | |
| | | | 18,750 | | | | 56,250 | | | | 0.76 | | | | 10/11/2016 | | | | (1 | ) |
| | | | — | | | | 98,500 | | | | 0.27 | | | | 07/14/2017 | | | | (1 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Mr. Crockford | | | 15,000 | | | | — | | | | 1.07 | | | | 7/1/2013 | | | | | |
| | | | 125,000 | | | | — | | | | 0.86 | | | | 1/1/2014 | | | | | |
| | | | 100,000 | | | | — | | | | 3.21 | | | | 4/1/2015 | | | | | |
| | | | 25,000 | | | | — | | | | 3.82 | | | | 5/25/2015 | | | | | |
| | | | 37,500 | | | | 12,500 | | | | 2.15 | | | | 1/16/2014 | | | | (1 | ) |
| | | | 56,250 | | | | 18,750 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 37,500 | | | | 37,500 | | | | 1.15 | | | | 4/15/2015 | | | | (1 | ) |
| | | | — | | | | 98,500 | | | | 0.27 | | | | 07/14/2017 | | | | (1 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| | | Number of
| | | | | | | | |
| | | Shares
| | Number of Shares
| | | | | | |
| | | Underlying
| | Underlying
| | | | | | |
| | | Unexercised
| | Unexercised
| | Option
| | | | |
| | | Options (#)
| | Options (#)
| | Exercise Price
| | Option
| | |
Name | | Exercisable | | Unexercisable | | ($) | | Expiration Date | | Note |
| |
| Mr. Finkelstein | | | 500,000 | | | | — | | | | 0.33 | | | | 1/1/2012 | | | | | |
| | | | 100,000 | | | | — | | | | 3.21 | | | | 4/1/2015 | | | | | |
| | | | 62,500 | | | | 62,500 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 31,250 | | | | 93,750 | | | | 1.15 | | | | 4/15/2015 | | | | (1 | ) |
| | | | 114,748 | | | | — | | | | 0.57 | | | | 4/10/2019 | | | | | |
| | | | — | | | | 125,000 | | | | 0.76 | | | | 10/11/2016 | | | | (1 | ) |
| Mr. Lyons | | | 133,332 | | | | 66,668 | | | | 3.10 | | | | 4/7/2015 | | | | (2 | ) |
| | | | 37,500 | | | | 37,500 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 18,750 | | | | 56,250 | | | | 1.50 | | | | 6/15/2015 | | | | (1 | ) |
| | | | 77,728 | | | | — | | | | 0.57 | | | | 4/10/2019 | | | | | |
| | | | — | | | | 75,000 | | | | 0.76 | | | | 10/11/2016 | | | | (1 | ) |
| Mr. Crockford | | | 15,000 | | | | — | | | | 1.07 | | | | 7/1/2013 | | | | | |
| | | | 125,000 | | | | — | | | | 0.86 | | | | 1/1/2014 | | | | | |
| | | | 70,000 | | | | 30,000 | | | | 3.21 | | | | 4/1/2015 | | | | (3 | ) |
| | | | 17,500 | | | | 7,500 | | | | 3.82 | | | | 5/25/2015 | | | | (3 | ) |
| | | | 25,000 | | | | 25,000 | | | | 2.15 | | | | 1/16/2014 | | | | (1 | ) |
| | | | 37,500 | | | | 37,500 | | | | 2.34 | | | | 3/15/2014 | | | | (1 | ) |
| | | | 18,750 | | | | 56,250 | | | | 1.15 | | | | 4/15/2015 | | | | (1 | ) |
| | |
| (1) | | This option vests in equal installments on the first four anniversaries of the grant date. In each case these options were granted seven years prior to the listed expiration dates. |
| |
| (2) | | This option vests in equal installments on the first six anniversaries of the grant date which was April 7, 2005. |
|
(3) | | This option vests on the first five anniversaries of the grant date in the following installments: 10%, 15%, 20%, 25%, 30%. In each case these options were granted ten years prior to the listed expiration dates. |
52
Post-Employment Compensation
We do not maintain any plans providing for payment or other benefits at, following, or in connection with retirement other than a 401(k) plan made available to all employees. In addition, we do not maintain any non-qualified deferred compensation plans.
57
Equity Compensation Plan Information
The following table provides information as of December 31, 20092010 about the securities authorized for issuance to our employees, directors and other eligible participants under our equity compensation plans, consisting of the Amended and Restated 2000 Stock Option and Incentive Plan and the 2010 Equity Incentive Plan.
| | | | | | | | | | | | | |
| | | | | | | | | | | Number of Securities | |
| | | | | | | | | | | Remaining Available for | |
| | | Number of Securities to | | | | | | | Future Issuance Under | |
| | | be Issued Upon Exercise | | | Weighted-Average Exercise | | | Equity Compensation Plans | |
| | | of Outstanding Options, | | | Price of Outstanding Options, | | | (Excluding Securities | |
| | | Warrants and Rights | | | Warrants and Rights | | | Reflected in Column (a)) | |
| Plan Category | | (a) | | | (b) | | | (c) | |
| Equity compensation plans approved by security holders | | | 5,348,863 | | | $ | 1.37 | | | | 4,327,500 | |
| | | | | | | | | | | | | |
| Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | |
| | | | | | | | | | | | | |
| Total | | | 5,348,863 | | | $ | 1.37 | | | | 4,327,500 | |
2010 Equity Incentive Plan
In May 2010 our Board adopted, and in July 2010, upon the recommendation of our Board, our stockholders approved the adoption of, the 2010 Equity Incentive Plan, or 2010 Plan. The following is a summary of the material terms of the 2010 Plan:
General
The 2010 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance stock awards and other forms of equity compensation, which we refer to collectively as “stock awards.” Additionally, the 2010 Plan provides for the grant of performance cash awards. Incentive stock options granted under the 2010 Plan are intended to qualify as “incentive stock options” within the meaning of Section 422 of the Internal Revenue Code, or the Code. Nonstatutory stock options granted under the 2010 Plan are not intended to qualify as incentive stock options under the Code. Incentive stock options may be granted only to our employees or to employees of certain of our affiliates. All other awards may be granted to our employees, including officers, non-employee directors, and consultants. See “Federal Income Tax Information” below for a discussion of the tax treatment of awards.
Purpose
The Board adopted the 2010 Plan to provide a means by which employees, directors and consultants of ours and certain of our affiliates may be given an opportunity to purchase our stock, to assist us in retaining the services of such persons, to secure and retain the services of persons capable of filling such positions and to provide incentives for such persons to exert
53
maximum efforts for our success and for the success of our affiliates.
Shares Available for Awards Under the 2010 Plan
The total number of shares of our common stock reserved for issuance under the 2010 Plan is 5,000,000 shares.
If a stock award granted under the 2010 Plan expires or otherwise terminates without being exercised in full, or is settled in cash, the shares of our common stock not acquired pursuant to the stock award will again become available for issuance under the 2010 Plan. Additionally, the following types of shares will be available for the grant of new stock awards under the 2010 Plan: (i) shares that are forfeited to or repurchased by us prior to becoming fully vested; (ii) shares withheld to satisfy income and employment withholding taxes; and (iii) shares tendered to us to pay the exercise price of an option.
Eligibility
All of our employees, directors and consultants, and those of our affiliates, are eligible to participate in the 2010 Plan and may receive all types of awards other than incentive stock options. Incentive stock options may be granted only to our employees and to employees of certain of our affiliates.
Administration
The Board administers the 2010 Plan. Subject to the provisions of the 2010 Plan, the Board has the power to construe and interpret the 2010 Plan and to determine the persons to whom and the dates on which awards will be granted, the number of shares of our common stock subject to each award, the time or times during the term of each award within which all or a portion of such award may be exercised, the exercise price, the type of consideration and other terms of the award.
The Board has the power to delegate its authority to administer the 2010 Plan to a committee consisting solely of two or more “non-employee directors” within the 2000meaning of Rule 16b-3 of the Exchange Act, and solely of two or more “outside directors” within the meaning of Section 162(m) of the Code. The Board has delegated administration of the 2010 Plan to the Compensation Committee. Except as explicitly stated otherwise, with respect to the 2010 Plan, the “Board” refers to any committee the Board appoints (including the Compensation Committee) as well as to the Board itself.
Repricing; Cancellation and Re-Grant of Stock Awards
Under the 2010 Plan, the Board does not have the authority to reprice any outstanding equity awards by reducing the exercise price of the stock award or to cancel any outstanding stock awards in exchange for cash or other stock awards without obtaining the approval of our stockholders within 12 months prior to the repricing or cancellation and re-grant event.
Options
Options may be granted pursuant to stock option agreements. The 2010 Plan permits the grant of options that qualify as incentive stock options and nonstatutory stock options. Individual stock option agreements may be more restrictive as to any or all of the permissible terms described in this section.
Exercise Price; Consideration.The exercise price of incentive stock options may not be less than 100% of the fair market value of the stock subject to the option on the date of the grant and, in some cases (see “Limitations” below), may not be less than 110% of such fair market value. The exercise price of nonstatutory stock options may not be less than 100% of the fair market value of the stock on the date of grant.
The Board will determine the acceptable forms of consideration for the purchase of common stock issued upon the exercise of a stock option, which may include cash or check, a broker-assisted cashless exercise, the tender of common stock previously owned by the participant, a net exercise of the option if it is a nonstatutory stock option, and other legal consideration approved by the Board.
Option Exercise.Options granted under the 2010 Plan may become exercisable in cumulative increments, or “vest,” at the rate specified in the option agreement as determined by the Board. Shares covered by different options granted under the 2010 Plan may be subject to different vesting schedules as the Board may determine. Vesting can be time-based or performance-based or can be a hybrid of performance-based and time-based vesting. The Board also has flexibility to provide for accelerated vesting of options and other equity awards as it deems appropriate.
Term.The maximum term of options granted under the 2010 Plan is ten years, except that in certain cases (see “Limitations” below) the maximum term is five years. Unless the terms of a participant’s stock option agreement provide
54
otherwise, if a participant’s service relationship with us, or any of our affiliates, ceases for any reason other than a termination for cause or a termination because of disability or death, the participant may exercise the vested portion of any option for a period of three months following termination of service. If a participant’s service relationship with us, or any of our affiliates, ceases due to disability or death or a participant dies within a specified period following termination of service, the participant or a beneficiary may exercise the vested portion of any option for a period of 12 months in the event of disability and 18 months in the event of death. Under the 2010 Plan, the option term may be further extended in the event that exercise of the option following termination of service is prohibited by applicable securities laws, or the sale of any common stock received upon exercise of the option would violate our insider trading policy. In no event, however, may an option be exercised beyond the expiration of its term. In the event of a termination of a participant’s service for “cause,” as defined in the 2010 Plan, the option will terminate on the termination date and the participant may not exercise the option following such termination.
Limitations. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive options that are exercisable for the first time by a participant during any calendar year under all of our stock plans may not exceed $100,000. The options or portions of options that exceed this limit are treated as nonstatutory stock options. No incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any affiliate unless:
| • | | the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant; and |
|
| • | | the term of the incentive stock option does not exceed five years from the date of grant. |
The aggregate maximum number of shares of our common stock that may be issued in respect of incentive stock options is 5,000,000.
In addition, no person may be granted stock awards covering more than 1,000,000 shares of our common stock under the 2010 Plan during any calendar year pursuant to stock options, stock appreciation rights and other equity awards whose value is determined by reference to an increase over an exercise or strike price of at least 100% of the fair market value on the date the stock award is granted.
Restricted Stock Awards
A restricted stock award is the grant of shares of our common stock to a participant that may, but need not, be subject to forfeiture or to a share repurchase option in our favor in accordance with a vesting scheduled determined by the Board. For example, some or all of the shares of common stock granted pursuant to a restricted stock award may be repurchased by us if a participant’s service with us or with any of our affiliates terminates before a specified date (that is, before the restricted stock award is fully vested). Restricted stock awards are granted pursuant to restricted stock award agreements. Restricted stock awards may be granted in consideration for cash, past or future services rendered to us or an affiliate or any other form of legal consideration.
Restricted Stock Unit Awards
A restricted stock unit award is a promise by us to issue shares of our common stock, or to pay cash equal to the value of shares of our common stock, equivalent to the number of units covered by the award at the time of vesting of the units or thereafter. Restricted stock unit awards are granted pursuant to restricted stock unit award agreements. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award entitles the recipient to receive cash, stock, a combination of cash and stock as deemed appropriate by the Board or any other form of consideration set forth in the restricted stock unit award agreement at a specified date (typically, upon vesting of the restricted stock units). Additionally, dividend equivalents may be credited in respect of shares covered by a restricted stock unit award. Except as otherwise provided in the applicable award agreement, restricted stock units that have not vested will be forfeited upon the termination of the participant’s service for any reason.
Stock Appreciation Rights
A stock appreciation right entitles the participant to a payment equal in value to the appreciation in the value of the underlying shares of our common stock for a predetermined number of shares over a specified period. Stock appreciation rights are granted pursuant to stock appreciation right agreements. Each stock appreciation right is denominated in common stock share equivalents. The board determines the strike price of each stock appreciation right, which may not be less than 100% of the fair market value of our common stock on the date of grant. A stock appreciation right entitles the recipient to
55
receive cash, stock, a combination of cash and stock as determined by the Board or any other form of consideration set forth in the stock appreciation right agreement upon exercise. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (a) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (b) the number of shares of common stock with respect to which the stock appreciation right is exercised. A stock appreciation right vests at the rate specified in the stock appreciation right agreement as determined by the Board. Stock appreciation rights are subject to the same conditions upon termination of a participant’s service and the same restrictions on transfer as stock options under the 2010 Plan.
| | | | | | | | | | | | | |
| | | | | | | | | Number of Securities
| |
| | | | | | | | | Remaining Available for
| |
| | | Number of Securities to
| | | | | | Future Issuance Under
| |
| | | be Issued Upon Exercise
| | | Weighted-Average Exercise
| | | Equity Compensation Plans
| |
| | | of Outstanding Options,
| | | Price of Outstanding Options,
| | | (Excluding Securities
| |
| | | Warrants and Rights
| | | Warrants and Rights
| | | Reflected in Column (a))
| |
Plan Category | | (a) | | | (b) | | | (c) | |
| |
| Equity compensation plans approved by security holders | | | 4,914,112 | | | $ | 1.53 | | | | 1,550,888 | |
| Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Total | | | 4,914,112 | | | $ | 1.53 | | | | 1,550,888 | |
Performance Awards
The 2010 Plan permits the grant of performance-based stock and cash awards that may qualify as performance-based compensation that is not subject to the $1,000,000 limitation on the income tax deductibility of compensation paid per covered executive officer imposed by Section 162(m) of the Code. To assure that the compensation attributable to performance-based awards will so qualify, the Compensation Committee can structure such awards so that the stock or cash will be issued or paid pursuant to such award only following the achievement of certain pre-established performance goals during a performance period designed by the Compensation Committee. The maximum amount covered by a performance award that may be granted to any individual in a calendar year (whether the grant, vesting or exercise is contingent upon the attainment during a performance period of the performance goals) may not exceed 1,000,000 shares of our common stock in the case of performance stock awards, or $500,000 in the case of performance cash awards.
In granting a performance award, the Compensation Committee will set a period of time (a “performance period”) over which the attainment of one or more goals (“performance goals”) will be measured for the purpose of determining whether the award recipient has a vested right in or to such award. Within the time period prescribed by Section 162(m) of the Code, at a time when the achievement of the performance goals remains substantially uncertain (typically before the 90th day of a performance period or the date on which 25% percent of the performance period has elapsed), the Compensation Committee will establish the performance goals, based upon one or more criteria (“performance criteria”) enumerated in the 2010 Plan and described below. As soon as administratively practicable following the end of the performance period, the Compensation Committee will certify in writing whether the performance goals have been satisfied.
The Compensation Committee may establish performance goals by selecting from one or more of the following performance criteria: (1) earnings (including earnings per share and net earnings); (2) earnings before interest, taxes and depreciation; (3) earnings before interest, taxes, depreciation and amortization; (4) total stockholder return; (5) return on equity or average stockholder’s equity; (6) return on assets, investment, or capital employed; (7) stock price; (8) margin (including gross margin); (9) income (before or after taxes); (10) operating income; (11) operating income after taxes; (12) pre-tax profit; (13) operating cash flow; (14) sales or revenue targets; (15) increases in revenue or product revenue; (16) expenses and cost reduction goals; (17) improvement in or attainment of working capital levels; (18) economic value added (or an equivalent metric); (19) market share; (20) cash flow; (21) cash flow per share; (22) share price performance; (23) debt reduction; (24) implementation or completion of projects or processes; (25) customer satisfaction; (26) stockholders’ equity; (27) capital expenditures; (28) debt levels; (29) operating profit or net operating profit; (30) workforce diversity; (31) growth of net income or operating income; (32) billings; (33) achievement of clinical trial milestones, such as patient enrollment or successful completion of the trial; (34) execution of a new license agreement; (35) receipt of a milestone payment under a license agreement; or (36) to the extent that an award is not intended to comply with Section 162(m) of the Code, other measures of performance selected by the Board or the Compensation Committee.
The Compensation Committee may establish performance goals on a company-wide basis, with respect to one or more business units, divisions, affiliates, or business segments, and in either absolute terms or relative to the performance of one or more comparable companies or the performance of one or more relevant indices. Unless specified otherwise (a) in the award agreement at the time the award is granted or (b) in such other document setting forth the performance goals at the time the goals are established, the Compensation Committee will appropriately make adjustments in the method of calculating the attainment of the performance goals as follows: (1) to exclude restructuring and/or other nonrecurring charges; (2) to exclude exchange rate effects, as applicable, for non-U.S. dollar denominated goals; (3) to exclude the effects of changes to generally accepted accounting principles; (4) to exclude the effects of any statutory adjustments to corporate tax rates; and (5) to exclude the effects of any “extraordinary items” as determined under generally accepted accounting principles.
Compensation attributable to performance-based awards under the 2010 Plan will qualify as performance-based compensation, provided that: (i) the award is granted by a compensation committee comprised solely of “outside directors,” (ii) the award is granted (or exercisable) only upon the achievement of an objective performance goal established in writing by the compensation committee while the outcome is substantially uncertain, and (iii) the compensation committee certifies in writing prior to the granting, payment or exercisability of the award that the performance goal has been satisfied.
56
Withholding Obligations
Unless prohibited in an individual award agreement, we may satisfy any federal, state or local tax withholding obligation relating to an award by causing the recipient to tender a cash payment, by withholding shares of common stock from the shares otherwise issuable, by withholding cash from an award settled in cash, by withholding payment from amounts otherwise payable, or by such other method as specified in the award agreement or by a combination of these means.
Changes to Capital Structure
In the event that there is a specified type of change in our capital structure, such as a stock split, the Board will make appropriate adjustments to (a) the class and maximum number of shares reserved under the 2010 Plan, (b) the class and maximum number of shares of our common stock that may be issued upon the exercise of incentive stock options, (c) the class and maximum number of shares of our common stock subject to equity awards that can be granted in a calendar year (as established under the 2010 Plan pursuant to Section 162(m) of the Code), and (d) the class, number of securities and the exercise price or strike price, if applicable, of all outstanding equity awards.
Corporate Transactions
In the event of certain significant corporate transactions, the following will occur with respect to outstanding stock awards under the 2010 Plan:
| • | | The Board will arrange for assumption, continuation, or substitution of a stock award by a surviving or acquiring entity (or its parent company) and assign any reacquisition or repurchase rights held by us in respect of common stock issued pursuant to awards to our successor (or its parent). |
|
| • | | With respect to stock awards that have not been assumed, continued or substituted, the vesting of such stock awards will be accelerated in full to a date prior to the effective date of the corporate transaction and any reacquisition or repurchase right held by us in respect of common stock issuable pursuant to such stock awards will lapse, and such stock awards will terminate if not exercised (if applicable) at or prior to the time of the corporate transaction. |
|
| • | | With respect to stock awards that have not been assumed, continued or substituted, such outstanding stock awards will terminate if not exercised at or prior to the time of the corporate transaction, and the Board may, in its discretion, make a payment, in such form as the Board may determine, equal in value to the excess, if any, of (a) the value of the property the holder would have received upon the exercise of the stock award, over (b) any exercise price payable by such holder in connection with such exercise. |
The Board need not take the same action with respect to all stock awards or portions of stock awards or with respect to all participants. The Board may take different actions with respect to the vested and unvested portions of a stock award.
For purposes of the 2010 Plan, a corporate transaction includes the consummation of any one or more of the following events: (i) a sale of all or substantially all of our consolidated assets; (ii) a sale of at least 50% of our outstanding securities; (iii) a merger or consolidation in which we are not the surviving corporation; or (iv) a merger or consolidation in which we are the surviving corporation but shares of our outstanding common stock are converted into other property by virtue of the transaction.
Change in Control
As of the effective time of a change in control, the vesting of all outstanding stock awards (and the exercisability of options and stock appreciation rights) will be accelerated in full and any reacquisition or repurchase rights held by us with respect to outstanding stock awards will lapse.
For purposes of the 2010 Plan, a change in control includes any one or more of the following events: (a) a person or group becomes the owner of more than 50% of the combined voting power of our outstanding securities; (b) a consummated merger or consolidation in which our stockholders immediately prior to the transaction do not own more than 50% of the combined voting power of the surviving entity or its parent company in substantially the same proportions as their ownership in us immediately prior to the transaction; (c) a consummated sale, lease, exclusive license or disposition of all or substantially all of our consolidated assets to an entity more than 50% of the combined voting power of which is not owned by our stockholders in substantially the same proportions as their ownership in us immediately prior to the transaction; or (d) certain changes in the composition of the Board. A change in control excludes: (i) the sale of securities to an investor or
57
group for the primary purpose of obtaining financing through our issuance of securities (including offering stock to the general public through a registration statement filed with the SEC); (ii) a change in the level of ownership held by a person or group beyond the designated threshold in section (a) above as a result of our repurchase or other acquisition of our voting securities; and (iii) a transaction for the purpose of changing our domicile.
Plan Amendments
The Board will have the authority to amend or terminate the 2010 Plan. However, no amendment or termination of the plan will adversely affect any rights under awards already granted to a participant unless agreed to by the affected participant. We will obtain stockholder approval of any amendment to the 2010 Plan as required by applicable law.
Plan Termination
Unless sooner terminated by the Board, the 2010 Plan will automatically terminate on July 13, 2020, the day before the tenth anniversary of the date the 2010 Plan was adopted by our stockholders.
Amended and Restated 2000 Stock Option and Incentive Plan
The Amended and Restated 2000 Stock Option and Incentive Plan, or 2000 Plan, providesprovided for grants of both incentive stock options and non-qualified stock options, as such terms are defined below, to participants. The 2000 Plan expired in December 2010, and no further grants may be made under the 2000 Plan. Options granted pursuant to the 2000 Plan remain outstanding in accordance with their terms. Participants in the 2000 Plan may includeincluded our employees, directors, consultants and advisors and those of our affiliates. The 2000 Plan is administered by the Compensation Committee of the Board. Unless otherwise restricted by the Board, the Compensation Committee has the authority and discretion to select participants in the 2000 Plan and to grant options under the 2000 Plan. The Compensation Committee is authorized under the 2000 Plan to fix the terms and conditions of all option awards. The exercise price for options is determined by the Compensation Committee, provided that the exercise price cannot be less than the fair market value of a share on the date of grant of the option. In the case of an incentive stock option granted to a ten percent owner, the exercise price must be at least equal to 110% of the fair market value of a share on the date of grant. The Compensation Committee may set the term of each option granted under the 2000 Plan, provided that the term cannot exceed ten years or five years in the case of an incentive stock option granted to a ten percent owner. The 2000 Plan also gives to the Compensation Committee the authority to determine vesting and exercisability of options granted under the 2000 Plan and to specify the method of payment of the exercise price.
There are currently 6,500,000 shares authorized for issuance under the 2000 Plan. Under the 2000 Plan, no single participant can receive options for more than 750,000 shares in any one year.
For purposes of the 2000 Plan, fair market value is defined to mean the per share closing price of the shares on the securities exchange on which the shares are listed or, if no such price information is reported, the fair market value on such date of a share as the Compensation Committee shall determine. The 2000 Plan generally provides that upon an option holder’soptionholder’s termination of service for any reason other than for cause or due to death or disability, the option holder’soptionholder’s options, to the extent vested and exercisable, can be exercised up until the earlier to occur of (i) three months following the termination of service or (ii) the expiration of the term of the option. Unless otherwise determined by the Compensation Committee, upon termination of service of an option holderoptionholder due to death or disability, the option holder’soptionholder’s options, to the extent vested and exercisable, can be exercised up until the earlier to occur of (i) one year following the termination of service on account of death or disability or (ii) the expiration of the term of the option. Upon termination of service of an option holderoptionholder for cause (as defined by the 2000 Plan), all of the option holder’soptionholder’s unexercised options shall immediately be forfeited.
The 2000 Plan provides that in certain events (including certain mergers or consolidations involving our company), option holdersoptionholders may have the right to elect to receive cash upon exercise of any option equal to the fair market value of the underlying stock less the exercise price of such option times the number of shares with respect to which the options are option exercised. The Compensation Committee in its discretion will determine whether such amounts are to be paid in cash, property or some combination. The 2000 Plan also provides that upon the occurrence of certain events that are treated as a “change in control,” all outstanding
58
options generally will become fully vested and exercisable (unless otherwise provided in an option holder’soptionholder’s award agreement).
Options granted under the 2000 Plan are restricted as to transferability. Generally, options only may be transferred by will or the laws of descent and distribution,distribution; however, non-qualified stock options also may be transferred by gift under certain circumstances and pursuant to certain domestic relations orders. Option holdersOptionholders may be required under the 2000 Plan to make certain investment representations in connection with the exercise of options to enable us to comply with federal and state securities laws. We may refuse delivery of shares under the 2000 Plan if the requested representations are not made by an option holderoptionholder or if the shares have not been registered by us on a stock exchange. At the time of exercise of options under the 2000 Plan, option holdersoptionholders may be required to pay any taxes associated with such exercise of the option that we are required to withhold. The 2000 Plan permits us to retain or sell shares that an option holderoptionholder otherwise would receive upon exercise of the option to cover the tax amounts required to be withheld.
No person has a right to be selected as a participant in the 2000 Plan or to be granted an option under the 2000 Plan. Participation in the 2000 Plan or the grant of an option under the 2000 Plan does not give any participant rights as an employee of or a consultant or advisor to us or the right to be retained in the employ of or as a consultant or advisor to us.
The Compensation Committee generally has the authority to amend, alter, suspend, discontinue, or terminate the 2000 Plan without the consent of stockholders or 2000 Plan participants. However, to the extent that an amendment to the 2000 Plan requires shareholder approval under any applicable federal or state law or regulation or the rules of any stock exchange, the Compensation Committee will seek stockholder approval. Unless otherwise terminated, the 2000 Plan will remain effective for a term of ten years from its effective date, or until December 15, 2010. The Compensation Committee may not amend, alter, suspend, discontinue or terminate any outstanding option without the consent of the participant. We currently intend to adopt a new equity incentive plan and submit it to stockholders for approval during 2010.
Federal Income Tax Information
Incentive Stock Options. Incentive stock options under the 2000 Plan are intended The information set forth below is only a summary and does not purport to be eligible for thecomplete. The information is based upon current federal income tax treatment accorded “incentive stock options” underrules and therefore is subject to change when those rules change. Because the Internal Revenue Code of 1986, as amended (the “Code”).
There generally are no federal income tax consequences to any participant may depend on his or her particular situation, each participant should consult the participant or to us by reasonparticipant’s tax adviser regarding the federal, state, local, and other tax consequences of the grant or exercise of an incentiveaward or the disposition of stock option. However, the exerciseacquired as a result of an incentive stock option may increaseaward. The 2000 Plan and 2010 Plan are not qualified under the participant’s alternative minimum tax liability, if any.
If a participant holds stock acquired through exerciseprovisions of an incentive stock option for more than two years from the date on which the option is granted and more than one year from the date on which the shares are transferred to the participant upon exerciseSection 401(a) of the option,Code and are not subject to any gain or loss on a disposition of such stock will be a long-term capital gain or loss.
Generally, if the participant disposes of the stock before the expiration of either of these holding periods (a “disqualifying disposition”), then at the time of disposition the participant will realize taxable ordinary income equal to the lesser of (i) the excessprovisions of the stock’s fair market valueEmployee Retirement Income Security Act of 1974. Our ability to realize the benefit of any tax deductions described below depends on the dateour generation of exercise overtaxable income as well as the exercise price, or (ii) the participant’s actual gain, if any, on the purchase and sale. Any additional gain or any loss upon the disqualifying disposition will be a capital gain or loss, which will be long-term or short-term depending on whether the stock was held for more than one year.
58
To the extent the participant recognizes ordinary income by reason of a disqualifying disposition, we will generally be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) of the Code, and the satisfaction of aour tax reporting obligation) to a corresponding business expense deduction in the tax year in which the disqualifying disposition occurs.obligations.
Nonstatutory Stock Options.Options Nonstatutory
Generally, there is no taxation upon the grant of a nonstatutory stock optionsoption where the option is granted under the 2000 Plan generally have the following federal income tax consequences.
59
There are no tax consequenceswith an exercise price equal to the participant or to us by reasonfair market value of the grant. Uponunderlying stock on the grant date. On exercise, of an options and acquisition of the stock, thea participant normally will recognize taxable ordinary income equal to the excess, if any, of the stock’s fair market value on the acquisition date of exercise of the option over the purchase price (the exercise price). However, ifprice. If the stockparticipant is employed by us, that income will be subject to certain types of vesting restrictions (an “early exercise” feature), the taxable eventwithholding tax. The participant’s tax basis in those shares will be delayed untilequal to their fair market value on the vesting restrictions lapse unless the participant elects to be taxed on receiptdate of exercise of the stock. With respect to employees, we are generally required to withhold from regular wages or supplemental wage payments an amount basedoption, and the participant’s capital gain holding period for those shares will begin on the ordinary income recognized.that date.
Subject to the requirement of reasonableness, the provisions of Section 162(m) of the Code and the satisfaction of a tax reporting obligation, we will generally be entitled to a business expensetax deduction equal to the taxable ordinary income realized by the participant.
Incentive Stock Options
Our equity incentive plans provide for the grant of stock options that qualify as “incentive stock options,” as defined in Section 422 of the Code. Under the Code, a participant generally is not subject to ordinary income tax upon the grant or exercise of an incentive stock option. If the participant holds a share of our common stock received on exercise of an incentive stock option for more than two years from the date the stock option was granted and more than one year from the date the stock option was exercised, which is referred to as the required holding period, the difference, if any, between the amount realized on a sale or other taxable disposition of that share and the participant’s tax basis in that share will be long-term capital gain or loss.
Upon If, however, a participant disposes of a share acquired on exercise of an incentive stock option before the end of the required holding period, which is referred to as a disqualifying disposition, the participant generally will recognize ordinary income in the year of the disqualifying disposition equal to the excess, if any, of the fair market value of the share on the date the incentive stock option was exercised over the exercise price. However, if the sales proceeds are less than the fair market value of the share on the date of exercise of the stock option, the amount of ordinary income recognized by the participant will not exceed the gain, if any, realized on the sale. If the amount realized on a disqualifying disposition exceeds the fair market value of the share on the date of exercise of the stock option, that excess will be short-term or long-term capital gain, depending on whether the holding period for the share exceeds one year.
For purposes of the alternative minimum tax, the amount by which the fair market value of a share of stock acquired on exercise of an incentive stock option exceeds the exercise price of that stock option generally will be an adjustment included in the participant’s alternative minimum taxable income for the year in which the stock option is exercised. If, however, there is a disqualifying disposition of the share in the year in which the stock option is exercised, there will be no adjustment for alternative minimum tax purposes with respect to that share. In computing alternative minimum taxable income, the tax basis of a share acquired on exercise of an incentive stock option is increased by the amount of the adjustment taken into account with respect to that share for alternative minimum tax purposes in the year the stock option is exercised.
We are not allowed an income tax deduction with respect to the grant or exercise of an incentive stock option or the disposition of a share acquired on exercise of an incentive stock option after the required holding period. If there is a disqualifying disposition of a share, however, we are allowed a deduction in an amount equal to the ordinary income includible in income by the participant, subject to Section 162(m) of the Code, and provided that amount constitutes an ordinary and necessary business expense for us and is reasonable in amount, and either the employee includes that amount in income or we timely satisfy our reporting requirements with respect to that amount.
Restricted Stock Awards
Generally, the recipient of a restricted stock award will recognize ordinary compensation income at the time the stock is received equal to the excess, if any, of the fair market value of the stock received over any amount paid by the recipient in exchange for the stock. If, however, the stock is not vested when it is received (for example, if the employee is required to work for a capitalperiod of time in order to have the right to sell the stock), the recipient generally will not recognize income until the stock becomes vested, at which time the recipient will recognize ordinary compensation income equal to the excess, if any, of the fair market value of the stock on the date it vests over any amount paid by the recipient in exchange for the stock. A recipient may, however, file an election with the Internal Revenue Service, within 30 days of his or her receipt of the stock award, to recognize ordinary compensation income as of the date the recipient receives the award equal to the excess, if any,
59
of the fair market value of the stock on the date the award is granted over any amount paid by the recipient in exchange for the stock.
The recipient’s tax basis for the determination of gain or loss equal toupon the difference between the selling price and the sumsubsequent disposition of shares acquired from stock awards will be the amount paid for such stockshares plus any amount recognized as ordinary income upon acquisition (or vesting) of the stock. Such gain or loss will be long-term or short-term depending on whetherrecognized either when the stock was held for more than one year. Slightly different rules may applyis received or when the stock becomes vested.
Subject to participants who acquire stock subject to certain repurchase options or who are subject to Section 16(b)the requirement of reasonableness, the Exchange Act.
Potential Limitation on Company Deductions.provisions of Section 162(m) of the Code deniesand the satisfaction of a tax reporting obligation, we will generally be entitled to a tax deduction equal to the taxable ordinary income realized by the recipient of the stock award.
Stock Appreciation Rights
If a stock appreciation right is granted under the 2010 Plan with a strike price equal to the fair market value of the underlying stock on the date of grant and the recipient may receive only the appreciation inherent in the stock appreciation right in shares of our common stock, the recipient will recognize ordinary compensation income equal to the fair market value of the stock received upon exercise of the stock appreciation right. If the recipient may receive the appreciation inherent in the stock appreciation right in cash or other property and the stock appreciation right has been structured to conform with the requirements of Section 409A of the Code, then the cash will be taxable as ordinary compensation income to the recipient at the time that the cash is received.
Subject to the requirement of reasonableness, the provisions of Section 162(m) of the Code, and the satisfaction of a tax reporting obligation, we will generally be entitled to a tax deduction equal to the taxable ordinary income realized by the recipient of the stock appreciation right.
Restricted Stock Units
Generally, the recipient of a restricted stock unit that is structured to conform to the requirements of Section 409A of the Code or an exception to Section 409A of the Code will recognize ordinary compensation income at the time the stock is received equal to the excess, if any, publicly held corporationof the fair market value of the shares of our common stock received over any amount paid by the recipient in exchange for compensationsuch shares. To conform to the requirements of Section 409A of the Code, the shares of our common stock subject to a restricted stock unit award may generally be delivered only upon one of the following events: a fixed calendar date (or dates), separation from service, death, disability or a change in control. If delivery occurs on another date, unless the restricted stock units otherwise comply with or qualify for an exception to the requirements of Section 409A of the Code, in addition to the tax treatment described above, the recipient will owe an additional 20% federal tax plus interest on any taxes owed.
The recipient’s tax basis for the determination of gain or loss upon the subsequent disposition of shares acquired from restricted stock units, will be the amount paid for such shares plus any ordinary income recognized when the stock is received.
Subject to certainthe requirement of reasonableness, the provisions of Section 162(m) of the Code and the satisfaction of a tax reporting obligation, we will generally be entitled to a tax deduction equal to the taxable ordinary income realized by the recipient of the stock award.
Section 162 Limitations
Compensation of persons who are our “covered employees” in a taxable yearis subject to the extent that compensation to such covered employee exceeds $1 million. It is possible that compensation attributable to awards, when combined with all other typestax deduction limits of compensation received by a covered employee from us, may cause this limitation to be exceeded in any particular year.
Certain kinds of compensation, including qualified “performance-based compensation,” are disregarded for purposesSection 162(m) of the Code. Awards that qualify as “performance-based compensation” are exempt from Section 162(m), thereby permitting us to claim the full federal tax deduction limitation. In accordance with Treasury Regulations issued underotherwise allowed for such compensation. The 2010 Plan is intended to enable the Board or the Compensation Committee to make awards, including cash performance awards, that will be exempt from the deduction limits of Section 162(m). Under Section 162(m), compensation attributable to stock options and stock appreciation rights will qualify as performance-based compensation if the award is granted(a) such awards are approved by a compensation committee comprisecomposed solely of “outside directors” and either (i)directors,” (b) the plan contains a per-employee limitation on the number of shares for which such awards may be granted during a specified period, (c) the per-employee limitation is approved by the stockholders,shareholders, and (d) the exercise or strike price of the award is no less than the fair market value of the stock on the date of grant, orgrant. Compensation attributable to restricted stock awards, restricted stock unit awards, performance awards and other stock-based awards will qualify as performance-based compensation, provided that (i) the award is approved by a compensation committee composed solely of “outside directors,” (ii) the award is granted, (or exercisable)becomes vested or is settled, as applicable, only upon the achievement (as certified in writing by the compensation committee) of an objective performance goal established in writing by the compensation committee while the
60
outcome is substantially uncertain, (iii) a committee of outside directors certifies in writing prior to the granting (or vesting or settlement) of the award that the performance goal has been satisfied, and (iv) prior to the granting (or vesting or settlement) of the award, the shareholders have approved the material terms of the award (including the class of employees eligible for such award, the business criteria on which the performance goal is based, and the award is approved by stockholders.maximum amount, or formula used to calculate the maximum amount, payable upon attainment of the performance goal).
6061
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a summary of transactions, and series of related transactions, since January 1, 20072008 to which we have been or will be a participant, in which the amount involved exceeded or will exceed one percent of the average of our total assets at year end for the last two completed fiscal years and in which any of our executive officers, directors or beneficial holders of more than five percent of our capital stock had or will have a direct or indirect material interest, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest, other than executive and director compensation arrangements, including the employment, termination of employment and change of control arrangements, which are described in the section of this prospectus entitled “Executive Compensation.”
Since January 1, 2007,2008, we have entered into foursix financing transactions within which Sigma-Tau and its affiliates have participated, as described below. As described above, Mauro Bove, one of our directors, is an officer of Sigma-Tau. Each of these transactions was approved by our Board of Directors and our audit committee, following disclosure of Mr. Bove’s potential interests in these transactions.
On February 29, 2008, we issued 2,500,000 shares of common stock to each of Chaumiere-Consultadoria e Servicos SDC Unipessoal LDA, or Chaumiere, which iswas an indirect wholly-owned subsidiary of an entity owned by Paolo Cavazza and members of his family, who own 38% of Sigma-Tau, and Inverlochy-Consultadoria e Servicos (S.U.) LDA, or Inverlochy, an entity wholly owned by Claudio Cavazza, who directly and indirectly owns 57% of Sigma-Tau, at a purchase price of $1.00 per share in a private placement. The purchase agreements provideprovided that the purchasers may not transfer the shares through December 31, 2010, except for transfers to affiliates, that we, rather than the purchasers, havehad all voting rights in respect of the shares until December 31, 2010, and that we had the right to repurchase the shares at a price of $2.00 per share until December 31, 2009, which right has expired, and that we have the rightup to repurchase the shares at a price of $2.50 per share until December 31, 2010.2010, which right has expired. We also issued warrants to each of Chaumiere and Inverlochy to purchase 500,000 shares of our common stock at an exercise price of $1.60 per share. The warrants have fully vested in full as of December 31, 2009 and arewere originally exercisable until December 31, 2010.2010; however, as described below, we have recently agreed to amend these warrants to extend their expiration dates to December 31, 2011.
On December 10, 2008, we issued 1,034,482 shares of common stock to each of Chaumiere and Inverlochy at a purchase price of $1.45 per share in a private placement. The purchase agreements provide that the purchasers may not transfer the shares through December 31, 2011, except for transfers to affiliates and that we, rather than the purchasers, have all voting rights in respect of the shares until December 31, 2011. We also issued warrants to each of Chaumiere and Inverlochy to purchase 372,552 shares of our common stock at an exercise price of $1.74 per share. The warrants were vested in full upon issuance and are exercisable until December 31, 2011.
On April 30, 2009, we issued 1,052,631 shares of common stock to Chaumiere at a purchase price of $0.57 per share in a private placement. The purchase agreement provides that Chaumiere may not transfer the shares through April 30, 2012 except for transfers to affiliates and that we, rather than Chaumiere, have all voting rights in respect to the shares through April 30, 2012. We also issued a warrant to Chaumiere to purchase 263,158 shares of our common stock at an exercise price of $0.91 per share. The warrant was fully vested upon issuance and is exercisable until April 30, 2012.
On October 15, 2009, we issued 1,219,512 shares of common stock to Chaumiere at a purchase price of $0.82 per share in a private placement. The purchase agreement provides that Chaumiere may not transfer the shares through September 30, 2012 except for transfers to affiliates and that we, rather than Chaumiere, have all voting rights in respect to the shares through September 30, 2012. We also issued a warrant to Chaumiere to purchase 609,756 shares of our common stock at an exercise price of $1.12 per share. The warrant was fully vested upon issuance and is exercisable until September 30, 2014.
On May 21, 2010, we completed a public offering of units, consisting of shares of our common stock and warrants to purchase common stock. Sinaf S.A., which participated in the offering, is a direct wholly-owned subsidiary of Aptafin S.p.A., or Aptafin. Aptafin is owned directly by Paolo Cavazza and members of his family. Sinaf purchased 240,000 units, consisting of 240,000 shares of common stock and warrants to purchase 96,000 shares of our common stock, for a purchase price of $0.41 per unit, in the public offering on the same terms and conditions as other investors participating in the public offering.
On June 29, 2010, Inverlochy merged with and into Taufin International S.A., or Taufin. Taufin is a direct wholly-owned subsidiary of Taufin SPA. Taufin SPA is owned directly by Claudio Cavazza. As a result of the merger, Taufin became the direct beneficial owner of the warrants and shares of common stock owned by Inverlochy immediately prior to the merger and Chaumiere merged with and into Sinaf, and Sinaf thereby became the direct beneficial owner of the warrants and shares of Common Stock owned by Chaumiere immediately prior to the merger.
62
On January 7, 2011, we issued 925,926 shares of common stock to Defiante, 1,296,296 shares to Taufin and 1,296,297 shares to Sinaf, all at a purchase price of $0.27 per share in a private placement. We also issued warrants to each of Defiante, Taufin and Sinaf to purchase 370,370 shares, 518,518 shares and 518,519 shares of our common stock, respectively, at an exercise price of $0.38 per share. The warrants will be exercisable on July 7, 2011 and thereafter until January 7, 2016. We also entered into an agreement with Defiante, Taufin and Sinaf to amend the terms of certain warrants held by them. Under the warrant amendment, all outstanding warrants held by Defiante, Taufin and Sinaf that were issued between March 2006 and December 2008, exercisable for an aggregate of 3,046,453 shares of common stock and with exercise prices between $1.60 per share and $4.06 per share, were amended to reduce their exercise prices to $0.38 per share and to extend their expiration dates to December 31, 2011.
Indemnification of Officers and Directors
Our restated certificate of incorporation limits the personal liability of directors for breach of fiduciary duty to the maximum extent permitted by the General Corporation Law of the State of Delaware, referred to herein as the DGCL. Our restated certificate of incorporation provides that no director will have personal
61
liability to us or to our stockholders for monetary damages for breach of fiduciary duty as a director. However, these provisions do not eliminate or limit the liability of any of our directors for any of the following:
| | |
| | • | | any breach of their duty of loyalty to us or our stockholders; |
| |
| | • | | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| |
| | • | | voting or assenting to unlawful payments of dividends or other distributions; or |
| |
| | • | | any transaction from which the director derived an improper personal benefit. |
Any amendment to or repeal of these provisions will not eliminate or reduce the effect of these provisions in respect of any act or failure to act, or any cause of action, suit or claim that would accrue or arise prior to any amendment or repeal or adoption of an inconsistent provision. If the DGCL is amended to provide for further limitations on the personal liability of directors of corporations, then the personal liability of our directors will be further limited in accordance with the DGCL.
Section 145 of the DGCL provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. Our amended and restated bylaws include such a provision. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful.
Section 145 of the DGCL also provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. Our amended and restated bylaws contain such a provision. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses that such officer or director has actually and reasonably incurred.
Expenses incurred by any indemnitee in defending or investigating a threatened or pending action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined that such indemnitee is not entitled to be indemnified by us. No advance will be made by us if a determination is reasonably and promptly made by our board of directors by a majority vote of a quorum of disinterested directors, or if such a quorum is not obtainable or, even if obtainable, a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, that, based upon the facts known to the board or counsel at the time such determination is made, such person did not meet the applicable standard of conduct in order
63
to be indemnified.
At present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
6264
PRINCIPAL STOCKHOLDERS
The following table sets forth the beneficial ownership of our common stock as of April 28, 2010 and as adjusted to reflect the sale of shares in this offeringJanuary 7, 2011 by:
| | |
| | • | | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock; |
| |
| | • | | each of our named executive officers; |
| |
| | • | | each of our directors; and |
| |
| | • | | all of our executive officers and directors as a group. |
The percentage ownership information shown in the table is based upon 60,406,82879,860,282 shares of common stock outstanding as of April 28, 2010 and 71,906,828 shares outstanding following the offering.
January 7, 2011.
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable on or before June 27, 2010,March 8, 2011, which is 60 days after April 28, 2010.January 7, 2011. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for persons listed in the table isc/o RegeneRx Biopharmaceuticals, Inc., 15245 Shady Grove Road, Suite 470, Rockville, Maryland 20850.
| | | | | | | | | |
| | | Number of | | | Percentage of | |
| | | Shares | | | Shares | |
| | | Beneficially | | | Beneficially | |
| Name of Beneficial Owner | | Owned | | | Owned | |
5% Stockholders: | | | | | | | | |
| Entities affiliated with Sigma-Tau Finanziaria, S.p.A. Via Sudafrica, 20, Rome, Italy 00144 | | | 33,997,378 | (1) | | | 40.5 | % |
Named Executive Officers and Directors: | | | | | | | | |
| J.J. Finkelstein | | | 2,330,886 | (2) | | | 2.9 | % |
| Allan L. Goldstein | | | 1,921,288 | (3) | | | 2.4 | % |
| R. Don Elsey | | | — | | | | * | |
| Joseph C. McNay | | | 1,544,635 | (4) | | | 1.9 | % |
| Mauro Bove | | | 204,655 | (5) | | | * | |
| L. Thompson Bowles | | | 132,343 | (6) | | | * | |
| C. Neil Lyons | | | 386,893 | (7) | | | * | |
| David R. Crockford | | | 408,750 | (8) | | | * | |
| All current directors and executive officers as a group (8 persons) | | | 6,929,450 | (9) | | | 8.4 | % |
| | | | | | | | | | | | | |
| | | Number of
| | Percentage of Shares
|
| | | Shares
| | Beneficially Owned |
| | | Beneficially
| | Before
| | After
|
Name of Beneficial Owner | | Owned | | Offering | | Offering |
| |
5% Stockholders: | | | | | | | | | | | | |
| Entities affiliated with Sigma-Tau Finanziaria, S.p.A. Via Sudafrica, 20, Rome, Italy 00144 | | | 30,142,859 | (1) | | | 46.9 | % | | | 41.9 | % |
Named Executive Officers and Directors: | | | | | | | | | | | | |
| J.J. Finkelstein | | | 2,299,636 | (2) | | | 3.8 | % | | | 3.2 | % |
| Allan L. Goldstein | | | 2,152,538 | (3) | | | 3.5 | % | | | 3.0 | % |
| Richard J. Hindin | | | 1,175,459 | (4) | | | 1.9 | % | | | 1.6 | % |
| Joseph C. McNay | | | 1,537,135 | (5) | | | 2.5 | % | | | 2.1 | % |
| Mauro Bove | | | 197,155 | (6) | | | * | | | | * | |
| L. Thompson Bowles | | | 124,843 | (7) | | | * | | | | * | |
| C. Neil Lyons | | | 348,143 | (8) | | | * | | | | * | |
| David R. Crockford | | | 396,250 | (9) | | | * | | | | * | |
| All current directors and executive officers as a group (8 persons) | | | 8,231,159 | (10) | | | 13.0 | % | | | 11.0 | % |
| | |
| * | | Represents beneficial ownership of less than 1%. |
65
| | |
| (1) | | Consists of 984,615 shares of common stock held of record held by Sigma-Tau Finanziaria, S.p.A. (“Sigma-Tau”); 12,011,18512,937,111 shares of common stock held of record and 589,481 shares of common stock issuable upon exercise of warrants held by Defiante Farmaceutica S.A. (“Defiante”), a subsidiary of Sigma-Tau, that are exercisable within 60 days of April 28, 2010; 5,052,582January 7, 2011; 6,348,878 shares of common stock held of record and 1,228,486 shares of common stock issuable upon exercise of warrants held by Inverlochy-Consultadoria e Servicos (S.U.) LDATaufin International S.A. (“Inverlochy”Taufin”), an entity wholly owned by Taufin SPA, which is owned directly by Claudio Cavazza, who |
63
| | |
| | directly and indirectly owns 57% of Sigma-Tau, that are exercisable within 60 days of April 28, 2010;January 7, 2011; and 8,175,1109,711,407 shares of common stock held of record and 2,101,4002,197,400 shares of common stock issuable upon exercise of warrants held by Chaumiere-Consultadoria e Servicos SDC Unipessoal LDASinaf S.A. (“Chaumiere”Sinaf”), an indirect wholly-owned subsidiary of Aptafin S.p.A., which is owned by Paolo Cavazza and members of his family, that are exercisable within 60 days of April 28, 2010.January 7, 2011. Paolo Cavazza directly and indirectly owns 38% of Sigma-Tau. The number of shares beneficially owned and the percentage of shares beneficially owned after the offering do not include any securities that affiliates of Sigma-Tau may purchase in this offering. |
| | |
| (2) | | Consists of 1,377,638 shares of common stock held of record by Mr. Finkelstein and 51,000 shares of common stock held of record by Mr. Finkelstein’s daughter over which Mr. Finkelstein shares voting and dispositive power. Also includes 870,998902,248 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010.January 7, 2011. |
| | |
| (3) | | Consists of 1,586,8461,336,846 shares of common stock held of record by Dr. Goldstein and 565,692584,442 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010.January 7, 2011. |
| | |
| (4) | | Consists of 967,710 shares of common stock held of record by Mr. Hindin and 207,749 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010. |
| | |
(5) | | Consists of 1,339,111 shares of common stock held of record by Mr. McNay and 198,024205,524 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010. |
| | January 7, 2011. |
(6) |
| (5) | | Consists of shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010.January 7, 2011. Mr. Bove is an officer of Sigma-Tau, but he has no beneficial ownership over the reported securities as he has no voting or dispositive power with respect to the securities held by Sigma-Tau and its affiliates described in Note 21 above. |
| | |
(7)(6) | | Consists of shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010. |
| | January 7, 2011. |
(8) |
| (7) | | Consists of 10,00030,000 shares of common stock held of record by Mr. Lyons and 338,143356,893 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010. |
| | January 7, 2011. |
(9) |
| (8) | | Consists of shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010. |
| | January 7, 2011. |
(10) |
| (9) | | Consists of 5,332,3054,134,595 shares of common stock held of record and 2,898,8542,794,855 shares of common stock issuable upon exercise of options exercisable within 60 days of April 28, 2010.January 7, 2011. |
6466
THE LINCOLN PARK TRANSACTION
General
On January 4, 2011, we entered into the Purchase Agreement, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Lincoln Park is committed to purchase up to an aggregate of $11,000,000 of our shares of common stock over the term of the Purchase Agreement.
As of January 7, 2011, there were 79,860,282 shares outstanding, of which 45,743,676 shares were held by non-affiliates (including the 958,333 shares already issued to Lincoln Park under the Purchase Agreement and 1,851,852 shares issued to Lincoln Park outside of the Purchase Agreement). If all of the 15,000,000 shares offered by Lincoln Park were issued and outstanding as of the date hereof, such shares would represent 15.8% of the total common stock outstanding, or 24.7% of the non-affiliates’ shares outstanding. The number of shares ultimately offered for sale by Lincoln Park is dependent upon the number of shares that we sell to Lincoln Park under the Purchase Agreement.
Pursuant to the Purchase Agreement, we filed a registration statement, of which this prospectus is a part, with regard to the sale by Lincoln Park of the common stock issuable under the Purchase Agreement. We do not have the right to commence any sales of our shares to Lincoln Park until the SEC has declared the registration statement effective. Thereafter, over approximately 30 months, we have the right but not the obligation to direct Lincoln Park to purchase up to 200,000 shares of common stock every third business day at a purchase price calculated by reference to the prevailing market price of our common stock without any fixed discount, subject to the floor price of $0.15 per share. There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of any sales of our common stock to Lincoln Park. Lincoln Park has no right to require any sales by us, but is obligated to make purchases from us as we direct in accordance with the Purchase Agreement. We can also accelerate the amount of common stock to be purchased under certain circumstances. There are no limitations on use of proceeds, financial or business covenants, restrictions on future funding, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. The Purchase Agreement may be terminated by us at any time, at our discretion, without any penalty or cost to us.
Purchase of Shares Under The Purchase Agreement
Under the Purchase Agreement, on any trading day selected by us, we may sell to Lincoln Park up to 200,000 shares of our common stock by delivering a purchase notice to Lincoln Park. The Purchase Price of such shares is equal to the lesser of: (i) the lowest sale price of our common stock on the purchase date; or (ii) the arithmetic average of the three lowest closing sale prices for our common stock during the twelve consecutive trading days ending on the trading day immediately preceding the purchase date. In addition, we may accelerate the amounts of purchases by an additional 200,000 shares of our common stock in the event the closing price of our stock is not below $0.35 per share on such day. The Purchase Price of such accelerated amounts will be the lesser of (i) the lowest sale price of our common stock on the purchase date and (ii) the lowest purchase price (as described above) during the previous 10 business days prior to the purchase date. The Purchase Price will be adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the trading day(s) used to compute the Purchase Price.
In consideration for entering into the Purchase Agreement, we issued to Lincoln Park 958,333 restricted shares of common stock (not included in this offering) as initial commitment shares and are required to issue up to 958,333 shares of common stock (included in this offering) as additional commitment shares on a pro rata basis as we require Lincoln Park to purchase shares under the Purchase Agreement over the term.
If we elect to issue more than the 15,000,000 shares offered under this prospectus, which we have the right but not the obligation to do, we must first register under the Securities Act any additional shares we may elect to sell to Lincoln Park before we can sell such additional shares, which could cause substantial dilution to our stockholders.
Minimum Purchase Price
Under the Purchase Agreement, we have set a floor price of $0.15 per share. Lincoln Park will not have the right nor the obligation to purchase any shares of our common stock in the event that the purchase price per share would be less than the floor price.
Events of Default
The Purchase Agreement contains a number of events constituting an “Event of Default,” including:
67
| • | | while any registration statement is required to be maintained effective pursuant to the terms of the registration rights agreement between us and Lincoln Park, the effectiveness of such registration statement lapses for any reason (including, without limitation, the issuance of a stop order) or is unavailable for sale of our shares of common stock in accordance with the terms of the registration rights agreement, and such lapse or unavailability continues for a period of twenty consecutive business days or for more than an aggregate of sixty business days in any 365-day period; |
|
| • | | the suspension from trading or failure of our common stock to be listed on our principal market for a period of three consecutive business days; |
|
| • | | the delisting of our common stock from our principal market, provided our common stock is not immediately thereafter trading on the OTC Bulletin Board, the NASDAQ Global Market, the NASDAQ Global Select Market, the New York Stock Exchange or the NASDAQ Capital Market; |
|
| • | | our transfer agent’s failure to issue to Lincoln Park shares of our common stock which Lincoln Park is entitled to receive under the Purchase Agreement within five business days after an applicable purchase date; |
|
| • | | any breach by us of the representations or warranties or covenants contained in the Purchase Agreement or any related agreements which could have a material adverse effect on us subject to a cure period of five business days; |
|
| • | | if we become insolvent or are generally unable to pay our debts as they become due; or |
|
| • | | any participation or threatened participation in insolvency or bankruptcy proceedings by or against us. |
Our Termination Rights
The Purchase Agreement may be terminated by us at any time, at our discretion, without any cost to us.
No Short-Selling or Hedging by Lincoln Park
Lincoln Park has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of our common stock during any time prior to the termination of the Purchase Agreement.
Effect of Performance of the Purchase Agreement on Our Stockholders
All 15,000,000 shares of common stock that are registered in this offering which may be sold by us to Lincoln Park under the Purchase Agreement are expected to be freely tradable. It is anticipated that shares registered in this offering will be sold over a period of up to 30 months from the date of this prospectus. The sale by Lincoln Park of a significant amount of shares registered in this offering at any given time could cause the market price of our common stock to decline and to be highly volatile. Lincoln Park may ultimately acquire all, some or none of the shares of common stock not yet issued but registered in this offering. After it has acquired such shares, it may sell all, some or none of such shares. Therefore, sales to Lincoln Park by us under the Purchase Agreement may result in substantial dilution to the interests of other holders of our common stock. However, we have the right to control the timing and amount of any sales of our shares to Lincoln Park and the Purchase Agreement may be terminated by us at any time at our discretion without any cost to us.
In connection with entering into the Purchase Agreement, we authorized the sale to Lincoln Park of up to $11,000,000 of our common stock exclusive of the 958,333 commitment shares issued, but not included in this offering, and the 958,333 additional commitment shares that may be issued and are part of this offering. The number of shares ultimately offered for sale by Lincoln Park under this prospectus is dependent upon the number of shares purchased by Lincoln Park under the Purchase Agreement. In the event we elect to issue more than the 15,000,000 shares offered hereby, we will be required to file a new registration statement and have it declared effective by the SEC. The following table sets forth the amount of proceeds we would receive from Lincoln Park from the sale of shares at varying purchase prices:
68
| | | | | | | | |
| | | | | | | Additional Proceeds from the Sale | |
| | | | | | | of | |
| | | | | Percentage of Outstanding | | Registered Shares | |
| Assumed Average | | Number of Registered | | Shares After Giving Effect to | | to Lincoln Park Under the | |
| Purchase Price | | Shares to be Issued if | | the | | Purchase Agreement | |
| ($) | | Full Purchase (1)(2) | | Issuance to Lincoln Park(3) | | ($) | |
| $0.15(4) | | 15,000,000 | | 15.8 | % | 2,220,976 | |
| $0.22(5) | | 15,000,000 | | 15.8 | % | 3,237,940 | |
| $0.50 | | 15,000,000 | | 15.8 | % | 7,186,933 | |
| $1.00 | | 11,958,333 | | 13.0 | % | 11,000,000 | |
| $1.50 | | 8,291,666 | | 9.4 | % | 11,000,000 | |
| | |
| (1) | | Although the Purchase Agreement provides that we may sell up to $11,000,000 of our common stock to Lincoln Park, we are only registering 15,000,000 shares to be purchased thereunder, which may or may not cover all such shares purchased by them under the Purchase Agreement, depending on the purchase price per share. As a result, we have included in this column only those shares which are registered in this offering. |
|
| (2) | | The number of registered shares to be issued includes a number of shares to be purchased at the applicable price plus the applicable number of pro rata additional commitment shares to be issued to Lincoln Park as a result of such purchase (but not the 958,333 initial commitment shares), although no additional proceeds will be attributable to such additional commitment shares. |
|
| (3) | | The denominator is based on 79,860,282 shares outstanding, and includes the 2,810,185 shares already owned by Lincoln Park not included in this offering, and the number of shares set forth in the adjacent column which includes the commitment fee issued pro rata up to the $11,000,000 of our stock if purchased by Lincoln Park. The numerator is based on the number of shares issuable under the Purchase Agreement at the corresponding assumed purchase price set forth in the adjacent column. |
|
| (4) | | Under the Purchase Agreement, we may not sell and Lincoln Park cannot purchase any shares in the event the price of our stock is below $0.15 per share. |
|
| (5) | | The closing price of our common stock on January 28, 2011. |
69
SELLING STOCKHOLDER
The shares of common stock being offered by the selling stockholder are those to be issued to Lincoln Park under the Purchase Agreement. We are registering the shares of common stock in order to permit Lincoln Park to offer the shares for resale from time to time. Lincoln Park is not a licensed broker-dealer or an affiliate of a licensed broker-dealer. Neither Lincoln Park nor any of its affiliates has held a position or office, or had any other material relationship, with us within the past three years.
We do not know when or in what amounts Lincoln Park may offer shares for sale. Lincoln Park may elect not to sell any or all of the shares offered by this prospectus. Because Lincoln Park may offer all or some of the shares, and because there are currently no agreements, arrangements or understandings with respect to the sale of any of the shares, we cannot estimate the number of the shares that will be held by Lincoln Park after completion of the offering. However, for purposes of this table, we have assumed that, after completion of the offering, all of the shares covered by this prospectus will be sold by Lincoln Park.
The following table presents information regarding Lincoln Park. The information concerning beneficial ownership has been taken from our stock transfer records and information provided by Lincoln Park. Beneficial ownership has been calculated in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares of common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable within 60 days. The warrants held by Lincoln Park are not exercisable within 60 days of the date of this prospectus and, therefore, the shares issuable upon exercise of those warrants have not been included in the beneficial ownership calculations.
| | | | | | | | | | | | | | | |
| | | | | | | | | | | Shares to be | | | |
| | | | | | | | | | | Sold | | | |
| | | | | | | | | | | in the Offering | | | |
| | | | | | | | | | | Assuming | | |
| | | | | | | Percentage of | | RegeneRx Issues | | Percentage of | |
| | | Shares Beneficially | | Outstanding Shares | | | Maximum No. | | Outstanding Shares | |
| | | Owned Before | | | Beneficially Owned | | | of Shares | | Beneficially Owned | |
| Selling Stockholder | | Offering | | | Before Offering | | | in the Offering | | After Offering | |
Lincoln Park Capital Fund, LLC(1) | | | 2,810,185 | | | | 3.5 | % | | 15,000,000 | (3) | | 3.0 | % |
| | |
| (1) | | Josh Scheinfeld and Jonathan Cope, the principals of Lincoln Park, are deemed to be beneficial owners of all of the shares of common stock owned by Lincoln Park. Messrs. Scheinfeld and Cope have shared voting and disposition power over the shares being offered under this prospectus. |
|
| (2) | | 2,810,185 shares of our common stock are already owned by Lincoln Park, which shares include (i) 958,333 shares of restricted common stock and (ii) 1,851,852 shares acquired by Lincoln Park in connection with the January 2011 registered direct offering of our securities. We may at our discretion elect to issue to Lincoln Park up to an additional 15,000,000 shares of our common stock under the Purchase Agreement, subject to the terms and provisions of such agreement, but Lincoln Park does not beneficially own any such shares that may be issued by us at our sole discretion and such shares are not included in determining the percentage of shares beneficially owned before the offering. |
|
| (3) | | Although the Purchase Agreement provides what we may sell up to $11,000,000 of our common stock to Lincoln Park, we are only registering 15,000,000 shares issuable under the Purchase Agreement on this registration statement. If we elect to issue more than the 15,000,000 shares offered by this prospectus, which we have the right but not the obligation to do, we must first register under the Securities Act any additional shares we may elect to sell to Lincoln Park before we can sell such additional shares. |
70
DESCRIPTION OF SECURITIES
As of the date of this prospectus, our certificate of incorporation authorizes us to issue 100,000,000200,000,000 shares of common stock, par value $0.001 per share, and 1,000,000 shares of preferred stock, par value $0.001 per share. As of March 31, 2010, 60,406,828January 7, 2011, 79,860,282 shares of common stock were outstanding and no shares of preferred stock were outstanding. Our board of directors and stockholders have approved an amendment to our restated certificate of incorporation to increase the authorized number of shares of common stock from 100,000,000 shares to 200,000,000 shares. We expect to effect this amendment to our restated certificate of incorporation following the completion of this offering.
As of March 31, 2010,January 7, 2011, we also had outstanding:
| | |
| | • | | options to purchase 4,914,1125,348,863 shares of our common stock at a weighted average exercise price of $1.53$1.37 per share; and |
| |
| | • | | warrants to purchase an aggregate of 7,933,85116,136,900 shares of our common stock at a weighted average exercise price of $2.01$0.89 per share. |
The following summary description of our capital stock is based on the provisions of our certificate of incorporation, including the certificate of designation for our Series A Participating Cumulative Preferred Stock described below, as well as our bylaws, our stockholder rights plan and the applicable provisions of the Delaware General Corporation Law. This information is qualified entirely by reference to the applicable provisions of our restated certificate of incorporation, as amended to date, our bylaws, as amended to date, our stockholder rights plan and the Delaware General Corporation Law. For information on how to obtain copies of our certificate of incorporation, bylaws and stockholder rights plan, which are exhibits to the registration statement of which this prospectus is a part, see “Where You Can Find Additional Information.”
Units
In this offering, we are offering 11,500,000 units, consisting in the aggregate of 11,500,000 shares of common stock and warrants to purchase 5,290,000 shares of common stock. Each unit consists of one share of common stock and 0.4 tradeable warrants to purchase common stock. The units will separate immediately and the common stock and the warrants will be issued separately. There will be no market for the units. We are also issuing a warrant to purchase up to an aggregate of 805,000 shares of our common stock at an exercise price of $ per share, which is equal to 110% of the public offering price per share, to the representative of the underwriters as underwriting compensation. The terms of this warrant are summarized below under “Underwriting — Representative’s Warrants.” This prospectus also relates to the offering of shares of our common stock upon exercise, if any, of the warrant.
Common Stock
Voting Rights.Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Our certificate of incorporation and bylaws do not provide for cumulative voting rights. Because of this, the holders of a majority of the shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends.Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation.In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences.Holders of common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to the common stock. The rights,
65
preferences and privileges of the holders of common stock are subject to and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
Fully Paid and Nonassessable.All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Certain Repurchase Rights and Voting Restrictions.
On October 15, 2009, we issued 1,219,512 shares of common stock and a warrant to purchase an additional 609,756 shares of common stock to Sigma-Tau. The purchaser agreed to vote the shares purchased in the transaction, and any additional shares issued pursuant to the exercise of the warrant issued in the transaction, at the direction of our Board until September 30, 2012.
On April 30, 2009, we issued 1,052,631 shares of common stock and a warrant to purchase an additional 263,158 shares of common stock to Sigma-Tau. The purchaser agreed to vote the shares purchased in the transaction, and any additional shares issued pursuant to the exercise of the warrant issued in the transaction, at the direction of our Board until April 30, 2012.
On December 10, 2008, we issued an aggregate of 2,068,964 shares of common stock and warrants to purchase an additional 745,104 shares of common stock to Sigma-Tau. The purchasers agreed to vote the shares purchased in the
71
transaction, and any additional shares issued pursuant to the exercise of the warrants issued in the transaction, at the direction of our Board until December 31, 2011.
On February 29, 2008, we issued an aggregate of 5,000,000 shares of common stock and warrants to purchase an additional 1,000,000 shares of common stock to Sigma-Tau. We may, in our sole discretion, repurchase the shares issued in the transaction at any time until December 31, 2010 for $2.50 per share. In addition, the purchasers agreed to vote the shares purchased in the transaction, and any additional shares issued pursuant to the exercise of the warrants issued in the transaction, at the direction of our Board until December 31, 2010.
On June 23, 2005, we issued an aggregate of 1,538,461 shares of common stock to Sigma-Tau. The purchasers agreed to assign the right to vote the shares issued in the transaction to us until June 23, 2010. At the end of this period, we, in our sole discretion, may repurchase for $5.00 per share the number of shares required to reduce the aggregate equity ownership of the purchasers to 30.1% of our outstanding common stock.
Warrants to Be Issued as Part of the UnitsPreferred Stock
The material terms and provisions of the warrants being offered pursuant to this prospectus are summarized below. This summary is subject to, and qualified in its entirety by, the form of warrant agreement and warrant certificate included as exhibits to the registration statement filed with the SEC of which this prospectus is a part. You should review copies of these items for a complete description of the terms and conditions applicable to the warrants.
Each whole warrant entitles the registered holder to purchase one share of our common stock at a price equal to $ , which represents 110% of the closing bid price per share of our common stock on the date of this prospectus. The warrants may only be exercised for cash. The warrants may be exercised beginning 30 days after original issuance and will expire five years from the date of issuance at 5:00 p.m., New York City time.
We may call the warrants for redemption as follows:
| | |
| • | at a price of $0.01 per share for each warrant at any time while the warrants are exercisable, so long as a registration statement relating to the common stock issuable upon exercise of the warrants is effective and current; |
|
| • | upon not less than 30 days’ prior written notice of redemption to each warrant holder; and |
66
| | |
| • | if, and only if, the last reported sale price of the common stock equals or exceeds $ per share, which is equal to 350% of the closing bid price of our common stock on the date of the prospectus, for any 20 trading days within a period of 30 consecutive trading days. |
If the foregoing conditions are satisfied and we call the warrants for redemption, each warrant holder will then be entitled to exercise his or her warrant prior to the date scheduled for redemption. However, there can be no assurance that the price of the common stock will exceed the call price or the warrant exercise price after the redemption call is made.
The warrants will be issued in registered form under a warrant agreement between American Stock Transfer & Trust Company, as warrant agent, and us. It is anticipated that the warrants will be quoted on the OTC Bulletin Board under the symbol “ ” promptly after the date of this prospectus.
The exercise price and number of shares of common stock issuable on exercise of the warrants may be adjusted in certain circumstances, including but not limited to in the event of a stock split, stock dividend, recapitalization, reorganization, merger or consolidation. However, the warrants will not be adjusted for the issuances of common stock or securities convertible or exercisable into common stock at a price below their respective exercise prices.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by certified check payable to us, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their warrants and received shares of common stock. After issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No warrants will be exercisable unless at the time of exercise a prospectus relating to common stock issuable upon exercise of the warrants is current and the common stock has been registered or qualified or deemed to be exempt under the securities laws of the state of residence of the holder of the warrants. Under the terms of the warrant agreement, we have agreed to meet these conditions and use our best efforts to maintain a current prospectus relating to common stock issuable upon exercise of the warrants until the expiration of the warrants. However, we cannot assure you that we will be able to do so, and if we do not maintain a current prospectus related to the common stock issuable upon exercise of the warrants, holders will be unable to exercise their warrants and we will not be required to settle any such warrant exercise. If the prospectus relating to the common stock issuable upon the exercise of the warrants is not current or if the common stock is not qualified or exempt from qualification in the jurisdictions in which the holders of the warrants reside, we will not be required to net cash settle or cash settle the warrant exercise, the warrants may have no value, the market for the warrants may be limited and the warrants may expire worthless.
Preferred Stock
Under our certificate of incorporation, our board of directors has the authority, without further action by the stockholders, to issue up to 1,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding. Our board of directors has designated 200,000 of the 1,000,000 authorized shares of preferred stock as Series A Participating Cumulative Preferred Stock, none of which shares are outstanding but which could be issued under the terms of the stockholder rights plan.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control
67
of our company and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock.
Stockholder Rights Plan.Our Board adopted a Rights Agreement, dated April 29, 1994, as amended, often referred to as a “poison pill,” as a tool to prevent an unsolicited takeover. In general, under our rights agreement, our Board has the discretion to issue certain rights to purchase our capital stock when a person acquires in excess of 25% of our outstanding common shares. These provisionsOur Board has exempted purchases by Sigma-Tau to date and purchases that may be made by Lincoln Park under the Purchase Agreement from the operation of our stockholder rights plan. Our stockholder rights plan may make it more difficult for stockholders to take corporate actions and may have the effect of delaying or preventing a change in control, even if such actions or change in control would be in your best interests.
Delaware Anti-takeover Law and Certain Provisions of Our Amended and Restated Certificate of Incorporation and Bylaws
Delaware law.We are subject to Section 203 of the Delaware General Corporation Law. Section 203 generally prohibits a public Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
| | |
| | • | | prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| |
| | • | | the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for this purpose shares owned by persons who are directors and also officers and shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| |
| | • | | on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| | |
| | • | | any merger or consolidation involving the corporation and the interested stockholder; |
| |
| | • | | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| |
| | • | | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| |
| | • | | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; and |
|
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
72
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Certificate of Incorporation and Bylaws.Provisions of our restated certificate of incorporation and amended and restated bylaws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock.
68
Among other things, our restated certificate of incorporation and amended and restated bylaws:
| | |
| | • | | permit our board of directors to issue up to 1,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in our control; |
| |
| | • | | provide that the authorized number of directors, which may not be less than three nor more than seven, may be changed only by resolution of the board of directors; |
| |
| | • | | provide that stockholders seeking to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
| |
| | • | | do not provide for cumulative voting rights, therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose; and |
| |
| | • | | provide that special meetings of our stockholders may be called only by the chairman of the board, our president or by the board of directors. |
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company. The transfer agent and registrar’s address is 6201 15th Street, Brooklyn, NY 11219.
NYSE Amex ListingOTC Bulletin Board Quotation
Our common stock is currently listedquoted on the NYSE AmexBulletin Board under the trading symbol “RGN.“RGRX.”
6973
UNDERWRITINGPLAN OF DISTRIBUTION
SubjectThe common stock offered by this prospectus is being offered by Lincoln Park, the selling stockholder.The common stock may be sold or distributed from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the terms and conditionsprevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the underwriting agreement between us and Maxim Group LLC, the sole book-running manager and sole representativecommon stock offered by this prospectus may be effected in one or more of the underwriters, each underwriter named below has severally agreed to purchase from us on a firm commitment basis the following respective number of units of common stock and warrants to purchase common stock opposite its name below, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus:methods:
| | • | | | |
Underwriter
| | Number of Unitsordinary brokers’ transactions; |
| |
Maxim Group LLC | • | | | | transactions involving cross or block trades; |
Boenning & Scattergood, Inc. | | | | |
| | • | | | through brokers, dealers, or underwriters who may act solely as agents; |
Total | | | 11,500,000 | |
| | • | | | “at the market” into an existing market for the common stock; |
The underwriting agreement provides for the purchase of a specific number of units by each of the underwriters. The underwriters’ obligations are several, which means that each underwriter is required to purchase a specified number of units, but is not responsible for the commitment of any other underwriter to purchase units.
Subject to the terms of the underwriting agreement, the underwriters have agreed to purchase all of the units offered by this prospectus (other than those covered by the over-allotment option described below) if any are purchased. Under the underwriting agreement, if an underwriter defaults in its commitment to purchase units, the commitments of non-defaulting underwriters may be increased or the underwriting agreement may be terminated, depending on the circumstances. The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the units are subject to the passing upon certain legal matters by counsel and to certain other conditions, such as confirmation of the accuracy of our representations and warranties made in the underwriting agreement about our financial condition and operations.
We have been advised by the representative of the underwriters that the underwriters propose to offer the units to the public at the public offering price set forth on the cover of this prospectus and to dealers at a price that represents a concession not in excess of $ per unit. The underwriters may allow, and these dealers may re-allow, a concession of not more than $ per unit to other dealers. After the securities are released for sale to the public, the underwriters may change the offering price and other selling terms at various times.
Commissions and Expenses
The following table summarizes the underwriting discounts and commissions we will pay to the underwriters.
| | | | | | | | | | | | |
| | • | | | Total Without
| | Total With
|
| | Fee per
| | Exercise of
| | Exercise of
|
| | Unit(2) | | Over-Allotment | | Over-Allotmentin other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents; |
| |
Public offering price | • | | $in privately negotiated transactions; or | | | | $ | | | | $ | |
| |
Underwriting discount(1) | • | | $ | | | | $ | | | | $ | | |
Proceeds, before expenses, to us | | $ | | | | $ | | | | $ | | |
| | |
(1) | | Does not include a corporate finance fee in the amount of 1%any combination of the gross proceeds payable to the representative of the underwriters for structuring the terms of the offering. No underwriting discount will be paid on units purchased by Sigma-Tau.foregoing. |
In order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the registration or qualification requirement is available and complied with.
| | |
(2) | | The fees shown do not include the warrant to purchase shares of common stock issuable to the representative of the underwriters at the closing. |
We estimate thatBrokers, dealers, underwriters, or agents participating in the total expensesdistribution of the offering, including registration, filing and listing fees, printing fees, legal and accounting expenses and transfer and warrantshares as agents may receive compensation in the form of commissions, discounts, or concessions from the selling shareholder and/or purchasers of the common stock for whom the broker-dealers may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of customary commissions.
Lincoln Park is an “underwriter” within the meaning of the Securities Act.
Neither we nor Lincoln Park can presently estimate the amount of compensation that any agent fees, and the 1% corporate finance fee payablewill receive. We know of no existing arrangements between Lincoln Park, any other stockholder, broker, dealer, underwriter, or agent relating to the representativesale or distribution of the underwriters, but excluding underwriting discounts and commissions, and not taking into accountshares offered by this prospectus. At the underwriters’ over-allotment option,time a particular offer of shares is made, a prospectus supplement, if required, will be approximately $500,000,distributed that will set forth the names of any agents, underwriters, or dealers and any compensation from the selling stockholder, and any other required information.
We will pay all of which are payable by us.
70
Over-Allotment Option
We have granted the underwriters an over-allotment option. This option, which is exercisable for upexpenses incident to 45 days after the dateregistration, offering, and sale of this prospectus, permits the underwritersshares to purchase a maximum of 1,725,000 additional units, consisting of an aggregate of 1,725,000 shares of common stock and warrants to purchase an aggregate of 690,000 shares of common stock, from us to cover over-allotments. If the underwriters exercise all or part of this option, they will purchase units covered by the option at the public offering price that appears on the cover pageother than commissions or discounts of this prospectus, less the underwriting discount. The underwriters, have severally agreed that, to the extent the over-allotment option is exercised, they will each purchase a number of additional units proportionate to the underwriter’s initial amount reflected in the foregoing table.
Representative’s Warrant
broker-dealers, or agents. We have also agreed to issue to the representative of the underwriters a warrant to purchase a number of shares of our common stock equal to an aggregate of seven percent (7%) of the shares of common stock underlying units sold in the offering (or 805,000 shares). The warrant will have an exercise price equal to $ per share, which is 110% of the public offering price. The warrant is exercisable commencing six months following the closing of this offering,indemnify Lincoln Park and will be exercisable for five years following the closing date of this offering. The warrant is not redeemable by us, and allows for “cashless” exercise. The warrant also provides for one demand registration during the five-year period following the closing of this offering and for unlimited “piggyback” registration rights with respect to the underlying shares during the five year period commencing six months after the effective date of this offering. Pursuant to the rules of the Financial Industry Regulatory Authority, Inc., or FINRA, and in particular Rule 5110(g)(1), the warrant (and underlying shares of common stock) issued to the representative of the underwriters may not be sold, transferred, assigned, pledged, or hypothecated, or the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective disposition of the securities by any person for a period of 180 days immediately following the date of delivery and payment for the units offered; provided, however, the warrant (and underlying shares) may be transferred to officers or directors of the representative of the underwriters and members of the underwriting syndicate and their affiliates as long as the warrant (and underlying shares) remain subject to the lockup.
The warrant contains anti-dilution terms that allow the underwriters to receive more shares or exercise at a lower price than originally agreed to upon at the time of the offering, provided that the public stockholders are proportionally affected by a stock split, stock dividend, or other similar event. The warrant will not provide for the underwriters to accrue cash dividends prior to the exercise or conversion of the warrant.
Lock-Up Agreements
Our executive officers, directors and certain of our stockholders have agreed to a90-day“lock-up” from the date of this prospectus of shares of our common stock that they beneficially own, including the issuance of common stock upon the exercise of currently outstanding options and options which may be issued. This means that, for a period of 90 days following the date of this prospectus, suchrelated persons may not offer, sell, pledge or otherwise dispose of these securities without the prior written consent of the representative of the underwriters. Thelock-up period described in the preceding paragraph will be extended if (1) during the last 17 days of thelock-up period we issue an earnings release or material news or a material event relating to us occurs or (2) prior to the expiration of thelock-up period we announce that we will release earnings results during the16-day period beginning on the last day of thelock-up period, in which case thelock-up period will be extended until the expiration of the18-day period beginning on the date of issuance of the earnings release or the occurrence of the material news or material event.
The representative of the underwriters has no present intention to waive or shorten thelock-up period; however, the terms of thelock-up agreements may be waived at its discretion. In determining whether to waive the terms of the lockup agreements, the representative of the underwriters may base its decision on its assessment of the relative strengths of the securities markets and companies similar to ours in general, and the trading pattern of, and demand for, our securities in general.
71
In addition, the underwriting agreement provides that we will not, for a period of 90 days following the date of this prospectus, offer, sell or distribute any of our securities, without the prior written consent of the representative of the underwriters.
Other Terms
We have advanced $50,000 to Maxim Group LLC, which represents a reasonable estimate of the actual accountable expenses Maxim Group LLC will incur in the offering. Maxim Group LLC shall only receive an accountable expense reimbursement if the offering is terminated. If the offering is consummated, Maxim Group LLC will not receive an expense reimbursement and will refund the advance to us at the closing of the offering.
We also have agreed that, upon successful completion of this offering, for a period of six (6) months from the closing of this offering, we will grant Maxim Group LLC the right of participation to act as lead managing underwriter and book runner or minimally as a co-lead manager and co-book runnerand/or co-lead placement agent with at least 50.0% of the economics; or, in the case of a three-handed deal 33.0% of the economics, for any and all future equity offerings as well as any convertible debt offerings undertaken during this period by us.
The underwriting agreement provides for indemnification between us and the underwriters against specified liabilities, including liabilities under the Securities Act,Act.
Lincoln Park and for contribution by us and the underwritersits affiliates have agreed not to payments that may be required to be made with respect to those liabilities. We have been advised that, in the opinion of the SEC, indemnification liabilities under the Securities Act is against public policy as expressed in the Securities Act, and is therefore, unenforceable.
This prospectus in electronic format may be made available on a website maintained by the representatives of the underwriters and may also be made available on a website maintained by other underwriters. The underwriters may agree to allocate a number of units to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives of the underwriters to underwriters that may make Internet distributions on the same basis as other allocations. In connection with the offering, the underwriters or syndicate members may distribute prospectuses electronically. No forms of electronic prospectus other than prospectuses that are printable as Adobe® PDF will be used in connection with this offering.
The underwriters have informed us that they do not expect to confirm sales of the securities offered by this prospectus to accounts over which they exercise discretionary authority without obtaining the specific approval of the account holder.
Stabilization
Until the distribution of the securities offered by this prospectus is completed, rules of the SEC may limit the ability of the underwriters to bid for and to purchase our common stock. As an exception to these rules, the underwriters may engage in transactions effected in accordance with Regulation M under the Securities Exchange Act of 1934 that are intended to stabilize, maintainany direct or otherwise affect the priceindirect short selling or hedging of our common stock or publicly traded warrants. The underwriters may engageduring the term of the Purchase Agreement.
We have advised Lincoln Park that while it is engaged in over-allotment sales, syndicate covering transactions, stabilizing transactions and penalty bidsa distribution of the shares included in accordancethis prospectus it is required to comply with Regulation M.
| | |
| • | Stabilizing transactions permit bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock and publicly traded warrants, so long as stabilizing bids do not exceed a specified maximum. |
|
| • | Over-allotment involves sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase, which creates a short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of common stock or warrants over-allotted by the underwriters is not greater than the number of shares of common stock or warrants that they may purchase in the over-allotment option. In a naked short position, the number of shares of common stock or warrants involved is greater than the number of shares of common stock or warrants in the over-allotment option. The underwriters may close out any |
72
| | |
| | covered short position by either exercising their over-allotment option or purchasing common stock or warrants in the open market. |
| | |
| • | Covering transactions involve the purchase of securities in the open market after the distribution has been completed in order to cover short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over-allotment option. If the underwriters sell more shares of common stock or warrants than could be covered by the over-allotment option, creating a naked short position, the position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares of common stock or publicly traded warrants in the open market after pricing that could adversely affect investors who purchase in this offering. |
|
| • | Penalty bids permit the underwriters to reclaim a selling concession from a selected dealer when the securities originally sold by the selected dealer are purchased in a stabilizing or syndicate covering transaction. |
These stabilizing transactions, covering transactionsM promulgated under the Exchange Act. With certain exceptions, Regulation M precludes the selling stockholder, any affiliated purchasers, and penalty bids may have the effect of raisingany broker-dealer or maintaining the market price of our common stock or publicly traded warrants or preventing or retarding a declineother person who participates in the market pricedistribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of our common stockthe distribution until the entire distribution is complete. Regulation M also prohibits any bids or publicly traded warrants. As a result,purchases made in order to stabilize the price of our common stock or publicly traded warrants may be higher than the price that might otherwise exista security in the open market.
Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the prices of our securities. These transactions may occur on the NYSE Amex or on any other trading market. If any of these transactions are commenced, they may be discontinued without notice at any time.
Foreign Regulatory Restrictions on Purchase of Units
We have not taken any action to permit a public offering of the units outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering of units andconnection with the distribution of the prospectus outside the United States.
European Economic Area. In relation to each Member Statethat security. All of the European Economic Area which has implementedforegoing may affect the Prospectus Directive (each, a relevant member state), with effect from and includingmarketability of the shares offered hereby this prospectus.
This offering will terminate on the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation date) an offer of securities to the public in that relevant member state prior to the publication of a prospectus in relation to the securities that have been approved by the competent authority in that relevant member state or, where appropriate, approved in another relevant member state and notified to the competent authority in that relevant member state, all in accordance with the Prospectus Directive, except that, with effect from and including the relevant implementation date, an offer of securities may be offered to the public in that relevant member state at any time:
| | |
| • | to any legal entity that is authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
|
| • | to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
|
| • | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of for any such offer; or |
|
| • | in any other circumstances which do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive. |
73
Each purchaser of securities described in this prospectus located within a relevant member state will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of Article 2(1)(e) of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to the public” in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the expression may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
Israel. The unitsshares offered by this prospectus have not been approvedsold by Lincoln Park or disapproved by the Israeli Securities Authority (ISA). The units may not be offered or sold, directly or indirectly, to the public in Israel. The ISA has not issued permits, approvals or licenses in connection with the offering of the units or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale, directly or indirectly, to the public of the units offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
United Kingdom. In the United Kingdom, the units offered by this prospectus are directed to and will only be available for purchase to a person who is an exempt person as referred to at paragraph (c) below and who warrants, represents and agrees that: (a) it has not offered or sold, will not offer or sell, any units offered by this prospectus to any person in the United Kingdom except in circumstances which do not constitute an offer to the public in the United Kingdom for the purposes of section 85 of the Financial Services and Markets Act 2000 (as amended) (“FSMA”); and (b) it has complied and will comply with all applicable provisions of FSMA and the regulations made thereunder in respect of anything done by it in relation to the units offered by this prospectus in, from or otherwise involving the United Kingdom; and (c) it is a person who falls within the exemptions to Section 21 of the FSMA as set out in The Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (“the Order”), being either an investment professional as described under Article 19 or any body corporate (which itself has or a group undertaking has a called up share capital or net assets of not less than £500,000 (if more than 20 members) or otherwise £5 million) or an unincorporated association or partnership (with net assets of not less than £5 million) or is a trustee of a high value trust or any person acting in the capacity of director, officer or employee of such entities as defined under Article 49(2)(a) to (d) of the Order, or a person to whom the invitation or inducement may otherwise lawfully be communicated or cause to be communicated. The investment activity to which this document relates will only be available to and engaged in only with exempt persons referred to above. Persons who are not investment professionals and do not have professional experience in matters relating to investments or are not an exempt person as described above, should not review nor rely or act upon this document and should return this document immediately. It should be noted that this document is not a prospectus in the United Kingdom as defined in the Prospectus Regulations 2005 and has not been approved by the Financial Services Authority or any competent authority in the United Kingdom.
Italy. This offering of the units has not been cleared by Consob, the Italian Stock Exchanges regulatory agency of public companies, pursuant to Italian securities legislation and, accordingly, no units may be offered, sold or delivered, nor may copies of this prospectus or of any other document relating toresold by Lincoln Park without restriction under Rule 144(b)(1)(i) under the units to be distributed in Italy, except (1) to professional investors (operatori qualificati); or (2) in circumstances which are exempted from the rules on solicitation of investments pursuant to Decree No. 58 and Article 33, first paragraph, of Consob Regulation No. 11971 of May 14, 1999, as amended. Any offer, sale or delivery of the securities or distribution of copies of this prospectus or any other document relating to the securities in Italy under (1) or (2) above must be (i) made by an investment firm, bank or financial intermediary permitted to conduct such activities in Italy in accordance with the Decree No. 58 and Legislative Decree No. 385 of September 1, 1993, or the Banking Act; and (ii) in compliance with Article 129 of the Banking Act and the implementing guidelines of the Bank of Italy, as amended from time to time, pursuant to which the issue or the offer of securities in Italy may need to be preceded and followed by an appropriate notice to be filed withSecurities Act.
74
the Bank of Italy depending,inter alia, on the aggregate value of the securities issued or offered in Italy and their characteristics; and (iii) in compliance with any other applicable laws and regulations.
Germany. The offering of the units is not a public offering in the Federal Republic of Germany. The units may only be acquired in accordance with the provisions of the Securities Sales Prospectus Act (Wertpapier-Verkaufsprospektgesetz), as amended, and any other applicable German law. No application has been made under German law to publicly market the securities in or out of the Federal Republic of Germany. The units are not registered or authorized for distribution under the Securities Sales Prospectus Act and accordingly may not be, and are not being, offered or advertised publicly or by public promotion. Therefore, this prospectus is strictly for private use and the offering is only being made to recipients to whom the document is personally addressed and does not constitute an offer or advertisement to the public. The units will only be available to persons who, by profession, trade or business, buy or sell securities for their own or a third party’s account.
France. The units offered by this prospectus may not be offered or sold, directly or indirectly, to the public in France. This prospectus has not been or will not be submitted to the clearance procedure of the Autorité des Marchés Financiers, or the AMF, and may not be released or distributed to the public in France. Investors in France may only purchase the securities offered by this prospectus for their own account and in accordance with articles L.411-1, L.441-2 and L.412-1 of the Code Monétaire et Financier and decreeno. 98-880 dated October 1, 1998, provided they are “qualified investors” within the meaning of said decree. Each French investor must represent in writing that it is a qualified investor within the meaning of the aforesaid decree. Any resale, directly or indirectly, to the public of the units offered by this prospectus may be effected only in compliance with the above mentioned regulations.
“Les actions offertes par ce document d’information ne peuvent pas être, directement ou indirectement, offertes ou vendues au public en France. Ce document d’information n’a pas été ou ne sera pas soumis au visa de l’Autorité des Marchés Financiers et ne peut être diffusé ou distribué au public en France. Les investisseurs en France ne peuvent acheter les actions offertes par ce document d’information que pour leur compte propre et conformément aux articles L.411-1, L.441-2 et L.412-1 du Code Monétaire et Financier et du décretno. 98-880 du 1 octobre 1998, sous réserve qu’ils soient des investisseurs qualifiés au sens du décret susvisé. Chaque investisseur doit déclarer par écrit qu’il est un investisseur qualifié au sens du décret susvisé. Toute revente, directe ou indirecte, des actions offertes par ce document d’information au public ne peut être effectuée que conformément à la réglementation susmentionnée.”
Switzerland. This prospectus may only be used by those persons to whom it has been directly handed out by the offeror or its designated distributors in connection with the offer described therein. The units are only offered to those personsand/or entities directly solicited by the offeror or its designated distributors, and are not offered to the public in Switzerland. This prospectus constitutes neither a public offer in Switzerland nor an issue prospectus in accordance with the respective Swiss legislation, in particular but not limited to Article 652A Swiss Code Obligations. Accordingly, this prospectus may not be used in connection with any other offer, whether private or public and shall in particular not be distributed to the public in Switzerland.
Norway. This prospectus has not been produced in accordance with the prospectus requirements laid down in the Norwegian Securities Trading Act 1997 as amended. This prospectus has not been approved or disapproved by, or registered with, neither the Oslo Stock Exchange nor the Norwegian Registry of Business Enterprises. This prospectus may not, either directly or indirectly be distributed to other Norwegian potential investors than the addressees without the prior consent of Vringo, Inc.
Denmark. This prospectus has not been prepared in the context of a public offering of securities in Denmark within the meaning of the Danish Securities Trading Act No. 171 of 17 March 2005 as amended from time to time or any Executive Orders issued on the basis thereof and has not been and will not be filed with or approved by or filed with the Danish Financial Supervisory Authority or any other public authorities in Denmark. The offering of units will only be made to persons pursuant to one or more of the exemptions set out in Executive Order No. 306 of 28 April 2005 on Prospectuses for Securities Admitted for Listing or Trade on a Regulated Market and on the First Public Offer of Securities exceeding EUR 2,500,000 or Executive
75
Order No. 307 of 28 April 2005 on Prospectuses for the First Public Offer of Certain Securities between EUR 100,000 and EUR 2,500,000, as applicable.
Sweden. Neither this prospectus nor the units offered hereunder have been registered with or approved by the Swedish Financial Supervisory Authority under the Swedish Financial Instruments Trading Act (1991:980) (as amended), nor will such registration or approval be sought. Accordingly, this prospectus may not be made available nor may the units offered hereunder be marketed or offered for sale in Sweden other than in circumstances which are deemed not to be an offer to the public in Sweden under the Financial Instruments Trading Act. This prospectus may not be distributed to the public in Sweden and a Swedish recipient of the prospectus may not in any way forward the prospectus to the public in Sweden.
British Virgin Islands. No shares, warrants or units of the Company shall be offered or sold, directly or indirectly, to the public or any member of the public in the British Virgin Islands.
RelationshipsLEGAL MATTERS
Certain of the underwriters or their affiliates have provided from time to time and may in the future provide investment banking, lending, financial advisory and other related services to us and our affiliates for which they have received and may continue to receive customary fees and commissions.
LEGAL MATTERS
The validity of the securities being offered by this prospectus will behas been passed upon for us by Cooley LLP, Reston, Virginia. The underwriters are being represented by Lowenstein Sandler PC, Roseland, New Jersey.
EXPERTS
Reznick Group P.C., independent registered public accounting firm, has audited our financial statements at December 31, 2009 and 2008, and for each of the two years in the period ended December 31, 2009, as set forth in their report, which includes an explanatory paragraph relating to our ability to continue as a going concern. We have included our financial statements in this prospectus and elsewhere in the registration statement of which it is a part in reliance on Reznick Group’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement onForm S-1 under the Securities Act, with respect to the securities being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to RegeneRx and the securities offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We are subject to the information reporting requirements of the Exchange Act, and we file reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website atwww.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at1-800-SEC-0330 for further information on the operation of the public reference facilities.
76
We also maintain a website atwww.regenerx.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR
SECURITIES ACT LIABILITY
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
7775
RegeneRx Biopharmaceuticals, Inc.
| | | | | |
| | | Page |
| |
| | | F-2 | |
| | | F-3 | |
| | | F-4 | |
| |
| | | F-5 | |
| | | F-6 | |
| | | F-7 | |
| | | F-18 | |
| | | F-19 | |
| | | F-20 | |
| | | F-21 | |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
RegeneRx Biopharmaceuticals, Inc.
We have audited the accompanying balance sheets of RegeneRx Biopharmaceuticals, Inc. (the “Company”) as of December 31, 2009 and 2008, and the related statements of operations, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2009. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of RegeneRx Biopharmaceuticals, Inc. as of December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1 to the financial statements, the Company has experienced negative cash flows from operations since inception and is dependent upon future financing in order to meet its planned operating activities. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ REZNICK GROUP, P.C.
Vienna, Virginia
March 31, 2010
F-2
RegeneRx Biopharmaceuticals, Inc.
| | | | | | | | | |
| | | December 31,
| | | December 31,
| |
| | | 2009 | | | 2008 | |
| |
ASSETS |
| Current assets | | | | | | | | |
| Cash and cash equivalents | | $ | 4,355,768 | | | $ | 5,655,367 | |
| Prepaid expenses and other current assets | | | 196,546 | | | | 236,477 | |
| | | | | | | | | |
| Total current assets | | | 4,552,314 | | | | 5,891,844 | |
| Property and equipment, net of accumulated depreciation of $98,171 and $81,623 | | | 8,492 | | | | 25,039 | |
| Other assets | | | 22,948 | | | | 5,693 | |
| | | | | | | | | |
| Total assets | | $ | 4,583,754 | | | $ | 5,922,576 | |
| | | | | | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 140,206 | | | $ | 70,554 | |
| Accrued expenses | | | 740,198 | | | | 1,255,358 | |
| | | | | | | | | |
| Total current liabilities | | | 880,404 | | | | 1,325,912 | |
| | | | | | | | | |
| Commitments | | | — | | | | — | |
| Stockholders’ equity | | | | | | | | |
| Preferred stock, $.001 par value per share, 1,000,000 shares authorized; no shares issued | | | — | | | | — | |
| Common stock, $.001 par value per share, 100,000,000 shares authorized; 60,406,828 and 53,622,491 issued and outstanding | | | 60,407 | | | | 53,623 | |
| Additional paid-in capital | | | 88,144,347 | | | | 82,550,585 | |
| Accumulated deficit | | | (84,501,404 | ) | | | (78,007,544 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 3,703,350 | | | | 4,596,664 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 4,583,754 | | | $ | 5,922,576 | |
| | | | | | | | | |
| | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2009 | | | 2008 | |
| ASSETS |
| Current assets | | | | | | | | |
| Cash and cash equivalents | | $ | 4,355,768 | | | $ | 5,655,367 | |
| Prepaid expenses and other current assets | | | 196,546 | | | | 236,477 | |
| | | | | | | |
| Total current assets | | | 4,552,314 | | | | 5,891,844 | |
| Property and equipment, net of accumulated depreciation of $98,171 and $81,623 | | | 8,492 | | | | 25,039 | |
| Other assets | | | 22,948 | | | | 5,693 | |
| | | | | | | |
| Total assets | | $ | 4,583,754 | | | $ | 5,922,576 | |
| | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 140,206 | | | $ | 70,554 | |
| Accrued expenses | | | 740,198 | | | | 1,255,358 | |
| | | | | | | |
| Total current liabilities | | | 880,404 | | | | 1,325,912 | |
| | | | | | | |
| Commitments | | | — | | | | — | |
| Stockholders’ equity | | | | | | | | |
| Preferred stock, $.001 par value per share, 1,000,000 shares authorized; no shares issued | | | — | | | | — | |
| Common stock, $.001 par value per share, 100,000,000 shares authorized; 60,406,828 and 53,622,491 issued and outstanding | | | 60,407 | | | | 53,623 | |
| Additional paid-in capital | | | 88,144,347 | | | | 82,550,585 | |
| Accumulated deficit | | | (84,501,404 | ) | | | (78,007,544 | ) |
| | | | | | | |
| Total stockholders’ equity | | | 3,703,350 | | | | 4,596,664 | |
| | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 4,583,754 | | | $ | 5,922,576 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-3
RegeneRx Biopharmaceuticals, Inc.
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2008 | |
| |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | |
| Operating expenses | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | |
| | | | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | |
| | | | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) |
| | | | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | |
| | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.12 | ) | | $ | (0.21 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding | | | 55,680,525 | | | | 50,967,617 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2008 | |
| Sponsored research revenue | | $ | — | | | $ | 168,412 | |
| Operating expenses | | | | | | | | |
| Research and development | | | 3,724,514 | | | | 7,149,808 | |
| General and administrative | | | 2,781,790 | | | | 3,805,346 | |
| | | | | | | |
| Total operating expenses | | | 6,506,304 | | | | 10,955,154 | |
| | | | | | | |
| Loss from operations | | | (6,506,304 | ) | | | (10,786,742 | ) |
| | | | | | | |
| Interest income | | | 12,444 | | | | 149,777 | |
| | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) |
| | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.12 | ) | | $ | (0.21 | ) |
| | | | | | | |
| Weighted average number of common shares outstanding | | | 55,680,525 | | | | 50,967,617 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-4
RegeneRx Biopharmaceuticals, Inc.
Years ended December 31, 2009 and 2008
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Accumulated
| | | | |
| | | | | | | | | | | | | | | Other
| | | Total
| |
| | | Common Stock | | | Additional
| | | Accumulated
| | | Comprehensive
| | | Stockholders’
| |
| | | Shares | | | Amount | | | Paid-in Capital | | | Deficit | | | Income/(loss) | | | Equity | |
| |
| Balance, December 31, 2007 | | | 46,553,527 | | | $ | 46,554 | | | $ | 73,513,292 | | | $ | (67,405,579 | ) | | $ | (1,543 | ) | | $ | 6,152,724 | |
| Cumulative effect of a change in accounting principle — ASC Topic 730 | | | — | | | | — | | | | — | | | | 35,000 | | | | — | | | | 35,000 | |
| Issuance of common stock, net of offering costs of $52,240 | | | 7,068,964 | | | | 7,069 | | | | 7,940,691 | | | | — | | | | — | | | | 7,947,760 | |
| Share-based compensation expense | | | — | | | | — | | | | 1,096,602 | | | | — | | | | — | | | | 1,096,602 | |
| Net loss | | | — | | | | — | | | | — | | | | (10,636,965 | ) | | | — | | | | (10,636,965 | ) |
| Unrealized gain on available for sale securities | | | — | | | | — | | | | — | | | | — | | | | 1,543 | | | | 1,543 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (10,635,422 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2008 | | | 53,622,491 | | | | 53,623 | | | | 82,550,585 | | | | (78,007,544 | ) | | $ | — | | | $ | 4,596,664 | |
| Issuance of common stock, net of offering costs of $447,933 | | | 6,784,337 | | | | 6,784 | | | | 4,845,282 | | | | — | | | | — | | | | 4,852,066 | |
| Share-based compensation expense | | | — | | | | — | | | | 748,480 | | | | — | | | | — | | | | 748,480 | |
| Net loss | | | — | | | | — | | | | — | | | | (6,493,860 | ) | | | — | | | | (6,493,860 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2009 | | | 60,406,828 | | | $ | 60,407 | | | $ | 88,144,347 | | | $ | (84,501,404 | ) | | $ | — | | | $ | 3,703,350 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Accumulated | | | | |
| | | | | | | | | | | | | | | | | | | Other | | | Total | |
| | | Common Stock | | | Additional | | | Accumulated | | | Comprehensive | | | Stockholders’ | |
| | | Shares | | | Amount | | | Paid-in Capital | | | Deficit | | | Income/(loss) | | | Equity | |
| Balance, December 31, 2007 | | | 46,553,527 | | | $ | 46,554 | | | $ | 73,513,292 | | | $ | (67,405,579 | ) | | $ | (1,543 | ) | | $ | 6,152,724 | |
| Cumulative effect of a change in accounting principle — ASC Topic 730 | | | — | | | | — | | | | — | | | | 35,000 | | | | — | | | | 35,000 | |
| Issuance of common stock, net of offering costs of $52,240 | | | 7,068,964 | | | | 7,069 | | | | 7,940,691 | | | | — | | | | — | | | | 7,947,760 | |
| Share-based compensation expense | | | — | | | | — | | | | 1,096,602 | | | | — | | | | — | | | | 1,096,602 | |
| Net loss | | | — | | | | — | | | | — | | | | (10,636,965 | ) | | | — | | | | (10,636,965 | ) |
| Unrealized gain on available for sale securities | | | — | | | | — | | | | — | | | | — | | | | 1,543 | | | | 1,543 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | (10,635,422 | ) |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2008 | | | 53,622,491 | | | | 53,623 | | | | 82,550,585 | | | | (78,007,544 | ) | | $ | — | | | $ | 4,596,664 | |
| Issuance of common stock, net of offering costs of $447,933 | | | 6,784,337 | | | | 6,784 | | | | 4,845,282 | | | | — | | | | — | | | | 4,852,066 | |
| Share-based compensation expense | | | — | | | | — | | | | 748,480 | | | | — | | | | — | | | | 748,480 | |
| Net loss | | | — | | | | — | | | | — | | | | (6,493,860 | ) | | | — | | | | (6,493,860 | ) |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2009 | | | 60,406,828 | | | $ | 60,407 | | | $ | 88,144,347 | | | $ | (84,501,404 | ) | | $ | — | | | $ | 3,703,350 | |
| | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-5
RegeneRx Biopharmaceuticals, Inc.
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2008 | |
| |
| Operating activities: | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 16,547 | | | | 19,396 | |
| Non-cash share-based compensation | | | 748,480 | | | | 1,096,602 | |
| Gain on settlement of accrued expenses | | | (100,000 | ) | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | — | | | | 26,951 | |
| Prepaid expenses and other current assets | | | 39,931 | | | | 66,767 | |
| Other assets | | | (17,255 | ) | | | — | |
| Accounts payable | | | 69,652 | | | | (203,007 | ) |
| Accrued expenses | | | (415,160 | ) | | | (940,150 | ) |
| | | | | | | | | |
| Net cash used in operating activities | | | (6,151,665 | ) | | | (10,570,406 | ) |
| | | | | | | | | |
| Investing activities: | | | | | | | | |
| Sales/maturities of short-term investments | | | — | | | | 4,581,135 | |
| | | | | | | | | |
| Net cash provided by investing activities | | | — | | | | 4,581,135 | |
| | | | | | | | | |
| Financing activities: | | | | | | | | |
| Net proceeds from issuance of common stock | | | 4,852,066 | | | | 7,947,760 | |
| | | | | | | | | |
| Net cash provided by financing activities | | | 4,852,066 | | | | 7,947,760 | |
| | | | | | | | | |
| Net (decrease) increase in cash and cash equivalents | | | (1,299,599 | ) | | | 1,958,489 | |
| | | | | | | | | |
| Cash and cash equivalents at beginning of year | | | 5,655,367 | | | | 3,696,878 | |
| | | | | | | | | |
| Cash and cash equivalents at end of year | | $ | 4,355,768 | | | $ | 5,655,367 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2008 | |
| Operating activities: | | | | | | | | |
| Net loss | | $ | (6,493,860 | ) | | $ | (10,636,965 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 16,547 | | | | 19,396 | |
| Non-cash share-based compensation | | | 748,480 | | | | 1,096,602 | |
| Gain on settlement of accrued expenses | | | (100,000 | ) | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | — | | | | 26,951 | |
| Prepaid expenses and other current assets | | | 39,931 | | | | 66,767 | |
| Other assets | | | (17,255 | ) | | | — | |
| Accounts payable | | | 69,652 | | | | (203,007 | ) |
| Accrued expenses | | | (415,160 | ) | | | (940,150 | ) |
| | | | | | | |
| Net cash used in operating activities | | | (6,151,665 | ) | | | (10,570,406 | ) |
| | | | | | | |
| Investing activities: | | | | | | | | |
| Sales/maturities of short-term investments | | | — | | | | 4,581,135 | |
| | | | | | | |
| Net cash provided by investing activities | | | — | | | | 4,581,135 | |
| | | | | | | |
| Financing activities: | | | | | | | | |
| Net proceeds from issuance of common stock | | | 4,852,066 | | | | 7,947,760 | |
| | | | | | | |
| Net cash provided by financing activities | | | 4,852,066 | | | | 7,947,760 | |
| | | | | | | |
| Net (decrease) increase in cash and cash equivalents | | | (1,299,599 | ) | | | 1,958,489 | |
| | | | | | | |
| Cash and cash equivalents at beginning of year | | | 5,655,367 | | | | 3,696,878 | |
| | | | | | | |
| Cash and cash equivalents at end of year | | $ | 4,355,768 | | | $ | 5,655,367 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-6
RegeneRx Biopharmaceuticals, Inc.
1. ORGANIZATION AND BUSINESS
| |
1. | ORGANIZATION AND BUSINESS |
Organization and Nature of Operations.RegeneRx Biopharmaceuticals, Inc. (the “Company”, “We”, “Us”, “Our”), a Delaware corporation, was incorporated in 1982. We are focused on the discovery and development of novel molecules to accelerate tissue and organ repair. Our operations are confined to one business segment: the development and marketing of product candidates based on Thymosin Beta 4 (“Tß4”), an amino acid peptide.
Management Plans to Address Operating Conditions.We have incurred net losses of $6.5 million and $10.6 million for the years ended December 31, 2009 and 2008, respectively. Since inception, and through December 31, 2009, we have an accumulated deficit of $84.5 million and we had cash and cash equivalents of $4.4 million as of December 31, 2009. Based on our operating plan, we believe that our cash and cash equivalents will fund our operations into the third quarter of 2010.
We anticipate incurring additional losses in the future as we continue to explore the potential clinical benefits of Tß4-based product candidates over multiple indications. We will need substantial additional funds in order to initiate any further preclinical studies or clinical trials, and to fund our operations beyond the third quarter of 2010. Accordingly, we will have a need for financing and are in the process of exploring various alternatives, including, without limitation, a public or private placement of our securities, debt financing or corporate collaboration and licensing arrangements or the sale of our company or certain of our intellectual property rights.
These factors raise substantial doubt about our ability to continue as a going concern. The accompanying financial statements have been prepared assuming that we will continue as a going concern. This basis of accounting contemplates the recovery of our assets and the satisfaction of our liabilities in the normal course of business.
Although we intend to continue to seek additional financing or a strategic partner, we may not be able to complete a financing or corporate transaction, either on favorable terms or at all. If we are unable to complete a financing or strategic transaction, we may not be able to continue as a going concern after our funds have been exhausted, and we could be required to significantly curtail or cease operations, file for bankruptcy or liquidate and dissolve. There can be no assurance that we will be able to obtain any sources of funding. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be forced to take any such actions.
In addition to our current operational requirements, we expect to continue to expend substantial funds to complete our planned product development efforts. Additionally, we continually refine our operating strategy and evaluate alternative clinical uses of Tß4. However, substantial additional resources will be needed before we will be able to achieve sustained profitability. Consequently, we continually evaluate alternative sources of financing such as the sharing of development costs through strategic collaboration agreements. There can be no assurance that our financing efforts will be successful, and if we are not able to obtain sufficient levels of financing, we would delay certain clinicaland/or research activities, and our financial condition would be materially and adversely affected. Even if we are able to obtain sufficient funding, other factors including competition, dependence on third parties, uncertainty regarding patents, protection of proprietary rights, manufacturing of peptides and technology obsolescence could have a significant impact on us and our operations.
To achieve profitability we must successfully conduct pre-clinical studies and clinical trials, obtain required regulatory approvals and successfully manufacture and market those pharmaceuticals we wish to commercialize. The time required to reach profitability is highly uncertain, and there can be no assurance that we will be able to achieve sustained profitability, if at all.
F-7
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
| |
2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Use of Estimates.The preparation of financial statements in conformity with accounting principles generally accepted in the United Stated of America (“U.S. GAAP”) requires management to make certain estimates and assumptions that affect the reported earnings, financial position and various disclosures. Critical accounting policies involved in applying our accounting policies are those that require management to make assumptions about matters that are highly uncertain at the time the accounting estimate was made and those for which different estimates reasonably could have been used for the current period. Critical accounting estimates are also those which are reasonably likely to change from period to period, and would have a material impact on the presentation of our financial condition, changes in financial condition or results of operations. Our most critical accounting estimates relate to accounting policies for clinical trial accruals and share-based arrangements. Management bases its estimates on historical experience and on various other assumptions that it believes are reasonable under the circumstances. Actual results could differ from these estimates.
Cash and Cash Equivalents.Cash and cash equivalents consist of cash and highly-liquid investments with original maturities of three months or less when acquired and are stated at cost that approximates their fair market value.
Concentration of Credit Risk.Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash, and cash equivalents. We limit our exposure to credit loss by placing our cash and cash equivalents with high quality financial institutions and, in accordance with our investment policy, in securities that are rated investment grade.
Property and Equipment.Property and equipment consists of office furniture and equipment, and is stated at cost and depreciated over the estimated useful lives of the assets (generally two to five years) using the straight-line method. Expenditures for maintenance and repairs which do not significantly prolong the useful lives of the assets are charged to expense as incurred. Depreciation expense was $16,547 and $19,396 for the years ended December 31, 2009 and 2008, respectively.
Impairment of Long-lived Assets.When we record long-lived assets our policy is to regularly perform reviews to determine if and when the carrying value of our long-lived assets becomes impaired. During the two years ended December 31, 2009 we did not report qualifying long-lived assets and therefore no impairment losses were recorded.
Sponsored Research Revenues.We account for non-refundable grants as “Sponsored research revenues” in the accompanying statements of operations. Revenues are recognized when the associated research has been performed and the related underlying costs are incurred.
Research and Development.Research and development (“R&D”) costs are expensed as incurred and include all of the wholly-allocable costs associated with our various clinical programs passed through to us by our outsourced vendors. Those costs include: manufacturing Tß4; formulation of Tß4 into the various product candidates; stability for both Tß4 and the various formulations; pre-clinical toxicology; safety and pharmacokinetic studies; clinical trial management; medical oversight; laboratory evaluations; statistical data analysis; regulatory compliance; quality assurance; and other related activities. R&D includes cash and non-cash compensation, employee benefits, travel and other miscellaneous costs of our internal R&D personnel, seven persons in total, who are wholly dedicated to R&D efforts. R&D also includes a pro-ration of our common infrastructure costs for office space and communications.
On January 1, 2008, pursuant to Accounting Standards Codification (“ASC”)730-20 (formerly EITF IssueNo. 07-3, “Accounting for Advance Payments for Goods or Services to Be Used in Future Research and Development Activities”), “Research and Development Costs,” we changed our accounting for non-refundable advance payments to acquire goods or pay for services that will be consumed or performed in a future period
F-8
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
in conducting research and development activities on behalf of the entity. Advance payments are recorded as an asset when the advance payments are made. Capitalized amounts are recognized as expense when the research and development activities are performed; that is, when the goods without alternative future use are acquired or the service is rendered. We determined that approximately $35,000 in qualifying transactions required capitalization as of January 1, 2008, and accordingly recognized a cumulative-effect adjustment to our accumulated deficit as of that date.
F-8
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
Cost of Preclinical Studies and Clinical Trials.We accrue estimated costs for preclinical studies based on estimates of work performed. We estimate expenses incurred for clinical trials that are in process based on patient enrollment and based on clinical data collection and management. Costs based on clinical data collection and management are recognized based on estimates of unbilled goods and services received in the reporting period. We monitor the progress of the trials and their related activities and adjust the accruals accordingly. Adjustments to accruals are charged to expense in the period in which the facts that give rise to the adjustment become known. In the event of early termination of a clinical trial, we would accrue an amount based on estimates of the remaining non-cancelable obligations associated with winding down the clinical trial.
Patent Costs.Costs related to filing and pursuing patent applications are recognized as general and administrative expenses as incurred since recoverability of such expenditures is uncertain.
Income Taxes.Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. We recognize the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company’s policy for recording interest and penalties associated with audits is that penalties and interest expense are recorded in “Income taxes” in the Company’s statements of operations.
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making that assessment. We recorded a full valuation allowance against all estimated net deferred tax assets at December 31, 2009 and 2008. We have significant net operating loss carryforwards to potentially reduce future federal and state taxable income, and research and experimentation tax credit carryforwards available to potentially offset future federal and state income taxes. Use of our net operating loss and research and experimentation credit carryforwards may be limited due to changes in our ownership as defined within Section 382 of the Internal Revenue Code.
Net Loss Per Common Share.Net loss per common share for the years ended December 31, 2009 and 2008, respectively, is based on the weighted-average number of shares of common stock outstanding during the periods. Basic and diluted loss per share are identical for all periods presented as potentially dilutive securities have been excluded from the calculation of the diluted net loss per common share because the inclusion of such securities would be antidilutive. The potentially dilutive securities include 12,847,963 shares and 9,366,590 shares in 2009 and 2008, respectively, reserved for the exercise of outstanding options and warrants.
F-9
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
Share-Based Compensation.We measure share-based compensation expense based on the grant date fair value of the awards which is then recognized over the period which service is required to be provided. We estimate the grant date fair value using the Black-Scholes option-pricing model (“Black-Scholes”). We recognized $748,480 and $1,096,602 in share-based compensation expense for the years ended December 31, 2009 and 2008, respectively.
Fair Value of Financial Instruments.The carrying amounts of our financial instruments, as reflected in the accompanying balance sheets, approximate fair value. Financial instruments consist of cash and cash equivalents, and accounts payable.
Recent Accounting Pronouncements.In February 2010, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (ASU 2010 — 09) to address potential practice issues associated with FASB ASC 855 (formerly SFAS 165), “Subsequent Events.” The ASU was effective upon issuance and eliminated the requirement for entities that file or furnish financial statements with the SEC to disclose the date through which subsequent events have been evaluated in originally issued and reissued financial statements. Other entities would continue to be required to disclose the date through which subsequent events have been evaluated; however, disclosures about the date would be required only in financial statements revised because of an error correction or retrospective application of U.S. GAAP. Our adoption of this standard changed our presentation of subsequent events when preparing our financial statements.
F-9
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
In September 2009, the FASB ratified ASU2009-13 (formerlyEITF 08-1), “Revenue Recognition” (ASC 605): Multiple-Deliverable Revenue Arrangements, the final consensus reached by the Emerging Issues Task Force that revised the authoritative guidance for revenue arrangements with multiple deliverables. The guidance addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting and how the arrangement consideration should be allocated among the separate units of accounting. The guidance will be effective for our fiscal year beginning January 1, 2011 with early adoption permitted. The guidance may be applied retrospectively or prospectively for new or materially modified arrangements. We currently do not have any multiple-deliverable revenue arrangements, accordingly, the adoption of the guidance will not have an impact on our financial statements.
In August 2009, the FASB issued ASUNo. 2009-05, “Fair Value Measurements and Disclosures (ASC 820) — Measuring Liabilities at Fair Value” (ASU2009-05). ASU2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset or the quoted prices for similar liabilities or similar liabilities when traded as assets. The guidance provided is effective for the first reporting period (including interim periods) beginning after issuance. Our adoption of ASU2009-05 did not impact our financial position or results of operations.
In June 2009, the FASB issued ASC 105 (formerly SFAS 168), “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles” (ASC 105). ASC 105 is now the source of authoritative U.S. GAAP recognized by the FASB to be applied by nongovernment entities. It also modifies the GAAP hierarchy to include only two levels of GAAP: authoritative and non-authoritative. ASC 105 is effective for financial statements issued for interim and annual periods ended after September 15, 2009. The adoption of this standard in 2009 changed how we reference various elements of U.S. GAAP when preparing our financial statement disclosures, but did not have an impact on our financial position or results of operations.
Other new pronouncements issued but not effective until after December 31, 2009 are not expected to have a significant effect on our financial position or results of operations.
Reclassifications.Certain account balances as of and for the year ended December 31, 2008 were reclassified to conform to current year presentation.
F-10
RegeneRx Biopharmaceuticals, Inc.
3. FAIR VALUE MEASUREMENTS
Notes to Financial Statements — (Continued)
| |
3. | FAIR VALUE MEASUREMENTS |
We adopted a new accounting standard that defines fair value and establishes a framework for fair value measurements effective January 1, 2008 for financial assets and liabilities and effective January 1, 2009 for non-financial assets and liabilities. This standard establishes a three-level hierarchy for fair value measurements. The hierarchy is based upon the transparency of inputs and the valuation of an asset or a liability as of the measurement date. The three levels of inputs are as follows:
| | |
| | • | | Level 1 — Quoted prices in active markets for identical assets and liabilities. |
| |
| | • | | Level 2 — Observable inputs other than quoted prices in active markets for identical assets and liabilities. |
| |
| | • | | Level 3 — Unobservable inputs. |
At December 31, 2009 and 2008, we held no qualifying liabilities, and our only qualifying assets that required measurement under the foregoing fair value hierarchy were money market funds and U.S. Treasury Bills included in Cash and Cash Equivalents valued at $4.4 million and $5.7 million, respectively, using Level 1 inputs.
F-10
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
4. LICENSES, INTELLECTUAL PROPERTY, AND RELATED PARTY TRANSACTIONS
| |
4. | LICENSES, INTELLECTUAL PROPERTY, AND RELATED PARTY TRANSACTIONS |
We have an exclusive, worldwide licensing agreement with the National Institutes of Health (“NIH”) for all claims to Tß4 within their broadly-defined patent application. In exchange for this exclusive worldwide license, we must make certain royalty and milestone payments to the NIH. Through December 31, 2009 we have complied with these requirements. No assurance can be given as to whether or when a patent will be issued, or as to any claims that may be included or excluded within the patent. We have also filed numerous additional patent applications covering various compositions, uses, formulations and other components of Tß4, as well as to novel peptides resulting from our research efforts. Some of these patents have issued, while many patent applications are still pending. Minimum annual maintenance fees for each of the years ended December 31, 2009 and 2008 were $25,000.
We have entered into a License and Supply Agreement (the “Agreement”) with Defiante Farmaceutica, S.A. (“Defiante”) a Portuguese company that is a wholly owned subsidiary of Sigma-Tau, S.p.A., an international pharmaceutical company and an affiliate of Sigma-Tau Finanziaria S.p.A., who together with its affiliates comprise our largest stockholder group (the “Sigma-Tau Group”). This Agreement grants to Defiante the exclusive right to use Tß4 to conduct research and development activities in Europe. Under the Agreement, we will receive fees and royalty payments based on a percentage of specified sales of Tß4-related products by Defiante. The term of the Agreement continues until the later of the expiration of any patents developed under the Agreement, the expiration of marketing rights, or December 31, 2016.
In furtherance of the licensed rights, Sigma-Tau Group funded and managed the RegeneRx-sponsored Phase II dermal wound healing clinical trials in venous stasis ulcers conducted in Italy and Poland that concluded in the first quarter of 2009.
F-11
RegeneRx Biopharmaceuticals, Inc.
5. COMPOSITION OF CERTAIN FINANCIAL STATEMENT CAPTIONS
Notes to Financial Statements — (Continued)
| |
5. | COMPOSITION OF CERTAIN FINANCIAL STATEMENT CAPTIONS |
Accrued expenses are comprised of the following:
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Accrued clinical research | | $ | 496,997 | | | $ | 944,283 | |
| Accrued professional fees | | | 122,590 | | | | 155,000 | |
| Accrued vacation | | | 35,300 | | | | 61,714 | |
| Accrued license fees | | | 30,000 | | | | — | |
| Accrued compensation | | | 28,995 | | | | 84,361 | |
| Other | | | 26,316 | | | | 10,000 | |
| | | | | | | |
| | | $ | 740,198 | | | $ | 1,255,358 | |
| | | | | | | |
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| |
| Accrued clinical research | | $ | 496,997 | | | $ | 944,283 | |
| Accrued professional fees | | | 122,590 | | | | 155,000 | |
| Accrued vacation | | | 35,300 | | | | 61,714 | |
| Accrued license fees | | | 30,000 | | | | — | |
| Accrued compensation | | | 28,995 | | | | 84,361 | |
| Other | | | 26,316 | | | | 10,000 | |
| | | | | | | | | |
| | | $ | 740,198 | | | $ | 1,255,358 | |
| | | | | | | | | |
6. EMPLOYEE BENEFIT PLANS
| |
6. | EMPLOYEE BENEFIT PLANS |
We have a defined contribution retirement plan that complies with Section 401(k) of the Internal Revenue Code (the “Code”). All employees of the Company are eligible to participate in the plan. The Company matches 100% of each participant’s voluntary contributions, subject to a maximum Company contribution of 4% of the participant’s compensation. The Company’s matching portion totaled $18,269 and $51,494 for the years ended December 31, 2009 and 2008, respectively. In order to conserve cash, the Company discontinued the matching contribution effective June 5, 2009 and reinstated it on March 1, 2010.
F-11
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
7. STOCKHOLDERS’ EQUITY
Shareholders Rights Plan.Our Board of Directors adopted a Rights Agreement, dated April 29, 1994, as amended, that is intended to discourage an unsolicited change in control of the Company. In general, if an entity acquires more than a 25% ownership interest in the Company without the endorsement of our Board of Directors, then our current stockholders (other than the acquiring entity) will be issued a significant number of new shares, the effect of which would dilute the ownership of the acquiring entity and could delay or prevent the change in control.
Registration Rights Agreements.In connection with the sale of certain equity instruments, we have entered into Registration Rights Agreements. Generally, these Agreements required us to file registration statements with the Securities and Exchange Commission to register common shares to permit re-sale of common shares previously sold under an exemption from registration or to register common shares that may be issued on exercise of outstanding warrants.
The Registration Rights Agreements usually require us to pay penalties for any failure or time delay in filing or maintaining the effectiveness of the required registration statements. These penalties are usually expressed as a fixed percentage, per month, of the original amount we received on issuance of the common shares, options or warrants. While to date we have not incurred any penalties under these agreements, if a penalty is determined to be probable we would recognize the amount as a contingent liability and not as a derivative instrument.
Common Stock.In February 2008, the Company sold 5,000,000 shares of its common stock at a price of $1.00 per share, raising net proceeds of $4,947,760 (the “February 2008 Private Placement”) from Sigma TauSigma-Tau Group. In connection with the February 2008 Private Placement, the Company also issued warrants to the investors. The warrants are exercisable for an aggregate of 1,000,000 shares of common stock at an exercise price of $1.60 per share. The warrants, which have a term of three years and an exercise price of $1.60 per
F-12
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
share, were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair market value of the warrants at the date of issuance was $0.3 million.
Under the terms of the February 2008 Private Placement, the Company may, in its sole discretion, repurchase the shares at any time between January 1, 2010 and December 31, 2010, for $2.50 per share. The Company’s repurchase right terminates after December 31, 2010. In addition, the investors have agreed to vote the shares, and any additional shares issued pursuant to the exercise of the warrants, as recommended by the Company’s Board of Directors until December 31, 2010.
In December 2008, the Company sold 2,068,964 shares of its common stock at a price of $1.45 per share, raising net proceeds of $3,000,000 (the “December 2008 Private Placement”) from Sigma TauSigma-Tau Group. In connection with the December 2008 Private Placement, the Company also issued warrants to the investors. The warrants are exercisable for an aggregate of 745,104 shares of common stock at an exercise price of $1.74 per share. The warrants, which have a term of three years and an exercise price of $1.74 per share, were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair market value of the warrants at the date of issuance was $0.4 million.
Under the terms of the December 2008 Private Placement, the investors have agreed to vote the shares, and any additional shares issued pursuant to the exercise of the warrants, as recommended by the Company’s Board of Directors until December 31, 2011.
On April 30, 2009 we issued 1,052,631 shares of common stock at a price of $0.57 per share, and warrants to purchase 263,158 shares of our common stock at $0.91 per share, to Sigma-Tau Group for gross proceeds of $600,000. The warrants, which have a term of three years and an exercise price of $0.91 per share, were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair market value of the warrants at the date of issuance was $0.1 million.
F-12
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
On October 5, 2009, we issued 4,512,194 shares of common stock and warrants to purchase 2,256,097 shares of our common stock in a registered direct offering to new institutional investors, for proceeds of approximately $3.3 million, net of approximately $400,000 of offering costs. The warrants, which have a term of five years and an exercise price of $1.12 per share, were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair market value of the warrants at the date of issuance was $1.0 million.
On October 15, 2009, we issued 1,219,512 shares of common stock and warrants to purchase 609,756 shares of our common stock to Sigma-Tau Group for gross proceeds of $1.0 million. The warrants, which become exercisable on April 15, 2010 and have a term through September 30, 2014, and an exercise price of $1.12 per share, were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair market value of the warrants at the date of issuance was $0.2 million.
Share-Based Compensation.We recognized $748,480 and $1,096,602 in stock-based compensation expense for the years ended December 31, 2009 and 2008, respectively. Given our current estimates of future forfeitures, we expect to recognize the compensation cost related to non-vested options as of December 31, 2009 of $723,000 over the weighted average remaining recognition period of 1.1 years.
2000 Stock Option and Incentive Plan, as amended.Our Board of Directors (the “Board”) and stockholders have approved the 2000 Stock Option and Incentive Plan under which the Board may grant options to purchase shares of our common stock. Options may only be granted to our directors, officers, employees, consultants or advisors, and no single participant can receive more than 450,000 shares in any one year. The exercise price and term of any grant are determined by the Board at the time of grant but the exercise price may not be less than the fair market value of our common stock on the date of the grant, and the term of the option shall not exceed ten years. As of December 31, 2009, there were 6,500,000 shares
F-13
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
reserved for issuance under the plan, of which 4,914,112 were outstanding and 1,550,888 were available for issuance.
The following summarizes share-based compensation expense for the years ended December 31, 2009 and 2008, which was allocated as follows:
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| Research and development | | $ | 369,814 | | | $ | 440,850 | |
| General and administrative | | | 378,666 | | | | 655,752 | |
| | | | | | | |
| | | $ | 748,480 | | | $ | 1,096,602 | |
| | | | | | | |
| | | | | | | | | |
| | | December 31, | |
| | | 2009 | | | 2008 | |
| |
| Research and development | | $ | 369,814 | | | $ | 440,850 | |
| General and administrative | | | 378,666 | | | | 655,752 | |
| | | | | | | | | |
| | | $ | 748,480 | | | $ | 1,096,602 | |
| | | | | | | | | |
F-13
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
The following summarizes stock option activity for the years ended December 31, 2009 and 2008:
| | | | | | | | | | | | | | | | | |
| | | | | | | Options Outstanding | |
| | | | | | | | | | | | | | | Weighted | |
| | | Shares | | | | | | | | | | | Average | |
| | | Available for | | | Number of | | | Exercise Price | | | Exercise | |
| | | Grant | | | Shares | | | Range | | | Price | |
| December 31, 2007 | | | 620,000 | | | | 3,545,000 | | | $ | 0.28 - $3.82 | | | $ | 1.80 | |
| Grants | | | (572,500 | ) | | | 572,500 | | | | 1.14 - 1.50 | | | | 1.23 | |
| Exercises | | | — | | | | — | | | | — | | | | — | |
| Cancellations | | | — | | | | — | | | | — | | | | — | |
| Newly authorized | | | 2,300,000 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| December 31, 2008 | | | 2,347,500 | | | | 4,117,500 | | | | 0.28 - 3.82 | | | | 1.72 | |
| Grants | | | (1,192,939 | ) | | | 1,192,939 | | | | 0.57 - 0.76 | | | | 0.64 | |
| Exercises | | | — | | | | — | | | | — | | | | — | |
| Cancellations | | | 396,327 | | | | (396,327 | ) | | | 0.57 - 2.59 | | | | 0.82 | |
| | | | | | | | | | | | | |
| December 31, 2009 | | | 1,550,888 | | | | 4,914,112 | | | $ | 0.28 - $3.82 | | | $ | 1.53 | |
| | | | | | | | | | | | | |
The following summarizes information about stock options outstanding at December 31, 2009:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Outstanding Options | | | Exercisable Options | |
| | | | | | | Weighted- | | | | | | | | | | | Weighted- | | | | |
| | | | | | | Average | | | Weighted- | | | | | | | Average | | | Weighted- | |
| | | Number of | | | Remaining | | | Average | | | Number of | | | Remaining | | | Average | |
| | | Shares | | | Contractual | | | Exercise | | | Shares | | | Contractual | | | Exercise | |
| Range of Exercise Prices | | Outstanding | | | Life (in Years) | | | Price | | | Exercisable | | | Life (in Years) | | | Price | |
| $0.28-$0.86 | | | 2,151,612 | | | | 4.5 | | | $ | 0.50 | | | | 1,724,112 | | | | 4.0 | | | $ | 0.43 | |
| $1.07-$1.93 | | | 827,500 | | | | 5.0 | | | $ | 1.31 | | | | 435,625 | | | | 4.6 | | | $ | 1.40 | |
| $2.02-$2.68 | | | 860,000 | | | | 4.3 | | | $ | 2.26 | | | | 323,750 | | | | 4.4 | | | $ | 2.31 | |
| $3.00-$3.82 | | | 1,075,000 | | | | 5.4 | | | $ | 3.19 | | | | 950,832 | | | | 5.4 | | | $ | 3.19 | |
| | | | | | | | | | | | | | | | | | | |
| | | | 4,914,112 | | | | | | | | | | | | 3,434,319 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| Intrinsic value of in-the-money options, using the December 31, 2009 closing price of $0.55 | | $ | 254,450 | | | | | | | | | | | $ | 254,450 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Outstanding Options | | | Exercisable Options | |
| | | | | | Weighted-
| | | | | | | | | Weighted-
| | | | |
| | | | | | Average
| | | Weighted-
| | | | | | Average
| | | Weighted-
| |
| | | Number of
| | | Remaining
| | | Average
| | | Number of
| | | Remaining
| | | Average
| |
| | | Shares
| | | Contractual
| | | Exercise
| | | Shares
| | | Contractual
| | | Exercise
| |
Range of Exercise Prices | | Outstanding | | | Life (in Years) | | | Price | | | Exercisable | | | Life (in Years) | | | Price | |
| |
| $0.28-$0.86 | | | 2,151,612 | | | | 4.5 | | | $ | 0.50 | | | | 1,724,112 | | | | 4.0 | | | $ | 0.43 | |
| $1.07-$1.93 | | | 827,500 | | | | 5.0 | | | $ | 1.31 | | | | 435,625 | | | | 4.6 | | | $ | 1.40 | |
| $2.02-$2.68 | | | 860,000 | | | | 4.3 | | | $ | 2.26 | | | | 323,750 | | | | 4.4 | | | $ | 2.31 | |
| $3.00-$3.82 | | | 1,075,000 | | | | 5.4 | | | $ | 3.19 | | | | 950,832 | | | | 5.4 | | | $ | 3.19 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | 4,914,112 | | | | | | | | | | | | 3,434,319 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Intrinsic value ofin-the-money options, using the December 31, 2009 closing price of $0.55 | | $ | 254,450 | | | | | | | | | | | $ | 254,450 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
F-14
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
Determining the Fair Value of Options.We use the Black-Scholes valuation model to estimate the fair value of options granted. Black-Scholes considers a number of factors, including the market price and
F-14
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
volatility of our common stock. We used the following forward-looking range of assumptions to value each stock option granted to employees, directors and consultants during the years ended December 31, 2009 and 2008:
| | | | | | |
| | | 2009 | | 2008 | | | | | | | | |
| | | 2009 | | 2008 | |
| Dividend yield | | 0.0% | | 0.0% | | | 0.0 | % | | | 0.0 | % |
| | | |
| Risk free rate of return | | 1.9 - 2.3% | | 0.8 - 3.7% | | | 1.9 - 2.3 | % | | | 0.8 - 3.7 | % |
| | | |
| Expected life in years | | 4.75 - 5.38 | | 1.00 - 4.75 | | 4.75 - 5.38 | | 1.00 - 4.75 | |
| Volatility | | 71 - 72% | | 68 - 82% | | | 71 - 72 | % | | | 68 - 82 | % |
| Forfeitures | | 2.61% | | — | | | 2.61 | % | | — | |
Our dividend yield assumption is based on the fact that we have never paid cash dividends and do not anticipate paying cash dividends in the foreseeable future. Our risk-free interest rate assumption is based on yields of U.S. Treasury notes in effect at the date of grant. Our expected life represents the period of time that options granted are expected to be outstanding and is calculated in accordance with the Securities and Exchange Commission (“SEC”) guidance provided in the SEC’s Staff Accounting Bulletin 107 (“SAB 107”), using a “simplified” method. The Company has used the simplified method and will continue to use the simplified method as it does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate an expected term. Our volatility assumption is based on reviews of the historical volatility of our common stock. We estimate forfeiture rates at the time of grant and adjust these estimates, if necessary, periodically based on the extent to which future actual forfeitures differ, or are expected to differ, from such estimates. Accordingly, we have estimated forfeiture percentages for the unvested portion of previously granted awards that remain outstanding at the date of adoption and for awards granted subsequent to the date of adoption. Forfeitures are estimated based on the demographics of current option holders and standard probabilities of employee turnover. Using Black-Scholes and these factors, the weighted average fair value of stock options granted to employees and directors was $0.39 for the year ended December 31, 2009 and $0.73 for the year ended December 31, 2008.
We do not record tax-related effects on stock-based compensation given our historical and anticipated operating experience and offsetting changes in our valuation allowance which fully reserves against potential deferred tax assets.
Warrants to Purchase Common Stock.
The following table summarizes our warrant activity for 2009 and 2008:
| | | | | | | | | | | | | |
| | | | | | | Warrants Outstanding | |
| | | | | | | | | | | Weighted | |
| | | | | | | | | | | Average | |
| | | Number of | | | Exercise Price | | | Exercise | |
| | | Shares | | | Range | | | Price | |
| December 31, 2007 | | | 3,522,544 | | | $ | 2.75 - $4.06 | | | $ | 3.26 | |
| Grants | | | 1,745,104 | | | | 1.60 - 1.74 | | | | 1.66 | |
| Exercises | | | — | | | | — | | | | — | |
| Cancellations | | | (18,558 | ) | | | 4.05 - 4.06 | | | | 4.05 | |
| | | | | | | | | | |
| December 31, 2008 | | | 5,249,090 | | | | 1.60 - 4.06 | | | | 2.80 | |
| Grants | | | 3,129,011 | | | | 0.91 - 1.12 | | | | 1.10 | |
| Exercises | | | — | | | | — | | | | — | |
| Cancellations | | | (444,250 | ) | | | 4.06 | | | | 4.06 | |
| | | | | | | | | | |
| December 31, 2009 | | | 7,933,851 | | | $ | 0.91 - $4.06 | | | $ | 2.01 | |
| | | | | | | | | | |
F-15
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
8. INCOME TAXES
Significant components of the Company’s deferred tax assets at December 31, 2009 and 2008 and related valuation reserves are presented below:
| | | | | | | | | | |
| | | December 31, | | | | | | | | | |
| | | 2009 | | 2008 | | | December 31, | |
| | | 2009 | | 2008 | |
| Deferred tax assets: | | | | | | | | | |
| Net operating loss carryforwards | | $ | 16,988,000 | | | $ | 18,370,000 | | | $ | 16,988,000 | | $ | 18,370,000 | |
| Research and development tax credit carryforward | | | 1,710,000 | | | | 1,628,000 | | | 1,710,000 | | 1,628,000 | |
| Charitable contribution carryforward | | | 37,000 | | | | 39,000 | | | 37,000 | | 39,000 | |
| Accrued vacation | | | 8,000 | | | | 12,000 | | | 8,000 | | 12,000 | |
| Accrued expenses | | | 163,000 | | | | 150,000 | | | 163,000 | | 150,000 | |
| Amortization | | | 5,000 | | | | 6,000 | | | 5,000 | | 6,000 | |
| Depreciation | | | 1,000 | | | | — | | | 1,000 | | — | |
| Stock option expense | | | 975,000 | | | | 919,000 | | | 975,000 | | 919,000 | |
| | | | | | | | | | | |
| | | | 19,887,000 | | | | 21,124,000 | | | 19,887,000 | | 21,124,000 | |
| Less — valuation allowance | | | (19,887,000 | ) | | | (21,123,000 | ) | | | (19,887,000 | ) | | | (21,123,000 | ) |
| | | | | | | | | | | |
| Net deferred tax asset | | | — | | | | 1,000 | | | — | | 1,000 | |
| Deferred tax liabilities: | | | | | | | | | |
| Depreciation | | | — | | | | (1,000 | ) | | — | | | (1,000 | ) |
| | | | | | | | | | | |
| Net deferred tax amounts | | $ | — | | | $ | — | | | $ | — | | $ | — | |
| | | | | | | | | | | |
A full valuation allowance has been provided at December 31, 2009 and 2008 to reserve for deferred tax assets, as it appears more likely than not that net deferred tax assets will not be realized.
At December 31, 2009, we had net operating loss carryforwards for income tax purposes of approximately $43.1 million, which are available to offset future federal and state taxable income, if any, and, research and development tax credit carryforwards of approximately $1.7 million. The carryforwards, if not utilized, will expire in increments through 2029.
The Code imposes substantial restrictions on the utilization of net operating losses and tax credits in the event of a corporation’s ownership change, as defined in Section 382 of the Code. During 2009, the Company completed a preliminary study to compute any limits on the net operating losses and credit carryforwards for purposes of Section 382. It was determined that the Company experienced a cumulative change in ownership, as defined by the regulations, in 2002. This change in ownership triggers an annual limitation on the Company’s ability to utilize certain U.S. federal and state net operating loss carryforwards and research tax credit carryforwards, resulting in the potential loss of approximately $9.8 million of net operating loss carryforwards and $0.2 million in research credit carryforwards. The Company has reduced the deferred tax assets associated with these carryforwards in its balance sheet at December 31, 2009 and 2008.
F-16
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
The provision for income taxes on earnings subject to income taxes differs from the statutory Federal rate at December 31, 2009 and 2008, due to the following:
| | | | | | | | | | |
| | | December 31, | | | | | | | | | |
| | | 2009 | | 2008 | | | December 31, | |
| | | 2009 | | 2008 | |
| Tax benefit at statutory rate | | $ | (2,213,000 | ) | | $ | (3,617,000 | ) | | $ | (2,213,000 | ) | | $ | (3,617,000 | ) |
| State taxes | | | (354,000 | ) | | | (579,000 | ) | | | (354,000 | ) | | | (579,000 | ) |
| Permanent M-1s | | | 339,000 | | | | 563,000 | | | 339,000 | | 563,000 | |
| Limited/expired net operating loss carryforwards | | | 3,546,000 | | | | 6,150,000 | | | 3,546,000 | | 6,150,000 | |
| Limited/expired research and development tax credit carryforward | | | 120,000 | | | | 284,000 | | | 120,000 | | 284,000 | |
| Research and development tax credit carryforward | | | (202,000 | ) | | | (504,000 | ) | | | (202,000 | ) | | | (504,000 | ) |
| Change in effective tax rate | | | — | | | | (455,000 | ) | | — | | | (455,000 | ) |
| Change in valuation allowance | | | (1,236,000 | ) | | | (1,842,000 | | | | (1,236,000 | ) | | (1,842,000 | |
| | | | | | | | | | | |
| | | $ | — | | | $ | — | | | $ | — | | $ | — | |
| | | | | | | | | | | |
As discussed in Note 2, we recognize the effect of income tax positions only if those positions more likely than not of being sustained. At December 31, 2009, and December 31, 2008 we had no gross unrecognized tax benefits. We do not expect any significant changes in unrecognized tax benefits over the next 12 months. In addition, we did not recognize any interest or penalties related to uncertain tax positions at December 31, 2009 and 2008.
9. COMMITMENTS
Lease.Our rent expense, related solely to office space, for 2009 and 2008 was $91,183 and $100,196, respectively. We are committed under an office space lease that expires on January 31, 2013 that requires the following approximate annual lease payments: $63,000, $94,000, $98,000 and $8,000 for the years ending December 31, 2010, through 2013, respectively.
Employment Continuity Agreements.We have entered into employment contracts with our executive officers which provide for severance if the executive is dismissed without cause or under certain circumstances after a change of control in our ownership. At December 31, 2009 these obligations, if triggered, could amount to a maximum of approximately $900,000 in the aggregate.
F-17
RegeneRx Biopharmaceuticals, Inc.
| | | | | | | | | |
| | | March 31,
| | | December 31,
| |
| | | 2010 | | | 2009 | |
| | | (Unaudited) | | | | |
| |
ASSETS |
| Current assets | | | | | | | | |
| Cash and cash equivalents | | $ | 3,189,990 | | | $ | 4,355,768 | |
| Prepaid expenses and other current assets | | | 225,166 | | | | 196,546 | |
| | | | | | | | | |
| Total current assets | | | 3,415,156 | | | | 4,552,314 | |
| Fixed assets, net of accumulated depreciation of $101,444 and $98,171 | | | 23,443 | | | | 8,492 | |
| Other assets | | | 17,255 | | | | 22,948 | |
| | | | | | | | | |
| Total assets | | $ | 3,455,854 | | | $ | 4,583,754 | |
| | | | | | | | | |
| |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 177,718 | | | $ | 140,206 | |
| Accrued expenses | | | 588,651 | | | | 740,198 | |
| | | | | | | | | |
| Total current liabilities | | | 766,369 | | | | 880,404 | |
| | | | | | | | | |
| Commitments | | | — | | | | — | |
| Stockholders’ equity | | | | | | | | |
| Preferred stock, $.001 par value per share, 1,000,000 authorized; no shares issued | | | — | | | | — | |
| Common stock, par value $.001 per share, 100,000,000 shares authorized; 60,406,828 issued and outstanding | | | 60,407 | | | | 60,407 | |
| Additional paid-in capital | | | 88,276,191 | | | | 88,144,347 | |
| Accumulated deficit | | | (85,647,113 | ) | | | (84,501,404 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 2,689,485 | | | | 3,703,350 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 3,455,854 | | | $ | 4,583,754 | |
| | | | | | | | | |
| | | | | | | | | |
| | | September 30, | | | December 31, | |
| | | 2010 | | | 2009 | |
| | | (Unaudited) | | | | | |
| ASSETS | | | | | | | | |
| Current assets | | | | | | | | |
| Cash and cash equivalents | | $ | 4,975,947 | | | $ | 4,355,768 | |
| Accounts receivable | | | 15,603 | | | | — | |
| Prepaid expenses and other current assets | | | 582,225 | | | | 196,546 | |
| | | | | | | |
| Total current assets | | | 5,573,775 | | | | 4,552,314 | |
| Fixed assets, net of accumulated depreciation of $105,655 and $98,171 | | | 24,640 | | | | 8,492 | |
| Other non-current assets | | | 17,255 | | | | 22,948 | |
| | | | | | | |
| Total assets | | $ | 5,615,670 | | | $ | 4,583,754 | |
| | | | | | | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities | | | | | | | | |
| Accounts payable | | $ | 605,138 | | | $ | 140,206 | |
| Accrued expenses | | | 544,732 | | | | 740,198 | |
| | | | | | | |
| Total current liabilities | | | 1,149,870 | | | | 880,404 | |
| | | | | | | |
| | | | | | | | | |
| Commitments | | | — | | | | — | |
| | | | | | | | | |
| Stockholders’ equity | | | | | | | | |
| Preferred stock, $.001 par value per share, 1,000,000 authorized; no shares issued | | | — | | | | — | |
| Common stock, par value $.001 per share, 200,000,000 shares authorized, 73,531,578 issued and outstanding as of September 30, 2010; 100,000,000 authorized, 60,406,828 issued and outstanding as of December 31, 2009 | | | 73,531 | | | | 60,407 | |
| Additional paid-in capital | | | 92,997,669 | | | | 88,144,347 | |
| Accumulated deficit | | | (88,605,400 | ) | | | (84,501,404 | ) |
| | | | | | | |
| Total stockholders’ equity | | | 4,465,800 | | | | 3,703,350 | |
| | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 5,615,670 | | | $ | 4,583,754 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-18
RegeneRx Biopharmaceuticals, Inc.
(unaudited)
| | | | | | | | | |
| | | Three Months Ended
| |
| | | March 31, | |
| | | 2010 | | | 2009 | |
| |
| Revenues | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | |
| Research and development | | | 470,434 | | | | 1,661,600 | |
| General and administrative | | | 678,068 | | | | 859,568 | |
| | | | | | | | | |
| Total operating expenses | | | 1,148,502 | | | | 2,521,168 | |
| | | | | | | | | |
| Loss from operations | | | (1,148,502 | ) | | | (2,521,168 | ) |
| | | | | | | | | |
| Interest income | | | 2,793 | | | | 6,518 | |
| | | | | | | | | |
| Net loss | | $ | (1,145,709 | ) | | $ | (2,514,650 | ) |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.02 | ) | | $ | (0.05 | ) |
| | | | | | | | | |
| Weighted average number of common shares outstanding | | | 60,406,828 | | | | 53,622,491 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Nine Months ended September 30, | |
| | | 2010 | | | 2009 | |
| Sponsored research revenues | | $ | 53,819 | | | $ | — | |
| | | | | | | | | |
| Operating expenses: | | | | | | | | |
| Research and development | | | 1,819,036 | | | | 3,064,248 | |
| General and administrative | | | 2,345,619 | | | | 2,161,539 | |
| | | | | | | |
| Total operating expenses | | | 4,164,655 | | | | 5,225,787 | |
| | | | | | | |
| Loss from operations | | | (4,110,836 | ) | | | (5,225,787 | ) |
| | | | | | | |
| Interest income | | | 6,840 | | | | 10,304 | |
| | | | | | | |
| Net loss | | $ | (4,103,996 | ) | | $ | (5,215,483 | ) |
| | | | | | | |
| | | | | | | | | |
| Basic and diluted net loss per common share | | $ | (0.06 | ) | | $ | (0.10 | ) |
| | | | | | | |
| | | | | | | | | |
| Weighted average number of common shares outstanding | | | 66,729,519 | | | | 54,216,430 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-19
RegeneRx Biopharmaceuticals, Inc.
(unaudited)
| | | | | | | | | |
| | | For the Three Months Ended
| |
| | | March 31, | |
| | | 2010 | | | 2009 | |
| |
| Operating activities: | | | | | | | | |
| Net loss | | $ | (1,145,709 | ) | | $ | (2,514,650 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 3,273 | | | | 4,467 | |
| Non-cash share-based compensation | | | 131,844 | | | | 266,628 | |
| Gain on settlement of accrued liabilities | | | (141,016 | ) | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Prepaid expenses and other current assets | | | (28,620 | ) | | | 72,405 | |
| Other assets | | | 5,693 | | | | — | |
| Accounts payable | | | 37,512 | | | | 301,075 | |
| Accrued expenses | | | (10,531 | ) | | | 119,016 | |
| | | | | | | | | |
| Net cash used in operating activities | | | (1,147,554 | ) | | | (1,751,059 | ) |
| | | | | | | | | |
| Investing activities: | | | | | | | | |
| Purchase of fixed assets | | | (18,224 | ) | | | — | |
| | | | | | | | | |
| Net cash used in investing activities | | | (18,224 | ) | | | — | |
| | | | | | | | | |
| Net decrease in cash and cash equivalents | | | (1,165,778 | ) | | | (1,751,059 | ) |
| | | | | | | | | |
| Cash and cash equivalents at beginning of period | | | 4,355,768 | | | | 5,655,367 | |
| | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 3,189,990 | | | $ | 3,904,308 | |
| | | | | | | | | |
| | | | | | | | | |
| | | Nine Months ended September 30, | |
| | | 2010 | | | 2009 | |
| Operating activities: | | | | | | | | |
| Net loss | | $ | (4,103,996 | ) | | $ | (5,215,483 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
| Depreciation and amortization | | | 7,484 | | | | 12,578 | |
| Non-cash share-based compensation | | | 361,195 | | | | 607,440 | |
| Gain on settlement of accrued liabilities | | | (141,016 | ) | | | — | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Accounts receivable | | | (15,603 | ) | | | — | |
| Prepaid expenses and other current assets | | | (385,679 | ) | | | (29,592 | ) |
| Other non-current assets | | | 5,693 | | | | — | |
| Accounts payable | | | 464,932 | | | | (13,804 | ) |
| Accrued expenses | | | (54,450 | ) | | | (481,319 | ) |
| | | | | | | |
| Net cash used in operating activities | | | (3,861,440 | ) | | | (5,120,180 | ) |
| | | | | | | |
| | | | | | | | | |
| Investing activities: | | | | | | | | |
| Purchase of fixed assets | | | (23,632 | ) | | | — | |
| | | | | | | |
| Net cash used in investing activities | | | (23,632 | ) | | | — | |
| | | | | | | |
| | | | | | | | | |
| Financing activities: | | | | | | | | |
| Net proceeds from issuance of common stock | | | 4,505,251 | | | | 573,683 | |
| | | | | | | |
| Net cash provided by financing activities | | | 4,505,251 | | | | 573,683 | |
| | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | 620,179 | | | | (4,546,497 | ) |
| | | | | | | |
| Cash and cash equivalents: | | | | | | | | |
| Beginning of period | | | 4,355,768 | | | | 5,655,367 | |
| | | | | | | |
| End of period | | $ | 4,975,947 | | | $ | 1,108,870 | |
| | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-20
RegeneRx Biopharmaceuticals, Inc.
For the three months ended March 31, 2010 and 2009 (Unaudited)
| |
1. | organization, business overview and basis of presentation1.ORGANIZATION, BUSINESS OVERVIEW AND BASIS OF PRESENTATION |
Organization and Nature of Operations.
RegeneRx Biopharmaceuticals, Inc. (the “Company”, “We”, “Us”, “Our”), a Delaware corporation, was incorporated in 1982. We are focused on the development of a novel therapeutic peptide, Thymosin Beta 4 (“Tß4”), for tissue and organ protection, repair and regeneration. Our operations are confined to one business segment: the development and marketing of product candidates based on Tß4.
Management Plans to Address Operating Conditions.
We incurred net losses of $6.5 million for the year ended December 31, 2009 and $1.1$4.1 million for the threenine months ended March 31,September 30, 2010. Since inception, and through March 31,September 30, 2010, we have an accumulated deficit of $85.6$88.6 million and we had cash and cash equivalents of $3.2$5.0 million as of March 31,September 30, 2010. Based on our operating plan and a recently awarded $733,438 cash grant more fully described below, we believe that our cash and cash equivalents as of March 31,September 30, 2010 will fund our operations into the thirdsecond quarter of 2010,2011, without additional capital. We anticipate incurring substantial future losses as we continue development of Tß4-based product candidates. Wecandidates, and will therefore need substantial additional funds in order to fund our operations beyondcapital resources.
During the third quarter ended June 30, 2010, we sold an aggregate of 2010.
We have filed a registration statement with the Securities and Exchange Commission (“SEC”) for the public offering13,124,750 shares of our common stock and warrants to purchase an additional 5,249,900 shares of our common stock.stock in a public offering for net proceeds of approximately $4.5 million. In May 2010, we were awarded a grant from the National Institutes of Health under which we may receive up to $3.0 million for development expenses that we may incur over a three-year period, as more fully described in Note 65 below. In October 2010, we were awarded a cash grant totaling $733,438 under Section 48D of the Internal Revenue Code, as more fully described in Note 8 below, in support of our research and we intend to applydevelopment initiatives for additional grant funding and tax credits set aside for biotechnology companies under recently enacted healthcare reform legislation.
our product candidates.
We maywill explore other funding alternatives, including, without limitation,such as additional government funding, public or private placements of our securities, debt financing, corporate collaborations and licensing arrangements or the sale of our company or certain of our intellectual property rights. If we are unable to complete the contemplated public offering or anotherobtain additional financing, or strategic transaction, we may not be able to continue as a going concern after our funds have been exhausted, and we could be required to significantly curtail or cease operations, file for bankruptcy or liquidate and dissolve.
These factors raise substantial doubt about our ability to continue as a going concern as of the date of the accompanying financial statements. The accompanying financial statements have been prepared assuming that we will continue as a going concern. This basis of accounting contemplates the recovery of our assets and the satisfaction of our liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be forced to take any such actions.
Even if we are able to obtain sufficient funding, other factors including competition, dependence on third parties, uncertainty regarding patents, protection of proprietary rights, manufacturing of peptides and technology obsolescence could have a significant impact on us and our operations.
F-21
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements
(Unaudited)
To achieve profitability we, or a strategic partner, must successfully conduct pre-clinical studies and clinical trials, obtain required regulatory approvals and successfully manufacture and market those pharmaceutical products we wish to commercialize. The time required to reach profitability is highly uncertain and there can be no assurance that we will be able to achieve sustained profitability, if at all.
Basis of Presentation.
The accompanying unaudited interim financial statements reflect, in the opinion of management, all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of our financial position, results of operations and cash flows for each period presented. These statements have been prepared without audit in accordance with U.S. generally accepted accounting principles (“GAAP”) and with the rules and regulations of the SEC,Securities and Exchange Commission (“SEC”) for interim financial statements. Accordingly, they do not include all of the information and footnotes required by GAAP. The accounting policies underlying our
F-21
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
For the three months ended March 31, 2010 and 2009 (Unaudited)
unaudited interim financial statements are consistent with those underlying our audited annual financial statements. These unaudited interim financial statements should be read in conjunction with the audited annual financial statements as of and for the year ended December 31, 2009, and related notes thereto, included in our Annual Report onForm 10-K for the year ended December 31, 2009 (the “Annual Report”).
The accompanying December 31, 2009 financial information was derived from our audited financial statements. Operating results for the three-monthnine-month period ended March 31,September 30, 2010 are not necessarily indicative of the results to be expected for the year ending December 31, 2010 or any other future period.
References in this Quarterly Report onForm 10-Q to “authoritative guidance” are to the Accounting Standards Codification issued by the Financial Accounting Standards Board (“FASB”) in June 2009.
Subsequent events have been evaluated through the filing date of these unaudited financial statements.
Use of Estimates.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates.
Sponsored Research Revenues.
We account for non-refundable grants as “Sponsored research revenues” in the accompanying statements of operations. Revenues are recognized when the associated research has been performed and the related underlying costs are incurred.
| |
2. | Net Loss per Common Share |
New Accounting Pronouncements.
In April 2010, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2010-17, “Revenue Recognition—Milestone Method,” which provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. Research or development arrangements frequently include payment provisions whereby all or a portion of the consideration is contingent upon milestone events such as successful completion of phases in a study or achieving a specific result from the research or development efforts. The amendments in this ASU provide guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. The ASU is effective for fiscal years and interim periods within those years beginning on or after June 15, 2010, with early adoption permitted. We are currently evaluating the impact, if any, of the application of this ASU on our financial condition and results of operations.
F-22
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements
(Unaudited)
In July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act was signed into law. This legislation includes an exemption for companies with less than $75 million in market capitalization from the requirement set forth in Section 404(b) of the Sarbanes-Oxley Act of 2002 to include an external auditor’s report on the effectiveness of a registrant’s internal control over financial reporting. As a result of the new legislation, our independent registered public accounting firm will not be required to issue an attestation report with respect to our internal control over financial reporting. However, we will continue to be subject to the requirement of Section 404 of the Sarbanes-Oxley Act of 2002 for our management to make an annual assessment of the effectiveness of our internal control over financial reporting.
2.NET LOSS PER COMMON SHARE
Net loss per common share for the three-month periodsnine-month period ended March 31,September 30, 2010 and 2009, respectively, is based on the weighted-average number of shares of common stock outstanding during the periods. Basic and diluted loss per share are identical for all periods presented, as potentially dilutive securities have been excluded from the calculation of the diluted net loss per common share because the inclusion of such securities would be antidilutive. The potentially dilutive securities include 12,847,96319,545,363 shares and 9,366,5909,998,860 shares for the threenine months ended March 31,September 30, 2010 and 2009, respectively, reserved for the exercise of outstanding options and warrants.
| |
3. | Stock Based Compensation3.STOCK-BASED COMPENSATION |
We recognized $131,844$361,195 and $266,628$607,440 in stock-based compensation expense for the threenine months ended March 31,September 30, 2010 and 2009, respectively. Given our current estimates of future forfeitures, we expect to recognize the compensation cost related to non-vested options as of March 31,September 30, 2010 of $590,780$447,364 over the weighted average remaining recognition period of 1.11.2 years.
We estimate the value of our stock option awards on the date of grant using the Black-Scholes option pricing model. We did not grant anyused the following forward-looking range of assumptions to value each stock optionsoption granted to employees and directors during the threenine months ended March 31,September 30, 2010 and 2009.2009:
| | | | | | | | | |
| | | 2010 | | | 2009 | |
| Dividend yield | | | 0.0 | % | | | 0.0 | % |
| Risk-free rate of return | | | 1.6 | % | | | 1.9 | % |
| Expected life in years | | | 4.75 | | | | 5.38 | |
| Volatility | | | 70 | % | | | 72 | % |
| Forfeiture rate | | | 2.6 | % | | | 2.6 | % |
F-23
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements
(Unaudited)
| |
4. | Income Taxes4.FAIR VALUE MEASUREMENTS |
As of March 31, 2010, there have been no material changes to our uncertain tax positions disclosures as provided in Note 8 of the Annual Report. We do not anticipate that total unrecognized tax benefits will significantly change prior to March 31, 2011.
| |
5. | Fair Value Measurements |
We have adopted authoritative guidance that defines fair value and establishes a framework for fair value measurements. This authoritative guidance established a three-level hierarchy for fair value measurements. The hierarchy is based upon the transparency of inputs and the valuation of an asset or a liability as of the measurement date. The three levels of inputs are as follows:
| • | | |
| • | Level 1 — Quoted prices in active markets for identical assets and liabilities. |
F-22
RegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements — (Continued)
For the three months ended March 31, 2010 and 2009 (Unaudited)
| | |
| • | • | Level 2 — Observable inputs other than quoted prices in active markets for identical assets and liabilities. |
| | |
| • | • | Level 3 — Unobservable inputs. |
At March 31,September 30, 2010, we held no qualifying liabilities, and our only qualifying assets that required measurement under the foregoing fair value hierarchy were money market fundsaccounts, insured bank deposits and U.S. Treasury Bills, included in Cash and Cash Equivalents valued at $0.2$5.0 million using Level 1 inputs.
| |
6. | Subsequent Events5.NIH GRANT |
On May 13, 2010, we were awarded a $3 million grant from the National Institutes of Health’s National Heart, Lung and Blood Institute to support and accelerate the clinical development of our product candidate RGN-352, an injectable formulation of Tß4 for patients who have suffered an acute myocardial infarction, commonly known as a heart attack. The award is beingwas issued under the American Reinvestment and Recovery Act of 2009. Subject to our compliance with the terms and conditions of the award, we are eligible to receive up to $3.0 million over a three-year period in cost reimbursements for direct costs incurred for the purposes set forth in the grant, as well as allocable indirect costs. We recorded sponsored research revenue of $53,819 for the nine months ended September 30, 2010 from this grant.
6.PUBLIC OFFERING
During the quarter ended June 30, 2010, we sold an aggregate of 13,124,750 shares of our common stock and warrants to purchase an additional 5,249,900 shares of our common stock for net proceeds of approximately $4.5 million. These securities were sold as units, with each unit consisting of one share of common stock and a warrant to purchase 0.4 shares of our common stock. Each unit was sold at a public offering price of $0.41.
Each warrant has a term of five years and represents the right to purchase one share of common stock at an exercise price of $0.56 per share. In the event the closing sale price of our common stock is at least $1.78 per share for any 20 trading days within a period of 30 consecutive trading days, we may call these warrants for redemption, at a redemption price of $0.01 per warrant, by providing at least 30 days notice to each warrant holder. The warrants were valued using the Black-Scholes option-pricing model as of the closing date and accounted for in permanent equity. The estimated fair value of the warrants at the date of issuance was approximately $725,000.
In addition, the representative of the underwriters in the public offering was granted a warrant to purchase 805,000 shares of our common stock at an exercise price of $0.45 per share. This warrant is exercisable beginning on November 17, 2010 and continuing until May 17, 2015. The representative’s warrant also provides for one demand registration until May 17, 2015. The representative’s warrant was also valued using the Black-Scholes option-pricing model as of the closing date and accounted for as a cost of the offering. The estimated fair value of the representative’s warrant at the date of issuance was approximately $112,000.
The public offering was made pursuant to a registration statement on Form S-1 (Registration No. 333-166146), which was declared effective by the SEC on May 17, 2010, and a final prospectus filed with the SEC on May 18, 2010.
F-23F-24
11,500,000 UnitsRegeneRx Biopharmaceuticals, Inc.
Notes to Financial Statements
(Unaudited)
7.2010 EQUITY INCENTIVE PLAN
On July 14, 2010, at the Company’s Annual Meeting of Stockholders, the Company’s stockholders approved the 2010 Equity Incentive Plan (the “2010 Plan”). The terms of the 2010 Plan provide for the discretionary grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, performance stock awards, other stock awards and performance cash awards to our employees, directors and consultants. At inception of the 2010 Plan, 5,000,000 shares of the Company’s common stock were reserved for future issuance.

8.SUBSEQUENT EVENT
Common StockOn October 29, 2010, we were notified by the Internal Revenue Service that we have been certified to receive a total cash grant of $733,438 related to the previously filed applications under the Qualifying Therapeutic Discovery Projects (Section 48D of the Internal Revenue Code). This amount will be recognized as revenue and is expected to be received in 2010.
Warrants
PROSPECTUS
Maxim Group LLC
Boenning & Scattergood, Inc.
, 2010
F-25
15,000,000 Shares
Common Stock
PROSPECTUS
, 2011
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13.Other Expenses of Issuance and Distribution.
| |
Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of the common stock being registered. All amounts shown are estimates except for the SEC registration fee, the FINRA filing feefee.
| | | | | |
| | | Amount to | |
| | | be Paid | |
| SEC registration fee | | $ | 383 | |
| Printing and engraving expenses | | $ | 15,000 | |
| Legal fees and expenses | | $ | 75,000 | |
| Accounting fees and expenses | | $ | 20,000 | |
| Transfer agent and registrar fees and expenses | | $ | 5,000 | |
| Miscellaneous expenses | | $ | 10,000 | |
| | | | |
| Total | | $ | 125,383 | |
| | | | |
Item 14.Indemnification of Directors and the NYSE Amex listing fee.Officers.
| | | | | |
| | | Amount to
| |
| | | be Paid | |
| |
| SEC registration fee | | $ | 798 | |
| FINRA filing fee | | | 1,619 | |
| NYSE Amex listing fee | | | 45,000 | |
| Corporate finance fee payable to the representative of the underwriters | | | 64,400 | |
| Printing and engraving expenses | | | 75,000 | |
| Legal fees and expenses | | | 250,000 | |
| Accounting fees and expenses | | | 25,000 | |
| Transfer agent and registrar fees and expenses | | | 5,000 | |
| Miscellaneous expenses | | | 10,000 | |
| | | | | |
| Total | | $ | 476,816 | |
| | | | | |
| |
Item 14. | Indemnification of Directors and Officers. |
We are incorporated under the laws of the State of Delaware. Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| | |
| | • | | transaction from which the director derives an improper personal benefit; |
| |
| | • | | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| |
| | • | | unlawful payment of dividends or redemption of shares; or |
| |
| | • | | breach of a director’s duty of loyalty to the corporation or its stockholders. |
Our restated certificate of incorporation includes such a provision.
Section 145 of the DGCL provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. Our amended and restated bylaws include such a provision. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful.
Section 145 of the DGCL also provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. Our amended and restated bylaws contain such a provision. The indemnity
II-1
may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses that such officer or director has actually and reasonably incurred.
Expenses incurred by any indemnitee in defending or investigating a threatened or pending action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined that such indemnitee is not entitled to be indemnified by us. No advance will be made by us if a determination is reasonably and promptly made by our board of directors by a majority vote of a quorum of disinterested directors, or if such a quorum is not obtainable or, even if obtainable, a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, that, based upon the facts known to the board or counsel at the time such determination is made, such person did not meet the applicable standard of conduct in order to be indemnified.
At present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
We plan to enter into an underwriting agreement that provides thatUnder the underwriters are obligated, under some circumstances,Purchase Agreement, we have agreed to indemnify Lincoln Park from and against claims and expenses in connection with the Purchase Agreement as a result of, or arising out of, or relating to our directors, officersmisrepresentation or breach of representations, warranties or covenants made by us, and controllingclaims brought or made against Lincoln Park and arising out of or resulting from the Purchase Agreement other than with respect to liabilities that directly and primarily result from the gross negligence or willful misconduct of Lincoln Park.
We have agreed to indemnify Lincoln Park and certain other persons against specifiedcertain liabilities in connection with the offering of shares of common stock covered by this registration statement, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Lincoln Park has agreed to indemnify us against liabilities under the Securities Act.Act that may arise from certain written information furnished to us by Lincoln Park specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Item 15.Recent Sales of Unregistered Securities.
| |
Item 15. | Recent Sales of Unregistered Securities. |
The following list sets forth information regarding all unregistered securities sold by us since January 1, 20072008 through the date of this registration statement.
1) In February 2008, we issued and sold 5,000,000 shares of common stock at a price of $1.00 per share, and warrants to purchase an aggregate of 1,000,000 shares of common stock at an exercise price of $1.60 per share, to two accredited investors for aggregate consideration of approximately $5.0 million.
2) In December 2008, we issued and sold 2,068,964 shares of common stock at a price of $1.45 per share, and warrants to purchase an aggregate of 745,104 shares of common stock at an exercise price of $1.74 per share, to two accredited investors for aggregate consideration of approximately $3.0 million.
3) On April 30, 2009 we issued and sold 1,052,631 shares of common stock at a price of $0.57 per share, and warrants to purchase an aggregate of 263,158 shares of common stock at an exercise price of $0.91 per share, to one accredited investor for aggregate consideration of $600,000.
4) On October 5, 2009, we issued and sold 4,512,194 shares of common stock at a price of $0.82 per share, and warrants to purchase an aggregate of 2,256,097 shares of common stock at an exercise price of $1.12 per share, to three accredited investors for aggregate consideration of $3.7 million.
5) On October 15, 2009, we issued and sold 1,219,512 shares of common stock at a price of $0.82 per share, and warrants to purchase an aggregate of 609,756 shares of common stock at an exercise price of $1.12 per share, to one accredited investor for aggregate consideration of $1.0 million.
6) On January 4, 2011, we issued and sold 958,333 shares of common stock to Lincoln Park. The consideration for this issuance was Lincoln Park’s commitment to purchase shares under the Purchase Agreement.
7) On January 7, 2011, we issued and sold an aggregate of 3,518,519 shares of common stock at a price per share of $0.27, and warrants to purchase an aggregate of 1,407,407 shares of common stock at an exercise price of $0.38 per share,
II-2
to three accredited investors for aggregate consideration of $950,000.
The offers, sales and issuances of the securities described in paragraphs (1) through (5)(7) were exempt from registration under the Securities Act under Section 4(2) of the Securities Act and Regulation D promulgated thereunder as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection
II-2
with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor as defined in Rule 501 promulgated under the Securities Act.
Item 16.Exhibits and Financial Statement Schedules.
(a) Exhibits
| | |
Item 16.Exhibit | Exhibits and Financial Statement Schedules. | |
| Number | | Description of Document |
| 3.1(1) | | Restated Certificate of Incorporation. |
| |
(a) 3.2(1) | Exhibits | Certificate of Amendment of Restated Certificate of Incorporation. |
|
| 3.3(1) | | Certificate of Amendment of Restated Certificate of Incorporation. |
|
| 3.4(2) | | Certificate of Amendment of Restated Certificate of Incorporation. |
|
| 3.5(1) | | Certificate of Designation of Series A Participating Cumulative Preferred Stock. |
|
| 3.6(3) | | Amended and Restated Bylaws adopted July 26, 2006. |
|
| 3.7(4) | | Amendment to Amended and Restated Bylaws. |
|
| 4.1(1) | | Specimen Common Stock Certificate. |
|
| 4.2(1) | | Specimen Rights Certificate. |
|
| 4.3(1) | | Rights Agreement, dated April 29, 1994, between the Company and American Stock Transfer & Trust Company, as Rights Agent. |
|
| 4.4(1) | | Amendment No. 1 to Rights Agreement, dated March 4, 2004, between the Company and American Stock Transfer & Trust Company, as Rights Agent. |
|
| 4.5(5) | | Form of Warrant Agreement. |
|
| 4.6(6) | | Form of Warrant Certificate. |
|
| 4.7(5) | | Form of Representative’s Warrant. |
|
| 4.8(7) | | Warrant issued to Lincoln Park. |
|
| 4.9(7) | | Form of Warrant issued to the Sigma-Tau Purchasers. |
|
| 4.10(7) | | Omnibus Warrant Amendment Agreement, dated as of January 5, 2011, by and among the Company and the Sigma-Tau Purchasers. |
|
| 5.1 | | Opinion of Cooley LLP. |
|
| 10.1+(8) | | Amended and Restated 2000 Stock Option Incentive Plan, as amended. |
|
| 10.2*(9) | | Patent License Agreement — Exclusive, dated January 24, 2001, between the Company and the U.S. Public Health Service. |
|
| 10.3+(10) | | Form of Stock Option Grant Notice and Stock Option Agreement under the 2010 Equity Incentive Plan. |
|
| 10.4*(11) | | Thymosin Beta 4 License and Supply Agreement, dated January 21, 2004, between the Company and Defiante Farmaceutica S.A. |
|
| 10.5(12) | | Lease by and between RegeneRx Biopharmaceuticals, Inc. and The Realty Associates Fund V, L.P., dated December 10, 2009. |
|
| 10.6+(13) | | Second Amended and Restated Employment Agreement, dated March 11, 2009, between the Company and Allan L. Goldstein, as amended. |
|
| 10.6+(14) | | Second Amended and Restated Employment Agreement, dated March 12, 2009, between the Company and J.J. Finkelstein, as amended. |
|
| 10.7+(14) | | Second Amended and Restated Employment Agreement, dated March 31, 2009, between the Company and C. Neil Lyons, as amended. |
|
| 10.8+(14) | | Second Amended and Restated Employment Agreement, dated March 31, 2009, between the Company and David Crockford. |
|
| 10.9+ (10) | | 2010 Equity Incentive Plan. |
| | | | | |
Exhibit
| | |
Number | | Description of Document |
| |
| | 1 | .1 | | Form of Underwriting Agreement. |
| | 3 | .1# | | Restated Certificate of Incorporation. |
| | 3 | .2# | | Certificate of Amendment of Restated Certificate of Incorporation. |
| | 3 | .3# | | Certificate of Amendment of Restated Certificate of Incorporation. |
| | 3 | .4# | | Certificate of Designation of Series A Participating Cumulative Preferred Stock. |
| | 3 | .5(1) | | Amended and Restated Bylaws adopted July 26, 2006. |
| | 3 | .6(2) | | Amendment to Amended and Restated Bylaws. |
| | 4 | .1# | | Specimen Common Stock Certificate. |
| | 4 | .2# | | Specimen Rights Certificate. |
| | 4 | .3# | | Rights Agreement, dated April 29, 1994, between the Company and American Stock Transfer & Trust Company, as Rights Agent. |
| | 4 | .4# | | Amendment No. 1 to Rights Agreement, dated March 4, 2004, between the Company and American Stock Transfer & Trust Company, as Rights Agent. |
| | 4 | .5 | | Form of Warrant Agreement. |
| | 4 | .6 | | Form of Warrant Certificate. |
| | 4 | .7 | | Form of Representative’s Warrant. |
| | 5 | .1 | | Opinion of Cooley LLP. |
| | 10 | .1+(3) | | Amended and Restated 2000 Stock Option and Incentive Plan, as amended. |
| | 10 | .2*(4) | | Patent License Agreement — Exclusive, dated January 24, 2001, between the Company and the U.S. Public Health Service. |
| | 10 | .3*(5) | | Thymosin Beta 4 License and Supply Agreement, dated January 21, 2004, between the Company and Defiante Farmaceutica S.A. |
| | 10 | .4(6) | | Lease by and between RegeneRx Biopharmaceuticals, Inc. and The Realty Associates Fund V, L.P., dated December 10, 2009. |
| | 10 | .5+(8) | | Second Amended and Restated Employment Agreement, dated March 11, 2009, between the Company and Allan L. Goldstein, as amended. |
| | 10 | .6+(7) | | Second Amended and Restated Employment Agreement, dated March 12, 2009, between the Company and J.J. Finkelstein, as amended. |
| | 10 | .7+(7) | | Second Amended and Restated Employment Agreement, dated March 31, 2009, between the Company and C. Neil Lyons, as amended. |
| | 10 | .8+(7) | | Second Amended and Restated Employment Agreement, dated March 31, 2009, between the Company and David Crockford. |
| | 10 | .1(9) | | Stock Purchase Agreement, dated as of June 23, 2005. |
| | 10 | .2(10) | | Form of Warrant to Purchase Common Stock, dated March 17, 2006. |
| | 10 | .3(11) | | Form of Warrant to Purchase Common Stock, dated December 18, 2006. |
| | 10 | .4(11) | | Registration Rights Agreement, dated as of December 15, 2006. |
| | 10 | .5(12) | | Securities Purchase Agreement, dated as of February 27, 2008. |
| | 10 | .6(12) | | Form of Warrant to Purchase Common Stock, dated February 29, 2008. |
| | 10 | .7(13) | | Securities Purchase Agreement, dated as of December 10, 2008. |
| | 10 | .8(13) | | Form of Warrant to Purchase Common Stock, dated December 10, 2008. |
II-3
| | | | | |
Exhibit
| | |
Number | | Description of Document |
| |
| | 10 | .9(14) | | Securities Purchase Agreement, dated as of April 13, 2009. |
| | 10 | .10(14) | | Form of Warrant to Purchase Common Stock, dated April 30, 2009. |
| | 10 | .11(15) | | Securities Purchase Agreement, dated as of September 30, 2009. |
| | 10 | .12(15) | | Form of Warrant to Purchase Common Stock, dated October 5, 2009. |
| | 10 | .13(16) | | Securities Purchase Agreement, dated as of September 30, 2009. |
| | 10 | .14(16) | | Form of Warrant to Purchase Common Stock, dated October 15, 2009. |
| | 23 | .1 | | Consent of Reznick Group, P.C., independent registered public accounting firm. |
| | 23 | .2 | | Consent of Cooley LLP (included in Exhibit 5.1). |
| | 24 | .1# | | Power of Attorney. Reference is made to Page II-6 of the Registration Statement onForm S-1 (FileNo. 333-166146) filed with the SEC on April 16, 2010. |
| | |
| Exhibit | | |
| Number | | Description of Document |
| 10.10(15) | | Stock Purchase Agreement, dated as of June 23, 2005. |
|
| 10.11(16) | | Form of Warrant to Purchase Common Stock, dated March 17, 2006. |
|
| 10.12(17) | | Form of Warrant to Purchase Common Stock, dated December 18, 2006. |
|
| 10.13(17) | | Registration Rights Agreement, dated as of December 15, 2006. |
|
| 10.14(18) | | Securities Purchase Agreement, dated as of February 27, 2008. |
|
| 10.15(18) | | Form of Warrant to Purchase Common Stock, dated February 29, 2008. |
|
| 10.16(19) | | Securities Purchase Agreement, dated as of December 10, 2008. |
|
| 10.17(19) | | Form of Warrant to Purchase Common Stock, dated December 10, 2008. |
|
| 10.18(20) | | Securities Purchase Agreement, dated as of April 13, 2009. |
|
| 10.19(20) | | Form of Warrant to Purchase Common Stock, dated April 30, 2009. |
|
| 10.20(21) | | Securities Purchase Agreement, dated as of September 30, 2009. |
|
| 10.21(21) | | Form of Warrant to Purchase Common Stock, dated October 5, 2009. |
|
| 10.22(22) | | Securities Purchase Agreement, dated as of September 30, 2009. |
|
| 10.23(22) | | Form of Warrant to Purchase Common Stock, dated October 15, 2009. |
|
| 10.24(7) | | Securities Purchase Agreement, dated as of January 5, 2011, by and between the Company and Lincoln Park |
|
| 10.25(7) | | Purchase Agreement, dated as of January 4, 2011, by and between the Company and Lincoln Park |
|
| 10.26(7) | | Registration Rights Agreement, dated as of January 4, 2011, by and between the Company and Lincoln Park |
|
| 10.27(7) | | Securities Purchase Agreement, dated as of January 5, 2011, by and between the Company and Defiante Farmaceutica S.A. |
|
| 10.28(7) | | Securities Purchase Agreement, dated as of January 5, 2011, by and between the Company and Taufin International S.A. |
|
| 10.29(7) | | Securities Purchase Agreement, dated as of January 5, 2011, by and between the Company and Sinaf S.A. |
|
| 23.1 | | Consent of Reznick Group, P.C., independent registered public accounting firm. |
|
| 23.2 | | Consent of Cooley LLP (included in Exhibit 5.1). |
|
| 24.1 | | Power of Attorney(see signature page). |
| | |
| (1) | | Filed as an exhibit to the registrant’s Registration Statement on Form S-1 (File No. 333-166146) filed with the Securities and Exchange Commission on April 16, 2010 and incorporated herein by reference. |
|
| (2) | | Filed as an exhibit to the registrant’s Registration Statement on Form S-8 (File No. 333-168252) filed with the Securities and Exchange Commission on July 21, 2010 and incorporated herein by reference. |
|
| (3) | | Filed as an exhibit to the registrant’s Quarterly Report onForm 10-Q for the quarter ended June 30, 2006 filed with the Securities and Exchange Commission on August 14, 2006 and incorporated herein by reference. |
| |
(2)(4) | | Filed as an exhibit to the registrant’s Registration Statement onForm S-8 (File No.333-152250) filed with the Securities and Exchange Commission on July 10, 2008 and incorporated herein by reference. |
| |
(3)(5) | | Filed as an exhibit to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on May 21, 2010 and incorporated herein by reference. |
|
| (6) | | Filed as an exhibit to the registrant’s Amendment No. 1 to Registration Statement on Form S-1 (File No. 333-166146) filed with the Securities and Exchange Commission on May 17, 2010 and incorporated herein by reference. |
|
| (7) | | Filed as an exhibit to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 7, 2011 and incorporated herein by reference. |
|
| (8) | | Filed as Annex A to the registrant’s definitive proxy statement on Schedule 14A filed with the Securities and Exchange Commission on May 9, 2008 and incorporated herein by reference. |
II-4
| | |
(4)(9) | | Filed as an exhibit to the registrant’s Annual Report onForm 10-KSB for the year ended December 31, 2000 (FileNo. 1-15070) filed with the Securities and Exchange Commission on April 2, 2001 and incorporated herein by reference. |
| |
(5)(10) | | Filed as an exhibit to the registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on July 20, 2010 and incorporated herein by reference. |
|
| (11) | | Filed as an exhibit to the registrant’s Registration Statement onForm SB-2 (File No.333-113417) filed with the Securities and Exchange Commission on March 9, 2004 and incorporated herein by reference. |
| |
(6)(12) | | Filed as an exhibit to the registrant’s Annual Report onForm 10-K for the year ended December 31, 2009 filed with the Securities and Exchange Commission on March 31, 2010 and incorporated herein by reference. |
| |
(7)(13) | | Filed as an exhibit to Amendment No. 1 the registrant’s Annual Report on Form 10-K/A for the year ended December 31, 2008 filed with the Securities and Exchange Commission on April 30, 2009 and incorporated herein by reference. |
|
| (14) | | Filed as an exhibit to the registrant’s Annual Report onForm 10-K for the year ended December 31, 2008 filed with the Securities and Exchange Commission on April 15, 2009 and incorporated herein by reference. |
| |
(8)(15) | | Filed as an exhibit to Amendment No. 1 the registrant’s Annual Report onForm 10-K/A for the year ended December 31, 2008 filed with the Securities and Exchange Commission on April 30, 2009 and incorporated herein by reference. |
|
(9) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on June 23, 2005 and incorporated herein by reference. |
| |
(10)(16) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on March 7, 2006 and incorporated herein by reference. |
| |
(11)(17) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on December 18, 2006 and incorporated herein by reference. |
| |
(12)(18) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on February 27, 2008 and incorporated herein by reference. |
| |
(13)(19) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on December 12, 2008 and incorporated herein by reference. |
| |
(14)(20) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on April 16, 2009 and incorporated herein by reference. |
| |
(15)(21) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on September 30, 2009 and incorporated herein by reference. |
II-4
| | |
(16)(22) | | Filed as an exhibit to the registrant’s Current Report onForm 8-K filed with the Securities and Exchange Commission on October 5, 2009 and incorporated herein by reference. |
| | |
| + | | Indicates management contract or compensatory plan. |
| | |
| * | | The registrant has been granted confidential treatment with respect to certain portions of this exhibit (indicated by asterisks), which have been filed separately with the Securities and Exchange Commission. |
(b) Financial Statement Schedules
| | |
| (b) | Financial Statement Schedules |
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
II-5
Item 17.Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
II-5
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-6
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this Amendment No. 2 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Rockville, State of Maryland, on the 17th31st day of May, 2010.
REGENERX BIOPHARMACEUTICALS, INC.
January, 2011.
| | | | |
| REGENERX BIOPHARMACEUTICALS, INC.
| |
| | By: | /s/ J.J. Finkelstein | |
| | J.J. Finkelstein | |
| | President and Chief Executive Officer | |
|
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints J.J. Finkelstein
President and Chief Executive Officer
C. Neil Lyons, and each of them, his true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments (including post-effective amendments) to this registration statement together with all schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, together with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as he might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act, this Amendment No. 2 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | | | |
| Signature | | Signature
Title | | Title
Date |
| | Date
|
| |
/s/ J.J. Finkelstein
J.J. Finkelstein | | President and Chief Executive Officer and Director(Principal Executive Officer) | | May 17, 2010January 31, 2011 |
| | | | | |
/s/ C. Neil Lyons
C. Neil Lyons | | Chief Financial Officer
(Principal Accounting and Financial Officer) | | May 17, 2010January 31, 2011 |
| | | | | |
*
/s/ Allan L. GoldsteinAllan L. Goldstein | | Chairman of the Board of Directors | | May 17, 2010January 31, 2011 |
| | | | | |
*/s/ R. Don ElseyR. Don Elsey Richard J. Hindin | | Director | | May 17, 2010January 31, 2011 |
| | | | | |
*
/s/ Joseph C. McNayJoseph C. McNay | | Director | | May 17, 2010January 31, 2011 |
| | | | | |
*
/s/ Mauro BoveMauro Bove | | Director | | May 17, 2010January 31, 2011 |
| | | | | |
*
/s/ L. Thompson BowlesL. Thompson Bowles | | Director | | May 17, 2010 |
| | | | |
*By: /s/ C. Neil Lyons
C. Neil LyonsAttorney-in-fact | | | | January 31, 2011 |
II-7