
Use these links to rapidly review the documentTABLE OF CONTENTSINDEX TO CONSOLIDATED FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on October 28, 2013June 25, 2014
Registration No. 333-191584333-196892
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
KARYOPHARM THERAPEUTICS INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) | 2834 (Primary Standard Industrial Classification Code Number) | 26-3931704 (I.R.S. Employer Identification No.) |
2 Mercer Road
Natick, MA 01760
(508) 975-4820
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
Michael G. Kauffman, M.D., Ph.D.
Chief Executive Officer
Karyopharm Therapeutics Inc.
2 Mercer Road
Natick, MA 01760
(508) 975-4820
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
| Copies to: | ||||
Steven D. Singer, Esq. Joshua D. Fox, Esq. Wilmer Cutler Pickering Hale and Dorr LLP 60 State Street Boston, MA 02109 Telephone: (617) 526-6000 | Christopher B. Primiano, Esq. Vice President, General Counsel Karyopharm Therapeutics Inc. 2 Mercer Road Natick, MA 01760 Telephone: (508) 975-4820 | Patrick O'Brien, Esq. Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199 Telephone: (617) 951-7000 | ||
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) | Smaller reporting company o |
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Amount to be Registered(1) | Proposed Maximum Offering Price Per Share(2) | Proposed Maximum Aggregate Offering Price(2) | Amount of Registration Fee(3) | ||||
|---|---|---|---|---|---|---|---|---|
Common Stock, par value $0.001 per share | 6,516,667 | $16.00 | $104,266,672 | $13,429.55 | ||||
| ||||||||
| Class of Securities to be Registered | Number of Shares to be Registered(1) | Proposed Maximum Offering Price Per Share(2) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee(3) | ||||
|---|---|---|---|---|---|---|---|---|
Common Stock, par value $0.0001 per share | 2,530,000 | $43.20 | $109,296,000 | $14,077.32 | ||||
| ||||||||
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We and the selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated October 28, 2013June 25, 2014
PROSPECTUS
5,666,6672,200,000 Shares

Common Stock
This is Karyopharm Therapeutics Inc.'s initial public offering. We are selling 5,666,667offering 2,000,000 shares of our common stock and the selling stockholders are offering 200,000 shares of our common stock. We will not receive any proceeds from the sale of shares by the selling stockholders.
We expect the public offering price to be between $14.00 and $16.00 per share. Currently, no public market exists for the shares. After pricing of the offering, we expect that the shares will tradeOur common stock is listed on theThe NASDAQ Global Select Market under the symbol "KPTI." The last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014 was $41.37 per share.
We are an "emerging growth company" under federal securities laws and are subject to reduced public company disclosure standards. See "Prospectus Summary—Implications of Being an Emerging Growth Company."
Investing in the common stock involves risks that are described in the "Risk Factors" section beginning on page 1015 of this prospectus.
| | Per Share | Total | |||||
|---|---|---|---|---|---|---|---|
Public offering price | $ | $ | |||||
Underwriting | $ | $ | |||||
Proceeds, before expenses, to us | $ | $ | |||||
Proceeds, before expenses, to selling stockholders | $ | $ |
The underwriters may also exercise their option to purchase up to an additional 850,000330,000 shares from us at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2013.2014.
| BofA Merrill Lynch | Leerink |
JMP Securities | Wedbush PacGrow Life Sciences | Oppenheimer & Co. |
The date of this prospectus is , 2013.2014.
| | Page | |||
|---|---|---|---|---|
Prospectus Summary | 1 | |||
Risk Factors | ||||
Cautionary Note Regarding Forward-Looking Statements | ||||
Use of Proceeds | ||||
Price Range of Common Stock | 19 | |||
Dividend Policy | ||||
Industry and Other Data | ||||
Capitalization | ||||
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
Principal and Selling Stockholders | ||||
Description of Capital Stock | ||||
Shares Eligible for Future Sale | ||||
Material U.S. Federal Tax Considerations for Non-U.S. Holders of Common Stock | ||||
Underwriting | ||||
Legal Matters | ||||
Experts | ||||
Where You Can Find More Information | ||||
| ||||
You should rely only on the information contained in or incorporated by reference in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We have not, the selling stockholders have not, and the underwriters have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We and the selling stockholders are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained or incorporated by reference in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock.
For investors outside the United States: We have not, the selling stockholders have not, and the underwriters have not, done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
This summary highlights information contained elsewhere in this prospectus or incorporated by reference into this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and our other filings with the Securities and Exchange Commission listed in the section of this prospectus entitled "Incorporation of Documents by Reference" and is qualified in its entirety by the more detailed information and consolidated financial statements included or incorporated by reference elsewhere in this prospectus. This summary does not contain all of the information that may be important to you. You should read and carefully consider the following summary together with the entire prospectus and the documents included herein by reference, including our consolidated financial statements and the notes thereto appearing elsewhere in this prospectusincorporated herein by reference and the matters discussed in the sections in this prospectus entitledunder "Risk Factors," "Selected Consolidated Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in each case appearing elsewhere in this prospectus or in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 incorporated by reference herein before deciding to invest in our common stock. Some of the statements in this prospectus constitute forward-looking statements that involve risks and uncertainties. See "Cautionary Note Regarding Forward-Looking Statements." Our actual results could differ materially from those anticipated in such forward-looking statements as a result of certain factors, including those discussed in the "Risk Factors" and other sections of this prospectus.prospectus and the documents incorporated herein by reference.
Except as otherwise indicated herein or as the context otherwise requires, references in this prospectus to "Karyopharm" "the Company," "we," "us" and "our" refer to Karyopharm Therapeutics Inc. and, where appropriate, its consolidated subsidiary, NPM Pharma Inc.subsidiaries.
Overview
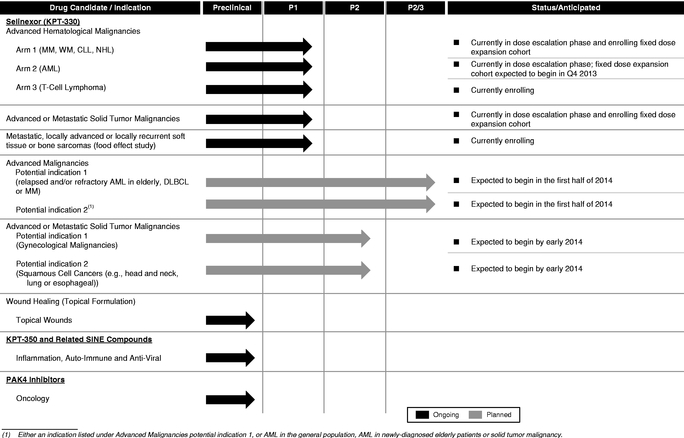
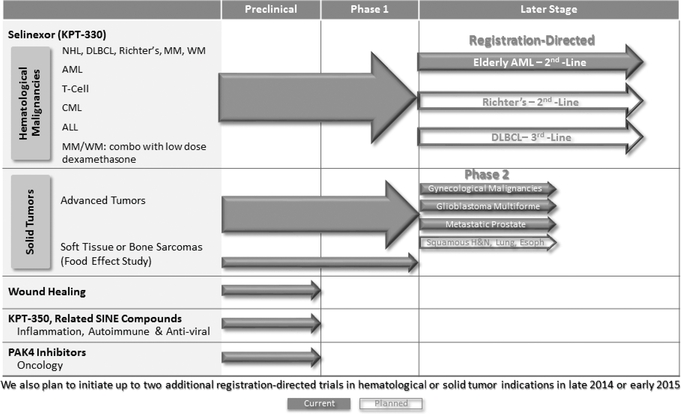
We are a clinical-stage pharmaceutical company founded in December 2008 by Dr. Sharon Shacham. We are focused on the discovery and development of novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases. Our scientific expertise is focused on the understanding of the regulation of intracellular transport between the nucleus and the cytoplasm. We have discovered and developedare developing wholly-owned, novel, small molecule,SelectiveInhibitors ofNuclearExport,electiveInhibitors ofNuclearExport, orSINE, compounds that inhibit the nuclear export protein XPO1. We have worldwide rights to these SINE compounds. Our lead drug candidate, Selinexor (KPT-330), is an XPO1 inhibitor being evaluated in multiple open-label Phase 1 and Phase 2 clinical trials in patients with heavily pretreated relapsed and/or refractory hematological and solid tumor malignancies. As of September 20, 2013,To date, we hadhave administered Selinexor to over 170330 patients across Phase 1 and Phase 2 clinical trials in these trials. Preliminary evidencehematologic and solid tumor indications. Evidence of anti-cancer activity has been observed in some patients and Selinexor has been sufficiently well-tolerated to allow many of these patients to remain on therapy for prolonged periods, including several who have remained on study for over 8-1212 months. During 2014, we plan to initiate registration-directed clinical trials in three different hematological malignancy indications. We plan to initiate up to two additional registration-directed trials in hematological or solid tumor indications in late 2014 or early 2015. These registration-directed trials are designed to serve as the basis for an application seeking regulatory approval for Selinexor in such indications. To our knowledge, no other XPO1 inhibitors are in clinical development at the present time.
One of the ways in which the cell regulates the function of a particular protein is by controlling the protein's location within the cell, as a specific function may only occur within a particular location in the cell. In healthy cells, nuclear transport, both into and out of the nucleus, is a normal and regular occurrence that is tightly regulated and requires specific carrier proteins to occur. XPO1 mediates the export of approximately 220 different mammalian cargo proteins, including the vast majority of tumor
suppressor proteins. Moreover, XPO1 appears to be the only nuclear exporter for most of these tumor suppressor proteins. Cancer cells have increased levels of XPO1, causing the increased export of these tumor suppressor proteins from the nucleus. Since the tumor suppressor proteins need to be located in the nucleus to promote programmed cell death, or apoptosis, XPO1 overexpression in cancer cells counteracts the natural apoptotic process that protects the body from cancer. Due to XPO1 inhibition by our SINE compounds, the export of tumor suppressor proteins is prevented, thereby leading to their accumulation in the nucleus whichnucleus. This subsequently reinitiates and amplifies their natural apoptotic function in cancer cells. This leads to the death of cancer cells through apoptosis with minimal effects on normal cells. The figure below depicts the process by which our SINE compounds inhibit the XPO1 nuclear export of tumor suppressor proteins.
Transient XPO1 Inhibition by SINE Compounds
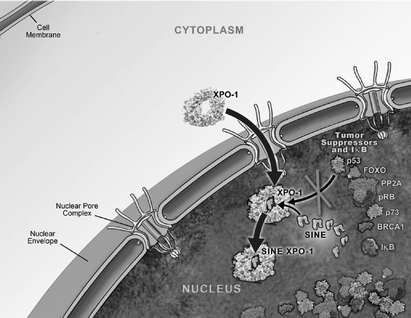
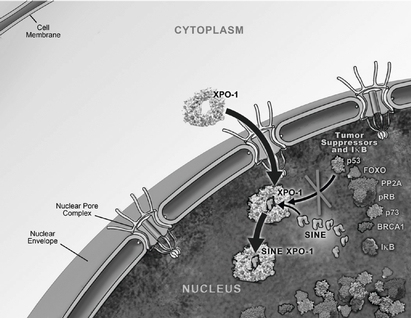
We are currently conducting multiple open-label clinical trials of Selinexor, including three open-label Phase 1 clinical trials, of Selinexor, the first in patients with various advanced hematological malignancies, the second in patients with various advanced or metastatic solid tumor malignancies and the third, a food effect study, in patients who have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. In these trials, we have observed preliminary evidence of anti-cancer activity of Selinexor across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. Our hematological malignancy trial consists of three arms.six arms, in which Selinexor is administered as a monotherapy in Arms 1-5 and in combination in Arm 6. Arm 1 includes patients with certain chronic B-cell malignancies, including non-Hodgkin's lymphoma, or NHL, chronic lymphocytic leukemia, or CLL, multiple myeloma, or MM and Waldenström's Macroglobulinemia, or WM; Arm 2 includes patients with acute myeloid leukemia, andor AML; Arm 3 includes patients with T-cell lymphomas. Inlymphomas; Arm 4 includes patients with chronic myeloid leukemia, or CML; Arm 5 includes patients with acute lymphocytic leukemia, or ALL; and Arm 6 includes patients on combination therapy who have MM and are taking low-dose dexamethasone (20mg) in combination with each biweekly dose of Selinexor.
Of the patients evaluated in our hematological malignancy trial as of September 20, 2013,May 13, 2014, we have observed complete responses or remissions, partial responses or remissions, minimal responses or stable disease in a number of these patients, all as determined in accordance with commonly accepted evaluation criteria for the specific indication. For example, partialPartial or minimal responses or stable disease have been observed in 74%70% of patients with relapsed and/or refractory chronic B-cell malignancies. In patients
with relapsed and/or refractory acute myeloid leukemia, or AML, as of September 20, 2013,May 13, 2014, we have observed complete remissions, partial remissions, morphologic leukemia-free state or stable disease in 47%49% of patients, some for longer than three months. In 45%Among eight patients with multiple myeloma treated with Selinexor in combination with low-dose dexamethasone (20mg twice weekly), complete, partial or minor responses were observed in 75% of patients as of June 5, 2014. Of the patients in the solid tumor malignancy trial evaluated as of September 20, 2013,May 13, 2014, we have observed partial responses or stable disease in 49%, all as determined in accordance with Response Evaluation Criteria In Solid Tumors, or RECIST.
Assuming continuedWe have initiated Phase 2 studies of Selinexor in several indications, including prostate cancer, gynecologic malignancies (ovarian, cervical and uterine cancers) and glioblastoma and we expect initial data during 2015. In addition, we expect to initiate a Phase 2 clinical trial in squamous head, neck or lung cancers during 2014. We have also initiated a Phase 1/2 study of Selinexor in combination with decitabine (Dacogen) in patients with AML and additional combination studies are expected to begin in 2014 and 2015.
We have initiated one registration-directed clinical trial and, assuming positive results from our ongoing Phase 1 clinical trials of Selinexor and based onpending regulatory feedback, we plan to initiate Phase 2/3registration-directed clinical trials of Selinexor in two canceradditional hematological malignancy indications in the first half ofduring 2014. Like Phase 2 clinicalWe refer to these trials our planned Phase 2/3 clinical trialsas registration-directed because they are controlled studies designed to further assess the efficacy of Selinexor and also possible adverse effects and safety risks of Selinexor. But, like Phase 3 clinical trials, the Phase 2/3 clinical trials are also multi-site trials designed to generate enough data to statistically evaluate the efficacy and safety of Selinexor and to potentially serve as the basis for an application seeking regulatory approval of Selinexor. On June 24, 2014, we announced the initiation of a registration-directed clinical trial for Selinexor in older patients with relapsed refractory AML. We expect to initiate registration-directed clinical trials in Richter's Syndrome (also called Richter's Transformation) during the middle of 2014 and in diffuse large B-cell lymphoma, or DLBCL, in the second half of 2014. Moreover, we plan to select theinitiate up to two additional registration-directed trials in hematological or solid tumor indications, for these Phase 2/3 clinical trials based on the results of our ongoing Phase 1 clinical trials of Selinexor, the level of unmet medical need and the competitive landscape. Assuming positive results from these Phase 2/3 clinical trials, wepotentially including multiple myeloma, in late 2014 or early 2015. We plan to seek regulatory approvals of Selinexor in North America and Europe in each such indication with respect to which we receive positive results and wepositive regulatory feedback. We may seek such approvals in other geographies. In
addition, we expect to initiate two Phase 2 clinical trials of Selinexor in solid tumor malignancies by early 2014. We intend to enter into collaborations for marketing and commercialization of Selinexor in particular geographies at an appropriate time.as well.
We believe that the XPO1-inhibiting SINE compounds that we have discovered and developed to date, including Selinexor, have the potential to provide a novel targeted therapy that enableenables tumor suppressor proteins to remain in the nucleus and promote apoptosis of cancer cells. Moreover, our SINE compounds spare normal cells, which, unlike cancer cells, do not have significant damage to their genetic material, and we believe this selectivity for cancer cells minimizes side effects. We believe that the oral administration of Selinexor and the lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in our Phase 1 clinical trials create the potential for its broad use across many cancer types, including both hematological and solid tumor malignancies. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus.
Another SINE compound, Verdinexor (KPT-335), which is closely related to Selinexor, is being developed for the treatment of pet dogs with lymphomas. The approval of drugs to treat animals is based on a New Animal Drug Application, or NADA, reviewed by the FDA's Center for Veterinary Medicine. Three of the four technical sections of the NADA for Verdinexor have been approved by the FDA, including Effectiveness, Safety and Environmental Impact. We anticipate obtaining a marketing collaborator to complete the final major technical section of the NADA, the Chemicals/Manufacturing/Controls section, which covers the commercial-scale manufacturing, or CMC. of Verdinexor. We believe that the approval of the effectiveness and safety sections of the NADA for Verdinexor for the treatment of dogs with lymphoma helps confirm the activity and tolerability of our novel SINE compounds for the treatment of cancers.
We are focused on building a leading oncology business. Karyopharm was founded by Sharon Shacham, Ph.D., M.B.A., our President and Chief Scientific Officer and President of Research and Development.Officer. We are led by Dr. Shacham and
Dr. Michael Kauffman, our Chief Executive Officer. Dr. Kauffman played a leadership role in the development and approval of Velcade® at Millennium Pharmaceuticals, and of Kyprolis® while serving as Chief Medical Officer at Proteolix and then Onyx Pharmaceuticals. Dr. Shacham has played a leadership role in the discovery and development of many novel drug candidates, which have been or are being tested in human clinical trials, prior to her founding of Karyopharm and while at Karyopharm.
In addition to cancer, we believe that our SINE compounds have the potential to provide therapeutic benefit in a number of additional indications, including autoimmune and inflammatory diseases, wound healing, HIV and influenza. We have discovered and are developing a pipeline of SINE compounds that have shown evidence of activity in preclinical models of inflammation, wound healing and viral infection. We may seek to enter into development, marketing and commercialization collaboration arrangements for our SINE compounds other than Selinexor in non-oncology indications globally.
The table below summarizes the current stages of development of our key human drug candidates and indications for which clinical trials are currently being conducted or indications that we expect to initially focus on for each candidate. We expect to initiate the planned clinical trials of Selinexor described below assuming continued positive results from our ongoing Phase 1 clinical trials and based onpending regulatory feedback. We also expect a number of investigator-sponsored trials, or ISTs, to be initiated for Selinexor in a variety of cancer indications over the next year. These ISTs could consist of single agent or combination studies with other agents in both hematological and solid tumor malignancies.
Our StrategyRecent Developments
As a clinical-stage pharmaceutical company focused on the discovery and development of orally available, novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases, the critical components ofWe recently presented updated data from our business strategy are to:
Risks Associatedafter multiple approved therapies and often after one or more experimental therapies. Anti-cancer activity has been observed with Our Businesstumor reductions and durable disease control across many hematologic and solid tumor malignancies. Several patients have remained on single-agent oral Selinexor for over one year. Adverse events observed in our most recent patient data are generally mild, responsive to standard supportive care and consistent with those previously reported in patients in our Phase 1 clinical trials.
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risksResponse data presented herein are discussed more fullyinterim unaudited data based on reports by physicians at the clinical trial sites. Responses in the "Risk Factors" section of this prospectus immediately following this prospectus summary. These risks includehematological trial are measured using commonly accepted evaluation criteria for the following:specific indication. Responses in the sold tumor trial are measured using RECIST. Responses include:
Disease control rate, or DCR, refers to the percentage of responses that are SD or better (MR or better in the case of MM) and overall response rate, or ORR, refers to the percentage of responses that are PR or better.
Advanced Hematological Malignancies
We have applied for, but not yet received, patent protection forrecently reported data from patients with non-Hodgkin's lymphoma, including diffuse large B-cell lymphoma and Richter's syndrome/transformation (Richter's), acute myeloid leukemia and multiple myeloma as part of our key drug candidates and if we are unable to obtain and maintain patent protection for our drug candidates and other discoveries, or if the scopeongoing Phase 1 clinical trial of Selinexor in patients with various advanced hematological malignancies. The primary objectives of the patent protection obtained is not sufficiently broad, our competitors could developPhase 1 trial are to determine the safety, tolerability and commercialize drugsrecommended Phase 2 dose of orally administered Selinexor. Patients were dosed 3 - 80mg/m2 of Selinexor orally over a four-week cycle, with lower doses initially given ten times per cycle and other discoveries similar or identicalhigher doses given twice weekly. Responses were evaluated every one to ours, and our ability to successfully commercialize our drug candidates and other discoveries may be adversely affected.two cycles.
Our Corporate InformationNon-Hodgkin's Lymphoma
WeFifty-one heavily pretreated patients with relapsed and/or refractory NHL, including DLBCL and Richter's, and mean prior treatment regimens of 4.1 were incorporated under the lawsenrolled in this arm of the StatePhase 1 clinical trial as of DelawareMay 13, 2014. Of this group, 43 patients were evaluable, five patients had tumors deemed non-evaluable and three patients had tumors pending evaluation. Among the 43 evaluable patients, the DCR was 74% across all doses of Selinexor and the ORR was 28%. Responses were observed across all subtypes of NHL, independent of genetic abnormalities, with durable cancer control observed across several patients who remained on study for longer than nine months. Responses in December 2008. Our executive offices43 evaluable patients are located at 2 Mercer Road, Natick, MA 01760, and our telephone number is (508) 975-4820. Our website address is www.karyopharm.com. The information contained in, or accessible through, our website does not constitute part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. The trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.shown below.
Implications of Being an Emerging Growth Company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
Generally, we may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1 billion in annual revenue, we have more than $700 million in market value of our stock held by non-affiliates or we issue more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
| ||
| ||
| ||
| ||
|
The number of shares of our common stock to be outstanding after this offering is based on 2,815,352 shares of our common stock issued and outstanding as of September 30, 2013, including 224,019 shares of unvested restricted stock subject to repurchase by us, and 19,114,241 additional shares of our common stock issuable upon the automatic conversion of all outstanding shares of our preferred stock and excludes:
Unless otherwise indicated, this prospectus reflects and assumes the following:
In addition, unless otherwise indicated, all information in this prospectus gives effect to the 1-for-3.3 reverse stock split of our common stock that was effected on October 25, 2013.
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table presents our summary consolidated financial data. We have derived the following summary of our statement of operations data for the years ended December 31, 2011 and 2012 from our audited consolidated financial statements appearing elsewhere in this prospectus. We have derived the summary of our statement of operations data for the six months ended June 30, 2012 and 2013 and the balance sheet data as of June 30, 2013 from our unaudited consolidated financial statements appearing elsewhere in this prospectus. The unaudited consolidated financial statements include, in the opinion of management, all adjustments that management considers necessary for the fair presentation of the financial information set forth in those statements. Our historical results are not necessarily indicative of the results that may be expected in the future. The summary of our consolidated financial data set forth below should be read together with our consolidated financial statements and the related notes to those statements, as well as "Management's Discussion and Analysis of Financial Condition and Results of Operations," appearing elsewhere in this prospectus.
| | Year Ended December 31, | Six Months Ended June 30, | Period from December 22, 2008 (Date of Inception) to June 30, 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
| | (in thousands, except share and per share amounts) | |||||||||||||||
Consolidated Statement of Operations Data: | ||||||||||||||||
Contract and grant revenue | $ | 152 | $ | 634 | $ | 567 | $ | 366 | $ | 1,245 | ||||||
Operating expenses: | ||||||||||||||||
Research and development | 8,623 | 14,095 | 7,432 | 11,025 | 35,408 | |||||||||||
General and administrative | 1,840 | 2,429 | 1,152 | 1,822 | 6,754 | |||||||||||
Total operating expenses | 10,463 | 16,524 | 8,584 | 12,847 | 42,162 | |||||||||||
Loss from operations | (10,311 | ) | (15,890 | ) | (8,017 | ) | (12,481 | ) | (40,917 | ) | ||||||
Interest income (expense), net | — | 2 | 2 | 1 | (185 | ) | ||||||||||
Net loss | $ | (10,311 | ) | $ | (15,888 | ) | $ | (8,015 | ) | $ | (12,480 | ) | $ | (41,102 | ) | |
Net loss per share applicable to common stockholders—basic and diluted | $ | (10.27 | ) | $ | (8.95 | ) | $ | (5.06 | ) | $ | (5.39 | ) | $ | (45.68 | ) | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders—basic and diluted | 1,004,144 | 1,775,323 | 1,584,607 | 2,315,331 | 899,836 | |||||||||||
Pro forma net loss per share applicable to common stockholders—basic and diluted | $ | (2.74 | ) | $ | (1.27 | ) | ||||||||||
Weighted-average number of common shares used in pro forma net loss per share applicable to common stockholders—basic and diluted | 5,799,420 | 9,796,943 | ||||||||||||||
Pro forma basic and diluted net loss per common share is calculated assuming (i) the settlement of all outstanding shares of our special participation stock into 12,121 shares of our common stock in July 2013 and (ii) the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 19,114,241 shares of common stock upon the closing of this offering, which includes (a) 1,000,000 shares of our Series B preferred stock issued in July 2013, (b) 8,636,362 shares of our Series B-1 preferred stock issued in July 2013, (c) 6,100,000 shares of our series A-2 preferred stock, 1,764,706 shares of our series A-3 preferred stock and 1,538,461 shares of our series A-4
preferred stock, all of which were issued in August 2013, and (d) 12,500,000 shares of our series B preferred stock that we issued in September 2013.
| | As of June 30, 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma(1) | Pro Forma as Adjusted(2) | |||||||
| | (in thousands) | |||||||||
Consolidated Balance Sheet Data: | ||||||||||
Cash and cash equivalents | $ | 17,667 | $ | 61,667 | $ | 138,117 | ||||
Working capital | 15,648 | 59,648 | 136,098 | |||||||
Total assets | 18,477 | 62,477 | 138,927 | |||||||
Total preferred stock and preferred stock subscription | 55,815 | — | — | |||||||
Total stockholders' equity (deficit) | (39,879 | ) | 59,936 | 136,386 | ||||||
InvestingBest Responses in our common stock involves a high degree of risk. Before investing in our common stock, you should consider carefully the risks described below, together with the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus. We believe the risks described below are the risks that are material to usNHL Patients as of the date of this prospectus. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related To Our Financial Position And Need For Additional Capital
We have incurred significant losses since inception. We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was $12.5 million, $15.9 million and $10.3 million for the six months ended June 30, 2013, and for the years ended December 31, 2012 and 2011, respectively. As of June 30, 2013, we had an accumulated deficit of $41.1 million. We have not generated any revenue to date from sales of any drugs and have financed our operations principally through private placements of our preferred stock. We have devoted substantially all of our efforts to research and development. Our lead drug candidate, Selinexor (KPT-330), is in Phase 1 clinical development and our other drug candidates for the treatment of human disease are in preclinical development. As a result, we expect that it will be several years, if ever, before we have a drug candidate ready for commercialization for the treatment of human disease. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:
To become and remain profitable, we must develop and eventually commercialize a drug or drugs with significant market potential, either on our own or with a collaborator. This will require us to be successful in a range of challenging activities, including completing preclinical studies and clinical trials of our drug candidates, obtaining marketing approval for these drug candidates, manufacturing, marketing and selling those drugs for which we may obtain marketing approval and establishing and managing any collaborations for the development, marketing and/or commercialization of our drug candidates. We may never succeed in these activities and, even if we do, may never generate revenues
that are significant or large enough to achieve profitability. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the company and could impair our ability to raise capital, maintain our research and development efforts, expand our business and/or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are an early-stage company. We were incorporated in December 2008 and commenced operations in the first half of 2009. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital, developing our platform, identifying potential drug candidates and conducting preclinical studies and early-stage clinical trials of our drug candidates. Our lead drug candidate is currently in Phase 1 clinical trials and all of our other drug candidates for the treatment of human disease are in preclinical development. We have not yet demonstrated our ability to successfully complete any late-stage clinical trials in humans, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial scale drug, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful drug commercialization. Typically, it takes about six to ten years to develop one new drug from the time it is in Phase 1 clinical trials to when it is available for treating patients. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, as a business with a short operating history, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
As we continue to build our business, we expect our financial condition and operating results may fluctuate significantly from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any particular quarterly or annual periods as indications of future operating performance.
We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our research and drug development programs or commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the clinical trials of, and seek marketing approval for, Selinexor and our other drug candidates. In addition, if we obtain marketing approval for any of our drug candidates, we expect to incur significant commercialization expenses related to drug sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of any collaborator that we may have at such time for any such drug. Furthermore, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and drug development programs or commercialization efforts.
We expect that our existing cash and cash equivalents, exclusive of any proceeds from this offering, will enable us to fund our operating expenses and capital expenditure requirements into early 2015. We expect that the net proceeds from this offering, together with our existing cash and cash
equivalents, will enable us to fund our operating expenses and capital expenditure requirements for at least the next 30 months. Our future capital requirements will depend on many factors, including:
Identifying potential drug candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve drug sales. In addition, our drug candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of drugs that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to our stockholders, including purchasers of common stock in this offering, restrict our operations or require us to relinquish rights to our drug candidates.
Until such time, if ever, as we can generate substantial revenues from the sale of drugs, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances and/or licensing arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities after the closing of this offering, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our future revenue streams, research programs or drug candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our research and drug development or commercialization efforts or grant rights to develop and market drug candidates that we would otherwise prefer to develop and market ourselves.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
As has been widely reported, global credit and financial markets have been experiencing extreme disruptions over the past several years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be compromised by economic downturns, a volatile business environment and unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or do not improve, it may make any necessary equity or debt financing more difficult to secure, more costly or more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could harm our growth strategy, financial performance and stock price and could require us to delay or abandon plans with respect to our business, including clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers or other third parties with which we conduct business may not survive these difficult economic times, which could directly affect our ability to attain our operating goals on schedule and on budget.
Risks Related to the Discovery, Development and Commercialization of Our Drug Candidates
We depend heavily on the success of our lead drug candidate Selinexor (KPT-330), which is currently in Phase 1 clinical trials. Our clinical trials of Selinexor may not be successful. If we are unable to commercialize Selinexor or experience significant delays in doing so, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources in the research and development of our lead drug candidate, Selinexor. Our ability to generate revenues from drugs that treat cancer and other diseases in humans, which we do not expect to occur for several years, if ever, will depend heavily on the successful development, regulatory approval and eventual commercialization of Selinexor.
We cannot commercialize drug candidates in the United States without first obtaining regulatory approval for the drug from the United States Food and Drug Administration, or FDA; similarly, we cannot commercialize drug candidates outside of the United States without obtaining regulatory approval from similar regulatory authorities outside of the United States. Even if Selinexor or another drug candidate were to successfully obtain approval from the FDA and non-U.S. regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If we are unable to obtain regulatory approval for Selinexor in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development, marketing and/or commercialization of Selinexor or any other drug candidate that we may discover, in-license, develop or acquire in the future. Furthermore, even if we obtain regulatory approval for Selinexor, we will still need to develop a commercial organization, or collaborate with a third party for the commercialization of Selinexor, establish commercially viable pricing and obtain approval for adequate reimbursement from third-party and government payors. If we or our commercialization collaborators are unable to successfully commercialize Selinexor, we may not be able to generate sufficient revenues to continue our business.
The results of previous clinical trials may not be predictive of future results, and the results of our current and planned clinical trials may not satisfy the requirements of the FDA or non-U.S. regulatory authorities.
We currently have no drugs approved for sale and we cannot guarantee that we will ever have marketable drugs. Clinical failure can occur at any stage of clinical development. Clinical trials may
produce negative or inconclusive results, and we or any future collaborators may decide, or regulators may require us, to conduct additional clinical trials or preclinical studies. We will be required to demonstrate with substantial evidence through well-controlled clinical trials that our drug candidates are safe and effective for use in a diverse population before we can seek regulatory approvals for their commercial sale. Success in early-stage clinical trials does not mean that future larger registration clinical trials will be successful because drug candidates in later-stage clinical trials may fail to demonstrate sufficient safety and efficacy to the satisfaction of the FDA and non-U.S. regulatory authorities despite having progressed through early-stage clinical trials. Drug candidates that have shown promising results in early-stage clinical trials may still suffer significant setbacks in subsequent registration clinical trials. Additionally, the outcome of preclinical studies and early-stage clinical trials may not be predictive of the success of later-stage clinical trials and interim results of a clinical trial are not necessarily indicative of final results.
In addition, the design of a clinical trial can determine whether its results will support approval of a drug and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We have limited experience in designing clinical trials and may be unable to design and conduct a clinical trial to support regulatory approval. Further, if our drug candidates are found to be unsafe or lack efficacy, we will not be able to obtain regulatory approval for them and our business would be harmed. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier clinical trials.
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same drug candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain regulatory approval to market our drug candidates.
Further, our drug candidates may not be approved even if they achieve their primary endpoints in Phase 3 clinical trials or registration trials. The FDA or non-U.S. regulatory authorities may disagree with our trial design and our interpretation of data from preclinical studies and clinical trials. In addition, any of these regulatory authorities may change requirements for the approval of a drug candidate even after reviewing and providing comments or advice on a protocol for a pivotal clinical trial that has the potential to result in approval by the FDA or another regulatory authority. In addition, any of these regulatory authorities may also approve a drug candidate for fewer or more limited indications that we request or may grant approval contingent on the performance of costly post-marketing clinical trials. In addition, the FDA or other non-U.S. regulatory authorities may not approve the labeling claims that we believe would be necessary or desirable for the successful commercialization of our drug candidates.
To date, we have had no discussions with the FDA or non-U.S. regulatory authorities regarding the design of our planned Phase 2/3 clinical trials for Selinexor. We plan to commence two Phase 2/3 clinical trials in the first half ofMay 13, 2014 and, assuming positive results, we plan to seek regulatory approvals of Selinexor in North America and Europe and we may seek such approvals in other geographies. Before obtaining regulatory approvals for the commercial sale of any drug candidate for a target indication, we must demonstrate with substantial evidence gathered in preclinical studies and well-controlled clinical studies, and, with respect to approval in the United States, to the satisfaction of the FDA, that the drug candidate is safe and effective for use for that target indication. There is no assurance that the FDA or non-U.S. regulatory authorities would consider our planned Phase 2/3 clinical trials to be sufficient to serve as the basis for approval of Selinexor for any indication. The FDA and non-U.S. regulatory authorities retain broad discretion in evaluating the results of our clinical trials and in determining whether the results demonstrate that Selinexor is safe and effective. If we are
required to conduct additional clinical trials of Selinexor prior to approval, including additional Phase 1 clinical trials that may be required prior to commencing either of our planned Phase 2/3 clinical trials, or an additional Phase 3 clinical trial following completion of our planned Phase 2/3 clinical trials, we will need substantial additional funds and there is no assurance that the results of any such additional clinical trials will be sufficient for approval.
The results to date in preclinical studies that conducted by us or our academic collaborators and in Phase 1 clinical trials that we are currently conducting include the response of tumors to Selinexor. We expect that the primary endpoint in any randomized pivotal trials of Selinexor will be either progression free survival, meaning the length of time on treatment until objective tumor progression, or overall survival. We have no clinical data in humans relating to the impact of Selinexor on overall survival; we are gathering information on progression free survival. We have no comparative clinical data between Selinexor and standard or supportive care. If Selinexor does not demonstrate a progression free or overall survival benefit, it will likely not be approved. In some instances, the FDA and other regulatory bodies have accepted overall response rate as a surrogate for a clinical benefit, and have granted regulatory approvals based on this or other surrogate endpoints. Overall response rate is defined as the portion of patients with tumor size reduction of a predefined amount for a minimum time period. For some types of cancer, following discussions with regulatory authorities, we may use overall response rate as a primary endpoint. If Selinexor does not demonstrate a sufficient overall response rate for a particular indication, it will likely not be approved for that indication.
We are very early in our development efforts and have only one drug candidate in clinical development. All of our other drug candidates are still in preclinical development. If we are unable to successfully develop and commercialize our drug candidates or experience significant delays in doing so, our business will be materially harmed.
We are very early in our development efforts and have only one drug candidate, Selinexor, in clinical development. The success of Selinexor and any of our other drug candidates will depend on several factors, including the following:
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our drug candidates, which would materially harm our business.
Our approach to the discovery and development of drug candidates that target Exportin 1, or XPO1, is unproven, and we do not know whether we will be able to develop any drugs of commercial value. If Selinexor is unsuccessful in proving that drug candidates targeting XPO1 have commercial value or experiences significant delays in doing so, our business may be materially harmed.
Our SINE compounds inhibit the nuclear export protein XPO1. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus. Despite promising results to date in preclinical studies of Selinexor that we have conducted and in Phase 1 clinical trials of Selinexor conducted by us or our academic collaborators, we may not succeed in demonstrating safety and efficacy of SINE compounds in our current and future human clinical trials. Any drug candidates that we develop may not effectively prevent the exportation of tumor suppressor and/or growth regulatory proteins from the nucleus in humans with a particular form of cancer. If Selinexor is unsuccessful in proving that drug candidates targeting the regulation of intracellular transport of XPO1 have commercial value or experiences significant delays in doing so, our business may be materially harmed and we may not be able to generate sufficient revenues to continue our business.
We may not be successful in our efforts to identify or discover additional potential drug candidates.
A key element of our strategy is to use our technology platform to build a pipeline of novel drug candidates. The drug discovery that we are conducting using our proprietary technology may not be successful in identifying compounds that are useful in treating cancer or other diseases. Our research programs may initially show promise in identifying potential drug candidates, yet fail to yield drug candidates for clinical development for a number of reasons, including:
Research programs to identify new drug candidates require substantial technical, financial and human resources. We may choose to focus our efforts and resources on a potential drug candidate that ultimately proves to be unsuccessful.
If we are unable to identify suitable compounds for preclinical and clinical development, we will not be able to obtain revenues from sale of drugs in future periods, which likely would result in significant harm to our financial position and adversely impact our stock price.
Clinical drug development is a lengthy and expensive process, with an uncertain outcome. If clinical trials of our drug candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our drug candidates.
Before obtaining marketing approval from regulatory authorities for the sale of our drug candidates, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our drug candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical studies and early-stage clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. For example, the results of our Phase 1 clinical trials of Selinexor to date are based on unaudited data provided by our clinical trial investigators. An audit of this data may change the conclusions drawn from this unaudited data provided by our clinical trial investigators indicating less promising results than we currently anticipate. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their drug candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their drugs.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our drug candidates, including:
If we are required to conduct additional clinical trials or other testing of our drug candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our drug candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
Our drug development costs will also increase if we experience delays in testing or marketing approvals. We do not know whether clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our drug candidates or allow our competitors to bring drugs to market before we do and impair our ability to successfully commercialize our drug candidates and may harm our business and results of operations.
If we experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our drug candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside of the United States. In addition, some of our competitors may have ongoing clinical trials for drug candidates that treat the same indications as our drug candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors' drug candidates.
Patient enrollment is affected by other factors, including:
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our drug candidates, which would cause the value of our company to decline and limit our ability to obtain additional financing.
If serious adverse or unacceptable side effects are identified during the development of our drug candidates or we observe limited efficacy of our drug candidates, we may need to abandon or limit the development of one or more of our drug candidates.
Our lead drug candidate Selinexor is in Phase 1 clinical development and our other drug candidates are in preclinical development. Their risk of failure is high. It is impossible to predict when or if any of our drug candidates will prove effective or safe in humans or will receive marketing approval. If our drug candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. For example, even though Selinexor has generally been well-tolerated by patients in our Phase 1 clinical trials to date, in some cases there were adverse events, some of which were serious. The most common drug-related adverse events, or AEs, were gastrointestinal, such as nausea, anorexia, diarrhea and vomiting, and fatigue. These side effects were generally mild or moderate in severity. The most common AEs that were Grade 3 or Grade 4, meaning they were more than mild or moderate in severity, were thrombocytopenia, or low count of platelets in the blood, and neutropenia, or low neutrophil counts. As of September 20, 2013, five patients withdrew from our Phase 1 solid tumor malignancy trial as a result of AEs. We do not gather data relating to patient withdrawals as a result of AEs for our other Phase 1 clinical trials. As of September 20, 2013, six patients across our Phase 1 clinical trials have experienced serious adverse events, or SAEs, deemed by us and the clinical investigator to be related to Selinexor. SAEs generally refer to AEs that result in death, are life threatening, require hospitalization or prolonging of hospitalization, or cause a significant and permanent disruption of normal life functions, congenital anomalies or birth defects, or require intervention to prevent such an outcome.
As a result of these adverse events or further safety or toxicity issues that we may experience in our clinical trials in the future, we may not receive approval to market any drug candidates, which could prevent us from ever generating revenue from the sale of drugs or achieving profitability. Results of our trials could reveal an unacceptably high severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our drug candidates for any or all targeted indications. Many compounds that initially showed promise in early-stage trials for treating cancer or other diseases have later been found to cause side effects that prevented further development of the compound.
The FDA or non-U.S. regulatory authorities may disagree with our and/or our clinical trial investigators' interpretation of data from clinical trials in determining if serious adverse or unacceptable side effects are drug-related.
We, and our clinical trial investigators, currently determine if serious adverse or unacceptable side effects are drug-related. The FDA or non-U.S. regulatory authorities may disagree with our or our clinical trial investigators' interpretation of data from clinical trials and the conclusion by us or our clinical trial investigators that a serious adverse effect or unacceptable side effect was not drug-related. The FDA or non-U.S. regulatory authorities may require more information, including additional preclinical or clinical data to support approval, which may cause us to incur additional expenses, delay or prevent the approval of one of our drug candidates, and/or delay or cause us to change our
commercialization plans, or we may decide to abandon the development or commercialization of the drug candidate altogether.
We may expend our limited resources to pursue a particular drug candidate or indication and fail to capitalize on drug candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and drug candidates that we identify for specific indications. As a result, we may forego or delay pursuit of opportunities with other drug candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial drugs or profitable market opportunities. Our spending on current and future research and development programs and drug candidates for specific indications may not yield any commercially-viable drugs. If we do not accurately evaluate the commercial potential or target market for a particular drug candidate, we may relinquish valuable rights to that drug candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such drug candidate.
Even if any of our drug candidates receive marketing approval, they may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
If any of our drug candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors and others in the medical community. For example, current cancer treatments like chemotherapy and radiation therapy are well-established in the medical community, and doctors may continue to rely on these treatments. If our drug candidates do not achieve an adequate level of acceptance, we may not generate significant revenues from sales of drugs and we may not become profitable. The degree of market acceptance of our drug candidates, if approved for commercial sale, will depend on a number of factors, including:
If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our drug candidates, we may not be successful in commercializing our drug candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale or marketing of pharmaceutical drugs. To date, we have not entered into a strategic collaboration that provides us with access to a collaborator's resources in selling or marketing drugs. To achieve commercial success for any approved drug for which sales and marketing is not the responsibility of any
strategic collaborator that we may have in the future, we must either develop a sales and marketing organization or outsource these functions to other third parties. In the future, we may choose to build a sales and marketing infrastructure to market or co-promote some of our drug candidates if and when they are approved, or enter into collaborations with respect to the sale and marketing of our drug candidates.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time-consuming and could delay any commercial launch of a drug candidate. If the commercial launch of a drug candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our drugs on our own include:
If we enter into arrangements with third parties to perform sales and marketing services, our revenues from the sale of drug or the profitability of these revenues to us are likely to be lower than if we were to market and sell any drugs that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our drug candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our drugs effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our drug candidates.
We face substantial competition, which may result in others discovering, developing or commercializing drugs before or more successfully than we do.
The discovery, development and commercialization of new drugs is highly competitive. We face competition with respect to our current drug candidates, and will face competition with respect to any drug candidates that we may seek to discover and develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of major pharmaceutical, specialty pharmaceutical and biotechnology companies that currently market and sell drugs or are pursuing the development of drugs for the treatment of cancer and the other disease indications for which we are developing our drug candidates, although we believe that to date, none of these competitive drugs and therapies currently in development are based on scientific approaches that are the same as our approach. Potential competitors also include academic institutions and governmental agencies and public and private research institutions.
We are initially focused on developing our current drug candidates for the treatment of cancer. There are a variety of available therapies marketed for cancer. In many cases, cancer drugs are administered in combination to enhance efficacy. Some of these drugs are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established therapies and are widely accepted by physicians, patients and third-party payors. Insurers and other third-party payors may also encourage the use of generic drugs. We expect that if our drug candidates are approved, they will be priced at a significant premium over competitive generic drugs. This may make it difficult for us to achieve our business strategy of using our drug candidates in combination with existing therapies or replacing existing therapies with our drug candidates.
Our competitors may develop drugs that are more effective, safer, more convenient or less costly than any that we are developing or that would render our drug candidates obsolete or non-competitive. Our competitors may also obtain marketing approval from the FDA or other regulatory authorities for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical studies, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or that may be necessary for, our programs.
Even if we are able to commercialize any drug candidates, the drugs may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drugs vary widely from country to country. In the United States, recently enacted legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or drug licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a drug in a particular country, but then be subject to price regulations that delay our commercial launch of the drug, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the drug in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more drug candidates, even if our drug candidates obtain marketing approval.
Our ability to commercialize any drugs successfully also will depend in part on the extent to which reimbursement for these drugs and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the healthcare industry in the United States and elsewhere is cost containment. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any drug that we commercialize
and, if reimbursement is available, we cannot be sure as to the level of reimbursement. Reimbursement may impact the demand for, or the price of, any drug candidate for which we obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any drug candidate for which we obtain marketing approval.
There may be significant delays in obtaining reimbursement for newly-approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside of the United States. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved drugs that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize drugs and our overall financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any drugs that we may develop.
We face an inherent risk of product liability exposure related to the testing of our drug candidates in human clinical trials and will face an even greater risk if we commercially sell any drugs that we may develop. If we cannot successfully defend ourselves against claims that our drug candidates or drugs caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
We currently hold clinical trial liability insurance coverage for up to $5.0 million, but that coverage may not be adequate to cover any and all liabilities that we may incur. We would need to increase our insurance coverage when we begin the commercialization of our drug candidates, if ever. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Verdinexor (KPT-335), our drug candidate for the treatment of pet dogs with newly-diagnosed and first time relapse lymphomas, is in Phase 2 clinical development. We expect that we will submit the safety and effectiveness sections of a New Animal Drug Application, or NADA, for Verdinexor to the FDA by early 2014. If this clinical trial does not produce positive results or if Verdinexor is not approved by the FDA, this may raise safety and efficacy concerns for Selinexor, as the anti-cancer activity and adverse event profile of Verdinexor in dogs with lymphomas provided support for our decision to move Selinexor into Phase 1 clinical trials.
As part of the drug discovery and development process, we have used spontaneously occurring pet dog cancers as a surrogate model for human malignancies. Dog lymphomas respond to chemotherapy in a manner similar to their human counterparts (human non-Hodgkin's lymphomas) and display a comparable genetic profile. The anti-cancer activity of our drug candidate Verdinexor (KPT-335) in a Phase 1 clinical trial in dogs with certain lymphomas provided support for our decision to move Selinexor, our closely-related human drug candidate, into Phase 1 clinical trials. We are currently conducting a Phase 2b clinical trial of Verdinexor in dogs with newly-diagnosed or first time relapse lymphomas. We have received a Minor Use / Minor Species, or MUMS, designation from the Center for Veterinary Medicine of the FDA for the treatment of newly-diagnosed or after first relapse lymphomas in dogs with Verdinexor. Our Phase 2b clinical trial is intended to support regulatory approval under the MUMS designation. We expect that we will submit the safety and effectiveness sections of a NADA for Verdinexor to the FDA by early 2014. If this clinical trial of Verdinexor fails to demonstrate safety and efficacy to the satisfaction of the FDA or does not otherwise produce positive results or if Verdinexor is not otherwise approved by the FDA for the indication with respect to which we are submitting an application, this may raise questions regarding Selinexor because we have used dog cancers as a surrogate model for human malignancies. In such an event, Verdinexor's clinical trial results may cause the FDA or non-U.S. regulatory authorities to require more information, including additional preclinical or clinical data to support approval of Selinexor. If the Phase 2b clinical trial of Verdinexor fails to demonstrate safety and efficacy to the satisfaction of the FDA or does not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of Verdinexor. In such an event, we also may not be able to realize our potential to generate revenue from the commercialization of Verdinexor, either on our own or with a collaborator.
Risks Related To Our Dependence On Third Parties
We expect to depend on third parties for the development, marketing and/or commercialization of our drug candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these drug candidates.
We intend to seek third-party collaborators for the development, marketing and/or commercialization of our drug candidates. For example, while we currently plan to conduct two Phase 2/3 clinical trials of Selinexor and make regulatory filings in North America and Europe with respect to the potential approval of Selinexor without a collaborator, we anticipate that we will seek to enter into a collaboration for marketing and commercialization of Selinexor at the appropriate time in the future. In addition, we intend to seek one or more collaborators to aid in the further development, marketing and/or commercialization of selected SINE compounds for inflammatory conditions, viral disorders and wound healing. Our likely collaborators for any collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. If we do enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development, marketing and/or commercialization of our drug candidates. Our ability to generate revenues from these arrangements will depend on our collaborators' abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our drug candidates pose the following risks to us:
Collaboration agreements may not lead to development or commercialization of drug candidates in the most efficient manner, or at all.
If we are not able to establish collaborations as we currently plan, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our drug candidates will require substantial additional cash to fund expenses. As noted above, we expect to collaborate with pharmaceutical and biotechnology companies for the development and/or commercialization of our drug candidates.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator's resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator's evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside of the United States, the potential market for the subject drug candidate, the costs and complexities of manufacturing and delivering such drug candidate to patients, the potential of competing drugs, the existence of uncertainty with respect to our ownership of intellectual property, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative drug candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our drug candidate.
We may also be restricted under then-existing collaboration agreements from entering into future agreements on certain terms with potential collaborators.
Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of such drug candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we may not be able to further develop our drug candidates or bring them to market and generate revenue from sales of drugs.
We rely on third parties to conduct our clinical trials and some aspects of our research and preclinical studies, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research or testing.
We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to conduct our clinical trials. We currently rely and expect to continue to rely on third parties to conduct some aspects of our research and preclinical studies. Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it would delay our drug development activities.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The European Medicines Agency and Health Canada also require us to comply with comparable standards. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties,
meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our drug candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our drug candidates.
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of such third parties could delay clinical development or marketing approval of our drug candidates or commercialization of our drugs, producing additional losses and depriving us of potential revenue from sales of drugs.
We intend to rely on third parties to conduct investigator-sponsored clinical trials of Selinexor and our other drug candidates. Any failure by a third party to meet its obligations with respect to the clinical development of our drug candidates may delay or impair our ability to obtain regulatory approval for Selinexor and our other drug candidates.
We intend to rely on academic and private non-academic institutions to conduct and sponsor clinical trials relating to Selinexor and our other drug candidates. We will not control the design or conduct of the investigator-sponsored trials, and it is possible that the FDA or non-U.S. regulatory authorities will not view these investigator-sponsored trials as providing adequate support for future clinical trials, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results.
Such arrangements will provide us certain information rights with respect to the investigator-sponsored trials, including access to and the ability to use and reference the data, including for our own regulatory filings, resulting from the investigator-sponsored trials. However, we do not have control over the timing and reporting of the data from investigator-sponsored trials, nor do we own the data from the investigator-sponsored trials. If we are unable to confirm or replicate the results from the investigator-sponsored trials or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development of our drug candidates. Further, if investigators or institutions breach their obligations with respect to the clinical development of our drug candidates, or if the data proves to be inadequate compared to the first-hand knowledge we might have gained had the investigator-sponsored trials been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected.
Additionally, the FDA or non-U.S. regulatory authorities may disagree with the sufficiency of our right of reference to the preclinical, manufacturing or clinical data generated by these investigator-sponsored trials, or our interpretation of preclinical, manufacturing or clinical data from these investigator-sponsored trials. If so, the FDA or other non-U.S. regulatory authorities may require us to obtain and submit additional preclinical, manufacturing, or clinical data before we may initiate our planned trials and/or may not accept such additional data as adequate to initiate our planned trials.
We contract with third parties for the manufacture of our drug candidates for preclinical studies and clinical trials and expect to continue to do so for clinical trials and ultimately for commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our drug candidates or drugs or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not have any manufacturing facilities or personnel. We currently rely, and expect to continue to rely, on third-party manufacturers for the manufacture of our drug candidates for preclinical studies and clinical trials under the guidance of members of our organization. To date, we have obtained starting materials for our supply of the current good manufacturing practices, or cGMP, bulk drug substance for our drug candidates from one third-party manufacturer. We have engaged a separate third-party manufacturer for fill-and-finish services. We do not have a long term supply
agreement with either of these third-party manufacturers, and we purchase our required drug supplies on a purchase order basis.
We expect to rely on third-party manufacturers or third-party collaborators for the manufacture of our drug candidates for clinical trials and ultimately for commercial supply of any of these drug candidates for which we or any of our future collaborators obtain marketing approval. We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
Third-party manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside of the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of drug candidates or drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our drugs and harm our business and results of operations.
Any drugs that we may develop may compete with other drug candidates and drugs for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply or a second source for cGMP bulk drug substance or fill-and-finish services. If our current contract manufacturers cannot perform as agreed, we may be required to replace those manufacturers. Although we believe that there are several potential alternative manufacturers who could manufacture our drug candidates, we may incur added costs and delays in identifying and qualifying any such replacement.
Our current and anticipated future dependence upon others for the manufacture of our drug candidates or drugs may adversely affect our future profit margins and our ability to commercialize any drugs that receive marketing approval on a timely and competitive basis.
Risks Related To Our Intellectual Property
If we are unable to obtain and maintain patent protection for our drug candidates and other discoveries, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize drugs and other discoveries similar or identical to ours, and our ability to successfully commercialize our drug candidates and other discoveries may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary drug candidates and other discoveries. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel drug candidates and other discoveries that are important to our business. To date, one patent has issued that relates to XPO1 inhibitors, other than our key drug candidates, and their use in targeted therapeutics. We cannot be certain that any patents will issue with claims that cover any of our key drug candidates or other discoveries or drug candidates.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our drug candidates or other discoveries, or which effectively prevent others from commercializing competitive drugs and discoveries. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, in some foreign jurisdictions, our ability to secure patents based on our filings in the United States may depend, in part, on our ability to timely obtain assignment of rights to the invention from the employees and consultants who invented the technology. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we were the first to make the inventions claimed in our patent or pending patent applications, or that we were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside of the United States, the first to file a patent application is entitled to the patent. In March 2013, the United States transitioned to a first-inventor-to-file system in which, assuming the other requirements for patentability are met, the first inventor to file a patent application is entitled to the patent. We may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, revocation, reexamination, or post-grant orinter partes review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our discoveries or drugs and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us
with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative discoveries or drugs in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical discoveries and drugs, or limit the duration of the patent protection of our discoveries and drug candidates. Given the amount of time required for the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents and other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, or may refuse to stop the other party from using the intellectual property at issue. An adverse result in any litigation could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of any future collaborators that we may have to develop, manufacture, market and sell our drug candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our drug candidates and technology, including interference proceedings before the U.S. Patent and Trademark Office. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. No litigation asserting such infringement claims is currently pending against us, and we have not been found by a court of competent jurisdiction to have infringed a third party's intellectual property rights; however, if we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our drug candidates and using our technology. However, we may not be able to obtain any required license on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same intellectual property licensed to us. We could be forced, including by court order, to cease commercializing the infringing intellectual property or drug or to cease using the infringing technology. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our drug candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee's former employer. Although we have no knowledge of any such claims being alleged to date, if such claims were to arise, litigation may be necessary to defend against any such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
Obtaining and maintaining our patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to the United States Patent and Trademark Office, or USPTO, and various foreign patent offices at various points over the lifetime of the patents and/or applications. We have systems in place to remind us to pay these fees, and we rely on our outside counsel to pay these fees when due. Additionally, the USPTO and various foreign patent offices require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply with such provisions, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If such an event were to occur, it could have a material adverse effect on our business.
If we do not successfully extend the term of patents covering our drug candidates under the Hatch-Waxman Amendments and similar foreign legislation, our business may be materially harmed.
Depending upon the timing, duration and conditions of FDA marketing approval, if any, of our drug candidates, one or more of our U.S. patents may be eligible for patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman
Amendments. The Hatch-Waxman Amendments permit a patent term extension of up to five years for a patent covering an approved product as compensation for effective patent term lost during product development and the FDA regulatory review process. However, we may not receive an extension if we fail to apply within applicable deadlines, fail to apply prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request.
In the United States, only a single patent can be extended for each FDA approval, and any patent can be extended only once, for a single product. Laws governing analogous patent term extensions in foreign jurisdictions vary widely, as do laws governing the ability to obtain multiple patents from a single patent family. Because both Selinexor and Verdinexor are protected by a single family of patents and applications, we may not be able to secure patent term extensions for both of these drug candidates in all jurisdictions where these drug candidates are approved, if ever.
If we are unable to obtain a patent term extension for a drug candidate or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that drug candidate, if any, in that jurisdiction will be shortened and our competitors may obtain approval to market competing products sooner. As a result, our revenue could be materially reduced.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for our drug candidates and other discoveries, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, outside scientific collaborators, contract research organizations, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. To the extent that we are unable to timely enter into confidentiality and invention or patent assignment agreements with our employees and consultants, our ability to protect our business through trade secrets and patents may be harmed. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside of the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We have not yet registered our trademarks. Failure to secure those registrations could adversely affect our business.
We have not yet registered our trademarks in the United States or other countries. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would, which could adversely affect our business. We have also not yet registered trademarks for any of our drug candidates in any jurisdiction. When we file trademark applications for our drug candidates those applications may not be allowed for registration, and registered trademarks may not be obtained, maintained or enforced. During trademark registration proceedings in the United States and foreign jurisdictions, we may receive rejections. We are given an opportunity to respond to those rejections, but we may not be able to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered
trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings.
In addition, any proprietary name we propose to use with our key drug candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed drug names, including an evaluation of potential for confusion with other drug names. If the FDA objects to any of our proposed proprietary drug names for any of our drug candidates, if approved, we may be required to expend significant additional resources in an effort to identify a suitable proprietary drug name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA.
Risks Related to Regulatory Approval and Other Legal Compliance Matters
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our drug candidates. As a result, we cannot predict when or if we, or any collaborators we may have in the future, will obtain marketing approval to commercialize a drug candidate.
The research, testing, manufacturing, labeling, approval, selling, marketing, promotion and distribution of drugs are subject to extensive regulation by the FDA and comparable foreign regulatory authorities, whose laws and regulations may differ from country to country. We are not permitted to market our drug candidates in the United States or in other countries until we, or any collaborators we may have in the future, receive approval of an NDA from the FDA or marketing approval from applicable regulatory authorities outside of the United States. Our drug candidates are in early stages of development and are subject to the risks of failure inherent in drug development. We have not submitted an application for or received marketing approval for any of our drug candidates in the United States or in any other jurisdiction. We have limited experience in conducting and managing the clinical trials necessary to obtain marketing approvals, including FDA approval of an NDA.
The process of obtaining marketing approvals, both in the United States and abroad, is a lengthy, expensive and uncertain process. It may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the drug candidates involved.
In addition, changes in marketing approval policies during the development period, changes in or the enactment or promulgation of additional statutes, regulations or guidance or changes in regulatory review for each submitted drug application, may cause delays in the approval or rejection of an application. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical studies, clinical trials or other studies and testing. In addition, varying interpretations of the data obtained from preclinical studies and clinical trials could delay, limit or prevent marketing approval of a drug candidate. Any marketing approval we, or any collaborators we may have in the future, ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved drug not commercially viable.
Any delay in obtaining or failure to obtain required approvals could materially adversely affect our ability or that of any collaborators we may have to generate revenue from the particular drug candidate, which likely would result in significant harm to our financial position and adversely impact our stock price.
Failure to obtain marketing approval in international jurisdictions would prevent our drug candidates from being marketed abroad.
In order to market and sell our drugs in the European Union and many other jurisdictions, we, and any collaborators we may have in the future, must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The marketing approval process outside of the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside of the United States, it is required that the drug be approved for reimbursement before the drug can be approved for sale in that country. We, and any collaborators we may have in the future, may not obtain approvals from regulatory authorities outside of the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside of the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA.
Even if we, or any collaborators we may have in the future, obtain marketing approvals for our drug candidates, the terms of approvals and ongoing regulation of our drugs may limit how we, or they, manufacture and market our drugs, which could materially impair our ability to generate revenue.
Once marketing approval has been granted, an approved drug and its manufacturer and marketer are subject to ongoing review and extensive regulation. We, and any collaborators we may have in the future, must therefore comply with requirements concerning advertising and promotion for any of our drug candidates for which we or they obtain marketing approval. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the drug's approved labeling. Thus, we, and any collaborators we may have in the future, may not be able to promote any drugs we develop for indications or uses for which they are not approved.
In addition, manufacturers of approved drugs and those manufacturers' facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMPs, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We, our contract manufacturers, our future collaborators and their contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with cGMPs.
Accordingly, assuming we, or our future collaborators, receive marketing approval for one or more of our drug candidates, we, and our future collaborators, and our and their contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control.
If we, and our future collaborators, are not able to comply with post-approval regulatory requirements, we, and our future collaborators, could have the marketing approvals for our drugs withdrawn by regulatory authorities and our, or our future collaborators', ability to market any future drugs could be limited, which could adversely affect our ability to achieve or sustain profitability. Further, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
Any of our drug candidates for which we, or our future collaborators, obtain marketing approval in the future could be subject to post-marketing restrictions or withdrawal from the market and we, and our future collaborators, may be subject to substantial penalties if we, or they, fail to comply with regulatory requirements or if we, or they, experience unanticipated problems with our drugs following approval.
Any of our drug candidates for which we, or our future collaborators, obtain marketing approval in the future, as well as the manufacturing processes, post-approval studies and measures, labeling, advertising and promotional activities for such drug, among other things, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a drug candidate is granted, the approval may be subject to limitations on the indicated uses for which the drug may be marketed or to the conditions of approval, including the requirement to implement a Risk Evaluation and Mitigation Strategy, which could include requirements for a restricted distribution system.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a drug. The FDA and other agencies, including the Department of Justice, or the DOJ, closely regulate and monitor the post-approval marketing and promotion of drugs to ensure that they are manufactured, marketed and distributed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers' communications regarding off-label use and if we, or our future collaborators, do not market any of our drug candidates for which we, or they, receive marketing approval for only their approved indications, we, or they, may be subject to warnings or enforcement action for off-label marketing. Violation of the Federal Food, Drug, and Cosmetic Act and other statutes, including the False Claims Act, relating to the promotion and advertising of prescription drugs may lead to investigations or allegations of violations of federal and state health care fraud and abuse laws and state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our drugs or their manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
Recently-enacted and future legislation may increase the difficulty and cost for us and our future collaborators to obtain marketing approval of and commercialize our drug candidates and affect the prices we, or they, may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our drug candidates, restrict or regulate post-approval activities and affect our ability, or the ability of our future collaborators, to profitably sell any drugs for which we, or they, obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or our future collaborators, may receive for any approved drugs.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products and could decrease the coverage and price that we, or our future collaborators, may receive for any approved drugs. While the MMA only addresses drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
More recently, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the PPACA.
Among the provisions of the PPACA of potential importance to our drug candidates are the following:
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for any of our drug candidates for which marketing approval is obtained.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue from sales of drugs, attain profitability, or commercialize our drug candidates.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our drug candidates, if any, may be. In addition, increased scrutiny by the United States Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us and our future collaborators to more stringent drug labeling and post-marketing testing and other requirements.
Our relationships with healthcare providers and physicians and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third party payors will play a primary role in the recommendation and prescription of any drugs for which we obtain marketing approval. Our future arrangements with third party payors, healthcare providers and physicians may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any drugs for which we obtain marketing approval. These include the following:
program or making false statements relating to healthcare matters, and, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms and technical safeguards, with respect to maintaining the privacy, security and transmission of individually identifiable health information;
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Our employees may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological and radioactive materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers' compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or commercialization efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Laws and regulations governing any international operations we may have in the future may preclude us from developing, manufacturing and selling certain drug candidates outside of the United States and require us to develop and implement costly compliance programs.
If we expand our operations outside of the United States, we must comply with numerous laws and regulations in each jurisdiction in which we plan to operate. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. The anti-bribery provisions of the FCPA are enforced primarily by the DOJ. The Securities and Exchange Commission, or SEC, is involved with enforcement of the books and records provisions of the FCPA.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the United States, or the sharing with certain non-U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. If we
expand our presence outside of the United States, it will require us to dedicate additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing, or selling certain drugs and drug candidates outside of the United States, which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial penalties, including suspension or debarment from government contracting. Violation of the FCPA can result in significant civil and criminal penalties. Indictment alone under the FCPA can lead to suspension of the right to do business with the U.S. government until the pending claims are resolved. Conviction of a violation of the FCPA can result in long-term disqualification as a government contractor. The termination of a government contract or relationship as a result of our failure to satisfy any of our obligations under laws governing international business practices would have a negative impact on our operations and harm our reputation and ability to procure government contracts. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA's accounting provisions.
Governments outside of the United States tend to impose strict price controls, which may adversely affect our revenues from the sales of drugs, if any.
In some countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug. To obtain reimbursement or pricing approval in some countries, we, or our future collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our drug to other available therapies. If reimbursement of our drugs is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be materially harmed.
Risks Related To Employee Matters And Managing Growth
Our future success depends on our ability to retain our President and Chief Executive Officer, our President of Research and Development and Chief Scientific Officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Michael Kauffman, M.D., Ph.D., our President, Chief Executive Officer and Chief Medical Officer, and Sharon Shacham, Ph.D., M.B.A., our President of Research and Development and Chief Scientific Officer, as well as the other principal members of our management and scientific teams. Although we have entered into formal employment agreements with Drs. Kauffman and Shacham, these agreements do not prevent them from terminating their employment with us at any time. We do not maintain "key person" insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
Michael Kauffman, M.D., Ph.D. and Sharon Shacham, Ph.D., M.B.A. are married. The separation or divorce of the couple in the future could adversely affect our business.
Dr. Kauffman, our President, Chief Executive Officer and Chief Medical Officer and member of our board of directors, and Dr. Shacham, our President of Research and Development and Chief Scientific Officer, are married. They are two of our executive officers and are a vital part of our operations. If they were to become separated or divorced or could otherwise not amicably work with each other, one of them may decide to cease his or her employment with us or it could negatively impact our working environment. Alternatively, their work performance may not be satisfactory if they become preoccupied with issues relating to their personal situation. In these cases, our business could be materially harmed.
We expect to expand our development, regulatory and future sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and, potentially, sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Our business and operations may be materially adversely affected in the event of computer system failures.
Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our drug candidates could be delayed.
Risks Related To Our Common Stock And This Offering
After this offering, our executive officers, directors and principal stockholders will maintain the ability to control all matters submitted to stockholders for approval.
Upon the closing of this offering, our executive officers, directors and stockholders who each owned more than 5% of our outstanding common stock before this offering will, in the aggregate, beneficially own shares representing approximately 70% of our capital stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws that will become effective upon the closing of this offering may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The initial public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent shares are issued under outstanding options, you will incur further dilution. Based on an assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover of this prospectus, you will experience immediate dilution of $10.05 per share, representing the difference between our pro forma as adjusted net tangible book value per share after giving effect to this offering and the assumed initial public offering price per share. In addition, purchasers of common stock in this offering will have contributed approximately
46% of the aggregate price paid by all purchasers of our stock but will own only approximately 21% of our common stock outstanding after this offering.
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. The initial public offering price for our common stock will be determined through negotiations with the underwriters. Although we have applied to list our common stock on The NASDAQ Global Market, an active trading market for our shares may never develop or be sustained following this offering. If an active market for our common stock does not develop or is not sustained, it may be difficult for you to sell shares you purchase in this offering without depressing the market price for the shares, or at all.
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our stock, the price of our stock could decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us or our business. We do not currently have and may never obtain research coverage by industry or financial analysts. If no or few analysts commence coverage of us, the trading price of our stock would likely decrease. Even if we do obtain analyst coverage, if one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our stock, which in turn could cause our stock price to decline.
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our common stock in this offering.
Our stock price is likely to be volatile. The stock market in general and the market for pharmaceutical and biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price. The market price for our common stock may be influenced by many factors, including:
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management's attention and our resources, which could harm our business.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our drug candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We are an "emerging growth company," and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements, only two years of audited financial statements and a correspondingly reduced "Management's Discussion and Analysis of Financial Condition and Results of Operations" disclosure, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In this prospectus, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, and particularly after we are no longer an "emerging growth company," we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and NASDAQ have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance. We estimate that our incremental costs resulting from operating as a public company may be between $2.0 million and $4.0 million per year.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, we will be required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. In the course of the preparation and external audit of our consolidated financial statements, we and our independent registered public accounting firm identified "significant deficiencies" in our internal control over financial reporting related to the insufficient staffing of our finance department. A significant deficiency is a deficiency, or combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of a company's financial reporting. Following the identification of these control deficiencies, we have taken actions and measures to improve our internal control over financial reporting by hiring additional employees and consultants at various appropriate levels. Our remediation efforts may not, however, enable us to avoid material weaknesses or other significant deficiencies in the future. There is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, of our common stock will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding 27,596,260 shares of common stock based on the number of shares outstanding as of September 30, 2013. This includes the shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, 21,929,593 shares are currently restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the "Shares Eligible for Future Sale" section of this prospectus. Moreover, after this offering, holders of an aggregate of 19,114,241 shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Underwriting" section of this prospectus.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. These statements include all matters that are not related to present facts or current conditions or that are not historical facts, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the "Risk Factors" section, that could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make.
You should read this prospectus, the documents that we reference in this prospectus and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus are made as of the date of this prospectus and we do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
We estimate that the net proceeds from our issuance and sale of 5,666,667 shares of our common stock in this offering will be approximately $76.5 million, assuming an initial public offering price of $15.00 per share, which is the midpoint of the range set forth on the cover of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares of our common stock in full, we estimate that our net proceeds will be approximately $88.3 million.
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) the net proceeds to us from this offering by $5.3 million, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, as follows:
This expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development, feedback from regulatory authorities, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our drug candidates, and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering.
Pending use of the proceeds as described above, we intend to invest the proceeds in a variety of capital preservation investments, including short-term, interest-bearing, investment-grade instruments and U.S. government securities.
We have not declared or paid any cash dividends on our capital stock since our inception. We intend to retain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry and general publications and research, surveys and studies conducted by third parties generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2013, as follows:
You should read this information in conjunction with our consolidated financial statements and the related notes appearing at the end of this prospectus and the "Management's Discussion and
Analysis of Financial Condition and Results of Operations" section, the "Selected Consolidated Financial Data" section and other financial information contained in this prospectus.
| | As of June 30, 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma | Pro Forma as Adjusted | |||||||
| | (in thousands, except share and per share data) | |||||||||
Cash and cash equivalents | $ | 17,667 | $ | 61,667 | $ | 138,117 | ||||
Preferred stock subscription | 13,980 | — | — | |||||||
Series A convertible preferred stock, par value $0.0001 per share; 20,937,500 shares authorized, 20,937,500 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 20,778 | — | — | |||||||
Series A-2 convertible preferred stock, par value $0.0001 per share; 6,100,000 shares authorized, no shares issued or outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | — | — | — | |||||||
Series A-3 convertible preferred stock, par value $0.0001 per share; 1,764,706 shares authorized, no shares issued or outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | — | — | — | |||||||
Series A-4 convertible preferred stock, par value $0.0001 per share; 1,538,461 shares authorized, no shares issued or outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | — | — | — | |||||||
Series B convertible preferred stock, par value $0.0001 per share; 30,000,000 shares authorized, 10,600,000 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 21,057 | — | — | |||||||
Special participation stock, par value $0.0001 per share; 10,000 shares authorized, 10,000 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | — | — | — | |||||||
Common stock, par value $0.0001 per share; 80,000,000 shares authorized, 2,450,510 shares issued and outstanding, actual; 80,000,000 shares authorized, 21,576,872 shares issued and outstanding, pro forma and 100,000,000 shares authorized, 27,243,539 shares issued and outstanding, pro forma as adjusted | — | 2 | 3 | |||||||
Additional paid-in capital | 1,223 | 101,036 | 177,485 | |||||||
Accumulated deficit | (41,102 | ) | (41,102 | ) | (41,102 | ) | ||||
Total stockholders' equity (deficit) | (39,879 | ) | 59,936 | 136,386 | ||||||
Total capitalization | $ | 15,936 | $ | 59,936 | $ | 136,386 | ||||
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, which is the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total stockholders' equity (deficit) and total capitalization on a pro forma as adjusted basis by approximately $5.3 million, assuming that the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The table above does not include:
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of June 30, 2013 was approximately $(39.9) million, or $(14.50) per share of common stock. Our historical net tangible book value is the amount of our total tangible assets less our total liabilities. Historical net tangible book value per share is our historical net tangible book value divided by the number of shares of common stock outstanding as of June 30, 2013.
Our pro forma net tangible book value as of June 30, 2013 was $59.9 million, or $2.74 per share of our common stock. Pro forma net tangible book value per share represents our total tangible assets less our total liabilities, divided by the number of shares of our common stock outstanding after giving effect to (i) the settlement of all outstanding shares of our special participation stock into 12,121 shares of our common stock in July 2013 and (ii) the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 19,114,241 shares of our common stock upon the closing of this offering, including (a) 1,000,000 shares of our series B preferred stock issued in July 2013, (b) 8,636,362 shares of our series B-1 preferred stock issued in July 2013, and receipt of proceeds therefrom, (c) 6,100,000 shares of our series A-2 preferred stock, 1,764,706 shares of our series A-3 preferred stock and 1,538,461 shares of our series A-4 preferred stock, all of which were issued in August 2013, and (d) 12,500,000 shares of our series B preferred stock that we issued in September 2013, and receipt of proceeds therefrom.
After giving effect to the sale of 5,666,667 shares of common stock that we are offering at an assumed initial public offering price of $15.00 per share, which is the midpoint of the range set forth on the cover of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of June 30, 2013 would have been approximately $136.3 million, or approximately $4.95 per share. This amount represents an immediate increase in pro forma net tangible book value of $2.21 per share to our existing stockholders and an immediate dilution in pro forma net tangible book value of approximately $10.05 per share to new investors purchasing shares of common stock in this offering. We determine dilution by subtracting the pro forma as adjusted net tangible book value per share after giving effect to this offering from the assumed initial public offering price per share.
The following table illustrates this dilution:
Assumed initial public offering price per share | $ | 15.00 | |||||
Historical net tangible book value per share as of June 30, 2013 | $ | (14.50 | ) | ||||
Increase per share attributable to the conversion of outstanding preferred stock | 17.24 | ||||||
Pro forma net tangible book value per share as of June 30, 2013 | 2.74 | ||||||
Increase per share attributable to this offering | 2.21 | ||||||
Pro forma as adjusted net tangible book value per share after this offering | $ | 4.95 | |||||
Dilution per share to new investors | $ | 10.05 | |||||
If the underwriters exercise their option to purchase additional shares of our common stock in full in this offering, the pro forma as adjusted net tangible book value per share after the offering would be $5.22, the increase in pro forma net tangible book value per share to existing stockholders would be $2.48 and the dilution per share to new investors would be $9.78 per share, in each case
assuming an initial public offering price of $15.00 per share, which is the midpoint of the range set forth on the cover of this prospectus.
A $1.00 increase (decrease) in the assumed initial public offering price of $15.00 per share, the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value after this offering by $5.3 million and the pro forma as adjusted net tangible book value per share after this offering by $0.20 and would increase (decrease) the dilution per share to new investors in this offering by $0.20 per share, in each case, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The following table summarizes, on a pro forma basis as of June 30, 2013, the differences between the number of shares purchased from us, the total consideration paid to us in cash and the average price per share paid by existing stockholders and to be paid by new investors. The calculation below is based on an assumed initial public offering price of $15.00 per share, which is the midpoint of the range set forth on the cover of this prospectus, before deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| | | | Total Consideration | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Shares Purchased | | ||||||||||||||
| | Average Price Per Share | |||||||||||||||
| | Number | Percent | Amount | Percent | ||||||||||||
Existing stockholders | 21,864,198 | 79 | % | $ | 100,211,000 | 54 | % | $ | 4.58 | |||||||
New investors | 5,666,667 | 21 | 85,000,000 | 46 | 15.00 | |||||||||||
Total | 27,530,865 | 100 | % | $ | 185,211,000 | 100 | % | |||||||||
If the underwriters exercise their option to purchase additional shares of our common stock in full:
The foregoing tables and calculations are based on the number of shares of our common stock outstanding as of June 30, 2013 after giving effect to (i) the settlement of all outstanding shares of our special participation stock into 12,121 shares of our common stock in July 2013 and (ii) the automatic conversion immediately prior to the closing of the offering of all outstanding shares of our preferred stock into an aggregate of 19,114,241 shares of our common stock, including (a) 1,000,000 shares of our series B preferred stock issued in July 2013, (b) 8,636,362 shares of our series B-1 preferred stock issued in July 2013, (c) 6,100,000 shares of our series A-2 preferred stock, 1,764,706 shares of our series A-3 preferred stock and 1,538,461 shares of our series A-4 preferred stock, all of which were issued in August 2013, and (d) 12,500,000 shares of our series B preferred stock that we issued in September 2013, and excludes:
To the extent any of these outstanding options is exercised, there will be further dilution to new investors. To the extent all of such outstanding options had been exercised as of June 30, 2013, the pro forma as adjusted net tangible book value per share after this offering would be $4.86, and dilution per share to new investors would be $10.14.
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated financial data in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and the related notes appearing elsewhere in this prospectus.
The consolidated statements of operations data for the years ended December 31, 2011 and 2012 and the consolidated balance sheet data as of December 31, 2011 and 2012 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of operations data for the six months ended June 30, 2012 and 2013 and the consolidated balance sheet data as of June 30, 2013 are derived from our unaudited consolidated financial statements included in this prospectus. The unaudited consolidated financial statements include, in the opinion of management, all adjustments that management considers necessary for the fair presentation of the financial information set forth in those statements. Our historical results are not necessarily indicative of the results to be expected in any future period.
| | Year Ended December 31, | Six Months Ended June 30, | Period from December 22, 2008 (Date of Inception) to June 30, 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
| | (in thousands, except share and per share data) | |||||||||||||||
Consolidated Statement of Operations Data: | ||||||||||||||||
Contract and grant revenue | $ | 152 | $ | 634 | $ | 567 | $ | 366 | $ | 1,245 | ||||||
Operating expenses: | ||||||||||||||||
Research and development | 8,623 | 14,095 | 7,432 | 11,025 | 35,408 | |||||||||||
General and administrative | 1,840 | 2,429 | 1,152 | 1,822 | 6,754 | |||||||||||
Total operating expenses | 10,463 | 16,524 | 8,584 | 12,847 | 42,162 | |||||||||||
Loss from operations | (10,311 | ) | (15,890 | ) | (8,017 | ) | (12,481 | ) | (40,917 | ) | ||||||
Interest income (expense), net | — | 2 | 2 | 1 | (185 | ) | ||||||||||
Net loss | $ | (10,311 | ) | $ | (15,888 | ) | $ | (8,015 | ) | $ | (12,480 | ) | $ | (41,102 | ) | |
Net loss per share applicable to common stockholders—basic and diluted | $ | (10.27 | ) | $ | (8.95 | ) | $ | (5.06 | ) | $ | (5.39 | ) | $ | (45.68 | ) | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders—basic and diluted | 1,004,144 | 1,775,323 | 1,584,607 | 2,315,331 | 899,836 | |||||||||||
Pro forma net loss per share applicable to common stockholders—basic and diluted | $ | (2.74 | ) | $ | (1.27 | ) | ||||||||||
Weighted-average number of common shares used in pro forma net loss per share applicable to common stockholders—basic and diluted | 5,799,420 | 9,796,943 | ||||||||||||||
Pro forma basic and diluted net loss per common share is calculated assuming (i) the settlement of all outstanding shares of our special participation stock into 12,121 shares of our common stock in July 2013 and (ii) the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 19,114,241 shares of common stock upon the closing of this offering, which
includes (a) 1,000,000 shares of our series B preferred stock issued in July 2013, (b) 8,636,362 shares of our Series B-1 preferred stock issued in July 2013, (c) 6,100,000 shares of our series A-2 preferred stock, 1,764,706 shares of our series A-3 preferred stock and 1,538,461 shares of our series A-4 preferred stock, all of which were issued in August 2013, and (d) 12,500,000 shares of our series B preferred stock that we issued in September 2013.
| | As of December 31, | | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | As of June 30, 2013 | |||||||||
| | 2011 | 2012 | ||||||||
| | (in thousands) | |||||||||
Consolidated Balance Sheet Data: | ||||||||||
Cash and cash equivalents | $ | 6,512 | $ | 391 | $ | 17,667 | ||||
Working capital | 4,749 | (976 | ) | 15,648 | ||||||
Total assets | 7,224 | 1,311 | 18,477 | |||||||
Total preferred stock and preferred stock subscription | 17,758 | 27,258 | 55,815 | |||||||
Total stockholders' deficit | (12,651 | ) | (27,877 | ) | (39,879 | ) | ||||
MANAGEMENT'S DISCUSSION AND ANALYSIS OFFINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our selected consolidated financial data and the consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and analysis contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under the section entitled "Risk Factors" and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
Overview
We are a clinical-stage pharmaceutical company founded in December 2008 by Dr. Sharon Shacham. We are focused on the discovery and development of novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases. Our scientific expertise is focused on the understanding of the regulation of intracellular transport between the nucleus and the cytoplasm. We have discovered and developed novel, small molecule,SelectiveInhibitors ofNuclearExport, orSINE, compounds that inhibit the nuclear export protein XPO1. We have worldwide rights to these SINE compounds. Our lead drug candidate, Selinexor (KPT-330), is an XPO1 inhibitor being evaluated in multiple open-label Phase 1 clinical trials in patients with heavily pretreated relapsed and/or refractory hematological and solid tumor malignancies. As of September 20, 2013, we had administered Selinexor to over 170 patients in these trials. Preliminary evidence of anti-cancer activity has been observed in some patients and Selinexor has been sufficiently well-tolerated to allow many of these patients to remain on therapy for prolonged periods, including several who have remained on study for over 8-12 months. To our knowledge, no other XPO1 inhibitors are in clinical development at the present time.
We have devoted substantially all of our efforts to research and development. We expect that it will be several years, if ever, before we have a drug candidate ready for commercialization for the treatment of human disease. To date, we have financed our operations primarily with the net proceeds from the private placements of our preferred stock.
Since inception, we have incurred significant operating losses. Our net loss was $12.5 million, $15.9 million and $10.3 million for the six months ended June 30, 2013, and for the years ended December 31, 2012 and 2011, respectively. As of June 30, 2013, we had an accumulated deficit of $41.1 million. We have not generated any revenue to date from sales of any drugs.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:
Financial Operations Overview
Revenue
To date, we have not generated any revenue from drug sales and do not expect to generate any revenue from drug sales for many years, if ever. Our ability to generate drug revenues will depend on the successful development and eventual commercialization of our drug candidates.
To date, our only revenue is from foundation and government grants and contracts.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts, and the development of our drug candidates, which include:
Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to us by our vendors on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development.
Since our research and development has been focused primarily on using our drug discovery and optimization platform to identify drug candidates, we have not historically tracked research and development costs by project. In addition, we use our employee and infrastructure resources across multiple research and development projects. We expect to track specific project costs when additional drug candidates enter clinical trials in humans.
The successful development of our drug candidates is highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the remainder of the development of these drug candidates. We are also unable to predict when, if ever, material net cash inflows will commence from any drug candidates. This is due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
A change in the outcome of any of these variables with respect to the development of any of our drug candidates would significantly change the costs and timing associated with the development of that drug candidate.
Research and development activities are central to our business model. Drug candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect research and development costs to increase significantly for the foreseeable future as our drug candidates progress in clinical trials. However, we do not believe that it is possible at this time to accurately project total program-specific expenses through commercialization. There are numerous factors associated with the successful commercialization of any of our drug candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development. Additionally, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in executive, finance and administrative functions. Other significant costs include facility costs not otherwise included in research and development expenses, legal fees relating to patent and corporate matters and fees for accounting and consulting services.
We anticipate that our general and administrative expenses will increase in the future to support continued research and development activities, potential commercialization of our drug candidates and increased costs of operating as a public company. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate that the increased costs associated with being a public company will include expenses related to services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which we have prepared in accordance with United States generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those described in greater detail below. We base our
estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our consolidated financial statements included elsewhere in this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Accrued Research and Development Expenses
As part of the process of preparing our consolidated financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. The significant estimates in our accrued research and development expenses include fees paid to contract research organizations, or CROs, and contract manufacturing organizations, or CMOs, in connection with research and development activities for which we have not yet been invoiced.
We base our expenses related to CROs and CMOs on our estimates of the services received and efforts expended pursuant to quotes and contracts with CROs and CMOs that conduct research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepayment accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in us reporting amounts that are too high or too low in any particular period.
Stock-based Compensation
We issue stock-based awards to employees and non-employees, generally in the form of stock options and restricted stock. We account for our stock-based awards in accordance with Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 718,Compensation—Stock Compensation, or ASC 718. ASC 718 requires all stock-based awards to employees, including grants of employee stock options and modifications to existing stock options, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values. We account for stock-based awards to non-employees in accordance with FASB ASC Topic 505-50,Equity-Based Payments to Non-Employees, which requires the fair value of the award to be re-measured at fair value as the award vests. We recognize the compensation cost of stock-based awards to employees on a straight-line basis over the vesting period of the award and by using an accelerated attribution model for awards to non-employees. Described below is the methodology we have utilized in measuring stock-based compensation expense. Following the consummation of this
offering, stock option and restricted stock values will be determined based on the quoted market price of our common stock.
We estimate the fair value of our options to employees and non-employees using the Black-Scholes option pricing model, which requires the input of highly subjective assumptions, including (a) the expected volatility of our stock, (b) the expected term of the option, (c) the risk-free interest rate, and (d) expected dividends. Due to the lack of a public market for the trading of our common stock and a lack of company specific historical and implied volatility data, we have based our estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. For these analyses, we have selected companies with comparable characteristics to ours including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the options. We compute the historical volatility data using the closing prices for the selected companies' shares during the equivalent period of the calculated expected term of our options. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available. We have estimated the expected term of our employee stock options using the "simplified" method, whereby the expected term equals the average of the vesting term and the original contractual term of the option. For non-employee stock options, we utilize the contractual term of the option. The risk-free interest rates for periods within the expected term of the option are based on the U.S. Treasury yield curve in effect during the period the options were granted.
We are also required to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from estimates. We use historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those options that are expected to vest. To the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates were revised. Stock-based compensation expense recognized in the financial statements is based on options that are ultimately expected to vest.
We have computed the fair value of employee and non-employee stock options at date of grant using the following assumptions:
| | Year Ended December 31, | Six Months Ended June 30, | ||||||
|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||
Expected volatility | 78%–79% | 75%–92% | 75%–81% | 83%–93% | ||||
Expected term (in years) | 6.25–10 | 6.25–10 | 6.25–10 | 6.25–10 | ||||
Risk-free interest rate | 1.18%–2.62% | 0.85%–1.76% | 1.10%–1.18% | 1.07%–2.44% | ||||
Expected dividend yield | 0.0% | 0.0% | 0.0% | 0.0% | ||||
The following table presents the grant dates, number of underlying shares and related exercise prices, of stock options granted between March 1, 2012 and October 24, 2013, along with the corresponding fair value per share utilized to calculate stock-based compensation expense:
Date of Grant | Number of Shares | Exercise Price Per Share | Common Stock Fair Value Per Share on Grant Date | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
April 18, 2012 | 12,424 | $ | 0.26 | $ | 0.26 | |||||
October 10, 2012 | 96,501 | $ | 1.49 | $ | 1.49 | |||||
December 6, 2012 | 60,151 | $ | 1.49 | $ | 2.87 | |||||
January 17, 2013 | 8,303 | $ | 1.49 | $ | 2.87 | |||||
February 25, 2013 | 18,181 | $ | 1.49 | $ | 2.87 | |||||
July 25, 2013 | 110,606 | $ | 4.75 | $ | 7.26 | |||||
September 3, 2013 | 1,131,666 | $ | 4.75 | $ | 10.40 | |||||
October 1, 2013 | 113,333 | $ | 5.64 | $ | 12.18 | |||||
Stock-based compensation related to stock options totaled approximately $264,000 for the year ended December 31, 2012 and $187,000 for the six months ended June 30, 2013. As of June 30, 2013, we had $323,000 of total unrecognized stock-based compensation expense, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of approximately 2.2 years. We expect the impact of our stock-based compensation expense for stock options granted to employees and non-employees to grow in future periods due to the potential increases in the value of our common stock and headcount.
Determination of the Fair Value of Common Stock on Grant Dates
We have historically granted stock options at exercise prices not less than the fair value of our common stock on the grant dates. As there has been no public market for our common stock to date, the estimated fair value of our common stock has been determined by our board of directors. As a private company with no active public market for our common stock, we have periodically determined for financial reporting purposes the estimated per share fair value of our common stock at various dates using contemporaneous valuations performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants Practice Aid,Valuation of Privately-Held Company Equity Securities Issued as Compensation, also known as the Practice Aid. We performed these contemporaneous valuations as of June 30, 2012, June 30, 2013 and July 21, 2013. In conducting the contemporaneous valuations, we considered all objective and subjective factors that we believed to be relevant for each valuation conducted, including our best estimate of our business condition, prospects and operating performance at each valuation date. Within the contemporaneous valuations performed, a range of factors, assumptions and methodologies were used. The significant factors included:
The dates of our contemporaneous valuations have not always coincided with the dates of our option grants. In determining the exercise prices of the options set forth in the table above, our board of directors considered, among other things, the most recent contemporaneous valuations of our common stock and our assessment of additional objective and subjective factors we believed were relevant as of the grant date. The additional factors considered when determining any changes in fair value between the most recent contemporaneous valuation and the grant dates included, when
available, the prices paid in recent transactions involving our equity securities, as well as our stage of development, our operating and financial performance and current business conditions.
In July 2013, based on our review of overall market conditions and the improving market for biopharmaceutical initial public offerings, our board of directors determined that a significant shift was occurring with respect to the valuation we could achieve in an IPO and directed our management to begin the process of preparing our company for an IPO. We selected underwriters and held an organizational meeting in August 2013. We believe these events increased the probability of an IPO scenario and therefore, in connection with the preparation of our consolidated financial statements, we re-assessed the fair value of our common stock for financial reporting purposes at interim dates between the contemporaneous valuations where there were stock option grants. Retrospective valuations were completed as of December 31, 2012 and March 31, 2013.
On October 23, 2013, the pricing committee of our board of directors determined the estimated price range for our initial public offering, after consultation with the underwriters. The estimated price range that was determined by the pricing committee of our board of directors implied a higher IPO valuation than we used in our contemporaneous common stock valuations. In connection with the process of determining the estimated price range, we re-assessed the fair value of our common stock for financial reporting purposes through retrospective valuations as of July 31, 2013, September 3, 2013 and September 30, 2013.
Common Stock Valuation Methodologies
These contemporaneous and retrospective valuations were prepared in accordance with the guidelines in the Practice Aid, which prescribes several valuation approaches for setting the value of an enterprise, such as the cost, market and income approaches, and various methodologies for allocating the value of an enterprise to its common stock. We generally used the market approach, in particular, the guideline company and precedent transaction methodologies, based on inputs from comparable public companies' equity valuations and comparable acquisition transactions, to estimate the enterprise value of our company.
Methods Used to Allocate Our Enterprise Value to Classes of Securities
In accordance with the Practice Aid, we considered the various methods for allocating the enterprise value across our classes and series of capital stock to determine the fair value of our common stock at each valuation date. The methods we considered consisted of the following:
We used two market approaches, the Recent Transactions Method and Guideline Initial Public Offering Transactions to estimate the value of our equity. In utilizing the Recent Transactions Method, we utilized an Option Pricing Method to estimate the equity value of our company, taking into account
the price paid for preferred stock acquired by investors. The Option Pricing Method treats common stock and preferred stock as call options on the enterprise's value, with exercise prices based on the liquidation preference of the preferred stock. The common stock has value only if the funds available for distribution to shareholders exceed the value of the liquidation preference at the time of a liquidity event (for example, merger or sale). The common stock is modeled as a call option that gives its owner the right but not the obligation to buy the underlying enterprise value at a predetermined exercise price. In the model, the exercise price is based on a comparison with the enterprise value rather than, as in the case of a "regular" call option, a comparison with a per-share stock price. Thus, common stock is considered to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the preferred stock is liquidated. Typically option-pricing models such as the Black-Scholes model or a form of a lattice model (e.g. binomial) would be used to price the call option. The Option Pricing Method considers the various terms of the stockholder agreements—including the level of seniority among the securities, dividend policy, conversion ratios, and cash allocations—upon liquidation of the enterprise. In addition, the method implicitly considers the effect of the liquidation preference as of the future liquidation date, not as of the valuation date. In addition, at various dates, we utilized a direct waterfall analysis to allocate the value to the respective classes of capital stock under the Guideline Initial Public Offering Transactions method. In each case, we applied probability weightings to the various methodologies based upon our assessment of our prospects of a sale/merger transaction or an initial public offering of our common stock.
June 30, 2012 Common Stock Valuation
For the contemporaneous valuation at June 30, 2012, we relied upon the back-solve method of the Option Pricing Method, or OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the weighted average purchase price per share for the series A-2 preferred stock and series A-3 preferred stock agreed to be purchased by investors per the terms of the Series A-2 and Series A-3 Preferred Stock Purchase Agreement. Given the proximity to the execution of the Series A-2 and Series A-3 Preferred Stock Purchase Agreement, we believe the weighted average per share purchase price of the series A-2 and series A-3 preferred stock provides an indication of the fair value of our equity as of June 30, 2012.
We estimated the time to liquidity as 1.0 year based on then-current plans and estimates of our board of directors and management regarding our likely need to raise additional capital within this timeframe. The risk free rate was estimated based on the 1-year yield on government bonds.
We applied a discount for lack of marketability of 30% to reflect the antidilution protection rights of the preferred stock, rights to participate in future financing rounds and the fact our shares are not actively traded on a stock exchange and are essentially illiquid securities. The resulting value, which represented the estimated fair value of our common stock as of June 30, 2012, was $1.49 per share.
| ||
| ||
| ||
| ||
|
December 31, 2012 Retrospective Common Stock Valuation
For the retrospective valuation at December 31, 2012, we used a hybrid of PWERM and the OPM, which we refer to as the hybrid method, with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred stock round to the IPO price for a group of drug development companies which completed IPOs in the two years preceding the appraisal date.
For the OPM scenario, we relied upon the back-solve method of the OPM. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on negotiations between our board of directors and investors regarding the pricing of our series A-3 preferred stock, which was subsequently agreed to be purchased by investors in February of 2013.
The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
December 31, 2012 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 15% | 85% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 1.25 yrs | 2.0 yrs | ||
Discount Rate—Common Stock | 30% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $5.21 | $3.47 |
In applying the market approach to estimate our future enterprise value under the IPO scenario, as described previously, it was assumed that a liquidity event would occur in 1.25 years. Given our development pipeline, as of the valuation date, the selected enterprise value in the IPO scenario was based on pre-money IPO market data for transactions between the minimum and the 25th percentile of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 2.0 years. The selected enterprise value utilized in the OPM scenario was based on the terms of the Series A-3 Preferred Stock Purchase Agreement executed in February 2013, pursuant to which the series A-3 preferred stock investors agreed to purchase shares of our series A-3 preferred stock. As a result, we used the back-solve method to determine the equity value of our common stock, taking into account the series A-3 preferred stock purchase price per share.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned an 85% weighting to the OPM scenario and 15% to the IPO scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of December 31, 2012, was $2.87 per share.
The estimated per share fair value of our common stock calculated in our valuation as of December 31, 2012 of $2.87 per share increased from the June 30, 2012 valuation of $1.49 per share primarily due to the following factors:
March 31, 2013 Retrospective Common Stock Valuation
For the retrospective valuation at March 31, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred stock round to the IPO price for a group of drug development companies which completed IPOs in the two years preceding the appraisal date.
For the OPM scenario, we relied upon the back-solve method of the OPM. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the terms of our series B preferred stock financing.
For the retrospective valuation at March 31, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transactions Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
March 31, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 40% | 60% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 1.0 yr | 1.75 yrs | ||
Discount Rate—Common Stock | 30% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $5.61 | $2.81 |
In applying the market approach to estimate our future enterprise value under the IPO scenario, as described previously, it was assumed that a liquidity event would occur in 1.0 year. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on pre-money IPO market data for transactions between the 25th percentile and the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.75 years. The selected enterprise value utilized in the OPM scenario was based on the terms of the series B preferred stock financing, including the terms of the shares of series B preferred stock purchased at the initial closing. The series B preferred stock investors were also obligated to purchase additional shares of our series B preferred stock at various dates in the future. We allocated the series B preferred stock purchase price among the purchased series B preferred shares and the two additional call options to acquire additional series B preferred shares. We used the back-solve method to determine the equity value of our common stock, taking into account the terms of the series B preferred stock financing.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 60% weighting to the OPM scenario and 40% to the IPO scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of March 31, 2013, was $3.43 per share.
The estimated per share fair value of our common stock calculated in our valuation as of March 31, 2013 of $3.43 per share increased from the December 31, 2012 valuation of $2.87 per share primarily due to the following factors:
June 30, 2013 Contemporaneous Common Stock Valuation
For the contemporaneous valuation at June 30, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred stock round to the IPO price for a group of drug development companies which completed IPOs in the three years preceding the appraisal date.
For the OPM scenario, we relied upon the back-solve method of the OPM. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the terms of the series B preferred stock financing.
For the valuation at June 30, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transactions Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
June 30, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 50% | 50% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.75 yrs | 1.50 yrs | ||
Discount Rate—Common Stock | 30% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $5.97 | $2.77 |
In applying the market approach to estimate our future enterprise value under the IPO scenario, as described previously, it was assumed that a liquidity event would occur in 0.75 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on pre-money IPO market data for transactions between the 25th percentile and the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.50 years. The selected enterprise value utilized in the OPM scenario was based on the terms of the series B preferred stock financing, including the terms of the shares of series B preferred stock purchased at the initial closing. The series B preferred stock investors were also obligated to purchase additional shares of our series B preferred stock at various dates in the future. We allocated the series B preferred stock purchase price among the purchased series B preferred shares and the two additional call options to acquire additional series B preferred shares. We used the back-solve method to determine the equity value of our common stock, taking into account the terms of the series B preferred stock financing.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 50% weighting
to the OPM scenario and 50% to the IPO scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of June 30, 2013, was $3.96 per share.
The estimated per share fair value of our common stock calculated in our valuation as of June 30, 2013 of $3.96 per share increased from the March 31, 2013 valuation of $3.43 per share primarily due to the following factors:
July 21, 2013 Common Stock Valuation
For the contemporaneous valuation at July 21, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred stock round to the IPO price for a group of drug development companies which completed IPOs in the three years preceding the appraisal date.
For the OPM scenario, we relied upon the back-solve method of the OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the terms of our series B-1 preferred stock financing.
For the contemporaneous valuation at July 21, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transactions Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
July 21, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 50% | 50% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.50 yrs | 1.50 yrs | ||
Discount Rate—Common Stock | 30% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $6.37 | $4.52 |
In applying the market approach to estimate our future enterprise value under the IPO exit scenario, as described previously, it was assumed that a liquidity event would occur in 0.50 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on pre-money IPO market data for transactions approximating the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.50 years. The selected enterprise value utilized in the OPM scenario was based on the terms of our series B-1 preferred stock financing. The series B-1 preferred stock investors purchased series B-1 preferred stock. We used the back-solve method to determine the equity value of the common stock, taking into account the series B-1 preferred stock financing.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 50% weighting to the OPM scenario and 50% to the IPO scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of July 21, 2013, was $4.75 per share.
The estimated per share fair value of our common stock calculated in our valuation as of July 21, 2013 of $4.75 per share increased from the June 30, 2013 valuation of $3.96 per share primarily due to the following factors:
July 31, 2013 Retrospective Common Stock Valuation
For the retrospective valuation at July 31, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred round to the IPO price for a group of drug development companies which completed IPOs in the 2 years preceding the appraisal date and considered several recently completed oncology IPOs in late July 2013.
For the OPM scenario, we relied upon the back-solve method of the OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the terms of our Series B-1 preferred stock financing with existing and new investors.
For the retrospective valuation at July 31, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transaction Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value
under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
July 31, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 50% | 50% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.29 yrs | 1.50 yrs | ||
Discount Rate—Common Stock | 30% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $11.35 | $4.52 |
In applying the market approach to estimate our future enterprise value under the IPO exit scenario, as described previously, it was assumed that a liquidity event would occur in 0.29 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on the pre-money IPO market data for transactions approximating the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.50 years. The selected enterprise value utilized in the OPM scenario was based on the recent Series B-1 preferred stock financing. The Series B-1 preferred stock investors purchased 8.2 million shares of Series B-1 preferred stock. We used the back-solve method to determine the equity value of our common stock, taking into account the Series B-1 preferred stock purchase price per share.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 50% weighting to the OPM scenario and 50% to the IPO scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of July 31, 2013, was $7.26 per share.
September 3, 2013 Retrospective Common Stock Valuation
For the retrospective valuation at September 3, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred round to the IPO price for a group of drug development companies which completed IPOs in the 2 years preceding the appraisal date and considered several recently completed oncology IPOs in July and August 2013.
For the OPM scenario, we relied upon the back-solve method of the OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the Series B-1 preferred stock financing with existing and new investors.
For the retrospective valuation at September 3, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transaction Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value
under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
September 3, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 65% | 35% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.21 yrs | 1.25 yrs | ||
Discount Rate—Common Stock | 25% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $14.29 | $4.52 |
In applying the market approach to estimate our future enterprise value under the IPO exit scenario, as described previously, it was assumed that a liquidity event would occur in 0.21 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on the pre-money IPO market data for transactions approximating the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.25 years. The selected enterprise value utilized in the OPM scenario was based on the recent Series B-1 preferred stock financing. We used the back-solve method to determine the equity value of our common stock, taking into account the Series B-1 preferred stock purchase price per share.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 65% weighting to the IPO scenario and 35% to the OPM scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of September 3, 2013, was $10.40 per share.
September 24, 2013 Common Stock Valuation
For the contemporaneous valuation at September 24, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred round to the IPO price for a group of drug development companies which completed IPOs in the two years preceding the appraisal date.
For the OPM scenario, we relied upon the back-solve method of the OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve for the equity value and corresponding value of the common stock based on the completed series B-1 preferred stock financing with existing and new investors.
For the contemporaneous valuation at September 24, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transactions Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value
under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
September 24, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 65% | 35% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.17 yrs | 1.25 yrs | ||
Discount Rate—Common Stock | 25% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $7.00 | $4.52 |
In applying the market approach to estimate our future enterprise value under the IPO exit scenario, as described previously, it was assumed that a liquidity event would occur in 0.17 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on the pre-money IPO market data for transactions approximating the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.25 years. The selected enterprise value utilized in the OPM scenario was based on the recent series B-1 preferred stock financing. The series B-1 preferred stock investors purchased 8.2 million shares of series B-1 preferred stock. We used the back-solve method to determine the equity value of the common stock, taking into account the series B-1 preferred stock financing.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 65% weighting to the IPO scenario and 35% to the OPM scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of September 24, 2013, was $5.64 per share.
The estimated per share fair value of our common stock calculated in our valuation as of September 24, 2013 of $5.64 per share increased from the July 21, 2013 valuation of $4.75 per share primarily due to the following factors:
September 30, 2013 Retrospective Common Stock Valuation
For the retrospective valuation at September 30, 2013, we used the hybrid method with one IPO scenario and one OPM scenario. As an indicator of value for the IPO scenario, we considered the increase in value, or step-up, from the most recent preferred round to the IPO price for a group of drug development companies which completed IPOs in the 2 years preceding the appraisal date and considered several recently completed oncology IPOs for the last three months ending September 30, 2013.
For the OPM scenario, we relied upon the back-solve method of the option pricing method, or OPM, which derives the implied equity value for one type of equity security from a contemporaneous transaction involving another type of equity security. We applied the OPM back-solve method to solve
for the equity value and corresponding value of the common stock based on the Series B-1 preferred stock financing with existing and new investors.
For the contemporaneous valuation at September 30, 2013, we used the Recent Transactions Method and the Guideline Initial Public Offering Method to determine the value of our equity. The Recent Transaction Method was used to determine the value of our company under a possible merger/sale transaction and the Guideline Initial Public Offering Method was used to estimate the equity value under the potential IPO scenario. The specific facts and circumstances considered by our board of directors in assessing these key valuation assumptions included those noted in the following table:
September 30, 2013 Major Assumptions | IPO | OPM | ||
|---|---|---|---|---|
Probability of Scenario | 80% | 20% | ||
Discount for Marketability | 0% | 30% | ||
Timeline to Liquidity | 0.17 yrs | 1.25 yrs | ||
Discount Rate—Common Stock | 25% | N/A | ||
Estimated per share Fair Value of Common Stock—Before Discounts | $14.42 | $4.52 |
In applying the market approach to estimate our future enterprise value under the IPO exit scenario, as described previously, it was assumed that a liquidity event would occur in 0.17 years. Given our development pipeline and clinical trials, as of the valuation date, the selected enterprise value in the IPO scenario was based on the pre-money IPO market data for transactions approximating the median of the observed range. The selected enterprise value contemplated our stage of development, amount of capital raised, depth of clinical candidates and number of partnerships/collaborations in comparison to the IPO transactions.
In applying the market approach to estimate our aggregate future enterprise value under the OPM scenario, as described previously, it was assumed that a liquidity event would occur in 1.25 years. The selected enterprise value utilized in the OPM scenario was based on the Series B-1 preferred stock financing. We used the back-solve method to determine the equity value of our common stock, taking into account the Series B-1 preferred stock purchase price per share.
We applied a discount for lack of marketability of 30% and 0% under the OPM and IPO scenarios, respectively. We assessed the probabilities of each transaction and assigned a 80% weighting to the IPO scenario and 20% to the OPM scenario based on our assessment of our development pipeline and market conditions. The resulting value, which represented the estimated fair value of our common stock as of September 30, 2013, was $12.18 per share.
Initial public offering price
In consultation with the underwriters for this offering, we determined the estimated price range for this offering, as set forth on the cover page of this prospectus. The midpoint of the price range is $15.00 per share. In comparison, our estimate of the fair value of our common stock was $12.18 per share as of September 30, 2013. We note that, as is typical in IPOs, the estimated price range for this offering was not derived using a formal determination of fair value, but was determined by negotiation between us and the underwriters. Among the factors that were considered in setting this range were the following:
The midpoint of the estimated price range for this offering reflects a significant increase over the estimated valuation as of September 30, 2013 of $12.18 per share. Investors should be aware of this difference and recognize that the price range for this offering is in excess of our prior valuations. Further, investors are cautioned not to place undue reliance on the valuation methodologies discussed above as an indicator of future stock prices. We believe the difference may be due to the following factors:
determine the fair value of our common stock and this methodology differs from the methodology used to determine the estimated price range for this offering. The estimated price range for this offering was not derived using a formal determination of fair value, but rather was determined by negotiation between us and the underwriters. In particular, the estimate of fair value of our common stock as of September 30, 2013 was not a factor in setting the estimated price range for this offering; and
Of the factors described above, we believe that the most important differences between our estimate of the fair value of our common stock as of September 30, 2013 and the midpoint of the estimated price range for this offering are the following:
For the period from September 30, 2013 to October 23, 2013, the date on which the pricing committee of our board of directors determined the estimated price range for this offering:
Investors should be cautioned that the midpoint of the price range set forth on the cover of this prospectus does not necessarily represent the fair value of our common stock, but rather reflects an estimate of the offer price determined in consultation with the underwriters.
There are significant judgments and estimates inherent in the determination of these valuations. These judgments and estimates include assumptions regarding our future performance, including the successful enrollment and completion of our clinical studies as well as the determination of the appropriate valuation methods. If we had made different assumptions, our stock-based compensation expense could have been different. The foregoing valuation methodologies are not the only methodologies available and they will not be used to value our common stock once this offering is complete. We cannot make assurances as to any particular valuation for our common stock. Accordingly, investors are cautioned not to place undue reliance on the foregoing valuation methodologies as an indicator of future stock prices.
JOBS Act
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of specified reduced disclosure
and other requirements that are otherwise applicable generally to public companies. These provisions include:
Generally, we may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1 billion in annual revenue, we have more than $700 million in market value of our stock held by non-affiliates or we issue more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Results of Operations
Comparison of Six Months Ended June 30, 2012 and 2013
The following table summarizes our results of operations for the six months ended June 30, 2012 and 2013, together with the changes in those items in dollars:
| | Six Months Ended June 30, | | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Dollar Change | |||||||||
| | 2012 | 2013 | ||||||||
| | (in thousands) | |||||||||
Contract and grant revenue | $ | 567 | $ | 366 | $ | (201 | ) | |||
Operating expenses: | ||||||||||
Research and development | 7,432 | 11,025 | 3,593 | |||||||
General and administrative | 1,152 | 1,822 | 670 | |||||||
Loss from operations | (8,017 | ) | (12,481 | ) | (4,464 | ) | ||||
Interest income | 2 | 1 | (1 | ) | ||||||
Net loss | $ | (8,015 | ) | $ | (12,480 | ) | $ | (4,465 | ) | |
Contract and Grant Revenue. We recorded revenue of $567,000 and $366,000 for the six months ended June 30, 2012 and 2013, respectively. The decrease is due to recognizing fewer milestones during the six months ended June 30, 2013.
Research and Development Expense. Research and development expense increased by $3.6 million to $11.0 million for the six months ended June 30, 2013 from $7.4 million for the six months ended June 30, 2012. The $3.6 million increase is primarily related to:
These increases are partially offset by a $431,000 decrease in collaboration expense and a $405,000 decrease in toxicology and efficacy studies.
General and Administrative Expense. General and administrative expense increased by $670,000 to $1.8 million for the six months ended June 30, 2013 from $1.2 million for the six months ended June 30, 2012. The $670,000 increase is primarily related to:
Comparison of Years Ended December 31, 2011 and 2012
The following table summarizes our results of operations for the years ended December 31, 2011 and 2012, together with the changes in those items in dollars:
| | Years Ended December 31, | | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Dollar Change | |||||||||
| | 2011 | 2012 | ||||||||
| | (in thousands) | |||||||||
Contract and grant revenue | $ | 152 | $ | 634 | $ | 482 | ||||
Operating expenses: | ||||||||||
Research and development | 8,623 | 14,095 | 5,472 | |||||||
General and administrative | 1,840 | 2,429 | 589 | |||||||
Loss from operations | (10,311 | ) | (15,890 | ) | (5,579 | ) | ||||
Interest income | — | 2 | 2 | |||||||
Net loss | $ | (10,311 | ) | $ | (15,888 | ) | $ | (5,577 | ) | |
Contract and Grant Revenue. Contract and grant revenue increased by $482,000 to $634,000 in 2012 from $152,000 in 2011. The increase in revenue was the result of a full year of revenue recognized in 2012 associated with a grant.
Research and Development Expense. Research and development expense increased by $5.5 million to $14.1 million in 2012 from $8.6 million in 2011. The $5.5 million increase is primarily related to:
These increases are partially offset by a $373,000 decrease in toxicology and efficacy studies.
General and Administrative Expense. General and administrative expense increased by $589,000 to $2.4 million for 2012 from $1.8 million for 2011. The $589,000 increase is primarily related to:
Liquidity and Capital Resources
Sources of Liquidity
To date, we have not generated any material revenues. We have financed our operations to date primarily through private placements of our preferred stock. As of June 30, 2013, we had $17.7 million in cash and cash equivalents. In July 2013, we received proceeds of $19.0 million from the sale and issuance of our series B-1 preferred stock. Also, we issued an additional 12.5 million shares of our series B preferred stock for an aggregate purchase price of $25.0 million in September 2013.
Cash Flows
The following table provides information regarding our cash flows for the years ended December 31, 2011 and 2012, and the six months ended June 30, 2012 and 2013:
| | Years Ended, December 31, | Six Months Ended, June 30, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | |||||||||
| | (in thousands) | ||||||||||||
Net cash used in operating activities | $ | (8,549 | ) | $ | (15,509 | ) | $ | (7,898 | ) | $ | (11,313 | ) | |
Net cash used in investing activities | (376 | ) | (121 | ) | (87 | ) | — | ||||||
Net cash provided by financing activities | 11,992 | 9,509 | 4,507 | 28,589 | |||||||||
Net increase (decrease) in cash and cash equivalents | $ | 3,067 | $ | (6,121 | ) | $ | (3,478 | ) | $ | 17,276 | |||
Net Cash Used in Operating Activities
The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital. Net cash used in operating activities was $7.9 million during the six months ended June 30, 2012 compared to $11.3 million during the six months ended June 30, 2013. The increase in cash used in operating activities during the six months ended June 30, 2013 was driven primarily by an increase in our net loss.
Net cash used in operating activities was $8.5 million for the year ended December 31, 2011 compared to $15.5 million for the year ended December 31, 2012. The increase in cash used in operating activities was driven primarily by an increase in our net loss and by changes in components of working capital, including a decrease in accounts payable and accrued expenses.
Net Cash Used in Investing Activities
Net cash used in investing activities was $87,000 during the six months ended June 30, 2012 compared to none during the six months ended June 30, 2013. The cash used in investing activities was for the purchase of property and equipment.
Net cash used in investing activities was $376,000 during the year ended December 31, 2011 compared to $121,000 during the year ended December 31, 2012. The cash used in investing activities was for the purchase of property and equipment.
Net cash provided by financing activities
Net cash provided by financing activities was $4.5 million during the six months ended June 30, 2012 compared to $28.6 million during the six months ended June 30, 2013. The increase in cash provided by financing activities during the six months ended June 30, 2013 was driven primarily by the proceeds from the sale of preferred stock.
Net cash provided by financing activities was $12.0 million during the year ended December 31, 2011 compared to $9.5 million during the year ended December 31, 2012. The cash provided by financing activities for both periods was primarily from proceeds from the sale of preferred stock and issuance of the preferred stock subscriptions.
Funding requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the clinical trials of, and assuming positive results of our clinical trials and based on regulatory feedback, if and when we seek marketing approval for, Selinexor and our other drug candidates. In addition, if we obtain marketing approval for any of our drug candidates, we expect to incur significant commercialization expenses related to drug sales, marketing, manufacturing and distribution to the extent that such sales, marketing, manufacturing and distribution are not the responsibility of any collaborator that we may have at such time for any such drug. Furthermore, upon the closing of this offering, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
We expect that the net proceeds from this offering, together with our existing cash and cash equivalents, will enable us to fund our operating expenses and capital expenditure requirements for at least the next 30 months. Our future capital requirements will depend on many factors, including:
Identifying potential drug candidates and conducting preclinical studies and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve drug sales. In addition, our drug candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of drugs that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans.
Contractual Obligations
The following table summarizes our significant contractual obligations as of December 31, 2012:
| | Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Total | Less Than 1 Year | 1-3 Years | 3-5 Years | More Than 5 Years | |||||||||||
| | (in thousands) | |||||||||||||||
Operating lease obligations(1) | $ | 201 | $ | 95 | $ | 106 | $ | — | $ | — | ||||||
Purchase obligations(2) | — | — | — | — | — | |||||||||||
Total contractual cash obligations | $ | 201 | $ | 95 | $ | 106 | $ | — | $ | — | ||||||
Royalty payments associated with our agreements have not been included in the above table of contractual obligations as we cannot reasonably estimate if or when they will occur. At this time, no royalty payments are probable of occurrence.
Multiple Myeloma Research Foundation
In July 2011, we entered into a research agreement with the Multiple Myeloma Research Foundation, or MMRF, for the research and development of small molecule XPO1 inhibitor compounds for the treatment of multiple myeloma. Pursuant to the research agreement, MMRF awarded us a $1 million grant, all of which has been paid to us based on our achievement of specified milestones. We own all inventions and other intellectual property that arose or will arise from the conduct of the research program, which we refer to as program inventions and program intellectual property, respectively.
If we, our affiliates, licensees or transferees commercialize products incorporating a program invention or program intellectual property, which we call research program products, we would be obligated to pay to MMRF mid-single-digit royalties as a percentage of worldwide net sales of research program products, including Selinexor, sold by us, our affiliates, licensees or transferees. If we out-license rights to a research program product, we are obligated to pay MMRF a percentage of certain payments we receive from our licensee for the grant of such rights. If we sell all or substantially all of our assets to one or more third parties who were not our stockholders on the effective date of the agreement, or if one or more third parties acquire more than fifty percent of our equity and payments are made directly to our stockholders for the sale of their shares of our stock, each of which we call a change of control, we will be obligated to pay to MMRF a percentage of the value we or our shareholders receive in connection with such change of control. The maximum aggregate amount we may be obligated to pay to MMRF for royalties, out-licensing our rights or as a result of a change of control is $6 million.
While this agreement has expired in accordance with its terms, our payment obligations survive the expiration of the agreement.
Off-Balance Sheet Arrangements
We did not have, during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined under applicable Securities and Exchange Commission rules.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2012 and June 30, 2013, we had cash and cash equivalents of $391,000 and $17.7 million, respectively. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in cash and cash equivalents. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% change in interest rates would not have a material effect on the fair market value of our investment portfolio.
We are also exposed to market risk related to change in foreign currency exchange rates. We contract with CROs and CMOs that are located in Canada and Europe, which are denominated in foreign currencies. We are subject to fluctuations in foreign currency rates in connection with these agreements. We do not currently hedge our foreign currency exchange rate risk. As of December 31, 2012 and June 30, 2013, we had payables and accrued expenses of $535,000 and $342,000, respectively, denominated in foreign currencies.
Overview
We are a clinical-stage pharmaceutical company founded in December 2008 by Dr. Sharon Shacham. We are focused on the discovery and development of novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases. Our scientific expertise is focused on the understanding of the regulation of intracellular transport between the nucleus and the cytoplasm. We have discovered and developed wholly-owned, novel, small molecule,SelectiveInhibitors ofNuclearExport, orSINE, compounds that inhibit the nuclear export protein XPO1. Our lead drug candidate, Selinexor (KPT-330), is an XPO1 inhibitor being evaluated in multiple open-label Phase 1 clinical trials in patients with heavily pretreated relapsed and/or refractory hematological and solid tumor malignancies. As of September 20, 2013, we had administered Selinexor to over 170 patients in these trials. Preliminary evidence of anti-cancer activity has been observed in some patients and Selinexor has been sufficiently well-tolerated to allow many of these patients to remain on therapy for prolonged periods, including several who have remained on study for over 8-12 months. To our knowledge, no other XPO1 inhibitors are in clinical development at the present time.
The nucleus contains a cell's genetic material, or DNA, and acts as the control center of the cell, while the cytoplasm is the intracellular compartment around the nucleus where numerous processes involving proteins and other molecules occur. One of the ways in which the cell regulates the function of a particular protein is by controlling the protein's location within the cell, as a specific function may only occur within a particular location. In healthy cells, nuclear transport, both into and out of the nucleus, is a normal and regular occurrence that is tightly regulated and requires specific carrier proteins to occur. There are seven known nuclear export proteins (Exportins 1 through 7), of which the most well-characterized is Exportin 1, or XPO1, also known as CRM1. XPO1 mediates the export of approximately 220 different mammalian cargo proteins, including the vast majority of tumor suppressor proteins. Moreover, XPO1 appears to be the only nuclear exporter for most of these tumor suppressor proteins. Tumor suppressor proteins are anti-cancer proteins which must be in the nucleus to carry out their main function of detecting damage to genetic material that may indicate cancer, and, subsequently, initiating programmed cell death, or apoptosis, of the damaged cells. Cancer cells have increased levels of XPO1, causing the increased export of these tumor suppressor proteins from the nucleus, and thus counteracting the natural apoptotic process that protects the body from cancer. Due to XPO1 inhibition by our SINE compounds, the export of tumor suppressor proteins is prevented, thereby leading to their accumulation in the nucleus. The accumulation of tumor suppressor proteins in the nucleus reinitiates and amplifies their natural apoptotic function in cancer cells. This leads to the death of cancer cells through apoptosis with minimal effects on normal cells.
We are focused on building a leading oncology business. We were founded in December 2008 by Dr. Sharon Shacham, who established the Company to focus on the discovery and development of small molecule inhibitors of nuclear export. Dr. Shacham has led our company since its inception, and now serves as our Chief Scientific Officer and President of Research and Development, and co-chair of our Scientific Advisory Board. Her computational drug discovery algorithms formed a critical part of the technological basis for our drug discovery and optimization platform, which was used for the discovery of Selinexor, our lead drug candidate. Dr. Shacham has played a leadership role in the discovery and development of many novel drug candidates, which have been or are being tested in human clinical trials, prior to her founding of Karyopharm and while at Karyopharm. Along with Dr. Shacham, we are led by Dr. Michael Kauffman, M.D., Ph.D., who joined the Company in January 2011 as President and Chief Executive Officer. Dr. Kauffman played a leadership role in the development and approval of Velcade® at Millenium Pharmaceuticals, and of Kyprolis® while serving as Chief Medical Officer at Proteolix and then Onyx Pharmaceuticals.
We believe that the XPO1-inhibiting SINE compounds that we have discovered and developed to date, including Selinexor, have the potential to provide a novel targeted therapy that enable tumor suppressor proteins to remain in the nucleus and promote apoptosis of cancer cells. Moreover, our SINE compounds spare normal cells, which, unlike cancer cells, do not have significant damage to their genetic material, and we believe this selectivity for cancer cells minimizes side effects. We believe that the oral administration of Selinexor and the lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in our Phase 1 clinical trials create the potential for its broad use across many cancer types, including both hematological and solid tumor malignancies. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus.
We are currently conducting three open-label Phase 1 clinical trials of Selinexor, the first in patients with various advanced hematological malignancies, the second in patients with various advanced or metastatic solid tumor malignancies and the third, a food effect study, in patients who have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. In these trials, we have observed preliminary evidence of anti-cancer activity of Selinexor across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. Assuming continued positive results from our ongoing Phase 1 clinical trials of Selinexor and based on regulatory feedback, we plan to initiate Phase 2/3 clinical trials of Selinexor in two cancer indications in the first half of 2014. Like Phase 2 clinical trials, our planned Phase 2/3 clinical trials are controlled studies designed to further assess the efficacy of Selinexor and also possible adverse effects and safety risks of Selinexor. But, like Phase 3 clinical trials, the Phase 2/3 clinical trials are also multi-site trials designed to generate enough data to statistically evaluate the efficacy and safety of Selinexor and to potentially serve as the basis for an application seeking regulatory approval of Selinexor. We plan to select the indications for these Phase 2/3 clinical trials based on the results of our ongoing Phase 1 clinical trials of Selinexor, the level of unmet medical need and the competitive landscape. Assuming positive results from these Phase 2/3 clinical trials, we plan to seek regulatory approvals of Selinexor in North America and Europe and we may seek such approvals in other geographies. In addition, we expect to initiate two Phase 2 clinical trials of Selinexor in solid tumor malignancies by early 2014. We intend to enter into collaborations for marketing and commercialization of Selinexor in particular geographies at an appropriate time.
We designed our Phase 1 clinical trials of Selinexor in relapsed and/or refractory hematological malignancies and relapsed and/or refractory solid tumor malignancies to evaluate the safety of Selinexor, to determine the Phase 2 clinical trial dose and dosing schedule and to evaluate preliminary anti-cancer activity of Selinexor. In patients evaluated in our hematological malignancy trial as of September 20, 2013, we have observed complete responses or remissions, partial responses or remissions, minimal responses or stable disease, all as determined in accordance with commonly accepted evaluation criteria for the specific indication. For example, partial or minimal responses or stable disease have been observed in 74% of patients with relapsed and/or refractory chronic B-cell malignancies. In patients with relapsed and/or refractory acute myeloid leukemia, we have observed complete remissions or stable disease in 47% of patients, some for longer than three months. In 45% of patients in the solid tumor malignancy trial evaluated as of September 20, 2013, we have observed partial responses or stable disease, all as determined in accordance with Response Evaluation Criteria In Solid Tumors, or RECIST.
In addition to cancer, we believe that our SINE compounds have the potential to provide therapeutic benefit in a number of additional indications, including autoimmune and inflammatory diseases, wound healing, HIV and influenza. We have discovered and are developing a pipeline of SINE compounds that have shown evidence of activity in preclinical models of inflammation, wound
healing and viral infection. We may seek to enter into development, marketing and commercialization collaboration arrangements for our SINE compounds other than Selinexor in non-oncology indications globally.
The table below summarizes the current stages of development of our key drug candidates and indications for which clinical trials are currently being conducted or indications that we expect to initially focus on for each candidate. We expect to initiate the planned clinical trials of Selinexor described below assuming continued positive results from our ongoing Phase 1 clinical trials and based on regulatory feedback. We also expect a number of investigator-sponsored trials, or ISTs, to be initiated for Selinexor in a variety of cancer indications over the next year. These ISTs could consist of single agent or combination studies with other agents in both hematological and solid tumor malignancies.

In addition, we are currently conducting a Phase 2b clinical trial of Verdinexor (KPT-335), a SINE compound that is closely-related to Selinexor, in pet dogs with newly-diagnosed or first relapse after chemotherapy lymphomas. Our Phase 2b clinical trial is intended to support regulatory approval under the MUMS designation. We plan to submit the safety and effectiveness sections of a New Animal Drug Application to the U.S. Food and Drug Administration for regulatory approval in such indication by early 2014 and, if we obtain such approval, to seek to enter into a collaboration with respect to the commercialization of Verdinexor.
The development of Selinexor, and our other drug candidates, including our other SINE compounds and PAK4 inhibitors, as well as Verdinexor, began with our proprietary drug discovery and optimization platform. We intend to continue using this platform, which includes expertise in computational chemistry, our proprietary virtual chemical library andin silico screening know-how, certain biochemical assays andin silico complexes of the structures of the target proteins bound with
our small molecules, and other trade secrets and know-how, for the discovery and optimization of additional drug candidates for cancer and other major diseases.
Our Strategy
As a clinical-stage pharmaceutical company focused on the discovery and development of orally available, novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases, the critical components of our business strategy are to:
Our Focus: Nuclear Transport
A human cell is divided into various compartments, including the nucleus and the cytoplasm. The nucleus contains a cell's genetic material, or DNA, and is the compartment where gene expression and consequently cellular function is regulated. The cytoplasm is the compartment around the nucleus where translation of gene transcripts, or mRNA, to proteins, assembly of proteins into cellular structural elements, and cellular metabolism of fats, carbohydrates, and proteins, occur. One of the ways in which the cell regulates the function of a particular protein is by controlling the protein's location within the cell, as a specific function may only occur within a particular location. Certain proteins, including tumor suppressor proteins and other growth regulatory proteins, need to be transported from the cytoplasm, where they are made, into the nucleus where they need to be located for their primary functions to occur. The nuclear pore is a complex gate between the nucleus and cytoplasm, closely regulating the import and export of most large molecules, called macromolecules, including many proteins, into and out of the nucleus. In healthy cells, nuclear transport processes of macromolecules in either direction through the nuclear pore are tightly regulated and require specific carrier proteins, including nuclear export proteins, to occur. There are seven known nuclear export proteins. The most well-characterized was discovered in 1999 and is called Exportin 1, or XPO1 (also called CRM1). XPO1 mediates the export of approximately 220 different mammalian cargo proteins, including some growth regulatory proteins and the vast majority of tumor suppressor proteins. Moreover, XPO1 appears to be the only nuclear exporter for most of these tumor suppressor proteins, including those generally referred to as p53, p73, FOXO, pRB, BRCA1 and PP2A.
Cancer is a disease characterized by unregulated cell growth. Cancer typically develops when DNA in normal cells begins to fail and genes that regulate cell growth become disrupted. Tumor suppressor proteins are an integral part of the body's natural defense mechanism in identifying and preventing cancer. They exert their effects on cancer cells once DNA damage is detected by promoting apoptosis. Tumor suppressor proteins can also have an anti-cancer effect by dampening unregulated cell growth and division. In addition to tumor suppressor proteins, cells contain growth regulatory proteins that, when located in the nucleus, are involved in ensuring that cells undergo cell division, or cell growth, only under appropriate circumstances, such as repairing wounds, increasing cell numbers to deal with damage to an organ, or replacing cells that have died through normal circumstances. Growth regulatory proteins are also exported from the nucleus by XPO1 in all cells. Examples of well-characterized growth regulatory proteins are p21, p27 and E2F4.
XPO1 is also the only exporter of the anti-inflammatory protein IkB, the inhibitor of NF-kB. NF-kB is known to play a role in cancer metastasis and resistance to chemotherapy as well as in many inflammatory and autoimmune diseases. Blockade of XPO1 leads to accumulation of IkB in the cell nucleus where it binds to and inhibits NF-kB. In this way, the inhibition of NF-kB may be beneficial in overcoming chemotherapy resistance and in treating autoimmune diseases.
Because tumor suppressor proteins need to be located in the nucleus in order to carry out their anti-cancer activities, their nuclear export, or exit from the nucleus, leads to their being unavailable in the nucleus to identify cancer cells and initiate their death. As XPO1 levels have been shown to be elevated by two- to four-fold in nearly all cancer cells compared to their normal cell counterparts, it appears that cancer cells have co-opted XPO1 to move tumor suppressor proteins out of the nucleus, thereby adversely affecting their ability to identify and initiate the death of cancer cells. Increased levels of XPO1 in cancer cells also lead to excessive nuclear export of growth regulatory proteins and allow cancer cells to divide continuously and inappropriately. Higher levels of XPO1 expression are also generally correlated with poor prognosis and/or resistance to chemotherapies. The figure below depicts the process by which XPO1 mediates the nuclear transport process.
XPO1 Mediation of Nuclear Transport
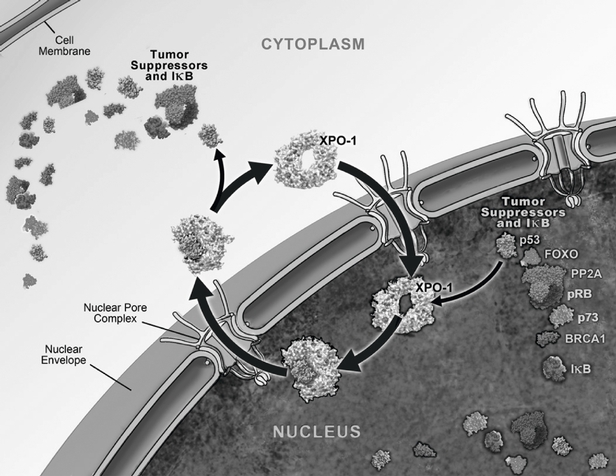
Our Approach: Targeting Nuclear Export with Selective Inhibitors of Nuclear Export, or SINE
Since the discovery of XPO1, a growing body of research has documented that the high levels of XPO1 found in cancer cells are associated with the transport of tumor suppressor proteins and growth regulatory proteins from their site of action in the nucleus into the cytoplasm, where their anti-cancer activity is minimal and they are ultimately degraded. The inhibition of XPO1 cargo binding has been studied for over ten years. XPO1 inhibitors block the nuclear export of tumor suppressor proteins and growth regulatory proteins, leading to accumulation of these proteins in the nucleus and enhancing their anti-cancer activity in the cell. The forced nuclear retention of these proteins can counteract a multitude of the oncogenic pathways that allow cancer cells with severe DNA damage to continue to grow and divide in an unrestrained fashion. One naturally occurring XPO1 inhibitor called
leptomycin B has been shown to have potent anti-cancer activityin vitro, but has been toxic to normal cells. These toxicities to normal cells have been observed in both animals and humans, which we believe are most likely caused by theirreversible nature of leptomycin B binding to XPO1. Because of its observed toxicities in animals and humans, to our knowledge, leptomycin B is no longer being developed.
Our lead drug candidates are first-in-class, oralSelectiveInhibitors ofNuclearExport, orSINE, compounds. We have discovered SINE compounds by applying our proprietary drug discovery and optimization platform to the recently published X-ray structure of XPO1. SINE compounds inhibit XPO1-mediated nuclear-cytoplasmic transport bytransiently binding to the XPO1 cargo binding site, meaning that they block XPO1 cargo binding over an extended period of time, but do not permanently do so. Transient XPO1 inhibition, or inhibition of approximately 12 to 24 hours, which corresponds to the inhibition period that we have observed to date with our SINE compounds, appears to be sufficient for nuclear retention and the increase of tumor suppressor proteins in the nucleus. During this period, the inhibition of XPO1 cargo binding enables tumor suppressor proteins to accumulate in the nucleus of cancer cells and perform their normal role of detecting DNA damage, thereby inhibiting a cancer cell's ability to divide and promoting apoptosis. Healthy cells also build up tumor suppressor proteins in the presence of a SINE compound, but are able to resume normal activity after transient XPO1 inhibition because they have an intact genome with minimal or no DNA damage. The figure below depicts the process by which SINE compounds inhibit the XPO1 nuclear export of tumor suppressor proteins.
Transient XPO1 Inhibition by SINE Compounds
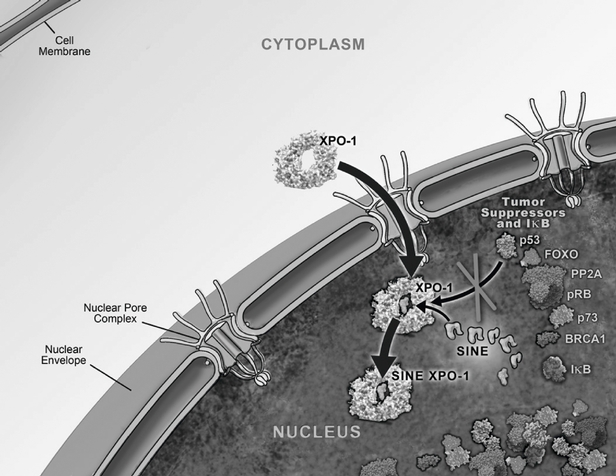
We believe that the XPO1-inhibiting SINE compounds that we have discovered and developed to date, including Selinexor, have the potential to provide a novel targeted therapy that enable tumor suppressor proteins to remain in the nucleus and promote apoptosis of cancer cells. Moreover, our SINE compounds spare normal cells, which, unlike cancer cells, do not have significant damage to their genetic material, and we believe this selectivity for cancer cells minimizes side effects. We believe that the oral administration of Selinexor and the lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in our Phase 1 clinical trials create the potential for its broad use across many cancer types, including both hematological and solid tumor malignancies. We believe that no currently approved cancer treatments or current clinical-stage cancer drug candidates are selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins in the nucleus.
In addition to cancer, we believe that our SINE compounds have the potential to provide therapeutic benefit in a number of additional indications, including autoimmune and inflammatory diseases, wound healing, HIV and influenza. We have discovered and are developing a pipeline of SINE compounds that have shown evidence of activity in preclinical models of inflammation, wound healing and viral infection. Specifically, our SINE compounds have shown potent evidence of anti-inflammatory activity in several animal models of inflammation, including systemic lupus erythematosis, multiple sclerosis and rheumatoid arthritis. Our SINE compounds have also shown evidence of activity as topical formulations in wound healing by accelerating wound closure and improving wound appearance in both mouse and pig models of surgical and/or knife wounds. In addition, in preclinical studies, our SINE compounds have shown evidence of activity against specific viruses which require XPO1 for their replication, including HIV and influenza.
Our Initial Indication: Cancer
Cancer is a leading cause of death worldwide, with approximately 580,000 people in the United States and 7.6 million people in the world projected to die of cancer in 2013 according to the American Cancer Society. The American Cancer Society also projects that approximately 1.7 million new cancer cases will be diagnosed in the United States in 2013. The International Agency for Research on Cancer projects that, by 2030, 20 million to 26 million people will be diagnosed with cancer, and 13 million to 17 million will die of cancer, each year worldwide.
The most common methods of treating patients with cancer are surgery, radiation and drug therapy. A cancer patient often receives treatment with a combination of these methods. Surgery and radiation therapy are particularly effective in patients in whom the disease is localized. Physicians generally use systemic drug therapies in situations in which the cancer has spread beyond the primary site or cannot otherwise be treated through surgery. In many cases, drug therapy entails the administration of several different drugs in combination. An early approach to cancer treatment was to develop drugs, referred to as cytotoxic drugs, that kill rapidly proliferating cancer cells through non-specific mechanisms, such as disrupting cell metabolism or causing damage to cellular components required for survival and rapid growth. While these drugs have been effective in the treatment of some cancers, they act in an indiscriminate manner, killing healthy cells, as well as cancer cells. Due to their mechanism of action, many cytotoxic drugs have a narrow dose range above which the toxicity causes unacceptable or even fatal levels of damage and below which the drugs are not effective in promoting cancer cell death. A different approach to pharmacological cancer treatment has been to develop drugs, referred to as targeted therapeutics, that target specific biological molecules in the human body that play a role in rapid cell growth and the spread of cancer. Targeted therapeutics are designed to specifically enable the death of cancer cells and spare normal cells, to improve efficacy, and to minimize side effects. The drugs are designed to either attack a target that causes uncontrolled growth of cancer cells because of either a specific genetic alteration primarily found in cancer cells, but not in
normal cells, or a target that cancer cells are more dependent on for their growth in comparison to normal cells.
Our SINE approach is a novel targeted therapeutic approach specifically focused on selectively targeting the restoration and increase in the levels of multiple tumor suppressor proteins and growth regulatory proteins in the nucleus. Unlike many other targeted therapeutic approaches which only work for a specific set of cancers or in a specific sub-group of patients, we believe there is evidence to suggest that our SINE compounds have the potential to provide therapeutic benefits across a broad range of both hematological and solid tumor malignancies and benefit a wide range of patients.
Our Drug Candidates
Our Lead Drug Candidate Selinexor (KPT-330)
Overview
Our lead drug candidate, Selinexor (KPT-330), is a wholly-owned, orally available, small molecule, potent SINE compound that specifically blocks XPO1 cargo binding. Selinexor inhibits the export of tumor suppressor proteins out of the nucleus. As a result, these proteins are retained in the nucleus where they can detect cancerous changes and promote the death of cancer cells. We are currently conducting three open-label Phase 1 clinical trials of Selinexor, the first in patients with heavily pretreated relapsed and/or refractory hematological malignancies, the second in patients with heavily pretreated relapsed and/or refractory solid tumor malignancies, and the third, a food effect study, in patients with metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. We have observed preliminary evidence of anti-cancer activity of Selinexor across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. We believe that the oral administration of Selinexor and lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in our ongoing Phase 1 clinical trials create the potential for its broad use across many cancer types. Assuming continued positive results from our ongoing Phase 1 clinical trials of Selinexor and based on regulatory feedback, we plan to initiate Phase 2/3 clinical trials in two cancer indications in the first half of 2014. We plan to select these indications based on the results of our ongoing Phase 1 clinical trials of Selinexor, the level of unmet medical need and the competitive landscape. Assuming positive results from these Phase 2/3 clinical trials, we plan to seek regulatory approvals of Selinexor in North America and Europe and we may seek such approvals in other geographies. In addition, we expect to initiate two Phase 2 clinical trials in solid tumor malignancies by early 2014. We intend to enter into collaborations for marketing and commercialization of Selinexor in particular geographies at an appropriate time.
In May 2012, we filed two investigational new drug applications, or INDs, with the U.S. Food and Drug Administration, one covering Selinexor in advanced hematological malignancies and the other covering Selinexor in advanced or metastatic solid tumor malignancies. The trials in patients with these two indications were initiated in mid-2012 and are being conducted at cancer centers in the United States, Canada and Denmark. In July 2013, we began enrollment in our third Phase 1 clinical trial of Selinexor, a food effect study that is being conducted in the United States and Canada. We also intend to gather additional safety and efficacy data regarding Selinexor as part of the food effect study. As of September 20, 2013, over 170 patients have received Selinexor in these three clinical trials.
Advanced Hematological Malignancies
Our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies continues to enroll patients with documented progressive disease at the time of enrollment. These patients have relapsed and/or refractory hematological malignancies, meaning that their cancers are no
longer responsive, or were never responsive, to treatment with approved and/or experimental therapies. These patients had received multiple previous treatments, which we refer to as heavily pretreated.
There are three arms to this clinical trial:
We designed this open-label Phase 1 clinical trial to evaluate the safety of Selinexor, to determine the Phase 2 clinical trial dose and dosing schedule and to evaluate preliminary anti-cancer activity of Selinexor. Selinexor is orally administered in escalating dose levels two to three times per week over a 28-day cycle. We expect to treat up to approximately 100 patients over the course of the initial dose escalation phase of this clinical trial, with approximately 60 patients expected to be evaluated in Arm 1 and approximately 40 patients expected to be evaluated in Arm 2. In addition, we expect to enroll approximately 12 patients in Arm 3. There are multiple ongoing and planned cohorts in each arm. Cohorts are designed to enroll at least three patients and, if dose limiting toxicities, or DLTs, are observed, to add an additional three patients to that cohort at that dose. If no DLTs are observed in three patients, or a DLT is only observed in one patient out of six, then three new patients will be added to a new cohort at an escalated dose. We expect to evaluate approximately 50 additional patients in fixed dose expansion cohorts in Arms 1 and 2 at the expected Phase 2 clinical trial dose.
Arm 1. As of September 20, 2013, a total of 49 patients on Arm 1 (20 with MM, two with WM, 19 with NHL and eight with CLL) have been enrolled at doses ranging from 3 mg/m2 to 45 mg/m2 at eight clinical centers in the United States, Canada and Denmark. We have observed preliminary evidence of anti-cancer activity in certain of these heavily pretreated patients. Potential responses include CR, which for CLL means complete remission and for the other indications listed means complete response, PR, which for CLL means partial remission and for the other indications listed means partial response, MR, which means minimal response for all indications listed and SD, which means stable disease for all indications listed, each as determined in accordance with commonly accepted evaluation criteria for the specific indication. 29 of the 39 patients (74%) evaluated as of September 20, 2013 in this arm have experienced PR, MR or SD. The distribution of these responses across indications as of September 20, 2013 was as follows: a partial response or partial remission in six patients, one in each of MM, DLBCL and MCL and three in CLL; a minimal response in six patients, four in MM and two in WM; and stable disease in 17 patients, seven in MM, two in DLBCL, one in MCL, five in FL and two in CLL. Eight of the 39 patients (21%) evaluated as of September 20, 2013 in this arm have experienced PD, including four patients with MM, two patients with DLBCL and two patients with transformed NHL. Ten patients had not yet been evaluated as of September 20, 2013.
Responses are shown in the table below for the 39 patients who had been evaluated as of September 20, 2013, each of whom received a dose between 3 mg/m2 to 35 mg/m2 per cycle. As of September 20, 2013, patients receiving a dose higher than 35 mg/m2 had not yet been evaluated. These responses are interim unaudited data based on site reports and are measured using commonly accepted evaluation criteria for the specific indication.
Responses in Arm 1 (Chronic B-Cell Malignancies) [3 mg/m2 to 35 mg/m2] as of September 20, 2013
Cancer | CR (%) | PR (%) | SD (%) | PD (%) | WC | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 (29%) | 1 | 1 | |||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| 1 | — | — | 1 (14%) | ||||||||||||||||||||
| 3 | 2 (67%) | 1 (33%) | — | 1 (33%) | 1 (33%) | — | 1 (33%) | ||||||||||||||||
Transformed | 3 | 1 (33%) | 1 (33%) | — | 1 (33%) | — | 2 (67%) | — | ||||||||||||||||
T-Cell | 4 | 3 (75%) | 1 (25%) | 1 (25%) | — | 2 (50%) | — | 1 (25%) | ||||||||||||||||
Richter's Syndrome | 5 | 5 (100%) | ||||||||||||||||||||||
— | 2 | 3 (60%) | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | ||||||||
Total | 32 (74%) | 20 (47%) | 7 (16%) | 4 (9%) | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | ||||||||
| | | | | | | | | | | | | | | | | | ||||||||
The following chart indicates the maximum percentage shrinkage of tumors across various subtypes of NHL relative to lymph node response only. One patient with Richter's transformation patient was taken off study and moved to transplantation given the response of his lymph nodes to Selinexor.
PD means progressive disease, as determined in accordance with commonly accepted evaluation criteriabaseline scans for the specific indication. Withdrew consent, or WC, means a patient withdrew frompatients depicted.
Among the trial before evaluation. Non-evaluable, or NE, means the patient's response could not be evaluated due to a number of potential factors, including when a patient fails to comply with therapeutic protocol for the trial. Patients who have not yet been evaluated are not included in the response data and are not considered NE.
Enrollment in this arm began in July 2012. In order to remain on study, patients must exhibit a response of SD or better at each evaluation, which typically occurs at the end of each 28-day dosing cycle. A response of SD represents a stabilization of the disease, as determined in accordance with commonly accepted evaluation criteria for the specific indication, over one dosing cycle, which we believe is an indicator of the anti-cancer effect of the drug candidate. As of September 20, 2013, 22 patients in this arm remain on study. As of September 20, 2013, five patients remained on study in this arm for longer than nine months, including two patients who have been on study for longer than ten months and one patient who has been on study for longer than 12 months. Four of five of these patients continue to remain on study as of September 20, 2013. No major organ toxicities have been observed in this arm to date.
The most common side effects in this arm, known as adverse events, or AEs, are Grade 1 or Grade 2 adverse events. Grade 1 and 2 adverse events are generally characterized as mild. Grade 3 adverse events are considered moderate and Grade 4 adverse events are considered severe. As of September 20, 2013, we have reports of AEs in 38 of the 49 patients enrolled in this arm and the AE prevalence percentages below are based upon 49 patients. Gastrointestinal adverse events and fatigue are the most common types of adverse events seen in Arm 1. As of September 20, 2013, the gastrointestinal events typically consist of nausea in 30 patients (61%), anorexia in 24 patients (49%), vomiting in 15 patients (31%) and diarrhea in 15 patients (31%). The gastrointestinal events are solely either Grade 1 or Grade 2 events and are generally responsive to standard supportive care. Grade 1 or
Grade 2 fatigue was observed in 19 patients (39%) in this arm as of September 20, 2013, with one additional patient (2%) showing Grade 3 fatigue. We have also observed Grade 3 or Grade 4 thrombocytopenia, or low count of platelets in the blood, in 12 patients (24%), with one additional patient (2%) showing Grade 1 thrombocytopenia. We have also observed Grade 3 or Grade 4 neutropenia, or low neutrophil counts, in 11 patients (22%), with three additional patients (6%) showing Grade 1 or Grade 2 neutropenia. We expect that thrombocytopenia and neutropenia are primarily a result of patients entering this arm with marked bone marrow suppression due to both disease and prior therapies. We do not gather data regarding the number of patients that have withdrawn from this arm as a result of AEs.
Due to the gastrointestinal events we observed earlier in the arm, we now instruct physicians to initiate supportive care and medications prior to patients beginning on Selinexor therapy. The supportive care consists primarily of focusing on maintaining caloric and fluid intake as well as the introduction of anti-nausea medication. We believe that we will see fewer and milder gastrointestinal events in the future as a result of the initiation of supportive care and medications prior to beginning Selinexor therapy. We believe supportive care and medications can also reduce fatigue and patients remaining in this arm for longer than two to four months typically do not show an increase in fatigue.
As of September 20, 2013, there have been 49 serious adverse events, or SAEs, reported in 21 patients in Arm 1. SAEs generally refer to AEs that result in death, are life threatening, require hospitalization or prolonging of hospitalization, or cause a significant and permanent disruption of normal life functions, congenital anomalies or birth defects, or require intervention to prevent such an outcome. SAEs may be attributed to Selinexor or deemed unrelated. Of the 49 SAEs reported, one SAE was deemed by us and the clinical investigator to be related to Selinexor. This SAE was Grade 2 blurred vision. This was not permanent and the patient recovered from this SAE. All patients in this arm at the time received eye examinations by an ophthalmologist and all new patients receive the same examination prior to beginning treatment in order to assess any changes in vision while on Selinexor therapy.
No DLTs were observed in the initial three dose cohorts ranging from 3 to 12 mg/m2. In cohort 4 (16.8 mg/m2), one of six patients experienced a DLT consisting of Grade 4 thrombocytopenia for five or more days without bleeding during the first cycle of dosing. Despite this DLT, which was resolved after temporarily withholding Selinexor, the treating physicians decided that the potential benefits to this patient of continued treatment with Selinexor outweighed the risks. This patient with MM remains on study for approximately 10 months as of September 20, 2013, with an MR. At the 23 mg/m2 dose level, one of six patients also experienced a DLT consisting of Grade 4 thrombocytopenia for five or more days without bleeding during the first cycle of dosing. Despite this DLT, which was resolved after temporarily withholding Selinexor, the treating physicians decided that the potential benefits to this patient of continued treatment with Selinexor outweighed the risks. This patient with FL was on study for 84 days with SD until PD. No DLTs were observed at the 30 mg/m2 or 35 mg/m2 dose levels.
In June 2013, we initiated a fixed dose expansion cohort of 35 mg/m2. We expect to evaluate approximately 10 patients with MM or WM and approximately 15 patients with DLBCL in this cohort.
Arm 2. As of September 20, 2013, a total of 34 patients with heavily pretreated relapsed and/DLBCL, ORR and DCR were nearly identical across all subtypes of DLBCL. There are two major subtypes of DLBCL, namely Germinal Center B-Cell, or refractory AML were enrolled in this armGCB and Activated B-Cell, or ABC, also called non-GCB. Many targeted therapies such as ibrutinib or lenalidomide show activity primarily against the majority of these patients are elderly, meaning 65 years of age or older. We have observed preliminary evidence of anti-cancer activity in certain of the patients in this arm. Potential responses include CR, which for AML means complete remission with complete blood count recovery, CR(i), which for AML means complete remission with incomplete blood count recovery, and SD. 15 of the 32 patients (47%) evaluatedABC subtype. However, as of September 20, 2013 in this arm have experienced a CR, CR(i) or SD. Three patients experienced a CR or CR(i). Two of these patients experienced a CR, while the other patient experienced a CR(i). As of September 20, 2013, 12 patients have experienced SD. 11 of the 32 patients (34%) evaluated as of September 20, 2013 in this arm have experienced PD. Two patients had not yet been evaluated as of September 20, 2013.
Responses are showndetailed in the table below forand consistent with the 32 patients who had been evaluated asbroadly applicable mechanism of September 20, 2013, eachaction of whom received a dose between 16.8 mg/m2 to 40 mg/m2 per cycle. These responses are interim unaudited data based on site reports and are measured using commonly accepted evaluation criteria for AML.Selinexor, Selinexor shows activity across both major subtypes.
Best Responses in Arm 2 (AML) [16.8 mg/m2 to 40 mg/m2]Diffuse Large B-Cell Patients as of September 20, 2013May 13, 2014
| DCR (%) | ORR (%) | CR (%) | SD (%) | PD (%) | WC (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 (27%) | 1 | 1 (9%) | |||||||||||||||
non-GCB | 4 | 3 (75%) | 1 (25%) | — | 1 (25%) | 2 | 1 (25%) | — | ||||||||||
Unknown | 6 | 4 (67%) | 2 (33%) | — | 2 (33%) | 2 (33%) | 2 (33%) | — |
Enrollment in this arm began in January 2013. In order to remain on study, patients must exhibit a response of SD or better at each evaluation, which typically occurs at the end of each 28-day dosing cycle. A response of SD represents a stabilization of the disease, as determined in accordance with commonly accepted evaluation criteria for AML, over one dosing cycle, which we believe is an indicator of the anti-cancer effect of the drug candidate. As of September 20, 2013, five patients in this arm remain on study. As of September 20, 2013, four patients remained on study in this arm for more than three months. Two of these patients continue to remain on study as of September 20, 2013. No major organ toxicities have been observed in this arm to date.
Gastrointestinal adverse events and fatigue are the most common types of AEs seen in Arm 2. As of September 20, 2013, we have reports of AEs in 30 of the 34 patients enrolled in this arm and the AE prevalence percentages below are based upon 34 patients. As of September 20, 2013, the gastrointestinal adverse events typically consist of nausea in 18 patients (53%), anorexia in 17 patients (50%), vomiting in 12 patients (35%) and weight loss in 7 patients (21%). The gastrointestinal events are primarily Grade 1 or Grade 2 events (93%) that are generally responsive to standard supportive care. Fatigue was observed in 16 patients in this arm (47%) as of September 20, 2013, including Grade 3 fatigue in two patients (6%) and Grade 1 or Grade 2 fatigue in 14 patients (41%). We have also observed Grade 4 thrombocytopenia in three patients (9%) and Grade 4 neutropenia in two patients (6%) in this arm as of September 20, 2013. We expect that the thrombocytopenia and neutropenia are primarily a result of patients entering this arm with marked bone marrow suppression due to both disease and prior therapies. We do not gather data regarding the number of patients that have withdrawn from this arm as a result of AEs.
As in Arm 1, we believe that we will see fewer and milder gastrointestinal events and reduced fatigue in the future as a result of the initiation of supportive care and medications prior to beginning Selinexor therapy.
As of September 20, 2013, there have been 49 SAEs reported in 20 patients in Arm 2. Of the 49 SAEs reported, one SAE was deemed by us and the clinical investigator to be related to Selinexor. This SAE was Grade 3 nausea combined with Grade 1 vomiting. This SAE was not permanent and the patient recovered from this SAE following supportive care.
As of September 20, 2013, no DLTs have been observed in this arm.
In the fourth quarter of 2013, we plan to enroll approximately 25 patients with AML, many of whom we expect to be heavily pretreated patients with relapsed and/or refractory AML, in a fixed dose expansion cohort of 40 mg/m2.
Arm 3. We are evaluating Selinexor in patients with heavily pretreated relapsed and/or refractory T-cell lymphoma in Arm 3 of this clinical trial. We began enrollment in August 2013 and have administered Selinexor to one patient in Arm 3 who had not yet been evaluated as of September 20, 2013.
In addition, a minority of DLBCL patients have "double-hit" disease because these tumors over-express the two oncogenes MYC and BCL2 (or BCL6). Double-hit DLBCL is particularly difficult to treat due in part to its resistance to multi-agent immunochemotherapy and many targeted agents. Of four patients with double-hit DLBCL, there was one CR, two patients with SD (with 43% and 45% lymph node reductions, respectively), and one PD. We believe, together, these data and the consistent data across DLBCL subtypes indicate that Selinexor has the potential to treat a broad range of subtypes of DLBCL, largely independent of the cell of origin or oncogenic drivers.
Among all NHL patients, Grade 3/4 adverse events occurring in more than three patients included thrombocytopenia (20%), neutropenia (16%) and hyponatremia (6%). The most common Grade 1/2 adverse events were nausea (51%), anorexia (41%) and fatigue (36%). There have been no dose limiting toxicities among NHL patients as of May 13, 2014. The maximum tolerated dose was not reached, but based on biological activity and data from additional studies, the intended Phase 2 or Phase 3 trial dose of oral Selinexor for NHL is 60mg/m2 twice weekly.
Acute Myeloid Leukemia
Sixty-five heavily pretreated patients with progressive, relapsed and/or refractory AML, most with three or more prior treatment regimens, were enrolled in this arm of the Phase 1 clinical trial as of May 13, 2014. These patients were dosed 16.8 - 70mg/m2 in a four-week cycle, with lower doses initially given ten times per cycle and higher doses given twice weekly. Of these 65 patients, two patients had tumors pending evaluation, and among the 63 other patients, the complete response rate with or without full hematologic recovery was 11%, the ORR was 16% and the DCR was 49%; 16 (25%) of the 63 patients with AML were non-evaluable but were included in the AML response rate calculation. Responses were observed across multiple genetic subtypes of AML. Higher doses of Selinexor were associated with greater reductions in bone marrow blast counts, which were also observed across different AML subtypes. Responses in 63 patients are shown below. Two patients had tumors pending evaluation.
Best Responses in AML Patients as of May 13, 2014
N | DCR (%) | ORR (%) | CR (%) | CR(i/p) (%) | PR (%) | MLFS (%) | SD (%) | PD (%) | NE (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
63 | 31 (49%) | 10 (16%) | 5 (8%) | 2 (3%) | 1 (2%) | 2 (3%) | 21 (33%) | 16 (25%) | 16 (25%) |
Grade 3/4 adverse events occurring in more than three patients included fatigue (18%), thrombocytopenia (15%), neutropenia (11%), and nausea (8%). The most common Grade 1/2 adverse events were diarrhea (82%), anorexia (78%), nausea (74%), and fatigue (65%). There have been no dose limiting toxicities in AML patients as of May 13, 2014 and the maximum tolerated dose is³70mg/m2 twice weekly. The intended Phase 2 or Phase 3 trial dose of oral Selinexor for AML is 55mg/m2 twice weekly.
Multiple Myeloma
As part of our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies, patients with multiple myeloma were treated with either single-agent Selinexor or Selinexor in combination with "low-dose" (20mg) dexamethasone, all dosed twice weekly. Forty-four patients with multiple myeloma whose disease was relapsed and/or refractory to all available classes of approved therapies, with a mean of 5.7 prior therapies, and progressing on study entry have been enrolled in this trial as of June 5, 2014. Of these 44 patients, 34 received single-agent Selinexor therapy and eight patients were treated with Selinexor in combination with dexamethasone. Among the 34 patients receiving single-agent Selinexor therapy, including six patients who were non-evaluable, best responses include one PR (3%), five MRs (15%), 16 SDs (47%) and six PDs (18%). It should also be noted that some patients treated with single agent Selinexor receive very low doses of dexamethasone
(£12mg with each dose of selinexor) or another steroid as part of supportive care. These very low doses of steroids are not known to have any anti-myeloma activity, particularly in the patients enrolled in this study, whose disease is refractory to steroids and multiple other agents.
Eight patients were treated with 45mg/m2 of oral Selinexor and 20mg of dexamethasone, each dosed twice weekly. Two additional patients have been treated with combination therapy but had not yet completed the first treatment cycle as of June 5, 2014. This dose of dexamethasone is the standard low dose dexamethasone (40mg weekly or 20mg twice weekly) used with other anti-myeloma drugs including lenalidomide or pomalidomide. The patients enrolled in this study had received a median of 5.5 prior lines of therapy. All had received prior therapy with a proteasome inhibitor, such as Kyprolis or Velcade, and an immunomodulatory agent, such as pomalidomide, steroids (typically two or more times) while seven of the eight patients also received stem cell transplantations. As of June 5, 2014, the best responses among the eight patients were one stringent complete response (sCR) (12.5%), three PRs (37.5%), two MRs (25%), one PD (12.5%) and one NE (12.5%). The clinical benefit response rate (sCR+PR+MR) was 75% and the ORR was 50%. Five of the six responding patients remain on study as of June 5, 2014. Approximately 12 additional patients with multiple myeloma may be dosed with Selinexor in combination with dexamethasone in this ongoing Phase 1 study. Responses in these eight patients are described below.
Patients with Relapsed/Refractory MM Treated with Twice Weekly
Oral Selinexor 45mg/m2 + Dexamethasone 20mg
| MM Type | Number of Prior therapies | Maximal Change | Response | Study Days | |||||
|---|---|---|---|---|---|---|---|---|---|
| IgG-k | 7 | -73% | PR | 122+ | |||||
| FLC-l | 5 | * | NE | 15 | |||||
| FLC-k | 3 | -53% | PR | 45 | |||||
| FLC-k | 5 | -98% | sCR | 107+ | |||||
| IgG-k | 7 | -81% | PR | 81+ | |||||
| IgG-k | 4 | * | PD | 38 | |||||
| IgA-k | 6 | -48% | MR | 51+ | |||||
| IgG-k | 7 | -32% | MR | 46+ |
Adverse events in patients receiving single-agent Selinexor were generally low-grade, consistent with events observed in patients with other hematological malignancies and responsive to standard supportive care. Compared with Selinexor given alone, fewer adverse events in patients receiving Selinexor in combination with dexamethasone were reported, particularly levels of nausea, consistent with dexamethasone's reduction in Selinexor's main side effects of nausea, anorexia, and fatigue.
In addition to patients with multiple myeloma achieving durable responses and disease control on Selinexor single-agent therapy, Selinexor with low dose dexamethasone showed activity with rapid M-protein reductions and good tolerability, even in patients with disease refractory to pomalidomide and/or carfilzomib. We believe that Selinexor may have synergistic potential with other therapies.
Advanced or Metastatic Solid Tumor Malignancies
OurWe recently reported data from our ongoing Phase 1 clinical trial of Selinexor in patients with advanced or metastatic solid tumor malignancies continues to enrollmalignancies. All patients entered the study with documented progressive disease at the timeadvanced or
Table of enrollment. These patients have heavily pretreatedContents
metastatic solid tumor cancers relapsed and/or refractory solid tumor malignancies. We have treated patients diagnosed with colorectal cancer, or CRC, squamous cell cancerafter multiple previous treatments and objectively progressing on study entry. The primary objectives of the head and neck or lung, ovarian cancer, cervical cancer, endometrial stromal sarcoma, or ESS, melanoma, glioblastoma, or GBM, pancreatic cancer, prostate cancer and other solid tumor malignancies. We designed this open-label Phase 1 clinicaldose escalation trial to evaluate the safety of Selinexor,are to determine the safety, tolerability and recommended Phase 2 clinical trial dose and dosing schedule and to evaluate preliminary anti-cancer activity of Selinexor. Selinexor is orally administered in escalating dose levels two to three times per week over each 28-day cycle. We expect to treat up to approximately 50Selinexor. These patients over the course of the initial dose escalation phase of this clinical trial. We expect to evaluate approximately 25 additional patients in fixed dose expansion cohorts of this trial at the expected Phase 2 clinical trial dose.
As of September 20, 2013, a total of 85 patients have been enrolled at six clinical centers in the United States, Canada and Denmark, and are being treated at doses ranging fromwere dosed 3 mg/- 85mg/m2 to 50 mg/m2. We have observed preliminary evidence of anti-cancer activity in certain of the patients in this trial. Potential responses include CR, PRoral Selinexor over a four-week cycle, with lower doses initially given ten times per cycle and SD, each as determined in accordance withhigher doses given twice weekly. Response Evaluation Criteria In Solid Tumors, or RECIST, the commonly accepted evaluation criteria for solid tumor malignancies. 35 of the 77 patients (45%) evaluated as of September 20, 2013 in this trial have experienced a PR or SD, including a PR in a patient with CRC and a PR in a patient with melanoma; 33 patients have experienced SD. 32 of the 77 patients (42%) evaluated as of September 20, 2013 in this trial have experienced PD. Eight patients had not yet been evaluated as of September 20, 2013.
Responses are shown in the table below for the 77 patients who had been evaluated as of September 20, 2013, each of whom received a dose between 3 mg/m2 to 50 mg/m2 per cycle. These responses are interim unaudited data based on site reports and are evaluatedwas done every two cycles in accordance with RECIST.
As of May 13, 2014, 129 patients were enrolled in this Phase 1 clinical trial. Enrolled patients had received a mean of 3.7 prior therapies. Of these patients, 106 were evaluable and 23 patients were non-evaluable or pending evaluation as of May 13, 2014. Of these evaluable patients, the DCR was 49%. PRs were observed in four patients, one each with colorectal cancer (KRAS mutant), melanoma (BRAFwt), ovarian adenocarcinoma and cervical cancer. SD was noted in 47 patients, with 17 patients (16%) experiencing SD for six months or longer. Eight of nine evaluable patients with hormone and chemotherapy refractory prostate cancer achieved stable disease and have remained on study for between 70 and 317 days. Among 14 evaluable patients with head and neck cancer, nine achieved stable disease with eight on study for 75 to over 401 days. Responses in 106 evaluable patients are shown below.
Best Responses in Advanced or Metastatic Solid Tumor Malignancies [3 mg/m2 to 50 mg/m2]Patients as of September 20, 2013May 13, 2014
Cancer Type | PR (%) | SD (%) | PD (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (3%) | |||||||||||||||
Head & Neck | — | |||||||||||||||
| ||||||||||||||||
| 7 | 1 | ||||||||||||||
Cervical | ||||||||||||||||
| ||||||||||||||||
| ||||||||||||||||
| 1 (20%) | 3 (60%) | 1 (20%) | |||||||||||||
Ovarian | 5 | 3 (60%) | 1 (20%) | 2 (40%) | ||||||||||||
| ||||||||||||||||
| ||||||||||||||||
GBM | — | — | — | 5 (100%) | ||||||||||||
Melanoma | 3 | 2 (67%) | 1 | 1 (33%) | 1 (33%) | |||||||||||
Sarcoma | 8 | 7 (88%) | — | 7 (88%) | 1 (12%) | |||||||||||
Other | 19 | 6 (32%) | — | 6 (32%) | 13 (68%) | |||||||||||
| | | | | | | | | | | | ||||||
Total | 4 | 54 (51%) | ||||||||||||||
| | | | | | | | | | | | ||||||
| | | | | | | | | | | | ||||||
Enrollment Side effects were generally low grade and typically gastrointestinal in nature, or fatigue. These common side effects decreased over time, in part due to prophylactic use of standard supportive care. Two DLTs were observed in solid tumor patients receiving 85 mg/m2 dosed twice weekly. Grade 3/4 adverse events occurring in six or more patients in the first cycle included fatigue hyponatremia (8%), fatigue (7%), and thrombocytopenia (7%). The most common Grade 1/2 adverse events in the first cycle were nausea (62%), fatigue (52%), anorexia (48%) and vomiting (35%). One was Grade 3 asymptomatic hyponatremia and the other was acute cerebellar syndrome that reversed over several weeks with markedly improving ataxia and dysarthria. No additional central nervous system toxicities were observed in any other patients receiving Selinexor to date. Major organ dysfunction or clinically significant cumulative toxicities have not been observed. The intended Phase 2 or Phase 3 trial dose of oral Selinexor in solid tumors is 65mg/m2 twice weekly.
Registration-Directed Trial Design
On June 24, 2014, we announced the initiation of our Phase 2 study of Selinexor in patients 60 years of age or older with relapsed or refractory acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy and/or transplantation. We have titled this trial begantheSelinexor in June 2012. In orderOlderPatients withRelapsed/RefractoryAML, or SOPRA, study. We also expect to remain on study, patients must exhibit a response of SD or better at each evaluation, which typically occurs following the completion of two 28-day dosing cycles. A response of SD represents a stabilization of the disease, as determined in accordance with RECIST, over one dosing cycle, which we believe is an indicator of the anti-cancerinitiate registration-
effect
directed clinical trials for Selinexor in Richter's Syndrome during the middle of the drug candidate. As of September 20, 2013, 21 patients2014 and in this clinical trial remain on study. As of September 20, 2013, seven patients have remained on study for more than six months, including one patient on study for longer than eight months, one patient on study for longer than 11 months and one patient on study for longer than 13 months. The patient who has remained on study for longer than thirteen months remains on study as of September 20, 2013. No major organ toxicities have been observed in this trial to date.
The graph below shows the change in tumor size for the 49 patients in this trial who had received a radiological disease assessment as of September 20, 2013. Each vertical barDLBCL in the graph representssecond half of 2014.
In SOPRA, 150 patients with AML which has relapsed after, or was refractory to, first line therapy will be randomized in a 2:1 fashion to Selinexor provided orally twice per week at a dose of 55mg/m2 versus one of four physician choices. Physician choices include best supportive care, or BSC, alone, or BSC plus either azacytidine (Vidaza), decitabine (Dacogen), or low dose cytosine arabinoside (LD-AraC). Overall survival is the percent changeprimary endpoint. SOPRA was designed based on data from the time when the patient entered the clinical trial until the largest tumor size reduction or smallest tumor size growth, as applicable, was measured for that patient in accordance with RECIST. The changes in tumor size are interim unaudited data based on independent radiological disease assessment at the treating center.
Percentage Change in Tumor Size asongoing Phase 1 study of September 20, 2013
The most common AEs associated with Selinexor in patients with advanced or metastatic solid tumorhematologic malignancies, are gastrointestinalincluding AML. SOPRA is expected to take approximately two years to complete.
Our registration-directed trial in nature or fatigue. AsRichter's Syndrome is designed as a single-arm trial in patients with CLL who experienced a transformation to Richter's Syndrome and subsequently relapsed after chemotherapy. The primary endpoint of September 20, 2013, we have reports of AEs in 68 of the 85 patients enrolled in this armtrial is ORR and the AE prevalence percentages below are based upon 85 patients. As of September 20, 2013, the gastrointestinal adverse events typically consist of nausea in 55 patients (65%), anorexia intrial is expected to enroll approximately 50 patients (59%), vomiting in 41 patients (48%), dysgeusia, orwho will be treated with Selinexor given at a distortion in the sensedose of taste, in 30 patients (35%), weight loss in 27 patients (32%) and diarrhea in 21 patients (25%). The gastrointestinal events are primarily Grade 1 or Grade 2 events (94%) that are generally responsive to standard supportive care. Fatigue was observed in 54 patients (64%) as of September 20, 2013, including Grade 3 fatigue in ten patients (12%) and Grade 1 or Grade 2 fatigue in 44 patients (52%). Anemia, or a decrease in red blood cell count, was observed in 20 patients (24%) as of September 20, 2013, including Grade 3 anemia in six patients (7%) and Grade 1 or Grade 2 anemia in 14 patients (16%). As of September 20, 2013, five patients have withdrawn from this trial as a result of AEs.
As in our hematological malignancy clinical trial, we believe that we will see fewer and milder gastrointestinal events and reduced fatigue in the future as a result of the initiation of supportive care and medications prior to beginning Selinexor therapy.
As of September 20, 2013, there have been 61 SAEs reported in 32 patients in this clinical trial, of which four were deemed related to Selinexor. These were one of each of the following: Grade 3 nausea, Grade 3 nausea and vomiting, Grade 3 weight loss and severe malnutrition and Grade 3 fatigue. All four patients who experienced these SAEs have recovered from these SAEs following supportive care.
As of September 20, 2013, no DLTs were observed in the initial six cohorts at doses ranging from 3 to 30 mg/m2. Of the three patients who received 10 doses per cycle at 40 mg/60mg/m2, there wereadministered orally two DLTs. One was Grade 3 anorexia with dehydration and fatigue, and the other was Grade 3 fatigue with Grade 1-2 anorexia. Although we believe that neither of these patients with DLTs received optimal supportive care, given the overall clinical picture, we made the decision to establish 10 dosestimes per cycle at 30 mg/m2 to be the maximum tolerated dose for advanced or metastatic solid tumor malignancy patients. As a result of our decision regarding the maximum tolerated dose, the dose of a third patient being treated at 40 mg/m2, who had tolerated therapy well, was also reduced to 30 mg/m2. We have also evaluated 10 additional patients at 10 doses per cycle at 30 mg/m2.week.
In addition, weOur registration-directed trial in DLBCL is designed as a single-arm trial in patients that are evaluating a dosing schedule of eight doses per cycle. Of the six patients receiving eight doses per cycle at 35 mg/m2, there was one DLT, which consisted of Grade 3 nausea, vomiting and fatigue with a diarrheal infection. At eight doses per cycle, a maximum tolerated dose has not yet been established. Currently, a dose of 50 mg/m2 at eight doses per cycle is being evaluated in patients.
Approximately 30 patients with CRC, prostate, ovarian, squamous (head and neck, lung and cervical) cancers, and malignant gliomas (glioblastoma multiforme and anaplastic astrocytomas) are being evaluated in a fixed dose expansion cohort of 35 mg/m2. Enrollment in this expansion cohort began in May 2013.
Metastatic, Locally Advanced or Locally Recurrent Soft Tissue or Bone Sarcomas (Food Effect Study)
In July 2013, we began enrollment in our third clinical trial of Selinexor, a Phase 1b open-label food effect study in heavily pretreated patients who have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. The trial is primarily designed to evaluate the effects of food and formulation (capsules and tablets) on the absorption of oral Selinexor. We also expect to gather additional safety and efficacy data regarding Selinexor in this trial. We are currently using the capsule formulation in our other Phase 1 clinical trials. As of September 20, 2013, three patients have been enrolled in this clinical trial. Two are evaluable for response but had not yet been evaluated as of September 20, 2013. Depending on the results of the food effect study, we may consider using the tablet formulation in future clinical trials of Selinexor. We plan to enroll up to approximately 20 patients in this study in the United States and Canada.
Clinical Development Plan
We have observed preliminary evidence of anti-cancer activity of Selinexor across a spectrum of patients with heavily pretreated relapsed and/or refractory cancers. Furthermore, severalto two lines of chemotherapy. The primary endpoint of this trial is ORR and the trial is expected to enroll approximately 150 patients have remainedwho will be treated with Selinexor given at a dose of 60mg/m2 in combination with low dose dexamethasone (12mg), each administered orally two times per week.
Our Strategy
As a clinical-stage pharmaceutical company focused on the discovery and development of orally available, novel first-in-class drugs directed against nuclear transport targets for the treatment of cancer and other major diseases, the critical components of our business strategy are to:
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the "Risk Factors" section of this prospectus immediately following this prospectus summary and in some cases, over one year. our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 incorporated by reference herein. These risks include the following:
Phase
Our Corporate Information
We were incorporated under the laws of the State of Delaware in two cancer indicationsDecember 2008. Our executive offices are located at 2 Mercer Road, Natick, MA 01760, and our telephone number is (508) 975-4820. Our website address is www.karyopharm.com. The information contained in, or accessible through, our website does not constitute part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. The trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
Implications of Being an Emerging Growth Company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the first halfJumpstart Our Business Startups Act of 2014. We plan to select these indications based on2012, or the results of our ongoing Phase 1 clinical trials of Selinexor, the level of unmet medical need and the competitive landscape. We expect at least one of those indications to be in heavily pretreated patients with AML (in the elderly), DLBCL or MM, each of which is described below. Other potential indications include AML in the general population, newly-diagnosed AML in elderly patients or solid tumor malignancies. Assuming positive results from these Phase 2/3 clinical trials, we plan to seek regulatory approvals of Selinexor in North America and Europe andJOBS Act. As an emerging growth company, we may seek such approvals in other geographies. In addition, we expect to initiate two Phase 2 clinical trials in solid tumor malignancies by early 2014, which may include gynecological malignancies and squamous cell cancers.
• Acute Myeloid Leukemia in Elderly Patients
Acute myeloid leukemia, or AML, in elderly populations remains a vexing clinical problem. AML is a cancer that starts in the bone marrow and in most cases quickly moves into the blood. The incidencetake advantage of AML dramatically increases after the age of 55. The American Cancer Society estimates that approximately 14,590 new cases of AML, most of which will be in adults, will be diagnosed in the United States in 2013. Given the shift in demographics in the population in the Western hemisphere, it is likely that an increased number of elderly individuals will be diagnosed with this form of cancer. Aside from a general increase in the incidence of AML in the general population, three additional patient populations are contributing to the increasing number of AML cases: an increasing number of older persons are developing a disease called myelodysplastic syndrome, or MDS, which can convert to AML; certain types of chemotherapy, such as alkylating agents used to treat Hodgkin's disease, breast cancer,specified reduced disclosure and other disorders, can increase the risk of developing AML later in life; and patients with chronic myelogenous leukemia treated long term with imatinib (Gleevec) and other drugs can have their disease reach an accelerated or blast phase, converting to AML.
About 40% of AML patients are young enough and fit enough to undergo bone marrow transplantation for their AML, and about 50% of these patients can be cured of their disease. Thoserequirements that are not cured,otherwise applicable generally to public companies. These provisions include:
Over the past two decades, many compounds have been evaluated in elderly patients with AML, but due to significant toxicities and/or lack of efficacy, none has been approved to date. Preclinical data on our SINE compounds from several groups at Dana-Farber Cancer Institute, Ohio State University and MD Anderson Cancer Center have shown preliminary evidence of anti-cancer activity of our SINE compounds against a set of AML cell lines with diverse genetics, as well as against leukemia stem (initiating) cells. In addition, in our Phase 1 clinical trial of Selinexor in patients with
advanced hematological malignancies, as described above,
Generally, we have observed preliminary evidence of anti-cancer activity of Selinexor in elderly patients with heavily pretreated relapsed and/or refractory AML. We have observed CRs, CR(i)s or SD in 47%may take advantage of these patients as of September 20, 2013 and, in many cases, the response has been maintained for longer than two months. We believe that these initial results suggest that Selinexor has the potential to demonstrate anti-cancer activity and tolerability in elderly patients with heavily pretreated AML. Based on the results observed to date in our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies and the expansion cohort in Arm 2 of this trial consisting primarily of elderly patients with AML that we expect will begin enrollment by the end of 2013, we may decide to initiate a Phase 2/3 clinical trial of Selinexor in elderly patients with AML in the first half of 2014.
• Diffuse Large B-Cell Lymphoma
NHL is a cancer that starts in cells called lymphocytes, which are part of the body's immune system. Lymphocytes are found in the lymph nodes and other lymphoid tissues (such as the spleen and bone marrow). According to the American Cancer Society, about 69,740 patients will be diagnosed with NHL in the United States in 2013. Diffuse large B-cell lymphoma, or DLBCL, is the most common of the aggressive NHLs, accountingexemptions for up to 30%five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1 billion in annual revenue, we have more than $700 million in market value of newly-diagnosed casesour stock held by non-affiliates or we issue more than $1 billion of NHLnon-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of certain reduced reporting burdens in this prospectus. Accordingly, the United States, accordinginformation contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the American Cancer Society. According to the Leukemia and Lymphoma Society, NHL rates, including DLBCL, have steadily increased 3 to 4% each year in the United States from 1973 to the mid-1990s.
These increases in NHL rates have been observed across all major demographic groups (except for the very young), without a clear cause. Such temporal increases in incidence of a particular form of cancer are atypical. Improved cancer reporting, more sensitive diagnostic techniques, particularly for borderline lesions, changes in classification of lymphoproliferative diseases, which are diseases where lymphocytes are produced in excessive quantities, and, in particular, the increasing occurrence of AIDS-associated DLBCL, have contributed to the escalation of incidence of this disease. Non-AIDS related NHL incidence rates have continued to increase, specifically the rates among females, older males and blacks. For the vast majority of patients, the etiology of DLBCL is unknown.
The fundamental treatment of DLBCL has changed little in the past two decades, with nosame new or targeted agents approved for this indication. Initial therapy with multiagent, or three to four, cytotoxic drugs in combination with the monoclonal antibody rituximab (Rituxan®) leads to responses in greater than 75% of patients. In patients who are less than 65 years old, and who have good organ function, high dose chemotherapy with stem cell transplantation can lead to cures in approximately 50% of these patients. Older patients relapsing after initial chemotherapy, and those relapsing after stem cell transplantation, have a very poor prognosis, and the expected survival of such patients is less than one year. Newer targeted agents suchrevised accounting standards as the BTK inhibitor ibrutinib and the immunomodulatory drug lenalidomide (Revlimid®) have shown activity in the immunoblastic (activated B cell) type of DLBCL in clinical trials, but responses are generally short-lived. Responses are much lower in the germinal center, or GC, type of DLBCL. Therefore, we believe that novel, well-tolerated drugs are needed for the treatment of relapsed DLBCL, particularly because ibrutinib and Revlimid have not been approved by the FDA for the treatment of DLBCL.
In our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies, Selinexor has shown preliminary evidence of anti-cancer activity in patients with DLBCL. As of September 20, 2013, three out of five patients (60%) evaluated with DLBCL have responded to treatment and the response has been maintained in these three patients for longer than two months. In June 2013, we initiated enrollment of an expansion cohort that we expect will include approximately 15 patients with DLBCL. If we are able to confirm the preliminary evidence of anti-cancer activity of Selinexor in the expansion cohort, we may decide to initiate a Phase 2/3 clinical trial in DLBCL in the first half of 2014.
• Multiple Myeloma
Multiple myeloma, or MM, is a hematological malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow, the presence of monoclonal immunoglobulin, or M protein, in the serum or urine, bone disease, kidney disease, and immunodeficiency. It is more common in elderly patients, with a median age at diagnosis of 65-70 years. In the United States, the American Cancer Society estimates that there will be approximately 22,350 new cases of MM in 2013. M protein, produced by most MM tumors, has been an established biomarker of the disease and the extent of the disease for over 30 years. More recently, the measurement of a fragment of the M protein, the free light chain, has been used as an additional biomarker of the disease and the extent of the disease in a subset of MM patients.
The treatment of MM has improved in the last 20 years due to the use of high-dose chemotherapy and autologous stem cell transplantation, and the subsequent introduction of the immunomodulatory agents thalidomide and lenalidomide and the proteasome inhibitor bortezomib. The median overall survival of MM patients, meaning the length of time an MM patient survives with the disease, has increased significantly in patients younger than 50 years old, with those patients experiencing a 10-year survival rate of around 40%, meaning that 40% of those patients are still alive after 10 years. However, despite the increased effectiveness of the first-line agents, the majority of patients will eventually relapse and become drug-resistant. Although a wide variety of new agents are being used in relapsed and/or refractory patients, including new proteasome inhibitors (carfilzomib, ixazomib, oprozomib, and marizomib), immunomodulatory drugs (pomalidomide), monoclonal antibodies (elotuzumab and daratumumab), a signal transduction modulator (perifosine), and histone deacetylase inhibitors (vorinostat and panobinostat), we believe that there remains a need for therapies in these relapsed and/or refractory patients that can improve the overall survival rate.
In our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies, Selinexor has shown preliminary evidence of anti-cancer activity in patients with MM. As of September 20, 2013, 17 MM patients had been treated and evaluated in our trial. 12 of the 17 patients (71%) with progressive MM on entry experienced either a PR, an MR or SD, while 4 of 17 (24%) had PD. The remaining patient withdrew from the trial. Five of these 12 patients experiencing either a PR, an MR or SD (42%) have a form of MM called light chain disease. Patients with light chain MM generally have a prognosis that is worse than patients with usual MM, where the myeloma protein is composed of both light and heavy chains. Light chain MM represents approximately 15% to 20% of MM cases and generally does not respond to therapies as well as the usual MM. Given the preliminary responses of patients with light chain MM observed to date in our Phase 1 clinical trial of Selinexor in patients with advanced hematological malignancies, and the unmet medical need in light chain MM, we may decide to initiate additional clinical trials of Selinexor in light chain MM. If we are able to confirm the preliminary evidence of anti-cancer activity of Selinexor in our expansion cohort of MM patients, including both usual and light chain MM, we may decide to initiate a Phase 2/3 clinical trial in MM in the first half of 2014.
• Other Cancer Indications
Selinexor has shown preliminary evidence of anti-cancer activity in all but one of the different types of advanced hematological malignancies evaluated in our Phase 1 trial. We expect to evaluate approximately 10 patients with MM or WM, approximately 15 patients with DLBCL and approximately 25 patients with AML in expansion cohorts of this trial. We have also observed preliminary evidence of anti-cancer activity of Selinexor in our Phase 1 clinical trial of patients with advanced or metastatic solid tumor malignancies. Patients with CRC, prostate, ovarian, squamous (head and neck, lung and cervical) cancers, and malignant gliomas (glioblastoma multiforme and anaplastic astrocytomas) are expected to be evaluated in the expansion cohort of this Phase 1 clinical trial of Selinexor. Enrollment in this expansion cohort began in May 2013. In July 2013, we began enrollment in our third clinical
trial of Selinexor, a Phase 1b open-label food effect study in heavily pretreated patients that have metastatic, locally advanced or locally recurrent soft tissue or bone sarcomas. In addition to the potential clinical trials in the indications described above, we may elect to evaluate one or more additional indications, including AML in the general population, newly-diagnosed AML in elderly patients or solid tumor malignancies, in various future clinical trials. We also expect to initiate two Phase 2 clinical trials in solid tumor malignancies, which may include gynecological malignancies and squamous cell cancers, by early 2014.
We expect a number of ISTs to be initiated in a variety of these indications over the next year. These ISTs could consist of single agent or combination studies with other agents in both hematological and solid tumor malignancies.
We believe that the oral administration of Selinexor and lack of cumulative or major organ toxicities observed to date in patients treated with Selinexor in our Phase 1 clinical trials create the potential for its broad use across many cancer types. We also plan to evaluate Selinexor in earlier lines of therapy, including front-line treatment, either alone or in combination with other therapies, and in maintenance settings following initial therapy.
Preclinical Studies
Selinexor was administered in efficacy studies to mice implanted with human tumors, or xenografts. We observed evidence of anti-cancer activity of Selinexor in mouse models of myeloma, MCL and T-cell acute lymphocytic leukemia xenografts. In addition, we observed anti-cancer activity of Selinexor, including survival advantages in models of orthotopic MM, and in several NHL xenografts, as well as in orthotopic leukemia models of AML, ALL and CLL. We have also observed evidence of anti-cancer activity of Selinexor in solid tumor xenografts including prostate, breast, neuroblastoma, melanoma, lung, glioblastoma, alveolar soft part sarcoma, colon and ovarian cancers. In addition, we performed preclinical studies of Selinexor in combination with paclitaxel, velboraf (B-raf inhibitor), irinotecan, topotecan and radiation therapy. In all of these preclinical studies of Selinexor in combination with other drugs, we observed evidence of additive and/or synergistic effects with inhibition of tumor growth. In addition, we have observed evidence of anti-cancer activity with Verdinexor, an oral SINE compound closely-related to Selinexor, in dogs with newly-diagnosed or first relapse after chemotherapy lymphomas.
Our Other Drug Candidates
KPT-350 and Related SINE Compounds
As described above, XPO1 mediates the nuclear export of many different cargo proteins. Several of these proteins play key roles in inflammation and related processes. Nuclear factorkB, or NF-kB, is a protein that plays very important roles in many types of inflammation. In cells, NF-kB can be inhibited by another protein called IkB, or Inhibitor of NF-kB, that binds to NF-kB and prevents NF-kB from binding to DNA and driving inflammation. When inflammation occurs, XPO1 transports IkB out of the nucleus into the cytoplasm where it cannot inhibit NF-kB activity. When KPT-350 or a similar SINE compound inhibits XPO1, IkB export to the cytoplasm is blocked and IkB accumulates in the nucleus. The IkB in the nucleus binds to NF-kB and blocks its inflammatory activity. KPT-350 or a similar SINE compound also increases the concentration of other inhibitors of NF-kB in the nucleus such as FOXO and COMMD1 proteins. Thus, XPO1 inhibition leads to potent, multifaceted inhibition of the inflammatory mediator NF-kB.
KPT-350 and similar SINE compounds have additional important anti-inflammatory activities such as activation of the proteins RXRg, PPARg and NRF2 (an anti-oxidant and neuroprotective protein). Finally, in human patients treated with Selinexor, we observed reductions in the numbers of eosinophils, which are white blood cellspublic companies that are associated with inflammation and allergies. Our SINEnot emerging growth companies.
compounds have shown broad evidence of anti-inflammatory activity across preclinical models of the following diverse autoimmune diseases: rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis. These observations suggest that SINE compounds have multiple anti-inflammatory effects. We are evaluating several SINE compounds, including KPT-350, in additional inflammatory models and preclinical safety studies.
PAK4 Inhibitors
In addition to our SINE compounds, we also investigate XPO1 cargo proteins and their role in cell cycle and division. As part of this investigation, we have identified several XPO1 cargo proteins whose inhibition leads to the selective death of cancer cells. One of the XPO1 cargo proteins that we identified was P21-activated kinase 4, or PAK4. PAK4 is a signaling protein regulating numerous fundamental cellular processes, including intracellular transport, cellular division, cell shape and motility, cell survival, immune defense and the development of cancer. PAK4 interacts with many key signaling molecules involved in cancer such as beta-catenin, CDC42, Raf-1, BAD and myosin light change. Based on this biology, we used our drug discovery and optimization platform to identify small molecule inhibitors of PAK4. Our PAK4 inhibitors have shown broad evidence of anti-cancer activity against hematological and solid tumor malignancies cells while showing minimal toxicity to normal cellsin vitro. In mouse xenograft studies, our PAK4 inhibitors given orally have shown evidence of anti-cancer activity and tolerability. If we confirm these preliminary results in future preclinical studies, we may initiate IND-enabling toxicology studies with one or more PAK4 inhibitors.
Verdinexor (KPT-335)
We have used spontaneously occurring dog cancers as a surrogate model for human malignancies. It is widely known that canine lymphomas respond to chemotherapy similarly to their human counterpart (human NHL) and display a comparable genetic profile. Lymphomas are one of the most common tumors in pet dogs. Lymphoma in dogs is very aggressive and, without treatment, the tumors are often fatal within weeks. The majority of dog lymphomas are DLBCL and most of the others are T-cell lymphomas. Given the similarities of dog and human lymphomas, prior to initiating clinical trials of Selinexor in humans, we investigated a closely-related, orally available SINE, Verdinexor (KPT-335), in dogs with lymphomas. We have received a Minor Use / Minor Species, or MUMS, designation from the Center for Veterinary Medicine, or CVM, of the FDA for the treatment of newly-diagnosed or first relapse after chemotherapy lymphomas in dogs with Verdinexor.
Several different dog tumor cell lines including those derived from lymphomas exhibited growth inhibition and apoptosisin vitro upon exposure to nanomolar concentrations of Verdinexor. A Phase 1 clinical trial of Verdinexor was performed in dogs with cancer, primarily with lymphoma. The maximum tolerated dose was 35 mg/m2 twice per week although biological activity was observed at 20 mg/m2. PR or SD, in each case for at least 4 weeks, was observed in nine out of 14 dogs (64%) with lymphoma with a median time to disease progression of 66 days (range of 35 to 256 days). We performed a dose expansion study in six dogs with lymphoma who were given 30 mg/m2 of Verdinexor three times per week; PR or SD was observed in four of the six dogs (67%) with a median time to disease progression of 83 days (range of 35 to 250 days). Side effects included anorexia, weight loss, vomiting and diarrhea and were manageable with dose modulation and supportive care. We conducted an owner observation-based survey and the data indicated that the overall quality of life did not change significantly in dogs treated with Verdinexor. Based on these findings, a Phase 2b clinical trial, intended to support regulatory approval under the MUMS designation in the United States, was performed in 58 pet dogs with either newly-diagnosed or first relapse after chemotherapy lymphomas. Verdinexor was administered initially at doses ranging from 25 mg/m2 to 30 mg/m2 two or three days per week. Minimal or no supportive care was given. 54 of the 58 dogs (93%) were evaluable for response, meaning they had tumors that could be measured, as of August 7, 2013. The total CRs and PRs of the 58 dogs was
29%, with 1 CR and 16 PRs. An additional 33 of 58 dogs (57%) experienced SD for at least four weeks. The total CRs, PRs, and SD were equal to 98% of the 54 evaluable dogs. The median time to disease progression was approximately five weeks, with 15 of the 54 evaluable dogs (or 28%) remaining on study for longer than eight weeks. A few dogs who have received Verdinexor in the Phase 1 or 2b studies remained on therapy for longer than eight months.
We expect that we will submit the safety and effectiveness sections of a NADA for Verdinexor to the FDA by early 2014. We expect to seek to enter into a collaboration with a third party for the commercialization of Verdinexor for dog lymphoma, if we obtain regulatory approval. We believe that Verdinexor, if approved, would represent the first oral, targeted therapy for the treatment of dog lymphoma.
The evidence of anti-cancer activity and adverse effect profile of our drug candidate Verdinexor in dogs with certain NHL, primarily B and T-cell lymphomas, provided support for our decision to move our closely-related drug candidate Selinexor into Phase 1 clinical trials in humans.
Our Drug Discovery and Optimization Platform
The development of Selinexor, and other drug candidates, including our other SINE compounds and PAK4 inhibitors, as well as Verdinexor, began with our proprietary drug discovery and optimization platform. We intend to continue using this platform, which includes expertise in computational chemistry, our proprietary virtual chemical library andin silico screening know-how, certain biochemical assays, andin silico complexes of the structures of the target proteins bound with our small molecules, and other trade secrets and know-how.
While our platform can be used to target many protein families, we are focused on the discovery and development of novel inhibitors of nuclear export, particularly those targeting XPO1 and XPO1 cargos. We identified our small molecule inhibitors by using structural insights from X-ray crystallography and molecular modeling approaches, coupled with virtual screening. Initially promising compounds were then evaluated with our proprietary platform to optimize them into drug candidates.
Our ideal drug candidates selectively bind to the target protein and do so in part by forming a covalent bond with a particular cysteine residue in the protein. Cysteine is one of the 20 amino acids that make up proteins and has been useful for forming covalent bonds with drug compounds. Like non-covalent drugs, our compounds selectively bind, or "fit" into a specific binding pocket in the target protein, and don't "fit" well into binding pockets of other proteins. Additionally, our drug candidates form a covalent bond, which introduces a second level of selectivity, meaning that our compounds are less likely to bind inappropriately compared with typical non-covalent drugs. In addition, because covalent drugs can be given infrequently (e.g., once a day or even less), there are potentially fewer off-target effects as there is less need to maintain high drug levels. For example, our compounds Selinexor and Verdinexor are given two to three times per week.
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary or intellectual property protection for our drug candidates, our core technologies, and other know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary or intellectual property rights. Our policy is to seek to protect our proprietary and intellectual property position by, among other methods, filing patent applications in the United States and in foreign jurisdictions related to our proprietary technology and drug candidates. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary and intellectual property position.
We file patent applications directed to the composition of matter and methods of use and manufacture for our drug candidates. As of September 30, 2013, we were the sole owner of one patent in the United States (issued August 20, 2013 as U.S. Patent No. 8,513,230 and having an expiration date of March 5, 2031) and we had 19 pending patent applications in the United States, one of which is co-owned with a third party, four pending international applications filed under the Patent Cooperation Treaty (PCT) and 15 pending patent applications in foreign jurisdictions. The PCT is an international patent law treaty that provides a unified procedure for filing a single initial patent application to seek patent protection for an invention simultaneously in each of the member states. Although a PCT application is not itself examined and cannot issue as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications. The technology underlying such pending patent applications has been developed by us and was not acquired from any in-licensing agreement.
The intellectual property portfolios for our key drug candidates as of September 30, 2013 are summarized below.
In addition to the patent portfolios covering our key drug candidates, as of September 30, 2013, our patent portfolio also includes one patent that was issued August 20, 2013 as U.S. Patent No. 8,513,230 and pending patent applications relating to other XPO1 inhibitors and their use in targeted therapeutics. We also filed three Intent to Use Trademark Applications on August 29, 2013 covering our name, our logo and the two used together.
The term of individual patents depends upon the legal term for patents in the countries in which they are obtained. In most countries, including the United States, the patent term is 20 years from the earliest filing date of a non-provisional patent application. In the United States, a patent's term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent. The term of a patent that covers a drug may also be eligible for patent term extension when FDA approval is granted, provided statutory and regulatory requirements are met. See "—Government Regulation—Patent Term Restoration and Extension" below for additional information on such extensions. In the future, if and when our drug candidates receive approval by the FDA or foreign regulatory authorities, we expect to apply for patent term extensions on issued patents covering those drugs, depending upon the length of the clinical trials for each drug candidate and other factors. There can be no assurance that any of our pending patent applications will issue or that we will benefit from any patent term extension or favorable adjustment to the term of any of our patents.
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify our proprietary and intellectual property position for our drug candidates and technologies will depend on our success in obtaining effective patent claims and enforcing those claims if granted. However, patent applications that we may file or license from third parties may not result in the issuance of patents. We also cannot predict the breadth of claims that may be allowed or enforced in our patents. Our issued patent and any issued patents that we may receive in the future may be challenged, invalidated or circumvented. For example, we cannot be certain of the priority of inventions covered by pending third-party patent applications. If third parties prepare and file patent applications that also claim technology or therapeutics to which we have rights, we may have to participate in interference proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us. In addition, because of the extensive time required for clinical development and regulatory review of a drug candidate we may develop, it is possible that, before any of our drug candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent.
In addition to patents, we rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our collaborators, scientific advisors, employees and consultants, and invention assignment agreements with our employees. We also have agreements with selected consultants, scientific advisors and collaborators requiring assignment of inventions. The confidentiality agreements are designed to protect our proprietary information and, in the case of agreements or clauses requiring invention assignment, to grant us ownership of technologies that are developed through our relationship with a third party.
With respect to our proprietary drug discovery and optimization platform, we consider trade secrets and know-how to be our primary intellectual property. Trade secrets and know-how can be difficult to protect. We anticipate that with respect to this technology platform, these trade secrets and know-how may over time be disseminated within the industry through independent development, the publication of journal articles describing the methodology, and the movement of personnel skilled in the art from academic to industry scientific positions.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any drug candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
There are a large number of companies developing or marketing treatments for cancer and the other indications on which we currently plan to initially focus, including many major pharmaceutical and biotechnology companies. However, to our knowledge, no other company has an XPO1 inhibitor in clinical development at the present time.
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or that may be necessary for, our programs.
The key competitive factors affecting the success of all of our drug candidates, if approved, are likely to be their efficacy, safety, convenience, price, the level of generic competition and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any drugs that we may develop. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic drugs. Generic drugs for the treatment of cancer and the other indications on which we currently plan to initially focus are currently on the market, and additional drugs are expected to become available on a generic basis over the coming years. If we obtain marketing approval for our drug candidates, we expect that they will be priced at a significant premium over competitive generic drugs.
The most common methods of treating patients with cancer are surgery, radiation and drug therapy. There are a variety of available drug therapies marketed for cancer. In many cases, these drugs are administered in combination to enhance efficacy. While our drug candidates may compete with many existing drugs and other therapies, to the extent they are ultimately used in combination with or as an adjunct to these therapies, our drug candidates will not be competitive with them. Some of the currently-approved drug therapies are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established therapies and are widely-accepted by physicians, patients and third-party payors.
In addition to currently-marketed therapies, there are also a number of drugs in late stage clinical development to treat cancer and the other indications on which we plan to initially focus. These
drugs in development may provide efficacy, safety, convenience and other benefits that are not provided by currently-marketed therapies. As a result, they may provide significant competition for any of our drug candidates for which we obtain marketing approval.
If our lead drug candidates are approved for the indications for which we currently plan to initially focus, they will compete with the therapies and currently-marketed drugs discussed below.
XPO1 Inhibitors
We have observed preliminary evidence of anti-cancer activity of our XPO1 inhibitor and lead drug candidate, Selinexor, across a spectrum of patients with advanced cancers who had received multiple previous treatments and, despite these treatments, had disease that was progressing at the time of enrollment in our clinical trials. Assuming continued positive results from our ongoing Phase 1 clinical trials of Selinexor and based on regulatory feedback, we plan to initiate Phase 2/3 clinical trials of Selinexor in two cancer indications in the first half of 2014. We plan to select these indications based on the results of our ongoing Phase 1 clinical trials of Selinexor, the level of unmet medical need and the competitive landscape. We expect at least one of those indications to be in heavily pretreated patients with AML (in the elderly), DLBCL or MM, each of which is described above. Other potential indications include relapsed and/or refractory AML in the general population, newly-diagnosed AML in elderly patients or solid tumor malignancies, which may include gynecological malignancies and squamous cell cancers.
Patients with AML typically are treated with intensive multi-agent chemotherapy and high risk patients who enter remission and have a matched donor often receive an allogeneic stem cell transplant. Elderly patients with AML are often treated with less intensive chemotherapy regimens or drugs called hypomethylating agents because usual chemotherapy has marked toxicities. Once elderly patients with AML experience disease progression while on their initial chemotherapy and/or hypomethylating agent, their expected survival is very poor. Because of their advanced age, multiple other medical conditions, and requirements for multiple other drugs, the treatment of relapsed and/or refractory AML in elderly persons is difficult. An IL3-toxin conjugate (Stemline Inc.) is being evaluated in elderly persons with relapsed and/or refractory AML. A number of other trials with existing anti-cancer drugs (often in combinations) are ongoing in this population.
The initial therapy for DLBCL typically consists of multiagent cytotoxic drugs in combination with the monoclonal antibody rituximab (Rituxan®). In patients with DLBCL who are not elderly and who have good organ function, high dose chemotherapy with stem cell transplantation is often used. Newer targeted agents such as the BTK inhibitor ibrutinib and the immunomodulatory drug lenalidomide (Revlimid®) have shown activity in immunoblastic (activated B cell) DLBCL. There are also a number of other widely-used anti-cancer agents that have broad labels which include NHL, and some of these are being evaluated alone or in combination for the treatment of patients with DLBCL that have relapsed after several different types of chemotherapy. Certain monoclonal antibodies similar to rituximab are also being evaluated in relapsed DLBCL.
Currently, there are three commonly-used targeted or novel agents approved in the U.S. for the treatment of patients with MM: Velcade®, Revlimid® and Thalomid®. Other approved agents include Kyprolis®, approved by the FDA on July 20, 2012, and Pomalyst®, approved by the FDA on February 8, 2013, each for the relapsed and/or refractory patient population. Other potentially competitive therapies are in clinical development for MM. Vorinistat, being developed by Merck & Co., and panobinostat, being developed by Novartis AG, are being studied in combination with bortezomib for relapsed myeloma, and elotuzumab is being developed by Abbott Laboratories.
Drug compounds currently in preclinical studies, if developed and approved, could also be competitive with our drug candidates, if approved. Kosan Biosciences Inc. (acquired by Bristol-Myers
Squibb Company) has evaluated compounds derived from leptomycin B in preclinical studies. CanBas Co., Ltd. has been developing a product referred to as CBS9106, a preclinical XPO1 inhibitor.
With respect to indications other than cancer, there are many currently-marketed therapies and drugs in late-stage clinical development to treat non-oncology indications on which we plan to initially focus development of our XPO1 inhibitors. However, to our knowledge, as in cancer, there are no other XPO1 inhibitors in clinical development for the treatment of any other diseases, including indications like autoimmune and inflammatory diseases or wound healing. There is no published information on the use of the preclinical compounds that have been developed by Kosan Biosciences or CanBas Co. in models other than cancer.
PAK4 Inhibitors
Our PAK4 inhibitors, if developed and approved, would compete with currently-marketed therapies and drugs in clinical development to treat cancer. However, there are currently no marketed therapies that selectively target PAK4. Pfizer Inc. developed PF-03758309, a non-selective PAK inhibitor, meaning that this compound inhibited several of the PAK family members, and not solely PAK4, through Phase 1 clinical development, but that compound had poor oral bioavailability and, to our knowledge, its development has been discontinued. We are aware that PAK4 biology is being evaluated preclinically by AstraZeneca plc and Genentech, Inc. (acquired by Roche Holding AG). We are not aware of any PAK4 inhibitors that are in clinical development at the present time.
Manufacturing
We do not have any manufacturing facilities or personnel. We currently rely, and expect to continue to rely, on third parties for the manufacture of our drug candidates for preclinical and clinical testing, as well as for commercial manufacture if our drug candidates receive marketing approval. We have engaged one third party manufacturer to obtain the active pharmaceutical ingredient for Selinexor for preclinical and clinical testing. We have engaged a separate third-party manufacturer for fill-and-finish services. We obtain our supplies from these manufacturers on a purchase order basis and do not have a long-term supply arrangement in place. We do not currently have arrangements in place for redundant supply. For all of our drug candidates, we intend to identify and qualify additional manufacturers to provide the active pharmaceutical ingredient and fill-and-finish services prior to seeking regulatory approval.
All of our drug candidates are small molecules and are manufactured in reliable and reproducible synthetic processes from readily available starting materials. The chemistry is amenable to scale up and does not require unusual equipment in the manufacturing process. We expect to continue to develop drug candidates that can be produced cost-effectively at contract manufacturing facilities.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, manufacture, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Review and Approval of Drugs in the United States
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining regulatory approvals and the
subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant and/or sponsor to a variety of administrative or judicial sanctions, including refusal by FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by FDA and the Department of Justice or DOJ or other governmental entities.
An applicant seeking approval to market and distribute a new drug product in the United States must typically undertake the following:
Preclinical Studies
Preclinical studies include laboratory evaluation of the purity and stability of the manufactured drug substance or active pharmaceutical ingredient and the formulated drug or drug product, as well asin vitro and animal studies to assess the safety of the drug for initial testing in humans and to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. The results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical studies, among other things, are submitted to FDA as part of an IND. Some long-term preclinical testing, such as animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted.
Human Clinical Studies in Support of an NDA
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among
other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. An IND automatically becomes effective 30 days after receipt by FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and FDA must resolve any outstanding concerns before the clinical trial can begin.
In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health for public dissemination on their ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug has been associated with unexpected serious harm to patients. The FDA will typically inspect one or more clinical sites to assure compliance with GCP and the integrity of the clinical data submitted.
Section 505(b)(2) NDAs
NDAs for most new drug products are based on two full clinical studies which must contain substantial evidence of the safety and efficacy of the proposed new product. These applications are submitted under Section 505(b)(1) of the FDCA. The FDA is, however, authorized to approve an
alternative type of NDA under Section 505(b)(2) of the FDCA. This type of application allows the applicant to rely, in part, on the FDA's previous findings of safety and efficacy for a similar product, or published literature. Specifically, Section 505(b)(2) applies to NDAs for a drug for which the investigations made to show whether or not the drug is safe for use and effective in use and relied upon by the applicant for approval of the application "were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted."
Thus, Section 505(b)(2) authorizes the FDA to approve an NDA based on safety and effectiveness data that were not developed by the applicant. NDAs filed under Section 505(b)(2) may provide an alternate and potentially more expeditious pathway to FDA approval for new or improved formulations or new uses of previously approved products. If the 505(b)(2) applicant can establish that reliance on the FDA's previous approval is scientifically appropriate, the applicant may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new drug candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Submission of an NDA to FDA
Assuming successful completion of required clinical testing and other requirements, the results of the preclinical and clinical studies, together with detailed information relating to the product's chemistry, manufacture, controls and proposed labeling, among other things, are submitted to FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to an application user fee, currently exceeding $2.1 million, and the sponsor of an approved NDA is also subject to annual product and establishment user fees, currently exceeding $104,000 per product and $554,600 per establishment. These fees are typically increased annually.
The FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after FDA's receipt of the submission to determine whether the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before FDA accepts it for filing. Once the submission is accepted for filing, FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review process of NDAs. Most such applications are meant to be reviewed within ten months from the date of filing, and most applications for "priority review" products are meant to be reviewed within six months of filing. The review process may be extended by FDA for three additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by FDA following the original submission.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
The FDA also may require submission of a REMS plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA is required to refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Fast Track Designation
The FDA is authorized to expedite the review of applications for new drug products that are intended, either alone or in combination with other products, for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the disease or condition. Under the fast track program, the sponsor of a drug candidate may request FDA to designate the product for a specific indication as a fast track product concurrent with or after the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for fast track designation within 60 days after receipt of the sponsor's request.
In addition to other benefits, such as the ability to use surrogate endpoints and have greater interactions with FDA, FDA may initiate review of sections of a fast track product's NDA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, FDA's time period goal for reviewing a fast track application does not begin until the last section of the NDA is submitted. In addition, the fast track designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapies
In 2012, Congress enacted the Food and Drug Administration Safety and Improvement Act or FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as "breakthrough therapies." Breakthrough therapies are defined as those intended, either alone or in combination with other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development.
Under FDASIA, FDA may take certain actions with respect to products designated as breakthrough therapies, including holding meetings with the sponsor and the review team throughout the development process; providing timely advice to and communication with the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking certain steps to design the clinical trials in an efficient manner.
Accelerated Approval
FDASIA also codified and expanded on FDA's accelerated approval regulations, under which FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit. Under FDASIA, FDA may also grant accelerated approval using a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
A surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. FDASIA lists the types of evidence that may be used to support a finding that an endpoint is reasonably likely to predict clinical benefit. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, would allow FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA.
FDA's Decision on an NDA
On the basis of the FDA's evaluation of the NDA and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
If the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug's safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly,
manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Hatch-Waxman Act, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application or ANDA to the agency. In support of such applications, a generic manufacturer may rely on the preclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference listed drug, or RLD.
Specifically, in order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug. At the same time, the FDA must also determine that the generic drug is "bioequivalent" to the innovator drug. Under the statute, a generic drug is bioequivalent to a RLD if "the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug. . . ."
Upon approval of an ANDA, the FDA indicates that the generic product is "therapeutically equivalent" to the RLD and it assigns a therapeutic equivalence rating to the approved generic drug in its publication "Approved Drug Products with Therapeutic Equivalence Evaluations," also referred to as the "Orange Book." Physicians and pharmacists consider an "AB" therapeutic equivalence rating to mean that a generic drug is fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, FDA's designation of an "AB" rating often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
The FDCA provides a period of five years of non-patent data exclusivity for a new drug containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA may not be filed with FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication.
Hatch-Waxman Patent Certification and the 30-Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant's product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, the applicant must certify with respect to each patent that:
A certification that the new product will not infringe the already approved product's listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicate that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, a NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in 2012, sponsors must also submit
pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, FDA, and FDA's internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity. This six-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA's request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot accept or approve another application.
Orphan Designation and Exclusivity
Under the Orphan Drug Act, FDA may designate a drug product as an "orphan drug" if it is intended to treat a rare disease or condition (generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a drug product available in the United States for treatment of the disease or condition will be recovered from sales of the product). A company must request orphan product designation before submitting a NDA. If the request is granted, FDA will disclose the identity of the therapeutic agent and its potential use. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product with orphan status receives the first FDA approval for the disease or condition for which it has such designation, the product will be entitled to orphan product exclusivity. Orphan product exclusivity means that FDA may not approve any other applications for the same product for the same indication for seven years, except in certain limited circumstances. Competitors may receive approval of different products for the indication for which the orphan product has exclusivity and may obtain approval for the same product but for a different indication. If a drug or drug product designated as an orphan product ultimately receives marketing approval for an indication broader than what was designated in its orphan product application, it may not be entitled to exclusivity.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, which permits a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of a NDA, plus the time between the submission date of a NDA and the ultimate approval date. Patent term
restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product's approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drugs for which approval is sought can only be extended in connection with one of the approvals. The USPTO reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
Review and Approval of Drug Products in the European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of drug products. Whether or not it obtains FDA approval for a product, the company would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of a European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favourable opinion. Clinical trial application must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a drug under European Union regulatory systems, an applicant must submit a marketing authorization application, or MAA, either under a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or the CHMP, established at the European Medicines Agency, or EMA, is responsible for conducting the initial assessment of a drug. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic
innovation. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
The decentralized procedure is available to applicants who wish to market a product in various European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state designated by the applicant, known as the reference member state. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application. Within 90 days of receiving the reference member state's assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, whose decision is binding on all member states.
Data and Market Exclusivity in the European Union
In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator's data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator's data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full MAA with a complete database of pharmaceutical test, pre-clinical tests and clinical trials and obtain marketing approval of its product.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by FDA and other government authorities. Sales of products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor's decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third-party reimbursement may
not be sufficient to maintain price levels high enough to realize an appropriate return on investment in product development.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third-party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider a product to be cost-effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals.
As a result, the marketability of any product which receives regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on managed care in the United States has increased and will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the Patient Protection and Affordable Care Act contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies' share of sales to federal health care programs. Even if favorable coverage and reimbursement status is attained for one or more products that receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular drug candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
Healthcare Law and Regulation
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with third-party payors and customers are subject to broadly applicable fraud and abuse and other
healthcare laws and regulations. Such restrictions under applicable federal and state healthcare laws and regulations, include the following:
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Review and Approval of Animal Drugs in the United States
In addition to pursuing approval of our drug candidates for use in human beings, we may also seek approval of certain drug candidates for veterinary applications. As with new drug products for human beings, new animal drugs may not be marketed in the United States until they have been approved by the FDA as safe and effective. The requirements and phases governing approval of a new animal drug are analogous to those for new human drugs. Specifically, the Center for Veterinary Medicine or CVM at FDA is responsible for determining whether a new veterinary product should be approved on the basis of a NADA filed by the applicant. A NADA must contain substantial evidence
of the safety and effectiveness of the animal drug, as well as data and controls demonstrating that the product will be manufactured and studied in compliance with, among other things, applicable cGMP and GLP practices.
To begin this process, an applicant must file an Investigational New Animal Drug application, or INAD, with the CVM. The applicant will hold a pre-development meeting with the CVM to reach general agreement on the plans for providing the data necessary to fulfill requirements for a NADA. In this context, an applicant must submit pivotal protocols to the CVM for review and concurrence prior to conducting the required studies. The applicant will gather and submit data on safety, efficacy and chemistry, manufacturing and controls or CMC to the CVM for review, as below:
| ||
|
| |
|
| |
Option to purchase additional shares | The underwriters have an option for a period of 30 days to purchase up to 330,000 additional shares of our common stock. | |
Use of proceeds | We intend to use the | |
We will | ||
See "Use of | ||
NASDAQ Global Select Market symbol | "KPTI" |
Once all
The number of shares of our common stock to be outstanding after this offering is based on 29,762,499 shares of our common stock issued and outstanding as of March 31, 2014, including 143,620 shares of unvested restricted stock subject to repurchase by us, and excludes:
Unless otherwise indicated, this prospectus reflects and assumes the following:
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table presents our summary consolidated financial data. We have derived the following summary of our statement of operations data for the years ended December 31, 2013, 2012 and 2011 from our audited consolidated financial statements incorporated by reference in this prospectus from our Annual Report on Form 10-K for the year ended December 31, 2013. We have been submitted and reviewedderived the following summary of our statement of operations data for each technical section—safety, efficacy and CMC—the CVM will issue a technical section complete letter as each section review is completed. When the three lettersmonths ended March 31, 2014 and 2013 from our unaudited consolidated financial statements incorporated by reference in this prospectus from our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2014. We have been issued,derived the applicant will compile a draftsummary of our balance sheet data as of March 31, 2014 from our unaudited consolidated financial statements incorporated by reference herein. Our historical results are not necessarily indicative of the Freedom of Information Summary, the proposed labeling, and all other relevant information, and submit these as an administrative NADA for CVM review. Generally, if there are no deficienciesresults that may be expected in the submission,future. The summary of our consolidated financial data set forth below should be read together with our consolidated financial statements and the NADArelated notes to those statements, as well as "Management's Discussion and Analysis of Financial Condition and Results of Operations," all included elsewhere or incorporated by reference in this prospectus. For more details on how you can obtain the documents incorporated by reference in this prospectus, see "Where You Can Find More Information" and "Incorporation of Documents by Reference" appearing elsewhere in this prospectus.
| | | | | Three Months Ended, March 31, | Period from December 22, 2008 (Inception) to March 31, 2014 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Year Ended December 31, | ||||||||||||||||||
| | 2013 | 2012 | 2011 | 2014 | 2013 | ||||||||||||||
| | (in thousands, except share and per share data) | ||||||||||||||||||
Consolidated Statement of Operations Data: | |||||||||||||||||||
Contract and grant revenue | $ | 387 | $ | 634 | $ | 152 | $ | 171 | $ | 233 | $ | 1,437 | |||||||
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | |||||||||||||||||||
Research and development | 28,452 | 14,095 | 8,623 | 10,979 | 4,965 | 63,814 | |||||||||||||
General and administrative | 5,885 | 2,429 | 1,840 | 2,904 | 879 | 13,721 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | 34,337 | 16,524 | 10,463 | 13,883 | 5,844 | 77,535 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | (33,950 | ) | (15,890 | ) | (10,311 | ) | (13,712 | ) | (5,611 | ) | (76,098 | ) | |||||||
Interest income (expense), net | 3 | 2 | — | 18 | — | (165 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | |
Net loss | $ | (33,947 | ) | $ | (15,888 | ) | $ | (10,311 | ) | $ | (13,694 | ) | $ | (5,611 | ) | $ | (76,263 | ) | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Net loss per share applicable to common stockholders—basic and diluted | $ | (5.59 | ) | $ | (8.95 | ) | $ | (10.27 | ) | $ | (0.46 | ) | $ | (2.52 | ) | $ | (24.70 | ) | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders—basic and diluted | 6,067,679 | 1,775,323 | 1,004,144 | 29,606,683 | 2,225,596 | 3,087,622 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | As of March 31, 2014 | ||||||
|---|---|---|---|---|---|---|---|
| | Actual | As Adjusted(1) | |||||
| | (in thousands) | ||||||
Consolidated Balance Sheet Data: | |||||||
Cash and cash equivalents | $ | 144,893 | 221,919 | ||||
Working capital | 142,551 | 219,577 | |||||
Total assets | 149,285 | 226,311 | |||||
Total stockholders' equity | 144,100 | 221,126 | |||||
Investing in our common stock involves a high degree of risk. Before investing in our common stock, you should consider carefully the risks described below, together with the other information contained in this prospectus and incorporated by reference in this prospectus, including the risk factors incorporated by reference from our Annual Report on Form 10-K for the year ended December 31, 2013, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 and other filings we make with the SEC. We believe the risks described below and incorporated by reference herein are the risks that are material to us as of the date of this prospectus. If any of the following risks or the risks incorporated by reference herein occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related To This Offering
Our executive officers, directors and principal stockholders maintain the ability to control all matters submitted to stockholders for approval.
As of June 1, 2014, our executive officers, directors and a small number of stockholders own more than a majority of our outstanding common stock. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
If you purchase shares of common stock in this offering, you will suffer immediate dilution of your investment.
The price of our common stock in this offering will be substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent shares are issued within fourunder outstanding options, you will incur further dilution. Based on an assumed public offering price of $41.37 per share, which is the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, you will experience immediate dilution of $34.41 per share, representing the difference between our as adjusted net tangible book value per share after giving effect to six months after submissionthis offering and the assumed public offering price per share.
An active trading market for our common stock may not be sustained following this offering.
Although our common stock is listed on The NASDAQ Global Select Market, an active trading market for our shares may not be sustained following this offering. If an active market for our common stock does not continue, it may be difficult for you to sell shares you purchase in this offering without depressing the market price for the shares, or at all. An inactive trading market for our common stock may also impair our ability to raise capital to continue to fund our operations by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
We have broad discretion in the use of our cash and cash equivalents, including the administrative NADA. Thisnet proceeds we receive in this offering, and may not use them effectively.
Our management has broad discretion to use our cash and cash equivalents, including the net proceeds we receive in this offering, to fund our operations and could spend these funds in ways that do not improve our results of operations or enhance the value of our common stock. The failure by
reviewour management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our common stock to decline and delay the development of our drug candidates. Pending their use to fund our operations, we may invest our cash and cash equivalents, including the net proceeds from this offering, in a manner that does not produce income or that loses value.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, of our common stock will be conducted according to timelines specified in the Animal Drug User Fee Act. The FDA's basissole source of gain for approving a NADA is documented in a Freedom of Information Summary. Post-approval monitoring of products is required by law, with reports being provided to the CVM's Surveillance and Compliance group. Reports of product quality defects, adverse events or unexpected results must also be produced in accordance with the relevant regulatory requirements.
Employees
As of September 30, 2013, we had 23 full-time employees, including a total of 11 employees with M.D. or Ph.D. degrees. Of these full-time employees, 18 employees are engaged in research and development activities. None of our employees is represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
Facilitiesstockholders.
We occupy approximately 7,743 square feethave never declared or paid cash dividends on our capital stock. We currently intend to retain all of officeour future earnings, if any, to finance the growth and laboratory spacedevelopment of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in Natick, Massachusettsthe near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. After this offering, we will have outstanding 31,762,499 shares of common stock based on 29,762,499 shares outstanding as of March 31, 2014. This assumes no exercise by the underwriters of their option to purchase additional shares and includes the shares that we and the selling stockholders are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our affiliates. Of the remaining shares, a majority are currently restricted as a result of securities laws, including under a leaseRule 144 or under lock-up agreements but will be able to be sold after the offering as described in the "Shares Eligible for Future Sale" section of this prospectus. Moreover, following this offering, based on information we have available to us, we believe holders of an aggregate of 17,819,662 shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that expires in January 31, 2015.we may file for ourselves or other stockholders. We believehave also registered shares of common stock that our facility is sufficient to meet our current needswe have issued upon the exercise of outstanding stock options, and that suitable additional space willwe may in the future issue, under our equity compensation plans. These can be available asfreely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and when needed.
Legal Proceedings
We are not currently a party to any material legal proceedings.the lock-up agreements described in the "Underwriting" section of this prospectus.
MANAGEMENTCAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Executive Officers This prospectus and Directorsthe documents incorporated by reference into it contain forward-looking statements that involve substantial risks and uncertainties. These statements include all matters that are not related to present facts or current conditions or that are not historical facts, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward- looking statements.
The following table sets forth words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the "Risk Factors" section and the other documents incorporated by reference herein, that could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make.
You should read this prospectus, the documents that we incorporate by reference or otherwise reference herein and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this prospectus and in the documents incorporated herein by reference are made as of October 1, 2013.
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
Michael G. Kauffman, M.D., Ph.D. Dr. Kauffman co-founded Karyopharm with Dr. Sharon Shacham in 2008 and has served as our President and Chief Executive Officer since January 2011, as Chief Medical Officer since December 2012, andwe do not assume any obligation to update any forward-looking statements, whether as a director since 2008. Prior to joining Karyopharm, he was Chief Medical Officerresult of Onyx Pharmaceuticals Inc., a biopharmaceutical company, from November 2009 to December 2010, which acquired Proteolix Inc. in November 2009 where he was Chief Medical Officer since November 2008, where he led the development of Kyprolis® (carfilzomib), a novel proteasome inhibitor approved in refractory myelomanew information, future events or otherwise, except as required by the FDA in July 2012. Prior to joining Onyx Pharmaceuticals, Dr. Kauffman was an operating partner at Bessemer Venture Partners from 2006 to 2008 where he led investments in biotechnology companies. Prior to that, he was President and Chief Executive Officer of Epix Pharmaceuticals, Inc., a biopharmaceutical company that underwent liquidation proceedings through an assignment for the benefit of creditors under Massachusetts law in 2009, from 2006 to 2008, and President and Chief Executive Officer of Predix Pharmaceuticals, Inc., a private biopharmaceutical company focused on G protein-coupled receptors (GPCR), from 2002 until its merger into Epix Pharmaceuticals in 2006, where he led the merger of Predix Pharmaceuticals and Epix Pharmaceuticals, oversaw the discovery and development of four new clinical candidates and led collaboration transactions with Amgen and GlaxoSmithKline. From March 2000 to September 2002, Dr. Kauffman was Vice President, Clinical at Millennium Pharmaceuticals, Inc., a biopharmaceutical company, where he led the Velcade® development program. From September 1997 to March 2000, Dr. Kauffman held a number of senior positions at Millennium Predictive Medicine, Inc., a biopharmaceutical company and a subsidiary of Millennium Pharmaceuticals, where he led the discovery and development of novel molecular diagnostics for major cancers including melanoma, and led transactions with Becton-Dickenson and Bristol Myers Squibb. From August 1995 to September 1997, Dr. Kauffman held a number of senior positions at Biogen Idec, Inc., a biopharmaceutical company, where he led the clinical development of anti-CD40L antibodies in autoimmune and inflammatory diseases, and acted as the main medical advisor to the Biogen business developmentlaw.
group. Dr. Kauffman
USE OF PROCEEDS
We estimate that the net proceeds to us from this offering will be approximately $77.0 million, based on an assumed public offering price of $41.37 per share, which is the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares of our common stock in full, we estimate that our net proceeds will be approximately $89.9 million after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We will not receive any of the proceeds from any sale of shares in this offering by the selling stockholders.
As of March 31, 2014, we had cash and cash equivalents of $144.9 million. We currently serves onintend to use the boardnet proceeds from this offering as follows:
This expected use of directorsnet proceeds from this offering represents our intentions based upon our current plans and the audit committee and compensation committee of Zalicus Inc. (formerly CombinatoRx Inc.), a public biopharmaceutical company, and on the board of directors and the compensation committee of Metamark Genetics Inc., a private molecular diagnostics company. Dr. Kauffman received his B.A. in Biochemistry from Amherst College, his M.D. and Ph.D. from Johns Hopkins Medical School, and trained in internal medicine and rheumatology at Beth Israel (now Beth Israel Deaconness Medical Center) and Massachusetts General Hospitals. He is board certified in internal medicine. We believe Dr. Kauffman's qualifications to serve on our board of directors include his extensive experiencebusiness conditions, which could change in the healthcare industryfuture as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development, feedback from regulatory authorities, the status of and results from clinical trials, as well his extensive knowledge of our company and its business since inception through service in multiple executive leadership positions and as a member of our board.
Sharon Shacham, Ph.D., M.B.A. Dr. Shacham founded Karyopharm in 2008 and has served as our Chief Scientific Officer and President of Research and Development since December 2012, as our Chief Scientific Officer and Head of Research and Development from October 2010 to December 2012 and as our President and Chief Executive Officer from October 2010 to January 2011. Dr. Shacham established the company to focus on the discovery and development of small molecule inhibitors of nuclear export and has led our scientific progress since inception. Her computational drug discovery algorithms formed a critical part of the technological basisany collaborations that we may enter into with third parties for our drug discoverycandidates, and optimization platform, which was used forany unforeseen cash needs. As a result, our management will retain broad discretion over the discoveryallocation of Selinexor, our lead drug candidate. Dr. Shacham co-chairs our Scientific Advisory Board. Priorthe net proceeds from this offering.
Pending use of the proceeds as described above, we intend to founding Karyopharm, from July 2000 to April 2009, she was Senior Vice Presidentinvest the proceeds in a variety of Drug Development at Epix Pharmaceuticals, Inc., which underwent liquidation proceedings through an assignment for the benefit of creditors under Massachusetts law in 2009,capital preservation investments, including short-term, interest-bearing, investment-grade instruments and Director, Algorithm and Software Development at Predix Pharmaceuticals Inc. which merged into Epix Pharmaceuticals in 2006, where she led the company's efforts in GPCR modeling, computational chemistry, lead optimization and development of clinical trials. Dr. Shacham received her B.Sc. in Chemistry, Ph.D. and M.B.A. from Tel Aviv University.
Paul Brannelly. Mr. Brannelly joined Karyopharm in June 2013 as Senior Vice President, Finance and Administration and has served as our Secretary and Treasurer since July 2013. Prior to joining Karyopharm, Mr. Brannelly served most recently as Vice President of Finance at Verastem, Inc., a biopharmaceutical company, from September 2011 to June 2013, Chief Financial Officer from November 2010 to September 2011, and as Treasurer and Secretary from November 2010 to June 2013, where he led the company through the initial public offering process and managed several successful financings. From January 2010 to September 2011, Mr. Brannelly held the position of Chief Financial Officer at the Longwood Fund, a venture capital firm aimed at investing in, managing and building healthcare companies, where he set up the financial and operational infrastructure following the closing of its first fund. From November 2005 to September 2009, he served as Vice President, Finance at Sirtris Pharmaceuticals, Inc., a biopharmaceutical company which GlaxoSmithKline plc purchased for $720 million in 2008, where he managed the S-1 preparation and due diligence process for Sirtris' initial public offering and managed the company's transition to being a public company. Mr. Brannelly started his biopharmaceutical career at Dyax Corporation from September 1999 to May 2002, and subsequently moved on to positions of increasing responsibility at Zalicus Inc. (formerly CombinatoRx Inc.) from May 2002 to November 2005, most recently as VP Finance and Treasurer, where he led Zalicus through the initial public offering process. Mr. Brannelly holds a Bachelors of Business Administration in Accounting from the University of Massachusetts at Amherst.
Garen G. Bohlin. Garen G. Bohlin has served as a member of our board of directors since October 2013. Since April 2012, Mr. Bohlin has focused exclusively on service on boards of directors and consulting. From January 2010 until his retirement in April 2012, he served as Executive Vice President of Constellation Pharmaceuticals, Inc., a biopharmaceutical company, where his responsibilities included consummating option-to-acquire and pre-negotiated merger agreements withU.S. government securities.
Genentech.
PRICE RANGE OF COMMON STOCK
Our common stock began trading on The NASDAQ Global Select Market under the symbol "KPTI" on November 6, 2013. Prior to Constellation Pharmaceuticals, Mr. Bohlin servedthat time, there was no public market for our common stock. The following table sets forth the high and low sale prices per share of our common stock, as Chief Operating Officerreported on The NASDAQ Global Select Market, for the periods indicated.
| | High | Low | |||||
|---|---|---|---|---|---|---|---|
Year Ended December 31, 2013 | |||||||
Fourth quarter (from November 6, 2013) | $ | 25.69 | $ | 15.50 | |||
Year Ending December 31, 2014 | |||||||
First quarter | $ | 47.87 | $ | 21.13 | |||
Second quarter (through June 24, 2014) | $ | 47.98 | $ | 23.86 | |||
On June 24, 2014, the last reported sale price of Sirtris Pharmaceuticals, a biotechnology company, from January 2006our common stock on The NASDAQ Global Select Market was $41.37 per share. As of the date of this prospectus, we had approximately 25 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
We have not declared or paid any cash dividends on our capital stock since our inception. We intend to December 2009, where he played key rolesretain future earnings, if any, to finance the operation and expansion of our business and do not anticipate paying any cash dividends in the overall management of Sirtris, its initial public offeringforeseeable future.
We obtained the industry, market and the sale of the company to GlaxoSmithKline. Mr. Bohlin was the founding Chief Executive Officer of Syntonix Pharmaceuticals, Inc., a biopharmaceutical company,competitive position data in this prospectus from 1999 through December 2008, where he played a key role in the overall management of Syntonix, positioning it for an eventual sale to Biogen Idec. Prior to Syntonix, Mr. Bohlin was Executive Vice President of Genetics Institute Inc., a biotechnology company, where he played a key role in overall management, its initial public offeringour own internal estimates and its sale to American Home Products/Wyeth, and a partner at Arthur Andersen & Co., a public accounting and consulting organization. Mr. Bohlin serves on the board of directors and audit committee of Tetraphase Pharmaceuticals, Inc., a public biopharmaceutical company, and serves on the board of directors and audit committees of private biotechnology companies Acusphere, Inc. and Precision Dermatology, Inc. He holds a B.S. in Accountingresearch as well as from the University of Illinois. We believe Mr. Bohlin's qualifications to serve on our board of directors include his extensive industry and board experience, including his audit committee experience, with publicly tradedgeneral publications and privately held biotechnology companies.
Barry E. Greene. Mr. Greene has served as a memberresearch, surveys and studies conducted by third parties. Industry and general publications and research, surveys and studies conducted by third parties generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of our board of directors since January 2013. Mr. Greene has served as President and Chief Operating Officer of Alnylam Pharmaceuticals, Inc., a biopharmaceutical company, since 2007, as its Chief Operating Officer since 2003, and from 2004 through 2005, as its Treasurer. Mr. Greene joined Alnylam in 2003, bringing over 15 years of experience in the healthcare industry and in consulting. Prior to Alnylam, he was General Manager of Oncology at Millennium Pharmaceuticals, Inc., a biopharmaceutical company, where he led the company's global strategy and execution for its oncology business, including strategic business direction and execution, culminating in the successful FDA approval and launch of VELCADE (bortezomib) in mid-2003. Prior to joining Millennium in 2001, Mr. Greene served as Executive Vice President and Chief Business Officer for Mediconsult.com, a healthcare consulting company. Prior to Mediconsult.com, Mr. Greene's experience included serving as Vice President of Marketing and Customer Services for AstraZeneca (formerly AstraMerck), a biopharmaceutical company; Vice President, Strategic Integration with responsibility for the AstraZeneca North American post-merger integration; and a partner of Andersen Consulting, a consulting company, where he was responsible for the pharmaceutical/biotechnology marketing and sales practice. Mr. Greene currently is a member of the board of directors of Acorda Therapeutics, Inc., a public biopharmaceutical company. Mr. Greene received his B.S. in Industrial Engineering from the University of Pittsburgh and serves as a Senior Scholar at Duke University, Fuqua School of Business. We believe Mr. Greene's qualifications to serve on our board of directors include his extensive experience in the healthcare and consulting industries as well his practical experience guiding new drugs through the commercialization process.
Deepa R. Pakianathan, Ph.D. Dr. Pakianathan has served as a member of our board of directors since April 2013. Since 2001, Dr. Pakianathan has been a Managing Member at Delphi Ventures, a venture capital firm focused on medical device and biotechnology investments, and leads the firm's biotechnology investment activities. From 1998 to 2001, Dr. Pakianathan was a senior biotechnology banker at JPMorgan, a global investment bank. Since 2004, Dr. Pakianathan has served on the board of directors of Alexza Pharmaceuticals, Inc., a public biopharmaceutical company, where she serves as a member of its compensation and nominating and governance committees. Since 2007, Dr. Pakianathan has served on the board of directors of Alder Biopharmaceuticals, Inc., a private biopharmaeutical company, where she serves as a member of its nominating and governance committee, and has served on the board of directors of NeurAxon, Inc., a private biopharmaceutical company, where she serves as a member of its compensation committee. Since 2008, Dr. Pakianathan has served on the board of directors of OncoMed Pharmaceuticals, Inc., a public biopharmaceutical company, where she serves as chair of its audit committee. From 2009 to March 2013, Dr. Pakianathan served on the board of directors of PTC Therapeutics, Inc., a public biopharmaceutical company.such information.
Dr. Pakianathan received a B.Sc. from the University of Bombay, India, a M.Sc. from
CAPITALIZATION
The Cancer Research Institute at the University of Bombay, India,following table sets forth our cash and an M.S.cash equivalents and Ph.D. from Wake Forest University. We believe Dr. Pakianathan's qualifications to serve on our board of directors include her experience as a venture capital investor in, and director of, multiple biotechnology companies, as well as her experience as a biotechnology investment banker.
Mansoor Raza Mirza, M.D. Dr. Mirza has served as a member of our board of directors since October 2010. He has also served as a scientific consultant to us since 2010. Dr. Mirza is Chief Oncologist at the Department of Oncology, Rigshopitalet—the Copenhagen University Hospital, Denmark. Dr. Mirza is both a medical and radiation oncologist, with a primary focus in non-surgical treatment of gynecologic cancers. His key academic goals are to promote clinical research, international trial collaboration and education, and he has broad experience in clinical protocol development, trial conduct and clinical trial regulations. Dr. Mirza is the author of several phase 1, 2 and 3 studies and serves as the chair of the Independent Data Safety Monitoring Committee of the OUTBACK trial, which is a large international cervical cancer trial. He is the senior author of national Danish guidelines for the management of endometrial, cervical, vulvar and non-epithelial ovarian cancers as wellcapitalization as of NSGO radio therapy guidelines for cervical and vulvar cancers. His other current appointments include service as President-Elect of the Nordic Society of Gynecologic Oncology (NSGO), Medical Director of the NSGO-Clinical Trial Unit, Vice-Chairman of the Danish Gynecological Cancer Society, Founding Executive Member of the Euroepean Network of Gynecologic Oncology Trials Group, and membership on the faculty of the European Society of Medical Oncology. He also serves on the board of directors of the Gynecologic Cancer Intergroup, a private organization promoting high quality clinical trials, and Metamark Genetics Inc., a private biopharmaceutical company. He holds a M.D., Diploma in Surgery and Diploma in Clinical Oncology from the Pirogov Moscow State Medical Institute as well as post-graduate education and certification in radiation and medical oncology from the University of Southern Denmark. We believe Dr. Mirza's qualifications to serve on our board of directors include his position as an expert in the non-surgical treatment of cancer, and gynecologic cancers in particular, and his knowledge of our company and its business through service on our board since October 2010.
Kenneth E. Weg. Mr. Weg has served as a member of our board of directors since February 2013. He has over 34 years of experience in the pharmaceutical industry with global biopharmaceutical companies Bristol-Myers Squibb Company and Merck & Co., Inc. From 1993 to 1998, he was President, Worldwide Medicines Group of Bristol-Myers Squibb, responsible for all ethical pharmaceuticals and over-the-counter medicines on a global basis. Mr. Weg also served as Vice-Chairman of the Board of Bristol-Myers Squibb from 1993 to 1998. He retired from Bristol-Myers Squibb in February 2001. Mr. Weg also served as non-Executive Chairman of Millennium Pharmaceuticals until that company was acquired by Takeda, Inc. in 2008. In addition, he has served as founder and Chairman of Metamark Genetics Inc., a private biotechnology company, since 2005. He holds a B.A. in English Literature from Dartmouth College and an M.B.A. from Columbia University. We believe Mr. Weg's qualifications to serve on our board of directors include his extensive leadership experience in the global pharmaceutical industry, including his extensive executive leadership at Bristol-Myers Squibb, and his experience as a member of the boards of directors of multiple biopharmaceutical companies.
Board Composition
Our board of directors is currently authorized to have eight members. Upon the closing of this offering, our board of directors will consist of six directors. In accordance with the terms of our certificate of incorporation and bylaws that will become effective upon the closing of this offering, our board of directors will be divided into three classes, class I, class II and class III, with members of each
class serving staggered three-year terms. Upon the closing of this offering, the members of the classes will be dividedMarch 31, 2014, as follows:
You should read this information in conjunction with our consolidated financial statements and the annual meetingrelated notes and the "Management's Discussion and Analysis of stockholdersFinancial Condition and Results of Operations" section, the "Selected Financial Data" section and other financial information in our Annual Report on Form 10-K for the year ended December 31, 2013 and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014, which are incorporated by reference in this prospectus.
| | As of March 31, 2014 | ||||||
|---|---|---|---|---|---|---|---|
| | Actual | As Adjusted | |||||
| | (in thousands, except share and per share data) | ||||||
Cash and cash equivalents | $ | 144,893 | $ | 221,919 | |||
| | | | | | | | |
Undesignated preferred stock, $0.0001 par value: 5,000,000 shares authorized and no shares issued or outstanding | — | — | |||||
Common stock, par value $0.0001 per share; 100,000,000 shares authorized, 29,618,879 shares issued and outstanding, actual; and 31,618,879 shares issued and outstanding, as adjusted | 3 | 3 | |||||
Additional paid-in capital | 220,360 | 297,386 | |||||
Deficit accumulated during the development stage | (76,263 | ) | (76,263 | ) | |||
| | | | | | | | |
Total stockholders' equity | 144,100 | 221,126 | |||||
| | | | | | | | |
Total capitalization | $ | 144,100 | $ | 221,126 | |||
| | | | | | | | |
| | | | | | | | |
The table above does not include:
Upon the expiration of the term of a class of directors, directors in that class will be eligible to be electedour common stock available for a new three-year term at the annual meeting of stockholders in the year in which their term expires. Our directors may be removed only for causepurchase by the affirmative vote of the holders of 75% or more of our stock entitled to vote thereon.
We have no formal policy regarding board diversity. Our priority in selection of board members is identification of members who will further the interests of our stockholders through his or her established record of professional accomplishment, the ability to contribute positively to the collaborative culture among board members, knowledge of our business and understanding of the competitive landscape.
Director Independence
The NASDAQ Listing Rules requires a majority of a listed company's board of directors to be comprised of independent directors within one year of listing. In addition, the NASDAQ Listing Rules require that, subject to specified exceptions, each member of a listed company's audit, compensation and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Under the NASDAQ Listing Rules, a director will only qualify as an independent director if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In October 2013, our board of directors undertook a review of the composition of our board of directors and its committees upon the closing of the offering and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors has determined that each of our directors, with the exception of Dr. Kauffman and Dr. Mirza, is an independent director as defined under the NASDAQ Listing Rules. Our board of directors also determined that Mr. Bohlin, Mr. Greene and Dr. Pakianathan, who will comprise our audit committee following this offering, Mr. Greene, Dr. Pakianathan and Mr. Weg, who will comprise our compensation committee following this offering, and Mr. Bohlin and Mr. Weg, who will be two of the members of our nominating and corporate governance committee following this offering, satisfy the independence standards for such committees established by the SEC and the NASDAQ Listing Rules, as applicable. In making such determinations, our board of directors considered the relationships that each such non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director. Dr. Kauffman does not qualify as an independent director under the standards established by the SEC and the NASDAQ Listing Rules, but will be a member of our nominating and corporate governance committee following this offering, under applicable exemptions in the NASDAQ Listing Rules. Under such exemptions, Dr. Kauffman will be permitted to serve on the nominating and corporate governance committee for up to one year following the consummation of this offering.
There are no family relationships among any of our directors or executive officers, except that Michael Kauffman, M.D., Ph.D., our President, Chief Executive Officer, Chief Medical Officer and a director, is married to Sharon Shacham, Ph.D., M.B.A., our Chief Scientific Officer and President of Research and Development.
Board Committees
Our board has established three standing committees—audit, compensation, and nominating and corporate governance—each of which will operate, upon the closing of this offering, under a charter that has been approved by our board. We intend to post current copies of each committee's charter on the Corporate Governance section of our website, www.karyopharm.com. The composition of each committee will be effective upon the closing of this offering.
Audit Committee
The members of our audit committee are Mr. Bohlin, Mr. Greene and Dr. Pakianathan. Mr. Bohlin is the chair of the audit committee. Our board of directors has determined that Mr. Bohlin qualifies as an audit committee financial expert within the meaning of SEC rules. In making this determination, our board has considered the formal education and nature and scope of his previous experience, coupled with past and present service on various audit committees. Our audit committee assists our board of directors in its oversight of our accounting and financial reporting process and the audits of our financial statements. Following this offering, our audit committee's responsibilities will include:
Compensation Committee
The members of our compensation committee are Mr. Greene, Dr. Pakianathan and Mr. Weg. Mr. Greene is the chair of the compensation committee. Our compensation committee assists our
board of directors in the discharge of its responsibilities relating to the compensation of our executive officers. Following this offering, the compensation committee's responsibilities will include:
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Mr. Bohlin, Dr. Kauffman and Mr. Weg. Mr. Weg is the chair of the nominating and corporate governance committee. Following this offering, the nominating and corporate governance committee's responsibilities will include:
Compensation Committee Interlocks and Insider Participation
During 2012, the members of our compensation committee were Dr. Mansoor Raza Mirza and our former director Ronald A. DePinho, who resigned from our board in October 2012. None of our executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director or member of our compensation committee during the fiscal year ended December 31, 2012. During 2012, no member of our compensation committee was a current or former officer or employee of Karyopharm or had any related person transaction involving Karyopharm, other than Dr. Mirza, who serves as a special scientific consultant to usunderwriters pursuant to a consulting agreement with an entity wholly-owned by Dr. Mirza. See "Certain relationships and related person transactions—Consulting Arrangements."
Code of Ethics and Code of Conduct
Upon the closing of this offering, we intend to adopt a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. We intend to post on our website, www.karyopharm.com, a current copy of the code and all disclosures that are required by law or NASDAQ stock market listing standards concerning any amendments to, or waivers from, any provision of the code.
This section discusses the material elements of our executive compensation policies and important factors relevant to an analysis of these policies. It provides qualitative information regarding the manner and context in which compensation is awarded to and earned by our executive officers named in the "Summary Compensation Table" below and is intended to place in perspective the information presented in the following tables and the corresponding narrative.
Summary Compensation Table
The following table sets forth information regarding compensation earned by our chief executive officer and our two other most highly compensated executive officers during the fiscal years ending December 31, 2011 and 2012. We refer to these executive officers as our named executive officers elsewhere in this prospectus.
Name and Principal Position | Year | Salary ($) | Option Awards ($)(1) | Non-Equity Incentive Plan Compensation ($)(2) | All Other Compensation ($)(3) | Total ($) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Michael G. Kauffman, M.D., Ph.D. | 2012 | 352,240 | — | 97,900 | 13,138 | 463,278 | |||||||||||||
President, Chief Executive Officer | 2011 | 340,000 | 21,522 | 59,500 | 9,541 | 430,563 | |||||||||||||
and Chief Medical Officer(4) | |||||||||||||||||||
Sharon Shacham, Ph.D., M.B.A. | 2012 | 300,000 | 5,460 | 138,800 | 12,060 | 456,320 | |||||||||||||
Chief Scientific Officer and | 2011 | 280,000 | 7,130 | 58,800 | 8,907 | 354,837 | |||||||||||||
President of Research and | |||||||||||||||||||
Development | |||||||||||||||||||
Alan T. Barber | 2012 | 205,600 | 7,695 | — | — | 213,295 | |||||||||||||
Acting Chief Financial Officer(5) | 2011 | 166,300 | 1,619 | — | — | 167,919 | |||||||||||||
Narrative Disclosure to Summary Compensation Table
Our compensation committee makes compensation decisions regarding our named executive officers or makes recommendations concerning executive compensation to our board of directors. In May 2013, our compensation committee engaged Arnosti Consulting, Inc., an independent compensation consultant, to provide comparative data on executive compensation practices in our industry and to advise on our executive compensation program generally. Although our board of directors and compensation committee consider the advice and recommendation of any independent compensation consultants as to our executive compensation program, the board of directors and compensation committee ultimately make their own decisions about these matters.
Base Salary
Base salaries are used to recognize the experience, skills, knowledge and responsibilities required of our named executive officers. Historically, base salaries for our named executive officers typically have been established through arm's length negotiation at the time the executive officer is appointed, taking into account the position for which the named executive officer is being considered and the named executive officer's qualifications, prior experience and prior salary. None of our named executive officers is currently party to an employment agreement or other service agreement that provides for automatic or scheduled increases in base salary. However, on an annual basis, our compensation committee reviews and evaluates, with input from our chief executive officer, the need for adjustment of the base salaries of each of our named executive officers, including our chief executive officer. In making decisions regarding salary increases, we may also draw upon the experience of members of our board of directors with other companies.
In 2012, we paid base salaries to Dr. Kauffman and Dr. Shacham of $352,240 and $300,000, respectively, and we paid aggregate consulting fees to Mr. Barber of $205,600, which fees were paid on an hourly basis. In 2011, we paid base salaries to Dr. Kauffman and Dr. Shacham of $340,000 and $280,000, respectively, and we paid aggregate consulting fees to Mr. Barber of $166,300, which fees were paid on an hourly basis.
Annual Performance-based Cash Incentives
We have designed our annual performance-based cash incentive program to emphasize pay-for-performance, on corporate and individual levels, and to reward our named executive officers for the preceding year's performance. Historically, each named executive officer has been eligible, at our board of directors' discretion, to receive an annual performance-based cash incentive, which we refer to as an annual cash incentive, in an amount corresponding to a percentage of his or her base salary. The target amount of the annual cash incentive has been determined, prior to the applicable fiscal year, by our board of directors, based upon the accomplishment of certain objective corporate milestones for the applicable fiscal year, upon the recommendation of the compensation committee, and the amount of the annual cash incentive paid to our named executive officers has been determined by our board of directors, upon a consideration of the level of achievement of these milestones and a subjective evaluation of individual performance, based upon the recommendation of the compensation committee. The final evaluation made by our board of directors did not involve a predetermined mathematical formula. Our compensation committee has authority to adjust the incentive percentage each year in connection with its review of our and the named executive officer's performance.
For 2012, the objective factors contributing to corporate performance were based on (i) the achievement of clinical and regulatory milestones, including effectiveness in the U.S. of an IND and authorization of a clinical trial application in Europe and Canada for the oral administration of Selinexor, recruitment of patients for human clinical trials, initiation of a Phase 2b clinical trial to support regulatory approval of Verdinexor and demonstration of activity of our SINE compounds in
certain indications, and (ii) the achievement of a financial milestone consisting of the receipt of non-dilutive funding during 2012. Based upon the company's achievement, on or before the Company's projected timeframe, of effectiveness of an IND in the U.S. and authorization of a clinical trial application in Europe and Canada for the oral administration of Selinexor and the successful recruitment of patients for human clinical trials, the board of directors determined that the company overachieved its expectations regarding corporate performance during 2012. The board of directors approved annual cash incentives for Drs. Kauffman and Shacham upon consideration of these corporate achievements along with subjective factors related to each named executive officer's individual performance, responsibilities and then existing compensation levels.
In 2012, we awarded cash incentives to Dr. Kauffman and Dr. Shacham in the amounts of $97,900 and $138,800, respectively. In 2011, we awarded cash incentives to Dr. Kauffman and Dr. Shacham in the amounts of $59,500 and $58,800, respectively. We did not award any cash incentives to Mr. Barber in 2011 or 2012.
Equity Incentive Awards
Our equity award program is the primary vehicle for offering long-term incentives to our named executive officers. While we do not currently have any equity ownership guidelines for our named executive officers, we believe that equity grants provide our named executive officers with a strong link to our long-term performance, create an ownership culture and help to align the interests of our named executive officers and our stockholders. We believe stock options provide meaningful incentives to our named executive officers to achieve increases in the value of our stock over time. In addition, the vesting feature of our equity grants contributes to executive retention by providing an incentive to our named executive officers to remain employed by us during the vesting period.
Prior to this offering, our named executive officers were eligible to participate in the 2010 Stock Incentive Plan, as amended, or the 2010 Plan. During 2012, all stock options granted to our named executive officers were granted pursuant to the 2010 Plan. Following the closing of this offering, our named executive officers and employees will be eligible to receive stock options and other stock-based awards pursuant to the 2013 Stock Incentive Plan, or the 2013 Plan.
Prior to this offering, we have used stock options to compensate our named executive officers in the form of initial grants in connection with the named executive officer's appointment to his or her position, generally on an annual basis thereafter, and also at various times, including concurrent with our preferred stock financings in order to address dilution to existing options attributable to such financings. The award of stock options to our named executive officers has been made upon the recommendation of the compensation committee and the approval of our board of directors. None of our named executive officers is currently party to an employment or other service agreement with us that provides for automatic award of stock options. We have historically granted stock options to our named executive officers with time-based and performance-based vesting, and stock options with an ability to early exercise. The options that we grant to our named executive officers with time-based vesting typically become exercisable as to 25% of the shares underlying the option on the first anniversary of the grant date, and as to an additional 1/48th of the shares underlying the option on a monthly basis thereafter. The options that we grant to our named executive officers with performance-based vesting become exercisable upon the attainment of certain operational milestone events recommended by the compensation committee and approved by our board of directors. The options that we grant to our named executive officers with an ability to early exercise are immediately exercisable in full, provided that any shares issued upon exercise will remain subject to the original vesting terms. In addition, such shares that are vested or unvested are subject to repurchase by us in the event such named executive officer ceases service to us or our successor in the capacity of an employee, officer, director or consultant for cause. Unvested shares are subject to repurchase by us in the event such named executive officer ceases service to us or our successor, other than for cause. For
all options, vesting rights cease upon, and exercise rights cease shortly after, termination of employment or other service except in the case of death or disability. Prior to the exercise of an option, the holder has no rights as a stockholder with respect to the shares subject to such option, including no voting rights and no right to receive dividends or dividend equivalents.
In November 2010, we issued awards of restricted stock pursuant to the 2010 Plan, which we refer to as founders shares, to compensate Dr. Kauffman and Dr. Shacham in connection with their service to us from inception to such date and their expected continued service following such date. The founders shares vested as to 25% of the shares underlying the awards upon the first sale of shares of our series A preferred stock in October 2010, and vest as to an additional 1/48th of the shares underlying the awards on a monthly basis thereafter.
In 2013, our board of directors granted stock options to our named executive officers, pursuant to our 2010 Plan, as follows:
Name | Date of Grant | Option Award (#) | Grant Date Fair Value ($) | ||||
|---|---|---|---|---|---|---|---|
Michael G. Kauffman, M.D., Ph.D. | 9/3/2013 | 480,303 | (1) | ||||
Sharon Shacham, Ph.D., M.B.A. | 9/3/2013 | 480,303 | (1) | ||||
Alan T. Barber | 7/25/2013 | 4,545 | (1) | ||||
Subsequent to the closing of this offering, we expect to enter into new employment agreements with each of our executive officers, which agreements will include adjustments to each executive officer's existing base salary and annual performance-based cash incentives.
2012 Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information concerning outstanding equity awards for each of our named executive officers at December 31, 2012:
| | Option Awards | Stock Awards | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested(1) ($) | ||||||||||||
Michael G. Kauffman, M.D., Ph.D. | 10,606 | 31,818 | (2) | $ | 0.26 | 12/14/2021 | ||||||||||||
| 33,333 | (3) | $ | 0.26 | 12/14/2021 | ||||||||||||||
| 57,524 | (4) | 862,860 | ||||||||||||||||
| �� | 356 | (5) | 5,340 | |||||||||||||||
| 178,622 | (6) | 2,679,330 | ||||||||||||||||
Sharon Shacham, Ph.D., M.B.A. | 23,272 | (7) | $ | 0.03 | 10/21/2020 | |||||||||||||
| 23,567 | (8) | $ | 0.03 | 11/1/2020 | ||||||||||||||
| 9,848 | 29,545 | (9) | $ | 0.26 | 12/14/2021 | |||||||||||||
| 30,303 | (10) | $ | 0.26 | 12/14/2021 | ||||||||||||||
| 176,042 | (11) | 2,640,630 | ||||||||||||||||
| 1,090 | (12) | 16,335 | ||||||||||||||||
Alan T. Barber | 2,020 | 4,040 | (13) | $ | 0.26 | 8/1/2021 | ||||||||||||
| 1,515 | (14) | $ | 0.26 | 12/14/2021 | ||||||||||||||
| 3,030 | (15) | $ | 1.49 | 12/6/2022 | ||||||||||||||
of such option and subject to repurchase by us in certain circumstances. As of December 31, 2012, 12,765 shares were vested and 10,802 unvested shares underlying the option are scheduled to vest in equal monthly installments through October 1, 2014.
Employment Agreements, Severance and Change in Control Arrangements
Subsequent to the closing of this offering, we expect to enter into new employment agreements with each of Dr. Kauffman and Dr. Shacham.
Historically, we have entered into employment agreements with each of our named executive officers, except for Mr. Barber, pursuant to which such named executive officers are employed "at will," meaning the executive or we may terminate the employment arrangement at any time. Such employment agreements establish the named executive officer's title, initial compensation arrangements, eligibility for benefits made available to employees generally and also provide for certain benefits upon termination of employment under specified conditions. The following summarizes such termination benefits under our named executive officers' existing employment agreements.
Benefits Provided Upon Termination Without Cause or for Good Reason Upon a Change in Control
Under the terms of the existing employment agreements we have entered into with each of Dr. Kauffman and Dr. Shacham, subject to the execution and effectiveness of a separation agreement that includes a release of claims against us, if such executive's employment is terminated by us without cause or by such executive for good reason, as defined in such employment agreements, after a change of control, as defined in such employment agreements, we will be obligated to pay (i) in the case of Dr. Kauffman, an amount equal to his then-current base salary for a period of eight months, or, in the case of Dr. Shacham, an amount equal to her then-current base salary for a period of six months, (ii) any earned but unpaid annual cash incentive and (iii) all other payments and benefits to which the executive was entitled on such date of termination under the terms of any applicable arrangement, plan or program in effect at the time of such termination.
We entered into a consulting agreement with Mr. Barber regarding his service to us as an independent contractor, which does not provide Mr. Barber with any employee benefits or any benefits upon termination of his service relationship with us. Our board of directors has authorized the immediate vesting of an option to purchase 6,060 shares of our common stock, which option was granted on August 1, 2011, upon the termination of Mr. Barber's service relationship with us.
Non-Disclosure Agreements
We have entered into non-disclosure and inventions assignment agreements with Dr. Kauffman and Dr. Shacham, and have entered into a consulting agreement with Mr. Barber which governs inventions assignment and confidential and proprietary information non-disclosure. Under the non-disclosure and inventions assignment agreements, Dr. Kauffman and Dr. Shacham have agreed (i) not to compete with us during his or her employment and for a period of twelve months after the termination of his or her employment; (ii) not to solicit our employees or customers during his or her employment and for a period of twelve months after the termination of his or her employment; (iii) to protect our confidential and proprietary information, and (iv) to assign to us related intellectual property that is developed during the course of his or her employment. Under the consulting agreement, Mr. Barber has agreed (i) not to solicit our employees during his service to us and for a period of twelve months after the termination of his service relationship with us; (ii) to protect our confidential and proprietary information and (iii) to assign to us related intellectual property that is developed during the course of his service to us.
Equity and Non-Equity Incentive Plans
2013 Stock Incentive Plan
Our board of directors has adopted, and our stockholders have approved the 2013 stock incentive plan, which we refer to as the 2013 Plan, which will become effective immediately prior to the closing of this offering. The 2013 Plan provides for the grant of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards, and other stock-based awards. The number of shares of our common stock that will be reserved for issuance under the 2013 Plan is the sum of (1) 969,696 shares plus (2) the number of shares (up to 2,126,377 shares) equal to the sum of the number of shares of our common stock then available for issuance under our 2010 Plan and the number of shares of our common stock subject to outstanding awards under our 2010 Plan that expire, terminate or are otherwise surrendered, cancelled, forfeited or repurchased by us at their original issuance price pursuant to a contractual repurchase right plus (3) an annual increase to be added on the first day of each fiscal year, beginning with the fiscal year ending December 31, 2014 and continuing until, and including, the fiscal year ending December 31, 2023, equal to the least of (A) 1,939,393 shares of our common stock, (B) 4% of the number of shares of our common stock outstanding on the first day of the fiscal year, and (C) an amount determined by our board of directors.
Our employees, officers, directors, consultants, and advisors, and employees, officers, directors, consultants, and advisors of any of our present or future parent or subsidiary corporations and any other business venture (including, without limitation, joint venture or limited liability company) in which we have a controlling interest, as determined by our board of directors, are eligible to be granted awards under the 2013 Plan. However, incentive stock options may only be granted to employees who are eligible to receive incentive stock options under the Internal Revenue Code.
The 2013 Plan will be administered by our board of directors. Pursuant to the terms of the 2013 Plan, our board of directors selects the recipients of awards and determines:
Our board of directors has delegated its powers under the 2013 Plan to our compensation committee. If our board of directors delegates authority to an officer to grant awards under the 2013 Plan, the officer will have the power to grant awards to all of our employees, except executive officers. Our board of directors will fix the terms of awards to be granted by such officer, including the exercise price of such awards (which may include a formula by which the exercise price will be determined) and the maximum number of shares subject to awards that the officer may grant.
In the event of any stock split, reverse stock split, stock dividend, recapitalization, combination of shares, reclassification of shares, spin-off or other similar change in capitalization or event, or any dividend or distribution to holders of our common stock other than an ordinary cash dividend, our board of directors is required by the 2013 Plan to make equitable adjustments, in a manner determined by our board, to:
In connection with a merger or other reorganization event (as defined in the 2013 Plan), our board of directors may take any one or more of the following actions as to all or any portion of any outstanding awards other than restricted stock, on such terms as it determines:
Our board of directors is not obligated under the 2013 Plan to treat all awards, all awards held by a participant, or all awards of the same type, identically in connection with a reorganization event.
In the case of certain restricted stock unit awards, no assumption or substitution will be permitted and the restricted stock unit awards will instead be settled in accordance with the terms of the applicable restricted stock unit agreement, and in certain circumstances, any unvested restricted stock unit awards will be terminated immediately prior to the consummation of the reorganization event without any payment in exchange therefor.
Upon the occurrence of a reorganization event other than our liquidation or dissolution, the repurchase and other rights with respect to outstanding awards of restricted stock will continue for the benefit of the successor company and will, unless our board of directors otherwise determines, apply to the cash, securities or other property into which our common stock was converted into or exchanged for pursuant to the reorganization event in the same manner and to the same extent as they applied to such restricted stock. Upon the occurrence of a reorganization event involving our liquidation or dissolution, all restrictions and conditions on all restricted stock then outstanding will automatically be deemed terminated or satisfied, unless otherwise provided in the instrument evidencing the award of restricted stock or any other agreement between a participant and us.
At any time, our board of directors may provide that any award under the 2013 Plan shall become immediately exercisable in whole or in part, free of some or all restrictions or conditions, or otherwise realizable in whole or in part, as the case may be.
In addition, the 2013 Plan provides that, notwithstanding the provisions of the plan that may apply upon a reorganization event and except as otherwise provided for in the instrument evidencing an option or award of restricted stock or any other agreement between us and the participant, upon the occurrence of a change in control event (as defined in the 2013 Plan) each option shall become immediately exercisable and each award of restricted stock shall become immediately free from all conditions and restrictions, if, in either case, the employment of the participant holding such award is terminated by us (or our acquiring or succeeding corporation) without cause (as defined in the 2013 Plan) or by the participant for good reason (as defined in the 2013 Plan), on or prior to the first anniversary of the date of the change in control event. Our board of directors may specify in an award at the time of grant the effect of a change in control event on any stock appreciation right, restricted stock unit or other stock-based award.
Except with respect to certain actions requiring stockholder approval under the Internal Revenue Code or the rules of the NASDAQ Stock Market, our board of directors may amend, modify or terminate any outstanding award under the 2013 Plan, including but not limited to, substituting therefor another award of the same or a different type, changing the date of exercise or realization, and converting an incentive stock option into a non-qualified stock option, subject to certain participant consent requirements. Unless our stockholders approve such action, the 2013 Plan provides that we may not (except as otherwise permitted in connection with a change in capitalization or reorganization event):
No award may be granted under the 2013 Plan after October 21, 2023, but awards previously granted may extend beyond that date. Our board of directors may amend, suspend or terminate the 2013 Plan or any portion thereof at any time, subject to certain stockholder approval requirements and limitations under the Internal Revenue Code.
2013 Employee Stock Purchase Plan
Our board of directors has adopted, and our stockholders have approved, the 2013 Employee Stock Purchase Plan, or the 2013 ESPP. The 2013 ESPP will be administered by our board of directors or by a committee appointed by our board of directors. The 2013 ESPP will initially provide participating employees with the opportunity to purchase an aggregate of 242,424 shares of our common stock. The number of shares of our common stock reserved for issuance under the 2013 ESPP will automatically increase on the first day of each fiscal year, commencing on January 1, 2015 and ending on December 31, 2023, in an amount equal to the least of (1) 484,848 shares of our common stock, (2) 1% of the total number of shares of our common stock outstanding on the first day of the applicable year, and (3) an amount determined by our board of directors.
All of our employees and employees of any of our designated subsidiaries, as defined in the 2013 ESPP, are eligible to participate in the 2013 ESPP, provided that:
No employee may purchase shares of our common stock under the 2013 ESPP and any of our other employee stock purchase plans in excess of $25,000 of the fair market value of our common stock (as of the date of the option grant) in any calendar year. In addition, no employee may purchase shares of our common stock under the 2013 ESPP that would result in the employee owning 5% or more of the total combined voting power or value of our stock.
We expect to make one or more offerings to our eligible employees to purchase stock under the 2013 ESPP beginning at such time as our board of directors may determine. Each offering will consist of a six-month offering period during which payroll deductions will be made and held for the purchase of our common stock at the end of the offering period. Our board of directors may, at its discretion, choose a different period of not more than 12 months for offerings.
On the commencement date of each offering period, each eligible employee may authorize up to a maximum of 15% of his or her compensation to be deducted by us during the offering period. Each employee who continues to be a participant in the 2013 ESPP on the last business day of the offering period will be deemed to have exercised an option to purchase from us the number of whole shares of our common stock that his or her accumulated payroll deductions on such date will pay for, not in excess of the maximum numbers set forth above. Under the terms of the 2013 ESPP, the purchase price shall be determined by our board of directors for each offering period and will be at least 85% of the applicable closing price of our common stock. If our board of directors does not make a determination of the purchase price, the purchase price will be 85% of the lesser of the closing price of our common stock on the first business day of the offering period or on the last business day of the offering period.
An employee may for any reason withdraw from participation in an offering prior to the end of an offering period and permanently draw out the balance accumulated in the employee's account. If an employee elects to discontinue his or her payroll deductions during an offering period but does not elect to withdraw his or her funds, funds previously deducted will be applied to the purchase of common stock at the end of the offering period. If a participating employee's employment ends before the last business day of an offering period, no additional payroll deductions will be made and the balance in the employee's account will be paid to the employee.
We will be required to make equitable adjustments to the number and class of securities available under the 2013 ESPP, the share limitations under the 2013 ESPP, and the purchase price for an offering period under the 2013 ESPP to reflect stock splits, reverse stock splits, stock dividends, recapitalizations, combinations of shares, reclassifications of shares, spin-offs and other similar changes in capitalization or events or any dividends or distributions to holders of our common stock other than ordinary cash dividends.
In connection with a merger or other reorganization event (as defined in the 2013 ESPP), our board of directors or a committee of our board of directors may take any one or more of the following actions as to outstanding options to purchase shares of our common stock under the 2013 ESPP on such terms as our board or committee determines:
determining the purchase price and where the number of shares that could be purchased is subject to the applicable limitations under the 2013 ESPP minus (2) the result of multiplying such number of shares by the purchase price; and/or
Our board of directors may at any time, and from time to time, amend or suspend the 2013 ESPP or any portion thereof. We will obtain stockholder approval for any amendment if such approval is required by Section 423 of the Internal Revenue Code. Further, our board of directors may not make any amendment that would cause the 2013 ESPP to fail to comply with Section 423 of the Internal Revenue Code. The 2013 ESPP may be terminated at any time by our board of directors. Upon termination, we will refund all amounts in the accounts of participating employees.
2010 Stock Incentive Plan
In July 2013, our board of directors adopted and our stockholders approved the amended and restated 2010 stock incentive plan, which we refer to as the 2010 Plan. The 2010 Plan provides for the grant of incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards, and other stock-based awards. Subject to adjustment in connection with changes in capitalization, the aggregate number of shares of our common stock that may be issued pursuant to awards granted under the 2010 Plan is 2,763,270 shares. No additional awards will be granted under the 2010 Plan following the closing of this offering, but awards outstanding under the 2010 Plan may extend beyond that date.
Our employees, officers, directors, consultants, and advisors, and employees, officers, directors, consultants, and advisors of any of our present or future parent or subsidiary corporations and any other business venture in which we have a direct or indirect significant interest, as determined by our board of directors in its sole discretion, are eligible to be granted awards under the 2010 Plan. However, incentive stock options may only be granted to our employees and employees of our present or future parent or subsidiary corporations. No participant may be granted awards under the 2010 Plan during any one fiscal year to purchase more than 646,718 shares of our common stock.
The 2010 Plan is administered by our board of directors. Pursuant to the terms of the 2010 Plan, our board of directors selects the recipients of awards and determines:
To the extent permitted by applicable law, our board of directors may delegate its powers under the 2010 Plan to one or more committees or subcommittees of our board or to one or more of our executive officers. If our board of directors delegates authority to an executive officer to grant awards under the 2010 Plan, our board of directors will fix the maximum number of awards to be
granted and the maximum number of shares issuable to any one participant pursuant to awards granted by such executive officer.
In the event of any stock split, reverse stock split, stock dividend, recapitalization, combination or reclassification of shares, spin-off or other similar change in capitalization or event, we are required by the 2010 Plan to make appropriate adjustments, in a manner determined by our board, to:
Upon the consummation of a merger or other event constituting an acquisition (as defined in the 2010 Plan), our board of directors or the board of directors of the surviving or acquiring entity shall, as to outstanding awards under the 2010 Plan (on the same basis or on different bases), either:
Unless otherwise determined by the applicable board, any repurchase rights or other rights that relate to an award shall continue to apply to consideration, including cash, that has been substituted, assumed or amended for an award in connection with an acquisition. We may require that all or any portion of such consideration payable in respect of an award in connection with an acquisition shall be held in escrow (including in an escrow pursuant to the agreement effecting such acquisition) in order to effectuate any continuing restrictions.
At any time, our board of directors may provide that any award under the 2010 Plan shall become immediately exercisable in full or in part or free of some or all restrictions or conditions, or otherwise realizable in full or in part, as the case may be.
Our board of directors may amend, modify or terminate any outstanding award under the 2010 Plan, including, but not limited to, substituting therefor another award of the same or a different type, changing the date of exercise or realization, and converting an incentive stock option to a nonstatutory stock option, subject to certain participant consent requirements. In addition, our board of directors may, without stockholder approval, amend any outstanding option to reduce the exercise price of such option or cancel any outstanding option and grant in substitution therefor new options covering the same or a different number of shares of our common stock and having a lower exercise price than the
cancelled options. Our board of directors may amend, suspend or terminate the 2010 Plan or any portion thereof at any time.
401(k) Retirement Plan
We maintain a 401(k) retirement plan that is intended to be a tax-qualified defined contribution plan under Section 401(k) of the Internal Revenue Code. In general, all of our U.S.-based employees are eligible to participate in the 401(k) plan, beginning on the first day of the month following commencement of their employment. The 401(k) plan includes a salary deferral arrangement pursuant to which participants may elect to reduce their current compensation by up to the statutorily prescribed limit, equal to $17,500 in 2013, and have the amount of the reduction contributed to the 401(k) plan.
2012 Director Compensation
Our director compensation program is intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors' interests with those of our stockholders. During and prior to 2012, we did not pay cash compensation to any non-employee director for his or her services as a director. The following table sets forth information concerning the total compensation for our non-employee directors during the fiscal year ended December 31, 2012:
Name(1) | Option Awards ($)(2) | All Other Compensation ($) | Total ($) | |||
|---|---|---|---|---|---|---|
Ronald DePinho, M.D.(3) | 18,826(4) | — | 18,826 | |||
Mansoor Raza Mirza, M.D. | 15,396(5) | 112,043(6) | 127,439 |
for his service on the board of directors, and an option to purchase 12,121 shares, which option had a fair value amount as of the grant date of $30,793, granted to Mirza Consulting in 2012 for such consulting and advisory services provided to us. As of December 31, 2012, Mirza Consulting held options to purchase 12,121 shares of our common stock.
Prior to this offering, the compensation of our non-employee directors was established through arm's length negotiation at the time the director was elected, taking into account the responsibilities of each director, such as committee service, and the director's qualifications and prior experience and industry data for such responsibilities. This compensation was reviewed and recommended by our compensation committee and approved by our board.
The stock options granted to our non-employee directors for service on our board have an exercise price equal to the fair market value of our common stock on the date of grant, expire ten years after the date of grant, and are subject to the director's continued service on our board. To the extent that a non-employee director has other responsibilities in addition to service on our board, such director may receive additional compensation to the extent as deemed appropriate by our board. Each member of our board of directors receives reimbursement for reasonable travel and other expenses incurred in connection with attending meetings of the board of directors, consistent with our travel expense reimbursement guidelines.
Dr. Mirza serves as a special scientific consultant to us pursuant to a consulting agreement with an entity wholly-owned by Dr. Mirza, for which he is paid (through such entity) a consulting fee of $16,000 per month, effective January 1, 2013. See "Certain relationships and related person transactions—Consulting Arrangements."
Director Compensation Guidelines Following this Offering
On October 8, 2013, our board, upon the recommendation of our compensation committee, established the following compensation guidelines for non-employee directors, effective upon the closing of this offering:
Limitation of Liability and Indemnification
Our certificate of incorporation, which will become effective upon the closing of this offering, limits the personal liability of directors for breach of fiduciary duty to the maximum extent permitted by the Delaware General Corporation Law and provides that no director will have personal liability to us or to our stockholders for monetary damages for breach of fiduciary duty as a director. However, these provisions do not eliminate or limit the liability of any of our directors:
Any amendment to or repeal of these provisions will not eliminate or reduce the effect of these provisions in respect of any act, omission or claim that occurred or arose prior to such amendment or repeal. If the Delaware General Corporation Law is amended to provide for further limitations on the personal liability of directors of corporations, then the personal liability of our directors will be further limited to the greatest extent permitted by the Delaware General Corporation Law.
In addition, our certificate of incorporation, which will become effective upon the closing of this offering, provides that we must indemnify our directors and officers and we must advance expenses, including attorneys' fees, to our directors and officers in connection with legal proceedings, subject to very limited exceptions.
We maintain a liability insurance policy that covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers. In addition, we have entered into indemnification agreements with all of our directors. These indemnification agreements require us, among other things, to indemnify each such director for certain expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by him in any action or proceeding arising out of his service as one of our directors.
Certain of our non-employee directors may, through their relationships with their employers, be insured and/or indemnified against certain liabilities incurred in their capacity as members of our board of directors.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, executive officers or persons controlling us, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
CERTAIN RELATIONSHIPSPRINCIPAL AND RELATED PERSON TRANSACTIONS
Since January 1, 2010, we have engaged in the following transactions with our directors and executive officers and holders of more than 5% of our voting securities and affiliates of our directors, executive officers and such 5% stockholders. We believe that all of the transactions described below were made on terms no less favorable to us than could have been obtained from unaffiliated third parties.
Preferred Stock Financings
Series B-1 Preferred Stock Financing
In July 2013, we issued and sold an aggregate of 8,636,362 shares of our series B-1 preferred stock, at a purchase price per share of $2.20, for an aggregate purchase price of $18,999,996, and immediately following such sale, Foresite Capital, which purchased 6,818,182 shares of our series B-1 preferred stock in this financing, became a beneficial owner of more than 5% of our voting securities.
The following table sets forth the number of shares of series B-1 preferred stock that were issued to holders of more than 5% of our voting securities at the time of such issuance and affiliates of such holders, in connection with the series B-1 preferred stock financing and the aggregate cash purchase price paid by such entities.
Purchaser | Shares of Series B-1 Preferred Stock | Purchase Price | |||||
|---|---|---|---|---|---|---|---|
Delphi Ventures and affiliates(1) | 909,091 | $ | 2,000,000 | ||||
Series B Preferred Stock Financing
Between April and September 2013, we issued and sold an aggregate of 24,100,000 shares of our series B preferred stock, at a purchase price per share of $2.00, for an aggregate purchase price of $48,200,000, and immediately following the first sale of shares of our series B preferred stock in April 2013, each of Plio Limited and Delphi Ventures, which purchased 3,000,000 and 2,500,000 shares, respectively, of our series B preferred stock, became a beneficial owner of more than 5% of our voting securities.
The following table sets forth the number of shares of series B preferred stock that were issued to holders of more than 5% of our voting securities at the time of such issuance, and affiliates of such holders, in connection with the series B preferred stock financing and the aggregate cash purchase price paid by such entities.
Purchaser | Shares of Series B Preferred Stock Issued to Date | Aggregate Purchase Price | |||||
|---|---|---|---|---|---|---|---|
Chione Ltd. | 6,000,000 | $ | 12,000,000 | ||||
Plio Limited | 9,000,000 | $ | 18,000,000 | ||||
Delphi Ventures and affiliates(1) | 5,000,000 | $ | 10,000,000 | ||||
Series A-4 Preferred Stock Financing
In February 2012, we entered into a stock purchase agreement for the purpose of issuing and selling up to an aggregate of 1,538,461 shares of our series A-4 preferred stock, at a purchase price per share of $1.30, for an aggregate purchase price of $1,999,999. We issued these shares in August 2013.
The following table sets forth the number of shares of series A-4 preferred stock that were issued to a holder of more than 5% of our voting securities at the time of such issuance in connection with the series A-4 preferred stock financing and the aggregate cash purchase price paid by such entity.
Purchaser | Shares of Series A-4 Preferred Stock | Purchase Price | |||||
|---|---|---|---|---|---|---|---|
Plio Limited | 1,538,461 | $ | 1,999,999 | ||||
Series A-2 and A-3 Preferred Stock Financing
In October 2011, we entered into a stock purchase agreement for the purpose of issuing and selling up to an aggregate of (i) 7,000,000 shares of our series A-2 preferred stock and (ii) 3,000,000 shares of our series A-3 preferred stock. Between October 2011 and February 2013, we became obligated to issue 6,100,000 shares of our series A-2 preferred stock at a purchase price per share of $1.15, for an aggregate purchase price of $7,015,000, and 1,764,706 shares of our series A-3 preferred stock at a purchase price per share of $1.70, for an aggregate purchase price of $3,000,000. We issued these shares in August 2013.
The following table sets forth the number of shares of series A-2 preferred stock and series A-3 preferred stock that were issued to a holder of more than 5% of our voting securities at the time of such issuance in connection with the series A-2 and series A-3 preferred stock financing and the aggregate cash purchase price paid by such entity.
Purchaser | Shares of Series A-2 Preferred Stock | Shares of Series A-3 Preferred Stock | Aggregate Purchase Price | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Chione Ltd. | 6,086,957 | 1,764,706 | $ | 10,000,001 | ||||||
Series A Preferred Stock Financing
Between October 2010 and January 2013, we issued and sold an aggregate of 20,937,500 shares of our series A preferred stock, at a purchase price per share of $1.00, for an aggregate purchase price of $20,937,500. Immediately following the first sale of shares of our series A preferred stock in October 2010, Chione Ltd., which purchased 5,000,000 shares of our series A preferred stock in such sale, became a beneficial owner of more than 5% of our voting securities.
The following table sets forth the number of shares of series A preferred stock that were issued to our directors, executive officers and holders of more than 5% of our voting securities at the time of such issuance in connection with the series A preferred stock financing and the aggregate cash purchase price paid by such persons and entities.
Purchaser | Shares of Series A Preferred Stock | Purchase Price | |||||
|---|---|---|---|---|---|---|---|
Michael G. Kauffman, M.D., Ph.D. | 437,500 | $ | 437,500 | ||||
Chione Ltd. | 15,000,000 | (1) | $ | 15,000,000 | |||
of our series A preferred stock listed in this table to Chione Ltd. after Chione Ltd. had become a holder of more than 5% of our voting securities.
Registration Rights
We are a party to a third amended and restated investors' rights agreement with the holders of our preferred stock, including certain of our directors, executive officers and 5% stockholders and their affiliates. The investor rights agreement provides these holders the right, following the completion of this offering, to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. See "Description of Capital Stock—Registration Rights" for additional information regarding these registration rights.
Severance and Change in Control Agreements
See the "Executive Compensation—Employment Agreements, Severance and Change in Control Arrangements" section of this prospectus for a further discussion of these arrangements.
Indemnification of Directors
Our certificate of incorporation, which will become effective upon the closing of this offering, limits the personal liability of directors for breach of fiduciary duty to the maximum extent permitted by the Delaware General Corporation Law and provides that no director will have personal liability to us or to our stockholders for monetary damages for any breach of fiduciary duty as a director. In addition, we have entered into indemnification agreements with each of our directors that that require us, among other things, to indemnify each director for certain expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by him or her in any action or proceeding arising out of his or her service as one of our directors. See the "Executive Compensation—Limitation of Liability and Indemnification" section of this prospectus for a further discussion of these arrangements.
Consulting Arrangements
We are party to a consulting agreement with Mirza Consulting, an entity that is wholly owned by Mansoor Raza Mirza, a member of our board of directors. Pursuant to this agreement, we pay Dr. Mirza (through such entity) a consulting fee of $16,000 per month, effective January 1, 2013. Prior to January 1, 2013, we paid Dr. Mirza (through such entity) a consulting fee of $10,000 per month. In December 2012, we issued an option to purchase 12,121 shares of our common stock to Mirza Consulting at an exercise price of $1.49 per share.
We are also a party to a consulting agreement with Dr. Tami Rashal, the sister of Dr. Sharon Shacham, our Chief Scientific Officer and President of Research and Development, and the sister-in-law of Michael Kauffman, our President and Chief Executive Officer, Chief Medical Officer and member of our board of directors. Pursuant to this agreement, we pay Dr. Rashal a consulting fee of $10,069 per month, effective May 1, 2013. Prior to May 1, 2013, we paid Dr. Rashal a consulting fee of $60 per hour, which fee was limited to $10,000 per year. In September 2013, we granted to Dr. Rashal an option to purchase 15,151 shares of our common stock at an exercise price of $4.75 per share.
Policies and Procedures for Related Person Transactions
On October 8, 2013, our board adopted written policies and procedures for the review of any transaction, arrangement or relationship in which we are a participant, the amount involved exceeds $120,000, and one of our executive officers, directors, director nominees or 5% stockholders (or their immediate family members), each of whom we refer to as a "related person," has a direct or indirect material interest.
If a related person proposes to enter into such a transaction, arrangement or relationship, which we refer to as a "related person transaction," the related person must report the proposed related person transaction to our principal financial officer. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by the board's audit committee. Whenever practicable, the reporting, review and approval will occur prior to effectiveness or consummation of the related person transaction. If advance review and approval is not practicable, the committee will review, and, in its discretion, may ratify the related person transaction. The policy also permits the chairman of the committee to review and, if deemed appropriate, approve proposed related person transactions that arise between committee meetings, subject to ratification by the committee at its next meeting. Any related person transactions that are ongoing in nature will be reviewed annually.
A related person transaction reviewed under the policy will be considered approved or ratified if it is authorized by the committee after full disclosure of the related person's interest in the transaction. As appropriate for the circumstances, the committee will review and consider:
The committee may approve or ratify the related person transaction only if the committee determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, our best interests. The committee may impose any conditions on the related person transaction that it deems appropriate.
In addition to the transactions that are excluded by the instructions to the SEC's related person transaction disclosure rule, the board has determined that the following transactions do not create a material direct or indirect interest on behalf of related persons and, therefore, are not related person transactions for purposes of this policy:
The policy provides that transactions involving compensation of executive officers shall be reviewed and approved by the compensation committee in the manner specified in its charter.
The following table sets forth information with respect to the beneficial ownership of our common stock, as of September 30, 2013May 31, 2014, by:
The column entitled "Shares Beneficially Owned Prior to Offering—Percentage" is based on a total of 21,929,59329,786,403 shares of our common stock outstanding as of September 30, 2013, assuming the automatic conversion of all outstanding shares of our preferred stock upon the closing of this offering, into an aggregate of 19,114,241 shares of our common stock upon the closing of this offering.May 31, 2014. The column entitled "Shares Beneficially Owned After Offering—Percentage" is based on shares of our common stock to be outstanding after this offering, including the2,000,000 shares of our common stock that we are selling in this offering, and 200,000 shares of our common stock that the selling stockholders are selling in this offering, each at an assumed public offering price of $41.37 per share, which was the last reported sale price of our common stock on The NASDAQ Global Select Market on June 24, 2014, but not including any additional shares issuable upon exercise of outstanding options.options or the underwriters' option to purchase additional shares.
The number of shares beneficially owned by each stockholder is determined under rules issued by the Securities and Exchange Commission and includes voting or investment power with respect to securities. Under these rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power. In computing the number of shares beneficially owned by an individual or entity and the percentage ownership of that person, shares of common stock subject to options or other rights held by such person that are currently exercisable or will become exercisable within 60 days of September 30, 2013,May 31, 2014, are considered outstanding, although such shares subject to options or other rights are not considered outstanding for purposes of computing the percentage ownership of any other person. Unless otherwise indicated, the address of all listed stockholders is c/o Karyopharm Therapeutics Inc., 2 Mercer Road, Natick, Massachusetts 01760. Each of the stockholders listed has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
The table below does not reflect any shares of our common stock that our directors, executive officers, 5% stockholders or their affiliated entities may purchase in this offering, including any of the reserved shares, as described in the "Underwriting" section of this prospectus.offering.
| | Shares Beneficially Owned Prior to Offering | Shares Beneficially Owned After Offering | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name of Beneficial Owner | Number | Percentage | Number | Percentage | |||||||||
5% Stockholders | |||||||||||||
Chione Ltd.(1) | 10,258,079 | 46.78 | % | 10,258,079 | 37.17 | % | |||||||
Plio Limited(2) | 3,193,473 | 14.56 | % | 3,193,473 | 11.57 | % | |||||||
Entities Affiliated with Foresite Capital(3) | 2,066,115 | 9.42 | % | 2,066,115 | 7.49 | % | |||||||
Entities Affiliated with Delphi Ventures(4) | 1,790,633 | 8.17 | % | 1,790,633 | 6.49 | % | |||||||
Named Executive Officers and Directors | |||||||||||||
Michael G. Kauffman, M.D., Ph.D.(5) | 1,951,080 | 8.85 | % | 1,951,080 | 7.04 | % | |||||||
Sharon Shacham, Ph.D., M.B.A.(6) | 1,951,080 | 8.85 | % | 1,951,080 | 7.04 | % | |||||||
Deepa R. Pakianathan, Ph.D.(7) | 1,790,633 | 8.17 | % | 1,790,633 | 6.49 | % | |||||||
Mansoor Raza Mirza, M.D.(8) | 40,838 | * | 40,838 | * | |||||||||
Alan T. Barber(9) | 6,218 | * | 6,218 | * | |||||||||
Barry E. Greene(10) | 1,956 | * | 1,956 | * | |||||||||
Kenneth E. Weg | 0 | * | 0 | * | |||||||||
All executive officers and directors as a group (8 persons)(11) | 3,854,361 | 17.53 | % | 3,854,361 | 13.94 | % | |||||||
| | Shares Beneficially Owned Prior to Offering | | Shares Beneficially Owned After Offering | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Number of Shares Offered | |||||||||||||||
Name of Beneficial Owner | Number | Percentage | Number | Percentage | ||||||||||||
5% Stockholders | ||||||||||||||||
Chione Ltd.(1) | 10,258,079 | 34.44% | — | 10,258,079 | 32.30 | % | ||||||||||
Entities Affiliated with Franklin Resources, Inc.(2) | 3,542,004 | 11.89% | — | 3,542,004 | 11.15 | % | ||||||||||
Plio Limited(3) | 3,193,473 | 10.72% | — | 3,193,473 | 10.05 | % | ||||||||||
Entities Affiliated with Foresite Capital(4) | 2,378,615 | 7.99% | — | 2,378,615 | 7.49 | % | ||||||||||
Entities Affiliated with FMR LLC(5) | 2,346,000 | 7.88% | — | 2,346,000 | 7.39 | % | ||||||||||
Entities Affiliated with Delphi Ventures(6) | 2,217,552 | 7.44% | — | 2,217,552 | 6.98 | % | ||||||||||
Named Executive Officers and Directors | ||||||||||||||||
Michael G. Kauffman, M.D., Ph.D.(7) | 1,836,821 | 6.17% | 100,000 | (15) | 1,636,821 | 5.15 | % | |||||||||
Sharon Shacham, Ph.D., M.B.A.(8) | 1,836,821 | 6.17% | 100,000 | (16) | 1,636,821 | 5.15 | % | |||||||||
Paul Brannelly | 66,136 | * | — | 66,136 | * | |||||||||||
Deepa R. Pakianathan, Ph.D.(9) | 2,217,552 | 7.44% | — | 2,217,552 | 6.98 | % | ||||||||||
Mansoor Raza Mirza, M.D.(10) | 69,478 | * | — | 69,478 | * | |||||||||||
Alan T. Barber(11) | 10,952 | * | — | 10,952 | * | |||||||||||
Barry E. Greene(12) | 5,301 | * | — | 5,301 | * | |||||||||||
Kenneth E. Weg(13) | 2,683 | * | — | 2,683 | * | |||||||||||
Garen Bohlin | 0 | * | — | 0 | * | |||||||||||
All executive officers and directors as a group (9 persons)(14) | 4,208,923 | 14.13% | 200,000 | 4,008,923 | 12.62 | % | ||||||||||
All selling stockholders | 1,836,821 | 6.17% | 200,000 | 1,636,821 | 5.15 | % | ||||||||||
the general partner of Delphi VIII and DBI VIII (together, the "Delphi VIII Funds") and may be deemed to have sole voting and dispositive power over the shares held by the Delphi VIII Funds. DMP VIII and each of James J. Bochnowski, David L. Douglass, Douglas A. Roeder and Deepa R. Pakianathan, Ph.D., the Managing Members of DMP VIII who may be deemed to share voting and dispositive power over the reported securities, disclaim beneficial ownership of the reported securities held by Delphi VIII Funds except to the extent of any pecuniary interest therein.
General
Following the closing of this offering, ourOur authorized capital stock will consistconsists of 100,000,000 shares of common stock, par value $0.0001 per share, and 5,000,000 shares of preferred stock, par value $0.0001 per share. The following description of our capital stock and provisions of our certificate of incorporation and by-laws are summaries and are qualified by reference to theour certificate of incorporation and by-laws that will become effective upon the closing of this offering.by-laws. Copies of these documents have been filed with the Securities and Exchange Commission as exhibits to our registration statement, of which this prospectus forms a part. The description of our common stock reflects changes to our capital structure that will occur upon the closing of this offering.
Common Stock
As of September 30, 2013,March 31, 2014, there were 21,929,59329,762,499 shares of our common stock outstanding and held of record by 40 stockholders, assuming the automatic conversion of all outstanding shares of preferred stock.approximately 25 stockholders.
Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and do not have cumulative voting rights. An election of directors by our stockholders shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election. Holders of common stock are entitled to receive proportionately any dividends as may be declared by our board of directors, subject to any preferential dividend rights of any series of preferred stock that we may designate and issue in the future.
In the event of our liquidation or dissolution, the holders of common stock are entitled to receive proportionately our net assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock. Holders of common stock have no preemptive, subscription, redemption or conversion rights. Our outstanding shares of common stock are, and the shares offered by us in this offering will be, when issued and paid for, validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our certificate of incorporation, that will become effective upon the closing of this offering, our board of directors is authorized to direct us to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock. Upon the closingAs of this offering,March 31, 2014, there will bewere no shares of preferred stock outstanding, and we have no present plans to issue any shares of preferred stock.
Options
As of September 30, 2013,March 31, 2014, options to purchase 1,775,5932,505,749 shares of our common stock at a weighted average exercise price of $3.30$9.21 per share were outstanding.
Registration Rights
We have entered into a third amended and restated investors' rights agreement, dated July 26, 2013, which we refer to as the investor rights agreement, with the former holders of shares of our preferred stock. Upon the completion of this offering, holdersHolders of a total of 19,114,24117,819,662 shares of our common stock as of September 30, 2013 willMarch 31, 2014 have the right to require us to register these shares under the Securities Act, and to participate in future registrations of securities by us, under the circumstances described below. After registration pursuant to these rights, these shares will become freely tradable without restriction under the Securities Act except that affiliates will need to comply with resale restrictions under Rule 144 of the Securities Act. If not otherwise exercised, the rights described below will expire five years after the closing of thisour initial public offering.
Demand Registration Rights
Beginning 180 days after the effective date of the registration statement of which this prospectus forms a part, subjectSubject to specified limitations set forth in the investor rights agreement, at any time, the holders of 50% or more of the then outstanding shares having rights under the investor rights agreement, which we refer to as registrable shares, may at any time demand in writing that we register all or a portion of the registrable shares under the Securities Act if the total amount of registrable shares registered have an anticipated aggregate offering price of at least $5,000,000. We are not obligated to file a registration statement pursuant to this provision on more than two occasions.
In addition, subject to specified limitations set forth in the investor rights agreement, at any time after we become eligible to file a registration statement on Form S-3, holders of registrable shares outstanding may demand that we register on Form S-3 the registrable shares held by them so long as the total amount of registrable shares being registered has an aggregate offering price of at least $1,000,000. We are not obligated to file a registration statement pursuant to this provision on more than two occasions in any 12-month period.
Incidental Registration Rights
If we propose to file a registration statement to register any of our securities under the Securities Act, either for our own account or for the account of any of our stockholders, other than pursuant to the demand registration rights described above and other than pursuant to a Form S-4 or Form S-8, the holders of our registrable shares are entitled to notice of registration and, subject to specified limitations set forth in the investor rights agreement, we will be required to use best efforts to register the registrable shares then held by them that they request that we register.
In the event that any registration in which the holders of registrable shares participate pursuant to our investor rights agreement is an underwritten public offering, we agree to enter into an underwriting agreement containing customary representations and warranties and covenants.
In the event that any registration in which the holders of registrable shares participate pursuant to our investor rights agreement is an underwritten public offering, we will use our best efforts to include the requested registrable shares to be included, but may be limited by market conditions.
Expenses
Pursuant to the investor rights agreement, we are required to pay all registration, qualification and filing fees, printing and accounting expenses, fees and expenses of one counsel to represent the selling stockholders and other reasonable direct costs for the selling stockholders (such selling stockholder expenses not to exceed $50,000), but excluding underwriting discounts, selling commissions and the fees and expenses of selling stockholders' own counsel (other than the counsel selected to represent all selling stockholders). We are not required to pay registration expenses if the registration
request under the investor rights agreement is withdrawn at the request of holders of a majority of the
registrable shares, unless such holders agree to forfeit their right to one demand registration, or the withdrawal is due to discovery of a materially adverse change in our business.
The investor rights agreement contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them.
Anti-Takeover Provisions
We are subject to Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, Section 203 prevents a publicly held Delaware corporation from engaging in a "business combination" with any "interested stockholder" for three years following the date that the person became an interested stockholder, unless the interested stockholder attained such status with the approval of our board of directors or unless the business combination is approved in a prescribed manner or the interested stockholder acquired at least 85% of our outstanding voting stock in the transaction in which it became an interested stockholder. A "business combination" includes, among other things, a merger or consolidation involving us and the "interested stockholder" and the sale of more than 10% of our assets. In general, an "interested stockholder" is any entity or person beneficially owning 15% or more of our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person.
Staggered Board; Removal of Directors
Our certificate of incorporation and our by-laws that will be effective following this offering divide our board of directors into three classes with staggered three-year terms. In addition, such certificate of incorporation and by-laws provide that, subject to the rights of holders of any series of preferred stock, a director may be removed only for cause and only by the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in an annual election of directors. Under our certificate of incorporation and by-laws, any vacancy on our board of directors, including a vacancy resulting from an enlargement of our board of directors, may be filled only by vote of a majority of our directors then in office. Furthermore, our certificate of incorporation provides that the authorized number of directors may be changed only by the resolutions of our board of directors.
The classification of our board of directors and the limitations on the removal of directors and filling of vacancies could make it more difficult for a third party to acquire, or discourage a third party from seeking to acquire, control of our company.
Super-Majority Voting
The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation's certificate of incorporation or by-laws, unless a corporation's certificate of incorporation or by-laws, as the case may be, requires a greater percentage. Our by-laws that will become effective upon the closing of this offering may be amended or repealed by a majority vote of our board of directors or the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any annual election of directors. In addition, the affirmative vote of the holders of at least 75% of the votes that all our stockholders would be entitled to cast in any annual election of directors is required to amend or repeal, or to adopt any provisions inconsistent with, any of the provisions of our certificate of incorporation described under "Staggered Board; Removal of Directors" above.
Stockholder Action; Special Meeting of Stockholders; Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our certificate of incorporation and our by-laws that will be effective following this offering provide that any action required or permitted to be taken by our stockholders at a duly called annual meeting or special meeting of stockholders may only be taken if it is properly brought before such meeting and may not be taken by written action in lieu of a meeting. Our certificate of incorporation and our by-laws that will be effective following this offering also provide that, except as otherwise required by law, special meetings of our stockholders can only be called by our board of directors, chairman of the board or chief executive officer. In addition, our by-laws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed nominations of candidates for election to our board of directors. Stockholders at an annual meeting may only consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of our board of directors, or by a stockholder of record on the record date for the meeting, who is entitled to vote at the meeting and who has delivered timely written notice in proper form to our secretary of the stockholder's intention to bring such business before the meeting. These provisions could have the effect of delaying until the next stockholder meeting stockholder actions that are favored by the holders of a majority of our outstanding voting stock. These provisions also could discourage a third party from making a tender offer for our common stock, because even if it acquired a majority of our outstanding voting stock, it would be able to take action as a stockholder, such as electing new directors or approving a merger, only at a duly called stockholders meeting and not by written consent.
Authorized But Unissued Shares
The authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of theThe NASDAQ Global Select Market. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could make more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will beis Computershare Trust Company, Inc.N.A.
NASDAQ Global Select Market
We have applied to have ourOur common stock is listed on theThe NASDAQ Global Select Market under the symbol "KPTI."
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was no public market for our common stock. Future sales of substantial amounts of common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock. Although we have applied to have ourOur common stock is listed on theThe NASDAQ Global Select Market we cannot assure you that there will be an active public market for our common stock.under the symbol "KPTI."
Upon the closing of this offering, we will have outstanding an aggregate of 27,596,26031,762,499 shares of common stock, based on the number of shares of our common stock outstanding as of March 31, 2014, assuming the issuance of 5,666,6672,000,000 shares of common stock offered by us in this offering and assuming no exercise by the underwriters of their option to purchase additional shares and no exercise of options after September 30, 2013.outstanding as of March 31, 2014. Of these shares, all shares sold in this offering, including both the shares sold by us and the shares sold by the selling stockholders, will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our "affiliates," as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
The remaining 21,929,59327,762,499 shares of common stock will be "restricted securities," as that term is defined in Rule 144 under the Securities Act, of which 21,766,9094,008,923 shares of common stock will further be subject to either restrictions on transfer under the lock-up agreements described below, or restrictions on transfer under stock optionbelow. The lock-up agreements or the investor rights agreement entered into between us and the holders of those shares,in connection with this offering restrict transfer for a period from the date of 180execution of the lock-up agreement to 90 days from the effectivenessdate of the registration statement of which this prospectus forms a part.underwriting agreement described under "Underwriting". Following the expiration of these restrictions, these shares will become eligible for public sale if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below.
In addition, of the 1,775,5932,505,749 shares of our common stock that were subject to stock options outstanding as of September 30, 2013,March 31, 2014, options to purchase 281,035359,420 shares of common stock were vested as of September 30, 2013March 31, 2014 and, upon exercise, these shares will be eligible for sale, subject to the lock-up agreements described belowin this section and Rules 144 and 701 under the Securities Act.
Lock-Up Agreements
We and each of our directors and executive officers and holders of substantially all of our outstanding capital stock,the selling stockholders, who collectively beneficially own 21,766,9094,208,923 shares of our common stock upon the closing of this offering, based on shares outstanding as of September 30,March 31, 2013, including 19,114,241 shares of our common stock issuable upon the automatic conversion of all outstanding shares of our preferred stock, without the prior written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated on behalf of the underwriters, will not, subject to limited exceptions, during the period ending 180from the date of execution of the lock-up agreement to 90 days from the effectiveness of the registration statement of which this prospectus forms a part, directly or indirectly:
whether any transaction described above is to be settled by delivery of our common stock or such other securities, in cash or otherwise.
The Company announced on June 14, 2014 that each of Drs. Kauffman and Shacham entered into a pre-arranged stock trading plan (a "10b5-1 Plan") pursuant to Rule 10b5-1 of the Securities Exchange Act of 1934, as amended. Pursuant to an agreement with the underwriters, each of Drs. Kauffman and Shacham have agreed to terminate their respective 10b5-1 Plan effective as of, and conditioned upon, the execution of the underwriting agreement related to this offering.
The lock-up restrictions are described in more detail in the section of this prospectus entitled "Underwriting."
Rule 144
Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our common stock for at least six months would be entitled to sell in "broker's transactions" or certain "riskless principal transactions" or to market makers, a number of shares within any three-month period that does not exceed the greater of:
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the Securities and Exchange Commission and NASDAQ concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the 90 days preceding a sale, and who has beneficially owned shares of our common stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Rule 701
In general, under Rule 701, any of an issuer's employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a qualified compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Actour initial public offering is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
The Securities and Exchange Commission has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the
Securities Exchange Act of 1934, as amended, or the Exchange Act, along with the shares acquired
upon exercise of such options, including exercises after an issuer becomes subject to the reporting requirements of the Exchange Act.
Equity Plans
We intend to file one or morehave filed a registration statementsstatement on Form S-8 under the Securities Act to register all shares of common stock subject to outstanding stock options and common stock issuable under our stock plans. We expect to file the registration statement covering shares offered pursuant to our stock plans, shortly after the date of this prospectus, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance with the resale provisions of Rule 144.
Registration Rights
Upon the closingAs of this offering,March 31, 2014, the holders of 19,114,24117,819,662 shares of our common stock or their transferees will beare entitled to various rights with respect to the registration of these shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, subject to the terms of the lock-up agreement to which the holder is subject and except for shares purchased by affiliates. See "Description of Capital Stock—Registration Rights" for additional information.
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
OF COMMON STOCK
The following is a general discussion of material U.S. federal income and estate tax considerations relating to the purchase, ownership and disposition of shares of our common stock by a non-U.S. holder. For purposes of this discussion, the term "non-U.S. holder"a non-U.S. holder means a beneficial owner of shares of our common stock that is not for U.S. federal income tax purposes, an individual, corporation (or other entity treated as a corporation), estate or trust other than:purposes:
This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their common stock through partnerships or other pass-through entities. A partner in a partnership or other pass-through entity that will hold our common stock should consult his, her or its own tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock through a partnership or other pass-through entity, as applicable.
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated or proposed thereunder and current administrative and judicial interpretations thereof, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any change could alter the tax consequences to non-U.S. holders described in this prospectus. In addition,There can be no assurance that the Internal Revenue Service, or the IRS, couldwill not challenge one or more of the tax consequences described in this prospectus.
We assume in this discussion that each non-U.S. holder holds shares of our common stock as a capital asset (generally, property held for investment).
This discussion does not address all aspects of U.S. federal income and estate taxation including the Medicare contribution tax, that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder's individual circumstances nor does it address any aspects of U.S. state, local or non-U.S. taxes, the alternative minimum tax, the Medicare tax on net investment income or, except as explicitly addressed herein, U.S. federal taxes other than income and estate taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax considerations that may be applicable to particular non-U.S. holders, such as:
In addition, thisThis discussion doesis for general information only and is not address the tax treatment of partnerships or persons who hold their sharesadvice. Accordingly, all prospective non-U.S. holders of our common stock through partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. A partner in a partnership or other pass-through entity that will hold shares of our common stock should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of our common stock through a partnership or other pass-through entity, as applicable.
Prospective investors should consult their own tax advisors regarding the U.S. federal, state, local and non U.S.non-U.S. income and other tax considerations of acquiring, holding and disposing of shares of our common stock.
DividendsDistributions on Common Stock
If we pay distributions of cash or property with respect to shares ofDistributions on our common stock those distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles and will be subject to withholding as described in the paragraphs below. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder's investment, up to such holder's tax basis in its shares of our common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below under the heading "Gain on Sale, Exchange or Other Taxable Disposition of Shares of Our Common Stock." Any such distributions will also be subject to the discussion below under the section titled "Withholding and Information Reporting Requirements—FATCA."
Subject to the exceptions described below, dividendsDividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence. If we determine, at a time reasonably close to the date of payment of a distribution on shares of our common stock, that the distribution will not constitute a dividend because we do not anticipate having current or accumulated earnings and profits, we intend not to withhold any U.S. federal income tax on the distribution as permitted by U.S. Treasury Regulations; otherwise, we intend to withhold the appropriate amount of U.S. federal income tax on the distribution.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States, and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. To obtain this exemption, a non-U.S. holder must generally providesatisfy certain disclosure and certification requirements, including providing us with a properly executed IRS Form W-8ECI properly certifying such exemption.W-8ECI. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code). Any U.S. effectively connected income received by a non-U.S. holder that is treated as a corporation for U.S. federal income tax purposes may also, under certain circumstances, be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder's country of residence.
A non-U.S. holder of shares of our common stock who claims the benefit of an applicable income tax treaty between the United States and such holder's country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-BEN-E (or applicable successor form) and satisfy applicable certification and other requirements. Non-U.S holders are urged to consult their own tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty or that is otherwise not subject to U.S. withholding tax may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS.
Gain on Sale, Exchange or Other Taxable Disposition of Shares of Our Common Stock
Subject to the discussion below under the section titled "Withholding and Information Reporting Requirements—FATCA," a non-U.S. holder generally will not be subject to U.S. federal income tax on gain realized on a sale, exchange or other taxable disposition of shares of our common stock unless:
Information Reporting and Backup Withholding Tax
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on shares of our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. These information reporting requirements apply even if withholding is not required. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a U.S.United States person (as defined in the Code) or is otherwise subject to an exemption in order to avoid backup withholding at the applicable rate with respect to dividends paid with respect to
shares of our common stock. Generally, a holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN (or other applicable Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a non-U.S. holder, or otherwise establishes an exemption. Dividends paid to non-U.S. holders subject to the U.S. federal withholding tax, as described above in "Dividends," generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding generally will apply to the payment of the proceeds of a disposition of shares of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or non-U.S., unless the holder certifies that it is not a U.S. person (as defined in the Code) and satisfies certain other requirements, or otherwise establishes an exemption. For information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker and dispositions otherwise effected through a non-U.S. office generally will not be subject to information reporting. Generally, backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected through a non-U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder's U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Withholding and Information Reporting Requirements—FATCA
Legislation enacted in March 2010, which is commonly referred to as "FATCA,"The Foreign Account Tax Compliance Act, or FATCA, generally will imposeimposes a U.S. federal withholding tax at a rate of 30% on payments to certain non-U.S. entities (including intermediaries), of dividends on, and theor gross proceeds from the sale or other disposition of, shares of our common stock paid to a foreign entity unless (i) if the foreign entity is a "foreign financial institution," such entities comply with a complicated U.S. information reporting,foreign entity undertakes certain due diligence, disclosurereporting, withholding, and certification regime. This new regime andobligations, (ii) if the foreign entity is not a "foreign financial institution," such foreign entity identifies certain of its requirements are different from, and in addition to,U.S. investors, if any, or (iii) the certification requirements described elsewhere in this discussion. Withholdingforeign entity is otherwise exempt under FATCA. Under applicable U.S. Treasury regulations, withholding under FATCA will only apply (1) to (1) payments of dividends with respect to shares ofon our common stock made after June 30, 2014, and (2) to payments of gross proceeds from a sale or other disposition of shares of our common stock made after December 31, 2016. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of the tax. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their own tax advisors regarding the possible impactimplications of these rulesthis legislation on their investment in shares ofour common stock and the entities through which they hold our common stock, including, any investment in shareswithout limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of our common stock made through another entity or an intermediary.the 30% withholding tax under FATCA.
U.S. Federal Estate Tax
Shares of our common stock owned or treated as owned at the time of death by an individual who is not a citizen or resident of the United States (as specially defined for U.S. federal estate tax purposes) are considered U.S. situs assets and will be included in the individual's gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise.
The preceding discussion of material U.S. federal tax considerations is for general information only. It is not tax advice. Prospective investors should consult their own tax advisors regarding the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of shares of our common stock, including the consequences of any proposed changes in applicable laws.
Merrill Lynch, Pierce, Fenner & Smith Incorporated and Leerink SwannPartners LLC are acting as representatives of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us, the selling stockholders and the underwriters, we and the selling stockholders have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us and the selling stockholders, the number of shares of common stock set forth opposite its name below.
| Underwriter | Number of Shares | |||
|---|---|---|---|---|
Merrill Lynch, Pierce, Fenner & Smith | ||||
Incorporated | ||||
Leerink | ||||
JMP Securities LLC | ||||
Wedbush Securities Inc | ||||
Oppenheimer & Co. Inc. | ||||
| | | | | |
Total | 2,200,000 | |||
| | | | | |
| | | | | |
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
We and the selling stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officers' certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representatives have advised us and the selling stockholders that the underwriters propose initially to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to us.us and to the selling stockholders. We will not receive any of the proceeds from the sale of the common stock by the selling stockholders. The information assumes either no exercise or full exercise by the underwriters of their option to purchase additional shares of our common stock.
| | Per Share | Without Option | With Option | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Public offering price | $ | $ | $ | ||||||||
Underwriting discount | $ | $ | $ | ||||||||
Proceeds, before expenses, to us | $ | $ | $ | ||||||||
Proceeds, before expenses, to the selling stockholders | $ | $ | — | ||||||||
The expenses of the offering to us, not including the underwriting discount, are estimated at $2.6 million$750,000. We have agreed to reimburse the underwriters for all expenses related to the clearing of this offering with the Financial Industry Regulatory Authority and are payable by us.
Tablethe qualification of Contentsour common stock under state securities laws (in an amount not to exceed in the aggregate $35,000).
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 30 days after the date of this prospectus, to purchase up to 850,000330,000 additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter's initial amount reflected in the above table.
Reserved Shares
At our request, the underwriters The selling stockholders have reserved for sale, at the initial public offering price, up to 283,333 of the shares offered by this prospectus for sale to our employees and other parties. If these persons purchase reserved shares, it will reduce the number of shares available for salenot granted such an option to the general public. Any reserved shares that are not so purchased will be offered by the underwriters to the general public on the same terms as the other shares offered by this prospectus.underwriters.
No Sales of Similar Securities
We, our executive officers and directors and substantially all of our other existing security holdersthe selling stockholders have agreed not to sell or transfer any common stock or securities convertible into or exchangeable or exercisable for common stock, for 18090 days after the date of this prospectus without first obtaining the written consent of Merrill Lynch, Pierce, Fenner & Smith Incorporated. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly:indirectly
This lock-up provision applies to common stock and to securities convertible into or exchangeable or exercisable for common stock. It also applies to common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
NASDAQ Global Select Market Listing
We expect the shares to be approved for listingOur common stock is listed on the NASDAQ Global Select Market subject to notice of issuance, under the symbol "KPTI."
Before this offering, there has been no public market for our common stock. The initial public offering price will be determined through negotiations between us and the representatives. In addition to prevailing market conditions, the factors to be considered in determining the initial public offering price are:
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
The underwriters do not expect to sell more than 5% of the shares in the aggregate to accounts over which they exercise discretionary authority.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common stock. However, the representatives may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover
positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. "Covered" short sales are sales made in an amount not greater than the underwriters' option described above. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase shares granted to them. "Naked" short sales are sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on the NASDAQ Global Select Market, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the
representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Passive Market Making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in the common stock on the NASDAQ Global Select Market in accordance with Rule 103 of Regulation M under the Exchange Act during a period before the commencement of offers or sales of common stock and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker's bid, that bid must then be lowered when specified purchase limits are exceeded. Passive market making may cause the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of those transactions. The underwriters and dealers are not required to engage in passive market making and may end passive market making activities at any time.
Electronic Distribution
In connection with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
Some of the underwriters and their affiliates have engaged in, and may in the future engage in, investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They have received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
A managing director of Leerink Partners LLC, one of the underwriters in this offering, has a 0.1% interest in Foresite Capital Fund I, L.P., who holds 1,585,743 shares of our common stock as of March 31, 2014.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area (each, a "Relevant Member State"), no offer of shares may be made to the public in that Relevant Member State other than:
Each person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a "qualified investor" within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representatives has been obtained to each such proposed offer or resale.
The Company, the representatives and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Relevant Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the underwriters have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the Company or the underwriters to publish a prospectus for such offer.
For the purpose of the above provisions, the expression "an offer to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States) and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are "qualified investors" (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the "Order") and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons"). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange ("SIX") or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes ("CISA"). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority ("DFSA"). This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
Notice to Prospective Investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission ("ASIC"), in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the "Corporations Act"), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons (the "Exempt Investors") who are "sophisticated investors" (within the meaning of section 708(8) of the Corporations Act), "professional investors" (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Japan
The shares have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, "Japanese Person" shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the "SFA"), (ii) to a relevant person pursuant to Section 275 (1)275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
The validity of the shares of common stock offered hereby will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, Boston, Massachusetts. Ropes & Gray LLP, Boston, Massachusetts, has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The consolidated balance sheets as of December 31, 2011 and December 31, 2012, the consolidatedfinancial statements of operations and cash flows for the years then ended and the consolidated statements of convertible preferred stock and stockholder's equity for the period from December 22, 2008 (date of inception) to December 31, 2012, appearingincorporated in this Prospectus and Registration Statementby reference to the Annual Report on Form 10-K for the year ended December 31, 2013 have been audited by McGladrey LLP, an independent registered public accounting firm, as stated in their report appearing elsewhereincorporated by reference herein, and are includedhave been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement or incorporated herein by reference are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. Upon completion of this offering, we will bestatement or incorporated herein by reference. We are required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Exchange Act. You may read and copy this information at the SEC's public preference room, which is located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that site iswww.sec.gov.
KARYOPHARM THERAPEUTICS INC.INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders ofKaryopharm Therapeutics Inc.
We have audited the accompanying consolidated balance sheets of Karyopharm Therapeutics Inc. (a development stage enterprise) (the Company) as of December 31, 2011 and 2012, the related consolidated statements of operations and cash flows for the years then ended and the statement of convertible preferred stock and stockholders' equity (deficit) for the period from December 22, 2008 (date of inception) to December 31, 2012. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Karyopharm Therapeutics Inc. (a development stage enterprise) as of December 31, 2011 and 2012, the consolidated results of its operations and its cash flows for the years then ended and its changes in convertible preferred stock and stockholders' equity (deficit) for the period from December 22, 2008 (date of inception) to December 31, 2012, in conformity with U.S. generally accepted accounting principles.
/s/ McGladrey LLP
Boston, MassachusettsSeptember 4, 2013, except for Note 11 as towhich the date is October 25, 2013
INCORPORATION OF DOCUMENTS BY REFERENCEKaryopharm Therapeutics Inc.(A Development Stage Company)Consolidated Balance Sheets(In thousands except share and per share data)
| | | | June 30, 2013 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | December 31, 2011 | December 31, 2012 | |||||||||||
| | Actual | Pro forma | |||||||||||
| | | | (unaudited) | (unaudited) | |||||||||
Assets | |||||||||||||
Current assets: | |||||||||||||
Cash and cash equivalents (NPM restricted December 31, 2011 and 2012 $0 and $12, respectively, June 30, 2013 actual and pro forma $1,031 (unaudited)) | $ | 6,512 | $ | 391 | $ | 17,667 | $ | 61,667 | |||||
Prepaid expenses and other current assets (NPM restricted December 31, 2011 and 2012 $7 and $485, repectively, June 30, 2013 actual and pro forma $328 (unaudited)) | 354 | 563 | 522 | 522 | |||||||||
Total current assets | 6,866 | 954 | 18,189 | 62,189 | |||||||||
Property and equipment, net | 328 | 327 | 258 | 258 | |||||||||
Deposits | 30 | 30 | 30 | 30 | |||||||||
Total assets | $ | 7,224 | $ | 1,311 | $ | 18,477 | $ | 62,477 | |||||
Liabilities, convertible preferred stock and stockholders' equity (deficit) | |||||||||||||
Current liabilities: | |||||||||||||
Accounts payable (NPM restricted December 31, 2011 and 2012 $0 and $499, respectively, June 30, 2013 actual and pro forma $731 (unaudited)) | $ | 1,142 | $ | 1,076 | $ | 1,728 | $ | 1,728 | |||||
Accrued liabilities (NPM restricted December 31, 2011 and 2012 $58 and $312, respectively, June 30, 2013 actual and pro forma $13 (unaudited)) | 759 | 764 | 778 | 778 | |||||||||
Deferred revenue | 200 | 66 | — | — | |||||||||
Other liabilities | 16 | 24 | 35 | 35 | |||||||||
Total current liabilities | 2,117 | 1,930 | 2,541 | 2,541 | |||||||||
Commitments and contingencies (Note 9) | |||||||||||||
Preferred stock subscription | 6,980 | 8,980 | 13,980 | — | |||||||||
Series A convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized—20,937,500 shares; Issued and outstanding—10,937,500 shares at December 31, 2011, 18,437,500 at December 31, 2012 and 20,937,500 at June 30, 2013 (unaudited), and no shares issued and outstanding pro forma (unaudited) (aggregate liquidation preference of $20.9 million) | 10,778 | 18,278 | 20,778 | — | |||||||||
Series A-2 convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized, Issued and Outstanding—no shares at December 31, 2011 and 2012, and Authorized—6,100,000 shares, Issued and outstanding—no shares at June 30, 2013 (unaudited), and no shares pro forma (unaudited) | — | — | — | — | |||||||||
Series A-3 convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized, Issued and Outstanding—no shares at December 31, 2011 and 2012, and Authorized—1,764,706 shares, Issued and outstanding—no shares at June 30, 2013 (unaudited), and no shares pro forma (unaudited) | — | — | — | — | |||||||||
Series A-4 convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized, Issued and Outstanding—no shares at December 31, 2011 and 2012, and Authorized—1,538,461 shares, Issued and outstanding—no shares at June 30, 2013 (unaudited), and no shares pro forma (unaudited) | — | — | — | — | |||||||||
Series B convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized—0 shares at December 31, 2012 and 30,000,000 at June 30, 2013 (unaudited); Issued and outstanding—no shares at December 31, 2012 and 10,600,000 at June 30, 2013 (unaudited), and no shares issued and outstanding pro forma (unaudited) (aggregate liquidation preference of $21.2 million) | — | — | 21,057 | — | |||||||||
Series B-1 convertible preferred stock, $0.0001 par value; | |||||||||||||
Authorized, Issued and Outstanding—no shares at December 31, 2011 and 2012, June 30, 2013 (unaudited) and pro forma (unaudited) | — | — | — | — | |||||||||
Special participation stock, $0.0001 par value; | |||||||||||||
Authorized, issued and outstanding—10,000 shares at December 31, 2011, December 31, 2012 and June 30, 2013 (unaudited); and no shares issued and outstanding pro forma (unaudited) | — | — | — | — | |||||||||
| 17,758 | 27,258 | 55,815 | — | ||||||||||
Stockholders' equity (deficit): | |||||||||||||
Common stock, $0.001 par value; | |||||||||||||
Authorized—35,000,000 shares at December 31, 2011 and 2012 and 80,000,000 shares at June 30, 2013 (unaudited); Issued and outstanding—1,228,206 shares at December 31, 2011, 2,123,388 shares at December 31, 2012, 2,450,510 shares at June 30, 2013 (unaudited); and 21,576,872 shares issued and outstanding pro forma (unaudited) | — | — | — | 2 | |||||||||
Additional paid-in-capital | 83 | 745 | 1,223 | 101,036 | |||||||||
Deficit accumulated during the development stage | (12,734 | ) | (28,622 | ) | (41,102 | ) | (41,102 | ) | |||||
Total stockholders' equity (deficit) | (12,651 | ) | (27,877 | ) | (39,879 | ) | 59,936 | ||||||
Total liabilities, convertible preferred stock and stockholders' equity (deficit) | $ | 7,224 | $ | 1,311 | $ | 18,477 | $ | 62,477 | |||||
See accompanying notes to consolidated financial statements.
Karyopharm Therapeutics Inc.(A Development Stage Company)Consolidated Statements of Operations(In thousands except share and per share data)
| | Years Ended December 31, | Six Months Ended June 30, | Period from December 22, 2008 (date of inception) to June 30, 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
| | | | (unaudited) | (unaudited) | (unaudited) | |||||||||||
Statement of Operations: | ||||||||||||||||
Contract and grant revenue | $ | 152 | $ | 634 | $ | 567 | $ | 366 | $ | 1,245 | ||||||
Operating expenses: | ||||||||||||||||
Research and development | 8,623 | 14,095 | 7,432 | 11,025 | 35,408 | |||||||||||
General and administrative | 1,840 | 2,429 | 1,152 | 1,822 | 6,754 | |||||||||||
Total operating expenses | 10,463 | 16,524 | 8,584 | 12,847 | 42,162 | |||||||||||
Loss from operations | (10,311 | ) | (15,890 | ) | (8,017 | ) | (12,481 | ) | (40,917 | ) | ||||||
Other income (expense): | ||||||||||||||||
Interest income | — | 2 | 2 | 1 | 3 | |||||||||||
Interest expense | — | — | — | — | (188 | ) | ||||||||||
Total other income (expense) | — | 2 | 2 | 1 | (185 | ) | ||||||||||
Net loss | $ | (10,311 | ) | $ | (15,888 | ) | $ | (8,015 | ) | $ | (12,480 | ) | $ | (41,102 | ) | |
Net loss per share applicable to common stockholders—basic and diluted | $ | (10.27 | ) | $ | (8.95 | ) | $ | (5.06 | ) | $ | (5.39 | ) | $ | (45.68 | ) | |
Weighted-average number of common shares used in net loss per share applicable to common stockholders—basic and diluted | 1,004,144 | 1,775,323 | 1,584,607 | 2,315,331 | 899,836 | |||||||||||
Pro forma net loss per share applicable to common stockholders—basic and diluted (unaudited) | $ | (2.74 | ) | $ | (1.27 | ) | ||||||||||
Weighted-average number of common shares used in pro forma net loss per share applicable to common stockholders—basic and diluted (unaudited) | 5,799,420 | 9,796,943 | ||||||||||||||
See accompanying notes to consolidated financial statements.
Karyopharm Therapeutics Inc.(A Development Stage Company)Consolidated Statements of Convertible Preferred Stock and Stockholders' Equity (Deficit)(In thousands except share and per share data)
| | | | Series A Convertible Preferred Shares | Series B Convertible Preferred Shares | | | | | | | | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Preferred Stock Subscription | Special Participation Shares | | | | Deficit Accumulated During the Development Stage | | |||||||||||||||||||||||||||||||||
| | Common Shares | | Total Shareholders' Equity (Deficit) | |||||||||||||||||||||||||||||||||||||
| | Additional Paid-In Capital | |||||||||||||||||||||||||||||||||||||||
| | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||||
Balance at December 22, 2008 (date of inception) | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||||
Net loss | (179 | ) | (179 | ) | ||||||||||||||||||||||||||||||||||||
Balance at December 31, 2009 | — | — | — | — | — | — | — | — | — | — | — | (179 | ) | (179 | ) | |||||||||||||||||||||||||
Proceeds from sale of restricted stock | — | — | — | — | — | — | — | — | — | — | 1 | — | 1 | |||||||||||||||||||||||||||
Vesting of restricted stock | — | — | — | — | — | — | — | — | 578,600 | — | — | — | — | |||||||||||||||||||||||||||
Issuance of common stock in connection with a service agreement | — | — | — | — | — | — | — | — | 151,515 | — | — | — | — | |||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock, net of issuance costs of $160 | — | — | 5,000,000 | 4,840 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Issuance of special participation stock | — | — | — | — | — | — | 10,000 | — | — | — | — | — | — | |||||||||||||||||||||||||||
Conversion of notes payable into Series A convertible preferred stock | — | — | 937,500 | 938 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | 46 | — | 46 | |||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | (2,244 | ) | (2,244 | ) | |||||||||||||||||||||||||
Balance at December 31, 2010 | — | — | 5,937,500 | 5,778 | — | — | 10,000 | — | 730,115 | — | 47 | (2,423 | ) | (2,376 | ) | |||||||||||||||||||||||||
Issuance of common stock in connection with a service agreement | — | — | — | — | — | — | — | — | 11,170 | — | — | — | — | |||||||||||||||||||||||||||
Vesting of restricted stock | — | — | — | — | — | — | — | — | 486,921 | — | — | — | — | |||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock | — | — | 5,000,000 | 5,000 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Proceeds from the sale of Series A-2 convertible preferred stock, net of issuance costs of $35 | 6,100,000 | 6,980 | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Proceeds from sale of restricted stock to advisors | — | — | — | — | — | — | — | — | — | — | 12 | — | 12 | |||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | 24 | — | 24 | |||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | (10,311 | ) | (10,311 | ) | |||||||||||||||||||||||||
Balance at December 31, 2011 | 6,100,000 | 6,980 | 10,937,500 | 10,778 | — | — | 10,000 | — | 1,228,206 | — | 83 | (12,734 | ) | (12,651 | ) | |||||||||||||||||||||||||
Vesting of restricted stock | — | — | — | — | — | — | — | — | 575,547 | — | — | — | — | |||||||||||||||||||||||||||
Exercise of stock options | — | — | — | — | — | — | — | — | 319,635 | — | 9 | — | 9 | |||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock | — | — | 7,500,000 | 7,500 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Proceeds from the sale of Series A-4 convertible preferred stock | 1,538,461 | 2,000 | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | — | 653 | — | 653 | |||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | (15,888 | ) | (15,888 | ) | |||||||||||||||||||||||||
Balance at December 31, 2012 | 7,638,461 | 8,980 | 18,437,500 | 18,278 | — | — | 10,000 | — | 2,123,388 | — | 745 | (28,622 | ) | (27,877 | ) | |||||||||||||||||||||||||
Vesting of restricted stock (unaudited) | — | — | — | — | — | — | — | — | 253,890 | — | — | — | — | |||||||||||||||||||||||||||
Exercise of stock options (unaudited) | — | — | — | — | — | — | — | — | 73,232 | — | 32 | — | 32 | |||||||||||||||||||||||||||
Issuance of Series A convertible preferred stock (unaudited) | — | — | 2,500,000 | 2,500 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Proceeds from the sale of Series A-3 convertible preferred stock (unaudited) | 1,764,706 | 3,000 | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Proceeds from the sale of Series B convertible preferred stock (unaudited) | 1,000,000 | 2,000 | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Issuance of Series B convertible preferred stock, net of issuance costs of $143 (unaudited) | — | — | — | — | 10,600,000 | 21,057 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Stock-based compensation expense (unaudited) | — | — | — | — | — | — | — | — | — | — | 446 | — | 446 | |||||||||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | — | — | — | — | — | — | (12,480 | ) | (12,480 | ) | |||||||||||||||||||||||||
Balance at June 30, 2013 (unaudited) | 10,403,167 | $ | 13,980 | 20,937,500 | $ | 20,778 | 10,600,000 | $ | 21,057 | 10,000 | $ | — | 2,450,510 | $ | — | $ | 1,223 | $ | (41,102 | ) | $ | (39,879 | ) | |||||||||||||||||
Conversion of special participation stock into common stock (unaudited) | — | — | — | — | — | — | (10,000 | ) | — | 12,121 | — | — | — | — | ||||||||||||||||||||||||||
Issuance of shares related to preferred stock subscription (unaudited) | (10,403,167 | ) | (13,980 | ) | — | — | — | — | — | — | 3,152,474 | — | 13,980 | — | 13,980 | |||||||||||||||||||||||||
Issuance and conversion of Series B and B-1 convertible preferred stock into common stock (unaudited) | — | — | — | — | — | — | — | — | 6,404,958 | 1 | 43,999 | 44,000 | ||||||||||||||||||||||||||||
Conversion of convertible preferred stock into common stock (unaudited) | — | — | (20,937,500 | ) | (20,778 | ) | (10,600,000 | ) | (21,057 | ) | — | — | 9,556,809 | 1 | 41,834 | — | 41,835 | |||||||||||||||||||||||
Pro forma balance at June 30, 2013 (unaudited) | — | $ | — | — | $ | — | — | $ | — | — | $ | — | 21,576,872 | $ | 2 | $ | 101,036 | $ | (41,102 | ) | $ | 59,936 | ||||||||||||||||||
See accompanying notes to consolidated financial statements.
Karyopharm Therapeutics Inc.(A Development Stage Company)Consolidated Statements of Cash Flows(In thousands)
| | Years Ended December 31, | Six Months Ended June 30, | | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Period from December 22, 2008 (date of inception) to June 30, 2013 | |||||||||||||||
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
| | | | (Unaudited) | (Unaudited) | (Unaudited) | |||||||||||
Operating activities | ||||||||||||||||
Net loss | $ | (10,311 | ) | $ | (15,888 | ) | $ | (8,015 | ) | $ | (12,480 | ) | $ | (41,102 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||||||
Depreciation and amortization | 83 | 122 | 58 | 69 | 275 | |||||||||||
Noncash consulting expenses | — | — | — | — | 700 | |||||||||||
Noncash interest expense on convertible notes | — | — | — | — | 188 | |||||||||||
Stock-based compensation expense | 24 | 653 | 173 | 446 | 1,169 | |||||||||||
Changes in operating assets and liabilities: | ||||||||||||||||
Prepaid expenses and other current assets | (99 | ) | (209 | ) | 58 | 41 | (522 | ) | ||||||||
Deposits | — | — | — | — | (30 | ) | ||||||||||
Accounts payable | 1,131 | (66 | ) | (78 | ) | 652 | 1,728 | |||||||||
Accrued expenses and other liabilities | 423 | 13 | (27 | ) | 25 | 813 | ||||||||||
Deferred revenue | 200 | (134 | ) | (67 | ) | (66 | ) | — | ||||||||
Net cash used in operating activities | (8,549 | ) | (15,509 | ) | (7,898 | ) | (11,313 | ) | (36,781 | ) | ||||||
Investing activities | ||||||||||||||||
Purchases of property and equipment | (376 | ) | (121 | ) | (87 | ) | — | (533 | ) | |||||||
Net cash used in investing activities | (376 | ) | (121 | ) | (87 | ) | — | (533 | ) | |||||||
Financing activities | ||||||||||||||||
Proceeds from issuance of common stock | 12 | 9 | 7 | 32 | 54 | |||||||||||
Proceeds from issuance of convertible notes | — | — | — | — | 250 | |||||||||||
Proceeds from issuance of preferred stock subscription | 6,980 | 2,000 | 2,000 | 5,000 | 13,980 | |||||||||||
Principal payments of convertible notes | — | — | — | — | (200 | ) | ||||||||||
Proceeds from sale of convertible preferred stock, net of issuance costs | 5,000 | 7,500 | 2,500 | 23,557 | 40,897 | |||||||||||
Net cash provided by financing activities | 11,992 | 9,509 | 4,507 | 28,589 | 54,981 | |||||||||||
Net increase (decrease) in cash and cash equivalents | $ | 3,067 | $ | (6,121 | ) | $ | (3,478 | ) | $ | 17,276 | $ | 17,667 | ||||
Cash and cash equivalents at beginning of period | 3,445 | 6,512 | 6,512 | 391 | — | |||||||||||
Cash and cash equivalents at end of period | $ | 6,512 | $ | 391 | $ | 3,034 | $ | 17,667 | $ | 17,667 | ||||||
Supplemental disclosure of noncash investing and financing activities: | ||||||||||||||||
Conversion of notes payable to preferred stock | $ | — | $ | — | $ | — | $ | — | $ | 750 | ||||||
Issuance of convertible notes in satisfaction of accrued expenses | $ | — | $ | — | $ | — | $ | — | $ | 700 | ||||||
See accompanying notes to consolidated financial statements.
Karyopharm Therapeutics Inc.(A Development Stage Company)Notes to Consolidated Financial Statements(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)(in thousands, except share and per share data)
Note 1. Organization and Operations
The Company
Karyopharm Therapeutics Inc. (the Company) is a clinical stage pharmaceutical company that was incorporated in Delaware on December 22, 2008 and has a principal place of business in Natick, Massachusetts. The Company was established to discover, develop and commercialize drugs to treat cancer and other major diseases.
The Company is in the development stage, and is devoting substantially all of its efforts to product research and development, initial market development and raising capital. The Company has not generated any product revenue related to its primary business purpose to date and is subject to a number of risks similar to those of other development stage life science companies, including dependence on key individuals, competition from other companies, the need for development of commercially viable products, and the need to obtain adequate additional financing to fund the development of its product candidates. The Company is also subject to a number of risks similar to other companies in the industry, including rapid technological change, regulatory approval of products, uncertainty of market acceptance of products, competition from substitute products and larger companies, the need to obtain additional financing, compliance with government regulations, protection of proprietary technology, dependence on third parties, product liability and dependence on key individuals.
The Company has generated an accumulated deficit of $41.1 million since inception. The Company has financed its operations primarily through private placements of its preferred stock. The Company has not completed development of any product candidate and has devoted substantially all of its financial resources and efforts to research and development, including preclinical and clinical development. The Company expects to continue to incur significant expenses and increasing operating losses for at least the next several years.
Liquidity
The Company believesSEC allows us to "incorporate by reference" information from other documents that its cash resourceswe file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of approximately $17.7 million at June 30, 2013, togetherthis prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the proceeds from the sale of Series B and Series B-1 Convertible Preferred Stock (Note 6) it received in the third quarter of 2013 will be sufficient to allow the Company to fund its current operating plan and continue as a going concern through at least the next 12 months. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital. The Company intends to pursue a public offering of its common stock to fund future operations. However, if the Company is unable to complete a sufficient public offering in a timely manner it would need to pursue other financing alternatives including private financing of debt or equity or collaboration agreements. There can be no assurances, however, that the current operating plan will be achieved or that additional funding will be available on terms acceptableSEC prior to the Company,date of this prospectus.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or at all.documents listed below that we have filed with the SEC.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013,our Annual Report on Form 10-K for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies
Unaudited Pro Forma Presentation
In September 2013, the Company's board of directors authorized the Company to submit a draft registration statement to the Securities and Exchange Commission permitting the Company to sell shares of its common stock to the public. The unaudited pro forma balance sheet as of June 30, 2013 reflects the settlement of all outstanding shares of the Company's special participation stock into 12,121 shares of common stock and the conversion of all of the Series A convertible preferred stock (Series A Preferred Stock), Series A-2 convertible preferred stock (Series A-2 Preferred Stock), the Series A-3 convertible preferred stock (Series A-3 Preferred Stock), Series A-4 convertible preferred stock (Series A-4 Preferred Stock), the Series B convertible preferred stock (Series B Preferred Stock), the 8,636,362 shares of Series B-1 Preferred Stock (collectively, the "Preferred Stock") that were issued in July 2013 and the 12,500,000 shares of Series B Preferred Stock that were issued in September 2013, into 19,114,241 shares of common stock.
Unaudited pro forma net loss per share is computed using the weighted-average number of shares of common stock outstanding after giving effect to the conversion of all Preferred Stock during thefiscal year ended December 31, 2012 and the six months ended June 30, 2013 into shares of the Company's common stock as if such conversion had occurred at the date the Company issued such shares, or committed to issue such shares, or the beginning of the applicable period, as appropriate.
Unaudited Interim Financial Data
The accompanying unaudited June 30, 2013 interim balance sheet, the statements of operations and cash flows for the six months ended June 30, 2012 and 2013 and the statement of convertible preferred stock and stockholders' equity (deficit) for the six months ended June 30, 2013, as well as the related interim information contained within the notes to the financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission for interim financial information. Accordingly, they do not include all of the information and the notes required by U.S. generally accepted accounting principles (GAAP) for complete financial statements. In the opinion of management, the unaudited interim financial statements reflect all adjustments, consisting of normal and recurring adjustments, necessary for the fair statement of the Company's financial position at June 30, 2013 and results of its operations and its cash flows for the six months ended June 30, 2012 and 2013. The results for the six months ended June 30, 2013 are not necessarily indicative of future results.
Segment Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment, which is the business of discovering, developing
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
and commercializing drugs to treat cancer and other major diseases. All of the Company's revenues are derived in the United States. All material long-lived assets of the Company reside in the United States.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses, net loss and related disclosures. On an ongoing basis, the Company's management evaluates its estimates, including estimates related to clinical trial accruals, stock-based compensation expense and reported amounts of revenues and expenses during the reported period. The Company bases its estimatesfiled on historical experience and other market-specific or other relevant assumptions that it believes to be reasonable under the circumstances. Actual results may differ from those estimates or assumptions. The Company utilizes significant estimates and assumptions in determining the fair value of its common stock. The Company utilized various valuation methodologies in accordance with the framework of the 2004 American Institute of Certified Public Accountants publication,Valuation of Privately-Held Company Equity Securities Issued as Compensation, to estimate the fair value of its common stock. Each valuation methodology includes estimates and assumptions that require the Company's judgment. These estimates and assumptions include a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector, the prices at which the Company sold shares of Preferred Stock, the superior rights and preferences of securities senior to the Company's common stock at the time and the likelihood of achieving a liquidity event, such as an initial public offering or a sale of the Company. Significant changes to the key assumptions used in the valuations could result in different fair values of common stock at each valuation date.
Concentrations of Credit Risk and Off-Balance Sheet Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are primarily cash and cash equivalents. The Company maintains its cash and cash equivalent balances in the form of money market accounts with financial institutions that management believes are creditworthy. The Company's cash and cash equivalent accounts, at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts. The Company believes it is not exposed to any significant credit risk on cash and cash equivalents. The Company has no financial instruments with off-balance-sheet risk of loss.
Principles of Consolidation
The consolidated financial statements include the accounts of Karyopharm Therapeutics Inc. (a Delaware corporation) and the accounts of NPM Pharma Inc. ("NPM", a Canadian corporation), which is a variable interest entity requiring consolidation.
NPM was formed in December 2011 and was 50% owned by the Company until it became a wholly-owned subsidiary in August 2013. NPM was established to procure research and development
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
services in connection with clinical pharmaceutical studies with Canadian vendors on the Company's behalf.
The Company follows the accounting guidance issued by the Financial Accounting Standards Board ("FASB") which requires an entity to consolidate other entities (variable interest entities) where there exists the obligation to absorb a majority of the other entity's expected losses, residual returns, or both. The Company has considered its relationships with NPM to determine whether it has a variable interest in that entity and if so, whether the Company is the primary beneficiary of the relationship.
In determining whether it is the primary beneficiary, the Company considers, among other things, whether it has the authority to direct the activities of the entity that most significantly impact the entity's economic performance, including determining or limiting the scope or purpose of the entity, or the obligation to absorb losses or right to receive benefits from the entity that could potentially be significant to the entity. The Company assesses its determination as the primary beneficiary on an ongoing basis. The Company has determined that it is the primary beneficiary of NPM due to its level of control over NPM's operations and its obligation to fund 100% of NPM's operations. As a result, the Company is required to consolidate NPM. The carrying value of the noncontrolling interest in NPM is immaterial, and the Company has not allocated any of NPM's losses to the noncontrolling interest due to its obligation to fund 100% of NPM's losses. All significant intercompany balances and transactions have been eliminated in consolidation.
Total NPM assets and liabilities reflected on the Company's balance sheet are as follows:
| | December 31, | June 30, 2013 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | Actual | Pro forma | |||||||||
Assets: | |||||||||||||
Cash and cash equivalents | $ | — | $ | 12 | $ | 1,031 | $ | 1,031 | |||||
Prepaid expenses and other current assets | 7 | 485 | 328 | 328 | |||||||||
Total assets | $ | 7 | $ | 497 | $ | 1,359 | $ | 1,359 | |||||
Liabilities: | |||||||||||||
Accounts payable | $ | — | $ | 499 | $ | 731 | $ | 731 | |||||
Accrued and other liabilities | 58 | 312 | 13 | 13 | |||||||||
Total liabilities | $ | 58 | $ | 811 | $ | 744 | $ | 744 | |||||
All assets of NPM are restricted to use to settle obligations of NPM. Liabilities of NPM are non-recourse to the Company.
In August 2013, the Company purchased for nominal consideration from the other shareholder the 50% not owned by the Company. As a result NPM is a wholly owned subsidiary of the Company as of this date.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents.
Fair Value Measurements
The Company's financial instruments consist principally of cash and cash equivalents, accounts payable and accrued liabilities. Fair value measurements are classified and disclosed in one of the following three categories:
Financial instruments measured at fair value as of December 31, 2011 and 2012 and June 30, 2013 are classified below based on the three fair value hierarchy tiers described above:
| | | Fair Value Measurements at Reporting Date Using | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Balance | Level 1 | Level 2 | Level 3 | |||||||||
December 31, 2011 | |||||||||||||
Money market funds, included in cash equivalents | $ | 6,340 | $ | — | $ | 6,340 | $ | — | |||||
December 31, 2012 | |||||||||||||
Money market funds, included in cash equivalents | $ | 2 | $ | — | $ | 2 | $ | — | |||||
June 30, 2013 | |||||||||||||
Money market funds, included in cash equivalents | $ | 14,102 | $ | — | $ | 14,102 | $ | — | |||||
The Company measures cash equivalents at fair value on a recurring basis. The fair value of cash equivalents is determined based on "Level 2" inputs.
Property and Equipment, Net
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the respective assets, generally three to five years. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful economic lives of the related assets.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
Revenue Recognition
The Company recognizes revenue in accordance with FASB Accounting Standards Codification ("ASC") Topic 605,Revenue Recognition. The Company recognizes revenue in accordance with the milestone method of revenue recognition for arrangements involving research or development or other performance obligations whereby a portion or all of the consideration is contingent upon achievement of milestone events. Under these provisions, arrangement consideration contingent upon achievement of a milestone is recognized by the Company in the period the milestone is met when the Company concludes that the milestone is substantive. At the inception of each applicable arrangement, the Company assesses each milestone and the consideration payable upon achievement of each milestone and concludes that the milestone is substantive if all of the following criteria are met: (i) the consideration is commensurate with the Company's performance or the enhanced value of a delivered item which is a direct result of the Company's performance to achieve the milestone, (ii) the consideration relates to past performance and there are no refund rights or other penalties related to the consideration based on completion of future performance and (iii) the consideration is reasonable relative to all the deliverables and payment terms within the arrangement. The related consideration for milestones that are considered substantive is recognized in its entirety in the period which the milestone is met.
The milestone method of revenue recognition was applicable to one research agreement which was executed during 2011. This research agreement includes payments upon the achievement of several development milestones as well as an upfront payment. The Company concluded that the upfront payment of $200 did not represent a substantive milestone. Accordingly, the Company recognized this amount on a straight line basis from the date when substantive services commenced through the estimated completion of the final milestone. As of December 31, 2011, none of the milestones had been met and no revenue was recognized. During the year ended December 31, 2012, the Company recognized $634 related to this research agreement, including $134 of the upfront payment. During the six month periods ended June 30, 2012 and June 30, 2013, the Company recognized $567 and $366 related to this agreement, including $67 and $66, respectively related to the upfront payment. For the period from December 22, 2008 (date of inception) to June 30, 2013, the Company recognized revenue of $1.0 million under this research agreement.
During 2010, the Company was awarded a federal grant in the amount of $245 under the Qualifying Therapeutic Discovery Project ("QTDP") related to research performed in 2009 and 2010 of which $93 and $152 was received and recognized in income during the years ended December 31, 2010 and December 31, 2011, respectively. For the period from December 22, 2008 (date of inception) to June 30, 2013, the Company recognized revenue of $245 related to the QTDP.
Organizational Costs
All organizational costs are expensed as incurred.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
Research and Development Expenses
Research and development costs are charged to expense as incurred and include, but are not limited to:
Costs for certain development activities, such as clinical trials, are recognized basedthe description of our common stock contained in our Registration Statement on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations, or information provided to the Company by its vendorsForm 8-A filed on their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development.
Comprehensive Loss
Comprehensive loss consists of net income or loss and changes in equity during a period from transactions and other events and circumstances generated from non-owner sources. The Company's net loss equals comprehensive loss for all periods presented.
Income Taxes
The Company uses the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and the tax reporting basis of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized. The Company has evaluated available evidence and concluded that the Company may not realize the benefit of its deferred tax assets; therefore a valuation allowance has been established for the full amount of the deferred tax assets. The Company's practice is to recognize interest and/or penalties related to income tax matters in income tax expense.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
Stock-Based Compensation Expense
The Company accounts for its stock-based compensation awards in accordance with FASB ASC Topic 718,Compensation—Stock Compensation ("ASC 718"). ASC 718 requires all stock-based payments to employees, including grants of employee stock options and restricted stock and modifications to existing stock options, to be recognized in the consolidated statements of operations and comprehensive loss based on their fair values. The Company uses the Black-Scholes option pricing model to determine the fair value of options granted.
Consistent with the guidance in FASB ASC Topic 505-50,Equity-Based Payments to Non-Employees, the fair value of each non-employee stock option is estimated at the date of grant using the Black-Scholes option pricing model with assumptions generally consistent with those used for employee stock options, with the exception of expected term, which is over the contractual life.
Compensation expense related to awards to employees is recognized on a straight-line basis based on the grant date fair value over the associated service period of the award, which is generally the vesting term. Awards to non-employees are adjusted through stock-based compensation expense as the award vests to reflect the current fair value of such awards, and expensed using an accelerated attribution model.
Recent Accounting Pronouncements
In February 2013, the FASB issued guidance to provide information about the amounts reclassified out of accumulated other comprehensive income ("AOCI") by component. An entity is required to present, either on the face of the financial statements or in the notes, significant amounts reclassified out of AOCI by the respective line items of net income, but only if the amount reclassified is required to be reclassified in its entirety in the same reporting period. For amounts that are not required to be reclassified in their entirety to net income, an entity is required to cross-reference to other disclosures that provide additional details about those amounts. On JanuaryNovember 1, 2013, the Company adopted this standard, which had no impact on its financial positionincluding any amendment or results of operations.
Subsequent Events
The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. The Company has completed an evaluation of all subsequent events through the date the financial statements were issued.
Net Loss per Common Share
Basic net loss per share is calculated by dividing the net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding for the period, without consideration for common stock equivalents or unvested restricted stock. Diluted net loss per
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 2. Summary of Significant Accounting Policies (Continued)
share is computed by dividing the net loss applicable to common stockholders by the weighted average number of common share equivalents outstanding for the period determined using the treasury-stock method. For purposes of this calculation, convertible preferred stock and stock options are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive.
The amounts in the table below were excluded from the calculation of diluted weighted-average shares outstanding, prior to the use of the treasury stock method, due to their anti-dilutive effect:
| | Year Ended December 31, | Six Months Ended June 30, | Period from December 22, 2008 (date of inception) to June 30, 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
Convertible preferred stock | 3,314,393 | 5,587,121 | 4,071,969 | 9,556,818 | 9,556,818 | |||||||||||
Special participation stock | 10,000 | 10,000 | 10,000 | 10,000 | 10,000 | |||||||||||
Outstanding stock options | 1,086,221 | 691,367 | 551,128 | 598,724 | 598,724 | |||||||||||
Unvested restricted stock | 938,143 | 541,218 | 916,327 | 287,326 | 287,326 | |||||||||||
Unaudited pro forma net loss per share is computed using the weighted-average number of shares of common stock outstanding after giving effect to the conversion of all Preferred Stock during the year ended December 31, 2012 and the six months ended June 30, 2013 into shares of the Company's common stock as if such conversion had occurred at the date the Company issued such shares, or committed to issue such shares, or the beginning of the applicable period, as appropriate.
Note 3. Property and Equipment
Property and equipment, net, consists of the following:
| | | December 31, | | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Estimated Useful Life Years | June 30, 2013 | ||||||||||
| | 2011 | 2012 | ||||||||||
Laboratory equipment | 4 | $ | 286 | $ | 314 | $ | 314 | |||||
Furniture and fixtures | 5 | 53 | 90 | 90 | ||||||||
Office and computer equipment | 3 | 44 | 85 | 85 | ||||||||
Leasehold improvements | Lease term | 29 | 44 | 44 | ||||||||
| 412 | 533 | 533 | ||||||||||
Less accumulated depreciation and amortization | (84 | ) | (206 | ) | (275 | ) | ||||||
Property and equipment, net | $ | 328 | $ | 327 | $ | 258 | ||||||
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 3. Property and Equipment (Continued)
Depreciation and amortization expense for the years ended December 31, 2011 and 2012 was $83 and $122, respectively. Depreciation and amortization expense for the six months ended June 30, 2012 and 2013 was $58 and $69, respectively. Depreciation and amortization expense for the period from December 22, 2008 (date of inception) to June 30, 2013 was $275.
Note 4. Research Agreement
In July 2011, the Company entered into a research agreement in which the Company received payments upon the achievement of certain milestones. The agreement requires the Company to pay royalties on product sales, up to a predetermined maximum. The Company must also pay a royalty on any sublicense income, up to a predetermined maximum.
Note 5. Accrued Liabilities
Accrued liabilities at December 31, 2011 and 2012 and June 30, 2013 consisted of the following:
| | December 31, | | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | June 30, 2013 | |||||||||
| | 2011 | 2012 | ||||||||
Payroll and employee-related costs | $ | 278 | $ | 302 | $ | 297 | ||||
Research and development costs | 404 | 333 | 259 | |||||||
Other | 77 | 129 | 222 | |||||||
Total | $ | 759 | $ | 764 | $ | 778 | ||||
Note 6. Stockholders' Equity
Authorized Capital Stock
As of June 30, 2013, the authorized capital stock of the Company consisted of 80,000,000 shares of common stock, $0.0001 par value per share, and 60,350,667 shares of Preferred Stock, of which 20,937,500 shares are designated Series A Preferred Stock at $0.0001 par value per share, 6,100,000 shares are designated Series A-2 Preferred Stock at $0.0001 par value per share, 1,764,706 shares are designated Series A-3 Preferred Stock at $0.0001 par value per share, 1,538,461 shares are designated Series A-4 Preferred Stock at $0.0001 par value per share, 30,000,000 shares are designated Series B Preferred Stock at $0.0001 par value per share and 10,000 shares are designated Special Participation Stock at $0.0001 par value per share.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 6. Stockholders' Equity (Continued)
Common Stock
In October 2010, the Company issued and sold 1,958,210 shares of its common stock, subject to restrictions that vest over time, to the founders of the Company for an aggregate cash consideration of $1. In October 2010, the Company entered into a service contract and, pursuant to the terms of such contract, the Company was obligated to issue shares. The Company issued 162,685 shares of common stock in connection with this service contract. In December 2011, the Company issued and sold 45,454 shares of its common stock, subject to restrictions that vest over time, to two advisors of the Company for an aggregate cash consideration of $12. In accordance with the terms of the restricted stock agreements, the Company has the right (but not an obligation) to repurchase, at the original purchase price, any unvested shares of the restricted common stock in the event of termination of the business relationship. Restricted shares are recorded as outstanding as they vest. There were 938,143, 541,218 and 287,326 shares of unvested restricted common stock at December 31, 2011, December 31, 2012 and June 30, 2013, respectively.
Preferred Stock
In October of 2010, the Company entered into an agreement to issue and sell 20,000,000 shares of Series A Preferred Stock at $1.00 per share, which was effectuated in two tranches. The first closing of the first tranche occurred in October 2010, in which the Company issued and sold 5,000,000 shares of Series A Preferred Stock for gross proceeds of $5.0 million. The remaining 5,000,000 shares of the first tranche were issued and sold in 2011 for gross proceeds of $5.0 million. In accordance with the terms of the Series A Preferred Stock Purchase Agreement, the Company issued and sold an additional 10,000,000 shares of Series A Preferred Stock in the second tranche, of which 2,500,000 shares were sold upon the achievement of a pre-defined milestone, and 2,500,000 shares were sold in each of the following three calendar quarters, for total cash consideration of $10.0 million. As discussed below, the Company met this milestone in June 2012.
In connection with the sale of Series A Preferred Stock, in October 2010, outstanding convertible notes in the amount of $750 were converted into 937,500 shares of Series A Preferred Stock with a value of $938. The difference between the carrying value of the convertible notes and the fair value of the shares issued pursuant to the contractual terms of the conversion of $188 was recorded as a beneficial conversion feature in 2010.
On October 31, 2011, the Company entered into a stock purchase agreement with an existing stockholderreport filed for the purpose of issuing and selling upupdating such description.
Any statement contained in a document incorporated or deemed to 7,000,000 shares of Series A-2 Preferred Stock and the sale of up to 3,000,000 shares of Series A-3 Preferred Stockbe incorporated by reference in two closings.
Pursuant to this agreement, the Company agreed to sell 6,100,000 shares of Series A-2 Preferred Stock at $1.15 per share for gross proceeds of $7.0 million. The Company accounted for this stock purchase as a preferred stock subscription as these shares were not issued until August 2013.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 6. Stockholders' Equity (Continued)
Upon meeting a pre-defined clinical milestone in February 2013, the Company agreed to sell 1,764,706 shares of Series A-3 Preferred Stock at $1.70 per share for gross proceeds of $3.0 million. The Company accounted for this stock purchase as a preferred stock subscription as these shares were not issued until August 2013.
In February 2012, the Company agreed to sell 1,538,461 shares of Series A-4 Preferred Stock at $1.30 per share for gross proceeds of $2.0 million. The Company accounted for this stock purchase as a preferred stock subscription as these shares were not issued until August 2013.
In June 2012, the Company achieved the milestone for the second tranche of the Series A Preferred Stock Agreement. In accordance with the terms of the Agreement, the Company sold 2,500,000 shares of Series A Preferred Stock in June 2012 for $2.5 million and sold 2,500,000 shares of Series A Preferred Stock in each of the following three calendar quarters.
Between April and July 2013, the Company issued and sold an aggregate of 11,600,000 shares of Series B Preferred Stock at a price per share of $2.00 for an aggregate purchase price of $23.2 million. Upon the earlier of (i) September 6, 2013 or (ii) immediately prior to the closing of a firm commitment underwritten initial public offering of the Company's securities pursuant to an effective registration statement filed with the SEC, certain of the Company's existing investors were obligated to purchase an additional 12,500,000 shares of the Company's Series B Preferred Stock at a price per share of $2.00, for gross proceeds of $25.0 million. Such shares were issued in September 2013.
In July 2013, the Company issued and sold an aggregate of 8,636,362 shares of Series B-1 Preferred Stock at a price per share of $2.20 for gross proceeds of $19.0 million.
The Company has evaluated the tranched nature of certain of its Preferred Stock financings, as well as its rights, preferences and privileges of each series of Preferred Stock and has concluded that there are no freestanding derivative instruments or any embedded derivatives requiring bifurcation. All issued and subscribed shares of Preferred Stock are classified outside of Stockholder's Equity (Deficit) due to the presence of deemed liquidation provisions in the underlying agreements. Proceeds received relating to the sale of Series A-2, A-3, A-4 and B Preferred Stock were classified as a subscription until the shares were delivered subsequent to June 30, 2013.
The rights, preferences, and privileges of Series A, A-2, A-3, A-4, B and B-1 Preferred Stock are listed below:
Conversion
Shares of Preferred Stock are initially convertible into common stock on a one-for-one basis, adjustable for certain dilutive events including stock splits. Conversion is at the option of the holders of Preferred Stock, although conversion is automatic upon the consummation of an initial public offering resulting in gross proceeds to the Company of at least $30.0 million at an offering price of at least $16.50 per share (as adjusted for certain dilutive events) before October 15, 2015 or thereafter at least
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 6. Stockholders' Equity (Continued)
$26.40 per share (as adjusted for certain dilutive events). In July 2013, the $16.50 per share offering price requirement was changed to $14.52 per share (as adjusted for certain dilutive events).
Dividends
Holders of the Preferred Stock are entitled to receive dividends in any year, as and if declared by the Board of Directors of the Company. No dividends have been declared since the Company's inception.
Liquidation Preference
Upon liquidation or dissolution, the Preferred Stockholders receive an amount equal to the greater of the original issue price per share for each respective series of Preferred Stock (as adjusted for certain dilutive events) plus any declared but unpaid dividends or the amount that would be payable had the shares been converted into common stock subject to the rights of the holders of the Special Participation Stock.
Voting Rights
Holders of the Preferred Stock are entitled to vote with the holders of common stock, and shall have one vote for each equivalent common share into which the Preferred Stock is convertible. A majority vote of the holders of a particular series of Preferred Stock (other than the Series B Preferred Stock, which requires a vote of at least 67% of the holders of such shares) is required in order to amend, alter or repeal rights, privileges or preferences of such series of Preferred Stock.
Special Participation Stock
Upon the sale of Series A Preferred Stock, the Company amended various restricted stock agreements to allow for the sale and issuance of 10,000 shares of special participation stock ("Special Participation Stock") for $0.0001 per share (par value) for nominal consideration. The fair value of these shares during 2011 and 2012 was immaterial. In accordance with the terms of the amended restricted stock agreements, the Company has the right (but not an obligation) to repurchase, at the original purchase price, any unvested shares of Special Participation Stock in the event of termination of the underlying business relationship. There were 4,556, 2,071 and 828 shares of unvested Special Participation Stock at December 31, 2011, 2012, and June 30, 2013, respectively.
In July 2013, the Company issued 12,121 shares of common stock in settlement of all outstanding shares of Special Participation Stock.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 6. Stockholders' Equity (Continued)
The rights, preferences, and privileges of the Special Participation Stock are listed below:
Dividends
Holders of the Special Participation Stock are entitled to receive dividends for any calendar year during which the Company has non-dilutive financing and an operating profit. The amount of dividendsprospectus will be equal to 10%deemed modified, superseded or replaced for purposes of operating profit allocated pro rata among the shares. No dividends have been recorded to date. In April 2013, such dividend provision was removed.
Liquidation Preference
Upon liquidation or dissolution, the Special Participation Stockholders would receive an amount equal to 15% of the Exit Profit. Exit Profit is defined as a multiple of the amount by which the proceeds from such liquidation or dissolution otherwise allocable to the shares of Series A Preferred Stock and Series A-2 Preferred Stock held by Chione Ltd. exceeds the original issue price per share for such shares. The multiple ranges from seven to eight times depending upon when such liquidation or dissolution occurs. After required distributions to all holders of the Company's Preferred Stock and the Special Participation Stock, any remaining assets available for distribution would be distributed among the common stockholders.
Voting Rights
Generally, the Special Participation Stock is non-voting, however, a majority vote of the holders of Special Participation Stock is required in order to amend, alter or repeal rights, privileges or preferences of the Special Participation Stock.
Note 7. Stock-based Compensation
During 2010, the Company established the 2010 Stock Incentive Plan (the "Plan"). As of June 30, 2013, the maximum number of 1,757,072 shares of the Company's authorized and available common stock may be issued in the form of stock options and other equity interests under the Plan. Under the terms of the Plan, options and other equity interests may be granted to employees, officers, directors, consultants and advisors of the Company. The exercise price of each stock option shall be the fair market value as determined in good faith by the Board of Directors (the Board) at the time each option is granted. The Company has granted service-based options under the Plan. Service-based option grants under the Plan generally vest as follows: 25% of the shares vest one calendar year from the vesting start date, 2.083% of the shares vest on the first day of each month thereafter. The options granted under the Plan generally expire in 10 years.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 7. Stock-based Compensation (Continued)
In connection with all share-based payment awards, total stock-based compensation expense recognized is as follows:
| | Year Ended December 31, | Six Months Ended June 30, | Period from December 22, 2008 (date of inception) to June 30, 2013 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | 2012 | 2013 | ||||||||||||
Research and development | $ | 24 | $ | 605 | $ | 154 | $ | 389 | $ | 1,064 | ||||||
General and administrative | — | 48 | 19 | 57 | 105 | |||||||||||
Total | $ | 24 | $ | 653 | $ | 173 | $ | 446 | $ | 1,169 | ||||||
Stock Options
Total expense related to employee and non-employee stock options for the years ended December 31, 2011 and 2012, and the six months ended June 30, 2012 and 2013, and the period from December 22, 2008 (date of inception) to June 30, 2013 was $17, $264, $78, $187 and $469, respectively
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 7. Stock-based Compensation (Continued)
The following table summarizes stock option activity for employees and nonemployees:
| | Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (years) | Aggregate Intrinsic Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Options outstanding at December 31, 2011 | 1,086,221 | $ | 0.10 | 9.2 | $ | 173 | |||||||
Granted | 176,047 | $ | 1.35 | ||||||||||
Exercised(1) | (498,257 | ) | $ | 0.03 | |||||||||
Forfeited | (72,644 | ) | $ | 0.20 | |||||||||
Options outstanding at December 31, 2012 | 691,367 | $ | 0.46 | 8.8 | $ | 1,666 | |||||||
Granted | 26,484 | $ | 1.49 | ||||||||||
Exercised | (73,232 | ) | $ | 0.40 | |||||||||
Forfeited | (45,895 | ) | $ | 1.49 | |||||||||
Options outstanding at June 30, 2013 | 598,724 | $ | 0.43 | 8.3 | $ | 2,111 | |||||||
Options vested or expected to vest at December 31, 2012(2) | 640,972 | $ | 0.50 | 8.8 | $ | 1,526 | |||||||
Options vested or expected to vest at June 30, 2013(2) | 554,583 | $ | 0.46 | 8.3 | $ | 1,944 | |||||||
Options exercisable at December 31, 2012 | 261,050 | $ | 0.26 | 8.3 | $ | 684 | |||||||
Options exercisable at June 30, 2013 | 251,580 | $ | 0.23 | 7.9 | $ | 942 | |||||||
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 7. Stock-based Compensation (Continued)
The total intrinsic value of stock options exercised in the years ended December 31, 2011 and 2012, the six month periods ended June 30, 2012 and 2013 and for the period December 22, 2008 (date of inception) to June 30, 2013 was $ 0, $115, $115, $212 and $327, respectively.
The Company estimates the fair value of each employee and non-employee stock award on the grant date using the Black-Scholes option-pricing model based on the following assumptions:
| | Year Ended December 31, | Six Months Ended June 30, 2013 | ||||
|---|---|---|---|---|---|---|
| | 2011 | 2012 | ||||
Weighted-average expected volatility | 78% - 79% | 75% - 92% | 83% - 93% | |||
Expected term (in years) | 6.25 - 10 | 6.25 - 10 | 6.25 - 10 | |||
Risk-free interest rate | 1.18% - 2.62% | 0.85% - 1.76% | 1.07% - 2.44% | |||
Expected dividend yield | 0% | 0% | 0% | |||
The Company uses the simplified method as prescribed by the Securities and Exchange Commission Staff Accounting Bulletin No. 107,Share-Based Payment, to calculate the expected term as it does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate the expected term for options granted to employees and utilizes the contractual term for options granted to non-employees. The expected term is applied to the stock option grant group as a whole, as the Company does not expect substantially different exercise or post-vesting termination behavior among its employee population. The computation of expected volatility is based on the historical volatility of a representative group of companies with similar characteristics to the Company, including early stage of product development and therapeutic focus. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected term of the stock options. Management estimates expected forfeitures based on data from a representative group of companies with similar characteristics to the Company and recognizes compensation costs only for those equity awards expected to vest.
Using the Black-Scholes option-pricing model, the weighted-average grant date fair values of options granted during the years ended December 31, 2011 and 2012, and the six month ended June 30, 2012 and 2013, and the period from December 22, 2008 (date of inception) to June 30, 2013 was $0.10, $1.19, $0.20, $2.41 and $0.17 per share, respectively.
As of December 31, 2012 and June 30, 2013 there was $351 and $323, respectively, of total unrecognized compensation cost related to employee and non-employee unvested stock options granted under the Plan. The total unrecognized compensation cost will be adjusted for future forfeitures. As of June 30, 2013, the Company expects to recognize that cost over a remaining weighted-average period of 2.2 years.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 7. Stock-based Compensation (Continued)
Restricted Stock
To date, the Company has granted 1,958,210 shares of restricted stock outside of the Plan and 45,454 shares of restricted stock under the Plan. The following table summarizes the status of the Company's non-vested restricted common shares:
| | Number of Shares | Weighted-Average Grant Date Fair Value | |||||
|---|---|---|---|---|---|---|---|
Non-vested at December 31, 2011 | 938,143 | $ | 0.05 | ||||
Granted | — | — | |||||
Cancelled | — | — | |||||
Vested | (575,547 | ) | 0.05 | ||||
Non-vested at December 31, 2012 | 362,596 | $ | 0.04 | ||||
Granted | — | — | |||||
Cancelled | — | — | |||||
Vested | (211,023 | ) | 0.04 | ||||
Non-vested at June 30, 2013 | 151,573 | $ | 0.05 | ||||
Total vested restricted stock, for the six months ended June 30, 2013, also includes 42,869 shares pursuant to the exercise of unvested stock options during the year ended December 31, 2012. As of June 30, 2012, December 31, 2012 and June 30, 2013, there are 221,491, 178,622 and 135,753 shares of non-vested restricted stock remaining from the exercise, respectively.
The total expense related to employee and non-employee restricted stock for the years ended December 31, 2011 and 2012, and the six months ended June 30, 2012 and 2013, and the period from December 22, 2008 (date of inception) to June 30, 2013 was $7, $389, $95, $259 and $700.
The aggregate fair value of restricted stock awards that vested during the years ended December 31, 2011 and 2012, and the six months ended June 30, 2012 and 2013, and the period from the December 22, 2008 (date of inception) to June 30, 2013 was $0.17, $1.16, $0.20, $3.33 and $1.12, respectively.
As of December 31, 2012 and June 30, 2013, there was $380 and $284, respectively, of total unrecognized compensation cost related to employee and non-employee non-vested restricted stock. As of June 30, 2013, the unrecognized expense is expected to be recognized over a weighted average period of 0.9 years.
Note 8. Income Taxes
The Company provides for income taxes under ASC Topic 740, Accounting for Income Taxes. Under ASC Topic 740, the liability method is used in accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 8. Income Taxes (Continued)
tax bases of assets and liabilities, and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
For the years ended December 31, 2011 and 2012 and for the six month periods ended June 30, 2012 and 2013, the Company did not record a current or deferred income tax expense or benefit.
The components of income (loss) before income taxes were as follows:
| | Year ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | |||||
Foreign | $ | — | $ | — | |||
U.S. | (10,311 | ) | (15,888 | ) | |||
Totals | $ | (10,311 | ) | $ | (15,888 | ) | |
Deferred taxes are recognized for temporary differences between the basis of assets and liabilities for financial statement and income tax purposes. The significant components of the Company's deferred tax assets are comprised of the following:
| | Year ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | |||||
Deferred tax assets: | |||||||
U.S. and state net operating loss carryforwards | $ | 4,694 | $ | 10,621 | |||
Research and development credits | 871 | 871 | |||||
Accruals and other temporary differences | 45 | 335 | |||||
Total deferred tax assets | 5,610 | 11,827 | |||||
Less valuation allowance | (5,610 | ) | (11,827 | ) | |||
Net deferred tax assets | $ | — | $ | — | |||
The Company has evaluated the positive and negative evidence bearing upon the realizability of its deferred tax assets. Based on the Company's history of operating losses, the Company has concluded that it is more likely than not that the benefit of its deferred tax assets will not be realized. Accordingly, the Company has provided a full valuation allowance for deferred tax assets as of December 31, 2011 and 2012. The valuation allowance increased approximately $6.2 million and $4.7 million during the years ended December 31, 2012 and December 31, 2011 respectively, due primarily to the generation of net operating losses during the period.
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 8. Income Taxes (Continued)
A reconciliation of income tax expense computed at the statutory federal income tax rate to income taxes as reflected in the financial statements is as follows:
| | Year ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2011 | 2012 | |||||
Federal income tax expense at statutory rate | 34.0 | % | 34.0 | % | |||
State income tax, net of federal benefit | 7.0 | % | 5.2 | % | |||
Permanent differences | (0.1 | )% | (0.1 | )% | |||
Research and development credit | 3.9 | % | 0.0 | % | |||
Other | 0.0 | % | 0.0 | % | |||
Change in valuation allowance | (44.8 | )% | (39.1 | )% | |||
Effective tax rate | — | % | — | % | |||
As of December 31, 2011 and 2012, the Company had U.S. federal net operating loss carryforwards of approximately $11.8 million and $27.1 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2032. As of December 31, 2011 and 2012, the Company also had U.S. state net operating loss carryforwards of approximately $12.2 million and $27.1 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2017.
As of December 31, 2011 and 2012, the Company had federal research and development tax credit carryforwards of approximately $665 and $665, respectively, available to reduce future tax liabilities which expire at various dates through 2032. As of December 31, 2011 and 2012, the Company had state research and development tax credit carryforwards of approximately $206 and $206, respectively, available to reduce future tax liabilities which expire at various dates through 2027.
Under the provisions of the Internal Revenue Code, the net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. Net operating loss and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders over a three-year period in excess of 50 percent, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has completed several financings since its inception which may have resulted in a change in control as defined by Sections 382 and 383 of the Internal Revenue Code, or could result in a change in control in the future.
The Company will recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2011 and 2012, the Company had no accrued interest or penalties
Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as of June 30, 2013, for the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(in thousands, except share and per share data)
Note 8. Income Taxes (Continued)
related to uncertain tax positions and no amounts have been recognized in the Company's statements of operations.
For all years through December 31, 2012, the Company generated research credits but has not conducted a study to document the qualified activities. This study may result in an adjustment to the Company's research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position for these years. A full valuation allowance has been provided against the Company's research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforwards and the valuation allowance.
The Company or its subsidiary files income tax returns in the United States, and various state and foreign jurisdictions. The federal, state and foreign income tax returns are generally subject to tax examinations for the tax years ended December 31, 2009 through December 31, 2012. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service, state or foreign tax authoritiesprospectus to the extent utilizedthat a statement contained in a future period.
Note 9. Commitments and Contingencies
Lease Commitmentsthis prospectus modifies, supersedes or replaces such statement.
The Company leases its facility under an operating lease that expiresYou may request, orally or in January 2015. In January 2012 and July 2013,writing, a copy of any or all of the Company amended its operating leasedocuments incorporated herein by reference. These documents will be provided to increase the rentable square footage. The Company has the option to renew the lease for an additional two year optionyou at then current market rates which may include escalations in rent payments during the new term. The Company recognizes rent expense on a straight-line basis over the non-cancelable lease term.
As of December 31, 2012, the minimum future rent payments under the lease agreement are as follows:
2013 | $ | 95 | ||
2014 | 98 | |||
2015 | 8 | |||
Total minimum lease payment | $ | 201 | ||
The Company recorded $70, $127, $59 and $63 in rent expense for the years ended December 31, 2011 and 2012 and the six month periods ended June 30, 2012 and 2013, respectively. Total rent expense for the period from December 22, 2008 (date of inception) to June 30, 2013 was $264.
no cost, by contacting: Investor Relations, Karyopharm Therapeutics Inc.(A Development Stage Company)
Notes to Consolidated Financial Statements (Continued)
(Information as, 2 Mercer Road, Natick, MA 01760, (508) 975-4820. In addition, copies of June 30, 2013, forany or all of the Six Months Ended June 30, 2012 and 2013 andthe Period from December 22, 2008 (Date of Inception) to June 30, 2013 is unaudited)
(documents incorporated herein by reference may be accessed at our website at www.karyopharm.com. The information contained in, thousands, except share and per share data)
Note 9. Commitments and Contingencies (Continued)
Litigation
From time to time the Company may face legal claims or actions in the normal course of business. The Company is not currently a party to any litigation and, accordingly,accessible through, our website does not have amounts recorded for any litigation-related matters.
Note 10. Related Party Transactions
The Company incurred expenses for consulting and contract research services with certain shareholdersconstitute part of $889, $109, $24 and $231 for the years ended December 31, 2011 and 2012 and the six months ended June 30, 2012 and 2013, respectively. There are no amounts included in accounts payable or accrued expenses at December 31, 2011 or 2012 or June 30, 2013.
Note 11. Subsequent Events
In October 2013, the Company's board of directors and stockholders approved a one-for-3.3 reverse stock split of the Company's common stock. All common stock share and per share amounts in the financial statements have been retroactively adjusted for all periods presented to give effect to the reverse stock split of our common stock, including reclassifying an amount equal to the reduction in par value to additional paid-in capital.this prospectus.
Through and including , 2013 (the 25th day after the date of this prospectus), all dealers effecting transactions in the Common Stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
5,666,6672,200,000 Shares

Common Stock
PROSPECTUS
BofA Merrill Lynch
Leerink SwannPartners
JMP Securities
Wedbush PacGrow Life Sciences
Oppenheimer & Co.
, 20132014
Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. ("FINRA") filing fee and the NASDAQ Global Stock Market listing fee.
| | Amount | Amount | ||||||
|---|---|---|---|---|---|---|---|---|
Securities and Exchange Commission registration fee | $ | 13,430 | $ | 14,812 | ||||
FINRA filing fee | 16,140 | $ | 17,750 | |||||
NASDAQ Global Stock Market listing fee | 150,000 | |||||||
Accountants' fees and expenses | 500,000 | 150,000 | ||||||
Legal fees and expenses | 1,550,000 | 400,000 | ||||||
Transfer Agent's fees and expenses | 15,000 | 20,000 | ||||||
Printing and engraving expenses | 250,000 | 125,000 | ||||||
Miscellaneous | 105,430 | 22,438 | ||||||
| | | | | |||||
Total expenses | $ | 2,600,000 | 750,000 | |||||
| | | | | |||||
| | | | | |||||
Item 14. Indemnification of Directors and Officers.
Section 102 of the General Corporation Law of the State of Delaware permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificate of incorporation that will be effective following this offering provides that no director of the Registrant shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the General Corporation Law of the State of Delaware prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the General Corporation Law of the State of Delaware provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Our certificate of incorporation that will be effective following this offering provides that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding whether civil, criminal, administrative or investigative (other than an action by or in the
II-1
right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (including any employee benefit plan) (all such persons being referred to as an "Indemnitee"), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees), liabilities, losses, judgments, fines (including excise taxes and penalties arising under the Employee Retirement Income Security Act of 1974), and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our certificate of incorporation that will be effective following this offering provides that we will indemnify any Indemnitee who was or is a party to any threatened, pending, or completed action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (including any employee benefit plan), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys' fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is fairly and reasonably entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys' fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with each of our directors. These indemnification agreements may require us, among other things, to indemnify our directors for some expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by a director in any action or proceeding arising out of his or her service as one of our directors, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
We maintain a liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act of 1933, as amended (the "Securities Act"), against certain liabilities.
II-2
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding shares of capital stock issued by us within the past three years. Also included is the consideration received by us for such shares and information relating
II-2
to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.
No underwriters were involved in the foregoing sales of securities. The securities described in this section (a) of Item 15 were issued to third parties in reliance upon, (i) with respect to the shares of special participation stock issued in November 2010, the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act, or in connection with qualified written compensatory plans or arrangements with our employees, directors, officers, consultants and advisors, in reliance on the exemption from registration provided by Rule 701 promulgated under the Securities Act, and (ii) with respect to the remaining shares described in this section (a) of Item 15, pursuant to Regulation D promulgated under the Securities Act or pursuant to Regulation S promulgated under the Securities Act, in each case related to transactions by an issuer not involving any public offering. All purchasers of shares of preferred stock described above represented to us in connection with their purchase that they were accredited investors, or with respect to the shares of special participation stock issued in November 2010, sophisticated investors with such knowledge and experience in financial matters as to be able to evaluate the merits and risks of an investment in such shares, and were acquiring the shares for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
II-3
II-3
The common stock described in this section (b) of Item 15 was issued pursuant to (i) with respect to the shares issued in July 2013, the exemption from the registration requirements of the Securities Act provided by Section 3(a)(9) of the Securities Act, (ii) with respect to the shares issued in December 2011, qualified written compensatory plans or arrangements with our employees, directors, officers, consultants and advisors, in reliance on the exemption from registration provided by Rule 701 promulgated under the Securities Act, or (iii) with respect to the shares issued in March 2011, and October 2010, in reliance on the exemption from registration provided by Section 4(2) under the Securities Act, relative to transactions by an issuer not involving any public offering. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
Since January 1, 2010,2011 and through March 31, 2014, we have issued to certain employees, directors and consultants options to purchase an aggregate of 2,649,6922,908,677 shares of common stock, of which options issued between January 1, 2011 and March 31, 2014, as of October 23, 2013, of which, as of October 23, 2013,March 31, 2014, options to purchase 636,893460,880 shares of common stock had been exercised, options to purchase 123,873113,836 shares had been forfeited and options to purchase 1,888,9262,505,749 shares of common stock remained outstanding at a weighted-average exercise price of $3.36$9.21 per share.
The issuance of stock options and the common stock issuable upon the exercise of such options as described in this section (c) of Item 15 were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption from the registration requirements of the Securities Act provided by Rule 701 promulgated under the Securities Act, or pursuant to Section 4(2) under the Securities Act. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
II-4
Item 16. Exhibits and Financial Statement Schedules.
The exhibits to this registration statement are listed in the Exhibit Index attached hereto and incorporated by reference herein.
�� The undersigned registrant hereby undertakes to provide to the underwriters, at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-4
The undersigned hereby undertakes that:
II-5
Pursuant to the requirements of the Securities Act, the registrant has duly caused this Amendment No. 1 to the Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the Town of Natick, Commonwealth of Massachusetts, on this 25th day of October, 2013.June, 2014.
| KARYOPHARM THERAPEUTICS INC. | ||||
By: | /s/ MICHAEL G. KAUFFMAN Michael G. Kauffman, M.D., Ph.D. Chief Executive Officer | |||
II-6
SIGNATURES AND POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 1 to the Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
Signature | Title | Date | ||||
|---|---|---|---|---|---|---|
| /s/ MICHAEL G. KAUFFMAN Michael G. Kauffman, M.D., Ph.D. | ||||||
/s/ PAUL BRANNELLY Paul Brannelly | Senior Vice President, Finance and Administration, | |||||
Garen G. Bohlin | Director | |||||
Barry E. Greene | Director | |||||
Deepa R. Pakianathan, Ph.D. | Director | |||||
Mansoor Raza Mirza, M.D. | Director | |||||
Kenneth E. Weg | Director |
| /s/ PAUL BRANNELLY Paul Brannelly, |
II-7
| Exhibit Number | Description of Exhibit | ||
|---|---|---|---|
| Underwriting Agreement | |||
| 1.1 | Underwriting Agreement | ||
| Articles of Incorporation and Bylaws | |||
| 3.1 | |||
| 3.2 | |||
| Instruments Defining the Rights of Security Holders, Including Indentures | |||
| 4.1 | Specimen Stock Certificate evidencing the shares of common | ||
| 4.2 | Third Amended and Restated Investors' Rights Agreement dated as of July 26, | ||
| Opinion re Legality | |||
| 5.1 | Opinion of Wilmer Cutler Pickering Hale and Dorr LLP | ||
| Material Contracts—Management Contracts and Compensatory Plans | |||
| 10.1 | 2010 Stock Incentive | ||
| 10.2 | Forms of Non-Qualified Stock Option Agreement under 2010 Stock Incentive | ||
| 10.3 | 2013 Stock Incentive | ||
| 10.4 | Form of Incentive Stock Option Agreement under 2013 Stock Incentive | ||
| 10.5 | Form of Nonstatutory Stock Option Agreement under 2013 Stock Incentive | ||
| 10.6 | 2013 Employee Stock Purchase | ||
| 10.7 | Employment Agreement dated December 6, 2010 between the Registrant and Michael Kauffman, M.D., Ph.D.(2) | ||
| 10.8 | Employment Agreement dated October 19, 2010, as amended on December 6, 2010, between the Registrant and Sharon Shacham, Ph.D., M.B.A.(2) | ||
| 10.9 | Employment Agreement dated May 29, 2013 between the Registrant and Paul | ||
| 10.10 | Consulting Agreement, dated as of October 28, 2010, between the Registrant and Alan T. | ||
| 10.11 | Consulting Agreement, dated as of September 1, 2012, between the Registrant and Mirza | ||
| 10.12 | Form of Indemnification Agreement between the Registrant and each of its | ||
| 10.13 | Offer Letter from the Registrant to Christopher Primiano dated March 3, 2014(4) | ||
| Material Contracts—Leases | |||
| Lease, dated as of December 10, 2010, as amended on January 10, 2012 and July 29, 2013, between the Registrant and Nivek Investments I, | |||
| LLC(3) | |||
II-8
| Exhibit Number | Description of Exhibit | ||
|---|---|---|---|
| Material Contracts—Research Agreements | |||
| 10.15† | Research Agreement, dated as of July 18, 2011, between the Registrant and the Multiple Myeloma Research Foundation, Inc.(3) | ||
| 10.16 | Office Lease Agreement between NS Wells Acquisition LLC and Karyopharm Therapeutics Inc., dated March 27, 2014(5) | ||
| Additional Exhibits | |||
| 21.1 | Subsidiaries of the | ||
| 23.1 | Consent of McGladrey LLP | ||
| 23.2 | Consent of Wilmer Cutler Pickering Hale and Dorr LLP (included in Exhibit 5.1) | ||
| 24.1 | * | Power of Attorney (included on signature page) | |
| 101.INS XBRL | ** | Instance Document(4) | |
| 101.SCH XBRL | ** | Schema Document(4) | |
| 101.CAL XBRL | ** | Calculation Linkbase Document(4) | |
| 101.LAB XBRL | ** | Labels Linkbase Document(4) | |
| 101.PRE XBRL | ** | Presentation Linkbase Document(4) | |
| 101.DEF XBRL | ** | Definition Linkbase Document(4) | |
II-9