
Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on April 3,May 11, 2015
Registration No. 333-202124
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 13
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ARCADIA BIOSCIENCES, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) | 2870 (Primary Standard Industrial Classification Code Number) | 81-0571538 (I.R.S. Employer Identification Number) |
202 Cousteau Place, Suite 105
Davis, CA 95618
(530) 756-7077
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant's Principal Executive Offices)
Eric J. Rey
President & Chief Executive Officer
202 Cousteau Place, Suite 105
Davis, CA 95618
(530) 756-7077
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent of Service)
| Copies to: | ||||
Karen A. Dempsey, Esq. Christopher J. Austin, Esq. Michael J. Hopp, Esq. Orrick, Herrington & Sutcliffe LLP The Orrick Building 405 Howard Street San Francisco, CA 94105 | Wendy S. Neal, Esq. Vice President & Chief Legal Officer 4222 East Thomas Road, Suite 245 Phoenix, AZ 85018 | Andrew S. Williamson, Esq. Charles S. Kim, Esq. David G. Peinsipp, Esq. Cooley LLP 101 California Street, 5th Floor San Francisco, California 94111 | ||
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) | Smaller reporting company o |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Explanatory note
As a result of filing a certificate of amendment to our first amended and restated certificate of incorporation with the Secretary of State of the State of Delaware on May 8, 2015, to effect the 1-for-4 reverse stock split, the Registrant is filing this Amendment No. 3 ("Amendment No. 3") to its Registration Statement on Form S-1 (File No. 333-202124) (the "Registration Statement") to reflect that the reverse stock split was effected on May 8, 2015, to file the unlegended audit opinion of Deloitte & Touche LLP, to file certain exhibits as indicated in Part II of Amendment No. 3 and to respond to additional comments from the Staff of the Securities and Exchange Commission received on May 8, 2015.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED APRIL 3,MAY 11, 2015
Preliminary Prospectus
7,150,000 Shares

Common Stock
This is the initial public offering of shares of common stock of Arcadia Biosciences, Inc. Prior to this offering, there has been no public market for our common stock. The initial public offering price of our common stock is expected to be between $$13.00 and $$15.00 per share.
We have applied to list ourOur common stock has been approved for listing on The NASDAQ Global Select Market under the symbol "RKDA."
The underwriters have an option to purchase a maximum of 1,072,500 additional shares of common stock from us.
We are an "emerging growth company" as that term is used in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. See "Risk Factors" beginning on page 12.
| | Price to Public | Underwriting Discounts and Commissions(1) | Proceeds to Arcadia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Per Share | $ | $ | $ | |||||||
| Total | $ | $ | $ | |||||||
Entities affiliated with certain of our existing stockholders have indicated an interest in purchasing up to an aggregate of approximately $10.0 million in shares of our common stock in this offering at the initial public offering price. However, because these indications of interest are not binding agreements or commitments to purchase, the underwriters could elect to sell more, fewer or no shares to any of these entities and any of these entities could elect to purchase more, fewer or no shares in this offering. The underwriters will receive the same underwriting discount on any sales to such entities as they will from other shares sold in this offering.
Delivery of the shares of common stock will be made on or about , 2015.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Credit Suisse | J.P. Morgan | Piper Jaffray |
The date of this prospectus is , 2015

TABLE OF CONTENTS
| | Page | |||
|---|---|---|---|---|
PROSPECTUS SUMMARY | 1 | |||
RISK FACTORS | 12 | |||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS | 37 | |||
USE OF PROCEEDS | 38 | |||
DIVIDEND POLICY | 38 | |||
CAPITALIZATION | 39 | |||
DILUTION | 41 | |||
SELECTED CONSOLIDATED FINANCIAL DATA | 43 | |||
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 45 | |||
INDUSTRY OVERVIEW | ||||
BUSINESS | ||||
MANAGEMENT | ||||
EXECUTIVE COMPENSATION | ||||
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS | ||||
PRINCIPAL STOCKHOLDERS | ||||
DESCRIPTION OF CAPITAL STOCK | ||||
SHARES ELIGIBLE FOR FUTURE SALE | ||||
MATERIAL U.S. FEDERAL TAX CONSEQUENCES TO NON-U.S. HOLDERS | ||||
UNDERWRITING | ||||
LEGAL MATTERS | ||||
EXPERTS | ||||
ADDITIONAL INFORMATION | ||||
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | F-1 | |||
You should rely only on the information contained in this prospectus or contained in any free writing prospectus filed with the Securities and Exchange Commission. Neither we nor the underwriters have authorized anyone to provide you with additional information or information different from that contained in this prospectus or in any free writing prospectus filed with the Securities and Exchange Commission. We are offering to sell, and seeking offers to buy, our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock.
Through and including , 2015 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
This prospectus includes statistical, market and industry data and forecasts that we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such publications. We obtained certain trait value data used in the "Prospectus Summary" and "Industry Overview" sections of this prospectus from a third-party report we commissioned Phillips McDougall to prepare. Phillips McDougall has filed a consent to be named in this prospectus. Certain estimates and forecasts involve uncertainties and risks and are subject to change based on various factors, including those discussed under the headings "Special Note Regarding Forward-Looking Statements" and "Risk Factors" in this prospectus.
i
Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who obtain this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside of the United States.
"Arcadia Biosciences," "Sonova" and "Sonova GLA Safflower Oil and design" are our registered trademarks in the United States and, in some cases, in certain other countries. Other trademarks and service marks that we own include: "Sonova 400" and "Sonova ULTRA." This prospectus also contains trademarks, service marks, and trade names of other companies. Solely for convenience, the trademarks, service marks and trade names referred to in this prospectus may appear without the ®, TM, or SM symbols, but such references do not constitute a waiver of any rights that might be associated with the respective trademarks, service marks, or trade names.
ii
This summary highlights selected information that is presented in greater detail elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, including the sections titled "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and the related notes included elsewhere in this prospectus, before deciding whether to purchase shares of our common stock.
Overview
We are a leading agricultural biotechnology trait company with an extensive and diversified portfolio of late-stage yield and product quality traits addressing multiple crops that supply the global food and feed markets. We have achieved this leadership position based on our development collaborations with global agricultural leaders, recognition by third parties, and our track record of success in trait development since our founding in 2002. Our traits are focused on high-value enhancements that increase crop yields by enabling plants to more efficiently manage environmental and nutrient stresses, and that enhance the quality and value of agricultural products. Our traits increase value not only for farmers, but also for users of agricultural products. Our target market is the $39.4 billion global seed market. Our goal is to increase the value of this market significantly by increasing yields, and to capture a portion of the increased value. There currently are more than 50 products in development incorporating our traits and there are 13 that have demonstrated efficacy in field trials, one that is in the process of completing the regulatory process, and one that is currently on the market.
Our crop yield traits are being utilized by our commercial partners to develop higher yielding seeds for the most widely grown global crops, including wheat, rice, soybean, corn, and sugarcane, as well as for other crops such as cotton, canola, turf, and trees. Our business model positions us at the nexus of basic research and commercial product development, as we apply our strong product development and regulatory capabilities to collaborate with, and leverage the skills and investments of, upstream basic research institutions and downstream commercial partners. We believe our approach significantly reduces risk and capital requirements, while simplifying and expediting the product development process. We also believe that our collaboration strategy leverages our internal capabilities, enabling us to capture much higher value than would otherwise be the case, and enabling commercial partners to develop and commercialize products more cost-effectively.
Our business model focuses on creating value by leveraging collaborator investments and capabilities upstream in basic research, and downstream in product development and commercialization. We bridge the gap between basic research and commercial development, reducing risk and adding value as a result. We reduce risk and avoid most of the costs associated with basic research by acquiring trait technologies that have already completed initial feasibility screening, thus achieving proof of concept, through basic research carried out elsewhere. We further develop these technologies by optimizing function and validating performance through intensive field trial testing in multiple crops. We then form collaborations with major seed and consumer product companies who develop and commercialize products incorporating our traits. In select instances, we may also work with our commercial partners to make any regulatory filings required to support commercial launch of the trait in order to increase our share of the value created by the trait. Field trial data to date in multiple major commodity crops has shown yield improvements attributable to our Nitrogen Use Efficiency, or NUE, trait of greater than 10%. For example, rice plants with our NUE trait, tested in independent field trials over three years from 2012 to 2014 in multiple environments, had an average yield improvement of 27% compared to controls.
By licensing later stage de-risked technologies to our commercial partners, we expect to achieve significantly greater value than generally earned for access to early stage traits. Our license agreements
typically include upfront and annual license fees, as well as multiple milestone payments for key product development stages such as demonstration of greenhouse efficacy, demonstration of field efficacy, regulatory submission, regulatory approval, and commercial launch. Following commercialization of a product utilizing one or more of our traits, we share in the value of the traits realized by our commercial partners. We believe that this broad and balanced approach diversifies and reduces risk, allowing us to address multiple end markets through strong established channels.
We have formed strategic partnerships and developed strong relationships with global agricultural leaders for development and commercialization of our traits in major crops and consumer products. Our collaborators include subsidiaries or affiliates of Limagrain (Vilmorin & Cie), Mahyco (Maharashtra Hybrid Seeds Company Limited), Dow AgroSciences, DuPont Pioneer (E.I. du Pont de Nemours and Company), SES Vanderhave, Genective (a joint venture between Limagrain and KWS SAAT), Scotts, U.S. Sugar, Abbott, Ardent Mills, Bioceres, and others. Additionally, in order to increase our participation in the value of two major crops, wheat and soybean, we have formed two joint ventures. Limagrain Cereal Seeds LLC is our joint venture with Limagrain for the development and commercialization of wheat products for North America. Limagrain is the world's fourth-largest seed company. Verdeca LLC is our joint venture with a wholly owned subsidiary of Bioceres for the development and deregulation of soybean traits globally. Bioceres is an agricultural investment and development company owned by approximately 230 shareholders, including some of South America's largest soybean growers. In April 2015, we entered into a collaboration agreement with Dow AgroSciences and Bioceres under which our Verdeca joint venture will collaborate with Dow AgroSciences on the development and deregulation of soybean traits on a global basis.
The strength of our internal capabilities and collaboration strategy enables us to quickly identify and develop valuable traits and bring them to market, as we have demonstrated through commercializing Sonova 400 GLA safflower oil in less than six years from technology acquisition to commercial launch. Sonova 400 GLA safflower oil is a key ingredient in multiple branded nutritional supplements marketed through GNC stores and other major U.S. retailers.
Our headquarters and primary research and development facilities are located in Davis, California. We have additional facilities in Seattle, Washington; American Falls, Idaho; and Phoenix, Arizona. As of March 31, 2015, we had 76 full-time employees.
Industry Background
In recent decades, agricultural biotechnology has been a major driving force for improving farm economics by introducing genetically modified, or GM, seeds, with traits that reduce the cost of managing crop biotic stresses such as weeds, insects, and microbial pests. The first agricultural biotechnology traits, herbicide tolerance and insect resistance, were developed primarily by companies with deep expertise and a long heritage in crop protection chemistry and pest management. Seeds with these traits have achieved rapid growth and strong commercial success, reaching market share in excess of 90% in key crops and countries as of 2013.
Next generation seed trait research is focused on the development of new technologies that address unmet needs such as abiotic stress tolerance and agricultural product quality. Abiotic plant stresses, or those caused by non-living factors such as heat, drought, flooding, salinity, and nutrient availability, can have a significantly greater negative impact on crop yield than biotic stresses. Successfully increasing crop yields by addressing these stresses potentially creates much greater value than created by the first wave of biotic stress management traits. Commercially available solutions to manage abiotic stresses are currently limited, but have been the focus of substantial innovation efforts. Agricultural product quality traits increase the value of crops to crop processors, food and feed manufacturers, and consumers by altering the performance of the harvested crop in end market products.
Innovative traits can provide significant additional value for farmers. Planting seed is a relatively low cost input for farmers, representing less than 10% of average total costs in 2013 according to the
U.S. Department of Agriculture, or USDA. GM seeds can provide farmers with increased profitability at a relatively low increase in operating costs by means of increased yields, reduced costs of inputs such as chemicals, or enhanced product quality. The historic success of increasing farm profits through the use of GM seeds has fueled the development of the agricultural biotechnology industry, and farmers have historically shared a portion of their economic benefit with the GM seed provider in the form of seed premiums.
The following table, based on a Phillips McDougall analysis that we commissioned, sets forth an estimated range of incremental value increase that may be attributed to the addition of a novel, newly developed trait in the most widely grown global crops and key growing regions. Incremental value increase refers to the total revenue potential for seed providers generated from the premium charged on biotechnology seeds due to the added value of the improved trait. This estimated incremental value increase, or trait commercial value, was calculated by multiplying the estimated number of acres per country that could be planted with a particular biotechnology trait by the estimated premiums that will be charged to growers by seed providers. The values displayed are in constant 2013 U.S. Dollar terms.
| | | Estimated Trait Commercial Value ($ Million)(1) | | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | | Key Growing Regions(2) | ||||||||
| | Crop | From | To | |||||||
Trait | | |||||||||
Nitrogen Use Efficiency | Corn | 1,285 | 2,205 | NAFTA, LATAM, China | ||||||
| Soybeans | 747 | 1,269 | NAFTA, LATAM | |||||||
| Cotton | 189 | 312 | NAFTA, LATAM, India+ | |||||||
| Canola | 142 | 227 | NAFTA, LATAM, India, China | |||||||
| Rice | 535 | 910 | India+, China, Asia | |||||||
| Wheat | 573 | 1,136 | NAFTA, Europe, India+, China, Australia | |||||||
| Sugarcane | 42 | 70 | NAFTA, LATAM, India, China | |||||||
| Total | 3,513 | 6,129 | ||||||||
Water Use Efficiency | Corn | 557 | 976 | NAFTA, LATAM, China | ||||||
| Soybeans | 373 | 642 | NAFTA, LATAM | |||||||
| Cotton | 97 | 176 | NAFTA, LATAM, India+ | |||||||
| Canola | 75 | 114 | NAFTA, LATAM, India, China | |||||||
| Rice | 269 | 535 | India+, China, Asia | |||||||
| Wheat | 487 | 569 | NAFTA, Europe, India+, China, Australia | |||||||
| Sugarcane | 25 | 53 | NAFTA, LATAM, India, China | |||||||
| Total | 1,883 | 3,065 | ||||||||
Salinity Tolerance | Soybeans | 373 | 523 | NAFTA, LATAM | ||||||
| Cotton | 97 | 176 | NAFTA, LATAM, India+ | |||||||
| Canola | 75 | 114 | NAFTA, LATAM, India, China | |||||||
| Rice | 269 | 535 | India+, China, Asia | |||||||
| Wheat | 429 | 569 | NAFTA, Europe, India+, China, Australia | |||||||
| Total | 1,243 | 1,917 | NAFTA, LATAM, India, China | |||||||
Heat Tolerance | Corn | 557 | 976 | NAFTA, LATAM, China | ||||||
| Soybeans | 373 | 523 | NAFTA, LATAM | |||||||
| Cotton | 97 | 176 | NAFTA, LATAM, India+ | |||||||
| Canola | 75 | 114 | NAFTA, LATAM, India, China | |||||||
| Rice | 269 | 535 | India+, China, Asia | |||||||
| Wheat | 429 | 569 | NAFTA, Europe, India+, China, Australia | |||||||
| Sugarcane | 21 | 41 | NAFTA, LATAM, India, China | |||||||
| Total | 1,821 | 2,934 | ||||||||
Herbicide Tolerance | Wheat | 417 | 571 | NAFTA, Europe, India+, China, Australia | ||||||
All Traits | All Crops | 8,878 | 14,616 | |||||||
The development of GM seed traits is currently concentrated in a limited number of large seed companies, including Monsanto, DuPont Pioneer, Syngenta, Limagrain, Dow AgroSciences, KWS SAAT, and Bayer CropScience. According to Phillips McDougall, the leading 11 seed and trait companies as a group invested $4.1 billion in seed and trait research and development in 2013.
Our Products and Pipeline
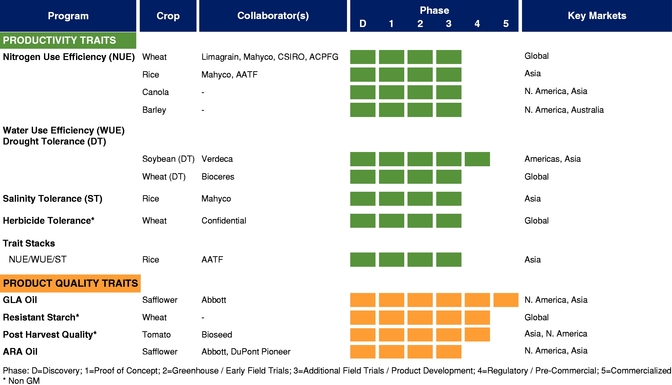
There currently are more than 50 products in development incorporating our traits and there are 13 that have demonstrated efficacy in field trials, one that is in the process of completing the regulatory process, and one that is on the market. We use both GM and non-GM technologies to develop our traits, which enables us to select the approach most suited for the particular trait, crop and market. Our agricultural yield traits are designed to substantially increase crop yields and farmer income. They do so either by improving efficiency in the use of key inputs, such as fertilizer and water, or by increasing tolerance to environmental stresses, such as drought, heat and salinity. Our existing portfolio of agricultural yield traits includes Nitrogen Use Efficiency, or NUE, Water Use Efficiency, or WUE, Drought Tolerance, Salinity Tolerance, Heat Tolerance, and Herbicide Tolerance. Field trial results have demonstrated significant yield improvements resulting from our agricultural yield traits in multiple crops and geographies.
Our agricultural product quality traits are designed to increase the value of harvested products by improving specific compositional qualities of oilseeds and grains. These traits include Enhanced Nutrition Grains and High Value Nutritional Oils, including Sonova 400 GLA safflower oil and Sonova Ultra GLA safflower oil, which we refer to as our Sonova products.
The table below summarizes our current commercial product and our pipeline of products that are in advanced stages of development or on the market, which corresponds to Phase 3 or higher in our product development cycle. The product development cycle is discussed in greater detail in "Industry Overview—Innovation and Commercialization Process in Biotech Seed Traits." The table also identifies the crops, collaborators, and markets that we and our collaborators are addressing with these products.
Our Strengths
We believe we are strategically positioned to capitalize on the need to increase crop yields and quality of agricultural products globally. Our competitive strengths include:
Our Growth Strategy
We believe that there are significant opportunities to grow our business globally by executing the following elements of our strategy:
traits, such as NUE, WUE, and Drought Tolerance, that are in advanced stages of development with our commercial partners and joint ventures.
Risks Associated with Our Business
Our business is subject to numerous risks, as more fully described in the section entitled "Risk Factors" immediately following this prospectus summary. You should read these risk factors before you invest in our common stock. For example, you should be aware of the following before investing in our common stock:
Corporate Information
We were incorporated in 2002 in Arizona and reincorporated in Delaware in March 2015. Our headquarters and primary research and development facilities are located at 202 Cousteau Place, Davis, CA 95618, and our telephone number is (530) 756-7077. Our corporate website address is www.arcadiabio.com. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to purchase our common stock. We have included our website address only as an inactive textural reference and do not intend it to be an active link to our website.
Unless the context otherwise requires, the terms "Arcadia Biosciences," "Arcadia," the "company," "we," "us," and "our" in this prospectus refer to Arcadia Biosciences, Inc. and its consolidated subsidiaries.
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenues during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting requirements that are otherwise applicable generally to public companies. These provisions include:
We will remain an emerging growth company until the earliest to occur of: the last day of the fiscal year in which we have more than $1.0 billion in annual revenues; the date we qualify as a "large accelerated filer," with at least $700 million of equity securities held by non-affiliates; the issuance, in any three-year period, by us of more than $1.0 billion in non-convertible debt securities; and the last day of the fiscal year ending after the fifth anniversary of our initial public offering. We may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We are choosing to irrevocably "opt out" of the extended transition periods available under the JOBS Act for complying with new or revised accounting standards, but we intend to take advantage of the other exemptions discussed above. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
The following information assumes that the underwriters do not exercise their option to purchase additional shares in the offering. See "Underwriting."
Common stock offered by us | 7,150,000 shares | |
Common stock to be outstanding after the offering | 38,036,754 shares | |
Option to purchase additional shares of common stock from us | The underwriters have an option to purchase a maximum of 1,072,500 additional shares of common stock from us. The underwriters can exercise this option at any time within 30 days from the date of this prospectus. | |
Use of proceeds | We intend to use the net proceeds from this offering for general corporate purposes, including working capital, capital expenditures, further development and commercialization of our products, and sales and marketing activities. We may also use a portion of the net proceeds to expand our business through investments in other complementary strategic joint ventures, products, and technologies, although we have no agreements or commitments to do so as of the date of this prospectus. | |
Listing |
| |
Risk factors | Investing in our common stock involves a high degree of risk. You should carefully read and consider the information set forth under "Risk Factors" and all other information in this prospectus before investing in our common stock. |
The number of shares of our common stock to be outstanding after this offering is based on 30,886,754 shares of our common stock outstanding as of December 31, 2014, and excludes:
Except as otherwise indicated, all information in this prospectus assumes:
Entities affiliated with certain of our existing stockholders have indicated an interest in purchasing up to an aggregate of approximately $10.0 million in shares of our common stock in this offering at the initial public offering price. However, because these indications of interest are not binding agreements or commitments to purchase, the underwriters could elect to sell more, fewer or no shares to any of these entities and any of these entities could elect to purchase more, fewer or no shares in this offering.
Summary Consolidated Financial Data
The following tables summarize our consolidated financial data and should be read together with our consolidated financial statements, the notes to our consolidated financial statements and "Management's Discussion and Analysis of Financial Condition and Results of Operations" contained elsewhere in this prospectus. We derived the summary consolidated statements of operations data for the years ended December 31, 2013 and 2014 from our audited consolidated financial statements included elsewhere in this prospectus. The consolidated balance sheet data as of December 31, 2014 is derived from our audited consolidated financial statements included elsewhere in this prospectus.
| | Year Ended December 31, | Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | 2013 | 2014 | ||||||||||
| | (in thousands, except share and per share amounts) | (in thousands, except share and per share amounts) | ||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||
Revenues: | ||||||||||||||
Product | $ | 1,102 | $ | 355 | $ | 1,102 | $ | 355 | ||||||
License | 1,625 | 2,325 | 1,625 | 2,325 | ||||||||||
Contract research and government grants | 3,751 | 4,302 | 3,751 | 4,302 | ||||||||||
| | ||||||||||||||
Total revenues | 6,478 | 6,982 | 6,478 | 6,982 | ||||||||||
Operating expenses: | ||||||||||||||
Cost of product revenues(1) | 673 | 1,997 | 673 | 1,997 | ||||||||||
Research and development(1) | 8,404 | 10,012 | 8,404 | 10,012 | ||||||||||
Selling, general, and administrative(1) | 7,967 | 10,126 | 7,967 | 10,126 | ||||||||||
| | ||||||||||||||
Total operating expenses | 17,044 | 22,135 | 17,044 | 22,135 | ||||||||||
| | ||||||||||||||
Loss from operations | (10,566 | ) | (15,153 | ) | (10,566 | ) | (15,153 | ) | ||||||
Interest expense | (626 | ) | (1,394 | ) | (626 | ) | (1,394 | ) | ||||||
Other income (expense), net | 5 | (597 | ) | 5 | (597 | ) | ||||||||
| | ||||||||||||||
Loss before income taxes and equity in loss of unconsolidated entity | (11,187 | ) | (17,144 | ) | (11,187 | ) | (17,144 | ) | ||||||
Income tax provision | (167 | ) | (263 | ) | (167 | ) | (263 | ) | ||||||
Equity in loss of unconsolidated entity | (1,841 | ) | (932 | ) | (1,841 | ) | (932 | ) | ||||||
| | ||||||||||||||
Net loss | (13,195 | ) | $ | (18,339 | ) | (13,195 | ) | $ | (18,339 | ) | ||||
Accretion of redeemable convertible preferred stock to redemption value | — | (3,738 | ) | — | (3,738 | ) | ||||||||
| | ||||||||||||||
Net loss attributable to common stockholders | $ | (13,195 | ) | $ | (22,077 | ) | $ | (13,195 | ) | $ | (22,077 | ) | ||
| | | |||||||||||||
Net loss per share attributable to common stockholders, basic and diluted(2) | $ | (1.61 | ) | $ | (2.68 | ) | $ | (6.43 | ) | $ | (10.71 | ) | ||
| | | |||||||||||||
Weighted-average number of shares used in per share calculations, basic and diluted(2) | 8,213,544 | 8,245,129 | 2,053,384 | 2,061,278 | ||||||||||
| | | |||||||||||||
Pro forma net loss per share attributable to common stockholders, basic and diluted(2) | $ | $ | (0.62 | ) | ||||||||||
| | | |||||||||||||
Weighted-average number of shares used in pro forma per share calculations, basic and diluted(2) | 29,595,148 | |||||||||||||
| | | |||||||||||||
| | Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | |||||
| | (in thousands) | ||||||
Research and development | $ | 414 | $ | 249 | |||
Selling, general, and administrative | 864 | 727 | |||||
| | | | | | | | |
Total stock-based compensation | $ | 1,278 | $ | 976 | |||
| | | | | | | | |
| | | | | | | | |
| | As of December 31, 2014 | As of December 31, 2014 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma(1) | Pro Forma As Adjusted(2)(3) | Actual | Pro Forma(1) | Pro Forma As Adjusted(2)(3) | ||||||||||||||
| | (in thousands) | (in thousands) | ||||||||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||||||
Cash and cash equivalents | $ | 16,571 | $ | 16,571 | $ | 16,571 | $ | 16,571 | $ | 115,299 | ||||||||||
Working capital | 7,426 | 7,426 | 7,426 | 7,426 | 107,209 | |||||||||||||||
Total assets | 24,889 | 24,889 | 24,889 | 24,889 | 123,617 | |||||||||||||||
Total indebtedness | 14,475 | 14,475 | 14,475 | 14,475 | 24,460 | |||||||||||||||
Redeemable convertible preferred stock | 34,098 | — | 34,098 | — | — | |||||||||||||||
Convertible preferred stock | 48,783 | — | 48,783 | — | — | |||||||||||||||
Common stock | — | 51 | 58 | |||||||||||||||||
Additional paid-in capital | 29,204 | 112,085 | 29,204 | 112,034 | 200,770 | |||||||||||||||
Accumulated deficit | (113,970 | ) | (113,970 | ) | (113,970 | ) | (113,970 | ) | (113,970 | ) | ||||||||||
Total stockholders' (deficit) equity | (84,766 | ) | (1,885 | ) | (84,766 | ) | (1,885 | ) | 86,858 | |||||||||||
Investing in our common stock involves a substantial risk of loss. You should carefully consider the risks and uncertainties described below and the other information in this prospectus before deciding whether to purchase shares of our common stock. If any of the following risks actually occur, our business, financial condition, or operating results could be materially adversely affected. This could cause the trading price of our common stock to decline, and you may lose part or all of your investment. See the section entitled "Special Note Regarding Forward-Looking Statements" elsewhere in this prospectus.
Risks Related to ourOur Business and ourOur Industry
We or our collaborators may not be successful in developing commercial products that incorporate our traits.
Our future growth depends on our ability to identify genes that will improve selected crop traits and license these genes to our collaborators to develop and commercialize seeds that contain the genes. Our long-term growth strategy is based on our expectation that revenues related to the sale of seeds containing our traits will comprise a significant portion of our future revenues. Pursuant to our collaboration agreements, we are entitled to share in the revenues from the sale of products that integrate our trait. We expect that it will take several years before the first seeds integrating our agricultural yield traits complete the development process and become commercially available for sale, resulting in revenues for us. However, the development process could take longer than we anticipate or could ultimately fail to succeed in commercialization for any of the following reasons:
If products containing our traits are never commercialized, or are commercialized on a slower timeline than we anticipate, our ability to generate revenues and become profitable, as well as our long-term growth strategy, would be materially and adversely affected.
Even if we or our collaborators are successful in developing commercial products that incorporate our traits, such products may not achieve commercial success.
Our long-term growth strategy is dependent upon our or our collaborators' ability to incorporate our traits into a wide range of crops with global scope. Even if we or our collaborators are able to develop commercial products that incorporate our traits, any such products may not achieve commercial success as quickly as we project, or at all, for one or more of the following reasons, among others:
Our financial condition and results of operations could be materially and adversely affected if any of the above were to occur.
Our product development cycle is lengthy and uncertain, and we may never earn revenues from the sale of products containing our traits.
Research and development in the seed, agricultural biotechnology, and larger agriculture industries is expensive and prolonged and entails considerable uncertainty. We and our collaborators may spend many years and dedicate significant financial and other resources, including the proceeds of this offering, developing traits that will never be commercialized. The process of discovering, developing, and commercializing a seed trait through either genetic modification or advanced breeding involves multiple phases, and it may require from six to thirteen years or more from discovery to commercialization. The length of the process may vary depending on one or more of the complexity of the trait, the particular crop, and the intended geographical market involved. This long product development cycle is in large part attributable to the nature-driven breeding period for a commercial product, as well as a lengthy regulatory process.
There are currently over 50 products in development incorporating our traits, each of which consists of the application of a specific seed trait to a specific crop. Although our Sonova products are on the market currently, we expect that it will take several years before the first products containing our agricultural yield traits complete the development process and become commercially available. However, we have little to no certainty as to which, if any, of these products will eventually reach commercialization in this timeframe or at all. Because of the long product development cycle and the complexities and uncertainties associated with agricultural biotechnology research, there is significant uncertainty as to whether we will ever generate revenues from the sale of products containing one of our traits and, even if such products reach commercialization, any resulting revenues may come at a later time than we currently anticipate.
We have a history of significant losses, which we expect to continue, and we may never achieve or maintain profitability.
We have incurred significant net losses since our formation in 2002 and expect to continue to incur net losses for the foreseeable future. We incurred net losses of $13.2 million and $18.3 million for the years ended December 31, 2013 and 2014, respectively. As of December 31, 2014, we had an
accumulated deficit of $114.0 million. We expect to continue to incur losses until we begin generating revenues from the sale of traits we are currently developing, which we expect will not occur for several years, if at all. Because we have incurred and will continue to incur significant costs and expenses for these efforts before we obtain any incremental revenues from the sale of seeds incorporating our traits, our losses in future periods could be even more significant. In addition, we may find our development efforts are more expensive than we anticipate or that they do not generate revenues in the time period we anticipate, which would further increase our losses. If we are unable to adequately control the costs associated with operating our business, including costs of development and commercialization of our traits, our business, financial condition, operating results, and prospects will suffer.
In addition, our ability to generate meaningful revenues and achieve and maintain profitability depends on our ability, alone or with strategic collaborators, to successfully complete the development of and complete the regulatory process to commercialize our traits. Most of our revenues since inception have consisted of upfront and milestone payments associated with our contract research and license agreements. Additional revenues from these agreements are largely dependent on successful development of our traits by us or our collaborators. To date, we have not generated any significant revenues from product sales other than from our Sonova products, and we do not otherwise anticipate generating revenues from product sales other than from sales of our Sonova products for the next several years. If products containing our traits fail to achieve market acceptance or generate significant revenues, we may never become profitable.
If ongoing or future field trials by us or our collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis.
The successful completion of field trials in United States and foreign locations is critical to the success of product development and marketing efforts for products containing our traits. If our ongoing or future field trials, or those of our collaborators, are unsuccessful or produce inconsistent results or unanticipated adverse effects on crops or on non-target organisms, or if we or our collaborators are unable to collect reliable data, regulatory review of products in development containing our traits could be delayed or commercialization of products in development containing our traits may not be possible. In addition, more than one growing season may be required to collect sufficient data to develop or market a product containing our traits, and it may be necessary to collect data from different geographies to prove performance for customer adoption. Even in cases where field trials are successful, we cannot be certain that additional field trials conducted on a greater number of acres, or in different crops or geographies, will be successful. Generally, our collaborators conduct these field trials or we pay third parties, such as farmers, consultants, contractors, and universities, to conduct field trials on our behalf. Poor trial execution or data collection, failure to follow required agronomic practices, regulatory requirements, or mishandling of products in development by our collaborators or these third parties could impair the success of these field trials.
Many factors that may adversely affect the success of our field trials are beyond our control, including weather and climatic variations, such as drought or floods, severe heat or frost, hail, tornadoes and hurricanes, pests and diseases, or acts of protest or vandalism. For example, if there was prolonged or permanent disruption to the electricity, climate control, or water supply operating systems in our greenhouses or laboratories, the crops in which we or our collaborators are testing our traits and the samples we or our collaborators store in freezers, both of which are essential to our research and development activities, could be severely damaged or destroyed, adversely affecting these activities and thereby our business and results of operations. Unfavorable weather conditions can also reduce both acreage planted and incidence, or timing of, certain crop diseases or pest infestations, each of which may halt or delay our field trials. We have also experienced crop failures in the past for then-unknown reasons, causing delays in our achievement of milestones and delivery of results and necessitating that we repeat the impacted field trials. Any field test failure we may experience may not be covered by
insurance and, therefore, could result in increased cost for the field trials and development of our traits, which may negatively impact our business and results of operations. Additionally, we are subject to U.S. Department of Agriculture, or USDA, regulations, which may require us to abandon a field trial or to purchase and destroy neighboring crops that are planted after our field trials have commenced. For example, while conducting early field trials for GLA safflower oil, we were forced to purchase and destroy an adjacent safflower crop when the placement of bee hives by a third party altered the required isolation distance between our crop and the neighboring crop, requiring us to either purchase and destroy the adjacent crop or abandon our field trial. In order to prevent the significant delays that would result from terminating our field trial, we decided to purchase and destroy the neighboring crop at a cost of approximately $30,000. Similar factors outside of our control can create substantial volatility relating to our business and results of operations.
Competition in traits and seeds is intense and requires continuous technological development, and, if we are unable to compete effectively, our financial results will suffer.
We face significant competition in the markets in which we operate. The markets for traits and agricultural-biotechnology products are intensely competitive and rapidly changing. In most segments of the seed and agricultural biotechnology market, the number of products available to consumers is steadily increasing as new products are introduced. At the same time, the expiration of patents covering existing products reduces the barriers to entry for competitors. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for products containing our traits. In addition, several of our competitors have substantially greater financial, marketing, sales, distribution, research and development, and technical resources than us, and some of our collaborators have more experience in research and development, regulatory matters, manufacturing, and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Our technologies may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our traits being developed.
We derive a significant portion of our current revenues from government agencies, which may not continue in the future and which may expose us to government audits and potential penalties.
We historically have derived a significant portion of our revenues from grants from U.S. government agencies. Such grants accounted for 44% and 50% of our total revenue in the years ended December 31, 2013 and 2014, respectively. For example, revenues from the U.S. Agency for International Development accounted for 26% and 36% of our total revenues in the years ended December 31, 2013 and 2014, respectively. Our ability to obtain grants is subject to the availability of funds under applicable government programs and approval of our applications to participate in such programs. The application process for these grants is highly competitive. We may not be successful in obtaining any additional grants. Once we successfully obtain a grant, the awarding U.S. government agency has the right to discontinue funding on such a grant at any time. The recent political focus on reducing spending at the U.S. federal and state levels may reduce the scope and amount of funds dedicated to seed and agricultural biotechnology innovations, if such funds continue to be available at all. To the extent that we are unsuccessful in obtaining any additional government grants in the future or if funding is discontinued on an existing grant, we would lose a significant source of our current revenues.
To the extent that we do not comply with the specific requirements of a grant, our expenses incurred may not be reimbursed and any of our existing grants or new grants that we may obtain in the future may be terminated or modified. In addition, our activities funded by our government grants may be subject to audits by U.S. government agencies. As part of an audit, these agencies may review our
performance, cost structures and compliance with applicable laws, regulations and standards, and the terms and conditions of the grant. An audit could result in a material adjustment to our results of operations and financial condition. Moreover, if an audit uncovers improper or illegal activities, we may also be subject to civil and criminal penalties and administrative sanctions, including termination of contracts, forfeiture of profits, suspension of payments, or fines, and we may be suspended or prohibited from doing business with the government. In addition, serious reputational harm or significant adverse financial effects could occur if allegations of impropriety are made against us, even if we are ultimately found to have done no wrong.
A significant portion of our revenues to date are from a limited number of strategic collaborations, and the termination of these collaborations would have a material adverse effect on our results of operations.
We derive a substantial amount of our revenues from a limited number of strategic collaborations, under which we generate revenues through licensing arrangements such as research and development payments, up-front payments, milestone payments, and, once a product is commercialized, a portion of the commercial value of the trait. In particular, revenues from Mahyco accounted for 23% and 29% of our total revenues in the years ended December 31, 2013 and 2014, respectively. A small number of commercial partners are expected to continue to account for a substantial amount of our revenues for the next several years. Our agreements with Mahyco are terminable by Mahyco at will upon 90 days' notice. The termination or non-renewal of our arrangements with Mahyco or our other commercial partners would have a material adverse effect on our business, financial condition, results of operations, and prospects.
We expect to derive a substantial portion of our future revenues from commercial products sold outside the United States, which subjects us to additional business risks.
A significant number of our research and collaboration agreements include products under development for markets outside the United States. Our collaborators' operations in these regions are subject to a variety of risks, including different regulatory requirements, uncertainty of contract and intellectual property rights, unstable political and regulatory environments, economic and fiscal instability, tariffs and other import and trade restrictions, restrictions on the ability to repatriate funds, business cultures accepting of various levels of corruption, and the impact of anti-corruption laws. These risks could result in additional cost, loss of materials, and delays in our commercialization timeline in international markets and have a negative effect on our operating results.
Revenues generated outside the United States could also be subject to increased difficulty in collecting delinquent or unpaid accounts receivables, adverse tax consequences, currency and exchange rate fluctuations, relatively high inflation, exchange control regulations, and governmental pricing directives. Acts of terror or war may impair our ability to operate in particular countries or regions and may impede the flow of goods and services between countries. Customers in these and other markets may be unable to purchase our products if their economies deteriorate, or it could become more expensive for them to purchase imported products in their local currency or sell their commodities at prevailing international prices, and we may be unable to collect receivables from such customers. If any of these risks materialize, our results of operations and profitability could be harmed.
We or our collaborators may fail to perform our respective obligations under contract research and collaboration agreements.
We are obligated under certain contract research agreements to perform research activities over a particular period of time. If we fail to perform our obligations under these agreements, in some cases our collaborators may terminate our agreements with them and in other cases our collaborators' obligations may be reduced and, as a result, our anticipated revenues may decrease. In addition, any of our collaborators may fail to perform their obligations under the diligence timelines in our
collaboration agreements, which may delay development and commercialization of products containing our traits and materially and adversely affect our future results of operations.
Furthermore, the various payments we receive from our collaborators are a significant source of our current revenues and are expected to be the largest source of our revenues in the future. If our collaborators do not make these payments, either due to financial hardship, disagreement under the relevant collaboration agreement, or for any other reason, our results of operations and business could be materially and adversely affected. If disagreements with a collaborator arise, any dispute with such collaborator may negatively affect our relationship with one or more of our other collaborators and may hinder our ability to enter into future collaboration agreements, each of which could negatively impact our business and results of operations.
Most of our collaborators have significant resources and development capabilities and may develop their own products that compete with or negatively impact the advancement or sale of products containing our traits.
Most of our collaborators are significantly larger than us and may have substantially greater resources and development capabilities. As a result, we are subject to competition from many of our collaborators, who could develop or pursue competing products and traits that may ultimately prove more commercially viable than our traits. In addition, former collaborators, by virtue of having had access to our proprietary technology, may utilize this insight for their own development efforts, despite the fact that our collaboration agreements prohibit such use. The development or launch of a competing product by a collaborator may adversely affect the advancement and commercialization of any traits we develop and any associated research and development and milestone payments and value-sharing payments we receive from the sale of products containing our traits.
We rely on third parties to conduct, monitor, support, and oversee field trials and, in some cases, to maintain regulatory files for those products in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our or our collaborators' ability to complete the regulatory process for or commercialize such products.
We rely on third parties, including farmers, to conduct, monitor, support, and oversee field trials. As a result, we have less control over the timing and cost of these trials than if we conducted these trials with our own personnel. If we are unable to maintain or enter into agreements with these third parties on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to conduct and complete our trials in the manner we anticipate. In addition, there is no guarantee that these third parties will devote adequate time and resources to our studies or perform as required by our contract or in accordance with regulatory requirements, including maintenance of field trial information regarding our products in development. If these third parties fail to meet expected deadlines, fail to transfer to us any regulatory information in a timely manner, fail to adhere to protocols, or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then field trials of our products in development may be extended or delayed with additional costs incurred, or our data may be rejected by the USDA, the U.S. Food and Drug Administration, or FDA, the U.S. Environmental Protection Agency, or EPA, or other regulatory agencies. Ultimately, we are responsible for ensuring that each of our field trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our responsibilities.
If our relationship with any of these third parties is terminated, we may be unable to enter into arrangements with alternative parties on commercially reasonable terms, or at all. Switching or adding farmers or other suppliers can involve substantial cost and require extensive management time and focus. In addition, there is a natural transition period when a new farmer or other third party commences work. As a result, delays may occur, which can materially impact our ability to meet our
desired development timelines. If we are required to seek alternative supply arrangements, the resulting delays and potential inability to find a suitable replacement could materially and adversely impact our business.
Our prospects for successful development and commercialization of our products are dependent upon the research, development, commercialization, and marketing efforts of our collaborators.
We primarily rely on third parties for research, development, commercialization, and marketing of our products and products in development. Other than as provided for in our collaboration agreements, we have no control over the resources, time and effort that our collaborators may devote to the development of products incorporating our traits, and have limited access to information regarding or resulting from such programs. We are dependent on our third party collaborators to fund and conduct the research and development of product candidates, to complete the regulatory process, and for the successful marketing and commercialization of one or more of such products or products in development. Such success will be subject to significant uncertainty.
Our ability to recognize revenues from successful collaborations may be impaired by multiple factors including:
If our collaborators do not perform in the manner we expect or fulfill their responsibilities in a timely manner, or at all, the development, regulatory, and commercialization process could be delayed, terminated, or otherwise unsuccessful. Conflicts between us and our collaborators may arise. In the event of termination of one or more of our collaboration agreements, it may become necessary for us to assume the responsibility for any terminated products or products in development at our own expense or seek new collaborators. In that event, we likely would be required to limit the size and scope of one or more of our independent programs or increase our expenditures and seek additional funding, which may not be available on acceptable terms or at all, and our business may be materially and adversely affected.
Our joint venture agreements could present a number of challenges that may have a material adverse effect on our business, financial condition, and results of operations.
We currently participate in two joint ventures, Limagrain Cereal Seeds LLC, which focuses on the development and commercialization of improved wheat seeds, and Verdeca LLC, which focuses on the development and deregulation of soybean traits, and we may enter into additional joint ventures in the future. Our joint venture arrangements may present financial, managerial, and operational challenges, including potential disputes, liabilities, or contingencies and may involve risks not otherwise present when operating independently, including:
The risks described above or the failure to continue any joint venture or joint development arrangement or to resolve disagreements with our current or future joint venture partners could materially and adversely affect our ability to transact the business that is the subject of such joint venture, which would in turn negatively affect our financial condition and results of operations.
We and our collaborators may disagree over our right to receive payments under our collaboration agreements, potentially resulting in costly litigation and loss of reputation.
Our ability to receive payments under our collaboration agreements depends on our ability to clearly delineate our rights under those agreements. We typically license our intellectual property to our collaborators, who then develop and commercialize seeds with improved traits. However, a collaborator may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights, or argue that our intellectual property does not cover, or add value to, their marketed product. If a dispute arises, it may result in costly patent office procedures and litigation, and our collaborator may refuse to pay us while the dispute is ongoing. Furthermore, regardless of any resort to
legal action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator and may also harm our reputation in the industry.
Even if we are entitled to payments from our collaborators, we may not actually receive these payments, or we may experience difficulties in collecting the payments to which we believe we are entitled. After our collaborators launch commercial products containing our licensed traits, we will need to rely on the good faith of our collaborators to report to us the sales they earn from these products and to accurately calculate the payments we are entitled to, a process that will involve complicated and difficult calculations. Although we seek to address these concerns in our collaboration agreements by reserving our right to audit financial records, such provisions may not be effective.
Our business is subject to various government regulations and if we or our collaborators are unable to timely complete the regulatory process for our products in development, our or our collaborators' ability to market our traits could be delayed, prevented or limited.
Our business is generally subject to two types of regulations: regulations that apply to how we and our collaborators operate and regulations that apply to products containing our traits. We apply for and maintain the regulatory permits necessary for our operations, particularly those covering our field trials, while we or our collaborators apply for and maintain regulatory approvals necessary for the commercialization of products containing our seed traits. The large-scale field trials that our collaborators conduct during advanced stages of product development are subject to regulations similar to those to which we are subject. Pursuant to our collaboration agreements, our collaborators also apply for the requisite regulatory approvals prior to commercialization of products containing our traits. In most of our key target markets, regulatory approvals must be received prior to the importation of genetically modified products. These regulatory processes may be complex; for example, the U.S. federal government's regulation of biotechnology is divided among the EPA, which regulates activity related to the use of plant pesticides and herbicides, the USDA, which regulates the import, field testing, and interstate movement of specific technologies that may be used in the creation of genetically modified plants, and the FDA, which regulates foods derived from new plant varieties. In addition to regulation by the U.S. government, products containing our biotech traits may be subject to regulation in each country in which such products are tested or sold. International regulations may vary from country to country and from those of the United States. The difference in regulations under U.S. law and the laws of foreign countries may be significant and, in order to comply with the laws of foreign countries, we may have to implement global changes to our products or business practices. Such changes may result in additional expense to us and either reduce or delay product development or sales. Additionally, we or our collaborators may be required to obtain certifications or approvals by foreign governments to test and sell the products in foreign countries.
The regulatory process is expensive and time-consuming, and the time required to complete the process is difficult to predict and depends upon numerous factors, including the substantial discretion of the regulatory authorities. Other than our Sonova products, neither we nor our collaborators have completed the regulatory process for any of our products in development. Our traits could require a significantly longer time to complete the regulatory process than expected, or may never gain approval, even if we and our collaborators expend substantial time and resources seeking such approval. A delay or denial of regulatory approval could delay or prevent our ability to generate revenues and to achieve profitability. For example, we are currently awaiting completion of the regulatory process for one of our Sonova products to be used in pet food, which has taken longer than expected. Changes in regulatory review policies during the development period of any of our traits, changes in, or the enactment of, additional regulations or statutes, or changes in regulatory review practices for a submitted product application may cause a delay in obtaining approval or result in the rejection of an application for regulatory approval. Regulatory approval, if obtained, may be made subject to limitations on the indicated uses for which we or our collaborators may market a product. These limitations could
adversely affect our potential revenues. Failure to comply with applicable regulatory requirements may, among other things, result in fines, suspensions of regulatory approvals, product recalls, product seizures, operating restrictions, and criminal prosecution. We have on certain occasions notified the USDA of instances of noncompliance with regulations. Although these occasions did not result in any enforcement actions, we may have occasions of noncompliance in the future that result in USDA or other governmental agency enforcement action.
Consumer resistance to genetically modified organisms may negatively affect our public image and reduce sales of seeds containing our traits.
We are active in the field of agricultural biotechnology research and development in seeds and crop protection, including GM seeds. Foods made from such seeds are not accepted by many consumers due to concerns over such products' effects on food safety and the environment. The high public profile of biotechnology in food production and lack of consumer acceptance of products to which we have devoted substantial resources could negatively affect our public image and results of operations. The current resistance from consumer groups, particularly in Europe, to GM crops not only limits our access to such markets but also has the potential to spread to and influence the acceptance of products developed through biotechnology in other regions of the world. For example, in the United States, organizations have advocated for the labeling of food products containing GM ingredients, three states (Connecticut, Maine, and Vermont) have passed GM labeling legislation, and more than 20 states introduced legislation or ballot initiatives in 2014 that would require GM labeling. These labeling-related initiatives have heightened consumer awareness of GM crops generally and may make consumers less likely to purchase food products containing GM ingredients, which could have a negative impact on the commercial success of products that incorporate our traits and materially and adversely affect our financial condition and results of operations.
Governmental restrictions on the production of GM crops may negatively affect our business and results of operations.
The production of certain GM crops is effectively prohibited in certain countries, including throughout the European Union, which limits our commercial opportunities and may influence regulators in other countries to limit or ban production of GM crops. Our GM crops are grown principally in North America, South America, and Australia, where there are fewer restrictions on the production of GM crops. If these or other countries where our GM crops are grown enact laws or regulations that ban the production of such crops or make regulations more stringent, we could experience a longer product development cycle for our products, encounter difficulty obtaining intellectual property protection, and may even have to abandon projects related to certain crops or geographies, any of which would negatively affect our business and results of operations. Furthermore, any changes in such laws and regulations or consumer acceptance of our GM crops could negatively impact our collaborators, who in turn might terminate or reduce the scope of their collaborations with us or seek to alter the financial terms of our agreements with them.
Changes in laws and regulations to which we are subject, or to which we may become subject in the future, may materially increase our costs of operation, decrease our operating revenues, and disrupt our business.
Laws and regulatory standards and procedures that impact our business are continuously changing. Responding to these changes and meeting existing and new requirements may be costly and burdensome. Changes in laws and regulations could:
Any of these events could have a material adverse effect on our business, results of operations, and financial condition. Legislators and regulators have increased their focus on plant biotechnology in recent years, with particular attention paid to GM crops.
Our future growth relies on the ability of our collaborators to commercialize and market our products in development, and any restrictions on such activities could materially and adversely impact our business and results of operations. Any changes in regulations in countries where GM crops are grown or imported could result in our collaborators being unable or unwilling to develop, commercialize, or sell products that incorporate our traits. Any changes to these existing laws and regulations may also materially increase our costs of operation, decrease our operating revenues, and disrupt our business. See "Business—Regulatory Matters."
The unintended presence of our traits in other products or plants may negatively affect us.
Trace amounts of our traits may unintentionally be found outside our containment area in the products of third parties, which may result in negative publicity and claims of liability brought by such third parties against us. Furthermore, in the event of an unintended dissemination of our genetically engineered materials to the environment or the presence of unintended but unavoidable trace amounts, sometimes called "adventitious presence," of our traits in conventional seed, or in the grain or products produced from conventional or organic crops, we could be subject to claims by multiple parties, including environmental advocacy groups, as well as governmental actions such as mandated crop destruction, product recalls, or additional stewardship practices and environmental cleanup or monitoring.
Loss of or damage to our germplasm collection would significantly slow our product development efforts.
We have developed and maintain a comprehensive collection of germplasm through strategic collaborations with leading institutions, which we utilize in our non-GM programs. Germplasm comprises collections of genetic resources covering the diversity of a crop, the attributes of which are inherited from generation to generation. Germplasm is a key strategic asset since it forms the basis of seed development programs. To the extent that we lose access to such germplasm because of the termination or breach of our collaboration agreements, our product development capabilities would be severely limited. In addition, loss of or damage to these germplasm collections would significantly impair our research and development activities. Although we restrict access to our germplasm at our research facilities to protect this valuable resource, we cannot guarantee that our efforts to protect our germplasm collection will be successful. The destruction or theft of a significant portion of our germplasm collection would adversely affect our business and results of operations.
We depend on our key personnel and, if we are not able to attract and retain qualified scientific and business personnel, we may not be able to grow our business or develop and commercialize our products.
We depend heavily on the skills, expertise and legacy knowledge of principal members of our management, including Eric J. Rey, our President and Chief Executive Officer, and Vic C. Knauf, our Chief Scientific Officer, the loss of whose services might significantly delay or prevent the achievement of our scientific or business objectives.
Additionally, the vast majority of our workforce is involved in research, development, and regulatory activities. Our business is therefore dependent on our ability to recruit and maintain a highly skilled and educated workforce with expertise in a range of disciplines, including molecular biology, biochemistry, plant genetics, agronomics, mathematics, agribusiness, and other subjects relevant to our operations. All of our current employees are at-will employees, and the failure to retain or hire skilled and highly educated personnel could limit our growth and hinder our research and development efforts.
Many of our employees have become or will soon become vested in a substantial number of stock options. Our employees may be more likely to leave us if the shares they own or the shares underlying their vested options have significantly appreciated in value relative to the original purchase prices of the shares or the exercise prices of the options. Further, our employees' ability to exercise those options and sell their stock in a public market after the closing of this offering may result in a higher than normal turnover rate.
Our development activities are currently conducted at a limited number of locations, which makes us susceptible to damage or business disruptions caused by natural disasters.
Our headquarters, certain research and development operations and our seed storage warehouse are located in Davis, California. We also conduct certain research and development operations and store certain biomaterials in Seattle, Washington. The safflower grain used in the production of our Sonova products is grown in several locations throughout Idaho and is stored in a single facility in Idaho. Our production of our Sonova products takes place at a single facility in Northern California, and the inventory is stored in a single cold storage facility in Northern California. We take precautions to safeguard our facilities, including insurance, health and safety protocols, and off-site storage of critical research results and computer data. However, a natural disaster, such as a fire, flood, or earthquake, could cause substantial delays in our operations, damage or destroy our equipment, inventory, or development projects, and cause us to incur additional expenses. The insurance we maintain against natural disasters may not be adequate to cover our losses in any particular case.
Interruptions in the production or transportation of raw materials used in our Sonova products could adversely affect our operations and profitability.
The production of our Sonova products requires that sufficient quantities of certain raw materials, including our GLA safflower grain grown in Idaho, be timely delivered to our service provider's production facility in Northern California. Our dependency upon timely deliveries means that interruptions or stoppages in such deliveries, or delays or limitations with respect to the production of such raw materials, could adversely affect our operations until alternative arrangements could be made. If we were unable to obtain the necessary raw materials for an extended period of time for any reason, our business, customer relations, and operating results could suffer.
Disruption to our IT system could adversely affect our reputation and have a material adverse effect on our business and results of operations.
Our technologies rely on our IT system to collect and analyze our genomic data, including TILLING and other experimental data, and manage our plant inventory system, which tracks every plant that we have ever produced. We can provide no assurance that our current IT system is fully
protected against third-party intrusions, viruses, hacker attacks, information, or data theft, or other similar threats. Furthermore, we store significant amounts of data and, though we are developing back-up storage for our stored data, we can not assure you that our back-up storage arrangements will be effective if it becomes necessary to rely on them.
If our IT system does not function properly or proves incompatible with new technologies, we could experience interruptions in data transmissions and slow response times, preventing us from completing routine research and business activities. Furthermore, disruption or failure of our IT system due to technical reasons, natural disaster, or other unanticipated catastrophic events, including power interruptions, storms, fires, floods, earthquakes, terrorist attacks, and wars could significantly impair our ability to deliver data related to our projects to our collaborators on schedule and materially and adversely affect the outcome of our collaborations, our relationships with our collaborators, our business, and our results of operations.
Our use of hazardous materials exposes us to potential liabilities.
Certain of our operations involve the storage and controlled use of hazardous materials, including herbicides and pesticides. This requires us to conduct our operations in compliance with applicable environmental and safety standards, and we cannot completely eliminate the risk of accidental contamination from hazardous materials. In the event of such contamination, we may be held liable for significant damages or fines, which could have a material adverse effect on our business and operating results.
Most of the licenses we grant to our collaborators to use our proprietary genes in certain crops are exclusive within certain jurisdictions, which limits our licensing opportunities.
Most of the licenses we grant our collaborators to use our proprietary genes in certain crops are exclusive within specified jurisdictions, so long as our collaborators comply with certain diligence requirements. That means that once genes are licensed to a collaborator in a specified crop or crops, we are generally prohibited from licensing those genes to any third party. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our product development initiatives with new collaborators, both of which could adversely affect our business and results of operations.
Our business model for discovery of genes is dependent on licensing patent rights from third parties, and any disruption of this licensing process could adversely affect our competitive position and business prospects.
Our business model involves acquiring technologies that have achieved proof of concept through rigorous development and testing by third-party basic researchers in order to avoid the significant risks and high costs associated with basic research. Only a small number of the genes we evaluate for acquisition are likely to provide viable commercial candidates and an even more limited number, if any, are likely to be commercialized by us or our collaborators. A failure by us to continue identifying genes that improve specific crop traits could make it difficult to grow our business. If we are unable to identify additional genes, we may be unable to develop new traits, which may negatively impact our ability to generate revenues.
If we are unable to enter into licensing arrangements to acquire rights to these potentially viable genes on favorable terms in the future, it may adversely affect our business. In addition, if the owners of the patents we license do not properly maintain or enforce the patents underlying such licenses, our competitive position and business prospects could be harmed. Without protection for the intellectual property we license, other companies might be able to offer substantially similar or identical products for sale, which could adversely affect our competitive business position and harm our business prospects.
If we fail to comply with our obligations under license agreements, our counterparties may have the right to terminate these agreements, in which event we may not be able to develop, manufacture, register, or market, or may be forced to cease developing, manufacturing, registering, or marketing, any product that is covered by these agreements or may face other penalties under such agreements. Such an occurrence could materially adversely affect the value of the applicable products to us and have an adverse effect on our business and result of operations.
Our success depends on our ability to protect our intellectual property and our proprietary technologies.
Our commercial success depends, in part, on our ability to obtain and maintain patent and trade secret protection for our proprietary technologies, our traits, and their uses, as well as our ability to operate without infringing upon the proprietary rights of others. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially and adversely affected and our business could be harmed.
We treat our proprietary technologies, including unpatented know-how and other proprietary information, as trade secrets. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with any third parties who have access to them, such as our consultants, independent contractors, advisors, corporate collaborators, and outside scientific collaborators. We also enter into confidentiality and invention or patent assignment agreements with employees and certain consultants. Any party with whom we have executed such an agreement could breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time consuming, and the outcome is unpredictable. In addition, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, or if we otherwise lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced and our business and competitive position could be harmed.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products in development.
As an agricultural biotechnology company, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents involves technological and legal complexity, and is costly, time consuming, and inherently uncertain. In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that may weaken or undermine our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, maintaining, and defending patents on products in development in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some
countries outside the United States are less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. For example, several countries outside the United States prohibit patents on plants and seeds entirely. In addition, we may at times license third-party technologies for which limited international patent protection exists and for which the time period for filing international patent applications has passed. Consequently, we are unable to prevent third parties from using intellectual property we develop or license in all countries outside the United States, or from selling or importing products made using our intellectual property in and into the jurisdictions in which we do not have patent protection. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and we may be unable to prevent such competitors from importing those infringing products into territories where we have patent protection, but where enforcement is not as strong as in the United States. These products may compete with our products in development and our patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions. Moreover, farmers or others in the chain of commerce may raise legal challenges to our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult to prevent or detect, and local regulators may choose to not enforce our intellectual property rights.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions where we have filed patent applications. The legal systems of certain countries have not historically favored the enforcement of patents or other intellectual property rights, which could hinder us from preventing the infringement of our patents or other intellectual property rights and result in substantial risks to us. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful or even cover our associated legal costs. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
If we or one of our collaborators are sued for infringing the intellectual property rights of a third party, such litigation could be costly and time consuming and could prevent us or our collaborators from developing or commercializing our products.
Our ability to generate significant revenues from our products depends on our and our collaborators' ability to develop, market and sell our products and utilize our proprietary technology without infringing the intellectual property and other rights of any third parties. In the United States and abroad there are numerous third-party patents and patent applications that may be applied toward our proprietary technology, business processes, or developed traits, some of which may be construed as containing claims that cover the subject matter of our products or intellectual property. Because of the rapid pace of technological change, the confidentiality of patent applications in some jurisdictions (including U.S. provisional patent applications), and the fact that patent applications can take many years to issue, there may be currently pending applications that are unknown to us that may later result in issued patents upon which our products in development or proprietary technologies infringe. Similarly, there may be issued patents relevant to our products in development of which we are not aware. These patents could reduce the value of the traits we develop or the genetically modified plants containing our traits or, to the extent they cover key technologies on which we have unknowingly relied, require that we seek to obtain licenses or cease using the technology, no matter how valuable to our business. We may not be able to obtain such a license on commercially reasonable terms. If any third party patent or patent application covers our intellectual property or proprietary rights and we are not
able to obtain a license to it, we and our collaborators may be prevented from commercializing products containing our traits.
As the agricultural biotechnology industry continues to develop, we may become party to, or threatened with, litigation or other adverse proceedings regarding intellectual property or proprietary rights in our technology, processes, or developed traits. Third parties may assert claims based on existing or future intellectual property rights and the outcome of any proceedings is subject to uncertainties that cannot be adequately quantified in advance. Any litigation proceedings could be costly and time consuming, and negative outcomes could result in liability for monetary damages, including treble damages and attorneys' fees, if we are found to have willfully infringed a patent. There is also no guarantee that we would be able to obtain a license under such infringed intellectual property on commercially reasonable terms or at all. A finding of infringement could prevent us or our collaborators from developing, marketing or selling a product or force us to cease some or all of our business operations. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel may be diverted as a result of these proceedings, which could have a material adverse effect on us. Claims that we have misappropriated the confidential information or trade secrets of third parties could similarly have a negative impact on our business.
Our results of operations will be affected by the level of royalty payments that we are required to pay to third parties.
We are a party to license agreements that require us to remit royalty payments and other payments related to in-licensed intellectual property. Under our in-license agreements, we may pay up-front fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. As our product sales increase, we may, from time to time, disagree with our third-party collaborators as to the appropriate royalties owed and the resolution of such disputes may be costly and may consume management's time. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
We are subject to governmental export and import controls that could impair our ability to compete in international markets due to licensing requirements and subject us to liability if we are not in compliance with applicable laws.
Our products and products in development are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department's Office of Foreign Assets Controls. Exports of our products and technology must be made in compliance with these laws and regulations. If we fail to comply with these laws and regulations, we and certain of our employees could be subject to substantial civil or criminal penalties, including the possible loss of export or import privileges; fines, which may be imposed on us and responsible employees or managers; and, in extreme cases, the incarceration of responsible employees or managers.
In addition, changes in our products or solutions or changes in applicable export or import laws and regulations may create delays in the introduction and sale of our products and solutions in international markets, prevent our customers from deploying our products and solutions or, in some cases, prevent the export or import of our products and solutions to certain countries, governments or persons altogether. Any change in export or import laws and regulations, shift in the enforcement or scope of existing laws and regulations, or change in the countries, governments, persons or technologies targeted by such laws and regulations, could also result in decreased use of our products and solutions, or in our decreased ability to export or sell our products and solutions to existing or potential
customers. Any decreased use of our products and solutions or limitation on our ability to export or sell our products and solutions would likely adversely affect our business, financial condition and results of operations.
We are subject to anti-corruption and anti-money laundering laws with respect to both our domestic and international operations, and non-compliance with such laws can subject us to criminal and civil liability and harm our business.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and possibly other anti-bribery and anti-money laundering laws in countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit us and our collaborators from authorizing, offering, or directly or indirectly providing improper payments or benefits to recipients in the public or private sector. We or our collaborators may have direct and indirect interactions with government agencies and state-affiliated entities and universities in the course of our business. We may also have certain matters come before public international organizations such as the United Nations. We use third-party collaborators, joint venture and strategic partners, law firms, and other representatives for regulatory compliance, patent registration, lobbying, deregulation advocacy, field testing, and other purposes in a variety of countries, including those that are known to present a high corruption risk such as India, China, and Latin American countries. We can be held liable for the corrupt or other illegal activities of these third-party collaborators, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize such activities. In addition, although we have implemented policies and procedures to ensure compliance with anti-corruption and related laws, there can be no assurance that all of our employees, representatives, contractors, partners, or agents will comply with these laws at all times. Noncompliance with these laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other enforcement actions, disgorgement of profits, significant fines, damages, other civil and criminal penalties or injunctions, suspension and debarment from contracting with certain governments or other persons, the loss of export privileges, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations, and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management's attention and resources and significant defense costs and other professional fees. Enforcement actions and sanctions could further harm our business, results of operations, and financial condition.
We may require additional financing in the future and may not be able to obtain such financing on favorable terms, if at all, which could force us to delay, reduce, or eliminate our research and development activities.
We will continue to need capital to fund our research and development projects and to provide working capital to fund other aspects of our business. If our capital resources are insufficient to meet our capital requirements, we will have to raise additional funds. If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we are able to raise additional debt financing, which will require the consent of our current debt holders, we may be subject to additional restrictive covenants that limit our operating flexibility. We may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, develop and commercialize products or technologies, or otherwise respond to competitive pressures could be significantly limited. If this happens, we may be forced to delay or terminate research and development programs or the commercialization of products or curtail operations. If adequate funds are not available, we will not be able to successfully execute on our business strategy or continue our business.
Adverse outcomes in future legal proceedings could subject us to substantial damages and adversely affect our results of operations and profitability.
We may become party to legal proceedings, including matters involving personnel and employment issues, personal injury, environmental matters, and other proceedings. Some of these potential proceedings could result in substantial damages or payment awards that exceed our insurance coverage. We will estimate our exposure to any future legal proceedings and establish provisions for the estimated liabilities where it is reasonably possible to estimate and where an adverse outcome is probable. Assessing and predicting the outcome of these matters will involve substantial uncertainties. Furthermore, even if the outcome is ultimately in our favor, our costs associated with such litigation may be material. Adverse outcomes in future legal proceedings or the costs and expenses associated therewith could have an adverse effect on our results of operations.
We may be required to pay substantial damages as a result of product liability claims for which insurance coverage is not available.
We are subject to product liability claims with respect to our Sonova products, and as additional products integrating our traits reach commercialization, product liability claims will increasingly be a commercial risk for our business, particularly as we are involved in the supply of biotechnological products, some of which may be harmful to humans and the environment. Product liability claims against us or our collaborators selling products that contain our traits, or allegations of product liability relating to seeds containing traits developed by us, could damage our reputation, harm our relationships with our collaborators, and materially and adversely affect our business, results of operations, financial condition, and prospects. Furthermore, while our collaboration agreements typically require that our collaborators indemnify us for the cost of product liability claims brought against us as a result of our collaborator's misconduct, such indemnification provisions may not always be enforced, and we may receive no indemnification if our own misconduct contributed to the claims.
We may seek to expand through acquisitions of and investments in other brands, businesses, and assets. These acquisition activities may be unsuccessful or divert management's attention.
We may consider strategic and complementary acquisitions of and investments in other agricultural biotechnology brands, businesses or other assets, and such acquisitions or investments are subject to risks that could affect our business, including risks related to:
We may not be able to identify opportunities or complete transactions on commercially reasonable terms, or at all, or actually realize any anticipated benefits from such acquisitions or investments. Similarly, we may not be able to obtain financing for acquisitions or investments on attractive terms. In addition, the success of any acquisitions or investments also will depend, in part, on our ability to integrate the acquisition or investment with our existing operations.
We will incur significantly increased costs and devote substantial management time as a result of operating as a public company.
As a public company, we will incur significant legal, accounting, and other expenses that we did not incur as a private company. For example, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and will be required to comply with the applicable requirements of the Sarbanes-Oxley Act and the Dodd-Frank Wall Street Reform and Consumer Protection Act, as well as rules and regulations subsequently implemented by the SEC and The NASDAQ Stock Market, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We expect that compliance with these requirements will increase our legal and financial compliance costs and will make some activities more time consuming and costly. In addition, we expect that our management and other personnel will need to divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act, which will increase when we are no longer an emerging growth company, as defined by the JOBS Act. We will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge and may need to establish an internal audit function. We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs. We also expect that operating as a public company will make it more expensive for us to obtain director and officer liability insurance on the terms that we would like. As a public company, it may be more difficult for us to attract and retain qualified people to serve on our board of directors, our board committees, or as executive officers.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
As of December 31, 2014, we had net operating loss carryforwards, or NOLs, for federal income tax reporting purposes of $98.1 million, which begin to expire in 2020, and state NOLs of $77.2 million, which began expiring in 2015. In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an "ownership change" is subject to limitations on its ability to utilize its NOLs to offset future taxable income. Our existing NOLs may be subject to limitations arising from previous ownership changes, and if we undergo an ownership change in connection with or after this offering, our ability to utilize NOLs could be further limited by Section 382 of the Code. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Code. Furthermore, our ability to utilize NOLs of companies that we may acquire in the future may be subject to limitations. There is also a risk that, due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing NOLs could expire or otherwise be unavailable to offset future income
tax liabilities. For these reasons, we may not be able to realize a tax benefit from the use of our NOLs, whether or not we obtain profitability.
We expect our operating results to vary significantly from quarter to quarter, which may cause our stock price to fluctuate widely.
We expect our quarterly operating results to fluctuate widely and unpredictably for the following reasons, among others:
Further, a large proportion of our costs are fixed, due in part to our significant research and development costs and general and administrative expenses. Thus, even a small decline in revenues could disproportionately affect our quarterly operating results and could cause such results to differ materially from expectations. Any unanticipated change in revenues or operating results is likely to cause our stock price to fluctuate since such changes reflect new information available to investors and analysts.
The estimates of market opportunity and forecasts of market growth included in this prospectus may prove to be inaccurate, and even if the market in which we compete achieves the forecasted growth, our business could fail to grow at similar rates, if at all.
Market opportunity estimates and growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. The estimates and forecasts in this prospectus relating to the size and expected growth of the seed and agricultural biotechnology market and the estimated range of incremental value increase that a novel, newly developed trait may produce may prove to be inaccurate. Even if the market in which we compete meets our size estimates and forecasted growth, our business could fail to grow at similar rates, if at all. For more information regarding our estimates of market opportunity, forecasts of market growth, and estimated trait commercial values included in this prospectus, see "Industry Overview."
Risks Related to Our Common Stock and this Offering
An active, liquid and orderly trading market for our common stock may not develop, our stock price may be volatile, and you may be unable to sell your shares at or above the offering price you paid.
Prior to this offering, there has not been a public market for our common stock. We cannot predict the extent to which a trading market will develop or how liquid that market might become. The initial public offering price for our common stock will be determined by negotiations between us and representatives of the underwriters and may not be indicative of prices that will prevail in the trading market after the offering closes. The market price of our common stock could be subject to wide
fluctuations in response to many risk factors listed in this section and others beyond our control, including:
Further, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. In addition, the stock prices of many seed and agricultural biotechnology companies have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions such as recessions, interest rate changes, or international currency fluctuations, may cause the market price of our common stock to decline. If the market price of our common stock after this offering does not exceed the initial public offering price, you may not realize any return on your investment and may lose some or all of your investment.
As a result of becoming a public company, we will be obligated to develop and maintain proper and effective internal control over financial reporting. We may not complete our analysis of our internal control over financial reporting in a timely manner, or these internal controls may not be determined to be effective, which may adversely affect investor confidence in our company and, as a result, the value of our common stock.
Pursuant to Section 404 of the Sarbanes-Oxley Act and the related rules adopted by the SEC and the Public Company Accounting Oversight Board, starting with the second annual report that we file with the SEC after the consummation of this offering, our management will be required to report on the effectiveness of our internal control over financial reporting. In addition, once we no longer qualify as an emerging growth company under the JOBS Act and lose the ability to rely on the exemptions related thereto, our independent registered public accounting firm will also need to attest to the effectiveness of our internal control over financial reporting under Section 404. We have not yet commenced the process of determining whether our existing internal controls over financial reporting systems are compliant with Section 404. This process will require the investment of substantial time and
resources, including by members of our senior management. As a result, this process may divert internal resources and take a significant amount of time and effort to complete. In addition, we cannot predict the outcome of this determination and whether we will need to implement remedial actions in order to implement effective internal control over financial reporting.
In connection with the preparation of our financial statements for the years ended December 31, 2013 and 2014, we identified certain internal control deficiencies that did not rise to the level of a material weakness, on an individual basis or in the aggregate, but which represented significant deficiencies in our internal control over financial reporting. One deficiency related to our information technology access controls and the other related to the timeliness of our accounting and disclosure procedures. We successfully remediated the deficiency relating to the timeliness of our accounting and disclosure procedures during the year ended December 31, 2014, but we can provide no assurance that we will not experience similar control deficiencies in the future. We are currently working to improve our technology access controls and strengthen our internal control environment. As a result, we may experience higher than anticipated operating expenses, as well as higher auditor fees during and after the implementation of these changes. If we are unable to implement any of the required changes to our internal control over financial reporting effectively or efficiently or are required to do so earlier than anticipated, it could adversely affect our operations, financial reporting, and results of operations and could result in an adverse opinion on internal controls from our independent registered public accounting firm.
Our stock price could decline due to the large number of outstanding shares of our common stock eligible for future sale.
Sales of substantial amounts of our common stock in the public market following this offering, or the perception that these sales could occur, could cause the market price of our common stock to decline. These sales could also make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate.
Upon completion of this offering, we will have 38,036,754 outstanding shares of common stock based on the number of shares outstanding as of March 31, 2015 and assuming no exercise of the underwriters' option to purchase additional shares and no exercise of outstanding options after .March 31, 2015. The 7,150,000 shares sold pursuant to this offering will be immediately tradable without restriction, excluding any shares sold under our reserved share program or to our directors or officers, which shares will become saleable beginning 181 days after the date of this prospectus. Of the remaining shares:
We and all of our directors and officers and substantially all of our security holders have agreed that, subject to certain exceptions, we and they will not, without the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC, on behalf of the underwriters, during the period ending 180 days after the date of this prospectus:
whether any transaction described above is to be settled by delivery of shares of our common stock or such other securities, in cash or otherwise. See "Underwriting."
Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC, on behalf of the underwriters, may, in their sole discretion and at any time, release all or any portion of the securities subject to lock-up agreement. After the closing of this offering, we intend to register approximately7,763,428 shares of common stock that have been reserved for future issuance under our stock incentive plans.
Insiders will continue to have substantial control over us after this offering, which could limit your ability to influence the outcome of key transactions, including a change of control.
Our directors, executive officers and each of our stockholders who own greater than 5% of our outstanding common stock and their affiliates, in the aggregate, will beneficially own approximately %79.5% of the outstanding shares of our common stock after this offering. As a result, these stockholders, if acting together, would be able to influence or control matters requiring approval by our stockholders, including the election of directors and the approval of mergers, acquisitions or other extraordinary transactions. They may have interests that differ from yours and may vote in a way with which you disagree and that may be adverse to your interests. This concentration of ownership may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and might affect the market price of our common stock.
Immediately following this offering, Moral Compass Corporation, our largest stockholder, will beneficially own approximately %56.5% of our outstanding common stock assuming no exercise of the underwriters' option to purchase additional shares and approximately %55.0% assuming full exercise of the underwriters' option to purchase additional shares, and Moral Compass Corporation and Mandala Capital together will beneficially own approximately %72.0% of our outstanding common stock assuming no exercise of the underwriters' option to purchase additional shares and approximately %70.0% assuming full exercise of the underwriters' option to purchase additional shares.shares, in each case without giving effect to any potential purchases of shares in this offering by Moral Compass Corporation. For so long as Moral Compass Corporation continues to own a significant percentage of our outstanding shares they will be able to significantly influence the composition of our board of directors and the approval of actions requiring stockholder approval. Accordingly, for such period of time, Moral Compass Corporation may be able to exercise control over our management, business plans, and policies, including the appointment and removal of our officers, and may be able to cause or prevent a change of control of our company or a change in the composition of our board of directors and could preclude any unsolicited acquisition of our company. This concentration of ownership could deprive you of an opportunity to receive a premium for your shares as part of a sale of our company and ultimately might affect the market price of our common stock.
Our management will have broad discretion over the use of the proceeds from this offering and may not apply the proceeds of this offering in ways that increase the value of your investment.
Our management will have broad discretion to use the net proceeds we receive from this offering and you will be relying on its judgment regarding the application of these proceeds. We expect to use the net proceeds from this offering as described under the heading "Use of Proceeds." However, management may not apply the net proceeds of this offering in ways that increase the value of your investment.
If you purchase shares of our common stock in this offering, you will experience substantial and immediate dilution.
If you purchase shares of our common stock in this offering, you will experience substantial and immediate dilution of $$11.72 per share, because the price that you pay will be substantially greater than the net tangible book value per share of the common stock that you acquire. This dilution is due in large part because our earlier investors paid substantially less than the initial public offering price when they purchased their shares of our capital stock. You will experience additional dilution upon the exercise of options to purchase common stock under our equity incentive plans, if we issue restricted stock to our employees under these plans, or if we otherwise issue additional shares of our common stock. See "Dilution."
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws and under Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could depress the trading price of our common stock by discouraging, delaying or preventing a change of control of our company or changes in our management that the stockholders of our company may believe advantageous. These provisions include:
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us make adverse changes to their recommendation regarding our stock, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
As an emerging growth company within the meaning of the Securities Act, we will utilize certain modified disclosure requirements, and we cannot be certain if these reduced requirements will make our common stock less attractive to investors.
We are an emerging growth company within the meaning of the rules under the Securities Act of 1933, as amended, or the Securities Act. We have in this prospectus utilized, and we plan in future filings with the SEC to continue to utilize, the modified disclosure requirements available to emerging growth companies, including reduced disclosure about our executive compensation and omission of compensation discussion and analysis, and an exemption from the requirement of holding a nonbinding advisory vote on executive compensation. In addition, we will not be subject to certain requirements of Section 404 of the Sarbanes-Oxley Act, including the additional testing of our internal control over financial reporting as may occur when outside auditors attest as to our internal control over financial reporting. As a result, our stockholders may not have access to certain information they may deem important. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We could remain an emerging growth company for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1.0 billion, (ii) the date that we become a large accelerated filer as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1.0 billion in non-convertible debt during the preceding three-year period.
Because we do not expect to pay any dividends for the foreseeable future, investors in this offering may be forced to sell their stock to realize a return on their investment.
We do not anticipate that we will pay any dividends to holders of our common stock for the foreseeable future. Any payment of cash dividends will be at the discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions including compliance with covenants under our debt agreements, and other factors that our board of directors may deem relevant. Our ability to pay dividends might be restricted by the terms of any indebtedness that we incur in the future. In addition, certain of our current outstanding debt agreements prohibit us from paying cash dividends on our common stock. Consequently, you should not rely on dividends to receive a return on your investment. See "Dividend Policy."
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of the federal securities laws, which statements involve substantial risks and uncertainties. Forward-looking statements generally relate to future events, our future financial or operating performance, growth strategies, anticipated trends in our industry, and our potential opportunities, plans, and objectives. In some cases, you can identify forward-looking statements because they contain words such as "may," "will," "should," "expects," "plans," "anticipates," "could," "intends," "target," "projects," "contemplates," "believes," "estimates," "predicts," "potential," or "continue" or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
We caution you that the foregoing list may not contain all of the forward-looking statements made in this prospectus.
You should not rely upon forward-looking statements as predictions of future events. We have based the forward-looking statements contained in this prospectus primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations and prospects. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties and other factors described in the section titled "Risk Factors" and elsewhere in this prospectus. Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time to time and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this prospectus. We cannot assure you that the results, events and circumstances reflected in the forward-looking statements will be achieved or occur, and actual results, events or circumstances could differ materially from those described in the forward-looking statements.
The forward-looking statements made in this prospectus relate only to events as of the date on which the statements are made. We undertake no obligation to update any forward-looking statements made in this prospectus to reflect events or circumstances after the date of this prospectus or to reflect new information or the occurrence of unanticipated events, except as required by law. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, or investments we may make.
We estimate that we will receive net proceeds from this offering of approximately $$88.7 million, or approximately $$102.7 million if the underwriters exercise their option in full to purchase additional shares of our common stock, based upon the assumed initial public offering price of $$14.00 per share (the midpoint of the estimated offering price range set forth on the cover page of this prospectus), after deducting the estimated underwriting discounts and commissions of 7% and estimated offering expenses of this offering.approximately $4.4 million.
Each $1.00 increase or decrease in the assumed initial public offering price of $$14.00 per share would increase or decrease the net proceeds that we receive from this offering by approximately $$6.6 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions.
We will have broad discretion over the use of the net proceeds in this offering. As of the date of this prospectus, we cannot specify all of the particular uses for the net proceeds from this offering. We currently intend to use the net proceeds to us from this offering primarily for general corporate purposes, including working capital, capital expenditures, further development and commercialization of our products, and sales and marketing activities. We may also use a portion of the net proceeds to expand our business through investments in or acquisitions of other complementary strategic joint ventures, businesses, products or technologies, including potential investments along with our seed company partners in product development and/or deregulation. We do not have any understandings, commitments, or agreements with respect to any such acquisitions or investments at this time.
We intend to invest the net proceeds in short- and intermediate-term interest-bearing obligations, investment-grade instruments, certificates of deposit or guaranteed obligations of the U.S. government, pending their use as described above.
Some of the other principal purposes of this offering are to create a public market for our common stock and increase our visibility in the marketplace. A public market for our common stock will facilitate future access to public equity markets and enhance our ability to use our common stock as a means of attracting and retaining key employees and as consideration for acquisitions.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any decision to declare and pay cash dividends in the future will be made at the discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions including compliance with covenants under our debt agreements, and other factors that our board of directors may deem relevant. Certain of our current outstanding debt agreements prohibit us from paying cash dividends on our commoncapital stock.
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2014:
You should read this table in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes included elsewhere in this prospectus.
| | As of December 31, 2014 | As of December 31, 2014 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Actual | Pro Forma | Pro Forma As Adjusted(1) | Actual | Pro Forma | Pro Forma As Adjusted(1) | ||||||||||||||
| | (in thousands, except share and per share data) | (in thousands, except share and per share data) | ||||||||||||||||||
Cash and cash equivalents | $ | 16,571 | $ | 16,571 | $ | $ | 16,571 | $ | 16,571 | $ | 115,299 | |||||||||
| | | |||||||||||||||||||
Total indebtedness | 14,475 | 14,475 | 14,475 | 14,475 | 24,460 | |||||||||||||||
| | ||||||||||||||||||||
Redeemable convertible preferred stock, no par value: 10,533,770 shares authorized and 9,822,283 issued and outstanding, actual; no shares authorized, issued and outstanding, pro forma and pro forma as adjusted | 34,098 | — | 34,098 | — | — | |||||||||||||||
Convertible preferred stock, no par value: 94,586,346 shares authorized, 93,540,163 shares issued and outstanding, actual; no shares authorized, issued and outstanding, pro forma and pro forma as adjusted | 48,783 | — | 48,783 | — | — | |||||||||||||||
Stockholders' equity (deficit): | ||||||||||||||||||||
Common stock: no par value, 140,000,000 shares authorized, 8,296,131 shares issued and outstanding, actual; par value per share: $0.001, shares authorized, shares issued and outstanding, pro forma; par value per share: $0.001, shares authorized, shares issued and outstanding, pro forma as adjusted | — | — | ||||||||||||||||||
Undesignated preferred stock, $0.001 par value per share: no shares authorized, issued and outstanding, actual and pro forma; shares authorized, no shares issued and outstanding, pro forma as adjusted | — | — | ||||||||||||||||||
Common stock: no par value, 140,000,000 shares authorized, 2,074,030 shares issued and outstanding, actual; par value per share: $0.001 par value, 400,000,000 shares authorized, 30,886,754 shares issued and outstanding, pro forma; $0.001 par value per share, 400,000,000 shares authorized, 38,036,754 shares issued and outstanding, pro forma as adjusted | — | 51 | 58 | |||||||||||||||||
Undesignated preferred stock, $0.001 par value per share, no shares authorized, issued and outstanding, actual and pro forma; 20,000,000 shares authorized, no shares issued and outstanding, pro forma as adjusted | — | — | — | |||||||||||||||||
Additional paid-in capital | 29,204 | 112,085 | 29,204 | 112,034 | 200,770 | |||||||||||||||
Accumulated deficit | (113,970 | ) | (113,970 | ) | (113,970 | ) | (113,970 | ) | (113,970 | ) | ||||||||||
| | ||||||||||||||||||||
Total stockholders' (deficit) equity | (84,766 | ) | (1,885 | ) | (84,766 | ) | (1,885 | ) | 86,858 | |||||||||||
| | ||||||||||||||||||||
Total capitalization | $ | 12,590 | $ | 12,590 | $ | $ | 12,590 | $ | 12,590 | $ | 111,318 | |||||||||
| | | |||||||||||||||||||
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering. Our pro forma net tangible book value as of December 31, 2014 was $$(4.6) million, or $$(0.15) per share of common stock. Pro forma net tangible book value per share represents total tangible assets less total liabilities, divided by the number of shares of common stock outstanding. After giving effect to our sale of our common stock in this offering at the initial public offering price of $$14.00 per share, and after deducting underwriting discounts and commissions and our estimated offering expenses, our pro forma as adjusted net tangible book value as of December 31, 2014 would have been $ ,$86.9 million, or $$2.28 per share. This represents an immediate increase in net tangible book value of $$2.43 per share to our existing stockholders and an immediate dilution of $$11.72 per share to new investors purchasing shares of common stock in this offering. The following table illustrates this dilution on a per share basis:
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
Initial public offering price per share | $ | 14.00 | |||||
Pro forma net tangible book value per share as of December 31, 2014 | $ | (0.15 | ) | ||||
Increase in net tangible book value per share attributable to new investors purchasing shares in this offering | 2.43 | ||||||
| | | | | | | | |
Pro forma net tangible book value per share after giving effect to this offering | 2.28 | ||||||
| | | | | | | | |
Dilution per share to new investors in this offering | $ | 11.72 | |||||
| | | | | | | | |
| | | | | | | | |
Each $1.00 increase or decrease in the assumed initial public offering price of $$14.00 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, would increase or decrease, as applicable, our pro forma as adjusted net tangible book value by $ ,$6.6 million, or approximately $$0.18 per share, and would increase or decrease, as applicable, dilution per share to new investors in this offering by $ ,$0.82, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions. In addition, to the extent any outstanding options or warrants are exercised, new investors would experience further dilution.
The following table presents on a pro forma basis, as of December 31, 2014, the differences between the existing stockholders and new investors with respect to the number of shares of common stock purchased from us, the total consideration paid to us and the average price per share paid or to be paid to us at an assumed initial public offering price of $$14.00 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, before deducting estimated underwriting discounts and commissions:
| | Shares Purchased | Total Consideration | | Shares Purchased | Total Consideration | | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Average Price Per Share | Average Price Per Share | ||||||||||||||||||||||||||||||
| | Number | Percent | Amount | Percent | Number | Percent | Amount | Percent | ||||||||||||||||||||||||
Existing stockholders | % | $ | % | $ | 30,886,754 | 81.2 | % | $ | 82,881,000 | 45.3 | % | $ | 2.68 | |||||||||||||||||||
New investors | 7,150,000 | 18.8 | 100,100,000 | 54.7 | 14.00 | |||||||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||
Total | 100.0 | % | $ | 100.0 | % | 38,036,754 | 100.0 | % | $ | 182,981,000 | 100.0 | % | ||||||||||||||||||||
| | | |||||||||||||||||||||||||||||||
Each $1.00 increase or decrease in the assumed initial public offering price of $$14.00 per share, which is the midpoint of the estimating offering price range set forth on the cover page of this prospectus, would increase or decrease, as applicable, the total consideration paid by new investors and total consideration paid by all stockholders by approximately $$7.2 million, assuming that the number of
of shares offered by us, as set forth on the cover page of this prospectus, remains the same and afterbefore deducting estimated underwriting discounts and commissions.
Except as otherwise indicated, the above discussion and tables assume no exercise of the underwriters' option to purchase additional shares. If the underwriters exercise their option to purchase additional shares in full, the total consideration paid by new investors and total consideration paid by all stockholders would (decrease) increase by approximately $ and $ per share, respectively, the pro forma as adjusted net tangible book value per share would be $ per share, and the dilution in pro forma as adjusted net tangible book value per share to new investors in this offering would be $ per share. Following such exercise, our existing stockholders would own %79.0% and our new investors would own %21.0% of the total number of shares of our common stock outstanding upon the closing of this offering.
The foregoing discussion and tables do not reflect any potential purchases by entities affiliated with certain of our existing stockholders that have indicated an interest in purchasing shares of our common stock in this offering, as described in "Underwriting" and elsewhere in this prospectus.
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated statements of operations data for the years ended December 31, 2013 and 2014 and the consolidated balance sheet data as of December 31, 2013 and 2014 are derived from our audited consolidated financial statements included elsewhere in this prospectus, and should be read together with our consolidated financial statements, the notes to our consolidated financial statements and "Management's Discussion and Analysis of Financial Condition and Results of Operations" contained elsewhere in this prospectus.
| | Year Ended December 31, | Year Ended December 31, | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | 2013 | 2014 | ||||||||||
| | (in thousands, except share and per share amounts) | (in thousands, except share and per share amounts) | ||||||||||||
Revenues: | ||||||||||||||
Product | $ | 1,102 | $ | 355 | $ | 1,102 | $ | 355 | ||||||
License | 1,625 | 2,325 | 1,625 | 2,325 | ||||||||||
Contract research and government grants | 3,751 | 4,302 | 3,751 | 4,302 | ||||||||||
| | ||||||||||||||
Total revenues | 6,478 | 6,982 | 6,478 | 6,982 | ||||||||||
Operating expenses: | ||||||||||||||
Cost of product revenues(1) | 673 | 1,997 | 673 | 1,997 | ||||||||||
Research and development(1) | 8,404 | 10,012 | 8,404 | 10,012 | ||||||||||
Selling, general, and administrative(1) | 7,967 | 10,126 | 7,967 | 10,126 | ||||||||||
| | ||||||||||||||
Total operating expenses | 17,044 | 22,135 | 17,044 | 22,135 | ||||||||||
| | ||||||||||||||
Loss from operations | (10,566 | ) | (15,153 | ) | (10,566 | ) | (15,153 | ) | ||||||
Interest expense | (626 | ) | (1,394 | ) | (626 | ) | (1,394 | ) | ||||||
Other income (expense), net | 5 | (597 | ) | 5 | (597 | ) | ||||||||
| | ||||||||||||||
Loss before income taxes and equity in loss of unconsolidated entity | (11,187 | ) | (17,144 | ) | (11,187 | ) | (17,144 | ) | ||||||
Income tax provision | (167 | ) | (263 | ) | (167 | ) | (263 | ) | ||||||
Equity in loss of unconsolidated entity | (1,841 | ) | (932 | ) | (1,841 | ) | (932 | ) | ||||||
| | ||||||||||||||
Net loss | (13,195 | ) | (18,339 | ) | (13,195 | ) | (18,339 | ) | ||||||
Accretion of redeemable convertible preferred stock to redemption value | — | (3,738 | ) | — | (3,738 | ) | ||||||||
| | ||||||||||||||
Net loss attributable to common stockholders | $ | (13,195 | ) | $ | (22,077 | ) | $ | (13,195 | ) | $ | (22,077 | ) | ||
| | | |||||||||||||
Net loss per share attributable to common stockholders, basic and diluted(2) | $ | (1.61 | ) | $ | (2.68 | ) | $ | (6.43 | ) | $ | (10.71 | ) | ||
| | | |||||||||||||
Weighted-average number of shares used in per share calculations, basic and diluted(2) | 8,213,544 | 8,245,129 | 2,053,384 | 2,061,278 | ||||||||||
| | | |||||||||||||
Pro forma net loss per share attributable to common stockholders, basic and diluted(2) | $ | $ | (0.62 | ) | ||||||||||
| | | |||||||||||||
Weighted-average number of shares used in pro forma per share calculations, basic and diluted(2) | 29,595,148 | |||||||||||||
| | | |||||||||||||
| | Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | |||||
| | (in thousands) | ||||||
Research and development | $ | 414 | $ | 249 | |||
Selling, general, and administrative | 864 | 727 | |||||
| | | | | | | | |
Total stock-based compensation | $ | 1,278 | $ | 976 | |||
| | | | | | | | |
| | | | | | | | |
| | As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | |||||
| | (in thousands) | ||||||
Consolidated Balance Sheet Data: | |||||||
Cash and cash equivalents | $ | 2,835 | $ | 16,571 | |||
Working capital (deficit) | (4,977 | ) | 7,426 | ||||
Total assets | 9,542 | 24,889 | |||||
Total indebtedness | 14,492 | 14,475 | |||||
Redeemable convertible preferred stock | — | 34,098 | |||||
Convertible preferred stock(1) | — | 48,783 | |||||
Additional paid-in capital | 78,334 | 29,204 | |||||
Accumulated deficit | (95,631 | ) | (113,970 | ) | |||
Total stockholder's deficit | (17,297 | ) | (84,766 | ) | |||
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes to those statements included elsewhere in this prospectus. In addition to historical financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under "Risk Factors" and elsewhere in this prospectus.
Overview
We are a leading agricultural biotechnology trait company with an extensive and diversified portfolio of late-stage yield and product quality traits addressing multiple crops that supply the global food and feed markets. Our traits are focused on high-value enhancements that increase crop yields by enabling plants to more efficiently manage environmental and nutrient stresses, and that enhance the quality and value of agricultural products. Our traits increase value not only for farmers, but also for users of agricultural products. Our target market is the $39.4 billion global seed market. Our goal is to increase the value of this market significantly by increasing yields in the more than $1.0 trillion market for the five largest global crops, and to capture a portion of the increased value. There currently are more than 50 products in development incorporating our traits and there are 13 in advanced stages of development or on the market.
Our crop yield traits are being utilized by our commercial partners to develop higher yielding seeds for the most widely grown global crops, including wheat, rice, soybean, corn, and sugarcane, as well as for other crops such as cotton, canola, turf, and trees. Our business model positions us at the nexus of basic research and commercial product development, as we apply our strong product development and regulatory capabilities to collaborate with, and leverage the skills and investments of, upstream basic research institutions and downstream commercial partners. We believe our approach significantly reduces risk and capital requirements, while simplifying and expediting the product development process. We also believe that our collaboration strategy leverages our internal capabilities, enabling us to capture much higher value than would otherwise be the case, and enabling our commercial partners to develop and commercialize products more cost-effectively.
We were incorporated in 2002 to pursue agricultural-based biotechnology business opportunities that improve the environment and human health, and in 2004, we entered into our first collaboration agreement with a potential commercial partner. In 2009, we completed the U.S. Food and Drug Administration, or FDA, regulatory process for our Sonova brand gamma linolenic acid safflower oil, called Sonova 400 GLA safflower oil, just six years after we first began developing the trait under a research and commercial agreement with Abbott. We introduced this product commercially in late 2010, and in 2014, we introduced Sonova Ultra GLA safflower oil, a more concentrated version of our Sonova 400 GLA safflower oil. We refer to these products as our Sonova products.
We have formed strategic partnerships and developed strong relationships with global agricultural leaders for development and commercialization of our traits in major crops and consumer products. Our collaborators include subsidiaries or affiliates of Limagrain (Vilmorin & Cie), Mahyco (Maharashtra Hybrid Seeds Company Limited), Dow AgroSciences, DuPont Pioneer (E.I. du Pont de Nemours and Company), SES Vanderhave, Genective (a joint venture between Limagrain and KWS SAAT), Scotts, U.S. Sugar, Abbott, Ardent Mills, Bioceres, and others. Additionally, in order to increase our participation in the value of two major crops, wheat and soybean, we have formed two joint ventures. Limagrain Cereal Seeds LLC is our joint venture with Limagrain for the development and commercialization of wheat products for North America. Verdeca LLC is our joint venture with
Bioceres for the development and deregulation of soybean traits globally. In April 2015, we entered into a collaboration agreement with Dow AgroSciences and Bioceres under which our Verdeca joint venture will collaborate with Dow AgroSciences on the development and deregulation of soybean traits on a global basis. We intend to enter into future collaboration agreements and joint ventures depending on our assessment of which structure provides the best ratio of risk to investment return.
The process of developing and commercializing innovative traits and seed products requires significant time and investment. Our business model focuses on creating value by leveraging collaborator investments and capabilities upstream in basic research, and downstream in product development and commercialization. We bridge the gap between basic research and commercial development, reducing risk and adding value as a result. We reduce risk and avoid most of the costs associated with basic research by acquiring trait technologies that have already completed initial feasibility screening, thus achieving proof of concept, through basic research carried out elsewhere. We further develop these technologies by optimizing function and validating performance through intensive field trial testing in multiple crops. We then form collaborations with major seed and consumer product companies that develop and commercialize products incorporating our traits. As a result of our expertise and this additional development work, we are positioned to capture significantly greater payments in our downstream license and collaboration agreements than we believe is otherwise typical in our industry.
In certain instances, we may also work to complete the regulatory process to support the commercial launch of products containing our traits. We would do this in order to obtain a greater share of the economics of the commercial product. We intend to pace any regulatory investments so that we only make such investments after the performance risk for the seed trait has been significantly reduced through extensive field testing. We may pursue regulatory investments in these instances if we believe that they will result in a highly positive rate of return due to increased payments from our commercial partners or joint ventures.
Our commercial strategy aims to balance our near-term revenue goals with long-term value capture. Our trait license agreements with our commercial partners contain two main types of financial components:
While we seek patent protection on our technologies and traits, we have structured our commercial agreements so that we receive our percentage of additional commercial value whether or not patent protection is in effect at any particular time or place. Nearly all of our agreements provide
that access to our traits, and our right to receive a share of commercial value, continue for a set number of years after products containing our traits are commercialized. While the exclusive rights afforded by patents may enable our commercial partners to realize greater commercial value attributable to our traits, our right to receive a portion of that increased commercial value is not dependent on the existence of patent rights in a particular geography.
Most of our agreements include the grant of exclusive rights to a particular trait for use in a particular crop within a defined geography. To date, we have not granted exclusive rights to all of our traits for use in a particular crop to a single partner and, likewise, we have not granted exclusive rights to utilize a particular trait in all crops to a single partner. Our approach to selecting commercial partners involves careful consideration of their market channels and capabilities to ensure that they are well matched to the trait, crop, and geography that form the foundation of our commercial relationship.
The process of discovering, developing, and commercializing a seed trait through either genetic modification or advanced breeding involves multiple phases and takes an average of 13 years from discovery to commercialization. The length of the process may vary depending on both the complexity of the trait and the type of crop involved. This long product development cycle is in large part attributable to the limitations of natural growing seasons and the impact of this on the time it takes to breed commercial seed products. For genetically modified, or GM, seeds, there is also a rigorous and lengthy regulatory process that operates in parallel to the later stages of the seed breeding process.
Since our inception, we have devoted substantially all of our efforts to research and development activities, including the discovery, development, and testing of our traits and products in development incorporating our traits. To date, we have not generated revenues from sales of commercial products, other than limited revenues from our Sonova products, and we do not anticipate generating any revenues from commercial product sales other than from sales of our Sonova products for at least the next three to five years. We do receive revenues from fees associated with the licensing of our traits to commercial partners. Our long-term business plan and growth strategy is based in part on our expectation that revenues from products that incorporate our traits will comprise a significant portion of our future revenues.
We have never been profitable and had an accumulated deficit of $114.0 million as of December 31, 2014. We incurred net losses of $13.2 million and $18.3 million for the years ended December 31, 2013 and 2014, respectively. We expect to incur substantial costs and expenses before we obtain any revenues from the sale of seeds incorporating our traits. As a result, our losses in future periods could become even more significant, and we will need substantial additional funding to support our operating activities.
Components of Our Statements of Operations Data
Revenues
We derive our revenues from product revenues, licensing agreements, contract research agreements, and government grants. We expect that over the next several years, a substantial majority of our revenues will consist of license revenues and contract research and government grant revenues until our product revenues increase with the introduction of our seed trait products to the market, if and when they are commercially available. Further, we expect that our license revenues will vary as we enter into new license agreements and with the timing of milestone payments and recognition of deferred upfront license fees under existing license agreements.
Product Revenues
Our product revenues to date have consisted solely of sales of our Sonova products. We generally recognize revenue from product sales upon delivery to our third-party distributors or customers. Our revenues will fluctuate depending on the timing of orders from our customers and distributors. Because some of our large customers and distributors order in bulk only one or two times a year, our product revenues may fluctuate significantly from period to period.
License Revenues
Our license revenues consist of up-front, nonrefundable license fees, annual license fees, and subsequent milestone payments that we receive under our research and license agreements. We generally recognize nonrefundable up-front license fees and guaranteed, time-based payments as revenue proportionally over the expected development period. We recognize annual license fees proportionally over the related term subject to cancellation provisions.
We recognize milestone payments as revenue when the related performance criteria are achieved. Milestones typically consist of significant stages of development for our traits in a potential commercial product, such as achievement of specific technological targets, completion of field trials, filing with regulatory agencies, completion of the regulatory process, and commercial launch of a product containing our traits. Given the seasonality of agriculture and time required to progress from one milestone to the next, achievement of milestones is inherently uneven, and our license revenues are likely to fluctuate significantly from period to period.
Contract Research and Government Grant Revenues
Contract research revenues consist of amounts earned from performing contracted research primarily related to breeding programs or the genetic engineering of plants for third parties. We generally recognize revenue as these services are provided. In addition, we are entitled to receive a portion of the revenues generated from sales of products that incorporate our seed traits. Products expected to result from such contract research are in various stages of the product development cycle and we do not expect to generate any revenues from the sale of any such products for at least the next three to five years.
We receive payments from government entities in the form of government grants. Government grant revenues are recognized as eligible research and development expenses are incurred. Our obligation with respect to these agreements is to perform the research on a best-efforts basis.
Operating Expenses
Cost of Product Revenues
Cost of product revenues relates to sale of our Sonova products and consists of in-licensing and royalty fees as well as the cost of raw materials, including inventory and third-party services costs related to procuring, processing, formulating, packaging, and shipping our Sonova products.
Research and Development Expenses
Research and development expenses consist of costs incurred in the discovery, development, and testing of our products and products in development incorporating our traits. These expenses consist primarily of employee salaries and benefits, fees paid to subcontracted research providers, fees associated with in-licensing technology, land leased for field trials, chemicals and supplies, and other external expenses. These costs are expensed as incurred. Additionally, we are required from time to time to make certain milestone payments in connection with the development of technologies in-licensed from third parties. We expense these milestone payments at the time the milestone is
achieved and deemed payable. We expect our research and development expenses to increase on an absolute dollar basis for the foreseeable future, although our research and development expenses may increase significantly if we choose to accelerate certain research and development programs or if we elect to take a greater role in the regulatory and commercialization process with respect to one or more of our seed traits or products in development incorporating our seed traits. Our research and development expenses may also fluctuate from period to period as a result of the timing of various research and development projects.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses consist primarily of employee costs, professional service fees, and overhead costs. In addition, in the first half of 2014, we also incurred costs of $2.1 million paid to an advisor related to our financing efforts that ultimately resulted in the issuance of our Series D preferred stock. Notwithstanding these costs of $2.1 million, we expect our selling, general, and administrative expenses to increase substantially for the foreseeable future as we begin to operate as a public company after the completion of this offering, although our selling, general, and administrative expenses may fluctuate from period to period.
Interest Expense
Interest expense consists of interest costs related to our outstanding borrowings of promissory notes and convertible promissory notes payable to related and non-related parties.
Other Income (Expense), Net
Other income (expense), net, consists of changes in the fair value of our derivative liabilities related to our convertible promissory notes, gains or losses from the sale or retirement of property and equipment, and interest income on our cash and cash equivalents.
Equity in Loss of Unconsolidated Entity
We use the equity method to account for our investment in Limagrain Cereal Seeds LLC, or LCS, a joint venture we formed with an affiliate of Limagrain and in which we hold a 35% interest. We account for LCS as an unconsolidated entity, as we exercise significant influence but do not have a controlling interest.
Income Tax Provision
Our income tax provision has not been historically significant, as we have incurred losses since our inception. As of December 31, 2014, we had federal and state net operating loss carryforwards of $98.1 million and $77.2 million, respectively, for which we have provided a full valuation allowance, as it is more likely that we will not realize the benefit of such net operating losses. The federal net operating loss carryforwards expire at various dates beginning in 2020 and the state net operating loss carryforwards expire at various dates beginning in 2015.
Results of Operations
Comparison of the Years Ended December 31, 2013 and 2014
| | Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | |||||
| | (in thousands) | ||||||
Revenues: | |||||||
Product | $ | 1,102 | $ | 355 | |||
License | 1,625 | 2,325 | |||||
Contract research and government grants | 3,751 | 4,302 | |||||
| | | | | | | | |
Total revenues | 6,478 | 6,982 | |||||
Operating expenses: | |||||||
Cost of product revenues | 673 | 1,997 | |||||
Research and development | 8,404 | 10,012 | |||||
Selling, general and administrative | 7,967 | 10,126 | |||||
| | | | | | | | |
Total operating expenses | 17,044 | 22,135 | |||||
| | | | | | | | |
Loss from operations | (10,566 | ) | (15,153 | ) | |||
Interest expense | (626 | ) | (1,394 | ) | |||
Other income (expense), net | 5 | (597 | ) | ||||
| | | | | | | | |
Loss before income taxes | (11,187 | ) | (17,144 | ) | |||
Income tax provision | (167 | ) | (263 | ) | |||
Equity in loss of unconsolidated entity | (1,841 | ) | (932 | ) | |||
| | | | | | | | |
Net loss | $ | (13,195 | ) | $ | (18,339 | ) | |
Accretion of redeemable convertible preferred stock to redemption value | — | (3,738 | ) | ||||
| | | | | | | | |
Net loss attributable to common stockholders | $ | (13,195 | ) | $ | (22,077 | ) | |
| | | | | | | | |
| | | | | | | | |
Revenues
Product revenues accounted for 17% and 5% of our total revenues for the years ended December 31, 2013 and 2014, respectively. Our product revenues from sales of our Sonova products decreased by $0.7 million, or 68%, in 2014 compared to 2013. The decrease in product revenues was primarily due to the timing of large orders, as there were no bulk orders for our Sonova products from distributors during 2014. The timing for bulk orders fluctuates greatly as distributors tend to order large quantities on an infrequent basis.
License revenues accounted for 25% and 33% of our total revenues for the years ended December 31, 2013 and 2014, respectively. Our license revenues increased by $0.7 million, or 43%, in 2014 compared to 2013. The increase in license revenues was primarily attributable to the timing of recognition of deferred upfront license fees.
Contract research and government grant revenues comprise a significant portion of our total revenues, accounting for 58% and 62% of our total revenues for the years ended December 31, 2013 and 2014, respectively. Our contract research and government grant revenues increased by $0.6 million, or 15%, in 2014 compared to 2013. The increase was primarily driven by increased revenues associated with new grants obtained in the second half of 2013 and recognized as revenue in 2014, as well as increased activity under existing grants.
Revenues from Mahyco accounted for 23% and 29% of our total revenues for 2013 and 2014, respectively. Revenues from the U.S. Agency for International Development accounted for 26% and 36% of our total revenues for 2013 and 2014, respectively.
Cost of Product Revenues
Cost of product revenues increased by $1.3 million, or 197%, in 2014 compared to 2013 due to a $1.7 million reserve for inventory recorded in the 2014. This reserve is primarily a result of changes in conditions of specific customers and regulatory delays related to the use of our Sonova products by certain new industries. The remaining decrease in the cost of product revenues is consistent with the decrease in product revenues.
Research and Development
Research and development expenses increased by $1.6 million, or 19%, in 2014 compared to 2013. The increase was primarily driven by an expense of $1.5 million recorded in 2014 relating to the September 2014 amendment of our Bioceres funding agreement pursuant to which we surrendered shares of Bioceres in exchange for a reduction of the annual commitment amount for 2014 and elimination of the 2015 commitment amount, as well as increased subcontracted services under various new and existing grants.
Selling, General, and Administrative
Selling, general, and administrative expenses increased by $2.2 million, or 27%, in 2014 compared to 2013. The increase was primarily due to costs of $2.1 million paid to an advisor upon the closing of our Series D preferred stock financing in 2014.
Interest Expense
Interest expense was $1.4 million for 2014, an increase of $0.8 million compared to $0.6 million for 2013. The increase was primarily due to interest expense related to debt issued to fund our operations, including our issuance of $3.6 million of promissory notes and $5.0 million of convertible promissory notes in the second half of 2013.
Other Income (Expense), Net
Other income (expense), net for 2014 primarily consisted of the increase in fair value of the derivative liabilities related to our convertible promissory notes that we entered into in the second half of 2013.
Accretion of Redeemable Convertible Preferred Stock to Redemption Value
Accretion of redeemable convertible preferred stock to redemption value was $3.7 million in 2014 as a result of our issuance of Series D preferred stock in the first half of 2014.
Seasonality
We and our commercial partners operate in different geographies around the world and conduct field trials that are used for data generation, which must be conducted during the appropriate growing seasons of particular crops and markets. Often, there is only one crop-growing season per year for certain crops and markets. Similarly, climate conditions and other variables on which sales of our products are dependent may vary from season to season and year to year. In particular, weather conditions, including natural disasters such as heavy rains, hurricanes, hail, floods, tornadoes, freezing conditions, drought, or fire, may affect the timing and outcome of field trials, which may delay milestone payments and the commercialization of products incorporating our seed traits. In the future, sales of commercial products that incorporate our seed traits will vary based on crop growing seasons and weather patterns in particular regions.
The level of seasonality in our business overall is difficult to evaluate at this time due to our relatively early stage of development, our relatively limited number of commercialized products, our expansion into new geographical markets, and our introduction of new products and traits.
Liquidity and Capital Resources
Since our inception, we have funded our operations primarily with the net proceeds from private placements of equity and debt securities, as well as proceeds from the sale of our Sonova products and payments under license agreements, contract research agreements, and government grants. Our principal use of cash is to fund our operations, which are primarily focused on progressing our agricultural yield and product quality seed traits through the regulatory process and to commercialization. This includes conducting replicated field trials, coordinating with our partners on their development programs, and collecting, analyzing, and submitting field trial data to regulatory authorities. As of December 31, 2014, we had cash and cash equivalents of $16.6 million.
In April 2015, we obtained additional debt financing in the form of a $20.0 million senior secured term loan facility as described below. We expectborrowed the entire $20.0 million under the term loan facility on the closing date of the term loan facility.
Our principal uses of cash are to incur additional losses related to the continued developmentfund our operations. We believe that our existing cash and expansion of our business, including research and development, seed production and operations, and sales and marketing. We cannot assure you that we will ever achieve profitability or, if we do, that wecash equivalents will be ablesufficient to sustain it.
We do not currently expect to generate significant revenues from sales ofmeet our seed trait products under developmentanticipated cash requirements for at least the next several years. We expect that research and development expenses will continue to constitute a significant portion of our operating expenses in the future, with the timing and magnitude of expenditures varying depending on the timing of various research and development projects. For example, as additional products in development incorporating our traits reach Phase 3 or later in the development process, we expect that the relative share of research and development expenses devoted to the regulatory process will increase. In addition, we can elect to increase our participation in, as well as our cost share of, the regulatory process in order to obtain a greater share of the economics of the commercial product. We may also use a portion of the proceeds from this offering to expand our pipeline of seed traits in development.12 months.
We believecurrently anticipate that the proceeds from this offering, together with our existing cash and future license and product revenues, including milestone revenues,we will be sufficientseek to fund our operations through the commercialization of our initial products incorporating our next generation seed traits.additional debt or equity financings. We may also consider entering into additional partner arrangements seek further contract research revenues, pursueor pursuing additional government grants, institute cost cutting measures, extend the maturity debt on existing debt, incurgrants. Our sale of additional debt, or sellequity would result in additional equity. We are currently in the process of exploring financing with various lenders.dilution to our stockholders. Our incurrence of additional debt would result in increased debt service obligations, and the instruments governing our debt could provide for additional operating and financing covenants that would restrict our operations. The sale of additional equity would result in dilution to our existing stockholders. If we are not able to secure adequate additional funding, we may be forced to reduce our spending, extend payment terms with our suppliers, liquidate assets, or suspend or curtail planned development programs. Any of these actions could harm our business, results of operations and financial condition.
Term Note, Related Party
In July 2012, we entered into a 36-month unsecured term note with Moral Compass Corporation, our controlling stockholder, in the amount of $8.0 million. The interest rate on the loan was prime plus 2%, with interest paid monthly in arrears, and the principal was due in full at maturity in July 2015. In November 2014, we amended this note to change the maturity date to the first to occur of (i) April 1, 2016, (ii) the date of an event of default, or (iii) a date designated by Moral Compass Corporation no earlier than the 20th day following our completion of an equity financing with gross proceeds to us of at least $50.0 million. In addition, the interest rate on the term loan remained at prime plus 2% through December 31, 2014, after which the rate increased to 11% per annum until maturity.
Table In April 2015, we entered into a new term loan facility and repaid the principal balance of Contents$8.0 million and accrued interest and prepayment fee of $148,000 on this note.
Promissory Notes
We entered into promissory notes in August 2013 and November 2013 in the amounts of $2.0 million and $1.1 million, respectively. The interest rate on the notes is fixed at 10% with principal and interest due in 36 equal monthly installments over the course of the three-year terms. Monthly principal and interest on the $2.0 million note is $65,000 and the three-year term ends in August 2016. Monthly principal and interest on the $1.1 million note is $35,000 and the three-year term ends in November 2016. In April 2015, we entered into a new term loan facility and repaid the aggregate outstanding principal balance of $1.6 million and accrued interest and prepayment fee of $44,000 on the promissory notes.
Convertible Promissory Notes
In September 2013, we entered into a note and warrant purchase agreement in the amount of $5.0 million with an affiliate of Mahyco, one of our commercial partners with which we have several research and license agreements. We issued a convertible promissory note under this agreement in exchange for $500,000 in September 2013 and issued a second convertible promissory note in exchange for $4.5 million in December 2013. The interest rate on the notes is prime plus 2%, compounded monthly over the course of the five-year terms ending in September and December 2018, respectively. At any time during the term, Mahyco may convert all or part of the aggregate outstanding balance of the notes (including principal and accrued but unpaid interest) into shares of our common stock at $4.13$16.52 per share. Mahyco has the right, at its option, to place another $5.0 million of convertible debt with us during the five-year term. Mahyco, at its option, may offset future fee payments to us due under any license agreements or contract research and development agreements with us against the outstanding balance of the note, including principal and accrued but unpaid interest. With the exception of such offset payments, no principal or interest is due until the end of the term. Under this note and warrant purchase agreement, we also issued Mahyco warrants to purchase 302,66575,666 shares of our common stock at an exercise price of $4.13$16.52 per share. The warrants were issued in December 2013, vested immediately, and remain exercisable throughout the five-year term.
Term Loans
In April 2015, we entered into a loan and security agreement with lenders that are affiliates of Tennenbaum Capital Partners, LLC. Obsidian Agency Services, Inc. acts as administrative agent for the lenders under this agreement. Under the agreement, the lenders committed to advance term loans in an aggregate principal amount of up to $20.0 million, and we borrowed the entire $20.0 million of term loan commitments on the loan closing date.
Under this loan and security agreement, interest on the term loans accrues at a rate per annum equal to the greater of (i) 9.0% and (ii) a fluctuating rate of interest equal to three-month LIBOR as in effect from time to time plus 8.74%. We are required to make interest-only payments under this agreement from the drawdown dates through April 30, 2016, subject to certain conditions for extension to October 31, 2016. After this date, we are required to make equal monthly payments of principal and interest so that all outstanding principal amounts and accrued interest will be repaid by November 1, 2018. This agreement provides for a right of prepayment with associated prepayment fees. Upon the maturity date or the date on which the term loans are prepaid in whole or in part, we will owe an additional end-of-term payment to the lenders.
The loan and security agreement includes customary covenants for credit facilities of this type. In addition, the agreement contains a financial covenant with respect to quarterly revenue targets or cash and cash equivalents on hand. As of the date of this prospectus, we are in compliance with such covenants. Our obligations under the loan agreement are secured by substantially all of our assets (excluding our intellectual property). The loan agreement includes customary events of default for credit facilities of this type. If any of these events of default occurs, the lenders may accelerate and declare to be immediately due and payable the outstanding principal amount of the term loans and our other payment obligations under the agreement, and in the case of a bankruptcy or insolvency event of default, the outstanding principal amount of the term loans and our other payment obligations under the loan agreement automatically are accelerated and become due and payable. In addition, if an event of default occurs and is continuing under the loan agreement, the lenders may exercise certain additional secured creditor remedies against us and against the assets securing our obligations under the agreement.
Cash Flows
The following table summarizes our cash flows for the periods indicated (in thousands):
| | Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2013 | 2014 | |||||
Net cash provided by (used in): | |||||||
Operating activities | $ | (9,745 | ) | $ | (14,787 | ) | |
Investing activities | (600 | ) | (1,591 | ) | |||
Financing activities | 7,830 | 30,114 | |||||
| | | | | | | | |
Net (decrease) increase in cash and cash equivalents | $ | (2,515 | ) | $ | 13,736 | ||
| | | | | | | | |
| | | | | | | | |
Cash Flows from Operating Activities
Cash used in operating activities for the year ended December 31, 2014 was $14.8 million. Our net loss of $18.3 million and decrease in net operating assets of $1.3 million were partly offset by non-cash charges of $1.5 million relating to the amendment of our Bioceres funding agreement, $0.9 million for equity in loss of unconsolidated entity, $1.0 million for stock-based compensation, $0.5 million for accretion of debt discount, $0.6 million change in fair value of derivative liabilities, and $0.4 million for depreciation and amortization.
Cash used in operating activities for the year ended December 31, 2013 was $9.7 million. Our net loss of $13.2 million and decrease in net operating assets of $0.1 million were partly offset by non-cash charges of $1.8 million for equity in loss of unconsolidated entity, $1.3 million for stock-based compensation, and $0.4 million for depreciation and amortization.
Cash Flows from Investing Activities
Cash used in investing activities for the year ended December 31, 2014 of $1.6 million consisted primarily of our investment in Bioceres in accordance with our agreements concerning Verdeca.
Cash used in investing activities for the year ended December 31, 2013 of $0.6 million consisted of our $0.5 million investment in Bioceres and $0.1 million in purchases of property and equipment.
Cash Flows from Financing Activities
Cash from financing activities for the year ended December 31, 2014 of $30.1 million was related to the $32.8 million of net proceeds from our issuance of Series D preferred stock in the first half of 2014 and offset by $2.7 million of payments on notes payable, convertible promissory notes, and deferred offering costs.
Cash from financing activities for the year ended December 31, 2013 of $7.8 million was primarily related to the proceeds from our issuance of notes payable, promissory notes, and convertible promissory notes and related common stock warrants issued to Mahyco of $8.6 million and offset by $0.7 million of our payments on notes payable and convertible promissory notes.
Contractual Obligations and Other Commitments
Our future contractual obligations at December 31, 2014 were as follows (in thousands):
| | Payments Due by Period(2)(3) | Payments Due by Period(2)(3)(4) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Less than 1 year | 1 to 3 Years | 3 to 5 Years | More than 5 years | Total | Less than 1 year | 1 to 3 Years | 3 to 5 Years | More than 5 years | Total | ||||||||||||||||||||||
Non-cancelable operating leases | $ | 623 | $ | 231 | $ | — | $ | — | $ | 854 | $ | 623 | $ | 231 | $ | — | $ | — | $ | 854 | ||||||||||||
Notes payable due to a related party | 841 | 8,219 | — | — | 9,060 | 841 | 8,219 | — | — | 9,060 | ||||||||||||||||||||||
Promissory notes payable | 1,196 | 902 | — | — | 2,098 | 1,196 | 902 | — | — | 2,098 | ||||||||||||||||||||||
Convertible promissory notes payable(1) | 274 | 593 | 5,169 | — | 6,036 | 274 | 593 | 5,169 | — | 6,036 | ||||||||||||||||||||||
| | ||||||||||||||||||||||||||||||||
Total contractual obligations | $ | 2,934 | $ | 9,945 | $ | 5,169 | $ | — | $ | 18,048 | $ | 2,934 | $ | 9,945 | $ | 5,169 | $ | — | $ | 18,048 | ||||||||||||
| | ||||||||||||||||||||||||||||||||
We are obligated to make future payments to related and unrelated parties under in-license agreements, including certain license fees, royalties, and milestone fees. In addition, certain royalty payments ranging from the low single digits to mid-teens are payable on net revenue amounts as defined in the in-licensing agreements. Milestone payments under these agreements may also be payable upon the successful development or implementation of various technologies. The amount and timing of these payments are uncertain and have been excluded from the above table.
Off-Balance Sheet Arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities, or variable interest entities.
Critical Accounting Polices and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management's judgments and estimates.
Revenue Recognition
We generate revenues through sales of product, license agreements, contract research agreements, and government grants. Revenue generated from our license agreements may include up-front, nonrefundable license fees, annual license fees, milestone payments, and future value-sharing payments subsequent to commercialization by our partners. We recognize revenue when the following criteria have been met: persuasive evidence of an arrangement with the customer exists, price and terms of the arrangement are fixed or determinable, delivery of the product has occurred or the service has been performed in accordance with the terms of the arrangement, and collectability is reasonably assured.
We generally recognize product revenues once passage of title has occurred. Shipping and handling costs charged to customers are recorded as revenues and included in cost of product revenues at the time the sale is recognized.
For revenue agreements with multiple-element arrangements, such as license and contract research agreements, we evaluate the arrangements to determine whether the deliverables can be separated or whether they must be accounted for as a single unit of accounting. This determination is generally based on whether any deliverable has stand-alone value to the customer. This analysis also establishes a selling price hierarchy for determining how to allocate arrangement consideration to identified units of accounting. When deliverables are separable, consideration received is allocated to the separate units of accounting based on the relative selling price method and the appropriate revenue recognition principles are applied to each unit. The selling price used for each unit of accounting is based on estimated selling price as neither vendor-specific nor third-party evidence is available. When we determine that an arrangement should be accounted for as a single unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized over the performance period.
We have determined that, at the inception of each license agreement, there is only one deliverable for the license for, access to, and assistance with the development of the specified intellectual property. We recognize revenue from upfront payments proportionally over the term of our estimated period of performance under the agreement. On a quarterly basis, we review our estimated period of performance for our license agreements based on the progress under the arrangement and account for the impact of any changes on a prospective basis. We recognize annual license fees proportionally over the related term subject to cancellation provisions.
We recognize revenue related to milestone payments when the contractually specified performance obligations are achieved. Performance obligations typically consist of significant milestones in the development life cycle of the related technology, such as achievement of specific technological targets,
successful results from field trials, filing for approval with regulatory agencies, approvals granted by regulatory agencies and commercial launch of a product utilizing the licensed technology.
Contract research revenue consists of amounts earned from performing contracted research activities for third parties. Activities performed are related to breeding programs or the genetic engineering of plants and are subject to an executed agreement. We generally recognize fees for research activities ratably over the contractually specified performance period.
Grant revenues are recognized as eligible research and development expenses are incurred using a proportional performance recognition methodology.
Deferred revenue represents the portion of payments received that has not been recognized.
Inventories
Inventory costs are tracked on a lot-identified basis, valued at the lower of cost or market and are included as cost of product sales when sold. We compare the cost of inventories with market value and write down inventories to market value, if lower. We provide for inventory reserves when conditions indicate that the selling price may be less than cost due to physical deterioration, obsolescence, changes in price levels, or other factors. Additionally, we provide reserves for excess and slow-moving inventory
to its estimated net realizable value. The reserves are based upon estimates about future demand from our customers and distributors and market conditions. Future events that could significantly influence our judgment and related estimates include conditions in target markets, introduction of new products or changes to current or future competitor products.
Stock-Based Compensation
We recognize compensation expense related to stock options granted to employees and directors based on the estimated fair value of the awards on the date of grant, net of estimated forfeitures. We estimate the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model. The grant date fair value of the stock-based awards is generally recognized on a straight-line basis over the requisite service period, which is generally the vesting period of the respective awards.
We recognize compensation expense for equity instruments issued to non-employees based on the estimated fair value of the equity instrument. The fair value of the non-employee awards is subject to re-measurement at each reporting period until services required under the arrangement are completed, which is the vesting date.
We recorded stock-based compensation expense related to equity awards of $1.3 million and $1.0 million for the years ended December 31, 2013 and 2014, respectively. This expense relates to equity awards made prior to January 1, 2013 and in the year ended December 31, 2014.
In February and March 2015, our Board of Directors approved the grant of options to purchase an aggregate of 1,230,000307,493 shares of common stock with an exercise price of $1.80$7.20 per share.
In determining the fair value of stock-based awards, we use the Black-Scholes option-pricing model and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine.
Expected Term—The expected term represents the period that stock-based awards are expected to be outstanding and was estimated based on historical and anticipated future exercise activity.
Expected Volatility—Since we are privately held and do not have any trading history for our common stock, the expected volatility was estimated based on the average historical volatilities of common stock of comparable publicly traded entities over a period equal to the expected term of the stock option grants. The comparable companies were chosen based on their similar size, stage in the life cycle, or area of specialty. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available.
Risk-Free Interest Rate—The risk-free interest rate is based on the U.S. Treasury zero coupon issues in effect at the time of grant for periods corresponding with the expected term of option.
Expected Dividend—We have never paid dividends on our common stock and have no plans to pay dividends on our common stock. Therefore, we used an expected dividend yield of zero.
In addition to the Black-Scholes assumptions, we estimate our forfeiture rate based on an analysis of our actual forfeitures and will continue to evaluate the adequacy of the forfeiture rate based on actual forfeiture experience, analysis of employee turnover behavior, and other factors. The impact from any forfeiture rate adjustment would be recognized in full in the period of adjustment and if the actual number of future forfeitures differs from our estimates, we might be required to record adjustments to stock-based compensation in future periods.
Historically, for all periods prior to this initial public offering, the fair value of the shares of common stock underlying our share-based awards were estimated on each grant date by our board of directors, which intended all stock options granted to be exercisable at a price per share not less than the per share fair market value of our common stock underlying those options on the date of grant. Given the absence of a public trading market for our common stock, our board of directors exercised
reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of our common stock, including our stage of development; progress of our research and development efforts; the rights, preferences and privileges of our preferred stock relative to those of our common stock; equity market conditions affecting comparable public companies and the lack of marketability of our common stock. To assist our board of directors in this determination and in order to set the exercise price of each stock option grant, our management informed them of the most recent available valuation analysis prior to the dates of grant.
For purposes of our February and March 2015 option grants, our board of directors determined the fair market value of our common stock after consideration of the factors listed above, including the contemporaneous independent third-party valuation as of September 15, 2014. The valuation uses the income approach and the market approach. The income approach estimates the fair value of a company based on the present value of the company's future estimated cash flows. These future cash flows are discounted to their present values using an appropriate discount rate, to reflect the risks inherent in the company achieving these estimated cash flows. The discount rate used in our valuation was based primarily on benchmark venture capital studies of other companies in similar stages of development. The market approach determines the fair value of the company by estimating the value of the business based on projecting a future value under an initial public offering scenario, referencing recent biotechnology initial public offerings, our 2014 Series D preferred stock transaction, and an estimate of value under a merger and acquisition scenario. The estimated enterprise value is then allocated to the common stock using both the Option Pricing Method, or OPM, and the Probability Weighted Expected Return Method, or PWERM, or the hybrid method. The hybrid method applies the PWERM utilizing the probability of two exit scenarios, an initial public offering or an acquisition, and the OPM was utilized in the continuing as a private company scenario.
For stock options and other equity awards after the completion of this offering, our board of directors will determine the fair value of each share of underlying common stock based on the closing price of our common stock as reported on The NASDAQ Global Select Market on the date of grant.
Income Taxes
We use the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
As of December 31, 2014, we had net operating loss carryforwards for federal income tax reporting purposes of $98.1 million, which begin to expire in 2020, and state net operating loss carryforwards of $77.2 million, which began expiring in 2015.
Quantitative and Qualitative Disclosures aboutAbout Market Risk
As of December 31, 2014, we had cash and cash equivalents of $16.6 million, which consisted primarily of bank deposits. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations of interest income have not been significant.
We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
Recent Accounting Pronouncements
In April 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update (ASU) ASU 2014-08,Presentation of Financial Statements (Topic 205) and Property, Plant, and Equipment (Topic 36), which amends the definition of a discontinued operation in ASC 205-20 and requires entities to provide additional disclosures about disposal transactions that do not meet the
discontinued-operations criteria. The revised guidance will change how entities identify and disclose information about disposal transactions under U.S. GAAP. The ASU applies to all entities and is effective for annual periods beginning after December 15, 2014 and interim periods thereafter, with early adoption permitted. We do not anticipate that the adoption of this ASU will change the presentation of our consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09,Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard will be effective for us beginning January 1, 2017. Early application is not permitted. The standard permits the use of either the retrospective or cumulative effect transition method. We are evaluating the effect that ASU 2014-09 will have on our consolidated financial statements and related disclosures. We have not yet selected a transition method nor have we determined the effect of the standard on our ongoing financial reporting.
In August 2014, the FASB issued ASU 2014-15,Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which provides guidance on determining when and how to disclose going-concern uncertainties in the consolidated financial statements. The new standard requires management to perform interim and annual assessments of an entity's ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if "conditions or events raise substantial doubt about the entity's ability to continue as a going concern." The ASU applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. We do not anticipate a material change to our consolidated financial statements upon the adoption of this ASU. However, we will be required to evaluate and determine if further disclosure is necessary at each balance sheet date.
In February 2015, the FASB issued ASU 2015-02,Consolidation (Topic 810): Amendments to the Consolidation Analysis. The amendments significantly change the consolidation analysis required under U.S. GAAP. Reporting entities will need to reevaluate all their previous consolidation conclusions. We do not anticipate that the adoption of this ASU will materially change the presentation of our consolidated financial statements.
Industry Overview and Macro Drivers
Global agricultural production continues to expand in response to a growing population, an increasing standard of living in emerging markets, and the resulting increased demand for higher-quality food. According to the United States Department of Agriculture, or USDA, the annual global food market was estimated to be approximately $4.0 trillion in 2012. This market was supplied by 164 crops that, according to the Food and Agriculture Organization of the United Nations, or FAO, generated approximately $2.6 trillion in annual farm revenue in 2012. Of these crops, the top five (rice, corn, wheat, soybean, and sugarcane) accounted for more than $1.0 trillion in annual farm revenue as of 2012. Global crop production utilizes several key inputs. According to MarketLine, the global fertilizer industry was $183.3 billion in 2012. According to Phillips McDougall, the global crop protection chemicals industry was $54.2 billion and the global seed industry was $39.4 billion in 2013.
The combination of population growth and rising incomes in emerging markets is expected to continue driving increased demand for higher value food, particularly meat. Growth in global meat consumption directly increases the need for agricultural crop products. In 2009, the FAO estimated that supplies of agricultural products will need to increase 60% by 2050 in order to meet global food demand. Due to constrained land and water resources, the FAO also estimated that approximately 90% of this increase will need to come from increased farm yield and increased farming intensity.
Over the last several decades, consecutive waves of innovation have provided new solutions for increasing agricultural yields. Improved irrigation techniques, enhanced mechanization, increasing use of fertilizers and crop protection chemicals, and improved seed varieties have contributed to significant increases in yield.
Traditional plant breeding techniques have resulted in a very long history of increasing crop yields. Over the past two decades, advances in plant biotechnology have led to the development and commercialization of novel characteristics, or traits, in crops. Seeds developed using biotechnology techniques rather than conventional plant breeding are generally known as biotech seeds. Biotech seeds that are developed using genetic modification techniques are known as genetically modified, or GM, seeds. There are also biotechnology techniques, such as marker assisted selection, that do not involve genetic modification and, therefore, do not result in GM seeds. The GM seed industry has historically focused on mitigating the negative yield impact of living, or biotic, plant stresses, such as insect pests, diseases, and weeds. To date, GM seed traits have generally been herbicide tolerance and insect resistance, and have primarily been commercialized by companies with significant crop protection chemical platforms. Seeds with these traits have achieved strong commercial success, reaching market share in excess of 90% in key crops and countries as of 2013. These traits, initially offered individually, are increasingly available in combinations, or stacks, leading to an increase in seed value and significant growth in the GM seed industry.
Phillips McDougall estimates that the market for GM seeds was approximately $20.1 billion in 2013, or approximately 51% of the total $39.4 billion seed market. According to Phillips McDougall, the total market for conventional seeds grew at a compound annual growth rate, or CAGR, of 3.6% between 2003 and 2013. During the same period, GM seed market growth was at a CAGR of 18.4%, far outpacing growth in the conventional seed market.
The graph below depicts the growth in value of GM and conventional seed markets from 2003 through 2013.
Source: Seed Industry Synopsis, Phillips McDougall, June 2014
The chart below shows the composition of the $20.1 billion GM seed market in 2013 by type of trait and illustrates the dependence on biotic stress traits as well as the significant market share of stacked biotic traits.
Source: Seed Traits, Phillips McDougall, July 2014
Abiotic plant stresses, or those caused by non-living factors such as heat, drought, flooding, salinity, and nutrient availability, can have a significantly greater negative impact on yield than biotic stresses. Commercially available solutions to manage abiotic stresses are currently limited, but have been the focus of recent innovation efforts. Seed companies are working to develop portfolios of abiotic traits such as nitrogen use efficiency, water use efficiency, drought tolerance, heat tolerance, salinity tolerance, and flooding tolerance, among others.
Innovative traits can provide significant additional value to farmers. Planting seed is a relatively low cost input for farmers, representing less than 10% of average total costs in 2013 according to the USDA. GM seeds can provide farmers with increased profitability at a relatively low increase in operating costs by means of increased yields, reduced cost of inputs such as chemicals, or enhanced product quality. The historic success of increasing farm profits through the use of GM seeds has fueled the development of the agricultural biotechnology industry, and farmers have historically shared a significant portion of their economic benefit with the GM seed provider in the form of seed premiums.
History of Innovation in the Seed Market
Historically, crop improvements came from conventional plant breeding approaches involving the selection and advancement of exceptional individual plants within a population. Because of the low frequency and generally low margin of improvement for any individual plant, this approach requires many generations and numerous years of breeding to develop a significantly improved variety. Plant biotechnology techniques, including genetic modification, have significantly enhanced the capabilities of plant breeders and facilitated the development of improved varieties with valuable traits. Other biotechnology techniques, including advanced methods of selection and screening, build on knowledge gained from genetic research and have also led to the development of valuable new traits at a faster pace.
Since the early 1990s, the number of research programs involving biotech plants has grown enormously. Crops containing GM traits were first introduced in 1995 and the total area planted with GM seeds has been steadily growing, both in industrial and developing countries, as illustrated by the chart below.
Source: Clive James, 2013, ISAAA
Herbicide tolerance and insect resistance traits were the first types of GM seeds to be successfully introduced, and the GM seeds on the market today are primarily focused on these two types of traits, both of which address biotic stress issues. There are also a few commercialized product quality traits available, most of which are focused on changing the fatty acid composition of plant oils produced by soybean and canola seeds. While research and innovation into traits addressing abiotic stresses, such as heat, drought, flooding, salinity, and nutrient availability, is ongoing, there were only three such traits on the market as of 2013 according to Phillips McDougall.
LibertyLink, introduced in 1995 by Bayer, and Roundup Ready, introduced in 1996 by Monsanto, provide resistance to broad spectrum herbicides and allow farmers to use these on their fields instead of complex and more expensive combinations of selective herbicides. More recently, the range of available herbicide tolerance traits has broadened, providing resistance to multiple herbicides, although many are available only for selected crops.
Since the introduction of herbicide tolerance and insect resistance traits in 1995 and 1996, respectively, many major seed companies have commercialized multiple seed products with these traits. The use of herbicide tolerant and insect resistant seeds enables farmers to reduce their use of herbicides and insecticides on crops. Some of the increased profit realized by farmers is shared with seed providers in the form of a premium paid for the improved seeds.
Biotech seeds, and specifically GM seeds, have been adopted by an increasing number of countries since their introduction in 1995. According to International Service for the Acquisition of Agri-Biotech Applications, or ISAAA, the global area of GM crops has increased more than 100-fold from 1.7 million hectares in 1996 to over 175 million hectares in 2013, making GM crops the highest growth crop technology in recent history. According to ISAAA, as of 2013, 27 countries have grown GM crops and more than half of the world's population, or 60%, live in those countries. The United States continues to be the leading producer of GM crops globally with 70.1 million hectares, and average
adoption level of 90% across all crops. Six developing countries—Brazil, Argentina, India, China, Paraguay, and South Africa—grew approximately 49% of global GM crops.
The graphic below identifies the 27 countries growing GM crops and the area grown in each country.
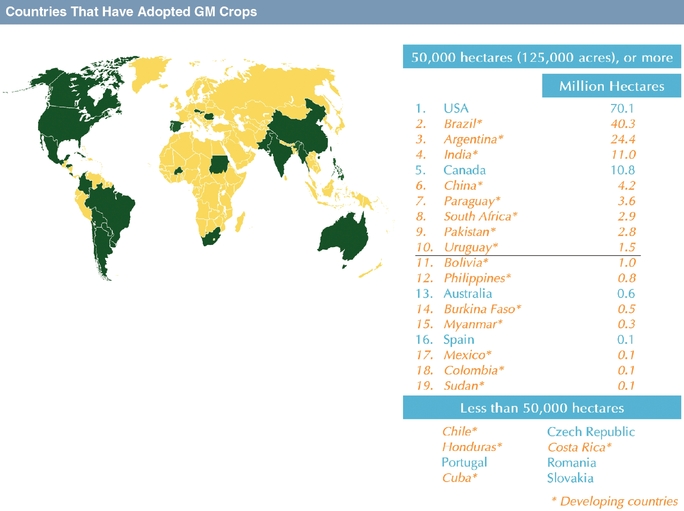
Source: Clive James, 2013. Global Status of Commercialized Biotech/GM Crops for 2013: ISAAA Brief 46.
In markets where GM seeds are commercialized, market penetration is significant by crop type, as illustrated in the table below.
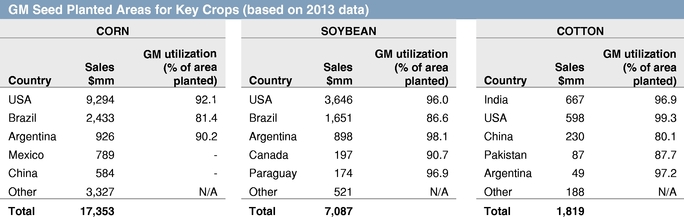
Source: Phillips McDougall, 2013
Next Generation Seed Traits
Next generation seed trait research is focused on the development of new technologies that address unmet needs such as abiotic stress tolerance and agricultural product quality. Seed traits are generally divided into two main categories, agricultural yield and product quality traits. Agricultural yield traits add value to farmers by decreasing production costs and increasing yield. Product quality traits increase the value of crops to crop processors, food and feed manufacturers and consumers by altering the composition of the harvested product.
Traits addressing abiotic stresses that limit crop yields have significant potential for increasing global yields and farmer profits. For example, conventional crops are inherently inefficient in the use of nitrogen fertilizer, the key yield-driving nutrient, and a $104.6 billion product within the $183.3 billion fertilizer industry. Research published in Plant Biotechnology Journal reported that only 30 to 50% of added nitrogen fertilizer is taken up by agricultural crops, with the remainder left unutilized and potentially becoming a significant environmental pollutant. Nitrogen use efficiency, or NUE, traits are designed to improve crop use of nitrogen and are expected to provide farmers with higher profits by increasing yields or decreasing the use of nitrogen fertilizer.
Other abiotic stresses such as heat, drought, flooding, salinity, and nutrient availability have significant negative impacts on yield and are largely unaddressed by currently available products. Abiotic stress tolerance traits are designed to reduce the negative impact of this natural response and significantly enhance yield potential. As illustrated in the chart below, yield losses from abiotic stresses significantly outweigh the impact of losses from biotic stresses and, as a result, the potential value of traits addressing abiotic stresses is expected to be significantly higher than the value of currently commercialized biotic stress traits.
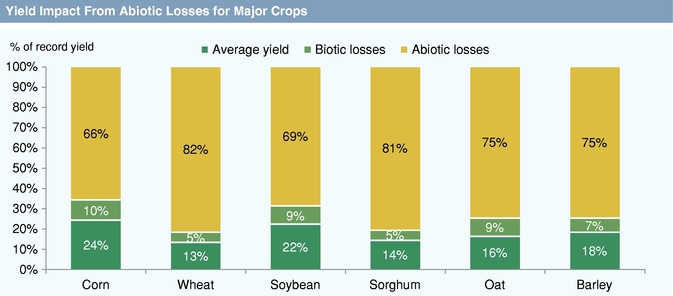
Source: Biochemistry and Molecular Biology of Plants, Buchanan, Gruissem, Jones, American Society of Plant Physiologists, 2000.
The GM seed industry has historically focused on crops and traits where the combination of large acreage and high input costs (such as pest and weed control chemical costs) create significant economic value for a trait. In contrast, a number of crops with very large planted areas and high potential for yield improvement remain largely unpenetrated by the GM seed industry today. These represent a large opportunity for market expansion with next generation seed traits. For example, wheat—the most widely grown global crop in 2013 according to the FAO—represents a large potential market for GM seed development. The same is true for rice, the third most widely grown global crop in 2013 according to the FAO.
The following table, based on a Phillips McDougall analysis that we commissioned, sets forth the estimated commercial value of key agricultural yield traits in the most widely grown global crops. Estimated trait commercial value refers to the total revenue potential for seed providers generated from the premium charged on biotechnology seeds due to the added value of the improved trait. The estimated trait commercial value was calculated by multiplying the estimated number of acres per country that could be planted with a particular biotechnology trait by the estimated premiums that will be charged to growers by seed providers. The values displayed are in constant 2013 U.S. Dollar terms.
| | | Estimated Trait Commercial Value ($ Million)(1) | | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Crop | Trait | From | To | Key Growing Regions(2) | ||||||
Corn | Nitrogen Use Efficiency | 1,285 | 2,205 | NAFTA, LATAM, China | ||||||
| Water Use Efficiency | 557 | 976 | ||||||||
| Heat Tolerance | 557 | 976 | ||||||||
| Crop Total | 2,399 | 4,157 | ||||||||
Soybeans | Nitrogen Use Efficiency | 747 | 1,269 | NAFTA, LATAM | ||||||
| Water Use Efficiency | 373 | 642 | ||||||||
| Salinity Tolerance | 373 | 523 | ||||||||
| Heat Tolerance | 373 | 523 | ||||||||
| Crop Total | 1,866 | 2,957 | ||||||||
Rice | Nitrogen Use Efficiency | 535 | 910 | India+, China, Asia | ||||||
| Water Use Efficiency | 269 | 535 | ||||||||
| Salinity Tolerance | 269 | 535 | ||||||||
| Heat Tolerance | 269 | 535 | ||||||||
| Crop Total | 1,342 | 2,515 | ||||||||
Wheat | Nitrogen Use Efficiency | 573 | 1,136 | NAFTA, Europe, India+, China, Australia | ||||||
| Water Use Efficiency | 487 | 569 | ||||||||
| Salinity Tolerance | 429 | 569 | ||||||||
| Heat Tolerance | 429 | 569 | ||||||||
| Herbicide Tolerance | 417 | 571 | ||||||||
| Crop Total | 2,335 | 3,414 | ||||||||
Cotton | Nitrogen Use Efficiency | 189 | 312 | NAFTA, LATAM, India+ | ||||||
| Water Use Efficiency | 97 | 176 | ||||||||
| Salinity Tolerance | 97 | 176 | ||||||||
| Heat Tolerance | 97 | 176 | ||||||||
| Crop Total | 480 | 840 | ||||||||
Canola | Nitrogen Use Efficiency | 142 | 227 | NAFTA, LATAM, India, China | ||||||
| Water Use Efficiency | 75 | 114 | ||||||||
| Salinity Tolerance | 75 | 114 | ||||||||
| Heat Tolerance | 75 | 114 | ||||||||
| Crop Total | 368 | 569 | ||||||||
Sugarcane | Nitrogen Use Efficiency | 42 | 70 | NAFTA, LATAM, India, China | ||||||
| Water Use Efficiency | 25 | 53 | ||||||||
| Heat Tolerance | 21 | 41 | ||||||||
| Crop Total | 88 | 164 | ||||||||
All Crops | All Traits | 8,878 | 14,616 | |||||||
Innovation and Commercialization Process in Biotech Seed Traits
Developing and integrating seed traits into commercial seeds using advanced breeding or biotechnology is a lengthy process. The length of the process may vary depending on the complexity of the trait and the type of crop involved. The development process for GM seed traits is divided into several discrete steps, or phases, which generally include discovery, validation, and development through field trials, regulatory review, and commercial launch of a GM seed product containing the trait. The following table summarizes these phases, indicates the timeframes that may be required to complete each phase, and provides an estimate of the average probability of commercial success at each phase. This table is substantially based on the development stages described in Monsanto Supplemental Information for Investors, April 6, 2011, which we refer to as the Monsanto Toolkit and which we believe has been adopted by other companies and research analysts as an industry standard for seed trait development. We believe that the Monsanto Toolkit framework has been adopted by the industry because the factors that affect the duration and probability of success of each stage in the GM trait research and development process are comparable across companies within the industry, due in part to the impact of plant biology on the development process and timeline. Although we believe the development stages described in the Monsanto Toolkit are generally applicable to trait research and development, whether conducted by Monsanto or by other companies, including us, the actual development time for any particular product and the probability of success will vary and is dependent on many factors, including those set forth in "Risk Factors."
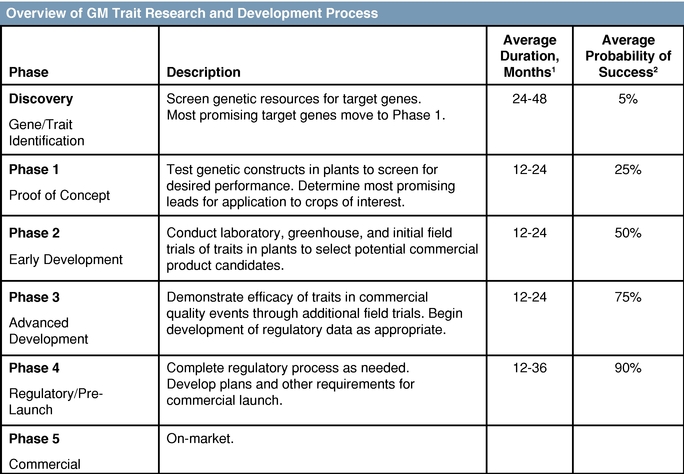
In the early stages of development, the process for developing seed traits is similar under both conventional and GM approaches. However, the two methods differ significantly in later phases of development. Completing regulatory review for GM seeds is a far more comprehensive and lengthy process than for conventionally developed seeds. However, the plant breeding required to develop commercial seed products based on GM technologies is typically carried out in parallel to the regulatory process, meaning that the regulatory process may not be rate-limiting in all situations.
Participation in different stages of the trait development and commercialization process carries different levels of economic benefits. Typically, companies that participate in later stages of the development process are able to retain a greater portion of the economic value attributable to the trait.
Competitive Landscape
The development of GM seed traits is concentrated in a limited number of large seed companies, including Monsanto, DuPont Pioneer, Syngenta, Limagrain, Dow AgroSciences, KWS SAAT, and Bayer CropScience. According to Phillips McDougall, the leading 11 seed and trait companies as a group invested $4.1 billion in seed and trait research and development in 2013. Many of these companies have programs that address both biotic and abiotic stress. Many of them also have extensive regulatory and commercialization capabilities and are able to take a trait from the gene discovery phase to commercialization on their own. Notwithstanding their in-house capabilities, many of these companies also regularly source traits from third parties.
A few small specialized biotechnology companies focus on the research and development of a select range of traits. These companies typically have limited development capabilities and do not have significant regulatory capabilities. They generally seek to partner with large seed companies, such as those identified above, in order to complete regulatory requirements for their technologies and bring products to market.
Overview
We are a leading agricultural biotechnology trait company with an extensive and diversified portfolio of late-stage yield and product quality traits addressing multiple crops that supply the global food and feed markets. Our traits are focused on high-value enhancements that increase crop yields by enabling plants to more efficiently manage environmental and nutrient stresses, and that enhance the quality and value of agricultural products. Our traits increase value not only for farmers, but also for users of agricultural products. There currently are more than 50 products in development incorporating our traits and there are 13 in advanced stages of development or on the market.
Our crop yield traits are being utilized by our commercial partners to develop higher yielding seeds for the most widely grown global crops, including wheat, rice, soybean, corn, and sugarcane, as well as for other crops such as cotton, canola, turf, and trees. Our business model positions us at the nexus of basic research and commercial product development, as we apply our strong product development and regulatory capabilities to collaborate with, and leverage the skills and investments of, upstream basic research institutions and downstream commercial partners. We believe our approach significantly reduces risk and capital requirements, while simplifying and expediting the product development process. We also believe that our collaboration strategy leverages our internal capabilities, enabling us to capture much higher value than would otherwise be the case, and enabling our commercial partners to develop and commercialize products more cost-effectively.
In recent decades, agricultural biotechnology has been a major driving force for improving farm economics by introducing genetically modified, or GM, seeds, with traits that reduce the cost of managing crop biotic stresses such as weeds, insects, and microbial pests. The first agricultural biotechnology traits, herbicide tolerance and insect resistance, were developed primarily by companies with deep expertise and a long heritage in crop protection chemistry and pest management. Seeds with these traits have achieved rapid growth and strong commercial success, reaching market share in excess of 90% in key crops and countries as of 2013.
We believe the next generation of advancements in agricultural biotechnology involves increasing yields by making crops which perform significantly better under a wide range of abiotic stresses, including drought, heat, salinity, and variable availability of key nutrients such as nitrogen. Our target market is the $39.4 billion global seed market. Our goal is to increase the value of this market significantly by increasing yields in the more than $1.0 trillion market for the five largest global crops, and to capture a portion of the increased value.
Our business model focuses on creating value by leveraging collaborator investments and capabilities upstream in basic research, and downstream in product development and commercialization. We bridge the gap between basic research and commercial development, reducing risk and adding value as a result. We reduce risk and avoid most of the costs associated with basic research by acquiring trait technologies that have already completed initial feasibility screening, thus achieving proof of concept, through basic research carried out elsewhere. We further develop these technologies by optimizing function and validating performance through intensive field trial testing in multiple crops. We then form collaborations with major seed and consumer product companies that develop and commercialize products incorporating our traits. In select instances, we also work with our commercial partners to make any regulatory filings required to support commercial launch of the trait in order to increase our share of the value created by the trait.
By licensing later stage de-risked technologies to our commercial partners, we expect to achieve significantly greater value than generally earned for access to early stage traits. Our license agreements typically include upfront and annual license fees, as well as multiple milestone payments for key product development stages such as demonstration of greenhouse efficacy, demonstration of field
efficacy, regulatory submission, regulatory approval, and commercial launch. Following commercialization of a product utilizing one or more of our traits, we share in the value of the traits realized by our commercial partners. We believe that this broad and balanced approach diversifies and reduces risk, allowing us to address multiple end markets through strong established channels.
There currently are more than 50 products in development incorporating our traits and there are 13 in advanced stages of development or on the market. We use both GM and non-GM technologies to develop our traits, which enables us to select the approach most suited for the particular trait, crop and market. Our agricultural yield traits are designed to substantially increase crop yields and farmer income. They do so either by improving efficiency in the use of key inputs, such as fertilizer and water, or by increasing tolerance to environmental stresses, such as drought, heat and salinity. Our existing portfolio of agricultural yield traits includes Nitrogen Use Efficiency, or NUE, Water Use Efficiency, or WUE, Drought Tolerance, Salinity Tolerance, Heat Tolerance, and Herbicide Tolerance. Field trial results have demonstrated significant yield improvements resulting from our agricultural yield traits in multiple crops and geographies. As one example, field trials in multiple environments conducted by an independent testing organization with our NUE trait in rice resulted in a consistent yield improvement that on average was 27% above the controls over a three-year period from 2012 to 2014. Rice is the world's most valuable crop, with a harvest value of $334.4 billion in 2012, and the third most widely grown crop, according to the FAO. Our agricultural product quality traits increase the value of harvested products by improving specific compositional qualities of oilseeds and grains. These traits include Enhanced Nutrition Grains and High Value Nutritional Oils, including Sonova 400 GLA safflower oil and Sonova Ultra GLA safflower oil, which we refer to as our Sonova products.
We have formed strategic partnerships and developed strong relationships with global agricultural leaders for development and commercialization of our traits in major crops and consumer products. Our collaborators include subsidiaries or affiliates of Limagrain (Vilmorin & Cie), Mahyco (Maharashtra Hybrid Seeds Company Limited), Dow AgroSciences, DuPont Pioneer (E.I. du Pont de Nemours and Company), SES Vanderhave, Genective (a joint venture between Limagrain and KWS SAAT), Scotts, U.S. Sugar, Abbott, Ardent Mills, Bioceres, and others. Additionally, in order to increase our participation in the value of two major crops, wheat and soybean, we have formed two joint ventures. Limagrain Cereal Seeds LLC is our joint venture with Limagrain for the development and commercialization of wheat products for North America. Limagrain is the world's fourth-largest seed company. Verdeca LLC is our joint venture with Bioceres for the development and deregulation of soybean traits globally. Bioceres is an agricultural investment and development company owned by approximately 230 shareholders, including some of South America's largest soybean growers. In April 2015, we entered into a collaboration agreement with Dow AgroSciences and Bioceres under which our Verdeca joint venture will collaborate with Dow AgroSciences on the development and deregulation of soybean traits on a global basis.
The strength of our internal capabilities and collaboration strategy enables us to quickly identify and develop valuable traits and bring them to market, as we have demonstrated through commercializing Sonova 400 GLA safflower oil in less than six years from technology acquisition to commercial launch. Sonova 400 GLA safflower oil is a key ingredient in multiple branded nutritional supplements marketed through GNC stores and other major U.S. retailers.
Our Strengths
We believe we are strategically positioned to capitalize on the need to increase crop yields and quality of agricultural products globally. Our competitive strengths include:
addressing opportunities to increase yield in the much larger market for agricultural products will dramatically expand the size of the GM seed market, and that we are well-positioned to take advantage of this with our portfolio of late-stage, high-value traits, and our ability to reduce
product development risk and leverage the capabilities of our licensees and partners. We believe the yield-enhancing benefits of our agricultural yield traits provide significant value to farmers, based on field trials to date. As one example, field trials conducted by an independent testing organization with our NUE trait in rice resulted in a consistent yield improvement that on average was 27% above the controls over a three-year period from 2012 to 2014. We carefully select our collaborators and partners and license our traits to leading seed and consumer product companies. This allows us to leverage their substantial development capabilities and market presence, creating a highly scalable and capital light platform.
McDougall. Our regulatory team's expertise in bringing traits through the regulatory process quickly and cost-effectively is a key differentiating factor. For example, by working closely with federal and state regulatory authorities, we have designed and implemented robust protocols for conducting field trials in California with GM rice. To our knowledge, we are the only company currently permitted to conduct such trials. Coupled with strong in-house intellectual property law
expertise, our technology development process has resulted in a portfolio of over 100 issued patents that are either owned or exclusively controlled by us.
Our Growth Strategy
We believe that there are significant opportunities to grow our business globally by executing the following elements of our strategy:
best-in-class technology and research and development capabilities that will enable us to continue advancing our position in the agricultural biotechnology marketplace.
Our Products and Product Development Pipeline
There currently are more than 50 products in development incorporating our traits and there are 13 in advanced stages of development or on the market. We use both GM and non-GM technologies to develop our traits, which enables us to select the approach most suited for the particular trait, crop, and market. Our agricultural yield traits are designed to substantially increase crop yields and farmer income. They do so either by improving efficiency in the use of key inputs, such as fertilizer and water, or by increasing tolerance to environmental stresses, such as drought, heat, and salinity. Our existing portfolio of agricultural yield traits includes NUE, WUE, Drought Tolerance, Salinity Tolerance, Heat Tolerance, and Herbicide Tolerance as further described below. Our traits are developed as individual offerings and as stacks that incorporate several different traits, and can be designed for use in a variety of crops and end markets.
The following table summarizes our current commercial product and our pipeline of products in development.
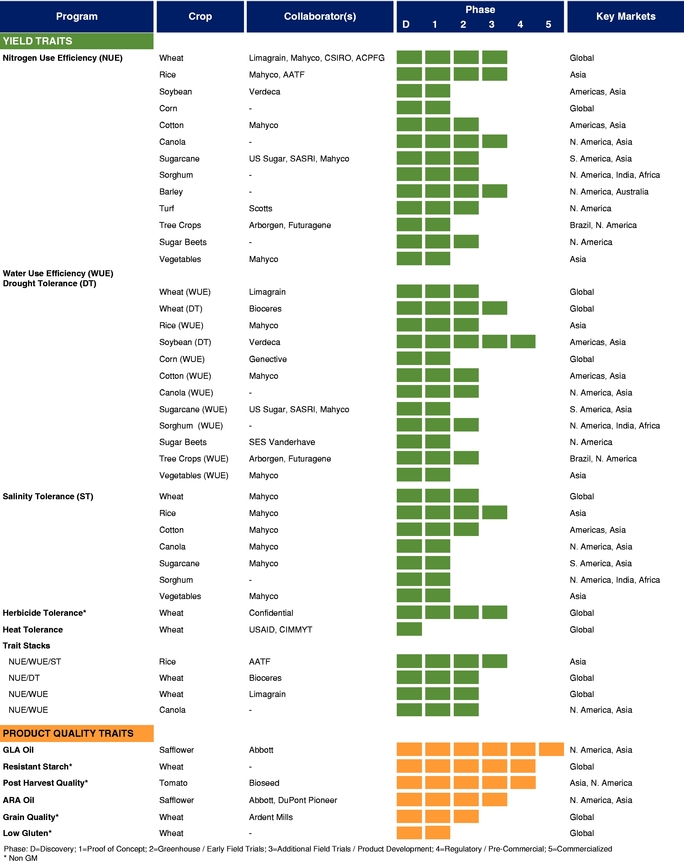
Agricultural Yield Traits
Nitrogen Use Efficiency (NUE)
Our NUE technology enables plants to absorb and utilize nitrogen fertilizer much more efficiently than conventional plants. This allows crops to achieve significantly higher yields under normally applied levels of nitrogen fertilizer, or to achieve the same yields as conventional crops while using 30 to 50% less nitrogen fertilizer.
Nitrogen fertilizer is a primary plant nutrient and key driver of crop yield. Nitrogen fertilizer is a significant component of crop production cost and was a $104.6 billion product within the $183.3 billion market for all fertilizers in 2012, according to an industry-specific report by MarketLine. Research published in Plant Biotechnology Journal reported that only 30 to 50% of added nitrogen fertilizer is taken up by agricultural crops, with the remainder left unutilized and potentially becoming a significant environmental pollutant.
Our NUE technology was originally discovered at the University of Alberta (Canada) and we hold an exclusive, global license to the technology for use in all crops, with unlimited sublicense rights. The first commercialization of crops with NUE technology is expected to occur within the next three to five years.
The target crops and markets for NUE include all major agricultural crops and markets. Our NUE technology has now been incorporated, or is under development by our commercial partners, in major global crops, including rice, wheat, soybean, cotton, canola, sugar beets, sugarcane, vegetables, turf grass, and multiple forestry species. Specific crops, collaborators, stages of development, and target markets for our NUE technology are shown in the following table.
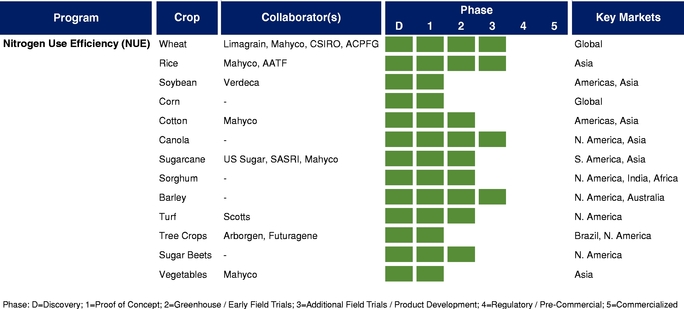
Field trial data to date in multiple major commodity crops has shown yield improvements attributable to our NUE trait of greater than 10%. For example, we and Limagrain have independently conducted field trials of NUE in wheat at multiple locations across multiple crop seasons, and the leading NUE wheat line has shown an average yield improvement relative to the control of 10%.
In another example, the International Center for Tropical Agriculture, or CIAT, an independent research organization, has conducted field trials of NUE in a major type of rice for three years (2012, 2013, and 2014) under both lowland (flood irrigated) and upland (rain irrigated) locations. Of the six NUE rice lines tested, two consistently showed significant yield benefits across all field trials and
treatments. The leading line out-yielded the control by an average of 27% over three years for the two locations and for three rates of nitrogen fertilizer in the lowland location. The table below summarizes the average yield increase over three years, relative to the control, for the two lead lines, as reported by CIAT.
NUE Rice Field Trial Results 2012-2014 (increase in grain yield)
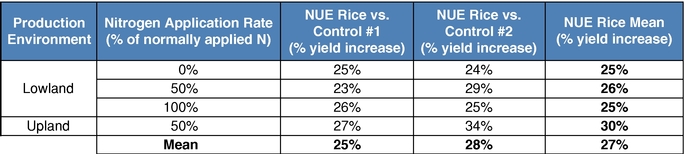
We have also created a methodology to quantify and document changes in greenhouse gas emissions resulting from changes in nitrogen use. This methodology, approved by the Intergovernmental Panel on Climate Change in 2012, is the first of its kind to link crop genetics with carbon emissions. We believe this may encourage the adoption of crops with NUE technology by enabling farmers to further increase revenue through the sale of carbon credits.
Water Use Efficiency (WUE)/Drought Tolerance
Our WUE trait technology enables plants to better tolerate two distinct types of stress: reduced or inconsistent water and severe drought. The WUE trait has been demonstrated to improve crop yield under conditions of episodic water stress and to help crops recover from severe drought conditions. A related but distinct technology, Drought Tolerance, helps plants maintain yields under conditions of prolonged water stress.
In 2012, the United Nations Educational, Scientific, and Cultural Organization, or UNESCO, reported that modern agriculture is highly water intensive, using approximately 70% of world water withdrawals. UNESCO also estimates that future global agricultural water consumption will increase by about 19% by 2050 and could be even higher if crop yields and the efficiency of agricultural production do not improve dramatically. The irregular availability of suitable water is one the leading causes of reduced crop yield globally. Loss due to drought in the United States, as reported to the USDA Risk Management Agency, averaged $4.7 billion from 2009 through 2013 and was $12.9 billion in 2012. Water-limiting conditions can result from prolonged drought, leading to severe reductions in crop yields, or can result from periodic dry conditions, leading to reduced crop yields. Whenever water limitations occur, economic losses and impairment of the food supply result.
Our WUE trait technology was jointly discovered by researchers at the University of California, Davis and Technion—Israel Institute of Technology. We hold an exclusive, global license to the technology, with sublicense rights, for use in all crops. Target crop markets for WUE technology include most major crops, such as rice, wheat, corn, soybean, sugarcane, cotton, and canola. Target geographies are global, based on regions where water availability can limit productivity in the target crops. Our Drought Tolerance technology was discovered by researchers at National Scientific and Technical Research Council (Argentina), and further developed by Bioceres, S.A. We hold an exclusive license to this technology for use in wheat globally outside of South America. Verdeca, our joint venture with Bioceres, Inc., holds exclusive global rights and is developing and commercializing this technology in soybeans.
Our WUE technology has now been incorporated, or is under development by our commercial partners, in major global and secondary crops, including those shown in the following table. Our Drought Tolerance technology is being applied in wheat, and soybeans with this technology are in the regulatory approval process in Argentina. Specific crops, collaborators, stages of development, and target markets for our WUE and Drought Tolerance technologies are shown in the following table.
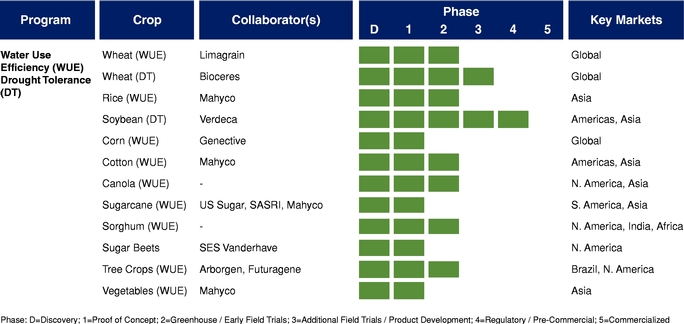
Greenhouse and field trials of our WUE traits have been completed in agronomic crops such as rice, cotton, peanuts and alfalfa. We are currently working with collaborators in additional crops, including wheat, sugar beets, sugarcane, and multiple tree species. Recent collaborator results in rice show significant yield improvement under water-limited conditions. Our Drought Tolerance technology is most advanced in soybeans under our Verdeca joint venture. Multiple seasons of field trials under reduced yield conditions that represent the average yield of soybean production in North and South America have shown yield improvements relative to controls of up to 14%, with no decrease in yield under optimal conditions, as illustrated in the following chart.
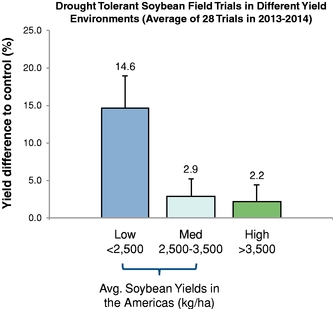
Salinity Tolerance
Our Salinity Tolerance trait allows plants to produce increased yields under conditions of elevated salinity and is applicable to a wide range of crops, including wheat, rice, soybean, cotton, and vegetables. Our salt-tolerant plants have also been demonstrated to bind excess salt from the soil into the plant, potentially providing the benefit of rehabilitating salinized land over time.
The global cost of lost crop yield to salt-induced land degradation is estimated to be $27.3 billion according to the United Nations Natural Resources Forum. Of the current 230.0 million hectares of irrigated land, 45.0 million hectares, or about 20%, are salt-affected. Crops grown under salt-affected conditions may be inhibited in two ways. First, the presence of salt in the soil reduces the ability of the plant to take up water, leading to reductions in growth rate. Second, if excessive amounts of salt enter the plant, there can be injury to the cells, which may cause further reductions in growth. Modern agriculture is highly water intensive and the ability to manage crops in saline environments will reduce agricultural demand on critical fresh water supplies.
Our most advanced Salinity Tolerance trait technology is being developed based on basic research conducted at the University of Toronto, the University of California, Davis, and the National Institute of Agrobiological Sciences (Japan), all of which have granted us exclusive licenses for all crops. We are conducting early stage research on additional salinity tolerance genes under a research funded agreement with the United States Agency for International Development, or USAID.
Target markets for the Salinity Tolerance trait are those areas where water or soil salinity decrease crop yield. Such areas occur globally where irrigation is prevalent, where ground water supplies are salinized due to seawater intrusion, and where soils are salinized due to mineral deposits. Such areas are common in North America, India, China, additional countries in Asia, Australia, and other major crop production countries. Our Salinity Tolerance trait has been licensed to partners for use in rice, wheat, corn, cotton, canola, sugarcane, and vegetable crops. Specific crops, collaborators, stages of development, and target markets for our Salinity Tolerance technologies are shown in the following table.
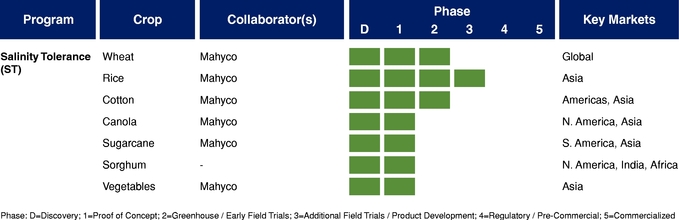
Development in rice and wheat is the most advanced of these crops, and our collaborators have reported successful trials showing yield increases over control varieties of more than 46% in rice, and up to 36% in wheat, under conditions of salinity stress, as shown in the following charts.
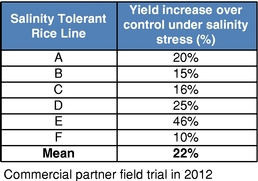 | 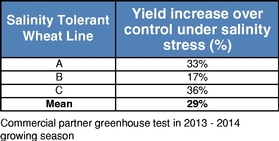 |
Heat Tolerance
Our Heat Tolerance technology program is carrying out discovery research funded by USAID in collaboration with the International Maize and Wheat Improvement Center, or CIMMYT, and the Indian National Bureau of Plant Genetic Resources, or NBPGR. Our work targets metabolic approaches to reduce the heat sensitivity of starch synthesis in wheat and increase membrane thermostability. We are pyramiding the CIMMYT-identified natural genetic diversity that affects membrane thermostability and induced genetic diversity in starch synthesis, developed by us, in order to improve wheat heat adaptation in a fundamental way.
Among major staple crops, global wheat yields may be the most impacted by climate change, according to a number of climate change models. And while wheat is the most drought-adapted of major crops, improving heat adaptation would make wheat a climate resilient staple. Developing countries are both significant producers and importers of wheat. According to CIMMYT, an estimated 1.2 billion poor people depend on wheat and 81% of wheat in the developing world is produced and consumed in the same country. At the same time, wheat accounts for 43% of food imports in developing countries, underscoring the importance of global wheat trade to food security. CIMMYT estimates that demand for wheat will increase by 60% by 2050 in developing countries. As we saw with the global food price crisis in 2008, poor yields in major wheat exporting countries such as Australia can have a significant impact on global prices.
Wheat has been shown to lose three to four percent of yield per degree Celsius above the optimum daytime temperature of 15° C. Since the 1980s, global wheat productivity is estimated to have been reduced by as much as five percent due to increasing temperature, and wheat yields in South Asia could decline about 50% by 2050. Recent research in India suggests that most crop models have underestimated the impact of extreme heat on yield losses by as much as 50%.
This technology is being developed in collaboration with CIMMYT and NBPGR, under funding provided by USAID and is currently in the discovery stage. The initial target crop for this technology is wheat, where the impacts from heat stress are among the most severe of all major crops. Target commercial geographies are global. It is expected that discoveries under this program are likely to lead to improvements in heat stability of major crops other than wheat as well.

Herbicide Tolerance
Our Herbicide Tolerance program is currently focused on wheat and we have developed a non-GM source of tolerance to glyphosate, a widely used non-selective herbicide. We believe that the discoveries under this program are likely to result in similar opportunities in other major crops.
According to ISAAA, from 1996 to 2013, herbicide tolerant crops consistently occupied the largest planting area of biotech crops. In 2013 alone, herbicide tolerant crops occupied 99.4 million hectares, or 57%, of the 175.2 million hectares of biotech crops planted globally. For the first 17 years of commercialization (1996 to 2012), benefits from herbicide tolerant crops were valued at $47.7 billion, which accounted for 41% of global biotech crop value. For 2012 alone, herbicide tolerant crops were valued at $6.6 billion or 35% of global biotech crop value.
Our Herbicide Tolerance technology is in Phase 3 of development and was developed using our proprietary non-GM research platform, TILLING, which enabled us to find and further develop valuable rare genes within our wheat genetic diversity collection. This work is fully funded by a collaborator who has the option to obtain a non-exclusive commercial license to this trait in certain countries. We retain the right to further license this technology to additional collaborators in global wheat markets.
Testing results to date show clear tolerance in multiple wheat lines to levels of glyphosate herbicide, which may be sufficient to control many weed species in wheat production. Individual glyphosate tolerant wheat lines are being combined via plant breeding to combine sources of tolerance and create products with increasing levels of tolerance.

Agronomic Trait Stacks
Trait stacks are combinations of multiple individual traits. Trait stacks can be made by using conventional plant breeding to cross plants with different traits, and can also be made by combining multiple traits in a molecular stack that is then inserted into a target crop. Our collaborators are generally allowed to combine multiple traits of ours either by breeding or molecular stacks. Deep portfolios of agronomic stress tolerance traits are rare in the industry, and the ability to pyramid multiples of such traits is even rarer. In order to validate the efficacy of particular trait stacks, we carry out our own research and field trials.
We have developed three molecular trait stacks, and have field-tested them in example crops, as shown in the table below. Efficacy of a trait stack in one crop suggests the probability that the stack will also work in other key crops. For example, our NUE and WUE trait stack is in Phase 2 of development for both wheat and canola and functions in both. The history of single traits functioning in multiple crops, along with the evidence of stacked traits working in more than one crop, suggest that
stacked traits are likely to function in multiple crops. Thus, we believe that our trait stacks have market opportunities well beyond the specific demonstration crops and geographies shown in the table.

Our most advanced and tested trait stack—the combination of NUE, WUE, and Salinity Tolerance—has been field tested in rice over multiple seasons. We have tested this trait stack under varying levels of nitrogen, water availability, and salinity. Rice plants with this stack out-yielded control plants by five to 22% under different levels of nitrogen fertilizer, by 19 to 32% under different types of water stress, and by 27 to 42% under high salinity conditions. The table below summarizes the results from a field trial conducted by us in 2012, with similar results obtained in a field trial conducted in 2013.
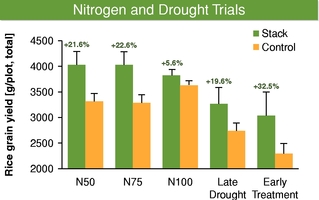 | 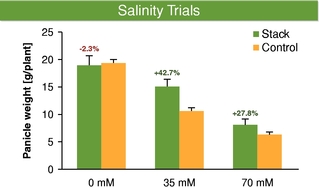 |
Agricultural Product Quality Traits
Gamma Linolenic Acid (GLA) Oil
Under the license agreement we have with Abbott, we developed a new source of vegetable oil with very high levels of the fatty acid gamma linolenic acid (GLA). Our GLA safflower oil product has the highest concentration of GLA available in any plant oil at 65%; conventional plant oils range from 10 to 22% GLA. We sell the oil to manufacturers of nutritional supplements, medical foods, and other products.
GLA has multiple clinically-demonstrated nutritional and medical benefits, including anti-inflammation, improved skin condition, and healthy weight management. Multiple parties had expressed commercial interest in incorporating an enhanced GLA oil into their food and medical products, where conventional sources of GLA are not sufficiently concentrated to deliver amounts that are cost and performance effective.
Against a commercial target of 40% GLA concentration, we developed, deregulated, and commercialized GLA safflower oil containing up to 65% GLA concentration in less than six years. This is significantly less than the 13 years it takes, on average, to commercialize a seed using advanced breeding or biotechnology, according to Phillips McDougall.
We produce GLA safflower oil by contracting with farmers in Idaho and process the seed under contract with a manufacturer in California to make refined oil. We sell GLA safflower oil under the brand name Sonova with multiple concentrations and formulations. Our markets are nutritional supplements, medical foods, and pet foods. Our key customers include significant participants in those markets, such as GNC, Lindora Nutrition, and others.

Arachidonic Acid (ARA) Oil
Our Arachidonic Acid (ARA) Oil has high levels of the fatty acid ARA, which is a key ingredient in more than 90% of U.S. infant nutrition products. ARA contributes to benefits such as fostering infant eye and brain development. We estimate the global market for ARA at $160 million and believe that our ARA product will cost significantly less than currently available sources of ARA.
Our ARA Oil is being developed under agreements with Abbott and DuPont Pioneer, each of which licensed intellectual property to us for this program. In exchange for licenses to intellectual property, these agreements provide product access rights to Abbott and DuPont Pioneer, as well as certain royalty payments on product sales to third parties.
Our ARA Oil is in Phase 3 of development. We have multiple safflower lines with oil compositions that offer the opportunity of being direct replacements for current sources of ARA in infant nutrition products.

Enhanced Quality Grains
We have multiple programs aimed at developing wheat and other small grains with improved nutritional qualities. One such program generated bread and pasta wheat lines with high levels of resistant starch. Resistant starch increases the fiber content of wheat and reduces glycemic index, which are both desirable nutritional qualities that are important in diabetes mitigation. A second program increases specific quality targets in wheat, and is funded by Ardent Mills, which combines the operations of ConAgra Mills and Horizon Milling, a Cargill-CHS joint venture. A third program, funded by the National Institutes of Health, or NIH, is aimed at reducing gluten in wheat and other grains. All three of these programs utilize our proprietary TILLING platform, and resulting products are non-GM.
Our resistant starch wheat provides a source of wheat with inherently high levels of resistant starch, increasing the fiber content of resulting food products without the need for fiber additives from other sources such as corn, potato and cassava. Resistant starch is a key product in two market segments: dietary fiber additives and modified starch additives. According to MarketsandMarkets, the fiber additives market was estimated to be $2.2 billion in 2013 and the modified starch market was estimated to be $12.8 billion in 2012. Major growth in these markets is being driven by the convenience health food sector and functional foods. Flour from our resistant starch wheat lines has resistant starch levels that are 12 to 20 times higher than the control wheat, and total dietary fiber, or TDF, that is
more than eight times higher than the control. Resistant starch wheat flour has been tested in applications in bread, where loaf quality was comparable to bread made with conventional wheat flour, and pasta, where it had the highest consumer preference rankings in tests carried out by a major consumer products company.
 |  |
Resistant starch wheat flour is currently being tested in a range of additional bakery products with industrial partners. We have several resistant starch wheat lines that are being evaluated for optimal quality and agronomic characteristics.
The gluten-free market was estimated to be $486.5 million in 2013 by Euromonitor. This figure only includes products that have been formulated to replace wheat flour and does not include products that are naturally gluten free or have undergone minor formulation changes. Consumers in this market are composed of people with celiac disease (approximately 1% of the population), people with non-celiac gluten intolerance (approximately 6% of the population) and people who choose to eat less gluten because they are in households with individuals with a gluten-free diet or choose to eat gluten-free food. Our wheat with reduced gluten will provide options for wheat products in the low gluten product category and additional options for blending wheat flour to meet the U.S. Food and Drug Administration, or FDA, standard for gluten-free products.

Post Harvest Quality
Our post harvest quality program for tomatoes has resulted in tomato lines with significantly increased post harvest storage life. These tomato lines were developed using our proprietary TILLING platform and are non-GM. Our early research program was funded by the U.S. Department of Defense, due to their interest in being able to procure quantities of fresh fruit with extended storage life for deployment on board ships and submarines and overseas. The global market for fresh tomatoes is estimated by the FAO at $84.5 billion per year. Our initial collaborator for this product is Bioseed, a
vegetable seed company based in India, and the product is in Phase 4 of development. Additional collaborations in North America are in development.

Our Product Development Capabilities
The diagram below illustrates the key steps in our technology identification and product development process.
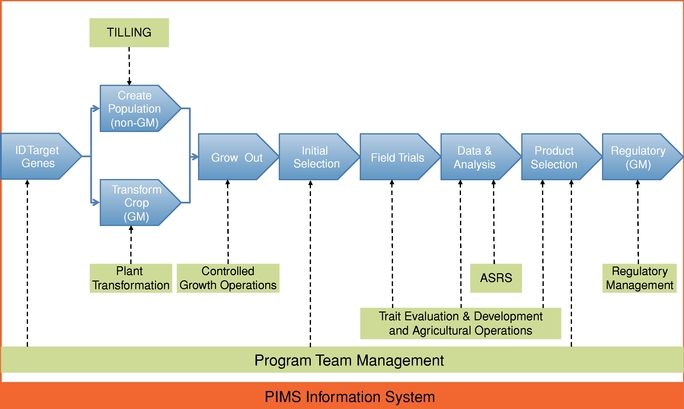
Identification of New Technology Programs
Because our business model is based on leveraging upstream investment in basic research to expand our product development pipeline, we actively seek out and participate in collaborative programs with external partners for the early-stage exploration and identification of promising plant technologies, particularly those related to abiotic stress tolerance in plants. The results of these collaborations directly feed innovation and often drive the progress of our ongoing programs. Some of these key early-stage collaborations include programs with the ARC Centre of Excellence in Plant Cell Walls (Australia), the University of Adelaide (Australia), CIMMYT (Mexico), the University of California, Davis (United States), Tulane University (United States), the University of California, Berkeley (United States), the International Center for Research in Semi-Arid Tropics (India), the Bangladesh Rice Research Institute (Bangladesh), and ICABIOGRAD (Indonesia). Many of these collaborative programs are funded by U.S. government grants that we have secured either ourselves or in connection with our collaboration partners, including grants by USAID, the NIH, the National Science Foundation, and the USDA.
Other early-stage technologies are introduced to us by commercial entities engaged in basic research who may be seeking to partner with us to advance their discoveries to further validation and product development. In some cases, such commercial entities are technology start-ups, and in other cases they include some of the largest companies involved in agricultural and food technology research.
We have a formal team and process for evaluating new technology opportunities. This team has multi-disciplinary membership, and reviews promising new technologies with regard to mission fit, scientific feasibility, intellectual property, business opportunity, and other considerations. Generally, we accept less than 10% of the potential opportunities we evaluate. Once a promising new trait technology has been accepted, we negotiate an agreement with the technology provider that, at a minimum, enables us to further evaluate the technology for a suitable period of time, or, in some cases, secures rights that enable full research and commercial exploitation of the technology.
Technology Evaluation
Our technology program teams include multiple Ph.D.-level scientists who are leaders in their respective fields. These teams contribute to the initial evaluation of new technologies and are responsible for development of technologies brought onboard. Each of our technology programs involves multiple gene, trait, and crop targets, and our process focuses on rapid development of the most promising combinations. In the development of any particular technology, we carry out a series of steps including the direct evaluation of target gene function, and the specific evaluation of results in key representative crop species. While common core scientific services are provided by specialized groups, the technology program team manages overall progress and remains directly involved throughout the development cycle, including by providing scientific and development support to our collaborators.
GM and Non-GM Product Development Platforms
Transformation—GM Traits. For projects involving GM traits, the genetic construct for insertion into plants is designed and built by the relevant program team, and then the gene transfer step is accomplished by our plant transformation functional group. This group consists of six members with more than 100 years of combined experience in plant transformation. The group has developed a complete physical and methodological infrastructure at our laboratory facility in Davis, California to efficiently transfer genetic materials into key crop species. Our plant transformation team has demonstrated transformation capabilities in all primary and some secondary agricultural crops, including rice (japonica, indica, and NERICA types), wheat, corn, canola, cotton, soybean, safflower, barley, sorghum, alfalfa, tomato, and grapes.
Targeting Induced Local Lesions in Genomes (TILLING)—Non-GM Traits. Our proprietary TILLING platform enables us to develop value-added crops without the use of GM methods. The TILLING platform is primarily managed by a dedicated team of scientists at our laboratory in Seattle, Washington. TILLING technology was originally invented by a member of our science team and utilizes specialized laboratory equipment to carry out high-throughput allele screening of DNA samples from genetic diversity populations created in major crops. Our populations include wheat, rice, soybean, canola, tomato, and lettuce. These populations include numerous native and induced gene function alterations, which can be discovered and exploited rapidly at low cost and with minimal regulatory requirements. While the TILLING approach is also practiced elsewhere, we believe that the combination of our specialized background in the technology, highly refined skills in developing and screening genetic diversity plant populations, and proprietary TILLING software makes us the leader in commercial applications of TILLING.
Controlled Growth Operation. Our controlled growth operations group manages our growth chamber facility, where plants transitioning out of the plant transformation group are grown under
precisely controlled conditions, and our greenhouse facility, consisting of 25,928 square feet of high quality greenhouse space, both at our headquarters in Davis, California. The controlled growth operations group uses these facilities to manage plant experiments and grow-outs under rigorously controlled conditions. They also carry out the initial seed increases and first stages of plant breeding for some projects. For certain projects, such as those relating to oil quality and resistant starch wheat, this group also manages crop breeding programs to develop plant varieties for the production of commercial products.
Field Trials and Commercial Production. Our trait evaluation and development group has extensive field and specialized statistical analytical capabilities, and is based in our Davis, California facility and has remote field operations in American Falls, Idaho and Brawley, California. The group also conducts field trials throughout the United States with specialized contractors, and elsewhere globally with our collaborators. This group also works actively with our commercial and joint venture partners to support their field trial execution and data analysis.
Our agricultural operations group manages late-stage and regulatory field trials and, in the case of GLA safflower, commercial crop production. Late-stage field trials are intended to develop extensive data on a limited number of potential commercial plant varieties. These trials may be used to test new varieties developed by our collaborators containing our traits, and to test our own commercial varieties for oil quality and grain quality programs. Similarly, regulatory trials develop data for use in submissions for regulatory review and may involve plant varieties developed by our collaborators or our own oil quality and grain quality programs.
Regulatory Data Generation. Our Analytical Services and Regulatory Science, or ASRS, group, located at our Davis, California facility, develops data for use in product selection and validation, certification of Sonova product specifications, and regulatory submissions. These data are generated from specialized analyses performed internally and externally under contract. The resulting data support regulatory submissions and provide core trait data to our collaborators for use in their crop-specific regulatory applications. The instrument-intensive work of the ASRS group provides automated DNA preparations, genomic blot analyses, lipid profiling, metabolomics, and protein purification services for us and our collaborators.
Biological Materials Inventory and Tracking. Our proprietary Pedigree and Inventory Management System, or PIMS, tracks the genetic, phenotypic, and location information for all of our plant materials. PIMS encompasses genetic elements such as genes and promoters, GM seeds, and plant material received by us, as well as seeds and plants developed by us and used in trait development. The performance of our plant materials is recorded through a variety of laboratory and field observations, and the data are stored within PIMS. The location of all plant materials is tracked throughout the plant life cycle. This includes specific seeds planted within a specific plot of a specific field trial, harvest, seed storage location, and use by, or distribution of plant material to, our collaborators or elsewhere. PIMS interfaces with our Biotechnology Quality Management System, or BQMS, to manage all movement and release of regulated GM plant materials. This ensures that all of our plant materials are accounted for, tracked, and inventoried, which enables us to maintain control over and documentation of all plant materials.
Regulatory Matters
Our regulatory management group provides regulatory services for all of our product development programs, as well as for joint ventures and selected collaborations. These services include establishing protocols, completing regulatory permits as necessary, and monitoring regulatory and stewardship compliance for all products at all stages.
Our regulatory group includes key employees who are directly responsible for leading all regulatory agency interactions and providing tactical and strategic regulatory direction. Our group collectively has more than 70 years of regulatory experience, with nearly 60 years of direct involvement in the development and approvals of GM crops. Members of our regulatory group were responsible for completing the first FDA and USDA deregulation of a GM whole food. The interactions and processes associated with these first USDA and FDA processes established benchmarks for the regulation of GM products that remain applicable today.
In addition to our core regulatory management group, we employ a group of scientists, our ASRS group, dedicated to data generation in support of regulatory filings. Our regulatory management and compliance activities encompass three broad categories: stewardship, authorization, and deregulation. In the United States, these activities are regulated by various government agencies, including the USDA, the FDA, and the U.S. Environmental Protection Agency, or EPA.
Stewardship
Stewardship, or the careful and responsible management of assets, forms the foundation of our regulatory compliance programs associated with GM plants. Our stewardship framework for GM plants is defined by government regulations and related internal policies. The USDA requirements and internal procedures for regulatory stewardship are embodied in our Biotechnology Quality Management System, or BQMS, which was developed by us and approved by the USDA Animal and Plant Health Inspection Service, Biotechnology Regulatory Service.
Our BQMS program was developed to address all conditions required under USDA authority to ensure containment of regulated plant material. The BQMS includes standard operating procedures, or SOPs, recording and reporting forms, instructions for managing all compliance related activities, and training requirements for all individuals handling GM plant materials. SOPs are highly detailed and consider all elements of each relevant activity or process. Each field trial site is accompanied by a field compliance guide and record containing multiple SOPs and associated forms for each activity. For example, a GM wheat trial requires 19 SOPs and associated verification forms.
Our BQMS is audited annually both internally and independently by an auditor trained and supervised by the USDA. Since our BQMS program was first recognized by the USDA in 2011, each annual independent audit has confirmed that our program is functioning as intended. Our BQMS manager has attended BQMS training programs at the request of the USDA to assist in training personnel at other companies, to share our experience and the SOPs that form the basis of our program.
Compliance with the specific parameters of regulatory requirements is only one element of stewardship. Additional activities within each functional group throughout the company are integral to the overall stewardship program. Each of our employees is trained on, and must comply with, relevant stewardship guidelines as defined and described in our BQMS.
Authorization
The USDA Biotechnology Regulatory Service, or BRS, has legal and regulatory authority over the movement and release of GM plants and seeds. "Movement" includes movement of regulated GM plant material between states and the importation of regulated GM plant material from outside the United States. "Release" includes field trials of any size and any other use of regulated GM plant material outside of contained greenhouses.
We have obtained over 180 authorizations from the BRS for the movement, importation or release of GM plants under development. General and specific conditions to maintain containment during all
activities associated with the movement or release are a requirement of each authorization. These conditions are defined and applied in the context of the BQMS.
Deregulation
Our business is subject to regulations related to agriculture, food, and the environment. Plant products produced using GM technology are subject to laws and regulations in countries where the plants are grown and in countries where the GM plant-derived food and feed are consumed by humans or animals. Commodity products utilizing our GM traits may require approvals in multiple countries prior to commercialization, whereas our identity-preserved GM products (for example, GLA safflower and resulting Sonova products) may require approvals only in the limited geographies where the products are marketed and sold. Such products must be appropriately channeled in the food and feed markets to ensure that the products are not exported to geographies where necessary approvals have not been obtained.
U.S. Regulatory Agencies
U.S. Department of Agriculture. We must obtain USDA authorizations and permits in order to conduct the field releases of regulated materials that are necessary to advance the development of GM crops. Obtaining such authorizations and permits is generally routine and delays impacting the planned movement or release of GM material are uncommon. The USDA provides detailed regulations and guidance for obtaining a so-called"Determination of Deregulated Status," which authorizes the commercial and uncontained growing of GM plants. For regulated GM plants, the USDA requires that a company petition the agency to demonstrate that the product is unlikely to pose a risk. Based on the information provided, the USDA prepares an Environmental Assessment, or EA, and/or an Environmental Impact Statement, or EIS, in order to make its determination. These procedures afford the public an opportunity to submit written comments on the draft EA or EIS for consideration by the USDA before the final version of the EA or EIS is published. For any GM plant product, there may be delays or requests for additional information based on the USDA's review or the public comments. Submissions received by the USDA from all applicants in August 2011 and thereafter averaged 27 months to completion; however, the USDA has announced proposed rules intended to significantly shorten this time period.
U.S. Food and Drug Administration. The FDA is responsible for food safety under the Federal Food, Drug and Cosmetic Act. The FDA recommended in its 1992Statement of Policy: Foods Derived from New Plant Varieties that developers of GM plant products consult with the agency about the safety of GM products under development. In 1996, the FDA provided additional guidance to the industry on procedures for these consultations. These procedures require a developer intending to commercialize a food or feed product derived from a GM plant to first meet with the agency to identify and discuss relevant safety, nutritional, and other regulatory issues regarding the product. Subsequently, the developer must submit to the FDA a scientific and regulatory assessment supporting proposed product safety. The FDA evaluates the submission and engages with the developer to resolve any questions, requests for additional data, or other informational requirements. Once the FDA has determined that all requirements have been satisfied, the FDA concludes the consultation process by issuing a letter to the developer acknowledging completion of the consultation process with the addition of the product to the list of completed consultations on the FDA website. The completed consultation acknowledges product safety for use as food and feed. To date, over 150 GM products have completed this process. This process may have delays if the FDA requires additional data and information for its consultation and to resolve any questions the FDA may have. The FDA completed nine consultations in 2013 and 2014, with consultation time periods ranging from 13 to 40 months and averaging 21 months from first submission to conclusion.
Environmental Protection Agency. Certain products may also be regulated by the EPA, including plants that contain a plant-incorporated protectant, such as a pesticides or herbicide, or plants engineered to be treated with industrial chemicals.
International Regulation
Commercialization of GM crops in the United States requires approvals in those jurisdictions into which resulting products will be imported. The laws and regulations for GM plant products are well defined in many commercially significant jurisdictions, including Australia, South America, India, and the European Union, and are evolving in others, such as Africa and China. Typically, our collaborators are responsible for obtaining all regulatory permits and approvals relevant to product development and commercialization in their licensed countries and for generating crop and transformation event-specific data required by jurisdictions of interest. We provide basic safety data on trait expression products in accordance with generally accepted standards and may serve as a regulatory consultant and participate in the design of regulatory data generation protocols and development of regulatory submissions beyond the basic safety data package. In certain countries, we may develop strategic business relationships or employ independent consultants with geography-specific knowledge and expertise to support and obtain required approvals.
Intellectual Property
We rely on patents and other proprietary right protections, including trade secrets and contractual protection of our proprietary know-how and confidential information, to preserve our competitive position.
As of December 31, 2014, we owned or exclusively controlled 115 issued patents and 44 pending patent applications worldwide. As of this date, we owned six and exclusively in-licensed 19 U.S. patents and we owned seven and exclusively in-licensed one pending non-provisional U.S. patent applications relating to our trait technologies and business methods. Also, as of this date, we owned 11 and exclusively in-licensed 79 foreign patents and owned 16 and exclusively in-licensed 20 pending foreign patent applications. With respect to all of the foregoing patent assets, our exclusive licenses afford us control over the prosecution and maintenance of the licensed patents and patent applications. These numbers do not include in-licensed patents for which we either do not have exclusive rights (such as certain enabling technology licenses), or for which we have exclusive rights only in a limited field of use and do not control prosecution and maintenance of the licensed patents.
As of December 31, 2014, we had nine registered trademarks in the United States. As of this date, we also had eight registered trademarks and had one trademark application pending in various other countries.
We also have entered into in-license agreements enabling the use and commercialization of our traits, including NUE, WUE, and Salinity Tolerance, and certain products that we have commercialized or are under development, including GLA safflower oil and ARA safflower oil. Under these licensing arrangements, we are obligated to pay royalty fees on sublicense revenue and net product sales ranging between low single digit percentages and percentages in the mid-teens, subject in certain cases to aggregate dollar caps. The exclusivity and royalty provisions of these agreements are generally tied to the expiration of underlying patents. After the termination of these provisions, we and our collaborators may continue to produce and sell products utilizing the technology under the expired patents. While third parties thereafter may develop products using the technology under the expired patents, in many cases, we have incremental patent rights covering our most important technologies, which we believe mitigate the impact of the expiration of these patents, or the related exclusivity provisions, on our business.
We also have numerous in-licenses relating to enabling technologies utilized in our development programs, such as transformation methods (e.g., Japan Tobacco, DuPont Pioneer), promoters (e.g., Dow, Louisiana State University) and selectable marker technologies (e.g., Bayer). These in-licenses are non-exclusive and include some combination of upfront and annual license fees, milestone fees, and commercial royalty obligations consisting of low single-digit percentages, or in one instance, a low single-digit dollars per acre fee.
Below is a summary of those in-license agreements that we believe are most significant for our product development programs.
University of Alberta. We hold an exclusive license from University of Alberta to the patent portfolio that formed the basis of our NUE program, which began in 2002. In exchange for an upfront license fee and royalties on sublicense revenues and net product sales (which are capped at an aggregate amount in the mid-seven figures), and subject to the University's right to perform academic research using the technology, we exclusively control all research, development, commercialization, and sublicensing of the patented technology globally for all crops.
Blue Horse Labs. In conjunction with a sponsored research and development agreement entered into in 2003, we obtained an exclusive license from Blue Horse Labs, an affiliate entity of our majority stockholder, Moral Compass Corporation, for technology related to several of our development programs. Under the sponsored research and development agreement, Blue Horse Labs has an ownership right in patents covering technology that was developed using Blue Horse Labs funds, including certain NUE and GLA safflower patents. In the corresponding license agreement, in exchange for a single-digit royalty on net revenues and management of all aspects of the patent portfolio, we exclusively control all research, development, commercialization, and sublicensing of the patented technology globally for all crops.
University of California. Our WUE technology was developed under an exclusive option agreement with the University of California, pursuant to which we exercised our right to secure an exclusive license in 2010. In exchange for an upfront license fee, license maintenance fees, and royalties on sublicense revenues and net product sales, we exclusively control all for-profit research, development, commercialization, and sublicensing of the patented technology globally for all crops.
University of Toronto. We hold an exclusive license from University of Toronto to the patent portfolio that forms the basis of our Salinity Tolerance program. In exchange for an upfront license fee, a low single-digit royalty on revenues, and payment of all costs associated with the patent portfolio, and subject to the University's right to use the technology for research and teaching purposes, we exclusively control all for-profit research, development, commercialization, and sublicensing of the patented technology globally for all crops.
Abbott. We entered into a license and development agreement with Abbott in 2003 under which we have been granted limited exclusive rights to Abbott's portfolio of U.S. and foreign patents relating to the development of plant-based sources of GLA, ARA, and essential fatty acids. Under this agreement, we provide Abbott with preferential access to commercial products from our GLA and ARA safflower programs, as well as the right to receive low single-digit royalty payments on product sales to third parties, in exchange for the licenses to Abbott's intellectual property rights.
Key Collaborations
Since our founding in 2002, we have established numerous trait collaborations and have developed deep relationships with industry-leading seed and consumer product companies. Our partnerships with global strategic seed and consumer product players enable us to further participate in the development and commercialization of innovative products that promise to play significant roles in improving global crop efficiency and enhancing human health. The results of these collaborations directly feed
innovation and drive the progress of our ongoing programs. Moreover, the expertise and opportunities created by the collaborations represent important assets to our business. While our collaboration-focused business model has resulted in numerous strategically significant relationships, below is a summary of selected collaborative partnerships that we view as key to the achievement of our near-term and mid-term business objectives.
Mahyco
We have multiple collaborative agreements with Mahyco covering more than 15 programs, using our most advanced traits in multiple major crops, crops, and have been working with Mahyco as a key partner since 2007. Our collaborations in NUE rice and salt tolerant rice are in advanced stages of development.
Under our various agreements relating to our NUE, WUE, and Salinity Tolerance traits, Mahyco has exclusive research and commercial rights in all licensed geographies and must timely meet certain diligence milestones in order to maintain their exclusivity. Each of our collaboration agreements with Mahyco includes an upfront technology access fee, technical and regulatory milestone fees, and, once products utilizing our traits are commercialized, we are entitled to receive a portion of the commercial value of seeds sold by Mahyco incorporating our traits. Mahyco is entitled to offset some of these fees against outstanding convertible promissory notes issued by us. Rights to new intellectual property developed under a collaboration agreement are owned by the inventing party or parties.
Vilmorin & Cie (Limagrain)
We selected Limagrain as our strategic partner and collaborator in wheat—the world's largest crop by area grown and the third most valuable at $186.4 billion annual value—due to their position as the leading global breeder and marketer of wheat seeds. In 2009, we executed an agreement with Limagrain under which we partnered to develop and commercialize NUE wheat in all countries of the world except Australia, India, Pakistan, Bangladesh and Sri Lanka. Under our agreement, Limagrain has exclusive research and commercial rights in all licensed geographies except North America and South America, in which we retained co-exclusive rights, and Limagrain must timely meet diligence milestones to maintain exclusivity. Our agreement with Limagrain includes an upfront technology access fee, annual maintenance fees, and technical and regulatory milestone fees, and once an NUE wheat product is commercialized, we are entitled to receive a portion of the commercial value of the trait in the marketplace. Limagrain owns rights to new intellectual property it develops that is based on our NUE technology, but Limagrain has agreed not to assert its rights in any way that limits our ability or our other collaborators' ability to use our NUE technology in crops other than wheat. We and Limagrain have since coordinated with collaborators in Australia to align development efforts in NUE wheat on a global basis.
In 2010, we further expanded our relationship with Limagrain from collaborator to stockholder and joint venture partner. Contemporaneously with Limagrain's $25 million equity investment in our company, we formed Limagrain Cereal Seeds LLC, a joint venture company focused on the development and commercialization of improved wheat seed in North America, of which a U.S. wholly owned subsidiary of Limagrain owns 65% and we own 35%. This joint venture strengthens a close strategic relationship between us and Limagrain, and increases the share of net trait value that we will recognize on traits commercialized in wheat.
As a key strategic partner, Limagrain has a right of first offer to license—on an arm's-length basis—new technologies that we develop or acquire that are applicable to wheat or barley. This right of first offer extends to Limagrain Cereal Seeds for the United States and Canada. Pursuant to the right of first offer, we formed a global collaboration with Limagrain and Limagrain Cereal Seeds in 2011 to develop and commercialize WUE wheat. Our agreement with Limagrain and Limagrain Cereal Seeds
includes an upfront technology access fee, technical and regulatory milestone fees, and, once a product is commercialized, we are entitled to receive a portion of the commercial value of the WUE trait in the marketplace.
Bioceres
In 2012, we partnered with Bioceres, an Argentina-based technology company, to form Verdeca LLC, a U.S.-based joint venture company engaged in the development and deregulation of soybean traits, of which we own 50%. We selected Bioceres as our partner in soybeans—the world's fourth largest crop by area grown and the fourth most valuable at $119 billion annual value—due to their desirable trait portfolio, their presence in key South American markets, and the significant presence of large soybean growers in their ownership structure.
Our joint venture agreement provides for each of the joint venture partners to license its trait technologies to Verdeca for use in soybeans, with product development and regulatory efforts equitably divided and managed by us and Bioceres under standalone service agreements that are executed annually. The first product in the Verdeca pipeline is a drought and abiotic stress tolerance trait that has already completed extensive validation trials and is now in the regulatory phase of development. This trait has been demonstrated to confer a seven to 16% yield advantage over conventional soybeans grown under the same suboptimal conditions. In April 2015, Verdeca received the first regulatory approval of its stress tolerance trait in soybeans in Argentina. This is the world's first regulatory approval of an abiotic stress tolerance trait in soybeans, which we believe is an important initial step in pursuing additional regulatory approvals that Verdeca intends to seek in multiple geographies globally.
Verdeca's pipeline also includes our NUE and WUE technologies, which are combined in a two-trait stack that will be further stacked with the initial drought and abiotic stress tolerance trait discussed above. Verdeca has successfully negotiated favorable market access in South America through established players and is working on adding market channel partners in the United States, India, and China.
In addition to those agreements with Bioceres directly associated with Verdeca, we also have negotiated exclusive access to Bioceres' drought and abiotic stress tolerance trait for use globally, outside of South America, in wheat. Our agreement with Bioceres provides for sharing of trait value once a product is commercialized.
In April 2015, we entered into a collaboration agreement with Dow AgroSciences and Bioceres under which our Verdeca joint venture will collaborate with Dow AgroSciences on the development and deregulation of soybean traits on a global basis.
Scientific Advisory Board
We maintain a scientific advisory board consisting of the members identified below. Our scientific advisory board meets on a quarterly basis and is comprised of industry and academic experts that have extensive experience in the analysis, research and development, and commercialization of biotech plants, including experience relating to discovery, transformation, and field trials. We consult with our scientific advisory board on a variety of matters pertaining to our current and future pipeline of products in development, including, for example, trait selection and development, transformation and TILLING methodologies, field trials, regulatory matters, and intellectual property evaluation.
We currently have scientific advisory board that consists of six members as follows:
Eduardo Blumwald, Ph.D. is a professor at the University of California, Davis. Dr. Blumwald's research program is multidisciplinary in nature, combining physiology, biochemistry, molecular biology, genomics, and proteomics. The general objectives of his work are: (i) the cellular and molecular mechanisms that regulate ion homeostasis in plants; (ii) the cellular and molecular mechanisms
mediating the responses of plants to abiotic stress (e.g., salt, drought, and heat); (iii) the biochemical and molecular basis of sugar and acid accumulation in fruits; and (iv) the development of genomic and proteomic resources for the improvement of fruit quality. Dr. Blumwald has worked closely with our scientists from the time of his former position with the University of Toronto.
Vicki Chandler, Ph.D. is Chief Program Officer, Science, at the Gordon and Betty Moore Foundation. She studied biochemistry for her undergraduate and doctoral degrees at the University of California, Berkeley, and the University of California, San Francisco, respectively. She then pursued
postdoctoral research at Stanford University and was on the faculty at the University of Oregon and the University of Arizona. Dr. Chandler's research on paramutation, an epigenetic process, has implications not only for corn, which she used for the majority of her research, but also for animal and human genetics and genetic diseases. Dr. Chandler is president of the Genetics Society of America, a member of the National Academy of Sciences, and a member of the National Science Board. Her many honors include the Presidential Young Investigator Award, Searle Scholar Award, and American Association for the Advancement of Science Fellow. She has served on advisory boards and panels for the National Research Council, National Science Foundation, Department of Energy, and National Institutes of Health. Dr. Chandler has chaired numerous conferences and served on the editorial boards of several journals, including Genetics, Plant Physiology, PNAS, and Science.
Luca Comai, Ph.D. is a professor at the Genome Center in University of California, Davis. Dr. Comai's lab is involved in two areas pertinent to breeding. In the first, they study genome regulation, hybridization, and heterosis responses in chromosome copy number variants and interspecific hybridization. In the second, they develop and make available to the plant community a functional genomic discovery tool called TILLING that allows targeted inactivation of genes in crop plants. The research combines plant genetics and genomics with the use of next-generation sequencing and bioinformatics to identify genes responsible for traits of interest as well as to discover and use natural and induced variation. Dr. Comai is known for his pioneering work creating glyphosate tolerant crops, and as a founding scientist in Calgene Pacific, Targeted Growth, Inc., and Tilligen.
Georges Freyssinet, Ph.D. is recently retired after many years in the plant biotechnology industry in France. He is the former CEO of RhoBio, a joint venture between Rhône-Poulenc Agro and Biogemma, and served as the Scientific Advisor for Life Sciences for the RP Group. Dr. Freyssinet is the former director of plant genomics for Aventis, which was later acquired by BayerCropScience. He joined Biogemma in 2003 to lead their genomic and bioinformatics platform, and in 2006 he joined the Scientific Direction of Groupe Limagrain, serving as Scientific Director from 2008-2011. Dr. Freyssinet is the founder and former CEO of LemnaGene, a biomanufacturing company, and the former CEO of Genective, a joint venture between Groupe Limagrain and KWS. Retired since 2014, he continues his independent consulting activities in plant biotechnology.
Jim Petersen, Ph.D. is Vice President for Research at Limagrain Cereal Seeds, a U.S. joint venture between Groupe Limagrain and our company, where he currently oversees all U.S. breeding operations. Prior to joining Limagrain, Dr. Peterson spent 27 years in public sector wheat research, including 12 years as the Krondstadt Professor of Wheat Breeding and Genetics at Oregon State University. Dr. Peterson served as Chair of the National Wheat Improvement Committee and is a recipient of the Weatherford Award for Entrepreneurship and Innovation from the College of Business at Oregon State University. He is noted for his fundamental research on wheat end-use quality and GxE interactions impacting quality. Dr. Peterson received his B.S. in agronomy from Washington State University and his M.S. and Ph.D. in agronomy and plant breeding from the University of Nebraska.
Peter Quail, Ph.D. is a professor of Plant and Microbial Biology at the University of California, Berkeley where he also serves as Research Director of the Plant Gene Expression Center (U.S. Department of Agriculture/Albany, California). Dr. Quail has been a pioneer in the study of phytochromes, photoreceptor proteins that play a major regulatory role in plant growth and
development. Dr. Quail was elected to the National Academy of Sciences in 2004, as a Fellow of the American Association of Science in 2004, and was the recipient of the Stephen Hales Prize, American Society of Plant Biologists, 2008. He received a B.S. and Ph.D. from the University of Sydney, Australia.
Competition
The markets for seed traits and agricultural biotechnology products are highly competitive, and we face significant direct and indirect competition in several aspects of our business. Competition for improving plant genetics comes from conventional and advanced plant breeding techniques, as well as from the development of advanced biotechnology traits. Other potentially competitive sources of improvement in crop yields include improvements in crop protection chemicals, fertilizer formulations, farm mechanization, other biotechnology, and information management. Programs to improve genetics and chemistry are generally concentrated within a relatively small number of large companies, while non-genetic approaches are underway with broader set of companies.
In general, we believe that our competitors generally fall into the following categories:
considered to compete with us as their products seek to improve crop yields, we believe that such products and our traits may be additive, or synergistic, to our future products in terms of increasing crop yields.
acquiring, analyzing, and acting upon data in ways that may improve farm economics via increased crop yield and more efficient management of crop production inputs. Technical approaches include weather prediction and monitoring, high-density field and crop imaging systems, precision field soil and yield mapping, and others. Companies focusing on this space include Trimble, Planet Labs, Ceres Imaging, Blue River, and others. While these products are potentially competitive with us for increasing crop yields, we believe that certain of these products could also be additive or synergistic with our traits.
We believe that we are uniquely positioned at the nexus of basic research and commercial product development. Unlike many companies in our space, we generally do not compete in the area of basic research. Our focus is on development and validation and, therefore, we provide a value-added link by which basic research can be brought to market. Public research institutions provide us with a source of innovative new technologies and traits and, while such basic research programs are competitive with in-house programs at the largest seed and technology companies, global public investment in basic research in 2008 at more than $31 billion was more than seven times greater than industry spending in 2013. We believe that these public programs are valuable and sustainable sources of new technologies for us and we have earned a reputation in our industry as a trustworthy and effective partner based on our demonstrated ability to manage the development and regulatory processes for GM seeds and capture additional value for ourselves and our basic research collaborators. While internal programs at the largest seed and technology companies are competitive with ours in some cases, we are technology providers to some of these companies, and we have numerous collaborations with many of them. To remain competitive, we plan to pursue multiple strategies, including further building our pipeline of new technologies from basic research programs, increasing the scope and range of our field testing activities, and continuing to protect our intellectual property rights in key jurisdictions globally.
Research and Development
As of March 31, 2015, we had 49 full-time employees dedicated to research and development, nine of whom are development and field personnel focused on demonstration and research field trials. Our research and development team has technical expertise in molecular biology, biochemistry, genetics and genetic engineering, analytical chemistry, plant physiology, plant virology, molecular pathogenesis, and
soil and water science. Our research and development activities are conducted principally at our Davis, California and Seattle, Washington facilities, with ongoing field trials conducted in American Falls, Idaho; Brawley, California; and numerous other locations throughout the United States and at locations managed by our collaborators worldwide. We have made, and will continue to make, substantial
investments in research and development. Our research and development expenses were $8.4 million and $10.0 million in the years ended December 31, 2013 and 2014, respectively.
Employees
As of March 31, 2015, we had 76 full-time employees, of whom 13 hold Ph.D. degrees. Approximately 49 employees are engaged in research and development activities, four in business development, three in regulatory management, and 20 in management, operations, accounting/finance, legal, and administration. We consider our employee relations to be good. None of our employees are represented by a labor union or collective bargaining agreement.
Facilities
Our corporate headquarters are located in Davis, California, in a facility consisting of approximately 20,775 square feet of office, laboratory, and growth chamber space under a lease that expires on June 30, 2015, pursuant to which we have an option to renew the lease for an additional three-year term. This facility accommodates research and development, operations, analytical services, regulatory, and administrative activities. We also lease approximately 4,381 square feet of office and laboratory space in Seattle, Washington, where our team of scientists executes our TILLING technology platform, under a lease that expires on December 31, 2015, with an option to renew for an additional one-year term. Our administrative offices in Phoenix, Arizona consist of 1,913 square feet under a lease that expires on December 31, 2016 and accommodate finance, legal, and other administrative activities, as well as sales and marketing activities for our Sonova products. We also lease greenhouse space and farm land for agricultural use in Northern California as well as farmland in Idaho. We also lease grain bin and office space in Idaho under a lease that expires on March 3, 2019.
We believe that our leased facilities are adequate to meet our current needs and that, if needed, suitable additional or alternative space will be available to accommodate our operations.
Legal Proceedings
We currently are not a party to any material litigation or other material legal proceedings. From time to time, we may be subject to legal proceedings and claims in the ordinary course of business.
The following table provides information regarding our executive officers, directors, and director nominees (ages as of March 31, 2015):
Name | Age | Position | |||
|---|---|---|---|---|---|
Eric J. Rey | 59 | President, Chief Executive Officer, and Director | |||
Vic C. Knauf, Ph.D. | 63 | Chief Scientific Officer | |||
Thomas P. O'Neil | 50 | Chief Financial Officer | |||
Steven F. Brandwein | 59 | Vice President of Finance and Administration | |||
Wendy S. Neal | 41 | Vice President and Chief Legal Officer | |||
Don Emlay | 73 | Vice President of Regulatory Affairs and Compliance | |||
Roger Salameh | 51 | Vice President of Business Development | |||
Zhongjin Lu, Ph.D. | 49 | Vice President of Product Development | |||
Darby E. Shupp | 39 | Chairman of the Board of Directors | |||
Peter Gajdos | 32 | Director | |||
Uday Garg | 36 | Director | |||
James R. Reis | 57 | Director | |||
Mark W. Wong | 65 | Director | |||
Matthew A. Ankrum | 45 | Director Nominee | |||
George F.J. Gosbee | 45 | Director Nominee | |||
Rajiv Shah, M.D. | 42 | Director Nominee | |||
Executive Officers
Eric J. Rey is one of our founders and has served as our President and Chief Executive Officer and a director of our company since August 2003. Mr. Rey has managed agricultural research, product development, and commercialization programs for more than 30 years, most of which have focused specifically on food, feed, and industrial products from agricultural biotechnology. Prior to founding our company, Mr. Rey worked as a partner with the Rockridge Group, a management consulting firm focused on the agricultural biotechnology industry. While at Rockridge, Mr. Rey managed the development of strategic partnerships for early-stage companies developing genomic, biopharmaceutical, nutraceutical, crop protection, animal nutrition and health, alternative crop, and industrial products. Prior to his work with Rockridge, Mr. Rey served as Vice President of Operations with Calgene Inc. for 17 years, including two years with Monsanto Company following its acquisition of Calgene. During his 17 years at Calgene and Monsanto, he was responsible for the establishment and management of the company's operational, product development, and agricultural infrastructure. Mr. Rey holds a B.S. in Plant Science from the University of California, Davis. We believe that Mr. Rey is qualified to serve as a member of our board of directors because of his operational and historical expertise gained from serving as our President and Chief Executive Officer. As one of our founders and the longest serving member of our board of directors, we also value his deep understanding of our business as it has evolved over time.
Vic C. Knauf, Ph.D. has served as our Chief Scientific Officer since June 2005. Dr. Knauf has 30 years of experience in agricultural product and technology development. Prior to joining our company, Dr. Knauf founded Anawah, Inc., a food and agricultural research company focused on the development of value-added whole foods, which we acquired in 2005. Before Anawah, Dr. Knauf served as a Director of Monsanto Food and Nutrition Research and Vice President of Research at Calgene, Inc. Dr. Knauf holds a B.S. in Biology from the New Mexico Institute of Mining and Technology and a M.S. and Ph.D. in Microbiology and Immunology from the University of Washington.
Thomas P. O'Neil has served as our Chief Financial Officer since March 2015. From January 2014 to July 2014, Mr. O'Neil served as Chief Financial Officer of Sorbent Therapeutics, Inc., a
biopharmaceutical company. From September 2011 to December 2013, Mr. O'Neil served as a consultant to Sorbent and a variety of health care and technology companies, providing financial planning and operational support. From December 2009 to August 2011, Mr. O'Neil served as Vice President of Finance & Administration of ChemGenex Pharmaceuticals Ltd., a biopharmaceutical company. From March 2007 to May 2009, Mr. O'Neil served as Vice President of Finance & Administration of Nodality, Inc., a biotechnology company. From October 1999 to February 2007, Mr. O'Neil performed several finance roles with Monogram Biosciences, Inc., which conducted its initial public offering in 2000 and was subsequently acquired and taken private in 2009. Mr. O'Neil holds a B.A. from Pomona College in International Relations and an M.B.A. from the University of California at Los Angeles.
Steven F. Brandwein has served as our Vice President of Finance and Administration since September 2002 and as our Treasurer since June 2014. He previously served as our Secretary from September 2002 until June 2014. Mr. Brandwein has more than 30 years of business, operations and international finance experience in agricultural biotechnology and a range of other industries. Prior to joining us, Mr. Brandwein served as CFO for Rulebase, Inc., an early-stage software company, where he led the finance, accounting, and human resources functions. Before joining Rulebase, Mr. Brandwein spent more than 10 years as an executive with Dial Corporation, including seven years based in London as the Controller for the company's European finance subsidiaries, and three years as an excise tax auditor with the U.S. Department of the Treasury. Mr. Brandwein holds a B.A. in International Relations from the University of Minnesota and a Masters in International Management from Thunderbird School of Global Management.
Wendy S. Neal has served as our Vice President and Chief Legal Officer since October 2008, and has served as our Secretary since June 2014. Ms. Neal has more than 15 years of experience in intellectual property and business law. Prior to joining our company, Ms. Neal was a partner in the Intellectual Property & Technology group at the law firm of Snell & Wilmer L.L.P. and served as our outside counsel. Prior to joining Snell & Wilmer, Ms. Neal worked with the patent team at GE Aircraft Engines and has served in technical roles at companies such as BP Oil, BP Chemicals, and Henkel Corporation. Ms. Neal has also served as Risk Policy Consultant to the American Institute of Chemical Engineers government relations team. Ms. Neal holds a B.S. in Chemical Engineering and a J.D. from the University of Cincinnati.
Don Emlay has served as our Vice President of Regulatory Affairs and Compliance since June 2004. Mr. Emlay has nearly 40 years of regulatory experience with consumer, industrial, and transgenic plant products. Prior to joining our company, Mr. Emlay was an independent consultant and provided counsel in the areas of research, product development, production, and regulatory procedures. Before his work as a consultant, Mr. Emlay served as Vice President of Regulatory Affairs with Calgene Inc. for 13 years, including three years with Monsanto Company following its acquisition of Calgene. Prior to Calgene and Monsanto, Mr. Emlay worked with Zoecon where he worked exclusively with the EPA in obtaining approvals for the first insect growth regulators to be registered for homeowner and pest control applications. Mr. Emlay holds a B.S. in Entomology from San Jose State University.
Roger Salameh has served as our Vice President of Business Development since November 2003. Mr. Salameh has more than 22 years of executive, managerial, and operations experience in agricultural, biotechnology, and food ingredients businesses. Prior to joining our company, Mr. Salameh was a consultant with Rockridge. Before joining Rockridge, Mr. Salameh served as director of business development at Monsanto Company. Prior to Monsanto, Mr. Salameh served as product manager for Calgene, Inc.'s genetically modified oils business. Mr. Salameh attended New York University where he studied Economics and Political Science.
Zhongjin Lu, Ph.D. has served as our Vice President of Product Development since May 2010. He previously served as our Director of Product Development and Plant Breeding since our inception.
Dr. Lu has 30 years of experience in agronomy, crop genetics and breeding, plant physiology, and agricultural biotechnology. Prior to joining our company, Dr. Lu was the Director of Plant Breeding and Senior Scientist at Seaphire International, Inc., a seawater-based agricultural company in Arizona.
Before his work with Seaphire, Dr. Lu worked for Monsanto Company where he was responsible for the project of salicornia genetic improvement for saline agriculture. Prior to Monsanto Company, Dr. Lu was associated with the Jiangsu Academy of Agricultural Sciences. Dr. Lu holds an M.S. in Plant Genetics and Breeding from Nanjing Agricultural University, China, and a Ph.D. in Plant Physiology from Technion—Israel Institute of Technology.
Each executive officer serves at the discretion of our board of directors and holds office until his or her successor is duly elected and qualified or until his or her earlier resignation or removal. There are no family relationships among any of our directors or executive officers.
Non-Employee Directors
Darby E. Shupp has served as chairman of our board of directors since June 2014 and as a director of our company since February 2010, and served as our treasurer from February 2010 until June 2014. Since January 2010, Ms. Shupp has served as Chief Financial Officer of Moral Compass Corporation, an investment company formed by Dr. John Sperling, the founder of Apollo Education Group, Inc. and one of our founders. Since February 2005, Ms. Shupp has been employed by various entities affiliated with Moral Compass Corporation. She previously worked for Deloitte LLP as an Audit Manager serving clients in the business services, manufacturing, and real estate industries. Ms. Shupp has served as a director of Apollo Education Group since March 2011. Ms. Shupp holds a B.S. in Accountancy from Arizona State University and is a Certified Public Accountant. We believe that Ms. Shupp is qualified to serve on our board of directors due to her management, accounting, and operational experience as an executive and director for public and private companies.
Peter Gajdos has served as a director of our company since May 2014. Since November 2014, Mr. Gajdos has served as a managing director and portfolio manager at Presidio Partners, a venture capital investment firm. From July 2013 to November 2014, Mr. Gajdos served as a managing director and portfolio manager at CMEA Capital, a venture capital investment firm. He co-founded AgoraSol LLC, a solar development company, in March 2013 and had an active role at the company from March 2013 until June 2013. From September 2012 to December 2012, Mr. Gajdos was a consultant at Silver Lake Kraftwerk, a technology investment firm. From June 2012 to August 2012, he was an intern at Warburg Pincus, a private equity investment firm. From April 2007 to July 2011, Mr. Gajdos worked as an associate at the Virgin Green Fund, a private equity investment firm. He has served as a director of CNano Technology Limited since August 2013. Mr. Gajdos served on the board of Reel Solar Inc. from September 2013 until its acquisition in April 2014. He holds a B.A. in International Business and Finance from the Kenan-Flagler Business School at the University of North Carolina-Chapel Hill and an M.B.A. from the Wharton School of Business at the University of Pennsylvania. We believe that Mr. Gajdos is qualified to serve as a member of our board of directors because of his extensive experience in the venture capital industry and his knowledge of technology companies. Pursuant to Presidio Partners policy, Mr. Gajdos has notified us that he plans to resign from our board of directors prior to the initial public offering.
Uday Garg has served as a director of our company since June 2014. Mr. Garg founded Mandala Capital, a private equity fund, in 2008 and has served as managing director and a director since its inception. As part of his duties at Mandala, Mr. Garg serves on the boards of various Mandala portfolio companies and affiliated investment vehicles. Previously, Mr. Garg was a portfolio manager at Duet Group, Altima Partners, and Amaranth Advisors. He began his career as an investment banker in the corporate finance and mergers and acquisitions department of Deutsche Bank. He holds a B.S. in Economics with a concentration in Finance from the Wharton School of Business at the University of Pennsylvania. Mr. Garg has considerable experience in the private equity industry and extensive
knowledge of the seed business in India, which provides our board of directors a useful perspective on our business strategy in India.
James R. Reis has served as a director of our company since August 2005. He also served as a director of Apollo Group, Inc. from March 2007 to January 2010. Since 2006, Mr. Reis has been employed by and served as Vice Chairman of Gainsco, Inc., an insurance company. Mr. Reis holds a B.S. in Accounting from St. John Fisher College in Rochester, New York and is a Certified Public Accountant (inactive). We believe that Mr. Reis is qualified to serve as a member of our board of directors due to his financial, accounting, and operational expertise from prior experience as an executive and director for public and private technology companies.
Mark W. Wong has served as a director of our company since May 2006. Mr. Wong was the Chief Executive Officer of Renewable Agricultural Energy Corporation, a private ethanol production company, from 2006 to 2007. From 1999 to 2005, Mr. Wong was the founder and Chief Executive Officer of Emergent Genetics, an international seed company sold to Monsanto Company in 2005. Prior to that time, Mr. Wong founded and managed a series of agricultural and biotechnology companies including Big Stone Partners, Agracetus Corporation, and Agrigenetics Corporation. Mr. Wong also worked as an engineer for FMC Corporation and Chemical Construction Corporation. Mr. Wong served as a director of BioFuel Energy Corp., a publicly traded ethanol company, from January 2008 until October 2014, and Chair from March 2010 to October 2014, when it was renamed Green Brick Partners following an acquisition and recapitalization transaction. Mr. Wong holds a B.S. in Chemical Engineering from Lehigh University and a M.B.A. from the Wharton School of Business at the University of Pennsylvania. Mr. Wong brings to our board of directors over 35 years' experience in the biotechnology and seed industries as a founder and manager. His service on a number of private and public company boards also provides an important perspective on corporate governance matters.
Director Nominees
Matthew A. Ankrum will become a director of the company in connection with this offering. In December 2010, Mr. Ankrum co-founded BodeTree LLC, a business-to-business subscription-based web application, where he continues to serve as Chairman of the company. From August 2008 to September 2012, Mr. Ankrum served as the head of strategy for Apollo Education Group, Inc. Prior to these positions, Mr. Ankrum served as a financial analyst and portfolio manager for Janus Capital Management, Lateef Investment Management, and William Blair & Company. Mr. Ankrum holds a B.A. in Finance from the University of Wisconsin, Madison and an M.B.A. from the University of Chicago. We believe Mr. Ankrum is qualified to serve on our board of directors due to his knowledge of technology companies and his management, financial, and operational experience with public and private companies.
George F.J. Gosbee will become a director of the company in connection with this offering. In May 2010, Mr. Gosbee founded the investment firm AltaCorp Capital Inc., where he currently serves as Chief Executive Officer and Chairman of the board of directors. Since 2013, Mr. Gosbee has been a co-owner of the Arizona Coyotes, a National Hockey League team. Prior to founding AltaCorp Capital, Mr. Gosbee founded Capital Global Inc., a global energy investment firm, where he served as President, Chief Executive Officer, and Chairman of the board of directors until its acquisition in 2009. Mr. Gosbee is a director and former Vice Chair of Alberta Investment Management Co, an investment fund. He also served as a director of Chrysler Group LLC from July 2009 to September 2011. He holds a Bachelor of Commerce from the University of Calgary, where he specialized in finance and petroleum land management. We believe Mr. Gosbee is qualified to serve on our board of directors due to his twenty years of experience in corporate finance, investment banking, and global capital markets. This experience will provide our board with valuable insights on financial and strategic planning matters.
Rajiv Shah, M.D. will become a director of the company in connection with this offering. Dr. Shah served as Administrator of the United States Agency for International Development, or USAID, from January 2010 to February 2015. Prior to his appointment at USAID, Dr. Shah served as Undersecretary
and Chief Scientist at the U.S. Department of Agriculture, during which time he created the National Institute for Food and Agriculture. Prior to working in government, Dr. Shah spent eight years at the Bill & Melinda Gates Foundation, where he led efforts in global health, agriculture, and financial services. Dr. Shah holds a B.S. from the University of Michigan, an M.Sc. in Health Economics from the Wharton School of Business at the University of Pennsylvania and an M.D. from the University of Pennsylvania School of Medicine. We believe Dr. Shah is qualified to serve as a member of our board of directors due to his experience in agriculture, government, and regulatory affairs.
Code of Business Conduct and Ethics
Our board of directors has adopted a code of business conduct and ethics that applies to all of our employees, including our Chief Executive Officer, Chief Financial Officer, other executive and senior financial officers, and our directors. Our code of business conduct and ethics will be posted on the investor relations page on our website. We intend to disclose any amendments to these documents, or waivers of their requirements, on our website or in filings under the Securities Exchange Act of 1934, or the Exchange Act, as required by the applicable rules and exchange requirements.
Board of Directors
Our business and affairs are managed under the direction of our board of directors. The number of directors will be fixed by our board of directors, subject to the terms of our amended and restated certificate of incorporation and amended and restated bylaws that will become effective upon the completion of this offering. Upon the completion of this offering, our board of directors will consist of eight directors, six of whom will qualify as "independent" under the listing standards of The NASDAQ Stock Market.
In accordance with our amended and restated certificate of incorporation and our amended and restated bylaws, upon the completion of this offering our board of directors will be divided into three classes with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Our directors will be divided among the three classes as follows:
Any increase or decrease in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors.
This classification of our board of directors may have the effect of delaying or preventing changes in control of our company.
Currently, Ms. Shupp serves on our board of directors as designee of entities affiliated with Moral Compass Corporation, Mr. Garg serves on our board of directors as designee of Mandala Capital, and Mr. Gajdos serves on our board of directors as designee of certain of our preferred stockholders, in each case pursuant to the provisions of a voting agreement among us and certain of our stockholders.
The voting agreement will terminate upon completion of this offering. For additional information, see "Certain Relationships and Related Party Transactions—Voting Agreement."
Director Independence
Under the rules of The NASDAQ Stock Market, independent directors must comprise a majority of a listed company's board of directors within a specified period of time after completion of such company's initial public offering. In addition, the rules of The NASDAQ Stock Market require that, subject to specified exceptions, each member of a listed company's audit, compensation, and nominating and governance committees be independent. Under the rules of The NASDAQ Stock Market, a director will only qualify as an "independent" director if, in the determination of that company's board of directors, that director does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, each member of the audit committee of a listed company may not, other than in his or her capacity as a member of such committee, the board of directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fees from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors has undertaken a review of its composition, the composition of its committees, and the independence of each director and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Based on information provided by each director concerning his or her background, employment, and affiliations, including family relationships, our board of directors has determined that each of Matthew A. Ankrum, Uday Garg, George F.J. Gosbee, James R. Reis, Rajiv Shah and Mark W. Wong does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is "independent" as that term is defined under the applicable rules and regulations of the SEC, and the listing standards of The NASDAQ Stock Market. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director, and the transactions involving them described in the section titled "Certain Relationships and Related Party Transactions."
Committees of the Board of Directors
Our board of directors has established an audit committee, a compensation committee, and a nominating and governance committee. The composition and responsibilities of each of the committees of our board of directors is described below. Members will serve on these committees until their resignation or until as otherwise determined by our board of directors.
Audit Committee
Effective upon the completion of this offering, our audit committee will consist of Matthew A. Ankrum, George F.J. Gosbee, and James R. Reis, with Mr. Reis serving as audit committee chair. Our board of directors has determined that each of the members of our audit committee satisfies the requirements for independence and financial literacy under the current listing standards of The NASDAQ Stock Market and SEC rules and regulations, including Rule 10A-3. Our board of directors has also determined that Mr. Ankrum and Mr. Reis are each an audit committee financial expert
within the meaning of Item 407(d) of Regulation S-K under the Securities Act of 1933, as amended, or the Securities Act. Our audit committee will be responsible for, among other things:
Our audit committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of The NASDAQ Stock Market and that will be available on our website upon completion of this offering. In accordance with and pursuant to Section 10A(i)(3) of the Exchange Act, our board of directors has delegated to Mr. Reis the authority to pre-approve any auditing and permissible non-auditing services to be performed by our registered independent public accounting firm, provided that all such decisions to pre-approve an activity are presented to the full audit committee at its first meeting following any such decision.
Compensation Committee
Effective upon the completion of this offering, our compensation committee will consist of Uday Garg, Rajiv Shah and Mark W. Wong, each of whom is a non-employee member of our board of directors, with Mr. Wong serving as compensation committee chair. Our board of directors has determined that each member of the compensation committee meets the requirements for independence under the listing standards of The NASDAQ Stock Market and SEC rules and regulations, is a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act, and is an outside director, as defined pursuant to Section 162(m) of the Internal Revenue Code of 1986, or the Code. The purpose of our compensation committee is to discharge the responsibilities of our board of directors relating to compensation of our executive officers. Our compensation committee will be responsible for, among other things:
Our compensation committee will operate under a written charter, to be effective on the date of this offering, that satisfies the applicable rules of the SEC and the listing standards of The NASDAQ Stock Market and that will be available on our website upon completion of this offering.
Nominating and Governance Committee
Effective upon the completion of this offering, our nominating and governance committee will consist of George F.J. Gosbee, Rajiv Shah, and Darby E. Shupp, each of whom is a non-employee member of our board of directors, with Ms. Shupp serving as nominating and governance committee chair. Our board of directors has determined that Mr. Gosbee and Dr. Shah meet the requirements for independence under the listing standards of The NASDAQ Stock Market and SEC rules and regulations. The NASDAQ Stock Market and SEC rules allow an issuer to, among other things, phase in, in connection with an initial public offering, the number of directors on its nominating and governance committee. Under these initial public offering phase-in rules, our nominating and governance committee must have at least one independent member at the time of listing, at least a majority of independent members within 90 days after listing, and all independent members within one year after listing. We plan to change the membership of our nominating and governance committee in the future to maintain compliance with the applicable phase-in requirements. Our nominating and governance committee will be responsible for, among other things:
The nominating and governance committee will operate under a written charter, to be effective upon the closing of this offering, that satisfies the applicable listing requirements and rules of The NASDAQ Stock Market and that will be available on our website upon completion of this offering.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee, or other board committee performing equivalent functions, of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Director Compensation
We currently provide cash compensation to two of our non-employee directors, Mr. Reis and Mr. Wong, which includes an annual retainer of $12,000, $1,200 per meeting for each in-person meeting attended, and $750 per meeting for each telephonic meeting attended. We also have a policy of reimbursing our directors for their reasonable out-of-pocket expenses in connection with their attendance at board and committee meetings. From time to time, we have granted stock options to certain of our non-employee directors as compensation for their services. Mr. Rey, who is also an employee, is compensated for his service as an employee and does not receive any additional compensation for his service on our board of directors.
The following table sets forth information regarding the compensation received by Mr. Reis and Mr. Wong during the fiscal year ended December 31, 2014.
Name | Fees Earned or Paid in Cash ($)(1) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | Fees Earned or Paid in Cash ($)(1) | Stock Awards ($) | Option Awards ($)(2) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
James R. Reis | $ | 19,050 | — | — | — | — | — | $ | 19,050 | $ | 19,050 | — | — | — | — | — | $ | 19,050 | ||||||||||||||||||||||||||
Mark W. Wong | 17,100 | — | — | — | — | — | 17,100 | 17,100 | — | — | — | — | — | 17,100 | ||||||||||||||||||||||||||||||
Additionally,
Director Name | Option Grant Date | Number of Options Granted(1) | Option Exercise Price Per Share ($)(2) | Option Expiration Date | Option Grant Date | Number of Options Granted(1) | Option Exercise Price Per Share ($)(2) | Option Expiration Date | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
James R. Reis | 5/2/2006 | 30,000 | (3) | $ | 0.11 | 12/31/2015 | 5/2/2006 | 7,500 | (3) | $ | 0.44 | 12/31/2015 | ||||||||||||||
| 7/1/2008 | 40,000 | (3) | 0.27 | 6/30/2018 | 7/1/2008 | 10,000 | (3) | 1.08 | 6/30/2018 | |||||||||||||||||
| 11/1/2009 | 20,000 | (3) | 0.56 | 10/31/2019 | 11/1/2009 | 5,000 | (3) | 2.24 | 10/31/2019 | |||||||||||||||||
| 1/1/2010 | 120,000 | (3) | 0.56 | 12/31/2019 | 1/1/2010 | 30,000 | (3) | 2.24 | 12/31/2019 | |||||||||||||||||
| 2/11/2015 | 20,000 | (4) | 1.80 | 2/10/2025 | 2/11/2015 | 5,000 | (4) | 7.20 | 2/10/2025 | |||||||||||||||||
Mark W. Wong | 5/2/2006 | 20,000 | (3) | 0.11 | 12/31/2015 | 5/2/2006 | 5,000 | (3) | 0.44 | 12/31/2015 | ||||||||||||||||
| 7/1/2008 | 40,000 | (3) | 0.27 | 6/30/2018 | 7/1/2008 | 10,000 | (3) | 1.08 | 6/30/2018 | |||||||||||||||||
| 11/1/2009 | 20,000 | (3) | 0.56 | 10/31/2019 | 11/1/2009 | 5,000 | (3) | 2.24 | 10/31/2019 | |||||||||||||||||
| 1/1/2010 | 120,000 | (3) | 0.56 | 12/31/2019 | 1/1/2010 | 30,000 | (3) | 2.24 | 12/31/2019 | |||||||||||||||||
| 2/11/2015 | 20,000 | (4) | 1.80 | 2/10/2025 | 2/11/2015 | 5,000 | (4) | 7.20 | 2/10/2025 | |||||||||||||||||
Following this offering, we will have a compensation policy applicable to our non-employee directors that is intended to compensate, incentivize, and retain them, consisting of the following:
remains a director), such director is granted an initial option to purchase 60,00015,000 shares of our common stock. This initial option will vest and become exercisable in three equal installments on
each of the first three anniversaries of the date of grant, subject to the director's continued service through each vesting date. The per share exercise price for the initial option shall be equal to the fair market value for a share of our common stock on the date of grant. An employee director who ceases to be an employee, but who remains a director, will not receive an option grant. Each of Messrs. Ankrum and Gosbee and Dr. Shah, who are joining our board in connection with this offering, will receive an initial option grant to purchase 60,00015,000 shares of our common stock with a per share exercise price equal to the initial public offering price, which will vest over three years.
Further, non-employee directors are reimbursed for the expenses they incur in connection with attending board and committee meetings.
Overview
The following discussion contains forward-looking statements that are based on our current plans, considerations, expectations, and determinations regarding future compensation programs. The actual amount and form of compensation and the compensation policies and practices that we adopt in the future may differ materially from currently planned programs as summarized below.
As an emerging growth company, we have opted to comply with the executive compensation disclosure rules applicable to "smaller reporting companies," as such term is defined in the rules promulgated under the Securities Act. The compensation provided to our named executive officers for 2013 and 2014 is detailed in the Summary Compensation Table and accompanying footnotes and narrative that follows this section.
Our named executive officers in 2013 and 2014 were:
Summary Compensation Table
The following table provides information regarding the total compensation awarded to, earned by, and paid to each of our named executive officers for the fiscal years ended December 31, 2013 and 2014:
Name and Principal Position | Year | Salary ($)(1) | Bonus ($)(2) | Stock Awards ($)(3) | Option Awards ($)(3) | Non-Equity Incentive Plan Compensation ($)(2) | All Other Compensation ($) | Total ($) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Eric J. Rey, | 2014 2013 | $ | 338,057 333,250 | — — | — — | $ | 108,612 — | — — | — — | $ | 446,669 333,250 | ||||||||||||||
Vic C. Knauf, | 2014 2013 | 261,503 235,000 | — — | — — | 54,306 — | — — | — — | 315,809 235,000 | |||||||||||||||||
Wendy S. Neal, | 2014 2013 | 405,995 378,216 | — — | — — | 54,306 — | — — | — — | 460,301 378,216 | |||||||||||||||||
statements included elsewhere in this prospectus. No stock awards or options awards were granted to the named executive officers during the 2013 fiscal year.
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding each unexercised stock option held by each of our named executive officers as of December 31, 2014:
| | Option Awards | Option Awards | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price ($) | Option Expiration Date | ||||||||||||||||||
Eric J. Rey | 850,000 | — | $ | 0.11 | 12/31/2015 | 212,500 | — | $ | 0.44 | 12/31/2015 | ||||||||||||||||
| 2,500,000 | — | 0.27 | 6/30/2018 | 625,000 | — | 1.08 | 6/30/2018 | |||||||||||||||||||
| 550,000 | — | 0.56 | 10/31/2019 | 137,500 | — | 2.24 | 10/31/2019 | |||||||||||||||||||
| 750,000 | — | 0.56 | 12/31/2019 | 187,500 | — | 2.24 | 12/31/2019 | |||||||||||||||||||
| 500,000 | — | 3.39 | 12/31/2020 | 125,000 | — | 13.56 | 12/31/2020 | |||||||||||||||||||
| 75,000 | 25,000 | (1) | 3.39 | 12/31/2022 | 18,750 | 6,250 | (1) | 13.56 | 12/31/2022 | |||||||||||||||||
| — | 100,000 | (2) | 1.53 | 10/29/2024 | — | 25,000 | (2) | 6.12 | 10/29/2024 | |||||||||||||||||
Vic C. Knauf | 190,000 | — | 0.11 | 12/31/2015 | 47,500 | — | 0.44 | 12/31/2015 | ||||||||||||||||||
| 1,010,000 | — | 0.27 | 6/30/2018 | 252,500 | — | 1.08 | 6/30/2018 | |||||||||||||||||||
| 212,000 | — | 0.56 | 10/31/2019 | 53,000 | — | 2.24 | 10/31/2019 | |||||||||||||||||||
| 300,000 | — | 0.56 | 12/31/2019 | 75,000 | — | 2.24 | 12/31/2019 | |||||||||||||||||||
| 75,000 | — | 3.39 | 12/31/2020 | 18,750 | — | 13.56 | 12/31/2020 | |||||||||||||||||||
| 75,000 | 25,000 | (1) | 3.39 | 12/31/2022 | 18,750 | 6,250 | (1) | 13.56 | 12/31/2022 | |||||||||||||||||
| — | 50,000 | (2) | 1.53 | 10/29/2024 | — | 12,500 | (2) | 6.12 | 10/29/2024 | �� | ||||||||||||||||
Wendy S. Neal | 200,000 | — | 0.27 | 6/30/2018 | 50,000 | — | 1.08 | 6/30/2018 | ||||||||||||||||||
| 125,000 | — | 0.56 | 10/31/2019 | 31,250 | — | 2.24 | 10/31/2019 | |||||||||||||||||||
| 200,000 | — | 0.56 | 12/31/2019 | 50,000 | — | 2.24 | 12/31/2019 | |||||||||||||||||||
| 75,000 | — | 3.39 | 12/31/2020 | 18,750 | — | 13.56 | 12/31/2020 | |||||||||||||||||||
| 75,000 | 25,000 | (1) | 3.39 | 12/31/2022 | 18,750 | 6,250 | (1) | 13.56 | 12/31/2022 | |||||||||||||||||
| — | 50,000 | (2) | 1.53 | 10/29/2024 | — | 12,500 | (2) | 6.12 | 10/29/2024 | |||||||||||||||||
Agreements with Current Named Executive Officers
Currently, all of our named executive officers are "at-will" employees and none of them have entered into a written employment agreement with us. However, in connection with this offering, we anticipate entering into new offer letters with each of our named executive officers that will set forth each executive officer's title, base salary, and bonus opportunity (each as detailed in the footnotes to the Summary Compensation Table above). The new offer letters will not change the "at-will" nature of
the executive officers' employment. In March 2015, Thomas O'Neil joined us as our Chief Financial Officer. His offer letter provides for an annual base salary of $280,000, a target bonus of 35% of base salary, the grant of an initial option to purchase 250,00062,500 shares of our common stock, which vests over four years, and an additional grant to be made at the time of the initial public offering.
Severance and Change in Control Agreements
In February 2015, our Board approved new severance and change in control agreements, or CIC Agreements, for each of our executive officers, the specific terms of which are discussed below. Each of the CIC Agreements expires by its terms on the third anniversary of the effective date of such agreement, which we expect to be on or around the completion of this offering.
Pursuant to the CIC Agreements, if we terminate an executive's employment with us for a reason other than cause (as defined in the CIC Agreements) or the executive's death or disability (as defined in the CIC Agreements) at any time other than during the twelve month period immediately following a change of control (as defined in the CIC Agreements), then such executive will receive the following severance benefits from the company: (i) severance in the form of base salary continuation for a period of six months (twelve months for Mr. Rey); and (ii) reimbursement for premiums paid for coverage pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended, or COBRA, for the executive and the executive's eligible dependents for up to six months (twelve months for Mr. Rey).
If during the twelve month period immediately following a change of control (as defined in the CIC Agreements), (x) we terminate an executive's employment with us for a reason other than cause (as defined in the CIC Agreements) or the executive's death or disability (as defined in the CIC Agreements), or (y) an executive resigns from such employment for good reason (as defined in the CIC Agreements), then, in lieu of the above described severance benefits, such executive shall receive the following severance benefits from the company: (i) severance in the form of base salary continuation for a period of twelve months (twenty-four months for Mr. Rey); (ii) reimbursement for premiums paid for coverage pursuant to COBRA, for the executive and the executive's eligible dependents for up to twelve months (twenty-four months for Mr. Rey); and (iii) vesting shall accelerate as to 100% of all of the executive's outstanding equity awards.
An executive's receipt of severance payments or benefits pursuant to a CIC Agreement is subject to the executive signing a release of claims in our favor and complying with certain restrictive covenants set forth in the CIC Agreement.
Each CIC Agreement contains a "better after-tax" provision, which provides that if any of the payments to an executive constitutes a parachute payment under Section 280G of the Code, the payments will either be (i) reduced or (ii) provided in full to the executive, whichever results in the executive receiving the greater amount after taking into consideration the payment of all taxes, including the excise tax under Section 4999 of the Code, in each case based upon the highest marginal rate for the applicable tax.
Pension Benefits
None of our named executive officers participate in or have account balances in qualified or nonqualified defined benefit plans sponsored by us.
Nonqualified Deferred Compensation
We do not maintain any nonqualified defined contribution or deferred compensation plans or arrangements for our named executive officers.
Employee Benefit and Stock Plans
2015 Omnibus Equity Incentive Plan
In April 2015, our board of directors approved our 2015 Omnibus Equity Incentive Plan, or the 2015 Plan. The 2015 Plan will become effective immediately prior to the effectiveness of the registration statement of which this prospectus forms a part, subject to the approval of our stockholders. Our 2015 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Code, to our employees and the employees of our subsidiaries, and for the grant of nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units, performance units, and performance shares to our employees, directors, and consultants and the employees and consultants of our subsidiaries.
The following summary of terms of the 2015 Plan is based on the terms of the 2015 Plan as approved by the board of directors, but the terms are not final until approved by our stockholders.
Authorized Shares. The maximum aggregate number of shares that may be issued under the 2015 Plan is 2,875,000 shares of our common stock plus (i) any shares that as of the completion of this offering, have been reserved but not issued pursuant to any awards granted under our 2006 Plan and are not subject to any awards granted thereunder, and (ii) any shares subject to awards under the 2006 Plan that otherwise would have been returned to the 2006 Plan on account of the expiration, cancellation or forfeiture of such awards, with the maximum number of shares to be added to the 2015 Plan pursuant to clauses (i) and (ii) above equal to shares as of .4,263,428 shares. In addition, the number of shares available for issuance under the 2015 Plan will be annually increased on the first day of each of our fiscal years beginning with the 2016 fiscal year, by an amount equal to the least of:
Shares issued pursuant to awards under the 2015 Plan that we repurchase or that are otherwise forfeited will become available for future grant under the 2015 Plan on the same basis as the award initially counted against the share reserve. In addition, to the extent that an award is paid out in cash rather than shares, such cash payment will not reduce the number of shares available for issuance under the 2015 Plan.
Award Limitations. The following limits apply to any awards granted under the 2015 Plan:
is greater. No individual may be granted more than one award of performance units or performance shares for a performance period.
Plan Administration. The 2015 Plan will be administered by our board of directors, which, at its discretion or as legally required, may delegate such administration to our compensation committee and/or one or more additional committees. In the case of awards intended to qualify as "performance-based compensation" within the meaning of Code Section 162(m), the compensation committee will consist of two or more "outside directors" within the meaning of Code Section 162(m).
Subject to the provisions of our 2015 Plan, the administrator has the power to determine the terms of awards, including the recipients, the exercise price, if any, the number of shares subject to each award, the fair market value of a share of our common stock, the vesting schedule applicable to the awards, together with any vesting acceleration, and the form of consideration, if any, payable upon exercise of the award and the terms of the award agreement for use under the 2015 Plan. The administrator also has the authority, subject to the terms of the 2015 Plan, to amend existing awards, to prescribe rules, and to construe and interpret the 2015 Plan and awards granted thereunder. In addition, the administrator cannot reduce the exercise price of options and initiate an option exchange program, whereby outstanding options are exchanged for options with a lower exercise price, without stockholder approval if the fair market value of such options has declined since the date such options were granted.
Stock Options. The administrator may grant incentive and/or nonstatutory stock options under our 2015 Plan; provided that incentive stock options are only granted to employees. The exercise price of such options must equal at least the fair market value of our common stock on the date of grant. The term of an option may not exceed ten years; provided, however, that an incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock, or of certain of our subsidiary corporations, may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value of our common stock on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the administrator. Subject to the provisions of our 2015 Plan, the administrator determines the vesting terms of options granted under the 2015 Plan. After the termination of service of an employee, director or consultant, the participant may exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in his or her option agreement. Generally, if termination is due to death or disability, the option will remain exercisable for twelve months. In all other cases, the option will generally remain exercisable for three months following the termination of service. However, in no event may an option be exercised later than the expiration of its term.
Stock Appreciation Rights. Stock appreciation rights may be granted under our 2015 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Subject to the provisions of our 2015 Plan, the administrator determines the terms of stock appreciation rights, including when such rights vest and become exercisable and whether to settle such awards in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted Stock. Restricted stock may be granted under our 2015 Plan. Restricted stock awards are grants of shares of our common stock that are subject to various restrictions, including restrictions on transferability and forfeiture provisions. Shares of restricted stock will vest and the restrictions on such shares will lapse in accordance with terms and conditions established by the administrator. Such terms may include, among other things, vesting upon the achievement of specific performance goals determined by the administrator and/or continued service. The administrator, in its sole discretion, may
accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards generally will have voting and cash dividend rights with respect to such shares upon grant without regard to vesting, unless the administrator provides otherwise. Shares of restricted stock that do not vest for any reason will be forfeited by the recipient and will revert to us.
Restricted Stock Units. Restricted stock units may be granted under our 2015 Plan, which may include the right to dividend equivalents, as determined in the discretion of the administrator. Each restricted stock unit granted is a bookkeeping entry representing an amount equal to the fair market value of one share of our common stock. The administrator determines the terms and conditions of restricted stock units including the vesting criteria, which may include achievement of specified performance criteria or continued service, and the form and timing of payment. The administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. The administrator determines in its sole discretion whether an award will be settled in stock, cash, or a combination of both.
Performance Units and Performance Shares. Performance units and performance shares may be granted under our 2015 Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the administrator are achieved and any other applicable vesting provisions are satisfied. The administrator will establish organizational or individual performance goals in its discretion, which, depending on the extent to which they are met, will determine the number and/or the value of performance units and performance shares to be paid out to participants. For purposes of such awards, the performance goals may be one or more of the following, as determined by the administrator: (i) sales or non-sales revenue; (ii) return on revenues; (iii) operating income; (iv) income or earnings including operating income; (v) income or earnings before or after taxes, interest, depreciation, and/or amortization; (vi) income or earnings from continuing operations; (vii) net income; (viii) pre-tax income or after-tax income; (ix) net income excluding amortization of intangible assets, depreciation and impairment of goodwill and intangible assets, and/or excluding charges attributable to the adoption of new accounting pronouncements; (x) raising of financing or fundraising; (xi) project financing; (xii) revenue backlog; (xiii) gross margin; (xiv) operating margin or profit margin; (xv) capital expenditures, cost targets, reductions and savings, and expense management; (xvi) return on assets (gross or net), return on investment, return on capital, or return on stockholder equity; (xvii) cash flow, free cash flow, cash flow return on investment (discounted or otherwise), net cash provided by operations, or cash flow in excess of cost of capital; (xviii) performance warranty and/or guarantee claims; (xix) stock price or total stockholder return; (xx) earnings or book value per share (basic or diluted); (xxi) economic value created; (xxii) pre-tax profit or after-tax profit; (xxiii) strategic business criteria, consisting of one or more objectives based on meeting specified market penetration or market share, completion of strategic agreements such as licenses, funded collaborations, joint ventures, acquisitions, and the like, geographic business expansion, objective customer satisfaction or information technology goals, and/or intellectual property asset metrics; (xxiv) objective goals relating to divestitures, joint ventures, mergers, acquisitions, and similar transactions; (xxv) objective goals relating to staff management, results from staff attitude and/or opinion surveys, staff satisfaction scores, staff safety, staff accident and/or injury rates, compliance headcount, performance management, and completion of critical staff training initiatives; (xxvi) objective goals relating to projects, including project completion timing and/or achievement of milestones, project budget, and technical progress against work plans; (xxvii) key regulatory objectives or milestones; and (xxviii) enterprise resource planning. Any criteria used may be measured, as applicable, (i) in absolute terms, (ii) in relative terms (including, but not limited to, any increase (or decrease) over the passage of time and/or any measurement against other companies or financial or business or stock index metrics particular to us), (iii) on a per share and/or share per capita basis, (iv) against our performance as a whole or against any of our affiliate(s), or a particular segment(s), a business unit(s) or a product(s) of ours or individual project company, (v) on a pre-tax or after-tax basis, and/or (vi) using an actual foreign exchange rate or on a foreign exchange neutral basis. After
the grant of a performance unit or performance share, the administrator, in its sole discretion, may reduce or waive any performance objectives or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the administrator prior to the grant date. Performance shares shall have an initial value equal to the fair market value of our common stock on the grant date. The administrator, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares, or in some combination thereof.
Transferability of Awards. Unless the administrator provides otherwise, our 2015 Plan generally does not allow for the transfer of awards and only the recipient of an option or stock appreciation right may exercise such an award during his or her lifetime.
Certain Adjustments. In the event of certain corporate events or changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the 2015 Plan, the administrator will make adjustments to one or more of the number and class of shares that may be delivered under the 2015 Plan and/or the number, class and price of shares covered by each outstanding award and the numerical share limits contained in the 2015 Plan.
Dissolution or Liquidation. In the event of our proposed winding up, liquidation, or dissolution, the administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or Change in Control. Our 2015 Plan provides that in the event of a merger or change in control (other than a winding up, dissolution, or liquidation), as defined under the 2015 Plan, each outstanding award will be treated as the administrator determines (including assumed, substituted, or cancelled), except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award, then such award will fully vest, all restrictions on such award will lapse, and all performance goals or other vesting criteria applicable to such award will be deemed achieved at 100% of target levels. In addition, if an option or stock appreciation right is not assumed or substituted in the event of a change in control, the administrator will notify the participant in writing or electronically that the option or stock appreciation right will be exercisable for a period of time determined by the administrator in its sole discretion, and the option or stock appreciation right will terminate upon the expiration of such period. Under our 2015 Plan, an award will be considered assumed if, following the change in control, the award confers the right to purchase or receive, for each share subject to the award immediately prior to the change in control, the consideration (whether stock, cash, or other securities or property) received in the change in control by our common stockholders (and if holders were offered a choice of consideration, the type of consideration chosen by the holders of a majority of the outstanding shares of our common stock). However, if the consideration received in the change in control is not solely common stock of the successor corporation or its parent, the administrator may, with the consent of the successor corporation, provide for the consideration to be received upon the exercise of an option or stock appreciation right or upon the payout of a restricted stock unit, performance unit, or performance share to be solely common stock of the successor corporation or its parent equal in fair market value to the per share consideration received by our common stockholders in the change in control.
Plan Amendment, Termination. Our board of directors has the authority to amend, suspend, or terminate the 2015 Plan provided such action does not impair the existing rights of any participant. Our 2015 Plan will automatically terminate on the tenth anniversary of the effective date of the 2015 Plan, unless we terminate it sooner.
IPO Grants. Immediately following the effective date of the registration statement of which this prospectus is a part, the named executive officers and certain other executives will receive stock option grants under the 2015 Plan, which we call the IPO Options, with the number of shares of our common
stock subject to each IPO Option determined by dividing a fixed dollar amount applicable to each individual by the Black-Scholes value of a share of our common stock as determined on the date of grant. The IPO Options will have a per share exercise price equal to the initial public offering price and shall vest as to 25% of the shares subject to the IPO Option on the 1-year anniversary of the date of grant and as to 1/48 of the shares subject to the IPO Options monthly thereafter, subject to the individual's continued service through each vesting date. The applicable dollar values of the IPO Options for the named executive officers are as follows: (i) $2,070,000 for Mr. Rey; (ii) $496,000 for Dr. Knauf; and (iii) $414,000 for Ms. Neal.
2006 Stock Plan
Our board of directors adopted, and our stockholders approved, our 2006 Stock Plan, or the 2006 Plan, on February 1, 2006. The 2006 Plan was last amended and restated on May 4, 2012. Our 2006 Plan provides for the grant of incentive stock options to our employees and the employees of our affiliates, and for the grant of nonstatutory stock options and stock purchase rights to our employees, directors, and consultants and the employees, directors, and consultants of our affiliates. No new awards will be granted under our 2006 Plan following this offering, but previously granted awards will continue to be subject to the terms and conditions of the 2006 Plan and the stock award agreements pursuant to which such awards were granted.
Authorized Shares. The maximum aggregate number of shares that may be issued under the 2006 Plan is 18,000,0004,500,000 shares of our common stock. The maximum aggregate number of shares that may be issued pursuant to incentive stock options under the 2006 Plan is 18,000,0004,500,000 shares of our common stock. As of DecemberMarch 31, 2014,2015, options to purchase 15,039,3634,062,181 shares of our common stock were outstanding and 2,040,058203,924 shares were available for future grants.
Plan Administration. Our board of directors, or a committee appointed by the board of directors, administers the 2006 Plan and any stock awards granted under the 2006 Plan. The administrator has the power and authority to determine the terms of the awards, including eligibility, the form of agreements for use under the 2006 Plan, the exercise price, the number of shares covered by each such award, the vesting schedule and exercisability of awards, and the form of consideration payable upon exercise. The administrator also has the power and authority to construe and interpret the terms of the 2006 Plan and awards granted pursuant to the 2006 Plan and to allow participants to satisfy their tax withholding obligations by electing to have us withhold shares to be issued upon exercise of an option or pursuant to a stock purchase right. In addition, the administrator has the authority to reduce the exercise price of options if the fair market value of such options has declined since the date such options were granted and initiate an option exchange program, whereby outstanding options are exchanged for options with a lower exercise price.
Stock Options. With respect to all stock options granted under the 2006 Plan, the exercise price of such options must equal at least the fair market value of our common stock on the date of grant. The term of an option may not exceed ten years; provided, however, that an incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock, or of certain of our subsidiary corporations, may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value of our common stock on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash or other methods of payment acceptable to the administrator. Subject to the provisions of the 2006 Plan, the administrator determines the vesting terms of options granted under the 2006 Plan). After the termination of service of an employee, director or consultant, the participant may exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in his or her option agreement. Unless the terms of the participant's option agreement provide otherwise, in the case of nonstatutory stock options, if the participant's service relationship with
us, or any of our affiliates, ceases for any reason other than disability, death, retirement or cause, the participant may generally exercise any vested options for a period of at least 30 days following the cessation of service and, if a participant's service relationship with us or any of our affiliates ceases due to disability or death, or a participant retires, the participant or legal representative may generally exercise any vested options at any time prior to the expiration of such options. Unless the terms of the participant's option agreement provide otherwise, in the case of incentive stock options, if the participant's service relationship with us, or any of our affiliates, ceases for any reason other than disability, death, retirement, or cause, the participant may generally exercise any vested options for a period of three months following the cessation of service and, if a participant's service relationship with us or any of our affiliates ceases due to disability or death, or a participant retires, the participant or legal representative may generally exercise any vested options for a period of 12 months in the event of disability or death and three months in the event of retirement. In no event may an option be exercised beyond the expiration of its term.
Stock Purchase Rights. Stock purchase rights are rights to purchase shares of our common stock that are either fully vested at grant or will vest in accordance with terms and conditions established by the administrator, in its sole discretion. The administrator will determine the number of shares that the participant may purchase, the price to be paid, and the time in which the participant must accept the offer. The offer must be accepted by execution of a restricted stock purchase agreement in the form determined by the administrator. The purchase price of stock purchased by a participant must not be less than 100% of the fair market value per share on the date of grant. Once a stock purchase right is exercised, the participant has all the rights of a stockholder.
Transferability of Awards. Unless otherwise determined by the administrator, the 2006 Plan generally does not allow for the sale or transfer of awards under the 2006 Plan other than by will or the laws of descent and distribution, and may be exercised during the lifetime of the participant only by such participant. Subject to compliance with all applicable laws, the administrator may in its discretion grant transferable nonstatutory stock options and stock purchase rights in accordance in accordance with the terms set forth in the applicable award agreement.
Certain Adjustments. In the event of certain changes made in our common stock, appropriate adjustments will be made in the number and class of shares that may be delivered under the 2006 Plan and/or the number, class and price of shares covered by each outstanding award and the numerical share limits contained in the 2006 Plan.
Dissolution or Liquidation. In the event of our proposed winding up, liquidation, or dissolution, the administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction. The administrator in its discretion may provide for the participant to exercise his or her options 15 days prior to such transaction, including shares that would not otherwise be exercisable, and provide for the lapsing of any repurchase rights with respect to shares purchased upon exercise of an option or stock purchase right.
Merger or Change in Control. In the event of a merger, consolidation, or the sale of substantially all of our assets, the 2006 Plan provides that the outstanding options and stock rights will be treated as set forth in the agreement of merger, consolidation, or asset sale, which shall provide for any of the following: (i) the assumption of the awards by the surviving corporation or its parent; (ii) the continuation of the awards by the company if the company is the surviving corporation; (iii) a cash settlement equal in the case of options to the difference between the amount paid for one share and the exercise price multiplied by the number of shares, vested or unvested or both, subject to the option, and in the case of stock purchase rights, the amount to be paid for one share multiplied by the number of vested or unvested shares determined by the company; or (iv) subject to the consummation of the transaction, the acceleration of the vesting of outstanding stock awards prior to the consummation of
the transaction, with notification to the holders of options that such options shall be exercisable for 15 days from the date of such notice and termination of awards after such period.
Plan Amendment, Termination. Our board of directors may at any time amend, alter, suspend or terminate the 2006 Plan, provided such action does not impair the existing rights of any participant. Our 2006 Plan will terminate in connection with, and contingent upon, the effectiveness of the registration statement of which this prospectus forms a part; provided that the 2006 Plan will continue to govern the terms and conditions of awards originally granted under the 2006 Plan. Following the consummation of our initial public offering, we expect to make future awards under our 2015 Plan.
2015 Employee Stock Purchase Plan
In April 2015, our board of directors approved our 2015 Employee Stock Purchase Plan, or the ESPP. The ESPP will become effective immediately prior to the effectiveness of the registration statement of which this prospectus forms a part, subject to the approval of our stockholders. Our executive officers and all of our other employees will be allowed to participate in our ESPP. In general, we intend to make offerings under the ESPP that qualify under Section 423 of the Code, but may make offerings that are not intended to qualify under Section 423 of the Code to the extent deemed advisable for designated subsidiaries outside the United States. Additionally, we may make separate offerings under the ESPP, each of which may have different terms, but each separate offering will be intended to comply with the requirements of Section 423 of the Code.
The following summary of terms of the ESPP is based on the terms of the ESPP as approved by the board of directors, but the terms are not final until approved by the stockholders.
Authorized Shares. A total of 625,000 shares of our common stock will be made available for sale under our ESPP. In addition, our ESPP provides for annual increases in the number of shares available for issuance under the ESPP on the first day of each fiscal year beginning with the 2016 fiscal year, equal to the least of:
Plan Administration. Our board of directors or a committee that has been duly authorized by our board of directors has full and exclusive authority to interpret the terms of the ESPP and determine eligibility.
Eligibility. All of our employees are eligible to participate if they are customarily employed by us or any participating subsidiary for more than 20 hours per week and more than five months in any calendar year. However, an employee may not be granted rights to purchase stock under our ESPP if such employee:
Offerings. Our ESPP is intended to qualify under Section 423 of the Code, and provides for consecutive, non-overlapping six-month offering periods. The offering periods generally start on the first trading day on or after February 1 and August 1 of each year, except for the first such offering period which will commence on the first trading day on or after the effective date of the registration
statement of which this prospectus is a part and will end on February 1, 2016. The administrator may, in its discretion, modify the terms of future offering periods.
Limitations. Our ESPP permits participants to purchase common stock through payroll deductions of up to 15% of their eligible compensation, which includes a participant's regular and recurring straight time gross earnings, payments for overtime, and shift premium, exclusive of payments for incentive compensation, bonuses and other similar compensation. A participant may purchase up to a maximum of 3,000750 shares of common stock during each six-month offering period.
Purchase of Stock. Amounts deducted and accumulated by the participant are used to purchase shares of our common stock at the end of each six-month offering period. The purchase price of the shares will be 15% of the lower of the fair market value of our common stock on the first trading day of the offering period or on the last day of the offering period. Participants may end their participation at any time during an offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon termination of employment with us.
Withdrawal. During an offering, a participant may cease making contributions and withdraw from the offering by delivering a notice of withdrawal and terminating his or her payroll deductions in such form as we may require. Upon such withdrawal, we will refund accumulated payroll deductions without interest to the employee, and such employee's right to participate in that offering will terminate. An employee's withdrawal from an offering will not affect such employee's eligibility to participate in subsequent offerings under the ESPP.
Transferability. A participant may not transfer rights granted under the ESPP other than by will, the laws of descent and distribution, or as otherwise provided under the ESPP.
Certain Adjustments. In the event of certain corporate events or changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under the ESPP, the administrator will make adjustments to one or more of the number and class of shares that may be delivered under the ESPP and/or the number, class, and price of shares covered by each outstanding purchase right and the numerical share limits contained in the ESPP.
Dissolution or Liquidation. In the event of our proposed winding up, liquidation, or dissolution, any offering period then in progress will be shortened by setting a new exercise date, and will terminate immediately prior to the consummation of such proposed dissolution or liquidation, unless provided otherwise by the administrator.
Merger or Change in Control. In the event of our merger or change of control, as defined under the ESPP, a successor corporation may assume or substitute each outstanding purchase right. If the successor corporation refuses to assume or substitute for the outstanding purchase rights, the offering period then in progress will be shortened, and a new exercise date will be set. The plan administrator will notify each participant in writing that the exercise date has been changed and that the participant's option will be exercised automatically on the new exercise date unless the participant has already withdrawn from the offering period.
Plan Amendment, Termination. Our ESPP will automatically terminate on the tenth anniversary of the effective date of the ESPP, unless we terminate it sooner. In addition, our board of directors has the authority to amend, suspend, or terminate our ESPP, except that, subject to certain exceptions described in the ESPP, no such action may adversely affect any outstanding rights to purchase stock under our ESPP.
Executive Incentive Bonus Plan
In April 2015, our board of directors approved the Executive Incentive Bonus Plan, or the Bonus Plan. The Bonus Plan will become effective immediately prior to the effectiveness of the registration statement of which this prospectus forms a part. The purpose of the Bonus Plan is to motivate and reward eligible officers and employees for their contributions toward the achievement of certain performance goals, with the intention that the incentives paid thereunder to certain of our executive officers be deductible during the applicable reliance period under Section 162(m) of the Code and the regulations and interpretations promulgated thereunder.
Administration. The Bonus Plan will be administered by the compensation committee, which shall have the discretionary authority to interpret the provisions of the Bonus Plan, including all decisions on eligibility to participate, the establishment of performance goals, the amount of awards payable under the plan, and the payment of awards.
Performance criteria. Commencing with our 2015 fiscal year, we expect the compensation committee to establish cash bonus targets and corporate performance metrics for a specific performance period (not to exceed 36 months) or fiscal year pursuant to the Bonus Plan. Corporate performance goals may be based on one or more of the following criteria, as determined by our compensation committee: (i) sales or non-sales revenue; (ii) return on revenues; (iii) operating income; (iv) income or earnings including operating income; (v) income or earnings before or after taxes, interest, depreciation, and/or amortization; (vi) income or earnings from continuing operations; (vii) net income; (viii) pre-tax income or after-tax income; (ix) net income excluding amortization of intangible assets, depreciation, and impairment of goodwill and intangible assets and/or excluding charges attributable to the adoption of new accounting pronouncements; (x) raising of financing or fundraising; (xi) project financing; (xii) revenue backlog; (xiii) gross margin; (xiv) operating margin or profit margin; (xv) capital expenditures, cost targets, reductions, and savings and expense management; (xvi) return on assets (gross or net), return on investment, return on capital, or return on stockholder equity; (xvii) cash flow, free cash flow, cash flow return on investment (discounted or otherwise), net cash provided by operations, or cash flow in excess of cost of capital; (xviii) performance warranty and/or guarantee claims; (xix) stock price or total stockholder return; (xx) earnings or book value per share (basic or diluted); (xxi) economic value created; (xxii) pre-tax profit or after-tax profit; (xxiii) strategic business criteria, consisting of one or more objectives based on meeting specified market penetration or market share, completion of strategic agreements such as licenses, funded collaborations, joint ventures acquisitions, and the like, geographic business expansion, objective customer satisfaction or information technology goals, or intellectual property asset metrics; (xxiv) objective goals relating to divestitures, joint ventures, mergers, acquisitions, and similar transactions; (xxv) objective goals relating to staff management, results from staff attitude and/or opinion surveys, staff satisfaction scores, staff safety, staff accident and/or injury rates, compliance, headcount, performance management, or completion of critical staff training initiatives; (xxvi) objective goals relating to projects, including project completion, timing and/or achievement of milestones, project budget, or technical progress against work plans; (xxvii) key regulatory objectives or milestones; and (xxviii) enterprise resource planning. Awards issued to participants who are not subject to the limitations of Code Section 162(m) or awards to participants that are not intended to comply with the requirements of Code Section 162(m) may, in either case, take into account other factors (including subjective factors). Performance goals may differ from participant to participant, performance period to performance period, and from award to award. Any criteria used may be measured, as applicable, (i) in absolute terms, (ii) in relative terms (including, but not limited to, any increase (or decrease) over the passage of time and/or any measurement against other companies or financial or business or stock index metrics particular to us), (iii) on a per share and/or share per capita basis, (iv) against our performance as a whole or against any of our affiliate(s), or a particular segment(s), a business unit(s) or a product(s) of ours or individual project company, (v) on a pre-tax or after-tax basis, and/or (vi) using an actual foreign exchange rate or on a foreign
exchange neutral basis. It is the intent that, starting in 2015, the compensation committee will establish corporate performance metrics that are both aggressive and obtainable and that the executive officers' performance at expected levels will provide the opportunity to achieve a meaningful number of the corporate goals and objectives. Following the end of the performance period, the compensation committee will approve the achievement of the corporate performance metrics and authorize the funding of the cash bonuses for that period.
Limits. Under the Bonus Plan, the maximum award that can be paid to a participant during any performance period is $ .$2,000,000. The total awards under the Bonus Plan may not exceed $$10,000,000 during any calendar year or $$30,000,000 during the applicable reliance period (within the meaning of Section 162(m)).
Plan Amendment, Termination. The compensation committee may terminate the Bonus Plan at any time, provided such termination shall not affect the payment of any awards accrued under the Bonus Plan prior to the date of the termination. The compensation committee may, at any time, or from time to time, amend or suspend and, if suspended, reinstate, the Bonus Plan in whole or in part; provided, however, that any amendment of the Bonus Plan shall be subject to the approval of our stockholders to the extent required to comply with the requirements of Section 162(m) of the Code, or any other applicable laws, regulations or rules.
401(k) Plan
We maintain a tax-qualified retirement plan that provides eligible U.S. employees with an opportunity to save for retirement on a tax-advantaged basis. Plan participants are able to defer eligible compensation subject to applicable annual Code limits. Each participant's pre-tax contributions are allocated to his or her individual account and are then invested in selected investment alternatives according to the participant's directions. We have the ability to make discretionary contributions to the 401(k) plan but have not done so to date. The 401(k) plan is intended to be qualified under Section 401(a) of the Code with the 401(k) plan's related trust intended to be tax exempt under Section 501(a) of the Code. As a tax-qualified retirement plan, contributions to the 401(k) plan and earnings on those contributions are not taxable to the employees until distributed from the 401(k) plan.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or executive officer when entering into the plan, without further direction from them. The director or executive officer may amend or terminate the plan in some circumstances. Our directors and executive officers may also buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
Furthermore, certain of our directors and executive officers hold options to purchase approximately 1.5 million shares of our common stock that will expire on December 31, 2015. A portion of these shares are not subject to the 180-day lock-up described in "Underwriting" and may be sold immediately after this offering by these directors and executive officers solely to cover applicable withholding taxes.
Limitation of Liability and Indemnification of Officers and Directors
Our amended and restated certificate of incorporation, to be effective upon the completion of this offering, will provide that we will indemnify our directors and officers, and may indemnify our employees and other agents, to the fullest extent permitted by the Delaware General Corporation Law,
which prohibits our amended and restated certificate of incorporation from limiting the liability of our directors for the following:
Any amendment to, or repeal of, these provisions will not eliminate or reduce the effect of these provisions in respect of any act, omission, or claim that occurred or arose prior to that amendment or repeal. If the Delaware General Corporation Law is amended to provide for further limitations on the personal liability of directors of corporations, then the personal liability of our directors will be further limited to the greatest extent permitted by the Delaware General Corporation Law.
Our amended and restated bylaws, to be effective upon the completion of this offering, will provide that we will indemnify, to the fullest extent permitted by law, any person who is or was a party or is threatened to be made a party to any action, suit, or proceeding by reason of the fact that he or she is or was one of our directors or officers or is or was serving at our request as a director or officer of another corporation, partnership, joint venture, trust, or other enterprise. Our amended and restated bylaws are expected to provide that we may indemnify to the fullest extent permitted by law any person who is or was a party or is threatened to be made a party to any action, suit, or proceeding by reason of the fact that he or she is or was one of our employees or agents or is or was serving at our request as an employee or agent of another corporation, partnership, joint venture, trust, or other enterprise. Our amended and restated bylaws will also provide that we must advance expenses incurred by or on behalf of a director or officer in advance of the final disposition of any action or proceeding, subject to very limited exceptions.
Further, prior to the closing of this offering, we expect to enter into indemnification agreements with each of our directors, director nominees, and executive officers that may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements will require us, among other things, to indemnify our directors and executive officers against liabilities that may arise by reason of their status or service. These indemnification agreements will also require us to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit, or proceeding. We believe that these agreements are necessary to attract and retain qualified individuals to serve as directors and executive officers.
The limitation of liability and indemnification provisions that are expected to be included in our amended and restated certificate of incorporation, amended restated bylaws, and in indemnification agreements that we enter into with our directors, director nominees, and executive officers may discourage stockholders from bringing a lawsuit against our directors and executive officers for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against our directors and executive officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder's investment may be harmed to the extent that we pay the costs of settlement and damage awards against directors and executive officers as required by these indemnification provisions. At present, we are not aware of any pending litigation or proceeding involving any person who is or was one of our directors, director nominees, officers, employees, or other agents or is or was serving at our request as a director, director nominee, officer, employee, or agent of another corporation, partnership, joint venture, trust or other enterprise, for which
indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
We have obtained insurance policies under which, subject to the limitations of the policies, coverage is provided to our directors and executive officers against loss arising from claims made by reason of breach of fiduciary duty or other wrongful acts as a director or executive officer, including claims relating to public securities matters, and to us with respect to payments that may be made by us to these directors and executive officers pursuant to our indemnification obligations or otherwise as a matter of law.
Certain of our non-employee directors may, through their relationships with their employers or affiliated entities, be insured or indemnified against certain liabilities incurred in their capacity as members of our board of directors. In our indemnification agreements with these non-employee directors, we have agreed that our indemnification obligations will be primary to any such other indemnification arrangements.
The underwriting agreement provides for indemnification by the underwriters of us and our officers, directors, and employees for certain liabilities arising under the Securities Act, or otherwise.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or persons controlling our company pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
In addition to the compensation arrangements, including employment, termination of employment and change in control arrangements, and indemnification arrangements, discussed, when required, in the sections titled "Management" and "Executive Compensation" and the registration rights described in the section titled "Description of Capital Stock—Registration Rights," the following is a description of each transaction since January 1, 2012 and each currently proposed transaction in which:
Series D Preferred Stock Financing
Between March and May 2014, we sold an aggregate of 9,822,283 shares of our Series D preferred stock at a purchase price of $3.36 per share for an aggregate purchase price of approximately $33.0 million.
In this transaction, Mandala Capital and its affiliates purchased 8,918,750 shares of our Series D redeemable convertible preferred stock at an aggregate purchase price of $30.0 million and were issued warrants to purchase 4,459,3751,114,843 shares of our common stock. Following this sale, Mandala Capital and its affiliates beneficially owned more than 5% of our outstanding capital stock. Uday Garg, a member of our board of directors, is managing director and a member of the board of directors of Mandala Capital and was designated by Mandala Capital to serve on our board of directors as their representative.
Vic C. Knauf, Ph.D., our Chief Scientific Officer, also participated in the Series D Preferred Stock Financing and purchased 3,268 shares of our Series D redeemable convertible preferred stock at an aggregate purchase price of $10,980 and was issued a warrant to purchase 1,634408 shares of our common stock.
CMEA Capital and its affiliates also participated in the Series D Preferred Stock Financing and purchased 462,802 shares of our Series D redeemable convertible preferred stock at an aggregate purchase price of $1.6 million and were issued warrants to purchase 231,40257,850 shares of our common stock. These shares were subsequently transferred from CMEA Capital via affiliated party transfer to Presidio Partners 2014, L.P. in November 2014. Peter Gajdos, a member of our board of directors, is a managing director and portfolio manager at Presidio Partners.
All purchasers of our Series D redeemable convertible preferred stock are entitled to specified registration rights. For more information regarding these registration rights, see "Description of Capital Stock—Registration Rights."
Moral Compass Corporation
In July 2012, we executed a term note in the principal amount of $8.0 million with Moral Compass Corporation, which holds more than 5% of our capital stock. Darby E. Shupp, the chairman of our board of directors, is the Chief Financial Officer of Moral Compass Corporation and has beneficial ownership of the shares held by Moral Compass Corporation. As of December 31, 2014, the principal balance outstanding under this term note was $8.0 million. We have paid $1.0 million in aggregate interest under this note through December 31, 2014, with such interest accruing at an annual rate equal to prime plus two percent. In November 2014, we amended this note to change the maturity date to the first to occur of (i) April 1, 2016, (ii) the date of an event of default, or (iii) a date designated by
Moral Compass Corporation no earlier than the 20th day following our completion of an equity financing with gross proceeds to us of at least $50.0 million. In addition, the interest rate on the term loan remained at prime plus 2% through December 31, 2014, after which the rate increased to 11% per annum until maturity. In April 2015, in connection with our entry into a new term facility loan, we repaid the principal balance of $8.0 million and accrued interest and prepayment fee of $148,000 on the note.
We executed an additional term note with Moral Compass Corporation in July 2013 in the principal amount of $500,000. The principal balance of $500,000 and accrued interest of $19,000 was repaid in December 2013.
We have a license agreement with Blue Horse Labs, Inc., or BHL, an affiliate of Moral Compass Corporation. Ms. Shupp, the chairman of our board of directors, is the Treasurer of BHL. Royalty fees due to BHL were $161,000 and $21,000 as of December 31, 2013 and 2014, respectively.
Investors' Rights Agreements
We have entered into investors' rights agreements with certain holders of our preferred stock, including entities affiliated with Moral Compass Corporation, Mandala Capital, and Vilmorin & Cie (Limagrain), which each hold more than 5% of our capital stock and of which certain of our directors are affiliated. Pursuant to these agreements, these holders are entitled to rights with respect to the registration of their shares following this offering under the Securities Act. For a more detailed description of these registration rights, see "Description of Capital Stock—Registration Rights."
Co-Sale Agreement
In March 2014, we entered into a co-sale agreement with certain holders of our preferred stock, including entities with which certain of our directors are affiliated and entities affiliated with Mandala Capital. This co-sale agreement grants certain of our investors the right of co-sale with respect to proposed transfers of our securities by certain stockholders. Such rights will terminate upon the completion of this offering.
Voting Agreement
In March 2014, we entered into a voting agreement under which certain holders of our capital stock, including entities with which certain of our directors are affiliated and entities affiliated with Mandala Capital, have agreed to vote their shares on certain matters, including with respect to the election of directors. The voting agreement will terminate upon the completion of this offering and thereafter none of our stockholders will have any special rights regarding the election or designation of members of our board of directors or the voting of our capital stock.
Collaboration Agreements
We have several agreements with Limagrain, which held more than 5% of our capital stock prior to this offering. We have a research and development agreement with Limagrain in wheat for our NUE trait that we entered into in 2009 and a second one in wheat for our WUE trait entered into in 2011. We have received $400,000 from Limagrain under these agreements from January 1, 2012 through December 31, 2014. We further expanded our relationship with Limagrain in 2010, when they made a $25.0 million equity investment in us and we entered into a joint venture focused on the development and commercialization of improved wheat seed in North America, of which a U.S. wholly owned subsidiary of Limagrain owns 65% and we own 35%. See "Business—Key Collaborations" and Note 4 of the notes to our consolidated financial statements for additional information about these arrangements.
Participation in This Offering
Entities affiliated with certain of our existing stockholders have indicated an interest in purchasing up to an aggregate of approximately $10.0 million in shares of our common stock in this offering at the initial public offering price. However, because these indications of interest are not binding agreements or commitments to purchase, the underwriters could elect to sell more, fewer or no shares to any of these entities and any of these entities could elect to purchase more, fewer or no shares in this offering.
Other Transactions
We have granted stock options and other equity awards to our executive officers and certain of our directors. For a description of these options and equity awards, see "Executive Compensation—Outstanding Equity Awards at Fiscal Year-End" and "Management—Director Compensation."
We have not entered into arrangements with any of our executive officers that provide for severance and change in control benefits. However, in connection with the completion of this offering, we anticipate entering into an agreement with each of our named executive officers that may provide for severance or change in control protections, in which case we will disclose the terms of such agreements once finalized.
Indemnification Agreements
We expect to enter into indemnification agreements with each of our directors, executive officers, and certain key employees prior to the completion of this offering. The indemnification agreements and our amended and rested certificate of incorporation and amended and restated bylaws will require us to indemnify our directors and officers to the fullest extent permitted by Delaware law. See "Executive Compensation—Limitation of Liability and Indemnification of Officers and Directors."
Policies and Procedures for Related Party Transactions
Our audit committee charter states that our audit committee is responsible for reviewing and approving in advance any related party transaction, which is a transaction between us and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 in any calendar year and in which a related person has or will have a direct or indirect interest. Our audit committee has adopted policies and procedures for review of, and standards for approval of, such a related party transaction. For purposes of these policies and procedures, a related person is defined as an executive officer, director, or nominee for director, including his or her immediate family members, or a beneficial owner of greater than 5% our common stock, in each case since the beginning of the most recently completed year. Prior to the creation of our audit committee, our full board of directors reviewed related party transactions, with any directors abstaining from matters in which the director had an interest.
We believe that we have executed all of the transactions set forth under the section entitled "Certain Relationships and Related Party Transactions" on terms no less favorable to us than we could have obtained from unaffiliated third parties. It is our intention to ensure that all future transactions between us and our officers, directors, and principal stockholders and their affiliates are approved by the audit committee of our board of directors and are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
The following table sets forth information known to us regarding the beneficial ownership of our common stock as of March 31, 2015, as adjusted to reflect the shares of common stock to be issued and sold by us in this offering, assuming no exercise of the underwriters' option to purchase additional shares, for:
Beneficial ownership of shares is determined under the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power. Except as indicated by footnote, and subject to applicable community property laws, we believe each person identified in the table has sole voting and investment power with respect to all shares of common stock beneficially owned by them. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 13(d) and 13(g) of the Securities Act.
Applicable percentage ownership before this offering is based on 30,912,508 shares of our common stock outstanding as of March 31, 2015, assuming the automatic conversion of all outstanding shares of our preferred stock into an aggregate of 28,834,728 shares of common stock effective upon the closing of this offering, including the conversion of each shareall outstanding shares of our existing preferred stock (other than our Series D preferred stock) into one share23,385,029 shares of common stock and the conversion of all outstanding shares of our Series D preferred stock into 5,449,699 shares of common stock based on an assumed initial public offering price of $$14.00 per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, as if this conversion had occurred as of DecemberMarch 31, 2014.2015. We have based our calculation of the percentage of beneficial ownership after this offering on 38,062,508 shares of our common stock outstanding after the completion of this offering. Shares of our common stock subject to stock options or warrants that are currently exercisable or exercisable within 60 days of DecemberMarch 31, 20142015 are deemed to be outstanding and to be beneficially owned by the person holding the stock option or warrant for the purpose of computing the number and percentage ownership of outstanding shares of that person. We did not deem these shares outstanding, however, for the purposes of computing the percentage ownership of any other person. Consequently, the denominator for calculating beneficial ownership percentages may be different for each beneficial owner.
Entities affiliated with certain of our existing stockholders have indicated an interest in purchasing up to an aggregate of approximately $10.0 million in shares of our common stock in this offering at the initial public offering price. However, because these indications of interest are not binding agreements or commitments to purchase, the underwriters could elect to sell more, fewer or no shares to any of these entities and any of these potential investors could elect to purchase more, fewer or no shares in this offering. The following table does not reflect any purchases by these entities in this offering.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o Arcadia Biosciences, Inc., 202 Cousteau Place, Davis, CA 95618.
directors of Mandala Capital, and a member of the board of directors of Mandala Agribusiness Fund. Mr. Garg, Dominic Redfern, Tej Gujadur, and Sheokumar Gujadur are members of the board of directors of Mandala Agribusiness Fund,
General The following description summarizes certain terms of our capital stock, as they are expected to be in effect upon the completion of this offering. We expect to adopt an amended and restated certificate of incorporation and amended and restated bylaws in connection with this offering, and this description summarizes the provisions that are expected to be included in such documents. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description of the matters set forth in this "Description of Capital Stock," you should refer to our amended and restated certificate of incorporation and amended and restated bylaws and investors' rights Upon the completion of this offering, our authorized capital stock will consist of 400,000,000 shares of common stock, $0.001 par value per share, and 20,000,000 shares of undesignated preferred stock, $0.001 par value per share. As of
Common Stock Dividend Rights Subject to preferences that may be applicable to our outstanding preferred stock, holders of common stock are entitled to receive ratably such dividends as may be declared by the board of directors out of funds legally available for that purpose. However, certain of our current outstanding debt agreements prohibit us from paying cash dividends on our common stock. See "Dividend Policy." Voting Rights The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders. No Preemptive or Similar Rights The common stock has no preemptive or conversion rights or other subscription rights. The outstanding shares of common stock are, and the shares of common stock to be issued upon completion of this offering will be, fully paid and non-assessable. Liquidation Rights In the event of liquidation, dissolution or winding up of the company, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to the prior distribution rights of any outstanding preferred stock. Preferred Stock After the completion of this offering, no shares of preferred stock will be outstanding. Undesignated Preferred Stock After the closing of this offering, the board of directors will have the authority, without further action by the stockholders, to issue up to 20,000,000 shares of preferred stock, $0.001 par value per share, in one or more series. The board of directors will also have the authority to designate the rights, preferences, privileges and restrictions of each such series, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences, and the number of shares constituting any series. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of the company without further action by the stockholders. The issuance of preferred stock with voting and conversion rights may also adversely affect the voting power of the holders of common stock. In certain circumstances, an issuance of preferred stock could have the effect of decreasing the market price of the common stock. As of the closing of the offering, no shares of preferred stock will be outstanding. We currently have no plans to issue any shares of preferred stock. Options As of Convertible Notes In September 2013 and December 2013, we issued convertible notes to Mahyco for an aggregate principal amount of $5.0 million pursuant to a note and warrant purchase agreement, or 2013 Note Agreement. At any time during the five-year term of the notes, Mahyco may convert all or part of the outstanding balance of the note (including principal and accrued but unpaid interest) into our common stock at Warrants As of In connection with the 2013 Note Agreement described above, we issued warrants to Mahyco International Pte Ltd. to purchase In connection with our Series D preferred stock financing, we issued warrants to the Series D preferred stock purchasers to purchase an aggregate of In connection with our Series D preferred stock financing, we issued warrants to Piper Jaffray & Co., a placement agent for our Series D preferred stock financing, to purchase outstanding upon completion of this offering. These warrants will expire on the later of April 8, 2019, May 5, 2019, and May 12, 2019, respectively, or the second anniversary of the completion of this offering. Registration Rights Following the completion of this offering, the holders of an aggregate of 31,162,866 shares of our common stock and certain holders of warrants exercisable for 1,227,783 shares of our common stock, or their permitted transferees, are entitled to rights with respect to the registration of these shares under the Securities Act. These rights are provided under the terms of two investors' rights agreements between us and the holders of these shares, and include demand registration rights, The registration rights terminate with respect to the registration rights of an individual holder on the earliest to occur of five years following the completion of this offering or such time as all registrable securities held by such holder can be sold in any 90-day period without registration in compliance with Rule 144 of the Act. Demand Registration Rights Following this offering, certain holders of our preferred and common stock may request that we effect a registration under the Securities Act covering the public offering and sale of all or part of such registrable securities held by such stockholders. Upon any such demand we must provide notice of such request to all other holders of registrable securities, and then effect the registration of such registrable securities that the initiating holders have requested to register together with all other registrable securities that any other holders of registrable securities have requested to register. Holders of registrable securities may only demand up to a maximum of two registrations Piggyback Registration Rights In connection with this offering, holders of registrable securities were entitled to, and the necessary percentage of holders waived, their rights to notice of this offering and to include their registrable securities in this offering. If we register any of our securities for public sale in another offering, including pursuant to any Short Form Registration Rights Following this offering, we may be obligated under our investors' rights Expenses of Registration We are obligated to pay certain registration expenses related to any demand, Anti-Takeover Effects of Provisions of the Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws Our amended and restated certificate of incorporation and our amended and restated bylaws, which will be in effect upon the completion of this offering, will contain certain provisions that could have the effect of delaying, deterring or preventing another party from acquiring control of us. These provisions and certain provisions of Delaware law, which are summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate more favorable terms with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us. Undesignated Preferred Stock As discussed above, our board of directors will have the ability to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management of our company. Limits on Ability of Stockholders to Act by Written Consent or Call a Special Meeting Our amended and restated certificate of incorporation will provide that our stockholders may not act by written consent, which may lengthen the amount of time required to take stockholder actions. As a result, a holder controlling a majority of our capital stock would not be able to amend our bylaws or remove directors without holding a meeting of our stockholders called in accordance with our bylaws. In addition, our amended and restated bylaws will provide that special meetings of the stockholders may be called only by the majority of our board of directors. Stockholders may not call a special meeting, which may delay the ability of our stockholders to force consideration of a proposal or for holders controlling a majority of our capital stock to take any action, including the removal of directors. Requirements for Advance Notification of Stockholder Nominations and Proposals Our amended and restated bylaws will establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of our board of directors or a committee of our board of directors. These provisions may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer's own slate of directors or otherwise attempting to obtain control of our company. Board Classification Upon the closing of the offering, our board of directors will be divided into three classes, one class of which is elected each year by our stockholders. The directors in each class will serve three-year terms. For more information on the classified board, see "Management—Board of Directors." A third party may be discouraged from making a tender offer or otherwise attempting to obtain control of us as it is more difficult and time consuming for stockholders to replace a majority of the directors on a classified board. No Cumulative Voting Our amended and restated certificate of incorporation and amended and restated bylaws will not permit cumulative voting in the election of directors. Cumulative voting allows a stockholder to vote a portion or all of its shares for one or more candidates for seats on the board of directors. Without cumulative voting, a minority stockholder may not be able to gain as many seats on our board of directors as the stockholder would be able to gain if cumulative voting were permitted. The absence of cumulative voting makes it more difficult for a minority stockholder to gain a seat on our board of directors to influence our board's decision regarding a takeover. Amendment of Charter and Bylaws Provisions The amendment of the above provisions of our amended and restated certificate of incorporation will require approval by holders of at least Delaware Anti-Takeover Statute We
Generally, a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. An interested stockholder is a person who, together with affiliates and associates, owns or, within three years prior to the determination of interested stockholder status, did own 15% or more of a corporation's outstanding voting stock. We expect the existence of this provision to have an anti-takeover effect with respect to transactions our board of directors does not approve in advance. We anticipate that Section 203 may also discourage attempts that might result in a premium over the market price for the shares of common stock held by stockholders. The provisions of Delaware law and the provisions of our amended and restated certificate of incorporation and amended and restated bylaws, as amended upon the completion of this offering, could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they might also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions might also have the effect of preventing changes in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders might otherwise deem to be in their best interests. Transfer Agent and Registrar Upon the completion of this offering, the transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company, LLC. The transfer agent and registrar's address is 6201 15th Avenue, Brooklyn, NY 11219. Listing
Prior to this offering, there has not been a public market for shares of our common stock. Future sales of substantial amounts of shares of our common stock, including shares issued upon the exercise or settlement of outstanding options and warrants, in the public market after this offering, or the possibility of these sales occurring, could cause the prevailing market price for our common stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our common stock will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future. Upon the completion of this offering, based on the number of shares of our capital stock outstanding as of The remaining 29,814,254 shares of our common stock outstanding after this offering are "restricted securities," as such term is defined in Rule 144 under the Securities Act. These were issued and sold by us in private transactions and are eligible for public sale only if registered under the Securities Act or sold in accordance with Rule 144 or Rule 701 under the Securities Act, each of which is discussed below. Holders of substantially all of our equity securities have entered into lock-up agreements with the underwriters under which they have agreed, subject to specific exceptions, not to sell any of our stock for a period of time following the date of this prospectus, as described below. As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, shares will be available for sale in the public market as follows:
Lock-up Agreements and Market Stand-Off Agreements We, all of our directors, officers and substantially all of our securityholders have entered into lock-up agreements that generally provide that these holders will not offer, pledge, sell, agree to sell, directly or indirectly, or otherwise dispose of any shares of common stock or any securities convertible into or exchangeable for shares of common stock without the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC for a period of 180 days from the date of this prospectus, subject to certain exceptions. Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC may, in their discretion, release any of the securities subject to these lock-up agreements at any time. In addition to the restrictions contained in the lock-up agreements described above, we have entered into agreements with stockholders that contain certain market stand-off provisions imposing restrictions on the ability of such stockholders to offer, sell, or transfer our equity securities for a period of 180 days following the date of this prospectus. Rule 144 In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell such shares without complying with the manner of sale, volume limitation, or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then such person is entitled to sell such shares without complying with any of the requirements of Rule 144. In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us. Rule 701 Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701. Registration Rights When this offering is complete, the holders of an aggregate of 31,162,866 shares of our common stock and certain holders of warrants exercisable for 1,227,783 shares of our common stock, or their transferees, will be entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of such registration. For a further description of these rights, see "Description of Capital Stock—Registration Rights." Registration Statement on Form S-8 We intend to file registration statements on Form S-8 under the Securities Act promptly after the completion of this offering to register all of the shares of our common stock issued or reserved for issuance under our 2006 Stock Plan, our 2015 Plan and our ESPP. The registration statements on Form S-8 are expected to become effective immediately upon filing, and shares of our common stock covered by these registration statements will be eligible for sale in the public market, subject to the Rule 144 limitations applicable to affiliates, vesting restrictions, and any applicable lock-up agreements and market standoff agreements. For a description of our equity compensation plans, see "Executive Compensation—Employee Benefit and Stock Plans."
This section summarizes certain material U.S. federal income tax considerations relating to the ownership and disposition of our common stock sold pursuant to this offering to a "non-U.S. holder" (as defined below). This summary does not provide a complete analysis of all potential tax considerations. The information provided below is based upon provisions of the Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions currently in effect. These authorities may change at any time, possibly on a retroactive basis, or the IRS might interpret the existing authorities differently. In either case, the U.S. federal income tax considerations of owning or disposing of our common stock could differ from those described below. As a result, we cannot assure you that the U.S. federal income tax considerations described in this discussion will not be challenged by the IRS or will be sustained by a court if challenged by the IRS. This summary does not address the tax considerations arising under the alternative minimum tax, the net investment income tax, the laws of any state, local or non-U.S. jurisdiction, or under U.S. federal gift and estate tax laws. In addition, this discussion does not address tax considerations applicable to an investor's particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes is a beneficial owner of our common stock, the tax treatment of a partner in the partnership or an owner of the entity will depend upon the status of the partner or other owner and the activities of the partnership or other entity. Accordingly, this summary does not address U.S. federal income tax considerations applicable to partnerships that hold our common stock, and partners in such partnerships should consult their tax advisors. INVESTORS CONSIDERING THE PURCHASE OF OUR COMMON STOCK SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME, GIFT AND ESTATE TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES OF FOREIGN, STATE OR LOCAL LAWS, AND TAX TREATIES. Non-U.S. Holder Defined For purposes of this summary, a "non-U.S. holder" is any holder of our common stock, other than an entity taxable as a partnership for U.S. federal income tax purposes that is not:
If you are a non-U.S. citizen who is an individual, you may, in many cases, be deemed to be a resident alien, as opposed to a nonresident alien, by virtue of being present in the United States for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in the current calendar year. For these purposes, all the days present in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Resident aliens are subject to U.S. federal income tax as if they were U.S. citizens. Such an individual is urged to consult his or her own tax advisor regarding the U.S. federal income tax consequences of the ownership, sale, exchange or other disposition of our common stock. Distributions We do not expect to declare or make any distributions on our common stock in the foreseeable future. If we do make any distributions on shares of our common stock, however, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a non-U.S. holder's adjusted tax basis in shares of our common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of our common stock. See "—Sale of Common Stock." Subject to the discussion below regarding the Foreign Account Tax Compliance Act, or FATCA, and backup withholding, any distribution made to a non-U.S. holder on our common stock that is not effectively connected with a non-U.S. holder's conduct of a trade or business in the United States will generally be subject to U.S. withholding tax at a 30% rate. The withholding tax might not apply, however, or might apply at a reduced rate, under the terms of an applicable income tax treaty between the United States and the non-U.S. holder's country of residence. You should consult your tax advisors regarding your entitlement to benefits under a relevant income tax treaty. Generally, in order for us or our paying agent to withhold tax at a lower treaty rate, a non-U.S. holder must certify its entitlement to treaty benefits. A non-U.S. holder generally can meet this certification requirement by providing an IRS Form W-8BEN, W-8BEN-E, any successor form to the IRS Form W-8BEN or W-8BEN-E, or appropriate substitute form to us or our paying agent. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the holder's behalf, the holder will be required to provide appropriate documentation to the agent. The holder's agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. If you are eligible for a reduced rate of U.S. federal withholding tax under an income tax treaty, you may obtain a refund or credit from the IRS of any excess amounts withheld by filing an appropriate claim for a refund with the IRS in a timely manner. Distributions received by a non-U.S. holder that are effectively connected with a U.S. trade or business conducted by the non-U.S. holder, and, if required by an applicable income tax treaty between the United States and the non-U.S. holder's country of residence, are attributable to a permanent establishment maintained by the non-U.S. holder in the United States, are not subject to such withholding tax. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI properly certifying such exemption. Such effectively connected distributions, although not subject to U.S. withholding tax, are generally taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition to the graduated tax described above, distributions received by corporate non-U.S. holders that are effectively connected with a U.S. trade or business of the corporate non-U.S. holder may also be subject to a branch profits tax equal to 30% of its effectively connected earnings and profits for the taxable year, as adjusted for certain items, although an applicable income tax treaty between the United States and the non-U.S. holder's country of residence might provide for a lower rate. Sale of Common Stock Subject to the discussion below regarding FATCA and backup withholding, non-U.S. holders will generally not be subject to U.S. federal income tax on any gains realized on the sale, exchange or other disposition of common stock unless:
The FIRPTA rules may apply to a sale, exchange or other disposition of our common stock if we are at the time of the disposition, or were within the shorter of the five-year period preceding the disposition and the non-U.S. holder's holding period, a "U.S. real property holding corporation," or USRPHC. In general, we would be a USRPHC if interests in U.S. real property comprised at least half of the value of our business assets. If we are or become a USRPHC, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests subject to the FIRPTA rules only if a non-U.S. holder actually owns or constructively holds more than 5% of our outstanding common stock. If any gain from the sale, exchange or other disposition of common stock, (1) is effectively connected with a U.S. trade or business conducted by a non-U.S. holder and (2) if required by an applicable income tax treaty between the United States and the non-U.S. holder's country of residence, is attributable to a permanent establishment (or, in the case of an individual, a fixed base) maintained by such non-U.S. holder in the United States, then the gain generally will be subject to U.S. federal income tax at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. If the non-U.S. holder is a corporation, under certain circumstances, that portion of its earnings and profits that is effectively connected with its U.S. trade or business, subject to certain adjustments, generally would be subject to a "branch profits tax." The branch profits tax rate is equal to 30% of its effectively connected earnings and profits for the taxable year, as adjusted for certain items, although an applicable income tax treaty between the United States and the non-U.S. holder's country of residence might provide for a lower rate. Backup Withholding and Information Reporting The Code and the Treasury regulations require those who make specified payments to report the payments to the IRS. Among the specified payments are dividends and proceeds paid by brokers to their customers. The required information returns enable the IRS to determine whether the recipient properly included the payments in income. This reporting regime is reinforced by "backup withholding" rules. These rules require the payors to withhold tax from payments subject to information reporting if the recipient fails to cooperate with the reporting regime by failing to provide his taxpayer identification number to the payor, furnishing an incorrect identification number, failing to report interest or dividends on his U.S. tax returns, or failing to otherwise establish an exemption to these rules. The backup withholding rate is currently 28%. The backup withholding rules do not apply to payments to corporations, whether domestic or foreign, provided that they establish such exemption. Payments to non-U.S. holders of dividends on common stock generally will not be subject to backup withholding, and payments of proceeds made to non-U.S. holders by a broker upon a sale of common stock will not be subject to information reporting or backup withholding, in each case so long as the non-U.S. holder certifies its nonresident status (and we or our paying agent do not have actual knowledge or reason to know the holder is a U.S. person or that the conditions of any other exemption are not, in fact, satisfied) or otherwise establishes an exemption. The certification procedures to claim treaty benefits described under "Distributions" above will generally satisfy the certification requirements necessary to avoid the backup withholding tax. We must report annually to the IRS any dividends paid to each non-U.S. holder and the tax withheld, if any, with respect to these dividends. Copies of these reports may be made available to tax authorities in the country where the non-U.S. holder resides. Backup withholding is not an additional tax. Any amounts withheld from a payment to a holder of common stock under the backup withholding rules can be credited against any U.S. federal income tax liability of the holder and may entitle the holder to a refund from the IRS, provided that the required information is furnished to the IRS in a timely manner. Foreign Account Tax Compliance Act (FATCA) FATCA imposes U.S. federal withholding tax of 30% on certain types of U.S. source "withholdable payments" (including dividends and the gross proceeds from the sale or other disposition of U.S. stock) to foreign financial institutions, which are broadly defined for this purpose, and other non-U.S. entities that fail to comply with certain certification and information reporting requirements regarding U.S. account holders or owners of such institutions or entities. The obligation to withhold under FATCA applies to any dividends on our common stock and is currently expected to apply to gross proceeds from the disposition of our common stock paid after December 31, 2016. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in our common stock. THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS. Under the terms and subject to the conditions contained in an underwriting agreement dated , we have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC are acting as representatives, the following respective numbers of shares of common stock:
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock in the offering if any are purchased, other than those shares covered by the option to purchase additional shares described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. We have granted to the underwriters a 30-day option to purchase The underwriters propose to offer the shares of common stock initially at the public offering price on the cover page of this prospectus and to selling group members at that price less a selling concession of $ per share. After the initial public offering the underwriters may change the public offering price and selling concession to broker-dealers. The following table summarizes the compensation we will pay:
We estimate that our total expenses for this offering, excluding the underwriting discounts and commissions, will be approximately Entities affiliated with certain of our existing stockholders have indicated an interest in purchasing up to an aggregate of approximately $10.0 million in shares of our common stock in this offering at the initial public offering price. However, because these indications of interest are not binding agreements or commitments to purchase, the underwriters could elect to sell more, fewer or no shares to any of these entities and any of these potential investors could elect to purchase more, fewer or no shares in this offering. The underwriters will receive the same underwriting discount on any sales to such potential investors. The underwriters have informed us that they do not expect sales to accounts over which the underwriters have discretionary authority to exceed 5% of the shares of common stock being offered. We have agreed that we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC for a period of 180 days after the date of this prospectus, except issuances pursuant to the exercise of employee stock options outstanding on the date hereof. Our officers, directors and substantially all of our existing securityholders have agreed that they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of Credit Suisse Securities (USA) LLC and J.P. Morgan Securities LLC for a period of 180 days after the date of this prospectus, subject to limited exceptions. We have agreed to indemnify the underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect. Prior to this offering, there has been no public market for our common stock. The initial public offering price will be determined by negotiations between us and the representatives of the underwriters. In determining the initial public offering price, we and the representatives of the underwriters expect to consider a number of factors including:
Neither we nor the underwriters can assure investors that an active trading market will develop for our common shares, or that the shares will trade in the public market at or above the initial public offering price. In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and passive market making in accordance with Regulation M under the Exchange Act.
option to purchase. The underwriters may close out any covered short position by either exercising their option to purchase additional shares and/or purchasing shares in the open market.
the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase additional shares through their option. If the underwriters sell more shares than could be covered by the option to purchase additional shares, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on The NASDAQ Global Select Market or otherwise and, if commenced, may be discontinued at any time. A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future. Piper Jaffray & Co. acted as placement agent in connection with our Series D preferred stock financing, under which we sold shares between March and May 2014, and received as compensation a cash fee of $2.0 million and warrants to purchase Selling Restrictions General Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful. United Kingdom This document is only being distributed to and is only directed at (i) persons who are outside the United Kingdom or (ii) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the "Order") or (iii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as "relevant persons"). The securities are only available to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such securities will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents. European Economic Area In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a "Relevant Member State"), from and including the date on which the European Union Prospectus Directive (the "EU Prospectus Directive") was implemented in that Relevant Member State (the "Relevant Implementation Date") an offer of securities described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
For the purposes of this provision, the expression an "offer of securities to the public" in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression "EU Prospectus Directive" means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression "2010 PD Amending Directive" means Directive 2010/73/EU. Hong Kong The shares may not be offered or sold by means of any document other than (i) in circumstances that do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances that do not result in the document being a "prospectus" within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), that is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted under the laws of Hong Kong) other than with respect to shares that are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder. Singapore This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA. Where the shares are subscribed or purchased under Section 275 by a relevant person that is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and where each beneficiary of which is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries' rights and interest in that corporation or trust shall not be transferable for six months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law. Switzerland This document, as well as any other material relating to the shares of our common stock, which are the subject of the offering contemplated by this prospectus, does not constitute an issue prospectus pursuant to Article 652a of the Swiss Code of Obligations. The shares will not be listed on the SIX Swiss Exchange and, therefore, the documents relating to the shares, including, but not limited to, this document, do not claim to comply with the disclosure standards of the listing rules of the SIX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SIX Swiss Exchange. The shares are being offered in Switzerland by way of a private placement, i.e., to a small number of selected investors only, without any public offer and only to investors who do not purchase the shares with the intention to distribute them to the public. The investors will be individually approached by us from time to time. Notice to Residents of Canada The distribution of the shares in Canada is being made only in the provinces of Ontario, Quebec and The validity of the shares of common stock offered hereby will be passed upon for us by Orrick, Herrington & Sutcliffe LLP, San Francisco, California. Certain legal matters in connection with this offering will be passed upon for the underwriters by Cooley LLP, San Francisco, California. The consolidated financial statements as of December 31, 2014 and 2013 and for each of the two years in the period ended December 31, 2014 included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein. Such consolidated financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing. We have filed with the SEC a registration statement on Form S-1, including exhibits and schedules filed with the registration statement, of which this prospectus is a part, under the Securities Act with respect to the shares of common stock we propose to sell in this offering. This prospectus does not contain all of the information set forth in the registration statement and exhibits and schedules to the registration statement. For further information with respect to our company and the shares of common stock to be sold in this offering, reference is made to the registration statement, including the exhibits and schedules thereto. Statements contained in this prospectus as to the contents of any contract or other document referred to in this prospectus are not necessarily complete and, where that contract or other document has been filed as an exhibit to the registration statement, each statement in this prospectus is qualified in all respects by the exhibit to which the reference relates. Copies of the registration statement, including the exhibits and schedules to the registration statement, may be examined without charge at the public reference room of the SEC, 100 F Street, N.E., Room 1580, Washington, DC 20549. Information about the operation of the public reference room may be obtained by calling the SEC at 1-800-SEC-0300. Copies of all or a portion of the registration statement can be obtained from the public reference room of the SEC upon payment of prescribed fees. Our SEC filings, including our registration statement, are also available to you, free of charge, on the SEC's website at http://www.sec.gov. As a result of this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with this law, will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other SEC information will be available for inspection and copying at the SEC's public reference facilities and the website referred to above. We also maintain a website at http://www.arcadiabio.com. When this offering is complete, you may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus or the registration statement of which it forms a part. ARCADIA BIOSCIENCES, INC.
The Board of Directors and Stockholders of We have audited the accompanying consolidated balance sheets of Arcadia Biosciences, Inc. and its subsidiary (the "Company") as of December 31, 2014 and 2013, and the related consolidated statements of operations, redeemable and convertible preferred stock and stockholders' deficit, and cash flows for each of the two years in the period ended December 31, 2014. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the financial statements based on our audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion. In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Arcadia Biosciences, Inc. and subsidiary at December 31, 2014 and 2013, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America. /s/ Deloitte & Touche LLP Phoenix, Arizona
See accompanying notes to consolidated financial statements.
See accompanying notes to consolidated financial statements. Arcadia Biosciences, Inc.
See accompanying notes to consolidated financial statements.
See accompanying notes to consolidated financial statements.
1. Organization, Description of Business and Liquidity Arcadia Biosciences, Inc. (the "Company"), was incorporated in the state of Arizona in 2002 and maintains its headquarters in Davis, California, with additional facilities in Seattle, Washington; Phoenix, Arizona; and American Falls, Idaho. The Company was reincorporated in Delaware in March 2015. The Company pursues agriculture-based biotechnology business opportunities that improve the environment and human health. The Company is an agricultural biotechnology trait company with an extensive and diversified portfolio of late-stage crop productivity and product quality traits addressing multiple crops that supply the global food and feed markets. Its traits are focused on high-value enhancements that increase crop yields by enabling plants to more efficiently manage environmental and nutrient stresses, and that enhance the quality and value of agricultural products. In February 2012, the Company formed Verdeca LLC ("Verdeca", see Note 5), which is jointly owned with Bioceres, Inc. ("Bioceres USA"), a U.S. wholly owned subsidiary of Bioceres, S.A. ("Bioceres"), an Argentine corporation. Bioceres is an agricultural investment and development cooperative. Verdeca was formed to develop and deregulate soybean varieties using both partners' agricultural technologies. The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the ordinary course of business. The Company has not yet achieved profitability. As of December 31, 2013 and 2014, the Company had an accumulated deficit of $95.6 million and $114.0 million, respectively, and expects to incur losses for the next several years. Since its inception, the Company has funded its operations primarily with the net proceeds from private placements of equity and debt securities, as well as proceeds from the sale of its products and payments under license agreements, contract research agreements and government grants. As of December 31, 2013 and 2014, the Company had cash and cash equivalents of $2.8 million and $16.6 million. In the first half of 2014, the Company received net proceeds of $32.8 million from its Series D redeemable convertible preferred stock offering. Management believes existing cash should be sufficient to fund its operations through December 31, 2015. In addition, the Company may, if necessary, institute cost cutting measures and can extend the maturity date on its $8.0 million loan in order to allow existing cash to fund operations for more than one year from the date of this report. Furthermore, the Company is in the process of exploring financing with various lenders. The Company expects to incur additional losses related to the continued development and expansion of its business, including research and development, seed production and operations, and sales and marketing. There is no assurance that profitable operations will be achieved or if achieved can be sustained on a continued basis. 2. Significant Accounting Policies Basis of Presentation and Principles of Consolidation The consolidated financial statements include the accounts of the Company and Verdeca LLC. All intercompany balances and transactions have been eliminated in consolidation. The Company prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP, and with the rules of the Securities and Exchange Commission. The Company uses a qualitative approach in assessing the consolidation requirement for variable interest entities ("VIEs"). This approach focuses on determining whether the Company has the power to direct the activities of the VIE that most significantly affect the VIE's economic performance
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) and whether the Company has the obligation to absorb losses, or the right to receive benefits, that could potentially be significant to the VIE. For all periods presented, the Company has determined that it is the primary beneficiary of Verdeca, which is a VIE. The Company evaluates its relationships with the VIEs upon the occurrence of certain significant events that affect the design, structure or other factors pertinent to the primary beneficiary determination. Unaudited Pro Forma Stockholders' Equity The pro forma stockholders' equity as of December 31, 2014 is presented as though all of the Company's outstanding convertible preferred stock had converted into shares of common stock upon the completion of an initial public offering ("IPO") of the Company's common stock. Use of Estimates The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions in the Company's consolidated financial statements and notes thereto. Significant estimates and assumptions made by management included the determination of the provision for income taxes, costs to complete government grants and research contracts, and the development period of revenue-generating technologies. Management bases its estimates on historical experience and on various other market-specific and relevant assumptions that management believes to be reasonable under the circumstances. Actual results could differ from those estimates. Cash and Cash Equivalents The Company considers any liquid investments with a stated maturity of three months or less at purchase to be cash equivalents. Cash and cash equivalents consist of cash on deposit with banks. The Company limits cash investments to financial institutions with high credit standings; therefore, management believes that there is no significant exposure to any credit risk in the Company's cash and cash equivalents. However, as of December 31, 2013 and 2014, a substantial portion of the Company's cash in depository accounts is in excess of the federal deposit insurance limits. Accounts Receivable Accounts receivable represents amounts owed to the Company from product sales, licenses and contract research and government grants. The carrying value of the Company's receivables represents estimated net realizable values. The Company generally does not require collateral and estimates any required allowance for doubtful accounts based on historical collection trends, the age of outstanding receivables, and existing economic conditions. If events or changes in circumstances indicate that specific receivable balances may be impaired, further consideration is given to the collectability of those balances and the allowance is recorded accordingly. Past-due receivable balances are written off when the Company's internal collection efforts have been unsuccessful in collecting the amounts due. The Company had no amounts reserved for doubtful accounts at December 31, 2013 and 2014 as the Company expected full collection of the accounts receivable balances as of each of these dates.
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) Customer Concentration Significant customers are those that represent greater than 10% of the Company's total revenues or gross accounts receivable balance at each respective balance sheet date. Customers representing greater than 10% of accounts receivable were as follows (in percentages):
Customers representing greater than 10% of total revenues were as follows (in percentages):
Property and Equipment Property and equipment acquisitions are recorded at cost. Provisions for depreciation are calculated using the straight-line method over the following average estimated useful lives of the assets:
Deferred Offering Costs Deferred offering costs, which consist of direct incremental legal, consulting, banking and accounting fees relating to the anticipated initial public offering ("IPO"), are capitalized. The deferred offering costs will be offset against IPO proceeds upon the consummation of the offering. In the event the offering is terminated, deferred offering costs will be expensed. The Company has recorded $2.8 million of deferred offering costs in other noncurrent assets on the consolidated balance sheet as of December 31, 2014. No amounts were deferred as of December 31, 2013.
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) Impairment of Long-Lived Assets The Company evaluates if events and circumstances have occurred that indicate the remaining estimated useful life of long-lived assets and identifiable intangible assets may warrant revision or that the remaining balance of these assets may not be recoverable. In evaluating for recoverability, the Company estimates the future undiscounted cash flows expected to result from the use of the assets and their eventual disposition. In the event that the balance of any asset exceeds the future undiscounted cash flow estimate, impairment is recognized based on the excess of the carrying amounts of the asset above its estimated fair value. As of December 31, 2013 and 2014, there was no impairment of the Company's long-lived assets. Stock-Based Compensation The Company measures its stock-based compensation awards made to employees and directors based on the estimated fair values of the awards and recognizes the compensation expense over the requisite service period. The Company has selected the Black-Scholes option-pricing model to estimate the fair value of its stock-based awards and compensation expense is recognized using the straight-line method. Stock-based compensation expense is based on the value of the portion of stock-based compensation awards that is ultimately expected to vest. As such, the Company's stock-based compensation expense is reduced for the estimated forfeitures at the date of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company accounts for compensation expense related to stock options granted to non-employees based on the fair values estimated using the Black-Scholes model. Stock options granted to non-employees are remeasured at each reporting date until the award is vested. Income Taxes The Company uses the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and the tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. Net Loss per Share Basic net loss per share, which excludes dilution, is computed by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock, such as stock options, convertible promissory notes, convertible preferred stock, redeemable convertible preferred stock and warrants, result in the issuance of common stock which share in the losses of the Company. Certain potential shares of common stock have been excluded from the computation of diluted net loss per share as their effect would be anti-dilutive. Such potentially dilutive shares are excluded when the effect would be to reduce the loss per share. The Company's convertible preferred stock are considered to be participating securities as they are entitled to participate in undistributed earnings with shares of common stock. Due to net
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) losses, there is no impact on earnings per share calculation in applying the two-class method since the participating securities have no legal requirement to share in any losses. Unaudited Pro Forma Net Loss per Share Pro forma basic and diluted net loss per share attributable to common stockholders has been computed to give effect to the conversion of all outstanding shares of the redeemable convertible preferred stock and the convertible preferred stock upon the closing of an IPO. Revenue Recognition Revenue is generated through product sales, license agreements, contract research agreements, and government grants. The Company recognizes revenue when the following criteria have been met: persuasive evidence of an arrangement with the customer exists; price and terms of the arrangement are fixed or determinable; delivery of the product has occurred or the service has been performed in accordance with the terms of the arrangement; and collectability is reasonably assured. For revenue agreements with multiple-element arrangements, such as license and contract agreements, the Company analyzes the arrangements to determine whether the deliverables can be separated or whether they must be accounted for as a single unit of accounting. This determination is generally based on whether any deliverable has stand-alone value to the customer. This analysis also establishes a selling price hierarchy for determining how to allocate arrangement consideration to identified units of accounting. When deliverables are separable, consideration received is allocated to the separate units of accounting based on the relative selling price method and the appropriate revenue recognition principles are applied to each unit. The selling price used for each unit of accounting is based on estimated selling price as neither vendor-specific nor third-party evidence is available. When the Company determines that an arrangement should be accounted for as a single unit of accounting, it must determine the period over which the performance obligations will be performed and revenue will be recognized over the performance period. Product Revenues Product revenues consist of sales of gamma linolenic acid ("GLA") safflower oil (i.e., SONOVA® brand GLA safflower oil). Product revenues are recognized once passage of title has occurred, contractually specified acceptance criteria have been met, and all other revenue recognition criteria have been met. Shipping and handling costs charged to customers are recorded as revenues and included in cost of product revenues at the time the sale is recognized. License Revenues The Company's license agreements generally includes up-front, nonrefundable license fees, annual license fees, and subsequent milestone payments. Upon commercialization of a product utilizing a licensed technology, the Company receives certain value-sharing payments associated with the incremental revenue attributable to the licensed technology. The Company has determined that, at the inception of each license agreement, there is only one deliverable for the license for, access to, and assistance with the development of the specified intellectual property. The up-front nonrefundable license fees are recognized as revenue proportionally
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) over the development period, which approximates the expected efforts by the Company. The development period is estimated based upon factors such as traits, nature of crops and geographies, which are used to establish the initial deferral period. The Company continually reviews such estimates based on progress toward product commercialization. If the deferral period estimate changes, the amount of revenue recognized during the period is adjusted to reflect the updated deferred balances as of the current period-end. The annual license fees are payable at the end of the annual period and such fees are not required to be paid if the agreement is cancelled prior to the due date. Therefore, the annual license fees are only recognized when they become due. The Company's license agreements generally include contingent milestone payments in the development life cycle of the related technology, such as achievement of specific technological targets, successful results from field trials, filing for approval with regulatory agencies, approvals granted by regulatory agencies and commercial launch of a product utilizing the licensed technology. The Company evaluates whether each milestone is substantive and at risk at the time the agreement is executed. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (i) the entity's performance to achieve the milestone or (ii) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity's performance to achieve the milestone; (b) the consideration relates solely to past performance; and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company generally considers non-refundable milestones that the Company expects to be achieved as a result of the Company's efforts during the period of the Company's performance obligations under the license agreement to be substantive and recognizes them as revenue upon the achievement of the milestone, assuming all other revenue recognition criteria are met. Once a product containing one or more of the Company's traits is commercialized, the Company is entitled to receive a portion of the incremental revenue that the trait generates for its commercial partner. These value-sharing payments will be recorded on the accrual basis when results are reliably measurable, collectability is reasonably assured, and all other revenue recognition criteria are met. Contract Research Revenues Contract research revenues consist of amounts earned from performing contracted research activities for third parties. Activities performed are related to breeding programs or the genetic engineering of plants and are subject to an executed agreement. Generally, fees for research and development activities are recognized as the services are performed over the performance period, as specified in the respective agreements, assuming all other revenue recognition criteria are met. Similar to the license agreements, under the contract research agreements, once a product containing one or more of the Company's traits is commercialized, the Company is entitled to receive a portion of the incremental revenue that the trait generates for its commercial partner. These value-sharing payments will be recorded on the accrual basis when results are reliably measurable, collectability is reasonably assured, and all other revenue recognition criteria are met. Government Grant Revenues The Company generates revenue from grant payments received from government entities for research and development activities over a contractually defined period. Revenues from government
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met. Revenues from government entities accounted for approximately 44% and 50% of the Company's total revenue recognized for the years ended December 31, 2013 and 2014, respectively. Unearned Revenue The Company defers revenue to the extent that cash received in conjunction with a license agreement, contract or grant exceeds the revenue recognized in accordance with Company policies. Research and Development Expenses Research and development expenses consist of costs incurred in the discovery, development, and testing of the Company's product candidates. These expenses consist primarily of employee salaries and benefits, stock-based compensation, fees paid to subcontracted research providers, fees associated with in-licensing technology, royalty agreements, land leased for field trials, chemicals and supplies and other external expenses. These costs are expensed as incurred. Additionally, as disclosed in Note 8, the Company is required from time to time to make certain milestone payments in connection with the development of technologies. These milestone payments are expensed at the time the milestone is achieved and deemed payable. SONOVA® GLA Safflower Oil Inventory Proprietary safflower plants are grown, producing seed with a high-GLA content. This seed is used for subsequent plantings or processed, and sold as GLA oil. Amounts inventoried consist primarily of fees paid to contracted cooperators to grow the crops and costs to process and store harvested seed. Inventory costs are tracked on a lot-identified basis, valued at the lower of cost or market, and are included as cost of product revenues when sold. The Company provides for inventory reserves when conditions indicate that the selling price may be less than cost due to physical deterioration, obsolescence, changes in price levels, or other factors. Additionally, the Company provides reserves for excess and slow-moving inventory on hand that is not expected to be sold to reduce the carrying amount of excess slow-moving inventory to its estimated net realizable value. The reserves are based upon estimates about future demand from the Company's customers and distributors and market conditions. The Company had inventory reserves of $0 and $1.7 million as of December 31, 2013 and 2014. During the year ended December 31, 2014, the Company recorded a reserve of $1.7 million against inventory primarily as a result of changes in conditions of specific customers and regulatory delays related to the use of its Sonova products by certain new industries. The inventories—current line item in the balance sheet consists of the cost of oil inventory forecasted to be sold in the next 12 months, as of the balance sheet date. The inventories—noncurrent line item consists of oil and seed inventory expected to be used in production or sold beyond the next 12 months, as of the balance sheet date.
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) Raw materials inventories consist primarily of seed production costs incurred by our contracted cooperators. Finished goods inventories consist of GLA oil that is available for sale. Inventories consist of the following (in thousands):
Investment in Unconsolidated Entity The equity method is used to account for the Company's investment in Limagrain Cereal Seeds LLC ("LCS"), an unconsolidated entity over which the Company exercises significant influence, but does not have a controlling interest. Under the equity method, the Company's share of the unconsolidated entity's loss is included in equity method loss in the statements of operations. See Note 4 for further discussion. No distributions were received in the years ended December 31, 2013 and 2014. As of December 31, 2014, the Company's investment in LCS has been reduced to $0 as a result of its equity method loss pick-up. The Company regularly reviews each of its investments for impairment by determining if the investment has sustained an other-than-temporary decline in its value, in which case the investment is written down to its fair value by a charge to earnings. Factors that are considered by the Company in determining whether an other-than-temporary decline in value has occurred include (i) the market value of the investment in relation to its cost basis, (ii) the financial condition of the investment, and (iii) the Company's intent and ability to retain the investment for a sufficient period of time to allow for recovery of the market value of the investment. As of December 31, 2013, there was no impairment of the Company's equity method investment. Cost Method Investment Investments in equity securities of companies in which the Company holds less than 20% voting interest and on which the Company does not have the ability to exercise significant influence are accounted for under the cost method. Contingent Liability Related to the Anawah Acquisition On June 15, 2005, the Company completed its agreement and plan of merger and reorganization with Anawah, Inc. ("Anawah" or "Sellers"), to purchase the Sellers' food and agricultural research company through a stock purchase. Pursuant to the merger with Anawah, and in accordance with the FASB Statement No. 141,Business Combinations, which was applicable at the time of the acquisition, the Company incurred a contingent liability not to exceed $5.0 million. This liability represents amounts to be paid to Anawah's previous stockholders for cash collected on revenue recognized by the Company upon commercial sale of certain specific products developed using technology acquired in the purchase. As of December 31, 2010, the Company ceased activities relating to three of the six Anawah
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) product programs and, as a result, reduced the contingent liability to $3.0 million. The Company believes the contingent liability is appropriate as it continues to pursue three development programs using this technology. Fair Value of Financial Instruments Fair value accounting is applied for all financial assets and liabilities and non-financial assets and liabilities that are recognized or disclosed at fair value in the consolidated financial statements on a recurring basis (at least annually). Assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels, which are directly related to the amount of subjectivity, associated with the inputs to the valuation of these assets or liabilities are as follows:
The carrying values of the Company's financial instruments, including cash equivalents, accounts receivable, and accounts payable, approximated their fair values due to the short period of time to maturity or repayment. The carrying values of the Company's promissory notes, convertible promissory notes, and notes payable approximate their fair values for the years ended December 31, 2013 and 2014 as the market rates currently available to the Company and other assumptions have not changed significantly. The Company's Level 3 liabilities measured and recorded on a recurring basis consist of derivative liabilities related to the convertible promissory note (see Note 7). The following table sets forth a summary of the changes in the fair value and other adjustments of these derivative liabilities (in thousands):
Notes to Consolidated Financial Statements (Continued) 2. Significant Accounting Policies (Continued) Recent Accounting Pronouncements In April 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update (ASU) ASU 2014-08,Presentation of Financial Statements (Topic 205) and Property, Plant, and Equipment (Topic 36), which amends the definition of a discontinued operation in ASC 205-20 and requires entities to provide additional disclosures about disposal transactions that do not meet the discontinued-operations criteria. The revised guidance will change how entities identify and disclose information about disposal transactions under U.S. GAAP. The ASU applies to all entities and is effective for annual periods beginning after December 15, 2014 and interim periods thereafter, with early adoption permitted. The Company does not anticipate that the adoption of this ASU will materially change the presentation of its consolidated financial statements. In May 2014, the FASB issued ASU 2014-09,Revenue from Contracts with Customers, which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. The new standard will be effective for the Company on January 1, 2017, which is the effective date for public companies. Non-public entities have an additional one year to adopt this standard. Early application is not permitted. The standard permits the use of either the retrospective or cumulative effect transition method. The Company is evaluating the effect that ASU 2014-09 will have on its consolidated financial statements and related disclosures. The Company has not yet selected a transition method nor has it determined the effect of the standard on its ongoing financial reporting. In August 2014, the FASB issued ASU 2014-15,Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which provides guidance on determining when and how to disclose going-concern uncertainties in the consolidated financial statements. The new standard requires management to perform interim and annual assessments of an entity's ability to continue as a going concern within one year of the date the consolidated financial statements are issued. An entity must provide certain disclosures if "conditions or events raise substantial doubt about the entity's ability to continue as a going concern." The ASU applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. The Company does not anticipate a material change to its consolidated financial statements upon the adoption of this ASU. However, it will be required to evaluate and determine if further disclosure is necessary at each balance sheet date. In February 2015, the FASB issued ASU 2015-02,Consolidation (Topic 810): Amendments to the Consolidation Analysis. The amendments significantly change the consolidation analysis required under U.S. GAAP. Reporting entities will need to reevaluate all their previous consolidation conclusions. The Company does not anticipate that the adoption of this ASU will materially change the presentation of its consolidated financial statements.
Notes to Consolidated Financial Statements (Continued) 3. Property and Equipment, Net Property and equipment, net, consisted of the following (in thousands):
The Company acquired laboratory equipment during 2013 under a capital lease. This equipment has a gross value of $117,000 and accumulated depreciation expense of $39,000 and $117,000 as of December 31, 2013 and 2014, respectively. This equipment is included in property and equipment and related depreciation is included in depreciation expense. Depreciation and amortization expense is $391,000 and $358,000 for the years ended December 31, 2013 and 2014, respectively. 4. Investment in Unconsolidated Entity Limagrain Cereal Seeds LLC The Company owns a 35% ownership position in LCS. The remaining 65% of LCS is owned by Vilmorin & Cie ("Limagrain"), a major global producer and marketer of field crop and vegetable seeds, through its wholly owned subsidiary, Vilmorin USA ("VUSA"). LCS improves and develops new wheat and barley varieties utilizing genetic and breeding resources, as well as advanced technologies from Limagrain and the Company. Funding for LCS comes from an initial pro rata equity investment from each partner and with subsequent financing in the form of debt from VUSA. As of December 31, 2014, the debt balance was $13.5 million with a maturity date of January 15, 2015. The maturity date was extended to April 15, 2015 and an additional $1.0 million was funded by VUSA, also due April 15, 2015. While it is the Company's expectation that VUSA will provide LCS with additional debt financing as needed, should additional capital in the form of equity be necessary to support the operations of LCS, the Company has the option to fund its pro rata share of such cash or elect to have its ownership percentage diluted.
Notes to Consolidated Financial Statements (Continued) 4. Investment in Unconsolidated Entity (Continued) Summarized condensed financial information related to the unconsolidated entity, accounted for using the equity method is as follows (in thousands):
5. Variable Interest Entity In February 2012, the Company formed Verdeca LLC, which is jointly owned with Bioceres, Inc. ("Bioceres"), a U.S. wholly owned subsidiary of Bioceres, S.A., an Argentine corporation. Bioceres, S.A. is an agricultural investment and development company owned by approximately 230 shareholders, including some of South America's largest soybean growers. Verdeca was formed to develop and deregulate soybean varieties using both partners' agricultural technologies. Both the Company and Bioceres incur expenses in support of specific activities agreed, as defined by joint work plans, which apply fair market value to each partner's activities. Unequal contributions of services are equalized by the partners through cash payments. Verdeca is not the primary obligor for these activities performed by the Company or Bioceres. An agreement executed in conjunction with the formation of Verdeca specifies that if Bioceres determines it requires cash to fund its contributed services (subject to certain annual limits), Bioceres, S.A. may elect to sell shares of its common stock to the Company for an amount not exceeding $5.0 million in the aggregate over a four-year period. The Company determined that its commitment to purchase common stock in Bioceres, S.A. as a means to provide capital to Verdeca resulted in a de facto agency relationship between the Company and Bioceres. The Company considers qualitative factors in assessing the primary beneficiary which include
Notes to Consolidated Financial Statements (Continued) 5. Variable Interest Entity (Continued) understanding the purpose and design of the VIE, associated risks that the VIE creates, activities that could be directed by the Company, and the expected relative impact of those activities on the economic performance of the VIE. Based on an evaluation of these factors, the Company concluded that it is the primary beneficiary of Verdeca. As a result of the agreement to fund future contributions by Bioceres, the Company purchased common stock of Bioceres, S.A. in the amount of $500,000 in January 2013, which is included in the cost method investment on the balance sheet as of December 31, 2013. Additional common stock purchases were made in the amount of $700,000 in January 2014, $250,000 in April 2014, and $500,000 in August 2014. The Company's remaining maximum commitment to purchase stock in Bioceres, S.A. under the original funding agreement amounted to $2.0 million for 2014 and $1.2 million for 2015. In September 2014, the Company and Bioceres, S.A. entered into an agreement to reduce the annual commitment for 2014 to $500,000 from the original $2.0 million and to eliminate the 2015 commitment amount of $1.2 million. In consideration for these amendments, the Company surrendered 1,832 shares of Bioceres, S.A. held by the Company. The Company recorded an expense of $1.5 million related to this agreement, which is classified as research and development expense in the consolidated statement of operations for the year ended December 31, 2014. In addition, the Company has a right to require Bioceres, S.A. to repurchase any shares of common stock then owned by the Company upon the occurrence of certain events specified in the agreement, and similarly, Bioceres, S.A. has the right to require the Company to sell back any shares of common stock owned by the Company under certain circumstances. Management has evaluated the carrying value of its investment in Bioceres and has determined there has been no impairment. Under the terms of the joint development agreement, the Company has incurred direct expenses and allocated overhead in the amount of $1.2 million and $1.0 million for the years ended December 31, 2013 and 2014, respectively. 6. Accounts Payable and Accrued Expenses Accounts payable and accrued expenses consisted of the following (in thousands):
Notes to Consolidated Financial Statements (Continued) 7. Debt Long-term Debt Long-term debt consisted of the following (in thousands):
In July 2012, a 36-month $8.0 million term note was executed with Moral Compass Corporation ("MCC"), the Company's largest stockholder (see Note 15), and is subordinate to the promissory notes and convertible promissory notes. The interest rate on the loan is prime plus 2%, with interest paid monthly in arrears. The principal is due in full at maturity in July 2015. On November 10, 2014, the Company and MCC entered into an amendment to the $8.0 million term loan under which the maturity date was amended to the first to occur of the following dates: (i) April 1, 2016, (ii) the date of an Event of Default, or (iii) a date designated by MCC, by notice to the Company, no earlier than the 20th day following consummation by the Company of an equity financing with gross proceeds to the Company of at least $50 million. In addition, the interest rate remains at prime plus 2% through December 31, 2014, and was amended to be 11% per annum thereafter until maturity. The balance of the note, inclusive of accrued interest, was approximately $8.0 million as of December 31, 2013 and 2014. Accrued interest of $36,000 and $36,000 are recorded in amounts due to related parties on the balance sheet as of December 31, 2013 and 2014, respectively. Promissory notes were executed with an unrelated party in August 2013 and November 2013 in the amounts of $2.0 million and $1.1 million, respectively. The interest rate on the notes is 10% with principal and interest due in 36 equal monthly installments over the course of the three-year terms. Monthly principal and interest on the $2.0 million note is $65,000 and the three-year term ends in August 2016. Monthly principal and interest on the $1.1 million note is $35,000 and the three-year term ends in November 2016. The balance of the promissory notes, inclusive of accrued interest, was $1.9 million as of December 31, 2014. In addition, in July 2013, the Company entered into a short-term loan agreement for $500,000 with MCC. The interest rate on the loan is 7.5%. The principal and related interest were paid in full in December 2013. Interest expense related to this loan was $19,000 in the year ended December 31, 2013. Convertible Promissory Notes A note and warrant purchase agreement was executed in September 2013, with Mahyco International Pte Ltd., ("Mahyco"), a licensee of the Company's technologies. The Company issued two notes under this agreement in the amounts of $500,000 in September 2013 and $4.5 million in December 2013, both of which are subordinate to the promissory notes. The interest on the notes is prime plus 2%, compounded monthly over the course of the five-year term ending September and
Notes to Consolidated Financial Statements (Continued) 7. Debt (Continued) December 2018, respectively, and is payable on the maturity date. At any time during the term, the lender may convert all or part of the outstanding balance of the note (including principal and accrued but unpaid interest) into common stock of the Company at At its option, Mahyco may offset future fee payments to the Company against the outstanding balance of the note (including principal and accrued but unpaid interest). Mahyco has the right to demand immediate settlement of a portion of the outstanding balance of the convertible promissory note, the amount of which shall be mutually agreed by the Company and the lender prior to such settlement. The Company recorded a derivative liability for the initial fair value of the settlement obligation. The derivative liability is valued using the binomial lattice option-pricing method. The lender has the right, at its option, to place another $5.0 million of convertible debt with the Company during the five-year term. The Company recorded a derivative liability for the initial fair value of the Company's obligation to issue the additional $5.0 million of convertible promissory notes. The derivative liability was valued using the Monte Carlo simulation method. Changes in the fair value of the derivative liabilities are recorded to other income (expense), net in the consolidated statement of operations. The Company also issued to the lender a warrant to purchase Minimum principal payments on the Company's outstanding debt, consisting of the term note, promissory notes and the convertible promissory notes, as of December 31, 2014 are as follows (in thousands):
Notes to Consolidated Financial Statements (Continued) 8. Commitments and Contingencies Leases The Company leases office and laboratory space, greenhouse space, grain storage bins, warehouse space, and equipment under operating lease agreements having initial lease terms ranging from three to five years, including certain renewal options available to the Company at market rates. The Company also leases land for field trials on a short-term basis. Future minimum payments under non-cancelable operating leases in effect as of December 31, 2014, are presented below (in thousands):
The Company acquired laboratory equipment under a capital lease during 2013. The remaining payments under the capital lease totaled $74,000 as of December 31, 2013, $72,000 of which was principal and $2,000 of which was interest. All amounts were paid as of December 31, 2014, and the Company subsequently purchased the equipment. Rent expense under all operating leases totaled $1.3 million and $1.0 million for the years ended December 31, 2013 and 2014, respectively. Legal Matters From time to time, in the ordinary course of business, the Company may become involved in certain legal proceedings. As of December 31, 2013 and 2014, the Company was not involved in any legal proceedings. Contracts The Company has entered into contract research agreements with unrelated parties that require the Company to pay certain funding commitments. The initial terms of these agreements range from one to three years in duration and in certain cases are cancelable. The Company licenses certain technologies via executed agreements ("In-Licensing Agreements") that are used to develop and advance the Company's own technologies. The Company has entered into various In-Licensing Agreements with related and unrelated parties that require the Company to pay certain license fees, royalties, and/or milestone fees. In addition, certain royalty payments ranging from 2% to 15% of net revenue amounts as defined in the In-Licensing Agreements will be due. There is no minimum payment for non-cancelable annual license fees due during the year ended December 31, 2014. Royalties on licensed revenue accrued as of December 31, 2013 and 2014, were $336,000 and $252,000, respectively. Royalties are included within research and development on the consolidated statements of operations. Milestone payments are contingent upon the successful development or implementation of various technologies. Payments for milestones yet to be achieved total $2.1 million as of December 31, 2014. The timing of the payments is not determinable at this time pending research and development
Notes to Consolidated Financial Statements (Continued) 8. Commitments and Contingencies (Continued) currently in progress; however, no significant payments were made during the years ended December 31, 2013 and 2014. The Company could be adversely affected by certain actions by the government as it relates to government contract revenue received in prior years. Government agencies, such as the Defense Contract Audit Agency routinely audit and investigate government contractors. These agencies review a contractor's performance under its agreements; cost structure; and compliance with applicable laws, regulations, and standards. The agencies also reviews the adequacy of, and a contractor's compliance with, its internal control systems and policies, including the contractor's purchasing, property, estimating, compensation, and management information systems. While the Company's management anticipates no adverse result from an audit, should any costs be found to be improperly allocated to a government agreement, such costs will not be reimbursed, or if already reimbursed, may need to be refunded. If an audit uncovers improper or illegal activities, civil and criminal penalties and administrative sanctions, including termination of contracts, forfeiture of profits, suspension of payments or fines, and suspension or prohibition from doing business with the government could occur. In addition, serious reputational harm or significant adverse financial effects could occur if allegations of impropriety were made against the Company. There are no current audits relating to government grant revenues in process. 9. Common Stock and Redeemable and Convertible Preferred Stock Common Stock As of December 31, 2014, the Company had reserved the following shares of common stock, on an as-converted basis, for future issuance as follows:
Redeemable and Convertible Preferred Stock Convertible preferred stock as of December 31, 2013 consisted of the following:
Notes to Consolidated Financial Statements (Continued) 9. Common Stock and Redeemable and Convertible Preferred Stock (Continued) Redeemable and convertible preferred stock as of December 31, 2014 consisted of the following:
On March 28, 2014, the Company entered into an agreement with certain investors to issue 9,822,283 shares of its Series D redeemable convertible preferred stock at an original issue price of $3.36 per share, and closings that were scheduled to be completed within 90 days from the initial close. The holders of the Series D redeemable convertible preferred stock also received warrants for the purchase of an aggregate of The Company also incurred transaction costs for an advisor of approximately $2.1 million, which includes a warrant for the purchase of In connection with the issuance of the Series D redeemable convertible preferred stock and the resulting amendment to the Articles of Incorporation, the Company reclassified the Series A, Series B and Series C convertible preferred stock outside of stockholders' deficit because, in the event of certain deemed liquidation events that are not solely within its control, the shares would become redeemable at the option of the holders. The Company did not adjust the carrying values of the Series A, Series B, Series C convertible preferred stock to the liquidation values of such shares, since a liquidation event was not probable at any of the balance sheet dates. Subsequent adjustments to increase or decrease the carrying values to the ultimate liquidation values will be made only if and when it becomes probable that such a liquidation event will occur. As the Series D convertible preferred stock is redeemable at the request of the holder on or after the eight-year anniversary from the original issuance date, the Company classified the Series D
Notes to Consolidated Financial Statements (Continued) 9. Common Stock and Redeemable and Convertible Preferred Stock (Continued) redeemable convertible preferred stock outside of stockholders' deficit because of the date certain redemption that is not within the control of the Company. The Company accretes the carrying value of the Series D redeemable convertible preferred stock to the redemption amount on the eighth anniversary using the interest method through periodic charges to additional paid-in capital, which amounted to $3.7 million for the year ended December 31, 2014. The redemption amount is the greater of (i) two times the original issue price of the Series D redeemable convertible preferred stock plus accrued and unpaid dividends through the redemption date, or (ii) the fair market value of the Series D redeemable convertible preferred stock. The redemption value also includes dividends which are payable in arrears upon redemption and aggregate to $97.0 million over the redemption period of eight years. The redemption amount of outstanding Series A, Series B and Series C convertible stock is equal to its liquidation value, or $1.00 per share. Significant provisions of the redeemable and convertible preferred stock are as follows: Liquidation Preferences—In the event of liquidation, dissolution, or winding-up (Liquidation Event), a merger, sale or a change in control (Deemed Liquidation Event), or an IPO of the Company (other than a Qualified IPO, as such term is defined below), each holder of Series D redeemable convertible preferred stock will be entitled to receive an initial liquidation amount of $3.36 per share plus accrued and unpaid dividends, which accrue and compound annually until paid ("Accrued Dividends"), before any payment shall be made, or any assets distributed, with respect to any Series A, Series B, or Series C convertible preferred stock or any common stock. Next, each holder of Series D redeemable convertible preferred stock will be entitled to receive, out of the assets of the Company, a liquidation amount of $3.36 per share and each holder of Series A, Series B, and Series C convertible preferred stock will be entitled to receive, out of the assets of the Company, a liquidation amount of $1.00 per share and, except that in the case of an IPO other than a Qualified IPO, only the holders of Series D redeemable convertible preferred stock will receive a liquidation amount. Finally, holders of common stock will receive a ratable portion of any assets remaining following the liquidation payments to holders of preferred stock described above. If, under the initial liquidation allocation described above, there is a shortfall in the amount to be paid to the holders of the Series D redeemable convertible preferred stock, then all assets of the Company will be distributed on a pro rata basis among all of the outstanding shares of Series D redeemable convertible preferred stock. If, under the subsequent liquidation allocation described above, there is a shortfall in the amount to be paid to the holders of preferred stock, then all assets of the Company will be distributed on a pro rata basis among all of the outstanding shares of preferred stock. A "Qualified IPO" is an IPO that meets certain requirements as to the Company's pre-money valuation, percentage of the Company capital stock that is sold in the offering, and the gross proceeds from the offering to the Company. In the event of a Qualified IPO, each holder of Series D redeemable convertible preferred stock will be entitled to receive Accrued Dividends in the form of, at the Company's election, a cash payment or shares of common stock. Voting Rights—All holders of preferred stock and common stock shall have the right to one vote on all matters concerning stockholders, including, but not limited to, the right to vote for members of the board of directors.
Notes to Consolidated Financial Statements (Continued) 9. Common Stock and Redeemable and Convertible Preferred Stock (Continued) Conversion Rights—The holders of preferred stock are subject to certain optional and mandatory conversion rights. (i) Optional Conversion Rights: Each share of Series A, Series B and Series C convertible preferred stock shall be convertible into common stock at any time at the option of the holder thereof. Such conversion will be on a share-for-share basis, one fully paid and non-assessable share of common stock for Subscription Rights—In the event that the Company issues new shares of stock to any party, the holders of preferred stock and certain holders of common stock carry the right to purchase additional shares of any such future stock issuance such that their existing ownership allocation remains intact. Any additional shares purchased by the existing stockholders would be at the same price as any other stockholders at that time. This right will not apply to shares of the Company's common stock issued in connection with the IPO and will terminate upon the consummation of the IPO. Dividends—The holders of record of shares of Series D redeemable convertible preferred stock are entitled to a 15% dividend that accrues annually until the five-year anniversary of the issuance date and a 20% dividend that accrues annually thereafter. Such dividends on the Series D redeemable convertible preferred stock are payable upon a liquidation event, an IPO of the Company, in the case of a redemption of the Series D redeemable convertible preferred stock, or if declared by the board of directors, at its discretion. Cumulative dividends (undeclared and unpaid) totaled $3.5 million ($0.35 per share) as of December 31, 2014. The Series A, Series B, and Series C convertible preferred stockholders are not entitled to receive dividends, except as the board of directors may declare in its discretion, provided that upon such declaration, each share of preferred stock outstanding shall receive a dividend at least equal, per share, to any cash dividend paid to any holders of common stock. In the event of a declaration of dividends by the board of directors, the Company's note holders have rights restricting the payment until the note holders are paid in full. Redemption—The Series D redeemable convertible preferred stock shall be redeemed at the option of the holders at any time on or after the eight-year anniversary or at the option of the Company, at any time on or after the four-year anniversary. The redemption amount shall be the greater of (i) two times the original issue price of the Series D redeemable convertible preferred stock plus accrued and unpaid dividends through the redemption date, or (ii) the fair market value of the Series D redeemable convertible preferred stock.
Notes to Consolidated Financial Statements (Continued) 10. Stock-Based Compensation 2006 Stock Incentive Plan In 2006, the Company authorized the 2006 Stock Plan ("2006 Plan"), which provides for the granting of stock options to executives, employees, and other service providers. The 2006 Plan was adopted on May 2, 2006, with an effective date of January 1, 2006, and, as amended, provides for A summary of activity under the Plan is as follows (in thousands, except exercise price and remaining contractual life data):
Aggregate intrinsic value represents the difference between the exercise price of the options and the estimated fair value of the Company's common stock determined by the board of directors for each of the respective periods. The intrinsic value of options exercised was $113,000 and $74,000 for the years ended December 31, 2013 and 2014, respectively. The estimated grant date fair value of options vested was $1.3 million and $976,000 during the years ended December 31, 2013 and 2014, respectively. As of December 31, 2014, there was $720,000 of unrecognized compensation cost related to unvested stock-based compensation grants that will be recognized over the weighted-average remaining recognition period of 1.53 years.
Notes to Consolidated Financial Statements (Continued) 10. Stock-Based Compensation (Continued) In determining the fair value of the stock-based awards, the Company uses the Black-Scholes option-pricing model and assumptions discussed below. Each of these inputs is subjective and generally requires significant judgment to determine. Expected Term—The expected term is the estimated period of time outstanding for stock options granted and was estimated based on historical, as well as anticipated future, exercise activity. Expected Volatility—Since the Company is privately held and does not have any trading history for its common stock, the expected volatility was estimated based on the average volatility for comparable publicly traded biotechnology companies over a period equal to the expected term of the stock option grants. When selecting comparable publicly traded biotechnology companies on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected life of the stock-based awards. The historical volatility data was computed using the daily closing prices for the selected companies' shares during the equivalent period of the calculated expected term of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available. Risk-Free Interest Rate—The risk-free interest rate is based on the interest rate of U.S. Treasuries of comparable maturities on the date the options were granted. Expected Dividend—The expected dividend yield is based on the Company's expectation of future dividend payouts to common stockholders. The fair value of stock option awards to employees was estimated at the date of grant using a Black-Scholes option-pricing model with the following weighted-average assumption:
No employee stock options were granted during the year ended December 31, 2013. The weighted-average, estimated grant-date fair value of employee stock options granted during the year ended December 31, 2014 was 11. Retirement Benefits The Company has a 401(k) retirement plan (the "Plan") available for participation by all regular full-time employees who have completed three months of service with the Company. The Company established the Plan in 2008. The Plan provides for a discretionary matching contribution equal to 50% of the amount of the employee's salary deduction, not to exceed 3% of the salary per employee. Highly compensated employees are excluded from receiving any discretionary matching contribution. Employees' rights to employer contributions vest on the one-year anniversary of their date of employment. The Company has the option to make discretionary matching contributions. The Company did not make discretionary matching contributions during the years ended December 31, 2013 or 2014.
Notes to Consolidated Financial Statements (Continued) 12. Income Taxes The components of loss before income taxes are as follows:
The components of the provision for income taxes for the years ended December 31, 2013 and 2014 are as follows (in thousands):
The Company operates in only one federal jurisdiction, the United States. The following is a reconciliation of the statutory federal income tax rate to the Company's effective tax rate is as follows:
The total income tax expense for the years ended December 31, 2013 and 2014 was $167,000 and $263,000 and is comprised of current, foreign taxes withheld by governmental agencies outside of the United States. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax
Notes to Consolidated Financial Statements (Continued) 12. Income Taxes (Continued) purposes, net operating loss carryforwards ("NOLs"), and other tax credits. Significant components of the Company's deferred tax assets are as follows (in thousands):
Realization of the deferred tax assets is dependent upon future taxable income, if any, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets has been offset by a valuation allowance. The net valuation allowance increased by $4.6 million and $6.3 million during the years ended December 31, 2013 and 2014, respectively. At December 31, 2014, the Company had federal and state NOLs aggregating approximately $98.1 million and $77.2 million, respectively. These federal NOLs will begin to expire in 2020 and these state NOLs will begin to expire in 2015, if not utilized. The Company's deferred tax asset at December 31, 2014 does not include $0.1 million of excess tax deductions from employee stock option exercises included in its NOLs. The Company's stockholders' deficit will be decreased by up to $0.1 million if and when the Company ultimately realizes these excess tax benefits. The Company evaluates its NOLs on an ongoing basis to determine if they may be limited by the Internal Revenue Code (IRC) Section 382. The Company has determined its federal NOLs at December 31, 2014 are subject to IRC Section 382 limitations. Of the $98.1 million generated, $7.2 million will not be available to be utilized within the carryforward period. The Company evaluates deferred tax assets, including the benefit from NOLs, to determine if a valuation allowance is required. Such evaluation is based on consideration of all available evidence using a "more likely than not" standard with significant weight being given to evidence that can be objectively verified. This assessment considers, among other matters, the nature, frequency, and severity of current and cumulative losses; forecasts of future profitability; the length of statutory carryforward periods; the Company's experience with operating losses; and tax-planning alternatives. The significant piece of objective negative evidence evaluated was the cumulative loss incurred over the three-year
Notes to Consolidated Financial Statements (Continued) 12. Income Taxes (Continued) period ended December 31, 2014. Given this evidence and the expectation to incur operating losses in the foreseeable future, a full valuation allowance has been recorded against net deferred tax asset. The Company will continue to maintain a full valuation allowance against the entire amount of its net deferred tax asset, until such time as the Company has determined that the weight of the objectively verifiable positive evidence exceeds that of the negative evidence and it is likely that the Company will be able to utilize all of its net deferred tax asset relating to its federal and state NOL carryforwards. Although the Company has established a full valuation allowance on its net deferred tax asset, it has not forfeited the right to carryforward tax losses up to 20 years and apply such tax losses against taxable income in such years, thereby reducing its future tax obligations. The Company is subject to taxation in the United States and various state jurisdictions. As of December 31, 2014, the Company's tax years for 2004 through 2014 are generally subject to examination by the tax authorities. On January 1, 2009, the Company adopted the provisions of ASC 740 related to accounting for uncertain tax positions and concluded there were no such positions associated with the Company requiring accrual of a liability. As of December 31, 2014, the Company has not accrued for any such positions. The Company is currently not under audit for federal or state purposes. The Company does not expect a significant change to occur within the next 12 months. 13. Net Loss per Share and Unaudited Pro Forma Net Loss per Share Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period and excludes any dilutive effects of stock-based awards and warrants. Diluted net loss per share attributable to common stockholders is computed giving effect to all potentially dilutive common shares, including common stock issuable upon exercise of stock options and warrants and conversion of convertible promissory notes, redeemable convertible preferred stock and convertible preferred stock. As the Company had net losses for the years ended December 31, 2013 and 2014, all potentially dilutive common shares were determined to be anti-dilutive. The following table sets forth the computation of net loss per share attributable to common stockholders (in thousands, except share and per share amounts):
Notes to Consolidated Financial Statements (Continued) 13. Net Loss per Share and Unaudited Pro Forma Net Loss per Share (Continued) Potentially dilutive securities that were not included in the diluted per share calculations because they would be anti-dilutive were as follows:
The Company has presented unaudited pro forma basic and diluted net loss per share attributable to common stockholders, which has been computed to give effect to the conversion of all shares of redeemable convertible preferred stock and convertible preferred stock into shares of common stock as if such conversion had occurred as of the beginning of the period presented. The terms of the Series D preferred stock provide that the ratio at which each share of Series D preferred stock automatically converts into shares of common stock in connection with the initial public offering is dependent upon the initial public offering price of the common stock. The number of shares of common stock to be issued upon the automatic conversion of all outstanding shares of Series D preferred stock is based on an assumed initial public offering price of
14. Segment and Geographic Information Management has determined that it has one business activity and operates in one segment as it only reports financial information on an aggregate and consolidated basis to its Chief Executive Officer, who is the Company's chief operating decision maker.
Notes to Consolidated Financial Statements (Continued) 14. Segment and Geographic Information (Continued) Revenues based on the location of the customers, are as follows (in percentages):
15. Related-Party Transactions The Company's related parties include MCC and Blue Horse Labs, Inc. ("BHL"), and Limagrain. BHL is deemed a related party as a result of its existing contractual relationship with the Company and that a Director of the Company also serves as the Treasurer of BHL and as an Officer and Director of MCC, the Company's controlling stockholder. Transactions with related parties are reflected in the consolidated financial statements under amounts due to or from related parties and notes payable to related party. Outlined below are details of agreements between the Company and its related parties: A term note was executed with MCC in July 2012 for $8.0 million (see Note 7). The principal balance is included in the December 31, 2013 and 2014 balance sheets as notes payable to related party and the related accrued interest is included in amounts due to related parties. An additional term note was executed with MCC in July 2013 for $500,000. The principal balance and $19,000 of interest was repaid in December 2013. Under a license agreement executed in 2003 and amended in 2009, BHL receives a single-digit royalty from the Company when revenue has been collected for certain intellectual property such as GLA, NUE monocot among others that has been licensed to third parties. Royalty fees due to BHL were $161,000 and $21,000 as of December 31, 2013 and 2014, respectively, and are included in the balance sheets as amounts due to related parties. License agreements were executed with Limagrain, a stockholder of the Company, in September 2009 and February 2011. The agreements license certain of the Company's traits to Limagrain and include up-front license fees, annual license fees, milestone fees and value-sharing payments. The Company recognized $144,000 and $191,000 of revenue under these agreements in the years ended December 31, 2013 and 2014, respectively. The amounts due from Limagrain were $100,000 and $0 as of December 31, 2013 and 2014, respectively. In addition to the investment in LCS as described in Note 4, Limagrain purchased
Notes to Consolidated Financial Statements (Continued) 16. Subsequent Events In connection with the issuance of the consolidated financial statements for the years ended December 31, 2013 and 2014, the Company has evaluated subsequent events through April 3, 2015, the date the consolidated financial statements were In February 2015, the Company's board of directors approved the grant of options to purchase an aggregate of In March 2015, the Company's board of directors approved the grant of an option to purchase an aggregate of In March 2015, the Company completed its reincorporation in the State of Delaware from the State of Arizona (the "Reincorporation"). In connection with the Reincorporation, each outstanding share of, or option or warrant to purchase, capital stock of the Arizona corporation was exchanged for an equivalent share of, or option or warrant to purchase, capital stock of the Delaware corporation. The shares were exchanged such that stockholders received one share of the Company's Delaware capital stock, par value $0.001 per share, for each share of the Company's Arizona capital stock, no par value. The Reincorporation will not result in any material change in the business, management, assets, liabilities, or net worth of the Company. In April 2015, the Company entered into a loan and security agreement, under which the Company incurred an aggregate principal amount of $20.0 million in term loan borrowings (the "Term Loans"). The Company is required to make interest-only payments under the Term Loans from the drawdown date through April 30, 2016, subject to certain conditions for extension to October 31, 2016. After this date, it is required to make equal monthly payments so that all outstanding principal amounts and accrued interest will be repaid by November 1, 2018. As part of the loan and security arrangement, the Company also issued the lenders warrants to purchase shares of its common stock which are exercisable in the event that an IPO is not completed prior to September 30, 2015. In April 2015, the Company's board of directors approved a certificate of amendment of the Company's amended and restated certificate of incorporation to effect a reverse split of the Company's issued and outstanding common stock at a one-for-four ratio. In May 2015, the Company's stockholders approved the certificate of amendment, and on May 8, 2015, the Company filed the certificate of amendment with the Secretary of State of the State of Delaware to effect the reverse split. The par value and authorized shares of common stock and convertible preferred stock were not adjusted as a result of the reverse split. All issued and outstanding common stock, options to purchase common stock and per share amounts contained in the financial statements have been retroactively adjusted to reflect the reverse stock split for all periods presented. The financial statements have also been retroactively adjusted to reflect a proportional adjustment to the conversion ratio for each series of redeemable convertible preferred stock and convertible preferred stock that will be effected in connection with the reverse stock split. In April 2015, the Company paid off its term note with MCC, repaying the principal balance of $8.0 million and accrued interest and prepayment fee of $148,000, and paid off its promissory notes with an unrelated party, repaying the aggregate outstanding principal balance of $1.6 million and accrued interest and prepayment fee of $44,000.
Item 13. Other Expenses of Issuance and Distribution The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the sale of common stock being registered. All amounts are estimates except the SEC registration fee, FINRA filing fee and The NASDAQ Global Select Market listing fee.
Item 14. Indemnification of Directors and Officers On completion of this offering, the Registrant's amended and restated certificate of incorporation will contain provisions that eliminate, to the maximum extent permitted by the General Corporation Law of the State of Delaware, the personal liability of the Registrant's directors and executive officers for monetary damages for breach of their fiduciary duties as directors or officers. The Registrant's amended and restated certificate of incorporation and bylaws will provide that the Registrant must indemnify its directors and executive officers and may indemnify its employees and other agents to the fullest extent permitted by the General Corporation Law of the State of Delaware. Sections 145 and 102(b)(7) of the General Corporation Law of the State of Delaware provide that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of a corporation against expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation. The Registrant has entered into indemnification agreements with its directors and executive officers, in addition to the indemnification provided for in its amended and restated certificate of incorporation and bylaws, and intends to enter into indemnification agreements with any new directors and executive officers in the future. The Registrant has purchased and intends to maintain insurance on behalf of each and any person who is or was a director or officer of the Registrant against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions. II-1 The Underwriting Agreement (Exhibit 1.1 hereto) provides for indemnification by the underwriters of the Registrant and its executive officers and directors, and by the Registrant of the underwriters, for certain liabilities, including liabilities arising under the Securities Act. Item 15. Recent Sales of Unregistered Securities Since January 1, 2012, the Registrant issued the following unregistered securities: Preferred Stock Issuances From March 2014 through May 2014, the Registrant sold to accredited investors in a series of closings an aggregate of 9,822,283 shares of our Series D preferred stock at a per share price of $3.36, for aggregate consideration of approximately $33.0 million. In connection with the Series D preferred stock financing, from March 2014 through May 2014, the Registrant issued to accredited investors warrants to purchase an aggregate of 4,911,145 shares of its common stock at an exercise price of $4.54 per share. In connection with the Series D preferred stock financing, from March 2014 through May 2014, the Registrant issued to its placement agent, an accredited investor, warrants to purchase 133,781 shares of its common stock at an exercise price of $3.36 per share as partial consideration for their services. Convertible Notes and Warrant Issuances From September 2013 through December 2013, the Registrant issued convertible notes to an accredited investor in the principal amount of $5.0 million. In December 2013, the Registrant issued to an accredited investor a warrant to purchase 302,665 shares of its common stock at an exercise price of $4.13 per share. 2006 Stock Plan-Related Issuances Since January 1, 2012, the Registrant granted its directors, employees, consultants and other service providers options to purchase an aggregate of 2,444,400 shares of common stock under its 2006 Stock Plan at exercise prices ranging from $1.53 to $3.39 per share. The Registrant has issued and sold an aggregate of A placement agent was used in connection with the Series D preferred stock financing described above. Otherwise, no underwriters were involved in the foregoing sales of securities. The issuances of the securities described above were deemed to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, or Regulation D promulgated thereunder, as transactions by an issuer not involving a public offering, or Regulation S of the Securities Act or Rule 701 promulgated under 3(b) of the Securities Act as transactions pursuant to compensation benefits plans and contracts relating to compensation. Item 16. Exhibits and Financial Statement Schedules (a) Exhibits
II-2
II-3
(b) Financial Statement Schedules Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto. The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. The undersigned registrant hereby undertakes that: (1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. (2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. II-4 Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Amendment No.
Pursuant to the requirements of the Securities Act of 1933, this Amendment No.
II-5
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|