Synergy CHC Corp.
(NameExact name of small business issuerregistrant as specified in its charter)
| Nevada | 99-0379440 | |||
(State or other incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer | ||
Identification No.) |
865 Spring Street
Westbrook, Maine 04092
(615) 939-9004
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices and principal place of business)
23 Dassan Island DriveJack Ross
Chief Executive Officer
c/o Synergy CHC Corp.
865 Spring Street
Westbrook, Maine 04092
(615) 939-9004
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
W. David Mannheim E. Peter Strand Michael K. Bradshaw, Jr. Nelson Mullins Riley & Scarborough LLP 4140 Parklake Avenue, Suite 200 Raleigh, NC 27612 (919) 329-3800 | C. Brophy Christensen, Jr., Esq. Jeeho M. Lee, Esq. O’Melveny & Myers LLP Two Embarcadero Center, 28th Floor San Francisco, CA 94111 (415) 984-8793 |
Approximate date of commencement of proposed sale to timethe public: As soon as practicable after the effective date of this Registration Statement becomes effective.
If any of the securities being registered on this formForm are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of "large“large accelerated filer," "accelerated” “accelerated filer,"” “smaller reporting company,” and "smaller reporting company"“emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee | ||||||
| Common stock, par value $0.00001 per share | $ | 69,000,000 | $ | 6,396.3 | ||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. Includes the offering price of any additional shares of common stock that the underwriters have the right to purchase to cover over-allotments. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
| Amount To Be | Offering Price | Aggregate | Registration Fee | |||||||||||||
| Securities to be Registered | Registered | Per Share | Offering Price | [1] | ||||||||||||
| Common Stock: | 1,000,000 | 0.04 | $ | 40,000 | $ | 1.57 | ||||||||||
The information in this offering. No exchange or over the counter market exists for our common stock. Our offering price per share was arbitrarily determined in order for us to raise $40,000.
| Offering Price | Expenses | Proceeds to Us | ||||||||||
| Per Share – Gross Proceeds | $ | 0.04 | $ | 0.005 | $ | 0.035 | ||||||
| Total – Gross Proceeds | $ | 40,000 | $ | 13,002 | $ | 26,998 | ||||||
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED OCTOBER 22, 2021 |
Shares
Common Stock

Synergy CHC Corp.
Synergy CHC Corp. is a non-contingent Offeringoffering shares of our common stock, par value $0.00001 per share. We currently estimate that the initial public offering price of our common stock will be between $ and there is no minimum number$ .
Prior to September 28, 2021, shares of Shares required to be sold.our common stock were quoted on the OTC Markets Group, Inc. Pink tier under the symbol “SNYR.” As a result of amendments to Exchange Act Rule 15c2-11, because we do not presently make current information publicly available, our common stock was shifted to the OTC Expert Market on September 28, 2021, which means that there are no longer publicly-available quotations of our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the symbol “SNYR.”
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 8.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
| (1) | The underwriters will receive compensation in addition to the discounts and commissions. See “Underwriting” beginning on page 56 for a description of compensation payable to the underwriters. |
We have granted a 30-day option to the representative of the underwriters to purchase up to additional shares of common stock solely to cover over-allotments, if any.
The underwriters are offering the shares for sale on a firm commitment basis. The underwriters expect to deliver the shares to purchasers on or about , 2021.
B. Riley Securities
The date of this prospectus is , 2021
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or in any free writing prospectus that we may provide to you in connection with this offering. Neither we nor any of the underwriters has authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus or in any such free writing prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We can provide no assurance as to the reliability of any other information that others may give you. Neither we nor any of the underwriters is making an offer to sell or seeking offers to buy these securities in any jurisdiction where or to any person to whom the offer or sale is not permitted. The information in this prospectus is accurate only as of the date on the front cover of this prospectus, and the information in any free writing prospectus that we may provide you in connection with this offering is accurate only as of the date of such free writing prospectus. Our business, financial condition, results of operations and prospects may have changed since those dates.
Trademarks
We own or have rights to various trademarks, service marks and trade names that we use in connection with the operation of our business. This prospectus may also contain trademarks, service marks and trade names of third parties, which are the property of their respective owners. Our use or display of third parties’ trademarks, service marks and trade names or products in this prospectus is not intended to, and does not imply a relationship with, or endorsement or sponsorship by us. Solely for convenience, the trademarks, service marks and trade names referred to in this prospectus may appear without the ®, TM or SM symbols, but the omission of such references is not intended to indicate, in any way, that we will assert, to the fullest extent under applicable law, our rights or the right of the applicable owner of these trademarks, service marks and trade names.
Market and Industry Data
Unless otherwise indicated, information contained in this prospectus concerning our industry, competitive position and the markets in which we operate is based on information from independent industry and research organizations, other third-party sources and management estimates. Management estimates are derived from publicly available information released by independent industry analysts and other third-party sources, as well as data from our internal research, and are based on assumptions we made upon reviewing such data, and our experience in, and knowledge of, such industry and markets, which we believe to be reasonable. In addition, projections, assumptions and estimates of the future performance of the industry in which we operate and our future performance are necessarily subject to uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
| i |
This summary highlights information contained in greater detail elsewhere in this prospectus and does not contain all of the information that you should consider before deciding to invest in our common stock. You should read the entire prospectus carefully, including the “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included in this prospectus, before making an investment decision. Some of the statements in this prospectus constitute forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.” Unless otherwise indicated in this prospectus, “Synergy CHC,” “we,” “us” and “our” refer to Synergy CHC Corp. and, where appropriate, its subsidiaries.
Our Company
We are a provider of consumer health care, beauty, and lifestyle products. Our current brand portfolio consists of two marquee brands, FOCUSfactor, a patented brain health supplement that has been clinically shown to improve memory, concentration and focus, and Flat Tummy, a lifestyle brand that provides a suite of nutritional products to help women achieve their weight management goals, and a developing brand, Hand MD, consisting of a full range of preventative, restorative, and anti-aging skin care products designed for the hands. During the year ended December 31, 2020, FOCUSfactor represented 62% of our net revenue, Flat Tummy was 17%, and Hand MD was 17%. During the six months ended June 30, 2021, FOCUSfactor represented 71% of our net revenue, Flat Tummy was 29%, and Hand MD was 0.2%. Our products are sold through some of the nation’s leading club, mass drug, and other retailers such as Costco, Amazon.com, Walmart, Walgreens, CVS, The Vitamin Shoppe, Target.com, H-E-B, and SuperValu.
We built our brand portfolio through strategic acquisitions. We acquired the FOCUSfactor brand in January 2015 for cash consideration of $6.0 million, including earnout. Our Hand MD brand was acquired for $1.5 million of our stock in August 2015. In November 2015, we acquired our second marquee brand – Flat Tummy – for AUD 10.0 million (or approximately $7.0 million), using a mix of cash and stock. Our capital structure following the acquisition of our key brands in 2015 was highly levered, and our focus was on paying our debt as we did not have the resources to grow our business.We have grown our FOCUSfactor brand from 2 SKUs at acquisition to 16 SKUs, and our Flat Tummy Brand from 1 SKU to 18 SKUs.
With the outbreak of the COVID-19 pandemic in North America during the first three months of 2020, we extended our Hand MD brand to produce and market a range of hand sanitizers. We deployed the cash flow from hand sanitizer sales for a television advertising strategy to drive growth in our flagship FOCUSfactor brand products. Our FOCUSfactor advertising campaigns have aired on major news and entertainment networks such as Fox News, CNN, MSNBC, TLC, and TNT, targeting adults 45 years of age and older. As a result, net revenue for the year ended December 31, 2020 was $40.2 million, an increase of $10.9 million, or 37.0%, over net revenue for the year ended December 31, 2019. Net revenue for the six months ended June 30, 2021 was $24.8 million, an increase of $6.9 million, or 38.3% over net revenue for the six months ended June 30, 2020. In particular, FOCUSfactor net revenue continued to benefit from our advertising strategy, increasing to $17.5 million for the six months ended June 30, 2021, a 124% increase over the same period in 2020.
Following the completion of this offering, we intend to use the proceeds from sales will be expended byto repay in full our outstanding debt, to accelerate the Company without escrow, minimums orgrowth of our current brands through advertising and otherwise, and to drive our acquisition strategy as we have a pipeline of potential near-term acquisition targets that we are eager to pursue. Given the success of our FOCUSfactor advertising campaigns – in which we have achieved approximately a $4 increase in gross revenue for each incremental $1 of advertising spend – we believe that we can meaningfully expand our FOCUSfactor campaigns. In addition, we have tailored strategies for our Flat Tummy and Hand MD brands to maximize our return on investment and leverage our experience with both traditional and digital marketing. Our asset-light business model, in which we partner with third-party manufacturers to produce our brand offerings, allows us to scale quickly and profitably while satisfying growing demand.
For the six months ended June 30, 2021, our net revenues, net income and Adjusted EBITDA were $24.8 million, $2.6 million and $3.5 million, respectively, representing an increase of 38.3%, 139.7%, and 13.4% over the same period in the prior fiscal year. During the year ended December 31, 2020, our net revenues, net income (loss) and Adjusted EBITDA were $40.2 million, $1.4 million and $4.7 million, respectively, as compared to $29.4 million, $(9.2) million and $4.2 million for the prior year.
Our Brands
Our flagship brand, FOCUSfactor, is a brain health nutritional supplement with over 15 years of heritage and a patented formula comprised of a proprietary blend of key brain supporting ingredients along with vitamins, minerals, and other conditions.
Our second marquee brand, Flat Tummy, consists of a range of lifestyle products and accessories including tea, shakes, lollipops, supplements, apparel, and exercise accessories. We also provide a Flat Tummy mobile app, which as of June 30, 2021 had 1.3 million unique downloads and is intended as a tool to promote the Flat Tummy lifestyle centered around general wellness and health. Our Flat Tummy brand consists of 18 SKUs and is sold direct to consumer through the Flat Tummy website and application, as well as through retailers such prospective investor.as Amazon.com and CVS.
Our Business
We were incorporated on December 29, 2010also own five additional, non-core brands. While we may elect to promote these brands and commercialize their products in the Statefuture, we have prioritized our key FOCUSfactor, Flat Tummy, and Hand MD brands, and management is focused on the growth of Nevada. Wethese core products.
Our net revenues by brand for the six months ended June 30, 2021 are an exploration stage corporation. An exploration stage corporationbelow:
Net Revenue by Brand for the Six Months Ended June 30, 2021(1)
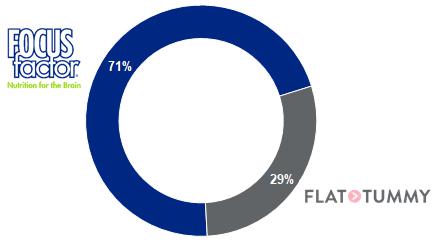
| (1) | Hand MD represented 0.2% of our net revenue for the six months ended June 30, 2021. |
In the United States, the U.S. Food and Drug Administration (the “FDA”) has regulatory oversight over our FOCUSfactor, Flat Tummy and Hand MD products. However, no formal FDA approval or registration is one engagedrequired because our products are classified as dietary supplements (all FOCUSfactor products and some Flat Tummy products), foods (some Flat Tummy products) or cosmetics (all Hand MD products except hand sanitizer, which must follow the OTC monograph). Hand MD hand sanitizer, provided it follows the conditions, uses, doses, labeling and testing in the search for mineral deposits or reservesOTC monograph, is generally recognized as safe and effective and can be marketed without FDA approval.
In Canada, Health Canada (“HC”) has oversight over our FOCUSfactor, Flat Tummy and Hand MD products. Our FOCUSfactor and Flat Tummy products are considered natural health products by HC so they each have a natural product number that was assigned by HC upon its review and approval. Hand MD products are considered cosmetics by HC, so notification filings are required which are not in eithersubject to review and approval, with the development or production stage. We intend to conduct exploration activities on the Shipman Diamond Projectexception of hand sanitizer, which is located 50 kilometers northeast of Prince Albert, Saskatchewan, Canadaconsidered a natural health product and 2 kilometers north ofhas been assigned a natural product number by HC. This natural product number represents the village of Shipman. Oro Capital Corporation has acquired a 100% interestproduct license that enables us to distribute hand sanitizer in Canada.
In the United Kingdom, both FOCUSfactor and Flat Tummy are considered food supplements that are regulated by the Food Standards Agency. There is no requirement for licensing or registering food supplement products in the Project. United Kingdom, and products must comply with relevant food law. Hand MD products are not currently sold in the United Kingdom.
In Australia, FOCUSfactor products are “Listed Medicines” that are regulated by the Therapeutic Goods Administration (“TGA”) and require an AUST L (Australia Listed Medicine) number. Flat Tummy products are classified as either Listed Medicines or meal replacements. Listed Medicines are regulated by the TGA while meal replacements are regulated under the Australia New Zealand Food Standards Code. Hand MD products are not currently sold in Australia.
Our Competitive Strengths
We intend to explore for diamond-bearing kimberlite on the property
Well-Positioned in Growing Categories Driven by Favorable Consumer Trends
An increased focus on health, beauty and wellness by consumers has served as a tailwind for our brands. The nutritional supplement market has experienced significant growth across a range of which we paid $6,250 for office rent, $6,250 for consulting fees,areas including immune health, brain health, heart health, sleep/stress, and $12,380 for legaloverall nutrition and accounting fees. We expect to incur additional expenses of approximately $10,000wellness as a result of becomingan aging population, increased obesity, pandemic concerns and a public company. Thesedesire for more natural solutions and treatments over prescription medication. Additionally, there is an increased expenses will bedemand on hand cleansing and hand sanitizing products due to the COVID-19 pandemic, which is also driving demand for hand moisturizing products to soothe dried, chapped hands due to frequent hand washing and sanitizing. We believe that we are well positioned to benefit from these favorable trends. Our FOCUSfactor, Flat Tummy and Hand MD brands have seen strong growth with net revenues up 38% year-over-year in the six months ended June 30, 2021. We believe our focus on lifestyle products has also benefited from the growth and prevalence of social media.
Clinically Shown Results Backed by Independent Study for FOCUSfactor
We believe FOCUSfactor is the only product in its category with both a patent and a clinical study to support the product claims for improved memory, concentration, and focus. FOCUSfactor has been tested in a single-center, randomized, double-blind, placebo-controlled, parallel group study to evaluate its effect on memory, concentration, and focus in healthy adults.
In this study, FOCUSfactor was tested on its entire 52-ingredient formulation rather than testing one or two ingredients within a formulation. FOCUSfactor was shown to provide a 44% increase in recall memory after six weeks of use versus placebo. This differentiates FOCUSfactor from other brain-health supplements and is a prime reason why FOCUSfactor has been placed in premier retailers.See “Business — FOCUSfactor Clinical Study” for additional information.
Experienced Management Team with Proven Track Record of Value Creation
Our executive team has a combined 90 years of experience in consumer marketing and distribution and has been instrumental in acquiring and building our core brands. Management has exercised strong financial discipline in its acquisition strategy, with a focus on acquiring brands at attractive valuations. For example, we acquired FOCUSfactor for approximately 3x trailing EBITDA. For the six months ended June 30, 2021, the year ended December 31, 2020 and the year ended December 31, 2019, FOCUSfactor generated net revenue of $17.5 million, $24.9 million and $18.9 million, respectively. Management’s philosophy is to acquire promising brands that fit within our health, beauty and lifestyle offerings, and apply our proven marketing and distribution strategies to develop brands to their full potential. We believe we are adept at identifying promising opportunities that build out and complement our core brand portfolio.
Premier Retail Partners
Our premier retail partners include Costco, Walmart, Amazon.com, Walgreens, and CVS. We sell products to these partners under their standard arrangements, which do not include a term or duration as sales under each vendor agreement are generally made on a purchase order basis, and do not include any termination provisions. Our partners provide a platform to expand the breadth of our current offerings through product line extensions and new product innovation. We continue to introduce new SKUs to our current retail partners, such as the addition of FOCUSfactor Gummies to our membership club channel. Additionally, the international footprint of certain of our various retail partners facilitates our geographic expansion plans.
Scalable and Flexible Asset-Light Model to Support Growth
Our focus is on brand management, marketing, product development and distribution, and we utilize contract manufacturing partners in order to produce our various brand offerings. The use of third-party manufacturing partners allows us to scale quickly, as we ensure that our partners have sufficient capacity to meet our demand needs. We also maintain multiple relationships with different contract manufactures, ensuring diversification of our manufacturing base and reducing the likelihood of supply bottlenecks or deficits that could potentially slow our growth.
Our Growth Strategy
We intend to drive growth and increased profitability in our business through these key elements of our strategy:
Broaden Media Advertising Strategy
We have experienced a significant acceleration in sales growth for the FOCUSfactor brand as a result of increased audit, legalour television advertising. We launched a national advertising campaign in August 2020, which has aired on major news and Edgar fees.
Acquire Brands which Complement Our Existing Portfolio
We will continue to evaluate acquisition opportunities that we believe fit well within our brand portfolio and create value for our stockholders, such as further retail expansion in nutraceuticals and market expansion in health and beauty. In spite of historical capital constraints, our opportunistic approach to acquisitions has resulted in a successful track record of identifying promising targets that align with our overall brand strategy in the health, beauty and lifestyle segments. With the proceeds from this offering, we expect to accelerate our acquisition strategy, focusing on acquisition targets that management believes have the greatest synergistic potential, enabling us to significantly grow our product offerings and reach.
Partner with Additional Leading Retailers to Expand the Reach of Our Products
We have established distribution relationships with premier retail partners, including Costco, Walmart, Amazon.com, Walgreens, CVS, The Vitamin Shoppe, Target.com, H-E-B and SuperValu. Based on the success of our products with these leading retail partners, we believe that we are well positioned to add new retailers that will enhance our distribution footprint. We believe we have expansion opportunities with food retailers, including those focused on health foods. We intend to introduce seven new SKUs across three potential retailers, which would potentially result in the addition of approximately 50,000 retail doors.
Diversify Our Geographic Presence through Entry into New Markets
We seek to accelerate our sales growth by expanding and further diversifying our geographic footprint. In the year ended December 31, 2020, markets outside of North America represented 0.5% of our total net revenues. Our goal is to increase our net revenues generated from new markets. As we target new international markets, our strategy is to develop highly competitive and differentiated products that are produced in-country for ease of entry, with support from our regulatory group and an in-country regulatory consultant to help expedite the approval process. We currently plan to enter the United Kingdom and Australia markets in 2022, initially with FOCUSfactor, followed by Flat Tummy and Hand MD. We then plan to expand our brands into Asian markets and Mexico in late 2022 and 2023.
| 3 |
Use Innovative Strategies to Boost Consumer Engagement
We have made investments in promoting apps for Flat Tummy and view this as a key aspect of growing our customer base and maintaining high levels of engagement. We have also focused on developing our social media presence, in particular through Instagram, in order to foster and grow our relationship with customers. Our brands appeal to both specific consumer needs as well as lifestyle choices and we seek to deepen our understanding of our customers and boost recognition of our brands through increased engagement.
Continue to Develop and Expand Our Current Brands
Our plan is to further develop and expand our brands by reaching a broader set of customers through advertising and product expansion. More specifically, we look to develop new products for our brands to satisfy the various customer segment opportunities (i.e. baby boomers, millennials, etc.) and satisfy various consumer needs as they relate to new and improved formulations, expanded and improved product benefits, alternative delivery formats and sizes. As we increase the product line-up behind our brands, we leverage our current retail distribution network by expanding our presence as well as adding incremental distribution with new retail partners. With a broader brand presence, we believe our advertising becomes even more efficient at driving sales velocity.
This is evidenced by our expanded FOCUSfactor product line, including gummies that are marketed to both adults and children and liquid energy and focus shots for a younger adult audience. Beginning in 2022, we plan to introduce additional FOCUSfactor products, including a “maximum strength” formula, an “ultimate” formula, ready-to-drink beverages and a nootropic line. The Flat Tummy brand has strategically added complementary products such as new shake options and supplements to appeal to a broader consumer group. In the fourth quarter of 2021, we plan to introduce a Flat Tummy superfruits gummy and an ashwagandha/calming gummy. Beginning in 2022, we plan to introduce additional Flat Tummy products, including three new protein shakes, a protein ready-to-drink beverage, hydration powder and preworkout powder. Additionally, we plan to employ this strategy of expanding our brands into international markets that include the United Kingdom, Australia and Asia, among others.
Marketing and Sales
Our targeted, consumer-driven marketing strategy has been key to building our brands and driving revenue growth. We manage dedicated marketing strategies for each of our brands in order to build deep connections with our customers.
FOCUSfactor. Our marketing strategy for FOCUSfactor is primarily focused on television advertising campaigns with leading national networks that appeal to the demographics of our wellness focused customer base. Our television advertising campaigns, launched in 2020, have coincided with strong sales results for the brand, with FOCUSfactor net revenue increasing 124% in the six months ended June 30, 2021 compared with the same period in 2020. In the year ended December 31, 2020, FOCUSfactor net revenue increased 38.1% year-over-year, to $24.9 million. As our flagship brand, FOCUSfactor accounted for 71% of our net revenue in the six months ended June 30, 2021, compared with 44% in the six months ended June 30, 2020, 62% in the year ended December 31, 2020 and 64% in the year ended December 31, 2019.
Flat Tummy. We employ a primarily online and social media driven strategy for our Flat Tummy brand. The brand is focused primarily on women. We employ campaigns to reach our core target segments through a mix of traditional online advertising as well as influencer-based marketing. In the six months ended June 30, 2021, Flat Tummy accounted for 29% of our net revenue, compared with 25% in the six months ended June 30, 2020, 17% in the year ended December 31, 2020 and 34% in the year ended December 31, 2019.
Hand MD. We mainly rely on social media and online advertising for our Hand MD brand. In the six months ended June 30, 2021, Hand MD accounted for 0.2% of our net revenue, compared with 28% in the six months ended June 30, 2020, 17% in the year ended December 31, 2020 and 1% for the year ended December 31, 2019.
Competition
The U.S. nutritional supplements retail industry is a large and highly fragmented industry with few barriers to entry. We compete against other specialty retailers, mass merchants, multi-level marketing organizations, mail-order and direct-to-consumer companies, and e-commerce companies. This market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the property,market. Certain of our competitors may have significantly greater financial, technical and marketing resources than we willdo, and may be able to adapt to changes in consumer preferences more quickly, devote greater resources to the marketing and sale of their products, or generate greater brand recognition. In addition, our competitors may be more effective and efficient in introducing new products.
Recent Developments
Certain Preliminary Estimated Financial Data
The following presents preliminary estimates of certain of our consolidated financial data for the three months ended September 30, 2021. Our consolidated financial statements as of and for the three months ended September 30, 2021 are not yet available and are subject to completion of our financial closing procedures. The following information reflects our preliminary estimates based on currently available information and is subject to change. We have to find alternative sources, likeprovided ranges, rather than specific amounts, for the preliminary estimated results for net income, EBITDA (a non-GAAP financial measure) and Adjusted EBITDA (a non-GAAP financial measure) described below primarily because we are still in the process of finalizing our financial and operating results as of and for the three months ended September 30, 2021 and, as a second public offering, a private placementresult, our final reported results may vary from the preliminary estimates. The preliminary estimated financial data set forth below have been prepared by, and are the responsibility of, securities, or loans from our officers or others. At the present time, wemanagement. Our auditors have not made any arrangements to raise additional cash, other than through this offering. If we need additional cash and can't raise it we will either have to suspend operations until we do raise the cash,audited, reviewed, compiled or cease operations entirely. Other than as described in this paragraph, we have no other financing plans.
As of January 31, 2013 (unaudited) | As of July 31, 2012 (audited) | |||||||
| Balance Sheet | ||||||||
| Total Assets | $ | - | 4,000 | |||||
| Total Liabilities | $ | 19,205 | 18,000 | |||||
| Stockholders’ Deficit | $ | (19,205 | ) | (14,000 | ) | |||
Three Months Ended September 30, 2021 | ||||||||
| (Unaudited; in thousands of dollars) | Low (Estimated) | High (Estimated) | ||||||
| $ | $ | |||||||
| EBITDA* | $ | $ | ||||||
| Adjusted EBITDA* | $ | $ | ||||||
| * | A non-GAAP financial measure. See reconciliation to net income below. |
The below table reconciles expected net income to expected EBITDA and expected Adjusted EBITDA for the three months ended September 30, 2021.
Three Months Ended September 30, 2021 | ||||||||
| (Unaudited; in thousands of dollars) | Low (Estimated) | High (Estimated) | ||||||
| $ | | |||||||
| $ | | |||||||
| Interest expense | ||||||||
| Taxes | ||||||||
| Depreciation and amortization | ||||||||
| EBITDA | $ | $ | ||||||
| Impairment of intangible assets | ||||||||
| Stock-based compensation | ||||||||
| Non-recurring expenses | ||||||||
| Bad debts | ||||||||
| Obsolete inventory | ||||||||
| Loss on foreign currency translation and transaction | ||||||||
| Adjusted EBITDA | $ | $ | ||||||
| (1) | ||
| (2) | ||
| (3) | ||
| (4) |
Summary Risk Factors
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. These risks are discussed more fully in the section titled “Risk Factors” following this prospectus summary. These risks include, but are not limited to, the following:
| ● | We operate in a highly competitive industry and our failure to compete effectively could materially and adversely affect our sales and growth prospects; | |
| ● | Our failure to appropriately respond to changing consumer preferences and demand for new products or product enhancements could significantly harm our relationship with customers and our product sales, as well as our financial condition and operating results; | |
| ● | Our sales growth is dependent upon maintaining our relationships with a small number of existing large customers, and the loss of any one such customer could materially adversely affect our business and financial performance; |
| ● | If our outside suppliers and manufacturers fail to supply products in sufficient quantities and in a timely fashion, our business could suffer; | |
| ● | The COVID-19 pandemic and associated responses could materially adversely affect our business, operating results, financial condition and prospects; | |
| ● | Adverse or negative publicity could cause our business to suffer; | |
| ● | We continue to explore new strategic initiatives, but we may not be able to successfully execute on, or realize the expected benefits from, the implementation of our strategic initiatives, and our pursuit of new strategic initiatives may pose significant costs and risks; | |
| ● | The nutritional supplement industry increasingly relies on intellectual property rights and although we seek to ensure that we do not infringe the intellectual property rights of others, there can be no assurance that third parties will not assert intellectual property infringement claims against us; | |
| ● | We plan to expand into additional international markets, which will expose us to significant operational risks; | |
| ● | We may experience product recalls, withdrawals or seizures, which could materially and adversely affect our business, financial condition and results of operations; | |
| ● | We and our suppliers are subject to numerous laws and regulations that apply to the manufacturing and sale of nutritional supplements, and compliance with these laws and regulations, as they currently exist or as modified in the future, may increase our costs, limit or eliminate our ability to sell certain products, subject us or our suppliers to the risk of enforcement action or litigation, or otherwise adversely affect our business, results of operations and financial condition; and | |
| ● | The other factors described in “Risk Factors.” |
Our Corporate Information
We were organized as a corporation under the laws of the State of Nevada on December 29, 2010 under the name “Oro Capital Corporation.” In April 2014, Synergy Strips Corp., a Delaware corporation, became our wholly-owned subsidiary, and we changed our name from “Oro Capital Corporation” to “Synergy Strips Corp.” In August 2015, we changed our name to “Synergy CHC Corp.” In January 2019, our other U.S. subsidiaries, Neuragen Corp., Sneaky Vaunt Corp., The Queen Pegasus Corp. and Breakthrough Products Inc., merged with and into the Company. In July 2021, we acquired Hand MD Corp. as a wholly-owned subsidiary.
We were a public reporting company until July 17, 2020, the date on which we filed a Form 15 to voluntarily suspend our duty to file reports under Sections 13 and 15(d) of the Exchange Act. As a result of this offering, we will become subject again to the information and reporting requirements of the Exchange Act and we will file periodic reports, proxy statements and other information with the SEC.
The address of our principal executive offices is currently 865 Spring Street, Westbrook, Maine 04092 and our phone number is (615) 939-9004. Our website is www.synergychc.com. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus.
1-for-Reverse Stock Split
Prior to the effective date of the registration statement of which this prospectus is a part, we will effect a 1-for- reverse stock split with respect to our common stock. Unless we indicate otherwise or the context otherwise requires, all information in this prospectus gives effect to this reverse stock split.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in the Exchange Act. We may take advantage of certain of the scaled disclosures available to smaller reporting companies so long as the market value of our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
| We have granted the underwriters a 30-day option to purchase up to an additional shares of our common stock | ||
| Use of proceeds | We We intend to | |
| Risk | You should read the | |
| Proposed Nasdaq Capital Market symbol | SNYR | |
| Insider Participation | Certain of our officers, directors and stockholders, including , and certain of their respective affiliates, have indicated an interest in participating in this offering at the public offering price. We anticipate that such persons will purchase in the aggregate approximately shares of common stock offered hereby. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters could determine to sell fewer shares to them than they indicated an interest in purchasing or sell no shares to them, and they could determine to purchase fewer shares than they indicated an interest in purchasing or purchase no shares in this offering. |
As of October 8, 2021, 89,889,074 shares of our common stock were outstanding. Unless we indicate otherwise or the context otherwise requires, all information in this prospectus:
| ● | assumes no exercise by the underwriters of their over-allotment option; | |
| ● | excludes 3,466,667 shares of common stock issuable upon the exercise of outstanding options at a weighted exercise price of $0.54 per share; | |
| ● | gives effect to a 1-for- reverse stock split with respect to our common stock, which will occur prior to the effective date of the registration statement of which this prospectus | |
| ● | excludes 12,058,333 shares |
SUMMARY HISTORICAL CONSOLIDATED FINANCIAL AND OTHER DATA
The following tables set forth our summary historical consolidated financial data as of, and for the periods ended on, the dates indicated.
The summary consolidated statements of operations data for the years ended December 31, 2020 and 2019 are derived from our audited consolidated financial statements and notes that are included elsewhere in this prospectus.
The summary condensed consolidated statements of operations data for the six months ended June 30, 2021 and 2020 and the summary consolidated balance sheet data as of June 30, 2021 are derived from our unaudited interim condensed consolidated financial statements and notes that are included elsewhere in this prospectus. We have prepared the unaudited condensed consolidated financial statements in accordance with generally accepted accounting principles (GAAP) and on the same basis as the audited consolidated financial statements. Our historical results are not necessarily indicative of our results in any future period and results from our interim period may not necessarily be indicative of the results of the entire year.
| For the six months ended | For the six months ended | For the year ended | For the year ended | |||||||||||||
| June 30, 2021 | June 30, 2020 | December 31, 2020 | December 31, 2019 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Statement of operations data: | ||||||||||||||||
| Revenue | $ | 24,761,974 | $ | 17,906,767 | $ | 40,226,865 | $ | 29,357,546 | ||||||||
| Cost of sales (including related party purchases of $0, $0, $0 and $4,847,626, respectively) | 6,794,344 | 6,593,446 | 14,578,865 | 9,137,602 | ||||||||||||
| Gross profit | 17,967,630 | 11,313,321 | 25,648,000 | 20,219,944 | ||||||||||||
| Total operating expenses | 14,505,045 | 8,736,985 | 22,609,745 | 28,176,054 | ||||||||||||
| Income (loss) from operations | 3,462,585 | 2,576,336 | 3,038,255 | (7,956,110 | ) | |||||||||||
| Total other expenses | 717,619 | 1,345,990 | 1,627,123 | 1,119,800 | ||||||||||||
| Net income (loss) before income taxes | 2,744,966 | 1,230,346 | 1,411,132 | (9,075,910 | ) | |||||||||||
| Income tax expense | (104,537 | ) | (128,996 | ) | - | (131,537 | ) | |||||||||
| Net income (loss) after tax | $ | 2,640,429 | $ | 1,101,350 | $ | 1,411,132 | $ | (9,207,447 | ) | |||||||
| Net income (loss) per share – basic and diluted | $ | 0.03 | $ | 0.01 | $ | 0.02 | $ | (0.10 | ) | |||||||
| Weighted average common shares outstanding, basic and diluted | 89,889,044 | 89,889,044 | 89,889,044 | 89,883,194 | ||||||||||||
| Adjusted EBITDA | $ | 3,507,083 | $ | 3,093,940 | $ | 4,680,161 | $ | 4,205,741 | ||||||||
Non-GAAP Financial Measures
We currently focus on Adjusted EBITDA to evaluate our business relationships and our resulting operating performance and financial position. Adjusted EBITDA is defined as EBITDA (net income plus interest expense, income tax expense, depreciation and amortization), further adjusted to exclude certain non-cash expenses and other adjustments as set forth below. We present Adjusted EBITDA because we consider it an important measure of our performance and a meaningful financial metric in assessing our operating performance from period to period by excluding certain items that we believe are not representative of our core business, such as certain non-cash items and other adjustments. We believe that Adjusted EBITDA, viewed in addition to, and not in lieu of, our reported results in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”), provides useful information to investors.
Six Months Ended June 30, 2021 | Six Months Ended June 30, 2020 | Year Ended December 31, 2020 | Year Ended December 31, 2019 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Net income (loss) | $ | 2,640,429 | $ | 1,101,350 | $ | 1,411,132 | $ | (9,207,447 | ) | |||||||
| Interest income | (24 | ) | (79 | ) | (104 | ) | (414 | ) | ||||||||
| Interest expense | 627,883 | 497,992 | 1,186,034 | 981,105 | ||||||||||||
| Taxes | 104,537 | 128,996 | – | 131,537 | ||||||||||||
| Depreciation and amortization | – | 95,686 | 148,697 | 1,342,689 | ||||||||||||
| EBITDA | $ | 3,372,825 | $ | 1,823,945 | $ | 2,745,759 | $ | (6,752,530 | ) | |||||||
| Impairment of intangible assets | – | – | 47,002 | 9,715,137 | ||||||||||||
| Stock-based compensation | – | 77,358 | 128,929 | 201,155 | ||||||||||||
| Non-recurring expenses | 393,965 | (1) | 1,014,846 | (2) | 1,263,427 | (3) | 493,924 | (4) | ||||||||
| Bad debts | – | – | 74,068 | 283,972 | ||||||||||||
| Obsolete inventory | – | – | 527,737 | 257,111 | ||||||||||||
| Loss on foreign currency translation and transaction | (259,707 | ) | 177,791 | (106,761 | ) | 6,972 | ||||||||||
| Adjusted EBITDA | $ | 3,507,083 | $ | 3,093,940 | $ | 4,680,161 | $ | 4,205,741 | ||||||||
| (1) | Consists of acquisition expenses with customers for shelf placement of $393,965. | |
| (2) | Consists of loan fees of $1,000,000 and acquisition expenses with customers for shelf placement of $14,846. | |
| (3) | Consists of loan fees of $1,000,000 and related financing costs of $61,000, leasehold renovations of $174,215 and acquisition expenses with customers for shelf placement of $28,212. | |
| (4) | Consists of acquisition expenses with customers for shelf placement of $261,801, customer return of $215,189 and inventory write off of $16,934. |
EBITDA and Adjusted EBITDA are considered non-GAAP financial measures. EBITDA represents earnings before interest, taxes, depreciation and amortization. Adjusted EBITDA represents EBITDA further adjusted to exclude the impact of higher-than-normal revenue change order activity and certain expenses and transactions that we believe are not representative of our core operating results; stock-based compensation; non-recurring expenses for acquisitions, such as one-time expenses with customers for shelf placement, and other fees and costs; and loss on foreign currency translation and transaction. Our definitions of EBITDA and Adjusted EBITDA might not be comparable to similarly titled measures reported by other companies.
June 30, 2021 | December 31, 2020 | December 31, 2019 | ||||||||||
| (Unaudited) | ||||||||||||
| Balance sheet data: | ||||||||||||
| Current assets | $ | 15,921,408 | $ | 12,587,089 | $ | 5,047,095 | ||||||
| Total assets | 15,921,408 | 12,587,089 | 5,190,629 | |||||||||
| Current liabilities | 15,717,469 | 14,739,196 | 10,147,064 | |||||||||
| Total liabilities | 17,258,150 | 16,271,192 | 10,393,980 | |||||||||
| Total stockholders’ deficit | (1,336,742 | ) | (3,684,103 | ) | (5,203,351 | ) | ||||||
| Total liabilities and stockholders’ deficit | $ | 15,921,408 | $ | 12,587,089 | $ | 5,190,629 | ||||||
| 7 |
An investment in our common stock involves a high degree of risk. You should carefully consider the following risks and all shares.
Risks Related to investOur Business, Strategy and Industry
We operate in a highly competitive industry and our common stock.failure to compete effectively could materially and adversely affect our sales and growth prospects.
The U.S. nutritional supplements retail industry is a large and highly fragmented industry with few barriers to entry. We discuss allcompete against other specialty retailers, mass merchants, multi-level marketing organizations, mail-order and direct-to-consumer companies, and e-commerce companies. This market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. As certain products become more mainstream, with broader distribution, we experience increased competition for those products. Increased competition from companies that distribute through retail, e-commerce or wholesale channels could have a material risks in the risk factors.
| market share. Our |
Our business is subject to changing consumer trends and preferences, including rapid and frequent changes in a default under the September 2011 agreement. While the agreement does not specify remediesdemand for default, Mr. Bain would have all of the remedies allowable under Canadian lawproducts, new product introductions and investorsenhancements. Our failure to accurately predict these trends could expect to lose their entire investment.
| accurately anticipate consumer needs; | ||
| ● | innovate and | |
| ● | successfully commercialize new products or product enhancements in | |
| ● | price our products competitively; | |
| ● | manufacture and | |
| ● | differentiate our |
If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could be rendered obsolete, which could negatively impact our revenues, financial condition, and operating results.
We have only one officer and director. he lacks formal training in financial accounting and management; however, hedepend on a small number of large retailers for a significant portion of our sales. Our sales growth is responsible fordependent upon maintaining our managerial and organizational structure which will include preparation of disclosure and accounting controls under the Sarbanes Oxley Act of 2002. When the disclosure and accounting controls referred to above are implemented, he will be responsible for the administration of them. Should he not have sufficient experience, he may be incapable of creating and implementing the controls which may cause us to be subject to sanctions and fines by the Securities and Exchange Commission, which ultimately could cause you to lose your investment.
Certain retailers make up a significant percentage of our products’ retail volume. For the year ended December 31, 2020, our top two customers, Costco and Walmart, accounted for 47% of our net revenue. We sell products to each of Costco and Walmart under their standard vendor agreements. These vendor agreements do not include a term or duration as sales under each vendor agreement are generally made on a purchase order basis, and do not include any termination provisions. The loss of sales of any of our products in a major retailer, or the Securities Actreduction of 1934, we may have to hire additional experienced personnel to assist us withpurchasing levels or the preparation thereof. The hiringcancellation of additional experienced personnel will result in additional expenses whichany business from a major retailer, could have a material adverse effect on our business results of operations and financial condition.
| 8 |
If our outside suppliers and manufacturers fail to supply products in sufficient quantities and in a timely fashion, our business could suffer.
Contract manufacturers produce all of our directorsproducts. Our contract manufacturers acquire all of the raw materials for manufacturing our products from third-party suppliers. We also depend on outside suppliers for the packaging materials for our products. In the event we were to lose any significant suppliers or contract manufacturers and thereby influencehave trouble in finding or directtransitioning to alternative suppliers or manufacturers, it could result in product shortages or product back orders, which could harm our policies. Although investorsbusiness. There can be no assurance that suppliers will be able to provide our contract manufacturers the raw materials in this offering will have paidthe quantities and at the appropriate level of quality that we request or at a significantly higher price for their shares, they will have littlethat we are willing to pay. We are also subject to delays caused by any interruption in the production of these materials including weather, disease, crop conditions, climate change, transportation interruptions and natural disasters or other catastrophic events. Our profit margins and timely product delivery are dependent upon the ability of our suppliers and contract manufacturers to supply us with products in a timely and cost-efficient manner. Our ability to enter new markets and sustain satisfactory levels of sales in each market depends on the ability of our suppliers and contract manufacturers to provide required levels of ingredients and products and to comply with all applicable regulations. The failure of our outside suppliers or manufacturers to supply ingredients or produce our products could materially adversely affect the decisionsour business operations. We believe we have dependable suppliers for all of management.
A downturn in the United States and elsewhere have been experiencing extreme volatility and disruption for more than 12 months, due in part to the financial stresses affecting the liquidity of the banking system and the financial markets generally. In 2009, this volatility and disruption has reached unprecedented levels. The consequences of a potential or prolonged recession may include a lower level of economic activity and uncertainty regarding mineral prices, the costs of operations, the cost of capital and commodity markets. While the ultimate outcome and impact of the current economic conditions cannot be predicted, a lower level of economic activity might result in a decline in energy consumption, which may adversely affect the price and market for diamonds, liquidity and future growth. Instability in the financial markets,economy, including as a result of recession or otherwise, also mayCOVID-19, could affect the costconsumer purchases of capital and our ability to raise capital.
We offer a buyerbroad selection of health and negotiate your own sale. Further, resaleswellness products. A downturn in the United States may require compliance with some state securities laws. There is no assurance that such compliance can be obtained or maintained. The Company does not presently plan to file with any state securities regulators.
Adverse or negative publicity could cause our business to suffer.
Our business depends, in part, on the public’s perception of our integrity and the safety and quality of our products. Any adverse publicity could negatively affect the public’s perception about our industry, our products, or our reputation and could result in a significant decline in our operations. Specifically, we are susceptible to adverse or negative publicity regarding:
| ● | the nutritional supplements industry; | |
| ● | skeptical consumers; | |
| ● | competitors; | |
| ● | the safety and quality of our products and/or our ingredients; | |
| ● | any recalls or adverse health consequences of our competitors’ products; | |
| ● | regulatory investigations of our products or our competitors’ products; and | |
| ● | scandals or regulatory investigations regarding the business practices or products of our competitors. |
We continue to explore new strategic initiatives, but we may not be able to successfully execute on, or realize the expected benefits from, the implementation of our strategic initiatives, and our pursuit of new strategic initiatives may pose significant costs and risks.
Our strategic initiatives are focused on, among other things, new product acquisition, new customer acquisition, improving the customer experience through the roll-out of initiatives including increasing customer engagement and personalization, improving the omni-channel experience (including in stores as well as through the internet and mobile devices), providing a relevant and inspiring product assortment and improving customer loyalty and retention. We also continually evaluate acquisition opportunities that we believe fit well within our brand portfolio and create value for our stockholders. Our future operating results are dependent, in part, on our management’s success in implementing these and other strategic initiatives, and as a result could divert management’s attention from our existing business as management focuses on developing these initiatives and related operations. Also, our short-term operating results could be unfavorably impacted by the opportunity and financial costs associated with the implementation of our strategic plans or the completion of any acquisitions, and we might not realize the benefits from such strategies. In addition, we may not be successful in achieving the intended objectives of the strategic initiatives (including acquisitions) in a timely manner or at all. We may choose to fund any acquisitions by way of (i) debt, which would subject us to additional covenant obligations and liquidity constraints, (ii) cash, which could divert working capital away from our existing business, or (iii) equity, which would result in dilution for existing stockholders, or any combination of the foregoing. There can also be no guarantee that we will be able to obtain debt on favorable terms, or at all.
As has been the case with our historical acquisition transactions, future business combinations could involve the acquisition of significant tangible and intangible assets, which could require us to record ongoing amortization expense with respect to identified intangible assets acquired. In addition, we may need to record write-downs from future impairments of identified tangible and intangible assets and goodwill. These and other similar accounting charges would reduce any future earnings or increase any losses. In future acquisitions, we could also incur debt to pay for acquisitions or issue additional equity securities as consideration, either of which could cause our stockholders to suffer significant dilution. Additionally, our ability to utilize net operating loss carryforwards, if any, acquired in any acquisitions may be significantly limited or unusable by us under “Risk Factors”Section 382 or other sections of the Internal Revenue Code (as has been the case with our net operating loss carryforwards attributable to the acquisition of Breakthrough Products, Inc.).
The nutritional supplement industry increasingly relies on intellectual property rights and matters described in this Prospectus generally. In lightalthough we seek to ensure that we do not infringe the intellectual property rights of these risks and uncertainties,others, there can be no assurance that the forward-looking statements containedthird parties will not assert intellectual property infringement claims against us, which claims may result in this Prospectus will in fact occur.
Recently it has become more and more common for suppliers and competitors to apply for patents or develop proprietary technologies and processes. We seek to ensure that we will be able to keep up with industry techniques and standards, that there will be no material adverse competitive or technological change in conditions in our business, that demand for our products will significantly increase, that our sole officer will remain employed as such, that our forecasts accurately anticipate market demand, and that there will be no material adverse change in our operations or business or in governmental regulations affecting us or our manufacturers and/or suppliers. The foregoing assumptions are based on judgments with respect to, among other things, future economic, competitive and market conditions, and future business decisions, alldo not infringe the intellectual property rights of which are difficult or impossible to predict accurately and many of which are beyond our control. Accordingly, although we believe that the assumptions underlying the forward-looking statements are reasonable, any such assumption could prove to be inaccurate and thereforeothers, but there can be no assurance that the results contemplated in forward-looking statementsthird parties will be realized. In addition, as disclosed elsewherenot assert intellectual property infringement claims against us. These developments could prevent us from offering or supplying competitive products or ingredients in the “Risk Factors” sectionmarketplace. They could also result in litigation or threatened litigation against us related to alleged or actual infringement of this prospectus, therethird-party rights. If an infringement claim is asserted or litigation is pursued, we may be required to obtain a license of rights, pay royalties on a retrospective or prospective basis or terminate our manufacturing and marketing of our products that are a numberalleged to have infringed. Litigation with respect to such matters could result in substantial costs and diversion of other risks inherent in our business and operations which could cause our operating results to vary markedly and adversely from prior results or the results contemplated by the forward-looking statements. Growth in absolute and relative amounts of cost of goods sold and selling, general and administrative expenses or the occurrence of extraordinary events could cause actual results to vary materially from the results contemplated by the forward-looking statements. Management decisions, including budgeting, are subjective in many respects and periodic revisions must be made to reflect actual conditions and business developments, the impact of which may cause us to alter marketing, capital investmentmanagement and other expenditures, which may also materially adversely affect our results of operations. In light of significant uncertainties inherent in the forward-looking information included in this prospectus, the inclusion of such information should not be regarded as a representation by us or any other person that our objectives or plans will be achieved.
We have numerous United States and foreign trademarks and service marks. There can be no assurance that the protection afforded by these trademarks and service marks will provide us with a competitive advantage or that we will be able to assert our intellectual property rights in infringement actions. We may be required to defend our intellectual property against such infringement, which could result in substantial costs and diversion of management and other resources. In addition, results of such litigation are difficult to predict and if we are not successful in defending our intellectual property rights, this could have a material adverse effect on our business, financial condition and results of operations.
If we are not able to adequately prevent disclosure of proprietary knowledge, the value of our products could be materially diminished.
Trade secrets are difficult to protect. We rely on trade secrets to protect our proprietary knowledge, especially where we do not believe patent protection is appropriate or obtainable, or where such patents would be difficult to enforce. We rely in part on confidentiality agreements to protect our trade secrets and other proprietary knowledge. We cannot guarantee that we have entered into such agreements with each party that may have had access to our proprietary knowledge, or that such agreements, even if in place, will not be circumvented. These agreements may not effectively prevent disclosure of proprietary knowledge and may not provide an adequate remedy in the event of unauthorized disclosure of such information. In addition, others may independently discover our trade secrets and proprietary knowledge, in which case we may have no right to prevent them from using such trade secrets or proprietary knowledge to compete with us. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could materially adversely affect our business, financial condition and results of operations.
International expansion will subject our business to additional economic and operational risks that could increase our costs and make it difficult to operate profitably.
One of our key growth strategies is to pursue international expansion. Expansion of our international operations may require significant expenditure of financial and management resources and result in increased administrative and compliance costs. As a result of such expansion, we will be increasingly subject to the risks inherent in conducting business internationally, including:
| ● | foreign currency fluctuations, which could result in reduced revenues and increased operating expenses; | |
| ● | longer or less predictable payment and sales cycles; | |
| ● | difficulty in collecting accounts receivable; | |
| ● | applicable foreign tax structures, including tax rates that may be higher than tax rates in the United States or taxes that may be duplicative of those imposed in the United States; | |
| ● | tariffs and trade barriers; | |
| ● | general economic and political conditions in each country; | |
| ● | inadequate intellectual property protection in foreign countries; | |
| ● | uncertainty regarding liability for information retrieved and replicated in foreign countries; | |
| ● | the difficulties and increased expenses of complying with a variety of foreign laws, regulations and trade standards; and | |
| ● | unexpected changes in regulatory requirements. |
As a result of these risks, we may be required to incur higher than expected costs to implement or we may not be able to achieve the expected benefits of our international strategy. If we are unsuccessful in this international expansion, we would be required to reevaluate our growth strategy, and we may have incurred substantial expenses and devoted significant management time and resources in pursuing international growth.
We may experience product recalls, withdrawals or seizures, which could materially and adversely affect our business, financial condition and results of operations.
We may be subject to product recalls, withdrawals or seizures if any of the products we sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution of those products. A significant recall, withdrawal or seizure of any of the products we manufacture or sell may require significant management attention, would likely result in substantial and unexpected costs and may materially and adversely affect our business, financial condition or results of operations. Furthermore, a recall, withdrawal or seizure of any of our products may adversely affect consumer confidence in our brands and thus decrease consumer demand for our products. As is common in the nutritional supplements industry, we rely on our contract manufacturers and suppliers to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our contract manufacturers and suppliers. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of those products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations.
Increases in the price or shortages of supply of key raw materials could materially and adversely affect our business, financial condition and results of operations.
Our products are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, it could result in a significant increase to us in the prices charged to us. Raw material prices may increase in the future and we may not be able to pass on those increases to customers who purchase our products. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our business, financial condition and results of operations.
We are subject to credit risk.
We are exposed to credit risk primarily on our accounts receivable. We provide credit to our customers in the ordinary course of our business and perform ongoing credit evaluations. While we believe that our exposure to concentrations of credit risk with respect to accounts receivable is mitigated by our large retail partner base, and we make allowances for doubtful accounts, we nevertheless run the risk of our customers not being able to meet their payment obligations, particularly in a future economic downturn. For instance, in 2019, payments from one of our customers were delayed, and in 2020, one of our customers entered Chapter 11 bankruptcy. If a material number of our customers were not able to meet their payment obligations, our results of operations could be harmed.
Natural disasters and unusually adverse weather conditions could cause permanent or temporary damage to our distribution centers, impair our ability to purchase, receive or replenish inventory or cause customer traffic to decline, all of which could result in lost sales and otherwise materially and adversely affect our results of operations.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods, earthquakes, tornadoes, high winds and other severe weather, could materially and adversely affect our operations and results of operations. To the extent these events result in the suspension of shipping by our distributors, closure of our corporate headquarters, or a significant number of the stores in which our products are sold, or to the extent they adversely affect one or more of our key suppliers, our operations and results of operations could be materially and adversely affected through an inability to make deliveries to stores and through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from suppliers, delay in the delivery of goods to our distribution centers or stores, the temporary reduction in the availability of products in our stores and disruption to our information systems, as noted above. These events also could have indirect consequences, such as increases in the cost of insurance, if they were to result in significant loss of property or other insurable damage.
Our e-commerce business is dependent on certain third parties. Changes in business practices or terms by such third parties could have a material adverse effect on our results of operations.
Our e-commerce business has several third-party relationships that contribute to our ability to generate revenue from a variety of online sources. These relationships may be dependent upon third-party tools, such as search engines, established business terms negotiated by us, or utilization of third-party marketplaces. If the economics of these relationships or the use of the third-party tools used to drive revenue change materially, this could affect our decision to maintain these relationships, and could result in lost sales and otherwise materially and adversely affect our financial performance.
If we do not successfully develop and maintain a relevant omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.
Omni-channel retailing is rapidly evolving, and we must keep pace with changing customer expectations and new developments by our competitors. Our customers are increasingly using computers, tablets, mobile phones, and other devices to shop online. As part of our omni-channel strategy, we have made and will continue to make technology investments to expand our online distribution. If we are unable to make, improve, or develop relevant customer-facing technology in a timely manner, our ability to compete and our business and results of operations could be materially and adversely affected. In addition, if our e-commerce businesses or our other customer-facing technology systems do not function as designed, we may experience a loss of customer confidence, lost sales, or data security breaches, any of which could materially and adversely affect our business and results of operations.
Our principal stockholders have the ability to significantly influence or control matters requiring a stockholder vote and other stockholders may not have the ability to influence corporate transactions.
Currently, our principal stockholders beneficially own approximately 77% of our outstanding common stock, and following this offering, assuming the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, will beneficially own approximately % of our outstanding common stock. As a result, they have the ability to determine the outcome on all matters requiring approval of our stockholders, including the election of directors and approval of significant corporate transactions.
We are highly dependent on our management team, and the loss of our senior executive officers or other key employees could harm our ability to implement our strategies, impair our relationships with customers and adversely affect our business, results of operations and growth prospects.
Our success depends, in large degree, on the skills of our management team and our ability to retain, recruit and motivate key officers and employees. Our active senior executive leadership team, including Jack Ross, Brendan Horning and Alfred Baumeler, have significant experience, and their knowledge and relationships would be difficult to replace. Leadership changes will occur from time to time, and we cannot predict whether significant resignations will occur or whether we will be able to recruit additional qualified personnel. Competition for senior executives and skilled personnel in our industry is intense, which means the cost of hiring, paying incentives and retaining skilled personnel may continue to increase.
We need to continue to attract and retain key personnel and to recruit qualified individuals to succeed existing key personnel to ensure the continued growth and successful operation of our business. In addition, we must attract and retain qualified personnel to continue to grow our business, and competition for such personnel can be intense. Our ability to effectively compete for senior executives and other qualified personnel by offering competitive compensation and benefit arrangements may be restricted by cash flow and other operational restraints. The loss of the services of any senior executive or other key personnel, or the inability to recruit and retain qualified personnel in the future, could have a material adverse effect on our business, financial condition or results of operations. In addition, to attract and retain personnel with appropriate skills and knowledge to support our business, we may offer a variety of benefits, which could reduce our earnings or have a material adverse effect on our business, financial condition or results of operations.
System security risks, data protection breaches, cyber-attacks and systems integration issues could disrupt our internal operations.
Experienced computer programmers and hackers may be able to penetrate our network security and misappropriate or compromise our confidential information or that of third parties, create system disruptions or cause shutdowns. Computer programmers and hackers also may be able to develop and deploy viruses, worms, and other malicious software programs that attack or otherwise exploit any security vulnerabilities of the products that we may sell in the future. Such disruptions could adversely impact our ability to fulfill orders and interrupt other processes. Delayed sales, lower profits, or lost customers resulting from these disruptions could adversely affect our financial results, stock price and reputation.
Legal and Regulatory Risks
Our products are subject to government regulation, both in the United States and abroad, which could increase our costs significantly and limit or prevent the sale of our products.
The manufacture, packaging, labeling, advertising, promotion, distribution and sale of our products are subject to regulation by numerous national and local governmental agencies in the United States and other countries. The primary regulatory bodies in the United States are the FDA and FTC, and we are also subject to similar regulatory bodies in all the countries in which we do business. Failure to comply with regulatory requirements may result in various types of penalties or fines. These include injunctions, product withdrawals, recalls, product seizures, fines and criminal prosecutions. Individual U.S. states also regulate nutritional supplements. A state may seek to interpret claims or products presumptively valid under federal law as illegal under that state’s regulations. For example, in February 2015, the New York Attorney General issued cease and desist letters to several national retailers regarding certain herbal supplements, and since that time both the New York Attorney General and other states’ Attorneys General have engaged in inquiries regarding the manufacture and sale of various supplements, and pursuant to such inquiries could seek to take actions against industry participants or amend applicable regulations in their state. In markets outside the United States, we are usually required to obtain approvals, licenses, or certifications from a country’s ministry of health or comparable agency, as well as labeling and packaging regulations, all of which vary from country to country. Approvals or licensing may be conditioned on reformulation of products or may be unavailable with respect to certain products or product ingredients. Any of these government agencies, as well as legislative bodies, can change existing regulations, or impose new ones, or could take aggressive measures, causing or contributing to a variety of negative consequences, including:
| ● | requirements for the reformulation of certain or all products to meet new standards; |
| ● | the recall or discontinuance of certain or all products; | |
| ● | additional record keeping; | |
| ● | expanded documentation of the properties of certain or all products; | |
| ● | expanded or different labeling; | |
| ● | adverse event tracking and reporting; and | |
| ● | additional scientific substantiation. |
Any or all of these requirements could have a material adverse effect on us. There can be no assurance that the regulatory environment in which we operate will not change or that such regulatory environment, or any specific action taken against us, will not result in a material adverse effect on us.
Congress and/or regulatory agencies may impose additional laws or regulations or change current laws or regulations, and state attorneys general may increase enforcement of existing or new laws, and compliance with new or changed governmental regulations, or any state attorney proceeding, could increase our costs significantly and materially and adversely affect our business, financial condition and results of operations.
From time to time, Congress, the FDA, the FTC, or other federal, state, local or foreign legislative and regulatory authorities may impose additional laws or regulations that apply to us, repeal laws or regulations that we consider favorable to us or impose more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations or to predict the effect that additional governmental regulation, when and if it occurs, would have on our business in the future. Those developments could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products (including products that we sell) not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or other new requirements. For example, in recent years, the FDA has issued warning letters to several cosmetic companies alleging improper claims regarding their cosmetic products. If the FDA determines that we have disseminated inappropriate drug claims for our products intended to be sold as cosmetics, we could receive a warning or untitled letter, be required to modify our product claims or take other actions to satisfy the FDA. Any developments of this nature could increase our costs significantly and could have a material adverse effect on our business, financial condition and results of operations.
Our failure to comply with regulations could result in substantial monetary penalties and could adversely affect our operating results.
The marketing and labeling of any food product in recent years has brought increased risk that consumers will bring class action lawsuits and that the FTC and/or state attorneys general will bring legal action concerning the truth and accuracy of the marketing and labeling of the product, seek removal of a product from the marketplace, and/or impose fines and penalties. Products that we sell carry express or implied statements relating to the ingredients or health and wellness related attributes of our products. For example, in May 2017, we were one of 45 brands that were warned by the FTC regarding influencer promotion of products on Instagram. The FTC warned the companies and influencers that any sponsored posts for a product must use clear language indicating that the post is a paid sponsorship. The lack of regulatory definition for many label statements has contributed to legal challenges against many supplements companies, and plaintiffs have commenced legal actions against several nutritional supplement companies, asserting false, misleading and deceptive advertising and labeling claims. As a result of such legal or regulatory challenges, consumers may avoid purchasing products from us or seek alternatives, even if the basis for the claim is unfounded.
The FTC has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. Failure by us to comply with applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
Even when unmerited, class claims, action by the FTC or state attorneys general enforcement actions can be expensive to defend and adversely affect our reputation with existing and potential customers and consumers and our corporate and brand image, which could have a material and adverse effect on our business, financial condition or results of operations. The number of private consumer class actions relating to false or deceptive advertising against nutritional supplement companies has increased in recent years. In addition, the FDA has aggressively enforced its regulations with respect to different types of product claims that may or may not be made for food products. These events could interrupt the marketing and sales of our products, severely damage our brand reputation and public image, increase our legal expenses, result in product recalls or litigation, and impede our ability to deliver our products in sufficient quantities or quality, which could result in a material adverse effect on our business, financial condition, results of operations and cash flows.
| 13 |
We may experience product liability claims and litigation to prosecute such claims, and although we maintain product liability insurance, which we believe to be adequate for our needs, there can be no assurance that our insurance coverage will be adequate or that we will be able to obtain adequate insurance coverage in the future. In addition, we may be subject to consumer fraud claims, including consumer class action claims regarding product labeling and advertising, and litigation to prosecute such claims; these claims are generally not covered by insurance.
We could face financial liability from product liability claims if the use of our products results in significant loss or injury. We can make no assurances that we will not be exposed to any future product liability claims. Such claims may include claims that our products contain contaminants or that we fail to provide or provide inadequate warnings concerning side effects or interactions of our products with other substances. Such claims may result in substantial settlement amounts or judgments against us, and may also require us to incur additional costs to change the packaging of our products to include adequate warning language or subject our products to additional testing. A product liability claim, regardless of its merit or ultimate outcome, could result in:
| ● | injury to our reputation; | |
| ● | decreased demand for our products; | |
| ● | diversion of management’s attention; | |
| ● | a change in the design, manufacturing process or the indications for which our marketed products may be used; | |
| ● | loss of revenue; and | |
| ● | an inability to commercialize product candidates. |
In addition, consumer fraud claims, including consumer class action claims regarding product labeling and advertising, are increasingly common as to food and dietary supplement products. If we face such claims, we may be forced to defend the action in the applicable courts. If such claims are found to be correct, this would have a material adverse effect on us and our reputation.
We carry insurance coverage in the types and amounts that we consider reasonably adequate to cover the risks we face from product liability claims. If insurance coverage is inadequate or unavailable or premium costs continue to rise, we may face additional claims not covered by insurance, and claims that exceed coverage limits or that are not covered could have a material adverse effect on us. Moreover, liability claims arising from a serious adverse event may in addition to increasing our costs through higher insurance premiums and deductibles, make it more difficult to secure adequate insurance coverage in the future. Because insurance is generally hard to obtain for such claims, these could have a material adverse effect on us.
We may experience Lanham Act claims by competitors, and litigation to prosecute such claims.
The Lanham Act empowers competitors to file suit regarding any promotional statements that the competitor believes to be false or misleading. If a competitor prevails, it could obtain monetary damages, including potentially treble damages and attorneys’ fees. A court can also order corrective advertising, or even a product recall if the offending claims are found on the product’s packaging and labeling. If we experience a Lanham Act claim filed against us, this could have a material adverse effect on us and on our products’ reputation.
If we fail to protect the integrity and security of customer-related and other confidential information, we could be exposed to litigation, increased costs and reputational damage, and our business, results of operations and financial condition could be materially and adversely affected.
The use of individually identifiable data by us, our customers, and others is regulated at the state, federal and international levels. Privacy and information security laws and regulations change from time to time, and there may not always be clear guidance from the respective governments and regulators regarding the interpretation of these laws and regulations, which may create the risk of an inadvertent violation. In addition, the increasing costs of compliance with those laws and regulations and related technology investments could materially and adversely affect our business and results of operations. Additionally, the success of our e-commerce operations depends upon the secure transmission of confidential information over public networks, including the use of cashless payments, and we use computers in substantially all other aspects of our business operations. Such uses give rise to cybersecurity risks, including security breach, espionage, system disruption, theft and inadvertent release of information. While we have taken significant steps to protect customers’ personal information, consumer preferences and credit card information, and other confidential information, including our employees’ private information and financial and strategic data about the Company and our business partners, our suppliers or others may undermine our security measures. As a result, unauthorized parties may obtain access to our data systems and misappropriate confidential data. Furthermore, because the methods used to obtain unauthorized access change frequently and may not be immediately detected, we may be unable to anticipate these methods or implement preventative measures, and our incident response efforts may not be entirely effective. Any preventative measures we implement may have the potential to negatively affect our relations with our customers or decrease activity on our websites or apps by making them less user-friendly. If our data security is compromised, it could have a material adverse effect on our reputation, results of operations and financial condition, materially increase the costs we incur to protect against those events in the future and subject us to additional legal risk and a competitive disadvantage and damage to our brand reputation. In addition, our customers could lose confidence in our ability to protect their personal information, which could cause them to stop using our websites or apps. We are reliant on third-party electronic payment systems and platforms, such as PayPal, Stripe, Amazon Pay, Afterpay and Shopify Payments, not only to protect the security of the information stored, but also to appropriately track and record data. Any failures or inadequacies in these third-party systems, even if unrelated to our business, could result in significant liability, could materially and adversely affect our reputation and business and could cause government agencies to enact additional regulatory requirements or to modify their enforcement or investigation activities.
| 14 |
Changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters could significantly affect our financial results.
U.S. generally accepted accounting principles and related pronouncements, implementation guidelines and interpretations with regard to a wide variety of matters that are relevant to our business, such as, but not limited to, revenue recognition, stock-based compensation, trade promotions, and income taxes are highly complex and involve many subjective assumptions, estimates and judgments by our management. Changes to these rules or their interpretation or changes in underlying assumptions, estimates or judgments by our management could significantly change our reported results.
Risks Related to this Offering and Ownership of Our Common Stock
An active, liquid trading market for our common stock does not currently exist and may not develop after this offering, and as a result, you may not be able to sell your common stock at or above the public offering price, or at all.
Prior to September 28, 2021, shares of our common stock were quoted on the OTC Markets Group, Inc. Pink tier under the symbol “SNYR.” Trading on the OTC Pink marketplace was infrequent and in limited volume. As a result of amendments to Exchange Act Rule 15c2-11, because we do not presently make current information publicly available, our common stock was shifted to the OTC Expert Market on September 28, 2021, which means that there are no longer publicly-available quotations of our common stock. Although we have applied to list our shares of common stock on Nasdaq in connection with this offering, an active trading market for shares of our common stock may never develop or be sustained following this offering. If an active trading market does not develop, you may have difficulty selling your shares of common stock at an attractive price, or at all. The public offering price for our common stock will be determined by negotiations between us and the representative of the underwriters and may not be indicative of prices that will prevail in the open market following this offering. Consequently, you may not be able to sell your common stock at or above the public offering price or at any other price or at the time that you would like to sell. An inactive market may also impair our ability to raise capital by selling our common stock and may impair our ability to expand our business by using our common stock as consideration in an acquisition.
You may be diluted by future issuances of common stock in connection with our incentive plans, acquisitions or otherwise; future sales of such shares in the public market, or the expectations that such sales may occur, could lower our stock price.
Our certificate of incorporation authorizes us to issue shares of our common stock and options, rights, warrants and appreciation rights relating to our common stock for the consideration and on the terms and conditions established by our Board of Directors (the “Board”) in its sole discretion. We could issue a significant number of shares of common stock in the future in connection with investments or acquisitions. Any of these issuances could dilute our existing stockholders, and such dilution could be significant. Moreover, such dilution could have a material adverse effect on the market price for the shares of our common stock.
A significant number of our total outstanding shares are restricted from immediate resale, but may be sold into the market in the near future. This could cause the market price of our common stock could declineto drop significantly, even if our business is doing well.
Subject to certain exceptions, without the prior written consent of B. Riley Securities, Inc., as representative of the underwriters, we, and you could lose allour officers and directors and our 1% and greater stockholders, during the period ending 180 days after the date of this prospectus, have agreed not to: (1) offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or partotherwise transfer or dispose of, your investment.
The market price of our common stock may decline significantly when the restrictions on resale by our existing stockholders lapse. A decline in the market price of our common stock might impede our ability to raise capital through the issuance of additional shares of common stock or other equity securities.
| 15 |
You will incur immediate dilution in the net tangible book value of the shares you purchase in this offering.
The public offering price of our common stock will be higher than the net tangible book value per share of outstanding common stock prior to completion of this offering. Based on our net tangible book value as of June 30, 2021 and upon the issuance and sale of unregistered securities referenced above, all transactions were exempt from registration pursuant to Regulation S promulgated undershares of common stock by us at the 1933 Act. In each instance, the purchaser had access to sufficient information regarding the Company so as to make an informed investment decision.
There are material limitations with respectmaking preliminary estimates of our financial results for the period ended September 30, 2021 prior to future eventsthe completion of our and our auditors’ financial performance. Forward-lookingreview procedures for such period.
The preliminary financial estimates contained in “Prospectus Summary — Recent Developments” are not a comprehensive statement of our financial results for the period ended September 30, 2021, and our auditors have not yet completed their review of such financial results. Our financial statements for the period ended September 30, 2021 will not be available until after this offering is completed and, consequently, will not be available to you prior to investing in this offering. Our actual financial results for the period ended September 30, 2021 may differ materially from the preliminary financial estimates we have provided as a result of the completion of our financial closing procedures, final adjustments, and other developments arising between now and the time that our financial results for such periods are often identified by words like: believe, expect, estimate, anticipate, intend, project and similar expressions, or words which, by their nature, refer to future events.finalized. Our preliminary estimated results also include non-GAAP financial measures. Neither such measures nor our estimates of GAAP results should be viewed as a substitute for interim financial statements prepared in accordance with GAAP. You should not place undue certaintyreliance on the preliminary estimates, and the preliminary estimates are not necessarily indicative of the results to be expected in the future. The preliminary financial data included herein has been prepared by, and are the responsibility of, management. RBSM LLP, our independent registered public accounting firm, has not audited, reviewed, compiled, or performed any procedures with respect to such preliminary estimates. Accordingly, RBSM LLP does not express an opinion or any other form of assurance with respect thereto.
We do not anticipate paying any cash dividends on our common stock in the foreseeable future.
We currently intend to retain our future earnings, if any, for the foreseeable future, to repay indebtedness and to fund the development and growth of our business. We do not intend to pay any dividends to holders of our common stock in the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of our Board taking into account various factors, including our business, operating results and financial condition, current and anticipated cash needs, plans for expansion, any legal or contractual limitations on our ability to pay dividends under our loan agreements or otherwise. As a result, if our Board does not declare and pay dividends, the capital appreciation in the price of our common stock, if any, will be your only source of gain on an investment in our common stock, and you may have to sell some or all of your common stock to generate cash flow from your investment.
If securities or industry analysts do not publish research or reports about our business, or if they downgrade their recommendations regarding our common stock, its trading price and volume could decline.
We expect the trading market for our common stock to be influenced by the research and reports that industry or securities analysts publish about us, our business or our industry. As a new public company, we do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our stock may be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline and our common stock to be less liquid. Moreover, if one or more of the analysts who cover us downgrades our stock or publishes inaccurate or unfavorable research about our business, or if our results of operations do not meet their expectations, our stock price could decline.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
We were a public reporting company until July 17, 2020, the date on which we filed a Form 15 to voluntarily suspend our duty to file reports under Sections 13 and 15(d) of the Exchange Act. At that time, because our common stock was not listed on a national securities exchange, we believed that the significant cost reductions associated with ceasing to be a reporting company would benefit us and our stockholders. After review, we felt that being on the over the counter market was not valuable due to the illiquidity and limited investor base. We now believe that listing our common stock on a national securities exchange, although accompanied by reporting requirements, is in our best interest because we expect such listing to improve the liquidity of our common stock, broaden the pool of investors that may be interested in investing in us, improve the attractiveness of using our stock as consideration for acquisitions and make our common stock a more attractive investment for institutional investors.
As a result of this offering, we will become subject to the reporting requirements of the Exchange Act, as amended, the Sarbanes-Oxley Act, the Dodd-Frank Act, and other applicable securities rules and regulations. Compliance with these rules and regulations involves significant legal and financial compliance costs, may make some activities more difficult, time-consuming or costly and may increase demand on our systems and resources. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, management’s attention may be diverted from other business concerns, which could adversely affect our business and operating results. We may need to hire more employees in the future or engage outside consultants, which will increase our costs and expenses.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us, and our business may be adversely affected.
Furthermore, although we are permitted to purchase and maintain insurance on behalf of our directors and officers, we do not currently do so and may have to expend significant funds to cover our commitments to indemnify our directors and officers. However, we are considering obtaining such insurance coverage with terms of coverage appropriate for a company of our size and nature.
We may be subject to additional regulatory burdens resulting from our public listing.
We are working with our legal, accounting and financial advisors to identify those areas in which changes should be made to our financial management control systems to manage our obligations as a public company listed on Nasdaq. These areas include corporate governance, corporate controls, disclosure controls and procedures and financial reporting and accounting systems. We have made, and will continue to make, changes in these and other areas, including our internal controls over financial reporting. However, we cannot assure holders of our common stock that these and other measures that we might take will be sufficient to allow us to satisfy our obligations as a public company listed on Nasdaq on a timely basis. In addition, compliance with reporting and other requirements applicable to public companies listed on Nasdaq will create additional costs for us and will require the time and attention of management. We cannot predict the amount of the additional costs that we might incur, the timing of such costs or the impact that management’s attention to these matters will have on our business.
Anti-takeover effects of certain provisions of Nevada state law hinder a potential takeover of us.
Though not now, we may be or in the future we may become subject to Nevada’s control share law. A corporation is subject to Nevada’s control share law if it has more than 200 stockholders, at least 100 of whom are stockholders of record and residents of Nevada, and it does business in Nevada or through an affiliated corporation. The law focuses on the acquisition of a “controlling interest,” which means the ownership of outstanding voting shares sufficient, but for the control share law, to enable the acquiring person to exercise the following proportions of the voting power of the corporation in the election of directors: (i) one-fifth or more but less than one-third; (ii) one-third or more but less than a majority; or (iii) a majority or more. The ability to exercise such voting power may be direct or indirect, as well as individual or in association with others.
The effect of the control share law is that the acquiring person, and those acting in association with it, obtains only such voting rights in the control shares as are conferred by a resolution of the stockholders of the corporation, approved at a special or annual meeting of stockholders. The control share law contemplates that voting rights will be considered only once by the other stockholders. Thus, there is no authority to strip voting rights from the control shares of an acquiring person once those rights have been approved. If the stockholders do not grant voting rights to the control shares acquired by an acquiring person, those shares do not become permanent non-voting shares. The acquiring person is free to sell its shares to others. If the buyers of those shares themselves do not acquire a controlling interest, their shares do not become governed by the control share law.
If control shares are accorded full voting rights and the acquiring person has acquired control shares with a majority or more of the voting power, any stockholder of record, other than an acquiring person, who has not voted in favor of approval of voting rights is entitled to demand fair value for the redemption of such stockholder’s shares.
Nevada’s control share law may have the effect of discouraging takeovers of the corporation.
In addition to the control share law, Nevada has a business combination law, which prohibits certain business combinations between Nevada corporations and “interested stockholders” for two years after the “interested stockholder” first becomes an “interested stockholder,” unless the corporation’s board of directors approves the combination in advance or thereafter by both the board of directors and 60% of the disinterested stockholders. For purposes of Nevada law, an “interested stockholder” is any person who is (i) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (ii) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then outstanding shares of the corporation. The definition of the term “business combination” is sufficiently broad to cover virtually any kind of transaction that would allow a potential acquiror to use the corporation’s assets to finance the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its other stockholders.
The effect of Nevada’s business combination law is to potentially discourage parties interested in taking control of us from doing so if it cannot obtain the approval of our board of directors.
We may experience fluctuations in our tax obligations and effective tax rate, which could materially and adversely affect our results of operations.
We are subject to U.S. federal and state income taxes and taxes in certain other non-U.S. jurisdictions. Tax laws, regulations and administrative practices in various jurisdictions may be subject to significant change, with or without advance notice, due to economic, political and other conditions, and significant judgment is required in evaluating and estimating our provision and accruals for these taxes. There are many transactions that occur during the ordinary course of business for which the ultimate tax determination is uncertain. Our effective tax rates could be affected by numerous factors, such as changes in tax, accounting and other laws, regulations, administrative practices, principles and interpretations, the mix and level of earnings in a given taxing jurisdiction or our ownership or capital structures. For example, the United States government may enact significant changes to the taxation of business entities including, among others, an increase in the corporate income tax rate, an increase in the tax rate applicable to certain income earned overseas and elimination of certain exemptions, and the imposition of minimum taxes or surtaxes on certain types of income. No specific United States tax legislation has been proposed at this time and the likelihood of these changes being enacted or implemented is unclear. We are currently unable to predict whether such changes will occur and, if so, the ultimate impact on our business. We urge investors to consult with their legal and tax advisers regarding implications of potential changes in U.S. tax laws on an investment in our common stock.
Our reverse stock split may not result in a proportional increase in the per share price of our common stock.
The effect of the reverse stock split on the market price for our common stock cannot be accurately predicted. In particular, we cannot assure you that the prices for shares of the common stock after the reverse stock split will increase proportionately to prices for shares of our common stock immediately before the reverse stock split. The market price of our common stock may also be affected by other factors which may be unrelated to the reverse stock split or the number of shares issued and outstanding.
Furthermore, even if the market price of our common stock does rise following the reverse stock split, we cannot assure you that the market price of our common stock immediately after the proposed reverse stock split will be maintained for any period of time. Moreover, because some investors may view the reverse stock split negatively, we cannot assure you that the reverse stock split will not adversely impact the market price of our common stock. There is also the possibility that liquidity may be adversely affected by the reduced number of shares which would be issued and outstanding when the reverse stock split is effected, particularly if the price per share of our common stock begins a declining trend after the reverse stock split is affected. Accordingly, our total market capitalization after the reverse stock split may be lower than the market capitalization before the reverse stock split.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes statements that express our opinions, expectations, beliefs, plans, objectives, assumptions or projections regarding future events or future results and therefore are, or may be deemed to be, “forward-looking statements.” All statements other than statements of historical facts contained in this prospectus may be forward-looking statements. These forward-looking statements can generally be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “continues,” “anticipates,” “expects,” “seeks,” “projects,” “intends,” “plans,” “may,” “will,” “would” or “should” or, in each case, their negative or other variations or comparable terminology. They appear in a number of places throughout this prospectus, and include statements regarding our intentions, beliefs or current expectations concerning, among other things, our results of operations, financial condition, liquidity, prospects, growth, strategies, future acquisitions and the industry in which we operate.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. We believe that these risks and uncertainties include, but are not limited to, those described in the “Risk Factors” section of this prospectus, which include, but are not limited to, risks related to the following:
| ● | our ability to compete in our industry, including against competitors that have significantly greater financial, technical and marketing resources than we do; | |
| ● | our ability to respond to customer preferences and successfully develop new and innovative products in a timely manner and effectively manage the introduction of new or enhanced products; | |
| ● | risks related to a loss of, or material cancellation, reduction, or delay in purchases by, one or more of our largest customers; | |
| ● | our outside suppliers and manufacturers failing to supply products in sufficient quantities and in a timely fashion; | |
| ● | the impact of the COVID-19 pandemic and associated responses on our business, operating results, financial condition and prospects; | |
| ● | our ability to execute on our strategic initiatives (including acquisitions); | |
| ● | our ability to maintain the reputation of our brands; | |
| ● | the risks related to consumers’ perception of the safety and quality of our products as well as similar products distributed by other companies in our industry; | |
| ● | the risks related to third parties asserting intellectual property infringement claims against us; | |
| ● | the risks related to our planned expansion into additional international markets; | |
| ● | the risks related to adverse economic conditions; | |
| ● | the risks related to catastrophic events; | |
| ● | our ability to retain key personnel, manage our business effectively, and continue to grow; | |
| ● | the impact of numerous laws and regulations that apply to the manufacture, sale, and manufacturing of nutritional supplements, and compliance with these laws and regulations, as they currently exist or as modified in the future, on us and our suppliers; | |
| ● | the risks related to product recalls; | |
| ● | the risks related to product liability claims and litigation to prosecute such claims; and | |
| ● | the other factors described in “Risk Factors.” |
These factors should not be construed as exhaustive and should be read with the other cautionary statements in this prospectus.
Although we base these forward-looking statements on assumptions that we believe are reasonable when made, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and industry developments may differ materially from statements made in or suggested by the forward-looking statements contained in this prospectus. The matters summarized under “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and elsewhere in this prospectus could cause our actual results to differ significantly from those contained in our forward-looking statements. In addition, even if our results of operations, financial condition and liquidity, and industry developments are consistent with the forward-looking statements contained in this prospectus, those results or developments may not be indicative of results or developments in subsequent periods.
In light of these risks and uncertainties, we caution you not to place undue reliance on these forward-looking statements, which applystatements. Any forward-looking statement that we make in this prospectus speaks only as of the date of such statement, and we undertake no obligation to update any forward-looking statement or to publicly announce the results of any revision to any of those statements to reflect future events or developments, except as required by applicable law. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless specifically expressed as such, and should only be viewed as historical data.
Assuming a public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus. These forward-looking states are subjectprospectus, we estimate that the net proceeds to certain risks and uncertainties that could cause actual results to differ materiallyus from historical results orthe sale of our predictions.
We believe it is likelyintend to use approximately $6,925,000 of the net proceeds as follows:
| ● | approximately $6,500,000 to repay a portion of amounts owed under our amended and restated loan agreement, as amended, with Knight Therapeutics (Barbados) Inc. (an owner of greater than 10% of our common stock), which bears interest at a rate of 12.5% per annum and has a maturity date of December 31, 2021, and to pay associated fees; and | |
| ● | approximately $425,000 to repay amounts due to Knight Therapeutics Inc. in connection with our acquisition of Neuragen in 2015, which amounts do not bear interest and have a maturity date of June 30, 2029. |
We intend to use the remaining net proceeds (which will be approximately $ million, or $ million if the underwriters exercise their over-allotment option in full) to support our organic growth, for advertising and for other general corporate purposes, including to fund potential future investments and acquisitions of companies that we will continuebelieve are complementary to have uncertainties surrounding our ability to continue as a going concern even ifbusiness and consistent with our growth strategy. Although we raise the maximum amount from this offering.
The amounts and timing of our explorationuse of the property. If we are unable to complete financing, wenet proceeds from this offering will vary depending on numerous factors. As a result, our management will have broad discretion over how these proceeds are used.
As a result of amendments to find alternative sources, which may include private placements of securities, debt or loans from our officers or others. At the present time, we have not made any arrangements to raise additional cash, other than this offering. If we need additional cash and can't raise it we will either have to suspend operations until we raise sufficient cash, or cease operations entirely. In that event investors will likely lose their entire investment in the Company. We presently have no other financing plans.
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “SNYR.” However, we cannot assure you that a liquid trading market for our common stock will ultimatelydevelop or be neededsustained after this offering. You may not be able to sell your shares quickly or at the market price if trading in our common stock is not active. See “Underwriting” for exploration. Ifmore information regarding our initial exploration proves positive results, we will expandarrangements with the exploration activities to include reverse circulation drilling. This is a less expensive form of drilling that does not allowunderwriters and the factors considered in setting the public offering price.
The following table sets forth, for the recoveryfiscal quarters indicated, the high and low sale prices per share of a tubeour common stock, without giving effect to our proposed reverse stock split. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or core of rock. The material is brought up from depth as a series of small chips of rock that are then baggedcommission, and sent in for analysis. This is a quicker and cheaper method of drilling, but doesmay not necessarily give one as much information about the underlying rocks. If warranted, core drilling would follow this stage.represent actual transactions.
| High | Low | |||||||
| Year Ending December 31, 2021 | ||||||||
| 3rd Quarter (through September 27, 2021) | $ | 0.170 | $ | 0.047 | ||||
| 2nd Quarter | $ | 0.100 | $ | 0.030 | ||||
| 1st Quarter | $ | 0.183 | $ | 0.034 | ||||
| Year Ended December 31, 2020 | ||||||||
| 4th Quarter | $ | 0.080 | $ | 0.028 | ||||
| 3rd Quarter | $ | 0.111 | $ | 0.040 | ||||
| 2nd Quarter | $ | 0.199 | $ | 0.025 | ||||
| 1st Quarter | $ | 0.150 | $ | 0.030 | ||||
| Year Ended December 31, 2019 | ||||||||
| 4th Quarter | $ | 0.190 | $ | 0.040 | ||||
| 3rd Quarter | $ | 0.270 | $ | 0.150 | ||||
| 2nd Quarter | $ | 0.290 | $ | 0.150 | ||||
| 1st Quarter | $ | 0.270 | $ | 0.135 | ||||
| 20 |
Since our officers and directors, however, our officers and directors are unwilling to make any commitment to loan us any significant amounts of money at this time. At the present time,inception, we have not madepaid any arrangementsdividends on our common stock, and we currently expect that, for the foreseeable future, all earnings, if any, will be retained for use in the development and operation of our business. In the future, our Board may decide, at its discretion, whether dividends may be declared and paid to raise additional cash. If we need additionalholders of our common stock.
The following table sets forth our cash and cannot raise it we will either have to suspend operations until we do raise the cash or cease operations entirely.
| ● | on an as adjusted basis to |
This table should be read in conjunction with, and is qualified in its entirety by reference to, “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes appearing elsewhere in this prospectus.
| As of June 30, 2021 | ||||||||
| Actual | As Adjusted | |||||||
| Cash and cash equivalents | $ | 966,398 | ||||||
| Capitalization: | ||||||||
| Current debt: | ||||||||
| Current portion of long-term notes payable, net of debt discount and debt issuance cost, related party | $ | 5,524,450 | – | |||||
Short term loan | 393,754 | – | ||||||
| Total current debt | 5,918,204 | – | ||||||
| Long-term debt: | ||||||||
| Note payable, net of debt discount and debt issuance cost, related party | 1,239,908 | – | ||||||
| Total long-term debt | 1,239,908 | – | ||||||
| Stockholders’ deficit: | ||||||||
| Common stock, $0.00001 par value; 300,000,000 shares authorized; 89,889,074 and , shares issued and outstanding, respectively | 899 | |||||||
| Additional paid in capital | 19,147,884 | |||||||
| Accumulated other comprehensive (loss) income | (302,517 | ) | ||||||
| Accumulated deficit | (20,183,008 | ) | ||||||
| Total stockholders’ deficit | (1,336,742 | ) | ||||||
| Total Capitalization | $ | 5,821,370 | ||||||
Unless we indicate otherwise, all information in this Capitalization section:
| ● | assumes no exercise by the underwriters of their over-allotment option; | |
| Preliminary review of assessment work | $ | 1,000.00 | ||
| Mobilization/demobilization of crew | $ | 1,000.00 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 2,000.00 | ||
| Detailed magnetometer survey, 7 line km | $ | 2,000.00 | ||
| Base station and instrument rental | $ | 1,000.00 | ||
| Consumables – flagging, pickets etc. | $ | 100.00 | ||
| Accommodation/meals, 3 men | $ | 400.00 | ||
| Drafting and report | $ | 1,500.00 | ||
| Contingencies | $ | 1,000.00 | ||
| TOTAL COST, PHASE 1 PROGRAM | $ | 10,000.00 |
| ● |
| Gross proceeds | $ | 40,000 | ||
| Offering expenses (1) | $ | 13,002 | ||
| Net proceeds | $ | 26,998 |
Annual Assessment Saskatchewan | $ | 3,072 | ||
| Consulting Services | $ | 5,000 | ||
| Preliminary review of assessment work | $ | 2,000 | ||
| Mobilization/demobilization of crew | $ | 2,000 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 4,000 | ||
| Detailed magnetometer survey, 7 line km | $ | 4,000 | ||
| Base station and instrument rental | $ | 1,000 | ||
| Consumables – flagging, pickets, etc. | $ | 100 | ||
| Accommodation/meals, 3 men | $ | 400 | ||
| Drafting and report | $ | 1,500 | ||
| Contingencies | $ | 1,000 | ||
| Telephone | $ | 200 | ||
| $ | 50 | |||
| Stationary | $ | 100 | ||
| Accounting and Legal | $ | 500 | ||
| SEC filing | $ | 250 | ||
| Repayment of loan and Advances | $ | 6 | ||
| Other expenses | $ | 1,826 | ||
| TOTAL | $ | 26,998 |
| Gross proceeds | $ | 30,000 | ||
| Offering expenses (1) | $ | 13,002 | ||
| Net proceeds | $ | 16,998 |
| The net proceeds will be used as follows: | ||||
Annual Assessment Saskatchewan | $ | 3,072 | ||
| Consulting Services | $ | 1,100 | ||
| Preliminary review of assessment work | $ | 1,500 | ||
| Mobilization/demobilization of crew | $ | 1,500 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 3,000 | ||
| Detailed magnetometer survey, 7 line km | $ | 3,000 | ||
| Base station and instrument rental | $ | 750 | ||
| Consumables – flagging, pickets, etc. | $ | 75 | ||
| Accommodation/meals, 3 men | $ | 300 | ||
| Drafting and report | $ | 1,125 | ||
| Contingencies | $ | 750 | ||
| Telephone | $ | 150 | ||
| $ | 38 | |||
| Stationary | $ | 75 | ||
| Accounting and Legal | $ | 375 | ||
| SEC filing | $ | 188 | ||
| Repayment of loan and Advances | $ | 0 | ||
| Other expenses | $ | 0 | ||
| TOTAL | $ | 16,998 |
| Gross proceeds | $ | 20,000 | ||
| Offering expenses (1) | $ | 13,002 | ||
| Net proceeds | $ | 6,998 |
| The net proceeds will be used as follows: | ||||
Annual Assessment Saskatchewan | $ | 3,072 | ||
| Consulting Services | $ | 0 | ||
| Preliminary review of assessment work | $ | 1,000 | ||
| Mobilization/demobilization of crew | $ | 1,000 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 1,000 | ||
| Detailed magnetometer survey, 7 line km | $ | 926 | ||
| Base station and instrument rental | $ | 0 | ||
| Consumables – flagging, pickets, etc. | $ | 0 | ||
| Accommodation/meals, 3 men | $ | 0 | ||
| Drafting and report | $ | 0 | ||
| Contingencies | $ | 0 | ||
| Telephone | $ | 0 | ||
| $ | 0 | |||
| Stationary | $ | 0 | ||
| Accounting and Legal | $ | 0 | ||
| SEC filing | $ | 0 | ||
| Repayment of loan and Advances | $ | 0 | ||
| Other expenses | $ | 0 | ||
| TOTAL | $ | 6,998 |
| Gross proceeds | $ | 10,000 | ||
| Offering expenses (1) | $ | 13,002 | ||
| Net proceeds | $ | -3,002 |
| Dilution | 100 | % | 75 | % | 50 | % | 25 | % | ||||||||
| Price per share | $ | 0.00001 | 0.0000075 | 0.000005 | 0.0000025 | |||||||||||
| Net tangible book value per share before offering | $ | 0 | 0 | 0 | 0 | |||||||||||
| Potential gain to existing shareholder | $ | 40,000 | 30,000 | 20,000 | 10,000 | |||||||||||
| Net tangible book value per share after offering | $ | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| Increase to present stockholder in net tangible book value per share | 0 | 0 | 0 | |||||||||||||
| after offering | $ | 0.02 | 0.015 | 0.01 | 0.005 | |||||||||||
| Capital contributions | $ | 50 | 37.5 | 25 | 12.5 | |||||||||||
| Number of shares outstanding before the offering | 5,000,000 | 3,750,000 | 2,500,000 | 1,250,000 | ||||||||||||
| Number of shares after offering assuming the sale of the maximum | 0 | 0 | 0 | |||||||||||||
| number of shares | 6,000,000 | 4,500,000 | 3,000,000 | 1,500,000 | ||||||||||||
| Percentage of ownership after offering | 80 | % | 0.6 | 0.4 | 0.2 | |||||||||||
| 0 | 0 | 0 | ||||||||||||||
| Purchasers of Shares in this Offering: | 0 | 0 | 0 | |||||||||||||
| 0 | 0 | 0 | ||||||||||||||
| 0 | 0 | 0 | ||||||||||||||
| Price per share | $ | 0.04 | 0.04 | 0.04 | 0.04 | |||||||||||
| Dilution per share | $ | 0.04 | 0.04 | 0.04 | 0.04 | |||||||||||
| Capital contributions | $ | 200,000 | 150,000 | 100,000 | 50,000 | |||||||||||
| Number of shares after offering held by public investors | 5,000,000 | 3,750,000 | 2,500,000 | 1,250,000 | ||||||||||||
| Percentage of capital contributions by existing shareholder | 0.00 | % | 0.000015 | 0.00001 | 0.000005 | |||||||||||
| Percentage of capital contributions by new investors | 100.00 | % | 0.749985 | 0.49999 | 0.249995 | |||||||||||
| Percentage of ownership after offering | 20 | % | 15 | % | 10 | % | 5 | % |
If you invest in our common stock in this offering, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after the closing of this offering.
Our historic net tangible book value of our common stock as of June 30, 2021 was approximately $(1.3 million), or $(0.01) per share, based on the number of shares of our common stock outstanding as of June 30, 2021. Historic net tangible book value per share represents our total tangible assets less our total liabilities, divided by the number of outstanding shares of common stock.
After giving effect to the 1-for- reverse stock split and the receipt of the net proceeds from our sale of shares of common stock in this offering at an assumed public offering price of $ per share (the midpoint of the range set forth on the cover page of this prospectus), after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2021, would have been $ million, or $ per share. This represents an immediate increase in as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to investors purchasing common stock in this offering.
We calculate dilution per share to new investors by subtracting the historic net tangible book value per share from the public offering price paid by the new investor. The broker-dealer also must provide, priorfollowing table illustrates the dilution to effecting any transactionnew investors on a per share basis:
| Assumed public offering price per share | $ | |||||||
| Historic net tangible book value per share as of June 30, 2021 | $ | (0.01 | ) | |||||
| Increase in net tangible book value per share attributable to new investors in this offering | ||||||||
| As adjusted net tangible book value per share after this offering | ||||||||
| Dilution in net tangible book value per share to new investors in this offering | $ |
Each $1.00 increase (decrease) in a pennythe assumed public offering price of $ would increase (decrease) our as adjusted net tangible book value per share after this offering by $ per share and the dilution to new investors by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1,000,000 shares in the number of shares of common stock offered by us would increase (decrease) the customer:
If the underwriters’ option to purchase additional shares to cover over-allotments is exercised in full, the as adjusted net tangible book value per share after giving effect to this offering would be $ per share, representing an immediate increase to existing stockholders of $ per share, and immediate dilution to new investors in this offering of $ per share.
The following table summarizes, as of June 30, 2021, on the as adjusted basis described above:
| the | ||
| Shares Purchased | Total Consideration | Average Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing stockholders | % | $ | % | $ | ||||||||||||||||
| New investors | % | % | ||||||||||||||||||
| Total | 100.0 | % | $ | 100.0 | % | $ | ||||||||||||||
A $1.00 increase (decrease) in the secondary market for our securities because it will be subjectassumed public offering price of $ per share would increase (decrease) total consideration paid by new investors by $ million and increase (decrease) the total consideration paid to these penny stock rules. Therefore, security holders may have difficulty selling those securities.
If the underwriters’ option to purchase additional shares to cover over-allotments is exercised in full, the number of shares held and the percentage of total consideration paid by the existing stockholders after this offering from bidding for or purchasing, or attemptingwould be reduced to induce any person to bid for or purchase any security which is% and %, respectively, and the subjectnumber of the distribution until the entire distribution is complete.
The value of the diamond extends far beyond the exquisite beauty that makes it popular for use in fine jewelry. The hardest substance known to man, diamonds can also withstand extreme pressure and shock, making them valuable for industrial use in tools for cutting, polishing, drilling and grinding. Flawed diamonds that are not suited for jewelry as well as synthetic diamonds are often designated for such manufacturing applications.
| Months from completing our public offering | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
Phase 1 - (Magnetic Survey) | ||||||||||||||||
Permitting | X | X | X | |||||||||||||
Field Work | X | |||||||||||||||
Summary Results | X | |||||||||||||||
Phase 2 - (Gravity Survey) | ||||||||||||||||
Field Work | X | |||||||||||||||
Summary Report | X | |||||||||||||||
Phase 3 - (Drilling) | ||||||||||||||||
Permitting | X | X | ||||||||||||||
Drill Mobilization Drilling | X | |||||||||||||||
Sample Analysis | X | |||||||||||||||
Final Report | X |
| Preliminary review of assessment work | $ | 1,000.00 | ||
| Mobilization/demobilization of crew | $ | 1,000.00 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 2,000.00 | ||
| Detailed magnetometer survey, 7 line km | $ | 2,000.00 | ||
| Base station and instrument rental | $ | 1,000.00 | ||
| Consumables – flagging, pickets etc | $ | 100.00 | ||
| Accommodation/meals, 3 men | $ | 400.00 | ||
| Drafting and report | $ | 1,500.00 | ||
| Contingencies | $ | 1,000.00 | ||
| TOTAL COST, PHASE 1 PROGRAM | $ | 10,000.00 |
| Review of previous work | $ | 1,500.00 | ||
| Mobilization/demobilization of crew | $ | 2,000.00 | ||
| Pace and compass/GPS lines, estimated 7 line km, 25 m spacing | $ | 3,000.00 | ||
| Detailed gravity survey, 7 line km | $ | 4,000.00 | ||
| Base station and instrument rental | $ | 2,000.00 | ||
| Consumables – flagging, pickets etc | $ | 1,000.00 | ||
| Accommodation/meals, 3 men | $ | 1,000.00 | ||
| Drafting and report | $ | 3,000.00 | ||
| Contingencies | $ | 2,500.00 | ||
| TOTAL COST, PHASE 2 PROGRAM | $ | 20,000.00 |
| Review of previous work | $ | 2,000.00 | ||
| Mobilization/demobilization of crew | $ | 10,000.00 | ||
| Diamond drilling, all inclusive $200 per meter, 1000 m total | $ | 200,000.00 | ||
| Drafting and report | $ | 10,000.00 | ||
| Contingencies | $ | 28,000.00 | ||
| TOTAL COST, PHASE 3 PROGRAM | $ | 250,000.00 |
| NAME | AGE | TITLE | DATE OF APPOINTMENT | PERCENT OF TIME DEVOTED | |||||
| Aaron, Danny | 49 | Chief Executive Officer | January 7, 2011 | 10 | % |
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||
Name and principal position (a) | Year (b) | Salary ($) (c) | Bonus ($) (d) | Stock Awards ($) (e) | Option Awards ($) (f) | Non-Equity Incentive Plan Compensation ($) (g) | Nonqualified Deferred Compensation Earnings ($) (h) | All Other Compensation (4) ($) (i) | Total ($) (j) | |||||||||||||||||||||||||
Danny Aaron | 2011 | $ | 0 | 0 | 0 | 0 | 0 | 0 | 1,750 | $ | 0 | |||||||||||||||||||||||
| CEO | 2012 | $ | 0 | 0 | 0 | 0 | 0 | 0 | 3,000 | $ | 0 | |||||||||||||||||||||||
| Name and Address of Beneficial Owner(1) | Number of shares Beneficially owned | Percentage | ||||||
| Executive Officers and Directors | ||||||||
| Danny Aaron | 5,000,000 | 100 | % | |||||
| Officers and Directors as a Group (1 person) | 5,000,000 | 100 | % | |||||
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our Articlesresults of Incorporation, Bylaws and the applicable statutes of the State of Nevada.
| January 31, 2013 | July 31, 2012 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | - | $ | 4,000 | ||||
| Deposit on Mining Claim | - | - | ||||||
| Total Current Assets | - | 4,000 | ||||||
| Mining Claims, net of impairment | - | - | ||||||
| Total Assets | $ | - | $ | 4,000 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable and Accrued Liabilities | $ | 4,205 | $ | 3,000 | ||||
| Due to Directors | 15,000 | 15,000 | ||||||
| Total Liabilities | 19,205 | 18,000 | ||||||
| Stockholders’ Deficit | ||||||||
| Common Stock (75,000,000 shares authorized, par value 0.00001, 5,000,000 shares issued and outstanding for both fiscal years 2011 and 2012) | 50 | 50 | ||||||
| Additional paid-in capital | 14,597 | 10,997 | ||||||
| Deficit accumulated during the exploration stage | (33,852 | ) | (25,047 | ) | ||||
| Total Stockholders’ Deficit | (19,205 | ) | (14,000 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | - | $ | 4,000 | ||||
Three Months Ended January 31, 2013 | Three Months Ended January 31, 2012 | Six Months Ended January 31, 2012 | Six Months Ended January 31, 2012 | Inception December 29, 2010 to January 31, 2013 | ||||||||||||||||
| Operating Expenses | ||||||||||||||||||||
| Consulting services | $ | 750 | $ | - | $ | 1,500 | $ | - | $ | 6,250 | ||||||||||
| General and administrative | - | - | 805 | - | 825 | |||||||||||||||
| Rent | 750 | - | 1,500 | - | 6,250 | |||||||||||||||
| Legal and accounting | 4,400 | - | 4,400 | - | 12,380 | |||||||||||||||
| Impairment of Mineral Claims | - | - | - | - | 6,000 | |||||||||||||||
| Imputed Interest Expense | 300 | - | 600 | - | 2,147 | |||||||||||||||
| Total Expenses | 6,200 | - | 8,805 | - | 33,852 | |||||||||||||||
| Net Loss | $ | (6,200 | ) | $ | - | $ | (8,805 | ) | $ | - | $ | (33,852 | ) | |||||||
| Net Loss Per Common Share – Basic and Diluted | $ | (0.00 | ) | $ | 0.00 | $ | (0.00 | ) | $ | 0.00 | ||||||||||
| Weighted Average Number of Common Shares Outstanding | 5,000,000 | 5,000,000 | 5,000,000 | 5,000,000 | ||||||||||||||||
Six Months Ended January 31, 2013 | Six Months Ended January 31, 2012 | Inception December 29, 2010 to January 31, 2013 | ||||||||||
| Operating Activities | ||||||||||||
| Net loss | $ | (8,805 | ) | $ | - | $ | (33,852 | ) | ||||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||||||
| Impairment of mineral claims | - | - | 6,000 | |||||||||
| Imputed interest expense | 600 | - | 2,147 | |||||||||
| Donated consulting services and rent expenses | 3,000 | - | 12,500 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts payable and accrued liabilities | 1,205 | - | 4,205 | |||||||||
| Net Cash Used by Operating Activities | (4,000 | ) | - | (9,000 | ) | |||||||
| Investing Activities | ||||||||||||
| Purchase of mining claims | - | - | (6,000 | ) | ||||||||
| Financing Activities | ||||||||||||
| Borrowings on debt-related party | - | - | 15,000 | |||||||||
| Increase (Decrease) in Cash | (4,000 | ) | - | - | ||||||||
| Cash - Beginning of Period | 4,000 | - | - | |||||||||
| Cash - End of Period | $ | - | $ | - | $ | - | ||||||
Supplemental Disclosure of Cash Flow Information Cash paid during the period for : | ||||||||||||
| Interest | $ | - | $ | - | $ | - | ||||||
| Income taxes | $ | - | $ | - | $ | - | ||||||
| Common Stock | Additional Paid-in | Deficit Accumulated During the Exploration | ||||||||||||||||||
| Shares | Amount | Capital | Stage | Total | ||||||||||||||||
| Balance at December 22, 2009 | - | $ | - | $ | - | $ | - | $ | - | |||||||||||
| Issuance of common stock to founders | 5,000,000 | 50 | (50 | ) | - | - | ||||||||||||||
| Donated consulting services and rent | - | - | 3,500 | - | 3,500 | |||||||||||||||
| Imputed interest expense | - | - | 347 | - | 347 | |||||||||||||||
| Net loss | - | - | - | (8,847 | ) | (8,847 | ) | |||||||||||||
| Balances at July 31, 2011 | 5,000,000 | 50 | 3,797 | (8,847 | ) | (5,000 | ) | |||||||||||||
| Donated services | - | - | 6,000 | - | 6,000 | |||||||||||||||
| Imputed interest expense | - | - | 1,200 | - | 1,200 | |||||||||||||||
| Net loss | - | - | - | (16,200 | ) | (16,200 | ) | |||||||||||||
| Balances at July 31, 2012 | 5,000,000 | $ | 50 | $ | 10,997 | $ | (25,047 | ) | $ | (14,000 | ) | |||||||||
| Donated services | - | - | 3,000 | - | 3,000 | |||||||||||||||
| Imputed interest expense | - | - | 600 | - | 600 | |||||||||||||||
| Net loss | - | - | - | (8,805 | ) | (8,805 | ) | |||||||||||||
| Balances at January 31, 2013 | 5,000,000 | $ | 50 | $ | 14,597 | $ | (33,852 | ) | $ | (19,205 | ) | |||||||||
Overview
We are presenteda provider of consumer health care, beauty, and lifestyle products. Our current brand portfolio consists of three core brands: FOCUSfactor, a patented brain health supplement that has been clinically shown to improve memory, concentration and focus; Flat Tummy, a lifestyle brand that provides a suite of nutritional products to help women achieve their weight management goals; and Hand MD, a full range of preventative, restorative, and anti-aging skin care products designed for the hands.
Our management’s discussion and analysis of our financial condition and results of operations are only based on our current business and should be read in conjunction with our audited Consolidated Financial Statements and accompanying notes thereto included elsewhere in this prospectus. Key factors affecting our results of operations include revenues, cost of sales, operating expenses and income and taxation.
Non-GAAP Financial Measures
We currently focus on Adjusted EBITDA to evaluate our business relationships and our resulting operating performance and financial position. Adjusted EBITDA is defined as EBITDA (net income plus interest expense, income tax expense, depreciation and amortization), further adjusted to exclude certain non-cash expenses and other adjustments as set forth below. We present Adjusted EBITDA because we consider it an important measure of our performance and a meaningful financial metric in assessing our operating performance from period to period by excluding certain items that we believe are not representative of our core business, such as certain non-cash items and other adjustments.
We believe that Adjusted EBITDA, viewed in addition to, and not in lieu of, our reported results in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”), provides useful information to investors.
| Six Months Ended June 30, 2021 | Six Months Ended June 30, 2020 | Year Ended December 31, 2020 | Year Ended December 31, 2019 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Net income (loss) | $ | 2,640,429 | $ | 1,101,350 | $ | 1,411,132 | $ | (9,207,447 | ) | |||||||
| Interest income | (24 | ) | (79 | ) | (104 | ) | (414 | ) | ||||||||
| Interest expense | 627,883 | 497,992 | 1,186,034 | 981,105 | ||||||||||||
| Taxes | 104,537 | 128,996 | – | 131,537 | ||||||||||||
| Depreciation and amortization | – | 95,686 | 148,697 | 1,342,689 | ||||||||||||
| EBITDA | $ | 3,372,825 | $ | 1,823,945 | $ | 2,745,759 | $ | (6,752,530 | ) | |||||||
| Impairment of intangible assets | – | – | 47,002 | 9,715,137 | ||||||||||||
| Stock-based compensation | – | 77,358 | 128,929 | 201,155 | ||||||||||||
| Non-recurring expenses | 393,965 | (1) | 1,014,846 | (2) | 1,263,427 | (3) | 493,924 | (4) | ||||||||
| Bad debts | – | – | 74,068 | 283,972 | ||||||||||||
| Obsolete inventory | – | – | 527,737 | 257,111 | ||||||||||||
| Loss on foreign currency translation and transaction | (259,707 | ) | 177,791 | (106,761 | ) | 6,972 | ||||||||||
| Adjusted EBITDA | $ | 3,507,083 | $ | 3,093,940 | $ | 4,680,161 | $ | 4,205,741 | ||||||||
| (1) | Consists of acquisition expenses with customers for shelf placement of $393,965. | |
| (2) | Consists of loan fees of $1,000,000 and acquisition expenses with customers for shelf placement of $14,846. | |
| (3) | Consists of loan fees of $1,000,000 and related financing costs of $61,000, leasehold renovations of $174,215 and acquisition expenses with customers for shelf placement of $28,212. | |
| (4) | Consists of acquisition expenses with customers for shelf placement of $261,801, customer return of $215,189 and inventory write off of $16,934. |
EBITDA and Adjusted EBITDA are expressedconsidered non-GAAP financial measures. EBITDA represents earnings before interest, taxes, depreciation and amortization. Adjusted EBITDA represents EBITDA further adjusted to exclude the impact of higher-than-normal revenue change order activity and certain expenses and transactions that we believe are not representative of our core operating results; stock-based compensation; non-recurring expenses for acquisitions, such as one-time expenses with customers for shelf placement, and other fees and costs; and loss on foreign currency translation and transaction. Our definitions of EBITDA and Adjusted EBITDA might not be comparable to similarly titled measures reported by other companies.
Results of Operations for the Six Months Ended June 30, 2021 and June 30, 2020
During both the six months ended June 30, 2021 and 2020, we focused on developing our currently owned brands into new markets and by product extensions. Our objective is to grow all four of our targeted verticals (Nutraceuticals, Over the Counter (OTC), Consumer Goods and Cosmeceuticals) to provide a balanced and synergistic portfolio that drives consumer demand via multiple channels. Our Nutraceuticals vertical consists of FOCUSfactor and Flat Tummy consumables, Over the Counter (OTC) consists of Neuragen products, Consumer Goods consists of Sneaky Vaunt and app revenue, and Cosmeceuticals consists of Hand MD products.
Revenue
For the six months ended June 30, 2021, we had revenue of $24,761,974 from sales of our products, as compared to revenue of $17,906,767 for the six months ended June 30, 2020. The revenue is comprised of the following categories:
| June 30, 2021 | June 30, 2020 | |||||||
| Nutraceuticals | $ | 24,549,101 | $ | 12,361,201 | ||||
| Over the Counter (OTC) | - | 22,311 | ||||||
| Consumer Goods | 168,047 | 565,756 | ||||||
| Cosmeceuticals | 44,826 | 4,957,499 | ||||||
| $ | 24,761,974 | $ | 17,906,767 | |||||
We had an increase in Nutraceuticals revenue in the six months ended June 30, 2021 as compared to the six months ended June 30, 2020 due to higher consumer sales across all of our distribution channels driven by our advertising campaigns and new products entering the market. The increase was due solely to sales volume increases, as prices for our products did not increase year-over-year. We had a decrease in Over the Counter revenue in 2021 as compared to 2020 due to a supply issue with Neuragen during the period. We had a decrease in Consumer Goods revenue in 2021 as compared to 2020 due to a shift in business focus away from our Sneaky Vaunt brand. We had a decrease in Cosmeceuticals revenue in 2021 as compared to 2020 due to sales of a hand sanitizer product to a retailer in 2020 that was not repeated in 2021. The sales to the retailer in 2020 were as a result of excessive demand and insufficient supply of hand sanitizers as a result of the COVID-19 outbreak. As the supply of hand sanitizers increased in 2021, the pricing to customers and resulting margins decreased and we decided to no longer pursue the sale of the product.
Cost of Revenue
For the six months ended June 30, 2021, our cost of revenue was $6,794,344. Our cost of revenue for the six months ended June 30, 2020, was $6,593,446. The cost of revenue is comprised of the following categories:
| June 30, 2021 | June 30, 2020 | |||||||
| Nutraceuticals | $ | 6,762,231 | $ | 3,633,453 | ||||
| Over the Counter (OTC) | - | - | ||||||
| Consumer Goods | 29,986 | 161,821 | ||||||
| Cosmeceuticals | 2,127 | 2,798,172 | ||||||
| $ | 6,794,344 | $ | 6,593,446 | |||||
We had an increase in Nutraceuticals in 2021 as compared to 2020 due to higher sales. We had a decrease in Consumer Goods in 2021 as compared to 2020 due to a decrease in sales. We had a decrease in Cosmeceuticals in 2021 as compared to 2020 due to sale of a hand sanitizer product to a retailer in 2020 that was not repeated in 2021.
Gross Profit
Gross profit was $17,967,631, or 73% of revenue, for the six months ended June 30, 2021, as compared to gross profit of $11,313,321, or 63% of revenue, for the same period in 2020, an increase of $6,654,309, or 59%. The increase in gross profit is directly related to the increase in net sales. The increase in gross profit margin in total is directly related to the mix of products being sold.Our Nutraceuticals gross profit margin remained largely stable with a slight increase in 2021 to 72% from 71%. Our Cosmeceuticals gross profit increased in 2021 to 95% from 44%, which is directly attributable to the high volume of hand sanitizer sales during 2020 at a lower margin.
Operating Expenses
Selling and Marketing Expenses
For the six months ended June 30, 2021, our selling and marketing expenses were $11,507,769 as compared to $5,640,397 for the six months ended June 30, 2020, which is primarily due to an increase in promotions and advertising, which had been reduced in response to COVID-19 during the six months ended June 30, 2020.
General and Administrative Expenses
For the six months ended June 30, 2021, our general and administrative expenses were $2,997,276. For the six months ended June 30, 2020, our general and administrative expenses were $3,043,044. The decrease is primarily due to improved management of operating costs.
Depreciation and Amortization Expenses
For the six months ended June 30, 2021, our depreciation and amortization expenses were $0 as compared to $53,544 for the six months ended June 30, 2020. The decrease is due to impairment of assets in 2020.
Other Income and Expenses
For the six months ended June 30, 2021 and 2020 we had other (income) and expense items of the following:
| Six months ended June 30, 2021 | Six months ended June 30, 2020 | |||||||
| Interest income | $ | (24 | ) | $ | (79 | ) | ||
| Other income | – | (181,849 | ) | |||||
| Other expense (loan success fee) | – | 1,000,000 | ||||||
| Interest expense | 627,883 | 497,992 | ||||||
| Remeasurement loss (gain) on translation of foreign subsidiary | 89,760 | (12,216 | ) | |||||
| Amortization of debt issuance cost | – | 42,142 | ||||||
| Total other expense | $ | 717,619 | $ | 1,345,990 | ||||
For the six months ended June 30, 2021, we had interest expense of $627,883 as compared to $497,992 for the six months ended June 30, 2020 due to interest on the Knight loan success fee during the entire period ended June 30, 2021 and our entry into the $500,000 working capital loan with Shopify Capital.
Net Income
For the six months ended June 30, 2021, our net income was $2,640,429 as compared to a net income of $1,101,350 for the six months ended June 30, 2020 due to higher revenue.
Results of Operations for the Years Ended December 31, 2020 and December 31, 2019
During both 2020 and 2019, we focused on developing our currently owned brands into new markets and by product extensions.
Revenue
For the year ended December 31, 2020, we had revenues of $40,226,865 from sales of our products, as compared to revenue of $29,357,546 for the year ended December 31, 2019. This is comprised of the following categories:
December 31, 2020 | December 31, 2019 | |||||||
| Nutraceuticals | $ | 31,902,291 | $ | 28,149,938 | ||||
| Over the Counter (OTC) | 22,310 | 62,359 | ||||||
| Consumer Goods | 1,201,317 | 706,688 | ||||||
| Cosmeceuticals | 7,100,947 | 438,561 | ||||||
| $ | 40,226,865 | $ | 29,357,546 | |||||
The increase in our Nutraceutical category was due to higher sales. The decrease in the Over the Counter (OTC) category was due to a supply issue with Neuragen during the year. The increase in the Consumer Goods category is due to normalization of business after the 2019 launch of our online application. The increase in the Cosmeceuticals category was due to the launch of a new Hand MD hand sanitizer product.
Cost of Sales
For the year ended December 31, 2020, our cost of sales was $14,578,865. Our cost of sales for the year ended December 31, 2019, was $9,137,602. This is comprised of the following categories:
December 31, 2020 | December 31, 2019 | |||||||
| Nutraceuticals | $ | 9,948,690 | $ | 8,943,967 | ||||
| Over the Counter (OTC) | 24,082 | - | ||||||
| Consumer Goods | 494,591 | 78,109 | ||||||
| Cosmeceuticals | 4,111,502 | 115,526 | ||||||
| $ | 14,578,865 | $ | 9,137,602 | |||||
The increase in our Nutraceutical category was due to higher revenue and a write off of obsolete inventory. The increase in Consumer Goods was due to the write off of obsolete inventory. The increase in Cosmeceuticals was due to higher Hand MD revenue and a write off of obsolete inventory. Management reviews inventory periodically and determines if any products have a limited useful life or are otherwise obsolete and should be written off.
Gross Profit
Gross profit was $25,648,000, or 64% of revenue for the year ended December 31, 2020, as compared to gross profit of $20,219,944 or 69% of revenue for the same period in 2019, an increase of $5,428,056 or 27%. The increase in gross profit is directly related to the increase in net sales. The decrease in gross profit margin is directly related to the mix of products being sold.
Operating Expenses
Selling and Marketing Expenses
For the year ended December 31, 2020, our selling and marketing expenses were $15,889,066 as compared to $11,471,652 for the year ended December 31, 2019. The increase is due to an increase in promotions and advertising, including $2,674,000 in media spending related to the launch of our national television advertising campaign for FOCUSfactor, with the balance representing in-store promotional and advertising support to drive sales at our retailers.
Bad debts
For the year ended December 31, 2020, our bad debts expenses were $74,068. For the year ended December 31, 2019, our bad debts expenses were $283,971. The decrease is due to the write off of a single customer in each of 2020 and 2019 as a result of a customer entering Chapter 11 bankruptcy and slow payment, respectively.
General and Administrative Expenses
For the year ended December 31, 2020, our general and administrative expenses were $6,499,344. For the year ended December 31, 2019, our general and administrative expenses were $5,493,433. The increase is due to higher operating costs, which correlate with an increase in revenue.
Impairment of Intangible Assets
For the year ended December 31, 2020, our impairment of intangible assets expenses were $47,002 as compared to $9,715,137 for the year ended December 31, 2019. The decrease is due to the impairment of goodwill and indefinite life intangible assets in 2019 as a result of management’s reevaluation of such intangible assets arising out of the purchase of FOCUSfactor and Flat Tummy.
Depreciation and Amortization Expenses
For the year ended December 31, 2020, our depreciation and amortization expenses were $100,265 as compared to $1,211,861 for the year ended December 31, 2019. The decrease is due to the impairment of intangible assets during 2019, thus lower amortization costs during 2020.
Other Income and Expenses
For the years ended December 31, 2020 and December 31, 2019, we had other (income) and expense items of the following:
| Year ended December 31, 2020 | Year ended December 31, 2019 | |||||||
| Interest income | $ | (104 | ) | $ | (414 | ) | ||
| Interest expense | 1,186,034 | 981,105 | ||||||
| Remeasurement (gain) loss on translation of foreign subsidiary | (457,955 | ) | 8,280 | |||||
| Amortization of debt issuance cost | 48,432 | 130,829 | ||||||
| Other income | (210,284 | ) | – | |||||
| Other expense (loan success fee) | 1,000,000 | – | ||||||
| Finance expense | 61,000 | – | ||||||
| Total | $ | 1,627,123 | $ | 1,119,800 | ||||
The increase in interest expense in 2020 was due to our May 2020 working capital loan. In 2020 we received SBA PPP Loan proceeds from the United States government and Cash Flow Boost from the Australian government related to COVID-19 relief payments, which are included in other income.
Income tax expense
For the year ended December 31, 2020, we incurred income tax expense of $0. For the year ended December 31, 2019 we incurred income tax expense of $131,537 relating to an accrual of estimated future taxes.
Net Income (Loss)
For the year ended December 31, 2020, our net income was $1,411,132. For the year ended December 31, 2019 our net loss was $9,207,447. This was due to increase in impairment of intangible assets during 2019.
Liquidity and Capital Resources
Overview
As of June 30, 2021, we had $966,398 cash on hand. In addition, we had $20,000,000 available for certain future acquisitions under our credit facility with Knight, and restricted cash of $100,000 which is held for credit card collateral.
Our sources of cash have historically consisted of proceeds from issuances of loans from related parties and revenues generated from operations. We believe the existing cash and cash equivalents and cash provided by sales of our products will be sufficient to meet working capital and capital expenditure needs for at least the next 12 months. Our future capital requirements will depend on many factors, including growth rate, the timing and extent of spending to support development efforts, the expansion of sales and marketing activities, the introduction of new and enhanced product offerings, and the continuing market acceptance of our products. In the future, we may enter into arrangements to acquire or invest in complementary businesses, services and technologies, including intellectual property rights.
We may be required to seek additional equity or debt financing, including to fund these acquisitions or investments. In the event that additional financing is required from outside sources, we may not be able to raise it on terms acceptable to us or at all. If we are unable to raise additional capital or generate cash flows necessary to expand operations and invest in new products, our ability to compete successfully could be reduced and our results of operations may be adversely impacted.
Short- and Long-Term Borrowings
On January 22, 2015, we entered into a Loan and Security Agreement (“Loan Agreement”) with Knight Therapeutics (Barbados) Inc. (“Knight”), pursuant to which Knight agreed to loan us $6,000,000, and which amount was borrowed at closing for the purpose of acquiring the FOCUSfactor business. At closing, we paid Knight an origination fee of $120,000 and a work fee of $60,000 and also paid $40,000 of Knight’s expenses associated with the loan. The loan bore interest at a rate of 15% per year; provided, however, that upon the occurrence of an equity or convertible equity offering by us of at least $1,000,000, the interest rate will drop to 13% per year. Interest accrues quarterly and is payable in arrears on March 31, June 30, September 30 and December 31 in each year, beginning on March 31, 2015. This loan was fully repaid in 2018.
On June 26, 2015, the Company, through its wholly owned subsidiary, Neuragen Corp. (“Neuragen”), issued a 0% promissory note in a principal amount of $950,000 in connection with an asset purchase agreement. The note requires $250,000 to be paid on or before June 30, 2016, and $700,000 to be paid in quarterly installments (beginning with the quarter ending September 30, 2015) equal to the greater of $12,500 or 5% of U.S. dollars.net sales, and 2% of U.S. net sales of Neuragen for 60 months thereafter. The Company’spayment of such amounts is secured by a security interest in certain assets, undertakings and property pursuant to the security agreement, which will be released upon receipt of total payments of $1,200,000. The balance at June 30, 2021 was $425,000.
On November 12, 2015, we entered into a First Amendment to Loan Agreement (“First Amendment”) with Knight, pursuant to which Knight agreed to loan us an additional $5,500,000, and which amount was borrowed at closing for the purpose of acquiring Breakthrough Products, Inc. and NomadChoice Pty Limited through stock purchase agreements. At closing, we paid Knight an origination fee of $110,000 and a work fee of $55,000 and also paid $24,000 of Knight’s expenses associated with the loan. The loan bears interest at a rate of 15% per year. The interest rate will decrease to 13% if we meet certain equity fundraising targets. The First Amendment matured on November 11, 2017. This loan was fully repaid in 2017.
On August 9, 2017, we entered into an Amended and Restated Loan Agreement with Knight, pursuant to which Knight agreed to loan us an additional $10,000,000, which amount was borrowed at closing for working capital purposes, and an ongoing credit facility of up to $20,000,000 for use in connection with future mergers and acquisitions. At closing, we paid Knight an origination fee of $200,000 and a work fee of $100,000 and also paid $100,000 of Knight’s expenses associated with the loan.
On May 8, 2020, we entered into a Third Amendment Agreement (the “Third Amendment”) to the Amended and Restated Loan Agreement with Knight, pursuant to which Knight agreed to loan us an additional $2,500,000 (the “Additional Loan”). We paid Knight a work fee of $36,000, and $25,000 for Knight’s legal costs and expenses incurred in connection with the Third Amendment. The Additional Loan had a maturity date of May 8, 2021 and bears interest at 12.5% per annum compounding quarterly. During the six months ended June 30, 2021, $2,000,000 of the Additional Loan was repaid and the remaining balance has been extended to a maturity date of December 31, 2021. The loan balance of the Additional Loan at June 30, 2021 was $500,000.
The terms of the $10,000,000 loaned in connection with the Amended and Restated Loan Agreement were modified by the Third Amendment. This tranche bears interest from May 8, 2020 at a rate equal to 12.5% per annum compounded quarterly. We agreed to pay a success fee in the amount of $1,000,000 with respect to this tranche, which shall be fully earned on May 8, 2020 and payable no later than August 31, 2022. The success fee shall bear interest at 12.5% per annum compounding quarterly. The loan has been extended to a maturity date of December 31, 2021. The loan balance at June 30, 2021 was $5,000,000.
As amended, the Amended and Restated Loan Agreement includes customary representations, warranties, and affirmative and restrictive covenants, including covenants to attain and maintain certain financial metrics (including maintenance at all times of a cash balance of $600,000, Adjusted EBITDA of at least $3,000,000 for the 12 months ending on June 30, 2020, Adjusted EBITDA of at least $4,000,000 for each 12 month period ending on the last day of each fiscal year-endquarter thereafter), and to not merge or dispose of assets, acquire other businesses (except for businesses substantially similar or complementary to our business, provided that the aggregate consideration to be paid does not exceed $100,000 and the acquired business guarantees our obligations under the Amended and Restated Loan Agreement, or for qualifying acquisitions financed under our $20,000,000 credit facility for future mergers and acquisitions, among other permitted acquisitions) or make capital expenditures in excess of $500,000. The Amended and Restated Loan Agreement also includes customary events of default, including payment defaults, breaches of covenants, change of control and material adverse effect defaults. Upon the occurrence of an event of default and during the continuation thereof, the principal amount of all loans under the Amended and Restated Loan Agreement will bear a default interest rate of an additional 5%.
During the year ended December 31, 2020, we received funding (the “PPP Loan”) through the U.S. Small Business Administration’s Paycheck Protection Program (“PPP”) of $163,542, which bears interest at 1% per annum, with monthly installments commencing on May 5, 2020 for 60 months through its maturity on May 5, 2025. We used all proceeds from the PPP Loan to maintain payroll and other allowable expenses. In July 2021, the PPP Loan was forgiven in full. Our subsidiary, Nomad Choice Pty Ltd, received the Cash Flow Boost from the Australian government. This assistance was received from March 2020 through September 2020 in the amount of $46,742 and does not need to be repaid.
On May 16, 2021, we entered into a loan agreement in the amount of $565,000 with Shopify Capital Inc. for an advancement of working capital from our online processing account. We received $500,000 from Shopify Capital Inc. and $65,000 of original issue discount. The loan bears a repayment rate of 14% of daily sales and we shall pay $94,167 every 60 days with a final payment due on May 12, 2022. The payment of such amounts is July 31.
Operating Activities
For the six months ended June 30, 2021, net cash provided by operating activities was $1,312,136, compared to net cash used in operating activities of $2,521,575 for the six months ended June 30, 2020. This increase in net cash provided by operating activities for the six months ended June 30, 2021 was primarily attributable to a decrease in accounts receivable and an increase in accounts payable and accrued expenses.
For the six months ended June 30, 2021, net cash provided by operating activities of $1,312,136 consisted of our net income of $2,640,429 adjusted by:
| Amortization of debt issuance cost | $ | 11,877 | ||
| Foreign currency transaction loss | (348,208 | ) | ||
| Remeasurement loss on translation of foreign subsidiary | 89,760 | |||
| Non cash implied interest | 17,442 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts receivable | 1,326,856 | |||
| Loan receivable, related party | (783,293 | ) | ||
| Inventory | (4,717,766 | ) | ||
| Prepaid expense | (80,210 | ) | ||
| Prepaid expense, related party | 44,545 | |||
| Income taxes receivable | 116,074 | |||
| Contract liabilities | (73,139 | ) | ||
| Accounts payable and accrued liabilities | 3,125,110 | |||
| Accounts payable, related party | (217,761 | ) |
For the six months ended June 30, 2020, net cash used by operating activities of $2,521,575 consisted of our net income of $1,101,350 adjusted by:
| Amortization of debt issuance cost | $ | 42,141 | ||
| Depreciation and amortization | 53,544 | |||
| Stock based compensation expense | 77,358 | |||
| Foreign currency transaction loss | 190,007 | |||
| Remeasurement loss on translation of foreign subsidiary | (12,216 | ) | ||
| Non cash implied interest | 9,298 | |||
| Reversal of allowance for doubtful accounts | (170,309 | ) | ||
| Gain on write-off of payables | (180,000 | ) | ||
| Accrual of loan success fee | 1,000,000 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts receivable | (3,610,852 | ) | ||
| Loan receivable, related party | (45,179 | ) | ||
| Inventory | (337,369 | ) | ||
| Prepaid expense | (344,414 | ) | ||
| Prepaid expense, related party | – | |||
| Income taxes receivable | 226,686 | |||
| Contract liabilities | 9,250 | |||
| Accounts payable and accrued liabilities | (791,573 | ) | ||
| Accounts payable, related party | 170,345 |
For the year ended December 31, 2020, we had net cash used in operating activities of $1,588,248 as compared to $2,946,350 of net cash provided by operating activities for the year ended December 31, 2019. The decrease was attributable to a decision in 2020 to purchase inventory and ship through a third party rather than shipping directly from our manufacturers, and the increase in accounts receivable and the increase in a related party loan receivable in 2020 due to higher revenues in the normal course of our growth.
For 2020, net cash used in operating activities of $1,588,248 consisted of our net income of $1,411,132 adjusted by:
| Amortization of debt issuance cost | $ | 48,432 | ||
| Depreciation and amortization | 100,265 | |||
| Stock based compensation expense | 128,929 | |||
| Impairment of intangible assets | 47,002 | |||
| Foreign currency transaction loss | 351,194 | |||
| Bad debts | 74,068 | |||
| Remeasurement loss on translation of foreign subsidiary | (457,955 | ) | ||
| Non cash implied interest | 36,455 | |||
| Reversal of allowance for doubtful accounts | (237,768 | ) | ||
| Gain on write-off of payables | (180,000 | ) | ||
| Accrual of loan success fee | 1,000,000 | |||
| Write-off of inventory | 527,737 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts receivable | (2,070,032 | ) | ||
| Loan and accounts receivable, related party | (2,028,524 | ) | ||
| Inventory | (2,133,145 | ) | ||
| Prepaid expenses | (558,346 | ) | ||
| Prepaid expense, related party | (492,741 | ) | ||
| Income taxes receivable | (280,304 | ) | ||
| Income taxes payable | 300,773 | |||
| Contract liabilities | 113,916 | |||
| Accounts payable and accrued liabilities | 3,251,816 | |||
| Accounts payable, related party | (541,152 | ) |
For 2019, net cash provided by operating activities of $2,946,350 consisted of our net loss of $9,207,447 adjusted by:
| Amortization of debt issuance cost | $ | 130,829 | ||
| Depreciation and amortization | 1,211,860 | |||
| Stock based compensation | 201,155 | |||
| Impairment of intangible assets | 9,715,137 | |||
| Foreign currency transaction loss | (1,308 | ) | ||
| Bad debts | 283,972 | |||
| Remeasurement loss on translation of foreign subsidiary | 8,280 | |||
| Non cash implied interest | 38,310 | |||
| Write-off of inventory | 257,111 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts receivable | 3,027,900 | |||
| Accounts receivable, related party | (277,432 | ) | ||
| Inventory | 552,158 | |||
| Prepaid expenses | 642,704 | |||
| Income taxes receivable | 135,072 | |||
| Contract liabilities | (41,823 | ) | ||
| Accounts payable and accrued expenses | (2,910,949 | ) | ||
| Accounts payable, related party | (819,179 | ) |
Investing Activities
For the six months ended June 30, 2021, net cash used in investing activities was $0, compared to net cash used in investing activities of $130,528 for the six months ended June 30, 2020.
For the years ended December 31, 2020 and 2019, we used net cash of $0 in investing activities.
Financing Activities
For the six months ended June 30, 2021, net cash used in financing activities was $1,618,123, compared to net cash used in financing activities of $1,975,000 for the six months ended June 30, 2020.
Financing activities during the six months ended June 30, 2021 and June 30, 2020:
| Six months ended June 30, 2021 | Six months ended June 30, 2020 | |||||||
| Advances from related party | $ | – | $ | 70,490 | ||||
| Repayments of advances to related party | – | (70,490 | ) | |||||
| Proceeds of note payable, related party | – | 2,500,000 | ||||||
| Proceeds of short term debt | 500,000 | – | ||||||
| Repayment of short term debt | (118,123 | ) | – | |||||
| Repayment of notes payable, related party | (2,000,000 | ) | (525,000 | ) | ||||
For the year ended December 31, 2020, financing activities provided $1,950,000, as compared to $2,050,000 used in financing activities for the year ended December 31, 2019. The increase was attributable to the issuance of a new note in 2020.
Financing activities during 2020:
| Proceeds of notes payable | $ | 2,500,000 | ||
| Repayment of notes payable | (550,000 | ) | ||
| Advances from related party | 74,629 | |||
| Repayments of advances to related party | (74,629 | ) |
Financing activities during 2019:
| Repayment of notes payable | $ | (2,050,000 | ) | |
| Advances from related party | 324,102 | |||
| Repayments of advances to related party | (324,102 | ) |
Key Near-Term Initiatives
During the remainder of 2021 and through 2022, we intend to organically grow our current product lines by developing and launching new products and expanding into new markets. Specifically, for Flat Tummy we are expecting to launch a superfruits gummy and an ashwagandha/calming gummy in the fourth quarter of 2021, and three new protein shakes, a protein ready-to-drink beverage, hydration powder and preworkout powder in 2022. For FOCUSfactor, we are working on developing four new products targeted to launch beginning in 2022, including a “maximum strength” formula, an “ultimate” formula, ready-to-drink beverages and a nootropic line. Another major initiative is increasing investment in our TV advertising campaign for our FOCUSfactor brand. Additionally, in the fourth quarter of 2021, we are planning the launch of three core FOCUSfactor products in the United Kingdom and Australia. Lastly, we intend to grow further through additional strategic acquisitions and we continue to evaluate opportunities and candidates that we believe fit well with our brand portfolio. With the proceeds from this offering, we expect to accelerate our acquisition strategy, in particular with candidates with whom we are in discussions. Certain of these acquisitions, if consummated, would require us to incur additional debt.
Off-Balance Sheet Arrangements
During the six months ended June 30, 2021, and during the years ended December 31, 2020 and 2019, we had no off-balance sheet arrangements.
Inflation
The effect of inflation on our operating results was not significant in the six months ended June 30, 2021 or the years ended December 31, 2020 and 2019.
Critical Accounting Policies
Management’s Discussion and Analysis of Financial Condition and Results of Operations discusses our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements in accordance with U.S. generally accepted accounting principles requires management to make estimates and assumptionsjudgments that affect the reported amounts of assets, liabilities, revenues and liabilities at the date of the financial statementsexpenses, and the reported amountsrelated disclosures of net revenuecontingent assets and expenses in the reporting period. We regularly evaluate ourliabilities. On an on-going basis, management evaluates its estimates and assumptionsjudgments, including those related to the useful liferevenue recognition and recoverability of long-lived assets, stock-based compensation and deferred income tax asset valuation allowances. We base ourallowance for doubtful accounts. Management bases its estimates and assumptionsjudgments on current facts, historical experience and on various other factors that we believeare believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions and conditions.
Management believes the following critical accounting policies, among others, affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Use of Estimates
In preparing the consolidated financial statements, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities as of the date of the statements of financial condition, and revenues and expenses for the years then ended. Actual results may differ significantly from those estimates. Significant estimates made by management include, but are not limited to, the assumptions used to calculate stock-based compensation, derivative liabilities and common stock issued for services. Significant estimates are assumptions about collection of accounts receivable, current income taxes, deferred income taxes valuation allowance, useful life of fixed and intangible assets, and assumptions used in Black-Scholes-Merton, or BSM, valuation methods, such as expected volatility, risk-free interest rate, and expected dividend rate. The actual results experienced by us July differ materiallyof any changes in accounting estimates are reflected in the financial statements in the period in which the changes become evident. Estimates and adverselyassumptions are reviewed periodically, and the effects of revisions are reflected in the period that they are determined to be necessary.
Revenue recognition
We recognize revenue in accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”). Revenues are recognized when control is transferred to customers in amounts that reflect the consideration we expect to be entitled to receive in exchange for those goods. Revenue recognition is evaluated through the following five steps: (i) identification of the contract, or contracts, with a customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied.
We recognize revenue upon shipment from our estimates. Tofulfillment centers. Certain of our distributors may also perform a separate function as a co-packer on our behalf. In such cases, ownership of and title to our products that are co-packed on our behalf by those co-packers who are also distributors, passes to such distributors when we are notified by them that they have taken transfer or possession of the extent there are material differences betweenrelevant portion of our estimatesfinished goods. Freight billed to customers is presented as revenues, and the actual results, our future resultsrelated freight costs are presented as cost of operationsgoods sold. Cancelled orders are refunded if not already dispatched, refunds are only paid if stock is damaged in transit, discounts are only offered with specific promotions and orders will be affected.
Revenues from sale of Hand MD hand sanitizers in 2020 were recognized in accordance with our revenue recognition policies which are based upon ASC 606. All product sales were initiated based upon the retailer’s purchase orders at a fixed transaction price and revenues recognized when the products were delivered and accepted by the customer.
Contract Liabilities - Deferred Revenue
Our contract liabilities consist of advance customer payments and deferred revenue. Deferred revenue results from transactions in which we have been paid for products by customers, but for which all revenue recognition criteria have not yet been met. Once all revenue recognition criteria have been met, the deferred revenues are recognized.
Income Taxes
We utilize FASB ASC 740, “Income Taxes,” which requires the recognition of deferred tax liabilitiesassets and assetsliabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Under this method, deferred tax liabilitiesassets and assetsliabilities are determined based on the difference between the financial statement and tax basesbasis of assets and liabilities usingand their financial reporting amounts based on enacted tax laws and statutory tax rates in effect forapplicable to the yearperiods in which the differences are expected to reverse. The Company computesaffect taxable income. A valuation allowance is recorded when it is “more likely-than-not” that a deferred tax asset benefits forwill not be realized.
We generated a deferred tax asset through net operating losses carried forward. The potential benefitloss carry-forward. However, a valuation allowance of net operating losses100% has not been recognized in these financial statements becauseestablished due to the Company cannot be assured it is more likely than not it will utilizeuncertainty of our realization of the net operating losses carriedloss carry forward prior to its expiration.
NomadChoice Pty Ltd, our wholly-owned foreign subsidiary, is subject to income taxes in future years.the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. We recognize liabilities for anticipated tax audit issues based on our current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Synergy CHC Inc. is a wholly-owned foreign subsidiary, is subject to income taxes in the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. We recognize liabilities for anticipated tax audit issues based on our current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Effect of Exchange Rate on Results
The Company reportsfunctional currency of one of our foreign subsidiaries (NomadChoice Pty Ltd.) is the U.S. Dollar. This foreign subsidiary maintains its records using local currency (Australian Dollar – “AUD”). All monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at period end exchange rates, non-monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at transaction day exchange rates. Income and expense items related to non-monetary items were translated at exchange rates prevailing during the transaction date and other incomes and expenses were translated using average exchange rate for the period. The resulting translation adjustments were recorded in statements of operations as Remeasurement gain or loss on translation of foreign subsidiary.
The functional currency of our other foreign subsidiary (Synergy CHC Inc.) is the Canadian Dollar (CAD). This foreign subsidiary maintains its records using local currency (CAD). All assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at period end exchange rates and stockholders’ equity is translated at the historical rates. Income and expense items were translated using average exchange rate for the period. The resulting translation adjustments, net loss per shareof income taxes, are reported as other comprehensive income and accumulated other comprehensive income in the stockholder’s equity in accordance with provisionsASC 220 – Comprehensive Income.
The exchange rates used to translate amounts in AUD and CAD into USD for the purposes of preparing the consolidated financial statements were as follows:
Balance sheet:
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Period-end AUD: USD exchange rate | $ | 0.7708 | $ | 0.7030 | ||||
| Period-end CAD: USD exchange rate | $ | 0.7854 | $ | 0.7699 | ||||
Income statement:
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| Average Yearly AUD: USD exchange rate | $ | 0.6902 | $ | 0.6954 | ||||
| Average Yearly CAD: USD exchange rate | $ | 0.7463 | $ | 0.7537 | ||||
Translation gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are translated into either Australian Dollars or Canadian Dollars, as the case may be, at the rate on the date of the FASB.transaction and included in the results of operations as incurred.
Our Company
We are a provider of consumer health care, beauty, and lifestyle products. Our current brand portfolio consists of two marquee brands, FOCUSfactor, a patented brain health supplement that has been clinically shown to improve memory, concentration and focus, and Flat Tummy, a lifestyle brand that provides a suite of nutritional products to help women achieve their weight management goals, and a developing brand, Hand MD, consisting of a full range of preventative, restorative, and anti-aging skin care products designed for the hands. During the year ended December 31, 2020, FOCUSfactor represented 62% of our net revenue, Flat Tummy was 17%, and Hand MD was 17%. During the six months ended June 30, 2021, FOCUSfactor represented 71% of our net revenue, Flat Tummy was 29%, and Hand MD was 0.2%. Our products are sold through some of the nation’s leading club, mass drug, and other retailers such as Costco, Amazon.com, Walmart, Walgreens, CVS, The provisions require dual presentationVitamin Shoppe, Target.com, H-E-B, and SuperValu.
We built our brand portfolio through strategic acquisitions. We acquired the FOCUSfactor brand in January 2015 for cash consideration of basic$6.0 million, including earnout. Our Hand MD brand was acquired for $1.5 million of our stock in August 2015. In November 2015, we acquired our second marquee brand – Flat Tummy – for AUD 10.0 million (or approximately $7.0 million), using a mix of cash and diluted loss per share. Basicstock. Our capital structure following the acquisition of our key brands in 2015 was highly levered, and our focus was on paying our debt as we did not have the resources to grow our business. We have grown our FOCUSfactor brand from 2 SKUs at acquisition to 16 SKUs, and our Flat Tummy Brand from 1 SKU to 18 SKUs.
With the outbreak of the COVID-19 pandemic in North America during the first three months of 2020, we extended our Hand MD brand to produce and market a range of hand sanitizers. We deployed the cash flow from hand sanitizer sales for a television advertising strategy to drive growth in our flagship FOCUSfactor brand products. Our FOCUSfactor advertising campaigns have aired on major news and entertainment networks such as Fox News, CNN, MSNBC, TLC, and TNT, targeting adults 45 years of age and older. As a result, net loss per share excludesrevenue for the impactyear ended December 31, 2020 was $40.2 million, an increase of common stock equivalents. Diluted$10.9 million, or 37.0%, over net loss per share utilizesrevenue for the average market price per share when applyingyear ended December 31, 2019. Net revenue for the treasury stock methodsix months ended June 30, 2021 was $24.8 million, an increase of $6.9 million, or 38.3% over net revenue for the six months ended June 30, 2020. In particular, FOCUSfactor net revenue continued to benefit from our advertising strategy, increasing to $17.5 million for the six months ended June 30, 2021, a 124% increase over the same period in determining common stock equivalents. As2020.
Following the completion of Januarythis offering, we intend to use the proceeds to repay in full our outstanding debt, to accelerate the growth of our current brands through advertising and otherwise, and to drive our acquisition strategy as we have a pipeline of potential near-term acquisition targets that we are eager to pursue. Given the success of our FOCUSfactor advertising campaigns – in which we have achieved approximately a $4 increase in gross revenue for each incremental $1 of advertising spend – we believe that we can meaningfully expand our FOCUSfactor campaigns. In addition, we have tailored strategies for our Flat Tummy and Hand MD brands to maximize our return on investment and leverage our experience with both traditional and digital marketing. Our asset-light business model, in which we partner with third-party manufacturers to produce our brand offerings, allows us to scale quickly and profitably while satisfying growing demand.
For the six months ended June 30, 2021, our net revenues, net income and Adjusted EBITDA were $24.8 million, $2.6 million and $3.5 million, respectively, representing an increase of 38.3%, 139.7%, and 13.4% over the same period in the prior fiscal year. During the year ended December 31, 20132020, our net revenues, net income (loss) and 2012, thereAdjusted EBITDA were no common stock equivalents outstanding.
Our Brands
Our flagship brand, FOCUSfactor, is requireda brain health nutritional supplement with over 15 years of heritage and a patented formula comprised of a proprietary blend of key brain supporting ingredients along with vitamins, minerals, and other nutrients. We believe FOCUSfactor is the only product in its category whose entire patented formula has been clinically shown to estimatesupport memory, concentration and focus. Our FOCUSfactor brand consists of 16 SKUs and is sold primarily through leading retailers in the fair valueUnited States, including Costco, Walmart, Amazon.com, Walgreens, and CVS, in addition to selling direct to consumer through the FOCUSfactor website. Across three of all financial instruments included on its balance sheetour key partners, we have increased the number of SKUs sold through the retailer from the single SKU available at the beginning of our relationship in 2015 or 2016. In addition, we have increased our presence in retail locations for these key partners, resulting in a significant increase in retail doors, being the number of SKUs multiplied by the number of retail locations for each retailer.
Our second marquee brand, Flat Tummy, consists of a range of lifestyle products and accessories including tea, shakes, lollipops, supplements, apparel, and exercise accessories. We also provide a Flat Tummy mobile app, which as of January 31, 2013June 30, 2021 had 1.3 million unique downloads and July 31, 2012.is intended as a tool to promote the Flat Tummy lifestyle centered around general wellness and health. Our Flat Tummy brand consists of 18 SKUs and is sold direct to consumer through the Flat Tummy website and application, as well as through retailers such as Amazon.com and CVS.
Our developing brand, Hand MD, is a beauty-focused brand consisting of an anti-aging skincare line formulated specifically for the hands. The Company’s financial instruments consistproduct line includes serums and creams for exfoliating, skin repair and rehydration as well as hand soaps and hand sanitizers. The Hand MD brand consists of cash. The Company considers8 SKUs and is sold online through the carrying value of such amountsHand MD website as well as through Amazon.com.
We also own five additional, non-core brands. While we may elect to promote these brands and commercialize their products in the financial statements to approximate their fair valuefuture, we have prioritized our key FOCUSfactor, Flat Tummy, and Hand MD brands, and management is focused on the growth of these core products.
Our net revenues by brand for the six months ended June 30, 2021 are below:
Net Revenue by Brand for the Six Months Ended June 30, 2021(1)

| (1) | Hand MD represented 0.2% of our net revenue for the six months ended June 30, 2021. |
Our Competitive Strengths
We believe that we have attributes that differentiate us from our competitors and provide us with significant competitive advantages. Our key competitive strengths include:
Well-Positioned in Growing Categories Driven by Favorable Consumer Trends
An increased focus on health, beauty and wellness by consumers has served as a tailwind for our brands. The nutritional supplement market has experienced significant growth across a range of areas including immune health, brain health, heart health, sleep/stress, and overall nutrition and wellness as a result of an aging population, increased obesity, pandemic concerns and a desire for more natural solutions and treatments over prescription medication. Additionally, there is an increased demand on hand cleansing and hand sanitizing products due to the short-term natureCOVID-19 pandemic, which is also driving demand for hand moisturizing products to soothe dried, chapped hands due to frequent hand washing and sanitizing. We believe that we are well positioned to benefit from these favorable trends. Our FOCUSfactor, Flat Tummy and Hand MD brands have seen strong growth with net revenues up 38% year-over-year in the six months ended June 30, 2021. We believe our focus on lifestyle products has also benefited from the growth and prevalence of social media.
Clinically Shown Results Backed by Independent Study for FOCUSfactor
We believe FOCUSfactor is the only product in its category with both a patent and a clinical study to support the product claims for improved memory, concentration, and focus. FOCUSfactor has been tested in a single-center, randomized, double-blind, placebo-controlled, parallel group study to evaluate its effect on memory, concentration, and focus in healthy adults.
In this study, FOCUSfactor was tested on its entire 52-ingredient formulation rather than testing one or two ingredients within a formulation. FOCUSfactor was shown to provide a 44% increase in recall memory after six weeks of use versus placebo. This differentiates FOCUSfactor from other brain-health supplements and is a prime reason why FOCUSfactor has been placed in premier retailers. See “ — FOCUSfactor Clinical Study” for additional information.
Experienced Management Team with Proven Track Record of Value Creation
Our executive team has a combined 90 years of experience in consumer marketing and distribution and has been instrumental in acquiring and building our core brands. Management has exercised strong financial discipline in its acquisition strategy, with a focus on acquiring brands at attractive valuations. For example, we acquired FOCUSfactor for approximately 3x trailing EBITDA. For the six months ended June 30, 2021, the year ended December 31, 2020 and the year ended December 31, 2019, FOCUSfactor generated net revenue of $17.5 million, $24.9 million and $18.9 million, respectively. Management’s philosophy is to acquire promising brands that fit within our health, beauty and lifestyle offerings, and apply our proven marketing and distribution strategies to develop brands to their full potential. We believe we are adept at identifying promising opportunities that build out and complement our core brand portfolio.
Premier Retail Partners
Our premier retail partners include Costco, Walmart, Amazon.com, Walgreens, and CVS. We sell products to these partners under their standard arrangements, which do not include a term or duration as sales under each vendor agreement are generally made on a purchase order basis, and do not include any termination provisions. Our partners provide a platform to expand the breadth of our current offerings through product line extensions and new product innovation. We continue to introduce new SKUs to our current retail partners, such as the addition of FOCUSfactor Gummies to our membership club channel. Additionally, the international footprint of certain of our various retail partners facilitates our geographic expansion plans.
Scalable and Flexible Asset-Light Model to Support Growth
Our focus is on brand management, marketing, product development and distribution, and we utilize contract manufacturing partners in order to produce our various brand offerings. The use of third-party manufacturing partners allows us to scale quickly, as we ensure that our partners have sufficient capacity to meet our demand needs. We also maintain multiple relationships with different contract manufactures, ensuring diversification of our manufacturing base and reducing the likelihood of supply bottlenecks or deficits that could potentially slow our growth.
Our Growth Strategy
We intend to drive growth and increased profitability in our business through these key elements of our strategy:
Broaden Media Advertising Strategy
We have experienced a significant acceleration in sales growth for the FOCUSfactor brand as a result of our television advertising. We launched a national advertising campaign in August 2020, which has aired on major news and entertainment networks such as Fox News, CNN, MSNBC, TLC, and TNT, targeting adults 45 years of age and older. Since initiating our advertising strategy, FOCUSfactor net revenue has nearly doubled at key retailers. Based on these recent television campaigns, we estimate that we have experienced approximately $4 of gross revenue growth for every incremental $1 of advertising spend. We believe that we have significant growth potential through increased advertising budgets, and we expect to deploy a portion of the proceeds from this offering to expand our advertising campaigns. In particular, we anticipate a coordinated expansion of our television advertising strategy as we focus on pushing additional SKUs within our retail sales partner network to continue to build brand awareness and increase reach for FOCUSfactor. We also plan to invest in online marketing to promote all of our brands, including social media and influencer driven marketing.
Acquire Brands which Complement Our Existing Portfolio
We will continue to evaluate acquisition opportunities that we believe fit well within our brand portfolio and create value for our stockholders, such as further retail expansion in nutraceuticals and market expansion in health and beauty. In spite of historical capital constraints, our opportunistic approach to acquisitions has resulted in a successful track record of identifying promising targets that align with our overall brand strategy in the health, beauty and lifestyle segments. With the proceeds from this offering, we expect to accelerate our acquisition strategy, focusing on acquisition targets that management believes have the greatest synergistic potential, enabling us to significantly grow our product offerings and reach.
Partner with Additional Leading Retailers to Expand the Reach of Our Products
We have established distribution relationships with premier retail partners, including Costco, Walmart, Amazon.com, Walgreens, CVS, The Vitamin Shoppe, Target.com, H-E-B and SuperValu. Based on the success of our products with these leading retail partners, we believe that we are well positioned to add new retailers that will enhance our distribution footprint. We believe we have expansion opportunities with food retailers, including those focused on health foods. We intend to introduce seven new SKUs across three potential retailers, which would potentially result in the addition of approximately 50,000 retail doors.
Diversify Our Geographic Presence through Entry into New Markets
We seek to accelerate our sales growth by expanding and further diversifying our geographic footprint. In the year ended December 31, 2020, markets outside of North America represented 0.5% of our total net revenues. Our goal is to increase our net revenues generated from new markets. As we target new international markets, our strategy is to develop highly competitive and differentiated products that are produced in-country for ease of entry, with support from our regulatory group and an in-country regulatory consultant to help expedite the approval process. We currently plan to enter the United Kingdom and Australia markets in 2022, initially with FOCUSfactor, followed by Flat Tummy and Hand MD. We then plan to expand our brands into Asian markets and Mexico in late 2022 and 2023. In the United Kingdom and Australia, we have established relationships with manufacturers who will be producing FOCUSfactor in each country by December 2021. In addition, we are developing our marketing plans in compliance with applicable law, and are initiating retailer meetings as we seek to gain distribution across retail channels. In Mexico, we are currently awaiting regulatory review of our FOCUSfactor labels by the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS). Following approval, we will establish in-country production with local manufacturers and begin connecting with retailers in Mexico. Our planned expansion into Asian markets is in early stages, and we are working with regulatory consultants to develop our plans.
| 33 |
Use Innovative Strategies to Boost Consumer Engagement
We have made investments in promoting apps for Flat Tummy and view this as a key aspect of growing our customer base and maintaining high levels of engagement. We have also focused on developing our social media presence, in particular through Instagram, in order to foster and grow our relationship with customers. Our brands appeal to both specific consumer needs as well as lifestyle choices and we seek to deepen our understanding of our customers and boost recognition of our brands through increased engagement.
Continue to Develop and Expand Our Current Brands
Our plan is to further develop and expand our brands by reaching a broader set of customers through advertising and product expansion. More specifically, we look to develop new products for our brands to satisfy the various customer segment opportunities (i.e. baby boomers, millennials, etc.) and satisfy various consumer needs as they relate to new and improved formulations, expanded and improved product benefits, alternative delivery formats and sizes. As we increase the product line-up behind our brands, we leverage our current retail distribution network by expanding our presence as well as adding incremental distribution with new retail partners. With a broader brand presence, we believe our advertising becomes even more efficient at driving sales velocity.
This is evidenced by our expanded FOCUSfactor product line, including gummies that are marketed to both adults and children and liquid energy and focus shots for a younger adult audience. Beginning in 2022, we plan to introduce additional FOCUSfactor products, including a “maximum strength” formula, an “ultimate” formula, ready-to-drink beverages and a nootropic line. The Flat Tummy brand has strategically added complementary products such as new shake options and supplements to appeal to a broader consumer group. In the fourth quarter of 2021, we plan to introduce a Flat Tummy superfruits gummy and an ashwagandha/calming gummy. Beginning in 2022, we plan to introduce additional Flat Tummy products, including three new protein shakes, a protein ready-to-drink beverage, hydration powder and preworkout powder. Additionally, we plan to employ this strategy of expanding our brands into international markets that include the United Kingdom, Australia and Asia, among others.
Marketing and Sales
Our targeted, consumer-driven marketing strategy has been key to building our brands and driving revenue growth. We manage dedicated marketing strategies for each of our brands in order to build deep connections with our customers.
FOCUSfactor. Our marketing strategy for FOCUSfactor is primarily focused on television advertising campaigns with leading national networks that appeal to the demographics of our wellness focused customer base. Our television advertising campaigns, launched in 2020, have coincided with strong sales results for the brand, with FOCUSfactor net revenue increasing 124% in the six months ended June 30, 2021 compared with the same period in 2020. In the year ended December 31, 2020, FOCUSfactor net revenue increased 38.1% year-over-year, to $24.9 million. As our flagship brand, FOCUSfactor accounted for 71% of our net revenue in the six months ended June 30, 2021, compared with 44% in the six months ended June 30, 2020, 62% in the year ended December 31, 2020 and 64% in the year ended December 31, 2019. The following charts demonstrate the significant growth in FOCUSfactor sales following the launch of our television advertising campaigns in August 2020, at a mass retailer (FOCUSfactor 90-count), a club store (FOCUSfactor 180-count) and a drug store (FOCUSfactor 60-count and FOCUSfactor Extra 60-count).

We also utilize in-store promotions along with online and social media advertising to promote our FOCUSfactor brand. We leverage the following online and social media assets as part of our marketing strategy:
| ● | Website: Our FOCUSfactor website helps to educate and inform consumers on our line of products. The website also serves as a direct-to-consumer sales channel for most FOCUSfactor products. |
| ● | Instagram: Our main social media platform is Instagram. As of June 30, 2021, we had 14,300 followers. |
| ● | FOCUSfactor – Brain Hub App: Early in 2021 we launched our Brain Hub app on the Android and Apple iOS platforms to provide an additional point of engagement with customers. The app contains a library of brain games and guided meditation sessions on topics related to mindfulness and brain health in order to keep consumers engaged. |
Flat Tummy. We employ a primarily online and social media driven strategy for our Flat Tummy brand. The brand is focused primarily on women. We employ campaigns to reach our core target segments through a mix of traditional online advertising as well as influencer-based marketing. In the six months ended June 30, 2021, Flat Tummy accounted for 29% of our net revenue, compared with 25% in the six months ended June 30, 2020, 17% in the year ended December 31, 2020 and 34% in the year ended December 31, 2019.
| ● | Website: Our Flat Tummy website acts as a platform for engagement with our customers. In addition to offering a direct-to-consumer sales channel for our products, we also host a lifestyle blog on our website with a focus on health and fitness. |
| ● | Instagram: Our primary social media platform is Instagram. As of June 30, 2021, we had 1.6 million followers. Our marketing strategy for Flat Tummy seeks to leverage our large online following to promote products from across the Flat Tummy brand. More recently we have engaged with social media influencers as a new strategy to promote our products. |
| ● | Facebook: As of June 30, 2021, we had 552,000 followers. We mainly use the platform to share promotions and to relay content and advertisements. |
| 34 |
| ● | Flat Tummy App: Our Flat Tummy app had 1.3 million unique downloads as of June 30, 2021, across both the Apple and Android platforms. The app provides customized workouts, nutrition information, and diet plans. The app is currently free to customers; however, we are exploring different strategies to monetize our large user base. |
Hand MD. We mainly rely on social media and online advertising for our Hand MD brand. In the six months ended June 30, 2021, Hand MD accounted for 0.2% of our net revenue, compared with 28% in the six months ended June 30, 2020, 17% in the year ended December 31, 2020 and 1% for the year ended December 31, 2019.
| ● | Website: We primarily sell Hand MD products direct to consumer through our website www.handmd.com. We also host a blog on our website to drive consumer engagement and education on our various product offerings. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus. |
| ● | Instagram: We mainly utilize Instagram as our social medial platform for engaging with Hand MD customers. |
FOCUSfactor Clinical Study
FOCUSfactor has been tested in a single-center, randomized, double-blind, placebo-controlled, parallel group study to evaluate its effect on memory, concentration, and focus in healthy adults. A total of 96 subjects were enrolled and randomized to one of the two treatment groups (FOCUSfactor and placebo). The study was sponsored by Factor Nutrition Labs, LLC, the developer of FOCUSfactor, and was conducted in 2011 at Cognitive Research Corporation (“CRC”) in Saint Petersburg, Florida. The sponsor, in consultation with CRC, was responsible for study design including selection of dose, eligibility criteria, efficacy and safety assessments, and vitamin/nutraceutical supply. CRC, a contract research organization, was responsible for data collection, database preparation, overall project management, site monitoring, data management, statistical analyses, and preparation of the final clinical study report.
The primary endpoint was sum recall for five trials of the Rey Auditory Verbal Learning Test (RAVLT), a standardized neuropsychological test of memory. The RAVLT is one of the most commonly used tests of memory in psychopharmacology research. The test was originally developed in the 1940s and has proven useful in evaluating verbal learning and memory, including proactive inhibition, retroactive inhibition, retention, encoding versus retrieval, and subjective organization. The standard RAVLT begins with a subject being read a list of 15 unrelated words at the rate of one word per second. The examiner then asks the subject to recall as many words as possible. This procedure is then repeated four more times with the same list of words and the number of correct responses is summed. This summed score was chosen as the primary outcome measure, or endpoint, for the current study.
The study demonstrated that, compared to placebo, FOCUSfactor improved abilities referred to as memory (i.e., short term memory), attention (e.g., focus), concentration and working memory in healthy adults. Following six weeks of treatment, subjects who received FOCUSfactor had a mean increase in recall of 6.5 words compared to 4.5 words for those who received placebo (t = -4.32, df = 87, p <0.001). The total words recalled over the five trials following six weeks of treatment (corrected for baseline score) was 51.9 words for subjects receiving FOCUSfactor compared to 49.7 words for subjects receiving placebo (t = -2.98, df = 87, p = 0.002). The significant effect on the RAVLT summed score supports the hypothesis that FOCUSfactor improves memory, attention (e.g. focus), and concentration. In addition, FOCUSfactor was found to be very well tolerated.
Hand MD Daily Dual Repair Clinical Study
Hand MD Daily Dual Repair has been tested in a single-center, single arm study to evaluate its effects on skin hydration, the appearance of hyperpigmentation/age spots, the appearance of skin radiance, and the appearance of fine lines and wrinkles of the hand area, among other objectives. A total of 51 healthy female subjects, 35 to 65 years of age, completed the clinical study. We sponsored the study, which was conducted in 2017 at BioScreen Testing Services, Inc. in Phoenix, Arizona. The experimental techniques used were: corneometer readings to measure electrical capacitance/hydration of the skin, cutometer readings to measure viscoelastic properties of the skin, software analysis of very high resolution images of the skin to measure roughness and smoothness, standardized photographs of the skin to evaluate fine lines and wrinkles, hyperpigmentation/age spots and skin radiance using the R.W. Johnson Pharmaceutical Research Scale for Evaluation of Photodamage, and self-assessment questionnaires. Baseline evaluations were made at the start of the study, after a five-day “washout” period. Subjects were instructed to use the test products as indicated, and evaluations were made after four weeks and eight weeks.
The tested product regimen provided the following statistically significant improvements after eight weeks of use: 100% of subjects demonstrated an increase in skin hydration, 71% demonstrated a decrease in appearance of hyperpigmentation/age spots, 88% of subjects demonstrated an increase in appearance of skin radiance, and 86% of subjects demonstrated a decrease in appearance of fine lines and wrinkles of the hand area. There were no adverse events reported during the study period.
Our History
We were organized as a corporation under the laws of the State of Nevada on December 29, 2010 under the name “Oro Capital Corporation.” In April 2014, Synergy Strips Corp., a Delaware corporation, became our wholly-owned subsidiary, and we changed our name from “Oro Capital Corporation” to “Synergy Strips Corp.” In August 2015, we changed our name to “Synergy CHC Corp.” In January 2019, our other U.S. subsidiaries, Neuragen Corp., Sneaky Vaunt Corp., The Queen Pegasus Corp. and Breakthrough Products Inc., merged with and into the Company. In July 2021, we acquired Hand MD Corp. as a wholly-owned subsidiary.
We were a public reporting company until July 17, 2020, the date on which we filed a Form 15 to voluntarily suspend our duty to file reports under Sections 13 and 15(d) of the Exchange Act. As a result of this offering, we will become subject again to the information and reporting requirements of the Exchange Act and we will file periodic reports, proxy statements and other information with the SEC.
Our Industry
The global nutritional supplement market is expected to grow at a compound annual growth rate (CAGR) of approximately 9.3% from 2018 to 2028 according to Inkwood Research. One of the drivers of this growth is the increasing availability of over-the-counter products as an alternative to prescription medication.
FOCUSfactor competes in the brain health supplement category. The global brain health supplements market was estimated to be $7.2 billion in 2020 and is expected to grow at a compound annual growth rate of 8.0% from 2021 to 2028, according to Grand View Research. FOCUSfactor’s growth in the year ended December 31, 2020 was 31% year-over-year, significantly outpacing the overall brain health supplement market. The industry is fragmented, with both global and domestic competitors, which gives us an opportunity to scale and continue to take market share.
Our Flat Tummy brand competes in the weight management and wellbeing market, which in 2019 was estimated to be a $20.6 billion global market, with forecasted growth of 7.0% annually from 2020 to 2025, according to ResearchAndMarkets.com.
Demographic trends and changing consumer habits, including a focus on reducing obesity prevalence, have been drivers of this market. We expect these trends will benefit the Flat Tummy brand and allow for new and innovative products to appeal to the changing market demographics.
COVID-19 has raised awareness of hand hygiene, which we view as supportive of our line of Hand MD products, including hand sanitizer. The global hand care market was estimated to be $12.4 billion in 2018, and it is expected to grow at a compound annual growth rate of 4.5% from 2019 to 2025, according to Grand View Research.
Research and Development
The development of new products is comprised of two distinct steps. First, our marketing team reviews new product opportunities by analyzing trends in the market as well as products offered by our competition and then develops preliminary new product concepts which include claims/benefits, delivery form, packaging, and pricing targets, among others. We then work with our third-party manufacturers and leverage their research and development to finalize our new product initiative (including formula and specifications), as these partners are experienced in product development and formulation. When we acquire a brand, we typically further expand the SKUs under that brand, through internal development and with our existing partners. Generally, we take ownership of the formulas and related intellectual property, unless the products use a generic formulation.
| 35 |
Manufacturing and Related Operations
We currently outsource the manufacturing of our products to third parties who have the necessary equipment and technology to provide the significant quantities required. The FOCUSfactor line is manufactured by Atrium Innovations and Vit-Best Nutrition and we have recently added Protab as a third source to support the growth of the brand. The Flat Tummy line uses various manufacturers such as Caraway Tea Company to make our teas, Vit-Best Nutrition to make our shakes and supplement capsules, Supplement Manufacturing Partner, Inc. to make our gummies, and Primrose Candy Company to make appetite suppression lollipops. Hand MD is manufactured by HealthSpecialty.
Distribution
Most of our revenues are generated through the retail channel, primarily due to our FOCUSfactor brand which is sold mainly through leading retailers. These retailers include club, mass, drug and other retailers such as Costco, Walmart, Amazon.com, Walgreens and CVS. All of our brands are also sold direct to consumer through their respective brand websites.
Competition
The U.S. nutritional supplements retail industry is a large and highly fragmented industry with few barriers to entry. We compete against other specialty retailers, mass merchants, multi-level marketing organizations, mail-order and direct-to-consumer companies, and e-commerce companies. This market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market.Certain of our competitors may have significantly greater financial, instruments.
Although there are many competing products on the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2012-04, “Technical Correctionsmarket across our product categories, FOCUSfactor is the only brain-health formulation with both a patent and Improvements” in Accounting Standards Update No. 2012-04. The amendments in this update coverclinical study to support its claim of improving memory, concentration and focus. FOCUSfactor’s competitors include a wide range of Topicsproducts, from targeted brain-enhancement supplements to indirect competitors such as energy drinks that claim to improve concentration. Our Flat Tummy and Hand MD brands all compete in well-established segments with a diverse range of competition both domestically and internationally.
Government Regulation
Domestic
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to regulation by one or more federal agencies, including the U.S. Food and Drug Administration (the “FDA”), the Federal Trade Commission (the “FTC”), the Consumer Product Safety Commission, the United States Department of Agriculture and the Environmental Protection Agency, and by various agencies of the states and localities in which our products are sold.However, no formal FDA approval or registration is required because our products are classified as dietary supplements (all FOCUSfactor products and some Flat Tummy products), foods (some Flat Tummy products) or cosmetics (all Hand MD products except hand sanitizer, which must follow the OTC monograph).
Food and Drug Administration
Dietary Supplements
The Dietary Supplement Health and Education Act of 1994 (“DSHEA”) amended the Federal Food, Drug, and Cosmetic Act (the “FDC Act”) to establish a new framework governing the composition, safety, labeling, manufacturing and marketing of dietary supplements. Generally, under the FDC Act, dietary ingredients (i.e., vitamins; minerals; herbs or other botanicals; amino acids; or dietary substances for use by humans to supplement diet by increasing total dietary intake; or any concentrate, metabolite, constituent, extract or combination of any of the above) that were marketed in the Accounting Standards Codification. These amendments include technical corrections and improvementsUnited States prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. “New” dietary ingredients (i.e., dietary ingredients that were not marketed in the United States before October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the Accounting Standards CodificationFDA unless the ingredient has been “present in the food supply as an article used for food” without being “chemically altered.” A new dietary ingredient notification must provide the FDA evidence of a “history of use or other evidence of safety” establishing that use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. The FDA may determine that a new dietary ingredient notification does not provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe.
Such a determination could prevent the marketing of such dietary ingredient. In 2011 and conforming amendments related2016, the FDA issued draft guidance setting forth recommendations for complying with the new dietary ingredient notification requirement. Although FDA guidance is non-binding and does not establish legally enforceable responsibilities, and companies are free to fair value measurements. The amendmentsuse an alternative approach if the approach satisfies the requirements of applicable laws and regulations, FDA guidance is a strong indication of the FDA’s view on the topic discussed in the guidance, including its position on enforcement. At this update will be effective for fiscal periods beginning after December 15, 2012. The adoption of ASU 2012-04time, it is not expecteddifficult to determine whether the 2016 draft guidance (which replaced the 2011 draft guidance), if finalized, would have a material impact on our operations. However, if the FDA were to enforce the applicable statutes and regulations in accordance with the draft guidance as written, such enforcement could require us to incur additional expenses, which could be significant, and negatively impact our business in several ways, including, but not limited to, enjoining the manufacturing of our products until the FDA determines that we are in compliance and can resume manufacturing, increasing our liability and reducing our growth prospects.
The FDA or other agencies could take actions against products or product ingredients that, in their determination, present an unreasonable health risk to consumers that would make it illegal for us to sell such products. In addition, the FDA could issue consumer warnings with respect to the products or ingredients in such products that we sell. Such actions or warnings could be based on information received through FDC Act-mandated reporting of serious adverse events.
We take a number of actions to ensure the products we sell comply with the FDC Act. As is common in our industry, we rely on our third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of such products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations. A removal or recall could also result in negative publicity and damage to our reputation that could reduce future demand for our products. In such case, we may attempt to offset any losses related to recalls and removals with reformulated or alternative products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall.
The FDC Act permits structure/function claims to be included in labels and labeling for dietary supplements without FDA pre-market approval. However, companies must have substantiation that the claims are “truthful and not misleading,” and must submit a notification with the text of the claims to the FDA no later than 30 days after marketing the dietary supplement with the claims. Permissible structure/function claims may describe how a particular nutrient or dietary ingredient affects the structure, function or general well-being of the body, or characterize the documented mechanism of action by which a nutrient or dietary ingredient acts to maintain such structure or function. The label or labeling of a product marketed as a dietary supplement may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat or prevent a disease (i.e. a disease claim). If the FDA determines that a particular structure/function claim is an unacceptable disease claim that causes the product to be regulated as a drug, a conventional food claim or an unauthorized version of a “health claim,” or, if the FDA determines that a particular claim is not adequately supported by existing scientific data or is false or misleading in any particular way, we would be prevented from using the claim and would have to update our product labels and labeling accordingly.
In addition, DSHEA provides that so-called “third-party literature,” e.g., “a publication, including an article, a chapter in a book, or an official abstract of a peer-reviewed scientific publication that appears in an article and was prepared by the author or the editors of the publication” supplements, when reprinted in its entirety, may be used “in connection with the sale of a dietary supplement to consumers” without the literature being subject to regulation as labeling. Such literature: (1) must not be false or misleading; (2) may not “promote” a particular manufacturer or brand of dietary supplement; (3) must present a balanced view or is displayed or presented with other such items on the same subject matter so as to present a balanced view of the available scientific information; (4) if displayed in an establishment, must be physically separate from the dietary supplements; and (5) should not have appended to it any information by sticker or any other method. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any continued dissemination could subject our product to regulatory action as an illegal drug.
In June 2007, pursuant to the authority granted by the FDC Act as amended by DSHEA, the FDA published detailed current Good Manufacturing Practice (“cGMP”) regulations that govern the manufacturing, packaging, labeling and holding operations of dietary supplement manufacturers. The cGMP regulations, among other things, impose significant recordkeeping requirements on manufacturers. The cGMP requirements are in effect for all dietary supplement manufacturers, and the FDA conducts inspections of dietary supplement manufacturers pursuant to these requirements. There remains considerable uncertainty with respect to the FDA’s interpretation of the regulations and their actual implementation in manufacturing facilities.
In addition, the FDA’s interpretation of the regulations governing dietary supplements will likely change over time as the agency becomes more familiar with the industry and the regulations. The failure of a manufacturing facility to comply with the cGMP regulations renders products manufactured in such facility “adulterated,” and subjects such products and the manufacturer to a variety of potential FDA enforcement actions. In addition, under the Food Safety Modernization Act (“FSMA”), which was enacted in January 2011, the manufacturing of dietary ingredients contained in dietary supplements will be subject to similar or even more burdensome manufacturing requirements, which will likely increase the costs of dietary ingredients and will subject suppliers of such ingredients to more rigorous inspections and enforcement. The FSMA will also require importers of food, including dietary supplements and dietary ingredients, to conduct verification activities to ensure that the food they might import meets applicable domestic requirements.
The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements, including powers to issue a public warning or notice of violation letter to a company, publicize information about illegal products, detain products intended for import, require the reporting of serious adverse events, require a recall of illegal or unsafe products from the market, and request the Department of Justice to initiate a seizure action, an injunction action or a criminal prosecution in United States courts.
The FSMA expands the reach and regulatory powers of the FDA with respect to the production and importation of food, including dietary supplements. The expanded reach and regulatory powers include the FDA’s ability to order mandatory recalls, administratively detain domestic products, and require certification of compliance with domestic requirements for imported foods associated with safety issues. FSMA also gave the FDA the authority to administratively revoke manufacturing facility registrations, effectively enjoining manufacturing of dietary ingredients and dietary supplements without judicial process. The regulation of dietary supplements may increase or become more restrictive in the future.
Hand Sanitizers
Hand sanitizers are classified as over-the-counter (“OTC”) drugs, which have specific regulatory requirements, including ingredient and manufacturing requirements. Under the OTC monograph system, selected OTC drugs are generally recognized as safe and effective and do not require the submission and approval of a new drug application. The FDA OTC monographs include well-known ingredients and specific requirements for permitted indications, required warnings and precautions, allowable combinations of ingredients and dosage levels. Products marketed under the OTC monograph system must conform to specific quality, formula and labeling requirements.
Cosmetics
The FDC Act, defines cosmetics as articles or components of articles intended for application to the human body to cleanse, beautify, promote attractiveness, or alter the appearance, with the exception of soap. The labeling of cosmetic products is subject to the requirements of the FDC Act, the Fair Packaging and Labeling Act, the Poison Prevention Packaging Act and other FDA regulations. Cosmetics are not subject to pre-market approval by the FDA; however, certain ingredients, such as color additives, must be pre-approved for the specific intended use of the product and are subject to certain restrictions on their use. If a company has not adequately substantiated the safety of its products or ingredients by, for example, performing appropriate toxicological tests or relying on already available toxicological test data, then a specific warning label is required. The FDA may, by regulation, require other warning statements on certain cosmetic products for specified hazards associated with such products. FDA regulations also prohibit or otherwise restrict the use of certain types of ingredients in cosmetic products.
In addition, the FDA requires that cosmetic labeling and claims be truthful and not misleading. Moreover, cosmetics may not be marketed or labeled for their use in treating, preventing, mitigating, or curing disease or other conditions or in affecting the structure or function of the body, as such claims would render the products to be a drug and subject to regulation as a drug. The FDA has issued warning letters to cosmetic companies alleging improper drug claims regarding their cosmetic products, including, for example, product claims regarding hair growth or preventing hair loss. In addition to FDA requirements, the FTC as well as state consumer protection laws and regulations can subject a cosmetics company to a range of requirements and theories of liability, including similar standards regarding false and misleading product claims, under which FTC or state enforcement or class-action lawsuits may be brought.
In the United States, the FDA has not promulgated regulations establishing cGMPs for cosmetics. However, FDA’s draft guidance on cosmetic cGMPs, most recently updated in June 2013, provides recommendations related to process documentation, recordkeeping, building and facility design, equipment maintenance and personnel, and compliance with these recommendations can reduce the risk that FDA finds such products have been rendered adulterated or misbranded in violation of applicable law. FDA also recommends that manufacturers maintain product complaint and recall files and voluntarily report adverse events to the agency. The FDA monitors compliance of cosmetic products through market surveillance and inspection of cosmetic manufacturers and distributors to ensure that the products are not manufactured under unsanitary conditions, or labeled in a false or misleading manner. Inspections also may arise from consumer or competitor complaints filed with the FDA. In the event the FDA identifies unsanitary conditions, false or misleading labeling, or any other violation of FDA regulation, FDA may request or a manufacturer may independently decide to conduct a recall or market withdrawal of product or to make changes to its manufacturing processes or product formulations or labels.
Federal Trade Commission
The FTC exercises jurisdiction over the advertising of dietary supplements and cosmetics and requires that all advertising to consumers be truthful and non-misleading. The FTC actively monitors the dietary supplement space and has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. These enforcement actions have resulted in consent decrees and significant monetary judgments against the companies and/or individuals involved. Regulators require a company to convey product claims clearly and accurately and further require marketers to maintain adequate substantiation for their claims. More specifically, the FTC requires such substantiation to be competent and reliable scientific evidence and requires a company to have a reasonable basis for the expressed and implied product claim before it disseminates an advertisement. A reasonable basis is determined based on the claims made, how the claims are presented in the context of the entire advertisement, and how the claims are qualified. The FTC’s standard for evaluating substantiation is designed to ensure that consumers are protected from false and/or misleading claims by requiring scientific substantiation of product claims at the time such claims are first made. The failure to have this substantiation violates the Federal Trade Commission Act.
Foreign
Our products sold in foreign countries are also subject to regulation under various national, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements and over-the-counter drugs. Government regulations in foreign countries may prevent or delay the introduction, or require the reformulation, of certain of our products.
In foreign markets, our regulatory department works with an in-country regulatory consultant group to guide us through the regulatory process needed to launch our product in a particular country such as Canada, the United Kingdom and Australia. For example, Canada and Australia require a product submission packet and approval from Health Canada (“HC”) and the Therapeutic Goods Administration (“TGA”), respectively. In the United Kingdom, on the other hand, no formal regulatory approval is needed. Launch timing varies by country. In the United States and United Kingdom, once a formula is established and labeling has been approved by our regulatory and legal advisors, the product can be launched upon production. The Australian approval process generally takes one to two weeks from the time the packet is submitted, while in Canada the approval process can take from two months to six months from submission.
In Canada, HC has oversight over our FOCUSfactor, Flat Tummy and Hand MD products. Our FOCUSfactor and Flat Tummy products are considered natural health products by HC so they each have a natural product number that was assigned by HC upon its review and approval. Hand MD products are considered cosmetics by HC, so notification filings are required which are not subject to review and approval, with the exception of hand sanitizer, which has been assigned a natural product number by HC.
In the United Kingdom, both FOCUSfactor and Flat Tummy are considered food supplements that are regulated by the Food Standards Agency. There is no requirement for licensing or registering food supplement products in the United Kingdom, and products must comply with relevant food law. Hand MD products are not currently sold in the United Kingdom.
In Australia, FOCUSfactor products are “Listed Medicines” that are regulated by the TGA and require an AUST L (Australia Listed Medicine) number. Flat Tummy products are classified as either Listed Medicines or meal replacements. Listed Medicines are regulated by the TGA while meal replacements are regulated under the Australia New Zealand Food Standards Code. Hand MD products are not currently sold in Australia.
New Legislation or Regulation
Legislation may be introduced which, if passed, would impose substantial new regulatory requirements on dietary supplements. We cannot determine what effect additional domestic or international governmental legislation, regulations, or administrative orders, when and if promulgated, would have on our business in the future. New legislation or regulations may require the reformulation of certain products to meet new standards, require the recall or discontinuance of certain products not capable of reformulation, impose additional record keeping or require expanded documentation of the properties of certain products, expanded or different labeling or scientific substantiation.
Intellectual Property
We own 18 trademarks that have been registered with the United States Patent and Trademark Office and have filed applications to register additional trademarks. In addition, we claim domestic trademark and service mark rights in numerous additional marks that we use. We own a number of trademark registrations in countries outside the United States. Federally registered trademarks in the United States have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. Most foreign trademark offices use similar trademark renewal processes. We regard our trademarks and other proprietary rights as valuable assets and believe they make a significant positive contribution to the marketing of our products.
We protect our legal rights concerning our trademarks by appropriate measures, which may include legal action. We possess a portfolio of both registered and unregistered (i.e., common law) trademarks. In certain circumstances, we seek and obtain registrations for our trademarks, which may confer certain advantages, and the decision to register a trademark is made on a case by case basis. We have registered and intend to register certain trademarks in certain limited jurisdictions outside the United States where our products are sold, but we may not register all or even some of our trademarks in every country in which we conduct business or intend to conduct business.
We own U.S. Patent 8,329,227 covering FOCUSfactor’s proprietary formulation “for enhanced mental function.” This patent was issued by the United States Patent and Trademark Office in December 2012 and expires in April 2025.
In addition to this intellectual property, we also rely on our proprietary knowledge and ongoing technological innovation to develop a competitive position in the market for our products. Each of our patents and know-how are integral to the conduct of our business and the loss of any could have a material adverse effect on our business.
Human Capital Management
We recognize that attracting, motivating and retaining passionate talent at all levels is vital to continuing our success. By improving employee retention and engagement, we also improve our ability to support our customers and protect the long-term interests of our stakeholders and stockholders. We invest in our employees through continuously improving benefits and various health and wellness initiatives, and offer competitive compensation packages, working to continuously improve fairness in internal compensation practices.
As of October 1, 2021, we had 39 full-time employees. We intend to grow our employee base in response to the demands and requirements of the business. We believe that the employer-employee relationships in our Company are positive. We have no labor union contracts.
Properties
The following table describes our principal properties leased as of October 20, 2021:
| Purpose | Location | Square Footage | ||
| Technology Center (1) | Halifax, NS | 8,500 | ||
| Main Office (2) | Westbrook, ME | 3,510 |
(1) Monthly rental payments are $25,000 Canadian Dollars per month on a month-to-month basis.
(2) Monthly rental payments are $4,290 per month on a month-to-month basis.
Legal Proceedings
From time to time, we are involved in various litigation matters arising in the ordinary course of our business. We do not believe that any of these matters, individually or in the aggregate, are currently material to us.
The following table sets forth certain information as of October 20, 2021 about our executive officers and members of our Board.
| Name | Age | Position | |||
| Jack Ross | 56 | Chief Executive Officer and Chairman | |||
| Brendan Horning | 38 | Chief Financial Officer | |||
| Alfred Baumeler(1) | 56 | President and Director Nominee | |||
| Jaime Fickett | 43 | Senior Vice President of Finance and Operations | |||
| J. Paul SoRelle | 65 | Director | |||
| Stephen Fryer | 83 | Director | |||
| Nitin Kaushal | 56 | Director Nominee |
| (1) | Alfred Baumeler will be appointed to the Board effective upon the closing of the offering described in this prospectus. |
Executive Officers
Jack Ross has served as our Chief Executive Officer and Chairman since October 2014, served as our Chief Financial Officer from August 2018 until July 2021 and from October 2014 until October 2017, and served as our President from May 2020 until January 2021 and from October 2014 until October 2017. Mr. Ross has also served as the Chief Executive Officer of Kenek Brands Inc., an executive consulting firm, since January 2014. In addition, Mr. Ross has served as the Chairman and Chief Executive Officer of Gowan Capital Inc. since May 2011. Mr. Ross’ significant leadership experience at various private and public companies led to the conclusion that he should serve as a member of our Board of Directors, in light of our business and structure.
Brendan Horning was appointed our Chief Financial Officer in July 2021. Since November 2019, Mr. Horning has also served as the President and director of Brendan Horning Incorporated. Prior to being appointed our Chief Financial Officer, Mr. Horning served as our Vice President of Investor Relations from February 2021 until July 2021 and from September 2016 until October 2019. From February 2013 to September 2016, Mr. Horning served as a partner of an accounting firm. Mr. Horning received a Bachelor of Commerce in Finance and Accounting from St. Mary’s University. Mr. Horning is also a chartered professional accountant in Canada and has completed the three-year Canadian Institute of Chartered Accountants in-depth tax program.
Alfred Baumeler has served as our President since January 2021 and has agreed to serve on our Board of Directors upon the completion of this offering. From August 2015 to December 2020, Mr. Baumeler served as our Executive Vice President of Sales and Marketing. From September 2012 to August 2015, Mr. Baumeler served as Senior Vice President of Marketing for Healthy Natural Systems/Core Science Medica (CSM)/Lifeagen, a nutritional supplement company. From January 2012 to August 2012, Mr. Baumeler was self-employed as a marketing consultant. From November 2009 to November 2011, Mr. Baumeler was the Senior Vice President and Chief Marketing Officer for Natrol, Inc., a manufacturer of vitamins, minerals and supplements. Mr. Baumeler earned his Bachelor of Science from Rutgers University in Engineering and his Master of Business Administration in Marketing and Finance from Columbia Business School. Mr. Baumeler’s 30 years of experience in consumer goods, of which 20 were in the nutritional supplement industry, led to the conclusion that he should serve as a member of our Board of Directors.
Jaime Fickett has served as our Senior Vice President of Finance and Operations since January 2015. From August 2006 to January 2015, Ms. Fickett served as Chief Financial Officer of Factor Nutrition Labs, LLC, a company that developed dietary supplements. From 1999 to 2006, Ms. Fickett was employed in the public accounting sector as an auditor. Ms. Fickett earned a Bachelor of Science in Accounting from the University of Maine. Ms. Fickett earned her Master of Science in Accounting from Southern New Hampshire University.
Directors and Director Nominees
J. Paul SoRelle was appointed as a director in December 2014. From 1999 to April 2020, Mr. SoRelle served as the President and Chief Executive Officer of Pioneer Press of Greeley, Inc., a commercial printing company. Since April 2020, Mr. SoRelle has continued to consult for Pioneer Press. Prior to joining Pioneer Press, Mr. SoRelle acquired RamStar, Inc. in November 1988 and served as President and CEO, as well as in other roles. RamStar, Inc. was acquired by International Thunderbird Gaming Corporation in 1994, where Mr. SoRelle served on the board of directors and as Executive Vice President until October 1996. In November 1982, Mr. SoRelle acquired three convenience stores, expanded to eight stores and sold them in 1988. Mr. SoRelle’s significant leadership experience at Pioneer Press of Greeley, Inc. led to the conclusion that he should serve as a member of our Board of Directors, in light of our business and structure.
Nitin Kaushal has agreed to serve on our Board of Directors upon the completion of this offering. Since March 2020, Mr. Kaushal has served as President of Anik Capital Corp. He retired from PricewaterhouseCoopers LLP (Canada), where he was a Managing Director in the Corporate Finance Practice from April 2012 until February 2020. He has over thirty years of experience in the financial services industry and has held senior roles in investment banking, venture capital and consulting firms including Desjardins Securities Inc., Orion Securities Inc., Vengate Capital, HSBC Securities Inc. and Gordon Capital and in the venture capital industry with MDS Capital Corp. Mr. Kaushal serves on the board of VieMed Healthcare, Inc., FSD Pharma, Inc., High Tide Inc., Delta 9 Cannabis Inc., PsyBio Therapeutics Corp. and Flower One Holdings Inc. Mr. Kaushal earned his Bachelor of Science in Chemistry from the University of Toronto and is a Chartered Accountant in Canada. Mr. Kaushal’s experience as a member of various boards of directors and as a Chartered Accountant provides the Board with additional perspective on financial reporting, governance and management issues and led to the conclusion that he should serve as a member of our Board of Directors.
Stephen Fryer was appointed as a director in December 2014. Since June 2003, Mr. Fryer has been the Chief Executive Officer and Managing Partner of SC Capital Partners, Inc., a private micro-market investment banking and private equity intermediary. Prior to joining SC Capital Partners, Inc., Mr. Fryer was Chief Executive Officer of Pen Interconnect from March 1998 to September 2002. From May 1989 to August 1997, Mr. Fryer was Managing Director of Ventana International, Ltd., a venture capital and private investment banking firm with operations and investors in the United States, Latin America, Europe and Asia. Mr. Fryer earned a B.S. in Mechanical Engineering, with a minor in Economics, from the University of Southern California. Mr. Fryer’s substantial experience in the investment banking industry, and his demonstrated skill in corporate finance, led to the conclusion that he should serve as a member of our Board of Directors, in light of our business and structure.
Corporate Governance
Composition of our Board of Directors
Our business and affairs are managed under the direction of our Board. The number of directors will be fixed by our Board, subject to the terms of our certificate of incorporation and amended and restated bylaws. Our Board currently consists of three directors (Mr. Ross, Mr. SoRelle and Mr. Fryer), and will consist of five directors (Mr. Ross, Mr. Baumeler, Mr. SoRelle, Mr. Fryer and Mr. Kaushal) upon the completion of this offering.
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable our Board to satisfy its oversight responsibilities effectively in light of our business and structure, the Board focuses primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business.
Corporate Governance Profile
We intend to structure our corporate governance in a manner we believe closely aligns our interests with those of our stockholders. Notable features of our corporate governance structure will include the following:
| ● | our Board will not be classified, with each of our directors subject to re-election annually; | |
| ● | we expect that a majority of our directors will satisfy the Nasdaq listing standards for independence; | |
| ● | generally, all matters to be voted on by stockholders will be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all stockholders present in person or represented by proxy, voting together as a single class; | |
| ● | we intend to comply with the requirements of the Nasdaq marketplace rules, including having committees comprised solely of independent directors; and | |
| ● | we do not have a stockholder rights plan. |
Our directors will stay informed about our business by attending meetings of our Board and its committees and through supplemental reports and communications. Our independent directors will meet regularly in executive sessions without the presence of our corporate officers or non-independent directors.
Role of the Board in Risk Oversight
The Board actively manages the Company’s risk oversight process and receives periodic reports from management on areas of material risk to the Company, including operational, financial, legal, and regulatory risks. The Board committees assist the Board in fulfilling its oversight responsibilities in certain areas of risk. The Audit Committee assists the Board with its oversight of the Company’s major financial risk exposures. The Compensation Committee assists the Board with its oversight of risks arising from the Company’s compensation policies and programs. The Corporate Governance and Nominating Committee assists the Board with its oversight of risks associated with board organization, board independence, and corporate governance. While each committee is responsible for evaluating certain risks and overseeing the management of those risks, the entire Board is regularly informed about the risks.
| 41 |
Director Independence
The Nasdaq marketplace rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominations committees be independent, or, if a listed company has no nominations committee, that director nominees be selected or recommended for the board’s selection by independent directors constituting a majority of the board’s independent directors. The Nasdaq marketplace rules further require that audit committee members satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act and that compensation committee members satisfy the independence criteria set forth in Rule 10C-1 under the Exchange Act.
Prior to the completion of this offering, our Board undertook a review of the independence of our directors and considered whether any director has a material relationship with us that could compromise that director’s ability to exercise independent judgment in carrying out that director’s responsibilities. Our Board has affirmatively determined that each of Messrs. SoRelle, Fryer and Kaushal qualify as an independent director, as defined under the applicable corporate governance standards of Nasdaq. These rules require that our Audit Committee be composed of at least three members, one of whom must be independent on the date of listing on Nasdaq, a majority of whom must be independent within 90 days of the effective date of the registration statement containing this prospectus, and all of whom must be independent within one year of the effective date of the registration statement containing this prospectus.
Board Leadership
The offices of the chairman of the Board and chief executive officer are currently combined. Mr. Ross serves as the Company’s chairman and chief executive officer. The Board believes that this structure is the most appropriate structure at this time for several reasons. Mr. Ross is responsible for the day-to-day operations of the Company and the execution of its strategies. Since these topics are an integral part of Board discussions, Mr. Ross is the director best qualified to chair those discussions. In addition, Mr. Ross’ experience and knowledge of the Company and the industry are critical to Board discussions and the Company’s success. The Board believes that Mr. Ross is well qualified to serve in the combined roles of chairman and chief executive officer and that Mr. Ross’ interests are sufficiently aligned with the stockholders he represents.
The Board does not have a lead independent director. To help ensure the independence of the Company’s Board, the independent directors of the Board intend to meet without members of management at various times during the year.
Board Committees
Our Board of Directors will establish an audit committee, a compensation committee, and a nominating and corporate governance committee, each of which will operate pursuant to a charter to be adopted by our Board of Directors and will be effective upon the effectiveness of the registration statement of which this prospectus is a part. Upon the effectiveness of the registration statement of which this prospectus is a part, the composition and functioning of all of our committees will comply with all applicable requirements of the Sarbanes-Oxley Act of 2002, Nasdaq and SEC rules and regulations.
Following the completion of this offering, the full text of our audit committee charter, compensation committee charter, and nominating and corporate governance charter will be posted on the investor relations portion of our website at www.synergychc.com. We do not incorporate the information contained on, or accessible through, our corporate website into this prospectus, and you should not consider it a part of this prospectus.
Audit Committee
Upon completion of this offering, Messrs. SoRelle, Fryer and Kaushal will serve on the Audit Committee, which will be chaired by Mr. Kaushal. The committee’s primary duties are to:
| ● | review and discuss with management and our independent auditor our annual and quarterly financial statements and related disclosures, including disclosure under “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the results of the independent auditor’s audit or review, as the case may be; | |
| ● | review our financial reporting processes and internal control over financial reporting systems and the performance, generally, of our internal audit function; | |
| ● | oversee the audit and other services of our independent registered public accounting firm and be directly responsible for the appointment, independence, qualifications, compensation and oversight of the independent registered public accounting firm, which reports directly to the Audit Committee; | |
| ● | provide an open means of communication among our independent registered public accounting firm, management, our internal auditing function and our Board; | |
| ● | review any disagreements between our management and the independent registered public accounting firm regarding our financial reporting; | |
| ● | prepare the Audit Committee report for inclusion in our proxy statement for our annual stockholder meetings; | |
| ● | establish procedures for complaints received regarding our accounting, internal accounting control and auditing matters; and | |
| ● | approve all audit and permissible non-audit services conducted by our independent registered public accounting firm. |
All members of our Audit Committee will meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the Nasdaq listing rules. Our Board of Directors has determined that Mr. Kaushal qualifies as an “audit committee financial expert” within the meaning of applicable SEC regulations. In making this determination, our Board of Directors considered the nature and scope of experience that Mr. Kaushal has previously had with public reporting companies. Our Board of Directors has determined that all of the directors that will become members of our audit committee upon the effectiveness of the registration statement of which this prospectus forms a part satisfy the relevant independence requirements for service on the Audit Committee set forth in the rules of the SEC and the Nasdaq listing rules. Both our independent registered public accounting firm and management will periodically meet privately with our Audit Committee.
Compensation Committee
Upon completion of this offering, Messrs. SoRelle, Fryer and Kaushal will serve on the Compensation Committee, which will be chaired by Mr. Kaushal. The committee’s primary duties are to:
| ● | approve corporate goals and objectives relevant to chief executive officer compensation and evaluate performance in light of those goals and objectives; | |
| ● | determine and approve executive officer compensation, including base salary and incentive awards; | |
| ● | make recommendations to the Board regarding compensation plans; and | |
| ● | administer our stock plan. |
Our Compensation Committee determines and approves all elements of executive officer compensation. It also provides recommendations to the Board with respect to non-employee director compensation. The Compensation Committee may not delegate its authority to any other person, other than to a subcommittee.
Our Board of Directors has determined that each member of the Compensation Committee is “independent” as defined in the applicable Nasdaq rules. Each member of our Compensation Committee will be a non-employee director, as defined in Rule 16b-3 promulgated under the Exchange Act, and an outside director, as defined pursuant to Section 162(m) of the Internal Revenue Code of 1986, as amended.
Nominating and Corporate Governance Committee
Upon completion of this offering, Messrs. SoRelle, Fryer and Kaushal will serve on the Nominating and Corporate Governance Committee, which will be chaired by Mr. Kaushal. The committee’s primary duties are to:
| ● | consider director nominees recommended by stockholders and recommend nominees for election as directors; | |
| ● | oversee the evaluation of the Board; | |
| ● | review our Board’s committee structure and composition and make recommendations; and | |
| ● | develop, recommend and oversee our corporate governance principles, including our Code of Business Ethics and Conduct. |
Code of Business Ethics and Conduct
Prior to the effectiveness of the registration statement of which this prospectus is a part, we will adopt a written code of business ethics and conduct that applies to our directors, officers, and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Following the effectiveness of the registration statement of which this prospectus is a part, a current copy of the code will be posted on the investor relations section of our website, which is located at www.synergychc.com. If we make any substantive amendments to, or grant any waivers from, the code of business ethics and conduct for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a Current Report on Form 8-K.
Legal Proceedings
To our knowledge, (i) no director or executive officer has been a director or executive officer of any business which has filed a bankruptcy petition or had a bankruptcy petition filed against it during the past ten years; (ii) no director or executive officer has been convicted of a criminal offense or is the subject of a pending criminal proceeding during the past ten years; (iii) no director or executive officer has been the subject of any order, judgment or decree of any court permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities during the past ten years; and (iv) no director or officer has been found by a court to have violated a federal or state securities or commodities law during the past ten years.
EXECUTIVE AND DIRECTOR COMPENSATION
We are a “smaller reporting company” under applicable SEC rules and are providing disclosure regarding our executive compensation arrangements pursuant to the rules applicable to emerging growth companies, which means that we are not required to provide a compensation discussion and analysis and certain other disclosures regarding our executive compensation. The following discussion relates to the compensation of our named executive officers for 2020, consisting of Jack Ross, our Chief Executive Officer, Chairman and former President and Chief Financial Officer, and our two other most highly compensated executive officers as of December 31, 2020, Alfred Baumeler, our President and former Executive Vice President – Sales and Marketing, and Jaime Fickett, our Senior Vice President of Finance and Operations.
Fiscal Year 2020 and 2019 Summary Compensation Table
The following Fiscal Year 2020 and 2019 Summary Compensation Table contains information regarding compensation for 2020 and 2019 that we paid to Mr. Ross and our two other most highly compensated executive officers as of December 31, 2020.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | All other compensation ($) | Total ($) | |||||||||||||||
| Jack Ross | 2020 | 1,080,000 | (3) | – | 30,000 | (4) | 1,110,000 | |||||||||||||
| Chief Executive Officer, Chairman, Former President and Former Chief Financial Officer (1) | 2019 | 824,413 | – | 18,000 | (5) | 842,413 | ||||||||||||||
| Alfred Baumeler | 2020 | 275,000 | 25,000 | 14,700 | (6) | 314,700 | ||||||||||||||
| President and Former Executive Vice President – Sales and Marketing (2) | 2019 | 250,000 | 75,000 | 11,100 | (7) | 336,100 | ||||||||||||||
| Jaime Fickett | 2020 | 255,000 | 25,000 | – | 280,000 | |||||||||||||||
| Senior Vice President of Finance and Operations | 2019 | 255,000 | 76,580 | – | 331,580 | |||||||||||||||
| (1) | Mr. Ross serves as our Chief Executive Officer and Chairman, served as our Chief Financial Officer from October 2014 until July 2021 and served as our President from May 2020 until January 2021. Mr. Ross is not an employee of the Company. | |
| (2) | Mr. Baumeler served as our Executive Vice President – Sales and Marketing until January 2021, when he was promoted to President. | |
| (3) | Consists of amounts paid to Kenek Brands Inc. pursuant to a consulting agreement. See “Consulting Agreement” below. | |
| (4) | Consists of a vehicle allowance of $2,500 per month. | |
| (5) | Consists of a vehicle allowance of $1,500 per month. | |
| (6) | Consists of a vehicle allowance of $600 per month and retirement allowance of $7,500. | |
| (7) | Consists of a vehicle allowance of $300 per month and retirement allowance of $7,500. |
Consulting Agreement
The following discussion relates to compensation arrangements on behalf of, and compensation paid by us to an entity controlled by Mr. Ross. We do not have an employment agreement with Mr. Baumeler or Ms. Fickett.
We are party to a Sales and Marketing Consultant and Distribution Agreement dated April 2, 2014 (the “Consulting Agreement”) with Kenek Brands Inc. (“Kenek”). Mr. Ross is the owner of Kenek and its sole officer and director. Pursuant to the Consulting Agreement, Kenek has been retained to serve as a consultant and advisor to us, including developing, expanding and managing sales, marketing and distribution and aiding in strategic direction of our sales and planning. As compensation for Kenek’s services, we pay Kenek $9,000 per month plus a 2% commission on all product sales on products that Kenek or its agents or brokers introduce to us. In addition, on April 2, 2014, we granted Kenek fully-vested options to purchase 1,000,000 shares of our common stock at an exercise price of $0.25 per share, which have expired. We have also agreed to reimburse Kenek for all out of pocket expenses incurred to perform the duties set out in the Consulting Agreement, including travel, meals and lodging. The Consulting Agreement had an initial period of one year and automatically renews for successive one-year periods unless terminated by either party upon 90 days’ prior written notice. For two years following termination, we have agreed to pay to Kenek a 1% commission on all product sales on products that Kenek or its agents or brokers introduced to us. See “Certain Relationships and Related Party Transactions” for additional information.
Fiscal Year 2020 Outstanding Equity Awards at Fiscal Year-End Table
The following table lists all of the outstanding equity awards held on December 31, 2020 by each of the Company’s named executive officers, before adjusting for the proposed 1-for- reverse stock split.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name | Number of (#) | Number of (#) | Equity (#) | Option ($) | Option expiration date | Number (#) | Market value of shares of units of stock that have not vested ($) | Equity incentive plan unearned shares, (#) | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||||||||||||||||||
| Jack Ross | – | – | – | – | – | – | – | – | – | |||||||||||||||||||||||||||
| Alfred Baumeler | 1,000,000 | – | – | 0.65 | 12/14/2025 | – | – | – | – | |||||||||||||||||||||||||||
| Jaime Fickett | 1,000,000 | – | – | 0.65 | 12/14/2025 | – | – | – | – | |||||||||||||||||||||||||||
Equity Incentive Plans
The following table summarizes information about our equity compensation plans as of December 31, 2020. All outstanding awards relate to our common stock.
| Plan category | Number of securities to be issued upon vesting of grants and exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plan approved by stockholders(1) | 3,466,667 | $ | 0.54 | 12,058,333 | ||||||||
| Equity compensation plan not approved by stockholders | – | $ | – | – | ||||||||
| Total | 3,466,667 | $ | 0.54 | 12,058,333 | ||||||||
| (1) | The Company’s 2014 Stock Incentive Plan was approved by stockholders in July 2015. |
Fiscal Year 2020 Director Compensation
Our named executive officer and director, Mr. Ross, did not receive any compensation for his service as a director for the year ended December 31, 2020. All compensation paid to Mr. Ross is set forth above under “—Fiscal Year 2020 and 2019 Summary Compensation Table.” The following table provides information regarding all compensation paid to our non-employee directors during the fiscal year ended December 31, 2020.
| Name | Fees earned or paid in cash | Stock awards | Option awards | Non-equity incentive plan compensation | Nonqualified deferred compensation earnings | All other compensation | Total | |||||||||||||||||||||
| Stephen Fryer | $ | 20,000 | - | - | - | - | - | $ | 20,000 | |||||||||||||||||||
| Paul SoRelle | $ | - | - | - | - | - | - | $ | - | |||||||||||||||||||
Director Compensation
We do not currently have a formal policy with respect to compensating non-employee directors for service as directors. Following the consummation of this offering, we anticipate that directors who are not also our officers or employees will receive compensation for their service on our board and committees thereof. The amount and form of such compensation has not yet been determined. Each non-employee director will be reimbursed for out-of-pocket expenses incurred in connection with attending board and committee meetings.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Following is a description of transactions since January 1, 2018, including currently proposed transactions to which we have been or are to be a party in which the amount involved exceeded or will exceed $120,000, and in which any of our directors (including nominees), executive officers or beneficial holders of more than 5% of our capital stock, or any immediate family members of and any entities affiliated with any such person, had or will have a direct or indirect material interest.
We may encounter business arrangements or transactions with businesses and other organizations in which one of our directors or executive officers, significant stockholders or their immediate families is a participant and the amount exceeds $120,000. We refer to these transactions as related party transactions. Related party transactions have the potential to create actual or perceived conflicts of interest between us and our directors, officers and significant stockholders or their immediate family members. All services listed below are provided at cost.
On April 2, 2014, we entered into a Sales and Marketing Consultant and Distribution Agreement, with Kenek Brands, Inc. (“Kenek”), a company owned by Jack Ross, our Chief Executive Officer. For the years ended December 31, 2020, 2019 and 2018, we accrued and paid consulting fees of $82,917, $57,917 and $57,917, respectively, per month to Kenek. We also pay a vehicle allowance of $2,500 per month (or $30,000 per year) to Mr. Ross. We expensed $1,110,000, $824,413 and $648,944, respectively, during the years ended December 31, 2020, 2019 and 2018 as consulting fees, advanced $425,000 in the manner of a prepaid bonus in the year ended December 31, 2020, and made payments totaling $1,110,000, $852,626 and $648,944 towards services to Kenek for the years ended December 31, 2020, 2019 and 2018, respectively. We expensed $862,296 during the six months ended June 30, 2021. As of June 30, 2021, December 31, 2020, December 31, 2019 and December 31, 2018, the total outstanding balance was $32,500, $0, $0 and $28,213, respectively.
On January 22, 2015, we entered into a Loan Agreement with Knight Therapeutics (Barbados) Inc. (“Knight”) (an owner of greater than 10% of our common stock), for the purchase of the FOCUSfactor assets. The loan bore interest at a rate of 15% per year; provided, however, that upon the occurrence of an equity or convertible equity offering by us of at least $1,000,000, the interest rate would drop to 13% per year. During the year ended December 31, 2018, the highest aggregate principal amount outstanding under this loan was $562,500. During the year ended December 31, 2018, $562,500 of principal and $4,611 of interest was paid, resulting in full repayment of this loan.
On June 26, 2015, we entered into a Security Agreement with Knight Therapeutics, Inc. (“Knight Therapeutics”) (an affiliate of an owner of greater than 10% of our outstanding common stock) through its wholly owned subsidiary Neuragen Corp., for the purchase of the assets of Knight Therapeutics, Inc. At June 30, 2021, December 31, 2020, December 31, 2019 and December 31, 2018, we owed $425,000, $425,000, $475,000 and $525,000, respectively, in relation to this agreement.
On August 18, 2015, we entered into a Consulting Agreement with Kara Harshbarger, the co-founder of Hand MD, LLC, pursuant to which she provided marketing and sales related services. Under the terms of the Consulting Agreement, we paid $120,000, $120,000 and $120,000 to Ms. Harshbarger for the years ended December 31, 2020, 2019 and 2018, respectively, and $60,000 for the six months ended June 30, 2021.
On August 9, 2017, we entered into an Amended and Restated Loan Agreement with Knight for a working capital loan. The loan bears interest at a rate of 10.5% per year. At December 31, 2020, 2019 and 2018, we owed Knight $5,000,000, $5,451,568 and $7,320,739, respectively, on this loan, net of debt issuance cost. Since January 1, 2018, the highest aggregate principal amount outstanding under this loan was $9,500,000 during the year ended December 31, 2018. During the years ended December 31, 2020, 2019 and 2018, $500,000, $2,000,000 and $500,000 of principal was paid, respectively. During the years ended December 31, 2020, 2019 and 2018, $737,595, $912,486 and $325,803 of interest was paid, respectively. During 2021, through June 30, 2021, no principal and $473,958 of interest was paid. As of October 20, 2021, the outstanding principal balance was $5,000,000.
On May 8, 2020, we entered into a Third Amendment Agreement to the Amended and Restated Loan Agreement with Knight, pursuant to which Knight agreed to loan us an additional $2.5 million for working capital. The loan bears interest at a rate of 12.5% per year. Pursuant to the Third Amendment Agreement, a loan success fee of $1,000,000 was earned by Knight and is payable in August 2022. The loan success fee bears interest at a rate of 12.5% per year. At December 31, 2020, we owed Knight $2,500,000 on this loan. Since May 8, 2020, the highest aggregate principal amount outstanding under this loan was $2,500,000. During the year ended December 31, 2020, no principal and $125,868 of interest was paid. During 2021, through June 30, 2021, no principal and $203,646 of interest was paid. As of October 20, 2021, the outstanding principal balance was $500,000.
On December 23, 2016, we entered into an agreement with Knight Therapeutics, Inc. for the distribution rights of FOCUSfactor in Canada. In conjunction with this agreement, we are required to pay Knight Therapeutics a distribution fee equal to 30% of gross sales for sales achieved through a direct sales channel and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight under this agreement is $100,000 Canadian dollars. On and after February 15, 2021, the agreement automatically renews for additional one-year terms unless either party gives notice of nonrenewal at least 180 days prior to the end of the then-current term. During the year ended December 31, 2020, we expensed $178,341 Canadian dollars ($133,094 U.S. Dollars). As of December 31, 2020, 2019 and 2018, the total outstanding balance was $178,341, $100,000 and $200,000 Canadian dollars, respectively. In U.S. Dollars, the total outstanding balance was $140,069, $70,295 and $152,834 as of December 31, 2020, 2019 and 2018, respectively.As of June 30, 2021, the total outstanding balance was $178,341 Canadian dollars ($143,885 U.S. Dollars).
On December 23, 2016, we entered into an agreement with Knight Therapeutics, Inc. for the distribution rights of Hand MD in Canada. In conjunction with this agreement, we are required to pay Knight Therapeutics a distribution fee equal to 60% of gross sales for sales achieved through a direct sales channel until the sales in the calendar year equal the threshold amount and then 40% of all such gross sales in such calendar year in excess of the threshold amount and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight Therapeutics under this agreement is $25,000 Canadian dollars. On and after February 15, 2021, the agreement automatically renews for additional one-year terms unless either party gives notice of nonrenewal at least 180 days prior to the end of the then-current term. During the year ended December 31, 2020, we expensed $385,689 Canadian dollars ($287,836 U.S. Dollars). As of December 31, 2020, 2019 and 2018, the total outstanding balance was $110,637, $25,000 and $25,000 Canadian dollars, respectively. In U.S. Dollars, the total outstanding balance was $86,894, $17,574 and $18,325 as of December 31, 2020, 2019 and 2018, respectively.As of June 30, 2021, the total outstanding balance was $110,637 Canadian dollars ($89,263 U.S. Dollars).
On January 1, 2017, we entered into agreements with Knight Therapeutics, Inc. for the distribution rights of Sneaky Vaunt in Canada, and with Knight Therapeutics (Barbados) Inc. for the distribution rights of Sneaky Vaunt in Israel, Romania, Russia and Sub-Saharan Africa. In conjunction with these agreements, for sales in Canada, we are required to pay Knight Therapeutics a distribution fee equal to 60% of gross sales until the sales in the calendar year equal the threshold amount and then 40% of gross sales in such calendar year in excess of the threshold amount. For sales in Israel, Romania, Russia and Sub-Saharan Africa, we are required to pay Knight Therapeutics (Barbados) a distribution fee equal to 60% of gross sales. On and after February 15, 2021, the agreements automatically renew for additional one-year terms unless either party gives notice of nonrenewal at least 180 days prior to the end of the then-current term. We expensed royalties under the agreements of $1,055, $4,867 and $16,066 for the years ended December 31, 2020, 2019 and 2018, respectively, related to Sneaky Vaunt. At December 31, 2020, 2019 and 2018, we owed $0, $246 and $5,906, respectively, in connection with the royalty distribution agreements. We expensed royalties under the agreements of $1,184 during the six months ended June 30, 2021. At June 30, 2021, we owed $1,079 in connection with the royalty distribution agreements.
We expensed royalties to Knight Therapeutics of $0 and $2,361 for the years ended December 31, 2019 and 2018, respectively, related to The Queen Pegasus, a former product. At December 31, 2019 and 2018, we owed Knight Therapeutics $0 and $193, respectively, in connection with a royalty distribution agreement.
We expensed $145,356, $14,801 and $250,000 for the years ended December 31, 2020, 2019 and 2018, respectively, and $631 for the six months ended June 30, 2021, to Hand MD Corp, related to a royalty agreement. As of June 30, 2021, December 31, 2020, December 31, 2019 and December 31, 2018, the Company owed Hand MD Corp. $0 in minimum future royalties.
On February 15, 2016, Nomad Choice Pty Ltd entered into agreements with Knight Therapeutics, Inc. for the distribution rights of Flat Tummy in Canada and with Knight Therapeutics (Barbados) Inc. for the distribution rights of Flat Tummy in Israel, Romania, Russia and Sub-Saharan Africa. In conjunction with these agreements, for sales in Canada, we are required to pay Knight Therapeutics a distribution fee equal to 60% of gross sales. For sales in Israel, Romania, Russia and Sub-Saharan Africa, we are required to pay Knight Therapeutics (Barbados) a distribution fee equal to 60% of gross sales. On and after February 15, 2021, the agreements automatically renew for additional one-year terms unless either party gives notice of nonrenewal at least 180 days prior to the end of the then-current term. We expensed royalties under the agreements of $169,552, $192,700 and $392,589 for the years ended December 31, 2020, 2019 and 2018, respectively, related to Flat Tummy. At December 31, 2020, 2019 and 2018, we owed $11,026, $5,528 and $109,329, respectively, in connection with the royalty distribution agreements. We expensed royalties under the agreements of $84,465 during the six months ended June 30, 2021. At June 30, 2021, we owed $25,576 in connection with the royalty distribution agreements.
Gale Bensussen, a former member of our Board of Directors, was an executive officer of a supplier to us during 2019. During the years ended December 31, 2019 and 2018, we acquired $4,847,626 and $4,392,245, of products from the supplier, respectively. We owed the supplier $956,438 and $1,775,617, respectively, at December 31, 2019 and 2018.
We entered into transactions with Black Otter, Inc., a related party 100% indirectly owned by Jack Ross, our Chief Executive Officer, during the years ended December 31, 2020 and 2019. The transactions were a pass through of expenses and reimbursements related to marketing. During the years ended December 31, 2020 and 2019, we received advances of $74,629 ($100,000 Canadian Dollars) and $324,102 ($430,000 Canadian Dollars), respectively, which were fully repaid. As of each of December 31, 2020 and 2019, there was $0 due or payable.
We entered into transactions with BoomBod Ltd., a related party 100% indirectly owned by Jack Ross, our Chief Executive Officer, during the six months ended June 30, 2021 and years ended December 31, 2020 and 2019. The transactions were a pass through and allocation of expenses and reimbursements related to marketing. As of June 30, 2021, December 31, 2020 and December 31, 2019 we were owed $3,089,249, $2,305,956 and $277,432, respectively.
We entered into transactions with Gowan Properties Inc., a related party 100% indirectly owned by Jack Ross, our Chief Executive Officer, during the year ended December 31, 2020. The transactions are related to rent expense of $25,000 Canadian Dollars per month for our Halifax, Nova Scotia technology center. During the year ended December 31, 2020, we expensed $275,000 Canadian Dollars ($205,485 U.S. Dollars) for rent expense. As of December 31, 2020, we had advanced $86,250 Canadian Dollars ($67,741 U.S. Dollars) for prepaid rent relating to 2021. During the six months ending June 30, 2021, we expensed $150,000 Canadian Dollars ($120,329 U.S. Dollars) for rent expense. As of June 30, 2021 we had advanced $28,750 Canadian Dollars ($23,196 U.S. Dollars) for prepaid rent.
Our only outstanding class of voting securities is our common stock. The following table sets forth information known to us about the beneficial ownership of our common stock on October 8, 2021 by (i) each current director and director nominee; (ii) each named executive officer; and (iii) all of our executive officers and directors as a group. Other than as set forth below, no person known to us beneficially owns 5% or more of the outstanding common stock as of October 8, 2021.
Unless otherwise indicated in the footnotes, each person listed in the following table has sole voting power and investment power over the common stock listed as beneficially owned by that person. The percentages reflect beneficial ownership immediately prior to and immediately after the completion of this offering and are based on 89,889,074 shares of our common stock outstanding as of October 8, 2021 and shares of our common stock outstanding after the completion of this offering after taking into account the reverse stock split described below. The percentages assume the underwriters do not exercise their option to purchase any additional shares. Unless otherwise indicated in the footnotes, the address for each listed person is Synergy CHC Corp., 865 Spring Street, Westbrook, Maine 04092. The information in the table gives effect to the 1-for- reverse stock split with respect to our common stock, which will occur prior to the effective date of the registration statement of which this prospectus is a part.
| After this Offering | |||||||||||||||||
| Prior to this Offering | If Underwriters’ Option is Not Exercised | If Underwriters’ Option is Exercised in Full | |||||||||||||||
| Shares Beneficially Owned(1) | Shares Beneficially Owned | Shares Beneficially Owned | |||||||||||||||
| Name of Beneficial Owner | Number | Percent | Number | Percent | Number | Percent | |||||||||||
| 5% Stockholders: | |||||||||||||||||
| Gowan Private Equity Inc. (2) | 43,780,750 | 48.7 | % | ||||||||||||||
| Knight Therapeutics (Barbados) Inc. (3) | 17,645,812 | 19.6 | % | ||||||||||||||
| Named executive officers, directors and director nominees: | |||||||||||||||||
| Jack Ross (4) | 51,993,005 | 57.8 | % | ||||||||||||||
| Alfred Baumeler (5) | 1,000,000 | 1.1 | % | ||||||||||||||
| Jaime Fickett (6) | 1,000,000 | 1.1 | % | ||||||||||||||
| J. Paul SoRelle (7) | 2,296,658 | 2.5 | % | ||||||||||||||
| Stephen Fryer | 500 | * | |||||||||||||||
| Nitin Kaushal | – | – | |||||||||||||||
| All executive officers and directors as a group (6 persons) | 56,290,163 | 60.6 | % | ||||||||||||||
| * | Less than 1% of the outstanding common stock. |
| (1) | Beneficial ownership as reported in the table has been determined in accordance with Rule 13d-3 under the Exchange Act and is not necessarily indicative of beneficial ownership for any other purpose. The number of shares of common stock shown as beneficially owned includes shares of common stock which may not be beneficially owned but over which a person would be deemed to exercise control or direction. The number of shares of common stock shown as beneficially owned includes shares of common stock subject to stock options exercisable and restricted stock units that were outstanding on October 8, 2021 and that will vest within 60 days of October 8, 2021. Shares of common stock subject to stock options exercisable and restricted stock units that will vest within 60 days after October 8, 2021 are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. | |
| (2) | This stockholder’s address is 156 Heddas Way, Fall River NS B2T 0J4, Canada. Consists of 43,780,750 shares of common stock owned by Gowan Private Equity Inc. Jack Ross is the Chief Executive Officer of Gowan Private Equity Inc. Decisions regarding the voting or disposition of the shares held by Gowan Private Equity Inc. are made by Mr. Ross. | |
| (3) | Consists of 17,645,812 shares of common stock. This stockholder’s address is Chancery House, High Street, Bridgetown, Barbados. Decisions regarding the voting or disposition of the shares held by Knight Therapeutics (Barbados) Inc. are made by Michel Loustric. | |
| (4) | This stockholder’s address is 156 Heddas Way, Fall River NS B2T 0J4, Canada. Consists of 3,378,572 shares of common stock owned by Jack Ross, 43,780,750 shares of common stock owned by Gowan Private Equity Inc., 3,208,649 shares of common stock owned by Dunhill Distribution Group and 1,625,034 shares of common stock owned by Gowan Capital Inc. Jack Ross is the Chief Executive Officer of Gowan Private Equity Inc., Dunhill Distribution Group, Inc. and Gowan Capital Inc. Decisions regarding the voting or disposition of the shares held by Gowan Private Equity Inc., Dunhill Distribution Group, Inc. and Gowan Capital Inc. are made by Mr. Ross. | |
| (5) | Consists of options to purchase 1,000,000 shares of common stock. | |
| (6) | Consists of options to purchase 1,000,000 shares of common stock. | |
| (7) | Consists of 1,296,658 shares of common stock owned by the SoRelle Family Partnership LLLP and an option to purchase 1,000,000 shares of common stock held by Mr. SoRelle. Decisions regarding the voting or disposition of the shares held by the SoRelle Family Partnership LLLP are made by its general partners, Mr. SoRelle, Carroll V. SoRelle, Carol S. Pederson and Merrie S. Foreman, and each general partner individually has voting and dispositive power over such shares. |
The following descriptions are summaries of the material terms of our certificate of incorporation and amended and restated bylaws as proposed to be in effect upon consummation of the offering. Reference is made to the more detailed provisions of, and the descriptions are qualified in their entirety by reference to, the certificate of incorporation and amended and restated bylaws, forms of which are filed with the SEC as exhibits to the registration statement of which this prospectus is a part, and applicable law.
General
Our authorized capital stock consists of 300,000,000 shares of common stock, par value $0.00001 per share, of which 89,889,074 shares are issued and outstanding as of October 8, 2021, held by approximately 37 stockholders of record, before giving effect to the 1-for- reverse stock split. Upon completion of this offering, there will be shares of common stock outstanding, after giving effect to the 1-for- reverse stock split.
Common Stock
Dividend Rights
The holders of outstanding shares of our common stock are entitled to receive dividends out of funds legally available at the times and in the amounts that our Board of Directors may determine.
Voting Rights
Each holder of our common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of stockholders. Cumulative voting for the election of directors is not provided for in our articles of incorporation, which means that the holders of a majority of our shares of common stock voted can elect all of the directors then standing for election.
Preemptive or Similar Rights
Our common stock is not entitled to preemptive rights and is not subject to conversion or redemption.
Liquidation Rights
Upon our liquidation, dissolution, or winding-up, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our common stock outstanding at that time after payment of other claims of creditors.
Anti-Takeover Effects of Certain Provisions of Nevada Law
Effect of Nevada Anti-takeover Statute. We are subject to Section 78.438 of the Nevada Revised Statutes, an anti-takeover law. In general, Section 78.438 prohibits a Nevada corporation from engaging in any business combination with any interested stockholder for a period of two years following the date that the stockholder became an interested stockholder, unless prior to that date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder, or if after the date that the stockholder becomes an interested stockholder the business combination is approved by the board of directors and by 60% of the voting power of all disinterested stockholders at either an annual or special meeting of the stockholders of the corporation. Section 78.439 provides that business combinations after the two-year period following the date that the stockholder becomes an interested stockholder may also be prohibited unless either approved by the corporation’s directors before the stock acquisition, or by a majority of the disinterested stockholders or unless the price and terms of the transaction meet other criteria set forth in the statute.
Section 78.416 defines “business combination” to include the following:
| ● | any merger or consolidation involving the corporation and the interested stockholder or any other corporation which is an affiliate or associate of the interested stockholder; | |
| ● | any sale, transfer, pledge or other disposition of the assets of the corporation involving the interested stockholder or any affiliate or associate of the interested stockholder if the assets transferred have a market value equal to 5% or more of all of the assets of the corporation or 5% or more of the value of the outstanding shares of the corporation or represent 10% or more of the earning power of the corporation; |
| ● | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to an interested stockholder, with a market value of 5% or more of the value of the outstanding shares of the corporation; | |
| ● | the adoption of a plan of liquidation proposed by or under any arrangement with the interested stockholder or any affiliate or associate of the interested stockholder; | |
| ● | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of voting shares of securities convertible into voting shares of the corporation beneficially owned by the interested stockholder or any affiliate or associate of the interested stockholder; or | |
| ● | the receipt by the interested stockholder or any affiliate or associate of the interested stockholder of the benefit, except proportionately as a stockholder of the corporation, of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 78.423 defines an interested stockholder as any entity or person beneficially owning, directly or indirectly, 10% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by any of these entities or persons.
Control Share Acquisitions. Sections 78.378 through 78.3793 of the Nevada Revised Statutes limit the voting rights of certain acquired shares in a corporation. The provisions apply to any acquisition of outstanding voting securities of a Nevada corporation that has 200 or more stockholders, at least 100 of which are Nevada residents, and conducts business in Nevada (an “issuing corporation”) resulting in ownership of one of the following categories of an issuing corporation’s then outstanding voting securities: (i) twenty percent or more but less than thirty-three percent; (ii) thirty-three percent or more but less than fifty percent; or (iii) fifty percent or more. The securities acquired in such acquisition are denied voting rights unless a majority of the security holders approve the granting of such voting rights. Unless an issuing corporation’s articles of incorporation or bylaws then in effect provide otherwise: (i) voting securities acquired are also redeemable in part or in whole by an issuing corporation at the average price paid for the securities within 30 days if the acquiring person has not given a timely information statement to an issuing corporation or if the stockholders vote not to grant voting rights to the acquiring person’s securities, and (ii) if outstanding securities and the security holders grant voting rights to such acquiring person, then any security holder who voted against granting voting rights to the acquiring person may demand the purchase from an issuing corporation, for fair value, all or any portion of his securities. These provisions do not apply to acquisitions made pursuant to the laws of descent and distribution, the enforcement of a judgment, or the satisfaction of a security interest, or made in connection with certain mergers or reorganizations.
Indemnification of Directors and Officers
Neither our articles of incorporation, nor our amended and restated bylaws, prevent us from indemnifying our officers, directors and agents to the extent permitted under the Nevada Revised Statutes (“NRS”). NRS Section 78.7502, provides that a corporation may indemnify any director, officer, employee or agent of a corporation against expenses, including fees, actually and reasonably incurred by him in connection with any defense to the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to Section 78.7502(1) or 78.7502(2), or in defense of any claim, issue or matter therein.
NRS 78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he: (a) is not liable pursuant to NRS 78.138; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
NRS Section 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he: (a) is not liable pursuant to NRS 78.138; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals there from, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
NRS Section 78.747 provides that except as otherwise provided by specific statute, no director or officer of a corporation is individually liable for a debt or liability of the corporation, unless the director or officer acts as the alter ego of the corporation. The court as a matter of law must determine the question of whether a director or officer acts as the alter ego of a corporation.
Our amended and restated bylaws will provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the NRS, as amended from time to time, subject to any permissible expansion or limitation of such indemnification, as may be set forth in any stockholders’ or directors’ resolution or by contract. Any repeal or modification of these provisions approved by our stockholders will be prospective only and will not adversely affect any limitation on the liability of any of our directors or officers existing as of the time of such repeal or modification. We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the NRS would permit indemnification.
We will enter into agreements with our officers and directors to provide contractual indemnification in addition to the indemnification provided for in our articles of incorporation and amended and restated bylaws.
We do not currently carry directors’ and officers’ insurance. However, we may in the future purchase a policy of directors’ and officers’ liability insurance that insures our officers and directors against the cost of defense, settlement or payment of a judgment in some circumstances and insures us against our obligations to indemnify our officers and directors.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against officers and directors pursuant to these indemnification provisions.
We believe that these provisions and the indemnity agreements are necessary to attract and retain talented and experienced officers and directors.
Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “SNYR.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is VStock Transfer LLC, 18 Lafayette Place, Woodmere, New York 11598.
| 52 |
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there are no publicly-available quotations of our common stock. Future sales of substantial amounts of our common stock in the public market, or the perception that such sales may occur, could adversely affect market prices prevailing from time to time. Further, because only a limited number of shares will be available for sale shortly after this offering due to existing contractual and legal restrictions on resale as described below, there may be sales of substantial amounts of our common stock in the public market after the restrictions lapse. This may adversely affect the prevailing market price and our ability to raise equity capital in the future.
Upon completion of this offering, shares of common stock will be outstanding. Of these shares, shares of our common stock (or shares if the underwriters exercise in full their option to purchase additional shares) sold in this offering will be freely transferable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. The remaining shares of our common stock outstanding are “restricted shares” as defined in Rule 144. Restricted shares may be sold in the public market only if registered under the Securities Act or if they qualify for an exemption from registration under Rule 144. As a result of the contractual 180-day lock-up period described below, the shares subject to lock-up agreements will be available for sale in the public market only after 180 days from the date of this prospectus (generally subject to resale limitations).
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell such securities, provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, the sale and (ii) we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months but who are our affiliates at the time of, or any time during the 90 days preceding, the sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of the following:
| ● | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| ● | the average weekly trading volume of our common stock on the Nasdaq Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale; |
provided, in each case, that we are subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Such sales both by affiliates and by non-affiliates must also comply with the manner of sale and notice provisions of Rule 144 to the extent applicable.
Lock-up Agreements
The Company, each of our directors and executive officers following this offering, and our 1% and greater stockholders, have agreed, subject to certain limited exceptions, not to offer, pledge, sell, contract to sell, grant any option to purchase, or otherwise dispose of our common stock or any securities convertible into or exchangeable or exercisable for common stock, or to enter into any hedge or other arrangement or any transaction that transfers, directly or indirectly, the economic consequence of ownership of the shares of our common stock for a period of 180 days after the date of this prospectus, without the prior written consent of B. Riley Securities, Inc., as representative of the underwriters. See “Underwriting—Lock-up Agreements.” The underwriters do not have any present intention or arrangement to release any shares of our common stock subject to lock-up agreements prior to the expiration of the 180-day lock-up period.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following is a summary of the material U.S. federal income tax consequences relating to the acquisition, ownership, and disposition of common stock acquired pursuant to this offering by non-U.S. holders (as defined below). This summary deals only with common stock held as a capital asset (within the meaning of Section 1221 of the Code) and does not discuss the U.S. federal income tax consequences applicable to a non-U.S. holder that is subject to special treatment under U.S. federal income tax laws, including, but not limited to: a dealer in securities or currencies; a broker-dealer; a financial institution; a qualified retirement plan, individual retirement plan, or other tax-deferred account; a regulated investment company; a real estate investment trust; a tax-exempt organization; an insurance company; a person holding common stock as part of a hedging, integrated, conversion, or straddle transaction or a person deemed to sell common stock under the constructive sale provisions of the Code; a trader in securities that has elected the mark-to-market method of tax accounting; an accrual method taxpayer subject to special tax accounting rules under Section 451(b) of the Code; an entity that is treated as a partnership for U.S. federal income tax purposes; a person that received such common stock in connection with services provided; qualified foreign pension funds as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds; a corporation that is subject to the accumulated earnings tax; a person that owns or has owned, actually or constructively, more than 5% of our common stock; a corporation organized outside the United States, any state thereof or the District of Columbia that is nonetheless treated as a U.S. taxpayer for U.S. federal income tax purposes; a person that is not a non-U.S. holder; a “controlled foreign corporation;” a “passive foreign investment company;” or a U.S. expatriate and former citizens or long-term residents of the United States.
This summary is based upon provisions of the Code, its legislative history, applicable U.S. Treasury regulations promulgated thereunder, published rulings, and judicial decisions, all as in effect as of the date hereof. We have not sought, and will not seek, any ruling from the Internal Revenue Service, or IRS, with respect to the tax consequences discussed herein, and there can be no assurance that the IRS will not take a position contrary to the tax consequences discussed below or that any position taken by the IRS would not be sustained. Those authorities may be repealed, revoked, or modified, perhaps retroactively, or may be subject to differing interpretations, which could result in U.S. federal income tax consequences different from those discussed below. This summary does not address all aspects of U.S. federal income tax, does not deal with all tax considerations that may be relevant to stockholders in light of their personal circumstances, and does not address any state, local, foreign, gift, Medicare, estate (except to the limited extent set forth herein), or alternative minimum tax considerations.
For purposes of this discussion, a “U.S. holder” is a beneficial holder of common stock that is for U.S. federal income tax purposes: an individual citizen or resident of the United States; a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; an estate the income of which is subject to U.S. federal income taxation regardless of its source; or a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (2) was in existence on August 20, 1996 and has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
For purposes of this discussion, a “non-U.S. holder” is a beneficial owner of common stock that is neither a U.S. holder nor a partnership (or any other entity or arrangement that is treated as a partnership) for U.S. federal income tax purposes regardless of its place of organization or formation. If a partnership (or an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes) holds common stock, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. A partner of a partnership holding common stock is urged to consult its own tax advisors.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS CONCERNING THE U.S. FEDERAL INCOME, ESTATE, AND OTHER TAX CONSEQUENCES OF ACQUIRING, OWNING, AND DISPOSING OF OUR COMMON STOCK IN LIGHT OF THEIR SPECIFIC SITUATIONS, AS WELL AS THE TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL, OR NON-U.S. TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS (INCLUDING THE U.S. FEDERAL ESTATE AND GIFT TAX LAWS) OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Distributions on Our Common Stock
Distributions with respect to common stock, if any, generally will constitute dividends for U.S. federal income tax purposes to the extent paid out of current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. Any portion of a distribution in excess of current or accumulated earnings and profits will be treated as a return of capital and will first be applied to reduce the holder’s tax basis in its common stock, but not below zero. Any remaining amount will then be treated as gain from the sale or exchange of the common stock and will be treated as described under “—Disposition of Our Common Stock” below.
Distributions treated as dividends that are paid to a non-U.S. holder, if any, with respect to shares of our common stock, will be subject to U.S. federal withholding tax at a rate of 30% (or such lower rate as may be specified in an applicable income tax treaty, provided the non-U.S. Holder furnishes a valid IRS Form W-8BEN or IRS Form W-8BEN-E (or applicable successor form or other documentation) to us or our paying agent certifying qualification for the lower treaty rate) of the gross amount of the dividends unless the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States subject to the discussion below regarding foreign accounts and backup withholding. A non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaties.
If a non-U.S. holder is engaged in a trade or business in the United States and dividends with respect to the common stock are effectively connected with the conduct of that trade or business and, if required by an applicable income tax treaty, are attributable to a U.S. permanent establishment, then although the non-U.S. holder will generally be exempt from the 30% U.S. federal withholding tax, provided certain certification requirements are satisfied, the non-U.S. holder will be subject to U.S. federal income tax on those dividends on a net income basis at regular graduated U.S. federal income tax rates in the same manner as if such holder were a resident of the United States. Any such effectively connected income received by a foreign corporation may, under certain circumstances, be subject to an additional branch profits tax equal to 30% (or lower applicable income tax treaty rate) of its effectively connected earnings and profits for the taxable year, as adjusted under the Code. To claim the exemption from withholding with respect to any such effectively connected income, the non-U.S. holder must generally furnish to us or our paying agent a properly executed IRS Form W-8ECI (or applicable successor form). In the case of a non-U.S. holder that is an entity, Treasury regulations and the relevant tax treaty provide rules to determine whether, for purposes of determining the applicability of a tax treaty, dividends will be treated as paid to the entity or to those holding an interest in that entity. If a non-U.S. holder holds stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. Such holder’s agent will then be required to provide certification to us or our paying agent.
Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain from a sale, exchange or other disposition of our stock unless:
| (a) | that gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a U.S. permanent establishment maintained by the non-U.S. holder); | |
| (b) | the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or | |
| (c) | we are or have been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2) for U.S. federal income tax purposes at any time during the shorter of the five-year period preceding the date of disposition or the holder’s holding period for our common stock, and certain other requirements are met. |
If a non-U.S. holder is described in clause (a) of the preceding paragraph, the non-U.S. holder will generally be subject to tax on the net gain derived from the disposition at the regular graduated U.S. federal income tax rates in the same manner as if such non-U.S. holder were a U.S. person, unless an applicable income tax treaty provides otherwise. In addition, a non-U.S. holder that is a corporation may be subject to the branch profits tax at a rate equal to 30% (or lower applicable income tax treaty rate) of its effectively connected earnings and profits, as adjusted for certain items.
If the non-U.S. holder is an individual described in clause (b) of the preceding paragraph, the non-U.S. holder will generally be subject to a flat 30% tax on the gain derived from the disposition, which may be offset by U.S.-source capital losses even though the non-U.S. holder is not considered a resident of the United States, provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
If the non-U.S. holder is described in clause (c) of the preceding paragraph, the non-U.S. holder will generally be subject to U.S. federal income tax in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax generally will not apply. Although there can be no assurance, we believe that we are not, and we do not anticipate becoming, a United States real property holding corporation for U.S. federal income tax purposes. Even if we are treated as a United States real property holding corporation, gain realized by a non-U.S. holder on a disposition of our common stock will not be subject to U.S. federal income tax so long as (1) the non-U.S. holder owned, directly, indirectly and constructively, no more than five percent of our common stock at all times within the shorter of (x) the five-year period preceding the disposition, or (y) the holder’s holding period, and (2) our common stock is regularly traded on an established securities market. There can be no assurance that our common stock will continue to qualify as regularly traded on an established securities market. If any gain on your disposition is taxable because we are a United States real property holding corporation and your ownership of our common stock exceeds five percent, you will be taxed on such disposition generally in the manner applicable to U.S. persons and in addition, a purchaser of your common stock may be required to withhold tax with respect to that obligation. Such withheld tax is not an additional tax but merely an advance payment, which may be credited against the tax liability of persons subject to such withholding or refunded to the extent it results in an overpayment of tax and the appropriate information is timely supplied to the IRS.
Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
U.S. Federal Estate Tax
The estate of a nonresident alien individual is generally subject to U.S. federal estate tax on property it is treated as the owner of, or has made certain life transfers of, having a U.S. situs. Because we are a U.S. corporation, our common stock will be U.S. situs property and therefore will be included in the taxable estate of a nonresident alien decedent for U.S. federal estate tax purposes, unless an applicable estate tax treaty between the United States and the decedent’s country of residence provides otherwise.
Information Reporting and Backup Withholding Tax
We report to our non-U.S. holders and the IRS certain information with respect to any dividends we pay on our common stock, including the amount of dividends paid during each fiscal year, the name and address of the recipient, and the amount, if any, of tax withheld. All distributions to holders of common stock are subject to any applicable withholding. Information reporting requirements apply even if no withholding was required because the distributions were effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business or withholding was reduced by an applicable income tax treaty. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. Under U.S. federal income tax law, interest, dividends, and other reportable payments may, under certain circumstances, be subject to “backup withholding” at the then applicable rate (currently, 24%). Backup withholding, however, generally will not apply to distributions on our common stock to a non-U.S. holder, provided the non-U.S. holder furnishes to us or our paying agent the required certification as to its non-U.S. status, such as by providing a valid IRS Form W-8BEN, IRS Form W-8BEN-E or IRS Form W-8ECI, or otherwise establishes an exemption. Notwithstanding the foregoing, backup withholding may apply if either we or our paying agent has actual knowledge, or reason to know, that the holder is a U.S. person that is not an exempt recipient. Backup withholding is not an additional tax but merely an advance payment, which may be credited against the tax liability of persons subject to backup withholding or refunded to the extent it results in an overpayment of tax and the appropriate information is timely supplied to the IRS.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, information reporting but not backup withholding will apply in a manner similar to dispositions effected through a U.S. office of a broker, if a non-U.S. holder sells our common stock through a non-U.S. office of a broker that has certain connections with the United States.
Withholding on Payments to Foreign Accounts
Certain withholding taxes may apply under Section 1471 through 1472 of the Code (which are commonly referred to as the Foreign Account Tax Compliance Act (“FATCA”)) to certain types of payments made to “foreign financial institutions” (as specially defined under these rules) and certain other non-U.S. entities if certification, information reporting and other specified requirements are not met. A 30% withholding tax may apply to “withholdable payments” if they are paid to a foreign financial institution or to a non-financial foreign entity, unless (a) the foreign financial institution undertakes certain diligence and reporting obligations and other specified requirements are satisfied, (b) the non-financial foreign entity either certifies it does not have any substantial U.S. owners or furnishes identifying information regarding each substantial U.S. owner and other specified requirements are satisfied or (c) the foreign financial institution or non-financial foreign entity otherwise qualified for an exemption from these rules.
“Withholdable payment” generally means any payment of interest, dividends, rents, and certain other types of generally passive income if such payment is from sources within the United States. U.S. Treasury Regulations proposed in December 2018 (and upon which taxpayers and withholding agents are entitled to rely until final regulations are issued) eliminate possible withholding under these rules on the gross proceeds from any sale or other disposition of our common stock, previously scheduled to apply beginning January 1, 2019. If the payee is a foreign financial institution, it must enter into an agreement with the U.S. Treasury requiring, among other things, that it undertake to identify accounts held by certain U.S. persons or U.S.-owned foreign entities, annually report certain information about such accounts and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements, or comply with comparable requirements under an applicable inter-governmental agreement between the United States and the foreign financial institution’s home jurisdiction. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. If an investor does not provide us with the information necessary to comply with these rules, it is possible that distributions to such investor that are attributable to withholdable payments, such as dividends, will be subject to the 30% withholding tax.
Holders should consult their own tax advisers regarding the implications of FATCA on their investment in our common stock.
We have entered into an underwriting agreement, dated , 2021, with B. Riley Securities, Inc., acting as the sole book-running manager (sometimes referred to as the “Representative”). Subject to the terms and conditions of the underwriting agreement, each of the underwriters named below has agreed to purchase, and we have agreed to sell to it, the number of shares of common stock listed next to its name at the public offering price, less the underwriting discounts and commissions, as set forth on the cover page of this prospectus and as indicated below:
| Underwriters | Number of Shares | |||
| B. Riley Securities, Inc. | ||||
| Total |
The underwriting agreement provides that the obligation of the underwriters to purchase all of the shares being offered to the public is subject to approval of legal matters by counsel and the satisfaction of other conditions. These conditions include, among others, the continued accuracy of representations and warranties made by us in the underwriting agreement, delivery of legal opinions and the absence of any material changes in our assets, business or prospects after the date of this prospectus. The underwriters are obligated to purchase all of our shares in this offering, other than those covered by the over-allotment option described below, if they purchase any of our shares.
The Representative of the underwriters has advised us that the underwriters propose to offer the common stock directly to the public at the public offering prices listed on the cover page of this prospectus and to selected dealers, who may include the underwriters, at the public offering price less a selling concession not in excess of $ per share for the common stock. After the completion of this offering, the underwriters may change the offering price and other selling terms.
Pursuant to the underwriting agreement, we have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments which the underwriters or other indemnified parties may be required to make in respect of any such liabilities.
Over-Allotment Option
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus, permits the underwriters to purchase a maximum of additional shares from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, each underwriter will be obligated to purchase its proportionate number of shares covered by the option at the public offering price that appears on the cover page of this prospectus, less the underwriting discounts and commissions.
Commissions and Expenses
The following table provides information regarding the amount of the underwriting discounts and commissions to be paid to the underwriters by us. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares to cover over-allotments, if any.
| Per Share | Total Without Over-Allotment | With Over-Allotment | |||||||
| Underwriting discounts and commissions paid by us | $ | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ | $ |
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $ million, which includes our legal, accounting and printing costs and various other fees associated with registration and listing of our common stock. We have agreed to reimburse the Representative for its reasonable out-of-pocket expenses actually incurred in the offering, other than fees and disbursements of legal counsel to the representative. We have agreed to reimburse reasonable fees and disbursements of the Representative’s legal counsel actually incurred up to $300,000 if the offering is not consummated. If the offering is not consummated within a specified period agreed to by and between B. Riley Securities, Inc. and us and any person purchases securities from us in a public offering pursuant to a registration statement filed with the SEC within six months thereafter, we will pay B. Riley Securities, Inc. a termination fee equal to 7.0% of the price paid by the purchaser of such securities, subject to FINRA Rule 5110(g)(5).
| 56 |
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Each of our directors and executive officers following this offering, and our 1% and greater stockholders have agreed to a 180-day “lock-up” from the date of this prospectus relating to shares of our common stock that they beneficially own. This means that, for a period of 180 days following the date of this prospectus, such persons may not offer, sell, pledge or otherwise dispose of these securities without the prior written consent of the representative, subject to certain exceptions.
The Representative may, in their sole discretion and at any time or from time to time, release all or any portion of the common stock or other securities subject to the lock-up agreement. Any determination to release any common stock would be based upon a number of factors at the time of determination, which may include the market price of the common stock, the liquidity of the trading market of the common stock, general market conditions, the number of shares of common stock or other securities proposed to be sold or otherwise transferred and the timing, purposes and terms of the proposed sale or other transfer. The Representative does not have any present intention, agreement or understanding, implicit or explicit, to release any of the shares of common stock or other securities subject to the lock-up agreements prior to the expiration of the lock-up period described above.
In addition, the underwriting agreement provides that, subject to certain exceptions, we will not, for a period of 180 days following the date of this prospectus, offer, sell or distribute any of our securities, without the prior written consent of the underwriters.
Right of First Refusal
We have agreed to grant the Representative, for the 12-month period following the closing of this offering, a right of first refusal to act as (i) lead underwriter and sole book runner in connection with any public offering of equity or equity-linked securities, sole placement agent in any private offering of equity, equity-linked securities, debt or debt-like securities or other capital markets financings and (ii) financial advisor in connection with any sale of all or a significant portion of our assets or capital stock, regardless of how structured and whether in one transaction or a series of transactions, as well as any transaction or disposition that results in the effective sale, transfer or other disposition of ownership or control over a significant portion of one or more of our principal businesses or operations (the “Right of First Refusal”), which right is exercisable in the representative’s sole discretion. In accordance with Financial Industry Regulation Authority (“FINRA”) Rule 5110(g)(6), such Right of First Refusal does not have a duration of more than three years from the commencement of sales in this offering or the termination date of the engagement between us and the representative.
Nasdaq Capital Market
We have applied to have our shares of common stock listed on the Nasdaq Capital Market under the symbol “SNYR.” Our application might not be approved and the completion of this offering is contingent upon such approval.
Stabilization
Until the distribution of the securities offered by this prospectus is completed, rules of the SEC may limit the ability of the underwriters to bid for and to purchase our common stock. As an exception to these rules, the underwriters may engage in transactions effected in accordance with Regulation M under the Exchange Act that are intended to stabilize, maintain or otherwise affect the price of our common stock. The underwriters may engage in over-allotment sales, syndicate covering transactions, stabilizing transactions and penalty bids in accordance with Regulation M:
| ● | Stabilizing transactions permit bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, so long as stabilizing bids do not exceed a specified maximum. |
| ● | Over-allotment involves sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase, which creates a short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of common stock over-allotted by the underwriters is not greater than the number of shares of common stock that they may purchase in the over-allotment option. In a naked short position, the number of shares of common stock involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of our common stock in the open market. |
| ● | Covering transactions involve the purchase of securities in the open market after the distribution has been completed in order to cover short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which they may purchase securities through the over-allotment option. If the underwriters sell more shares of common stock than could be covered by the over-allotment option, creating a naked short position, the position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in this offering. |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a selected dealer when the securities originally sold by the selected dealer are purchased in a stabilizing or syndicate covering transaction. |
These stabilizing transactions, covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market.
Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the prices of our common stock. These transactions may occur on any trading market. If any of these transactions are commenced, they may be discontinued without notice at any time.
Electronic Prospectus
This prospectus may be made available in electronic format on Internet sites or through other online services maintained by the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. Other than this prospectus in electronic format, any information on the underwriters’ or their affiliates’ websites and any information contained in any other website maintained by the underwriters or any affiliate of the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Notice to Prospective Investors in Canada (Alberta, British Columbia, Manitoba, Ontario and Québec Only)
This document constitutes an “exempt offering document” as defined in and for the purposes of applicable Canadian securities laws. No prospectus has been filed with any securities commission or similar regulatory authority in Canada in connection with the offer and sale of shares of common stock described herein (the “Securities”). No securities commission or similar regulatory authority in Canada has reviewed or in any way passed upon this document or on the merits of the Securities and any representation to the contrary is an offence.
Canadian investors are advised that this document has been prepared in reliance on section 3A.3 of National Instrument 33-105 Underwriting Conflicts (“NI 33-105”). Pursuant to section 3A.3 of NI 33-105, this document is exempt from the requirement that the issuer and the underwriters in the offering provide Canadian investors with certain conflicts of interest disclosure pertaining to “connected issuer” and/or “related issuer” relationships as may otherwise be required pursuant to subsection 2.1(1) of NI 33-105.
Resale Restrictions
The offer and sale of the Securities in Canada are being made on a private placement basis only and are exempt from the requirement that the issuer prepare and file a prospectus under applicable Canadian securities laws. Any resale of Securities acquired by a Canadian investor in this offering must be made in accordance with applicable Canadian securities laws, which may vary depending on the relevant jurisdiction, and which may require resales to be made in accordance with Canadian prospectus requirements, a statutory exemption from the prospectus requirements, in a transaction exempt from the prospectus requirements or otherwise under a discretionary exemption from the prospectus requirements granted by the applicable local Canadian securities regulatory authority. These resale restrictions may under certain circumstances apply to resales of the Securities outside of Canada.
Representations of Purchasers
Each Canadian investor who purchases the Securities will be deemed to have represented to the issuer, the underwriters and each dealer from whom a purchase confirmation is received, as applicable, that the investor (i) is purchasing as principal, or is deemed to be purchasing as principal in accordance with applicable Canadian securities laws, for investment only and not with a view to resale or redistribution; (ii) is an “accredited investor” as such term is defined in section 1.1 of National Instrument 45-106 Prospectus Exemptions or, in Ontario, as such term is defined in section 73.3(1) of the Securities Act (Ontario); and (iii) is a “permitted client” as such term is defined in section 1.1 of National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations.
| 58 |
Taxation and Eligibility for Investment
Any discussion of taxation and related matters contained in this document does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a Canadian investor when deciding to purchase the Securities and, in particular, does not address any Canadian tax considerations. No representation or warranty is hereby made as to the tax consequences to a resident, or deemed resident, of Canada of an investment in the Securities or with respect to the eligibility of the Securities for investment by such investor under relevant Canadian federal and provincial legislation and regulations.
Rights of Action for Damages or Rescission
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Personal Information
We and the representative hereby notify prospective Canadian purchasers that: (a) we may be required to provide personal information pertaining to the purchaser as required to be disclosed in Schedule I of Form 45-106F1 under NI 45-106 (including its name, address, telephone number, email address, if provided, and the number and type of securities purchased, the total purchase price paid for such securities, the date of the purchase and specific details of the prospectus exemption relied upon under applicable securities laws to complete such purchase) (“personal information”), which Form 45-106F1 may be required to be filed by us under NI 45-106, (b) such personal information may be delivered to the securities regulatory authority or regulator in accordance with NI 45-106, (c) such personal information is being collected indirectly by the securities regulatory authority or regulator under the authority granted to it under the securities legislation of the applicable legislation, (d) such personal information is collected for the purposes of the administration and enforcement of the securities legislation of the applicable jurisdiction, and (e) the purchaser may contact the applicable securities regulatory authority or regulator by way of the contact information provided in Schedule 2 to Form 45-106F1. Prospective Canadian purchasers that purchase securities in this offering will be deemed to have authorized the indirect collection of the personal information by each applicable securities regulatory authority or regulator, and to have acknowledged and consented to such information being disclosed to the Canadian securities regulatory authority or regulator, and to have acknowledged that such information may become available to the public in accordance with requirements of applicable Canadian laws.
Language of Documents
Upon receipt of this document, each Canadian investor hereby confirms that it has expressly requested that all documents evidencing or relating in any way to the sale of the Securities described herein (including for greater certainty any purchase confirmation or any notice) be drawn up in the English language only. Par la réception de ce document, chaque investisseur canadien confirme par les présentes qu’il a expressément exigé que tous les documents faisant foi ou se rapportant de quelque manière que ce soit à la vente des valeurs mobilières décrites aux présentes (incluant, pour plus de certitude, toute confirmation d’achat ou tout avis) soient rédigés en anglais seulement.
Notice to Prospective Investors in the European Economic Area and the United Kingdom
In relation to the Member States of the European Economic Area and the United Kingdom (each, a “Relevant State”), no offer of shares of our common stock which are the subject of the offering contemplated by this prospectus to the public may be made in that Relevant State other than:
| ● | to any legal entity that is a qualified investor as defined in the Prospectus Regulation; |
| ● | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Regulation), subject to obtaining the prior consent of the relevant representative or representatives nominated by us for any such offer; or |
| ● | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of shares of our common stock described in this prospectus shall result in a requirement for the publication of a prospectus, by us or any of the underwriters, pursuant to Article 3 of the Prospectus Regulation.
Each purchaser of shares of our common stock described in this prospectus located within a Relevant State will be deemed to have represented, acknowledged and agreed that (1) it is a “qualified investor” within the meaning of the Prospectus Regulation; and (2) in the case of any shares of common stock acquired by it as a financial intermediary as that term is used in Article 5(1) of the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares of common stock acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer to the public other than their offer or resale in a Relevant State to qualified investors, as that term is defined in the Prospectus Regulation, or in circumstances in which the prior consent of the underwriters has been given to the offer or resale; or where shares of common stock have been acquired by it on behalf of persons in any Relevant State other than qualified investors, the offer of those shares of common stock to it is not treated under the Prospectus Regulation as having been made to such persons. For purposes of this provision, the expression an “offer to the public” in relation to the shares of our common stock in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares of our common stock to be offered so as to enable an investor to decide to purchase or subscribe to the shares and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
We and the underwriters have not authorized and do not authorize the making of any offer of shares of our common stock through any financial intermediary on their behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated in this prospectus. Accordingly, no purchaser of the shares of our common stock, other than the underwriters, is authorized to make any further offer of the shares on behalf of us or the underwriters. References to the Prospectus Regulation includes, in relation to the UK, the Prospectus Regulation as it forms part of UK domestic law by virtue of the European Union (Withdrawal) Act 2018.
The above selling restriction is in addition to any other selling restrictions set out below.
Additional Notice to Prospective Investors in the United Kingdom
The communication of this prospectus and any other document or materials relating to the issue of the shares of our common stock offered hereby is not being made, and such documents and/or materials have not been approved, by an authorized person for the purposes of section 21 of the United Kingdom’s Financial Services and Markets Act 2000, as amended, or the FSMA. Accordingly, such documents and/or materials are not being distributed to, and must not be passed on to, the general public in the United Kingdom. The communication of such documents and/or materials as a financial promotion is only being made to those persons in the United Kingdom who have professional experience in matters relating to investments and who fall within the definition of investment professionals (as defined in Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Financial Promotion Order), or who fall within Article 49(2)(a) to (d) of the Financial Promotion Order, or who are any other persons to whom it may otherwise lawfully be made under the Financial Promotion Order (all such persons together being referred to as “relevant persons”). In the United Kingdom, the shares of our common stock offered hereby are only available to, and any investment or investment activity to which this prospectus relates will be engaged in only with, relevant persons. Any person in the United Kingdom that is not a relevant person should not act or rely on this prospectus or any of its contents. Any invitation or inducement to engage in investment activity (within the meaning of Section 21 of the FSMA) in connection with the issue or sale of the shares of our common stock may only be communicated or caused to be communicated in circumstances in which Section 21(1) of the FSMA does not apply to us.
All applicable provisions of the FSMA must be complied with in respect to anything done by any person in relation to the shares of our common stock in, from or otherwise involving the United Kingdom.
Notice to Prospective Investors in Switzerland
This document is not intended to constitute an offer or solicitation to purchase or invest in the securities. The securities may not be publicly offered, directly or indirectly, in Switzerland within the meaning of the Swiss Financial Services Act (“FinSA”) and no application has or will be made to admit the securities to trading on any trading venue (exchange or multilateral trading facility) in Switzerland. Neither this document nor any other offering or marketing material relating to the securities constitutes a prospectus pursuant to the FinSA, and neither this document nor any other offering or marketing material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of the securities.
The validity of the shares of common stock offered hereby will be passed upon for us by Nelson Mullins Riley & Scarborough LLP, Raleigh, North Carolina. O’Melveny & Myers LLP is acting as counsel for the underwriters.
The consolidated financial statements of Synergy CHC Corp. as of December 31, 2020 and 2019 and for the years then ended included in this prospectus have been so included in reliance on the reports of RBSM LLP, an independent registered public accounting firm, which are included herein, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement.
Upon completion of this offering, we will be subject to the information and periodic requirements of the Exchange Act and, in accordance therewith, file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov. We also maintain a website at www.synergychc.com. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC.
| 61 |
Synergy CHC Corp.
Condensed Consolidated Balance Sheets
| June 30, 2021 | December 31, 2020 | |||||||
| (Unaudited) | ||||||||
| Assets | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 966,398 | $ | 1,565,453 | ||||
| Restricted cash | 100,000 | 100,000 | ||||||
| Accounts receivable, net | 2,053,230 | 3,380,086 | ||||||
| Loan receivable (related party) | 3,089,249 | 2,305,956 | ||||||
| Prepaid expenses including related party amount of $448,196 and $492,741, respectively) | 1,112,475 | 1,237,230 | ||||||
| Income taxes receivable | 415,844 | 531,918 | ||||||
| Inventory, net | 8,184,212 | 3,466,446 | ||||||
| Total Current Assets | 15,921,408 | 12,587,089 | ||||||
| Total Assets | $ | 15,921,408 | $ | 12,587,089 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued liabilities (including related party payable of $292,303 and $510,064, respectively) | $ | 9,750,601 | $ | 7,101,700 | ||||
| Contract liabilities | 48,664 | 121,803 | ||||||
| Short term loans payable, net of debt discount | 393,754 | - | ||||||
| Current portion of long-term debt, net of debt discount and debt issuance cost, related party | 5,524,450 | 7,515,693 | ||||||
| Total Current Liabilities | 15,717,469 | 14,739,196 | ||||||
| Long-term Liabilities: | ||||||||
| Note payable, net of debt discount and debt issuance cost, related party | 1,239,908 | 1,231,223 | ||||||
| Income taxes payable | 300,773 | 300,773 | ||||||
| Total Long-term Liabilities | 1,540,681 | 1,531,996 | ||||||
| Total Liabilities | 17,258,150 | 16,271,192 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Deficit: | ||||||||
| Common stock, $0.00001 par value; 300,000,000 shares authorized; 89,889,074 shares issued and outstanding | 899 | 899 | ||||||
| Additional paid in capital | 19,147,884 | 19,147,884 | ||||||
| Accumulated other comprehensive loss | (302,517 | ) | (9,449 | ) | ||||
| Accumulated deficit | (20,183,008 | ) | (22,823,437 | ) | ||||
| Total stockholders’ deficit | (1,336,742 | ) | (3,684,103 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 15,921,408 | $ | 12,587,089 | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-2 |
Synergy CHC Corp.
Unaudited Condensed Consolidated Statements of Income and Comprehensive Income
| For the six months ended | ||||||||
| June 30, 2021 | June 30, 2020 | |||||||
| Revenue | $ | 24,761,974 | $ | 17,906,767 | ||||
| Cost of sales | 6,794,344 | 6,593,446 | ||||||
| Gross profit | 17,967,630 | 11,313,321 | ||||||
| Operating expenses | ||||||||
| Selling and marketing | 11,507,769 | 5,640,397 | ||||||
| General and administrative | 2,997,276 | 3,043,044 | ||||||
| Depreciation and amortization | - | 53,544 | ||||||
| Total operating expenses | 14,505,045 | 8,736,985 | ||||||
| Income from operations | 3,462,585 | 2,576,336 | ||||||
| Other (income) expenses | ||||||||
| Interest income | (24 | ) | (79 | ) | ||||
| Other income | - | (181,849 | ) | |||||
| Other expense (loan success fee) | - | 1,000,000 | ||||||
| Interest expense | 627,883 | 497,992 | ||||||
| Remeasurement loss on translation of foreign subsidiary | 89,760 | (12,216 | ) | |||||
| Amortization of debt issuance cost | - | 42,142 | ||||||
| Total other expenses | 717,619 | 1,345,990 | ||||||
| Net income before income taxes | 2,744,966 | 1,230,346 | ||||||
| Income tax expense | 104,537 | 128,996 | ||||||
| Net income after tax | $ | 2,640,429 | $ | 1,101,350 | ||||
| Net income per share – basic | $ | 0.03 | $ | 0.01 | ||||
| Net income per share – diluted | $ | 0.03 | $ | 0.01 | ||||
| Weighted average common shares outstanding | ||||||||
| Basic | 89,889,044 | 89,889,044 | ||||||
| Diluted | 89,889,044 | 89,889,044 | ||||||
| Comprehensive income: | ||||||||
| Net income | $ | 2,640,429 | $ | 1,101,350 | ||||
| Foreign currency translation adjustment | (293,068 | ) | 174,191 | |||||
| Comprehensive income (loss) | $ | 2,347,361 | $ | 1,275,541 | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-3 |
Synergy CHC Corp.
Unaudited Condensed Consolidated Statements of Stockholders’ Deficit
| Common stock | Additional Paid in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Income | Deficit | Deficit | |||||||||||||||||||
| Balance as of December 31, 2019 | 89,889,074 | $ | 899 | $ | 19,018,955 | $ | 11,364 | �� | $ | (24,234,569 | ) | $ | (5,203,351 | ) | ||||||||||
| Fair value of vested stock options | 77,358 | 77,358 | ||||||||||||||||||||||
| Foreign currency translation gain | 174,191 | 174,191 | ||||||||||||||||||||||
| Net income | 1,101,350 | 1,101,350 | ||||||||||||||||||||||
| Balance as of June 30, 2020 | 89,889,074 | $ | 899 | $ | 19,096,313 | $ | 185,555 | $ | (23,133,219 | ) | $ | (3,850,452 | ) | |||||||||||
| Common stock | Additional Paid in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Loss | Deficit | Deficit | |||||||||||||||||||
| Balance as of December 31, 2020 | 89,889,074 | $ | 899 | $ | 19,147,884 | $ | (9,449 | ) | $ | (22,823,437 | ) | $ | (3,684,103 | ) | ||||||||||
| Foreign currency translation loss | (293,068 | ) | (293,068 | ) | ||||||||||||||||||||
| Net income | 2,640,429 | 2,640,429 | ||||||||||||||||||||||
| Balance as of June 30, 2021 | 89,889,074 | $ | 899 | $ | 19,147,884 | $ | (302,517 | ) | $ | (20,183,008 | ) | $ | (1,336,742 | ) | ||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-4 |
Synergy CHC Corp.
Unaudited Condensed Consolidated Statements of Cash Flows
| For the six months ended | ||||||||
| June 30, 2021 | June 30, 2020 | |||||||
| Cash Flows from Operating Activities | ||||||||
| Net income | $ | 2,640,429 | $ | 1,101,350 | ||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | – | 53,544 | ||||||
| Amortization of debt issuance cost | 11,877 | 42,141 | ||||||
| Stock based compensation expense | – | 77,358 | ||||||
| Remeasurement loss (gain) on translation of foreign subsidiary | 89,760 | (12,216 | ) | |||||
| Foreign currency transaction gain (loss) | (348,208 | ) | 190,007 | |||||
| Non cash implied interest | 17,442 | 9,298 | ||||||
| Reversal of allowance for doubtful accounts | – | (170,309 | ) | |||||
| Gain on write-off of payables | – | (180,000 | ) | |||||
| Accrual of loan success fee | – | 1,000,000 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | 1,326,856 | (3,610,852 | ) | |||||
| Loan receivable, related party | (783,293 | ) | 45,179 | |||||
| Inventory | (4,717,766 | ) | (337,369 | ) | ||||
| Prepaid expense | 80,210 | (344,414 | ) | |||||
| Prepaid expense, related party | 44,545 | – | ||||||
| Income taxes receivable | 116,074 | 226,686 | ||||||
| Accounts payable and accrued liabilities | 3,125,110 | (791,573 | ) | |||||
| Accounts payable, related party | (217,761 | ) | 170,345 | |||||
| Contract liabilities | (73,139 | ) | 9,250 | |||||
| Net cash provided by (used in) operating activities | 1,312,136 | (2,521,575 | ) | |||||
| Cash Flows from Investing Activities | ||||||||
| Payments for acquisition of fixed assets | – | (130,528 | ) | |||||
| Net cash used in investing activities | – | (130,528 | ) | |||||
| Cash Flows from Financing Activities | ||||||||
| Advances from related party | – | 70,490 | ||||||
| Repayments of advances to related party | – | (70,490 | ) | |||||
| Proceeds from notes payable, related party | – | 2,500,000 | ||||||
| Proceeds from short term debt | 500,000 | – | ||||||
| Repayment of short term debt | (118,123 | ) | – | |||||
| Repayment of notes payable, related party | (2,000,000 | ) | (525,000 | ) | ||||
| Net cash (used in) provided by financing activities | (1,618,123 | ) | 1,975,000 | |||||
| Effect of exchange rate on cash, cash equivalents and restricted cash | (293,068 | ) | 174,191 | |||||
| Net decrease in cash, cash equivalents and restricted cash | (599,055 | ) | (502,912 | ) | ||||
| Cash, Cash Equivalents and restricted cash, beginning of year | 1,665,453 | 1,324,514 | ||||||
| Cash, Cash Equivalents and restricted cash, end of period | $ | 1,066,398 | $ | 821,602 | ||||
| Supplemental Disclosure of Cash Flow Information: | ||||||||
| Cash paid during the period for: | ||||||||
| Interest | $ | 873,564 | $ | 466,245 | ||||
| Income taxes | $ | – | $ | – | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
| F-5 |
Synergy CHC Corp.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Nature of the Business
Synergy CHC Corp. (“Synergy”, “we”, “us”, “our” or the “Company”) (formerly Synergy Strips Corp.) was incorporated on December 29, 2010 in Nevada under the name “Oro Capital Corporation.” On April 21, 2014, the Company changed its fiscal year end from July 31 to December 31. On April 28, 2014, the Company changed its name to “Synergy Strips Corp.”. On August 5, 2015, the Company changed its name to “Synergy CHC Corp.”
The Company is a consumer health care company that is in the process of building a portfolio of best-in-class consumer product brands. Synergy’s strategy is to grow its portfolio both organically and by further acquisition.
Effective January 1, 2019 the Company has merged the U.S. Subsidiaries (Neuragen Corp., Breakthrough Products Inc., Sneaky Vaunt Corp., and The Queen Pegasus Corp.) into the parent company.
Synergy is the sole owner of two subsidiaries: NomadChoice Pty Ltd. and Synergy CHC Inc. and the results have been consolidated in these statements.
Note 2 – Summary of Significant Accounting Policies
General
The accompanying condensed consolidated financial statements as of June 30, 2021 and December 31, 2020 and for the six months ended June 30, 2021 and 2020 are unaudited. These unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) for interim financial information and are presented in accordance with the requirements of Rule S-X of the Securities and Exchange Commission (the “SEC”). Accordingly, they do not include all the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the six months ended June 30, 2021 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2021. The unaudited condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements as of and for the year ended December 31, 2020 and footnotes thereto.
Basis of Presentation
The unaudited condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
All amounts referred to in the notes to the consolidated financial statements are in United States Dollars ($) unless stated otherwise.
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
| F-6 |
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Significant estimates are assumptions about collection of accounts receivable, current income taxes, deferred income taxes valuation allowance, useful life of fixed and intangible assets, and assumptions used in Black-Scholes-Merton, or BSM, valuation methods, such as expected volatility, risk-free interest rate, and expected dividend rate. The results of any changes in accounting estimates are reflected in the financial statements in the period in which the changes become evident. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the period that they are determined to be necessary.
Reclassification
Certain amounts in prior periods have been reclassified to conform to current period presentation. These reclassifications had no effect on the previously reported net loss.
Cash and Cash Equivalents
The Company considers all cash on hand and in banks, including accounts in book overdraft positions, certificates of deposit and other highly-liquid investments with maturities of three months or less, when purchased, to be cash and cash equivalents. As of June 30, 2021, the Company had no cash equivalents. The Company maintains its cash and cash equivalents in banks insured by the Federal Deposit Insurance Corporation (FDIC) in accounts that at times may be in excess of the federally insured limit of $250,000 per bank. The Company minimizes this risk by placing its cash deposits with major financial institutions. At June 30, 2021, the uninsured balance amounted to $722,362.
Restricted Cash
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the statement of financial position that sum to the total of the same such amounts shown in the statement of cash flows.
| June 30, 2021 | December 31, 2020 | June 30, 2020 | ||||||||||
| Cash and cash equivalents | $ | 966,398 | $ | 1,565,453 | $ | 721,602 | ||||||
| Restricted cash | 100,000 | 100,000 | 100,000 | |||||||||
| Total cash, cash equivalents, and restricted cash shown in the statement of cash flows | $ | 1,066,398 | $ | 1,665,453 | $ | 821,602 | ||||||
Amounts included in restricted cash represent amounts held for credit card collateral.
Capitalization of Fixed Assets
The Company capitalizes expenditures related to property and equipment, subject to a minimum rule, that have a useful life greater than one year for: (1) assets purchased; (2) existing assets that are replaced, improved or the useful lives have been extended; or (3) all land, regardless of cost. Acquisitions of new assets, additions, replacements and improvements (other than land) costing less than the minimum rule in addition to maintenance and repair costs, including any planned major maintenance activities, are expensed as incurred.
Revenue Recognition
The Company recognizes revenue in accordance with the Financial Accounting Standards Board’s (“FASB”), Accounting Standards Codification (“ASC”) ASC 606, Revenue from Contracts with Customers (“ASC 606”). Revenues are recognized when control is transferred to customers in amounts that reflect the consideration the Company expects to be entitled to receive in exchange for those goods. Revenue recognition is evaluated through the following five steps: (i) identification of the contract, or contracts, with a customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied.
| F-7 |
The Company recognizes revenue upon shipment from its fulfillment centers. Certain of our distributors may also perform a separate function as a co-packer on our behalf. In such cases, ownership of and title to our products that are co-packed on our behalf by those co-packers who are also distributors, passes to such distributors when we are notified by them that they have taken transfer or possession of the relevant portion of our finished goods. Freight billed to customers is presented as revenues, and the related freight costs are presented as cost of goods sold. Cancelled orders are refunded if not already dispatched, refunds are only paid if stock is damaged in transit, discounts are only offered with specific promotions and orders will be refilled if lost in transit. The Company recognizes revenue for its digital products in the month the download by the customer occurs.
Contract Assets
The Company does not have any contract assets such as work-in-process. All trade receivables on the Company’s condensed consolidated balance sheet are from contracts with customers.
Contract Costs
Costs incurred to obtain a contract are capitalized unless short term in nature. As a practical expedient, costs to obtain a contract that are short term in nature are expensed as incurred. The Company does not have any contract costs capitalized as of June 30, 2021 or December 31, 2020.
Contract Liabilities
The Company’s contract liabilities consist of advance customer payments. Contract liability results from transactions in which the Company has been paid for products by customers, but for which all revenue recognition criteria have not yet been met. Once all revenue recognition criteria have been met, the contract liabilities are recognized.
| June 30, 2021 | December 31, 2020 | |||||||
| Beginning balance | $ | 121,803 | $ | 7,887 | ||||
| Additions | 48,664 | 121,803 | ||||||
| Recognized as revenue | (121,803 | ) | (7,887 | ) | ||||
| Ending balance | $ | 48,664 | $ | 121,803 | ||||
Accounts receivable
Accounts receivable are generally unsecured. The Company establishes an allowance for doubtful accounts receivable based on the age of outstanding invoices and management’s evaluation of collectability. Accounts are written off after all reasonable collection efforts have been exhausted and management concludes that likelihood of collection is remote. Any future recoveries are applied against the allowance for doubtful accounts. As of both June 30, 2021 and December 31, 2020 the allowance for doubtful accounts was $46,204.
Advertising Expense
The Company expenses marketing, promotions and advertising costs as incurred. Such costs are included in selling expense in the accompanying unaudited condensed consolidated statements of income.
Research and Development
Costs incurred in connection with the development of new products and processing methods are charged to general and administrative expenses as incurred.
Income Taxes
The Company utilizes FASB ASC 740, “Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the tax basis of assets and liabilities and their financial reporting amounts based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. A valuation allowance is recorded when it is “more likely-than-not” that a deferred tax asset will not be realized.
| F-8 |
The Company generated a deferred tax asset through net operating loss carry-forward. However, a valuation allowance of 100% has been established due to the uncertainty of the Company’s realization of the net operating loss carry forward prior to its expiration.
NomadChoice Pty Ltd, the Company’s wholly-owned foreign subsidiary, is subject to income taxes in the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. The company recognizes liabilities for anticipated tax audit issues based on the Company’s current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Synergy CHC Inc. is a wholly-owned foreign subsidiary, is subject to income taxes in the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. The company recognizes liabilities for anticipated tax audit issues based on the Company’s current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Net Earnings (Loss) Per Common Share
The Company computes earnings per share under ASC subtopic 260-10, Earnings Per Share. Basic earnings (loss) per share is computed by dividing the net income (loss) attributable to the common stockholders (the numerator) by the weighted average number of shares of common stock outstanding (the denominator) during the reporting periods. Diluted earnings per share is computed by increasing the denominator by the weighted average number of additional shares that could have been outstanding from securities convertible into common stock (using the “treasury stock” method), unless their effect on net loss per share is anti-dilutive. As of June 30, 2021, and 2020, options to purchase 3,466,667 and 4,666,667 shares of common stock, respectively, were outstanding.
The following is a reconciliation of the number of shares used in the calculation of basic earnings per share and diluted earnings per share for the six months ended June 30, 2021 and 2020:
| For the six months ended | ||||||||
| June 30, 2021 | June 30, 2020 | |||||||
| Net income after tax | $ | 2,640,429 | $ | 1,101,350 | ||||
| Weighted average common shares outstanding | 89,889,074 | 89,889,074 | ||||||
| Incremental shares from the assumed exercise of dilutive stock options | - | - | ||||||
| Dilutive potential common shares | 89,889,074 | 89,889,074 | ||||||
| Net earnings per share: | ||||||||
| Basic | $ | 0.03 | $ | 0.01 | ||||
| Diluted | $ | 0.03 | $ | 0.01 | ||||
The following securities were not included in the computation of diluted net earnings per share as their effect would have been antidilutive:
| 2021 | 2020 | |||||||
| Options to purchase common stock | 3,466,667 | 4,666,667 | ||||||
| F-9 |
Fair Value Measurements
The Company measures and discloses the fair value of assets and liabilities required to be carried at fair value in accordance with ASC 820, Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a framework for measuring fair value, and enhances fair value measurement disclosure.
ASC 820 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. ASC 820 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes three levels of inputs that may be used to measure fair value:
Level 1 - Quoted prices for identical assets or liabilities in active markets to which we have access at the measurement date.
Level 2 - Inputs other than quoted prices within Level 1 that are observable for the asset or liability, either directly or indirectly.
Level 3 - Unobservable inputs for the asset or liability.
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
As of June 30, 2021, the Company has determined that there were no assets or liabilities measured at fair value.
Inventory
Inventory consists of raw materials, components and finished goods. The Company’s inventory is stated at the lower of cost (FIFO cost basis) or net realizable value. Finished goods include the cost of labor to assemble the items.
Stock-Based Compensation
ASC 718, “Compensation – Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
Foreign Currency Translation
The functional currency of one of the Company’s foreign subsidiaries (Nomadchoice Pty Ltd.) is the U.S. Dollar. The Company’s foreign subsidiary maintains its records using local currency (Australian Dollar). All monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at quarter end exchange rates, non-monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at transaction day exchange rates. Income and expense items related to non-monetary items were translated at exchange rates prevailing during the transaction date and other incomes and expenses were translated using average exchange rate for the period. The resulting translation adjustments were recorded in statements of income as Remeasurement gain or loss on translation of foreign subsidiary.
| F-10 |
The functional currency of the Company’s other foreign subsidiary (Synergy CHC Inc.) is the Canadian Dollar (CAD). The Company’s foreign subsidiary maintains its records using local currency (CAD). All assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at period end exchange rates and stockholders’ equity is translated at the historical rates. Income and expense items were translated using average exchange rate for the period. The resulting translation adjustments, net of income taxes, are reported as other comprehensive income and accumulated other comprehensive income in the stockholder’s equity in accordance with ASC 220 – Comprehensive Income.
The exchange rates used to translate amounts in AUD and CAD into USD for the purposes of preparing the consolidated financial statements were as follows:
Balance sheet:
| June 30, 2021 | December 31, 2020 | |||||||
| Period-end AUD: USD exchange rate | $ | 0.7495 | $ | 0.7708 | ||||
| Period-end CAD: USD exchange rate | $ | 0.8068 | $ | 0.7854 | ||||
Income statement:
| June 30, 2021 | June 30, 2020 | |||||||
| Average Quarterly AUD: USD exchange rate | $ | 0.7712 | $ | 0.6572 | ||||
| Average Quarterly CAD: USD exchange rate | $ | 0.8022 | $ | 0.7331 | ||||
Translation gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are translated into either Australian Dollars or Canadian Dollars, as the case may be, at the rate on the date of the transaction and included in the results of operations as incurred.
Concentrations of Credit Risk
In the normal course of business, the Company provides credit terms to its customers; however, collateral is not required. Accordingly, the Company performs credit evaluations of its customers and maintains allowances for possible losses which, when realized, were within the range of management’s expectations. From time to time, a higher concentration of credit risk exists on outstanding accounts receivable for a select number of customers due to individual buying patterns.
Warehousing costs
Warehouse costs include all third party warehouse rent fees and are charged to selling and marketing expenses as incurred. Any additional costs relating to assembly or special pack-outs of the Company’s products are charged to cost of sales.
Product display costs
All displays manufactured and purchased by the Company are for placement of product in retail stores. This also includes all costs for display execution and setup and retail services are charged to cost of sales and expensed as incurred.
Cost of Sales
Cost of sales includes the purchase cost of products sold and all costs associated with getting the products into the retail stores including buying and transportation costs.
Debt Issuance Costs
Debt issuance costs consist primarily of arrangement fees, professional fees and legal fees. These costs are netted off with the related loan and are being amortized to interest expense over the term of the related debt facilities.
| F-11 |
Gain (Loss) on Modification/Extinguishment of Debt
In accordance with ASC 470, a modification or an exchange of debt instruments that adds or eliminates a conversion option that was substantive at the date of the modification or exchange is considered a substantive change and is measured and accounted for as extinguishment of the original instrument along with the recognition of a gain or loss. Additionally, under ASC 470, a substantive modification of a debt instrument is deemed to have been accomplished with debt instruments that are substantially different if the present value of the cash flows under the terms of the new debt instrument is at least 10 percent different from the present value of the remaining cash flows under the terms of the original instrument. A substantive modification is accounted for as an extinguishment of the original instrument along with the recognition of a gain or loss.
Shipping Costs
Shipping and handling costs billed to customers are recorded in sales. Shipping costs incurred by the company are recorded in selling and marketing expenses.
Related parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests.
Segment Reporting
Segment identification and selection is consistent with the management structure used by the Company’s chief operating decision maker to evaluate performance and make decisions regarding resource allocation, as well as the materiality of financial results consistent with that structure. Based on the Company’s management structure and method of internal reporting, the Company has one operating segment. The Company’s chief operating decision maker does not review operating results on a disaggregated basis; rather, the chief operating decision maker reviews operating results on an aggregate basis.
Presentation of Financial Statements – Going Concern
Going Concern Evaluation
In connection with preparing unaudited condensed consolidated financial statements for the six months ended June 30, 2021, management evaluated whether there were conditions and events, considered in the aggregate, that raised substantial doubt about the Company’s ability to continue as a going concern within one year from the date that the financial statements are issued.
The Company considered the following:
| ● | At June 30, 2021, the Company had an accumulated deficit of $20,183,008. | |
| ● | During 2021, the Company extended the due date of its outstanding loans of $5,500,000 to December 31, 2021. |
Ordinarily, conditions or events that raise substantial doubt about an entity’s ability to continue as a going concern relate to the entity’s ability to meet its obligations as they become due.
| F-12 |
The Company evaluated its ability to meet its obligations as they become due within one year from the date that the financial statements are issued by considering the following:
| ● | The Company received $500,000 through a working capital loan. | |
| ● | During the six months ended June 30, 2021, the Company repaid $2,000,000 of loans. | |
| ● | The Company generated net income of $2,640,429 for the six months ended June 30, 2021. | |
| ● | At June 30, 2021, the Company had working capital surplus of $203,939, which includes loans receivable from related party of $3,089,249, prepaid expenses to related party of $448,196 offset by loans payable to related party of $5,524,450 and contract liabilities of $48,664. | |
| ● | Revenue increase in 2021 as compared to 2020 of $6,855,207. | |
| ● | During the six months ended June 30, 2021, the Company’s operating activities provided cash of $1,312,136. | |
| ● | The Company has a line of credit facility of $20 million available from its current lender for future mergers and acquisitions. | |
| ● | The Company may sell its common stock to raise additional capital. The offering price would be determined between the Company and the underwriters based on market conditions at the time of the offering. |
Management concluded that above factors alleviates doubts about the Company’s ability to generate enough cash from operations and other available sources to satisfy its obligations for the next twelve months from the issuance date.
The Company will take the following actions if it starts to trend unfavorably to its internal profitability and cash flow projections, in order to mitigate conditions or events that would raise substantial doubt about its ability to continue as a going concern:
| ● | Raise additional capital through line of credit and/or loans financing for future mergers and acquisition, which may be impacted by the recent outbreak of COVID-19. | |
| ● | Implement additional restructuring and cost reductions. | |
| ● | Raise additional capital through a private placement, which may be impacted by the recent outbreak of COVID-19. |
As of September 14, 2021 and June 30, 2021, the Company had $796,985 and $1,066,398, respectively, in cash and cash equivalents.
Impact of COVID-19
The outbreak of COVID-19, which has been declared by the World Health Organization to be a pandemic, has spread across the globe and is impacting worldwide economic activity. A pandemic, including COVID-19, or other public health epidemic poses the risk that the Company or its employees, suppliers, and other partners may be prevented from conducting business activities at full capacity for an indefinite period of time, including due to spread of the disease within these groups or due to shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the impact that COVID-19 could have on the Company’s business, the continued spread of COVID-19 and the measures taken by the governments of countries affected and in which the Company operates could disrupt the operation of the Company’s business. The COVID-19 outbreak and mitigation measures may also have an adverse impact on global economic conditions, which could have an adverse effect on the Company’s business and financial condition, including on its potential to conduct financings on terms acceptable to the Company, if at all. In addition, the Company may take temporary precautionary measures intended to help minimize the risk of the virus to its employees, including temporarily requiring all employees to work remotely, and discouraging employee attendance at in-person work-related meetings, which could negatively affect the Company’s business. The extent to which the COVID-19 outbreak impacts the Company’s results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of the virus and the actions to contain its impact. COVID-19 did not have a material effect on the Consolidated Statements of Operations or the Consolidated Balance Sheets included in this prospectus.
The CARES Act includes, among other things, provisions relating to payroll tax credits and deferrals, net operating loss carryback periods, alternative minimum tax credits and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also established a Paycheck Protection Program (“PPP”), whereby certain small business are eligible for a loan to fund payroll expenses, rent and related costs. We had received funding under the PPP, and all of that as indicated in our Consolidated Statement of Operations has been forgiven. Our subsidiary, Nomad Choice Pty Ltd received the cash flow boost from the Australian Government due to the global pandemic. This assistance was received from March 2020 through to September 2020 (refer to Note 10).
| F-13 |
Recent Accounting Pronouncements
ASU 2019-12
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which provides guidance in certain areas of accounting for income taxes. ASU 2019-12 is effective for fiscal years beginning after December 15, 2020. Early adoption is permitted. The Company is in the process of evaluating the impact of this standard on its consolidated financial statements and related disclosures. The adoption of the new standard did not have any impact on the Company’s consolidated financial statements.
ASU 2016-13
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments and subsequent amendment to the initial guidance: ASU 2018-19 (collectively, Topic 326). ASU 2016-13 amends the impairment model by requiring entities to use a forward-looking approach based on expected losses to estimate credit losses on certain types of financial instruments, including trade receivables. ASU 2016-13 is effective for fiscal years beginning after December 15, 2022, with early adoption permitted. The adoption of the new standard is not expected to have any impact on the Company’s consolidated financial statements.
There were various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company’s financial position, results of operations or cash flows.
Note 3 – Inventory
Inventory consists of finished goods, components and raw materials. The Company’s inventory is stated at the lower of cost (FIFO cost basis) or net realizable value.
The carrying value of inventory consisted of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Finished goods | $ | 7,621,780 | $ | 3,205,557 | ||||
| Components | 88,666 | 63,910 | ||||||
| Inventory in transit | 355,641 | 171,861 | ||||||
| Raw materials | 118,125 | 25,118 | ||||||
| Total inventory | $ | 8,184,212 | $ | 3,466,446 | ||||
On January 22, 2015, inventory was pledged to Knight Therapeutics under the Loan Agreement (see note 10). As of June 30, 2021 and December 31, 2020, $355,641 and $171,861, respectively, of the Company’s inventory was in transit. During the year ended December 31, 2020 $527,737 of expiring and slow-moving inventory was written off to cost of sales.
Note 4 – Accounts Receivable
Accounts receivable, net of allowances for sales returns and doubtful accounts, consisted of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Trade accounts receivable | $ | 2,099,434 | $ | 3,426,290 | ||||
| Less allowances | (46,204 | ) | (46,204 | ) | ||||
| Total accounts receivable, net | $ | 2,053,230 | $ | 3,380,086 | ||||
During the year ended December 31, 2020, the Company charged $74,068 to bad debt expense and created an allowance for doubtful accounts. During the year ended December 31, 2020, the Company wrote-off accounts receivable of $74,068. During 2020, the Company has reduced the allowance for doubtful accounts by $237,768 relating to allowance for doubtful accounts created in 2019.
| F-14 |
Note 5 – Prepaid Expenses
Prepaid expenses consisted of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Advances for inventory | $ | 363,389 | $ | 362,310 | ||||
| Media | 67,647 | 46,749 | ||||||
| Insurance | 8,234 | 22,951 | ||||||
| Deposits | 16,397 | 12,099 | ||||||
| Rent, related party | 23,196 | 67,741 | ||||||
| Promotion - Bloggers | 66,970 | 113,887 | ||||||
| License agreement | 89,444 | 51,111 | ||||||
| Software subscriptions | 22,149 | - | ||||||
| Components | 9,156 | 73,305 | ||||||
| Prepaid bonus, related party | 425,000 | 425,000 | ||||||
| Miscellaneous | 20,893 | 62,077 | ||||||
| Total | $ | 1,112,475 | $ | 1,237,230 | ||||
Note 6 – Concentration of Credit Risk
Cash and cash equivalents
The Company maintains its cash and cash equivalents in banks insured by the Federal Deposit Insurance Corporation (FDIC) in accounts that at times may be in excess of the federally insured limit of $250,000 per bank. The Company minimizes this risk by placing its cash deposits with major financial institutions. At June 30, 2021 and December 31, 2020, the uninsured balances amounted to $722,362 and $1,386,413, respectively.
Accounts receivable
As of June 30, 2021, three customers accounted for 77% of the Company’s accounts receivable. As of December 31, 2020, four customers accounted for 86% of the Company’s accounts receivable.
Major customers
For the six months ended June 30, 2021, three customers accounted for approximately 67% of the Company’s net revenue. For the six months ended June 30, 2020, four customers accounted for approximately 58% of the Company’s net revenue. For the year ended December 31, 2020, two customers accounted for approximately 47% of the Company’s net revenue. Substantially all of the Company’s business is with companies in North America.
Accounts payable
As of June 30, 2021 and December 31, 2020, two vendors accounted for 64% and 59%, respectively, of the Company’s accounts payable.
Major suppliers
For the six months ended June 30, 2021, two suppliers accounted for approximately 42% of the Company’s purchases. For the six months ended June 30, 2020, two suppliers accounted for approximately 49% of the Company’s purchases. For the year ended December 31, 2020, three suppliers accounted for approximately 46% of the Company’s purchases. Substantially all of the Company’s business is with suppliers in the United States.
| F-15 |
Note 8 – Related Party Transactions
The Company accrued and paid consulting fees of $125,000 for five months to a company owned by Mr. Jack Ross, Chief Executive Officer of the Company. The Company expensed $862,296 during the six months ended June 30, 2021. The Company also paid six months of a vehicle allowance of $2,500 per month. As of June 30, 2021, the total outstanding balance was $32,500 for consulting fees and reimbursements.
On June 26, 2015, the Company entered into a Security Agreement with Knight Therapeutics, Inc., (owner of greater than 10% shares of the Company) through its wholly owned subsidiary Neuragen Corp., for the purchase of Knight Therapeutics, Inc.’s assets. At both June 30, 2021 and December 31, 2020, the Company owed Knight $425,000 in relation to this agreement. The Company recorded present value of future payments of $264,358 as of June 30, 2021 (see Note 10).
On August 18, 2015, the Company entered into a Consulting Agreement with Kara Harshbarger, the co-founder of Hand MD, LLC, pursuant to which she will provide marketing and sales related service. The Company pays Ms. Harshbarger $10,000 a month for one year unless the Consulting Agreement is terminated earlier by either party. The Company has extended this agreement on a month to month basis. Hand MD, LLC is a 50% owner in Hand MD Corp. The Company expensed $60,000 through payroll for the six months ended June 30, 2021. As of June 30, 2021, the total outstanding balance was $0.
The Company has entered into transactions with a related party controlled by the CEO. The transactions were a pass through and allocation of expenses and reimbursements. As of June 30, 2021 and December 31, 2020 the Company was owed $3,089,249 and $2,305,956, respectively.
On August 9, 2017, the Company entered into a Loan Agreement with Knight Therapeutics (Barbados) Inc., a related party (owner of greater than 10% shares of the Company), for a working capital loan. At June 30, 2021 and December 31, 2020, the Company owed Knight $5,000,000 on this loan. During the year ended December 31, 2020 a loan success fee of $1,000,000 was earned by Knight and recorded as a long-term liability, payable in August 2022.
On May 8, 2020, the Company entered into a Loan Agreement with Knight Therapeutics (Barbados) Inc., a related party, for working capital loan. At June 30, 2021 and December 31, 2020, the Company owed Knight $500,000 and $2,500,000, respectively, on this loan (see Note 10).
On December 23, 2016, we entered into an agreement with Knight Therapeutics for the distribution rights of FOCUSfactor in Canada. In conjunction with this agreement, we are required to pay Knight a distribution fee equal to 30% of gross sales for sales achieved through a direct sales channel and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight under this agreement is $100,000 Canadian dollars and is calculated and accrued for annually. During the year ended December 31, 2020, the Company expensed was $178,341 Canadian dollars (US Dollars $133,094). As of both June 30, 2021 and December 31, 2020, the outstanding balance was $178,341 Canadian dollars. In US Dollars, the total outstanding balance was $140,069 as of December 31, 2020. In US Dollars the total outstanding balance was $143,885 as of June 30, 2021.
On December 23, 2016, we entered into an agreement with Knight Therapeutics for the distribution rights of Hand MD into Canada. In conjunction with this agreement, we are required to pay Knight a distribution fee equal to 60% of gross sales for sales achieved through a direct sales channel until the sales in the calendar year equal the threshold amount and then 40% of all such gross sales in such calendar year in excess of the threshold amount and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight under this agreement is $25,000 Canadian dollars and is calculated and accrued for annually. During the year ended December 31, 2020, the Company expensed was $385,689 Canadian dollars (US Dollars $287,836). As of both June 30, 2021 and December 31, 2020, the total outstanding balance was $110,637 Canadian dollars. In US Dollars, the total outstanding balance was $86,894 as of December 31, 2020. In US Dollars, the total outstanding balance was $89,263 as of June 30, 2021.
The Company expensed royalty of $84,465 during the six months ended June 30, 2021. At June 30, 2021, the Company, owed Knight Therapeutics $25,576 in connection with a royalty distribution agreement.
The Company expensed royalty of $1,184 during the six months ended June 30, 2021. At June 30, 2021 the Company owed Knight Therapeutics $1,079 in connection with a royalty distribution agreement for Sneaky Vaunt.
| F-16 |
The Company expensed and paid $631 during the six months ended June 30, 2021 to Hand MD, Corp, related to a royalty agreement. At June 30, 2021, the Company owed Hand MD Corp. $0 in minimum future royalties.
The Company entered into transactions with a related party controlled by the CEO during the six months ended June 30, 2020. The transactions were a pass through of expenses and reimbursements. During the six months ended June 30, 2020, the Company received advances of $70,490 ($100,000 Canadian Dollars), which was fully repaid. As of June 30, 2020, there was $0 due or payable.
The Company entered into transactions with a related party controlled by the CEO during the six months ended June 30, 2021 and 2020. The transactions are related to rent expense of $25,000 Canadian Dollars per month. During the six months ended June 30, 2021, the Company expensed $150,000 Canadian Dollars ($120,329 US Dollars) for rent expense. During the six months ended June 30, 2020, the Company expensed $75,000 Canadian Dollars ($54,984 US Dollars) for rent expense. As of June 30, 2021, the Company has advanced $28,750 Canadian Dollars ($23,196 US Dollars) for prepaid rent. As of December 31, 2020, the Company has advanced $86,250 Canadian Dollars ($67,741 US Dollars) for prepaid rent relating to 2021.
Note 9 – Accounts Payable and Accrued Liabilities
As of June 30, 2021, and December 31, 2020, accounts payable and accrued liabilities consisted of the following:
| June 30, 2021 | December 31, 2020 | |||||||
| Accrued payroll | $ | 232,144 | $ | 180,374 | ||||
| Legal/Professional fees | 11,616 | 17,924 | ||||||
| Commissions | 881,681 | 516,491 | ||||||
| Manufacturers | 5,933,732 | 3,149,773 | ||||||
| Promotions | 639,669 | 1,292,262 | ||||||
| Accounting Fees | 9,121 | 2,926 | ||||||
| Interest, related party | – | 271,528 | ||||||
| Management fee, related party | 32,500 | – | ||||||
| Customers | 80,168 | 144,142 | ||||||
| Royalties, related party | 259,803 | 238,536 | ||||||
| Warehousing | 63,061 | 64,140 | ||||||
| Sales taxes | 738,271 | 899,852 | ||||||
| Payroll taxes | 25,669 | 31,703 | ||||||
| Severance Accrual | 270,333 | 270,333 | ||||||
| Inventory | 512,476 | – | ||||||
| Others | 60,357 | 21,716 | ||||||
| Total | $ | 9,750,601 | $ | 7,101,700 | ||||
The Company has not filed its State & Local Income/Franchise tax returns in States it is required to file. The Company has estimated and accrued for its sales tax liability at $140,519 and $197,483 for the parent entity as of June 30, 2021 and December 31, 2020, respectively.
Note 10 – Notes Payable
The Company’s loans payable at June 30, 2021 and December 31, 2020 are as follows:
| June 30, 2021 | December 31, 2020 | |||||||
| Loans payable | $ | 7,211,235 | $ | 8,746,916 | ||||
| Unamortized debt issuance cost and original issuance discount | (53,123 | ) | – | |||||
| Total | 7,158,112 | 8,746,916 | ||||||
| Less: Current portion, related party | (5,524,450 | ) | (7,515,693 | ) | ||||
| Less: Current portion, other | (393,754 | ) | – | |||||
| Long-term portion, related party | $ | 1,239,908 | $ | 1,231,223 | ||||
| F-17 |
$950,000 June 26, 2015 Security Agreement:
On June 26, 2015, the Company issued a 0% promissory note in a principal amount of $950,000 in connection with an Asset Purchase Agreement. The note requires $250,000 to be paid on or before June 30, 2016, and $700,000 to be paid in quarterly installments (beginning with the quarter ended September 30, 2015) equal to the greater of $12,500 or 5% of U.S. net sales, and 2% of U.S. net sales of Neuragen for 60 months thereafter. The payment of such amounts is secured by a security interest in certain assets, undertakings and property (“Collateral”) pursuant to the Security Agreement, which will be released upon receipt of total payments of $1.2 million.
The Company recorded present value of future payments of $264,358 and $246,916 as of June 30, 2021 and December 31, 2020, respectively. At June 30, 2021 and December 31, 2020, the Company owed Knight $425,000 in relation to this agreement. The Company recorded imputed interest expense of $17,442 and $9,298 for the six months ended June 30, 2021 and 2020, respectively.
$10,000,000 August 9, 2017 Loan:
On August 9, 2017, we entered into a Second Amendment to Loan Agreement (“Second Amendment”) with Knight, pursuant to which Knight agreed to loan us an additional $10 million, and an ongoing credit facility of up to $20 million, and which amount was borrowed at closing (the “Financing”) for working capital purposes. At closing, we paid Knight an origination fee of $200,000 and a work fee of $100,000 and also paid $100,000 of Knight’s expenses associated with the Loan.
Additional Tranches under the Loan Agreement are available to the Company until August 9, 2022 provided that no event of default exists. Each Additional Tranche must be for a minimum amount of $1.0 million, may only be used to finance qualified acquisitions (as defined in the Loan Agreement), and can be denied in Knight’s absolute discretion. If an Additional Tranche is denied, the Company can effect a qualified acquisition through a special purpose entity with such special purpose entity being entitled to obtain financing from third parties so long as such financing does not adversely affect Knight or Knight’s rights under the Loan Agreement. Upon the closing of any Additional Tranche, the Company will pay Knight an origination fee equal to 2% of the Additional Tranche, a work fee equal to 1% of the amount of the Additional Tranche, and reimburse Knight for its expenses incurred in connection with its consideration of any Additional Tranche (whether or not advanced).
The Loan bears interest at 10.5% per annum. The amended Loan Agreement matures on August 8, 2020 and (b) the date that Knight, in its discretion, accelerates the Company’s obligations due to an event of default.
On the Maturity Date of the Third Tranche and every Additional Tranche (or upon the acceleration of each such loan), the Company must pay Knight a success fee (the “Success Fee”) of that number of Company common shares equal to 10% of the loan, divided by the lesser of (a) $1.50, (b) the lowest price at which any common shares were issued by the Company in any offering or equity financing or other transaction between the Closing Date and the date the Success Fee is due, and (c) the current market price on the date the Success Fee is due. The Company may also pay the Success Fee in cash pursuant to the terms of the Loan Agreement.
The Loan Agreement includes customary representations, warranties, and affirmative and restrictive covenants, including covenants to attain and maintain certain financial metrics, and to not merge or dispose of assets, acquire other businesses (except for businesses substantially similar or complementary to the Company’s business, and provided that the aggregate consideration to be paid does not exceed $100,000 and the acquired business guarantees the Company’s obligations under the Loan Agreement) or make capital expenditures in excess of $500,000. The Loan Agreement also includes customary events of default, including payment defaults, breaches of covenants, change of control and material adverse effect defaults. Upon the occurrence of an event of default and during the continuation thereof, the principal amount of all loans under the Loan Agreement will bear a default interest rate of an additional 5%.
The Company’s obligations and liabilities under the Loan Agreement are secured and unconditionally guaranteed by certain of the Company’s wholly owned subsidiaries as provided in the Loan Agreement.
| F-18 |
We have met all the covenants except for the TTM EBITDA of $5 million during the period ending March 31, 2018. Default Interest rate of 5% (from 10.5% to 15.5%) applies in accordance to our current agreement and will be in effect starting April 1, 2018 and will be in effect until the $5 million TTM EBITDA covenant is achieved. We entered into Loan Amendment Agreement on May 14, 2018, the interest rate was reduced to 13% due to reducing payroll expenses. Also, Synergy will maintain Focus Factor Net Sales as measured on a year-end basis of at least USD $15 million for each fiscal year starting with December 31, 2017.
We have amended our covenants under our loan agreement on March 27, 2019. The new covenants are as follows: we will maintain a minimum EBITDA of $1,900,000 for the twelve months ending on December 31, 2018, $2,500,000 for the twelve months ending March 31, 2019, $3,500,000 for the twelve months ending June 30, 2019 and $5,000,000 for the twelve months period ending on last day of each fiscal quarters thereafter. We shall maintain a net debt to TTM EBITDA ratio of no more than 8:1 for the twelve month period ending on December 31, 2018 until March 31, 2019 and shall maintain a net debt to TTM EBITDA ratio of no more than 6:1 thereafter. We shall maintain at all times a positive cash balance of $575,000 for the three month period ending December 31, 2018, $750,000 for the three month period ending March 31, 2019 and $1,000,000 thereafter. The default interest rate of 2.5% applies (from 13% to 15.5%) in accordance to our current agreement and will be in effect as of October 1, 2018 to June 30, 2019. Effective June 30, 2019 the interest rate reverted back to 10.5%.
The Company also recorded deferred financing costs of $452,869 with respect to the above loan. The Company recognized amortization of deferred financing costs of $0 and $42,141 during the six months ended June 30, 2021 and 2020, respectively. Unamortized debt issuance cost as of both June 30, 2021 and December 31, 2020 amounted to $0.
On May 8, 2020, the Company entered into a Third Amendment Agreement (the “Third Amendment”) to the Amended and Restated Loan Agreement (the “Loan Agreement”) with Knight Therapeutics (Barbados) Inc. (“Knight”), pursuant to which Knight agreed to loan the Company an additional $2.5 million (the “Additional Loan”). That same day (the “Closing”), the Company paid Knight a work fee of $36,000, and $25,000 for Knight’s legal costs and expenses incurred in connection with the Third Amendment. The Third Amendment amends the original loan agreement that the Company and Knight entered into in January 2015 and subsequently amended (as amended, the “Original Loan Agreement”). The Additional Loan matures on May 8, 2021 (the “TA Maturity Date”) and bears interest at 12.5% per annum compounding quarterly. On the TA Maturity Date, the Company will pay Knight a success fee (the “Success Fee”) of $83,250. The Success Fee is payable in cash or stock as set forth in the Loan Agreement. The Third Amendment includes customary representations, warranties, and affirmative and restrictive covenants, including covenants to attain and maintain certain financial metrics, including an undertaking to maintain at all times a cash balance of $600,000 and EBITDA of $3,000,000 for the twelve months ended June 30, 2020 and $4,000,000 for the twelve month period ending on the last day of each fiscal quarter thereafter.
Terms of the $10,000,000 August 9, 2017 loan (Third Tranche) (see note 10) were modified in the Third amendment. Third tranche shall bear interest from May 8, 2020 at a rate equal to 12.5% per annum compounded quarterly. The Company shall pay success fee in the amount of $1,000,000 with respect to the Third Tranche, which shall be fully earned on May 8, 2020 and payable no later than August 31, 2022. Third Tranche success fee shall bear interest at 12.5% per annum compounding quarterly. The loan has been extended to a maturity date of December 31, 2021. Because these amendments were considered not substantive changes, the Company accounted for the modifications as modification of debt.
The Company recognized interest expense of $500,868 and $466,246 during the six months ended June 30, 2021 and 2020, respectively. Accrued interest was $0 and $271,528 as of June 30, 2021 and December 31, 2020, respectively. The loan balances, including success fee, at June 30, 2021 and December 31 2020 were $6,500,000 and $8,500,000, respectively.
| F-19 |
The PPP Loan and Financial Assistance:
In June 2020, the American Institute of Certified Public Accountants in conjunction with the Financial Accounting Standards Board developed Technical Question and Answer (“TQA”) 3200.18, “Borrower Accounting for a Forgivable Loan Received Under the Small Business Administration Paycheck Protection Program”, which is intended to provide clarification on how to account for loans received from the PPP. TQA 3200.18 states that an entity may account for PPP loans under ASC 470, “Debt” or, if the entity is expected to meet PPP eligibility criteria and the PPP loan is expected to be forgiven, the entity may account for the loans under IAS 20, “Accounting for Government Grants and Disclosure of Government Assistance”. The Company has elected to account for PPP loan proceeds under IAS 20 as allowed by TQA 3200.18.
The CARES Act includes, among other things, provisions relating to payroll tax credits and deferrals, net operating loss carryback periods, alternative minimum tax credits and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also established a Paycheck Protection Program (“PPP”), whereby certain small business are eligible for a loan to fund payroll expenses, rent and related costs. We had received funding under the PPP of $163,542 and bears interest at 1% per annum, with monthly installments commencing on May 5, 2020 for 60 months through its maturity on May 5, 2025. The Company used all proceeds from the PPP Loan to maintain payroll and other allowable expenses. As a result, management believes that the Company has met the PPP eligibility criteria for forgiveness and has concluded that the loan represents, in substance, a government grant that is expected to be forgiven in its entirety. As such, in accordance with International Accounting Standards (“IAS”) 20, “Accounting for Government Grants and Disclosure of Government Assistance,” the Company has recognized the entire PPP Loan amount of $163,542 as other income, which is included in other non-operating income (expense) in the consolidated statement of income for the six months ended June 30, 2020. Our subsidiary, Nomad Choice Pty Ltd received the cash flow boost from the Australian Government due to the global pandemic. This assistance was received from March 2020 through to June 2020 of $18,307 which is included in other non-operating income (expense) in the consolidated statement of income for the six months ending June 30, 2020.
$565,000 May 16, 2021 Loan:
On May 16, 2021, the Company entered into a loan agreement of $565,000 with Shopify Capital Inc. for an advancement of working capital from its online processing account. The Company received $500,000 from Shopify Capital Inc. and $65,000 was an original issue discount. The loan bears a repayment rate of 14% of daily sales and the Company shall pay $94,167 every 60 days with a final payment due on May 12, 2022.
The payment of such amounts is secured by a security interest in certain assets, undertakings and property pursuant to the Security Agreement, which will be released upon receipt of total payments of $565,000.
The Company recognized amortization original issue discount of $11,877, which is included in interest expense in the statement of income during the six months ended June 30, 2021. The outstanding loan balance at June 30, 2021 was $393,754.
Note 11 – Stockholders’ Equity
The total number of shares of all classes of capital stock which the Company is authorized to issue is 300,000,000 shares of common stock with $0.00001 par value.
As of both June 30, 2021 and December 31, 2020, there were 89,889,074 shares of the Company’s common stock issued and outstanding.
Note 12 – Commitments & Contingencies
Litigation:
From time to time the Company may become a party to litigation in the normal course of business. Management believes that there are no current legal matters that would have a material effect on the Company’s financial position or results of operations.
| F-20 |
Note 13 – Stock Options
The following table summarizes the options outstanding, option exercisability and the related prices for the shares of the Company’s common stock issued to employees and consultants under a stock option plan at June 30, 2021:
| Options Outstanding | Options Exercisable | |||||||||||||||||
| Exercise Prices ($) | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price ($) | Number Exercisable | Weighted Average Exercise Price ($) | |||||||||||||
| $ | 0.25 – 0.70 | 3,466,667 | 3.96 | $ | 0.54 | 3,446,667 | $ | 0.54 | ||||||||||
The stock option activity for the six months ended June 30, 2021 is as follows:
| Options Outstanding | Weighted Average Exercise Price | |||||||
| Outstanding at December 31, 2020 | 3,466,667 | $ | 0.54 | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Expired or canceled | - | - | ||||||
| Outstanding at June 30, 2021 | 3,466,667 | $ | 0.54 | |||||
Stock-based compensation expense related to vested options was $0 and $77,358 during the six months ended June 30, 2021 and 2020, respectively, which is a component of general and administrative expense in the statement of operations. The Company determined the value of share-based compensation for options vesting during the year ended December 31, 2017 using the Black-Scholes fair value option-pricing model with the following weighted average assumptions: estimated fair value of Company’s common stock of $0.48-0.50, risk-free interest rate of 1.95-1.99%, volatility of 116-117%, expected lives of 10 years, and dividend yield of 0%. Stock options outstanding as of June 30, 2021, as disclosed in the above table, have an intrinsic value of $0. As of June 30, 2021, unamortized stock-based compensation costs related to options was $0.
Note 14 – Segments
Segment identification and selection is consistent with the management structure used by the Company’s chief operating decision maker to evaluate performance and make decisions regarding resource allocation, as well as the materiality of financial results consistent with that structure. Based on the Company’s management structure and method of internal reporting, the Company has one operating segment. The Company’s chief operating decision maker does not review operating results on a disaggregated basis; rather, the chief operating decision maker reviews operating results on an aggregate basis.
Net sales attributed to customers in the United States and foreign countries for the six months ended June 30, 2021 and 2020 were as follows:
| June 30, 2021 | June 30, 2020 | |||||||
| United States | $ | 23,928,636 | $ | 12,737,461 | ||||
| Foreign countries | 833,338 | 5,169,306 | ||||||
| $ | 24,761,974 | $ | 17,906,767 | |||||
Foreign countries primarily consist of sales in Canada.
| F-21 |
The Company’s net sales by product group for the six months ended June 30, 2021 and 2020 were as follows:
| June 30, 2021 | June 30, 2020 | |||||||
| Nutraceuticals | $ | 24,549,101 | $ | 12,361,201 | ||||
| Over the Counter (OTC) | - | 22,311 | ||||||
| Consumer Goods | 168,047 | 565,756 | ||||||
| Cosmeceuticals | 44,826 | 4,957,499 | ||||||
| $ | 24,761,974 | $ | 17,906,767 | |||||
(1) Net sales for any other product group of similar products are less than 10% of consolidated net sales.
The Company’s net sales by major sales channel for the six months ended June 30, 2021 and 2020 were as follows:
| June 30, 2021 | June 30, 2020 | ||||||||
| Online | $ | 10,163,673 | $ | 6,538,289 | |||||
| Retail | 14,598,301 | 11,368,478 | |||||||
| $ | 24,761,974 | $ | 17,906,767 | ||||||
Note 15 – Income Taxes
Income tax expense was $104,537 for the six months ended June 30, 2021, compared to $128,996 for the same period in 2020. The current provision is attributable to Australian operations and the current tax rate in effect in that country.
The total deferred tax asset is calculated by multiplying a domestic federal (US) 21% marginal tax rate by the cumulative net operating loss carryforwards (“NOL”). The domestic marginal tax rate does not include any state & local marginal tax rate attributable to the Company. The Company currently has estimated NOLs, which expire through 2035. Management has determined based on all the available information that a 100% valuation reserve is required.
For U.S. purposes, the Company has not completed its evaluation of NOL utilization limitations under Internal Revenue Code, as amended (the “Code”) Section 382/383, change of ownership rules. If the Company has had a change in ownership, the NOLs would be limited or eliminated, as to the amount that could be utilized each year, based on the Code. NOLs attributable to Breakthrough Products, Inc., which are the majority of the Company’s domestic NOLs are Separate Return Limitation Year (SRLY) NOLs. Such losses may generally not be available for use (limited or eliminated).
The Company has not filed its State & Local Income/Franchise tax returns in States it is required to file for the last few years, so such returns and liability remain open. The Company does not expect this to be a significant liability.
Note 16 – Subsequent Events
Management evaluated all activities of the Company through the issuance date of the Company’s unaudited condensed consolidated financial statements and concluded that except as noted below, no subsequent events have occurred that would require adjustment or disclosure into the unaudited condensed consolidated financial statements.
Subsequent to June 30, 2021 the Company has entered into an agreement to acquire the outstanding 50% of Hand MD Corp. whereby Synergy will become the 100% owner. The consideration of $1,700,000 will be disbursed as follows: $500,000 within 10 business days of the execution of the agreement, $400,000 on or before the six month anniversary of the agreement, $400,000 on or before the twelve month anniversary of the agreement and $400,000 on or before the eighteen month anniversary of the agreement. The first payment of $500,000 was made in July 2021.
| F-22 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Synergy CHC Corp. and subsidiaries
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Synergy CHC Corp. and subsidiaries (the Company) as of December 31, 2020 and 2019, and the related statements of operations, other comprehensive income (loss), stockholders’ (deficit) equity, and cash flows for each of the years in the two year period ended December 31, 2020, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the two year period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments.
We determined that there are no critical audit matters.
| /s/ RBSM LLP | |
| We have served as the Company’s auditor since 2014. | |
| New York, NY | |
| August 5, 2021 |
| F-23 |
Consolidated Balance Sheets
| December 31, 2020 | December 31, 2019 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 1,565,453 | $ | 1,224,514 | ||||
| Restricted cash | 100,000 | 100,000 | ||||||
| Accounts receivable, net | 3,380,086 | 1,146,354 | ||||||
| Loan receivable (related party) | 2,305,956 | 277,432 | ||||||
| Prepaid expenses (including related party amount of $492,741 and $0, respectively) | 1,237,230 | 186,143 | ||||||
| Income taxes receivable | 531,918 | 251,614 | ||||||
| Inventory, net | 3,466,446 | 1,861,038 | ||||||
| Total Current Assets | 12,587,089 | 5,047,095 | ||||||
| Fixed assets, net | - | 135,898 | ||||||
| Intangible assets, net | - | 7,636 | ||||||
| Total Assets | $ | 12,587,089 | $ | 5,190,629 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable and accrued liabilities (including related party payable of $510,064 and $1,051,216, respectively) | $ | 7,101,700 | $ | 4,674,064 | ||||
| Contract liabilities | 121,803 | 7,887 | ||||||
| Current portion of long-term notes payable, net of debt discount and debt issuance cost, related party | 7,515,693 | 5,465,113 | ||||||
| Total Current Liabilities | 14,739,196 | 10,147,064 | ||||||
| Long-term Liabilities: | ||||||||
| Notes payable, net of debt discount and debt issuance cost, related parties | 1,231,223 | 246,916 | ||||||
| Income taxes payable | 300,773 | – | ||||||
| Total long-term liabilities | 1,531,996 | 246,916 | ||||||
| Total Liabilities | 16,271,192 | 10,393,980 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Deficit: | ||||||||
| Common stock, $0.00001 par value; 300,000,000 shares authorized; 89,889,074 and 89,889,074, shares issued and outstanding, respectively | 899 | 899 | ||||||
| Additional paid in capital | 19,147,884 | 19,018,955 | ||||||
| Accumulated other comprehensive (loss) income | (9,449 | ) | 11,364 | |||||
| Accumulated deficit | (22,823,437 | ) | (24,234,569 | ) | ||||
| Total stockholders’ deficit | (3,684,103 | ) | (5,203,351 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 12,587,089 | $ | 5,190,629 | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-24 |
Consolidated Statements of Operations and Other Comprehensive Income (Loss)
| For the year Ended | For the year ended | |||||||
| December 31, 2020 | December 31, 2019 | |||||||
| Revenue | $ | 40,226,865 | $ | 29,357,546 | ||||
| Cost of Sales (including related party purchases of $0 and $4,847,626, respectively) | 14,578,865 | 9,137,602 | ||||||
| Gross Profit | 25,648,000 | 20,219,944 | ||||||
| Operating expenses | ||||||||
| Selling and marketing | 15,889,066 | 11,471,652 | ||||||
| General and administrative | 6,499,344 | 5,493,433 | ||||||
| Reserve for bad debts | 74,068 | 283,971 | ||||||
| Impairment of assets | 47,002 | 9,715,137 | ||||||
| Depreciation and amortization | 100,265 | 1,211,861 | ||||||
| Total operating expenses | 22,609,745 | 28,176,054 | ||||||
| Income (loss) from operations | 3,038,255 | (7,956,110 | ) | |||||
| Other (income) expenses | ||||||||
| Interest income | (104 | ) | (414 | ) | ||||
| Interest expense | 1,186,034 | 981,105 | ||||||
| Remeasurement (gain) loss on translation of foreign subsidiary | (457,955 | ) | 8,280 | |||||
| Amortization of debt issuance cost | 48,432 | 130,829 | ||||||
| Other income | (210,284 | ) | - | |||||
| Other expenses (loan success fee) | 1,000,000 | - | ||||||
| Finance expenses | 61,000 | - | ||||||
| Total other expenses | 1,627,123 | 1,119,800 | ||||||
| Net income (loss) before income taxes | 1,411,132 | (9,075,910 | ) | |||||
| Income tax expense | – | (131,537 | ) | |||||
| Net income (loss) after tax | $ | 1,411,132 | $ | (9,207,447 | ) | |||
| Net income (loss) per share – basic and diluted | $ | 0.02 | $ | (0.10 | ) | |||
| Weighted average common shares outstanding | ||||||||
| Basic and Diluted | 89,889,044 | 89,883,194 | ||||||
| Comprehensive income (loss): | ||||||||
| Net income (loss) | $ | 1,411,132 | $ | (9,207,447 | ) | |||
| Foreign currency translation adjustment | (20,813 | ) | (167,752 | ) | ||||
| Comprehensive income (loss) | $ | 1,390,319 | $ | (9,375,199 | ) | |||
The accompanying notes are an integral part of these consolidated financial statements
| F-25 |
Consolidated Statements of Stockholders’ (Deficit) Equity
| Common stock | Additional Paid in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | (Deficit) | |||||||||||||||||||
| Balance as of December 31, 2018 | 89,862,683 | $ | 899 | $ | 18,817,800 | $ | 179,116 | $ | (15,027,122 | ) | $ | 3,970,693 | ||||||||||||
| Fair value of vested stock options | 161,570 | 161,570 | ||||||||||||||||||||||
| Common stock issued for Per-fekt settlement | 26,391 | 39,585 | 39,585 | |||||||||||||||||||||
| Foreign currency translation loss | (167,752 | ) | (167,752 | ) | ||||||||||||||||||||
| Net loss | (9,207,447 | ) | (9,207,447 | ) | ||||||||||||||||||||
| Balance as of December 31, 2019 | 89,889,074 | $ | 899 | $ | 19,018,955 | $ | 11,364 | $ | (24,234,569 | ) | $ | (5,203,351 | ) | |||||||||||
| Fair value of vested stock options | 128,929 | 128,929 | ||||||||||||||||||||||
| Foreign currency translation loss | (20,813 | ) | (20,813 | ) | ||||||||||||||||||||
| Net income | 1,411,132 | 1,411,132 | ||||||||||||||||||||||
| Balance as of December 31, 2020 | 89,889,074 | $ | 899 | $ | 19,147,884 | $ | (9,449 | ) | $ | (22,823,437 | ) | $ | (3,684,103 | ) | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements
| F-26 |
Consolidated Statements of Cash Flows
| For the year Ended | For the year ended | |||||||
| December 31, 2020 | December 31, 2019 | |||||||
| Cash Flows from Operating Activities | ||||||||
| Net income (loss) | $ | 1,411,132 | $ | (9,207,447 | ) | |||
| Adjustments to reconcile net income (loss) to net cash (used in) provided by operating activities: | ||||||||
| Amortization of debt issuance cost | 48,432 | 130,829 | ||||||
| Depreciation and amortization | 100,265 | 1,211,860 | ||||||
| Stock based compensation expense | 128,929 | 201,155 | ||||||
| Impairment of intangible assets | 47,002 | 9,715,137 | ||||||
| Foreign currency transaction loss | 351,194 | (1,308 | ) | |||||
| Bad debts | 74,068 | 283,972 | ||||||
| Remeasurement loss on translation of foreign subsidiary | (457,955 | ) | 8,280 | |||||
| Non cash implied interest | 36,455 | 38,310 | ||||||
| Reversal of allowance for doubtful accounts | (237,768 | ) | - | |||||
| Gain on write-off of payables | (180,000 | ) | - | |||||
| Accrual of loan success fee | 1,000,000 | - | ||||||
| Write-off of inventory | 527,737 | 257,111 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (2,070,032 | ) | 3,027,900 | |||||
| Loan and accounts receivable, related party | (2,028,524 | ) | (277,432 | ) | ||||
| Inventory | (2,133,145 | ) | 552,158 | |||||
| Prepaid expenses | (558,346 | ) | 642,704 | |||||
| Prepaid expense, related party | (492,741 | ) | - | |||||
| Income taxes receivable | (280,304 | ) | 135,072 | |||||
| Income taxes payable | 300,773 | - | ||||||
| Contract liabilities | 113,916 | (41,823 | ) | |||||
| Accounts payable and accrued liabilities | 3,251,816 | (2,910,949 | ) | |||||
| Accounts payable, related party | (541,152 | ) | (819,179 | ) | ||||
| Net cash (used in) provided by operating activities | (1,588,248 | ) | 2,946,350 | |||||
| Cash Flows from Investing Activities | ||||||||
| Net cash used in investing activities | - | - | ||||||
| Cash Flows from Financing Activities | ||||||||
| Advances from related party | 74,629 | 324,102 | ||||||
| Repayments of advances to related party | (74,629 | ) | (324,102 | ) | ||||
| Proceeds from notes payable | 2,500,000 | - | ||||||
| Repayment of notes payable | (550,000 | ) | (2,050,000 | ) | ||||
| Net cash provided by (used in) financing activities | 1,950,000 | (2,050,000 | ) | |||||
| Effect of exchange rate on cash, cash equivalents and restricted cash | (20,813 | ) | (167,752 | ) | ||||
| Net increase in cash, cash equivalents and restricted cash | 340,939 | 728,598 | ||||||
| Cash, Cash Equivalents and restricted cash, beginning of year | 1,324,514 | 595,916 | ||||||
| Cash, Cash Equivalents and restricted cash, end of year | $ | 1,665,453 | $ | 1,324,514 | ||||
| Supplemental Disclosure of Cash Flow Information: | ||||||||
| Cash paid during the period for: | ||||||||
| Interest | $ | 805,048 | $ | 939,711 | ||||
| Income taxes | $ | - | $ | 38,919 | ||||
| Supplemental Disclosure of Non-cash Investing and Financing Activities: | ||||||||
| Accrual of loan success fee | $ | 1,000,000 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements
| F-27 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Nature of the Business
Synergy CHC Corp. (“Synergy”, “we”, “us”, “our” or the “Company”) (formerly Synergy Strips Corp.) was incorporated on December 29, 2010 in Nevada under the name “Oro Capital Corporation.” On April 21, 2014, the Company changed its fiscal year end from July 31 to December 31. On April 28, 2014, the Company changed its name to “Synergy Strips Corp.”. On August 2012,5, 2015, the Company changed its name to “Synergy CHC Corp.”
The Company is a consumer health care company that is in the process of building a portfolio of best-in-class consumer product brands. Synergy’s strategy is to grow its portfolio both organically and by further acquisition.
Effective January 1, 2019 the Company has merged the U.S. Subsidiaries (Neuragen Corp., Breakthrough Products Inc., Sneaky Vaunt Corp., and The Queen Pegasus Corp.) into the parent company.
Synergy is the sole owner of two subsidiaries: NomadChoice Pty Ltd. and Synergy CHC Inc. and the results have been consolidated in these statements.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“US GAAP”).
All amounts referred to in the notes to the consolidated financial statements are in United States Dollars ($) unless stated otherwise.
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Reclassification
Certain amounts in prior periods have been reclassified to conform to current period presentation. These reclassifications had no effect on the previously reported net loss.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates. At December 31, 2020 and 2019 significant estimates included are assumptions about collection of accounts receivable, current income taxes, deferred income taxes valuation allowance, useful life of fixed and intangible assets, impairment analysis of goodwill and intangible assets, estimates used in the fair value calculation of stock based compensation, assumptions used in Black-Scholes-Merton, or BSM, valuation methods, such as expected volatility, risk-free interest rate, and expected dividend rate. The results of any changes in accounting estimates are reflected in the financial statements in the period in which the changes become evident. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the period that they are determined to be necessary.
Cash and Cash Equivalents
The Company considers all cash on hand and in banks, including accounts in book overdraft positions, certificates of deposit and other highly-liquid investments with maturities of three months or less, when purchased, to be cash and cash equivalents. As of December 31, 2020, and 2019, the Company had no cash equivalents. The Company maintains its cash and cash equivalents in banks insured by the Federal Deposit Insurance Corporation (FDIC) in accounts that at times may be in excess of the federally insured limit of $250,000 per bank. The Company minimizes this risk by placing its cash deposits with major financial institutions. At December 31, 2020 and 2019, the uninsured balances amounted to $1,386,413 and $947,312, respectively.
| F-28 |
Restricted Cash
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the statement of financial position that sum to the total of the same such amounts shown in the statement of cash flows.
| December 31, 2020 | December 31, 2019 | |||||||
| Cash and cash equivalents | $ | 1,565,453 | $ | 1,224,514 | ||||
| Restricted cash | 100,000 | 100,000 | ||||||
| Total cash, cash equivalents, and restricted cash shown in the statement of cash flows | $ | 1,665,453 | $ | 1,324,514 | ||||
Amount included in restricted cash represents the amount held for credit card collateral.
Capitalization of Fixed Assets
The Company capitalizes expenditures related to property and equipment, subject to a minimum rule, that have a useful life greater than one year for: (1) assets purchased; (2) existing assets that are replaced, improved or the useful lives have been extended; or (3) all land, regardless of cost. Acquisitions of new assets, additions, replacements and improvements (other than land) costing less than the minimum rule in addition to maintenance and repair costs, including any planned major maintenance activities, are expensed as incurred.
Intangible Assets
We evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. All of our intangible assets are subject to amortization except intellectual property of $1,450,000 acquired as part of an Asset Purchase Agreement entered into with Factor Nutrition Labs LLC on January 22, 2015, $10,000 acquired as part of an Asset Purchase Agreement entered into with Perfekt Beauty Holdings LLC and CDG Holdings, LLC (“Perfekt”) on June 21, 2017 and $50,000 acquired as an Asset Purchase entered into with Cocowhite on May 22, 2018. Intangible assets are amortized on a straight line basis over the useful lives. During the year ended December 31, 2019, the Company fully impaired intellectual property related to Focus Factor and charged to operations impairment loss of $1,450,000.
Long-lived Assets
Long-lived assets include equipment and intangible assets other than those with indefinite lives. We assess the carrying value of our long-lived asset groups when indicators of impairment exist and recognize an impairment loss when the carrying amount of a long-lived asset is not recoverable when compared to undiscounted cash flows expected to result from the use and eventual disposition of the asset.
Indicators of impairment include significant underperformance relative to historical or projected future operating results, significant changes in our use of the assets or in our business strategy, loss of or changes in customer relationships and significant negative industry or economic trends. When indications of impairment arise for a particular asset or group of assets, we assess the future recoverability of the carrying value of the asset (or asset group) based on an undiscounted cash flow analysis. If carrying value exceeds projected, net, undiscounted cash flows, an additional analysis is performed to determine the fair value of the asset (or asset group), typically a discounted cash flow analysis, and an impairment charge is recorded for the excess of carrying value over fair value. During the year ended December 31, 2019, the Company fully impaired intangible assets and charged to operations impairment loss of $471,897.
Goodwill
An asset purchase is accounted for under the purchase method of accounting. Under that method, assets and liabilities of the business acquired are recorded at their estimated fair values as of the date of the acquisition, with any excess of the cost of the acquisition over the estimated fair value of the net tangible and intangible assets acquired recorded as goodwill. As of December 31, 2019 our qualitative analysis of goodwill indicated potential impairment, thus the Company chose to fully impair goodwill and charged to operations impairment loss of $7,793,240.
Revenue Recognition
The Company recognizes revenue in accordance with the Financial Accounting Standards Board’s (“FASB”), Accounting Standards Codification (“ASC”) ASC 606, Revenue from Contracts with Customers (“ASC 606”). Revenues are recognized when control is transferred to customers in amounts that reflect the consideration the Company expects to be entitled to receive in exchange for those goods. Revenue recognition is evaluated through the following five steps: (i) identification of the contract, or contracts, with a customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied.
| F-29 |
The Company recognizes revenue upon shipment from its fulfillment centers. Certain of our distributors may also perform a separate function as a co-packer on our behalf. In such cases, ownership of and title to our products that are co-packed on our behalf by those co-packers who are also distributors, passes to such distributors when we are notified by them that they have taken transfer or possession of the relevant portion of our finished goods. Freight billed to customers is presented as revenues, and the related freight costs are presented as cost of goods sold. Cancelled orders are refunded if not already dispatched, refunds are only paid if stock is damaged in transit, discounts are only offered with specific promotions and orders will be refilled if lost in transit. The Company recognizes revenue for its digital products in the month the download by the customer occurs.
Contract Assets
The Company does not have any contract assets such as work-in-process. All trade receivables on the Company’s consolidated balance sheet are from contracts with customers.
Contract Costs
Costs incurred to obtain a contract are capitalized unless short term in nature. As a practical expedient, costs to obtain a contract that are short term in nature are expensed as incurred. The Company does not have any contract costs capitalized as of December 31, 2020 or 2019.
Contract Liabilities
The Company’s contract liabilities consist of advance customer payments. Contract liability results from transactions in which the Company has been paid for products by customers, but for which all revenue recognition criteria have not yet been met. Once all revenue recognition criteria have been met, the contract liabilities are recognized.
| December 31, 2020 | December 31, 2019 | |||||||
| Beginning balance | $ | 7,887 | $ | 49,709 | ||||
| Additions | 121,803 | 7,887 | ||||||
| Recognized as revenue | (7,887 | ) | (49,709 | ) | ||||
| Ending balance | $ | 121,803 | $ | 7,887 | ||||
Accounts receivable
Accounts receivable are generally unsecured. The Company establishes an allowance for doubtful accounts receivable based on the age of outstanding invoices and management’s evaluation of collectability. Accounts are written off after all reasonable collection efforts have been exhausted and management concludes that likelihood of collection is remote. Any future recoveries are applied against the allowance for doubtful accounts. As of December 31, 2020 and 2019, allowance for doubtful accounts was $46,204 and $283,971, respectively.
Advertising Expense
The Company expenses marketing, promotions and advertising costs as incurred. Such costs are included in selling and marketing expense in the accompanying consolidated statements of operations.
Research and Development
Costs incurred in connection with the development of new products and processing methods are charged to general and administrative expenses as incurred.
Income Taxes
The Company utilizes FASB ASC 740, “Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the tax basis of assets and liabilities and their financial reporting amounts based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. A valuation allowance is recorded when it is “more likely-than-not” that a deferred tax asset will not be realized.
| F-30 |
The Company generated a deferred tax asset through net operating loss carry-forward. However, a valuation allowance of 100% has been established due to the uncertainty of the Company’s realization of the net operating loss carry forward prior to its expiration.
NomadChoice Pty Ltd, the Company’s wholly-owned subsidiary is subject to income taxes in the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. The company recognizes liabilities for anticipated tax audit issues based on the Company’s current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Synergy CHC Inc. is a wholly-owned foreign subsidiary, is subject to income taxes in the jurisdictions in which it operates. Significant judgment is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. The company recognizes liabilities for anticipated tax audit issues based on the Company’s current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Net Earnings (Loss) Per Common Share
The Company computes earnings per share under ASC subtopic 260-10, Earnings Per Share. Basic earnings (loss) per share is computed by dividing the net income (loss) attributable to the common stockholders (the numerator) by the weighted average number of shares of common stock outstanding (the denominator) during the reporting periods. Diluted earnings per share is computed by increasing the denominator by the weighted average number of additional shares that could have been outstanding from securities convertible into common stock (using the “treasury stock” method), unless their effect on net income per share is anti-dilutive. As of December 31, 2020, and 2019, options to purchase 3,466,667 and 6,166,667 shares of common stock, respectively, were outstanding.
The following is a reconciliation of the number of shares used in the calculation of basic and diluted earnings (loss) per share for the years ending December 31, 2020, and 2019:
| For the year ending | ||||||||
| December 31, 2020 | December 31, 2019 | |||||||
| Net income (loss) after tax | $ | 1,411,132 | $ | (9,207,447 | ) | |||
| Weighted average common shares outstanding | 89,889,044 | 89,883,194 | ||||||
| Incremental shares from the assumed exercise of dilutive stock options | - | - | ||||||
| Dilutive potential common shares | 89,889,044 | 89,883,194 | ||||||
| Net earnings (loss) per share: | ||||||||
| Basic | $ | 0.02 | $ | (0.10 | ) | |||
| Diluted | $ | 0.02 | $ | (0.10 | ) | |||
The following securities were not included in the computation of diluted net earnings per share as their effect would have been antidilutive:
| For the year ending | ||||||||
| December 31, 2020 | December 31, 2019 | |||||||
| Options to purchase common stock | 3,466,667 | 6,166,667 | ||||||
Fair Value Measurements
The Company measures and discloses the fair value of assets and liabilities required to be carried at fair value in accordance with ASC 820, Fair Value Measurements and Disclosures. ASC 820 defines fair value, establishes a framework for measuring fair value, and enhances fair value measurement disclosure.
| F-31 |
ASC 825 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. ASC 825 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825 establishes three levels of inputs that may be used to measure fair value:
– Level 1 - Quoted prices for identical assets or liabilities in active markets to which we have access at the measurement date.
– Level 2 - Inputs other than quoted prices within Level 1 that are observable for the asset or liability, either directly or indirectly.
– Level 3 - Unobservable inputs for the asset or liability.
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
As of both December 31, 2020 and 2019, the Company has determined that there were no assets or liabilities measured at fair value.
Inventory
Inventory consists of raw materials, components and finished goods. The Company’s inventory is stated at the lower of cost (FIFO cost basis) or net realizable value. Finished goods include the cost of labor to assemble the items.
Stock-Based Compensation
ASC 718, “Compensation – Stock Compensation,” prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
Foreign Currency Translation
The functional currency of one of the Company’s foreign subsidiaries (NomadChoice Pty Ltd.) is the U.S. Dollar. The Company’s foreign subsidiary maintains its records using local currency (Australian Dollar – “AUD”). All monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at period end exchange rates, non-monetary assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at transaction day exchange rates. Income and expense items related to non-monetary items were translated at exchange rates prevailing during the transaction date and other incomes and expenses were translated using average exchange rate for the period. The resulting translation adjustments, net of income taxes, were recorded in statements of operations as Remeasurement gain or loss on translation of foreign subsidiary.
The functional currency of the Company’s other foreign subsidiary (Synergy CHC Inc.) is the Canadian Dollar (CAD). The Company’s foreign subsidiary maintains its records using local currency (CAD). All assets and liabilities of the foreign subsidiary were translated into U.S. Dollars at period end exchange rates and stockholders’ equity is translated at the historical rates. Income and expense items were translated using average exchange rate for the period. The resulting translation adjustments, net of income taxes, are reported as other comprehensive income and accumulated other comprehensive income in the stockholder’s equity in accordance with ASC 220 – Comprehensive Income.
The exchange rates used to translate amounts in AUD and CAD into USD for the purposes of preparing the consolidated financial statements were as follows:
Balance sheet:
| December 31, 2020 | December 31, 2019 | |||||||
| Period-end AUD: USD exchange rate | $ | 0.7708 | $ | 0.7030 | ||||
| Period-end CAD: USD exchange rate | $ | 0.7854 | $ | 0.7699 | ||||
| F-32 |
Income statement:
| December 31, 2020 | December 31, 2019 | |||||||
| Average Yearly AUD: USD exchange rate | $ | 0.6902 | $ | 0.6954 | ||||
| Average Yearly CAD: USD exchange rate | $ | 0.7463 | $ | 0.7537 | ||||
Translation gains and losses that arise from exchange rate fluctuations from transactions denominated in a currency other than the functional currency are translated into either Australian Dollars or Canadian Dollars, as the case may be, at the rate on the date of the transaction and included in the results of operations as incurred.
Concentrations of Credit Risk
In the normal course of business, the Company provides credit terms to its customers; however, collateral was not required. Accordingly, the Company performed credit evaluations of its customers and maintained allowances for possible losses which, when realized, were within the range of management’s expectations. From time to time, a higher concentration of credit risk existed on outstanding accounts receivable for a select number of customers due to individual buying patterns.
Warehousing costs
Warehouse costs include all third party warehouse rent fees and are charged to selling and marketing expenses as incurred. Any additional costs relating to assembly or special pack-outs of the Company’s products are charged to cost of sales.
Product display costs
All displays manufactured and purchased by the Company are for placement of product in retail stores. This also includes all costs for display execution and setup and retail services are charged to cost of sales and expensed as incurred.
Cost of Sales
Cost of sales includes the purchase cost of products sold, all costs associated with getting the products into the retail stores including buying and transportation costs and the hosting of our online Application.
Debt Issuance Costs
Debt issuance costs consist primarily of arrangement fees, professional fees and legal fees. These costs were netted off with the related loan and were being amortized to interest expense over the term of the related debt facilities.
Gain (Loss) on Modification/Extinguishment of Debt
In accordance with ASC 470, a modification or an exchange of debt instruments that adds or eliminates a conversion option that was substantive at the date of the modification or exchange is considered a substantive change and is measured and accounted for as extinguishment of the original instrument along with the recognition of a gain or loss. Additionally, under ASC 470, a substantive modification of a debt instrument is deemed to have been accomplished with debt instruments that are substantially different if the present value of the cash flows under the terms of the new debt instrument is at least 10 percent different from the present value of the remaining cash flows under the terms of the original instrument. A substantive modification is accounted for as an extinguishment of the original instrument along with the recognition of a gain or loss.
Shipping Costs
Shipping and handling costs billed to customers are recorded in sales. Shipping costs incurred by the company are recorded in selling and marketing expenses.
Related parties
Parties are considered to be related to the Company if the parties that, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests.
| F-33 |
Segment Reporting
Segment identification and selection is consistent with the management structure used by the Company’s chief operating decision maker to evaluate performance and make decisions regarding resource allocation, as well as the materiality of financial results consistent with that structure. Based on the Company’s management structure and method of internal reporting, the Company has one operating segment. The Company’s chief operating decision maker does not review operating results on a disaggregated basis; rather, the chief operating decision maker reviews operating results on an aggregated basis.
Presentation of Financial Statements – Going Concern
Going Concern Evaluation
In connection with preparing consolidated financial statements for the year ended December 31, 2020, management evaluated whether there were conditions and events, considered in the aggregate, that raised substantial doubt about the Company’s ability to continue as a going concern within one year from the date that the consolidated financial statements are issued.
The Company considered the following:
| ● | At December 31, 2020, the Company had an accumulated deficit of $22,823,437. | |
| ● | At December 31, 2020, the Company had working capital deficit of $2,152,107. | |
| ● | During 2020, the Company extended the due date of its outstanding loan to December 31, 2020 and subsequently extended it until December 31, 2021. |
Ordinarily, conditions or events that raise substantial doubt about an entity’s ability to continue as a going concern relate to the entity’s ability to meet its obligations as they become due.
The Company evaluated its ability to meet its obligations as they become due within one year from the date that the financial statements are issued by considering the following:
| ● | Revenue increased in 2020 by $10,869,319. | |
| ● | The Company had net income of $1,411,132 in 2020 as opposed to a net loss of $9,207,447 in 2019. | |
| ● | The Company raised $2.5 million via debt financing during the year ended December 31, 2020. | |
| ● | In 2020, the Company repaid $0.5 million of loans. | |
| ● | Working capital deficit of $2,152,107 at December 31, 2020, includes loans payables to related party of $7,515,693 and deferred revenue of $121,803. | |
| ● | The Company has line of credit facility of $20 million available from its current lender for future mergers and acquisition. | |
| ● | The Company has the option of selling its common stock to raise additional capital. The offering price will be determined between us and the underwriters based on market conditions at the time of the offering. |
Management concluded that above factors alleviates doubts about the Company’s ability to generate enough cash from operations and other available sources to satisfy its obligations for the next twelve months from the issuance date.
The Company will take the following actions if it starts to trend unfavorably to its internal profitability and cash flow projections, in order to mitigate conditions or events that would raise substantial doubt about its ability to continue as a going concern:
| ● | Raise additional capital through line of credit and/or loans financing for future mergers and acquisition, which may be impacted by the outbreak of COVID-19. | |
| ● | Implement additional restructuring and cost reductions. | |
| ● | Raise additional capital through a private placement, which may be impacted by the outbreak of COVID-19. |
At July 27, 2021 and December 31, 2020, the Company had $2,700,863 and $1,665,453, respectively in cash and cash equivalents and restricted cash.
Impact of COVID-19
The outbreak of COVID-19, which has been declared by the World Health Organization to be a pandemic, has spread across the globe and is impacting worldwide economic activity. A pandemic, including COVID-19, or other public health epidemic poses the risk that the Company or its employees, suppliers, and other partners may be prevented from conducting business activities at full capacity for an indefinite period of time, including due to spread of the disease within these groups or due to shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the impact that COVID-19 could have on the Company’s business, the continued spread of COVID-19 and the measures taken by the governments of countries affected and in which the Company operates could disrupt the operation of the Company’s business. The COVID-19 outbreak and mitigation measures may also have an adverse impact on global economic conditions, which could have an adverse effect on the Company’s business and financial condition, including on its potential to conduct financings on terms acceptable to the Company, if at all. In addition, the Company may take temporary precautionary measures intended to help minimize the risk of the virus to its employees, including temporarily requiring all employees to work remotely, and discouraging employee attendance at in-person work-related meetings, which could negatively affect the Company’s business. The extent to which the COVID-19 outbreak impacts the Company’s results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of the virus and the actions to contain its impact. COVID-19 did not have a material effect on the consolidated statements of operations or the consolidated balance sheets included in this prospectus.
| F-34 |
The CARES Act includes, among other things, provisions relating to payroll tax credits and deferrals, net operating loss carryback periods, alternative minimum tax credits and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also established a Paycheck Protection Program (“PPP”), whereby certain small business are eligible for a loan to fund payroll expenses, rent and related costs. We had received funding under the PPP, and all of that as indicated in our Consolidated Statement of Operations has been forgiven. Our subsidiary, Nomad Choice Pty Ltd received the cash flow boost from the Australian Government due to the global pandemic. This assistance was received from March 2020 through to September 2020 (refer to Note 12).
Recent Accounting Pronouncements
ASU 2019-12
In December 2019, the FASB issued ASU 2012-03, “Technical Amendments2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which provides guidance in certain areas of accounting for income taxes. ASU 2019-12 is effective for fiscal years beginning after December 15, 2020. Early adoption is permitted. The Company is in the process of evaluating the impact of this standard on its consolidated financial statements and Corrections to SEC Sections: Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin (SAB) No. 114, Technical Amendments Pursuant to SEC Release No. 33-9250, and Corrections Related to FASB Accounting Standards Update 2010-22 (SEC Update)” in Accounting Standards Update No. 2012-03. This update amends various SEC paragraphs pursuant to the issuance of SAB No. 114.related disclosures. The adoption of ASU 2012-03the new standard is not expected to have a materialany impact on ourthe Company’s consolidated financial position or results of operations.
ASU 2018-13
In July 2012,August 2018, the FASB issued ASU 2012-02, “Intangibles2018-13, Fair Value Measurement (Topic 820): Disclosure Framework – GoodwillChanges in Disclosure Requirements for Fair Value Measurement, which removes, modifies and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment”adds certain disclosure requirements in Accounting Standards Update No. 2012-02. This update amendsTopic 820 “Fair Value Measurement”. ASU 2011-08, Intangibles – Goodwill and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment and permits an entity first to assess qualitative factors to determine whether it2018-13 is more likely than not that an indefinite-lived intangible asset is impaired as a basis for determining whether it is necessary to perform the quantitative impairment test in accordance with Subtopic 350-30, Intangibles - Goodwill and Other - General Intangibles Other than Goodwill. The amendments are effective for annual and interim impairment tests performed for fiscal years beginning after SeptemberDecember 15, 2012.2019, including interim periods within those fiscal years. Early adoption is permitted. Adoption of this new standard did not have any impact on the Company’s consolidated financial statements.
ASU 2017-04, Intangibles - Goodwill and other (Topic 350)
In January 2017, the FASB issued Accounting Standards Update (“ASU”) 2017-04 Intangibles - Goodwill and other, which simplifies the test for goodwill impairment. This Update eliminates Step 2 from the goodwill impairment test. In computing the implied fair value of goodwill under Step 2, an entity had to perform procedures to determine the fair value at the impairment testing date of its assets and liabilities (including unrecognized assets and liabilities) following the procedure that would be required in determining the fair value of the assets acquired and liabilities assumed in a business combination. Instead an entity should perform its annual, or interim, goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value, however the loss recognized should not exceed the total amount of goodwill allocated to the reporting unit. The amendments in this Update are effective for the Company for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. Early adoption is permitted, including adoption in an interim period. Adoption of this new standard did not have any impact on the Company’s consolidated financial statements.
ASU 2016-15
In August 2016, the FASB issued AS 2016-15, Classification of Certain Cash Receipts and Cash Payments, which clarifies how certain cash receipts and cash payments are presented and classified in the statement of cash flows. The effective date for annualASU 2016-15 is for fiscal years beginning after December 15, 2018, and interim periods within fiscal years beginning after December 15, 2019. Early adoption is permitted. Adoption of this new standard did not have any impact on the Company’s consolidated financial statements.
ASU 2016-13
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments and subsequent amendment to the initial guidance: ASU 2018-19 (collectively, Topic 326). ASU 2016-13 amends the impairment tests performed asmodel by requiring entities to use a forward-looking approach based on expected losses to estimate credit losses on certain types of a date before July 27, 2012, if a public entity’s financial statementsinstruments, including trade receivables. ASU 2016-13 is effective for the most recent annual or interim period have not yet been issued or, for nonpublic entities, have not yet been made available for issuance.fiscal years beginning after December 15, 2022, with early adoption permitted. The adoption of ASU 2012-02the new standard is not expected to have a materialany impact on ourthe Company’s consolidated financial position or results of operations.statements.
| F-35 |
ASU 2016-02
In December 2011,February 2016, the FASB issued ASU 2011-12, “Deferral201602, “Leases” (“ASU 201602”). This guidance, as amended by subsequent ASU’s on the topic, improves transparency and comparability among companies by recognizing right of use (ROU) assets and lease liabilities on the Effective Datebalance sheet and by disclosing key information about leasing arrangements. ASU No. 2016-02 is effective for Amendmentspublic business entities for annual periods, including interim periods within those annual periods, beginning after December 15, 2018, with early adoption permitted. We adopted ASU No. 2016-02 in our fiscal year beginning January 1, 2019 and used the optional transition method provided by the FASB in ASU No. 2018-10, “Codification Improvements to Topic 842, Leases” and ASU No. 2018-11, “Leases (Topic 842): Targeted Improvements”, with no restatement of comparative periods. The Company notes there was no impact on adoption as the leases entered into by the Company were for less than 12 month terms.
The new standard provides optional practical expedients in transition. We will only elect the package of practical expedients where, under the new standard, prior conclusions about lease identification, lease classification and initial direct costs do not need to be reassessed. The new standard also provides practical expedients for ongoing accounting where we elected the practical expedients on adoption and did not record any ROU asset with terms of less than 12 months.
There were various updates recently issued, most of which represented technical corrections to the Presentation of Reclassifications of Items out of Accumulated Other Comprehensive Income” in Accounting Standards Update No. 2011-05. This update defers the requirementaccounting literature or application to present items thatspecific industries and are reclassified from accumulated other comprehensive income to net income in both the statement of income where net income is presented and the statement where other comprehensive income is presented. The adoption of ASU 2011-12 is not expected to a have a material impact on ourthe Company’s financial position, or results of operations.
Note 3 –MINING CLAIM
The Company intends to conduct exploration activitiesutilizes FASB ASC 740, “Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the Shipman Diamond Project,difference between the tax basis of assets and liabilities and their financial reporting amounts based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. A valuation allowance is located 50 kilometers northeast of Prince Albert, Saskatchewan, Canada and 2 kilometers north of the village of Shipman. Oro Capital Corporation has acquiredrecorded when it is “more likely-than-not” that a 100% interest in the Project.
Deferred income taxes July arise from temporary differences resulting from income and expense items reported for financial accounting and tax purposes in different periods. Deferred taxes are classified as current or non-current, depending on the classification of assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-currentnoncurrent depending on the periods in which the temporary differences are expected to reverse. The companyCompany does not have any uncertain tax positions.
For U.S. purposes, the Company has not completed its evaluation of NOL utilization limitations under Internal Revenue Code, as amended (the “Code”) Section 382/383, change of ownership rules. If the Company has had a change in ownership, the NOLs would be limited or eliminated, as to the amount that could be utilized each year, based on the Code. NOLs attributable to Breakthrough Products, Inc., which are the majority of the Company’s domestic NOLs are Separate Return Limitation Year (SRLY) NOLs. Such losses may generally not be available for use (limited or eliminated).
The Company currently has net operating loss carryforwards aggregating $33,852 (2012: $25,047), which expire through 2030.not filed its State & Local Income/Franchise tax returns in States it is required to file, as such returns and liability remain open. The deferredCompany does not expect this to be a significant liability.
The table below summarizes the differences between the U.S. statutory federal rate and the Company’s estimated effective tax asset related torate for the carryforwards has been fully reserved.years ended December 31, 2020 and 2019:
| December 31, 2020 | December 31, 2019 | |||||||
| U.S. Statutory Rate | 21 | % | (21 | )% | ||||
| Impairment of intangible assets | - | 23 | % | |||||
| Amortization of intangible assets | - | 3 | % | |||||
| AU/CA rate in excess of U.S. effective rate | 22 | % | - | % | ||||
| Carryback of Australian tax loss | - | (2 | )% | |||||
| Utilization of U.S. net operating losses | - | (2 | )% | |||||
| Increase in valuation allowance | 13 | % | - | |||||
| Other | 16 | % | - | |||||
| Utilization of Australian and Canadian NOL | (72 | )% | - | |||||
| Total provision for income taxes | - | 1 | % | |||||
| F-36 |
The Company has deferred income tax assets, which have been fully reserved, as follows as of JanuaryDecember 31, 2013:
| 2013 | 2012 | |||||||
| Deferred tax assets | $ | 11,848 | $ | 8,766 | ||||
| Valuation allowance for deferred tax assets | (11,848 | ) | (8,766 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
| December 31, 2020 | December 31, 2019 | |||||||
| Net operating Losses | $ | 6,404,580 | $ | 8,275,000 | ||||
| Accrued severance (from 2019 still unpaid) | 56,700 | - | ||||||
| Obsolete inventory | 110,880 | - | ||||||
| Nonstatutory stock options | 515,319 | - | ||||||
| Success fee | 210,000 | - | ||||||
| Other | 24,150 | - | ||||||
| True up of prior year nonstatutory stock options | - | 916,673 | ||||||
| True up of prior year net operating loss | - | (2,055,690 | ) | |||||
| Deferred tax asset | 7,321,629 | 7,135,983 | ||||||
| Valuation allowance for deferred tax assets | (7,321,629 | ) | (7,135,983 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
Tax expense was $0 and $131,537 for 2020 and 2019, respectively.
The Company also has net operating loss carryforwards of approximately $30,000,000 and approximately $33,000,000 (United States and Canada) included in the deferred tax asset table above for 2020 and 2019, respectively, the majority attributable to the acquisition of Breakthrough Products, Inc. However, due to limitations of carryover attributes and separate return limitation year rules, it is unlikely the company will benefit from the NOLs and thus Management has determined a 100% valuation reserved is required. Further, the Company has not completed an evaluation of the NOLs attributable to Breakthrough Products, Inc. at the date of this report.
Note 4 – Accounts Receivable
Accounts receivable, net of allowances for doubtful accounts, consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Trade accounts receivable | $ | 3,426,290 | $ | 1,430,326 | ||||
| Less allowances | (46,204 | ) | (283,972 | ) | ||||
| Total accounts receivable, net | $ | 3,380,086 | $ | 1,146,354 | ||||
During the years ended December 31, 2020 and 2019, the Company charged $74,068 and $283,972, respectively, to bad debt expense and created an allowance for doubtful accounts. During the year ended December 31, 2020, the Company wrote-off accounts receivable of $74,068. During 2020, the Company has reduced the allowance for doubtful accounts by $237,768 relating to allowance for doubtful accounts created in 2019.
Note 5 – FAIR VALUE MEASUREMENTSPrepaid Expenses
At December 31, 2020 and 2019, prepaid expenses consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Advances for inventory | $ | 362,310 | $ | 9,071 | ||||
| Media | 46,749 | - | ||||||
| Insurance | 22,951 | 16,763 | ||||||
| Deposits | 12,099 | 10,234 | ||||||
| Rent, related party | 67,741 | - | ||||||
| Promotion - Bloggers | 113,887 | - | ||||||
| License agreement | 51,111 | - | ||||||
| Software subscriptions | - | 9,536 | ||||||
| Components | 73,305 | - | ||||||
| Prepaid bonus, related party | 425,000 | - | ||||||
| Promotions | - | 122,626 | ||||||
| Miscellaneous | 62,077 | 17,913 | ||||||
| Total | $ | 1,237,230 | $ | 186,143 | ||||
| F-37 |
Note 6 – Concentration of Credit Risk
Cash and cash equivalents
The Company adopted ASC No. 820-10 (ASC 820-10)maintains its cash and cash equivalents in banks insured by the Federal Deposit Insurance Corporation (FDIC) in accounts that at times may be in excess of the federally insured limit of $250,000 per bank. The Company minimizes this risk by placing its cash deposits with major financial institutions. At December 31, 2020 and 2019, the uninsured balance amounted to $1,386,413 and $947,312, respectively.
Accounts receivable
As of December 31, 2020 and 2019, four and two customers accounted for 86% and 52%, Fair Value Measurements. ASC 820-10 relates to financial assets and financial liabilities.
Major customers
For the year ended December 31, 2020, two customers accounted for measuring fair value in accounting principles generally acceptedapproximately 47% of the Company’s net revenue. For the year ended December 31, 2019, two customers accounted for approximately 51% of the Company’s net revenue. Substantially all of the Company’s business is with companies in the United StatesStates.
Accounts payable
As of America (GAAP)December 31, 2020 and 2019, two vendors accounted for 59% and 73%, respectively, of the Company’s accounts payable. This includes a related party vendor in 2019.
Major suppliers
For the year ended December 31, 2020, three suppliers accounted for approximately 46% of the Company’s purchases. For the year ended December 31, 2019, two suppliers accounted for approximately 40% of the Company’s purchases. Substantially all of the Company’s business is with suppliers in the United States. This includes purchases from related party supplier for 2019.
Note 7 – Inventory
Inventory consists of finished goods, components and expands disclosures about fair value measurements.raw materials. The provisions of this standard apply to other accounting pronouncements that require or permit fair value measurements and are to be applied prospectively with limited exceptions.
The carrying value of inventory consisted of the single sourcefollowing:
| December 31, 2020 | December 31, 2019 | |||||||
| Finished goods | $ | 3,205,557 | $ | 1,554,013 | ||||
| Components | 63,910 | 264,518 | ||||||
| Inventory in transit | 171,861 | 42,507 | ||||||
| Raw materials | 25,118 | - | ||||||
| Total inventory | $ | 3,466,446 | $ | 1,861,038 | ||||
As of January 22, 2015, inventory was pledged to Knight under the Loan Agreement (see note 12). As of December 31, 2020 and 2019, $171,861 and $42,507, respectively, of the Company’s inventory was in GAAPtransit. During the years ended December 31, 2020 and 2019, $527,737 and $257,111, respectively, of expiring and slow-moving inventory was written off to cost of sales.
Note 8 – Fixed Assets and Intangible Assets
As of December 31, 2020 and 2019, fixed assets and intangible assets consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Property and equipment | $ | 566,445 | $ | 566,445 | ||||
| Less accumulated depreciation | (525,617 | ) | (430,547 | ) | ||||
| Less accumulated impairment | (40,828 | ) | - | |||||
| Fixed assets, net | $ | - | $ | 135,898 | ||||
| F-38 |
Depreciation expense for the definitionyears ended December 31, 2020 and 2019 was $98,230 and $133,873, respectively. During the year ended December 31, 2020, the Company fully impaired remaining assets of fair value, except$40,828.
| December 31, 2020 | December 31, 2019 | |||||||
| FOCUSfactor intellectual property | $ | 1,450,000 | $ | 1,450,000 | ||||
| Per-fekt intellectual property | - | - | ||||||
| Cocowhite intellectual property | - | - | ||||||
| Intangible assets subject to amortization | 5,388,230 | 5,388,230 | ||||||
| Less accumulated amortization | (4,910,159 | ) | (4,908,696 | ) | ||||
| Less accumulated impairment | (1,928,071 | ) | (1,921,898 | ) | ||||
| Intangible assets, net | $ | - | $ | 7,636 | ||||
Amortization expense for the fair valueyears ended December 31, 2020 and 2019 was $2,035 and $1,077,987, respectively. Impairment of leased property as definedintangible assets for the years ended December 31, 2020 and 2019 related to intangible assets from brands purchased in SFAS 13. ASC 820-10 establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs) and (2) an entity’s own assumptions, about market participant assumptions, that are developed based on the best information available in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy under ASC 820-10 are described below:
During the sixth monthsyear ended JanuaryDecember 31, 20132019, the Company recognized a totalfully impaired goodwill of $3,000 (2012: $0) for rent$7,793,240. During the year ended December 31, 2020, the Company fully impaired remaining intangible assets of $6,173.
Note 9 – Related Party Transactions
The Company accrued and services from directors for rent at $250paid consulting fees of $82,917 per month and at $250 per month for consulting services providedthrough December 2020 to a company owned by the President and DirectorMr. Jack Ross, Chief Executive Officer of the Company. These transactions are recorded at the exchange amount which is the amount agreed to by the transacting parties.
On June 26, 2015, the Company entered into a Security Agreement with Knight Therapeutics, Inc., a related party (owner of greater than 10% shares of the Company), through its wholly owned subsidiary Neuragen Corp., for the purchase of Knight Therapeutics, Inc.’s assets. At December 31, 2019 and 2018, the Company owed Knight $425,000 and $475,000 in relation to this agreement (see Note 11). The Company recorded present value of future payments of $246,916 and $260,461 as of December 31, 2020 and 2019, respectively.
On August 18, 2015, the Company entered into a Consulting Agreement with Kara Harshbarger, the co-founder of Hand MD, LLC, pursuant to which she will provide marketing and sales related service. The Company will pay Ms. Harshbarger $10,000 a month for one year unless the Consulting Agreement is terminated earlier by either party. The Company decided to extend the contract on a month to month basis. Hand MD, LLC is a 50% owner in Hand MD Corp. The Company expensed $120,000 through payroll for each of the years ended December 31, 2020 and 2019. As of December 31, 2020 and 2019, the total outstanding balance was $0.
The Company entered into transactions with a related party controlled by the CEO during the years ended December 31, 2020 and 2019. The transactions were a pass through and allocation of expenses and reimbursements. As of December 31, 2020 and 2019 the Company was owed $2,305,956 and $277,432, respectively.
On August 9, 2017, the Company entered into a Loan Agreement with Knight Therapeutics (Barbados) Inc., a related party (owner of greater than 10% shares of the Company), for a working capital loan. At December 31, 2020 and 2019, the Company owed Knight $5,000,000 and $5,451,568, respectively, on this loan, net of debt issuance cost (see Note 11). During the year ended December 31, 2020 a loan success fee of $1,000,000 was earned by Knight and recorded as a long-term liability, payable in August 2022.
On May 8, 2020, the Company entered into a Loan Agreement with Knight Therapeutics (Barbados) Inc., a related party, for working capital loan. At December 31, 2020, the Company owed Knight $2,500,000 on this loan (see Note 11).
On December 23, 2016, we entered into an agreement with Knight Therapeutics for the distribution rights of FOCUSFactor in Canada. In conjunction with this agreement, we are required to pay Knight a distribution fee equal to 30% of gross sales for sales achieved through a direct sales channel and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight under this agreement is $100,000 Canadian dollars. During the year ended December 31, 2020, the Company expensed was $178,341 Canadian dollars (US Dollars $133,094). As of December 31, 2020 and 2019, the total outstanding balance was $178,341 and $100,000 Canadian dollars, respectively. In US Dollars, the total outstanding balance was $140,069 and $70,295 as of December 31, 2020 and 2019, respectively.
| F-39 |
On December 23, 2016, we entered into an agreement with Knight Therapeutics for the distribution rights of Hand MD into Canada. In conjunction with this agreement, we are required to pay Knight a distribution fee equal to 60% of gross sales for sales achieved through a direct sales channel until the sales in the calendar year equal the threshold amount and then 40% of all such gross sales in such calendar year in excess of the threshold amount and 5% of gross sales for sales achieved through retail sales. The minimum due to Knight under this agreement is $25,000 Canadian dollars. During the year ended December 31, 2020, the Company expensed was $385,689 Canadian dollars (US Dollars $287,836). As of December 31, 2020 and 2019, the total outstanding balance was $110,637 and $25,000 Canadian dollars, respectively. In US Dollars, the total outstanding balance was $86,894 and $17,574 as of December 31, 2020 and 2019, respectively.
The Company expensed royalty of $1,055 and $4,867 for the years ended December 31, 2020 and 2019, respectively. At December 31, 2020 and 2019, the Company owed Knight Therapeutics $0 and $246, respectively in connection with a royalty distribution agreement.
The Company expensed commissions of $0 and $9,065 for the years ended December 31, 2020 and 2019, respectively. At both December 31, 2020 and 2019, the Company owed Founded Ventures, owned by a shareholder in the Company, $0 in connection with a commission agreement.
The Company expensed commissions of $0 and $644 for the years ended December 31, 2020 and 2019, respectively. At both December 31, 2020 and 2019, the Company owed Founded Ventures $0 in connection with a commission agreement.
The Company expensed and paid $145,356 and $14,801 for the years ended December 31, 2020 and 2019, respectively, to Hand MD, Corp, related to a royalty agreement. As of both December 31, 2020 and 2019, the Company owed Hand MD Corp. $0 in minimum future royalties.
The Company expensed royalty of $169,552 and $192,700 for the years ended December 31, 2020 and 2019, respectively. At December 31, 2020 and 2019, the Company owed Knight Therapeutics $11,026 and $5,528, respectively, in connection with a royalty distribution agreement.
A member of the Company’s Board of Directors was an executive officer of a supplier to the Company during 2019. During the year ended December 31, 2019, the Company acquired $4,847,626 of products from the supplier and is included in cost of sales. The Company owed the supplier $956,438 December 31, 2019.
The Company entered into transactions with a related party controlled by the CEO during the years ended December 31, 2020 and 2019. The transactions were a pass through of expenses and reimbursements. During the years ended December 31, 2020 and 2019, the Company received advances of $74,629 ($100,000 Canadian Dollars) and $324,102 ($430,000 Canadian Dollars), respectively, which were fully repaid. As of both December 31, 2020 and 2019, there was $0 due or payable.
The Company entered into transactions with a related party controlled by the CEO during the year ended December 31, 2020. The transactions are related to rent expense of $25,000 Canadian Dollars per month. During the year ended December 31, 2020 the Company expensed $275,000 Canadian Dollars ($205,485 US Dollars) for rent expense. As of December 31, 2020 the Company has advanced $86,250 Canadian Dollars ($67,741 US Dollars) for prepaid rent relating to 2021.
Note 10 – Accounts Payable and Accrued Liabilities
As of December 31, 2020 and 2019, accounts payable and accrued liabilities consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Accrued payroll | $ | 180,374 | $ | 110,536 | ||||
| Legal fees | 17,924 | 68,098 | ||||||
| Commissions | 516,491 | 229,657 | ||||||
| Manufacturers (including related party payable of $0 and $956,438, respectively) | 2,447,404 | 2,082,256 | ||||||
| Promotions | 1,292,262 | 1,312,541 | ||||||
| Accounting Fees | 2,926 | 10,873 | ||||||
| Interest, related party | 271,528 | - | ||||||
| Customers | 144,142 | 26,206 | ||||||
| Royalties, related party | 238,536 | 93,643 | ||||||
| Warehousing | 64,140 | 13,746 | ||||||
| Sales taxes | 899,852 | 313,985 | ||||||
| Payroll taxes | 734,072 | 90,500 | ||||||
| Severance Accrual | 270,333 | 270,333 | ||||||
| Related Party Reimbursements | - | 1,135 | ||||||
| Others | 21,716 | 50,555 | ||||||
| Total | $ | 7,101,700 | $ | 4,674,064 | ||||
| F-40 |
The Company has not filed its State & Local Income/Franchise tax returns in States it is required to file. The Company has estimated and accrued for its sales tax liability at $197,483 and $273,855 for the parent entity as of December 31, 2020 and 2019, respectively.
Note 11 – Notes Payable, related party
The Company’s notes payable at December 31, 2020 and 2019 are as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Notes payable | $ | 8,746,916 | $ | 5,760,461 | ||||
| Unamortized debt issuance cost | - | (48,432 | ) | |||||
| Total | 8,746,916 | 5,712,029 | ||||||
| Less: Current portion | (7,515,693 | ) | (5,465,113 | ) | ||||
| Long-term portion | $ | 1,231,223 | $ | 246,916 | ||||
$950,000 June 26, 2015 Security Agreement:
On June 26, 2015, the Company, through its wholly owned subsidiary, Neuragen Corp. (“Neuragen”), issued a 0% promissory note in a principal amount of $950,000 in connection with an Asset Purchase Agreement. The note requires $250,000 to be paid on or before June 30, 2016, and $700,000 to be paid in quarterly installments (beginning with the quarter ending September 30, 2015) equal to the greater of $12,500 or 5% of U.S. net sales, and 2% of U.S. net sales of Neuragen for 60 months thereafter. The payment of such amounts is secured by a security interest in certain assets, undertakings and property (“Collateral”) pursuant to the Security Agreement, which will be released upon receipt of total payments of $1.2 million.
Company recorded present value of future payments of $246,916 and $260,461 as of December 31, 2020 and 2019, respectively. At December 31, 2020 and 2019, the Company owed Knight $425,000 and $475,000 in relation to this agreement. The Company recorded interest expense of $36,455 and $38,310 for the year ended December 31, 2020 and 2019, respectively. The Company made payments of $50,000 during both 2020 and 2019.
$10,000,000 August 9, 2017 Loan:
On August 9, 2017, we entered into a Second Amendment to Loan Agreement (“Second Amendment”) with Knight, pursuant to which Knight agreed to loan us an additional $10 million, and an ongoing credit facility of up to $20 million, and which amount was borrowed at closing (the “Financing”) for working capital purposes. At closing, we paid Knight an origination fee of $200,000 and a work fee of $100,000 and also paid $100,000 of Knight’s expenses associated with the Loan.
Additional Tranches under the Loan Agreement are available to the Company until August 9, 2022 provided that no event of default exists. Each Additional Tranche must be for a minimum amount of $1.0 million, may only be used to finance qualified acquisitions (as defined in the Loan Agreement), and can be denied in Knight’s absolute discretion. If an Additional Tranche is denied, the Company can effect a qualified acquisition through a special purpose entity with such special purpose entity being entitled to obtain financing from third parties so long as such financing does not adversely affect Knight or Knight’s rights under the Loan Agreement. Upon the closing of any Additional Tranche, the Company will pay Knight an origination fee equal to 2% of the Additional Tranche, a work fee equal to 1% of the amount of the Additional Tranche, and reimburse Knight for its expenses incurred in connection with its consideration of any Additional Tranche (whether or not advanced).
The Loan bears interest at 10.5% per annum. The amended Loan Agreement matures on August 8, 2020 and (b) the date that Knight, in its discretion, accelerates the Company’s obligations due to an event of default.
On the Maturity Date of the Third Tranche and every Additional Tranche (or upon the acceleration of each such loan), the Company must pay Knight a success fee (the “Success Fee”) of that number of Company common shares equal to 10% of the loan, divided by the lesser of (a) $1.50, (b) the lowest price at which any common shares were issued by the Company in any offering or equity financing or other transaction between the Closing Date and the period from December 29, 2010 (Inception)date the Success Fee is due, and (c) the current market price on the date the Success Fee is due. The Company may also pay the Success Fee in cash pursuant to July 31, 2012 and July 31, 2011. These financial statements are the responsibilityterms of the Company's management. Our responsibilityLoan Agreement.
| F-41 |
The Loan Agreement includes customary representations, warranties, and affirmative and restrictive covenants, including covenants to attain and maintain certain financial metrics, and to not merge or dispose of assets, acquire other businesses (except for businesses substantially similar or complementary to the Company’s business, and provided that the aggregate consideration to be paid does not exceed $100,000 and the acquired business guarantees the Company’s obligations under the Loan Agreement) or make capital expenditures in excess of $500,000. The Loan Agreement also includes customary events of default, including payment defaults, breaches of covenants, change of control and material adverse effect defaults. Upon the occurrence of an event of default and during the continuation thereof, the principal amount of all loans under the Loan Agreement will bear a default interest rate of an additional 5%.
The Company’s obligations and liabilities under the Loan Agreement are secured and unconditionally guaranteed by certain of the Company’s wholly-owned subsidiaries as provided in the Loan Agreement.
We have met all the covenants except for the TTM EBITDA of $5 million during the period ending March 31, 2018. Default Interest rate of 5% (from 10.5% to 15.5%) applies in accordance to our current agreement and will be in effect starting April 1, 2018 and will be in effect until the $5 million TTM EBITDA covenant is achieved. We entered into Loan Amendment Agreement on May 14, 2018, the interest rate was reduced to express13% due to reducing payroll expenses. Also, Synergy will maintain Focus Factor Net Sales as measured on a year-end basis of at least USD $15 million for each fiscal year starting with December 31, 2017.
We have amended our covenants under our loan agreement on March 27, 2019. The new covenants are as follows: we will maintain a minimum EBITDA of $1,900,000 for the twelve months ending on December 31, 2018, $2,500,000 for the twelve months ending March 31, 2019, $3,500,000 for the twelve months ending June 30, 2019 and $5,000,000 for the twelve months period ending on last day of each fiscal quarters thereafter. We shall maintain a net debt to TTM EBITDA ratio of no more than 8:1 for the twelve month period ending on December 31, 2018 until March 31, 2019 and shall maintain a net debt to TTM EBITDA ratio of no more than 6:1 thereafter. We shall maintain at all times a positive cash balance of $575,000 for the three month period ending December 31, 2018, $750,000 for the three month period ending March 31, 2019 and $1,000,000 thereafter. The default interest rate of 2.5% applies (from 13% to 15.5%) in accordance to our current agreement and was in effect as of October 1, 2018 to June 30, 2019. Effective June 30, 2019 the interest rate referred back to 10.5%.
The Company also recorded deferred financing costs of $452,869 with respect to the above loan. The Company recognized amortization of deferred financing costs of $48,432 and $130,829 during the years ended December 31, 2020 and 2019, respectively. Unamortized debt issuance cost as of December 31, 2020 and 2019 amounted to $0 and $48,432, respectively.
On May 8, 2020, the Company entered into a Third Amendment Agreement (the “Third Amendment”) to the Amended and Restated Loan Agreement (the “Loan Agreement”) with Knight Therapeutics (Barbados) Inc. (“Knight”), pursuant to which Knight agreed to loan the Company an opinionadditional $2.5 million (the “Additional Loan”). That same day (the “Closing”), the Company paid Knight a work fee of $36,000, and $25,000 for Knight’s legal costs and expenses incurred in connection with the Third Amendment. The Third Amendment amends the original loan agreement that the Company and Knight entered into in January 2015 and subsequently amended (as amended, the “Original Loan Agreement”). The Additional Loan matures on May 8, 2021 (the “TA Maturity Date”) and bears interest at 12.5% per annum compounding quarterly. On the TA Maturity Date, the Company will pay Knight a success fee (the “Success Fee”) of $83,250. The Success Fee is payable in cash or stock as set forth in the Loan Agreement. The Third Amendment includes customary representations, warranties, and affirmative and restrictive covenants, including covenants to attain and maintain certain financial metrics, including an undertaking to maintain at all times a cash balance of $600,000 and EBITDA of $3,000,000 for the twelve months ended June 30, 2020 and $4,000,000 for the twelve month period ending on the last day of each fiscal quarter thereafter.
Terms of the $10,000,000 August 9, 2017 loan (Third Tranche) (see note 10) was modified in the Third amendment. Third tranche shall bear interest from May 8, 2020 at a rate equal to 12.5% per annum compounded quarterly. The Company shall pay success fee in the amount of $1,000,000 with respect to the Third Tranche, which shall be fully earned on May 8, 2020 and payable no later than August 31, 2022. Third Tranche success fee shall bear interest at 12.5% per annum compounding quarterly. The loan has been extended to a maturity date of December 31, 2021. Because these financial statements basedamendments were considered not substantive changes, the Company accounted for the modifications as modification of debt.
The Company recognized interest expense of $995,760 and $912,486 during the years ended December 31, 2020 and 2019, respectively. Accrued interest was $271,528 and $0 as of December 31, 2020 and 2019, respectively. The loan balances, including success fee, at December 31, 2020 and 2019 were $8,500,000 and $5,500,000, respectively.
| F-42 |
The PPP Loan and Financial Assistance:
In June 2020, the American Institute of Certified Public Accountants in conjunction with the Financial Accounting Standards Board developed Technical Question and Answer (“TQA”) 3200.18, “Borrower Accounting for a Forgivable Loan Received Under the Small Business Administration Paycheck Protection Program”, which is intended to provide clarification on our auditshow to account for loans received from the PPP. TQA 3200.18 states that an entity may account for PPP loans under ASC 470, “Debt” or, if the entity is expected to meet PPP eligibility criteria and the PPP loan is expected to be forgiven, the entity may account for the loans under IAS 20, “Accounting for Government Grants and Disclosure of Government Assistance”.
The CARES Act includes, among other things, provisions relating to payroll tax credits and deferrals, net operating loss carryback periods, alternative minimum tax credits and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also established a Paycheck Protection Program (“PPP”), whereby certain small business are eligible for a loan to fund payroll expenses, rent and related costs. We conducted our auditshad received funding under the PPP of $163,542 and bears interest at 1% per annum, with monthly installments commencing on May 5, 2020 for 60 months through its maturity on May 5, 2025. The Company used all proceeds from the PPP Loan to maintain payroll and other allowable expenses. As a result, management believes that the Company has met the PPP eligibility criteria for forgiveness and has concluded that the loan represents, in substance, a government grant that is expected to be forgiven in its entirety. As such, in accordance with the standardsInternational Accounting Standards (“IAS”) 20, “Accounting for Government Grants and Disclosure of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
| July 31, 2012 | July 31, 2011 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 4,000 | $ | 4,000 | ||||
| Deposit on Mining Claim | - | 6,000 | ||||||
| Total Current Assets | 4,000 | 10,000 | ||||||
| Mining Claims, net of impairment | - | - | ||||||
| Total Assets | $ | 4,000 | $ | 10,000 | ||||
| LIABILITIES AND STOCKHOLDER’S DEFICIT | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable and Accrued Liabilities | $ | 3,000 | $ | - | ||||
| Due to Director | 15,000 | 15,000 | ||||||
| Total Liabilities | 18,000 | 15,000 | ||||||
| Stockholder’s Deficit | ||||||||
| Common Stock (75,000,000 shares authorized, par value 0.00001, 5,000,000 shares issued and outstanding for both fiscal years 2011 and 2012) | 50 | 50 | ||||||
| Additional paid-in capital | 10,997 | 3,797 | ||||||
| Deficit accumulated during the exploration stage | (25,047 | ) | (8,847 | ) | ||||
| Total Stockholder’s Deficit | (14,000 | ) | (5,000 | ) | ||||
| Total Liabilities and Stockholder’s Deficit | $ | 4,000 | $ | 10,000 | ||||
Fiscal Year Ended July 31, 2012 | Fiscal Year Ended July 31, 2011 | Inception December 29, 2010 to July 31, 2012 | ||||||||||
| Operating Expenses | ||||||||||||
| Consulting services | $ | 3,000 | $ | 1,750 | $ | 4,750 | ||||||
| General and administrative | - | 20 | 20 | |||||||||
| Rent | 3,000 | 1,750 | 4,750 | |||||||||
| Legal and accounting | 3,000 | 4,980 | 7,980 | |||||||||
| Impairment of Mineral Claims | 6,000 | - | 6,000 | |||||||||
| Interest Expense | 1,200 | 347 | 1,547 | |||||||||
| Total Expenses | 16,200 | 8,847 | 25,047 | |||||||||
| Net Loss | $ | (16,200 | ) | $ | (8,847 | ) | $ | (25,047 | ) | |||
| Net Loss Per Common Share – Basic and Diluted | $ | (0.00 | ) | $ | (0.00 | ) | ||||||
| Weighted Average Number of Common Shares Outstanding | 5,000,000 | 5,000,000 | ||||||||||
Fiscal Year Ended July 31, 2012 | Fiscal Year Ended July 31, 2011 | Inception December 29, 2010 to July 31, 2012 | ||||||||||
| Operating Activities | ||||||||||||
| Net loss | $ | (16,200 | ) | $ | (8,847 | ) | $ | (25,047 | ) | |||
| Adjustments to reconcile net loss to cash used in operating activities: | ||||||||||||
| Impairment of mineral claims | 6,000 | - | 6,000 | |||||||||
| Imputed interest expense | 1,200 | 347 | 1,547 | |||||||||
| Donated consulting services and rent expenses | 6,000 | 3,500 | 9,500 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts payable and accrued liabilities | 3,000 | - | 3,000 | |||||||||
| Net Cash Used by Operating Activities | - | (5,000 | ) | (5,000 | ) | |||||||
| Investing Activities | ||||||||||||
| Deposit of mining claims | - | (6,000 | ) | (6,000 | ) | |||||||
| Financing Activities | ||||||||||||
| Borrowings on debt-related party | - | 15,000 | 15,000 | |||||||||
| Increase (Decrease) in Cash | - | 4,000 | 4,000 | |||||||||
| Cash - Beginning of Period | 4,000 | - | - | |||||||||
| Cash - End of Period | $ | 4,000 | $ | 4,000 | $ | 4,000 | ||||||
| Supplemental Disclosure of Cash Flow Information Cash paid during the period for : | ||||||||||||
| Interest | $ | - | $ | - | $ | - | ||||||
| Income taxes | $ | - | $ | - | $ | - | ||||||
| Common Stock | Additional Paid-in | Deficit Accumulated During the Exploration | ||||||||||||||||||
| Shares | Amount | Capital | Stage | Total | ||||||||||||||||
| Balance at December 22, 2010 | - | $ | - | $ | - | $ | - | $ | - | |||||||||||
| Issuance of common stock to founders | 5,000,000 | 50 | (50 | ) | - | - | ||||||||||||||
| Donated consulting services and rent | - | - | 3,500 | - | 3,500 | |||||||||||||||
| Imputed interest | - | - | 347 | - | 347 | |||||||||||||||
| Net loss | - | - | - | (8,847 | ) | (8,847 | ) | |||||||||||||
| Balances at July 31, 2011 | 5,000,000 | 50 | 3,797 | (8,847 | ) | (5,000 | ) | |||||||||||||
| Donated consulting services and rent | - | - | 6,000 | - | 6,000 | |||||||||||||||
| Imputed interest | - | - | 1,200 | - | 1,200 | |||||||||||||||
| Net loss | - | - | - | (16,200 | ) | (16,200 | ) | |||||||||||||
| Balances at July 31, 2012 | 5,000,000 | $ | 50 | $ | 10,997 | $ | (25,047 | ) | $ | (14,000 | ) | |||||||||
Note 12 – Stockholders’ Equity
The total number of shares of all classes of capital stock which the differences are expectedCompany is authorized to reverse. The Company computes tax asset benefits for net operating losses carried forward. The potential benefit of net operating losses has not been recognized in these financial statements because the Company cannot be assured itissue is more likely than not it will utilize the net operating losses carried forward in future years.
During the average market price per share when applyingyear ended December 31, 2019, the treasury stock method in determiningCompany issued 26,391 shares of its common stock equivalents. valued at $39,585 in full and final settlement on the Perfekt transaction.
As of Julyboth December 31, 20122020, and 2011,2019, there were no89,889,074 shares of the Company’s common stock equivalentsissued and outstanding.
Note 13 – Commitments and Contingencies
Litigation:
From time to ASC No. 820, “Fair Value Measurements and Disclosures”,time the Company is requiredmay become a party to estimate the fair value of all financial instruments included on its balance sheet as of July 31, 2012 and 2011. The Company’s financial instruments consist of cash. The Company considers the carrying value of such amountslitigation in the financial statements to approximate their fair value due to the short-term naturenormal course of these financial instruments.
Other Commitments
During the FASB issued ASU 2012-03, “Technical Amendments and Corrections to SEC Sections: Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin (SAB) No. 114, Technical Amendments Pursuant to SEC Release No. 33-9250, and Corrections Related to FASB Accounting Standards Update 2010-22 (SEC Update)” in Accounting Standards Update No. 2012-03. This update amends various SEC paragraphs pursuant toyear ended December 31, 2019 the issuance of SAB No. 114. The adoption of ASU 2012-03 is not expected to haveCompany received a material impact on our financial position or results of operations.
Note 14 – Stock Options
The following table summarizes the changes in options outstanding and the related prices for the shares of the Company’s common stock issued to employees and consultants under a stock option plan at December 31, 2020:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Exercise Price ($) | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price ($) | Number Exercisable | Weighted Average Exercise Price ($) | |||||||||||||||||
| $ | 0.25 – 0.70 | 3,466,667 | 4.46 | $ | 0.54 | 3,466,667 | $ | 0.54 | ||||||||||||||
| F-43 |
The stock option activity for the year ended December 31, 2020 is as followsfollows:
| Options Outstanding | Weighted Average Exercise Price | |||||||
| Outstanding at December 31, 2018 | 7,166,667 | $ | 0.50 | |||||
| Granted | - | - | ||||||
| Exercised | - | - | ||||||
| Expired or canceled | (1,000,000 | ) | (0.25 | ) | ||||
| Outstanding at December 31, 2019 | 6,166,667 | 0.54 | ||||||
| Granted | ||||||||
| Exercised | ||||||||
| Expired or canceled | (2,700,000 | ) | (0.53 | ) | ||||
| Outstanding at December 31, 2020 | 3,466,667 | $ | 0.54 | |||||
Stock-based compensation expense related to vested options was $128,929 and $161,570 during the years ended December 31, 2020 and 2019, respectively. The Company determined the value of share-based compensation for options vesting during the year ended December 31, 2017 using the Black-Scholes fair value option-pricing model with the following weighted average assumptions: estimated fair value of Company’s common stock of $0.48-0.50, risk-free interest rate of 1.95-1.99%, volatility of 116-117%, expected lives of 10 years, and dividend yield of 0%. Stock options outstanding as of JulyDecember 31, 2012:
| 2012 | 2011 | |||||||
| Deferred tax assets | $ | 8,516 | $ | 3,008 | ||||
| Valuation allowance for deferred tax assets | (8,516 | ) | (3,008 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
Note 15 – FAIR VALUE MEASUREMENTS
Segment identification and selection is consistent with the management structure used by the Company’s chief operating decision maker to evaluate performance and make decisions regarding resource allocation, as well as the materiality of financial results consistent with that structure. Based on the Company’s management structure and method of internal reporting, the Company has one operating segment. The Company adopted ASC No. 820-10 (ASC 820-10), Fair Value Measurements. ASC 820-10 relatesCompany’s chief operating decision maker does not review operating results on a disaggregated basis; rather, the chief operating decision maker reviews operating results on an aggregated basis.
Net sales attributed to financial assets and financial liabilities.
| December 31, 2020 | December 31, 2019 | |||||||
| United States | $ | 30,683,330 | $ | 27,295,126 | ||||
| Foreign countries | 9,543,535 | 2,062,420 | ||||||
| $ | 40,226,865 | $ | 29,357,546 | |||||
Foreign country sales primarily consist of fair value, exceptsales in Canada.
The Company’s net sales by product group for the fair value of leased propertyyears ended December 31, 2020 and 2019 were as defined in SFAS 13. ASC 820-10 establishes a fair value hierarchy that distinguishes between (1) market participant assumptions developed based on market data obtained from independent sources (observable inputs)follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Nutraceuticals | $ | 31,902,291 | $ | 28,149,938 | ||||
| Over the Counter (OTC) | 22,310 | 62,359 | ||||||
| Consumer Goods | 1,201,317 | 706,688 | ||||||
| Cosmeceuticals | 7,100,947 | 438,561 | ||||||
| $ | 40,226,865 | $ | 29,357,546 | |||||
| (1) | Net sales for any other product group of similar products are less than 10% of consolidated net sales. |
The Company’s net sales by major sales channel for the years ended December 31, 2020 and (2) an entity’s own assumptions, about market participant assumptions, that are developed based on the best information available2019 were as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Online | $ | 9,923,825 | $ | 10,382,593 | ||||
| Retail | 30,303,040 | 18,974,953 | ||||||
| $ | 40,226,865 | $ | 29,357,546 | |||||
Long-lived assets (net) attributable to operations in the circumstances (unobservable inputs). The fair value hierarchy consists of three broad levels, which gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1)United States and the lowest priority to unobservable inputs (Level 3). The three levels of the fair value hierarchy under ASC 820-10 are described below:
| December 31, 2020 | December 31, 2019 | |||||||
| United States | $ | - | $ | - | ||||
| Foreign countries | - | 7,636 | ||||||
| $ | - | $ | 7,636 | |||||
Note 16 – Prior to the current year, our president had advanced $15,000 in the form on a non-interest bearing loan. An imputed interest of $1,200 was, therefore, deemed to have been incurred in the current fiscal year ended on July 31, 2012 and an imputed interest of $347 was incurred in the fiscal year ended July 31, 2011, which was calculated using an interest rate of 8% (eight percent) which is the interest rate that was payable on comparable notes and advances that we have recently incurred. For the period December 29, 2010 (inception) through July 31, 2012, Oro Capital has incurred a total of $1,547 on imputed interest expenses.
The Company had no reportableevaluated its December 31, 2020 consolidated financial statements for subsequent events after July 31, 2012 through the date the consolidated financial statements were issued.
Subsequent to December 31, 2020, the Company has made $2,000,000 in payments on the outstanding loans.
Subsequent to December 31, 2020, the Company has received verification of full forgiveness of their disbursement under the PPP Loan program.
Subsequent to December 31, 2020, the Company has entered into an agreement to acquire the outstanding 50% of Hand MD Corp. whereby Synergy will become the 100% owner. The consideration of $1,700,000 will be disbursed as follows: $500,000 within 10 business days of the execution of the agreement, $400,000 on or before the six month anniversary of the agreement, $400,000 on or before the twelve month anniversary of the agreement and $400,000 on or before the eighteen month anniversary of the agreement. The first payment of $500,000 was made in July 2021.
| F-44 |
Shares of Common Stock

Synergy CHC Corp.
PRELIMINARY PROSPECTUS
B. Riley Securities
, 2021
Through and including , 2021 (25 days after the date of this prospectus), all dealers that effect transactions in our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, paid or payable by us in and Disagreements with Accountants on Accounting and Financial Issues.
Amount Paid or to be Paid | ||||
| SEC registration fee | $ | 7,995 | ||
| FINRA filing fee | 13,438 | |||
| Nasdaq listing fee | 75,000 | |||
| Printing and engraving expenses | * | |||
| Legal fees and expenses | * | |||
| Accounting fees and expenses | * | |||
| Blue sky fees and expenses | * | |||
| Transfer agent and registrar fees and expenses | * | |||
| Miscellaneous expenses | * | |||
| Total | $ | * | ||
* To be provided by amendment.
| Item 14. | Indemnification of Directors and Officers |
Neither our articles of incorporation, providenor our amended and restated bylaws, prevent us from indemnifying our officers, directors and agents to the extent permitted under the Nevada Revised Statutes (“NRS”). NRS Section 78.7502, provides that we willa corporation may indemnify any director, officer, employee or agent of a corporation against expenses, including attorneys’ fees, actually and reasonably incurred by him in connection with any defense to the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to Section 78.7502(1) or 78.7502(2), or in defense of any claim, issue or matter therein.
NRS 78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or fiduciaryis or was serving at the request of our company to the fullest extent permittedcorporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by applicable law. Nevada law permits a Nevada corporation to indemnify its directors, officers, employees and agents against liabilities and expenses they may incur in such capacitieshim in connection with anythe action, suit or proceeding in which they may be involved, if (i) such director or officerhe: (a) is not liable pursuant to the corporationNRS 78.138; or its stockholders due to the fact that his or her acts or omissions constituted a breach of his or her fiduciary duties as a director or officer and the breach of those duties involved intentional misconduct, fraud or a knowing violation of law, or (ii) he or she(b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of our company, or that with respect to any criminal action or proceeding, he or she had no reasonable cause to believe that his or her conduct was unlawful.
NRS Section 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of anythe corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendre or its equivalent willif he: (a) is not of itself, create a presumption that the person is liable pursuant to Nevada Revised Statutes 78.138NRS 78.138; or did not act(b) acted in good faith and in a manner which such personhe reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals there from, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
NRS Section 78.747 provides that except as otherwise provided by specific statute, no director or officer of a corporation is individually liable for a debt or liability of the corporation, unless the director or officer acts as the alter ego of the corporation. The court as a matter of law must determine the question of whether a director or officer acts as the alter ego of a corporation.
| II-1 |
Our amended and restated bylaws will provide that we will indemnify our companydirectors, officers, employees and with respectagents to the extent and in the manner permitted by the provisions of the NRS, as amended from time to time, subject to any criminal actionpermissible expansion or proceeding, had reasonable cause to believe thatlimitation of such person's conduct was unlawful.
| Registration Fees* | $ | 2 | ||
| Federal Taxes | — | |||
| State Taxes | — | |||
| Legal Fees and Expenses | 7,000 | |||
| Transfer Agent and Printing | 500 | |||
| Blue Sky Fees | 0 | |||
| Accounting Fees and Expenses | 5,000 | |||
| Miscellaneous (1) | 500 | |||
| Total | $ | 13,002 |
We will enter into indemnification agreements with each of our officers and $14,597directors a form of which is filed as an exhibit to this Registration Statement.
These agreements will require us to indemnify these individuals to the fullest extent permitted under Nevada law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
The proposed form of underwriting agreement filed as Exhibit 1.1 to this Registration Statement provides for the six months ended January 31, 2013.
| Item 15. | Recent Sales of Unregistered Securities |
In the three years preceding the filing of this registration statement, we have issued the following securities that were not registered under the Securities Act:
On January 28, 2019, we issued 26,391 shares of our common stock for investment purposesto Malk Group, Inc. in full and not withfinal settlement of our purchase of all of the assets and the assumption of certain liabilities of Perfekt Beauty Holdings LLC (“Perfekt Beauty”), pursuant to the Asset Purchase Agreement dated June 21, 2017 by and among us, Perfekt Beauty and CDG Holdings, LLC. The shares of common stock were sold in a view to any public resale or other distribution in violation oftransaction exempt from registration under the Securities Act of 1933, or the securities lawsas amended (the “Securities Act”), in reliance on Section 4(a)(2) thereof. The shares of any state. In addition, theour common stock certificate representing these shares contained a legend that they are restricted securities under the Securities Act of 1933. These securities may not be offered or sold in the United States in the absence of an effectiveabsent registration statement or exemption from the registration requirements under the Securities Act.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
The following documents are filed as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
| II-2 |
| # | Denotes a management contract or compensatory plan or arrangement. | |
| + | Certain confidential information contained in this agreement has been omitted because it is not material and would be competitively harmful if publicly disclosed. | |
| * | Filed herewith. | |
| ** | To be filed | |
(b) Financial Statement Schedules
All schedules have been omitted because the information required to be set forth in the schedules is either not applicable or is shown in the financial statements or notes thereto.
| Item 17. | Undertakings |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the small business issuerregistrant pursuant to the foregoing provisions, or otherwise, the small business issuerregistrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
The undersigned registrant hereby undertakes:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-3 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all the requirements of filing ofduly caused this Registration Statement on Form S-1 and authorized this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Cape TownWestbrook, State of Maine, on May 17, 2013.
| By: | /s/ Jack Ross | |
| Jack Ross | ||
| Chief Executive Officer and Chairman | ||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jack Ross and Brendan Horning and each of them, his or her true and lawful attorneys-in-fact and agents with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by the registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, his, hers or their substitute or substitutes, may lawfully do or cause to be done or by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | ||
| /s/ Jack Ross | Chief Executive Officer and Chairman | October 22, 2021 | ||
| Jack Ross | (principal executive officer) | |||
| /s/ Brendan Horning | Chief Financial Officer | October 22, 2021 | ||
| Brendan Horning | (principal financial officer) | |||
| /s/ Alfred Baumeler | President and Director Nominee | October 22, 2021 | ||
| Alfred Baumeler | ||||
| /s/ Jaime Fickett | October 22, 2021 | |||
| Jaime Fickett | (principal accounting officer) | |||
| /s/ J. Paul SoRelle | Director | October 22, 2021 | ||
| J. Paul SoRelle | ||||
| /s/ Stephen Fryer | October 22, 2021 | |||
| Stephen Fryer |
| II-4 |