As filed with the Securities and Exchange Commission on September 18, 2008
June 28, 2022
Registration No. 333-150468
================================================================================
333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Pre-Effective
Amendment No. 4
to
Form S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
EASY ENERGY, INC.
(Exact
Raphael Pharmaceutical Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 2833 | 26-0204284 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification |
4 Lui Paster
Tel Aviv-Jaffa, Israel 6803605
Telephone: (972) 52-775-5072
(Address, including zip code, and telephone number, including area code, of registrant'sregistrant’s principal executive offices)
EASTBIZ.COM INC.
5348 Vegas Drive
Las Vegas, NV 89108
Tel: (702) 442-1166
(Name,
Shlomo Pilo
Chief Executive Officer
4 Lui Paster
Tel Aviv-Jaffa, Israel 6803605
Telephone: (972) 52-775-5072
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copy
Copies to:
Edwin L. Miller Jr., Esq.
Zysman, Aharoni, Gayer & Co.
Sullivan & Worcester LLP
One Post Office Square
Boston, Massachusetts 02110
Telephone: (617) 338-2800
Fax: (617) 338-2880
Oded Har-Even, Esq. Sullivan & Worcester LLP |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement becomes effective.
registration statement.
If any of the securities being registered on this formForm are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: [X]
box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of "large“large accelerated filer," "accelerated filer"” “accelerated filer,” “smaller reporting company” and "smaller
reporting company"“emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
Large Accelerated Filer [ ] Accelerated Filer [ ]
Non-accelerated Filer [ ]
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☐ | ||
If an emerging growth company, [X]
(Doindicate by check market if the registrant has elected not check ifto use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a Smaller reporting company)
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES
AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE
A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT
SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE
SECURITIES ACT, OR UNTIL THIS REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON
SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SAID
SECTION 8(A)further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), MAY DETERMINE.
================================================================================
SUBJECT TO COMPLETION, DATED ____________, 2008
THE INFORMATION CONTAINED IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED.
THE SELLING STOCKHOLDERS MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION
STATEMENT FILED WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION IS
EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS
NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR
SALE IS NOT PERMITTED.
EASY ENERGY, INC.
PROSPECTUS
20,638,273 SHARES OF COMMON STOCK
may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and it is not soliciting an offer to buy these securities, in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED JUNE 28, 2022 |
Raphael Pharmaceutical Inc.
Resale of 1,667,393 Shares of Common Stock
This prospectus relates to the resale by the selling security holders named in this prospectus or their permitted transferees (the “Selling Securityholders”) of up to the public by certain selling
shareholders of Easy Energy, Inc. of:
* Up to 750,0001,667,393 shares of our common stock, par value $0.01 per share (“Common Stock”) issued in aone or more private placement on
March 27, 2008.
* Up to 882,353placements.
The shares of our common stock issued in a private placement on
March 10, 2008.
* Up to 3,000,000 shares of our common stock whichCommon Stock that may be issued upon the
exercise of warrants issued in connection with the private placement on
March 10, 2008.
* Up to 300,000 shares of our common stock issued in a private placement on
March 3, 2008.
* Up to 1,000,000 shares of our common stock which may be issued upon the
exercise of warrants issued in connection with the private placement on
March 3, 2008.
* Up to 3,676,480 shares of our common stock issued in a private placement on
February 28, 2008.
* Up to 11,029,440 shares of our common stock which may be issued upon the
exercise of warrants issued in connection with the private placement on
February 28, 2008.
The warrants issued on March 10, 2008 entitle the holder to purchase one
additional share of our common stock at an exercise price of $0.27 for a period
of five years from the closing date.
The warrants issued on March 3, 2008 entitle the holder to purchase one
additional share of our common stock at an exercise price of $0.15 for a period
of five years from the closing date.
The warrants issued on February 28, 2008 entitle the holder the purchase one
share of our common stock at an exercise price of $0.27 for a period of five
years.
The shares were acquiredsold by the selling stockholders directly from our company
in private transactions that were exempt from the registration requirements of
the Securities Act of 1933. The selling stockholders may offerSelling Securityholders are collectively referred to sell the
shares of common stock being offered in this prospectus at fixed prices, at
prevailing market prices atas the time of sale, at varying prices or at negotiated
prices. Our common stock is quoted on the OTC Bulletin Board under the symbol
"ESYE.OB". On September 12, 2008 the closing bid price for one share of our
common stock on the OTC Bulletin Board was $0.16.
We will not receive any proceeds from the resale of shares of common stock by
the selling stockholders, although we may receive proceeds of up to $3,937,949
if all of the warrants are exercised. We will pay for all costs associated with
this registration statement and prospectus.
The selling shareholders may be deemed to be "underwriters," as such term is
defined in the Securities Act.
OUR BUSINESS IS SUBJECT TO MANY RISKS, AND AN INVESTMENT IN OUR COMMON STOCK
WILL ALSO INVOLVE A HIGH DEGREE OF RISK. YOU SHOULD INVEST IN OUR COMMON STOCK
ONLY IF YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. YOU SHOULD CAREFULLY
CONSIDER THE VARIOUS RISK FACTORS DESCRIBED BEGINNING ON PAGE 3 BEFORE INVESTING
IN OUR COMMON STOCK.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES
COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS
PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A
CRIMINAL OFFENCE.
EASY ENERGY, INC.
PROSPECTUS
20,638,273 SHARES OF COMMON STOCK
TABLE OF CONTENTS
Page Number
-----------
PROSPECTUS SUMMARY 1
RISK FACTORS 3
FORWARD-LOOKING STATEMENTS 9
USE OF PROCEEDS 9
DETERMINATION OF OFFERING PRICE 9
DILUTION 9
SELLING SHAREHOLDERS 9
PLAN OF DISTRIBUTION 12
INTEREST OF NAMED EXPERTS AND COUNSEL 13
DESCRIPTION OF BUSINESS 13
DESCRIPTION OF PROPERTY 16
DESCRIPTION OF SECURITIES 16
LEGAL PROCEEDINGS 17
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS 17
MANAGEMENT'S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION 18
APPLICATION OF CRITICAL ACCOUNTING POLICIES 21
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE 22
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS 22
EXECUTIVE COMPENSATION 24
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT 25
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS 27
DISCLOSURE OF SEC POSITION OF INDEMNIFICATION FOR SECURITIES
ACT LIABILITIES 27
EXPERTS 28
WHERE YOU CAN FIND MORE INFORMATION 28
FINANCIAL STATEMENTS 29
As used in this prospectus, the terms "we", "us", "our" and "Easy Energy" mean
Easy Energy, Inc., unless otherwise indicated.
All dollar amounts refer to U.S. dollars unless otherwise indicated.
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus.
Because this is a summary, it may not contain all of the information that you
should consider before receiving a distribution of our common stock. You should
read this entire prospectus carefully. We are a development stage company that
has only recently begun operations. We have not generated any revenues from our
intended business activities, and we do not expect to generate revenues in the
near future. We may never generate revenues. We have minimal assets and have
incurred losses since inception.
CORPORATE BACKGROUND
Easy Energy, Inc. was incorporated under the laws of the State of Nevada on May
17, 2007. We have not generated any revenue to date and are a development stage
company. We currently have no employees other than our President and Secretary
who are also our only board members. We plan to develop a novel, man-powered
charger solution for the problems related to the ongoing power requirements of
small hand-carried battery-powered personal electronic devices. On August 20,
2007 we filed a patent application (Application No.: 11/841,046) with the United
States Patent and Trademark Office. Prior to our incorporation, Mr. Guy Ofir,
our President and Director developed a prototype of the patent. On January 29,
2008, we announced on the completion of the fully working prototype of the
man-powered charger solution for battery powered small hand-carried devices.
The Company's principal business plan is to manufacture and market the product
and/or seek third party entities interested in licensing the rights to
manufacture and market the man-powered charger. Our target market will be
consumers of disposable and rechargeable batteries, those who heavily depend on
their portable devices, especially cell phone users, and those who are looking
for "green" energy sources.
Our principal executive office is located at Suite 105 - 5348 Vegas Dr., Las
Vegas, NV 89108. Our telephone number is (702) 442-1166. We also have an office
in Israel at 26 Ga'aton Blvd., Nahariya 22401 Israel, Tel. No. +972-4-988 8314 .
We do not have any subsidiaries. The address of our resident agent is
Eastbiz.com Inc, 5348 Vegas Dr, Las Vegas, Nevada, U.S.A., 89108.
Due to the uncertainty of our ability to meet our current operating and capital
expenses, in their report on our audited financial statements for the period
ended March 31, 2008 our independent auditors included an explanatory paragraph
regarding concerns about our ability to continue as a going concern. Our
financial statements contain additional note disclosures describing the
circumstances that lead to this disclosure by our independent auditors.
NUMBER OF SHARES BEING OFFERED
This prospectus covers the resale by the selling stockholders named in this
prospectus of up to 20,638,273 shares of our common stock. The offered shares
were acquired by the selling stockholders in several private placement
transactions. All of these transactions were exempt from the registration
requirements of the Securities Act of 1933. The selling stockholders may offer
to sell the shares of common stock being offered in this prospectus at fixed
prices, at prevailing market prices at the time of sale, at varying prices or at
negotiated prices. Our common stock is presently traded on the OTC Bulletin
Board under the symbol "ESYE.OB". Please see the Plan of Distribution section at
page 11 of this prospectus for a detailed explanation of how the common shares
may be sold.
NUMBER OF SHARES ISSUED AND OUTSTANDING
There were 93,186,070 shares of our common stock issued and outstanding as at
September 12, 2008.
1
USE OF PROCEEDS“Offered Securities.” We will not receive any of the proceeds from the sale by the Selling Securityholders of the sharesOffered Securities. See “Use of Proceeds” beginning on page 29 of this prospectus. We will bear all costs, expenses and fees in connection with the registration of the Offered Securities, including with regard to compliance with state securities or “blue sky” laws. The Selling Securityholders will bear all commissions and discounts, if any, attributable to their sale of the Offered Securities, except as otherwise expressly set forth under “Plan of Distribution” beginning on page 62 of this prospectus.
This prospectus describes the general manner in which the Offered Securities may be offered and sold. If necessary, the specific manner in which the Offered Securities may be offered and sold will be described in one or more supplements to this prospectus. Any prospectus supplement may add, update or change information contained in this prospectus. You should carefully read this prospectus, and any applicable prospectus supplement before you invest in any of our common stock being offeredsecurities.
The Selling Securityholders may offer, sell or distribute Offered Securities publicly or through private transactions. If the Selling Securityholders use underwriters, dealers or agents to sell Offered Securities, we will name them and describe their compensation in a prospectus supplement. The price to the public of those securities and the net proceeds the Selling Securityholders expect to receive from that sale will also be set forth in a prospectus supplement.
No public market currently exists for saleour Common Stock. We have applied to have our Common Stock quoted on OTC Pink Market, and upon approval of such application, we intend to have our Common Stock quoted on the OTCQB operated by OTC Markets Group, Inc. We cannot assure you that our Common Stock will ever be quoted on the OTC Pink Market or OTCQB. Until such time that our Common Stock is quoted on the OTC Pink Market, OTCQB or another existing market, if ever, the Common Stock to be sold by the Selling Securityholders from time to time will be at a fixed price of $2.70 per share and thereafter at prevailing market prices or privately negotiated prices. Consequently, if you purchase Common Stock in this offering you may not be able to sell your Common Stock in any organized marketplace and you may be limited to selling stockholders, althoughyour Common Stock privately. Accordingly, an investment in our Common Stock is an illiquid investment.
See “Risk Factors” beginning on page 6of this prospectus for a discussion of information that should be considered in connection with the ownership of our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of the prospectus is , 2022.
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or a supplement to this prospectus. We have not authorized anyone to provide you with different information. This prospectus is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus or any supplement to this prospectus is accurate as of any date other than the date on the front cover of those documents.
i
PROSPECTUS SUMMARY
This summary highlights information contained in other parts of this prospectus. Because it is a summary, it does not contain all of the information that you should consider in making your investment decision. Before investing in our Common Stock, you should read the entire prospectus carefully, including our consolidated financial statements and the related notes included in this prospectus and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
On May 14, 2021, Raphael Pharmaceutical Ltd., an Israeli company, and Easy Energy, Inc., a Nevada corporation, completed a share exchange agreement, or the share exchange, pursuant to which the shareholders of Raphael Pharmaceutical Ltd. became the holders of 90% of the issued and outstanding share capital of Easy Energy, Inc., while Easy Energy, Inc.’s shareholders hold, following the share exchange, 10% of Easy Energy, Inc. On May 19, 2021, as agreed by the parties to the share exchange, Easy Energy, Inc. changed its name to Raphael Pharmaceutical Inc. Unless otherwise mentioned or unless the context requires otherwise, when used in this prospectus, the terms “Raphael,” “Company,” “we,” “us,” and “our” refer to Raphael Pharmaceutical Inc. and its subsidiary, Raphael Pharmaceutical Ltd., or Raphael Israel. References to Easy Energy are to Easy Energy, Inc. Unless otherwise mentioned or unless the context requires otherwise, the information provided in this prospectus relates to Raphael Israel.
Our Company
History
The Company was incorporated in the State of Nevada in May 2007 and was formerly known as Easy Energy, Inc. On May 14, 2021, Easy Energy and Raphael Israel completed the Share Exchange, as a result of which, Raphael Israel’s shareholders own 90% of our Company and Easy Energy’s shareholders hold the remaining 10%. Raphael Israel was incorporated in 2019 in the State of Israel and has focused to date on developing its lead product candidate for the treatment of rheumatoid arthritis, or RA. Easy Energy did not have any ongoing business or operations before the Share Exchange and following the Share Exchange we adopted Raphael Israel’s business plan.
Overview
We are a pharmaceutical drug research and development company focused on the discovery and clinical development of life-improving drug therapies based on cannabinoids, including cannabidiol, or CBD, oil. Unless indicated otherwise, we plan on using oil derived from CBD strains with low levels of Tetrahydrocannabinol, or THC. All references to the use of CBD in our product candidates refer to CBD strains with less than 0.3% of THC.
We are currently in the pre-clinical development stage for our lead product candidate, our RA product candidate for the treatment of RA. In addition, we are aiming to develop a pharmaceutical drug product for the treatment of hyperinflammatory syndrome inflammation related to COVID-19, or our COVID-19 product candidate, which may be based on data or studies related to our RA product candidate. We successfully completed a preclinical study for the RA product candidate at Rambam Hospital as well as a mice model trial.
On February 9, 2022, we filed an application for a clinical trial with the Medical Cannabis Unit of the Ministry of Health of Israel, or MOH. On February 16, 2022 we submitted an application with the Helsinki Committee at Rambam Hospital for a clinical trial in COVID-19 patients. We plan to submit such applications for our RA product during 2022.
Our goal is to become a leader in development of CBD oil-based pharmaceutical drug products for the treatment of indications in which we believe there is a high unmet medical need in a range of disorders, including those related to inflammation in the body, including RA and COVID-19.
In order to achieve our goal, we have and will continue to build an experienced team of senior executives and scientists, with experience in all facets of pharmaceutical research and development, drug formulation, clinical trial execution and regulatory submissions. We intend to leverage the knowledge of our team in order to complete the clinical trials needed to receive approvals of our product candidates from applicable regulatory authorities.
Initially, we intend to obtain approvals for our product candidates from the U.S. Food and Drug Administration, or FDA, and the Medical Cannabis Unit of the Israeli Ministry of Health, or MOH. Upon obtaining FDA approvals, or in the event that we are not successful in obtaining such approvals, we intend to apply for European Medicines Agency, or EMA, and other countries’ governmental regulatory agencies approvals for our product candidates. If we are successful in obtaining FDA approvals for our product candidates, we intend to enter into royalty agreements with good manufacturing practice, or GMP, approved medical manufactures and distributors, having them using our medical formulas for the purpose of growing, cultivating, manufacturing, and distributing Raphael Pharmaceutical medical indications in their designated territories.
Our discovery platform currently focuses the use of CBD oil, one of the cannabinoids in cannabis plants, as the active pharmaceutical ingredient, or API, for our RA product candidate and COVID-19 product candidate. Research results published in 2018 (“Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age”) has shown that there may be benefits to treading medical conditions, or their effects, with cannabinoids, and more specifically, with CBD, which may help reduce chronic pain by impacting endocannabinoid receptor activity, reducing inflammation and interacting with neurotransmitters. This research has also shown that CBD may have neuroprotective properties, and could have the ability to (i) reduce anxiety and depression, (ii) alleviate cancer-related symptoms, (iii) reduce acne and (iv) benefit heart health.
Over the last few years, pharmaceutical drug products that include parts of the cannabis plant have begun to receive regulatory approvals for use in patients suffering from certain disorders. In light of the past regulatory approvals for other pharmaceutical drug products and, more specifically, the potential beneficial effects of CBD and other parts of the cannabis plant, we believe that a drug discovery platform based on CBD may offer new and differentiated treatment options for patients.
After two successful years of pre-clinical research and studies at the laboratories of Rambam Hospital, in January 2021, we commenced a pre-clinical trial in mice for our RA product candidate that we expect will take four to five months. Following the completion of this pre-clinical trial, we intend on submitting an Investigational New Drug, or IND, application to the FDA and MOH. See “– Research and Clinical Development Strategy – Clinical Development Plan” for additional information on the ongoing pre-clinical trial and our planned clinical trial for our RA product candidate. In addition, with respect to our COVID-19 product candidate, our clinical research partners have been focused on the effect of cannabinoids and cannabis extracts on immune cells which induce acute inflammation. This study will begin in the pre-clinical level in immune cell models and, subject to positive results that exhibit downregulation of pro-inflammatory cytokines by cannabis extract, the study was completed successfully. Following the completion of the pre-clinical study, a mice model was conducted to analyze for acute inflammation, which resembles the immunopathology of COVID-19. The mice model was successfully completed and we have registered for a clinical trial in patients with the MOH.
As a pharmaceutical research and clinical development company we do not own or operate, and currently do not intend on creating an in-house team to manufacture and commercialize our pharmaceutical drug products, if any, that receive regulatory approval allowing for commercialization. We currently rely, and expect to continue to rely, on third parties for the manufacturing of our product candidates for preclinical and clinical testing, as well as for commercial manufacturing of any pharmaceutical drug products for which we may receive proceedsregulatory approval. Subject to the receipt of such regulatory approvals, we intend on cooperating with manufacturers and other third parties to manufacture and commercialize approved pharmaceutical drug products.
Product Pipeline
We have begun development for our RA and COVID-19 product candidates, which are in the pre-clinical stage.
Assuming that we successfully complete the clinical development of our RA and COVID-19 product candidates, we intend to then turn our attention to the clinical development of cannabinoid-based drug products for the treatment of certain oncology indications. Unlike our RA and COVID-19 product candidate, the use of our cannabinoid based drug products for the treatment of certain oncology indications will require specific dosing and potentially, a different regulatory pathway than our existing product candidates.
We intend to apply for FDA approval, subject to the completion of the applicable clinical trials, for our RA product candidate as well as our COVID-19 product candidate using the FDA’s regulatory pathway for drug products.
Research and Clinical Development Strategy
Research and clinical development of our pharmaceutical drug product candidates is our core business. We are currently focused on developing innovative cannabinoid-based medical indications that we aim to push through Phase 2A and Phase 2B approval from the MOH, FDA and EMA.
The research efforts that have been conducted to date by the team at Rambam Med-Tech Ltd., or Rambam MT, are aimed at revealing the mechanism which structures the activity of cannabinoids in human cells and organs, while applying a variety of disease models. We are using our PCR method to identify different receptors to cannabinoids and which we believe will allow us to identify which cannabinoids receptors are participating in the downregulation of inflammation. We believe that this strategy will allow us to identify the cannabinoids components of a chosen strain in order to accurately administer cannabinoids targeted to the treatment of patients.
Competition and Competitive Position
We face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates for which we complete clinical development successfully and for which we receive marketing approval may compete with existing therapies and new therapies that may become available in the future. However, we believe, specifically with respect to our competitors not using cannabis in their pharmaceutical drug products that our use of, and experience with, cannabinoids provides us with a potential competitive advance. Our research has shown that the use of cannabinoids for the treatment of RA is justified based on its positive effect on pain, fatigue, sleep problems and its potential safety profile. Growing evidence on the anti-inflammatory effect of cannabinoids provide more strong ground for their use in the treatment of RA. We believe that our RA product candidate, if approved for commercialization by regulators, will be available to patients at a lower price than that of other available treatments.
Cultivation of our API
In October 2020, we entered into an agreement with Way of Life Cannabis Ltd., or Wolc, pursuant to which Raphael Israel is scheduled to be provided with up to $3,937,949 if all15 liters of warrantsCBD oil, from a strain of cannabis of our selection, during a term of 18 months, to be provided in two to three deliveries of between one to seven liters of CBD oil. We believe that our current agreement with Wolc will provide us with sufficient amounts of CBD oil in order to complete our clinical development and for initial sales of our pharmaceutical drug products.
Manufacturing
We do not own or operate, and currently have no plans to establish, any manufacturing facilities for final manufacture. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical and clinical testing, as well as for commercial manufacturing of any pharmaceutical drug products for which we receive regulatory approval.
Commercialization Plan
Subject to the receipt of regulatory approval to commercialize our pharmaceutical product candidates, our goal is to distribute our approved formulas to good manufacturing practice, or GMP, approved medical cannabis manufacturers and global medical cannabis distributors. Depending on the expertise of the distributors, we expect the licensing agreements to provide us with royalty-based payments for the sale of each of our approved pharmaceutical drug products.
Summary Risk Factors
The risk factors described below are exercised. We will
incur all costsa summary of the principal risk factors associated with this registration statement and prospectus.
SUMMARY FINANCIAL DATA
Thean investment in us. These are not the only risks we face. You should carefully consider these summary financial data presented below is derived from and should be readrisk factors, together with the risk factors set forth in
conjunction with our audited financial statements from May 17, 2007 (date of
inception) to June 30, 2008, including the notes to those financial statements
which are included elsewhere in this prospectus along with the section entitled "Plan of Operation"“Risk Factors” beginning on page 216.
| ● | We have a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future. |
| ● | We have not generated revenue from any product candidate and may never be profitable. |
| ● | We expect that we will need to raise substantial additional funding before we can expect to complete the development of our RA product candidate or any other product candidate. This additional financing may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product candidate development efforts or other operations. |
| ● | The lack of an existing trading market could adversely impact our ability to raise working capital and adversely impact our ability to continue operations. |
| ● | We are heavily dependent on the success of our product candidates, which are in various stages of pre-clinical development. We cannot give any assurance that any of our product candidates will proceed to clinical development or that they will receive regulatory approval, which is necessary before they can be commercialized. |
| ● | The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable. If we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed. |
| ● | Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future study results. |
| ● | We hold no patents on our products, and our business employs proprietary technology (know-how) and information may be difficult to protect and/or infringe on the intellectual property rights of third parties. |
| ● | We manage our business through a small number of employees and key consultants. |
| ● | We will need to expand our organization and we may experience difficulties in recruiting needed additional employees and consultants, which could disrupt our operations. |
| ● | We rely on third parties to conduct our preclinical and, in the future, clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed. |
| ● | We will rely on third parties to grow and provide us with our active pharmaceutical ingredient, or API, and formulations. Our business could be harmed if those third parties fail to provide us with sufficient quantities of our needed supplies, or fail to do so at acceptable quality levels or prices. |
| ● | We rely on third parties to supply and manufacture our product candidates, and we expect to continue to rely on third parties to manufacture our products, if approved. The development of such product candidates and the commercialization of any products, if approved, could be stopped, delayed, or made less profitable if any such third party fails to provide with sufficient quantities of product candidates or products, or fails to do so at acceptable quality levels or prices, or fails to maintain or achieve satisfactory regulatory compliance. |
| ● | We and our collaborators and contract manufacturers are subject to significant regulation with respect to manufacturing our product candidates. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity. |
| ● | Our executive officer, directors and certain stockholders who are beneficial owners of 5% or more of our outstanding Common Stock possess the majority of our voting power, and through this ownership, have the ability to control our Company and our corporate actions. |
| ● | Investors may have difficulty in reselling their shares due to the lack of market or state Blue Sky laws. |
| ● | Because we previously had our registration with the SEC revoked and because our business operations resulted from public by means of a “reverse merger,” we may not be able to attract the attention of major brokerage firms. |
| ● | As a former shell company, resales of shares of our restricted Common Stock in reliance on Rule 144 of the Securities Act are subject to the requirements of Rule 144(i). |
| ● | Our headquarters and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic and military instability in Israel. |
Corporate Information
The mailing address of our principal executive office is 4 Lui Paster, Tel Aviv-Jaffa, Israel 6803605 and the telephone number is Telephone: (972) 52-775-5072. The website address is www.raphaelpharmaceutical.com. The information found on the website is not part of, and is not incorporated into, this prospectus.
As at
BALANCE SHEET INFORMATION June 30, 2008
------------------------- --------------
Cash $ 595,446
Total Assets $ 970,446
Liabilities $ 300
From May 17, 2007
(date of inception) to
STATEMENT OF OPERATIONS INFORMATION June 30, 2008
----------------------------------- --------------
Working Capital $ 970,146
Expenses $ 1,480,045
Total Number of Issued Shares of Common Stock 93,186,070
Net Gain (Loss) $ (1,474,354)
2
THE OFFERING
| Common Stock offered by the Selling Securityholders | We are registering 1,667,393 shares of Common Stock to be offered from time to time by the Selling Securityholders, issued in private placements which had multiple closings between April 2021 and May 2022. | |
| Terms of the offering | The Selling Securityholders will determine when and how they will dispose of the Common Stock, registered under this prospectus for resale. For additional information concerning the offering, see “Plan of Distribution” beginning on page 62. | |
| Risk factors | Before investing in our securities, you should carefully read and consider the information set forth in “Risk Factors” beginning on page 6 of this prospectus. | |
| Use of proceeds | We will not receive any of the proceeds from the sale of Offered Securities by the Selling Securityholders. | |
| Trading market and symbol | Our Common Stock is not listed for quotation on any exchange or market system and there is no established public trading market for our Common Stock. We have applied to have our Common Stock quoted on OTC Pink Market, and upon approval of such application, we intend to apply to list the Common Stock on OTCQB under the symbol “RAPH”. We cannot assure you that our Common Stock will ever be quoted on the OTC Pink Market or OTCQB. Consequently, if you purchase Common Stock in this offering you may not be able to sell your Common Stock in any organized marketplace and you may be limited to selling your Common Stock privately. Accordingly, an investment in our Common Stock is an illiquid investment. |
RISK FACTORS
An investment in our common stock involvessecurities carries a numbersignificant degree of very significant risks.risk. You should carefully consider the following risks, and uncertainties in addition
toas well as the other information contained in this prospectus, including our historical financial statements and related notes included elsewhere in evaluatingthis prospectus, before you decide to purchase our companysecurities. Any one of these risks and itsuncertainties has the potential to cause material adverse effects on our business, before purchasing sharesprospects, financial condition and operating results which could cause actual results to differ materially from any forward-looking statements expressed by us and a significant decrease in the value of our company's common stock. Our business,
operating results and financial condition couldCommon Stock. Refer to “Cautionary Statement Regarding Forward-Looking Statements.”
We may not be seriously harmed due tosuccessful in preventing the material adverse effects that any of the following risks.risks and uncertainties may cause. These potential risks and uncertainties may not be a complete list of the risks and uncertainties facing us. There may be additional risks and uncertainties that we are presently unaware of, or presently consider immaterial, that may become material in the future and have a material adverse effect on us. You could lose all or parta significant portion of your investment due to any of these risks.
RISKS RELATED TO OUR BUSINESS
OUR SUCCESS IS HEAVILY DEPENDENT ON PROTECTING OUR INTELLECTUAL PROPERTY RIGHTS.
risks and uncertainties.
Risks Related to Our Financial Condition and Capital Requirements
We relyhave a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future.
We are an emerging pharmaceutical research and clinical development company with a limited operating history in our industry. Before the Share Exchange, we did not have any operations dating back to 2011. To date, we have focused almost exclusively on developing our RA product candidate. Raphael Israel has funded its operations to date primarily through proceeds from investors and from its own resources. Other than the funding that Easy Energy received, and which was lent to (invested in Raphael Israel after the closing of the Share Exchange), Easy Energy has had no sources of funding since ceasing its operations in 2011. We have only a combination of patent, copyright, trademark,limited operating history in our current industry upon which you can evaluate our business and trade secret
protections to protect our proprietary technology. Our success will, in part,
depend on ourprospects. In addition, we have limited experience and have not yet demonstrated an ability to obtain trademarkssuccessfully overcome many of the risks and patents. We have recently
submitted provisional patent applications, but we cannot ensure that any patents
will issue from those applications. We cannot assure you thatuncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the patents issued
to us will not be challenged, invalidated, or circumvented, or that the rights
granted under those registrations will provide competitive advantages to us.
We also rely on trade secrets and new technologies to maintain our competitive
position. Althoughpharmaceutical industry. To date, we have entered into confidentiality agreementsnot generated revenue from the sale of our product candidates (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” for additional information). We incurred losses in each year since its inception and, while operational, we have never been profitable and have incurred losses since inception. We expect to continue to incur losses until, and if, a product candidate that we are developing, such as our RA product candidate, receives approval from regulators for commercialization. Our net loss attributable to holders of our ordinary shares for the years ended December 31, 2021 and the three months period ended March 31, 2022 was approximately $1.62 million and $185,000, respectively. As of June 28, 2022, we had an accumulated deficit of approximately $3.0 million. Substantially all of our operating losses resulted from costs incurred in connection with our employeesdevelopment program and consultants, we cannot be certain that others will not gain accessfrom general and administrative costs associated with our operations.
We expect our research and development expenses to these trade secrets. Others may independently develop substantially
equivalent proprietary informationincrease in connection with our planned preclinical and techniques or otherwise gain access to
our trade secrets.
IF WE ARE UNABLE TO ADEQUATELY PROTECT OUR INTELECTUAL PROPERTY, THIRD PARTIES
MAY BE ABLE TO USE OUR TECHNOLOGY, WHICH COULD ADVSERSLY AFFECT OUR ABILITY TO
COMPETE IN THE MARKET.
Our commercial success will depend in part on our ability to obtain and maintain
patent protection on our products, and successfully defend these patents and
technologies against third-party challenges. In particular, On August 20, 2007,
we filed a provisional patent application (Application No.: 11/841,046) with the
United States Patent and Trademark Office, which is still pending. In doing so,
we have secured a filing date in the United States Trademark and Patent Office.
In order to extend the potential patent protection beyond the US, we are
required to file an international patent application via the Patent Cooperation
Treaty ("PCT") no later than August 19, 2008. On June 30, 2008, we have filed
the international patent application via the PCT. This international filing will
enable us to file a national patent application in any PCT country until
February 19, 2009 and still claim the benefit of the priority date of August 20,
2007. After February 29, 2009, we will be required to file a separate patent
application in each PCT country, in which we would like to have a patent
protection. There is no certainty that such applications will be approved.clinical trials. In addition, even if we obtain marketing approval for any current or future product candidate, we will likely incur significant business development expenses as we seek to commercialize such applications are approved, they may not be sufficiently
broad to prevent others from practicing our technologies or from developing
competing products.products, as well as continued research and development expenses. Furthermore, others may independently develop similar or
alternative technologies or design around our patented technologies. Patents we
use may be challenged or invalidated or may fail to provide us with any
competitive advantage. Moreover, in certain parts of the world, such as in
China, western companies are adversely affected by poor enforcement of
intellectual property rights.
WE MAY BE EXPOSED TO LIABILITY FOR INFRINGING INTELLECTUAL PROPERTY RIGHTS OF
OTHER COMPANIES.
Our success will, in part, depend on our ability to operate without infringing
on the proprietary rights of others. Although we have conducted searches and are
not aware of any patents and trademarks which our products or their use might
infringe, we cannot be certain that infringement has not or will not occur. We
could incur substantial costs, in addition to the great amount of time lost, in
defending any patent or trademark infringement suits or in asserting any patent
or trademark rights, in a suit with another.
WE HAVE LIMITED OPERATING HISTORY AND HAVE SUSTAINED LOSSES SINCE INCEPTION,
WHICH WE EXPECT TO CONTINUE INTO THE FUTURE.
3
We were incorporated on May 17, 2007, and have very limited operations to date.
We have not realized any revenues to date. Our product is under development and
is not ready for commercial sale. We have no operating history at all upon which
an evaluation of our future success or failure can be made. Our net loss from
inception to June 30, 2008 is $1,474,354 Based upon our proposed plans, we expect to incur additional costs associated with operating as a reporting company, which we estimate will be at least tens of thousands of dollars annually. As a result, we expect to continue to incur significant and increasing operating losses in future periods. This will happen because
there are substantial costsfor the foreseeable future. Because of the numerous risks and expensesuncertainties associated with the development,
testing and marketing of our product. Based on arrangements made with potential
distributors and the development stage of our product, we believe that we are at
least 6-8 months away from generating our first revenues. We may be wrong and
may fail to generate revenues in the future. If we cannot attract a significant
number of users, we will not be able to generate any significant revenues or
income. Failure to generate revenues will cause us to go out of business because
we will not have the money to pay our ongoing expenses.
In particular, additional capital may be required in the event that:
- the actual expenditures required to be made are at or above the higher
range of our estimated expenditures;
- we incur unexpected costs in completing the development of our product
or encounter any unexpected technical or other difficulties;
- we incur delays and additional expenses as a result of technology
failure;
-developing pharmaceutical products, we are unable to create a substantial market for our product; or
- we incur any significant unanticipated expenses.
The occurrencepredict the extent of any of the aforementioned events could adversely affectfuture losses or when we will become profitable, if at all.
We expect to continue to incur significant losses until we are able to commercialize our product candidates, which we may not be successful in achieving. We anticipate that our expenses will increase substantially if and as we:
| ● | continue the research and development of our RA product candidate or any other product candidate; | |
| ● | expand the scope of our current clinical studies for our RA product candidate or any other product candidate; | |
| ● | seek regulatory and marketing approvals for our product candidates that successfully complete clinical studies; |
| ● | seek to maintain, protect, and expand our intellectual property portfolio; | |
| ● | seek to attract and retain skilled personnel; and | |
| ● | create additional infrastructure to support our operations as a reporting company and our product candidate development and planned future commercialization efforts. |
We have not generated revenue from any product candidate yet and may never be profitable.
Our ability to become profitable depends upon our ability to meetgenerate revenue. We do not expect to generate significant revenue unless or until we obtain marketing approval of, and commercialize, our business plans and achieve a profitable level of operations.
IF WE ARE UNABLE TO OBTAIN THE NECESSARY FINANCING TO IMPLEMENT OUR BUSINESS
PLAN WE WILL NOT HAVE THE MONEY TO PAY OUR ONGOING EXPENSES AND WE MAY GO OUT OF
BUSINESS.RA product candidate or any other product candidate that we may seek to develop. Our budgeted expenditures for the next twelve months are $922,000 of which we
have prepaid $200,000 for our research and development costs which are included
in the statement of operations under product development and prepaid $50,000 in
Consulting Fees which is included in the balance sheet and we anticipate needing
approximately $21,243.32 for expenses associated with this Registration
Statement (See ITEM 13 "Other Expenses if Issuance and Distribution").
Therefore, we presently have a budgeted shortfall of $32,503.68.
Because we have not generated anyability to generate future revenue from product candidate sales depends heavily on our business,success in many areas, including but not limited to:
| ● | obtaining favorable results from and progress the pre-clinical and clinical development of our product candidate(s); | |
| ● | developing and obtaining regulatory approval for registration studies protocols for our product candidate(s); |
| ● | subject to successful completion of registration and clinical trials of our RA product candidate, applying for and obtaining marketing approval; | |
| ● | establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate (in amount and quality) products, and at acceptable costs, to support market demand for our product candidates, if marketing approval is received; |
| ● | identifying, assessing, acquiring and/or developing new product candidate(s); | |
| ● | accurately identifying demand for our product candidate(s); | |
| ● | continued consumer interest in treatments to the symptoms of RA; | |
| ● | obtaining market acceptance of our product candidates, if approved for marketing, as viable treatment options; | |
| ● | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; and | |
| ● | attracting, hiring and retaining qualified personnel. |
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing on reasonable terms or at all.
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. In addition, the report of our independent registered public accounting firm on our audited financial statements for each of the two years ended December 31, 2021 and we are 6-8
months away2020 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our audited financial statements do not include any adjustments that might result from being inthe outcome of the uncertainty regarding our ability to continue as a position to generate revenues, we will needgoing concern. This going concern opinion could materially limit our ability to raise additional funds forthrough the future developmentissuance of our business and to respond to
unanticipated requirementsequity or expenses. Management estimates that our current
cash balances will be exhausted by April 2009 provided we do not have any
unanticipated expenses. We do not currently have any arrangements for financing
and we can provide no assurance to investors we will be able to find such
financing.
Our ability to successfully develop our product and to eventually produce and
sell it to generate operating revenues dependsdebt securities or otherwise. Further reports on our ability to obtain the
necessary financing to implement our business plan. Given that we have no
operating history, no revenues and only losses to date, wefinancial statements may not be able to
achieve this goal, and if this occurs we will not be able to pay for our
operations and we may go out of business. We will likely need to issue
additional equity securities in the future to raise the necessary funds. We do
not currently have any arrangements for additional financing and we can provide
no assurance to investors we will be able to find such financing if further
funding is required. Obtaining additional financing would be subject to a number
of factors, including investor acceptance of our product and our business model.
The issuance of additional equity securities by us would result in a significant
dilution in the equity interests of our current stockholders. The resale of
shares by our existing shareholders pursuant to this prospectus may result in
significant downward pressure on the price of our common stock and cause
negative impact on our ability to sell additional equity securities. Obtaining
loans will increase our liabilities and future cash commitments.
There can be no assurance that capital will continue to be available if
necessary to meet future funding needs or, if the capital is available, that it
will be on terms acceptable to us. If we are unable to obtain financing in the
amounts and on terms deemed acceptable to us, we may be forced to scale back or
cease operations, which might result in the loss of some or all of your
investment in our common stock.
IF OUR ESTIMATES RELATED TO EXPENDITURES ARE ERRONEOUS OUR BUSINESS WILL FAIL
AND YOU WILL LOSE YOUR ENTIRE INVESTMENT.
4
Our success is dependent in part upon the accuracy of our management's estimates
of expenditures, which are currently budgeted at $922,000, of which $250,000 has
been prepaid for the next 12 months for our business plan andinclude an additional
$21,243.32. (See "Plan of Operation".) If such estimates are erroneous or
inaccurate we may not be able to carry out our business plan, which could, in a
worst-case scenario, result in the failure of our business and you losing your
entire investment.
OUR BUSINESS MODEL MAY NOT BE SUFFICIENT TO ENSURE OUR SUCCESS IN OUR INTENDED
MARKET.
Our survival is currently dependent upon the success of our efforts to gain
market acceptance of one product that ultimately represents a small sector in
the overall charger industry. Should our services be too narrowly focused or
should the target market not be as responsive as we anticipate, we may not have
in place alternate products or services that we can offer to ensure our
survival.
IF WE ARE UNABLE TO COMPLETE THE DEVELOPMENT OF OUR PRODUCT WE WILL NOT BE ABLE
TO GENERATE REVENUES AND YOU WILL LOSE YOUR INVESTMENT.
We have not completed the development of our proposed product and we have no
definitive contracts or licenses for the sale or use of our product. The success
of our proposed business will depend on its completion and the acceptance of our
product by the general public. Achieving such acceptance will require
significant marketing investment. Our product, once developed and tested, may
not be accepted by our customers at sufficient levels to support our operations
and build our business. If the proposed product that we will develop is not
accepted at sufficient levels, our business will fail.
WE ARE DEPENDENT ON REVENUES GENERATED BY A SOLE PRODUCT AND THUS WE ARE SUBJECT
TO MANY ASSOCIATED RISKS.
Our revenue will be generated through the sale of our man-powered charger.
Unless we expand our product offerings to include related or other products, our
likely source of revenues for the foreseeable future will continue to be
generated by the man-powered charger. Accordingly, 100% of our revenue will be
dependent upon the sale of our sole product.
If potential users are satisfied with other means for charging their cell phone
battery we may not be able to sell our product.
* If technological developments render man-powered chargers obsolete,
our business could fail;
* our patent application is unsuccessful, our business could fail.
Thus, we may expand our financial resources on marketing and advertising without
generating concomitant revenues. If we cannot generate sufficient revenues to
cover our overhead, manufacturing and operating costs, we will fail.
THERE ARE LOW BARRIERS TO ENTRY INTO THE MAN-POWERED CHARGER INDUSTRY AND, AS A
RESULT, MANY COMPANIES MAY BE ABLE TO COMPETE WITH US ON LIMITED FINANCIAL
RESOURCES.
Our product does not require large capital expenditures for its development or
manufacture. As a result, barriers to entering this industry may be low. If the
intellectual property protectionexplanatory paragraph with respect to the man-powered charger product
does not prove effective, a competitor with limited financial resources may be
able to successfully compete with us.
BECAUSE OUR EXECUTIVE OFFICERS AND DIRECTORS LIVE OUTSIDE OF THE UNITED STATES,
YOU MAY HAVE NO EFFECTIVE RECOURSE AGAINST THEM FOR MISCONDUCT AND MAY NOT BE
ABLE TO ENFORCE JUDGMENT AND CIVIL LIABILITIES AGAINST THEM. INVESTORS MAY NOT
BE ABLE TO RECEIVE COMPENSATION FOR DAMAGES TO THE VALUE OF THEIR INVESTMENT
CAUSED BY WRONGFUL ACTIONS BY OUR DIRECTORS AND OFFICERS.
Both of our directors and officers live outside of the United States. Mr. Guy
Ofir, our President and a director is a national and a resident of Israel, and
all or a substantial portion of his assets are located outside of the United
States. Mr. Emanuel Cohen, our Secretary, Treasurer and a director is a national
and a resident of Israel, and all or a substantial portion of his assets are
5
located outside of the United States. As a result, it may be difficult for
investors to enforce within the United States any judgments obtained against our
directors or officers, or obtain judgments against them outside of the United
States that are predicated upon the civil liability provisions of the securities
laws of the United States or any state thereof. Investors may not be able to
receive compensation for damages to the value of their investment caused by
wrongful actions by our directors and officers.
BECAUSE WE HAVE TWO DIRECTORS, DEADLOCKS MAY OCCUR IN OUR BOARD'S
DECISION-MAKING PROCESS, WHICH MAY DELAY OR PREVENT CRITICAL DECISIONS FROM
BEING MADE.
Since we currently only have an even number of directors, deadlocks may occur
when such directors disagree on a particular decision or course of action. Our
Articles and By-Laws do not contain any mechanisms for resolving potential
deadlocks. While our directors are under a duty to act in the best interest of
our company, any deadlocks may impede the further development of our business in
that such deadlocks may delay or prevent critical decisions regarding our
development.
BECAUSE OUR EXECUTIVE OFFICERS ARE EMPLOYED ELSEWHERE, THEY WILL BE UNABLE TO
DEVOTE THEIR SERVICES TO OUR COMPANY ON A FULL TIME BASIS AND THE PERFORMANCE OF
OUR BUSINESS MAY SUFFER, OUR BUSINESS COULD FAIL AND INVESTORS COULD LOSE THEIR
ENTIRE INVESTMENT.
Mr. Guy Ofir, our President and director is employed elsewhere and he will be
unable to devote his services to our company on a full time basis. Mr. Guy Ofir
currently devotes approximately 30 to 40 hours a week to our company. Mr.
Emanuel Cohen, our Secretary, Treasurer and a director, is employed elsewhere
and he will be unable to devote his services to our company on a full time
basis. Mr. Emanuel Cohen currently devotes 15 to 20 hours a week to our company.
As a result, the management of our company could under-perform, our business
could fail and investors could lose their entire investment.
OUR EXECUTIVE OFFICERS HAVE NO EXPERIENCE OR TECHNICAL TRAINING IN THE
DEVELOPMENT, MAINTENANCE AND MARKETING OF MAN-POWERED CHARGER OR IN OPERATING
BUSINESSES THAT SELL PRODUCTS OR SERVICES TO WHOLESALES. THIS COULD CAUSE THEM
TO MAKE INEXPERIENCED OR UNINFORMED DECISIONS THAT HAVE BAD RESULTS FOR OUR
COMPANY. AS A RESULT, OUR OPERATIONS COULD SUFFER IRREPARABLE HARM AND MAY CAUSE
US TO SUSPEND OR CEASE OPERATIONS, WHICH COULD CAUSE INVESTORS TO LOSE THEIR
ENTIRE INVESTMENT.
Mr. Guy Ofir, our President and director and Mr. Emanuel Cohen, our Secretary,
Treasurer and director, have no experience or technical training in the
development, maintenance and marketing of man-powered charger or in operating
businesses that sell products or services to wholesales. Due to their lack of
experience and knowledge in these areas, our executive officers could make the
wrong decisions regarding the development, operation and marketing of our
products and the operation of our business, which could lead to irreparable
damage to our business. Consequently, our operations could suffer irreparable
harm from mistakes made by our executive officers and we may have to suspend or
cease operations, which could cause investors to lose their entire investment.
WE DEPEND HEAVILY ON MR. GUY OFIR AND MR. EMANUEL COHEN. THE LOSS OF EITHER
PERSON WILL HAVE A SUBSTANTIAL NEGATIVE EFFECT ON OUR BUSINESS AND MAY CAUSE OUR
BUSINESS TO FAIL.
We depend entirely on Mr. Ofir and Mr. Cohen for all of our operations. The loss
of either person will have a substantial negative effect on the company and may
cause our business to fail. Our officers did not receive any compensation for
their services and it is highly unlikely that they will receive any compensation
unless and until we generate substantial revenues.
We do not have any employment agreements or maintain key person life insurance
policies on our officers. If our officers do not devote sufficient time towards
our business, we may never be able to effectuate our business plan.
BECAUSE OUR EXECUTIVE OFFICERS CONTROL A LARGE PERCENTAGE OF OUR COMMON STOCK,
THEY HAVE THE ABILITY TO INFLUENCE MATTERS AFFECTING OUR SHAREHOLDERS.
6
Our executive officers, in the aggregate, beneficially own approximately 43% of
the issued and outstanding shares of our common stock. As a result, they have
the ability to influence matters affecting our shareholders, including the
election of our directors, the acquisition or disposition of our assets, and the
future issuance of our shares. Because our executive officers control such
shares, investors may find it difficult to replace our management if they
disagree with the way our business is being operated.
WE HAVE A GOING CONCERN OPINION FROM OUR AUDITORS, INDICATING THE POSSIBILITY
THAT WE MAY NOT BE ABLE TO CONTINUE TO OPERATE.
The Company has incurred net losses of $1,474,354 for the period from May 17,
2007 (inception) to June 30, 2008. We anticipate generating losses for the next
12 months. Therefore, we may be unable to continue operations in the future as a
going concern. No adjustment has been made in the accompanying financial
statements to the amounts and classification of assets and liabilities which
could result should we be unable to continue as a going concern. If we cannot continue as a viable entity,going concern, our shareholdersinvestors may lose some or all of their entire investment in our Common Stock. Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through debt or equity financing. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the Company.
OUR INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM HAS EXPRESSED SUBSTANTIAL
DOUBT ABOUT OUR ABILITY TO CONTINUE AS A GOING CONCERN, WHICH MAY HINDER OUR
ABILITY TO OBTAIN FUTURE FINANCING.
Forscope of, or eliminate research or development plans for, or commercialization efforts with respect to our products.
We expect that we will need to raise substantial additional funding before we can expect to complete the perioddevelopment of our RA product candidate or any other product candidate. This additional financing may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product candidate development efforts or other operations.
As of December 31, 2022, our cash and cash equivalent were approximately $0.15 million and we had a working capital of $0.13 million and an accumulated deficit of $2.67 million]. Based upon our currently expected level of operating expenditures, we expect that our existing cash and cash equivalents will only be sufficient to fund operations through June 2022. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future funding requirements will depend on many factors, including but not limited to:
| ● | our clinical trial results; | |
| ● | the cost, timing and outcomes of seeking marketing approval of any product candidate for which we may seek marketing approval and the costs associated with our plans to have third parties commercialize any approved product candidate(s); |
| ● | the cost of filing and prosecuting patent applications and the cost of defending patents, if any; | |
| ● | development of other early-stage development product candidates; | |
| ● | subject to receipt of marketing approval, revenue received from sales of approved products, if any, in the future; | |
| ● | any product liability or other lawsuits related to our products; | |
| ● | the expenses needed to attract and retain skilled personnel; and | |
| ● | the costs associated with being a reporting company. |
Any additional fundraising efforts may divert our management from May 17, 2007 (inception)their day-to-day activities, which may adversely affect our ability to March 31, 2008,develop and commercialize our independent
registered public accounting firm has expressed substantial doubt aboutproduct candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all, during or after the COVID-19 pandemic. Moreover, the terms of any financing may adversely affect the holdings or the rights of holders of our securities and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the trading price of our shares to decline. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the development or commercialization, if any, of any product candidates or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
The lack of an existing trading market could adversely impact our ability to raise working capital and adversely impact our ability to continue as a going concern. Such doubt was expressed as a resultoperations.
Our Common Stock is not currently traded on any national securities exchange and is not quoted on any over-the-counter market (see “Risks Related to Ownership of Our Securities – Investors may have difficulty in reselling their shares due to the lack of market or state Blue Sky laws” for additional information). Because our recurring losses and cash flow deficiencies since our inception. We continue
to experience net losses. Our ability to continue as a going concernCommon Stock is subject
to our ability to generate a profit and/or obtain necessary funding from outside
sources, including obtaining additional funding from the sale of our securities,
future sales of our product or obtaining loans and grants from various financial
institutions whenever possible. Ifconsidered illiquid, we continue to incur losses,may find it will become
increasinglymore difficult for us to achieve our goals and there can be no assurance
that our business plan will materialize.
WE WILL BE HEAVILY DEPENDENT ON CONTRACTING WITH THIRD PARTY FIRM(S) TO
MANUFACTURE COMPONENTS FOR US. IF WE ARE UNABLE TO LOCATE, HIRE AND RETAIN THESE
FIRM(S), OUR BUSINESS WILL FAIL.
We intend to hire a third party firm(s) to manufacture the components of our
product. Should we be unable to contract qualified third parties firm(s) to
manufacture because we are unable to find them, are unable to attract them to
our company or are unable to afford them, we will never become profitable and
our business will fail.
RISKS ASSOCIATED WITH OUR COMMON STOCK
BECAUSE WE CAN ISSUE ADDITIONAL COMMON SHARES, PURCHASERS OF OUR COMMON STOCK
MAY INCUR IMMEDIATE DILUTION AND MAY EXPERIENCE FURTHER DILUTION.
We are authorized to issue up to 1,000,000,000 common shares, of which
93,186,070 are issued and outstanding. Our board of directors has the authority
to cause our company to issue additional shares of common stock without the
consent of any of our shareholders. Consequently, our shareholders may
experience dilution in their ownership of our company in the future.
A DECLINE IN THE PRICE OF OUR COMMON STOCK COULD AFFECT OUR ABILITY TO RAISE
FURTHER WORKING CAPITAL AND ADVERSELY IMPACT OUR ABILITY TO CONTINUE OPERATIONS.
A prolonged decline in the price of our common stock could result in a reduction
in the liquidity of our common stock and a reduction in our ability to raise capital. Because a significant portion of our operations has been and will be
financed through the sale of equity securities, a decline in the price of our
common stock could be especially detrimental to our liquidity and our
operations. Such reductions may force us to reallocate funds from other planned
uses and may have a significant negative effect on our business plans and
operations, including our ability to develop new products and continue our
current operations. If our stock price declines, we can offer no assurance that
we will be able to raise additional capital or generate funds from operations
sufficient to meet our obligations. If we are unable to raise sufficient capital in the future, we may not be able to have the resources to continue our normal operations.
7
The
Risks Related to Product Development, Regulatory Approval and Commercialization
We are heavily dependent on the success of our product candidates, which are in various stages of pre-clinical development. We cannot give any assurance that any of our product candidates will proceed to clinical development or that they will receive regulatory approval, which is necessary before they can be commercialized.
Since its inception, Raphael Israel has invested substantially all of its efforts and financial resources to design and develop its product candidates, including conducting preclinical studies and providing general and administrative support for these operations. Our future success is dependent on our ability to continue these operations, and more specifically, to successfully complete pre-clinical and clinical development of our product candidates, which will require coordination from applicable regulatory agencies, obtain regulatory approval for, and then successfully commercialize one or more product candidates for which we receive regulatory approval. We currently generate no revenue from, and we may never be able to generate revenue.
Our RA product candidate and the product candidate that we are seeking to develop for the treatment of inflammation associated with COVID-19 are both in pre-clinical development and will require clinical development (and in some cases additional pre-clinical development), management of non-clinical, clinical and manufacturing activities, regulatory approval, obtaining adequate manufacturing supply, building of a commercial organization and significant marketing efforts before we generate any revenue from product candidate sales. It may be months or years before a pivotal study is initiated, if at all. Any clinical trials in the United States will require the approval of an Investigational New Drug, or IND, application by the FDA, and we cannot assure that we will obtain such approval in a timely manner, or at all. We are not permitted to market priceor promote any of our product candidates before we receive regulatory approval from the FDA or comparable foreign regulatory authorities, and we may never receive such regulatory approval for any of our product candidates.
We as a company have never submitted marketing applications to the FDA or comparable foreign regulatory authorities. We cannot be certain that any of our product candidates will be successful in clinical studies or receive regulatory approval or what regulatory pathway the regulatory authorities shall designate for our common stockproduct candidates. Further, our product candidates may not receive regulatory approval even if they are successful in clinical studies. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations.
We generally plan to seek regulatory approval to commercialize our product candidates in the United States, the European Union, Israel and other foreign countries. To obtain regulatory approvals we must comply with the numerous and varying regulatory requirements of such countries regarding safety, efficacy, chemistry, manufacturing and controls, clinical studies, commercial sales, pricing and distribution of our product candidates. Even if we are successful in obtaining approval in one jurisdiction, we cannot ensure that we will obtain approval in any other jurisdictions. If we are unable to obtain approval for our product candidates in multiple jurisdictions, our revenue and results of operations would be negatively affected.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable. If we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable, typically takes many years following the commencement of clinical studies and depends upon numerous factors. In addition, approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, which may cause delays in the approval or the decision not to approve an application. We have not obtained regulatory approval for any product candidate, and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval.
Applications for our product candidates could fail to receive regulatory approval for many reasons, including but not limited to the following:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical studies; |
| ● | we may be unable to demonstrate to the FDA or comparable foreign regulatory authorities that a product candidate’s safety-benefit ratio for its proposed indication is acceptable; | |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical studies; | |
| ● | the data collected from clinical studies of our product candidates may not be sufficient to support the submission of a new drug application, or NDA, in the United States or elsewhere; | |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes, test procedures and specifications or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and | |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process, as well as the unpredictability of the results of clinical studies, may result in our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business, results of operations and prospects.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical study process. The results of pre-clinical studies and early clinical studies, if any are commenced, of our product candidates may not be predictive of the results of later-stage clinical studies. Product candidates that have shown promising results in early-stage clinical studies may still suffer significant setbacks in subsequent advanced clinical studies. There is a high failure rate for drugs proceeding through clinical studies, and product candidates in later stages of clinical studies may fail to show the desired safety and efficacy traits despite having progressed satisfactorily through preclinical studies and initial clinical studies. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical studies due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier studies. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses. We do not know whether any Phase 1, Phase 2, Phase 3 (if any) or other clinical studies we may conduct will demonstrate consistent or adequate efficacy and safety sufficient to obtain regulatory approval to market our product candidates.
We may find it difficult to enroll patients in our clinical studies. Difficulty in enrolling patients could delay or prevent clinical studies of our product candidates.
Identifying and qualifying patients to participate in clinical studies of our product candidates is critical to our success. The timing of our clinical studies depends in part on the speed at which we can recruit patients to participate in testing our product candidates, and we may experience delays in our clinical studies if we encounter difficulties in enrollment.
Additionally, the process of finding patients may prove costly. We also may not be able to identify, recruit and enroll a sufficient number of patients to complete our clinical studies because of the perceived risks and benefits of the product candidate under study, the availability and efficacy of competing therapies and clinical studies, the proximity and availability of clinical study sites for prospective patients and the patient referral practices of physicians. If patients are unwilling to participate in our studies for any reason, the timeline for recruiting patients, conducting studies and obtaining regulatory approval of potential product candidates will be delayed.
In addition, the COVID-19 pandemic may affect the operations of the FDA and other health authorities, which could result in delays of reviews and approvals. The evolving COVID-19 pandemic may directly or indirectly impact the pace of enrollment in future clinical trials, if any, as patients may avoid or may not be able to travel to healthcare facilities and physicians’ offices unless due to a health emergency. Additionally, such facilities and offices have been and may continue to be required to focus limited resources on non-clinical trial matters, including treatment of COVID-19 patients, thereby decreasing availability, in whole or in part, for clinical trial services. We also do not yet know if, as a result of the COVID-19 pandemic, people who would otherwise enroll in our or other clinical trials would elect not to participate. The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to change.
If we experience delays in the completion or termination of any clinical study of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product candidate revenue from any of these product candidates could be delayed or prevented. In addition, any delays in completing our clinical studies will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product candidate sales and generate revenue. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical studies may also ultimately lead to the denial of regulatory approval of our product candidates.
We may encounter substantial delays in our clinical studies, or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is expensive, time consuming and uncertain as to outcome. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing, and our future clinical studies may not be successful. Events that may prevent successful or timely completion of clinical development include but are not limited to:
| ● | inability to generate sufficient preclinical, toxicology or other in vivo or in vitro data to support the initiation of human clinical studies; | |
| ● | delays in reaching a consensus with regulatory agencies on study design; | |
| ● | delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites; | |
| ● | delays in obtaining required Institutional Review Board, or IRB, approval at each clinical study site; | |
| ● | imposition of a clinical hold by regulatory agencies, after review of an IND, application, or equivalent application, or an inspection of our clinical study operations or study sites; | |
| ● | delays in recruiting suitable patients to participate in our clinical studies; | |
| ● | difficulty collaborating with patient groups and investigators; | |
| ● | failure by our CROs, other third parties or us to adhere to clinical study requirements; | |
| ● | failure to perform in accordance with the FDA’s Good Clinical Practices, or GCP, requirements, or applicable regulatory guidelines in other countries; | |
| ● | delays in having patients complete participation in a study or return for post-treatment follow-up; | |
| ● | patients dropping out of a study; | |
| ● | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; | |
| ● | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; | |
| ● | the cost of clinical studies of our product candidates being greater than we anticipate; | |
| ● | clinical studies of our product candidates producing negative or inconclusive results, which may result in us deciding, or regulators requiring us, to conduct additional clinical studies or abandon product candidate development programs; and |
| ● | delays in manufacturing, testing, releasing, validating or importing/exporting sufficient stable quantities of our product candidates for use in clinical studies or the inability to do any of the foregoing. |
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue. We may also be affected byrequired to conduct additional safety, efficacy and comparability studies before we will be allowed to start clinical studies with our repurposed drugs. Clinical study delays could also shorten any periods during which our product candidates have patent protection and may allow our competitors to bring product candidates to market before we do, which could impair our ability to meetobtain orphan exclusivity and successfully commercialize our product candidates and may harm our business and results of operations.
Our product candidates may cause undesirable side effects or exceed expectationshave other properties that could delay or prevent their regulatory approval, limit the commercial profile of analystsan approved label or investors.result in significant negative consequences following marketing approval, if any.
The use of dronabinol has been associated with seizures, paranoia, rapid heart rate and unusual thoughts and behaviors. Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical studies and could result in a more restrictive marketing label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities. Potential side effects of our cannabinoid-based treatments may include: asthenia, palpitations, tachycardia, vasodilation/facial flush, abdominal pain, nausea, vomiting, amnesia, anxiety/nervousness, ataxia, confusion, depersonalization, dizziness, euphoria, hallucinations, paranoid reaction, somnolence and abnormal thinking. Results of our studies may identify unacceptable severity and prevalence of these or other side effects. In such an event, our studies could be suspended or terminated, and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny or withdraw approval of our product candidates for any or all targeted indications.
Drug-related side effects could affect patient recruitment, the ability of enrolled patients to complete the study or result in potential product candidate liability claims.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such product candidates, a number of potentially significant negative consequences could result, including but not limited to:
| ● | regulatory authorities may withdraw approvals of such product candidate; | |
| ● | regulatory authorities may require additional warnings on the label; | |
| ● | we may be required to create a Risk Evaluation and Mitigation Strategy, or REMS, plan, which could include a medication guide outlining the risks of such side effects for distribution to patients, a communication plan for healthcare providers and/or other elements to assure safe use; | |
| ● | we could be sued and held liable for harm caused to patients; and | |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
Even if we obtain regulatory approval for a product candidate, our product candidates will remain subject to regulatory scrutiny.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States. In addition, manufacturers and manufacturers’ facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP, regulations and Quality System Regulation, or QSR. As such, we and our contract manufacturers will be subject to continual review and inspections to assess compliance with cGMP, QSR and adherence to commitments made in any NDA. Accordingly, we and others with whom we work must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we receive for our product candidates may also be subject to limitations on the approved indicated uses for which the product candidate may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials and surveillance to monitor the safety and efficacy of the product candidate. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with requirements concerning advertising and promotion for our product candidates. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product candidate’s approved label. As such, we may not promote our product candidates for indications or uses for which they do not have FDA approval. The holder of an approved NDA must also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product candidate, product candidate labeling or manufacturing process. We could also be asked to conduct post-marketing clinical studies to verify the safety and efficacy of our product candidates in general or in specific patient subsets. If original marketing approval were obtained via the accelerated approval pathway, we could be required to conduct a successful post-marketing clinical study to confirm clinical benefit for our product candidates. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of marketing approval. Furthermore, any new legislation addressing drug safety issues could result in delays in product candidate development or commercialization or increased costs to assure compliance. Foreign regulatory authorities impose similar requirements.
If a regulatory agency discovers previously unknown problems with a product candidate, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product candidate is manufactured, or disagrees with the promotion, marketing or labeling of a product candidate, such regulatory agency may impose restrictions on that product candidate or us, including requiring withdrawal of the product candidate from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| ● | issue warning letters; | |
| ● | impose civil or criminal penalties; | |
| ● | suspend or withdraw regulatory approval; | |
| ● | suspend any of our ongoing clinical studies; | |
| ● | refuse to approve pending applications or supplements to approved applications submitted by us; | |
| ● | impose restrictions on our operations, including closing our contract manufacturers’ facilities; or | |
| ● | seize or detain product candidates, or require a product candidate recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our product candidates. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected.
We are subject to numerous complex regulations and failure to comply with these regulations, or the cost of compliance with these regulations, may harm our business.
The research, testing, development, manufacturing, quality control, approval, labeling, packaging, storage, recordkeeping, promotion, advertising, marketing, distribution, possession and use of our product candidates, among other things, are subject to regulation by numerous governmental authorities in the United States and elsewhere. The FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act of 1938, or the FDC Act, and implementing regulations. Noncompliance with any applicable regulatory requirements can result in refusal to approve product candidates for marketing, warning letters, product candidate recalls or seizure of product candidates, total or partial suspension of production, prohibitions or limitations on the commercial sale of product candidates or refusal to allow the entering into of federal and state supply contracts, fines, civil penalties and/or criminal prosecution. Additionally, the FDA and comparable governmental authorities have the authority to withdraw product candidate approvals that have been previously granted. Moreover, the regulatory requirements relating to our product candidates may change from time to time and it is impossible to predict what the impact of any such changes may be.
We are developing product candidates that are controlled substances as defined in the Controlled Substances Act of 1970, or CSA, which establishes, among other things, certain registration, production quotas, security, recordkeeping, reporting, import, export and other requirements administered by the Drug Enforcement Administration, or the DEA. The active ingredient in our product candidates is CBD, which, when derived from cannabis, is a Schedule V controlled substance, meaning that any drug containing it cannot be marketed before it receives regulatory approval from the FDA.
The manufacture, shipment, storage, sale and use, among other things, of controlled substances that are pharmaceutical product candidates are subject to a high degree of regulation. The DEA also conducts periodic inspections of registered establishments that handle controlled substances. Facilities that conduct research, manufacture, distribute, import or export controlled substances must be registered to perform these activities and have the security, control and inventory mechanisms required by the DEA to prevent drug loss and diversion. Failure to maintain compliance, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, results of operations, financial condition and prospects. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to suspend or revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
Individual states also have controlled substances laws. Though state controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule our product candidates as well. While some states automatically schedule a drug when the DEA does so, other states schedule drugs through rulemaking or a legislative action. State scheduling may delay commercial sale of any product candidate for which we obtain federal regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product candidate. We or our partners must also obtain separate state registrations, permits or licenses in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
If the market opportunities for our product candidates are smaller than we believe they are, our revenue may be adversely affected, and our business may suffer.
Our projections of both the number of people who have our target diseases, as well as the subset of people with these expectations,diseases who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, surveys of clinics, patient foundations or market research and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these diseases. The number of patients may turn out to be lower than expected. The effort to identify patients with diseases we seek to treat is in early stages, and we cannot accurately predict the number of patients for whom treatment might be possible. Additionally, the potentially addressable patient population for each of our product candidates may be limited or may not be amenable to treatment with our product candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business.
We face intense competition and rapid technological change and the possibility that our competitors may discover, develop or commercialize therapies that are similar, more advanced or more effective than ours, which may adversely affect our financial condition and our ability to successfully commercialize our product candidates.
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to our product candidates.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may have an advantage in marketing their approved products and may obtain regulatory approval of their product candidates before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less expensive than ours, and may also be more successful than us in manufacturing and marketing their products. These advantages could materially impact our ability to develop and commercialize our product candidates successfully.
Our product candidates may also compete with medical and recreational marijuana, in markets where the recreational and/or medical use of marijuana is legal. There is support in the United States for further legalization of marijuana. In markets where recreational and/or medical marijuana is not legal, our product candidates may compete with marijuana purchased in the illegal drug market. We cannot assess the extent to which patients may utilize marijuana obtained illegally for the treatment of the indications for which we are developing our product candidates.
Even if we successfully develop our product candidates, and obtain marketing approval for them, other treatments or therapeutics may be preferred and we may not be successful in commercializing our product candidates or in bringing them to market.
Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. As a result, these companies may obtain regulatory approval more rapidly than we are able to and may be more effective in selling and marketing their products as well. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing on an exclusive basis, products that are more effective or less costly than any product candidate that we may develop, or achieve earlier patent protection, regulatory approval, product commercialization and market penetration than we do. Additionally, technologies developed by our competitors may render our potential product candidates uneconomical or obsolete, and we may not be successful in marketing our product candidates against competitors.
The commercial success of any current or future product candidate will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Even with the requisite approvals from the FDA and comparable foreign regulatory authorities, the commercial success of our product candidates will depend in part on the medical community, patients and third-party payors accepting our product candidates as medically useful, cost-effective and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors and others in the medical community. The degree of market acceptance of any of our product candidates, if minor,approved for commercial sale, will depend on a number of factors, including:
| ● | the safety and efficacy of the product as demonstrated in clinical studies and potential advantages over competing treatments; | |
| ● | the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling; | |
| ● | the clinical indications for which approval is granted; | |
| ● | relative convenience and ease of administration; | |
| ● | the cost of treatment, particularly in relation to competing treatments; | |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |
| ● | the strength of marketing and distribution support and timing of market introduction of competitive products; | |
| ● | publicity concerning our products or competing products and treatments; and | |
| ● | sufficient third-party insurance coverage and reimbursement. |
Even if a potential product displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the product will not be fully known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the product candidates may require significant resources and may never be successful. If our product candidates are approved but fail to achieve an adequate level of acceptance by physicians, patients, third-party payors and others in the medical community, we will not be able to generate sufficient revenue to become or remain profitable.
The insurance coverage and reimbursement status of newly-approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
The pricing, coverage and reimbursement of our product candidates, if approved, must be adequate to support our commercial infrastructure. Our per-patient prices must be sufficient to recover our development and manufacturing costs and potentially achieve profitability. Accordingly, the availability and adequacy of coverage and reimbursement by governmental and private payors are essential for most patients to be able to afford expensive treatments such as ours, assuming approval. Sales of our product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of our product candidates will be paid for by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government authorities, private health insurers and other third-party payors. If coverage and reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about coverage and reimbursement for new drugs are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new drug will be covered and reimbursed under Medicare. Private payors tend to follow the coverage reimbursement policies established by CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for products such as ours.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for medicinal products, but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, was passed. The Affordable Care Act is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and the health insurance industry, impose new taxes and fees on the healthcare industry and impose additional health policy reforms. This law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states once the provision is effective. Further, the law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners. While the U.S. Supreme Court upheld the constitutionality of most elements of the Affordable Care Act in 2012, other legal challenges are still pending final adjudication in several jurisdictions. In addition, Congress has also proposed a number of legislative initiatives, including possible repeal of the Affordable Care Act. At this time, it remains unclear whether there will be any changes made to the Affordable Care Act, whether to certain provisions or its entirety. We can provide no assurance that the Affordable Care Act, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. In 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year. In 2013, the 2% Medicare payment reductions went into effect. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
Risks Related to our Business and Operations
We hold no patents on our products, and our business employs proprietary technology (know-how) and information may be difficult to protect and/or infringe on the intellectual property rights of third parties.
Raphael has no patents and therefore, as a result of the Share Exchange, we did not acquire any patents. As such, we currently rely on trade secrets, proprietary know-how, and technology methods that we seek to protect, in part, by confidentiality agreements. We cannot assure you that these agreements will not be breached, that we will have adequate remedies for any breach, or that our trade secrets and proprietary know-how will not otherwise become known or be independently discovered by others. We currently do not hold patents from the United States Patent and Trademark Office, or USPTO.
We cannot assure you that the patents of others will not have an adverse effect on our ability to conduct our business, that any of our trade secrets and applications will be protected, that we will develop additional proprietary technology (know-how) or methods that is defensible against theft or will provide us with competitive advantages or will not be challenged by third parties.
We manage our business through our three executive officers and key consultants and are highly dependent upon our Chief Technology Officer.
Key service providers are serving as our Chief Executive Officer, Chief Financial Officer and Chief Technology Officer and other scientific services consultants. Due to the lean nature of our operations and our current operations which mainly consist of outsourcing services, we are highly dependent upon our Chief Technology Officer. If our Chief Technology Officer, and to a lesser extent, any key consultants, resigns, this could adversely affect our ability to execute our business plan and harm our operating results. We do not currently carry “key person” insurance on the lives of members of management.
We will need to expand our organization and we may experience difficulties in recruiting needed additional employees and consultants, which could disrupt our operations.
As our development and commercialization plans and strategies develop and because we are so leanly staffed, we will need additional managerial, operational, sales, marketing, financial, legal and other resources. The competition for qualified personnel in the pharmaceutical field is intense. Due to this intense competition, we may be unable to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
We may not be successful in our efforts to identify, license or discover additional product candidates.
Although a substantial amount of our effort will focus on the continued clinical testing, potential approval and commercialization of our existing product candidates, the success of our business also depends in part upon our ability to identify, license or discover additional product candidates. Our research programs or licensing efforts may fail to yield additional product candidates for clinical development for a number of reasons, including but not limited to the following:
| ● | our research or business development methodology or search criteria and process may be unsuccessful in identifying potential product candidates; | |
| ● | we may not be able or willing to assemble sufficient resources to acquire or discover additional product candidates; | |
| ● | our product candidates may not succeed in preclinical or clinical testing; | |
| ● | our potential product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval; | |
| ● | competitors may develop alternatives that render our product candidates obsolete or less attractive; | |
| ● | product candidates we develop may be covered by third parties’ patents or other exclusive rights; | |
| ● | the market for a product candidate may change during our program so that such a product may become unreasonable to continue to develop; | |
| ● | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and | |
| ● | a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors. |
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, or we may not be able to identify, license or discover additional product candidates, which would have a material adverse effect on our business and could potentially cause us to cease operations. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we obtain FDA approval for any of our product candidates and begin commercializing those products in the United States, our operations may be directly or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| ● | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs; | |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
| ● | the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; | |
| ● | HIPAA, as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; | |
| ● | the federal physician sunshine requirements under the Affordable Care Act requires manufacturers of drugs, devices and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and | |
| ● | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States or Israel.
Other than our headquarters and other operations which are located in Israel (as further described below), we currently have limited international operations, but our business strategy incorporates potentially significant international expansion, particularly in anticipation of approval of our product candidates. We plan to maintain sales representatives and conduct physician and patient association outreach activities, as well as clinical trials, outside of the United States and Israel. Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; | |
| ● | failure by us to obtain regulatory approvals for the use of our products in various countries; | |
| ● | additional potentially relevant third-party patent rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; |
| ● | difficulties in staffing and managing foreign operations; | |
| ● | complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; | |
| ● | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, or FCPA, its books and records provisions or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
Our research and development activities and our third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials, including the components of our product candidates and other hazardous compounds. We and our manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts, business operations and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. We do not currently carry biological or hazardous waste insurance coverage.
The use of any of our product candidates could result in product liability or similar claims that could be expensive, damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability or similar claims. The pharmaceutical industry has historically been litigious, and we face financial exposure to product liability or similar claims if the use of any of our products were to cause or contribute to injury or death. There is also the possibility that defects in the design or manufacture of any of our products might necessitate a product recall. Although we plan to maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Reliance on Third Parties
We rely on third parties to conduct our preclinical and, in the future, clinical studies and perform other tasks for us. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or comply with regulatory requirements, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We have relied upon and plan to continue to rely upon third-party CROs to monitor and manage data for our ongoing preclinical and clinical programs. We rely on these parties for execution of our preclinical and, in the future, clinical studies, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities. We and our CROs and other vendors will be required to comply with current cGMP, GCP, QSR and Good Laboratory Practices, or GLP, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, and comparable foreign regulatory authorities for any product candidates that move into clinical development. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, study sites and other contractors. If we or any of our CROs or vendors fail to comply with applicable regulations, the clinical data generated in our clinical studies may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical studies before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical studies comply with GCP regulations. In addition, our clinical studies must be conducted with product candidates which are produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical studies, which would delay the regulatory approval process.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. In addition, our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our on-going clinical, nonclinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical studies may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. CROs may also generate higher costs than anticipated. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Switching or adding additional CROs involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which could materially impact our ability to meet our desired clinical development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
We will rely on third parties to grow and provide us with our active pharmaceutical ingredient, or API, and formulations. Our business could be harmed if those third parties fail to provide us with sufficient quantities of our needed supplies, or fail to do so at acceptable quality levels or prices.
We do not have the infrastructure or capability internally to manufacture the API formulations, and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. We plan to rely on third parties for such supplies. In addition, we are reliant on one third party for the CBD that we intend to use in our preclinical studies, which, has caused us to rely on their cooperation to meet their contractual obligations, but there can be no guarantee that we are able to meet such obligations. In addition, there may be a need to identify alternate manufacturers to prevent a possible disruption of our clinical studies. Any significant delay or discontinuity in the supply of these components could considerably delay completion of our preclinical studies, product candidate testing and potential regulatory approval of our product candidates, which could harm our business and results of operations.
We and our collaborators and contract manufacturers are subject to significant regulation with respect to manufacturing our product candidates. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity.
All entities involved in the preparation of therapeutics for clinical studies or commercial sale, including our existing contract manufacturers for our product candidates, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or a product candidate used in late-stage clinical studies must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational product candidates and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our product candidates that may not be detectable in final product testing. We, our collaborators or our contract manufacturers must supply all necessary documentation in support of an NDA, or Marketing Authorization Application, or MAA, on a timely basis and must adhere to GLP and cGMP QSR regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. Some of our contract manufacturers have never produced a commercially approved pharmaceutical product and therefore have not obtained the requisite regulatory authority approvals to do so. The facilities and quality systems of some or all of our collaborators and third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our product candidates or any of our other potential product candidates. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our product candidates or our other potential product candidates or the associated quality systems for compliance with the regulations applicable to the activities being conducted. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the regulatory requirements. If these facilities do not pass a pre-approval plant inspection, regulatory approval of the product candidates may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever.
The regulatory authorities also may, at any time following approval of a product candidate for sale, if ever, audit the manufacturing facilities of our collaborators and third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product candidate specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us or a third party to implement, and that may include the temporary or permanent suspension of a clinical study or commercial sales, or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
If we, our collaborators, or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA or other applicable regulatory authority can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new drug product, withdrawal of an approval or suspension of production. As a result, our business, financial condition and results of operations may be materially harmed.
Additionally, if supply from one approved manufacturer is interrupted, an alternative manufacturer would need to be qualified through an NDA or MAA amendment, or equivalent foreign regulatory filing, which could result in further delay. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause us to incur higher costs and, if we commence clinical development, could cause the delay or termination of clinical studies, regulatory submissions, required approvals or commercialization of our product candidates. Furthermore, if our suppliers fail to meet contractual requirements and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical studies may be delayed or we could lose potential revenue.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to develop and manufacture our product candidates, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business.
Risks Related to Ownership of Our Securities
Our executive officer, directors and certain stockholders who are beneficial owners of 5% or more of our outstanding Common Stock possess the majority of our voting power, and through this ownership, have the ability to control our Company and our corporate actions.
Following the Share Exchange, our current executive officer, directors and certain stockholders who are beneficial owners of 5% or more of our outstanding Common Stock hold approximately 53% of the issued and outstanding voting power of the Company’s outstanding shares. These persons have a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions. As such, our directors and executive officer may have the power, acting alone or together, to prevent or cause a change in control; therefore, without their consent we could be prevented from entering into transactions that could be beneficial to us. The interests of our executive officer may give rise to a conflict of interest with the Company and the Company’s shareholders.
In addition, we have a number of stockholders who are beneficial owners of more than 5% of our outstanding Common Stock, as of June 28, 2022, including our Chief Executive Officer who beneficially owns approximately 26% of our issued and outstanding shares, and as such, also may have the ability to prevent us from entering into transactions that could be beneficial to us and/or other shareholders. In addition, we have three additional non-affiliated stockholders who are beneficial owners of more than 5% of our outstanding Common Stock. Although none of these non-affiliated stockholders currently have a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions, obtaining their vote on certain matters may be necessary to effect certain actions that our management and directors otherwise deem to be in the best interests of the Company.
For additional details concerning beneficial ownership of our securities, please refer to the section below entitled “Beneficial Ownership of the Company’s Common Stock” and with respect to voting power, please refer to the section below entitled “Description of Securities.”
Investors may have difficulty in reselling their shares due to the lack of market or state Blue Sky laws.
Our Common Stock is not currently traded on any national securities exchange and is not quoted on any over-the-counter market. No market may ever develop for our Common Stock, or if developed, may not be sustained in the future. The holders of our shares of Common Stock and persons who desire to purchase them in any trading market that might develop in the future should be aware that there might be significant state law restrictions upon the ability of investors to resell our shares. Accordingly, even if we are successful in having the shares available for trading on the over-the-counter markets, investors should consider any secondary market for our Common Stock to be a limited one.
We may seek coverage and publication of information regarding our Company in an accepted publication, which permits a “manual exemption.” This manual exemption permits a security to be distributed in a particular state without being registered if the company issuing the security has a listing for that security in a securities manual recognized by the state. However, it is not enough for the security to be listed in a recognized manual. The listing entry must contain (1) the names of issuers, officers, and directors, (2) an issuer’s balance sheet, and (3) a profit and loss statement for either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. We may not be able to secure a listing containing all of this information. Furthermore, the manual exemption is a non-issuer exemption restricted to secondary trading transactions, making it unavailable for issuers selling newly issued securities. Most of the accepted manuals are those published in Standard and Poor’s, Moody’s Investor Service, Fitch’s Investment Service, and Best’s Insurance Reports, and many states expressly recognize these manuals. A smaller number of states declare that they “recognize securities manuals” but do not specify the recognized manuals. Accordingly, our Common Stock should be considered totally illiquid, which inhibits investors’ ability to resell their shares
Our Common Stock may never be listed on a major stock exchange.
While we may seek the listing of our Common Stock on a national or other securities exchange at some time in the future, we currently do not satisfy the initial listing standards and cannot ensure that we will be able to satisfy such listing standards or that our Common Stock will be accepted for listing on any such exchange. Should we fail to satisfy the initial listing standards of such exchanges, or our Common Stock is otherwise rejected for listing, the trading price of our common stock.
TRADING OF OUR STOCK MAY BE RESTRICTED BY THE SECURITIES AND EXCHANGE
COMMISSION'S PENNY STOCK REGULATIONS, WHICH MAY LIMIT A STOCKHOLDER'S ABILITY TO
BUY AND SELL OUR STOCK.
Common Stock could suffer, the trading market for our Common Stock may be less liquid, and our Common Stock price may be subject to increased volatility.
As a former shell company, resales of shares of our restricted Common Stock in reliance on Rule 144 of the Securities Act are subject to the requirements of Rule 144(i).
We previously were a “shell company” and, as such, sales of our securities pursuant to Rule 144 under the Securities Act of 1933, as amended, cannot be made unless, among other things, at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 as amended, as applicable, during the preceding 12 months, other than Form 8-K reports. Because, as a former shell company, the reporting requirements of Rule 144(i) will apply regardless of holding period, restrictive legends on certificates for shares of our Common Stock cannot be removed except in connection with an actual sale that is subject to an effective registration statement under, or an applicable exemption from the registration requirements of, the Securities Act of 1933, as amended. Because our unregistered securities cannot be sold pursuant to Rule 144 unless we continue to meet such requirements, any unregistered securities we issue will have limited liquidity unless we continue to comply with such requirements.
The securities issued in connection with the Share Exchange are restricted securities and may not be transferred in the absence of registration or the availability of a resale exemption.
The shares of Common Stock being issued in connection with the Share Exchange are being issued in reliance on an exemption from the registration requirements under Section 4(a)(2) of the Securities Act. Consequently, these securities will be subject to restrictions on transfer under the Securities Act and may not be transferred in the absence of registration or the availability of a resale exemption. In particular, in the absence of registration, such securities cannot be resold to the public until certain requirements under Rule 144 promulgated under the Securities Act have been satisfied, including certain holding period requirements. As a result, a purchaser who receives any such securities issued in connection with the Share Exchange Commissionmay be unable to sell such securities at the time or at the price or upon such other terms and conditions as the purchaser desires, and the terms of such sale may be less favorable to the purchaser than might be obtainable in the absence of such limitations and restrictions.
We do not plan to declare or pay any dividends to our stockholders in the near future.
We have not declared any dividends in the past, and we do not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors and will depend upon, among other things, the results of operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend.
The requirements of being a reporting company may strain our resources and distract management.
As a reporting company, we are subject to the reporting requirements of the Exchange Act and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). These requirements are extensive. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting.
We may incur significant costs associated with our public company reporting requirements and costs associated with applicable corporate governance requirements. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. This may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition and results of operations. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
If in the future there is a trading market for our Common Stock, “penny stock” rules may make buying or selling our Common Stock difficult.
The SEC has adopted regulations whichthat generally define "penny stock"a penny stock to be any equity security that has a market price (as
defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. Our securities are covered by the penny
stockThese rules which impose additional sales practice requirements on
broker-dealers who sellrequire that any broker-dealer that recommends our Common Stock to persons other than establishedprior customers and "accredited investors". The term "accredited investor" refers generally to
institutions with assets in excess of $5,000,000 or individuals with a net worth
in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 jointly
with their spouse. The penny stock rules require a broker-dealer,accredited investors, must, prior to a
transaction in a penny stock not otherwise exempt from the rules, to deliver a
standardized risk disclosure document in a form prepared by the Securities and
Exchange Commission, which provides information about penny stocks and the
nature and level of risks in the penny stock market. The broker-dealer also must
provide the customer with current bid and offer quotations for the penny stock,
the compensation of the broker-dealer and its salesperson in the transaction and
monthly account statements showing the market value of each penny stock held in
the customer's account. The bid and offer quotations, and the broker-dealer and
salesperson compensation information, must be given to the customer orally or in
writing prior to effecting the transaction and must be given to the customer in
writing before or with the customer's confirmation. In addition, the penny stock
rules require that prior to a transaction in a penny stock not otherwise exempt
from these rules, the broker-dealer mustsale, make a special written suitability determination
that the penny stock is a suitable investment for the purchaser and receive the purchaser'spurchaser’s written agreement to execute the transaction. These disclosure requirements
may haveUnless an exception is available, the effect of reducingregulations require the level of trading activity in the secondary
market for the stock that is subjectdelivery, prior to theseany transaction involving a penny stock, rules. Consequently,
these penny stock rules may affect the ability of broker-dealers to trade our
securities. We believe thata disclosure schedule explaining the penny stock rulesmarket and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage investor interestbroker-dealers from effecting transactions in andour Common Stock, which could severely limit the marketabilitymarket price and liquidity of our commonCommon Stock.
The sales practice requirements of the Financial Industry Regulatory Authority, or FINRA, may also limit a stockholder’s ability to buy and sell our stock.
NASD SALES PRACTICE REQUIREMENTS MAY ALSO LIMIT A STOCKHOLDER'S ABILITY TO BUY
AND SELL OUR STOCK.
In addition to the "penny stock"“penny stock” rules described above, the National Association
of Securities Dealers (NASD)FINRA has adopted rulesRule 2111 that require that in recommending
an investment to a customer,requires a broker-dealer mustto have reasonable grounds for believing that thean investment is suitable for that customer.a customer before recommending the investment. Prior to recommending speculative low pricedlow-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer'scustomer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the NASDFINRA believes that there is a high probability that speculative low pricedlow-priced securities will not be suitable for at least some customers. The NASDFINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common
stock,the Company’s Common Stock, which may limit your ability to buy and sell ourthe Company’s stock and have an adverse effect on the market for our shares.
OUR COMMON STOCK IS ILLIQUID AND THE PRICE OF OUR COMMON STOCK MAY BE NEGATIVELY
IMPACTED BY FACTORS WHICH ARE UNRELATED TO OUR OPERATIONS.
Our common stock
We have not yet determined whether our existing internal controls over financial reporting are in compliance with Section 404 of the Sarbanes-Oxley Act.
We are not currently trades onrequired to comply with the rules of the SEC implementing Section 404 of the Sarbanes-Oxley Act (“Section 404”) and therefore are not required to make a limited basisformal assessment of the effectiveness of our internal control over financial reporting for that purpose. Compliance with the SEC’s rules implementing Sections 302 and 404 of the Sarbanes-Oxley Act, which will require management to certify financial and other information in our annual reports and provide an annual management report on the OTC Bulletin Board.
Tradingeffectiveness of control over financial reporting. Though we will be required to disclose material changes in internal control over financial reporting on an annual basis, we will not be required to make our first annual assessment of our stockinternal control over financial reporting pursuant to Section 404 until the year following our first annual report required to be filed with the SEC. Additionally, as we do not qualify as an emerging growth company, we are required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. We have begun the OTC Bulletin Boardprocess of evaluating the adequacy of our accounting personnel staffing level and other matters related to our internal control over financial reporting. Despite our efforts, there is frequently thin and
highly volatile. Therea risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is no assurance thateffective as required by Section 404. If we identify one or more material weaknesses once we are a sufficient market will developpublic company, it could result in an adverse reaction in the stock,financial markets due to a loss of confidence in which case it could be difficult for shareholders to sell their
stock. Thethe reliability of our financial statements. As a result, the market price of our commonCommon Stock could be negatively affected, and we could become subject to investigations by the stock exchange on which our securities are listed, the SEC or other regulatory authorities, which could fluctuate substantiallyrequire additional financial and management resources.
Risks Related to our Operations in Israel
Our headquarters and other significant operations are located in Israel, and, therefore, our results may be adversely affected by political, economic and military instability in Israel.
Our executive offices are located in Tel Aviv-Jaffa, Israel. In addition, the majority of our officers and directors are residents of Israel. If these or any future facilities in Israel were to be damaged, destroyed or otherwise unable to operate, whether due to a varietywar, acts of factors, including market perceptionhostility, earthquakes, fire, floods, hurricanes, storms, tornadoes, other natural disasters, employee malfeasance, terrorist acts, power outages or otherwise, or if performance of our research and development is disrupted for any other reason, such an event could delay our clinical trials or, if our product candidates are approved and we choose to manufacture all or any part of them internally, jeopardize our ability to achievemanufacture our planned growth, quarterly operatingproducts as promptly as our prospective customers will likely expect, or possibly at all. If we experience delays in achieving our development objectives, or if we are unable to manufacture an approved product within a timeframe that meets our prospective customers’ expectations, our business, prospects, financial results and reputation could be harmed.
Political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and groups in its neighboring countries, Hamas (an Islamist militia and political group that has historically controlled the Gaza Strip) and Hezbollah (an Islamist militia and political group based in Lebanon). In addition, several countries, principally in the Middle East, restrict doing business with Israel, and additional countries may impose restrictions on doing business with Israel and Israeli companies whether as a result of hostilities in the region or otherwise. Any hostilities involving Israel, terrorist activities, political instability or violence in the region or the interruption or curtailment of trade or transport between Israel and its trading partners could adversely affect our operations and results of operations and the market price of our competitors, trading volumeordinary shares.
Our commercial insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East. Although the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or, if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our common stock,business, financial condition and results of operations.
Our operations are subject to currency and interest rate fluctuations.
Although our functional currency is the U.S. dollar, and our financial records are maintained in U.S. dollars, we also incur expenses in Euros and New Israeli Shekels. In the future, we expect that a substantial portion of our revenues will be generated in U.S. dollars, Euros and other foreign currencies, although we currently incur a significant portion of our expenses in currencies other than U.S. dollars, mainly New Israeli Shekels. As a result, we are affected by foreign currency exchange fluctuations through both translation risk and transaction risk, and our financial results may be affected by fluctuations in the exchange rates of currencies in the countries in which our prospective product candidates may be sold. We currently partially hedge our foreign currency exchange rate risk to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies.
It may be difficult to enforce a judgment of a U.S. court against us and our executive officers and directors and the Israeli experts named in this prospectus in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our executive officers and directors and these experts.
We were incorporated in Israel. Substantially all of our executive officers and directors reside outside of the United States, and all of our assets and most of the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court. See “Enforceability of Civil Liabilities” for additional information on your ability to enforce a civil claim against us and our executive officers or directors named in this prospectus.
General Risk Factors
Future changes in general conditionsfinancial accounting standards or practices may cause adverse unexpected financial reporting fluctuations and affect reported results of operations.
A change in the economy and the
financial marketsaccounting standards or other developments affecting our competitors or us. In
addition, the stock market is subject to extreme price and volume fluctuations.
This volatility has hadpractices can have a significant effect on our reported results and may even affect our reporting of transactions completed before the market pricechange is effective. New accounting pronouncements and varying interpretations of securities
issued by many companies for reasons unrelatedaccounting pronouncements have occurred and may occur in the future. Changes to their operating performanceexisting rules or the questioning of current practices may adversely affect our reported financial results or the way we conduct business.
Raising additional capital would cause dilution to our existing shareholders, and could havemay affect the same effect on our common stock.
WE DO NOT INTEND TO PAY DIVIDENDS ON ANY INVESTMENT IN THE SHARES OF STOCK OF
OUR COMPANY AND THERE WILL BE FEWER WAYS FOR INVESTORS TO MAKE A GAIN ON ANY
INVESTMENT IN OUR COMPANY.
rights of existing shareholders.
We have never paid any cash dividendsmay seek additional capital through a combination of private and currently do not intend to pay any
dividends for the foreseeable future.public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we requireraise additional funding currently not provided forcapital through the issuance of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a shareholder.
Failure in our financing plan,information technology systems, including by cybersecurity attacks or other data security incidents, could significantly disrupt our funding sources
may prohibitoperations.
Our operations depend, in part, on the paymentcontinued performance of our information technology systems. Our information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. Failure of our information technology systems could adversely affect our business, profitability and financial condition. Although we have information technology security systems, a successful cybersecurity attack or other data security incident could result in the misappropriation and/or loss of confidential or personal information, create system interruptions, or deploy malicious software that attacks our systems. It is possible that a cybersecurity attack might not be noticed for some period of time. The occurrence of a dividend. Because we do not intendcybersecurity attack or incident could result in business interruptions from the disruption of our information technology systems, or negative publicity resulting in reputational damage with our shareholders and other stakeholders and/or increased costs to declare
dividends, any gain on an investmentprevent, respond to or mitigate cybersecurity events. In addition, the unauthorized dissemination of sensitive personal information or proprietary or confidential information could expose us or other third parties to regulatory fines or penalties, litigation and potential liability, or otherwise harm our business.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their shares have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
The requirements associated with being a reporting company will require significant company resources and management attention.
We are subject to the reporting requirements of the Exchange Act. The Exchange Act requires that we file periodic reports with respect to our business and financial condition and maintain effective disclosure controls and procedures and internal control over financial reporting. In addition, subsequent rules implemented by the SEC may also impose various additional requirements on reporting companies. We estimate that these expenses will be at least several tens of thousand dollars annually. Further, the need to come through an
increaseestablish the corporate infrastructure demanded of a reporting company may divert management’s attention from implementing our development plans. We have made changes to our corporate governance standards, disclosure controls and financial reporting and accounting systems to meet our reporting obligations. The measures we take, however, may not be sufficient to satisfy our obligations as a public company, which could subject us to fines, sanctions and other regulatory action and potentially civil litigation.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The Company makes forward-looking statements in this prospectus, including in the stock's price. Thisstatements incorporated herein by reference. Forward-looking statements provide our current expectations or forecasts of future events. Forward-looking statements include statements about our expectations, beliefs, plans, objectives, intentions, assumptions and other statements that are not historical facts. Words or phrases such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “will” or similar words or phrases, or the negatives of those words or phrases, may never happen and investors may lose all
of their investment in our company.
8
PLEASE READ THIS PROSPECTUS CAREFULLY. YOU SHOULD RELY ONLY ON THE INFORMATION
CONTAINED IN THIS PROSPECTUS. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH
DIFFERENT INFORMATION. YOU SHOULD NOT ASSUME THAT THE INFORMATION PROVIDED BY
THE PROSPECTUS IS ACCURATE AS OF ANY DATE OTHER THAN THE DATE ON THE FRONT OF
THIS PROSPECTUS.
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements which relate to future
events or our future financial performance. In some cases, you can identify forward-looking statements, by terminology such as "may", "will" ,"intend"
"should", "expects", "plans", "anticipates", "believes", "estimates",
"predicts", "potential" or "continue" orbut the negativeabsence of these terms or other
comparable terminology. words does not necessarily mean that a statement is not forward-looking. Examples of forward-looking statements in this prospectus include, but are not limited to, statements regarding our disclosure concerning our operations, cash flows and financial position.
Forward-looking statements appear in a number of places in this prospectus including, without limitation, in the sections entitled “Management’s Discussion and Analysis of Financial Conditions and Results of Operations,” and “Business.” The risks and uncertainties include, but are not limited to:
| ● | the regulatory pathways that we may elect to utilize in seeking U.S. Food and Drug Administration, or FDA, European Medicines Agency, or EMA, and other regulatory approvals, if any; |
| ● | obtaining (and the cost thereof) FDA and EMA approval of, or other regulatory action in Europe or the United States and elsewhere with respect to our product candidates; |
| ● | the commercial launch and future sales of our product candidates and our advancement of product candidates for other indications in our pipeline; |
| ● | the potential cost of our rheumatoid arthritis product candidate, or RA and RA product candidate, respectively, for patients; |
| ● | our expectations regarding the timing of commencing clinical trials; |
| ● | our expectations regarding the supply of the active pharmaceutical ingredient for our product candidates; |
| ● | third-party payor reimbursement for our product candidates; |
| ● | our estimates regarding anticipated expenses, capital requirements and our needs for additional financing; |
| ● | completion and receiving favorable results of clinical trials for our product candidates; |
| ● | the filing by us, and the subsequent issuance of patents to us, by the U.S. Patent and Trademark Office and other governmental patent agencies; |
| ● | our expectations regarding the impact of the COVID-19 pandemic, including on our planned clinical trials, operations and financial position; and |
These statements are only current predictions and involveare subject to known and unknown risks, uncertainties and other factors including the risks in the
section entitled "Risk Factors" beginning on page 3, that may cause our or our industry'sindustry’s actual results, levels of activity, performance or achievements to be materially different from anythose anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the section entitled “Risk Factors” and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements expressed or implied by these forward-looking statements.
While these forward-looking statements, and any assumptions upon which they are
based, are made in good faith and reflect our current judgment regarding the
direction of our business, actual results will almost always vary, sometimes
materially, from any estimates, predictions, projections, assumptions or other
future performance suggested herein.achievements. Except as required by applicable law, including the securities laws of the United States, we do not intendare under no duty to update or revise any of the forward-looking statements, to conform these statements to actual
results. The safe harbour for forward-looking statements provided inwhether as a result of new information, future events or otherwise, after the Private
Securities Litigation Reform Actdate of 1995 does not apply to the offering made in
this prospectus.
USE OF PROCEEDS
All of the shares of Common Stock offered by the Selling Securityholders pursuant to this prospectus will be sold by the Selling Securityholders for their respective accounts. We will not receive any of the proceeds from these sales. All proceeds from the sale of the sharesCommon Stock will be paid directly to the Selling Securityholders.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our common stock being offeredfinancial condition and results of operations should be read in conjunction with the consolidated financial statements and the related notes included elsewhere herein and in our consolidated financial statements.
In addition to our consolidated financial statements, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. See “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors” for sale bya discussion of the selling stockholders, althoughuncertainties, risks and assumptions associated with these statements.
Overview
We are a pharmaceutical drug research and development company focused on the discovery and clinical development of life-improving drug therapies based on cannabinoids, including CBD oil. Unless indicated otherwise, we plan on using oil derived from CBD strains with low levels of THC. All references to the use of CBD in our product candidates refer to CBD strains with less than 0.3% of THC.
We are currently in the pre-clinical development stage for our lead product candidate, our RA product candidate for the treatment of RA. In addition, we are aiming to develop our COVID-19 product candidate, which may be based on data or studies related to our RA product candidate. Our goal is to become a leader in development of CBD oil-based pharmaceutical drug products for the treatment of indications in which we believe there is a high unmet medical need in a range of disorders, including those related to inflammation in the body, including RA and COVID-19.
In order to achieve our goal, we have and will continue to build an experienced team of senior executives and scientists, with experience in all facets of pharmaceutical research and development, drug formulation, clinical trial execution and regulatory submissions. We intend to leverage the knowledge of our team in order to complete the clinical trials needed to receive proceedsapprovals of upour product candidates from applicable regulatory authorities.
Initially, we intend to $3,937,949obtain approvals for our product candidates from the FDA and MOH. Upon obtaining FDA approvals, or in the event that we are not successful in obtaining such approvals, we intend to apply for EMA and other countries’ governmental regulatory agencies approvals for our product candidates. If we are successful in obtaining FDA approvals for our product candidates, we intend to enter into royalty agreements with GMP approved medical manufactures and distributors, having them using our medical formulas strains for the purpose of growing, cultivating, manufacturing, and distributing Raphael Pharmaceutical medical indications in their designated territories.
Components of Operating Results
Operating Expenses
Our current operating expenses consist of two components – research and development expenses, and general and administrative expenses. To date, we have not generated any revenues. We do not expect to receive any revenue from our product candidate unless and until we obtain regulatory approval and commercialize our product candidate or enter into agreements with third parties to commercialize them. There can be no assurance that we will receive such regulatory approvals, and if our product candidate is approved, that we will be successful in commercializing them.
Research and Development Expenses
Research and development activities are our primary focus. Products in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will increase as we prepare for, and commence, registrational clinical trials of our RA and COVID-19 product candidates. A key activity in progressing our product candidates toward registrational trials is the development of large-scale manufacturing processes that are tailored specifically to our product candidate. In order to confirm the suitability of a new manufacturing facility and/or process, numerous experiments are needed. Moreover, the regulatory requirements in preparation for manufacturing a drug to be used in a registrational trial or for commercial use involve validation activities and extensive updates to our regulatory files, all of which are lengthy and costly activities. For these reasons, the warrantsdevelopment of manufacturing processes currently represents the largest portion of our research and development expenses. Research and development expenses include, but are exercised. not limited to, the following:
| ● | employee-related expenses, such as salaries and share-based compensation; |
| ● | expenses of developing manufacturing processes; |
| ● | expenses relating to outsourced and contracted services, such as external laboratories and consulting and advisory services; |
| ● | costs associated with pre-clinical activities; |
| ● | patent application and maintenance expenses; |
| ● | expenses incurred in operating our laboratories and small-scale equipment; and |
| ● | clinical development expenses. |
General and Administrative Expenses
General and administrative expenses consist primarily of employee related expenses, including salaries, benefits, and equity-based compensation, for personnel in executive, finance, accounting, business development and human resources functions. Other significant costs include facility costs not otherwise included in research and development expenses, legal fees relating to patent and corporate matters, and fees for accounting and consulting services.
We expectanticipate that our general and administrative expenses will increase in the future to usesupport continued research and development activities, potential commercialization of our product candidates and increased costs of operating as a public company. These increases will likely include increased costs related to the proceeds received fromhiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. We also anticipate increased expenses related to the exercisereimbursements of the warrants, if any,
for general working capital purposes. We will incur all costs associated with
this registration statement and prospectus. Our company estimates that the total
costs that will be incurred by our companythird-party patent related expenses in connection with the ongoing interference proceeding with respect to certain of our in-licensed intellectual property. Additionally, we anticipate increased costs associated with being a public company, including expenses related to services associated with maintaining compliance with SEC requirements and insurance costs.
Comparison of the Year Ended December 31, 2021 to the Year Ended December 31, 2020
Results of Operations
| For the Year Ended December 31, | For the Year Ended December 31, | |||||||
| 2021 | 2020 | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | $ | (776,000 | ) | $ | (461,000 | ) | ||
| General and administrative expenses | (853,000 | ) | (245,000 | ) | ||||
| Operating loss | (1,629,000 | ) | (706,000 | ) | ||||
| Financial Income (expense), net | 4,000 | (12,000 | ) | |||||
| Net loss and comprehensive loss | $ | (1,625,000 | ) | $ | (718,000 | ) | ||
| Basic and diluted net loss per share | $ | (0.15 | ) | $ | (0.07 | ) | ||
| Weighted average number of shares of ordinary shares used in computing basic and diluted net loss per share | 11,171,704 | 9,459,253 | ||||||
Research and Development Expenses
Our research and development expenses totaled $776,000 for the year ended December 31, 2021, representing an increase of $315,000, or 68%, compared to $461,000 for the year ended December 31, 2020. The increase was primarily attributable to the increase in our expenses to our consultants and service providers, which resulted from the increase in research activity, mainly with Rambam Hospital.
General and Administrative Expenses
Our general and administrative expenses totaled $853,000 for the year ended December 31, 2021, representing an increase of $608,000, or 248%, compared to $245,000 for the year ended December 31, 2020. The increase was primarily attributable to increase in our fees for our consultants and professional services which was as a result of increase in our activity and being a public Company.
Operating Loss
As a result of the foregoing, our operating loss totaled $1,629,000 for the year ended December 31, 2021, representing an increase of $923,000, or 130%, compared to $706,000 for the year ended December 31, 2020.
Financial income (expense), net
We recognized financing income, net of $4,000 for the year ended December 31, 2021, representing a decrease of $16,000, or 133%, compared to finance expenses of $12,000 for the year ended December 31, 2020. The decrease was primarily attributable to exchange rate differences between the U.S. dollar and the New Israeli Shekel.
Net and Comprehensive Loss
As a result of the foregoing, our loss totaled $1,625,000 for the year ended December 31, 2021, representing an increase of $907,000, or 126%, compared to $718,000 for the year ended December 31, 2020.
Comparison of the Three Months Ended March 31, 2022 to the Three Months Ended March 31, 2021
Results of Operations
| For the Three Months Ended March 31, | For the Three Months Ended March 31, | |||||||
| 2022 | 2021 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | $ | (86,000 | ) | $ | (169,000 | ) | ||
| General and administrative expenses | (89,000 | ) | (83,000 | ) | ||||
| Operating loss | (175,000 | ) | (252,000 | ) | ||||
| Financial expense, net | (10,000 | ) | (3,000 | ) | ||||
| Net loss and comprehensive loss | $ | (185,000 | ) | $ | (255,000 | ) | ||
| Basic and diluted net loss per share | $ | (0.01 | ) | $ | (0.02 | ) | ||
| Weighted average number of shares of ordinary shares used in computing basic and diluted net loss per share | 13,035,371 | 9,459,253 | ||||||
Research and Development Expenses
Our research and development expenses totaled $86,000 for the three months ended March 31, 2022, representing a decrease of $83,000, or 49%, compared to $169,000 for the three months ended March 31, 2021. The decrease was primarily attributable to the decrease in pre-clinical trial expenses.
General and Administrative Expenses
Our general and administrative expenses totaled $89,000 for the three months ended March 31, 2022, representing a increase of $6,000, or 7%, compared to $83,000 for the three months ended March 31, 2021. The increase was primarily due to lower professional services expenses in 2021.
Operating Loss
Our operating loss totaled $175,000 for the three months ended March 31, 2022, representing a decrease of $77,000, or 30%, compared to $252,000 for the three months ended March 31, 2021.
Financial expense
We recognized financial expense of $10,000 for the three months ended March 31, 2022, representing an increase of $7,000, or 233%, compared to $3,000 for the three months ended March 31, 2021. The increase was primarily due to an increase in exchange rate differences.
Net Loss
As a result of the foregoing, our loss totaled $185,000 for the three months ended March 31, 2022, representing a decrease of $70,000, or 27%, compared to $255,000 for the three months ended March 31, 2021.
Critical Accounting Estimates
Our consolidated financial statements are prepared in accordance with US GAAP. There are no critical accounting estimates for the years ended December 31, 2021 and 2020.
Liquidity and Capital Resources
The Company has funded its operations to date primarily through equity financing and the issuance of a loan. Additional funding will be required to complete the Company’s research and development and clinical trials, to attain regulatory approvals, to begin the commercialization efforts of the Company’s product and to achieve a level of sales adequate to support the Company’s cost structure. As of December 31, 2021, we had $0.15 million in cash and cash equivalents, and have invested most of our available cash funds in ongoing cash accounts.
Overview
The table below presents our cash flows for the periods indicated:
| For the Year Ended December 31, | For the Year Ended December 31, | For the Three Months Ended March 31, | For the Three Months Ended March 31, | |||||||||||||
| 2021 | 2020 | 2022 | 2021 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Cash used in operating activities | $ | (1,419,000 | ) | $ | (779,000 | ) | (82,000 | ) | (129,000 | ) | ||||||
| Cash provided by financing activities | $ | 1,477,000 | $ | 824,000 | 10,000 | 44,000 | ||||||||||
| Net increase (decrease) in cash and cash equivalents | $ | 45,000 | $ | 45,000 | (72,000 | ) | (85,000 | ) | ||||||||
Net cash used in operating activities was $1,419,000 for the year ended December 31, 2021, compared with net cash used in operating activities of $779,000 for the year ended December 31, 2020. The $640,000 increase in the net cash used in operating activities during 2021, compared to 2020, was primarily the result of $907,000 increase in net loss which was offset by an increase of $234,000 in other account payables and accrued expenses which derived mainly from the increase in Company’s activity.
Net cash used in operating activities was $82,000 for the three months period ended March 31, 2022, compared with net cash used in operating activities of $129,000 for the same period in 2021. The $47,000 decrease in the net cash used in operating activities during 2022, compared to 2021, was primarily the result of $70,000 decrease in net loss.
Net cash used in investing activities for the year ended December 31, 2021 and for the year ended December 31, 2020 was zero.
Net cash used in investing activities for the three months period ended March 31, 2022 and for the same period in 2021 was zero.
Net cash provided by financing activities for the year ended December 31, 2021 was $1,477,000 compared to $824,000 for the year ended December 31, 2020. The increase of $653,000 in net cash provided by financing activities during 2021 compared to 2020 was mainly due to increase of $823,000 in issuance of shares of Common Stock which was offset by a decrease of $183,000 in receipt of a loan.
Net cash provided by financing activities for the three months period ended March 31, 2022 was $10,000 compared to $44,000 for the same period in 2021. The decrease in net cash provided by financing activities during 2022 compared to 2021 was mainly due to increase in proceeds from issuance of Common Stock at the amount of $10,000 which was offset by a receipt of a loan in 2021 at the amount of $44,000.
Current Outlook
We have financed our operations to date primarily through proceeds from founder’s capital and issuance of shares and warrants. We have incurred losses and generated negative cash flows from operations since inception. To date we have not generated revenue, and we do not expect to generate significant revenues from the sale of our products in the near future.
We do not believe that our current cash on hand will be sufficient to fund our projected operating requirements. This raises substantial doubt about our ability to continue as a going concern. At this time, there is no guarantee that we will be able to obtain an adequate level of financial resources required for the short and long-term support of our operations or that we will be able to obtain additional financing as needed, or meet the conditions of such financing, or that the costs of such financing may not be prohibitive. These conditions raise substantial doubt about our ability to continue as a going concern for a period within one year from the date of the financial statements included elsewhere in this prospectus.
As of December 31, 2021, our cash and cash equivalents were $0.15 million. We believe that our existing cash and cash equivalents will be sufficient to fund our projected cash requirements through June 2022. Therefore, we will require significant additional financing in the near future to fund our operations. We currently anticipate that we will require approximately $0.6 million for research and development activities over the course of the next 12 months. We also anticipate that we will require approximately $1.4 million for capital expenditures over such 12-month period, which consists primarily of expenditures for pre-clinical trials, mice model trials and clinical trials and general Company operating costs.
In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned. Our future capital requirements will depend on many factors, including:
| ● | our research and development efforts, including our ability to finish research and development projects or product development within the allotted or expected timeline; |
| ● | the cost, timing and outcomes of seeking to commercialize our products in a timely manner; |
| ● | our ability to generate cash flows; |
| ● | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| ● | government regulation in our industry, and more specifically, the costs and timing of obtaining regulatory approval or permits to launch our technology in various geographical markets; and |
| ● | the costs of, and timing for, strengthening our manufacturing agreements for production of our wave energy systems. |
In addition to the foregoing, based on our current assessment, we do not expect any material impact on our long-term liquidity due to the COVID-19 pandemic. However, we will continue to assess the effect of the pandemic to our operations. The extent to which the COVID-19 pandemic will impact our business and operations will depend on future developments that are highly uncertain and cannot be predicted with confidence, such as the duration of the COVID-19 pandemic, any restrictions on the ability of hospitals and trial sites to conduct trials that are not designed to address the COVID-19 pandemic and the perceived effectiveness of actions taken in Israel, the United States and Europe and other countries to contain and treat the disease. While the potential economic impact brought by COVID-19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing our ability to access capital in the future. In addition, a recession or long-term market correction resulting from the spread of COVID-19 could materially affect our business and the value of our Common Stock.
Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through our existing cash, cash equivalents and short-term deposits, loans, or debt or equity financings. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate research or development plans for, or commercialization efforts with respect to, one or more applications of our products. This may raise substantial doubts about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
Rambam Research Agreement
On July 17, 2019, we entered into a sponsored research agreement, or the Research Agreement, with Rambam MT, pursuant to which the Company agreed to fund a research project, to be performed by Rambam MD, with a research plan aimed at identifying the effects of different cannabis strains on the function of immune cells. On October 28, 2020, the Company and Rambam MT agreed to expand the research plan to study the anti-inflammatory activities of cannabis extracts in an RA mouse model. On February 15, 2021, the Company and Rambam MT agreed to further expand the research plan to study the effect of cannabis extracts on the immunopathology of the COVID-19 disease. The Sponsored Researched Agreement is for an initial term of 48 months.
Pursuant to the Research Agreement, we agreed to pay Rambam $1.4 million in four equal payments, due on the first day of August on each successive year from 2019 through 2022. Furthermore, in accordance with the terms of the Research Agreement, we and Rambam MT will have joint ownership of any IP created as a result of research programs covered by such agreement. In connection with the Research Agreement, Rambam MT agreed not to work, study or develop any technologies with other entities that compete with our work with Rambam MT for our COVID-19 product candidate or RA product candidate for a term of three and seven years, respectively, from the end of the parties’ collaboration with respect to the COVID-19 product candidate and seven years from the end of the term of the Research Agreement with respect to the RA product candidate.
Subject to commercial sales of any product candidate using the intellectual property created as a part of the research covered by such agreement, Raphael Israel is required to pay Rambam MT a royalty in an amount equal to 6% of all net sales, subject to certain deductions, such as taxes paid by any purchaser, transportation and shipping costs, and other customary deductions.
As of June 28, 2022, the Company has made three of the four equal payments due pursuant to the Research Agreement, for a total amount of $1,050,000.
Way of Life Cannabis Agreement
In October 2020, Raphael Israel entered into an engagement agreement with Wolc, pursuant to which, subject to its completing the Share Exchange with Easy Energy, Raphael Israel will be provided with up to 15 liters of CBD oil, from a strain of cannabis during a term of 18 months, to be provided in two to three deliveries of between one to seven liters of CBD oil. In accordance with Raphael Israel’s agreement with Wolc, Raphael Israel has agreed to issue to certain persons affiliated with Wolc 3% of Raphael’s issued and outstanding share capital as of the date of the Share Exchange, to be provided in three equal issuances; provided, however, that such persons may elect to receive a cash payment of $100,000 instead of any one issuance of Raphael’s shares. In addition to the issuance of shares, Raphael Israel has also agreed to pay Wolc a royalty fee equal to 15% of the net royalties generated from sales of Raphael Israel’s pharmaceutical drug products that are developed at Rambam hospital in Israel.
Except for the above, we have not engaged in any off-balance sheet arrangements, such as the use of unconsolidated subsidiaries, structured finance, special purpose entities or variable interest entities.
We do not believe that our off-balance sheet arrangements and commitments have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
BUSINESS
Overview
We are a pharmaceutical drug research and development company focused on the discovery and clinical development of life-improving drug therapies based on cannabinoids, including cannabidiol, or CBD, oil. Unless indicated otherwise, we plan on using oil derived from CBD strains with low levels of Tetrahydrocannabinol, or THC. All references to the use of CBD in our product candidates refer to CBD strains with less than 0.3% of THC.
We are currently in the pre-clinical development stage for our lead product candidate, our RA product candidate for the treatment of RA. In addition, we are aiming to develop a pharmaceutical drug product for the treatment of hyperinflammatory syndrome inflammation related to COVID-19, or our COVID-19 product candidate, which may be based on data or studies related to our RA product candidate. We successfully completed a preclinical study for the RA product candidate at Rambam Hospital as well as a mice model trial.
On February 9, 2022, we filed an application for a clinical trial with the Medical Cannabis Unit of the Ministry of Health of Israel, or MOH. On February 16, 2022 we submitted an application with the Helsinki Committee at Rambam Hospital for a clinical trial in COVID-19 patients. The Company plans to submit such applications for our RA product during 2022.
Our goal is to become a leader in development of CBD oil-based pharmaceutical drug products for the treatment of indications in which we believe there is a high unmet medical need in a range of disorders, including those related to inflammation in the body, including RA and COVID-19.
In order to achieve our goal, we have and will continue to build an experienced team of senior executives and scientists, with experience in all facets of pharmaceutical research and development, drug formulation, clinical trial execution and regulatory submissions. We intend to leverage the knowledge of our team in order to complete the clinical trials needed to receive approvals of our product candidates from applicable regulatory authorities.
Initially, we intend to obtain FDA approvals for our product candidates. Upon obtaining FDA approvals, or in the event that we are not successful in obtaining such approvals, we intend to apply for EMA and other countries’ governmental regulatory agencies approvals for our product candidates. If we are successful in obtaining FDA approvals for our product candidates, we intend to enter into royalty agreements with GMP approved medical manufactures and distributors, having them using our medical formulas for the purpose of growing, cultivating, manufacturing, and distributing Raphael Pharmaceutical medical indications in their designated territories.
Our discovery platform currently focuses the use of CBD oil, one of the cannabinoids in cannabis plants, as the active pharmaceutical ingredient, or API, for our RA product candidate and COVID-19 product candidate. Research results published in 2018 (“Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age”) has shown that there may be benefits to treading medical conditions, or their effects, with cannabinoids, and more specifically, with CBD, which may help reduce chronic pain by impacting endocannabinoid receptor activity, reducing inflammation and interacting with neurotransmitters. This research has also shown that CBD may have neuroprotective properties, and could have the ability to (i) reduce anxiety and depression, (ii) alleviate cancer-related symptoms, (iii) reduce acne and (iv) benefit heart health.
Over the last few years, pharmaceutical drug products that include parts of the cannabis plant have begun to receive regulatory approvals for use in patients suffering from certain disorders, as highlighted below.
| ● | Nabiximols, better known under the tradename Sativex, is a botanical mouth spray consisting of natural THC and CBD extracts, that received approval in the United Kingdom in 2010 for the alleviation of multiple sclerosis, or MS, symptoms like spasticity, pain and overactive bladder. |
| ● | Dronabinol, better known under the name Marinol, contains mainly THC and is a partial agonist of the cannabinoid receptor type 1, or CB1, in the nervous system and a partial agonist of the cannabinoid receptor type 2, or CB2, in the periphery that activates appetite, mood, cognition, memory and perception. Dronabinol received FDA-approval for use in United States in 1985 for treatment of anorexia in acquired immunodeficiency syndrome, or AIDS, patients and for the prevention of chemotherapy-induced nausea and vomiting, or CINV. A Lack of randomized controlled trials, or RCTs, makes a recommendation for usage of dronabinol as a third-line treatment for CINV difficult. Dronabinol in the form of an oral tablet is known under the trade name Namisol. It has high bioavailability and a long shelf life and is indicated for MS, chronic pain and behavioral disturbances in dementia patients. |
| ● | Nabilone, better known under the tradename Cesamet, contains primarily THC, is approved for use as an anti-emetic and adjunctive analgesic for neuropathic pain, CINV and treatment for anorexia in AIDS patients in Canada, Mexico, the UK and the United States. Its main usage today is as adjunct medicine for chronic pain management. |
In light of the past regulatory approvals for other pharmaceutical drug products and, more specifically, the potential beneficial effects of CBD and other parts of the cannabis plant, we believe that a drug discovery platform based on CBD may offer new and differentiated treatment options for patients. Prior regulatory approvals of other companies’ pharmaceutical drug products do not serve as an indication as to the ability or likelihood that we receive regulatory approval to commercialize any of our product candidates.
After two successful years of pre-clinical research and studies at the laboratories of Rambam Hospital, in January 2021, we commenced a pre-clinical trial in mice for our RA product candidate that we expect will take four to five months. Following the completion of this pre-clinical trial, we intend on submitting an Investigational New Drug, or IND, application to the FDA and MOH. See “– Research and Clinical Development Strategy – Clinical Development Plan” for additional information on the ongoing pre-clinical trial and our planned clinical trial for our RA product candidate. In addition, with respect to our COVID-19 product candidate, our clinical research partners have been focused on the effect of cannabinoids and cannabis extracts on immune cells which induce acute inflammation. This study will begin in the pre-clinical level in immune cell models and, subject to positive results that exhibit downregulation of pro-inflammatory cytokines by cannabis extract, the study was completed successfully. Following the completion of the pre-clinical study, a mice model was conducted to analyze for acute inflammation, which resembles the immunopathology of COVID-19. The mice model was successfully completed and we have registered for a clinical trial in patients with the MOH.
As a pharmaceutical research and clinical development company we do not own or operate, and currently do not intend on creating an in-house team to manufacture and commercialize our pharmaceutical drug products, if any, that receive regulatory approval allowing for commercialization. We currently rely, and expect to continue to rely, on third parties for the manufacturing of our product candidates for preclinical and clinical testing, as well as for commercial manufacturing of any pharmaceutical drug products for which we may receive regulatory approval. Subject to the receipt of such regulatory approvals, we intend on cooperating with manufacturers and other third parties to manufacture and commercialize approved pharmaceutical drug products.
Product Pipeline
Currently, we have begun development for our RA and COVID-19 product candidates, which are in the pre-clinical stage.
Assuming that we successfully complete the clinical development of our RA and COVID-19 product candidates, we intend to then turn our attention to the clinical development of cannabinoid-based drug products for the treatment of certain oncology indications. Unlike our RA and COVID-19 product candidate, the use of our cannabinoid based drug products for the treatment of certain oncology indications will require specific dosing and potentially, a different regulatory pathway than our existing product candidates.
We intend to apply for MOH approval, as well as the FDA and EMA approvals right afterwards, subject to the completion of the applicable clinical trials, for our RA product candidate as well as our COVID-19 product candidate using the FDA’s regulatory pathway for drug products.
Indications and Market
Rheumatoid Arthritis
RA is an autoimmune disease of unknown cause characterized by inflammation in multiple joints, including synovial inflammation with hyperplasia. Inflammation is also associated with reduced hemoglobin (anemia) and reduced albumin and changes in levels of cholesterol and triglycerides. In addition to the inflammation associated with RA, studies, including a 2018 publication entitled, “Cartilage and bone damage in rheumatoid arthritis,” patients suffering from RA generally also suffer from chronic pain, fatigue, progressive joint damage, disability, hyperplasia, production of autoantibodies such as rheumatoid factor and anti–citrullinated protein antibody, cartilage damage and bone erosions.
Research has shown that in about 30% of RA patients, current conventional synthetic and biologic disease modifying anti-rheumatic drugs and targeting molecules may fail or induce only partial responses, both of which we believe are insufficient for patients suffering from RA. Using disease modified anti-rheumatic drugs, or DMARD, based treatments, as shown in a 2017 study from Sohita Dhillon, patients tend to report at follow-up meetings that pain relief is unsatisfactory and although there is an initial improvements in the average pain score, a plateau may be reached beyond which DMARDs are not able to resolve RA pain. As a result, we believe that RA patients need ongoing therapy as RA relapses are frequent. During RA flareups, patients experience acute and chronic pain, fatigue, sleep disturbances, and morning stiffness which significantly reduces their quality of life. Furthermore, damage is accumulated by long-term disease which also interferes with pain, fatigue and quality of life.
All types of pain (acute or chronic, widespread or local and nociceptive) have been reported in RA. Patients with RA may develop fibromyalgia, or FM, especially with long-term disease. Concomitant FM is a key factor for discordance between PRO and clinical outcomes in assessment of RA patients including in RCTs. Peripheral sensitization, induced by local inflammation or damage, and pain augmentation by the central nervous system, or CNS, both drives the pain problems in RA patients. Anxiety or depression, impaired sleep and fatigue all contribute to pain sensitization in RA patients. As noted in the study, “Tackling Pain Associated with Rheumatoid Arthritis: Proton-Sensing Receptors,” some RA patients have allodynia and peripheral neuropathies that contribute to refractory chronic pain.
We believe that clinical studies on the use of cannabinoids in rheumatic conditions, and particularly RA, are logically advocated as possible positive effectors of the inflammatory pathway of RA, as well as symptomatic pain relievers that may have the potential to also improve fatigue, sleep disorder and tolerability of DMARDs. Through our Sponsored Research Agreement (as further detailed below), we believe that we have arrived at an understanding as to how cannabinoids influence inflammation. Applying immune cells models in our pre-clinical research, we identified specific strains of cannabis which reduce the capacity of the immune cells to communicate during inflammation, thus decreasing their capacity to participate in chronic inflammation. For a deeper understanding of the mechanism in which cannabinoids effect inflammation, we developed a unique, real time-Polymerase chain reaction, or RT-PCR, method to identify 10 different receptors to cannabinoids, both in human and mice models. We believe that this technology will allow us to identify which cannabinoids receptors are participating in the downregulation of inflammation, which we believe will help us develop our RA product candidate.
In 2015 alone, research conducted by the NIH National Library of Medicine showed that RA affected about 24.5 million people as of 2015, which reflected between 0.5% and 1% of adults in the developed world, with an additional 5 to 50 per 100,000 people developing the condition each year. It is believed that onset is most frequent during middle age and women are affected 2.5 times as frequently as men. Further research indicates that RA resulted in 38,000 deaths in 2013, up from 28,000 deaths in 1990.
Hyperinflammatory Syndrome Related to COVID-19
Since the first emergence of COVID-19 in December 2019 in Wuhan, China, COVID-19 has spread across more than 200 countries across the world and over 112 million cases of the virus have been reported. Most patients develop only mild symptoms of COVID-19; however, some develop severe symptoms including dyspnea, hypoxia and lung involvement which requires hospitalization. Based on research, we believe that most of the severe COVID-19 symptoms are related to hyperinflammation caused by failure of resolution of the immunological response to the infection similar as observed in cytokine release syndrome. Treatments with anti-inflammatory or anti-viral drugs are still experimental or have not reduced mortality. Moreover, they are administered only to inpatients in a severe disease state. As a result of the foregoing, the necessity of finding new anti-inflammatory therapies that have the potential to prevent deterioration of the symptoms is heightened.
Research and Clinical Development Strategy
Research and clinical development of our pharmaceutical drug product candidates is our core business. We are currently focused on developing innovative cannabinoid-based medical indications that we aim to push through Phase 2A and Phase 2B approval from both the FDA and EMA.
The research efforts that have been conducted to date by the team at Rambam Med-Tech Ltd., or Rambam MT, are aimed at revealing the mechanism which structures the activity of cannabinoids in human cells and organs, while applying a variety of disease models. We are using our PCR method to identify different receptors to cannabinoids and which we believe will allow us to identify which cannabinoids receptors are participating in the downregulation of inflammation. We believe that this strategy will allow us to identify the cannabinoids components of a chosen strain in order to accurately administer cannabinoids targeted to the treatment of patients.
Research Agreement with Rambam MT
On July 17, 2019, we entered into a sponsored research agreement, or the Research Agreement, with Rambam MT, pursuant to which the Company agreed to fund a research project, to be performed by Rambam MD, with a research plan aimed at identifying the effects of different cannabis strains on the function of immune cells. On October 28, 2020, the Company and Rambam MT agreed to expand the research plan to study the anti-inflammatory activities of cannabis extracts in an RA mouse model. On February 15, 2021, the Company and Rambam MT agreed to further expand the research plan to study the effect of cannabis extracts on the immunopathology of the COVID-19 disease. The Sponsored Researched Agreement is for an initial term of 48 months.
Pursuant to the Research Agreement, we agreed to pay Rambam $1.4 million in four equal payments, due on the first day of August on each successive year from 2019 through 2022. Furthermore, in accordance with the terms of the Research Agreement, we and Rambam MT will have joint ownership of any IP created as a result of research programs covered by such agreement. In connection with the Research Agreement, Rambam MT agreed not to work, study or develop any technologies with other entities that compete with our work with Rambam MT for our COVID-19 product candidate or RA product candidate for a term of three and seven years, respectively, from the end of the parties’ collaboration with respect to the COVID-19 product candidate and seven years from the end of the term of the Research Agreement with respect to the RA product candidate.
Subject to commercial sales of any product candidate using the IP created as a part of the research covered by such agreement, we are required to pay Rambam MT a royalty in an amount equal to 6% of all net sales, subject to certain deductions, such as taxes paid by any purchaser, transportation and shipping costs, and other customary deductions.
As of June 28, 2022, the Company has made three of the four equal payments due pursuant to the Research Agreement, for a total amount of $1,050,000.
We and Rambam MT are currently focused on characterizing the activity of cannabinoids in RA and in hyperinflammatory syndrome related to COVID-19. RA is a long-term autoimmune disorder, and as such, the research conducted by Rambam MT has focused on identifying the effect of cannabinoids on inflammatory processes related to RA, while, with respect to our COVID-19 product candidate, their research has been focused on the effect of cannabinoids and cannabis extracts on immune cells which induce acute inflammation.
Pre-Clinical Studies for RA Product Candidate
In Vitro Study
Pursuant to the Research Agreement, the team at Rambam MT established a study in order to determine which cannabis strains extracts may affect inflammation. The lab applied an in vitro system, allowing them to screen a variety of cannabis derived oil extracts and their influence on cytokine secretion, which is a type of response to injury and infection in the body.
The researchers employed human THP1 cells, which can be induced to differentiate macrophages (specialized cells involved in the detection, phagocytosis and destruction of bacteria and other harmful organisms) and to secrete cytokines (which is aimed as serving as a bridge for cross-communication with other innate immune cells). Using this system, our partners from Rambam MT have established a variety of cannabinoids and studied their influence on pro-inflammatory and anti-inflammatory cytokines. Most interestingly the study has identified a non-psychoactive cannabis strain, which we refer to as “CAN1.” Our study showed that CAN1 reduced TNFα and IL-6 secretion but increased the secretion of the anti-inflammatory cytokine IL-10, while also reducing the secretion of the pro-inflammatory chemokine IL-8, as highlighted in Figure 1 below. Based on the results from this pre-clinical study, we believe that CAN1 strain, may be a potential anti-inflammatory agent with the ability to influence both activation and migration of cells during inflammation.

Figure 1. CAN1 downregulates pro-inflammatory cytokines (TNFα, IL-8, IL-6) and upregulates the anti-inflammatory counterpart IL-10.
The results in Figure 1 above reflect the results from our in vitro study of THP1 cells (0.25*10^6/ml) incubated with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) to induce THP1 cells to differentiate to macrophages. for 48 hours. Then, cells were washed twice with phosphate buffer saline (PBS) and treated with Can1 (1 µg/ml) in medium free serum for 24 hours. Then, cells were washed twice with PBS and incubated with LPS (Lipopolysaccharides) to induce cytokines secretion (1 µg/ml) overnight. Supernatants were collected and frozen. Levels of TNFα, IL-8, IL-6 and IL-10 were determined by the enzyme-linked immunosorbent assay (ELISA).
Pre-Clinical Study in Mice
In January 2021, we commenced a pre-clinical study in mice. Mice in our pre-clinical study are being treated with cannabis strains that we previously identified in our in vitro study and other prior research as potential candidates in the cell models. Following the treatment, we expect to examine the ability of the treatment to modulate the immune function, specifically in the case of chronic inflammation, in order to optimize treatment for RA.
This pre-clinical study is expected to be focused on the following results.
| ● | Aim 1: Evaluating the immune modulatory properties of different cannabis strains related to the immunopathology (i.e., the immune responses) in RA; |
| ● | Aim 2: Demonstrating the immunomodulatory properties of specific cannabis extracts on a mouse model for RA; |
| ● | Aim 3: Elucidating the mechanisms of action, or MOA, of cannabinoids that are involved in the regulation of inflammation in RA; and |
| ● | Aim 4: Establishing a phase 1 and 2 clinical trial experiment in compliance with FDA and EMA rules and regulations to study the effect of cannabis-based medical indication on RA. |
In addition, this pre-clinical study is expected to enable us to examine how we manufacture the API, the dosage design, analytical and bioanalytical method development and validation, metabolism and pharmacokinetics, toxicology, both safety and genetic toxicology and possibly safety pharmacology; and good manufacturing practice, or GMP, manufacture and documentation of drug product for use in clinical trials.
Aim 1. Evaluating the immune modulation properties of different cannabis strains
Rheumatic diseases, including RA, are characterized by chronic inflammation that affects the connective tissue or supporting structures of the body, most commonly the joints, but also tendons, ligaments, bones, and muscles, while certain rheumatic diseases can also have an adverse effect on certain organs. RA, the latter of which means “joint inflammation,” encompasses more than 100 different disorders, including, for example, join inflammation disorders, including RA, psoriatic arthritis, or PsA, systemic lupus erythematosus, or SLE, or lupus, gout and ankylosing spondylitis, or AS. Therefore, a major goal for therapies of rheumatic diseases is the application of agents with immune modulation properties with the ability to suppress effector autoimmune cells and to increase the activity of regulatory immune cells.
It is our belief, based on the research conducted by our partners, and that or our industry peers, that cannabinoids have immunomodulatory properties, although the exact effects are not fully comprehended. Together with the team at the Medical Cannabis Research and Innovation Center, we intend on working to identify and characterize the types of cannabis strains as well as specific cannabinoids with immune modulatory function that lie in the usage of cannabis in the treatment of rheumatic diseases. We aim to do this by evaluating the immune regulatory properties of different cannabis strains on a variety of subsets of immune cells including lymphocytes and myeloid cells, which we plan to have purified from the rodent model of rheumatic disease and from healthy humans or humans diagnosed with RA. For each isolated immune cell type, we expect to evaluate the impact of cannabis on a variety of functions, such as cytokine and chemokine secretion, proliferation and migration.
Aim 2. Demonstrating the immune modulatory properties of specific cannabis extract on mouse models for RA.
Cannabis is not an isolated substance; it contains a plethora of biologically active substances. The most common substances are THC and CBD. Today more than 140 cannabinoids are known to be expressed in the plant. In addition to cannabinoids, the plants contain flavonoids and terpenes. This greatly complicates our ability to understand the effects of cannabis on the physiology because the different substances may have different (and even contradictory) effects. Therefore, the use of different cannabis varieties with diverse ingredients may produce distinct and unexpected results.
In the current study, we expect to examine whether cannabis is a potential treatment for RA in the in vivo mouse model of RA. We expect to test particular inflammation markers, such as the cytokines tumor necrosis factor alpha, interferon gamma, monocyte chemoattractant protein-1, or MCP-1/CCL2 and Interleukin-6 during the onset of RA disease and investigate the effects of the different cannabis strains on particular inflammatory disease markers. We then intend on comparing the effects of cannabis strains with high CBD-concentrations to those with high THC-concentrations in order to be able to better understand the main effects of the major ingredients of each of CBD and THC. Furthermore, we intend on conducting a pharmacokinetic analysis of the cannabinoid profile in the mouse blood. To this end, we expect to expose mice to a chronic controlled administration of known doses of cannabis extracts from different strains. Following the exposure, we intend on measuring the effect of chronic exposure over time and doses on RA markers. We believe that this information, together with additional data that we collect from our clinical studies, will enable us to complete the clinical development of a cannabis-based treatment for patients with RA.
Aim 3. Elucidating the MOA of cannabinoids that are involved in the regulation of inflammation in RA.
Upon activation, it is the cannabinoid receptors, or CBrs, in the endocannabinoid system, or ECS, which initiate numerous regulatory functions in a mammal. CBrs have been found in a variety of species including human, monkey, pig, dog, rat and mouse. The discovery of membrane receptors found in the brain, central nervous system as well as peripheral tissues and organs that bind cannabimimetic compounds was a critical turning point that paved the way towards the pharmacological understanding of cannabis-derived compounds.
The most studied CBrs are CB1 and CB2; both belong to the G protein-coupled receptors (these cell surface receptors act like an inbox for messages in the form of light energy, peptides, lipids, sugars, and proteins), or GPCR, family. GPCRs constitute a large protein family of receptors that detect molecules outside the cell and activate internal signal transduction pathways and cellular responses. GPCRs, are called seven-transmembrane receptors because they pass through the cell membrane seven times. Heterotrimeric G proteins are activated by GPCRs and are made up of three subunits, α, β and γ. G proteins are divided into four main classes: Gαs, Gαi, Gαq and Gα12. These proteins are activated depending on the ability of the G protein α-subunit, or Gα, to cycle between an inactive guanosine diphosphate, or GDP, bound conformation and an active guanosine triphosphate, or GTP, bound conformation that can modulate the activity of downstream effector-proteins. Additional receptors have been shown to bind cannabinoids: G protein-coupled receptor 55, or GPR55, several transient receptor potential, or TRP, channels (TRPV1, TRPV2, TRPA1, TRPM8), and glycine receptors.
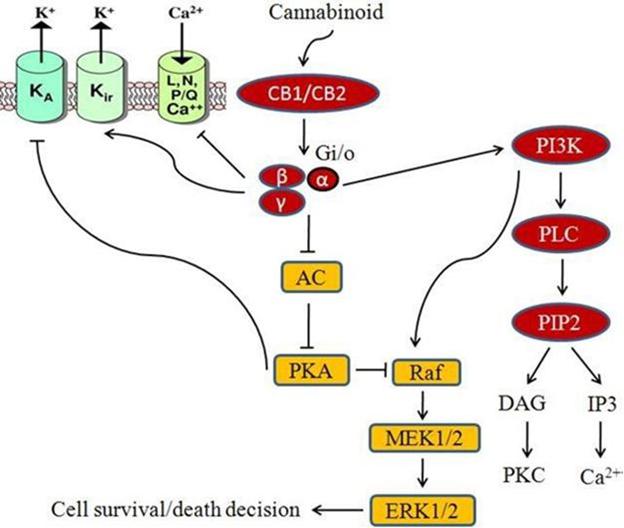
Figure 2. CB1 downstream signaling network. Adapted from Chakravarti et al., 2014
Since most of the biological properties related to phytocannabinoids, a type of natural cannabinoid, rely on their interactions with receptors of the endocannabinoid system, it is crucial to define which receptors are expressed and activated in the target cells. As a result, we have developed a system with the capacity to identify ten cannabinoid receptors simultaneously and measure their expression levels using quantitative real-time PCR. We have applied this method in examining cells of the immune system, and more specifically, in monocytes, before and after differentiation to macrophages, or after stimulation to secrete cytokines. Using this methodology, we were able to identify a differential expression pattern of the receptors under different conditions.
To obtain a deep understanding on the mechanism of action of the cannabis strains, we will apply our research system and establish a unique study to research the response of 10 different receptors to Cannabis in RA patients, which aims to identify the specific cannabinoids receptors on the cells of RA patients. The data from this study is expected to subsequently be used to set up a system for analyzing and matching the specific cannabis treatment to the specific cannabinoids receptors that are expressed in the patient’s cells. We believe that if cannabinoid treatments correspond to the receptors in the patient’s cells, the treatment may be more accurate for treating that specific patient’s RA symptoms.
Aim 4. Establish a Phase 1 & 2B clinical trial according to FDA rules and regulations to study the effect of cannabis-based medical indications on RA.
Our goal for the treatment of RA is to achieve disease remission or low disease activity, or LDA. LDA is measured with several assessment metrics that are intended to measure the effect of the treatment on a number of physical phenomena that are connected to RA. These metrics include, but are not limited to: (i) Disease Activity Score based on assessment of 28 joints, or DAS28, patient’s assessment, (ii) erythrocyte sedimentation rate (a common hematology test, and is a non-specific measure of inflammation) or C-reactive protein, or CRP, test, which is a blood test that measures the CRP in a person’s blood, (iii) SDAI and CDAI (Simple and Complex Disease Activity Indices) as compared to DAS28, (iv) patient’s and physician’s assessment, including global assessment scores, or PtGA and PhGA, respectively (v) pain visual analogue scale and (vi) health assessment questionnaire disability index, or HAQ-DI.
Clinical Development Plan for RA Product Candidate
We intend to utilize the knowledge and results that we obtain from our pre-clinical study to establish, initially a Phase 1 clinical trial, which we expect will take approximately 3 months, followed by a Phase 2 clinical trial to study the effect of cannabis-based medical intervention on RA, which we expect will take approximately 24 months, following which, we hope to receive FDA approval to allow for the commercialization of our RA product candidate. Specifically, we intend to study the changes in RA patients’ status after cannabis compounds were added to their stable conventional treatment. We currently plan that our partners at Rambam MT will carry out these trials at the Rambam Health Care Campus in Haifa, Israel.
Our hypothesis is that in patients with RA, the regular addition of cannabinoids in a defined dosage to basic treatment of RA might add to the patients’ quality of life. We intend to measure quality of life by scores for fatigue using the fatigue severity scale, pain using the visual analogue scale, the Pittsburgh Sleep Quality Index, PtGA, PhGA, HAQ-DI and the 6-Item Short Form Health Survey. We also propose that cannabinoids may influence the disease status. This may be measured by DAS28, SDAI, CDAI, CRP, ESR, albumin and Hb levels and also by levels of key cytokines and chemokines. As part of these clinical studies, we currently plan to assess changes in RA patients’ status after cannabis compounds were added to their stable conventional treatment.
Pre-Clinical Studies for Treatment of Hyperinflammatory Syndrome Related to COVID-19
We are studying the potential of cannabis extracts to downregulate the hyperinflammation and the immunopathology in COVID-19 patients. Our partners at Rambam MT have been conducting research on the use of cannabis to treat disorders with widespread inflammatory responses, such as RA and COVID-19. We hope that by decoding the cannabinoid mechanism of action during inflammatory storms, we can treat inflammation associated with COVID-19 where conventional drugs and other therapies have failed.
At the outbreak of the COVID-19 pandemic, members of the Rambam MT team directed their efforts and experience to join the world-wide battle against the COVID-19 pandemic. Their research found that cannabinoids contribute to the sophisticated fabric network of intercellular communications. Based on this research, we believe, that if we understand how cannabinoid components are used in intercellular communication, we can help influence this communication in the event of a disease, to disrupt it or empower the communication to convey desired messages.
In order to properly understand cannabis’ effects on COVID-19, the Rambam MT team compiled its Biobank database. In generating the Biobank database, the Rambam MT team found what they believed to be a safe way to separate the white blood cells, including the immune cells, from verified patients. We believe that this is crucial as blood samples are the most accessible resource for continuous sampling (allowing for the understanding of biological processes during the disease) and to develop vaccines and drugs for treatment of the condition.
In February 2021, we entered into an agreement with Rambam MT for investigation of CBD oil extracts as a potential treatment for COVID-19 related inflammation. In accordance therewith, the Rambam MT team is investigating the effect of cannabis extracts oil on the inflammation response of immune cells derived from COVID-19 patients as well as a deep study on the capacity of cannabis extract oil to reduce acute inflammation and lung inflammation in mouse models which resembles the immunopathology of COVID-19.
This study will begin in the pre-clinical level in immune cell models and, subject to positive results that exhibit downregulation of pro-inflammatory cytokines by cannabis extract, the study is expected to continue to a mouse model to analyze for acute inflammation, which resembles the immunopathology of COVID-19. We believe that our strategy to investigate the response of ex-vivo immune cells to cannabis extract together with the analysis of the in-vivo model for acute and lung inflammations, will allow us to identify the medical cannabis extracts, if any, that have the potential to treat patients with COVID-19 related hyperinflammatory syndrome.
Competition and Competitive Position
The pharmaceutical industry is characterized by rapidly advancing technologies and intense competition. While we believe that our knowledge, experience and scientific resources provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates for which we complete clinical development successfully and for which we receive marketing approval may compete with existing therapies and new therapies that may become available in the future.
Many of our competitors have far greater marketing and research capabilities than us. We also face potential competition from academic institutions, government agencies and private and public research institutions, among others, which may in the future develop products to treat those diseases that we currently or, in the future, seek to treat. All of these companies and institutions may have product candidates in development that are or may become superior to our RA product candidate or any other product candidate that we may seek to develop. Our commercial opportunity would be reduced significantly if our competitors develop and commercialize products that are safer, more effective, more convenient, have fewer side effects or are less expensive than our product candidates.
In addition, although our product candidates may, if approved, be considered advantageous to existing therapies, such as the use of corticosteroids and DMARDs for the treatment of RA, our target market may continue to use existing therapies.
However, we do believe, specifically with respect to our competitors not using cannabis in their pharmaceutical drug products that our use of, and experience with, cannabinoids provides us with a potential competitive advance. Our research has shown that the use of cannabinoids for the treatment of RA is justified based on its positive effect on pain, fatigue, sleep problems and its potential safety profile. Growing evidence on the anti-inflammatory effect of cannabinoids provide more strong ground for their use in the treatment of RA.
Although the use of any part of the cannabis plant in pharmaceutical drug products was once non-existent or minimal, in addition to approved pharmaceutical drug products that use parts of the cannabis plant (see “Overview” for additional information), we are aware that there is at least one plan for a multicenter randomized control trial on the use of medical cannabidiol in Danish patients with RA and Ankylosing Spondylitis (inflammatory joint and spine disease), as previously published in an issue of BMJ Open in 2019. As the medical benefits of cannabis become more well-known, we believe that we may face more competition from both new startup pharmaceutical and biotechnology companies and from well-funded and experienced organizations and it is therefore imperative that we face as few delays as possible in our pre and clinical development plan or we may otherwise face more competition.
The following table highlights the estimated cost that RA patients incur on an annual basis based on a 2017 report from the Canadian Agency for Drugs and Technologies in Health.
| Drug Product | Strength | Dose Form | Price ($) | Recommended Dose | Annual Drug Cost ($) | |||||
| Sarilumab (Kevzara) | 150 mg/1.14 mL 200 mg/1.14 mL | Pre-filled syringe | 700.0000 | 200 mg SC every two weeks | 18,200 | |||||
| Abatacept SC (Orencia) | 125 mg/mL | Pre-filled syringe | 366.1000 | 125 mg weekly | 19,037 | |||||
| Abatacept IV (Orencia) | 250 mg/15 mL | Vial | 490.0500 | Patients < 60 kg: 500 mg Patients 60 to 100 kg: 750 mg Patients > 100 kg: 1,000 mg 500 to 1,000 mg at weeks 0, 2, and 4 then every 4 weeks | Year 1: 20,582 Thereafter: 19,112 | |||||
| Adalimumab SC (Humira) | 40 mg/0.8 mL | Pre-filled syringe or pen | 769.9700 | 40 mg every other week | 20,019 | |||||
| Anakinra (Kineret) | 100 mg | Pre-filled syringe | 48.0571 | 100 mg daily | 17,493 | |||||
| Certolizumab pegol (Cimzia) | 200 mg/mL | Pre-filled syringe | 664.5100 | 400 mg at weeks 0, 2 and 4 then 200 mg every 2 weeks | Year 1: 19,271 Thereafter: 17,277 | |||||
| Etanercept (Enbrel) | 25 mg | Vial | 202.9300 | 50 mg weekly or two 25 mg doses on same day every week or every 3 or 4 days | 21,105 | |||||
| 50mg/mL | Pre-filled syringe or auto-injector | 405.9850 | 21,111 | |||||||
| Entanercept (Brenzys) | 50 mg/mL | Pre-filled syringe | 305.0000d | 50 mg weekly | 15,860 | |||||
| Golimumab SC (Simponi) | 50 mg/0.5 mL | Pre-filled syringe or auto-injector | 1,555.17 | 50 mg monthly | 18,662 | |||||
| Golimumab IV (Simponi) | 50 mg/4 mL | Vial | 849.5000b | 2 mg/kg at weeks 0 and 4, then every 8 weeks thereafter | Year 1:17,829 Thereafter: 16,565 | |||||
| Infliximab (Remicade) | 100 mg | Vial | 987.5600 | 3 mg/kg at weeks 0, 2, and 6, then every 8 weeks thereafter
Depending on clinical response, dose can be increased to 10 mg/kg and/or up to every four weeks | Year 1: 23,701 Thereafter: 19,257 10 mg/kg every 4 weeks: $102,706 annually | |||||
| Infliximab (Inflectra) | 100 mg | Vial | 525.0000 | Year 1: 12,600b Thereafter: 10,238b 10 mg/kg every 4 weeks: $54,600 annually15 | ||||||
| Rituximab (Rituxan) | 100 mg/10 mL 500 mg/50 mL | Vial | 466.3200 2,331.61 | A course consists of 1,000 mg infusions at weeks 0 and 2. Reassess for retreatment at week 26, no sooner than 16 weeks after previous | 18,653 assumes 2 courses Per course: 9,326 | |||||
| Tocilizumab SC (Actemra) | 162 mg/0.9 mL | Pre-filled syringe | 355.0000 | Patients < 100 kg: 162 mg SC every two weeks, increasing to weekly based on clinical response. Patients ≥ 100 kg: 162 mg SC weekly | Every two weeks: 9,230 Weekly: 18,460 | |||||
| Tocilizumab IV (Actemra) | 80 mg/4 mL 200 mg/10mL 400 mg/20 mL | Vial | 180.8100 452.0300 904.0600 | 4 mg/kg every 4 weeks followed by an increase to 8 mg/kg based on clinical response | 4 mg/kg: 10,577 8 mg/kg: 17,629 | |||||
| Tofacitinib (Xeljanz) | 5 mg | Tablet | 23.5585 | 5 mg p.o. twice daily | 17,151 |
IV = intravenous; p.o. = orally; SC = subcutaneous.
Although there can be no guarantee, we believe that our RA product candidate, if approved for commercialization by regulators, will be available to patients at a lower price than that of other available treatments.
With respect to our development of a product candidate to treat inflammation associated with COVID-19, we will face competition from major pharmaceutical companies that have developed or that will develop vaccines, along with other companies and organizations that have or will develop therapies or pharmaceutical drug products aimed at treating the underlying symptoms of COVID-19.
Cultivation of our API
In October 2020, we entered into an engagement agreement with Way of Life Cannabis Ltd., or Wolc, pursuant to which, subject to its completing the Share Exchange, Raphael Israel is scheduled to be provided with up to 15 liters of CBD oil, from a strain of cannabis of our selection, during a term of 18 months, to be provided in two to three deliveries of between one to seven liters of CBD oil. In accordance with the agreement with Wolc, we have agreed to issue to certain persons affiliated with Wolc 3% of our issued and outstanding share capital as of the date of the Share Exchange, to be provided in three equal issuances; provided, however, that such persons may elect to receive a cash payment of $100,000 instead of any one issuance of our shares. In addition to the issuance of shares, we have also agreed to pay Wolc a royalty fee equal to 15% of net income royalties generated from sales of our pharmaceutical drug products that are developed at Rambam hospital in Israel.
At this time, we only require a limited amount of our API for our studies and trials and, to date, we have received oil extracted from high CBD strains, from Wolc in the amounts that we require in order to conduct our pre-clinical trials. Pursuant to our agreement with Wolc, pending FDA approval of any of our product candidates, Wolc is expected to transfer seeds used for the FDA-approved product candidate to growers in California, Colorado and Oklahoma. Wolc is a fully licensed Israeli cannabis company focused on growing, cultivating and manufacturing cannabis medical oil, located in Aviel, Israel. As an owner and operator of green houses for growing organic cannabis plants and a GMP manufacturing facility located in Netanya, Israel, we intend to utilize, pursuant to an agreement between the parties, Wolc for the growing and cultivation of the CBD oil needed for our product candidates.
We believe that our current agreement with Wolc will provide us with sufficient amounts of CBD oil in order to complete our clinical development and for initial sales of our pharmaceutical drug products. In the future, as our demand for pharmaceutical products grows, if ever, we may need to find additional partners that may provide us with sufficient amounts of CBD oil and/or amend or terminate our engagement with Wolc.
Manufacturing
We do not own or operate, and currently have no plans to establish, any manufacturing facilities for final manufacture. We currently rely, and expect to continue to rely, on third parties for the manufacture of our product candidates for preclinical and clinical testing, as well as for commercial manufacturing of any pharmaceutical drug products for which we receive regulatory approval.
Commercialization Plan
Subject to the receipt of regulatory approval to commercialize our pharmaceutical product candidates, our goal is to distribute our approved formulas to good manufacturing practice, or GMP, approved medical cannabis manufacturers and global medical cannabis distributors. Depending on the expertise of the distributors, we expect the licensing agreements to provide us with royalty-based payments for the sale of each of our approved pharmaceutical drug products. In Israel, pursuant to our agreement with Wolc, the parties are expected to negotiate an exclusive distribution agreement in Israel, pursuant to which Wolc will be the exclusive supplier of any approved pharmaceutical drug products of the Company in Israel.
Although we expect government regulation of pharmaceutical products derived from cannabis to develop over the next few years, we may be limited in the manner in which we commercialize our product candidates. We fully intend on being fully complaint with local and state-wide government regulations and therefore we expect to enter into licensing agreements with vendors only if such vendor may legally distribute our product candidate within the region for which they have obtained a license from us to sell our pharmaceutical product.
Intellectual Property
We do not currently have any patents, and currently rely on our know-how and trade secrets. However, subject to the completion of our pre-clinical trial for our RA product candidate, and prior to the completion of our clinical development plan, we intend on seeking patent protection in the United States and/or internationally for such product candidate and, potentially for other product candidates that we may seek to develop. Our policy is to pursue, maintain and defend patent rights developed internally and to protect the technology, inventions and improvements that are commercially important to the development of our business.
Governmental Regulation
Government authorities in the United States, at the federal, state and local levels, and in other countries and jurisdictions, including the EU, extensively regulate, among other things, the research, development, testing, manufacture, sales, pricing, reimbursement, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of biopharmaceutical products. The processes for obtaining marketing approvals in the United States and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
We are a research and development company collaborating with our partners at Rambam MT to research and develop our COVID-19 and RA product candidates. We do not grow or cultivate cannabis and we have no physical connection to the raw cannabis materials, which is shipped directly to Rambam MT. Pre-clinical research, animal models and clinical trials are sponsored by us through our agreement with Rambam MT. Such research and trials are being done by Rambam MT’s medical team, researchers, doctors and professors.
We do not own or operate, and currently have no plans to establish, any manufacturing facilities for final manufacturing of our products. We do not distribute, and we have no plans to distribute, our products. Once we receive regulatory approval for our products, we intend to license to our future candidate partners the rights to commercialize our medical formulas. Our future candidate partners will be responsible for the manufacturing, distributing, promoting, and marketing of medical indications. We intend to engage with candidate partners that are GMP approved professionals, well established and experienced medical manufacturers and distributors in the U.S. and other countries.
Our future candidate partners will be entirely responsible and liable for regulatory compliance, including but not limited to cannabis growing and cultivation, GMP manufacturing, distribution, advertising and promotional regulations, marketing, labeling, post-market approval reporting and record keeping.
We intend to hire and train quality assurance professional that will inspect periodically the facilities of our future candidate partners as well as the methods of production, marketing and distribution under applicable governmental regulatory guidelines.
U.S. Cannabis Market
The emergence of the legal cannabis sector in the United States, both for medical and adult use, has been rapid as more states adopt regulations for its production and sale. A majority of Americans now live in a state where cannabis is legal in some form and almost a quarter of the population lives in states in which both medical and recreational use is permitted as a matter of, and in accordance with, applicable state and local laws. According to Fortune Business Insights, the global legal marijuana market is anticipated to reach a value of US$97.35 billion by the end of 2026 from US$10.60 billion in 2018. The market is predicted to rise at a compounded annual growth rate of 32.6% during the period 2019 to 2026.
The use of cannabis and cannabis derivatives to treat or alleviate the symptoms of a wide variety of chronic conditions, while not recognized by the FDA, has been accepted by a majority of citizens with a growing acceptance by the medical community. A review of the research, published in 2015 in the Journal of the American Medical Association, found solid evidence that cannabis can treat pain and muscle spasms. The pain component is particularly important, because other studies have suggested that cannabis can replace pain patients’ use of highly addictive, potentially deadly opiates. Although hemp, defined as cannabis and derivatives of cannabis with not more than 0.3% THC, has been descheduled from the Controlled Substances Act, the FDA has regulatory oversight over foods, drugs, cosmetics containing cannabis under the Food, Drug and Cosmetics Act of 1938. All references to the use of CBD in our product candidates refer to CBD strains with less than 0.3% of THC. It is possible that as the federal and state agencies legalize certain products, the FDA may issue rules and regulations, including good manufacturing practices related to the growth, cultivation, harvesting and processing of such products, even if they are not marketed as drugs. It is possible that the FDA would require that facilities where medical-use cannabis is grown to register with the FDA and comply with certain federally prescribed regulations, certifications, testing, or other requirements. The potential impact on the cannabis industry is uncertain and could include the imposition of new costs, requirements, and prohibitions.
Although we are not currently engaged and do not expect to be engaged in the production or distribution of medical marijuana products, the FDA has jurisdiction over our flower, oil, vape and edible products, among others. The FDA is currently taking action in the form of Warning Letters, but may also take more extreme enforcement such as recalls, disgorgement or penalties.
Polls conducted throughout the United States consistently show overwhelming support for the legalization of medical cannabis, together with strong majority support for the full legalization of recreational adult-use cannabis. According to a Pew Research Center survey, as of November 11, 2019, “Around nine-in-ten Americans favor legalization for recreational or medical purposes” and “Only 8% say it should not be legal.” These are large increases in public support over the past 40 years in favor of legal cannabis use.
As of the date of this prospectus, cannabis is legal in some form in a total of 36 states, the District of Columbia, Guam, Puerto Rico and the U.S. Virgin Islands. On the recreational side, there are currently 15 states, plus the District of Columbia and four U.S. territories, in which the recreational sale of cannabis has been approved. These states include Oregon, Washington, Nevada, California, Colorado, Massachusetts, Michigan, Vermont, Alaska, Illinois, Maine, New Jersey, Arizona, South Dakota and Montana. With respect to medical marijuana, as more research centers study the effects of cannabis-based products in treating or addressing therapeutic needs, and assuming that research findings demonstrate that such products are effective in doing so, management believes that the size of the U.S. medical cannabis market will also continue to grow as more States expand their medical marijuana programs and new states legalize medical marijuana.
Notwithstanding that 36 states and the District of Columbia have now legalized adult-use and/or medical cannabis, cannabis remains illegal under U.S. federal law with cannabis listed as a Schedule I drug under the Controlled Substance Act, or CSA.
Government Regulation and Product Approval
We are a preclinical to early clinical stage pharmaceutical company that intends to engage third parties to test, register and license the rights to commercialize our products in the United States and other jurisdictions. Such third parties may be subject to extensive regulation by various regulatory authorities. The primary regulatory agency in the United States is the FDA and in Europe it is the EMA. Along with these two, there are other federal, state, and local regulatory agencies. In the United States, the Federal Food, Drug, and Cosmetic Act, or the FDCA, and its implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for, and marketing of, our products in other countries.
Generally, our activities outside the United States will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Approval in the United States, Canada, or Europe does not assure approval by other regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way through the EMA but country specific regulation remains essential in many respects. A major difference in Europe, when compared to Canada and the United States, is with the approval process. In Europe, there are different procedures that can be used to gain marketing authorization in the European Union. The first procedure is referred to as the centralized procedure and requires that a single application be submitted to the EMA and, if approved, allows marketing in all countries of the European Union. The centralized procedure is mandatory for certain types of medicines and optional for others. The second procedure is referred to as national authorization and has two options; the first is referred to as the mutual recognition procedure and requires that approval is gained from one member state, after which a request is made to the other member states to mutually recognize the approval, whilst the second is referred to as the decentralized procedure which requires a member state to act as the reference member state through a simultaneous application made to other member states.
The process of obtaining regulatory marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources and may not be successful. See “Risk Factors” for additional information.
U.S. Government Regulation
The FDA is the main regulatory body that controls pharmaceuticals in the United States, and its regulatory authority is based in the FDCA. Pharmaceutical products are also subject to other federal, state and local statutes. A failure to comply explicitly with any requirements during the product development, approval, or post approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an Institutional Review Board of a hold on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
The steps required before a new drug may be marketed in the United States generally include:
| ● | completion of preclinical studies, animal studies and formulation studies in compliance with the FDA’s GLP regulations; | |
| ● | submission to the FDA of an IND application to support human clinical testing in the United States; | |
| ● | approval by an IRB at each clinical site before each trial may be initiated; | |
| ● | performance of adequate and well-controlled clinical trials in accordance with federal regulations and with GCP regulations to establish the safety and efficacy of the investigational product candidate for each target indication; | |
| ● | submission of a new drug application, or NDA, to the FDA; | |
| ● | satisfactory completion of an FDA Advisory Committee review, if applicable; | |
| ● | satisfactory completion of an FDA inspection of the manufacturing facilities at which the investigational product candidate is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate; and | |
| ● | FDA review and approval of the NDA. |
Clinical Trials and the FDA Approval Process
An IND is a request for authorization from the FDA to administer an investigational product candidate to humans. This authorization is required before interstate shipping and administration of any new drug product to humans in the United States that is not the subject of an approved NDA. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin. Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease under study, under the supervision of qualified investigators following GCPs, an international standard intended to protect the rights and health of patients with the disease under study and define the roles of clinical trial sponsors, administrators and monitors. Clinical trials are conducted under protocols that detail the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. Each protocol involving testing on patients in the United States and subsequent protocol amendments must be submitted to the FDA as part of the IND. Rambam MT has not yet submitted an IND in the United States for any clinical programs.
The clinical investigation of an investigational product candidate is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or some may be combined. The three phases of clinical investigation are as follows:
| ● | Phase I. Phase I includes the initial introduction of an investigation product candidate into humans. Phase I clinical trials may be conducted in patients with the target disease or condition, or in healthy volunteers. These studies are designed to evaluate the safety, metabolism, pharmacokinetics, or “PK”, and pharmacologic actions of the investigational product candidate in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational product candidate’s PK and pharmacological effects may be obtained to inform the design of Phase II clinical trials. The total number of participants included in Phase I clinical trials varies but is generally in the range of 20 to 80. We expect that it will take approximately 3 months for Rambam MT to complete Phase I. |
| ● | Phase II. Phase II includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product candidate for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product candidate. Phase II clinical trials are typically well controlled, closely monitored, conducted in a limited subject population and usually involving no more than several hundred participants. Rambam MT is planning to divide Phase II into two parts: |
| ○ | Phase IIa. Phase IIa will include a randomized, double-blind, placebo-controlled, multiple ascending dose study in Israel to determine the maximum CBD extract administered sublingually to assess the safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy for at least 4 weeks in RA patients in the presence of concurrent active therapies, such as non-steroidal anti-inflammatory drugs, or NSAIDs, and steroids. We expect that Phase IIa will take approximately 6 months. | ||
| ○ | Phase IIb. Phase IIb will include IND submission for randomized, multi center double blinded, placebo-controlled dose response finding, or DRF, study for at least 12 weeks with either CBD extract or placebo administered sublingually in the presence of concurrent active therapies such as NSAIDs and steroids. This study will include 300-400 patients (80-100 patients per cohort) to study safety and efficacy of the product in active RA patients. We expect that Phase IIb will take approximately 18 months. |
| ● | Phase III. Phase III clinical trials are controlled clinical trials conducted in an expanded subject population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product candidate has been obtained, are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product candidate, and to provide an adequate basis for drug approval. Phase III clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase III clinical trials to demonstrate the efficacy of the drug. |
The decision to terminate development of an investigational product candidate may be made by either a health authority body, such as the FDA or IRB/ethics committees, or by a company for various reasons. The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor or the clinical monitoring board. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of development can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration statementof ongoing clinical trials of Product Candidates on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
New Drug Applications
In order to obtain approval to market a drug in the United States, a marketing application must be submitted to the FDA that provides data establishing the safety and effectiveness of the product candidate for the proposed indication. The application includes all relevant data available from pertinent preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company sponsored clinical trials intended to test the safety and effectiveness of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational product candidate to the satisfaction of the FDA. In most cases, the NDA must be accompanied by a substantial user fee; there may be some instances in which the user fee is waived. The FDA will initially review the NDA for completeness before it accepts the NDA for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review Product Candidates are reviewed within ten to twelve months. The FDA can extend this review by three months to consider certain late submitted information or information intended to clarify information already provided in the submission. The FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP. The FDA may refer applications for novel Product Candidates that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. Product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of certain FDA regulated products, including prescription drugs, are required to register and disclose certain clinical trial information (though not specifically required for Phase I trials) on a public website maintained by the U.S. National Institutes of Health, or “NIH”. Information related to the product, patient population, phase of investigation, study sites and investigator, and other aspects of the clinical trial is made public as part of the registration. Sponsors are also obligated to disclose the results of these trials after completion. Disclosure of the results of these trials can be delayed until the product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the design and progress of our development programs.
Advertising and Promotion
The FDA and other federal regulatory agencies closely regulate the marketing and promotion of drugs through, among other things, standards and regulations for direct-to-consumer advertising, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. A product cannot be commercially promoted before it is approved. After approval, product promotion can include only those claims relating to safety and effectiveness that are consistent with the labeling (package insert) approved by the FDA. Healthcare providers are permitted to prescribe drugs for “off-label” uses — that is, uses not approved by the FDA and, therefore, not described in the drug’s labeling — because the FDA does not regulate the practice of medicine. However, FDA regulations impose stringent restrictions on manufacturers’ communications regarding off-label uses.
Post-Approval Regulations
After regulatory approval of a drug is obtained, a company is required to comply with a number of post-approval requirements. For example, as a condition of approval of an NDA, the FDA may require post-marketing testing, including Phase IV clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. In addition, as a holder of an approved NDA, a company would be required to report adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with requirements concerning advertising and promotional labeling for any of its products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to assure and preserve the long-term stability of the drug or biological product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural and substantive record keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon a company and any third-party manufacturers that a company may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Controlled Substances
The CSA and its implementing regulations establish a “closed system” of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA, which is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
Facilities that research, manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s). For example, separate registrations are required for importation and manufacturing activities, and each registration authorizes which schedules of controlled substances the registrant may handle. However, certain coincident activities are permitted without obtaining a separate DEA registration, such as distribution of controlled substances by the manufacturer that produces them.
The DEA categorizes controlled substances into one of five schedules — Schedule I, II, III, IV, or V— with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently “accepted medical use” in treatment in the United States and lack accepted safety for use under medical supervision. They may be used only in federally approved research programs and may not be marketed or sold for dispensing to patients in the United States. Pharmaceutical products having a currently accepted medical use that are otherwise approved for marketing may be listed as Schedule II, III, IV or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence. The regulatory requirements are more restrictive for Schedule II substances than Schedule III substances. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist in most situations, and cannot be refilled. Once FDA has approved a medical use for Schedule I drugs, the DEA must reschedule the drug. For example, after FDA approval for Epidiolex®, a purified CBD oil, for the treatment of two rare forms of epilepsy, DEA placed it in Schedule V. Further, on April 6, 2020, GW Pharma announced that Epidiolex® was descheduled by the DEA and is no longer considered a controlled substance.
The DEA inspects all manufacturing facilities to review security, record keeping, reporting and handling prior to issuing a controlled substance registration. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. Manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics.
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA’s estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. The quotas apply equally to the manufacturing of the API and production of dosage forms.
The states also maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State Authorities, including Boards of Pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on our business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Cannabinoids as a Controlled Substance
Cannabinoids are subject to the United Nations Single Convention on Narcotic Drugs (1961) adopted by numerous countries globally, which prohibits the production and supply of specific drugs, except for scientific and research purposes. Under the current UN definition, Cannabis extracts and tinctures are controlled substances. Individual countries (and sometimes jurisdictions within countries) are rapidly changing how they interpret and apply the international rules. Currently there is a broad spectrum of legal statuses based on strength, source and intended use. We are closely monitoring these changes. We expect that there may be different requirements in each region where we have clinical sites.
Several Cannabis-related drugs were placed in lower schedules once they were approved as drugs. For example, the US DEA reduced Epidiolex® (CBD) to Schedule V after it was approved for treatment of two rare forms of childhood epilepsy. In April 2020, the DEA descheduled Epidiolex® entirely.
The passage of the Farm Bill in December 2018 legalized the cultivation of hemp in the United States and the production of hemp-derived non-THC cannabinoids, removing these products from the CSA. Our products use oil extracted from CBD strains, containing <0.3% THC.
We plan to engage third parties to conduct clinical trials for our product candidates outside the United States, subject to regulatory approval. As a result, such third parties will also be subject to controlled substance laws and regulations from the various other regulatory agencies in other countries where we develop, manufacture or commercialize our product candidates in the future.
Marketing Exclusivity
Upon NDA approval of a new chemical entity, which for this purpose is defined as a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA cannot approve any abbreviated new drug application, or ANDA, seeking approval of a generic version of that drug. Certain changes to the scope of an approval for a drug, such as the addition of a new indication to the package insert, are associated with a three-year period of exclusivity during which the FDA cannot approve an ANDA for a generic drug that includes the change. A Section 505(b)(2) NDA may be eligible for three-year marketing exclusivity, assuming the NDA includes reports of new clinical studies (other than bioequivalence studies) essential to the approval of the NDA.
An ANDA may be submitted one year before marketing exclusivity expires if a Paragraph IV certification is filed. In this case, the 30 months stay, if applicable, runs from the end of the five-year marketing exclusivity period. If there is no listed patent in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Additionally, six months of marketing exclusivity in the United States is available under Section 505A of the FDCA if, in response to a written request from the FDA, a sponsor submits and the agency accepts requested information relating to the use of the approved drug in the pediatric population. This six-month pediatric exclusivity period is added to any existing patent or non-patent exclusivity period for which the drug product is eligible.
Patent Term Extension
The term of a patent that covers an FDA approved drug may be eligible for patent-term extension, which provides patent-term restoration as compensation for the patent term lost during the FDA regulatory review process. The United States Federal Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent-term extension of up to five years beyond the expiration of the patent. The length of the patent-term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug.
European and Other International Government Regulation
In addition to regulations in the United States and Canada, our third-party licensees will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, such third-party licensees must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Some countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the clinical trial application is approved in accordance with a country’s requirements, clinical trial development may proceed.
The UK was previously in a transition period until December 31, 2020, during which time it continued to abide by the EU regulatory processes; however, they may adopt different or additional procedures.
To obtain regulatory approval to commercialize a new drug under EU regulatory systems, it is required to submit a MAA. The MAA is similar to the NDA, with the exception of, among other things, country-specific document requirements.
For other countries outside of the EU, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. Internationally, clinical trials are generally required to be conducted in accordance with GCP, applicable regulatory requirements of each jurisdiction and the medical ethics principles that have their origin in the Declaration of Helsinki.
Compliance
During all phases of development (pre- and post-marketing), failure to comply with applicable regulatory requirements may result in administrative or judicial sanctions. These sanctions could include the FDA’s imposition of a clinical hold on trials, refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, product detention or refusal to permit the import or export of products, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect.
Other Special Regulatory Procedures
Priority Review (United States) and Accelerated Assessment (European Union)
Based on results of the Phase III clinical trial(s) submitted in an NDA, upon the request of an applicant, a priority review designation may be granted to a product by the FDA, which sets the target date for FDA action on the application at six months from the FDA’s decision on priority review application, or eight months from the NDA filing. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a safe and effective therapy where no satisfactory alternative therapy exists, or a significant improvement compared to marketed products is possible. If criteria are not met for priority review, the standard FDA review period is ten months from the FDA’s decision on priority review application, or 12 months from the NDA filing. The priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the Centralized Procedure in the European Union, the maximum timeframe for the evaluation of a MAA is 210 days (excluding “clock stops,” when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or “CHMP”). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, which takes into consideration: the seriousness of the disease (e.g., disabling or life-threatening diseases); the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days.
Accelerated Approval
Under the FDA’s accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit. This approval mechanism is provided for under 21CRF314 Subpart H and 21CRF601 Subpart E. In this case, clinical trials are conducted in which a surrogate endpoint is used as the primary outcome for approval. A surrogate endpoint is reasonably likely to predict clinical benefit, or an effect on a clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. This surrogate endpoint substitutes for a direct measurement of how a patient feels, functions, or survives and is considered reasonably likely to predict clinical benefit. Such surrogate endpoints may be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase IV or post-approval clinical trials to confirm the effect on the clinical endpoint. When the Phase IV commitment is successfully completed, the biomarker is deemed to be a surrogate endpoint. Failure to conduct required post-approval studies or confirm a clinical benefit during post-marketing studies, could lead the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to additional regulation by various federal, state and local authorities in addition to the FDA, including, among others, the Centers for Medicare and Medicaid Services, other divisions of HHS, the DOJ, and individual United States Attorney offices within the DOJ and state and local governments.
Compliance with Environmental Laws
Other than our ongoing research and development, our only operations consist of our pre-clinical trial being in conducted in Israel for RA, which may further our COVID-19 product candidate. At this time, compliance with environmental laws in Israel, where we conduct our operations, has not been a burden and has not required from us the use of material resources or capital expenditures.
Employees
As of June 28, 2022, we do not have any employees. Our officers are engaged through consulting agreements.
Corporate Information
Easy Energy was incorporated under the laws of the State of Nevada in May 2017. On May 14, 2021, Raphael Israel and Easy Energy completed the Share Exchange, pursuant to which we changed our name to Raphael Pharmaceutical Inc. Our registered address is 4 Lui Paster, Tel Aviv-Jaffa, Israel 6803605. Our website address is https://www.raphaelpharmaceutical.com/. Information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus, and you should not consider information on our website to be part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
The SEC also maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our Common Stock as of June 28, 2022 by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of at least 5% of our outstanding Common Stock; |
| ● | each of our directors and executive officers; and |
| ● | all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to Common Stock. Percentage of shares beneficially owned is based on 13,945,540 shares of Common Stock outstanding on June 28, 2022.
Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is: 4 Lui Paster, Tel Aviv-Jaffa, Israel 6803605.
| No. of Shares Beneficially Owned | Percentage Owned | |||||||
| Holders of more than 5% of our voting securities: | ||||||||
| Haim Castro | 1,255,325 | 9 | % | |||||
| Directors and executive officers: | ||||||||
| Shlomo Pilo | 3,783,701 | 27 | % | |||||
| Guy Ofir | 575,263 | 4 | % | |||||
| Professor Joseph Press, M.D. | 0 | 0 | % | |||||
| Dr. Igal Louria Hayon | 0 | 0 | % | |||||
| Dr. Yehuda Eliya | 0 | 0 | % | |||||
| All directors and executive officers as a group (5 persons) | 4,358,964 | 30 | % | |||||
MANAGEMENT
The following sets forth information regarding our executive officers and the members of our Board of Directors as of June 28, 2022.
All directors hold office for one-year terms until the election and qualification of their successors. Officers are appointed by our Board of Directors and serve at the discretion of our Board of Directors.
| Name | Age | Position(s) | ||
| Professor Joseph Press, M.D. | 68 | Director and Chairman of the Board | ||
| Shlomo Pilo | 68 | Chief Executive Officer and Director | ||
| Guy Ofir | 49 | Chief Financial Officer and Director | ||
| Dr. Igal Louria Hayon | 49 | Chief Technology Officer and Director | ||
| Dr. Yehuda Eliya | 48 | Director |
Professor Joseph Press M.D., has been a director and our Chairman of the Board since the Share Exchange. Since 2008, Professor Press has been the Chief Executive Officer of Schneider Children’s Medical Center of Israel. Before that, from 2002 to 2007, Professor Press was Chairman of Soroka University Medical Center in Israel.
Shlomo Pilo, has been our Chief Executive Officer and a member of our board of directors since the Share Exchange and previously served in such capacity with Raphael Israel from July 2019. In addition, following the founding of Sheffa Enterprises Inc. in 2009, Mr. Pilo is also engaged in providing food brokering services, representing seven food manufacturers in Europe and North America. Before founding Sheffa Enterprises Inc., Mr. Pilo served as VP Sales & Marketing of Alle Processing Corporation, the world’s largest Glatt Kosher food manufacturer.
Guy Ofir, has been our Chief Financial Officer and a member of our board of directors since September 27, 2021. Mr. Ofir is a qualified lawyer in Israel and the owner of “Guy Ofir Adv” law firm since 2000, with a main practice in civil and business law. In 2007 he established and registered a company named Easy Energy Inc. that developed a green energy patent. Mr. Ofir owns an investment company, which has invested in land and construction in Romania since 2005.
Dr. Igal Louria-Hayon, has been our Chief Technology Officer and member of our board of directors since September 27, 2021. Dr. Louria-Hayon serves as a scientific director of the Medical Cannabis Research and Innovation Center at Rambam Health Care Campus and Head of the Leukemia & Immunotherapy Research Laboratory in the Clinical Research Institute at Rambam. In addition, Dr. Louria-Hayon served as Senior Research Fellow in the Technion’s Cannabinoid Research Laboratory.
Dr. Yehuda Eliya, has been a member of our board of directors since September 27, 2021. Dr. Eliya is a partner at one of the largest accounting firms in Israel and the owner of a law firm. Dr. Eliya is the Vice President of the Chamber of Internal Auditors in Israel. In addition, Dr. Eliya is a member of the presidency of ZAKA, a series of volunteer community emergency response teams in Israel, a parliamentary adviser to the Republic of Abkhazia and serves as a director in several non-profit organizations in Israel.
Scientific Advisory Board
Professor Alexandra Balbir-Gurman, Frontline Director of Clinical Trials (Rambam) and Director of the B. Shine Rheumatology Unit at Rambam Health Care Campus, a member of the Executive Committee of the Israeli Society of Rheumatology, an active member of the Scleroderma Research Group (EUSTAR) and a member of the EULAR Target US initiative.
Dr. Shachar Eduardo, Frontline Director of Clinical Trials (Rambam), is the Director of the Clinical Immunology Unit at Rambam Health Care Campus.
Prof. Shai Israeli, Head of the Division of Pediatric Hematology-Oncology Center, Schneider Children’s’ Medical Center of Israel (affiliated to Tel Aviv University), is a member of the executive Board of the European Hematology Association (EHA).
Family Relationships
There are no family relationships among the directors and officers of the Company.
EXECUTIVE COMPENSATION
We currently have no written employment agreements with any of our officers and directors. We have entered into a written consulting agreements with our executive officers, including our Chief Executive Officer who is also a member of our board of directors.
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our named executive officers for the fiscal years ended December 31, 2021 and 2020.
Summary Compensation Table
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Shlomo Pilo | 2021 | 120,000 | - | - | - | - | 120,000 | |||||||||||||||||||||
| 2020 | 110,000 | - | - | - | - | 110,000 | ||||||||||||||||||||||
| Guy Ofir | 2021 | 75,000 | - | - | - | - | 75,000 | |||||||||||||||||||||
| 2020 | - | - | - | - | - | - | ||||||||||||||||||||||
| Dr. Igal Louria-Hayoun | 2021 | 115,500 | 115,500 | |||||||||||||||||||||||||
| 2020 | 72,500 | 72,500 | ||||||||||||||||||||||||||
Our Chief Executive Officer provides services to the Company pursuant to a consulting agreement, entered into on June 1, 2020, by and between the Company and Sheffa Enterprises, Inc., a New Jersey corporation, or Sheffa. Pursuant to the terms thereof, we pay Sheffa $10,000 per month, payable in quarterly installments, for management services. The agreement is in effect until December 31, 2022; provided, however that the Company may terminate the agreement prior to the termination date by providing 120 days’ advance written notice and paying Sheffa a termination fee equal to the lesser of (i) $1 million, or (ii) the monthly management fee paid or payable to Sheffa for the remaining term of the agreement. Furthermore, as disclosed in the agreement, the Company agreed to indemnify Sheffa against and in respect of any losses arising out of or due to the operation of the business by the Company, its affiliates, agents, servants and/or employees.
Our Chief Financial Officer provides services to our Company pursuant to an arrangement, which has not been reduced to a written agreement between the Company and Guy Ofir & Co. S.R.L, a Romanian corporation owned by our Chief Financial Officer. Pursuant to this arrangement, we paid our Chief Financial Officer $75,000 for his services for the fiscal year ended December 31, 2021.
Our Chief Technology Officer provides services to our Company pursuant to a consulting agreement, as amended, by and between Dr. Igal Louria Hayoun and Raphael Israel. Pursuant to the terms thereof, Dr. Hayoun provides consulting services the the Company to engage with an array of science consultants and to coordinate collaborations with hospitals on medical cannabis research. Raphael Israel pay Dr. Hayoun $9,000 per month, and agreed to pay him 15% of the Company’s net income from worldwide sales of any of Raphael Israel’s cannabis-based medical indications treating COVID-19.
As of June 28, 2022, there were no outstanding equity awards to any of our executive officers or the members of our board of directors. The Company has no stock option, retirement, pension, or profit sharing programs for the benefit of directors, officers or other employees, but our board of directors may recommend adoption of one or more such programs in the future.
See “Certain Relationships and Related Party Transactions, and Director Independence” below for additional information.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Share Exchange
In connection with the Share Exchange, certain of our shareholders and directors received shares of our Company as a result of shares that they held in Raphael Israel.
Consulting Agreements
We have entered into a written consulting agreements with our executive officers, including our Chief Executive Officer who is also on our board of directors.
Our Chief Executive Officer provides services to our Company pursuant to a consulting agreement between Raphael Israel and Sheffa Enterprises, Inc., a New Jersey corporation. Pursuant to the terms thereof, we pay our Chief Executive Officer $10,000 per month, payable in quarterly installments. The agreement is in effect until December 31, 2022; provided, however that Raphael Israel may terminate the agreement prior to the termination date by providing 120 days’ advance written notice and paying our Chief Executive Officer a termination fee equal to the lesser of $1 million of the monthly management fee paid or payable to the Chief Executive Officer for the remaining term of the agreement. Furthermore, as disclosed in the agreement, Raphael Israel has undertaken to indemnify Sheffa Enterprises, Inc. against and in respect of any and losses arising out of or due to the operation of the business by Raphael Israel, its affiliates, agents, servants and/or employees.
Our Chief Technology Officer provides services to our Company pursuant to a consulting agreement, as amended, by and between Dr. Igal Louria Hayoun and Raphael Israel. Pursuant to the terms thereof Dr. Hayoun provides consulting services the the Company to engage with an array of science consultants and to coordinate collaborations with hospitals on medical cannabis research. Raphael Israel pay Dr. Hayoun $9,000 per month and agreed to pay him 15% of the Company’s net royalties income from worldwide sales of any of Raphael Israel’s cannabis-based medical indications treating COVID-19.
Raphael Israel has entered into a consulting agreement with Professor Joseph Press, who is also a member of our board of directors, pursuant to which Prof. Press provides Raphael Israel with medical research services for $3,000 per month.
Indemnification Agreements
We have entered into indemnification agreements with our directors pursuant to which we agreed to indemnify each director for any liability he or she may incur by reason of the fact that he or she serves as our director, to the maximum extent permitted by law.
Policies and Procedures for Related-Party Transactions
Our Company does not have any formal written policies or procedures for related party transactions, however in practice, our board of directors’ reviews and approves all related party transactions and other matters pertaining to the integrity of management, including potential conflicts of interest and adherence to standards of business conduct. We have two independent directors on our board of directors. See “—Director Independence” for further information.
Director Independence
None of our securities are listed or trade on any securities or currency exchange or other established public trading market. However, the members of our board of directors have reviewed their relationship with the Company in conjunction with Nasdaq Listing Rule 5605(a)(2) that provides that an “independent director” is ‘a person other than an executive officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.’ Based upon information requested from and provided by each director concerning their background, employment and affiliations, including family relationships, our board of directors has affirmatively determined that each of Prof. Press and Dr. Eliya has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq rules and the SEC. Our members of the board of directors have determined that Dr. Louria-Hayon, Mr. Ofir and Mr. Pilo do not qualify as independent directors pursuant to the standards described above.
Our Company does not have a separately designated audit, nominating or compensation committee or committee performing similar functions; therefore, our full board of directors currently serves in these capacities.
SELLING SECURITYHOLDERS
Up to 1,667,393 shares of Common Stock may be offered for resale, from time to time, by the Selling Securityholders under this prospectus.
The following tables set forth, with respect to each Selling Securityholder, the number of shares of Common Stock (i) known to us to be beneficially owned as of June 28, 2022, (ii) being offered hereby and (iii) beneficially owned after giving effect to the sale by such Selling Securityholder of all of its Offered Securities. The immediately following table also sets forth the percentage of Common Stock beneficially owned by a Selling Securityholder after giving effect to the sale by such Selling Securityholder of all Offered Securities, based on 13,945,540 shares of Common Stock outstanding as of June 28, 2022.
The Selling Securityholders are not making any representation that any shares of Common Stock covered by this prospectus will be approximately $21,243.32.
DETERMINATION OF OFFERING PRICE
The selling shareholdersoffered for sale. Because each Selling Securityholder may dispose of all, none or some portion of their securities, no estimate can be given as to the number of securities that will determine at what price theybe beneficially owned by a Selling Securityholder upon termination of this offering. In addition, the Selling Securityholders may sellhave sold, transferred or otherwise disposed of their securities in transactions exempt from the offered
shares,registration requirements of the Securities Act after the date on which the information in the table is presented.
We may amend or supplement this prospectus from time to time in the future to update or change this Selling Securityholders list and such salesthe securities that may be maderesold.
| Number of Shares of Common Stock Beneficially | Number of Shares of Common Stock Offered | Shares of Common Stock Beneficially Owned After Completion of the Offering(1) | ||||||||||||||
| Name | Owned(1) | Hereby | Number | Percentage | ||||||||||||
| Haim Kastro | 1,255,325 | 54,000 | 1,201,325 | 8.6 | % | |||||||||||
| Rachel Mellul | 460,000 | 370,000 | 90,000 | * | ||||||||||||
| Arie Kalmanzon | 414,370 | 36,000 | 378,370 | 2.7 | % | |||||||||||
| Avner Josef | 279,185 | 90,000 | 189,185 | 1.3 | % | |||||||||||
| Doron Fishbin | 270,000 | 270,000 | 0 | * | ||||||||||||
| Amir Nahmani | 190,000 | 190,000 | 0 | * | ||||||||||||
| Erel Pilo | 141,889 | 141,889 | 0 | * | ||||||||||||
| Leeor Pilo | 141,889 | 141,889 | 0 | * | ||||||||||||
| Mary Rabinovitch | 139,593 | 45,000 | 94,593 | * | ||||||||||||
| Amnon Yosef | 139,593 | 45,000 | 94,593 | * | ||||||||||||
| Cheni Zabzarsky | 136,750 | 103,000 | 33,750 | * | ||||||||||||
| Adi Kaplan | 46,148 | 22,500 | 23,648 | * | ||||||||||||
| Joseph Gueta | 45,000 | 45,000 | 0 | * | ||||||||||||
| Hay Amira | 35,000 | 35,000 | 0 | * | ||||||||||||
| Guy Mizrahi | 30,000 | 30,000 | 0 | * | ||||||||||||
| Vladimir Elkin | 30,000 | 30,000 | 0 | * | ||||||||||||
| Josef Guy Mosseri | 18,115 | 18,115 | 0 | * | ||||||||||||
| * | Less than 1%. |
| (1) | The amounts and percentages of Common Stock beneficially owned are determined in accordance with the SEC’s rules, pursuant to which a person is deemed to be a “beneficial owner” of a security if that person has or shares voting or investment power or has the right to acquire such power within 60 days through exercise of any option, warrant or other right. Securities that can be so acquired are deemed to be outstanding for purposes of computing such person’s ownership percentage, but not for purposes of computing any other person’s percentage. Under these rules, more than one person may be deemed beneficial owner of the same securities, and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest. Except as otherwise indicated in these footnotes, each of the beneficial owners has, to our knowledge, sole voting and investment power with respect to the indicated shares of Common Stock. |
PLAN OF DISTRIBUTION
We are registering the resale of Common Stock offered by this prospectus on behalf of the Selling Securityholders. The Selling Securityholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling Common Stock received after the date of this prospectus from a Selling Securityholder as a gift, pledge, limited liability company or partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their securities on any stock exchange, market or trading facility on which such securities are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale or at negotiated prices.
DILUTION
The common stock to be sold by the selling stockholders is the 5,608,833 shares
of common stock that are currently issued and outstanding. Accordingly, there
will be no dilution to our existing stockholders.
SELLING SHAREHOLDERS
The following table sets forth the number of shares beneficially owned by the
selling stockholders and certain other information regarding such stockholders,
as of June 30, 2008.
The selling stockholders acquired their securities (1) through a private
transaction exempt from registration pursuant to Regulation S of the Securities
Act of 1933, as amended (the "Act"), pursuant to which we sold to the Meitav
entities listed below (the "Meitav Entities") on February 8, 2008, 367,647
units, each unit being offered for $1.70, for aggregate gross proceeds of
$625,001. Each unit consisted of (i) ten shares of our common stock and (ii)
thirty Class A Warrants. Each Class A Warrant entitles the holder thereof to
purchase one share of common stock at an exercise price of $0.27 per share,
expiring five years from the date of purchase of the Warrant; (2) as
compensation for legal services and services in connection with the facilitation
of the financing of the Company by the Meitav Entities and Tailor Made Capital
Ltd. ("TMC"). In connection therewith, on March 3, 2008, we issued Mr. Victor
Tshuva, 300,000 shares of restricted common stock for an aggregate price of
$3,000 and warrants to purchase 1,000,000 shares of our common stock at an
exercise price of $0.15 for a period of five years; (3) as a commitment fee of
9
882,353 shares of the Company's common stock and a warrant to purchase 3,000,000
shares of the Company's common stock to TMC for arranging the equity line
pursuant to the March 10, 2008 agreement; and (4) 750,000 shares of our common
stock as a consideration for investor relations services to be provided by
Falcon Financial Services LLC.
The shares offered by this prospectusSelling Securityholders may be offered from time to time by the
selling stockholders. The following table assumes that the selling stockholders
will sell all of the shares being offered for their respective accounts by this
prospectus. However, the selling stockholders may sell some, all or none of
their shares of our common stock offered by this prospectus and we are unable to
determine the exact number of shares that actually will be sold. We do not know
how long the selling stockholders will hold the shares of our common stock
before selling them, and we currently have no agreements, arrangements or
understandings withuse any of the selling stockholders regarding the sale of any of
the shares of our common stock. The information in the table below is current
only as of the date of this prospectus. None of the selling shareholders had or
have any material relationship with our company or any of its affiliates within
the past three years. To our knowledge, there is also no affiliation among
Victor Tshuva, Falcon Financial Services LLC, the Meitav Entities, TMC and their
beneficial owners. None of the selling shareholders is a broker-dealer or an
affiliate of a broker-dealer. Each shareholder is an adult.
We may require the selling shareholders to suspend the sales of the securities
offered by this prospectus upon the occurrence of any event that makes any
statement in this prospectus or the related registration statement untrue in any
material respect or that requires the changing of statements in these documents
in order to make statements in those documents not misleading.
In the following table, except as noted below, we have determined the number and
percentage of shares beneficially owned in accordance with Rule 13d-3 of the
Exchange Act, and this information does not necessarily indicate beneficial
ownership for any other purpose. Except as otherwise indicated in the footnotes
below, we believe that each of the selling stockholders named in this table has
sole voting and investment power over the shares of our common stock indicated.
In determining the number of shares of our common stock beneficially owned by a
person and the percentage ownership of that person, we include any shares as to
which the person has sole or shared voting power or investment power, as well as
any shares registered hereby that are subject to outstanding warrants held by
that person and any shares subject to outstanding warrants or options that are
currently exercisable or exercisable within 60 days after March 31, 2008.
Applicable percentages are based on 93,186,070 shares of our common stock
outstanding on March 31, 2008.
Beneficial Ownership Beneficial Ownership
Prior to this Offering(1) After Offering
---------------------------- -----------------------
Number of
Shares Issuable Number of Number
Number of Upon Exercise Shares Being of
Name of Selling Stockholder Shares of Warrants Offered Shares Percent(**)
- --------------------------- ------ ----------- ------- ------ -----------
Victor Tshuva 300,000 1,000,000 1,300,000 0 0
Meitav Gemel Ltd
Meitav Tagmulim Clalit(2) 1,394,120 4,182,360 5,576,480 0 0
Meitav Gemel Ltd
Meitav Histalmut Clalit(2) 947,060 2,841,180 3,788,240 0 0
Meitav Gemel Ltd
Meitav Pizuim Clalit(2) 302,940 908,820 1,211,760 0 0
Meitav Gemel Ltd
Meitav Tagmulim CPI(2) 70,590 211,770 282,360 0 0
10
Meitav Gemel Ltd
Meitav Tagmulim Nis(2) 26,470 79,410 105,880 0 0
Meitav Gemel Ltd
Meitav Tagmulim Shares(2) 67,650 202,950 270,600 0 0
Meitav Gemel Ltd
Meitav Histalmut CPI(2) 23,530 70,590 94,120 0 0
Meitav Gemel Ltd
Meitav Histalmut Nis(2) 5,880 17,640 23,520 0 0
Meitav Gemel Ltd
Meitav Histalmut Shares(2) 38,240 114,720 152,960 0 0
Meitav Gemel Ltd
Meitav Chisachon Gemel(2) 229,410 688,230 917,640 0 0
Meitav Gemel Ltd
Meitav Chisachon Histalmut(2) 164,710 494,130 658,840 0 0
Meitav Gemel Ltd
Meitav Chisachon Pizuim(2) 33,530 100,590 134,120 0 0
Meitav Gemel Ltd
Meitav Yerushalayim Gemel Bound 85(2) 11,180 33,540 44,720 0 0
Meitav Gemel Ltd
Meitav Yerushalayim Gemel Zahav(2) 12,350 37,050 49,400 0 0
Meitav Gemel Ltd
Meitav Yerushalayim Histalmut Bound 85(2) 4,710 14,130 18,840 0 0
Meitav Gemel Ltd
Meitav Yerushalayim Histalmut Zahav(2) 4,710 14,130 18,840 0 0
Meitav Pension Ltd
Meitav Pensia Makifa(2) 58,820 176,460 235,280 0 0
Meitav Pension Ltd
Meitav Pensia Clalit(2) 2,350 7,050 9,400 0 0
Meitav Mishan Ltd
Meitav Gemel Plus(2) 205,880 617,640 823,520 0 0
Meitav Mishan Ltd
Meitav Histalmut Plus(2) 55,880 167,640 223,520 0 0
Meitav Mishan Ltd
Meitav Pizuim Plus(2) 16,470 49,410 65,880 0 0
Tailor Made Capital(2) 882,353 3,000,000 3,882,353 0 0
Meitav Entities and Tailor Made
Capital as a group (2) 4,558,833 14,029,440 18,588,273(3) 0 0
Falcon Financial Services LLC 750,000 -- 750,000 0 0
---------- ---------- ---------- ----- -----
TOTAL 5,608,833 15,029,440 20,638,273 0 0
========== ========== ========== ===== =====
- ----------
* Less than one percent.
** These figures assume that the selling stockholders will sell all of their
shares available for sale during the effectiveness of the registration
statement that includes this prospectus. The selling shareholders are not
required to sell their shares.
(1) "Prior to this Offering" means prior to the offering by the selling
stockholders of the securities registered under this prospectus for resale.
11
(2) An entity controlled by Meitav Investment House Ltd, ("Meitav") which as
reported on Schedule 13G filed on March 19, 2008, is beneficially owned by
Messrs. Zvi Stepak and Shlomo Simanovsky through intermediary entities.
Messrs. Zvi Stepak and Shlomo Simanovsky may exercise shared voting and
investment powers with respect to all shares owned by Meitav and the Meitav
Entities.
(3) In an appendix to the warrant issued by the Company to the Meitav Entities
and TMC the following exercise limitations have been agreed to: the company
shall not effect the exercise of the warrant and the holder shall not have
the right to exercise any portion of the warrant to the extent that after
giving effect to such issuance after exercise, such holder along with its
affiliates (which include all of Meitav Entities and TMC) shall have more
than 9.99% of the number of shares of common stock outstanding of the
Company. This provision however, may be waived by the holder at its
election upon not less than 61 days' notice to the Company.
PLAN OF DISTRIBUTION
The selling stockholders may, from time to time, sell all or a portion of the
shares of common stock on any market upon which the common stock may be listed
or quoted (currently the National Association of Securities Dealers OTC Bulletin
Board in the United States), in privately negotiated transactions or otherwise.
Such sales may be at fixed prices prevailing at the time of sale, at prices
related to the market prices or at negotiated prices.
The distribution of such shares may be effected by the selling stockholders in one or more of the following methods:
* ordinary brokers transactions, which may include long or short sales,
* transactions involving cross or block trades on anymethods when disposing of their securities or market where our common stock is trading,
* interests therein:
| ● | in market transactions, including transactions on a national securities exchange or quotations service or OTC market; |
| ● | in privately negotiated transactions; |
| ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| ● | in a block trade in which a broker-dealer will attempt to sell a block of securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| ● | through the settlement of short sales (including short sales “against the box”), in each case subject to compliance with the Securities Act and other applicable securities laws; |
| ● | through one or more underwriters in a public offering on a firm commitment or best-efforts basis; |
| ● | an exchange distribution in accordance with the rules of the applicable exchange, if any; |
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | broker-dealers may agree with the Selling Securityholders to sell a specified number of such securities at a stipulated price per security; |
| ● | directly to one or more purchasers; |
| ● | in other ways not involving market makers or established trading markets; |
| ● | by pledge to secure debts and other obligations; |
| ● | through agents; or |
| ● | in any combination of the above or by any other legally available means. |
The Selling Securityholders may, from time to time, pledge or grant a security interest in some or all of the securities owned by brokers, dealersthem and, if they default in the performance of their secured obligations, the pledgees or underwriters as principalsecured parties may offer and resale
by such purchasers forsell their own accounts pursuantsecurities, from time to time, under this prospectus, or under an amendment to this prospectus "atunder Rule 424(b)(3) or other applicable provision of the market"Securities Act amending the list of Selling Securityholders to include the pledgee, transferee or through market makers or into an existing market
for the common stock,
*other successors in interest as Selling Securityholders under this prospectus. The Selling Securityholders also may transfer their securities in other ways not involving market makers or established trading
markets, including direct sales to purchasers or sales effected
through agents
* through transactionscircumstances, in options, swapswhich case the transferees, pledgees or other derivatives (whether
exchange listed or otherwise), or
* any combination of the foregoing.
In addition,successors in interest will be the selling stockholdersbeneficial owners for purposes of this prospectus.
In connection with the sale of our securities or interests therein, the Selling Securityholders may enter into hedging transactions with broker-dealers whoor other financial institutions, which may in turn engage in short sales if short sales were permitted, of sharesour securities in the course of hedging the positions they assume with the selling
shareholders.assume. The selling stockholdersSelling Securityholders may also sell their securities short and deliver these securities to close out their short positions, or loan or pledge such securities to broker-dealers that in turn may sell these securities. The Selling Securityholders may also enter into option or other transactions with broker-dealers thator other financial institutions or the creation of one or more derivative securities which require the delivery byto such broker-dealers or other financial institutions of the shares,securities offered by this prospectus, which sharessecurities such broker-dealers or other financial institutions may be resold thereafterresell pursuant to this prospectus.
Brokers, dealers, underwritersprospectus (as supplemented or agents participating inamended to reflect such transaction).
The aggregate proceeds to the distributionSelling Securityholders from the sale of the shares may receive compensation insecurities offered by them will be the formpurchase price of the security less discounts concessions or commissions, if any. Each of the Selling Securityholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of their securities to be made directly or through agents. We will not receive any of the proceeds from the selling shareholders and/resale of securities being offered by the Selling Securityholders named herein. However, we will receive proceeds from the exercise of the Warrants if they are exercised by a holder thereof.
The Selling Securityholders also may resell all or a portion of their securities in open market transactions in reliance upon Rule 144 under the purchasersSecurities Act, provided that they meet the criteria and conform to the requirements of shares for
whom such broker-dealers may act as agent or to whom they may sell as principal,
or both (which compensation as to a particular broker-dealer may be in excess of
customary commissions). that rule.
The selling shareholdersSelling Securityholders and any broker-dealers actingthat act in connection with the sale of the shares hereunder maysecurities might be deemed to be underwriters“underwriters” within the meaning of Section 2(11)2(a)(11) of the Securities Act, of 1933, and any commissions received by themsuch broker-dealers and any profit realized by them on the resale of sharesthe securities sold by them while acting as principals may be deemed underwriting compensation under the
Securities Act of 1933. The amount of such compensation cannot be estimated at
this time. We know of no existing arrangements between the selling shareholders
and any other shareholder, broker, dealer, underwriter or agent relating to the
sale or distribution of the shares.
We can provide no assurance that all or any of the common stock offered will be
sold by the selling stockholders named in this prospectus. The estimated costs
of this Offering are $21,243.32. We are bearing all costs relating to the
registration of the common stock. The selling stockholders, however, will pay
any commissions or other fees payable to brokers or dealers in connection with
any sale of the common stock.
The selling stockholders named in this prospectus must comply with the
requirements of the Securities Act and the Exchange Act in the offer and sale of
the common stock. The selling stockholders and any broker-dealers who execute
sales for the selling stockholders maymight be deemed to be an "underwriter" withinunderwriting discounts or commissions under the meaningSecurities Act.
To the extent required, the securities to be sold, the names of the Securities Act in connection with such sales. In particular,
during such times asSelling Securityholders, the selling stockholders may be deemed to be engaged in a
distributionrespective purchase prices and public offering prices, the names of the common stock, and therefore be considered to be an
underwriter, they must comply with applicable law and may among other things:
12
1. Not engage in any stabilization activities in connection with our
common stock;
2. Furnish each brokeragent, dealer or dealer through which common stock may be
offered, such copies of this prospectus, as amended from time to time,
as may be required by such broker or dealer; and
3. Not bid for or purchase any of our securities or attempt to induce any
person to purchase any of our securities other than as permitted under
the Exchange Act.
If an underwriter is selected in connection with this Offering, an amendment
will be filed to identify the underwriter, disclose the arrangements with the underwriter, and we will file the underwriting agreement as an exhibitany applicable commissions or discounts with respect to this
prospectus.
The selling stockholders should be aware that the anti-manipulation provisions
of Regulation M under the Exchange Act will apply to purchases and sales of
shares of common stock by the selling stockholders, and that there are
restrictions on market-making activities by persons engaged in the distribution
of the shares. Under Regulation M, the selling stockholders or their agents may
not bid for, purchase, or attempt to induce any person to bid for or purchase,
shares of our common stock while such selling stockholder is distributing shares
covered by this prospectus. Accordingly, the selling stockholders are not
permitted to cover short sales by purchasing shares while the distribution is
taking place. The selling stockholders are advised that if a particular offer of
common stock is towill be made on terms constituting a material change from the
information set forth above with respect to the Plan of Distribution, then, to
the extent required,in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the accompanying registration statement must be filed with the SEC.
INTEREST OF NAMED EXPERTS AND COUNSEL
No expert or counsel named inthat includes this prospectus as having prepared or certified
any part of this prospectus or having given an opinion upon the validity of the
securities being registered or upon other legal matters in connection with the
registration or offering of the common stock was employed on a contingency basis
or had, or is to receive, in connection with the offering, a substantial
interest, directly or indirectly, in the registrant. Nor was any such person
connected with the registrant as a promoter, managing or principal underwriter,
voting trustee, director, officer or employee.
DESCRIPTION OF BUSINESS
OVERVIEW OF THE COMPANY
We are a development stage company in the business of developing battery
charging solutions for small hand-carried devices. We were incorporated on May
17, 2007, in the State of Nevada. We have never declared bankruptcy, have never
been in receivership, and have never been involved in any legal action or
proceedings.
OBJECTIVES
Our product is a man-powered generator for recharging cellular phones and small
batteries. We plan to develop a palm-sized device named YoGen, intended to
provide a quick recharge for cell phones and other personal electronic devices
operating on AA or AAA batteries. Prior to incorporation, our President and
Director, Mr. Guy Ofir, started developing a prototype of the man-powered
generator. We will focus our efforts on developing a product that will be rugged
enough to withstand constant use. It will be shockproof with a suspension system
that maintains the unit in an available state of readiness but not in the user's
way. We plan to keep the controls of the product as user-friendly as possible
with the design of the casing intended to appeal to personal electronic
gadget-lovers in general and more specifically to the younger market segment
which is the largest consumer of disposable batteries.
Our President and Director, Mr. Guy Ofir, purchased the rights to the design and
the patent application in a private transaction. Mr. Guy Ofir transferred those
rights to the company at no cost to us.
We plan to complete the development of the man-powered charger solution for
battery powered small hand-carried devices. On August 20, 2007, we filed a
patent application (Application No.: 11/841,046) with the United States Patent
and Trademark Office. By August 19, 2008, in order to extend the potential
patent protection beyond the United States, we needed to file an international
patent application via the PCT. Indeed, we made such filing on June 30, 2008.
The international filing enables us to file a national patent application in any
PCT country until February 19, 2009 and still claim the benefit of the priority
date of August 20, 2007. The Company's principal business plan is to complete
13
the development of the prototype and then manufacture and market the product and
seek third party entities interested in licensing the rights to manufacture and
market the man-powered charger. We are currently engaged in completion of the
final prototype of the YoGen, including the final design that we will then sell
in the market, before passing the certification procedure required to commence
mass production.
To enable the power capability and provide minutes of operation without fatigue
the YoGen device will generate 20 watts of peak power for each cord pull. It is
designed as a very low profile device fitting into the overall size of 45mm x
70mm x 18mm. A novel design uses a combination of a permanent magnet disk rotor
and integrated stator coils without an iron core and an attached mechanical
drive contribute to the high power and efficiency of the generator. To keep the
unit flat, a simple speed multiplication concept is designed instead of complex
gear mechanism. Furthermore, the magnitude of the inertia extends the effective
generation cycle after the pulling cycle has ceased, thus making for more
effective charging. Designed as a belt attachment, it can be operated with
either hand, adding further to its ergonomics capabilities by dividing and
lowering the required human strength.
Our Secretary, Treasurer and Director, Mr. Emanuel Cohen, will be in charge of
minimizing the size of our product and complete a series of quality tests.
Our product package will consist of a disk generator and the mechanical system
to activate it, an intermittent accumulator battery, an electronic system, a
strong protective casing and a strap for suspending it from a belt.
In the future, if we generate revenue from the sale of our man-powered slim
charger, we plan to sell accessories such as adapters for common hand-held
electrical devices.
TECHNICAL BRIEF:
Controls for
safe charging/ Applicable for
State of up-scaling
Time to recharge 10% Ease of usage internal and for larger
Power capacity of typical 1000 Ergonomic external battery application
Autonomy Level Capability mA/hr Li-ion Battery design indications laptops
- -------------- ---------- -------------------- ------ ----------- -------
Complete autonomy 20 watts max 6-10 minutes Ergonomic Internal and Yes
+ wall charger from the device internal design for arm external Tandem design of
battery pulling. batteries 2 generators
charging with common
~ 2 minutes to replace Belt attached indicating shaft will
the energy in the to enable LEDS produce up to
internal battery by single hand 40 watts
the hand generator charging
Cellular phones consume electric power at a rate which fits to cell antennas,
and the like. For example, cell phone power consumption for conversation mode
(50% talking / 50% listening) near to a cellular antenna is approximately 0.5
watts. Since nominal YoGen output is 5 watts (ten times higher), for every five
minutes of cell phone usage under these conditions, our device can replace the
energy consumed in 30 seconds. This data only applies to short conversations
(typically up to 5 minutes) because during short conversations, a cell phone
battery's state-of-charge does not change significantly. For example, a half
hour conversation under these conditions shall require about 3 minutes to be
brought to its pre-conversation charge state. In all cases, the charge is
proportional to incremental levels of effort.
The efficiency with which our YoGen device recharges cell phone is significantly
dependent on the phone's dedicated built-in charging controller which regulates
maximum charging current. For a majority of up-to-date cellular phone models,
these controllers permit a charging current supplying about 5 watts, which is
the output of our device. A few older cellular phone models permit less of a
charge. For those phones, YoGen is equipped with a switch for a "lower power"
mode.
As indicated before, the YoGen device will generate 20 watts of peak power for
each cord pull. 20W is a peak instant power which an internal alternator can
generate when the cord is pulled very quickly. It is a pure technical
characteristic which was possible to measure only in a laboratory by accessing
YoGen's internal points. We have not used any external third party testing in
connection with this process.
14
YoGen is based on a novel axial-field synchronous generator (alternator)
producing an alternating voltage of frequency and amplitude which are
proportional to its rotation speed. Since it is driven by reciprocating
pull-release hand movement and is outputting energy during rotation, its voltage
and frequency vary considerably through the period.
Cellular phone battery controllers require a definite voltage and internal
impedance of chargers to allow them to charge the batteries. YoGen includes an
electronic converter outputting a stabilized DC voltage falling under load to
emulate required impedance. YoGen has 2 modes: "nominal power" (with nominal
impedance) and "lower power" (with higher impedance).
In its basic format, YoGen includes an internal 400/800 mAh Li-ione buffer
(back-up) battery considerably widening the stabilized power output from zero
pulling speed (the battery gives power) up to pulling high speed (the battery
receives power).
The foregoing statistics are based solely on our own limited internal testing
and have not been verified by an outside testing agency. We are now working to
validate these results in external laboratories. To date, we have produced three
prototypes of our device, each with an internal battery but without a "lower
power" option. These prototypes have been tested in our laboratory with
approximately 15 cellular phone models. Our results confirm the statistics
contained above, provided that the pulling process is done correctly. We are now
in the process of creating the final prototype of the YoGen, including the final
design, that we will then sell in the market.
MARKETING STRATEGY
We plan to market our product in the United States by establishing a network of
wholesalers who can promote our product to the retail market such as 7-Eleven,
Wal-Mart and other high-traffic locations and points-of-sale which cater to
electronic accessories such as Office Depot, Radio Shack and Circuit City.
We plan to offer our product at a comparable price to other re-charging systems,
we will stress advantages such as clean energy source, use with a wide array of
products and dependability. The end-user should be able to purchase a unit for
$30 to $50 depending on packaging and features.
Our President and Director, Mr. Guy Ofir, will be in charge of executing the
marketing plan.
We have budgeted $250,000 for this purpose.
THE MARKET OPPORTUNITY
Our target market consists of the following market segments: cellular phones,
which are our main initial market, laptops and notebooks, mobile hand held
computers: PDAs, GPS devices and smart phones and digital still cameras and
camcorders., all of which are markets with tremendous growth opportunities.
COMPETITION
Competition within the re-charger systems industry is intense. Many of our
competitors have longer operating histories, greater financial, sales, marketing
and technological resources and longer established client relationships than we
do. Solutions currently marketed for the problem Easy Energy addresses include
several different groups of products, each with its own advantage and
disadvantage. Although there are several other ways of recharging batteries for
a cell phone, the only ones we know of that are in direct competition are the
hand-held human powered chargers. Such competitors include:
IST Design Inc.'s Sidewinder hand charger has a crank-operated mechanism.
Sidewinder is designed and produced by ElectroHiFi, LLC as the SOS Charger.
Sidewinder is claimed to generate 6 minutes of talk time for every 2 minutes of
turning at 2 revolutions per second. The unit currently sells for $19.95 to
$24.95.
Aladdinpower Inc. has a hand-squeeze generator, meant primarily for cell phones,
which has been on the market since 2002. Although it claims to be useable with
any rechargeable battery, it produces only 1.6 watts, which may not be effective
in recharging a cell phone. It is currently priced at around $60.
Freeplay Energy plc has developed Freeplay Freecharge, a Freecharge mobile phone
charge, available in the market since 2002. This product has ergonomic features,
including reverse winding. It claims that talk time of 2-3 minutes (depending on
the mobile phone used), or several hours of standby time, can be achieved with
15
45 seconds of winding. Its internal battery is a rechargeable NiMH battery pack,
3.6 Volts, 1300 mAh. It is currently priced at $59.95 per unit.
There are other products in the market, but normally, such products are
unbranded copies of the above mentioned designs.
We seek to differentiate ourselves by providing a slimmer and lighter generator
at a competitive price.
DESCRIPTION OF PROPERTY
Our executive and head office is located at Suite 105 - 5348 Vegas Dr., Las
Vegas, NV 89108. Our office is rented on a month to month sub-lease at a cost of
$50 per month. We also have an office in Israel at 26 Ga'aton Blvd., Nahariya
22401, which is provided to us free of charge by our directors. We believe that
our office space and facilities are sufficient to meet our present needs and do
not anticipate any difficulty securing alternative or additional space, as
needed, on terms acceptable to us.
prospectus.
DESCRIPTION OF SECURITIES
COMMON STOCK
We are authorized
The following summary of the terms of our Common Stock does not purport to issue 1,000,000,000 common shares with a par valuebe complete and is subject to and qualified in its entirety by reference to our articles of $0.00001. Asincorporation and bylaws.
Common Stock
The holders of July 1, 2008 there were 93,186,070outstanding shares of our common stock
issuedCommon Stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and outstanding.in such amounts as the board from time to time may determine. Holders of Common Stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for election. The Common Stock are not entitled to pre-emptive rights and are not subject to conversion or redemption. Upon liquidation, dissolution or winding up of the corporation,Company, the holders of
common stock are entitled to share ratably in all net assets legally available for distribution to shareholders after payment to creditors. The common stock is not
convertible or redeemable and has no pre-emptive, subscription or conversion
rights. Therestockholders are no conversion, redemption, sinking fund or similar provisions
regardingdistributable ratably among the common stock. Each outstanding shareholders of common stock is entitled
to one vote on all matters submitted to a vote of shareholders. There are no
cumulative voting rights.
Each shareholder is entitled to receive the dividends as may be declared by our
directors out of funds legally available for dividends and, in the event of
liquidation, to share pro rata in any distribution of our assetsCommon Stock after payment of liabilities. liquidation preferences, if any, on any outstanding payment of other claims of creditors. Each outstanding common share is duly and validly issued, fully paid and non-assessable.
Our directors are not obligated to declare a dividend. Any
future dividends will be subject to the discretion of our directorsTransfer Agent and will
depend upon, among other things, future earnings, the operating and financial
condition of our company, our capital requirements, general business conditions
and other pertinent factors. It is not anticipated that dividends will be paid
in the foreseeable future.
There are no provisions in our articles of incorporation or our bylaws that
would delay, defer or prevent a change in control of our company.
PREFERRED STOCK
We are authorized to issue 50,000,000 shares of preferred stock with a par value
of $0.0001. As of September 12, 2008 there were no preferred shares issued and
outstanding.
WARRANTS
As of September 12, 2008, there were outstanding warrants to purchase 3,000,000
shares of our common stock at an exercise price of $0.27 per share, which were
issued in conjunction with the Private Placement we undertook in March 10, 2008.
These warrants expire on March 10, 2013.
As of September 12, 2008, there were outstanding warrants to purchase 1,000,000
shares of our common stock at an exercise price of $0.15 per share, which were
issued in conjunction with the Private Placement we undertook in March 3, 2008.
These warrants expire on March 3, 2013.
As of September 12, 2008, there were outstanding warrants to purchase 11,029,440
shares of our common stock at an exercise price of $0.27 per share, which were
issued in conjunction with the Private Placement we undertook in February 28,
2008. These warrants expire on February 28, 2013.
16
TRANSFER AGENT AND REGISTRAR
We have appointed the followingWarrant Agent
The transfer agent for our shares of common stock:
HolladayCommon Stock is Pacific Stock Transfer Inc., 2939 North 67th Place,Company, 6725 Via Austi Pkwy, Suite C, Scottsdale, AZ
85251, Telephone: (480) 481-3940; Facsimile: (480) 481-3941. The transfer agent
is responsible for all record-keeping and administrative functions in connection
with the common shares.
LEGAL PROCEEDINGS
We know300, Las Vegas, Nevada 89119.
Special meeting of no material, existing or pending legal proceedings against our
company, nor are we involved as a plaintiff in any material proceeding or
pending litigation. There are no proceedings in which anystockholders
Our Bylaws provide that special meetings of our directors,
Officersstockholders may be called only by a majority vote of our Board of Directors, or affiliates,by our President, or any registered or beneficial shareholder, is an
adverse party or has a material interest adverse to our interest.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
Our stock is listed for quotation on the OTC Bulletin Board under the trading
symbol "ESYE.OB". Our common shares initially began trading on the OTC Bulletin
Board on December 26, 2007. The following table sets forth, for the periods
indicated, the high and low closing prices for each quarter within the last
fiscal year ended December 31, 2007 and subsequent interim period as reported by the quotation service operated by the OTC Bulletin Board. All quotations for the
OTC Bulletin Board reflect inter-dealer prices, without retail mark-up,
mark-down or commission and may not necessarily represent actual transactions.
Year 2007 High Low
--------- ---- ---
Fourth Quarter $0.30 $0.15
Year 2008 High Low
--------- ---- ---
First Quarter $0.30 $0.15
Second Quarter $0.30 $0.12
HOLDERS
On September 12 2008, the closing price of our common stock as reported on the
OTC Bulletin Board was $0.12 per share. On September 12, 2008, we had
approximately 62 holders of recordsa majority of commonthe outstanding shares of capital stock and 93,186,070of the Company, the holders of which are entitled to vote on matters that are to be voted on at such meeting.
Rule 144
Pursuant to Rule 144, a person who has beneficially owned restricted shares of our common stock were issued and outstanding, plus an additional 15,029,440 shares
issuable upon the exercise of outstanding warrants. SomeCommon Stock for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been one of our shares are held
in brokers' accounts, soaffiliates at the time of, or at any time during the three months preceding, a sale and (ii) we are unablesubject to give an accurate statementthe Exchange Act periodic reporting requirements for at least three months before the sale and have filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months (or such shorter period as we were required to file reports) preceding the sale.
Persons who have beneficially owned restricted shares of our Common Stock for at least six months but who are our affiliates at the time of, or at any time during the three months preceding, a sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of shareholders.
DIVIDEND POLICY
We havesecurities that does not declared or paid any cash dividends since inception. We do not
intend to pay any cash dividends inexceed the foreseeable future. Although there are
no restrictions that limit our ability to pay dividends on our common stock, we
intend to retain future earnings for use in our operations and the expansion of
our business. Our future dividend policy will be determined from time to timegreater of:
| ● | 1% of the total number of shares of Common Stock then outstanding; or |
| ● | the average weekly reported trading volume of the Common Stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by our Boardaffiliates under Rule 144 are also limited by manner of Directors.
Tosale provisions and notice requirements and to the extent that we require additional funding our funding sources may
prohibitavailability of current public information about us.
Restrictions on the paymentUse of a dividend. Because we doRule 144 by Shell Companies or Former Shell Companies
Rule 144 is not intend to declare
dividends, any gain on an investment in our company will need to come through an
increase in the stock's price, which may never happen.
EQUITY COMPENSATION PLAN INFORMATION
As of July 1, 2008 and as of December 31, 2007, the end of our most recently
completed fiscal year, our company did not have any equity compensation plan.
17
MANAGEMENT'S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION
THE FOLLOWING DISCUSSION OF OUR PLAN OF OPERATION SHOULD BE READ IN CONJUNCTION
WITH THE FINANCIAL STATEMENTS AND RELATED NOTES THAT APPEAR ELSEWHERE IN THIS
PROSPECTUS. THIS DISCUSSION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE
RISKS AND UNCERTAINTIES. OUR ACTUAL RESULTS COULD DIFFER MATERIALLY FROM THOSE
ANTICIPATED IN THESE FORWARD-LOOKING STATEMENTS AS A RESULT OF VARIOUS FACTORS,
INCLUDING THOSE DISCUSSED IN "RISK FACTORS" BEGINNING ON PAGE 2 OF THIS
PROSPECTUS. ALL FORWARD-LOOKING STATEMENTS SPEAK ONLY AS OF THE DATE ON WHICH
THEY ARE MADE. WE UNDERTAKE NO OBLIGATION TO UPDATE SUCH STATEMENTS TO REFLECT
EVENTS THAT OCCUR OR CIRCUMSTANCES THAT EXIST AFTER THE DATE ON WHICH THEY ARE
MADE.
OVERVIEW
We are a development stage company with limited operations and no revenues from
our business operations. Our auditors have issued a going concern opinion. This
means that our auditors believe there is substantial doubt that we can continue
as an on-going businessavailable for the next twelve months. We do not anticipateresale of securities initially issued by shell companies (other than business combination related shell companies) or issuers that we
will generate significant revenues until we have completed the development and
manufacturing of our product. We plan to develop a man-powered charger solution
for battery powered small hand-carried devices.
On August 20, 2007 we filed a patent application (Application No.: 11/841,046)
with the United States Patent and Trademark Office. Prior to our incorporation,
Mr. Guy Ofir, Our President and Director started developing a prototype of the
man-powered generator. This prototype is a "successor" of several practically
validated approaches suggested by its inventors as outlined below:
- Charger shall be powerful enough to provide a significantly higher
talk-to-charge time ratio (current models operate at about 1:1);
- Charger shall include a converter providing both utilization of input
mechanical energy through the entire speed range and stabilized
electric output;
- Charger shall have an option to include a back-up internal battery;
- Charger size shall be not greater than those of today's cell-phones
and should be as thin as possible; and
- Mechanical input shall be of a reciprocating pull-release type since
such a hand application allows for extended use without tiredness.
This product is based on a novel dedicated application of the unique high
power-density planar alternator technology developed and tested by one of the
inventors.
This technology allows for implementation of these principles since and makes it
possible to provide an extremely slim and unique compact package containing all
charger components, which include: mechanical transmission electronic converter,
back-up battery and the alternator itself.
The technology of the proposed product was invented by Alexander Sromin and
Michael Fridhendler. Mr. Sromin is an alumnus of the Academy for Aviation &
Space Instruments in St. Petersburg, Russia with 22 years of research and
development experience related to a wide range of electric machines and
electromechanical systems. His professional scope covers compact permanent
magnet motors, actuators (including linear, rotating, reciprocating, multi-axis,
etc.) and generators in the range from MEMS up to 100 KW. He is an author of
more than 30 articles and inventor of 10 inventions. Mr. Friedhendler graduated
from The Technion University in Haifa, Israel. He has 25 years of experience in
multi-disciplinary hi-tech ventures promotion and technological support. He
succeeded in numerous application projects in the field of mobile and internet
GPS and J2ME software projects among others. His expertise relates to
electronics, communications, cellular technology, internet and video data
transfer.
Both Mr. Sromin and Mr. Fridhendler worked for Pipera Technologies Ltd.
("Pipera"), a company wholly owned and fully funded by our president and
director, Mr. Ofir, and during the course of their work, developed the
technology of the proposed product. Mr. Sromin and Mr. Fridhendler have been assistedat any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| ● | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| ● | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| ● | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports; and |
| ● | at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company. |
The Company filed its Form 10 with SEC on July 29, 2021, as amended by Mr. Roman Lanzet that serves as production manager at Pipera. Mr.
Sromin, Mr. FridhendlerAmendment No. 1 and Mr. Lanzet assigned their rights in the technology
of the productAmendment No. 2 to the Company for no consideration pursuant to the terms of the
assignment agreement dated August 15, 2007Form 10 filed by the Company as exhibit 10.3on September 2, 2021 and September 23, 2021, respectively.
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion of the material U.S. federal income tax considerations generally applicable to the Company's registration statement on Form SB-2 filed on October 24, 2007.
The prototypeacquisition, ownership and disposition of our Common Stock. This discussion is limited to certain U.S. federal income tax considerations to beneficial owners of our securities who hold the securities as a capital asset within the meaning of Section 1221 of the YoGen product was created by Pipera. Pipera's employeesU.S. Internal Revenue Code of 1986, as amended (the “Code”). This discussion does not describe all of the tax consequences that workedmay be relevant to you in light of your particular circumstances, including the alternative minimum tax, the Medicare contribution tax on certain investment income and the different consequences that may apply if you are subject to special rules that apply to certain types of investors, such as:
| ● | financial institutions or financial services entities; | |
| ● | broker-dealers; | |
| ● | insurance companies; | |
| ● | governments or agencies or instrumentalities thereof; | |
| ● | regulated investment companies; | |
| ● | real estate investment trusts; | |
| ● | expatriates or former long-term residents of the United States; | |
| ● | persons that actually or constructively own five percent or more of our voting shares; | |
| ● | persons that acquired our securities pursuant to an exercise of employee share options, in connection with employee share incentive plans or otherwise as compensation; | |
| ● | dealers or traders subject to a mark to market method of accounting with respect to the securities; | |
| ● | persons holding the securities as part of a “straddle,” hedge, constructive sale, conversion or other integrated or similar transaction; | |
| ● | U.S. holders (as defined below) whose functional currency is not the U.S. dollar; | |
| ● | persons subject to special tax accounting rules under Section 451(b) of the Code; partnerships or other pass through entities for U.S. federal income tax purposes; and | |
| ● | tax exempt entities. |
If you are a partnership for U.S. federal income tax purposes, the U.S. federal income tax treatment of your partners will generally depend on the creation of such prototype included mainly Mr. Sromin and Mr.
Fridhendler.
18
On January 29, 2008 we announced the completionstatus of the fully working prototypepartners and your activities.
This discussion is based on the Code and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury regulations as of the man-powered charger solution for battery powered small hand-carried
devices. Such prototype is designed for testingdate hereof, changes to any of which subsequent to the principles behinddate of this prospectus may affect the product. The Company's principal business plan is to completetax consequences described herein. This discussion does not address any aspect of state, local or non-U.S. taxation, or any U.S. tax law other than the manufacturing
design, mainly minimizing the product and improving human engineeringU.S. federal income tax (such as gift, estate or Medicare contribution taxes) or except as discussed below, any tax reporting obligations of the
product and then manufacture and market the product and / or seek third party
entities interested in licensing the rights to manufacture and market our
product. We estimate that we could begin the manufacturing of the products
during the third quarter of 2008. We have selected a contractor to assist us
with this process. We estimate the costs to be incurred by the time we have an
operating manufacturing line ready for mass production to be at approximately
$400,000.
Our business objectives are:
- To complete the designholder of our product.
- To engage third parties firm(s) to manufacture the components ofsecurities. This discussion also assumes that any distribution made (or deemed made on our
product.
- To set up an assembly line. - To be a leading provider of man-powered
charger.
- To execute our marketing plan.
Our goals over the next 12 months are:
- Develop and manufacture a first product suited to cellular phone use.
- Explore potential distributors for our product.
ESTIMATED EXPENSES FOR THE NEXT TWELVE MONTH PERIOD:
We have not sought, and March of 2008. Cash
used in operations amountedwill not seek, a ruling from the Internal Revenue Service (the “IRS”) as to $502,243 representedany U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a loss of $1,444,242 plus
an increase in prepaid expenses from the previous balance sheet of $37,500 and
offset by non-cash adjustments for contributed capital and common stock and
warrants issued for services totaling $960,083.
As indicated above, our estimated working capital requirements and projected
operating expenses for the next twelve month period total $922,000, of which
$250,000 has been prepaid. We anticipate that such funds will not be sufficient
to pay our estimated expenses for the next twelve month period. We intend to
fulfill any additional cash requirement through the sale of either equity or
debt. Historically we have financed our operation through the sale of equity. On
August 27, 2007, we closed a private placement for 30,333,190 common shares at a
price of $0.003 per share, or an aggregate of $91,000. On February 28, 2008, we
commenced a private placement offering of 367,647.6 units, each unit being
offered for $1.70, for aggregate gross proceeds of $625,001. Each unit consisted
of (i) ten common stock shares, (ii) thirty Class A Warrant. Each Class A
Warrant entitles the holder thereof to purchase one share of common stock at an
exercise price of $0.27 per share, expiring five years from the date of
purchase. This offering was made to non-U.S. persons in offshore transactions
pursuant to the exemption from registration provided by Regulation S of the
Securities Act. We agreed to register the shares and warrants issued in this
transaction on a registration statement. On that same date, we also sold and
issued 208,333 common stock shares under Rule 903 of Regulation S of the Act to
an accredited investor for the aggregate purchase price of US $50,000, or a
purchase price of US $0.24 per share.
On March 10, 2008, we entered into a Securities Purchase Agreement and a
Registration Rights Agreement with Tailor Made Capital, Ltd. ("TMC") relating to
the issuance to TMC of 882,353 shares of our common stock, par value $0.0001 per
share, and a warrant to purchase up to 3,000,000 shares of the Company's common
stock at a price of $0.27 per share (the "Warrant"). The Warrant shall be in
effect for five years from the date that the Company's common stock is initially
listed or quoted for trading on a trading market. The Securities Purchase
Agreement further provided that, at the Company's demand, TMC will purchase up
to an additional $1,000,000 of shares of the Company's common stock commencing
immediately after the date that the shelf registration of the Company's shares
that are subject to the Securities Purchase Agreement is declared effective (the
"Put").We agreed to file a registration statement to register all of the shares
of common stock to be issued pursuant to the Securities Purchase Agreement,
including those shares issuable upon the exercise of the Warrant and the Put.
On March 25, 2008, we entered into a subscription agreement under which we
undertook to issue 2,000,000 shares for a cash payment of $50,000 by an
"accredited investor" as defined in Rule 501 of Regulation D under the
Securities Act of 1933, and, in consideration for services provided, warrants to
purchase 1,000,000 shares of the Company's common stock at an exercise price of
$0.10 for a period of three years. On March 27, 2008, (pursuant to an agreement
dated January 16, 2008) we issued 4,285,714 common stock shares to an
"accredited investor" for the aggregate purchase price of US $300,000, purchase
price of $0.07 per share.
20
We have used our stock as form of consideration for certain services provided to
us and intend to continue to do so in selected contracts from time to time.
There are no assurances that we will be able to obtain funds required for our
continued operation. Therecourt. Moreover, there can be no assurance that additional financingfuture legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
THIS DISCUSSION IS ONLY A SUMMARY OF THE U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES. EACH PROSPECTIVE INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL, AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS AND ANY APPLICABLE TAX TREATIES.
U.S. Holders
This section applies to you if you are a “U.S. holder.” A U.S. holder is a beneficial owner of our securities that is, for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized in or under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or | |
| ● | a trust if (i) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (ii) it has in effect a valid election to be treated as a U.S. person. |
Taxation of Distributions. If we pay cash distributions to U.S. holders of shares of our Common Stock, such distributions generally will be availabletreated as a dividend for U.S. federal income tax purposes to us when neededthe extent the distribution is paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in our Common Stock. Any remaining excess will be treated as gain realized on the sale or other disposition of the Common Stock and will be treated as described under “U.S. holders — Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities” below.
Dividends we pay to a U.S. holder that is a taxable corporation generally will qualify for the dividends received deduction if available,the requisite holding period is satisfied. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate U.S. holder generally will constitute “qualified dividends” that will be subject to tax at the maximum tax rate accorded to long-term capital gains.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities. Upon a sale or other taxable disposition of our securities which, in general, would include a redemption of Common Stock or warrants, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. holder’s adjusted tax basis in such securities. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period for the securities so disposed of exceeds one year. Long-term capital gains recognized by non-corporate U.S. holders will be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to various limitations that are not described herein because a discussion of such limitations depends on each U.S. holder’s particular facts and circumstances.
Generally, the amount of gain or loss recognized by a U.S. holder is an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. holder’s adjusted tax basis in its securities so disposed of. A U.S. holder’s adjusted tax basis in its Common Stock or warrants generally will equal the U.S. holder’s acquisition cost less, in the case of a share of Common Stock, any prior distributions treated as a return of capital.
Information Reporting and Backup Withholding. In general, information reporting requirements may apply to dividends paid to a U.S. holder and to the proceeds of the sale or other disposition of our securities, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it canis subject to backup withholding (and such notification has not been withdrawn).
Any amounts withheld under the backup withholding rules will be obtainedallowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Non-U.S. Holders
This section applies to you if you are a “Non-U.S. holder.” A Non-U.S. holder is a beneficial owner of our securities who or that is, for U.S. federal income tax purposes:
| ● | a non-resident alien individual, other than certain former citizens and residents of the United States subject to U.S. tax as expatriates; | |
| ● | a foreign corporation; or | |
| ● | an estate or trust that is not a U.S. holder; |
but does not include an individual who is present in the United States for 183 days or more in the taxable year of disposition.
If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the sale or other disposition of a security.
Taxation of Distributions. In general, any distributions we make to a Non-U.S. holder of shares of our Common Stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States, we will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on commercially reasonable terms.an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its shares of our Common Stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as gain realized from the sale or other disposition of the Common Stock, which will be treated as described under “Non-U.S. holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities” below. In addition, if we determine that we are classified as a “United States real property holding corporation” (see “Non-U.S. holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities” below), we will withhold 15% of any distribution that exceeds our current and accumulated earnings and profits.
The withholding tax does not apply to dividends paid to a Non-U.S. holder who provides a Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular U.S. federal income tax as if the Non-U.S. holder were a U.S. resident, subject to an applicable income tax treaty providing otherwise. A Non-U.S. corporation receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower treaty rate).
Possible Constructive Distributions. Under certain circumstances, a Non-U.S. holder may be deemed to have received a constructive dividend (see “U.S. holders” above). Any such constructive distribution deemed received will be treated, and therefore generally be subject to withholding, in the same manner as an actual dividend received (and may be subject to information reporting), as discussed above under “Non-U.S. Holders — Taxation of Distributions.” Because a constructive dividend received by a Non-U.S. holder would not give rise to any cash from which any applicable withholding tax could be satisfied, if we or the withholding agent pays withholding taxes on the Non-U.S. holder’s behalf with respect to amounts which are includible in the Non-U.S. holder’s income but which are not paid in cash, we or the withholding agent may withhold any such withholding tax from any other payments owed to the Non-U.S. holder or other assets, including cash payments of interest payable on the notes, shares of Common Stock or cash payable upon conversion, or proceeds from a sale subsequently paid or credited to the Non-U.S. holder. If we are not able to obtain additional financingdeduct, or such withholding agent deducts, any such amount from interest payments on a timely basis, weNon-U.S. holder’s notes under these circumstances, such Non-U.S. holder should consult its own tax advisor as to whether it can obtain a refund for all or a portion of any tax withheld.
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Our Securities. A Non-U.S. holder generally will not be ablesubject to meetU.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of our securities unless:
| ● | the gain is effectively connected with the conduct of a trade or business by the Non-U.S. holder within the United States (and, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. holder); or | |
| ● | we are or have been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. holder held our securities, and, in the case where shares of our Common Stock are regularly traded on an established securities market, the Non-U.S. holder has owned, directly or constructively, more than 5% of our Common Stock at any time within the shorter of the five-year period preceding the disposition or such Non-U.S. holder’s holding period for the shares of our Common Stock. There can be no assurance that our Common Stock will be treated as regularly traded on an established securities market for this purpose. |
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to tax at generally applicable U.S. federal income tax rates as if the Non-U.S. holder were a U.S. resident. Any gains described in the first bullet point above of a Non-U.S. holder that is a foreign corporation may also be subject to an additional “branch profits tax” at a 30% rate (or lower treaty rate).
If the second bullet point above applies to a Non-U.S. holder, gain recognized by such holder on the sale, exchange or other disposition of our securities will be subject to tax at generally applicable U.S. federal income tax rates. In addition, a buyer of our securities from such holder may be required to withhold U.S. federal income tax at a rate of 15% of the amount realized upon such disposition. We will be classified as a U.S. real property holding corporation if the fair market value of our “U.S. real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other obligationsassets used or held for use in a trade or business, as they
become duedetermined for U.S. federal income tax purposes.
Information Reporting and weBackup Withholding. Information returns will be forced to scale downfiled with the IRS in connection with payments of dividends and the proceeds from a sale or perhaps even cease the
operationother disposition of our business.
Givensecurities. A Non-U.S. holder may have to comply with certification procedures to establish that we areit is not a development stage companyUnited States person in order to avoid information reporting and backup withholding requirements. The certification procedures required to claim a reduced rate of withholding under a treaty will satisfy the certification requirements necessary to avoid the backup withholding as well. The amount of any backup withholding from a payment to a Non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
FATCA Withholding Taxes
Provisions commonly referred to as “FATCA” impose withholding of 30% on payments of dividends (including constructive dividends) on our securities, and sales or other disposition proceeds from our securities to “foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other Non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied, or an exemption applies (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). If FATCA withholding is imposed, a beneficial owner of the payment that is not generateda foreign financial institution (or that is a foreign financial institution entitled to a reduced rate of withholding tax with respect to such payment under an income tax treaty) generally may be entitled to a refund or credit of any revenuesamounts withheld by filing a U.S. federal income tax return and providing certain other information to date, our cash flow projections arethe IRS (which may entail significant administrative burden). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to numerous
contingencies and risk factors beyond our control, including market acceptancedifferent rules. Notwithstanding the foregoing, the IRS has issued proposed regulations, upon which taxpayers may generally rely, that exclude gross proceeds from the sale or other disposition of our products, competitionsecurities from well-funded competitors,the application of the withholding tax imposed under FATCA. Prospective investors should consult their tax advisers regarding the effects of FATCA on their investment in our securities.
LEGAL MATTERS
The validity of the securities offered by this prospectus will be passed upon for us by Sullivan & Worcester LLP.
EXPERTS
The financial statements of the Company as of and our abilityfor the years ended December 31, 2021 and 2020 included in this prospectus have been so included in reliance on the reports (which contains an explanatory paragraph relating to manage our expected growth. We can offer no assurance that our company will
generate cash flow sufficient to meet our cash flow projections or that our
expenses will not exceed our projections. If our expenses exceed estimates, we
will require additional monies during the next twelve months to execute our
business plan.
There is substantial doubt about ourCompany’s ability to continue as a going concern as the continuation of our business is dependent upon obtaining further long-term
financing, successful development of our technologies into a marketable product
and successful and sufficient market acceptance of our products once developed
and, finally, achieving a profitable level of operations. The issuance of
additional equity securities by us could resultdescribed in significant dilution of the
equity interests of our current stockholders. Obtaining commercial loans,
assuming those loans would be available, will increase our liabilities and
future cash commitments.
GOING CONCERN
DueNote 1B to the uncertaintyfinancial statements) of our ability to meet current operating and capital
expenses, our independent auditors included an explanatory paragraph regarding
substantial doubt about our ability to continue asBrightman Almagor Zohar & Co., a going concern in their
audit report for the period ended March 31, 2008.
PURCHASE OF SIGNIFICANT EQUIPMENT
We do not expect to purchase any significant equipment over the twelve months.
EMPLOYEES
Currently our only employees are our directors and officers. We do not expect
any other material changesfirm in the number of employees over the next 12 months.
OFF-BALANCE SHEET ARRANGEMENTS
Our company does not have any off-balance sheet arrangements, including any
outstanding derivative financial statements, off-balance sheet guarantees,
interest rate swap transactions or foreign currency contracts. Our company does
not engage in trading activities involving non-exchange traded contracts.
APPLICATION OF CRITICAL ACCOUNTING POLICIES
Our financial statements and accompanying notes are prepared in accordance with
generally accepted accounting principles used in the United States. Preparing
financial statements requires management to make estimates and assumptions that
affect the reported amounts of assets, liabilities, revenue, and expenses. These
estimates and assumptions are affected by management's application of accounting
policies. We believe that understanding the basis and nature of the estimates
and assumptions involved with the following aspects of our financial statements
is critical to an understanding of our financials.
ACCOUNTING BASIS
These financial statements are prepared on the accrual basis of accounting in
conformity with accounting principles generally accepted in the United States of
America.
21
EARNINGS PER SHARE
In February 1997, the FASB issued SFAS No. 128, "Earnings Per Share", which
specifies the computation, presentation and disclosure requirements for earnings
(loss) per share for entities with publicly held common stock. SFAS No. 128
supersedes the provisions of APB No. 15, and requires the presentation of basic
earnings (loss) per share and diluted earnings (loss) per share. The Company has
adopted the provisions of SFAS No. 128 effective its inception.
The basic earnings (loss) per share is calculated by dividing the Company's net
income available to common shareholders by the weighted average number of common
shares during the year. The diluted earnings (loss) per share is calculated by
dividing the Company's net income (loss) available to common shareholders by the
diluted weighted average number of shares outstanding during the year. The
diluted weighted average number of shares outstanding is the basic weighted
number of shares adjusted as of the first of the year for any potentially
dilutive debt or equity.
DIVIDENDS
The Company has not yet adopted any policy regarding payment of dividends. No
dividends have been paid during the periods shown.
CASH EQUIVALENTS
The Company considers all highly liquid investments with maturity of three
months or less when purchased to be cash equivalents.
INCOME TAXES
The Company provides for income taxes under Statement of Financial Accounting
Standards No. 109, "Accounting for Income Taxes" and FIN 48. SFAS No. 109
requires the use of an asset and liability approach in accounting for income
taxes.
SFAS No. 109 requires the reduction of deferred tax assets by a valuation
allowance if, based on the weight of available evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. No
provision for income taxes is included in the statement due to its immaterial
amount, net of the allowance account, based on the likelihood of the Company to
utilize the loss carry-forward.
USE OF ESTIMATES
The preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of revenue and expenses during
the reporting period. Actual results could differ from those estimates.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS
ON ACCOUNTING AND FINANCIAL DISCLOSURE
We engaged the firm of Moore & Associates Chartered to audit our financial
statements for the period ended March 31, 2008. There has been no change in the
accountants and no disagreements with Moore & Associates Chartered, on any
matter of accounting principles or practices, financial statement disclosure, or
auditing scope procedure.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
All directors of our company hold office until the next annual meeting of the
shareholders or until their successors have been elected and qualified. The
executive officers of our company are appointed by our board of directors and
hold office until their death, resignation or removal from office. Our directors
and executive officers, their ages, positions held, and duration as such, are as
follows:
22
Position Held Date First Elected
Name with the Company Age or Appointed
---- ---------------- --- ------------
Guy Ofir President and Director 35 May 17, 2007
Emanuel Cohen Secretary, Treasurer and Director 58 May 17, 2007
BUSINESS EXPERIENCE:
The following is a brief account of the education and business experience of
each director and executive officer during at least the past five years,
indicating each person's business experience, principal occupation during the
period, and the name and principal business of the organization by which they
were employed.
MR. GUY OFIR
Mr. Guy Ofir has been serving as our President and a member of our Board of
Directors since May 17, 2007. The term of his office is for one year and is
renewable on an annual basis.
Mr. Ofir is an attorney and owns a law firm in Israel which specializes in
corporate and international law. His law firm employs several lawyers and
represents over 100 companies.
In addition to his work as a lawyer, he also manages investments and companies
in Romania. His main company (Guy Ofir & Co. SRL) deals with land and properties
in Bucharest.
MR. EMANUEL COHEN
Mr. Cohen has been serving as our Secretary, Treasurer and a member of our Board
of Directors since May 17, 2007. The term of his office is for one year and is
renewable on an annual basis.
Mr Cohen is a major shareholder and a director of several privately owned
companies in Israel & in the United States. His specialialty includes land,
properties & fabrics. (Amitex & Emday Ltd- one of the biggest Israeli fabric
companies ). He is also a shareholder in private companies which hold land &
properties in Israel. - (Lev Hazom Ltd), (Hafia Zamin Ltd), (Leved Adi
Properties Ltd) & (Mashko Ltd).
In addition to his activities in Europe & Israel, he is also a shareholder in
the following companies which hold land & properties in the USA .- (Echo
investments LLC), (Bilou Capital investment LLC) & (Eden Associated LLC).
Previously he was an officer in Israel's largest bank, Bank of Israel (Israel
Hapoalim Bank).
FAMILY RELATIONSHIPS:
There are no family relationships among our directors or executive officers.
INVOLVEMENT IN CERTAIN LEGAL PROCEEDINGS
Our directors, executive officer and control person have not been involved in
any of the following events during the past five years:
1. any bankruptcy petition filed by or against any business of which such
person was a general partner or executive officer either at the time
of the bankruptcy or within two years prior to that time;
2. Any conviction in a criminal proceeding or being subject to a pending
criminal proceeding (excluding traffic violations and other minor
offences');
3. Being subject to any order, judgment, or decree, not subsequently
reversed, suspended or vacated, of any court of competent
jurisdiction, permanently or temporarily enjoining, barring,
suspending or otherwise limiting his involvement in any type of
business, securities or banking activities; or
23
4. being found by a court of competent jurisdiction (in a civil action),
the Commission or the Commodity Futures Trading Commission to have
violated a federal or state securities or commodities law, and the
judgment has not been reversed, suspended, or vacated.
COMMITTEES OF THE BOARD:
All proceedings of the board of directors for the year ended December 31, 2007
were conducted by resolutions consented to in writing by board of directors and
filed with the minutes of the proceedings of the director. Our company currently
does not have nominating, compensation or audit committees or committees
performing similar functions nor does our company have a written nominating,
compensation or audit committee charter. Our officers and directors believe that
it is not necessary to have such committees, at this time, because the functions
of such committees can be adequately performed by the board of directors.
Our company does not have any defined policy or procedural requirements for
shareholders to submit recommendations or nominations for directors. The board
of directors believes that, given the stage of our development, a specific
nominating policy would be premature and of little assistance until our business
operations develop to a more advanced level. Our company does not currently have
any specific or minimum criteria for the election of nominees to the board of
directors and we do not have any specific process or procedure for evaluating
such nominees. Our board of directors, as the case may be, will assess all
candidates, whether submitted by management or shareholders, and make
recommendations for election or appointment.
A shareholder who wishes to communicate with our board of directors may do so by
directing a written request addressed to our President and director, Guy Ofir,
at the address appearing on the first page of this prospectus.
AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that we do not have a board member that
qualifies as an "audit committee financial expert" as defined in Item 401(e) of
Regulation S-B, nor do we have a Board member that qualifies as "independent" as
the term is used in Item 7(d)(3)(iv)(B) of Schedule 14A under the Securities
Exchange Act of 1934, as amended, and as defined by Rule 4200(a)(14) of the NASD
Rules.
We believe that our board of directors is capable of analyzing and evaluating
our financial statements and understanding internal controls and procedures for
financial reporting. The board of directors of our company does not believe that
it is necessary to have an audit committee because management believes that the
functions of an audit committee can be adequately performed by our board of
directors. In addition, we believe that retainingDeloitte global network, an independent director who
would qualify as an "audit committee financial expert" would be overly costly
and burdensome and is not warranted in our circumstancesregistered public accounting firm, given the stage of our
development and the fact that we have not generated any positive cash flows from
operations to date.
CONFLICT OF INTEREST
None of our officers or directors is subject to a conflict of interest.
EXECUTIVE COMPENSATION
No executive officer of our company received an annual salary and bonus that
exceeded $100,000 during the period from May 17, 2007 (date of inception) to
June 30, 2008.
24
Non-Equity Nonqualified
Incentive Deferred All
Name and Plan Compen- Other
Principal Stock Option Compen- sation Compen-
Position Year(3) Salary($) Bonus($) Awards($) Awards($) sation($) Earnings($) sation($) Totals($)
-------- ------- --------- -------- --------- --------- --------- ----------- --------- ---------
Guy Ofir 2007 Nil Nil Nil Nil Nil Nil Nil Nil
President and 2008 Nil Nil Nil Nil Nil Nil Nil Nil
director(1)
Emanuel Cohen 2007 Nil Nil Nil Nil Nil Nil Nil Nil
Secretary, 2008 Nil Nil Nil Nil Nil Nil Nil Nil
Treasurer and
director(2)
- ----------
(1) Guy Ofir became our President and a director of our company, on May 17,
2007.
(2) Emanuel Cohen became our Secretary, Treasurer and a director of our
company, on May 17, 2007.
(3) We were incorporated on May 17, 2007.
STOCK OPTIONS AND STOCK APPRECIATION RIGHTS
Since May 17, 2007 (date of inception) to our the period ended June 30, 2008, we
have not granted any stock options or stock appreciation rights to any of our
directors or executive officers.
COMPENSATION OF DIRECTORS
There are no arrangements pursuant to which directors are or will be compensated
in the future for any services provided as a director, unless and until we begin
to realize revenues and become profitable in our business operations.
EMPLOYMENT CONTRACTS AND TERMINATION OF EMPLOYMENT AND CHANGE IN CONTROL
ARRANGEMENTS
We have not entered into any employment agreement or consulting agreements with
our directors and executive officers. There are no arrangements or plans in
which we provide pension, retirement or similar benefits for directors or
executive officers. Our directors and executive officers may receive stock
options at the discretion of our board of directors in the future. We do not
have any material bonus or profit sharing plans pursuant to which cash or
non-cash compensation is or may be paid to our directors or executive officers,
except that stock options may be granted at the discretion of our board of
directors.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of April 22, 2008 certain information with
respect to the beneficial ownership of our common stock by each shareholder
known by us to be the beneficial owner of more than 5% of our common stock and
by our current directors and executive officers. The shareholder has sole voting
and investment power with respect to the shares of common stock, except as
otherwise indicated. Beneficial ownership consists of a direct interest in the
shares of common stock, except as otherwise indicated.
25
Name and Address Amount and Nature of Percent of
of Beneficial Owner Title of Class(1) Beneficial Ownership Class(2)
- ------------------- ----------------- -------------------- --------
Guy Ofir Common Shares 20,000,000 21.5%
40 Baz St., Karmiel 20100 Direct
Israel
Emanuel Cohen Common Shares 20,000,000 21.5%
51 Bilu St., Direct
Raanana, Israel
Shamir Benita Common Shares 5,000,000 5.4%
8 Nafetali Ben Eferaim St. Direct
Dira 21
Rehovot, Israel
Albert Glinoviecki Common Shares 5,000,000 5.4%
Rehov Dov Fromer 19 Direct
Kiryat Shemuel
Israel
Meir Duke(3) Common Shares and 7,285,714 7.8%
12300 Highgrove Ct, Common Shares Direct
Raistertown, MD Warrants
USA
Meitav Entities and TMC(4), Common Shares and
4 Berkowitz St. Common Shares
Tel Aviv, Israel Warrants 18,588,273 9.99%(5)
Directors and Officers Common Shares 40,000,000 43%
as a group (2 persons)
- ----------
(1) Beneficial ownership is determined in accordance with SEC rules and
generally includes voting or investment power with respect to securities.
Shares of common stock subject to options, warrants and convertible
preferred stock currently exercisable or convertible, or exercisable or
convertible within sixty (60) days, would be counted as outstanding for
computing the percentage of the person holding such options or warrants but
not counted as outstanding for computing the percentage of any other
person.
(2) Based on 93,186,070 shares issued and outstanding as of July 1, 2008.
(3) Includes 1,000,000 shares issuable upon exercise of outstanding common
stock purchase warrants. This information is based solely on Schedule 13D
filed by the beneficial owner on April 16, 2008, describing the holdings of
the beneficial owner as of April 7, 2008.
(4) An entity controlled by Meitav Investment House Ltd, ("Meitav") which as
reported on Schedule 13G filed on March 19, 2008, is beneficially owned by
Messrs. Zvi Stepak and Shlomo Simanovsky through intermediary entities.
Messrs. Zvi Stepak and Shlomo Simanovsky may exercise shared voting and
investment powers with respect to all shares owned by Meitav and the Meitav
Entities. Includes 14,029,440 shares issuable upon exercise of outstanding
common stock purchase warrants. This information is based solely on
Schedule 13G filed by the benecial owner on March 19, 2008, describing the
holdings of the beneficial owner as of March 10, 2008.
(5) In an appendix to the warrant issued by the Company to the Meitav Entities
and TMC the following exercise limitations have been agreed to: the company
shall not effect the exercise of the warrant and the holder shall not have
the right to exercise any portion of the warrant to the extent that after
giving effect to such issuance after exercise, such holder along with its
affiliates (which include all of Meitav Entities and TMC) shall have more
than 9.99% of the number of shares of common stock outstanding of the
Company. This provision however, may be waived by the holder at its
election upon not less than 61 days' notice to the Company.
26
CHANGES IN CONTROL
We are unaware of any contract, or other arrangement or provision of our
Articles of Incorporation or Bylaws, the operation of which may at a subsequent
date result in a change of control of our company.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Except as described below, no director, executive officer, principal shareholder
holding at least 5% of our common shares, or any family member thereof, had any
material interest, direct or indirect, in any transaction, or proposed
transaction, during the period ended March 31, 2008, in which the amount
involved in the transaction exceeded or exceeds the lesser of $120,000 or one
percent of the average of our total assets at the year end for the last three
completed fiscal years.
On May 17, 2007 Mr. Ofir and Cohen each purchased 20,000,000 shares of our
common stock for $0.00005 per share, or $1,000 each, for an aggregate of $2,000.
The promoters of our company are Guy Ofir, our President and director and
Emanuel Cohen, our Secretary, Treasurer and director.
We have determined that neither Mr. Guy Ofir nor Mr. Emanuel Cohen are
independent directors, as that term is used in Rule 4200(a)(15) of the Rules of
the Financial Industry Regulatory Authority.
During the period ended March 31, 2008, the Company paid $200,000 in product
development costs to a company wholly owned by the president of the Company and
its director, Mr. Ofir.
The Company's directors provide office space free of charge. The Company has
recorded the estimated fair value of the office space of $1,000 per month as a
contribution to capital.
DISCLOSURE OF SEC POSITION OF
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our Bylaws provide that we will indemnify an officer, director, or former
officer or director, to the full extent permitted by law. Insofar as
indemnification for liabilities arising under the Securities Act may be
permitted to our directors, officers and controlling persons pursuant to the
foregoing provisions, or otherwise, we have been advised that in the opinion of
the SEC such indemnification is against public policy as expressed in the
Securities Act, and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities, other
than the payment by us of expenses incurred or paid by one of our directors,
officers, or controlling persons in the successful defense of any action, suit
or proceeding, is asserted by one of our directors, officers, or controlling
persons in connection with the securities being registered, we will, unless in
the opinion of our counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question whether such
indemnification is against public policy as expressed in the Securities Act, and
we will be governed by the final adjudication of such issue.
27
EXPERTS
The financial statements of Easy Energy included in this registration statement
have been audited by Moore & Associates Chartered, to the extent and for the
period set forth in their report (which contains an explanatory paragraph
regarding our company's ability to continue as a going concern) appearing
elsewhere in the registration statement, and are included in reliance upon such
report given upon the authority of said firm as experts in auditing and accounting.
Zysman, Aharoni, Gayer & Co./Sullivan & Worcester LLP, One Post Office Square,
Boston, Massachusetts 02110 has provided an opinion on the validity of the
shares of our common stock that are the subject of this prospectus.
WHERE YOU CAN FIND MOREADDITIONAL INFORMATION
We are subject to the filing requirements of the Exchange Act. Therefore, we file annual, quarterly and currentperiodic reports, proxy statements and other information with the SEC. You may readSecurities and copy anyExchange Commission. The Securities and Exchange Commission maintains a website (www.sec.gov) that contains reports, proxy and information statements, orand other information regarding issuers that file electronically.
We make our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to such reports filed or furnished pursuant to Section 13(a) or 15(d) of the Company files at the SEC's public reference room at 100 F
Street, NE, N.W., Washington, D.C., 20549. Please call the SEC at 1-800-SEC-0330
for further informationExchange Act available free of charge through a link on the operationInvestors section of the public reference room. Our SEC
filings are also available to the public from commercial document retrieval
services and through the web site maintained by the SECour website located at www.sec.gov.
As allowed by SEC rules, this prospectus does not contain all the information
you can find in the registration statement or the exhibits filed with or
incorporated by reference into the registration statement. Whenever a reference
is made in this prospectus to an agreement or other document of the Company, be
aware that such reference is not necessarily complete and that you should refer
to the exhibits thatwww.raphaelpharmaceutical.com as soon as reasonably practicable after they are filed with or incorporated by reference intofurnished to the registration statement for a copy of the agreement or other document. You may
review a copy of the registration statement at the SEC's public reference room
in Washington, D.C., as well as through the web site maintained by the SEC at
www.sec.gov.
You should read this prospectusSecurities and any prospectus supplement together with the
registration statement and the exhibits filed with or incorporated by reference
into the registration statement.Exchange Commission. The information contained in or accessible through our website or contained on other websites is not a part of, and is not incorporated into, this prospectus
speaksprospectus.
For further information about us, please visit our main website located at www.raphaelpharmaceutical.com. Information contained on these websites is not a part of this prospectus.
You should rely only as of its date unlesson the information specifically indicates that
another date applies.contained in this prospectus. We have not authorized any personanyone to give any information or to make any
representations that differ from, or add to, the information discussed in this
prospectus.provide you with different information. Therefore, if anyone gives you different or additional information, you should not rely on it. We maintain a websiteThe information contained in this prospectus is correct as of its date. It may not continue to be correct after this date.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of Raphael Pharmaceutical Inc.
Opinion on the Internet at www.easy-energy.biz. Our websiteFinancial Statements
We have audited the accompanying consolidated balance sheets of Raphael Pharmaceutical Inc. and its subsidiary (the “Company”) as of December 31, 2021 and 2020, the related consolidated statements of comprehensive loss, changes in shareholders’ deficit and cash flows for each of the two years in the period ended December 31, 2021, and the information included onrelated notes (collectively referred to as the “financial statements”). In our website is not partopinion, the financial statements present fairly, in all material respects, the financial position of this prospectus.
We have filed with the SecuritiesCompany as of December 31, 2021 and Exchange Commission a registration
statement on Form S-1, under2020 and the Securities Act with respect toresults of its operations and its cash flows for each of the securities
offered under this prospectus. This prospectus, which forms a part of that
registration statement, does not contain all information includedtwo years in the registration statement. Certain information is omitted and you should refer to
the registration statement and its exhibits. Our filings and the registration
statement can also be reviewed by accessing the SEC's website at
http://www.sec.gov.
NO FINDER, DEALER, SALES PERSON OR OTHER PERSON HAS BEEN AUTHORIZED TO GIVE ANY
INFORMATION OR TO MAKE ANY REPRESENTATION IN CONNECTION WITH THIS OFFERING OTHER
THAN THOSE CONTAINED IN THIS PROSPECTUS AND, IF GIVEN OR MADE, SUCH INFORMATION
OR REPRESENTATION MUST NOT BE RELIED UPON AS HAVING BEEN AUTHORIZED BY OUR
COMPANY. THIS PROSPECTUS DOES NOT CONSTITUTE AN OFFER TO SELL OR A SOLICITATION
OF AN OFFER TO BUY ANY OF THE SECURITIES OFFERED HEREBY BY ANYONE IN ANY
JURISDICTION IN WHICH SUCH OFFER OR SOLICITATION IS NOT AUTHORIZED OR IN WHICH
THE PERSON MAKING SUCH OFFER OR SOLICITATION IS NOT QUALIFIED TO DO SO OR TO ANY
PERSON TO WHOM IT IS UNLAWFUL TO MAKE SUCH OFFER OR SOLICITATION.
28
RESTATED FINANCIAL STATEMENTS
JUNE 30, 2008
INDEX
Our restated financial statements are stated in United States Dollars (US$) and
are prepared in conformity with generally accepted accounting principles of the
United States of America.
The following audited restated financial statements pertaining to Easy Energy
Inc. are filed as part of this registration statement:
Page
Number
------
Restated Financial Statements
Restated Balance Sheets F-1
Restated Statements of Operations F-2
Restated Statement of Stockholders' Equity F-3
Restated Statements of Cash Flow F-4
Notes to audited Restated Financial Statements F-5
29
EASY ENERGY INC.
(A Development Stage Company)
Restated Balance Sheets
June 30, 2008
June 30, December 31,
2008 2007
----------- -----------
(unaudited)
ASSETS
Current:
Cash and bank accounts $ 595,446 $ 72,688
Prepaid expenses 37,500 --
Prepaid expenses - stock related 337,500 --
----------- -----------
Total current assets 970,446 72,688
----------- -----------
Total Assets $ 970,446 $ 72,688
=========== ===========
CURRENT LIABILITIES
Current:
Due to director $ 300 $ 300
----------- -----------
Total Current Liabilities 300 300
----------- -----------
STOCKHOLDERS` EQUITY
Preferred stock authorized -
50,000,000 shares with a par value of $0.0001
None issued or outstanding
Common stock authorized -
1,000,000,000 shares with a par value of $0.00001
Common stock issued and outstanding -
93,186,070 common shares (December 31, 2007: 80,333,190) 932 803
Additional paid in capital 2,635,917 101,697
Subscriptions received 19,416 --
Deferred offering costs - stock related (211,765) --
Deficit accumulated during the development stage (1,474,354) (30,112)
----------- -----------
Total Stockholders' Equity 970,146 72,388
----------- -----------
Total Liabilities and Stockholders' Equity $ 970,446 $ 72,688
=========== ===========
The accompanying notes are an integral part of these financial statements
F-1
EASY ENERGY INC.
(A Development Stage Company)
Restated Statements of Operations
(Unaudited)
May 17, 2007 May 17, 2007
Three Months Six Months (Inception) to (Inception) to
June 30, June 30, June 30, June 30,
2008 2008 2007 2008
------------ ------------ ------------ ------------
Revenue $ -- $ -- $ -- $ --
Expenses
General and Administrative 4,475 4,475 29,780 34,255
Filing Fee 1,600 1,600 1,000 2,600
Product Development 130,000 330,000 -- 330,000
Professional fees 143,910 1,113,190 -- 1,113,190
------------ ------------ ------------ ------------
Total Expenses 279,985 1,449,265 30,780 1,480,045
------------ ------------ ------------ ------------
Other Income
Interest income 3,763 5,023 668 5,691
------------ ------------ ------------ ------------
Total Other Income 3,763 5,023 668 5,691
------------ ------------ ------------ ------------
Net loss before income taxes (276,222) (1,444,242) (30,112) (1,474,354)
Provision for Income Taxes -- -- -- --
------------ ------------ ------------ ------------
Net loss for the period $ (276,222) $ (1,444,242) $ (30,112) $ (1,474,354)
============ ============ ============ ============
Basic and Diluted (Loss) per Share a $ (0.02) a
------------ ------------ ------------
Weighted Average Number of Shares 93,186,070 87,783,730 50,000,000
------------ ------------ ------------
- ----------
(a) = Less than $0.01 per share
The accompanying notes are an integral part of these financial statements
F-2
EASY ENERGY INC.
(A Development Stage Company)
Restated Statements of Cash Flows
(Unaudited)
May 17, 2007 May 17, 2007
Six Months (Inception) to (Inception) to
June 30, June 30, June 30,
2008 2007 2008
----------- ----------- -----------
Operating Activities
Net loss $(1,444,242) $ (30,112) $(1,474,354)
Adjustments to reconcile net loss to
cash used in operating activities:
Contributed capital 3,000 7,500 10,500
Common stock and warrants issued for services 957,083 -- 957,083
Changes in operating assets and liabilities:
(Increase) in prepaid expenses (37,500) -- (37,500)
Increase (Decrease) in subscriptions received 19,416 -- 19,416
----------- ----------- -----------
Net Cash (Used) by Operating Activities (502,243) (22,612) (524,855)
----------- ----------- -----------
Financing Activities
Cash from sale of stock 1,025,001 95,000 1,120,001
Due to shareholder -- 300 300
----------- ----------- -----------
Cash Provided by Financing Activities 1,025,001 95,300 1,120,301
----------- ----------- -----------
Net Increase in Cash 522,758 72,688 595,446
Cash, Beginning of Period 72,688 -- --
----------- ----------- -----------
Cash, End of Period $ 595,446 $ 72,688 $ 595,446
=========== =========== ===========
Non-cash activities:
Contributed capital $ 3,000 $ -- $ 3,000
Stock and warrants issued for services $ 957,083 $ -- $ 957,083
Supplemental Information:
Interest Paid $ -- $ -- $ --
Income Taxes Paid $ -- $ -- $ --
The accompanying notes are an integral part of these financial statements
F-3
EASY ENERGY INC.
(A Development Stage Company)
Restated Statements of Stockholders` Equity
Common Shares
-------------------------- Additional
Issued Paid In
Shares Amount Capital Subscriptions
------ ------ ------- -------------
Balance, May 17, 2007 (date of inception) -- $ -- $ -- $ --
Issued to founders on May 17, 2007 @ $0.0005 40,000,000 400 1,600 --
Private placement May 17, 2007 @ $0.0002 10,000,000 100 1,900 --
Private placement August 17, 2007 @ $0.003 30,333,190 303 90,697 --
Contributed capital -- -- 7,500 --
Net loss -- -- -- --
---------- ---------- ---------- ----------
Balance, December 31, 2007 80,333,190 803 101,697 --
Private placement January 16, 2008 @ $0.07 4,285,714 43 299,957 --
Private placement February 28, 2008 @ $0.17 3,676,480 37 624,964 --
Private placement February 28, 2008 @ $0.24 208,333 2 49,998 --
Shares for services March 3, 2008 @$0.24 300,000 3 71,997 --
Shares for services March 10, 2008 @ $0.24 882,353 9 211,756 --
Private placement March 25, 2008 @ $0.02 2,000,000 20 599,980 --
Shares for services March 27, 2008 @ $0.07 1,500,000 15 449,985 --
Contributed capital -- -- 3,000 --
Fair value of warrants granted -- -- 222,583 --
Subscriptions received -- -- -- 19,416
Net loss -- -- -- --
---------- ---------- ---------- ----------
BALANCE, JUNE 30, 2008 93,186,070 $ 932 $2,635,917 $ 19,416
========== ========== ========== ==========
Deficit
Accumulated
Deferred During
Offering Development
Costs Stage Total
----- ----- -----
Balance, May 17, 2007 (date of inception) $ -- $ -- $ --
Issued to founders on May 17, 2007 @ $0.0005 -- -- 2,000
Private placement May 17, 2007 @ $0.0002 -- -- 2,000
Private placement August 17, 2007 @ $0.003 -- -- 91,000
Contributed capital -- -- 7,500
Net loss -- (30,112) (30,112)
---------- ---------- ----------
Balance, December 31, 2007 -- (30,112) 72,388
Private placement January 16, 2008 @ $0.07 -- -- 300,000
Private placement February 28, 2008 @ $0.17 -- -- 625,001
Private placement February 28, 2008 @ $0.24 -- -- 50,000
Shares for services March 3, 2008 @$0.24 -- -- 72,000
Shares for services March 10, 2008 @ $0.24 (211,765) -- --
Private placement March 25, 2008 @ $0.02 -- -- 600,000
Shares for services March 27, 2008 @ $0.07 -- -- 450,000
Contributed capital -- -- 3,000
Fair value of warrants granted -- -- 222,583
Subscriptions received (Unaudited) -- -- 19,416
Net loss (Unaudited) -- (1,444,242) (1,444,242)
---------- ---------- ----------
BALANCE, JUNE 30, 2008 (Unaudited) $ (211,765) $(1,474,354) $ 970,146
========== ========== ==========
The accompanying notes are an integral part of these financial statements
F-4
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 andperiod ended December 31, 2007
NOTE 1 - NATURE OF OPERATIONS
The Company was originally incorporated under the laws of the state of Nevada on
May 17, 2007. The Company has limited operations and in accordance with SFAS #7,
is considered a development stage company, and has had no revenues from
operations to date.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
ACCOUNTING BASIS
These financial statements are prepared on the accrual basis of accounting2021, in conformity with accounting principles generally accepted in the United States of America. The Company was incorporated on May 17, 2007, and therefore does not
have comparable numbers for the period ended June 30, 2007. Accordingly, the
Company is presenting the statement of operations from May 17, 2007 through
December 31, 2007 for comparison purposes. The accompanying financial statements
have been prepared by the Company without audit. In the opinion of management,
all adjustments (which include only normal recurring adjustments) necessary to
present fairly the financial position, results of operations and cash flows at
June 30, 2008 and for all periods presented have been made.
Certain information and footnote disclosures normally included in financial
statements prepared in accordance with accounting principles generally accepted
in the United States of America have been condensed or omitted. It is suggested
that these condensed financial statements be read in conjunction with the
financial statements and notes thereto included in the Company's December 31,
2007 audited financial statements. The results of operations for the period
ended June 30, 2008 are not necessarily indicative of the operating results for
the full years.
EARNINGS PER SHARE
In February 1997, the FASB issued SFAS No. 128, "Earnings Per Share", which
specifies the computation, presentation and disclosure requirements for earnings
(loss) per share for entities with publicly held common stock. SFAS No. 128
supersedes the provisions of APB No. 15, and requires the presentation of basic
earnings (loss) per share and diluted earnings (loss) per share. The Company has
adopted the provisions of SFAS No. 128 effective its inception.
The basic earnings (loss) per share is calculated by dividing the Company's net
income available to common shareholders by the weighted average number of common
shares during the year. The diluted earnings (loss) per share is calculated by
dividing the Company's net income (loss) available to common shareholders by the
diluted weighted average number of shares outstanding during the year. The
diluted weighted average number of shares outstanding is the basic weighted
number of shares adjusted as of the first of the year for any potentially
dilutive debt or equity.
DIVIDENDS
The Company has not yet adopted any policy regarding payment of dividends. No
dividends have been paid during the periods shown.
CASH EQUIVALENTS
The Company considers all highly liquid investments with maturity of three
months or less when purchased to be cash equivalents.
INCOME TAXES
The Company provides for income taxes under Statement of Financial Accounting
Standards No. 109, "Accounting for Income Taxes" and FIN 48. SFAS No. 109
requires the use of an asset and liability approach in accounting for income
taxes.
SFAS No. 109 requires the reduction of deferred tax assets by a valuation
allowance if, based on the weight of available evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. No
provision for income taxes is included in the statement due to its immaterial
amount, net of the allowance account, based on the likelihood of the Company to
utilize the loss carry-forward.
F-5
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
USE OF ESTIMATES
The preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of revenue and expenses during
the reporting period. Actual results could differ from those estimates.
NOTE 3 - GOING CONCERN
The accompanying financial statements have been prepared assuming that the
Company will continue as a going concern. The Company has net losses for the
period from inception to June 30, 2008 of $1,444,242 and the Company has not
established revenue sufficient to cover its operating expenses. These conditions
raise substantial doubt about the Company's ability to continue as a going
concern.
Managements' plan is to complete the design of the Company's product, to engage
third parties firms to manufacture the components of the product, develop and
manufacture a first product suited to cellular phone use and explore potential
distributors for our product. The Company had approximately $595,000 on hand as
of June 30, 2008, management anticipates that such funds will not be sufficient
to pay our estimated expenses for the next twelve month period. Management
expects to start generating revenue within 8-10 months but has no assurance that
such revenues shall be generated and in what amounts. Management intends to
fulfill any additional cash requirement through the sale of either equity or
debt. However, the Company has not identified the source of additional cash and
there is no guarantee that such funds will be available or if available that the
terms will be acceptable to the Company.
The Company's continuation as a going concern is dependent on its ability to
complete and market its product and to obtain additional financing as may be
required and ultimately to attain profitability. The financial statements do not
include any adjustments that might result from the outcome of this uncertainty.
NOTE 4 - DEFERRED OFFERING COSTS - STOCK RELATED
On March 10, 2008 the Company entered into agreements relating to the issuance
of 882,353 shares of the Company's common stock, par value $0.00001 per share,
and a warrant to purchase up to 3,000,000 shares of the Company's common stock
at a price of $0.27 per share.
The shares issued to date as part of this agreement have been charged to
deferred financing cost until such time the Company exercises its option to
demand an additional $1,000,000 of financing from the financer. At such time,
the deferred financing charges will be offset against the financing.
NOTE 5 - CAPITAL STOCK
COMMON SHARES - AUTHORIZED
The company has 1,000,000,000 common shares authorized at a par value of
$0.00001 per share and 50,000,000 shares of preferred stock at a par value of
$0.0001 per shares. All common stock have equal voting rights, are
non-assessable and have one vote per share. Voting rights are not non-cumulative
and, therefore, the holders of more than 50% of the stock could, if they choose
to do so, elect all the directors of the company.
F-6
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 5 - CAPITAL STOCK (CONTINUED)
ISSUED AND OUTSTANDING -
On May 17, 2007 (inception), the Company issued 40,000,000 shares of its common
stock to its Directors for cash of $2,000. See Note 5.
On May 17, 2007, the Company closed a private placement for 10,000,000 common
shares at a price of $0.0002 per share, or an aggregate of $2,000. The Company
accepted subscription from two offshore non-affiliated investors.
On August 27, 2007, the Company closed a private placement for 30,333,190 common
shares at a price of $0.003 per share, or an aggregate of $91,000. The Company
accepted subscription from forty offshore non-affiliated investors.
On February 8, 2008, the Company changed it number of authorized shares of
Common Stock from 100,000,000 to 1,000,000,000 and provide for a ten for one
forward split of the Registrant's shares of common stock outstanding.
On February 28, 2008, the Company commenced a private placement offering of
367,647.6 units, each unit being offered for $1.70, for aggregate gross proceeds
of $625,001. Each unit consists of (i) ten common stock shares, (ii) thirty
Class A Warrant. Each Class A Warrant entitles the holder thereof to purchase
one share of common stock at an exercise price of $0.27 per share, expiring five
years from the date of purchase. This offering is being made to non-U.S. persons
in offshore transactions pursuant to the exemption from registration provided by
Regulation S of the Securities Act.
On February 28, 2008, the Company completed a subscription agreement pursuant to
which it sold and issued 208,333 common stock shares under Rule 903 of
Regulation S of the Act of 1933 (the "Act") to an accredited investor for the
aggregate purchase price of US $50,000, purchase price US $0.24 per share.
On March 3, 2008, the Company signed a subscription agreement in which it
undertook to issue to its legal counsel 300,000 shares of restricted common
stock valued at $0.24 per share based upon the Regulation S offering completed
at approximately the same date for an aggregate price of $72,000 for legal
services provided and warrants to purchase 1,000,000 shares of the Company's
common stock at an exercise price of $0.15 for a period of five years. The
warrants were valued in accordance with SFAS 123R using the assumptions
described below and resulted in an expense of $1,031.
On March 10, 2008, the Company entered into a Securities Purchase Agreement and
a Registration Rights Agreement with Tailor Made Capital, Ltd. ("TMC") relating
to the issuance to TMC of 882,353 shares of the Company's common stock, par
value $0.0001 per share, and a warrant to purchase up to 3,000,000 shares of the
Company's common stock at a price of $0.27 per share (the "Warrant"). The shares
were issued for deferred stock offering costs and valued at $0.24 per share
based upon the Regulation S offering completed at approximately the same date
for an aggregate price of $211,765. The warrant shall be in effect for five
years from the date that the Company's common stock is initially listed or
quoted for trading on a trading market. The warrants were valued in accordance
with SFAS 123R using the assumptions described below and resulted in an expense
of $8,770. On March 25, 2008, we entered into a subscription agreement under
which we undertook to issue 2,000,000 shares for cash payment of $50,000 by an
"accredited investor" as defined in Rule 501 of Regulation D under the
Securities Act of 1933, and, in consideration for services provided, warrants to
purchase 1,000,000 shares of the Company's common stock at an exercise price of
$0.10 for a period of three years. The shares were valued at the trading price
on the date of issuance of $0.30 for an additional expense of $550,000. The
warrants were valued using the Black-Scholes model at $213,813.
On March 27, 2008(pursuant to an agreement dated January 16, 2008),, we issued
4,285,714 common stock shares to an "accredited investor" as defined in Rule 501
of Regulation D under the Securities Act of 1933 for the aggregate purchase
price of US $300,000, purchase price of $0.07 per share.
On March 27, 2008, the Company entered into a consulting services agreement of
which the consultant will provide certain investor and market relations
consulting services to the Company in consideration of the Company's issuance of
F-7
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 5 - CAPITAL STOCK (CONTINUED)
ISSUED AND OUTSTANDING (CONTINUED) -
1,500,000 restricted common shares and the sum of $100,000 in cash. The common
shares were valued at the trading price on the date of issuance of $0.30 per
share for a total of $450,000. The expense will be amortized over the one year
service agreement beginning April 1, 2008.
WARRANTS OUTSTANDING -
Date Issued Number of Warrants Exercise Price Expiry Date
- ----------- ------------------ -------------- -----------
February 28, 2008 11,029,428 $ 0.27 February 28, 2013
March 3, 2008 1,000,000 $ 0.15 March 3, 2013
March 10, 2008 3,000,000 $ 0.27 March 10, 2013
March 25, 2008 1,000,000 $ 0.10 March 25, 2008
The value allocated to the warrants was estimated using the Black-Scholes option
pricing model with the following assumptions: dividend yield of 0%, expected
volatility of 100%, risk-free interest rate of 3.94% and expected lives of
3years to 5 years. The volatility was determined based upon the weekly trading
price of the stock from the date of inception through March 31, 2008. Common
stock issued for services was valued at the price of the shares issued for cash
on or close to the date of issuance.
NOTE 6 - RELATED PARTY TRANSACTIONS
The Company's directors provide office space free of charge. The Company has
recorded the estimated value of the office space of $1,000 per month as a
contribution to capital. The Company recorded a contribution to capital of
$3,000 and $7,500 during the periods ended March 31, 2008 and December 31, 2007,
respectively. The officers and directors of the Company are involved in other
business activities and may, in the future, become involved in other business
opportunities. If a specific business opportunity becomes available, such
persons may face a conflict in selecting between the Company and their other
business interests. The Company has not formulated a policy for the resolution
of such conflicts.
On May 17, 2007 (inception), the Company issued 40,000,000 shares of its common
stock to its Directors for cash of $2,000. See Note 4.
During the period ended March 31, 2008, the Company paid $200,000 in product
development costs to a company wholly owned by the president and director of the
Company.
NOTE 7 - INCOME TAXES
The Company provides for income taxes under Statement of Financial Accounting
Standards No. 109, ACCOUNTING FOR INCOME TAXES. SFAS No. 109 requires the use of
an asset and liability approach in accounting for income taxes. Deferred tax
assets and liabilities are recorded based on the differences between the
financial statement and tax bases of assets and liabilities and the tax rates in
effect currently.
SFAS No. 109 requires the reduction of deferred tax assets by a valuation
allowance if, based on the weight of available evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. In the
Company's opinion, it is uncertain whether they will generate sufficient taxable
income in the future to fully utilize the net deferred tax asset. Accordingly, a
valuation allowance equal to the deferred tax asset has been recorded. The total
deferred tax asset is $95,881, which is calculated by multiplying a 22%
estimated tax rate by the cumulative NOL of $435,824.
F-8
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENTS
Below is a listing of the most recent accounting standards SFAS 150-162 and
their effect on the Company.
STATEMENT NO. 150 - ACCOUNTING FOR CERTAIN FINANCIAL INSTRUMENTS WITH
CHARACTERISTICS OF BOTH LIABILITIES AND EQUITY (ISSUED 5/03)
This Statement establishes standards for how an issuer classifies and measures
certain financial instruments with characteristics of both liabilities and
equity. The application of SFAS No. 150 did not have any effect on the Company's
financial statements.
STATEMENT NO. 151 - INVENTORY COSTS-AN AMENDMENT OF ARB NO. 43, CHAPTER 4
(ISSUED 11/04)
This statement amends the guidance in ARB No. 43, Chapter 4, INVENTORY PRICING,
to clarify the accounting for abnormal amounts of idle facility expense,
freight, handling costs, and wasted material (spoilage). Paragraph 5 of ARB 43,
Chapter 4, previously stated that "...under some circumstances, items such as
idle facility expense, excessive spoilage, double freight and re-handling costs
may be so abnormal ass to require treatment as current period charges...." This
Statement requires that those items be recognized as current-period charges
regardless of whether they meet the criterion of "so abnormal." In addition,
this Statement requires that allocation of fixed production overheads to the
costs of conversion be based on the normal capacity of the production
facilities. The application of SFAS No. 151 did not have any effect on the
Company's financial statements.
STATEMENT NO. 152 - ACCOUNTING FOR REAL ESTATE TIME-SHARING TRANSACTIONS (AN
AMENDMENT OF FASB STATEMENTS NO. 66 AND 67)
This Statement amends FASB Statement No. 66, ACCOUNTING FOR SALES OF REAL
ESTATE, to reference the financial accounting and reporting guidance for real
estate time-sharing transactions that is provided in AICPA Statement of Position
(SOP) 04-2, ACCOUNTING FOR REAL ESTATE TIME-SHARING TRANSACTIONS.
This Statement also amends FASB Statement No. 67, Accounting FOR COSTS AND
INITIAL RENTAL OPERATIONS OF REAL ESTATE PROJECTS, states that the guidance for
(a) incidental operations and (b) costs incurred to sell real estate projects
does not apply to real estate time-sharing transactions. The accounting for
those operations and costs is subject to the guidance in SOP 04-2. The
application of SFAS No. 152 did not have any effect on the Company's financial
statements.
STATEMENT NO. 153 - EXCHANGES OF NON-MONETARY ASSETS (AN AMENDMENT OF APB
OPINION NO. 29)
The guidance in APB Opinion No. 29, ACCOUNTING FOR NON-MONETARY TRANSACTIONS, is
based on the principle that exchanges of non-monetary assets should be measured
based on the fair value of the assets exchanged. The guidance in that Opinion,
however, includes certain exceptions to the principle. This Statement amends
Opinion 29 to eliminate the exception for non-monetary exchanges of similar
productive assets and replaces it with a general exception for exchanges of
non-monetary assets that do not have commercial substance. A non-monetary
exchange has commercial substance if the future cash flows of the entity are
expected to change significantly as a result of the exchange. The Company has
applied SFAS No. 153 to the changes made in the financial statements as of March
31, 2008 and December 31, 2007.
STATEMENT NO. 154 - ACCOUNTING CHANGES AND ERROR CORRECTIONS (A REPLACEMENT OF
APB OPINION NO. 20 AND FASB STATEMENT NO. 3)
This Statement replaces APB Opinion No. 20, ACCOUNTING CHANGES, and FASB
Statement No. 3, REPORTING ACCOUNTING CHANGES IN INTERIM FINANCIAL STATEMENTS,
and changes the requirements for the accounting for and reporting of a change in
F-9
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENT (CONTINUED)
accounting principle. This Statement applies to all voluntary changes in
accounting principle. It also applies to changes required by an accounting
pronouncement in the unusual instance that the pronouncement does not include
specific transition provisions. When a pronouncement includes specific
transition provisions, those provisions should be followed. The Company has
applied SFAS No. 154 to the changes made in the financial statements as of March
31, 2008 and December 31, 2007.
SFAS NO. 155 - ACCOUNTING FOR CERTAIN HYBRID FINANCIAL INSTRUMENTS-AN AMENDMENT
OF FASB STATEMENTS NO. 133 AND 140
This statement amends FASB Statements No. 140, Accounting for Transfers and
Servicing of Financial Assets and Extinguishments of Liabilities. This statement
resolves issues addressed in Statement 133 Implementation Issue No. D1,
Application of Statement 133 to Beneficial Interests in Securitized Financial
Assets. This statement is effective for all financial instruments acquired or
issued after
the beginning of an entity's first fiscal year that begins after September 15,
2006. The application of SFAS No. 155 did not have any effect on the Company's
financial statements.
SFAS NO. 156 - ACCOUNTING FOR SERVICING OF FINANCIAL ASSETS-AN AMENDMENT OF FASB
STATEMENT NO. 140
This statement amends FASB Statement No. 140 with respect to the accounting for
separately recognized servicing liabilities. An entity should adopt this
statement as of the beginning of its first fiscal year that begins after
September 15, 2006. The application of SFAS No. 156 did not have any effect on
the Company's financial statements.
SFAS NO. 157 - FAIR VALUE MEASUREMENTS
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements, which
defines fair value, establishes a framework for measuring fair value in GAAP,
and expands disclosures about fair value measurements. SFAS No. 157 does not
require any new fair value measurements, but provides guidance on how to measure
fair value by providing a fair value hierarchy used to classify the source of
the information. This statement is effective for us beginning May 1, 2008. The
application of SFAS No. 157 will not have any effect on the Company's financial
statements.
SFAS NO. 158 - EMPLOYERS' ACCOUNTING FOR DEFINED BENEFIT PENSION AND OTHER
POSTRETIREMENT PLANS-AN AMENDMENT OF FASB STATEMENTS NO. 87, 88, 106, AND
132(R))
This statement improves the financial reporting by requiring an employer to
recognize the overfunded or underfunded status of a defined benefit
postretirement plan (other than a multiemployer plan) as an asset or liabilities
in its statement of financial positions and to recognize changes in that funded
status in the year in which the changes occur through comprehensive income of a
business entity. This statement also improves financial reporting by requiring
an employer to measure the funded status of a plan as of the date of its
year-end statement of financial position, with limited exceptions. The
application of SFAS No. 158 did not have any effect on the Company's financial
statements.
SFAS NO. 159 - THE FAIR VALUE OPTION FOR FINANCIAL ASSETS AND FINANCIAL
LIABILITIES-INCLUDING AN AMENDMENT OF FASB STATEMENT NO. 115
This statement permits entities to choose to measure many financial instruments
and certain items at fair value. The objective is to improve the financial
reporting by providing entities with the opportunity to mitigate volatility in
F-10
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
June 30, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENT (CONTINUED)
reported earnings caused by measuring related assets and liabilities differently
without having to apply complex hedge accounting provisions. This statement is
expected to expand the use of fair value measurement objectives for accounting
for financial instruments. This statement is effective as of the beginning of an
entity's first fiscal year that begins after November 15, 2007. The application
of SFAS No. 159 did not have any effect on the Company's financial statements.
SFAS NO. 160 - NON-CONTROLLING INTEREST IN CONSOLIDATED FINANCIAL STATEMENTS- AN
AMENDMENT OF ARB NO. 51
This statement amends ARB 51 to establish accounting and reporting standards for
the non-controlling interest in a subsidiary and for the deconsolidation of a
subsidiary. It also changes the way the consolidated income statement is
presented for non-controlling interest. This statement improves comparability by
eliminating diversity of methods. This statement also requires expanded
disclosure. The application of SFAS No. 160 did not have any effect on the
Company's financial statements.
SFAS NO. 161
This statement is intended to enhance the disclosure requirements for derivative
instruments and hedging activities as required by SFAS 133. The application of
SFAS No. 161 did not have any effect on the Company's financial statements.
SFAS 162
This statement identifies the sources of accounting principles and the framework
for selecting the principles to be used in the preparation of financial
statements for entities that are presented in conformity with GAAP hierarchy.
The application of SFAS No. 162 did not have any effect on the Company's
financial statements.
The past and future adoption of these Statements did not have and is not
expected to have a material effect on the Company's current financial position,
results or operations, or cash flows.
F-11
RESTATED FINANCIAL STATEMENTS
MARCH 31, 2008
INDEX
Our restated financial statements are stated in United States Dollars (US$) and
are prepared in conformity with generally accepted accounting principles of the
United States of America.
The following audited restated financial statements pertaining to Easy Energy
Inc. are filed as part of this registration statement:
Page
Number
------
Restated Financial Statements
Report of Independent Registered Public Accounting Firm F-13
Restated Balance Sheets F-14
Restated Statements of Operations F-15
Restated Statements of Cash Flow F-16
Restated Statement of Stockholders' Equity F-17
Notes to audited Restated Financial Statements F-18
F-12
MOORE & ASSOCIATES, CHARTERED
ACCOUNTANTS AND ADVISORS
PCAOB REGISTERED
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Easy Energy, Inc.
(A Development Stage Company)
We have audited the accompanying restated balance sheets of Easy Energy, Inc. (A
Development Stage Company) as of March 31, 2008 and December 31, 2007, and the
related restated statements of operations, stockholders' equity and cash flows
for the three months ended March 31, 2008, since inception on May 17, 2007
through December 31, 2007 and since inception on May 17, 2007 through March 31,
2008. These financial statements are the responsibility of the Company's
management. Our responsibility is to express an opinion on these financial
statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audits to obtain reasonable assurance about whether the
financial statements are free of material misstatement. An audit includes
examining, on a test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the accounting
principles used and significant estimates made by management, as well as
evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
In our opinion, the restated financial statements referred to above present
fairly, in all material respects, the financial position of Easy Energy, Inc. (A
Development Stage Company) as of March 31, 2008 and December 31, 2007, and the
related restated statements of operations, stockholders' equity and cash flows
for the three months ended March 31, 2008, since inception on May 17, 2007
through December 31, 2007 and since inception on May 17, 2007 through March 31,
2008, in conformity with accounting principles generally accepted in the United
States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 31b to the financial statements, the Company has netCompany’s lack of revenues and accumulated operating losses of $1,199,637 which raisesraise substantial doubt about its ability to continue as a going concern. Management'sManagement’s plans concerningin regard to these matters are also described in Note 3.1b. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Moore & Associates, Chartered
- ----------------------------------------
Moore & Associates Chartered
Las Vegas, Nevada
July
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.

Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Changes in Ordinary Share Capital – refer to note 7 2008
2675 S. Jones Blvd. Suite 109, Las Vegas, NV 89146
(702) 253-7499 FAX (702) 253-7501
F-13
EASY ENERGY, INC.
(A Development Stage Company)
Restated Balance Sheets
Marchto the consolidated financial statements
Critical Audit Matter Description
During the year ended December 31, 2008
2021, the Company raised equity in the aggregate amount of $1,290 thousands, through equity transactions, by issuing shares and warrants.
We deemed our audit over the allocation, classification and accuracy of equity transactions as challenging from a perspective of audit effort in comparison to our audit as a whole, because of the amount of equity transactions and due to the magnitude of total amounts raised. As such, we believe this represents a critical audit matter.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to equity transactions included the following, among others:
| ● | We read the agreements and analyzed the terms of the Company’s equity transactions. |
| ● | We evaluated the interpretation and application of the relevant accounting guidance in relation to the appropriateness of the equity classification of warrants issued in these transactions. |
| ● | We agreed the consideration received from the transactions to the respective bank |
| ● | We compared the shares |
/s/ Brightman Almagor Zohar & Co.
Certified Public Accountants
A Firm in the Deloitte Global Network
Tel Aviv, Israel
March 30, 2022
We have served as the Company’s auditor since 2021.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
U.S dollars in thousands (except for share and per share data)
| As of December 31, | ||||||||||||
| Note | 2021 | 2020 | ||||||||||
| Assets | ||||||||||||
| Current assets: | ||||||||||||
| Cash and cash equivalents | $ | 153 | $ | 95 | ||||||||
| Other current assets | 3 | 269 | 228 | |||||||||
| Total current assets | 422 | 323 | ||||||||||
| Total assets | $ | 422 | $ | 323 | ||||||||
| Liabilities and stockholders’ equity (deficit) | ||||||||||||
| Current liabilities: | ||||||||||||
| Other accounts payable and accrued expenses | 4 | 272 | - | |||||||||
| Payable to related party | 22 | 9 | ||||||||||
| Loan | 5 | - | 357 | |||||||||
| Total current liabilities | 294 | 366 | ||||||||||
| Stockholders’ equity (deficit): | ||||||||||||
| Common stock, $0.01 par value: | ||||||||||||
| Authorized: 21,020,560 shares; | ||||||||||||
| Issued and outstanding: 12,970,540 and 9,459,253 as of December 31, 2021 and December 31, 2020, respectively | 130 | 95 | ||||||||||
| Additional paid-in capital | 2,665 | 905 | ||||||||||
| Accumulated deficit | (2,667 | ) | (1,043 | ) | ||||||||
| Total stockholders’ equity (deficit) | 128 | (43 | ) | |||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 422 | $ | 323 | ||||||||
The accompanying notes are an integral part of thesethe condensed consolidated financial statements
F-14
EASY ENERGY,statements.
RAPHAEL PHARMACEUTICAL INC. (A Development Stage Company)
Restated Statements of Operations
For the three months ended March 31, 2008AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S dollars in thousands (except for share and the period ended
May 17, 2007 (Date of inception) to March 31, 2008
per share data)
| For the year ended December 31, | ||||||||||
| Note | 2021 | 2020 | ||||||||
| Research and development expenses | 10a | $ | 776 | $ | 461 | |||||
| General and administrative expenses | 10b | 853 | 245 | |||||||
| Operating loss | 1,629 | 706 | ||||||||
| Total financial expense, net | 10c | (4 | ) | 12 | ||||||
| Net loss and comprehensive loss | 1,625 | 718 | ||||||||
| Basic and diluted net loss per share | 0.15 | 0.07 | ||||||||
| Weighted average number of common shares used in computing basic and diluted net loss per share (*) | 11,171,704 | 9,459,253 | ||||||||
| (*) | Number of |
The accompanying notes are an integral part of thesethe condensed consolidated financial statements
F-15
EASY ENERGY,statements.
RAPHAEL PHARMACEUTICAL INC. (A Development Stage Company)
Restated Statements of Cash Flows
For the three months ended March 31, 2008AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES STOCKHOLDERS’ IN EQUITY (DEFICIT)
U.S dollars in thousands (except for share and the period ended
May 17, 2007 (Date of inception) to March 31, 2008
per share data)
| Common shares | Additional paid-in | Accumulated | Total | |||||||||||||||||
| Number | Amount | capital | Deficit | equity | ||||||||||||||||
| Balance as of January 1, 2020 (**) | 9,459,253 | $ | 95 | $ | 438 | $ | (325 | ) | $ | 208 | ||||||||||
| Issuance of common stock | - | (* | ) | 467 | - | 467 | ||||||||||||||
| Net loss | - | - | - | (718 | ) | (718 | ) | |||||||||||||
| Balance as of December 31, 2020 | 9,459,253 | $ | 95 | $ | 905 | $ | (1,043 | ) | $ | (43 | ) | |||||||||
| Common stock | Additional paid-in | Accumulated | Total | |||||||||||||||||
| Number | Amount | capital | Deficit | equity | ||||||||||||||||
| Balance as of January 1, 2021 (**) | 9,459,253 | $ | 95 | $ | 905 | $ | (1,043 | ) | $ | (43 | ) | |||||||||
| Issuance of common stock and warrants | 2,460,259 | 25 | 1,265 | - | 1,290 | |||||||||||||||
| Effect of reverse recapitalization transaction (Notes 1a and 7b) | 1,051,028 | 10 | 496 | - | 506 | |||||||||||||||
| Net loss | - | - | - | (1,625 | ) | (1,625 | ) | |||||||||||||
| Balance as of December 31, 2021 | 12,970,540 | $ | 130 | $ | 2,666 | $ | (2,668 | ) | $ | 128 | ||||||||||
| (*) | Represents amount less than $1 |
| (**) | Number of shares has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization transaction and to |
The accompanying notes are an integral part of thesethe consolidated financial statements
F-16
EASY ENERGY,statements.
RAPHAEL PHARMACEUTICAL INC. (A Development Stage Company)
Restated Statements of Stockholders` Equity
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S dollars in thousands (except for share and per share data)
| Year Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (1,625 | ) | $ | (718 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Changes in: | ||||||||
| Other current assets | (41 | ) | (70 | ) | ||||
| Related parties | 13 | 9 | ||||||
| Other accounts payables and accrued expenses | 234 | - | ||||||
| Net cash used in operating activities | (1,419 | ) | (779 | ) | ||||
| Cash flows from financing activities | ||||||||
| Receipt of a loan | 174 | 357 | ||||||
| Proceeds from issuance of common stock and warrants | 1,290 | 467 | ||||||
| Cash acquired in the reverse recapitalization (*) | 13 | - | ||||||
| Net cash provided by financing activities | 1,477 | 824 | ||||||
| Change in cash and cash equivalents | 58 | 45 | ||||||
| Cash and cash equivalents at the beginning of the year | 95 | 50 | ||||||
| Cash and cash equivalents at the end of the year | $ | 153 | $ | 95 | ||||
| Non cash supplement | ||||||||
| Non cash issuance costs | $ | 102 | - | |||||
| (*) Cash acquired in the reverse recapitalization: | ||||||||
| Current assets (excluding cash and cash equivalents) | (531 | ) | - | |||||
| Current liabilities | 38 | - | ||||||
| Reverse recapitalization effect on equity | 506 | - | ||||||
| Cash acquired in connection with reverse recapitalization transaction | 13 | - | ||||||
The accompanying notes are an integral part of thesethe consolidated financial statements
F-17
EASY ENERGY,statements.
RAPHAEL PHARMACEUTICAL INC. (A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and December 31, 2007
NOTE 1 - NATURE OF OPERATIONS
The Company was originally incorporated under the laws of the state of Nevada on
May 17, 2007. The Company has limited operations and in accordance with SFAS #7,
is considered a development stage company, and has had no revenues from
operations to date.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
ACCOUNTING BASIS
These financial statements are prepared on the accrual basis of accounting in
conformity with accounting principles generally accepted in the United States of
America. The Company was incorporated on May 17, 2007, and therefore does not
have comparable numbers for the period ended March 31, 2007. Accordingly, the
Company is presenting the statement of operations from May 17, 2007 through
December 31, 2007 for comparison purposes. per share data)
| NOTE 1:- | GENERAL |
| a. | Raphael Pharmaceutical Inc (formerly Easy Energy, Inc.) (the “Company”) was incorporated under the laws of the State of Nevada on May 17, 2007. The Company is headquartered in Tel Aviv-Jaffa, Israel. From April 1, 2011 until December 31, 2019, the Company was not active. On October 8, 2020, the Company and its stockholders entered into a Share Exchange Agreement (the “Share Exchange”) with an Israeli pharmaceutical company (“Raphael”), according to which, among other matters, all shareholders of Raphael will sell and convey the entire holdings in Raphael to the Company such that following the Share Exchange, the shareholders of Raphael will hold 90% of the issued and outstanding common stock of the Company, and the existing shareholders of the Company will hold the remaining 10% of the issued and outstanding common stock. On May 14, 2021, the Company’s board of directors and stockholders approved a 1-for-100 reverse split of the Company’s common stock, which was implemented and became effective as of May 14, 2021. The reverse split combined each one hundred (100) shares of the Company’s issued and outstanding Common stock into one share of common stock. No fractional shares were issued in connection with the reverse split, and any fractional shares resulting from the reverse split were rounded up to the nearest whole share. On May 14, 2021, Raphael and the Company, completed the Share Exchange pursuant to which 9,459,253 common stock were issued to the shareholders of Raphael so that they became the holders of 90% of the issued and outstanding common stock of the Company immediately after the Share Exchange while the Company’s shareholders hold, following the Share Exchange, 1,051,028 common stock which represents 10% of the Company. On May 19, 2021, as agreed by the parties to the Share Exchange, the Company changed its name to Raphael Pharmaceutical Inc. Following such Share Exchange, Raphael’s activities are the sole activities of the Company. The Share Exchange was accounted for as a reverse recapitalization which is outside the scope ASC 805, “Business Combinations” (“ASC 805”), as the Company, the legal acquirer, is considered a non-operating public shell, and is therefore not a business as defined in ASC 805. As the shareholders of Raphael received the largest ownership interest in the Company, Raphael was determined to be the “accounting acquirer” in the Share Exchange. As a result, the historical financial statements of the Company were replaced with the financial statement of Raphael for all periods presented. | |
| b. | Going concern and management plans |
The accompanying consolidated financial statements have been prepared byon a going-concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. Since its inception, the Company without audit. Inhas devoted substantially all of its efforts to research and development, clinical trials, and raising capital. The Company is still in its development and pre-clinical stage and has not yet generated revenues. The extent of the opinionCompany’s future operating losses and the timing of becoming profitable are uncertain. As of December 31, 2021, the Company’s accumulated deficit was $2,668, the net loss for the year then ended was $1,625 and the net cash used in operating activities was $1,419. The Company has funded its operations to date primarily through equity financing and the issuance of a loan. Additional funding will be required to complete the Company’s research and development and clinical trials, to attain regulatory approvals, to begin the commercialization efforts of the Company’s product and to achieve a level of sales adequate to support the Company’s cost structure.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 1:- | GENERAL (Cont.) |
Management’s plans include, but are not limited to, raising capital in the United States. There can be no assurance that it will be able to successfully raise additional financing, including in a public offering, or obtain additional financing on a timely basis or on terms acceptable to the Company, or at all.
Management expects that the Company will continue to generate losses from the development, clinical development and regulatory activities of its product, which will result in negative cash flow from operating activity. This has led management all adjustments (which include only normal recurring adjustments) necessary to present fairlyconclude that substantial doubt about the financial position, results of operations andCompany’s ability to continue as a going concern exists in the event that additional funding does not occur. If such sufficient financing is not received timely, the Company will not have sufficient cash flows at
March 31, 2008 and for all periods presentedliquidity to finance its business operations as currently contemplated and would then need to pursue a plan to license its assets, seek to be acquired by another entity, cease operations and/or seek bankruptcy protection. The Company’s financial statements do not reflect any adjustments that might result from the outcome of this uncertainty.
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES |
| a. | Basis of presentation: |
The consolidated financial statements have been made.
Certain information and footnote disclosures normally included in financial
statements prepared in accordance with accounting principles generally accepted in the United States of America have been condensed or omitted. It is suggested
that these condensed(“U.S. GAAP”).
The consolidated financial statements be read in conjunction withinclude the financial statements and notes thereto included in the Company's December 31,
2007 audited financial statements. The results of operations for the period
ended March 31, 2008 are not necessarily indicative of the operating results for
the full years.
EARNINGS PER SHARE
In February 1997, the FASB issued SFAS No. 128, "Earnings Per Share", which
specifies the computation, presentation and disclosure requirements for earnings
(loss) per share for entities with publicly held common stock. SFAS No. 128
supersedes the provisions of APB No. 15, and requires the presentation of basic
earnings (loss) per share and diluted earnings (loss) per share. The Company has
adopted the provisions of SFAS No. 128 effective its inception.
The basic earnings (loss) per share is calculated by dividing the Company's net
income available to common shareholders by the weighted average number of common
shares during the year. The diluted earnings (loss) per share is calculated by
dividing the Company's net income (loss) available to common shareholders by the
diluted weighted average number of shares outstanding during the year. The
diluted weighted average number of shares outstanding is the basic weighted
number of shares adjusted as of the first of the year for any potentially
dilutive debt or equity.
DIVIDENDS
The Company has not yet adopted any policy regarding payment of dividends. No
dividends have been paid during the periods shown.
CASH EQUIVALENTS
The Company considers all highly liquid investments with maturity of three
months or less when purchased to be cash equivalents.
INCOME TAXES
The Company provides for income taxes under Statement of Financial Accounting
Standards No. 109, "Accounting for Income Taxes" and FIN 48. SFAS No. 109
requires the use of an asset and liability approach in accounting for income
taxes.
SFAS No. 109 requires the reduction of deferred tax assets by a valuation
allowance if, based on the weight of available evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. No
provision for income taxes is included in the statement due to its immaterial
amount, net of the allowance account, based on the likelihoodaccounts of the Company to
utilize the loss carry-forward.
F-18
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 and December 31, 2007
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
USE OF ESTIMATES
its subsidiary. Intercompany accounts and transactions have been eliminated upon consolidation.
| b. | Use of estimate in preparation of financial statements: |
The preparation of consolidated financial statements in conformity with accounting principles
generally accepted in the United States of AmericaU.S. GAAP requires management to make estimates, judgments and assumptions that affect the amounts reported in the financial statements and accompanying notes. The Company evaluates on an ongoing basis its assumptions. The Company’s management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the datedates of the financial statements, and the reported amounts of revenue and expenses during the reporting period.periods. Actual results could differ from those estimates.
| c. | Financial statements in United States dollars: |
The Company’s functional currency is the U.S. dollar (“dollar” or “$”) since the dollar is the currency of the primary economic environment in which the Company has operated and expects to continue to operate in the foreseeable future. Transactions and balances denominated in dollars are presented at their original amounts. Transactions and balances denominated in currencies other than dollars have been re-measured to dollars. All transaction gains and losses from re-measurement of monetary balance sheet items denominated in currencies other than dollars are reflected in the statements of comprehensive loss as financial expenses, net.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| d. | Cash and cash equivalents: |
Cash equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less as of the date acquired and that are exposed to insignificant risk of change in value.
| e. | Fair value measurements: |
The carrying values of Company’s financial assets and liabilities, including cash and cash equivalents, other current assets, accounts payable and accrued expenses, payable to related party and loan approximate their fair value due to the short-term maturity of these instruments.
| f. | Research and development expenses: |
Research and development expenses are charged to the statements of comprehensive loss as incurred.
| g. | Income taxes: |
The Company accounts for income taxes in accordance with ASC 740, “Income Taxes”. ASC 740 prescribes the use of the liability method whereby deferred tax assets and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances in respect of deferred tax assets are provided for, if necessary, to reduce deferred tax assets to amounts more likely than not to be realized. As of December 31, 2021, and 2020, the Company had a full valuation allowance on its deferred tax assets.
| h. | Basic and diluted net loss per share: |
Earnings or loss per share (“EPS”) is the amount of earnings attributable to each share of common stock. For convenience, the term is used to refer to either earnings or loss per share. EPS is computed pursuant to ASC 260-10-45. Pursuant to ASC 260-10-45-10 through 260-10-45-16 Basic EPS is computed by dividing income available to common stockholders (the numerator) by the weighted-average number of common shares outstanding (the denominator) during the period. Loss available to common stockholders shall be computed by deducting both the dividends declared in the period on preferred stock (whether or not paid) from loss from operating loss (if that amount appears in the statements of comprehensive loss) and also from net loss. The computation of diluted EPS is similar to the computation of basic EPS except that the denominator is increased to include the number of additional common shares that would have been outstanding if the dilutive potential common stock had been issued during the period to reflect the potential dilution that could occur from common shares issuable through contingent shares issuance arrangement, stock options or warrants.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The net loss per share and the weighted average number of shares used in computing basic and diluted net loss per share is as follows:
| For the Year Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Numerator: | ||||||||
| Net loss applicable to common stockholders | $ | (1,625 | ) | $ | (718 | ) | ||
| Denominator: | ||||||||
| Number of shares of common stock used in computing basic and diluted net loss per share | 11,171,704 | 9,459,253 | ||||||
| Net loss of shares of common, basic and diluted | $ | (0.15 | ) | $ | (0.07 | ) | ||
| i. | Leases |
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842) (“ASU 2016-02”). ASU 2016-02 requires lessees to recognize their leases contracts as assets and liabilities in the financial statements. Furthermore, the ASU requires the Company to continue recognizing expenses but recognize expenses on their statements of comprehensive loss in a manner similar to current lease accounting. The amendments in this ASU are effective January 1, 2019. In July 2018, the FASB issued ASU 2018-11, Leases - Targeted Improvements, to allow a company to elect an optional modified retrospective transition method that applies the new lease requirements through a cumulative-effect adjustment in the period of adoption.
Effective January 2019, the Company adopted the new lease accounting standard. The Company elected to apply the practical expedients permitted under the transition guidance within the new standard. As such, there was no impact on the Company’s financial statements as a result of adopting ASU 2016-02. See note 6a for more details.
| j. | Recently issued accounting pronouncements |
In June 2016, the FASB issued ASU 2016-13, “Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments” (“ASU 2016-13”). ASU 2016-13 replaces the current incurred loss model guidance with a new method that reflects expected credit losses. Under this guidance, an entity would recognize an allowance for credit losses equal to its estimate of expected credit losses on financial assets measured at amortized cost. In November 2019, the FASB extended the effective date of ASU 2016-13 for smaller reporting companies. As a result, ASU 2016-13 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2022, with early adoption permitted. The standard is not expected to have a significant impact on the Company’s consolidated financial statements.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
In May 2021, the Financial Accountings Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2021-04, “Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging— Contracts in Entity’s Own Equity (Subtopic 815- 40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options” (“ASU 2021-04”). The guidance is effective for the Company on January 1, 2022. The Company is currently evaluating the impact of adopting this standard
| NOTE 3:- | OTHER CURRENT ASSETS |
| As of December 31, | ||||||||
| 2021 | 2020 | |||||||
| Receivables from governmental authorities | $ | 92 | $ | 27 | ||||
| Prepaid expenses | 177 | 201 | ||||||
| $ | 269 | $ | 228 | |||||
| NOTE 4:- | OTHER ACCOUNTS PAYABLE AND ACCRUED EXPENSES |
| As of December 31, | ||||||||
| 2021 | 2020 | |||||||
| Account payables | $ | 107 | $ | 9 | ||||
| Accrued expenses | 165 | - | ||||||
| $ | 272 | $ | 9 | |||||
| NOTE 5:- | LOAN |
In August 31, 2020, the Company and Raphael signed an investment agreement according to which the Company will raise up to $950 from investors. Under the agreement, the funds which the Company raised were transferred to Raphael for its current activity. The funds transferred to Raphael by the Company were considered as a loan which bears no interest and has no repayment date. Based on the agreement, the loan will become an equity interest once the Share Exchange is complete. As of part of the Share Exchange, the loan was converted into stockholders’ equity without any issuance of shares and recorded as part of the Company’s additional paid in capital
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 6:- | CONTINGENT LIABILITIES AND COMMITMENTS |
| a. | Lease commitments: |
Starting July 14, 2019, the Company began renting its offices from a third party for a rental monthly fee of approximately $3 per month. The rent period is for a period of one month which renews on a monthly basis.
Effective July 14, 2019, the Company adopted the new lease accounting standard. The Company elected to apply the practical expedients permitted under the transition guidance within the new standard, and the Company also elected not to apply the recognition requirements in the lease standard to short-term leases (less than 12 months) as of the adoption date. As such, there was no impact on the Company’s financial statements as a result of adopting ASU 2016-02.
| b. | Rambam research agreement |
The Company’s research and development efforts relating to its COVID-19 and rheumatoid arthritis (“RA”) product candidates are being conducted by Rambam Med-Tech Ltd., or Rambam MT, a part of the Rambam Health Care Campus in Israel, in accordance with a Sponsored Research Agreement (the “Research Agreement”), that was entered into by the Company and Rambam MT in July 2019, to be in effect for a period of 48 months and which includes a non-compete extension option for an additional 48 months of research and development. Pursuant to the Research Agreement, the Company agreed to pay Rambam MT $1.4 million in four equal milestone payments, due on the first day of August on each successive year from 2019 through 2022. Furthermore, in accordance with the terms of the Research Agreement, the Company and Rambam MT will have joint ownership of any IP created as a result of research programs covered by such agreement. In addition, subject to commercial sales of any product candidate using the IP created as a part of the research covered by the Research Agreement, the Company is required to pay Rambam MT a royalty in an amount equal to 6% of all net sales, subject to certain deductions, such as taxes paid by any purchaser, transportation and shipping costs, and other customary deductions.
As of December 31, 2021 the Company paid $1,050 (three out of four milestones’ payments).
| c. | Way of Life Cannabis research agreement |
In October 2020, Raphael Israel entered into an engagement agreement with Wolc, pursuant to which, subject to its completing the Share Exchange with Easy Energy, Raphael Israel will be provided with up to 15 liters of CBD oil, from a strain of cannabis during a term of 18 months, to be provided in two to three deliveries of between one to seven liters of CBD oil. In accordance with Raphael Israel’s agreement with Wolc, Raphael Israel has agreed to issue to certain persons affiliated with Wolc 3% of Raphael’s issued and outstanding share capital as of the date of the Share Exchange, to be provided in three equal issuances; provided, however, that such persons may elect to receive a cash payment of $100 instead of any one issuance of Raphael’s shares. In addition to the issuance of shares, Raphael Israel has also agreed to pay Wolc a royalty fee equal to 15% of the net royalties generated from sales of Raphael Israel’s pharmaceutical drug products that are developed at Rambam hospital in Israel.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 6:- | CONTINGENT LIABILITIES AND COMMITMENTS (Cont.) |
| d. | Consulting agreement with the Chief Executive Officer |
In June 2019, the Company entered into an agreement with Sheffa Enterprises Inc, a company wholly owned by the Company’s Chief Executive Officer (“CEO”). According to the terms of the agreement, the CEO will provide consulting services to the Company until December 31, 2022 (“termination date”). The fees for his services will be $10 per month. Furthermore, the Company may terminate the agreement prior to the termination date by providing 120 days’ advance written notice and paying the CEO a termination fee equal to the lesser of $1,000 or the monthly fee agreed, for the remaining term of the agreement.
| e. | Consulting agreement with the Chief Technology Officer |
In September 2019, the Company entered into an agreement with its Chief Technology Officer (“CTO”). According to the terms of the agreement, the CTO will provide consulting services to the Company that include engaging with an array of science consultants and coordinating collaborations with hospitals on medical cannabis research. The fees for his services will be $9 per month. Furthermore, the CTO will receive 15% of the Company’s net royalties’ from worldwide sales of any of the Company’s cannabis-based medical indications treating COVID-19, once the Company starts generating revenues.
NOTE 3 7:- GOING CONCERN
STOCKOLDERS’ EQUITY
| a. | Between January and September 2021, the Company raised $578 (net of issuance expenses of $52) and issued 1,632,509 shares of common stock. An amount of $160 was received before the Share Exchange (see Note 1a) and an amount of $470 was received after the Share Exchange. |
| b. | On May 14, 2021, the Company, completed a Share Exchange pursuant to which the Company issued 9,459,253 common stock to the shareholders of Raphael, and the shareholders of Raphael became the holders of 90% of the issued and outstanding common stock of the Company while the Company’s shareholders hold, immediately following the Share Exchange, 1,051,028 common stock, which represents 10% of the Company (see also Note 1a). |
| c. | In October through December 2021, the Company raised $820 and issued 715,250 shares of common stock and 441,000 warrants to purchase common stock in private placement. The warrants are exercisable at an exercise price of $1.12-$1.25 per share into common stock and expire in November and December 2022. As part of the fund raising, the Company issued 112,500 common stock to certain third parties as a finder fee. As such, an amount of $102 was recorded as non cash issuance costs. |
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 8:- | RELATED PARTIES BALANCES AND TRANSACTIONS |
| A. | Balances |
The following related party payables are included in accounts payable and accrued expenses.
| As of December 31, | ||||||||
| 2021 | 2020 | |||||||
| Payables to related party - Officers (*) | 22 | 10 | ||||||
| 22 | 10 | |||||||
| (*) | Relates to Chief Executive Officer’s and Chief Financial Officer’s services |
| B. | Transactions |
| Year ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Consulting services (*) | 120 | 110 | ||||||
| Legal fee (**) | 75 | - | ||||||
| 195 | 110 | |||||||
| (*) | Including salary expenses to Company’s CEO. |
For further details on the consulting agreement with Company’s CEO, refer to Note 6d.
| (**) | Including legal services provided to Company’s subsidiary by Company’s Chief Financial Officer with respect to an agreement between the Company and its Chief Financial Officer. |
| NOTE 9:- | TAXES ON INCOME |
| a. | As of December 31, 2021, the Company had U.S. federal net operating loss carryforwards of approximately $3,912 available to reduce future taxable income. There is a limitation on the amount of taxable income that can be offset by carryforwards after a change in control (generally greater than a 50% change in ownership). Losses from 2018 and forward that can only offset 80% of taxable income in a future year. |
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 9:- | TAXES ON INCOME (Cont.) |
Income tax rates applicable to the Company in 2021 and 2020 was 21%.
| b. | Foreign income tax: |
| 1. | Income tax rates: |
Presented hereunder are the income tax rates relevant to the Company’s Israeli subsidiary
| 2021 - 23% |
| 2020 - 23% |
| 2. | The Company’s Israeli subsidiary have estimated total available carryforward operating tax losses for Israeli income tax purposes of approximately $1,843 as of December 31, 2021. |
| c. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows:
| As of December 31, | ||||||||
| 2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forward | $ | 5,746 | $ | 687 | ||||
| Deferred tax asset before valuation allowance | 1,243 | 158 | ||||||
| Valuation allowance | (1,243 | ) | (158 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that all or some portion of the deferred tax assets will not be realized.
The ultimate realization of the deferred tax assets is dependent upon the generation of future taxable income during the periods in which temporary differences are deductible and net operating losses are utilized. Based on consideration of these factors, the Company recorded a full valuation allowance as of December 31, 2021 and 2020.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S dollars in thousands (except for share and per share data)
| NOTE 9:- | TAXES ON INCOME (Cont.) |
| d. | Reconciliation of the theoretical tax expense to the actual tax expense: |
The main reconciling item between the statutory tax rate of the Company and the effective tax rate is the recognition of valuation allowance in respect of deferred taxes relating to accumulated net operating losses carried forward due to the uncertainty of the realization of such deferred taxes.
| Year ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Net loss, as reported in the consolidated statements of comprehensive loss | $ | 1,625 | $ | 718 | ||||
| Statutory tax rate | 21 | % | 21 | % | ||||
| Computed “expected” tax income | 341 | 151 | ||||||
| Valuation allowance | (341 | ) | (151 | ) | ||||
| Taxes on income | $ | - | $ | - | ||||
| NOTE 10:- | SELECTED STATEMENTS OF COMPREHENSIVE LOSS DATA |
| a. | Research and development expenses: |
| For the Year Ended December 31 | ||||||||
| 2021 | 2020 | |||||||
| Subcontractors and consultants | $ | 765 | $ | 455 | ||||
| Laboratory services | 11 | 6 | ||||||
| $ | 776 | $ | 461 | |||||
| b. | General and administrative expenses: |
| For the Year Ended December 31 | ||||||||
| 2021 | 2020 | |||||||
| Professional services | $ | 697 | $ | 94 | ||||
| Consulting services | 120 | 110 | ||||||
| Rent and office maintenance | 31 | 32 | ||||||
| Others | 5 | 9 | ||||||
| $ | 853 | $ | 245 | |||||
| c. | Financial expenses, net: |
| For the Year Ended December 31 | ||||||||
| 2021 | 2020 | |||||||
| Bank fees | 2 | 1 | ||||||
| Exchange rate differences | (6 | ) | 11 | |||||
| Total financial expenses, net | $ | (4 | ) | $ | 12 | |||
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED INTERIM BALANCE SHEETS
U.S dollars in thousands (except for share and per share data)
| As of March 31, | As of December 31, | |||||||
| 2022 | 2021 | |||||||
| (Unaudited) | (Audited) | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 81 | $ | 153 | ||||
| Other current assets | 99 | 269 | ||||||
| Total current assets | 180 | 422 | ||||||
| Total assets | $ | 180 | $ | 422 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Other account payables and accrued expenses | 76 | 272 | ||||||
| Payable to related party | 1 | 22 | ||||||
| Total current liabilities | 77 | 294 | ||||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.01 par value: | ||||||||
| Authorized: 21,020,560 shares at March 31, 2022 and December 31, 2021; | ||||||||
| Issued and outstanding: 13,290,540 and 12,970,540 at March 31, 2022 and December 31, 2021, respectively; | 133 | 130 | ||||||
| Additional paid-in capital | 2,823 | 2,665 | ||||||
| Accumulated deficit | (2,853 | ) | (2,667 | ) | ||||
| Total stockholders’ equity | 103 | 128 | ||||||
| Total liabilities and stockholders’ equity | $ | 180 | $ | 422 | ||||
The accompanying notes are an integral part of the condensed consolidated interim financial statements.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF COMPREHENSIVE LOSS
U.S dollars in thousands (except for share and per share data)
Three months ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Unaudited | ||||||||
| Research and development expenses | $ | 86 | $ | 169 | ||||
| General and administrative expenses | 89 | 83 | ||||||
| Operating loss | 175 | 252 | ||||||
| Total financial expense | 10 | 3 | ||||||
| Net loss | $ | 185 | $ | 255 | ||||
| Basic and diluted net loss per share | $ | 0.01 | $ | 0.02 | ||||
| Weighted average number of shares of common stock used in computing basic and diluted net loss per share (*) | 13,035,371 | 9,459,253 | ||||||
| (*) | Number of shares has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization transaction and to reflect adjustment to the share par value (refer to Note 1a). |
The accompanying notes are an integral part of the condensed consolidated interim financial statements.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (UNAUDITED)
U.S dollars in thousands (except for share and per share data)
| Common stock | Additional paid-in | Accumulated | ||||||||||||||||||
| Number | Amount | capital | Deficit | Total equity | ||||||||||||||||
| Balance as of January 1, 2022 | 12,970,540 | $ | 130 | $ | 2,666 | $ | (2,668 | ) | $ | 128 | ||||||||||
| Issuance of common stock and warrants | 320,000 | 3 | 157 | - | 160 | |||||||||||||||
| Net loss | - | - | - | (185 | ) | (185 | ) | |||||||||||||
| Balance as of March 31, 2022 | 13,290,540 | $ | 133 | $ | 2,823 | $ | (2,853 | ) | $ | 103 | ||||||||||
| Common stock | Additional paid-in | Accumulated | ||||||||||||||||||
| Number | Amount | capital | Deficit | Total equity | ||||||||||||||||
| Balance as of January 1, 2021 (*) | 9,459,253 | $ | 95 | $ | 905 | $ | (1,043 | ) | $ | (43 | ) | |||||||||
| Net loss | - | - | - | (255 | ) | (255 | ) | |||||||||||||
| Balance as of March 31, 2021 (*) | 9,459,253 | $ | 95 | $ | 905 | $ | (1,298 | ) | $ | (298 | ) | |||||||||
| (*) | Number of shares has been retroactively adjusted based on the equivalent number of shares received by the accounting acquirer in the reverse recapitalization transaction and to reflect adjustment to the share par value (refer to Note 1a). |
The accompanying notes are an integral part of the condensed consolidated interim financial statements.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF CASH FLOWS
U.S dollars in thousands (except for share and per share data)
| Three months ended March 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (185 | ) | $ | (255 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Changes in: | ||||||||
| Other current assets | 170 | 99 | ||||||
| Account payables | (67 | ) | 27 | |||||
| Net cash used in operating activities | (82 | ) | (129 | ) | ||||
| Cash flows from investing activities | ||||||||
| - | - | |||||||
| Net cash provided by investing activities | - | - | ||||||
| Cash flows from financing activities | ||||||||
| Issuance of common stock and warrants | 10 | - | ||||||
| Receipt of a loan | - | 44 | ||||||
| Net cash provided by financing activities | 10 | 44 | ||||||
| Change in cash and cash equivalents | (72 | ) | (85 | ) | ||||
| Cash and cash equivalents at the beginning of the period | 153 | 95 | ||||||
| Cash and cash equivalents at the end of the period | $ | 81 | $ | 10 | ||||
| Non cash supplement | ||||||||
| Issuance of common stock and warrants | $ | 150 | - | |||||
The accompanying notes are an integral part of the condensed consolidated interim financial statements.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (UNAUDITED)
U.S dollars in thousands (except for share and per share data)
| NOTE 1:- | GENERAL |
| a. | Raphael Pharmaceutical Inc (formerly Easy Energy, Inc.) (the “Company”) was incorporated under the laws of the State of Nevada on May 17, 2007. The Company is headquartered in Tel Aviv-Jaffa, Israel. From April 1, 2011 until December 31, 2019, the Company was not active. On October 8, 2020, the Company and its stockholders entered into a Share Exchange Agreement (the “Share Exchange”) with an Israeli pharmaceutical company (“Raphael”), according to which, among other matters, all shareholders of Raphael will sell and convey the entire holdings in Raphael to the Company such that following the Share Exchange, the shareholders of Raphael will hold 90% of the issued and outstanding common stock of the Company, and the existing shareholders of the Company will hold the remaining 10% of the issued and outstanding common stock. On May 14, 2021, the Company’s board of directors and stockholders approved a 1-for-100 reverse split of the Company’s common stock, which was implemented and became effective as of May 14, 2021. The reverse split combined each one hundred (100) shares of the Company’s issued and outstanding Common stock into one share of common stock. No fractional shares were issued in connection with the reverse split, and any fractional shares resulting from the reverse split were rounded up to the nearest whole share. On May 14, 2021, Raphael and the Company, completed the Share Exchange pursuant to which 9,459,253 common stock were issued to the shareholders of Raphael so that they became the holders of 90% of the issued and outstanding common stock of the Company immediately after the Share Exchange while the Company’s shareholders hold, following the Share Exchange, 1,051,028 common stock which represents 10% of the Company. On May 19, 2021, as agreed by the parties to the Share Exchange, the Company changed its name to Raphael Pharmaceutical Inc. Following such Share Exchange, Raphael’s activities are the sole activities of the Company. The Share Exchange was accounted for as a reverse recapitalization which is outside the scope ASC 805, “Business Combinations” (“ASC 805”), as the Company, the legal acquirer, is considered a non-operating public shell, and is therefore not a business as defined in ASC 805. As the shareholders of Raphael received the largest ownership interest in the Company, Raphael was determined to be the “accounting acquirer” in the Share Exchange. As a result, the historical financial statements of the Company were replaced with the financial statement of Raphael for all periods presented. | |
| b. | Going concern and management plans |
The accompanying financial statements have been prepared assumingon a going-concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. Since its inception, the Company has devoted substantially all of its efforts to research and development, clinical trials, and raising capital. The Company is still in its development and clinical stage and has not yet generated revenues. The extent of the Company’s future operating losses and the timing of becoming profitable are uncertain. As of March 31, 2022, the Company’s accumulated deficit was $2,853. The Company has funded its operations to date primarily through equity financing and the issuance of a loan. Additional funding will be required to complete the Company’s research and development and clinical trials, to attain regulatory approvals, to begin the commercialization efforts of the Company’s product and to achieve a level of sales adequate to support the Company’s cost structure.
RAPHAEL PHARMACEUTICAL INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (UNAUDITED)
U.S dollars in thousands (except for share and per share data)
| NOTE 1:- | GENERAL (Cont.) |
Management’s plans include, but are not limited to, raising capital in the United States. There can be no assurance that it will be able to successfully raise additional financing, including in a public offering, or obtain additional financing on a timely basis or on terms acceptable to the Company, or at all.
Management expects that the Company will continue as a going concern. The Companyto generate losses from the development, clinical development and regulatory activities of its product, which will result in negative cash flow from operating activity. This has net losses for the
period from inceptionled management to March 31, 2008 of $1,199,637 and the Company has not
established revenue sufficient to cover its operating expenses. These conditions
raiseconclude that substantial doubt about the Company'sCompany’s ability to continue as a going concern.
Managements' planconcern exists in the event that additional funding does not occur. If such sufficient financing is to completenot received timely, the design of the Company's product, to engage
third parties firms to manufacture the components of the product, develop and
manufacture a first product suited to cellular phone use and explore potential
distributors for our product. The Company had approximately $725,000 on hand as
of March 31, 2008, management anticipates that such funds will not have sufficient cash flows and liquidity to finance its business operations as currently contemplated and would then need to pursue a plan to license its assets, seek to be sufficient
to pay our estimated expenses for the next twelve month period. Management
expects to start generating revenue within 8-10 months but has no assurance that
such revenues shall be generated and in what amounts. Management intends to
fulfill any additional cash requirement through the sale of either equity acquired by another entity, cease operations and/or debt. However, the Company has not identified the source of additional cash and
there is no guarantee that such funds will be available or if available that the
terms will be acceptable to the Company.seek bankruptcy protection. The Company's continuation as a going concern is dependent on its ability to
complete and market its product and to obtain additional financing as may be
required and ultimately to attain profitability. TheCompany’s financial statements do not includereflect any adjustments that might result from the outcome of this uncertainty.
NOTE 4 - DEFERRED OFFERING COSTS - STOCK RELATED
On March 10, 2008
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES |
These unaudited interim financial statements should be read in conjunction with the audited financial statements and accompanying notes for the year ended December 31, 2021. The significant accounting policies applied in the annual financial statements of the Company entered into agreements relating to the issuanceas of 882,353 shares of the Company's common stock, par value $0.00001 per share,
and a warrant to purchase up to 3,000,000 shares of the Company's common stock
at a price of $0.27 per share.
December 31, 2021, are applied consistently in these interim financial statements.
| NOTE 3:- | UNAUDITED INTERIM FINANCIAL STATEMENTS |
The shares issued to date as part of this agreementaccompanying unaudited condensed consolidated interim financial statements have been charged to
deferred financing cost until such time the Company exercises its option to
demand an additional $1,000,000 of financing from the financer. At such time,
the deferred financing charges will be offset against the financing.
NOTE 5 - CAPITAL STOCK
COMMON SHARES - AUTHORIZED
The company has 1,000,000,000 common shares authorized at a par value of
$0.00001 per share and 50,000,000 shares of preferred stock at a par value of
$0.0001 per shares. All common stock have equal voting rights, are
non-assessable and have one vote per share. Voting rights are not non-cumulative
and, therefore, the holders of more than 50% of the stock could, if they choose
to do so, elect all the directors of the company.
F-19
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 and December 31, 2007
NOTE 5 - CAPITAL STOCK (CONTINUED)
ISSUED AND OUTSTANDING -
On May 17, 2007 (inception), the Company issued 40,000,000 shares of its common
stock to its Directors for cash of $2,000. See Note 5.
On May 17, 2007, the Company closed a private placement for 10,000,000 common
shares at a price of $0.0002 per share, or an aggregate of $2,000. The Company
accepted subscription from two offshore non-affiliated investors.
On August 27, 2007, the Company closed a private placement for 30,333,190 common
shares at a price of $0.003 per share, or an aggregate of $91,000. The Company
accepted subscription from forty offshore non-affiliated investors.
On February 8, 2008, the Company changed it number of authorized shares of
Common Stock from 100,000,000 to 1,000,000,000 and provide for a ten for one
forward split of the Registrant's shares of common stock outstanding.
On February 28, 2008, the Company commenced a private placement offering of
367,647.6 units, each unit being offered for $1.70, for aggregate gross proceeds
of $625,001. Each unit consists of (i) ten common stock shares, (ii) thirty
Class A Warrant. Each Class A Warrant entitles the holder thereof to purchase
one share of common stock at an exercise price of $0.27 per share, expiring five
years from the date of purchase. This offering is being made to non-U.S. persons
in offshore transactions pursuant to the exemption from registration provided by
Regulation S of the Securities Act.
On February 28, 2008, the Company completed a subscription agreement pursuant to
which it sold and issued 208,333 common stock shares under Rule 903 of
Regulation S of the Act of 1933 (the "Act") to an accredited investor for the
aggregate purchase price of US $50,000, purchase price US $0.24 per share.
On March 3, 2008, the Company signed a subscription agreement in which it
undertook to issue to its legal counsel 300,000 shares of restricted common
stock valued at $0.24 per share based upon the Regulation S offering completed
at approximately the same date for an aggregate price of $72,000 for legal
services provided and warrants to purchase 1,000,000 shares of the Company's
common stock at an exercise price of $0.15 for a period of five years. The
warrants were valuedprepared in accordance with SFAS 123R usingU.S. generally accepted accounting principles (“GAAP”) for interim financial information and with the assumptions
described belowinstructions to Form 10-Q and resulted in an expenseArticle 10 of $1,031.
On March 10, 2008,U.S. Securities and Exchange Commission Regulation S-X. Accordingly, they do not include all the Company entered intoinformation and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments considered necessary for a Securities Purchase Agreement and
a Registration Rights Agreement with Tailor Made Capital, Ltd. ("TMC") relatingfair presentation have been included (consisting only of normal recurring adjustments except as otherwise discussed). For further information, reference is made to the issuance to TMC of 882,353 shares ofconsolidated financial statements and footnotes thereto included in the Company's common stock, par
value $0.0001 per share, and a warrant to purchase up to 3,000,000 shares of the
Company's common stock at a price of $0.27 per share (the "Warrant"). The shares
were issued for deferred stock offering costs and valued at $0.24 per share
based upon the Regulation S offering completed at approximately the same date
for an aggregate price of $211,765. The warrant shall be in effect for five
years from the date that the Company's common stock is initially listed or
quoted for tradingCompany’s Annual Report on a trading market. The warrants were valued in accordance
with SFAS 123R using the assumptions described below and resulted in an expense
of $8,770. On March 25, 2008, we entered into a subscription agreement under
which we undertook to issue 2,000,000 shares for cash payment of $50,000 by an
"accredited investor" as defined in Rule 501 of Regulation D under the
Securities Act of 1933, and, in consideration for services provided, warrants to
purchase 1,000,000 shares of the Company's common stock at an exercise price of
$0.10 for a period of three years. The shares were valued at the trading price
on the date of issuance of $0.30 for an additional expense of $550,000. The
warrants were valued using the Black-Scholes model at $213,813.
On March 27, 2008(pursuant to an agreement dated January 16, 2008), we issued
4,285,714 common stock shares to an "accredited investor" as defined in Rule 501
of Regulation D under the Securities Act of 1933Form 10-K for the aggregate purchase
price of US $300,000, purchase price of $0.07 per share.
On March 27, 2008, the Company entered into a consulting services agreement of
which the consultant will provide certain investor and market relations
consulting services to the Company in consideration of the Company's issuance of
F-20
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 andyear ended December 31, 2007
NOTE 5 - CAPITAL STOCK (CONTINUED)
ISSUED AND OUTSTANDING (CONTINUED) -
1,500,000 restricted common shares and2021. Operating results for the sum of $100,000 in cash. The common
shares were valued at the trading price on the date of issuance of $0.30 per
share for a total of $450,000. The expense will be amortized over the one year
service agreement beginning April 1, 2008.
WARRANTS OUTSTANDING -
Date Issued Number of Warrants Exercise Price Expiry Date
- ----------- ------------------ -------------- -----------
February 28, 2008 11,029,428 $ 0.27 February 28, 2013
March 3, 2008 1,000,000 $ 0.15 March 3, 2013
March 10, 2008 3,000,000 $ 0.27 March 10, 2013
March 25, 2008 1,000,000 $ 0.10 March 25, 2008
The value allocated to the warrants was estimated using the Black-Scholes option
pricing model with the following assumptions: dividend yield of 0%, expected
volatility of 100%, risk-free interest rate of 3.94% and expected lives of
3years to 5 years. The volatility was determined based upon the weekly trading
price of the stock from the date of inception through March 31, 2008. Common
stock issued for services was valued at the price of the shares issued for cash
on or close to the date of issuance.
NOTE 6 - RELATED PARTY TRANSACTIONS
The Company's directors provide office space free of charge. The Company has
recorded the estimated value of the office space of $1,000 per month as a
contribution to capital. The Company recorded a contribution to capital of
$3,000 and $7,500 during the periodsthree months ended March 31, 2008 and2022 are not necessarily indicative of the results that may be expected for the year ending December 31, 2007,
respectively. The officers and directors of the Company are involved in other
business activities and may, in the future, become involved in other business
opportunities. If a specific business opportunity becomes available, such
persons may face a conflict in selecting between the Company and their other
business interests. The Company has not formulated a policy for the resolution
of such conflicts.
On May 17, 2007 (inception), the Company issued 40,000,000 shares of its common
stock to its Directors for cash of $2,000. See Note 4.
During the period ended March 31, 2008, the Company paid $200,000 in product
development costs to a company wholly owned by the president and director of the
Company.
NOTE 7 - INCOME TAXES
The Company provides for income taxes under Statement of Financial Accounting
Standards No. 109, ACCOUNTING FOR INCOME TAXES. SFAS No. 109 requires the use of
an asset and liability approach in accounting for income taxes. Deferred tax
assets and liabilities are recorded based on the differences between the
financial statement and tax bases of assets and liabilities and the tax rates in
effect currently.
SFAS No. 109 requires the reduction of deferred tax assets by a valuation
allowance if, based on the weight of available evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. In the
Company's opinion, it is uncertain whether they will generate sufficient taxable
income in the future to fully utilize the net deferred tax asset. Accordingly, a
valuation allowance equal to the deferred tax asset has been recorded. The total
deferred tax asset is $263,920, which is calculated by multiplying a 22%
estimated tax rate by the cumulative NOL of $1,199,637.
F-21
EASY ENERGY,2022.
RAPHAEL PHARMACEUTICAL INC. (A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENTS
Below is a listing of the most recent accounting standards SFAS 150-162 and
their effect on the Company.
STATEMENT NO. 150 - ACCOUNTING FOR CERTAIN FINANCIAL INSTRUMENTS WITH
CHARACTERISTICS OF BOTH LIABILITIES AND EQUITY (ISSUED 5/03)
This Statement establishes standards for how an issuer classifies and measures
certain financial instruments with characteristics of both liabilities and
equity. The application of SFAS No. 150 did not have any effect on the Company's
financial statements.
STATEMENT NO. 151 - INVENTORY COSTS-AN AMENDMENT OF ARB NO. 43, CHAPTER 4
(ISSUED 11/04)
This statement amends the guidance in ARB No. 43, Chapter 4, INVENTORY PRICING,
to clarify the accounting for abnormal amounts of idle facility expense,
freight, handling costs, and wasted material (spoilage). Paragraph 5 of ARB 43,
Chapter 4, previously stated that "...under some circumstances, items such as
idle facility expense, excessive spoilage, double freight and re-handling costs
may be so abnormal ass to require treatment as current period charges...." This
Statement requires that those items be recognized as current-period charges
regardless of whether they meet the criterion of "so abnormal." In addition,
this Statement requires that allocation of fixed production overheads to the
costs of conversion be based on the normal capacity of the production
facilities. The application of SFAS No. 151 did not have any effect on the
Company's financial statements.
STATEMENT NO. 152 - ACCOUNTING FOR REAL ESTATE TIME-SHARING TRANSACTIONS (AN
AMENDMENT OF FASB STATEMENTS NO. 66 AND 67)
This Statement amends FASB Statement No. 66, ACCOUNTING FOR SALES OF REAL
ESTATE, to reference the financial accounting and reporting guidance for real
estate time-sharing transactions that is provided in AICPA Statement of Position
(SOP) 04-2, ACCOUNTING FOR REAL ESTATE TIME-SHARING TRANSACTIONS.
This Statement also amends FASB Statement No. 67, Accounting FOR COSTS AND
INITIAL RENTAL OPERATIONS OF REAL ESTATE PROJECTS, states that the guidance for
(a) incidental operations and (b) costs incurred to sell real estate projects
does not apply to real estate time-sharing transactions. The accounting for
those operations and costs is subject to the guidance in SOP 04-2. The
application of SFAS No. 152 did not have any effect on the Company's financial
statements.
STATEMENT NO. 153 - EXCHANGES OF NON-MONETARY ASSETS (AN AMENDMENT OF APB
OPINION NO. 29)
The guidance in APB Opinion No. 29, ACCOUNTING FOR NON-MONETARY TRANSACTIONS, is
based on the principle that exchanges of non-monetary assets should be measured
based on the fair value of the assets exchanged. The guidance in that Opinion,
however, includes certain exceptions to the principle. This Statement amends
Opinion 29 to eliminate the exception for non-monetary exchanges of similar
productive assets and replaces it with a general exception for exchanges of
non-monetary assets that do not have commercial substance. A non-monetary
exchange has commercial substance if the future cash flows of the entity are
expected to change significantly as a result of the exchange. The Company has
applied SFAS No. 153 to the changes made in the financial statements as of March
31, 2008 and December 31, 2007.
STATEMENT NO. 154 - ACCOUNTING CHANGES AND ERROR CORRECTIONS (A REPLACEMENT OF
APB OPINION NO. 20 AND FASB STATEMENT NO. 3)
This Statement replaces APB Opinion No. 20, ACCOUNTING CHANGES, and FASB
Statement No. 3, REPORTING ACCOUNTING CHANGES INSUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (UNAUDITED)
U.S dollars in thousands (except for share and changes the requirements for the accounting for and reporting of a change in
F-22
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENT (CONTINUED)
accounting principle. This Statement applies to all voluntary changes in
accounting principle. It also applies to changes required by an accounting
pronouncement in the unusual instance that the pronouncement does not include
specific transition provisions. When a pronouncement includes specific
transition provisions, those provisions should be followed. The Company has
applied SFAS No. 154 to the changes made in the financial statements as of March
31, 2008 and December 31, 2007.
SFAS NO. 155 - ACCOUNTING FOR CERTAIN HYBRID FINANCIAL INSTRUMENTS-AN AMENDMENT
OF FASB STATEMENTS NO. 133 AND 140
This statement amends FASB Statements No. 140, Accounting for Transfers and
Servicing of Financial Assets and Extinguishments of Liabilities. This statement
resolves issues addressed in Statement 133 Implementation Issue No. D1,
Application of Statement 133 to Beneficial Interests in Securitized Financial
Assets. This statement is effective for all financial instruments acquired or
issued after
the beginning of an entity's first fiscal year that begins after September 15,
2006. The application of SFAS No. 155 did not have any effect on the Company's
financial statements.
SFAS NO. 156 - ACCOUNTING FOR SERVICING OF FINANCIAL ASSETS-AN AMENDMENT OF FASB
STATEMENT NO. 140
This statement amends FASB Statement No. 140 with respect to the accounting for
separately recognized servicing liabilities. An entity should adopt this
statement as of the beginning of its first fiscal year that begins after
September 15, 2006. The application of SFAS No. 156 did not have any effect on
the Company's financial statements.
SFAS NO. 157 - FAIR VALUE MEASUREMENTS
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements, which
defines fair value, establishes a framework for measuring fair value in GAAP,
and expands disclosures about fair value measurements. SFAS No. 157 does not
require any new fair value measurements, but provides guidance on how to measure
fair value by providing a fair value hierarchy used to classify the source of
the information. This statement is effective for us beginning May 1, 2008. The
application of SFAS No. 157 will not have any effect on the Company's financial
statements.
SFAS NO. 158 - EMPLOYERS' ACCOUNTING FOR DEFINED BENEFIT PENSION AND OTHER
POSTRETIREMENT PLANS-AN AMENDMENT OF FASB STATEMENTS NO. 87, 88, 106, AND
132(R))
This statement improves the financial reporting by requiring an employer to
recognize the overfunded or underfunded status of a defined benefit
postretirement plan (other than a multiemployer plan) as an asset or liabilities
in its statement of financial positions and to recognize changes in that funded
status in the year in which the changes occur through comprehensive income of a
business entity. This statement also improves financial reporting by requiring
an employer to measure the funded status of a plan as of the date of its
year-end statement of financial position, with limited exceptions. The
application of SFAS No. 158 did not have any effect on the Company's financial
statements.
SFAS NO. 159 - THE FAIR VALUE OPTION FOR FINANCIAL ASSETS AND FINANCIAL
LIABILITIES-INCLUDING AN AMENDMENT OF FASB STATEMENT NO. 115
This statement permits entities to choose to measure many financial instruments
and certain items at fair value. The objective is to improve the financial
reporting by providing entities with the opportunity to mitigate volatility in
F-23
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Restated Financial Statements
March 31, 2008 and December 31, 2007
NOTE 8 - RECENT ACCOUNTING PRONOUNCEMENT (CONTINUED)
reported earnings caused by measuring related assets and liabilities differently
without having to apply complex hedge accounting provisions. This statement is
expected to expand the use of fair value measurement objectives for accounting
for financial instruments. This statement is effective as of the beginning of an
entity's first fiscal year that begins after November 15, 2007. The application
of SFAS No. 159 did not have any effect on the Company's financial statements.
SFAS NO. 160 - NON-CONTROLLING INTEREST IN CONSOLIDATED FINANCIAL STATEMENTS- AN
AMENDMENT OF ARB NO. 51
This statement amends ARB 51 to establish accounting and reporting standards for
the non-controlling interest in a subsidiary and for the deconsolidation of a
subsidiary. It also changes the way the consolidated income statement is
presented for non-controlling interest. This statement improves comparability by
eliminating diversity of methods. This statement also requires expanded
disclosure. The application of SFAS No. 160 did not have any effect on the
Company's financial statements.
SFAS NO. 161
This statement is intended to enhance the disclosure requirements for derivative
instruments and hedging activities as required by SFAS 133. The application of
SFAS No. 161 did not have any effect on the Company's financial statements.
SFAS 162
This statement identifies the sources of accounting principles and the framework
for selecting the principles to be used in the preparation of financial
statements for entities that are presented in conformity with GAAP hierarchy.
The application of SFAS No. 162 did not have any effect on the Company's
financial statements.
The past and future adoption of these Statements did not have and is not
expected to have a material effect on the Company's current financial position,
results or operations, or cash flows.
NOTE 9 - RESTATED FINANCIAL STATEMENTS
The Company's financial statements were restated to correct the valuation of
shares and warrants issued for services of $280,711 and $-0- at fair value,
record contributed office rent of $3,000 and $7,500 at fair value and record
prepaid expenses of $50,000 and $-0-, as of and for the periods ended March 31,
2008 and December 31, 2007, respectively. A summary of the changes is presented
below:
BALANCE SHEET
March 31, 2008
As revised Original
---------- --------
ASSETS
Total current assets $1,225,747 $ 775,747
---------- ----------
Total Assets $1,225,747 $ 775,747
========== ==========
LIABILITIES
Current:
Due to director $ 300 $ 300
F-24
EASY ENERGY, INC.
(A Development Stage Company)
Notes to Financial Statements
March 31, 2008
NOTE 9 - RESTATED FINANCIAL STATEMENTS (CONTINUED)
per share data)
| NOTE 4:- | SHAREHOLDERS’ EQUITY |
| a. | On March |
| b. | On March 15, 2022, the Company issued 270,000 shares |
| NOTE 5:- | SUBSEQUENT EVENTS |
| a. | On April 28, 2022, the Company signed an agreement to raise $50 and to issue 300,000 shares of common stock |
| b. | On April 28, 2022 the Company and a certain investor signed a finder fee agreement according to which the Company will issue to the investor 70,000 shares of common stock and 100,000 warrants to purchase common stock at an exercise price of $0.5 per share. The warrants will be exercisable until April 30, 2024. The agreement will become effective once the investor will provide the Company with an equity investment of $550. |
| c. | On May 2, 2022, the Company signed several agreements to raise $250 and to issue 250,000 shares of common stock and 100,000 warrants to purchase common stock at an exercise price of $1.13 per share to certain investors of the Company. The warrants are exercisable until April 30, 2024. The funds were received in May 2022. In addition, it was agreed that the Company will issue 250,000 shares of common stock and 100,000 warrants to purchase common stock at an exercise price of $1.13 per share to a |
PART II -
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM
Item 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
Other Expenses of Issuance and Distribution.
The following table sets forth the costs andvarious expenses payable by usto be incurred in connection with the issuance and distributionregistration of the securities being registered hereunder. No expenses shallhereby, all of which will be borne by the selling shareholders.
SEC registration fees $ 243.32
Legalus.
| SEC registration fee | $ | 408.57 | ||
| Transfer agent’s fees and expenses | $ | 900 | ||
| Printing expenses | $ | 1,000 | ||
| Legal fees and expenses | $ | 6,000 | ||
| Accounting fees and expenses | $ | 10,000 | ||
| Miscellaneous | $ | - | ||
| Total expenses | $ | 18,308.57 |
Item 14. Indemnification of Directors and accounting fees $20,000.00 (1)
Miscellaneous $ 1,000.00 (1)
----------
TOTAL $21,243.32 (1)
- ----------
(1) We have estimated these amounts.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Our officers and directors are indemnified as provided by the Officers.
Nevada Revised Statutes, (the "NRS"), our Articles of Incorporation and our Bylaws.
INDEMNIFICATION
Chapter 78 ofor the NRS, pertaining to private corporations,78.138(7) provides that, we are
requiredsubject to indemnify our officerslimited statutory exceptions and directorsunless the articles of incorporation or an amendment thereto (in each case filed on or after October 1, 2003) provide for greater individual liability, a director or officer is not individually liable to the extent that they are
successful in defendinga corporation or its stockholders or creditors for any actions or claims brought against themdamages as a result of servingany act or failure to act in that position, including criminal, civil, administrativehis or investigative actions and actions brought byher capacity as a director or on behalf of Easy Energy.
Chapter 78 ofofficer unless it is proven that: (i) the NRS further provides that we are permittedact or failure to indemnify our
officers and directors for criminal, civil, administrative or investigative
actions brought against them by third parties and for actions brought by or on
behalf of Easy Energy, even if they are unsuccessful in defending that action,
unless the officer or director's:
(a) action or inactionact constituted a breach of his or her fiduciary duties as a director or officer;officer and (b)(ii) the breach of those duties involved intentional misconduct, fraud or a knowing violation of law.
However,
NRS 78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding if the person (i) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to actions broughtany criminal action or proceeding, had no reasonable cause to believe the conduct was unlawful. NRS 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by the person in connection with the defense or settlement of the action or suit if the person (a) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation. To the extent that a director, officer, employee or agent of a corporation has been successful on behalfthe merits or otherwise in defense of Easy Energyany such action, suit or proceeding, or in defense of any claim, issue or matter therein, the corporation shall indemnify him or her against our officersexpenses, including attorneys’ fees, actually and reasonably incurred by him or directors, we areher in connection with the defense. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendere or its equivalent, does not, permittedof itself, create a presumption that the person is liable pursuant to indemnify our officersNRS 78.138 or directors where they aredid not act in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, or that, with respect to any criminal action or proceeding, he or she had reasonable cause to believe that the conduct was unlawful. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after the exhaustion of all appeals therefrom, to be liable to usthe corporation or for amounts paid in settlement to Easy Energy,the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
II-1
NRS 78.751(1) provides that any discretionary indemnification pursuant to NRS 78.7502 (unless ordered by a court determinesor advanced pursuant to NRS 78.751(2)), may be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made (i) by the stockholders; (ii) by the board of directors by majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding; (iii) if a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion; or (iv) if a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion. NRS 78.751(2) provides that the corporation’s articles of incorporation or bylaws, or an agreement made by the corporation, may provide that the expenses of officers and directors incurred in defending a civil or directorscriminal action, suit or proceeding must be paid by the corporation as they are incurred and in advance of the final disposition of the action, suit or proceeding, upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that the director or officer is not entitled to be indemnified.
indemnified by the corporation.
Under the NRS, the indemnification pursuant to NRS 78.7502 and advancement of expenses authorized in or ordered by a court pursuant to NRS 78.751:
| ● | Does not exclude any other rights to which a person seeking indemnification or advancement of expenses may be entitled under the articles of incorporation or any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, for either an action in the person’s official capacity or an action in another capacity while holding office, except that indemnification, unless ordered by a court pursuant to NRS 78.7502 or for the advancement of expenses made pursuant to NRS 78.751(2), may not be made to or on behalf of any director or officer if a final adjudication establishes that the director’s or officer’s acts or omissions involved intentional misconduct, fraud or a knowing violation of the law and was material to the cause of action; and |
| ● | Continues for a person who has ceased to be a director, officer, employee or agent and inures to the benefit of the heirs, executors and administrators of such a person. |
A right to indemnification or to advancement of expenses arising under a provision of the articles of incorporation or any bylaw is not eliminated or impaired by an amendment to such provision after the occurrence of the act or omission that is the subject of the civil, criminal, administrative or investigative action, suit or proceeding for which indemnification or advancement of expenses is sought, unless the provision in effect at the time of such act or omission explicitly authorizes such elimination or impairment after such action or omission has occurred.
Our Articles and Bylawsgoverning documents provide that to the fullest extent permitted under the NRS (including, without limitation, to the fullest extent permitted under NRS 78.7502 and 78.751(3)) and other applicable law, that we willshall indemnify our directors and officers toin their respective capacities as such and in any and all other capacities in which any of them serves at our request.
Item 15. Recent Sales of Unregistered Securities.
Set forth below are the fullest extent not prohibitedsales of all securities by Nevada law; provided, however,
that we shall not be required to indemnify any director or officer in connection
with any proceeding (or part thereof) initiated by such person unless: (a) such
indemnification is expressly required to be made by law; (b) the proceeding was
authorized by our Board of Directors; (c) such indemnification is provided by
us, in our sole discretion, pursuant to the powers vested in us under Nevada
law; or (d) such indemnification is required to be made pursuant to the bylaws.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
The following sets forth certain information concerning securitiesCompany since May 2019, which were sold or issued by us since our inception on May 17, 2007 withoutnot registered under the Securities Act. The Company believes that each of such issuances was exempt from registration under the Securities Act of 1933 (the "Act") in reliance on exemptions from such
registration requirements:
II-1
On March 27, 2008, we entered into a consulting services agreement of which the
consultant will provide certain investor and market relations consulting
services to the Company in consideration of the Company's issuance of 1,500,000
restricted common shares and the sum of $100,000. The issuance was exempt under
Regulation D under the Act and/or Section 4(2) of the Act.
On March 27, 2008 (pursuant to an agreement dated January 16, 2008), we issued
4,285,714 common stock shares to an "accredited investor" as defined in Rule 501
of Regulation D under the Act for the aggregate purchase price of $300,000, or
$0.07 per share.
On March 25, 2008, we entered into a subscription agreement under which we
undertook to issue 2,000,000 shares for cash payment of $50,000 by an
"accredited investor" as defined in Rule 501 of Regulation D under the Act, and,
in consideration for services provided, warrants to purchase 1,000,000 shares of
the Company's common stock at an exercise price of $0.10 per share for a period
of three years.
On March 10, 2008, we entered into a Securities Purchase Agreement and a
Registration Rights Agreement with Tailor Made Capital, Ltd. ("TMC") relating to
the issuance to TMC of 882,353 shares of the Company's common stock, par value
$0.0001 per share, and a warrant to purchase up to 3,000,000 shares of the
Company's common stock at a price of $0.27 per share (the "Warrant"). The
Warrant shall be in effect for five years from the date that the Company's
common stock is initially listed or quoted for trading on a trading market. The
Securities Purchase Agreement further provides that, at the Company's demand,
TMC will purchase up to an additional $1,000,000 of shares of the Company's
common stock commencing immediately after the date that the shelf registration
of the Company's shares that are subject to the Securities Purchase Agreement is
declared effective. These sales were exempt under Regulation S and/or Regulation
D under the Act and/or Section 4(2) of the Act.
On March 3, 2008, we entered into a Regulation S Subscription Agreement pursuant
to which we issued under Rule 903 of Regulation S of the Act to our then legal
counsel 300,000 shares of restricted common stock for an aggregate price of
$3,000 for services provided and warrants to purchase 1,000,000 shares of the
Company's common stock at an exercise price of $0.15 per share for a period of
five years.
On February 28, 2008, we entered into a Regulation S Subscription Agreement
pursuant to which we sold and issued 208,333 common stock shares under Rule 903
of Regulation S of the Act to an accredited investor for the aggregate purchase
price of $50,000, or $0.24 per share.
On February 28, 2008, we sold 367,647 units, each unit being offered for $1.70,
for aggregate gross proceeds of $625,001 Each unit consisted of (i) ten common
stock shares, (ii) thirty Class A Warrants. Each Class A Warrant entitles the
holder thereof to purchase one share of common stock at an exercise price of
$0.27 per share, expiring five years from the date of purchase. This offering
was made to non-U.S. persons in offshore transactions pursuant to the exemption
from registration provided by Regulation S of the Act.
On August 17, 2007 we issued 30,033,319 shares of our common stock to forty non
U.S. subscribers at an offering price of $0.003 per share for gross offering
proceeds of $91,000 in an offshore transaction pursuant to an exemption from
registration under Rule 903 of Regulation S of the Act.
On May 17, 2007 we issued 40,000,000 shares of our common stock to two executive
officers of our company, at an offering price of $ 0.00005 per share for gross
offering proceeds of $2,000 in an offshore transaction pursuant to an exemption
from registration under Regulation S of the Act.
On May 17, 2007 we issued 10,000,000 shares of our common stock to two
subscribers at an offering price of $0.0002 per share for gross offering
proceeds of $2,000 in an offshore transaction pursuant to Regulation S4(a)(2) of the Securities Act of 1933.
All of the above issuances and sales were exempt underand/or Regulation S and/or
Regulation D under the Act and/or Section 4(2)Securities Act.
Between June 2019 and June 2022, we issued and sold an aggregate of the Act.
II-2
ITEM 16. EXHIBITS.
The following Exhibits are filed with this prospectus:
Exhibit
Number Description
------ -----------
3.1 Amended Articles3,812,747 shares of Incorporation (incorporated by reference to
exhibit 3.1 of the registrant's registration statement on Form
SB-2 filed on October 24, 2007).
3.2 Bylaws (incorporated by reference to exhibit 3.2 of the
registrant's registration statement on Form SB-2 filed on October
24, 2007).
4.1 Form of share certificate (incorporated by reference to exhibit
4.1 of the registrant's registration statement on form SB-2 filed
on October 24, 2007.)
4.2** Form ofCommon Stock, Purchase Warrant dated February 28, 2008.
4.3** Appendix to Stock Purchase Warrant dated February 28, 2008.
5.1*** Opinion of Zysman, Aharoni, Gayer & Co./ Sullivan & Worcester LLP
10.1 Form of subscription agreement (incorporated by reference to
exhibit 10.1 of the registrant's registration statement on Form
SB-2 filed on October 24, 2007).
10.2 Promissory Note of registrant to Guy Ofir (incorporated by
reference to exhibit 10.2 of the registrant's registration
statement on Form SB-2 filed on October 24, 2007).
10.3 Assignment of patent application (incorporated by reference to
exhibit 10.3 of the registrant's registration statement on Form
SB-2 filed on October 24, 2007).
10.4** Subscription Agreement dated February 28, 2008 between the
Registrant and the Meitav Entities.
10.5 Securities Purchase Agreement dated March 10, 2008 between the
registrant and Tailor Made Capital, Ltd. (incorporated by
reference to exhibit 4.1 of the registrant's Current Report on
Form 8-K filed on March 24, 2008, as amended on July 7, 2008).
10.6 Registration Rights Agreement dated March 10, 2008 between the
registrant and Tailor Made Capital, Ltd. (incorporated by
reference to exhibit 4.2 of the Current Report on Form 8-K filed
on March 24, 2008, as amended on July 7, 2008).
10.7** Investment Agreement dated January 16, 2008 between the
registrant and Meir Duke.
23.1* Consent of Moore & Associates Chartered
23.2*** Consent of Zysman, Aharoni,Gayer & Co./ Sullivan & Worcester LLP,
included in Exhibit 5.1.
24.1 Power of Attorney (included on the signature page of the
registrant's registration statement number 333-150468 on Form S-1
filed with the Securities and Exchange Commission on April 25,
2008).
- ----------
* Filed herewith.
** Incorporated by reference to the corresponding exhibit of Easy Energy,
Inc.'s registration statement number 333-150468 on Form S-1, pre effective
amendment #2, filed with the Securities and Exchange Commission on July 7,
2008.
*** Incorporated by reference to the corresponding exhibit of Easy Energy,
Inc.'s registration statement number 333-150468 on Form S-1, pre effective
amendment #3, filed with the Securities and Exchange Commission on August
13, 2008.
II-3
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a
post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the
Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after
the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate,
represent a fundamental change in the information set forth in the
registration statement. Notwithstanding the foregoing, any increase or
decrease in the volume of securities offered (if the total dollar value of
securities offered wouldwere not exceed that which was registered) and any
deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Commission
pursuant to Rule 424(b) if, in the aggregate, the changes in volume and
price represent no more than a 20 percent change in the maximum aggregate
offering price set forth in the "Calculation of Registration Fee" table in
the effective registration statement; and
(iii) To include any material information with respect to the plan of
distribution not previously disclosed in the registration statement or any
material change to such information in the registration statement;
(2) That, for the purpose of determining any liabilityregistered under the Securities Act, to investors and service providers for aggregate proceeds of 1933, each such post-effective amendment shall be deemed to be a new
registration statement relatingapproximately $2,225,160 in gross proceeds.
On May 14, 2021, pursuant to the securities offered therein,Share Exchange, Easy Energy issued an aggregate of 9,459,252 shares of its common stock to the Raphael Israel shareholders in exchange for their shares of Raphael Israel.
II-2
Item 16. Exhibits and the
offering of such securities at that time shall be deemed to be the initial bona
fide offering thereof.
Financial Statement Schedules
| (a) | Exhibits. |
EXHIBIT INDEX
| * | Filed herewith. |
| (b) | Financial Statements. See page F-1 for an index to the financial statements and schedules included in the registration statement. |
II-3
Item 17. Undertakings
| (a) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| (b) | The undersigned registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
II-4
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of a post-effective amendment any of the securities being registered which remain unsold atfollowing communications, the termination of the
offering.
(4) That, for the purpose of determining liability under the Securities Act
of 1933 to any purchaser:
Each prospectus filed by theundersigned registrant pursuant to Rule 424(b) as part ofwill be a registration statement relating to an offering, other than registration
statements relying on rule 430B or other than prospectuses filed in
reliance on Rule 430A, shall be deemed to be part of and included in the
registration statement as of the date it is first used after effectiveness.
Provided, however, that no statement made in a registration statement or
prospectus that is part of the registration statement or made in a document
incorporated or deemed incorporated by reference into the registration
statement or prospectus that is part of the registration statement will, as
to a purchaser with a time of contract of sale prior to such first use,
supersede or modify any statement that was made in the registration
statement or prospectus that was part of the registration statement or made
in any such document immediately prior to such date of first use.
Insofar as indemnification for liabilities arising under the Securities Act
of 1933 may be permitted to directors, officers and controlling persons of the
registrant pursuantseller to the foregoing provisions, or otherwise, the registrant
has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Act and is,
therefore, unenforceable. In the event that a claim for indemnification against
such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is asserted by such
director, officer or controlling person in connection with the securities being
registered, the registrant will, unless in the opinion of its counsel the matter
has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public
policy as expressed in the Actpurchaser and will be governed by the final adjudication ofconsidered to offer or sell such issue.
II-4
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act, 1933, the registrantRegistrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this registration statementRegistration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the cityTel Aviv-Jaffa, Israel, on this 28th day of Las Vegas in the state of
Nevada, on September 18, 2008.
EASY ENERGY, INC.
By: /s/ Guy Ofir
-------------------------------------------------
Guy Ofir, President and Director
(Principal Executive Officer, Principal Financial
Officer and Principal Accounting Officer)
June, 2022.
| Raphael Pharmaceutical Inc. | ||
| By | /s/ Shlomo Pilo | |
| Shlomo Pilo | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act, of 1933, this registration
statementRegistration Statement has been signed below by the following persons in the capacities and on the datesdate indicated.
By: /s/ Guy Ofir
-------------------------------------------------
Guy Ofir, President and Director
(Principal Executive Officer, Principal Financial
Officer and director)
Dated: September 18, 2008
By: *
-------------------------------------------------
Emanuel Cohen, Secretary, Treasurer and Director
Dated: September 18, 2008
* By: /s/ Guy Ofir
-------------------------------------------------
Guy Ofir
(Attorney-in-Fact)
II-5
| Signature | Title | Date | ||
| /s/ Professor Joseph Press, M.D. | Chairman of the Board of Directors | June 28, 2022 | ||
| Professor Joseph Press, M.D. | ||||
| /s/ Shlomo Pilo | Chief Executive Officer | June 28, 2022 | ||
| Shlomo Pilo | (Principal Executive Officer) and Director | |||
| /s/ Guy Ofir | Chief Financial Officer | June 28, 2022 | ||
| Guy Ofir | (Principal Financial Officer) and Director | |||
| /s/ Igal Louria Hayon | Chief Technology Officer and Director | June 28, 2022 | ||
| Igal Louria Hayon | ||||
| /s/ Yehuda Eliya | Director | June 28, 2022 | ||
| Yehuda Eliya |
II-6