INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The Commission allows us to “incorporate by reference” into this prospectus the information we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus, and information in documents that we subsequently file with the SEC. The addressCommission will automatically update and supersede information in this prospectus. We incorporate by reference into this prospectus the documents listed below and any future filings made by us with the Commission under Section 13(a), 13(c), 14 or 15(d) of the website is www.sec.gov.
CONSOLIDATED FINANCIAL STATEMENTS
Index
Amyris, Inc.
ReportExchange Act (other than those documents or the portions of Independent Registered Public Accounting Firm
To the Board of Directorsthose documents furnished and Stockholders of
Amyris, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of convertible preferred stock, redeemable noncontrolling interest and deficit, and of cash flows present fairly, in all material respects, the financial position of Amyris, Inc. and its subsidiaries at December 31, 2009 and December 31, 2008, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statementsnot filed in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2Commission rules) prior to the consolidated financial statements, the Company changed the manner in which it accounts for noncontrolling interest in 2009.
/s/ PricewaterhouseCoopers LLP
San Jose, California
April 16, 2010, except for the tenth
paragraphtermination of Note 19 to the
consolidated financial statements
as to which the date is
June 22, 2010
Amyris, Inc.
Consolidated Balance Sheets
In Thousands, Except Share and Per Share Amounts
| | | | | | | | | | | | | | | | |
| | | December 31,
2008 | | | December 31,
2009 | | | June 30,
2010 | | | Pro Forma
Stockholders’
Equity as of
June 30,
2010 | |
| | | | | | | | | (Unaudited) | | | (Unaudited) | |
Assets | | | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 17,899 | | | $ | 19,188 | | | $ | 112,242 | | | | | |
Short-term investments | | | 19,291 | | | | 48,022 | | | | 95,303 | | | | | |
Accounts receivable | | | 787 | | | | 1,372 | | | | 3,082 | | | | | |
Inventories | | | 1,420 | | | | 2,298 | | | | 2,728 | | | | | |
Prepaid expenses and other current assets | | | 1,556 | | | | 3,983 | | | | 3,664 | | | | | |
| | | | | | | | | | | | | | | | |
Total current assets | | | 40,953 | | | | 74,863 | | | | 217,019 | | | | | |
Property and equipment, net | | | 41,565 | | | | 42,560 | | | | 44,242 | | | | | |
Restricted cash | | | 2,748 | | | | 4,506 | | | | 4,520 | | | | | |
Long-term investments | | | 12,950 | | | | — | | | | 7,995 | | | | | |
Other assets | | | 607 | | | | 230 | | | | 31,212 | | | | | |
| | | | | | | | | | | | | | | | |
Total assets | | $ | 98,823 | | | $ | 122,159 | | | $ | 304,988 | | | | | |
| | | | | | | | | | | | | | | | |
Liabilities, Convertible Preferred Stock, Redeemable Noncontrolling Interest and Equity (Deficit) | | | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 2,617 | | | $ | 1,709 | | | $ | 2,507 | | | | | |
Deferred revenue | | | — | | | | 378 | | | | 565 | | | | | |
Accrued and other current liabilities | | | 5,083 | | | | 10,445 | | | | 11,959 | | | | | |
Capital lease obligation, current portion | | | 557 | | | | 2,251 | | | | 2,810 | | | | | |
Debt, current portion | | | 340 | | | | 9,018 | | | | 1,260 | | | | | |
| | | | | | | | | | | | | | | | |
Total current liabilities | | | 8,597 | | | | 23,801 | | | | 19,101 | | | | | |
Capital lease obligation, net of current portion | | | 3,041 | | | | 4,977 | | | | 4,605 | | | | | |
Long-term debt, net of current portion | | | 2,809 | | | | 4,362 | | | | 4,173 | | | | | |
Convertible preferred stock warrant liability | | | 2,132 | | | | 2,740 | | | | 3,281 | | | $ | — | |
Deferred rent, net of current portion | | | 12,154 | | | | 8,828 | | | | 8,450 | | | | | |
Deferred revenue | | | — | | | | — | | | | 1,412 | | | | | |
Restructuring liability | | | — | | | | 4,486 | | | | 4,335 | | | | | |
Other liabilities | | | 797 | | | | 1,553 | | | | 1,822 | | | | | |
| | | | | | | | | | | | | | | | |
Total liabilities | | | 29,530 | | | | 50,747 | | | | 47,179 | | | | | |
| | | | | | | | | | | | | | | | |
Commitments and contingencies (Note 5) | | | | | | | | | | | | | | | | |
Convertible preferred stock—$0.0001 par value, 16,104,641 authorized as of December 31, 2008, 21,080,641 shares authorized as of December 31, 2009 and 30,963,903 shares authorized as of June 30, 2010 (unaudited); 13,681,658 and 18,365,222 shares issued and outstanding as of December 31, 2008 and 2009, respectively (aggregate liquidation value of $126,225 and $185,566 as of December 31, 2008 and 2009, respectively); 28,487,517 shares issued and outstanding as of June 30, 2010 (unaudited) (aggregate liquidation value of $370,255) no shares issued and outstanding, pro forma (unaudited) | | | 121,436 | | | | 179,651 | | | | 391,411 | | | | — | |
Redeemable noncontrolling interest | | | — | | | | 5,506 | | | | 12,248 | | | | — | |
| | | | |
Stockholders’ Equity (Deficit): | | | | | | | | | | | | | | | | |
Common stock— $0.0001 par value; 28,000,000 and 33,000,000 shares authorized as of December 31, 2008 and 2009, respectively and 70,000,000 authorized as of June 30, 2010; 5,015,576 and 5,114,205 shares issued and outstanding as of December 31, 2008 and 2009, respectively and 5,271,249 shares issued and outstanding as of June 30, 2010 (unaudited); 70,000,000 shares authorized, 35,133,225 shares issued and outstanding, pro forma (unaudited) | | | 1 | | | | 1 | | | | 1 | | | | 4 | |
Additional paid-in capital | | | 3,164 | | | | 5,366 | | | | 9,758 | | | | 416,695 | |
Accumulated other comprehensive income (loss) | | | (468 | ) | | | 1,336 | | | | 916 | | | | 916 | |
Accumulated deficit | | | (55,989 | ) | | | (120,448 | ) | | | (156,544 | ) | | | (156,544 | ) |
| | | | | | | | | | | | | | | | |
Total Amyris, Inc. stockholders’ equity (deficit) | | | (53,292 | ) | | | (113,745 | ) | | | (145,869 | ) | | | 261,071 | |
| | | | | | | | | | | | | | | | |
Noncontrolling interest | | | 1,149 | | | | — | | | | 19 | | | | 19 | |
| | | | | | | | | | | | | | | | |
Total equity (deficit) | | | (52,143 | ) | | | (113,745 | ) | | | (145,850 | ) | | $ | 261,090 | |
| | | | | | | | | | | | | | | | |
Total liabilities, convertible preferred stock, redeemable noncontrolling interest and equity (deficit) | | $ | 98,823 | | | $ | 122,159 | | | $ | 304,988 | | | | | |
| | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
Amyris, Inc.
Consolidated Statements of Operations
In Thousands, Except Share and Per Share Amounts
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | | | | | | | | | | (Unaudited) | |
Revenues | | | | | | | | | | | | | | | | | | | | |
Product sales | | $ | — | | | $ | 10,680 | | | $ | 61,689 | | | $ | 21,527 | | | $ | 19,982 | |
Collaborative research services | | | 6,046 | | | | 3,008 | | | | 2,919 | | | | 1,367 | | | | 1,401 | |
Government grants | | | 138 | | | | 204 | | | | — | | | | — | | | | 4,974 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenues | | | 6,184 | | | | 13,892 | | | | 64,608 | | | | 22,894 | | |
| 26,357
|
|
Cost and operating expenses | | | | | | | | | | | | | | | | | | | | |
Cost of product sales | | | — | | | | 10,364 | | | | 60,428 | | | | 20,875 | | | | 20,132 | |
Research and development | | | 8,662 | | | | 30,306 | | | | 38,263 | | | | 17,510 | | | | 23,591 | |
Sales, general and administrative | | | 10,522 | | | | 16,622 | | | | 23,558 | | | | 9,274 | | | | 18,902 | |
Restructuring and asset impairment charges | | | — | | | | — | | | | 5,768 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total cost and operating expenses | | | 19,184 | | | | 57,292 | | | | 128,017 | | |
| 47,659
|
| | | 62,625 | |
| | | | | | | | | | | | | | | | | | | | |
Loss from operations | | | (13,000 | ) | | | (43,400 | ) | | | (63,409 | ) | | | (24,765 | ) | | | (36,268 | ) |
Other income (expense): | | | | | | | | | | | | | | | | | | | | |
Interest income | | | 1,178 | | | | 1,378 | | | | 448 | | | | 329 | | | | 562 | |
Interest expense | | | (28 | ) | | | (377 | ) | | | (1,218 | ) | | | (563 | ) | | | (760 | ) |
Other income (expense), net | | | 76 | | | | (144 | ) | | | (621 | ) | | | 221 | | | | (60 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total other income (expense) | | | 1,226 | | | | 857 | | | | (1,391 | ) | | | (13 | ) | | | (258 | ) |
| | | | | | | | | | | | | | | | | | | | |
Loss before income taxes | | | (11,774 | ) | | | (42,543 | ) | | | (64,800 | ) | | | (24,778 | ) | | | (36,526 | ) |
Benefit from income taxes | | | — | | | | (207 | ) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net loss | | $ | (11,774 | ) | | $ | (42,336 | ) | | $ | (64,800 | ) | | $ | (24,778 | ) | | $ | (36,526 | ) |
Loss attributable to noncontrolling interest | | | — | | | | (472 | ) | | | (341 | ) | | | (221 | ) | | | (430 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net loss attributable to Amyris, Inc. stockholders | | $ | (11,774 | ) | | $ | (41,864 | ) | | $ | (64,459 | ) | | $ | (24,557 | ) | | $ | (36,096 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net loss per share of common stock attributable to Amyris, Inc. stockholders, basic and diluted | | $ | (3.28 | ) | | $ | (9.91 | ) | | $ | (13.56 | ) | | $ | (5.27 | ) | | $ | (7.17 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average shares of common stock outstanding used in computing net loss per share of common stock, basic and diluted | | | 3,592,932 | | | | 4,223,533 | | | | 4,753,085 | | | | 4,661,704 | | | | 5,034,163 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma net loss per share of common stock attributable to Amyris, Inc. stockholders, basic and diluted (unaudited) | | | | | | | | | | $ | (3.16 | ) | | | | | | $ | (1.36 | ) |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average shares of common stock outstanding used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) | | | | | | | | | | | 20,279,433 | | | | | | | | 26,583,633 | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
Amyris, Inc.
Consolidated Statements of Convertible Preferred Stock, Redeemable Noncontrolling Interest and Deficit
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Convertible
Preferred Stock | | | Redeemable
Noncontrolling
Interest | | | | | Common Stock | | Additional
Paid-in
Capital | | | Accumulated
Deficit | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Noncontrolling
Interest | | | Total
Deficit | |
| (In Thousands, Except Share and Per Share Amounts) | | Shares | | Amount | | | | | Shares | | | Amount | | | | | |
January 1, 2007 | | 2,992,176 | | $ | 6,397 | | | $ | — | | | | | 4,503,917 | | | $ | 1 | | $ | 50 | | | $ | (2,351 | ) | | $ | — | | | $ | — | | | $ | (2,300 | ) |
Issuance of Series A convertible preferred stock at $2.174 per share for cash and services, net of issuance costs of $21 | | 6,482,824 | | | 14,073 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of Series B convertible preferred stock at $24.88 per share for cash and services, net of issuance costs of $89 | | 1,517,093 | | | 37,656 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock upon exercise of stock options, net of restricted stocks | | — | | | — | | | | — | | | | | 361,723 | | | | — | | | 219 | | | | — | | | | — | | | | — | | | | 219 | |
Repurchase of common stock | | — | | | — | | | | — | | | | | (20,625 | ) | | | — | | | (2 | ) | | | — | | | | — | | | | — | | | | (2 | ) |
Stock-based compensation | | — | | | — | | | | — | | | | | — | | | | — | | | 546 | | | | — | | | | — | | | | — | | | | 546 | |
Components of other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized gain on investments | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | 10 | | | | — | | | | 10 | |
Net loss | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | (11,774 | ) | | | — | | | | — | | | | (11,774 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (11,764 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2007 | | 10,992,093 | | | 58,126 | | | | — | | | | | 4,845,015 | | | | 1 | | | 813 | | | | (14,125 | ) | | | 10 | | | | — | | | | (13,301 | ) |
Issuance of Series B convertible preferred stock at $24.88 per share for cash, net of issuance costs of $80 | | 150,724 | | | 3,670 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of Series B-1 convertible preferred stock at $25.26 per share for cash, net of issuance costs of $2,746 | | 2,538,841 | | | 61,385 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of warrants in connection with issuance of Series B-1 convertible preferred stock | | — | | | (1,745 | ) | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock upon exercise of stock options, net of restricted stocks | | — | | | — | | | | — | | | | | 172,059 | | | | — | | | 326 | | | | — | | | | — | | | | — | | | | 326 | |
Repurchase of common stock | | — | | | — | | | | — | | | | | (1,498 | ) | | | — | | | (2 | ) | | | — | | | | — | | | | — | | | | (2 | ) |
Stock-based compensation | | — | | | — | | | | — | | | | | — | | | | — | | | 2,027 | | | | — | | | | — | | | | — | | | | 2,027 | |
Sale of noncontrolling interest | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | 1,621 | | | | 1,621 | |
Components of other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized gain on investments | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | 77 | | | | — | | | | 77 | |
Foreign currency translation adjustment | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | (555 | ) | | | — | | | | (555 | ) |
Net loss | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | (41,864 | ) | | | — | | | | (472 | ) | | | (42,336 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (42,814 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2008 | | 13,681,658 | | | 121,436 | | | | — | | | | | 5,015,576 | | | | 1 | | | 3,164 | | | | (55,989 | ) | | | (468 | ) | | | 1,149 | | | | (52,143 | ) |
Issuance of Series B-1 convertible preferred stock at $25.26 per share for cash, net of issuance costs of $103 | | 76,880 | | | 1,840 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of Series C convertible preferred stock at $12.46 per share for cash, net of issuance costs of $956 | | 4,606,684 | | | 56,443 | | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of warrants in connection with issuance of Series B-1 convertible preferred stock | | — | | | (68 | ) | | | — | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock upon exercise of stock options, net of restricted stock | | — | | | — | | | | — | | | | | 127,515 | | | | — | | | 284 | | | | — | | | | — | | | | — | | | | 284 | |
Repurchase of common stock | | — | | | — | | | | — | | | | | (28,886 | ) | | | — | | | (9 | ) | | | — | | | | — | | | | — | | | | (9 | ) |
Stock-based compensation | | — | | | — | | | | — | | | | | — | | | | — | | | 3,299 | | | | — | | | | — | | | | — | | | | 3,299 | |
Proceeds from redeemable noncontrolling interest | | — | | | — | | | | 5,626 | | | | | — | | | | — | | | — | | | | — | | | | — | | | | — | | | | — | |
Purchase of noncontrolling interest | | — | | | — | | | | — | | | | | — | | | | — | | | (1,372 | ) | | | — | | | | — | | | | (928 | ) | | | (2,300 | ) |
Components of other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized loss on investments | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | (84 | ) | | | — | | | | (84 | ) |
Foreign currency translation adjustment | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | | — | | | | 1,888 | | | | — | | | | 1,888 | |
Net loss | | — | | | — | | | | (120 | ) | | | | — | | | | — | | | — | | | | (64,459 | ) | | | — | | | | (221 | ) | | | (64,680 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (62,876 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
December 31, 2009 | | 18,365,222 | | $ | 179,651 | | | $ | 5,506 | | | | | 5,114,205 | | | $ | 1 | | $ | 5,366 | | | $ | (120,448 | ) | | $ | 1,336 | | | $ | — | | | $ | (113,745 | ) |
Amyris, Inc.
Consolidated Statements of Convertible Preferred Stock, Redeemable Noncontrolling Interest and Deficit—(Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Convertible
Preferred Stock | | | Redeemable
Noncontrolling
Interest | | | | | Common Stock | | Additional
Paid-in
Capital | | Accumulated
Deficit | | | Accumulated
Other
Comprehensive
Income (Loss) | | | Noncontrolling
Interest | | | Total
Deficit | |
| (In Thousands, Except Share and Per Share Amounts) | | Shares | | Amount | | | | | Shares | | | Amount | | | | | |
December 31, 2009 | | 18,365,222 | | $ | 179,651 | | | $ | 5,506 | | | | | 5,114,205 | | | $ | 1 | | $ | 5,366 | | $ | (120,448 | ) | | $ | 1,336 | | | $ | — | | | $ | (113,745 | ) |
Issuance of Series C convertible preferred stock at $12.46 per shares for cash, net of issuance costs of $5 (unaudited) | | 295,981 | | | 3,683 | | | | — | | | | | — | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Issuance of Series C-1 convertible preferred stock at $17.56 per shares for cash, net of issuance costs of $68 (unaudited) | | 2,724,766 | | | 47,779 | | | | — | | | | | — | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Issuance of Series D convertible preferred stock at $18.75 per shares for cash and deferred charge asset of $27,909, net of issuance costs of $258 (unaudited) | | 7,101,548 | | | 160,805 | | | | — | | | | | — | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Issuance of warrants in connection with issuance of Series C convertible preferred stock (unaudited) | | — | | | (507 | ) | | | — | | | | | — | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Issuance of common stock upon exercise of stock options, net of restricted stock (unaudited) | | — | | | — | | | | — | | | | | 22,707 | | | | — | | | 90 | | | — | | | | — | | | | — | | | | 90 | |
Repurchase of common stock (unaudited) | | — | | | — | | | | — | | | | | (10,367 | ) | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Shares issued from restricted stock unit settlement (unaudited) | | — | | | — | | | | — | | | | | 144,704 | | | | — | | | — | | | — | | | | — | | | | — | | | | — | |
Stock-based compensation (unaudited) | | — | | | — | | | | | | | | | — | | | | — | | | 4,302 | | | — | | | | | | | | | | | | 4,302 | |
Proceeds from noncontrolling interest (unaudited) | | — | | | — | | | | 7,041 | | | | | — | | | | — | | | — | | | — | | | | — | | | | 28 | | | | 28 | |
Components of other comprehensive income (loss) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized loss on investments (unaudited) | | — | | | — | | | | — | | | | | — | | | | — | | | — | | | — | | | | (3 | ) | | | — | | | | (3 | ) |
Foreign currency translation adjustment (unaudited) | | — | | | — | | | | 121 | | | | | — | | | | — | | | — | | | — | | | | (417 | ) | | | — | | | | (417 | ) |
Net loss (unaudited) | | — | | | — | | | | (420 | ) | | | | — | | | | — | | | — | | | (36,096 | ) | | | — | | | | (9 | ) | | | (36,105 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive loss (unaudited) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (36,525 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
June 30, 2010 | | 28,487,517 | | $ | 391,411 | | | $ | 12,248 | | | | | 5,271,249 | | | $ | 1 | | $ | 9,758 | | $ | (156,544 | ) | | $ | 916 | | | $ | 19 | | | $ | (145,850 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
Amyris, Inc.
Consolidated Statements of Cash Flows
In Thousands
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | | | | | | | | | | (Unaudited) | |
Operating activities | | | | | | | | | | | | | | | | | | | | |
Net loss. | | $ | (11,774 | ) | | $ | (42,336 | ) | | $ | (64,800 | ) | | $ | (24,778 | ) | | $ | (36,526 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | | | | | |
Convertible preferred stock issued for services | | | 255 | | | | — | | | | — | | | | — | | | | — | |
Convertible preferred stock warrants | | | — | | | | 274 | | | | 62 | | | | — | | | | 33 | |
Depreciation and amortization | | | 634 | | | | 2,627 | | | | 5,775 | | | | 2,847 | | | | 3,421 | |
Loss on disposal of property and equipment | | | — | | | | 144 | | | | 12 | | | | — | | | | — | |
Loss on the sale of investments | | | — | | | | — | | | | — | | | | — | | | | (1 | ) |
Stock-based compensation | | | 546 | | | | 2,027 | | | | 3,299 | | | | 1,177 | | | | 4,302 | |
Amortization of premium (discount) on investments | | | 402 | | | | (400 | ) | | | 191 | | | | (30 | ) | | | 378 | |
Change in fair value of convertible preferred stock warrant liability | | | — | | | | 114 | | | | 445 | | | | (333 | ) | | | 34 | |
Restructuring and asset impairment charges | | | — | | | | — | | | | 356 | | | | — | | | | — | |
Other noncash expenses | | | — | | | | — | | | | 219 | | | | 157 | | | | 40 | |
Changes in assets and liabilities: | | | | | | | | | | | | | | | | | | | | |
Accounts receivable, net | | | — | | | | (787 | ) | | | (585 | ) | | | (5,042 | ) | | | (1,710 | ) |
Inventories | | | — | | | | (1,420 | ) | | | (878 | ) | | | (3,330 | ) | | | (430 | ) |
Prepaid expenses and other assets | | | (103 | ) | | | (1,943 | ) | | | 972 | | | | (536 | ) | | | (1,266 | ) |
Accounts payable | | | 114 | | | | 1,278 | | | | (997 | ) | | | (34 | ) | | | 735 | |
Restructuring | | | — | | | | — | | | | 5,078 | | | | — | | | | (359 | ) |
Accrued and other long-term liabilities | | | 2,303 | | | | 2,114 | | | | 4,470 | | | | 496 | | | | 1,440 | |
Deferred revenue | | | (1,903 | ) | | | (1,355 | ) | | | 378 | | | | 1,430 | | | | 1,599 | |
Deferred rent | | | — | | | | 784 | | | | 285 | | | | (407 | ) | | | (275 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash used in operating activities | | $ | (9,526 | ) | | $ | (38,879 | ) | | $ | (45,718 | ) | | $ | (28,383 | ) | | $ | (28,585 | ) |
| | | | | | | | | | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | | | | | | | | | |
Purchase of short-term investments | | $ | (120,901 | ) | | $ | (48,153 | ) | | $ | (47,996 | ) | | $ | (8,284 | ) | | $ | (81,429 | ) |
Maturities of short-term investments | | | 88,258 | | | | 52,746 | | | | 31,690 | | | | 20,500 | | | | 18,061 | |
Sales of short-term investments | |
| 2,950
|
| | | 8,905 | | | | 250 | | | | — | | | | 15,708 | |
Purchases of long-term investments | | | (8,750 | ) | | | (6,200 | ) | | | — | | | | — | | | | (7,995 | ) |
Sales and maturities of long-term investments | | | — | | | | 2,000 | | | | — | | | | — | | | | — | |
Change in restricted cash | | | (736 | ) | | | (1,962 | ) | | | (1,758 | ) | | | (260 | ) | | | (14 | ) |
Purchase of property and equipment, net of disposals | | | (2,464 | ) | | | (21,996 | ) | | | (7,608 | ) | | | (4,256 | ) | | | (4,608 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | $ | (41,643 | ) | | $ | (14,660 | ) | | $ | (25,422 | ) | | $ | 7,700 | | | $ | (60,277 | ) |
| | | | | | | | | | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | | | | | | | | | |
Proceeds from issuance of convertible preferred stock, net of issuance costs | | $ | 51,474 | | | $ | 65,055 | | | $ | 58,283 | | | $ | 1,845 | | | $ | 184,616 | |
Proceeds from issuance of common stock, net of repurchases | | | 217 | | | | 586 | | | | 113 | | | | 2 | | | | 70 | |
Purchase of noncontrolling interest | | | — | | | | — | | | | (2,300 | ) | | | (2,300 | ) | | | — | |
Proceeds from equipment financing | | | — | | | | 1,220 | | | | 4,763 | | | | 4,280 | | | | 1,446 | |
Principal payments on capital leases | | | (152 | ) | | | (378 | ) | | | (1,134 | ) | | | (447 | ) | | | (1,258 | ) |
Proceeds from debt | | | — | | | | — | | | | 9,643 | | | | 8,643 | | | | — | |
Principal payments on debt | | | — | | | | (125 | ) | | | (985 | ) | | | (276 | ) | | | (8,498 | ) |
Deferred offering costs | | | — | | | | — | | | | — | | | | — | | | | (1,446 | ) |
Proceeds from sale of noncontrolling interest | | | — | | | | 1,621 | | | | 3,090 | | | | — | | | | 7,069 | |
| | | | | | | | | | | | | | | | | | | | |
Net cash provided by financing activities | | $ | 51,539 | | | $ | 67,979 | | | $ | 71,473 | | | $ | 11,747 | | | $ | 181,999 | |
| | | | | | | | | | | | | | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | $ | — | | | $ | (555 | ) | | $ | 956 | | | $ | 214 | | | $ | (83 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | 370 | | | | 13,885 | | | | 1,289 | | | | (8,722 | ) | | | 93,054 | |
Cash and cash equivalents at beginning of period | | | 3,644 | | | | 4,014 | | | | 17,899 | | | | 17,899 | | | | 19,188 | |
| | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 4,014 | | | $ | 17,899 | | | $ | 19,188 | | | $ | 9,177 | | | $ | 112,242 | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
Amyris, Inc.
Consolidated Statements of Cash Flows—(Continued)
In Thousands
| | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | Six Months Ended June 30, | |
| | | 2007 | | 2008 | | 2009 | | | 2009 | | | 2010 | |
| | | | | | | | | | (Unaudited) | |
Supplemental disclosures of cash flow information: | | | | | | | | | | | | | | | | | | |
Cash paid for interest | | $ | 18 | | $ | 320 | | $ | 1,204 | | | $ | 557 | | | $ | 760 | |
| | | | | | | | | | | | | | | | | | |
Cash paid for income taxes, net of refunds | | $ | — | | $ | — | | $ | 27 | | | $ | 30 | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | | | | | | | | | | | |
Convertible preferred stock issued for services | | $ | 255 | | $ | — | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Stock receivable for noncontrolling interest | | $ | — | | $ | — | | $ | 2,536 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Additions to property and equipment under capital lease obligations | | $ | 807 | | $ | 2,101 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Additions to property and equipment under notes payable | | $ | — | | $ | 3,274 | | $ | 1,038 | | | $ | 677 | | | $ | 211 | |
| | | | | | | | | | | | | | | | | | |
Additions to property and equipment under tenant improvement allowances | | $ | — | | $ | 11,370 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Acquisitions of assets under accounts payable | | $ | 411 | | $ | 731 | | $ | 20 | | | $ | 29 | | | $ | 529 | |
| | | | | | | | | | | | | | | | | | |
Financing of insurance premium under notes payable | | $ | — | | $ | — | | $ | 378 | | | $ | 378 | | | $ | 101 | |
| | | | | | | | | | | | | | | | | | |
Change in unrealized gain (loss) on investments | | $ | 10 | | $ | 77 | | $ | (84 | ) | | $ | (85 | ) | | $ | (3 | ) |
| | | | | | | | | | | | | | | | | | |
Asset retirement obligation | | $ | — | | $ | 470 | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | |
Warrants issued in connection with the issuance of convertible preferred stock | | $ | — | | $ | 1,745 | | $ | 68 | | | $ | 68 | | | $ | 507 | |
| | | | | | | | | | | | | | | | | | |
Accrued deferred offering costs | | $ | — | | $ | — | | $ | — | | | $ | — | | | $ | 1,546 | |
| | | | | | | | | | | | | | | | | | |
Accrued Series D preferred stock issuance costs | | $ | — | | $ | — | | $ | — | | | $ | — | | | $ | 258 | |
| | | | | | | | | | | | | | | | | | |
Financing of rent payments under notes payable | | $ | — | | $ | — | | $ | — | | | $ | — | | | $ | 239 | |
| | | | | | | | | | | | | | | | | | |
Deferred charge asset related to issuance of Series D preferred stock | | $ | — | | $ | — | | $ | — | | | $ | — | | | $ | 27,909 | |
| | | | | | | | | | | | | | | | | | |
See accompanying notes to the consolidated financial statements.
Amyris, Inc.
Notes to Consolidated Financial Statements
1. The Company and Basis of Presentation
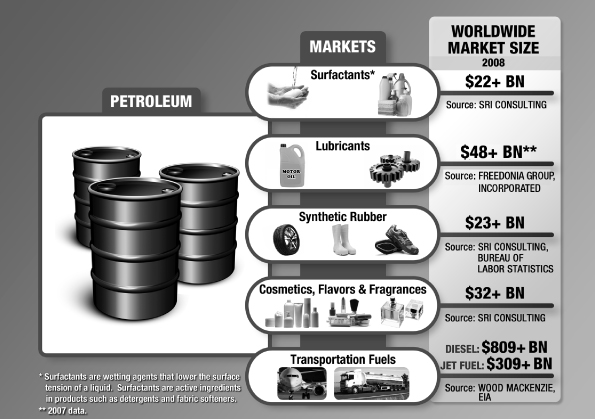
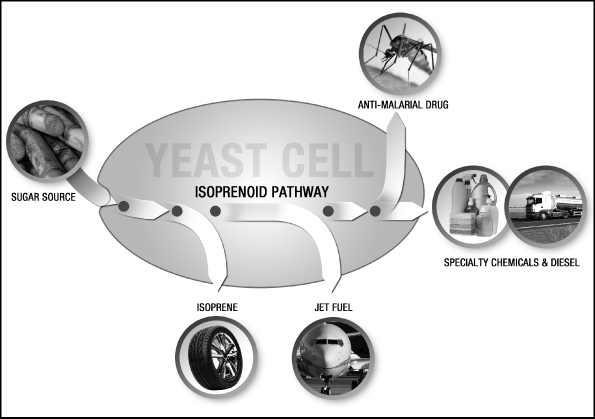
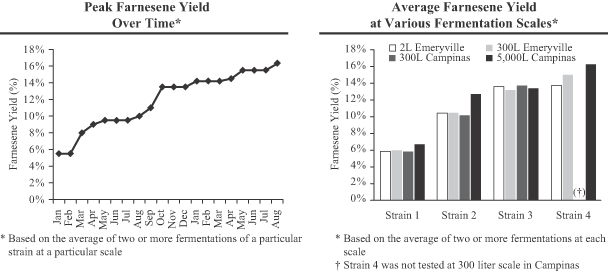
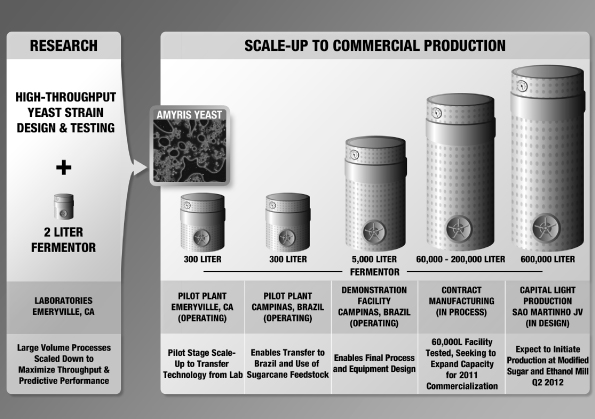
Amyris, Inc. (the “Company”) was incorporated in the State of California on July 17, 2003 for the purpose of leveraging breakthroughs in synthetic biology to develop and provide renewable compounds for a variety of markets. The Company is currently building and applying its industrial synthetic biology platform to provide alternatives to select petroleum-sourced products used in specialty chemical and transportation fuel markets worldwide. The Company’s first commercialization efforts have been focused on a molecule called farnesene, which forms the basis for a wide range of products varying from specialty chemical applications to transportation fuels, such as diesel. While the Company’s platform is able to use a wide variety of feedstocks, the Company has focused initially on Brazilian sugarcane. The Company intends to secure access to this feedstock and to expand its production capacity by working with existing sugar and ethanol mill owners to build new, adjacent bolt-on facilities at their existing mills in return for a share of the higher gross margin the Company believes it will realize from the sale of our renewable products. The Company’s first such arrangement is its joint venture with Usina São Martinho (see Note 19).
In February 2008, the Company incorporated a wholly owned subsidiary, Amyris Fuels, Inc., a Delaware corporation, which is engaged in marketing and distribution of certain fuels. In September 2008, the Company established a wholly owned subsidiary, Amyris Fuels, LLC, a Delaware limited liability company into which it merged Amyris Fuels, Inc. In March 2008, the Company formed a subsidiary, Amyris Pesquisa e Desenvolvimento de Biocombustíveis Ltda., for the purpose of manufacturing and trading transportation fuels from sugarcane feedstock in Brazil and abroad (see Note 16). In March 2008, the Company sold a 30% interest to Crystalsev and the subsidiary was renamed Amyris-Crystalsev Pesquisa e Desenvolvimento de Biocombustiveis Ltda. (“ACB”). The Company invested $3.8 million of cash for a 70% interest in ACB, and Crystalsev contributed $1.6 million of cash for the remaining 30% interest.
In April 2009, the Company re-purchased Crystalsev’s 30% interest in ACB for $2.3 million resulting in Amyris Brasil becoming a wholly-owned subsidiary again. The purchase of the noncontrolling interest was treated as an equity transaction and the fair value of the consideration paid of $2.3 million was recorded as a reduction of the carrying value of the noncontrolling interest and additional paid-in capital. In December 2009, ACB was renamed Amyris Brasil S.A.
On December 22, 2009, the Company sold a 4.8% interest in Amyris Brasil for BRL$10.0 million. The redeemable noncontrolling interest is reported in the mezzanine equity section of the consolidated balance sheet because the Company is subject to a contingent put option under which it may be required to repurchase an interest in Amyris Brasil from the noncontrolling interest holder.
In March 2010, the Company sold an incremental 3.4% interest in Amyris Brasil for BRL $3.0 million (unaudited). In May 2010, Amyris Brasil sold an additional 1,111,111 shares of its stock, an incremental 4.07% interest, for BRL $10.0 million (unaudited).
In 2010, the Company was awarded a $24.3 million “Integrated Bio-Refinery” grant from the U.S. Department of Energy (“DOE”). Under this grant, the Company is required to fund an additional $10.6 million in cost sharing expenses. According to the terms of the DOE grant, the Company is required to maintain a cash balance of $8.7 million, calculated as a percentage of the total project costs, to cover potential contingencies and cost overruns. These funds are not legally restricted but they must be available and unrestricted during the term of the project. The Company’s obligation for this cost share is contingent on reimbursement for project costs incurred. As of December 31, 2009, no amounts had been provided from the DOE. During the six months ended June 30, 2010 the Company recognized $5.0 million (unaudited) in revenue under this grant.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
2. Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and includeoffering, including all adjustments necessary for the fair presentation of the Company’s consolidated financial position, results of operations and cash flows for the periods presented. The consolidated financial statements include the accounts of the Company, wholly- owned subsidiaries and one majority-owned subsidiary. All intercompany accounts and transactions have been eliminated in consolidation. In preparing the consolidated financial statements, management must make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as offilings made after the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Principals of Consolidations
The consolidated financial statements of the Company include the accounts of Amyris, Inc., its subsidiaries and variable interest entity (“VIE”) where the Company is considered the primary beneficiary, after elimination of intercompany accounts and transactions. Disclosure regarding the Company’s participation in VIE is included in Note 16.
Unaudited Interim Financial Information
The consolidated financial statements as of June 30, 2010, and for the six months ended June 30, 2010 and June 30, 2009 are unaudited. All disclosures as of June 30, 2010, and for the six month periods ended June 30, 2009 and June 30, 2010, presented in the notes to the financial statements are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all normal recurring adjustments necessary for a fair statement of the Company’s financial position as of June 30, 2010 and results of operations and cash flows for the six months ended June 30, 2009 and June 30, 2010. The results of operations for the six months ended June 30, 2010 are not necessarily indicative of the results to be expected for the year ended December 31, 2010 or for other interim periods or for future years.
Unaudited Pro Forma Information
The June 30, 2010 unaudited pro forma stockholders’ equity has been prepared assuming that upon the completion of a qualifying initial public offering (i) all of the Company’s convertible preferred stock outstanding will automatically convert into shares of common stock, (ii) the Company’s convertible preferred stock warrants will become warrants for common stock (see Note 10), and (iii) shares of Amyris Brasil held by third parties will convert into shares of the Company’s common stock (see Note 16). The June 30, 2010 unaudited pro forma stockholders’ equity reflects the (i) the conversion of all 28,487,517 outstanding shares of preferred stock into 29,000,821 shares of common stock, the reclassification of the preferred stock warrant liability to additional paid-in capital and reclassification of redeemable noncontrolling interest to common stock and additional paid-in capital immediately prior to the completion of the initial public offering.
Significant Risks and Uncertainties
The Company is subject to certain risks and uncertainties that could have a material and adverse effect on the Company’s future financial position or results of operations. Factors that could affect the Company’s future operating results and cause actual results to vary materially from expectations include, but are not limited to, limited operating history, delays or greater than anticipated expenses associated with the completion of new production facilities and the time to complete scale up of production following completion of a new production
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
facility, disruptions in the production process at any facility where we produce our products, the timing, size and mix of sales to customers for our products, fluctuations in foreign currency exchange rates, gains or losses associated with our hedging activities, especially in Amyris Fuels, fluctuations in the price of and demand for ethanol, as well as petroleum-based and other products for which our products are alternatives, seasonal production and sale of our products, the effects of competitive pricing pressures, including decreases in average selling prices of our products, unanticipated expenses associated with changes in governmental regulations and environmental, health and safety requirements, reductions or changes to existing fuel and chemical regulations and policies, departure of executives or other key management employees, our ability to use our net operating loss carry forwards to offset future taxable income, our ability to integrate businesses that we may acquire and risks associated with the international aspects of our business.
Certain products developed by the Company may require approvals from the Environmental Protection Agency or other United States or international regulatory agencies prior to commercial sales. There can be no assurance the Company’s future products will receive the necessary approvals. If the Company was denied approval or approval was delayed, it may have a material adverse impact on the Company.
To achieve profitable operations, the Company must successfully develop, manufacture and market its products. There can be no assurance that any such products can be developed or manufactured at an acceptable cost and with appropriate performance characteristics, or that such products will be successfully marketed. These factors could have a material adverse effect upon the Company’s future financial results.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents, short-term and long-term investments, accounts receivable, derivatives and financial instruments. The Company places its cash equivalents and investments with high credit quality financial institutions and, by policy, limits the amount of credit exposure with any one financial institution. Deposits held with banks may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
The Company’s accounts receivable are derived from customers located in the United States. The Company performs ongoing credit evaluation of its customers, does not require collateral, and maintains allowances for potential credit losses on customer accounts when deemed necessary. To date, there have been no such losses and the Company has not recorded an allowance for doubtful accounts.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Customers representing greater than 10% of accounts receivable were as follows (in percentages):
| | | | | | |
| | | December 31, | | June 30, |
Customers AR | | 2008 | | 2009 | | 2010 |
| | | | | | | (Unaudited) |
Customer A | | 10 | | * | | 15 |
Customer B | | 15 | | * | | * |
Customer C | | * | | 51 | | * |
Customer D | | 12 | | * | | * |
Customer E | | * | | 17 | | * |
Customer F | | 14 | | * | | * |
Customer G | | 20 | | * | | * |
Customer H | | 26 | | * | | * |
Customer I | | * | | * | | 15 |
Customer J | | * | | * | | 23 |
Customer K | | * | | * | | 11 |
Customers representing greater than 10% of revenues were as follows (in percentages):
| | | | | | |
| | | Years Ended
December 31, | | Six Months Ended
June 30, |
Customers | | 2008 | | 2009 | | 2010 |
| | | | | | | (Unaudited) |
Customer A | | 33 | | 33 | | * |
Customer B | | 30 | | * | | * |
Customer C | | * | | 22 | | 40 |
Customer D | | 21 | | * | | * |
Customer E | | * | | * | | 12 |
The Company is exposed to counterparty credit risk on all of its derivative commodity instruments. The Company has established and maintains strict counterparty credit guidelines and enters into agreements only with counterparties that are investment grade or better. The Company does not require collateral under these agreements.
Fair Value of Financial Instruments
The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. Financial instruments are primarily comprised of commercial paper, government bonds and notes, auction rate securities (“ARS”), rights to sell its ARS (“Put Option”), derivatives and convertible preferred stock warrants. Where available, fair value is based on or derived from observable market prices or other observable inputs. Where observable prices or inputs are not available valuation models are applied. These valuation techniques involve some level of management estimation and judgment, the degree of which is dependent on the price transparency for the instruments or market and the instruments’ complexity.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The carrying amounts of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, prepaid expenses, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities, and market interest rates if applicable. Based on the borrowing rates currently available to the Company for debt with similar terms, the carrying value of the notes payable and credit facility approximates its fair value. The carrying amount of the convertible preferred stock warrant liability represents its estimated fair value.
Cash and Cash Equivalents
All highly liquid investments purchased with an original maturity date of three months or less at the date of purchase are considered to be cash equivalents. Cash and cash equivalents consist of money market funds, commercial paper, U.S. Government agency securities and various deposit accounts.
Investments
Investments with original maturities greater than 90 days that mature less than one year from the consolidated balance sheet date are classified as short-term investments. The Company classifies investments as short-term or long-term based upon whether such assets are reasonably expected to be realized in cash or sold or consumed during the normal cycle of business. The Company invests its excess cash balances primarily in short- term investment grade commercial paper, U.S. Government agency securities and notes and ARS. The Company classifies all of its investments, other than ARS, as available-for-sale and records such assets at estimated fair value in the consolidated balance sheets, with unrealized gains and losses, if any, reported as a component of accumulated other comprehensive income (loss) in stockholders’ deficit. Debt securities are adjusted for amortization of premiums and accretion of discounts and such amortization and accretion are reported as a component of interest income. Realized gains and losses and declines in value that are considered to be other than temporary are recognized in the statements of operations. The cost of securities sold is determined on the specific identification method. There were no significant realized gains or losses from sales of debt securities during the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010 (unaudited). As of December 31, 2008 and 2009 and June 30, 2010, the Company did not have any other-than-temporary declines in the fair value of its debt securities.
The Company classifies the ARS as trading securities and records all changes in fair value as component of other income (expense), net. The underlying securities have stated or contractual maturities that are generally greater than one year. The Company estimates the fair value of the ARS using a discounted cash flow model incorporating assumptions that market participants would use in their estimates of fair value. The Company has a Put Option to sell its ARS at par value. The Company has accounted for the Put Option as a freestanding financial instrument and elected to record it at fair value with changes in fair value recorded as a component of other income (expense), net (see Note 3).
Restricted Cash
Cash accounts that are restricted as to withdrawal or usage are presented as restricted cash. As of December 31, 2008 and 2009 and June 30, 2010, the Company had $2.7 million, $4.5 million and $4.5 million (unaudited), respectively, of restricted cash held by a bank in certificate of deposits as collateral for certain of its facility and capital lease agreements.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Inventories
Inventories, which consist of ethanol, are stated at the lower of cost or market. Cost is computed on a first-in, first-out basis. Inventory costs include costs such as transportation and storage costs incurred in bringing the inventory to its existing location.
Derivative Instruments
The Company is exposed to market risks related to price volatility of ethanol. The Company makes limited use of derivative instruments, which include futures positions on the New York Mercantile Exchange and the CME/Chicago Board of Trade. The Company does not engage in speculative derivative activities, and the majority of the Company’s activity in derivative commodity instruments is intended to manage the financial risk posed by physical transactions and inventory. Changes in the fair value of the derivative contracts are recognized currently in the consolidated statements of operations as specific hedge accounting criteria are not met.
Asset Retirement Obligations
The fair value of the asset retirement obligation is recognized in the period in which it is incurred if a reasonable estimate of fair value can be made. In addition, the associated asset retirement cost is added to the carrying amount of the associated asset and this additional carrying amount is amortized over the life of the asset. The Company’s asset retirement obligations are associated with its commitment to return property subject to the operating lease in Brazil to its original condition upon lease termination.
As of December 31, 2008 and 2009 and June 30, 2010 the Company has recorded asset retirement obligations of $499,000, $746,000 and $762,000 (unaudited), respectively. The related leasehold improvements are being amortized to depreciation expense over the term of the lease or the useful life of the assets, whichever is shorter. Related amortization expense was $0, $106,000 and $175,000 for the years ended December 31, 2007, 2008 and 2009, respectively, and $102,000 (unaudited) for the six months ended June 30, 2010.
Property and equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation and amortization is computed using the straight-line method over the estimated useful lives of the related assets. Maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the balance sheet and any resulting gain or loss is reflected in operations in the period realized.
Leasehold improvements are amortized on a straight-line basis over the terms of the lease, or the useful life of the assets, whichever is shorter. Depreciation and amortization periods for the Company’s property and equipment are as follows:
| | |
Furniture and office equipment
| | 5 years |
Computers and software
| | 3-5 years |
Research and laboratory equipment
| | 7 years |
Computers and software includes internal-use software that is acquired, internally developed or modified to meet the Company’s internal needs. Amortization commences when the software is ready for its intended use and the amortization period is the estimated useful life of the software, generally three to five years. Capitalized costs primarily include contract labor and payroll costs of the individuals dedicated to the development of internal-use
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
software. Capitalized software totaled approximately $0, $0.6 million, and $1.1 million as of December 31, 2007, 2008 and 2009, respectively, and $1.4 million (unaudited) as of June 30, 2010 related to software development costs pertaining to the installation of a new financial reporting system.
Impairment of Long-Lived Assets
The Company periodically reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset are impaired or the estimated useful lives are no longer appropriate. If indicators of impairment exist and the undiscounted projected cash flows associated with such assets are less than the carrying amount of the asset, an impairment loss is recorded to write the asset down to their estimated fair values. Fair value is estimated based on discounted future cash flows. Impairment charges of $0, $0 and $3,075,000 were recorded during the years ended December 31, 2007, 2008 and 2009, respectively, and zero (unaudited) for six months ended June 30, 2010.
Convertible Preferred Stock Warrant Liability
The Company accounts for its freestanding warrants for shares of the Company’s convertible preferred stock that are contingently redeemable as liabilities at fair value on the consolidated balance sheets. The warrants are subject to re-measurement at each balance sheet date and the change in fair value, if any, is recognized as other income (expense), net. The Company will continue to adjust the liability for changes in fair value until the earlier of (i) exercise of the warrants, (ii) conversion into warrants to purchase common stock, or (iii) expiration of the warrants. Upon conversion, the convertible preferred stock warrant liability will be reclassified to additional paid-in capital.
Convertible Preferred Stock
The holders of the Company’s outstanding convertible preferred stock, voting or consenting together as a separate class, control the vote of the Company’s stockholders. As a result, the holders of all series of the Company’s convertible preferred stock can force a change in control that would trigger liquidation. As redemption of the convertible preferred stock through liquidation is outside the control of the Company, all shares of convertible preferred stock have been presented outside of stockholders’ deficit in the Company’s consolidated balance sheets. All series of convertible preferred stock are collectively referred to in the consolidated financial statements as convertible preferred stock.
Noncontrolling Interest and Redeemable Noncontrolling Interest
As of January 1, 2009, the Company adopted the new accounting standard which establishes accounting and reporting standards for noncontrolling interests in consolidated financial statements. These provisions require that the carrying value of noncontrolling interests to be removed from the mezzanine equity section of the consolidated balance sheet and reclassified as equity, and that consolidated net income be recast to include net income attributable to the noncontrolling interests. The standard requires retrospective presentation and disclosure of existing noncontrolling interests. Accordingly, the Company presented noncontrolling interests as a separate component of equity (deficit) and has also presented net loss attributable to the noncontrolling interest in the consolidated statement of operations. Upon adoption, the noncontrolling interest of $1.1 million was reclassified to a component of total equity (deficit) in the consolidated balance sheet from the mezzanine equity section.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
In accordance with accounting and reporting standards for redeemable equity instruments, a noncontrolling interest with redemption features (“redeemable noncontrolling interest”), such as a put option, that is not solely within the control of the Company, is required to be reported in the mezzanine equity section of the consolidated balance sheet.
Changes in noncontrolling interest ownership that do not result in a change of control and where there is a difference between fair value and carrying value are accounted for as equity transactions.
On April 14, 2010, the Company entered into a joint venture with Usina São Martinho. The carrying value of noncontrolling interest from this joint venture is recorded in the equity section of the consolidated balance sheet (see Note 8).
Revenue Recognition
The Company recognizes revenue from the sale of ethanol and delivery of research and development services and governmental grants. Ethanol sales consists of sales to customers through purchase from third-party suppliers in which the Company takes physical control of the ethanol and accepts risk of loss. Revenue is recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the fee is fixed or determinable and collectability is reasonably assured.
If sales arrangements contain multiple elements, the Company evaluates whether the components of each arrangement represent separate units of accounting. To date the Company has determined that all revenue arrangements should be accounted for as a single unit of accounting.
Product Sales
The Company sells ethanol under short-term agreements at prevailing market prices. Revenues are recognized, net of discounts and allowances, once passage of title and risk of loss has occurred and contractually specified acceptance criteria have been met, provided all other revenue recognition criteria have also been met.
Collaborative Research Services
Revenue from collaborative research services is recognized as the services are performed consistent with the performance requirements of the contract. In cases where the planned levels of research services fluctuate over the research term, the Company recognizes revenue using the proportionate performance method based upon actual efforts to date relative to the amount of expected effort to be incurred by the Company. When up-front payments are received and the planned levels of research services do not fluctuate over the research term, revenue is recorded on a ratable basis over the arrangement term, up to the amount of cash received. When up-front payments are received and the planned levels of research services fluctuate over the research term, revenue is recorded using the proportionate performance method, up to the amount of cash received. Where arrangements include milestones that are determined to be substantive and at risk at the inception of the arrangement, revenue is recognized upon achievement of the milestone and is limited to those amounts whereby collectability is reasonably assured.
Government grants
Government grants are agreements that generally provide cost reimbursement for certain types of expenditures in return for research and development activities over a contractually defined period. Revenues
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
from government grants are recognized in the period during which the related costs are incurred, provided that the conditions under which the government grants were provided have been met and only perfunctory obligations are outstanding.
Cost of Product Sales
Cost of product sales consists primarily of cost of purchased ethanol, terminal fees paid for storage and handling, transportation costs between terminals and changes in the fair value of the derivative commodity instruments.
Shipping and handling costs charged to customers are recorded as revenues. Shipping costs are included in cost of product revenues. Such charges were not significant in any of the periods presented.
Costs of Start-Up Activities
Start-up activities are defined as those one-time activities related to opening a new facility, introducing a new product or service, conducting business in a new territory, conducting business with a new class of customer or beneficiary, initiating a new process in an existing facility, commencing some new operation or activities related to organizing a new entity All the costs associated with a potential site are expensed, until the site is considered viable by management, at which time costs would be considered for capitalization based on authoritative accounting literature.
Research and Development
Research and development costs are expensed as incurred and include costs associated with research performed pursuant to collaborative agreements. Research and development costs consist of direct and indirect internal costs related to specific projects as well as fees paid to other entities that conduct certain research activities on the Company’s behalf.
Income taxes
The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company’s assets and liabilities and their financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. A valuation allowance is provided against deferred tax assets unless it is more likely than not that they will be realized.
Effective January 1, 2007, the Company adopted the accounting guidance for uncertainties in income taxes, which prescribes a recognition threshold and measurement process for recording uncertain tax positions taken, or expected to be taken in a tax return, in the consolidated financial statements. Additionally, the guidance also prescribes new treatment for the derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has a less than 50% likelihood of being sustained.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Currency Translation
The functional currency of the Company’s majority-owned subsidiary in Brazil is the Brazilian real. Accordingly, asset and liability accounts of those operations are translated into United States dollars using the current exchange rate in effect at the balance sheet date and equity accounts are translated into United States dollars using historical rates. The revenues and expenses are translated using the average exchange rates in effect during the period, and gains and losses from foreign currency translation adjustments are included as a component of accumulated other comprehensive income (loss) in the consolidated balance sheets.
Comprehensive Income (Loss)
Comprehensive income (loss) represents all changes in stockholders’ deficit except those resulting from investments or contributions by stockholders. The Company’s unrealized gains and losses on available-for-sale securities and foreign currency translation adjustments represent the components of comprehensive income (loss) excluded from the Company’s net loss and have been disclosed in the consolidated statements of convertible preferred stock, redeemable noncontrolling interest and deficit for all periods presented.
The components of accumulated other comprehensive income (loss) are as follows (in thousands):
| | | | | | | | | | | | | | |
| | | December 31, | | June 30, |
| | | 2008 | | | 2009 | | 2009 | | | 2010 |
| | | | | | | | (Unaudited) |
Foreign currency translation adjustment | | $ | (555 | ) | | $ | 1,333 | | $ | (337 | ) | | $ | 916 |
Accumulated unrealized gain (loss) on investment | | | 87 | | | | 3 | | | (2 | ) | | | — |
| | | | | | | | | | | | | | |
Total accumulated other comprehensive income (loss) | | $ | (468 | ) | | $ | 1,336 | | $ | (339 | ) | | $ | 916 |
| | | | | | | | | | | | | | |
Stock-Based compensation
The Company accounts for stock-based compensation arrangements with employees using a fair value method which requires the recognition of compensation expense for costs related to all stock-based payments including stock options. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant using an option pricing model. The Company uses the Black-Scholes pricing model to estimate the fair value of options granted that are expensed on a straight-line basis over the vesting period.
The Company accounts for stock options issued to nonemployees based on the estimated fair value of the awards using the Black-Scholes option pricing model. The Company accounts for restricted stock units, issued to nonemployees based on the estimated fair value of the Company’s common stock. The measurement of stock-based compensation is subject to periodic adjustments as the underlying equity instruments vest, and the resulting change in value, if any, is recognized in the Company’s consolidated statements of operations during the period the related services are rendered.
Net Loss per Share and Unaudited Pro Forma Net Loss per Share of Common Stock
Basic net loss per share of common stock is computed by dividing the Company’s net loss attributable to Amyris, Inc. stockholders by the weighted-average number of shares of common stock outstanding during the period. Diluted net loss per share of common stock is computed by giving effect to all potentially dilutive securities, including stock options, restricted stock units, warrants and convertible preferred stock. Basic and diluted net loss per share of common stock attributable to Amyris, Inc. stockholders was the same for all periods
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
presented as the inclusion of all potentially dilutive securities outstanding was anti-dilutive. As such, the numerator and the denominator used in computing both basic and diluted net loss are the same for each period presented.
The calculations for the unaudited pro forma basic and diluted net loss per share of common stock attributable to Amyris, Inc. stockholders assume the conversion of all outstanding shares of convertible preferred stock into shares of common stock, as if the conversions had occurred at the beginning of the period or the issuance date for Series B-1, Series C, Series C-1 and Series D convertible preferred stock issued during the year ended December 31, 2009 and the six months ended June 30, 2010 (unaudited), and the conversion of Amyris Brasil shares held by third parties. Also, the numerator in the pro forma basic and diluted net loss per share calculation has been adjusted to remove gains and losses resulting from re-measurements of the convertible preferred stock warrant liability as these measurements would no longer be required when the convertible preferred stock warrants become warrants to purchase shares of the Company’s common stock.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following table presents the calculation of historical and pro forma basic and diluted net loss per share of common stock attributable to Amyris, Inc. stockholders (in thousands, except share and per share amounts):
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | Six Months Ended
June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | | | | | | | | | | (Unaudited) | |
Actual: | | | | | | | | | | | | | | | | | | | | |
Numerator: | | | | | | | | | | | | | | | | | | | | |
Net loss attributable to Amyris, Inc. stockholders | | $ | (11,774 | ) | | $ | (41,864 | ) | | $ | (64,459 | ) | | $ | (24,557 | ) | | $ | (36,096 | ) |
| | | | | | | | | | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | | | | | | | | | |
Weighted-average shares of common stock outstanding used in computing net loss per share of common stock, basic and diluted | | | 3,592,932 | | | | 4,223,533 | | | | 4,753,085 | | | | 4,661,704 | | | | 5,034,163 | |
| | | | | | | | | | | | | | | | | | | | |
Net loss per share of common stock attributable to Amyris, Inc. stockholders, basic and diluted | | $ | (3.28 | ) | | $ | (9.91 | ) | | $ | (13.56 | ) | | $ | (5.27 | ) | | $ | (7.17 | ) |
| | | | | | | | | | | | | | | | | | | | |
Pro Forma: | | | | | | | | | | | | | | | | | | | | |
Numerator: | | | | | | | | | | | | | | | | | | | | |
Net loss attributable to Amyris, Inc. stockholders | | | | | | | | | | $ | (64,459 | ) | | | | | | $ | (36,096 | ) |
| | | | | |
Less: Change in fair value of convertible preferred stock warrant liability (unaudited) | | | | | | | | | | | (445 | ) | | | | | | | (34 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net loss used in computing pro forma net loss per share of common stock attributable to Amyris, Inc. stockholders, basic and diluted (unaudited) | | | | | | | | | | $ | (64,014 | ) | | | | | | $ | (36,062 | ) |
| | | | | | | | | | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | | | | | | | | | |
Weighted-average shares of common stock outstanding used in computing net loss per share of common stock, basic and diluted | | | | | | | | | | | 4,753,085 | | | | | | | | 5,034,163 | |
| | | | | |
Add: Pro forma adjustment to reflect weighted-average effect of assumed conversion of convertible preferred stock (unaudited) | | | | | | | | | | | 15,517,824 | | | | | | | | 21,049,293 | |
| | | | | |
Add: Pro forma adjustment to reflect weighted-average effect of conversion of Amyris Brasil shares (unaudited) | | | | | | | | | | | 8,524 | | | | | | | | 500,177 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted-average shares of common stock used in computing pro forma net loss per share of common stock, basic and diluted (unaudited) | | | | | | | | | | | 20,279,433 | | | | | | | | 26,583,633 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma net loss per share of common stock attributable to Amyris, Inc. stockholders, basic and diluted (unaudited) | | | | | | | | | | $ | (3.16 | ) | | | | | | $ | (1.36 | ) |
| | | | | | | | | | | | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following outstanding shares of potentially dilutive securities were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive:
| | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
Convertible preferred stock (as converted basis)* | | 11,171,110 | | 14,185,660 | | 18,878,526 | | 14,271,843 | | 29,000,821 |
Period-end stock options to purchase common stock | | 2,628,910 | | 3,628,169 | | 4,446,894 | | 3,513,088 | | 6,275,730 |
Period-end common stock subject to repurchase | | 964,525 | | 505,035 | | 132,038 | | 254,543 | | 47,695 |
Convertible preferred stock warrants (as converted basis)* | | — | | 129,272 | | 146,447 | | 133,580 | | 195,604 |
Period-end restricted stock units | | — | | 50,000 | | 50,000 | | 50,000 | | 31,568 |
| | | | | | | | | | |
Total | | 14,764,545 | | 18,498,136 | | 23,653,905 | | 18,223,054 | | 35,551,418 |
| | | | | | | | | | |
| * | | The convertible preferred stock and convertible preferred stock warrants were computed on an as converted basis using the conversion ratios in effect as of June 30, 2010 for all periods presented. See Note 9 for conversion ratios. |
Recent accounting pronouncements
In June 2009, the FASB issued a new accounting standard that requires a qualitative approach to identifying a controlling financial interest in a VIE and requires ongoing assessment of whether an interest in a VIE makes the holder the primary beneficiary of the VIE. The new accounting standard is effective for the Company on January 1, 2010. The adoption of the standard had no impact on the Company’s consolidated financial position, results of operations or cash flows.
In October 2009, the FASB issued a new accounting standard that changes the accounting for arrangements with multiple deliverables. Specifically, the new accounting standard requires an entity to allocate arrangement consideration at the inception of an arrangement to all of its deliverables based on their relative selling prices. In addition, the new standard eliminates the use of the residual method of allocation and requires the relative-selling-price method in all circumstances in which an entity recognizes revenue for an arrangement with multiple deliverables. In October 2009, the FASB also issued a new accounting standard that changes revenue recognition for tangible products containing software and hardware elements. Specifically, if certain requirements are met, revenue arrangements that contain tangible products with software elements that are essential to the functionality of the products are scoped out of the existing software revenue recognition accounting guidance and will be accounted for under these new accounting standards. Both standards will be effective for the Company in the first quarter of 2011. Early adoption is permitted. The Company is currently assessing the impact that the adoption of these standards will have on its consolidated financial statements.
In January 2010, the FASB issued an amendment to an accounting standard which requires new disclosures for fair value measures and provides clarification for existing disclosure requirements. Specifically, this amendment require an entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfers; and to disclose separately information about purchases, sales, issuances and settlements in the reconciliation for fair value measurements
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
using significant unobservable inputs, or Level 3 inputs. This amendment clarifies existing disclosure requirements for the level of disaggregation used for classes of assets and liabilities measured at fair value and requires disclosure about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements using Level 2 and Level 3 inputs. The adoption of this amendment will not impact the Company’s consolidated financial statements.
In April 2010, the FASB issued an accounting standard update related to revenue recognition under the milestone method. The standard provides guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. Research or development arrangements frequently include payment provisions whereby a portion or all of the consideration is contingent upon milestone events such as successful completion of phases in a study or achieving a specific result from the research or development efforts. The amendments in this standards provide guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. The standard is effective for fiscal years and interim periods within those years beginning on or after June 15, 2010, with early adoption permitted, and applies to milestones achieved on or after that time. The Company is currently evaluating the impact of the implementation of this guidance on its consolidated financial statements.
3. Fair Value of Financial Instruments
Assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels which are directly related to the amount of subjectivity associated with the inputs to the valuation of these assets or liabilities are as follows:
Level 1—Observable inputs, such as quoted prices in active markets for identical assets or liabilities.
Level 2—Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following tables set forth the Company’s financial instruments that were measured at fair value on a recurring basis by level within the fair value hierarchy. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and considers factors specific to the asset or liability. As of December 31, 2008, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows (in thousands):
| | | | | | | | | | | | |
| | | Quoted Prices
in Active
Markets for
Identical Assets
(Level 1) | | Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | | Balance as of
December 31,
2008 |
Financial Assets | | | | | | | | | | | | |
Money market funds | | $ | 7,784 | | $ | — | | $ | — | | $ | 7,784 |
Commercial paper | | | — | | | 2,850 | | | — | | | 2,850 |
U.S. Government agency securities | | | — | | | 16,441 | | | — | | | 16,441 |
Auction rate securities | | | — | | | — | | | 10,907 | | | 10,907 |
Put Option | | | — | | | — | | | 2,043 | | | 2,043 |
| | | | | | | | | | | | |
Total financial assets | | $ | 7,784 | | $ | 19,291 | | $ | 12,950 | | $ | 40,025 |
| | | | | | | | | | | | |
Financial Liabilities | | | | | | | | | | | | |
Derivative liabilities | | $ | — | | $ | 45 | | $ | — | | $ | 45 |
Convertible preferred stock warrant liability | | | — | | | — | | | 2,132 | | | 2,132 |
| | | | | | | | | | | | |
Total financial liabilities | | $ | — | | $ | 45 | | $ | 2,132 | | $ | 2,177 |
| | | | | | | | | | | | |
As of December 31, 2009, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows (in thousands):
| | | | | | | | | | | | |
| | | Quoted Prices
in Active
Markets for
Identical
Assets
(Level 1) | | Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | | Balance as of
December 31,
2009 |
Financial Assets | | | | | | | | | | | | |
Money market funds | | $ | 12,479 | | $ | — | | $ | — | | $ | 12,479 |
U.S. Government agency securities | | | — | | | 35,322 | | | — | | | 35,322 |
Auction rate securities | | | — | | | — | | | 11,235 | | | 11,235 |
Put Option | | | — | | | — | | | 1,465 | | | 1,465 |
Derivative assets | | | — | | | 13 | | | — | | | 13 |
| | | | | | | | | | | | |
Total financial assets | | $ | 12,479 | | $ | 35,335 | | $ | 12,700 | | $ | 60,514 |
| | | | | | | | | | | | |
Financial Liabilities | | | | | | | | | | | | |
Convertible preferred stock warrant liability | | $ | — | | $ | — | | $ | 2,740 | | $ | 2,740 |
| | | | | | | | | | | | |
Total financial liabilities | | $ | — | | $ | — | | $ | 2,740 | | $ | 2,740 |
| | | | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
As of June 30, 2010, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows (in thousands):
| | | | | | | | | | | | | |
| | | Quoted Prices in
Active Markets for
identical Assets
(Level 1) | | Other
Observable
Inputs
(Level 2) | | Significant
Unobservable
Inputs
(Level 3) | | | Balance as of
June 30,
2010 |
| | | (Unaudited) |
Financial Assets | | | | | | | | | | | | | |
Money market funds and deposit accounts | | $ | 106,573 | | $ | — | | $ | — | | | $ | 106,573 |
U.S. Government agency securities | | | — | | | 101,048 | | | — | | | | 101,048 |
Auction rate securities | | | — | | | — | | | 4,750 | | | | 4,750 |
Derivative assets | | | — | | | 230 | | | — | | | | 230 |
| | | | | | | | | | | | | |
Total financial assets | | $ | 106,573 | | $ | 101,278 | | $ | 4,750 | | | $ | 212,601 |
| | | | | | | | | | | | | |
Financial Liabilities | | | | | | | | | | | | | |
Convertible preferred stock warrant liability | | $ | — | | $ | — | | $ | 3,281 | | | $ | 3,281 |
| | | | | | | | | | | | | |
Total financial liabilities | | $ | — | | $ | — | | $ | 3,281 | | | $ | 3,281 |
| | | | | | | | | | | | | |
The change in the fair value of the Level 3 investments is summarized below (in thousands):
| | | | | | | | |
| | | Auction Rate
Securities | | | Put Option | |
Fair value as of December 31, 2007 | | $ | 18,750 | | | $ | — | |
Unrealized loss recorded in other income (expense), net | | | (2,043 | ) | | | — | |
Recognition of the Put Option | | | — | | | | 2,043 | |
Net purchases and sales/ maturities | | | (5,800 | ) | | | — | |
| | | | | | | | |
Fair value as of December 31, 2008 | | | 10,907 | | | | 2,043 | |
Redemption at par | | | (250 | ) | | | — | |
Change in fair value recorded in other income (expense), net | | | 578 | | | | (578 | ) |
| | | | | | | | |
Fair value as of December 31, 2009 | | | 11,235 | | | | 1,465 | |
Redemption at par (unaudited) | | | (7,950 | ) | | | — | |
Change in fair value recorded in other income (expense), net (unaudited) | | | 1,465 | | | | (1,465 | ) |
| | | | | | | | |
Fair value at June 30, 2010 (unaudited) | | $ | 4,750 | | | $ | — | |
| | | | | | | | |
The change in the fair value of the convertible preferred stock warrant liability is summarized below:
| | | | |
Fair value as of December 31, 2007 | | $ | — | |
Fair value of warrants issued | | | 2,018 | |
Change in fair value recorded in other income (expense), net | | | 114 | |
| | | | |
Fair value as of December 31, 2008 | | | 2,132 | |
Fair value of warrants issued | | | 336 | |
Fair value of cancelled award | | | (173 | ) |
Change in fair value recorded in other income (expense), net | | | 445 | |
| | | | |
Fair value as of December 31, 2009 | | | 2,740 | |
Fair value of warrant issued (unaudited) | | | 507 | |
Change in fair valued recorded in other income (expense), net (unaudited) | | | 34 | |
| | | | |
Fair value as of June 30, 2010 (unaudited) | | $ | 3,281 | |
| | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The Company’s investment portfolio includes ARS, which are issued principally by student loan entities and rated AAA by a major credit rating agency. ARS are structured to provide liquidity via an auction process that resets the applicable interest rate at predetermined calendar intervals, usually every 28 days. The underlying securities have stated or contractual maturities that are generally greater than one year. Typically, the carrying value of ARS approximates fair value due to the frequent resetting of the interest rates. In February 2008, auctions failed for $12.95 million in par value of ARS that the Company held because sell orders exceeded buy orders. These failures are not believed to be a credit issue, but rather caused by a lack of liquidity. The funds associated with these failed auctions may not be accessible until the issuer calls the security, a successful auction occurs, a buyer is found outside of the auction process, or the security matures.
The Company received notification from UBS AG (“UBS”), issued in connection with a settlement entered into between UBS and certain regulatory agencies, offering to repurchase all of the Company’s ARS holdings at par value. The Company formally accepted the settlement offer and entered into a repurchase agreement with UBS in November 2008. By accepting the agreement, the Company (1) received the right (“Put Option”) to sell its ARS at par value to UBS between June 30, 2010 and July 2, 2012; and (2) gave UBS the right to purchase the ARS from the Company any time after the acceptance date as long as the Company receives the full par value.
As of December 31, 2009, the Company had $12.7 million par value of ARS (fair value of $11.2 million). During the year ended December 31, 2009 and the six months ended June 30, 2009 and 2010, a total of $250,000, $0 (unaudited) and $7.9 million (unaudited), respectively, of the ARS held by the Company were called at par by the issuer; therefore no realized losses were recognized on these securities. The Put Option was exercised on June 30, 2010 to sell the remaining ARS of $4.8 million (unaudited) at par value and was subsequently settled. As of June 30, 2010, those remaining ARS that had been sold but had not yet been settled as of such date are included in short term investments and valued at par.
During the year ended December 31, 2008, the Company made an election to transfer the ARS from available-for-sale to trading securities. The transfer to trading securities reflects the Company’s intent to exercise the Put Option during the period from June 30, 2010 to June 30, 2012. Prior to entering into the agreement, the Company’s intent was to hold the ARS until the market recovered. At the time of transfer, the unrealized loss on the ARS was $2.0 million. Prior to the transfer, this unrealized loss was included in accumulated other comprehensive income (loss). Upon transfer of the ARS from available-for-sale to trading securities, the Company immediately recognized an unrealized loss of $2.0 million, included in other income (expense), net, for the amount of the unrealized loss not previously recognized in earnings. The Company accounted for the Put Option as a freestanding financial instrument and recorded it at fair value. This allowed any changes in the fair value of the Put Option to be offset with changes in the fair value of the related ARS in the Company’s consolidated statements of operations. As a result, $2.0 million was initially recorded as a credit to other income (expense), net for the fair value of the Put Option.
The Company estimates the fair value of the ARS using a discounted cash flow model incorporating assumptions that market participants would use in their estimates of fair value. Some of these assumptions include estimates for interest rates, timing and amount of cash flows and expected holding periods of the ARS. The Company estimates the fair value of the Put Option using the expected value that the Company will receive from UBS which was calculated as the difference between the fair value and the par value of the ARS as of the option exercise date. This value is discounted by using UBS’s credit default swap rate to account for the credit considerations of the counterparty risk. The Company will reassess the fair values in future reporting periods based on several factors, including continued failure of auctions, failure of investments to be redeemed, deterioration of credit ratings of investments, market risk and other factors.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
As of December 31, 2009, the Company has classified its ARS as short-term investments and they were classified as long-term investments as of December 31, 2008 based on its intention to liquidate the investments on June 30, 2010.
Derivative Instruments
The Company utilizes derivative financial instruments to mitigate its exposure to certain market risks associated with its ongoing operations. The primary objective for holding derivative financial instruments is to manage commodity price risk. The Company’s derivative instruments principally include ethanol futures. All derivative commodity instruments are recorded at fair value on the consolidated balance sheets. None of the Company’s derivative instruments are designated as a hedging instrument. Changes in the fair value of these non-designated hedging instruments are recognized in cost of product sales in the consolidated statements of operations.
Derivative instruments measured at fair value as of December 31, 2008, 2009 and June 30, 2010, and their classification on the consolidated balance sheets and consolidated statements of operations, are presented in the following tables (in thousands) except contract amounts:
| | | | | | | | | | | | | | | |
| | | Asset/Liability as of December 31, | | Asset/Liability as of
June 30, 2010 |
| | | 2008 | | 2009 | | (Unaudited) |
Type of Derivative Contract | | Quantity of Short
Contracts | | Fair Value | | Quantity of Short
Contracts | | Fair Value | | Quantity of Short
Contracts | | Fair Value |
Regulated fixed price futures contracts, included in prepaid expenses and other current assets | | — | | $ | — | | 57 | | $ | 13 | | 112 | | $ | 230 |
Regulated fixed price futures contracts, included in accrued and other current liabilities | | 35 | | $ | 45 | | — | | $ | — | | — | | $ | — |
| | | | | | | | | | | | |
Type of Derivative Contract | | Income
Statement Classification | | Years Ended December 31, | | | Six Months
Ended
June 30, 2010 |
| | | 2008 | | 2009 | | |
| | | | | Gains (Losses) Recognized | | | Gain (Losses)
Recognized |
| | | | | | | | (Unaudited) |
Regulated fixed price futures contracts | | Cost of Product Sales | | $ | 752 | | $ | (1,910 | ) | | $ | 844 |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
4. Balance Sheet Components
Investments
The following table summarizes the Company’s investments as of December 31, 2008 (in thousands):
| | | | | | | | | | |
| | | December 31, 2008 |
| | | Amortized
Cost | | Unrealized Gain
(Loss) | | | Fair Value |
Short-term investments | | | | | | | | | | |
Commercial paper | | $ | 2,841 | | $ | 9 | | | $ | 2,850 |
U.S. Government agency securities | | | 16,363 | | | 78 | | | | 16,441 |
| | | | | | | | | | |
Total short-term investments | | $ | 19,204 | | $ | 87 | | | $ | 19,291 |
| | | | | | | | | | |
Long-term investments | | | | | | | | | | |
Auction rate securities | | $ | 12,950 | | $ | (2,043 | ) | | $ | 10,907 |
Put Option | | | — | | | 2,043 | | | | 2,043 |
| | | | | | | | | | |
Total long-term investments | | $ | 12,950 | | $ | — | | | $ | 12,950 |
| | | | | | | | | | |
The following table summarizes the Company’s investments as of December 31, 2009 (in thousands):
| | | | | | | | | | |
| | | December 31, 2009 |
| | | Amortized
Cost | | Unrealized Gain
(Loss) | | | Fair Value |
Short-term investments | | | | | | | | | | |
U.S. Government agency securities | | $ | 35,319 | | $ | 3 | | | $ | 35,322 |
Auction rate securities | | | 12,700 | | | (1,465 | ) | | | 11,235 |
Put Option | | | — | | | 1,465 | | | | 1,465 |
| | | | | | | | | | |
Total short-term investments | | $ | 48,019 | | $ | 3 | | | $ | 48,022 |
| | | | | | | | | | |
The following table summarizes the Company’s investment as of June 30, 2010 (in thousands):
| | | | | | | | | | |
| | | June 30, 2010 |
| | | Amortized
Cost | | Unrealized Gain
(Loss) | | | Fair Value |
| | | | | (Unaudited) | | | |
Short-term investments | | | | | | | | | | |
U.S. Government agency securities | | $ | 90,551 | | $ | 2 | | | $ | 90,553 |
Auction rate securities | | | 4,750 | | | — | | | | 4,750 |
| | | | | | | | | | |
Total short-term investments | | $ | 95,301 | | $ | 2 | | | $ | 95,303 |
| | | | | | | | | | |
Long-term investments | | | | | | | | | | |
U.S. Government agency securities | | $ | 7,997 | | $ | (2 | ) | | $ | 7,995 |
| | | | | | | | | | |
Total long-term investments | | $ | 7,997 | | $ | (2 | ) | | $ | 7,995 |
| | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Property and Equipment
Property and equipment, net is comprised of the following (in thousands):
| | | | | | | | | | | | |
| | | December 31, | | | June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| | | | | | | | | (Unaudited) | |
Leasehold improvements | | $ | 31,182 | | | $ | 29,575 | | | $ | 29,637 | |
Research and laboratory equipment | | | 9,422 | | | | 16,904 | | | | 18,562 | |
Computers and software | | | 1,159 | | | | 1,472 | | | | 2,958 | |
Furniture and office equipment | | | 1,355 | | | | 1,468 | | | | 1,625 | |
Construction in progress | | | 1,998 | | | | 2,158 | | | | 3,870 | |
| | | | | | | | | | | | |
| | | 45,116 | | | | 51,577 | | | | 56,652 | |
Less: accumulated depreciation and amortization | | | (3,551 | ) | | | (9,017 | ) | | | (12,410 | ) |
| | | | | | | | | | | | |
Property and equipment, net | | $ | 41,565 | | | $ | 42,560 | | | $ | 44,242 | |
| | | | | | | | | | | | |
Depreciation and amortization expense was $634,000, $2.6 million and $5.8 million for the years ended December 31, 2007, 2008 and 2009, respectively. Depreciation and amortization expense was $2.8 million (unaudited) and $3.4 million (unaudited) for the six months ended June 30, 2009 and 2010, respectively.
Property and equipment includes $4.1 million, $8.9 million and $10.3 million (unaudited) of research and laboratory equipment and furniture and office equipment under capital leases as of December 31, 2008 and 2009 and June 30, 2010, respectively. Accumulated amortization of assets under capital leases totaled $626,000, $2.1 million and $3.0 million (unaudited) as of December 31, 2008 and 2009 and June 30, 2010, respectively.
Other Assets
Other assets are comprised of the following (in thousands):
| | | | | | | | | |
| | | December 31,
2008 | | December 31,
2009 | | June 30,
2010 |
| | | | | | | (Unaudited) |
Deferred charge asset(1) | | $ | — | | $ | — | | $ | 27,909 |
Deferred offering costs | | | — | | | — | | | 2,992 |
Non-current deposits and other | | | 607 | | | 230 | | | 311 |
| | | | | | | | | |
Total other assets | | $ | 607 | | $ | 230 | | $ | 31,212 |
| | | | | | | | | |
| (1) | | The deferred charge asset relates to the collaboration agreement between the Company and Total (see Note 8). |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Accrued and Other Current Liabilities
Accrued and other current liabilities are comprised of the following (in thousands):
| | | | | | | | | |
| | | December 31, | | June 30, |
| | | 2008 | | 2009 | | 2010 |
| | | | | | | (Unaudited) |
Professional services | | $ | 1,635 | | $ | 2,411 | | $ | 5,225 |
Property and equipment | | | 731 | | | — | | | — |
Accrued vacation | | | 636 | | | 1,471 | | | 1,842 |
Payroll and related expenses | | | 604 | | | 1,573 | | | 2,647 |
Tax-related liabilities | | | 431 | | | 330 | | | 432 |
Deferred rent, current portion | | | 100 | | | 893 | | | 758 |
Refundable exercise price on early exercise of stock options | | | 262 | | | 101 | | | 82 |
Refundable deposits | | | — | | | 2,177 | | | — |
Restructuring charge, current portion | | | — | | | 592 | | | 384 |
Other | | | 684 | | | 897 | | | 589 |
| | | | | | | | | |
Total accrued and other current liabilities | | $ | 5,083 | | $ | 10,445 | | $ | 11,959 |
| | | | | | | | | |
5. Commitments and Contingencies
Capital Leases
In March 2008, the Company executed an equipment financing agreement intended to cover certain qualifying research and laboratory hardware and software. In January 2009, the agreement was amended to increase the financing amount. During the years ended December 31, 2008 and 2009, the Company financed certain purchases of hardware equipment and software of approximately $3.3 million and $4.8 million, respectively. Pursuant to the equipment financing agreement, the Company financed the equipment with the transactions representing capital leases. Accordingly, fixed assets and capital lease liabilities were recorded at the present values of the future lease payments of $3.1 million and $6.9 million during the years ended December 31, 2008 and December 31, 2009. The incremental borrowing rates used to determine the present values of the future lease payments was 9.5%. Capital lease obligations expire at various dates, with the latest maturity in April 2013.
In connection with the capital lease entered into in 2008, the Company issued a warrant to purchase shares of the Company’s convertible preferred stock (see Note 10).
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The recorded balance of capital lease obligations as of December 31, 2008 and 2009 and June 30, 2010 was $3.6 million, $7.2 million and $7.4 million (unaudited), respectively. The Company recorded interest expense in connection with its capital leases of $18,000, $175,000, $751,000, $430,000 (unaudited) for the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010, respectively. Future minimum payments under capital leases as of December 31, 2009, are as follows (in thousands):
| | | | |
| | | Capital Leases | |
Years ending December 31: | | | | |
2010 | | $ | 2,961 | |
2011 | | | 2,905 | |
2012 | | | 2,394 | |
2013 | | | 271 | |
2014 | | | — | |
Thereafter | | | — | |
| | | | |
Total future minimum lease payments | | | 8,531 | |
Less: executory costs | | | (19 | ) |
| | | | |
Net minimum lease payments | | | 8,512 | |
Less: amount representing interest | | | (1,284 | ) |
| | | | |
Present value of minimum lease payments | | | 7,228 | |
Less: current maturities | | | (2,251 | ) |
| | | | |
Long-term portion | | $ | 4,977 | |
| | | | |
Operating Leases
The Company has noncancelable operating lease agreements for office, research and development and manufacturing space in the United States and Brazil.
In August 2007, the Company entered into an operating lease for its new headquarters in Emeryville, California, with a term of ten years commencing in May 2008. As part of the operating lease agreement, the Company received a tenant improvements allowance of $11.4 million. The Company recorded the allowance as deferred rent and associated expenditures as leasehold improvements that are being amortized over the shorter of their useful life or the terms of the lease.In connection with the operating lease, the Company elected to defer a portion of the monthly base rent due under the lease and entered into notes payable agreements with the lessor for the purchase of certain tenant improvements (see Note 6).
In addition, the Company leases a facility in Brazil pursuant to a noncancelable operating lease that expires in May 2013 and the Company leases office space in the United States under noncancelable operating leases that expire at various dates, with the latest expiration in May 2018.
The Company recognizes rent expense on a straight-line basis over the noncancelable lease term and records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. Where leases contain escalation clauses, rent abatements, and/or concession, such as rent holidays and landlord or tenant incentives or allowances, the Company applies them in the determination of straight-line rent expense over the lease term. Rent expense for the years ended December 31, 2007, 2008 and 2009 was $1.0 million, $2.9 million and $3.6 million. Rent expense was $2.0 million (unaudited) and $1.5 million (unaudited) for the six months ended June 30, 2009 and 2010, respectively.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
As of December 31, 2009, future minimum rental payments under the operating leases are as follows (in thousands):
| | | |
| | | Operating Leases |
Years ending December 31: | | | |
2010 | | $ | 3,161 |
2011 | | | 4,021 |
2012 | | | 4,669 |
2013 | | | 4,328 |
2014 | | | 4,051 |
Thereafter | | | 14,517 |
| | | |
Total future minimum lease payments | | $ | 34,747 |
| | | |
Amyris Brasil Transactions
On December 22, 2009, the Company entered into an investment and stockholders’ agreement with third-party investors (“Investors”) to sell a 4.8% equity interest in Amyris Brasil. In the quarter ended March 31, 2010, Amyris Brasil sold an incremental 3.4% (unaudited) equity interest in Amyris Brasil to investors. Under the terms of the agreements, the Company is required to make additional capital investments in Amyris Brasil worth at least BRL $50.0 million. In May 2010, Amyris Basil sold an additional 1,111,111 shares of its stock, an incremental 4.07%, interest for BRL$10.0 million (unaudited) (see Note 16).
Guarantor Arrangements
The Company has agreements whereby it indemnifies its officers and directors for certain events or occurrences while the officer or director is, or was, serving at the Company’s request in such capacity. The term of the indemnification period is for the officer or director’s lifetime. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a director and officer insurance policy that limits its exposure and enables the Company to recover a portion of any future amounts paid. As a result of its insurance policy coverage, the Company believes the estimated fair value of these indemnification agreements is minimal. Accordingly, the Company had no liabilities recorded for these agreements as of December 31, 2008 and 2009.
Other Matters
The Company may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. The Company accrues amounts, to the extent they can be reasonably estimated, that it believes are adequate to address any liabilities related to legal proceedings and other loss contingencies that the Company believes will result in a probable loss. While there can be no assurances as to the ultimate outcome of any legal proceeding or other loss contingency involving the Company, management does not believe any pending matter will be resolved in a manner that would have a material adverse effect on the Company’s consolidated financial position, results of operations or cash flows.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
6. Debt
Debt is comprised of the following (in thousands):
| | | | | | | | | | | | |
| | | December 31, | | | June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| | | | | | | | | (Unaudited) | |
Credit facility | | $ | — | | | $ | 8,343 | | | $ | 327 | |
Notes payable | | | — | | | | 4,038 | | | | 4,117 | |
Loans payable | | | 3,149 | | | | 999 | | | | 989 | |
| | | | | | | | | | | | |
Total debt | | | 3,149 | | | | 13,380 | | | | 5,433 | |
Less: current portion | | | (340 | ) | | | (9,018 | ) | | | (1,260 | ) |
| | | | | | | | | | | | |
Long-term debt | | $ | 2,809 | | | $ | 4,362 | | | $ | 4,173 | |
| | | | | | | | | | | | |
Credit Facility
In January 2009, the Company entered into a credit facility with UBS associated with student loan auction rate securities holdings. In March and April 2009, the Company drew down $8.1 million and $0.5 million on the credit facility. The credit facility is collateralized by the ARS held with the bank. The credit facility bears a variable interest rate of LIBOR plus 1.25% and the weighted average borrowing rate under the credit facility was 1.32% and 1.30% (unaudited) as of December 31, 2009 and June 30, 2010, respectively. As of December 31, 2009 and June 30, 2010, the total amount outstanding under the credit facility was $8.3 million and $0.3 million (unaudited), respectively.
Notes Payable
During the period between May 2008 and October 2008, the Company entered into notes payable agreements with the lessor of its headquarters under which the total amount of $3.3 million was borrowed for the purchase of tenant improvements, bearing an interest rate of 9.5% per annum and to be repaid over a period of 55 to 120 months. As of December 31, 2008 and 2009 and June 30, 2010, a principal amount of $3.1 million, $2.8 million and $2.6 million (unaudited) was outstanding under these notes payable, respectively.
During the period between January 2009 and December 2009, the Company entered into notes payable agreements with a service provider in connection with its software implementation under which the total amount of $1.2 million was borrowed for the payment of implementation services and software licenses, bearing an interest rate of 8.53% per annum and to be repaid over a period of 69 (unaudited) to 83 months. As of December 31, 2009 and June 30, 2010 (unaudited), a principal amount of $1.1 million was outstanding under these notes payable.
In July 2009, the Company entered into a notes payable agreement of $378,000 with its insurance provider. The notes payable are payable in monthly principal and interest installments of $45,300 through March 2010. The note payable accrues interest at 6%. As of December 31, 2009 and June 30, 2010, a principal amount of $125,000 and zero (unaudited) was outstanding under the notes payable, respectively.
In March 2010, the Company entered into a notes payable agreement of $101,000 (unaudited) with its insurance provider. The notes are payable in monthly principal and interest installments of $11,000 (unaudited) through November 2010. The note payable accrues interest at 5.50% (unaudited). As of June 30, 2010 a principal amount of $57,000 (unaudited) was outstanding under these notes payable.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
In February 2010, the Company entered into a notes payable agreement of $239,000 (unaudited) with its landlord. The notes are payable in monthly principal and interest installments of $31,000 from June 2010 through January 2011. The notes payable accrue interest at 10.50%. As of June 30, 2010, a principal amount of $210,000 (unaudited) was outstanding under these notes payable.
In April 2010, the Company entered into a notes payable agreement of $182,000 (unaudited) with a financial institution to finance a software purchase. The notes are payable in equal installments of principal and interest starting in May 2010 through April 2012. The notes payable accrues interest at 10%. As of June 30, 2010, a principal amount of $168,000 (unaudited) was outstanding under these notes payable.
Loans Payable
In August 2009, the Company entered into a loans payable agreement with the lessor of its headquarters under which $750,000 was borrowed. The loan is payable in monthly installments of interest only and unpaid interest and principle is payable in December 2011. Interest accrues at an interest rate of 10.5%. As of December 31, 2009 and June 30, 2010 (unaudited), a principal amount of $750,000 was outstanding under the loan. The notes payable agreement is secured by a $750,000 letter of credit.
In December 2009, the Company entered into a loan payable agreement with the lessor of its Emeryville, pilot plant under which the total amount of $250,000 was borrowed, bearing an interest rate of 10% per annum and to be repaid over a period of 96 months. As of December 31, 2009 and June 30, 2010, a principal amount of $249,000 and $239,000 (unaudited) was outstanding under the loan, respectively.
Future minimum payments under the debt agreements as of December 31, 2009 are as follows (in thousands):
| | | | | | | | | | | | |
| | | Credit Line | | | Notes Payable | | | Loans Payable | |
Years ending December 31: | | | | | | | | | | | | |
2010 | | $ | 8,398 | | | $ | 993 | | | $ | 123 | |
2011 | | | — | | | | 863 | | | | 866 | |
2012 | | | — | | | | 861 | | | | 45 | |
2013 | | | — | | | | 682 | | | | 45 | |
2014 | | | — | | | | 592 | | | | 45 | |
Thereafter | | | — | | | | 1,406 | | | | 134 | |
| | | | | | | | | | | | |
Total minimum payments | | | 8,398 | | | | 5,397 | | | | 1,258 | |
Less: interest | | | (55 | ) | | | (1,359 | ) | | | (259 | ) |
| | | | | | | | | | | | |
Present value of future minimum payments | | | 8,343 | | | | 4,038 | | | | 999 | |
Less: current portion | | | (8,343 | ) | | | (653 | ) | | | (22 | ) |
| | | | | | | | | | | | |
Noncurrent portion of debt | | $ | — | | | $ | 3,385 | | | $ | 977 | |
| | | | | | | | | | | | |
Letters of Credit
In November 2008, the Company entered into an uncommitted facility letter (the “Credit Agreement”) with a financial institution to finance the purchase and sale of fuel and for working capital requirements, as needed. In October 2009, the agreement was amended to decrease the maximum amount that the Company may borrow under such facility. The Credit Agreement, as amended, provides an aggregate maximum availability up to the lower of $20.0 million and the borrowing base as defined in the agreement, and is subject to a sub-limit of $5.7 million for the issuance of letters of credit and a sub-limit of $20 million for short-term cash advances for product purchases. Amyris has a parent guarantor for the payment of the outstanding balance under the Credit
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Agreement. Outstanding advances bear an interest rate at the Company’s option of the bank’s prime rate plus 1.0% or the bank’s cost of funds plus 3.5%. As of December 31, 2009, the Company had sufficient borrowing base levels to draw down up to a total of $2.8 million in short term cash advances and $4.6 million available for letters of credit in addition to those outstanding as of December 31, 2009. As of July 2, 2010 the Company could draw $541,000 (unaudited) in short term advances and had $3.3 million (unaudited) available for letters of credit in addition to those outstanding at June 30, 2010. As of December 31, 2008 and 2009, and June 30, 2010 the Company had no outstanding advances and had $0.7 million, $1.1 million and $2.4 million (unaudited) in outstanding letters of credit under the Credit Agreement.
To the extent that amounts under the Credit Agreement remain unused, while the Credit Agreement is in effect and for so long thereafter as any of the obligations under the Credit Agreement are outstanding, the Company will pay an annual commitment fee of $300,000. The Credit Agreement requires compliance with certain customary covenants that require maintenance of certain specified financial ratios and conditions. As of December 31, 2009, the Company was in compliance with its financial covenants under the Credit Agreement. The Credit Agreement is collateralized by a first priority security interest in certain of the Company’s present and future assets.
In November 2009, the Company entered into an irrevocable standby letter of credit agreement for up to $4.5 million. As of June 30, 2010 eight letters of credit had been issued totaling $4.3 million (unaudited) as security for certain facility and capital leases, each of which expires on November 9, 2010 and will be automatically extended for two one-year periods. As of December 31, 2009, the Company was in compliance with all financial covenants in the letter of credit agreements. In connection with the letter of credit agreements, the Company maintained a deposit balance with the financial institution, which amounted to $4.5 million as of December 31, 2009 and June 30, 2010 (unaudited), respectively. The availability of the letter of credit is dependent upon maintaining the cash deposits.
7. Income Taxes
The components of the provision for (benefit from) income taxes are as follows for the years ended December 31, 2007, 2008 and 2009 (in thousands):
| | | | | | | | | | |
| | | 2007 | | 2008 | | | 2009 |
Current: | | | | | | | | | | |
Federal | | $ | — | | $ | — | | | $ | — |
State | | | — | | | (207 | ) | | | — |
Foreign | | | — | | | — | | | | — |
| | | | | | | | | | |
Total current provision (benefit) | | | — | | | (207 | ) | | | — |
| | | | | | | | | | |
Deferred: | | | | | | | | | | |
Federal | | | — | | | — | | | | — |
State | | | — | | | — | | | | — |
Foreign | | | — | | | — | | | | — |
| | | | | | | | | | |
Total deferred provision (benefit) | | | — | | | — | | | | — |
| | | | | | | | | | |
Total provision for (benefit from) income taxes | | $ | — | | $ | (207 | ) | | $ | — |
| | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
A reconciliation between the statutory federal income tax and the Company’s effective tax rates as a percentage of loss before income taxes is as follows:
| | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
Statutory tax rate | | 34.0 | % | | 34.0 | % | | 34.0 | % |
State tax rate, net of federal benefit | | 6.8 | | | 3.5 | | | 5.5 | |
Incentive stock option stock compensation | | (0.6 | ) | | (0.5 | ) | | (0.4 | ) |
Federal R&D credit | | 1.5 | | | 0.1 | | | 1.0 | |
Other | | (3.4 | ) | | 1.1 | | | 1.2 | |
Change in valuation allowance | | (38.3 | ) | | (37.8 | ) | | (41.3 | ) |
| | | | | | | | | |
Effective income tax rate | | 0 | % | | 0.4 | % | | 0 | % |
| | | | | | | | | |
Temporary differences and carryforwards that gave rise to significant portions of deferred taxes are as follows (in thousands):
| | | | | | | | |
| | | December 31,
2008 | | | December 31,
2009 | |
Net operating loss carry forwards | | $ | 19,879 | | | $ | 37,118 | |
Fixed assets | | | — | | | | 108 | |
Research and development credits | | | 208 | | | | 2,409 | |
Accruals and reserves | | | 552 | | | | 1,240 | |
Stock compensation | | | 719 | | | | 1,602 | |
Other | | | — | | | | 5,322 | |
| | | | | | | | |
Total deferred tax assets | | | 21,358 | | | | 47,799 | |
Fixed assets | | | (296 | ) | | | — | |
Other | | | (43 | ) | | | — | |
| | | | | | | | |
Total deferred tax liabilities | | | (339 | ) | | | — | |
| | | | | | | | |
Net deferred tax asset prior to valuation allowance | | | 21,019 | | | | 47,799 | |
Less: Valuation allowance | | | (21,019 | ) | | | (47,799 | ) |
| | | | | | | | |
Net deferred tax assets (liabilities) | | $ | — | | | $ | — | |
| | | | | | | | |
Due to uncertainties surrounding the realization of deferred tax assets through future taxable income, the Company has provided a full valuation allowance and, therefore, has not recognized any benefits from the net operating losses and other deferred tax assets. The valuation allowance increased $4.5 million, $16.1 million and $26.8 million during the years ended December 31, 2007, 2008 and 2009, respectively.
Recognition of deferred tax assets is appropriate when realization of such assets is more likely than not. Based upon the weight of available evidence, the Company believes it is not yet more likely than not that the net deferred tax assets will be fully realizable. Accordingly, the Company has provided a full valuation allowance against its net deferred tax assets as of December 31, 2009.
As of December 31, 2009, the Company had federal and California net operating loss carryforwards of approximately $95.8 million and $67.4 million available to reduce future taxable income, if any, respectively.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The federal net operating loss carryforward begins expiring in 2025, and the California net operating loss carryforward begins expiring in 2015. The Company also had federal and California state research and development credit carryforwards of approximately $2.0 million and $2.1 million at December 31, 2009. The federal credits will expire starting 2024 if not utilized. The California tax credits can be carried forward indefinitely.
The Tax Reform Act of 1986 limits the use of net operating loss and tax credit carryforwards in certain situations where equity transactions result in a change of ownership as defined by Internal Revenue Code Section 382. In the event the Company should experience an ownership change, as defined, utilization of its United States net operating loss carryforwards and tax credits could be limited.
Effective January 1, 2007, the Company adopted the accounting guidance on uncertainties in income taxes. A reconciliation of the beginning and ending amounts of unrecognized tax benefits since the adoption of accounting guidance on uncertainty in income taxes is as follows:
| | | |
January 1, 2007 | | $ | 41 |
Increases in balances related to tax provisions taken during current period | | | 108 |
| | | |
December 31, 2007 | | | 149 |
Increases in balances related to tax provisions taken during current period | | | 423 |
| | | |
December 31, 2008 | | | 572 |
Increases in balances related to tax provisions taken during current period | | | 460 |
| | | |
December 31, 2009 | | $ | 1,032 |
| | | |
The Company’s policy is to include interest and penalties related to unrecognized tax benefits within the provision for taxes. Management determined that no accrual for interest and penalties was required as of December 31, 2009.
As of December 31, 2009, the Company’s total unrecognized tax benefits were $1 million, of which none of the tax benefits, if recognized, would affect the effective income tax rate due to the valuation allowance that currently offsets deferred tax assets. The Company does not anticipate the total amounts of unrecognized income tax benefits will significantly increase or decrease in the next 12 months.
The Company’s primary tax jurisdiction is the United States. For United States federal and state tax purposes, tax years 2003 through 2009 remain open and subject to tax examination by the appropriate federal or state taxing authorities. Brazil tax years 2008 and 2009 remain open and subject to examination.
8. Research and Development Activities
One World Health
Research and Collaboration Agreement
In November 2004, the Company entered into a research and collaboration agreement (the “Agreement”) with the Institute for One World Health (“IOWH”), a California nonprofit corporation, and the Regents of the University of California, Berkeley (“UC Berkeley”). Per the agreement, IOWH agreed to work in partnership with UC Berkeley and the Company. UC Berkeley agreed to conduct basic research to aid in the engineering of a microbe to make a biosynthetic precursor of artemisinin, currently the most effective treatment for malaria. The Company will optimize the strain for industrial fermentation and develop a follow-on chemical synthesis for artemisinin using the precursor. IOWH agreed to perform the drug development and regulatory work to
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
demonstrate the bioequivalence of microbially produced artemisinin derivative to the drug’s natural form. The Company will receive no payments from the eventual use of the technology towards the production of antimalarial drugs for use in the developing world. The Agreement, however, allows the Company to develop and maintain ownership rights over a platform technology that is applicable towards the production of many other compounds.
During the year ended December 31, 2007, the Company received $4.1 million under this agreement. The Company recorded deferred revenue of $1.4 million and $0 million as of December 31, 2007 and 2008.
Exclusive Development and Commercialization Agreement
In November 2004, the Company entered into an exclusive development and commercialization agreement, as amended in January 2008, with IOWH and sanofi-aventis (the “Parties”) under which the Parties agreed to develop and commercialize specific products (“anti-malarias”) in the IOWH field and territory (countries in the developing world). Under this amended agreement, the Company will grant IOWH and its affiliates a worldwide, exclusive, royalty-free license to develop and commercialize such anti-malaria products in those developing world countries.
During the year ended December 31, 2007, 2008 and 2009, the Company received $0, $1.7 million and $1.3 million related to these agreements. During the six months ended June 30, 2009 and 2010, the Company received $1.3 million (unaudited) and $0 (unaudited) related to these agreements.
Auburn University Professional Services Contract
During the year ended December 31, 2008, the Company entered into a professional services contract (the “Contract”) with Auburn University. Per the contract, the Company will provide a portion of the activities required to plant, grow, harvest, and analyze a sugarcane nursery in Alabama. During the years ended December 31, 2008 and 2009, the Company recognized revenue of $204,000 and $0 related to this arrangement.
Sanofi Chimie Development Services Agreement
In June 2009, the Company entered into a development services agreement with Sanofi Chimie, under which the Company agreed to perform molecular biology development services related to strain improvement on behalf of Sanofi Chimie. Under the terms of the agreement, Sanofi Chimie provided the Company with an up-front payment of $1.5 million which is being recognized on a ratable basis over the research period under the arrangement. The Company is also eligible to receive milestone payments totaling $1.5 million and a bonus payment of $0.3 million if a specified target is met. Milestones to be earned under the agreement have been determined to be at risk and substantive at the inception of the arrangement and are expected to be recognized upon achievement of the milestone and when collectability is reasonably assured. Research and development revenue of $1.6 million, including milestone revenue was recognized under the agreement during the year ended December 31, 2009 and $1.4 million (unaudited) was recognized under the agreement in the six months ended June 30, 2010. The Company recorded deferred revenue of $378,000 as of December 31, 2009 and $0 (unaudited) as of June 30, 2009 and 2010.
Total (unaudited)
In June 2010, the Company entered into a technology license, development, research and collaboration agreement (“collaboration agreement”) with Total Gas & Power USA Biotech, Inc., an affiliate of Total S.A. (“Total”). The agreement sets forth the terms for the research, development, production and commercialization of certain to be determined chemical and/or fuel products made through the use of the Company’s synthetic biology platform. The collaboration agreement establishes a multiphased process through which projects are identified,
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
screened, studied for feasibility, and ultimately selected as a project for development of an identified lead compound using an identified microbial strain. Under the terms of the collaboration agreement, Total will fund up to the first $50.0 million in research and development costs for the selected projects; thereafter the parties will share such costs equally. Once a development project has commenced, the parties are obligated to work together exclusively to develop the lead compound during the project development phase. After a development project is completed, the Company and Total expect to form one or more joint ventures to commercialize any products that are developed, with costs and profits to be shared on an equal basis, provided that if Total has not achieved profits from sales of a joint venture product equal to the amount of funding it provided for development plus an agreed upon rate of return within three years of commencing sales, then Total will be entitled to receive all profits from sales until this rate of return has been achieved. Each party has certain rights to independently produce commercial quantities of these products under certain circumstances, subject to paying royalties to the other party. Total has the right of first negotiation with respect to exclusive commercialization arrangements the Company would propose to enter into with certain third parties, as well as the right to purchase any of the Company’s products on terms no less favorable than those offered to or received by the Company from third parties in any market where Total or its affiliates have a significant market position. The collaboration agreement has an initial term of 12 years.
In June 2010, concurrent with the collaboration agreement, the Company issued 7,101,548 shares of Series D preferred stock to Total for aggregate proceeds of approximately $133 million at a per share price of $18.75, which was lower that the per share fair value of common stock as determined by management and the Board of Directors. Due to the fact the collaboration agreement and the issuance of shares to Total occurred concurrently, the terms of both the collaboration agreement and the issuance of preferred stock were evaluated to determine whether their separately stated pricing was equal to the fair value of services and preferred stock. The Company determined that the fair value of Series D preferred stock was $22.68 at the time of issuance, and therefore, the Company measured the preferred stock initially at its fair value with a corresponding reduction in the consideration for the services under the collaboration agreement. As revenue from collaboration agreement will be generated over a period of time based on the performance requirements, the Company recorded the difference between the fair value and consideration received for Series D preferred stock of $27.9 million as deferred charge asset within other assets at the time of issuance which will be recognized as a reduction to revenue in proportion to the total estimated revenue under the collaboration agreement.
Total is required to pay up to $14.3 million of additional cash consideration to the Company for the purchase of the Series D preferred stock if and to the extent the offering price in the Company’s initial public offering exceeds an agreed upon threshold.
9. Convertible Preferred Stock
Under the Company’s amended and restated articles of incorporation, the Company’s convertible preferred stock is issuable in series.
A summary of convertible preferred stock issued and outstanding as of December 31, 2008 is as follows (in thousands, except share data):
| | | | | | | | | | | | | |
| | | December 31, 2008 |
| | | Shares
Authorized | | Shares
Issued and
Outstanding | | Liquidation
Preference per
Share | | Liquidation
Amount | | Carrying
Value |
Series A | | 9,475,000 | | 9,475,000 | | $ | 2.174 | | $ | 20,599 | | $ | 20,470 |
Series B | | 1,929,641 | | 1,667,817 | | | 24.88 | | | 41,495 | | | 41,326 |
Series B-1 | | 4,700,000 | | 2,538,841 | | | 25.26 | | | 64,131 | | | 59,640 |
| | | | | | | | | | | | | |
Total | | 16,104,641 | | 13,681,658 | | | | | $ | 126,225 | | $ | 121,436 |
| | | | | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
A summary of convertible preferred stock issued and outstanding as of December 31, 2009 is as follows (in thousands, except share data):
| | | | | | | | | | | | | |
| | | December 31, 2009 |
| | | Shares
Authorized | | Shares
Issued and
Outstanding | | Liquidation
Preference per
Share | | Liquidation
Amount | | Carrying
Value |
Series A | | 9,475,000 | | 9,475,000 | | $ | 2.174 | | $ | 20,599 | | $ | 20,470 |
Series B | | 1,929,641 | | 1,667,817 | | | 24.88 | | | 41,495 | | | 41,326 |
Series B-1 | | 4,700,000 | | 2,615,721 | | | 25.26 | | | 66,073 | | | 61,412 |
Series C | | 4,976,000 | | 4,606,684 | | | 12.46 | | | 57,399 | | | 56,443 |
| | | | | | | | | | | | | |
Total | | 21,080,641 | | 18,365,222 | | | | | $ | 185,566 | | $ | 179,651 |
| | | | | | | | | | | | | |
A summary of convertible preferred stock issued and outstanding as of June 30, 2010 is as follows (in thousands, except share data);
| | | | | | | | | | | | | |
| | | June 30, 2010 |
| | | Shares
Authorized | | Shares
Issued and
Outstanding | | Liquidation
Preference per
Share | | Liquidation
Amount | | Carrying
Value |
| | | (Unaudited) |
Series A | | 9,475,000 | | 9,475,000 | | $ | 2.174 | | $ | 20,599 | | $ | 20,470 |
Series B | | 1,929,641 | | 1,667,817 | | | 24.88 | | | 41,495 | | | 41,326 |
Series B-1 | | 4,700,000 | | 2,615,721 | | | 25.26 | | | 66,073 | | | 61,412 |
Series C | | 4,976,000 | | 4,902,665 | | | 12.46 | | | 61,087 | | | 59,619 |
Series C-1 | | 2,781,714 | | 2,724,766 | | | 17.56 | | | 47,847 | | | 47,779 |
Series D | | 7,101,548 | | 7,101,548 | | | 18.75 | | | 133,154 | | | 160,805 |
| | | | | | | | | | | | | |
Total | | 30,963,903 | | 28,487,517 | | | | | $ | 370,255 | | $ | 391,411 |
| | | | | | | | | | | | | |
The convertible preferred stock is recorded at fair value on the dates of issuance, net of issuance costs. The convertible preferred stock is classified outside of stockholders’ equity (deficit) because the shares contain liquidation features that are not solely within the control of the Company.
The rights, preferences and privileges of the convertible preferred stock are as follows:
Voting
Except as required by applicable law, each share of convertible preferred stock has voting rights equal to an equivalent number of shares of common stock into which it is convertible and votes together as one class with the common stock.
As long as at least 20% of the originally issued shares of the convertible preferred stock remain outstanding, consent of the holders of at least the majority of the convertible preferred stock are required for any action that includes among others (i) alters or changes the rights, preferences or privileges of the common stock and convertible preferred stock; (ii) results in the redemption of any shares of convertible preferred stock or common stock; (iii) results in a liquidation or other corporate reorganization; (iv) amends or waives any provision of the Company’s amended and restated articles of incorporation; (v) increases or decreases the authorized size of the Board of Directors; and (vi) results in the payment or declaration of any dividend on any shares of common or convertible preferred stock.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Dividends
The holders of the convertible preferred stock are entitled to receive, in preference to any dividend on the common stock, noncumulative dividends, on a pari passu basis, at the rate of 8% of the original issue price per annum on each outstanding share of Series A, B, B-1, C, C-1 and D convertible preferred stock. Such dividends are payable when, as and if declared by the Board of Directors. No dividends have been declared to date.
Conversion Rights
Each share of preferred stock is convertible, at the option of the holder, at any time, into shares of common stock determined by dividing the issuance price per share by the conversion price in effect at the time of the conversion. The per share conversion prices are subject to adjustment for antidilution, stock splits and reclassifications. The per share conversion prices of Series A, B, B-1, C, C-1 and D convertible preferred stock are $2.174, $22.25, $22.53, $12.46, $17.56 and $18.75, respectively. Accordingly, the conversion ratios for the Series A, B, B-1, C, C-1 and D convertible preferred stock to common stock are 1:1, 1:1.118, 1:1.121, 1:1, 1:1 and 1:1, respectively.
Each share of Series A, B, B-1, C, C-1 and D convertible preferred stock shall automatically be converted into a number of shares of common stock upon the earlier to occur of (i) the date specified by the written consent or agreement of the holders of a majority of the then outstanding shares of Series A, B, B-1, C, C-1 and D convertible preferred stock, each such series voting together as a single class, or (ii) immediately upon the completion of the sale of the Company’s common stock in a firm commitment, underwritten public offering registered under the Securities Act of 1933, which results in aggregate proceeds to the Company equal to at least $30.0 million.
The Series D preferred stock has a contingently adjustable conversion price such that: (i) in the event of a qualified initial public offering, or qualified IPO, on or before September 30, 2010 with an offering price less than $21.75, the conversion price will be reduced to the offering price divided by 1.16 and (ii) in the event of a qualified IPO after September 30, 2010 with an offering price less than $24.38, the conversion price will be reduced to the offering price divided by 1.30 (unaudited).
The consent of the holders of a majority of the Series D preferred stock is required if the conversion of the shares of Series D preferred stock into shares of common stock is effected in connection with a qualified acquisition or qualified asset sale in which the value of the consideration to be received in respect to the share or shares of common stock issuable upon conversion of a share of Series D preferred stock is less than $28.13 per share (unaudited).
Liquidation Rights
In the event of any liquidation, dissolution, or winding up of the Company, the holders of the Series A, B, B-1, C and C-1 convertible preferred stock shall be entitled to receive, prior and in preference to any distribution of any of the assets or surplus funds of the Company to the holders of the common stock, an amount equal to $2.174, $24.88, $25.26, $12.46, $17.56 and $18.75 per share plus any declared but unpaid dividends on such share (the “Liquidation Preference”). After the payment of the Liquidation Preference, all remaining assets available for distribution, if any, shall be distributed ratably among the holders of the common stock. If available assets are insufficient to pay the full Liquidation Preference, the available assets will be distributed to the holders of Series A, B, B-1, C, C-1 and D convertible preferred stock, in proportion to the preferential amount each such holder is otherwise entitled to receive.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
A merger, consolidation, sale or lease of all or substantially all of the assets of the Company which will result in the Company’s stockholders immediately prior to such transaction not holding at least 50% of the voting power of the surviving entity, shall be deemed to be a liquidation, dissolution or winding up. Upon this event, holders of all shares of Series A, B, B-1, C, C-1 and D convertible preferred stock shall receive the greater of (i) their liquidation preference including any declared but unpaid dividends as of the liquidation date or (ii) the amount that would have been payable had all shares of each such series been converted into common stock immediately prior to this event.
Redemption Rights
The convertible preferred stock is not redeemable.
The Company performs ongoing assessmentsof all terms and features of its convertible preferred stock in order to identify any potential embedded features that would require bifurcation or any beneficial conversion features. As part of this analysis, the Company assessed the economic characteristics and risks of its convertible preferred stock, including conversion, liquidation and redemption features, as well as dividend and voting rights. Based on the Company’s determination that each series of its convertible preferred stock is an “equity host,” the Company determined that the features of the convertible preferred stock are most closely associated with an equity host and, although the convertible preferred stock includes conversion features, such conversion features do not require bifurcation as a derivative liability. The Company also determined that the conversion option with a contingent reduction in the conversion price, upon occurrence of certain dilutive events, is a potential contingent beneficial conversion feature. In accordance with certain antidilution provisions contained in the Series B and B-1 convertible preferred stock agreements, issuances of Series C, and C-1 convertible preferred stock resulted in an antidilution adjustment of the conversion prices for the Series B and B-1 convertible preferred stock during the year ended December 31, 2009 and six months ended June 30, 2010. As a result, the Company performed a calculation to determine if a beneficial conversion feature was triggered for the Series B and B-1 convertible preferred stock at each issuance of Series C, and C-1 convertible preferred stock. The fair value of common stock, as determined by management and the Board of Directors, on the corresponding issuance dates of Series B and B-1 convertible preferred stock in each instance was below the adjusted conversion prices. Therefore, no beneficial conversion feature was identified. The Company will continue to evaluate if a beneficial conversion feature needs to be recorded upon each subsequent adjustment of the conversion price based upon the difference between the adjusted conversion price and the fair market value of common stock at the original issuance date.
10. Warrants for Convertible Preferred Stock
The Company had the following unexercised convertible preferred stock warrants (in thousands, except for share data):
| | | | | | | | | | | | | |
| | | Exercise
Price per
Share | | Shares as of
December 31, | | Fair Value as of
December 31, |
Underlying Stock | | | |
| | | 2008 | | 2009 | | 2008 | | 2009 |
Series B Convertible Preferred Stock | | $ | 24.88 | | 12,628 | | 2,580 | | $ | 237 | | $ | 48 |
Series B-1 Convertible Preferred Stock | | $ | 25.26 | | 102,724 | | 106,567 | | | 1,895 | | | 2,267 |
Series C Convertible Preferred Stock | | $ | 12.46 | | — | | 24,101 | | | — | | | 425 |
| | | | | | | | | | | | | |
Total | | | | | 115,352 | | 133,248 | | $ | 2,132 | | $ | 2,740 |
| | | | | | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
| | | | | | | | |
| | | Exercise
Price per
Share | | Shares as of
June 30, | | Fair Value
as of
June 30, |
| | | | 2010 | | 2010 |
| | | | | (Unaudited) | | |
Series B Convertible Preferred Stock | | $ | 24.88 | | 2,580 | | $ | 39 |
Series B-1 Convertible Preferred Stock | | $ | 25.26 | | 106,567 | | | 1,898 |
Series C Convertible Preferred Stock | | $ | 12.46 | | 73,258 | | | 1,344 |
| | | | | | | | |
Total | | | | | 182,405 | | $ | 3,281 |
| | | | | | | | |
Series B
In 2008, in connection with consulting services, the Company issued a warrant to purchase 2,580 shares of the Company’s Series B convertible preferred stock at an exercise price of $24.88 per share. The warrant is immediately exercisable and expires upon the earlier of (i) five years from the effective date; (ii) one year from the effective date of a public offering; or (iii) upon a change of control. The Company estimated the initial fair value of the warrant as of the date of issuance to be $39,000 and was recorded as consulting expense. The fair value was based on the contractual term of the warrants of five years, risk-free interest rate of 3.5%, expected volatility of 70% and 0% expected dividend yield.
In 2008, in connection with a capital lease agreement, the Company issued a warrant to purchase 10,048 shares of the Company’s Series B convertible preferred at an exercise price of $24.88 per share. The warrant was immediately exercisable and was due to expire upon the earlier of (i) ten years from the effective date; (ii) one year from the effective date of a public offering; or (iii) a merger event. The Company estimated the initial fair value of the warrant to be $196,000 and was recorded as other assets. The fair value was based on the contractual term of the warrants of ten years, risk-free interest rate of 4.04%, expected volatility of 70% and 0% annual dividend rate. In September 2009, the Company cancelled the warrant and issued a warrant to purchase 10,048 shares of the Company’s Series C convertible preferred stock.
Series B-1
In 2008, in connection with the Company’s issuance of Series B-1 convertible preferred stock, the Company issued warrants to purchase 100,715 shares of the Company’s Series B-1 convertible preferred stock at an exercise price of $25.26 per share to the placement agent. The warrants are immediately exercisable and expire seven years from the effective date. The Company estimated the initial fair value of these warrants as of the issuance dates to be $1.7 million and was recorded as a reduction to the carrying value of the Series B-1 convertible preferred stock. The fair value was based on the contractual term of the warrants of seven years, risk-free interest rates from 2.9% to 3.7%, expected volatility from 70% to 75%, and 0% expected dividend yield.
In 2008, in connection with an operating lease, the Company issued a warrant to purchase 2,009 shares of the Company’s Series B-1 convertible preferred stock at an exercise price of $25.26 per share. The warrant is exercisable immediately and expires upon the earlier of (i) ten years from the effective date; (ii) an underwritten public offering; or (iii) upon a change of control. The Company estimated the initial fair value of the warrant as of the date of issuance to be $40,000 and was recorded as rent expense. The fair value was based on the contractual term of the warrants of ten years, risk-free interest rate of 4.0%, expected volatility of 70% and 0% expected dividend yield.
In 2009, in connection with the Company’s issuance of Series B-1 convertible preferred stock, the Company issued a warrant to purchase 3,843 shares of the Company’s Series B-1 convertible preferred stock at an exercise
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
price of $25.26 to the placement agent. The warrants are exercisable immediately and expire seven years from the effective date. The Company estimated the fair value of the warrant as of the date of issuance to be $68,000 and was recorded as a reduction to the carrying value of the Series B-1 convertible preferred stock. The fair value was based on the contractual term of the warrants of seven years, risk-free interest rate of 2.3%, expected volatility of 90% and 0% expected dividend yield.
Series C
In September 2009, the Company cancelled the warrant to purchase 10,048 shares of the Company’s Series B convertible preferred stock and issued a warrant to purchase 16,075 shares of the Company’s Series C convertible preferred stock with an exercise price of $12.46 per share. In connection with a capital lease arrangement, the Company issued a warrant to purchase 8,026 shares of the Company’s Series C convertible preferred stock at an exercise price of $12.46 per share. The warrants are exercisable immediately and expire upon the earlier of (i) September 2019; (ii) one year from the effective date of a public offering; or (iii) a merger event. The Company estimated the initial fair value of warrants as of the date of issuance to be $269,000 and was recorded as other assets and amortized over the capital lease draw-down period. The fair value was based on the contractual term of the warrants of ten years, risk-free interest rate of 3.3%, expected volatility of 97%, and 0% expected dividend yield.
In January 2010, in connection with the Company’s issuance of Series C convertible preferred stock, the Company issued a warrant to purchase 49,157 shares of the Company’s Series C convertible preferred stock at an exercise price of $12.46 per share to the placement agent. The warrant is exercisable immediately and expires seven years from the effective date. The Company estimated the fair value of the warrant as of the date of issuance to be $507,000 which was recorded as a reduction to the carrying value of the Series C convertible preferred stock. The fair value was based on the contractual term of the warrant of seven years, risk-free interest rate of 3.39%, expected volatility of 98% and 0% expected dividend yield.
The change in the fair value of convertible preferred stock warrants resulted in a charge to other expense in the amount of $114,000 and $445,000 during the years ended December 31, 2008 and 2009. For the six months ended June 30, 2009 and 2010, the Company recorded a gain of $333,000 (unaudited) and a loss of $34,000 (unaudited), respectively as an offset to other expense to reflect the change in the fair value of the warrants. The Company determined the fair value of the warrants as of December 31, 2008 and 2009 and June 30, 2009 and 2010 using the Black-Scholes option pricing model with the following assumptions:
| | | | | | | | |
| | | December 31, | | June 30, |
| | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | (Unaudited) |
Expected dividend yield | | 0% | | 0% | | 0% | | 0% |
Risk-free interest rate | | 1.6%-2.3% | | 1.7%-3.9% | | 1.15%-3.53% | | 1.0-3.84% |
Contractual term (in years) | | 4-9.5 | | 3-9.8 | | 3.5-9.25 | | 2.5-9.5 |
Expected volatility | | 80% | | 98%-111% | | 90%-105% | | 97%-113% |
Each of these warrants includes a cashless exercise provision which permits the holder of the warrant to elect to exercise the warrant without paying the cash exercise price, and receive a number of shares determined by multiplying (i) the number of shares for which the warrant is being exercised by (ii) the difference between the fair market value of the stock on the date of exercise and the warrant exercise price, and dividing such by (iii) the fair market value of the stock on the date of exercise. As of December 31, 2009 and June 30, 2010, these warrants were outstanding and exercisable.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
11. Common Stock
The Articles of Incorporation authorize the Company to issue 33,000,000 and 70,000,000 (unaudited) shares of common stock as of December 31, 2009 and June 30, 2010, respectively. Holders of the Company’s common stock are entitled to dividends as and when declared by the Board of Directors, subject to the rights of holders of all classes of stock outstanding having priority rights as to dividends. There have been no dividends declared to date. The holder of each share of common stock is entitled to one vote.
12. Restricted stock
Pursuant to restricted stock agreements with the Company’s founders, the Company has the right, but not the obligation, to repurchase all or any portion of the unvested shares of common stock upon termination of employment at the original purchase price per share. The repurchase rights with respect to founders stock lapse over the vesting period, which ranges from 68 to 100 months. As of December 31, 2008 and 2009 and June 30, 2010, 267,711, 52,084 and 0 (unaudited) restricted shares of common stock held by the Company’s founders were subject to repurchase by the Company.
13. Stock Based Compensation
Employee Equity Plan
In 2005, the Company established its 2005 Stock Option Plan (the “Plan”) which provides for the granting of common stock options, restricted stock units, restricted stock and stock purchase rights awards to employees and consultants of the Company. The Plan allows for time-based or performance-based vesting for the awards. Options granted under the Plan may be either incentive stock options (“ISOs”) or nonqualified stock options (“NSOs”). ISOs may be granted only to Company employees (including officers and directors who are also employees). NSOs may be granted to Company employees and consultants.
Options under the Plan may be granted for periods of up to ten years. All options issued to date have had a ten year life. The exercise price of an ISO and NSO shall not be less than 100% of the estimated fair value of the shares on the date of grant, as determined by the Board of Directors. The exercise price of an ISO and NSO granted to a 10% stockholder shall not be less than 110% of the estimated fair value of the underlying stock on the date of grant as determined by the Board of Directors. The Company’s options generally vest over four to five years.
In December 2009, the Company issued 10,000 shares of restricted stock to an employee with performance-based vesting conditions. The performance based vesting conditions require the achievement of certain operational performance criteria as a condition of vesting for such award within six months following the date of grant. As of December 31, 2009, the Company assessed the probability of achieving the performance conditions and determined it was not probable that the performance condition will be satisfied. No compensation cost was recorded during the year ended December 31, 2009 or the six months ended June 30, 2010 (unaudited).On April 30, 2010, these shares of restricted stock were cancelled.
No income tax benefit has been recognized relating to stock-based compensation expense and no tax benefits have been realized from exercised stock options.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Stock Option Activity
The Company’s stock option, restricted stock units and restricted stock grant activity and related information for the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010 was as follows:
| | | | | | | | | | | | | | |
| | | Shares
Available
for Grant | | | Number
of Stock
Options
Outstanding | | | Weighted-
Average
Exercise
Price | | Weighted-
Average
Remaining
Contractual
Life (Years) | | Aggregate
Intrinsic
Value |
| | | | | | | | | | | | | (in thousands) |
Outstanding—January 1, 2007 | | 386,800 | | | 247,200 | | | $ | 0.22 | | 6.96 | | $ | — |
| | | | | |
Additional shares authorized | | 3,662,700 | | | — | | | | — | | | | | |
Options granted | | (2,792,060 | ) | | 2,792,060 | | | | 1.65 | | | | | |
Options exercised | | — | | | (361,723 | ) | | | 0.60 | | | | | |
Options cancelled | | 48,627 | | | (48,627 | ) | | | 0.20 | | | | | |
Shares repurchased | | 20,625 | | | — | | | | 0.10 | | | | | |
| | | | | | | | | | | | | | |
Outstanding—December 31, 2007 | | 1,326,692 | | | 2,628,910 | | | | 1.69 | | 8.81 | | | 5,891 |
| | | | | |
Additional shares authorized | | 1,390,000 | | | — | | | | | | | | | |
Options granted | | (1,289,549 | ) | | 1,289,549 | | | | 3.93 | | | | | |
Restricted stock units granted | | (50,000 | ) | | — | | | | — | | | | | |
Options exercised | | — | | | (172,059 | ) | | | 3.42 | | | | | |
Options cancelled | | 118,231 | | | (118,231 | ) | | | 2.37 | | | | | |
Shares repurchased | | 1,498 | | | — | | | | 1.16 | | | | | |
| | | | | | | | | | | | | | |
Outstanding—December 31, 2008 | | 1,496,872 | | | 3,628,169 | | | | 2.38 | | 8.22 | | | 21,764 |
| | | | | |
Additional shares authorized | | — | | | — | | | | — | | | | | |
Options granted | | (1,109,553 | ) | | 1,109,553 | | | | 4.31 | | | | | |
Restricted stock granted | | (10,000 | ) | | — | | | | — | | | | | |
Options exercised | | — | | | (117,515 | ) | | | 1.04 | | | | | |
Options cancelled | | 173,313 | | | (173,313 | ) | | | 2.98 | | | | | |
Shares repurchased | | 28,886 | | | — | | | | 0.32 | | | | | |
| | | | | | | | | | | | | | |
Outstanding—December 31, 2009 | | 579,518 | | | 4,446,894 | | | | 2.87 | | 8.19 | | | 28,661 |
| | | | | |
Additional shares authorized (unaudited) | | 1,526,272 | | | — | | | | | | | | | |
Options granted (unaudited) | | (1,924,764 | ) | | 1,924,764 | | | | 12.07 | | | | | |
Restricted stock units granted (unaudited) | | (126,272 | ) | | — | | | | — | | | | | |
Options exercised (unaudited) | | — | | | (22,707 | ) | | | 3.10 | | | | | |
Options cancelled (unaudited) | | 73,221 | | | (73,221 | ) | | | 5.62 | | | | | |
Shares repurchased (unaudited) | | 367 | | | — | | | | 0.01 | | | | | |
Restricted stock cancelled (unaudited) | | 10,000 | | | — | | | | — | | | | | |
| | | | | | | | | | | | | | |
Outstanding—June 30, 2010 (unaudited) | | 138,342 | | | 6,275,730
|
| | $ | 5.90 | | 8.25 | | $ | 93,276 |
| | | | | | | | | | | | | | |
Vested and expected to vest—June 30, 2010 (unaudited) | | | | | 6,023,810 | | | $ | 5.77 | | 8.22 | | $ | 90,310 |
Exercisable—June 30, 2010 (unaudited) | | | | | 2,434,383 | | | $ | 2.87 | | 7.47 | | $ | 43,561 |
The aggregate intrinsic value of options exercised under the Plan was $73,000, $26,000 and $308,000 for the years ended December 31, 2007, 2008 and 2009 and $332,000 for the six months ended June 30, 2010 (unaudited), determined as of the date of option exercise.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following table summarizes information about stock options outstanding as of December 31, 2009:
| | | | | | |
| | | Options Outstanding | | Options Exercisable |
Exercise Price | | Number of Options | | Weighted-Average
Remaining
Contractual Life
(Years) | | Number of Options |
$0.10 | | 35,500 | | 5.95 | | 34,374 |
$0.20 | | 1,200 | | 6.33 | | 1,200 |
$0.28 | | 1,180,400 | | 7.10 | | 706,686 |
$1.50 | | 273,660 | | 7.57 | | 186,633 |
$3.93 | | 1,846,591 | | 8.11 | | 760,537 |
$4.31 | | 1,109,543 | | 9.69 | | 208,353 |
| | | | | | |
| | 4,446,894 | | 8.19 | | 1,897,783 |
| | | | | | |
The following table summarizes information about stock options outstanding as of June 30, 2010 (unaudited):
| | | | | | |
| | | Options Outstanding | | Options Exercisable |
Exercise Price | | Number of Options | | Weighted-Average
Remaining
Contractual Life
(Years) | | Number of Options |
$0.10 | | 32,500 | | 5.47 | | 32,500 |
$0.20 | | 1,200 | | 5.84 | | 1,200 |
$0.28 | | 1,171,700 | | 6.60 | | 825,676 |
$1.50 | | 273,660 | | 7.08 | | 204,559 |
$3.93 | | 1,840,471 | | 7.63 | | 934,562 |
$4.31 | | 1,057,481 | | 9.14 | | 312,411 |
$9.32 | | 1,161,039 | | 9.47 | | 86,109 |
$14.28 | | 228,700 | | 9.72 | | 24,416 |
$20.41 | | 508,979 | | 9.81 | | 12,950 |
| | | | | | |
| | 6,275,730 | | 8.25 | | 2,434,383 |
| | | | | | |
At December 31, 2008, 930,152 options were vested and exercisable under the plan with a weighted average exercise price of $1.27 per share.
Common Stock Subject to Repurchase
Historically, the Company allowed employees to exercise options prior to vesting. The Company has the right to repurchase at the original purchase price any unvested (but issued) common shares upon termination of service of an employee. The consideration received for an exercise of an option is considered to be a deposit of the exercise price and the related dollar amount is recorded as a liability. The shares and liability are reclassified into equity on a ratable basis as the award vests. The Company recorded a liability in accrued expenses of $262,000, $101,000 and $82,000 (unaudited) relating to 237,324, 69,954 and 47,695 (unaudited) options that were exercised and unvested as of December 31, 2008 and 2009 and June 30, 2010, respectively. These shares were subject to a repurchase right held by the Company and are included in issued and outstanding shares as of December 31, 2008 and 2009 and June 30, 2010, respectively.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Stock-Based Compensation
Stock-based compensation expense related to options and restricted stock units granted to employees and nonemployees was allocated to research and development expense and sales, general and administrative expense as follows (in thousands):
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
Research and development | | $ | 117 | | $ | 632 | | $ | 773 | | $ | 276 | | $ | 876 |
Sales, general and administrative | | | 429 | | | 1,395 | | | 2,526 | | | 901 | | | 3,426 |
| | | | | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 546 | | $ | 2,027 | | $ | 3,299 | | $ | 1,177 | | $ | 4,302 |
| | | | | | | | | | | | | | | |
Employee Stock–Based Compensation
During the years ended December 31, 2007, 2008 and 2009, the Company granted 2,531,360, 1,289,549 and 1,089,053 stock options to employees with a weighted average grant date fair value of $1.65, $5.83 and $4.31 per share and during the six months ended June 30, 2010 the Company granted 1,889,764 options to employees with a weighted average grant date fair value of $12.86 (unaudited). As of December 31, 2009 and June 30, 2010, there was unrecognized compensation costs of $6.7 million and $23.8 million (unaudited) related to these stock options. The Company expects to recognize those costs over a weighted average period of 3.4 years as of June 30, 2010. Future option grants will increase the amount of compensation expense to be recorded in these periods.
Compensation expense was recorded for stock-based awards granted to employees based on the grant date estimated fair value (in thousands):
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
Research and development | | $ | 38 | | $ | 501 | | $ | 765 | | $ | 273 | | $ | 818 |
Sales general and administrative | | | 268 | | | 849 | | | 1,822 | | | 659 | | | 1,998 |
| | | | | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 306 | | $ | 1,350 | | $ | 2,587 | | $ | 932 | | $ | 2,816 |
| | | | | | | | | | | | | | | |
The Company sells ethanol procured from third parties and relies on contracted third parties for the transportation and storage of products. Accordingly, the Company does not have any dedicated headcount included in cost of product sales. The six employees of Amyris Fuels are involved in sales, general and administrative functions and their entire compensation including stock compensation expense is recorded in sales, general and administrative expenses.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The Company estimated the fair value of employee stock options using the Black-Scholes option pricing model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards. The fair value of employee stock options was estimated using the following weighted-average assumptions:
| | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
Expected dividend yield | | 0% | | 0% | | 0% | | * | | 0% |
Risk-free interest rate | | 3.9%-4.7% | | 3.2% | | 2.8% | | * | | 2.8%-2.91% |
Expected term (in years) | | 6.0 | | 6.0 | | 6.0 | | * | | 6.0 |
Expected volatility | | 70% | | 70% | | 97% | | * | | 98% |
* | | There were no stock option grants during the six months ended June 30, 2009. |
Expected Dividend Yield—The Company has never paid dividends and does not expect to pay dividends.
Risk-Free Interest Rate—The risk-free interest rate is based on the United States Treasury yield curve in effect at the time of grant for zero coupon United States Treasury notes with maturities approximately equal to the option’s expected term.
Expected Term—Expected term represents the period that the Company’s stock-based awards are expected to be outstanding. The Company’s assumptions about the expected term have been based on that of companies that have similar industry, life cycle, revenue and market capitalization.
Expected Volatility—The expected volatility was based on the historical stock volatilities of several of the Company’s publicly listed comparable companies over a period equal to the expected terms of the options, as the Company does not have any trading history to use the volatility of its own common stock.
Fair Value of Common Stock—The fair value of the shares of common stock underlying the stock options has historically been determined by the Board of Directors. Because there has been no public market for the Company’s common stock, the Board of Directors has determined fair value of the common stock at the time of grant of the option by considering a number of objective and subjective factors including valuation of comparable companies, sales of convertible preferred stock to unrelated third parties, operating and financial performance, the lack of liquidity of capital stock and general and industry specific economic outlook, amongst other factors. The fair value of the underlying common stock shall be determined by the Board of Directors until such time as the Company’s common stock is listed on an established stock exchange or national market system.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Information regarding the Company’s stock option grants during the year ended December 31, 2009 and the six months ended June 30, 2010, including the grant date; the number of stock options issued with each grant; the exercise price, which equals the grant date fair value of the underlying common stock for each grant of stock options, is summarized as follows:
| | | | | | |
Grant Date | | Number of Options
Granted | | Exercise Price and Fair
Value of Underlying
Common Stock | |
September 14, 2009 | | 965,153 | | $ | 4.31 | |
October 27, 2009 | | 144,400 | | $ | 4.31 | |
January 7, 2010 | | 1,178,810 | | $ | 9.32 | |
March 19, 2010 | | 236,500 | | $ | 14.28 | |
April 20, 2010 | | 509,454 | | $ | 20.41 | * |
| * | | Underlying fair value was $20.40 on the date of grant |
Forfeiture Rate—The Company estimates its forfeiture rate based on an analysis of its actual forfeitures and will continue to evaluate the adequacy of the forfeiture rate based on actual forfeiture experience, analysis of employee turnover behavior, and other factors. The impact from a forfeiture rate adjustment will be recognized in full in the period of adjustment, and if the actual number of future forfeitures differs from that estimated by the Company, the Company may be required to record adjustments to stock-based compensation expense in future periods.
Each of the inputs discussed above is subjective and generally requires significant management and director judgment to determine.
Nonemployee Stock–Based Compensation
During the years ended December 31, 2007, 2008 and 2009, the Company granted options to purchase 260,700, 0 and 20,500 shares of common stock to nonemployees in exchange for services. During the six months ended June 30, 2010, the Company granted 35,000 options to purchase common stock to nonemployees. Compensation expense of $240,000, $636,000 and $238,000 was recorded during the years ended December 31, 2007, 2008 and 2009 for stock-based awards granted to nonemployees. Compensation expense of $201,000 (unaudited) and $190,000 (unaudited) was recorded for the six months ended June 30, 2009 and 2010. The nonemployee options were valued using the Black-Scholes option pricing model with the following weighted-average assumptions:
| | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
Expected dividend yield | | 0% | | 0% | | 0% | | 0% | | 0% |
Risk-free interest rate | | 3.9%-4.8% | | 1.52%-4.1% | | 1.8%-3.6% | | 1.82%-3.72% | | 3.42%-3.85% |
Contractual term (in years) | | 9.7 | | 7.0-9.2 | | 6.0-10.0 | | 6.0-9.0 | | 7.2-10.0 |
Expected volatility | | 70% | | 70%-75% | | 90%-98% | | 90%-94% | | 98% |
During 2008, the Company entered into a related party transaction with a venture capital group to provide strategic advisory services to Amyris and its majority owned subsidiary Amyris Brasil. One of its directors is also a member of the Company’s Board of Directors. As compensation for the advisory services to be rendered, the Company granted 50,000 restricted stock units with a grant date fair value of $8.38 during the year ended December 31, 2008. No additional restricted stock units were granted during the year ended December 31, 2009.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
In the three months ended March 31, 2010, 126,272 restricted stock units were granted to the same related party. The restricted stock units vest quarterly and become fully vested in July 2010. Compensation expense of $41,000 and $466,000 was recorded for the years ended December 31, 2008 and 2009. Compensation expense of $44,000 (unaudited) and $1,296,000 (unaudited) was recorded for the six months ended June 30, 2009 and 2010, respectively.
Shares Reserved for Future Issuance
As of December 31, 2009 and June 30, 2010 the Company had reserved shares of common stock for issuance as follows:
| | | | |
| | | December 31, 2009 | | June 30, 2010 |
| | | | | (Unaudited) |
Issuance of stock options | | 5,026,412 | | 6,414,072 |
Restricted stock units | | 50,000 | | 31,568 |
Conversion of convertible preferred stock* | | 18,878,526 | | 29,000,821 |
Issuance upon exercise of convertible preferred stock warrants | | 146,447 | | 195,604 |
| | | | |
| | 24,101,385 | | 35,642,065 |
| | | | |
| * | | The convertible preferred stock and convertible preferred stock warrants were computed on an as converted basis using the conversion ratios in effect as of June 30, 2010 for all periods presented. See Note 9 for conversion ratios. |
14. Employee Benefit Plan
The Company established a 401(k) Plan to provide tax deferred salary deductions for all eligible employees. Participants may make voluntary contributions to the 401(k) Plan up to 90% of their eligible compensation, limited by certain Internal Revenue Service restrictions. The Company does not match employee contributions.
15. Related Party Transactions
The Company has entered into a license agreement with University of California, Berkeley. A co-founder and advisor to the Company is a professor at the University of California, Berkeley. The Company paid the advisor $15,000, $30,000, $34,000 and $15,000 during the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010, respectively.
Crystalsev Comércio E Representação Ltda. (“Crystalsev”) was issued 40,193 shares of Series B convertible preferred stock by contributing $1.0 million during the Series B convertible preferred stock financing in March 2008 (see Note 16).
During 2008, the Company entered into an agreement with a venture capital group to provide strategic advisory services to Amyris and its majority owned subsidiary Amyris Brasil. One of its directors is also a member of the Company’s Board of Directors (see Note 13).
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
16. Noncontrolling Interest
Redeemable Noncontrolling Interest
In February 2008, the Company formed a subsidiary Amyris Pesquisa e Desenvolvimento de Biocombustíveis, Ltda. In March 2008, the Company sold a 30% interest to Crystalsev and the subsidiary was renamed Amyris-Crystalsev Pesquisa e Desenvolvimento de Biocombustíveis Ltda. (“ACB”). The Company invested $3.8 million of cash for 70% interest in ACB and Crystalsev contributed $1.6 million of cash for the remaining 30% interest.
In April 2009, the Company re-purchased Crystalsev’s 30% interest in ACB for $2.3 million resulting in ACB once again becoming a wholly-owned subsidiary. The purchase of the noncontrolling interest was treated as an equity transaction and the fair value of the consideration paid of $2.3 million was recorded as a reduction of the carrying value of the noncontrolling interest and additional paid-in capital. In December 2009, ACB was renamed Amyris Brasil S.A.
In December 2009, Amyris Brasil sold 1,111,111 shares of Amyris Brasil for a 4.8% interest in Amyris Brasil for BRL$10.0 million. The redeemable noncontrolling interest is reported in the mezzanine equity section of the consolidated balance sheet because the Company is subject to a contingent put option under which it may be required to repurchase an interest in Amyris Brasil from the noncontrolling interest holder. The Company has recognized a noncontrolling interest for December 22, 2009 to December 31, 2009 in the consolidated balance sheets and statements of operations. The Company has also recognized a receivable of $2.5 million for which payment was received in January 2010.
In March 2010, Amyris Brasil sold an additional 853,333 shares of its stock, an incremental 3.4% interest, for BRL $3.0 million (unaudited). In May 2010, Amyris Basil sold an additional 1,111,111 shares of its stock, an incremental 4.07% interest, for BRL$10.0 million (unaudited).
Under the terms of the agreements with these Amyris Brasil Investors, the Company has the right to require the investors to convert their shares of Amyris Brasil into shares of common stock at a 1:0.28 conversion ratio. The Company intends to exercise this right prior to the completion of this offering, and as a result will issue 861,155 shares of their common stock to these investors.
Under the terms of the agreements, the Company is required to make additional capital investments in Amyris Brasil worth at least BRL $50.0 million. The Company also entered into a contingent put option with the Investors in the event that Amyris Brasil receives a binding offer from one or more third parties willing to acquire control of Amyris Brasil through a change of control event that will entitle the Investors to sell their shares to the Company. Under the terms of the put option, the Investors will have the right to receive from the Company its pro rate share of the total put option price. The put option price is defined as being the lower of (i) the amount invested by the Investors in Amyris Brasil plus 8% per year; and (ii) the total amount of proceeds actually received by the Company from a third party acquirer or from Amyris Brasil, in connection with a change of control event relating to the Company.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following table provides a rollforward of the redeemable noncontrolling interest recorded as mezzanine equity:
| | | | |
Beginning balance as of December 31, 2008 | | $ | — | |
Proceeds from redeemable noncontrolling interest | | | 5,626 | |
Net loss | | | (120 | ) |
| | | | |
Ending balance as of December 31, 2009 | | | 5,506 | |
Proceeds from redeemable noncontrolling interest (unaudited) | | | 7,041 | |
Foreign currency translation adjustment (unaudited) | | | 121 | |
Net loss (unaudited) | | | (420 | ) |
| | | | |
Ending balance as of June 30, 2010 (unaudited) | | $ | 12,248 | |
| | | | |
Noncontrolling interest (unaudited)
On April 14, 2010, the Company entered into a joint venture with Usina São Martinho to build the facility in Brazil dedicated to the production of Amyris renewable products. The joint venture, SMA Indústria Química S.A., (“SMA”) is owned 50% by Amyris and 50% by Usina São Martinho and is located in Pradópolis, São Paulo state (see Note 19). SMA is a VIE pursuant to the accounting guidance for consolidating VIE because the amount of total equity investment at risk is not sufficient to permit SMA to finance its activities without additional subordinated financial support, as well as because commercialization agreement provides a substantive minimum price guarantee. Under the terms of the joint venture agreement, Amyris directs the design and construction activities, as well as production and distribution. In addition, Amyris has the obligation to fund the design and construction activities until commercialization is achieved. Subsequent to the construction phase, both parties equally fund SMA for the term of the joint venture. Based on those factors, the Company was determined to have the power to direct the activities that most significantly impact SMA’s economic performance and the obligation to absorb losses and the right to receive benefits. Accordingly, the financial results of SMA are included in the Company’s consolidated financial statements and amounts pertaining to Usina São Martinho’s interest in SMA are reported as noncontrolling interests in subsidiaries. The Company’s investment in SMA does not have a significant impact on consolidated its financial position, results of operations or cash flows as of June 30, 2010.
17. Restructuring
In June 2009, the Company initiated a restructuring plan to reduce its cost structure. The restructuring plan resulted in the consolidation of the Company’s headquarter facility located in Emeryville, California, which is under an operating lease. The Company ceased using a certain part of its headquarter facility in August 2009. The Company recorded approximately $5.4 million of restructuring charges associated with the facility lease costs after the operations ceased. In addition, as a result of the consolidation of the headquarter facility, the Company recorded approximately $3.1 million related to asset impairments and reversed $2.7 million related to deferred rent associated with the leased facility.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
The following table summarizes the liability and utilization by cost type associated with the restructuring (in thousands):
| | | | | | | | | | | | | | | | |
| | | Exit Costs | | | Asset
Impairments | | | Deferred Rent
Write-Off | | | Total | |
Accrued restructuring as of December 31, 2008 | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
Additional accruals | | | 5,412 | | | | 3,075 | | | | (2,719 | ) | | | 5,768 | |
Cash payments | | | (534 | ) | | | — | | | | — | | | | (534 | ) |
Accretion expense | | | 200 | | | | — | | | | — | | | | 200 | |
Non-cash settlements | | | — | | | | (3,075 | ) | | | 2,719 | | | | (356 | ) |
Accrued restructuring as of December 31, 2009 | | | 5,078 | | | | — | | | | — | | | | 5,078 | |
Cash payments (unaudited) | | | (643 | ) | | | | | | | | | | | (643 | ) |
Accretion expense (unaudited) | | | 284 | | | | | | | | | | | | 284 | |
| | | | | | | | | | | | | | | | |
Accrued restructuring as of June 30, 2010(unaudited) | | | 4,719 | | | | — | | | | — | | | | 4,719 | |
Less current portion (unaudited) | | | (384 | ) | | | | | | | | | | | (384 | ) |
| | | | | | | | | | | | | | | | |
Long-term portion as of June 30, 2010(unaudited) | | $ | 4,335 | | | $ | — | | | $ | — | | | $ | 4,335 | |
| | | | | | | | | | | | | | | | |
The restructuring accrual for facility-related leases (net of estimated sublease proceeds) will be paid over the respective facility lease term through May 2018.
18. Reporting Segments
The chief operating decision maker for the Company is the Chief Executive Officer. The Chief Executive Officer reviews financial information presented on a consolidated basis, accompanied by information about revenue by geographic region, for purposes of allocating resources and evaluating financial performance. The Company has one business activity and there are no segment managers who are held accountable for operations, operating results or plans for levels or components below the consolidated unit level. Accordingly, the Company has determined that it has a single reporting segment and operating unit structure.
Revenues by geography are based on the billing address of the customer. The following tables set forth revenue and long-lived assets by geographic area (in thousands):
Revenues
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, | | Six Months Ended
June 30, |
| | | 2007 | | 2008 | | 2009 | | 2009 | | 2010 |
| | | | | | | | | (Unaudited) |
United States | | $ | 6,184 | | $ | 13,892 | | $ | 64,608 | | $ | 22,894 | | $ | 26,357 |
| | | | | | | | | | | | | | | |
Total | | $ | 6,184 | | $ | 13,892 | | $ | 64,608 | | $ | 22,894 | | $ | 26,357 |
| | | | | | | | | | | | | | | |
Long-Lived Assets
| | | | | | | | | |
| | | December 31,
2008 | | December 31,
2009 | | June 30,
2010 |
| | | | | | | (Unaudited) |
United States | | $ | 39,995 | | $ | 34,868 | | $ | 34,845 |
Brazil | | | 1,570 | | | 7,692 | | | 9,397 |
| | | | | | | | | |
Total | | $ | 41,565 | | $ | 42,560 | | $ | 44,242 |
| | | | | | | | | |
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
19. Subsequent Events
In January 2010, the Company raised $3.7 million before issuance costs, by issuing 295,981 shares of Series C convertible preferred stock at $12.46 per share.
In March 2010, the Company raised $47.8 million before issuance costs, by issuing 2,724,766 shares of Series C-1 convertible preferred stock at $17.56 per share.
In March 2010, Amyris Brasil received an additional $1.7 million investment from an investor.
On April 13, 2010, the board of directors of the Company approved the filing of a registration statement with the Securities and Exchange Commission for an initial public offering of the Company’s common stock.
On April 14, 2010, the Company entered into a joint venture with Usina São Martinho,prior to produce Amyris renewable products. The joint venture, SMA Indústria Química S.A., was created to build the first facility in Brazil fully dedicated to the production of Amyris renewable products. The new company will be located at the Usina São Martinho mill in Pradópolis, São Paulo state. The joint venture has a 20 year initial term.
Amyris plans to provide genetically engineered yeast to enable the joint venture to produce farnesene, a molecule which may be used as an ingredient in a wide range of consumer and industrial products, including detergents, cosmetics, perfumes and industrial lubricants.
The joint venture will be governed by a four member board of directors, of which the Company and São Martinho will each appoint two members. A three member executive committee, of which we appoint two members one of which will be the plant manager who is the most senior executive, will be responsible for managing construction and operation of the facility.
Under the joint venture agreements, Amyris will grant a royalty-free license to the JV. Amyris will fund the construction costs of the new facility, which is estimated to total between $80 million to $100 million, of which São Martinho will reimburse up to 61.8 million Brazilian reais (approximately $36 million (unaudited), based on a September 3, 2010 exchange rate) after we commence production. Post commercialization, Amyris will market and distribute the Amyris renewable products. São Martinho will sell feedstock and provide certain other services to the joint venture. The cost of the feedstock to the joint venture is a price that is based on the average return that Usina São Martinho could receive from the production of its current products, sugar and ethanol. We are required to purchase the output of the joint venture for the first four years at a price that guarantees the return of Usina São Martinho’s investment plus a fixed interest rate. After this four year period, the price is set to guarantee a break-even price to the joint venture plus an agreed upon return.
Amyris will have 50% ownership in the JV. We have identified the JV as a VIE. We expect to be the Primary Beneficiary and consequently intend to consolidate the joint venture’s operations in our financial statements.
Reincorporation in Delaware
On June 10, 2010, the Company reincorporated in Delaware and, in connection therewith, increased its authorized number of shares of common and preferred stock to 61,862,355 and 23,862,355, respectively, and established the par value of each share of common and preferred stock to be $0.0001. In connection with the reincorporation, common stock and additional paid-in capital amounts in these financial statements have been adjusted to reflect the par value of common stock shares. All share information included in these financial statements has been adjusted to reflect this reincorporation.
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
Unaudited
In April 2010, the Company filed a registration statement with the Securities and Exchange Commission for a proposed initial public offering.
In June 2010, the Company entered into a term sheet with Cosan S.A. for the formation of a joint venture to develop and commercialize farnesene-based specialty chemicals for industrial and automotive applications. Subject to the joint venture meeting certain milestones, the rights granted by Amyris for these products would be exclusive to the joint venture.
In June 2010, the Company entered into a collaboration agreement with M&G Finanziaria S.R.L. Under the terms of the collaboration agreement each party bears its own costs incurred for the collaboration milestones. The agreement also establishes the terms under which M&G may purchase farnesene from the Company for use in M&G’s polyethylene terephthalate, or PET, resins to be incorporated into containers for food, beverages and other products.
In June 2010, the Company entered into a supply agreement with The Procter & Gamble Company that establishes terms under which P&G may purchase farnesene from the Company for use in P&G’s products. The terms of the agreement call for non-refundable development fees payable to the Company in addition to payments for purchase of farnesene. At this time, P&G does not have an obligation to purchase a specified quantity.
In June 2010, the Company entered into an agreement with Shell Western Supply and Trading Limited (“Shell), a subsidiary of Royal Dutch Shell plc, that contemplates the Company’s sale of certain minimum quantities of Company diesel fuel to Shell, commencing 18 months after the Company provides notice of election to sell under this agreement, and running for two years after the date specified in such notice, up to the end of March 2016 at the latest, with an option to renew for a further year. At this time, Shell does not have an obligation to purchase a specific quantity, or any, product under this agreement, and the Company is not obligated to sell specific quantities to Shell, but the parties will become subject to obligations to purchase and sell to the extent that formal notices and orders are submitted under the agreement in the future.
In June 2010, the Company entered into an agreement with Soliance for the development and commercialization of farnesene-based squalane for use as an ingredient in cosmetics products. The parties anticipate the formation of a joint venture for the production of squalane and the development of squalane formulations for these products, with Soliance to act as the distributor. Initial production of farnesene for conversion to squalane is expected to be completed at a Soliance facility in France, with production of squalane to commence as early as the second half of 2010, dependent upon the parties’ determination that production efficiency goals can be achieved at full scale production.
In June 2010, the Company entered into a joint manufacturing agreement with Biomin to utilize a portion of its Brazilian manufacturing facilities to produce our products commencing in 2011. The joint manufacturing agreement requires the acquisition of certain equipment to be used exclusively for the manufacturing of the product. Under the terms of the agreement Amyris will procure and contract the engineering activities and the necessary equipment for the manufacturing of our products.
In June 2010, the Company board of directors approved the 2010 Equity Incentive Plan, which will become effective upon the completion of the initial public offering of their common stock. A total of 4,200,000 shares of common stock were initially reserved for future issuance under the 2010 Equity Incentive Award Plan, and any shares of common stock reserved for future grant or issuance under our 2005 Stock Option/Stock Issuance Plan
Amyris, Inc.
Notes to Consolidated Financial Statements—(Continued)
but which remain unissued at the time of the effectiveness of the registration statementstatement. We hereby incorporate by reference the following documents (other than the portions of those documents furnished and not filed in which these financial statements are includedaccordance with Commission rules):
| • | | Our Annual Report onForm10-K for the fiscal year ended December 31, 2018, filed with the Commission on October 1, 2019, as amended by Amendment No. 1 onForm10-K/A filed with the Commission on October 4, 2019; |
| • | | Our Definitive Proxy Statement onSchedule 14A, filed with the Commission on October 10, 2019; |
| • | | Our Quarterly Reports on Form10-Q for the quarters ended March 31, 2019, June 30, 2019 and September 30, 2019, filed with the Commission onOctober 7, 2019,October 7, 2019 andNovember 12, 2019, respectively; |
| • | | Our Current Reports on Form8-K filed with the Commission onJanuary 2, 2019,January 7, 2019,February 22, 2019,March 18, 2019 (solely with respect to Item 1.02),April 9, 2019 (solely with respect to Item 3.01),April 10, 2019,April 11, 2019 (solely with respect to Items 1.01, 2.03 and 4.02),April 16, 2019,April 17, 2019,April 19, 2019,April 30, 2019,May 14, 2019,May 16, 2019 (four filings),May 17, 2019,May 20, 2019,June 4, 2019 (solely with respect to Item 5.02),June 17, 2019,June 20, 2019,June 28, 2019,July 9, 2019,July 11, 2019,July 15, 2019 (solely with respect to Items 1.01 and 2.03),July 19, 2019,July 24, 2019,July 25, 2019,July 29, 2019,August 1, 2019,August 2, 2019,August 14, 2019,August 20, 2019,August 27, 2019,September 4, 2019,September, 11, 2019,September 23, 2019,October 7, 2019 (solely with respect to Item 3.01),October 16, 2019,October 30, 2019,November 1, 2019,November 6, 2019 andNovember 12, 2019; and |
| • | | The description of our common stock contained in our registration statement onForm8-A filed with the Commission on September 24, 2010, including any amendment or report filed for the purpose of updating such description. |
We will be addedprovide to each person, including any beneficial holder, to whom a prospectus is delivered, at no cost to the sharesrequestor, upon written or oral request, a copy of any or all information that has been incorporated by reference in this prospectus but not delivered with this prospectus, including any exhibits that are specifically incorporated by reference in that information. You may request a copy of these filings by sending ane-mail request to be reserved under our 2010 Equity Incentive Plan.Amyris Investor Relations at investor@amyris.com, calling (510)740-7481, or writing to Amyris Investor Relations at 5885 Hollis Street, Suite 100, Emeryville, California 94608.
In June 2010,Copies of these filings are also available free of charge through a link on the Company board of directors approved the 2010 Employee Stock Purchase Plan which will become effective upon the completion of the initial public offeringInvestors section of our common stock. A total of 168,627 shares of common stock were initially reserved for future issuance under the 2010 Employee Stock Purchase Plan.
In July 2010, the Company entered into a First Amendmentwebsite located at www.amyris.com (under “Financial Information — SEC Filings”) as soon as reasonably practicable after they are filed with or furnished to the Investment Agreement dated December 22, 2009 between the CompanyCommission. The information contained in or accessible through our website or contained on other websites is not a part of, and entities affiliated with the Stratus Group. Under the terms of the amendment, Amyris, Inc. reaffirmed its requirement to invest the amounts that were contemplated in the original agreement and added a requirement that such contributions are to be made before December 31, 2010 (see Note 16).is not incorporated into, this prospectus.