
As filed with the Securities and Exchange Commission on July 3, 2013January 10, 2024.
Registration No. 333-188838333-275443
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 4 to
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
EVOKE PHARMA, INC.
(Exact name of registrant as specified in its charter)
Delaware | 2834 | 20-8447886 | ||
(State or other jurisdiction of
| (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
12555 High Bluff Drive,
420 Stevens Avenue, Suite 385230
San Diego,Solana Beach, CA 9213092075
(760) 487-1255Telephone: (858) 345-1494
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
David A. Gonyer, R.Ph.
President and Chief Executive Officer and Director
Evoke Pharma, Inc.
12555 High Bluff Drive,420 Stevens Avenue, Suite 385230
San Diego,Solana Beach, CA 9213092075
(760) 487-1255Telephone: (858) 345-1494
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| ||||
Matthew T. Bush Cheston J. Larson
Latham
San Diego, CA 92130 (858) 523-5400 |
|
New York, (212) | ||
Approximate date of commencement of proposed sale to the public:As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this formForm are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨☒
If this formForm is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
If this formForm is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
If this formForm is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”filer,” “smaller reporting company,” and “smaller reporting“emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| ||||
Title of Each Class of Securities To Be Registered | Proposed Maximum Aggregate | Amount of Registration Fee(2) | ||
Common Stock, $0.0001 par value per share(3) | $23,000,000 | $3,137.20 | ||
Representative’s Warrant to Purchase Common Stock(4) | — | — | ||
Common Stock Underlying Representative’s Warrant(3)(5) | 920,000 | 125.49 | ||
Total | $23,920,000 | $3,262.69(6) | ||
| ||||
| ||||
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities or the solicitation ofand it is not soliciting an offer to buy these securities in any state in which suchjurisdiction where the offer solicitation or sale is not permitted.
PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED |
SharesUp to 8,333,000 Common Stock Units
Each Common Stock Unit Consisting of One Share of Common Stock
One Series A Warrant to Purchase One Share of Common Stock
One Series B Warrant to Purchase One Share of Common Stock
One Series C Warrant to Purchase One Share of Common Stock
Up to 24,999,000 Shares of Common Stock Underlying the Series A Warrants, Series B Warrants and Series C Warrants
and
Up to 8,333,000 PFW Units
Each PFW Unit Consisting of One Pre-Funded Warrant to Purchase One Share of Common Stock
One Series A Warrant to Purchase One Share of Common Stock
One Series B Warrant to Purchase One Share of Common Stock
One Series C Warrant to Purchase One Share of Common Stock
Up to 33,332,000 Shares of Common Stock Underlying the Pre-Funded Warrants, Series A Warrants, Series B Warrants and Series C Warrants

Evoke Pharma, Inc.

This isWe are also offering to each purchaser whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, up to 8,333,000 pre-funded warrant units (the “PFW Units” and together with the Common Stock Units, the “Units”), in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding common stock. Each PFW Unit consists of: (i): pre-funded warrants to purchase one share of our common stock (the “Pre-Funded Warrants”), (ii) a firm commitment initial public offeringSeries A Warrant to purchase one share of our common stock, (iii) a Series B Warrant to purchase one share of our common stock, and (iv) a Series C Warrant to purchase one share of our common stock. NoThe Common Warrants included in the PFW Units are identical to the Common Warrants included in the Common Stock Units. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%, 14.99%, or 19.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.0001 per share of common stock. The public market currently exists foroffering price per PFW Unit is equal to the public offering price per Common Stock Unit less $0.0001. Each Pre-Funded Warrant will be exercisable upon issuance and will expire when exercised in full. We are also offering the shares of our common stock. Westock that are issuable from time to time upon exercise of the Pre-Funded Warrants. For each PFW Unit we sell, the number of Common Stock Units we are offering allwill be decreased on a one-for-one basis.
Because a Series A Warrant, Series B Warrant and Series C Warrant are being sold together in this offering with each Common Stock Unit and, in the alternative, each PFW Unit, the number of Series A Warrants, Series B Warrants and Series C Warrants sold in this offering will not change as a result of a change in the mix of the Common Stock Units and PFW Units sold. The shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance; provided that a Series C Warrant may not be separated from the corresponding Series B Warrant until such Series B Warrant has been exercised.
Neither the Common Stock Units nor the PFW Units will be issued or certificated. The Common Stock Units, the PFW Units, the shares of common stock, offered by this prospectus. We expect the public offering price to be between $Common Warrants, the Pre-Funded Warrants and $ per share.
We have applied to list ourshares of common stock underlying the Common Warrants and Pre-Funded Warrants are sometimes collectively referred to herein as the “securities.”
Our common stock is listed on The NASDAQNasdaq Capital Market under the symbol “EVOK.”“EVOK”. The closing price of our common stock on January 9, 2024, as reported by The Nasdaq Capital Market, was $0.90 per share. There is no established public trading market for the Pre-Funded Warrants or the Common Warrants, and we do not expect a market to develop. We do not intend to apply for listing of the Pre-Funded Warrants or the Common Warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants and the Common Warrants will be limited.
The public offering price per Common Stock Unit and any PFW Units, and the accompanying Common Warrants will be determined by us at the time of pricing, may be at a discount to the current market price of our common stock, and the recent market price of our common stock used throughout this prospectus may not be indicative of the final public offering price of the Common Stock Units or PFW Units.
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus and the documents incorporated by reference into this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
We are an emerging growth companya “smaller reporting company” as that term is used in the Jumpstart Our Business Startups Act of 2012,defined under federal securities law and as such, we have elected to take advantage ofcomply with certain reduced public company reporting requirements for this prospectus and future filingsavailable to smaller reporting companies. See the section titled “Prospectus Summary — Implications of Being a Smaller Reporting Company.”
Before investing in our common stock, you should carefully read the discussion of “Risk Factors” beginning on page9.
Neither the Securities and Exchange Commission nor any other state securities commission has approved or disapproved of these securities or determined ifpassed upon the adequacy or accuracy of this prospectus is truthful or complete.prospectus. Any representation to the contrary is a criminal offense.
| Per Common | Per PFW | ||||||||||||
Stock Unit | Unit | Total | ||||||||||||
Public offering price | $ | $ | $ | |||||||||||
Underwriting discounts and commissions(1) | $ | $ | $ | |||||||||||
Proceeds to | $ | $ | ||||||||||||
$ |
We have granted a 30-day option
(1) In addition to the representativeunderwriting discount above, we have also agreed to pay the underwriters a cash fee of 5.0% of the underwritersaggregate gross proceeds received upon the exercise of the Series B Warrants. Does not include 0.75% management fee payable to purchase upthe representatives of the underwriters. See “Underwriting” beginning on page 49 of this prospectus for additional information regarding the compensation payable to additional shares of common stock solely to cover over-allotments, if any.the underwriters
The underwriters are offering the common stock as set forth under “Underwriting.” Delivery ofexpect to deliver the shares will be madeto purchasers on or about , 2013.2024.
Aegis Capital Corp
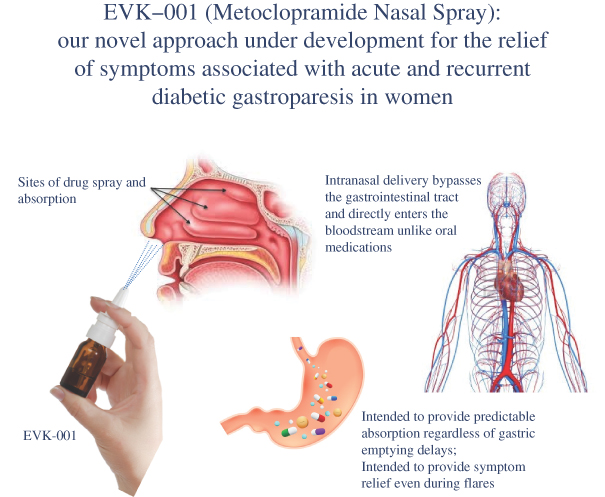
TABLE OF CONTENTSBook-Running Managers
Craig-Hallum | Laidlaw & Company (UK) Ltd. |
____________________________
The date of this prospectus is , 2024.
TABLE OF CONTENTS
Page | ||||
1 | ||||
9 | ||||
13 | ||||
14 | ||||
15 | ||||
16 | ||||
18 | ||||
| ||||
24 | ||||
| 40 | |||
Material U.S. Federal Income Tax Consequences | 42 | |||
49 | ||||
53 | ||||
54 | ||||
55 | ||||
| 56 | |||
____________________________
Neither we nor the underwriters have authorized anyone to provide you withany information that is different from thator to make any representations other than those contained in or incorporated by reference in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may authorizegive you. This prospectus is an offer to be deliveredsell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in or made available to you. When you make a decision about whether to invest in our common stock, you should not rely upon any information other than the informationincorporated by reference in this prospectus or in any applicable free writing prospectus that we may authorize to be deliveredis current only as of its date, regardless of its time of delivery or made available to you. Neither the delivery of this prospectus nor theany sale of our common stock meanssecurities. Our business, financial condition, results of operations and prospects may have changed since that date.
To the extent there is a conflict between the information contained in this prospectus, oron the one hand, and the information contained in any free writing prospectus is correct afterdocument incorporated by reference filed with the Securities and Exchange Commission (“SEC”) before the date of this prospectus, or such free writing prospectus. This prospectus is not an offer to sell oron the solicitation of an offer to buyother hand, you should rely on the shares of common stock in any circumstances under which the offer or solicitation is unlawful.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Special Note Regarding Forward-Looking Statements.”
We use our registered trademark, EVOKE PHARMA, in this prospectus. This prospectus also includes trademarks, tradenamesIf any statement in a document incorporated by reference is inconsistent with a statement in another document incorporated by reference having a later date, the statement in the document having the late date modifies or supersedes the earlier statement.
We have not and service marksthe underwriters have not taken any action that are the propertywould permit this offering or possession or distribution of other organizations. Solely for convenience, trademarks and tradenames referred to in this prospectus appear without the® and ™ symbols, but those references are not intended to indicate, in any way,jurisdiction where action for that we will not assert,purpose is required, other than in the United States. Persons who have come into possession of this prospectus in a jurisdiction outside the United States are required to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
We further note that the representations, warranties and covenants made by us in any agreement that is incorporated by reference or filed as an exhibit to the fullest extent under applicable law,registration statement of which this prospectus is a part were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our rights or that the applicable owner will not assert its rights, to these trademarks and tradenames.
i
PROSPECTUS SUMMARY
ThisThe following summary highlights information contained elsewhere in this prospectus.prospectus and in documents incorporated by reference. This summary doesis not complete and may not contain all of the information you should consider before investing in our common stock.securities. You should read this entire prospectus and the documents incorporated by reference in this prospectus carefully, especially the sectionrisks of investing in this prospectus entitledour securities discussed under the heading “Risk Factors” beginning on page 9factors,” and our financial statements and the related notes thereto appearing at the end ofincorporated by reference in this prospectus before making an investment decision. As used in this prospectus, unlessExcept as otherwise indicated herein or as the context otherwise requires, references in this prospectus and the documents incorporated by reference in this prospectus to “Evoke Pharma,” “Evoke,” “the Company,” “we,” “us,” “our,” “our company”“us” and “Evoke”“our” refer to Evoke Pharma, Inc.
Overview
Overview
We are a specialty pharmaceutical company focused primarily on the development and commercialization of drugs to treat gastrointestinal or GI,(“GI”) disorders and diseases. We areSince our inception, we have devoted our efforts to developing EVK-001, a metoclopramideour sole product, Gimoti® (metoclopramide) nasal spray, the first and only nasally-administered product indicated for the relief of symptoms associatedin adults with acute and recurrent diabetic gastroparesisgastroparesis. In June 2020, we received approval from the U.S. Food and Drug Administration (“FDA”) for our 505(b)(2) New Drug Application for Gimoti (the “Gimoti NDA”). We launched commercial sales of Gimoti in women with diabetes mellitus. the United States in October 2020 through our commercial partner, Eversana Life Science Services, LLC (“Eversana”).
Diabetic gastroparesis is a GI disorder afflictingaffecting millions of suffererspatients worldwide, in which thefood in an individual’s stomach takes too long to empty its contents resulting in serious digestive system symptoms. Metoclopramide is the only product currently approved in the United States to treat gastroparesis, and is currently available only in oral and intravenous forms. EVK-001 is a novel formulation of this drug, designed to provide systemic delivery of metoclopramide through intranasal administration.
Gastroparesis
Gastroparesis is a condition of delayed gastric emptying in the absence of mechanical obstruction. Gastroparesis results in food remaining in the stomach for a longer time than normal, yielding a variety of symptoms. serious GI symptoms and systemic metabolic complications. The gastric delay caused by gastroparesis can also compromise absorption of orally administered medications. In May 2023, we reported results from a study conducted by Eversana which showed diabetic gastroparesis patients taking Gimoti had significantly fewer physician office visits, emergency department visits, and inpatient hospitalizations compared to patients taking oral metoclopramide. This overall lower health resource utilization reduced patient and payor costs by approximately $15,000 during a six-month time period for patients taking Gimoti compared to patients taking oral metoclopramide.
Gastroparesis is a common problemfrequently occurs in individuals with diabetes, but is also is observed in patients with prior gastric surgery, a preceding infectious illness, pseudo-obstruction, collagen vascular disorders and anorexia nervosa. In some patients with gastroparesis, no cause can be identified, which is referred to as idiopathic gastroparesis. According to the American Motility Society Task Force on Gastroparesis, the prevalence of gastroparesis is estimated to be up to 4% of the United States population. SymptomsSigns and symptoms of gastroparesis may include nausea, vomiting,early satiety, bloating, prolonged fullness, upper abdominal pain, bloating, early satiety, lackvomiting and retching. Patients may experience any combination of appetite,signs and weight loss. The disorder cansymptoms with varying frequency and degrees of severity.
In addition, we believe the increased use of GLP-1 (glucagon-like peptide-1) agonists could increase the number of people suffering from gastroparesis. GLP-1 receptor agonists affect glucose control through several mechanisms, including enhancement of glucose-dependent insulin secretion, slowed gastric emptying, and reduction of postprandial glucagon and food intake. Slow gastric emptying may potentially lead to considerable pain and discomfort, poor nutrition, impaired glycemic control and diminished quality of life. Accordingsymptoms similar to gastroparesis. Although definitive evidence attributing GLP-1 agonists specifically to causing gastroparesis is limited, a 2008recent study published in theAmerican Journal of Gastroenterology, it is estimatedthe American Medical Association found that hospitalization costsuse of GLP-1 agonists for weight loss compared with use of bupropion-naltrexone was associated with increased risk of pancreatitis, bowel obstruction and gastroparesis. While these adverse events from GLP-1 agonists are rare, we believe it could have an impact on the gastroparesis exceed $3.5 billion annually.market, considering the increased use, the large population expected to be treated and the incidence rate.
Patients with diabetic gastroparesis may experience impaired glucose control due to unpredictable gastric emptying and altered absorption of orally administered drugs, which may affect the severity of their signs and symptoms. Any combination of issues or signs and symptoms may cause complications such as malnutrition, esophagitis, and Mallory‑Weiss tears. Gastroparesis adversely affects the lives of patients with the disease, resulting in decreased social interaction, poor work functionality, and the development of anxiety and/or depression.
EVK-001: Metoclopramide Nasal Spray
We believe intranasalnasal spray administration has the potential to offerprovide our target population of diabetic gastroparesis patients with a preferred treatment option because,over the tablet formulation for several important reasons: (1) unlike oral
1
metoclopramide EVK-001tablets which may be absorbed erratically due to gastroparesis itself, Gimoti is designed to effectively bypass the digestive system andto allow for more predictable absorption without needing to determine if a patient’s stomach is functioning; (2) during episodes of vomiting, Gimoti may provide predictable drug administration of our proprietaryabsorption through the nasal mucosa; and (3) for gastroparesis patients experiencing nausea and are not wanting to swallow a pill or water, a nasal spray formulation acrossmay be better tolerated than an oral medication.
In January 2020, we entered into a commercial services agreement with Eversana (as amended to date, the thin mucosa“Eversana Agreement”) for the commercialization of Gimoti. Pursuant to the Eversana Agreement, Eversana commercializes and distributes Gimoti in the nasal cavity. Intranasal drug delivery effectively bypassesUnited States. Eversana also manages the gut, unlike oral formulations which might be delayed in absorption duemarketing of Gimoti to gastroparesis itself. For patients suffering from nausea and vomiting, EVK-001 is designed to allow for rapid and predictable drug administration.
We have evaluated EVK-001 in a multicenter, randomized, double-blind, placebo-controlled parallel group, dose-ranging Phase 2b clinical trial in 287 patients with diabetic gastroparesis where EVK-001 was observed to be effective in improving the most prevalent and clinically relevant symptoms associated with gastroparesis in women while exhibiting a favorable safety profile.
We plan to initiate a Phase 3 trial of EVK-001 in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in the second half of 2013. We anticipate receiving topline data from this trial in late 2014 or early 2015. We will need to successfully complete this trial,targeted health care providers, as well as a thorough QT study, which
- 2 -
is an evaluationthe sales and distribution of cardiac safety, before we are able to submit a new drug application, or NDA, to the U.S. Food and Drug Administration, or FDA, for EVK-001. Approval of an NDA is required in order for us to commercially market EVK-001Gimoti in the United States. In addition, based2020, we borrowed $5 million from Eversana pursuant to a revolving credit facility (the “Eversana Credit Facility”) which expires on our discussions withDecember 31, 2026, unless terminated earlier pursuant to its terms. The maturity date of all amounts, including interest, borrowed under the FDA, and to assess safety of EVK-001 in men, we plan to conduct a similar companion study for safety and efficacy in diabetic men with gastroparesis. We anticipate this trialEversana Credit Facility will be conducted concurrently with90 days after the Phase 3 trial in women. The completionexpiration or earlier termination of the male companion trialEversana Agreement. As of September 30, 2023, there were approximately $60.4 million in cumulative unreimbursed commercialization costs under the agreement (“Cumulative Deferred Costs”), to be payable only as net product profits are recognized, or upon certain termination events as described below. The Eversana Agreement terminates on December 31, 2026, unless terminated earlier pursuant to its terms. Among other reasons, either party has the right to terminate the Eversana Agreement upon 30 days prior written notice if the net profit under the agreement is not requirednegative for submissionany two consecutive calendar quarters (the “Net Profit Quarterly Termination Right”). As of September 30, 2023, either party had the right to exercise the Net Profit Quarterly Termination Right, which either party could have done until November 29, 2023, which was the end of the NDA for EVK-001; however, we expect to include safety data from this trial in our NDA submission for EVK-001.
At this time, due to60-day period following the risks inherent in the drug development process, we are unable to estimate with any certainty the costs we will incur in the continued development of EVK-001 for potential commercialization. However, we currently estimate the costs to complete our Phase 3 clinical trial in women, our companion clinical trial in men and a thorough QT study of EVK-001 will be approximately $15.0 million.
Our Strategy
Our objective is to develop and bring to market products to treat acute and chronic GI motility disorders that are not satisfactorily treated with current therapies and that represent significant market opportunities. Our business strategy is to:
continue development and pursue regulatory approval for EVK-001;
seek partnerships to accelerate and maximize the potential for EVK-001;
explore building in-house capabilities to potentially commercialize EVK-001 in the United States;
explore regulatory approval of EVK-001 outside the United States; and
evaluate the development and/or commercialization of other therapies for GI motility disorders.
Risks Related to Our Business
Our ability to implement our business strategy is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. These risks include, among others:
Our business is entirely dependent on the success of a single product candidate, EVK-001, which has not yet entered a Phase 3 clinical trial; and we cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, EVK-001.
The results observed in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in our Phase 2b clinical trial of EVK-001 may not be predictiveend of the safety and efficacy results in our planned Phase 3 clinical trial.
We believe, based on our current operating plan, that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will only be sufficient to fund our operations for approximately 12 months after the date of this prospectus.
We will require substantial additional funding, including to complete our planned Phase 3 clinical trial of EVK-001 as well as finance additional development requirements, and may be unable to raise capital when needed, which would force us to suspend our planned Phase 3 clinical trial and otherwise delay, reduce or eliminate our development program for EVK-001.
We have no approved products and no product revenue to date, and we may never become profitable. Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern.
- 3 -
We face significant competition from other pharmaceutical companies, and we anticipate that EVK-001, if approved, would compete directly with metoclopramide, erythromycin and domperidone.quarter. Each of these products is available under various trade names sold by several major pharmaceutical companies, including generic manufacturers.
It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights; any impairment of our intellectual property rights would materially affect our business.
We currently have only two full-time employees, and therefore rely andparty will continue to rely on outsourcing arrangementshave the option to exercise this termination right for many of our activities, including clinical development and supply of EVK-001.
Corporate Information
Our principal executive offices are located at 12555 High Bluff Drive, Suite 385, San Diego, CA 92130, and our telephone number is (760) 487-1255. Our website address iswww.evokepharma.com. The information contained in, or accessible through, our website does not constitute part of this prospectus.
Implications of being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations in this prospectus;
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act;
reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We may take advantage of these provisions until the last day of our fiscal year60-day period following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended, or the Securities Act, which such fifth anniversary will occur in 2018. However, if certain events occur prior to the end of future quarters so long as the net profit under the agreement remains negative for consecutive quarters. Either party may also terminate the Eversana Agreement upon a change of control of our ownership. In the event that we initiate such five-year period,termination, we shall pay to Eversana a one-time payment equal to all of Eversana’s unreimbursed costs (including the Cumulative Deferred Costs) plus a portion of Eversana’s commercialization costs incurred in the 12 months prior to termination. If Eversana initiates such termination following a change of control, none of the Cumulative Deferred Costs incurred by Eversana will be due from Evoke. If Eversana terminates the agreement due to an uncured material breach by us or if we terminate the Eversana Agreement in certain circumstances, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.0 billionexercise the Net Profit Quarterly Termination Right, we have agreed to reimburse Eversana for its unreimbursed commercialization costs for the prior twelve-month period and certain other costs. Upon expiration of the agreement, none of the Cumulative Deferred Costs incurred by Eversana will be due from Evoke. Upon expiration or we issue more than $1.0 billiontermination of non-convertible debt in any three-year period,the agreement, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligationsretain all profits from product sales and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
- 4 -
The Offering
|
|
|
|
|
|
The number of shares of our common stock to be outstanding after this offering is based on 3,681,752 shares of our common stock outstanding as of March 31, 2013 and excludes:
123,250 shares of common stock issuable upon exercise of stock options outstanding as of March 31, 2013, at a weighted-average exercise price of $0.40 per share;
510,000 shares of our common stock reserved for future issuance under our 2013 Equity Incentive Award Plan, which will become effective on the day prior to the public trading date of our common stock;
30,000 shares of common stock reserved for future issuance under our 2013 Employee Stock Purchase Plan, which will become effective on the day prior to the public trading date of our common stock;
22,000 shares of common stock issuable upon exercise of warrants outstanding as of March 31, 2013, at a weighted-average exercise price of $7.50 per share; and
shares of common stock issuable upon exercise of a warrant to be issued to the representative in connection with this offering, at an exercise price per share equal to 175% of the public offering price.
Unless otherwise indicated, this prospectus reflects and assumes the following:
the filing of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws, which will occur immediately prior to the closing of this offering;
the automatic conversion of all outstanding shares of our Series A convertible preferred stock into 2,439,002 shares of our common stock immediately prior to the closing of the offering;
the adjustment of outstanding warrants to purchase shares of our Series A convertible preferred stock into warrants to purchase 22,000 shares of common stock upon the closing of this offering;
a one-for-five reverse stock split of our common stock to be effected before the completion of this offering;
- 5 -
no exercise of the outstanding options or warrants described above; and
no exercise by the underwriters of their option to purchase additional shares of our common stock to cover over-allotments, if any.
- 6 -
Summary Financial Data
The following tables set forth a summary of our historical financial data as of, and for the period ended on, the dates indicated. We have derived the statements of operations data for the years ended December 31, 2011 and 2012 and the balance sheet data as of December 31, 2012 from our audited financial statements appearing elsewhere in this prospectus. We have derived the statements of operations data for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 and balance sheet data as of March 31, 2013 from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and, in the opinion of management, reflect all adjustments, consisting only of normal recurring adjustments, necessary to fairly state our financial position as of March 31, 2013 and results of operations for the three months ended March 31, 2012 and 2013. You should read this data together with our financial statements and related notes appearing elsewhere in this prospectus and the sections in this prospectus entitled “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results for any prior period are not necessarily indicative of our future results.
| Years Ended December 31, | Three Months Ended March 31, | Period From January 29, 2007 (Inception) to March 31, 2013 | ||||||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | $ | 1,844,044 | $ | 1,165,645 | $ | 297,285 | $ | 110,981 | $ | 16,102,510 | ||||||||||
General and administrative | 570,524 | 836,781 | 205,137 | 221,049 | 3,525,582 | |||||||||||||||
Purchase of in-process research and development | — | — | — | — | 650,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total operating expenses | 2,414,568 | 2,002,426 | 502,422 | 332,030 | 20,278,092 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Loss from operations | (2,414,568 | ) | (2,002,426 | ) | (502,422 | ) | (332,030 | ) | (20,278,092 | ) | ||||||||||
Total other income (expense) | 13,324 | (15,102 | ) | 2,264 | (161,962 | ) | (70,903 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss and comprehensive loss | $ | (2,401,244 | ) | $ | (2,017,528 | ) | $ | (500,158 | ) | $ | (493,992 | ) | $ | (20,348,995 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss per common share, basic and diluted(1) | $ | (2.18 | ) | $ | (1.79 | ) | $ | (0.45 | ) | $ | (0.44 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted-average shares used to compute basic and diluted net loss per share(1) | 1,102,625 | 1,124,000 | 1,118,375 | 1,133,375 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Pro forma net loss per common share, basic and diluted (unaudited)(1) | $ | (0.57 | ) | $ | (0.10 | ) | ||||||||||||||
|
|
|
| |||||||||||||||||
Weighted-average shares used to compute pro forma net loss per common share, basic and diluted (unaudited)(1) | 3,563,002 | 3,572,377 | ||||||||||||||||||
|
|
|
| |||||||||||||||||
- 7 -
| As of March 31, 2013 | ||||||||||||
| Actual | Pro Forma(1) | Pro Forma As Adjusted(1)(2) | ||||||||||
Balance Sheet Data: | ||||||||||||
Cash and cash equivalents | $ | 1,669,518 | $ | 1,669,518 | $ | |||||||
Working capital (deficit) | 1,001,053 | 1,001,053 | ||||||||||
Total assets | 1,680,018 | 1,680,018 | ||||||||||
Current liabilities (including warrant liability) | 668,465 | 439,465 | ||||||||||
Long-term debt, net of debt discount | 2,936,607 | 2,936,607 | ||||||||||
Convertible preferred stock | 18,225,166 | — | ||||||||||
Deficit accumulated during the development stage | (20,348,995 | ) | (20,348,995 | ) | ||||||||
Total stockholders’ deficit | (20,150,220 | ) | (1,696,054 | ) | ||||||||
- 8 -
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this prospectus, including our financial statements and the related notes and “Management’s Discussion and Analysis of Results of Operations and Financial Condition,” before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Related to our Business, including the Development, Regulatory Approval and Potential Commercialization of our Product Candidate, EVK-001
Our business is entirely dependent on the success of a single product candidate, EVK-001, which has not yet entered a Phase 3 clinical trial. We cannot be certain that we will be able to obtain regulatory approval for, or successfully commercialize, EVK-001.
We have only one product candidate: EVK-001, a metoclopramide nasal spray to treat female patients with symptoms associated with acute and recurrent diabetic gastroparesis. We are entirely dependent on successful continued development and regulatory approval of this product candidate for our future business success. We have invested, and will continue to invest, a significant portion of our time and financial resources in the development of EVK-001. We will need to raise sufficient funds for, and successfully enroll and complete, our planned Phase 3 clinical trial of EVK-001, which we intend to commence in the second half of 2013. The future regulatory and commercial success of this product candidate is subject to a number of risks, including the following:
we may not have sufficient financial and other resources to complete the Phase 3 clinical trial;
we may not be able to provide acceptable evidence of safety and efficacy for EVK-001;
the results of our planned clinical trials may not confirm the positive results of earlier clinical trials, particularly because we will utilize a modified patient report outcomes, or PRO, instrument for our planned Phase 3 clinical trial compared to our Phase 2b clinical trial;
variability in patients, adjustments to clinical trial procedures and inclusion of additional clinical trial sites;
the results of our clinical trial may not meet the level of statistical or clinical significance required by the U.S. Food and Drug Administration, or FDA, for marketing approval;
we may be required to undertake additional clinical trials and other studies of EVK-001 before we can submit a new drug application, or NDA, to the FDA or receive approval of the NDA;
patients in our clinical trials may die or suffer other adverse effects for reasons that may or may not be related to EVK-001, such as dysgeusia, headache, diarrhea, nasal discomfort, tremor, myoclonus, somnolence, rhinorrhea, throat irritation, and fatigue;
if approved, EVK-001 will compete with well-established products already approved for marketing by the FDA, including oral and intravenous forms of metoclopramide, the same active ingredient in the nasal spray for EVK-001;
we may not be able to obtain, maintain and enforce our patents and other intellectual property rights; and
we may not be able to obtain and maintain commercial manufacturing arrangements with third-party manufacturers or establish commercial-scale manufacturing capabilities.
- 9 -
Of the large number of drugs in development in this industry, only a small percentage result in the submission of an NDA to the FDA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market EVK-001, any such approval may be subject to limitations on the indicated uses for which we may market the product.
We will require substantial additional funding and may be unable to raise capital when needed, which would force us to suspend our Phase 3 clinical trial and otherwise delay, reduce or eliminate our development program for EVK-001.
Our operations have consumed substantial amounts of cash since inception. To date, our operations have been primarily financed through the proceeds from the sale of our common and preferred stock, and borrowings under our loan and financing agreements with Silicon Valley Bank and a prior lender. We believe, based on our current operating plan, that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our operations for approximately 12 months after the date of this prospectus, although there can be no assurance in that regard. Because we expect our planned Phase 3 clinical trial of EVK-001 to commence in the second half of 2013 with an approximately 12 month enrollment period, we will need to obtain additional funds to complete this trial as well as finance any additional development requirements requested by the FDA.
Our estimates of the amount of cash necessary to fund our activities may prove to be wrong, and we could spend our available financial resources much faster than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
the rate of progress and cost of our Phase 3 clinical trial and any other clinical requirements for EVK-001;
the timing of regulatory approval, if granted, of EVK-001 or any other product candidates;
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights associated with EVK-001;
the costs and timing of completion of outsourced commercial manufacturing supply arrangements for EVK-001;
costs associated with any other product candidates that we may develop, in-license or acquire;
the effect of competing technological and market developments; and
the terms and timing of any collaborative, licensing, co-promotion or other arrangements that we may establish.
The results observed in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in our Phase 2b clinical trial of EVK-001 may not be predictive of the safety and efficacy results in our planned Phase 3 clinical trial.
A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in earlier-stage development. We currently plan to commence one Phase 3 clinical trial in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in the second half of 2013. Our Phase 2b clinical trial of EVK-001 for the treatment of diabetic gastroparesis showed statistically significant improvement in clinically meaningful endpoints in female patients. This was a pre-specified analyses of the primary efficacy endpoint performed on a gender subgroup of the intent to treat, or ITT population. Due to a large placebo response in male patients, EVK-001 did not achieve the primary endpoint in the ITT population for all subjects in this Phase 2b clinical trial.
This risk may be particularly significant for us because the primary endpoint in our planned Phase 3 clinical trial is not identical to the primary endpoint used in our Phase 2b trial. In our Phase 2b clinical trial, the primary
- 10 -
endpoint was the Gastroparesis Cardinal Symptom Index Daily Diary, or GCSI-DD, a PRO instrument. The GCSI-DD is a composite of clinically relevant diabetic gastroparesis symptoms which patients rate according to severity. Based on our discussions with the FDA, the primary endpoint for our Phase 3 trial will be the Gastroparesis Symptom Assessment, or GSA, which is a PRO instrument derived from the GCSI-DD. We have analyzed our Phase 2b data utilizing the GSA’s methodology. Although we observed statistically significant and nearly identical statistical improvement in the GSA compared to the GCSI-DD in females in our Phase 2b trial, we cannot assure you that our Phase 3 trials will achieve positive results.
A number of factors could contribute to a lack of favorable safety and efficacy results in our planned Phase 3 trial. For example:
a multicenter trial could result in increased variability due to varying site characteristics, such as local standards of care;
a multicenter trial could result in increased variability due to varying patient characteristics including demographic factors, health status, underlying reason for disease state and concomitant medications; and
diagnosis of diabetic gastroparesis by physicians, including use of gastric emptying tests, could select for a patient population that differs from those patients included within previous clinical trials.
If we are not able to obtain regulatory approval for EVK-001, we will not be able to commercialize this product candidate and our ability to generate revenue will be limited.
We have not submitted an NDA or received regulatory approval to market any product candidates in any jurisdiction. We are not permitted to market EVK-001 in the United States until we receive approval of an NDA for the product candidate in a particular indication from the FDA. To date, we have completed one Phase 2 clinical trial for EVK-001 in diabetic subjects with gastroparesis and acquired the results from a separate Phase 2 clinical trial in diabetic patients with gastroparesis. In the Phase 2 clinical trial that we performed ourselves, which concluded in 2011, EVK-001 failed to meet the primary endpoint for the trial. Although an overall improvement in symptoms was observed in EVK-001-treated patients with diabetic gastroparesis compared to placebo in this second Phase 2 clinical trial, the difference was not statistically significant due to a high placebo response among male subjects. The earlier Phase 2 clinical trial performed by Questcor Pharmaceuticals, Inc., or Questcor, was a multicenter, randomized, open-label, parallel design study. This head-to-head study compared the efficacy and safety of two doses of metoclopramide nasal spray, 10 mg and 20 mg, with the FDA-approved 10 mg metoclopramide tablet. Although data from the earlier Phase 2 clinical trial will be referenced in the EVK-001 NDA, the open-label study design limits the importance of the efficacy results in the NDA.
We currently plan to commence one Phase 3 clinical trial in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in the second half of 2013. There is no guarantee that this Phase 3 clinical trial or any other future trials will be successful or that regulators will agree with our assessment of the clinical trials for EVK-001 conducted to date. In addition, we have only limited experience in filing the applications necessary to gain regulatory approvals and expect to rely on consultants and third party contract research organizations to assist us in this process. The FDA and other regulators have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional clinical trials, or preclinical or other studies.
Varying interpretation of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Furthermore, we have acquired our rights to EVK-001 from Questcor who acquired its rights from a predecessor. Thus, much of the preclinical and a portion of the clinical data relating to EVK-001 that we would expect to submit in an NDA for EVK-001 was obtained from studies conducted before we owned the rights to the product candidate and, accordingly, was prepared and managed by others. These predecessors may not have applied the same resources and given the same attention to this development program as we would have if we had been in control from inception.
- 11 -
EVK-001 and the activities associated with its development and potential commercialization, including its testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain regulatory marketing approval for EVK-001 will prevent us from commercializing the product candidate, and our ability to generate revenue will be materially impaired.
The FDA may impose requirements on our clinical trials that are difficult to comply with, which could harm our business.
The requirements that the FDA may impose on clinical trials for EVK-001 are uncertain. We currently plan to conduct one Phase 3 trial in adult female subjects with diabetic gastroparesis, which we believe will be sufficient for NDA submission. We plan to initiate the four-week, multicenter, randomized, double-blind, placebo-controlled, parallel Phase 3 clinical trial to evaluate the efficacy, safety and population pharmacokinetics of EVK-001 in adult female subjects with diabetic gastroparesis in the second half of 2013. Although we believe successful results from this single Phase 3 clinical trial will be sufficient to allow us to submit an NDA for EVK-001, it is possible the FDA will require additional clinical testing before submission or approval of the NDA. In addition, based on discussions with the FDA, we also plan to conduct a similar study for safety and efficacy in adult male subjects with diabetic gastroparesis. If we are unable to comply with the FDA’s requirements, we will not be able to obtain approval for EVK-001 and our business will suffer.
Any termination or suspension of, or delays in the commencement or completion of, our planned Phase 3 clinical trial could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects.
Delays in the commencement or completion of our planned Phase 3 clinical trial for EVK-001 could significantly affect our product development costs. We do not know whether our planned trial will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
the FDA failing to grant permission to proceed and placing the clinical trial on hold;
subjects failing to enroll or remain in our trial at the rate we expect;
subjects choosing an alternative treatment for the indication for which we are developing EVK-001, or participating in competing clinical trials;
subjects experiencing severe or unexpected drug-related adverse effects;
a facility manufacturing EVK-001 or any of its components being ordered by the FDA or other government or regulatory authorities to temporarily or permanently shut down due to violations of current Good Manufacturing Practices, or cGMP, or other applicable requirements, or infections or cross-contaminations of product candidate in the manufacturing process;
any changes to our manufacturing process that may be necessary or desired;
third-party clinical investigators losing their license or permits necessary to perform our clinical trials, not performing our clinical trials on our anticipated schedule or consistent with the clinical trial protocol, Good Clinical Practice and regulatory requirements, or other third parties not performing data collection and analysis in a timely or accurate manner;
inspections of clinical trial sites by the FDA or the finding of regulatory violations by the FDA or an institutional review board, or IRB, that require us to undertake corrective action, result in suspension or termination of one or more sites or the imposition of a clinical hold on the entire trial, or that prohibit us from using some or all of the data in support of our marketing applications;
- 12 -
third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case we may need to find a substitute contractor, and we may not be able to use some or any of the data produced by such contractors in support of our marketing applications; or
one or more IRBs refusing to approve, suspending or terminating the trial at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial.
Product development costs will increase if we have delays in testing or approval of EVK-001 or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for reexamination, which may impact the costs, timing or successful completion of a clinical trial. If we experience delays in completion of or if we, the FDA or other regulatory authorities, the IRB, or other reviewing entities, or any of our clinical trial sites suspend or terminate any of our clinical trials, the commercial prospects for our product candidate may be harmed and our ability to generate product revenues will be delayed. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate. Also if one or more clinical trials are delayed, our competitors may be able to bring products to market before we do, and the commercial viability of EVK-001 could be significantly reduced.
Final marketing approval for EVK-001 by the FDA or other regulatory authorities for commercial use may be delayed, limited, or denied, any of which would adversely affect our ability to generate operating revenues.
After the completion of our Phase 3 clinical trial and, assuming the results of the trial are successful, the submission of an NDA, we cannot predict whether or when we will obtain regulatory approval to commercialize EVK-001 and we cannot, therefore, predict the timing of any future revenue. Because EVK-001 is our only product candidate this risk is particularly significant for us. We cannot commercialize EVK-001 until the appropriate regulatory authorities have reviewed and approved the applications for this product candidate. We cannot assure you that the regulatory agencies will complete their review processes in a timely manner or that we will obtain regulatory approval for EVK-001. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action or changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. If marketing approval for EVK-001 is delayed, limited or denied, our ability to market the product candidate, and our ability to generate product sales, would be adversely affected.
Even if we obtain marketing approval for EVK-001, it could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidate, when and if EVK-001 is approved.
Even if U.S. regulatory approval is obtained, the FDA may still impose significant restrictions on EVK-001’s indicated uses or marketing or impose ongoing requirements for potentially costly and time consuming post-approval studies, post-market surveillance or clinical trials. EVK-001 will also be subject to ongoing FDA requirements governing the labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of safety and other post-market information. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents. If we or a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requesting recall or withdrawal of the product from the market or suspension of manufacturing.
- 13 -
If we or the manufacturing facilities for EVK-001 fail to comply with applicable regulatory requirements, a regulatory agency may:
issue warning letters or untitled letters;
seek an injunction or impose civil or criminal penalties or monetary fines;
suspend or withdraw regulatory approval;
suspend any ongoing clinical trials;
refuse to approve pending applications or supplements or applications filed by us;
suspend or impose restrictions on operations, including costly new manufacturing requirements; or
seize or detain products, refuse to permit the import or export of product, or request us to initiate a product recall.
The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and generate revenue.
The FDA has the authority to require a risk evaluation and mitigation strategy plan as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. In March 2009, the FDA informed drug manufacturers that it will require a REMS for metoclopramide drug products. The FDA’s authority to take this action is based on risk management and post market safety provisions within the FDAAA. The REMS consists of a Medication Guide, elements to assure safe use (including an education program for prescribers and materials for prescribers to educate patients), and a timetable for submission of assessments of at least six months, 12 months, and annually after the REMS is approved. We intend to submit a REMS at the time of the NDA submission for EVK-001.
In addition, if EVK-001 is approved, our product labeling, advertising and promotion would be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. If we receive marketing approval for EVK-001, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
Even if we receive regulatory approval for EVK-001, we still may not be able to successfully commercialize it and the revenue that we generate from its sales, if any, will be limited.
EVK-001’s commercial success will depend upon the acceptance of the product candidate by the medical community, including physicians, patients and health care payors. The degree of market acceptance of our product candidate will depend on a number of factors, including:
demonstration of clinical efficacy and safety compared to other more-established products;
the limitation of our targeted patient population to women-only;
limitations or warnings contained in any FDA-approved labeling, including the potential boxed warning on all metoclopramide product labels concerning the chance of tardive dyskinesia, or TD, for patients taking these products;
- 14 -
acceptance of a new formulation by health care providers and their patients;
the prevalence and severity of any adverse effects;
new procedures or methods of treatment that may be more effective in treating or may reduce the incidences of diabetic gastroparesis;
pricing and cost-effectiveness;
the effectiveness of our or any future collaborators’ sales and marketing strategies;
our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors; and
the willingness of patients to pay out-of-pocket in the absence of third-party coverage.
If EVK-001 is approved, but does not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not generate sufficient revenue, and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of EVK-001 may require significant resources and may never be successful. In addition, our ability to successfully commercialize our product candidate will depend on our ability to manufacture our products, differentiate our products from competing products and defend the intellectual property of our products.
It will be difficult for us to profitably sell EVK-001 if reimbursement is limited.
Market acceptance andgenerated modest sales of our product candidate will depend on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors have been challenging the prices charged for products. They may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted marketing approval. This trend may impact the reimbursement for treatments for GI disorders especially, including EVK-001, as physicians typically focus on symptoms rather than underlying conditions when treating patients with these disorders and drugs are often prescribed for uses outside of their approved indications. In instances where alternative products are available, it may be required that those alternative treatment options are tried before reimbursement is available for EVK-001. Although EVK-001 is a novel nasal spray formulation of metoclopramide, this is the same active ingredient that is already available in other treatments for gastroparesis that are already widely available at generic prices. We cannot be sure that reimbursement will be available for our EVK-001 and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, this product candidate. In addition, in certain foreign countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize our product candidate.
We rely and will continue to rely on outsourcing arrangements for many of our activities, including clinical development and supply of EVK-001.
We have only two full-time employees and, as a result, we rely on outsourcing arrangements for a significant portion of our activities, including clinical research, data collection and analysis and manufacturing as well as function as a public company. We may have limited control over these third parties and we cannot guarantee that they will perform their obligations in an effective and timely manner.
- 15 -
We expect to retain a contract research organization, or CRO, to conduct our planned Phase 3 clinical trial of EVK-001. We will be required to reach agreement on acceptable terms with prospective CROs as well as clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites. We will need assistance from our CRO in obtaining IRB approval at each clinical trial site and will rely on our CRO to recruiting suitable patients to participate the proposed trial.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We do not own or operate manufacturing facilities for the production of any component of EVK-001, including metoclopramide, the nasal spray device or associated bottle, nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, drug substance and drug product for our clinical trials. For EVK-001, we are currently using, and relying on, single suppliers and single manufacturers for starting materials, the final drug substance and nasal spray delivery device. Although potential alternative suppliers and manufacturers for some components have been identified, we have not qualified these vendors to date. If we were required to change vendors, it could result in a failure to meet regulatory requirements or projected timelines and necessary quality standards for successful manufacturing of the various required lots of material for our development and commercialization efforts.
We do not have any current contractual relationships for the manufacture of commercial supplies of EVK-001. If EVK-001 is approved for sale by any regulatory agency, we intend to enter into agreements with third-party contract manufacturers for commercial production. The number of third-party manufacturers with the expertise, required regulatory approvals and facilities to manufacture bulk drug substance on a commercial scale is limited. We have identified one manufacturer for potentially providing commercial supplies of EVK-001; however no alternative providers have been identified to date. If we are unable to come to terms on commercial supplier with this manufacturer, we would have to find replacements, which could delay the commercialization of our product candidate.
In addition, our reliance on third party CROs and contract manufacturing organizations, or CMOs, entails further risks including:
non-compliance by third parties with regulatory and quality control standards;
breach by third parties of our agreements with them;
termination or non-renewal of an agreement with third parties; and
sanctions imposed by regulatory authorities if compounds supplied or manufactured by a third party supplier or manufacturer fail to comply with applicable regulatory standards.
We face substantial competition, which may result in others selling their products more effectively than we do, and in others discovering, developing or commercializing product candidates before, or more successfully, than we do.
Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of EVK-001. We anticipate that EVK-001, if approved, would compete directly with metoclopramide, erythromycin and domperidone, each of which is available under various trade names sold by several major pharmaceutical companies, including generic manufacturers. Metoclopramide is the only molecule currently approved in the United States to treat gastroparesis. Metoclopramide is generically-available and indicated for the relief of symptoms associated with acute and recurrent diabetic gastroparesis, without the limitation of use in women only.
- 16 -
Many of our potential competitors have substantially greater financial, technical and personnel resources than we have. In addition, many of these competitors have significantly greater commercial infrastructures than we have. We will not be able to compete successfully unless we successfully:
assure health care providers, patients and health care payors that EVK-001 is beneficial compared to other products in the market;
obtain patent and/or other proprietary protection for EVK-001;
obtain and maintain required regulatory approvals for the product candidate; and
collaborate with others to effectively market, sell and distribute EVK-001.
Established competitors may invest heavily to quickly discover and develop novel compounds that could make our product candidate obsolete. In addition to our EVK-001 product candidate, we are aware of other development candidates in clinical development. Any of these product candidates could advance through clinical development faster than EVK-001 and, if approved, could attain faster and greater market acceptance than our product candidate. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
We have no sales, marketing or distribution capabilities currently and we will have to invest significant resources to develop these capabilities.
Currently, we have no internal sales, marketing or distribution capabilities. If EVK-001 ultimately receives regulatory approval, we may not be able to effectively market and distribute the product candidate. We will have to invest significant amounts of financial and management resources to develop internal sales, distribution and marketing capabilities, some of which will be committed prior to any confirmation that EVK-001 will be approved. We may not be able to hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms or at all. Even if we determine to perform sales, marketing and distribution functions ourselves, we could face a number of additional related risks, including:
inability to attract and build an effective marketing department or sales force;
the cost of establishing a marketing department or sales force may exceed our available financial resources and the revenues generated by EVK-001 or any other product candidates that we may develop, in-license or acquire; and
our direct sales and marketing efforts may not be successful.
If we fail to attract and retain senior management and key commercial personnel, we may be unable to successfully complete the development of EVK-001 and commercialize this product candidate.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and commercial personnel. We are highly dependent upon our senior management team composed of two individuals: David Gonyer, our President and Chief Executive Officer, and Matt D’Onofrio, our Executive Vice President and Chief Business Officer. The loss of services of either of these individuals could delay or prevent the successful development of EVK-001 or the commercialization of this product candidate, if approved.
We will need to hire and retain qualified personnel. We could experience problems in the future attracting and retaining qualified employees. For example, competition for qualified personnel in the biotechnology and pharmaceuticals field is intense, particularly in the San Diego, California area where we are headquartered. We may not be able to attract and retain quality personnel on acceptable terms who have the expertise we need to sustain and grow our business.
- 17 -
We may encounter difficulties in managing our growth and expanding our operations successfully.
Because we currently have only two full-time employees, we will need to grow our organization substantially to continue the development and pursue the potential commercialization of EVK-001 as well as function as a public company. As we seek to advance EVK-001, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Future growth will impose significant added responsibilities on members of management and require us to retain additional internal capabilities. Our future financial performance and our ability to commercialize EVK-001 and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, clinical and regulatory, financial, administrative and sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize EVK-001 and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for EVK-001, restrict or regulate post-approval activities and affect our ability to profitably sell our product candidate, assuming we obtain marketing approval.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of EVK-001, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Modernization Act, or MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
In early 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, beginning in 2011, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners. We will not know the full effects of the
- 18 -
Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
If we market products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties.
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include false claims statutes and anti-kickback statutes. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Over the past few years, several pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. Most states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
Federal legislation and actions by state and local governments may permit re-importation of drugs from foreign countries into the United States, including foreign countries where the drugs are sold at lower prices than in the United States, which could materially adversely affect our operating results and our overall financial condition.
We may face competition in the United States for EVK-001, if approved, from lower priced products from foreign countries that have placed price controls on pharmaceutical products. This risk may be particularly applicable to drugs such as EVK-001. The U.S. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, contains provisions that may change U.S. importation laws and expand pharmacists’ and wholesalers’ ability to import lower priced versions of an approved drug and competing products from Canada, where there are government price controls. These changes to U.S. importation laws will not take effect unless and until the Secretary of Health and Human Services certifies that the changes will pose no additional risk to the public’s health and safety and will result in a significant reduction in the cost of products to consumers. The Secretary of Health and Human Services has not yet announced any plans to make this required certification.
- 19 -
A number of federal legislative proposals have been made to implement the changes to the U.S. importation laws without any certification, and to broaden permissible imports in other ways. Even if the changes do not take effect, and other changes are not enacted, imports from Canada and elsewhere may continue to increase due to market and political forces, and the limited enforcement resources of the FDA, U.S. Customs and Border Protection and other government agencies. For example, Pub. L. No. 111-83, which was signed into law in October 2009 and provides appropriations for the Department of Homeland Security for the 2010 fiscal year, expressly prohibits U.S. Customs and Border Protection from using funds to prevent individuals from importing from Canada less than a 90-day supply of a prescription drug for personal use, when the drug otherwise complies with the Federal Food, Drug, and Cosmetic Act, or FDCA. Further, several states and local governments have implemented importation schemes for their citizens and, in the absence of federal action to curtail such activities, we expect other states and local governments to launch importation efforts.
The importation of foreign products that compete with EVK-001 could negatively impact our revenue and profitability, possibly materially.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of EVK-001.
We face an inherent risk of product liability as a result of the clinical testing of EVK-001 and will face an even greater risk if we commercialize the product candidate. For example, we may be sued if EVK-001 allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product candidate, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts.
In particular, products containing metoclopramide have been reported to cause side effects, including TD. It is possible that a patient taking EVK-001 will be found to experience a variety of side effects. In 2009, the FDA required a boxed warning on all metoclopramide product labels concerning the chance of TD for patients taking these products. We expect that the label for EVK-001, if approved, will likely contain a similar warning regarding TD. Several manufactures of metoclopramide products have been sued by patients regarding TD.
If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidate. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
decreased demand for EVK-001;
injury to our reputation;
withdrawal of clinical trial participants;
costs to defend the related litigation;
a diversion of management’s time and our resources;
substantial monetary awards to trial participants or patients;
product recalls, withdrawals or labeling, marketing or promotional restrictions;
loss of revenue;
the inability to commercialize EVK-001; and
a decline in our stock price.
- 20 -
We may form strategic alliances in the future, and we may not realize the benefits of such alliances.
We may form strategic alliances, create joint ventures or collaborations or enter into licensing arrangements with third parties that we believe will complement or augment our existing business, including for the continued development or commercialization of EVK-001. These relationships or those like them may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for EVK-001 because third parties may view the risk of success in our planned Phase 3 clinical trial as too significant or the commercial opportunity for our product candidate as too limited. We cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors and consultants and collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development program for EVK-001 and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture EVK-001 and conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidate could be delayed.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce our EVK-001. Our ability to obtain clinical supplies of EVK-001 could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. Our operations are located in San Diego, California near major earthquake faults and fire zones. The ultimate impact on us, our significant suppliers and our general infrastructure of being located near major earthquake faults and fire zones and being consolidated in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster.
If we fail to develop and commercialize other product candidates, we may be unable to grow our business.
As part of our growth strategy, we plan to evaluate the development and/or commercialization of other therapies for GI motility disorders. Similar to our initial focus on gastroparesis, we will evaluate opportunities to in-license or acquire other product candidates as well as commercial products to treat patients suffering from predominantly GI disorders, seeking to identify areas of high unmet medical needs with limited treatment options. These other product candidates will require additional, time-consuming development efforts prior to commercial sale, including preclinical studies, extensive clinical trials and approval by the FDA and applicable
- 21 -
foreign regulatory authorities. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development, including the possibility that the drug candidate will not be shown to be sufficiently safe and/or effective for approval by regulatory authorities. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or be more effective than other commercially available alternatives.
If we engage in an acquisition, reorganization or business combination, we will incur a variety of risks that could adversely affect our business operations or our stockholders.
From time to time we have considered, and we will continue to consider in the future, strategic business initiatives intended to further the development of our business. These initiatives may include acquiring businesses, technologies or products or entering into a business combination with another company. If we do pursue such a strategy, we could, among other things:
issue equity securities that would dilute our current stockholders’ percentage ownership;
incur substantial debt that may place strains on our operations;
spend substantial operational, financial and management resources in integrating new businesses, technologies and products; and
assume substantial actual or contingent liabilities.
We may be unable to maintain sufficient product liability insurance.
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. We currently carry product liability insurance covering our clinical studies. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. If we determine that it is prudent to increase our product liability coverage due to the commercial launch of any product, we may be unable to obtain such increased coverage on acceptable terms or at all. Our insurance policies also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Risks Relating to Our Intellectual Property
It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. Any impairment of our intellectual property rights would materially affect our business.
We place considerable importance on obtaining patent protection for new technologies, products and processes because our commercial success will depend, in large part, on obtaining patent protection for new technologies, products and processes, successfully defending these patents against third-party challenges and successfully enforcing our patents against third party competitors. To that end, we have acquired and will file applications for patents covering formulations containing or uses of EVK-001 or our proprietary processes as well as other intellectual property important to our business. One of our patents related to EVK-001 was acquired from Questcor. This method of use patent was not written by us or our attorneys, and we did not have control over the drafting and prosecution of these patents. Further, Questcor and other predecessors might not have given the same attention to the drafting and prosecution of these patents and applications as we would have if we had been the owners of the patent and application and had control over the drafting and prosecution.
- 22 -
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain and involves complex legal and factual questions for which legal principles remain unresolved. In recent years patent rights have been the subject of significant litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our predecessors were the first to make the inventions claimed in our owned and licensed patents or pending patent applications, or that we or our predecessors were the first to file for patent protection of such inventions One or more of these factors could possibly result in findings of invalidity or unenforceability of one or more of the patents we own.
The patent rights we own covering EVK-001 are limited to specific methods of use and formulations of metoclopramide. As a result, our ability to market EVK-001 may be limited by the lack of patent protection for the active ingredient itself and other metoclopramide formulations may be developed by competitors. The active ingredient in EVK-001 is metoclopramide. No patent protection is available for metoclopramide itself. As a result, competitors who develop and receive required regulatory approval for competing products using the same active ingredient as EVK-001 may market their competing products so long as they do not infringe any of the method or formulation patents owned by us.
Others have filed, and in the future are likely to file, patent applications covering products and technologies that are similar, identical or competitive to ours, or important to our business. We cannot be certain that any patent application owned by a third party will not have priority over patent applications filed or in-licensed by us, or that we will not be involved in interference, opposition or invalidity proceedings before U.S. or foreign patent offices.
We have focused our intellectual property efforts on the United States. To the extent that our patent portfolio differs from country to country outside the United States, this may make protecting EVK-001 as a product outside the United States even more difficult and unpredictable. Various countries maintain their own standards and interpretation of intellectual property law, potentially creating additional patent risk beyond even that experienced within the United States.
We also rely on trade secrets to protect technology in cases when we believe patent protection is not appropriate or obtainable. However, trade secrets are difficult to protect. While we require employees, consultants and other contractors to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary information. Our research collaborators and scientific advisors may have rights to publish data and information in which we have rights. If we cannot maintain the confidentiality of our technology and other confidential information in connection with our collaborators and advisors, our ability to receive patent protection or protect our proprietary information may be imperiled.
Claims by third parties that we infringe their proprietary rights may result in liability for damages or prevent or delay our developmental and commercialization efforts.
The biotechnology industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Because patent applications are maintained in secrecy until the application is published, we may be unaware of third party patents that may be infringed by commercialization of EVK-001. In addition, identification of third party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Any claims of patent infringement asserted by third parties would be time consuming and could likely:
result in costly litigation;
- 23 -
divert the time and attention of our technical personnel and management;
cause development delays;
prevent us from commercializing EVK-001 until the asserted patent expires or is held finally invalid or not infringed in a court of law;
require us to develop non-infringing technology; or
require us to enter into royalty or licensing agreements.
Although no third party has asserted a claim of infringement against us, others may hold proprietary rights that could prevent EVK-001 from being marketed. Any patent-related legal action against us claiming damages or seeking to enjoin commercial activities relating to our product candidate or processes could subject us to potential liability for damages and could require us to obtain a license to continue to manufacture or market EVK-001, or, if no such license were available on commercially viable terms, could require us to cease manufacturing and marketing of EVK-001. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign our product candidate or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing EVK-001, which could harm our business, financial condition and operating results. Whatever the outcome, any patent litigation would be costly and time consuming, could be distracting to our management, and could have a material adverse effect on our business.
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ and consult with individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject in the future to claims that our employees or consultants are subject to a continuing obligation to their former employers or clients (such as non-competition or non-solicitation obligations) or claims that our employees, our consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Our Financial Position and Need for Capital
Our recurring losses from operations have raised substantial doubt regarding our ability to continue as a going concern.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern, and as a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2012 with respect to this uncertainty. This going concern opinion could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise. Future reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern. We have incurred significant losses since our inception and have never been profitable, and it is possible we will never achieve profitability. We have devoted our resources to developing our product candidate, but it cannot be marketed until regulatory approvals have been obtained. If we successfully complete this offering, based upon our currently expected level of operating expenditures, we expect to be able to fund our operations for approximately the next year. This period could be shortened if there are any significant increases in planned spending on our EVK-001 development
- 24 -
program or more rapid progress of our planned Phase 3 clinical trial than anticipated. There is no assurance that other financing will be available when needed to allow us to continue as a going concern. The perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations.
We have incurred significant operating losses since inception, and we expect to incur losses for the foreseeable future. We may never become profitable or, if achieved, be able to sustain profitability.
We have incurred significant operating losses since we were founded in 2007 and expect to incur significant losses for the next several years as we begin our Phase 3 clinical trial for EVK-001. Our net loss for the year ended December 31, 2012 and the three months ended March 31, 2013 was $2.0 million and $0.5 million, respectively. As of December 31, 2012 and March 31, 2013, we had an accumulated deficit of $19.9 million and $20.3 million, respectively. Losses have resulted principally from costs incurred in our clinical trials, research and development programs and from our general and administrative expenses. In the future, we intend to continue to conduct research and development, clinical testing, regulatory compliance activities and, if EVK-001 is approved, sales and marketing activities that, together with anticipated general and administrative expenses, will likely result in our incurring further significant losses for the next several years.
We currently generate no revenue from sales, and we may never be able to commercialize EVK-001 or other marketable drugs. As a result, there can be no assurance that we will ever generate revenues or achieve profitability, which could impair our ability to sustain operations or obtain any required additional funding. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
If we fail to obtain the capital necessary to fund our operations, we will be unable to successfully develop and commercialize EVK-001.
We will require substantial future capital in order to complete the remaining clinical development for EVK-001 and to potentially commercialize this product candidate. The amount and timing of any expenditure needed to implement our development and commercialization programs will depend on numerous factors, including:
the initiation, progress, costs, results of and timing of our clinical development program for EVK-001, including our planned Phase 3 clinical trial;
the need for, and the progress, costs and results of, any additional clinical trials of EVK-001 we may initiate based on the results of our planned clinical trials or discussions with the FDA, including any additional trials the FDA or other regulatory agencies may require evaluating the safety of EVK-001;
the outcome, costs and timing of seeking and obtaining regulatory approvals from the FDA, and any similar regulatory agencies;
the timing and costs associated with manufacturing EVK-001 for clinical trials and other studies and, if approved, for commercial sale;
our need and ability to hire additional management, development and scientific personnel;
the cost to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights;
the timing and costs associated with establishing sales and marketing capabilities;
market acceptance of EVK-001;
the extent to which we are required to pay milestone or other payments under our Questcor asset purchase agreement and the timing of such payments;
- 25 -
the costs of acquiring, licensing or investing in additional businesses, products, product candidates and technologies; and
our need to implement additional internal systems and infrastructure, including financial and reporting systems.
Some of these factors are outside of our control. We do not expect our existing capital resources together with the net proceeds from this offering to be sufficient to enable us to fund the completion of our Phase 3 clinical trial and remaining development program through commercial introduction. We expect that we will need to raise additional funds in the near future.
We may seek additional funding through collaboration agreements and public or private financings. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. In addition, the issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline.
If we are unable to obtain funding on a timely basis, we will be unable to complete the planned Phase 3 clinical trial for EVK-001 and may be required to significantly curtail all of our activities. We also could be required to seek funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to our product candidate or some of our technologies or otherwise agree to terms unfavorable to us.
The terms of our secured debt facility require us to meet certain operating and financial covenants and place restrictions on our operating and financial flexibility. If we raise additional capital through debt financing, the terms of any new debt could further restrict our ability to operate our business.
We have a $3.0 million loan and security agreement with Silicon Valley Bank that is secured by a lien covering substantially all of our assets, excluding intellectual property. As of March 31, 2013, the outstanding principal balance of the Silicon Valley Bank loan was $3.0 million. The loan agreement contains customary affirmative and negative covenants and events of default. The affirmative covenants include, among others, covenants requiring us to maintain our legal existence and governmental approvals, deliver certain financial reports and maintain insurance coverage. The negative covenants include, among others, restrictions on transferring collateral, changing our business, incurring additional indebtedness, engaging in mergers or acquisitions, paying dividends or making other distributions, making investments and creating other liens on our assets, in each case subject to customary exceptions. If we default under the loan agreement, the lender may accelerate all of our repayment obligations and take control of our pledged assets, potentially requiring us to renegotiate our agreement on terms less favorable to us or to immediately cease operations. Further, if we are liquidated, the lender’s right to repayment would be senior to the rights of the holders of our common stock to receive any proceeds from the liquidation. The lenders could declare a default upon the occurrence of any event that they interpret as a material adverse change as defined under the loan agreement, thereby requiring us to repay the loan immediately or to attempt to reverse the declaration of default through negotiation or litigation. Any declaration by the lender of an event of default could significantly harm our business and prospects and could cause the price of our common stock to decline. If we raise any additional debt financing, the terms of such additional debt could further restrict our operating and financial flexibility.
Our ability to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments is limited by provisions of the Internal Revenue Code, and may be subject to further limitation as a result of the transactions contemplated by this offering.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. As a result of this offering, our most recent
- 26 -
private placement and other transactions that have occurred over the past three years, we may have experienced, or may upon completion of this offering experience, an “ownership change.” We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As of December 31, 2012, we had federal and state net operating loss carryforwards of approximately $18.6 million and $18.2 million, respectively, and federal research and development credits of $0.5 million which could be limited if we experience an “ownership change.”
Risks Related to Our Common Stock and this Offering
An active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. Although we anticipate that our common stock will be approved for listing on The NASDAQ Capital Market, an active trading market for our shares may never develop or be sustained following this offering. If the market does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at a price that is attractive to you or at all. In addition, an inactive market may impair our ability to raise capital by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration, which, in turn, could materially adversely affect our business.
The trading price of the shares of our common stock could be highly volatile, and purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the initial public offering price. The market price for our common stock may be influenced by many factors, including:
our ability to enroll patients in our planned Phase 3 clinical trial;
results of the clinical trial, and the results of trials of our competitors or those of other companies in our market sector;
regulatory developments in the United States and foreign countries;
variations in our financial results or those of companies that are perceived to be similar to us;
changes in the structure of healthcare payment systems, especially in light of current reforms to the U.S. healthcare system;
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
market conditions in the pharmaceutical and biotechnology sectors and issuance of securities analysts’ reports or recommendations;
sales of our stock by insiders and 5% stockholders;
trading volume of our common stock;
general economic, industry and market conditions other events or factors, many of which are beyond our control;
additions or departures of key personnel; and
intellectual property, product liability or other litigation against us.
- 27 -
In addition, in the past, stockholders have initiated class action lawsuits against biotechnology and pharmaceutical companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources, which could have a material adverse effect on our business, financial condition and results of operations.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
variations in the level of expenses related to our EVK-001 development program;
addition or termination of clinical trials;
any intellectual property infringement lawsuit in which we may become involved;
regulatory developments affecting EVK-001; and
our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements.
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
We may allocate the net proceeds from this offering in ways that you and other stockholders may not approve.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. We expect to use the net proceeds from this offering for research and development activities for EVK-001, including our planned Phase 3 clinical trial of EVK-001, and for working capital and other general corporate purposes. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering and the concurrent private placement in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
You will suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase.
The initial public offering price of our common stock is substantially higher than the net tangible book value per share of our outstanding common stock immediately after the completion of this offering. Purchasers of common stock in this offering will experience immediate dilution of approximately $ per share in net tangible book value of the common stock. In the past, we issued options and warrants to acquire common stock at prices significantly below the initial public offering price. To the extent these outstanding options and warrants are ultimately exercised, investors purchasing common stock in this offering will sustain further dilution. For a further description of the dilution that you will experience immediately after this offering, see “Dilution.”
- 28 -
Because a small number of our existing stockholders own a majority of our voting stock, your ability to influence corporate matters will be limited.
Following the completion of this offering, our executive officers, directors and greater than 5% stockholders, in the aggregate, will own approximately % of our outstanding common stock. As a result, such persons, acting together, will have the ability to control our management and affairs and substantially all matters submitted to our stockholders for approval, including the election and removal of directors and approval of any significant transaction. These persons will also have the ability to control our management and business affairs. This concentration of ownership may have the effect of delaying, deferring or preventing a change in control, impeding a merger, consolidation, takeover or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our business, even if such a transaction would benefit other stockholders.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include:
authorizing the issuance of “blank check” preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval;
limiting the removal of directors by the stockholders;
creating a staggered board of directors;
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders;
eliminating the ability of stockholders to call a special meeting of stockholders;
permitting our board of directors to accelerate the vesting of outstanding option grants upon certain transactions that result in a change of control; and
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Although we believe these provisions collectively provide for an opportunity to obtain greater value for stockholders by requiring potential acquirors to negotiate with our board of directors, they would apply even if an offer rejected by our board were considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
We do not intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our common stock and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, our loan and security agreement with Silicon Valley Bank currently prohibits us from paying dividends on our equity securities, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to
- 29 -
stockholders will therefore be limited to the appreciation of their stock. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could significantly reduce the market price of our common stock and impair our ability to raise adequate capital through the sale of additional equity securities.
Based on shares of common stock outstanding as of March 31, 2013, upon the closing of this offering, we will have outstanding a total of shares of common stock, assuming no exercise of the underwriters’ overallotment option and no exercise of outstanding options and warrants. Of these shares, only the shares of common stock sold in this offering by us, plus any shares sold upon exercise of the underwriters’ overallotment option, will be freely tradable without restriction in the public market immediately following this offering. Aegis Capital Corp. however, may, in its sole discretion, permit our officers, directors and other stockholders who are subject to these lock-up agreements to sell shares prior to the expiration of the lock-up agreements.
We expect that the lock-up agreements pertaining to this offering will expire 180 days from the date of this prospectus. After the lock-up agreements expire, up to an additional shares of common stock will be eligible for sale in the public market of which shares are held by directors, executive officers and other affiliates and will be subject to volume limitations under Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). In addition, shares of common stock that are either subject to outstanding options or reserved for future issuance under our employee benefit plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
After this offering, as of March 31, 2013, holders of 2,984,752 shares of our common stock, which includes 2,439,002 shares issuable upon the automatic conversion of our Series A convertible preferred stock immediately prior to the closing of this offering, will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the 180-day lock-up agreements described above. In addition, holders of 10,000 shares of common stock issuable upon the exercise of a warrant will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the 180-day lock-up agreements described above. See “Description of Capital Stock—Registration Rights.” Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five
- 30 -
years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, which will require, among other things, that we file with the Securities and Exchange Commission, or the SEC, annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC, and The NASDAQ Stock Market to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, in 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation related provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Recent legislation permits smaller “emerging growth companies” to implement many of these requirements over a longer period and up to five years from the pricing of this offering. We intend to take advantage of this new legislation but cannot guarantee that we will not be required to implement these requirements sooner than budgeted or planned and thereby incur unexpected expenses. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. The increased costs will decrease our net income or increase our consolidated net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the
- 31 -
amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
- 32 -
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions described under the sections in this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act of 1933, as amended.
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
- 33 -
We estimate that the net proceeds to us from the sale of the common stock that we are offering will be approximately $million (or $ million if the underwriters exercise their over-allotment option in full), assuming an initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase (decrease) in the assumed initial public offering price of $per share would increase (decrease) the net proceeds to us from this offering by approximately $million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1.0 million in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million, assuming the assumed initial public offering price stays the same.
We intend to use $ of the net proceeds of this offering for research and development activities for EVK-001, including our planned Phase 3 clinical trial of EVK-001, and the remainder for working capital and other general corporate purposes. We may also use a portion of the net proceeds to in-license, acquire or invest in complementary businesses or products; however, we have no current commitments or obligations to do so. Pending use of the proceeds as described above, we intend to invest the net proceeds of this offering in short-term, interest-bearing, investment-grade securities or certificates of deposit. We do not intend to use any of the net proceeds from this offering to repay our outstanding debt with Silicon Valley Bank.
We believe that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, will be sufficient to fund our operations through approximately 12 months after the date of this prospectus, although there can be no assurance in that regard. In particular, we believe that the net proceeds from this offering will allow us to commence our planned Phase 3 clinical trial of EVK-001. Because we expect this clinical trial to commence in the second half of 2013 with an approximately 12 month enrollment period, we will need to obtain additional funds to complete this trial as well as finance any additional development requirements requested by the FDA.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress of our clinical trials and other development efforts for EVK-001, as well as the amount of cash used in our operations. We therefore cannot estimate the amount of net proceeds to be used for the purposes described above. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds. Pending the uses described above, we plan to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
- 34 -
We have never declared or paid any cash dividends on our capital stock. We intend to retain future earnings, if any, to finance the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. In addition, unless waived, the terms of our loan and security agreement with Silicon Valley Bank limit our ability to pay cash dividends. Any future determination related to dividend policy will be made at the discretion of our board of directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the board of directors deems relevant, and subject to the restrictions contained in our current or future financing instruments.
- 35 -
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2013 as follows:
on an actual basis;
on a pro forma basis to reflect (1) the automatic conversion of all outstanding shares of our Series A convertible preferred stock into 2,439,002 shares of our common stock prior to the closing of this offering, (2) the adjustment of our outstanding warrants to purchase Series A convertible preferred stock into warrants to purchase 22,000 shares of our common stock immediately prior to the closing of this offering and the resultant reclassification of our convertible preferred stock warrant liability to additional paid-in capital, a component of stockholders’ equity (deficit), and (3) the filing of our amended and restated certificate of incorporation immediately prior to the closing of this offering; and
on a pro forma as adjusted basis to give further effect to our issuance and sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, and the payment of retention bonuses to our executive officers in an aggregate amount of $355,000 which will become payable upon the closing of this offering.
The pro forma and pro forma as adjusted information below is illustrative only, and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our financial statements and the related notes appearing at the end of this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information contained in this prospectus.
| As of March 31, 2013 | ||||||||||||
| Actual | Pro Forma | Pro Forma As Adjusted(1) | ||||||||||
Cash and cash equivalents | $ | 1,669,518 | $ | 1,669,518 | $ | |||||||
|
|
|
|
|
| |||||||
Capitalization: | ||||||||||||
Warrant liability | $ | 229,000 | $ | — | ||||||||
Long-term debt, net of debt discount | 2,936,607 | 2,936,607 | ||||||||||
Series A convertible preferred stock, $0.0001 par value per share; 12,305,068 shares authorized, 12,195,068 shares issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted | 18,225,166 | — | ||||||||||
Common stock, $0.0001 par value per share; 20,000,000 shares authorized, 1,242,750 shares issued and outstanding, actual; 100,000,000 shares authorized, pro forma and pro forma as adjusted; 3,681,752 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted | 124 | 368 | ||||||||||
Preferred stock, $0.0001 par value per share; no shares authorized, issued and outstanding, actual; 10,000,000 shares authorized, no shares issued or outstanding, pro forma and pro forma as adjusted | — | — | — | |||||||||
Additional paid-in capital | 198,651 | 18,652,573 | ||||||||||
Deficit accumulated during the development stage | (20,348,995 | ) | (20,348,995 | ) | ||||||||
|
|
|
|
|
| |||||||
Total stockholders’ equity (deficit) | (20,150,220 | ) | (1,696,054 | ) | ||||||||
|
|
|
|
|
| |||||||
Total capitalization | $ | 1,240,553 | $ | 1,240,553 | $ | |||||||
|
|
|
|
|
| |||||||
- 36 -
The number of shares in the table above excludes, as of March 31, 2013:
123,250 shares of common stock issuable upon exercise of stock options outstanding as of March 31, 2013, at a weighted-average exercise price of $0.40 per share;
510,000 shares of our common stock reserved for future issuance under our 2013 Equity Incentive Award Plan, which will become effective on the day prior to the public trading date of our common stock;
30,000 shares of common stock reserved for future issuance under our 2013 Employee Stock Purchase Plan, which will become effective on the day prior to the public trading date of our common stock;
22,000 shares of common stock issuable upon exercise of warrants outstanding as of March 31, 2013, at a weighted-average exercise price of $7.50 per share; and
shares of common stock issuable upon exercise of a warrant to be issued to the representative in connection with this offering, at an exercise price per share equal to 175% of the public offering price.
- 37 -
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
As of March 31, 2013, we had a historical net tangible book value (deficit) of $(20,150,220), or $(16.21) per share of common stock, based on 1,242,750 shares of common stock outstanding at March 31, 2013. Our historical net tangible book value represents total tangible assets less total liabilities at March 31, 2013.
On a pro forma basis, after giving effect to the automatic conversion of all outstanding shares of our Series A convertible preferred stock into 2,439,002 shares of our common stock immediately prior to the closing of this offering and the reclassification of our convertible preferred stock warrant liability to additional paid-in capital, a component of stockholders’ equity (deficit), our pro forma net tangible book value (deficit) as of March 31, 2013 would have been approximately $(1,696,054), or approximately $(0.46) per share of our common stock.
After giving further effect to the sale of shares of common stock that we are offering at an assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, and the payment of retention bonuses to our executive officers in an aggregate amount of $355,000 which will become payable upon the closing of this offering, our pro forma as adjusted net tangible book value as of March 31, 2013 would have been approximately $ million, or approximately $ per share. This amount represents an immediate increase in pro forma net tangible book value of $ per share to our existing stockholders and an immediate dilution in pro forma net tangible book value of approximately $ per share to new investors purchasing shares of common stock in this offering.
Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the initial public offering price per share paid by new investors. The following table illustrates this dilution:
Assumed initial public offering price per share | $ | |||||||
Historical net tangible book value (deficit) per share as of March 31, 2013 | $ | (16.21 | ) | |||||
Pro forma increase in historical net tangible book value (deficit) per share attributable to the pro forma transactions described in preceding paragraphs | 15.75 | |||||||
|
| |||||||
Pro forma net tangible book value (deficit) per share as of March 31, 2013 | (0.46 | ) | ||||||
Increase in pro forma net tangible book value per share attributable to this offering | ||||||||
|
| |||||||
Pro forma as adjusted net tangible book value per share after this offering | $ | |||||||
|
| |||||||
Dilution per share to new investors | $ | |||||||
|
|
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value per share after this offering by approximately $ , and dilution in pro forma net tangible book value per share to new investors by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and the estimated offering expenses payable by us. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) our pro forma as adjusted net tangible book value per share after this offering by approximately $ per share and decrease (increase) the dilution to investors participating in this offering by approximately $ per share, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and the estimated offering expenses payable by us.
- 38 -
If the underwriters exercise their over-allotment option to purchase additional shares of our common stock in full in this offering, the pro forma as adjusted net tangible book value after the offering would be $ per share, the increase in pro forma net tangible book value per share to existing stockholders would be $ per share and the dilution per share to new investors would be $ per share, in each case assuming an initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus.
The following table summarizes on the pro forma as adjusted basis described above, as of March 31, 2013, the differences between the number of shares purchased from us, the total consideration paid to us in cash and the average price per share that existing stockholders and new investors paid. The calculation below is based on the assumed initial public offering price of $ per share, the midpoint of the price range listed on the cover page of the prospectus, before deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
Existing stockholders | % | $ | % | $ | ||||||||||||||
New investors | ||||||||||||||||||
|
|
|
|
|
|
| ||||||||||||
Total | 100 | % | $ | 100 | % | |||||||||||||
|
|
|
|
|
|
| ||||||||||||
The foregoing tables and calculations exclude:
123,250 shares of common stock issuable upon exercise of stock options outstanding as of March 31, 2013, at a weighted-average exercise price of $0.40 per share;
510,000 shares of our common stock reserved for future issuance under our 2013 Equity Incentive Award Plan, which will become effective on the day prior to the public trading date of our common stock;
30,000 shares of common stock reserved for future issuance under our 2013 Employee Stock Purchase Plan, which will become effective on the day prior to the public trading date of our common stock;
22,000 shares of common stock issuable upon exercise of warrants outstanding as of March 31, 2013, at a weighted-average exercise price of $7.50 per share; and
shares of common stock issuable upon exercise of a warrant to be issued to the representative in connection with this offering, at an exercise price per share equal to 175% of the public offering price.
To the extent any of these outstanding options or warrants is exercised, there will be further dilution to new investors. If all of such outstanding options and warrants had been exercised as of March 31, 2013, the pro forma as adjusted net tangible book value per share after this offering would be $ , and total dilution per share to new investors would be $ .
If the underwriters exercise their over-allotment option to purchase additional shares of our common stock in full in this offering:
the percentage of shares of common stock held by existing stockholders will decrease to approximately % of the total number of shares of our common stock outstanding after this offering; and
the number of shares held by new investors will increase to , or approximately % of the total number of shares of our common stock outstanding after this offering.
- 39 -
You should read the following selected financial data in conjunction with our financial statements and the related notes thereto appearing elsewhere in this prospectus and in the section of this prospectus entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. We have derived the statements of operations data for the years ended December 31, 2011 and 2012 and the balance sheet data as of December 31, 2011 and 2012 from our audited financial statements appearing elsewhere in this prospectus. We have derived the statements of operations data for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 and balance sheet data as of March 31, 2013 from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and, in the opinion of management, reflect all adjustments, consisting only of normal recurring adjustments, necessary to fairly state our financial position as of March 31, 2013 and results of operations for the three months ended March 31, 2012 and 2013. Our historical results for any prior period are not necessarily indicative of the results to be expected in any future period.
Years Ended December 31, | Three Months Ended March 31, | Period From January 29, 2007 (Inception) to March 31, 2013 | ||||||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | $ | 1,844,044 | $ | 1,165,645 | $ | 297,285 | $ | 110,981 | $ | 16,102,510 | ||||||||||
General and administrative | 570,524 | 836,781 | 205,137 | 221,049 | 3,525,582 | |||||||||||||||
Purchase of in-process research and development | — | — | — | — | 650,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total operating expenses | 2,414,568 | 2,002,426 | 502,422 | 332,030 | 20,278,092 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Loss from operations | (2,414,568 | ) | (2,002,426 | ) | (502,422 | ) | (332,030 | ) | (20,278,092 | ) | ||||||||||
Total other income (expense) | 13,324 | (15,102 | ) | 2,264 | (161,962 | ) | (70,903 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss and comprehensive loss | $ | (2,401,244 | ) | $ | (2,017,528 | ) | $ | (500,158 | ) | $ | (493,992 | ) | $ | (20,348,995 | ) | |||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net loss per common share, basic and diluted(1) | $ | (2.18 | ) | $ | (1.79 | ) | $ | (0.45 | ) | $ | (0.44 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted-average shares used to compute basic and diluted net loss per share(1) | 1,102,625 | 1,124,000 | 1,118,375 | 1,133,375 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Pro forma net loss per common share, basic and diluted (unaudited)(1) | $ | (0.57 | ) | $ | (0.10 | ) | ||||||||||||||
|
|
|
| |||||||||||||||||
Weighted-average shares used to compute pro forma net loss per common share, basic and diluted (unaudited)(1) | 3,563,002 | 3,572,377 | ||||||||||||||||||
|
|
|
| |||||||||||||||||
- 40 -
| As of December 31, | As of March 31, 2013 | |||||||||||
| 2011 | 2012 | |||||||||||
| (unaudited) | ||||||||||||
Balance Sheet Data: | ||||||||||||
Cash and cash equivalents | $ | 865,876 | $ | 116,013 | $ | 1,669,518 | ||||||
Working capital (deficit) | 570,835 | (454,396 | ) | 1,001,053 | ||||||||
Total assets | 905,335 | 116,013 | 1,680,018 | |||||||||
Current liabilities (including warrant liability) | 334,500 | 570,409 | 668,465 | |||||||||
Long-term debt, net of debt discount | — | 979,792 | 2,936,607 | |||||||||
Convertible preferred stock | 18,225,166 | 18,225,166 | 18,225,166 | |||||||||
Deficit accumulated during the development stage | (17,837,475 | ) | (19,855,003 | ) | (20,348,995 | ) | ||||||
Total stockholders’ deficit | (17,654,331 | ) | (19,659,354 | ) | (20,150,220 | ) | ||||||
- 41 -
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes and other financial information included elsewhere in this prospectus. Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this prospectus for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a specialty pharmaceutical company focused primarily on the development of drugs to treat gastrointestinal, or GI, disorders and diseases. We are developing EVK-001, a metoclopramide nasal spray for the relief of symptoms associated with acute and recurrent diabetic gastroparesis in women with diabetes mellitus. Diabetic gastroparesis is a GI disorder afflicting millions of sufferers worldwide, in which the stomach takes too long to empty its contents resulting in serious digestive system symptoms. Metoclopramide is the only product currently approved in the United States to treat gastroparesis, and is currently available only in oral and intravenous forms. EVK-001 is a novel formulation of this drug, designed to provide systemic delivery of metoclopramide through intranasal administration.
We have evaluated EVK-001 in a multicenter, randomized, double-blind, placebo-controlled parallel group, dose-ranging Phase 2b clinical trial in 287 patients with diabetic gastroparesis where EVK-001 was observed to be effective in improving the most prevalent and clinically relevant symptoms associated with gastroparesis in women while exhibiting a favorable safety profile. We plan to initiate a Phase 3 trial of EVK-001 in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in the second half of 2013.
We have no products approved for sale, and we have not generated any revenue from product sales or other arrangements. We have primarily funded our operations through the sale of our convertible preferred stock and borrowings under our loan and security agreements.Gimoti. We have incurred losses in eachevery year since our inception. Our net losses were $2.4 million and $2.0 million for the years ended December 31, 2011 and 2012, respectively, and $0.5 million for the three months ended March 31, 2013. As of December 31, 2012 and March 31, 2013, we had an accumulated deficit of $19.9 million and $20.3 million, respectively. Substantially all of ourThese operating losses resulted from expenses incurred in connection with advancing EVK-001Gimoti through development activities, from pre-commercialization and commercialization costs and from other general and administrative costs associated with operating our operations.business. We expect to continue to incur significant expenses and increasing operating losses for at leastuntil revenues from the next several years.
Our recurring losses from operations, negative cash flows and insufficient working capital raise substantial doubt aboutsales of Gimoti exceed our ability to continue as a going concern. In its report on our financial statements for the year ended December 31, 2012, our independent registered public accounting firm included an explanatory paragraph.expenses, if ever. We may never become profitable, or if we do, we may not be able to sustain profitability on a recurring basis.
Questcor Asset Purchase Agreement
We acquired all worldwide rights, data, patents and other related assets associated with EVK-001 from Questcor Pharmaceuticals in June 2007. We paid to Questcor $650,000 in the form of an upfront payment, and will be required to make additional milestone payments totaling up to $52.0 million. These milestones include up to $5.0 million in payments if EVK-001 achieves the following development targets:
$0.5 million upon the initiation of the first patient dosing in our planned Phase 3 clinical trial for EVK-001;
$1.5 million upon the FDA’s acceptance for review of an NDA for EVK-001; and
$3.0 million upon the FDA’s approval of EVK-001.
The remaining $47.0 million in milestone payments depend on EVK-001’s commercial success and will only apply if EVK-001 receives regulatory approval. In addition, we will be required to pay to Questcor a low single digit royalty on net sales of EVK-001. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering EVK-001, which is expected to occur in 2030.
- 42 -
Financial Operations OverviewStrategy
Research and Development Expenses
We expense all research and development expenses as they are incurred. Research and development expenses primarily include:
clinical trial and regulatory-related costs;
expenses incurred under agreements with contract research organizations, or CROs, investigative sites and consultants that conduct our clinical trials;
manufacturing and stability testing costs and related supplies and materials;
employee-related expenses, including salaries, benefits, travel and stock-based compensation expense;
All of our research and development expenses to date have been incurred in connection with EVK-001. We expect our research and development expenses to increase for the foreseeable future as we advance EVK-001 through clinical development, including the conduct of our planned Phase 3 clinical trial. The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. We are unable to estimate with any certainty the costs we will incur in the continued development of EVK-001. However, we currently estimate the costs to complete our Phase 3 clinical trial in women, our companion clinical trial in men and a thorough QT study of EVK-001 will be approximately $15.0 million. Clinical development timelines, the probability of success and development costs can differ materially from expectations. We may never succeed in achieving marketing approval for our product candidate.
The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
per patient trial costs;
the number of sites included in the trials;
the countries in which the trials are conducted;
the length of time required to enroll eligible patients;
the number of patients that participate in the trials;
the number of doses that patients receive;
the cost of comparative agents used in trials;
the drop-out or discontinuation rates of patients;
potential additional safety monitoring or other studies requested by regulatory agencies;
the duration of patient follow-up; and
the efficacy and safety profile of the product candidate.
We do not expect EVK-001 to be commercially available, if at all, for the next few years.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related benefits, including stock-based compensation. Our general and administrative expenses consisted of payroll expenses for our two full-time employees during the two-year period ended December 31, 2012 and the three months ended March 31, 2012 and 2013. Other general and administrative expenses include professional fees for auditing, tax, patent costs and legal services.
- 43 -
We expect that general and administrative expenses will increase in the future as we expand our operating activities and incur additional costs associated with being a publicly-traded company and maintaining compliance with exchange listing and Securities and Exchange Commission requirements. These increases will likely include higher consulting costs, legal fees, accounting fees, directors’ and officers’ liability insurance premiums and fees associated with investor relations.
Total Other Income (Expense)
Total other income (expense) consists primarily of interest income we earn on interest-bearing accounts and money market funds for cash and cash equivalents, interest expense incurred on our outstanding debt and changes in the fair value of our warrant liability and preferred stock purchase right liability.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States (GAAP). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, as well as the reported revenues and expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our financial statements appearing elsewhere in this prospectus, we believe that the following accounting policies are the most critical for fully understanding and evaluating our financial condition and results of operations.
Accrued Research and Development Expenses
As part of the process of preparing financial statements, we are required to estimate and accrue expenses, the largest of which are research and development expenses. This process involves the following:
communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost;
estimating and accruing expenses in our financial statements as of each balance sheet date based on facts and circumstances known to us at the time; and
periodically confirming the accuracy of our estimates with selected service providers and making adjustments, if necessary.
Examples of estimated research and development expenses that we accrue include:
fees paid to CROs in connection with toxicology studies and clinical studies;
fees paid to investigative sites in connection with clinical studies;
fees paid to contract manufacturing organizations in connection with the production of clinical study materials; and
professional service fees for consulting and related services.
- 44 -
We base our expense accruals related to clinical studies on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors, such as the successful enrollment of patients, site initiation and the completion of clinical study milestones. Our service providers invoice us monthly in arrears for services performed. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. To date, we have not experienced significant changes in our estimates of accrued research and development expenses after a reporting period. However, due to the nature of estimates, we cannot assure you that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our clinical studies and other research activities.
Estimated Fair value of Convertible Preferred Stock Warrants
Freestanding warrants for the purchase of convertible preferred stock that is either subject to a put right or redeemable are classified as liabilities on the balance sheet at their estimated fair value. At the end of each reporting period, changes in estimated fair value during the period are recorded as a component of other income (expense). We will continue to adjust the carrying value of these warrants until the earlier of the exercise of the warrants or the completion of a liquidity event, including the completion of an initial public offering, or IPO, at which time the liabilities will be reclassified to stockholders’ deficit. As of December 31, 2012 and March 31, 2013, we had outstanding warrants exercisable to purchase 70,000 and 110,000 shares, respectively, of our Series A convertible preferred stock. We estimate the fair values of the convertible preferred stock warrants using the Black-Scholes option pricing model based on inputs as of the valuation measurement dates for the estimated fair value of the underlying convertible preferred stock, the remaining contractual terms of the warrants, risk-free interest rates, expected dividend rates and the estimated volatility of the price of the convertible preferred stock. The consummation of this offering will result in the conversion of our Series A convertible preferred stock into common stock. Upon such conversion, the preferred stock warrants will be reclassified to stockholders’ equity (deficit) and will no longer be subject to remeasurement.
Other Accounting Policies
Although our stock-based compensation expense may be significant in future periods, we have not issued any stock awards since February 2011 and have not recorded a significant amount of stock-based compensation expense during the periods presented. See Note 2—Summary of Significant Accounting Policies—Stock-Based Compensation Expense to our audited financial statements included elsewhere in this prospectus.
Other Information
Net Operating Loss Carryforwards
As of December 31, 2012, we had federal and California tax net operating loss carryforwards of approximately $18.6 million and $18.2 million, respectively. The federal and California net loss carryforwards will begin to expire in 2028 and 2018, respectively, unless previously utilized. As of December 31, 2012, we also had federal and California research and development tax credit carryforwards of $525,000 and $428,000, respectively. The federal research and development tax credit carryforwards will begin to expire in 2028. The California research and development tax credit carryforwards are available indefinitely.
Under Section 382 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We have not completed our analysis to determine what, if any, impact any prior ownership change has had on our ability to utilize our net operating loss carryforwards.
- 45 -
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board (PCAOB) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (a) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more, (b) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering, (c) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years or (d) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
Results of Operations
Comparison of Three Months Ended March 31, 2012 and 2013
The following table summarizes the results of our operations for the three months ended March 31, 2012 and 2013:
| Three Months Ended March 31, | Increase/ (Decrease) | |||||||||||
| 2012 | 2013 | |||||||||||
Research and development | $ | 297,285 | $ | 110,981 | $ | (186,304 | ) | |||||
General and administrative | 205,137 | 221,049 | 15,912 | |||||||||
Other income (expense): | ||||||||||||
Interest income | 764 | 1,353 | 589 | |||||||||
Interest expense | — | (39,315 | ) | (39,315 | ) | |||||||
Change in fair value of warrant liability | 1,500 | (124,000 | ) | (125,500 | ) | |||||||
Total other income (expense) | 2,264 | (161,962 | ) | (164,226 | ) | |||||||
Research and Development Expenses.Research and development expenses were $0.3 million for the three months ended March 31, 2012, compared to $0.1 million for the three months ended March 31, 2013. The decrease of $0.2 million is primarily related to the decrease of $0.3 million in compensation costs related to the election of our board of directors to not pay 2012 bonuses and from finalizing clinical development-related costs as we completed the Phase 2 clinical trial for EVK-001, and an increase of $0.1 million due to the initiation of manufacturing of clinical trial materials for phase 3.
General and Administrative Expenses.General and administrative expenses were $0.2 million for the three months ended March 31, 2012, compared to $0.2 million for the three months ended March 31, 2013.
Other Income (Expense).Other income (expense) was approximately $2,000 for the three months ended March 31, 2012 and primarily consisted of interest income and a minor decline in the fair value of our warrant
- 46 -
liability. Other income (expense) was $(162,000) for the three months ended March 31, 2013 and primarily consisted of $39,000 of interest expense related to advances under our loan and security agreement and $124,000 of expense related to the increase in fair value of our outstanding warrant liability.
Comparison of Fiscal Years Ended December 31, 2011 and 2012
The following table summarizes the results of our operations for the fiscal years ended December 31, 2011 and 2012:
| Years Ended December 31, | Increase/ (Decrease) | |||||||||||
| 2011 | 2012 | |||||||||||
Research and development | $ | 1,844,044 | $ | 1,165,645 | $ | (678,399 | ) | |||||
General and administrative | 570,524 | 836,781 | 266,257 | |||||||||
Other income (expense): | ||||||||||||
Interest income | 10,696 | 1,690 | (9,006 | ) | ||||||||
Interest expense | (2,872 | ) | (24,042 | ) | (21,170 | ) | ||||||
Change in fair value of warrant liability | 5,500 | 7,250 | 1,750 | |||||||||
Total other income (expense) | 13,324 | (15,102 | ) | (28,426 | ) | |||||||
Research and Development Expenses. Research and development expenses were $1.8 million for the year ended December 31, 2011, compared to $1.2 million for the year ended December 31, 2012. The decrease of $0.7 million is primarily related to the decrease in development-related costs as we finalized the Phase 2 clinical trial for EVK-001 and engaged with the FDA for Phase 3 planning.
General and Administrative Expenses. General and administrative expenses were $0.6 million for the year ended December 31, 2011, compared to $0.8 million for the year ended December 31, 2012. The increase of $0.3 million is primarily related to an increase in accruals for bonus payments to our officers in 2012.
Other Income (Expense). Other income (expense) was $13,000 for the year ended December 31, 2011 and primarily consisted of interest income. Other income (expense) was $(15,000) for the year ended December 31, 2012 and primarily consisted of $24,000 of interest expense related to advances under our loan and security agreement, offset by $2,000 of interest income and $7,000 of other income related to the decrease in fair value of our outstanding warrant liability.
Liquidity and Capital Resources
We have funded our operations primarily from the sale of convertible equity securities and borrowings under our loan and security agreements. Through March 31, 2013, we have received $17.7 million in net proceeds from the sale of our Series A convertible preferred stock and net proceeds of $3.0 million under our current loan and security agreement. We have incurred losses since inception and negative cash flows from operating activities. As of March 31, 2013, we had approximately $1.7 million in cash and cash equivalents, working capital of $1.0 million and an accumulated deficit of $20.3 million.
In June 2012, we entered into a $3.0 million loan and security agreement with Silicon Valley Bank which is collateralized by our personal property. Interest on advances under the agreement is at a fixed interest rate equal to 4.50%. The loan and security agreement contains only non-financial covenants. Advances under the loan and security agreement have an interest-only period through December 31, 2013 and a 24-month payback period commences in January 2014. As of March 31, 2013, we had drawn down the entire $3.0 million available under the agreement to fund working capital and have no credit available for future borrowings. In connection with the loan and security agreement, we issued a warrant to Silicon Valley Bank which is immediately exercisable for an aggregate of 60,000 shares of our Series A convertible preferred stock, at an exercise price of $1.50 per share. The warrant will be adjusted to provide for the purchase of an aggregate of 12,000 shares of our common stock at an exercise price of $7.50 per share immediately prior to the closing of this offering.
- 47 -
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. In the near-term, we anticipate that our expenses will increase substantially as we:
initiate significant clinical trials associated with EVK-001, including our planned Phase 3 clinical trial that we plan to initiate in the second half of 2013;
hire additional staff, including clinical, scientific, operational, financial and management personnel; and
to maintain, expand and protect our intellectual property portfolio.
To fund further operations we will need to raise additional capital. The expected net proceeds from this offering will not be sufficient for us to complete our planned Phase 3 clinical trial of EVK-001 or any additional development requirements requested by the FDA, or, if applicable, to prepare for commercialization of EVK-01 should we receive product approval. At this time, due to the risks inherent in the drug development process, we are unable to estimate with any certainty the costs we will incur in the continued development of EVK-001 for potential commercialization. However, we currently estimate the costs to complete our Phase 3 clinical trial in women, our companion clinical trial in men and a thorough QT study of EVK-001 will be approximately $15.0 million. Accordingly, we will continue to require substantial additional capital beyond the expected proceeds from this offering to continue our clinical development and potential commercialization activities. The amount and timing of our future funding requirements will depend on many factors, including the pace and results of our clinical development efforts. We anticipate that we will seek to fund our operations through public or private equity or debt financings or other sources, such as potential collaboration arrangements. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies.
The following table summarizes of our cash flows for the years ended December 31, 2011 and 2012 and the three months ended March 31, 2012 and 2013:
| Years Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||
Net cash used in operating activities | $ | (2,893,108 | ) | $ | (1,749,863 | ) | $ | (447,573 | ) | $ | (446,495 | ) | ||||
Net cash provided by (used in) financing activities | (277,779 | ) | 1,000,000 | — | 2,000,000 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Net (decrease) increase in cash and cash equivalents | $ | (3,170,887 | ) | $ | (749,863 | ) | $ | (447,573 | ) | $ | 1,553,505 | |||||
|
|
|
|
|
|
|
| |||||||||
Operating Activities. Net cash used in operating activities was $2.9 million for the year ended December 31, 2011, compared to net cash used in operating activities of $1.8 million for the year ended December 31, 2012. Net cash used in operating activities was $0.4 million for both the three months ended March 31, 2012 and 2013. In all periods the primary use of cash was to fund our net loss.
Financing Activities. Net cash used in financing activities was $0.3 million for the year ended December 31, 2011 compared to net cash provided by financing activities of $1.0 million for the year ended December 31, 2012. In 2011 we paid down our outstanding balances under our original loan and security agreement while we took advances on our current loan and security agreement in 2012. During the three months ended March 31, 2013 our sole financing activity was to draw $2.0 million under our loan and security agreement to fund working capital requirements. We had no financing activity during the three months ended March 31, 2012.
The report of our independent registered public accounting firm on our audited consolidated financial statements for the year ended December 31, 2012 includes an explanatory paragraph stating that our recurring losses from operations and working capital deficit raise substantial doubt about our ability to continue as a going concern. If we are unable to obtain additional financing on commercially reasonable terms, our business, financial condition and results of operations will be materially adversely affected and we may be unable to continue as a going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements.
- 48 -
We believe that our existing cash and cash equivalents as of March 31, 2013, together with interest thereon, and the estimated net proceeds from this offering, will be sufficient to meet our anticipated cash requirements for approximately 12 months after the date of this prospectus. However, our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
the initiation, progress, costs, results of and timing of our clinical development program for EVK-001, including our planned Phase 3 clinical trial;
the need for, and the progress, costs and results of, any additional clinical trials of EVK-001 we may initiate based on the results of our planned clinical trials or discussions with the FDA, including any additional trials the FDA or other regulatory agencies may require evaluating the safety of EVK-001;
the outcome, costs and timing of seeking and obtaining regulatory approvals from the FDA, and any similar regulatory agencies;
the timing and costs associated with manufacturing EVK-001 for clinical trials and other studies and, if approved, for commercial sale;
our need and ability to hire additional management, development and scientific personnel;
the cost to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights;
the timing and costs associated with establishing sales and marketing capabilities;
market acceptance of EVK-001;
the extent to which we are required to pay milestone or other payments under our Questcor asset purchase agreement and the timing of such payments;
the costs of acquiring, licensing or investing in additional businesses, products, product candidates and technologies; and
our need to implement additional internal systems and infrastructure, including financial and reporting systems.
Off-Balance Sheet Arrangements
Through March 31, 2013, we have not entered into and did not have any relationships with unconsolidated entities or financial collaborations, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purpose.
Contractual Obligations
Our most significant clinical trial expenditures are to CROs. The contracts with CROs generally are cancellable, with notice, at our option and do not have any cancellation penalties.
Our long-term debt obligation consists of amounts we are obligated to repay under our loan and security agreement with Silicon Valley Bank, of which we have drawn the full amount of $3.0 million as of January 31, 2013. Unless principal is paid in advance, we are required to make an aggregate of $135,000 of interest-only
- 49 -
payments in 2013. In January 2014 we are required to begin making the first of 24 monthly principal and interest payments of $131,024, such that the loan balance will be fully repaid in December 2015. We will incur a total of $144,570 of interest charges in 2014 and 2015.
As of December 31, 2012 and March 31, 2013, we had no operating lease commitments.
Quantitative and Qualitative Disclosure about Market Risk
Interest Rate Fluctuation Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. However, because of the short-term nature of our cash and cash equivalents, a sudden change in market interest rates would not be expected to have a material impact on our financial condition and/or results of operations.
Our long-term debt bears interest at a fixed rate and therefore has minimal exposure to changes in interest rates.
Foreign Currency Exchange Risk
To date, all of our contractual obligations have been denominated in U.S. dollars. In the future, we may contract with organizations to manufacture drug product, active pharmaceutical ingredient, container closure system materials as well as CROs and investigational sites in foreign countries. We may therefore become subject to fluctuations in foreign currency rates in connection with these agreements.
Inflation Risk
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation has had a material effect on our business, financial condition or results of operations during the years ended December 31, 2011 and 2012 and the three months ended March 31, 2012 and 2013.
- 50 -
Overview
We are a specialty pharmaceutical company focused primarily on the development of drugs to treat gastrointestinal, or GI, disorders and diseases. We are developing EVK-001, a metoclopramide nasal spray for the relief of symptoms associated with acute and recurrent diabetic gastroparesis in women with diabetes mellitus. Diabetic gastroparesis is a GI disorder afflicting millions of sufferers worldwide, in which the stomach takes too long to empty its contents resulting in serious digestive system symptoms. Metoclopramide is the only product currently approved in the United States to treat gastroparesis, and is currently available only in oral and intravenous forms. EVK-001 is a novel formulation of this drug, designed to provide systemic delivery of metoclopramide through intranasal administration.
Gastroparesis is a condition of delayed gastric emptying in the absence of mechanical obstruction. Gastroparesis results in food remaining in the stomach for a longer time than normal, yielding a variety of symptoms. Gastroparesis is a common problem in individuals with diabetes, but also is observed in patients with prior gastric surgery, a preceding infectious illness, pseudo-obstruction, collagen vascular disorders and anorexia nervosa. According to the American Motility Society Task Force on Gastroparesis, the prevalence of gastroparesis is estimated to be up to 4% of the United States population. Symptoms of gastroparesis include nausea, vomiting, abdominal pain, bloating, early satiety, lack of appetite, and weight loss. The disorder can lead to considerable pain and discomfort, poor nutrition, impaired glycemic control and diminished quality of life. According to a 2008 study published in theAmerican Journal of Gastroenterology, it is estimated that hospitalization costs associated with gastroparesis exceed $3.5 billion annually.
We believe intranasal administration has the potential to offer our target gastroparesis patients a preferred treatment option because, unlike oral metoclopramide, EVK-001 is designed to effectively bypass the digestive system and allow for more predictable drug administration of our proprietary nasal spray formulation across the thin mucosa in the nasal cavity. Intranasal drug delivery effectively bypasses the gut, unlike oral formulations which might be delayed in absorption due to gastroparesis itself. For patients suffering from nausea and vomiting, EVK-001 is designed to allow for rapid and predictable drug administration.
We have evaluated EVK-001 in a multicenter, randomized, double-blind, placebo-controlled parallel group, dose-ranging Phase 2b clinical trial in 287 patients with diabetic gastroparesis where EVK-001 was observed to be effective in improving the most prevalent and clinically relevant symptoms associated with gastroparesis in women while exhibiting a favorable safety profile. We plan to initiate a Phase 3 trial of EVK-001 in female patients with symptoms associated with acute and recurrent diabetic gastroparesis in the second half of 2013. We anticipate receiving topline data from this trial in late 2014 or early 2015. We will need to successfully complete this trial, as well as a thorough QT, or TQT, study, which is an evaluation of cardiac safety, before we are able to submit a new drug application, or NDA, to the U.S. Food and Drug Administration, or FDA, for EVK-001. FDA approval of the NDA is required in order for us to commercially market EVK-001 in the United States. In addition, based on our discussions with the FDA, we plan to conduct a similar study for safety and efficacy in male patients with symptoms associated with acute and recurrent diabetic gastroparesis to assess the safety and efficacy of EVK-001 in men. We anticipate this trial will be conducted concurrently with the Phase 3 trial in women. The completion of the male companion trial is not required for submission of the NDA for EVK-001; however, we expect to include safety data from this trial in our NDA submission for EVK-001.
At this time, due to the risks inherent in the drug development process, we are unable to estimate with any certainty the costs we will incur in the continued development of EVK-001 for potential commercialization. However, we currently estimate the costs to complete our Phase 3 clinical trial in women, our companion clinical trial in men and a TQT study of EVK-001 will be approximately $15.0 million.
- 51 -
Business Strategy
Our objective is to develop and bring to market products to treat acute and chronic GI motility disorders that are not satisfactorily treated with current therapies and that represent significant market opportunities. Our business strategy is to:
|
|
|
|
|
The Gastrointestinal Market
The health of the GI system has a major effect on an individual’s daily activities and quality of life. A retrospective review published by the National Institute of Diabetes and Digestive and Kidney Diseases estimated that in 2004 there were more than 72 million ambulatory care visits with a diagnosis of a GI disorder
In 2004, the total cost of prescription drugsallow us to directly market Gimoti in the United States was $12.3 billion,States. We have engaged Eversana to utilize its internal sales organization, along with other commercial functions, for market access, marketing, distribution, and over half of this cost ($7.7 billion) was associated with drugs prescribed for Gastroesophageal Reflux Disease, or GERD. Peptic Ulcer disease, hepatitis C, IBS and IBD were major contributorsother related patient support services. If Eversana terminates the agreement, our plan moving forward to the remaining drug cost. Historically GI product development efforts have focused on indications with the largest patient populations such as GERD, constipation, peptic ulcers and irritable bowel syndrome, or IBS. Ascommercialize Gimoti is to initially employ a result, limited innovation has occurred in other segments of the GI market, such as upper GI motility disorders, even though these disorders affect several million patients worldwide. Consequently, due to the limited treatment options available for upper GI motility disorders,primarily digital marketing strategy. In addition, we believe there ismay hire a substantial market opportunity for us to address significant unmet medical needs, initially for diabetic gastroparesis.
GI Motility Disorders
Motility disorders are one of the most common GI disorders. Motility disorders affect the orderly contractions or relaxation of the GI tract which move contents forward and prevent backwards egress. This is important in the normal movement of food through the GI tract. Motility disorders are sometimes referred to as functional GI disorders to highlight that many abnormalities in gut function can occur even when anatomic structures appear normal. Functional GI disorders affect the upper and lower GI tract and include gastroparesis, GERD, functional dyspepsia, constipation and IBS. It has been estimated by the International Foundation for
- 52 -
Functional Gastrointestinal Disorders that one in four people in the United States suffer from functional GI disorders, having symptoms such as abdominal pain, nausea, vomiting, constipation, diarrhea, bloating, decreased appetite, early satiety, swallowing difficulties, heartburn and/or incontinence.
Gastroparesis
Gastroparesis is a debilitating, chronic condition that has a significant impact on patients’ lives. It is characterized by slow or delayed gastric emptying and evidence of gastric retention in the absence of mechanical obstruction. Muscular contractions in the stomach, which move food into the intestine, may be too slow, out of rhythm or cease altogether. The following graph depicts the timing associated the emptying of solids in patients with diabetic gastroparesis compared to normal individuals:
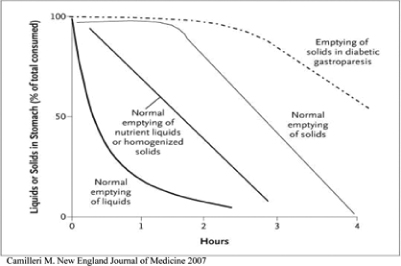
The stomach is a muscular sac between the esophagus and the small intestine where the digestion of food begins. The stomach makes acids and enzymes referred to as gastric juices which are mixed with food by the churning action of the stomach muscles. Peristalsis is the contraction and relaxation of the stomach muscles to physically breakdown food and propel it forward. The crushed and mixed food is liquefied to form chyme and is pushed through the pyloric canal into the small intestine in a controlled and regulated manner.
In gastroparesis, the stomach does not perform these functions normally causing characteristic symptoms that include nausea, vomiting, early satiety, bloating, and abdominal pain. As a result of these symptoms, patients may limit their food and liquid intake leading to poor nutrition and dehydration with the patient ultimately requiring hospitalization. If left untreated or not adequately treated, gastroparesis causes significant acute and chronic medical problems, including additional diabetic complications resulting from poor glucose control.
Gastroparesis in the Hospital Setting
When patients experience a flare of their gastroparesis symptoms that cannot be adequately managed by oral medications, they may be hospitalized for hydration, parenteral nutrition, and correction of abnormal blood glucose electrolyte levels. In this setting, intravenous metoclopramide is the first line of treatment. Typically, these diabetic patients with severe gastroparesis symptoms remain in the hospital until they are stabilized and able to be effectively treated with oral metoclopramide. These hospitalizations are costly and expose patients to increased risks, including hospital-acquired infections.
- 53 -
Theselect number of patients with gastroparesis that require hospitalization duedirect sales representatives to their disease is growing, according to a study published in the American Journalcommercialize Gimoti.
2
A study of patients in clinics at the University of Pittsburgh Medical Center between January 2004 and December 2008 published in the Journal of Gastroenterology and Hepatology, showed that patients with diabetic or post-surgical gastroparesis had significantly more emergency room visits than other gastroparesis groups. The study reinforced the view that gastroparesis constitutes a significant burden for patients and the healthcare system, with more than one-third of patients requiring hospitalization. The number of emergency room visits and annual days of inpatient treatment were comparable to patients with Crohn’s disease. The study indicated that patients received an average of 6.7 prescriptions on admission. Eighty percent of the patients identified in the University of Pittsburgh study were women.
Etiology
Gastroparesis can be a manifestation of many systemic illnesses, arise as a complication of select surgical procedures, or develop due to unknown causes. Any disease inducing neuromuscular dysfunction of the GI tract can result in gastroparesis, with diabetes being one of the leading known causes. In a 2007 study published in Current Gastroenterology Reports, 29% of gastroparesis cases were found in association with diabetes, 13% developed as a complication of surgery and 36% were due to unknown causes. According to the American Motility Society Task Force on Gastroparesis, up to 4% of the U.S. population experiences symptomatic manifestations of gastroparesis. As the incidence of diabetes rises worldwide, the prevalence of gastroparesis is expected to rise correspondingly.
The most common identified cause of gastroparesis is diabetes mellitus typically have long-standing and often poorly controlled diabetes. The underlying mechanism of diabetic gastroparesis is unknown; although, it is thought to be related in part to neuropathic changes in the vagus nerve and/or the myenteric plexus. Prolonged elevated serum glucose levels are also associated with vagus nerve damage. The vagus nerve controls the movement of food through the digestive tract and when it is damaged, forward movement of food through the GI tract is delayed. The prevalence of diabetes in the United States is rapidly rising with the Centers for Disease Control estimating that one in ten adults currently sufferinitiation timing pending additional feedback from the disease. Sedentary lifestyles, poor dietary habitsFDA.
According to a study published in the Journal of Gastrointestinal and Liver Diseases in July 2010, between 25% and 55% of Type 1 and 15% and 30% of Type 2 diabetics suffer from symptoms associated with the condition and diabetics are 29% of the total gastroparesis population. A 2007 study published in Current Gastroenterology Reports states that approximately 36% of gastroparesis patients suffer from idiopathic gastroparesis. The development of idiopathic gastroparesis is thought to be related to loss of myenteric ganglion cells in the distal large bowel (myenteric hypoganglionosis) and reduction in the interstitial cells of Cajal, which help control contraction of the smooth muscle in the GI tract. Post-surgical gastroparesis is a smaller subset of the total patient pool and accounts for approximately 13% of all cases of the disease, according to a 2007 study published in Current Gastroenterology Reports. Post-surgical gastroparesis is often associated with peptic ulcer surgery, bariatric procedures or esophageal procedures and is thought to result from damage/desensitization of the vagus nerve.
- 54 -
Prevalence
In 2011, the American Diabetes Association estimated that diabetes affects approximately 26 million people of all ages in the United States, equating to about 8.3% of the U.S. population. Based on prevalence data,maximize the potential gastroparesis patient pool infor Gimoti.
Unmet Needs in Gastroparesis Treatment
Market research and physician interviews demonstrate that existing treatment options for diabetic gastroparesis are inadequate and there is a high level of interest in effective outpatient options for managing patients with gastroparesis symptoms. The market is currently served by oral and intravenous metoclopramide, and the oral disintegrating tablet, or ODT, formulation of metoclopramide (Metozolv® ODT). Due to the limited availability of FDA-approved treatments for gastroparesis, physicians resort to using medications “off-label” in an attempt to address individual symptoms experienced by patients. Off-label therapies are pharmaceuticals prescribed by physicians for an unapproved indication or in an unapproved age group, unapproved dose or unapproved form of administration. Examples of drugs used without FDA approval in gastroparesis include; erythromycin, domperidone, and Botox® injected via endoscopic procedure directly into the lower gastric sphincter. Previously-approved drugs, such as cisapride and tegaserod, are no longer commercially available in the United States because of safety concerns.
EVK-001 is a non-oral, promotility and anti-emetic treatment that we believe hasGimoti, including the potential to significantly improveexplore regulatory approval outside the standardUnited States.
Financial Update
Our Solution: EVK-001 (Metoclopramide Nasal Spray)
We are developing EVK-001, a dopamine antagonist / mixed 5-HT3 antagonist / 5-HT4 agonist with promotility and anti-emetic effects,financial statements for the relief of symptoms associated with acutequarter and recurrent diabetic gastroparesisyear ended December 31, 2023, will not be available until after this offering is completed and consequently will not be available to you prior to investing in women with diabetes mellitus. Since its approvalour securities offered in 1980, oralthis offering. Based upon preliminary estimates and intravenous metoclopramide have been the only products approved in the United Statesinformation available to treat gastroparesis. EVK-001 is a novel formulation of metoclopramide offering systemic delivery by intranasal administration.
We are developing the intranasal formulation of metoclopramide to provide our targeted patients with acute or recurrent symptoms of diabetic gastroparesis with a product that can be systemically deliveredus as an alternative to the oral or intravenous routes of administration. Intranasal delivery is possible because the mucosa of the nasal cavity is single epithelial cell layer which is well vascularizeddate of this registration statement, we expect to report that we had approximately $4.7million of cash and allows metoclopramide molecules to be transferred directly to the systemic circulation. There is no first pass liver metabolism required prior to onsetcash equivalents as of action. Since gastroparesis is a disease that blocks or slows the movement of the contents of the stomach to the small intestine, oral drug administration is often compromised. Unlike the oral tablet formulation of metoclopramide, we believe that EVK-001 may be tolerated even when patients are experiencing nausea and vomiting. The intranasal formulation may also provide a predictable and consistent means of delivering metoclopramide in patients with delayed gastric emptying and/or frequent vomiting.
December 31, 2023. We believe that if approved EVK-001 could also offer an alternative route of administration for female patients with severe symptoms of diabetic gastroparesis, who typically receive the intravenous formulation of metoclopramide. A nasal spray formulation of metoclopramide could offer an alternative route of administration
- 55 -
for female patients with severe symptoms of diabetic gastroparesis receiving the parenteral formulation of metoclopramide. Following hospitalization for intravenous metoclopramide, a nasal spray formulation would also provide a non-oral optionhave not yet completed our quarter-end financial close process for the transitionquarter ended December 31, 2023. This estimate of our cash and cash equivalents as of December 31, 2023 is preliminary, has not been audited and is subject to an outpatient treatment.
Phase 2b Clinical Trial
We have evaluated EVK-001 inchange upon completion of our financial statement closing procedures. Additional information and disclosure would be required for a multicenter, randomized, double-blind, placebo-controlled parallel group, dose-ranging Phase 2b clinical trial in 287 subjects (79% female) with diabetic gastroparesis. Subjects in the trial were between the agesmore complete understanding of 18our financial position and 75, with a history of diabetes (type I and type II) and diabetic gastroparesis, who had a baseline modified Gastroparesis Cardinal Symptom Index Daily Diary, or mGCSI-DD, of³ 2 and£ 4 for the seven days prior to randomization on the drug or placebo.
In this trial, EVK-001 demonstrated effectiveness in reducing the most common and clinically relevant symptoms associated with gastroparesis in women, while exhibiting a favorable safety profile. EVK-001 was shown to provide a statistically significant clinical benefit as defined by a reduction in the symptoms of gastroparesis as measured by the mGCSI-DD in women (p<0.025). Male subjects treated with EVK-001 showed some improvement in gastroparesis symptoms, but did not show a statistically significant difference compared to placebo. Due to these results in men, the primary objective of statistical significance in the overall population was not achieved (p=0.15).
We believe this Phase 2b trial is the largest ever conducted in a diabetic gastroparesis population for any approved metoclopramide dosage forms (oral tablet, orally disintegrating tablet and intravenous). Previous metoclopramide studies enrolled small numbers of subjects and did not evaluate gender. Fewer than 150 subjects were enrolled across all studies included in the NDA for Reglan. The results of the Phase 2b trial are consistentoperations as of December 31, 2023. Our independent registered public accounting firm has not audited, reviewed or performed any procedures with what is known about gender effects in other GI motility disorders. GI motility and functional GI disorders, including gastroparesis, are more common in females than in males. Also, healthy females generally have slower gastric emptying rates. In a study conducted at Temple University, Parkman et al have shown that gastric emptying of solid food in normal young women is slower than in age-matched men, even in the first 10 days of the menstrual cycle when estrogen and progesterone levels are low, and that the delay in gastric emptying of solids in women appears to be primarily due to altered distal gastric motor function. One explanation may be that less vigorous antral contractions may contribute to slower breakdown of food particles and thus delay the rate of emptying.
Gastrointestinal disorders present differently in males and females and responses to therapy vary by gender. There is general consensus among thought leaders in GI motility that women have a higher prevalence of symptoms, their neural and sensory pathways differ, and hormones, such as estrogen and progesterone, play a role. While the EVK-001 Phase 2b trial is the first report of a gender-based difference in response to metoclopramide among subjects with diabetic gastroparesis, gender effects have been reported in drug studies for other GI disorders, such as irritable bowel syndrome, or IBS. For example, products such as Lotronex® (alosetron), Zelnorm® (tegaserod) and Amitiza® (lubiprostone) were approved by FDA based on effectiveness in women, but not in men.
Phase 2b Trial Design
The Phase 2b clinical trial consisted of up to a 23-day screening period and a seven-day washout period, followed by 28 days of treatment with study drug. We evaluated two dosage strengths of EVK-001: 10 mg and 14 mg; as well as placebo. The study drug was administered for the 28-day treatment period as a single intranasal spray four times daily, 30 minutes before meals and at bedtime. Subjects recorded the severity of their gastroparesis symptoms in a telephonic diary using an interactive voice response system once each day. The symptoms were analyzed using a patient reported outcomes instrument, the Gastroparesis Cardinal Symptom Index Daily Diary, or GCSI-DD, developed for collecting and analyzing data to evaluate the effectiveness of treatments for gastroparesis.
The GCSI-DD contains nine symptoms (nausea, retching, vomiting, stomach fullness, not able to finish a normal sized meal, feeling excessively full after meal, loss of appetite, bloating, and stomach or belly visibly
- 56 -
larger) grouped in three subscales. The daily score is calculated as a mean of three subscale means. Additional symptoms collected in the daily diary included; abdominal pain, abdominal discomfort, number of hours of nausea, number of episodes of vomiting, and overall severity of gastroparesis symptoms. In close collaboration with FDA and its Study Endpoint and Labeling Division, these additional symptom data were used to further refine the patient reported outcome instrument. The result is a mGCSI-DD comprised of four symptoms (nausea, early satiety, bloating, and upper abdominal pain) rated from zero (none) to five (very severe). The instrument has been optimized to detect symptom variability on a severity continuum from nausea to vomiting.
Phase 2b Efficacy Results
Two patient reported outcome endpoints (mGCSI-DD and GCSI-DD) were examined in the intention-to-treat population based the protocol design and FDA communications:
The primary efficacy endpoint was the change from seven-day baseline to Week 4 of the treatment period in the mGCSI-DD total score (mean of four symptoms).
The second efficacy endpoint analyzed was the change from seven-day baseline to Week 4 of the treatment period in the GCSI-DD total score (mean of three subset means with a total of nine symptoms).
Although an overall improvement in symptoms was observed in EVK-001-treated patients with diabetic gastroparesis compared to placebo, the difference was not statistically significant due to a high placebo response among male subjects. However, statistically significant improvement in gastroparesis symptoms was observed in female subjects with diabetic gastroparesis as measured by the mGCSI-DD and GCSI-DD total scores for both doses of EVK-001 compared to the placebo. The beneficial effect of treatment in females appears to be uniform. The results are consistent across the overall endpoints, the individual components, and the two dose groups.
The observed differences in efficacy were based on gender and were not due to severity of baseline disease, or other demographic characteristics. No statistically significant differences were observed in efficacy between the 10 mg and 14 mg EVK-001 doses; thus the 10 mg dose was considered the lowest effective dose in this study. The table below summarizes thep-values observed for both doses of EVK-001 compared to placebo in the Phase 2b clinical trial across all subjects and for male and female patients separately.
EVK-001 Phase 2b Clinical Trial
Gastroparesis Study Endpoint PointsP-Value Summary
(EVK-001 vs. Placebo: Change from Baseline to Week 4)
| EVK-001 10 mg p-values | EVK-001 14 mg p-values | |||||||
mGCSI-DD Total Score (per FDA guidance)(1) |
| |||||||
All Subjects | 0.1504 | 0.3005 | ||||||
Females | 0.0247 | 0.0215 | ||||||
Males | 0.4497 | 0.2174 | ||||||
GCSI-DD Total Score (per trial protocol)(2) |
| |||||||
All Subjects | 0.2277 | 0.5266 | ||||||
Females | 0.0485 | 0.0437 | ||||||
Males | 0.4054 | 0.0972 | ||||||
P-values for pairwise comparisons are obtained from an ANCOVA model with effects for treatment group and Baseline value as a covariate.
- 57 -
The table below summarizes the key data from the trial across all subjects and for female and male payments separately:
EVK-001 Phase 2b Clinical Trial
Primary Endpoint: Mean mGCSI-DD Total Score Change
from Baseline to Week 4 by All Subjects and Gender
(intent-to-treat, last observation carried forward on treatment)
Time Point | Placebo (N=95) | Metoclopramide 10 mg IN (N=96) | Metoclopramide 14 mg IN (N=96) | |||
ALL SUBJECTS | ||||||
Baseline(1) | ||||||
N | 95 | 96 | 96 | |||
Mean (SD) | 2.8 (0.57) | 2.9 (0.60) | 2.8 (0.62) | |||
Week 4 | ||||||
N | 95 | 96 | 96 | |||
Mean (SD) | 1.8 (1.00) | 1.6 (1.06) | 1.7 (0.90) | |||
Change from Baseline to Week 4 | ||||||
N | 95 | 96 | 96 | |||
Mean (SD) | -1.0 (0.89) | -1.2 (1.18) | -1.2 (0.94) | |||
Difference of Least Square Means (95% CI) | -0.20 (-0.47, 0.07) | -0.14 (-0.42, 0.13) | ||||
Pairwisep-value vs. Placebo(2) | 0.1504 | 0.3005 | ||||
Difference of Least Square Means (95% CI) | 0.06 (-0.22, 0.33) | |||||
Pairwisep-value vs. Metoclopramide 10 mg(2) | 0.6830 | |||||
FEMALES | ||||||
Baseline(1) | ||||||
N | 68 | 65 | 70 | |||
Mean (SD) | 2.7 (0.54) | 2.9 (0.62) | 2.9 (0.62) | |||
Week 4 | ||||||
N | 68 | 65 | 70 | |||
Mean (SD) | 1.9 (1.02) | 1.6 (1.08) | 1.7 (0.94) | |||
Change from Baseline to Week 4 | ||||||
N | 68 | 65 | 70 | |||
Mean (SD) | -0.8 (0.79) | -1.2 (1.18) | -1.3 (0.98) | |||
Difference of Least Square Means (95% CI) | -0.38 (-0.71, -0.05) | -0.38 (-0.71, -0.06) | ||||
Pairwisep-value vs. Placebo(2) | 0.0247 | 0.0215 | ||||
Difference of Least Square Means (95% CI) | -0.00 (-0.33, 0.32) | |||||
Pairwisep-value vs. Metoclopramide 10 mg(2) | 0.9864 | |||||
MALES | ||||||
Baseline(1) | ||||||
N | 27 | 31 | 26 | |||
Mean (SD) | 2.9 (0.63) | 2.8 (0.54) | 2.5 (0.56) | |||
Week 4 | ||||||
N | 27 | 31 | 26 | |||
Mean (SD) | 1.4 (0.84) | 1.6 (1.05) | 1.7 (0.79) | |||
Change from Baseline to Week 4 | ||||||
N | 27 | 31 | 26 | |||
Mean (SD) | -1.4 (0.98) | -1.2 (1.21) | -0.9 (0.78) | |||
Difference of Least Square Means (95% CI) | 0.18 (-0.30, 0.66) | 0.32 (-0.19, 0.83) | ||||
Pairwisep-value vs. Placebo(2) | 0.4497 | 0.2174 | ||||
Difference of Least Square Means (95% CI) | 0.14 (-0.35, 0.63) | |||||
Pairwisep-value vs. Metoclopramide 10 mg(2) | 0.5805 |
- 58 -
Phase 2b Safety Observations
In the Phase 2b clinical trial, EVK-001 10 mg and 14 mg doses were well-tolerated and no differences in the safety profiles were observed between the two doses administered. No serious adverse events occurred related to study treatment. In addition, there were no clinically-meaningful differences observed in clinical laboratory parameters, physical examination findings, or electrocardiogram recordings. Adverse events that occurred more commonly in both EVK-001 10 mg and 14 mg doses compared to placebo (³2% difference between treated compared to placebo groups) were dysgeusia, headache, nasal discomfort, rhinorrhea, throat irritation, fatigue, hypoglycemia, and hyperglycemia.
Treatment-Emergent Adverse Events Reported by More than 2 Subjects in Any Treatment Group
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
| ||||||||||||
Phase 1 Comparative Bioavailability Bridging Study
Our Phase 1 clinical trial of EVK-001 was an open-label, four-treatment, four-period, four-sequence crossover study conducted at a single study center. Forty healthy volunteers were enrolled and randomly assigned to one of four treatment sequences. After an overnight fast, subjects received a single dose of each of the metoclopramide treatments (10 mg EVK-001, 20 mg EVK-001, 10 mg oral tablet, and 5 mg/mL injection) in random sequence with a seven-day washout period between doses. Thirty nine subjects received at least one dose of metoclopramide. The pharmacokinetic analysis population consisted of 37 subjects who received all four treatments and two subjects who received three of the four treatments.
After intranasal administration of the 10 mg and 20 mg doses of EVK-001, mean plasma metoclopramide concentrations increased in a dose-related manner, as did mean values for Cmax and AUC(inf). The absolute bioavailability of EVK-001 after intranasal administration was comparable for the 10 mg (47.4%) and 20 mg
- 59 -
(52.5%) doses as were the bioavailabilities relative to the oral tablet (60.1% and 66.5%, respectively). The graphs below illustrate the mean plasma concentrations of the active ingredient in the two doses of EVK-001 as well as the oral and injection forms.
EVK-001 Phase 1 Clinical Trial
Mean Plasma Concentrations of Metoclopramide
(15 minute intervals 0-2h)
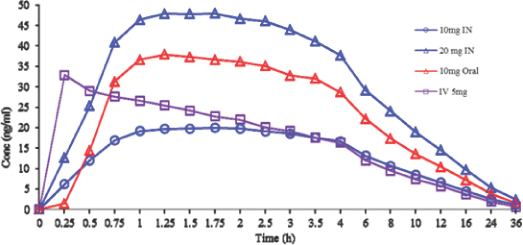
Prior Development
From 1985 to present, we or our predecessors have conducted 24 clinical studies to evaluate the safety and pharmacokinetic profile of nasal spray formulations of metoclopramide in healthy volunteers and the safety, efficacy, pharmacokinetic and pharmacodynamic profile of metoclopramide nasal spray in patients. A total of 1,045 patients have been dosed in these studies with intranasal formulations of metoclopramide at doses ranging from 10 mg to 80 mg. Metoclopramide nasal spray was initially developed by Nastech Pharmaceutical Company, Inc. in precursor formulations to EVK-001 and subsequently acquired and developed by Questcor Pharmaceuticals, Inc. or Questcor.
We acquired rightsrespect to this product candidate from Questcor in 2007. We then optimized the acquired formulationpreliminary result and, accordingly, does not express an opinion or any other form of metoclopramide nasal spray to improve stability and remove inactive ingredients to improve the palatability and tolerability of EVK-001 for patients. We also developed the current formulation with excipients that are at or below the levels listed in the FDA’s Inactive Ingredient Database for intranasal products. We evaluated the current formulation of EVK-001 in 229 patients in our completed Phase 1 and Phase 2 clinical trials and intend to evaluate the same formulation in our proposed Phase 3 clinical trial. Similarly, the nasal spray pump used in our completed Phase 1 and Phase 2 clinical trials was identical and will also be used in our proposed Phase 3 clinical trial.
The primary container closure system for EVK-001 is comprised of an amber glass vial directly attached to a pre-assembled spray pump unit with a protection cap. Each multi dose sprayer system comes preassembled and capable of delivering a 30 day supply (120 doses at 4 doses per day.) The sprayer is a standardized metered sprayer technology utilized in other nasal spray products as well as the amber vial.
- 60 -
Our Planned Four-Week Phase 3 Clinical Trial in Female Subjects with Diabetic Gastroparesisassurance about it.
Based on discussions with the FDA, we plan to conduct one Phase 3 trial in women, which we believe will be sufficient for NDA submission. We plan to initiate the four-week, multicenter, randomized, double-blind, placebo-controlled, parallel Phase 3 clinical trial to evaluate the efficacy, safety and population pharmacokinetics of EVK-001 in adult female subjects with diabetic gastroparesis in the second half of 2013. We plan to enroll approximately 200 patients at approximately 60 sites across the United States. The trial population will consist of female diabetic patients with gastroparesis, identified by the presence of relevant symptoms and delayed gastric emptying. Female subjects with diabetic gastroparesis meeting the protocol-specified entry criteria will be studied in a parallel-group design with randomization in a 1:1 ratio to EVK-001 10 mg or placebo administered as a single intranasal spray four times daily; 30 minutes before meals and at bedtime.
Based on our discussions with the FDA, we plan to use specific symptoms from a composite score, the Gastroparesis Symptom Assessment, or GSA, as a patient-reported outcomes instrument to assess efficacy in this patient population. The primary efficacy endpoint for this Phase 3 clinical trial will be based upon a change from baseline in total composite score of the specific symptoms included in the GSA. We anticipate receiving topline data from this trial in late 2014 or early 2015. Also based on discussions with FDA, and to assess safety in men, we plan to conduct a similar and concurrent companion study for safety and efficacy in diabetic men with gastroparesis. The trial design will include an early stop for futility. The FDA has agreed that completion of the male companion study is not required for submission of the NDA seeking approval of EVK-001 for use in women. Whether the male study stops early for futility or continues to enroll, safety data from the male companion study will be included in the NDA for an approval in women. We also plan to conduct the required TQT study of EVK-001 prior to NDA submission.
Intellectual Property and Proprietary Rights
Overview
We are building an intellectual property portfolio for EVK-001Gimoti in the United States and abroad. We seek patent protection in the United States and internationally for our product candidate, its methods of use and processes for its manufacture, and for other technologies, where appropriate. Our policy is to actively seek to protect our proprietary position by, among other things, filing patent applications in the United States and abroad relating to proprietary technologies that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position. We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our technology.
Our business success will depend significantly on our ability to obtainto:
Patent Portfolio
Patent Portfolio
Our patent portfolio currently includesconsists of patents and patent applications, including the following U.S. patents and applications:
patent applications as of December 31, 2023:
•
U.S. Patent 5,760,086—Nasal Administration for the Treatment of Delayed Onset Emesis. This patent expires in 2016.
U.S. Patent 8,334,281—Patents 8,334,281; 11,020,361, 11,628,150, and 11,813,231 - Nasal Formulations of Metoclopramide. This patent expires in 2030.These patents are expected to expire no earlier than 2030, 2029, 2029, and 2029,
3
- 61 -
We have also been granted European and Canadian patents in the European Union for the method of use of metoclopramide via nasal delivery for gastroparesis.pharmaceutical compositions comprising metoclopramide. These patents provide protection through 2021.are expected to expire no earlier than 2029. We have also receivedbeen granted European, Japanese, Russian and Mexican patents infor the European Union covering the intranasal use of intranasal metoclopramide for delayed onset emesis.treating diabetic gastroparesis in human females. These patents offer protection through 2016.are expected to expire no earlier than 2032. Two additional PTC patent applications have been filed related to more recent clinical trial findings.
The United States patent system permits the filing of provisional and non-provisional patent applications. A non-provisional patent application is examined by the U.S. Patent and Trademark Office, or USPTO, and can mature into
Patents have a patent once the USPTO determines that the claimed invention meets the standards for patentability. A provisional patent application is not examined for patentability, and automatically expires 12 months after its filing date. As a result, a provisional patent application cannot mature into a patent. The requirements for filing a provisional patent application are not as strict as those for filing a non-provisional patent application. Provisional applications are often used, among other things, to establish an earlier filing date for a subsequent non-provisional patent application. The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application.limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent’s termpatent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be lengthenedavailable, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidate are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Risks Affecting Our Business
Our business is subject to a number of risks, including risks that may prevent us from achieving our business objectives or may adversely affect our business, financial condition, results of operations, cash flows and prospects that you should consider before making a decision to invest in our securities. These risks are discussed more fully in the section titled “Risk Factors” beginning on page 10 of this prospectus, and include the following:
4
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We may take advantage of certain of the scaled disclosures available to smaller reporting companies such as including: (i) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act of 2002, as amended; (ii) scaled executive compensation disclosures; and (iii) the requirement to provide only two years of audited financial statements, instead of three years.
Corporate Information
We were formed under the laws of the state of Delaware in January 2007. Our principal executive offices are located at 420 Stevens Avenue, Suite 230, Solana Beach, California 92075, and our telephone number is (858) 345-1494.
Our website address is www.evokepharma.com. The information on our website is not part of this prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website.
5
THE OFFERING
Common stock offered by us | Up to 8,333,000 shares of our common stock plus: (i) up to 8,333,000 shares of our common stock underlying the Series A Warrants, (ii) 8,333,000 shares of our common stock underlying the Series B Warrants, and (iii) 8,333,000 shares of our common stock underlying the Series C Warrants; based on an assumed combined public offering price of $0.90 per Common Stock Unit, which is the last reported sale price of our common stock on The Nasdaq Capital Market on January 9, 2024, and assuming no sale of any PFW Units. |
Pre-Funded Warrants offered by us | We are also offering to each purchaser whose purchase of Common Stock Units in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, up to 8,333,000 PFW Units, in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding common stock. Each PFW Unit consists of: (i): a Pre-Funded Warrant to purchase one share of our common stock, (ii) a Series A Warrant to purchase one share of our common stock, (iii) a Series B Warrant to purchase one share of our common stock, and (iv) a Series C Warrant to purchase one share of our common stock. Subject to limited exceptions, a holder of Pre-Funded Warrants will not have the right to exercise any portion of its Pre-Funded Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%, 14.99%, or 19.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-Funded Warrant will be exercisable upon issuance for one share of our common stock and will expire when exercised in full. The purchase price of each PFW Unit will equal the public offering price per Common Stock Unit less $0.0001, and the exercise price of each Pre-Funded Warrant will be $0.0001 per share. This offering also relates to the shares of common stock issuable upon exercise of any Pre-Funded Warrants sold in this offering. The exercise price and number of shares of common stock issuable upon exercise will be subject to certain further adjustments as described herein. See “Description of Units” on page 18 of this prospectus. For each PFW Unit we sell, the number of Common Stock Units we are offering will be decreased on a one-for-one basis. Because a Series A Warrant, Series B Warrant, and Series C Warrant are being sold together in this offering with each Common Stock Unit and, in the alternative, each PFW Unit, the number of Series A Warrants, Series B Warrants and Series C Warrants sold in this offering will not change as a result of a change in the mix of the Common Stock Units and PFW Units sold. |
6
Common Warrants offered by us | Each Common Stock Unit or PFW Unit purchased in this offering, as the case may be, will include: (i) a Series A Warrant to purchase one share of our common stock, (ii) a Series B Warrant to purchase one share of our common stock, and (iii) a Series C Warrant to purchase one share of our common stock. The Series A Warrants and the Series C Warrants will have an exercise price per share of $ . The Series B Warrants will have an exercise price per share equal to $ , which is equal to 100% of the price per Common Stock Unit sold in the offering. The Series A Warrants and Series B Warrants are exercisable immediately, subject to certain limitations described herein. The Series C Warrants may only be exercised to the extent and in proportion to a holder of the Series C Warrants exercising its corresponding Series B Warrants. The Series A Warrants will expire five years from the closing date of this offering. The Series B Warrants will expire nine months from the closing date of this offering. The Series C Warrants will also expire nine months from the closing date of this offering, provided that to the extent and in proportion to a holder of the Series C Warrants exercising its corresponding Series B Warrants included in the Common Stock Unit, such Series C Warrant will expire five years from the closing date of this offering. The shares of common stock in the Common Stock Units or the Pre-Funded Warrants in the PFW Units, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance, provided that a Series C Warrant may not be separated from the corresponding Series B Warrant until such Series B Warrant has been exercised. This offering also relates to the offering of the shares of common stock issuable upon exercise of the Common Warrants. The exercise price and number of shares of common stock issuable upon exercise will be subject to certain further adjustments as described herein See “Description of Units” on page 18 of this prospectus. Each Common Warrant is exercisable for one share of our common stock (subject to adjustment as provided therein) at any time at the option of the holder, provided that the holder will be prohibited from exercising its Common Warrant for shares of our common stock if, as a result of such exercise, the holder, together with its affiliates, would own more than 4.99% (or, at the election of the purchaser, 9.99%, 14.99%, or 19.99%) of the total number of shares of our common stock then issued and outstanding. However, any holder may increase such percentage to any other percentage not in excess of 19.99%, provided that any increase in such percentage shall not be effective until 61 days after such notice to us. |
Common stock to be outstanding immediately after this offering | 11,676,070 shares, assuming no sale of any Pre-Funded Warrants and assuming none of the Common Warrants issued in this offering are exercised. |
7
Use of proceeds | We estimate that our net proceeds from this offering will be approximately $6.3 million at an assumed combined public offering price of $0.90 per Common Stock Unit, which was the closing price of our common stock on The Nasdaq Capital Market on January 9, 2024, after deducting estimated offering expenses payable by us. We intend to use the net proceeds we receive from this offering, together with our existing cash and cash equivalents, for working capital and general corporate purposes, including for commercialization activities. We may also use a portion of the net proceeds, together with our existing cash and cash equivalents, to in-license, acquire, or invest in complementary businesses, technologies, products or assets; however, we have no current commitments or obligations to do so. See “Use of Proceeds” for additional information. |
Risk factors | See “Risk factors” beginning on page 10 of this prospectus and other information included and incorporated by reference in this prospectus for a discussion of factors that you should carefully consider before deciding to invest in our securities. |
Market symbol andtrading | Our common stock is listed on The Nasdaq Capital Market under the symbol “EVOK”. There is no established trading market for the Common Warrants or the Pre-Funded Warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the Common Warrants or Pre-Funded Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Common Warrants and Pre-Funded Warrants will be limited. |
The number of shares of our common stock that will be outstanding immediately after this offering as shown above is based on 3,343,070 shares outstanding as of September 30, 2023. The number of shares outstanding as of September 30, 2023 as used throughout this prospectus, unless otherwise indicated, excludes:
Unless otherwise indicated, all information in this prospectus assumes or gives effect to:
8
RISK FACTORS
You should consider carefully the risks described below and discussed under the section captioned “Risk Factors” contained in our Annual Report on Form 10-K for the year ended December 31, 2022, as amended by our Quarterly Reports on Form 10-Q as may be further amended, supplemented or superseded from time to time by our subsequent filings under the Exchange Act which compensatesare incorporated by reference in this prospectus in their entirety, together with other information in this prospectus and the information and documents incorporated by reference in this prospectus, and any free writing prospectus that we have authorized for use in connection with this offering before you make a patenteedecision to invest in our securities. If any of the following events actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. This could cause the trading price of our common stock to decline and you may lose all or part of your investment. The risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business operations.
Risks Related to this Offering
Even if this offering is successful, we will require substantial additional funding and may be unable to raise capital when needed, which would force us to liquidate, dissolve or otherwise wind down our operations.
Our operations have consumed substantial amounts of cash since inception. We believe, based on our current operating plan, that our cash and cash equivalents as well as future cash flows from net sales of Gimoti, together with the net proceeds from this offering, will be sufficient to fund our operations for at least the next twelve months. This estimate of cash runway could be shortened if there are any significant increases in planned spending on commercialization activities, including for marketing and manufacturing of Gimoti, and our selling, general and administrative delayscosts to support operations, or as a result of any termination of the Eversana Agreement. As of September 30, 2023, we and Eversana each have the right to exercise the Net Profit Quarterly Termination Right and terminate the Eversana Agreement, which right either party may exercise for a 60-day period following the end of the quarter. We and Eversana will continue to have the option to exercise this termination right for the 60-day period following the end of future quarters so long as the net profit under the agreement remains negative for consecutive quarters. If the Net Profit Quarterly Termination Right is exercised, the outstanding principal and interest under the Eversana Credit Facility would be due within 90 days after the effective date of such termination. This would materially and adversely affect our near-term liquidity needs and cash runway. Even if this offering is successful, we anticipate that we will be required to raise additional funds through debt, equity or other forms of financing, such as potential collaboration arrangements, to fund future operations and continue as a going concern. There can be no assurance that we will be able to raise additional funds on acceptable terms, or at all. Because our business is entirely dependent on the success of Gimoti, if we are unable to secure additional financing, successfully commercialize Gimoti or identify and execute on other commercialization or strategic alternatives for Gimoti or our Company, we will be required to curtail all of our activities and may be required to liquidate, dissolve or otherwise wind down our operations. Any of these events could result in a complete loss of your investment in our securities.
Our estimates of the amount of cash necessary to fund our activities may prove to be wrong and we could spend our available financial resources much faster than we currently expect. Our future funding requirements will depend on many factors, including, but not limited to:
9
We are authorized to issue up to 50,000,000 shares of common stock. As of December 31, 2023, we had 3,343,070 shares of common stock outstanding and have reserved an aggregate of 1,317,451 shares of common stock for issuance under our equity incentive award plan and employee stock purchase plan. Assuming we sell all of the Common Stock Units or PFW Units we propose to sell in this offering, we will have a very limited number of remaining unreserved and authorized shares available for issuance, which will impact our ability to raise additional funds in the future.
Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. Furthermore, the issuance of additional shares or other securities by us, or the possibility of such issuance, may cause the market price of our shares to decline and dilute the holdings of our existing stockholders. If we raise additional funds by incurring debt, the terms of the debt may involve significant cash payment obligations, as well as covenants and specific financial ratios that may restrict our ability to operate our business. We cannot provide any assurance that our existing capital resources, even after taking into account the proceeds of this offering, will be sufficient to enable us to continue the commercialization of Gimoti or to otherwise continue as a going concern.
If you purchase our securities sold in this offering, you will experience immediate and substantial dilution in the net tangible book value of your shares. In addition, we may issue additional equity or convertible debt securities in the future, which may result in additional dilution to investors.
Based on an assumed public offering price of $0.90 per Common Stock Unit, the last reported sale price of our common stock on The Nasdaq Capital Market on January 9, 2024 ,and our as adjusted net tangible book value per share as of September 30, 2023, if you purchase securities in this offering, you will experience a decrease of $0.44 per share in the net tangible book value of the common stock you purchase representing the difference between our as adjusted net tangible book value per share after giving effect to this offering and the assumed public offering price per share of common stock. The exercise of outstanding stock options and warrants, including those sold in this offering, will, however, result in dilution of your investment. In addition, to the extent we need to raise additional capital in the future and we issue additional shares of common stock or securities exercisable, convertible or exchangeable for our common stock, our then existing stockholders may experience dilution and the new securities may have rights senior to those of our common stock offered in this offering. See the section titled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this offering.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of our existing cash and cash equivalents and the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether such proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of our existing cash and cash equivalents and the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our existing cash and cash equivalents and the net proceeds from this offering in ways that ultimately increase the value of your investment. The failure by our management to
10
apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
The sale of our common stock in this offering, including any shares issuable upon exercise of any Pre-Funded Warrants or Common Warrants, and any future sales of our common stock, or the perception that such sales could occur, may depress our stock price and our ability to raise funds in new stock offerings.
We may from time-to-time issue additional shares of common stock at a discount from the current trading price of our common stock. As a result, our stockholders would experience immediate dilution upon the purchase of any shares of our common stock sold at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. Sales of shares of our common stock in this offering, including any shares issuable upon exercise of any Pre-Funded Warrants or Common Warrants issued in this offering and in the public market following this offering, or the perception that such sales could occur, may lower the market price of our common stock and may make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all.
There is no public market for the Pre-Funded Warrants or Common Warrants being offered in this offering.
There is no established public trading market for the Pre-Funded Warrants or Common Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants or Common Warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the Pre-Funded Warrants and the Common Warrants will be limited.
We may not receive any additional funds upon the exercise of the Pre-Funded Warrants or Common Warrants.
Each Pre-Funded Warrant may be exercised by way of a cashless exercise, meaning that the holder may not pay a cash purchase price upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the Pre-Funded Warrants. Accordingly, we may not receive any additional funds upon the exercise of the Pre-Funded Warrants.
Each Common Warrant (other than the Series B Warrant) may be exercised by way of a cashless exercise if at the time of exercise hereof there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of our common stock issuable upon exercise of the Common Warrants to the holder.
Significant holders or beneficial holders of our common stock may not be permitted to exercise Pre-Funded Warrants that they hold.
A holder of a Pre-Funded Warrant will not be entitled to exercise any portion of any Pre-Funded Warrants which, upon giving effect to such exercise, would cause the aggregate number of shares of our common stock beneficially owned by the USPTOholder (together with its affiliates) to exceed 4.99% (or, at the election of the purchaser, 9.99%, 14.99%, or 19.99%) of the number of shares of our common stock outstanding immediately after giving effect to the exercise. Such percentage may be increased or decreased by written notice by the holder of the Pre-Funded Warrants to any other percentage not in grantingexcess of 9.99%, 14.99%, or 19.99%. Such increase or decrease will not be effective until the sixty-first (61st) day after such notice is delivered to us. As a patent.result, you may not be able to exercise your Pre-Funded Warrants for shares of our common stock at a time when it would be financially beneficial for you to do so. In viewsuch circumstance you could seek to sell your Pre-Funded Warrants to realize value, but you may be unable to do so in the absence of an established trading market for the Pre-Funded Warrants.
The Common Warrants being offered may not have value.
The Series A Warrants and the Series C Warrants will have an exercise price of $ . The Series B Warrants will have an exercise price per share equal to $ , which is equal to 100% of the price per Common Stock Unit sold in the offering. The Series A Warrants will expire five years from the closing date of this offering. The Series B
11
Warrants will expire nine months from the closing date of this offering. The Series C Warrants will also expire nine months from the closing date of this offering, provided that to the extent and in proportion to a holder of the Series C Warrants exercising its corresponding Series B Warrants included in the Common Stock Unit, such Series C Warrant will expire five years from the closing date of this offering. Upon such dates, the Common Warrants will expire and have no further value. In the event that the market price of our common stock does not exceed the exercise price of the Common Warrants during the period when they are exercisable, the Common Warrants may not have any value.
Holders of Pre-Funded Warrants and Common Warrants purchased in this offering will have no rights as common stockholders until such holders exercise their Pre-Funded Warrants or Common Warrants and acquire our common stock.
Until holders of Pre-Funded Warrants or Common Warrants acquire shares of our common stock upon exercise of such warrants, holders of Pre-Funded Warrants and Common Warrants will have no rights with respect to the shares of our common stock underlying such Pre-Funded Warrants and Common Warrants. Upon exercise of the Pre-Funded Warrants and Common Warrants, the holders will be entitled to exercise the rights of a recent court decision,common stockholder only as to matters for which the USPTOrecord date occurs after the exercise date.
If we fail to meet all applicable Nasdaq Capital Market requirements and Nasdaq determines to delist our common stock, the delisting could adversely affect the market liquidity of our common stock and the market price of our common stock could decrease.
Our common stock is under greater scrutiny regarding its calculations where the USPTO erredlisted on The Nasdaq Capital Market. In order to maintain our listing, we must meet minimum financial and other requirements, including requirements for a minimum amount of capital, a minimum closing bid price per share of $1.00 and continued business operations so that we are not characterized as a “public shell company.”
On May 24, 2023, we received a written notice from Nasdaq indicating that, based on our stockholders’ equity of $2.1 million as of March 31, 2023, as reported in calculating the patent term adjustmentour Quarterly Report on Form 10-Q for the patentsquarter ended March 31, 2023, we were not in question denyingcompliance with the patenteeminimum stockholders’ equity requirement for continued listing on The Nasdaq Capital Market under Nasdaq Listing Rule 5550(b)(1) (the “Minimum Stockholders’ Equity Requirement”). As required by Nasdaq, we submitted our plan to regain compliance with the Minimum Stockholders’ Equity Requirement and Nasdaq granted us an extension until November 20, 2023 to regain compliance. Following notice on November 21, 2023 from Nasdaq that we had not met the Minimum Stockholders’ Equity Requirement, we requested a hearing before the Nasdaq Hearings Panel (the “Hearings Panel”) and on December 9, 2023, Nasdaq notified the Company that the hearing was scheduled for February 15, 2024. Any further action by Nasdaq will be stayed pending the conclusion of the hearing process. There can be no assurance that the Hearings Panel will grant our request for continued listing or that we will be able to evidence compliance prior to the expiration of any extension that may be granted by the Hearings Panel. If the Hearings Panel does not grant our request for continued listing, we may be subject to delisting from The Nasdaq Capital Market. After giving effect to this offering, we expect to meet the Minimum Stockholders’ Equity Requirement on a pro forma basis, although there can be no assurances that we will regain compliance with the Minimum Stockholders’ Equity Requirement to the satisfaction of Nasdaq currently or in any future periods, even applying the expected proceeds of this offering, or meet the other Nasdaq continued listing requirements. Further, even if we regain compliance with the Minimum Stockholders’ Equity Requirement, we may not be able to maintain compliance which may cause Nasdaq to delist our shares.
In the event that our common stock is delisted from the Nasdaq Capital Market and is not eligible for quotation or listing on another market or exchange, trading of our common stock could be conducted only in the over-the-counter market. In such event, it could become more difficult to dispose of, or obtain accurate price quotations for, our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
12
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein contain forward-looking statements. All statements other than statements of historical facts contained in this prospectus and the documents incorporated by reference herein are forward-looking statements, including statements regarding our future results of operations and financial position, business strategy, commercial activities to be conducted by Eversana, prospective products, product approvals, the pricing and reimbursement for Gimoti, future regulatory developments, research and development costs, the timing and likelihood of commercial success, the potential to develop future product candidates, plans and objectives of management for future operations, continued compliance with Nasdaq listing requirements, and future results of current and anticipated products. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. This prospectus and the documents incorporated by reference herein also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus and the documents incorporated by reference herein are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions, which we discuss in greater detail in the documents incorporated by reference herein, including under the heading “Risk Factors” and elsewhere in this prospectus. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. Given these risks and uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained in this prospectus or the documents incorporated by reference herein, whether as a result of any new information, future events, changed circumstances or otherwise. For all forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
13
USE OF PROCEEDS
We estimate the net proceeds from this offering will be approximately $6.3 million based on an assumed public offering price of $0.90 per Common Stock Unit, which was the closing price of our common stock on The Nasdaq Capital Market on January 9, 2024, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. These estimates exclude the proceeds, if any, from the exercise of Common Warrants and Pre-Funded Warrants sold in this offering, if any.
Each $0.15 increase (decrease) in the assumed public offering price of $0.90 per Common Stock Unit, would increase (decrease) the net proceeds to us by approximately $1.1 million, assuming that the number of Common Stock Units and PFW Units offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 1,000,000 shares in the number of Common Stock Units (or PFW Units) offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us by approximately $0.8 million, assuming the assumed public offering price per Common Stock Unit and PFW Unit remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds from this offering, together with our existing cash and cash equivalents, for working capital and general corporate purposes, including for commercialization activities. We may also use a portion of the patent termnet proceeds, together with our existing cash and cash equivalents, to which it was entitled. Alternatively,in-license, acquire, or invest in complementary businesses, technologies, products or assets; however, we have no current commitments or obligations to do so.
We believe, based on our current operating plan, that our cash and cash equivalents as well as future cash flows from net sales of Gimoti, together with the net proceeds from this offering, will be sufficient to fund our operations for the next 12 months. However, the amounts and timing of our actual expenditures will depend on numerous factors, including the amount of future Gimoti sales, any unexpected increases in planned spending on commercialization activities, including as a patent’s term may be shortened if a patent is terminally disclaimed over another patent.
The effective filing dateresult of a non-provisional patent application is used by the USPTO to determine what information is prior art when it considers the patentability of a claimed invention. If certain requirements are satisfied, a non-provisional patent application can claim the benefitany termination of the filing dateEversana Agreement or otherwise for marketing and manufacturing of an earlier filed provisional patent application. As a result, the filing date accorded by the provisional patent application may supersede information that otherwise could preclude the patentability of an invention.
Other Intellectual Property Rights
We currently have a registered trademark for EVOKE PHARMAGimoti, and our general and administrative costs to support operations, and other factors described under “Risk Factors” in this prospectus and in the United States.
Confidential Informationdocuments incorporated by reference herein, as well as the amount of cash used in our operations. We therefore cannot estimate with certainty the amount of net proceeds to be used for the purposes described above. We may find it necessary or advisable to use the net proceeds for other purposes, and Inventions Assignment Agreements
We require our employees and consultants to execute confidentiality agreements uponwe will have broad discretion in the commencement of employment, consulting or collaborative relationships with us. These agreements provide that all confidential information developed or made known during the courseapplication of the relationship with us be kept confidentialnet proceeds. Pending the uses described above, we plan to invest the net proceeds from this offering in short- and not disclosedintermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
14
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock. We intend to third parties except in specific circumstances.
Inretain future earnings, if any, to finance the caseoperation of employees, the agreements provide that all inventions resulting from work performed for us, utilizing our property or relating to our business and conceived or completed by the individual during employment shall be our exclusive property to the extent permitted by applicable law. Our consulting agreements also provide for assignment to us of any intellectual property resulting from services performed for us.
Sales and Marketing
We are initially seeking to commercialize EVK-001 in the United States alone, or in partnership with pharmaceutical companies that have established development and sales and marketing capabilities. Our strategy for EVK-001, if approved, will be to establish EVK-001 as the prescription product of choice for diabetic gastroparesis in women. If the product candidate is approved, our expectation is that EVK-001 would initially be
- 62 -
sold to gastrointestinal and internal medicine specialists, primary care physicians, and select health care providers. We may also utilize contract sales forces to assist in the marketing of EVK-001 to approved patient population.
Manufacturing
We do not own or operate manufacturing facilities for the production of EVK-001, nor do we have plans to develop our own manufacturing operationsanticipate paying any cash dividends in the foreseeable future. We currently depend on third-party contract manufacturers for allAny future determination related to our dividend policy will be made at the discretion of our required raw materials, drug substanceboard of directors after considering our financial condition, results of operations, capital requirements, business prospects and finished product forother factors our preclinical researchboard of directors deems relevant, and clinical trials. We do notsubject to the restrictions contained in any future financing instruments.
15
DILUTION
If you invest in our securities in this offering, your ownership interest will be immediately diluted to the extent of the difference between the assumed public offering price per Common Stock Unit and the as adjusted net tangible book value per share of our common stock immediately after this offering.
Historical net tangible book value (deficit) per share is determined by dividing our total tangible assets less our total liabilities by the total number of shares of common stock outstanding. Our historical net tangible book value (deficit) as of September 30, 2023 was approximately ($0.9) million, or ($0.26) per share, based on 3,343,070 shares of common stock outstanding as of that date.
After giving effect to the sale of 8,333,000 Common Stock Units in this offering, at an assumed public offering price of $0.90 per Common Stock Unit, which was the closing price of our common stock on the Nasdaq Capital Market on January 9, 2024, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2023 would have any current contractual relationships for the manufacturebeen approximately $5.4 million, or $0.46 per share. This represents an immediate increase in as adjusted net tangible book value of commercial supplies$0.72 per share to our existing stockholders and an immediate dilution of EVK-001. If EVK-001 is approved by any regulatory agency, we intend$0.44 per share to enter into agreements with third-party contract manufacturers for the commercial production at that time. We currently utilize a third-party consultant, which we engage on an as-needed, hourly basis, to managenew investors purchasing shares of our manufacturing contractors.
Competitionsecurities in this offering.
The pharmaceutical industry is characterized by intense competition and rapid innovation. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. We believe the key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety and tolerability profile, reliability, convenience of dosing, price and reimbursement.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining FDA approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition asfollowing table illustrates this dilution to new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render our drugs non-competitive or obsolete.
We expect that, if approved, EVK-001 will compete directly with metoclopramide oral, erythromycin and domperidone asinvestors on a treatment for gastroparesis. Metoclopramide is the only product currently approved in the United States to treat gastroparesis. Metoclopramide is available from a number of generic pharmaceutical manufacturers as well in branded form in the United States under the tradename Reglan® from Ani Pharmaceuticals.
Previously, Propulsid® (cisapride) and Zelnorm® (tegaserod) were prescribed off-label by physicians to treat gastroparesis. Propulsid® (cisapride) was approved for use in the treatment of dyspepsia and GERD. Zelnorm® (tegaserod) was approved for use in IBS and idiopathic chronic constipation. Both of these products have been withdrawn from the market because of cardiac safety issues.
- 63 -
Salix Pharmaceuticals launched an orally dissolving tablet formulation of metoclopramide in 2009. Other programs in the gastroparesis pipeline include new chemical entities in earlier-stage clinical trials. In addition to our EVK-001 product candidate, we are aware of three other development candidates. All are in Phase 2 clinical development.
per share basis:
| $ | 0.90 |
| |||||||
| ||||||||||
|
| (0.26 | ) |
| ||||||
| 0.72 | |||||||||
| 0.46 | |||||||||
| ||||||||||
RM-131 is a small-peptide analog of ghrelin, a hormone produced in the stomach that stimulates gastrointestinal activity. The compound is being developed for GI motility disorders and has shown efficacy in surgical and opiate-induced ileus in animal models due to a direct prokinetic effect. RM-131 reverses body weight loss in cachexia models.
Two other ghrelin analogs that were previously being developed by Tranzyme Pharma, an intravenous ghrelin agonist, ulimorelin, in post-operative ileus and a different oral ghrelin agent, TZP-102, in diabetic gastroparesis. Development of both product candidates has been discontinued after ulimorelin was unsuccessful in two Phase 3 studies and TZP-102 was unsuccessful in two Phase 2b trials.
GSK962040 is a selective non-peptide motilin receptor agonist under development for the treatment of conditions associated with slow rates of gastric emptying. Motilin is an endogenous peptide, produced mainly in the duodenum, whose physiological action is mediated by motilin receptors located on enteric neurons, peripheral terminals of the vagus, and on the smooth muscle of the gut. Motilin and non-peptide agonists of motilin receptors increase gastric emptying and may offer a new approach to the treatment of delayed gastric emptying conditions.
Erythromycin, is a motilin receptor agonist and is frequently used off-label in the treatment of gastroparesis. Erythromycin is well known to induce nausea and vomiting across all indications and is particularly associated with exacerbated nausea when used in gastroparesis. Repeated administration of macrolides is also linked to desensitization of the motilin receptor and tachyphylaxis. Extended dosing with antibiotics can lead to the development of resistant organisms as well as pathologic changes in intestinal flora.
TD-5108, also called Velusetrag, is a 5-HT4 receptor agonist compound under development for the treatment of gastroparesis by Theravance in collaboration with Alfa Wassermann S.p.A. Previously, TD-5108 was under development for chronic constipation.
Tegaserod, another 5-HT4 agonist, was approved in the United States and other countries for treatment of chronic idiopathic constipation and IBS-C. In 2007, Tegaserod was removed from the market in the United States by the FDA for cardiac safety concerns.
One additional medication, Motilium (domperidone), a dopamine receptor modulator, is not FDA-approved, but is available in the United States through various compounding pharmacies under a specific FDA restricted-access program. The safety and efficacy of Motilium as a promotility agent is not fully established.
- 64 -
Questcor Acquisition Agreement
We acquired all worldwide rights, data, patents and other related assets associated with EVK-001 from Questcor in June 2007. We paid to Questcor $650,000 in the form of an upfront payment, and will be required to make additional milestone payments totaling up to $52.0 million. These milestones include up to $5.0 million in payments if EVK-001 achieves the following development targets:
$0.5 million upon the initiation of the first patient dosing in our planned Phase 3 clinical trial for EVK-001;
$1.5 million upon the FDA’s acceptance for review of an NDA for EVK-001; and
$3.0 million upon the FDA’s approval of EVK-001.
The remaining $47.0 million in milestone payments depend on EVK-001’s commercial success and will only apply if EVK-001 receives regulatory approval. In addition, we will be required to pay to Questcor a low single digit royalty on net sales of EVK-001. Our obligation to pay such royalties will terminate upon the expiration of the last patent right covering EVK-001, which is expected to occur in 2030.
Government Regulation
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The FFDCA and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable FDA or other requirements may subject a company to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending applications, a clinical hold, warning letters, recall or seizure of products, partial or total suspension of production, withdrawal of the product from the market, injunctions, fines, civil penalties or criminal prosecution.
FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States. The process required by the FDA before a drug may be marketed in the United States generally involves:
completion of pre-clinical laboratory and animal testing and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations;
submission to the FDA of an IND for human clinical testing which must become effective before human clinical trials may begin in the United States;
approval by an independent institutional review board, or IRB, at each clinical trial site before each trial may be initiated;
performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug product for each intended use;
satisfactory completion of an FDA pre-approval inspection of the facility or facilities at which the product is manufactured to assess compliance with the FDA’s cGMP regulations, and for devices and device components, the QSR, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity;
submission to the FDA of an NDA;
satisfactory completion of an FDA advisory committee review, if applicable; and
FDA review and approval of the NDA.
The pre-clinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. Pre-clinical tests include laboratory evaluation of product chemistry, formulation, stability and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The results of pre-clinical tests, together with manufacturing information, analytical data and a proposed clinical trial protocol and other information, are submitted as part of an IND to the FDA. Some pre-clinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the
- 65 -
FDA, unless the FDA, within the 30-day time period, raises concerns or questions relating to one or more proposed clinical trials and places the clinical trial on a clinical hold, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, our submission of an IND may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development.
Further, an independent IRB, covering each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and informed consent information for subjects before the trial commences at that site and it must monitor the study until completed. The FDA, the IRB, or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk or for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Sponsors of clinical trials generally must register and report, at the NIH-maintained website ClinicalTrials.gov, key parameters of certain clinical trials. For purposes of an NDA submission and approval, human clinical trials are typically conducted in the following sequential phases, which may overlap or be combined:
| $ | 0.44 | |||||||||
|
|
|
The resultsNasdaq Capital Market on January 9, 2024, would increase (decrease) the as adjusted net tangible book value by approximately $1.1 million, or $0.10 per, share and increase (decrease) the dilution per share to new investors by $0.05 per share, assuming the number of product development, pre-clinical studiesCommon Stock Units offered by us, as set forth on the cover page of this prospectus, remains the same, and clinical trialsafter deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We may also increase or decrease the number of shares of common stock and accompanying Common Warrants we are submittedoffering. Each increase (decrease) of 1,000,000 shares in the number of Common Stock Units we are offering would increase (decrease) our as adjusted net tangible book value by approximately $0.8 million, or $0.03 per share, and decrease (increase) the dilution per share to new investors participating in this offering by $0.03 per share, assuming that the FDAassumed public offering price per share for each Common Stock Unit remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The as part of an NDA. NDAs must also contain extensiveadjusted information relating to the product’s pharmacology, CMCdiscussed above is illustrative only and proposed labeling, among other things.
Under federal law, the submission of most NDAs is additionally subject to a substantial application user fee, and the manufacturer and/or sponsor under an approved NDA are also subject to annual product and establishment user fees. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filingchange based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. Inactual public offering price, number of shares and other terms of this event, the NDA must be resubmitted with the additional information and is subject to payment of additional user fees. The resubmitted application is also subject to review before the FDA accepts it for filing.
- 66 -
OnceThe foregoing tables and calculations (other than the submission has been accepted for filing, the FDA begins an in-depth substantive review. Under the Prescription Drug User Fee Act, or PDUFA, the FDA agrees to specific performance goals for NDA review time through a two-tiered classification system, Standard Review and Priority Review. Standard Review NDAs have a goal of being completed within a ten-month timeframe. A Priority Review designation is given to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. The goal for completing a Priority Review is six months. However, the FDA does not always complete its review within these timelines and the Agency’s review can take substantially longer.
It is likely that our product candidates will be granted a Standard Review. The review process may be extended by the FDA for three additional months to consider certain information or obtain clarification regarding information already provided in the submission. The FDA may refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations carefully when making decisions. In addition, for combination products, the FDA’s review may include the participation of both the FDA’s Center for Drug Evaluation and Research and the FDA’s Center for Devices and Radiological Health, which may complicate or prolong the review.
Before approving an NDA, the FDA may inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilitieshistorical net tangible book value calculation) are in compliance with cGMP, and if applicable, QSR, requirements and are adequate to assure consistent production of the product within required specifications. Additionally, the FDA will typically inspect one or more clinical sites to assure compliance with GCP before approving an NDA.
After the FDA evaluates the NDA and, in some cases, the related manufacturing facilities, it may issue an approval letter or a Complete Response Letter, or CRL, to indicate that the review cycle for an application is complete and that the application is not ready for approval. CRLs generally outline the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when the deficiencies have been addressed to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety problems are identified after the product reaches the market. In addition, the FDA may require post-approval testing, including Phase 4 studies, and surveillance programs to monitor the effect of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on 3,343,070 shares of common stock outstanding as of September 30, 2023, and exclude:
Assuming the approved indicationsPre-Funded Warrants were immediately and fully exercised, this would result in accordance with the provisionsan as adjusted net tangible book value per share after this offering of the approved label,$ million, or $ per share of common stock, which represents a dilution per share to new investors of $ , and even if the FDA approves a product, it may limit the approved indications for use for the product or impose other conditions, including labeling or distribution restrictions or other risk-management mechanisms. Further, if there are any modificationsan increase in net tangible book value per share to the drug, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approvalexisting stockholders of a new or supplemental NDA, which may require us to develop additional data or conduct additional pre-clinical studies and clinical trials.$ .
Post-Approval Requirements16
Once an NDA is approved, a product will be subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to drug/device listing, recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product.
- 67 -
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and generally require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may suspend, restrict or withdraw the approval, require a product recall, or impose additional restrictions or limitations if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
fines, warning letters or holds on post-approval clinical trials;
refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals;
product seizure or detention, or refusal to permit the import or export of products; or
injunctions or the imposition of civil or criminal penalties.
The FDA may require post-approval studies and clinical trials if the FDA finds that scientific data, including information regarding related drugs, deem it appropriate. The purpose of such studies would be to assess a known serious risk or signals of serious risk related to the drug or to identify an unexpected serious risk when available data indicate the potential for a serious risk. The FDA may also require a labeling change if it becomes aware of new safety information that it believes should be included in the labeling of a drug.
The Food and Drug Administration Amendments Act of 2007 gave the FDA the authority to require a Risk Evaluation and Mitigation Strategy, or REMS, from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks. In determining whether a REMS is necessary, FDA must consider the size of the population likely to use the drug, the seriousness of the disease or condition to be treated, the expected benefit of the drug, the duration of treatment, the seriousness of known or potential adverse events, and whether the drug is a new molecular entity. If the FDA determines a REMS is necessary, the drug sponsor must agree to the REMS plan at the time of approval. A REMS may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate health care providers of the drug’s risks, limitations on who may prescribe or dispense the drug, or other measures that the FDA deems necessary to assure the safe use of the drug. In addition, the REMS must include a timetable to assess the strategy at 18 months, three years, and seven years after the strategy’s approval. The FDA may also impose a REMS requirement on a drug already on the market if the FDA determines, based on new safety information, that a REMS is necessary to ensure that the drug’s benefits outweigh its risks.
In March 2009, the FDA informed drug manufacturers that it will require a REMS for metoclopramide drug products. The FDA’s authority to take this action is based on risk management and post market safety provisions within the FDAAA. The REMS consists of a Medication Guide, elements to assure safe use (including an education program for prescribers and materials for prescribers to educate patients), and a timetable for submission of assessments of at least six months, 12 months, and annually after the REMS is approved. We intend to submit a REMS at the time of the NDA submission for EVK-001.
- 68 -
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market, and the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities, and promotional activities involving the internet. While physicians may prescribe for off-label uses, manufacturers may only promote for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. Indeed, the FDA has very broad enforcement authority under the FFDCA, and failure to abide by these regulations can result in penalties, including the issuance of a warning letter directing entities to correct deviations from FDA standards, a requirement that future advertising and promotional materials are pre-cleared by the FDA, and state and federal civil and criminal investigations and prosecutions.
The distribution of prescription pharmaceutical products is also subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution, including a drug pedigree which tracks the distribution of prescription drugs.
Section 505(b)(2) New Drug Applications
As an alternate path to FDA approval for modifications to formulations or uses of products previously approved by the FDA, an applicant may submit an NDA under Section 505(b)(2) of the FFDCA. Section 505(b)(2) was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Amendments, and permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The applicant may rely upon published literature and the FDA’s findings of safety and effectiveness based on certain pre-clinical or clinical studies conducted for an approved product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
To the extent that any outstanding options or warrants described above are exercised, new options are issued, or we issue additional shares of common stock or other equity or convertible debt securities in the future, there will be further dilution to investors participating in this offering.
17
DESCRIPTION OF UNITS
We are offering up to 8,333,000 Common Stock Units, each consisting of: (i) one share of our common stock, (ii) a Section 505(b)(2) NDA relies on studies conducted forSeries A Warrant to purchase one share of our common stock, (iii) a previously approved drug product, the applicant is requiredSeries B Warrant to certifypurchase one share of our common stock, and (iv) a Series C Warrant to purchase one share of our common stock. The Series A Warrants, Series B Warrants and Series C Warrants are collectively referred to the FDA concerning any patents listed foras the approved product in the Orange Book. Specifically, the applicant must certify for each listed patent that (1) the required patent information has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (4) the listed patent is invalid, unenforceable or will not be infringed by the new product. A certification that the new product will not infringe the already approved product’s listed patent or that such patent is invalid is known as a Paragraph IV certification. If the applicant does not challenge the listed patents through a Paragraph IV certification, the Section 505(b)(2) NDA application will not be approved until all the listed patents claiming the referenced product have expired. The Section 505(b)(2) NDA application also will not be accepted or approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a New Chemical Entity, listed in the Orange Book for the referenced product, has expired.
If the 505(b)(2) NDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the referenced NDA and patent holders once the 505(b)(2) NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a legal challenge to the Paragraph IV certification. Under the FFDCA, the filing of a patent infringement lawsuit within 45 days of their receipt of a Paragraph IV certification in most cases automatically prevents the FDA from approving the Section 505(b)(2) NDA for 30 months, or until a court decision or settlement finding that the patent is invalid, unenforceable or not infringed, whichever is earlier. The court also has the ability to shorten or lengthen the
- 69 -
30 month stay if either party is found not to be reasonably cooperating in expediting the litigation. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its product only to be subject to significant delay and patent litigation before its product may be commercialized.
The 505(b)(2) NDA applicant also may be eligible for its own regulatory exclusivity period, such as three-year exclusivity. The first approved 505(b)(2) applicant for a particular condition of approval, or change to a marketed product, such as a new extended release formulation for a previously approved product, may be granted three-year Hatch-Waxman exclusivity if one or more clinical studies, other than bioavailability or bioequivalence studies, was essential to the approval of the application and was conducted/sponsored by the applicant. Should this occur, the FDA would be precluded from making effective any other application for the same condition of use or for a change to the drug product that was granted exclusivity until after that three-year exclusivity period has run. Additional exclusivities may also apply.
Additionally, the 505(b)(2) NDA applicant may have relevant patents in the Orange Book, and if it does, can initiate patent infringement litigation against those applicants that challenge such patents, which could result in a thirty-month stay delaying those applicants.
Manufacturing Requirements“Common Warrants.”
We and our third-party manufacturers must comply with applicable FDA regulations relating to FDA’s cGMP regulations and, if applicable, QSR requirements. The cGMP regulations include requirements relating to, among other things, organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements to the satisfaction of the FDA pursuant to a pre-approval inspection before we can use them to manufacture our products. We and our third-party manufacturers are also subject to periodic unannounced inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including, among other things, warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties.
Other Regulatory Requirements
We are also subjectoffering to various laws and regulations regarding laboratory practices, the experimental useeach purchaser whose purchase of animals, and the use and disposal of hazardous or potentially hazardous substancesCommon Stock Units in connection with our research. In each of these areas, as above, the FDA has broad regulatory and enforcement powers, including, among other things, the ability to levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals, any one or more of which could have a material adverse effect on us.
Employees
We currently have two full time employees and several consultantsthis offering would otherwise result in the regulatory, clinical, manufacturingpurchaser, together with its affiliates and finance areas, which we engage on an as-needed, hourly basis. We intend to increasecertain related parties, beneficially owning more than 4.99% of our employee base uponoutstanding common stock immediately following the closingconsummation of this offering, and the commencementopportunity to purchase, if the purchaser so chooses, up to 8,333,000 PFW Units, in lieu of Common Stock Units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the holder, 9.99%, 14.99%, or 19.99%) of our Phase 3 clinical trial for EVK-001. We expect thatoutstanding common stock. Each PFW Unit consists of: (i): a numberPre-Funded Warrant to purchase one share of consultants previously engaged in developmentour common stock, (ii) a Series A Warrant to purchase one share of EVK-001 will participateour common stock, (iii) a Series B Warrant to purchase one share of our common stock, and (iv) a Series C Warrant to purchase one share of our common stock. The Common Warrants included in the ongoing clinicalPFW Units are identical to the Common Warrants included in the Common Stock Units
Common Stock
Voting Rights
Under the terms of our Certificate of Incorporation, holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, including the election of directors, and manufacturing developmentdo not have cumulative voting rights. Accordingly, the holders of a majority of the outstanding shares of common stock entitled to vote in any election of directors can elect all of the directors standing for the product candidate.election, if they so choose, other than any directors that holders of any preferred stock we may issue may be entitled to elect.
Facilities
We currently have no facilities.Dividend Rights
Legal Proceedings
We are not currently a partySubject to preferences that may be applicable to any legal proceedings.
- 70 -
Executive Officers and Directors
The following table sets forth the name, age and position of eachthen outstanding preferred stock, holders of our executive officers and directorscommon stock are entitled to receive ratably those dividends, if any, as of July 1, 2013.
|
|
| ||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
Executive Officers
David A. Gonyer, R.Ph. is one of our co-founders and has served as our President and Chief Executive Officer and as a member of our board of directors since March 2007. From January 2004 to June 2007, Mr. Gonyer served as Vice President, Strategic and Product Development of Medgenex, Inc., a subsidiary of Victory Pharma, Inc. a biopharmaceutical company focused on acquiring, developing and marketing products to treat pain and related conditions. From April 2000 to December 2004, Mr. Gonyer was a founder and Vice President of Sales and Marketing at Xcel Pharmaceuticals, Inc., a specialty pharmaceutical focused on neurological disorders. From December 1996 to April 2000, Mr. Gonyer served as Director of Marketing at Elan/Dura Pharmaceuticals, Inc. From 1987 to 1996, Mr. Gonyer held a broad range of management positions in commercial operations, alliance/partnership management, and regional sales at Eli Lilly & Company. Mr. Gonyer serves as a member ofmay be declared by the board of directors out of Neurelis, Inc., a privately held neurological specialty pharmaceutical company. Mr. Gonyer is a Registered Pharmacist and holds a B.Sc. in Pharmacy from Ferris State University School of Pharmacy. As onelegally available funds.
Liquidation Rights
In the event of our co-founders and having served as our Chief Executive Officer since March 2007, Mr. Gonyer’s extensive knowledgeliquidation, dissolution or winding up, the holders of our business, as well as over 25 years of experiencecommon stock will be entitled to share ratably in the pharmaceutical industry, including executive leadership in several pharmaceutical companies, contributedassets legally available for distribution to our boardstockholders after the payment of directors’ conclusion that he should serve as a director of our company.
Matthew J. D’Onofrio is one of our co-founders and has served as our Executive Vice President, Chief Business Officer since 2010 and as our Executive Vice President, Corporate Development, Treasurer and Secretary since March 2007. Mr. D’Onofrio has over 20 years of experience in both large and small pharmaceutical firms. Prior to founding Evoke, Mr. D’Onofrio was Vice President, Business Developmentor provision for Victory Pharma, a growing specialty pharma company based in San Diego. From 2002 to 2005, Mr. D’Onofrio led efforts to acquire marketed brands for the growing sales force. Earlier, Mr. D’Onofrio was previously Director and Head of West Coast Business Development at Vertex Pharmaceuticals, a biotechnology company, directing partnership efforts associated with the La Jolla research facility as well as other corporate assets. Mr. D’Onofrio also held various commercial roles of increasing responsibility over a decade at Eli Lilly & Company, including significant experience in worldwide corporate business development. During his licensing career, Mr. D’Onofrio has developed and executed license and investment relationships across a wide collection of disease states and technologies. Mr. D’Onofrio earned a B.S. in Chemistry from San Diego State University and an M.B.A. (Finance) from the Marshall School of Business, University of Southern California.
- 71 -
Non-Employee Directors
Cam L. Garner is one of our co-founders and has served as chairman of our board of directors since June 2007. Mr. Garner co-founded specialty pharmaceutical companies Zogenix Pharmaceuticals, Cadence Pharmaceuticals, Inc., Somaxon Pharmaceuticals, Inc., Elevation Pharmaceuticals, Inc., DJ Pharma, Verus Pharmaceuticals, Inc., Xcel Pharmaceuticals, Inc. and Meritage Pharma, Inc. He has served as chairman of Zogenix, Cadence, Verus, Elevation and Meritage since August 2006, May 2004, November 2002, December 2007 and February 2008, respectively. Xcel was acquired in March 2005 by Valeant Pharmaceuticals International, DJ Pharma was sold to Biovail in 2000 and Elevation was acquired by Sunovion Pharmaceuticals Inc. in September 2012. He was Chief Executive Officer of Dura Pharmaceuticals, Inc. from 1989 to 1995 and its Chairman and Chief Executive Officer from 1995 to 2000 until it was sold to Elan in November 2000. Mr. Garner also serves on the board of directors of Aegis Therapeutics, Inc., Cadence Pharmaceuticals, Inc., Meritage Pharma, Inc., Neurelis, Inc., and Zogenix, Inc. Mr. Garner earned his B.A. in Biology from Virginia Wesleyan College and an M.B.A. from Baldwin-Wallace College. As one of our co-founders and having served as our chairman since June 2007, Mr. Garner’s extensive knowledge of our business and history, experience as a board member of multiple publicly-traded and privately-held companies, and expertise in developing, financing and providing strong executive leadership to numerous biopharmaceutical companies contributed to our board of directors’ conclusion that he should serve as a director of our company.
Todd C. Brady, M.D., Ph.D. has served as a member of our board of directors since June 2007. Dr. Brady is an Entrepreneur in Residence at Domain Associates, a leading healthcare venture capital firm, a position he has held since 2013. From 2004 to 2013, he was a Principal at Domain Associates. He is President and Chief Executive Officer of Aldexa Therapeutics and is a member of the Board of Directors of Novadigm Therapeutics, ParinGenix, Sebacia, Aldexa Therapeutics and Asmacure. Prior to joining Domain, Dr. Brady was co-founder and CEO of Phenome Sciences, a biotechnology firm he merged with Xanthus Pharmaceuticals (acquired by Antisoma), where he was later Executive Vice President of Strategic Development and Planning. Dr. Brady also worked as head of business development and medical director at Aderis Pharmaceuticals (acquired by Schwarz Pharma, now part of UCB). While at Xanthus and Aderis, Dr. Brady was a medical consultant on numerous pre-clinical programs and clinical programs in Phases I through IV. Earlier in his career, Dr. Brady was a senior associate at CB Health Ventures (now Excel Medical Ventures), a healthcare venture capital fund. Dr. Brady holds an M.D. from Duke University Medical School, a Ph.D. from Duke University Graduate School, and an A.B. from Dartmouth College. Dr. Brady’s extensive knowledge of our business and history, experience as a board member of multiple companies and expertise in strategic development contributed to our board of directors’ conclusion that he should serve as a director of our company.
Scott L. Glenn is one of our co-founders and has served as a member of our board of directors since June 2007. Mr. Glenn is the founder of and has been the Managing Partner of Windamere Venture Partners since its inception in 1999. Mr. Glenn is the past Chairman or founder of Prometheus Laboratories, Inc., Santarus Inc., DexCom, Cadence Pharmaceuticals, NovaCardia Inc., Somaxon Pharmaceuticals, Zogenix Pharmaceuticals, SpineWave, Verus Pharmaceuticals Conception Technologies, and currently serves on the board of directors of Planet Biopharmaceuticals. Prior to Mr. Glenn’s involvement in venture capital, he was the President and CEO of Quidel Corporation and simultaneously was a founder of La Jolla Pharmaceuticals. Prior to Quidel, Mr. Glenn held various management positions including Division General Manager with Allergan. Mr. Glenn holds a Bachelor of Science degree in Finance and Accounting from California State University at Fullerton. As one of our co-founders and having served on our board since June 2007, Mr. Glenn’s extensive knowledge of our business and history, experience as a board member of multiple publicly-traded and privately-held companies and expertise in developing, financing and providing strong executive leadership to numerous biopharmaceutical companies contributed to our board of directors’ conclusion that he should serve as a director of our company.
Malcolm R. Hill, Pharm.D. has served as a member of our board of directors since June 2007. Dr. Hill has more than 20 years of academic and pharmaceutical industry experience in new product assessment and clinical trial design and execution, with a special emphasis in pediatrics and drug delivery systems. Dr. Hill has been a Senior Vice President of Research and Development at Meritage Pharma since 2008 and was a member of the
- 72 -
senior management team at Dura Pharmaceuticals, where he served as a vice president and corporate officer. At Dura, Dr. Hill was responsible for all clinical development activities related to the Spiros® dry powder inhaler, including numerous asthma programs. Dr. Hill’s academic career includes his position at the National Jewish Medical and Research Center, and he has also served as an assistant professor in the Schools of Medicine and Pharmacy at the University of Colorado. Dr. Hill has published more than 80 articles on the topics of clinical pharmacology and pharmacokinetics, and the treatment of pediatric asthma and related conditions. Dr. Hill earned his Pharm.D. from the University of Southern California and completed a post-doctoral program at the Veterans Administration Medical Center, San Diego, as well as a research fellowship in the Schools of Medicine and Pharmacy at the University of Florida Health Sciences Center. Dr. Hill’s experience as a founder of a private pharmaceutical firm, strong background in clinical and product development and substantial knowledge of the pharmaceutical industry contributed to our board of directors’ conclusion that he should serve as a director of our company.
Ann D. Rhoads has served as a member of our board of directors since June 2013. Currently, Ms. Rhoads is the Executive Vice President and Chief Financial Officer of Zogenix, Inc., a publicly-traded pharmaceutical company, and has served in that capacity since March 2010. From 2000 through the end of 2009, Ms. Rhoads served as the Chief Financial Officer of Premier, Inc., a healthcare supply management company. From 1998 to 2000, she was Vice President, Strategic Initiatives at Premier, Inc., and from 1993 to 1998, she was a Vice President of The Sprout Group, an institutional venture capital firm. Ms. Rhoads holds a B.S. in Finance from the University of Arkansas and a M.B.A. from the Harvard Graduate School of Business Administration. Ms. Rhoads currently serves on the board of directors of Globus Medical Inc. and previously served on the board of directors of Novellus Systems, Inc. from 2003 until 2012. Ms. Rhoads’ experience as the chief financial officer of a publicly-traded pharmaceutical company and as a member of the board of directors of a publicly-traded company brings to our board of directors and the committees of our board of directors valuable financial skills and expertise, which qualify her to serve as an “audit committee financial expert” on our audit committee, and significant executive management experience and leadership skills, as well as a strong understanding of corporate governance principles, all of which contributed to our board of directors’ conclusion that she should serve as a director of our company.
Kenneth J. Widder, M.D. has served as a member of our board of directors since June 2007. Dr. Widder has 32 years of experience working with biomedical companies. Dr. Widder has been a General Partner with Latterell Venture Partners since 2007 and serves on the boards of Meritage Pharma Inc., Naurex Inc., Vision of Children and the San Diego Museum of Art. Dr. Widder has founded seven companies and was Chairman/CEO of five of these companies. His last company, Sytera Inc. merged with Sirion Therapeutics, an ophthalmology specialty pharmaceutical company. Prior to Sytera, Dr. Widder co-founded and was the initial CEO of NovaCardia, a company acquired by Merck. Prior to NovaCardia, Dr. Widder founded and was Chairman/CEO of Santarus Inc., which developed and currently markets Zegerid, a rapid onset proton pump inhibitor for esophageal reflux disease. Additionally, Dr. Widder was Chairman and CEO of Converge Medical, a medical device company developing a suture less anastamosis system for vein grafts in coronary bypass surgery. Dr. Widder started his career as a founder, Chairman and CEO of Molecular Biosystems, where he was responsible for the development and approval of Albunex and Optison, the first two ultrasound contrast agents to be approved in the U.S. Dr. Widder is an inventor on over 30 patents and patent applications and has authored or co-authored over 25 publications. Dr. Widder holds an M.D. from Northwestern University and trained in pathology at Duke University. Dr. Widder’s extensive knowledge of our business and history, experience as a board member of multiple publicly-traded and privately-held companies and expertise in developing and financing contributed to our board of directors’ conclusion that he should serve as a director of our company.
Board Composition and Election of Directors
Director Independence
Our board of directors currently consists of seven members. Our board of directors has determined that all of our directors,debts and other than Mr. Gonyer,liabilities, subject to the prior rights of any preferred stock then outstanding.
Rights and Preferences
Holders of our common stock have no preemptive or conversion rights or other subscription rights and there are independent directorsno redemption or sinking funds provisions applicable to the common stock. The rights, preferences and privileges of holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in accordance with the listing requirementsfuture.
Fully Paid and Nonassessable
All outstanding shares of our common stock are duly authorized, validly issued, fully paid and nonassessable.
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
18
- 73 -
The NASDAQ Capital Market. The NASDAQ independence definition includes a seriesSome provisions of objective tests, including that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
Classified Board of Directors
In accordance with the terms ofDelaware law, our amended and restated certificate of incorporation and our amended and restated bylaws contain provisions that will go into effect immediately priorcould make the following transactions more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or
could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions which provide for payment of a premium over the closingmarket price for our shares.
These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of this offering,us to first negotiate with our board of directors. We believe that the benefits of the increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Undesignated Preferred Stock
The ability of our board of directors, will be divided into three classeswithout action by the stockholders, to issue up to 5,000,000 shares of undesignated preferred stock with staggered, three-year terms. At each annualvoting or other rights or preferences as designated by our board of directors could impede the success of any attempt to change control of us. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Stockholder Meetings
Our amended and restated bylaws provide that a special meeting of stockholders may be called only by our chairperson of the successorsboard, chief executive officer or president, or by a resolution adopted by a majority of our board of directors.
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, whose terms then expire will be elected to serve fromother than nominations made by or at the timedirection of election and qualification until the third annual meeting following election. Effective uponboard of directors or a committee of the closingboard of this offering, our directors will be divided among the three classes as follows:directors.
the Class I directors will be Mr. Gonyer and Drs. Brady and Widder, and their terms will expire at our first annual meetingElimination of stockholders following this offering;Stockholder Action by Written Consent
the Class II directors will be Messrs. Garner and Glenn, and their terms will expire at our second annual meeting of stockholders following this offering; and
the Class III directors will be Dr. Hill and Ms. Rhoads, and their terms will expire at our third annual meeting of stockholders following this offering.
Our amended and restated certificate of incorporation that will go into effect immediately priorand amended and restated bylaws eliminate the right of stockholders to the closing of this offering will provide that the authorized numberact by written consent without a meeting.
Staggered Board
Our board of directors is divided into three classes. The directors in each class serve for a three-year term, one class being elected each year by our stockholders. This system of electing and removing directors may be changed only by resolutiontend to discourage a third-party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the boarddirectors.
Removal of directors. Any additional directorships resulting from an increase in the numberDirectors
Our amended and restated certificate of directors will be distributed among the three classes soincorporation provides that as nearly as possible, each class will consist of one-third of the directors. The divisionno member of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of our company. Our directors may be removed onlyfrom office by our stockholders except for cause and, in addition to any other vote required by law, upon the affirmative voteapproval of not less than 66 2/3% of the holderstotal voting power of at least 66 2/3%all of our outstanding voting stock then entitled to vote in the election of directors.
Stockholders Not Entitled to Cumulative Voting
Board Leadership StructureOur amended and restated certificate of incorporation does not permit stockholders to cumulate their votes in the election of directors. Accordingly, the holders of a majority of the outstanding shares of our common stock
Our board19
entitled to vote in any election of directors is currently led by its chairman, Cam L. Garner. Our board of directors recognizes that it is important to determine an optimal board leadership structure to ensure the independent oversight of management as the company continues to grow. We separate the roles of chief executive officer and chairmancan elect all of the board in recognitiondirectors standing for election, if they choose, other than any directors that holders of our preferred stock may be entitled to elect.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the differences betweenDGCL, which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the two roles.date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The chief executive officer is responsible for setting the strategic direction for the company and the day-to-day leadership and performanceexistence of the company, while the chairman ofthis provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors provides guidance to the chief executive officer and presides over meetingsdirectors.
Amendment of Charter Provisions
The amendment of any of the full board of directors. We believe that this separation of responsibilities provides a balanced approach to managingabove provisions, except for the board of directors and overseeing the company.
Our board of directors has concluded that our current leadership structure is appropriate at this time. However,provision making it possible for our board of directors will continue to periodically reviewissue preferred stock, would require approval by holders of at least 66 2/3% of the total voting power of all of our leadership structureoutstanding voting stock.
The provisions of Delaware law, our amended and restated certificate of incorporation and our amended and restated bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may make such changesalso inhibit temporary fluctuations in the future as it deems appropriate.
- 74 -
Role of Board in Risk Oversight Process
Our board of directors has responsibility for the oversight of the company’s risk management processes and, either as a whole or through its committees, regularly discusses with management our major risk exposures, their potential impact on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board to understand the company’s risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, strategic and reputational risk.
The audit committee reviews information regarding liquidity and operations, and oversees our management of financial risks. Periodically, the audit committee reviews our policies with respect to risk assessment, risk management, loss prevention and regulatory compliance. Oversight by the audit committee includes direct communication with our external auditors, and discussions with management regarding significant risk exposures and the actions management has taken to limit, monitor or control such exposures. The compensation committee is responsible for assessing whether any of our compensation policies or programs has the potential to encourage excessive risk-taking. The nominating/corporate governance committee manages risks associated with the independence of the board, corporate disclosure practices, and potential conflicts of interest. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire board is regularly informed through committee reports about such risks. Matters of significant strategic risk are considered by our board as a whole.
Board Committees and Independence
Our board has established three standing committees—audit, compensation and nominating and corporate governance—each of which operates under a charter that has been approved by our board.
Our board has determined that all of the members of each of the board’s three standing committees are independent as defined under the rules of The NASDAQ Capital Market. In addition, all members of the audit committee meet the independence requirements contemplated by Rule 10A-3 under the Securities Exchange Act of 1934, or the Exchange Act.
Audit Committee
The audit committee’s main function is to oversee our accounting and financial reporting processes, internal systems of control, independent registered public accounting firm relationships and the audits of our financial statements. This committee’s responsibilities include, among other things:
selecting and engaging our independent registered public accounting firm;
evaluating the qualifications, independence and performance of our independent registered public accounting firm;
approving the audit and non-audit services to be performed by our independent registered public accounting firm;
reviewing the design, implementation, adequacy and effectiveness of our internal controls and our critical accounting policies;
discussing with management and the independent registered public accounting firm the results of our annual audit and the review of our quarterly unaudited financial statements;
reviewing, overseeing and monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to financial statements or accounting matters;
reviewing with management and our auditors any earnings announcements and other public announcements regarding our results of operations;
- 75 -
preparing the report that the SEC requires in our annual proxy statement;
reviewing and approving any related party transactions and reviewing and monitoring compliance with our code of conduct and ethics; and
reviewing and evaluating, at least annually, the performance of the audit committee and its members including compliance of the audit committee with its charter.
The members of our audit committee are Mr. Glenn, Ms. Rhoads and Dr. Widder. Ms. Rhoads serves as the chairperson of the committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and The NASDAQ Capital Market. Our board of directors has determined that Ms. Rhoads is an “audit committee financial expert” as defined by applicable SEC rules and has the requisite financial sophistication as defined under the applicable NASDAQ rules and regulations. Our board of directors has determined each of Mr. Glenn, Ms. Rhoads and Dr. Widder is independent under the applicable rules of the SEC and The NASDAQ Capital Market. Upon the listingmarket price of our common stock on The NASDAQ Capital Market,that often result from actual or rumored hostile takeover attempts. These provisions may also have the audit committee will operate under a written charter that satisfieseffect of preventing changes in the applicable standards of the SEC and The NASDAQ Capital Market.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and recommends corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and recommends to our board of directors the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our equity plan. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance by the compensation committee with its charter.
The members of our compensation committee are Mr. Garner, Dr. Brady and Ms. Rhoads. Mr. Garner serves as the chairperson of the committee. Our Board has determined that each of Mr. Garner, Dr. Brady and Ms. Rhoads is independent under the applicable rules and regulations of The NASDAQ Capital Market, is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act and is an “outside director” as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or Section 162(m). Upon the listing of our common stock on The NASDAQ Capital Market, the compensation committee will operate under a written charter, which the compensation committee will review and evaluate at least annually.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Pre-Funded Warrants
General
The term “pre-funded” refers to the fact that the purchase price of directors. In addition, the nominating and corporate governance committeePre-Funded Warrants in this offering includes almost the entire exercise price that will be paid under the Pre-Funded Warrants, except for a nominal remaining exercise price of $0.0001. The purpose of the Pre-Funded Warrants is responsible for overseeing our corporate governance policies and reporting and making recommendations to our boardenable investors that may have restrictions on their ability to beneficially own more than 4.99% (or, at the election of directors concerning governance matters. The memberssuch purchaser, 9.99%, 14.99%, or 19.99%) of our nominating and corporate governance committee are Drs. Brady, Hill and Widder. Dr. Brady serves as the chairman of the committee. Our board has determined that each of Drs. Brady, Hill and Widder is independent under the applicable rules and regulations of The NASDAQ Capital Market relating to nominating and corporate governance committee independence. Upon the listing of ouroutstanding common stock on The NASDAQ Capital Market, the nominating and corporate governance committee will operate under a written charter, which the nominating and corporate governance committee will review and evaluate at least annually.
- 76 -
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has ever been one of our officers or employees. None of our executive officers currently serves, or has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Board Diversity
Upon consummation of this offering, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
personal and professional integrity, ethics and values;
experience in corporate management, such as serving as an officer or former officer of a publicly-held company;
development or commercialization experience in large pharmaceutical companies;
experience as a board member or executive officer of another publicly-held company;
strong finance experience;
diversity of expertise and experience in substantive matters pertaining to our business relative to other board members;
diversity of background and perspective, including with respect to age, gender, race, place of residence and specialized experience;
conflicts of interest; and
practical and mature business judgment.
Currently, our board of directors evaluates, and following the consummation of this offering the opportunity to invest capital into the Company without triggering their ownership restrictions, by receiving Pre-Funded Warrants in lieu of shares of our common stock which would result in such ownership of more than 4.99%, 9.99%, 14.99%, or 19.99%, as applicable, and receiving the ability to exercise their option to purchase the shares underlying the Pre-Funded Warrants at a nominal price at a later date.
The following is a brief summary of certain terms and provisions of the Pre-Funded Warrants being offered by us. The following description is not complete and is subject in all respects to the provisions of the Pre-Funded Warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of Pre-Funded Warrant for a complete description of the terms of the Pre-Funded Warrants.
Exercise Price
Pre-Funded Warrants will evaluate, each individualhave an exercise price of $0.0001 per share. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the contextevent of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Exercisability
20
The Pre-Funded Warrants are immediately exercisable and do not expire until exercised in full. The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full of the boardexercise price in immediately available funds for the number of directorsshares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below).
Exercise Limitations
The Pre-Funded Warrants may not be exercised by the holder to the extent that the holder, together with its affiliates, would beneficially own, after such exercise more than 4.99% of the shares of our common stock then outstanding (including for such purpose the shares of our common stock issuable upon such exercise). However, any holder may increase or decrease such beneficial ownership limitation upon notice to us, provided that such limitation cannot exceed 19.99%, and provided that any increase in the beneficial ownership limitation shall not be effective until 61 days after such notice is delivered. Purchasers of Pre-Funded Warrants in this offering may also elect prior to the issuance of the Pre-Funded Warrants to have the initial exercise limitation set at 9.99%, 14.99%, or 19.99% of our outstanding shares of common stock.
Cashless Exercise
In lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder of a Pre-Funded Warrant may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants.
Transferability
Subject to applicable laws, the Pre-Funded Warrants may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the objectiveappropriate instruments of assembling a group that can best maximize the successtransfer.
Fractional Shares
No fractional shares of the business and represent stockholder interests throughcommon stock will be issued upon the exercise of sound judgment using its diversitythe Pre-Funded Warrants. Rather, the number of experienceshares of common stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in these various areas.respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market for the Pre-Funded Warrants, and we do not expect a market to develop. We do not intend to list the Pre-Funded Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants will be limited.
Rights as a Stockholder
Except as otherwise provided in the Pre-Funded Warrants or by virtue of the holders’ ownership of shares of our common stock, the holders of warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until such warrant holders exercise their Pre-Funded Warrants.
Fundamental Transactions
CodeIn the event of Business Conducta fundamental transaction, as described in the Pre-Funded Warrants and Ethicsgenerally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding common stock, upon consummation of such a fundamental transaction, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded
We21
Warrants the kind and amount of securities, cash or other property that the holders would have adoptedreceived had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the Pre-Funded Warrants.
Waivers and Amendments
No term of the Pre-Funded Warrants may be amended or waived without the written consent of the holder of such Pre-Funded Warrant.
Common Warrants
General
The following is a written codebrief summary of business conductcertain terms and ethics that appliesprovisions of the Common Warrants being offered by us. The following description is not complete and is subject in all respects to the provisions of each series of Common Warrant, the forms of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the forms of each series of Common Warrant for a complete description of the terms and conditions of the Common Warrants.
Exercise Price
Each Series A Warrant and Series C Warrant offered hereby has an initial exercise price per share equal to $ . The exercise price of the Series A Warrant and the Series C Warrant will be determined at the time of pricing based on negotiations with the underwriters. Each Series B Warrant offered hereby has an assumed initial exercise price per share equal to $ (representing 100% of the price per share of the common stock sold in the offering). The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our directors,stockholders.
Exercisability
The Series A Warrants are immediately exercisable and will expire five years following the date of issuance. The Series B Warrants are immediately exercisable and will expire nine months following the date of issuance. The Series C Warrants will become exercisable only to the extent and in proportion to a holder of the Series C Warrants exercising its corresponding Series B Warrants included in a Unit. The Series C Warrant will expire nine months following the date of issuance, provided that to the extent and in proportion to a holder of the Series C Warrants exercising its corresponding Series B Warrants included in a Unit, such Series C Warrant will expire five years following the date of issuance. Each series of Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full of the exercise price in immediately available funds for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below).
Exercise Limitations
The Common Warrants may not be exercised by the holder to the extent that the holder, together with its affiliates, would beneficially own, after such exercise more than 4.99% of the shares of our common stock then outstanding (including for such purpose the shares of our common stock issuable upon such exercise). However, any holder may increase or decrease such beneficial ownership limitation upon notice to us, provided that such limitation cannot exceed 19.99%, and provided that any increase in the beneficial ownership limitation shall not be effective until 61 days after such notice is delivered. Purchasers of Common Warrants in this offering may also elect prior to the issuance of the Common Warrants to have the initial exercise limitation set at 9.99%, 14.99%, or 19.99% of our outstanding shares of common stock.
Cashless Exercise
22
If at the time of exercise hereof there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of the shares of common stock issuable upon exercise of the Series A or Series C Warrants, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Series A Warrants and Series C Warrants.
The Series B Warrants may only be exercised for cash.
Transferability
Subject to applicable laws, a Series A Warrant may be transferred at the option of the holder upon surrender of the Series A Warrant to us together with the appropriate instruments of transfer. A Series B Warrant may not be transferred during its term except with our consent. A Series C Warrant may not be transferred during its term except with our consent; provided that a Series C Warrant may be transferred without our consent after the corresponding Series B Warrant included in a Unit has been exercised, but only to the extent and in proportion to the exercise of such corresponding Series B Warrant. A Series C Warrant that is transferable may be transferred at the option of the holder upon surrender of the Series C Warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Common Warrants. Rather, the number of shares of common stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market for the Common Warrants, and we do not expect a market to develop. We do not intend to list the Common Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Common Warrants will be limited.
Rights as a Stockholder
Except as otherwise provided in the Common Warrants or by virtue of the holders’ ownership of shares of our common stock, the holders of warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until such warrant holders exercise their Common Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Common Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of more than 50% of the voting power represented by our outstanding common stock, upon consummation of such a fundamental transaction, the holders of the Common Warrants will be entitled to receive upon exercise of the Common Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Common Warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the Common Warrants.
Notwithstanding the foregoing, in the event of a fundamental transaction, we or a successor entity shall, at the holder’s option, exercisable at any time concurrently or within thirty (30) days following the consummation of a fundamental transaction, purchase the Common Warrant by paying to the holder an amount equal to the Black Scholes Value (as defined in each Common Warrant) of the remaining unexercised portion of the Common Warrant on the date of the fundamental transaction. If the fundamental transaction is not within our control, the holders of the Common Warrants will only be entitled to receive from us or a successor entity the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Common Warrant, that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether
23
that consideration is in the form of cash, stock or any combination thereof, or whether the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
Waivers and Amendments
No term of the Common Warrants may be amended or waived without the written consent of the holder of such Common Warrant.
24
EXECUTIVE AND DIRECTOR COMPENSATION
Overview
Our named executive officers and employees, includingfor 2023, which consist of our principal executive officer principal financial officer, principal accounting officer or controller, or persons performing similar functions. Upon completion of this offering,during 2023 and our code of business conduct and ethics will be available under the Investor Relations—Corporate Governance section of our website at www.evokepharma.com. In addition, we intend to post on our website all disclosures that are required by law or the listing standards of The NASDAQ Capital Market concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
- 77 -
EXECUTIVE AND DIRECTOR COMPENSATION
This section discusses the material components of the executive compensation program for ourtwo next most highly compensated executive officers who are named in the “2012 Summary Compensation Table” below. In 2012, our named executive officers and their positions were as follows:
during 2023, were:
•
•
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations, and determinations regarding future compensation programs. Actual compensation programs that we adopt following the completionclosing of this offering may differ materially from the currently planned programs summarized in this discussion.
2012 Summary Compensation Table
The following table sets forth information concerningregarding compensation earned with respect to the fiscal years ended December 31, 2022 and 2023 by our named executive officers.
Summary Compensation Table
The following table shows information regarding the compensation ofearned by our named executive officers during the fiscal yearyears ended December 31, 2012:2023 and 2022:
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||||
David A. Gonyer, R.Ph. | 2012 | 309,000 | — | — | — | — | — | 309,000 | ||||||||||||||||||||||||
President and Chief Executive Officer | ||||||||||||||||||||||||||||||||
Matthew J. D’Onofrio | 2012 | 268,000 | — | — | — | — | — | 268,000 | ||||||||||||||||||||||||
Executive Vice President, Chief Business Officer | ||||||||||||||||||||||||||||||||
Name and | Year | Salary | Bonus | Option | All Other | Total $ | ||||||||||||||||||
David A. Gonyer | 2023 | 595,000 | - | 94,500 | 71,309 | 760,809 | ||||||||||||||||||
Chief Executive Officer | 2022 | 570,000 | 171,000 | 78,488 | 65,534 | 885,022 | ||||||||||||||||||
Matthew J. D'Onofrio | 2023 | 450,000 | - | 78,000 | 48,121 | 576,121 | ||||||||||||||||||
President, Chief | 2022 | 417,000 | 93,825 | 44,850 | 46,285 | 601,960 | ||||||||||||||||||
Operating Officer, Secretary and Treasurer | ||||||||||||||||||||||||
Marilyn R. Carlson, D.M.D., M.D. | 2023 | 420,000 | - | 47,250 | 24,989 | 492,239 | ||||||||||||||||||
Chief Medical Officer | 2022 | 402,000 | 90,450 | 44,850 | 25,688 | 562,988 | ||||||||||||||||||
25
Narrative Disclosure to Summary Compensation TablesTable
Annual Base Salary
In general, base salaries for our named executive officers are initially established through arm’s length negotiation at the time the executive is hired, taking into account such executive’s qualifications, experience and prior salary. Base salaries of our named executive officers are approved and reviewed annually by our compensation committee and adjustments to base salaries are based on the scope of an executive’s responsibilities, individual contribution, prior experience and sustained performance. Decisions regarding salary increases may take into account an executive officer’s current salary, equity ownership, and the amounts paid to an executive officer’s peers inside our company by conducting an internal analysis, which compares the pay of an executive officer to other members of the management team. Base salaries are also reviewed in the case of promotions or other significant changes in responsibility. Base salaries are not automatically increased if the board of directors and compensation committee believe that other elements of the named executive officer’s compensation are more appropriate in light of our stated objectives. This strategy is consistent with our intent of offering compensation that is both cost-effective, competitive and contingent on the achievement of performance objectives.
The actual base salaries paid to all of our named executive officers for 2023 are set forth in the “Summary Compensation Table” above.
In February 2023, our compensation committee approved base salary increases for 2023 for Messrs. Gonyer and D’Onofrio and Dr. Carlson to $595,000, $450,000, and $420,000, respectively. These base salary increases represented adjustments of approximately 4.4 %, 7.9% and 4.5%, respectively.
The base salaries of our named executive officers continue to be below the median level of similarly-situated executives for our peer group of companies.
Annual Bonuses
Each named executive officer is also eligible for a performance bonus based upon the achievement of certain corporate performance goals and objectives approved by our compensation committee and board of directors.
Bonuses are set based on a percentage of the executive’s base salary as of the end of the bonus year and are expected to be paid out in the first quarter of the following year. The target levels for 2023 executive bonuses were as follows: 60% for our Chief Executive Officer, 50% for our President and Chief Operating Officer, and 45% for our Chief Medical Officer. The executive bonuses are 100% based on the achievement of corporate objectives that are set each year by the board of directors and the compensation committee. All final bonus payments to our named executive officers are determined by our compensation committee. The actual bonuses awarded in any year, if any, may be more or less than the target, depending on individual performance and the achievement of corporate objectives and may also vary based on other factors at the discretion of the compensation committee.
For 2023, the corporate performance objectives for our named executive officers were related to corporate financial objectives. Bonuses for any one year are usually determined and paid in the first quarter of the following year. Accordingly, bonus compensation for our named executive officers for 2023 has not yet been determined as of the date of filing of this prospectus.
The overall achievement level was then used to determine each named executive officer’s bonus. The bonuses paid to our named executive officers for 2023 are set forth in the “Summary Compensation Table” above.
Equity-Based Incentive Awards
The goals of our long-term, equity-based incentive awards are to align the interests of our named executive officers and other employees, non-employee directors and consultants with the interests of our stockholders. Because vesting is based on continued employment, our equity-based incentives also encourage the retention of our named executive officers through the vesting period of the awards. In determining the size of the long-term equity incentives to be awarded to our named executive officers, we take into account a number of internal factors, such as the relative
26
job scope, the value of existing long-term incentive awards, individual performance history, prior contributions to us and the size of prior grants. Our compensation committee reviews competitive market data prepared by APA in connection with its grant of long-term equity incentive awards to the named executive officers, but such awards are not determined by reference to any specific target level of compensation or benchmarking. Based upon these factors, the compensation committee determines the size of the long-term equity incentives at levels it considers appropriate to create a meaningful opportunity for reward predicated on the creation of long-term stockholder value. We have not granted any equity awards other than stock options to date.
To reward and retain our named executive officers in a manner that best aligns employees’ interests with stockholders’ interests, we use stock options as the primary incentive vehicles for long-term compensation. We believe that stock options are an effective tool for meeting our compensation goal of increasing long-term stockholder value by tying the value of the stock options to our future performance. Because employees are able to profit from stock options only if our stock price increases relative to the stock option’s exercise price, we believe stock options provide meaningful incentives to employees to achieve increases in the value of our stock over time.
The exercise price of each stock option grant is the fair market value of our common stock on the grant date, as determined by our board of directors from time to time. Stock option awards granted to our named executive officers generally vest on a monthly basis over a four-year period. From time to time, our compensation committee may, however, determine that a different vesting schedule is appropriate.
In February 2023, our named executive officers were granted stock options to purchase shares of our common stock. Specifically, Messrs. Gonyer and D’Onofrio and Dr. Carlson were granted options to purchase 30,000, 25,000, and 15,000 shares of our common stock, respectively. The options for Mr. Gonyer and Dr. Carlson vest on a monthly basis over a four-year period commencing January 1, 2023, while the options for Mr. D'Onofrio vest on the four-year anniversary of the grant date, subject to the reporting person’s continued service to the issuer through each such vesting date.
We have had no program, plan or practice pertaining to the timing of stock option grants to named executive officers coinciding with the release of material non-public information. Stock options granted to our named executive officers may be subject to accelerated vesting in certain circumstances. For additional discussion, please see “Employment Agreements.”
27
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth specified information concerning unexercised stock options and unvested stock awards for each of the named executive officers outstanding as of December 31, 2023:
Option Awards | ||||||||||||||||
Name | Grant | Number of Securities Underlying Unexercised Options (#) Exercisable(1)(2)(3)(4) (5) | Number of Securities Underlying Unexercised Options (#) Unexercisable(1)(2)(3)(4)(5) | Option | Option Expiration Date | |||||||||||
David A. Gonyer, R.Ph. | 6/17/2019 | 4,417 | - | 7.44 | 3/5/2025 | |||||||||||
6/17/2019 | 3,750 | - | 7.44 | 1/27/2026 | ||||||||||||
6/17/2019 | 14,062 | - | 7.44 | 1/25/2027 | ||||||||||||
6/17/2019 | 14,062 | - | 7.44 | 2/7/2028 | ||||||||||||
6/17/2019 | 14,062 | - | 7.44 | 2/5/2029 | ||||||||||||
2/28/2020 | 25,107 | 1,976 | 14.76 | 2/27/2030 | ||||||||||||
1/27/2021 | 25,216 | 9,367 | 32.16 | 1/26/2031 | ||||||||||||
2/2/2022 | 6,986 | 7,597 | 6.60 | 2/1/2032 | ||||||||||||
2/8/2023 | 6,875 | 23,125 | 4.01 | 2/7/2033 | ||||||||||||
Matthew J. D'Onofrio | 6/17/2019 | 4,417 | - | 7.44 | 3/5/2025 | |||||||||||
6/17/2019 | 3,281 | - | 7.44 | 1/27/2026 | ||||||||||||
6/17/2019 | 9,375 | - | 7.44 | 1/25/2027 | ||||||||||||
6/17/2019 | 9,375 | - | 7.44 | 2/7/2028 | ||||||||||||
6/17/2019 | 9,375 | - | 7.44 | 2/5/2029 | ||||||||||||
2/28/2020 | 15,450 | 1,216 | 14.76 | 2/27/2030 | ||||||||||||
1/27/2021 | 18,228 | 6,772 | 32.16 | 1/26/2031 | ||||||||||||
2/2/2022 | 3,992 | 4,341 | 6.60 | 2/1/2032 | ||||||||||||
2/8/2023 | - | 25,000 | 4.01 | 2/7/2033 | ||||||||||||
Marilyn R. Carlson | 6/17/2019 | 4,687 | - | 7.44 | 3/5/2025 | |||||||||||
D.M.D., M.D. | 6/17/2019 | 2,109 | - | 7.44 | 1/27/2026 | |||||||||||
6/17/2019 | 6,250 | - | 7.44 | 1/25/2027 | ||||||||||||
6/17/2019 | 7,812 | - | 7.44 | 2/7/2028 | ||||||||||||
6/17/2019 | 7,812 | - | 7.44 | 2/5/2029 | ||||||||||||
2/28/2020 | 15,450 | 1,216 | 14.76 | 2/27/2030 | ||||||||||||
1/27/2021 | 18,228 | 6,772 | 32.16 | 1/26/2031 | ||||||||||||
2/2/2022 | 3,992 | 4,341 | 6.60 | 2/1/2032 | ||||||||||||
2/8/2023 | 3,437 | 11,563 | 4.01 | 2/7/2023 | ||||||||||||
28
Employment Arrangements with our Executive Officers
Employment Agreements with Messrs. Gonyer and D'Onofrio
We have entered into employment agreements with each of Messrs. Gonyer and D’Onofrio, our named executive officers, whichD’Onofrio. Pursuant to the employment agreements, were most recently amended and restatedeach executive's base salary is subject to review each year at the sole discretion of the compensation committee. The executives are eligible to earn an annual cash performance bonus under the company’s bonus plan or plans applicable to senior executives. The annual cash performance bonus payable is based on the achievement of individual and/or company performance goals to be determined in June 2013.good faith by the compensation committee.
Pursuant to each of the employment agreements, if we terminate such officer’s employment without cause (as defined below), such officer resigns for good reason (as defined below) or such officer’s employment is terminated as a result of his or her death or following his or her permanent disability, the executive officer or his or her estate, as applicable, is entitled to the following payments and benefits: (1) his fully earned but unpaid base salary through the date of termination at the rate then in effect, plus all other amounts under any compensation plan or practice to which he or she is entitled; (2) a lump sum cash payment in an amount equal to 12 months of his base salary as in effect immediately prior to the date of termination; (3) a lump sum cash payment in an amount equal to his or her bonus (as defined below) for the year in which the termination of his employment occurs, prorated for the period of his service during such year, provided that the officer shall not be entitled to receive such amount in the event that his termination results from his discharge by us without cause prior to a change in control (as defined below); (4) a lump sum cash payment in an amount equal to the cost of the continuation of health benefits for a period of 12 months following the date of termination; (5) a lump sum cash payment in an amount equal to the cost of his life insurance premiums for a period of 12 months following the date of termination; (6) solely in the event of the officer’s termination by us without cause or by the officer for good reason, a lump sum cash payment in an amount equal to $15,000 for outplacement services;service; and (7) the automatic acceleration of the vesting and exercisability of outstanding unvested stock awards as to the number of stock awards that would have vested over the 12-month period following termination had such executive officer remained continuously employed by us during such period. In the event an officer’s termination without cause or resignation for good reason occurs
- 78 -
within three months prior to the occurrence of a change in control or within 12 months following a change in control, all of his outstanding unvested stock awards will accelerate and become fully vested on the later of (1) the date of termination or (2) the date of such change in control.
Employment Agreement with Dr. Carlson
We have also entered into an employment agreement with Dr. Carlson. Pursuant to the employment agreement, Dr. Carlson agrees to devote 80% of her productive time and efforts to the performance of her duties as Chief Medical Officer. Pursuant to the employment agreement, Dr. Carlson’s base salary is subject to review each year at the sole discretion of the compensation committee. Dr. Carlson is also eligible to earn an annual cash performance bonus under the company’s bonus plan or plans applicable to senior executives. The annual cash performance bonus payable is based on the achievement of individual and/or Company performance goals to be determined in good faith by the
29
compensation committee. The company also pays Dr. Carlson a taxable monthly payment equal to the monthly premium Dr. Carlson pays for healthcare coverage under Medicare, in an amount not to exceed $2,000 per month.
Pursuant to the employment agreement, if we terminate Dr. Carlson’s employment without cause (as defined below) or Dr. Carlson resigns for good reason (as defined below), Dr. Carlson is entitled to the following payments and benefits: (1) fully earned but unpaid base salary through the date of termination at the rate then in effect, plus all other amounts under any compensation plan or practice to which she is entitled; (2) a lump sum cash payment in an amount equal to her monthly base salary as in effect immediately prior to the date of termination for a period of nine months; (3) a lump sum cash payment in an amount equal to her bonus for the year in which the termination of her employment occurs, prorated for the period of her service during such year, provided that Dr. Carlson shall not be entitled to receive such amount in the event that her termination results from her discharge by the company without cause prior to a change in control (as defined below); and (4) a taxable monthly payment in an amount equal to her monthly healthcare coverage costs under Medicare as in effect immediately prior to the date of termination, in an amount not to exceed $2,000 per month, for a period of nine months.
In the event Dr. Carlson’s termination without cause or resignation for good reason occurs within three months prior to the occurrence of a change in control or within 12 months following a change in control, all of her outstanding unvested stock awards will accelerate and become fully vested on the later of (1) the date of termination or (2) the date of such change in control.
Dr. Carlson’s employment agreement also contains standard confidentiality, non-competition and non-solicitation covenants.
Defined Terms for Purposes of Employment Agreements
For purposes of the employment agreements with the named executive officers, “cause” generally means an executive officer’s (1) commission of an act of fraud, embezzlement or dishonesty that has a material adverse impact on us or any successor or affiliate of ours; (2) conviction of, or entry into a plea of “guilty” or “no contest” to, a felony; (3) unauthorized use or disclosure of our confidential information or trade secrets or any successor or affiliate of ours that has a material adverse impact on any such entity; (4) gross negligence, insubordination or material violation of any duty of loyalty, or any other material misconduct on the part of the executive officer; (5) ongoing and repeated failure or refusal to perform or neglect of his or her duties as required by his or her employment agreement, which failure, refusal or neglect continues for 15 days following his or her receipt of written notice from our board of directors stating with specificity the nature of such failure, refusal or neglect; or (6) breach of any material provision of his or her employment agreement.
For purposes of the employment agreements with the named executive officers, “good reason” generally means (1) other than for Dr. Carlson, a change in the executive officer’s status, position or responsibilities that, in the executive officer’s reasonable judgment, represents a substantial and material reduction in the status, position or responsibilities as in effect immediately prior thereto; the assignment to the executive officer of any duties or responsibilities that, in the executive officer’s reasonable judgment, are materially inconsistent with such status, position or responsibilities; or any removal of the executive officer from or failure to reappoint or reelect the executive officer to any of such positions, except in connection with the termination of the executive officer’s employment for cause (as defined above), as a result of his or her permanent disability or death, or by the executive officer other than for good reason; (2) with respect to Dr. Carlson, a material diminution in her authority, duties or responsibilities; (3) a material reduction in the executive officer’s annual base salary, except in connection with a general reduction in the compensation of our or any successor’s or affiliate’s personnel with similar status and responsibilities; (3)(4) our or any successor’s or affiliate’s requirement the executive officer (without the executive officer’s consent) be based at any place outside a 50-mile radius of his or her placement of employment as of the effective date of the employment agreement, except for reasonably required travel for our or any successor’s or affiliate’s business that is not materially greater than such travel requirements prior to the effective date of the employment agreement; (4)(5) any material breach by us or any successor or affiliate of obligations to the executive officer under the employment agreement; (5)(6) other than for Dr. Carlson, any purported termination of the executive officer’s employment or service relationship for cause (as defined above) by us or any successor or affiliate that is not in accordance with the definition of cause; or (6)(7) other than for Dr. Carlson, a change in control (as defined below).
30
For purposes of the employment agreements with the named executive officers, “bonus” generally means an amount equal to the greater of (1) the executive officer’s target bonus for the fiscal year in which the date of termination occurs; or (2) the bonus awarded to the executive officer for the fiscal year prior to the date of termination (which bonus shall be annualized to the extent the executive officer was not employed for the entire fiscal year prior to the date of termination). If any portion of the bonus awarded to the executive officer consisted of securities or other property, the fair market value thereof shall be determined in good faith by our board of directors.
For purposes of the employment agreements with the named executive officers, a “change in control” hasgenerally means:
Annual Cash Performance Bonus
In addition to base salaries, the employment agreements described above provide that each of Messrs. Gonyer and D’Onofrio are eligible to earn an annual cash performance bonus determined on the basis of the executive officers’ and/or our attainment of financial or other performance criteria. For 2012, the target bonus level for Mr. Gonyer was 50% of his base salary and the target bonus level for Mr. D’Onofrio was 40% of his base salary.
- 79 -
The ultimate determination as to whether annual cash performance bonuses are paid is within the sole discretion of our compensation committee. For 2012, the corporate performance objectives related to progress towards business and clinical development goals. In May 2013, in order to conserve cash and without regard to the progress made towards the achievement of such objectives, our compensation committee elected not to pay a cash performance bonus to either Mr. Gonyer or Mr. D’Onofrio for fiscal year 2012.
Retention Letters
In March 2012 we entered into retention agreements with each of Messrs. Gonyer and D’Onofrio, which agreements were amended in May 2013. Pursuant to such agreements, and notwithstanding anything contained in the employment agreements, provided that each of Messrs. Gonyer and D’Onofrio continues employment with us through the applicable payment dates, they will be entitled to receive $225,000 and $130,000, respectively, in connection with certain retention events. For purposes of the retention agreements, “retention event” generally means (1) a change in control (as defined in the employment agreements described above) or (2) the consummation of a public or private equity financing in which investors purchase shares of our common or preferred stock, including the consummation of this offering. If we terminate the executive officer’s employment without cause or the executive officer resigns for good reason (each as defined in the employment agreements described above, provided, however, that good reason shall not include clauses six and seven described above) prior to the date on which the executive officer receives the full retention amount, and without regard to whether a retention event has occurred prior to the date of termination, the executive officer will be entitled to receive any unpaid portion of the retention amount on the date that is 60 days following the date of termination.
Equity Compensation
We offer stock options to our named executive officers as the long-term incentive component of our compensation program. Our stock options allow employees to purchase shares of our common stock at a price per share equal to the fair market value of our common stock on the date of grant and may or may not be intended to qualify as “incentive stock options” for U.S. federal income tax purposes. In the past, our board of directors has determined the fair market value of our common stock based upon inputs including valuation reports prepared by third-party valuation firms from time to time. Generally, the stock options we grant vest in equal monthly installments over 48 months, subject to the employee’s continued employment with us on the vesting date. We also generally offer our employees the opportunity to “early exercise” their unvested stock options by purchasing shares underlying the unvested portion of an option subject to our right to repurchase any unvested shares for the lesser of the exercise price paid for the shares and the fair market value of the shares on the date of the holder’s termination of service if the employee’s service with us terminates prior to the date on which the options are fully vested.
Stock options granted to our named executive officers may be subject to accelerated vesting in certain circumstance. For additional discussion, please see “—Employment Agreements” above and “—Change in Control Benefits” below.
Neither of our named executive officers received stock option awards in 2012.
We have adopted a 2013 Incentive Award Plan, to be effective on the day prior to the public trading date of our common stock, referred to below as the 2013 Plan, in order to facilitate the grant of cash and equity incentives to directors, employees (including our named executive officers) and consultants of our company and certain of its affiliates and to enable our company and certain of its affiliates to obtain and retain services of these individuals, which is essential to our long-term success. For additional information about the 2013 Plan, please see the section titled “Incentive Award Plans” below.
Other Elements of Compensation
Retirement Plans
We currently maintain a 401(k) retirement savings plan that allows eligible employees to defercontribute a portion of their compensation, within limits prescribed by the Internal Revenue Code, on a pre-tax basis through
- 80 -
contributions to the plan. Our named executive officers are eligible to participate in the 401(k) plan. Currently, we match contributions made by participants in the 401(k) plan up to a specified percentage, and these matching contributions are fully vested as of the date on which the contribution is made. We believe that providing a vehicle for tax-deferred retirement savings through our 401(k) plan and making fully vested matching contributions, adds to the overall desirability of our executive compensation package and further incentivizes our named executive officers in accordance with our compensation policies.
Employee BenefitsHealth and Welfare Benefits; Perquisites
Our named executive officers are eligible to participate in our health and welfare plans. We reimburse Dr. Carlson for her health care premiums and pay for the health and welfare benefits for our other named executive officers and our other four employees. We do not provide our named executive officers with any other significant perquisites or other personal benefits.
No Tax Gross-Ups
Clawback Policy
We do not make gross-up paymentsmaintain a compensation recovery policy that is compliant with the Nasdaq Listing Rules, as required by the Dodd-Frank Act.
Equity Incentive Plans
The principal features of our equity incentive plans are summarized below. These summaries are qualified in their entirety by reference to cover our named executive officers’ personal income taxes that may pertain to anythe actual text of the compensation paid or provided by our company.applicable plan, each of which is filed as an exhibit to the registration statement of which this prospectus is a part.
2013 Equity Incentive Plan
31
Change in Control BenefitsPurpose.
Our named executive officers may become entitled to certain benefits or enhanced benefits in connection with a change in controlThe purpose of our company. Each2013 Equity Incentive Plan (the “2013 Plan”) is to promote our success and enhance our value by linking the individual interests of the members of the board of directors and our employees and consultants to those of our named executive officers’ employment agreements entitles themstockholders and by providing such individuals with an incentive for outstanding performance to accelerated vestinggenerate superior returns to our stockholders. The 2013 Plan is further intended to provide us flexibility in our ability to motivate, attract, and retain the services of all outstanding equity awards, as well as certain other benefits,members of the board of directors, our employees and our consultants upon their termination without cause or their resignation for good reason within three months priorwhose judgment, interest, and special effort the successful conduct of our operation is largely dependent.
Securities Subject to the occurrence of a change in control or within 12 months following a change in control of our company. In addition, the occurrence of a change in control constitutes “good reason” for our named executive officers’ resignation. For additional discussion, please see “—Employment Agreements” above.
2013 Plan. Outstanding Equity Awards at 2012 Fiscal Year-End
The following table summarizes the number of shares of common stock underlying outstanding equity incentive plan awards for each named executive officer asAs of December 31, 2012.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||||
Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($)(2) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(2) | ||||||||||||||||||||||||||||
David A. Gonyer, R.Ph. | 2/9/11 | 72,000 | — | — | 0.40 | 2/8/21 | — | — | — | — | ||||||||||||||||||||||||||||
| 11/18/10 | — | — | — | — | — | 14,375 | (3) | — | — | |||||||||||||||||||||||||||||
| 9/14/07 | — | — | — | — | — | — | — | 41,250 | (4) | |||||||||||||||||||||||||||||
| 8/3/07 | 40,000 | — | — | 0.29 | 8/2/17 | — | — | — | — | |||||||||||||||||||||||||||||
Matthew J. D’Onofrio | 2/9/11 | 46,000 | — | — | 0.40 | 2/8/21 | — | — | — | |||||||||||||||||||||||||||||
| 11/18/10 | — | — | — | — | — | 14,375 | (3) | — | — | |||||||||||||||||||||||||||||
| 9/14/07 | — | — | — | — | — | — | — | 13,750 | (5) | |||||||||||||||||||||||||||||
| 8/3/07 | 20,000 | — | — | 0.29 | 8/2/17 | — | — | — | — | |||||||||||||||||||||||||||||
- 81 -
Director Compensation
2012 Director Compensation Table
The following table sets forth information for the year ended December 31, 2012 regarding the compensation awarded to, earned by or paid to our non-employee directors who served on our board2023, a total of directors during 2012. Employees of our company who also serve as directors do not receive additional compensation for their performance of services as directors.
Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||
Cam Garner | 100,000 | — | — | — | 100,000 | |||||||||||||||||
Todd C. Brady, M.D., Ph.D. | — | — | — | — | — | |||||||||||||||||
Scott Glenn | — | — | — | — | — | |||||||||||||||||
Malcolm R. Hill, PharmD. | — | — | — | — | — | |||||||||||||||||
Ken Widder, M.D. | — | — | — | — | — | |||||||||||||||||
The table below shows the aggregate numbers of option awards (exercisable and unexercisable) held as of December 31, 2012 by each non-employee director who was serving as of December 31, 2012.
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
Our board of directors has approved a compensation program for our non-employee directors, to be effective concurrently with this offering, that consists of annual retainer fees and/or long-term equity awards. Each non-employee director will receive an annual cash retainer of $17,500 for his or her services. In addition, the chair of our board of directors will receive an additional annual cash retainer of $17,500, the chair of the audit committee will receive an additional annual cash retainer of $7,500, the chair of the compensation committee will receive an additional annual cash retainer of $5,000 and the chair of the nominating and corporate governance committee will receive an additional annual cash retainer of $3,500. Audit committee members will receive an additional cash retainer of $3,750, compensation committee members will receive an additional annual cash retainer of $2,500 and nominating and corporate governance committee members will receive an additional annual cash retainer of $1,750. The non-employee directors will receive initial grants of options to purchase 18,0001,172,070 shares of our common stock vesting over three years, upon election to the board of directors or, for our current directors, the initial public trading date of our common stock, and thereafter annual grants of options to purchase 9,000 shares of our common stock, vesting on the first anniversary of the date of grant.
- 82 -
Incentive Award Plans
2013 Incentive Award Plan
Concurrently with this offering, we intend to establish the Evoke Pharma, Inc. 2013 Equity Incentive Award Plan, or the 2013 Plan. Our board of directors adopted the 2013 Plan on June 7, 2013, and we expect our stockholders to approve the 2013 Plan prior to the completion of this offering. The 2013 Plan will become effective on the day prior to the public trading date of our common stock. The material terms of the 2013 Plan, as it is currently contemplated, are summarized below.
Authorized Shares. A total of 510,000 shares of our common stock will initially be reservedwere authorized for issuance under the 2013 Plan. In addition, commencing on January 1, 2024 and on each January 1 thereafter during the ten-year term of the 2013 Plan, through and including January 1, 2033, the aggregate number of shares available for issuance under the 2013 Plan willshall be annually increased on the first dayby that number of each of our fiscal years during the term of the 2013 Plan, beginning with the 2014 fiscal year, by an amount equal to the least of:
300,000 shares;
5% of the outstanding shares of our common stock asequal to the lesser of:
such other•
The 2013 Plan will also provide for an aggregate limitnumber of 3,510,000 shares of common stock that may be issued or transferred pursuant to ISOs under the 2013 Plan overmay not exceed an aggregate of 50,000,000 shares. All of the course of its ten-year term.
Shares issued pursuant to awardsforegoing share numbers may be adjusted for changes in our capitalization and certain corporate transactions, as described below under the 2013 Planheading “Adjustments.”
To the extent that we repurchase or that are forfeited, as well as shares used to pay the exercise price of an award lapses, expires, is forfeited or is settled for cash, any shares subject to satisfy the tax withholding obligations related to an award will, becometo the extent of such lapse, expiration, forfeiture or cash settlement, be available for future grant or sale under the 2013 Plan. In addition, shares of common stock which are delivered by the holder or withheld by us in payment of the grant or exercise price or tax withholding obligation of any award under the 2013 Plan will again be available for future grant or sale under the 2013 Plan. If any shares of restricted stock are forfeited by a participant or repurchased by us pursuant to the extent2013 Plan, such shares shall again be available for future grant or sale under the 2013 Plan. Any shares subject to a Stock Appreciation Right, or a SAR, that are not issued in connection with the stock settlement of the SAR on exercise thereof shall again be available for the grant of an award is paid outpursuant to the 2013 Plan. The payment of dividend equivalents in cash rather thanin conjunction with any outstanding awards shall not be counted against the shares such cash payment will not reduce the number of sharesstock available for issuance under the 2013 Plan.plan.
Plan Administration. Administration. The compensation committee of our board of directors will administeradministers the 2013 Plan (except with respect to any award granted to “independent directors” (as defined in the 2013 Plan),non-employee directors, which must be administered by our full board of directors). Following the completion of this offering, toTo administer the 2013 Plan, our compensation committee must consist solely of at least two members of our board of directors, each of whom is a “non-employee director” for purposes of Rule 16b-3 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, an “independent director” under the rules of the NASDAQ Stock Market and, with respect to awards that are intended to constitute performance-based compensation under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, an “outside director” for purposes of Section 162(m).Act. Subject to the terms and conditions of the 2013 Plan, our compensation committee has the authority to select the persons to whom awards are to be made, to determine the type or types of awards to be granted to each person, the number of awards to grant, the number of shares to be subject to such awards, and the terms and conditions of such awards, and to make all other determinations and decisions and to take all other actions necessary or advisable for the administration of the 2013 Plan. Our compensation committee is also authorized to establish, adopt, amend or revise rules relating to administration of the 2013 Plan. Our board of directors may at any time revest in itself the authority to administer the 2013 Plan.
Eligibility. Eligibility. Options, stock appreciation rights, or SARs, restricted stock and other awards under the 2013 Plan may be granted to individuals who are then our officers or employees or are the officers or employees of any of our subsidiaries. Such awards may also be granted to our non-employee directors and consultants, but only employees may be granted incentive stock options,ISOs. The sum of any cash compensation, or ISOs. As of December 31, 2012, there were five non-employee directorsother compensation, and two employees who would have been eligible for awards under the 2013 Plan had it been in effect on such date. At such time after the completion of this offering when we are subject to the requirements of Section 162(m)value (determined as of the Code, the maximum numbergrant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of shares that may be subject to awards granted under the 2013 Plan to any individual in any calendar year cannot exceed 510,000 and the maximum amount that may be
- 83 -
paid to a participant in cash during any calendar year with respect to one or more cash based awards under the 2013 Plan is $5,000,000. In addition, the maximum number of shares that may be subject to awards granted under the 2013 Plan to anynon-employee director as compensation for services as a non-employee director during any calendar year shall be 150,000.under the 2013 Plan may not exceed $400,000. The board of directors may make exceptions to this limit for individual non-employee directors in extraordinary circumstances, as the board of directors may determine in its discretion, provided that the non-employee director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving non-employee directors.
32
Awards. Awards. The 2013 Plan provides that our compensation committee (or the board of directors, in the case of awards to non-employee directors) may grant or issue stock options, SARs, restricted stock, restricted stock units, dividend equivalents, stock payments and performance awards, or any combination thereof. Our compensation committee (or the board of directors, in the case of awards to non-employee directors) will consider each award grant subjectively, considering factors such as the individual performance of the recipient and the anticipated contribution of the recipient to the attainment of our long-term goals. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
•
•
•
•
•
- 84 -
|
•
•
33
Stock payments may be authorized by our compensation committee (or our board of directors, in the case of awards to non-employee directors) in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation arrangement, made in lieu of all or any part of compensation, including bonuses, that would otherwise be payable to employees, consultants or members of our board of directors.
Transferability of Awards. Unless the administrator provides otherwise, our 2013 Plan generally does not allow for the transfer of awards and only the recipient of an option or SAR may exercise such an award during his or her lifetime.
Qualified Performance-Based Compensation. The compensation committee may designate employees as “covered employees” whose compensation for a given fiscal year may be subject to the limit on deductible compensation imposed by Section 162(m) of the Code. Compensation. The compensation committee may grant to such covered employeeseligible recipients restricted stock, dividend equivalents, stock payments, restricted stock units, cash bonuses and other stock-based awards that are paid, vest or become exercisable upon the attainment of company performance criteria which are related to one or more of the following performance criteria as applicablerelating to our performance, or the performance of a division, business unit or an individual:individual, and may include but are not limited to the following: operating or other costs and expenses, improvements in expense levels, cash flow (including, but not limited to, operating cash flow and free cash flow), return on assets, return on capital, stockholders’ equity, return on stockholders’ equity, total stockholder return, return on sales, gross or net profit or operating margin, working capital, net earnings (either before or after interest, taxes, depreciation and amortization), gross or net sales or revenue, net income (either before or after taxes), adjusted net income, operating earnings, earnings per share of stock, adjusted earnings per share of stock, price per share of stock, regulatory body approval for commercialization of a product, capital raised in financing transactions or other financing milestones, market recognition (including but not limited to awards and analyst ratings), financial ratios, implementation or completion of critical projects, market share, economic value, comparisons with various stock market indices, and implementation, completion or attainment of objectively determinable objectives relating to research, development, regulatory, commercial or strategic milestones or development. These performance criteria may be measured in absolute terms or as compared to performance in an earlier period or as compared to any incremental increase or decrease or as compared to results of a peer group or to market performance indicators or indices.
The compensation committee may provide that one or more objectively determinable adjustments will be made to one or more of the performance goals established for any performance period. Such adjustments may include one or more of the following: items related to a change in accounting principle, items relating to financing activities, expenses for restructuring or productivity initiatives, other non-operating items, items related to acquisitions, items attributable to the business operations of any entity acquired by us during the performance period, items related to the disposal of a business or segment of a business, items related to discontinued operations that do not qualify as a segment of a business under applicable accounting standards, items attributable to any stock dividend, stock split, combination or exchange of shares occurring during the
- 85 -
performance period, any other items of significant income or expense which are determined to be appropriate adjustments, items relating to unusual or extraordinary corporate transactions, events or developments, items related to amortization of acquired intangible assets, items that are outside the scope of our core, on-going business activities, items related to acquired in-process research and development, items relating to changes in tax laws, items relating to major licensing or partnership arrangements, items relating to asset impairment charges, items relating to gains and losses for litigation, arbitration or contractual settlements, or items relating to any other unusual or nonrecurring events or changes in applicable laws, accounting principles or business conditions.
Forfeiture, Recoupment and Clawback Provisions. Provisions. Pursuant to its general authority to determine the terms and conditions applicable to awards under the 2013 Plan, the compensation committee has the right to provide, in an award agreement or otherwise, that an award shall be subject to the provisions of any recoupment or clawback policies implemented by us, including, without limitation, any recoupment or clawback policies adopted to comply with the requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act and any rules or regulations promulgated thereunder.
Adjustments. Adjustments. If there is any stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of our assets to stockholders, or any other change affecting the shares of our common stock or the share price of our common stock other than an equity restructuring (as defined in the 2013 Plan), the plan administrator maywill make such equitable adjustments, if any, as the plan administrator in its discretion may deem appropriate to reflect such change with respect to (1) the aggregate number and type of shares that may be issued under the 2013 Plan (including, but not limited to, adjustments of the number of shares available under the 2013 Planplan and the maximum number of shares which may be subject to one or more awards to a participant pursuant to the 2013 Planplan during any calendar year), (2) the number and kind of shares, or other securities or property, subject to outstanding awards, (3) the number and kind of shares, or other securities or property, for which automatic grants are to be subsequently made to new and continuing non-employee directors, (4) the terms and conditions of any outstanding awards (including, without limitation, any applicable performance targets or criteria with respect
34
thereto), and (5) the grant or exercise price per share for any outstanding awards under the 2013 Plan. If there is any equity restructuring, (1) the number and type of securities subject to each outstanding award and the grant or exercise price per share for each outstanding award, if applicable, will be proportionately adjusted, and (2) the plan administrator will make proportionate adjustments to reflect such equity restructuring with respect to the aggregate number and type of shares that may be issued under the 2013 Plan (including, but not limited to, adjustments of the number of shares available under the 2013 Planplan and the maximum number of shares which may be subject to one or more awards to a participant pursuant to the 2013 Planplan during any calendar year). Adjustments in the event of an equity restructuring will not be discretionary. Any adjustment affecting an award intended as “qualified performance-based compensation” will be made consistent with the requirements of Section 162(m) of the Code. The plan administrator also has the authority under the 2013 Plan to take certain other actions with respect to outstanding awards in the event of a corporate transaction, including provision for the cash-out, termination, assumption or substitution of such awards.
Corporate Transactions. Transactions. In the event of a change in control where the acquirer does not assume or substitute awards granted under the 2013 Plan, awards issued under the 2013 Plan will be subject to accelerated vesting such that 100% of the awards will become vested and exercisable or payable, as applicable. Under the 2013 Plan, a change in control is generally defined as:
•
- 86 -
|
•
•
•
•
•
Amendment and Termination. of the 2013 Plan. Our compensation committee or board of directors has the authority tomay terminate, amend suspend or terminatemodify the 2013 Plan at any time.Plan. However, stockholder approval of any amendment to the 2013 Plan will be obtained to the extent necessary and desirable to comply with any applicable law, regulation or stock exchange rule. Additionally, stockholder approval is required within 12 months of an increase inrule, or for any amendment to the maximum2013 Plan that increases the number of shares issuableavailable under the 2013 Plan or that may be issued to an individual in any calendar year. Except as necessary to comply with Section 409A of the Code, no amendment, suspension or termination of the 2013 Plan will impair the rights or obligations of a holder under an award theretofore granted, unless such award expressly so provides or such holder consents. If not terminated earlier by our compensation committee or board of directors, the 2013 Plan will terminate in March 2033 on the tenth anniversary of the date it was approvedof its initial approval by our board of directors.
35
Repricing Permitted. Permitted. Our compensation committee (or the board of directors, in the case of awards to non-employee directors) shall havehas the authority, without the approval of our stockholders, to authorize the amendment of any outstanding award to reduce its price per share and to provide that an award will be canceled and replaced with the grant of an award having a lesser price per share.
Securities Laws and Federal Income Taxes. The 2013 Plan is designed to comply with various securities and federal tax laws as follows:
Securities Laws. The 2013 Plan is intended to conform to all provisions of the Securities Act of 1933, as amended, and the Exchange Act and any and all regulations and rules promulgated by the SEC thereunder, including, without limitation, Rule 16b-3. The 2013 Plan will be administered, and awards will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Federal Income Tax Consequences. The material federal income tax consequences of the 2013 Plan under current federal income tax law are summarized in the following discussion, which deals with the general tax
- 87 -
principles applicable to the 2013 Plan. The following discussion is based upon laws, regulations, rulings and decisions now in effect, all of which are subject to change. Foreign, state and local tax laws, and employment, estate and gift tax considerations are not discussed due to the fact that they may vary depending on individual circumstances and from locality to locality.
Stock Options and Stock Appreciation Rights. A 2013 Plan participant generally will not recognize taxable income and we generally will not be entitled to a tax deduction upon the grant of a stock option or stock appreciation right. The tax consequences of exercising a stock option and the subsequent disposition of the shares received upon exercise will depend upon whether the option qualifies as an ISO as defined in Section 422 of the Code. The 2013 Plan permits the grant of options that are intended to qualify as ISOs as well as options that are not intended to so qualify; however, ISOs generally may be granted only to our employees and employees of our parent or subsidiary corporations, if any. Upon exercising an option that does not qualify as an ISO when the fair market value of our stock is higher than the exercise price of the option, a 2013 Plan participant generally will recognize taxable income at ordinary income tax rates equal to the excess of the fair market value of the stock on the date of exercise over the purchase price, and we (or our subsidiaries, if any) generally will be entitled to a corresponding tax deduction for compensation expense, in the amount equal to the amount by which the fair market value of the shares purchased exceeds the purchase price for the shares. Upon a subsequent sale or other disposition of the option shares, the participant will recognize a short-term or long-term capital gain or loss in the amount of the difference between the sales price of the shares and the participant’s tax basis in the shares.
Upon exercising an ISO, a 2013 Plan participant generally will not recognize taxable income, and we will not be entitled to a tax deduction for compensation expense. However, upon exercise, the amount by which the fair market value of the shares purchased exceeds the purchase price will be an item of adjustment for alternative minimum tax purposes. The participant will recognize taxable income upon a sale or other taxable disposition of the option shares. For federal income tax purposes, dispositions are divided into two categories: qualifying and disqualifying. A qualifying disposition generally occurs if the sale or other disposition is made more than two years after the date the option was granted and more than one year after the date the shares are transferred upon exercise. If the sale or disposition occurs before these two periods are satisfied, then a disqualifying disposition generally will result.
Upon a qualifying disposition of ISO shares, the participant will recognize long-term capital gain in an amount equal to the excess of the amount realized upon the sale or other disposition of the shares over their purchase price. If there is a disqualifying disposition of the shares, then the excess of the fair market value of the shares on the exercise date (or, if less, the price at which the shares are sold) over their purchase price will be taxable as ordinary income to the participant. If there is a disqualifying disposition in the same year of exercise, it eliminates the item of adjustment for alternative minimum tax purposes. Any additional gain or loss recognized upon the disposition will be recognized as a capital gain or loss by the participant.
We will not be entitled to any tax deduction if the participant makes a qualifying disposition of ISO shares. If the participant makes a disqualifying disposition of the shares, we should be entitled to a tax deduction for compensation expense in the amount of the ordinary income recognized by the participant.
Upon exercising or settling a SAR, a 2013 Plan participant will recognize taxable income at ordinary income tax rates, and we should be entitled to a corresponding tax deduction for compensation expense, in the amount paid or value of the shares issued upon exercise or settlement. Payments in shares will be valued at the fair market value of the shares at the time of the payment, and upon the subsequent disposition of the shares the participant will recognize a short-term or long-term capital gain or loss in the amount of the difference between the sales price of the shares and the participant’s tax basis in the shares.
Restricted Stock and Restricted Stock Units. A 2013 Plan participant generally will not recognize taxable income at ordinary income tax rates and we generally will not be entitled to a tax deduction
- 88 -
|
Dividend Equivalents, Stock Payment Awards and Cash-Based Awards. A 2013 Plan participant will not recognize taxable income and we will not be entitled to a tax deduction upon the grant of dividend equivalents, stock payment awards or cash-based awards until cash or shares are paid or distributed to the participant. At that time, any cash payments or the fair market value of shares that the participant receives will be taxable to the participant at ordinary income tax rates and we should be entitled to a corresponding tax deduction for compensation expense. Payments in shares will be valued at the fair market value of the shares at the time of the payment, and upon the subsequent disposition of the shares, the participant will recognize a short-term or long-term capital gain or loss in the amount of the difference between the sales price of the shares and the participant’s tax basis in the shares.
Section 409A of the Code. Certain types of awards under the 2013 Plan may constitute, or provide for, a deferral of compensation under Section 409A. Unless certain requirements set forth in Section 409A are complied with, holders of such awards may be taxed earlier than would otherwise be the case (e.g., at the time of vesting instead of the time of payment) and may be subject to an additional 20% federal income tax (and, potentially, certain interest penalties). To the extent applicable, the 2013 Plan and awards granted under the 2013 Plan will be structured and interpreted to comply with Section 409A and the Department of Treasury regulations and other interpretive guidance that may be issued pursuant to Section 409A.
Section 162(m) Limitation. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including base salary, annual bonus, stock option exercises and non-qualified benefits paid) for certain executive officers exceeds $1 million (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any one year. However, under Section 162(m), the deduction limit does not apply to certain “performance-based compensation” if an independent compensation committee determines performance goals and if the material terms of the performance-based compensation are disclosed to and approved by our stockholders. In particular, stock options and SARs will satisfy the “performance-based compensation” exception if the awards are made by a qualifying compensation committee, the plan sets the maximum number of shares that can be granted to any person within a specified period and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date. Under a Section 162(m) transition rule for compensation plans of corporations which are privately held and which become publicly held in an initial public offering, certain awards under the 2013 Plan will not be subject to Section 162(m) until a specified transition date, which is the earlier of (1) the material modification of the 2013 Plan, (2) the issuance of all employer stock and other compensation that has been allocated under the 2013 Plan, or (3) the first
- 89 -
|
We have attempted to structure the 2013 Plan in such a manner that, after the transition date, the compensation attributable to stock options and SARs which meet the other requirements of Section 162(m) will not be subject to the $1 million limitation. We have not, however, requested a ruling from the Internal Revenue Service or an opinion of counsel regarding this issue.
2007 Equity Incentive Plan
On May 30, 2007, our board of directors approved the Evoke Pharma, Inc. 2007 Equity Incentive Plan, or the 2007 Plan.
A total of 450,000 shares of our common stock are reserved for issuance under the 2007 Plan. As of December 31, 2012, 450,000 shares of our common stock were subject to outstanding option awards and zero shares of our common stock remained available for future issuance. After the effective date of the 2013 Plan, no additional awards will be granted under the 2007 Plan.
Administration. The compensation committee of our board of directors administers the 2007 Plan, except with respect to any award granted to non-employee directors (as defined in the 2007 Plan), which must be administered by our full board of directors. Subject to the terms and conditions of the 2007 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the type or types of awards to be granted to each person, determine the number of awards to grant, determine the number of shares to be subject to such awards, and the terms and conditions of such awards, and make all other determinations and decisions and to take all other actions necessary or advisable for the administration of the 2007 Plan. The plan administrator is also authorized to establish, adopt, amend or revise rules relating to administration of the 2007 Plan, subject to certain restrictions.
Eligibility. Options, SARs, restricted stock and other awards under the 2007 Plan may be granted to individuals who are then our employees, consultants and members of our board of directors and our subsidiaries. Only employees may be granted ISOs.
Awards. The 2007 Plan provides that our administrator may grant or issue stock options, restricted stock, restricted stock units, SARs, dividend equivalents, stock payments, or any combination thereof. The administrator considers each award grant subjectively, considering factors such as the individual performance of the recipient and the anticipated contribution of the recipient to the attainment of our long-term goals. Each award is set forth in a separate agreement with the person receiving the award and indicates the type, terms and conditions of the award.
NQSOs provide for the right to purchase shares of our common stock at a specified price which may not be less than the fair market value of a share of stock on the date of grant, and usually will become exercisable (at the discretion of our compensation committee or the board of directors, in the case of awards to non-employee directors) in one or more installments after the grant date, subject to the participant’s continued employment or service with us and/or subject to the satisfaction of performance targets established by our Our compensation committee (or the board of directors, in the case of awards to non-employee directors). NQSOs may be granted for also has the authority, without the approval of our stockholders, to amend any term specified by our compensation committee (oroutstanding award to increase the board of directors, in the case of awardsprice per share or to non-employee directors), but the term may not exceed ten years.
- 90 -
ISOs are designed to complycancel and replace an award with the provisionsgrant of an award having a price per share that is greater than or equal to the price per share of the Internal Revenue Code and are subject to specified restrictions contained in the Internal Revenue Code applicable to ISOs. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of common stock on the date of grant, may only be granted to employees, must expire within a specified period of time following the optionee’s termination of employment, and must be exercised within the ten years after the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) more than 10% of the total combined voting power of all classes of our capital stock on the date of grant, the 2007 Plan provides that the exercise price must be at least 110% of the fair market value of a share of common stock on the date of grant and the ISO must expire on the fifth anniversary of the date of its grant.
Restricted stock may be granted to participants and made subject to such restrictions as may be determined by the administrator. Typically, restricted stock may be repurchased by us at the original purchase price or, if no cash consideration was paid for such stock, forfeited for no consideration if the conditions or restrictions are not met, and the restricted stock may not be sold or otherwise transferred to third parties until restrictions are removed or expire. Recipients of restricted stock, unlike recipients of options, may have voting rights and may receive dividends, if any, prior to when the restrictions lapse.
Restricted stock units may be awarded to participants, typically without payment of consideration or for a nominal purchase price, but subject to vesting conditions including continued employment or performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold or otherwise transferred or hypothecated until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until some time after the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied and the shares have been issued.
SARs typically will provide for payments to the holder based upon increases in the price of our common stock over the exercise price of the SAR. There are no restrictions specified in the 2007 Plan on the exercise of SARs or the amount of gain realizable therefrom. The administrator may elect to pay SARs in cash or in common stock or in a combination of both.
Dividend equivalents may be awarded to participants and represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by the stock options, SARs or other awards held by the participant.
Stock payments may be authorized by the administrator in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation arrangement, made in lieu of all or any part of compensation, including bonuses, that would otherwise be payable to employees, consultants or members of our board of directors.
Corporate Transactions. In the event of a change of control where the acquiror does not assume awards granted under the 2007 Plan, awards issued under the 2007 Plan will be subject to accelerated vesting such that 100% of the awards will become vested and exercisable or payable, as applicable, immediately prior to the change in control. Under the 2007 Plan, a change of control is generally defined as:award.
a transaction or series of related transactions whereby any person or entity or related group of persons or entities (other than us, our subsidiaries, an employee benefit plan maintained by us or any of our subsidiaries or a person or entity that, prior to such transaction, directly or indirectly controls, is controlled by, or is under common control with, us) directly or indirectly acquires beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act) of more than 50% of the total combined voting power of our securities outstanding immediately after such acquisition;
during any two-year period, individuals who, at the beginning of such period, constitute our board of directors together with any new director(s) whose election by our board of directors or nomination for
- 91 -
|
our consummation (whether we are directly or indirectly involved through one or more intermediaries) of (1) a merger, consolidation, reorganization, or business combination, (2) the sale or other disposition of all or substantially all of our assets or (3) the acquisition of assets or stock of another entity, in each case other than a transaction that results in our voting securities outstanding immediately before the transaction continuing to represent, directly or indirectly, at least a majority of the combined voting power of the successor entity’s outstanding voting securities immediately after the transaction, and after which no person or entity beneficially owns voting securities representing 50% or more of the combined voting power of the acquiring company that is not attributable to voting power held in the company prior to such transaction; or
the approval by our stockholders of a liquidation or dissolution of our company.
Amendment and Termination of the 2007 Plan. Our board of directors may terminate, amend or modify the 2007 Plan. However, stockholder approval of any amendment to the 2007 Plan must be obtained to the extent necessary and desirable to comply with any applicable law, regulation or stock exchange rule, or for any amendment to the 2007 Plan that increases the number of shares available under the 2007 Plan. The administrator may, with the consent of the affected option holders, cancel any or all outstanding awards under the 2007 Plan and grant new awards in substitution. If not terminated earlier by the compensation committee or the board of directors, the 2007 Plan will terminate on May 30, 2017.
Securities Laws and Federal Income Taxes. The 2007 Plan is designed to comply with applicable securities laws in the same manner as described above in the description of the 2013 Plan under the heading “—2013 Equity Incentive Award Plan—Securities Laws and Federal Income Taxes—Securities Laws.” The general federal tax consequences of awards under the 2007 Plan are the same as those described above in the description of the 2013 Plan under the heading “—2013 Equity Incentive Award Plan—Securities Laws and Federal Income Taxes—Federal Income Tax Consequences.”
2013 Employee Stock Purchase Plan
Concurrently with this offering, we intend to establish the Evoke Pharma, Inc.
Purpose. The purpose of our 2013 Employee Stock Purchase Plan or(the “ESPP”) is to assist our eligible employees in acquiring a stock ownership interest in our company and to help our eligible employees provide for their future security and to encourage them to remain in our employment.
Securities Subject to the ESPP. Our boardAs of directors adopted the ESPP on June 7, 2013, and we expect our stockholders to approve the ESPP prior to the completion of this offering. The ESPP will become effective on the day prior to the public trading date of our common stock. Our executive officers and all of our other employees will be allowed to participate in our ESPP, subject to the eligibility requirements described below. The material terms of the ESPP, as it is currently contemplated, are summarized below. Our board of directors is still in the process of developing, approving and implementing the ESPP and, accordingly, this summary is subject to change.
ADecember 31, 2023, a total of 30,000145,381 shares of our common stock will initially be reservedare authorized for issuance under ourthe ESPP. In addition, commencing on January 1, 2024 and on each January 1 thereafter through and including January 1, 2033, the aggregate number of shares available for issuance under the ESPP willshall be annually increased on the first dayby that number of each fiscal year during the term of the ESPP, beginning with the 2014 fiscal year, by an amount equal to the least of:
30,000 shares;
1% of the outstanding shares of our common stock asequal to the lesser of:
such other•
The ESPP will also provide for an aggregate limit
Not withstanding the foregoing, the number of 330,000 shares of common stock that may be issued or transferred pursuant to awards under the ESPP during the termmay not exceed an aggregate of 10,000,000 shares. All of the ESPP.
- 92 -
Administration. Our board of directors or its committee has full and exclusive authority to interpret the terms of the ESPP and determine eligibility. Our compensation committee will beis the initial administrator of the ESPP.
Eligibility. Our employees are eligible to participate in the ESPP if they are customarily employed by us or any participating subsidiary for at least 20 hours per week and more than five months in any calendar year.year on the day prior to the first day of the offering period. However, an employee may not be granted rights to purchase stock under our ESPP if such employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power or value of all classes of our common or other class of stock.
Eligible employees become participants in the ESPP by enrolling and authorizing payroll deductions by the deadline established by the administrator prior to the relevant offering date. Directors who are not employees are not eligible to participate. Employees who choose not to participate, or are not eligible to participate at the start of an offering period but who become eligible thereafter, may enroll in any subsequent offering period.
Participation in an Offering.
36
After an employee authorizes us to deduct a certain percentage of his or her compensation for the purchase of shares under the ESPP, we will make such deductions from his or her paycheck each pay period during an offering period and hold the accumulated amounts in a bank account until the completion of the offering period. An employee will not receive any interest on the amounts of compensation that we accumulate for the purchase of shares under the ESPP. We may use all funds held by us under the ESPP for any corporate purpose, and we are not obligated to segregate such funds.
A participant may not transfer rights grantedAdjustments.
In the event of any stock dividend, stock split, combination or exchange of shares, merger, consolidation, spin-off, recapitalization, distribution of company assets to stockholders (other than normal cash dividends), or any other corporate event affecting our common stock, the number of shares reserved under the ESPP other thanand the price per share and number of shares of our common stock covered by each outstanding right may be adjusted proportionately. Such adjustments will be made by the lawsadministrator of descentthe ESPP, whose determination in that respect will be final, binding and distribution or as otherwise provided under the ESPP.conclusive.
In the event of certain significant transactions or a change in control (as defined in the ESPP), the compensation committeeadministrator of the ESPP may provide for (1) either the replacement or termination of outstanding rights in exchange for cash, (2) the assumption or substitution of outstanding rights by the successor or survivor corporation or parent or subsidiary thereof, if any, (3) the adjustment in the number and type of shares of stock subject to outstanding rights, (4) the use of participants’ accumulated payroll deductions to purchase stock on a new purchase date prior to the next purchase date and termination of any rights under ongoing offering periods or (5) the termination of all outstanding rights. Under
Transferability. A participant may not transfer rights granted under the ESPP a change in control hasother than by will, the same definitionlaws of descent and distribution or as given to such term inotherwise provided under the 2013 Plan.ESPP.
Amendment and Termination. The compensation committeeadministrator of the ESPP may amend, suspend or terminate the ESPP. However, stockholder approval of any amendment to the ESPP will be obtained for any amendment which changes the aggregate number or type of shares that may be sold pursuant to rights under the ESPP, changes the corporations or classes of corporations whose employees are eligible to participate in the ESPP or changes the ESPP in any manner
37
that would cause the ESPP to no longer be an employee stock purchase plan within the meaning of Section 423(b) of the Code. The ESPP will terminate no later than the tenth anniversary of the ESPP’s initial adoptioncontinue in effect until it is terminated by our board of directors.
- 93 -
Securities Laws. The ESPP has been designed to comply with various securities laws in the same manner as described above in the description of the 2013 Plan.Non-EmployeeDirector Compensation
Federal Income Taxes. The material federal income tax consequences of the ESPP under current federal income tax law are summarized in the following discussion, which deals with the general tax principles applicable to the ESPP. The following discussion is based upon laws, regulations, rulings and decisions now in effect, all of which are subject to change. Foreign, state and local tax laws, and employment, estate and gift tax considerations are not discussed due to the fact that they may vary depending on individual circumstances and from locality to locality.
The ESPP, and the right of participants to make purchases thereunder, is intended to qualify under the provisions of Section 423 of the Code. Under the applicable Code provisions, no income will be taxable to a participant until the sale or other disposition of the shares purchased under the ESPP. This means that an eligible employee will not recognize taxable income on the date the employee is granted an option under the ESPP (i.e., the first day of the offering period). In addition, the employee will not recognize taxable income upon the purchase of shares. Upon such sale or disposition, the participant will generally be subject to tax in an amount that depends upon the length of time such shares are held by the participant prior to disposing of them. If the shares are sold or disposed of more than two years from the first day of the offering period during which the shares were purchased and more than one year from the date of purchase, or if the participant dies while holding the shares, the participant (or his or her estate) will recognize ordinary income measured as the lesser of (1) the excess of the fair market value of the shares at the time of such sale or disposition over the purchase price or (2) an amount equal to 15% of the fair market value of the shares as of the first day of the offering period. Any additional gain will be treated as long-term capital gain. If the shares are held for the holding periods described above but are sold for a price that is less than the purchase price, there is no ordinary income and the participating employee has a long-term capital loss for the difference between the sale price and the purchase price.
If the shares are sold or otherwise disposed of before the expiration of the holding periods described above, the participant will recognize ordinary income generally measured as the excess of the fair market value of the shares on the date the shares are purchased over the purchase price and we will be entitled to a tax deduction for compensation expense in the amount of ordinary income recognized by the employee. Any additional gain or loss on such sale or disposition will be long-term or short-term capital gain or loss, depending on how long the shares were held following the date they were purchased by the participant prior to disposing of them. If the shares are sold or otherwise disposed of before the expiration of the holding periods described above but are sold for a price that is less than the purchase price, the participant will recognize ordinary income equal to the excess of the fair market value of the shares on the date of purchase over the purchase price (and we will be entitled to a corresponding deduction), but the participant generally will be able to report a capital loss equal to the difference between the sales price of the shares and the fair market value of the shares on the date of purchase.
Limitations of Liability and Indemnification Matters
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by the Delaware General Corporation Law, which prohibits our amended and restated certificate of incorporation from limiting the liability of our directors for the following:
any breach of the director’s duty of loyalty to us or our stockholders;
acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
unlawful payment of dividends or unlawful stock repurchases or redemptions; or
any transaction from which the director derived an improper personal benefit.
- 94 -
Our amended and restated certificate of incorporation and our amended and restated bylaws also provide that if Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended. This limitation of liability does not apply to liabilities arising under the federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated certificate of incorporation and our amended and restated bylaws also provide that we shall have the power to indemnify our employees and agents to the fullest extent permitted by law. Our amended and restated bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity, regardless of whether our amended and restated bylaws would permit indemnification. We have obtained directors’ and officers’ liability insurance.
We have entered into separate indemnification agreements with our directors and executive officers, in addition to indemnification provided for in our amended and restated certificate of incorporation and amended and restated bylaws. These agreements, among other things, provide for indemnification of our directors and executive officers for expenses, judgments, fines and settlement amounts incurred by this person in any action or proceeding arising out of this person’s services as a director or executive officer or at our request. We believe that these provisions in our amended and restated certificate of incorporation and amended and restated bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and executive officers.
The above description of the indemnification provisions of our amended and restated certificate of incorporation, our amended and restated bylaws and our indemnification agreements is not complete and is qualified in its entirety by reference to these documents, each of which is incorporated by reference as an exhibit to this registration statement.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
- 95 -
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
The following includes a summary of transactions since January 1, 2010 to which we have been a party in which the amount involved exceeded or will exceed $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under “Executive and Director Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
Preferred Stock Financings
From June 2007 through June 2010, we issued and sold to investors an aggregate of 12,195,068 shares of our Series A convertible preferred stock at a purchase price of $1.50 per share, for aggregate consideration of approximately $18.3 million.
The participants in this Series A convertible preferred stock financing included the following directors and holders of more than 5% of our capital stock or entities affiliated with them. The following table presents the number of shares issued to these related parties in these financings. Each share of Series A convertible preferred stock identified in the following table will be automatically converted into of a share of our common stock immediately prior to the closing of this offering.
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
Some of our directors are associated with our principal stockholders as indicated in the table below:
|
| |
| ||
|
Investor Rights Agreement
We entered into an investor rights agreement in June 2007 with the holders of our convertible preferred stock, including entities with which certain of our directors are affiliated. This agreement provides for certain rights relating to the registration of their shares of common stock issuable upon conversion of their convertible preferred stock, a right of first refusal to purchase future securities sold by us and certain additional covenants made by us. Except for the registration rights (including the related provisions pursuant to which we have agreed to indemnify the parties to the investor rights agreement), all rights under this agreement will terminate upon
- 96 -
completion of this offering. The registration rights will continue following this offering and will terminate seven years following the completion of this offering, or for any particular holder with registration rights, at such time following this offering when all securities held by that stockholder subject to registration rights may be sold pursuant to Rule 144 under the Securities Act. See “Description of Capital Stock—Registration Rights” for additional information.
Voting Agreement
We entered into a voting agreement in June 2007 by and among us and certain of our stockholders, pursuant to which the following directors were each elected to serve as members on our board of directors and, as of the date of this prospectus, continue to so serve: Drs. Brady, Widder and Hill and Messrs. Garner, Gonyer, and Glenn. Pursuant to the voting agreement, Mr. Gonyer, as our Chief Executive Officer, was initially selected to serve on our board of directors as a representative of holders of our common stock, as designated by a majority of our common stockholders. Drs. Brady and Widder were initially selected to serve on our board of directors as representatives of holders of our previously outstanding preferred stock, as designated by Domain Partners VII, L.P., and Latterell Venture Partners, L.P., respectively.
The voting agreement will terminate upon the closing of this offering, and members previously elected to our board of directors pursuant to this agreement will continue to serve as directors until they resign, are removed or their successors are duly elected by holders of our common stock. The composition of our board of directors after this offering is described in more detail under “Management—Board Composition and Election of Directors.”
Employment Agreements
We have entered into employment agreements with the following executive officers: David A. Gonyer, R.Ph. our President and Chief Executive Officer; Matthew J. D’Onofrio, MBA, our Executive Vice President, Chief Business Officer. For more information regarding these agreements, see the section of this prospectus entitled “Executive and Director Compensation—Narrative to Compensation Table.”
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers prior to the closing of this offering. These agreements, among other things, require us or will require us to indemnify each director (and in certain cases their related venture capital funds) and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
Our amended and restated certificate of incorporation and our amended and restated bylaws provide that we will indemnify each of our directors and officers to the fullest extent permitted by the Delaware General Corporation Law. Further, we have entered into indemnification agreements with each of our directors and officers, and we have purchased a policy of directors’ and officers’ liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment under certain circumstances. For further information, see “Executive and Director Compensation—Limitations of Liability and Indemnification Matters.”
Stock Option Grants to Executive Officers and Directors
We have granted stock options to our executive officers and certain of our directors as more fully described in the section entitled “Executive and Director Compensation.”
- 97 -
Policies and Procedures for Related Person Transactions
Our board of directors has adopted a written related person transaction policy, to be effective upon the consummation of this offering, setting forth the policies and procedures for the review and approval or ratification of related-person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
- 98 -
The following table sets forth information for the year ended December 31, 2023 regarding the compensation awarded to, earned by or paid to our non-employee directors who served on our board of directors during 2023. Employees of our company who also serve as a director do not receive additional compensation for their performance of services as a director.
Director | Fees Earned or Paid in Cash ($) | Option | All Other | Total | |||||||||||
Cam Garner | 73,000 | 29,948 | (2) | — | 102,948 | ||||||||||
Todd C. Brady, M.D., Ph.D. | 54,750 | 24,675 | (3) | — | 79,425 | ||||||||||
Malcolm R. Hill, Pharm.D. | 54,500 | 24,675 | (4) | — | 79,175 | ||||||||||
Vickie W. Reed | 60,000 | 25,308 | (5) | — | 85,308 | ||||||||||
Kenneth J. Widder, M.D. | 53,500 | 24,043 | (6) | — | 77,543 | ||||||||||
38
The table below shows the aggregate numbers of option awards (exercisable and unexercisable) held as of December 31, 2023 by each non-employee director who was serving as of December 31, 2023.
Director | Options Exercisable at | Options Unexercisable at | ||||||
Cam Garner | 29,458 | 17,750 | ||||||
Todd C. Brady, M.D., Ph.D. | 26,018 | 14,625 | ||||||
Malcolm R. Hill, Pharm.D. | 24,645 | 14,625 | ||||||
Vickie W. Reed | 8,722 | 16,944 | ||||||
Kenneth J. Widder, M.D. | 25,580 | 14,250 | ||||||
For the year ended December 31, 2023, each non-employee director received $45,000 for his or her service. Additionally, the chair of our board of directors received an annual cash retainer of $20,000, the chair of the audit committee received an additional annual cash retainer of $15,000, the chair of the compensation committee received an additional annual cash retainer of $8,000, and the chair of the nominating and corporate governance committee received an additional annual cash retainer of $5,500. Audit committee members received an additional cash retainer of $5,750, compensation committee members received an additional annual cash retainer of $4,000 and nominating and corporate governance committee members received an additional annual cash retainer of $2,750.
Each non-employee director who is newly elected or appointed to the board of directors will receive an initial grant of options to purchase 5,833 shares of our common stock, vesting in three equal annual installments on each of the first three anniversaries of the date of grant, upon such election or appointment to the board of directors. Effective January 2023, our board of directors amended the compensation program for our non-employee directors. Under the amended program, non-employee directors receive annual grants of options on the date of each annual meeting of stockholders as follows: each non-employee director, options to purchase 12,000 shares; chair of our board of directors, an additional grant of options to purchase 3,500 shares; chair of the audit committee, an additional grant of options to purchase 3,000 shares; chair of the compensation committee, an additional 2,250 shares; and chair of the nominating and corporate governance committee, an additional grant of options to purchase 1,500 shares. Audit committee members received an additional grant of options to purchase 1,500 shares; members of the compensation committee received an additional grant of 1,125 shares; and the members of the nominating and corporate governance committee received an additional grant of options to purchase 750. All of the annual grants will vest on the first anniversary of the date of grant. The remaining terms of the director compensation program as in effect prior to this amendment remain unchanged.
39
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information regarding the beneficial ownership of ourthe Company’s common stock as of MarchDecember 31, 2013, and as adjusted to reflect the sale of shares of common stock in this offering, by:
each of our named executive officers;
each of our directors;
all of our executive officers and directors as a group; and
2023 by (i) each person or group of affiliated persons known by us to beneficially own more thanat least 5% of our common stock.stock, (ii) each of our named executive officers, (iii) each of our directors and (iv) all of our directors and executive officers as a group.
The numberfollowing table gives effect to the shares of sharescommon stock issuable within 60 days of December 31, 2023 upon the exercise of all options and other rights beneficially owned by each stockholderthe indicated stockholders on that date. Beneficial ownership is determined in accordance with Rule 13d-3 promulgated under rules issued bySection 13 of the SEC. Under these rules,Exchange Act and includes voting and investment power with respect to shares. Percentage of beneficial ownership includes any shares as to which a person has sole or shared voting power or investment power. Applicable percentage ownership is based on 3,681,7523,343,070 shares of common stock outstanding on MarchDecember 31, 2013, which gives effect2023. Except as otherwise noted below, each person or entity named in the following table has sole voting and investment power with respect to the conversion of all outstanding shares of our convertible preferred stock into shares of common stock. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options, warrantsthat he, she or other rights held by such person that are currently exercisable or will become exercisable within 60 days of March 31, 2013 are considered outstanding, although these shares are not considered outstanding for purposes of computing the percentage ownership of any other person.it beneficially owns.
Unless otherwise indicated below, the address offor each beneficial owner listed below is c/o Evoke Pharma, Inc., 12555 High Bluff Drive,420 Stevens Avenue, Suite 385, San Diego,230, Solana Beach, CA 92130.92075. We believe, based on information provided to us that each of the stockholders listed below has sole voting and investment power with respect to the shares beneficially owned by the stockholder unless noted otherwise, subject to community property laws where applicable.
Name of Beneficial Owner | Shares Beneficially Owned Prior to Offering |
| Shares Beneficially Owned After to Offering | ||||
| Number |
| Percentage |
| Number |
| Percentage |
Executive Officers and Directors |
|
|
|
|
|
|
|
David A. Gonyer, R.Ph.(1) | 147,279 |
| 4.3% |
| 147,279 |
| 4.2% |
Matthew J. D'Onofrio(2) | 95,409 |
| 2.8% |
| 95,409 |
| 2.8% |
Marilyn R. Carlson, D.M.D., M.D.(3) | 76,808 |
| 2.3% |
| 76,808 |
| 2.3% |
Cam L. Garner(4) | 57,424 |
| 1.7% |
| 57,424 |
| 1.7% |
Todd C. Brady, M.D., Ph.D.(5) | 26,351 |
| * |
| 26,351 |
| * |
Malcolm R. Hill, Pharm.D.(6) | 26,249 |
| * |
| 26,249 |
| * |
Vickie W. Reed(7) | 8,722 |
| * |
| 8,722 |
| * |
Kenneth J. Widder, M.D.(8) | 25,580 |
| * |
| 25,580 |
| * |
All executive officers and directors as a group (8 persons)(9) | 463,822 |
| 12.5% |
| 463,822 |
| 12.5% |
| Shares Beneficially Owned | Shares Beneficially Owned | |||||||||||
| Prior to Offering | After Offering | |||||||||||
Name of Beneficial Owner | Number | Percentage | Number | Percentage | ||||||||
5% or Greater Stockholders | ||||||||||||
Funds affiliated with Domain Associates, L.L.C.(1) | 1,138,583 | 30.9 | % | |||||||||
One Palmer Square | ||||||||||||
Princeton, NJ 08542 | ||||||||||||
Funds affiliated with LVP GP III, LLC(2) | 1,138,579 | 30.9 | % | |||||||||
1 Embarcadero Center, Suite 4050 | ||||||||||||
San Francisco, CA 94111 | ||||||||||||
Executive Officers and Directors | ||||||||||||
David A. Gonyer, R.Ph.(3) | 517,000 | 13.8 | % | |||||||||
Matthew J. D’Onofrio(4) | 241,000 | 6.5 | % | |||||||||
Cam L. Garner(5) | 375,605 | 10.2 | % | |||||||||
Todd C. Brady, M.D., Ph.D. | — | — | ||||||||||
Scott L. Glenn(6) | 120,855 | 3.3 | % | |||||||||
Malcolm R. Hill, Pharm.D.(7) | 19,250 | * | ||||||||||
Ann D. Rhoads | — | — | ||||||||||
Kenneth J. Widder, M.D.(2) | 1,138,583 | 30.9 | % | |||||||||
All executive officers and directors as a group (8 persons)(8) | 2,412,289 | 63.4 | % | |||||||||
|
- 99 -* Less than 1%
(1) Includes (a) 23,416 shares held by a family trust of which Mr. Gonyer and his spouse are co-trustees and (b) 118,965 shares Mr. Gonyer has |
- 100 -
General
Following the closing of this offering, our authorized capital stock will consist of 100,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. The following description summarizes some of the terms of our amended and restated certificate of incorporation and amended and restated bylaws, our outstanding warrants, the investors’ rights agreement and of the Delaware General Corporation Law. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our amended and restated certificate of incorporation, amended and restated bylaws, warrants and investors’ rights agreement, copies of which have been filed or incorporated by reference as exhibits to the registration statement of which the prospectus is a part, as well as the relevant provisions of the Delaware General Corporation Law.
Common Stock
As of March 31, 2013, there were 3,681,752 shares of our common stock outstanding and held of record by 15 stockholders, assuming the automatic conversion of all outstanding shares of our convertible preferred stock into 2,439,002 shares of common stock, which we expect to automatically occur immediately prior to the closing of this offering. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders, including the election of directors, and do not have cumulative voting rights. Accordingly, the holders of a majority of the outstanding shares of common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they so choose, other than any directors that holders of any preferred stock we may issue may be entitled to elect. Subject to preferences that may be applicable to any then outstanding preferred stock, holders of common stock are entitled to receive ratably those dividends, if any, as may be declared by the board of directors out of legally available funds. In the event of our liquidation, dissolution or winding up, the holders of common stock will be entitled to share ratably in the assets legally available for distribution to stockholders after the payment of or provision for all of our debts and other liabilities, subject to the prior rights of any preferred stock then outstanding. Holders of common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking funds provisions applicable to the common stock. All outstanding shares of common stock are, and the common stock to be outstanding upon completion of this offering will be, duly authorized, validly issued, fully paid and nonassessable. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our amended and restated certificate of incorporation, which will become effective immediately prior to the closing of this offering, our board of directors has the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the dividend, voting and other rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. We have no current plans to issue any shares of preferred stock.
- 101 -
Options
As of March 31, 2013, options to purchase 123,250 shares of our common stock were outstanding under our 2007 equity incentive plan, of which 69,167 were vested and all of which were exercisable as of that date.
Warrants
In February 2008, in connection with the closing of a debt facility, we issued a warrant to Square 1 Bank, which warrant is immediately exercisable for an aggregate of 50,000 shares of our Series A convertible preferred stock, at an exercise price of $1.50 per share. Immediately prior to the closing of this offering, this warrant will become exercisable for 10,000 shares of common stock at an exercise price of $7.50 per share. This warrant will expire three years from the effective date of the registration statement of which this prospectus is a part, which is , 2016.
In June 2012, in connection with the closing of a debt facility, we issued a warrant to Silicon Valley Bank, which warrant is immediately exercisable for an aggregate of 60,000 shares of our Series A convertible preferred stock, at an exercise price of $1.50 per share. Immediately prior to the closing of this offering, this warrant will become exercisable for 12,000 shares of common stock at an exercise price of $7.50 per share. This warrant will expire 10 years from the date of grant, which is June 1, 2022.
Each of the above warrants has a net exercise provision under which their holders may, in lieu of payment of the exercise price in cash, surrender the warrant and receive, a net amount of shares of our common stock based on the fair market value of our common stock at the time of the net exercise of the warrant after deduction of the aggregate exercise price. These warrants also contain provisions for the adjustment of the exercise price and the aggregate number of shares issuable upon the exercise of the warrants in the event of stock dividends, stock splits, reorganizations and reclassifications and consolidations.
We have agreed to issue to the representative of the underwriters in this offering warrants to purchase up to shares of our common stock, with a per share exercise price equal to 175% of the public offering price. In addition, the warrants provide for registration rights upon request, in certain cases. The demand registration right provided will not be greater than five years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(H)(iv). The piggyback registration right provided will not be greater than seven years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(H)(v). See “Underwriting—Representative’s Warrants” section of this prospectus for a description of these warrants.
Registration Rights
In addition to the registration rights granted with respect to the representative’s warrant described above, as of March 31, 2013, holders of 2,984,752 shares of our common stock, which includes 2,439,002 shares issuable upon the automatic conversion of our Series A convertible preferred stock immediately prior to the closing of this offering, will be entitled to the following rights with respect to the registration of such shares for public resale under the Securities Act, pursuant to an investor rights agreement by and among us and certain of our stockholders, subject to the 180-day lock-up agreements described in the “Shares Eligible for Future Sale—Lock-Up Agreements” section of this prospectus. In addition, holders of 10,000 shares of common stock issuable upon the exercise of a warrant will be entitled to the following rights with respect to the registration of such shares for public resale under the Securities Act, subject to the 180-day lock-up agreements described in the “Shares Eligible for Future Sale—Lock-Up Agreements” section of this prospectus. The registration of shares of common stock as a result of the following rights being exercised would enable holders to trade these shares without restriction under the Securities Act when the applicable registration statement is declared effective.
Demand Registration Rights
If at any time beginning six months after this offering the holders of at least a majority of the registrable securities request in writing that we effect a registration with respect to their shares in an offering with an
- 102 -
anticipated aggregate offering price of at least $5.0 million, we may be required to register their shares. We are obligated to effect at most two registrations for the holders of registrable securities in response to these demand registration rights. If the holders requesting registration intend to distribute their shares by means of an underwriting, the managing underwriter of such offering will have the right to limit the numbersacquire pursuant to outstanding options which are immediately exercisable within 60 days of December 31, 2023.
Piggyback Registration Rights
If at any time after this offering we propose to register any shares of our common stock under the Securities Act, subject to certain exceptions, the holders of registrable securities will be entitled to notice of the registration and to include their shares of registrable securities in the registration. If our proposed registration involves an underwriting, the managing underwriter of such offering will haveMr. D’Onofrio has the right to limit the number of shares to be underwritten for reasons related to the marketing of the shares.
Form S-3 Registration Rights
If at any time after we become entitled under the Securities Act to register our shares on Form S-3 a holder of registrable securities requests in writing that we register their shares for public resale on Form S-3 and the reasonably anticipated price to the public of the offering is $1.0 million or more, we will be required to use our best efforts to effect such registration; provided, however, that we will not be required to effect such a registration if, within the preceding 12 months, we have already effected two registrations on Form S-3 for the holders of registrable securities.
Expenses
Ordinarily, other than underwriting discounts and commissions, we will be required to pay all expenses incurred by us related to any registration effectedacquire pursuant to outstanding options which are immediately exercisable within 60 days of December 31, 2023.
40
The registration rights terminate upon the earlier of seven years after the effective date of the registration statement
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
Some provisions of Delaware law, our amended and restated certificate of incorporation and our amended and restated bylaws contain provisions that could make the following transactions more difficult: an acquisition of us by means of a tender offer; an acquisition of us by means of a proxy contest or otherwise; or the removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions which provide for payment of a premium over the market price for our shares.
These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seekingright to acquire controlpursuant to outstanding options which are immediately exercisable within 60 days of us to first negotiate with our board of directors. We believe thatDecember 31, 2023.
- 103 -
December 31, 2023.
The ability of our board of directors, without action by the stockholders, to issue up to 10,000,000
Stockholder Meetings
Our amended and restated bylaws provide that a special meeting of stockholders may be called only by our chairman of the board, chief executive officer or president, or by a resolution adopted by a majority of our board of directors.
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals to be brought before a stockholder meeting and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors.
Elimination of Stockholder Action by Written Consent
Our amended and restated certificate of incorporation and amended and restated bylaws eliminateDr. Hill has the right of stockholders to act by written consent without a meeting.
Staggered Board
Our board of directors is divided into three classes. The directors in each class will serve for a three-year term, one class being elected each year by our stockholders. For more information on the classified board, see “Management—Board Composition and Election of Directors.” This system of electing and removing directors may tendacquire pursuant to discourage a third-party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors.
Removal of Directors
Our amended and restated certificate of incorporation provides that no member of our board of directors may be removed from office by our stockholders except for cause and, in addition to any other vote required by law, upon the approval of not less than 66 2/3% of the total voting power of all of our outstanding voting stock then entitled to vote in the election of directors.
Stockholders Not Entitled to Cumulative Voting
Our amended and restated certificate of incorporation does not permit stockholders to cumulate their votes in the election of directors. Accordingly, the holders of a majority of the outstanding shares of our common stock entitled to vote in any election of directors can elect all of the directors standing for election, if they choose, other than any directors that holders of our preferred stock may be entitled to elect.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits persons deemed to be “interested stockholders” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person
- 104 -
who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors.
Amendment of Charter Provisions
The amendment of any of the above provisions, except for the provision making it possible for our board of directors to issue preferred stock, would require approval by holders of at least 66 2/3% of the total voting power of all of our outstanding voting stock.
The provisions of Delaware law, our amended and restated certificate of incorporation and our amended and restated bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition of our board and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be .
NASDAQ Capital Market
We have applied to have our common stock listed on The NASDAQ Capital Market under the symbol “EVOK.”
- 105 -
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was no public market for our common stock. Future sales of substantial amounts of common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock. Although we have applied to have our common stock listed on The NASDAQ Capital Market, we cannot assure you that there will be an active public market for our common stock.
Based on the number of shares of our common stock outstanding as of March 31, 2013 and assuming (1) the issuance of shares in this offering, and (2) the conversion of all outstanding shares of our convertible preferred stock into 2,439,002 shares of our common stock, which we expect to automatically occur immediately prior to the closing of the offering, (3) no exercise of the underwriters’ over-allotment option to purchase additional shares of common stock, and (5) no exercise of outstanding options or warrants, we will havewhich are immediately exercisable within 60 days of December 31, 2023.
Of these shares, all shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act. Shares purchased by our affiliates would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
The remaining shares of common stock will be “restricted securities,” as that term is defined in Rule 144 under the Securities Act. These restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rule 144 or 701 under the Securities Act, each of which is summarized below. We expect that substantially all of these shares will be subject to the 180-day lock-up period under the lock-up agreements described below.
In addition, of the 123,250 shares of our common stock that were subject to stock options outstanding as of MarchDecember 31, 2013, options to purchase 69,167 of such shares of common stock were vested as of such date and, upon exercise, these shares will be eligible for sale subject to the lock-up agreements described below and Rules 144 and 701 under the Securities Act.
2023.
We, each of our directors and executive officers and holders of substantially all of our outstanding shares of common stock have agreed that, without the prior written consent of Aegis Capital Corp. on behalf of the underwriters, we and they will not, subject to limited exceptions, during the period ending 180 days after the date of this prospectus, subject to extension in specified circumstances:
offer, pledge, sell or contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock;
enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock or any securities convertible into or exchangeable or exercisable for shares of our common stock, whether such transaction is to be settled by delivery of shares of our common stock or such other securities, in cash or otherwise;
make any demand for or exercise any right with respect to the registration of any shares of our common stock or any securities convertible into or exchangeable or exercisable for shares of our common stock; or
publicly announce an intention to do any of the foregoing.
The lock-up restrictions, specified exceptions and the circumstances under which the 180-day lock-up period may be extended are described in more detail under “Underwriting.”
- 106 -
Upon the expiration of the lock-up period, substantially all of the shares subject to such lock-up restrictions will become eligible for sale, subject to the limitations discussed above.
Rule 144
Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours, or who was an affiliate at any time during the 90 days before a sale, who has beneficially owned shares of our common stock for at least six months would be entitled to sell in “broker’s transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three-month period that does not exceed the greater of:
1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; or
the average weekly trading volume in our common stock on The NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale.
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the Securities and Exchange Commission and The NASDAQ Capital Market concurrently with either the placing of a sale order with the broker or the execution of a sale directly with a market maker.
Non-Affiliate Resales of Restricted Securities
In general, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the three months preceding a sale, and who has beneficially owned shares of our common stock for at least six months but less than a year, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell under Rule 144(b)(1) without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Rule 701
In general, under Rule 701, any of an issuer’s employees, directors, officers, consultants or advisors who purchases shares from the issuer in connection with a compensatory stock or option plan or other written agreement before the effective date of a registration statement under the Securities Act is entitled to sell such shares 90 days after such effective date in reliance on Rule 144. An affiliate of the issuer can resell shares in reliance on Rule 144 without having to comply with the holding period requirement, and non-affiliates of the issuer can resell shares in reliance on Rule 144 without having to comply with the current public information and holding period requirements.
Equity Plan
We intend to file one or more registration statements on Form S-8 under the Securities Act to register allIncludes 381,449 shares of common stock subject to outstanding stock options and common stock issued or issuable under our stock plan. We expect to file the registration statement covering shares offered pursuant to our stock plan shortly after the datewhich are immediately exercisable within 60 days of this prospectus, permitting the resale of such shares by non-affiliates in the public market without restriction under the Securities Act and the sale by affiliates in the public market subject to compliance with the resale provisions of Rule 144.
- 107 -41
Registration Rights
Based on the number of shares of our convertible preferred stock outstanding as of March 31, 2013 and assuming the automatic conversion of all outstanding shares of our convertible preferred stock into 2,439,002 shares of our common stock immediately prior to the closing of the offering, the holders of 2,984,752 shares of common stock or their transferees will be entitled to various rights with respect to the registration of these shares under the Securities Act upon the closing of this offering. In addition, holders of 10,000 shares of common stock issuable upon the exercise of a warrant will be entitled to various rights with respect to the registration of these shares under the Securities Act and upon the closing of this offering. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates. See “Description of Capital Stock—Registration Rights” for additional information. Shares covered by a registration statement will be eligible for sale in the public market upon the expiration or release from the terms of the lock-up agreement.
- 108 -
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS OF COMMON STOCK
The following discussion is a summary of thecertain material United StatesU.S. federal income tax consequences to non-U.S. holders (as defined below) of the acquisition,purchase, ownership and disposition of ourthe shares of common stock and Pre-Funded Warrants and accompanying Common Warrants or components thereof, which we refer to collectively as the “Securities,” issued pursuant to this offering. This discussion isoffering, but does not purport to be a complete analysis of all potential tax effects. The effects of the potential United Statesother U.S. federal income tax consequences relating thereto, nor does it address anylaws, such as estate and gift tax consequences orlaws, and any tax consequences arising under anyapplicable state, local or foreign tax laws or any other United States federal tax laws.are not discussed. This discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), United States Treasury regulationsRegulations promulgated thereunder, (“Treasury Regulations”), judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (“IRS”), all as in effect as of the date of this offering. These authorities may change possiblyor be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively resulting in United States federal income tax consequences different from those discussed below. No ruling has been ora manner that could adversely affect a holder of the Securities. We have not sought and will be soughtnot seek any rulings from the IRS with respect toregarding the matters discussed below, and therebelow. There can be no assurance that the IRS or a court will not take a contrary position regarding the tax consequences of the acquisition,purchase, ownership orand disposition of our common stock, or that any such contrary position would not be sustained by a court.the Securities.
This discussion is limited to non-U.S. holders who purchase our common stock issued pursuant to this offering and whothat hold our common stockthe Securities as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all of the United StatesU.S. federal income tax consequences that may be relevant to a particular holder in light of such holder’s particular circumstances. This discussion alsocircumstances, including the impact of the alternative minimum tax or the unearned income Medicare contribution tax. In addition, it does not consider any specific facts or circumstances that may beaddress consequences relevant to holders subject to specialparticular rules, under the United States federal income tax laws, including, without limitation:
banks, thrifts, insurance companies and other financial institutions;
tax-exempt organizations;
partnerships, S corporations or other pass-through entities;
real estate investment trusts and regulated investment companies;
brokers, dealers or traders in securities, commodities or currencies;
United StatesU.S. expatriates and certain former citizens or long-term residents of the United States;
“controlled foreign corporations,” “passive foreign investment companies” or corporations that accumulate earnings to avoid U.S. federal income tax;
persons that own, or are deemed to own, more than 5% of our outstanding common stock (except toholding the extent specifically set forth below);
persons deemed to sell our common stock under the constructive sale provisions of the Code;
persons subject to the alternative minimum tax;
persons that hold our common stockSecurities as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment;
•
•
If a partnership (or other entity taxedor arrangement treated as a partnership for United StatesU.S. federal income tax purposes) holds our common stock,the Securities, the tax treatment of a partner in the partnership generally will depend on the status of the partner, and upon the activities of the partnership. partnership and certain determinations made at the partner level.
42
Accordingly, partnerships that hold our common stockholding the Securities and the partners in such partnerships are urged toshould consult their tax advisors regarding the specific United StatesU.S. federal income tax consequences to them of acquiring, owning or disposing of our common stock.
- 109 -
PROSPECTIVETHIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT INTENDED AS LEGAL OR TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDINGWITH RESPECT TO THE PARTICULAR UNITED STATESAPPLICATION OF THE U.S. FEDERAL INCOME TAX CONSEQUENCESLAWS TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK,THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF THE SECURITIES ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR FOREIGN TAX LAWS, ANY OTHER UNITED STATES FEDERAL TAX LAWS ANDNON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Allocation of Purchase Price
Each share of common stock or Pre-Funded Warrant, as applicable, and accompanying Common Warrants will be treated for U.S. federal income tax purposes as an investment unit consisting of one share of our common stock or Pre-Funded Warrant, as applicable, and accompanying Series A Warrant, Series B Warrant and Series C Warrant. In determining their tax basis for the common stock or Pre-Funded Warrant and the Common Warrants constituting an investment unit, holders of Securities should allocate their purchase price for the investment unit between the common stock or Pre-Funded Warrant, as applicable, and each such Common Warrant on the basis of their relative fair market values at the time of issuance. The Company does not intend to advise holders of the Securities with respect to this determination, and holders of the Securities are advised to consult their tax and financial advisors with respect to the relative fair market values of the common stock or Pre-Funded Warrant, as applicable, and the accompanying Common Warrants for U.S. federal income tax purposes.
Treatment of Pre-Funded Warrants
Although not free from doubt, a Pre-Funded Warrant should be treated as a share of our common stock for U.S. federal income tax purposes, and a holder of Pre-Funded Warrants should generally be taxed in the same manner as a holder of common stock, as described below. Accordingly, no gain or loss should be recognized (other than with respect to cash paid in lieu of a fractional share) upon the exercise of a Pre-Funded Warrant (except in the case of a cashless exercise, the treatment of which for U.S. federal income tax purposes is not clear) and, upon exercise, the holding period of a Pre-Funded Warrant should carry over to the share of common stock received. Similarly, the tax basis of the Pre-Funded Warrant should carry over to the share of common stock received upon exercise, increased by the exercise price of $0.0001. The discussion below assumes the characterization described above is respected for U.S. federal income tax purposes. Holders should consult their tax advisors regarding the risks associated with the acquisition of Pre-Funded Warrants pursuant to this offering (including alternative characterizations).
Tax Considerations Applicable to U.S. Holders
Definition of Non-U.S.a U.S. Holder
For purposes of this discussion, a non-U.S. holder“U.S. holder” is any beneficial owner of our common stockthe Securities that, is not a “U.S. person” or a partnership for United StatesU.S. federal income tax purposes. A U.S. personpurposes, is or is treated as any of the following:
•
•
•
•
43
Distributions on Our Common Stock
As described in the section entitled “Dividend Policy,” we do not anticipate declaring or payingexpect to pay any cash dividends to holders ofon our commoncapital stock in the foreseeable future. However, if we do make distributions of cash or other property distributions on our common stock or Pre-Funded Warrants (other than certain distributions of common stock), such distributions generally will constitute dividends for United States federal income tax purposes to the extent paid fromout of our current or accumulated earnings and profits, as determined under United Statesfor U.S. federal income tax principles. Amounts not treated aspurposes. Dividends received by a corporate U.S. holder may be eligible for a dividends for United States federal income tax purposesreceived deduction, subject to applicable limitations. Dividends received by certain non-corporate U.S. holders, including individuals, are generally taxed at the lower applicable capital gains rate provided certain holding period and other requirements are satisfied. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital and will first be applied against and reduce a non-U.S.U.S. holder’s adjusted tax basis in theits common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below in the section relating to the sale or disposition of our common stock or Pre-Funded Warrants, as applicable.
Sale or Other Taxable Disposition of Common Stock or Pre-Funded Warrants
Upon the sale, exchange or other taxable disposition of the common stock or Pre-Funded Warrants, a U.S. holder generally will recognize capital gain or loss equal to the difference between (i) the amount of cash and the fair market value of any property received upon the sale, exchange or other taxable disposition and (ii) such U.S. holder’s adjusted tax basis in the common stock or Pre-Funded Warrant. Such capital gain or loss will be long-term capital gain or loss if the U.S. holder’s holding period in such common stock or Pre-Funded Warrant is more than one year at the time of the sale, exchange or other taxable disposition. Long-term capital gains recognized by certain non-corporate U.S. holders, including individuals, generally will be subject to reduced rates of U.S. federal income tax. The deductibility of capital losses is subject to certain limitations.
Sale or Other Disposition, Exercise or Expiration of Common Warrants
Upon the sale or other disposition of a Common Warrant (other than by exercise), a U.S. holder will generally recognize capital gain or loss equal to the difference between the amount realized on the sale or other disposition and the U.S. holder’s tax basis in the Common Warrant. This capital gain or loss will be long-term capital gain or loss if the U.S. holder’s holding period in such Common Warrant is more than one year at the time of the sale or other disposition. The deductibility of capital losses is subject to certain limitations.
In general, a U.S. holder will not be required to recognize income, gain or loss upon exercise of a Common Warrant for its exercise price (except to the extent the U.S. holder receives a cash payment for a such fractional share that would otherwise have been issuable upon exercise of the Common Warrant, which will be treated as a sale as described above under “—Dispositions“Sale or Other Taxable Disposition of Our Common Stock” below.Stock or Pre-Funded Warrants”). A U.S. holder’s tax basis in a share of common stock received upon exercise of Common Warrants will be equal to the sum of (i) the U.S. holder’s tax basis in the Common Warrants exchanged therefor and (ii) the exercise price of such Common Warrants. A U.S. holder’s holding period in the shares of common stock received upon exercise will commence on the day after such U.S. holder exercises the Common Warrants. Although there is no direct legal authority as to the U.S. federal income tax treatment of an exercise of a Common Warrant on a cashless basis, we intend to take the position that such exercise will not be taxable, either because the exercise is not a gain realization event or because it qualifies as a tax-free recapitalization. In the former case, the holding period of the shares of common stock received upon exercise of Common Warrants should commence on the day after the Common Warrants are exercised. In the latter case, the holding period of the shares of common stock received upon exercise of Common Warrants would include the holding period of the exercised Common Warrants. However, our position is not binding on the IRS, and the IRS may treat a cashless exercise of a Common Warrant as a taxable exchange. U.S. holders are urged to consult their tax advisors as to the consequences of an exercise of a Common Warrant on a cashless basis, including with respect to their holding period and tax basis in the common stock received.
If a Common Warrant expires without being exercised, a U.S. holder will recognize a capital loss in an amount equal to such holder’s tax basis in the Common Warrant. Such loss will be long-term capital loss if, at the time of the expiration, the U.S. holder’s holding period in such Common Warrant is more than one year. The deductibility of capital losses is subject to certain limitations.
44
Constructive Dividends on Common Warrants or Pre-Funded Warrants
As described in the section entitled “Dividend Policy,” we do not expect to pay any cash dividends on our capital stock in the foreseeable future. However, if, at any time during the period in which a U.S. holder holds Common Warrants or Pre-Funded Warrants, we were to pay a taxable dividend to our stockholders and, in accordance with the anti-dilution provisions of the Common Warrants or Pre-Funded Warrants, the exercise price thereof were decreased, that decrease would be deemed to be the payment of a taxable dividend to a U.S. holder of the Common Warrants or Pre-Funded Warrants, as applicable, to the extent of our earnings and profits, notwithstanding the fact that such holder will not receive a cash payment. If the exercise price is adjusted in certain other circumstances or other adjustments are made (or in certain circumstances, there is a failure to make adjustments), such adjustments may also result in the deemed payment of a taxable dividend to a U.S. holder. In addition, a holder of a Common Warrant or Pre-Funded Warrant may, in some circumstances, be deemed to have received a distribution subject to U.S. federal income tax as a result of an adjustment or the non-occurrence of an adjustment to the exercise price or number of shares of common stock issuable upon exercise of the Common Warrant or Pre-Funded Warrant. U.S. holders should consult their tax advisors regarding the proper treatment of any adjustments to the Common Warrants and Pre-Funded Warrants.
We are currently required to report the amount of any deemed distributions on our website or to the IRS and to holders not exempt from reporting. The IRS has proposed regulations addressing the amount and timing of deemed distributions, as well as obligations of withholding agents and filing and notice obligations of issuers in respect of such deemed distributions. If adopted as proposed, the regulations would generally provide that (i) the amount of a deemed distribution is the excess of the fair market value of the right to acquire stock immediately after the exercise price adjustment over the fair market value of the right to acquire stock (after the exercise price adjustment) without the adjustment, (ii) the deemed distribution occurs at the earlier of the date the adjustment occurs under the terms of the instrument and the date of the distribution of cash or property that results in the deemed distribution and (iii) we are required to report the amount of any deemed distributions on our website or to the IRS and to all holders (including holders that would otherwise be exempt from reporting). The final regulations will be effective for deemed distributions occurring on or after the date of adoption, but holders and withholding agents may rely on them prior to that date under certain circumstances.
Information Reporting and Backup Withholding
A U.S. holder may be subject to information reporting and backup withholding when such holder receives payments on the common stock or Pre-Funded Warrants or Common Warrants (including constructive dividends) or receives proceeds from the sale or other taxable disposition of common stock, Pre-Funded Warrants, or Common Warrants. Certain U.S. holders are exempt from backup withholding, including corporations and certain tax-exempt organizations. A U.S. holder will be subject to backup withholding if such holder is not otherwise exempt and such holder:
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS. U.S. holders should consult their tax advisors regarding their qualification for an exemption from backup withholding and the procedures for obtaining such an exemption.
Tax Considerations Applicable to Non-U.S. Holders
45
For purposes of this discussion, a “non-U.S. holder” is a beneficial owner of the Securities that is neither a U.S. holder nor an entity treated as a partnership for U.S. federal income tax purposes.
Distributions
As described in the section entitled “Dividend Policy,” we do not expect to pay any cash dividends on our capital stock in the foreseeable future. However, if we do make distributions of cash or property (other than certain distributions of common stock) on our common stock or Pre-Funded Warrants, such will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a non-U.S. holder’s adjusted tax basis in its common stock or Pre-Funded Warrants, but not below zero. Any excess will be treated as capital gain and will be treated as described below in the section relating to the sale or disposition of our common stock, Pre-Funded Warrants or Common Warrants. Because we may not know the extent to which a distribution is a dividend for U.S. federal income tax purposes at the time it is made, for purposes of the withholding rules discussed below we or the applicable withholding agent may treat the entire distribution as a dividend.
Subject to the discussion below on backup withholding and foreign accounts, dividends paid to a non-U.S. holder of our common stock or Pre-Funded Warrants that are not effectively connected with the non-U.S. holder’s conduct of a United States trade or business conducted by such non-U.S. holder generallywithin the United States will be subject to United StatesU.S. federal withholding tax at a rate of 30% of the gross amount of the dividends or(or such lower rate specified by an applicable income tax treaty.treaty).
Non-U.S. holders will be entitled to a reduction in or an exemption from withholding on dividends as a result of either (a) an applicable income tax treaty or (b) the non-U.S. holder holding our common stock or Pre-Funded Warrants in connection with the conduct of a trade or business within the United States and dividends being effectively connected with that trade or business. To receiveclaim such a reduction in or exemption from withholding, the non-U.S. holder must provide the applicable withholding agent with a properly executed (a) IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) claiming an exemption from or reduction of the withholding tax under the benefit of a reducedan income tax treaty rate, abetween the United States and the country in which the non-U.S. holder must furnish to usresides or our paying agent a validis established, or (b) IRS Form W-8BEN (or applicable successor form) certifying suchW-8ECI stating that the dividends are not subject to withholding tax because they are effectively connected with the conduct by the non-U.S. holder’s qualification forholder of a trade or business within the reduced rate. This certificationUnited States, as may be applicable. These certifications must be provided to us or our payingthe applicable withholding agent prior to the payment of dividends and must be updated periodically. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, who then will be required to provide certification to us or our paying agent, either directly or through other intermediaries. Non-U.S. holders that do not timely provide us or our payingthe applicable withholding agent with the required certification, but that qualify for a reduced rate under an applicable income tax treaty, rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisors regarding possible entitlement to benefits under a tax treaty.
If dividends paid to a non-U.S. holder holds our common stock in connection with the conduct of a trade or business in the United States, and dividends paid on the shares of our common stock are effectively connected with such non-U.S. holder’s United States trade or business (and, if required by an applicable tax treaty, are attributable to a permanent establishment maintained by the non-U.S. holder in the United States), the non-U.S. holder will be exempt from United States federal withholding tax. To claim the exemption, the non-U.S. holder must generally
- 110 -
furnish to us or our paying agent a valid IRS Form W-8ECI (or applicable successor form), certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States. Any dividends paid on our common stock that are effectively connected with a non-U.S. holder’s United States trade or business (and, if required by an applicable income tax treaty, attributable tothe non-U.S. holder maintains a permanent establishment maintained byin the United States to which such dividends are attributable), then, although exempt from U.S. federal withholding tax (provided the non-U.S. holder inprovides appropriate certification, as described above), the United States) generallynon-U.S. holder will be subject to United StatesU.S. federal income tax on such dividends on a net income basis inat the same manner as if such non-U.S. holder were aregular U.S. person and, forfederal income tax rates. In addition, a non-U.S. holder that is a corporation also may be subject to a branch profits tax equal toat a rate of 30% (or such lower rate specified by an applicable income tax treaty) ofon its effectively connected earnings and profits for the taxable year that are attributable to such dividends, as adjusted for certain items. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaties that may provide for different rules.treaty.
Exercise of Common Warrants or Pre-Funded Warrants
Dispositions of Our Common Stock
Subject to the discussion below regarding backup withholding, aA non-U.S. holder generally will not be subject to United StatesU.S. federal income tax on the exercise of Common Warrants or Pre-Funded Warrants into shares of common stock. However, if a cashless exercise of Common Warrants or Pre-Funded Warrants results in a taxable exchange, as described in “–Treatment of Pre-Funded Warrants” and “–Tax Considerations Applicable to U.S. Holders – Sale or Other Disposition, Exercise or Expiration of Common Warrants,” the rules described below under “Sale or Other Disposition of Common Stock, Pre-Funded Warrants or Common Warrants” would apply.
Sale or Other Disposition of Common Stock, Pre-Funded Warrants or Common Warrants
46
Subject to the discussions below on backup withholding and foreign accounts, a non-U.S. holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock, Pre-Funded Warrants or Common Warrants unless:
•
•
•
Unless an applicable treaty provides otherwise, the gainGain described in the first bullet point above will generally will be subject to United StatesU.S. federal income tax on a net income basis inat the same manner as if such non-U.S. holder were aregular U.S. person.federal income tax rates. A non-U.S. holder that is a foreign corporation also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable tax treaty) of its effectively connected earnings and profits for the taxable year, as adjusted for certain items. Non-U.S. holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Gain described in the second bullet point above generally will be subject to United States federal income tax at a flatrate of 30% rate (or such lower rate specified by an applicable income tax treaty), but on such effectively connected gain, as adjusted for certain items.
A non-U.S. holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on any gain derived from the disposition, which may be offset by United States sourcecertain U.S.-source capital losses of the non-U.S. holder (even though the individual is not considered a resident of the United States), provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe that we are not currently and we do not anticipate becoming a USRPHC. However, becauseBecause the determination of whether we are a USRPHC depends on the fair market value of our United States real property interestsUSRPIs relative to the fair market value of our other business assets and our non-U.S. real property interests, however, there can be no assurance that we are not a USRPHC or will not become a USRPHCone in the future. In
Non-U.S. holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Constructive Dividends on Common Warrants or Pre-Funded Warrants
As described in the eventsection entitled “Dividend Policy,” we do become a USRPHC, as long asnot expect to pay any cash dividends on our commoncapital stock is regularly traded on an established securities market, our common stock will be treated as a U.S. real property interest only with respect to a non-U.S. holder that actually or constructively held more than 5% of our common stockin the foreseeable future. However, if at any time during the shorter of (i)period in which a non-U.S. holder holds Common Warrants or Pre-Funded Warrants we were to pay a taxable dividend to our stockholders and, in accordance with the five-year period ending on the dateanti-dilution provisions of the saleCommon Warrants or dispositionPre-Funded Warrants, the exercise price of the warrants were decreased, that decrease would be deemed to be the payment of a taxable dividend to a non-U.S. holder to the extent of our earnings and profits, notwithstanding the fact that such holder will not receive a cash payment. If the exercise price is adjusted in certain other circumstances (or in certain circumstances, there is a failure to make adjustments), such adjustments may also result in the deemed payment of a taxable dividend to a non-U.S. holder. Any resulting withholding tax attributable to deemed dividends may be collected from other amounts payable or distributable to the non-U.S. holder. Non-U.S. holders should consult their tax advisors regarding the proper treatment of any adjustments to the Common Warrants and Pre-Funded Warrants.
Information Reporting and Backup Withholding
Subject to the discussion below on foreign accounts, a non-U.S. holder will not be subject to backup withholding with respect to distributions on our common stock or (ii) the non-U.S. holder’s holding period for our common stock. If gain on the sale or other taxable disposition of our common stock were subjectPre-Funded Warrants we make to taxation under the third bullet point above, the non-U.S. holder would be subject to regular United States federal income tax(including constructive dividends with respect to Common Warrants and Pre-Funded Warrants), provided the applicable withholding agent does not have actual knowledge or reason to know such gain in generally the same manner asholder is a U.S. person.
- 111 -
Information Reporting and Backup Withholding
Generally, we must report annually to the IRS and to each non-U.S. holder the amount of dividends paid to such non-U.S. holderUnited States person and the amount, if any, of tax withheld with respect to those dividends. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. Under certain circumstances, the Code imposes backup withholding on certain reportable payments. Backup withholding, however, generally will not apply to payments of dividends to a non-U.S. holder of our common stock provided the non-U.S. holder furnishes to us or our paying agent the required certification as tocertifies its non-U.S. status, such as by providing a valid IRS Form W-8BEN, W-8BEN-E or IRS Form W-8ECI, or otherwise establishes an exemption. Notwithstandingother applicable certification. However, information returns generally will be filed with the foregoing,IRS in connection with any distributions (including deemed distributions) made on our common stock, Pre-Funded Warrants and Common
47
Warrants to the non-U.S. holder, regardless of whether any tax was actually withheld. Copies of these information returns may also be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is established.
Information reporting and backup withholding may apply if either weto the proceeds of a sale or other taxable disposition of our paying agent hascommon stock, Pre-Funded Warrants or Common Warrants within the United States, and information reporting may (although backup withholding generally will not) apply to the proceeds of a sale or other taxable disposition of our common stock, Pre-Funded Warrants or Common Warrants outside the United States conducted through certain U.S.-related financial intermediaries, in each case, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder on IRS Form W-8BEN or W-8BEN-E, or other applicable form (and the payor does not have actual knowledge or reason to know that the holderbeneficial owner is a U.S. person that is notperson), or such owner otherwise establishes an exempt recipient.
Unlessexemption. Proceeds of a disposition of our common stock, Pre-Funded Warrants or Common Warrants conducted through a non-U.S. holder complies with certification procedures to establish that it isoffice of a non-U.S. broker generally will not a U.S. person, information returns may be filed with the IRS in connection with, and the non-U.S. holder may be subject to backup withholding on the proceeds from, a sale or other disposition of our common stock. The certification procedures described in the above paragraph will satisfy these certification requirements as well.information reporting.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s United StatesU.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may applybe imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act (“FATCA”)) on certain types of payments made to “foreignnon-U.S. financial institutions” (as specially defined under those rules)institutions and certain other non-U.S. entities. The failure to comply with additional certification, information reporting and other specified requirements could result inSpecifically, a withholding tax being imposed on payments of dividends and sales proceeds to foreign intermediaries and certain non-U.S. holders. A 30% withholding tax ismay be imposed on dividends (including deemed dividends) paid on our common stock, Pre-Funded Warrants or Common Warrants, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of our common stock, Pre-Funded Warrants or Common Warrants paid to a “foreign financial institution” or a “non-financial foreign financial institution or to a foreign non-financial entity,entity” (each as defined in the Code), unless (i)(1) the foreign financial institution undertakes certain diligence and reporting obligations, (ii)(2) the non-financial foreign non-financial entity either certifies it does not have any substantial“substantial United States ownersowners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (iii)(3) the foreign financial institution or non-financial foreign non-financial entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements clause in clause (i)(1) above, it must enter into an agreement with the United StatesU.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States personspersons” or United“United States-owned foreign entities,entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Recently issued finalUnder the applicable Treasury Regulations provide that such rules willand administrative guidance, withholding under FATCA generally applyapplies to payments of dividends (including deemed dividends). Because we may not know the extent to which a distribution is a dividend for U.S. federal income tax purposes at the time it is made, onfor purposes of these withholding rules we or after January 1, 2014 andthe applicable withholding agent may treat the entire distribution as a dividend. While withholding under FATCA would have applied also to payments of gross proceeds from athe sale or other disposition of our common stock, Pre-Funded Warrants or Common Warrants on or after January 1, 2017. 2019, proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Prospective investors should consult their tax advisors regarding the potential application of these withholding provisions.
48
- 112 -UNDERWRITING
Aegis Capital Corp. is acting as the sole book-running manager of the offering and as representative of the underwriters, or the “Representative.” We have entered into an underwriting agreement datedwith Craig-Hallum Capital Group LLC and Laidlaw & Company (UK) Ltd. (the “Representatives”), as the daterepresentatives of the underwriters named below and the book-running managers of this prospectus, with the Representative.offering. Subject to the terms and conditions of thean underwriting agreement between us and the Representative, we have agreed to sell to each underwriter named belowthe underwriters, and each underwriter named below has severally and not jointlythe underwriters have agreed to purchase, from us, at the public offering price per share less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stockour securities listed next to its name in the following table:
| Number of Shares and Accompanying Warrants | |
| ||
Laidlaw & Company (UK) Ltd. | ||
Total |
The underwriters are committed to purchase all of the shares of common stocksecurities offered by us other than those covered by the option to purchase additional shares described below, if they purchaseit purchases any shares.securities. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations, and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the shares,above securities, subject to prior sale, when, as, and if issued to and accepted by them,it, subject to approval of legal matters by theirits counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel, or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus, permits the underwriters to purchase a maximum of additional shares (15% of the shares sold in this offering) from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, they will purchase shares covered by the option at the public offering price per share that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the public will be $
Discounts, Commissions, and the total net proceeds, before expenses, to us will be $ .Expenses
Discount.
The following table shows the public offering price,per share and total underwriting discountdiscounts and proceeds, before expenses,commissions to us. The information assumes either no exercise or full exercise bybe paid to the underwriters of their over-allotment option.underwriters.
Per | Per PFW Unit | Total | ||||||||||
Public offering price | $ | $ | $ | |||||||||
Underwriting discounts and commissions | ||||||||||||
| $ | $ | $ | |||||||||
Proceeds before expenses to us | $ | $ | $ | |||||||||
____________
(1) Reflects underwriting discounts and commissions to the underwriters of $ , or eight percent (8%) per share. We have also agreed to issue the representatives of the underwriters or their designees warrants to purchase a number of shares of common stock equal to 5% of the securities sold in this offering, to pay the warrant solicitation fee described below, and to reimburse the underwriters for certain offering expenses. In addition, we have agreed to pay a management fee to the representatives equal to 0.75% of the gross proceeds received in this offering, which is not included in the underwriting discounts and commissions.
The underwriters propose to offer the sharessecurities offered by us to the public at the public offering price per share set forth on the cover of this prospectus. In addition, the underwriters may offer some of the sharessecurities to other securities dealers at such price less aan expected concession of $10% of the purchase price per share. If all of the shares offered by us are not sold at the public offering price, per share, the underwritersRepresentatives may change the offering price per share and other selling terms by means of a supplement to this prospectus.
- 113 -49
We have paid an expense deposit of $25,000 to the Representative, which will be applied against the accountable expenses that will be paid by us to the Representative in connection with this offering. The underwriting agreement provides that in the event the offering is terminated, the $25,000 expense deposit paid to the Representative will be returned to us to the extent that offering expenses are not actually incurred by the Representative.
We have also agreed to payreimburse the Representative’sunderwriters for their expenses relating to the offering, including (a) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed an aggregate of $15,000; (b) all filing fees incurred in clearingconnection with this offering, with FINRA; (c) all fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under state securities laws, or “blue sky” laws, or under the securities laws of foreign jurisdictions designated by the underwriters (including reasonable fees and disbursements of blue sky counsel not to exceed $10,000); (d) $21,775 for the underwriters’ use of Ipreo’s book-building, prospectus tracking and compliance software for this offering; and (e) up to $20,000$125,000, including fees of the Representative’s actual accountable road show expenses for the offering.
underwriters' counsel. We estimate that the total expenses of the offering payable by us, excluding the total underwriting discountsdiscount and commissions,reimbursement of fees of underwriters' counsel, will be approximately $ $600,000.
Warrant Solicitation Fee
Upon any exercise of the Series B Warrants issued in this offering, we have agreed to pay the underwriters a cash fee equal to 5.0% of the aggregate gross proceeds received upon the exercise of the Series B Warrants in accordance with the Financial Industry Regulatory Authority (“FINRA”) Rule 5110(g)(10).
Discretionary Accounts.Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements.Pursuant to certain “lock-up” agreements, we, our executive officers and directors, and holders of our outstanding shares of common stock have agreed, subject to certain exceptions, not to offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of or announce
Representatives' Warrants
Upon the intention to otherwise dispose of, or enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic risk of ownership of, directly or indirectly, engage in any short selling of any common stock or securities convertible into or exchangeable or exercisable for any common stock, whether currently owned or subsequently acquired, without the prior written consent of the underwriter, for a period of 180 days after the dateclosing of this prospectus.
The lock-up period described in the preceding paragraph will be automatically extended if: (1) during the last 17 days of the restricted period,offering, we issue an earnings release or announce material news or a material event; or (2) prior to the expiration of the lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the lock-up period, in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the 18-day period beginning on the date of the earnings release, unless the Representative waives this extension in writing; provided, however, that this lock-up period extension shall not apply to the extent that FINRA has amended or repealed NASD Rule 2711(f)(4), or has otherwise provided written interpretive guidance regarding such rule, in each case, so as to eliminate the prohibition of any broker, dealer, or member of a national securities association from publishing or distributing any research report, with respect to the securities of an emerging growth company (as defined in the JOBS Act) prior to or after the expiration of any agreement between the broker, dealer, or member of a national securities association and the emerging growth company or its shareholders that restricts or prohibits the sale of securities held by the emerging growth company or its shareholders after the initial public offering date.
Representative’s Warrants. We have also agreed to issue to the Representative, at the closing of this offering,Representatives or their designees warrants (the “Representative Warrants”) to purchase 4%a number of shares of Common Stock equal to five percent (5%) of the aggregate number of shares of common stock sold in this offering. The Representative Warrants are exercisable at a per share price equal to 175% of the public offering price per share of common stockUnits sold in this offering commencing on a date which is one year(the “Representatives' Warrants”). The Representatives' Warrants will be exercisable six months from the effective date of the offering under this prospectusregistration statement and expiringwill expire five years from the effective date thereof. The exercise price of the offering.warrants is equal to 165% of the offering price of the price per share of the Units offered hereby. The warrantsRepresentatives' Warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1)5110(e) of FINRA. The RepresentativeRepresentatives (or permitted assignees under Rule 5110(g)(1)5110(e)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants,
- 114 -
nor will itthey engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Representative Warrantswarrants or the underlying securities for a period of 180 days from the effective date of the offering in compliance with FINRA Rule 5110(g)(1).offering. In addition, the Representative Warrantswarrants provide for registration rights upon request, in certain cases. The one-time demand registration right provided will not be greater than five years from the effective date of the offeringregistration statement in compliance with FINRA Rule 5110(f)(2)(H)(iv)5110(g)(8)(C). The unlimited piggyback registration right provided will not be greater than seven years from the effective date of the offeringregistration statement in compliance with FINRA Rule 5110(f)(2)(H)(v)5110(g)(8)(D). We will bear all fees and expenses attendant to registering the securities issuable on exercise of the Representative Warrantswarrants other than underwriting commissions incurred and payable by the holders.
Right
Lock-Up Agreements
Our executive officers and directors have agreed with the Representatives to be subject to a lock-up period of First Refusal.We granted90 days following the representativedate of closing of the offering pursuant to this prospectus. This means that, during the lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our Common Stock, subject to certain customary exceptions. The Representatives may, in their sole discretion and without notice, waive the terms of any of these lock-up agreements. We have also agreed, in the underwriting agreement, that without prior written notice to the Representatives, we will not offer, sell, issue or otherwise transfer or dispose of our securities for 90 days following the closing of this offering, subject to certain customary exceptions.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Price Stabilization, Short Positions, and Penalty Bids
In connection with this offering, each underwriter may engage in transactions that stabilize, maintain, or otherwise affect the price of our securities. Specifically, such underwriter may over-allot in connection with this offering by selling more securities than are set forth on the cover page of this prospectus. This creates a short position in our
50
securities for such underwriter’s own accounts. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by such underwriter is not greater than the number of securities that it may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. To close out a short position, such underwriter may elect to exercise all or part of the over-allotment option. Such underwriter may also elect to stabilize the price of our securities or reduce any short position by bidding for, and purchasing, securities in the open market.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing a security in this offering because the underwriter repurchases that security in stabilizing or short covering transactions.
Finally, each underwriter may bid for, and purchase, shares of our securities in market-making transactions, including “passive” market-making transactions as described below.
These activities may stabilize or maintain the market price of our securities at a periodprice that is higher than the price that might otherwise exist in the absence of eight months afterthese activities. The underwriters are not required to engage in these activities and may discontinue any of these activities at any time without notice. These transactions may be affected on NASDAQ, in the effectiveness ofover-the-counter market, or otherwise.
In connection with this offering, the underwriters and selling group members, if any, or their affiliates may engage in passive market-making transactions in our Common Stock immediately prior to the commencement of sales in this offering, in accordance with Rule 103 of Regulation M under the Exchange Act. Rule 103 generally provides that:
NASDAQ Listing. We have applied to listthe passive market maker’s average daily trading volume in our common stock on The NASDAQ Capital Market under the symbol “EVOK.”during a specified two-month prior period or 200 shares, whichever is greater, and must be discontinued when that limit is reached; and
Electronic Offer, Sale and Distribution of Shares.A
This prospectus in electronic format may be made available on the websites or through other online services maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The Representative may agree to allocate a number of shares to underwriters and selling group members for sale toby their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.affiliates. Other than thethis prospectus in electronic format, the information on thesethe underwriters’ websites and any information contained in any other websites maintained by an underwriter is not part of nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriterthe underwriters in itstheir capacity as underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids. In order to facilitate
Other than the offering of our common stock,prospectus in electronic or printed format, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. In connection with the offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchasesinformation on the open market to cover positions createdunderwriters’ website and any information contained in any other website maintained by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in the offering. “Covered” short sales are sales made in an amountunderwriter is not greater than the underwriters’ option to purchase additional shares of common stock in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of common stock in the open market. In determining the source of shares of common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. “Naked” short sales are sales in excesspart of the over-allotment option. The underwriters must close out any naked short positionprospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids forus or purchases of shares of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As result, the price of our common stock maytheir capacity as underwriters and should not be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our common stock, including the
- 115 -Certain Relationships
imposition of penalty bids. This means that if the representative of the underwriters purchases common stock in the open market in stabilizing transactions or to cover short sales, the representative can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Other Relationships. Certain of the underwritersRepresentatives and their affiliates have provided,engaged in, and may in the future provide, variousengage in, investment banking, commercial banking and other financial services forcommercial dealings in the ordinary course of business with us andor our affiliates for which theyaffiliates. The Representatives have received, andor may in the future receive, customary fees. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwritersfees and commissions for any further services.these transactions.
Offer restrictions outside
Offers Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The
51
securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.Listing
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
- 116 -
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
(a) to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purposeOur common stock is solely to invest in securities;
(b) to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements);
(c) to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii)��a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
- 117 -
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ—$$—Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and
in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended.
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and
in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws.
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
- 118 -
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard toNasdaq Capital Market under the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.symbol “EVOK”.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.52
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
- 119 -
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
- 120 -
The validity of the shares of common stock offered herebyby this prospectus will be passed upon for us by Latham & Watkins LLP, San Diego, California. Sichenzia Ross Friedman FerenceCertain legal matters relating to the offering will be passed upon for the underwriters by Sheppard, Mullin, Richter & Hampton LLP, New York, New York has acted as counsel for the underwriters in connection with certain legal matters related to this offering.York.
53
EXPERTS
Ernst & Young LLP, independent registered public accounting firm, has audited ourThe financial statements atas of December 31, 20112022 and 2012,2021 and for each of the two years in the period ended December 31, 2012,2022 incorporated by reference in this prospectus and forin the period from January 29, 2007 (inception) to December 31, 2012,registration statement have been so incorporated in reliance on the report of BDO USA, LLP (n/k/a BDO USA P.C.), an independent registered public accounting firm, incorporated herein by reference, given on the authority of said firm as set forthexperts in theirauditing and accounting. The report (whichon the financial statements contains an explanatory paragraph describing conditions that raise substantial doubt about ourregarding the Company’s ability to continue as a going concern as described in Note 1 to the financial statements). We have included our financial statements in the prospectus and elsewhere in the registration statement in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.concern.
54
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange CommissionSEC a registration statement on Form S-1 under the Securities Act of 1933, as amended, with respect to the shares of common stocksecurities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer youreference is made to the registration statement and the exhibits and schedules filed thereto.therewith. Statements contained in this prospectus regardingconcerning the contents of any contract or any other document are not necessarily complete, please see the copy of the contract or document that ishas been filed for the complete contents of that contract or document. Each statement in this prospectus relating to a contract or document filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibitexhibit. The exhibits to the registration statement. Upon completionstatement should be reviewed for the complete contents of this offering, we will be required to file periodic reports, proxy statementsthese contracts and other information with the Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934. You may read and copy this information at the Public Reference Room of the Securities and Exchange Commission, 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference rooms by calling the Securities and Exchange Commission at 1-800-SEC-0330. documents.
The Securities and Exchange Commission alsoSEC maintains an Interneta website that contains reports, proxy and information statements and other information aboutregarding registrants like us, that file electronically with the Securities and Exchange Commission.SEC. The address is www.sec.gov.
We also maintain a website at www.evokepharma.com. The reference to our website address does not constitute incorporation by reference of that site iswww.sec.gov.
- 121 -
(A Development Stage Company)
IndexYou may also request a copy of these filings, at no cost to Financial Statements
| ||||
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholdersyou, by writing or telephoning us at the following address:
Evoke Pharma, Inc.
We have audited the accompanying balance sheets of Evoke Pharma, Inc. (a development stage company), as of December 31, 2011 and 2012, and the related statements of operations and comprehensive loss, convertible preferred stock and stockholders’ deficit and cash flows for the years then ended and for the periodAttn: Corporate Secretary
420 Stevens Avenue, Suite 230
Solana Beach, CA 92075
Telephone: (858) 345-1494
55
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” information from January 29, 2007 (inception) to December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards requireother documents that we plan and perform the auditfile with it, which means that we can disclose important information to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engagedyou by referring you to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates madethose documents. The information incorporated by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Evoke Pharma, Inc. at December 31, 2011 and 2012, and the results of its operations and its cash flows for the two years then ended and for the period from January 29, 2007 (inception) to December 31, 2012, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s recurring losses from operations and insufficient working capital raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Ernst & Young LLP
San Diego, California
April 19, 2013, except for the last paragraph of Note 8, as to which the date is July XX, 2013.
The foregoing report is in the form that will be signed upon the effectiveness of the reverse stock split as described in the last paragraph of Note 8 to the financial statements.
/s/ Ernst & Young LLP
San Diego, California
July 3, 2013
Evoke Pharma, Inc.
(A Development Stage Company)
| December 31, | March 31, | Pro Forma March 31, 2013 | ||||||||||||||
| 2011 | 2012 | 2013 | ||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
Assets | ||||||||||||||||
Current assets: | ||||||||||||||||
Cash and cash equivalents | $ | 865,876 | $ | 116,013 | $ | 1,669,518 | ||||||||||
Prepaids and other assets | 39,459 | — | — | |||||||||||||
|
|
|
|
|
| |||||||||||
Total current assets | 905,335 | 116,013 | 1,669,518 | |||||||||||||
Other assets | — | — | 10,500 | $ | — | |||||||||||
|
|
|
|
|
| |||||||||||
Total assets | $ | 905,335 | $ | 116,013 | $ | 1,680,018 | ||||||||||
|
|
|
|
|
| |||||||||||
Liabilities, convertible preferred stock and stockholders’ deficit | ||||||||||||||||
Current liabilities: | ||||||||||||||||
Accounts payable and accrued expenses | $ | 88,712 | $ | 96,798 | $ | 272,458 | ||||||||||
Accrued compensation | 206,788 | 417,611 | 167,007 | |||||||||||||
Warrant liability | 39,000 | 56,000 | 229,000 | $ | — | |||||||||||
|
|
|
|
|
| |||||||||||
Total current liabilities | 334,500 | 570,409 | 668,465 | |||||||||||||
Long-term debt, net of debt discount | — | 979,792 | 2,936,607 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total liabilities | 334,500 | 1,550,201 | 3,605,072 | |||||||||||||
Series A convertible preferred stock, $0.0001 par value: | ||||||||||||||||
Authorized shares—12,245,068 at December 31, 2011 and 12,305,068 at December 31, 2012 and March 31, 2013 (unaudited); issued and outstanding shares - 12,195,068 at December 31, 2011 and 2012 and March 31, 2013 (unaudited); liquidation preference - $12,292,600 at December 31, 2011 and 2012 and March 31, 2013 (unaudited); no shares issued and outstanding, pro forma (unaudited) | 18,225,166 | 18,225,166 | 18,225,166 | — | ||||||||||||
Stockholders’ deficit: | ||||||||||||||||
Common stock, $0.0001 par value; authorized shares—20,000,000 at December 31, 2011 and 2012 and March 31, 2013 (unaudited); issued and outstanding shares - 1,242,750 at December 31, 2011 and 2012 and March 31, 2013 (unaudited); 3,681,752 shares issued and outstanding, pro forma (unaudited) | 124 | 124 | 124 | 368 | ||||||||||||
Additional paid-in capital | 183,020 | 195,525 | 198,651 | 18,652,573 | ||||||||||||
Deficit accumulated during the development stage | (17,837,475 | ) | (19,855,003 | ) | (20,348,995 | ) | (20,348,995 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total stockholders’ deficit | (17,654,331 | ) | (19,659,354 | ) | (20,150,220 | ) | $ | (1,696,054 | ) | |||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities, convertible preferred stock and stockholders’ deficit | $ | 905,335 | $ | 116,013 | $ | 1,680,018 | ||||||||||
|
|
|
|
|
| |||||||||||
See accompanying notes.
Evoke Pharma, Inc.
(A Development Stage Company)
Statements of Operations and Comprehensive Loss
| Years Ended December 31, | Period From January 29, 2007 (Inception) to December 31, 2012 | Three Months Ended March 31, | Period From January 29, 2007 (Inception) to March 31, 2013 | |||||||||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||
Research and development | $ | 1,844,044 | $ | 1,165,645 | $ | 15,991,529 | $ | 297,285 | $ | 110,981 | $ | 16,102,510 | ||||||||||||
General and administrative | 570,524 | 836,781 | 3,304,533 | 205,137 | 221,049 | 3,525,582 | ||||||||||||||||||
Purchase of in-process research and development | — | — | 650,000 | — | — | 650,000 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses | 2,414,568 | 2,002,426 | 19,946,062 | 502,422 | 332,030 | 20,278,092 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Loss from operations | (2,414,568 | ) | (2,002,426 | ) | (19,946,062 | ) | (502,422 | ) | (332,030 | ) | (20,278,092 | ) | ||||||||||||
Other income (expense): | ||||||||||||||||||||||||
Interest income | 10,696 | 1,690 | 213,852 | 764 | 1,353 | 215,205 | ||||||||||||||||||
Interest expense | (2,872 | ) | (24,042 | ) | (205,942 | ) | — | (39,315 | ) | (245,257 | ) | |||||||||||||
Change in fair value of preferred stock purchase right | — | — | (188,587 | ) | — | — | (188,587 | ) | ||||||||||||||||
Change in fair value of warrant liability | 5,500 | 7,250 | 27,736 | 1,500 | (124,000 | ) | (96,264 | ) | ||||||||||||||||
Grant income | — | — | 244,000 | — | — | 244,000 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total other income (expense) | 13,324 | (15,102 | ) | 91,059 | 2,264 | (161,962 | ) | (70,903 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net loss and comprehensive loss | $ | (2,401,244 | ) | $ | (2,017,528 | ) | $ | (19,855,003 | ) | $ | (500,158 | ) | $ | (493,992 | ) | $ | (20,348,995 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net loss per common share, basic and diluted | $ | (2.18 | ) | $ | (1.79 | ) | $ | (0.45 | ) | $ | (0.44 | ) | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
Weighted-average shares used to compute basic and diluted net loss per share | 1,102,625 | 1,124,000 | 1,118,375 | 1,133,375 | ||||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||||||
Pro forma net loss per common share, basic and diluted (unaudited) | $ | (0.57 | ) | $ | (0.10 | ) | ||||||||||||||||||
|
|
|
| |||||||||||||||||||||
Weighted-average shares used to compute pro forma net loss per common share, basic and diluted (unaudited) | 3,563,002 | 3,572,377 | ||||||||||||||||||||||
|
|
|
| |||||||||||||||||||||
See accompanying notes.
Evoke Pharma, Inc.
(A Development Stage Company)
Statements of Convertible Preferred Stock and Stockholders’ Deficit
| Series A Convertible Preferred Stock | Common Stock | Additional Paid-In Capital | Deficit Accumulated During the Development Stage | Total Stockholders’ Deficit | ||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
Balance at January 29, 2007 (inception) | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
Issuance of restricted common stock for cash to founders at $0.005 per share | — | — | 916,000 | 92 | 4,488 | — | 4,580 | |||||||||||||||||||||||
Issuance of Series A convertible preferred stock at $1.50 per share for cash and the conversion of $250,000 of bridge notes and $42,538 of accrued interest, net of issuance costs of $218,037 | 4,195,067 | 6,074,501 | — | — | — | — | — | |||||||||||||||||||||||
Initial fair value of preferred stock purchase rights issued in connection with Series A financing | — | (848,257 | ) | — | — | — | — | — | ||||||||||||||||||||||
Estimated fair value of exercised purchase right of $0.04 per share | — | 80,819 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock upon exercise of stock options | — | — | 266,750 | 26 | 77,332 | — | 77,358 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 5,458 | — | 5,458 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (2,009,591 | ) | (2,009,591 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2007 | 4,195,067 | 5,307,063 | 1,182,750 | 118 | 87,278 | (2,009,591 | ) | (1,922,195 | ) | |||||||||||||||||||||
Issuance of Series A convertible preferred stock at $1.50 per share for cash, net of issuance costs of $1,855 | 4,000,000 | 5,998,145 | — | — | — | — | — | |||||||||||||||||||||||
Estimated fair value of purchase rights upon completion of final preferred stock investment | — | 956,025 | — | — | — | — | — | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 16,184 | — | 16,184 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (3,227,664 | ) | (3,227,664 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2008 | 8,195,067 | 12,261,233 | 1,182,750 | 118 | 103,462 | (5,237,255 | ) | (5,133,675 | ) | |||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 17,803 | — | 17,803 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (5,159,638 | ) | (5,159,638 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2009 | 8,195,067 | 12,261,233 | 1,182,750 | 118 | 121,265 | (10,396,893 | ) | (10,275,510 | ) | |||||||||||||||||||||
Issuance of Series A convertible preferred stock at $1.50 per share for cash, net of issuance costs of $36,069 | 4,000,001 | 5,963,933 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock upon exercise of stock options | — | — | 60,000 | 6 | 23,994 | — | 24,000 | |||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 15,056 | — | 15,056 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (5,039,338 | ) | (5,039,338 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2010 | 12,195,068 | 18,225,166 | 1,242,750 | 124 | 160,315 | (15,436,231 | ) | (15,275,792 | ) | |||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 22,705 | — | 22,705 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (2,401,244 | ) | (2,401,244 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2011 | 12,195,068 | 18,225,166 | 1,242,750 | 124 | 183,020 | (17,837,475 | ) | (17,654,331 | ) | |||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | 12,505 | — | 12,505 | |||||||||||||||||||||||
Net loss | — | — | — | — | — | (2,017,528 | ) | (2,017,528 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at December 31, 2012 | 12,195,068 | 18,225,166 | 1,242,750 | 124 | 195,525 | (19,855,003 | ) | (19,659,354 | ) | |||||||||||||||||||||
Stock-based compensation expense (unaudited) | — | — | — | — | 3,126 | — | 3,126 | |||||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | (493,992 | ) | (493,992 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
Balance at March 31, 2013 (unaudited) | 12,195,068 | $ | 18,225,166 | 1,242,750 | $ | 124 | $ | 198,651 | $ | (20,348,995 | ) | $ | (20,150,220 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||
See accompanying notes.
Evoke Pharma, Inc.
(A Development Stage Company)
| Years Ended December 31, | Period From January 29, 2007 (Inception) to December 31, 2012 | Three Months Ended March 31, | Period From January 29, 2007 (Inception) to March 31, 2013 | |||||||||||||||||||||
| 2011 | 2012 | 2012 | 2013 | |||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
Operating activities | ||||||||||||||||||||||||
Net loss | $ | (2,401,244 | ) | $ | (2,017,528 | ) | $ | (19,855,003 | ) | $ | (500,158 | ) | $ | (493,992 | ) | $ | (20,348,995 | ) | ||||||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||||||||||
Stock-based compensation expense | 22,705 | 12,505 | 89,711 | 3,126 | 3,126 | 92,837 | ||||||||||||||||||
Non-cash interest | 2,426 | 4,042 | 106,066 | — | 5,815 | 111,881 | ||||||||||||||||||
Change in fair value of purchase right liability | — | — | 188,587 | — | — | 188,587 | ||||||||||||||||||
Change in fair value of warrant liability | (5,500 | ) | (7,250 | ) | (27,736 | ) | (1,500 | ) | 124,000 | 96,264 | ||||||||||||||
Changes in operating assets and liabilities: | ||||||||||||||||||||||||
Prepaid expenses and other assets | 76,353 | 39,459 | — | 39,459 | (10,500 | ) | (10,500 | ) | ||||||||||||||||
Accounts payable and accrued expenses | (587,848 | ) | 218,909 | 514,409 | 11,500 | (74,944 | ) | 439,465 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net cash used in operating activities | (2,893,108 | ) | (1,749,863 | ) | (18,983,966 | ) | (447,573 | ) | (446,495 | ) | (19,430,461 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Financing activities | ||||||||||||||||||||||||
Proceeds from convertible promissory note | — | — | 250,000 | — | — | 250,000 | ||||||||||||||||||
Proceeds from bank line of credit and loan advances | — | 1,000,000 | 3,500,000 | — | 2,000,000 | 5,500,000 | ||||||||||||||||||
Payment on bank line of credit | (277,779 | ) | (2,500,000 | ) | — | — | (2,500,000 | ) | ||||||||||||||||
Proceeds from issuance of common stock | — | — | 4,580 | — | — | 4,580 | ||||||||||||||||||
Proceeds from the issuance of preferred stock and purchase rights, net | — | — | 17,744,041 | — | — | 17,744,041 | ||||||||||||||||||
Proceeds from the exercise of stock options | — | — | 101,358 | — | — | 101,358 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net cash (used in) provided by financing activities | (277,779 | ) | 1,000,000 | 19,099,979 | — | 2,000,000 | 21,099,979 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net (decrease) increase in cash and cash equivalents | (3,170,887 | ) | (749,863 | ) | 116,013 | (447,573 | ) | 1,553,505 | 1,669,518 | |||||||||||||||
Cash and cash equivalents at beginning of period | 4,036,763 | 865,876 | — | 865,876 | 116,013 | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Cash and cash equivalents at end of period | $ | 865,876 | $ | 116,013 | $ | 116,013 | $ | 418,303 | $ | 1,669,518 | $ | 1,669,518 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Supplemental disclosures of cash flow information | ||||||||||||||||||||||||
Interest paid | $ | 1,346 | $ | 20,000 | $ | 99,876 | $ | — | $ | 25,750 | $ | 125,626 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Noncash financing activities | ||||||||||||||||||||||||
Conversion of convertible promissory note and accrued interest to Series A Convertible Preferred Stock | $ | — | $ | — | $ | 292,538 | $ | — | $ | — | $ | 292,538 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Issuance of Series A Convertible Preferred Stock warrants | $ | — | $ | 24,250 | $ | 59,486 | $ | — | $ | 49,000 | $ | 108,486 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
1. Organization and Basis of Presentation
Evoke Pharma, Inc. (the “Company”) was incorporated in the state of Delaware on January 29, 2007 (inception). The Company is a privately held specialty pharmaceutical company focused primarily on the development of drugs to treat gastroenterological disorders and disease.
As of December 31, 2012 and March 31, 2013, the Company has devoted substantially all of its efforts to product development, raising capital and building infrastructure, and has not realized revenues from its planned principal operations. Accordingly, the Companyreference is considered to be in the development stage.
The accompanying financial statements have been prepared on the going concern basis, which assumes that the Company will continue to operate as a going concern and which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcomepart of this uncertainty. Management’s plansprospectus. Information in regardthis prospectus supersedes information incorporated by reference that we filed with the SEC prior to these matters are focused on raising additional capital or other financing.
As reflected in the accompanying financial statements, the Company has a limited operating history and the sales and income potential of the Company’s business are unproven. The Company has experienced net losses since its inception and, as of December 31, 2012 and March 31, 2013, had an accumulated deficit of $19,855,003 and $20,348,995, respectively. The Company had working capital (deficit) of $(454,396) and $1,001,053, respectively, as of December 31, 2012 and March 31, 2013.
Based on the Company’s current plan of expenditures on its development program, preparation for commercialization, and other operating costs, the Company believes that its current capital will not be sufficient to fund its planned operations for at least 12 months from the date of the financial statements. These issues raise substantial doubt about the ability of the Company to continue as a going concern. The Company expects to continue to incur net losses for at least the next several years. Over that period, the Company will need to raise additional debt or equity financing to fund its development.
If the Company is not able to secure adequate additional funding, the Company may be forced to make reductions in spending, extend payment terms with suppliers, and/or suspend or curtail planned programs. Any of these actions could materially harm the Company’s business, results of operations, financial condition and future prospects.
2. Summary of Significant Accounting Policies
Use of Estimates
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statementsthis prospectus. We incorporate by reference into this prospectus and the reported amountsregistration statement of expenses duringwhich this prospectus is a part the reporting period. Actual results could differ from those estimates.
Evoke Pharma, Inc.information or documents listed below that we have filed with the SEC:
(Information as of March 31, 2013 and thereafter andour Annual Report on Form 10-K for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Unaudited Interim Financial Information
The accompanying interim balance sheet as of March 31, 2013 and the statements of operations and comprehensive loss and cash flows for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 and the statements of convertible preferred stock and stockholders’ deficit for the three months ended March 31, 2013 and the related footnote disclosures are unaudited. These unaudited interim financial statements have been prepared in accordance with GAAP. In management’s opinion, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, which include only normal recurring adjustments, necessary for the fair presentation of the Company’s financial position as of March 31, 2013 and its results of operations and comprehensive loss and its cash flows for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013. The results for the three months ended March 31, 2013 are not necessarily indicative of the results expected for the full fiscal year or any other interim period.
Unaudited Pro Forma Balance Sheet Information
The unaudited pro forma balance sheet information as of March 31, 2013 assumes the automatic conversion of all outstanding shares of convertible preferred stock into 2,439,002 shares of common stock upon the completion of a qualifying initial public offering (“IPO”) of the Company’s common stock. In addition, the pro forma information assumes reclassification of the preferred stock warrant liability to additional paid-in capital upon completion of the IPO, as the warrants become common stock warrants that are not subject to remeasurement. Shares of common stock issued in such IPO and any related net proceeds are excluded from the pro forma information.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment operating in the United States.
Fair Value of Financial Instruments
The carrying amounts of accounts payable and accrued liabilities are considered to be representative of their respective fair values because of the short-term nature of those instruments. Based on the borrowing rates currently available to the Company for loans with similar terms, the Company believes that the fair value of long-term debt approximates its carrying value. The carrying amount of the warrant liability represents fair value.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash equivalents. Cash and cash equivalents include cash in readily available checking and savings accounts.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Research and Development Expenses
All research and development costs are expensed as incurred and primarily include costs paid to third-party contractors to perform research, conduct clinical trials and develop drug materials and delivery devices.
Concentrations of Risk
The Company relies on third-party manufacturers for the production of its drug candidate. If the third-party manufacturers are unable to continue manufacturing the Company’s drug candidate, or if the Company loses one of its sole source suppliers used in its manufacturing processes, the Company may not be able to meet clinical trial supply demand for its product candidate and the development of the product candidate could be materially and adversely affected.
Preferred Stock Warrant Liability
Warrants for shares that are puttable and warrants for shares that are contingently redeemable are classified as liabilities on the accompanying balance sheets and carried at their estimated fair value. At the end of each reporting period, any changes in fair value are recorded as a component of interest and other income, net. We will continue to adjust the carrying value of the warrants until the earlier of the exercise of the warrants or the completion of a liquidation event, including the completion of an IPO, at which time the liabilities will be reclassified to stockholders’ deficit.
Stock-Based Compensation
Share-based payments to employees, including grants of employee stock options and restricted stock, are recognized in the financial statements based on their grant date fair values in accordance with the applicable accounting guidance. For the years ended December 31, 2011 and 2012, the three months ended March 31, 2012 and 2013 and the period ended January 29, 2007 (inception) to March 31, 2013, the Company recognized $19,200, $12,505, $3,126, $3,126 and $71,178, respectively, in stock-based compensation expense associated with equity awards granted to employees and members of the board of directors of the Company.
For nonemployees, the Company accounts for stock-based compensation in accordance with Accounting Standards Codification (“ASC”) 505-50,Equity-Based Payments to Non-Employees. Equity instruments awarded to nonemployees are periodically remeasured as the underlying awards vest unless the instruments are fully vested, immediately exercisable and nonforfeitable on the date of grant. For the years ended December 31, 2011 and 2012, the three months ended March 31, 2012 and 2013 and the period of January 29, 2007 (inception) to March 31, 2013, the Company recognized $3,505, $0, $0, $0 and $21,659, respectively, in stock-based compensation expense related to equity awards granted to nonemployees.
At December 31, 2012 and March 31, 2013 there was $25,049 and $21,923, respectively, of unrecognized stock-based compensation expense related to unvested employee equity awards to be recognized over a weighted-average period of approximately two years.
The Company grants stock options to purchase common stock to employees with exercise prices equal to the value of the underlying stock, as determined by the board of directors on the date the equity award was granted. The board of directors determined the fair value of the underlying common stock by considering a number of
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
factors, including historical and projected financial results, the risks the Company faced at the time, the preferences of the Company’s preferred stockholders and the lack of liquidity of the Company’s common stock.
The fair value of each option award is estimated on the date of grant using the Black-Scholes Merton valuation model using the appropriate forfeiture rate, risk-free interest rate, expected term and volatility assumptions. The expected life of options was calculated using the simplified method. The simplified method calculates the life as the average of the contractual term and the vesting period of the option. The Company did not have a readily available market and therefore estimates the volatility rate based on comparable companies. The risk-free interest rate is based upon U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards. The Company granted 118,000 options in 2011 and no options in 2012 and the three months ended March 31, 2013.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740,Income Taxes. Under ASC 740, deferred tax assets and liabilities reflect the future tax consequences of the differences between the financial reporting and tax basis of assets and liabilities using current enacted tax rates. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
The Company’s policy related to accounting for uncertainty in income taxes prescribes a recognition threshold and measurement attributed criteria for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for common stock equivalents and adjusted for the weighted-average number of common shares outstanding that are subject to repurchase. The Company has excluded 140,125, 118,750, 124,375 and 109,375 weighted-average shares subject to repurchase from the weighted-average number of common shares outstanding for the years ended December 31, 2011 and 2012 and the three months ended March 31, 2012 and 2013, respectively. Diluted net loss per share is calculated by dividing the net loss by the weighted-average number of common share equivalents outstanding for the period determined using the treasury-stock method. Dilutive common stock equivalents are comprised of convertible preferred stock, warrants for the purchase of convertible preferred stock and options outstanding under the Company’s equity incentive plan. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding due to the Company’s net loss position.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Unaudited Pro Forma Net Loss Per Share
The following table summarizes our unaudited pro forma net loss per share:
| Year Ended December 31, 2012 | Three Months Ended March 31, 2013 | |||||||
Numerator | ||||||||
Net loss | $ | (2,017,528 | ) | $ | (493,992 | ) | ||
Change in fair value of warrant liability | (7,250 | ) | 124,000 | |||||
|
|
|
| |||||
Pro forma net loss | $ | (2,024,778 | ) | $ | (369,992 | ) | ||
|
|
|
| |||||
Denominator | ||||||||
Shares used to compute net loss per common share, basic and diluted | 1,124,000 | 1,133,375 | ||||||
Add: Pro forma adjustments to reflect assumed weighted-average effect of conversion of convertible preferred stock | 2,439,002 | 2,439,002 | ||||||
|
|
|
| |||||
Shares used to compute pro forma net loss per common share, basic and diluted | 3,563,002 | 3,572,377 | ||||||
|
|
|
| |||||
Pro forma net loss per common share, basic and diluted (unaudited) | $ | (0.57 | ) | $ | (0.10 | ) | ||
|
|
|
| |||||
Comprehensive Income (Loss)
ASC 220,Comprehensive Income, defines comprehensive income (loss) as a change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and comprehensive loss were the same for all periods presented.
3. Fair Value Measurements
The following tables present information about the Company’s financial liabilities measured at fair value on a recurring basis as of December 31, 2011 and 2012 and March 31, 2013, and indicate the fair value hierarchy of the valuation techniques utilized by the Company to determine such fair value. As a basis for categorizing inputs, the Company uses a three-tier fair value hierarchy, which prioritizes the inputs used to measure fair value from market based assumptions to entity specific assumptions:
Level 1: Observable inputs such as quoted prices in active markets;
Level 2: Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The Company’s Level 3 financial liabilities consist of warrant liabilities related to warrants to purchase preferred stock. All warrants are being measured at fair value utilizing the Black-Scholes option pricing model.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
The fair value of the outstanding preferred stock warrants at December 31, 2011 and 2012 and March 31, 2013 was estimated using the Black-Scholes option pricing model with the following assumptions:
| December 31, 2011 | December 31, 2012 | March 31, 2013 | ||||||||||
Assumed risk-free interest rate | 0.36 | % | 0.25% - 1.78 | % | 0.25% - 1.87 | % | ||||||
Assumed volatility | 80 | % | 80 | % | 80 | % | ||||||
Expected warrant life | 3.08 years | 2.08 - 9.50 years | 1.80 - 9.20 years | |||||||||
Expected dividend yield | — | % | — | % | — | % | ||||||
Liabilities measured at fair value on a recurring basis as of March 31, 2013 are as follows:
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Balance as of March 31, 2013 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
Liabilities | ||||||||||||||||
Preferred stock warrant liability | $ | 229,000 | $ | — | $ | — | $ | 229,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities | $ | 229,000 | $ | — | $ | — | $ | 229,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Liabilities measured at fair value on a recurring basis as of December 31, 2012 are as follows:
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Balance as of December 31, 2012 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
Liabilities | ||||||||||||||||
Preferred stock warrant liability | $ | 56,000 | $ | — | $ | — | $ | 56,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities | $ | 56,000 | $ | — | $ | — | $ | 56,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Liabilities measured at fair value on a recurring basis as of December 31, 2011 are as follows:
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
| Balance as of December 31, 2011 | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
Liabilities | ||||||||||||||||
Preferred stock warrant liability | $ | 39,000 | $ | — | $ | — | $ | 39,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities | $ | 39,000 | $ | — | $ | — | $ | 39,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
The following table is a reconciliation of all the Company’s liabilities measured using significant unobservable inputs (Level 3) for the year ended December 31, 2012 and2022 filed with the three monthsSEC on March 21, 2023;
| Warrant Liability | ||||
Fair value measurement at December 31, 2011 | $ | 39,000 | ||
Warrants issued in connection with loan and security agreement | 24,250 | |||
Change in fair value of warrant liability | (7,250 | ) | ||
|
| |||
Fair value measurement at December 31, 2012 | 56,000 | |||
Warrants issued in connection with loan and security agreement | 49,000 | |||
Change in fair value of warrant liability | 124,000 | |||
|
| |||
Fair value measurement at March 31, 2013 | $ | 229,000 | ||
|
| |||
In 2008,
We will furnish without charge to you, on written or oral request, a warrant to purchase 50,000 sharescopy of Series A convertible preferred stock (“Series A Convertible Preferred Stock”) at an exercise price of $1.50 per share, which warrant expires three years from the effective dateany or all of the registration statementdocuments incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to Evoke Pharma, Inc., Attn: Corporate Secretary, 420 Stevens Avenue, Suite 230, Solana Beach, CA 92075; Telephone: (858) 345-1494.
You also may access these filings on our website at www.evokepharma.com. We do not incorporate the IPO. The loaninformation on our website into this prospectus or any supplement to this prospectus and security agreement was repaid in fullyou should not consider any information on, or that can be accessed through, our website as part of December 31, 2011.
In June 2012, the Company entered into a $3.0 million loan and security agreement collateralized by the Company’s personal property. Interest on advances under the agreement is at a fixed interest rate equalthis prospectus or any supplement to 4.50%. The loan and security agreement contains only non-financial covenants. Advances under the loan and security agreement have an interest-only period through December 31, 2013 and a 24-month payback period commences in January 2014.
As of December 31, 2012 and March 31, 2013, the Company had $2.0 million and $0, respectively, in available credit under the loan and security agreement. Total interest incurred under the loan and security agreement for the year ended December 31, 2012 and the three months ended March 31, 2013 (excluding amortization of debt discount) was $20,000 and $33,500, respectively.
In connectionthis prospectus (other than those filings with the loan and security agreement,SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a warrant was issueddocument incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for sharespurposes of Series A Convertible Preferred Stock that is exercisable in whole, or in part, at any time until the expiration date of June 1, 2022. During July 2012, the Company drew down $1.0 million under the loan and security agreement and the warrant became exercisable for 20,000 shares of Series A Convertible Preferred Stock at an exercise price of $1.50 per share. During January 2013, the Company drew down the remaining $2.0 million under the loan and security agreement and the warrant became exercisable for an additional 40,000 shares of Series A Convertible Preferred Stock at an exercise price of $1.50 per share.
The initial $24,250 fair value of the 20,000 warrant shares earned in July 2012 and the initial $49,000 fair value of the warrant shares earned in January 2013 were recorded as a debt discount and are amortized to interest expense over the term of the loan on the effective interest method. As of December 31, 2012 and March 31, 2013, the Company had unamortized debt discount of $20,208 and $63,393, respectively, relatedthis prospectus to the initial fair value of the warrant.
Evoke Pharma, Inc.extent that a statement contained in this prospectus modifies, supersedes or replaces such statement.
(A Development Stage Company)56
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
The initial fair value of warrants earned in 2012 and 2013 was estimated using the Black-Scholes option pricing model with the following assumptions:
July 2012 | January 2013 | |||||||
Assumed risk-free interest rate | 1.43 | % | 1.86 | % | ||||
Assumed volatility | 80 | % | 80 | % | ||||
Expected warrant life | 10 years | 9.5 years | ||||||
Expected dividend yield | — | % | — | % | ||||
The aggregate advances under the loan and security agreement and unamortized discount as of December 31, 2012 and March 31, 2013 are as follows:
| December 31, 2012 | March 31, 2013 | |||||||
Aggregate advances under loan and security agreement | $ | 1,000,000 | $ | 3,000,000 | ||||
Less unamortized discount | (20,208 | ) | (63,393 | ) | ||||
|
|
|
| |||||
Long-term debt, net of debt discount | $ | 979,792 | $ | 2,936,607 | ||||
|
|
|
| |||||
5. Acquisition of Technology
In June 2007, the Company purchased from Questcor Pharmaceuticals, Inc. (“Questcor”) all rights and patents to a development program for the Company’s EVK-001 product candidate, for an upfront payment of $650,000 which was expensed as in-process research and development. In addition to the upfront payment, the Company will be required to make additional milestone payments totaling up to $52,000,000. These milestones include up to $5,000,000 in payments if EVK-001 achieves the following development targets:
$500,000 upon the initiation of the first patient dosing in the Company’s planned Phase 3 clinical trial for EVK-001;
$1,500,000 upon the U.S. Food and Drug Administration’s (the “FDA”) acceptance for review of a new drug application for EVK-001; and
$3,000,000 upon the FDA’s approval of EVK-001.
The remaining $47,000,000 in milestone payments depend on EVK-001’s commercial success and will only apply if EVK-001 receives regulatory approval. In addition, the Company will be required to pay to Questcor a low single digit royalty on net sales of EVK-001. The Company’s obligation to pay such royalties will terminate upon the expiration of the last patent right covering EVK-001, which is expected to occur in 2030.
6. Convertible Preferred Stock and Stockholders’ Deficit
Convertible Preferred Stock
The Company’s convertible preferred stock has been classified as temporary equity in the accompanying balance sheets instead of in stockholders’ deficit in accordance with authoritative guidance for the classification and measurement of potentially redeemable securities. Upon certain change in control events that are outside of the Company’s control, including liquidation, sale or transfer of control of the Company, holders of the convertible preferred stock can cause its redemption.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
During June and October 2007, the Company sold an aggregate of 4,000,000 shares of Series A Convertible Preferred Stock at $1.50 per share for gross proceeds of $6,000,000 in cash. In addition, $250,000 in convertible promissory notes issued in an earlier bridge financing and $42,538 in accrued interest thereon converted into 195,067 shares of Series A Convertible Preferred Stock. In connection with the Series A Convertible Preferred Stock issuance, $848,257 of the proceeds were allocated to the preferred stock purchase right liability, and the Company incurred $218,037 of offering costs.
As part of the October 2007 Series A Convertible Preferred Stock transaction, the preferred stock purchase right liability for the second closing was revalued with the $4,132 increase in fair value recorded as other expense on the statement of operations and the then fair value of $80,819 was reclassified to Series A Convertible Preferred Stock. At December 31, 2007, the preferred stock purchase right liability for the third closing was revalued with the $68,955 increase in fair value recorded as other expense on the statement of operations.
During November 2008, the Company sold an additional 4,000,000 shares of Series A Convertible Preferred Stock for gross proceeds of $6,000,000 in cash. In connection with this financing, the Company incurred $1,855 of offering costs. As part of the Series A Convertible Preferred Stock transaction, the preferred stock purchase right liability for the third closing was revalued with the $115,500 increase in fair value recorded as other expense on the statement of operations and the then fair value of $956,025 was reclassified to Series A Convertible Preferred Stock.
During June 2010, the Company sold an additional 4,000,001 shares of Series A Convertible Preferred Stock for gross proceeds of $6,000,002 in cash. In connection with this financing, the Company incurred $36,069 of offering costs.
The holders of the Series A Convertible Preferred Stock are entitled to receive noncumulative dividends at a rate of $0.12 per share per annum. The preferred stock dividends are payable when and if declared by the board of directors. As of December 31, 2012 and March 31, 2013, the board of directors has not declared any dividends. The Series A Convertible Preferred Stock dividends are payable in preference and in priority to any dividends on common stock.
The holders of the Series A Convertible Preferred Stock are entitled to receive liquidation preferences at the rate of $1.50 per share, plus all declared and unpaid dividends. Liquidation payments to the holders of Series A Convertible Preferred Stock have priority and are made in preference to any payments to the holders of common stock.
The shares of Series A Convertible Preferred Stock are convertible into one share of common stock for each five shares of preferred stock, at the option of the holder, subject to certain anti-dilutive adjustments. Each share of Series A Convertible Preferred Stock is automatically converted into common stock immediately upon the earlier of (i) the Company’s sale of its common stock in a firm commitment underwritten public offering pursuant to a registration statement under the Securities Act of 1933, as amended, in which the per share price is at least $22.50 (as adjusted), and the gross cash proceeds are at least $25,000,000 or (ii) the date specified by written consent or agreement of the holders of not less than 66.66% of the then outstanding shares of Series A Convertible Preferred Stock.
The holders of Series A Convertible Preferred Stock are entitled to one vote for each share of common stock into which such Series A Convertible Preferred Stock could then be converted; and with respect to such vote, such holder shall have full voting rights and powers equal to the voting rights and powers of the holders of common stock.
The holders of the Series A Convertible Preferred Stock with greater than 250,000 shares are entitled to elect one member each to the Company’s board of directors.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Common Stock
During March 2007, in conjunction with the founding of the Company, 916,000 shares of its common stock were issued to the founders at a price of $0.005 per share.
Stock Options
The Company adopted the 2007 Equity Incentive Plan (the “Plan”) in May 2007 under which 450,000 shares of common stock are reserved for issuance to employees, nonemployee directors and consultants of the Company. The Plan provides for the grant of incentive stock options, nonstatutory stock options, phantom stock and rights to purchase restricted stock to eligible recipients. Recipients of incentive stock options shall be eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the Plan is ten years. The options generally vest over four years or upon achieving predetermined corporate milestones. As of December 31, 2012 and March 31, 2013, no options remain available for future grant under the Plan.
The following table summarizes stock option transactions under the Plan:
| Options Outstanding | Weighted- Average Exercise Price | |||||||
Outstanding at December 31, 2011 | 123,250 | $ | 0.40 | |||||
Granted | — | — | ||||||
Exercised | — | — | ||||||
|
| |||||||
Outstanding at December 31, 2012 and March 31, 2013 | 123,250 | $ | 0.40 | |||||
|
| |||||||
Exercisable at December 31, 2012 and March 31, 2013 | 123,250 | $ | 0.40 | |||||
|
| |||||||
The shares of common stock issued from the exercise of stock options are restricted and vest over time or on the achievement of certain milestones. The Plan permits the early exercise of options but the Company has the option to repurchase any unvested shares at the original purchase price (the exercise price paid by the Purchaser) upon any voluntary or involuntary termination (“Repurchase Option”). Any unvested shares immediately vest in the event of termination for reasons other than cause, and vesting accelerates in the event of a merger, sale, or other change in control of the Company. Of the total 326,750 stock options exercised, 200,500, 215,500 and 219,250 were vested as of December 31, 2011 and 2012 and March 31, 2013, respectively.
Since the inception of the Company, 272,000 stock options were issued with an exercise price of $0.29 and 178,000 stock options were issued with an exercise price of $0.40, resulting in a weighted-average exercise price of $0.33 per share.
As of December 31, 2012 and March 31, 2013, the Company had 123,250 options outstanding, with a weighted-average remaining contractual term of 7.84 years and 7.59 years, respectively.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following at December 31, 2011 and 2012 and March 31, 2013:
| December 31, | March 31, 2013 | |||||||||||
| 2011 | 2012 | |||||||||||
Conversion of preferred stock | 2,439,002 | 2,439,002 | 2,439,002 | |||||||||
Stock options issued and outstanding | 123,250 | 123,250 | 123,250 | |||||||||
Authorized for future option grants | — | — | — | |||||||||
Warrants for convertible preferred stock | 10,000 | 14,000 | 22,000 | |||||||||
|
|
|
|
|
| |||||||
| 2,572,252 | 2,576,252 | 2,584,252 | ||||||||||
|
|
|
|
|
| |||||||
7. Income Taxes
On January 1, 2009, the Company adopted authoritative guidance relating to the accounting for uncertainty in income taxes. The guidance clarified the recognition threshold and measurement attributes for financial statement disclosure of tax positions taken, or expected to be taken, on a tax return. The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain tax position will not be recognized if it has a less than 50% likelihood of being sustained. On the date of adoption, there were no unrecognized tax benefits.
As of December 31, 2011 and 2012, there were no unrecognized tax benefits included in the balance sheets that would, if recognized, affect the Company’s effective tax rate. The Company has not recognized any interest and penalties related to income taxes in the balance sheets or statements of operations. The Company is subject to taxation in the U.S. and state jurisdictions. As of December 31, 2012, the Company’s tax years beginning 2007 to date are subject to examination by taxing authorities.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Pursuant to Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (“IRC”), annual use of the Company’s net operating loss and research and development credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has not completed an IRC Section 382/383 analysis regarding the limitation of net operating loss and research and development credit carryforwards. Until this analysis has been completed, the Company has removed the deferred tax assets for net operating losses of approximately $6.7 million and a research and development credit of approximately $773,000 generated through 2012 from its deferred tax asset schedule, and has recorded a corresponding decrease to its valuation allowance. When this analysis is finalized, the Company plans to update its unrecognized tax benefits accordingly. The Company does not expect this analysis to be completed within the next 12 months and, as a result, the Company does not expect that the unrecognized tax benefits will change within 12 months of this reporting date. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized tax benefits will not impact the Company’s effective tax rate. Significant components of the Company’s deferred tax assets at December 31, 2011 and 2012 are as follows:
| December 31, | ||||||||
| 2011 | 2012 | |||||||
Deferred tax assets: | ||||||||
Acquired technology | $ | 181,000 | $ | 164,000 | ||||
Other, net | 86,000 | 203,000 | ||||||
|
|
|
| |||||
Total deferred tax assets | 267,000 | 367,000 | ||||||
Less valuation allowance | (267,000 | ) | (367,000 | ) | ||||
|
|
|
| |||||
Net deferred tax assets | $ | — | $ | — | ||||
|
|
|
| |||||
At December 31, 2012, the Company has federal and state net operating loss carryforwards of approximately $18.6 million and $18.2 million, respectively. The federal and state loss carryforwards begin to expire in 2028 and 2018, respectively, unless previously utilized. The Company also has federal and state research tax credit carryforwards of approximately $525,000 and $428,000, respectively. The federal research credit carryforwards will begin expiring in 2028 unless previously utilized. The state research credit will carry forward indefinitely.
8. Subsequent Events (unaudited)
Approval of 2013 Equity Incentive Award Plan
On June 7, 2013, the Company’s board of directors adopted the 2013 Equity Incentive Award Plan (the “2013 Plan”), and the Company expects its stockholders to approve the 2013 Plan prior to the effectiveness of the IPO. The 2013 Plan will become effective on the day prior to the effectiveness of the IPO. Under the 2013 Plan, the Company may grant stock options, stock appreciation rights, restricted stock, restricted stock units and other awards to individuals who are then employees, officers, non-employee directors or consultants of the Company or its subsidiaries. A total of 510,000 shares of common stock will initially be reserved for issuance under the 2013 Plan. In addition, the number of shares of common stock available for issuance under the 2013 Plan will be annually increased on the first day of each fiscal year during the term of the 2013 Plan, beginning with the 2014 fiscal year, by an amount equal to the least of: (i) 300,000 shares; (ii) five percent of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; or (iii) such other amount as the Company’s board of directors may determine.
Evoke Pharma, Inc.
(A Development Stage Company)
Notes to Financial Statements
(Information as of March 31, 2013 and thereafter and for the three months ended March 31, 2012 and 2013 and the period from January 29, 2007 (inception) to March 31, 2013 is unaudited)
Approval of the Employee Stock Purchase Plan
On June 7, 2013, the Company’s board of directors adopted the Employee Stock Purchase Plan (the “ESPP”), and the Company expects its stockholders to approve the ESPP prior to the effectiveness of the IPO. The ESPP will become effective on the day prior to the effectiveness of the IPO. The ESPP permits participants to purchase common stock through payroll deductions of up to 20% of their eligible compensation. A total of 30,000 shares of common stock will initially be reserved for issuance under the ESPP. In addition, the number of shares of common stock available for issuance under the ESPP will be annually increased on the first day of each fiscal year during the term of the ESPP, beginning with the 2014 fiscal year, by an amount equal to the least of: (i) 30,000 shares; (ii) one percent of the outstanding shares of common stock as of the last day of the immediately preceding fiscal year; or (iii) such other amount as the Company’s board of directors may determine.
Reverse Stock Split
On June 7, 2013, the Company’s board of directors approved an amendment to the restated certificate of incorporation to effect a one-for-five reverse stock split of the Company’s common stock (the “Reverse Stock Split”). The par value and the authorized shares of the common and convertible preferred stock were not adjusted as a result of the Reverse Stock Split. All issued and outstanding common stock and the conversion ratio of the convertible preferred stock have been retroactively adjusted to reflect this Reverse Stock Split for all periods presented. The Reverse Stock Split is expected to be effected prior to the effectiveness of the IPO.
SharesEvoke Pharma, Inc.
Common Stock


PROSPECTUS
PROSPECTUSBook-Running Managers
CRAIG-HALLUM
LAIDLAW & COMPANY (UK) LTD.
Until , 2013
Aegis Capital Corp
You should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. The information in this prospectus is accurate only as of2024 (25 days after the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of these securities.
Until prospectus), 2013 (25 days after the commencement of this offering) all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealers’ obligation to deliver a prospectus when acting as underwritersan underwriter and with respect to their unsold allotments or subscriptions.
Part II , 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
II-1
Item 13. Other Expenses of Issuance and Distribution.
The following table indicatessets forth the costs and expenses, to be incurredother than the underwriting discounts and commissions, payable by the registrant in connection with the offering described in this registration statement, other than underwriting discounts and commissions, all of which will be paid by us.the securities being registered. All amounts are estimatedestimates except for the Securities and Exchange CommissionSEC registration fee and the Financial IndustryInstitution Regulatory Authority, Inc., or FINRA,Association (“FINRA”) filing fee and The NASDAQ Capital Market listing fee.
Item |
| Amount paid or to be paid | |
SEC registration fee |
| $ | 5,092 |
FINRA listing fee |
|
| 5,000 |
Legal fee and expenses |
|
| 400,000 |
Accounting fees and expenses |
|
| 180,000 |
Transfer agent and registrar fees and expenses |
|
| 5,000 |
Miscellaneous expenses |
|
| 4,908 |
Total |
| $ | 600,000 |
| Amount | ||||
Securities and Exchange Commission registration fee | $ | 3,263 | ||
FINRA filing fee | * | |||
NASDAQ Capital Stock Market listing fee | * | |||
Accountants’ fees and expenses | * | |||
Legal fees and expenses | * | |||
Blue Sky fees and expenses | * | |||
Transfer Agent’s fees and expenses | * | |||
Printing and engraving expenses | * | |||
Miscellaneous | * | |||
|
| |||
Total expenses | $ | * | ||
|
| |||
II-2
Item 14. Indemnification of Directors and Officers.
|
Section 102 of the General Corporation Law of the State of DelawareDGCL permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our certificateCertificate of incorporationIncorporation provides that no director of the RegistrantCompany shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the General Corporation Law of the State of DelawareDGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the General Corporation Law of the State of DelawareDGCL provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
II-1
Our certificate of incorporation providesBylaws provide that we will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our certificate of incorporation providesBylaws also provide that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
We have entered into indemnification agreements with each of our directors and officers. These indemnification agreements may require us, among other things, to indemnify our directors and officers for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers, or any of our subsidiaries or any other company or enterprise to which the person provides services at our request.
II-3
We maintain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In anyAny underwriting agreement or distribution agreement that we enter into with any underwriters or agents involved in connection with the offering or sale of common stock beingany securities registered hereby themay require such underwriters will agreeor dealers to indemnify under certain conditions, us, some or all of our directors ourand officers and our controlling persons, who control us within the meaning ofif any, for specified liabilities, which may include liabilities under the Securities Act of 1933, as amended, or Securities Act, against certain liabilities.1933.
II-4
Item 15. Recent Sales |
Set forth below is information regarding shares of capital stock issued by us since our inception in January 2010. Also included is the consideration received by us for such shares and information relating to the section of the Securities Act, or rule of the Securities and Exchange Commission, under which exemption from registration was claimed.Unregistered Securities.
None.
II-2II-5
(a) Exhibits. The following documents are filed as exhibits to this registration statement.
_____________
Exhibit Number | Description of Exhibit | |
1.1* | ||
3.1 | Amended and Restated Certificate of Incorporation of the Company(incorporated by reference to | |
3.2 | Certificate of | |
3.2 | Amended and Restated Bylaws of the Company (incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on September 30, 2013.) | |
4.1 | Form of the Company’s Common Stock Certificate(incorporated by reference to the Company’s Amendment No. 3 to Registration Statement on Form S-1 filed with the SEC on August 16, 2013.) | |
4.2** | ||
4.3* | Form of Series A | |
4.4* | Form of | |
4.5* | ||
5.1* | ||
10.1# | Form of Indemnity Agreement for | |
10.2# | Employment Agreement, effective as of | |
10.3# | Evoke Pharma, Inc. 2013 Equity Incentive Award Plan, as amended and restated effective May 10, 2023(incorporated by reference to the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on March 29, 2023.) | |
10.4† | Commercial Services Agreement, dated as of January 21, 2020, between the Company and Eversana Life Science Services, LLC(incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 12, 2020.) | |
10.5† | 3PL Agreement between the Company and Eversana Life Science Services, LLC dated August 27, 2020(incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 12, 2020.) | |
10.6# | Non-Employee Director Compensation Policy, as Amended and Restated Effective February 8, 2023 (incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 10, 2023.) | |
No underwriters were involved in the foregoing sales of securities. The securities described in this section (a) of Item 15 were issued to investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering, to the extent an exemption from such registration was required. All purchasers of shares of convertible preferred stock described above represented to us in connection with their purchase that they were accredited investors and were acquiring the shares for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.II-6
The stock options and the common stock issuable upon the exercise of such options as described in this section (b) of Item 15 were issued pursuant to written compensatory plans or arrangements with our employees and directors, in reliance on the exemption from the registration requirements of the Securities Act provided by Rule 701 promulgated under the Securities Act or the exemption set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering. All recipients either received adequate information about us or had access, through employment or other relationships, to such information.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. All certificates representing the issued shares of capital stock described in this Item 15 included appropriate legends setting forth that the securities had not been registered and the applicable restrictions on transfer.
II-3
Exhibit Number | Description of Exhibit | |
10.7† | ||
10.8† | Amendment No. 2 to the Commercial Services Agreement, dated | |
10.9# | ||
Amended and Restated Employment Agreement, effective as of June 7, 2013, between the | ||
10.10# | Evoke Pharma, Inc. 2013 | |
10.11† | Loan Agreement, dated as of January 21, 2020, between the Company and Eversana Life Science Services, LLC | |
10.12† | Master Supply Agreement dated as of May 11, 2016 by and between the Company and Cosma S.p.A.(incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 15, 2016.) | |
10.13 | Third Amendment to Standard Office Lease dated December 15, 2020 between the Company and SB Corporate Centre III-IV, LLC. (i | |
10.14# | 2013 Equity Incentive Award Plan and form of option agreement thereunder(incorporated by reference to the Company’s Amendment No. 4 to Registration Statement on Form S-1 filed with the SEC on August 30, 2013.) | |
10.15# | ||
Amended and Restated Retention Letter, dated May 22, 2013, between the | ||
10.16# | Amended and Restated Retention Letter, dated May 22, 2013, between the | |
10.17† | ||
Asset Purchase Agreement, dated as of June 1, 2007, | ||
10.18† | Manufacturing Services Agreement dated November 7, 2017, between the Company and Patheon UK Limited(incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 7, 2018.) | |
10.19 | Standard Office Lease, dated as of December 19, 2016, between the Company and SB Corporate Centre III-IV, LLC(incorporated by reference to the Company’s Annual Report on Form 10-K filed with the SEC on March 15, 2017.) | |
II-7
II-4† Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.
* Filed herewith
** Previously filed.
(b) Financial Statement Schedules.statement schedules. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statementsFinancial Statements or notes thereto.
II-8
Item 17. Undertakings.
The undersigned registrantRegistrant hereby undertakesundertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to providethis registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the underwriter,plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the closing specifiedtermination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting agreement, certificates inmethod used to sell the securities to the purchaser, if the securities are offered or sold to such denominationspurchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and registered inwill be considered to offer or sell such names assecurities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the underwriterundersigned registrant;
II-9
(iii) The portion of any other free writing prospectus relating to permit prompt deliverythe offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to eachthe purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(ii) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(iii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-10
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrantRegistrant has duly caused this Amendment No. 2 to Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego,Solana Beach, State of California, on this 3rd day of July, 2013.January 10, 2024.
EVOKE PHARMA, INC. | ||
By: | /s/ David A. Gonyer | |
Name: | David A. Gonyer, R.Ph. | |
Title: | Chief Executive Officer and Director | |
Pursuant to the requirements of the Securities Act of 1933, this Amendment No. 2 to Registration Statement on Form S-1 has been signed below by the following persons on behalf of the Registrant and in the capacities heldand on the dates indicated.
| Title | Date | ||
/s/ David A. Gonyer | Chief Executive Officer and Director | January 10, 2024 | ||
David A. Gonyer, R.Ph. |
| |||
/s/ Matthew J. D’Onofrio | President, Chief Operating Officer, | January 10, 2024 | ||
Matthew J. D’Onofrio |
| |||
* | Chairman of the Board of Directors | January 10, 2024 | ||
Cam L. Garner | ||||
* | Director | January 10, 2024 | ||
Todd C. Brady, M.D., Ph.D. | ||||
* | Director | January 10, 2024 | ||
Malcolm R. Hill, Pharm.D. | ||||
* | Director | January 10, 2024 | ||
Vickie W. Reed | ||||
* | Director | |||
January 10, 2024 | ||||
|
| |||
| Kenneth J. Widder, M.D. | ||||
* Pursuant to power of attorney. By: /s/ David A. Gonyer | ||
| ||
Exhibit Index
Name: David A. Gonyer
|
| |
Title: Attorney-in-Fact
II-11