
As filed with the Securities and Exchange Commission on July 22, 2013February 24, 2015
Registration No. 333-189395333-201731
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO.Amendment No. 1
TOto
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT
OF 1933
OPEXA THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Texas | 2834 | 76-0333165 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Neil K. Warma
President and Chief Executive Officer
OPEXA THERAPEUTICS, INC.
2635 Technology Forest Blvd.
The Woodlands, Texas 77381
(281) 272-9331
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent for Service)
Copies to:
Mike Hird, Esq. Gabriella A. Lombardi, Esq. Patty M. DeGaetano, Esq. Pillsbury Winthrop Shaw Pittman LLP 12255 El Camino Real, Suite 300 San Diego, CA 92130 (619) 234-5000 |
Lili Taheri, Esq. Loeb &
New York, NY (212) |
Approximate date of commencement of proposed sale to the public:As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company x | |||||
(Do not check if a smaller reporting company) | ||||||||
CALCULATION OF REGISTRATION FEE
| ||||
| Title of Each Class of Securities To Be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee(2)(4) | ||
Common Stock, $0.01 par value(3) | $17,250,000 | $2,352.90 | ||
| ||||
| ||||
| ||||||||
Title of each class of securities to be registered | Amount to be Registered | Proposed Maximum Per Unit | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee(5) | ||||
Units, each consisting of one share of common stock, par value $0.01 per share (“Common Stock”) and one warrant to purchase one share of common stock (“Units”) | 28,776,419 | $0.70 | $20,143,493 | $2,340.67 | ||||
Non-transferable Rights to purchase Units(2) | – | – | – | – | ||||
Common Stock included as part of the Units | 28,776,419 | Included with Units above | Included with Units above | – | ||||
Warrants included as part of the Units(3) | 28,776,419 | Included with Units above | Included with Units above | – | ||||
Common Stock issuable upon exercise of the Warrants included in the Units(1)(4) | 28,776,419 | $1.50 | $43,164,629 | $5,015.73 | ||||
Total | $63,308,122 | $7,356.40 | ||||||
| ||||||||
| ||||||||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) |
| (2) | Non-transferable Rights to subscribe for Units are being issued without consideration. Pursuant to Rule 457(g) under the Act, no separate registration fee is required for the Rights because the Rights are being registered in the same registration statement as the common stock of the Registrant underlying the Rights. |
| (3) | Pursuant to Rule |
| (4) | In addition to the shares of Common Stock set forth in this table, pursuant to Rule 416 under the |
| Previously |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED |
9,300,000 Shares
Common Stock

We are offering 9,300,000 sharesdistributing to holders of our common stock. Ourstock and to holders of certain of our outstanding warrants who are entitled to participate in this offering pursuant to the terms of such warrants (Series L), at no charge,non-transferable subscription rights to purchase units. Each unit, which we refer to as a Unit, consists of one share of common stock is listed onand one tradable warrant representing the NASDAQ Capital Market under the symbol “OPXA.” On July 19, 2013, the last reported sales price for ourright to purchase one share of common stock, was $1.61which we refer to as the Warrants. We refer to the offering that is the subject of this prospectus as the Rights Offering. In the Rights Offering, you will receive one subscription right for each share of common stock or each share of common stock underlying our Series L warrants owned at 5:00 p.m., Eastern Time, on March 13, 2015, the record date of the Rights Offering, or the Record Date. The common stock and the Warrants comprising the Units will separate upon the exercise of the rights and the Units will not trade as a separate security. The subscription rights will not be tradable.
Each subscription right will entitle you to purchase one Unit, which we refer to as the Basic Subscription Right, at a subscription price per share.Unit of $0.70, which we refer to as the Subscription Price. The Warrants entitle the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through their expiration three years from the date of issuance. If you exercise your Basic Subscription Rights in full, and other shareholders or warrant holders do not fully exercise their Basic Subscription Rights, you will be entitled to an over-subscription privilege to purchase a portion of the unsubscribed Units at the Subscription Price, subject to proration and ownership limitations, which we refer to as the Over-Subscription Privilege. Each subscription right consists of a Basic Subscription Right and an Over-Subscription Privilege, which we refer to as the Subscription Right.
The Subscription Rights will expire if they are not exercised by 5:00 p.m., Eastern Time, on April 8, 2015. We may extend the Rights Offering for additional periods in our sole discretion. Once made, all exercises of Subscription Rights are irrevocable.
We have not entered into any standby purchase agreement or other similar arrangement in connection with the Rights Offering. The Rights Offering is being conducted on a best-efforts basis and there is no minimum amount of proceeds necessary to be received in order for us to close the Rights Offering.
We have engaged Maxim Group LLC and National Securities Corporation to act as dealer-managers in the Rights Offering.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 14 in21 of this prospectus. You should carefully consider these risk factors, as well as the information contained in this prospectus, before you invest.
Continental Stock Transfer & Trust Company will serve as the Subscription Agent for the Rights Offering. The Subscription Agent will hold the funds we receive from subscribers until we complete, abandon or terminate the Rights Offering. Advantage Proxy, Inc. will serve as Information Agent for the Rights Offering. If you want to participate in this Rights Offering and you are the record holder of your shares or Series L warrants, we recommend that you submit your subscription documents to the Subscription Agent well before the deadline. If you want to participate in this Rights Offering and you hold shares through your broker, dealer, bank, or other nominee, you should promptly contact your broker, dealer, bank, or other nominee and submit your subscription documents in accordance with the instructions and within the time period provided by your broker, dealer, bank, or other nominee. For a detailed discussion, see “The Rights Offering – The Subscription Rights.”
Our board of directors reserves the right to terminate the Rights Offering for any reason any time before the closing of the Rights Offering. If we terminate the Rights Offering, all subscription payments received will be returned within 10 business days, without interest or penalty. We expect the Rights Offering to expire on or about April 8, 2015, subject to our right to extend the Rights Offering as described above.
Our common stock is listed on The NASDAQ Capital Market, or NASDAQ, under the symbol “OPXA.” On February 20, 2015, the last reported sale price of our common stock was $0.73 per share. We have applied to list the Warrants on NASDAQ following their issuance under the symbol “OPXAW.” The Subscription Rights are non-transferrable and will not be listed for trading on NASDAQ or any other stock exchange or market. You are urged to obtain a current price quote for our common stock before exercising your Subscription Rights.
| Per Unit | Total(2) | |||||||
Subscription price | $ | 0.70 | $ | 20,143,493 | ||||
Dealer-Manager fees and expenses (1) | $ | 0.05 | $ | 1,560,403 | ||||
Proceeds to us, before expenses | $ | 0.65 | $ | 18,583,090 | ||||
| (1) | In connection with this Rights Offering, we have agreed to pay fees to the dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and (c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to reimburse the dealer-managers for their expenses as follows: (i) up to $50,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is less than $6 million; and (ii) up to $100,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is at least $6 million. See “Plan of Distribution.” |
| (2) | Assumes the Rights Offering is fully subscribed, but excludes proceeds from the exercise of Warrants included within the Units. |
Our board of directors is making no recommendation regarding your exercise of the Subscription Rights. You should carefully consider whether to exercise your Subscription Rights before the expiration date. You may not revoke or revise any exercises of Subscription Rights once made unless we terminate the Rights Offering.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Dealer-Managers
| Maxim Group LLC | ||||||||
| ||||||||
| ||||||||
| National Securities Corp. | |||||||
We have granted a 30-day option to the representative of the underwriters to purchase up to 1,395,000 additional shares of common stock solely to cover over-allotments, if any.
The underwriters expect to deliver our shares of common to purchasers in this offering on or about , 2013.
Aegis Capital Corp
The date of this Prospectus is , 2013
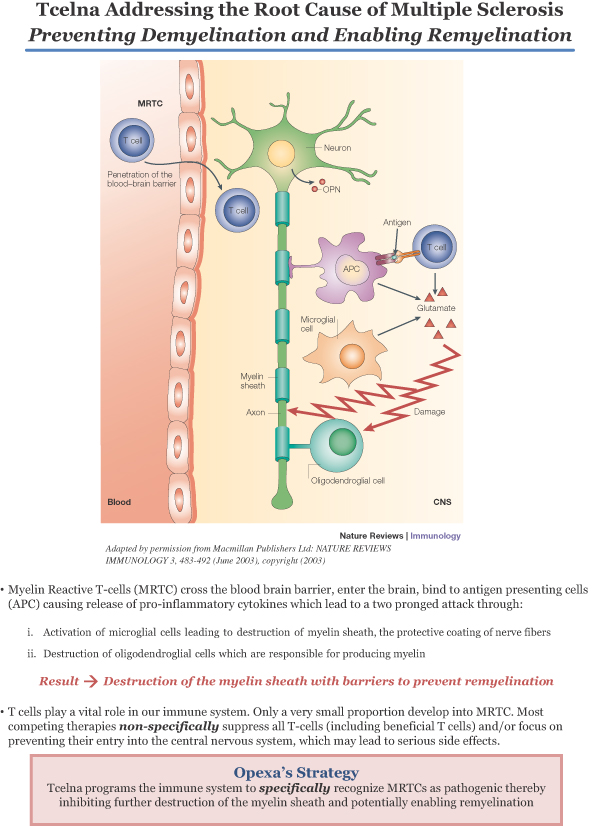
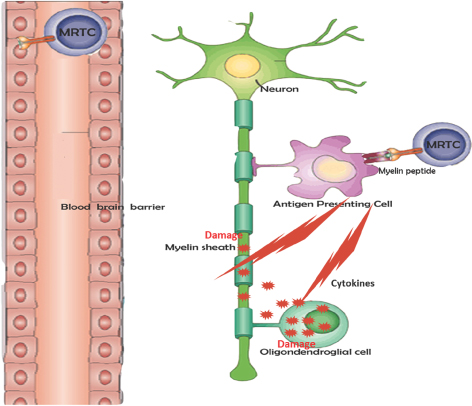
Degrade Myelin and Damage Myelin Producing Cells
Adapted by permission from Macmillan Publishers Ltd: NATURE REVIEWS
IMMUNOLOGY 3, 483-492 (June 2003), copyright (2003)
| ¡ | Destruction of myelin sheath, the protective coating of nerve fibers |
| ¡ | Destruction of oligodendroglial cells, which are responsible for producing myelin |
Opexa’s Strategy Tcelna programs the immune system tospecifically recognize MRTCs as pathogenic thereby inhibiting further destruction of the myelin sheath and potentially enabling remyelination |
| Page |
| 1 | |||
Market Price of our Common Stock and Related Stockholder Matters | ||||
| ||||
ABOUT THIS PROSPECTUS
The registration statement we filed with the Securities and Exchange Commission, or SEC, includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with the additional information described under the headingheadings “Where You Can Find More Information,”Information” and “Incorporation by Reference” before making your investment decision.
You should rely only on the information provided in this prospectus or in a prospectus supplement or amendment thereto. We have not authorized anyone else to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any state where the offer or sale is not permitted. You should assume that the information in this prospectus is accurate only as of the date hereof. Our business, financial condition, results of operations and prospects may have changed since that date.
Unless the context otherwise requires, references in this prospectus to “Opexa,” “the Company,” “we,” “us” and “our” refer to Opexa Therapeutics, Inc. Tcelna® is a registered trademark of Opexa and ImmPathTM® and Precision ImmunotherapyTM® are service marks of Opexa. All other product and company names are trademarks of their respective owners.
i
QUESTIONS AND ANSWERS RELATING TO THE RIGHTS OFFERING
The following are examples of what we anticipate will be common questions about the Rights Offering. The answers are based on selected information included elsewhere in this prospectus. The following questions and answers do not contain all of the information that may be important to you and may not address all of the questions that you may have about the Rights Offering. This prospectus and the documents incorporated by reference into this prospectus contain more detailed descriptions of the terms and conditions of the Rights Offering and provides additional information about us and our business, including potential risks related to the Rights Offering, the Units offered hereby, and our business. We urge you to read this entire prospectus and the documents incorporated by reference into this prospectus.
Why are we conducting the Rights Offering?
We are conducting the Rights Offering to raise additional capital:
What is the Rights Offering?
We are distributing, at no charge, to record holders of our common stock and to holders of certain of our outstanding warrants (Series L) who are entitled to participate in this Rights Offering pursuant to the terms of such warrants, non-transferable Subscription Rights to purchase Units at a price of $0.70 per whole Unit. The Subscription Rights will not be tradable. Each Unit consists of one share of common stock and one Warrant representing the right to purchase one share of common stock. Upon closing of the Rights Offering, the common stock and Warrants will immediately separate. We have applied to list the Warrants on NASDAQ under the symbol “OPXAW.” You will receive one Subscription Right for each share of common stock or each share of common stock underlying our Series L warrants that you owned as of 5:00 p.m., Eastern Time, on the Record Date. Each Subscription Right entitles the record holder or holder of a Series L warrant to a Basic Subscription Right and an Over-Subscription Privilege.
What are the Basic Subscription Rights?
For each whole share you owned or whole share underlying our Series L warrants you owned as of the Record Date, you will receive one Basic Subscription Right, which gives you the opportunity to purchase one share of our common stock and to receive one Warrant to purchase one additional share of our common stock for a price of $0.70 per Unit. For example, if you owned 100 shares of common stock as of the Record Date, you will receive 100 Subscription Rights and will have the right to purchase 100 shares of our common stock and Warrants to purchase 100 shares of our common stock for $0.70 per whole Unit (or a total payment of $70.00). You may exercise all or a portion of your Basic Subscription Rights or you may choose not to exercise any Basic Subscription Rights at all.
If you are a record holder or a holder of Series L warrants, the number of shares you may purchase pursuant to your Basic Subscription Rights is indicated on the enclosed Rights Certificate. If you hold your
shares in the name of a broker, dealer, bank, or other nominee who uses the services of the Depository Trust Company, or DTC, you will not receive a Rights Certificate. Instead, DTC will issue one Subscription Right to your nominee record holder for each share of our common stock that you own as of the Record Date. If you are not contacted by your nominee, you should contact your nominee as soon as possible.
What is the Over-Subscription Privilege?
If you exercise your Basic Subscription Rights in full, you may also choose to exercise your Over-Subscription Privilege to purchase a portion of any Units that the other record holders and applicable warrant holders do not purchase through the exercise of their Basic Subscription Rights. You should indicate on your Rights Certificate, or the form provided by your nominee if your shares are held in the name of a nominee, how many additional Units you would like to purchase pursuant to your Over-Subscription Privilege.
Subject to stock ownership limitations, if sufficient Units are available, we will seek to honor your Over-Subscription request in full. If Over-Subscription requests exceed the number of Units available, however, we will allocate the available Units pro-rata among the record holders and applicable warrant holders exercising the Over-Subscription Privilege in proportion to the number of shares of our common stock each of those record holders owned or the number of shares of our common stock underlying our Series L warrants held by each of those warrant holders on the Record Date, relative to the number of shares owned or shares underlying Series L warrants on the Record Date by all record holders and Series L warrant holders exercising the Over-Subscription Privilege. If this pro-rata allocation results in any record holders or Series L warrant holders receiving a greater number of Units than the record holder or Series L warrant holder subscribed for pursuant to the exercise of the Over-Subscription Privilege, then such record holder or Series L warrant holder will be allocated only that number of Units for which the record holder or Series L warrant holder oversubscribed, and the remaining Units will be allocated among all other record holders and Series L warrant holders exercising the Over-Subscription Privilege on the same pro rata basis described above. The proration process will be repeated until all Units have been allocated. See “The Rights Offering—Limitation on the Purchase of Units” for a description of certain stock ownership limitations.
To properly exercise your Over-Subscription Privilege, you must deliver to the Subscription Agent the subscription payment related to your Over-Subscription Privilege before the Rights Offering expires. See “The Rights Offering—The Subscription Rights—Over-Subscription Privilege.” To the extent you properly exercise your Over-Subscription Privilege for an amount of Units that exceeds the number of unsubscribed Units available to you, any excess subscription payments will be returned to you within 10 business days after the expiration of the Rights Offering, without interest or penalty.
Continental Stock Transfer & Trust Company, our Subscription Agent for the Rights Offering, will determine the Over-Subscription allocation based on the formula described above.
What are the terms of the Warrants?
Each Warrant entitles the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through their expiration three years from the date of issuance. The Warrants will be exercisable for cash, or, solely during any period when a registration statement for the exercise of the Warrants is not in effect, on a cashless basis. We may redeem the Warrants for $0.01 per Warrant if our common stock closes above $2.50 per share for 10 consecutive trading days.
Will fractional shares be issued upon exercise of Subscription Rights or upon the exercise of Warrants?
No. We will not issue fractional shares of common stock in the Rights Offering. Rights holders will only be entitled to purchase a number of Units representing a whole number of shares of common stock, rounded down to the nearest whole number of Units a holder would otherwise be entitled to purchase. Any excess
subscription payments received by the Subscription Agent will be returned within 10 business days after expiration of the Rights Offering, without interest or penalty. Similarly, no fractional shares of common stock will be issued in connection with the exercise of a Warrant. Instead, for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, the holder will be entitled to a cash payment equal to the pro-rated per share market price of the common stock on the last trading day preceding the exercise.
What effect will the Rights Offering have on our outstanding common stock?
Based on 28,234,751 shares of common stock outstanding as of February 20, 2015, as well as Series L warrants to purchase 541,668 shares of common stock outstanding on that date, assuming no other transactions by us involving our common stock prior to the expiration of the Rights Offering, if the Rights Offering is fully subscribed, 57,011,170 shares of our common stock will be issued and outstanding and Warrants to purchase an additional 28,776,419 shares of our common stock will be outstanding stock (excluding the 3,046,801 currently outstanding warrants). The exact number of shares and Warrants that we will issue in this Rights Offering will depend on the number of Units that are subscribed for in the Rights Offering.
How was the Subscription Price determined?
In determining the Subscription Price, the directors considered, among other things, the following factors:
In conjunction with the review of these factors, the board of directors also reviewed our history and prospects, including our past and present earnings and cash requirements, our prospects for the future, the outlook for our industry and our current financial condition. The board of directors also believed that the Subscription Price should be designed to provide an incentive to our current shareholders and holders of our Series L warrants to participate in the Rights Offering and exercise their Basic Subscription Right and their Over-Subscription Privilege.
The Subscription Price does not necessarily bear any relationship to any established criteria for value. You should not consider the Subscription Price as an indication of actual value of our company or our common stock. We cannot assure you that the market price of our common stock will not decline during or after the Rights Offering. You should obtain a current price quote for our common stock before exercising your Subscription Rights and make your own assessment of our business and financial condition, our prospects for the future, and the terms of this Rights Offering. Once made, all exercises of Subscription Rights are irrevocable.
Am I required to exercise all of the Basic Subscription Rights I receive in the Rights Offering?
No. You may exercise any number of your Basic Subscription Rights, or you may choose not to exercise any Basic Subscription Rights. If you do not exercise any Basic Subscription Rights, the number of shares of our common stock or number of shares underlying our Series L warrants you own will not change. However, if you
choose to not exercise your Basic Subscription Rights in full, your proportionate ownership interest in our company will decrease. If you do not exercise your Basic Subscription Rights in full, you will not be entitled to exercise your Over-Subscription Privilege.
How soon must I act to exercise my Subscription Rights?
If you received a Rights Certificate and elect to exercise any or all of your Subscription Rights, the Subscription Agent must receive your completed and signed Rights Certificate and payment for both your Basic Subscription Rights and any Over-Subscription Privilege you elect to exercise, including final clearance of any uncertified check, before the Rights Offering expires on April 8, 2015, at 5:00 p.m., Eastern Time. If you hold your shares in the name of a broker, dealer, custodian bank, or other nominee, your nominee may establish a deadline before the expiration of the Rights Offering by which you must provide it with your instructions to exercise your Subscription Rights, along with the required subscription payment.
May I transfer my Subscription Rights?
No. The Subscription Rights may be exercised only by the shareholders to whom they are distributed, and they may not be sold, transferred, assigned or given away to anyone else, other than by operation of law. As a result, Rights Certificates may be completed only by the shareholder who receives the certificate. The Subscription Rights will not be listed for trading on any stock exchange or market.
Will our directors and executive officers participate in the Rights Offering?
To the extent they hold common stock as of the Record Date, our directors and executive officers will be entitled to participate in the Rights Offering on the same terms and conditions applicable to other Rights holders. While none of our directors or executive officers has entered into any binding commitment or agreement to exercise Subscription Rights received in the Rights Offering, certain directors and executive officers have indicated an interest in participating (although the number of Units represented by such indications of interest would not be material to the overall Rights Offering).
Has the board of directors made a recommendation to shareholders and warrant holders regarding the Rights Offering?
No. Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. Stockholders and holders of our Series L warrants who exercise Subscription Rights will incur investment risk on new money invested. We cannot predict the price at which our shares of common stock will trade after the Rights Offering. On February 20, 2015, the closing price of our common stock was $0.73 per share. The market price for our common stock may be above the Subscription Price or may be below the Subscription Price. If you exercise your Subscription Rights, you may not be able to sell the underlying shares of our common stock or Warrants in the future at the same price or a higher price. You should make your decision based on your assessment of our business and financial condition, our prospects for the future, the terms of the Rights Offering and the information contained in this prospectus. See “Risk Factors” for discussion of some of the risks involved in investing in our securities.
How do I exercise my Subscription Rights?
If you are a shareholder of record (meaning you hold your shares of our common stock in your name and not through a broker, dealer, bank, or other nominee) or a holder of our Series L warrants and you wish to participate in the Rights Offering, you must deliver a properly completed and signed Rights Certificate, together with payment of the Subscription Price for both your Basic Subscription Rights and any Over-Subscription Privilege you elect to exercise, to the Subscription Agent before 5:00 p.m., Eastern Time, on April 8, 2015. If you are exercising your Subscription Rights through your broker, dealer, bank, or other nominee, you should promptly contact your broker, dealer, bank, or other nominee and submit your subscription documents and
payment for the Units subscribed for in accordance with the instructions and within the time period provided by your broker, dealer, bank or other nominee.
What if my shares are held in “street name”?
If you hold your shares of our common stock in the name of a broker, dealer, bank, or other nominee, then your broker, dealer, bank, or other nominee is the record holder of the shares you own. The record holder must exercise the Subscription Rights on your behalf. Therefore, you will need to have your record holder act for you.
If you wish to participate in this Rights Offering and purchase Units, please promptly contact the record holder of your shares. We will ask the record holder of your shares, who may be your broker, dealer, bank, or other nominee, to notify you of this Rights Offering.
What form of payment is required?
You must timely pay the full Subscription Price for the full number of Units you wish to acquired pursuant to the exercise of Subscription Rights by delivering to the Subscription Agent a:
If you send payment by personal uncertified check, payment will not be deemed to have been delivered to the Subscription Agent until the check has cleared.
If you send a payment that is insufficient to purchase the number of Units you requested, or if the number of Units you requested is not specified in the forms, the payment received will be applied to exercise your Subscription Rights to the fullest extent possible based on the amount of the payment received.
When will I receive my new shares of common stock and Warrants?
The Subscription Agent will arrange for the issuance of the common stock and Warrants as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. All shares and Warrants that you purchase in the Rights Offering will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of these securities if you are a holder of record of shares or Series L warrants. If you hold your shares in the name of a broker, dealer, bank, or other nominee, DTC will credit your account with your nominee with the securities you purchase in the Rights Offering.
After I send in my payment and Rights Certificate to the Subscription Agent, may I cancel my exercise of Subscription Rights?
No. Exercises of Subscription Rights are irrevocable unless the Rights Offering is terminated, even if you later learn information that you consider to be unfavorable to the exercise of your Subscription Rights. You should not exercise your Subscription Rights unless you are certain that you wish to purchase Units at the Subscription Price.
How much will our company receive from the Rights Offering?
Assuming that all 28,776,419 Units are sold in the Rights Offering, we estimate that the proceeds from the Rights Offering will be approximately $17.8 million, based on the Subscription Price of $0.70 per Unit, after deducting fees and expenses payable to the dealer-managers, and after deducting other expenses payable by us and excluding any proceeds received upon exercise of any Warrants.
Are there risks in exercising my Subscription Rights?
Yes. The exercise of your Subscription Rights involves risks. Exercising your Subscription Rights involves the purchase of additional shares of our common stock and Warrants to purchase common stock and you should consider this investment as carefully as you would consider any other investment. We cannot assure you that the market price of our common stock will exceed the Subscription Price, nor can we assure you that the market price of our common stock will not further decline during or after the Rights Offering. We also cannot assure you that you will be able to sell shares of our common stock or Warrants purchased in the Rights Offering at a price equal to or greater than the Subscription Price. In addition, you should carefully consider the risks described under the heading “Risk Factors” for discussion of some of the risks involved in investing in our securities.
Can the board of directors terminate or extend the Rights Offering?
Yes. Our board of directors may decide to terminate the Rights Offering at any time and for any reason before the expiration of the Rights Offering. We also have the right to extend the Rights Offering for additional periods in our sole discretion. We do not presently intend to extend the Rights Offering. We will notify shareholders and warrant holders if the Rights Offering is terminated or extended by issuing a press release.
If the Rights Offering is not completed or is terminated, will my subscription payment be refunded to me?
Yes. The Subscription Agent will hold all funds it receives in a segregated bank account until completion of the Rights Offering. If we do not complete the Rights Offering, all subscription payments received by the Subscription Agent will be returned within 10 business days after the termination or expiration of the Rights Offering, without interest or penalty. If you own shares in “street name,” it may take longer for you to receive your subscription payment because the Subscription Agent will return payments through the record holder of your shares.
How do I exercise my Rights if I live outside the United States?
The Subscription Agent will hold Rights Certificates for shareholders having addresses outside the United States. To exercise Subscription Rights, foreign shareholders must notify the Subscription Agent and timely follow other procedures described in the section entitled “The Rights Offering – Foreign Shareholders.”
What fees or charges apply if I purchase shares in the Rights Offering?
We are not charging any fee or sales commission to issue Subscription Rights to you or to issue shares or Warrants to you if you exercise your Subscription Rights. If you exercise your Subscription Rights through a broker, dealer, custodian bank, or other nominee, you are responsible for paying any fees your broker, dealer, bank, or other nominee may charge you.
What are the U.S. federal income tax consequences of exercising my Subscription Rights?
For U.S. federal income tax purposes, we do not believe you should recognize income or loss in connection with the receipt or exercise of Subscription Rights in the Rights Offering. You should consult your tax advisor as to the tax consequences of the Rights Offering in light of your particular circumstances. For a detailed discussion, see “Material U.S. Federal Income Tax Consequences.”
To whom should I send my forms and payment?
If your shares are held in the name of a broker, dealer, bank, or other nominee, then you should send your subscription documents and subscription payment to that broker, dealer, bank, or other nominee. If you are the record holder or a holder of our Series L warrants, then you should send your subscription documents, Rights Certificate, and subscription payment to the Subscription Agent hand delivery, first class mail or courier service to:
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
Telephone: (917) 262-2378
You or, if applicable, your nominee are solely responsible for completing delivery to the Subscription Agent of your subscription documents, Rights Certificate and payment. You should allow sufficient time for delivery of your subscription materials to the Subscription Agent and clearance of payment before the expiration of the Rights Offering at 5:00 p.m. Eastern Time on April 8, 2015.
Whom should I contact if I have other questions?
If you have other questions or need assistance, please contact the Information Agent:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
Who are the dealer-managers?
Maxim Group LLC and National Securities Corporation will act as dealer-managers for the Rights Offering. Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will use their best efforts to solicit the exercise of Subscription Rights. We have agreed to pay the dealer-managers for certain fees for acting as dealer-managers and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares of common stock or Warrants.
This summary contains basic information about us and this offering. Because it is a summary, it does not contain all of the information that you should consider before investing. Before you decide to invest in our common stock,Units, you should read this entire prospectus carefully, including the section entitled “Risk Factors,”Factors” and our consolidated financial statements and the related notes.any information incorporated by reference herein.
Our Business
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS) as well as other autoimmune diseases such as neuromyelitis optica (NMO). This therapy isThese therapies are based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM® by aligning the interests of patients, employees and shareholders. Information related to our product candidate,candidates, Tcelna®, and OPX-212, is preliminary and investigative. Tcelna hasand OPX-212 have not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is a chronic, often disablingan inflammatory autoimmune disease that affectsof the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves.nerves, with a clinically heterogeneous and unpredictable course that persists for decades. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. In addition to demyelination, the neuropathology of MS is characterized by variable loss of oligodendroglial cells and axonal degeneration and manifests in neurological deficits. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. This inflammatory, demyelinating, autoimmune disease has varied clinical presentations, ranging from relapses and remissions (relapsing remitting MS, or RRMS) to slow accumulation of disability with or without relapses (secondary progressive MS, or SPMS). There are approximately 450,000 MS patients in North America and over 2,000,000 patients worldwide according to estimates from The progress, severity and specific symptomsNational MS Society. The portion of the MS are unpredictable and vary from one personpatient population that can be classified as SPMS is estimated by various industry sources to another. be between 30-45% of the total MS patient population.
We believe that our lead product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths where demyelination has occurred (remyelination).
Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of Secondary Progressive MS (SPMS)SPMS and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Opexa was incorporated in Texas in March 1991. Our principal executive offices
In addition to our ongoing clinical development of Tcelna, we announced on September 8, 2014, that we are located at 2635 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600.
T-Cell Therapy and Tcelna
Tcelna is a novelalso developing OPX-212 as an autologous T-cell immunotherapy in Phase IIb clinical development for the treatment of NMO. NMO is an autoimmune disorder in which immune system cells and antibodies attack and destroy astrocytic/myelin cells in the optic nerves and the spinal cord leading to demyelination and loss of axons. There are currently no FDA-approved therapies for NMO, other than to treat an attack while it is happening, to reduce symptoms and to prevent relapses. OPX-212 is specifically tailored to each patient’s immune response to a protein, aquaporin-4, which is the targeted antigen in NMO. In NMO, the immune system recognizes aquaporin-4 as foreign, thus triggering the attack. We believe a mechanism of action of OPX-212 may be to reduce the number and/or regulate aquaporin-4 reactive T-cells (ARTCs), thereby reducing the frequency of clinical relapses and subsequent progression in disability. See “—NMO – OPX-212” below for more information on our development plans for OPX-212 in NMO.
Multiple Sclerosis—Background
MS is a disease that is more common in females than males (2:1) between the ages of 20 and 40, with a peak onset of approximately 25 years of age. MS frequently causes impairment of motor, sensory, coordination and balance, visual, and/or cognitive functions, as well as urinary (bladder) or bowel dysfunction and symptoms of fatigue. The identified autoimmune mechanisms directed at myelin tissue of the CNS may play an important role in the pathogenesis of MS. Epidemiologic studies suggest that a variety of genetic, immunologic, and environmental factors including viral infections may play a role in defining the etiology and in triggering the onset and progression of MS.
At the onset of MS, approximately 85% of MS patients with SPMS. Ithave RRMS. Without disease-modifying medication, one-half of these RRMS patients will develop steadily progressive disease, SPMS, within 10 years, increasing to 90% within 25 years of MS diagnosis. The MS drug market was approximately $13 billion in 2012 and is also positionedforecasted to enter Phase III clinicalreach as much as $16 billion by 2015.
MS remains a challenging autoimmune disease to treat because the pathophysiologic mechanisms are diverse, and the chronic, unpredictable course of the disease makes it difficult to determine whether the favorable effects of short-term treatment will be sustained. Therapies that are easy to use and can safely prevent or stop the progression of disease represent the greatest unmet need in MS.
In recent years, the understanding of MS pathogenesis has evolved to comprise an initial, T-cell-mediated inflammatory activity followed by selective demyelination (erosion of the myelin coating of the nerve fibers) and then neurodegeneration. The discovery of disease-relevant immune responses has accelerated the development of targeted therapeutic products for the treatment of patients with relapsing remitting MS (RRMS), subjectthe early stages of MS. Some subjects, who have the appropriate genetic background, have increased susceptibility for the in vivo activation and expansion of MRTCs. These MRTCs may remain dormant, but at some point they are activated in the periphery, thus enabling them to cross the blood-brain barrier and infiltrate the healthy tissue of the brain and spinal cord. The cascade of pathogenic events leads to demyelination of protrusions from nerve cells called axons, which causes nerve impulse transmissions to diffuse into the tissue resulting in disability to the availability of sufficient resources.individual.
Tcelna for MS
We believe that Tcelna works selectively on the MRTCs by harnessing the body’s natural immune defense system and feedback mechanisms to deplete these T-cells and induce favorable immune regulatory responses by rebalancing the immune system. Tcelna is a personalized therapyimmunotherapy that is specifically tailored to each patient’s disease profile. Tcelna is manufactured by using ImmPathTM®, our proprietary
method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and
proteolipid protein (PLP), expanding these MRTCs to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. These attenuated MRTCs are then injected subcutaneously into the returnbody in therapeutic dosages. The body recognizes specific T-cell receptor molecules of these expanded, irradiated T-cells back toMRTCs as immunogenic and initiates an immune response reaction against them, resulting in the patient. These attenuated T-cellsdepletion and/or immunosuppression of circulating MRTCs carrying the peptide-specific T-cell receptor molecules. In addition, we believe that T-cell activation molecules on the surface of the activated MRTCs promote anti-inflammatory responses. We believe that because the therapy uses an individual’s own cells, the only direct identifiable side effect observed thus far is injection site reactions which typically are reintroduced into the patient via subcutaneous injection to triggerminor and generally clear within 24 hours.
Tcelna Clinical Development Program
Tcelna is a therapeutic immune system response.
Abili-T Trial: Phase IIb Clinical Studynovel T-cell immunotherapy in Patients with SPMS
In September 2012, we announced the initiation of a Phase IIb clinical trialdevelopment for the treatment of patients with SPMS. It is also positioned to enter Phase III clinical development for the treatment of patients with RRMS, subject to the availability of sufficient resources or a strategic partnering commitment.
The Tcelna clinical development program spans studies conducted by Baylor College of Medicine and by Opexa.
Summary of Phase I Dose Escalation Study in MS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administering a primary series of four treatments at one of three dose levels administered at baseline and weeks 4, 8 and 12. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. Data from the Phase I study evaluating the Expanded Disability Status Scale (EDSS) showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings were published in Clinical Immunology (2009) 131, 202-215.
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The purpose of this extension study was to continue evaluating the efficacy, safety and tolerability of Tcelna in patients with SPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to EvaluateRRMS and SPMS with repeated administration of Tcelna. Results of the Efficacy and Safetystudy provide evidence of the effectiveness of Tcelna in Subjectsthe treatment of MS with Secondary Progressiverepeated dosing. Improvements from baseline at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations, or annualized relapse rate (ARR). Evaluation of the Multiple Sclerosis Impact Scale (MSIS-29) component scores suggests a trend for Tcelna therapy in the improvement of physical and psychological parameters assessed by the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a result of Tcelna treatment.
Subjects showed statistically significant reduction over baseline in the MRTC counts for each time point through month nine of the extension study. Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has been nameda substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the “Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study did not show statistical significance in SPMS patients who demonstrateits primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using magnetic resonance imaging (MRI) scans summed at various points in the study), the study showed compelling evidence of disease progressionefficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or without associated relapses.high risk Clinically Isolated Syndrome. The trial is expectedinclusion criteria for TERMS was an EDSS score of 0 to enroll 180 patients who have Expanded Disability Status Scale (EDSS) scores between 3.0 and 6.0 at approximately 30 leading clinical sites in the U.S. and Canada. According to the study protocol, patients will receive two annual courses of Tcelna treatment consisting5.5. Patients received a total of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24.
The primary efficacy endpoint of Key results from the TERMS trial is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression as measured by EDSS, changes in EDSS, time to sustained progression, annualized relapse rate (ARR), change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the Multiple Sclerosis Quality of Life Inventory (MSQLI), magnetic resonance imaging (MRI) measures and immune monitoring metrics are also being collected.included:
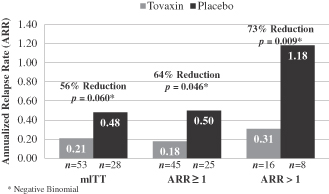
As part ofIn the Abili-T trial, we are undertaking a comprehensive immune monitoring program formodified intent to treat patient population consisting of all patients enrolledwho received at least one dose of study product and had at least one MRI scan at week 28 or later (n=142), the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARR for Tcelna as compared to placebo in the study. The goals of this program aregeneral population;
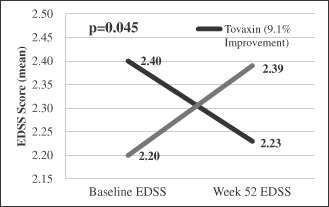
| • | In a prospective group of patients with more active disease (ARR>1, n=50), Tcelna demonstrated a 55% reduction in ARR as compared to placebo, an 88% reduction in whole brain atrophy and a statistically significant improvement in disability (EDSS) compared to placebo (p<0.045) at week 52 during the 24-week period following the administration of the full course of treatment; and |
We remain committed to further understand the biology behind the mechanism of action for Tcelna and to possibly identify novel biomarkers that are dominant in the pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers. We believe that the blinded data, which will be analyzed during the course of the trial, may potentially signal responders and non-responders. Directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression. A summary of pro-inflammatory and anti-inflammatory biomarkers to be studied, with their potential outcomes, is set forth in the graphic below.
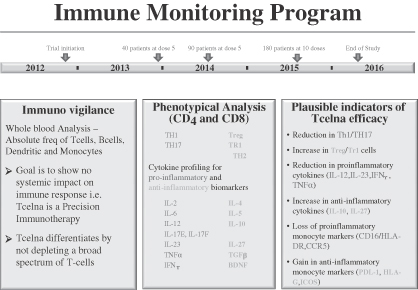
As of July 19, 2013, the Abili-T clinical trial has randomized 65 patients. A scheduled Data Safety Monitoring Board meeting took place during the week of May 20, 2013, and a recommendation was made to continue the study. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016.
During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., for the Option (see “—Option and License Agreement with Merck Serono”) as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that up to $3.6 million of the proceeds from the offering could be used for the repayment of convertible debt and at least $9.8 million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expenses of our operations during such development and for general corporate purposes. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. We believe we have sufficient liquidity to
support our clinical trial activities into the fourth quarter of 2013. Given our need for substantial amounts of capital to continue the Abili-T clinical study in North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financings, that will be necessary to complete the Abili-T study and to support our operations during the pendency of such study.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement with Merck. Pursuant to the agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS.
Under the terms of the agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights to use for other indications outside of MS.
Based upon the achievement of development milestones by Merck for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck elects to develop and commercializeadvancing Tcelna in RRMS we would be eligible to receive milestone payments aggregating up to $40 million based uponat a later date assuming the achievement by Merckavailability of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval andsufficient resources or a strategic partnering commitment. For Opexa, however, SPMS is commercialized by Merck, we would be eligible to receive royalties pursuant to a tiered structure at rates ranging from 8% to 15% of annual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an applicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine fromarea which we originally licensed related technology. If we were to exercise an option to co-fund certain of Merck’s development, the royalty rates payable by Merck would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of such milestones achievable at annual net sales targets in excess of $1 billion.believe represents a higher unmet medical need.
SPMS Overview and Tcelna Mechanism of Action
SPMS is characterized by a steady accrual of irreversible disability, despite, in some cases, relapses followed by remissions or clinical plateaus. Older age at onset of MS diagnosis is the strongest predictor of conversion to SPMS. Males have a shorter time to conversion to SPMS compared with females. Available immunomodulating and immunosuppressive therapies used for RRMS have not been effective in SPMS. In clinical trials, these therapies have demonstrated anti-inflammatory properties as measured by the reduction in number and volume of contrast-enhancing or acutely inflammatory CNS lesions most commonly seen in patients
with RRMS. The typical SPMS patient, however, has little or no radiographic evidence of acute inflammation. It is commonly observed that contrast-enhancing CNS lesions are uncommon among these patients, despite a clearly deteriorating neurologic course.
The lack of effect of conventional MS therapeutics in SPMS suggests that the cerebral deterioration characterizing progressive disease may be driven by factors other than acute inflammation. For instance, the immunopathology of SPMS is more consistent with a transition to a chronic T-cell dependent inflammatory type, which may encompass the innate immune response and persistent activation of microglia cells. Meningeal follicles close to cortical gray matter lesions suggests that adaptive immune responses involving antibody and complement contribute to progression in SPMS. Furthermore, chronic MRTCs may be contributing to the development of both innate and adaptive immune responses persisting in the CNS.
Radiographic features that stand out among patients with SPMS include significantly more atrophy of gray matter compared with RRMS patients. Of note, long-term disability in MS in general appears more closely correlated to gray matter atrophy than to white matter inflammation. Such atrophy may be suggestive of progressive clinical disability. Both clinically and radiographically, SPMS represents a disease process with certain features distinct from those of RRMS, and one with extremely limited treatment options.
Tcelna immunotherapy in SPMS may reduce the drivers of this chronic disease by down-regulating anti-myelin immunity through priming regulatory responses that may act in the periphery as well as within the CNS. We believe that our clinical results show therapeutic subcutaneous dosing of 30-45 million cells of Tcelna stimulates host reactivity to the over-represented MRTCs and, as a consequence, a dominant negative regulatory T-cell response is induced leading to down-regulation of similar endogenous disease-causing MRTCs.
We believe that Tcelna has the potential to induce an up-regulation of regulatory cells, such as Foxp3+ Treg cells and IL-10 secreting Tr1 cells, which may effect a reduction in inflammation and provide neuroprotection should they gain entry to the CNS. Data from our TERMS study showed statistically significant changes from baseline (p=0.02) in Foxp3+ Treg cells for the subset of Tcelna patients who had ARR>1. >1. The placebo arm for this subset was not statistically different from its baseline levels. Three SPMS patients from prior clinical studies, whose blood samples were analyzed to measure Tr1 cells prior to treatment and post treatment, showed an increase in the levels of Tr1 cells from non-detectable levels to the range of healthy donor samples. These three patients who had relapses in the preceding 12-24 month period remained relapse free during the 52-week assessment period and also showed a 57% to 67% reduction in MRTCs.
Current Treatment Options for SPMS
Only one product, mitoxantrone, is currently approved for the indication of SPMS.SPMS in the U.S. However, as ofsince 2005, this drug carries a black box warning, due to significant risks of decreased systolic function, heart failure, and leukemia. The American Academy of Neurology has issued a report indicating that these risks are even higher than suggested in the original report leading to the black box warning. Hence, a safe and effective treatment for SPMS remains a significant unmet medical need.
Tcelna Clinical Overview in SPMS
In multiple previously conducted clinical trials for the treatment of patients with MS (which have been weighted significantly toward patients with RRMS), Tcelna has demonstrated one of the safest side effect profiles for any marketed or development-stage MS therapy, as well as encouraging efficacy signals. A total of 144 MS patients have received Tcelna in previously conducted Opexa trials for RRMS and SPMS. The therapy has been well-tolerated in all subjects and has demonstrated an excellent overall safety profile. The most common side effect is mild to moderate irritation at the site of injection, which is typically resolved in 24 hours. Tcelna has been administered to a total of 36 subjects with SPMS across three previous clinical studies.
In a pooled assessment of data from 36 SPMS patients treated in Phase I open label studies at the Baylor College of Medicine completed in 1998 and in Opexa sponsoredOpexa-sponsored studies completed in 2006 and 2007,
approximately 80% of the 35 SPMS patients who completed two years of treatment showed disease stabilization
as measured by EDSS following two years of treatment with Tcelna, with the other 20% showing signs of progression. This compares to historical control data which showed a progression rate of 40% in SPMS patients (as reported in ESIMS Study published in Hommes Lancet 2004). The 10 SPMS patients in Opexa sponsored studies showed a substantial reduction in ARR at two years from 0.5 to an ARR less than 0.1. Only 1 out of the 10 patients experienced one episode of relapse during the two years of assessment. This same cohort showed no worsening of physical or psychological condition (key quality of life indicators as measured by the MS Impact Scale) after two years of treatment with Tcelna. Additionally, there were no reported serious adverse events (SAEs) in any of the patients. Based on preliminary data suggesting stabilized or improved disability among SPMS subjects receiving Tcelna, we believe that further development of this product candidate in SPMS is warranted.
Summary ofAbili-T Trial: Phase I Dose EscalationIIb Clinical Study in MSPatients with SPMS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administeringIn September 2012, we announced the initiation of a primary series of four treatments at one of three dose levels administered at baseline and weeks four, eight, and twelve. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. The follow-on TERMS Phase IIb clinical study provided further encouraging signs of efficacy in ARR and Multiple Sclerosis Impact Scale (MSIS-29). Data from the Phase I study evaluating the EDSS showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings, which demonstrate that administration of Tcelna induces a reduction in MRTCs, were published in Clinical Immunology (2009) 131, 202-215.
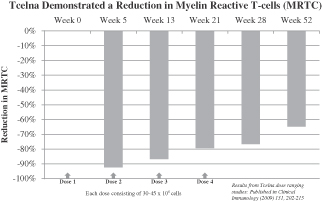
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The
purpose of this extension study was to continue evaluating the efficacy, safety and tolerabilitytrial of Tcelna in patients with RRMSSPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to Evaluate the Efficacy and SPMS with repeated administration of Tcelna. Results of the study provide evidence of the effectivenessSafety of Tcelna in Subjects with Secondary Progressive Multiple Sclerosis and has been named the treatment“Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study in SPMS patients who demonstrate evidence of MSdisease progression with repeated dosing. Improvements from baselineor without associated relapses. The trial is being conducted at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations (ARR). Evaluation of MSIS-29 component scores suggests a trend for Tcelna therapyapproximately 35 leading clinical sites in the improvement of physicalU.S. and psychological parameters assessed byCanada and has enrolled patients who have Expanded Disability Status Scale (EDSS) scores between 3.0 and 6.0. According to the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a resultstudy protocol, patients are receiving two annual courses of Tcelna treatment.
Subjects showed statistically significant improvement over baseline in the MRTC counts for each time point through month nine of the extension study. These results indicate that Tcelna treatment results in a statistically significant impact on these cells.
Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has a substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the study did not show statistical significance in its primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using MRI scans summed at various points in the study), the study showed compelling evidence of efficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or high risk Clinically Isolated Syndrome. Patients received a totalconsisting of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24. Key results from
The primary efficacy endpoint of the TERMS trial included:is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression as measured by EDSS, changes in EDSS, time to sustained progression, ARR, change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the Multiple Sclerosis Quality of Life Inventory (MSQLI), MRI measures and immune monitoring metrics are also being collected.
InAs part of the modified intent to treat patient population consisting ofAbili-T trial, we are undertaking a comprehensive immune monitoring program for all patients who received at least one doseenrolled in the study. The goals of study product and had at least one MRI scan at week 28 or later (n=142),this program are to further understand the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARRbiology behind the mechanism of action for Tcelna as comparedand to placebopossibly identify novel biomarkers that are dominant in the general population;
|
pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers and biomarker data is being gathered during the course of the trial on a blinded basis. We believe that directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement with Ares Trading SA (“Merck Serono”), a wholly owned subsidiary of Merck Serono S.A. Pursuant to the agreement, Merck Serono has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck Serono prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS. Under the terms of the agreement, we received an upfront payment
Inof $5 million for granting the Option. If the Option is exercised, Merck Serono would pay us an upfront license fee of $25 million unless Merck Serono is unable to advance directly into a retrospective analysisPhase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck Serono), in patients naïvewhich event the upfront license fee would be $15 million. After exercising the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to previous disease modifying treatment,co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the results showed that patients, when treated withmanufacture of Tcelna, hadand rights to use for other indications outside of MS.
Based upon the achievement of development milestones by Merck Serono for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a 56%milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck Serono elects to 73% reduction in ARR versus placebo for the various subsetsdevelop and p values ranged from 0.009 to 0.06.
We remain committed to further advancingcommercialize Tcelna in RRMS, we would be eligible to receive milestone payments aggregating up to $40 million based upon the achievement by Merck Serono of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval and is commercialized by Merck Serono, we would be eligible to receive royalties pursuant to a tiered structure at a later date assuming the availabilityrates ranging from 8% to 15% of sufficient resources. For Opexa, however, SPMSannual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an areaapplicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine from which we believe represents a higher unmet medical need. Depending uponoriginally licensed related technology. If we were to exercise an option to co-fund certain of Merck Serono’s development, the outcomeroyalty rates payable by Merck Serono would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of further feasibility analyses, the T-cell platform may have applicationssuch milestones achievable at annual net sales targets in development treatments for other autoimmune disorders such as rheumatoid arthritis, Type 1 diabetes, and orphan indications such as myasthenia gravis. The primary focusexcess of Opexa remains the$1 billion.
NMO – OPX-212
In addition to our ongoing clinical development of Tcelna, we announced on September 8, 2014 that we are also developing OPX-212 as an autologous T-cell immunotherapy for the treatment of NMO. NMO is an autoimmune disorder in SPMS.which immune system cells and antibodies attack astrocytes leading to the secondary destruction of nerve cells (axons) in the optic nerves and the spinal cord. OPX-212 is specifically tailored to each patient’s immune response to a protein, aquaporin-4 expressed by astrocytes, which is the targeted antigen in NMO. In NMO, the immune system recognizes aquaporin-4 as foreign, thus triggering the attack. We believe a mechanism of action of OPX-212 may be to reduce the number and/or regulate aquaporin-4 reactive T-cells (ARTCs), thereby reducing the frequency of clinical relapses and subsequent progression in disability.
Patients with NMO present with acute, often severe, attacks of blindness in one or both eyes followed within days or weeks by varying degrees of paralysis in the arms and legs. Most patients have relapsing attacks (separated by months or years with partial recovery), with usually sequential index episodes of optic neuritis (ON) and myelitis. A relapsing course is more frequent in women, and nearly 90% of patients are female (typically late middle-aged). It is estimated that there are approximately 4,800 cases of NMO in the U.S. NMO has a worldwide estimated prevalence of 1-2 people per 100,000 population.
There are currently no FDA-approved therapies for NMO. An initial attack is usually treated with a combination of corticosteroids and/or by plasma exchange to limit the severity of the attack. Although not approved for NMO, some physicians may utilize an immunosuppressant such as Rituximab as long-term therapy to provide protection from increasing neurological impairments through relapse.
We expect to manufacture OPX-212 using ImmPath, our proprietary method for the production of an autologous T-cell product, which comprises the collection of a blood product from the NMO patient and the expansion of ARTCs from the blood product. Upon completion of the manufacturing process, ARTCs are cryopreserved in dose-equivalents until required for use. On demand, a dose-equivalent is thawed, formulated and attenuated by irradiation before being returned to the patient for subcutaneous injection, with the express purpose of inducing a regulatory immune response to reduce the frequency and/or function of pathogenic ARTCs.
We initiated development activities for OPX-212, our drug development candidate for NMO, earlier this year and have achieved a number of regulatory and early development milestones to date. These include conducting a pre-Investigational New Drug application (pre-IND) meeting with the U.S. FDA and performing in-house manufacturing runs with NMO patient samples. We are continuing with preclinical development activities and expect to file an IND for OPX-212 with the FDA by mid-2015. We believe OPX-212 for NMO will qualify for Orphan drug designation, and we also expect to apply for Fast Track designation.
We believe part of the value of our T-cell platform comes from the ability to move relatively quickly and cost effectively into new autoimmune diseases. We do not expect our ongoing preclinical development activities related to the NMO program to materially affect the Company’s cash burn through IND submission. Assuming successful completion of the preclinical development activities and submission and acceptance of the IND by the FDA and/or CTA by Health Canada, we may thereafter advance into clinical development with a Phase 1/2 proof-of-concept study. We intend to evaluate various options to fund such clinical study, including public or private capital raises as well as potential partnership and out-licensing activities.
Other Opportunities
Our proprietary T-cell technology has enabled us to develop intellectual property and a comprehensive sample database that may enable discovery of novel biomarkers associated with MS.
We have developed (and, in part, licensed from the University of Chicago) a proprietary adult stem cell technology to produce monocyte-derived stem cells (MDSC) from blood. These MDSC can be derived from a patient’s monocytes, expandedex vivo, and then administered to the same patient. Our initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for thein vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus. The diabetes program is in an early (pre-clinical) development stage.
Risks Associated with our Business
We are a development stage company and have generated minimal revenues to date. Since our inception, we have incurred substantial losses. Our business and our ability to execute our business strategy are subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus, and among these important risks are the following:
We will be required to raise significant additional capital in the near-term, and our ability to obtain funding is uncertain. If sufficient capital is not available, we may not be able to continue our operations as proposed (including any Phase IIb clinical trial initiated or ongoing for Tcelna), which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
We have a history of operating losses and do not expect to be profitable in the foreseeable future.
Our business is at an early stage of development. We are largely dependent on the success of our product candidate, Tcelna, and we cannot be certain that Tcelna will receive regulatory approval or be successfully commercialized.
We might be unable to service our debt due to a lack of cash flow or otherwise fail to comply with terms of the convertible secured promissory notes or related agreements and might be subject to default. The convertible secured promissory notes are secured by a pledge of all of our assets. The issuance of securities Depending upon the conversionoutcome of such notes and/orfurther feasibility analysis, the exerciseT-cell platform may have applications in developing treatments for other autoimmune disorders. While the primary focus of warrants issued in tandem with such notes will result in significant dilution for our shareholders.
We have provided Merck withOpexa remains the Option, which provides Merck with the opportunity, if exercised, to control the development and commercialization of Tcelna in MS.
We will need regulatory approvalsSPMS, as well as our development plans for any product candidate, including Tcelna, priorOPX-212 in NMO, we continue to introduction toinvestigate the market, which will require successful testing in clinical trials. Clinical trials are subject to extensive regulatory requirements, and are very expensive, time-consuming and difficult to design and implement. Any product candidate, suchexpansion of the T-cell platform into other autoimmune diseases as Tcelna, may fail to achieve necessary safety and efficacy endpoints during clinical trials in which case we will be unable to generate revenue from the commercialization and salewell as potential in-licensing of our products.other novel technologies.
We will rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that may hamper our ability to successfully develop and commercialize any product candidate, including Tcelna.
We are dependent upon our management team and a small number of employees.
Corporate Information
We were incorporated in Texas in March 1991. Our principal executive offices are located at 2653 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600. Our website address is www.opexatherapeutics.com. The information contained on, or that can be accessed through, our website is not part of this prospectus.
|
|
|
|
up to $3.6 million for potential repayment of certain outstanding indebtedness; and
at least $9.8 million to fund further clinical development of Tcelna and the Phase IIb study in patients with SPMS as well as the ongoing expenses of our operations during such development and for general corporate purposes.
|
|
Unless we indicate otherwise, all information in this prospectus:
is based on 8,114,790 shares of common stock outstanding as of July 19, 2013:
excludes 1,089,500 shares of common stock issuable upon the exercise of stock options outstanding at July 19, 2013 with a weighted average exercise price of $4.46 per share;
excludes 3,069,113 shares of common stock issuable upon the exercise of outstanding warrants at July 19, 2013 with a weighted average exercise price of $4.12 per share;
excludes 1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding at July 19, 2013 are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock;
excludes 48,720 shares of common stock available for future grants under our 2010 Stock Incentive Plan at July 19, 2013;
excludes any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park; and
assumes no exercise by the underwriters of the option to purchase up to 1,395,000 additional shares of common stock from us to cover over-allotments, if any.
Holders of our July 2012 convertible secured promissory notes may purchase shares in this offering and pay the purchase price for such shares in the form of cancellation of principal amount and/or accrued interest on any such promissory note. In such an instance, the net cash proceeds from this offering would be reduced and, equally, the amount of such proceeds that would be needed to repay the indebtedness represented by the notes would also be reduced.
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated statements of operations data for the years ended December 31, 20122014 and 20112013 have been derived from our audited consolidated financial statements that are included in this prospectus. The summary consolidated statements of operations data for the three-month periods ended March 31, 2013 and 2012 and the consolidated balance sheet data as of March 31, 2013 is derived from our unaudited consolidated financial statements that are included indocuments incorporated by reference into this prospectus. The historical financial data presented below is not necessarily indicative of our financial results in future periods, and the results for the three-month period ended March 31, 2013 are not necessarily indicative of our operating results to be expected for the full fiscal year ending December 31, 2013 or any other period.periods. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”Operations” in the documents incorporated by reference into this prospectus. Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP. Our unaudited consolidated financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments, consisting of normal and recurring adjustments that we consider necessary for a fair presentation of the financial position and results of operations as of and for such periods.
| For the year ended December 31, | For the three months ended March 31, | |||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||
Option revenue | $ | — | $ | — | $ | 220,100 | $ | — | ||||||||
Research and development | 6,318,476 | 3,340,038 | 1,621,366 | 1,490,097 | ||||||||||||
General and administrative | 2,508,541 | 2,406,269 | 1,102,435 | 816,196 | ||||||||||||
Depreciation and amortization | 303,677 | 210,252 | 78,311 | 67,355 | ||||||||||||
Loss on disposal of fixed assets | 3,097 | 9,686 | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating loss | (9,133,791 | ) | (5,966,245 | ) | (2,582,012 | ) | (2,373,648 | ) | ||||||||
Interest income | 280 | 932 | 1,874 | 136 | ||||||||||||
Gain on derivative instruments | 552,978 | — | — | — | ||||||||||||
Other income and expense, net | — | — | 37,910 | — | ||||||||||||
Interest expense | (350,300 | ) | (3,135 | ) | (1,635,254 | ) | (487 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (4,177,482 | ) | $ | (2,373,999 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted loss per share | $ | (1.54 | ) | $ | (1.06 | ) | $ | (0.58 | ) | $ | (0.41 | ) | ||||
Weighted average shares outstanding | 5,785,372 | 5,633,124 | 7,239,102 | 5,762,028 | ||||||||||||
The following table presents consolidated balance sheets data as of March 31, 2013 on:
| For the year ended December 31, | ||||||||
| 2014 | 2013 | |||||||
Consolidated Statements of Operations Data: | ||||||||
Revenue: | ||||||||
Option revenue | $ | 1,271,895 | $ | 1,266,611 | ||||
Research and development | 12,118,629 | 9,181,090 | ||||||
General and administrative | 3,833,370 | 3,670,769 | ||||||
Depreciation and amortization | 387,779 | 335,597 | ||||||
Loss on disposal of fixed assets | — | 2,161 | ||||||
|
|
|
| |||||
Operating loss | (15,067,883) | (11,923,006) | ||||||
Interest income | 15,456 | 14,985 | ||||||
Other income and expense, net | 2,147 | 37,910 | ||||||
Loss on extinguishment of debt | — | (2,518,912) | ||||||
|
|
|
| |||||
Interest expense | (1,983) | (2,267,302) | ||||||
|
|
|
| |||||
Net loss | $ | (15,052,263) | $ | (16,656,325) | ||||
|
|
|
| |||||
Basic and diluted loss per share | $ | (0.54) | $ | (1.25) | ||||
Weighted average shares outstanding | 27,821,056 | 13,332,350 | ||||||
an actual basis; and
a pro forma as adjusted basis, giving effect to the sale by us of 9,300,000 shares of common stock in this offering at an assumed public offering price of $1.61 per share which is the last reported sale price of our common stock on July 19, 2013, after deducting the underwriting discount and estimated offering expenses.
| December 31, 2014 | ||||
Consolidated Balance Sheet Data: | ||||
Cash and cash equivalents | $ | 9,906,373 | ||
Other current assets | 758,943 | |||
Property & equipment, net | 1,098,104 | |||
Other long-term assets | 38,939 | |||
Total assets | 11,802,359 | |||
Total current liabilities | 3,132,424 | |||
Deferred revenue | 1,230,748 | |||
Total stockholders’ equity | 7,439,187 | |||
The pro forma as adjusted information set forth below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our consolidated financial statements and notes thereto included in this prospectus.SUMMARY OF THE RIGHTS OFFERING
| As of March 31, 2013 | ||||||||
| Actual | Pro Forma As Adjusted(1) | |||||||
Consolidated Balance Sheet Data: | ||||||||
Cash and cash equivalents | $ | 7,834,336 | $ | 21,214,226 | ||||
Other current assets | 1,166,430 | 1,166,430 | ||||||
Fixed assets, net | 1,189,328 | 1,189,328 | ||||||
Restricted cash | 500,000 | 500,000 | ||||||
Deferred financing costs, net | 158,540 | 158,540 | ||||||
Other long-term assets | 104,027 | 104,027 | ||||||
Total assets | 10,952,661 | 24,332,551 | ||||||
Total current liabilities | 3,157,125 | 3,157,125 | ||||||
Notes payable, net | 376,627 | 376,627 | ||||||
Deferred revenue | 3,384,552 | 3,384,552 | ||||||
Total stockholders’ equity | 4,034,357 | 17,414,247 | ||||||
Securities to be offered |
| Holders of our currently outstanding Series L warrants (which expire February 11, 2017) will also be entitled, pursuant to the terms of such Series L warrants, to receive one Subscription Right to purchase one Unit for every share of common stock with respect to which such Series L warrants are exercisable as of the Record Date. |
Size of offering | 28,776,419 Units, each consisting of one share of common stock and one Warrant representing the right to purchase one share of common stock. |
Subscription Price | $0.70 per Unit. |
Warrants | Each Warrant entitles the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share |
Record Date | 5:00 p.m., Eastern Time, March 13, 2015. |
Subscription Right | Each Subscription Right consists of a Basic Subscription Right and an Over-Subscription Privilege. |
Basic Subscription Rights | Basic Subscription Right will entitle you to purchase one Unit at the Subscription Price. |
Over-Subscription Privilege | If you exercise your Basic Subscription Rights in full, you may also choose to purchase a portion of any Units that are not purchased by our other shareholders or holders of Series L warrants through the exercise of their Basic Subscription Rights. You may subscribe for additional Units pursuant to this Over-Subscription Privilege, subject to proration and stock ownership limitations described elsewhere. |
Expiration date | The Subscription Rights will expire at 5:00 p.m., Eastern Time, on April 8, 2015. We reserve the right to extend the expiration date in our sole discretion. |
Procedure for exercising Subscription Rights | To exercise your Subscription Rights, you must take the following steps: |
| If you are a record holder of our common stock or a holder of Series L warrants, you must deliver payment and a properly completed Rights Certificate to the Subscription Agent to be received before 5:00 p.m., Eastern Time, on April 8, 2015. You may deliver the documents and payments by first class mail or courier service. If you use first class mail for this purpose, we recommend using registered mail, properly insured, with return receipt requested. |
| If you are a beneficial owner of shares |
Delivery of shares and Warrants | As soon as |
DTC will credit your account with your nominee with the securities you purchased in the Rights Offering. |
Non-transferability of Subscription Rights | The Subscription Rights may not be sold, transferred, assigned or given away to anyone. The Subscription Rights will not be listed for trading on any stock exchange or market. |
Transferability of Warrants | The Warrants will be separately transferable following their issuance and through their expiration three years from the issuance date. |
No board recommendation | Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. You are urged to make your decision to invest based on your own assessment of our business and the Rights Offering. Please see “Risk Factors” for a discussion of some of the risks involved in investing in our securities. |
No revocation | All exercises of Subscription Rights are irrevocable, even if you later learn of information that you consider to be unfavorable to the exercise of your Subscription Rights. |
Use of proceeds | Assuming the exercise of Subscription Rights to purchase all 28,776,419 Units of the Rights Offering, after deducting fees and expenses and excluding any proceeds received upon exercise of any Warrants, we estimate the net proceeds of the Rights Offering will be approximately $17.8 million, based on the |
Cash and Cash Equivalents at June 30, 2013
AsMaterial U.S. federal income tax consequences
For U.S. federal income tax purposes, we do not believe you should recognize income or loss upon receipt or exercise of June 30, 2013, we had cash and cash equivalents of approximately $5.0 million, excluding $500,000 of restricted cash which is subjecta Subscription Right. You should consult your own tax advisor as to a deposit control agreement in favor of the holders of our July 2012 convertible notes.tax
consequences of the Rights Offering in light of your particular circumstances. See “Material U.S. Federal Income Tax Consequences.” |
Extension and termination | Although we do not presently intend to do so, we may extend the Rights Offering for additional time in our sole discretion. Our board of directors may for any reason terminate the Rights Offering at any time before the completion of the Rights Offering. |
Subscription Agent | Continental Stock Transfer & Trust Company. |
Information Agent | Advantage Proxy, Inc. |
Questions | If you have any questions about the Rights Offering, please contact the Subscription Agent, Continental Stock Transfer & Trust Company, at (917) 262-2378, or the Information Agent, Advantage Proxy, Inc. at (877) 870-8565 (toll free) or (206) 870-8565 (collect). |
Market for common stock | Our common stock is listed on NASDAQ under the symbol “OPXA.” See “Market Price of our Common Stock and Related Stockholder Matters.” |
Risk factors | Before you exercise your Subscription Rights to purchase Units, you should be aware that there are risks associated with your investment, and you should carefully read and consider risks described in the section captioned “Risk Factors” together with all of the other information included in this prospectus. |
Dealer-Managers | Maxim Group LLC and National Securities Corporation. |
Distribution arrangements | Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will use their best efforts to solicit the exercise of Subscription Rights. We have agreed to pay the dealer-managers certain fees for acting as dealer-managers and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares of common stock or Warrants. |
Investing in our common stocksecurities involves a high degree of risk. You should consider the following risk factors, as well as other information contained in this prospectus, before deciding to invest in our common stock.securities. The following factors affect our business, our intellectual property, the industry in which we operate and our securities. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known or which we consider immaterial as of the date hereof may also have an adverse effect on our business. If any of the matters discussed in the following risk factors were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially adversely affected, the market price of our common stocksecurities could decline and you could lose all or part of your investment in our securities.
Risks Related to Our Business
We will be required to raise significant additional capital, in the near-term, and our ability to obtain funding is uncertain. If sufficient capital is not available, we may not be able to continue our operations as proposed (including anythe Phase IIb clinical trial initiated or ongoing for Tcelna)Tcelna, or any study planned for OPX-212 in NMO), which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
As of MarchDecember 31, 2013 and June 30, 2013,2014, we had cash and cash equivalents of $7,834,336 and approximately $5.0 million, respectively.$9.9 million. During 2012, we closed a private offering in July 2012 consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds (of which $500,000 is held in a controlled account, and $500,000 was released to us in January 2013).proceeds. These convertible secured notes are due July 25, 2014, and to date, notes in the aggregate principal amount of $900,000 have beenwere converted into shares of Series A convertible preferred stock, which in turn, have been converted intoequity during 2013 and an aggregate of 288,2292,002,926 shares of common stock.stock were issued. From November 2012 through January 2013, we sold an aggregate of 390,000 shares of our common stock to Lincoln Park for gross proceeds of $523,709 pursuant to our $1.5 million purchase agreement with Lincoln Park. We closed a private offering of unsecured convertible promissory notes and warrants to purchase common stock in January 2013 which generated $650,000 in gross proceeds. Upon receipt of the upfront payment from Merck Serono in February 2013, we repaid $550,000 principal amount plus accrued interest of the January 2013 notes and converted the remaining $100,000 principal amount into shares of common stock pursuant to the investor’s election to convert into equity. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to a sales agreement executed on September 6, 2012 with Brinson Patrick Securities Corporation acting as sales agent under an “at-the-market” (ATM) program, for gross proceeds of $536,417. On February 4, 2013, we entered into an Option and License Agreement with Merck Serono pursuant to which we granted the Option to Merck Serono to acquire an exclusive, worldwide (excluding Japan) license to our Tcelna program for the treatment of MS in consideration for an upfront payment of $5 million. On February 11, 2013, we closed on an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3,250,002. As part$3.25 million, or net proceeds of thatapproximately $3.0 million after deducting commissions and offering expenses. On August 13, 2013, we closed an offering of 12 million shares of common stock for gross proceeds of $18 million, or net proceeds of approximately $16.2 million after deducting underwriting discounts and commissions and offering expenses, and we granted the underwriters a 30-day option to purchase up to an additional 1.8 million shares of common stock to cover over-allotments. During September 2013, the underwriters of the August 2013 underwritten public offering exercised the over-allotment option granted to them which resulted in the issuance of an additional 900,000 shares of common stock for gross proceeds of $1.35 million, or net proceeds of approximately $1.2 million after deducting underwriting discounts and commissions and offering Lincoln Park did not participate in,expenses. On December 23, 2013, we agreed notclosed an offering of 4,738,000 shares of common stock including the full exercise of the over-allotment option granted to sellthe underwriters, raising gross proceeds of approximately $8.1 million or net proceeds of approximately $7.3 million after deducting the underwriting discounts and commission and offering expenses. Between March 5, 2014 and December 31, 2014, we generated gross and net proceeds including amortization of deferred financing costs of $674,126 and $648,175, respectively, on sales of an aggregate of 518,412 shares of our common stock under an amendment to our ATM sales agreement pursuant to our purchase agreements with Lincoln Park or under our “at-the-market” program forwhich Meyers Associates, L.P. (doing business as Brinson Patrick, a perioddivision of 120 days following that offering (or June 11, 2013).Meyers Associates, L.P.) became the new sales agent.
Our operating cash burn rate during the three12 months ended MarchDecember 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study,2014 was approximately $775,000$1.2 million per month. During this three-month period, the pace of on-boarding clinical sitesWe reached our enrollment target for the Abili-T clinicaltrial in June 2014, and a total of 190 patients have been enrolled in this two-year study. Costs associated with the ongoing Abili-T trial and the potential commencement of a Phase 1/2 proof-of-concept study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. Significant activitiesOPX-212 in the conduct of the Abili-T clinical trial are expected toNMO may result in substantial increasesan increase in our monthly operating cash burn during the balance of 2013. Based onin 2015. However, costs associated with our projectedongoing preclinical and manufacturing activities for OPX-212 should not materially affect our cash burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. However, the Abili-T clinical study in North America of Tcelnathrough IND submission, which is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016.
The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it.mid-2015. We will need to secure significant additional resources to complete the Abili-T trial of Tcelna in SPMS and, if initiated, to conduct a Phase 1/2 proof-of-concept study of OPX-212 in NMO, as well as to support our operations during the pendencycourse of the trial.trials. We believe that we have sufficient liquidity to support our current clinical activities for the Abili-T trial of Tcelna in SPMS, to continue planned preclinical development activities for OPX-212 in NMO with a possible IND submission by mid-2015, and for general operations to sustain the Company and support such activities into the fourth quarter of 2015. Any other costs, however, such as costs associated with the initiation and completion of a Phase 1/2 proof-of-concept study of OPX-212 in NMO or with pursuing additional disease indications for our T-cell technology or other research or development programs, would of course shorten this period.
Given our need for substantial amounts of capital to complete the Phase IIbAbili-T clinical study forof Tcelna in SPMS and to initiate and complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financing transactions, that will be necessary to complete the Phase IIb studystudies and to support our operations during the pendency of such study. These opportunities and alternatives may include one or more additional financing transactions.studies. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. If we are unable to obtain additional funding for operations inbeyond the immediate future,projected runway, we will be forced to suspend or terminate ourany ongoing clinical trial for Tcelna,trials, which may require us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws.
Assuming we are able to achieve financing which is sufficient to support the Phase IIb study of Tcelna in SPMS and to support our operations during the pendency of such study, we are also exploring a pivotal Phase III clinical study of Tcelna in RRMS. Any such study of Tcelna in RRMS would also depend upon the availability of sufficient resources.
Other than the $1.5 million purchase agreement and the $15$15.0 million purchase agreement we entered into with Lincoln Park on November 5, 2012 and November 2, 2012, respectively, each of which is subject to certain limitations and conditions, we have no sources of debt or equity capital committed for funding and we must rely upon best efforts third-party debt or equity funding. We can provide no assurance that we will be successful in any funding effort. The timing and degree of any future capital requirements will depend on many factors, including:
our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing;
the accuracy of the assumptions underlying our estimates for capital needs in 20132014 and beyond as well as for the clinical study of Tcelna;
scientific progress in our research and development programs;
the magnitude and scope of our research and development programs;
our progress with preclinical development and clinical trials;
the time and costs involved in obtaining regulatory approvals;
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and
the number and type of product candidates that we pursue.
If we raise additional funds by issuing equity securities, (including pursuant to the $1.5 million purchase agreement and the $15 million purchase agreement with Lincoln Park), shareholders may experience substantial dilution. Debt financing, if available, may involve restrictive covenants that may impede our ability to operate our business. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our shareholders. There is no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations.
If we are unable to obtain additional funding to support our current clinical trial activities beyond the projected runway, we may not be able to continue or complete the Phase IIb clinical study of Tcelna in SPMS
and, if initiated, to continue or complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, or otherwise continue our operations as proposed, which may require us to
modify our business plan or curtail various aspects of our operations. If we are unable to maintain an adequate level of capital, it may be necessary to cease operations or seek relief under applicable bankruptcy laws. In such event, our shareholders may lose a portion or even all of their investment.
We may experience delays in our clinical trial enrollment, which could result in increased costs to us.
Once a clinical trial has begun, recruitment and enrollment of subjects may be slower than we anticipate. In addition, clinical trials may take longer than we anticipate if we are required, or believe it is necessary, to enroll additional subjects. Our ongoing Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. These estimated costs are partially a function of the pace of clinical trial enrollment, and should enrollment timelines get delayed beyond our current expectation, the estimated costs are likely to increase due to the additional operational expenses. Similarly, should additional patients be enrolled in the trial, the costs are likely to increase.
We may make changes to discretionary R&D investments that may have an impact on costs.
We are presently complementing the Abili-T clinical trial with an immune monitoring program. Expenses associated with the immune monitoring program are incurred at our discretion and are not required to satisfy any FDA-mandated criteria. Consequently, we may make changes to the parameters that are being analyzed, and these changes may result in either increased or decreased expenses for the study.
We may also incur discretionary expenses related to Phase I, Phase II and/or Phase III development programs, manufacturing scale-up/automation and technology transfer, in the future.research on additional indications and business development activities. There is no assurance that any such future expenses would be recovered by us.
Funding from our purchase agreements with Lincoln Park and our ATM facility may be limited or be insufficient to fund our operations or to implement our strategy.
Under our $1.5 million purchase agreement and our $15$15.0 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. However, in connection with our February 2013 common stock and warrant offering, we agreed not to sell shares under the purchase agreements with Lincoln Park for a period of 120 days after that offering (or June 11, 2013). From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement, and we issued an aggregate of 56,507 initial commitment shares and 3,585 additional commitment shares in connection therewith. There can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the $1.5 million purchase agreement and the $15$15.0 million purchase agreement contain limitations, restrictions, requirements, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us, including that the closing price of our stock is at least $1.00 and that Lincoln Park own no more than 4.99% of our common stock under the $1.5 million purchase agreement or no more than 9.99% of our common stock under the $15$15.0 million purchase agreement.agreement, and the requirement to keep current the prospectus included as part of the Form S-1 registration statement relating to the $15.0 million purchase agreement (which is not current as of this date). In addition, under the applicable rules of the NASDAQ Capital Market, if we seek to issue shares which may be aggregated with shares sold to Lincoln Park under the $1.5 million purchase agreement and the $15$15.0 million purchase agreement in excess of 1,151,829 shares or 19.99% of the total common stock outstanding as of the date of the $15$15.0 million purchase agreement, we may be required to seek shareholder approval in order to be in compliance with the NASDAQ Capital Market rules.
The extent to which we rely on Lincoln Park as a source of funding will depend on a number of factors, including the amount of working capital needed, the prevailing market price of our common stock and the extent
to which we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we would need to secure another source of funding. Even if
We will need to keep current the offering prospectus relating to the new ATM Agreement with Brinson Patrick, a division of Meyers Associates, L.P., in order to use the program to sell shares of our common stock. The number of shares and price at which we may be able to sell all $16.5 million of common stockshares under the purchase agreements with Lincoln Park, we will still need additional capitalnew ATM Agreement may be limited due to fully implementmarket conditions and other factors beyond our business, operating plans and development plans as of the date hereof, including to complete the Phase IIb clinical study of Tcelna in patients with SPMS and to conduct our operations through the expected completion date of such study.control.
We have a history of operating losses and do not expect to be profitable in the foreseeable future.
We have not generated any profits since our entry into the biotechnology business and we have incurred significant operating losses. We expect to incur additional operating losses for the foreseeable future. We have
not received, and we do not expect to receive for at least the next several years, any revenues from the commercialization of any potential products. We do not currently have any sources of revenues as of the date hereof and may not have any in the foreseeable future.
There is substantial doubt as to our ability to continue as a going concern, which may make it more difficult for us to raise capital.
Our consolidated financial statements as of March 31, 2013 and for the three-month period then ended were prepared assuming that we will continue as a going concern, meaning that we will continue in operation for the foreseeable future and will be able to realize assets and discharge liabilities in the ordinary course of operations. We recognized $220,100 in revenue during the three-month period ended March 31, 2013 related to the $5 million upfront payment from Merck received in February 2013 in connection with the Option and License Agreement. We expect to continue recording revenue related to the $5 million upfront payment from Merck over the exclusive option period based on the expected completion term of the Abili-T clinical trial. However, we do not currently generate any additional revenues resulting in cash receipts, nor do we expect to generate revenues during the remainder of 2013 resulting in cash receipts. Our cash burn rate during the three months ended March 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study, was approximately $775,000 per month. Significant activities in the conduct of the clinical trial are expected to result in substantial increases in our monthly cash burn during the balance of 2013. Based on our projected burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. In the absence of significant additional funding, there is substantial doubt about our ability to continue as a going concern. This may make it more difficult for us to raise funds. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures or to generate revenue. If we are unable to obtain additional financing for our operations, we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations, cease operations or seek relief under applicable bankruptcy laws. In such event, investors may lose a portion or all of their investment. Our consolidated financial statements contain no adjustment for the outcome of this uncertainty.
Our business is at an early stage of development. We are largely dependent on the success of our lead product candidate, Tcelna, and we cannot be certain that Tcelna will receive regulatory approval or be successfully commercialized.
Our business is at an early stage of development. We do not have any product candidates that have completed late-stage clinical trials nor do we have any products on the market. We have only one product candidate, Tcelna, which has progressed to the stage of being studied in human clinical trials in the United States. In September 2012, weWe announced the initiation of a Phase IIb study of Tcelnaearly development activities with our second pipeline candidate, OPX-212 in patients with SPMS. We are still in the very early stages of identifying and conducting research on any other potential products.NMO. Tcelna, and any other potential products, including OPX-212, will require regulatory approval prior to marketing in the United States and other countries. Obtaining such approval requires significant research and development and preclinical and clinical testing. We may not be able to develop any products, to obtain regulatory approvals, to continue clinical
development of Tcelna, to enter clinical trials (or any development activities) for any other product candidates (such as OPX-212) or to commercialize any products. Tcelna, and any other potential products (such as OPX-212), may prove to have undesirable or unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits or to achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production.
We might be unable to service our debt due to a lack of cash flow or otherwise fail to comply with terms of the convertible secured promissory notes or related agreements and might be subject to default. The convertible secured promissory notes are secured by a pledge of all of our assets. The issuance of securities upon the conversion of such notes and/or the exercise of warrants issued in tandem with such notes will result in significant dilution for our shareholders.
On July 25, 2012, we closed a private offering consisting of 12% convertible secured notes and warrants to purchase shares of common stock which generated approximately $4.1 million in gross proceeds ($500,000 of which is held in a controlled account). The notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually in either cash or registered shares of common stock at our election. The notes are secured by substantially all of our assets and are convertible into a new class of non-voting Series A convertible preferred stock. The notes can be converted into Series A convertible preferred stock at the option of the investors at a price of $100.00 per share, subject to certain limitations and adjustments. Additionally, we can elect to convert the notes into Series A convertible preferred stock if (i) our common stock closes at or above $10.00 per share for 20 consecutive trading days or (ii) we achieve certain additional funding milestones to continue our clinical trial program. These milestones include (x) executing a strategic agreement with a partner or potential partner by which we will receive a minimum of $5 million to partially fund, or an option to partner with us for, our Phase II clinical trial for Tcelna in patients with SPMS and (y) receiving a minimum of $25 million in additional capital (including the note offering proceeds) from any partner, potential partner or any other source. The Series A convertible preferred stock accrues dividends at the rate of 8% per annum, which are cumulative and payable semi-annually in either cash or registered shares of the common stock at our election. The Series A convertible preferred stock is convertible into shares of our common stock at the option of the holders at a price of $3.1225 per share, subject to certain limitations and adjustments. Additionally, we can elect to convert the Series A convertible preferred stock into common stock if our common stock closes at or above $16.00 per share for 20 consecutive trading days. To date, secured promissory notes in the aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock which, in turn, have been converted into an aggregate of 288,229 shares of common stock. No shares of Series A convertible preferred stock are outstanding as of the date hereof.
As a result of anti-dilution adjustments since the closing of the July 2012 secured promissory note financing, up to 1,020,007 shares of common stock are issuable if all of the 12% convertible secured promissory notes outstanding as of the date hereof are converted to Series A convertible preferred stock and such stock is then converted into shares of our common stock. The noteholders were granted certain registration rights for the shares of common stock underlying the notes and the warrants issued in July 2012.
The warrants have a five-year term and, as a result of anti-dilution adjustments since the closing of the July 2012 secured promissory note financing, (i) have an adjusted exercise price of $2.56 per share and (ii) are exercisable for an aggregate of 1,436,121 shares of common stock. We can redeem the warrants at $0.01 per share if our common stock closes at or above $10.00 per share for 20 consecutive trading days.
As part of the security interest in all of our assets granted to the noteholders, $500,000 of the proceeds is maintained as of the date hereof in a controlled account. This amount was previously $1 million; however, in January 2013 we issued the noteholders five-year warrants to acquire an aggregate of 187,500 shares of our common stock at an exercise price of $1.21 per share in exchange for the reduction of such amount.
If we do not make the required payments when due, either at maturity, or at applicable installment payment dates, or if we breach other terms of the convertible secured notes or related agreements, the noteholders could elect to declare all amounts outstanding, together with accrued and unpaid interest, to be immediately due and payable. Even if we were able to prepay the full amount in cash, any such repayment could leave us with little or no working capital for our business. If we are unable to repay those amounts, the noteholders will have a first claim on our assets pledged under the convertible secured notes. If the noteholders should attempt to foreclose on the collateral, it is unlikely that there would be any assets remaining after repayment in full of such secured indebtedness. Any default under the convertible secured notes and resulting foreclosure would have a material adverse effect on our financial condition and our ability to continue our operations.
We have provided Merck Serono with the Option, which provides Merck Serono with the opportunity, if exercised, to control the development and commercialization of Tcelna in MS.
In February 2013, we granted the Option to Merck.Merck Serono. The Option permits Merck Serono to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck Serono prior to or upon completion of our ongoing Phase IIb trial of Tcelna in patients with SPMS. If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS. In consideration for the Option, we received an upfront payment of $5 million and may be eligible to receive an option exercise fee as well as milestone and royalty payments based on achievement of development and commercialization milestones. The rights we have relinquished to our product candidate Tcelna, including development and commercialization rights, may harm our ability to generate revenues and achieve or sustain profitability.
If Merck Serono exercises the Option, we would become reliant on Merck’sMerck Serono’s resources and efforts with respect to Tcelna in MS. In such an event, Merck Serono may fail to develop or effectively commercialize Tcelna for a variety of reasons, including that Merck:Merck Serono:
does not have sufficient resources or decides not to devote the necessary resources due to internal constraints such as limited cash or human resources;
decides to pursue a competitive potential product;
cannot obtain the necessary regulatory approvals;
determines that the market opportunity is not attractive; or
cannot manufacture or obtain the necessary materials in sufficient quantities from multiple sources or at a reasonable cost.
If Merck Serono does not exercise the Option, we may be unable to enter into a collaboration with any other potential partner on acceptable terms, if at all. We face competition in our search for partners from other
organizations worldwide, many of whom are larger and are able to offer more attractive deals in terms of financial commitments, contribution of human resources, or development, manufacturing, regulatory or commercial expertise and support.
If Merck Serono does not exercise the Option, and we are not successful in attracting another partner and entering into collaboration on acceptable terms, we may not be able to complete development of or commercialize any product candidate, including Tcelna. In such event, our ability to generate revenues and achieve or sustain profitability would be significantly hindered and we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations or cease operations.
We will need regulatory approvals for any product candidate, including Tcelna, prior to introduction to the market, which will require successful testing in clinical trials. Clinical trials are subject to extensive regulatory requirements, and are very expensive, time-consuming and difficult to design and implement. Any product candidate, such as Tcelna, may fail to achieve necessary safety and efficacy endpoints during clinical trials in which case we will be unable to generate revenue from the commercialization and sale of our products.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous FDA requirements, and must otherwise comply with federal, state and local requirements and policies of the medical institutions where they are conducted. The clinical trial process is also time-consuming. We estimate thatreached our enrollment target for the Phase IIb clinicalAbili-T trial in North AmericaJune 2014, and a total of our lead product candidate, Tcelna,190 patients have been enrolled in SPMS will complete enrollment by late 2013 or early 2014, with the resultingthis two-year study. We expect top-line data expectedfor Tcelna to be available in the firstsecond half of 2016. In addition, we anticipate that at least a pivotal Phase III clinical trial would be necessary before an application could be submitted for approval of Tcelna for SPMS. Failure can occur at any stage of the trials, and problems could be encountered that would cause us or Merck Serono (in the event the Option is exercised) to be unable to initiate a trial, or to abandon or repeat a clinical trial.
The commencement and completion of clinical trials, including the continuation and completion of the Phase IIb clinical trial of Tcelna in SPMS, may be delayed or prevented by several factors, including:
FDA or IRB objection to proposed protocols;
discussions or disagreement with the FDA over the adequacy of trial design to potentially demonstrate effectiveness, and subsequent design modifications;
unforeseen safety issues;
determination of dosing issues, epitope profiles, and related adjustments;
lack of effectiveness during clinical trials;
slower than expected rates of patient recruitment;
product quality problems (e.g., sterility or purity);
challenges to patient monitoring, retention and data collection during or after treatment (for example,(e.g., patients’ failure to return for follow-up visits)visits or to complete the trial, detection of epitope profiles in subsequent visits, etc.); and
failure of medical investigators to follow our clinical protocols.
In addition, we, Merck Serono with respect to Tcelna (if the Option is exercised) or the FDA (based on its authority over clinical studies) may delay a proposed investigation or suspend clinical trials in progress at any time if it appears that the study may pose significant risks to the study participants or other serious deficiencies are identified. Prior to approval of any product candidate, the FDA must determine that the data demonstrate safety and effectiveness. The large majority of drug candidates that begin human clinical trials fail to demonstrate the desired safety and efficacy characteristics.
Furthermore, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols, or otherwise modify our intended course of clinical development, to reflect these changes. This, too, may impact the costs, timing or successful completion of a clinical trial. In light of widely publicized
events concerning the safety risk of certain drug products, regulatory authorities, members of Congress, the U.S. Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products, and establishment of risk management programs that may, for instance, restrict distribution of drug products. The increased attention to drug safety issues may result in a more cautious approach by the FDA to clinical trials. Data from clinical trials may receive greater scrutiny with respect to safety, which may make the FDA or other regulatory authorities more likely to terminate clinical trials before
completion or require longer or additional clinical trials that may result in substantial additional expense and a delay or failure in obtaining approval or approval for a more limited indication than originally sought.
Even if regulatory approval is obtained for any product candidate, such as Tcelna, any such approval may be subject to limitations on the indicated uses for which it may be marketed. Our ability to generate revenues from the commercialization and sale of any potential products, whether directly or through any development arrangement (such as where Merck Serono exercises the Option) will be limited by any failure to obtain or limitation on necessary regulatory approvals.
If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates.
We will rely on third parties to conduct our clinical trials and perform data collection and analysis, which may result in costs and delays that may hamper our ability to successfully develop and commercialize any product candidate, including Tcelna.
Although we have participated in the design and management of our past clinical trials, we do not have the ability to conduct clinical trials directly for any product candidate, including Tcelna. We will need to rely on contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials and to perform data collection and analysis, including the Phase IIb trial of Tcelna in patients with SPMS.
Our clinical trials may be delayed, suspended or terminated if:
any third party upon whom we rely does not successfully carry out its contractual duties or regulatory obligations or meet expected deadlines;
licenses needed from third parties for manufacturing in order to conduct Phase III trials or to conduct commercial manufacturing, if applicable, are not obtained;
any such third party needs to be replaced; or
the quality or accuracy of the data obtained by the third party is compromised due to its failure to adhere to clinical protocols or regulatory requirements or for other reasons.
Failure to perform by any third party upon whom we rely may increase our development costs, delay our ability to obtain regulatory approval and prevent the commercialization of any product candidate, including Tcelna. While we believe that there are numerous alternative sources to provide these services, we might not be able to enter into replacement arrangements without delays or additional expenditures if we were to seek such alternative sources.
If we fail to identify and license or acquire other product candidates, we will not be able to expand our business over the long term.
We have targetedfocused on MS as the first disease to be pursued off our T-cell platform technology.technology, and we announced the initiation of NMO as the second disease we are pursuing. As a platform technology, there exists the potential to address other autoimmune diseases with the technology. MinimalEarly development activities including
preclinical and manufacturing activities have been initiated in OPX-212. The work in NMO is modest compared to the effort that has been done outsidecommitted to the lead MS indication. Our business over the long term is substantially dependent on our ability to develop, license or acquire product candidates and further develop them for commercialization. The success of this strategy depends upon our ability to expand our existing platform or identify, select and acquire the right product candidates. We have limited experience identifying, negotiating and implementing economically viable product candidate acquisitions or licenses, which is a lengthy and complex process. Also, the market for licensing and acquiring product candidates is intensely competitive, and many of our competitors have greater resources than we do. We may not have the requisite capital resources to consummate product candidate acquisitions or licenses that we identify to fulfill our strategy.
Moreover, any product candidate acquisition that we do complete will involve numerous risks, including:
difficulties in integrating the development program for the acquired product candidate into our existing operations;
diversion of financial and management resources from existing operations;
risks of entering new potential markets or technologies;
inability to generate sufficient funding to offset acquisition costs; and
delays that may result from our having to perform unanticipated preclinical trials or other tests on the product candidate.
We are dependent upon our management team and a small number of employees.
Our business strategy is dependent upon the skills and knowledge of our management team. If any critical employee leaves, we may be unable on a timely basis to hire suitable replacements to operate our business effectively. We also operate with a very small number of employees and thus have little or no backup capability for their activities. The loss of the services of any member of our management team or the loss of just a few other employees could have a material adverse effect on our business and results of operations.
If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on licenses from third parties. These third party license agreements impose obligations on us, such as payment obligations and obligations diligently to pursue development of commercial products under the licensed patents. We may also need to seek additional licenses as we move into Phase III trials and, if applicable, the commercial stage of operations. These licenses may require increased payments to the licensors. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation, our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology platform could be adversely affected.
Our research and manufacturing facility is not large enough to manufacture product candidates, such as Tcelna, for certain clinical trials or, if such clinical trials are successful, commercial applications.
We conduct our research and development in a 10,200 square foot facility in The Woodlands, Texas, which includes an approximately 1,200 square foot suite of three rooms for the manufacture of T-cell therapies. We believe our facility should have the capacity to support full clinical development of Tcelna in North American trials for SPMS.SPMS and, if applicable, a Phase 1/2 proof-of-concept study of OPX-212 in NMO. It is not sufficient, however, to support clinical trials outside North America including Europe and Asia, if required, or the commercial launch of Tcelna.Tcelna or any other product candidate. In this case, we would need to expand our
manufacturing staff and facility, obtain a new facility, contract with corporate collaborators or other third parties to assist with future drug production and commercialization, or defer to Merck Serono (in the event the Option is exercised) to address manufacturing requirements.
In the event that we decide to establish a commercial-scale manufacturing facility, we will require substantial additional funds and will be required to hire and train significant numbers of employees and comply with applicable regulations, which are extensive. We do not have funds available for building a manufacturing facility, and we may not be able to build a manufacturing facility that both meets regulatory requirements and is sufficient for our commercial-scale manufacturing.
We may arrange with third parties for the manufacture of our future products, if any. However, our third-party sourcing strategy may not result in a cost-effective means for manufacturing our future products. If we
employ third-party manufacturers, we will not control many aspects of the manufacturing process, including compliance by these third parties with current Good Manufacturing Practice (cGMP)cGMP and other regulatory requirements. We further may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
Problems with our manufacturing process or with a manufacturing facility (whether ours or a third party’s) could result in the failure to produce, or a delay in producing, adequate supplies of a product candidate such as Tcelna. A number of factors could cause interruptions or delays, including equipment malfunctions or failures, destruction or damage to a manufacturing facility due to natural disasters or otherwise, contamination of materials, changes in regulatory requirements or standards that require modifications to our manufacturing process, action by a regulatory agency or by a manufacturer (whether us or a third party) that results in the halting or slowdown of production due to regulatory issues, any third-party manufacturer going out of business or failing to produce as contractually required, or other similar factors.
Difficulties, delays or interruptions in the manufacture and supply of a product candidate such as Tcelna could require us to stop treating patients in our clinical development of such product candidate and/or require a halt to or suspension of, or otherwise adversely affect, a clinical trial, thus increasing our costs and damaging our reputation. If a product candidate such as Tcelna is approved, difficulties, delays or interruptions in the manufacture and supply of such product candidate could cause a delay in or even halt or suspend the commercialization of such product candidate, potentially causing a partial or complete loss of revenue or market share.
Tcelna is manufactured using our proprietary ImmPath® technology for the production of an autologous T-cell immunotherapy utilizing a patient’s own blood. Our manufacturing process may raise development issues that may not be resolvable, regulatory issues that could delay or prevent approval, or personnel issues that may prevent the further development or commercialization, if approved, of any product candidate such as Tcelna.
Tcelna is based on our novel T-cell immunotherapy platform, ImmPath, which produces an autologous T-cell immunotherapy utilizing a patient’s own blood. OPX-212 is expected to be similarly produced. The manufacture of living T-cell products requires specialized facilities, equipment and personnel which are different than the resources required for manufacturing chemical or biologic compounds. Scaling-out the manufacture of living cell products to meet demands for commercialization will require substantial amounts of such specialized facilities, equipment and personnel, especially where, as is the case for Tcelna and expected to be the case for OPX-212, the products are personalized and must be made for each patient individually. Because our manufacturing processes are complex, require facilities and personnel that are not widely available in the industry, involve equipment and training with long lead times, and the establishment of new manufacturing facilities is subject to a potentially lengthy regulatory approval process, alternative qualified production capacity may not be available on a timely basis or on reasonably terms, if at all. In addition, not many consultants or
advisors in the industry have relevant experience and can provide guidance or assistance because active immune therapies such as Tcelna are fundamentally a new category of product in two major ways: (i) the product consists of living T-cells, not chemical or biologic compounds; and (ii) the product is personalized. There can be no assurance that manufacturing problems will not arise in the future which we may not be able to resolve or which may cause significant delays in development or, if any product candidate such as Tcelna is approved, commercialization.
Regulatory approval of product candidates such as Tcelna that are manufactured using novel manufacturing processes such as ours can be more expensive and take longer than other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to a lack of experience with them. FDA approval of personalized immunotherapy products has been limited to date. This lack of experience and precedent may lengthen the regulatory review process, require that additional studies or clinical trials be conducted, increase development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization, or lead to significant post-approval limitations or restrictions.
In addition, the novel nature of product candidates such as Tcelna also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
If any product we may eventually have is not accepted by the market or if users of any such product are unable to obtain adequate coverage of and reimbursement for such product from government and other third-party payors, our revenues and profitability will suffer.
In the instance of Tcelna, if Merck Serono exercises the Option then our ability to achieve revenue will be dependent upon the efforts and success of Merck Serono in developing and commercializing Tcelna. Our ability to successfully commercialize any product we may eventually have, to the extent applicable, and/or our ability to receive any revenue associated with Tcelna in the event Merck Serono exercises the Option, will depend in significant part on the extent to which appropriate coverage of and reimbursement for such product and any related treatments are obtained from governmental authorities, private health insurers and other organizations, such as health maintenance organizations, or HMOs. Third-party payors are increasingly challenging the prices charged for medical products and services. We cannot provide any assurances that third-party payors will consider any product cost-effective or provide coverage of and reimbursement for such product, in whole or in part.
Uncertainty exists as to the coverage and reimbursement status of newly approved medical products and services and newly approved indications for existing products. Third-party payors may conclude that any product is less safe, less clinically effective, or less cost-effective than existing products, and third-party payors may not approve such product for coverage and reimbursement. If adequate coverage of and reimbursement for any product from third-party payors cannot be obtained, physicians may limit how much or under what circumstances they will prescribe or administer them. Such reduction or limitation in the use of any such product would cause sales to suffer. Even if third-party payors make reimbursement available, payment levels may not be sufficient to make the sale of any such product profitable.
In addition, the trend towards managed health care in the United States and the concurrent growth of organizations such as HMOs, which could control or significantly influence the purchase of medical services and products, may result in inadequate coverage of and reimbursement for any product we may eventually have. Many third-party payors, including in particular HMOs, are pursuing various ways to reduce pharmaceutical costs, including, for instance, the use of formularies. The market for any product depends on access to such formularies, which are lists of medications for which third-party payors provide reimbursement. These formularies are increasingly restricted, and pharmaceutical companies face significant competition in their efforts to place their products on formularies of HMOs and other third-party payors. This increased competition has led to a downward pricing pressure in the industry. The cost containment measures that third-party payors are instituting could have a material adverse effect on our ability to operate profitably.
Any product candidate, such as Tcelna, if approved for sale, may not gain acceptance among physicians, patients and the medical community, thereby limiting our potential to generate revenues.
Even if a product candidate, such as Tcelna, is approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product candidate by physicians, healthcare professionals and third-party payors, and our profitability and growth, will depend on a number of factors, including:
demonstration of efficacy;
relative convenience and ease of administration;
the prevalence and severity of any adverse side effects;
availability and cost of alternative treatments, including cheaper generic drugs;
pricing and cost effectiveness, which may be subject to regulatory control;
effectiveness of sales and marketing strategies for the product and competition for such product;
the product labeling or product insert required by the FDA or regulatory authority in other countries; and
the availability of adequate third-party insurance coverage or reimbursement.
If any product candidate does not provide a treatment regimen that is as beneficial as the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance and our ability to generate revenues from that product candidate would be substantially reduced.
We have incurred, and expect to continue to incur, increased costs and risks as a result of being a public company.
As a public company, we are required to comply with the Sarbanes-Oxley Act of 2002, or SOX, as well as rules and regulations implemented by the SEC and The NASDAQ Stock Market (NASDAQ). Changes in the laws and regulations affecting public companies, including the provisions of SOX and rules adopted by the SEC and by NASDAQ, have resulted in, and will continue to result in, increased costs to us as we respond to their requirements. Given the risks inherent in the design and operation of internal controls over financial reporting, the effectiveness of our internal controls over financial reporting is uncertain. If our internal controls are not designed or operating effectively, we may not be able to conclude an evaluation of our internal control over financial reporting as required or we or our independent registered public accounting firm may determine that our internal control over financial reporting was not effective. In addition, our registered public accounting firm may either disclaim an opinion as it relates to management’s assessment of the effectiveness of our internal controls or may issue an adverse opinion on the effectiveness of our internal controls over financial reporting. Investors may lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and which could affect our ability to run our business as we otherwise would like to. New rules could also make it more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the coverage that is the same or similar to our coverage as of the date hereof.current coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees and as executive officers. We cannot predict or estimate the total amount of the costs we may incur or the timing of such costs to comply with these rules and regulations.
Under the corporate governance standards of NASDAQ, a majority of our Board of Directors and each member of our Audit Committeeand Compensation Committees must be an independent director. If any vacancies on our Board or our Audit Committeeor Compensation Committees occur that need to be filled by independent directors, we may encounter difficulty in attracting qualified persons to serve on our Board and, in particular, our Audit Committee. If we fail to attract and retain the required number of independent directors, we may be subject to SEC enforcement proceedings and delisting of our common stock from the NASDAQ Capital Market.
Any acquisitions that we make could disrupt our business and harm our financial condition. We expect to evaluate potential strategic acquisitions of complementary businesses, products or technologies on a global geographic footprint. We may also consider joint ventures, licensing and other collaborative projects. We may not be able to identify appropriate acquisition candidates or strategic partners, or successfully negotiate, finance or integrate acquisitions of any businesses, products or technologies. Furthermore, the integration of any acquisition and management of any collaborative project may divert our management’s time and resources from our core business and disrupt our operations. We do not have any experience with acquiring companies, or with acquiring products outside of the United States. Any cash acquisition we pursue would potentially divert the cash we have on our balance sheet from our present clinical development programs. Any stock acquisitions would dilute our shareholders’ ownership. While we from time to time evaluate potential collaborative projects and acquisitions of businesses, products and technologies, and anticipate continuing to make these evaluations, we have no present commitments or agreements with respect to any acquisitions or collaborative projects. Risks Related to Doing Business Internationally We plan to do business internationally, which may prove to be difficult and fraught with economic, regulatory and political issues. We may acquire or in-license foreign companies or technologies or commercialize our T-cell or stem cell platform in countries where the business, economic and political climates are very different from those of the United States. We may not be aware of some of these issues and it may be difficult for a U.S. company to overcome these issues and ultimately become profitable. Certain foreign countries may favor businesses that are owned by nationals of those countries as opposed to foreign-owned business operating locally. As a small company, we may not have the resources to engage in the negotiation and time-consuming work needed to overcome some of these potential issues. Risks Related to Our Intellectual Property
Patents obtained by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential products, such as Tcelna.
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make or use our potential products, such as Tcelna, and such claims are ultimately
determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we (or, in the event the Option is exercised, Merck Serono with respect to Tcelna) may not be able to develop any affected product candidate commercially. There can be no assurance that we will not be obliged to defend ourselves (or, in the event the Option is exercised, Merck Serono with respect to Tcelna) in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
If we are unable to obtain patent protection and other proprietary rights, our operations will be significantly harmed.
Our ability to compete effectively is dependent upon obtaining patent protection relating to our technologies. The patent positions of pharmaceutical and biotechnology companies, including ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether pending patent applications for our technology will result in the issuance of patents, or if any issued patents will
provide significant protection or commercial advantage or will be circumvented by others. Since patent applications are secret until the applications are published (usually 18 months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that the inventors of our owned or licensed intellectual property rights were the first to make the inventions at issue or that any patent applications at issue were the first to be filed for such inventions. There can be no assurance that patents will issue from pending patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
For our licensed intellectual property, we have limited control over the amount or timing of resources that are devoted to the prosecution of certain of such intellectual property. Due to this lack of control and general uncertainties in the patent prosecution process, we cannot be sure that any licensed patents will result from licensed applications or, if they do, that they will be maintained. Issued U.S. patents require the payment of maintenance fees to continue to be in force. We rely on licensorsa third party payor to do this and their failure to do so could result in the forfeiture of patents not timely maintained. Many foreign patent offices also require the payment of periodic annuities to keep patents and patent applications in good standing. As we may not maintain direct control over the payment of all such annuities, we cannot assure you that our licensorsthird party payor will timely pay such annuities and that the granted patents and pending patent applications will not become abandoned. In addition, we or our licensors may have selected a limited amount of foreign patent protection, and therefore applications have not been filed in, and foreign patents may not have been perfected in, all commercially significant countries.
The patent protection of product candidates, such as Tcelna, involves complex legal and factual questions. To the extent that it would be necessary or advantageous for any of our licensors to cooperate or lead in the enforcement of our licensed intellectual property rights, we cannot control the amount or timing of resources such licensors devote on our behalf or the priority they place on enforcing such rights. We may not be able to protect our intellectual property rights against third party infringement, which may be difficult to detect. Additionally, challenges may be made to the ownership of our intellectual property rights, our ability to enforce them, or our underlying licenses.
We cannot be certain that any of the patents issued to us or to our licensors will provide adequate protection from competing products. Our success will depend, in part, on whether we or our licensors can:
obtain and maintain patents to protect our product candidates such as Tcelna;
obtain and maintain any required or desirable licenses to use certain technologies of third parties, which may be protected by patents;
protect our trade secrets and know-how;
operate without infringing the intellectual property and proprietary rights of others;
enforce the issued patents under which we hold rights; and
develop additional proprietary technologies that are patentable.
The degree of future protection for our proprietary rights (owned or licensed) is uncertain. For example:
we or our licensor might not have been the first to make the inventions covered by pending patent applications or issued patents owned by, or licensed to, us;
we or our licensor might not have been the first to file patent applications for these inventions;
others may independently develop similar or alternative technologies or duplicate any of the technologies owned by, or licensed to, us;
it is possible that none of the pending patent applications owned by, or licensed to, us will result in issued patents;
any patents under which we hold rights may not provide us with a basis for commercially viable products, may not provide us with any competitive advantages or may be challenged by third parties as invalid, or unenforceable under U.S. or foreign laws; or
any of the issued patents under which we hold rights may not be valid or enforceable or may be circumvented successfully in light of the continuing evolution of domestic and foreign patent laws.
Confidentiality agreements with employees and others may not adequately prevent disclosure of our trade secrets and other proprietary information and may not adequately protect our intellectual property, which could limit our ability to compete.
We rely in part on trade secret protection in order to protect our proprietary trade secrets and unpatented know-how. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. We also typically obtain agreements from these parties which provide that inventions conceived by the party in the course of rendering services to us will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. Further, we have limited control, if any, over the protection of trade secrets developed by our licensors. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time consuming and costly, and an unfavorable outcome could harm our business.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, T-cells, and other technologies potentially relevant to or required by our product candidatecandidates such as Tcelna. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware of a number of patent applications and patents claiming use of modified cells to treat disease, disorder or injury.
There is significant litigation in our industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, as of the date hereof, and are not aware of any
such threatened litigation, we may be exposed to future litigation by third parties based on claims that our product candidates, such as Tcelna, or their methods of use, manufacturing or other technologies or activities infringe the intellectual property rights of such third parties. If our product candidates, such as Tcelna, or their methods of manufacture are found to infringe any such patents, we may have to pay significant damages or seek licenses under such patents. We have not conducted comprehensive searches of patents issued to third parties relating to Tcelna.Tcelna or OPX-212. Consequently, no assurance can be given that third-party patents containing claims covering Tcelna its methodor OPX-212, their methods of use or manufacture do not exist or have not been filed and will not be issued in the future. Because some patent applications in the United States may be maintained in secrecy until the patents are issued, and because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, we cannot be certain that others have not filed patent applications that will mature into issued patents that relate to our current or future product candidates that could have a material effect in developing and commercializing one or more of our product candidates. A patent holder could prevent us from importing, making, using or selling the patented compounds. We may need to resort to litigation to enforce our intellectual property rights or to determine the scope and validity of third-party proprietary rights. Similarly, we may be subject to claims that we have inappropriately used or disclosed trade secrets or other proprietary information of third parties. If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. Some of our competitors may be able to sustain the costs of complex intellectual property litigation more effectively than we can because they have substantially greater resources. We may not be able to afford the costs of litigation. Any legal action against us or our collaborators could lead to:
payment of actual damages, royalties, lost profits, potentially treble damages and attorneys’ fees, if we are found to have willfully infringed a third party’s patent rights;
injunctive or other equitable relief that may effectively block our ability to further develop, commercialize and sell our products;
we or our collaborators having to enter into license arrangements that may not be available on commercially acceptable terms if at all; or
significant cost and expense, as well as distraction of our management from our business.
As a result, we could be prevented from commercializing current or future product candidates.
Risks Related to Our Industry
We are subject to stringent regulation of our product candidates, such as Tcelna, which could delay development and commercialization.
We, our third-party contractors, suppliers and partners (such as Merck Serono, in the event the Option is exercised, with respect to Tcelna), and our product candidates, such as Tcelna, are subject to stringent regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. None of our product candidates can be marketed in the United States until it has been approved by the FDA. No product candidate of ours has been approved, and we may never receive FDA approval for any product candidate. Obtaining FDA approval typically takes many years and requires substantial resources. Even if regulatory approval is obtained, the FDA may impose significant restrictions on the indicated uses, conditions for use and labeling of such products. Additionally, the FDA may require post-approval studies, including additional research and development and clinical trials. These regulatory requirements may limit the size of the market for the product or result in the incurrence of additional costs. Any delay or failure in obtaining required approvals could substantially reduce our ability to generate revenues.
In addition, both before and after regulatory approval, we, our partners and our product candidates, such as Tcelna, are subject to numerous FDA requirements covering, among other things, testing, manufacturing, quality control, labeling, advertising, promotion, distribution and export. The FDA’s requirements may change and additional government regulations may be promulgated that could affect us, our partners and our product
candidates, such as Tcelna. Given the number of recent high profile adverse safety events with certain drug products, the FDA may require, as a condition of approval, costly risk management programs, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events, preapproval of promotional materials and restrictions on direct-to-consumer advertising. Furthermore, heightened Congressional scrutiny on the adequacy of the FDA’s drug approval process and the agency’s efforts to assure the safety of marketed drugs resulted in the enactment of legislation addressing drug safety issues, the FDA Amendments Act of 2007. This legislation provides the FDA with expanded authority over drug products after approval and the FDA’s exercise of this authority could result in delays or increased costs during the period of product development, clinical trials and regulatory review and approval, and increased costs to assure compliance with new post-approval regulatory requirements. We cannot predict the likelihood, nature or extent of government regulation that may arise from this or future legislation or administrative action, either in the United States or abroad.
In order to market any of our products outside of the United States, we and our strategic partners and licensees must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods and the time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States. Approval by the FDA does not automatically lead to the approval of authorities outside of the United States and, similarly, approval by other regulatory authorities outside the United States will not automatically lead to FDA approval. In addition, regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. Our product
candidates, such as Tcelna, may not be approved for all indications that we request, which would limit uses and adversely impact our potential royalties and product sales. Such approval may be subject to limitations on the indicated uses for which any potential product may be marketed or require costly, post-marketing follow-up studies.
If we fail to comply with applicable regulatory requirements in the United States and other countries, among other things, we may be subject to fines and other civil penalties, delays in approving or failure to approve a product, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, interruption of manufacturing or clinical trials, injunctions and criminal prosecution, any of which would harm our business.
We may need to change our business practices to comply with health care fraud and abuse regulations, and our failure to comply with such laws could adversely affect our business, financial condition and results of operations.
If Merck Serono exercises the Option, Merck Serono would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. Otherwise, if we are successful in achieving approval to market one or more of our product candidates, our operations will be directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and False Claims Act. These laws may impact, among other things, our proposed sales, marketing, and education programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. The Anti-Kickback Statute is broad
and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Department of Health and Human Services, Office of Inspector General, or OIG, to issue a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, sometimes known as “relators” or, more commonly, as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing of qui tam actions has increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a False Claims Act action. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties. Various states have also enacted laws modeled after the federal False Claims Act.
In addition to the laws described above, the Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines or imprisonment.
Beginning August 1, 2013, the Physician Payments Sunshine Act (the “Sunshine Act”), which is part of the Patient Protection and Affordable Care Act, requires manufacturers of drugs, medical devices, biologicals or medical supplies that participate in U.S. federal health care programs to track and then report certain payments and items of value given to U.S. physicians and U.S. teaching hospitals (defined as “Covered Recipients”). The Sunshine Act requires that manufacturers collect this information on a yearly basis and then report it to Centers for Medicare & Medicaid Services by the 90th day of each subsequent year.
If our operations are found to be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
If our competitors develop and market products that are more effective than our product candidates, they may reduce or eliminate our commercial opportunities.
Competition in the pharmaceutical industry, particularly the market for MS products, is intense, and we expect such competition to continue to increase. We face competition from pharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies, in the United States and abroad. Our competitors have products that have been approved or are in advanced development and may succeed in developing drugs that are more effective, safer and more affordable or more easily administered than ours, or that achieve patent protection or commercialization sooner than our products. Our most significant competitors are fully integrated pharmaceutical companies and more established biotechnology companies. These companies have significantly greater capital resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals, and marketing than we currently do. However, smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaboration arrangements with large pharmaceutical and established biotechnology companies. In addition to
the competitors with existing products that have been approved, many of our competitors are further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or further product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
Our competitors may also develop alternative therapies that could further limit the market for any products that we may develop.
Rapid technological change could make our products obsolete.
Biopharmaceutical technologies have undergone rapid and significant change, and we expect that they will continue to do so. As a result, there is significant risk that our product candidates, such as Tcelna, may be rendered obsolete or uneconomical by new discoveries before we recover any expenses incurred in connection with their development. If our product candidates, such as Tcelna, are rendered obsolete by advancements in biopharmaceutical technologies, our future prospects will suffer.
Consumers may sue us for product liability, which could result in substantial liabilities that exceed our available resources and damage our reputation.
Developing and commercializing drug products entails significant product liability risks. Liability claims may arise from our and our collaborators’ use of products in clinical trials and the commercial sale of those products.
In the event that any of our product candidates becomes an approved product and is commercialized, consumers may make product liability claims directly against us and/or our partners (such as Merck Serono, in the event the Option is exercised, with respect to Tcelna), and our partners or others selling these products may seek contribution from us if they incur any loss or expenses related to such claims. We have insurance that covers clinical trial activities. We believe our insurance coverage as of the date hereof is reasonably adequate at this time. However, we will need to increase and expand this coverage as we commence additional clinical trials, as well as larger scale trials, and if any product candidate is approved for commercial sale. This insurance may be prohibitively expensive or may not fully cover our potential liabilities. Our inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the regulatory approval or commercialization of products that we or one of our collaborators develop. Product liability claims could have a material adverse effect on our business and results of operations. Liability from such claims could exceed our total assets if we do not prevail in any lawsuit brought by a third party alleging that an injury was caused by one or more of our products.
HealthGovernment controls and health care reform measures could adversely affect our business.
The business and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payors to contain or reduce the costs of health care. In the United States and in foreign jurisdictions, there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the health care system. For example, in some foreign countries, particularly in Europe, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of any product candidate, such as Tcelna, to other thanavailable therapies. If reimbursement of any product candidate such as Tcelna, if approved, is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability in such country. In the United States, pricingthe Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) changed the way Medicare covers and pays for pharmaceutical products. The legislation established Medicare Part D, which expanded Medicare coverage for outpatient prescription drug purchases by the elderly but provided authority for limiting the number of drugs is subjectthat will be covered in any therapeutic class. The MMA also introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. Any negotiated prices for any product candidate such as Tcelna, if approved, covered by a Part D prescription drug plan will likely be lower than the prices that might otherwise be obtained outside of the Medicare Part D prescription drug plan. Moreover, while Medicare Part D applies only to government control,drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and we expectpayment limitations in setting their own payment rates. Any reduction in payment under Medicare Part D may result in a similar reduction in payments from non-governmental payors.
The United States and several other jurisdictions are considering, or have already enacted, a number of legislative and regulatory proposals to implement similar controlschange the healthcare system in ways that could affect our ability to sell any product candidate such as Tcelna, if approved. Among policy-makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to continue. healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. There have been, and likely will continue to be, legislative and regulatory proposals at the
federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may adversely affect: the demand for any product candidate such as Tcelna, if approved; the ability to set a price that we believe is fair for any product candidate such as Tcelna, if approved; our ability to generate revenues and achieve or maintain profitability; the level of taxes that we are required to pay; and the availability of capital.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the ACA), became law in the United States. The goal of the ACA is to reduce the cost of healthcare and substantially change the way healthcare is financed by both governmental and private insurers. The ACA may result in downward pressure on pharmaceutical reimbursement, which could negatively affect market acceptance of any product candidate such as Tcelna, if approved. Provisions of the ACA relevant to the pharmaceutical industry include the following: an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts on negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability; expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; new requirements under the federal Open Payments program and its implementing regulations; and expansion of healthcare fraud and abuse laws, including the federal False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance. In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several types of providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding.
Another example of reform that could affect our business is drug reimportation into the United States (i.e., the reimportation of approved drugs originally manufactured in the United States back into the United States from other countries where the drugs were sold at lower prices). Initiatives in this regard could decrease the price we or any potential collaborators receive for our product candidates if they are ever approved for sale, adversely affecting our future revenue growth and potential profitability. Moreover, the pendency or approval of such proposals could result in a decrease in our stock price or adversely affect our ability to raise capital or to obtain strategic partnerships or licenses.
Risks Related to Our Securities
There is currently a limited market for our securities, and any trading market that exists in our securities may be highly illiquid and may not reflect the underlying value of our net assets or business prospects.
Although our common stock is traded on the NASDAQ Capital Market, there is currently a limited market for our securities and there can be no assurance that an active market will ever develop. Investors are cautioned not to rely on the possibility that an active trading market may develop.
Our stock may be delisted from NASDAQ, which could affect its market price and liquidity.
We are required to meet certain qualitative and financial tests (including a minimum stockholders’ equity requirement of $2.5 million and bid price for our common stock of $1.00 per share)share and a minimum stockholders’ equity of $2.5 million), as well as certain corporate governance standards, to maintain the listing of our common stock on the NASDAQ Capital Market,Market. It is possible that we could fail to satisfy one or more of these requirements. Since November 2014, our stock has continued to trade below the minimum bid price continued listing requirement, and our common stock is in jeopardy of being delisted. On November 26, 2012,December 5, 2014, we received a staff deficiency letter from NASDAQ indicating that our common stock failed to comply with the minimum bid price requirement because it closed below the $1.00 minimum closing bid price for 30 consecutive trading days. The notice further stated that we will be provided a period of 180 calendar days to regain compliance. If our common stock maintains a closing bid price of $1.00 per share or more for a minimum of 10 consecutive business days (or such longer period of time as the NASDAQ staff may require in some circumstances, but generally not more than 20 consecutive business days) before June 3, 2015, we will achieve compliance with the listing standards. If our common stock does not achieve compliance with the minimum bid price by June 3, 2015, we may be eligible for an additional 180 day grace period so long as we continue to meet the other listing standards and provide timely notice of our intention to cure the deficiency. However, if it appears to the NASDAQ staff that we will not be able to cure the deficiency, or if we do not meet the other listing standards, NASDAQ could provide notice that our stock will become subject to delisting.
We previously received a similar staff deficiency letter in February 2012 indicating that our common stock failed to comply with the minimum bid price requirement because it traded below the $1.00 minimum closing bid price for 30 consecutive trading days, and after an initial and an extended grace period, and implementation of a one-for-four reverse stock split of our common stock on December 14, 2012, we regained compliance with the $1.00 minimum closing bid price listing standard and NASDAQ notified us that the matter was closed in January 2013. We also received a staff deficiency letter in November 2012 notifying us that the stockholders’ equity of $2,339,285 as reported in our Quarterly Report on Form 10-Q for the period ended September 30, 2012 was below the minimum stockholders’ equity of $2.5 million required for continued listing on NASDAQ. We were provided 45 calendar days, or until January 10, 2013, to submit a plan to regain compliance with the minimum stockholders’ equity standard. We submitted such a plan and it was accepted, with NASDAQ thus granting us an extension until May 15, 2013 to evidence compliance with the minimum stockholders’ equity standard. IfUpon executing the plan, we had failed to evidence compliance upon filing ofattained the Quarterly Report on Form 10-Q for the period ended March 31, 2013, we could have been subject to delisting. Ournecessary stockholders’ equity as of March 31, 2013 was $4,034,357,level and we subsequently received notice from NASDAQ that we had regained compliance with the listing standard and the matter was closed in May 2013.
While we are exercising diligent efforts to maintain the listing of our common stock on NASDAQ, there can be no assurance that we will be able to maintain compliance with the stockholder’s equity standard.
It is also possible that we could fail to satisfy another NASDAQ requirement for continued listing of our stock, such as the minimum bid price, stockholder’s equity or other listing standards in the market value or number of publicly held shares or number of shareholders, or a corporate governance requirement. For example, during portions of 2008 and 2009, our stockholders’ equity was below the continued listing standard requirement of $2.5 million and the bid price for our common stock was below $1.00 per share for periods of time, and our common stock was in jeopardy of being delisted. Additionally, during 2010 and 2011, the trading price of our common stock was minimally above $1.00 per share for certain periods of time, and our stock closed below $1.00 per share from December 2011 through part of December 2012. In February 2012, we received a staff deficiency letter from NASDAQ indicating that our common stock failed to comply with the minimum bid price requirement because it traded below the $1.00 minimum closing bid price for 30 consecutive trading days, and after an initial and an extended grace period, and implementation of a one-for-four reverse stock split of our common stock on December 14, 2012, we regained compliance with the $1.00 minimum closing bid price listing standard and NASDAQ notified us that the matter was closed in January 2013. However, there can be no assurance that the closing bid price of our common stock will continue to stay above the minimum continued listing standard.
future. We may receive additional future notices from NASDAQ that we have failed to meet its requirements, and proceedings to delist our stock could be commenced. In such event, NASDAQ rules permit us to appeal any delisting determination to a NASDAQ Hearings Panel. If we are unable to maintain or regain compliance in a timely manner and our common stock is delisted, it could be more difficult to buy or sell our common stock and obtain accurate quotations, and the price of our stock could suffer a material decline. Delisting may also impair our ability to raise capital.
As ourOur share price is volatile, and you may not be able to resell our shares at a profit or at all.
The market prices for securities of biopharmaceutical and biotechnology companies, and early-stage drug discovery and development companies like us in particular, have historically been highly volatile and may
continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
the development status of any drug candidates, such as Tcelna, including clinical study results and determinations by regulatory authorities with respect thereto;
the initiation, termination, or reduction in the scope of any collaboration arrangements (such as developments involving Merck Serono and the Option, Agreement, including a decision by Merck Serono to exercise or not exercise the Option) or any disputes or developments regarding such collaborations;
announcements of technological innovations, new commercial products or other material events by our competitors or us;
disputes or other developments concerning our proprietary rights;
changes in, or failure to meet, securities analysts’ or investors’ expectations of our financial performance;
additions or departures of key personnel;
discussions of our business, products, financial performance, prospects or stock price by the financial and scientific press and online investor communities;
public concern as to, and legislative action with respect to, the pricing and availability of prescription drugs or the safety of drugs and drug delivery techniques;
regulatory developments in the United States and in foreign countries; or
dilutive effects of sales of shares of common stock by us or our shareholders, including Lincoln Park, and sales of common stock acquired upon exercise or conversion by the holders of warrants, options or convertible notes.
Broad market and industry factors, as well as economic and political factors, also may materially adversely affect the market price of our common stock.
We may be or become the target of securities litigation, which is costly and time-consuming to defend.
In the past, following periods of market volatility in the price of a company’s securities or the reporting of unfavorable news, security holders have often instituted class action litigation. If the market value of our securities experience adverse fluctuations and we become involved in this type of litigation, regardless of the outcome, we could incur substantial legal costs and our management’s attention could be diverted from the operation of our business, causing our business to suffer.
Our “blank check” preferred stock could be issued to prevent a business combination not desired by management or our majority shareholders.
Our articles of incorporation authorizecharter authorizes the issuance of “blank check” preferred stock with such designations, rights and preferences as may be determined by our Board of Directors without shareholder approval. Our preferred stock could be utilized as a method of discouraging, delaying, or preventing a change in our control and as a method of preventing shareholders from receiving a premium for their shares in connection with a change of control.
Future sales of our common stock insecurities could cause dilution, and the public marketsale of such securities, or the perception that such sales may occur, could lowercause the price of our stock price.to fall.
In July 2012, we closed a private offering consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds, of which notes in the aggregate principal amount of $900,000 have beenwere converted into shares of Series A convertible preferred stock which, in
turn, have beenwere converted into an aggregate of 288,229 shares of common stock.stock during February 2013. The remaining notes were converted into an aggregate of 1,714,697 shares of common stock at $1.91 per share on September 24, 2013. From November 2012 through January 2013, we sold an aggregate of 390,000 shares to Lincoln Park pursuant to the $1.5 million purchase agreement and issued an additional 56,507 shares as initial commitment shares and 3,585 shares as additional commitment shares. In January 2013, we issued $650,000 principal amount of unsecured convertible promissory notes of which $100,000 was converted into 77,034 shares of common stock at $1.298125 per share during February 2013 and the remaining $550,000 of principal amount plus accrued interest was repaid.repaid during February 2013. Purchasers of such notes also received five-year warrants
to acquire an aggregate of 243,750 shares of our common stock at an exercise price of $1.24 per share. Pursuant to a Sales Agreement executed on September 6, 2012, we have registered for sale up to 1,000,000 shares of common stock through Brinson Patrick Securities Corporation acting as sales agent in an “at-the-market” program. In February 2013, we sold an aggregate of 167,618 shares of our common stock pursuant to such at-the-market programthe ATM Agreement for gross proceeds of $536,417. On February 11, 2013, we closed on an offering of 1,083,334 shares of common stock and warrants to purchase 541,668 shares of common stock for gross proceeds of $3,250,002. There can be no assurance that$3.25 million, or net proceeds of approximately $3.0 million after deducting commissions and offering expenses. On August 13, 2013, we closed an offering of 12 million shares of common stock for gross proceeds of $18 million, or net proceeds of approximately $16.2 million after deducting underwriting discounts and commissions and offering expenses, and we granted the underwriters a 30-day option to purchase up to an additional 1.8 million shares of common stock to cover over-allotments. During September 2013, the underwriters of the August 2013 underwritten public offering exercised the over-allotment option granted to them which resulted in the issuance of an additional 900,000 shares of common stock for gross proceeds of $1.35 million, or net proceeds of approximately $1.2 million after deducting underwriting discounts and commissions and offering expenses. On December 23, 2013, we closed an offering of 4,738,000 shares of common stock, including the full exercise of the over-allotment option granted to the underwriters, raising gross proceeds of $8.1 million or net proceeds of approximately $7.3 million after deducting the underwriting discounts and commission and offering expenses. Between March 5, 2014 and December 31, 2014, we generated gross and net proceeds including amortization of deferred financing costs of $674,126 and $648,175, respectively, on sales of an aggregate of 518,412 shares of our capital raising efforts will be able to attractcommon stock under the capital needed to execute on our business plan and sustain our operations.new ATM Agreement.
Sales of a substantial number of additional shares of our common stock, as well as securities convertible into or exercisable for common stock, could result in the public market couldsubstantial dilution to our shareholders and cause the market price of our common stock to decline. An aggregate of 8,114,79028,234,751 shares of common stock were outstanding as of July 19, 2013.December 31, 2014. As of such date, another (i) 1,089,5002,423,253 shares of common stock were issuable upon exercise of outstanding options and (ii) 3,069,1133,046,801 shares of common stock were issuable upon the exercise of outstanding warrants, and (iii) 1,020,007 shares were issuable if all of the outstanding 12% convertible secured promissory notes were converted to Series A convertible preferred stock and such stock was then converted into shares of common stock.
warrants. A substantial majority of the outstanding shares of our common stock are freely tradable without restriction or further registration under the Securities Act of 1933.
We may sell additional shares of common stock, as well as securities convertible into or exercisable for common stock, in subsequent public or private offerings. We may also issue additional shares of common stock, as well as securities convertible into or exercisable for common stock, to finance future acquisitions. Among other requirements, we will need to raise significant additional capital in order to complete the Phase IIb clinical study of Tcelna in SPMS, and, if initiated, to complete a Phase 1/2 proof-of-concept study of OPX-212 in NMO, and this may require us to issue a substantial amount of securities (including common stock as well as securities convertible into or exercisable for common stock). WeThere can be no assurance that our capital raising efforts will be able to attract the capital needed to execute on our business plan and sustain our operations. Moreover, we cannot predict the size of future issuances of our common stock, as well as securities convertible into or exercisable for common stock, or the effect, if any, that future issuances and sales of our securities will have on the market price of our common stock. Sales of substantial amounts of our common stock, as well as securities convertible into or exercisable for common stock, including shares issued in connection with an acquisition or securing funds to complete our clinical trial plans, or the perception that such sales could occur, may result in substantial dilution and may adversely affect prevailing market prices for our common stock.
The sale or issuance of our common stock to Lincoln Park may cause dilution and the sale of the shares of common stock acquired by Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
Under the $1.5 million purchase agreement and $15$15.0 million purchase agreement with Lincoln Park, we may direct Lincoln Park to purchase up to $16.5 million of shares of common stock, subject to certain limitations and conditions, over a 30-month period. However, in conjunction with our February 2013 offering of common stock and warrants, we agreed not to sell shares under the purchase agreements with Lincoln Park for a period of 120 days after that offering (or June 11, 2013). We have sold an aggregate of 390,000 shares to date under the $1.5 million purchase agreement. Additionally, we issued Lincoln Park 56,507 shares of common stock as initial commitment shares and have issued an aggregate of 3,585 additional commitment shares, and may in the future issue up to an additional 109,428 shares of common stock as additional commitment shares, as a fee for its commitment to purchase the shares under the $1.5 million purchase agreement and the $15 million purchase agreement. The number of shares ultimately offered for sale by Lincoln Park is dependent upon the number of shares purchased by Lincoln Park under the purchase agreements. Depending on market liquidity at the time, sales of shares we issue to Lincoln Park may cause the trading price of our common stock to decline.
Subject to certain conditions, we generally have the right to control the timing and amount of any sales of our shares to Lincoln Park, except that, pursuant to the terms of our agreements with Lincoln Park, we would be unable to sell shares to Lincoln Park if and when the market price of our common stock is below $1.00 per share or if Lincoln Park would own more than 4.99% of our common stock for stock sold to it under the $1.5 million purchase agreement or 9.99% of our common stock for stock sold to it under the $15$15.0 million purchase agreement. The purchase price for the shares that we may sell to Lincoln Park will fluctuate based on the price of our common stock and other factors determined by us. As such, Lincoln Park may ultimately purchase all, some or none of the shares of our common stock issuable pursuant to the purchase agreements after the date hereof
and, after it has acquired shares, Lincoln Park may sell all, some or none of those shares. Therefore, sales to Lincoln Park by us pursuant to either or both of the purchase agreements could result in substantial dilution to the interests of other holders of our common stock. Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales, could cause the trading price of our common stock to decline and could make it more difficult for us to sell equity or equity-related securities in the future.
We presently do not intend to pay cash dividends on our common stock.
We currently anticipate as of the date hereof that no cash dividends will be paid on the common stock in the foreseeable future. While our dividend policy will be based on the operating results and capital needs of the business, it is anticipated that all earnings, if any, will be retained to finance the future expansion of our business.
Our shareholders may experience substantial dilution in the value of their investment if we issue additional shares of our capital stock.
Our charter allows us to issue up to 100,000,000 shares of our common stock and to issue and designate the rights of, without shareholder approval, up to 10,000,000 shares of preferred stock. In connection with the July 25, 2012 convertible note financing, 80,000 shares of preferred stock were designated as non-voting Series A convertible preferred stock. In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share paid by other investors, and dilution to our shareholders could result. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors, and investors purchasing shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by other investors.
We may issue debt and equity securities or securities convertible into equity securities, any of which may be senior to our common stock as to distributions and in liquidation, which could negatively affect the value of our common stock.
In the future, we may attempt to increase our capital resources by entering into debt or debt-like financing that is unsecured or secured by up to all of our assets, or by issuing additional debt or equity securities, which could include issuances of secured or unsecured commercial paper, medium-term notes, senior notes, subordinated notes, guarantees, preferred stock, hybrid securities, or securities convertible into or exchangeable for equity securities. In the event of our liquidation, our lenders and holders of our debt and preferred securities would receive distributions of our available assets before distributions to the holders of our common stock. Because our decision to incur debt and issue securities in future offerings may be influenced by market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings or debt financings. Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future.
For example, on July 25, 2012, we closed a private offering consisting of convertible secured notes and warrants to purchase common stock which generated approximately $4.1 million in gross proceeds. The notes
mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually, payable in either cash or registered shares of common stock. The notes are secured by substantially all of our tangible and intangible assets, and $500,000 of the proceeds from the note offering is being held in a controlled account as part of the security interest granted to the noteholders. This amount was previously $1 million; however, in January 2013 we issued the noteholders five-year warrants to acquire an aggregate of 187,500 shares of our common stock at an exercise price of $1.21 per share in exchange for the reduction of such amount. The notes are convertible into a new class of non-voting Series A convertible preferred stock at a conversion price of $100.00, subject to certain limitations and adjustments. The Series A convertible preferred stock accrues cumulative dividends at the rate of 8% per annum, payable in either cash or registered shares of common stock, and carries a $100.00 per share liquidation preference. The Series A convertible preferred stock is convertible into common stock at a conversion price of $3.1225, subject to certain limitations and adjustments. As a result of antidilution adjustments since the closing of the July 2012 financing: (i) up to 1,020,007 shares of common stock are issuable if all the outstanding principal balance as of the date hereof of $3,185,000 of 12% convertible secured promissory notes is converted to Series A convertible preferred stock and such stock is then converted into shares of common stock; and (ii) the warrants issued to the purchasers of the convertible secured promissory notes are exercisable at an adjusted exercise price of $2.56 per share for an aggregate of 1,436,121 shares of common stock. As of July 19, 2013, secured promissory notes with an aggregate principal amount of $900,000 have been converted into shares of Series A convertible preferred stock which, in turn, have been converted into an aggregate of 288,229 shares of our common stock.
Our management has significant flexibility in using our current available cash and the net proceeds from this offering.offering
In addition to general corporate purposes (including working capital, research and development, business development and operational purposes), we currently intend to use our current available cash and the net proceeds from this offering as well as the net proceeds from the sale of any common stock that we may sell to Lincoln Park under our purchase agreements from time to time, including the $5 million proceeds received from Merck in February 2013 and the approximately $3.25 million in gross proceeds from the February 2013 registered offering of common stock and warrants,(i) to continue ourfunding the ongoing Phase IIbAbili-T clinical study of Tcelna in SPMS.
The Phase IIb clinical studypatients with SPMS, and (ii) to continue preclinical and manufacturing activities for OPX-212 in North America of Tcelna is expectedpatients with NMO, and if such activities are successful, to complete enrollment of 180 patients by late 2013 or early 2014,file an IND application with the resultingFDA to initiate a Phase 1/2 proof-of-concept study. We reached our enrollment target for the Abili-T trial in June 2014, and a total of 190 patients have been enrolled in this two-year study. We expect top-line data expectedfor Tcelna to be available in the firstsecond half of 2016. During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. We used the $650,000 gross proceeds from the January 2013 private offering of convertible unsecured promissory notes and warrants as bridge financing to continue our Phase IIb clinical study while continuing discussions with Merck regarding the February 2013 Option and License Agreement. We repaid the principal balance of the January 2013 notes with $550,000 in cash and $100,000 was converted into 77,034 shares of common stock. Our existing resources are not adequate to permit us to complete ourthe ongoing Phase IIbAbili-T clinical study of Tcelna in patients with SPMS or, the majorityif initiated, to conduct a Phase 1/2 proof-of-concept study of it.OPX-212 in NMO. We will
need to secure significant additional resources to complete the trialongoing Abili-T clinical study of Tcelna in patients with SPMS and, if initiated, to conduct a Phase 1/2 proof-of-concept study of OPX-212 in NMO and to support our operations during the pendencycourse of the trial.trials.
Depending on future developments and circumstances, we may use some of our available cash or the net proceeds from this offering for other purposes.purposes which may have the potential to decrease the forecasted cash runway. Notwithstanding our intention tocurrent intentions regarding use of our available cash for further clinical studies of Tcelna,and the net proceeds from this offering, our management will have significant flexibility in using our available cash.with respect to such use. The actual amounts and timing of expenditures will vary significantly depending on a number of factors, including the amount and timing of cash used in our operations and our research and development efforts. Management’s failure to use these funds effectively would have an adverse effect on the value of our common stock and could make it more difficult and costly to raise funds in the future.
Risks Related to the Rights Offering
YouWe will incur substantial expenses in connection with the Rights Offering, which may experience immediate dilution innot return adequate value if the book value per shareRights Offering is ultimately not consummated or successful.
The estimated expenses for the Rights Offering are approximately $750,000, excluding fees and expenses of the common stock you purchase.dealer-managers that we have engaged to assist us with the Rights Offering. If the registration statement of which this prospectus is a part is not declared effective, the Rights Offering is not commenced or the Rights Offering is not ultimately consummated or successful, we will incur these expenses nonetheless. In addition, we have agreed to pay fees to the dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and (c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; and (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to reimburse the dealer-managers for their expenses as follows: (i) up to $50,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is less than $6 million; and (ii) up to $100,000 if the gross proceeds received by us directly from exercises of the Subscription Rights is at least $6 million.
Your interest in our company may be diluted as a result of this Rights Offering.
BecauseShareholders who do not fully exercise their Subscription Rights should expect that they will, at the completion of this offering, own a smaller proportional interest in our company than would otherwise be the case had they fully exercised their Basic Subscription Right and Over-Subscription Privilege. Further, the shares issuable upon the exercise of the Warrants to be issued pursuant to the Rights Offering will dilute the ownership interest of shareholders not participating in this offering or holders of Warrants issued pursuant to this offering who have not exercised them.
Further, because the price per share of our common stockUnit being offered may be substantially higher than the net tangible book value per share of our common stock, you may suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. If you purchase shares of common stockUnits in this offering at the current market value,Subscription Price, you may suffer immediate and substantial dilution in the net tangible book value of the common stock. See “Dilution” in this prospectus for a more detailed discussion of the dilution which may incur in connection with this offering.
Completion of the Rights Offering is not subject to us raising a minimum offering amount.
Completion of the Rights Offering is not subject to us raising a minimum offering amount and therefore proceeds may be insufficient to meet our objectives, thereby increasing the risk to investors in this offering, including investing in a company that continues to require capital.
This Rights Offering may cause the trading price of our common stock to decrease.
The Subscription Price, together with the number of shares of common stock we propose to issue and ultimately will issue if this Rights Offering is completed, may result in an immediate decrease in the market price of our common stock. This decrease may continue after the completion of this Rights Offering. If that occurs, you may have committed to buy shares of common stock in the Rights Offering at a price greater than the prevailing market price. We cannot predict the effect, if any, that the availability of shares for future sale represented by the Warrants issued in connection with the Rights Offering will have on the market price of our common stock from time to time. Further, if a substantial number of Subscription Rights are exercised and the holders of the shares received upon exercise of those Subscription Rights or the related Warrants choose to sell some or all of the shares underlying the Subscription Rights or the related Warrants, the resulting sales could depress the market price of our common stock. Following the exercise of your Subscription Rights you may not be able to sell your common stock at a price equal to or greater than the Subscription Price.
You could be committed to buying shares of common stock above the prevailing market price.
Once you exercise your Subscription Rights, you may not revoke such exercise even if you later learn information that you consider to be unfavorable to the exercise of your Subscription Rights. The market price of our shares of common stock may decline prior to the expiration of this offering or a Subscribing Rights holder may not be able to sell shares of common stock purchased in this offering at a price equal to or greater than the Subscription Price. Until shares of our common stock are delivered upon expiration of the Rights Offering, you will not be able to sell or transfer the shares of our common stock that you purchase in the Rights Offering. Any such delivery will occur as soon as practicable after the Rights Offering has expired, payment for the shares of common stock and attached Warrants subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected.
If we terminate this offering for any reason, we will have no obligation other than to return subscription monies as soon as practicable.
We may decide, in our sole discretion and for any reason, to cancel or terminate the Rights Offering at any time prior to the expiration date. If this offering is cancelled or terminated, we will have no obligation with respect to Subscription Rights that have been exercised except to return as soon as practicable, without interest, the subscription payments deposited with the Subscription Agent. If we terminate this offering and you have not exercised any Subscription Rights, such Subscription Rights will expire worthless.
Our common stock price may be volatile as a result of this Rights Offering.
The trading price of our common stock may fluctuate substantially. The price of the common stock that will prevail in the market after this offering may be higher or lower than the Subscription Price depending on many factors, some of which are beyond our control and may not be directly related to our operating performance. These factors include, but are not limited to, the following:
Additionally, the stock market historically has experienced significant price and volume fluctuations. These fluctuations are often unrelated to the operating performance of particular companies. These broad market fluctuations may cause declines in the trading price and market value of our common stock.
We cannot assure you that the trading price of our common stock will not decline after you elect to exercise your Subscription Rights. If that occurs, you may have committed to buy shares of common stock in the Rights Offering at a price greater than the prevailing market price and could have an immediate unrealized loss. Moreover, we cannot assure you that, following the exercise of your Subscription Rights, you will be able to sell your common stock at a price equal to or greater than the Subscription Price, and you may lose all or part of your investment in our common stock. Until shares are delivered upon expiration of the Rights Offering, you will not be able to sell the shares of our common stock that you purchase in the Rights Offering. Shares of our common stock purchased will be issued as soon as practicable after the Rights Offering has expired, payment for the shares of common stock and attached Warrants subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. We will not pay you interest on funds delivered to the Subscription Agent pursuant to your exercise of Subscription Rights.
Because we do not have any formal commitments from any of our shareholders to participate in the Rights Offering, the net proceeds we receive from the Rights Offering may be lower than we currently anticipate.
We do not have any formal commitments from any of our shareholders to participate in the Rights Offering, and we cannot assure you that any of our shareholders or warrant holders will exercise all or any part of their Basic Subscription Rights or their Over-Subscription Privilege. If our shareholders or warrantholders that may acquire Subscription Rights subscribe for fewer shares of our common stock than we currently anticipate, the net proceeds we receive from the Rights Offering could be significantly lower than we currently expect.
The Subscription Price determined for this offering is not an indication of the fair value of our common stock.
In determining the Subscription Price, our board of directors considered a number of factors, including, but not limited to, the price at which our stockholders might be willing to participate in the Rights Offering, the value of the Warrant being issued as a component of the Unit, historical and current trading prices for our common stock, the amount of proceeds desired, the potential need for liquidity and capital, potential market conditions, and the desire to provide an opportunity to our shareholders to participate in the Rights Offering. In conjunction with its review of these factors, our board of directors also reviewed a range of discounts to market value represented by the subscription prices in various prior Rights Offerings by other public companies. The Subscription Price does not necessarily bear any relationship to the book value of our assets, results of operations, cash flows, losses, financial condition or any other established criteria for value. You should not consider the Subscription Price as an indication of the fair value of our common stock. After the date of this prospectus, our common stock may trade at prices above or below the Subscription Price.
If you do not act on a timely basis and follow subscription instructions, your exercise of Subscription Rights may be rejected.
Holders of Subscription Rights who desire to purchase shares of our common stock and attached Warrants in this offering must act on a timely basis to ensure that all required forms and payments are actually received by the Subscription Agent prior to 5:00 p.m., New York City time, on the expiration date, unless extended. If you are a beneficial owner of shares of common stock and you wish to exercise your Subscription Rights, you must act promptly to ensure that your broker, dealer, custodian bank, trustee or other nominee acts for you and that all required forms and payments are actually received by your broker, dealer, custodian bank, trustee or other nominee in sufficient time to deliver such forms and payments to the Subscription Agent to exercise the Subscription Rights granted in this offering that you beneficially own prior to 5:00 p.m., New York City time on the expiration date, as may be extended. We will not be responsible if your broker, dealer, custodian bank, trustee or other nominee fails to ensure that all required forms and payments are actually received by the Subscription Agent prior to 5:00 p.m., New York City time, on the expiration date.
If you fail to complete and sign the required subscription forms, send an incorrect payment amount, or otherwise fail to follow the subscription procedures that apply to your exercise in this Rights Offering, the Subscription Agent may, depending on the circumstances, reject your subscription or accept it only to the extent of the payment received. Neither we nor the Subscription Agent undertakes to contact you concerning an incomplete or incorrect subscription form or payment, nor are we under any obligation to correct such forms or payment. We have the sole discretion to determine whether a subscription exercise properly follows the subscription procedures.
You may not receive all of the Units for which you subscribe.
Holders who fully exercise their Basic Subscription Rights will be entitled to subscribe for an additional number of Units. Over-Subscription Privileges will be allocated pro rata among Rights holders who over-subscribed, based on the number of over-subscription Units to which they have subscribed. We cannot guarantee that you will receive any or the entire amount of Units for which you over-subscribed. If the prorated amount of Units allocated to you in connection with your Over-Subscription Privilege is less than your Over-Subscription Request, then the excess funds held by the Subscription Agent on your behalf will be returned to you, without interest, as soon as practicable after the Rights Offering has expired and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected, and we will have no further obligations to you.
Unless we otherwise agree in writing, a person or entity, together with related persons or entities, may not exercise Subscription Rights (including Over-Subscription Privileges) to purchase Units that, when aggregated with their existing ownership, would result in such person or entity, together with any related persons or entities, owning in excess of 19.9% of our issued and outstanding shares of common stock following the closing of the transactions contemplated by this Rights Offering. If the amount of shares allocated to you is less than your subscription request, then the excess funds held by the Subscription Agent on your behalf will be returned to you, without interest, as soon as practicable after the Rights Offering has expired and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected, and we will have no further obligations to you.
If you make payment of the Subscription Price by personal check, your check may not clear in sufficient time to enable you to purchase shares in this Rights Offering.
Any personal check used to pay for shares and Warrants to be issued in this Rights Offering must clear prior to the expiration date of this Rights Offering, and the clearing process may require five or more business days. If you choose to exercise your Subscription Rights, in whole or in part, and to pay for shares and Warrants by personal check and your check has not cleared prior to the expiration date of this Rights Offering, you will not have satisfied the conditions to exercise your Subscription Rights and will not receive the shares and Warrants you wish to purchase.
The receipt of Subscription Rights may be treated as a taxable distribution to you.
We believe the distribution of the Subscription Rights in this Rights Offering should be a non-taxable distribution to holders of shares of common stock and our Series L warrants under Section 305(a) of the Internal Revenue Code of 1986, as amended (the “Code”). Please see the discussion on the “Material U.S. Federal Income Tax Consequences” below. This position is not binding on the IRS, or the courts, however. If this Rights Offering is deemed to be part of a “disproportionate distribution” under Section 305 of the Code, your receipt of Subscription Rights in this offering may be treated as the receipt of a taxable distribution to you equal to the fair market value of the Subscription Rights. Any such distribution would be treated as dividend income to the extent of our current and accumulated earnings and profits, if any, with any excess being treated as a return of capital to the extent thereof and then as capital gain. Each holder of shares of common stock and each holder of a Series L warrant is urged to consult his, her or its own tax advisor with respect to the particular tax consequences of this Rights Offering.
The Subscription Rights are not transferable, and there is no market for the Subscription Rights.
You may not sell, transfer, assign or give away your Subscription Rights. Because the Subscription Rights are non-transferable, there is no market or other means for you to directly realize any value associated with the Subscription Rights. You must exercise the Subscription Rights to realize any potential value from your Subscription Rights.
Absence of a public trading market for the Warrants may limit your ability to resell the Warrants.
There is no established trading market for the Warrants to be issued pursuant to this offering, and the Warrants may not be widely distributed. We have applied to list the Warrants for trading on NASDAQ under the symbol “OPXAW,” but there can be no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet minimum listing criteria to be accepted for listing on NASDAQ or that a market will develop for the Warrants. Even if a market for the Warrants does develop, the price of the Warrants may fluctuate and liquidity may be limited. If the Warrants are not accepted for listing on NASDAQ or if a market for the Warrants does not develop, then purchasers of the Warrants may be unable to resell the Warrants or sell them only at an unfavorable price for an extended period of time, if at all. Future trading prices of the Warrants will depend on many factors, including:
The market price of our common stock may never exceed the exercise price of the Warrants issued in connection with this offering.
The Warrants being issued in connection with this offering become exercisable upon issuance and will expire three years after issuance. We cannot provide you any assurance that the market price of our common stock will ever exceed the exercise price of the Warrants prior to their date of expiration. Any Warrants not exercised by their date of expiration will expire worthless and we will be under no further obligation to the Warrant holder.
The Warrants may be redeemed on short notice. This may have an adverse impact on their price.
We may redeem the Warrants for $0.01 per Warrant once the closing price of our common stock has equaled or exceeded $2.50 per share, subject to adjustment, for 10 consecutive trading days. If we give notice of redemption, you will be forced to sell or exercise your Warrants or accept the redemption price. The notice of redemption could come at a time when it is not advisable or possible for you to exercise the Warrants. As a result, you would be unable to benefit from owning the Warrants being redeemed.
The dealer-managers are not underwriting, nor acting as placement agents of, the Subscription Rights or the securities underlying the Subscription Rights.
Maxim Group LLC and National Securities Corporation will act as dealer-managers for this Rights Offering. Under the terms and subject to the conditions contained in the dealer-manager agreement, the dealer-managers will provide marketing assistance in connection with this offering. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants being issued in this offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares or Warrants. The dealer-managers will not be subject to any liability to us in rendering the services contemplated by the dealer-manager agreement except for any act of bad faith or gross negligence by the dealer-managers. The services of the dealer-managers to us in this connection cannot be construed as any assurance that this offering will be successful.
Our ability to use net operating loss carryovers to reduce future tax payments may be limited.
As of December 31, 2014, we had net operating loss carryforwards (NOLs) for federal income tax purposes of approximately $70 million. We generally are able to carry NOLs forward to reduce taxable income in future years. These NOLs begin to expire at December 31, 2025. However, our ability to utilize the NOLs is subject to the rules of Section 382 of the Internal Revenue Code. Section 382 generally restricts the use of NOLs after an “ownership change.” An ownership change occurs if, among other things, the stockholders (or specified groups of stockholders) who own or have owned, directly or indirectly, five (5%) percent or more of our common stock or are otherwise treated as five (5%) percent stockholders under Section 382 and the regulations promulgated thereunder increase their aggregate percentage ownership of our stock by more than 50 percentage points over the lowest percentage of the stock owned by these stockholders over a three-year rolling period. In the event of an ownership change, Section 382 imposes an annual limitation on the amount of taxable income a corporation may offset with NOL carryforwards. This annual limitation is generally equal to the product of the value of our stock on the date of the ownership change, multiplied by the long-term tax-exempt rate published monthly by the Internal Revenue Service. Any unused annual limitation may be carried over to later years until the applicable expiration date for the respective NOL carryforwards.
The rules of Section 382 are complex and subject to varying interpretations. Because of our numerous capital raises, which have included the issuance of various classes of convertible securities and warrants, uncertainty exists as to whether we may have undergone an ownership change in the past or will undergo one as a result of the Rights Offering. Even if the Rights Offering does not cause an ownership change, it may increase the likelihood that we may undergo an ownership change in the future. Based on our recent stock prices, we believe any ownership change would severely limit our ability to utilize the NOLs. Accordingly, no assurance can be given that our NOLs will be fully available. As a result, we could pay taxes earlier and in larger amounts than would be the case if the NOLs were available to reduce the federal income taxes without restriction.
Since the Warrants are executory contracts, they may have no value in a bankruptcy or reorganization proceeding.
In the event a bankruptcy or reorganization proceeding is commenced by or against us, a bankruptcy court may hold that any unexercised Warrants are executory contracts that are subject to rejection by us with the approval of the bankruptcy court. As a result, holders of the Warrants may, even if we have sufficient funds, not be entitled to receive any consideration for their Warrants or may receive an amount less than they would be entitled to if they had exercised their Warrants prior to the commencement of any such bankruptcy or reorganization proceeding.
This prospectus and the documents incorporated by reference herein contain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. When used in this prospectus, the words “expects,” “believes,” “hopes,” “anticipates,” “estimates,” “may,” “could,” “intends,” “exploring,” “evaluating,” “progressing,” “proceeding” and similar expressions are intended to identify forward-looking statements. These forward-looking statements include statements in this prospectus under the headings “Our Company,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These forward-looking statements do not constitute guarantees of future performance. Investors are cautioned that statements which are not strictly historical statements, including, without limitation, statements regarding current or future financial payments, costs, returns, royalties, performance and position, plans and objectives for future operations, plans and objectives for product development, plans and objectives for present and future clinical trials and results of such trials, plans and objectives for regulatory approval, litigation, intellectual property, product development, manufacturing plans and performance, management’s initiatives and strategies, and the development of the Company’s product candidate,candidates, Tcelna (imilecleucel-T), and OPX-212, constitute forward-looking statements. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated. These risks and uncertainties include, but are not limited to, those risks discussed in “Risk Factors,” as well as, without limitation, risks associated with:
market conditions;
our capital position;
the rights and preferences provided to the Series A convertible preferred stock and investors in the convertible secured notes we issued in July 2012 (including a secured interest in all of our assets);
our ability to compete with larger, better financed pharmaceutical and biotechnology companies;
new approaches to the treatment of our targeted diseases;
our expectation of incurring continued losses;
our uncertainty of developing a marketable product;
our ability to raise additional capital to continue our development programs (including to undertake and complete any ongoing or further clinical studies for Tcelna), including in this regard our ability to satisfy various conditions required to access the financing potentially available under the purchase agreements with Lincoln Park (such as the minimum closing price for our common stock and the requirement for an ongoing trading market for our stock)Tcelna or OPX-212);
our ability to maintain compliance with NASDAQ listing standards;
the success of our clinical trials (including the Phase IIb trial for Tcelna in secondary progressive MSSPMS which, depending upon results, may determine whether Merck Serono elects to exercise its Option);
whether Merck Serono exercises its Option and, if so, whether we receive any development or commercialization milestone payments or royalties from Merck Serono pursuant to the Option;
our dependence (if Merck Serono exercises its Option) on the resources and abilities of Merck Serono for the further development of Tcelna;
the efficacy of Tcelna for any particular indication, such as for relapsing remitting MS or secondary progressive MS;
our ability to develop and commercialize products;
our ability to obtain required regulatory approvals;
our compliance with all Food and Drug Administration regulations;
our ability to obtain, maintain and protect intellectual property rights (including for Tcelna)Tcelna and OPX-212);
the risk of litigation regarding our intellectual property rights or the rights of third parties;
the success of third party development and commercialization efforts with respect to products covered by intellectual property rights that we may license or transfer;
our limited manufacturing capabilities;
our ability to hire and retain skilled personnel;
our volatile stock price; and
other risks detailed in our filings with the SEC.
These forward-looking statements speak only as of the date made. We assume no obligation or undertaking to update any forward-looking statements to reflect any changes in expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. You should, however, review additional disclosures we make in our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the SEC.
WeAssuming that all 28,776,419 Units are subscribed for in the Rights Offering, we estimate that the net proceeds from the sale of the 9,300,000 shares of common stock in the offering at an assumed offering price of $1.61 per shareRights Offering will be approximately $13.4$17.8 million, based on the Subscription Price of $0.70 per Unit, after deducting the underwriting discountexpenses relating to this offering payable by us estimated at approximately $2.3 million, including dealer-manager fees and estimated offering expenses or $15.5 million if the underwritersand excluding any proceeds received upon exercise their over-allotment option in full.of any Warrants.
We currently expectintend to use the net proceeds from this offering as follows:the exercise of Subscription Rights:
up to $3.6 million for potential repayment of certain outstanding indebtedness; and
at least $9.8 million to fund furthercontinue funding the ongoing Phase IIb “Abili-T” clinical developmentstudy of Tcelna and the Phase IIb study in patients with SPMS as well assecondary progressive multiple sclerosis (SPMS);
With respect to the potential repayment of certain outstanding indebtedness, we currently plan to use up to $3.6 million of our available cash or proceeds from this offering to repay our 12% convertible secured promissory notes, subject to the right of the noteholders to convert any unpaid principal amount of the notes into shares of our Series A convertible preferred stock. The notes were originally issued in July 2012 and mature on July 25, 2014. Noteholders may purchase shares in this offering and pay the purchase price for such shares in the form of cancellation of principal amount and/or accrued interest on any such promissory note. In such an instance, the net cash proceeds from this offering would be reduced and, equally, the amount of such proceeds that would be needed to repay the indebtedness represented by the notes would also be reduced.
Our management will retain broad discretion as to the allocation of the net proceeds from this offering. Until we use the net proceeds of this offering, we intend to invest the funds in short-term, interest bearing investments. Any proceeds received by us from the exercise of Warrants will be used for the same purposes and in the same manner.
The following table presents our cash, cash equivalents and capitalization, as of MarchDecember 31, 2013:2014:
on an actual basis; and
on a pro forma as adjusted basis to give effect to the sale by us in this Rights Offering of 9,300,000maximum of 28,776,419 Units (consisting of 28,776,419 shares of our common stock and Warrants to purchase an aggregate of 28,776,419 shares of common stock in this offeringupon exercise), at an assumed public offering pricethe Subscription Price of $1.44 per share,$0.70, and our receipt of the net proceeds from that sale after deducting the underwriting discount and estimated offering expenses.
Note: AsThe table below does not reflect the exercise of June 30, 2013, we had cash and cash equivalents of approximately $5.0 million, excluding $500,000 of restricted cash subject to a deposit control agreementWarrants issued in favor ofconnection with the holders of our July 2012 convertible notes.
Rights Offering. The pro forma as adjusted information set forth below is illustrative only and will be adjusted based on the actual public offering price and other termsnumber of this offering determined at pricing.Units sold. You should read this information in conjunction with our consolidated financial statements and notes thereto included inincorporated by reference into this prospectus.
| As of March 31, 2013 | ||||||||
| Actual | Pro Forma As Adjusted(1) | |||||||
| (Unaudited) | ||||||||
Cash and cash equivalents | $ | 7,834,336 | $ | 21,214,226 | ||||
|
|
|
| |||||
Long-term liabilities | $ | 3,761,179 | $ | 3,761,179 | ||||
Stockholders’ equity: | ||||||||
Preferred stock, no par value, 10,000,000 shares authorized; none issued and outstanding, actual or pro forma as adjusted | — | — | ||||||
Common stock, $0.01 par value, 100,000,000 shares authorized; | 79,916 | 172,916 | ||||||
Additional paid-in capital | 117,743,543 | 131,030,433 | ||||||
Deficit accumulated during the development stage | (113,789,102 | ) | (113,789,102 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | $ | 4,034,357 | $ | 17,414,247 | ||||
|
|
|
| |||||
| As of December 31, 2014 | ||||||||
| Actual | Pro Forma As Adjusted | |||||||
| (Unaudited) | ||||||||
Cash and cash equivalents | $ | 9,906,373 | $ | 27,741,727 | ||||
|
|
|
| |||||
Long-term liabilities | $ | 1,230,748 | $ | 1,230,748 | ||||
Stockholders’ equity: | ||||||||
Preferred stock, no par value, 10,000,000 shares authorized; none issued and outstanding, actual or pro forma as adjusted | — | — | ||||||
Common stock, $0.01 par value, 100,000,000 shares authorized; | 282,348 | 570,112 | ||||||
Actual – 28,234,751 shares issued and outstanding; | ||||||||
Pro forma as adjusted – 57,011,170 shares issued and outstanding | ||||||||
Additional paid-in capital | 148,477,047 | 166,024,637 | ||||||
Accumulated deficit | (141,320,208) | (141,320,208) | ||||||
|
|
|
| |||||
Total stockholders’ equity | $ | 7,439,187 | $ | 25,274,541 | ||||
|
|
|
| |||||
The information above is as of MarchDecember 31, 20132014 and excludes:
123,231 shares of common stock issued to the noteholders of the July 2012 convertible notes as payment of accrued interest through June 30, 2013;
1,089,5002,423,253 shares of common stock issuable upon the exercise of stock options outstanding at July 19, 2013December 31, 2014 with a weighted average exercise price of $4.46$2.92 per share;
3,069,1133,046,801 shares of common stock issuable upon the exercise of outstanding warrants at July 19, 2013December 31, 2014 with a weighted average exercise price of $4.12$4.08 per share;
1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding at July 19, 2013 are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock;
48,7201,509,479 shares of common stock available for future grants under our 2010 Stock Incentive Plan at July 19, 2013;
any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park; and
any shares issued upon the exercise by the underwriters of the option to purchase up to 1,395,000 additional shares of common stock from us to cover over-allotments, if any.
Our net tangible book value as of March 31, 2013 was approximately $4,034,357 or $0.50 per share, based on 7,991,559 shares of our common stock outstanding on that date. Net tangible book value per share is determined by dividing our total tangible assets (total assets less intangible assets), less total liabilities, by the number of shares of our common stock outstanding.
After giving effect to our assumed sale of all 9,300,000 shares of our common stock in this offering at an assumed public offering price of $1.61 per share, our as adjusted net tangible book value as of March 31, 2013 would have been approximately $17,414,247, or $1.01 per share of common stock. This represents an immediate increase in net tangible book value of $0.51 per share to existing shareholders and immediate dilution in net tangible book value of $0.60 per share to new investors participating in this offering at the assumed offering price. The following table illustrates this dilution on a per share basis:
Assumed public offering price per share | $ | 1.61 | ||||||
Net tangible book value per share as of March 31, 2013, before this offering | $ | 0.50 | ||||||
Increase in pro forma net tangible book value per share attributable to new investors | $ | 0.51 | ||||||
Net tangible book value per share as of March 31, 2013, after giving effect to this offering | $ | 1.01 | ||||||
Dilution per share to new investors | $ | 0.60 |
The information above is as of March 31, 2013 and excludes:
123,231 shares of common stock issued to the noteholders of the July 2012 convertible notes as payment of accrued interest through June 30, 2013;
1,089,500 shares of common stock issuable upon the exercise of stock options outstanding at July 19, 2013 with a weighted average exercise price of $4.46 per share;
3,069,113 shares of common stock issuable upon the exercise of outstanding warrants at July 19, 2013 with a weighted average exercise price of $4.12 per share;
1,020,007 shares of common stock issuable if all of the 12% convertible secured promissory notes outstanding at July 19, 2013 are converted to Series A convertible preferred stock and such stock is then converted into shares of common stock;
48,720 shares of common stock available for future grants under our 2010 Stock Incentive Plan at July 19, 2013; and
any additional shares of common stock that we may issue to Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to a $1,500,000 purchase agreement and a $15,000,000 purchase agreement we entered into on November 5, 2012 and November 2, 2012, respectively, which provides that, upon the terms and subject to the conditions and limitation set forth therein, Lincoln Park is committed to purchase up to an aggregate of an additional $15.9 million of shares of our common stock over the term of the purchase agreements, should we elect to sell shares to Lincoln Park.
If
Purchasers of our common stock in the underwriters’ overallotment optionRights Offering (and upon exercise of the Warrants issued pursuant to this Rights Offering) will experience an immediate dilution of the net tangible book value per share of our common stock. Our net tangible book value as of December 31, 2014 was approximately $5,543,201, or $0.20 per share of our common stock (based upon 28,234,751 shares of our common stock outstanding). Net tangible book value per share is exercised,equal to our adjustedtotal net tangible book value, which is our total tangible assets less our total liabilities, divided by the number of shares of our outstanding common stock. Dilution per share equals the difference between the amount per share paid by purchasers of shares of common stock in the Rights Offering and the net tangible book value per share of our common stock immediately after the Rights Offering.
Based on the sale by us in this Rights Offering of a maximum of 28,776,419 Units (consisting of 28,776,419 shares of our common stock and Warrants to purchase an aggregate of 28,776,419 shares of common stock upon exercise), at the Subscription Price of $0.70 per Unit, and after deducting estimated offering expenses and dealer-manager fees and expenses payable by us of $2,308,139, and the application of the estimated $17,835,354 of net proceeds from the Rights Offering, our pro forma net tangible book value following the offering will be $1.04as of December 31, 2014 would have been approximately $23,378,555, or $0.41 per share, and the dilution to new investorsshare. This represents an immediate increase in the offering will be $0.57 per share.
A $0.10 increase or decrease in the assumed public offering price per share would increase or decrease our pro forma as adjusted net tangible book value to existing shareholders of $0.21 per share afterand an immediate dilution to purchasers in the Rights Offering of $0.29 per share.
The following table illustrates this offering by approximately $0.05, and increase or decreaseper-share dilution (assuming a fully subscribed for Rights Offering of 28,776,419 Units at the Subscription Price of $0.70 per share to new investors by approximately $0.05, after deducting the underwriting discount and estimated offering expenses payable by us and assuming noUnit, but excluding any issuance of shares of common stock upon exercise of Warrants):
Subscription Price | $ | 0.70 | ||||||
Net tangible book value per share as of December 31, 2014, before Rights Offering | $ | 0.20 | ||||||
Increase in net tangible book value per share attributable to Rights Offering | 0.21 | |||||||
Pro forma net tangible book value per share as of December 31, 2014, after giving effect to Rights Offering | 0.41 | |||||||
Dilution in net tangible book value per share to purchasers | $ | 0.29 | ||||||
The information above is as of December 31, 2014 and excludes:
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock is traded on theThe NASDAQ Capital Market under the symbol “OPXA.” Our common stock has, from time to time, traded on a limited, sporadic and volatile basis. The table below shows the high and low sales prices for our common stock for the periods indicated, as reported by Bloomberg.
| Price Ranges(1) | ||||||||
| High | Low | |||||||
Fiscal Quarter Ended March 31, 2013 | ||||||||
First Quarter | $ | 5.19 | $ | 1.09 | ||||
Fiscal Year Ended December 31, 2012 | ||||||||
First Quarter | $ | 4.60 | $ | 2.80 | ||||
Second Quarter | 3.04 | 1.21 | ||||||
Third Quarter | 3.48 | 1.16 | ||||||
Fourth Quarter | 3.36 | 1.07 | ||||||
Fiscal Year Ended December 31, 2011 | ||||||||
First Quarter | $ | 11.96 | $ | 8.11 | ||||
Second Quarter | 9.20 | 6.00 | ||||||
Third Quarter | 7.00 | 4.44 | ||||||
Fourth Quarter | 6.12 | 3.56 | ||||||
| Price Ranges | ||||
| High | Low | |||
Fiscal Quarter Ended March 31, 2015 | ||||
First Quarter (through February 20, 2015) | $0.90 | $0.68 | ||
Fiscal Year Ended December 31, 2014 | ||||
First Quarter | $2.20 | $1.64 | ||
Second Quarter | 1.93 | 1.26 | ||
Third Quarter | 1.71 | 0.85 | ||
Fourth Quarter | 1.07 | 0.70 | ||
Fiscal Year Ended December 31, 2013 | ||||
First Quarter | $5.19 | $1.09 | ||
Second Quarter | 2.44 | 1.44 | ||
Third Quarter | 3.70 | 1.25 | ||
Fourth Quarter | 2.56 | 1.65 | ||
The closing price of our common stock on July 19, 2013February 20, 2015 was $1.61$0.73 per share. We implemented a 1-for-4 reverse stock splitAs of our common stock on December 14, 2012. There areFebruary 20, 2015, there were approximately 200175 holders of record of our common stock, excluding shareholders for whom shares are held in “nominee” or “street name.”
We have never declared or paid any cash dividends on our common stock and we do not intend to pay cash dividends in the foreseeable future. We expect as of the date hereof to retain any future earnings to fund the operation and expansion of our business.
SELECTED CONSOLIDATED FINANCIAL DATATHE RIGHTS OFFERING
The following selected consolidated statementsSubscription Rights
We are distributing to the record holders and to holders of operations dataour outstanding Series L warrants (who are entitled to participate in this offering pursuant to the terms of such warrants), at no charge, non-transferable Subscription Rights to purchase one Unit at a subscription price of $0.70 per Unit. Each Basic Subscription Right will entitle you to purchase one share of our common stock and a Warrant for the years ended December 31, 2012 and 2011 have been derived from our audited consolidated financial statements that are included in this prospectus. The summary consolidated statementspurchase of operations data for the three-month periods ended March 31, 2013 and 2012 and the consolidated balance sheet data as of March 31, 2013 is derived from our unaudited consolidated financial statements that are included in this prospectus. The historical financial data presented below is not necessarily indicativeone additional share of our financial results in future periods, and the results for the three-month period ended March 31, 2013 are not necessarily indicativecommon stock at an exercise price of our operating results to be expected for the full fiscal year ending December 31, 2013 or any other period. You should read the summary consolidated financial data in conjunction with those financial statements and the accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our consolidated financial statements are prepared and presented in accordance with United States generally accepted accounting principles, or U.S. GAAP. Our unaudited consolidated financial statements have been prepared on a basis consistent with our audited financial statements and include all adjustments, consisting of normal and recurring adjustments that we consider necessary for a fair presentation of the financial position and results of operations as of and for such periods.
| For the year ended December 31, | For the three months ended March 31, | |||||||||||||||
| 2012 | 2011 | 2013 | 2012 | |||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||
Option revenue | $ | — | $ | — | $ | 220,100 | $ | — | ||||||||
Research and development | 6,318,476 | 3,340,038 | 1,621,366 | 1,490,097 | ||||||||||||
General and administrative | 2,508,541 | 2,406,269 | 1,102,435 | 816,196 | ||||||||||||
Depreciation and amortization | 303,677 | 210,252 | 78,311 | 67,355 | ||||||||||||
Loss on disposal of fixed assets | 3,097 | 9,686 | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating loss | (9,133,791 | ) | (5,966,245 | ) | (2,582,012 | ) | (2,373,648 | ) | ||||||||
Interest income | 280 | 932 | 1,874 | 136 | ||||||||||||
Gain on derivative instruments | 552,978 | — | — | — | ||||||||||||
Other income and expense, net | — | — | 37,910 | — | ||||||||||||
Interest expense | (350,300 | ) | (3,135 | ) | (1,635,254 | ) | (487 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (4,177,482 | ) | $ | (2,373,999 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Basic and diluted loss per share | $ | (1.54 | ) | $ | (1.06 | ) | $ | (0.58 | ) | $ | (0.41 | ) | ||||
Weighted average shares outstanding | 5,785,372 | 5,633,124 | 7,239,102 | 5,762,028 | ||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||
Cash and cash equivalents | $ | 592,004 | $ | 7,109,215 | $ | 7,834,336 | $ | 4,677,956 | ||||||||
Other current assets | 1,077,546 | 124,773 | 1,166,430 | 1,005,756 | ||||||||||||
Fixed assets, net | 1,265,041 | 1,029,236 | 1,189,328 | 1,390,674 | ||||||||||||
Restricted cash | 1,000,000 | — | 500,000 | — | ||||||||||||
Deferred financing costs, net | 211,479 | — | 158,540 | — | ||||||||||||
Other long-term assets | — | — | 104,027 | — | ||||||||||||
Total assets | 4,146,070 | 8,263,224 | 10,952,661 | 7,074,386 | ||||||||||||
Total current liabilities | 885,975 | 1,067,860 | 3,157,125 | 2,047,601 | ||||||||||||
Notes payable, net | 376,763 | — | 376,627 | — | ||||||||||||
Deferred revenue | — | — | 3,384,552 | — | ||||||||||||
Total stockholders’ equity | 2,883,332 | 7,195,364 | 4,034,357 | 5,026,785 | ||||||||||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Our results of operations and cash flows should be read in conjunction with our unaudited consolidated financial statements and notes thereto and the audited financial statements and the notes thereto included elsewhere in this prospectus.
Business Overview
Unless otherwise indicated, we use “Opexa,” “the Company,” “we,” “our” and “us” to refer to the businesses of Opexa Therapeutics, Inc.
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS). This therapy is based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM by aligning the interests of patients, employees and shareholders. Information related to our product candidate, Tcelna™, is preliminary and investigative. Tcelna has not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is a chronic, often disabling disease that affects the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. The progress, severity and specific symptoms of MS are unpredictable and vary(i) $0.50 per share from one person to another. We believe that our product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths were demyelination has occurred (remyelination).
Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of Secondary Progressive MS (SPMS) and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Critical Accounting Policies
General.Our discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from
other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our most significant judgments and estimates used in preparation of our financial statements.
Revenue Recognition. We adopted the provisions of FASB ASC 605, Revenue Recognition. ASC 605 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) consideration is fixed or determinable; and (4) collectability is reasonably assured.
We evaluated the Option and License Agreement (the “Merck Agreement”) with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., and determined that the $5 million upfront payment from Merck has “stand-alone value.” Opexa’s continuing performance obligations, in connection with the $5 million payment, include the execution and completion of the Abili-T clinical trial in SPMS using commercially reasonable efforts at our own costs. As a “stand-alone value” term in the Merck Agreement, the $5 million upfront payment is determined to be a single unit of accounting, and is recognized as revenue on a straight-line basis over the exclusive option period based on the expected completion term of the Abili-T clinical trial in SPMS.
Stock-Based Compensation.We adopted the provisions of FASB ASC 718 which establishes accounting for equity instruments exchanged for employee service. We utilize the Black-Scholes option pricing model to estimate the fair value of employee stock based compensation at the date of grant, which requiresissuance through June 30, 2016 and (ii) $1.50 per share from July 1, 2016 through the inputexpiration three years from the date of highly subjective assumptions, including expected volatility and expected life. Changes in these inputs and assumptions can materially affect the measure of estimated fair valueissuance. Each record holder will receive one Subscription Right for each whole share of our share-based compensation. These assumptions are subjective and generally require significant analysis and judgment to develop. When estimating fair value, somecommon stock owned by such record holder as of the assumptionsRecord Date. Each holder of an outstanding Series L warrant will be basedreceive one Subscription Right for each whole share of our common stock into which such Series L warrant is exercisable on the Record Date. Each Subscription Right entitles the record holder or determined from, external dataholder of a Series L warrant to a Basic Subscription Right and other assumptions may be derived fromanOver-Subscription Privilege.
Basic Subscription Rights
Your Basic Subscription Rights will entitle you to purchase one share of our historical experience with stock-based payment arrangements. The appropriate weightcommon stock and a Warrant to place on historical experience is a matterpurchase one share of judgment, based on relevant facts and circumstances.
We estimated volatility by considering historicalour common stock. For example, if you owned 100 shares of common stock volatility. We have opted to use the simplified method for estimating expected term of options as equal to the midpoint between the vesting period and the contractual term.
Research and Development.The costs of materials and equipment or facilities that are acquired or constructed for research and development activities and that have alternative future uses are capitalized as tangible assets when acquired or constructed. The cost of such materials consumed in research and development activities and the depreciation of such equipment or facilities used in those activities are research and development costs. However, the costs of materials, equipment, or facilities acquired or constructed for research and development activities that have no alternative future uses are considered research and development costs and are expensed at the time the costs are incurred.
Results of Operations and Financial Condition
Comparison of the Three Months Ended March 31, 2013 withRecord Date, you will receive 100 Subscription Rights and will have the Three Months Ended March 31, 2012
Revenue.Revenue of $220,100 related to the $5 million upfront payment from Merck in conjunction with the Option and License Agreement was recognized for the three months ended March 31, 2013. No revenues were recognized during the three months ended March 31, 2012.
Research and Development Expenses.Research and development expenses were $1,621,366 for the three months ended March 31, 2013, compared with $1,490,097 for the three months ended March 31, 2012. The increase in expenses is primarily due to an increase of staff to conduct increased development activities, an increase in the procurement and use of supplies for our product manufacturing and development operations and the engagement of clinical sites for the clinical study of Tcelna in SPMS, and was partially offset by decreases in the use of consultants and contract development costs.
General and Administrative Expenses.General and administrative expenses for the three months ended March 31, 2013 were $1,102,435, compared with $816,196 for the three months ended March 31, 2012. The increase in expense is due to an increase in capital financing expenses and a one-time severance charge, and was partially offset by a decrease in legal expense.
Depreciation and Amortization Expenses.Depreciation and amortization expenses for the three months ended March 31, 2013 were $78,311, compared with $67,355 for the three months ended March 31, 2012. The increase in expense is due to increase in depreciation for laboratory and manufacturing equipment acquired during 2012 to support increased development activities.
Interest Expense. Interest expense was $1,635,254 for the three months ended March 31, 2013, compared to $487 for the three months ended March 31, 2012. The increase in interest expense was primarily related to the amortized debt discount and interest on both the July 25, 2012 convertible secured promissory notes and the January 23, 2013 convertible promissory notes and the amortization of the financing fees over the life of the notes. Interest expense for the three months ended March 31, 2012 related solely to the financing of insurance premiums.
Interest Income. Interest income was $1,874 for the three months ended March 31, 2013, compared to $136 for the three months ended March 31, 2012.
Other Income. Other income of $37,910 for the three months ended March 31, 2013 was related to the extinguishment of membership interests in the mutual insurance company that we participated in for our product liability insurance through January 1, 2013. We recorded no other income for the three months ended March 31, 2012.
Net loss. We had a net loss for the three months ended March 31, 2013 of approximately $4.18 million, or $0.58 per share (basic and diluted), compared with a net loss of approximately $2.37 million or $0.41 per share (basic and diluted) for the three months ended March 31, 2012. The increased net loss is primarily related to increases in compensation and severance costs, capital financing expenses, the procurement and use of supplies for both our laboratory and our product manufacturing operations, the engagement of clinical sites for the clinical study of Tcelna in SPMS, depreciation expense and interest expense, and was partially offset by an increases in revenue and other income and decreases in legal expenses, consulting costs and contract development costs.
Comparison of Year Ended December 31, 2012 with the Year Ended December 31, 2011
Net Sales.We recorded no commercial revenues for the years ended December 31, 2012 and 2011.
Research and Development Expenses.Research and development expenses were $6,318,476 for the year ended December 31, 2012, compared to $3,340,038 for the year ended December 31, 2011. The increase in expenses was primarily due to an increases in staff to conduct increased development activities, the procurement and use of supplies used in both our laboratory and product manufacturing operations, the engagement of consultants and the costs of subject participation in our Phase IIb clinical study, facilities costs and stock compensation expense, and was partially offset by a decrease in legal costs. We have made and expect to continue to make substantial investments in research and development in order to develop and market our technology. We expense research and development costs as incurred. Acquired research and development that has no alternative future use is expensed when acquired. Property, plant and equipment for research and development that has an alternative future use is capitalized and the related depreciation is expensed.
General and Administrative Expenses.Our general and administrative expenses were $2,508,541 for the year ended December 31, 2012, as compared to $2,406,269 for the year ended December 31, 2011. The increase in expense is due to increases in legal expense, capital financing activities, stock compensation expense and facilities costs, and was partially offset by a reduction in professional service fees.
Depreciation and Amortization Expenses. Depreciation and amortization expenses were $303,677 for the year ended December 31, 2012, as compared to $210,252 for the year ended December 31, 2011. The increase in expense is due to an increase in depreciation for facility build-out costs incurred during the first half of 2011, an increase in depreciation for laboratory and manufacturing equipment acquired during 2011 and 2012 to support increased development activities and an increase in depreciation for information technology equipment to replace and upgrade obsolete equipment.
Interest Expense. Interest expense was $350,300 for the year ended December 31, 2012, compared to $3,135 for the year ended December 31, 2011. The increase in interest expense was primarily related to the non-cash amortized debt discount and interest on the July 25, 2012 convertible notes and the amortization of the financing fees over the life of the notes. Interest expense for the year ended December 31, 2011 related solely to the financing costs on insurance policies and the loan payable on an equipment line.
Interest Income. Interest income was $280 for the year ended December 31, 2012, compared to $932 for the year ended December 31, 2011.
Net Loss. We had a net loss for the year ended December 31, 2012 of $8,930,833, or $1.54 per share (basic and diluted), compared with a net loss of $5,968,448, or $1.06 per share (basic and diluted), for the year ended December 31, 2011. The increase in net loss is primarily due to increases in research and development, general and administrative, depreciation and interest expenses.
Liquidity and Capital Resources
Historically, we have financed our operations primarily from the sale of debt and equity securities. As of June 30, 2013, we had cash and cash equivalents of approximately $5.0 million, excluding $500,000 of restricted cash which is subject to a deposit control agreement in favor of the holders of our July 2012 convertible notes.
During January 2013, we closed a private offering consisting of convertible notes and Series J warrantsright to purchase common stock which generated $650,000 in gross proceeds, of which we repaid $550,000 and converted $100,000 to 77,034100 shares of our common stock during February 2013.
Also during January 2013, we sold 125,000 shares of our common stock to Lincoln Park for gross proceeds of $142,400 under a $1.5 million purchase agreement entered into in November 2012. We also entered into a $15 million purchase agreement and registration rights agreement with Lincoln Park in November 2012. Pursuant to these agreements, we have the right to sell to Lincoln Park an aggregate of up to $16.5 million in shares of our common stock, subject to certain conditions and limitations. Under the terms and subject to the conditions of the purchase agreements, Lincoln Park is obligatedWarrants to purchase up to an aggregate of $16.5 million in shares of common stock (subject to certain limitations) from time to time over a 30-month period. We may direct Lincoln Park, at our sole discretion and subject to certain conditions, to purchase up to 100,000 shares of common stock in regular purchases, increasing to amounts of up to 300,000 shares depending upon the closing sale price of our common stock. In addition, we may direct Lincoln Park to purchase additional amounts as accelerated purchases. The purchase price of shares of common stock related to the future funding will be based on the prevailing market prices of such shares at the time of sales (or over a period of up to 12 business days leading up to such time), but in no event will shares be sold to Lincoln Park on a day the common stock closing price is less than the adjusted minimum floor price of $1.00. As of July 19, 2013, we have sold 390,000 shares to Lincoln Park under the $1.5 million purchase agreement for gross proceeds of $523,709, and we have a remaining commitment amount of $15,976,291 available to us through Lincoln Park purchase agreements. However, there can be no assurance that we will be able to receive any or all of the additional funds from Lincoln Park because the purchase agreements contain limitations, restrictions, requirements, events of default and other provisions that could limit our ability to cause Lincoln Park to buy common stock from us.
Pursuant to a waiver executed in February 2013 by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 convertible promissory notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced from $1 million to $500,000.
In February 2013, we entered into the Merck Agreement. Pursuant to this agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon our completion of the Phase IIb trial. Under the terms of the Merck Agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS.
Also during February 2013, we sold an aggregate of 167,618100 shares of our common stock for gross proceeds$0.70 per whole Unit, or a total payment of $536,417, pursuant$70.00. You may exercise all or a portion of your Basic Subscription Rights, or you may choose not to a sales agreement entered into with Brinson Patrick Securities Corporationexercise any of your Basic Subscription Rights. If you do not exercise your Basic Subscription Rights in September 2012full, you will not be entitled to exercise your Over-Subscription Privilege.
Over-Subscription Privilege
If you exercise your Basic Subscription Rights in connection withfull, you may also choose to exercise your Over-Subscription Privilege. Subject to proration and stock ownership limitations, if applicable, we will seek to honor the implementationOver-Subscription Privilege requests in full. If Over-Subscription Privilege requests exceed the number of an “at-the-market” offering program. PursuantUnits available, however, we will allocate the available Units pro rata among the record holders and Series L warrant holders exercising the Over-Subscription Privilege in proportion to the sales agreement, we may sellnumber of shares of our common stock directly intoeach of those record holders owned or the open market from timenumber of shares underlying Series L warrants held by each of those warrant holders on the Record Date, relative to time depending upon market demand, through our sales agent,the number of shares owned or underlying Series L warrants on the Record Date by all record holders and Series L warrant holders exercising the Over-Subscription Privilege. If this pro rata allocation results in transactions deemedany record holder or Series L warrant holder receiving a greater number of Units than the record holder or Series L warrant holder subscribed for pursuant to be an “at-the-market” offering as defined in Rule 415the exercise of the Securities ActOver-Subscription Privilege, then such record holder or Series L warrant holder will be allocated only that number of 1933. Units for which the record holder or Series L warrant holder oversubscribed, and the remaining Units will be allocated among all other record holders and Series L warrant holders exercising the Over-Subscription Privilege on the same pro rata basis described above. The proration process will be repeated until all Units have been allocated.
Continental Stock Transfer & Trust Company, the Subscription Agent for the Rights Offering, will determine the over-subscription allocation based on the formula described above.
To the extent the aggregate subscription payment of the actual number of unsubscribed Units available to you pursuant to the Over-Subscription Privilege is less than the amount you actually paid in connection with the exercise of the Over-Subscription Privilege, you will be allocated only the number of unsubscribed Units available to you, and any excess subscription payments will be returned to you, without interest or penalty, with 10 business days after expiration of the Rights Offering.
We have registered upcan provide no assurances that you will actually be entitled to 1,000,000purchase the number of Units issuable upon the exercise of your Over-Subscription Privilege in full at the expiration of the Rights Offering. We will not be able to satisfy any requests for Units pursuant to the Over-Subscription Privilege if all of our shareholders and Series L warrant holders exercise their Basic Subscription Rights in full, and we will only honor an Over-Subscription Privilege to the extent sufficient Units are available following the exercise of Basic Subscription Rights.
Our Participating Warrant Holders
The terms and conditions of our currently outstanding Series L warrants to purchase shares of our common stock for potential sale underentitle the holders of these warrants to participate in this program. AsRights Offering. None of July 19, 2013, 167,618our other currently outstanding warrants is entitled to receive Subscription Rights in this offering.
The Series L warrants were originally issued in a private placement of shares had been soldof our common stock and 832,382 shares remain available for future sale under the sales agreement.
We closedwarrants on February 11, 2013. Series L warrants to purchase an offering in February 2013aggregate of 1,083,334541,668 shares of common stock at an exercise price of $3.00 per share are currently outstanding. These warrants expire on February 11, 2017. The Series L warrants provide that the holders are entitled to participate in a rights offering as if each of such warrants had been exercised immediately prior to the record date for a rights offering. As a result, holders of our Series L warrants are receiving Subscription Rights for an aggregate of 541,668 Units in connection with this Rights Offering.
Limitation on the Purchase of Units
You may only purchase the number of whole Units purchasable upon exercise of the number of Basic Subscription Rights distributed to you in the Rights Offering, plus the Over-Subscription Privilege, if any. Accordingly, the number of Units that you may purchase in the Rights Offering is limited by the number of shares of our common stock you held on the Record Date or the number of shares underlying your outstanding warrants and by the extent to which other shareholders and warrant holders exercise their Basic Subscription Rights and Over-Subscription Privileges, which we cannot determine prior to completion of the Rights Offering. However, due to stock exchange restrictions, we will not issue Units in the Rights Offering to the extent that a holder would beneficially own, together with any other person with whom such holder’s securities may be aggregated under applicable law, more than 19.9% of our outstanding shares of common stock.
Subscription Price
The Subscription Price is $0.70 per Unit. The Subscription Price does not necessarily bear any relationship to our past or expected future results of operations, cash flows, current financial condition, or any other established criteria for value. No change will be made to the Subscription Price by reason of changes in the trading price of our common stock or other factor prior to the expiration of this Rights Offering.
Determination of Subscription Price
In the determining the Subscription Price, the board of directors considered a variety of factors including those listed below:
The Subscription Price does not necessarily bear any relationship to any established criteria for value. No valuation consultant or investment banker has opined upon the fairness or adequacy of the Subscription Price. You should not consider the Subscription Price as an indication of actual value of our company or our common stock. You should not assume or expect that, after the Rights Offering, our shares of common stock will trade at or above the Subscription Price in any given time period. The market price of our common stock may decline during or after the Rights Offering. We cannot assure you that you will be able to sell the shares of our common stock purchased during the Rights Offering at a price equal to or greater than the Subscription Price. You should obtain a current price quote for our common stock before exercising your Subscription Rights and make your own assessment of our business and financial condition, our prospects for the future, and the terms of this Rights Offering. Once made, all exercises of Subscription Rights are irrevocable.
No Recombination
The common stock and Warrants comprising the Units will separate upon the exercise of the Subscription Rights, and the Units will not trade as a separate security. Holders may not recombine shares of common stock and Warrants to receive a Unit.
Non-Transferability of Subscription Rights
The Subscription Rights are non-transferable (other than by operation of law) and, therefore, you may not sell, transfer, assign or give away your Subscription Rights to anyone. The Subscription Rights will not be listed for trading on any stock exchange or market.
Expiration Date; Extension
The subscription period, during which you may exercise your Subscription Rights, expires at 5:00 p.m., Eastern Time, on April 8, 2015, which is the expiration of the Rights Offering. If you do not exercise your Subscription Rights before that time, your Subscription Rights will expire and will no longer be exercisable. We will not be required to issue shares to you if the Subscription Agent receives your Rights Certificate or your subscription payment after that time. We have the option to extend the Rights Offering in our sole discretion, although we do not presently intend to do so. We may extend the Rights Offering by giving oral or written notice to the Subscription Agent before the Rights Offering expires. If we elect to extend the Rights Offering, we will issue a press release announcing the extension no later than 9:00 a.m., Eastern Time, on the next business day after the most recently announced expiration date of the Rights Offering.
If you hold your shares of common stock in the name of a broker, dealer, custodian bank or other nominee, the nominee will exercise the Subscription Rights on your behalf in accordance with your instructions. Please note that the nominee may establish a deadline that may be before 5:00 p.m., Eastern Time, on April 8, 2015, which is the expiration date that we have established for the Rights Offering.
Termination
We may terminate the Rights Offering at any time and for any reason prior to the completion of the Rights Offering. If we terminate the Rights Offering, we will issue a press release notifying shareholders, warrant holders and the public of the termination.
Return of Funds upon Completion or Termination
The Subscription Agent will hold funds received in payment for shares in a segregated account pending completion of the Rights Offering. The Subscription Agent will hold this money until the Rights Offering is completed or is terminated. To the extent you properly exercise your Over-Subscription Privilege for an amount of Units that exceeds the number of unsubscribed Units available to you, any excess subscription payments will be returned to you within 10 business days after the expiration of the Rights Offering, without interest or penalty. If the Rights Offering is terminated for any reason, all subscription payments received by the Subscription Agent will be returned within 10 business days, without interest or penalty.
Shares of Our Common Stock Outstanding After the Rights Offering
Based on 28,234,751 shares of common stock outstanding as of December 31, 2014, as well as Series L warrants to purchase 541,668 shares of common stock for gross proceeds of $3,250,002. As part ofoutstanding on that offering, we agreed not to sell shares pursuant to our purchase agreements with Lincoln Park or under our “at-the-market” program for a period of 120 days following such February 2013 offering (or June 11, 2013).
Our cash burn rate during the three months ended March 31, 2013, inclusive of the cost of our ongoing Abili-T clinical study, was approximately $775,000 per month. During this three-month period, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Merck for the Option as well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. Significant activities in the conduct of the Abili-T clinical trial are expected to result in substantial increases in our monthly cash burn during the balance of 2013. Based on our projected burn rate increasing to an average of $1.2 million per month for the remainder of 2013, we believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013.
We currently intend to continue to use our available cash to fund general corporate purposes (including working capital and operational purposes) and continue the ongoing Abili-T clinical study of Tcelna in SPMS. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with resulting top-line data expected to be available in the first half of 2016. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that at least $9.8 million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expenses of our operations during such development and for general corporate purposes. We will need to secure significant additional resources to continue and complete the trial and support our operations during the pendency of the trial. If we are unable to obtain additional funding
for operations, we will be forced to suspend or terminate our current ongoing clinical trial for Tcelna, which may require us to modify our current business plan and curtail various aspects of our operations, as well as implement significant cost-reduction measures or potentially cease operations.
There can be no assurance that any such financing arrangements can be consummated on acceptable terms, if at all. Given our need for substantial amounts of capital to continue the Abili-T clinical study in North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the additional resources, including one or more financings, that will be necessary to complete the ongoing Phase IIb study and to support ongoing operations during the pendency of such study.
If Merck does not exercise the Option to acquire the exclusive, worldwide (excluding Japan) license of our Tcelna program for MS, or if we are not successful in attracting another partner and entering into a collaboration on acceptable terms, we may not be able to complete development of or commercialize any product candidate, including Tcelna. In particular, we may be unable to undertake, or complete, the planned Phase III clinical study of Tcelna in SPMS. In such event, our ability to generate revenues and achieve or sustain profitability would be significantly hindered and we may not be able to continue operations as proposed, requiring us to modify our business plan, curtail various aspects of our operations or cease operations.
We do not maintain any external lines of credit. Should we need any additional capital in the future beyond the purchase agreements with Lincoln Park and our at-the-market program, management will be reliant upon “best efforts” debt or equity financings. As our prospects for funding, if any, develop during the fiscal year, we will assess our business plan and make adjustments accordingly. Although we have successfully funded our operations to date by attracting additional investors in our equity and debt securities, there is no assurance that our capital raising efforts will be able to attract additional necessary capital for our operations in the future.
Assuming we are able to achieve financing which is sufficient to continue the Abili-T study in North America and to support our operations during the pendency of such study, we are also able to concurrently manage a pivotal Phase III clinical study in RRMS in North America in our present facility. Any such RRMS studies would also depend upon the availability of sufficient resources.
Off-Balance Sheet Arrangements
None.
Inflation
We believe that inflation has not had a material impact on our results of operations for the two years ended December 31, 2012 and 2011, since inflation rates have generally remained at relatively low levels and our operations are not otherwise uniquely affected by inflation concerns.
Recent Accounting Pronouncements
On July 1, 2009, the FASB officially launched the FASB Accounting Standards Codification, which has become the single official source of authoritative, nongovernmental U.S. Generally Accepted Accounting Principles, in addition to guidance issued by the Securities and Exchange Commission. The codification supersedes all prior FASB, AICPA, EITF, and related literature. The codification, which is effective for interim and annual periods ending after September 15, 2009, is organized into approximately 90 accounting topics. The FASB no longer issues new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, amendments to the codification are made by issuing “Accounting Standards Updates.”
There were various other accounting standards and interpretations issued during 2012 and 2011, none of which are expected to have a material impact on our financial position, operations or cash flows.
For the three months ended March 31, 2013, there were no accounting standards or interpretations issued that are expected to have a material impact on our financial position, operations or cash flows.
Overview
Opexa is a biopharmaceutical company developing a personalized immunotherapy with the potential to treat major illnesses, including multiple sclerosis (MS). This therapy is based on our proprietary T-cell technology. Our mission is to lead the field of Precision ImmunotherapyTM by aligning the interests of patients, employees and shareholders. Information related to our product candidate, Tcelna®, is preliminary and investigative. Tcelna has not been approved by the U.S. Food and Drug Administration (FDA) or other global regulatory agencies for marketing.
MS is an inflammatory autoimmune disease of the central nervous system (CNS), which is made up of the brain, spinal cord and optic nerves, with a clinically heterogeneous and unpredictable course that persists for decades. MS attacks the covering surrounding nerve cells, or myelin sheaths, leading to loss of myelin (demyelination) and nerve damage. In addition to demyelination, the neuropathology of MS is characterized by variable loss of oligodendroglial cells and axonal degeneration and manifests in neurological deficits. Symptoms may be mild, such as numbness in the limbs, or severe, such as paralysis or loss of vision. This inflammatory, demyelinating, autoimmune disease has varied clinical presentations, ranging from relapses and remissions (relapsing remitting MS, or RRMS) to slow accumulation of disability with or without relapses (secondary progressive MS, or SPMS). There are approximately 450,000 MS patients in North America and over 2,000,000 patients worldwide according to estimates from The National MS Society. The SPMS patient population is estimated by various industry sources to be between 30-45% of the total MS patient population.
We believe that our product candidate, Tcelna, has the potential to fundamentally address the root cause of MS by stopping the demyelination process and in supporting the generation of new myelin sheaths where demyelination has occurred (remyelination). Tcelna is an autologous T-cell immunotherapy that is currently being developed for the treatment of SPMS and is specifically tailored to each patient’s immune response profile to myelin. Tcelna is designed to reduce the number and/or functional activity of specific subsets of myelin-reactive T-cells (MRTCs) known to attack myelin. This technology was originally licensed from Baylor College of Medicine in 2001.
Tcelna is manufactured using our proprietary method for the production of an autologous T-cell product, which comprises the collection of blood from the MS patient and the expansion of MRTCs from the blood. Upon completion of the manufacturing process, an annual course of therapy consisting of five doses is cryopreserved. At each dosing time point, a single dose of Tcelna is formulated and attenuated by irradiation before returning the final product to the clinical site for subcutaneous administration to the patient.
Tcelna has received Fast Track designation from the FDA in SPMS, and we believe it is positioned as a potential first-to-market personalized T-cell therapy for MS patients. The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drug candidates intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs.
Opexa was incorporated in Texas in March 1991. Our principal executive offices are located at 2635 Technology Forest Blvd., The Woodlands, Texas 77381, and our telephone number is (281) 775-0600.
Multiple Sclerosis—Background
MS is a disease that is more common in females than males (2:1) between the ages of 20 and 40, with a peak onset of approximately 25 years of age. MS frequently causes impairment of motor, sensory, coordination and balance, visual, and/or cognitive functions, as well as urinary (bladder) or bowel dysfunction and symptoms of fatigue. The identified autoimmune mechanisms directed at myelin tissue of the CNS may play an important role in the pathogenesis of MS. Epidemiologic studies suggest that a variety of genetic, immunologic, and environmental factors including viral infections may play a role in defining the etiology and in triggering the onset and progression of MS.
At the onset of MS, approximately 85% of MS patients have RRMS. Without disease-modifying medication, one-half of these RRMS patients will develop steadily progressive disease, SPMS, within 10 years, increasing to 90% within 25 years of MS diagnosis. The MS drug market was approximately $13 billion in 2012 and is forecasted to reach as much as $16 billion by 2015.
MS remains a challenging autoimmune disease to treat because the pathophysiologic mechanisms are diverse, and the chronic, unpredictable course of the disease makes it difficult to determine whether the favorable effects of short-term treatment will be sustained. Therapies that are easy to use and can safely prevent or stop the progression of disease represent the greatest unmet need in MS.
In recent years, the understanding of MS pathogenesis has evolved to comprise an initial, T-cell-mediated inflammatory activity followed by selective demyelination (erosion of the myelin coating of the nerve fibers) and then neurodegeneration. The discovery of disease-relevant immune responses has accelerated the development of targeted therapeutic products for the treatment of the early stages of MS. Some subjects, who have the appropriate genetic background, have increased susceptibility for thein vivo activation and expansion of MRTCs. These MRTCs may remain dormant, but at some point they are activated in the periphery, thus enabling them to cross the blood-brain barrier and infiltrate the healthy tissue of the brain and spinal cord. The cascade of pathogenic events leads to demyelination of protrusions from nerve cells called axons, which causes nerve impulse transmissions to diffuse into the tissue resulting in disability to the individual.
Tcelna for MS
We believe that Tcelna works selectively on the MRTCs by harnessing the body’s natural immune defense system and feedback mechanisms to deplete these T-cells and induce favorable immune regulatory responses by rebalancing the immune system. Tcelna is manufactured by isolating the MRTCs from the blood, expanding them to a therapeutic doseex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. These attenuated MRTCs are then injected subcutaneously into the body in therapeutic dosages. The body recognizes specific T-cell receptor molecules of these MRTCs as immunogenic and initiates an immune response reaction against them, resulting in the depletion and/or immunosuppression of circulating, myelin reactive T-cells carrying the peptide-specific T-cell receptor molecules. In addition, we believe that T-cell activation molecules on the surface of the activated MRTCs, that constitute the Tcelna product, promote anti-inflammatory responses. Because the therapy uses an individual’s own cells, the only direct identifiable side effect observed thus far is injection site reactions which typically are minor and generally clear within 24 hours.
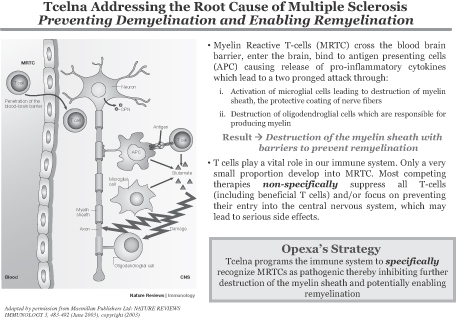
Tcelna Clinical Development Program
Tcelna is a novel T-cell immunotherapy in Phase IIb clinical development for the treatment of patients with SPMS. It is also positioned to enter Phase III clinical development for the treatment of patients with RRMS, subject to the availability of sufficient resources. Tcelna is a personalized immunotherapy that is specifically tailored to each patient’s disease profile. Tcelna is manufactured using ImmPathTM, our proprietary method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG) and proteolipid protein (PLP), and the return of these expanded, irradiated T-cells back to the patient. These attenuated T-cells are reintroduced into the patient via subcutaneous injection to trigger a therapeutic immune system response.
The Tcelna clinical development program spans studies conducted at Baylor College of Medicine and Opexa conducted clinical studies. A summary of the various Tcelna clinical studies is shown below:
| Phase | Completion Dates | Population | Total N | Treatment Duration | Tcelna | Placebo | ||||||
| Baylor | 1998 | RRMS, PPMS, SPMS (26) | 114 | Up to 24 months | 114 | |||||||
| Phase I dose escalation | 2006 | RRMS (5) SPMS (6) | 16 | 12 | 16 | — | ||||||
Phase I/II Open label retreatment | 2007 | RRMS (9) SPMS (4) | 13 | 12 | 13 | — | ||||||
Phase IIb TERMS | 2008 | RRMS, CIS | 150 | 12 | 100 | 50 | ||||||
| Phase IIb extension OLTERMS | 2008 | RRMS, CIS | 38 | At least one dose post TERMS | 15 from placebo arm | |||||||
Phase IIb Abili-T | 1st Half 2016* | SPMS | 180* | 24* | 90* | 90* |
Summary of Phase I Dose Escalation Study in MS
A Phase 1 dose escalation study completed in 2006 was conducted in patients with both RRMS and SPMS who were intolerant or unresponsive to current approved therapies for MS. The open-label, dose escalation study evaluated safety and clinical benefit by administering a primary series of four treatments at one of three dose levels administered at baseline and weeks four, eight and twelve. Results of the efficacy analyses provide some evidence of the effectiveness of Tcelna in the treatment of MS. Data from the Phase I study evaluating the Expanded Disability Status Scale (EDSS) showed improvements in some subjects in comparison to baseline for weeks 20 and 28.
Subjects showed statistically significant improvement in overall reduction of MRTC counts over baseline at all visits through week 52 for subjects receiving 30-45 million cells per dose, as assessed by total MRTC count percentage changes. These data indicate that Tcelna treatment causes a depletion or immunomodulation of these cells, most obvious at time points closer to the injections. These findings, which demonstrate that administration of Tcelna induces a reduction in MRTCs, were published in Clinical Immunology (2009) 131, 202-215.
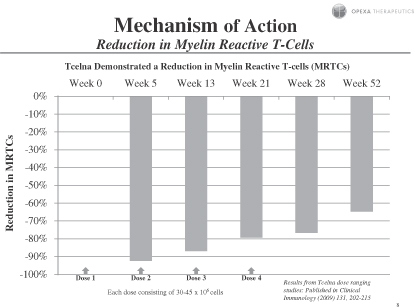
Overall, results of the safety analyses indicate that treatment with Tcelna is well-tolerated. Reported adverse events were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. In conclusion, data from this study suggest that Tcelna is safe for the treatment of MS.
Summary of Phase I/IIA Clinical Trial Data in MS
The second clinical study performed by Opexa was an open-label extension study completed in 2007 to treat patients who were previously treated with T-cell immunotherapy but who saw a rebound in MRTC activity. The purpose of this extension study was to continue evaluating the efficacy, safety and tolerability of Tcelna in patients with RRMS and SPMS with repeated administration of Tcelna. Results of the study provide evidence of the effectiveness of Tcelna in the treatment of MS with repeated dosing. Improvements from baseline at both week 28 and week 52 of the extension study were observed for the frequency of MS exacerbations (ARR). Evaluation of the Multiple Sclerosis Impact Scale (MSIS-29) component scores suggests a trend for Tcelna therapy in the improvement of physical and psychological parameters assessed by the MSIS-29. The EDSS score analysis revealed an upward trend for the percentage of subjects that reported improvement and sustained improvement over baseline as a result of Tcelna treatment.
Subjects showed statistically significant improvement over baseline in the MRTC counts for each time point through month nine of the extension study. These results indicate that Tcelna treatment results in a statistically significant impact on these cells.
Overall, results of the safety analyses indicate that repeated treatment with Tcelna is well-tolerated. Reported adverse events (AEs) were mostly mild or moderate in intensity. Mild injection site reactions were observed but all resolved rapidly without treatment. Furthermore, results from this study suggest that repeated dosing of Tcelna has a substantive effect in reduction of ARR in subjects with MS and was well-tolerated.
Summary of TERMS Phase IIb Clinical Trial Data in RRMS
Tovaxin for Early Relapsing Multiple Sclerosis (TERMS) was a Phase IIb clinical study of Tcelna in RRMS patients completed in 2008. Although the study did not show statistical significance in its primary endpoint (the cumulative number of gadolinium-enhanced brain lesions using MRI scans summed at various points in the study), the study showed compelling evidence of efficacy in various clinical and other MRI endpoints.
The TERMS study was a multi-center, randomized, double blind, placebo-controlled trial in 150 patients with RRMS or high risk Clinically Isolated Syndrome. The inclusion criteria for TERMS was an EDSS score of 0 to 5.5. Patients received a total of five subcutaneous injections at weeks 0, 4, 8, 12 and 24. Key results from the TERMS trial included:
In the modified intent to treat patient population consisting of all patients who received at least one dose of study product and had at least one MRI scan at week 28 or later (n=142), the ARR for Tcelna-treated patients was 0.214 as compared to 0.339 for placebo-treated patients, which represented a 37% decrease in ARR for Tcelna as compared to placebo in the general population;
|

In a retrospective analysis in patients naïve to previous disease modifying treatment, the results showed that patients, when treated with Tcelna, had a 56% to 73% reduction in ARR versus placebo for the various subsets and p values ranged from 0.009 to 0.06.

We remain committed to further advancing Tcelna in RRMS at a later date, assuming the availability of sufficient resources. For Opexa, however, SPMS is an area which we believe represents a higher unmet medical need.
SPMS Overview and Tcelna Mechanism of Action
SPMS is characterizedno other transactions by a steady accrual of irreversible disability, despite, in some cases, relapses followed by remissions or clinical plateaus. Older age at onset of MS diagnosis is the strongest predictor of conversion to SPMS. Males have a shorter time to conversion to SPMS compared with females. Available immunomodulating and immunosuppressive therapies used for RRMS have not been effective in SPMS. In clinical trials, these therapies have demonstrated anti-inflammatory properties as measured by the reduction in number and volume of contrast-enhancing or acutely inflammatory CNS lesions most commonly seen in patients with RRMS. The typical SPMS patient, however, has little or no radiographic evidence of acute inflammation. It is commonly observed that contrast-enhancing CNS lesions are uncommon among these patients, despite a clearly deteriorating neurologic course.
The lack of effect of conventional MS therapeutics in SPMS suggests that the cerebral deterioration characterizing progressive disease may be driven by factors other than acute inflammation. For instance, the immunopathology of SPMS is more consistent with a transition to a chronic T-cell dependent inflammatory type, which may encompass the innate immune response and persistent activation of microglia cells. Meningeal follicles close to cortical gray matter lesions suggests that adaptive immune responsesus involving antibody and complement contribute to progression in SPMS. Furthermore, chronic MRTCs may be contributing to the development of both innate and adaptive immune responses persisting in the CNS.
Radiographic features that stand out among patients with SPMS include significantly more atrophy of gray matter compared with RRMS patients. Of note, long-term disability in MS in general appears more closely correlated to gray matter atrophy than to white matter inflammation. Such atrophy may be suggestive of progressive clinical disability. Both clinically and radiographically, SPMS represents a disease process with certain features distinct from those of RRMS, and one with extremely limited treatment options.
Tcelna immunotherapy in SPMS may reduce the drivers of this chronic disease by down-regulating anti-myelin immunity through priming regulatory responses that may act in the periphery as well as within the CNS. We believe that our clinical results show therapeutic subcutaneous dosing of 30-45 million cells of Tcelna stimulates host reactivity to the over-represented MRTCs and, as a consequence, a dominant negative regulatory T-cell response is induced leading to down-regulation of similar endogenous disease-causing MRTCs.
We believe that Tcelna has the potential to induce an up-regulation of regulatory cells, such as Foxp3+ Treg cells and IL-10 secreting Tr1 cells, which may effect a reduction in inflammation and provide neuroprotection should they gain entry to the CNS. Data from our TERMS study showed statistically significant changes from baseline (p=0.02) in Foxp3+ Treg cells for the subset of Tcelna patients who had ARR>1. The placebo arm for this subset was not statistically different from its baseline levels. Three SPMS patients from prior clinical studies, whose blood samples were analyzed to measure Tr1 cells prior to treatment and post treatment, showed an increase in the levels of Tr1 cells from non-detectable levels to the range of healthy donor samples. These three patients who had relapses in the preceding 12-24 month period remained relapse free during the 52-week assessment period and also showed a 57% to 67% reduction in MRTCs.
Current Treatment Options for SPMS
Only one product, mitoxantrone, is currently approved for the indication of SPMS in the US. However, since 2005, this drug carries a black box warning, due to significant risks of decreased systolic function, heart failure, and leukemia. The American Academy of Neurology has issued a report indicating that these risks are even higher than suggested in the original report leading to the black box warning. Hence, a safe and effective treatment for SPMS remains a significant unmet medical need.
Tcelna Clinical Overview in SPMS
In multiple previously conducted clinical trials for the treatment of patients with MS (which have been weighted significantly toward patients with RRMS), Tcelna has demonstrated one of the safest side effect profiles for any marketed or development-stage MS therapy, as well as encouraging efficacy signals. A total of 144 MS patients have received Tcelna in previously conducted Opexa trials for RRMS and SPMS. The therapy has been well-tolerated in all subjects and has demonstrated an excellent overall safety profile. The most common side effect is mild to moderate irritation at the site of injection, which is typically resolved in 24 hours. Tcelna has been administered to a total of 36 subjects with SPMS across three previous clinical studies.
In a pooled assessment of data from 36 SPMS patients treated in Phase I open label studies at the Baylor College of Medicine completed in 1998 and in Opexa-sponsored studies completed in 2006 and 2007, approximately 80% of the 35 SPMS patients who completed two years of treatment showed disease stabilization as measured by EDSS following two years of treatment with Tcelna, with the other 20% showing signs of progression. This compares to historical control data which showed a progression rate of 40% in SPMS patients (as reported in ESIMS Study published in Hommes Lancet 2004). The 10 SPMS patients in Opexa sponsored studies showed a substantial reduction in ARR at two years from 0.5 to an ARR less than 0.1. Only 1 out of the 10 patients experienced one episode of relapse during the two years of assessment. This same cohort showed no worsening of physical or psychological condition (key quality of life indicators as measured by the MS Impact Scale) after two years of treatment with Tcelna. Additionally, there were no reported serious adverse events (SAEs) in any of the patients. Based on preliminary data suggesting stabilized or improved disability among SPMS subjects receiving Tcelna, we believe that further development of this product candidate in SPMS is warranted.
Abili-T Trial: Phase IIb Clinical Study in Patients with SPMS
In September 2012, we announced the initiation of a Phase IIb clinical trial of Tcelna in patients with SPMS. The trial is entitled: A Phase II Double-Blind, Placebo Controlled Multi-Center Study to Evaluate the Efficacy and Safety of Tcelna in Subjects with Secondary Progressive Multiple Sclerosis and has been named the “Abili-T” trial. The Abili-T trial is a double-blind, 1:1 randomized, placebo-controlled study in SPMS patients who demonstrate evidence of disease progression with or without associated relapses. The trial is expected to enroll 180 patients who have EDSS scores between 3.0 and 6.0 at approximately 30 leading clinical sites in the U.S. and Canada. According to the study protocol, patients will receive two annual courses of Tcelna treatment consisting of five subcutaneous injections per year at weeks 0, 4, 8, 12 and 24.
The primary efficacy endpoint of the trial is the percentage of brain volume change (whole brain atrophy) at 24 months. Study investigators will also measure several important secondary outcomes commonly associated with MS including sustained disease progression measured by EDSS, changes in EDSS, time to sustained progression, ARR, Change in Multiple Sclerosis Functional Composite (MSFC) assessment of disability and Change in Symbol Digit Modality Test. Data on certain exploratory endpoints such as quality of life metrics as measured by the MS Quality of Life Inventory (MSQLI), magnetic resonance imaging (MRI) measures and immune monitoring metrics are also being collected. The annual treatment and efficacy assessment schedule is shown below:
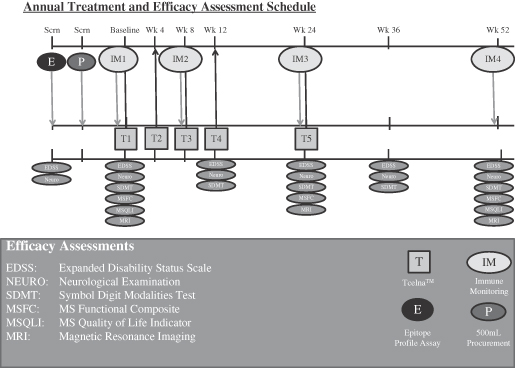
Immune Monitoring Program. As part of the Abili-T trial, we are undertaking a comprehensive immune monitoring program for all patients enrolled in the study. The goals of this program are to further understand the biology behind the mechanism of action for Tcelna and to possibly identify novel biomarkers that are dominant in the pathophysiology of SPMS patients. The program encompasses an analysis of various pro-inflammatory and anti-inflammatory biomarkers. We believe that the blinded data, which will be analyzed during the course of the trial, may signal responders and non-responders. Directional movement of certain biomarkers, when corroborated with final clinical trial data, may be indicative of responders and disease stabilization or progression. It is hypothesized that directional movement higher of the anti-inflammatory biomarkers (listed on the right side in the middle column of the graphic below) may be indicative of clinical efficacy. Similarly, it is hypothesized that a direction movement lower of pro-inflammatory markers (listed on the left side in the middle column of the graphic below) may be indicative of a reduction in inflammation and, consequently, clinical efficacy.

As a prelude to the immune monitoring program, Opexa characterized the status of patients entering the trial at baseline and compared the data sets to those of healthy donors. This baseline data was recently presented at the 2013 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) meeting. The poster showed that SPMS subjects have a reduced frequency of IL-10 secreting TR1 cells and the frequency of nTregs may be reduced in SPMS patients. Although the level of Foxp3 expression in SPMS patients is equivalent to that of healthy donors, the data suggest that SPMS is associated with lower levels of PDL1 and HLA-G expressing monocytes. The impact of Tcelna treatment will be studied over time on a blinded basis as part of the immune monitoring program, and compared to patients receiving placebo post trial completion, to better illustrate the mechanism of action for Tcelna therapy.
As of July 19, 2013, the Abili-T clinical trial has randomized 65 patients. A scheduled Data Safety Monitoring Board meeting took place during the week of May 20, 2013, and a recommendation was made to continue the study. The Abili-T clinical study in North America of Tcelna is expected to complete enrollment of 180 patients by late 2013 or early 2014, with the resulting top-line data expected to be available in the first half of 2016.
During the first quarter of 2013, the pace of on-boarding clinical sites for the Abili-T clinical study was tempered pending the completion of our negotiations with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., for the Option (see “—Option and License Agreement with Merck Serono”) as
well as financial considerations. Upon receipt of the upfront payment of $5 million for granting the Option, we were able to refocus on execution of the Abili-T clinical trial, including enrollment. The future costs of the study, which have been impacted by a slowed rate of enrollment prior to receipt of the upfront payment of $5 million for granting the Option, as well as the ongoing expenses of our operations through the expected completion date of the study and release of top-line data, are estimated as of June 30, 2013 to be between $30-$32 million. Our existing resources are not adequate to permit us to complete such study or the majority of it. We anticipate that at least $9.8 million of the proceeds from the offering will be applied to funding the continuation of the clinical study as well as the ongoing expense of our operations during such development and for general corporate purposes. We will need to secure significant additional resources to complete the trial and support our operations during the pendency of the trial. There can be no assurance that any such financings or potential opportunities and alternatives can be consummated on acceptable terms, if at all. We believe we have sufficient liquidity to support our clinical trial activities into the fourth quarter of 2013. Given our need for substantial amounts of capital to continue the Abili-T clinical study in North America of Tcelna in SPMS, we intend to continue to explore potential opportunities and alternatives to obtain the significant additional resources, including one or more additional financings, that will be necessary to complete the Abili-T study and to support our operations during the pendency of such study.
Option and License Agreement with Merck Serono
On February 4, 2013, we entered into an Option and License Agreement (“Merck Agreement”) with Merck. Pursuant to the Merck Agreement, Merck has an option (the “Option”) to acquire an exclusive, worldwide (excluding Japan) license of our Tcelna program for the treatment of MS. The Option may be exercised by Merck prior to or upon completion of our ongoing Abili-T trial of Tcelna in patients with SPMS.
Under the terms of the Merck Agreement, we received an upfront payment of $5 million for granting the Option. If the Option is exercised, Merck would pay us an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the Option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights to use for other indications outside of MS. Based upon the achievement of development milestones by Merck for Tcelna in SPMS, we would be eligible to receive one-time milestone payments totaling up to $70 million as follows: (i) milestone payments aggregating $35 million if Tcelna is submitted for regulatory approval and commercialized in the United States; (ii) milestone payments aggregating $30 million if Tcelna is submitted for regulatory approval in Europe and commercialized in at least three major countries in Europe; and (iii) a milestone payment of $5 million if Tcelna is commercialized in certain markets outside of the United States and Europe. If Merck elects to develop and commercialize Tcelna in RRMS, we would be eligible to receive milestone payments aggregating up to $40 million based upon the achievement by Merck of various development, regulatory and first commercial sale milestones.
If Tcelna receives regulatory approval and is commercialized by Merck, we would be eligible to receive royalties pursuant to a tiered structure at rates ranging from 8% to 15% of annual net sales, with step-ups over such range occurring when annual net sales exceed $500 million, $1 billion and $2 billion. Any royalties would be subject to offset or reduction in various situations, including if third party rights are required or if patent protection is not available in an applicable jurisdiction. We would also be responsible for royalty obligations to certain third parties, such as Baylor College of Medicine from which we originally licensed related technology. If we were to exercise an option to co-fund certain of Merck’s development, the royalty rates payable by Merck would be increased to rates ranging from 10% to 18%. In addition to royalty payments, we would be eligible to receive one-time commercial milestones totaling up to $85 million, with $55 million of such milestones achievable at annual net sales targets in excess of $1 billion.
Other Opportunities
Our proprietary T-cell technology has enabled us to develop intellectual property and a comprehensive sample database that may enable discovery of novel biomarkers associated with MS. Depending upon the outcome of further feasibility analysis, the T-cell platform may have applications in developing treatments for other autoimmune disorders such as rheumatoid arthritis, Type 1 diabetes, and orphan indications such as myasthenia gravis. The primary focus of Opexa remains the development of Tcelna in SPMS.
We have developed (and, in part, licensed from the University of Chicago) a proprietary adult stem cell technology to produce monocyte-derived stem cells (MDSC) from blood. These MDSC can be derived from a patient’s monocytes, expanded ex vivo, and then administered to the same patient. Our initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for thein vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus. The diabetes program is in an early (pre-clinical) development stage.
Tcelna Manufacturing
We manufacture Tcelna in our own current Good Manufacturing Practice (cGMP) facility. Over 850 Tcelna preparations have been successfully manufactured at our facilities.
Tcelna is a personalized autologous immunotherapy that is not only manufactured for every individual subject but also is tailored to match each subject’s evolving disease profile as defined by T-cell profiling against myelin antigens. In preparing Tcelna, the subject is pre-screened with our proprietary Epitope Profiling Assay (EPA) for immunodominant anti-myelin T-cell responses against specific peptides by assaying peripheral blood mononuclear cell (PBMC) reactivity against 109 peptides tested in pools of six derived from MBP, MOG and PLP. The EPA takes approximately 14 days to conduct and report data.
Using up to six pre-selected peptide pools, the MRTC lines to each pool are expanded to therapeutic levels, mixed and cryopreserved until time for final formulation. The manufacturing and quality control process spans approximately 35 days. Prior to injection, the MRTCs are thawed, formulated and attenuated (by irradiation) to render them unable to replicate but viable for therapy. These attenuated T-cells are administered in a defined schedule of five subcutaneous injections. Patients will be treated with a new, personalized treatment series (five subcutaneous injections) each year based on their altered disease profile or epitope shift and the re-manufacture of a new Tcelna product representing the emerging immunodominant T-cell response to myelin.
If Merck exercises its Option to acquire an exclusive, worldwide license for our Tcelna program for the treatment of MS, we retain certain rights with respect to the manufacture of Tcelna.
Personalized Therapy
The clinical symptoms of MS are the result of an immune attack against the myelin sheaths that insulate nerves in the brain and spinal cord that constitute the CNS. A subset of white cells, called T-cells, is the primary orchestrator of this immunity. Tcelna is an immunotherapy representing an enriched source of the patient’s own MRTCs that are used to invoke a protective response to limit further damage to the myelin sheaths within the patient’s CNS. Immunity to myelin in terms of the specificity of T-cells for myelin proteins varies between individuals. Therefore, Tcelna is further personalized by screening the immune response, and detecting those proteins that are preferentially targeted by T-cells on a per patient basis. This is achieved using protein fragments, called peptides, from the three major myelin proteins (MOG, MBP and PLP) as targets to finely map immunity to myelin. A limited number of peptides are chosen to which immunity appears greatest, and the Tcelna product is manufactured against these peptides. Thus, Tcelna is not only manufactured for each patient, but it is also tailored against each patient’s personalized T-cell immune response to myelin. In preparing Tcelna for a patient, the patient-specific MRTCs are expanded from a unit of whole blood using the selected myelin peptides in the presence of growth factors. Once sufficient numbers of T-cells have been propagated to support
the clinical dosing regimen, they are frozen down as individual Tcelna doses. Prior to clinical use, a frozen Tcelna dose is thawed, formulated, and attenuated (by irradiation) to render the T-cells unable to replicate, but viable for therapy. After quality control and quality assurance, each individual dose is shipped on request overnight to the clinical site for administration over a defined schedule of five subcutaneous injections. Patients will be treated with a new Tcelna series (five subcutaneous injections) each year based on their altered disease profile or epitope shift.
Tcelna Safety and Tolerability
We believe that Tcelna treatment selectively targets, depletes and/or down-regulates the pathogenic T-cell population. It is not a general immune suppressant and, accordingly, it is not associated with the serious side effects seen by those MS treatments that function by systemically suppressing the immune system. In clinical trials conducted to date, there have been no SAEs associated with Tcelna treatment. We believe that this favorable safety profile may be an important advantage as patient compliance represents a significant challenge due to serious side effects associated with many currently available and in development MS treatments.
Licenses, Patents and Proprietary Rights
We believe that proprietary protection of our technologies is critical to the development of our business. We will continue to protect our intellectual property through patents and other appropriate means. We rely upon trade-secret protection for certain confidential and proprietary information and take active measures to control access to that information. We currently have non-disclosure agreements with all of our employees, consultants, vendors, advisory board members and contract research organizations.
The initial T-cell technology on which Tcelna is based was originally discovered by researchers at Baylor College of Medicine in Houston, Texas. Baylor granted Opexa an exclusive, worldwide right and license to commercially exploit such technology, which includes rights to issued patents and pending patent applications owned by Baylor. Opexa has since expanded the development of technology related to Tcelna and T-cell technology and has filed patent applications with respect thereto, from which several patents have issued (including with respect to the specificity and veracity of antigens that have been discovered). There is also substantial proprietary know-how surrounding the Tcelna development and manufacturing processes that remains a trade secret. Consequently, we consider barriers to entry, relative to Tcelna for the treatment of MS, to be high.
Our patent portfolio tracks our scientific development programs in autoimmune disease treatments, with an initial focus on MS. We believe that our scientific platform is adaptable in that any T-cell dependent autoimmune disease with known specific antigens, such as rheumatoid arthritis, may be a candidate for treatment, and we believe that our patent strategy is readily extendable to address these additional indications.
Competition
The development of therapeutic agents for human disease is intensely competitive. Major pharmaceutical companies currently offer a number of pharmaceutical products to treat MS and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. We expect competition to increase. We believe that our most significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Some of our primary competitors in the current treatment of, and in the development of treatments for, MS include Biogen-Idec, Elan, Merck-Serono (which is an affiliate of the entity that holds the Option), Teva, Bayer/Schering AG and Novartis.
Sales and Marketing
If Merck exercises its Option to acquire an exclusive, worldwide license for our Tcelna program for the treatment of MS and pays us an upfront license fee, Merck would be solely responsible for funding future commercialization activities for Tcelna in MS, although we would retain an option to co-fund certain development in exchange for increased royalty rates. We would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS. We would consider partnering with large biotech and pharmaceutical companies, if and when applicable, to assist with marketing and sales of an MS T-cell therapy in Japan as well as to assist with marketing and sales in indications beyond MS.
If Merck does not exercise its Option, we may choose to partner with large biotech or other pharmaceutical companies for sales and marketing, if and when applicable, or alternatively develop our own sales force to market our MS cell therapy products in the U.S. Given the concentration of MS treatment among a relatively small number of specialized neurologists in the U.S., we believe that a modest size sales force would be sufficient to market an MS product in the U.S.
Government Regulation
Our research and development activities and the future manufacturing and marketing of our potential products are, and will be, subject to regulation for safety and efficacy by a number of governmental authorities in the U.S. and other countries.
In the U.S., pharmaceuticals, biologicals and medical devices are subject to FDA regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the Public Health Service Act, as amended, the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing in human subjects, manufacture, safety, efficacy, labeling, storage, export, record keeping, approval, marketing, advertising and promotion of our potential products. Product development and approval within this regulatory framework take a number of years and involve significant uncertainty combined with the expenditure of substantial resources.
FDA Approval Process
We will need to obtain FDA approval of any therapeutic product we plan to market and sell. The FDA will only grant marketing approval if it determines that a product is both safe and effective. The testing and approval process will require substantial time, effort and expense. The steps required before our products may be marketed in the U.S. include:
Preclinical Laboratory and Animal Tests. Preclinical tests include laboratory evaluation of the product candidate and animal studies in specific disease models to assess the potential safety and efficacy of the product candidate as well as the quality and consistency of the manufacturing process.
Submission to the FDA of an Investigational New Drug Application, or IND, Which Must Become Effective Before U.S. Human Clinical Trials May Commence. The results of the preclinical tests are submitted to the FDA, and the IND becomes effective 30 days following its receipt by the FDA, as long as there are no questions, requests for delay or objections from the FDA. The sponsor of an IND must keep the FDA informed during the duration of clinical studies through required amendments and reports, including adverse event reports.
Adequate and Well-Controlled Human Clinical Trials to Establish the Safety and Efficacy of the Product Candidate.Clinical trials, which test the safety and efficacy of the product candidate in humans, are conducted in accordance with protocols that detail the objectives of the studies, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Any product candidate administered in a U.S. clinical trial must be manufactured in accordance with cGMP.
The protocol for each clinical study must be approved by an independent Institutional Review Board, or IRB, at the institution at which the study is conducted, and the informed consent of all participants must be
obtained. The IRB will consider, among other things, the existing information on the product candidate, ethical factors, the safety of human subjects, the potential benefits of the therapy and the possible liability of the institution.
Clinical development is traditionally conducted in three sequential phases, which may overlap:
|
Phase II involves studies in a limited population of patients with the disease or condition under study to (i) determine the efficacy of the product candidates for specific targeted indications and populations, (ii) determine optimal dosage and dosage tolerance and (iii) identify possible and common adverse effects and safety risks. (Phase II may divided into Phase IIa and Phase IIb studies to address these issues.) When a dose is chosen and a candidate product is found to have preliminary evidence of effectiveness, and to have an acceptable safety profile in Phase II evaluations, Phase III trials begin.
Phase III trials are undertaken to develop additional safety and efficacy information from an expanded patient population, generally at multiple study sites. This information obtained is used to develop a better understanding of the risks and benefits of the product candidate and to determine appropriate labeling for use.
Based on clinical trial progress and results, the FDA may request changes or may require discontinuance of the trials at any time if significant safety issues arise.
Submission to the FDA of Marketing Authorization Applications and FDA Review.The results of the preclinical studies and clinical studies are submitted to the FDA as part of marketing approval authorization applications such as New Drug Applications (NDAs) or Biologics License Applications (BLAs). The FDA will evaluate such applications for the demonstration of safety and effectiveness. A BLA is required for biological products subject to licensure under the Public Health Service Act and must show that the product is safe, pure and potent. In addition to preclinical and clinical data, the BLA must contain other elements such as manufacturing materials, stability data, samples and labeling. FDA approval of a BLA is required prior to commercial sale or shipment of a biologic. A BLA may only be approved once the FDA examines the product and inspects the manufacturing establishment to assure conformity to the BLA and all applicable regulations and standards for biologics.
The time for approval may vary widely depending on the specific product candidate and disease to be treated, and a number of factors, including the risk/benefit profile identified in clinical trials, the availability of alternative treatments, and the severity of the disease. Additional animal studies or clinical trials may be requested during the FDA review period, which might add substantially to the review time.
The FDA’s marketing approval for a product is limited to the treatment of a specific disease or condition in specified populations in certain clinical circumstances, as described on the approved labeling. The approved use is known as the “indication.” After the FDA approves a product for the initial indication, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require post-marketing testing (Phase IV studies) and surveillance to monitor for adverse effects, which could involve significant expense. The FDA may also elect to grant only conditional approval.
Ongoing Compliance Requirements
Even after product approval, there are a number of ongoing FDA regulatory requirements, including:
Registration and listing;
Regulatory submissions relating to changes in an NDA or BLA (such as the manufacturing process or labeling) and annual reports;
Adverse event reporting;
Compliance with advertising and promotion restrictions that relate to drugs and biologics; and
Compliance with GMP and biological product standards (subject to FDA inspection of facilities to determine compliance).
Other Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other present and potential future foreign, federal, state and local regulations. For instance, product manufacturing establishments located in certain states also may be subject to separate regulatory and licensing requirements.
Outside the U.S., we will be subject to regulations that govern the import of drug products from the U.S. or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for products. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursements vary widely from country to country.
Research and Development
Research and development expenses for the year ended December 31, 2012 were approximately $6.3 million, mainly reflecting the costs of preparation, initiation and operation of the Abili-T clinical trial for Tcelna in Multiple Sclerosis. Research and development expenses for the year ended December 31, 2011 were approximately $3.3 million, mainly reflecting the costs of preparation for the Abili-T clinical trial for Tcelna.
Organizational History
We are a development-stage company and have a limited operating history. Our predecessor company for financial reporting purposes was formed on January 22, 2003 to acquire rights to an adult stem cell technology. In November 2004, we acquired Opexa Pharmaceuticals, Inc. and its MS treatment technology. Currently, we remain focused on developing our T-cell technology for MS. To date, we have not generated any commercial revenues from operations. As we continue to execute our business plan, we expect our development and operating expenses to increase.
Employees
As of July 19, 2013, we had 31 full-time employees. We believe that our relations with our employees are good. None of our employees is represented by a union or covered by a collective bargaining agreement.
As of the date of this prospectus, our directors and executive officers are as follows:
|
| |||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
Neil K. Warma has served as President and Chief Executive Officer since June 2008 and as a Director since September 2008. He also previously served as our Acting Chief Financial Officer from March 2009 to August 2012. From July 2004 to September 2007, Mr. Warma served as president and chief executive officer of Viron Therapeutics Inc., a privately-held clinical stage biopharmaceutical company. From 2000 to 2003 Mr. Warma was co-founder and president of MedExact USA, Inc., an Internet company providing clinical information and services to physicians and pharmaceutical companies. From 1992 to 2000, Mr. Warma held senior positions of increasing responsibility at Novartis Pharmaceuticals (previously Ciba-Geigy Ltd.) at its corporate headquarters in Basel, Switzerland. While at Novartis, Mr. Warma served as the Head of International Pharma Policy & Advocacy and in senior management within global marketing where he worked on the international launch of a gastrointestinal product. Mr. Warma obtained an honors degree specializing in Neuroscience from the University of Toronto and an International M.B.A. from the Schulich School of Management at York University in Toronto. As our President and Chief Executive Officer, Mr. Warma is directly involved in all aspects of our operations. He has extensive experience in corporate business development within the biopharmaceutical industry, in addition to executive leadership and management experience.
Karthik Radhakrishnan has served as Chief Financial Officer since March 2013. Mr. Radhakrishnan joined Opexa with over 10 years of healthcare capital markets experience and most recently was a Vice President at ING Investment Management in New York. While at ING from 2007 to 2012, he was responsible for healthcare investments in the small & small-mid cap core/growth products that are part of the Fundamental Equity product line. Previously he was the senior analyst at Eagle Asset Management from 2005 to 2007, responsible for large cap growth healthcare. Prior to this, Mr. Radhakrishnan served in various analyst positions including Senior Analyst at The Dow Chemical Company where he worked from 2002 to 2005. Mr. Radhakrishnan served as a member of the Board of Trustees at Cares Foundation, a non-profit organization serving the Congenital Adrenal Hyperplasia community from 2008 to 2011. Mr. Radhakrishnan is a CFA charter holder and has an MBA degree from the University of Michigan, a Masters in Engineering from the State University of New York and a Bachelor’s degree from the Indian Institute of Technology.
Donna R. Rill was appointed as our Chief Development Officer in April 2013 and has served as Senior Vice President of Operations and Quality Systems since January 2009. From November 2004 until January 2009, she served as Vice President of Operations. From April 2003 to November 2004, she was the director of quality systems and process development at Opexa Pharmaceuticals, Inc. From November 1997 to April 2003, she was the director of translational research for the Center for Cell & Gene Therapy at Baylor College of Medicine. Ms. Rill has worked to design and qualify GMP Cell & Gene Therapy Laboratories, GMP Vector Production facilities, and Translational Research Labs at St. Jude Children’s Research Hospital, Texas Children’s Hospital, and Baylor College of Medicine. Ms. Rill received her B.S. in Medical Technology from the University of Tennessee, Memphis.
David E. Jordenhas served as a Director since August 2008 and served as our Acting Chief Financial Officer from August 2012 until March 2013. Mr. Jorden has also served as Executive Chairman for Cytomedix, Inc. since February 2012 and previously as an executive board member since October 2008. In June 2013, he was appointed Interim Chief Executive Officer for Nanospectra Biosciences, Inc., a private company focused on
nanoparticle based therapies for tissue ablation. Mr. Jorden previously served as vice president with Morgan Stanley in its Wealth Management group where he was responsible for equity portfolio management for high net worth individuals since 2003. Prior to Morgan Stanley, Mr. Jorden served as vice president and chief financial officer of Genometrix, Inc., a private genomics/life sciences company focused on high-throughput microarray applications from March 2000 to September 2002. Mr. Jorden was a principal with Fayez Sarofim & Co. prior to joining Genometrix. Mr. Jorden earned a MBA from Kellogg School of Management at Northwestern University in 1989 and a BBA from the University of Texas/Austin in 1984. He currently serves as a director of Cytomedix, Inc., PLx Pharma, Inc. and Nanospectra Biosciences, Inc. Mr. Jorden holds Chartered Financial Analyst and Certified Public Accountant designations. He has extensive experience in various aspects of corporate finance and accounting for public companies including capital formation and deployment.
Gail J. Maderis has served as a Director since October 2011. Ms. Maderis has served as President and CEO of BayBio (Bay Area Bioscience Association), an independent, non-profit trade association serving the life sciences industry in Northern California, since October 2009 and joined BayBio’s board in 2004. From July 2003 to June 2009, Ms. Maderis served as President and CEO of FivePrime Therapeutics, Inc., a biotechnology company focused on the discovery and development of innovative protein and antibody drugs, and served as a director until 2010. Prior to that, Ms. Maderis held general management positions at Genzyme Corporation from 1997 to 2003, including founder and president of Genzyme Molecular Oncology, a publicly traded division of Genzyme, and corporate vice president of Genzyme Corporation. Ms. Maderis has served as a director of NovaBay Pharmaceuticals, Inc. since October 2010. Ms. Maderis has been a member of several private company boards, and currently serves on The Mayor’s Biotech Advisory Council of San Francisco, as well as the HBS Healthcare Initiative board. Ms. Maderis received a B.S. degree in business from the University of California at Berkeley and an M.B.A. from Harvard Business School. Ms. Maderis has extensive experience as a senior executive of life sciences companies, giving her valuable operational and industry experience and leadership skills, as well as an extensive network of contacts related to financing, partnering and support services in the biotech industry and visibility into business and policy trends that impact the biopharmaceutical industry.
Michael S. Richmanhas served as a Director since June 2006. Mr. Richman has served as president and chief executive officer of Amplimmune, Inc. since July 2008. Mr. Richman served as president and chief operating officer of Amplimmune, Inc. from May 2007 to July 2008. From April 2002 to May 2007, Mr. Richman served as executive vice president and chief operating officer of MacroGenics, Inc. Mr. Richman joined MacroGenics, Inc. in 2002 with approximately 20 years’ experience in corporate business development within the biotechnology industry. Mr. Richman served as a director of Cougar Biotechnology from June 2006 to July 2009. Mr. Richman obtained his B.S. in Genetics/Molecular Biology at the University of California at Davis and his MSBA in International Business at San Francisco State University. He has extensive experience in business development and strategic planning for life science companies, as well as executive leadership and management experience.
Scott B. Seamanhas served as a Director of since April 2006. Mr. Seaman has served for over five years as (i) the executive director and treasurer of the Albert and Margaret Alkek Foundation of Houston, Texas, a private foundation primarily supporting institutions in the Texas Medical Center in Houston, Texas, (ii) the chief financial officer of Chaswil Ltd., a private family management company, (iii) secretary and treasurer of M & A Properties Inc., a ranching and real estate concern, and (iv) director of Somebody Cares America. In March, 2013, Mr. Seaman was elected a director of Gradalis, Inc., a privately held clinical stage biotechnology company developing cancer-focused immunotherapies. In April 2009, Mr. Seaman became the Managing Member of ICT Development LLC which is the Managing Member of ICT Holdings LLC, an energy services supplier for which he serves as president. From January 2003 to April 2009, Mr. Seaman served as chairman and from July 2004 to April 2009, as president of ICT Management Inc., the general partner of Impact Composite Technology Ltd., a composite industry supplier. From October 2007 to December 2010, Mr. Seaman served on the board of GeneExcel, Inc., a privately held biotechnology company. From May 2004 to December 2010, Mr. Seaman served as a Member of the Investment Committee of Global Hedged Equity Fund LP, a hedge fund. Mr. Seaman received a bachelor’s degree in business administration from Bowling Green State University and is a certified public accountant. Mr. Seaman has extensive experience in overall financial management and corporate development, combined with operational and corporate governance experience.
Executive Officer Compensation
The following table sets forth certain information concerning compensation earned by or paid to certain persons who we refer to as our “Named Executive Officers” for services provided for the fiscal year ended December 31, 2012. Our Named Executive Officers include persons who (i) served as our principal executive officer or acted in a similar capacity during 2012, (ii) were serving at fiscal year-end as our two most highly compensated executive officers, other than the principal executive officer, whose total compensation exceeded $100,000, and (iii) if applicable, up to two additional individuals for whom disclosure would have been provided as a most highly compensated executive officer, but for the fact that the individual was not serving as an executive officer at fiscal year-end.
2012 Summary Compensation Table
Name and Principal Position | Year | Salary | Bonus | Options Awards(1) | All Other Compensation | Total | ||||||||||||||||||
Neil K. Warma | 2012 | $ | 396,550 | $ | 50,000 | $ | 688,684 | $ | 100 | $ | 1,135,334 | |||||||||||||
President, Chief Executive Officer, Acting Chief Financial Officer(2) | 2011 | $ | 385,000 | $ | 50,000 | $ | 115,051 | — | $ | 550,051 | ||||||||||||||
Donna R. Rill | 2012 | $ | 220,000 | $ | 15,000 | $ | 154,152 | $ | 250 | $ | 389,402 | |||||||||||||
Senior Vice President of Operations and Quality Systems | 2011 | $ | 200,000 | $ | 15,000 | $ | 38,350 | — | $ | 253,350 | ||||||||||||||
Jaye L. Thompson, Ph.D.(3) | 2012 | $ | 220,000 | $ | 10,000 | $ | 154,152 | $ | 50 | $ | 384,202 | |||||||||||||
Senior Vice President of Clinical Development and Regulatory Affairs | 2011 | $ | 200,000 | $ | 15,000 | $ | 38,350 | — | $ | 253,350 | ||||||||||||||
Executive Employment Agreements
Neil K. Warma. We entered into an employment agreement on June 16, 2008 with Neil K. Warma pursuant to which he serves as our President and Chief Executive Officer. Pursuant to the agreement, which automatically renews for 12-month periods, Mr. Warma is currently paid $396,550 per year. In addition, Mr. Warma is entitled to the following: (i) an annual cash bonus of up to 50% of his base salary based upon milestones to be agreed upon; and (ii) a one-time payment of $50,000 cash and 6,250 shares of our common stock prior to be issued if and when the closing bid price of our common stock equals or exceeds $16.00 for 20 consecutive trading days. In addition, we provide Mr. Warma with our standard benefits and insurance coverage as generally provided to our management, as well as contractual indemnification rights by reason of his service as an officer and employee. If his employment is terminated by the Board without cause, as defined in the agreement, Mr. Warma will be entitled to receive a severance payment equal to 12 months of his base salary plus a payment equal to 30% of base salary in lieu of any potential bonus, in addition any earned but unpaid bonus. In addition, vesting of stock options will accelerate in full. We will also reimburse Mr. Warma for COBRA expenses for a 12-month period, subject to a cap equal to Opexa’s standard contribution to employee health benefits. Upon the effectiveness of a
change in control, as defined in the agreement, Mr. Warma will receive 18 months of salary and COBRA reimbursement and a payment equal to 45% of base salary in lieu of any potential bonus, in addition to any earned but unpaid bonus. In addition, all vesting of options will accelerate in full. Any payment or benefit Mr. Warma might receive upon a change of control which would constitute a “parachute payment” under Section 280Gexpiration of the Internal Revenue CodeRights Offering, and if the Rights Offering is fully subscribed, we will be reduced so as not to trigger excise tax under Section 4999 of such Code. Mr. Warma’s agreement also provides that for a 12-month period following his termination of employment, he will not engage or participate in any competitive business or solicit or recruit any of Opexa’s employees. The severance and change of control benefits are subject to Mr. Warma executing and delivering a general release and waiver of claims in favor of Opexa.
Donna R. Rill. We entered into an amended and restated employment agreement with Donna R. Rill on April 21, 2010 which is effective as of April 1, 2010, pursuant to which Ms. Rill serves as our Senior Vice President of Operations and Quality Systems. This agreement superseded Ms. Rill’s prior agreement. Ms. Rill is currently compensated at the rate of $220,000 per annum and is eligible to receive an annual discretionary bonus of up to 20% of her base salary per 12-month period, based on the achievement of objectives as determined by Opexa’s Board and Chief Executive Officer. In addition, Ms. Rill receives our standard benefits and insurance coverage as generally provided to our management, as well as contractual indemnification rights by reason of her service as an officer and employee. Ms. Rill’s employment may be terminated at any time voluntarily by her or without cause (as defined in the agreement) by the Board. If her employment is terminated by the Board without cause, Ms. Rill will be entitled to receive a severance payment equal to six months of her base salary and vesting for any unvested stock options will accelerate by six additional months. The severance benefits are subject to Ms. Rill having been continuously employed through the termination event, executing and delivering a general release and waiver of claims in favor of Opexa, not being in breach of the employment agreement or Opexa’s proprietary information and inventions agreement, and not engaging in any activity which is competitive with Opexa during the term of the employment agreement or while receiving the severance benefits. The timing of any payments to Ms. Rill under the employment agreement is subject to applicable requirements of Section 409A of the Code and the related Treasury Regulations.
Jaye L. Thompson.Until the termination of her employment on March 29, 2013, Jaye L. Thompson, Ph.D., was employed as our Senior Vice President of Clinical Development and Regulatory Affairs pursuant to an amended and restated employment agreement dated June 27, 2011. This agreement superseded Dr. Thompson’s prior agreement dated November 16, 2009. Dr. Thompson was compensated at the rate of $220,000 per annum and was eligible to receive an annual discretionary bonus of up to 20% of her base salary per 12-month period, based upon the achievement of objectives as determined by Opexa’s Board and Chief Executive Officer. In addition, Dr. Thompson received our standard benefits and insurance coverage as generally provided to our management. The agreement provided that Dr. Thompson’s employment may be terminated at any time voluntarily by her or without cause (as defined in the agreement) by the Board. If her employment was terminated by the Board without cause, Dr. Thompson was entitled to receive a severance payment equal to six months of her base salary. In addition, in the event of a change of control (as defined in the agreement) and Dr. Thompson’s employment is terminated without cause or Dr. Thompson resigned for good reason (as defined in the agreement) within 12 months of such change of control, Dr. Thompson was entitled to receive a severance payment equal to six months of her base salary and all unvested equity awards would immediately vest in full and become exercisable pursuant to their terms. The severance benefits were subject to Dr. Thompson having been continuously employed through the termination event, executing and delivering a general release and waiver of claims in favor of Opexa, not being in material breach of the employment agreement or Opexa’s proprietary information and inventions agreement, and not engaging in any activity which is competitive with Opexa during the term of the employment agreement or while receiving the severance benefits. The timing of any payments to Dr. Thompson under the employment agreement is subject to applicable requirements of Section 409A of the Code and the related Treasury Regulations.
We entered into a termination agreement, waiver, and release with Dr. Thompson as of March 29, 2013, and her employment terminated on such date. On June 7, 2013, we amended and restated the termination agreement,
pursuant to which she received a severance payment in the form of a lump sum payment of $60,000 in satisfaction of any and all severance consideration or other amounts owed to Dr. Thompson through her termination date. In consideration for such severance payment, Dr. Thompson provided us with a general release and waiver of any and all claims. The consulting agreement entered into with Dr. Thompson in connection with her departure was mutually terminated.
2012 Grants of Plan Based Awards
The following table presents information regarding stock options granted during the fiscal year ended December 31, 2012 pursuant to our 2010 Stock Incentive Plan to our Named Executive Officers.
Estimated Future Payouts Under Equity Incentive Plan Awards(1)
Name | Grant Date | Threshold | Target | Maximum | All Other Option Awards: Number of Securities Underlying Options(3) | Exercise Price of Option Awards | Grant Date Fair Value of Options | |||||||||||||||||||||
Neil K. Warma | 01/06/12 | — | 139,593 | 139,593 | $ | 3.80 | $ | 528,612 | (2) | |||||||||||||||||||
| 01/06/12 | 43,623 | $ | 3.80 | $ | 160,071 | (4) | ||||||||||||||||||||||
Donna R. Rill | 01/06/12 | — | 31,408 | 31,408 | $ | 3.80 | $ | 118,937 | (2) | |||||||||||||||||||
| 01/06/12 | 9,597 | $ | 3.80 | $ | 35,215 | (4) | ||||||||||||||||||||||
Jaye L. Thompson | 01/06/12 | — | 31,408 | 31,408 | $ | 3.80 | $ | 118,937 | (2) | |||||||||||||||||||
| 01/06/12 | 9,597 | $ | 3.80 | $ | 35,215 | (4) | ||||||||||||||||||||||
2012 Outstanding Equity Awards at Fiscal Year-End
The following table presents information regarding outstanding equity awards at December 31, 2012 for each of the Named Executive Officers.
| Option Awards | Option Exercise Price | Option Expiration Date | ||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | ||||||||||||||
Neil K. Warma | 62,500 | — | $ | 4.04 | 06/16/18 | |||||||||||
| 37,500 | — | $ | 0.88 | 01/16/19 | ||||||||||||
| 25,000 | — | $ | 8.20 | 11/30/19 | ||||||||||||
| 10,937 | 7,813 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 10,906 | 32,717 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 3,635 | 135,958 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
Donna R. Rill | 1,500 | — | $ | 28.00 | 12/05/15 | |||||||||||
| 5,845 | — | $ | 20.00 | �� | 04/20/16 | |||||||||||
| 8,000 | — | $ | 21.88 | 06/18/17 | ||||||||||||
| 750 | — | $ | 4.36 | 05/06/18 | ||||||||||||
| 8,250 | — | $ | 4.68 | 06/26/18 | ||||||||||||
| 10,000 | — | $ | 0.88 | 01/16/19 | ||||||||||||
| 2,099 | — | $ | 1.88 | 02/06/19 | ||||||||||||
| 12,500 | — | $ | 8.20 | 11/30/19 | ||||||||||||
| 3,646 | 2,604 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 2,399 | 7,198 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 872 | 30,536 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
Jaye L. Thompson | 12,500 | — | $ | 8.20 | 11/30/19 | |||||||||||
| 3,646 | 2,604 | (1) | $ | 6.24 | 01/04/21 | |||||||||||
| 2,399 | 7,198 | (1) | $ | 3.80 | 01/06/22 | |||||||||||
| 872 | 30,536 | (2) | $ | 3.80 | 01/06/22 | |||||||||||
2012 Director Compensation
The following table presents summary information regarding compensation of the non-employee members of our Board of Directors who served during any part of the fiscal year ended December 31, 2012.
Name | Fees Earned or Paid in Cash | Options Awards(1) | Total | |||||||||
David E. Jorden(2) | $ | 37,500 | (3) | $ | 27,857 | (4)(5) | $ | 65,357 | ||||
Gail J. Maderis | — | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
Michael S. Richman | — | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
Scott B. Seaman | — | $ | 27,857 | (4)(5) | $ | 27,857 | ||||||
Standard Compensation Arrangements
Employee directors do not receive any compensation for services as a member of our Board. We reimburse our directors for travel and lodging expenses in connection with their attendance at Board and committee meetings. As compensation for their services on our Board, in 2012 our non-employee directors were issued options to purchase shares of Opexa common stock in lieu of cash compensation. Each option is granted with an exercise price equal to the fair market value of Opexa’s common stock on the date of grant and is issued either fully vested or with a vesting schedule over a period of time up to one year (or up to two years in the case of an initial grant to a new director). In addition, we paid a quarterly retainer of $15,000 in cash to David E. Jorden while he served as the chair of our Audit Committee until August 15, 2012. As of August 15, 2012, we discontinued payment of a quarterly retainer to the Audit Committee chair.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of July 19, 2013, the number and percentage of outstanding shares of our common stock beneficially owned by: (a) each person who is known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock; (b) each of our directors; (c) each of our named executive officers; and (d) all current directors and executive officers, as a group. As of July 19, 2013, there were 8,114,790have 57,011,170 shares of common stock issued and outstanding. All numbersoutstanding, and Warrants to purchase an additional 28,776,419 shares of our common stock (excluding the 3,046,801 currently outstanding warrants). The exact number of shares and Warrants that we will issue in this offering will depend on the number of Units that are subscribed for in the tableRights Offering.
Methods for Exercising Subscription Rights
The exercise of Subscription Rights is irrevocable and may not be cancelled or modified. You may exercise your Subscription Rights as follows:
Subscription by Record Holders
If you are a shareholder of record or a holder of Series L warrants, the footnotes thereto have been adjustednumber of Units you may purchase pursuant to reflectyour Subscription Rights in indicated on the 1-for-4 reverse stock splitenclosed Rights Certificate. You may exercise your Subscription Rights by properly completing and executing the Rights Certificate and forwarding it, together with your full payment, to the Subscription Agent at the address given below under “Subscription Agent,” to be received before 5:00 p.m., Eastern Time, on April 8, 2015.
Subscription by Beneficial Owners
If you are a beneficial owner of shares of our common stock that was implemented December 14, 2012.
Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act. Under this rule, certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire shares (for example, upon exercise of an option or warrant) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares is deemed to include the amount of shares beneficially owned by such person by reason of such acquisition rights. As a result, the percentage of outstanding shares of any person as shownregistered in the following table doesname of a broker, dealer, custodian bank, or other nominee, you will not necessarily reflect the person’s actual voting power at any particular date.
To our knowledge, except as indicated in the footnotesreceive a Rights Certificate. Instead, we will issue one Subscription Right to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect tosuch nominee record holder for all shares of common stock shown as beneficially owned by them.
Beneficial Ownership Table
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
|
|
|
TRANSACTIONS WITH RELATED PERSONS
Since January 1, 2010, we have engaged in the following reportable transactions with our directors, executive officers, beneficial holders of more than 5% of our voting securities, and affiliates or their immediately family members. We believe that all of these transactions were on terms as favorable as could have been obtained from unrelated third parties.
Entities affiliated with director Scott B. Seaman invested an aggregate of $1.3 million principal amount in our 12% convertible secured promissory notes which were issued in a private offering of notes and warrants to purchase shares of our common stock held by such nominee at the Record Date. If you are not contacted by your nominee, you should promptly contact your nominee in order to subscribe for shares in the Rights Offering and follow the instructions provided by your nominee.
To properly exercise your Over-Subscription Privilege, you must deliver the subscription payment related to your Over-Subscription Privilege before the Rights Offering expires. Because we will not know the total number of unsubscribed Units before the Rights Offering expires, if you wish to maximize the number of shares you purchase pursuant to a Note Purchase Agreement dated July 25, 2012. Anyour Over-Subscription Privilege, you will need to deliver payment in an amount equal to the aggregate subscription payment for the maximum number of $4.085 millionUnits that you wish to purchase.
Payment Method
Payments must be made in principal amountfull in U.S. currency by personal check, certified check or bank draft, or by wire transfer, and payable to “Continental Stock Transfer & Trust Company, as Subscription Agent for Opexa Therapeutics, Inc.” You must timely pay the full subscription payment, including payment for the Over-
Subscription Privilege, for the full number of notes was originally issued in the offering, of which $3.185 million is currently outstanding and $900,000 was converted into shares of Series A convertible preferred stock and such shares were then converted into 288,229 shares of common stock. The notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually on June 30 and December 31, commencing December 31, 2012, in either cash or registered shares of our common stock you wish to acquired pursuant to the exercise of Subscription Rights by delivering a:
If you elect to exercise your Subscription Rights, you should consider using a wire transfer or certified check drawn on a U.S. bank to ensure that the Subscription Agent receives your funds before the Rights Offering expires. If you send a personal check, payment will not be deemed to have been received by the Subscription Agent until the check has cleared. The clearinghouse may require five or more business days to clear a personal check. Accordingly, holders who wish to pay the Subscription Price by means of a personal check should make payment sufficiently in advance of the expiration of the Rights Offering to ensure that the payment is received and clears by that date. If you send a certified check, payment will be deemed to have been received by the Subscription Agent immediately upon receipt of such instrument.
You should read the instruction letter accompanying the Rights Certificate carefully and strictly follow it.DO NOT SEND RIGHTS CERTIFICATES OR PAYMENTS DIRECTLY TO US. We will not consider your subscription received until the Subscription Agent has received delivery of a properly completed and duly executed Rights Certificate and payment of the full subscription payment.
The method of delivery of Rights Certificates and payment of the subscription payment to the Subscription Agent will be at the risk of the holders of Subscription Rights. If sent by mail, we recommend that you send those certificates and payments by registered mail, properly insured, with return receipt requested, or by overnight courier, and that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance of payment before the Rights Offering expires.
Missing or Incomplete Subscription Forms or Payment
If you fail to complete and sign the Rights Certificate or otherwise fail to follow the subscription procedures that apply to the exercise of your Subscription Rights before the Rights Offering expires, the Subscription Agent will reject your subscription or accept it to the extent of the payment received. Neither we nor our election. Subscription Agent undertakes any responsibility or action to contact you concerning an incomplete or incorrect subscription form, nor are we under any obligation to correct such forms. We have the sole discretion to determine whether a subscription exercise properly complies with the subscription procedures.
If you send a payment that is insufficient to purchase the number of shares you requested, or if the number of shares you requested is not specified in the forms, the payment received will be applied to exercise your Subscription Rights to the fullest extent possible based on the amount of the payment received. Any excess subscription payments received by the Subscription Agent will be returned, without interest or penalty, within 10 business days following the expiration of the Rights Offering.
Issuance of common stock and Warrants
The notesshares of common stock and Warrants that are securedpurchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of these securities if you are a holder of record of
shares or Series L warrants. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the securities you purchased in the Rights Offering.
Subscription Agent
The Subscription Agent for the Rights Offering is Continental Stock Transfer & Trust Company. The address to which Rights Certificates and payments should be mailed or delivered by substantially allovernight courier is provided below. If sent by mail, we recommend that you send documents and payments by registered mail, properly insured, with return receipt requested, and that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance or payment before the Rights Offering expires. Do not send or deliver these materials to us.
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
Telephone: (917) 262-2378
If you deliver the Rights Certificates in a manner different than that described in this prospectus, we may not honor the exercise of your Subscription Rights.
Information Agent
You should direct any questions or requests for assistance concerning the method of subscribing for the shares of our tangible and intangible assets, and $500,000common stock or for additional copies of this prospectus to the proceeds is presently heldInformation Agent as follows:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
No Fractional Shares
We will not issue fractional shares of common stock in the Rights Offering. Rights holders will only be entitled to purchase a controlled account. Alkek & Williams Ventures Ltd. acts as the collateral agent for the noteholders. The notes are convertible intonumber of Units representing a new class of non-voting Series A convertible preferred stock, subject to certain limitations and adjustments, which is ultimately convertible into common stock. The warrants have an exercise price of $2.56 per share, a five-year term and are exercisable for 112.5% of thewhole number of shares of common stock, intorounded down to the nearest whole number of Units a holder would otherwise be entitled to purchase. Any excess subscription payments received by the Subscription Agent will be returned within 10 business days after expiration of the Rights Offering, without interest or penalty. Similarly, no fractional shares of common stock will be issued in connection with the exercise of a Warrant. Instead, for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, the holder will be entitled to a cash payment equal to the pro-rated per share market price of the common stock on the last trading day preceding the exercise.
Notice to Brokers and Nominees
If you are a broker, dealer, bank, or other nominee holder that holds shares of our common stock for the account of others on the Record Date, you should notify the beneficial owners of the shares for whom you are the nominee of the Rights Offering as soon as possible to learn their intentions with respect to exercising their Subscription Rights. If a beneficial owner of our common stock so instructs, you should complete the Rights Certificate and submit it to the Subscription Agent with the proper subscription payment by the expiration date. You may exercise the number of Subscription Rights to which all beneficial owners in the notesaggregate otherwise would have been entitled had they been direct holders of our common stock on the Record Date, provided that you, as a nominee record holder, make a proper showing to the Subscription Agent by submitting the form entitled “Nominee Holder Certification,” which is provided with your Rights Offering materials. If you did not receive this form, you should contact our Subscription Agent to request a copy.
Validity of Subscriptions
We will resolve all questions regarding the validity and form of the exercise of your Subscription Rights, including time of receipt and eligibility to participate in the Rights Offering. Our determination will be final and binding. Once made, subscriptions are ultimately convertible,irrevocable; we will not accept any alternative, conditional, or contingent subscriptions. We reserve the absolute right to reject any subscriptions not properly submitted or the acceptance of which would be unlawful. You must resolve any irregularities in connection with your subscriptions before the expiration date of the Rights Offering, unless we waive them in our sole discretion. Neither we nor the Subscription Agent is under any duty to notify you or your representative of defects in your subscriptions. A subscription will be considered accepted, subject to certain limitationsour right to withdraw or terminate the Rights Offering, only when the Subscription Agent receives a properly completed and adjustments. The investorsduly executed Rights Certificate and any other required documents and the full subscription payment including final clearance of any personal check. Our interpretations of the terms and conditions of the Rights Offering will be final and binding.
Stockholder Rights
You will have certain registrationno rights relating toas a holder of the shares of our common stock you purchase in the Rights Offering until shares are issued in book-entry form or your account at your broker, dealer, bank, or other nominee is credited with the shares of our common stock purchased in the Rights Offering. Holders of Warrants issued in connection with the Rights Offering will not have rights as holders of our common stock until such Warrants are exercised and the shares of common stock underlying the Series A convertible preferred stock and the warrants.
Convertible secured notes and warrants to purchase common stock wereWarrants are issued to investors affiliatedthe holder.
Foreign Shareholders
We will not mail this prospectus or Rights Certificates to shareholders with Mr. Seaman inaddresses that are outside the following amounts:
| Principal Amount of Note | Number of Shares Subject to Warrant | |||||||
Alkek & Williams Ventures Ltd. | $ | 500,000 | 175,781 | |||||
Albert and Margaret Alkek Foundation | $ | 300,000 | 105,469 | |||||
DLD Family Investments, LLC | $ | 500,000 | 175,781 | |||||
See footnotes 4, 6 and 8United States or that have an army post office or foreign post office address. The Subscription Agent will hold these Rights Certificates for their account. To exercise Subscription Rights, our foreign shareholders must notify the Subscription Agent prior to 5:00 p.m., Eastern Time, on April 3, 2015, the third business day prior to the “Beneficial Ownership Table” forexpiration date, of your exercise of Subscription Rights and provide evidence satisfactory to us, such as a descriptionlegal opinion from local counsel, that the exercise of such Subscription Rights does not violate the laws of the relationship betweenjurisdiction in which such shareholder resides and among Mr. Seaman and each of these investors, each of whom is alsopayment by a beneficial owner of more than 5% of our outstanding common stock.
Directors and executive officers David E. Jorden and Neil K. Warma also participatedU.S. bank in U.S. dollars before the note offering and invested $115,000 and $15,000, respectively, and were issued warrants to purchase 40,429 and 5,273 shares, respectively.
While the Audit Committee of our Board of Directors is generally responsible for oversight and review of any related person transactions, an independent special committee of our Board reviewed and negotiated the termsexpiration of the convertible secured note offering and recommendedoffer. If no notice is received by such time or the evidence presented is not satisfactory to us, the Subscription Rights represented thereby will expire.
No Revocation or Change
Once you submit the Rights Certificate or have instructed your nominee of your subscription request, you are not allowed to revoke or change the exercise or request a refund of monies paid. All exercises of Subscription Rights are irrevocable, even if you learn information about us that the offeringyou consider to be approved on behalf of Opexa and our Board of Directors.
We issued an aggregate of 163,224 shares of common stock to holders of the July 2012 notes in payment of accrued interest on December 31, 2012, of which entities affiliated with Mr. Seaman were issued an aggregate of 51,943 shares. Mr. Jorden and Mr. Warma were issued 4,595 and 600 shares, respectively. We also issued an aggregate of 123,231 shares of common stock in payment of accrued interest on June 30, 2013, of which entities affiliated with Mr. Seaman were issued an aggregate of 50,297 shares. Mr. Jorden and Mr. Warma were also issued 4,450 and 581 shares, respectively.
Mr. Jorden and Charles E. Sheedy, who is the beneficial owner of more than 5% of our outstanding common stock, participated in a private offering on January 23, 2013 withunfavorable. You should not exercise your Subscription Rights unless you are certain other accredited investors who purchased an aggregate of $650,000 in principal amount of our unsecured convertible promissory notes and warrantsthat you wish to purchase shares of our common stock. Messrs. Jorden and Sheedy each invested $100,000 in the offering and each received a warrant to purchase 37,500 shares of common stock. The notes were originally scheduled to mature on January 23, 2014 and accrued interest at the rateSubscription Price.
U.S. Federal Income Tax Treatment of 12% per annum, compounded annually. Interest was payable quarterly beginning March 31, 2013 in cash. The notes were convertible into common stock at the optionRights Distribution
For U.S. federal income tax purposes, we do not believe holders of the investors at a price of $1.298125 per share, subject to certain limitations. Fifty percent of the initial principal amount (less any amount of such principal that has otherwise been prepaid or converted) was payable by us five business days following our receipt of an aggregate of at least $5 million in proceeds from the sale of our equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. The remaining principal was payable five business days following our receipt of an aggregate of at least $7.5 million in proceeds from the sale of our equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. Upon receipt of the upfront payment of $5 million from Merck in February 2013, we repaid $550,000 principal amount of the notes plus accrued interest and converted the remaining $100,000 principal amount into shares of our common stock or Series L warrants should recognize income or loss upon receipt or exercise of a Subscription Right. See “Material U.S. Federal Income Tax Consequences.”
No Recommendation to Rights Holders
Our board of directors is not making a recommendation regarding your exercise of the Subscription Rights. Stockholders who exercise Subscription Rights risk investment loss on money invested. We cannot assure you that the market price of our common stock will reach or exceed the Subscription Price, and even if it does so, that it will not decline during or after the Rights Offering. We also cannot assure you that you will be
able to sell shares of our common stock or Warrants purchased in the Rights Offering at a price equal to or greater than the Subscription Price. You should make your investment decision based on your assessment of our business and financial condition, our prospects for the future and the terms of this Rights Offering. Please see “Risk Factors” for a discussion of some of the risks involved in investing in our common stock.
Fees and Expenses
We will pay all fees charged by the Subscription Agent and the Information Agent, and by the dealer-managers. You are responsible for paying any other commissions, fees, taxes or other expenses incurred in connection with the exercise of your Subscription Rights.
Listing
The Subscription Rights may not be sold, transferred, assigned or given away to anyone, and will not be listed for trading on any stock exchange or market. We have applied to have the Warrants listed for trading on NASDAQ under the symbol “OPXAW,” however, there is no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet the minimum listing criteria to be accepted for listing on NASDAQ. The shares of our common stock, including the shares to be issued in the Rights Offering and the shares underlying the Warrants to be issued in the Rights Offering, are traded on NASDAQ under the symbol “OPXA.”
Important
Do not send Rights Certificates directly to us. You are responsible for choosing the payment and delivery method for your Rights Certificate and you bear the risks associated with such delivery. If you choose to deliver your Rights Certificate and payment by mail, we recommend that you use registered mail, properly insured, with return receipt requested. We also recommend that you allow a sufficient number of days to ensure delivery to the Subscription Agent and clearance of payment prior to the expiration time.
Distribution Arrangements
Maxim Group LLC and National Securities Corp. are the dealer-managers for the Rights Offering. The dealer-managers will provide marketing assistance and advice to us in connection with the Rights Offering and will use their best efforts to solicit the exercise of Subscription Rights and participation in the Over-Subscription Privilege. The dealer-managers are not underwriting or placing any of the Subscription Rights or the shares of our common stock or Warrants to be issued in the Rights Offering and do not make any recommendation with respect to such Subscription Rights (including with respect to the exercise or expiration of such Subscription Rights), shares or Warrants. We have agreed to pay the dealer-managers certain fees and to reimburse the dealer-managers for certain out-of-pocket expenses incurred in connection with this offering. See “Plan of Distribution.”
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following discussion is a summary of material U.S. federal income tax consequences relating to the receipt and exercise (or expiration) of the Subscription Rights acquired through the Rights Offering and the ownership and disposition of shares of our common stock and Warrants received upon exercise of the Subscription Rights or Warrants.
This summary deals only with Subscription Rights acquired through the Rights Offering, shares of our common stock and Warrants acquired upon exercise of Subscription Rights and shares of our common stock acquired upon exercise of the Warrants, in each case, that are held as capital assets by a beneficial owner. This discussion does not address all aspects of U.S. federal income taxation that may be relevant to such beneficial owners in light of their personal circumstances. This discussion also does not address tax consequences to holders that may be subject to special tax rules, including, without limitation, insurance companies, real estate investment trusts, regulated investment companies, grantor trusts, tax-exempt organizations, employee stock purchase plans, partnerships and other pass-through entities, persons holding Subscription Rights, shares of our common stock, Series L warrants or Warrants as part of a hedging, integrated, conversion or constructive sale transaction or a straddle, financial institutions, brokers, dealers in securities or currencies, traders that elect to mark-to-market their securities, persons that acquired Subscription Rights, shares of our common stock, Series L warrants or Warrants in connection with employment or other performance of services, U.S. Holders (as defined below) that have a functional currency other than the U.S. dollar, U.S. expatriates, and certain former citizens or residents of the United States. In addition, the discussion does not describe any tax consequences arising out of the tax laws of any state, local or foreign jurisdiction, or any U.S. federal tax considerations other than income taxation (such as Medicare contribution taxation or estate, generation skipping or gift taxation).
The discussion below is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Code, and regulations, rulings and judicial decisions thereunder, as of the date hereof, and such authorities may be repealed, revoked or modified, perhaps retroactively. We have not sought, and will not seek, any rulings from the Internal Revenue Service, or the IRS, regarding the matters discussed below. There can be no assurance that the IRS or a court will not take positions concerning the tax consequences of the receipt of Subscription Rights acquired through the Rights Offering by persons holding shares of our common stock or Series L warrants, the exercise (or expiration) of the Subscription Rights, the acquisition, ownership and disposition of shares of our common stock and the acquisition, ownership and disposition (or expiration) of Warrants acquired upon exercise of the Subscription Rights that are different from those discussed below.
As used herein, a “U.S. Holder” means a beneficial owner of shares of our common stock, Series L warrants, Subscription Rights, shares of our common stock and Warrants acquired upon exercise of Subscription Rights or shares of our common stock acquired upon exercise of Warrants, as the case may be, that is for U.S. federal income tax purposes: (1) an individual who is a citizen or resident of the United States; (2) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any state thereof or the District of Columbia; (3) an estate the income of which is subject to U.S. federal income taxation regardless of its source; or (4) a trust (a) the administration of which is subject to the primary supervision of a court within the United States and one or more United States persons as described in Section 7701(a)(30) of the Code have authority to control all substantial decisions of the trust, or (b) that has a valid election in effect to be treated as a United States person. A “Non-U.S. Holder” is such a beneficial owner (other than an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes) that is not a U.S. Holder.
If any entity or arrangement that is treated as a partnership for U.S. federal income tax purposes is such a beneficial owner, the U.S. federal income tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. Holders that are partnerships (and partners in such partnerships) are urged to consult their own tax advisors.
HOLDERS OF SHARES OF OUR COMMON STOCK AND SERIES L WARRANTS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES UNDER FEDERAL ESTATE AND GIFT TAX LAWS, FOREIGN, STATE, AND LOCAL LAWS AND TAX TREATIES OF THE RECEIPT, OWNERSHIP AND EXERCISE OF SUBSCRIPTION RIGHTS AND THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF SHARES OF OUR COMMON STOCK AND WARRANTS ACQUIRED UPON EXERCISE OF SUBSCRIPTION RIGHTS AND SHARES OF OUR COMMON STOCK ACQUIRED UPON EXERCISE OF WARRANTS.
Tax Consequences to U.S. Holders
Taxation of Subscription Rights
Receipt of Subscription Rights
Although the authorities governing transactions such as this Rights Offering are complex and do not speak directly to the consequences of certain aspects of this Rights Offering, including the inclusion of the right to purchase Warrants in the Subscription Rights (rather than the right to purchase only shares of our common stock), the distribution of Subscription Rights to Series L warrant holders and the effects of the Over-Subscription Privilege, we do not believe your receipt of Subscription Rights pursuant to an investor’s electionthe Rights Offering should be treated as a taxable distribution with respect to convert into equity. The warrants have an exercise price of $1.24 per share, a five-year term and are exercisable for a maximum of an aggregate of 243,750your existing shares of common stock or Series L warrants for U.S. federal income tax purposes. Section 305(a) of the Code states that a shareholder’s taxable income does not include in-kind stock dividends; however, the general non-recognition rule in Section 305(a) is subject to exceptions in Section 305(b), which include “disproportionate distributions.” A disproportionate distribution is a distribution or a series of distributions, including deemed distributions, that has the effect of the receipt of cash or other property by some shareholders or holders of debt instruments convertible into stock and an increase in the proportionate interest of other shareholders in a corporation’s assets or earnings and profits. During the last 36 months, we have not made any distributions of cash or non-stock property with respect to: (i) our common stock or (ii) our options or warrants to acquire common stock. Within the past 36 months, we did, however, make a payment in cash of the accrued interest on certain limitations.previously outstanding convertible notes upon their retirement. Currently we do not have any convertible debt outstanding nor do we currently intend to issue any convertible debt or pay any dividends on our common stock (other than the issuance of the Subscription Rights in connection with this offering). Further, the payment of cash interest upon retirement of the previously outstanding convertible notes and the distribution of the Subscription Rights hereunder are unrelated isolated transactions which are not part of a plan to increase any shareholder’s proportionate interest in our earnings and profits or assets.
Pursuant to a waiver executedOur position regarding the tax-free treatment of the Subscription Right distribution is not binding on the IRS, or the courts. If this position is finally determined by the IRS or a court to be incorrect, whether on the basis that the issuance of the Subscription Rights is a “disproportionate distribution” or otherwise, the fair market value of the Subscription Rights would be taxable to holders of in excess of two-thirdsour common stock as a dividend to the extent of the principal amountholder’s pro rata share of our current and accumulated earnings and profits, if any, with any excess being treated as a return of capital to the extent thereof and then as capital gain. Although no assurance can be given, it is anticipated that we will not have current and accumulated earnings and profits through the end of 2015. Further, if our position is incorrect, the treatment of holders of Series L warrants in that case is not clear, and it may differ from the treatment of the outstanding July 2012 notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, we issued warrantsSubscription Right distribution to the holders of our common stock. The Series L warrant holders may be treated in a manner similar to holders of our common stock but it is possible that they may be subject to different and adverse U.S. federal income tax consequences.
The following discussion is based upon the July 2012 notestreatment of the Subscription Right issuance as a non-taxable distribution with respect to purchaseyour existing shares of common stock or Series L warrants for U.S. federal income tax purposes.
Tax Basis in the Subscription Rights
If the fair market value of the Subscription Rights you receive is less than 15% of the fair market value of your existing shares of common stock or Series L warrants (with respect to which the Subscription Rights are distributed) on the date you receive the Subscription Rights, the Subscription Rights will be allocated a zero basis for U.S. federal income tax purposes, unless you elect to allocate your basis in your existing shares of common stock or Series L warrants between your existing shares of common stock or Series L warrants and the Subscription Rights in proportion to the relative fair market values of the existing shares of common stock or Series L warrants and the Subscription Rights determined on the date of receipt of the Subscription Rights. If you choose to allocate basis between your existing common shares or Series L warrants and the Subscription Rights, you must make this election on a statement included with your timely filed tax return (including extensions) for the taxable year in which you receive the Subscription Rights. Such an aggregateelection is irrevocable.
However, if the fair market value of 187,500the Subscription Rights you receive is 15% or more of the fair market value of your existing shares of common stock or Series L warrants on the date you receive the Subscription Rights, then you must allocate your basis in your existing shares of common stock or Series L warrants between those shares or Series L warrants and the Subscription Rights you receive in proportion to their fair market values determined on the date you receive the Subscription Rights.
The fair market value of the Subscription Rights on the date that the Subscription Rights are distributed is uncertain, and we have not obtained, and do not intend to obtain, an appraisal of the fair market value of the Subscription Rights on that date. In determining the fair market value of the Subscription Rights, you should consider all relevant facts and circumstances, including any difference between the Subscription Price of the Subscription Rights and the trading price of our shares of common stock on the date that the Subscription Rights are distributed, the exercise price of the Series L warrants, the length of the period during which the Subscription Rights may be exercised and the fact that the Subscription Rights are non-transferable.
Exercise of Subscription Rights
Generally, you will not recognize gain or loss upon the exercise of a Subscription Right in the Rights Offering. Your adjusted tax basis, if any, in the Subscription Right plus the Subscription Price should be allocated between the new common share and Warrant acquired upon exercise of the Subscription Right in proportion to their relative fair market values on the exercise date. This allocation will establish your initial tax basis for U.S. federal income tax purposes in your new common shares and Warrants. The holding period of a share of common stock or Warrant acquired upon exercise of a Subscription Right in the Rights Offering will begin on the date of exercise.
If you exercise a Subscription Right received in the Rights Offering after disposing of the shares of our common stock or Series L warrants with respect to which such Subscription Right is received, then certain aspects of the tax treatment of the exercise of the Subscription Right are unclear, including (1) the allocation of the tax basis between the shares of common stock or Series L warrants previously sold and the Subscription Right, (2) the impact of such allocation on the amount and timing of gain or loss recognized with respect to the shares of our common stock or Series L warrants previously sold, and (3) the impact of such allocation on the tax basis of the shares of our common stock and Series L warrants acquired upon exercise of the Subscription Right. If you exercise a Subscription Right received in the Rights Offering after disposing of shares of our common stock or Series L warrants with respect to which the Subscription Right is received, you should consult with your tax advisor.
Expiration of Subscription Rights
If you allow Subscription Rights received in the Rights Offering to expire, you should not recognize any gain or loss for U.S. federal income tax purposes, and you should re-allocate any portion of the tax basis in your existing common shares or Series L warrants previously allocated to the Subscription Rights that have expired to the existing common shares or Series L warrants.
Taxation of Warrants
Sale or other Taxable Disposition of Warrants
Upon the sale, exchange or other taxable disposition of a Warrant, in general, you will recognize taxable gain or loss measured by the difference, if any, between (i) the amount of cash and the fair market value of any property received upon such taxable disposition, and (ii) your adjusted tax basis in the Warrant as allocated pursuant to the rules discussed above. Your gain or loss generally will be capital gain or loss and generally will be long-term capital gain or loss if, at the time of the sale or other disposition, your holding period for the Warrant is more than one year. The deductibility of capital losses is subject to limitations.
Exercise of Warrants
Upon the exercise of a Warrant for cash, in general, you will not recognize gain or loss for U.S. federal income tax purposes, except to the extent you receive a cash payment for any such fractional share that would otherwise have been issuable upon exercise of the Warrant. Your initial tax basis in common stock received will equal your adjusted tax basis in the Warrant exercised, increased by the amount of cash paid to exercise the Warrant and decreased by the adjusted tax basis allocable to any fractional share that would otherwise have been issuable upon exercise of the Warrant. Your holding period for the shares of our common stock received on exercise generally will commence on the day of exercise.
In certain circumstances, the Warrants will be exercisable on a cashless basis. The tax consequences of a cashless exercise are not clear and could differ from the consequences described above, including the possibility that a cashless exercise could be a taxable event. You should consult your tax advisor regarding the tax consequences of a cashless exercise of a Warrant.
Expiration of Warrants
If you allow a Warrant to expire, you will generally recognize a loss for U.S. federal income tax purposes equal to the adjusted tax basis of the Warrant. In general, such a loss will be a capital loss, and will be a short-term or long-term capital loss depending on your holding period for the Warrant.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to you if, and to the extent that, such adjustment has the effect of increasing your proportionate interest in our earnings and profits or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “Taxation of Common Shares – Distributions” below.
Taxation of Common Shares
Distributions
Distributions with respect to shares of our common stock acquired upon exercise of Subscription Rights or upon exercise of Warrants will be taxable as dividend income when actually or constructively received to the extent of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes.
Dividend income received by certain non-corporate U.S. Holders with respect to shares of our common stock generally will be “qualified dividends” subject to preferential rates of U.S. federal income tax, provided that the U.S. Holder meets applicable holding period and other requirements. Subject to similar exceptions for short-term and hedged positions, dividend income on our shares of common stock paid to U.S. Holders that are domestic corporations generally will qualify for the dividends-received deduction. To the extent that the amount of a distribution exceeds our current and accumulated earnings and profits, such distribution will be treated first as a tax-free return of capital to the extent of your adjusted tax basis in such shares of our common stock and thereafter as capital gain.
Dispositions
If you sell or otherwise dispose of shares of common stock acquired upon exercise of Subscription Rights or upon exercise of Warrants in a taxable transaction, you will generally recognize capital gain or loss equal to the difference between the amount realized and your adjusted tax basis in the shares. Such capital gain or loss will be long-term capital gain or loss if your holding period for such shares is more than one year at the time of disposition. Long-term capital gain of a non-corporate U.S. Holder is generally taxed at preferential rates of U.S. federal income tax. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
You may be subject to information reporting and/or backup withholding with respect to the gross proceeds from the disposition of Warrants, shares of our common stock acquired through the exercise of Subscription Rights or through the exercise of Warrants, or dividend payments. Backup withholding (currently at the rate of 28%) may apply under certain circumstances if you (1) fail to furnish your social security or other taxpayer identification number, or TIN, (2) furnish an incorrect TIN, (3) fail to report interest or dividends properly, or (4) fail to provide a certified statement, signed under penalty of perjury, that the TIN provided is correct, that you are not subject to backup withholding and that you are a U.S. person on IRS Form W-9 or Substitute Form W-9. Any amount withheld from a payment under the backup withholding rules is allowable as a credit against (and may entitle you to a refund with respect to) your U.S. federal income tax liability, provided that the required information is timely furnished to the IRS. Certain persons are exempt from information reporting and backup withholding, including corporations and financial institutions, provided that they demonstrate this fact, if requested. You are urged to consult your own tax advisor as to your qualification for exemption from backup withholding and the procedure for obtaining such exemption.
Tax Consequences to Non-U.S. Holders
Taxation of the Subscription Rights
Receipt, Exercise and Expiration of the Subscription Rights
The discussion assumes that the receipt of Subscription Rights will be treated as a nontaxable distribution. See “Tax Consequences to U.S. Holders – Taxation of Subscription Rights – Receipts of Subscription Rights” above. You will not be subject to U.S. federal income tax (or any withholding thereof) on the receipt, exercise or expiration of the Subscription Rights.
Exercise and Expiration of Warrants and Certain Adjustments to Warrants
Exercise of Warrants
In general, a Non-U.S. Holder will not recognize gain or loss for U.S. federal income tax purposes upon exercise of a Warrant, except to the extent the Non-U.S. Holder receives a cash payment for any such fractional share that would otherwise have been issuable upon exercise of the Warrant, which will be treated as a sale subject to the rules described under “Sale or Other Disposition of Common Stock or Warrants” below.
Expiration of Warrants
In general, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a Warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is present 183 days or more in the taxable year of disposition and certain other conditions are met.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of Warrant shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of the Warrants, may be treated as a constructive distribution to a Non-U.S. Holder of the Warrants if, and to the extent that, such adjustment has the effect of increasing such Non-U.S. Holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “– Taxation of Distributions on Common Shares” below.
Taxation of Distributions on Common Shares
Any distributions of cash or property made with respect to our common stock generally will be subject to withholding tax to the extent paid out of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes, if any, at a rate of 30% (or a lower rate prescribed in an applicable income tax treaty). In order to obtain a reduced withholding tax rate, if applicable, you will be required to provide an IRS Form W-8BEN or IRS Form W-8BEN-E, as applicable, certifying your entitlement to benefits under a treaty. In addition, you will not be subject to withholding tax if you provide an IRS Form W-8ECI certifying that the distributions are effectively connected with your conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment within the United States); instead, you generally will be subject to U.S. federal income tax, net of certain deductions, with respect to such income at the same rates applicable to U.S. persons, and if you are a corporation, a “branch profits tax” of 30% (or a lower rate prescribed in an applicable income tax treaty) also may apply to such effectively connected income.
Non-U.S. Holders may be required to periodically update their IRS Forms W-8.
Sale or Other Disposition of Our Common Stock or Warrants
In general, you will not be subject to U.S. federal income tax on any gain realized on a sale of shares of our common stock, or Warrants unless:
Gain that is effectively connected with your conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable to a permanent establishment within the United States) generally
will be subject to U.S. federal income tax, net of certain deductions, at the same rates applicable to U.S. persons. If you are a corporation, a “branch profits tax” of 30% (or a lower rate prescribed in an applicable income tax treaty) also may apply to such effectively connected gain.
A domestic corporation is treated as a USRPHC if the fair market value of its United States real property interests equals or exceeds 50% of the sum of (1) the fair market value of its United States real property interests, (2) the fair market value of its non-United States real property interests and (3) the fair market value of any other of its assets which are used or held for use in a trade or business. We believe that we are not currently, and have not been within the relevant testing period, a USRPHC. However, no assurance can be given that we will not become a USRPHC in the future. If we are a USRPHC or become a USRPHC in the future, a Non-U.S. Holder may still not be subject to U.S. federal income tax on a sale or other disposition if an exception for 5% or less shareholders applies. You are urged to consult your own tax advisor regarding the U.S. federal income tax considerations that could result if we are, or become, a USRPHC and with respect to the exception for 5% or less shareholders.
Information Reporting and Backup Withholding
Distributions on our common stock and the amount of tax withheld, if any, with respect to such distributions will generally be subject to information reporting. If you comply with certification procedures to establish that you are not a United States person, additional information reporting and backup withholding should not apply to distributions on our common stock and information reporting and backup withholding should not apply to the proceeds from a sale or other disposition of Warrants or shares of our common stock. The warrantsamount of any backup withholding will generally be allowed as a refund or credit against your U.S. federal income tax liability, provided that the required information is timely furnished to the IRS.
FATCA
Legislation enacted in 2010 and commonly referred to as FATCA may impose withholding taxes on certain types of payments made to “foreign financial institutions” and certain other non-U.S. entities. The legislation imposes a 30% withholding tax on dividends on shares of our common stock and on or after January 1, 2017, the gross proceeds from the sale or other disposition of our shares of common stock or Warrants received by a foreign financial institution unless the foreign financial institution enters into an agreement with the U.S. Treasury to among other things, undertake to identify accounts held by certain U.S. persons or U.S.-owned foreign entities, annually report certain information about such accounts and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. In addition, the legislation imposes a 30% withholding tax on the same types of payments to a non-financial foreign entity unless the entity certifies that it does not have any substantial U.S. owners or furnishes identifying information regarding each substantial U.S. owner. Foreign financial institutions located in jurisdictions that have an exercise priceintergovernmental agreement with the United States governing FATCA may be subject to different rules. Depending on your circumstances, you may be entitled to a refund or credit in respect of $1.21 per sharesome or all of this withholding. However, even if you are entitled to have any such withholding refunded, the required procedures could be cumbersome and a five-year term. Entities affiliated with Mr. Seaman were issued warrants to purchase an aggregatesignificantly delay your receipt of 59,670 shares, and Messrs. Jorden and Warma were issued warrants to purchase 5,278 and 688 shares, respectively.any withheld amounts. Prospective investors should consult their tax advisors regarding this legislation.
THE PRECEDING DISCUSSION OF MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES IS NOT TAX ADVICE. HOLDERS OF SHARES OF OUR COMMON STOCK AND SERIES L WARRANTS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES UNDER FEDERAL ESTATE AND GIFT TAX LAWS, FOREIGN, STATE, AND LOCAL LAWS AND TAX TREATIES OF THE RECEIPT, OWNERSHIP AND EXERCISE OF SUBSCRIPTION RIGHTS AND THE ACQUISITION, OWNERSHIP, AND DISPOSITION OF SHARES OF OUR COMMON STOCK AND WARRANTS ACQUIRED UPON EXERCISE OF SUBSCRIPTION RIGHTS AND SHARES OF OUR COMMON STOCK ACQUIRED UPON EXERCISE OF WARRANTS.
DESCRIPTION OF COMMON STOCKSECURITIES
Common Stock
This section describes the general terms and provisions of the shares of our common stock, $0.01 par value. This description is only a summary and is qualified in its entirety by reference to the description of our common stock included in our restated certificate of formation and our amended and restated bylaws which have been filed as exhibits to the registration statement of which this prospectus is a part. You should read our restated certificate of formation and our amended and restated bylaws for additional information before you buy any of our common stock or other securities. See “Where You Can Find More Information.Information” and “Incorporation by Reference.”
We have 100,000,000 shares of authorized common stock. As of July 19, 2013,December 31, 2014, there were 8,114,79028,234,751 shares of common stock issued and outstanding.outstanding, as well as Series L warrants to purchase 541,668 shares of common stock outstanding on that date. Each holder of common stock is entitled to one vote for each share of common stock held on all matters submitted to a vote of shareholders. We have not provided for cumulative voting for the election of directors in our restated certificate of formation. This means that the holders of a majority of the shares voted can elect all of the directors then standing for election. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the amounts that our board of directors may determine from time to time. Upon our liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any outstanding preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and nonassessable, and the shares of common stock offered, when issued, will be fully paid and nonassessable.
The shares of common stock that are purchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of shares if you are a holder of record of shares or Series L warrants. The Subscription Agent will arrange for the issuance of the common stock as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the common stock you purchased in the Rights.
Preferred Stock
We have 10,000,000 shares of authorized preferred stock, no par value, none of which were issued or outstanding as of December 31, 2014. Of this amount, 80,000 shares have been designated Series A convertible preferred stock. We may issue preferred stock, in series, with such designations, powers, preferences and other rights and qualifications, limitations or restrictions as our board of directors may authorize, without further action by our stockholders.
Warrants
Warrants Included in Units Issuable in the Rights Offering
The Warrants to be issued as a part of this Rights Offering will be designated as our “Series M” warrants. These Warrants will be separately transferable following their issuance and through their expiration three years from the date of issuance. The Warrants entitle the holder to purchase one share of common stock at an exercise price of (i) $0.50 per share from the date of issuance through June 30, 2016 and (ii) $1.50 per share
from July 1, 2016 through their expiration. We have applied to list the Warrants for trading on NASDAQ under the symbol “OPXAW,” however, there is no assurance that a sufficient number of Subscription Rights will be exercised so that the Warrants will meet the minimum listing criteria to be accepted for listing on NASDAQ. The common stock underlying the Warrants, upon issuance, will also be traded on NASDAQ under the symbol “OPXA.”
All Warrants that are purchased in the Rights Offering as part of the Units will be issued in book-entry, or uncertificated, form meaning that you will receive a direct registration (DRS) account statement from our transfer agent reflecting ownership of Warrants if you are a holder of record of shares or Series L warrants. The Subscription Agent will arrange for the issuance of the Warrants as soon as practicable after the expiration of the Rights Offering, payment for the Units subscribed for has cleared, and all prorating calculations and reductions contemplated by the terms of the Rights Offering have been effected. If you hold your shares of common stock in the name of a custodian bank, broker, dealer, or other nominee, DTC will credit your account with your nominee with the Warrants you purchased in the Rights Offering.
The Warrants will be exercisable for cash, or solely during any period when a registration statement for the exercise of the Warrants is not in effect, on a cashless basis.
We may call the Warrants for redemption, in whole and not in part, at a price of $.01 per Warrant at any time while the Warrants are exercisable, upon not less than 30 days’ prior written notice of redemption to each Warrant holder, and if, and only if, the closing price of the common stock equals or exceeds $2.50 per share, subject to adjustment, for 10 consecutive trading days.
The exercise price of the Warrants and the number of shares of common stock issuable upon exercise of the Warrants are subject to adjustment in certain circumstances, including a stock split of, stock dividend on, or a subdivision, combination or recapitalization of the common stock. Upon the merger, consolidation, sale of substantially all of our assets, or other similar transaction, the holders of Warrants shall, at the option of the company, be required to exercise the Warrants immediately prior to the closing of the transaction, or such Warrants shall automatically expire. Upon such exercise, the holders of Warrants shall participate on the same basis as the holders of common stock in connection with the transaction.
The Warrants do not confer upon the holder any voting or any other rights of a shareholder of the Company. Upon notice to the Warrants holders, we have the right at any time and from time to time, to reduce the exercise price or to extend the Warrants termination date.
The Warrants will be issued pursuant to a warrant agreement by and between us and Continental Stock Transfer & Trust Company, the warrant agent.
Other Currently Outstanding Warrants
We have the following series of warrants currently outstanding, some of which contain a provision for adjustment of the warrants upon completion of this Rights Offering:
Series | Expiration Date | Shares of Common Stock Underlying Warrants | Exercise Price | Potential Warrant Adjustment | ||||||
| Series A | 6-15-15 | 223,119 | $10.20 | Warrant exercise price is subject to adjustment if the Subscription Price is less than the daily volume weighted average price (VWAP) of the common stock on the Record Date, based on the number of shares outstanding, the number of additional shares offered and the VWAP. | ||||||
| Series H | 2-11-16 | 414,649 | $10.44 | None | ||||||
| Series I | 7-25-17 | 1,436,115 | $2.56 | None | ||||||
| Series J | 1-23-18 | 243,750 | $1.24 | Warrant exercise price is subject to adjustment, based on fair market value of the Subscription Rights, market price of the common stock prior to the Record Date, and number of shares outstanding. | ||||||
| Series K | 1-29-18 | 187,500 | $1.21 | Warrant exercise price is subject to adjustment, based on fair market value of the Subscription Rights, market price of the common stock prior to the Record Date, and number of shares outstanding. | ||||||
| Series L | 2-11-17 | 541,668 | $3.00 | Warrant exercise price and number of shares issuable upon exercise of the warrant are subject to adjustment, based on the number of shares outstanding before and after the offering. | ||||||
|
| |||||||||
TOTAL | 3,046,801 | |||||||||
|
| |||||||||
Certain Provisions of our Charter and Bylaws
Certain provisions of our restated certificate of formation and our amended and restated bylaws described below may have the effect of delaying, deferring or discouraging another party from acquiring control of us. Our restated certificate of formation and amended and restated bylaws provided that:
Our board of directors is authorized to issue preferred stock without shareholder approval; and
We will indemnify officers and directors against losses that they may incur in investigations and legal proceedings resulting from their services to us, which may include services in connection with takeover defense measures.
Transfer Agent
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, 17 Battery Place, New York, New York 10004.
UNDERWRITINGPLAN OF DISTRIBUTION
Aegis Capital Corp. is actingOn or about March 16, 2015, we will distribute the Subscription Rights, Rights Certificates and copies of this prospectus to the holders of our common stock and Series L warrants on the Record Date. Subscription Rights holders who wish to exercise their Subscription Rights and purchase Units must complete the Subscription Rights Certificate and return it with payment for the shares to the Subscription Agent at the following address:
Continental Stock Transfer & Trust Company
17 Battery Place—8th Floor
New York, NY 10004
Attn: Corporate Actions Department
See “The Rights Offering—Methods for Exercising Subscription Rights.”
If you have any questions, you should contact our Information Agent for the Rights Offering:
Advantage Proxy, Inc.
(877) 870-8565 (toll free)
(206) 870-8565 (collect)
Other than as described in this prospectus, we do not know of any existing agreements between any shareholder, broker, dealer, underwriter or agent relating to the sole managersale or distribution of the underlying common stock.
Maxim Group LLC and National Securities Corp. are the dealer-managers of this Rights Offering. In such capacity, such dealer-managers will provide marketing assistance and advice to us in connection with this offering and as representativewill solicit the exercise of Subscription Rights and participation in the Over-Subscription Privilege. The dealer-managers are not underwriting or placing any of the underwriters,Subscription Rights or the Representative. We have entered into an underwriting agreement dated , 2013shares of our common stock or Warrants being issued in this offering and do not make any recommendation with the Representative. Subjectrespect to such Subscription Rights (including with respect to the terms and conditionsexercise or expiration of the underwriting agreement,such Subscription Rights), shares or Warrants.
In connection with this Rights Offering, we have agreed to sellpay fees to each underwriter named belowthe dealer-managers as follows: (i) to Maxim Group LLC, a cash fee equal to (a) 1% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is less than $6 million; (b) 4% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million but less than $10 million; and each underwriter named below has severally(c) 4.5% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $10 million; and (ii) to National Securities Corporation, a cash fee equal to 2.75% of the gross proceeds received by us directly from exercises of the Subscription Rights if the amount of such gross proceeds is at least $6 million. We have also agreed to purchase, atreimburse the public offering price lessdealer-managers for their expenses (including, without limitation, legal fees) as follows: (i) up to $50,000 if the underwriting discount set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| ||
| ||
| ||
The underwriters are committed to purchase all the shares of common stock offeredgross proceeds received by us other than those covered by the option to purchase additional shares described below, if they purchase any shares. The obligationsdirectly from exercises of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representationsSubscription Rights is less than $6 million; and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for(ii) up to 30 days after$100,000 if the date of this prospectus, permits the underwriters to purchase a maximum of 1,395,000 additional shares (15%gross proceeds received by us directly from exercises of the shares sold in this offering) from usSubscription Rights is at least $6 million. We advanced $20,000 to cover over-allotments, if any. IfMaxim Group LLC against reimbursement of accountable expenses upon their engagement as a dealer-manager (the “Advance”). Any portion of the underwriters exercise all or part of this option, they will purchase shares covered by the option at the public offering price per share that appears on the cover page of this prospectus, less the underwriting discount. If this option is exercised in full, the total price to the publicAdvance will be $17,218,950 and the total net proceeds, before expenses, to us will be $16,013,624.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
Discount
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| ||||||||||||
| ||||||||||||
| ||||||||||||
|
The underwriters propose to offer the shares offered by us to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the shares to other securities dealers at such price less a concession of $ per share. If all of the shares offered by us are not sold at the public offering price, the Representative may change the offering price and other selling terms by means of a supplement to this prospectus.
We have paid an expense deposit of $10,000 to the Representative, which will be applied against accountable expenses and reimbursedreturned to us to the extent it is not actually incurred. The Representative will also be entitled to a non-accountable expense allowance of $50,000 that will be paid by us in connection with this offering; provided that if less than $10 million in gross proceeds is raised in the offering, the non-accountable expense allowance will be reduced to $20,000.
We have also agreed to payindemnify the Representative’s expenses relating to the offering, including (a) all fees, expensesdealer-managers and disbursements relating to background checks of our officers and directors in an amount not to exceed $5,000 in the aggregate (unless we raise a minimum of $10 million in gross proceeds, and in such event, plus 50% of the additional documented expenses for background checks subject to a maximum addition of $5,000); (b) all fees, expenses and disbursements relating to the registration, qualification or exemption of securities offeredtheir respective affiliates against certain liabilities arising under the securities lawsSecurities Act of such states and other jurisdictions designated by the Representative, up to a maximum of $5,000; (c) the cost of all mailing and printing of underwriting documents; (d) upon successfully completing this offering, $21,775 for the underwriters’ use of certain software for this offering; and (e) upon successfully completing this offering, up to $20,000 of the representative’s actual accountable road show expenses for the offering.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, will be approximately $545,000.
Discretionary Accounts
1933. The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
Lock-Up Agreements
Pursuant to certain “lock-up” agreements, we, our directors and officers and certain holders of 5% or more of our outstanding shares of common stock have agreed, for a period ending 90 days from the date of the final prospectus for the offering, not to (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, encumber, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our securities or any securities convertible into or exercisable or exchangeable for shares of our common stock owned or acquired on or prior to the closing date of this offering (including any shares of common stock acquired after the closing date of this offering upon the conversion, exercise or exchange of such securities); (2) file or caused to be filed any registration statement relating to the offering of any shares of our capital stock; or (3) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction described in clause (1), (2) or (3) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, except for certain exceptions and limitations.
The above restrictions do not apply to (i) shares solddealer-managers’ participation in this offering (ii)is subject to customary conditions contained in the issuancedealer-manager agreement, including the receipt by the dealer-managers of shares of capital stock upon the exercise of stock options or warrants or the conversion of any outstanding security or in payment
of accrued interest on our 12% convertible secured promissory notes issued in July 2012, (iii) the issuance of stock options, restricted stock or other equity-based compensation awards under any employee benefit or equity incentive plan, (iv) the filing of a registration statement on Form S-8, (v) securities issued in connection with a transaction that includes a commercial relationship (including but not limited to joint ventures, marketing or distribution arrangements, option or collaboration agreements or intellectual property license agreements) or any acquisition of assets or not less than a majority or controlling portion of the equity of another entity; provided that the aggregate number of shares or securities issued pursuant to clause (v) does not exceed 10% of the total number of outstanding shares of common stock immediately following the issuance and sale of the shares in this offering, and (vi) the issuance of shares of common stock under our existing at-the-market and equity line programs prior to the registration statement for this offering being declared effective.
Right of First Refusal
Subject to certain limited exceptions, until six months from the effective date of this offering, the Representative has a right of first refusal to act as sole book-running manager for any public or private equity or public debt offerings in which we or anyan opinion of our successors or subsidiaries may engage during that period.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically.counsel. The representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
Stabilizing transactions permit bids to purchase securities so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the securities while the offering is in progress.
Over-allotment transactions involve sales by the underwriters of securities in excess of the number of securities the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriters is not greater than the number of securities that they may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing securities in the open market.
Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriters will consider, among other things, the price of securities available for purchase in the open market as compared with the price at which they may purchase securities through exercise of the over-allotment option. If the underwriters sell more securities than could be covered by exercise of the over-allotment option and, therefore, have a naked
|
Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the s securities originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our securities or preventing or retarding a decline in the market price of our securities. As a result, the price of our securities in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our securities. These transactions may be effected on The NASDAQ Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive Market Making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common stock on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships
Certain of the underwritersdealer-managers and their affiliates may provide to us from time to time in the future provide variousin the ordinary course of their business certain financial advisory, investment banking and other financial services for us and our affiliates for which they may in the futurewill be entitled to receive customary fees.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in
substance that by accepting this offer, the offeree represents that the offereeEach of Maxim Group LLC and National Securities Corporation is such a person as set forth in clause (i) above,broker-dealer and unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area—Belgium, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of common stock will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of common stock has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any
implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority, or the ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and
in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended.
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and
in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws.
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1member of the Financial Instruments and Exchange LawIndustry Regulatory Authority, Inc. The principal business address of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIELMaxim Group LLC is 405 Lexington Avenue, New York, New York 10174, and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefitprincipal business address of any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan thatNational Securities Corporation is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.410 Park Avenue, 14th Floor, New York, New York 10022.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
NASDAQ Capital Market Listing
Our common stock is listed on the NASDAQ Capital Market under the symbol “OPXA.”
The financial statements of Opexa as of December 31, 2012,2014, and for the years ended December 31, 20122014 and 2011 included2013, incorporated in this prospectus by reference to our Annual Report on Form 10-K for the year ended December 31, 2014 have been so included in reliance on the report ofaudited by MaloneBailey, LLP, an independent registered public accounting firm, and are incorporated by reference in reliance upon their report dated March 28, 2013, appearing elsewhere in this prospectus,February 20, 2015, given upon such firm’s authority as experts in auditing and accounting.
The validity of any securities offered by this prospectus will be passed upon for us by Pillsbury Winthrop Shaw Pittman LLP, San Diego, California. Certain legal matters in connection with this offering will be passed upon for the underwritersThe dealer-managers are being represented by Kramer Levin NaftalisLoeb & FrankelLoeb LLP, New York, New York.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 with the SEC under the Securities Act of 1933, as amended. This prospectus is part of the registration statement but the registration statement includes additional information and exhibits. We file annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy the registration statement and any document we file with the SEC at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The website address is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only.
The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the same care that you read this prospectus. We have filed with the SEC, and incorporate by reference in this prospectus:
We are not, however, incorporating, in each case, any documents or information that we are deemed to furnish and not file in accordance with SEC rules.
You may request a copy of any or all of the documents incorporated by reference but not delivered with this prospectus, at no cost, by writing or telephoning us at the following address and number: Investor Relations, Opexa Therapeutics, Inc., 2635 Technology Forest Blvd., The Woodlands, Texas 77381, telephone (281) 775-0600. We will not, however, send exhibits to those documents, unless the exhibits are specifically incorporated by reference in those documents. We also maintain a website at www.opexatherapeutics.com. However, the information on our website is not part of this prospectus and should not be relied upon with respect to this offering.
| AEs | Adverse events. |
| ||
ARR | Annualized relapse rate. |
| Attenuation | Process of irradiation that renders | |
| Axon | The long projection of nerve cells. | |
| Biomarker | Is a molecule that is present (or absent) from a particular cellular type. This facilitates the characterization of a cell type and their identification. A biomarker can be used to identify a cell population, make a diagnostic, measure the progress of disease or the effects of treatment. | |
| BLA | Biologics license application. | |
| ||
| Blood-brain barrier | Is a separation of circulating blood from the brain extracellular fluid in the central nervous system. | |
| cGMP | Current good manufacturing practice. | |
| CNS | Central nervous system. | |
| Cryopreserved | Storage of cells in a frozen state in liquid nitrogen. | |
| ||
Demyelination | Destruction of myelin sheath. |
| Dose | Number of attenuated MRTCs per vial. | |
| EDSS | Expanded disability status scale. | |
| EPA | An Epitope Profiling Essay is a proprietary assay developed by Opexa to identify the patient specific antigens that might be responsible for triggering the myelin sheath destruction pathway. | |
| Epitope | Also known as antigenic determinant is the part of an antigen that is recognized by the immune system, specifically by antibodies, B-cells, or T-cells. | |
| FDA | U.S. Food and Drug Administration. | |
| Immunotherapy | Is a medical term defined as the “treatment of disease by inducing, enhancing, or suppressing an immune response.” | |
| ImmPath® | Our proprietary method for the production of a patient-specific T-cell immunotherapy which encompasses the collection of blood from the MS patient, isolation of peripheral blood mononuclear cells, generation of an autologous pool of MRTCs raised against selected peptides from MBP, MOG and PLP, expanding these MRTCs to a therapeutic dose ex-vivo, and attenuating them with gamma irradiation to prevent DNA replication and thereby cellular proliferation. | |
| Lymphocytes | A kind of white blood cell in the vertebrate immune system, specifically, landmark of the adaptive immune system. Lymphocytes can be divided into large lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells (NK cells). Small lymphocytes consist of T-cells and B-cells. | |
| MBP | Myelin basic protein is a protein believed to be important in the process of myelination of nerves in the nervous system. MBP maintain the correct structure of myelin, interacting with the lipids in the myelin membrane. | |
|
|
| Multiple sclerosis is a disease that involves an immune system attack against the central nervous system (brain, spinal cord, and optic nerves). The disease is thought to be triggered in a genetically susceptible individual by a combination of one or more environmental factors. The day-to-day actions of humans are facilitated by neurons as they send electrical impulses at very high speeds to different parts of the body. The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known |
as myelin or myelin sheath. In the case of multiple sclerosis, the body’s own immune system attacks the myelin sheath. When this happens the electrical impulses from the brain could be slowed down, distorted or stopped resulting in weakness, lack of coordination, fatigue, vision problems, numbness or paralysis. The diagnosis of multiple sclerosis is usually through an MRI scan which helps identify any scar tissue in the brain or the de-myelinated neurons. |
| MOG | Myelin oligodendrocyte glycoprotein is a glycoprotein believed to be important in the process of myelinization of nerves in the central nervous system. In humans this protein is encoded by the MOG gene. It is speculated to serve as a necessary “adhesion molecule” to provide structural integrity to the myelin sheath and is known to develop late on the oligodendrocyte. | |
| MRTCs | Myelin-reactive T-cells are a subset of T-cells that are associated with the destruction of the myelin sheath. | |
| MSFC | Multiple sclerosis functional composite. | |
| MSIS | Multiple sclerosis impact scale. | |
| MSQLI | Multiple sclerosis quality of life inventory. | |
| Myelin or myelin sheath | The ability of the neurons to function properly is determined by the fatty tissue insulation surrounding the axons known as myelin or myelin sheath. | |
| NDA | New drug application. | |
| ||
Oligodendroglial cells | Are a type of brain cell whose main functions are to provide support and to insulate the axons in the central nervous system. |
| PBMC | Peripheral blood mononuclear cell. | |
| Peptide | Short chains of amino acids linked by amide bonds. | |
| PLP | In a myelin sheath, as the layers of myelin wraps come together, proteolipid protein will bind itself and tightly hold the cellular membranes together. | |
| Precision Immunotherapy | A service mark owned by Opexa. | |
| ||
Regulatory T-Cells | Treg cells are a sub population of T cells which modulate the immune system. Animal models have suggested that modulation of Tregs can treat autoimmne diseases. |
| Remyelination | Remyelination is the process propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. | |
RRMS | Relapsing remitting multiple sclerosis. People with this type of MS experience clearly defined attacks of worsening neurologic function. These attacks—which are called relapses, flare-ups, or exacerbations—are followed by partial or complete recovery periods (remissions), during which no disease progression occurs. Approximately 85% of people are initially diagnosed withrelapsing-remitting MS. |
| SAEs | Serious adverse events. | |
| SPMS | Secondary progressive multiple sclerosis. Following an initial period of relapsing-remitting MS, many people develop a secondary-progressive disease course in which the disease worsens more steadily, with or without occasional flare-ups, minor recoveries (remissions), or plateaus. Before the disease-modifying medications became available, approximately 50% of people with relapsing-remitting MS developed this form of the disease within 10 years. | |
| T-cells or T-lymphocytes | Are a type of lymphocytes (itself a type of white blood cells) that play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B-cells and natural killer cells (NK-cells), by the presence of a T-cell receptor on the cell surface. They are called T-cells because they mature in the thymus. There are several subsets of T-cells, each with a distinct function. | |
| Tcelna® | Imilecleucel-T, formerly known as Tovaxin®. | |
| TERMS | Tovaxin for Early Relapsing Multiple Sclerosis was a Phase IIb clinical study of Tcelna in relapsing remitting multiple sclerosis patients completed in 2008. | |
| Whole brain atrophy | Brain atrophy is a condition in which cells in the brain are lost, or the connections between them are damaged. In the brain, losing neurons is highly undesirable, as loss of brain tissue can cause a variety of neurological and cognitive problems. Patients with brain atrophy may develop seizures, dementia, and aphasias. In focal cerebral atrophy, the damage is concentrated on a particular area of the brain, which means that the functions of that area of the brain can become impaired. Generalized brain atrophy involves the whole brain, and may be associated with a range of problems. | |
Sources: National MS Society, Wikipedia, Opexa documents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
OPEXA THERAPEUTICS, INC.
| ||||
| ||||
| ||||
| ||||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Opexa Therapeutics, Inc.
(a development stage company)
The Woodlands, Texas
We have audited the accompanying consolidated balance sheets of Opexa Therapeutics, Inc., (a development stage company), as of December 31, 2012 and 2011 and the related consolidated statements of expenses, changes in stockholders’ equity and cash flows for each of the years then ended and for the period from January 22, 2003 (Inception) through December 31, 2012. These financial statements are the responsibility of Opexa’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatements. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Opexa as of December 31, 2012 and 2011 and the results of its operations and its cash flows for each of the years then ended and for the period from January 22, 2003 (Inception) through December 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
/s/ MALONEBAILEY, LLP
www.malonebailey.com
Houston, Texas
March 28, 2013
OPEXA THERAPEUTICS, INC.
(a development stage company)
| December 31, 2012 | December 31, 2011 | |||||||
| Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 592,004 | $ | 7,109,215 | ||||
Other current assets | 1,077,546 | 124,773 | ||||||
|
|
|
| |||||
Total current assets | 1,669,550 | 7,233,988 | ||||||
Property & equipment, net of accumulated depreciation of $1,494,510 and $1,193,601, respectively | 1,265,041 | 1,029,236 | ||||||
Restricted cash | 1,000,000 | — | ||||||
Deferred financing costs, net of amortization of $58,639 and $0, respectively | 211,479 | — | ||||||
|
|
|
| |||||
Total assets | $ | 4,146,070 | $ | 8,263,224 | ||||
|
|
|
| |||||
| Liabilities and Stockholders’ Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 412,096 | $ | 491,315 | ||||
Accrued expenses | 473,879 | 576,545 | ||||||
|
|
|
| |||||
Total current liabilities | 885,975 | 1,067,860 | ||||||
Long term liabilities: | ||||||||
Convertible debt, net of unamortized discount of $3,136,342 and $0, respectively | 318,658 | — | ||||||
Convertible debt – related parties, net of unamortized discount of $571,895 and $0, respectively | 58,105 | — | ||||||
|
|
|
| |||||
Total liabilities | 1,262,738 | 1,067,860 | ||||||
|
|
|
| |||||
Commitments and contingencies | — | — | ||||||
Stockholders’ equity: | ||||||||
Preferred stock, no par value, 10,000,000 shares authorized, none issued and outstanding | — | — | ||||||
Common stock, $0.01 par value, 100,000,000 shares authorized, 6,249,369 and 5,762,028 shares issued and outstanding | 62,494 | 57,621 | ||||||
Additional paid in capital | 112,432,458 | 107,818,530 | ||||||
Deficit accumulated during the development stage | (109,611,620 | ) | (100,680,787 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | 2,883,332 | 7,195,364 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 4,146,070 | $ | 8,263,224 | ||||
|
|
|
| |||||
See accompanying summary of accounting policies and notes to consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF EXPENSES
Years ended December 31, 2012 and 2011 and the
Period from January 22, 2003 (Inception) to December 31, 2012
| 2012 | 2011 | Inception through 2012 | ||||||||||
Research and development | $ | 6,318,476 | $ | 3,340,038 | $ | 76,497,351 | ||||||
General and administrative | 2,508,541 | 2,406,269 | 30,117,616 | |||||||||
Depreciation | 303,677 | 210,252 | 1,650,158 | |||||||||
Loss on disposal of fixed assets | 3,097 | 9,686 | 513,345 | |||||||||
|
|
|
|
|
| |||||||
Operating loss | (9,133,791 | ) | (5,966,245 | ) | (108,778,470 | ) | ||||||
Interest income | 280 | 932 | 1,358,697 | |||||||||
Other income and expense, net | — | — | 661,146 | |||||||||
Gain on extinguishment of debt | — | — | 1,612,440 | |||||||||
Gain on derivative instruments | 552,978 | — | 1,941,826 | |||||||||
Gain on sale of technology | — | — | 3,000,000 | |||||||||
Interest expense | (350,300 | ) | (3,135 | ) | (9,407,259 | ) | ||||||
|
|
|
|
|
| |||||||
Net loss | $ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (109,611,620 | ) | |||
|
|
|
|
|
| |||||||
Basic and diluted loss per share | $ | (1.54 | ) | $ | (1.06 | ) | ||||||
Weighted average shares outstanding | 5,785,372 | 5,633,124 | ||||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
Period from January 22, 2003 (Inception) through December 31, 2012
| Common Stock | Additional Paid in Capital | Accumulated Deficit | Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
Shares issued for cash | 131,250 | $ | 65,625 | $ | (64,625 | ) | $ | — | $ | 1,000 | ||||||||||
Shares repurchased and cancelled | (42,656 | ) | (21,328 | ) | 21,003 | — | (325 | ) | ||||||||||||
Discount related to beneficial conversion feature | — | — | 28,180 | — | 28,180 | |||||||||||||||
Discount on warrants attached to debt | — | — | 28,180 | — | 28,180 | |||||||||||||||
Net loss | — | — | — | (126,003 | ) | (126,003 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2003 | 88,594 | 44,297 | 12,738 | (126,003 | ) | (68,968 | ) | |||||||||||||
Shares issued for: | ||||||||||||||||||||
cash | 562 | 281 | 8,719 | — | 9,000 | |||||||||||||||
services | 51,625 | 25,813 | 823,187 | — | 849,000 | |||||||||||||||
license | 6,067 | 3,033 | 424,042 | — | 427,075 | |||||||||||||||
reverse merger with Sportan | 24,934 | 12,467 | (160,200 | ) | — | (147,733 | ) | |||||||||||||
acquisition of Opexa | 62,500 | 31,250 | 23,718,750 | — | 23,750,000 | |||||||||||||||
additional shares attached to convertible debt | 4,025 | 2,012 | 286,354 | — | 288,366 | |||||||||||||||
conversion of convertible notes | 15,187 | 7,594 | 240,776 | — | 248,370 | |||||||||||||||
Shares cancelled | (2,000 | ) | (1,000 | ) | 1,000 | — | — | |||||||||||||
Discount related to beneficial conversion feature | — | — | 855,849 | — | 855,849 | |||||||||||||||
Discount on warrants attached to debt | — | — | 1,848,502 | — | 1,848,502 | |||||||||||||||
Option expense | — | — | 123,333 | — | 123,333 | |||||||||||||||
Net loss | — | — | — | (31,411,736 | ) | (31,411,736 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2004 | 251,494 | 125,747 | 28,183,050 | (31,537,739 | ) | (3,228,942 | ) | |||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 97,362 | 48,681 | 5,297,536 | — | 5,346,217 | |||||||||||||||
convertible debt | 152,756 | 76,378 | 7,573,068 | — | 7,649,446 | |||||||||||||||
debt | 575 | 288 | 160,712 | — | 161,000 | |||||||||||||||
license | 7,298 | 3,649 | 1,864,735 | — | 1,868,384 | |||||||||||||||
services | 6,000 | 3,000 | 1,009,400 | — | 1,012,400 | |||||||||||||||
Discount related to beneficial conversion feature | — | — | 831,944 | — | 831,944 | |||||||||||||||
Discount on warrants attached to debt | — | — | 1,433,108 | — | 1,433,108 | |||||||||||||||
Option expense | — | — | 2,487,741 | — | 2,487,741 | |||||||||||||||
Warrant expense | — | — | 2,373,888 | — | 2,373,888 | |||||||||||||||
Transition of warrants from equity instruments to liability instruments | — | — | (10,658,496 | ) | — | (10,658,496 | ) | |||||||||||||
Net loss | — | — | — | (14,856,724 | ) | (14,856,724 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2005 | 515,485 | 257,743 | 40,556,686 | (46,394,463 | ) | (5,580,034 | ) | |||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 1,150,000 | 575,000 | 20,578,519 | — | 21,153,519 | |||||||||||||||
debt | 8,707 | 4,354 | 175,646 | — | 180,000 | |||||||||||||||
Option expense | — | — | 2,749,617 | — | 2,749,617 | |||||||||||||||
Warrant expense | — | — | 1,568,966 | — | 1,568,966 | |||||||||||||||
Net loss | — | — | — | (12,649,170 | ) | (12,649,170 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2006 | 1,674,192 | 837,097 | 65,629,434 | (59,043,633 | ) | 7,422,898 | ||||||||||||||
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY—(Continued)
Period from January 22, 2003 (Inception) through December 31, 2012
| Common Stock | Additional Paid in Capital | Accumulated Deficit | Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
Cumulative change in derivative liability | — | — | 10,658,496 | (4,001,820 | ) | 6,656,676 | ||||||||||||||
Option expense | — | — | 1,876,103 | — | 1,876,103 | |||||||||||||||
Warrant expense | — | — | 845,275 | — | 845,275 | |||||||||||||||
Net loss | — | — | — | (14,667,367 | ) | (14,667,367 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2007 | 1,674,192 | 837,097 | 79,009,308 | (77,712,820 | ) | 2,133,585 | ||||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 1,375,968 | 687,984 | 7,963,595 | — | 8,651,579 | |||||||||||||||
services | 11,300 | 5,650 | 43,315 | — | 48,965 | |||||||||||||||
Issuance of warrants for cash | — | — | 603,850 | — | 603,850 | |||||||||||||||
Option expense | — | — | 1,901,570 | — | 1,901,570 | |||||||||||||||
Net loss | — | — | — | (11,852,152 | ) | (11,852,152 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2008 | 3,061,460 | 1,530,731 | 89,521,638 | (89,564,972 | ) | 1,487,397 | ||||||||||||||
Cumulative effect of change in accounting principle | (1,976,457 | ) | 1,755,622 | (220,835 | ) | |||||||||||||||
Par value adjustment | — | (1,500,117 | ) | 1,500,117 | — | — | ||||||||||||||
Reduction in derivative liability | — | — | 587,609 | — | 587,609 | |||||||||||||||
Discount on convertible notes | — | — | 439,493 | — | 439,493 | |||||||||||||||
Discount on warrants | — | — | 37,453 | — | 37,453 | |||||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 637,500 | 6,375 | 4,682,790 | — | 4,689,165 | |||||||||||||||
exercise of options | 15,100 | 151 | 63,453 | — | 63,604 | |||||||||||||||
exercise of warrants | 154,991 | 1,550 | 1,073,385 | — | 1,074,935 | |||||||||||||||
Option expense | — | — | 650,249 | — | 650,249 | |||||||||||||||
Net loss | — | — | — | (1,433,922 | ) | (1,433,922 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2009 | 3,869,051 | 38,690 | 96,579,730 | (89,243,272 | ) | 7,375,148 | ||||||||||||||
Conversion of convertible notes | 690,045 | 6,900 | 1,373,191 | — | 1,380,091 | |||||||||||||||
Shares issued for: | ||||||||||||||||||||
services | 13,750 | 138 | 64,212 | — | 64,350 | |||||||||||||||
exercise of options | 35,380 | 354 | 109,287 | — | 109,641 | |||||||||||||||
exercise of warrants | 8,500 | 85 | (85 | ) | — | — | ||||||||||||||
Option expense | — | — | 508,550 | — | 508,550 | |||||||||||||||
Net loss | — | — | — | (5,469,067 | ) | (5,469,067 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2010 | 4,616,726 | 46,167 | 98,634,885 | (94,712,339 | ) | 3,968,713 | ||||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 1,132,726 | 11,328 | 8,606,829 | — | 8,618,157 | |||||||||||||||
services | 12,576 | 126 | 86,902 | — | 87,028 | |||||||||||||||
Option expense | — | — | 489,914 | — | 489,914 | |||||||||||||||
Net loss | — | — | — | (5,968,448 | ) | (5,968,448 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2011 | 5,762,028 | 57,621 | 107,818,530 | (100,680,787 | ) | 7,195,364 | ||||||||||||||
Write off of derivative liability | — | — | 1,761,657 | — | 1,761,657 | |||||||||||||||
Discount related to beneficial conversion feature | — | — | 1,497,634 | — | 1,497,634 | |||||||||||||||
Shares issued for: | ||||||||||||||||||||
initial commitment on Lincoln Park $1.5 million share purchase agreement | 56,507 | 565 | 148,566 | — | 149,131 | |||||||||||||||
cash, net of offering costs | 267,610 | 2,676 | 331,294 | — | 333,970 | |||||||||||||||
accrued interest | 163,224 | 1,632 | 184,051 | 185,683 | ||||||||||||||||
Option expense | — | — | 690,726 | — | 690,726 | |||||||||||||||
Net loss | — | — | — | (8,930,833 | ) | (8,930,833 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2012 | 6,249,369 | $ | 62,494 | $ | 112,432,458 | $ | (109,611,620 | ) | $ | 2,883,332 | ||||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
Years ended December 31, 2012 and 2011 and the
Period from January 22, 2003 (Inception) to December 31, 2012
| 2012 | 2011 | Inception through 2012 | ||||||||||
Cash flows from operating activities | ||||||||||||
Net loss | $ | (8,930,833 | ) | $ | (5,968,448 | ) | $ | (109,611,620 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||
Stock payable for acquired research and development | — | — | 112,440 | |||||||||
Stock issued for acquired research and development | — | — | 26,286,589 | |||||||||
Stock issued for services | — | 87,028 | 2,061,743 | |||||||||
Stock issued for debt in excess of principal | — | — | 109,070 | |||||||||
Amortization of discount on notes payable due to warrants and beneficial conversion feature | 104,032 | — | 6,856,730 | |||||||||
Gain on extinguishment of debt | — | — | (1,612,440 | ) | ||||||||
Depreciation | 303,677 | 210,252 | 1,650,158 | |||||||||
Amortization of debt financing costs | 58,639 | — | 583,017 | |||||||||
Option and warrant expense | 690,726 | 489,914 | 16,265,933 | |||||||||
Gain on derivative instruments | (552,978 | ) | — | (1,941,826 | ) | |||||||
Loss on disposal of fixed assets | 3,097 | 9,686 | 513,345 | |||||||||
Changes in: | ||||||||||||
Other current assets | (611,607 | ) | (39,248 | ) | (1,153,053 | ) | ||||||
Accounts payable – third parties and related parties | (71,410 | ) | (13,028 | ) | (160,651 | ) | ||||||
Accrued expenses | 83,018 | 234,923 | 605,238 | |||||||||
|
|
|
|
|
| |||||||
Net cash used in operating activities | (8,923,639 | ) | (4,988,921 | ) | (59,435,327 | ) | ||||||
|
|
|
|
|
| |||||||
Cash flows from investing activities | ||||||||||||
Purchase of property & equipment | (550,389 | ) | (296,949 | ) | (2,222,199 | ) | ||||||
Restricted cash | (1,000,000 | ) | — | (1,000,000 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (1,550,389 | ) | (296,949 | ) | (3,222,199 | ) | ||||||
|
|
|
|
|
| |||||||
Cash flows from financing activities | ||||||||||||
Common stock and warrants sold for cash, net of offering costs | 381,309 | 8,618,157 | 49,453,797 | |||||||||
Common stock repurchased and canceled | — | — | (325 | ) | ||||||||
Proceeds from exercise of warrants and options | — | — | 1,248,588 | |||||||||
Proceeds from third party debt | 3,455,000 | — | 12,738,184 | |||||||||
Proceeds from related party debt | 630,000 | — | 630,000 | |||||||||
Deferred financing and offering costs | (509,492 | ) | — | (509,492 | ) | |||||||
Repayments on loan payable | — | (35,607 | ) | (311,222 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 3,956,817 | 8,582,550 | 63,249,530 | |||||||||
|
|
|
|
|
| |||||||
Net change in cash and cash equivalents | (6,517,211 | ) | 3,296,680 | 592,004 | ||||||||
Cash and cash equivalents at beginning of period | 7,109,215 | 3,812,535 | — | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of period | $ | 592,004 | $ | 7,109,215 | $ | 592,004 | ||||||
|
|
|
|
|
| |||||||
Cash paid for: | ||||||||||||
Income tax | $ | — | $ | — | $ | — | ||||||
Interest | 1,946 | 3,135 | 155,109 | |||||||||
NON-CASH TRANSACTIONS | ||||||||||||
Issuance of common stock to Sportan shareholders | — | — | 147,733 | |||||||||
Issuance of common stock for accrued interest | 185,683 | — | 789,287 | |||||||||
Issuance of warrants to placement agent | — | — | 37,453 | |||||||||
Conversion of notes payable to common stock | — | — | 7,709,980 | |||||||||
Conversion of accrued liabilities to common stock | — | — | 197,176 | |||||||||
Conversion of accounts payable to note payable | — | — | 93,364 | |||||||||
Discount on convertible notes relating to: | ||||||||||||
Warrants | 2,314,635 | — | 5,974,372 | |||||||||
Beneficial conversion feature | 1,497,634 | — | 3,303,153 | |||||||||
Stock attached to notes | — | — | 1,287,440 | |||||||||
Fair value of derivative instrument | — | — | 4,680,220 | |||||||||
Derivative reclassified to equity | 1,761,657 | — | 2,349,266 | |||||||||
Unpaid additions to property and equipment | 7,812 | 136,266 | 144,078 | |||||||||
Amortization of deferred offering costs to paid-in capital | 47,339 | — | 47,339 | |||||||||
Shares issued as deferred offering costs | 149,131 | — | 149,131 | |||||||||
See accompanying summary of accounting policies and notes to consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Business Overview and Summary of Accounting Policies
Description of Business. Opexa Therapeutics, Inc. (“Opexa” or “the Company”) was initially incorporated as Sportan United Industries, Inc. (“Sportan”) in Texas in March 1991. In June 2004, PharmaFrontiers Corp. (“PharmaFrontiers”) was acquired by Sportan in a transaction accounted for as a reverse acquisition. PharmaFrontiers’ stockholders were issued Sportan shares in exchange for all of the outstanding common shares of PharmaFrontiers. Concurrent with the transaction, Sportan changed its name to PharmaFrontiers. During its development stage as a biopharmaceutical company, PharmaFrontiers acquired the worldwide exclusive license to a stem cell technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory operated by the University of Chicago, in which adult multi-potent stem cells are derived from monocytes obtained from the patient’s own blood (the “Stem Cell License”). A patent application was filed in November 2003 with the United States Patent and Trade Office regarding the technology involved in the Stem Cell License. The initial focus for this technology is the further development of this monocyte-derived stem cell technology as a platform for thein vitro generation of highly specialized cells for potential application in autologous cell therapy for patients with diabetes mellitus (the “Diabetes Program”).
In October 2004, PharmaFrontiers acquired all of the outstanding stock of Opexa Pharmaceuticals, Inc. (“Opexa Pharmaceuticals”), a biopharmaceutical company that previously acquired the exclusive worldwide license from Baylor College of Medicine to an patient specific, autologous T-cell immunotherapy, Tcelna™ (formerly known as Tovaxin®), for the initial treatment of multiple sclerosis (MS). In June 2006, the Company changed its name to Opexa Therapeutics, Inc. from PharmaFrontiers Corp. and, in January 2007, Opexa Therapeutics, Inc., the parent, merged with its wholly owned subsidiary, Opexa Pharmaceuticals with Opexa Therapeutics, Inc. being the surviving company.
In August 2009, Opexa entered into an exclusive agreement with Novartis Institutes for BioMedical Research, Inc. (“Novartis”) whereby Novartis acquired Opexa’s rights to the Stem Cell License and associated technology platform and had full responsibility for funding and carrying out all research, development and commercialization activities. Opexa received an upfront cash payment of $3 million at the time the agreement was entered into and subsequently received $0.5 million as a technology transfer fee milestone. In November 2011, Opexa re-acquired the stem cell assets from Novartis in consideration for releasing Novartis with respect to any further payment obligations owed to Opexa by Novartis. In connection with the re-acquisition of the stem cell assets, a related license agreement with the University of Chicago was re-assigned to Opexa. Opexa and the University of Chicago entered into a Fourth Amended and Restated License Agreement in connection with such assignment to Opexa.
In September 2012, Opexa initiated a Phase IIb clinical trial of Tcelna in patients with secondary progressive MS (“SPMS”). Previously, in September 2008, the Company completed a Phase IIb clinical study of Tcelna in the relapsing-remitting MS (“RRMS”) indication.
Opexa operates in a highly regulated and competitive environment. The manufacturing and marketing of pharmaceutical products require approval from, and are subject to, ongoing oversight by the Food and Drug Administration, or FDA, in the United States, by the European Medicines Agency, or EMA, in the E.U. and by comparable agencies in other countries. Obtaining approval for a new therapeutic product is never certain and may take many years and may involve expenditure of substantial resources. Tcelna is in development stage and Opexa has not applied for a Biologics License Application (BLA) for Tcelna with the FDA nor a similar regulatory licensure in any other country, and thus Tcelna is not approved to be marketed in any country.
Development Stage Company.Opexa is considered to be in development stage and has had no commercial revenues to date.
Reverse Stock Split. In June, 2006, Opexa effected a one-for-ten reverse stock split of its common stock.
On December 14, 2012, Opexa effected a one-for-four reverse stock split of its common stock (the “1:4 Reverse Stock Split”) which decreased the number of common shares issued and outstanding from approximately 23.6 million shares to approximately 5.9 million shares as of December 14, 2012. The number of authorized shares of common stock and preferred stock remained the same following the 1:4 Reverse Stock Split.
Unless otherwise noted, impacted amounts included in the consolidated financial statements and notes thereto have been retroactively adjusted for the stock splits as if such stock splits occurred on the first day of the first period presented. Impacted amounts include shares of common stock issued and outstanding, shares underlying convertible promissory notes, warrants and stock options, shares reserved, conversion prices of convertible securities, exercise prices of warrants or options, and loss per share. There was no impact on preferred or common stock authorized resulting from the 1:4 Reverse Stock Split.
Principals of Consolidation.The financial statements include the accounts of Opexa and its former wholly-owned subsidiary, Opexa Pharmaceuticals through December 31, 2006. All intercompany accounts and transactions have been eliminated.
The consolidated financial statements include the accounts of Opexa and its wholly owned subsidiary, Opexa Hong Kong Limited (“Opexa Hong Kong”). Opexa Hong Kong was formed in the Hong Kong Special Administrative Region during 2012 in order to facilitate potential development collaborations in the pan-Asian region. Presently, Opexa Hong Kong has not entered into any agreements and has not recognized any revenues as of December 31, 2012. All intercompany transactions and balances between Opexa and Opexa Hong Kong are eliminated in consolidation.
Use of Estimates in Financial Statement Preparation.The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Certain Risks and Concentrations.Opexa is exposed to risks associated with foreign currency transactions insofar as it has used U.S. dollars to fund Opexa Hong Kong’s bank account denominated in Hong Kong dollars. As the net position of the unhedged Opexa Hong Kong bank account fluctuates, Opexa’s earnings may be negatively affected. In addition, the reported carrying value of the Company’s Hong Kong dollar-denominated assets and liabilities that remain in Opexa Hong Kong will be affected by fluctuations in the value of the U.S. dollar as compared to the Hong Kong dollar. Opexa currently does not utilize forward exchange contracts or any type of hedging instruments to hedge foreign exchange risk as Opexa believes that its overall exposure is relatively limited. As of December 31, 2012, Opexa Hong Kong reported cash and cash equivalents of $3,902 in converted U.S. dollars and does not have any reported liabilities in the consolidated balance sheets.
Cash and Cash Equivalents.For purposes of the statements of cash flows, cash equivalents include all highly liquid investments with original maturities of three months or less. The primary objectives for the fixed income investment portfolio are liquidity and safety of principal. Investments are made with the objective of achieving the highest rate of return consistent with these two objectives. Opexa’s investment policy limits investments to certain types of instruments issued by institutions primarily with investment grade credit ratings and places restrictions on maturities and concentration by type and issuer.
Supplies Inventory.Reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study are recorded as other current assets. The inventory of these reagents and supplies are determined at the lower of cost or market value with cost determined under the first-in first-out (FIFO) method.
Long-lived Assets.Property and equipment are stated on the basis of historical cost less accumulated depreciation. Depreciation is provided using the straight-line method over the estimated useful lives of the assets. Major renewals and improvements are capitalized, while minor replacements, maintenance and repairs are charged to current operations. Impairment losses are recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amount.
Deferred costs.Opexa incurs costs in connection with a debt or equity offering or in connection with the proceeds pursuant to an execution of a strategic agreement. These costs are recorded as deferred offering or deferred financing costs in the consolidated balance sheets. Such costs may consist of legal, accounting, underwriting fees and other related items incurred through the date of the debt or equity offering or the date of the execution of the strategic agreement. Costs in connection with a debt offering are amortized to interest expense over the term of the note instrument. Costs in connection with the execution of a strategic agreement in which an initial upfront payment is received are offset to the gain recognized in the Consolidated Statements of Expenses. Additional paid in capital includes costs recorded as an offset to proceeds in connection with the completion of an equity offering.
Income Taxes.Income tax expense is based on reported earnings before income taxes. Deferred income taxes reflect the impact of temporary differences between assets and liabilities recognized for financial reporting purposes and such amounts recognized for tax purposes, and are measured by applying enacted tax rates in effect in years in which the differences are expected to reverse. A valuation allowance is recorded to reduce the net deferred tax asset to zero because it is more likely than not that the deferred tax asset will not be realized. The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained upon an examination.
Stock-Based Compensation.Opexa accounts for share-based awards issued to employees and non-employees in accordance with FASB ASC 718. Accordingly, employee share-based payment compensation is measured at the grant date, based on the fair value of the award, and is recognized as an expense over the requisite service period (generally the vesting is over a 3-year period). Additionally, share-based awards to non-employees are expensed over the period in which the related services are rendered at their fair value.
Research and Development.Research and development expenses are expensed in the consolidated statements of expenses as incurred in accordance with FASB ASC 730,Research and Development. Research and development expenses include salaries, related employee expenses, clinical trial expenses, research expenses, consulting fees, and laboratory costs. In instances in which the Company enters into agreements with third parties for research and development activities, Opexa may prepay fees for services at the initiation of the contract. Opexa records the prepayment as a prepaid asset in the consolidated balance sheets and amortizes the asset into research and development expense in the consolidated statements of operations over the period of time the contracted research and development services are performed. Other types of arrangements with third parties may be fixed fee or fee for service, and may include monthly payments or payments upon completion of milestones or deliverables. Opexa expenses the costs of licenses of patents and the prosecution of patents until the issuance of such patents and the commercialization of related products is reasonably assured. Research and development expense for the years ended December 31, 2012 and 2011 was $6,318,476 and $3,340,038, respectively.
Foreign Currency Translation and Transaction Gains and Losses.Opexa records foreign currency translation adjustments and transaction gains and losses in accordance with FASB ASC 830,Foreign Currency Matters. For the Company’s operations that have a functional currency other than the U.S. dollar, gains and
losses resulting from the translation of the functional currency into U.S. dollars for financial statement presentation are not included in determining net loss, but are accumulated in the cumulative foreign currency translation adjustment account as a separate component of stockholders’ equity, except for intercompany transactions that are of a short-term nature with Opexa Hong Kong that are consolidated, combined or accounted for by the equity method in Opexa’s consolidated financial statements. Opexa Hong Kong has transactions in Hong Kong dollars. Opexa records transaction gains and losses in its consolidated statements of operations related to the recurring measurement and settlement of such transactions. For the year ended December 31, 2012, Opexa did not record any gains and losses resulting from the translation of the functional currency into U.S. dollars and thus did not report any cumulative foreign currency translation adjustments in stockholders’ equity in the consolidated balance sheets.
Net Loss per Share.Basic and diluted net loss per share is calculated based on the net loss attributable to common shareholders divided by the weighted average number of shares outstanding for the period excluding any dilutive effects of options, warrants, unvested share awards and convertible securities.
Note 2. Cash and Cash Equivalents
At December 31, 2012, Opexa invested approximately $24,500 in a money market fund investing exclusively in high-quality, short-term money market instruments consisting of U.S. government obligations and repurchase agreements collateralized by the U.S. Government. While this fund seeks current income while preserving capital and liquidity, the fund is subject to risk, including U.S. government obligations risk, and is not federally insured or guaranteed by or obligations of the Federal Deposit Insurance Corporation or any other agency. For the 12 months ended December 31, 2012, the money market fund recognized an average market yield of 0.01%. Interest income of $280 was recognized for the year ended December 31, 2012 in the statements of expenses.
At December 31, 2011, Opexa invested approximately $7.0 million in a money market account with an average market yield of 0.01%. Interest income of $932 was recognized for the year ended December 31, 2011 in the statements of expenses.
Opexa issued a total of $4,085,000 in principal amount of convertible secured promissory notes to related parties and third parties on July 25, 2012 (see Note 6 and Note 7). As part of the security interest granted by Opexa to the investors, $1.0 million of the proceeds are required to be maintained in an account subject to a deposit account control agreement while the notes are outstanding. As of December 31, 2012, the $1.0 million balance in the controlled account is reported as restricted cash in the consolidated balance sheets. Subsequent to December 31, 2012, the restricted cash was reduced to $500,000 (see Note 14).
Note 3. Other Current Assets
Other current assets consisted of the following at December 31, 2012 and 2011:
Description | 2012 | 2011 | ||||||
Supplies inventory | $ | 604,179 | $ | — | ||||
Deferred offering costs | 341,166 | — | ||||||
Prepaid expenses | 132,201 | 124,773 | ||||||
|
|
|
| |||||
| $ | 1,077,546 | $ | 124,773 | |||||
|
|
|
| |||||
Supplies inventory at December 31, 2012 includes reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study. Opexa expects to amortize these prepaid reagents and supplies to research and development costs in the consolidated statements of expenses over the course of the clinical study.
Deferred offering costs at December 31, 2012 include costs incurred from third parties in connection with the implementation of an at-the-market program (“ATM Agreement”) in September 2012 pursuant to which Opexa may sell shares of its common stock from time to time depending upon market demand through a sales agent in transactions deemed to be an “at-the-market” offering as defined in Rule 415 of the Securities Act of 1933. As of December 31, 2012, the costs of $101,972 in connection with the implementation of the ATM Agreement were capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of any shares of common stock under the ATM Agreement, the capitalized costs will be offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at December 31, 2012 also include costs incurred from third parties in connection with the implementation of a $1.5 million Purchase Agreement and a $15 million Purchase Agreement (collectively, the “Purchase Agreements”) in November 2012 pursuant to which Opexa has the right to sell to Lincoln Park Capital Fund, LLC (“Lincoln Park”) an aggregate of up to $16.5 million in shares of its common stock, subject to certain conditions and limitations. As of December 31, 2012, the remaining costs of $216,198 in connection with the implementation of the Purchase Agreements remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the Purchase Agreements, the capitalized costs are offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at December 31, 2012 also include costs incurred from third parties in connection with the Option and License Agreement entered into with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A., on February 4, 2013. Under the terms of the Agreement, the Company received an upfront payment of $5 million on February 20, 2013. As of December 31, 2012, the remaining costs of $22,996 in connection with the Option and License Agreement remained capitalized and are included in other current assets in the consolidated balance sheets.
Note 4. Property and Equipment
Property and equipment consisted of the following at December 31, 2012 and 2011:
Description | Life | 2012 | 2011 | |||||||||
Computer equipment | 3 years | $ | 121,129 | $ | 99,603 | |||||||
Office furniture and equipment | 5-7 years | 274,438 | 251,170 | |||||||||
Software | 3 years | 149,867 | 96,097 | |||||||||
Laboratory equipment | 7 years | 1,020,158 | 994,994 | |||||||||
Leasehold improvements | 10 years | 622,772 | 603,445 | |||||||||
Manufacturing equipment | 7 years | 571,187 | 177,528 | |||||||||
|
|
|
| |||||||||
Subtotal | 2,759,551 | 2,222,837 | ||||||||||
Less: accumulated depreciation | (1,494,510 | ) | (1,193,601 | ) | ||||||||
|
|
|
| |||||||||
Property and equipment, net | $ | 1,265,041 | $ | 1,029,236 | ||||||||
|
|
|
| |||||||||
Property and equipment is carried at cost less accumulated depreciation. Depreciation is calculated by the straight-line method over the estimated useful life of three to ten years, depending upon the type of equipment, except for leasehold improvements which are amortized using the straight-line method over the remaining lease term or the life of the asset, whichever is shorter. The cost of repairs and maintenance is charged as an expense as incurred. Depreciation expense totaled $303,677 and $210,252 for the years ended December 31, 2012 and 2011, respectively.
Note 5. Income Taxes
Opexa uses the liability method, where deferred tax assets and liabilities are determined based on the expected future tax consequences of temporary differences between the carrying amounts of assets and liabilities for financial and income tax reporting purposes.
At December 31, 2012 and 2011, Opexa had approximately $69 million and approximately $61 million of unused net operating losses, respectively, available for carry forward to future years. The unused net operating losses begin to expire at December 31, 2024. At December 31, 2012 and 2011, Opexa’s deferred tax asset resulting from its cumulative NOLs amounted to $23,678,228 and $20,876,592, respectively which is covered by a full valuation allowance due to uncertainty of Opexa’s ability to generate future taxable income necessary to realize the related deferred tax asset.
Note 6. Convertible Promissory Notes
On July 25, 2012, Opexa issued a total of $4,085,000 in principal amount of secured convertible promissory notes (“Notes”) to third parties and related parties (collectively, the “Noteholders”), of which an aggregate of $630,000 was issued to related parties (See Note 7). The Notes mature on July 25, 2014 and accrue interest at the rate of 12% per annum, compounded annually. Interest is payable semi-annually on June 30 and December 31 in either cash or registered shares of common stock, at Opexa’s election. The Notes are secured by substantially all of Opexa’s assets and are convertible into a new class of non-voting Series A convertible preferred stock. The Notes can be converted into Series A convertible preferred stock at the option of the investors at a price of $100.00 per share, subject to certain limitations and adjustments. Additionally, Opexa can elect to convert the Notes into Series A convertible preferred stock if (i) Opexa’s common stock closes at or above $10.00 per share for 20 consecutive trading days or (ii) Opexa achieves certain additional funding milestones to continue its clinical trial program. These milestones include (x) executing a strategic agreement with a partner or potential partner by which Opexa will receive a minimum of $5 million to partially fund, or an option to partner with Opexa for, its Phase II clinical trial for Tcelna in patients with SPMS and (y) receiving a minimum of $25 million in additional capital (including the Note offering proceeds) from any partner, potential partner or any other source.
The Series A convertible preferred stock accrues dividends at the rate of 8% per annum, which are cumulative and payable semi-annually on June 30 and December 31 in either cash or registered shares of common stock at Opexa’s election. The Series A convertible preferred stock has a liquidation preference of $100.00 per share, entitling holders to payment from the assets of the Company available for distribution to its shareholders before any payment is made to the holders of the common stock. The Series A convertible preferred stock participates in any dividends or other distributions on shares of common stock (other than dividends payable in shares of common stock) along with the common stock. As a result of anti-dilution adjustments following the November 2012 sale of shares of Opexa’s common stock, the Series A convertible preferred stock is convertible into shares of the Company’s common stock at a price of $3.12 per share (the floor price), subject to certain limitations and conditions, and up to 1,308,236 shares of common stock were issuable if all 12% convertible secured promissory notes issued in the July 2012 financing and outstanding at December 31, 2012 were converted to Series A convertible preferred stock and such stock is then converted into common stock. Additionally, Opexa can elect to convert the Series A convertible preferred stock into common stock if the Company’s common stock closes at or above $16.00 per share for 20 consecutive trading days. As of December 31, 2012, no shares of Series A convertible preferred stock were currently outstanding. Subsequent to December 31, 2012, $900,000 in principal amount of the notes were converted into shares of Series A convertible preferred stock and such shares were then converted into common stock (see Note 14).
As part of the security interest in all of the Company’s assets granted to the Noteholders, $1.0 million of the proceeds is maintained in a controlled account (see Note 2). Subsequent to December 31, 2012, the restricted cash was reduced to $500,000 (see Note 14). The Noteholders were granted certain registration rights for the shares of underlying common stock.
If the Company does not make the required payments when due, either at maturity, or at applicable installment payment dates, or if the Company breaches other terms of the convertible secured notes or related agreements, the Noteholders could elect to declare all amounts outstanding, together with accrued and unpaid interest, to be immediately due and payable. Even if the Company was able to prepay the full amount in cash, any
such repayment could leave the Company with little or no working capital for its business. If the Company is unable to repay those amounts, the Noteholders will have a first claim on Opexa’s assets pledged under the convertible secured notes. If the Noteholders should attempt to foreclose on the collateral, it is unlikely that there would be any assets remaining after repayment in full of such secured indebtedness. Any default under the convertible secured notes and resulting foreclosure would have a material adverse effect on Opexa’s financial condition and the Company’s ability to continue its operations.
The Notes were analyzed at issuance for a beneficial conversion feature and Opexa concluded that a beneficial conversion feature exists. The beneficial conversion feature was measured using the commitment-date stock price and was determined to be $1,497,634, of which $230,969 was attributable to related parties. This amount was recorded as a debt discount and is amortized to interest expense in the consolidated statements of expenses over the term of the Notes. Opexa also analyzed the Notes for derivative accounting consideration and determined that derivative accounting does not apply.
In connection with the issuance of the Notes, Opexa also issued Series I warrants to the Noteholders to initially purchase an aggregate of 957,422 shares of Opexa’s common stock at $5.00 per share, subject to certain limitations and adjustments. The warrants have a five-year term and are exercisable six months from the date of issuance, or January 25, 2013. As a result of anti-dilution adjustments, the number of warrant shares for which the Series I warrants are exercisable increased to an aggregate increase of 1,436,121 shares of Opexa’s common stock at an adjusted exercise price of $2.56 per share, subject to further certain limitations and adjustments. As a result, Opexa accounted for these reset provisions in accordance with FASB ASC 815-40, which requires Opexa to record the warrants as a derivative liability at the grant date and to record changes in fair value relating to the warrants at each subsequent balance sheet date (see Note 8). Opexa can redeem the warrants at $0.01 per share if its common stock closes at or above $10.00 per share for 20 consecutive trading days.
The initial fair value of the warrant liabilities of $2,314,635, together with the beneficial conversion feature of $1,497,634 were recognized as a debt discount and are amortized to interest expense in the consolidated statements of expenses over the term of the Notes using the effective interest method. The amortized debt discount for the year-ended December 31, 2012 was $104,032 and Opexa recognized $552,978 as a derivative gain in the consolidated statements of expenses due to the change in fair value of the liability. The unamortized discount as of December 31, 2012 amounted to $3,708,237.
The following table provides a summary of the changes in convertible debt – third parties, net of unamortized discount, during 2012:
Balance at December 31, 2011 | $ | — | ||
July 25, 2012 Notes, face value | 3,455,000 | |||
Discount on beneficial conversion feature of Notes at issuance | (1,266,665 | ) | ||
Discount on fair value of Series I warrant liability at issuance | (1,957,665 | ) | ||
Amortization of debt discount to interest expense through December 31, 2012 | 87,988 | |||
|
| |||
Balance at December 31, 2012 | $ | 318,658 | ||
|
|
Note 7. Related Party Transactions
Investors in the July 25, 2012 Note offering included two members of Opexa’s Board of Directors and entities affiliated with a third director. Opexa issued an aggregate of $630,000 in principal amount of Notes to the two directors and an entity for which a third director reports beneficial ownership of Opexa securities. In connection with the issuance of such Notes, Opexa also issued warrants to purchase an aggregate of 221,483
shares of common stock. The fair value of the warrants was $356,969. Opexa also determined the Notes contained a beneficial conversation feature with fair value of $230,969. Opexa recorded a total of $587,939 as debt discount associated with the Notes issued to the related parties and amortized $16,044 as interest expense in the consolidated statements of expenses for the year ended December 31, 2012.
On August 15, 2012, Opexa appointed director David E. Jorden as its Acting Chief Financial Officer. As a non-employee officer of Opexa, Mr. Jorden receives cash compensation of $100,000 per annum for his service. For the period of August 15, 2012 through December 31, 2012, cash compensation totaling $37,500 was earned by Mr. Jorden and is reported in general and administrative expense in the consolidated statements of expenses. As of December 31, 2012, cash compensation totaling $8,333 was due to Mr. Jorden and is included in accounts payable in the consolidated balance sheets.
The following table provides a summary of the changes in convertible debt – related parties, net of unamortized discount, during 2012:
Balance at December 31, 2011 | $ | — | ||
July 25, 2012 Notes, face value | 630,000 | |||
Discount on beneficial conversion feature of Notes at issuance | (230,970 | ) | ||
Discount on fair value of Series I warrant liability at issuance | (356,969 | ) | ||
Amortization of debt discount to interest expense through December 31, 2012 | 16,044 | |||
|
| |||
Balance at December 31, 2012 | $ | 58,105 | ||
|
|
Note 8. Fair Value of Derivative Financial Instruments
The carrying value of cash and cash equivalents, receivables, accounts payable and accrued expenses in the consolidated balance sheets approximates their fair values because of the short-term nature of these instruments. The carrying value of the Notes in the consolidated balance sheets approximates fair value since the related rate of interest approximates current market rates. Management believes Opexa is not exposed to significant interest or credit risks arising from these financial instruments.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value maximize the use of observable inputs and minimize the use of unobservable inputs. Opexa utilizes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable.
Level 1—Quoted prices in active markets for identical assets or liabilities. These are typically obtained from real-time quotes for transactions in active exchange markets involving identical assets.
Level 2—Quoted prices for similar assets and liabilities in active markets; quoted prices included for identical or similar assets and liabilities that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets. These are typically obtained from readily-available pricing sources for comparable instruments.
Level 3—Unobservable inputs, where there is little or no market activity for the asset or liability. These inputs reflect the reporting entity’s own beliefs about the assumptions that market participants would use in pricing the asset or liability, based on the best information available in the circumstances.
FASB ASC 815, “Accounting for Derivatives and Hedging Activities” (“FASB ASC 815”) specifies that a contract that would otherwise meet the definition of a derivative, but is both (a) indexed to its own stock and
(b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. FASB ASC 815 provides a new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer’s own stock, including evaluating the instrument’s contingent exercise and settlement provisions, and thus able to qualify for the FASB ASC 815-10 scope exception. It also clarifies the impact of foreign-currency-denominated strike prices and market-based employee stock option valuation instruments on the evaluation. Initially, Opexa evaluated all of its financial instruments and determined that the Series I warrants associated with the July 2012 Note financing (see Note 6) qualified for treatment under FASB ASC 815. Consequently, the Company recorded a derivative liability of $2,314,635 upon issuance of the warrants and a corresponding discount on the convertible debt. On November 8, 2012, it was determined that the floor for resetting the exercise price was met and no further adjustments to the exercise price of the Series I warrants would occur. Therefore, the Series I warrants were considered indexed to the company’s stock and qualified for the scope exception under FASB ASC 815-10 allowing for a transfer from liability classification to equity classification. Consequently, the remaining derivative liability of $1,761,657 at November 8, 2012 was written off to additional paid in capital.
The following table provides a summary of the changes in fair value, including net transfers in and/or out, of the derivative financial instruments, measured at fair value on a recurring basis using significant unobservable inputs (Level 3):
| ||||
| ||||
| ||||
| ||||
| ||||
The fair value of the derivative liabilities are calculated at the time of issuance using the Lattice option pricing model with Monte Carlo simulation. Opexa records a derivative liability for the calculated value. Changes in the fair value of the derivative liabilities are reported in other income (expense) in the consolidated statements of expenses. The variables used in the Lattice option pricing model for the derivative liabilities during the year ended December 31, 2012 include:
| July 25, 2012 | September 30, 2012 | November 8, 2012 | ||||||||||
Market value of common stock on measurement date | $ | 2.56 | $ | 2.70 | $ | 1.96 | ||||||
Projected exercise price | $ | 5.00 | $ | 4.52 | $ | 4.56 | ||||||
Risk free interest rate | 0.56% | 0.56% | 0.72% | |||||||||
Warrant lives in years | 5 | 4.88 | 4.71 | |||||||||
Expected volatility | 193% | 193% | 194% | |||||||||
Expected dividend yield | 0% | 0% | 0% | |||||||||
Offering price range | $ | 2.56-$6.56 | $ | 2.72-$6.72 | $ | 2.56-$6.56 | ||||||
Note 9. Commitments and Contingencies
In October 2005, Opexa entered into a ten-year lease for its office and research facilities. The facility including the property is leased for a term of ten years with two options for an additional five years each at the then prevailing market rate. Future minimum lease payments under the non-cancellable operating lease are $157,896 for 2013, $157,896 for 2014 and $118,422 for 2015. Rent expense in the consolidated statements of expenses was approximately $136,000 for each of the years ended December 31, 2012 and 2011.
Note 10. Significant Contractual Service and Milestone Agreements
In February 2012, Opexa entered into an agreement with Pharmaceutical Research Associates, Inc. (“PRA”), a contract research organization, in which PRA will provide Opexa with services related to the design, implementation and management of Opexa’s ongoing Phase IIb clinical trial program in SPMS (the “PRA Agreement”). Under the terms of the PRA Agreement, Opexa made upfront cash payments to PRA of $543,766. Future payments by Opexa to PRA under the PRA Agreement are based on the achievement of certain time and performance milestones as presented in the PRA Agreement. In December 2012, Opexa entered into an Amendment #1 to Task Order #1 (the “Amendment”) with PRA in which Opexa agreed to reimburse PRA for additional services and pass-through expenses incurred while performing out-of-scope work. Under the terms of the Amendment, an upfront cash payment of $37,605 is to be made to PRA as payment for certain out-of-scope tasks performed by PRA and future payments by Opexa to PRA under the PRA Agreement on the achievement of certain time milestones in the Amendment. Total payments to PRA during 2012, which were charged to expense, amounted to $1,382,236. Unless terminated by either party without cause on 60 days prior notice or on shorter notice with cause, the initial term of the PRA Agreement is for four years and automatically renews for successive one year terms.
During 2012, Opexa entered into individual Clinical Trial Agreements with 18 clinical institutions (the “Institutions”) across the U.S. and 18 principal investigators (the “Investigators”) acting within their employment or agent positions within their clinical institution. Under the terms of each Clinical Trial Agreement, each of the Investigators will identify and recruit subjects with SPMS meeting certain enrollment requirements and conduct clinical research in conjunction with Opexa’s Phase IIb clinical study, and each of the Institutions will provide appropriate resources and facilities so the Institution’s Investigator can conduct Opexa’s Phase IIb clinical study in a timely and professional manner and according to the terms of the Clinical Trial Agreement. Under the terms of each Clinical Trial Agreement, Opexa paid an upfront cash payment to each Institution for start-up and other costs which were charged directly to expense. Future payments by Opexa to the Institutions during the term of each Clinical Trial Agreement are based on the achievement of certain performance milestones as presented in each Clinical Trial Agreement. Unless terminated by Opexa without cause with 30 days’ notice, or unless terminated by the Institution, Investigator or Opexa for health or safety reasons, the initial term of the Clinical Trial Agreements with each Institution and Investigator is for the duration of their enrolled subjects in the Phase IIb clinical study.
Note 11. Equity
Summary information regarding equity related transactions for the years ended December 31, 2011 and December 31, 2012 is as follows:
During 2011, equity related transactions were as follows:
In January 2011, 96,189 shares of common stock were sold under the Continuous Offering Program Agreement dated May 14, 2010 (the “2010 ATM Agreement”) for net proceeds of $1,066,286. Compensation and fees totaling $10,826 was paid to the placement agent with respect to the shares sold. The 2010 ATM Agreement was subsequently terminated by Opexa on February 7, 2011.
In February 2011, an aggregate of 1,036,622 units were sold in a public offering, with each unit consisting of one share of common stock and a warrant to purchase four-tenths (0.40) of a share of common stock, at a price to the public of $2.05 per unit, for gross proceeds of $8,500,325. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants were exercisable immediately upon issuance, have a five-year term and an exercise price of $10.44 per share. Net proceeds from this offering were approximately $7,551,891 after deducting underwriting discounts and commissions and other estimated offering expenses. The offering closed on February 11, 2011.
12,576 shares of common stock valued at their fair value of $87,028 were issued to a consultant in exchange for services.
During 2012, equity related transactions were as follows:
In November 2012, Opexa entered into two purchase agreements with Lincoln Park pursuant to which the Company has the right to sell to Lincoln Park an aggregate of up to $16.5 million in shares of common stock, subject to certain conditions and limitations. As consideration for its commitment to purchase shares of common stock pursuant to the $1.5 million purchase agreement, Opexa issued to Lincoln Park 56,507 shares of common stock with a fair value of $149,131.
In November and December 2012, 265,000 shares of common stock were sold and 2,610 additional commitment shares were issued to Lincoln Park for net proceeds of $333,970.
In December 2012, 163,224 shares of common stock were issued to the Noteholders of the July 2012 Notes as payment of accrued interest.
Note 12. Options and Warrants
On September 2, 2010, the Board adopted the Opexa Therapeutics, Inc. 2010 Stock Incentive Plan (“the 2010 Plan”) for the granting of equity incentive awards to employees, directors and consultants of Opexa. The 2010 Plan was approved by the Company’s stockholders on October 19, 2010. The 2010 Plan is the successor to and continuation of Opexa’s June 2004 Compensatory Stock Option Plan (the “2004 Plan”). The 2004 Plan reserved a maximum of 575,000 shares of common stock for issuance pursuant to incentive stock options and nonqualified stock options granted to employees, directors and consultants. Awards were made as either incentive stock options or nonqualified stock options, with the Board having discretion to determine the number, term, exercise price and vesting of grants made under the 2004 Plan. All outstanding equity awards granted under the 2004 Plan continue to be subject to the terms and conditions as set forth in the agreements evidencing such stock awards and the terms of the 2004 Plan, but no additional awards will be granted under the 2004 Plan subsequent to approval of the 2010 Plan. Under the 2010 Plan, the total number of shares of common stock reserved for issuance consists of 625,000 shares plus the number of shares subject to stock options outstanding under the 2004 Plan that are forfeited or terminate prior to exercise and would otherwise be returned to the share reserves under the 2004 Plan and any reserved shares not issued or subject to outstanding grants, up to a maximum of 793,204 shares. The 2010 Plan provides for the grant of incentive stock options or nonqualified stock options, as well as restricted stock, stock appreciation rights, restricted stock units and performance awards that may be settled in cash, stock or other property. The Board of Directors or Compensation Committee, as applicable, administers the 2010 Plan and has discretion to determine the recipients, the number and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting. Subject to a limitation on repricing without stockholder approval, the Board or Compensation Committee, as applicable, may also determine the exercise price of options granted under the 2010 Plan.
Employee Options:
During 2011, options to purchase 43,750 shares of common stock were granted by Opexa to its employees at an exercise price of $6.24. These options have a term of ten years and vest over three years. Fair value of $268,451 was recorded using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for options issued during the year ended December 31, 2011 include (1) discount rate of 3.36%, (2) expected term of six years, (3) expected volatility of 192% and (4) zero expected dividends.
During 2011, options to purchase 18,750 shares were forfeited and cancelled.
Opexa recorded $304,024 stock-based compensation expense to management and employees during 2011. Unamortized stock-based compensation expense as of December 31, 2011 amounted to $364,064.
During 2012, options to purchase an aggregate of 107,832 shares were granted to employees, at exercise prices ranging from $1.80 to $3.80. These options have terms of ten years and have a vesting schedule of three years. Fair value of $381,020 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate range of 1.40% and 1.98%, (2) expected term of 5.25 to 7 years, (3) expected volatility range of 180% and 183% and (4) zero expected dividends.
During 2012, options to purchase an aggregate of 254,756 shares were granted to senior management, based on the achievement of future performance-based, strategic milestone objectives, at an exercise price of $3.80. These options have terms of ten years and have vesting schedules of three years commencing after the two specific milestone objectives have been individually met. Fair value of $964,715 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate of 1.98%, (2) expected term of ten years, (3) expected volatility of 183% and (4) zero expected dividends. As of December 31, 2012, one of the two specific milestone objectives had been individually met and an aggregate of 82,009 shares granted to senior management commenced vesting during 2012.
During 2012, options to purchase 4,678 shares were forfeited and cancelled.
Opexa recorded $549,150 stock-based compensation expense to management and employees during 2012, which included the related expense for the options that are expected to vest based on achievement of their related performance conditions. Unamortized stock compensation expense as of December 31, 2012 amounted to $1,142,135.
Non-Employee Options:
During 2011, options to purchase 32,407 shares of common stock were granted by Opexa to its consultants and directors at exercise prices ranging from $3.80 to $7.12. These options have terms of two to ten years, and have vesting dates that vary from either full or partial vesting at date of grant to full vesting within one to two years of the date of grant. Fair value of $196,783 was recorded using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for options issued during the year ended December 31, 2011 include (1) discount rate range of 0.25% to 3.50%, (2) expected term of two to five and one-quarter years, (3) expected volatility of 85%—198% and (4) zero expected dividends.
Opexa recorded $185,890 stock-based compensation expense to consultants and directors during 2011. Unamortized stock-based compensation expense as of December 31, 2011 amounted to $19,658.
During 2012, an option to purchase an aggregate of 18,750 shares was granted to Opexa’s non-employee Acting Chief Financial Officer at an exercise price of $2.04 in connection with his appointment. This option has a term of ten years, with one-third of the shares vesting immediately, one-third of the shares vesting on December 31, 2012 and the remaining one-third of the shares vesting at the earlier of June 30, 2013 or the appointment of a permanent chief financial officer. Fair value of $37,096 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for this option include (1) discount rate of 1.80%, (2) expected term of 5.25 years, (3) expected volatility of 185% and (4) zero expected dividends.
During 2012, options to purchase an aggregate of 30,600 shares were granted to directors for service on Opexa’s Board at an exercise price of $3.76. Options to purchase an aggregate of 10,000 shares have terms of 10 years, with 50% of the shares vesting immediately and 50% vesting one year from the date of grant. Options to purchase the remaining 20,600 shares will expire on the earlier of 10 years or a change in control of the Company, with 50% of the shares vesting immediately and 50% vesting on December 31, 2012. Fair value of $111,428 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for these options include (1) discount rate of 2.03%, (2) expected term of 5.25 years, (3) expected volatility of 186% and (4) zero expected dividends.
During 2012, options to purchase 25,563 shares were forfeited and cancelled.
Opexa recorded $141,576 of stock-based compensation expense to consultants and directors during 2012. Unamortized stock compensation expense as of December 31, 2012 amounted to $14,770.
Broker and Investor Warrants:
During 2011, warrants to purchase 671,972 shares were forfeited.
In connection with Opexa’s February 2011 public offering, Opexa issued warrants to purchase an aggregate of 414,650 shares of common stock to the investors at an exercise price of $10.44 per share. These warrants have a term of five years and were immediately exercisable.
During 2012, warrants to purchase 464,584 shares were forfeited.
In connection with Opexa’s July 25, 2012 private offering of the Notes (see Note 6), Opexa issued warrants to purchase an aggregate of 1,436,121 shares of common stock at a current adjusted exercise price of $2.56 per share, subject to certain limitations and adjustments. These warrants have a term of five years and are initially exercisable on January 25, 2013.
At December 31, 2011, the aggregate intrinsic value of the outstanding options and warrants was $227,567 and $435,913, respectively. At December 31, 2012, the aggregate intrinsic value of the outstanding options and warrants was $13,846 and $57,891, respectively.
Summary information regarding options and warrants from December 31, 2006 is as follows:
| Options | Weighted Average Exercise Price | Warrants | Weighted Average Exercise Price | |||||||||||||
Outstanding at December 31, 2006 | 190,426 | $ | 45.92 | 917,590 | $ | 78.04 | ||||||||||
Year ended December 31, 2007: | ||||||||||||||||
Granted | 73,475 | 21.12 | — | — | ||||||||||||
Forfeited and canceled | (4,336 | ) | 30.96 | — | — | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding at December 31, 2007 | 259,565 | $ | 39.16 | 917,590 | $ | 78.04 | ||||||||||
Year ended December 31, 2008: | ||||||||||||||||
Granted | 160,100 | 4.50 | 1,682,209 | 7.84 | ||||||||||||
Forfeited and canceled | (31,617 | ) | 24.41 | — | — | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding at December 31, 2008 | 388,048 | $ | 25.88 | 2,599,799 | $ | 32.60 | ||||||||||
Year ended December 31, 2009: | ||||||||||||||||
Granted | 193,583 | 3.83 | 801,143 | 6.68 | ||||||||||||
Exercised | (15,193 | ) | 4.21 | (179,691 | ) | 6.64 | ||||||||||
Forfeited and canceled | (85,605 | ) | 42.22 | (52,082 | ) | 20.00 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding at December 31, 2009 | 480,833 | $ | 14.80 | 3,169,169 | $ | 27.72 | ||||||||||
Year ended December 31, 2010: | ||||||||||||||||
Granted | 38,138 | 8.34 | 1,966 | 8.00 | ||||||||||||
Exercised | (36,284 | ) | 3.56 | (17,102 | ) | 8.40 | ||||||||||
Forfeited and canceled | (97,171 | ) | 36.62 | (289,160 | ) | 117.60 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding at December 31, 2010 Year ended December 31, 2011: Granted Exercised Forfeited and canceled Outstanding at December 31, 2011 Year ended December 31, 2012: Granted Exercised Forfeited and canceled Outstanding at December 31, 2012 Options Weighted
Average
Exercise
Price Warrants Weighted
Average
Exercise
Price 385,516 $ 8.60 2,864,873 $ 11.00 76,157 6.29 414,649 10.44 — — — — (18,750 ) 20.00 (671,972 ) 23.72 442,923 $ 7.71 2,607,550 $ 6.66 411,938 3.68 1,436,121 2.56 — — — — (30,241 ) 11.80 (464,584 ) 6.12 824,620 $ 5.54 3,579,087 $ 5.64
Summary of options outstanding and exercisable as of December 31, 2012 is as follows:
Range of Exercise Prices | Weighted Average Remaining Contractual Life (years) | Number of Options Outstanding | Number of Options Exercisable | |||||||||
$0.88 to $4.99 | 5.84 | 599,706 | 258,337 | |||||||||
5.00 to 9.99 | 1.58 | 178,054 | 157,221 | |||||||||
10.00 to 39.20 | 0.21 | 46,860 | 46,860 | |||||||||
|
|
|
|
|
| |||||||
$0.88 to $39.20 | 7.63 | 824,620 | 462,418 | |||||||||
|
|
|
|
|
| |||||||
Summary of warrants outstanding and exercisable as of December 31, 2012 is as follows:
Range of Exercise | Weighted Average Remaining Contractual Life (years) | Number of Warrants Outstanding | Number of Warrants Exercisable | |||||||||
$0.18 to $4.99 | 1.86 | 1,850,102 | 413,981 | |||||||||
5.00 to 9.99 | 0.04 | 1,068,905 | 1,068,905 | |||||||||
10.00 to 10.44 | 0.53 | 660,080 | 660,080 | |||||||||
|
|
|
|
|
| |||||||
$0.18 to $10.44 | 2.43 | 3,579,087 | 2,142,966 | |||||||||
|
|
|
|
|
| |||||||
Note 13. Licenses and Gain on Extinguishment of Debt
University of Chicago License Agreement
In 2004, Opexa entered into an agreement with the University of Chicago (“University”) for the worldwide license to technology developed at Argonne National Laboratory, a U.S. Department of Energy Laboratory operated by the University. The license was later amended granting Opexa an exclusive, non-transferable worldwide license to the University’s stem cell technology. In consideration for the license and amendment, Opexa paid the University a total of $232,742 and issued the University 53,462 shares of common stock valued at $2,295,461. Opexa also agreed to pay the University $1.5 million and to issue the University 21,623 shares of Opexa common stock. In April 2007, the $1.5 million cash payment obligation was extended until July 31, 2007 and the obligation to issue shares of Opexa’s common stock was extended until July 31, 2007, with $112,440 accrued as of June 30, 2007.
In July 2007, Opexa entered into a second amended and restated license agreement with the University that eliminated the obligations under the prior agreement for the payment of $1.5 million due July 31, 2007 and the obligation to issue 21,623 shares of Opexa common stock. These obligations were recorded as an intangible asset, with the liabilities recorded as a notes payable—current portion of $1.5 million and a stock payable of $112,440. As a result of the amendment and restatement of the license agreement with the University, $1,612,440 was reported as a gain on extinguishment of liability. Opexa applied the accounting guidance related to transfers and servicing of financial assets and extinguishments of liabilities as well as the guidance on debtor’s accounting for a modification or exchange of debt instruments. In August 2009, the University of Chicago license agreement was assigned to Novartis as part of Opexa’s sale of its stem cell technology platform to Novartis, and effective November 2, 2011, the license agreement was re-assigned to Opexa and the license agreement was amended and restated, as further described below.
Stem Cell Technology Agreement
In August 2009, Opexa entered into an exclusive agreement with Novartis for the further development of its stem cell technology. This technology, which has generated preliminary data, was in early preclinical development. Under the terms of the agreement, Novartis acquired the stem cell technology from Opexa and Novartis had full responsibility for funding and carrying out all research, development and commercialization activities. Opexa received an upfront cash payment of $3 million at the time the agreement was entered into and subsequently received $0.5 million as a technology transfer milestone fee.
In November 2011, Opexa re-acquired the stem cell assets from Novartis in consideration for releasing Novartis with respect to any further payment obligations owed to Opexa by Novartis In connection with the re-acquisition of the stem cell assets, a related license agreement with the University of Chicago was re-assigned to Opexa. Opexa and the University of Chicago entered into a Fourth Amended and Restated License Agreement in connection with such assignment to Opexa.
Note 14. Subsequent Events
In January 2013, 125,000 shares of common stock were sold and 975 additional commitment shares were issued to Lincoln Park under the $1.5 million purchase agreement for net proceeds of $142,400.
On January 23, 2013, Opexa closed a private offering consisting of convertible notes (the “January 2013 Notes”) and warrants to purchase shares of common stock for gross proceeds of $650,000 of which $100,000 was from a related party. The January 2013 Notes were scheduled to mature on January 23, 2014 and accrued interest at the rate of 12% per annum, compounded annually. The January 2013 Notes were convertible into common stock at the option of the investors at a price of $1.30 per share, subject to certain limitations. The principal balance plus accrued was payable within five business days of the receipt by Opexa of an aggregate of at least $7.5 million in proceeds from the sale of its equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna. On February 26, 2013, following the receipt of proceeds in excess of $7.5 million, Opexa paid principal and interest totaling $567,368 to holders of the January 2013 Notes and issued 77,034 shares of common stock to one holder of the January 2013 Notes who elected to convert the principal into common stock.
The warrants related to the January 2013 Notes financing have an exercise price of $1.24 per share, a five-year term and are exercisable for a maximum of 243,750 shares of common stock, subject to certain limitations. The Company can redeem the warrants at $0.01 per share if the Company’s common stock closes at or above $10.00 per share for 20 consecutive trading days.
Pursuant to the convertible secured promissory note financing effected by Opexa on July 25, 2012 (the “July 2012 Notes”), $1.0 million of the gross proceeds are maintained in a segregated account and subject to a deposit control agreement while the July 2012 Notes are outstanding. Pursuant to a waiver executed by the holders of in
excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock. The warrants have an exercise price of $1.21 per share and a five-year term. The Company can redeem the warrants at $0.01 per underlying share of common stock if the common stock closes at or above $10.00 per share for 20 consecutive trading days.
In February 2013, three of the holders of the July 2012 Notes elected to convert an aggregate of $900,000 of the July 2012 Notes into shares of the Company’s Series A convertible preferred stock with further immediate conversion into shares of the Company’s common stock. Accordingly, the Company issued an aggregate of 288,229 shares of common stock to the holders.
In February 2013, Opexa sold an aggregate of 167,618 shares of common stock under the ATM Agreement dated September 6, 2012 for gross proceeds of $536,417. Under the ATM Agreement, Opexa may sell an aggregate of up to 1,000,000 shares of common stock from time to time through the placement agent with a commission equal to 3% of the gross proceeds. Opexa paid compensation and fees totaling $16,105 to the placement agent with respect to the shares sold.
On February 4, 2013, Opexa entered into an option and license agreement with Merck. Pursuant to the agreement, Merck has an option to acquire an exclusive, worldwide (excluding Japan) license of the Company’s Tcelna program for the treatment of multiple sclerosis. Under the terms of the agreement, the Company received an upfront payment of $5 million on February 20, 2013.
On February 11, 2013, Opexa sold an aggregate of 1,083,334 units in a registered offering, with each unit consisting of one share of common stock and a warrant to purchase half (0.5) a share of common stock, at a price of $3.00 per unit, for gross proceeds of $3,250,002. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants are exercisable immediately upon issuance, have a four-year term and an exercise price of $3.00 per share. A fee of 6.0% of the gross proceeds was paid to the placement agent.
OPEXA THERAPEUTICS, INC.
(a development stage company)
(unaudited)
| March 31, 2013 | December 31, 2012 | |||||||
| Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 7,834,336 | $ | 592,004 | ||||
Other current assets | 1,166,430 | 1,077,546 | ||||||
|
|
|
| |||||
Total current assets | 9,000,766 | 1,669,550 | ||||||
Property & equipment, net of accumulated depreciation of $1,572,821 and $1,494,510, respectively | 1,189,328 | 1,265,041 | ||||||
Restricted cash | 500,000 | 1,000,000 | ||||||
Deferred financing costs, net of amortization of $111,578 and $58,639, respectively | 158,540 | 211,479 | ||||||
Other long-term assets | 104,027 | — | ||||||
|
|
|
| |||||
Total assets | $ | 10,952,661 | $ | 4,146,070 | ||||
|
|
|
| |||||
| Liabilities and Stockholders’ Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 830,658 | $ | 412,096 | ||||
Accrued expenses | 931,119 | 473,879 | ||||||
Deferred revenue | 1,395,348 | — | ||||||
|
|
|
| |||||
Total current liabilities | 3,157,125 | 885,975 | ||||||
Long term liabilities: | ||||||||
Convertible promissory notes, net of discount of $2,253,590 and $3,136,342, respectively | 301,410 | 318,658 | ||||||
Convertible promissory notes-R/P, net of discount of $554,783 and $571,895 | 75,217 | 58,105 | ||||||
Deferred revenue | 3,384,552 | — | ||||||
|
|
|
| |||||
Total liabilities | 6,918,304 | 1,262,738 | ||||||
|
|
|
| |||||
Commitments and contingencies | — | — | ||||||
Stockholders’ equity: | ||||||||
Preferred stock, no par value, 10,000,000 shares authorized, none issued and outstanding | — | — | ||||||
Common stock, $0.01 par value, 100,000,000 shares authorized, 7,991,559 and 6,249,369 shares issued and outstanding | 79,916 | 62,494 | ||||||
Additional paid in capital | 117,743,543 | 112,432,458 | ||||||
Deficit accumulated during the development stage | (113,789,102 | ) | (109,611,620 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | 4,034,357 | 2,883,332 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 10,952,661 | $ | 4,146,070 | ||||
|
|
|
| |||||
See accompanying notes to unaudited consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
| Three Months Ended March 31, 2013 | Three Months Ended March 31, 2012 | Inception through March 31, 2013 | ||||||||||
Revenue: | ||||||||||||
Option Revenue | $ | 220,100 | $ | — | $ | 220,100 | ||||||
Research and development | 1,621,366 | 1,490,097 | 78,118,717 | |||||||||
General and administrative | 1,102,435 | 816,196 | 31,220,051 | |||||||||
Depreciation and amortization | 78,311 | 67,355 | 1,728,469 | |||||||||
Loss on disposal of assets | — | — | 513,345 | |||||||||
|
|
|
|
|
| |||||||
Operating loss | (2,582,012 | ) | (2,373,648 | ) | (111,360,482 | ) | ||||||
Interest income | 1,874 | 136 | 1,360,571 | |||||||||
Other income and expense, net | 37,910 | — | 699,056 | |||||||||
Gain on extinguishment of debt | — | — | 1,612,440 | |||||||||
Gain on derivative instruments | — | — | 1,941,826 | |||||||||
Gain on sale of technology | — | — | 3,000,000 | |||||||||
Interest expense | (1,635,254 | ) | (487 | ) | (11,042,513 | ) | ||||||
|
|
|
|
|
| |||||||
Net loss | $ | (4,177,482 | ) | $ | (2,373,999 | ) | $ | (113,789,102 | ) | |||
|
|
|
|
|
| |||||||
Basic and diluted loss per share | $ | (0.58 | ) | $ | (0.41 | ) | N/A | |||||
Weighted avg shares outstanding – Basic and diluted | 7,239,102 | 5,762,028 | N/A | |||||||||
See accompanying notes to unaudited consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(unaudited)
| Common Stock | Additional Paid in Capital | Accumulated Deficit | Total | |||||||||||||||||
| Shares | Par | |||||||||||||||||||
Balances at December 31, 2012 | 6,249,369 | $ | 62,494 | $ | 112,432,458 | $ | (109,611,620 | ) | $ | 2,883,332 | ||||||||||
Conversion of convertible notes | 365,263 | 3,652 | 996,348 | — | 1,000,000 | |||||||||||||||
Discount related to beneficial conversion feature | — | — | 141,829 | — | 141,829 | |||||||||||||||
Discount on warrants attached to debt | — | — | 195,969 | — | 195,969 | |||||||||||||||
Shares issued for: | ||||||||||||||||||||
cash, net of offering costs | 1,376,927 | 13,770 | 3,565,752 | — | 3,579,522 | |||||||||||||||
Warrant expense | — | — | 219,553 | — | 219,553 | |||||||||||||||
Option expense | — | — | 191,634 | — | 191,634 | |||||||||||||||
Net loss | — | — | — | (4,177,482 | ) | (4,177,482 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at March 31, 2013 | 7,991,559 | $ | 79,916 | $ | 117,743,543 | $ | (113,789,102 | ) | $ | 4,034,357 | ||||||||||
See accompanying notes to unaudited consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
| Three Months Ended March 31, | Inception through March 31, 2013 | |||||||||||
| 2013 | 2012 | |||||||||||
Cash flows from operating activities | ||||||||||||
Net loss | $ | (4,177,482 | ) | $ | (2,373,999 | ) | $ | (113,789,102 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||
Stock payable for acquired research and development | — | — | 112,440 | |||||||||
Stock issued for acquired research and development | — | — | 26,286,589 | |||||||||
Stock issued for services | — | — | 2,061,743 | |||||||||
Stock issued for debt in excess of principal | — | — | 109,070 | |||||||||
Amortization of discount on notes payable due to warrants and beneficial conversion feature | 1,237,662 | — | 8,094,392 | |||||||||
Loss/(Gain) on extinguishment of debt | — | — | (1,612,440 | ) | ||||||||
Depreciation | 78,311 | 67,355 | 1,728,469 | |||||||||
Amortization of debt financing costs | 52,939 | — | 635,956 | |||||||||
Option expense | 411,187 | 205,420 | 16,677,120 | |||||||||
Loss/(Gain) on derivative instruments | — | — | (1,941,826 | ) | ||||||||
Loss on disposition of fixed assets | — | — | 513,345 | |||||||||
Changes in: | ||||||||||||
Deferred revenue | 4,779,900 | — | 4,779,900 | |||||||||
Other current assets | (115,589 | ) | (880,983 | ) | (1,268,642 | ) | ||||||
Accounts payable - third parties and related parties | 418,562 | 1,022,532 | 257,911 | |||||||||
Accrued expenses | 457,240 | (225,743 | ) | 1,062,478 | ||||||||
Other assets | (104,027 | ) | — | (104,027 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash provided by (used in) operating activities | 3,038,703 | (2,185,418 | ) | (56,396,624 | ) | |||||||
|
|
|
|
|
| |||||||
Cash flows from investing activities | ||||||||||||
Purchase of property & equipment | (2,598 | ) | (245,841 | ) | (2,224,797 | ) | ||||||
Restricted cash | 500,000 | — | (500,000 | ) | ||||||||
|
|
|
|
|
| |||||||
Net cash provided by (used in) investing activities | 497,402 | (245,841 | ) | (2,724,797 | ) | |||||||
|
|
|
|
|
| |||||||
Cash flows from financing activities | ||||||||||||
Common stock and warrants sold for cash, net of offering costs | 3,623,738 | — | 53,077,535 | |||||||||
Common stock repurchased and canceled | — | — | (325 | ) | ||||||||
Proceeds from exercise of warrants and options | — | — | 1,248,588 | |||||||||
Proceeds from third party debt | 550,000 | — | 13,288,184 | |||||||||
Proceeds from related party debt | 100,000 | — | 730,000 | |||||||||
Deferred financing and offering costs | (17,511 | ) | — | (527,003 | ) | |||||||
Repayment on related party notes payable | (100,000 | ) | — | (100,000 | ) | |||||||
Repayments on notes payable | (450,000 | ) | — | (761,222 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 3,706,227 | — | 66,955,757 | |||||||||
|
|
|
|
|
| |||||||
Net change in cash and cash equivalents | 7,242,332 | (2,431,259 | ) | 7,834,336 | ||||||||
Cash and cash equivalents at beginning of period | 592,004 | 7,109,215 | — | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of period | $ | 7,834,336 | $ | 4,677,956 | $ | 7,834,336 | ||||||
|
|
|
|
|
| |||||||
Cash paid for: Income tax Interest NON-CASH TRANSACTIONS Issuance of common stock to Sportan shareholders Issuance of common stock for accrued interest Issuance of warrants to placement agent Conversion of notes payable to common stock Conversion of accrued liabilities to common stock Conversion of accounts payable to note payable Discount on convertible notes relating to: Warrants Beneficial conversion feature Stock attached to notes Fair value of derivative instrument Derivative reclassified to equity Unpaid additions to property and equipment Amortization of deferred offering costs to paid-in capital Shares issued as deferred offering costs Three Months Ended
March 31, Inception
through
March 31, 2013 2013 2012 $ — $ — $ — 18,647 487 173,756 — — 147,733 — — 789,287 — — 37,453 1,000,000 — 8,709,980 — — 197,176 — — 93,364 — — 195,969 — 6,170,341 141,829 — 3,444,982 — — 1,287,440 — — 4,680,220 — — 2,349,266 — 182,952 — 45,450 — 92,789 1,234 — 150,365
See accompanying notes to unaudited consolidated financial statements
OPEXA THERAPEUTICS, INC.
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
Note 1. Basis of Presentation
The accompanying interim unaudited consolidated financial statements of Opexa Therapeutics, Inc. (“Opexa” or the “Company”), a development stage company, have been prepared in accordance with accounting principles generally accepted in the United States of America and the rules of the Securities and Exchange Commission (“SEC”) and should be read in conjunction with the audited financial statements and notes thereto contained in Opexa’s latest Annual Report filed with the SEC on Form 10-K. In the opinion of management, all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of financial position and the results of operations for the interim periods presented have been reflected herein. The results of operations for interim periods are not necessarily indicative of the results to be expected for the full year. Notes to the financial statements that would substantially duplicate the disclosure contained in the audited financial statements for the most recent fiscal year as reported in Form 10-K have been omitted.
The accompanying consolidated financial statements include the accounts of Opexa and its wholly owned subsidiary, Opexa Hong Kong Limited (“Opexa Hong Kong”). All intercompany balances and transactions have been eliminated in the consolidation.
Note 2. Significant Accounting Polices
Revenue Recognition. Opexa recognizes revenue in accordance with FASB ASC 605, Revenue Recognition. ASC 605 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) consideration is fixed or determinable; and (4) collectability is reasonably assured.
On February 4, 2013, Opexa entered into an Option and License Agreement (the “Merck Agreement”) with Ares Trading SA (“Merck”), a wholly owned subsidiary of Merck Serono S.A. Pursuant to the terms, Merck has an option to acquire an exclusive, worldwide (excluding Japan) license of the Company’s Tcelna™ program for the treatment of multiple sclerosis (“MS”). Tcelna is currently in a Phase IIb clinical trial in patients with Secondary Progressive MS (“SPMS”). The option may be exercised by Merck prior to or upon the Company’s completion of the Phase IIb Trial.
Opexa received an upfront payment of $5 million for granting the option. If the option is exercised, Merck would pay the Company an upfront license fee of $25 million unless Merck is unable to advance directly into a Phase III clinical trial of Tcelna for SPMS without a further Phase II clinical trial (as determined by Merck), in which event the upfront license fee would be $15 million. After exercising the option, Merck would be solely responsible for funding development, regulatory and commercialization activities for Tcelna in MS, although the Company would retain an option to co-fund certain development in exchange for increased royalty rates. The Company would also retain rights to Tcelna in Japan, certain rights with respect to the manufacture of Tcelna, and rights outside of MS.
Opexa evaluated the Merck Agreement and determined that the $5 million upfront payment from Merck has “stand-alone value”. Opexa’s continuing performance obligations, in connection with the $5 million payment, include the execution and completion of the Phase IIb clinical trial in SPMS using commercially reasonable efforts at the Company’s own costs. As a “stand-alone value” term in the Merck Agreement, the $5 million upfront payment is determined to be a single unit of accounting, and is recognized as revenue on a straight-line basis over the exclusive option period based on the expected completion term of the Phase IIb clinical trial in SPMS. Opexa includes the unrecognized portion of the $5 million as deferred revenue on the consolidated balance sheets.
Cash and Cash Equivalents. Opexa considers all highly liquid investments with an original maturity of three months or less, when purchased, to be cash equivalents. Investments with maturities in excess of three months but less than one year are classified as short-term investments and are stated at fair market value.
Opexa primarily maintains cash balances on deposit in accounts at a U.S.-based financial institution. The aggregate cash balance on deposit in these accounts is insured by the Federal Deposit Insurance Corporation up to $250,000. Opexa’s cash balances on deposit in these accounts may, at times, exceed the federally insured limits. Opexa has not experienced any losses in such accounts.
At March 31, 2013, Opexa invested approximately $7.7 million in a savings account. For the three months ended March 31, 2013, the savings account recognized an average market yield of 0.25%. Interest income of $1,874 was recognized for the three months ended March 31, 2013 in the consolidated statements of expenses.
Note 3. Other Current Assets
Other current assets consisted of the following at March 31, 2013 and December 31, 2012:
Description | March 31, 2013 | December 31, 2012 | ||||||
Supplies inventory | $ | 491,377 | $ | 604,179 | ||||
Deferred offering costs | 309,969 | 341,166 | ||||||
Prepaid expenses | 365,084 | 132,201 | ||||||
|
|
|
| |||||
| $ | 1,166,430 | $ | 1,077,546 | |||||
|
|
|
| |||||
Supplies inventory at March 31, 2013 and December 31, 2012 includes reagents and supplies that will be used to manufacture Tcelna and placebo product in Opexa’s Phase IIb clinical study. Opexa expects to amortize these prepaid reagents and supplies to research and development costs in the consolidated statements of expenses over the course of the clinical study.
Deferred offering costs at March 31, 2013 and December 31, 2012 include costs incurred from third parties in connection with the implementation of an at-the-market program (“ATM Agreement”) in September 2012 pursuant to which Opexa may sell shares of its common stock from time to time depending upon market demand through a sales agent in transactions deemed to be an “at-the-market” offering as defined in Rule 415 of the Securities Act of 1933. As of March 31, 2013, the remaining costs of $88,446 in connection with the implementation of the ATM Agreement remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the ATM Agreement, the remaining capitalized costs are offset against the proceeds of such sales of shares of common stock.
Deferred offering costs at March 31, 2013 also include costs incurred from third parties in connection with the implementation of a $1.5 million purchase agreement and a $15 million purchase agreement (collectively, the “purchase agreements”) in November 2012 pursuant to which Opexa has the right to sell to Lincoln Park Capital Fund, LLC (“Lincoln Park”) an aggregate of up to $16.5 million in shares of its common stock, subject to certain conditions and limitations. As of March 31, 2013, the remaining costs of $221,523 in connection with the implementation of the purchase agreements remained capitalized and are included in other current assets in the consolidated balance sheets. Upon the sales of shares of common stock under the purchase agreements, the remaining capitalized costs are offset against the proceeds of such sales of shares of common stock.
Prepaid expenses at March 31, 2013 include advance payments totaling $220,374 made to vendors and consultants for the conduct of the Phase IIb clinical trial in SPMS.
Note 4. Restricted Cash
Pursuant to the July 2012 Note financing, $1.0 million of the gross proceeds were initially required to be maintained in a segregated account and subject to a deposit control agreement while the July 2012 Notes are outstanding. Pursuant to a waiver executed by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock (see Note 11). As of March 31, 2013, the $500,000 balance in the controlled account is reported as restricted cash in the consolidated balance sheets.
Note 5. Deferred Financing Costs
Deferred financing costs at March 31, 2013 consist of costs incurred from third parties in conjunction with the July 2012 Notes. The costs in connection with the debt financing were capitalized and are amortized to interest expense over the term of the related debt. As of March 31, 2013, the unamortized deferred financing costs totaling $158,540 are reported as deferred financing costs in the consolidated balance sheets. During the quarter ended March 31, 2013, Opexa amortized $52,939 of deferred financing costs as interest expense.
Note 6. Other Assets
Other long-term assets at March 31, 2013 consist of legal costs incurred from third parties in conjunction with the Merck Agreement. These costs were capitalized and are amortized to general and administrative expenses on the consolidated statements of operations in conjunction with the recognition of revenue on a straight-line basis over the exclusive option period based on the term of the Phase IIb clinical trial in SPMS. During the quarter ended March 31, 2013, Opexa amortized $6,765 of legal costs to general and administrative expenses on the consolidated statements of operations. Opexa included the current portion of the legal costs of $42,887 in other current assets on the consolidated balance sheets and the long term portion of the legal costs of $104,027 in other long-term assets on the consolidated balance sheets.
Note 7. Convertible Promissory Notes
On January 23, 2013, Opexa closed a private offering consisting of convertible notes (the “January 2013 Notes”) and warrants to purchase shares of common stock for gross proceeds of $650,000 of which $100,000 was from a related party (see Note 8). The January 2013 Notes are scheduled to mature on January 23, 2014 and accrue interest at the rate of 12% per annum, compounded annually. The January 2013 Notes are convertible into common stock at the option of the investors at a price of $1.30 per share, subject to certain limitations. The principal balance plus accrued interest is payable within five business days of the receipt by Opexa of an aggregate of at least $7.5 million in proceeds from the sale of its equity securities and/or as payments from one or more partners or potential partners in return for granting a license, other rights, or an option to license or otherwise acquire rights with respect to Tcelna.
The January 2013 Notes were analyzed at issuance for a beneficial conversion feature and Opexa concluded that a beneficial conversion feature exists. The beneficial conversion feature was measured using the commitment-date stock price and was determined to be $141,829 of which $21,820 was attributable to the related party. Opexa also analyzed the Notes for derivative accounting consideration and determined that derivative accounting does not apply.
In connection with the issuance of the January 2013 Notes, Opexa also issued Series J warrants to purchase an aggregate of 243,750 shares of Opexa’s common stock (see Note 11), subject to certain limitations and adjustments. The relative fair value of the warrant liabilities of $195,969, together with the beneficial conversion feature of $141,829 were recognized as a debt discount and are amortized to interest expense in the consolidated statements of expenses over the term of the January 2013 Notes using the effective interest method.
On February 26, 2013, following the receipt of $3.25 million in gross proceeds during February 2013 from the sale of common stock and warrants to purchase shares of common stock, and following the receipt of the upfront payment of $5 million from Merck on February 20, 2013, Opexa paid principal and interest totaling $567,368 to holders of the January 2013 Notes, of which $100,000 was to a related party, and issued 77,034 shares of common stock to one holder of the January 2013 Notes who elected to convert the principal of $100,000.
During the quarter ended March 31, 2013, the debt discount of $337,798 in connection with the January 2013 Notes was fully amortized to interest expense.
In February 2013, three of the third party holders of the July 2012 Notes elected to convert their principal amounts of $900,000 into shares of the Company’s Series A convertible preferred stock with further immediate conversion into 288,229 shares of the Company’s common stock. As of March 31, 2013, an aggregate of $3,185,000 in principal amount of the July 2012 Notes to third parties and related parties is reported as long term liabilities in the consolidated balance sheets, of which $630,000 was issued to related parties (see Note 8).
The following table provides a summary of the changes in convertible debt—third parties, net of unamortized discount, during the quarter ended March 31, 2013:
Balance at December 31, 2012 | $ | 318,658 | ||
January 2013 Notes, face value | 550,000 | |||
Discount on beneficial conversion feature of January 2013 Notes at issuance | (120,009 | ) | ||
Discount on fair value of Series J warrant liability at issuance | (165,820 | ) | ||
Repayment of January 23, 2013 Notes | (450,000 | ) | ||
Conversion of January 23, 2013 Notes into common stock | (100,000 | ) | ||
Conversion of July 25, 2012 Notes into common stock | (900,000 | ) | ||
Amortization of debt discount to interest expense through March 31, 2013 | 1,168,581 | |||
|
| |||
Balance at March 31, 2013 | $ | 301,410 | ||
|
|
Note 8. Related Party Transactions
Investors in the January 2013 Notes offering included one member of Opexa’s Board of Directors who was issued a note with a principal amount of $100,000 (see Note 7).
The following table provides a summary of the changes in convertible debt—related parties, net of unamortized discount, during the quarter ended March 31, 2013:
Balance at December 31, 2012 | $ | 58,105 | ||
January 2013 Notes, face value | 100,000 | |||
Discount on beneficial conversion feature of January 2013 Notes at issuance | (21,820 | ) | ||
Discount on fair value of Series J warrant liability at issuance | (30,149 | ) | ||
Repayment of January 23, 2013 Notes | (100,000 | ) | ||
Amortization of debt discount to interest expense through March 31, 2013 | 69,081 | |||
|
| |||
Balance at March 31, 2013 | $ | 75,217 | ||
|
|
For the quarter ended March 31, 2013, cash compensation totaling $25,000 earned by Director David E. Jorden for his service as Opexa’s Acting Chief Financial Officer is reported in general and administrative expense in the consolidated statements of expenses. Concurrent with the appointment of Karthik Radhakrishnan as Chief Financial Officer on March 29, 2013, Mr. Jorden resigned as the Company’s Acting Chief Financial Officer but will continue in his role as director on Opexa’s Board. As of March 31, 2013, cash compensation totaling $8,333 was due to Mr. Jorden and is included in accounts payable in the consolidated balance sheets.
Note 9. Equity
For the quarter ended March 31, 2013, equity related transactions were as follows:
In January 2013, 125,000 shares of common stock were sold and 975 additional commitment shares were issued to Lincoln Park under the $1.5 million purchase agreement for net proceeds of $142,400.
365,263 shares of common stock were issued in connection with the conversion of the January 2013 and July 2012 Notes (see Note 7).
In February 2013, Opexa sold an aggregate of 167,618 shares of common stock under the ATM Agreement for gross proceeds of $536,417.
On February 11, 2013, Opexa sold an aggregate of 1,083,334 units in a registered offering, with each unit consisting of one share of common stock and a warrant to purchase half (0.5) a share of common stock, at a price of $3.00 per unit, for gross proceeds of $3,250,002. The shares of common stock and warrants were immediately separable and were issued separately such that no units were issued. The warrants are exercisable immediately upon issuance, have a four-year term and an exercise price of $3.00 per share. A fee of 6.0% of the gross proceeds was paid to the placement agent.
For the quarter ended March 31, 2013, $350,530 was netted against additional paid in capital as stock offering costs.
Note 10. Stock-Based Compensation
Stock Options
The 2010 Stock Incentive Plan (the “2010 Plan”) provides for the grant of equity incentive awards to employees, directors and consultants of Opexa in the form of incentive stock options or nonqualified stock options, as well as restricted stock, stock appreciation rights, restricted stock units and performance awards that may be settled in cash, stock or other property. The 2010 Plan is the successor to and continuation of Opexa’s June 2004 Compensatory Stock Option Plan (the “2004 Plan”). A total of 625,000 shares of common stock are authorized to be issued for awards made under the 2010 Plan through September 2020, plus (i) the number of shares subject to stock options outstanding under the 2004 Plan that are forfeited or terminate prior to exercise and would otherwise be returned to the share reserves under the 2004 Plan and (ii) any reserved shares under the 2004 Plan that were not issued or subject to outstanding grants. In addition, shares subject to awards granted under the 2010 Plan that terminate or expire before being exercised or settled will become available for grant under the 2010 Plan. As of March 31, 2013, options to purchase an aggregate of 903,289 shares were issued and outstanding.
Opexa accounts for share-based compensation, including options and nonvested shares, according to the provisions of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718, “Share Based Payment.” During the three months ended March 31, 2013, Opexa recognized option expense of $191,634 which includes the related expense for the options that are expected to vest based on achievement of their related performance conditions (see below). Unamortized stock compensation expense as of March 31, 2013 amounted to $1,126,780.
Stock Option Activity
A summary of stock option activity for the three months ended March 31, 2013 is presented below:
| Number of Shares | Wtd. Avg. Exercise Price | Wtd. Avg. Remaining Contract Term (# years) | Intrinsic Value | |||||||||||||
Outstanding at January 1, 2013 | 824,620 | $ | 5.54 | |||||||||||||
Granted | 125,000 | 2.34 | ||||||||||||||
Exercised | — | — | ||||||||||||||
Forfeited and canceled | (46,331 | ) | 4.73 | |||||||||||||
|
|
|
| |||||||||||||
Outstanding at March 31, 2013 | 903,289 | $ | 5.14 | 7.7 | $ | 104,769 | ||||||||||
Exercisable at March 31, 2013 | 485,124 | $ | 6.63 | 6.5 | $ | 100,730 | ||||||||||
Option awards are granted with an exercise price equal to the market price of Opexa’s stock at the date of issuance, generally have a ten-year life, and have various vesting dates that range from no vesting or partial vesting upon date of grant to full vesting on a specified date. Opexa estimates the fair value of stock options using the Black-Scholes option-pricing model and records the compensation expense ratably over the service period.
During the three months ended March 31, 2013, an option to purchase an aggregate of 125,000 shares was granted to an employee at an exercise price of $2.34. This option has a term of ten years and has a vesting schedule of three years. Fair value of $285,226 was calculated using the Black-Scholes option-pricing model. Variables used in the Black-Scholes option-pricing model for the option issued to an employee during the three months ended March 31, 2013 include (1) discount rate of 1.87%, (2) expected term of 5.25 years, (3) expected volatility of 194% and (4) zero expected dividends.
During the three months ended March 31, 2013, options to purchase 46,331 shares were forfeited and cancelled.
Warrant Activity
A summary of warrant activity for the three months ended March 31, 2013 is presented below:
| Number of Shares | Wtd. Avg. Exercise Price | Wtd. Avg. Remaining Contract Term (# years) | Intrinsic Value | |||||||||||||
Outstanding at January 1, 2013 | 3,579,087 | $ | 5.64 | |||||||||||||
Granted | 972,918 | 2.21 | ||||||||||||||
Exercised | — | — | ||||||||||||||
Forfeited and canceled | (1,129,359 | ) | 7.65 | |||||||||||||
|
|
|
| |||||||||||||
Outstanding at March 31, 2013 | 3,422,646 | $ | 4.01 | 3.5 | $ | 480,000 | ||||||||||
Exercisable at March 31, 2013 | 3,422,646 | $ | 4.01 | 3.5 | $ | 480,000 | ||||||||||
In connection with the January 2013 Notes (see Note 7), investors were issued five-year warrants to purchase up to an aggregate of 243,750 shares of common stock, at an exercise price of $1.24 per share. The estimated relative fair value of the investor warrants was $195,969 and was calculated using the Black-Scholes valuation model. The following assumptions were used: (1) no expected dividends, (2) risk free interest rate of 0.76%, (3) expected volatility of 191% and (4) expected life of five years. Opexa can redeem the warrants at $0.01 per share if the Company’s common stock closes at or above $10.00 per share for 20 consecutive trading days.
Pursuant to a waiver executed by the holders of in excess of two-thirds (66-2/3%) of the principal amount of the outstanding July 2012 Notes and accepted by Opexa, the amount of the cash subject to the deposit control agreement was reduced to $500,000 on January 29, 2013. In exchange for such waiver, the Company issued warrants to the holders of the July 2012 Notes to purchase an aggregate of 187,500 shares of the Company’s common stock. The warrants have an exercise price of $1.21 per share and a five-year term. The estimated fair value of the warrants was $219,553 and was calculated using the Black-Scholes valuation model. The following assumptions were used: (1) no expected dividends, (2) risk free interest rate of 0.90%, (3) expected volatility of 191% and (4) expected life of five years. Opexa can redeem the warrants at $0.01 per underlying share of common stock if the common stock closes at or above $10.00 per share for 20 consecutive trading days.
In connection with the February 2013 registered offering (See Note 10), Opexa issued warrants to the investors on February 11, 2013 to purchase an aggregate of 541,668 shares of common stock at an exercise price of $3.00 per share. These warrants have a term of four years and were immediately exercisable.
Note 11. Subsequent Events
Subsequent to March 31, 2013, Opexa granted its directors, officers and employees options to purchase an aggregate of 207,822 shares of common stock with a fair value of $356,335 at an exercise price of $1.75.
Subsequent to March 31, 2013, Opexa issued 123,231 shares of common stock to the Noteholders of the July 2012 Notes as payment of accrued interest through June 30, 2013.
9,300,000 Shares
Common Stock

PROSPECTUS
Aegis Capital CorpSubscription Rights to Purchase Up to 28,776,419 Units
Consisting of an Aggregate of Up to 28,776,419 Shares of Common Stock
and Warrants to Purchase Up to 28,776,419 Shares of Common Stock
at a Subscription Price of $0.70 Per Unit
Dealer-Managers
Maxim Group LLC
National Securities Corp.
, 20132015
Through and including , 2013 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
Information Not Required In Prospectus
Item 13. Other Expenses of Issuance and Distribution.
The following is a statement of estimated expenses in connection with the issuance and distribution of the securities being registered, other than the underwriting discount.excluding dealer-manager fees. All expenses incurred with respect to the registration of the common stock will be borne by us. All amounts are estimates except the SEC registration fee and the FINRA filing fee.
| Amount to be Paid | ||||
SEC Registration Fee | $ | 2,353 | ||
FINRA Filing Fee | 3,088 | |||
NASDAQ Fee | 65,000 | |||
Printing Expenses | 75,000 | |||
Legal Fees and Expenses | 225,000 | |||
Accounting Fees and Expenses | 15,000 | |||
Blue Sky Fees and Expenses | 5,000 | |||
Transfer Agent and Registrar Fees and Expenses | 5,000 | |||
Miscellaneous Expenses | 149,559 | |||
|
| |||
| $ | 545,000 | |||
|
| |||
Amount to be Paid | ||
SEC Registration Fee | $ 7,524 | |
FINRA Filing Fee | 10,212 | |
NASDAQ Fee | 76,000 | |
Printing Expenses | 125,000 | |
Legal Fees and Expenses | 285,000 | |
Accounting Fees and Expenses | 16,500 | |
Subscription Agent, Information Agent and Warrant Agent Fees and Expenses | 27,500 | |
Miscellaneous Expenses | 175,000 | |
| $747,736 | ||
Item 14. Indemnification of Directors and Officers.
Section 8.101 of the Texas Corporation Law (or the TCA) authorizes the Registrant to indemnify certain persons, including any person who was, is or is threatened to be made a named defendant or respondent in a threatened, pending or completed action or other proceeding, because the person is or was a director or officer, against judgments and reasonable expenses actually incurred by the person in connection with the threatened, pending or completed action or other proceeding. The Registrant is required by Section 8.051 of the TCA to indemnify a director or officer against reasonable expenses actually incurred by him or her in connection with a threatened, pending, or completed action or other proceeding in which he or she is a named defendant or respondent because he or she is or was a director or officer if he or she has been wholly successful, on the merits or otherwise, in the defense of the action or proceeding.
The Registrant’s restated certificate of formation provides that none of its directors shall be personally liable to the Registrant or its shareholders for monetary damages for an act or omission in such director’s capacity as a director; provided, however, that the liability of such director is not limited to the extent that such director is found liable for (i) a breach of the director’s duty of loyalty to the Registrant or its shareholders, (ii) an act or omission not in good faith that constitutes a breach of duty of the director to the Registrant or an act or omission that involves intentional misconduct or a knowing violation of the law, (iii) a transaction from which the director received an improper benefit, whether or not the benefit resulted from an action taken within the scope of the director’s office, or (iv) an act or omission for which the liability of the director is expressly provided by an applicable statute.
The Registrant’s restated certificate of formation and amended and restated bylaws provide that the Registrant shall indemnify its officers, directors, agents and any other persons to the fullest extent permitted by applicable law. The Registrant’s directors and officers are covered by insurance indemnifying them against certain liabilities which might be incurred by them in their capacities as such. Pursuant to terms of their employment contracts, certain of the Registrant’s officers are entitled to indemnification in their capacity as such and to the fullest extent permitted by applicable law.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of the Registrant in which indemnification would be required or permitted. The Registrant is not aware of any threatened litigation or proceeding which may result in a claim for such indemnification.
II-1
Item 15. Recent Sales of Unregistered Securities.Securities.
The following is a summary of all securities that we have sold within the past three years without registration under the Securities Act of 1933, as amended. The transaction amount and the price of securities listed below have been adjusted to reflect the 1-for-4 reverse split which was implemented on December 14, 2012.
On August 18, 2010, we issued 13,750 restricted shares of common stock to a consultant for professional services. The common stock was issued in reliance on the exemption from registration contained in Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder.
On June, 23, 2010, we issued an aggregate of 665,045 shares of common stock to the holders of our 10% convertible promissory notes, consisting of (i) 626,000 shares pursuant to the conversion of all currently outstanding notes with an aggregate outstanding principal balance of $1,252,000 and (ii) 39,045 shares in payment of one-half of the accrued and unpaid interest on said notes of $78,091. On May 6, 2010, we issued 25,000 shares of common stock to a holder of a note pursuant to the conversion of such note with a principal balance of $50,000. The common stock was issued in reliance on the exemptions from registration contained in Sections 3(a)(9) and 4(2) of the Securities Act and the rules and regulations promulgated thereunder.
On April 18, 2011, we issued 12,576 restricted shares of common stock to a consultant for professional services. The common stock was issued in reliance on the exemption from registration contained in Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder.
On July 25, 2012, we completed a private offering of $4,085,000 in principal amount of convertible secured promissory notes and Series I warrants to purchase an aggregate of 1,436,121 shares of common stock, subject to certain adjustments, with an exercise price of $2.56 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. In February 2013, we issued an aggregate of 288,229 shares of common stock to three noteholders who elected to convert such notes into shares of Series A convertible preferred stock with further immediate conversion into common stock.
On November 2, 2012, we completed a private placement to Lincoln Park Capital Fund, LLC pursuant to which we have the right to sell to Lincoln Park up to $15,000,000 in shares of common stock, subject to certain limitations, from time to time over the 30-month period commencing on the date that a registration statement covering the resale of the shares is declared effective by the SEC. The issuance and sale was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based on the offering of such securities to one investor, the lack of any general solicitation or advertising in connection with such issuance, and the representationrepresentations of such investor that it was an accredited investor and that it was purchasing the shares for its own account and without a view to distribute them.
On January 23, 2013, we completed a private offering of $650,000 in principal amount of unsecured convertible promissory notes and Series J warrants to purchase an aggregate of 243,750 shares of common stock, subject to certain adjustments, with an exercise price of $1.24 per share. The offers and sales were made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor. On February 26, 2013, we issued 77,034 shares of common stock to a noteholder who elected to convert such note into common stock.
On January 30, 2013, we issued Series K warrants to purchase an aggregate of 187,500 shares of common stock, at an exercise price of $1.21 per share, to the holders of our July 2012 convertible secured promissory notes in exchange for a waiver and reduction in the cash held in a controlled account as security for repayment of the notes. The issuance was made without registration under the Securities Act in reliance on the exemptions provided by Section 4(2) of the Act and Regulation D promulgated thereunder based upon representations made by the investors, each of whom was an accredited investor.
II-2
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
(b) Financial statement schedules
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings.Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the
II-3
registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-3
(5) That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(ii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Amendment No. 1 to Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of The Woodlands, State of Texas, on July 22, 2013.February 24, 2015.
| OPEXA THERAPEUTICS, INC. | ||
| By: | /s/ Neil K. Warma | |
Neil K. Warma | ||
President and Chief Executive Officer | ||
POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Name | Title | Date | ||
/s/ Neil K. Warma Neil K. Warma | President, Chief Executive Officer and Director (Principal Executive Officer) | |||
/s/ Karthik Radhakrishnan Karthik Radhakrishnan | Chief Financial Officer (Principal Financial and Accounting Officer) | |||
*
| Director | February 24, 2015 | ||
Hans-Peter Hartung, M.D. |
| February 24, 2015 | ||
* Gail J. Maderis | Director |
| ||
* Michael S. Richman | Director |
| ||
* Scott B. Seaman | Director | |||
| *By: | /s/ Neil K. Warma | |
Neil K. Warma | ||
Attorney-in-Fact |
II-5
EXHIBIT INDEX
Exhibit | Description | |
| 1.1* | Form of Dealer-Manager Agreement by and between Opexa Therapeutics, Inc., Maxim Group LLC and National Securities Corporation. | |
| ||
| ||
| ||
| Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
3.2 | Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
3.3 | Certificate of Amendment of the Restated Certificate of Formation of Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on December 14, 2012). | |
3.4 | Amended and Restated By-laws, as amended (incorporated by reference to Exhibit 3.3 to the Company’s Annual Report on | |
4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.7 to the Company’s Registration Statement on Form S-3 filed on November 13, 2009, File No. 333-163108). | |
4.2 | ||
| ||
| ||
| ||
| ||
| Form of Securities Purchase Agreement dated as of December 9, 2009 by and between Opexa Therapeutics, Inc. and each investor signatory thereto for Unit offering of Common Stock and Series A and Series B Warrants (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| Form of Common Stock Purchase Warrant for Series A and Series B Warrants (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed December 10, 2009). | |
| Form of Series H Warrant issued on February 11, 2011 (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed February 8, 2011). | |
|
| |
| Form of Series I Warrant issued on July 25, 2012 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on July 26, 2012). | |
| Form of Series J Warrant issued on January 23, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 23, 2013). | |
| Form of Series K Warrant issued on January 30, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 30, 2013). | |
| Form of Series L Warrant issued on February 11, 2013 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| Form of Securities Purchase Agreement, dated as of February 7, 2013, by and between Opexa Therapeutics, Inc. and each investor signatory thereto (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 7, 2013). | |
| ||
| 4.11** | Form of Series M Warrant underlying the Units. | |
| 4.12** | Form of Warrant Agreement | |
4.13* | Form of Subscription Agent Agreement between Opexa Therapeutics, Inc. and Continental Stock Transfer & Trust Company. | |
| 5.1** | Opinion of Pillsbury Winthrop Shaw Pittman LLP. | |
| Description | |
| 10.1+ | Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit B to the Company’s Definitive Information Statement on Schedule 14C filed on June 29, 2004, File No. 000-25513). | |
10.2+ | Certificate of Amendments to the Opexa Therapeutics, Inc. June 2004 Compensatory Stock Option Plan (incorporated by reference to Exhibit 10.15 of the Company’s Annual Report on Form 10-K filed March 5, 2010). | |
10.3+ | Opexa Therapeutics, Inc. 2010 Stock Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 12, 2013). | |
| 10.4+ | Form of Notice of Stock Option Grant and Stock Option Agreement for awards granted under the 2010 Stock Incentive Plan, as amended and restated (incorporated by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q filed August 14, 2014). | |
| 10.5+ | Employment Agreement dated June 16, 2008 by and between Opexa Therapeutics, Inc. and Neil K. Warma (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 18, 2008). | |
| Amended and Restated Employment Agreement entered into on April 21, 2010 by and between Opexa Therapeutics, Inc. and Donna R. Rill (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed April 27, 2010). | |
| Offer Letter, effective March 29, 2013, by and between Opexa Therapeutics, Inc. and Karthik Radhakrishnan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 1, 2013). | |
| 10.8 | License Agreement dated September 5, 2001 by and between Opexa Therapeutics, Inc. and Baylor College of Medicine (incorporated by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-KSB filed April 15, 2005, File No. 000-25513). | |
| Lease dated August 19, 2005 by and between Opexa Therapeutics, Inc. and Dirk D. Laukien (incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-KSB filed March 31, 2006, File No. 000-25513). | |
| License Agreement dated January 13, 2006 by and between Opexa Therapeutics, Inc. and Shanghai Institute for Biological Services (incorporated by reference to Exhibit 10.23 to the Company’s Registration Statement on Form SB-2 (Amendment No. 1) filed February 9, 2006, File No. 333-126687). | |
| ||
| ||
| ||
|
| |
| ||
| ||
| ||
| ||
| ||
| Sales Agreement, dated September 6, 2012, by and between Opexa Therapeutics, Inc. and Brinson Patrick Securities Corporation (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 7, 2012). | |
| First Amendment to Sales Agreement, dated March 5, 2014, by and among Opexa Therapeutics, Inc., Meyers Associates, L.P. (doing business as Brinson Patrick, a division of Meyers Associates, L.P.) and Brinson Patrick Securities Corporation (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K files on March 5, 2014). | |
| 10.13 | $ | |
| $ | |
| Registration Rights Agreement, dated as of November 2, 2012, by and between Opexa Therapeutics, Inc. and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed November 5, 2012). | |
| Description | |
| 10.16# | ||
| ||
| Option and License Agreement, dated February 4, 2013, by and between Ares Trading SA, a wholly owned subsidiary of Merck Serono S.A., and Opexa Therapeutics, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 5, 2013). | |
| ||
| List of Subsidiaries (incorporated by reference to Exhibit 21.1 to the Company’s Annual Report on Form 10-K filed on March 29, 2013). | |
23.1* | Consent of Independent Registered Public Accounting Firm MaloneBailey, LLP. | |
| 23.2** | Consent of Pillsbury Winthrop Shaw Pittman LLP, | |
|
| |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| 99.7* | Form of Notice of Important Tax Information. | |
| * | Filed herewith |
| ** | Previously filed |
| + | Management contract or compensatory plan or arrangement |
| Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |