
As filed with the Securities and Exchange Commission on August 28,December 5, 2023.
Registration No. 333-272110
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 47 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Advanced Biomed Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 8071 | 87-2177170 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
689-87 Xiaodong Road, Yongkang District
Tainan, Taiwan
Tel: 886-6-3121716
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Cogency Global Inc.
122 East 42nd Street, 18th Floor
New York, NY 10168
(212) 947-7200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of all communications to:
Fang Liu, Esq. VCL Law LLP | William S. Rosenstadt, Esq. Mengyi “Jason” Ye, Esq. | |
| 1945 Old Gallows Road | Ortoli Rosenstadt LLP | |
| Suite | 366 Madison Avenue, 3rd Floor | |
| Vienna, VA 22182 | New York, NY 10017 | |
| Telephone: (703) 919-7285 | Telephone: (212) 588-0022 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer ☐ | Accelerated Filer ☐ | Non-Accelerated Filer ☒ | Smaller Reporting Company ☒ | |||
| Emerging Growth Company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall hereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the SEC is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated August 28,December 5, 2023
PRELIMINARY PROSPECTUS
Advanced Biomed Inc.

25,000,000 Shares of Common Stock
This prospectus relates to the offer and sale of 25,000,000 shares of common stock, par value $0.001 per share, of Advanced Biomed Inc. We expect that the initial public offering price will be between $4.00 to $5.00 per share of common stock. Prior to this offering, there has been no public market for our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the symbol “ADVB.” We believe that upon the completion of the offering contemplated by this prospectus, we will meet the standards for listing on the Nasdaq Capital Market. We cannot guarantee that we will be successful in listing our common stock on the Nasdaq Capital Market; however, we will not complete this offering unless we are so listed.
Advanced Biomed Inc. (“Advanced Biomed”) is not an operating company but a holding company incorporated in the State of Nevada. Substantially all of the business operations are conducted in Taiwan by our Taiwan subsidiary. And we also have a subsidiary in Hong Kong and a Shanghai subsidiary in Mainland China. Shares of common stock offered in this offering are shares of a U.S. holding company, which does not conduct operations. As used in this prospectus, “we,” “us,” “our” or “the Company” refers to Advanced Biomed, the U.S. holding company. While none of our PRC Subsidiaries operates with a variable interest entity (“VIE”) structure, the Chinese regulatory authorities could disallow our current operating structure, which would likely result in a material change in our operations and/or a material change in the value of the securities we are registering for sale, including that it could cause the value of such securities to significantly decline or become worthless. See “Risk Factors — Changes in the policies, regulations, rules, and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon our ability to operate profitably in the PRC.”; and “Risk Factors — The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale.”
Although the majority of our operations are not conducted in Mainland China, we face various legal and operational risks and uncertainties relating to our Shanghai subsidiary, Shanghai Sglcell Biotech Co., Ltd., and similar legal and operational risks and uncertainties also apply to our holding company in Hong Kong. The Chinese government may intervene or influence the operation of our Shanghai subsidiary and Advanced Biomed HK and exercise significant oversight and discretion over the conduct of their business and may intervene in or influence their operations at any time, or may exert more control over securities offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and/or the value of our common stock. Further, any actions by the Chinese government to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.
| 2 |
Recently, the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, adopting new measures to extend the scope of cybersecurity reviews, and expanding the efforts in anti-monopoly enforcement. As advised by our PRC counsel, AllBright Law Offices (“AllBright”), we do not believe that we are directly subject to these regulatory actions or statements, as our Shanghai subsidiary does not have a VIE structure and their operations are not subject to cybersecurity review requirements, or involve any type of restricted industry. Because these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative rule making bodies in China will respond to them, or what existing or new laws or regulations will be modified or promulgated, if any, or the potential impact such modified or new laws and regulations will have on our subsidiaries’ daily business operations or ability to accept foreign investments and list on an U.S. exchange. On December 24, 2021, nine government agencies jointly issued the Opinions on Promoting the Healthy and Sustainable Development of Platform Economy, which provides that, among others, monopolistic agreements, abuse of dominant market position and illegal concentration of business operators in the field of platform economy will be strictly investigated and punished in accordance with the relevant laws. We do not hold a dominant market position in our product markets and we have not entered into any monopolistic agreement. We have not received any inquiry from the relevant governmental authorities. the Cyberspace Administration of China (“CAC”), together with 12 other Chinese regulatory authorities, released the final version of the Revised Measures for Cybersecurity Review, or the Revised Cybersecurity Measures, in December 2021, which took effect on February 15, 2022. Pursuant to the Revised Cybersecurity Measures, critical information infrastructure operators procuring network products and services and online platform operators carrying out data processing activities, which affect or may affect national security, shall conduct a cybersecurity review pursuant to the provisions therein. In addition, online platform operators possessing personal information of more than one million users seeking to be listed on foreign stock markets must apply for a cybersecurity review. On November 14, 2021, the CAC published the Draft Regulations on the Network Data Security Administration (Draft for Comments) (the “Security Administration Draft”), which provides that data processing operators engaging in data processing activities that affect or may affect national security must be subject to cybersecurity review by the relevant Cyberspace Administration of the PRC. As advised by AllBright, we do not believe that we are an “online platform operator” within the meaning of the Revised Cybersecurity Measures, and, we currently do not have over one million users’ personal information and do not anticipate that we will be collecting over one million users’ personal information in the foreseeable future. In addition, we are also not subject to Security Administration Draft if the Security Administration Draft are enacted as proposed, since we currently do not collect data that affects or may affect national security and we do not anticipate that we will be collecting data that affects or may affect national security in the foreseeable future.
On December 24, 2021, China Securities Regulatory Commission (the “CSRC”) issued the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing of Securities by Domestic Enterprises (the “Draft Administrative Provisions”) and the Measures for the Overseas Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Draft Filing Measures”), collectively, the Draft Overseas Listing Rules. On February 17, 2023, the CSRC released Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies with five interpretive guidelines (the “Trial Measures”) which came into effect on March 31, 2023. Pursuant to the Trial Measures, a PRC domestic company that seeks to offer and list securities in overseas markets, either in direct or indirect overseas offering, shall fulfill the filing procedure with the CSRC as per requirement of the Trial Measures, submit relevant materials that contain a filing report and a legal opinion, and provide truthful, accurate and complete information on the shareholder and etc. Direct overseas offering and listing by domestic companies refers to such overseas offering and listing by a joint-stock company incorporated domestically. Any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect offering and listing in overseas market and, therefore, be subject to filing requirement: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in the Mainland China, or its main places of business are located in the Mainland China, or the senior managers in charge of its business operation and management are mostly Chinese citizens or domiciled in the Mainland China. The determination as to whether or not an overseas offering and listing by domestic companies is indirect, shall be made on substance over form basis. As of the date of this prospectus, as advised by AllBright, we do not believe that we are required to obtain the approval from or complete the filing with the CSRC for this offering and thus we have not submitted an application for approval for this offering with the CSRC pursuant to the Trial Measures based on the factfacts that (i) we are a holding company incorporated in the State of Nevada, not a company incorporated under the PRC law; (ii) based on the report on the Shanghai subsidiary from a third party accounting firm in China, the Shanghai subsidiary contributed less than 50% to our operating revenue, total profit, total assets or net assets as documented in the audited consolidated financial statements for the fiscal year ended June 30, 2023; (iii) the business activities are primarily conducted in Taiwan, with minimal operation in Mainland China, and (iv) most of our officers and directors are non-Chinese citizens or domiciled outside Mainland China. Thus, we do not meet the explicit conditions set out in the Trial Measures to determine whether an overseas offering shall be deemed as a direct or an indirect overseas offering and listing by a domestic company. However, as the Trial Measures was newly published, there are substantial uncertainties as to the implementation and interpretation, and the CSRC may take a view that is contrary to our understanding of the Trial Measures. If we are required by the CSRC to submit and complete the filing procedures of this offering and listing, we cannot assure you that we will be able to complete such filings in a timely manner, or even at all. Any failure by us to comply with such filing requirements under the Trial Measures may result in rectification, warnings, and a fine between RMB 1 million and RMB 10 million on our Shanghai subsidiary, which could adversely and materially affect our business operations and financial outlook, and significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or such shares to become worthless.
As of the date of this prospectus, these new laws and guidelines have not impacted our ability to conduct our business, accept foreign investments, or continue to list on a U.S. or other foreign exchange; however, if (i) we inadvertently conclude that permissions or approvals are not required from applicable PRC authorities, (ii) applicable laws, regulations, or interpretations change, and we are required to obtain such permissions or approvals in the future, or (iii) we fail to file or were denied permission from the PRC authorities to this offering, any follow-up offerings or transactions, our ability to conduct our business may be materially impacted, and we will not be able to continue listing on any U.S. exchange, continue to offer securities to investors, the interest of the investors may be materially and adversely affected and our common stock may significantly decrease in value or become worthless. To date, there are uncertainties in the interpretation and enforcement of these new laws and guidelines, which could materially and adversely impact our business and financial outlook and may impact our ability to accept foreign investments, or continue to list on a U.S. or other foreign exchange. See “Risk Factors — The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale;” “Risk Factors — If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us, such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless”; and “Risk Factors — The M&A Rules and certain PRC regulations may make it more difficult for us to pursue growth through acquisitions.”
| 3 |
Cash may be transferred within our organization in the following manners: (i) Advanced Biomed may transfer funds to our subsidiaries, including our Shanghai subsidiary, by way of capital contributions or loans, through intermediate holding subsidiaries or otherwise; (ii) we and our intermediate holding subsidiaries may provide loans to our operating subsidiaries and vice versa; and (iii) our subsidiaries, including our Shanghai subsidiary, may make dividends or other distributions to us through intermediate holding companies or otherwise. As of the date of this prospectus, Advanced Biomed made seven capital contributions to Advanced Biomed Taiwan and Advanced Biomed HK to support their research and development.
| Date | Receiving Entity | Amount (US$) | ||
| June 29, 2022 | Advanced Biomed Taiwan | 2,500,000 | ||
| October 11, 2022 | Advanced Biomed Taiwan | 86,000 | ||
| October 24, 2022 | Advanced Biomed HK | 100,000 | ||
| October 26, 2022 | Advanced Biomed HK | 500,000 | ||
| November 7, 2022 | Advanced Biomed Taiwan | 122,000 | ||
| December 2, 2022 | Advanced Biomed HK | 110,000 | ||
| December 14, 2022 | Advanced Biomed Taiwan | 85,000 |
Other than the transfers in the table above, we have not made any distribution of dividends or assets, cash transfers, capital contributions or loans among the holding company or any of our subsidiaries. As advised by AllBright, our PRC counsel, PRC laws, regulations and judicial interpretations thereof do not prohibit using cash generated from one subsidiary to fund another subsidiary’s operations by way of short term interest free loans. In the future, cash proceeds raised from overseas financing activities, including this offering, may be transferred by us to our Taiwan and Hong Kong subsidiaries and Shanghai subsidiary via capital contributions or shareholder loans. As of the date of this prospectus, Advanced Biomed has not made dividend or other distributions to our shareholders. Advanced Biomed may pay dividends to our shareholders subject to our ability to service our debts as they become due and provided that our assets will exceed our liabilities after the payment of such dividends. As a holding company, Advanced Biomed may rely on dividends and other distributions on equity paid by our subsidiaries for our cash and liquidity requirements, including payment of any debt we may incur outside of China and our expenses. If any of our subsidiaries incurs debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to us. To the extent cash or assets in the business is in the PRC or a PRC subsidiary, the cash or assets may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on our or our subsidiaries’ ability by the PRC government to transfer cash or assets or distribute earnings within our group or to U.S. investors. PRC laws and regulations applicable to our Shanghai subsidiary permit payments of dividends only out of their retained earnings, if any, determined in accordance with applicable accounting standards and regulations. Our Shanghai subsidiary may pay dividends only out of their respective accumulated after-tax profits as determined in accordance with PRC accounting standards and regulations. In addition, our subsidiaries are required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund certain statutory reserve funds, until the aggregate amount of such funds reaches 50% of its registered capital. At its discretion, a wholly foreign-owned enterprise may allocate a portion of its after-tax profits to discretionary funds. These reserve funds and discretionary funds are not distributable as cash dividends. Furthermore, dividends paid by our Shanghai subsidiary to theirits parent companies will be subject to a 10% withholding tax, which can be reduced to 5% if certain requirements are met. The PRC government also imposes restrictions on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. As such, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any, or transfer cash within our group, across border, or to U.S. investors. Additionally, current Taiwan regulations only permit our Taiwan subsidiary to pay dividends to its shareholders out of its accumulated profits, and Advanced Biomed Taiwan must set aside at least 10% of its accumulated profits each year and use it to make up previous losses, if any. The statutory reserve cannot be distributed as cash dividends. As of the date of this prospectus, no dividends, transfers, or distributions have been made within our group or to shareholders. We presently intend to retain all earnings to fund our operations and business expansions and have no plan to distribute earnings to shareholders. We do not anticipate paying dividends or other distributions to our shareholders, including U.S. investors, in the foreseeable future. See the relevant discussions in “Risk Factors — Risks Related to Doing Business in China” on page 39; “Risk Factors — PRC regulation on loans to, and direct investment in, PRC entities by offshore holding companies and governmental control in currency conversion may delay or prevent us from making loans to or making additional capital contributions to our Shanghai subsidiary” on page 43; “Risk Factors — Our Shanghai subsidiary are subject to restrictions on paying dividends or making other payments to us, which may restrict our ability to satisfy our liquidity requirements in the future” on page 43; and “Risk Factors — Advanced Biomed Taiwan is subject to restrictions on paying dividend or making other payments to us, which may restrict our ability to satisfy its liquidity requirements” on page 37.”
| 4 |
Our common stock may be prohibited from trading on a national exchange or “over-the-counter” markets under the Holding Foreign Companies Accountable Act (the “HFCAA”) if the Public Company Accounting Oversight Board (“PCAOB”) determines it is unable to inspect or investigate completely our auditors for three consecutive years beginning in 2021. Further, on June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act (“AHFCAA”). If the AHFCAA is enacted into law, it would amend the HFCAA and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchanges if its auditor is not subject to PCAOB inspections or complete investigations for two consecutive years instead of three. On December 29, 2022, a legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to HFCAA, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCAA from three years to two. Pursuant to the HFCAA, the PCAOB issued a Determination Report on December 16, 2021, which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in mainland China and Hong Kong because of positions taken by the authorities in those jurisdictions. In addition, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. On August 26, 2022, the PCAOB signed a Statement of Protocol Agreement with the CSRC and the Ministry of Finance (the “MOF”) of the PRC governing inspections and investigations of audit firms based in China or Hong Kong. On December 15, 2022, the PCAOB announced in the 2022 Determination its determination that the PCAOB was able to secure complete access to inspect and investigate accounting firms headquartered in mainland China and Hong Kong, and the PCAOB Board voted to vacate previous determinations to the contrary. Should the PCAOB again encounter impediments to inspections and investigations in mainland China or Hong Kong as a result of positions taken by any authority in either jurisdiction, including by the CSRC or the MOF, the PCAOB will make determinations under the HFCAA as and when appropriate. Our auditor, WWC, P.C., is headquartered in California and, as a PCAOB-registered public accounting firm, is required to undergo regular inspections by the PCAOB to assess its compliance with the laws of the U.S. and professional standards. WWC, P.C. has been subject to PCAOB inspections and is not among the PCAOB-registered public accounting firms headquartered in the PRC or Hong Kong that are subject to PCAOB’s determination of having been unable to inspect or investigate completely. Notwithstanding the foregoing, if it is later determined that the PCAOB is unable to inspect or investigate our auditor completely, if there is any regulatory change or step taken by PRC regulators that does not permit WWC, P.C. to provide audit documentations located in China or Hong Kong to the PCAOB for inspection or investigation, or the PCAOB expands the scope of the Determination so that we are subject to the HFCAA, as the same may be amended, you may be deprived of the benefits of such inspection. Any audit reports not issued by auditors that are completely inspected or investigated by the PCAOB, or a lack of PCAOB inspections of audit work undertaken in China that prevents the PCAOB from regularly evaluating our auditors’ audits and their quality control procedures, could result in a lack of assurance that our financial statements and disclosures are adequate and accurate, which could result in limitation or restriction to our access to the U.S. capital markets, and trading of our securities, including trading on the national exchange and trading on “over-the-counter” markets, may be prohibited under the HFCAA and our securities may be delisted by an exchange. See “Risk Factors — Recent joint statement by the SEC and the PCAOB, rule changes by Nasdaq, and the Holding Foreign Companies Accountable Act all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our continued listing or future offerings of our securities in the U.S.”
We are an “emerging growth company” under applicable U.S. federal securities laws and are eligible for reduced public company reporting requirements.
| 5 |
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 23.
| Per Share | Total(3)(4) | |||||||
| Public offering price | $ | 4.50 | $ | 112,500,000 | ||||
| Underwriting discounts and commissions (1)(2) | $ | 0.2475 | $ | 6,187,500 | ||||
| Proceeds to us, before expenses(2) | $ | 4.2525 | $ | 106,312,500 | ||||
(1) We have agreed to pay the underwriters a commission equal to 5.50% of the gross proceeds of the offering. Underwriting discounts and commissions do not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering payable to the representative of the underwriters. We have also agreed to reimburse certain accountable expenses to the representative, including, but no limited to, the representative’s legal fees, background check expenses and other expenses related to the offering, up to $200,000.
(2) We have also agreed to issue to the representative of underwriters warrants to purchase 250,000 shares of common stock (or 287,500 shares of common stock if the representative of underwriters exercises its over-allotment option in full) equal to 1% of the shares sold in this offering, at a per share exercise price equal to 150% of the per share offering price. The representative’s warrants are exercisable at any time and from time to time, in whole or in part, during the four-and-a-half-year period commencing six months following the date of commencement of sales of securities issued in this offering. For a description of other terms of the representative’s warrants and other compensation to be received by the underwriters, see “Underwriting” beginning on page 115.
(3) Excludes fees and expenses payable to the representative. The maximum amount of the representative’s expenses that we are required to reimburse related to this offering is set forth in the section entitled “Underwriting.”
(4) Assumes that the underwriters do not exercise any portion of their over-allotment option as described below.
This offering is being conducted on a firm commitment basis. The underwriters are obligated to take and pay for all of the shares offered by this prospectus if any such shares are taken. The underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option to purchase additional shares of common stock.
We have granted a 45-day option to the underwriters to purchase up to 3,750,000 additional shares of common stock (equal to 15% of the shares sold in the offering) at the same offering price to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the Shares to purchasers on or about , 2023.

The date of this prospectus is , 2023
| 6 |
TABLE OF CONTENTS
Through and including , 2023 (25 days after the commencement of this offering), all dealers effecting transaction in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
| 7 |
MARKET, INDUSTRY AND OTHER DATA
About this Prospectus
You should rely only on the information contained in this prospectus and any free writing prospectus we may authorize to be delivered to you. We have not, and the underwriters have not, authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus and any related free writing prospectus. We and the underwriters take no responsibility for, and can provide no assurances as to the reliability of, any information that others may give you. This prospectus is not an offer to sell, nor is it seeking an offer to buy, these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is only accurate as of the date of this prospectus, regardless of the time of delivery of this prospectus and any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside of the United States: Neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside of the United States who come into possession of this prospectus and any free writing prospectus must inform themselves about and observe any restrictions relating to this offering and the distribution of this prospectus outside of the United States. See “Underwriting— Offer restrictions outside the United States” on page 118.
Industry and Market Data
This prospectus includes estimates regarding market and industry data. Unless otherwise indicated, information concerning our industry and the markets in which we operate, including our general expectations, market position, market opportunity, and market size, are based on our management’s knowledge and experience in the markets in which we operate, together with currently available information obtained from various third-party sources, including publicly available information, industry reports and publications, surveys, our customers, trade and business organizations, and other contacts in the markets in which we operate. Some data is also based on our good faith estimates. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors.” These and other factors could cause results to differ materially from those expressed in these publications.
| 8 |
This summary contains basic information about us and the offering contained elsewhere in this prospectus. Because it is a summary, it does not contain all the information that you should consider before investing in our securities. You should read and carefully consider the entire prospectus before making an investment decision, especially the information presented under the headings “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements,” “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and all other information included in this prospectus in its entirety before you decide whether to purchase any shares offered by this prospectus.
Unless the context requires otherwise, the words “we,” “us,” “our,” “our company,” “the Company,” and “Advanced Biomed” refer to Advanced Biomed Inc., a holding company incorporated in the State of Nevada.
Our Company
Our Mission
We are committed to the application research of integrating semiconductor technology and biotechnology. Through the enrichment, capture, and identification of circulating tumor cells and related tumor marker cells in the field of liquid biopsy, we aim to provide cancer patients with rapid and affordable assay products and services. These services include early screening and detection, diagnosis and staging, treatment selection, and patient outcome interventions for cancer.
Overview
We are a holding company incorporated in the State of Nevada. We operate through Advanced Biomed Inc. (Taiwan) (“Advanced Biomed Taiwan”) and Advanced Biomed HK Limited (“Advanced Biomed HK”). Advanced Biomed Taiwan is responsible for the main operation and the design and development of the company's primary technologies and products. Since our establishment in 2014, we have been focusing on the integration of multiple interdisciplinary technologies and established our own microfluidic technology platform. Utilizing the physical and molecular biological characteristics of tumor cells, we have developed various advanced and original research through the joint application of semiconductor technology and biotechnology. This includes complex precision structures, dielectric detection, functional microfluidic biochips, microfluidic integrated semiconductor sensors, related application modules, and key components of medical testing equipment. We have also developed a series of medical testing equipment and related products by integrating various functions of microfluidic modules, automation software, and hardware. Our technologies and products can be used for early screening and detection, diagnosis and staging, and treatment of cancer through the detection of circulating tumor cells and related tumor markers in blood samples, capture of single circulating tumor cells, and single-cell sorting and determination. These products provide assistance in treatment selection and patient prognosis intervention once the required licenses and approvals have been obtained. Advanced Biotech HK is our first localized operation company, mainly responsible for market operation and management in China, localized production, product registration, and future local market sales of our products in accordance with relevant local regulations in China. Our Shanghai subsidiary owns some of our R&D equipment and patents and will be responsible for operations related to clinical trials in Mainland China through contract research organizations.organizations (“CROs”). In the future, we will also establish operation centers in countries and regions in North America and Europe.
Our devices, A+Pre, AC-1000, A+CellScan, and A+SCDrop, and three corresponding microfluidic biochips, A+Pre Chip and AC-1000 CTC Enrichment Chip and A+CellScan Chip, are designed to provide rapid and affordable assay products and services to cancer patients. Among them, A+Pre is mainly used to reduce the viscosity of blood samples, and AC-1000 is used to complete the separation and enrichment of circulating tumor cells (“CTCs”) and tumor-related targeted cells in blood samples. The A+CellScan is mainly used for fluorescent labeling and automatic scanning judgment of targeted cells while A+SCDrop preserves the original viability of single cells.
Additionally, we have finished the research and development stage for four matching immunostaining kits, A+CTCE, A+CTCM, A+EMT and A+CM, and submitted registration applications in China. The immunostaining kit use antibodies combined with fluorescent groups of different colors to bind to specific proteins on the cell surface or inside the cells. The presence and intensity of fluorescent signals can be observed through a separate fluorescent imaging system, and the expression of the target protein and the cell type can be judged and determined accordingly. Different cell types can be distinguished using multiplexed combined staining with different antibodies. The A+CTCE kit is mainly used to identify epithelial circulating tumor cells, the A+CTCM kit is used to identify mesenchymal circulating tumor cells, the A+EMT kit is mainly used to identify epithelial-to-mesenchymal circulating tumor cells, and the A+CM kit is used to identify tumor-associated macrophages (cancer-associated macrophage-like cells).
We also developed a product for early screening of lung cancer, the A+LCGuard Lung Cancer Early Screening Kit (“A+LCGuard”), which is used to assist in the determination of benign and malignant pulmonary nodules. From August 2020 to September 2022, we finalized the research, design, and development of A+LCGuard. A+LCGuard is a Class III medical device and is required to conduct clinical trials before completing the registration process. We plan to start A+LCGuard’s clinical trials in March 2024, which are expected to be completed in the first half of 2025, and the registration declaration will be made afterwards.
| 9 |
All of our products must go through three steps to receive the required clearance from the National Medical Products Administration of China (“NMPA”) before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. At the registration application stage, we have to assemble all the required application materials, complete clinical trials (if required by NMPA), and work with an NMPA accredited third-party organization to examine our products in accordance with NMPA rules. NMPA will review our application during the registration review period and may request additional information before officially approving or denying our applications. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; the four matching immunostaining kits and A+SCDrop are under registration review; A+CellScan, A+CellScan Chip, and A+LCGuard are ready to start their registration applications. As of the date of this prospectus, we have not applied for similar clearances from other jurisdictions.
We participated in a scientific research project at Shanghai Pulmonary Hospital from July 17, 2019 to December 2021, and completed a total of 123 case studies to test A+Pre, AC-1000 and A+LCGuard. In the study, we selected 123 individuals, and among them, 75 were surgical patients with nodular changes or shadows in the lungs reported by imaging studies and 48 healthy patients without lung nodules reported by imaging studies. 7ml blood samples were taken from test subjects either before the clinical operation (for cancer patients) or after the physical examination (for healthy individuals), and A+Pre, AC-1000, and A+LCGuard kits were used to determine whether there were circulating tumor cells and other tumor markers in the blood samples. Finally, the pathological and physical examination results of the tested individuals were compared with the test results of our products. Our test results achieved 96% sensitivity and 99.9% specificity, which provides the research and development basis for our products. Specifically, A+Pre and AC-1000 were at the research and development stage, and we completed their effectiveness and performance indicators testing through this project. At the same time, A+LCGuard finished its feasibility and functional verification testing. All three products were tested together throughout the entire project.
All of our products must be approved by applicable regulatory authorities before being sold to customers. A+Pre and A+CellScan can work with third-party products to achieve their designed objectives. AC-1000 and A+SCDrop may be used together with other devices according to different application scenarios below. For the A+LCGuard early screening kit, it has to be used in combination with A+Pre and AC-1000. Our four staining kits, A+CTCE, A+CM, A+CTCM, and A+EMT, can be used independently or with third-party products. A+Pre, AC-1000, and A+CellScan require the use of our supporting microfluidic chips.
| · | For the analysis of high-viscosity blood samples: A+Pre can be independently used for pretreatment, retaining the original cell activity while preventing blood samples from clogging the equipment pipeline after entering the detection equipment. |
| · | For the identification and counting application of circulating tumor cells: blood samples are diluted with A+Pre, and then AC-1000 is used to separate and enrich circulating tumor cells and related tumor markers. The enriched samples are stained, calibrated, and finally identified and counted. We can provide this service to the public if using third-party staining reagents already on the market in China. However, we will officially roll out this service once our in-house developed staining reagents, A+CTCE, A+CTCM, A+EMT and A+CM, complete the registration process. The identification and counting of circulating tumor cells and related tumor marker cells can provide auxiliary references for relevant clinical applications. |
| · | The capture of circulating tumor cells: we follow the same process as the identification of circulating tumor cells to obtain enriched samples with A+Pre and AC-1000, and then the samples are captured and separated by A+SCDrop to isolate single circulating tumor cells. This service can provide tumor cells with high purity and high activity. |
| · | For early screening of lung cancer: peripheral blood samples of the subjects are first obtained, and the target cells are enriched and captured sequentially by A+Pre and AC-1000. After that, A+LCGuard performs cell fluorescence staining on the enriched samples to determine the number of targeted cells, and finally makes a judgment. |
Due to the different regulatory requirements for the marketing of medical device products and in-vitro diagnostics (“IVD”) products in various regions/countries, it is necessary to complete the registration application and obtain the corresponding license in accordance with the local regulations before engaging in commercial activities in the respective regions/countries (“localization registration”). Afterward, marketing and sales can be carried out. We follow the principle of modularization when design and develop all of our products and equipment so that products and equipment can be produced locally to meet different regulatory requirements. Based on the current development of the early tumor screening and preventive treatment industry and the characteristics of the products we are planning to register and apply in the future, we have adopted the operation model of centralized research and development and localized management. We have started the registration process with the NMPA in China for all of our products. Later on, the Company may establish subsidiaries in the United States and Europe to produce products and carry out product registration. To achieve that, our products must be cleared by the United States Food and Drug Administration and go through the conformity assessment process to obtain the Conformite Europeenne marking (“CE marking”) from competent authority in each European Union member state.
We are looking for suitable locations in the states of California and Washington for our planned expansion to the North America market. We aim to complete site selection and personnel recruitment in the United States by the end of 2023 and start product registration, testing and production in 2024. Our US subsidiary will be responsible for the production and registration of our equipment and related products in the US. Production, testing, and clinical trials in our US market will be conducted in accordance with US regulations, and clinical data from trials conducted in China will not be used to establish product standards. In addition, we also plan to break into the European market by establishing a United Kingdom subsidiary, which is expected to start its operation in 2024, and conduct localized management and operations in accordance with European regulations. In 2025, we will start the localized registration of our IVD products in Europe. As of the date of this prospectus, we have not conducted any clinical trials for our products.
However, as of the date of this prospectus, we have not commenced sales of our products nor have any revenue-generating products and do not expect sales of revenue-generating product candidates until we have completed clinical development, submitted regulatory filings, and received applicable regulatory approvals for candidate products. Due to differences in regulatory and clinical registration requirements, we may not be able to obtain device and product approvals or provide product service on time. We expect to be in a state of continuous loss for the next two to three years.
| 10 |
Market Opportunities in Early Cancer Detection Industry
The early cancer detection market has huge potential. Early tumor screening and related diagnosis are the most active directions in the industry. According to Grand View Research, Inc.’s market analysis report, Liquid Biopsy Market Size, Share & Trends Analysis Report, the global liquid biopsy market size was valued at USD 8,937.68 million in 2022 and is anticipated to grow at a CAGR of 12.46% from 2023 to 2030, resulting in sales worth USD 22,865.56 million in 2030. The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue in US dollars. It uses the bottom-up approach for market sizing, analyzing key regional markets, dynamics, and trends for various services, and end-uses. The forecast of the global market is calculated by integrating the regional markets’ amounts. The report has also considered factors including impact of COVID-19 up to 2023, supply chain disruptions and demand dynamics. According to the report, liquid biopsy is a revolutionary technique that has created various opportunities that were previously unexplored. It aids in detection and isolation of circulating tumor DNA, exosomes, and circulating tumor cells and is a source of proteomics and genomics information in cancer patients. It is an easy, rapid, and minimally invasive test for cancer genetic status based on circulating tumor cells, circulating tumor DNA, and other tumor-derived substances in blood plasma samples. Rapid development in digital Polymerase Chain Reaction (PCR) and NGS-based technology has improved accuracy of liquid biopsy. It can be performed repeatedly for disease monitoring and is anticipated to help overcome limitations of tissue biopsies. It is worth noting that these are estimates of the global market, and we intend to initially focus on developing our cancer screening market in China and plan to expand our operations to other markets in the following years.
Our Oncology Detection Solutions
The current market uses positive antibody-labeling selection to capture CTCs out of the blood by a specific epithelial cell adhesion molecule (EpCAM). This method could detect tumor cells in the patient’s blood for diagnosing lung, prostate, pancreatic, and breast cancers. However, this technology costs approximately $1,000 per chip, and the processing time per patient is up to 12 hours. Besides, antibody-based methods such as immunomagnetic methods are highly dependent on antigen expression of CTC. Some CTCs may show low or no EpCAM expression on the cell membrane, and thus cannot be effectively captured using the proposed biomarkers and makes it difficult to detect cancer in the early stage. More importantly, the capture of dying or dead CTCs cannot provide meaningful information to doctors for diagnosis or treatment.
Our products use pure physical mechanisms (antigen-independent) and can effectively enrich and detect the CTCs with high or low antigen expressions with high viability. Among them, A+Pre is mainly used to reduce the viscosity of blood samples, and AC-1000 is used to complete the separation and enrichment of circulating tumor cells (“CTCs”) and tumor-related targeted cells in blood samples. The A+CellScan is mainly used for fluorescent labeling and automatic scanning judgment of targeted cells while A+SCDrop preserves the original viability of single cells.
For three corresponding microfluidic biochips, A+Pre Chip and AC-1000 CTC Enrichment Chip have been cleared by NMPA and can be mass-produced and sold to customers. A+CellScan Chip is expected to start the registration application by the end of 2023. The A+CellScan Chip and A+CellScan will enrich our product chain by upgrading our immunochromogenic kits to tumor cell assay equipment. Specifically, A+CellScan, together with A+Pre and AC-1000, can serve as a liquid biopsy IVD product to accelerate downstream assay and reduce the amount of labor required for assaying tumor cells.
We use our own product features combined with different application scenarios to achieve the corresponding work objectives. To identify CTCs in liquid biopsy, blood samples are first diluted through equipment A+Pre, then AC-1000 is used to separate and enrich circulating tumor cells, and then the obtained cells are stained for identification and counting. We have been cleared by NMPA to provide this service to customers in China and plan to do so once our matching immunostaining kits pass the NMPA’s registration review. The counting of circulating tumor cells can provide auxiliary reference for clinical diagnosis, treatment evaluation, prognosis evaluation, recurrence, and metastasis detection, etc. In the future, we can also use our A+LCGuard early screening kit combined with two devices, A+Pre and AC-1000, for early screening of lung cancer. In addition, we use A+SCDrop together with A+Pre and AC-1000 to complete the capture of a single circulating tumor cell, which retains the original activity of the cell and can provide high-purity, high-activity tumor cells for relevant clinical applications. It can be applied to various applications such as single-cell sequencing, whole gene sequencing, protein sequencing, new drug development, cancer biomarker research, individualized diagnosis, and individualized drug sensitivity testing. The above product applications need to be approved by the local regulatory authorities before they can be provided to customers.
| 11 |
Commercialization Preparation
We are committed to the development of microfluidic chips, reagents and detectors for capturing circulating tumor cells in blood. We have integrated various complex precision structures, dielectric detection and functional microfluidic biochips. Our devices include a variety of expensive semiconductor manufacturing and precision micro-manufacturing related equipment. We signed a research project equipment use contract with Taiwan Semiconductor Research Institute of National Applied Research Laboratories (“TSRI”) and used their semiconductor manufacturing equipment and precision micro-nano processing equipment for chip technology research and development such as concept presentation of each R&D process, cross-scale composite structure production, rapid wafer trial production, material testing, and thin film production.
As for the designed microfluidic detector, since 2020, we have developed various functional microfluidic biomedical testing devices for microfluidic modules, automation software, and hardware. We also designed, manufactured and processed an increasing number of key components of our testing devices. All of our products must go through three steps to receive the required clearance from the National Medical Products Administration of China (“NMPA”) before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. At the registration application stage, we have to assemble all the required application materials, complete clinical trials (if required by NMPA), and work with an NMPA accredited third-party organization to examine our products in accordance with NMPA rules. NMPA will review our application during the registration review period and may request additional information before officially approving or denying our applications. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; A+SCDrop, A+CellScan, A+CellScan Chip, and A+LCGuard are at the registration application stage; the four matching immunostaining kits are under registration review. As of the date of this prospectus, we have not applied for similar clearances from other jurisdictions.
Although none of the chips has been mass produced as of the date of this prospectus, we have been cooperating with the injection molding machine manufacturer Riva and the mold manufacturer Unimold to conduct pre-mass production trial test for our A+Pre Chip and AC-1000 Enrichment Chip. During the mass production trial test, we examine the following factors:
| · | whether the tested chip can work with its corresponding product, and |
| · | whether chips’ flatness, roughness, water leakage, critical size and thickness match the original design. |
A Chip is ready for mass-production if each individual production line can produce at least 2,500 pieces per month and pass the quality control examination. During the quality control examination, one chip out of every 48 chips will be randomly selected for verification and must achieve designed objectives when working with their corresponding products and meet all the product specifications to pass the examination. The product specifications are listed in the table below.
| Flatness | Roughness | No Water leakage | Critical size compared with original design | Thickness | |
| A+Pre Chip | ΔF < 0.1 mm | ΔR<1.5 um /mm2 | Under 2 bar | within 5% | 2 mm ±10% |
| AC-1000 Enrichment Chip | ΔF < 0.3 mm | ΔR < 1.5 um/mm2 | Under 2 bar | within 5% | 1.5 mm ±10% |
Upon completion of the pre-mass production trial test, all of the randomly selected A+Pre Chips and AC-1000 Enrichment Chips can work with their corresponding A+Pre and AC-1000 products and meet or exceed the production specifications.
One of every 48 chips was randomly selected and tested for performance verification by using A549 lung cancer cells spiked into human blood. By the end ofIn May 2023, a total of 20 A+Pre chips were tested, with a result of average tumor cell recovery rate of 94%, which exceeded the target rate of 90%, with the highest and lowest rates of 99% and 91.6%, respectively. The liquid and blood cells removal rate by A+Pre chip was between 90-92.5%, which is in line with the target rate of 90-94%. A total of 12 AC-1000 chips were examined and the average recovery rate is 79.5%, which exceeded the target rate of 75%, with the highest and lowest recovery rates of 88.6% and 76.8%, respectively. The blood cells depletion rate was >2.5 logs, exceeding the target rate of 2 logs, with the highest and lowest rate of 4 and 2.5 logs, respectively.
The specifications of the randomly selected A+Pre and AC-1000 chips produced during the pre-mass production trial test can be found in the table below:
| Flatness | Roughness | No Water leakage | Critical size compared with original design | Thickness | |
| A+Pre Chip | ΔF < 0.05 mm | ΔR<1.5 um /mm2 | Under 2 bar | within 3% | 2 mm ±5% |
| AC-1000 Enrichment Chip | ΔF < 0.08 mm | ΔR < 1 um/mm2 | Under 2 bar | within 5% | 1.5 mm ±10% |
Riva is a mold manufacturing and injection molding machine manufacturer. We have purchased a Riva injection molding machine at the end of October 2022 and completed the mass production trial test for AC-1000 chips, and the production samples met our product specifications. The special structure mold core we made combined with the Riva injection molding machine has repeatedly produced hundreds of pieces of AC-1000 chips. The quality of the products is consistent and meet the product specifications of the microstructure characteristics. The operation on the equipment has achieved the expected efficiency and cell enrichment efficiency. Each equipment is expected to produce 2,000 pieces AC-1000 chips per month.
Unimold is a mold manufacturer and an ISO9001 plastic injection molding foundry. We cooperate with Unimold to embed our specially manufactured mold core for A+Pre Chip into the mold cavity, which will be employed at our future production facility. Although there is no formal cooperation agreement between Unimold and us, Unimold produces custom-made molds for us on a make-to-order basis. Each time, we first discuss the mold specification with a Unimold representative, then receive an offer from Unimold with the price, and Unimold will produce the customized mold for us to test. To protect our intellectual property, Unimold and us entered into a Non-Disclosure Agreement (the “NDA”) with a term of three years. The NDA prohibits both parties from disclosing confidential information without authorization and hiring the other party’s employees during the term of the NDA or within two years of the termination of the NDA. The NDA is governed by the laws of Taiwan, and any disputes arising from the NDA must be resolved before Taiwan Tainan District Court. The chips produced by Unimold’s mold have met the specifications set by us, and a batch of small-scale trial production of 5,000 pieces was carried out in October 2022. Our A+Pre is operating to expected efficacy and performance specifications using the in-house produced A+Pre Chip. One injection-type production equipment is expected to produce 50,000 pieces of A+Pre Chip per month.
Our Platform
We have built our microfluidic technology platform to integrate research and development, design, and manufacture of biochips and microfluidic chips. The platform combines our patented chip technology and will enable localized operations of a variety of microfluidic chips, biosensors made by semiconductor fabrication technology, and integrated application patented technology. Each geographic territory will establish clean rooms for chip production in compliance with local regulations to meet local market demand. While performing its designed duties, our microfluidic technology platform can provide customized services to third parties for a fee. We envision providing services such as OEM production of microfluidic chips, micro-electromechanical components, biochips, sensors, and other components, customized product design, and commissioned development and research services to customers. Different from the general ICIntegrated Circuit (“IC”) wafer OEM production, our production is based on our micro-nano manufacturing technology platform developed by professionals in various fields that integrates cross-field knowledge, including Micro Electro Mechanical Systems (MEMS), lithography-assisted micromachining (LIGA), semiconductor process, and soft materials such as silicone gel and polydimethylsiloxane. We boast the ability to develop, design, and manufacture micro-electromechanical components and sensors, including three-dimensional microstructures or micro-optical-electromechanical integrated components. The material of the microstructure can be multi-layer stacking of alloys and insulating materials, which may be used in a wide range of fields, such as pressure or environment detection, MEMS oscillators, optical actuators, biomedical components, passive components, silicon optical integration platforms, and microfluidic structures.
| 12 |
The platform integrates our AC-1000 and A+SCDrop devices. Our rare cell enrichment device AC-1000 integrates the A+Pre chip and AC-1000 CTC enrichment chip. AC-1000 uses semiconductor nano ultra-sensitive biosensors and patented microfluidic chip technology to isolate rare cells with complete cell activity. It has great potential to be applied to routine liquid biopsy and companion diagnosis in the future. AC-1000 can satisfy different applications with corresponding special chip products. Our products also have the potential to provide application services in tumor screening, auxiliary diagnosis, treatment evaluation, prognosis evaluation, recurrence and metastasis detection, individualized medication guidance, and companion diagnosis. In terms of tumor screening, we have developed a complete set of service models for early screening of lung cancer, and plan to conduct large-scale clinical trials in March 2024. Our application services for the identification of CTCs have matured, and the identification of CTCs can be used for tumor screening, auxiliary diagnosis, and treatment evaluation, etc. Our CTCs single cell capture device A+SCDrop is used in combination with A+Pre and AC-1000. It preserves the original activity of single cells and polymer microfluidic chips combined with cell dielectric sensing technology. With the technical advantages combined with our IVD kit products, A+SCDrop can be used in a variety of applications, such as single-cell sequencing, whole gene sequencing, protein sequencing, new drug development, cancer biomarker research, individualized diagnosis, individualized drug susceptibility testing, and other aspects of individualized precision medicine. We are also working on prognosis assessment, recurrence and metastasis detection, individualized medicine, and companion diagnosis.
Although no services have been provided to customers yet, we believe the application services using our products and devices will be an essential part of our future operations.
Competitive Strengths
Although we have not received any regulatory approvals necessary to commercialize our products, we believe that the following competitive strengths enable us to compete effectively in and capitalize on the growing oncology detection market:
| · | Own microfluidic technology platform. We can quickly complete the product development and improvement we need on our own platform. |
| · | Proprietary Ultra-Sensitive Biosensor Technology. Our self-developed proprietary semiconductor nano ultra-sensitive biosensor technology integrates various composite precision structures, dielectric detection and functional microfluidic biochips to complete the microfluidic chips and reagents for capturing circulating tumor cells in a patient’s blood. Our technology also enables a fast and inexpensive method for early cancer diagnosis because we mainly rely on self-developed equipment and products, including the microfluidic chips and the related reagents. Specifically, with the start and expansion of mass-production, the cost and price of microfluidic chips will drop multiple times. Also, since we estimate that we will enrich all the targeted cells, the amount of reagents required in the process will decrease correspondingly. Additionally, we are able to complete the detection and analysis in a short period of time while ensuring the accuracy of the results and reducing the death number of targeted cells throughout the process by employing our microfluidic technology platform, which uses self-developed microfluidic chips to separate and detect CTCs. By using automatic and efficient microfluidic chips, we can reduce human errors when capturing, releasing, counting, and detecting CTCs. For example, AC-1000 and its corresponding chip can achieve high throughput (800-1000 drops/s) and high flow rate (>0.7ml/hr) while completely removing red blood cells in peripheral blood samples within 30 minutes. Due to the non-destructive nature of the rare cell enrichment system, it can maintain the original characteristics of the desired target cells through purely physical and high-purity enrichment processes, improving the accuracy of the results and reducing the number of target cells that die during the entire process. |
| · | On Track to Commercialization. All of our products must go through three steps to receive the required clearance from NMPA before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; A+SCDrop, A+CellScan, A+CellScan Chip, and A+LCGuard are at the registration application stage; the four matching immunostaining kits are under registration review. We expect to submit the A+CellScan, A+CellScan Chip and A+SCDrop’s registrations to the NMPA by the end of 2023. We have been cooperating with the injection molding machine manufacturer Riva Machinery Co., Ltd. (“Riva”) and the mold manufacturer Unimold Technology Inc. (“Unimold”) to conduct mass production mode testing and trial production. Although none of the chips has been mass-produced as of the date of this prospectus, we have purchased a Riva injection molding machine at the end of October 2022 and completed the mass production trial test of AC-1000 CTC Enrichment chip. We also cooperate with Unimold to embed our specially manufactured mold core for A+Pre Chip into the mold cavity made by Unimold. The chips produced have met the specifications set by us, and a batch of small-scale trial production of 5,000 pieces has been carried out in October 2022. Our equipment is operating to expected efficacy and performance specifications using in-house produced chips. Moreover, the prototype of A+CellScan and its corresponding chip have completed the performance study. You can find more information on page 74 under the heading “A+CellScan.” We are refining the production method of A+CellScan chip and
|
| · | Multi-Disciplinary Management Team. We have a multi-disciplinary management team, an R&D team and strategic cooperation units composed of interdisciplinary and cross-field professionals and well-known experts. The R&D team has the ability to combine semiconductor/integrated circuits and biomedical expertise. Our team has accumulated valuable experience from chip development and design, manufacturing, mass production, design and development of detectors, research and development of system modules, and the operation of clinical laboratory personnel. |
| 13 |
Growth Strategy
We will strive to be a leading provider of precision oncology detection solutions by the following growth strategies:
| ● | Increase the market penetration of our oncology auxiliary products and expand our product portfolio to actively focus on in vitro early diagnosis, rapid evaluation of chemotherapy drugs, individualized treatment including clinical screening of drugs, detection of drug resistance, and monitoring of tumor recurrences. |
| ● | Develop cancer screening market in China and further expand to other regions. We will initially focus on developing our cancer screening market in China. We also plan to expand to North American and European market by setting up subsidiaries and localize production and operation to meet specific market demand and compliance requirements in the relevant market. |
| ● | Expand our R&D to strength and develop pipeline products. In the future, we will actively promote the research and development, application and registration of other cancer early screening products |
Corporate History and Structure
We were incorporated in Nevada in July 2021 as a holding company. We started operations in 2014 through Advanced Biomed Taiwan as a research and development center for technology research and product development. In August 2021, we established Advanced Biomed HK to integrate market development and commercialization in the PRC. On January 1, 2022, Advanced Biomed HK acquired 100% equity interest of Shanghai Sglcell and its subsidiaries from its shareholders for a consideration of RMB 12 million. In March 2022, Advanced Biomed HK established Sglcell (Huangshan) Biotech Co., Ltd.
In July 2022, we consummated a reorganization pursuant to which we acquired 100% equity interest of Advanced Biomed Taiwan, making it our wholly owned subsidiary. On November 7, 2022, we obtained the approval of the Investment Commission of the Ministry of Economic Affairs (“Taiwan Investment Commission”) for the reorganization, the Issue No. of which is “經審一字第11100116890號”. Additionally, the Bureau of Economic Development of Tainan City Government has also approved the reorganization in accordance with the Taiwan Company Act on December 26, 2022.
On June 8, 2023, pursuant to the Share Transfer Agreement entered into by Ting Wang and Haifeng Zhang, who are independent third-party individuals, and Shanghai Sglcell on June 2, 2023, Shanghai Sglcell transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to Ting Wang and Haifeng Zhang at aggregate consideration of RMB500,000 (approximately US$72,780) without any other obligations arising from the transfers.
On June 9, 2023, pursuant to the Share Transfer Agreement entered into by Ting Wang and Haifeng Zhang, who are independent third-party individuals, and Shanghai Sglcell on May 31, 2023, Shanghai Sglcell transferred its wholly owned subsidiary, Shandong Sglcell Medical Devices Co., Ltd., to Ting Wang and Haifeng Zhang at zero consideration without any other obligations arising from the transfers.
On June 15, 2023, pursuant to the Share Transfer Agreement entered into by Quantum Capital (Hong Kong) Limited, who is an independent third-party corporation, and Advanced Biomed HK on June 9, 2023, Advanced Biomed HK transferred its wholly owned subsidiary, Sglcell (Huangshan) Biotech Co., Ltd., to Quantum Capital (Hong Kong) Limited at zero consideration without any other obligations arising from the transfers.
As of the date of this prospectus, our Shanghai subsidiary is the only operating entity we have in the PRC and owns all of our patents and some of our R&D equipment and patents.while the rest of our R&D equipment are owned by Advanced Biomed Inc. (Taiwan). Our Shanghai subsidiary will also be responsible for operations related to clinical trials in Mainland China through contract research organizations.CROs.
| 14 |
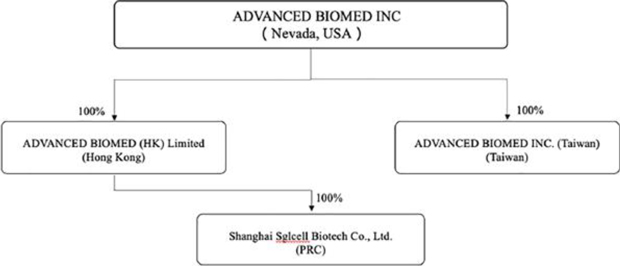
Corporate Structure
The chart below depicts the corporate structure of the Company as of the date of this prospectus.
Corporate Information
The Company was incorporated in the State of Nevada on July 16, 2021.
Our principal executive offices are located at No. 689-87, Xiaodong Rd., Yongkang Dist., Tainan City 710, Taiwan. Our telephone number is 886-6-3121716. We maintain a corporate website at www.advanbiomed.com. Information on our website, and any downloadable files found there, are not part of this prospectus and should not be relied upon with respect to this offering.
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”). An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies in the United States. These provisions include:
| · | a requirement to have only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure in this prospectus; |
| · | reduced executive compensation disclosure; and |
| · | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
We may choose to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
| 15 |
We may take advantage of these provisions for up to five years or such an earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.235 billion in annual revenue, have more than $700 million in the market value of our shares held by non-affiliates, or issue more than $1 billion of non-convertible debt over a three-year period.
Transfers of Cash to and from Our Subsidiaries
Cash may be transferred within our organization in the following manners: (i) Advanced Biomed may transfer funds to our subsidiaries, including our Shanghai subsidiary, by way of capital contributions or loans, through intermediate holding subsidiaries or otherwise; (ii) we and our intermediate holding subsidiaries may provide loans to our operating subsidiaries and vice versa; and (iii) our subsidiaries, including our Shanghai subsidiary, may make dividends or other distributions to us through intermediate holding companies or otherwise. As of the date of this prospectus, Advanced Biomed made seven capital contributions to Advanced Biomed Taiwan and Advanced Biomed HK to support their research and development.
| Date | Receiving Entity | Amount (US$) | ||
| June 29, 2022 | Advanced Biomed Taiwan | 2,500,000 | ||
| October 11, 2022 | Advanced Biomed Taiwan | 86,000 | ||
| October 24, 2022 | Advanced Biomed HK | 100,000 | ||
| October 26, 2022 | Advanced Biomed HK | 500,000 | ||
| November 7, 2022 | Advanced Biomed Taiwan | 122,000 | ||
| December 2, 2022 | Advanced Biomed HK | 110,000 | ||
| December 14, 2022 | Advanced Biomed Taiwan | 85,000 |
Other than the transfers in the table above, we have not made any distribution of dividends or assets, cash transfers, capital contributions or loans among the holding company or any of our subsidiaries. As advised by AllBright, our PRC counsel, PRC laws, regulations and judicial interpretations thereof do not prohibit using cash generated from one subsidiary to fund another subsidiary’s operations by way of short term interest free loans. In the future, cash proceeds raised from overseas financing activities, including this offering, may be transferred by us to our Taiwan and Hong Kong subsidiaries and Shanghai subsidiary via capital contributions or shareholder loans. As of the date of this prospectus, Advanced Biomed has not made dividend or other distributions to our shareholders. Advanced Biomed may pay dividends to our shareholders subject to our ability to service our debts as they become due and provided that our assets will exceed our liabilities after the payment of such dividends. As a holding company, Advanced Biomed may rely on dividends and other distributions on equity paid by our subsidiaries for our cash and liquidity requirements, including payment of any debt we may incur outside of China and our expenses. If any of our subsidiaries incurs debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to us. To the extent cash or assets in the business is in the PRC or athe Shanghai subsidiary, the cash or assets may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on our or our subsidiaries’ ability by the PRC government to transfer cash or assets or distribute earnings within our group or to U.S. investors. PRC laws and regulations applicable to our Shanghai subsidiary permit payments of dividends only out of their retained earnings, if any, determined in accordance with applicable accounting standards and regulations. Our Shanghai subsidiary may pay dividends only out of their respective accumulated after-tax profits as determined in accordance with PRC accounting standards and regulations. In addition, our subsidiaries are required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund certain statutory reserve funds, until the aggregate amount of such funds reaches 50% of its registered capital. At its discretion, a wholly foreign-owned enterprise may allocate a portion of its after-tax profits to discretionary funds. These reserve funds and discretionary funds are not distributable as cash dividends. Furthermore, dividends paid by our Shanghai subsidiary to theirits parent companies will be subject to a 10% withholding tax, which can be reduced to 5% if certain requirements are met. The PRC government also imposes restrictions on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. As such, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any, or transfer cash within our group, across border, or to U.S. investors. Additionally, current Taiwan regulations only permit our Taiwan subsidiary to pay dividends to its shareholders out of its accumulated profits, and Advanced Biomed Taiwan must set aside at least 10% of its accumulated profits each year and use it to make up previous losses, if any. The statutory reserve cannot be distributed as cash dividends. As of the date of this prospectus, no dividends, transfers, or distributions have been made within our group or to shareholders. We presently intend to retain all earnings to fund our operations and business expansions and have no plan to distribute earnings to shareholders. We do not anticipate paying dividends or other distributions to our shareholders, including U.S. investors, in the foreseeable future. See the relevant discussions in “Risk Factors — Risks Related to Doing Business in China” on page 39; “Risk Factors — PRC regulation on loans to, and direct investment in, PRC entities by offshore holding companies and governmental control in currency conversion may delay or prevent us from making loans to or making additional capital contributions to our Shanghai subsidiary” on page 43; “Risk Factors — Our Shanghai subsidiary are subject to restrictions on paying dividends or making other payments to us, which may restrict our ability to satisfy our liquidity requirements in the future” on page 43; and “Risk Factors — Advanced Biomed Taiwan is subject to restrictions on paying dividend or making other payments to us, which may restrict our ability to satisfy its liquidity requirements” on page 37
Risk Factor Summary
Risks Related to Our Business and Industry
|
Risks Associated with Government Regulations
Risks Associated with Our Testing and the Manufacture and Supply of Our Products
Risks Relating to Our Financial Prospects and Need for Additional Capital
Risks Related to Doing Business in Taiwan
Risks Related to Doing Business in China
Risks Related to this Offering
|
Although the majority of our operations are not conducted in Mainland China, we face various legal and operational risks and uncertainties relating to our Shanghai subsidiary, Shanghai Sglcell Biotech Co., Ltd., and similar legal and operational risks and uncertainties also apply to our holding company in Hong Kong. The Chinese government may intervene or influence the operation of our Shanghai subsidiary and Advanced Biomed HK and exercise significant oversight and discretion over the conduct of their business and may intervene in or influence their operations at any time, or may exert more control over securities offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and/or the value of our common stock. Further, any actions by the Chinese government to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.
Recently, the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, adopting new measures to extend the scope of cybersecurity reviews, and expanding the efforts in anti-monopoly enforcement. As advised by our PRC counsel, AllBright, we do not believe that we are directly subject to these regulatory actions or statements, as our Shanghai subsidiary does not have a VIE structure and their operations are not subject to cybersecurity review requirements, or involve any type of restricted industry. Because these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative rule making bodies in China will respond to them, or what existing or new laws or regulations will be modified or promulgated, if any, or the potential impact such modified or new laws and regulations will have on our subsidiaries’ daily business operations or ability to accept foreign investments and list on an U.S. exchange. In On December 24, 2021, nine government agencies jointly issued the Opinions on Promoting the Healthy and Sustainable Development of Platform Economy, which provides that, among others, monopolistic agreements, abuse of dominant market position and illegal concentration of business operators in the field of platform economy will be strictly investigated and punished in accordance with the relevant laws. We do not hold a dominant market position in our product markets and we have not entered into any monopolistic agreement. We have not received any inquiry from the relevant governmental authorities. the Cyberspace Administration of China (“CAC”), together with 12 other Chinese regulatory authorities, released the final version of the Revised Measures for Cybersecurity Review, or the Revised Cybersecurity Measures, in December 2021, which took effect on February 15, 2022. Pursuant to the Revised Cybersecurity Measures, critical information infrastructure operators procuring network products and services and online platform operators carrying out data processing activities, which affect or may affect national security, shall conduct a cybersecurity review pursuant to the provisions therein. In addition, online platform operators possessing personal information of more than one million users seeking to be listed on foreign stock markets must apply for a cybersecurity review. The Revised Cybersecurity Measures apply to companies going abroad for secondary listing, dual primary listing and other new foreign listings and subject to the reporting requirements. On November 14, 2021, the CAC published the Draft Regulations on the Network Data Security Administration (Draft for Comments) (the “Security Administration Draft”), which provides that data processing operators engaging in data processing activities that affect or may affect national security must be subject to cybersecurity review by the relevant Cyberspace Administration of the PRC. As advised by AllBright, we do not believe that we are an “online platform operator” within the meaning of the Revised Cybersecurity Measures, and, we currently do not have over one million users’ personal information and do not anticipate that we will be collecting over one million users’ personal information in the foreseeable future. In addition, we are also not subject to Security Administration Draft if the Security Administration Draft are enacted as proposed, since we currently do not collect data that affects or may affect national security and we do not anticipate that we will be collecting data that affects or may affect national security in the foreseeable future.
On December 24, 2021, China Securities Regulatory Commission (the “CSRC”) issued the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing of Securities by Domestic Enterprises (the “Draft Administrative Provisions”) and the Measures for the Overseas Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Draft Filing Measures”), collectively, the Draft Overseas Listing Rules. On February 17, 2023, the CSRC released Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies with five interpretive guidelines (the “Trial Measures”) which came into effect on March 31, 2023. Pursuant to the Trial Measures, a PRC domestic company that seeks to offer and list securities in overseas markets, either in direct or indirect overseas offering, shall fulfill the filing procedure with the CSRC as per requirement of the Trial Measures, submit relevant materials that contain a filing report and a legal opinion, and provide truthful, accurate and complete information on the shareholder and etc. Direct overseas offering and listing by domestic companies refers to such overseas offering and listing by a joint-stock company incorporated domestically. Any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect offering and listing in overseas market and, therefore, be subject to filing requirement: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in the Mainland China, or its main places of business are located in the Mainland China, or the senior managers in charge of its business operation and management are mostly Chinese citizens or domiciled in the Mainland China. The determination as to whether or not an overseas offering and listing by domestic companies is indirect, shall be made on substance over form basis. As of the date of this prospectus, we do not believe that we are required to obtain the approval from or complete the filing with the CSRC for this offering and thus we have not submitted an application for approval for this offering with the CSRC pursuant to the Trial Measures based on the fact that we do not meet the explicit conditions set out in the Trial Measures to determine whether an overseas offering shall be deemed as an indirect overseas offering and listing by a domestic company. However, as the Trial Measures was newly published, there are substantial uncertainties as to the implementation and interpretation, and the CSRC may take a view that is contrary to our understanding of the Trial Measures. If we are required by the CSRC to submit and complete the filing procedures of this offering and listing, we cannot assure you that we will be able to complete such filings in a timely manner, or even at all. Any failure by us to comply with such filing requirements under the Trial Measures may result in rectification, warnings, and a fine between RMB 1 million and RMB 10 million on our Shanghai subsidiary, which could adversely and materially affect our business operations and financial outlook, and significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or such shares to become worthless.
As of the date of this prospectus, these new laws and guidelines have not impacted our ability to conduct its business, accept foreign investments, or continue to list on a U.S. or other foreign exchange; however, if (i) we inadvertently conclude that permissions or approvals are not required from applicable PRC authorities, (ii) applicable laws, regulations, or interpretations change, and we are required to obtain such permissions or approvals in the future, or (iii) we fail to file or were denied permission from the PRC authorities to this offering, any follow-up offerings or transactions, our ability to conduct our business may be materially impacted, and we will not be able to continue listing on any U.S. exchange, continue to offer securities to investors, the interest of the investors may be materially adversely affected and our common stock may significantly decrease in value or become worthless. To date, there are uncertainties in the interpretation and enforcement of these new laws and guidelines, which could materially and adversely impact our business and financial outlook and may impact our ability to accept foreign investments, or continue to list on a U.S. or other foreign exchange. See “Risk Factors — The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale”; “Risk Factors — If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us, such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless”; and “Risk Factors — The M&A Rules and certain PRC regulations may make it more difficult for us to pursue growth through acquisitions.”
Prospectus Conventions
Except where the context otherwise requires and for purposes of this prospectus only:
| ||
Unless otherwise noted, all translations from NTD to U.S. dollars and from U.S. dollars to RMB in this prospectus are made at NTD29.74 to US$1.00, the exchange rate set forth in the H.10 statistical release of the Federal Reserve Board on June 30, 2022. We make no representation that any NTD or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, the rates stated below, or at all. On June 30, 2022, the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Board for New Taiwan Dollar was NTD29.74 to US$1.00.
The industry in which we operate is subject to a high degree of uncertainty and risk due to variety of factors, including those described in the “Risk Factors” section. These and other factors could cause results to differ materially from those expressed in these publications and reports.
THE OFFERING
| ||
| ||
(1) The number of shares of common stock to be outstanding immediately after this offering is based upon 100,000,000 shares outstanding as of the date of this prospectus.
(2) Except as otherwise indicated, the number of shares of common stock presented in this prospectus excludes shares of our common stock issuable if the underwriters exercise their over-allotment option and shares of our common stock underlying warrants to be issued to the representative of the underwriters in connection with this offering.
SUMMARY CONSOLIDATED FINANCIAL DATA
The following selected historical statements of operations for the fiscal years ended June 30, 2022 and 2021 and nine months period ended March 31, 2023 and 2022, and balance sheet data as of June 30, 2022 and 2021 and March 31, 2023 have been derived from our audited consolidated financial statements for those periods included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. You should read this data together with our consolidated financial statements and related notes appearing elsewhere in this prospectus as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” appearing elsewhere in the prospectus.
Selected Consolidated Results of Operations Data
| For the years ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| US$ | US$ | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (1,619,531 | ) | (1,734,620 | ) | ||||
| General and administrative expenses | (754,840 | ) | (102,320 | ) | ||||
| Impairment of intangible assets | (1,617,974 | ) | - | |||||
| Total operating expenses | (3,992,345 | ) | (1,836,940 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 243 | 28 | ||||||
| Other expense, net | (35,620 | ) | (246 | ) | ||||
| Total other expense, net | (35,377 | ) | (218 | ) | ||||
| Income before tax expense | (4,027,722 | ) | (1,837,158 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | (4,027,722 | ) | (1,837,158 | ) | ||||
| For the nine-month periods ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (895,011 | ) | (1,045,243 | ) | ||||
| General and administrative expenses | (1,157,047 | ) | (246,606 | ) | ||||
| Total operating expenses | (2,052,058 | ) | (1,291,849 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 122 | 301 | ||||||
| Other expense, net | (269 | ) | (37,300 | ) | ||||
| Total other expense, net | (147 | ) | (36,999 | ) | ||||
| Income before tax expense | ||||||||
| Income tax expense | - | - | ||||||
| Net loss | (2,052,205 | ) | (1,328,848 | ) | ||||
Selected Consolidated Balance Sheet Data
| As of June 30, | ||||||||
| 2022 | 2021 | |||||||
| US$ | US$ | |||||||
| Cash | 4,783,864 | 65,922 | ||||||
| Working capital | 2,015,999 | 885,147 | ||||||
| Total assets | 6,713,499 | 1,006,373 | ||||||
| Total liabilities | 3,221,903 | 30,131 | ||||||
| Total stockholders’ equity | 3,491,596 | 976,242 | ||||||
| As of March 31, 2023 | As of June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Cash | 2,529,498 | 4,783,864 | ||||||
| Working capital | 1,026,004 | 2,015,999 | ||||||
| Total assets | 4,416,783 | 6,713,499 | ||||||
| Total liabilities | 3,031,645 | 3,221,903 | ||||||
| Total stockholders’ equity | 2,385,138 | 3,491,596 | ||||||
Investing in our securities involves a high degree of risk. Before making any investment decision, you should consider carefully the following risks and other information in this prospectus, including our consolidated financial statements and related notes. The risks and uncertainties we describe are not the only ones facing us. Additional risks and uncertainties that we are unaware of or that we believe are not material at the time could also materially adversely affect our business, financial condition or results of operations. In any case, the value of our common stock could decline, and you could lose all or part of your investment. Please also see the section entitled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to Our Business and Industry
We are an early stage cancer diagnostics company with a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are at the early stage of commercializing our business. We have not started manufacturing or sales of any products or services. Our limited operating history may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty.
Taiwan and China’s precision oncology detection market is still in its early stage of development and rapidly evolving, and companies operating in this industry face a variety of risks. We may not have sufficient experience or resources to address risks frequently encountered in this industry, which include, among other things, our potential failure to:
We may not be able to develop or commercialize early cancer detection products and |
If we are unsuccessful in addressing any one or moreour results of operations may suffer” on page 26; “As of the date of this prospectus, we have not conducted any clinical trials for our products. Uncertainties or failures in clinical trials of these risks, our business, financial condition and results of operations could be adversely affected.
We may be unable to develop and commercialize our early cancer detection devices, chips or immunochromogenic kits on a timely basis, or at all.
We are developing early cancer detection products and may develop and commercialize our cancer diagnostics products from time to time in the future. Developing early cancer detection and new cancer diagnostics products is a lengthy and complex process. New products may take time to commercialize, and their launch could be delayed or may not be successful.
Our product development process involves various risks, and we may not be able to develop and commercialize any early cancer detection products or new precision oncology detection products on a timely basis, or at all. A product candidate that appears promising in the early phases of development may fail to reach the market for a number of reasons. For example:
| our product candidates could materially and adversely affect our business operations” on page 27; and “We are an early stage cancer diagnostics company with a limited operating history, which may |
In addition, our competitors may develop and commercialize competing products faster than we are able to, in which case our results of operations could be adversely affected.
If we fail to keep up with industry and technology developments in a timely and cost-effective manner, we may be unable to compete effectively and our business and prospects could suffer.
Cancer early detection market is characterized by rapid changes, including technological and scientific breakthroughs, increasing amounts of data, frequent introductions of new tests, constant emergence of alternative diagnostic methods, and evolving industry standards. If we are not able to keep pace with these advances and increased customer expectations as a result of these advances and capture new market opportunities that develop as a result of these advances, our proprietary technologies could be rendered obsolete, our existing products and services and products and services we are developing could be rendered less clinically effective, and our future operations and prospects could suffer. To remain competitive, we must continuously upgrade our existing products and services and launch new products and services, to keep pace with these developments. We cannot assure you that these efforts will be successful.
In addition, we must expend significant resources in order to continuously upgrade our existing products and services or launch new ones to keep pace with industry and technological advances. We may never realize a return on investment on these efforts, especially if the improved or new products and services fail to perform as expected, in which case our business, financial condition and results of operations could be adversely affected.
If our products or services do not perform as expected, our operating results, reputation and business could suffer.
Our success depends on the market confidence that we can provide reliable, high-quality cancer early detection products and services that will provide physicians with real-time clinically actionable diagnostic information. However, there is no assurance that our products and services will perform as expected at all times. Our tests may fail to accurately detect gene variants or incompletely or incorrectly identify the significance of genomic alterations, or contain other errors or mistakes due to a variety of reasons (such as malfunction of our laboratory equipment and degraded liquid biopsy or tissue samples provided by our delivery service providers), which may result in negative perception of our tests. In addition, inaccurate results or misunderstandings of, or inappropriate reliance on, the diagnostic information our tests provide could lead to, or be associated with, side effects or adverse events in patients who use our tests, including treatment-related death, and could lead to termination of our services or claims against us.
We face risks related to natural disasters, health epidemics, civil and social disruption and other outbreaks, which could significantly disrupt our operations. In particular, the COVID-19 outbreak in China and worldwide has adversely affected, and may continue to adversely affect, our business, results of operations and financial condition.
We are vulnerable to social and natural catastrophic events that are beyond our control, such as natural disasters, health epidemics, and other catastrophes, which may materially and adversely affect our business. Since December 2019, a novel strain of coronavirus, or COVID-19, has become widespread in China and around the world. In March 2020, the World Health Organization declared COVID-19 a pandemic, given its threat beyond a public health emergency of international concern that the organization had declared in January 2020. Since the beginning of 2020, China, Taiwan and many other countries have taken various restrictive measures to contain the virus’ spread, such as quarantines, travel restrictions and home office policies to avoid large-scale population movement and gathering. These restrictions had certain degree of impact on our research and development and production plan in Taiwan and China. COVID-19 has reduced the availability of labor and led to difficulties for employees to return to work and constraints of service chain interruption. Because of the uncertainty surrounding the COVID-19 outbreak, the business disruption and the related financial impact related to the outbreak of and response to the coronavirus cannot be reasonably estimated at this time.
Recently, the COVID-19 situation has improved in Taiwan and China. With Taiwan relaxing its mask mandate on April 17, 2023, and China no longer enforcing the “zero-COVID” policy, our research and development have started to recover. However, the potential downturn brought by the COVID-19 outbreak is difficult to assess or predict and the full impact of the virus on our operations will depend on many factors beyond our control. The extent to which the COVID-19 outbreak impacts our business, results of operations and financial condition remains uncertain, and we are closely monitoring its impact on us. Our business, results of operations, financial conditions and prospects could be materially adversely affected to the extent that COVID-19 harms the Chinese and global economy in general, and the trading price of our shares of common stock may be adversely affected. To the extent the COVID-19 pandemic and the outbreak of other health epidemics adversely affect our business and financial results, they may also have the effect of heightening many of the other risks described in this “Risk Factors” section.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
Cancer early detection and screening market has become increasingly competitive, and we expect this competition to intensify further in the future. Some of our existing and potential future competitors may have longer operating histories, larger customer bases, more expansive brand recognition and deeper market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities, and more favorable terms from suppliers. As a result, they may be able to respond more quickly to changes in customer requirements or preferences, develop faster, better and more expansive advancements for their technologies and tests, create and implement more successful strategies for the promotion and sale of their tests, adopt more aggressive pricing policies for their tests, secure supplies from vendors on more favorable terms or devote substantially more resources to infrastructure and system development. In addition, competitors may be acquired by, receive investments from or enter into other commercial relationships with larger, well-established and well-financed companies as the use of precision oncology detection increases. In addition, if we expand into international markets in the future, we could face competition from the companies in these markets.
If we are unable to compete successfully with current and future competitors for these or any other reasons, we may be unable to increase market acceptance and sales volume of our tests, which could prevent us from maintaining or increasing our revenue levels or achieving or sustaining profitability or could otherwise negatively affect our performance.
Our future success depends on our ability to promote our brand and protect our reputation. If we are unable to effectively promote our brand, our business may be adversely affected.
We believe that enhancing and maintaining awareness of our trademarks欣戈赛and sglcell is critical to achieving widespread acceptance of our microfluidic biochip products, gaining trust for our testing devices and attracting customers. Successful promotion of our brand depends largely on the quality of the products and services we offer and the effectiveness of our branding and marketing efforts. Currently, we rely primarily on our own sales and marketing team to promote our brand and our precision oncology detection products and testing services. We expect that our branding and marketing efforts will require us to incur significant expenses and devote substantial resources. We cannot guarantee that our sales and marketing efforts will be successful. Brand promotion activities may not lead to increased revenue in the near term, and, even if they do, any revenue increases may not offset the expenses we incur to promote our brand. Our failure to establish and promote our brand and any damage to our reputation will hinder our growth. In addition, our reputation may be undermined as a result of the negative publicity about our company or our industry in general. If precision oncology detection products or services provided by us or our competitors do not perform to customers’ expectations, it may result in lower confidence in precision oncology detection in general, which may in turn impair our operating results and our reputation. You can find more information regarding our intellectual properties in “Business – Intellectual Property.”
Failure to attract and retain our senior management and other key employees could adversely affect our business.
Our future success is significantly dependent upon the continued service of our senior management, such as Dr. Yi Lu, our chairman of the board of directors and chief executive officer. If we lose their services, we may not be able to locate suitable or qualified replacements, and we may incur additional expenses to recruit new senior management team members, which could severely disrupt our business and growth. In addition, if these personnel join our competitors or form a competing business, our business and prospects could be adversely affected.
Our research and development activities and laboratory operations depend upon our ability to attract and retain highly skilled scientists and technicians. We are also in strong need of sales and marketing personnel with the relevant technology background and industry expertise in order to effectively conduct our sales and marketing activities and increase our hospital network. We face intense competition for qualified individuals from numerous biotechnology and pharmaceutical companies, universities, governmental entities and other research institutions. We may be unable to attract and retain suitably qualified individuals, and our failure to do so could adversely affect our business.
If we experience difficulties in recruiting subjects for clinical trials, our clinical development activities may be delayed or otherwise adversely affected.
Completion of a clinical trial on time according to the trial plan depends, among other things, on our ability to recruit a sufficient number of subjects to participate in the trial until the end of the trial. We may encounter difficulties in recruiting subjects for clinical trials for a variety of reasons, including the base and nature of the subject population and the eligibility criteria for subjects defined in the trial plan. Our clinical trials will likely compete with other clinical trials, which will reduce the number and categories of subjects we can recruit, as some subjects who may choose to participate in our trials may instead choose to participate in trials conducted by other companies. Due to the limited number of qualified clinical investigators and clinical trial sites, we expect that some of our clinical trials will be conducted at the same clinical trial sites used by some of our competitors, which will result in subjects eligible to participate in our clinical trials at those clinical trial sites decrease in the number of people. Even if our clinical trials are able to enroll a sufficient number of subjects, delays in enrolling subjects may increase costs or affect the timing or outcomes of planned clinical trials, hindering the completion of those trials and hindering our progress adversely affect the ability of product candidates to develop.
If we do not launch new products in a timely manner, our products may become obsolete and our results of operations may suffer.
Technology in the cancer screening industry is constantly changing, new products are emerging, and industry standards continue to evolve. If new and improved products are not introduced in a timely manner, our products may become technologically obsolete or vulnerable to competition, and our revenue and results of operations could be impaired. Even if we develop new or improved products, our ability to bring those products to market may be limited by a variety of factors, including regulatory approvals and market demand. We invest significant financial and other resources in our research and development activities. The research and development process are lengthy and uncertain. The products we are currently developing may not be able to complete the development process or obtain the regulatory or other approvals required to bring such products to market in a timely manner or at all.
Technological innovations often require significant time and investment before their commercial viability can be determined. We may not have the necessary financial resources to fund all such projects. In addition, even if we are able to successfully develop new or improved products, those products may not generate revenue in excess of development costs or achieve desirable financial returns and may become obsolete or due to changes in customer preferences or the introduction of advanced technology or functionality by competitors. products or other factors resulting in a decline in competitiveness.
As of the date of this prospectus, we have not conducted any clinical trials for our products. Uncertainties or failures in clinical trials of our product candidates could materially and adversely affect our business operations.
We must conduct various clinical trials to demonstrate the sensitivity and specificity of our test prior to regulatory approval for marketing of our product candidates and depending on the type of our relevant product candidates, clinical trials may require large-scale prospective clinical studies, and such studies are far more rigorous and expensive than other existing tests or auxiliary diagnostic products. We have planned to conduct a multicenter registry trial in China to evaluate the performance of the pulmonary nodule benign and malignant determination kit. We may encounter a variety of contingencies while conducting or as a result of clinical trials that may delay or prevent us from obtaining regulatory approval or commercializing our product candidates, including but not limited to:
-Regulatory agencies, institutional review boards or ethics committees may refuse to authorize us or investigators to conduct clinical trials or conduct clinical trials at the intended trial site;
-We are unable to reach agreement on admissible terms with prospective contract research institutions and hospitals (as trial centers), which may require extensive negotiation and may vary significantly between different contract research institutions and hospitals (as trial centers);
-Production issues, including issues with the quality of production, supply, or obtaining sufficient quantities of the product candidate for use in clinical trials;
-Insufficient testing capacity to meet the needs of clinical trials;
-Our products fail to demonstrate superior results than competitors' or alternative products (if applicable);
-Clinical trials of our product candidates may fail to demonstrate the expected cancer screening sensitivity and specificity, and we may decide or regulatory authorities may require us to conduct additional clinical trials or abandon product development programs;
-The number of subjects required for clinical trials of our product candidates may be larger than expected, recruitment may be insufficient or slower than we expect, or the rate of subjects dropping out of trials may be higher than expected;
-Our third-party contractors may fail to comply with regulatory requirements or perform in a timely manner or at all of their contractual obligations to us;
-We may have to suspend or terminate clinical trials of our product candidates for a variety of reasons, including the discovery of a lack of clinical response or other unexpected characteristics; and preliminary or interim results of clinical trials may not be indicative of final results.
There is no assurance that such trials will be completed in a timely or cost-effective manner, or that they will result in a commercially viable product. If clinical trials of any of our product candidates are delayed or terminated, the commercial prospects of that product candidate will be impaired and our ability to generate revenue from any such product candidate will be delayed. In addition, any delay in the completion of our clinical trials would increase our costs, slow down the development and approval process of our product candidate, and impair our ability to initiate product sales and generate related revenue for that product candidate. The occurrence of any of the above events could materially damage our business, financial condition and prospects.
We have contracts with third parties for the future manufacturing, supply and registration applications of certain of our products, which supply could become limited or interrupted or may not be of satisfactory quality and quantity.
We currently rely on third parties in the PRC for certain supplying, registration applications and future manufacturing of certain of our products. Any such reliance may increase the risk that we will not have sufficient quantities of our products, or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts. Furthermore, all the contract manufacturers in the PRC for our products are subject to extensive PRC laws and regulations. These PRC laws and regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants, or to inadvertent changes in the properties or stability of our products.
Risks Associated with Government Regulations
We may be adversely affected by uncertainties and changes in China's regulations on the cancer screening industry, and the lack of necessary approvals, licenses, registrations or filings in relation to our business may adversely affect our business, results of operations and prospects material adverse effects.
Due to the relatively short history of the cancer screening industry in China, a comprehensive regulatory framework to regulate the industry has not yet been established. The possibility cannot be ruled out that certain industry practices, which we have also adopted, may be deemed to fail to fully comply with the existing laws and regulations in the PRC. As China's laws and regulations on medical devices are still developing, and it is uncertain whether new laws, regulations or interpretations will be promulgated or adopted in the future, we cannot assure you that our early stage cancer detection devices will not be construed as non-compliance with applicable laws and regulations in the future. If the PRC government issues clear requirements for the approval of our devices, we intend to take the necessary steps to comply with those requirements. Our business and results of operations may be adversely affected if we fail to comply with existing or future requirements or are found to be otherwise non-compliant in the conduct of our business.
If we fail to obtain, complete or maintain the required regulatory approvals, licenses, registrations or filings, or if there is a delay in obtaining, complete or maintain the required regulatory approvals, licenses, registrations or filings, we will not be able to commercialize our product candidates change, and our ability to generate revenue will be significantly compromised.
Due to the relatively short history of the cancer screening industry in China, a comprehensive regulatory framework to regulate the industry has not yet been established. However, the significant aspects of the development and commercialization of our products have been strictly regulated in China. The process of obtaining regulatory approvals and complying with applicable laws and regulations requires significant time and financial resources. Applicants may face administrative or judicial sanctions if they fail to comply with applicable regulations during the product development process, the approval process, or at any time after approval. Such sanctions may include regulatory refusals to approve pending applications, revocation of approvals, revocation of licenses, clinical restrictions, voluntary or compulsory product recalls, product seizures, suspensions of production or distribution in whole or in part, injunctions, fines, refusals to government contracts, compensation, Hand over money or civil or criminal penalties. Failure to comply with these regulations may affect our business, financial business conditions and prospects are materially and adversely affected.
Before obtaining regulatory approval for commercial sale of some of our products, we must demonstrate its effectiveness in a well-controlled clinical trial and, for approval in China, must be satisfied by NMPA that the product candidate is safe and effective for its intended use, and appropriate manufacturing and testing facilities, procedures and controls are in place. For now, only A+LCGuard is required by NMPA to complete clinical trial. Obtaining regulatory approval is a long, expensive and uncertain process, and approval may not be obtained. When we submit a registration application to NMPA, it will decide whether to accept or reject the submitted registration application. We cannot be certain that any submission will be approved by NMPA for registration review. NMPA may also slow down, suspend or terminate the review of our application, and any such circumstance will prolong the registration process for our products. Our product candidates may not receive regulatory approval for a number of reasons, including:
Regulatory requirements and guidelines may also change, and we may, among other things, need to revise clinical trial plans submitted to applicable regulatory authorities to reflect such changes. We may make revisions that necessitate resubmission of the clinical trial proposal to the Institutional Review Board or Ethics Committee for reconsideration, which may affect the outcome of the clinical trial.
The process of developing, obtaining regulatory approval, and commercializing medical device candidates is long, complex, and costly, both within China and abroad. Even if our product candidates are successful in obtaining regulatory approval, such approval may impose significant restrictions on the approved use, or require product labels to contain precautions or warnings, or require expensive and time-consuming post-approval clinical trials or surveillance as approval conditions of. If we are unable to obtain regulatory approvals for our product candidates in one or more jurisdictions, or any approvals come with significant restrictions, our target market will be reduced and our ability to realize the full market potential of our product candidates will be impaired. In addition, we may not be able to obtain sufficient financing or generate sufficient revenue and cash flow to continue developing any other product candidates in the future.
Our products and any future products will be subject to ongoing regulatory obligations and ongoing regulatory scrutiny, which may result in significant additional expenses, and if we fail to comply with regulatory requirements or if there are unexpected issues with our products and/or product candidates, we may will be punished.
Our regulatory agency-approved testing services, products and any other product candidates are subject to and will be subject to compliance with manufacturing, testing, labelling, packaging, storage, advertising, promotion, sampling, record keeping, post-marketing studies, submission of safety, efficacy and other ongoing regulatory requirements for post-listing materials, and other requirements from regulatory authorities in the PRC and/or other jurisdictions. Our testing and manufacturing facilities are subject to numerous regulatory requirements from the China Food and Drug Administration (“CFDA”) and/or other similar authorities. Accordingly, we have been and will be subject to ongoing scrutiny and inspection by regulatory agencies to assess our compliance with applicable laws and regulations and our commitments made in any application materials submitted to the CFDA or other authorities. Therefore, we must continue to invest time, money and effort in all aspects of regulatory compliance.
Regulatory approvals for our products and any approvals we obtain for product candidates are subject to and may be limited by the use of our marketable products. Our approvals may also be subject to other conditions that may result in the need for potentially costly post-market testing and surveillance to monitor the safety and efficacy of our products or product candidates. Such restrictions and conditions may adversely affect the commercial potential of our products.
After our product candidates are approved for commercialization, certain changes to the products, such as changes in manufacturing processes and additional labeling claims, may require additional review and approval by the CFDA and/or similar regulatory authorities. Regulatory approval of any of our product candidates may also be withdrawn. If we fail to maintain compliance with these ongoing regulatory requirements or if problems arise after the product is launched, the CFDA or similar regulatory authorities may seek to enforce a consent order or withdraw marketing approval. Late discovery of our products or product candidates or issues previously unknown to us in our manufacturing process may lead to revisions to approved labels or regulations to add new safety information; conduct clinical studies to evaluate new safety risk; or impose distribution restrictions or other restrictions. Other potential consequences include (among others):
The CFDA and other regulatory authorities strictly supervise the listing, labelling, advertising and promotion of medical products and services introduced to the market. Our products and testing services may only be marketed for their approved uses in accordance with approved labels. The CFDA and other regulatory authorities actively enforce laws and regulations that prohibit the promotion of off-label uses, and companies found to improperly promote off-label uses may bear significant responsibility. The policies of the CFDA and other regulatory authorities may change and other government regulations may be issued to prevent, limit or delay regulatory approval of our product candidates. Given the changing regulatory environment, we cannot predict the likelihood, nature or scope (whether in China or abroad) of government policies or regulations that may result from future legislative or administrative actions. If we are slow or unable to adapt to changes in existing regulations or adopt new regulations or policies, or if we are unable to maintain regulatory compliance, we may lose any regulatory approvals we have obtained and may not be able to achieve or maintain profitability.
If our existing and new products fail to meet the quality standards required by applicable laws, our business and reputation could be damaged, and our revenue and profitability could be materially and adversely affected.
Our production and manufacturing processes are subject to certain quality standards. We have established quality control and assurance systems and adopted standardized operating procedures to prevent quality issues related to our products and operating processes. For further details of our quality control and assurance system, please refer to “Business — Quality Control System”. Although we have quality control and assurance systems and procedures, we cannot eliminate the risk of product defects or malfunctions. Quality defects may not be detected or remedied due to a number of factors, many of which are beyond our control, including:
In addition, our failure to detect quality defects in our products or to prevent such defective products from being delivered to end users may result in injury or death, product recalls or withdrawals, revocation of licenses or fines by regulatory agencies, product and professional liability or other problems, which could seriously damage our reputation and business, expose us to the risk of liability, and materially and adversely affect our revenue and profitability.
In addition to the Chinese market, we are planning to expand to North American and European markets. We cannot give any assurance that any of our products will receive regulatory approval in North America or Europe, which is necessary before they can be commercialized.
We cannot be certain that any of our product candidates will be successful in clinical studies or receive regulatory approval. Applications for our products could fail to receive regulatory approval for many reasons, including but not limited to the following:
| · |
Currently, we plan to seek regulatory approval to commercialize our product candidates in the U.S., the EU and in additional countries where we obtain commercial and IP rights. To obtain regulatory approval in other countries, we must comply with numerous and varying regulatory requirements of such other countries regarding safety, efficacy, chemistry, manufacturing and controls, clinical studies, commercial sales, pricing, marketing and distribution of our products. Even if we are successful in obtaining approval in one jurisdiction, we cannot ensure that we will obtain approval in any other jurisdictions. Failure to obtain marketing authorization for our products will result in our being unable to market and sell such products, which would materially adversely affect our business, financial condition and results of operations. If we fail to obtain approval in any jurisdiction, the geographic market for our products could be limited.
Failure to obtain the broad market acceptance or maintain a good reputation necessary for our current products and any future products could materially and adversely affect our results of operations and profitability.
The commercial success of our current and future products depends on their market acceptance, especially among customers, hospitals and physicians. As a diagnostic method recently developed and introduced into the Chinese market, our products may not be widely recognized by the intended target customers, doctors or end users. If our products and any future approved product candidates fail to gain sufficient market acceptance from physicians, end users, third-party payers and other industry players, our product sales will be adversely affected. In addition, customers, physicians, end users and third-party payers may prefer other new products over our products. If our products and product candidates fail to achieve an adequate level of recognition, we may not generate significant revenue and may not be profitable. The failure of our products, product candidates and our testing services to achieve an adequate level of recognition or to increase market visibility could adversely affect our financial condition, business and results of operations. Once approved for commercial sale, the market acceptance of our products and product candidates and their services will depend on a number of factors, including:
If any of our commercialized products and services fail to gain market acceptance among doctors, end users, hospitals or other customers, or if we fail to maintain good relationships with them, we will not be able to generate significant revenue. Our ability to market our products and product candidates may be limited by regulatory approval requirements, restrictions on approved uses, inherent patterns of clinical practice, uncertainty about third-party compensation or other factors. Even if our products gain market acceptance, we may not be able to maintain market acceptance all the time if new products or technologies are introduced that are more popular, cost-effective or make our products obsolete. We believe that maintaining and enhancing our brand image and increasing the market awareness of our company and our products are critical to gaining broad recognition for our services and products, strengthening our relationships with existing customers and our ability to attract new customers. The successful promotion of our brand will largely depend on our ability to continue to provide high-quality products, as well as our research and development efforts. However, there is no guarantee that our branding activities and research and development efforts will be successful or contribute to our growth. In addition, even if such activities increase revenue, such revenue may not be sufficient to offset the increase in expenses we incur.
There is no guarantee that our products will be covered by the National Medical Insurance Program in China.
China maintains a National Medical Insurance Program that can reimburse certain residents in urban cities and rural areas for, among other things, fees associated with diagnostic and treatment devices and diagnostic tests covered by such Program through the basic medical insurance scheme.
As of the date of this prospectus, our products are not covered by any national or provincial medical insurance programs. Although we strive to make our products covered by the national medical insurance programs in the future, there is no guarantee that the responsible government agency, the National Healthcare Security Administration, will approve our applications. Furthermore, even if our products may be covered by the Program in the future, uncertainties still exist around reimbursement coverage and rates as we need to negotiate such terms with government entities in the PRC, and we cannot guarantee that we will receive favorable coverage and rates. In the absence of reimbursement or favorable coverage and rates from the National Medical Insurance Program, end users have to bear all or the majority part of their expenses to use our products, which may reduce consumers’ interest in our products or cause our products to be less competitive when compared with other products that are covered by such Program.
We have relatively limited experience in product promotion and sales. There is no assurance that we will be able to successfully commercialize our products and, as a result, our revenue and profitability could be materially and adversely affected.
We have relatively limited experience in launching and commercializing product candidates and in the sales and marketing of products, and limited experience in market analysis or managing sales teams for product candidates. As a result, our ability to successfully commercialize our product candidates may involve more inherent risks, require more time and cost more than would be the case if we were a company with extensive experience in launching product candidates.
The market size of existing and future products has not been precisely established and may be lower than our estimates, and we may not fully grasp the target population of our products.
Our estimates of the total addressable market and target population for our existing products and product candidates are based on a number of internal and third-party estimates, including but not limited to the size of the target population, the number of people at higher risk of developing cancer, and the availability of the hypothetical price at which the established market sells the relevant candidate product. Although we believe that our assumptions and data relating to estimates are reasonable, such assumptions and estimates may not be correct, and the conditions supporting our assumptions or estimates may change at any time, reducing the accuracy of forecasts for these relevant factors. Accordingly, our estimates of the total addressable market for current or future products may prove to be incorrect. If the target population that will benefit from our products, the price at which we can sell our products or the total addressable market for our products is lower than our estimates, it could harm our sales growth and adversely affect our business.
Risks Associated with Our Testing and the Manufacture and Supply of Our Products
Delays in the completion and obtaining regulatory approvals of our manufacturing facilities, or damage, destruction or interruption of production at such facilities may delay our development plans or commercialization efforts.
Our future manufacturing facilities will be global. The facility may incur unanticipated expenses due to a number of factors including regulatory requirements. Our manufacturing facilities are subject to ongoing periodic inspections by various NMPA or other comparable regulatory agencies to ensure compliance with current drug manufacturing practices. Failure to comply with applicable regulations may also result in us being subject to penalties, including fines, injunctions, civil penalties, requests to suspend or stop one or more of our clinical trials, failure to obtain regulatory approvals for our product candidates, delays, Suspension or withdrawal of approvals, supply interruptions, revocation of licenses, seizure or recall of products or product candidates, operational restrictions and criminal prosecutions, any of which can cause harm to our business.
Our facilities may be damaged or rendered inoperable due to physical damage caused by fire, flood, earthquake, typhoon, tornado, power outage, telecommunications failure, intrusion and similar events. If our production facilities or equipment are damaged or destroyed, we may not be able to replace our production capacity quickly or cheaply or at all. In the event of temporary or long-term damage to facilities or equipment, we may not be able to transfer manufacturing to third parties. Even if we could transfer manufacturing to a third party, that transfer could be expensive and time-consuming, especially since the new facility has to meet the necessary regulatory requirements and we have to obtain regulatory approvals before selling any products manufactured at that facility. This event could delay our clinical trials or reduce sales of our products. Any disruption to our manufacturing operations at our manufacturing facilities may result in us being unable to meet our clinical trial or commercialization needs. Any disruption to our ability to manufacture products or product candidates in a timely manner could materially damage our business, financial condition and results of operations. We currently insure against damage to our property and equipment for an amount we believe is reasonable. However, our insurance coverage may not compensate us or may not be sufficient to cover any expenses or losses we may incur. In the event of a catastrophic event or the failure of our production facilities or processes, we may not be able to meet our requirements for products and product candidates.
The manufacturing and testing process of our products is complex and subject to strict quality control. If we or any of our suppliers or logistics partners experience manufacturing, logistics or quality problems, including as a result of natural disasters, our business may be damaged.
Partly due to stringent regulatory requirements, the manufacturing and testing process for products is complex and subject to strict quality control. Additionally, quality is paramount as product or test defects can have serious and costly consequences. Manufacturing and testing processes can go wrong for a number of reasons, including equipment failure, non-compliance with codes and procedures, raw material issues, software issues, sample contamination or human error. In addition, if contaminants are found in our product or product candidate supply, or in our production and testing facilities, such production and testing facilities may need to be shut down for an extended period of time to investigate the contamination and remediate it. Stability and other issues may arise in the future related to the manufacture and testing of our products or product candidates. While well-managed, disruptions can occur during the introduction of new equipment and systems to replace aging equipment, as well as production line transfers and expansions. As we increase market penetration, we may face unexpected surges in demand for our products, which could put pressure on our production capacity or testing capabilities. If these problems arise, or if we otherwise fail to meet our internal quality standards or the standards of the CFDA or other applicable regulatory agencies, including detailed record-keeping requirements, our reputation may be damaged and we Safety warnings or recalls may apply, we may incur product and professional liability and other costs, product approvals may be delayed, and our business may otherwise be adversely affected. In addition, our manufacturing, testing and warehousing facilities, as well as those of our suppliers and logistics partners, may be severely damaged by earthquakes, hurricanes, volcanoes, fires and other natural disasters or catastrophic circumstances, could materially and adversely affect our business.
Security threats to our information technology infrastructure and unauthorized use of data by third parties could expose us to liability or damage our reputation and business.
Our information technology systems store and process a variety of sensitive data, including our proprietary business information, as well as patients’ personal data such as health information and personally identifiable information.
It is essential that our information technology infrastructure remains secure and is perceived by hospitals, patients and our research partners to be secure. Despite our security measures, we may face cyber-attacks that attempt to penetrate our network security, sabotage or otherwise disable our research, tests and services, misappropriate our proprietary business information or cause interruptions of our internal systems and services. Any cyber-attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and research partners, and expose us to significant financial liabilities.
Moreover, we may not be able to prevent third parties from illegally obtaining and misappropriating personal data of the tested patients that we collect. Concerns about data leakage or unauthorized use of data by third parties, even if unfounded, could damage our reputation and negatively affect our results of operations.
If we are unable to effectively protect our intellectual property, our business and competitive position would be harmed.
We rely on patents, software copyrights, trademarks, trade secrets and other intellectual property rights protection and contractual restrictions to protect our products, services and technologies. We have registered a number of patents and trademarks in China and the United States. However, such protection is limited and may not adequately protect our rights.
We may also be subject to infringement claims by third parties. We may be subject to fines and other legal or administrative sanctions, and it may also be costly to defend such claims. In addition, competitors could purchase our products and attempt to replicate and/or improve some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, and design their devices and tests around our protected technologies or develop their own competitive technologies that fall outside of our intellectual property rights.
Monitoring unauthorized disclosure and uses of our trade secrets is difficult, and we do not know whether the steps we have taken to prevent such disclosure and uses are, or will be, adequate. If we resort to litigation to enforce or protect our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and financial resources, while the outcome would be unpredictable, and any remedy may be inadequate. Our contractual agreements may be breached by our counterparties, and there may not be adequate remedies available to us for any such breach. In addition, our trade secrets may be leaked or otherwise become available to, or be independently discovered by, our competitors, and we would have no right to prevent others from using them. Moreover, if a party having an agreement with us has an overlapping or conflicting obligation to a third party, our rights in and to certain intellectual property could be undermined. If we fail to effectively protect our intellectual property, our competitive position and prospects could be adversely affected.
We may be subject to intellectual property infringement or misappropriation claims by third parties, which may force us to incur substantial legal expenses and, if determined adversely against us, could disrupt our business.
We cannot be certain that our products, tests and technologies do not or will not infringe patents, software copyrights, trademarks or other intellectual property rights held by third parties, especially when they are in China, as the validity, enforceability and scope of intellectual property rights protection in China are uncertain and still evolving. From time to time, we may be subject to legal proceedings and claims alleging infringement of patents, trademarks or copyrights, or misappropriation of creative ideas or formats, or other infringement of proprietary intellectual property rights. Any such proceedings and claims could result in significant costs to us and divert the time and attention of our management and technical personnel from the operation of our business. These types of claims could also potentially adversely impact our reputation and our ability to conduct business and raise capital, even if we are ultimately absolved of all liability. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more devices or tests and could result in a substantial award of damages against us. In addition, since we sometimes indemnify our customers or collaboration partners, we may have additional liability in connection with any infringement or alleged infringement of third-party intellectual property. Intellectual property litigation can be very expensive, and we may not have the financial means to defend ourselves or our customers or collaboration partners.
Because patent applications can take many years to issue, there may be pending applications, some of which are unknown to us, that may result in issued patents upon which our products, tests or proprietary technologies may infringe. Moreover, we may fail to identify issued patents of relevance or incorrectly conclude that an issued patent is invalid or not infringed by our technology or any of our devices or tests. There is a substantial amount of litigation involving patents and other intellectual property rights in our industry. If a third-party claims that we infringe upon a third-party’s intellectual property rights, we may have to:
We may be subject to litigation and other claims and legal proceedings, and may not always be successful in defending ourselves against these claims or proceedings.
We may be subject to lawsuits and other claims in the ordinary course of our business. We may from time to time be subject to lawsuits and other legal proceedings brought by our customers, competitors, employees, business partners, investors, other shareholders of the companies we invest, or other entities against us in the ordinary course of our business. We may also be subject to regulatory proceedings in the ordinary course of our business. We may not be successful in defending ourselves, and the outcomes of these lawsuits and proceedings may be unfavorable to us. Lawsuits and regulatory proceedings against us may also generate negative publicity that significantly harms our reputation, which may adversely affect our customer base, market position and our relationships with our research partners and other business partners. In addition to the related costs, managing and defending litigation and other legal proceedings and related indemnity obligations can significantly divert our management’s attention from operating our business. We may also need to pay damages or settle lawsuits or other claims with a substantial amount of cash, negatively affecting our liquidity. As a result, our business, financial condition and results of operations could be adversely affected.
Risks Relating to Our Financial Prospects and Need for Additional Capital
| · | We have not generated positive cash income and any failure to raise additional capital will significantly hinder our ability to continue our operations and developing new products. See “Risk Factors – Risks Relating to Our Financial Prospects and Need for Additional Capital – We incurred net losses for the years ended June 30, 2023 and 2022, and for the three months period ended September 30, 2023, and may not be able to generate sufficient operating cash flows and working capital to continue as a going concern. Failure to manage our liquidity and cash flows may materially and adversely affect our financial condition and results of operations. As a result, we may need additional capital, and financing may not be available on terms acceptable to us, or at all” on page 35; “We require substantial funding for our operations. If we cannot raise sufficient additional capital on acceptable terms, our business, financial condition and prospects may be adversely affected” on page 35; and “Raising additional capital may lead to dilution of shareholdings by our existing shareholders, restrict our operations, and may further result in fair value loss adversely affecting our financial results” on page 36. |
We incurred net losses for the years ended June 30, 2022 and 2021 and nine months period ended March 31, 2023 and 2022 and may not be able to generate sufficient operating cash flows and working capital to continue as a going concern. Failure to manage our liquidity and cash flows may materially and adversely affect our financial condition and results of operations. As a result, we may need additional capital, and financing may not be available on terms acceptable to us, or at all.
We incurred net losses of US$4,027,722 and US$1,837,158 for the years ended June 30, 2022 and 2021 and US$2,052,205 and US$1,328,848 for the nine months period ended March 31, 2023 and 2022, respectively.
We can offer no assurance that we will operate profitably or that we will generate positive cash flows in the next twelve months, given our substantial expenses in relation to our revenue at this stage of our Company. Inability to collect our accounts receivable in a timely and sufficient manner, or the inability to offset our expenses with adequate revenue, may adversely affect our liquidity, financial condition and results of operations. Although we believe that our cash on hand will be sufficient to meet our anticipated working capital requirements and capital expenditures in the ordinary course of business for the next 12 months, we cannot assure you this will be the case.
If and when we are unable to meet our working capital requirements and various operating needs, we may need to raise additional funds for our operations and such funds may not be available on commercially acceptable terms, if at all. If we are unable to raise funds on acceptable terms, we may not be able to execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements. This may seriously harm our business, financial condition and results of operations. If we are unable to achieve or maintain profitability, the market price of our shares may significantly decrease. In the event that the Company requires additional funding to finance its operations, the Company’s major shareholders have indicated their intent and ability to provide such financial support, however, there is no assurance such funding will be available when the Company needs it in the future.
We have recorded negative cash flows from operating activities historically and may have a current liabilities position in the future.
We have experienced significant cash outflow from operating activities since our inception. We had net cash outflow in operating activities of U$0.7 million and US$0.5 million for the years ended June 30, 2022 and 2021 and US$3.1 million for the nine-month period ended March 31, 2023, respectively. The cost of continuing operations could further reduce our cash position, and an increase in our net cash outflow from operating activities could adversely affect our operations by reducing the amount of cash available to meet the cash needs for operating our business and to fund our investments in our business expansion.
Although we had net current assets of US$1.0 million as of March 31, 2023, we cannot guarantee that we will not have a net current liabilities position in future, which would expose us to liquidity risk. Our future liquidity and ability to make additional capital investments necessary for our operations and business expansion will depend primarily on our ability to maintain sufficient cash and to obtain adequate external financing. There can be no assurance that we will be able to obtain any sources of financing.
We require substantial funding for our operations. If we cannot raise sufficient additional capital on acceptable terms, our business, financial condition and prospects may be adversely affected.
We require substantial capital to fund our existing operations, commercialize new products, expand our business and pursue strategic investments. In particular, we require substantial capital to:
Based on our current business plan, we believe our cash and cash equivalents, together with our financing activities, our initial public offering and private placement will be sufficient to meet our current and anticipated needs for general corporate purposes for at least the next 12 months. If our available cash balances are insufficient to satisfy our liquidity requirements, in particular, for the development and commercialization of our products, we may seek to obtain further funding through public or private equity offerings, debt financings or other sources.
Further financing may not be available to us on acceptable terms, or at all. If we fail to raise capital as and when needed it would have a negative impact on our financial condition and our ability to pursue our business strategy. In addition, if we raise funds by issuing debt securities or incurring additional borrowings, the terms of debt securities issued or borrowings could impose significant restrictions on our operations, and we may be unable to repay the indebtedness when due. If we raise funds by issuing equity securities, your investment in our company could be diluted.
Raising additional capital may lead to dilution of shareholdings by our existing shareholders, restrict our operations, and may further result in fair value loss adversely affecting our financial results.
We may seek additional funding through a combination of equity and debt financings and collaborations. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of existing holders of our shares will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our existing shareholders.
The incurrence of additional indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license IP rights and other operating restrictions that could adversely impact our ability to conduct our business.
We have only a limited accounting personnel and have corrected an error in application of the accounting principle for business combinations accounted for an acquisition of an entity during the year ended June 30, 2022.
Prior to the completion of this offering, we have been a private company with limited accounting personnel mainly engage in financial reporting. On January 1, 2022, the Company through Advanced Biomed HK Limited acquired 100% equity interest of Shanghai Sglcell Biotech Co., Ltd. The Company carefully considered the appropriate accounting guidance in accordance with its application of the accounting principle related to accounting for business combinations. However, we did not engage outside experts to assist in the detailed analysis.
Upon further assessment, the Company has corrected an error in its application of the accounting principle for business combinations where the Company previously incorrectly accounted for an acquisition of an entity as a business combination; the Company has amended its consolidated financial statements for the year ended June 30, 2022 to account for the transaction as purchase of assets.
Management concluded that the above correction identified in connection with the accounting treatment for the acquisition was a significant deficiency that did not rise to the level of a material weakness. The deficiency identified was narrow as it focused on the interpretation of accounting literature to a specific transaction structure which is not expected to be recurring nature. For future material acquisitions, the Company will engage outside experts to assist in the transaction, including legal and accounting advisors, to advise on complex accounting issues in order to prevent potential differences in the accounting for business combinations. As a result, the Company does not believe that the significant deficiency rose to the level of a material weakness.
Risks Related to Doing Business in Taiwan
| · | Our subsidiary in Taiwan is subject to risks related to complying with Taiwan laws and regulations. See “Risk Factors – Risks Related to Doing Business in Taiwan –Advanced Biomed Taiwan is subject to restrictions on paying dividend or making other payments to us, which may restrict our ability to satisfy its liquidity requirements” on page 37; and “Taiwanese investors holding more than 10% of Advanced Biomed common stock will be subject to Taiwan regulations on investment or technical cooperation in China for its investment or technical cooperation in China” on page 38. | |
| · | The geopolitical tension between Taiwan and China that could negatively affect our business and hence the value of your investment. See “Risk Factors – Risks Related to Doing Business in Taiwan – We face economic and political risks associated with doing business in Taiwan, particularly due to the geopolitical tension between Taiwan and China that could negatively affect our business and hence the value of your investment” on page 37. | |
| · | The imposition of foreign exchange restrictions in Taiwan may have an adverse effect on foreign investors’ abilities to acquire securities of a Taiwan company, including the shares of our subsidiaries in Taiwan, or to repatriate the interest, dividends or sale proceeds from those securities. See “Risk Factors – Risks Related to Doing Business in Taiwan – The imposition of foreign exchange restrictions in Taiwan may have an adverse effect on foreign investors’ abilities to acquire securities of a Taiwan company, including the shares of our subsidiaries in Taiwan, or to repatriate the interest, dividends or sale proceeds from those securities” on page 38. |
Risks Related to Doing Business in China
| · | We, through our Shanghai subsidiary, are subject to unique legal risks, and the enforcement of Chinese laws and regulations can change quickly with little advance notice. See “Risk Factors – Risks Related to Doing Business in China – Changes in the policies, regulations, rules, and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon our ability to operate profitably in the PRC” on page 39; Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and the Company” on page 41; and “You may experience difficulties in effecting service of legal process, enforcing foreign judgments, or bringing actions in PRC against us” on page 42. |
| · | The Chinese government may intervene or influence our operations at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and/or the value of our securities we are registering to offer and/or significantly limit or completely hinder our abilities to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. See “Risk Factors – Risks Related to Doing Business in China – The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale” on page 39, “If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us, such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.” on page 40. |
| · | You may face difficulties enforcing liabilities against our directors and officers in China. See “Risk Factors – Risks Related to Doing Business in China – Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and the Company” on page 41 and “You may experience difficulties in effecting service of legal process, enforcing foreign judgments, or bringing actions in PRC against us” on page 42. |
Risks Related to this Offering
| · | You may experience extreme stock price volatility. See “Risk Factors – Risks Related to this Offering –The market price of our common stock may be volatile or may decline regardless of our operating performance, and you may not be able to resell your shares at or above the public offering price” on page 51. |
Although the majority of our operations are not conducted in Mainland China, we face various legal and operational risks and uncertainties relating to our Shanghai subsidiary, Shanghai Sglcell Biotech Co., Ltd., and similar legal and operational risks and uncertainties also apply to our holding company in Hong Kong. The Chinese government may intervene or influence the operation of our Shanghai subsidiary and Advanced Biomed HK and exercise significant oversight and discretion over the conduct of their business and may intervene in or influence their operations at any time, or may exert more control over securities offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and/or the value of our common stock. Further, any actions by the Chinese government to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.
Recently, the PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, adopting new measures to extend the scope of cybersecurity reviews, and expanding the efforts in anti-monopoly enforcement. As advised by our PRC counsel, AllBright, we do not believe that we are directly subject to these regulatory actions or statements, as our Shanghai subsidiary does not have a VIE structure and their operations are not subject to cybersecurity review requirements, or involve any type of restricted industry. Because these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative rule making bodies in China will respond to them, or what existing or new laws or regulations will be modified or promulgated, if any, or the potential impact such modified or new laws and regulations will have on our subsidiaries’ daily business operations or ability to accept foreign investments and list on an U.S. exchange. In On December 24, 2021, nine government agencies jointly issued the Opinions on Promoting the Healthy and Sustainable Development of Platform Economy, which provides that, among others, monopolistic agreements, abuse of dominant market position and illegal concentration of business operators in the field of platform economy will be strictly investigated and punished in accordance with the relevant laws. We do not hold a dominant market position in our product markets and we have not entered into any monopolistic agreement. We have not received any inquiry from the relevant governmental authorities. the Cyberspace Administration of China (“CAC”), together with 12 other Chinese regulatory authorities, released the final version of the Revised Measures for Cybersecurity Review, or the Revised Cybersecurity Measures, in December 2021, which took effect on February 15, 2022. Pursuant to the Revised Cybersecurity Measures, critical information infrastructure operators procuring network products and services and online platform operators carrying out data processing activities, which affect or may affect national security, shall conduct a cybersecurity review pursuant to the provisions therein. In addition, online platform operators possessing personal information of more than one million users seeking to be listed on foreign stock markets must apply for a cybersecurity review. The Revised Cybersecurity Measures apply to companies going abroad for secondary listing, dual primary listing and other new foreign listings and subject to the reporting requirements. On November 14, 2021, the CAC published the Draft Regulations on the Network Data Security Administration (Draft for Comments) (the “Security Administration Draft”), which provides that data processing operators engaging in data processing activities that affect or may affect national security must be subject to cybersecurity review by the relevant Cyberspace Administration of the PRC. As advised by AllBright, we do not believe that we are an “online platform operator” within the meaning of the Revised Cybersecurity Measures, and, we currently do not have over one million users’ personal information and do not anticipate that we will be collecting over one million users’ personal information in the foreseeable future. In addition, we are also not subject to Security Administration Draft if the Security Administration Draft are enacted as proposed, since we currently do not collect data that affects or may affect national security and we do not anticipate that we will be collecting data that affects or may affect national security in the foreseeable future.
On December 24, 2021, China Securities Regulatory Commission (the “CSRC”) issued the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing of Securities by Domestic Enterprises (the “Draft Administrative Provisions”) and the Measures for the Overseas Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Draft Filing Measures”), collectively, the Draft Overseas Listing Rules. On February 17, 2023, the CSRC released Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies with five interpretive guidelines (the “Trial Measures”) which came into effect on March 31, 2023. Pursuant to the Trial Measures, a PRC domestic company that seeks to offer and list securities in overseas markets, either in direct or indirect overseas offering, shall fulfill the filing procedure with the CSRC as per requirement of the Trial Measures, submit relevant materials that contain a filing report and a legal opinion, and provide truthful, accurate and complete information on the shareholder and etc. Direct overseas offering and listing by domestic companies refers to such overseas offering and listing by a joint-stock company incorporated domestically. Any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect offering and listing in overseas market and, therefore, be subject to filing requirement: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in the Mainland China, or its main places of business are located in the Mainland China, or the senior managers in charge of its business operation and management are mostly Chinese citizens or domiciled in the Mainland China. The determination as to whether or not an overseas offering and listing by domestic companies is indirect, shall be made on substance over form basis. As of the date of this prospectus, as advised by AllBright, we do not believe that we are required to obtain the approval from or complete the filing with the CSRC for this offering and thus we have not submitted an application for approval for this offering with the CSRC pursuant to the Trial Measures based on the facts that (i) we are a holding company incorporated in the State of Nevada, not a company incorporated under the PRC law; (ii) based on the report on the Shanghai subsidiary from a third party accounting firm in China, the Shanghai subsidiary contributed less than 50% to our operating revenue, total profit, total assets or net assets as documented in the audited consolidated financial statements for the fiscal year ended June 30, 2023; (iii) the business activities are primarily conducted in Taiwan, with minimal operation in Mainland China, and (iv) most of our officers and directors are non-Chinese citizens or domiciled outside Mainland China. Thus, we do not meet the explicit conditions set out in the Trial Measures to determine whether an overseas offering shall be deemed as an indirect overseas offering and listing by a domestic company. However, as the Trial Measures was newly published, there are substantial uncertainties as to the implementation and interpretation, and the CSRC may take a view that is contrary to our understanding of the Trial Measures. If we are required by the CSRC to submit and complete the filing procedures of this offering and listing, we cannot assure you that we will be able to complete such filings in a timely manner, or even at all. Any failure by us to comply with such filing requirements under the Trial Measures may result in rectification, warnings, and a fine between RMB 1 million and RMB 10 million on our Shanghai subsidiary, which could adversely and materially affect our business operations and financial outlook, and significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or such shares to become worthless.
As of the date of this prospectus, these new laws and guidelines have not impacted our ability to conduct its business, accept foreign investments, or continue to list on a U.S. or other foreign exchange; however, if (i) we inadvertently conclude that permissions or approvals are not required from applicable PRC authorities, (ii) applicable laws, regulations, or interpretations change, and we are required to obtain such permissions or approvals in the future, or (iii) we fail to file or were denied permission from the PRC authorities to this offering, any follow-up offerings or transactions, our ability to conduct our business may be materially impacted, and we will not be able to continue listing on any U.S. exchange, continue to offer securities to investors, the interest of the investors may be materially adversely affected and our common stock may significantly decrease in value or become worthless. To date, there are uncertainties in the interpretation and enforcement of these new laws and guidelines, which could materially and adversely impact our business and financial outlook and may impact our ability to accept foreign investments, or continue to list on a U.S. or other foreign exchange. See “Risk Factors — The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale”; “Risk Factors — If the Chinese government chooses to exert more oversight and control over offerings that are conducted overseas and/or foreign investment in us, such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless”; and “Risk Factors — The M&A Rules and certain PRC regulations may make it more difficult for us to pursue growth through acquisitions.”
| 17 |
Prospectus Conventions
Except where the context otherwise requires and for purposes of this prospectus only:
| · | “Advanced Biomed Taiwan” refers to Advanced Biomed Inc. (Taiwan); |
| · | “Advanced Biomed HK” refers to Advanced Biomed HK Limited; |
| · | “China” or “PRC” refer to the People’s Republic of China, including Hong Kong, Macau and Taiwan; however the only time such jurisdictions are not included in the definition of PRC and China is when we reference to the specific laws that have been adopted by the PRC; The term “Chinese” has a correlative meaning for the purpose of this prospectus; The term “Mainland China” refers to the People’s Republic of China, excluding Hong Kong, Macau and Taiwan; |
| · | “CRO” refers to contract research organization, a type of third-party entity that is engaged by medical device research and manufacturing companies to conduct all or part of the required scientific or medical trials before a medical device can be sold to the public; | |
| · | “Common stock” prior to the completion of this offering refers to shares our common stock of par value US$0.001 per share; | |
| · | “NMPA” refers to National Medical Products Administration, formerly known as China Food and Drug Administration; | |
| · | “NTD” or “NT$” refers to New Taiwan Dollar, the legal currency of Taiwan; | |
| · | “RMB” or “Renminbi” refers to the legal currency of the People’s Republic of China; | |
| · | “Shanghai Sglcell” or “Shanghai subsidiary” refers to Shanghai Sglcell Biotech Co., Ltd., a company incorporated in the People’s Republic of China and our wholly foreign owned entity 100% owned by Advanced Biomed HK; and | |
| · | “Taiwan” refers to Taiwan, Penghu, Kinmen, Matsu, and any other areas under the effective control of the government of Republic of China; |
| · | “US$,” “$” or “U.S. dollars” refers to the legal currency of the United States; and |
| · | “we,” “us,” “our,” “our company,” and “our group” refer to Advanced Biomed Inc., a Nevada company and its subsidiaries. |
| 18 |
Unless otherwise noted, all translations from NTD to U.S. dollars and from U.S. dollars to RMB in this prospectus are made at NTD31.70 to US$1.00, the exchange rate set forth in the H.10 statistical release of the Federal Reserve Board on September 30, 2023. We make no representation that any NTD or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, the rates stated below, or at all. On September 30, 2023, the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Board for New Taiwan Dollar was NTD31.70 to US$1.00.
The industry in which we operate is subject to a high degree of uncertainty and risk due to variety of factors, including those described in the “Risk Factors” section. These and other factors could cause results to differ materially from those expressed in these publications and reports.
| 19 |
THE OFFERING
| Shares of common stock to be offered: | 25,000,000 shares of common stock (or 28,750,000 shares of common stock if the representative of the underwriters exercises its over-allotment option in full) on a firm commitment basis. | |
| Offering price per share: | The purchase price for the common stock being sold in this offering is expected to be between $4.00 and $5.00 per share of common stock. | |
| Shares of common stock outstanding prior to this offering (1) | 100,000,000 shares | |
| Shares of common stock outstanding after this offering (1)(2) | 125,000,000 shares | |
| Lock-up agreement | We have agreed that, without the prior written consent of the underwriters, subject to certain exceptions, we will not, for a period of six (6) months after the date of the prospectus, sell, transfer or dispose of any common stock. All of our directors, officers, and shareholders holding more than 5% of our common stock as of the effective date of this prospectus, have agreed not to sell, transfer or dispose of any common stock for a period of six months from the date of the prospectus, subject to certain exceptions. | |
| Proposed Nasdaq Capital Market symbol | We have applied to list our common stock on the Nasdaq Capital Market under the symbol “ADVB”. | |
| Underwriter’s over-allotment option | We have granted the underwriters an option for 45 days from the date of this prospectus to purchase up to an additional 3,750,000 shares of our common stock at the public offering price, less the underwriting discounts and commissions, to cover over-allotments, if any. | |
Representative’s warrants | Upon the closing of this offering, we will issue to the representative of the underwriters warrants entitling the representative of the underwriters to purchase 250,000 shares of common stock (or 287,500 shares of common stock if the representative of the underwriters exercises its over-allotment option in full) equal to 1% of the aggregate number of shares of common stock sold in this offering. The representative’s warrants are exercisable at any time and from time to time, in whole or in part, during the four and a half year period commencing six months following the date of commencement of sales of securities issued in this offering, at a price per share equal to 150% of the per share offering price. For additional information, please refer to the “Underwriting” section beginning on page 115. | |
| Use of proceeds | We estimate that we will receive net proceeds, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, of approximately $105 million from this offering assuming no exercise of the representative’s warrants and completion of the offering. We intend to use the net proceeds of this offering as follows after we complete the remittance process: |
| ● | Approximately 80% for invitro diagnosis (IVD) clinical trials, chip design and development, upgrade and construction of facilities, and expansion to other markets; | ||
| ● | 10% for marketing and sales; and | ||
| ● | 10% for general working capital. | ||
| See “Use of Proceeds.” | |||
| 20 |
| Transfer Agent | Transhare Corporation | |
| Risk factors | See “Risk Factors” below and the other information included in this prospectus for a discussion of factors that you should consider carefully before deciding to invest in our securities. |
(1) The number of shares of common stock to be outstanding immediately after this offering is based upon 100,000,000 shares outstanding as of the date of this prospectus.
(2) Except as otherwise indicated, the number of shares of common stock presented in this prospectus excludes shares of our common stock issuable if the underwriters exercise their over-allotment option and shares of our common stock underlying warrants to be issued to the representative of the underwriters in connection with this offering.
| 21 |
SUMMARY CONSOLIDATED FINANCIAL DATA
The following selected historical statements of operations for the fiscal years ended June 30, 2023 and 2022 and three-month periods ended September 30, 2023 and 2022, and balance sheet data as of September 30, 2023, June 30, 2023 and 2022 have been derived from our audited consolidated financial statements and unaudited interim condensed consolidated financial statements for those periods included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future. You should read this data together with our consolidated financial statements and related notes appearing elsewhere in this prospectus as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” appearing elsewhere in the prospectus.
Selected Consolidated Results of Operations Data
| For the years ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (1,383,181 | ) | (1,619,531 | ) | ||||
| General and administrative expenses | (1,688,042 | ) | (754,840 | ) | ||||
| Impairment of intangible assets | - | (1,617,974 | ) | |||||
| Total operating expenses | (3,071,223 | ) | (3,992,345 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 21,402 | 243 | ||||||
| Other expense, net | (682,956 | ) | (35,620 | ) | ||||
| Total other expense, net | (661,554 | ) | (35,377 | ) | ||||
| Income before tax expense | (3,732,777 | ) | (4,027,722 | ) | ||||
| Income tax expense | - | |||||||
| Net loss | (3,732,777 | ) | (4,027,722 | ) | ||||
| For the three-month periods ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (169,185 | ) | (116,605 | ) | ||||
| General and administrative expenses | (271,060 | ) | (292,014 | ) | ||||
| Total operating expenses | (440,245 | ) | (408,619 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 14 | 443 | ||||||
| Other expense, net | (331,085 | ) | (740,538 | ) | ||||
| Total other expense, net | (331,071 | ) | (740,095 | ) | ||||
| Income before tax expense | (771,316 | ) | (1,148,714 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | (771,316 | ) | (1,148,714 | ) | ||||
Selected Consolidated Balance Sheet Data
| As of September 30, | As of June 30, | |||||||||||
| 2023 | 2023 | 2022 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Cash | 2,587,218 | 2,622,279 | 4,783,864 | |||||||||
| Working capital | (861,792 | ) | (489,595 | ) | 2,015,999 | |||||||
| Total assets | 4,448,724 | 4,599,977 | 6,713,499 | |||||||||
| Total liabilities | 3,643,580 | 3,294,389 | 3,221,903 | |||||||||
| Total stockholders’ equity | 805,144 | 1,305,588 | 3,491,596 | |||||||||
| 22 |
Investing in our securities involves a high degree of risk. Before making any investment decision, you should consider carefully the following risks and other information in this prospectus, including our consolidated financial statements and related notes. The risks and uncertainties we describe are not the only ones facing us. Additional risks and uncertainties that we are unaware of or that we believe are not material at the time could also materially adversely affect our business, financial condition or results of operations. In any case, the value of our common stock could decline, and you could lose all or part of your investment. Please also see the section entitled “Cautionary Note Regarding Forward-Looking Statements.”
Risks Related to Our Business and Industry
We are an early stage cancer diagnostics company with a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are at the early stage of commercializing our business. We have not started manufacturing or sales of any products or services. Our limited operating history may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty.
Taiwan and China’s precision oncology detection market is still in its early stage of development and rapidly evolving, and companies operating in this industry face a variety of risks. We may not have sufficient experience or resources to address risks frequently encountered in this industry, which include, among other things, our potential failure to:
| ● | acquire and retain customers and increase adoption of our precision oncology detection products and services by hospitals, physicians, patients, pharmaceutical companies and others in the medical community; |
| ● | timely respond to changing market conditions and keep up with evolving industry and technological standards and regulatory developments; |
| ● | obtain and maintain the regulatory approvals required for us to further market and sell our precision oncology detection products and services and commercialize our early cancer detection products and services; |
| ● | manage our relationships with our suppliers, customers and research partners; |
| ● | protect proprietary technologies and intellectual property rights; and |
| ● | attract, train, motivate and retain research and development and other qualified personnel. |
If we are unsuccessful in addressing any one or more of these risks, our business, financial condition and results of operations could be adversely affected.
We may be unable to develop and commercialize our early cancer detection devices, chips or immunochromogenic kits on a timely basis, or at all.
We are developing early cancer detection products and may develop and commercialize our cancer diagnostics products from time to time in the future. Developing early cancer detection and new cancer diagnostics products is a lengthy and complex process. New products may take time to commercialize, and their launch could be delayed or may not be successful.
Our product development process involves various risks, and we may not be able to develop and commercialize any early cancer detection products or new precision oncology detection products on a timely basis, or at all. A product candidate that appears promising in the early phases of development may fail to reach the market for a number of reasons. For example:
| 23 |
| ● | our product candidates may fail to demonstrate clinical utility, or the development process may produce negative or inconclusive results, and we may decide, or regulators may require us to conduct additional clinical trials or we may decide to abandon our development programs; |
| ● | our employees, or third-party clinical investigators, medical institutions and CROs, may fail to comply with their contractual duties or obligations or meet expected deadlines, and if the quality, completeness or accuracy of the clinical data they obtain are compromised due to any failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated; |
| ● | we may fail to obtain approvals for our product candidates from relevant regulatory authorities; and |
| ● | failure to generate additional data and insights from our existing products to advance the research and development of new products as quickly, or at all. |
In addition, our competitors may develop and commercialize competing products faster than we are able to, in which case our results of operations could be adversely affected.
If we fail to keep up with industry and technology developments in a timely and cost-effective manner, we may be unable to compete effectively and our business and prospects could suffer.
Cancer early detection market is characterized by rapid changes, including technological and scientific breakthroughs, increasing amounts of data, frequent introductions of new tests, constant emergence of alternative diagnostic methods, and evolving industry standards. If we are not able to keep pace with these advances and increased customer expectations as a result of these advances and capture new market opportunities that develop as a result of these advances, our proprietary technologies could be rendered obsolete, our existing products and services and products and services we are developing could be rendered less clinically effective, and our future operations and prospects could suffer. To remain competitive, we must continuously upgrade our existing products and services and launch new products and services, to keep pace with these developments. We cannot assure you that these efforts will be successful.
In addition, we must expend significant resources in order to continuously upgrade our existing products and services or launch new ones to keep pace with industry and technological advances. We may never realize a return on investment on these efforts, especially if the improved or new products and services fail to perform as expected, in which case our business, financial condition and results of operations could be adversely affected.
If our products or services do not perform as expected, our operating results, reputation and business could suffer.
Our success depends on the market confidence that we can provide reliable, high-quality cancer early detection products and services that will provide physicians with real-time clinically actionable diagnostic information. However, there is no assurance that our products and services will perform as expected at all times. Our tests may fail to accurately detect gene variants or incompletely or incorrectly identify the significance of genomic alterations, or contain other errors or mistakes due to a variety of reasons (such as malfunction of our laboratory equipment and degraded liquid biopsy or tissue samples provided by our delivery service providers), which may result in negative perception of our tests. In addition, inaccurate results or misunderstandings of, or inappropriate reliance on, the diagnostic information our tests provide could lead to, or be associated with, side effects or adverse events in patients who use our tests, including treatment-related death, and could lead to termination of our services or claims against us.
We face risks related to natural disasters, health epidemics, civil and social disruption and other outbreaks, which could significantly disrupt our operations. In particular, the COVID-19 outbreak in China and worldwide has adversely affected, and may continue to adversely affect, our business, results of operations and financial condition.
We are vulnerable to social and natural catastrophic events that are beyond our control, such as natural disasters, health epidemics, and other catastrophes, which may materially and adversely affect our business. Since December 2019, a novel strain of coronavirus, or COVID-19, has become widespread in China and around the world. In March 2020, the World Health Organization declared COVID-19 a pandemic, given its threat beyond a public health emergency of international concern that the organization had declared in January 2020. Since the beginning of 2020, China, Taiwan and many other countries have taken various restrictive measures to contain the virus’ spread, such as quarantines, travel restrictions and home office policies to avoid large-scale population movement and gathering. These restrictions had certain degree of impact on our research and development and production plan in Taiwan and China. COVID-19 has reduced the availability of labor and led to difficulties for employees to return to work and constraints of service chain interruption. Because of the uncertainty surrounding the COVID-19 outbreak, the business disruption and the related financial impact related to the outbreak of and response to the coronavirus cannot be reasonably estimated at this time.
| 24 |
Recently, the COVID-19 situation has improved in Taiwan and China. With Taiwan relaxing its mask mandate on April 17, 2023, and China no longer enforcing the “zero-COVID” policy, our research and development have started to recover. However, the potential downturn brought by the COVID-19 outbreak is difficult to assess or predict and the full impact of the virus on our operations will depend on many factors beyond our control. The extent to which the COVID-19 outbreak impacts our business, results of operations and financial condition remains uncertain, and we are closely monitoring its impact on us. Our business, results of operations, financial conditions and prospects could be materially adversely affected to the extent that COVID-19 harms the Chinese and global economy in general, and the trading price of our shares of common stock may be adversely affected. To the extent the COVID-19 pandemic and the outbreak of other health epidemics adversely affect our business and financial results, they may also have the effect of heightening many of the other risks described in this “Risk Factors” section.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenue or achieve and sustain profitability.
Cancer early detection and screening market has become increasingly competitive, and we expect this competition to intensify further in the future. Some of our existing and potential future competitors may have longer operating histories, larger customer bases, more expansive brand recognition and deeper market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities, and more favorable terms from suppliers. As a result, they may be able to respond more quickly to changes in customer requirements or preferences, develop faster, better and more expansive advancements for their technologies and tests, create and implement more successful strategies for the promotion and sale of their tests, adopt more aggressive pricing policies for their tests, secure supplies from vendors on more favorable terms or devote substantially more resources to infrastructure and system development. In addition, competitors may be acquired by, receive investments from or enter into other commercial relationships with larger, well-established and well-financed companies as the use of precision oncology detection increases. In addition, if we expand into international markets in the future, we could face competition from the companies in these markets.
If we are unable to compete successfully with current and future competitors for these or any other reasons, we may be unable to increase market acceptance and sales volume of our tests, which could prevent us from maintaining or increasing our revenue levels or achieving or sustaining profitability or could otherwise negatively affect our performance.
Our future success depends on our ability to promote our brand and protect our reputation. If we are unable to effectively promote our brand, our business may be adversely affected.
We believe that enhancing and maintaining awareness of our trademarks欣戈赛and sglcell is critical to achieving widespread acceptance of our microfluidic biochip products, gaining trust for our testing devices and attracting customers. Successful promotion of our brand depends largely on the quality of the products and services we offer and the effectiveness of our branding and marketing efforts. Currently, we rely primarily on our own sales and marketing team to promote our brand and our precision oncology detection products and testing services. We expect that our branding and marketing efforts will require us to incur significant expenses and devote substantial resources. We cannot guarantee that our sales and marketing efforts will be successful. Brand promotion activities may not lead to increased revenue in the near term, and, even if they do, any revenue increases may not offset the expenses we incur to promote our brand. Our failure to establish and promote our brand and any damage to our reputation will hinder our growth. In addition, our reputation may be undermined as a result of the negative publicity about our company or our industry in general. If precision oncology detection products or services provided by us or our competitors do not perform to customers’ expectations, it may result in lower confidence in precision oncology detection in general, which may in turn impair our operating results and our reputation. You can find more information regarding our intellectual properties in “Business – Intellectual Property.”
| 25 |
Failure to attract and retain our senior management and other key employees could adversely affect our business.
Our future success is significantly dependent upon the continued service of our senior management, such as Dr. Yi Lu, our chairman of the board of directors and chief executive officer. If we lose their services, we may not be able to locate suitable or qualified replacements, and we may incur additional expenses to recruit new senior management team members, which could severely disrupt our business and growth. In addition, if these personnel join our competitors or form a competing business, our business and prospects could be adversely affected.
Our research and development activities and laboratory operations depend upon our ability to attract and retain highly skilled scientists and technicians. We are also in strong need of sales and marketing personnel with the relevant technology background and industry expertise in order to effectively conduct our sales and marketing activities and increase our hospital network. We face intense competition for qualified individuals from numerous biotechnology and pharmaceutical companies, universities, governmental entities and other research institutions. We may be unable to attract and retain suitably qualified individuals, and our failure to do so could adversely affect our business.
If we experience difficulties in recruiting subjects for clinical trials, our clinical development activities may be delayed or otherwise adversely affected.
Completion of a clinical trial on time according to the trial plan depends, among other things, on our ability to recruit a sufficient number of subjects to participate in the trial until the end of the trial. We may encounter difficulties in recruiting subjects for clinical trials for a variety of reasons, including the base and nature of the subject population and the eligibility criteria for subjects defined in the trial plan. Our clinical trials will likely compete with other clinical trials, which will reduce the number and categories of subjects we can recruit, as some subjects who may choose to participate in our trials may instead choose to participate in trials conducted by other companies. Due to the limited number of qualified clinical investigators and clinical trial sites, we expect that some of our clinical trials will be conducted at the same clinical trial sites used by some of our competitors, which will result in subjects eligible to participate in our clinical trials at those clinical trial sites decrease in the number of people. Even if our clinical trials are able to enroll a sufficient number of subjects, delays in enrolling subjects may increase costs or affect the timing or outcomes of planned clinical trials, hindering the completion of those trials and hindering our progress adversely affect the ability of product candidates to develop.
If we do not launch new products in a timely manner, our products may become obsolete and our results of operations may suffer.
Technology in the cancer screening industry is constantly changing, new products are emerging, and industry standards continue to evolve. If new and improved products are not introduced in a timely manner, our products may become technologically obsolete or vulnerable to competition, and our revenue and results of operations could be impaired. Even if we develop new or improved products, our ability to bring those products to market may be limited by a variety of factors, including regulatory approvals and market demand. We invest significant financial and other resources in our research and development activities. The research and development process are lengthy and uncertain. The products we are currently developing may not be able to complete the development process or obtain the regulatory or other approvals required to bring such products to market in a timely manner or at all.
Technological innovations often require significant time and investment before their commercial viability can be determined. We may not have the necessary financial resources to fund all such projects. In addition, even if we are able to successfully develop new or improved products, those products may not generate revenue in excess of development costs or achieve desirable financial returns and may become obsolete or due to changes in customer preferences or the introduction of advanced technology or functionality by competitors. products or other factors resulting in a decline in competitiveness.
| 26 |
As of the date of this prospectus, we have not conducted any clinical trials for our products. Uncertainties or failures in clinical trials of our product candidates could materially and adversely affect our business operations.
We must conduct various clinical trials to demonstrate the sensitivity and specificity of our test prior to regulatory approval for marketing of our product candidates and depending on the type of our relevant product candidates, clinical trials may require large-scale prospective clinical studies, and such studies are far more rigorous and expensive than other existing tests or auxiliary diagnostic products. We have planned to conduct a multicenter registry trial in China to evaluate the performance of the pulmonary nodule benign and malignant determination kit. We may encounter a variety of contingencies while conducting or as a result of clinical trials that may delay or prevent us from obtaining regulatory approval or commercializing our product candidates, including but not limited to:
-Regulatory agencies, institutional review boards or ethics committees may refuse to authorize us or investigators to conduct clinical trials or conduct clinical trials at the intended trial site;
-We are unable to reach agreement on admissible terms with prospective CROs and hospitals (as trial centers), which may require extensive negotiation and may vary significantly between different CROs and hospitals (as trial centers);
-Production issues, including issues with the quality of production, supply, or obtaining sufficient quantities of the product candidate for use in clinical trials;
-Insufficient testing capacity to meet the needs of clinical trials;
-Our products fail to demonstrate superior results than competitors' or alternative products (if applicable);
-Clinical trials of our product candidates may fail to demonstrate the expected cancer screening sensitivity and specificity, and we may decide or regulatory authorities may require us to conduct additional clinical trials or abandon product development programs;
-The number of subjects required for clinical trials of our product candidates may be larger than expected, recruitment may be insufficient or slower than we expect, or the rate of subjects dropping out of trials may be higher than expected;
-Our third-party contractors may fail to comply with regulatory requirements or perform in a timely manner or at all of their contractual obligations to us;
-We may have to suspend or terminate clinical trials of our product candidates for a variety of reasons, including the discovery of a lack of clinical response or other unexpected characteristics; and preliminary or interim results of clinical trials may not be indicative of final results.
There is no assurance that such trials will be completed in a timely or cost-effective manner, or that they will result in a commercially viable product. If clinical trials of any of our product candidates are delayed or terminated, the commercial prospects of that product candidate will be impaired and our ability to generate revenue from any such product candidate will be delayed. In addition, any delay in the completion of our clinical trials would increase our costs, slow down the development and approval process of our product candidate, and impair our ability to initiate product sales and generate related revenue for that product candidate. The occurrence of any of the above events could materially damage our business, financial condition and prospects.
We have contracts with third parties for the future manufacturing, supply and registration applications of certain of our products, which supply could become limited or interrupted or may not be of satisfactory quality and quantity.
We currently rely on third parties in the PRC for certain supplying, registration applications and future manufacturing of certain of our products. Any such reliance may increase the risk that we will not have sufficient quantities of our products, or such quantities at an acceptable cost or quality, which could delay, prevent or impair our development or commercialization efforts. Furthermore, all the contract manufacturers in the PRC for our products are subject to extensive PRC laws and regulations. These PRC laws and regulations govern manufacturing processes and procedures, including record keeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants, or to inadvertent changes in the properties or stability of our products.
| 27 |
Risks Associated with Government Regulations
We may be adversely affected by uncertainties and changes in China's regulations on the cancer screening industry, and the lack of necessary approvals, licenses, registrations or filings in relation to our business may adversely affect our business, results of operations and prospects material adverse effects.
Due to the relatively short history of the cancer screening industry in China, a comprehensive regulatory framework to regulate the industry has not yet been established. The possibility cannot be ruled out that certain industry practices, which we have also adopted, may be deemed to fail to fully comply with the existing laws and regulations in the PRC. As China's laws and regulations on medical devices are still developing, and it is uncertain whether new laws, regulations or interpretations will be promulgated or adopted in the future, we cannot assure you that our early stage cancer detection devices will not be construed as non-compliance with applicable laws and regulations in the future. If the PRC government issues clear requirements for the approval of our devices, we intend to take the necessary steps to comply with those requirements. Our business and results of operations may be adversely affected if we fail to comply with existing or future requirements or are found to be otherwise non-compliant in the conduct of our business.
If we fail to obtain, complete or maintain the required regulatory approvals, licenses, registrations or filings, or if there is a delay in obtaining, complete or maintain the required regulatory approvals, licenses, registrations or filings, we will not be able to commercialize our product candidates change, and our ability to generate revenue will be significantly compromised.
Due to the relatively short history of the cancer screening industry in China, a comprehensive regulatory framework to regulate the industry has not yet been established. However, the significant aspects of the development and commercialization of our products have been strictly regulated in China. The process of obtaining regulatory approvals and complying with applicable laws and regulations requires significant time and financial resources. Applicants may face administrative or judicial sanctions if they fail to comply with applicable regulations during the product development process, the approval process, or at any time after approval. Such sanctions may include regulatory refusals to approve pending applications, revocation of approvals, revocation of licenses, clinical restrictions, voluntary or compulsory product recalls, product seizures, suspensions of production or distribution in whole or in part, injunctions, fines, refusals to government contracts, compensation, Hand over money or civil or criminal penalties. Failure to comply with these regulations may affect our business, financial business conditions and prospects are materially and adversely affected.
Before obtaining regulatory approval for commercial sale of some of our products, we must demonstrate its effectiveness in a well-controlled clinical trial and, for approval in China, must be satisfied by NMPA that the product candidate is safe and effective for its intended use, and appropriate manufacturing and testing facilities, procedures and controls are in place. For now, only A+LCGuard is required by NMPA to complete clinical trial. Obtaining regulatory approval is a long, expensive and uncertain process, and approval may not be obtained. When we submit a registration application to NMPA, it will decide whether to accept or reject the submitted registration application. We cannot be certain that any submission will be approved by NMPA for registration review. NMPA may also slow down, suspend or terminate the review of our application, and any such circumstance will prolong the registration process for our products. Our product candidates may not receive regulatory approval for a number of reasons, including:
| ● | The clinical trial results cannot reach the level of statistical significance required for approval or the clinical trial cannot be conducted in accordance with regulatory regulations or clinical trial plan; |
| ● | Approval policy or regulatory changes that make our preclinical and clinical data insufficient to obtain approval or require us to revise our clinical trial plan; |
| ● | Regulatory requirements requiring additional analysis, reporting, data, non-clinical studies and clinical trials, or questions regarding interpretation of data and results and the emergence of new information about our product candidates or other products; and/or |
| ● | The relevant authority refuses to approve a pending application submitted by us or a supplementary application to an approved application, or suspends, withdraws or withdraws the approval. |
| 28 |
Regulatory requirements and guidelines may also change, and we may, among other things, need to revise clinical trial plans submitted to applicable regulatory authorities to reflect such changes. We may make revisions that necessitate resubmission of the clinical trial proposal to the Institutional Review Board or Ethics Committee for reconsideration, which may affect the outcome of the clinical trial.
The process of developing, obtaining regulatory approval, and commercializing medical device candidates is long, complex, and costly, both within China and abroad. Even if our product candidates are successful in obtaining regulatory approval, such approval may impose significant restrictions on the approved use, or require product labels to contain precautions or warnings, or require expensive and time-consuming post-approval clinical trials or surveillance as approval conditions of. If we are unable to obtain regulatory approvals for our product candidates in one or more jurisdictions, or any approvals come with significant restrictions, our target market will be reduced and our ability to realize the full market potential of our product candidates will be impaired. In addition, we may not be able to obtain sufficient financing or generate sufficient revenue and cash flow to continue developing any other product candidates in the future.
Our products and any future products will be subject to ongoing regulatory obligations and ongoing regulatory scrutiny, which may result in significant additional expenses, and if we fail to comply with regulatory requirements or if there are unexpected issues with our products and/or product candidates, we may will be punished.
Our regulatory agency-approved testing services, products and any other product candidates are subject to and will be subject to compliance with manufacturing, testing, labelling, packaging, storage, advertising, promotion, sampling, record keeping, post-marketing studies, submission of safety, efficacy and other ongoing regulatory requirements for post-listing materials, and other requirements from regulatory authorities in the PRC and/or other jurisdictions. Our testing and manufacturing facilities are subject to numerous regulatory requirements from the China Food and Drug Administration (“CFDA”) and/or other similar authorities. Accordingly, we have been and will be subject to ongoing scrutiny and inspection by regulatory agencies to assess our compliance with applicable laws and regulations and our commitments made in any application materials submitted to the CFDA or other authorities. Therefore, we must continue to invest time, money and effort in all aspects of regulatory compliance.
Regulatory approvals for our products and any approvals we obtain for product candidates are subject to and may be limited by the use of our marketable products. Our approvals may also be subject to other conditions that may result in the need for potentially costly post-market testing and surveillance to monitor the safety and efficacy of our products or product candidates. Such restrictions and conditions may adversely affect the commercial potential of our products.
After our product candidates are approved for commercialization, certain changes to the products, such as changes in manufacturing processes and additional labeling claims, may require additional review and approval by the CFDA and/or similar regulatory authorities. Regulatory approval of any of our product candidates may also be withdrawn. If we fail to maintain compliance with these ongoing regulatory requirements or if problems arise after the product is launched, the CFDA or similar regulatory authorities may seek to enforce a consent order or withdraw marketing approval. Late discovery of our products or product candidates or issues previously unknown to us in our manufacturing process may lead to revisions to approved labels or regulations to add new safety information; conduct clinical studies to evaluate new safety risk; or impose distribution restrictions or other restrictions. Other potential consequences include (among others):
| ● | Restrictions on the listing or manufacture of our products, withdrawal of products from the market, or voluntary or mandatory product recalls; |
| ● | Fines, untitled letters or warning letters, or suspension of clinical trials; |
| ● | The CFDA or similar regulatory authorities refuse to approve our pending applications or supplements to approved applications, or suspend or revoke licensing approval or withdraw approval; |
| 29 |
| ● | Product seizure or detention, or denial of permission to import or export our products and candidate products; and/or |
| ● | Injunction or Civil or Criminal Penalty |
The CFDA and other regulatory authorities strictly supervise the listing, labelling, advertising and promotion of medical products and services introduced to the market. Our products and testing services may only be marketed for their approved uses in accordance with approved labels. The CFDA and other regulatory authorities actively enforce laws and regulations that prohibit the promotion of off-label uses, and companies found to improperly promote off-label uses may bear significant responsibility. The policies of the CFDA and other regulatory authorities may change and other government regulations may be issued to prevent, limit or delay regulatory approval of our product candidates. Given the changing regulatory environment, we cannot predict the likelihood, nature or scope (whether in China or abroad) of government policies or regulations that may result from future legislative or administrative actions. If we are slow or unable to adapt to changes in existing regulations or adopt new regulations or policies, or if we are unable to maintain regulatory compliance, we may lose any regulatory approvals we have obtained and may not be able to achieve or maintain profitability.
If our existing and new products fail to meet the quality standards required by applicable laws, our business and reputation could be damaged, and our revenue and profitability could be materially and adversely affected.
Our production and manufacturing processes are subject to certain quality standards. We have established quality control and assurance systems and adopted standardized operating procedures to prevent quality issues related to our products and operating processes. For further details of our quality control and assurance system, please refer to “Business — Quality Control System”. Although we have quality control and assurance systems and procedures, we cannot eliminate the risk of product defects or malfunctions. Quality defects may not be detected or remedied due to a number of factors, many of which are beyond our control, including:
| ● | Manufacturing error; |
| ● | Technical or mechanical failures in the manufacturing process; |
| ● | Human error or malfeasance by our quality control personnel; |
| ● | Third party intervention; and/or |
| ● | The raw materials we produce or purchase have quality problems. |
In addition, our failure to detect quality defects in our products or to prevent such defective products from being delivered to end users may result in injury or death, product recalls or withdrawals, revocation of licenses or fines by regulatory agencies, product and professional liability or other problems, which could seriously damage our reputation and business, expose us to the risk of liability, and materially and adversely affect our revenue and profitability.
In addition to the Chinese market, we are planning to expand to North American and European markets. We cannot give any assurance that any of our products will receive regulatory approval in North America or Europe, which is necessary before they can be commercialized.
We cannot be certain that any of our product candidates will be successful in clinical studies or receive regulatory approval. Applications for our products could fail to receive regulatory approval for many reasons, including but not limited to the following:
| · | the Food and Drug Administration (FDA), the competent authority of individual member states of the European Union where we plan to offer our products, or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical studies; |
| · | the population studied in the clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| · | the FDA, the competent authority of individual member states of the European Union where we plan to offer our products, or comparable foreign regulatory authorities may disagree with our interpretation of data from nonclinical or clinical studies; |
| · | the data collected from clinical studies of our products in China may not be sufficient or accepted to obtain regulatory approval in the U.S. or elsewhere; |
| · | we may be unable to demonstrate to the FDA, the competent authority of individual member states of the European Union where we plan to offer our products, or comparable foreign regulatory authorities that a product’s benefit-risk ratio for its proposed indication is acceptable; |
| · | the FDA, the competent authority of individual member states of the European Union where we plan to offer our products, or other regulatory authorities may fail to approve the manufacturing processes, test procedures and specifications, or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| · | the approval policies or regulations of the FDA, the competent authority of individual member states of the European Union where we plan to offer our products, or comparable foreign regulatory authorities may change significantly in a manner rendering our clinical data insufficient for approval. |
Currently, we plan to seek regulatory approval to commercialize our product candidates in the U.S., the EU and in additional countries where we obtain commercial and IP rights. To obtain regulatory approval in other countries, we must comply with numerous and varying regulatory requirements of such other countries regarding safety, efficacy, chemistry, manufacturing and controls, clinical studies, commercial sales, pricing, marketing and distribution of our products. Even if we are successful in obtaining approval in one jurisdiction, we cannot ensure that we will obtain approval in any other jurisdictions. Failure to obtain marketing authorization for our products will result in our being unable to market and sell such products, which would materially adversely affect our business, financial condition and results of operations. If we fail to obtain approval in any jurisdiction, the geographic market for our products could be limited.
Failure to obtain the broad market acceptance or maintain a good reputation necessary for our current products and any future products could materially and adversely affect our results of operations and profitability.
The commercial success of our current and future products depends on their market acceptance, especially among customers, hospitals and physicians. As a diagnostic method recently developed and introduced into the Chinese market, our products may not be widely recognized by the intended target customers, doctors or end users. If our products and any future approved product candidates fail to gain sufficient market acceptance from physicians, end users, third-party payers and other industry players, our product sales will be adversely affected. In addition, customers, physicians, end users and third-party payers may prefer other new products over our products. If our products and product candidates fail to achieve an adequate level of recognition, we may not generate significant revenue and may not be profitable. The failure of our products, product candidates and our testing services to achieve an adequate level of recognition or to increase market visibility could adversely affect our financial condition, business and results of operations. Once approved for commercial sale, the market acceptance of our products and product candidates and their services will depend on a number of factors, including:
| 30 |
| ● | Physicians, end users and hospitals consider our products and product candidates to be safe and effective; |
| ● | Potential and visible advantages of our products and product candidates over other alternatives; |
| ● | Our continuous cooperation with existing commercialization channels; |
| ● | We further validate the product's capabilities through clinical studies and accompanying publications; |
| ● | The timing and scope of the approval of our other cancer screening products by the CFDA; |
| ● | Our ability to maintain laboratory accreditation, accreditation and regulatory approval and to complete required inspections; |
| ● | Defects or errors that lead to negative reports about our or our competitors' testing and technology influences; |
| ● | Changes in government policies or guidelines regarding cancer screening; |
| ● | Development of cancer treatments that may diminish or reduce the need for cancer screening; |
| ● | Our competitors accelerate R&D progress; |
| ● | The effectiveness of our sales and marketing efforts. |
If any of our commercialized products and services fail to gain market acceptance among doctors, end users, hospitals or other customers, or if we fail to maintain good relationships with them, we will not be able to generate significant revenue. Our ability to market our products and product candidates may be limited by regulatory approval requirements, restrictions on approved uses, inherent patterns of clinical practice, uncertainty about third-party compensation or other factors. Even if our products gain market acceptance, we may not be able to maintain market acceptance all the time if new products or technologies are introduced that are more popular, cost-effective or make our products obsolete. We believe that maintaining and enhancing our brand image and increasing the market awareness of our company and our products are critical to gaining broad recognition for our services and products, strengthening our relationships with existing customers and our ability to attract new customers. The successful promotion of our brand will largely depend on our ability to continue to provide high-quality products, as well as our research and development efforts. However, there is no guarantee that our branding activities and research and development efforts will be successful or contribute to our growth. In addition, even if such activities increase revenue, such revenue may not be sufficient to offset the increase in expenses we incur.
There is no guarantee that our products will be covered by the National Medical Insurance Program in China.
China maintains a National Medical Insurance Program that can reimburse certain residents in urban cities and rural areas for, among other things, fees associated with diagnostic and treatment devices and diagnostic tests covered by such Program through the basic medical insurance scheme.
As of the date of this prospectus, our products are not covered by any national or provincial medical insurance programs. Although we strive to make our products covered by the national medical insurance programs in the future, there is no guarantee that the responsible government agency, the National Healthcare Security Administration, will approve our applications. Furthermore, even if our products may be covered by the Program in the future, uncertainties still exist around reimbursement coverage and rates as we need to negotiate such terms with government entities in the PRC, and we cannot guarantee that we will receive favorable coverage and rates. In the absence of reimbursement or favorable coverage and rates from the National Medical Insurance Program, end users have to bear all or the majority part of their expenses to use our products, which may reduce consumers’ interest in our products or cause our products to be less competitive when compared with other products that are covered by such Program.
We have relatively limited experience in product promotion and sales. There is no assurance that we will be able to successfully commercialize our products and, as a result, our revenue and profitability could be materially and adversely affected.
We have relatively limited experience in launching and commercializing product candidates and in the sales and marketing of products, and limited experience in market analysis or managing sales teams for product candidates. As a result, our ability to successfully commercialize our product candidates may involve more inherent risks, require more time and cost more than would be the case if we were a company with extensive experience in launching product candidates.
| 31 |
The market size of existing and future products has not been precisely established and may be lower than our estimates, and we may not fully grasp the target population of our products.
Our estimates of the total addressable market and target population for our existing products and product candidates are based on a number of internal and third-party estimates, including but not limited to the size of the target population, the number of people at higher risk of developing cancer, and the availability of the hypothetical price at which the established market sells the relevant candidate product. Although we believe that our assumptions and data relating to estimates are reasonable, such assumptions and estimates may not be correct, and the conditions supporting our assumptions or estimates may change at any time, reducing the accuracy of forecasts for these relevant factors. Accordingly, our estimates of the total addressable market for current or future products may prove to be incorrect. If the target population that will benefit from our products, the price at which we can sell our products or the total addressable market for our products is lower than our estimates, it could harm our sales growth and adversely affect our business.
Risks Associated with Our Testing and the Manufacture and Supply of Our Products
Delays in the completion and obtaining regulatory approvals of our manufacturing facilities, or damage, destruction or interruption of production at such facilities may delay our development plans or commercialization efforts.
Our future manufacturing facilities will be global. The facility may incur unanticipated expenses due to a number of factors including regulatory requirements. Our manufacturing facilities are subject to ongoing periodic inspections by various NMPA or other comparable regulatory agencies to ensure compliance with current drug manufacturing practices. Failure to comply with applicable regulations may also result in us being subject to penalties, including fines, injunctions, civil penalties, requests to suspend or stop one or more of our clinical trials, failure to obtain regulatory approvals for our product candidates, delays, Suspension or withdrawal of approvals, supply interruptions, revocation of licenses, seizure or recall of products or product candidates, operational restrictions and criminal prosecutions, any of which can cause harm to our business.
Our facilities may be damaged or rendered inoperable due to physical damage caused by fire, flood, earthquake, typhoon, tornado, power outage, telecommunications failure, intrusion and similar events. If our production facilities or equipment are damaged or destroyed, we may not be able to replace our production capacity quickly or cheaply or at all. In the event of temporary or long-term damage to facilities or equipment, we may not be able to transfer manufacturing to third parties. Even if we could transfer manufacturing to a third party, that transfer could be expensive and time-consuming, especially since the new facility has to meet the necessary regulatory requirements and we have to obtain regulatory approvals before selling any products manufactured at that facility. This event could delay our clinical trials or reduce sales of our products. Any disruption to our manufacturing operations at our manufacturing facilities may result in us being unable to meet our clinical trial or commercialization needs. Any disruption to our ability to manufacture products or product candidates in a timely manner could materially damage our business, financial condition and results of operations. We currently insure against damage to our property and equipment for an amount we believe is reasonable. However, our insurance coverage may not compensate us or may not be sufficient to cover any expenses or losses we may incur. In the event of a catastrophic event or the failure of our production facilities or processes, we may not be able to meet our requirements for products and product candidates.
The manufacturing and testing process of our products is complex and subject to strict quality control. If we or any of our suppliers or logistics partners experience manufacturing, logistics or quality problems, including as a result of natural disasters, our business may be damaged.
Partly due to stringent regulatory requirements, the manufacturing and testing process for products is complex and subject to strict quality control. Additionally, quality is paramount as product or test defects can have serious and costly consequences. Manufacturing and testing processes can go wrong for a number of reasons, including equipment failure, non-compliance with codes and procedures, raw material issues, software issues, sample contamination or human error. In addition, if contaminants are found in our product or product candidate supply, or in our production and testing facilities, such production and testing facilities may need to be shut down for an extended period of time to investigate the contamination and remediate it. Stability and other issues may arise in the future related to the manufacture and testing of our products or product candidates. While well-managed, disruptions can occur during the introduction of new equipment and systems to replace aging equipment, as well as production line transfers and expansions. As we increase market penetration, we may face unexpected surges in demand for our products, which could put pressure on our production capacity or testing capabilities. If these problems arise, or if we otherwise fail to meet our internal quality standards or the standards of the CFDA or other applicable regulatory agencies, including detailed record-keeping requirements, our reputation may be damaged and we Safety warnings or recalls may apply, we may incur product and professional liability and other costs, product approvals may be delayed, and our business may otherwise be adversely affected. In addition, our manufacturing, testing and warehousing facilities, as well as those of our suppliers and logistics partners, may be severely damaged by earthquakes, hurricanes, volcanoes, fires and other natural disasters or catastrophic circumstances, could materially and adversely affect our business.
| 32 |
Security threats to our information technology infrastructure and unauthorized use of data by third parties could expose us to liability or damage our reputation and business.
Our information technology systems store and process a variety of sensitive data, including our proprietary business information, as well as patients’ personal data such as health information and personally identifiable information.
It is essential that our information technology infrastructure remains secure and is perceived by hospitals, patients and our research partners to be secure. Despite our security measures, we may face cyber-attacks that attempt to penetrate our network security, sabotage or otherwise disable our research, tests and services, misappropriate our proprietary business information or cause interruptions of our internal systems and services. Any cyber-attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and research partners, and expose us to significant financial liabilities.
Moreover, we may not be able to prevent third parties from illegally obtaining and misappropriating personal data of the tested patients that we collect. Concerns about data leakage or unauthorized use of data by third parties, even if unfounded, could damage our reputation and negatively affect our results of operations.
If we are unable to effectively protect our intellectual property, our business and competitive position would be harmed.
We rely on patents, software copyrights, trademarks, trade secrets and other intellectual property rights protection and contractual restrictions to protect our products, services and technologies. We have registered a number of patents and trademarks in China and the United States. However, such protection is limited and may not adequately protect our rights.
We may also be subject to infringement claims by third parties. We may be subject to fines and other legal or administrative sanctions, and it may also be costly to defend such claims. In addition, competitors could purchase our products and attempt to replicate and/or improve some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, and design their devices and tests around our protected technologies or develop their own competitive technologies that fall outside of our intellectual property rights.
Monitoring unauthorized disclosure and uses of our trade secrets is difficult, and we do not know whether the steps we have taken to prevent such disclosure and uses are, or will be, adequate. If we resort to litigation to enforce or protect our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and financial resources, while the outcome would be unpredictable, and any remedy may be inadequate. Our contractual agreements may be breached by our counterparties, and there may not be adequate remedies available to us for any such breach. In addition, our trade secrets may be leaked or otherwise become available to, or be independently discovered by, our competitors, and we would have no right to prevent others from using them. Moreover, if a party having an agreement with us has an overlapping or conflicting obligation to a third party, our rights in and to certain intellectual property could be undermined. If we fail to effectively protect our intellectual property, our competitive position and prospects could be adversely affected.
| 33 |
We may be subject to intellectual property infringement or misappropriation claims by third parties, which may force us to incur substantial legal expenses and, if determined adversely against us, could disrupt our business.
We cannot be certain that our products, tests and technologies do not or will not infringe patents, software copyrights, trademarks or other intellectual property rights held by third parties, especially when they are in China, as the validity, enforceability and scope of intellectual property rights protection in China are uncertain and still evolving. From time to time, we may be subject to legal proceedings and claims alleging infringement of patents, trademarks or copyrights, or misappropriation of creative ideas or formats, or other infringement of proprietary intellectual property rights. Any such proceedings and claims could result in significant costs to us and divert the time and attention of our management and technical personnel from the operation of our business. These types of claims could also potentially adversely impact our reputation and our ability to conduct business and raise capital, even if we are ultimately absolved of all liability. Moreover, third parties making claims against us may be able to obtain injunctive relief against us, which could block our ability to offer one or more devices or tests and could result in a substantial award of damages against us. In addition, since we sometimes indemnify our customers or collaboration partners, we may have additional liability in connection with any infringement or alleged infringement of third-party intellectual property. Intellectual property litigation can be very expensive, and we may not have the financial means to defend ourselves or our customers or collaboration partners.
Because patent applications can take many years to issue, there may be pending applications, some of which are unknown to us, that may result in issued patents upon which our products, tests or proprietary technologies may infringe. Moreover, we may fail to identify issued patents of relevance or incorrectly conclude that an issued patent is invalid or not infringed by our technology or any of our devices or tests. There is a substantial amount of litigation involving patents and other intellectual property rights in our industry. If a third-party claims that we infringe upon a third-party’s intellectual property rights, we may have to:
| ● | seek to obtain licenses that may not be available on commercially reasonable terms, if at all; |
| ● | abandon any product alleged or held to infringe, or redesign our products or processes to avoid potential assertion of infringement; |
| ● | pay substantial damages including, in exceptional cases, treble damages and attorneys’ fees, if a court decides that the device, test or proprietary technology at issue infringes upon or violates the third-party’s rights; |
| ● | pay substantial royalties or fees or grant cross-licenses to our technology; and |
| ● | defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
We may be subject to litigation and other claims and legal proceedings, and may not always be successful in defending ourselves against these claims or proceedings.
We may be subject to lawsuits and other claims in the ordinary course of our business. We may from time to time be subject to lawsuits and other legal proceedings brought by our customers, competitors, employees, business partners, investors, other shareholders of the companies we invest, or other entities against us in the ordinary course of our business. We may also be subject to regulatory proceedings in the ordinary course of our business. We may not be successful in defending ourselves, and the outcomes of these lawsuits and proceedings may be unfavorable to us. Lawsuits and regulatory proceedings against us may also generate negative publicity that significantly harms our reputation, which may adversely affect our customer base, market position and our relationships with our research partners and other business partners. In addition to the related costs, managing and defending litigation and other legal proceedings and related indemnity obligations can significantly divert our management’s attention from operating our business. We may also need to pay damages or settle lawsuits or other claims with a substantial amount of cash, negatively affecting our liquidity. As a result, our business, financial condition and results of operations could be adversely affected.
| 34 |
Risks Relating to Our Financial Prospects and Need for Additional Capital
We incurred net losses for the years ended June 30, 2023 and 2022 and the three-month period ended September 30, 2023, and may not be able to generate sufficient operating cash flows and working capital to continue as a going concern. Failure to manage our liquidity and cash flows may materially and adversely affect our financial condition and results of operations. As a result, we may need additional capital, and financing may not be available on terms acceptable to us, or at all.
We incurred net losses of $3,732,777, $4,027,722, and $771,316 for the years ended June 30, 2023 and 2022 and three-month period ended September 30, 2023, respectively. Our ability to continue as a going concern depends upon our ability to develop, register and obtain regulatory approval for commercial sell of our products to generate positive operating cash flows. For the financial year ended June 30, 2023 and the three-month period ended September 30, 2023, the Company reported net loss of $3,732,777 and $771,316, respectively. As of June 30, 2023 and September 30, 2023, the Company’s working capital deficit was $489,595 and $861,792, respectively. In addition, the Company had net cash outflows of $2,963,625 and $619,138 from operating activities for the financial year ended June 30, 2023 and three-month period ended September 30, 2023. These conditions give rise to substantial doubt as to whether the Company will be able to continue as a going concern. Management has commenced a strategy to raise debt and equity. However, there can be no certainty that these additional financings will be available on acceptable terms or at all. If management is unable to execute this plan, there would likely be a material adverse effect on the Company’s business.
We can offer no assurance that we will operate profitably or that we will generate positive cash flows in the next twelve months, given our substantial expenses in relation to our revenue at this stage of our Company. Inability to collect our accounts receivable in a timely and sufficient manner, or the inability to offset our expenses with adequate revenue, may adversely affect our liquidity, financial condition and results of operations. Although we believe that our cash on hand will be sufficient to meet our anticipated working capital requirements and capital expenditures in the ordinary course of business for the next 12 months, we cannot assure you this will be the case.
If and when we are unable to meet our working capital requirements and various operating needs, we may need to raise additional funds for our operations and such funds may not be available on commercially acceptable terms, if at all. If we are unable to raise funds on acceptable terms, we may not be able to execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements. This may seriously harm our business, financial condition and results of operations. If we are unable to achieve or maintain profitability, the market price of our shares may significantly decrease. In the event that the Company requires additional funding to finance its operations, the Company’s major shareholders have indicated their intent and ability to provide such financial support, however, there is no assurance such funding will be available when the Company needs it in the future.
We have recorded negative cash flows from operating activities historically and may have a current liabilities position in the future.
We have experienced significant cash outflow from operating activities since our inception. We had net cash outflow in operating activities of US$3.0 million and US$0.7 million for the years ended June 30, 2023 and 2022, respectively. We had net cash outflow of US$0.6 million in operating activities for the three-month period ended September 30, 2023. The cost of continuing operations could further reduce our cash position, and an increase in our net cash outflow from operating activities could adversely affect our operations by reducing the amount of cash available to meet the cash needs for operating our business and to fund our investments in our business expansion.
We had net current liabilities of US$0.86 million as of September 30, 2023. We cannot guarantee that we will turnaround into a net current assets position in future, which would expose us to liquidity risk. Our future liquidity and ability to make additional capital investments necessary for our operations and business expansion will depend primarily on our ability to maintain sufficient cash and to obtain adequate external financing. There can be no assurance that we will be able to obtain any sources of financing.
We require substantial funding for our operations. If we cannot raise sufficient additional capital on acceptable terms, our business, financial condition and prospects may be adversely affected.
We require substantial capital to fund our existing operations, commercialize new products, expand our business and pursue strategic investments. In particular, we require substantial capital to:
| ● | advance our early cancer detection technologies and develop early cancer detection product candidates; |
| ● | increase our sales and marketing efforts to drive market adoption of our products and services and address competitive developments; |
| ● | seek regulatory and marketing approvals for our tests; |
| ● | maintain, expand and protect our intellectual property portfolio; |
| 35 |
| ● | hire and retain additional personnel, such as scientists and sales and marketing personnel; |
| ● | develop, acquire and improve operational, financial and management information systems; |
| ● | add equipment and physical infrastructure to support our research and development programs; |
| ● | finance general and administrative expenses; and |
| ● | operate as a public company. |
Based on our current business plan, we believe our cash and cash equivalents, together with our financing activities, our initial public offering will be sufficient to meet our current and antitcipated needs for general corporate purposes for at least the next 12 months. If our available cash balances are insufficient to satisfy our liquidity requirements, in particular, for the development and commercialization of our products, we may seek to obtain further funding through public or private equity offerings, debt financings or other sources.
Further financing may not be available to us on acceptable terms, or at all. If we fail to raise capital as and when needed it would have a negative impact on our financial condition and our ability to pursue our business strategy. In addition, if we raise funds by issuing debt securities or incurring additional borrowings, the terms of debt securities issued or borrowings could impose significant restrictions on our operations, and we may be unable to repay the indebtedness when due. If we raise funds by issuing equity securities, your investment in our company could be diluted.
Raising additional capital may lead to dilution of shareholdings by our existing shareholders, restrict our operations, and may further result in fair value loss adversely affecting our financial results.
We may seek additional funding through a combination of equity and debt financings and collaborations. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of existing holders of our shares will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our existing shareholders.
The incurrence of additional indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in certain additional restrictive covenants, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license IP rights and other operating restrictions that could adversely impact our ability to conduct our business.
We have only a limited accounting personnel and have corrected an error in application of the accounting principle for business combinations accounted for an acquisition of an entity during the year ended June 30, 2022.
Prior to the completion of this offering, we have been a private company with limited accounting personnel mainly engage in financial reporting. On January 1, 2022, the Company through Advanced Biomed HK Limited acquired 100% equity interest of Shanghai Sglcell. The Company carefully considered the appropriate accounting guidance in accordance with its application of the accounting principle related to accounting for business combinations. However, we did not engage outside experts to assist in the detailed analysis.
Upon further assessment, the Company has corrected an error in its application of the accounting principle for business combinations where the Company previously incorrectly accounted for an acquisition of an entity as a business combination; the Company has amended its consolidated financial statements for the year ended June 30, 2022 to account for the transaction as purchase of assets.
Management concluded that the above correction identified in connection with the accounting treatment for the acquisition was a significant deficiency that did not rise to the level of a material weakness. The deficiency identified was narrow as it focused on the interpretation of accounting literature to a specific transaction structure which is not expected to be recurring nature. For future material acquisitions, the Company will engage outside experts to assist in the transaction, including legal and accounting advisors, to advise on complex accounting issues in order to prevent potential differences in the accounting for business combinations. As a result, the Company does not believe that the significant deficiency rose to the level of a material weakness.
Risks Related to Doing Business in Taiwan
Advanced Biomed Taiwan does not currently own any trademark or patent.
No trademark or patent has been registered by Advanced Biomed Taiwan so far in Taiwan. It also has no pending intellectual property right contracts, including license, transfer or sublicense. Its primary business is research and development of various advanced and innovative microfluidic biochip technologies.
Based on Article 7 of Taiwan’s Patent Act, where a fund provider appoints another party to conduct research and development, the ownership of the right to apply for a patent and the patent right in connection with the outcome of such research and development shall be vested in the party as mutually agreed upon in an agreement between both parties, or such rights shall be vested in the inventor, utility model creator or designer in the absence of such agreement. However, the fund provider shall be entitled to exploit such invention, utility model or design. Advanced Biomed Taiwan signed a research project equipment use contract with TSRI and used their semiconductor manufacturing equipment and precision micro-nano processing equipment for chip technology research and development such as concept presentation of each R&D process, cross-scale composite structure production, rapid wafer trial production, material testing, and thin film production. Both parties agree to keep the test data, process and materials confidential. Advanced Biomed Taiwan agrees that TSRI may quote the test data in its research paper with the consent of Advanced Biomed Taiwan. The contract also provides that any intellectual property rights of one party should only be used in the test by licensing to the other party and that any intellectual property rights derived from the test will belong to the developer. Both parties will jointly own the intellectual property right if it cannot be clearly identified who the developer is. The ownership of patent application rights and patent rights may be obscure in such practice and raise disputes on who the developers are. We cannot assure you that the patents owned or applied by Advanced Biomed Taiwan will not be subject to disputes under Taiwan’s Patent Act.
| 36 |
We face economic and political risks associated with doing business in Taiwan, particularly due to the geopolitical tension between Taiwan and China that could negatively affect our business and hence the value of your investment.
Currently, we rely on Advanced Biomed Taiwan for microfluidic biochip technology and its application in precision medicine in the field of oncology. Accordingly, our business, financial condition and results of operations and the market price of our securities may be affected by changes in governmental policies, taxation, growth rate, inflation rate or interest rates and by social instability and diplomatic and social developments in or affecting Taiwan. In particular, the unique political status of Taiwan and its internal political movement cause sustained tension between China and Taiwan. Past developments related to the interactions between China and Taiwan, especially in relation to trade activities such as bans on exports of goods from time to time, have on occasions depressed the transactions and business operations of certain Taiwanese companies and overall economic environment. We cannot predict whether there will be escalation of the tensions between China and Taiwan which would lead to new bans or tariffs on exports or even conflict. Any conflict which threatens the military, political or economic stability in Taiwan could have a material adverse effect on our current or future business and financial conditions and results of operations.
Advanced Biomed Taiwan is subject to restrictions on paying dividend or making other payments to us, which may restrict our ability to satisfy its liquidity requirements.
As a company incorporated under the laws of the State of Nevada structured as a holding company, we may need dividends and other distributions on equity from our Taiwan subsidiary to satisfy our liquidity requirements. Current Taiwan regulations permit our Taiwan subsidiary to pay dividends to its shareholders only out of its accumulated profits, if any, which shall first make up previous losses and set aside at least 10% of its accumulated profits each year. These reserves are not distributable as cash dividends. Furthermore, if our Taiwan subsidiary incurs debt on its own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us. Any limitation on the ability of our Taiwan subsidiary to distribute dividends or to make payments to us may restrict our ability to satisfy our liquidity requirements. In addition, the dividend payments by our Taiwan subsidiary to us shall be subject to the withholding tax of 21% since January 1, 2018.
| 37 |
Advanced Biomed Taiwan is subject to foreign exchange control imposed by Taiwan authorities, which may affect the paying dividends, repatriating the interest or making other payments to us.
Currently Taiwan regulates only those foreign exchange transactions that involve the conversion of the New Taiwan Dollar into foreign currencies. Pursuant to the relevant provisions of Taiwan Foreign Exchange Control Act, foreign exchange transactions of a value of NTD 500,000 or more shall be declared to the Central Bank of Republic of China (Taiwan). Further, for a remittance by a company as follows, relevant testimonials shall be submitted and such remittance shall be subject to the approval of the Central Bank of Taiwan: (i) a single remittance of an amount over USD 1 million; or (ii) annual accumulated settlement amount of foreign exchange purchased or sold has exceeded USD 50 million. Nevertheless, Taiwan government may impose further foreign exchange restrictions in certain emergency situations, where Taiwan government experiences extreme difficulty in stabilizing the balance of payments or where there are substantial disturbances in the financial and capital markets in Taiwan. If the dividend payments or other payments by our Taiwan subsidiary and branches to us involves the currency conversion from New Taiwan Dollar to US Dollar, such conversion would be subject to the foregoing foreign exchange control imposed by Taiwan authority.
Taiwan laws and regulations of loans to and direct investment in Taiwan entities by offshore holding companies may delay or prevent us from using the proceeds of this offering to make loans or additional contributions to Advanced Biomed Taiwan, which could materially and adversely affect our ability to fund and expand our business.
We are an offshore holding company conducting our R&D operations substantially in Taiwan through our Advanced Biomed Taiwan. We may make loans to Advanced Biomed Taiwan, or we may make additional capital contributions to Advanced Biomed Taiwan, or we may establish new Taiwan subsidiaries and make capital contributions to these new Taiwan subsidiaries, or we may acquire offshore entities with business operations in Taiwan in an offshore transaction.
Most of these ways are subject to Taiwan regulations and approvals or registration. For example, investment, including lending long-term loans, in Taiwan entities require Foreign Investment Approval (as defined below) from the Taiwan Investment Commission. Furthermore, foreign entities are prohibited from investing in some industries which are relating to national security and environmental protection, as specified in the negative list provided by Taiwan authority.
Advanced Biomed Taiwan will collaborate and share its R&D efforts with us and our Shanghai subsidiary and is subject to Taiwan regulations on investment or technical cooperation in China. It may affect its technical cooperation with the Shanghai subsidiary and more seriously its acquisition by Advanced Biomed.
As the cross-strait relations become more and more sensitive recently, Taiwan authorities incline to prohibit Taiwan technology companies from selling their subsidiaries or assets to mainland Chinese investors to prevent leak of sensitive technologies, including semiconductors. Pursuant to the Taiwan Permission Regulations for Investment or Technical Cooperation in the PRC and the Review Principles for Investments or Technical Cooperation in China (“Permission Regulations”), an investment or technical cooperation made by a Taiwanese investor in China is subject to the restrictions thereunder and requires the approval by the competent Taiwan authority, Taiwan Investment Commission. The restrictions under the Permission Regulations include a negative list in which investment or technical cooperation is prohibited. Currently, Advanced Biomed Taiwan’s technical cooperation with the Shanghai subsidiary is not on such negative list. However, we cannot preclude the possibility that the negative list will be amended to restrict Advanced Biomed Taiwan’s cooperation with the Shanghai subsidiary.
Taiwanese investors holding more than 10% of Advanced Biomed common stock will be subject to Taiwan regulations on investment or technical cooperation in China for its investment or technical cooperation in China.
Under the Permission Regulations, for an investment made by a Taiwanese individual or entity (“Taiwanese Investor”) in a “third region” company which conducts the investments or technical cooperation in China defined therein and such Taiwanese Investor (i) acts as director, supervisor, manager or equivalent position or (ii) has a shareholding or capital contribution of 10% or more in such third region company, the investment in such a third region company would also be deemed a defined investment in China and therefore be subject to the Permission Regulations.
Therefore, for our future investment or technical cooperation in China, our Taiwanese shareholders holding 10% or more of Advanced Biomed common stock will need to apply for the foreign investment approval with the competent Taiwan authority, the Taiwan Investment Commission in accordance with the Permission Regulations.Regulations (the “Foreign Investment Approval”). There are restrictions on the investment or technical cooperation with China, including, without limitation, the annual investment amount in China shall be capped at USD 5 million per year for Taiwan individuals or NTD 80 million or 60% of the higher of its stand-along net worth or consolidated net worth for a Taiwan small-medium enterprise. Your indirect investment in the PRC via the Company under the Permission Regulations will be calculated on the portion of your shareholdings in the Company. If your aggregate investments in the PRC exceed the annual ceiling amount, the Taiwan Investment Commission will reject your application for the exceeding investment in the PRC. If the Taiwanese Investor fails to obtain applicable approvals from the Taiwan Investment Commission in respect of its investment in China, an administrative fine ranging NTD 50 thousand to 25 million or imprisonment may be imposed.
The imposition of foreign exchange restrictions in Taiwan may have an adverse effect on foreign investors’ abilities to acquire securities of a Taiwan company, including the shares of our subsidiaries in Taiwan, or to repatriate the interest, dividends or sale proceeds from those securities.
The Taiwan government may impose foreign exchange restrictions in certain emergency situations, including situations where there are sudden fluctuations in interest rates or exchange rates, where the Taiwan government experiences extreme difficulty in stabilizing the balance of payments or where there are substantial disturbances in the financial and capital markets in Taiwan. These restrictions may require foreign investors to obtain the Taiwan government’s approval before acquiring securities of a Taiwan company, including the shares of our subsidiaries in Taiwan, repatriating the interest or dividends from those securities or repatriating the proceeds from the sale of those securities.
Our operations are subject to the effects of a rising rate of inflation.
Taiwan has recently experienced historically high levels of inflation. Although the inflation rate peaked in June 2022 at 3.59%, we observed an upward trend in the third quarter of 2023, with the October figure at 3.05%. If the inflation rate continues to remain at a relatively high level, for example, due to increases in the costs of labor and supplies, it will affect our expenses, such as employee compensation and research and development charges. Additionally, Taiwan is experiencing an acute workforce shortage, which, in turn, has created a very competitive wage environment that may increase our operating costs. To the extent inflation results in rising interest rates and has other adverse effects on the market, it may adversely affect our consolidated financial condition and results of operations.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments, or bringing actions in Taiwan against us.
As a company incorporated under the laws of the State of Nevada, it may be difficult for you to enforce judgments obtained in U.S. courts based on civil liability provisions of the U.S. federal securities laws against us in Taiwan. In addition, there is uncertainty as to whether the courts of Taiwan would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the U.S. or any state, if the following circumstances occurs:
| · | Where the foreign court lacks jurisdiction pursuant to the Taiwan laws; |
| · | Where a default judgment is rendered against the losing defendant, except in the case where the notice or summons of the initiation of action had been legally served in a reasonable time in the foreign country or had been served through judicial assistance provided under the Taiwan laws; |
| · | Where the performance ordered by such judgment or its litigation procedure is contrary to Taiwan public policy or morals; or |
| · | Where there exists no mutual recognition between the foreign country and the Taiwan. |
| 38 |
Risks Related to Doing Business in China
A downturn in China or global economy, and economic and political policies of the PRC could materially and adversely affect our business.
Due to the different regulatory requirements for the marketing of medical device products and invitro diagnosis (“IVD”) products in different regions/countries, it is necessary to complete the registration application and obtain the corresponding license in accordance with the local regulations before engaging in commercial activities in the respective regions/countries (“localization registration”). Afterwards, marketing and sales can be carried out. All the products and equipment we research and develop are designed following the principle of modularization so that products and equipment can be produced locally to meet different regulatory requirements. The Chinese market has a great potential and is our one of the main markets in the future. At present, we have applied for product registration in China in accordance with relevant Chinese laws and regulations. Meanwhile, we have established our Shanghai subsidiary in Mainland China. Accordingly, our business, prospects, financial condition and results of operations may be influenced by political, economic and social conditions in the PRC generally and by continued economic growth in the PRC as a whole. The Chinese economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the Chinese economy has experienced significant growth over the past decades, growth has been uneven, both geographically and among various sectors of the economy, and we cannot assure you that such growth is sustainable. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall Chinese economy but may have a negative effect on us. For example, the Chinese government may direct resources to industries other than the one we operate in and potentially reduce the investors’ interest in our business.
Economic conditions in China are sensitive to global economic conditions. Any prolonged slowdown in the global or Chinese economy may affect potential clients’ confidence in financial market as a whole and have a negative impact on our business, results of operations and financial condition. Additionally, continued turbulence in the international markets may adversely affect our ability to access the capital markets to meet liquidity needs.
Changes in the policies, regulations, rules, and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon our ability to operate profitably in the PRC.
Since we our Shanghai subsidiary is located in Mainland China, economic, political and legal developments in the PRC will affect our business, financial condition, results of operations and prospects. Policies, regulations, rules, and the enforcement of laws of the PRC government can have significant effects on economic conditions in the PRC and the ability of businesses to operate profitably. Our ability to operate profitably in the PRC may be adversely affected by changes in policies by the PRC government, including changes in laws, regulations or their interpretation, particularly those dealing with the Internet, including censorship and other restriction on material which can be transmitted over the Internet, security, intellectual property, money laundering, taxation and other laws that affect our ability to operate business in China.
The Chinese government may intervene in or influence our operations in the PRC at any time or may exert more control over offerings conducted overseas and/or foreign investment in us, which could result in a material change in our operations and and/or the value of the securities we are registering for sale.
The Chinese government has significant oversight and discretion over the conduct of our business and may intervene or influence our operations in the PRC as the government deems appropriate to further regulatory, political and societal goals. The Chinese government has recently published new policies that significantly affected certain industries such as the education and Internet industries, and we cannot rule out the possibility that it will in the future release regulations or policies regarding our industry that could require us to seek permission from Chinese authorities to commence to operate our business, which may adversely affect our business, financial condition and results of operations. Furthermore, recent statements made by the Chinese government have indicated an intent to increase the government’s oversight and control over offerings of companies with significant operations in China that are to be conducted in foreign markets, as well as foreign investment in PRC-based issuers. There is no guarantee that we will not be subject to such direct influence or intervention in the future due to changes in laws or other unforeseeable reasons. There is always a risk that the Chinese government may, in the future, seek to affect operations of any company with any level of operations in China. Any such action, once taken by the Chinese government, could cause the value of our common stock to significantly decline or become worthless. In addition, if we were to become subject to the direct intervention or influence of the PRC government at any time due to changes in laws or other unforeseeable reasons, it may require a material change in our operations and/or result in increased costs necessary to comply with existing and newly adopted laws and regulations or penalties for any failure to comply.
| 39 |
If the Chinese government were to impose new requirements for approval from the PRC authorities to issue our common stock to foreign investors or list on a foreign exchange, such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless.
Recent statements by the Chinese government have indicated an intent to exert more oversight and control over offerings that are conducted overseas and/or foreign investments in PRC based issuers. PRC has recently promulgated new rules that require companies collecting or holding large amounts of data to undergo a cybersecurity review prior to listing in foreign countries, a move that will significantly tighten oversight over PRC-based internet giants. The Measures for Cybersecurity Review (2021 version) was promulgated on December 28, 2021 and became effective on February 15, 2022. These measures specify that any “online platform operators” controlling the personal information of more than one million users which seek to list on a foreign stock exchange are subject to prior cybersecurity review.
On November 14, 2021, the Cyberspace Administration of China (the “CAC”) published the Draft Regulations on the Network Data Security Administration (Draft for Comments) (the “Security Administration Draft”), which provides that data processing operators engaging in data processing activities that affect or may affect national security must be subject to cybersecurity review by the relevant Cyberspace Administration of the PRC. According to the Security Administration Draft, data processing operators shall apply for a cybersecurity review by the relevant Cyberspace Administration of the PRC under certain circumstances, such as (i) mergers, restructurings, and divisions of Internet platform operators that hold large amount of data relating to national security, economic development, or public interest which affects or may affect the national security, (ii) overseas listings of data processors that process personal data for more than one million individuals, (iii) Hong Kong listings of data processors that affect or may affect national security, and (iv) other data processing activities that affect or may affect the national security. The deadline for public comments on the Security Administration Draft was December 13, 2021.
The PRC Data Security Law, which was promulgated by the Standing Committee of the National People's Congress (the “SCNPC”) on June 10, 2021 and took effect on September 1, 2021, requires data collection to be conducted in a legitimate and proper manner, and stipulates that, for the purpose of data protection, data processing activities must be conducted based on data classification and hierarchical protection system for data security.
On August 20, 2021, the SCNPC promulgated the Personal Information Protection Law of the People’s Republic of China, or the Personal Information Protection Law, which integrates the scattered rules with respect to personal information rights and privacy protection and took effect on November 1, 2021.
Our business does not involve the collection of user data, implicate cybersecurity, or involve any other type of restricted industry. Based on the advice of PRC counsel and our understanding of currently applicable PRC laws and regulations, our registered public offering in the U.S. is not subject to the review or prior approval of the CAC .CAC. As of the date of this prospectus, we have not received any notice from any authorities identifying the operating entities as CIIOs or requiring us to go through cybersecurity review or network data security review by the CAC. Uncertainties still exist, however, due to the possibility that laws, regulations, or policies in the PRC could change rapidly in the future. Any future action by the PRC government expanding the categories of industries and companies whose foreign securities offerings are subject to review by the CAC could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and could cause the value of such securities to significantly decline or be worthless.
On February 17, 2023, the CSRC released Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies with five interpretive guidelines (the “Trial Measures”), which came into effect on March 31, 2023. Pursuant to the Trial Measures, a PRC domestic company that seeks to offer and list securities in overseas markets, either in direct or indirect overseas offering, shall fulfill the filing procedure with the CSRC and report relevant information to the CSRC. Direct overseas offering and listing by domestic companies refers to such overseas offering and listing by a joint-stock company incorporated domestically. Any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect offering and listing in overseas market and, therefore, be subject to filing requirement: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in the Mainland China, or its main places of business are located in the Mainland China, or the senior managers in charge of its business operation and management are mostly Chinese citizens or domiciled in the Mainland China. The determination as to whether or not an overseas offering and listing by domestic companies is indirect, shall be made on substance over form basis. As of the date of this prospectus, as advised by AllBright, we do not believe that we are required to obtain the approval from or complete the filing with the CSRC for this offering and thus we have not submitted an application for approval for this offering with the CSRC pursuant to the Trial Measures based on the factfacts that (i) we are a holding company incorporated in the State of Nevada, not a company incorporated under the PRC law; (ii) based on the report on the Shanghai subsidiary from a third party accounting firm in China, the Shanghai subsidiary contributed less than 50% to our operating revenue, total profit, total assets or net assets as documented in the audited consolidated financial statements for the fiscal year ended June 30, 2023; (iii) the business activities are primarily conducted in Taiwan, with minimal operation in Mainland China, and (iv) most of our officers and directors are non-Chinese citizens or domiciled outside Mainland China. Thus, we do not meet the explicit conditions set out in the Trial Measures to determine whether an overseas offering shall be deemed as an indirect overseas offering and listing by a domestic company. However, as the Trial Measures was newly published, there are substantial uncertainties as to the implementation and interpretation, and the CSRC may take a view that is contrary to our understanding of the Trial Measures. If we are required by the CSRC to submit and complete the filing procedures of this offering and listing, we cannot assure you that we will be able to complete such filings in a timely manner, or even at all. Any failure by us to comply with such filing requirements under the Trial Measures may result in rectification, warnings, and a fine between RMB 1 million and RMB 10 million on our Shanghai subsidiary, which could adversely and materially affect our business operations and financial outlook, and significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or such shares to become worthless.
On February 24, 2023, the CSRC, together with other PRC government authorities, released the Provisions on Strengthening the Confidentiality and Archives Administration Related to the Overseas Securities Offering and Listing by Domestic Enterprises (the “Confidentiality and Archives Administration Provisions”), which come into effect on March 31, 2023. The Confidentiality and Archives Administration Provisions require, among others, that PRC domestic enterprises seeking to offer and list securities in overseas markets, either directly or indirectly, shall establish the confidentiality and archives system, and shall complete approval and filing procedures with competent authorities, if such PRC domestic enterprises or their overseas listing entities provide or publicly disclose documents or materials involving state secrets and work secrets of PRC government agencies to relevant securities companies, securities service institutions, overseas regulatory agencies and other entities and individuals. It further stipulates that providing or publicly disclosing documents and materials which may adversely affect national security or public interests, and accounting files or copies of important preservation value to the state and society shall be subject to corresponding procedures in accordance with relevant laws and regulations. As of the date of this prospectus, our Shanghai subsidiary has established the confidentiality and archives system and we are not subject to the approval to the competent authorities since we do not possess any documents or materials involving state secrets and work secrets of PRC government agencies.
We have been closely monitoring regulatory developments in PRC regarding any necessary approvals from the CSRC or other PRC governmental authorities required for overseas listings, including this offering. As of the date of this prospectus, we have not received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC or other PRC governmental authorities. However, there remains significant uncertainty as to the enactment, interpretation and implementation of regulatory requirements related to overseas securities offerings and other capital markets activities, which could materially and adversely impact our business and financial outlook and may impact our ability to accept foreign investments, or continue to list on a U.S. or other foreign exchange.
| 40 |
Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to you and the Company.
Our operations are subject to various PRC laws and regulations generally applicable to companies in the PRC. The PRC legal system is based on written statutes. Unlike common law systems, it is a system in which legal cases have limited value as precedents. In the late 1970s, the PRC government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past four decades has significantly increased the protections afforded to various forms of foreign or private-sector investment in the PRC.
However, the PRC has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in the PRC. In particular, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until sometime after the violation. Such uncertainties, including uncertainty over the scope and effect of our contractual, property (including intellectual property) and procedural rights, and any failure to respond to changes in the regulatory environment in the PRC could materially and adversely affect our business and impede our ability to continue our operation in the PRC.
In addition, if certain PRC laws and regulations were to become applicable to us in the future, the application of such laws and regulations may have a material adverse impact on our business, financial condition and results of operations, any of which may cause the value of our securities to significantly decline or become worthless. In addition, the laws and regulations in the PRC are evolving, and their enactment timetable, interpretation and implementation involve significant uncertainties. To the extent any PRC laws and regulations become applicable to our business, it may be subject to the risks and uncertainties associated with the legal system in the PRC, including with respect to the enforcement of laws and the possibility of changes of rules and regulations with little or no advance notice.
| 41 |
You may experience difficulties in effecting service of legal process, enforcing foreign judgments, or bringing actions in PRC against us.
As a company incorporated under the laws of the State of Nevada, it may be difficult for you to enforce judgments obtained in U.S. courts based on civil liability provisions of the U.S. federal securities laws against us in the PRC. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts against us or such persons predicated upon the civil liability provisions of the securities laws of the U.S. or any state.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between PRC and the country where the judgment is made or on principles of reciprocity between jurisdictions. PRC does not have any treaties or other forms of written arrangement with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security, or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
Non-compliance with the PRC Labor Contract Law and other labor-related regulations in the PRC may adversely affect our business and our results of operations.
We have been subject to stricter regulatory requirements in terms of entering into labor contracts with our employees and paying various statutory employee benefits, including pensions, housing fund, medical insurance, work-related injury insurance, unemployment insurance and childbearing insurance to designated government agencies for the benefit of our employees.
Pursuant to the PRC Labor Contract Law, or the Labor Contract Law, which was released on January 1, 2008, was amended on December 28, 2012 and became effective on July 1, 2013 employees have the right, among others, to enter into written labor contracts, minimum wages, paying remuneration, determining the term of employees’ probation and unilaterally terminating labor contracts. In the event that we decide to terminate some of our employees or otherwise change our employment or labor practices, the Labor Contract Law and its implementation rules may limit our ability to effect those changes in a desirable or cost-effective manner, which could adversely affect our business and results of operations. In addition, according to the PRC Social Insurance Law, which was released on July 1, 2011, amended on December 29, 2018 and became effective on the same day, and the Administrative Regulations on the Housing Provident Funds, which was released on April 3, 1999, amended on March 24, 2019 and became effective on the same day, companies operating in the PRC are required to participate in pension insurance, work-related injury insurance, medical insurance, unemployment insurance, maternity insurance and housing provident fund plans, and the employers must pay all or a portion of the social insurance premiums and housing provident funds for their employees. We believe our current practice complies with the Labor Contract Law and its amendments. However, the relevant governmental authorities may take a different view and impose fines on us.
As the interpretation and implementation of these laws and regulations are still evolving, our employment practices may not at all times be deemed in compliance with the new laws and regulations. If we incur significant liabilities in connection with labor disputes or investigations, our businesses and results of operations may be adversely affected.
Changes in the PRC’s economic, political or social conditions or government policies could have a material adverse effect on our PRC businesses and operations.
The PRC’s economy differs from the economies of the PRC’s counterpart countries in many respects, including the level of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the PRC government has implemented measures since the late 1970s emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets, and the establishment of improved corporate governance in business enterprises, which are generally viewed as a positive development for foreign business investment, a substantial portion of productive assets in the PRC is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. The PRC government also exercises significant control over the PRC’s economic growth through allocating resources, controlling payments of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
While the PRC’s economy has experienced significant growth over the past decades, such growth has been uneven, both geographically and among various sectors of the economy, and the rate of growth has been slowing down. In addition, in the past, the PRC government has implemented certain measures to control the pace of economic growth. These measures may cause decreased economic activity, which in turn could lead to a low demand for our products and services.
| 42 |
Our Shanghai subsidiary are subject to restrictions on paying dividends or making other payments to us, which may restrict our ability to satisfy our liquidity requirements in the future.
Although currently our Shanghai subsidiary are inactive, in the future, we may need dividends and other distributions on equity from our Shanghai subsidiary to satisfy our liquidity requirements. Current PRC regulations permit our Shanghai subsidiary to pay dividends to their respective shareholders only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, such companies are required to set aside at least 10% of its accumulated profits each year, if any, to fund certain reserve funds until the total amount set aside reaches 50% of its registered capital. our Shanghai subsidiary may also, at the respective subsidiary’s discretion, allocates a portion of its after-tax profits based on its articles of association and PRC accounting standards to certain reserve funds. These reserves are not distributable as cash dividends. Furthermore, if our Shanghai subsidiary incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us. Any limitation on the ability of our Shanghai subsidiary to distribute dividends or to make payments to us may restrict our ability to satisfy our future liquidity requirements.
In addition, the Enterprise Income Tax Law and its implementation rules provide that a withholding tax rate of up to 10% will be applicable to dividends payable by PRC companies to non-PRC-resident enterprises unless otherwise exempted or reduced according to treaties or arrangements between the PRC central government and governments of other countries or regions where the non-PRC-resident enterprises are incorporated.
If we are deemed by the PRC tax authorities as a PRC tax resident enterprise for tax purposes, any dividends we pay to our non-PRC resident shareholders may be regarded as China-sourced income and as a result, may be subject to PRC withholding tax at a rate of up to 10.0%. Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, or the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be reduced to 5% if a Hong Kong resident enterprise owns no less than 25% of a PRC entity. However, the 5% withholding tax rate does not automatically apply and certain requirements must be satisfied, including, without limitation, that (a) the Hong Kong entity must be the beneficial owner of the relevant dividends; and (b) the Hong Kong entity must directly hold no less than 25% share ownership in the PRC entity during the 12 consecutive months preceding its receipt of the dividends. In practice, a Hong Kong entity must obtain a tax resident certificate from the Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot be certain that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to any dividends to be paid by our Shanghai subsidiary to our Hong Kong subsidiary, Advanced Biomed HK. Our Shanghai subsidiary currently does not have any plan to declare and pay dividends, and we have not applied for the tax resident certificate from the relevant Hong Kong tax authority.
We can give no assurance that we will declare dividends of any amounts, at any rate or at all in the future. The declaration of future dividends, if any, will be at the discretion of our board of directors and will depend upon our future operations and earnings, capital requirements, general financial conditions, legal and contractual restrictions and other factors that our board of directors may deem relevant.
PRC regulation on loans to, and direct investment in, PRC entities by offshore holding companies and governmental control in currency conversion may delay or prevent us from making loans to or making additional capital contributions to our Shanghai subsidiary.
Any funds we transfer to the Shanghai subsidiary, either as a shareholder loan or as an increase in registered capital, are subject to approval by or registration with relevant governmental authorities in China. According to the relevant PRC regulations on foreign invested enterprises in China, capital contributions to our Shanghai subsidiary are subject to the registration with the State Administration for Market Regulation or its local counterpart and registration with a local bank authorized by the State Administration of Foreign Exchange (“SAFE”). In addition, (i) any foreign loan procured by our Shanghai subsidiary is required to be registered with the SAFE or its local branches and (ii) any of our Shanghai subsidiary may not procure loans which exceed the difference between its total investment amount and registered capital or, as an alternative, only procure loans subject to the calculation approach and limitation as provided by the People’s Bank of China. Additionally, any medium or long-term loans to be provided by us to the Shanghai subsidiary must be registered with the National Development and Reform Commission and SAFE or its local branches. We may not be able to obtain these government approvals or complete such registrations in a timely manner, or at all, with respect to future capital contributions or loans by us to our Shanghai subsidiary. If we fail to receive such approvals or complete such registration or filing, our ability to use the proceeds of future offerings to capitalize our PRC operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business.
| 43 |
SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming the Administration of Foreign Exchange Settlement of Capital of Foreign-invested Enterprises, or SAFE Circular 19, effective on June 1, 2015 and was amended on December 30, 2019, in replacement of the Circular on the Relevant Operating Issues Concerning the Improvement of the Administration of the Payment and Settlement of Foreign Currency Capital of Foreign-Invested Enterprises, the Notice from the State Administration of Foreign Exchange on Relevant Issues Concerning Strengthening the Administration of Foreign Exchange Businesses, and the Circular on Further Clarification and Regulation of the Issues Concerning the Administration of Certain Capital Account Foreign Exchange Businesses. According to SAFE Circular 19, the flow and use of the RMB capital converted from foreign currency-denominated registered capital of a foreign-invested company is regulated such that RMB capital may not be used for the issuance of RMB entrusted loans, the repayment of inter-enterprise loans or the repayment of bank loans that have been transferred to a third party. Although SAFE Circular 19 allows RMB capital converted from foreign currency-denominated registered capital of a foreign-invested enterprise to be used for equity investments within the PRC, it also reiterates the principle that RMB converted from the foreign currency-denominated capital of a foreign-invested company may not be directly or indirectly used for purposes beyond its business scope. Thus, it is unclear whether the SAFE will permit such capital to be used for equity investments in the PRC in actual practice. The SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or SAFE Circular 16, effective on June 9, 2016, which reiterates some of the rules set forth in SAFE Circular 19, but changes the prohibition against using RMB capital converted from foreign currency-denominated registered capital of a foreign-invested company to issue RMB entrusted loans to a prohibition against using such capital to grant loans to non-associated enterprises. Violations of SAFE Circular 19 and SAFE Circular 16 could result in administrative penalties. SAFE Circular 19 and SAFE Circular 16 may significantly limit our ability to transfer any foreign currency it holds, including the net proceeds from the offering to our Shanghai subsidiary, which may adversely affect our liquidity and our ability to fund and expand our business in the PRC. On October 23, 2019, SAFE issued the Notice of the State Administration of Foreign Exchange on Further Facilitating Cross-border Trade and Investment, or “SAFE Circular 28,” which, among other things, expanded the use of foreign exchange capital to domestic equity investment area. Non-investment foreign-funded enterprises are allowed to lawfully make domestic equity investments by using their capital if (i) such investments do not violate the current Negative List and (ii) the domestic investment projects are authentic and are in compliance with relevant regulations. However, since SAFE Circular 28 is newly promulgated, it is unclear how SAFE and competent banks will carry it out in practice.
In light of the various requirements imposed by PRC regulations on loans to, and direct investment in, PRC entities by offshore holding companies, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans or future capital contributions by us to our Shanghai subsidiary. If we fail to complete such registrations or obtain such approvals, our ability to capitalize or otherwise fund our PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
PRC regulations relating to offshore investment activities by PRC residents may subject our PRC resident beneficial owners or our Shanghai subsidiary to liability or penalties, limit our ability to inject capital into our Shanghai subsidiary, limit our Shanghai subsidiary’s ability to increase its registered capital or distribute profits to us, or may otherwise adversely affect us.
In July 2014, SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment Through Special Purpose Vehicles, or SAFE Circular 37, to replace the Notice on Relevant Issues Concerning Foreign Exchange Administration for Domestic Residents’ Financing and Roundtrip Investment Through Offshore Special Purpose Vehicles, or SAFE Circular 75, which ceased to be effective upon the promulgation of SAFE Circular 37. SAFE Circular 37 requires PRC residents (including PRC individuals and PRC corporate entities) to register with SAFE or its local branches in connection with their direct or indirect offshore investment activities. SAFE Circular 37 may be applicable to any offshore acquisitions that we make in the future.
Under SAFE Circular 37, PRC residents who make, or have prior to the implementation of SAFE Circular 37 made, direct or indirect investments in offshore special purpose vehicles, or SPVs, will be required to register such investments with SAFE or its local branches. In addition, any PRC resident who is a direct or indirect shareholder of an SPV is required to update its filed registration with the local branch of SAFE with respect to that SPV, to reflect any material change. Moreover, any subsidiary of such SPV in China is required to urge the PRC resident shareholders to update their registration with the local branch of SAFE. If any PRC shareholder of such SPV fails to make the required registration or to update the previously filed registration, the subsidiary of such SPV in China may be prohibited from distributing its profits or the proceeds from any capital reduction, share transfer or liquidation to the SPV, and the SPV may also be prohibited from making additional capital contributions into its subsidiary in China. On February 13, 2015, the SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration Policy on Direct Investment, or SAFE Notice 13, which became effective on June 1, 2015. Under SAFE Notice 13, applications for foreign exchange registration of inbound foreign direct investments and outbound overseas direct investments, including those required under SAFE Circular 37, will be filed with qualified banks instead of SAFE. The qualified banks will directly examine the applications and accept registrations under the supervision of SAFE.
As of the date of this prospectus, none of our shareholders are subject to the SAFE Circular 37. We may not be informed of the identities of all the PRC residents holding direct or indirect interest in our company, however, and we have no control over any of our future beneficial owners. Thus, we cannot provide any assurance that our current or future PRC resident beneficial owners will comply with our request to make or obtain any applicable registrations or continuously comply with all registration procedures set forth in these SAFE regulations. Such failure or inability of our PRC residents beneficial owners to comply with these SAFE regulations may subject us or our PRC resident beneficial owners to fines and legal sanctions, restrict our cross-border investment activities, or limit our Shanghai subsidiary’s ability to distribute dividends to or obtain foreign-exchange-dominated loans from us, or prevent us from being able to make distributions or pay dividends, as a result of which our business operations and our ability to distribute profits to you could be materially and adversely affected.
Recent joint statement by the SEC and the PCAOB, rule changes by Nasdaq, and the Holding Foreign Companies Accountable Act all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our continued listing or future offerings of our securities in the U.S.
On April 21, 2020, SEC Chairman Jay Clayton and PCAOB Chairman William D. Duhnke III, along with other senior SEC staff, released a joint statement highlighting the risks associated with investing in companies based in or have substantial operations in emerging markets including China. The joint statement emphasized the risks associated with lack of access for the PCAOB to inspect auditors and audit work papers in China and higher risks of fraud in emerging markets.
On May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply minimum offering size requirement for companies primarily operating in “Restrictive Market”, (ii) adopt a new requirement relating to the qualification of management or board of director for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant or listed company based on the qualifications of the company’s auditors.
On May 20, 2020, the U.S. Senate passed the Holding Foreign Companies Accountable Act requiring a foreign company to certify it is not owned or controlled by a foreign government if the PCAOB is unable to audit specified reports because the company uses a foreign auditor not subject to PCAOB inspection. If the PCAOB is unable to inspect the company’s auditors for three consecutive years, the issuer’s securities are prohibited to trade on a national exchange. On December 2, 2020, the U.S. House of Representatives approved the Holding Foreign Companies Accountable Act. On December 18, 2020, the Holding Foreign Companies Accountable Act was signed into law.
| 44 |
On March 24, 2021, the SEC announced the adoption of interim final amendments to implement the submission and disclosure requirements of the Holding Foreign Companies Accountable Act. In the announcement, the SEC clarifies that before any issuer will have to comply with the interim final amendments, the SEC must implement a process for identifying covered issuers. The announcement also states that the SEC staff is actively assessing how best to implement the other requirements of the Holding Foreign Companies Accountable Act, including the identification process and the trading prohibition requirements.
On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if passed by the U.S. House of Representatives and signed into law, would reduce the number of consecutive non-inspection years required for triggering the prohibitions under the Holding Foreign Companies Accountable Act from three years to two. On December 29, 2022, a legislation entitled “Consolidated Appropriations Act, 2023” (the “Consolidated Appropriations Act”), was signed into law by President Biden. The Consolidated Appropriations Act contained, among other things, an identical provision to HFCAA, which reduces the number of consecutive non-inspection years required for triggering the prohibitions under the HFCAA from three years to two.
On September 22, 2021, the PCAOB adopted a final rule implementing the Holding Foreign Companies Accountable Act, which provides a framework for the PCAOB to use when determining, as contemplated under the Holding Foreign Companies Accountable Act, whether the board of directors of a company is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction.
On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the Holding Foreign Companies Accountable Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. The final amendments are effective on January 10, 2022. The SEC will begin to identify and list Commission-Identified Issuers on its website shortly after registrants begin filing their annual reports for 2021.
On December 16, 2021, PCAOB announced the PCAOB Holding Foreign Companies Accountable Act determinations (the “PCAOB determinations”) relating to the PCAOB’s inability to inspect or investigate completely registered public accounting firms headquartered in China of the PRC or Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in the PRC or Hong Kong.
On August 26, 2022, PCAOB signed a Statement of Protocol with the CSRC and the Ministry of Finance of the People’s Republic of China governing inspections and investigations of audit firms based in China and Hong Kong.
On December 15, 2022, the PCAOB announced in the 2022 Determination its determination that the PCAOB was able to secure complete access to inspect and investigate accounting firms headquartered in mainland China and Hong Kong, and the PCAOB Board voted to vacate previous determinations to the contrary.
Should the PCAOB again encounter impediments to inspections and investigations in mainland China or Hong Kong as a result of positions taken by any authority in either jurisdiction, including by the CSRC or the MOF, the PCAOB will make determinations under the HFCAA as and when appropriate. The inability of the PCAOB to conduct inspections of auditors in PRC makes it more difficult to evaluate the effectiveness of these accounting firm’s audit procedures or quality control procedures as compared to auditors outside of PRC that are subject to the PCAOB inspections, which could cause investors and potential investors in our Common stock to lose confidence in our audit procedures and reported financial information and the quality of our financial statements.
Our auditor WWC, P.C., the independent registered public accounting firm that issues the audit report included elsewhere in this prospectus, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards Our auditor, WWC, P.C. is headquartered in San Mateo, California and has been inspected by the PCAOB on a regular basis, with the last inspection conducted in December 2021. It is not subject to the determinations announced by the PCAOB on December 16, 2021 or the Statement of Protocol with the China Securities Regulatory Commission and the Ministry of Finance of the People's Republic of China on August 26, 2022. However, if the PCAOB is unable to inspect our accounting firm in the future, our common stock may be delisted from or prohibited from trading on a national securities exchange.
The recent developments would add uncertainties to our offering and we cannot assure you whether Nasdaq or regulatory authorities would apply additional and more stringent criteria to us since we are an emerging growth company and substantially all of our operations are conducted in China. Furthermore, the Consolidated Appropriations Act reduces the period for foreign companies to comply with PCAOB audits to two consecutive years instead of three, thus reducing the time period for triggering the prohibition on trading, and this ultimately could result in our common stock being delisted by an exchange.
| 45 |
The M&A Rules and certain PRC regulations may make it more difficult for us to pursue growth through acquisitions.
Among other things, the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, adopted by six PRC regulatory agencies in 2006 and amended in 2009, and some other regulations and rules concerning mergers and acquisitions, established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex. Such regulation requires, among other things, that the Ministry of Commerce of the PRC, or the MOFCOM, be notified in advance of any change-of-control transaction in which a foreign investor acquires control of a PRC domestic enterprise or a foreign company with substantial PRC operations, if certain thresholds under the Provisions on Thresholds for Prior Notification of Concentrations of Undertakings, issued by the State Council in 2008, are triggered. Moreover, the Anti-Monopoly Law promulgated by the Standing Committee of the National People’s Congress which became effective in 2008 requires that transactions which are deemed concentrations and involve parties with specified turnover thresholds must be cleared by the Anti-Monopoly Bureau of State Administration for Market Regulation, or the Anti-Monopoly Bureau, before they can be completed. Therefore, our acquisitions of other entities that we make in the future (whether by ourselves or our subsidiaries) and that meets the thresholds for clearance, may be required to be report to and approved by the anti-monopoly law enforcement agency in the PRC, and we may be subject to penalty including but not limited to a fine of no more than RMB500,000 if we fail to comply with such requirement. In addition, PRC national security review rules which became effective in September 2011 require acquisitions by foreign investors of PRC companies engaged in military related or certain other industries that are crucial to national security be subject to security review before consummation of any such acquisition. On December 19, 2020, the Measures for the Security Review for Foreign Investment was jointly issued by National Development and Reform Commission (“NDRC”) and MOFCOM and took effect from January 18, 2021. The Measures for the Security Review for Foreign Investment specified provisions concerning the security review mechanism on foreign investment, including the types of investments subject to review, review scopes and procedures, among others.
| 46 |
It may be difficult for overseas shareholders and/or regulators to conduct investigations or collect evidence within the PRC.
Shareholder claims or regulatory investigation that are common in the United States generally are difficult to pursue as a matter of law or practicality in the PRC. For example, in the PRC, there are significant legal and other obstacles to providing information needed for regulatory investigations or litigation initiated outside the PRC or otherwise with respect to foreign entities. Although the authorities in the PRC may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such cooperation with the securities regulatory authorities in the Unities States may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, according to Article 177 of the PRC Securities Law, which became effective in March 2020, or Article 177, the securities regulatory authority of the State Council may collaborate with securities regulatory authorities of other countries or regions in order to monitor and oversee cross border securities activities. Article 177 further provides that no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC, and that PRC entities and individuals are not allowed to provide documents or materials related to securities business activities to overseas agencies without prior consent of the securities regulatory authority of the State Council and the competent departments of the State Council. While detailed interpretation of or implementation rules under Article 177 have yet to be promulgated, the inability for an overseas securities regulator to directly conduct investigation or evidence collection activities within the PRC may further increase difficulties faced by you in protecting your interests.
| 47 |
Risks Related to our Common Stock
Our common stock may not develop an active trading market and the price and trading volume of our shares may fluctuate significantly.
Following this offering, our common stock will be listed on the Nasdaq Capital Market. We cannot predict whether investor interest in us will lead to the development of an active and liquid trading market. In addition, no assurances can be given regarding when, and if, we will be able to list on a national exchange, including whether or not we will be able to meet applicable listing standards for any such exchange. If an active trading market does not develop, holders of our shares of common stock may have difficulty selling our shares that may now be owned or may be purchased later. In addition, until we are able to be listed on a national exchange, the number of investors willing to hold or acquire our shares may be reduced, we may receive decreased news and analyst coverage, and we may be limited in our ability to issue additional securities or obtain additional financing in the future on terms acceptable to us, or at all. Even if an active trading market develops for our shares, the market price of our shares may be highly volatile and could be subject to wide fluctuations. In addition, the trading volume of our shares may fluctuate and cause significant price variations to occur.
| 48 |
We do not anticipate paying cash dividends on our common stock in the foreseeable future.
We do not anticipate paying cash dividends in the foreseeable future. Presently, we intend to retain all our earnings, if any, to finance development and expansion of our business. Consequently, your only opportunity to achieve a positive return on your investment in us will be if the market price of our common stock appreciates.
Our Chairman Dr. Yi Lu and Chief Executive Officer Dr. Hung To Pau collectively own a majority of our outstanding shares of common stock and could significantly influence the outcome of our corporate matters.
Our Chairman Dr. Yi Lu beneficially owns 33.54% of our outstanding shares of common stock, and Chief Executive Officer Dr. Hung To Pau beneficially owns 17.62% of our outstanding shares of common stock. As a result, Messrs. Lu and Pau are collectively able to exercise significant influence over all matters that require us to obtain shareholder approval, including the election of directors to our board and approval of significant corporate transactions that we may consider, such as a merger or sale of our company or its assets. This concentration of ownership in our shares by an executive officer and controlling shareholders will limit other shareholders’ ability to influence corporate matters and may have the effect of delaying or preventing a third party from acquiring control over us.
The price of our common stock may be volatile or may decline regardless of our operating performance, and stockholders may not be able to resell their shares.
The market price of our stock may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| ● | actual or anticipated fluctuations in our revenue and other operating results; |
| ● | the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| ● | actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; |
| ● | announcements by us or our competitors of significant products, acquisitions, strategic partnerships, joint ventures, or capital commitments; |
| ● | price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole; |
| ● | lawsuits threatened or filed against us; and |
| ● | other events or factors, including those resulting from health pandemics, war or incidents of terrorism, or responses to these events. |
In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of securities of many companies. Stock prices of many companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies.
| 49 |
Risks Related to this Offering
If you purchase common stock in this offering, you will experience immediate dilution in the common stock included in the common stock you purchase. You will experience further dilution if we issue additional equity securities in future financing transactions.
Purchasers of common stock in this offering will pay a price per share of common stock included in the common stock you purchase that exceeds the net tangible book value per share of our common stock. Investors participating in this offering will incur immediate and substantial dilution. Giving effect to our receipt of approximately $[ ]$105 million of estimated net proceeds, after deducting underwriting discounts and commissions and estimated offering expenses payable by us from our sale of common stock in this offering at an assumed public offering price of $[ ]$4.50 per share, our pro forma as adjusted net tangible book value as of JuneSeptember 30, 20222023 would have been $[ ]$0.85 per share. This amount represents an immediate increase in net tangible book value of $[ ]$0.84 per share of our common stock to existing stockholders and an immediate dilution in net tangible book value of $[ ]$3.65 per share of our common stock to new investors purchasing in this offering. In addition, you could experience further dilution if the warrants issued in this offering are exercised. See the section entitled “Dilution” below for a more detailed illustration of the dilution you would incur if you purchase common stock in this offering.
If we issue additional common stock, or securities convertible into or exchangeable or exercisable for common stock, our stockholders, including investors who purchase shares of common stock in this offering, may experience additional dilution, and any such issuances may result in downward pressure on the price of our common stock. We also cannot assure you that we will be able to sell shares or other securities in any future offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders.
Nasdaq may apply additional and more stringent criteria for our initial and continued listing because we plan to have a small public offering and insiders will hold a large portion of the company’s listed securities.
Nasdaq Listing Rule 5101 provides Nasdaq with broad discretionary authority over the initial and continued listing of securities in Nasdaq and Nasdaq may use such discretion to deny initial listing, apply additional or more stringent criteria for the initial or continued listing of particular securities, or suspend or delist particular securities based on any event, condition, or circumstance that exists or occurs that makes initial or continued listing of the securities on Nasdaq inadvisable or unwarranted in the opinion of Nasdaq, even though the securities meet all enumerated criteria for initial or continued listing on Nasdaq. In addition, Nasdaq has used its discretion to deny initial or continued listing or to apply additional and more stringent criteria in the instances, including but not limited to: (i) where the company engaged an auditor that has not been subject to an inspection by the Public Company Accounting Oversight Board (“PCAOB”), an auditor that PCAOB cannot inspect, or an auditor that has not demonstrated sufficient resources, geographic reach, or experience to adequately perform the company’s audit; (ii) where the company planned a small public offering, which would result in insiders holding a large portion of the company’s listed securities. Nasdaq was concerned that the offering size was insufficient to establish the company’s initial valuation, and there would not be sufficient liquidity to support a public market for the company; and (iii) where the company did not demonstrate sufficient nexus to the U.S. capital market, including having no U.S. shareholders, operations, or members of the board of directors or management. Our public offering will be relatively small, and our company’s insiders will hold a large portion of the company’s listed securities. Nasdaq might apply the additional and more stringent criteria for our initial and continued listing, which might cause delay or even denial of our listing application.
| 50 |
If we cannot satisfy, or continue to satisfy, the initial listing requirements and other rules of Nasdaq Capital Market, our securities may not be listed or may be delisted, which could negatively impact the price of our securities and your ability to sell them.
We will seek to have our securities approved for listing on the Nasdaq Capital Market upon consummation of this offering. We cannot assure you that we will be able to meet those initial listing requirements at that time. Even if our securities are listed on the Nasdaq Capital Market, we cannot assure you that our securities will continue to be listed on the Nasdaq Capital Market.
In addition, following this offering, in order to maintain our listing on the Nasdaq Capital Market, we will be required to comply with certain rules of Nasdaq Capital Market, including those regarding minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. Even if we initially meet the listing requirements and other applicable rules of the Nasdaq Capital Market, we may not be able to continue to satisfy these requirements and applicable rules. If we are unable to satisfy the Nasdaq Capital Market criteria for maintaining our listing, our securities could be subject to delisting.
If the Nasdaq Capital Market does not list our securities or subsequently delists our securities from trading, we could face significant consequences, including:
| ● | limited availability for market quotations for our securities; |
| ● | reduced liquidity with respect to our securities; |
| ● | a determination that our common stock is a “penny stock,” which will require brokers trading in our Ordinary Share to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our Ordinary Share; |
| ● | limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
The market price of our common stock may be volatile or may decline regardless of our operating performance, and you may not be able to resell your shares at or above the public offering price.
Recently, there have been instances of extreme stock price run-ups followed by rapid price declines and strong stock price volatility with a number of recent initial public offerings, especially among companies with relatively smaller public floats. The public offering price for our common stock will be determined through negotiations between the underwriters and us and may vary from the market price of our common stock following our public offering. In particular, our common stock may be subject to rapid and substantial price volatility, low volumes of trades and large spreads in bid and ask prices, given that we will have relatively small public floats after this offering. Such volatility, including any stock-run up, may be unrelated to our actual or expected operating performance, financial condition or prospects.
We cannot assure you that the public offering price of our common stock, or the market price following our public offering, will equal or exceed prices in privately negotiated transactions of our shares that have occurred from time to time prior to our public offering. The market price of our common stock may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| ● | actual or anticipated fluctuations in our revenue and other operating results; |
| ● | the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| ● | actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; |
| ● | announcements by us or our competitors of significant services or features, technical innovations, acquisitions, strategic relationships, joint ventures, or capital commitments; |
| ● | price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole; |
| ● | lawsuits threatened or filed against us; and |
| 51 |
| ● | other events or factors, including those resulting from war or incidents of terrorism, or responses to these events. |
| ● | In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. Stock prices of many companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In the past, stockholders have filed securities class action litigation following periods of market volatility. In the event that we were to become involved in securities litigation, it could subject us to substantial costs, divert resources and the attention of management from our business, and adversely affect our business. |
Holders of our common stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our common stock. As a result of this volatility, investors may experience losses on their investment in our common stock. Furthermore, the potential extreme volatility may confuse the public investors of the value of our stock, distort the market perception of our stock price and our company’s financial performance and public image, negatively affect the long-term liquidity of our common stock, regardless of our actual or expected operating performance. If we encounter such volatility, including any rapid stock price increases and declines seemingly unrelated to our actual or expected operating performance and financial condition or prospects, it will likely make it difficult and confusing for prospective investors to assess the rapidly changing value of our common stock and understand the value thereof.
We have broad discretion in the use of the net proceeds from our public offering and may not use them effectively.
To the extent (i) we raise more money than required for the purposes explained in the section titled “Use of Proceeds” or (ii) we determine that the proposed uses set forth in that section are no longer in the best interests of our Company, we cannot specify with any certainty the particular uses of such net proceeds that we will receive from our public offering. Our management will have broad discretion in the application of such net proceeds, including working capital, possible acquisitions, and other general corporate purposes, and we may spend or invest these proceeds in a way with which our stockholders disagree. The failure by our management to apply these funds effectively could harm our business and financial condition. Pending their use, we may invest the net proceeds from our public offering in a manner that does not produce income or that loses value. As of the date of this Prospectus, Managementprospectus, the management team has not determined the types of businesses that the Company will target or the terms of any potential acquisition.
| 52 |
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding common stock in the public marketplace could reduce the price of our common stock.
The market price of our shares could decline as a result of sales of substantial amounts of our shares in the public market or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of our common stock. shares will be outstanding immediately after this offering if the firm commitment is completed and the underwriters do not exercise their over-allotment option and shares if exercised in full. All of the shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. The remaining shares will be “restricted securities” as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. See “Shares Eligible for Future Sale” On page 111.
We will incur additional costs as a result of becoming a public company, which could negatively impact our net income and liquidity.
Upon completion of this offering, we will become a public company. As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, Sarbanes-Oxley and rules and regulations implemented by the SEC and the Nasdaq Capital Market require significantly heightened corporate governance practices for public companies. We expect that these rules and regulations will increase our legal, accounting and financial compliance costs and will make many corporate activities more time-consuming and costly.
We do not expect to incur materially greater costs as a result of becoming a public company than those incurred by similarly sized U.S. public companies. In the event that we fail to comply with these rules and regulations, we could become the subject of a governmental enforcement action, investors may lose confidence in us and the market price of our common stock could decline.
The obligation to disclose information publicly may put us at a disadvantage to competitors that are private companies.
Upon completion of this offering, we will be a publicly listed company in the United States. As a publicly listed company, we will be required to file annual reports with the Securities and Exchange Commission. In some cases, we will need to disclose material agreements or results of financial operations that we would not be required to disclose if we were a private company. Our competitors may have access to this information, which would otherwise be confidential. This may give them advantages in competing with our company. Similarly, as a U.S.-listed public company, we will be governed by U.S. laws that our competitors, which are mostly private Chinese companies, are not required to follow. To the extent compliance with U.S. laws increases our expenses or decreases our competitiveness against such companies, our public listing could affect our results of operations.
| 53 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
The Information in this prospectus includes “forward-looking statements”. All statements, other than statements of historical fact included in this prospectus, regarding our strategy, future operations, financial position, estimated revenues and losses, projected costs, prospects, plans and objectives of management are forward-looking statements. When used in this prospectus, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “project” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. When considering forward-looking statements, you should keep in mind the risk factors and other cautionary statements described under the heading “Risk Factors” included in this prospectus. These forward-looking statements are based on our current expectations and assumptions about future events and are based on currently available information as to the outcome and timing of future events. Nevertheless, and despite the fact that management’s expectations and estimates are based on assumptions management believes to be reasonable and data management believes to be reliable, our actual results, performance or achievements are subject to future risks and uncertainties, any of which could materially affect our actual performance. These forward-looking statements include, but are not limited to, statements about our future financial performance, including the following:
| ● | our ability to generate revenue and profit; |
| ● | our ability to expand our business model; |
| ● | our ability to manage or expand operations; |
| ● | our ability to maintain adequate control of our expenses as we seek to grow; |
| ● | our ability to establish or protect our intellectual property; |
| ● | the impact of significant government regulations in Taiwan and PRC; |
| ● | our ability to implement marketing and sales strategies and adapt and modify them as needed; and |
| ● | our implementation of required financial, accounting and disclosure controls and procedures and related corporate governance policies. |
Although the forward-looking statements included herein, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein. Except as required by applicable law, including by the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
| 54 |
After deducting the underwriting discounts and commissions and estimated expenses of this offering payable by us, we expect net proceeds from this offering of approximately $105 million based on an assumed offering price of $4.50 per share (which is the midpoint of the price range set forth on the cover page of this prospectus), assuming no exercise of the underwriters’ over-allotment option. If the underwriters fully exercise the over-allotment option, net proceeds from this offering of approximately $121 million. The net proceeds from this offering must be remitted to Taiwan and Hong Kong before we will be able to use the funds to grow our business. The procedure to remit funds may take several months after completion of this offering, and we will be unable to use the offering proceeds in Taiwan and Hong Kong until remittance is completed.
We intend to use the net proceeds of this offering as follows after we complete the remittance process:
| ● | Approximately 80% for IVD clinical trials, chip design and development, laboratory building, and our planned expansion to the U.S. and European market; |
| ● | Approximately 10 % for marketing and sales; and |
| ● | Approximately 10% for general working capital. |
We estimate that 80% of the proceeds of this Offering will be sufficient for us to complete IVD clinical trials, warp up chip development and production preparation, upgrade our current laboratories and build new facilities without raising additional capital.
Specifically, we believe 40% of the proceeds will be enough to cover the entire clinical trials of IVD products, which are set to start in China in March 2024 and are expected to complete within one year. The trial aims to involve 5,000 individuals, and the average cost is estimated to be approximately $6,500 per person. Other expenses include experimental equipment, reagents, CRO fees, clinical trial fees, testing fees, subject fees, etc.
We will allocate 20% of the total proceeds to support us in completing the design, development, registration, and production preparation of our products and chips.
Finally, 20% of the overall proceeds will be used to modernize our laboratories in PRC, Hong Kong, and Taiwan and finish building new laboratories and production facilities in the U.S. and the United Kingdom.
The precise amounts and percentage of proceeds we devote to particular categories of activity and their priority of use will depend on prevailing market and business conditions as well as on the nature of particular opportunities that may arise from time to time. Accordingly, we reserve the right to change the use of proceeds that we presently anticipate and describe herein. Pending remitting the offering proceeds to Taiwan, we intend to invest our net proceeds in short-term, interest bearing, and investment-grade obligations.
| 55 |
We have never declared or paid any cash dividends on our shares and we do not anticipate paying any cash dividends on our shares in the foreseeable future. It is presently intended that we will retain our earnings for future operations and expansion.
Within the organization, investor cash inflows have all been received by Advanced Biomed Inc., the parent Nevada entity. Cash to fund Advanced Biomed’s operations is transferred from: (i) the Nevada parent to its operating companies through capital contributions; and (ii) operating companies to other operating companies through capital contributions.
As a holding company, Advanced Biomed Inc. may rely on dividends and other distributions on equity paid by its subsidiaries for its cash and financing requirements. If any of Advanced Biomed’s subsidiaries incur debt on its own behalf in the future, the instruments governing such debt may restrict their ability to pay dividends to Advanced Biomed Inc. As of the date of this prospectus, neither Advanced Biomed Inc. nor any of its subsidiaries have ever paid dividends or made distributions.
Advanced Biomed had a net loss in fiscal year 20222023 and does not expect to distribute earnings in the near future. Going forward, Advanced Biomed intends to continue to invest profit generated from its business operations to invest in new markets or business lines.
As of March 31,September 30, 2023, the following cash transfers have been made from the holding company Advanced Biomed Inc. to its subsidiaries to support research and development in Taiwan and Hong Kong:
| · | On June 29, 2022, Advanced Biomed made a $2.5 million capital contribution to Advanced Biomed Taiwan; | |
| · | On October 11, 2022, Advanced Biomed made a $86,000 capital contribution to Advanced Biomed Taiwan; | |
| · | On October 24, 2022, Advanced Biomed made a $100,000 capital contribution to Advanced Biomed HK; | |
| · | On October 26, 2022, Advanced Biomed made a $500,000 capital contribution to Advanced Biomed HK; | |
| · | On November 7, 2022, Advanced Biomed made a $122,000 capital contribution to Advanced Biomed Taiwan; | |
| · | On December 2, 2022, Advanced Biomed made a $110,000 capital contribution to Advanced Biomed HK; | |
| · | On December 14, 2022, Advanced Biomed made a $85,000 capital contribution to Advanced Biomed |
Advanced Biomed’s subsidiaries have not made any dividend distributions to the holding company Advanced Biomed. Advanced Biomed has not made any dividend distribution to its U.S. or non-U.S. shareholders.
Besides the aforementioned paragraph “Advanced Biomed Taiwan is subject to foreign exchange control imposed by Taiwan authorities, which may affect the paying dividends, repatriating the interest or making other payments to us.”, NTD is freely convertible into other currencies. As result, Advanced Biomed Taiwan has the ability to use their potential future NTD net profits to pay dividends to Advanced Biomed after deducting tax withheld at source. The proceeds of this offering from Advanced Biomed to Advanced Biomed Taiwan should be approved by Taiwan Investment Commissions.
RMB is not freely convertible into other currencies. As result, any restriction on currency exchange may limit the ability of Advanced Biomed’s Shanghai subsidiary to use their potential future RMB revenues to pay dividends to Advanced Biomed. The PRC government imposes controls on the convertibility of RMB into foreign currencies and, in certain cases, the remittance of currency out of China. Shortages in availability of foreign currency may then restrict the ability of our Shanghai subsidiary to remit sufficient foreign currency to our offshore entities for our offshore entities to pay dividends or make other payments or otherwise to satisfy our foreign-currency- denominated obligations. Currently, our Shanghai subsidiary does not purchase any foreign currency for settlement. However, if such needs arise in the future, the State Administration of Foreign Exchange of China (“SAFE”) and other relevant PRC governmental authorities may limit or eliminate our ability to purchase foreign currencies in the future to settle transactions. The PRC government may continue to strengthen its capital controls, and additional restrictions and substantial vetting processes may be instituted by SAFE for cross-border transactions. Any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in RMB to fund our business activities outside of PRC, pay dividends in foreign currencies to holders of our securities or to obtain foreign currency through debt or equity financing for our subsidiaries.
Based on the current corporate structure, Advanced Biomed does not believe that there are restrictions and limitations on its ability to: (i) remit offering proceeds to its subsidiaries outside China; (ii) distribute earnings from its businesses, including subsidiaries outside China, to the parent company and U.S. investors; and (iii) settle amounts owed.
| 56 |
The following table sets forth our cash and cash equivalents and total capitalization as of JuneSeptember 30, 2022 and March 31, 2023:
| ● | on an actual basis; and |
| ● | a pro forma basis giving effect to the sale of 25,000,000 shares of common stock in this offering at an assumed offering price of $4.50 per share (which is the midpoint of the price range set forth on the cover page of this prospectus), after deducting the commissions and discounts payable to the underwriter and estimated offering expenses payable by us (assuming the over-allotment is not exercised). |
The pro forma information below is illustrative only and our capitalization following the completion of this offering is subject to adjustment based on the offering price of our common stock and other terms of this offering determined at pricing. You should read this table in conjunction with our financial statements and notes thereto included in this prospectus, and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| As of June 30, 2022 | As of September 30, 2023 | |||||||||||||||
| Actual | Pro Forma Adjusted for this Offering | Actual | Pro Forma Adjusted for this Offering | |||||||||||||
| Cash | $ | 4,783,864 | $ | $ | 2,587,218 | $ | 107,664,935 | |||||||||
| Long-term debt | - | - | - | |||||||||||||
| Stockholders’ equity: | ||||||||||||||||
| common stock, $0.00025 par value per share, 500,000,000 shares authorized; 99,000,000 shares issued and outstanding on an actual basis; [ ] shares of common stock issued and outstanding on a pro forma basis* | 24,750 | |||||||||||||||
| common stock, $0.001 par value per share, 500,000,000 shares authorized; 100,000,000 shares issued and outstanding on an actual basis; 125,000,000 shares of common stock issued and outstanding on a pro forma basis* | 100,000 | 100,000 | ||||||||||||||
| Additional paid-in capital | 12,657,007 | 13,593,365 | 118,671,082 | |||||||||||||
| Accumulated deficits | (9,459,020 | ) | (13,963,113 | ) | (13,963,113 | ) | ||||||||||
| Accumulated other comprehensive income | 268,859 | 1,074,892 | 1,074,892 | |||||||||||||
| Stockholders’ equity | 3,491,596 | 805,144 | 105,882,861 | |||||||||||||
| Non-controlling interests | - | - | - | |||||||||||||
| Total stockholders’ equity | 3,491,596 | 805,144 | 105,882,861 | |||||||||||||
| Total capitalization | $ | 3,491,596 | $ | $ | 805,144 | $ | 105,882,861 | |||||||||
| As of March 31, 2023 | ||||||||
| Actual | Pro Forma Adjusted for this Offering | |||||||
| Cash | $ | 2,529,498 | $ | |||||
| Long-term debt | - | |||||||
| Stockholders’ equity: | ||||||||
| common stock, $0.00025 par value per share, 500,000,000 shares authorized; 100,000,000 shares issued and outstanding on an actual basis; [ ] shares of common stock issued and outstanding on a pro forma basis* | 25,000 | |||||||
| Additional paid-in capital | 13,656,757 | |||||||
| Accumulated deficits | (11,511,225 | ) | ||||||
| Accumulated other comprehensive income | 214,606 | |||||||
| Stockholders’ equity | 2,385,138 | |||||||
| Non-controlling interests | - | |||||||
| Total stockholders’ equity | 2,385,138 | |||||||
| Total capitalization | $ | 2,385,138 | $ | |||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
If the underwriters’ over-allotment option to purchase additional shares of common stock from us was exercised in full, pro forma (i) common stock would be 128,750,000 shares, (ii) additional paid-in capital would be $135,908,945,$134,617,957, (iii) total stockholders’ equity would be $124,644,513$121,829,736 and (iv) total capitalization would be $124,644,513,$121,829,736 after deducting the underwriting discount and commission payable to the underwriter and estimated offering expenses payable by us in the amount of $7,115,625.$8,350,408.
Each $1.00 increase or decrease in the assumed initial public offering price per share of $4.50 (which is the midpoint of the price range set forth on the cover page of this prospectus) would increase or decrease, as applicable, the pro forma as adjusted amount of each of additional paid-in capital, total shareholders’ equity and total capitalization by approximately $1,375,000, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| 57 |
The dilution in net tangible book value per share to new investors, represents the difference between the amount per share paid by in purchasers of shares this offering and the pro forma net tangible book value per share immediately after completion of this offering. After giving effect to the sale of the 25,000,000 shares being sold pursuant to this offering at an assumed public offering price of $4.50 per share (without over-allotment option) and after deducting the underwriting discount and commission payable by us in the amount of $6,187,500 and estimated offering expenses in the amount of approximately $979,783,$1,234,783, our pro forma as adjusted net tangible book value as of March 31,September 30, 2023 would be approximately $0.87$0.85 per share of common stock. This represents an immediate increase in net tangible book value of $0.85$0.84 per share to existing shareholders and an immediate and substantial dilution in net tangible book value of $3.64$3.65 per share to new investors purchasing shares in this offering.
If the underwriters exercise their over-allotment option in full, our pro forma as adjusted net tangible book value as of March 31,September 30, 2023 would be approximately $0.96$0.95 per share of common stock, the increase in net tangible book value of $0.94 per share to existing shareholders and an immediate and substantial dilution in net tangible book value of $3.54$3.55 per share to new investors purchasing shares in this offering.
The following table sets forth the estimated net tangible book value per share of common stock after the offering and the dilution to persons purchasing common stock based on the foregoing offering assumptions.
Offering Over- Option | Offering Exercise of Over- Option | Offering Over- Option | Offering Exercise of Over- Option | |||||||||||||
| Assumed public offering price per share | $ | 4.50 | $ | 4.50 | $ | 4.50 | $ | 4.50 | ||||||||
| Net tangible book value per share as of June 30, 2022 | $ | 0.02 | $ | 0.02 | ||||||||||||
Net tangible book value per share as of September 30, 2023 | $ | 0.01 | $ | 0.01 | ||||||||||||
| Increase in pro forma net tangible book value per share attributable to price paid by new investors | $ | 0.85 | $ | 0.94 | $ | 0.84 | $ | 0.94 | ||||||||
| Pro forma net tangible book value per share after this offering | $ | 0.87 | $ | 0.96 | $ | 0.85 | $ | 0.95 | ||||||||
| Dilution in pro forma net tangible book value per share to new investors in this offering | $ | 3.63 | $ | 3.54 | $ | 3.65 | $ | 3.55 | ||||||||
The following table sets forth, on an as adjusted basis as of JuneSeptember 30, 2022,2023, the difference between the number of common stock purchased from us, the total consideration paid, and the average price per share paid by our existing shareholders and by new public investors before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, using an assumed public offering price of $4.50 per share:
| Shares Purchased | Total Consideration | Average Price Per | ||||||||||||||||||
| Number | Percent | Amount | Percent | Share | ||||||||||||||||
| Existing shareholders | 100,000,000 | 80 | % | 13,681,757 | 11 | % | $ | 0.14 | ||||||||||||
| New investors from public offering | 25,000,000 | 20 | % | 112,500,000 | 89 | % | 4.50 | |||||||||||||
| Total | 125,000,000 | 100 | % | 126,181,757 | 100 | % | $ | 1.01 | ||||||||||||
After giving effect to the sale of common stock in this offering by us, if the underwriters do not exercise their over-allotment option, our existing shareholders would own 80% and purchasers of common stock in this offering would own 20% of the total number of shares of common stock outstanding upon completion of this offering.
| 58 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND
RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and analysis and other parts of this prospectus contain forward-looking statements based upon current beliefs, plans and expectations that involve risks, uncertainties and assumptions. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus. You should carefully read the “Risk Factors” section of this prospectus to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements.
Overview
We are a holding company incorporated in the State of Nevada. We operate through Advanced Biomed Inc. (Taiwan) (“Advanced Biomed Taiwan”) and Advanced Biomed HK Limited (“Advanced Biomed HK”). Advanced Biomed Taiwan is responsible for the main operation and the design and development of the company's primary technologies and products. Since our establishment in 2014, we have been focusing on the integration of multiple interdisciplinary technologies and established our own microfluidic technology platform. Utilizing the physical and molecular biological characteristics of tumor cells, we have developed various advanced and original research through the joint application of semiconductor technology and biotechnology. This includes complex precision structures, dielectric detection, functional microfluidic biochips, microfluidic integrated semiconductor sensors, related application modules, and key components of medical testing equipment. We have also developed a series of medical testing equipment and related products by integrating various functions of microfluidic modules, automation software, and hardware. Our technologies and products can be used for early screening and detection, diagnosis and staging, and treatment of cancer through the detection of circulating tumor cells and related tumor markers in blood samples, capture of single circulating tumor cells, and single-cell sorting and determination. These products provide assistance in treatment selection and patient prognosis intervention once the required licenses and approvals have been obtained. Advanced Biotech HK is our first localized operation company, mainly responsible for market operation and management in China, localized production, product registration, and future local market sales of our products in accordance with relevant local regulations in China. Our Shanghai subsidiary owns some of our R&D equipment and patents and will be responsible for operations related to clinical trials in Mainland China through CROs. In the future, we will also establish operation centers in countries and regions in North America and Europe.
Our devices, A+Pre, AC-1000, A+CellScan, and A+SCDrop, and three corresponding microfluidic biochips, A+Pre Chip and AC-1000 CTC Enrichment Chip and A+CellScan Chip, are designed to provide rapid and affordable assay products and services to cancer patients. Among them, A+Pre is mainly used to reduce the viscosity of blood samples, and AC-1000 is used to complete the separation and enrichment of circulating tumor cells (“CTCs”) and tumor-related targeted cells in blood samples. The A+CellScan is mainly used for fluorescent labeling and automatic scanning judgment of targeted cells while A+SCDrop preserves the original viability of single cells.
Additionally, we have finished the research and development stage for four matching immunostaining kits, A+CTCE, A+CTCM, A+EMT and A+CM, and submitted registration applications in China. The immunostaining kit use antibodies combined with fluorescent groups of different colors to bind to specific proteins on the cell surface or inside the cells. The presence and intensity of fluorescent signals can be observed through a separate fluorescent imaging system, and the expression of the target protein and the cell type can be judged and determined accordingly. Different cell types can be distinguished using multiplexed combined staining with different antibodies. The A+CTCE kit is mainly used to identify epithelial circulating tumor cells, the A+CTCM kit is used to identify mesenchymal circulating tumor cells, the A+EMT kit is mainly used to identify epithelial-to-mesenchymal circulating tumor cells, and the A+CM kit is used to identify tumor-associated macrophages (cancer-associated macrophage-like cells).
We also developed a product for early screening of lung cancer, the A+LCGuard Lung Cancer Early Screening Kit (“A+LCGuard”), which is used to assist in the determination of benign and malignant pulmonary nodules. From August 2020 to September 2022, we finalized the research, design, and development of A+LCGuard. A+LCGuard is Class III medical devices and is required to conduct clinical trials before completing the registration process. We plan to start A+LCGuard’s clinical trials in March 2024, which are expected to be completed in the first half of 2025, and the registration declaration will be made afterwards.
All of our products must go through three steps to receive the required clearance from the NMPA before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. At the registration application stage, we have to assemble all the required application materials, complete clinical trials (if required by NMPA), and work with an NMPA accredited third-party organization to examine our products in accordance with NMPA rules. NMPA will review our application during the registration review period and may request additional information before officially approving or denying our applications. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; A+SCDrop, A+CellScan, A+CellScan Chip, and A+LCGuard are at the registration application stage; the four matching immunostaining kits are under registration review. As of the date of this prospectus, we have not applied for similar clearances from other jurisdictions.
| 59 |
We participated in a scientific research project at Shanghai Pulmonary Hospital from July 17, 2019 to December 2021, and completed a total of 123 case studies to test A+Pre, AC-1000 and A+LCGuard. In the study, we selected 123 individuals, and among them, 75 were surgical patients with nodular changes or shadows in the lungs reported by imaging studies and 48 healthy patients without lung nodules reported by imaging studies. 7ml blood samples were taken from test subjects either before the clinical operation (for cancer patients) or after the physical examination (for healthy individuals), and A+Pre, AC-1000, and A+LCGuard kits were used to determine whether there were circulating tumor cells and other tumor markers in the blood samples. Finally, the pathological and physical examination results of the tested individuals were compared with the test results of our products. Our test results achieved 96% sensitivity and 99.9% specificity, which provides the research and development basis for our products. Specifically, A+Pre and AC-1000 were at the research and development stage, and we completed their effectiveness and performance indicators testing through this project. At the same time, A+LCGuard finished its feasibility and functional verification testing. All three products were tested together throughout the entire project.
All of our products must be approved by applicable regulatory authorities before being sold to customers. A+Pre and A+CellScan can work with third-party products to achieve their designed objectives. AC-1000 and A+SCDrop may be used together with other devices according to different application scenarios below. For the A+LCGuard early screening kit, it has to be used in combination with A+Pre and AC-1000. Our four staining kits, A+CTCE, A+CM, A+CTCM, and A+EMT, can be used independently or with third-party products. A+Pre, AC-1000, and A+CellScan require the use of our supporting microfluidic chips.
| · | For the analysis of high-viscosity blood samples: A+Pre can be independently used for pretreatment, retaining the original cell activity while preventing blood samples from clogging the equipment pipeline after entering the detection equipment. | |
| · | For the identification and counting application of circulating tumor cells: blood samples are diluted with A+Pre, and then AC-1000 is used to separate and enrich circulating tumor cells and related tumor markers. The enriched samples are stained, calibrated, and finally identified and counted. We can provide this service to the public if using third-party staining reagents already on the market in China. However, we will officially roll out this service once our in-house developed staining reagents, A+CTCE, A+CTCM, A+EMT and A+CM, complete the registration process. The identification and counting of circulating tumor cells and related tumor marker cells can provide auxiliary references for relevant clinical applications. | |
| · | The capture of circulating tumor cells: we follow the same process as the identification of circulating tumor cells to obtain enriched samples with A+Pre and AC-1000, and then the samples are captured and separated by A+SCDrop to isolate single circulating tumor cells. This service can provide tumor cells with high purity and high activity. | |
| · | For early screening of lung cancer: peripheral blood samples of the subjects are first obtained, and the target cells are enriched and captured sequentially by A+Pre and AC-1000. After that, A+LCGuard performs cell fluorescence staining on the enriched samples to determine the number of targeted cells, and finally makes a judgment. |
Due to the different regulatory requirements for the marketing of medical device products and in-vitro diagnostics (“IVD”) products in various regions/countries, it is necessary to complete the registration application and obtain the corresponding license in accordance with the local regulations before engaging in commercial activities in the respective regions/countries (“localization registration”). Afterward, marketing and sales can be carried out. We follow the principle of modularization when design and develop all of our products and equipment so that products and equipment can be produced locally to meet different regulatory requirements. Based on the current development of the early tumor screening and preventive treatment industry and the characteristics of the products we are planning to register and apply in the future, we have adopted the operation model of centralized research and development and localized management. We have started the registration process with the NMPA in China for all of our products. Later on, the Company may establish subsidiaries in the United States and Europe to produce products and carry out product registration. To achieve that, our products must be cleared by the United States Food and Drug Administration and go through the conformity assessment process to obtain the Conformite Europeenne marking (“CE marking”) from competent authority in each European Union member state.
We are looking for suitable locations in the states of California and Washington for our planned expansion to the North America market. We aim to complete site selection and personnel recruitment in the United States by the end of 2023 and start product registration, testing and production in 2024. Our US subsidiary will be responsible for the production and registration of our equipment and related products in the US. Production, testing, and clinical trials in our US market will be conducted in accordance with US regulations, and clinical data from trials conducted in China will not be used to establish product standards. In addition, we also plan to break into the European market by establishing a United Kingdom subsidiary, which is expected to start its operation in 2024, and conduct localized management and operations in accordance with European regulations. In 2025, we will start the localized registration of our IVD products in Europe. As of the date of this prospectus, we have not conducted any clinical trials for our products.
However, as of the date of this prospectus, we have not commenced sales of our products nor have any revenue-generating products and do not expect sales of revenue-generating product candidates until we have completed clinical development, submitted regulatory filings, and received applicable regulatory approvals for candidate products. Due to differences in regulatory and clinical registration requirements, we may not be able to obtain device and product approvals or provide product service on time. We expect to be in a state of continuous loss for the next two to three years.
Results of Operations
For the years ended June 30, 2023 and 2022 and 2021 and nine months periodthree-month periods ended March 31,September 30, 2023 and 2022, the Company conducted its business through Advanced Biomed Taiwan and Shanghai Sglcell’s subsidiaries and Sglcell (Huangshan) Biotech Co., Ltd. as research and development centers for technology research and product development.
| 60 |
Comparison of Results of Operations for the Fiscal Years Ended June 30, 20222023 and 20212022
| Years Ended June 30, 2022, | Years Ended June 30, | |||||||||||||||||||||||||||||||
| 2022 | 2021 | Change | 2023 | 2022 | Change | |||||||||||||||||||||||||||
| Operating expenses: | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||
| Research and development | (1,619,531 | ) | (1,734,620 | ) | 115,089 | 7 | % | (1,383,181 | ) | (1,619,531 | ) | 236,350 | 15 | % | ||||||||||||||||||
| General and administrative expenses | (754,840 | ) | (102,320 | ) | (652,520 | ) | (638 | )% | (1,688,042 | ) | (754,840 | ) | (933,202 | ) | (124 | )% | ||||||||||||||||
| Impairment of intangible assets | (1,617,974 | ) | - | (1,617,974 | ) | nm | % | - | (1,617,974 | ) | 1,617,974 | nm | % | |||||||||||||||||||
| Total operating expenses | (3,992,345 | ) | (1,836,940 | ) | (2,155,405 | ) | (117 | )% | (3,071,223 | ) | (3,992,345 | ) | 921,122 | 23 | % | |||||||||||||||||
| Interest income | 243 | 28 | 215 | nm | % | 21,402 | 243 | 21,159 | nm | % | ||||||||||||||||||||||
| Other expense, net | (35,620 | ) | (246 | ) | 35,374 | nm | % | (682,956 | ) | (35,620 | ) | (647,336 | ) | nm | % | |||||||||||||||||
| Net loss | (4,027,722 | ) | (1,837,158 | ) | (2,190,564 | ) | (119 | )% | (3,732,777 | ) | (4,027,722 | ) | 294,945 | 7 | % | |||||||||||||||||
Comparison of Results of Operations for the Three Months Periods Ended September 30, 2023 and 2022
Three-month Periods Ended September 30 | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Operating expenses: | $ | $ | $ | |||||||||||||
| Research and development | (169,185) | (116,605) | (52,580 | ) | 45% | |||||||||||
| General and administrative expenses | (271,060) | (292,014) | 20,954 | (7)% | ||||||||||||
| Total operating expenses | (440,245) | (408,619) | (31,626) | 8 | % | |||||||||||
| Interest income | 14 | 443 | (429) | (97) | % | |||||||||||
| Other expense, net | (331,085) | (740,538) | (409,453) | 55 | % | |||||||||||
| Net loss | (771,316) | (1,148,714) | 377,398 | 33 | % | |||||||||||
Since our inception, we do not have any products approved for sale, we have not generated any revenue from the sale of products, and we do not expect to generate revenue from the sale of our product candidates until we complete clinical development, submit regulatory filings and receive approvals from the applicable regulatory bodies for such product candidates, if ever. Our main activities through JuneSeptember 30, 20222023 have been re-organizational and capital raising activities and the research and development of three automated devices A+Pre, AC-1000 and A+SCDrop. We have initially applied these devices in clinic trials.
Comparison of Results of Operations for the Nine Months Period Ended March 31, 2023 and 2022
| Nine-month Periods Ended March 31 | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Operating expenses: | $ | $ | $ | |||||||||||||
| Research and development | (895,011 | ) | (1,045,243 | ) | 150,332 | 14 | % | |||||||||
| General and administrative expenses | (1,157,047 | ) | (246,606 | ) | (910,441 | ) | (369 | )% | ||||||||
| Total operating expenses | (2,052,058 | ) | (1,291,849 | ) | (760,208 | ) | (59 | )% | ||||||||
| Interest income | 122 | 301 | (178) | nm | % | |||||||||||
| Other expense, net | (269 | ) | (37,300 | ) | 37,876 | nm | % | |||||||||
| Net loss | (2,052,205 | ) | (1,328,848 | ) | (723,357 | ) | 54 | % | ||||||||
For the nine-month periods ended March 31, 2023 and 2022, we incurred research and development expenses of $0.9 million and $2.7 million, respectively. The research and development expenses decreased by approximately $1.8 million or 67% for the nine-month periods ended March 31, 2023 mainly due to increase in research and development activities.
Our research and development expenses are primarily related to research and development of microfluidic biochip technology and its application in precision medicine in the field of oncology, including early cancer screening and detection, diagnosis and staging, treatment selection, and patient prognosis.
For the years ended June 30, 20222023 and 2021,2022, we incurred research and development expenses of $1.6$1.4 million and $1.7$1.6 million, respectively. The research and development expenses decreased by approximately $0.1$0.2 million or 7%15% for the fiscal year ended June 30, 20222023 mainly due to slow down of clinical development activities as all of the Company’s products are in the regulatory registration process, and the clinical trial for A+LCGuard will not start until March 2024, which is expected to end in June 2025.
For the three-month periods ended September 30, 2023 and 2022, we incurred research and development activities as a resultexpenses of lockdowns$0.2 million and movement restrictions$0.1 million, respectively. The research and development expenses increased by approximately $0.1 million or 45% for the three-month periods ended September 30, 2023 mainly due to increase in in response to COVID-19.research and development activities.
Our general and administrative expenses primarily consist of (i) staff cost; (ii) depreciation and amortization; (iii) office supplies and upkeep expenses; (iv) travelling and entertainment; (v) legal and professional fees; (vi) property and related expenses; (vii) insurance; and (viii) miscellaneous expenses. The following table sets forth the breakdown of our general and administrative expenses for the years ended June 30, 2023 and 2022 and 2021:
| Years Ended June 30, 2022, | ||||||||||||||||
| 2022 | 2021 | Change | ||||||||||||||
| $ | $ | $ | ||||||||||||||
| Staff costs | 311,311 | - | 311,311 | nm | % | |||||||||||
| Depreciation and amortization | 130,066 | 57,850 | 72,216 | 125 | % | |||||||||||
| Travelling and entertainment | 30,229 | 5,545 | 24,684 | 445 | % | |||||||||||
| Legal and professional fees | 7,553 | - | 7,553 | nm | % | |||||||||||
| Property and related expenses | 142,150 | 10,835 | 131,315 | nm | % | |||||||||||
| Office supplies and upkeep expenses | 20,335 | 17,931 | 2,404 | 13 | % | |||||||||||
| Miscellaneous expenses | 113,196 | 10,159 | 103,037 | nm | % | |||||||||||
| 754,840 | 102,320 | 652,520 | 638 | % | ||||||||||||
The following table sets forth the breakdown of our general and administrative expenses for the nine months periodthree-month periods ended March 31,September 30, 2023 and 2022:
| Nine-month Periods Ended March 31 | Years Ended June 30, | |||||||||||||||||||||||||||||||
| 2023 | 2022 | Change | 2023 | 2022 | Change | |||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||
| Staff costs | 795,489 | 218,512 | 576,977 | 264 | % | 971,767 | 311,311 | 660,456 | 212 | % | ||||||||||||||||||||||
| Depreciation and amortization | 104,224 | 327 | 103,897 | nm % | 136,755 | 130,066 | 6,689 | 5 | % | |||||||||||||||||||||||
| Travelling and entertainment | 45,883 | 6,982 | 38,901 | 557 | % | 57,129 | 30,229 | 26,900 | 89 | % | ||||||||||||||||||||||
| Legal and professional fees | 66,249 | - | 66,249 | nm % | 380,000 | 7,553 | 372,447 | nm | % | |||||||||||||||||||||||
| Property and related expenses | 117,778 | 15,268 | 102,510 | 671 | % | 104,381 | 142,150 | (37,769) | (27) | % | ||||||||||||||||||||||
| Office supplies and upkeep expenses | 7,883 | 652 | 7,231 | Nm % | 11,146 | 20,335 | (9,189) | (45) | % | |||||||||||||||||||||||
| Miscellaneous expenses | 19,540 | 4,865 | 14,676 | 302 | % | 26,864 | 113,196 | (86,332) | (76) | % | ||||||||||||||||||||||
| 1,157,047 | 246,306 | 910,441 | 369 | % | 1,688,042 | 754,840 | 933,202 | 124 | % | |||||||||||||||||||||||
Three-month Periods Ended September 30, | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Staff costs | 160,214 | 164,054 | (3,840) | (2) | % | |||||||||||
| Depreciation and amortization | 26,933 | 51,184 | (24,251) | (47) | % | |||||||||||
| Travelling and entertainment | 4,164 | 21,636 | (17,472) | (81) | % | |||||||||||
| Property and related expenses | 16,350 | 49,038 | (32,688) | (67) | % | |||||||||||
| Office supplies and upkeep expenses | 663 | 6,102 | (5,439) | (89) | % | |||||||||||
| Miscellaneous expenses | 62,736 | - | 62,736 | nm | % | |||||||||||
| 271,060 | 292,014 | (20,954) | (7) | % | ||||||||||||
For the years ended June 30, 2023 and 2022 and 2021 and nine-monththree-month periods ended March 31,September 30, 2023 and 2022, our general and administrative expenses amounted to $0.7$1.7 million, $0.1$0.8 million, $1.2$0.3 million and $0.2$0.3 million, respectively. The increase in administrative expenses by approximately $0.6 million or 638% and $0.9 million or 369%124% for the year ended June 30, 2022 and for the period ended March 31, 2023, respectively, is mainly attributable to the increase in the staff costs, depreciation and amortization, service and maintenance fee, office supplies and upkeep expenses, travelling and entertainment, property and related expenses arising from the inclusion of the operations of the Shanghai Sglcell and its subsidiaries subsequent to the acquisition by Advanced Biomed HK which took effect on January 1, 2022, and Sglcell (Huangshan) Biotech Co., Ltd. subsequent to the establishment by Advanced Biomed HK which took effect on March 4, 2022.
For the year ended June 30, 2022, an impairment allowance of approximately US$1.6 million was recognized at the balance sheet date for the intangible assets related to the patents arising from the assets purchase on January 1, 2022 as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
For the years ended June 30, 2023 and 2022, our other expense, net amounted to $0.7 million and $0.04 million respectively. The increase in other expense, net by approximately $0.6 million for the year ended June 30, 2023, is mainly attributable to loss on disposal of subsidiaries of approximately $0.2 million and the exchange loss resulted from revaluation of the foreign currency balances of approximately $0.4 million.
For the three-months period ended September 30, 2023 and 2022, our other expense, net amounted to $0.3 million and $0.7 million respectively. The decrease in other expense, net by approximately $0.4 million for the three-month period ended September 30, 2023, is mainly attributable to the lower fluctuation of foreign currency balances which resulted in lower exchange loss during the three-month period.
Net Loss for the Year/PeriodYear
As a result of the foregoing, our loss from operations for the year ended June 30, 20222023 was $4.0$3.7 million, compared to a loss from operations of $1.8$4.0 million for the year ended June 30, 2021.2022.
Our loss from operations for the nine-month periodsthree-month period ended March 31,September 30, 2023 was $2.1$0.8 million, compared to a loss from operations of $1.1 million for the nine monthsthree-month period ended March 31,September 30, 2022.
Off Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures, or capital resources that is material to investors.
| 61 |
Liquidity and Capital Resources
Our liquidity and working capital requirements are primarily related to our research and development and operating expenses. Historically, we have met our working capital and other liquidity requirements primarily through a combination of cash generated from shareholders’ advances to the company. Going forward, we expect to fund our working capital and other liquidity requirements from various sources, including but not limited to cash generated from our operations, loans from banking facilities, the net proceeds from this offering and other equity and debt financings as and when appropriate.
The Company’s ability to continue as a going concern depends upon its ability to develop, register and obtain regulatory approval for commercial sell of its products to generate positive operating cash flows. For the financial year ended June 30, 2023 and the three-month period ended September 30, 2023, the Company reported net loss of $3,732,777 and $771,316, respectively. As of June 30, 2023 and September 30, 2023, the Company’s working capital deficit was $489,595 and $861,792, respectively. In addition, the Company had net cash outflows of $2,963,625 and $619,138 from operating activities for the financial year ended June 30, 2023 and the three-month period ended September 30, 2023. These conditions give rise to substantial doubt as to whether the Company will be able to continue as a going concern.
As circumstances warrant and to sustain its ability to support the Company’s operating activities, the Company may consider supplementing its available sources of funds through the following sources:
| ● | cash generated from operations; and | |
| �� | ● | financial support from the Company’s related party and shareholders as well as third parties. |
Management has commenced a strategy to raise debt and equity. Our Chief Executive Officer, Hung To Pau, Ph.D. has paid the IPO costs and certain expenditures, amounted US$1,027,077, on behalf of the Company and committed to the disbursement of the remaining IPO costs. Advanced Biomed Inc. (Taiwan) entered into an unsecured, interest-free loan with Yi Lu, our Chairman of the Board, of NTD3,578,212 (approximately $110,987) for general working capital in January 2023. As of September 30, 2023, the loan balance due to Yi Lu amounted to $110,987. Advanced Biomed Inc. (Taiwan) entered into four unsecured, interest-free loans with Well Fancy Development Ltd amounting to NTD2,967,700 (approximately $92,050), NTD2,989,873 (approximately US$92,738), NTD4,270,200 (approximately $132,450), and NTD1,945,000 (approximately $60,329), NTD 1,858,120 (approximately $57,634), and NTD 2,912,200 (approximately $90,329) for general working capital in August 2022, April 2023, May 2023, June 2023, July 2023 and August 2023 respectively. As of September 30, 2023 June 2023, the loan balance due to Well Fancy Development Ltd totally amounted to $525,530 and amounting to $95,950 for general working capital and $396,905, respectively. Shanghai Sglcell entered into three unsecured, interest-free loans with Shanghai Junfu Electronic Technology Co., Ltd. amounting to RMB 500,000 (approximately $68,530) for general working capital in April 2023, May 2023, and June 2023, respectively. As of September 30, 2023, the loan balance due to Shanghai Junfu Electronic Technology Co., Ltd. in total amounted to $205,592.
While we acknowledge that unforeseen circumstances or changes in market conditions could impact our liquidity, we remain committed to monitoring our financial health and will take necessary actions to secure additional financing if needed. In the event of unforeseen circumstances that disrupt the above-mentioned financial projection and strategies, the Company believes that our existing cash $2,587,218 as of September 30, 2023 will be sufficient to meet our research and development and operating expenditures for a minimum period of 4 months until March 31, 2024 from the date of this prospectus.
However, there can be no certainty that these additional financings will be available on acceptable terms or at all. If management is unable to execute this plan, there would likely be a material adverse effect on the Company’s business. All of these factors raise substantial doubt about the ability of the Company to continue as a going concern. The consolidated financial statements for the financial years ended June 30, 2023 and 2022 have been prepared on a going concern basis and do not include any adjustments to reflect the possible future effects on the recoverability and classifications of assets or the amounts and classifications of liabilities that may result from the inability of the Company to continue as a going concern.
Material Cash Requirements
Our cash requirements consist primarily of day-to-day operating expenses, research and development expenses, capital expenditures and contractual obligations with respect to facility leases and other operating leases. We lease our R&D centre and office. We expect to make future payments on existing leases from cash generated from operations.
We had the following contractual obligations and lease commitments as of June 30, 2023:
| Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
| Operating lease commitment | 235,373 | 216,567 | 18,806 | - | - | |||||||||||||||
| Total obligations | 235,373 | 216,567 | 18,806 | - | - | |||||||||||||||
We had the following contractual obligations and lease commitments as of September 30, 2023:
| Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
| Operating lease commitment | 180,024 | 179,544 | 480 | - | - | |||||||||||||||
| Total obligations | 180,024 | 179,544 | 480 | - | - | |||||||||||||||
Working Capital
| June 30, | June 30, | June 30, | June 30, | |||||||||||||||||||||
| 2022 | 2021 | Change | 2023 | 2022 | Change | |||||||||||||||||||
| Total current assets | $ | 5,005,711 | $ | 913,497 | $ | 4,092,214 | $ | 2,785,988 | $ | 5,005,711 | $ | (2,219,723 | ) | |||||||||||
| Total current liabilities | (2,989,712 | ) | (28,350 | ) | (2,961,362 | ) | (3,275,583 | ) | (2,989,712 | ) | (285,871 | ) | ||||||||||||
| Net current assets | $ | 2,015,999 | $ | 885,147 | $ | 1,130,852 | ||||||||||||||||||
| Net current (liabilities) assets | $ | (489,595 | ) | $ | 2,015,999 | $ | (2,505,594 | ) | ||||||||||||||||
| March 31, | June 30, | |||||||||||
| 2023 | 2022 | Change | ||||||||||
| Total current assets | $ | 2,986,800 | $ | 5,005,711 | $ | (2,018,911 | ) | |||||
| Total current liabilities | (1,960,796 | ) | (2,989,712 | ) | 1,028,916 | |||||||
| Net current assets | $ | 1,026,004 | $ | 2,015,999 | $ | (989,995 | ) | |||||
Working Capital
| September 30, | June 30, | |||||||||||
| 2023 | 2023 | Change | ||||||||||
| Total current assets | 2,781,308 | 2,785,988 | (4,680 | ) | ||||||||
| Total current liabilities | 3,643,100 | 3,275,583 | 367,517 | |||||||||
| Net current liabilities | (861,792) | (489,595) | (372,197 | ) | ||||||||
We believeAs of September 30, 2023 and June 30, 2023, the Company’s working capital deficit was $861,792 and $489,595. Management has commenced a strategy to raise debt and equity, including financial supports from the Company’s related party and shareholders as well as third parties, which will enable that we have sufficient working capital for our requirements for at least the next 124 months from the date of this prospectus, in the absence of unforeseen circumstances, taking into account the cash and financial resources presently available to us, including cash and additional paid-up capital received subsequent balance sheet date June 30, 2022 and March 31, 2023.us.
Cash flows
The following table summarizes our cash flows for the fiscal years ended June 30, 2021 and 2022 and the nine months ended March 31, 2023 and 2022:
| Nine-month periods ended March 31, | Years ended June 30, | Years ended June 30, | |||||||||||||||||||||||
| 2023 | 2022 | 2022 | 2021 | 2023 | 2022 | ||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||||||
| Cash and cash equivalents at beginning of the year/period | 4,783,864 | 65,922 | 65,922 | 65,705 | |||||||||||||||||||||
| Cash and cash equivalents at beginning of the year | 4,783,864 | 65,922 | |||||||||||||||||||||||
| Net cash provided by/(used in) from operating activities | (3,137,745 | ) | 788,948 | (741,450 | ) | (473,975 | ) | ||||||||||||||||||
| Net cash provided used in from operating activities | (2,963,625 | ) | (741,450 | ) | |||||||||||||||||||||
| Net cash used in investing activities | (62,368 | ) | (807,435 | ) | (1,083,684 | ) | (25,442 | ) | (263,009 | ) | (1,083,684 | ) | |||||||||||||
| Net cash provided financing activities | 1,000,000 | - | 6,097,131 | 697,164 | 458,137 | 6,097,131 | |||||||||||||||||||
| Foreign currency effect | (54,253 | ) | 241,661 | 445,945 | (197,530 | ) | 606,912 | 445,945 | |||||||||||||||||
| Net increase in cash and cash equivalents | (2,254,366 | ) | 223,174 | 4,717,942 | 217 | (2,161,585 | ) | 4,717,942 | |||||||||||||||||
| Cash and cash equivalents as at end of the year/period | 2,254,366 | 289,096 | 4,783,864 | 65,922 | |||||||||||||||||||||
| Cash and cash equivalents as at end of the year | 2,622,279 | 4,783,864 | |||||||||||||||||||||||
The following table summarizes our cash flows for the three-month period ended September 30, 2023 and 2022:
| Three-month period ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| $ | $ | |||||||
| Cash and cash equivalents at beginning of the period | 2,622,279 | 4,783,864 | ||||||
| Net cash provided used in operating activities | (619,138 | ) | (2,104,262 | ) | ||||
| Net cash provided financing activities | 313,206 | 490,137 | ||||||
| Foreign currency effect | 270,871 | 354,390 | ||||||
| Net decrease in cash and cash equivalents | (35,061 | ) | (1,259,735 | ) | ||||
| Cash and cash equivalents as at end of the period | 2,587,218 | 3,524,129 | ||||||
Cash Flow from Operating Activities
During the years ended June 30, 20212023 and 2022 and the nine months periodthree-month periods ended March 31,September 30, 2023 and 2022, and 2023, the operating activities were primarily comprised the research and development activities, staff costs and administrative expenses.
Our net cash used in operating activities primarily reflected our net loss, as adjusted for non-operating items, such as non-cash depreciation and amortization and effects of changes in working capital such as decrease in prepaid expenses and other current assets and increase or decrease in accounts payables, accruals and other current liabilities.
For the year ended June 30, 2021, our net cash used in operating activities was approximately $0.5 million, which primarily reflected our net loss of approximately $1.8 million, as positively adjusted by (i) the decrease in prepaid expenses and other current assets of approximately $1.3 million.
For the year ended June 30, 2022, our net cash used in operating activities was approximately $0.7 million, which primarily reflected our net loss of approximately $4.0 million, as primarily adjusted by the (i) non-cash depreciation and amortization of approximately $0.1 million, (ii) impairment of intangible assets of approximately $1.6 million, positively offset by (iii) increase in accounts payable, accrual and other current liabilities of approximately $0.9 million and positively adjusted by (iv) decrease in prepaid expenses and other current assets of approximately $0.6 million.
| 62 |
For the nine-month periodyear ended March 31, 2022,June 30, 2023, our net cash generatedused in operating activities was approximately $0.7$3.0 million, which primarily reflected our net loss of approximately $1.3$3.7 million, as primarily adjusted by the (i) non-cash depreciation and amortization of approximately $0.01$0.4 million, positively adjustedoffset by (ii) increase in accounts payable, accrual and other current liabilities of approximately $2.1$0.4 million and (iii) decreaseincrease in prepaid expenses and other current assets of approximately $0.05$0.2 million.
For the nine-monththree-month period ended March 31, 2023,September 30, 2022, our net cash used in operating activities was approximately $3.1$2.1 million, which primarily reflected our net loss of approximately $2.1$1.1 million, as primarily adjusted by the (i) non-cash depreciation and amortization of approximately $0.2 million, negatively adjusted by (ii) decrease in accounts payable, accrual and other current liabilities of approximately $1.02$0.9 million and (iii) increase in prepaid expenses and other current assets of approximately $0.2 million.
For the three-month period ended September 30, 2023, our net cash used in operating activities was approximately $0.6 million, which primarily reflected our net loss of approximately $0.8 million, as primarily adjusted by the (i) non-cash depreciation and amortization of approximately $0.1 million and (ii) increase in accounts payable, accrual and other current liabilities of approximately $0.1 million.
Cash Flow from Investing Activities
Our cash flows used in investing activities primarily consisted of (i) the purchase of intangible assets; (ii) the purchase of equipment, furniture and fixtures and leasehold improvements; (iii) purchase of right-of-use assets; and (iv)(iii) acquisition of assets of the subsidiaries.
For the year ended June 30, 2021, our net cash used in investing activities was approximately $25,442 primarily due to the purchase of office equipment.
For the year ended June 30, 2022, our net cash used in investing activities was approximately $1.1 million, primarily attributable to the purchase of equipment, furniture and fixtures and leasehold improvements of approximately $0.9 million, purchase of intangible assets of approximately $0.4 million and positively adjusted by the acquisition of subsidiaries totalling of approximately $0.2 million.
For the nine-month periodyear ended March 31, 2022,June 30, 2023, our net cash used in investing activities was approximately $0.2$0.26 million, primarily dueattributable to the payment for security deposits and other non-current assets and purchase of equipment, furniture and fixtures and leasehold improvements of approximately $0.6 million.$0.21 million and net cash outflow of approximately $0.05 million from the disposal of subsidiaries.
For the nine-monththree-month period ended March 31,September 30, 2023, our net cash usedwe did not engage in any investing activities was approximately $0.06 primarily due to the payment for security deposits and other non-current assets.activities.
Cash Flow from Financing Activities
Our cash flows from financing activities primarily consists of proceeds from issuance of shares.
For the year ended June 30, 2021, our Company recorded net cash generated from financing activities of approximately $0.7 million, which was mainly attributable to the proceeds from issuance of shares of approximately $0.7 million.
For the year ended June 30, 2022, our Company recorded net cash generated from financing activities of approximately $6.1 million, which was mainly attributable to the proceeds from issuance of shares of approximately $6.1 million.
For the nine-monthyear ended June 30, 2023, our Company recorded net cash generated from financing activities of approximately $0.4 million, which was mainly attributable to the proceeds from issuance of shares of approximately $1 million and offsetting the payment of deferred initial public offering costs of approximately $0.6 million.
For the three-month period ended March 31, 2023,September 30, 2022, our Company recorded net cash generated from financing activities of approximately $1 million, which was mainly attributable to the proceeds from issuance of shares of approximately $1 million and offsetting the payment of deferred initial public offering costs of approximately $0.5 million.
For the three-month period ended September 30, 2023, our Company recorded net cash generated from financing activities of approximately $0.3 million, which was mainly attributable to the advances from Company’s major shareholders and advances from Company’s major shareholders have controlling equity interest in.
Critical Accounting Policies and Estimates
Our financial statements and accompanying notes have been prepared in accordance with U.S. GAAP. The preparation of these financial statements and accompanying notes requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis of making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial conditions and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments. While our significant accounting policies are more fully described in Note 23 to the consolidated financial statements included elsewhere in this prospectus, we believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements.
We are an “emerging growth company” as defined under the federal securities laws and, as such, will be subject to reduced public company reporting requirements. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act, for complying with new or revised accounting standards. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act. As a result of our election, our financial statements may not be comparable to those of companies that comply with public company effective dates.
| 63 |
Use of Estimates and Assumptions
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. We base our estimates on historical experience, when available, and on other various assumptions that are believed to be reasonable under the circumstances. Actual results could differ significantly from those estimates under different assumptions and conditions. Significant accounting estimates reflected in our consolidated financial statements include the useful lives for equipment and intangible assets, fair value of financial instruments, assumptions used in assessing right of use assets, impairment of equipment and intangible assets. We continue to evaluate these estimates and assumptions that we believe to be reasonable under the circumstances. We rely on these evaluations as the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from these estimates. Some of our accounting policies require higher degrees of judgment than others in their application. We believe critical accounting policies as disclosed in this prospectus reflect the more significant judgments and estimates used in preparation of our consolidated financial statements.
The following accounting policies are significant to the preparation of our consolidated financial statements. The areas involving a higher degree of judgement or complexity, or areas where estimates and assumptions are significant to the financial statements are disclosed in Critical, Accounting Judgements and Key Sources of Estimation Uncertainty.
Intangible assets, net
The Company’s intangible assets are stated at cost less accumulated amortization and impairment, if any, and amortized on a straight-line basis over the estimated useful lives of the assets. The Company also re-evaluates the periods of depreciation to determine whether subsequent events and circumstances warrant revised estimates of useful lives.
| Category | Estimated useful lives | |
| Software | 3 years | |
| Patents | 6 years |
Patents represent the estimated fair value assigned to finite-lived intangible assets acquired in a transaction that is accounted for as an acquisition of assets rather than a business combination are initially recognized in accordance with other application GAAP. Any consideration transferred in excess of the fair value of the assets acquired is allocated to each asset acquired on a relative fair value basis. Amortization is computed using the straight-line method over the estimated useful lives of the respective finite-lived intangible assets, generally six years. Intangible assets are reviewed for impairment at least annually or more frequently if indicators of potential impairment exist. The Company reviews finite-lived intangible assets for impairment at least annually or more frequently if events or changes in circumstances indicate that the carrying value of the assets might not be recoverable. An impairment allowance of approximately US$1.6 mil was recognized at the balance sheet date for the intangible assets related to the patents as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
Once an impairment is determined, the actual impairment recognized is the difference between the carrying amount and the fair value as estimated using one of the following approaches: income, cost and/or market. Assets which are to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
The carrying amount of a long-lived asset or asset group is considered impaired when the anticipated undiscounted cash flows from such asset or asset group is less than its carrying amount. In that event, a loss is recorded in “Impairment of long-lived assets” on our Consolidated Statements of Operations and Comprehensive Income (Loss) based on the amount by which the carrying amount exceeds the fair value of the long-lived asset or asset group. Fair value, using the income approach, is determined primarily using a discounted cash flow model that uses the estimated cash flows associated with the asset or asset group under review, discounted at a rate commensurate with the risk involved. Fair value, utilizing the cost approach, is determined based on the replacement cost of the asset reduced for, among other things, depreciation and obsolescence. Fair value, utilizing the market approach, benchmarks the fair value against the carrying amount. As of September 30, 2023, June 30, 2023 and 2022, the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell. Impairment allowance made was approximately US$1.6 million as of September 30, 2023, June 30, 2023 and 2022, respectively, as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
Property, plant and equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation and impairment, if any, and depreciated on a straight-line basis over the estimated useful lives of the assets. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its intended use. The estimated useful lives are as follows:
| Category | Estimated useful lives | |
| Lab equipment | 3 to 5 years | |
| Computer equipment | 3 to 5 years | |
| Furniture and fixtures | 3 to 5 years | |
| Leasehold improvements | 3 years |
Expenditure for repair and maintenance costs, which do not materially extend the useful lives of the assets, are charged to expenses as incurred, whereas the expenditure for major renewals and betterment that substantially extends the useful lives of property and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the costs, accumulated depreciation and impairment with any resulting gain or loss recognized in the consolidated statements of income. The Company also re-evaluates the periods of depreciation to determine whether subsequent events and circumstances warrant revised estimates of useful lives.
Cash
Cash consist of cash on hand, the Company’s demand deposit placed with financial institutions, which have original maturities of less than three months and unrestricted as to withdrawal and use. Deposits are held at highly liquid and well capitalized financial institutions. As of March 31,June 30, 2023, bank and cash balances of $2,529,498$2,555,451 and $66,828 were maintained at financial institutions.institutions in Taiwan and the PRC. As of September 30, 2023, cash balances of $2,487,005 and $100,213 were maintained at financial institutions in Taiwan and the PRC. PRC and Taiwan laws and regulations impose certain restrictions on our ability to transfer cash between countries and between our subsidiaries. See “Prospectus Summary — Transfers of Cash to and from Our Subsidiaries” for detailed discussions. Risk of loss is not expected by management. A hypothetical 10% change in average interest rates during 20232024 would not have a material impact in annual interest income.
| 64 |
Impairment of long-lived assets
Long-lived assets, including equipment and intangible assets with finite lives are reviewed for impairment whenever events or changes in circumstances (such as a significant adverse change to market conditions that will impact the future use of the assets) indicate that the carrying value of an asset may not be recoverable. We assess the recoverability of the assets based on the undiscounted future cash flows the assets are expected to generate and recognize an impairment loss when estimated undiscounted future cash flows expected to result from the use of the asset plus net proceeds expected from disposition of the asset, if any, are less than the carrying value of the asset. If an impairment is identified, we would reduce the carrying amount of the asset to its estimated fair value based on a discounted cash flows approach or, when available and appropriate, to comparable market values. As of March 31, 2022 andSeptember 30, 2023, and June 30, 2021 and 2022, except for the impairment loss on intangible assets recognisedrecognized as of June 30, 2023 and 2022, no further impairment of long-lived assets was recognized.
Once an impairment is determined, the actual impairment recognized is the difference between the carrying amount and the fair value as estimated using one of the following approaches: income, cost and/or market. Assets which are to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
The carrying amount of a long-lived asset or asset group is considered impaired when the anticipated undiscounted cash flows from such asset or asset group is less than its carrying amount. In that event, a loss is recorded in “Impairment of long-lived assets” on our Consolidated Statements of Operations and Comprehensive Income (Loss) based on the amount by which the carrying amount exceeds the fair value of the long-lived asset or asset group. Fair value, using the income approach, is determined primarily using a discounted cash flow model that uses the estimated cash flows associated with the asset or asset group under review, discounted at a rate commensurate with the risk involved. Fair value, utilizing the cost approach, is determined based on the replacement cost of the asset reduced for, among other things, depreciation and obsolescence. Fair value, utilizing the market approach, benchmarks the fair value against the carrying amount.
Operating leases
The Company adopted ASC 842 on July 1, 2020. The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liability, and operating lease liability, non-current in the Company’s consolidated balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. When determining the lease term, the Company includes options to extend or terminate the lease when it is reasonably certain that it will exercise that option, if any. As the Company’s leases do not provide an implicit rate, the Company used an incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company has elected to adopt the following lease policies in conjunction with the adoption of ASU 2016-02: (i) for leases that have lease terms of 12 months or less and does not include a purchase option that is reasonably certain to exercise, the Company elected not to apply ASC 842 recognition requirements; and (ii) the Company elected to apply the package of practical expedients for existing arrangements entered into prior to July 1, 2020 to not reassess (a) whether an arrangement is or contains a lease, (b) the lease classification applied to existing leases, and(c) initial direct costs.
Fair Value Measurement
The accounting standard regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires disclosure of the fair value of financial instruments held by the Company.
The accounting standards define fair value, establish a three-level valuation hierarchy for disclosures of fair value measurement and enhance disclosure requirements for fair value measures. The three levels are defined as follows:
· Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
· Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
· Level 3 inputs to the valuation methodology are unobservable and significant to the fair value.
Financial instruments included in current assets and current liabilities are reported in the consolidated balance sheets at face value or cost, which approximate fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rates of interest. The Company accounts for bank loans and lease payables at amortized cost and has elected NOT to account for them under the fair value hierarchy. Fair value estimates are made at a specific point in time based on relevant market information about the financial instrument. These estimates are subjective in nature and involve uncertainties and matters of significant judgment and, therefore, cannot be determined with precision. Changes in assumptions could significantly affect the estimates.
As of MarchJune 30, 2023 and 2022 and JuneSeptember 30, 20222023 and 2021,2022, the carrying amount for cash and cash equivalents, trade accounts receivable (net of allowance for credit losses) and current liabilities (excluding the “Current portion of long-term debt and finance lease obligations”) was equal to or approximated fair value due to their short-term nature or proximity to current market rates.
The fair values of private debt are based on, among other things, available trade information, and/or an analysis in which we evaluate market conditions, related securities, various public and private offerings, and other publicly available information. In performing this analysis, we make various assumptions regarding, among other things, credit spreads, and the impact of these factors on the value of the debt securities. See Note 10 for the fair value of our long-term debt.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist of cash on hand, the Company’s demand deposit placed with financial institutions and other receivables. Bank and cash balances are maintained with high credit quality institutions, the composition and maturities of which are regularly monitored by management. As of March 31,June 30, 2023, bank and cash balances of $2.5 million were maintained at financial institutions in Taiwan and China, of which approximately $2.5 million was subject to credit risk. As of September 30, 2023, bank and cash balances of $2.6 million were maintained at financial institutions in Taiwan and China, of which approximately $2.5 million was subject to credit risk. While management believes that these financial institutions are of high credit quality, it also continually monitors their credit worthiness. The maximum exposure to credit risk is the carrying amounts of cash and bank balances presented on the consolidated statements of financial position.
Liquidity Risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they become due. The Company’s policy is to ensure that it has sufficient cash to meet its liabilities when they become due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Company’s reputation. A key risk in managing liquidity is the degree of uncertainty in the cash flow projections. If future cash flows are fairly uncertain, the liquidity risk increases.
Impact of Inflation
The types of inflationary pressures that affected the Company has primarily related to research and development costs, staff salaries and related costs. Inflation in Taiwan and China has not materially affected our profitability and operating results. However, we can provide no assurance that we will be unaffected by higher inflation rates in Taiwan and China or globally in the future.
Seasonality
We have not observed any significant seasonal trends. Our directors believe that there is no apparent seasonality factor affecting the industry that our Company is operating in.
| 65 |
Trend Information
Other than as disclosed elsewhere in this prospectus, we are not aware of any trends, uncertainties, demands, commitments, or events that are reasonably likely to have a material effect on our profitability, liquidity, or capital resources, or that would cause reported financial information not necessarily indicative of future operating results or financial condition.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
We are not exposed to interest rate risk while we have no bank loans outstanding.
As of March 31,September 30, 2023, bank and cash balances of approximately $2.5$2.6 million were maintained at financial institutions. A hypothetical 10% decrease in average interest rates during 20232024 would not have a material impact in annual interest income.
Credit Risk
Credit risk is the potential financial loss to the Company resulting from the failure of a customer or a counterparty to settle its financial and contractual obligations to the Company, as and when they fall due. As the Company does not hold any collateral, the maximum exposure to credit risk is the carrying amounts of other receivables (exclude prepayments), financial instrument and cash presented on the consolidated statements of financial position. The Company has no other financial assets which carry significant exposure to credit risk.
Foreign Exchange Risk
Our reporting currency is the United States Dollar. The Company cannot guarantee that the current exchange rate will remain steady; therefore there is a possibility that the Company could post the same amount of profit for two comparable periods and because of the fluctuating exchange rate actually post higher or lower profit depending on exchange rate of NT$ and RMB converted to US$ on that date. The exchange rate could fluctuate depending on changes in political and economic environments without notice. However, the Company assessed that a hypothetical 10% decrease in exchange rates during 20232024 would not have a material impact in exchange gain/loss.
Critical, Accounting Judgements and Key Sources of Estimation Uncertainty
There are no critical judgements, apart from those involving estimation (see below) that the management has made in the process of applying the Group’s accounting policy and that has the most significant effect on the amounts recognized in the financial statements.
Key sources of estimation uncertainty
The key assumptions concerning the future and other key sources of estimation uncertainty at the end of the reporting period, that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year, are disclosed below:
Impairment of intangible assets
The Company reviews finite-lived intangible assets for impairment at least annually or more frequently if events or changes in circumstances indicate that the carrying value of the assets might not be recoverable. Accounting standards require that if the sum of the future cash flows expected to result from a company's asset, undiscounted and without interest charges, is less than the reported value of the asset, an asset impairment must be recognized in the financial statements. The amount of impairment to recognize is calculated by subtracting the fair value of the asset from the reported value of the asset. As disclosed in note 6 to the consolidated financial statements for the year ended June 30, 2022, the intangible asset related to acquired patents through the acquisition of Shanghai Sglcell Biotech Co., Ltd. represent approximately 24% of our total assets as of June 30, 2022.
The Company believe that the accounting estimate related to asset impairment is a "critical accounting estimate" because: (1) it is susceptible to change from period to period because it requires company management to make assumptions about future sales and cost of sales over the life of the products arising from the patents acquired (generally six years); and (2) the impact that recognizing an impairment would have on the assets reported on our balance sheet as well as our net loss would be material. Management's assumptions about future sales prices and future sales volumes of the products arising from the patents acquired require significant judgment because actual sales prices and volumes have fluctuated in the past and are expected to continue to do so. Management has discussed the development and selection of this critical accounting estimate with our board of directors and the board has reviewed the company'sCompany's disclosure relating to it in this MD&A. An impairment allowance of approximately US$1.6 mil was recognized at the balance sheet date for the intangible assets related to the patents as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
In estimating future sales, we use our internal budgets. We develop our budgets based on estimated sales data for new products, planned timing of new product launches, customer commitments related to newly developed products. We have tested the patents acquired for impairment and as of June 30, 2022 we determined that, based on our assumptions, the sum of the expected future cash flows, undiscounted and without interest charges, below the reported value and therefore we have recognized an impairment allowance of approximately US$1.6 mil as of June 30, 2022 for the intangible assets related to the patents acquired as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
The management has assessed that risk related to using different assumptions and analyse their sensitivity to change based on outcomes that are deemed reasonably likely to occur and concluded any change in the would not have affected our liquidity and capital resources with the impairment allowance recognised as of JuneSeptember 30, 2022,2023, because we are still in positive net current assets and stockholders’ equityliabilities as of JuneSeptember 30, 2022 and March 31, 2023.
Recent accounting pronouncements
The Company considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued. Under the Jump start Our Business Start-ups Act of 2012, as amended (the “JOBS Act”), the Company meets the definition of an emerging growth company, or EGC, and has elected the extended transition period for complying with new or revised accounting standards, which delays the adoption of these accounting standards until they would apply to private companies.
In May 2020, the Financial Accounting Standard Board (“FASB”) issued ASU 2020-05, which is an update to ASU Update No. 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments”, which introduced the expected credit losses methodology for the measurement of credit losses on financial assets measured at amortized cost basis, replacing the previous incurred loss methodology. The amendments in Update 2016-13 added Topic 326, Financial Instruments — Credit Losses, and made several consequential amendments to the Codification. Update 2016-13 also modified the accounting for available-for-sale debt securities, which must be individually assessed for credit losses when fair value is less than the amortized cost basis, in accordance with Subtopic 326-30, Financial Instruments — Credit Losses — Available-for-Sale Debt Securities. The amendments in this Update address those stakeholders’ concerns by providing an option to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost basis. For those entities, the targeted transition relief will increase comparability of financial statement information by providing an option to align measurement methodologies for similar financial assets. Furthermore, the targeted transition relief also may reduce the costs for some entities to comply with the amendments in Update 2016-13 while still providing financial statement users with decision-useful information. In November 2020, the FASB issued ASU No. 2020-10, which to update the effective date of ASU No. 2016-02 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. The Company is currently evaluating the impact of this new standard on Company’s consolidated financial statements and related disclosures.
| 66 |
In October 2021, the FASB issued ASU 2021-08, Codification Improvements to Subtopic 310-20, Receivables — Nonrefundable Fees and Other Costs. The amendments in this Update represent changes to clarify the Codification. The amendments make the Codification easier to understand and easier to apply by eliminating inconsistencies and providing clarifications. ASU 2021-08 is effective for the Company for annual and interim reporting periods beginning July 1, 2021. Early application is not permitted. All entities should apply the amendments in this Update on a prospective basis as of the beginning of the period of adoption for existing or newly purchased callable debt securities. These amendments do not change the effective dates for Update 2017-08. The Company is currently evaluating the impact of this new standard on Company’s consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The amendments in this Update represent changes to clarify the Codification or correct unintended application of guidance that are not expected to have a significant effect on current accounting practice or create a significant administrative cost to most entities. The amendments in this Update affect a wide variety of Topics in the Codification and apply to all reporting entities within the scope of the affected accounting guidance. ASU 2021-10 is effective for annual periods beginning after December 15, 2021 for public business entities. Early application is permitted. The amendments in this Update should be applied retrospectively. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
Except as mentioned above, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s consolidated balance sheets, statements of income and comprehensive income and statements of cash flows.
| 67 |
This section includes estimates regarding market and industry data. Unless otherwise indicated, information concerning our industry and the markets in which we operate is based on a research report commissioned by us and independently prepared by Grand View Research Inc. (“Grand View”), our management’s knowledge and experience, together with currently available information obtained from various third-party sources, including publicly available information, industry reports and publications, surveys, our customers, trade and business organizations, and other contacts in the markets in which we operate. Some data is also based on our good faith estimates. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section entitled “Risk Factors.” These and other factors could cause the estimates to differ materially from those expressed in these publications.
Cancer is the second leading cause of death in the world, and the number of deaths and cases is increasing year by year. The World Health Organization's International Agency for Research on Cancer (IARC) has released the Biennial Report 2020-2021, which shows 19.29 million new cancer cases worldwide and 10.0 million cancer deaths in 2020. Among the 2020 new cancer cases, there were 4.57 million new cancer cases in China, accounting for 23.7% of the world. Since China is the most populous country in the world, the number of new cancer cases far exceeds that of other countries in the world. The top ten countries with new cancer cases are: China 4.57 million, the United States 2.28 million, India 1.32 million, Japan 1.03 million, Germany 630,000, Brazil 590,000, Russia 590,000, France 470,000, the United Kingdom 460,000, and Italy 420,000. IARC predicts that by 2040 cancer incidence will almost double, to 30.2 million new cases, with 16.3 million cancer deaths worldwide predicted in 2040.
According to Grand View’s market analysis report, Liquid Biopsy Market Size, Share & Trends Analysis Report, the global liquid biopsy market size was valued at USD 8,937.68 million in 2022 and is anticipated to grow at a CAGR of 12.46% from 2023 to 2030, resulting in sales worth USD 22,865.56 million in 2030. The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue in US dollars. It uses the bottom-up approach for market sizing, analyzing key regional markets, dynamics, and trends for various services, and end-uses. The forecast of the global market is calculated by integrating the regional markets’ amounts. The report has also considered factors including impact of COVID-19 up to 2023, supply chain disruptions and demand dynamics. According to the report, liquid biopsy is a revolutionary technique that has created various opportunities that were previously unexplored. It aids in detection and isolation of circulating tumor DNA, exosomes, and circulating tumor cells and is a source of proteomics and genomics information in cancer patients. It is worth noting that these are estimates of the global market, and we intend to initially focus on developing our cancer screening market in China and will expand our operations to other markets in the following years.
In recent years, from the diversification of early tumor screening and early detection technologies to the transformation and application of products based on different re-examination technologies, the early cancer screening industry is on the fast track of development and has broad prospects. Liquid biopsy is a diagnostic technology for tumor analysis which collects body fluid samples such as blood, effusion, urine, and saliva and detects biomarkers in the body fluid samples. Liquid biopsy technology has been widely used in lung cancer, breast cancer, prostate cancer, head and neck squamous cell carcinoma, colorectal cancer, gastrointestinal cancer, leukemia and other tumors, musculoskeletal system and connective tissue diseases. It provides molecular-level diagnosis for tumor patients, enabling tumor patients to receive more precise treatments.
Traditional Screening vs. Liquid Biopsy Screening
Traditional cancer screening methods mainly focus on imaging screening (color Doppler, CT, MRI), endoscopy screening (gastroscopy, colonoscopy), tumor marker screening (Prostate specific antigen, alpha fetoprotein), which are highly invasive with poor sensitivity, low compliance and other defects. The 5-year survival rate of lung cancer with the highest mortality rate in the world is only 15%-18%. But if lung cancer can be detected and treated early, the 5-year survival rate can exceed 80%.
The vast majority of early-stage lung cancers are asymptomatic, and most of the lung cancer patients who have symptoms and seek medical attention are in the advanced stages. However, early-stage lung cancers with ground-glass nodules are all missed by chest X-ray screening; early-stage lung cancers with soft-tissue nodules are too small, and a considerable part of them are missed by chest X-ray examination. Only intermediate and advanced lung cancer can be detected by chest X-ray. Low-dose helical CT can detect tiny tumors less than 1 cm in diameter and plays a much greater role in early lung cancer screening. For people who are under the age of 40 and have no family history of lung cancer, it is generally not recommended to do low-dose spiral CT. But if a chest X-ray is included in the physical examination, it is better to do a low-dose spiral CT than a chest X-ray, because the chest X-ray will miss more than 80% of early lung cancer. Many people are very concerned after finding pulmonary nodules in their physical examination and choose to perform CT examinations multiple times. It is not advisable by most doctors as CT examination has a certain amount of radiation and its use should be minimized.
Compared with the existing cancer screening technology, liquid biopsy technology for early cancer screening has three advantages of "non-invasive", "efficient" and "accurate". The test samples of liquid biopsy are usually blood, saliva, urine and other body fluids. The sampling process is convenient and fast, and it is less invasive to the human body and has good compliance. The entire testing process is simple to operate, less dependent on medical resources, and samples are highly accessible for multiple sampling. The testing cycle is usually 1-2 weeks. Compared with traditional screening methods, liquid biopsy technology has significantly improved sensitivity and specificity. It can also enable doctors to evaluate the overall tumor situation for early detection of tumors. In the future, it will be a clear path for large-scale adoption of liquid biopsy technology as a tool for early cancer screening and diagnosis, which has obvious benefits to patients and can reduce overall medical costs. Further, the detection results can effectively guide the clinical decision-making process for oncologists. In conclusion, the future of liquid biopsy technology can increase the options for molecular diagnosis of tumors and enhance the personalized treatment of tumors.
| 68 |
Market Opportunities in Early Cancer Detection Industry
The early cancer detection market has huge potential. Early tumor screening and related diagnosis are the most active directions in the industry. According to Grand View Research, Inc.’s market analysis report, Liquid Biopsy Market Size, Share & Trends Analysis Report, the global liquid biopsy market size was valued at USD 8,937.68 million in 2022 and is anticipated to grow at a CAGR of 12.46% from 2023 to 2030, resulting in sales worth USD 22,865.56 million in 2030. The report provides market value for the base year 2022 and a yearly forecast till 2030 in terms of revenue in US dollars. It uses the bottom-up approach for market sizing, analyzing key regional markets, dynamics, and trends for various services, and end-uses. The forecast of the global market is calculated by integrating the regional markets’ amounts. The report has also considered factors including impact of COVID-19 up to 2023, supply chain disruptions and demand dynamics. According to the report, liquid biopsy is a revolutionary technique that has created various opportunities that were previously unexplored. It aids in detection and isolation of circulating tumor DNA, exosomes, and circulating tumor cells and is a source of proteomics and genomics information in cancer patients. It is an easy, rapid, and minimally invasive test for cancer genetic status based on circulating tumor cells, circulating tumor DNA, and other tumor-derived substances in blood plasma samples. Rapid development in digital Polymerase Chain Reaction (PCR) and NGS-based technology has improved accuracy of liquid biopsy. It can be performed repeatedly for disease monitoring and is anticipated to help overcome limitations of tissue biopsies. It is worth noting that these are estimates of the global market, and we intend to initially focus on developing our cancer screening market in China and plan to expand our operations to other markets in the following years.
China’s Cancer Early Screening Market
In recent years, China has gradually focused on the national and local cancer screening and early diagnosis and early treatment in high-incidence areas for high-incidence cancer types. The screening methods are becoming more and more diverse. Imaging and endoscopy are mainly used for early screening of tumors. Liquid biopsy is used for in vitro diagnosis by collecting body fluids (such as blood, saliva, sweat, feces, urine and secretions) that represent the patient's disease type. Currently, common detection items include circulating tumor cells, circulating tumor DNA, and tumor cell-derived exosomes. China’s early screening market is growing steadily, and the scale is expected to exceed USD 23.6 billion in three years. The early tumor screening market in China has huge potential. The annual medical cost of malignant tumors in China exceeds 220 billion yuan, which has become an important expenditure for families and medical insurance funds. According to a report released by Frost & Sullivan, the cost of cancer treatment in China is expected to increase to US$351.7 billion in 2023 and US$592 billion in 2030. Single cancer products cover a wide range of cancer types, with the largest number of products for early screening of intestinal cancer types. Among the early screening products based on gene technology, the products targeting intestinal cancer are the most numerous, followed by liver cancer and cervical cancer. Lung cancer and esophageal cancer are the most common types of cancer, and there are fewer products for early screening. Since almost everyone is the target group, pan-cancer early screening products breed a huge market. Pan-cancer early screening products require a larger amount of data, and companies need to invest more time and money to obtain sufficient data to continuously improve product medical statistical indicators and improve product performance.
Traditional tumor screening tests are in short supply, and the demand for early screening products is on the rise. Compared with replacing the existing clinical diagnosis technology of early tumor screening, the more important clinical significance of early tumor screening is to help medical resources allocate and utilize more rationally. Laboratory developed tests, or LDT has helped accelerate market education and build clinical confidence. On March 18, 2021, China’s CFDA released on its official website the revised "Regulations on the Supervision and Administration of Medical Devices", requiring LDT to be used under the following three conditions: 1. there is no in vitro diagnostic reagent of the same variety listed in China; 2. the device is based on self-research and development; and 3. the device will be used under the guidance of licensed physicians. In addition to continuing the sales and service model of LDT, the regulations allow the certified products to be directly sold to hospitals or laboratories with corresponding testing qualifications in the form of test kits. Such a new sales method can reduce sample transportation, laboratory costs and labor costs, thereby reducing marginal costs and making it easier to cope with the rapidly expanding market coverage.
| 69 |
Overview
We are a holding company incorporated in the State of Nevada. We operate through Advanced Biomed Inc. (Taiwan) (“Advanced Biomed Taiwan”) and Advanced Biomed HK Limited (“Advanced Biomed HK”). Advanced Biomed Taiwan is responsible for the main operation and the design and development of the company's primary technologies and products. Since our establishment in 2014, we have been focusing on the integration of multiple interdisciplinary technologies and established our own microfluidic technology platform. Utilizing the physical and molecular biological characteristics of tumor cells, we have developed various advanced and original research through the joint application of semiconductor technology and biotechnology. This includes complex precision structures, dielectric detection, functional microfluidic biochips, microfluidic integrated semiconductor sensors, related application modules, and key components of medical testing equipment. We have also developed a series of medical testing equipment and related products by integrating various functions of microfluidic modules, automation software, and hardware. Our technologies and products can be used for early screening and detection, diagnosis and staging, and treatment of cancer through the detection of circulating tumor cells and related tumor markers in blood samples, capture of single circulating tumor cells, and single-cell sorting and determination. These products provide assistance in treatment selection and patient prognosis intervention once the required licenses and approvals have been obtained. Advanced Biotech HK is our first localized operation company, mainly responsible for market operation and management in China, localized production, product registration, and future local market sales of our products in accordance with relevant local regulations in China. Our Shanghai subsidiary owns some of our R&D equipment and patents and will be responsible for operations related to clinical trials in Mainland China through CROs. In the future, we will also establish operation centers in countries and regions in North America and Europe.
Our devices, A+Pre, AC-1000, A+CellScan, and A+SCDrop, and three corresponding microfluidic biochips, A+Pre Chip and AC-1000 CTC Enrichment Chip and A+CellScan Chip, are designed to provide rapid and affordable assay products and services to cancer patients. Among them, A+Pre is mainly used to reduce the viscosity of blood samples, and AC-1000 is used to complete the separation and enrichment of circulating tumor cells (“CTCs”) and tumor-related targeted cells in blood samples. The A+CellScan is mainly used for fluorescent labeling and automatic scanning judgment of targeted cells while A+SCDrop preserves the original viability of single cells.
Additionally, we have finished the research and development stage for four matching immunostaining kits, A+CTCE, A+CTCM, A+EMT and A+CM, and submitted registration applications in China. The immunostaining kit use antibodies combined with fluorescent groups of different colors to bind to specific proteins on the cell surface or inside the cells. The presence and intensity of fluorescent signals can be observed through a separate fluorescent imaging system, and the expression of the target protein and the cell type can be judged and determined accordingly. Different cell types can be distinguished using multiplexed combined staining with different antibodies. The A+CTCE kit is mainly used to identify epithelial circulating tumor cells, the A+CTCM kit is used to identify mesenchymal circulating tumor cells, the A+EMT kit is mainly used to identify epithelial-to-mesenchymal circulating tumor cells, and the A+CM kit is used to identify tumor-associated macrophages (cancer-associated macrophage-like cells).
We also developed a product for early screening of lung cancer, the A+LCGuard Lung Cancer Early Screening Kit (“A+LCGuard”), which is used to assist in the determination of benign and malignant pulmonary nodules. From August 2020 to September 2022, we finalized the research, design, and development of A+LCGuard. We plan to start A+LCGuard’s clinical trials in March 2024, which are expected to be completed in the first half of 2025, and the registration declaration will be made afterwards.
All of our products must go through three steps to receive the required clearance from the NMPA before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. At the registration application stage, we have to assemble all the required application materials, complete clinical trials (if required by NMPA), and work with an NMPA accredited third-party organization to examine our products in accordance with NMPA rules. NMPA will review our application during the registration review period and may request additional information before officially approving or denying our applications. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; A+SCDrop, A+CellScan, A+CellScan Chip, and A+LCGuard are at the registration application stage; the four matching immunostaining kits are under registration review. A+LCGuard is a Class III medical device and is required to conduct clinical trials before completing the registration process. As of the date of this prospectus, we have not applied for similar clearances from other jurisdictions.
We participated in a scientific research project at Shanghai Pulmonary Hospital from July 17, 2019 to December 2021, and completed a total of 123 case studies to test A+Pre, AC-1000 and A+LCGuard. In the study, we selected 123 individuals, and among them, 75 were surgical patients with nodular changes or shadows in the lungs reported by imaging studies and 48 healthy patients without lung nodules reported by imaging studies. 7ml blood samples were taken from test subjects either before the clinical operation (for cancer patients) or after the physical examination (for healthy individuals), and A+Pre, AC-1000, and A+LCGuard kits were used to determine whether there were circulating tumor cells and other tumor markers in the blood samples. Finally, the pathological and physical examination results of the tested individuals were compared with the test results of our products. Our test results achieved 96% sensitivity and 99.9% specificity, which provides the research and development basis for our products. Specifically, A+Pre and AC-1000 were at the research and development stage, and we completed their effectiveness and performance indicators testing through this project. At the same time, A+LCGuard finished its feasibility and functional verification testing. All three products were tested together throughout the entire project.
| 70 |
All of our products must be approved by applicable regulatory authorities before being sold to customers. A+Pre and A+CellScan can work with third-party products to achieve their designed objectives. AC-1000 and A+SCDrop may be used together with other devices according to different application scenarios below. For the A+LCGuard early screening kit, it has to be used in combination with A+Pre and AC-1000. Our four staining kits, A+CTCE, A+CM, A+CTCM, and A+EMT, can be used independently or with third-party products. A+Pre, AC-1000, and A+CellScan require the use of our supporting microfluidic chips.
| · | For the analysis of high-viscosity blood samples: A+Pre can be independently used for pretreatment, retaining the original cell activity while preventing blood samples from clogging the equipment pipeline after entering the detection equipment. |
| · | For the identification and counting application of circulating tumor cells: blood samples are diluted with A+Pre, and then AC-1000 is used to separate and enrich circulating tumor cells and related tumor markers. The enriched samples are stained, calibrated, and finally identified and counted. We can provide this service to the public if using third-party staining reagents already on the market in China. However, we will officially roll out this service once our in-house developed staining reagents, A+CTCE, A+CTCM, A+EMT and A+CM, complete the registration process. The identification and counting of circulating tumor cells and related tumor marker cells can provide auxiliary references for relevant clinical applications. |
| · | The capture of circulating tumor cells: we follow the same process as the identification of circulating tumor cells to obtain enriched samples with A+Pre and AC-1000, and then the samples are captured and separated by A+SCDrop to isolate single circulating tumor cells. This service can provide tumor cells with high purity and high activity. |
| · | For early screening of lung cancer: peripheral blood samples of the subjects are first obtained, and the target cells are enriched and captured sequentially by A+Pre and AC-1000. After that, A+LCGuard performs cell fluorescence staining on the enriched samples to determine the number of targeted cells, and finally makes a judgment. |
Due to the different regulatory requirements for the marketing of medical device products and in-vitro diagnostics (“IVD”) products in various regions/countries, it is necessary to complete the registration application and obtain the corresponding license in accordance with the local regulations before engaging in commercial activities in the respective regions/countries (“localization registration”). Afterward, marketing and sales can be carried out. We follow the principle of modularization when design and develop all of our products and equipment so that products and equipment can be produced locally to meet different regulatory requirements. Based on the current development of the early tumor screening and preventive treatment industry and the characteristics of the products we are planning to register and apply in the future, we have adopted the operation model of centralized research and development and localized management. We have started the registration process with the NMPA in China for all of our products. Later on, the Company may establish subsidiaries in the United States and Europe to produce products and carry out product registration. To achieve that, our products must be cleared by the United States Food and Drug Administration and go through the conformity assessment process to obtain the Conformite Europeenne marking (“CE marking”) from competent authority in each European Union member state.
We are looking for suitable locations in the states of California and Washington for our planned expansion to the North America market. We aim to complete site selection and personnel recruitment in the United States by the end of 2023 and start product registration, testing and production in 2024. Our US subsidiary will be responsible for the production and registration of our equipment and related products in the US. Production, testing, and clinical trials in our US market will be conducted in accordance with US regulations, and clinical data from trials conducted in China will not be used to establish product standards. In addition, we also plan to break into the European market by establishing a United Kingdom subsidiary, which is expected to start its operation in 2024, and conduct localized management and operations in accordance with European regulations. In 2025, we will start the localized registration of our IVD products in Europe. As of the date of this prospectus, we have not conducted any clinical trials for our products.
However, as of the date of this prospectus, we have not commenced sales of our products nor have any revenue-generating products and do not expect sales of revenue-generating product candidates until we have completed clinical development, submitted regulatory filings, and received applicable regulatory approvals for candidate products. Due to differences in regulatory and clinical registration requirements, we may not be able to obtain device and product approvals or provide product service on time. We expect to be in a state of continuous loss for the next two to three years.
Corporate History
We were incorporated in Nevada in July 2021 as a holding company. We started operations in 2014 through Advanced Biomed Taiwan as a research and development center for technology research and product development. In August 2021, we established Advanced Biomed HK as the wholly owned subsidiary of Advanced Biomed Taiwan to integrate market development and commercialization in the PRC. On January 1, 2022, Advanced Biomed HK acquired 100% equity interest of Shanghai Sglcell from its shareholders for a consideration of RMB 12 million. In March 2022, Advanced Biomed HK established Sglcell (Huangshan) Biotech Co., Ltd.
| 71 |
In July 2022, we consummated a reorganization pursuant to which we acquired 100% equity interest of Advanced Biomed Taiwan, making it our wholly owned subsidiary. On November 7, 2022, we obtained the approval of Taiwan Investment Commission for the reorganization, the Issue No. of which is “經審一字第11100116890號”. Additionally, the Bureau of Economic Development of Tainan City Government has also approved the reorganization in accordance with the Taiwan Company Act on December 26, 2022.
On June 8, 2023, pursuant to the Share Transfer Agreement entered into by Ting Wang and Haifeng Zhang, who are independent third-party individuals, and Shanghai Sglcell on June 2, 2023, Shanghai Sglcell transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to Ting Wang and Haifeng Zhang at aggregate consideration of RMB500,000 (approximately US$72,780) without any other obligations arising from the transfers.
On June 9, 2023, pursuant to the Share Transfer Agreement entered into by Ting Wang and Haifeng Zhang, who are independent third-party individuals, and Shanghai Sglcell on May 31, 2023, Shanghai Sglcell transferred its wholly owned subsidiary, Shandong Sglcell Medical Devices Co., Ltd., to Ting Wang and Haifeng Zhang at zero consideration without any other obligations arising from the transfers.
On June 15, 2023, pursuant to the Share Transfer Agreement entered into by Quantum Capital (Hong Kong) Limited, who is an independent third-party corporation, and Advanced Biomed HK on June 9, 2023, Advanced Biomed HK transferred its wholly owned subsidiary, Sglcell (Huangshan) Biotech Co., Ltd., to Quantum Capital (Hong Kong) Limited at zero consideration without any other obligations arising from the transfers.
As of the date of this prospectus, our Shanghai subsidiary is the only operating entity we have in the PRC and owns all of our patents and some of our R&D equipment and patents.while the rest of our R&D equipment are owned by Advanced Biomed Inc. (Taiwan). Our Shanghai subsidiary will also be responsible for operations related to clinical trials in Mainland China through contract research organizations.CROs.
Corporate Structure
The chart below depicts the corporate structure of the Company as of the date of this prospectus.

Our Oncology Detection Solutions
The current market uses positive antibody-labeling selection to capture CTCs out of the blood by a specific epithelial cell adhesion molecule (EpCAM). This method could detect tumor cells in the patient’s blood for diagnosing lung, prostate, pancreatic, and breast cancers. However, this technology costs approximately $1,000 per chip, and the processing time per patient is up to 12 hours. Besides, antibody-based methods such as immunomagnetic methods are highly dependent on antigen expression of CTC. Some CTCs may show low or no EpCAM expression on the cell membrane, and thus cannot be effectively captured using the proposed biomarkers and makes it difficult to detect cancer in the early stage. More importantly, the capture of dying or dead CTCs cannot provide meaningful information to doctors for diagnosis or treatment.
Our products use pure physical mechanisms (antigen-independent) and can effectively enrich and detect the CTCs with high or low antigen expressions with high viability. Among them, A+Pre is mainly used to reduce the viscosity of blood samples, and AC-1000 is used to complete the separation and enrichment of circulating tumor cells (“CTCs”) and tumor-related targeted cells in blood samples. The A+CellScan is mainly used for fluorescent labeling and automatic scanning judgment of targeted cells while A+SCDrop preserves the original viability of single cells.
For three corresponding microfluidic biochips, A+Pre Chip and AC-1000 CTC Enrichment Chip have been cleared by NMPA and can be mass-produced and sold to customers. A+CellScan Chip is expected to start the registration application by the end of 2023. The A+CellScan Chip and A+CellScan will enrich our product chain by upgrading our immunochromogenic kits to tumor cell assay equipment. Specifically, A+CellScan, together with A+Pre and AC-1000, can serve as a liquid biopsy IVD product to accelerate downstream assay and reduce the amount of labor required for assaying tumor cells.
We use our own product features combined with different application scenarios to achieve the corresponding work objectives. To identify CTCs in liquid biopsy, blood samples are first diluted through equipment A+Pre, then AC-1000 is used to separate and enrich circulating tumor cells, and then the obtained cells are stained for identification and counting. We have been cleared by NMPA to provide this service to customers in China and plan to do so once our matching immunostaining kits pass the NMPA’s registration review. The counting of circulating tumor cells can provide auxiliary reference for clinical diagnosis, treatment evaluation, prognosis evaluation, recurrence, and metastasis detection, etc. In the future, we can also use our A+LCGuard early screening kit combined with two devices, A+Pre and AC-1000, for early screening of lung cancer. In addition, we use A+SCDrop together with A+Pre and AC-1000 to complete the capture of a single circulating tumor cell, which retains the original activity of the cell and can provide high-purity, high-activity tumor cells for relevant clinical applications. It can be applied to various applications such as single-cell sequencing, whole gene sequencing, protein sequencing, new drug development, cancer biomarker research, individualized diagnosis, and individualized drug sensitivity testing. The above product applications need to be approved by the local regulatory authorities before they can be provided to customers.
| 72 |
A+Pre
A+Pre is a fully automated sample preparation system that enables to pre-concentrate diluted blood sample and it is able to deplete approximately 90 % blood cells in 10 minutes and reduce sample volume with a lower blood cell density (hematocrit approximately 4%). A+Pre provides dual channels to simultaneously process two patients’ samples per run, and 8 samples could be done in an hour. A+Pre is easy to operate with two simple steps: 1. dilute the samples by 10-fold; 2. insert the samples and A+Pre chips into A+Pre system and press ‘START’. Our A+Pre sample-pretreatment could provide clotting-free and clogging-free samples for downstream microfluidic applications.
A+Pre Chip
Blood sample includes several billions blood cells and millions white blood cells with a hematocrit of approximately 45% (“Hct”). The high-density blood cells in microfluidic chip typically induces clotting or clogging in microchannel and microstructs, causing trail failure or unstable system. Sample dilution can effectively reduce the clotting and clogging. However, dilution may increase sample volume and prolong the processing time for downstream precise separation and makes analysis more difficult.
A+Pre chip utilizes microfluidic inertial forces to rapidly pre-enrich (deplete approximately 90% blood cells) and pre-concentrate (reduce over 90% sample volume) the diluted blood sample in 10 minutes. We can dilute a 5ml blood sample with approximately 45% by a factor of 10 and obtain a 50ml blood sample. After concentration and enrichment using the A+Pre chip, the total volume of the 50ml blood sample can be reduced by 10-13 times to around 4 ml while maintaining low red blood cell density (Hct approximately 4.5%), and we can recover more than 90% of the targeted tumor cells. It reduces blood cell density of oncology patients to make it possible for clotting-free downstream precise separation, A+Pre treated sample provide AC-1000 with high purity rare tumor cells in 20 minutes (12 mL/hour). A+Pre chip plays an important role to reduce sample volume and density to make downstream applications much faster and more stable.
 | 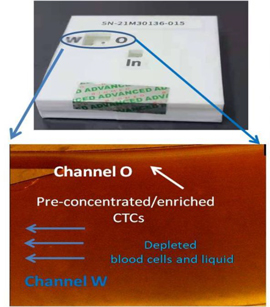 |
| A+Pre | A+Pre Chip |
| 73 |
AC-1000
AC-1000 is a rare cell enrichment device to solve the problem of detection of hypercoagulable state samples of tumor patients. Our rare cell enrichment system uses semiconductor fabrication method to implant high-aspect-ratio functional microstructures inside the microchannel and patented microfluidic chip technology to separate rare cells with complete cell activity. The system is convenient and efficient. We take 5 mL of blood from a patient and the whole process takes only 20 minutes. Compatible to A+Pre, AC-1000 also provides dual channels to simultaneously process two patients’ samples per run, and 8 samples could be done with high-purity enrichment of tumor cells in one and half hours.
The blood sample enriched by AC-1000 can maintain the capture rate of 76-90% of the targeted cells, and the isolated product after blood sample separation can achieve a leukocyte removal rate of 99.9%, which is convenient for downstream detection. Because the rare cell enrichment system is non-destructive, highly active and label-free, it can carry out pure physical and high-purity enrichment, completely maintain the original characteristics of the desired target cells and improve accuracy. What we get is highly active cells, which can be used for different applications in the future, and can be used for tumor screening, auxiliary diagnosis, treatment evaluation, prognosis evaluation, recurrence and metastasis detection, individualized medication guidance, and companion diagnosis. Our rare cell enrichment system is low-cost, and the cost will be further reduced after mass production. It has great potential to be applied to routine liquid biopsy and companion diagnostic platforms in the future.
AC-1000 CTC Enrichment Chip
AC-1000 chip utilizes a hybrid mechanism with microfluidics induced lateral displacement to combine the difference in cell deformability and to remove all of red blood cells (“RBCs”) and deplete over three logs White Blood Cell (“WBCs”). The remaining cells, including few WBCs and rare cell-based cancer biomarkers, are all sorted out.
 |  |
A+SCDrop
A+SCDrop is a CTCs single cell capture device. It preserves the original viability of single cells. The polymer microfluidic chip combines the cell dielectric sensing technology, without the need for antibody fluorescence calibration and image recognition. CTC sorting combined with AC-1000 achieves tandem for complete label-free live cell sorting. A+SCDrop can sort single circulating tumor cells into nanoliter droplet collection samples while maintaining single cell biological activity: a. Precise counting of CTCs while separating and capturing single cells; b. In vitro culture and expansion of non-destructive Single-CTC; drug sensitivity test; c. Single-CTC encapsulated in oil droplets, single-cell sequencing or genetic screening d. Single CTCs were independently sorted into 96-well plates in suspension liquid mode for CTC proteomic study.

A+CellScan
Currently, A+CellScan are developed as an analyzer with immunostaining chip for reducing hands-on time, manual discrimination, and accelerating immunostaining/analysis of the AC-1000 product. A+CellScan provides a novel approach for rapid on-chip immunofluorescence staining, which may take approximately 30 minutes, and artificial intelligence analysis of enriched sample for detecting, identification, and counting of rare CTCs/clusters from white blood cells with four color immune-fluorescent images. The prototype we designed can (a) autofocus for capturing and analyzing images of four colors fluoresces in A+CellScan chip, (b) perform on-chip immunostaining, and (c) automatically count tumor cells and labeling them on each image. We also completed A+CellScan’s performance study, which examine (i) its autofocus ability and precision for automatically capturing and analyzing cell images, (ii) whether it can detect and analyze 3-1000 cancer cells in <106 WBCs, and (iii) its maximum on-chip immunostaining ability. Performance study (i) to (iii) were completed in December 2022, January 2023, and March 2023, respectively. During the performance study, we have thoroughly evaluated A+CellScan’s autofocus precision in capturing cell images. Out of 6,400 cell pictures that were captured through a non-stop and fully automated process, less than 2 pictures were found to be out-of-focus. We also analyzed the presence of 3-1,000 cancer cells stained with fluorescence within samples containing 104, 105, and 106 WBCs, resulting in reliable detection of cancer cells. We have optimized the antibody concentrations for CK+, DAPI, and CD14/45 combination, resulting in a maximum on-chip immunostaining ability with high specificity.
A+CellScan Chip
Conventional immuno-staining is labor-intensive with multiple processes, and its reaction is based on diffusion-dominated mechanism, thus causing a long operating time (about 4-5 hours per sample). A+CellScan chip consists of fluid device and 5 μm micro-pores membrane that is used as a filter to trap nucleated cells on the membrane surface form the through-flow. Only fluid and RBCs are allowed to pass through the pores. Captured cells are kept in wells during flowing staining and washing. The active through-flow plays two important roles: 1. carrying antibodies to cells rapidly, thus accelerating the reaction rate of specific binding between reporting antibody and cells; 2. simplifying the operation processes in reagent spiking and repeated washing steps. A+CellScan chip stains rare cells such as CTCs and CTC clusters without cell loss during fixation, permeabilization, blocking, incubation and washing. The device provides flexibility to process up to eight samples per run. On-chip immunostaining only need 30 minutes for one sample, and batch process for eight samples can be completed in one and half hours. After cell staining, A+CellScan and A+CellScan chips automatically captures cell images and analyzes the stained cells in 30 minutes. Compared to conventional methods, A+CellScan chip significantly reduces the total detection time by 4-5 hours per 1 sample.
 | 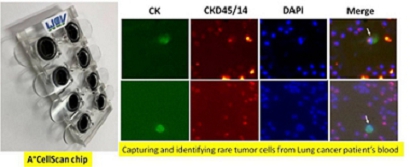 |
| 74 |
The following diagrams illustrate the procedures using our devices for diagnostic detection of blood samples from oncology patients:
Diagram 1: A+Pre reduces blood density of oncology patients.

For the analysis of high-viscosity blood samples, the A+Pre equipment can be used for pretreatment, retaining the original cell activity while preventing blood samples from clogging the equipment pipeline after entering the detection equipment.
Diagram 2: AC-1000 completes targeted cell enrichment work.

A+Pre and AC-1000 equipment can be combined for the identification and counting application of circulating tumor cells purposes. First, blood samples are diluted with A+Pre, and then AC-1000 is used to separate and enrich circulating tumor cells and related tumor markers. The enriched samples are stained, calibrated, and finally identified and counted. A+Pre combined with AC-1000 provide the purified solution of cell-based cancer biomarkers, which can be easily identified, analyzed and tested. The total purification process can be completed in only 40 minutes. A+Pre combined with AC-1000 capture multi-cell-based cancer markers simultaneously with a high recovery rate (76~90%), and therefore making it possible to diagnose cancer and predict cancer progression in very early stage. This application service can already be commercialized in the Chinese market. Calibration and staining currently use staining reagents that are already on the market. Once our products are registered, we will start using our own developed reagents. The identification and counting of circulating tumor cells and related tumor marker cells can provide auxiliary reference for relevant clinical applications.
| 75 |
Diagram 3: A+SCDrop completes single targeted cell capture.
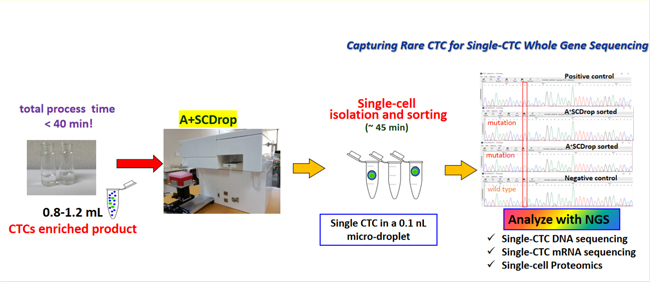
The capture of circulating tumor cells requires the use of A+Pre, AC-1000, and A+SCDrop in combination. First, the user needs to follow the same process as the identification of circulating tumor cells to obtain enriched samples, and then the samples are captured and separated by A+SCDrop equipment to isolate single circulating tumor cells. This combined application can provide tumor cells with high purity and high activity.
Products
Four Immunochromogenic Kits
The four immunochromogenic kits, A+CTCE, A+CM, A+CTCM and A+EMT, are currently under NMPA registration review.

For the four immunochromogenic kits, the basic principle is to use antibodies combined with different colored fluorophores to bind to specific proteins on the cell surface or inside the cells and observe the presence or absence of fluorescent signals through a fluorescent imaging system (to be obtained separately). Afterwards, the expression of the target protein can be examined, and the cell type can be determined accordingly. Different cell types can be distinguished using multiplexed combined staining with different antibodies. The A+CTCE kit is mainly used to identify epithelial circulating tumor cells, and is determined by immunochromogenicity of EpCAM, CK (pan), CD45, and CD14 antibodies. The A+CTCM kit is used to identify mesenchymal circulating tumor cells based on the immunochromogenicity of Vimentin, CD45, and CD14 antibodies. A+EMT is mainly used to identify epithelial-to-mesenchymal circulating tumor cells, which are determined by immunochromogenicity of Cell-Surface Vimentin (CSV), CD45 antibody, and CD14 antibody. A+CM is used to identify tumor-associated macrophages (cancer-associated macrophage-like cells) based on immunochromogenicity of PD-L1, CD45, and CD14 antibodies.
With the assistance of the four kits, targets are stained using antibodies conjugated with different fluorophores that specifically bind to antigens in cells. In immunohistochemical reaction or in situ hybridization reaction, it is combined with the primary antigen antibody, and the target is marked by staining. It is suitable for the staining of blood cells enriched by A+Pre and AC-1000 hemocytometer. We transfer the cell suspension separated and obtained by A+Pre and AC-1000 into a centrifuge tube, use the kits to stain the cell suspension, and observe and count the fluorescently stained cells under a fluorescence microscope or a fluorescence scanner. Observed under a fluorescence microscope, all nuclei are stained blue, and the cell membrane of leukocytes is red, and the color of the cell membrane we observe under the fluorescence microscope is the marked color, which is the target cell we want to obtain.
Product in the Pipeline
Our A+LCGuard was designed for early screening of lung cancer. In order to detect lung cancer, peripheral blood samples of the subjects are first obtained, and the target cells are enriched and captured sequentially by A+Pre and AC-1000. Then, A+LCGuard performs cell fluorescence staining on the enriched samples to determine the number of targeted cells, and finally makes a judgment. We completed A+LCGuard’s research and development in China between August 2020 and September 2022. A+LCGuard is a Class III medical device and is required to conduct clinical trials before completing the registration process. We plan to start A+LCGuard’s clinical trials in March 2024, which are expected to be completed in the first half of 2025, and the registration declaration will be made afterwards.
| 76 |
The following diagrams illustrate the procedures using our detection solutions for CTC enriched samples:
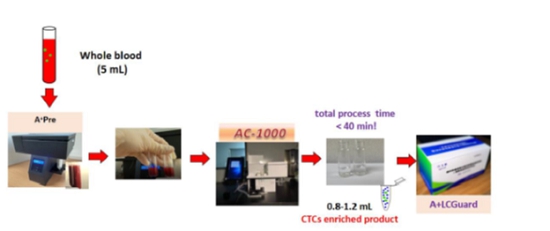
We participated in scientific research projects at Shanghai Pulmonary Hospital from July 17, 2019 to December 2021, and completed a total of 123 case studies. In the study, we selected 123 individuals, and among them, 75 were surgical patients with nodular changes or shadows in the lungs reported by imaging studies and 48 healthy patients without lung nodules reported by imaging studies. 7ml blood samples were taken from test subjects either before the clinical operation (for cancer patients) or after the physical examination (for healthy individuals), and A+Pre, AC-1000, and A+LCGuard kits were used to determine whether there were circulating tumor cells and other tumor markers in the blood samples. Finally, the pathological and physical examination results of the tested individuals were compared with the test results of our products. Our test results achieved 96% sensitivity and 99.9% specificity, which provides the research and development basis for our kit products.
Below are two comparable blood testing methods in the current market. We have not tested the sensitivity of our products against these two comparable blood testing products in head-to-head comparisons.
Universal Diagnostics (UDX), a Spanish vitro diagnostic company, announced the preliminary research data of its liquid biopsy method for early detection of lung cancer at the International Association for the Study of Lung Cancer 2020 World Conference. In a preliminary study, UDX detected 37 lung cancer patients and 71 asymptomatic control subjects with a panel of 10 methylation sites. The results showed that lung cancer patients could be detected with a sensitivity of 73% (27/37) with 90% (64/71) specificity.
The “Aifeiming” gene methylation detection kit (PCR-fluorescent probe method) by Beijing Akron Medical Technology Co., Ltd. won the NMPA Class III certification. The clinical sensitivity of this product is 86.83%.
Quality Control System
We have established quality control system in accordance with the following regulations:
| 1. | Medical Device Quality Management System for Regulatory Requirements |
| 2. | Quality Management System Requirements |
| 3. | Regulations on the Supervision and Administration of Medical Devices |
| 4. | Measures for the Supervision and Administration of Medical Device Production |
| 5. | Appendix of Good Manufacturing Practice for Medical Devices In Vitro Diagnostic Reagents |
| 6. | Guiding Principles for Clinical Trial of In Vitro Diagnostic Reagents |
Our quality control system includes quality manuals specifically designed for each product, procedure documents, supporting documents and quality records. We implement, maintain and improve the quality control system to ensure that product quality can be effectively and compliantly controlled throughout the entire process of procurement, production, formation and realization. Specifically, our quality control team (“QC”) consists of three members who are responsible to examine the entire production process from raw materials to finished products. QC also collaborate with various internal departments of the Company to conduct periodic review on all aspects of our research, development, and production process and provide their recommendations to the management to ensure our products and processes adhere to the regulations apply to us.
Commercialization Preparation
We are committed to the development of microfluidic chips, reagents and detectors for capturing circulating tumor cells in blood. We have integrated various complex precision structures, dielectric detection and functional microfluidic biochips. Our devices include a variety of expensive semiconductor manufacturing and precision micro-manufacturing related equipment. We signed a research project equipment use contract with TSRI and used their semiconductor manufacturing equipment and precision micro-nano processing equipment for chip technology research and development such as concept presentation of each R&D process, cross-scale composite structure production, rapid wafer trial production, material testing, and thin film production.
The contract between Advanced Biomed Taiwan and TSRI was executed on August 1, 2022 and expired on July 31, 2023. The total cost of the study, including tax, is NTD 1,300,000. Advanced Biomed Taiwan will pay the research fee to the National Institute of Experimental Research in two installments. The first installment of NTD 650,000 was paid on September 23, 2022, and the second installment of NTD 650,000 was paid on April 18, 2023. The agreement can be unilaterally terminated only if a party fails to fulfill its obligations, if Advanced Biomed Taiwan declares bankruptcy, or if its business activities are disrupted by the government. The agreement can only be terminated by mutual agreement in all other circumstances. The parties agree to take the Taiwan Taipei District Court as the jurisdiction court of first instance for any disputes arising from the execution of this agreement, which shall be subject to the laws of Taiwan.
As for the designed microfluidic detector, since 2020, we have developed various functional microfluidic biomedical testing devices for microfluidic modules, automation software, and hardware. We also designed, manufactured and processed an increasing number of key components of our testing devices. All of our products must go through three steps to receive the required clearance from the NMPA before they can be sold to customers. The three steps are research and development, registration application, and registration review, which must be done in that order. At the registration application stage, we have to assemble all the required application materials, complete clinical trials (if required by NMPA), and work with an NMPA accredited third-party organization to examine our products in accordance with NMPA rules. NMPA will review our application during the registration review period and may request additional information before officially approving or denying our applications. Currently, A+Pre and AC-1000 and their corresponding chips have been cleared by the NMPA; A+SCDrop, A+CellScan, A+CellScan Chip, and A+LCGuard are at the registration application stage; the four matching immunostaining kits are under registration review. A+LCGuard is a Class III medical device and is required to conduct clinical trials before completing the registration process. As of the date of this prospectus, we have not applied for similar clearances from other jurisdictions.
| 77 |
Although none of the chips has been mass produced as of the date of this prospectus, we have been cooperating with the injection molding machine manufacturer Riva and the mold manufacturer Unimold to conduct pre-mass production trial test for our A+Pre Chip and AC-1000 Enrichment Chip. During the mass production trial test, we examine the following factors:
| · | whether the tested chip can work with its corresponding product, and |
| · | whether chips’ flatness, roughness, water leakage, critical size and thickness match the original design. |
A Chip is ready for mass-production if each individual production line can produce at least 2,500 pieces per month and pass the quality control examination. During the quality control examination, one chip out of every 48 chips will be randomly selected for verification and must achieve designed objectives when working with their corresponding products and meet all the product specifications to pass the examination. The product specifications are listed in the table below.
| Flatness | Roughness | No Water leakage | Critical size compared with original design | Thickness | |
| A+Pre Chip | ΔF < 0.1 mm | ΔR<1.5 um /mm2 | Under 2 bar | within 5% | 2 mm ±10% |
| AC-1000 Enrichment Chip | ΔF < 0.3 mm | ΔR < 1.5 um/mm2 | Under 2 bar | within 5% | 1.5 mm ±10% |
Upon completion of the pre-mass production trial test, all of the randomly selected A+Pre Chips and AC-1000 Enrichment Chips can work with their corresponding A+Pre and AC-1000 products and meet or exceed the production specifications.
One of every 48 chips was randomly selected and tested for performance verification by using A549 lung cancer cells spiked into human blood. By the end ofIn May 2023, a total of 20 A+Pre chips were tested, with a result of average tumor cell recovery rate of 94%, which exceeded the target rate of 90%, with the highest and lowest rates of 99% and 91.6%, respectively. The liquid and blood cells removal rate by A+Pre chip was between 90-92.5%, which is in line with the target rate of 90-94%. A total of 12 AC-1000 chips were examined and the average recovery rate is 79.5%, which exceeded the target rate of 75%, with the highest and lowest recovery rates of 88.6% and 76.8%, respectively. The blood cells depletion rate was >2.5 logs, exceeding the target rate of 2 logs, with the highest and lowest rate of 4 and 2.5 logs, respectively.
The specifications of the randomly selected A+Pre and AC-1000 chips produced during the pre-mass production trial test can be found in the table below:
| Flatness | Roughness | No Water leakage | Critical size compared with original design | Thickness | |
| A+Pre Chip | ΔF < 0.05 mm | ΔR<1.5 um /mm2 | Under 2 bar | within 3% | 2 mm ±5% |
| AC-1000 Enrichment Chip | ΔF < 0.08 mm | ΔR < 1 um/mm2 | Under 2 bar | within 5% | 1.5 mm ±10% |
Riva is a mold manufacturing and injection molding machine manufacturer. We have purchased a Riva injection molding machine at the end of October 2022 and completed the mass production trial test for AC-1000 chips, and the production samples met our product specifications. The special structure mold core we made combined with the Riva injection molding machine has repeatedly produced hundreds of pieces of AC-1000 chips. The quality of the products is consistent and meet the product specifications of the microstructure characteristics. The operation on the equipment has achieved the expected efficiency and cell enrichment efficiency. Each equipment is expected to produce 2,000 pieces AC-1000 chips per month.
Unimold is a mold manufacturer and an ISO9001 plastic injection molding foundry. We cooperate with Unimold to embed our specially manufactured mold core for A+Pre Chip into the mold cavity, which will be employed at our future production facility. Although there is no formal cooperation agreement between Unimold and us, Unimold produces custom-made molds for us on a make-to-order basis. Each time, we first discuss the mold specification with a Unimold representative, then receive an offer from Unimold with the price, and Unimold will produce the customized mold for us to test. To protect our intellectual property, Unimold and us entered into a Non-Disclosure Agreement (the “NDA”) with a term of three years. The NDA prohibits both parties from disclosing confidential information without authorization and hiring the other party’s employees during the term of the NDA or within two years of the termination of the NDA. The NDA is governed by the laws of Taiwan, and any disputes arising from the NDA must be resolved before Taiwan Tainan District Court. The chips produced by Unimold’s mold have met the specifications set by us, and a batch of small-scale trial production of 5,000 pieces was carried out in October 2022. Our A+Pre is operating to expected efficacy and performance specifications using the in-house produced A+Pre Chip. One injection-type production equipment is expected to produce 50,000 pieces of A+Pre Chip per month.
Our Platform
We have built our microfluidic technology platform to integrate research and development, design, and manufacture of biochips and microfluidic chips. The platform combines our patented chip technology and will enable localized operations of a variety of microfluidic chips, biosensors made by semiconductor fabrication technology, and integrated application patented technology. Each geographic territory will establish clean rooms for chip production in compliance with local regulations to meet local market demand. While performing its designed duties, our microfluidic technology platform can provide customized services to third parties for a fee. We envision providing services such as OEM production of microfluidic chips, micro-electromechanical components, biochips, sensors, and other components, customized product design, and commissioned development and research services to customers. Different from the general IC wafer OEM production, our production is based on our micro-nano manufacturing technology platform developed by professionals in various fields that integrates cross-field knowledge, including Micro Electro Mechanical Systems (MEMS), lithography-assisted micromachining (LIGA), semiconductor process, and soft materials such as silicone gel and polydimethylsiloxane. We boast the ability to develop, design, and manufacture micro-electromechanical components and sensors, including three-dimensional microstructures or micro-optical-electromechanical integrated components. The material of the microstructure can be multi-layer stacking of alloys and insulating materials, which may be used in a wide range of fields, such as pressure or environment detection, MEMS oscillators, optical actuators, biomedical components, passive components, silicon optical integration platforms, and microfluidic structures.
The platform integrates our AC-1000 and A+SCDrop devices. Our rare cell enrichment device AC-1000 integrates the A+Pre chip and AC-1000 CTC enrichment chip. AC-1000 uses semiconductor nano ultra-sensitive biosensors and patented microfluidic chip technology to isolate rare cells with complete cell activity. It has great potential to be applied to routine liquid biopsy and companion diagnosis in the future. AC-1000 can satisfy different applications with corresponding special chip products. Our products also have the potential to provide application services in tumor screening, auxiliary diagnosis, treatment evaluation, prognosis evaluation, recurrence and metastasis detection, individualized medication guidance, and companion diagnosis. In terms of tumor screening, we have developed a complete set of service models for early screening of lung cancer, and plan to conduct large-scale clinical trials in March 2024. Our application services for the identification of CTCs have matured, and the identification of CTCs can be used for tumor screening, auxiliary diagnosis, and treatment evaluation, etc. Our CTCs single cell capture device A+SCDrop is used in combination with A+Pre and AC-1000. It preserves the original activity of single cells and polymer microfluidic chips combined with cell dielectric sensing technology. With the technical advantages combined with our IVD kit products, A+SCDrop can be used in a variety of applications, such as single-cell sequencing, whole gene sequencing, protein sequencing, new drug development, cancer biomarker research, individualized diagnosis, individualized drug susceptibility testing, and other aspects of individualized precision medicine. We are also working on prognosis assessment, recurrence and metastasis detection, individualized medicine, and companion diagnosis.
Although no services have been provided to customers yet, we believe the application services using our products and devices will be an essential part of our future operations.
| 78 |
Competitive Strengths
Although we have not received any regulatory approvals necessary to commercialize our products, we believe that the following competitive strengths enable us to compete effectively in and capitalize on the growing oncology detection market:
| · | Our microfluidic technology |
| · | Proprietary Ultra-Sensitive Biosensor |
| · | On Track to |
| 79 |
| ● | Multi-Disciplinary Management |
Growth Strategy
We will strive to be a leading provider of precision oncology detection solutions by the following growth strategies:
| ● | Increase the market penetration of our oncology auxiliary products and expand our product portfolio to actively focus on in vitro early diagnosis, rapid evaluation of chemotherapy drugs, individualized treatment including clinical screening of drugs, detection of drug resistance, and monitoring of tumor recurrences. |
| ● | Develop cancer screening market in China and further expand to other regions. We will initially focus on developing our cancer screening market in China. We also plan to expand to North American and European market by setting up subsidiaries and localize production and operation to meet specific market demand and compliance requirements in the relevant market. |
| ● | Expand our R&D to strength and develop pipeline products. In the future, we will actively promote the research and development, application and registration of other cancer early screening products. |
Intellectual Property
As of March 31,September 30, 2023, we had registered or applied with China Patent and Trademark Office 8 patents on novel separation technologies for antibody-free and label-free enrichment of rare cell-based cancer biomarkers (such as CTC, CTC cluster, tumor marker expressed cells) from very dense blood cells, 6 patents on high throughput droplet microfluidic chips for nano-liter scale reagent preparation and single-cell, 3 patents for the device of water phase single-cell isolation and capturing for single cell applications, 6 patents on single-CTC detection/discrimination and delivery for single-CTC sorting and downstream single-CTC analysis, and 3 patents on target cells pre-concentration and enrichment. Meanwhile, we also applied 2 of abovementioned patents with PCT and 2 of abovementioned patents with the U.S., and one of the U.S. application has been granted on October 11, 2022. As of the date of this prospectus, we own 14 patents and additional 12 patent applications are pending. Several smart and key chip fabrication techniques/methods with mass production level are protected by commercial confidential to reduce exposed risk.
Our commercial success depends in part on our ability to obtain and maintain proprietary or intellectual property protection for our detection solutions and other commercially important products, technologies, invention and know-how, to operate without infringing, misappropriating or otherwise violating the proprietary or intellectual property rights of others and to prevent others from infringing, misappropriating or otherwise violating our proprietary or intellectual property rights. Our patent strategy is focused on seeking coverage for our core technologies and specific follow-on applications, implementations for detecting and monitoring cancer by determining genomic alterations and evaluating the status of specific biomarkers in liquid biopsy or tissue samples. In addition, we file for patent protection on our on-going research and development, particularly into early-stage cancer screening.
We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary and intellectual property position. We generally require our R&D personnel to enter into confidentiality agreements. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except under specific circumstances. In the case of our R&D personnel, the agreements provide that all of the technology which is conceived by the individual during the course of employment is our exclusive intellectual property. Furthermore, as a matter of company policy, our R&D personnel have entered into agreements that generally require disclosure and assignment to us of ideas, developments, discoveries and inventions made by them which relate to their employment with us.
| 80 |
| Patent Number | Patent Type* | Patent Description | Jurisdiction | Status | Expiration Date | Product Application | ||||||
| ZL201821544689.4 | Utility Models | Target cells pre-concentration and enrichment | China | Granted | 09/20/2028 | A+Pre | ||||||
| ZL201811418768.5 | Invention | Target cells pre-concentration and enrichment | China | Granted | 11/26/2038 | A+Pre | ||||||
| 201811098975.7 | Invention | Target cells pre-concentration and enrichment | China | Pending | 09/20/2038 | A+Pre | ||||||
| PCT/CN2018/103116 | Invention | Single-CTC detection/discrimination | International | Granted | N/A | A+SCDrop chip | ||||||
| US11467081B216/639,117 | Invention | Single-CTC detection/discrimination | USA | Granted | 08/30/2038 | A+SCDrop chip | ||||||
| ZL201821544716.8 | Utility Models | Single-CTC detection/discrimination | China | Granted | 09/20/2028 | A+SCDrop chip | ||||||
| ZL201821544498.8 | Utility Models | Single-CTC detection/discrimination | China | Granted | 09/20/2028 | A+SCDrop chip | ||||||
| 201811099053.8 | Invention | Single-CTC detection/discrimination | China | Pending | 09/20/2038 | A+Pre Chip, AC-1000 CTC Enrichment Chip, and A+CellScan Chip | ||||||
| ZL201810381143.X | Invention | Single-CTC detection/discrimination | China | Granted | 04/25/2038 | A+SCDrop chip | ||||||
| PCT/CN2018/103171 | Invention | Separation technologies | International | Granted | N/A | AC-1000 | ||||||
| US20200086320A1 16/617,634 | Invention | Separation technologies | USA | Pending | 08/30/2038 | AC-1000 | ||||||
| ZL201821544718.7 | Utility Models | Separation technologies | China | Granted | 09/20/2028 | AC-1000 | ||||||
| ZL201821544717.2 | Utility Models | Separation technologies | China | Granted | 09/20/2028 | AC-1000 | ||||||
| 201811098985.0 | Invention | Separation technologies | China | Pending | 09/20/2038 | AC-1000 | ||||||
| 201811098981.2 | Invention | Separation technologies | China | Pending | 09/20/2038 | AC-1000 | ||||||
| 201810169100.5 | Invention | Separation technologies | China | Pending | 02/28/2038 | AC-1000 | ||||||
| 202110753142.5 | Invention | Separation technologies | China | Pending | 02/28/2038 | AC-1000 | ||||||
| ZL201921425592.6 | Utility Models | Droplet microfluidics | China | Granted | 08/29/2029 | A+SCDrop | ||||||
| 201910807870.2 | Invention | Droplet microfluidics | China | Pending | 08/30/2038 | A+SCDrop | ||||||
| ZL201821961715.3 | Utility Models | Droplet microfluidics | China | Granted | 11/26/2028 | A+CellScan Chip & A+SCDrop | ||||||
| ZL201821544526.6 | Utility Models | Droplet microfluidics | China | Granted | 09/20/2028 | A+SCDrop | ||||||
| 201811470094.3 | Invention | Droplet microfluidics | China | Pending | 11/26/2038 | A+SCDrop chip | ||||||
| 201811099052.3 | Invention | Droplet microfluidics | China | Pending | 09/20/2038 | A+SCDrop | ||||||
| ZL201821562851.5 | Utility Models | Single-cell isolation and capturing | China | Granted | 09/20/2028 | A+Pre Chip, AC-1000 CTC Enrichment Chip, and A+CellScan Chip | ||||||
| 201811098968.7 | Invention | Single-cell isolation and capturing | China | Pending | 09/20/2038 | A+SCDrop chip | ||||||
| 201811098887.7 | Invention | Single-cell isolation and capturing | China | Pending | 09/20/2038 | A+SCDrop chip |
* Chinese Patent Law defines three different types of patents for protecting invention-creation: invention, utility model, and design. “Invention” means any new technical solution proposed for a product, a process or the improvement thereof. “Utility Model” means any new technical solution proposed for the shape, the structure, or their combination, of a product, which is fit for practical use. “Design” means, with respect to an overall or partial product, any new design of the shape, the pattern, or their combination, or the combination of the colour with shape or pattern, which is rich in an aesthetic appeal and is fit for industrial application.
| 81 |
As of March 31,September 30, 2023, we had registered 8 trademarks in China, including 欣戈赛and sglcell.
We also own 11 registered domain names in China, including our official website, www.advanbiomed.com.
| Item | ||||
| 1 | 欣戈赛 | |||
| 2 | sglcell | |||
| Item | Domain Names | |||
| 1 | advanbiomed.com | |||
| 2 | sglcell.cn | |||
| 3 | sglcell.net | |||
| 4 | sglcell.com | |||
| 5 | singlecellbiomed.com.cn | |||
| 6 | singlecellbiomed.cn | |||
| 7 | singlecellbiomed.com | |||
| 8 | cell-biomed.com | |||
| 9 | abi-cell.com | |||
| 10 | abi-singlecell.com | |||
| 11 | yitiancell.com | |||
Employees
As of March 31,September 30, 2023, we had 40 employees. Most of our employees are located in mainland China, with a small number located in Taiwan. The following table sets forth the number of our employees by function as of March 31,September 30, 2023.
| As of March 31, 2023 | As of September 30, 2023 | |||||||||||||||
| Number | % of Total Employees | Number | % of Total Employees | |||||||||||||
| Functions: | ||||||||||||||||
| Technology, Research and Development | 21 | 53 | % | 21 | 53 | % | ||||||||||
| Medical Affairs | 5 | 12 | % | 5 | 12 | % | ||||||||||
| Operations and Quality Assurance | 4 | 10 | % | 4 | 10 | % | ||||||||||
| Sales and Marketing | 4 | 10 | % | 4 | 10 | % | ||||||||||
| General and Administration | 6 | 15 | % | 6 | 15 | % | ||||||||||
| Total number of employees | 40 | 100.0 | % | 40 | 100.0 | % | ||||||||||
Our R&D personnel are imperative to our technological development. They are responsible for the early stage of biochip design and production, design improvement and optimization, self-developed microfluidic system, design and development of biomedical testing instruments, and establishment of biochip mass production mode. They also test the electrical safety of our medical devices, assist us to prepare FDA medical device regulatory certification and registration application documents. The R&D personnel are also responsible for the training of clinical laboratory personnel, the training of assembly, testing and maintenance of medical equipment production line personnel, and future chip mass production and packaging production.
Our Shanghai subsidiary is required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance and other statutory benefits and a housing provident fund. Our Shanghai subsidiary also enters into standard labor contracts with its employees. The labor contract typically includes a confidentiality covenant that requires employees to protect the company confidential information during their employment. In addition, employees are prohibited from entering into another employment relationship with a third party, which may adversely affect our business.
Advisors and Industry-Academia Cooperation
We focus on the research and development of various advanced and original microfluidic biochips and strive to provide tumor precision diagnosis and treatment technology.technology and early cancer detection service. In order to strengthen our strategic development and research, we have worked with the following advisors (“Advisors”) and the National Taiwan University (the “NTU”) pursuant to certain advisory agreements (“Advisors”):and industry-academia cooperation agreements:
Advisors
Lin Jianhuang is currently President of Taipei Medical University, Director of National Applied Research Laboratory, Director of Biotechnology and Pharmaceutical Industry Research Institute, Executive Director of Taiwan Pharmacological Society, and Director of International Cooperation Foundation. Dr. Lin is mainly engaged in the field of molecular pharmacology signal transduction new drug development.
| 82 |
Zhou Caicun Director of the Institute of Oncology, Tongji University School of Medicine, Director of the Department of Oncology. Director of Oncology Department of Shanghai Pulmonary Hospital, Director of Lung Cancer Immunology Research Office, Director of Clinical Pharmacology Institute. Mainly engaged in early diagnosis of lung cancer, comprehensive treatment, targeted therapy and individualized therapy, molecular imaging of tumor, phage vaccine.
The Advisors attend quarterly meetings and provide professional advice to the Company. Advisors make recommendations and offer guidance on the Company's long-term development strategy and major decisions, including providing consultation and suggestions on the Company's development plans, research directions, scientific partnerships, and academic exchanges based on their abundant clinical experience to help the Company stay on the right track. For example, we used to focus on counting tumor cells, and our Advisors suggested to capture active single cells for further clinical application. Based on the suggestion, we eventually developed A+SCDrop. Furthermore, our Advisors suggested that we adopt the method of taking blood samples at multiple times, as the number of targeted cells contained in samples at different times is different, so as to ensure the accuracy of the obtained results. Accordingly, we adjusted our a clinical plan regarding our prospective clinical trials.
Below are the material terms of the advisory agreements (the “Advisory Agreement”):
Terms. The Advisory Agreement for Mr. Lin Jianhuang went into effect on November 1, 2022, and will end on October 31, 2025, unless terminated earlier in accordance with the provisions of this agreement.the Advisory Agreement. The Advisory Agreement for Mr. Lin Jianhuang may be renewed if the parties start the renewal process three months prior to the end of this agreement and agree on the terms and conditions of the new agreement. The Advisory Agreement for Mr. Zhou Caicun went into effect on July 1, 2022, and will end on June 30, 2025, unless terminated earlier in accordance with the provisions of this agreement.the Advisory Agreement. The Advisory Agreement for Mr. Zhou Caicun may be renewed if the parties start the renewal process one months prior to the end of this agreement and agree on the terms and conditions of the new agreement.
Services. The Advisor is hired by us as its advisor (“Advisor”) and provide consulting service to us regarding our development plans and strategies.
Compensation. We will reimburse Mr. Zhou Caicun any work-related expenses. We will reimburse Mr. Lin Jianhuang any work-related expenses and we agreed to grant Mr. Lin 0.5% of the total number of shares of this Offering. The common stock will be distributed evenly among Mr. Lin’s three years of service.
Termination. This Advisory Agreement can only be modified by both parties’ written consents and will remain in effect if either Party does not agree on the proposed modification. This Advisory Agreement is terminated once the end date has been reached or in accordance with the terms and conditions of this Advisory Agreement.
Industry-Academia Cooperation
Advanced Biomed Taiwan entered into two industry-academia cooperation agreements with the National Taiwan University under identical terms and conditions for identifying novel biomarkers for early detection of lung cancer.
Below are the material terms of the industry-academia cooperation agreements (the “Cooperation Agreement”):
Terms. The Cooperation Agreement went into effect on April 15, 2023, and will end on April 14, 2024, unless terminated earlier in accordance with the provisions of the Cooperation Agreement.
Scopes. Research and development works stipulated in the Cooperation Agreements focus on improving the accuracy of early lung cancer detection and exploring more novel biomarkers in that regard.
Payment. Advanced Biomed Taiwan is required to pay the NTU NTD 6 million in two equal installments, with the first one been paid on April 24, 2023. The second installment of NTD 3 million was due on October 15, 2023. We will promptly transfer the second installment to the NTU once it issues an official fee notification to us.
Ownership. The parties recognize that the services provided by the advisor are advisory and consulting in nature and do not involve actual research activities. The NTU shall own the copyrights of any and all reports and works produced under this Cooperation Agreement, while Advanced Biomed Taiwan has the license to use any and all reports and works produced under this Cooperation Agreement freely.
Termination. Either party to the Cooperation Agreement has the power to terminate such agreement if the NTU cannot perform its duties and obligations due to force majeure events or if Advanced Biomed Taiwan wishes to terminate the cooperation relationship, provided that the NTU shall refund Advanced Biomed Taiwan any portion of the payment to the extent not actually spent.
Property
We currently lease three properties for our Taiwan R&D center, which covers an area of 450 square meters. The lease of Haihuan Street expires on May 29, 2024; the lease of 689-86 Xiaodong Street expires on March 31, 2024; the lease of 689-87 Xiaodong Street expires on November 1, 2024. Pursuant to an equipment use contract with the adjacent TSRI, Taiwan R&D center can use the TSRI’s various semiconductor manufacturing equipment manufacturing and precision micro-nano processing equipment to carry out concept presentation and cross-scale composite structure of each R&D process, chip technology research and development, rapid chip trial production, material testing, and thin film production. We believe that we will be able to obtain adequate facilities, principally through leasing, to accommodate our future expansion.
Shanghai office is located in Shanghai Bay Valley Science and Technology Park (“Bay Valley”), adjacent to Shanghai Pulmonary Hospital and Fudan University Life Research Institute. The leased area is 1267.98 square meters, and the lease term is from October 16, 2021 to October 15, 2024. Bay Valley is structured to meet the unique needs of emerging companies.
The geographic location of our Taiwan R&D center provides access to supply of graduates from the local Chenggong University for R&D personnel. Its vicinity to Taiwan Semiconductor Research Center, Yongkang Industrial Zone, and Chengda Hospital enables us to form a good research and development ecosystem for biomedical testing of our devices and chips. The location also enables us to integrate nearby resources of the semiconductor micro-manufacturing related facilities, precision processing industry community, hospital biological treatment and preservation facilities and other resources required for our research and development process.
Licenses and Approvals
The following table sets forth licenses and approvals that our subsidiaries are required to obtain for our operations in Taiwan and China as of the date of this prospectus:
| Name | Licenses and Approvals | PRC and Taiwan Regulatory Authority | Expiration Date | ||||
| Advanced Biomed Taiwan | Business Registration Certificate | Tainan City Government | indefinite | ||||
| Advanced Biomed HK | Certificate of Incorporation | Registrar of Companies, Hong Kong Special Administrative Region | indefinite |
| 83 |
| Business Registration Certificate | Registrar of Companies, Hong Kong Special Administrative Region | August 9, | |||||
| Shanghai Sglcell | Business License | Shanghai Administration for Market Regulation | indefinite |
Legal Proceedings
We may from time to time be subject to legal and administrative proceedings and claims that arise in the ordinary course of business. We are not aware of any pending or threatened material legal or administrative proceedings against us. We do not believe that any claims exist where the outcome of such matters would have a material adverse effect on our consolidated financial position, operating results or cash flows. However, there can be no assurance such legal proceedings will not have a material impact on future results.
| 84 |
Regulations
This section summarizes the principal Taiwan and PRC laws, rules and regulations related to our business and operations.
REGULATIONS IN TAIWANRegulations in Taiwan
Regulations Relating to Taiwan Companies Going to The Mainland to Engage in Technical Cooperation
On March 1, 1993, the Ministry of Economic Affairs promulgated the Regulations Governing the Examination of Investment or Technical Cooperation in Mainland China (“Examination of Investment or Technical Cooperation”) which became effective immediately. The regulations were amended and took effect on December 30, 2020. On March 1, 1993, the Ministry of Economic Affairs also promulgated the Regulations Governing the Approval of Investment or Technical Cooperation in Mainland China (“Approval of Investment or Technical Cooperation”). The regulations were amended and took effect on April 21, 2020.
If a Taiwan company intends to go to mainland China for technical cooperation, in principle, it must submit an application to the Taiwan Investment Commission in advance. According to the instructions of the Taiwan Investment Commission, the average review time for each case is 15-30 days. During the review, the competent authority will consider factors such as the impact on the core competitiveness of other Taiwanese companies, the layout of R&D and innovation, and the infringement of intellectual property rights of other companies to comprehensively determine whether to approve or not. If a Taiwan company and a Chinese company engage in technical cooperation without permission, the competent authority may impose a fine of not less than NTD$NTD 50,000 but not more than NTD$NTD 25,000,000 in accordance with Paragraph 1 of Article 86 of the Taiwan -Mainland People's Relations Act, and may terminate it within a time limit, or require the companies to make corrections. Those who do not comply or make corrections within the time limit may be punished continuously. However, if a Taiwan company goes to mainland China to engage in technical cooperation that does not involve substantial technology outflow, for example, if the Chinese company has no substantial involvement in product design and related R&D processes, it is not necessary to apply to the Investment Review Committee in advance. The competent authority may make alterations to the general and prohibited technical cooperation projects either by conducting regular reviews annually or by conducting special reviews on a case-by-case basis.
Regulations Relating to Exchange Control or Currency Regulations or any Registration Requirements Under Anti-Money Laundering Laws
On October 23, 1996, the Ministry of Justice promulgated the Money Laundering Control Act (the “Act”), which became effective on April 23, 1997. The Act was amended and became effective on November 7, 2018. The Act is enacted to prevent money laundering activities and combat-related crimes; bolster anti-money laundering systems; maintain financial stability; increase transparency in money flows; and strengthen international cooperation.
Pursuant to the Act, inward and outward remittances involving currency exchange between NTD and another foreign currency are subject to an annual ceiling of: USD $5 million for individuals and USD $50 million for businesses. Any total annual remittance exceeding these thresholds requires approval by the Central Bank of the Republic of China (CBC). Individual foreign exchange transactions over NTD500,000NTD 500,000 must be reported to the CBC.
There are no other foreign exchange controls on trade, insurance, and authorized investment transactions, and no limit on repatriation of capital or profits from portfolio investments. There are also no registration requirements under anti-money laundering laws.
Regulations Relating to Anti-bribery or Corruption
Bribery and corruption of public officials in Taiwan are mainly regulated by the Anti-Corruption Act. The Anti-Corruption Act was promulgated by the Ministry of Justice and took effect on July 15, 1963. It was later amended on June 22, 2016 and became effective on July 1, 2016. The Anti-Corruption Act lists various behaviors that constitute bribery of public officials. Public officials who commit bribery may be punished by up to life imprisonment together with a fine of up to NTD100NTD 100 million. Any private individual who commits bribery of public officials may be punished by up to seven years of imprisonment together with a fine of up to NTD3NTD 3 million.
| 85 |
Hospitality accepted by a public official is only allowed under limited circumstances specified in the Integrity and Ethics Directions for Civil Servants. The value of gifts received by a public official cannot exceed NTD500.NTD 500. Gifts received due to visits to companies cannot exceed NTD3,000.NTD 3,000. The total value of gifts received from the same source in one year cannot exceed NTD10,000.NTD 10,000.
The Ethical Corporate Management Best Practice Principles were promulgated by the Taipei Exchange and became effective on September 3, 2010. The principles were amended and took effect on May 23, 2019. These principles are applicable to companies, their subsidiaries, any foundation to which the companies’ direct or indirect contribution of funds exceeds 50 percent of the total funds received, and other institutions or juridical persons which are substantially controlled by such companies. The purpose of the principles is to foster a corporate culture of ethical management and sound development and offer a reference framework for establishing good commercial practices.
Regulations Relating to employment relationships
The Employment Service Act was promulgated by the Ministry of Labor and became effective on May 8, 1992. It was amended and became effective on November 28, 2018. The Act is enacted to promote the employment of nationals with a view to enhance social and economic development.
Labor Standards Act was promulgated by the Ministry of Labor and became effective on July 30, 1984. It was amended and took effect on June 10, 2020. The Act is enacted to provide minimum standards for working conditions, protect workers' rights and interests, strengthen employee-employer relationships and promote social and economic development.
Regulations Relating to Intellectual Property
Patents
The Patent Act was promulgated by the Ministry of Economic Affairs and took effect on May 29, 1944. It was amended and took effect on May 4, 2022. The Patent Act is formulated to encourage, protect and utilize the creations of invention, utility model and design in order to promote industrial development.
Trademarks
The Trademark Act was promulgated by the Ministry of Economic Affairs and became effective on May 6, 1930. Article 68, 70, 95, 96 and 97 were amended on May 4, 2022,, which shall take effect on the date designated by Executive Yuan. This Act is enacted for protection of the rights of trademark, certification mark, collective membership mark, collective trademark and the interests of consumers, maintenance of fair competition, and promotion of development of the industry and commerce.
Copyright
The Copyright Act was promulgated by the Ministry of Economic Affairs and took effect on May 14, 1928. It was amended and took effect on June 15, 2022. This Act is specifically enacted for the purposes of protecting the rights and interests of authors with respect to their works, balancing different interests for the common good of society, and promoting the development of national culture. Matters not provided for herein shall be governed by the provisions of other acts.
Trade Secrets
The Trade Secrets Act was promulgated by the Ministry of Economic Affairs and became effective on January 17, 1996. It was amended and took effect on January 15, 2020. This Act is enacted to protect trade secrets, maintain industrial ethics and order in competition, and balance societal and public interests. Matters not provided for in this Act shall be governed by other laws.
Regulations Relating to Data Protection
The Personal Data Protection Act was promulgated by the National Development Council on August 11, 1995 and became effective on January 17, 1996. It was amended on December 30, 2015, and became effective on March 15, 2016. The Personal Data Protection Act (hereinafter,(hereinafter, the “PDPA”) is enacted to regulate the collection, processing and use of personal data so as to prevent harm on personality rights, and to facilitate the proper use of personal data.
| 86 |
Regulations on Taxation
The Income Tax Act was promulgated by the Ministry of Finance and became effective on February 17, 1943. It was amended on April 28, 2021 and became effective on July 1, 2021. Income tax is classified into consolidated income tax and profit-seeking enterprise income tax. For any individual having income from sources in the Republic of China, consolidated income tax shall be levied in accordance with this Act on his income derived from sources in the Republic of China.
The Tax Collection Act was promulgated by the Ministry of Finance and became effective on October 22, 1976. It was amended on December 17, 2021 and became effective on January 1, 2022. Collection of taxes shall be governed by this Act. Where a profit-seeking enterprise ceases to exist after a merger, the surviving or newly incorporated profit-seeking enterprise shall pay in full the taxes originally payable by the dissolved enterprise prior to the merger.
Major taxes on corporations:corporations include:
1. Corporate income tax (“CITCIT”)”)
If the total taxable income of a profit-seeking enterprise is NTD 120,000 or less, the profit-seeking enterprise is exempt from tax. If the total taxable income of a profit-seeking enterprise is more than NTD120,000 but not more than NTD 200,000, the income tax payable is computed based on the following formula: (Profit-NTD120,000) x 50%. As for a profit-seeking enterprise with the total taxable income of more than NTD 200,000, the income tax rate shall be: Profit x 20%.
2.
Profit retention tax
An additional profit retention tax of 5% is imposed on any current earnings of a corporation that remain undistributed by the end of the following year.
Regulations Relating to Bio Industry in Taiwan
Act for the Development of Biotech and Pharmaceutical Industry
In 2007, Act for the Development of Biotech and Pharmaceutical Industry (the “Statute”) was enacted in order to promote the development of the biotech and pharmaceutical industry in Taiwan so that it can bring about changes in the economic structure of the country.
By 2022, the scope of the Statute covers industries that deals in new drugs, new dosage forms, high-risk medical devices, regenerative medicine, precision medicine, digital medicine, innovative technology platforms dedicated to biotech and pharmaceutical industry and other strategic biotech and pharmaceutical products used by human beings, animals and plants.
The Statute permits biotech and new pharmaceutical companies to offset up to 30% of their expenses for personnel training and R&D against enterprise income tax over a period of five years. Up to five percent (5%) of the annual investment amount may be credited against the profit-seeking enterprise income tax payable by it in the then current year from the first year the biotech and pharmaceutical company has payable profit-seeking income tax; or up to three percent (3%) of the annual investment amount may be credited against the profit-seeking enterprise's income tax payable in each of the three years from the first year the biotech and pharmaceutical company has payable profit-seeking income tax. In addition, based on Article 5, up to 25% of the research and development investment of the biotech companies that engage in research, development, and manufacturing may be deducted from the amount of profit-seeking enterprise income tax payable for each year within five years from the year in which the payable profit-seeking enterprise income tax is incurred with the annual deduction capped at 50% of the tax.
The Statute provides for tax incentives to high-level professionals and technology investors to participate in company operations and R&D (Art. 7), to elaborate, up to 20% of the price paid for the shares may be deducted from the payable profit-seeking enterprise income tax for each year within five years from the year a payable profit-seeking enterprise income tax is incurred with the annual deduction capped at 50% of the tax. Moreover, if the senior professional staff or technology investors of the biotech companies receive newly issued stock or stock options as a result of reward or due to technology invested as capital stock and have held shares or stock options, been employed, or provided technical services cumulatively for two years, they may choose to be taxed at the lower of the “transfer price” or the “current price or price at which the stock was acquired”.
| 87 |
Finally, researchers of schools and institutions who are the main technology providers of the start-up biotech companies are exempted from the prohibitions against business operation and part-time employment and the restrictions on shareholding percentage under Article 34 of the Act Governing the Appointment of Educators and Articles 13 and 14 of the Civil Servants Work Act.
REGULATIONS IN THERegulations in the PRC
Regulations Relating to Medical Devices in the PRC
Regulations Relating to Classification of Medical Devices
Pursuant to the Regulations on the Supervision and Administration of Medical Devices promulgated on January 4, 2000, effective on June 1, 2014, amended by the State Council on May 4, 2017 and now effective, and then amended on February 9, 2021 but not yet effective until June 1, 2021 (“Regulation on Supervision and Administration of Medical Devices”), the China Food and Drug Administration (“CFDA”) of the State Council shall be responsible for the national administration and supervision of medical devices of the PRC and its local counterparts take charge of the local administration and supervision of medical devices of the PRC.
Under this regulation, medical devices have been classified into three categories based on the degree of risk. Class I medical devices shall refer to those devices with low level of risks and whose safety and effectiveness can be ensured through routine administration. Class II medical devices shall refer to those devices with moderate risks that must be strictly controlled and regulated to ensure their safety and effectiveness. Class III medical devices shall refer to those devices with relatively high risks that must be strictly controlled and regulated through special measures to ensure their safety and effectiveness.
Currently, the products we are approved by NMPA to manufacture and sell are Class I medical device.
Registration and Filings of Medical Devices
Pursuant to the Regulations on the Supervision and Administration of Medical Devices and the Administrative Measures for the Registration of Medical Devices promulgated by CFDA on July 30, 2014 and came into effect on October 1, 2014 (“the Supervision and Administration of Medical Devices” was amended and came into effect on May 4, 2017. Then it was amended on February 9, 2021 and came into effect on June 1, 2021), Class I medical devices are subject to filing administration, and Class II and Class III medical devices are subject to pre-approval registration administration. A registration certificate for Class II and Class III medical devices are issued upon approval, which is valid for five years and may be renewed six months prior to its expiration date. In addition, a registrant or record-filing party of medical devices may manufacture medical devices on its own or entrust enterprises that meet the provisions hereof and have corresponding conditions to manufacture medical devices. In the event of entrusted manufacture of medical devices, the registrant or record-filing party of medical devices shall be responsible for the quality of medical devices manufactured under entrustment, and strengthen management of the manufacturing activities of the entrusted manufacturers to ensure that they carry out manufacturing in accordance with statutory requirements. The registrant or record-filing party of medical devices shall enter into an entrustment agreement with the entrusted manufacturer to specify the rights, obligations and responsibilities of both parties. The entrusted manufacturer shall organize manufacture of medical devices in accordance with laws and regulations, GMP, compulsory standards, product technical requirements and the entrustment agreement, be responsible for the manufacturing activities and accept the supervision of the entrusting party.
Clinical trials are not required for the filing of the Class I medical devices, but necessary for the registration of Class II and Class III medical device with certain exceptions.
Among our products, A+Pre and AC-1000 are Class I medical devices and have passed NMPA registration review. A+CellScan, A+CellScan Chip, and A+SCDrop are Class II medical devices but do not need to conduct clinical trials. A+LCGuard is Class III medical device and is required to conduct clinical trials before completing the registration process. We have already started the registration process and plan to start clinical trials in March 2024. The trials are expected to end in June 2025, and we anticipate that we will obtain the required registration certificate by the end of 2026.
Production License for Medical Devices
Pursuant to the Regulation on the Supervision and Administration of Medical Devices promulgated on July 30, 2014 and came into effect on October 1, 2014, as amended in 2017 and came into effect on May 4, 2017 (amended on February 9, 2021, came into effect on June 1, 2021), and the Administrative Measures on the Production Supervision of Medical Devices promulgated on July 30, 2014 and came into effect on October 1, 2014, as amended in 2017 and came into effect on November 11, 2017, manufacturers engaged in the manufacturing of Class I medical devices are subject to production filing administration and receive production filing certificates upon satisfaction of filing requirements; while those engaged in the manufacturing of Class II and Class III medical devices are subject to pre-approval licensing administration and receive medical device production licenses upon receipt of approval for licensing. A medical device production license is valid for five years and may be renewed six months prior to its expiration date.
Before obtaining production licenses from local government authorities, our product must pass NMPA’s registration review. Among our products, A+Pre and AC-1000 and their corresponding chips have passed NMPA registration review, A+CellScan, A+CellScan Chip, and A+SCDrop are at the registration application stage, the four matching immunostaining kits are under NMPA registration review. A+LCGuard is required to conduct clinical trials before completing the registration process. We have already started the registration process and plan to start clinical trials in March 2024. The trials are expected to end in June 2025, and we anticipate that we will obtain the required registration certificate by the end of 2026.
| 88 |
In addition, a manufacturer of medical devices shall satisfy the following conditions:
(1) possessing production sites, environmental conditions, production equipment and professional technicians that are suitable for such medical device produced;
| · | possessing production sites, environmental conditions, production equipment and professional technicians that are suitable for such medical device produced; |
(2) possessing organizations or professional examination staff and examination equipment that carry out quality examination for such medical device produced;
| · | possessing organizations or professional examination staff and examination equipment that carry out quality examination for such medical device produced; |
(3) formulating a management system which ensures the quality of such medical device;
| · | formulating a management system which ensures the quality of such medical device; |
(4) having capability of after-sale services that is suitable for such medical device produced;
| · | having capability of after-sale services that is suitable for such medical device produced; and |
(5) satisfying the requirements as prescribed in production R&D and production technique documents.
| · | satisfying the requirements as prescribed in production R&D and production technique documents. |
As of the date of this prospectus, we have obtained the license for the production of the Class I medical device.
Production and Quality Management of Medical Devices
Pursuant to the Administrative Measures on the Supervision of the Production of Medical Devices promulgated on December 29, 2014 and came into effect on March 1, 2015, as amended in 2017 and came into effect on November 17, 2017, and the Standards on Production and Quality Management of Medical Devices promulgated by the CFDA on December 29, 2014 and came into effect on March 1, 2015, an enterprise engaged in the production of medical devices shall establish and effectively maintain a quality control system in accordance to the requirements of the Standards on Production and Quality Management of Medical Devices. The enterprise engaged in the production of medical devices shall regularly conduct comprehensive self-inspection on the operation of quality management system and submit this report to the local food and drug supervision and administration authorities before the end of every year. The enterprise shall also establish its procurement control procedure and assess its suppliers by establishing an examination system to ensure the purchased products are in compliance with the statutory requirements. The enterprise shall apply risk management to the whole process of design and development, production, sales and after-sale services.
Pursuant to The Notice of Four Guidelines including On-site Inspection Guidelines for the standards on Production and Quality Management of Medical Devices promulgated by the CFDA on September 25, 2015 and came into effect on September 25, 2015, during the course of on-site verification of the registration of medical devices and on-site inspection of production license t(including change production license), the inspection team shall, in accordance with the guidelines, issue recommended conclusions for on-site inspections, which shall be divided into “Passed,” “Failed” and “Reassessment after rectification.” During the supervision and inspection, if it is found that the requirements of the key items or ordinary items that may have direct impact on product quality are not satisfied, the enterprise shall suspend production and go through rectification. If it is found that the requirements of the ordinary items are not satisfied, and it does not directly affect product quality, the enterprise shall rectify in a prescribed time. The regulatory authorities will examine and verify the recommended conclusions and on-site inspection materials submitted by the inspection group and issue the final inspection results.
Good Clinical Practice for Medical Devices
On March 1, 2016, the CFDA and the National Health and Family Planning Commission jointly promulgated the Good Clinical Practice for Medical Devices, which became effective as of June 1, 2016. The regulation includes full procedures of clinical trial of medical devices, including, among others, the protocol design, conduction, monitoring, verification, inspection, and data collection, recording, analysis and conclusion and reporting procedure of a clinical trial.
| 89 |
For conducting clinical trials of medical devices, an applicant shall organize to formulate scientific and reasonable clinical trial protocol based on the categories, risks and intended use of the medical devices for the clinical study. The applicant shall be responsible for organizing to develop and revise of the researcher’s manual, clinical trial protocol, informed consent form, case report form, relevant standard operating procedures and other relevant documents, and shall be responsible for organizing necessary trainings for the clinical trials. The applicant shall select the clinical trial institutions and its researchers from the qualified medical device clinical trial institutions according to the characteristics of the medical devices to be used in the clinical study.
As an applicant for clinical trials of medical devices, we are responsible for initiating, applying, organizing and monitoring such clinical trials, and shall be responsible for the authenticity and reliability of the clinical trials.
Operation License for Medical Device
Pursuant to the Regulations on the Supervision and Administration of Medical Devices and the Administrative Measures on the Operation Supervision of Medical Devices, promulgated on July 30, 2014 and came into effect on October 1, 2014 (amended on November 17, 2017, came into effect on November 17, 2017), filing and licensing are not required for the operation of Class I medical devices. Operators engaged in the operation of Class II medical devices are subject to filing administration and will receive medical device operation filing certificate upon satisfaction of filing requirement, while operators engaged in the operation of Class III medical devices are subject to pre-approval licensing administration and will receive medical device operation license upon receipt of approval for licensing. A medical device operation license is valid for five years and may be renewed six months prior to its expiration date
To engage in business operations of medical devices, the following requirements shall be met:
| Having a quality control institution or staff corresponding to the business scope and scale, and the staff shall have relevant education or professional titles certified by the |
| Having an operation and storage premises corresponding to the business scope and |
| Having storage conditions corresponding to the business scope and scale; warehouses are not required if all storage is commissioned to other operators of medical |
| Having a quality control system corresponding to the medical devices |
| Possessing the capability of professional guidance, technical training and after-sale service corresponding to the medical devices it operates; or it has come into an agreement on technical support with a relevant institution. |
An enterprise to be engaged in business operations of Category III medical devices shall also have a computerized information management system compliant with quality standards to ensure traceability of products. An enterprise to be engaged in business operations of Category I or Category II medical devices is encouraged to set up such a system.
Special Procedures for Examination and Approval of Innovative Medical Devices
On October 2017, the General Office of the CPC Central Committee and the General Office of the State Council issued the Opinions on Deepening the Reform of the Evaluation and Approval Systems and Encouraging Innovation on Drugs and Medical Devices, which aims to encourage the innovation for medical devices.
Pursuant to the Opinions, the priority review and approval will be applicable to innovative medical devices supported by the National Science and Technology Major Projects and the National Key R&D Program of China, and the clinical trials of which having been conducted by the National Clinical Research Center and approved by the management department of National Clinical Research Center. Pursuant to the Special Procedures for Examination and Approval of Innovative Medical Devices which were promulgated by the NMPA on November 2, 2018 and came into effect on December 1, 2018, special procedures shall be applicable to the examination and approval for medical devices in the following circumstances:
| 90 |
| if the applicant legally owns the invention patent of the core technology of the product through its technological innovation activities in the PRC, or legally obtained the invention patent or the right of use thereof through transfer in the PRC, and that the interval between the date of application for the special examination and approval of innovative medical devices to the date of authorized publication should not exceed five years; or the patent administration department of the State Council has disclosed the application for the invention patent of the core technology and the Patent Search and Consultation Center of the National Intellectual Property Administration of the PRC has issued the patent search report setting out the novelty and innovation of the core technology solution of the product; |
| the applicant has developed the prototype product and completed the preliminary research under a true and controllable process that generated complete and traceable data; and |
| the product has major working mechanism or mechanism of action which is the first of its kind in the PRC, has fundamental improvement in product performance or safety compared with similar products, is of an internationally leading standard in terms of techniques and has significant clinical value. The Center for Medical Device Evaluation of the NMPA should give priority to the innovative medical devices in their technical review upon receiving the registration application, after which the NMPA will give priority to the product in their administrative approval. |
Advertisements of Medical Devices
Pursuant to the Regulations on Tentative Measures for the Censorship of Advertisement for Drugs, Medical Devices, Dietary Supplements, Food Formula for Special Medical Purpose promulgated by SAMR on December 24, 2019 and came into effect on March 1, 2020, the State Administration for Market Regulation is responsible for organizing and guiding the review of advertisements for drugs, medical devices, health foods and formula foods for special medical purposes. The administrations for market regulation and drug administrations (hereinafter referred to as the “advertisement review authorities”) of all provinces, autonomous regions and centrally administered municipalities shall be responsible for the review of advertisements for drugs, medical devices, health food and formula food for special medical purposes, and may entrust other administrative authorities to implement review of advertisements pursuant to the law.
The validity period of the advertisement approval number for drugs, medical devices, health food and formula food for special medical purposes shall be consistent with the shortest validity period of the product registration certificate, filing certificate or production license. If no valid period is prescribed in the product registration certificate, filing certificate or production license, the valid period of the advertisement approval number shall be two years.
Advertisements for drugs, medical devices, health food and formula food for special medical purposes shall be true and legitimate and shall not contain any false or misleading contents. Advertisers shall be responsible for the veracity and legitimacy of the contents of advertisements for drugs, medical devices, health food and formula food for special medical purposes.
National Medical Insurance Program
The National Medical Insurance Program (NMIP) was adopted pursuant to the Decision of the State Council on the Establishment of the Urban Employee Basic Medical Insurance Program issued by the State Council on December 14, 1998, under which all employers in urban cities are required to enroll their employees in the Urban Employee Basic Medical Insurance Program and the insurance premium is jointly contributed by the employers and employees. Pursuant to the Opinions on the Establishment of the New Rural Cooperative Medical System forwarded by the General Office of the State Council on January 16, 2003, China launched the New Rural Cooperative Medical System to provide medical insurance for rural residents in selected areas which has since spread to the whole nation. The State Council promulgated the Guiding Opinions of the State Council about the Pilot Urban Resident Basic Medical Insurance on July 10, 2007, under which urban residents of the pilot district, rather than urban employees, may voluntarily join Urban Resident Basic Medical Insurance. In 2015, the PRC government announced the Outline for the Planning of the National Medical and Health Service System (2015-2020) which aims to establish a basic medical and health care system that covers both rural and urban citizens by 2020. On January 3, 2016, the State Council issued the Opinions on Integrating the Basic Medical Insurance Systems for Urban and Rural Residents to integrate the Urban Resident Basic Medical Insurance and the New Rural Cooperative Medical System and the establishment of a unified Basic Medical Insurance for Urban and Rural Residents, which will cover all urban and rural non-working residents expect for rural migrant workers and persons in flexible employment arrangements who participate in the basic medical insurance for urban employees. The National Healthcare Security Administration (NHSA) is the responsible agency that makes the final determination on whether a product may be covered by the NMIP.
With regard to reimbursement for medical devices and diagnostic tests, the Notice of Opinion on the Diagnosis and Treatment Management, Scope and Payment Standards of Medical Service Facilities Covered by the National Urban Employees Basic Medical Insurance Scheme (Lao She Bu Fa [1999] No. 22) prescribes the coverage of diagnostic and treatment devices and diagnostic tests where part of the fees is paid through the basic medical insurance scheme. It also includes a negative list that precludes certain devices and medical services from governmental reimbursement, which includes (i) examination and treatment items by applying large medical devices such as positron emission tomography (PET), electron beam CT, ophthalmic excimer laser therapy instruments, etc.; (ii) rehabilitation appliances such as spectacles, artificial dentures, artificial eyes, artificial limbs and hearing aids, etc.; (iii) medical devices for health care, massage, physical examination and treatment for own use; and (iv) disposable materials for medical use that cannot be charged separately as stipulated by price authorities of all provinces. Detailed reimbursement coverage and rate for medical devices and medical services (including diagnostic tests and kits) are subject to each province’s local policies.
Therefore, every covered individual is eligible to request reimbursement from the NMIP for medical expenses, but the reimbursement rate and coverage are determined on a case-by-case basis. Based on our understanding of applicable PRC laws and regulations, the reimbursement rate and coverage for certain products are different for each individual and are subject to the following factors:
| Whether the product is covered by the NMIP or whether the product is part of a procedure or service that is covered by the NMIP. If not covered, the NMIP may deny the request for reimbursement or reduce the reimbursement rate and coverage; |
| The region and status of individuals seeking reimbursement. NMIP varies in different regions and types, leading to different reimbursement ratios. For instance, the basic medical insurance for urban employees generally covers around 50% to 80% of basic medical expenses, while the rural resident medical insurance typically covers around 60% to 80%; |
| Hospital level. The reimbursement ratio of NMIP is related to the level and grade of hospitals performing services. For example, a level 3 hospital’s service is generally more expensive than primary healthcare institutions, and the reimbursement ratio for level 3 hospitals is lower than for primary healthcare institutions. Typically, an individual who received services from a primary care provider can get a relatively high reimbursement rate and coverage for medical expenses; |
| Individual payment status. The reimbursement ratio of NMIP is also related to the individual payment status. If an individual contributes more and for a longer period of time, the reimbursement ratio will correspondingly increase; and |
| Special diseases or major illnesses. For certain special diseases or major illnesses, the reimbursement ratio of medical insurance may be correspondingly increased, or special medical subsidies or assistance may be provided to alleviate the financial burden on patients by NHSA. |
As of the date of this prospectus, our products are not covered by any national or provincial medical insurance programs. Currently, A+LCGuard is the only product we are applying for NMIP coverage. There is no fixed timeline to get a product covered by NMIP, as the application process involves multiple procedures and interactions with NHSA that are beyond the applicant’s control. To our knowledge, the application process may take one to two years to complete. Currently, A+LCGuard is the only product we are applying for NMIP coverage. Although we strive to make our products covered by the NMIP in the future, there is no guarantee that the NHSA will approve our applications.
| 91 |
Export Registration
Pursuant to Measures for the Supervision and Administration of Medical Device Production promulgated by the CFDA and amended on November 11, 2017, CFDA, in accordance with the spirit of the Notice of Guo Ban Fa [94] No. 66 of the State Council, conducts inspections of safety and legality of the exported products manufactured by domestic enterprises, grants legitimate production license in China (if these products are sold within Chinese territory) and files the relevant product information by its branches at the level of a districted city for recordation. In accordance with international practice, the quality of exported medical devices is mainly supervised by the importing countries. However, some importing countries/regions may require exporting enterprises to provide Medical Device Product Export Sales Certificates issued by the CFDA. Pursuant to Announcement on Issuing the Provisions on the Administration of Medical Device Product Export Sales Certificates, promulgated by the CFDA and effective on September 1, 2015, such exporting enterprises may apply to the provincial departments of the CFDA at the places where enterprises are located for Medical Device Product Export Sales Certificates.
The premise of obtaining Medical Device Product Export Sales Certificates is that the relevant production enterprises have obtained medical device product registration certificates and production licenses or have undergone the formalities for recordation and production recordation of medical device products in China. The valid period of Medical Device Product Export Sales Certificates, except being specified for one time use, shall not expire after the earliest deadline of any certificate among various certificates submitted by the enterprise amid the application materials, and shall be no longer than two years. Where the relevant materials submitted by an enterprise change, the enterprise shall report to the certificate issuing department in a timely manner. Where the relevant materials change, or the Medical Device Product Export Sales Certificate still needs to be used after its expiration, the enterprise shall apply for a new Medical Device Product Export Sales Certificate. Where the CFDA find that any relevant enterprises fail to meet the requirements of relevant regulations on production, they shall downgrade the credit ratings of such enterprises to lower levels; or, when any enterprises are considered failing to meet the requirements for issuance of certificates anymore, or the relevant materials submitted by the enterprises change, the provincial CFDA departments shall notify the relevant information in a timely manner.
Regulations Relating to M&A Rules and Overseas Listings
On August 8, 2006, six PRC regulatory agencies, including the China Securities Regulatory Commission, or the CSRC, adopted the Regulations on Mergers of Domestic Enterprises by Foreign Investors, or the M&A Rules, which became effective on September 8, 2006 and was amended on June 22, 2009. Foreign investors shall comply with the M&A Rules when they purchase equity interests of a domestic company or subscribe the increased capital of a domestic company, thus changing the nature of the domestic company into a foreign-invested enterprise; or when the foreign investors establish a foreign-invested enterprise in the PRC, purchase the assets of a domestic company and operate the assets; or when the foreign investors purchase the asset of a domestic company, establish a foreign-invested enterprise by injecting such assets and operate the assets. The M&A Rules purport, among other things, to require offshore special purpose vehicles formed for overseas listing purposes through acquisitions of PRC domestic companies and controlled by PRC companies or individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange.
| 92 |
According to the Anti-Monopoly Law which took effect as of August 1, 2008, where the concentration of business operators reaches the filing thresholds stipulated by the State Council, business operators shall file a declaration with the SAMR, and no concentration shall be implemented until the SAMR clears the anti-monopoly filing. Pursuant to the Notice of the General Office of the State Council on the Establishment of the Security Review System for Mergers and Acquisitions of Domestic Enterprises by Foreign Investors and the Security Review Rules issued by the General Office of the State Council on February 3, 2011 and became effective on March 3, 2011, mergers and acquisitions by foreign investors that raise “national defense and security” concerns, and mergers and acquisitions through which foreign investors may acquire de facto control over domestic enterprises that raise “national security” concerns, are subject to strict review by the PRC government authorities. On August 25, 2011, the MOFCOM issued the Provisions of the Ministry of Commerce for the Implementation of the Security Review System for Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, which provides that if a foreign investor’s merger or acquisition of a domestic enterprise falls within the scope of security review specified in the Notice of the General Office of the State Council on the Establishment of the Security Review System for Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, the foreign investor shall file an application with MOFCOM for security review. Whether a foreign investor’s merger or acquisition of a domestic enterprise falls within the scope of security review or not shall be determined based on the substance and actual influence of the merger or acquisition transaction. No foreign investor is allowed to substantially avoid the security review in any way, including but not limited to, holding shares on behalf of others, trust arrangements, multi-level reinvestment, leasing, loans, contractual control, or overseas transactions.
On February 17, 2023, the CSRC released Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Measures”) which came into effect on March 31, 2023. Pursuant to Trial Measures, a PRC domestic company that seeks to offer and list securities in overseas markets, either in direct or indirect overseas offering, shall fulfill the filing procedure with the CSRC as per requirement of the Trial Measures, submit relevant materials that contain a filing report and a legal opinion, and provide truthful, accurate and complete information on the shareholder and etc. Direct overseas offering and listing by domestic companies refers to such overseas offering and listing by a joint-stock company incorporated domestically. Any overseas offering and listing made by an issuer that meets both the following conditions will be determined as indirect offering and listing in overseas market and, therefore, be subject to filing requirement: (i) 50% or more of the issuer’s operating revenue, total profit, total assets or net assets as documented in its audited consolidated financial statements for the most recent accounting year is accounted for by domestic companies; and (ii) the main parts of the issuer’s business activities are conducted in the Mainland China, or its main places of business are located in the Mainland China, or the senior managers in charge of its business operation and management are mostly Chinese citizens or domiciled in the Mainland China. The determination as to whether or not an overseas offering and listing by domestic companies is indirect, shall be made on substance over form basis. In addition, initial public offerings or listings in overseas markets or subsequent securities offerings and listing of an issuer in overseas market other than where it has offered and listed shall be filed with the CSRC within 3 working days after the relevant application is submitted overseas. Subsequent securities offerings of an issuer in the same overseas market where it has previously offered and listed securities shall be filed with the CSRC within 3 working days after such offering is completed. A PRC domestic company that seeks to directly or indirectly list its domestic assets in overseas markets through single or multiple acquisitions, share swaps, transfers of shares or other means, shall also fulfil the filing procedure as prescribed above. Furthermore, upon the occurrence of any of the material events specified below after an issuer has offered and listed securities in an overseas market, the issuer shall submit a report thereof to CSRC within 3 working days after the occurrence and public disclosure of the event: (i) change of control; (ii) investigations or sanctions imposed by overseas securities regulatory agencies or other relevant competent authorities; (iii) change of listing status or transfer of listing segment; (iv) voluntary or mandatory delisting. Where an issuer’s main business undergoes material changes after overseas offering and listing, and is therefore beyond the scope of business stated in the filing documents, such issuer shall submit to the CSRC an ad hoc report and a relevant legal opinion issued by a domestic law firm within 3 working days after occurrence of the changes. However, the PRC Companies already listed overseas before effectiveness of Trial Measures are not required to submit any records with the CSRC until they have successive refinancing demand or other filing requirement. A six-month transition will be given to PRC domestic enterprises that have obtained the approval of overseas regulatory bodies or exchanges since implementation date of the Trial Measure but have not completed the overseas listing. If they fail to complete the overseas listing within six months, they should file records with CSRC according to the requirements. The Trial Measure further stipulate that CSRC may order rectification, issue warnings, and impose a fine between RMB 1 million and RMB 10 million if an applicant fails to fulfill the filing requirements with the CSRC or conducts an overseas offering or listing in violation of the Trial Measure.
On February 24, 2023, the CSRC, together with other PRC government authorities, released the Provisions on Strengthening the Confidentiality and Archives Administration Related to the Overseas Securities Offering and Listing by Domestic Enterprises (the “Confidentiality and Archives Administration Provisions”), which come into effect on March 31, 2023. The Confidentiality and Archives Administration Provisions require, among others, that PRC domestic enterprises seeking to offer and list securities in overseas markets, either directly or indirectly, shall establish the confidentiality and archives system, and shall complete approval and filing procedures with competent authorities, if such PRC domestic enterprises or their overseas listing entities provide or publicly disclose documents or materials involving state secrets and work secrets of PRC government agencies to relevant securities companies, securities service institutions, overseas regulatory agencies and other entities and individuals. It further stipulates that providing or publicly disclosing documents and materials which may adversely affect national security or public interests, and accounting files or copies of important preservation value to the state and society shall be subject to corresponding procedures in accordance with relevant laws and regulations.
| 93 |
Regulations Relating to Foreign Investment
Investment activities in the PRC by foreign investors are principally governed by the Industry Guidelines of Encouraged Foreign Investment, or the Industry Guidelines, effective on January 27, 2021, and the Special Administrative Measures for Entrance of Foreign Investment (Negative List), or the Negative List, most recently amended on December 27, 2021 and effective on January 1, 2022, and together with the PRC Foreign Investment Law, which took effect on January 1, 2020, and its respective implementation rules and ancillary regulations. The Industry Guidelines and the Negative List lay out the basic framework for foreign investments in China, classifying businesses into three categories with regard to foreign investments: “encouraged”, “restricted” and “prohibited”. Industries not listed in the Industry Guidelines or the Negative List are generally deemed as falling into a fourth category “permitted” unless specifically restricted by other PRC laws. The Negative List specifies that Investment in Internet news service, Internet publishing service, Internet audio-visual program service, cyber culture operation (except for music) and Internet information dissemination service (except for contents opened up in China’s WTO commitments) shall be prohibited.
According to the PRC Foreign Investment Law, foreign investments shall enjoy pre-entry national treatment, except for those foreign-invested entities that operate in industries deemed to be either “restricted” or “prohibited” in the “negative list.” While foreign investors shall refrain from investing in any of the foreign “prohibited” industries, foreign-invested entities operating in foreign “restricted” industries shall require market entry clearance and other approvals from relevant PRC governmental authorities. Furthermore, the PRC Foreign Investment Law provides that foreign-invested enterprises that have been established before the implementation of PRC Foreign Investment Law according to the then existing laws regulating foreign investments may maintain their structure and corporate governance within five years after the implementation of the PRC Foreign Investment Law.
On December 19, 2020, MOFCOM and NDRC released the Measures for the Security Review of Foreign Investments, which took effect on January 18, 2021. For foreign investments within the following scope, foreign investors or the relevant parties in China (hereinafter referred to collectively as the “parties concerned”) shall take the initiative to declare to the office of the working mechanism prior to implementation of the investments:…(II) investments in important agricultural products, important energy and resources, important equipment manufacturing, important infrastructure, important transport services, important cultural products and services, important information technology and Internet products and services, important financial services, key technologies and other important fields relating to national security, and obtaining the actual controlling stake in the investee enterprise. Prior to a decision made by the office of the working mechanism, the parties concerned shall not make the investment. The parties concerned shall not make the investment unless the office of the working mechanism decides that security review is not required. Where the declared foreign investment affects national security, a decision on prohibiting the investment shall be made. Foreign-invested entities of the group have businesses that conduct Internet services, but not related to national security within the scope of the regulations above.
On December 26, 2019, the State Council promulgated the Regulations for Implementing the PRC Foreign Investment Law, which took effect on January 1, 2020. The implementation regulations further clarified that the State encourages and promotes foreign investments, protects the lawful rights and interests of foreign investors, regulates foreign investment administration, continues to optimize foreign investment environment, and advances a higher-level opening.
On December 30, 2019, MOFCOM and SAMR jointly promulgated the Measures for Information Reporting on Foreign Investment, which became effective on January 1, 2020. Pursuant to the Measures for Information Reporting on Foreign Investment, where a foreign investor carries out investment activities in China directly or indirectly, the foreign investor or the foreign-invested enterprise shall submit the investment information to the competent commerce department.
| 94 |
Regulations relating to Anti-Monopoly and Competition
On September 11, 2020, the Anti-Monopoly Commission of the State Council issued Anti-Monopoly Compliance Guideline for Business Operators, which requires business operators to establish anti-monopoly compliance management systems under the PRC Anti-Monopoly Law to manage anti-monopoly compliance risks.
On August 17, 2021, the State Administration for Market Regulation, or the SAMR, issued a discussion draft of Provisions on the Prohibition of Unfair Competition on the Internet, under which business operators should not use data or algorithms to hijack traffic or influence users’ choices, or use technical means to illegally capture or use other business operators’ data. Furthermore, business operators are not allowed to (i) fabricate or spread misleading information to damage the reputation of competitors, or (ii) employ marketing practices such as fake reviews or use coupons or “red envelopes” to entice positive ratings.
On February 7, 2021, the Anti-Monopoly Commission of the State Council published Anti-Monopoly Guidelines for the Internet Platform Economy Sector that specified circumstances where an activity of an internet platform will be identified as monopolistic act as well as concentration filing procedures for business operators. According to the PRC Anti-Monopoly Law, if a business operator carries out a concentration in violation of the law, the relevant authority shall order the business operator to terminate the concentration, dispose of the shares or assets or transfer the business within a specified time limit, or take other measures to restore the pre-concentration status, and impose a fine of up to RMB500,000.
On October 23, 2021, the Standing Committee of the National People’s Congress issued a discussion draft of the amended Anti-Monopoly Law, which proposes to increase the fines for illegal concentration of business operators to no more than ten percent of its last year’s sales revenue if the concentration of business operator has or may have an effect of excluding or limiting competitions, or a fine of up to RMB5 million if the concentration of business operator does not have an effect of excluding or limiting competition. The draft also proposes that the relevant authority shall investigate a transaction where there is any evidence that the concentration has or may have the effect of eliminating or restricting competitions, even if such concentration does not reach the filing threshold.
Regulations Relating to Cybersecurity and Privacy Protection
The PRC Constitution states that PRC law protects the freedom and privacy of communications of citizens and prohibits infringement of these rights. In recent years, PRC government authorities have enacted legislation on the Internet use to protect personal information from any unauthorized disclosure. Under the Several Provisions on Regulating the Market Order of Internet Information Services which was promulgated by MIIT on December 29, 2011, an Internet content service operator may not collect any user personal information or provide any such information to third parties without the consent of a user, unless otherwise stipulated by laws and administrative regulations. An Internet content service operator must expressly inform the users of the method, content and purpose of the collection and processing of such user personal information and may only collect such information necessary for the provision of its services. An Internet content service operator is also required to properly keep the user personal information, and in case of any leak or likely leak of the user personal information, the Internet content service operator must take immediate remedial measures and, in severe circumstances, to make an immediate report to the telecommunication regulatory authority.
In addition, the Decision on Strengthening Network Information Protection, which was promulgated by the Standing Committee of NPC on December 28, 2012, provides that electronic information that is able to identify personal identities of citizens or is concerned with personal privacy of citizens is protected by law and shall not be unlawfully obtained or provided. Internet content service operators collecting or using personal electronic information of citizens shall specify purposes, manners and scopes of information collection and use, obtain the consent of citizens concerned, and strictly keep confidential personal information collected. Internet content service operators are prohibited from disclosing, tampering with, damaging, selling or illegally providing others with personal information collected. Technical and other measures are required to be taken by Internet content service operators to prevent personal information collected from unauthorized disclosure, damage or being lost. Internet content service operators are subject to legal liability, including warnings, fines, confiscation of illegal gains, revocation of licenses or filings, closing of websites concerned, public security administration punishment, criminal liabilities, or civil liabilities, if they violate relevant provisions on Internet privacy.
| 95 |
Pursuant to the Order for the Protection of Telecommunication and Internet User Personal Information which was promulgated by MIIT on July 16, 2013, any collection and use of users’ personal information must be subject to the consent of the users, abide by the principles of legality, rationality and necessity and be within the specified purposes, methods and scopes. Pursuant to the Ninth Amendment to the Criminal Law which was issued by the Standing Committee of NPC on August 29, 2015 and became effective on November 1, 2015, any Internet service provider that fails to fulfill obligations to manage information and network security as required by applicable laws and refuses to rectify upon orders from government authorities, will be subject to the criminal penalty if such failure (i) causes dissemination of illegal information in large scale; (ii) causes user information leaks resulting in severe consequences; (iii) causes serious loss of evidence to criminal investigations; or (iv) implicates other severe circumstances. Moreover, any individual or entity that (i) sells or provides personal information to others in violation of applicable laws, or (ii) steals or illegally obtains any personal information, in either case implicating severe circumstances, will be subject to the criminal penalty. The PRC government, however, has the power and authority to order Internet content service operators to turn over personal information if an Internet user posts any prohibited content or engages in illegal activities on the Internet.
To further regulate cybersecurity and privacy protection, the PRC Cybersecurity Law which was promulgated by the Standing Committee of NPC on November 7, 2016 and took effect on June 1, 2017, provides that: subject to certain exceptions, (i) to collect and use personal information, network operators must follow the principles of legitimacy, rightfulness, and necessity, disclose their rules of data collection and use, clearly express the purposes, means, and scope of collecting and using the information, and obtain the consent of the persons whose data is gathered; (ii) network operators can neither gather personal information unrelated to the services they provide, nor gather or use personal information in violation of the provisions of laws and administrative regulations or the scopes of consent given by the persons whose data is gathered, and must dispose of personal information they have saved in accordance with the provisions of laws and administrative regulations and agreements reached with users; (iii) network operators cannot divulge, tamper with, or damage the personal information they have collected, and cannot provide the personal information to others without the consent of persons whose data is collected. According to the PRC Cybersecurity Law, personal information refers to all kinds of information that are recorded electronically or that can otherwise be used to independently identify or be combined with other information to identify natural persons’ personal information, including but not limited to natural persons’ names, dates of birth, identification numbers, biologically identified personal information, addresses, and telephone numbers. Any Internet information services provider that violates these privacy protection requirements under the PRC Cybersecurity Law and related laws and regulations may be ordered to turn in illegal gains generated from unlawful operations and pay a fine of no less than one but no more than ten times of the illegal gains and may be ordered to cease the relevant business operations when the violation is serious.
On June 28, 2016, the CAC issued the Administrative Provisions on Mobile Internet Applications Information Services, which became effective on August 1, 2016, to further strengthen the regulation of the mobile app information services. Pursuant to these provisions, owners or operators of mobile apps that provide information services are required to be responsible for information security management, establish and improve the protective mechanism for user information, observe the principles of legality, rightfulness and necessity, and expressly state the purpose, method and scope of, and obtain user consent to, the collection and use of users’ personal information.
On May 8, 2017, the Supreme People’s Court and the Supreme People’s Procuratorate issued the Interpretations of the Supreme People’s Court and the Supreme People’s Procuratorate on Several Issues Concerning the Application of Law in the Handling of Criminal Cases Involving Infringement of Citizens’ Personal Information, or the Personal Information Interpretations, which became effective on June 1, 2017. The Personal Information Interpretations provides more practical conviction and sentencing criteria for the infringement of citizens’ personal information.
On January 23, 2019, the PRC Office of the Central Cyberspace Affairs Commission and other three authorities jointly issued the Circular on the Special Campaign of Correcting Unlawful Collection and Usage of Personal Information via Apps. Pursuant to this circular, (i) app operators are prohibited from collecting any personal information irrelevant to their services; (ii) information collection and usage policy should be presented in a simple and clear way, and such policy should be consented by the users voluntarily, and; (iii) authorization from users should not be obtained by coercing users with default or bundling clauses or making consent a condition of service. App operators violating these rules can be ordered by authorities to correct their noncompliance within a given period of time, be publicly reported, or ordered to quit its operation or cancel its business license or operational permits.
| 96 |
On April 10, 2019, the Ministry of Public Security promulgated the Guidelines for Internet Personal Information Security Protection, which establishes the management mechanism, security technical measures and business workflows for personal information security protection. On August 22, 2019, the CAC promulgated the Provisions on the Cyber Protection of Children’s Personal Information which requires, among others, that network operators who collect, store, use, transfer and disclose personal information of children under the age of 14 shall establish special rules and user agreements for the protection of children’s personal information, inform the children’s guardians in a noticeable and clear manner, and shall obtain the consent of the children’s guardians.
On November 28, 2019, the CAC, MIIT, the Ministry of Public Security and SAMR jointly promulgated the Measures for the Determination of the Collection and Use of Personal Information by Apps in Violation of Laws and Regulations, which provides guidance for the regulatory authorities to identify the illegal collection and use of personal information through mobile apps, and for the app operators to conduct self-examination and self-correction and social supervision by citizens.
On May 28, 2020, the NPC approved the Civil Code of the PRC or the Civil Code, which came into effect on January 1, 2021. Pursuant to the Civil Code, the personal information of a natural person shall be protected by the law. Any organization or individual that needs to obtain personal information of others shall obtain such information legally and ensure the safety of such information, and shall not illegally collect, use, process or transmit personal information of others, or illegally purchase or sell, provide or make public personal information of others. Furthermore, information processors shall not divulge or tamper with personal information collected or stored by them; without the consent of a natural person, information processors shall not illegally provide personal information of such person to others, except for information that has been processed so that specific persons cannot be identified and that cannot be restored. In addition, an information processor shall take technical measures and other necessary measures to ensure the security of the personal information that is collected and stored and to prevent the information from being divulged, tampered with or lost; where personal information has been or may be divulged, tampered with or lost, the information processor shall take remedial measures in a timely manner, inform the natural person concerned in accordance with the provisions and report the case to the relevant competent department.
On August 20, 2021, the SCNPC adopted the Personal Information Security Law, which took effect on November 1, 2021. The Personal Information Protection Law includes the basic rules for personal information processing, the rules for cross-border provision of personal information, the rights of individuals in personal information processing activities, the obligations of personal information processors, and the legal responsibilities for illegal collection, processing, and use of personal information. As the first systematic and comprehensive law specifically for the protection of personal information in the PRC, the Personal Information Protection Law provides, among others, that (i) an individual’s consent shall be obtained to use sensitive personal information, such as biometric characteristics and individual location tracking, (ii) personal information operators using sensitive personal information shall notify individuals of the necessity of such use and impact on the individual’s rights, and (iii) where personal information operators reject an individual’s request to exercise his or her rights, the individual may file a lawsuit with a People’s Court.
On November 14, 2021, the CAC published the Regulations of Internet Data Security Management (Draft for Comments), which further regulate the internet data processing activities and emphasize the supervision and management of network data security, and further stipulate the obligations of internet platform operators, such as to establish a system for disclosure of platform rules, privacy policies and algorithmic strategies related to data. Specifically, the draft regulations require data processors to, among others, (i) adopt immediate remediation measures when finding that network products and services they use or provide have security defects and vulnerabilities, or threaten national security or endanger public interest, and (ii) follow a series of detailed requirements with respect to processing of personal information, management of important data and proposed overseas transfer of data. In addition, the draft regulations require data processors handling important data or the data processors to be listed overseas to complete an annual data security assessment and file a data security assessment report to applicable regulators. Such annual assessment, as required by the draft regulations, would encompass areas including, but not limited to, the status of important data processing, data security risks identified and the measures adopted, the effectiveness of data protection measures, the implementation of national data security laws and regulations, data security incidents that occurred and their handling, and a security assessment with respect to sharing and provision of important data overseas. As of the date of this prospectus, the draft regulations have been released for public comment only and have not been formally adopted. The final provisions and the timeline for its adoption are subject to changes and uncertainties.
| 97 |
Currently, our business does not involve the collection of user data, implicate cybersecurity, or involve any other type of restricted industry. Based on the advice of PRC counsel and our understanding of currently applicable PRC laws and regulations, our registered public offering in the U.S. is not subject to the review or prior approval of the CAC.
Regulations Relating to Intellectual Property Rights
Patent
Patents in the PRC are principally protected under the Patent Law of the PRC. The duration of a patent right is either 10 years or 15 year or 20 years from the date of application, depending on the type of patent right. The Patent Law of the PRC and its implementation rules provide for three types of patents, namely, “invention”, “utility model” and “design”. Invention patents are valid for twenty years, utility model patents are valid for fifteen years, while design patents are valid for ten years, from the date of application. The Chinese patent system adopts a “first-to-file” principle, which means that where more than one person files a patent application for the same invention, a patent will be granted to the person who files the application first. To be patentable, invention or utility models must meet three criteria: novelty, inventiveness and practicability. A third party must obtain consent or a proper license from the patent owner to use the patent. Otherwise, the use constitutes an infringement of the patent rights.
Copyright
Copyright in the PRC, including copyrighted software, is principally protected under the Copyright Law of the PRC and related rules and regulations. Under the Copyright Law, promulgated in September 1990, implemented in June 1991, amended in October 2001, February 2010 and November 2020, and effective on June 1, 2021 the term of protection for copyrighted software is 50 years. The Regulation on the Protection of the Right to Communicate Works to the Public over Information Networks, as most recently amended on January 30, 2013, provides specific rules on fair use, statutory license, and a safe harbor for use of copyrights and copyright management technology and specifies the liabilities of various entities for violations, including copyright holders, libraries and Internet service providers.
Trademark
Registered Trademarks are protected by the PRC Trademark Law which was adopted by the Standing Committee of NPC on August 23, 1982 and most recently amended on April 23, 2019 as well as the Implementation Regulation of the PRC Trademark Law which was adopted by the State Council on August 3, 2002 and amended on April 29, 2014. The Trademark Office of the National Intellectual Property Administration under SAMR handles trademark registrations and grants a term of ten years to registered trademarks which may be renewed for consecutive ten-year periods upon request by the trademark owner. For licensed use of a registered trademark, the licensor shall file record of the licensing of the said trademark with the Trademark Office, otherwise it may not defend against a bona fide third party. The PRC Trademark Law has adopted a “first-to-file” principle with respect to trademark registration. Where a trademark for which a registration has been made is identical or similar to another trademark which has already been registered or been subject to a preliminary examination and approval for use on the same kind of or similar commodities or services, the application for registration of such trademark may be rejected. Any person applying for the registration of a trademark may not prejudice the existing right first obtained by others, nor may any person register in advance a trademark that has already been used by another party and has already gained a “sufficient degree of reputation” through such party’s use.
Under PRC law, any of the following acts will be deemed as an infringement to the exclusive right to use a registered trademark: (i) use of a trademark that is the same as or similar to a registered trademark for identical or similar goods without the permission of the trademark registrant; (ii) sale of any goods that have infringed the exclusive right to use any registered trademark; (iii) counterfeit or unauthorized production of the label of another’s registered trademark, or sale of any such label that is counterfeited or produced without authorization; (iv) change of any trademark of a registrant without the registrant’s consent, and selling goods bearing such replaced trademark on the market; or (v) other acts that have caused any other damage to another’s exclusive right to use a registered trademark.
| 98 |
According to the PRC Trademark Law, in the event of any of the foregoing acts, the infringing party will be ordered to stop the infringement immediately and may be imposed a fine; the counterfeit goods will be confiscated. The infringing party may also be held liable for the right holder’s damages, which will be equal to the losses suffered by the right holder as a result of the infringement, including reasonable expenses incurred by the right holder for stopping the infringement, or the gains obtained by the infringing party if the losses are difficult to be ascertained. If both gains and losses are difficult to be ascertained, the damages may be determined by referring to the amount of royalties for the license of such trademarks, which will be one to five times of the royalties in the case of any serious infringement with malicious intent. If the gains, losses and royalties are all difficult to be ascertained, the court may render a judgment awarding damages no more than RMB5 million. Notwithstanding the above, if a distributor does not know that the goods it sells infringe another’s registered trademark, it will not be liable for infringement provided that the seller shall prove that the goods are lawfully obtained and identify its supplier.
Domain Name
Domain names are protected under the Administrative Measures on Internet Domain Names promulgated by the MIIT on August 24, 2017 and effective as of November 1, 2017. Domain name registrations are handled through domain name service agencies established under the relevant regulations, and applicants become domain name holders upon successful registration.
Regulations Relating to Foreign Exchange
Regulations on Foreign Currency Exchange
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations, most recently amended on August 5,2008, 2008. Under PRC foreign exchange regulations, payments of current account items, such as profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. By contrast, approval from or registration with appropriate government authorities is required where RMB is to be converted into foreign currency and remitted out of China to pay capital account items, such as direct investments, repayment of foreign currency-denominated loans, repatriation of investments and investments in securities outside of China.
On November 19,2012, SAFE promulgated the Circular of Further Improving and Adjusting Foreign Exchange Administration Policies on Foreign Direct Investment, or Circular 59, which substantially amends and simplifies the current foreign exchange procedure. Pursuant to Circular 59, the opening of various special purpose foreign exchange accounts, such as pre-establishment expenses accounts, foreign exchange capital accounts and guarantee accounts, the reinvestment of RMB proceeds derived by foreign investors in the PRC, and remittance of foreign exchange profits and dividends by a foreign-invested enterprise to its foreign shareholders no longer require the approval or verification of SAFE, and multiple capital accounts for the same entity may be opened in different provinces, which was not possible previously. In 2013, SAFE specified that the administration by SAFE or its local branches over direct investment by foreign investors in the PRC must be conducted by way of registration and banks must process foreign exchange business relating to the direct investment in the PRC based on the registration information provided by SAFE and its branches. In February 2015, SAFE promulgated the Notice on Further Simplifying and Improving the Administration of the Foreign Exchange Concerning Direct Investment, or SAFE Notice 13. Instead of applying for approvals regarding foreign exchange registrations of foreign direct investment and overseas direct investment from SAFE, entities and individuals may apply for such foreign exchange registrations from qualified banks. The qualified banks, under the supervision of SAFE, may directly review the applications and conduct the registration.
In March 2015, SAFE promulgated the Circular of the SAFE on Reforming the Management Approach regarding the Settlement of Foreign Capital of Foreign-invested Enterprise, or Circular 19, which expands a pilot reform of the administration of the settlement of the foreign exchange capitals of foreign-invested enterprises nationwide. Circular 19 replaced both the Circular of the SAFE on Issues Relating to the Improvement of Business Operations with Respect to the Administration of Foreign Exchange Capital Payment and Settlement of Foreign-invested Enterprises, or Circular 142, and the Circular of the SAFE on Issues concerning the Pilot Reform of the Administrative Approach Regarding the Settlement of the Foreign Exchange Capitals of Foreign-invested Enterprises in Certain Areas, or Circular 36. Circular 19 allows all foreign-invested enterprises established in the PRC to settle their foreign exchange capital on a discretionary basis according to the actual needs of their business operation, provides the procedures for foreign invested companies to use RMB converted from foreign currency-denominated capital for equity investments and removes certain other restrictions that had been provided in Circular 142. However, Circular 19 continues to prohibit foreign-invested enterprises from, among other things, using RMB funds converted from their foreign exchange capital for expenditure beyond their business scope and providing entrusted loans or repaying loans between non-financial enterprises. SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming and Standardizing the Foreign Exchange Settlement Management Policy of Capital Account, or Circular 16, effective June 2016, which reiterates some of the rules set forth in Circular 19. Circular 16 provides that discretionary foreign exchange settlement applies to foreign exchange capital, foreign debt offering proceeds and remitted foreign listing proceeds, and the corresponding RMB capital converted from foreign exchange may be used to extend loans to related parties or repay inter-company loans (including advances by third parties). However, there are substantial uncertainties with respect to Circular 16’s interpretation and implementation in practice. Circular 19 or Circular 16 may delay or limit us from using the proceeds of offshore offerings to make additional capital contributions to our Shanghai subsidiary and any violations of these circulars could result in severe monetary or other penalties.
| 99 |
In January 2017, SAFE promulgated the Circular on Further Improving Reform of Foreign Exchange Administration and Optimizing Genuineness and Compliance Verification, or Circular 3, which stipulates several capital control measures with respect to the outbound remittance of profits from domestic entities to offshore entities, including (i) banks must check whether the transaction is genuine by reviewing board resolutions regarding profit distribution, original copies of tax filing records and audited financial statements, and (ii) domestic entities must retain income to account for previous years’ losses before remitting any profits. Moreover, pursuant to Circular 3, domestic entities must explain in detail the sources of capital and how the capital will be used, and provide board resolutions, contracts and other proof as a part of the registration procedure for outbound investment.
On October 23, 2019, SAFE issued Circular of the State Administration of Foreign Exchange on Further Promoting the Facilitation of Cross-border Trade and Investment, or the Circular 28, which took effect on the same day. Circular 28 allows non-investment foreign-invested enterprises to use their capital funds to make equity investments in China, provided that such investments do not violate the effective special entry management measures for foreign investment (negative list) and the target investment projects are genuine and in compliance with laws.
Regulation on Foreign Debt
A loan made by a foreign entity as direct or indirect shareholder in a FIE is considered to be foreign debt in China and is regulated by various laws and regulations, including the Regulation of the People’s Republic of China on Foreign Exchange Administration, the Interim Provisions on the Management of Foreign Debts, the Statistical Monitoring of Foreign Debts Tentative Provisions, the Detailed Rules for the Implementation of Provisional Regulations on Statistics and Supervision of External Debt, and the Administrative Measures for Registration of Foreign Debts. Under these rules and regulations, a shareholder loan in the form of foreign debt made to a PRC entity does not require the prior approval of SAFE. However, such foreign debt must be registered with and recorded by SAFE or its local branches within fifteen (15) business days after entering into the foreign debt contract. Pursuant to these rules and regulations, the maximum amount of the aggregate of (i) the outstanding balance of foreign debts with a term not longer than one year, and (ii) the accumulated amount of foreign debts with a term longer than one year, of a FIE shall not exceed the difference between its registered total investment and its registered capital, or Total Investment and Registered Capital Balance.
On January 12, 2017, the People’s Bank of China, or PBOC, promulgated the Notice of the People’s Bank of China on Matters concerning the Macro-Prudential Management of Full-Covered Cross-Border Financing, or PBOC Circular 9, which sets forth an upper limit for PRC entities, including FIEs and domestic enterprises, regarding their foreign debts. Pursuant to PBOC Circular 9, the outstanding cross-border financing of an enterprise (the outstanding balance drawn, here and below) shall be calculated using a risk-weighted approach, or Risk-Weighted Approach, and shall not exceed the specified upper limit, namely: risk-weighted outstanding cross-border financing £ the upper limit of risk-weighted outstanding cross-border financing. Risk-weighted outstanding cross-border financing =∑ outstanding amount of RMB and foreign currency denominated cross-border financing * maturity risk conversion factor * type risk conversion factor +∑ outstanding foreign currency denominated cross-border financing * exchange rate risk conversion factor. Maturity risk conversion factor shall be 1 for medium- and long-term cross-border financing with a term of more than one year and 1.5 for short-term cross-border financing with a term of one year or less than one year. Type risk conversion factor shall be 1 for on-balance-sheet financing and 1 for off-balance-sheet financing (contingent liabilities) for the time being. Exchange rate risk conversion factor shall be 0.5. The PBOC Circular 9 further provides that the upper limit of risk-weighted outstanding cross-border financing for enterprises, or Net Asset Limits, shall be 200% of its net assets. The PBOC Circular 9 does not supersede the Interim Provisions on the Management of Foreign Debts, but rather serves as a supplement to it. PBOC Circular 9 provided for a one-year transitional period, or the Transitional Period, from its promulgation date for FIEs, during which period FIEs could choose to calculate their maximum amount of foreign debt based on either (i) the Total Investment and Registered Capital Balance, or (ii) the Risk-Weighted Approach and the Net Asset Limits. Under the PBOC Circular 9, after the Transitional Period ends on January 11, 2018, the PBOC and SAFE will determine the cross-border financing administration mechanism for the foreign-invested enterprises after evaluating the overall implementation of PBOC Circular 9. In addition, according to PBOC Circular 9, a foreign loan must be filed with SAFE through the online filing system of SAFE after the loan agreement is signed and at least three business days prior to the borrower withdraws any amount from such foreign loan.
| 100 |
Regulations Relating to Dividend Distributions
According to the PRC Company Law and Foreign Investment Law, each of our Shanghai subsidiary, as a foreign invested enterprise, or FIE, is required to draw 10% of its after-tax profits each year, if any, to fund a common reserve, which may stop drawing its after-tax profits if the aggregate balance of the common reserve has already accounted for over 50% of its registered capital. These reserves are not distributable as cash dividends. Furthermore, under the EIT Law, which became effective in January 2008, the maximum tax rate for the withholding tax imposed on dividend payments from PRC foreign invested companies to their overseas investors that are not regarded as “resident” for tax purposes is 20%. The rate was reduced to 10% under the Implementing Regulations for the EIT Law issued by the State Council. However, a lower withholding tax rate might be applied if there is a tax treaty between China and the jurisdiction of the foreign holding companies, such as tax rate of 5% in the case of Hong Kong companies that holds at least 25% of the equity interests in the foreign-invested enterprise, and certain requirements specified by PRC tax authorities are satisfied.
Regulations Relating to Employment, Social Insurance and housing fund
The Labor Law and The Labor Contract Law provide requirements concerning employment contracts between an employer and its employees. If an employer fails to enter into a written employment contract with an employee within one year from the date on which the employment relationship is established, the employer must rectify the situation by entering into a written employment contract with the employee and pay the employee twice the employee’s salary for the period from the day following the lapse of one month from the date of establishment of the employment relationship to the day prior to the execution of the written employment contract. All employers must comply with local minimum wage standards. The Labor Contract Law and its implementation rules also require compensation to be paid upon certain terminations, which significantly affects the cost of reducing workforce for employers. In addition, if an employer intends to enforce a non-compete provision with an employee in an employment contract or non-competition agreement, it has to compensate the employee on a monthly basis during the term of the restriction period after the termination or ending of the labor contract. Employers in most cases are also required to provide a severance payment to their employees after their employment relationship are terminated. Violations of the PRC Labor Contract Law and the PRC Labor Law may result in the imposition of fines and other administrative and criminal liability in the case of serious violations.
Enterprises in China are required by PRC laws and regulations to participate in certain employee benefit plans, including social insurance funds, namely a pension plan, a medical insurance plan, an unemployment insurance plan, a work-related injury insurance plan and a maternity insurance plan, and a housing provident fund, and contribute to the plans or funds in amounts equal to certain percentages of salaries, including bonuses and allowances, of the employees as specified by the local government from time to time at locations where they operate their businesses or where they are located. According to the Social Insurance Law, an employer that fails to make social insurance contributions may be ordered to pay the required contributions within a stipulated deadline and be subject to a late fee. If the employer still fails to rectify the failure to make social insurance contributions within the stipulated deadline, it may be subject to a fine ranging from one to three times the amount overdue. According to the Regulations on Management of Housing Fund, an enterprise that fails to make housing fund contributions may be ordered to rectify the noncompliance and pay the required contributions within a stipulated deadline; otherwise, an application may be made to a local court for compulsory enforcement.
| 101 |
Regulations on Taxes
Enterprise Income Tax
Under the Enterprise Income Tax Law of the PRC, or the EIT Law, which became effective on January 1, 2008 and was subsequently amended on February 24, 2017 and December 29, 2018, and its implementing rules, enterprises are classified as resident enterprises and non-resident enterprises. PRC resident enterprises typically pay an enterprise income tax at the rate of 25% while non-PRC resident enterprises without any branches in the PRC should pay an enterprise income tax in connection with their income from the PRC at the tax rate of 10%. An enterprise established outside of the PRC with its “de facto management bodies” located within the PRC is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a PRC domestic enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define a de facto management body as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise. Enterprises qualified as “High and New Technology Enterprises” are entitled to a 15% enterprise income tax rate rather than the 25% uniform statutory tax rate. The preferential tax treatment continues as long as an enterprise can retain its “High and New Technology Enterprise” status.
The EIT Law and the implementation rules provide that an income tax rate of 10% should normally be applicable to dividends payable to investors that are “non-resident enterprises,” and gains derived by such investors, which (a) do not have an establishment or place of business in the PRC or (b) have an establishment or place of business in the PRC, but the relevant income is not effectively connected with the establishment or place of business to the extent such dividends and gains are derived from sources within the PRC. Such income tax on the dividends may be reduced pursuant to a tax treaty between China and other jurisdictions. Pursuant to the Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation on Income, or the Double Tax Avoidance Arrangement, and other applicable PRC laws, if a Hong Kong resident enterprise is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements under such Double Tax Avoidance Arrangement and other applicable laws, the 10% withholding tax on the dividends the Hong Kong resident enterprise receives from a PRC resident enterprise may be reduced to 5% upon receiving approval from in-charge tax authority. However, based on the Notice on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties issued on February 20, 2009 by the SAT, if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment; and based on the Announcement on Relevant Issues Concerning the “Beneficial Owners” in Tax Treaties issued on February 3, 2018 by the SAT and effective from April 1, 2018, which replaces the Notice on the Interpretation and Recognition of Beneficial Owners in Tax Treaties and the Announcement on the Recognition of Beneficial Owners in Tax Treaties by the SAT, comprehensive analysis based on the stipulated factor therein and actual circumstances shall be adopted when recognizing the “beneficial owner” and agents and designated wire beneficiaries are specifically excluded from being recognized as “beneficial owners.”
On January 17, 2019, the State Taxation Administration issued the notice on the scope of small-scale and low-profit corporate income tax preferential policies of the Ministry of Finance and the State Administration of Taxation (“MOF and SAT”), [2019] No. 13 for small-scale and low-profit enterprises whose annual taxable income is less than RMB1,000,000 (including RMB1,000,000), approximately $142,209, their income is reduced by 25% to the taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 5%. While for the portion of annual taxable income exceeding RMB1,000,000, approximately $142,209, but not more than RMB3,000,000, approximately $426,627, the income is reduced by 50% to the taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 10%. MOF and SAT [2021] No.12 provides an enterprise income tax rate of 2.5% on a small-scale and low-profit enterprises whose annual taxable income less than RMB1,000,000, approximately $142,209, from January 1, 2021 to December 31, 2022. MOF and SAT [2022] No.13 also provides an enterprise income tax rate of 5% on a small-scale and low-profit enterprises whose annual taxable income more than RMB1,000,000, approximately $142,209, but not more than RMB3,000,000, approximately $426,627, from January 1, 2022 to December 31, 2024. The qualifications of small-scale and low-profit enterprises were examined annually by the Tax Bureau. All of the Company’s Shanghai subsidiary met the criteria of small-scale and low-profit enterprises.
| 102 |
Value-added Tax
Pursuant to applicable PRC tax regulations, any entity or individual conducting business in the service industry used to be generally required to pay a business tax at the rate of 5% on the revenues generated from providing such services. However, if the services provided are related to technology development and transfer, such business tax may be exempted subject to approval by the relevant tax authorities. Whereas, pursuant to the Provisional Regulations on Value-Added Tax of the PRC and its implementation regulations, unless otherwise specified by relevant laws and regulations, any entity or individual engaged in the sales of goods, provision of processing, repairs and replacement services and importation of goods into China is generally required to pay a value-added tax, or VAT, for revenues generated from sales of products, while qualified input VAT paid on taxable purchase can be offset against such output VAT.
In November 2011, the Ministry of Finance and the State Administration of Taxation promulgated the Pilot Plan for Imposition of Value-Added Tax to Replace Business Tax. In March 2016, the Ministry of Finance and the State Administration of Taxation further promulgated the Notice on Fully Promoting the Pilot Plan for Replacing Business Tax by Value-Added Tax, which became effective on May 1, 2016. Pursuant to the pilot plan and relevant notices, VAT is generally imposed in lieu of business tax in the modern service industries, including the VATS, on a nationwide basis. VAT of a rate of 6% applies to revenue derived from the provision of some modern services. Certain small taxpayers under PRC law are subject to reduced value-added tax at a rate of 3%. Unlike business tax, a taxpayer is allowed to offset the qualified input VAT paid on taxable purchases against the output VAT chargeable on the modern services provided.
On April 4, 2018, the Ministry of Finance and the State Administration of Taxation issued the Notice on Adjustment of VAT Rates, which came into effect on May 1, 2018. According to the abovementioned notice, the taxable goods previously subject to VAT rates of 17% and 11%, respectively, become subject to lower VAT rates of 16% and 10%, respectively, starting from May 1, 2018. Furthermore, according to the Announcement on Relevant Policies for Deepening Value-added Tax Reform jointly promulgated by the Ministry of Finance, the State Administration of Taxation and the General Administration of Customs, which became effective on April 1, 2019, the taxable goods previously subject to VAT rates of 16% and 10%, respectively, become subject to lower VAT rates of 13% and 9%, respectively, starting from April 1, 2019. Under Provisional Regulations of the People’s Republic of China on Value-added Tax, amended and effective on November 19, 2017, for entities that are VAT small taxpayers, VAT is levied at a levy rate of 3%. On February 29, 2020, the State Administration of Taxation issued the Announcement on Taxation Matters to Support Individual Businesses in Resumption of Business, during the COVID-19, the small taxpayers are allowed to enjoy the preferred tax policy, tax rate from 3% to 1% for the period from March 1, 2020 to December 31, 2021.
| 103 |
The following table sets forth information regarding our executive officers and directors as of the date of this prospectus.
| Name | Age | Position with the Company | ||
| Yi Lu, Ph.D. | Chairman of the Board, Chief Science Officer, and President | |||
| Hung To Pau, Ph.D. | Chief Executive Officer, director and Secretary | |||
| Mingze Yin | Chief Financial Officer and Treasurer | |||
| Steven I-Fang Cheng, Ph.D. | Chief Technology Officer | |||
| Alfred Lee Ming Sung | 65 | Independent Director Nominee and Chair of the Audit Committee | ||
| Cheang I Kei | 29 | Independent Director Nominee and Chair of the Compensation Committee | ||
| Mengxing Tang, | 50 | Independent Director Nominee and Chair of the Nominating and Corporate Governance Committee |
Dr. Yi Lu has served as our Chief Science Officer and Chairman of the Board since November 2022. He has been the Chairman and Chief Science Officer of Advanced Biomed Taiwan since June 2014. He was the Chairman of HFC Semiconductor from January 2020 to October 2022 and was responsible for the company’s strategic development. Dr. Lu previously served as Chairman of Nextchip Semiconductor Corporation, a semiconductor foundry specializing in integrated circuit production, from February 2016 to December 2019 and led the company to launch the first 12-inch integrated circuit production line in Anhui Province with an investment of RMB 20 billion. From January 2004 to December 2007, he was the senior executive of Cypress Semiconductor, overseeing the company’s business operation. From May 2001 to January 2004, he was the founder, Chairman and CEO of Cascade Semiconductor, which was acquired by Cypress Semiconductor Corporation (Nasdaq: CY), and managed the overall operations of the company. Dr. Lu received a Doctor of Philosophy from the University of Florida in 1994. Dr. Lu has a wealth of experience in successful entrepreneurship and has extensive experience in the fields of technology management, market management, and operations. He has a clear strategic orientation and has established a clear strategic plan for us. We believe Dr. Lu’s significant experience in the United States and China qualifies him to serve as a member of our board of directors.
Dr. Hung To Pau has served as our Chief Executive Officer and a director since November 2022. He has been the Chairman of Shanghai Sglcell Biotech Co., Ltd. since November 2019 and oversees business operations and strategic development. From January 2017 to October 2019, Dr. Pau held positions as chairman of Take Chance HK Development Ltd., where he led the senior executives to expand the company’s business operations. From January 2017 to date, He was also a director at Well Fancy Development Ltd. and worked on areas such as corporate governance and strategic development. From 1994 to 2008, Dr. Pau worked at Shanghai Changzheng Hospital and managed its R&D department. He graduated from the Second Military Medical University with a Ph.D. in Immunology in 2008. He also obtained EMBA from Shanghai Jiaotong University in 2011. Dr. Pau has solid medical research and development experience as well as sufficient practical experience in managing a company’s overall and long-term development and its core competitiveness. We believe he will play a pivotal role in steering our long-term development and achieving future business goals.
Mr. Mingze Yin has served as our Chief Financial Officer since November 2022. He has over 11 years of experience in the financial and investment banking industry. He has been the Chief Financial Officer of Shanghai Sglcell Biotech Co., Ltd. since January 2021 and oversees its finance department. From March 2017 to December 2021, Mr. Yin was Director of Shanghai Guangdian Asset Management Co., Ltd., where he participated in all major corporate decisions and financial compliance matters. From February 2020 to November 2020, he was an independent director of TMSR Holding Company Limited (Nasdaq: TMSR) and a member of its Audit Committee, Nominating and Governance Committee, and Compensation Committee. From February 2018 to November 2018, Mr. Yin was the investors relations manager of Planet Green Holdings Corp. (NYSE: PLAG). From November 2015 to February 2017, Mr. Yin was a senior manager of the Investment Banking Department of Zhongshan Securities Co., Ltd. From October 2012 to October 2015, he was z senior audit manager of BDO China Shu Lun Pan Certified Public Accountants LLP. Mr. Yin obtained his Bachelor of Management from Jiangsu Haiyang University in 2011.
Dr. Steven I-Fang Cheng has served as our Chief Technology Officer since November 2022. Since 2014, Dr. Cheng joined Advanced Biomed Taiwan. as a co-founder and CTO. Since 2013, he was an associate research fellow (Principal Investigator) in TSRI. His professional research expertise includes biomicrofluidics, biosensors, cancer diagnosis and infectious detection, micro/nano fabrication technology, on-chip drug screening, electrokinetics/electrical analysis and their biomedical applications, patent design and implantation. He received his Master of Science degree from Department of Biomedical Engineering of National Cheng Kung University in Taiwan in 2007 and received his Ph.D. degree from Institute of Nanotechnology & Microsystems Engineering at National Cheng Kung University in 2010. He has great experience in cross-disciplinary integration development and novel techniques/products creation on biomedical research. He also has great professional capability and personality characteristic to lead and co-operate with our top researchers from many different fields.
Alfred Lee Ming Sung will serve as our independent director and the Chair of the Audit Committee immediately upon the effectiveness of our registration statement on Form S-1, of which this prospectus is a part. Mr. Sung has served as an executive director and the Chief Financial Officer of Modern Living Investments Holdings Limited, a public company listed on the Hong Kong Stock Exchange (stock code: 8426), and has run its finance department since 2017. He’s also been an independent non-executive director at Harbour Equine Holdings Ltd., a Hong Kong-listed company (stock code: 8377), since 2017. Mr. Sung founded Alfred Sung & Co., CPA, a Hong Kong accounting firm, in 1991. We value Mr. Sung’s experience in managing public companies and financial management and are confident in his ability to serve as an independent director of our company. Mr. Sung is a chartered accountants in Australia and received his bachelor’s degree in economics from La Trobe University in 1983.
Cheang I Kei will serve as our independent director and the Chair of the Compensation Committee immediately upon the effectiveness of our registration statement on Form S-1, of which this prospectus is a part. Ms. Cheang has been the legal senior manager at Luso International Banking Ltd. since October 2022. She served as the legal manager at Bank of China (Macau) from May 2019 to October 2022. She was the legal assistant at Luso International Banking Ltd. between August 2016 and May 2019. Ms. Cheang is experienced in corporate governance matters and day to day administrative affairs, which we believe makes her a suitable member to our Board. Ms. Cheang received her bachelor’s degree in Chinese-English translation and interpretation from Macau Polytechnic University in 2016.
Dr. Mengxing Tang will serve as our independent director and the Chair of the Nominating and Corporate Governance Committee immediately upon the effectiveness of our registration statement on Form S-1, of which this prospectus is a part. Dr. Tang has been a professor, the Chair of Biomedical Imaging, and the Director at Ultrasound Laboratory for Imaging and Sensing (ULIS) at Imperial College London in the United Kingdom since September 2018. He has authored over 100 peer-reviewed SCI journal papers, secured research funding of over 9 million pounds since 2006, and served in various management roles, including Director of Imperial Network of Excellence, Department Management Committee, Director of Teaching, and Co-Director of Research. We believe Dr. Tang’s strong academic background and management experience make him an ideal member of our Board of Directors. Dr. Tang received his Ph.D. in computer sciences and engineering from De Montfort University.
| 104 |
Family Relationships
There are no family relationships, or other arrangements or understandings between or among any of the directors or executive officer.
Board of Directors
All directors hold office until the next annual meeting of shareholders and until their successors have been duly elected and qualified. Directors are elected at the annual meetings to serve for one-year terms. Officers are elected by, and serve at the discretion of, the board of directors. Our board of directors shall hold meetings on at least a quarterly basis.
The board of directors has determined to comply with the Nasdaq Listing Rules with respect to certain corporate governance matters. We also intend to comply with the requirements of Rule 10A-3 under the Securities Exchange Act of 1934 within the applicable time frame.
Director Independence
The board of directors has reviewed the independence of our directors, applying the Nasdaq independence standards. Based on this review, the board of directors determined that Alfred Lee Ming Sung, Cheang I Kei, and Mengxing Tang are independent within the meaning of the Nasdaq rules. In making this determination, our board of directors considered the relationships that each of these non-employee directors has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence. As required under applicable Nasdaq rules, we anticipate that our independent directors will meet on a regular basis as often as necessary to fulfill their responsibilities, including at least annually in executive session without the presence of non-independent directors and management.
Board Committees
Upon effectiveness of the registration statement, our board of directors will establish standing committees in connection with the discharge of its responsibilities. These committees include an Audit Committee, a Compensation Committee and a Corporate Governance and Nominating Committee. Our board of directors will adopt written charters for each of these committees. Our board of directors may establish other committees as it deems necessary or appropriate from time to time.
Audit Committee
Upon effectiveness of the registration statement, we will establish an audit committee which will be composed of three of our independent directors: Alfred Lee Ming Sung (Chairman), Cheang I Kei and Mengxing Tang. It is anticipated that the Board will determine that Alfred Lee Ming Sung qualifies as the Audit Committee financial expert as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
According to its charter, the Audit Committee consists of at least three members, each of whom shall be a non-employee director who has been determined by the Board to meet the independence requirements of Nasdaq, and also Rule 10A-3(b)(1) of the SEC, subject to the exemptions provided in Rule 10A-3(c). The Audit Committee Charter describes the primary functions of the Audit Committee, including the following:
| · | Oversee the Company’s accounting and the financial reporting processes; |
| · | Oversee audits of the Company’s financial statements; |
| · | Review and discuss with management the Company’s audited financial statements and review with management and the Company’s independent registered public accounting firm the Company’s financial statements prior to the filing with the SEC of any report containing such financial statements; |
| · | Discuss policies with respect to risk assessment and risk management, and discuss the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures; |
| · | Review major changes to the Company’s auditing and accounting principles and practices as suggested by the Company’s independent registered public accounting firm, internal auditors or management; and |
| · | Take, or recommend that the board take, appropriate action to oversee and ensure the independence of the Company’s independent registered public accounting firm. |
| 105 |
Compensation Committee
Upon effectiveness of the registration statement, we will establish a compensation committee, which will be composed of three of our independent directors: Cheang I Kei (Chair), Alfred Lee Ming Sung, and Mengxing Tang. The Compensation Committee will be responsible for, among other matters:
| · | reviewing and approving employment agreements and other similar arrangements between us and our executive officers; |
| · | reviewing and approving, or recommending to the board of directors to approve the compensation of our CEO and other executive officers and directors reviewing key employee compensation goals, policies, plans and programs; and |
| · | appointing and overseeing any compensation consultants or advisors. |
Corporate Governance and Nominating Committee
Upon effectiveness of the registration statement, we will establish a corporate governance and nominating committee, which will be composed of three of our independent directors: Mengxing Tang (Chair), Alfred Lee Ming Sung, and Cheang I Kei. The Corporate Governance and Nominating Committee will be responsible for, among other matters:
| · | reviewing and making recommendations regarding the structure and composition of our board and the board committees; |
| · | evaluating the independence of directors and director nominees; |
| · | developing and recommending to the board corporate governance principles and practices; |
| · | reviewing and monitoring the Company’s code of business conduct; and |
| · | overseeing the evaluation of the Company’s management. |
Code of Ethics
We will adopt a new code of business conduct (the “code of business conduct”) that applies to all directors, executive officers and employees. It will be available on our website upon the effectiveness of the registration statement of which this prospectus forms a part. Our code of business conduct is a “code of ethics,” as defined in Item 406(b) of Regulation S-K. Copies of the code of business conduct and charters for each of our board committees will be provided without charge upon request from us and will be posted on our company website. We will make any legally required disclosures regarding amendments to, or waivers of, provisions of our code of ethics on our Internet website.
Involvement in Certain Legal Proceedings
To our knowledge, there are no material proceedings to which any of our directors, officers or affiliates of the Company is a party adverse to the Company or has a material interest adverse to the Company.
Outstanding Equity Awards
There were no outstanding equity awards as of JuneSeptember 30, 2022.2023.
| 106 |
Equity Compensation Plan Information
Effective March 30, 2023, our Stock InventiveIncentive Plan (the “2023 Plan”) was approved by our Board of Directors. Under the 2023 Plan, the Board of Directors may grant options or purchase rights to purchase common stock to officers, employees, and other persons who provide services to us or any related company. The participants to whom awards are granted, the type of awards granted, the number of shares covered for each award, and the purchase or exercise price, conditions and other terms of each award are determined by the Board of Directors, except that the term of the options shall not exceed 10 years. A total of 15 million shares of our common stock are subject to the 2023 Plan and maybe either a qualified or non-qualified stock option. The shares issued for the 2023 Plan may be either treasury or authorized and unissued shares. As of the date of this prospectus, we have granted no stock options to purchase any shares of our common stock under the 2023 Plan.
Compensation of Directors and Executive Officers
No directors’ or executive officers’ compensation was paid during the year ended June 30, 2021. Other than our CFO, no directors’ or executive officers’ compensation was paid during the year ended June 30, 2022. For the year ended June 30, 2022, we paid $20,000 to our CFO. Other than our CFO, no directors’ or executive officers’ compensation was paid during the year ended June 30, 2023. For the year ended June 30, 2023, we paid $40,000 to our CFO. No directors’ or officers’ compensation was paid during the three-month period ended September 30, 2023.
| 107 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Since July 1, 2019, there has not been any transaction or series of similar transactions to which we were, or will be, a party in which the amount involved exceeded, or will exceed, the lesser of (i) $120,000 or (ii) one percent of the average of our total assets for the last two completed fiscal years, and in which any director, executive officer, holder of five percent or more of any class of our capital stock or any member of the immediate family of, or entities affiliated with, any of the foregoing persons, had, or will have, a direct or indirect material interest except for the followings:-
| Name of Related Party | Relationship to Us | ||
| Yi Lu, Ph.D. | Chairman of the Board, Chief Science Officer, and President of the Company | ||
| Hung To Pau, Ph.D. | Chief Executive Officer, director and Secretary of the Company | ||
| Steven I-Fang Cheng, Ph.D. | Chief Technology Officer of the Company | ||
| Chen-Yi Lee | Chen-Yi Lee is the sole director and the controlling person of Advance On Ventures Limited, which owns 10.92% equity interest in the Company and has sole voting and dispositive power over shares beneficially owned by Advance On Ventures Limited. | ||
| Advance On Ventures Limited | Shareholder of the Company | ||
| Well Fancy Development Ltd | CEO-Hung To Pau is the director and shareholder of the entity | ||
| Shanghai Junfu Electronic Technology Co., Ltd. | CEO-Hung To Pau is the legal person and shareholder of the entity |
On July 16, 2021, the Company issued 8,000,000 shares to Dr. Hung To Pau. On March 15, 2022, Dr. Hung To Pau transferred all of his 8,000,000 shares to Sglcell Ltd,Ltd., an exempted company incorporated under the law of Cayman Islands, the sole shareholder of which is Dr. Hung To Pau for a total consideration of $8,000. On June 8, 2022, Sglcell LtdLtd. transferred all of its 8,000,000 shares to Dr. Yi Lu for a total consideration of $8,000.
Shares Issued to Advanced Biomed Taiwan Holders/Employees
On August 12, 2022, we issued additional 385,000 shares to Dr. Yi Lu, and 257 shares to Chen-Yi Lee while Dr. Yi Lu and Chen-Yi Lee transferred 2,998,000 shares and 2,000 shares owned by them respectively in Advanced Biomed Inc. (Taiwan), representing in aggregate 100% of the issued share capital of Advanced Biomed Inc. (Taiwan), to the Company pursuant to the share exchange agreement the Company entered into with Dr. Yi Lu and Chen-Yi Lee.
On October 24, 2022, we issued 365,352 shares for no consideration to Chen-Yi Lee, who is an employee of Advanced Biomed Taiwan, for past services to Advanced Biomed Taiwan. On October 24, 2022, we also issued 2,730,000 shares for no consideration to Advance On Ventures Limited, a company incorporated under the law of British Virgin Islands (the “Ventures Limited”), the beneficial owners of which are employees of Advanced Biomed Taiwan for past services to Advanced Biomed Taiwan. We issued 4,405,625 shares, 2,193,750 shares, 2,060,000 shares, 1,511,250 shares, 1,243,750 shares, 1,230,000 shares respectively to Dr. Hung To Pau, Yimin Jin, Xiaoyuan Luo, Nanzhen Shen, Jian Wang and Qiang Chen pursuant to the Debt-For-Equity Exchange Agreement the Company entered into with the abovementioned shareholders on June 30, 2022 to settle debt of a total amount of NTD 174,020,033 and RMB 22,200,000 (approximately $9.04 million).
In the ordinary course of business, during the three-month period ended September 2023 and fiscal years ended June 30, 2023 and 2022, the Company was involved in certain transactions, either at cost or current market prices, and on the normal commercial terms with related parties. The following table provides the transactions with these parties for the years as presented (for the portion of such period that they were considered related):
As of September 30, 2023 | June 30, 2023 | June 30, 2022 | ||||||||||
| US$ | US$ | US$ | ||||||||||
| Amount due to related parties – major shareholders | ||||||||||||
| Name of related party | ||||||||||||
| Hung To Pau, Ph.D. (1) | 1,027,077 | 933,471 | — | |||||||||
| Yi Lu, Ph.D. (2) | 110,987 | 114,907 | — | |||||||||
| Chen-Yi Lee (3) | 4,549 | 4,299 | — | |||||||||
| Amount due to related parties – related corporations | ||||||||||||
| Name of related party | ||||||||||||
| Well Fancy Development Ltd (4) | 621,480 | 396,905 | — | |||||||||
| Shanghai Junfu Electronic Technology Co., Ltd. (5) | 205,592 | 206,897 | — | |||||||||
| 1. | Payments of IPO costs paid on behalf of Advanced Biomed Inc. |
| 2. | Advanced Biomed Inc.(Taiwan) entered into an unsecured, interest-free loan to Yi Lu amounting to NTD 3,578,212 (approximately US$110,987) for general working capital in January 2023. As of September 30, 2023, June 30, 2023 and 2022, the loan balance due to Yi Lu amounted to US$110,987, US$114,907 and nil, respectively. |
| 3. | Payments of expenses on behalf of Advanced Biomed Inc. (Taiwan). |
| 4. | Advanced Biomed Inc.(Taiwan) entered into four unsecured, interest-free loans to Well Fancy Development Ltd amounting to NTD 2,967,700 (approximately US$92,050), NTD 2,989,873 (approximately US$92,738), NTD 4,270,200 (approximately US$132,450), and NTD 1,945,000 (approximately US$60,329), NTD 1,858,120 (approximately US$57,634), and NTD 2,912,200 (approximately US$90,329) for general working capital in August 2022, April 2023, May 2023, June 2023 and July 2023, and August 2023, respectively. As of September 30, 2023, the loan balance due to Well Fancy Development Ltd totally amounted to US$525,530 and amounted to US$95,950 for general working capital. As of June 30, 2023 and 2022, the loan balance due to Well Fancy Development Ltd totally amounted to US$396,905 and nil, respectively. |
| 5. | Shanghai Sglcell Biotech Co., Ltd entered into three unsecured, interest-free loans to Shanghai Junfu Electronic Technology Co., Ltd. amounting to RMB 500,000 (approximately US$68,530) for general working capital in April 2023, May 2023, and June 2023, respectively. As of September 30, 2023, June 30, 2023 and 2022, the loan balance due to Shanghai Sglcell Biotech Co., Ltd totally amounted to US$205,592, US$206,897 and nil, respectively. |
| 108 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
As of the date of this prospectus, there are 100,000,000 shares of common stock outstanding. The following table sets forth certain information known to us with respect to the beneficial ownership of common stock as of that date by (i) each of our directors, (ii) each of our executive officers, (iii) all of our directors and executive officers as a group, and (iv) each person, or group of affiliated persons, whom we know to beneficially own more than 5% of our common stock.
We have determined beneficial ownership in accordance with the rules of the SEC, which generally define beneficial ownership to include any shares over which a person exercises sole or shared voting or investment power. Such determination is not necessarily indicative of beneficial ownership for any other purpose. Unless otherwise indicated, we believe, based on the information furnished to us, that all persons named in the table have sole voting and investment power with respect to all shares beneficially owned by them. None of the stockholders listed in the table are a broker-dealer or an affiliate of a broker dealer. Applicable percentage ownership prior to the offering is based on [100,000,000] shares of common stock outstanding as of the date of this prospectus. The table also lists the percentage ownership after this offering based on [125,000,000] shares of common stock outstanding immediately after the completion of this offering, assuming no exercise of the underwriters’ over-allotment option to purchase additional shares of common stock from us in this offering.
| Prior to Offering | After Offering | Prior to Offering | After Offering | |||||||||||||||||||||||||||||
| Name and Address of Beneficial Owner(1) | Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares(2) | Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares(3) | Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares(2) | Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares(3) | ||||||||||||||||||||||||
| Directors and Officers | ||||||||||||||||||||||||||||||||
| Yi Lu | 33,540,000 | 33.54 | % | 33,540,000 | 26.83 | % | 33,540,000 | 33.54 | % | 33,540,000 | 26.83 | % | ||||||||||||||||||||
| Hung To Pau | 17,622,500 | 17.62 | % | 17,622,500 | 14.10 | % | 17,622,500 | 17.62 | % | 17,622,500 | 14.10 | % | ||||||||||||||||||||
| Mingze Yin | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Steven I-Fang Cheng(4) | 3,712,800 | 3.71 | % | 3,712,800 | 2.97 | % | 3,712,800 | 3.71 | % | 3,712,800 | 2.97 | % | ||||||||||||||||||||
| Alfred Lee Ming Sung | - | - | - | �� | - | |||||||||||||||||||||||||||
| Cheang I Kei | - | - | - | - | ||||||||||||||||||||||||||||
| Mengxing Tang | - | - | - | - | ||||||||||||||||||||||||||||
| All officers and directors as a group (four persons) | 54,875,300 | 54.88 | % | 54,875,300 | 43.90 | % | 54,875,300 | 54.88 | % | 54,875,300 | 43.90 | % | ||||||||||||||||||||
| 5% Shareholders | ||||||||||||||||||||||||||||||||
| Advance On Ventures Limited(5) | 10,920,000 | 10.92 | % | 10,920,000 | 8.74 | % | 10,920,000 | 10.92 | % | 10,920,000 | 8.74 | % | ||||||||||||||||||||
| Yimin Jin | 8,775,000 | 8.78 | % | 8,775,000 | 7.02 | % | 8,775,000 | 8.78 | % | 8,775,000 | 7.02 | % | ||||||||||||||||||||
| Xiaoyuan Luo | 8,240,000 | 8.24 | % | 8,240,000 | 6.59 | % | 8,240,000 | 8.24 | % | 8,240,000 | 6.59 | % | ||||||||||||||||||||
| Nanzhen Shen | 6,045,000 | 6.05 | % | 6,045,000 | 4.84 | % | 6,045,000 | 6.05 | % | 6,045,000 | 4.84 | % | ||||||||||||||||||||
| (1) | Unless otherwise indicated, the business address of each of the individuals is 689-87 Xiaodong Road, Yongkang District, Tainan, Taiwan. | |
| (2) | Based on 100,000,000 shares issued and outstanding as of the date of this prospectus. | |
| (3) | Based on [125,000,000] shares issued and outstanding immediately after this offering. | |
| (4) | Represents 3,712,800 shares beneficially owned by Mr. Steven I-Fang Cheng through Advance On Ventures Limited, a company incorporated in the British Virgin Islands, in which Mr. Cheng owns 34% equity interests. The registered address of Advance on Ventures Limited is Wickhams Cay II, Road Town, Tortola, VG1110, British Virgin Islands. | |
| (5) | Chen-Yi Lee is the sole director and |
On June 6, 2022, we entered into an investment agreement with Hanyu Assets Co., Ltd. (“Hanyu”). Under this agreement, Hanyu will invest US$2.5 million in the Company to acquire a 2.5% equity interest in the Company after the transaction, and Hanyu must pay US$2.5 million before June 30, 2022 to the Company’s designated bank account. Hanyu transferred US$2.5 million to us on June 29, 2023. This agreement can only be terminated by mutual agreement in writing. The formation, validity, interpretation, performance, and settlement of disputes arising from this agreement will be governed by the laws of the State of New York.
On June 6, 2022, we entered into an investment agreement with Newlink Technology Inc. (“Newlink”). Under this agreement, Newlink will invest HK$8,000,000 to acquire a 1% equity interest in the Company after the transaction, and Newlink must pay HK$8,000,000 before September 10, 2022, to the Company’s designated bank account. We received the payment from Newlink on September 13, 2022. This agreement can only be terminated by mutual agreement in writing. The formation, validity, interpretation, performance, and settlement of disputes arising from this agreement will be governed by the laws of the State of New York.
After receiving the fund from Newlink and Hanyu, we issued 625,000 shares to Hanyu Assets Co. Ltd. and 250,000 shares to Newlink Technology Inc. on October 25, 2022.
On June 30, 2022, we entered into a Debt-for-Equity Exchange Agreement with Pau Hung To, Jin Yimin, Luo Xiaoyuan, Shen Nanzhen, Wang Jian, and Chen Qiang (collectively, the “Creditors”) to settle certain debts owed to the Creditors. Pursuant to the agreement, we issued 12,644,375 common stock to the Creditors to completely settle the debt between the Creditors and us. Any disputes arising from this agreement must be submitted to China International Economic and Trade Arbitration Commission’s Shanghai Office for arbitration. The agreement cannot be amended unless all parties agree in writing.
| 109 |
The following description summarizes important terms of our securities. For a complete description, you should refer to our certificate of incorporation and bylaws, forms of which are incorporated by reference to the exhibits to the registration statement of which this prospectus is a part, as well as the relevant portions of the Nevada law. References to our certificate of incorporation and bylaws are to our certificate of incorporation and our bylaws, respectively, each of which will become effective upon completion of this offering.
General
We are authorized to issue 500,000,000 shares of common stock, par value $0.001 per share. On May 16, 2023, we effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, we now have 100,000,000 common stock issued and outstanding as of the date hereof.
Common Stock
Each share of our common stock is entitled to one vote on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required by law, the holders of common stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority of the votes entitled to be cast by all shares of common stock that are present in person or represented by proxy. Holders of common stock representing a majority of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders. Our Articles of Incorporation do not provide for cumulative voting in the election of directors. Holders of common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock.
Non-cumulative Voting
Holders of shares of our common stock do not have cumulative voting rights; meaning that the holders of 50.1% of the outstanding shares, voting for the election of directors, can elect all of the directors to be elected, and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
Cash Dividends
As of the date of this prospectus, we have not paid any cash dividends to stockholders. The declaration of any future cash dividend will be at the discretion of our Board and will depend upon our earnings, if any, our capital requirements and financial position, our general economic conditions, and other pertinent conditions. It is our present intention not to pay any cash dividends in the foreseeable future, but rather to reinvest earnings, if any, in our business operations.
Exchange Listing
We have applied to list our common stock on the Nasdaq Capital Market under the trading symbol “ADVB.” This offering will occur only if our listing application is approved.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Transhare Corporation, with an address at Bayside Center 1, 17755 North US Highway 19, Suite 140, Clearwater, FL 33764, telephone number is (303) 662-1112.
Indemnification of Officers and Directors
Pursuant to our Articles of Incorporation as amended, and Bylaws, we may indemnify an officer or director who is made a party to any proceeding, including a lawsuit, because of his position, if he acted in good faith and in a manner he reasonably believed to be in or not opposed to our best interest, provided, however, that (i) we will not indemnify such person against expenses incurred in connection with an action if he is threatened but does not become a party unless the incurring of such expenses was authorized by the board of directors and (ii) we will not indemnify against any amount paid in settlement unless our board of directors has consented to such settlement.
| 110 |
An officer or director is not entitled to indemnification against costs or expenses incurred in connection with any action, commenced by such person against us or any person who is or was a director, officer, fiduciary, employee or agent of our company unless and to the extent that the officer or directors is successful on the merits in any such proceeding as to which such person is to be indemnified, we must indemnify him against all expenses incurred, including attorney’s fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or directors is judged liable, only by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Nevada.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for directors, executive officers, or persons controlling us, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
SHARES ELIGIBLE FOR FUTURE SALE
Before the closing of this offering, there has been no public market for our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the trading symbol “ADVB.” We make no prediction as to the effect, if any, that market sales of our common stock or the availability of our common stock for sale will have on the market price of common stock prevailing from time to time. Nevertheless, sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect the market price of our common stock and could impair our future ability to raise capital through the sale of equity securities.
Upon the completion of this offering, we will have an aggregate of 125,000,000 shares of common stock outstanding, assuming the underwriters do not exercise their over-allotment option. Of the outstanding common stock, all of the shares of common stock sold in this offering will be freely tradable, except that any common stock purchased by “affiliates” (as that term is defined in Rule 144 under the Securities Act), may only be sold in compliance with the limitations described below. After this offering, 100,000,000 shares of common stock will be deemed “restricted securities” as defined in Rule 144. Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144 or Rule 701, promulgated under the Securities Act, which rules are summarized below.
As a result of the contractual restrictions described below and the provisions of Rule 144 and Rule 701, the restricted shares will be available for sale in the public market as follows: shares of common stock will be eligible for sale upon the expiration of the lock-up agreements, described below, beginning 180 days after the completion of the offering subject to extension in certain circumstances.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell such shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then such person is entitled to sell such shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
| · | 1% of the number of shares of common stock then outstanding, which will equal approximately shares immediately after this offering; or |
| · | the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, any of our employees, directors, officers, consultants or advisors who purchase shares from us in connection with a compensatory stock or option plan or other written agreement before the effective date of this offering are entitled to resell such shares 90 days after the effective date of this offering in reliance on Rule 144, without having to comply with the holding period requirements or other restrictions contained in Rule 701.
The SEC has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after the date of this prospectus. Securities issued in reliance on Rule 701 are restricted securities and, subject to the contractual restrictions described above, beginning 90 days after the date of this prospectus, may be sold by persons other than affiliates, subject only to the manner of sale provisions of Rule 144 and by affiliates under Rule 144 without compliance with its one-year minimum holding period requirement.
| 111 |
Lock-up Agreements
See “Underwriting—Lock-up Agreements.”
TAXATION
The following discussion of material Taiwan, PRC and United States federal income tax consequences of an investment in our common stock is based upon laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change. This discussion does not deal with all possible tax consequences relating to an investment in our common stock, such as the tax consequences under state, local and other tax laws. To the extent that the discussion relates to matters of Taiwan tax law, it represents the opinion of Wiseteam Law Firm, our Taiwan counsel. To the extent that the discussion relates to matters of PRC tax law, it represents the opinion of AllBright, our PRC counsel.
Taiwan Taxation
The following is a general summary of the principal Taiwan tax consequences of the ownership and disposition of our common stock by and to a non-resident individual or non-resident entity holder (referred to herein as a “Non-Taiwan Holder”). As used in the preceding sentence, a “non-resident individual” is generally a foreign national who owns our common stock and is not physically present in Taiwan for 183 days or more during any calendar year, and a “non-resident entity” is a corporation or a non-corporate body that owns our common stock and is organized under the laws of a jurisdiction other than Taiwan. Holders should consult their tax advisors concerning the Taiwan tax consequences of holding our common stock and the laws of any relevant taxing jurisdiction to which they are subject.
Capital gains from the sale or disposal of our common stock
Sale or disposal of the common stock of a Nevada company is generally not regarded as the sale of Taiwan securities; thus, any gains generated therefrom by Non-Taiwan Holders are not subject to Taiwan income tax.
Securities Transaction Tax
Sale of the common stock of a Nevada company by Non-Taiwan Holders is generally not subject to Taiwan securities transaction tax.
People’s Republic of China Taxation
Enterprise Income Tax
Under the Enterprise Income Tax Law of the PRC, or the EIT Law, which became effective on January 1, 2008 and was subsequently amended on February 24, 2017 and December 29, 2018, and its implementing rules, enterprises are classified as resident enterprises and non-resident enterprises. PRC resident enterprises typically pay an enterprise income tax at the rate of 25% while non-PRC resident enterprises without any branches in the PRC should pay an enterprise income tax in connection with their income from the PRC at the tax rate of 10%. An enterprise established outside of the PRC with its “de facto management bodies” located within the PRC is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a PRC domestic enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define a de facto management body as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise. Enterprises qualified as “High and New Technology Enterprises” are entitled to a 15% enterprise income tax rate rather than the 25% uniform statutory tax rate. The preferential tax treatment continues as long as an enterprise can retain its “High and New Technology Enterprise” status.
The EIT Law and the implementation rules provide that an income tax rate of 10% should normally be applicable to dividends payable to investors that are “non-resident enterprises,” and gains derived by such investors, which (a) do not have an establishment or place of business in the PRC or (b) have an establishment or place of business in the PRC, but the relevant income is not effectively connected with the establishment or place of business to the extent such dividends and gains are derived from sources within the PRC. Such income tax on the dividends may be reduced pursuant to a tax treaty between China and other jurisdictions. Pursuant to the Arrangement Between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation on Income, or the Double Tax Avoidance Arrangement, and other applicable PRC laws, if a Hong Kong resident enterprise is determined by the competent PRC tax authority to have satisfied the relevant conditions and requirements under such Double Tax Avoidance Arrangement and other applicable laws, the 10% withholding tax on the dividends the Hong Kong resident enterprise receives from a PRC resident enterprise may be reduced to 5% upon receiving approval from in-charge tax authority. However, based on the Notice on Certain Issues with Respect to the Enforcement of Dividend Provisions in Tax Treaties issued on February 20, 2009 by the SAT, if the relevant PRC tax authorities determine, in their discretion, that a company benefits from such reduced income tax rate due to a structure or arrangement that is primarily tax-driven, such PRC tax authorities may adjust the preferential tax treatment; and based on the Announcement on Relevant Issues Concerning the “Beneficial Owners” in Tax Treaties issued on February 3, 2018 by the SAT and effective from April 1, 2018, which replaces the Notice on the Interpretation and Recognition of Beneficial Owners in Tax Treaties and the Announcement on the Recognition of Beneficial Owners in Tax Treaties by the SAT, comprehensive analysis based on the stipulated factor therein and actual circumstances shall be adopted when recognizing the “beneficial owner” and agents and designated wire beneficiaries are specifically excluded from being recognized as “beneficial owners.”
| 112 |
On January 17, 2019, the State Taxation Administration issued the notice on the scope of small-scale and low-profit corporate income tax preferential policies of the Ministry of Finance and the State Administration of Taxation (“MOF and SAT”), [2019] No. 13 for small-scale and low-profit enterprises whose annual taxable income is less than RMB1,000,000 (including RMB1,000,000), approximately $142,209, their income is reduced by 25% to the taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 5%. While for the portion of annual taxable income exceeding RMB1,000,000, approximately $142,209, but not more than RMB3,000,000, approximately $426,627, the income is reduced by 50% to the taxable income, and enterprise income tax is paid at 20% tax rate, which is essentially resulting in a favorable income tax rate of 10%. MOF and SAT [2021] No.12 provides an enterprise income tax rate of 2.5% on a small-scale and low-profit enterprises whose annual taxable income less than RMB1,000,000, approximately $142,209, from January 1, 2021 to December 31, 2022. MOF and SAT [2022] No.13 also provides an enterprise income tax rate of 5% on a small-scale and low-profit enterprises whose annual taxable income more than RMB1,000,000, approximately $142,209, but not more than RMB3,000,000, approximately $426,627, from January 1, 2022 to December 31, 2024. The qualifications of small-scale and low-profit enterprises were examined annually by the Tax Bureau. All of the Company’s Shanghai subsidiary met the criteria of small-scale and low-profit enterprises.
Value-added Tax
Pursuant to applicable PRC tax regulations, any entity or individual conducting business in the service industry used to be generally required to pay a business tax at the rate of 5% on the revenues generated from providing such services. However, if the services provided are related to technology development and transfer, such business tax may be exempted subject to approval by the relevant tax authorities. Whereas, pursuant to the Provisional Regulations on Value-Added Tax of the PRC and its implementation regulations, unless otherwise specified by relevant laws and regulations, any entity or individual engaged in the sales of goods, provision of processing, repairs and replacement services and importation of goods into China is generally required to pay a value-added tax, or VAT, for revenues generated from sales of products, while qualified input VAT paid on taxable purchase can be offset against such output VAT.
In November 2011, the Ministry of Finance and the State Administration of Taxation promulgated the Pilot Plan for Imposition of Value-Added Tax to Replace Business Tax. In March 2016, the Ministry of Finance and the State Administration of Taxation further promulgated the Notice on Fully Promoting the Pilot Plan for Replacing Business Tax by Value-Added Tax, which became effective on May 1, 2016. Pursuant to the pilot plan and relevant notices, VAT is generally imposed in lieu of business tax in the modern service industries, including the VATS, on a nationwide basis. VAT of a rate of 6% applies to revenue derived from the provision of some modern services. Certain small taxpayers under PRC law are subject to reduced value-added tax at a rate of 3%. Unlike business tax, a taxpayer is allowed to offset the qualified input VAT paid on taxable purchases against the output VAT chargeable on the modern services provided.
On April 4, 2018, the Ministry of Finance and the State Administration of Taxation issued the Notice on Adjustment of VAT Rates, which came into effect on May 1, 2018. According to the abovementioned notice, the taxable goods previously subject to VAT rates of 17% and 11%, respectively, become subject to lower VAT rates of 16% and 10%, respectively, starting from May 1, 2018. Furthermore, according to the Announcement on Relevant Policies for Deepening Value-added Tax Reform jointly promulgated by the Ministry of Finance, the State Administration of Taxation and the General Administration of Customs, which became effective on April 1, 2019, the taxable goods previously subject to VAT rates of 16% and 10%, respectively, become subject to lower VAT rates of 13% and 9%, respectively, starting from April 1, 2019. Under Provisional Regulations of the People’s Republic of China on Value-added Tax, amended and effective on November 19, 2017, for entities that are VAT small taxpayers, VAT is levied at a levy rate of 3%. On February 29, 2020, the State Administration of Taxation issued the Announcement on Taxation Matters to Support Individual Businesses in Resumption of Business, during the COVID-19, the small taxpayers are allowed to enjoy the preferred tax policy, tax rate from 3% to 1% for the period from March 1, 2020 to December 31, 2021.
United States Federal Income Tax Considerations
The following is a discussion of United States federal income tax considerations relating to the acquisition, ownership, and disposition of our common stock by a U.S. Holder, as defined below, that acquires our common stock in this offering and holds our common stock as “capital assets” (generally, property held for investment) under the United States Internal Revenue Code of 1986, as amended (the “Code”). This discussion is based upon existing United States federal income tax law, which is subject to differing interpretations or change, possibly with retroactive effect. No ruling has been sought from the Internal Revenue Service (the “IRS”) with respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court will not take a contrary position. This discussion does not address all aspects of United States federal income taxation that may be important to particular investors in light of their individual circumstances, including investors subject to special tax rules (such as, for example, certain financial institutions, insurance companies, regulated investment companies, real estate investment trusts, broker-dealers, traders in securities that elect mark-to-market treatment, partnerships and their partners, tax-exempt organizations (including private foundations)), investors who are not U.S. Holders, investors that own (directly, indirectly, or constructively) 10% or more of our voting shares, investors that hold their common stock as part of a straddle, hedge, conversion, constructive sale or other integrated transaction), or investors that have a functional currency other than the U.S. dollar, all of whom may be subject to tax rules that differ significantly from those summarized below. In addition, this discussion does not address any tax laws other than the United States federal income tax laws, including any state, local, alternative minimum tax or non-United States tax considerations, or the Medicare tax. Each potential investor is urged to consult its tax advisor regarding the United States federal, state, local and non-United States income and other tax considerations of an investment in our common stock.
| 113 |
General
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of our common stock that is, for United States federal income tax purposes, (i) an individual who is a citizen or treated as a tax resident of the United States, (ii) a corporation (or other entity treated as a corporation for United States federal income tax purposes) created in, or organized under the laws of, the United States or any state thereof or the District of Columbia, (iii) an estate the income of which is includible in gross income for United States federal income tax purposes regardless of its source, or (iv) a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise elected to be treated as a United States person under the Code.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) is a beneficial owner of our common stock, the tax treatment of a partner in the partnership will depend upon the status of the partner and the activities of the partnership. Partnerships and partners of a partnership holding our common stock are urged to consult their tax advisors regarding an investment in our common stock.
The discussion set forth below is addressed only to U.S. Holders that purchase common stock in this offering. Prospective purchasers are urged to consult their own tax advisors about the application of the U.S. federal income tax rules to their particular circumstances as well as the state, local, foreign and other tax consequences to them of the purchase, ownership and disposition of our common stock.
Taxation of Dividends and Other Distributions on our Common Stock
Subject to the passive foreign investment company rules discussed below, the gross amount of distributions made by us to you with respect to the common stock (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the common stock are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a passive foreign investment company (as discussed below) for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Under U.S. Internal Revenue Service authority, common stock are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on Nasdaq. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our common stock, including the effects of any change in law after the date of this prospectus.
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your common stock, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
Taxation of Dispositions of Common Stock
You will recognize taxable gain or loss on any sale, exchange or other taxable disposition of a share equal to the difference between the amount realized (in U.S. dollars) for the share and your tax basis (in U.S. dollars) in the common stock. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the common stock for more than one year, you may be eligible for reduced tax rates on any such capital gains. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
Dividend payments with respect to our common stocks and proceeds from the sale, exchange or redemption of our may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or who is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. U.S. Holders are urged to consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information. We do not intend to withhold taxes for individual shareholders. However, transactions effected through certain brokers or other intermediaries may be subject to withholding taxes (including backup withholding), and such brokers or intermediaries may be required by law to withhold such taxes.
| 114 |
In connection with this offering, we will enter into an underwriting agreement with Univest Securities, LLC, as representative (the “Representative”) of the underwriters named below. The Representative may retain other brokers or dealers to act as sub-agents or selected dealers on their behalf in connection with this offering. Subject to the terms and conditions of the underwriting agreement, each underwriter will agree to purchase from us, on a firm commitment basis, the number of shares of common stock set forth opposite its name below, at the offering price less the underwriting discounts set forth on the cover page of this prospectus:
| Underwriter | Number of Common stock | |||
| Univest Securities, LLC | 25,000,000 | |||
The underwriters are committed to purchase all the shares of common stock offered by this prospectus if they purchase any shares pursuant to the underwriting agreement. The underwriters are not obligated to purchase the shares covered by the underwriter’s over-allotment option as described below. The underwriting agreement provides that the obligation of the underwriters to purchase all of the shares of common stock being offered to the public is subject to specific conditions, including the absence of any material adverse change in our business or in the financial markets and the receipt of certain legal opinions, certificates and letters from us, our counsel and the independent auditors. The underwriters reserve the right to withdraw, cancel, or modify offers to the public and to reject orders in whole or in part. In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments the underwriters may be required to make in respect thereof.
Underwriting Commissions and Discounts and Expenses
The following table shows the price per share and total public offering price, underwriting discounts, and proceeds before expenses to us, assuming a $4.50 per share offering price, which is the midpoint of the price range set forth on the cover page of this prospectus. The total amounts are shown assuming both no exercise and full exercise of the over-allotment option.
| Total | ||||||||||||
Per Share | Without Over-Allotment | With Over-Allotment | ||||||||||
| Public offering price | $ | 4.50 | $ | 112,500,000 | $ | 129,375,000 | ||||||
| Underwriting discounts and commissions (5.5%) | $ | 0.2475 | $ | 6,187,500 | $ | 7,115,625 | ||||||
| Proceeds, before expenses, to us | $ | 4.2525 | $ | 106,312,500 | $ | 122,259,375 | ||||||
We have agreed to pay all expenses relating to the offering, including, without limitation: (a) all filing fees and communication expenses relating to the registration of the securities of the Company with the SEC; (b) all fees and expenses relating to the listing of the common stock on Nasdaq Capital Market; (c) all fees associated with the review of the offering by FINRA; (d) all fees, expenses and disbursements relating to background checks of the Company’s officers and directors; (e) the registration, qualification or exemption of shares offered under “blue sky” securities laws or the securities laws of other jurisdictions designated by the Representative; (f)(e) all fees, expenses and disbursements relating to the registration, qualification or exemption of the securities of the Company under the securities laws of such foreign jurisdictions; (g)(f) the costs of mailing and printing the offering materials; (h)(g) the costs of preparing, printing and delivering certificates representing the shares offered in the offering; (i)(h) fees and expenses of the transfer agent for such shares; (j)(i) stock transfer and/or stamp taxes, if any, payable upon our transfer of the securities to the Representative; (k)(j) the fees and expenses of our accountants; (l)(k) the fees and expenses of the Company’s legal counsel and other agents and representatives; (m)(l) the fees and expenses of the Representative’s legal counsel; (n) the costs associated with bound volumes of the offering materials as well as commemorative mementos and lucite tombstones, each of which the Company or its designee will provide within a reasonable time after the closing of offering in such quantities as the Representative may reasonably request; and (o) the reasonable cost for roadshow meetings and the preparation of a power point presentation.counsel.
| 115 |
The Company will pay the reasonable and documented out-of-pocket expenses of the Representative actually incurred (including, but not limited to reasonable and documented fees and expenses of due diligence and its legal counsel)counsel; all fees, expenses and disbursements relating to background checks of the Company’s officers and directors; and the reasonable cost for roadshow meetings) up to $200,000. The Company has agreed to pay expense advance to be applied against such out-of-pocket accountable expenses in the amount of $100,000, according to the following schedule: (a) $50,000 upon signing of the exclusive engagement agreement with the Representative dated as of March 23, 2023 and (b) $50,000 upon the receipt of the “No Objection Letter” for this offering from FINRA. As of the date of this prospectus, we have not paid any advance expense to the Representative. The advance expense will be returned to us to the extent such out-of-pocket accountable expenses are not actually incurred in accordance with FINRA Rule 5110(g)(4)(A). In addition, we also agreed to pay a non-accountable expense allowance to the Representative equal to 1.0% of the gross proceeds received at the completion of the offering.
Representative’s Warrants
Upon completion of this offering, we have agreed to issue warrants to the Representative or its designees to purchase 250,000 shares of common stock (or 287,500 shares of common stock if the Representative exercises its over-allotment option in full) equal to 1% of the total number of shares of common stock sold in this offering (including any shares sold in the offering to cover over-allotments) at an exercise price equal to 150% of the offering price of the common stock sold in this offering. The underwriter warrants are exercisable at any time and from time to time, in whole or in part, during the four and a half year period commencing six months following the date of commencement of sales of the securities issued in this offering. The warrants are not redeemable by us. The Representative’s warrants and the shares underlying the warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to FINRA Rule 5110(e)(1)(A). The Representative (or permitted assignees under the FINRA Rule 5110(e)) may not sell, transfer, assign, pledge, or hypothecate the Representative’s warrants or the shares underlying the Representative’s warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Representative’s warrants or the underlying shares for a period of 180 days following the date of commencement of sales of the securities issued in this offering except as permitted by FINRA Rule 5110(e)(2). The Representative or its designees will also be entitled to one demand registration of the sale of the shares underlying the Representative’s warrants at our expense with a duration of no more than five (5) years following the commencement of sales of securities issued in this offering as permitted by FINRA Rule 5110(g)(8)(C), and unlimited “piggyback” registration rights with a duration of no more than seven (7) years following the commencement of sales of securities issued in this offering as permitted by FINRA Rule 5110(g)(8)(D). The Representative’s warrants will provide for adjustment in the number and price of such warrants and the shares underlying such warrants in the event of recapitalization, merger, or other structural transaction to prevent mechanical dilution.
Over-Allotment Option
We have granted to the Representative an option, exercisable not later than 45 days after the closing date of this offering, to purchase up to 3,750,000 additional shares of common stock equal to 15% of the number of shares of common stock sold in this offering at a price per share equal to the public offering price, less the underwriting discount. The Representative may exercise the option solely to cover over-allotments, if any, made in connection with this offering. If any additional shares of common stock are purchased pursuant to the over-allotment option, the underwriters will offer these shares of common stock on the same terms as those on which the other securities are being offered hereby.
Right of First Refusal
We have agreed to grant the Representative, for a period of eighteen (18) months from the closing of this offering, whether or not the engagement with the Representative is terminated, a right of first refusal to provide investment banking services to us on an exclusive basis in all matters for which investment banking services are sought by the Company, including, without limitation, (a) acting as lead or joint-lead manager for any underwritten public offering; (b) acting as lead or joint book-runner and/or lead or joint placement agent, initial purchaser in connection with any private offering of securities of the Company; and (c) acting as financial advisor in connection with any sale or other transfer by the Company, directly or indirectly, of a majority or controlling portion of its capital stock or assets to another entity, any purchase or other transfer by another entity, directly or indirectly, of a majority or controlling portion of the capital stock or assets of the Company, and any merger or consolidation of the Company with another entity, provided, however, that such right shall be subject to FINRA Rule 5110(g). Any decision by the Representative to act in any such capacity shall be contained in separate agreements, which agreements would contain, among other matters, provisions for customary fees for transactions of similar size and nature, as may be mutually agreed upon, and indemnification of the Representative and shall be subject to general market conditions. In accordance with FINRA Rule 5110(g)(6)(A), such right of first refusal shall not have a duration of more than three years from the date of commencement of sales of the securities issued in this offering or the termination date of the engagement between us and the Representative.
Lock-Up Agreements
We have agreed that, without the prior written consent of the Representative, subject to certain exceptions, we will not, for a period of six (6) months after the date of the prospectus:
| · | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; | |
| · | file or caused to be filed any registration statement with the SEC relating to the offering of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, | |
| · | complete any offering of debt securities of the Company, other than entering into a line of credit with a traditional bank or, | |
| · | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the shares of common stock, |
| 116 |
whether any such transaction described above is to be settled by delivery of shares of common stock or such other securities, in cash or otherwise.
In addition, for a period of six months after the date of the prospectus, our directors, executive officers, and any holders of 5% or more of the outstanding shares of common stock as of the effective date of the registration statement of which this prospectus is a part and certain other shareholders, without the prior written consent of the Representative, subject to limited exceptions, not to (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any common stock or any securities convertible into or exercisable or exchangeable for common stock, or (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more underwriters participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on, or that can be accessed through, any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of, and is not incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriters, and it should not be relied upon by investors.
Pricing of this Offering
We have applied to list the common stock on the Nasdaq Capital Market under the symbol “ADVB.” We believe that upon the completion of the offering contemplated by this prospectus, we will meet the standards for listing on the Nasdaq Capital Market or other national exchange. However, there can be no assurance that the common stock will be approved for listing on the Nasdaq Capital Market. We will not consummate and close this offering without obtaining the approval from Nasdaq Capital Market. We do not intend to apply to list the Representative’s warrants on any security exchange.
The public offering price for the common stock will be determined through negotiations between us and the Representative. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the Representative believe to be comparable to us, estimate of our business potential and earning prospects, the present state of our development, and other factors deemed relevant. The offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the shares of common stock sold in the public offering. The values of such shares of common stock are subject to change as a result of market conditions and other factors. We offer no assurances that the offering price will correspond to the price at which our shares of common stock will trade in the public market subsequent to this offering or that an active trading market for our shares will develop and continue after this offering.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of shares of common stock during and after this offering, including:
| · | stabilizing transactions; | |
| · | short sales; | |
| · | purchases to cover positions created by short sales; | |
| · | imposition of penalty bids; and |
| · | syndicate covering transactions. |
| 117 |
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of shares of common stock while this offering is in progress. Stabilization transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. These transactions may also include making short sales of the common stock, which involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering and purchasing shares of common stock on the open market to cover short positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position by either exercising their option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option.
Naked short sales are short sales made in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of common stock in the open market that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the Representative has repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
These stabilizing transactions, short sales, purchases to cover positions created by short sales, the imposition of penalty bids and syndicate covering transactions may have the effect of raising or maintaining the market price of shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result of these activities, the price of our shares of common stock may be higher than the price that otherwise might exist in the open market. The underwriters may carry out these transactions on the Nasdaq Capital Market, in the over-the-counter market or otherwise. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the shares. Neither we, nor any of the underwriters make any representation that the underwriters will engage in these stabilization transactions or that any transaction, once commenced, will not be discontinued without notice.
Other Relationships
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and their affiliates may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In the ordinary course of their various business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own accounts and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of our Company. The underwriters and their respective affiliates may also make investment recommendations and/or publish or express independent research views in respect to such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who come into possession of this prospectus are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
| 118 |
Australia. This document has not been lodged with the Australian Securities & Investments Commission and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
(a) you confirm and warrant that you are either:
(i) “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act 2001 (Cth) of Australia, or the Corporations Act;
(ii) “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to the company which complies with the requirements of section 708(8)(c)(i) or
(ii) of the Corporations Act and related regulations before the offer has been made;
(iii) person associated with the company under section 708(12) of the Corporations Act; or
(iv) “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act;
and to the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act, any offer made to you under this document is void and incapable of acceptance; and
(b) you warrant and agree that you will not offer any of the Common stock issued to you pursuant to this document for resale in Australia within 12 months of those Common stock being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
Canada. The Common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted customers, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the Common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (“NI 33-105”), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Cayman Islands. This prospectus does not constitute an invitation or offer to the public in the Cayman Islands of the Common stock, whether by way of sale or subscription. The underwriters have not offered or sold, and will not offer or sell, directly or indirectly, any Common stock in the Cayman Islands.
| 119 |
European Economic Area. In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any shares which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State unless the prospectus has been approved by the competent authority in such Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that an offer to the public in that Relevant Member State of any shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| · | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| · | to any legal entity which has two or more of (i) an average of at least 250 employees during the last financial year; (ii) a total balance sheet of more than €43,000,000 and (iii) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| · | by the underwriters to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than “qualified investors” as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives for any such offer; or |
| · | in any other circumstances falling within Article 3(2) of the Prospectus Directive; provided that no such offer of shares shall result in a requirement for the publication by us or any representative of a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive. |
Any person making or intending to make any offer of shares within the EEA should only do so in circumstances in which no obligation arises for us or the underwriters to produce a prospectus for such offer. Neither we nor the underwriters have authorized, nor do they authorize, the making of any offer of shares through any financial intermediary, other than offers made by the underwriters which constitute the final offering of shares contemplated in this prospectus.
For the purposes of this provision, and your representation below, the expression an “offer to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase any shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Each person in a Relevant Member State who receives any communication in respect of, or who acquires any shares under, the offer of shares contemplated by this prospectus will be deemed to have represented, warranted and agreed to and with us and the underwriters that:
| · | it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and | |
| · | in the case of any shares acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the shares acquired by it in the offering have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than “qualified investors” (as defined in the Prospectus Directive), or in circumstances in which the prior consent of the representatives has been given to the offer or resale; or (ii) where shares have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of those shares to it is not treated under the Prospectus Directive as having been made to such persons. |
| 120 |
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Hong Kong. The Common stock may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the Common stock may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to Common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder.
Malaysia. The shares have not been and may not be approved by the securities commission Malaysia, or SC, and this document has not been and will not be registered as a prospectus with the SC under the Malaysian capital markets and services act of 2007, or CMSA. Accordingly, no securities or offer for subscription or purchase of securities or invitation to subscribe for or purchase securities are being made to any person in or from within Malaysia under this document except to persons falling within any of paragraphs 2(g)(i) to (xi) of schedule 5 of the CMSA and distributed only by a holder of a capital markets services license who carries on the business of dealing in securities and subject to the issuer having lodged this prospectus with the SC within seven days from the date of the distribution of this prospectus in Malaysia. The distribution in Malaysia of this document is subject to Malaysian laws. Save as aforementioned, no action has been taken in Malaysia under its securities laws in respect of this document. This document does not constitute and may not be used for the purpose of a public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the approval of the SC or the registration of a prospectus with the SC under the CMSA.
Japan. The Common stock have not been and will not be registered under the Financial Instruments and Exchange Law of Japan, and Common stock will not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to any exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
People’s Republic of China. This prospectus has not been and will not be circulated or distributed in the PRC, and Common stock may not be offered or sold, and will not be offered or sold to any person for re-offering or resale, directly or indirectly, to any resident of the PRC except pursuant to applicable laws and regulations of the PRC.
Singapore. This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of our Common stock may not be circulated or distributed, nor may our Common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or SFA, (ii) to a relevant person or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
| 121 |
Where our Common stock are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor as defined in Section 4A of the SFA) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor; shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the Common stock under Section 275 of the SFA, except: (1) to an institutional investor (for corporations under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, debentures and units of shares and debentures of that corporation or such rights and interest in that trust are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is or will be given for the transfer; or (3) where the transfer is by operation of law.
Taiwan The Common stock have not been and will not be registered or filed with, or approved by, the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be offered or sold in Taiwan through a public offering or in circumstances which constitute an offer within the meaning of the Securities and Exchange Act of Taiwan or relevant laws and regulations that require a registration, filing, or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer or sell the Common stock in Taiwan.
United Kingdom. An offer of the Common stock may not be made to the public in the United Kingdom within the meaning of Section 102B of the Financial Services and Markets Act 2000, as amended, or the FSMA, except to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities or otherwise in circumstances that do not require the publication by the company of a prospectus pursuant to the Prospectus Rules of the Financial Services Authority, or the FSA.
An invitation or inducement to engage in investment activity (within the meaning of Section 21 of FSMA) may only be communicated to persons who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 or in circumstances in which Section 21 of FSMA does not apply to the company.
All applicable provisions of the FSMA with respect to anything done by the underwriters in relation to the shares must be complied with in, from or otherwise involving the United Kingdom.
| 122 |
We are represented by VCL Law LLP with respect to U.S. federal and New York State law in connection with this offering. Ortoli Rosenstadt LLP has acted as counsel to the Representative. The validity of the common stock offered in this offering and legal matters as to Nevada law will be passed upon for us by Sherman & Howard LLC. Certain legal matters as to Taiwan law will be passed upon for us by Wiseteam Law Firm. Certain legal matters as to PRC law will be passed upon for us by AllBright Law Offices. VCL Law LLP may rely upon Wiseteam Law Firm with respect to matters governed by Taiwan law and upon AllBright Law Offices with respect to matters governed by PRC law.
The consolidated balance sheets of Advanced Biomed Inc. as of September 30, 2023, June 30, 20222023 and 20212022 and the related consolidated statements of operations and comprehensive income (loss), stockholders’ equity (deficit) and cash flows for the three-month periods and years then ended appearing in this registration statement of which this prospectus forms a part have been so included in reliance on the report of WWC, P.C, an independent registered public accounting firm, appearing elsewhere in this prospectus, given the authority of such firm as experts in accounting and auditing.
ENFORCEABILITY OF CIVIL LIABILITIES
We are organized under the laws of the state of Nevada. Substantially all of our assets are located outside the United States. In addition, most of our officers and directors, and officersincluding director nominees, are nationals or residents of jurisdictions other than the United States, and all or a substantial portion of their assets are located outside the United States. Specifically, our Chairman of the Board is a U.S. citizen and lives in Taiwan; Our CEO and director, CTO and CFO live in and are residents of Hong Kong, Taiwan, and mainland China, respectively. Furthermore, none of our three independent director nominees are residents of the United States. Specifically, Mengxing Tang is a Chinese citizen residing in the United Kingdom, Alfred Lee Ming Sung is an Australian citizen living in Hong Kong, and Cheang I Kei is a resident of Macau. As a result, it may be difficult for investors to effect service of process within the United States upon us or these persons, or to enforce judgments obtained in U.S. courts against us or them, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States. It may also be difficult for you to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors.
| 123 |
We have appointed Cogency Global Inc. as our agent to receive service of process in any action against us in any U.S. federal or state court arising out of this offering or any purchase or sale of securities in connection with this offering. The address of our agent is 122 East 42nd Street, 18th Floor, New York, NY 10168.
In Hong Kong, foreign judgments can be enforced under statute under the Foreign Judgments (Reciprocal Enforcement) Ordinance or under common law. The Foreign Judgments (Reciprocal Enforcement) Ordinance is a registration scheme for the recognition and enforcement of foreign judgments based on reciprocity but the United States is not a designated country under the Foreign Judgments (Reciprocal Enforcement) Ordinance. As a result, a judgment rendered by a court in the United States, including as a result of administrative actions brought by regulatory authorities, such as the SEC, and other actions, will not be enforced by the Hong Kong courts under the statutory regime. However, the common law permits an action to be brought upon a foreign judgment. That is to say, a foreign judgment itself may form the basis of a cause of action since the judgment may be regarded as creating a debt between the parties to it. In a common law action for enforcement of a foreign judgment in Hong Kong, the enforcement is subject to various conditions, including but not limited to, that the foreign judgment is a final judgment conclusive upon the merits of the claim, the judgment is for a liquidated amount in a civil matter and not in respect of taxes, fines, penalties, or similar charges, the proceedings in which the judgment was obtained were not contrary to natural justice, and the enforcement of the judgment is not contrary to public policy of Hong Kong. Such a judgment must be for a fixed sum and must also come from a “competent” court as determined by the private international law rules applied by the Hong Kong courts. The defenses that are available to a defendant in a common law action brought on the basis of a foreign judgment include lack of jurisdiction, breach of natural justice, fraud, and contrary to public policy. However, a separate legal action for debt must be commenced in Hong Kong in order to recover such debt from the judgment debtor.
AllBright Law Offices, our counsel as to Chinese law, has advised us that there is uncertainty as to whether Chinese courts would (1) recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States, or (2) entertain original actions brought in China against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States. Furthermore, AllBright Law Offices have advised us that, as of the date of this prospectus, no treaty or other form of reciprocity exists between the United States and China governing the recognition and enforcement of judgments.
AllBright Law Offices has advised us that the recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedure Law. Chinese courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedure Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. AllBright Law Offices has advised us further that under Chinese law, courts in China will not recognize or enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of Chinese law or national sovereignty, security or social public interest. As there exists no treaty or other form of reciprocity between China and the United States governing the recognition and enforcement of judgments as of the date of this prospectus, including those predicated upon the liability provisions of the United States federal securities laws, there is uncertainty whether and on what basis a Chinese court would enforce judgments rendered by United States courts.
Wiseteam Law Firm, our counsel as to Taiwan law, has informed us that any final judgment obtained against us, our directors or executive officers, or our Taiwan subsidiary in any court other than the courts of Taiwan in respect of any legal suit or proceeding will be enforced by the courts of Taiwan without further review of the merits only if the court of Taiwan in which enforcement is sought is satisfied that: (i) the court rendering the judgment had jurisdiction over the subject matter according to the laws of Taiwan; (ii) the judgment and the legal procedures resulting in the judgment were not contrary to the public order or good morals of Taiwan; (iii) if the judgment was rendered by default by the court rendering the judgment, (a) we or such persons were duly served within a reasonable time in the jurisdiction of such court in accordance with the laws and regulations of such jurisdiction or (b) process was served on us or such persons with judicial assistance of Taiwan; and (iv) judgments of the courts of Taiwan would be recognized and enforceable in the jurisdiction of the court rendering the judgment on a reciprocal basis. Moreover, Wiseteam Law Firm has advised us that a party seeking to remit money in the process of enforcing a foreign judgment in Taiwan would, except under limited circumstances, be required to obtain foreign exchange approval from the Central Bank of the Republic of China (Taiwan) for the remittance out of Taiwan of any amounts recovered in respect of such judgment denominated in a currency other than New Taiwan Dollars.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 (including the exhibits, schedules and amendments to the registration statement) under the Securities Act with respect to the shares of our common stock offered by this prospectus. This prospectus does not contain all the information set forth in the registration statement. For further information with respect to us and the shares of our common stock to be sold in this offering, we refer you to the registration statement. Statements contained in this prospectus as to the contents of any contract, agreement or other documents to which we make reference are not necessarily complete. In each instance, we refer you to the copy of such contract, agreement or other document filed as an exhibit to the registration statement.
We file annual, quarterly and current reports, and other information with the SEC. Our filings with the SEC are available to the public on the SEC’s website at http://www.sec.gov. The information we file with the SEC or contained on or accessible through our corporate web site or any other web site that we may maintain is not part of this prospectus or the registration statement of which this prospectus is a part. You may read and copy this information at the Public Reference Room of the SEC located at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room. The SEC maintains an internet site that contains periodic and current reports, information statements and other information regarding issuers that file electronically with the SEC. The address of the SEC’s website is http://www.sec.gov.
| 124 |
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
INDEX TO UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS


REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Stockholders of |
Advanced Biomed Inc.
Results of Review of Interim Financial Information
We have reviewed the accompanying unaudited interim condensed consolidated balance sheet of Advanced Biomed Inc. and its subsidiaries (collectively the “Company”) as of March 31,September 30, 2023, and the related unaudited interim condensed consolidated statements of operations and comprehensive loss, stockholders’ (deficit) equity, and cash flows for the nine-monththree-month periods ended March 31,September 30, 2023 and 2022, and the related notes (collectively referred to as the “unaudited interim condensed consolidated financial statements”). Based on our reviews, we are not aware of any material modifications that should be made to the accompanying interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the condensed consolidated balance sheet of the Company as of June 30, 2022,2023, and the related statements of operations and comprehensive loss, stockholders’ equity and cash flows for the year then ended (not presented herein); and in our report dated May 19, 2023, except for Notes 8 and 11 for which the date is June 20, 2023 and Note 12 for which the date is August 11,October 27, 2023, we expressed an unqualified opinion with an emphasis of mattera paragraph indicating that there was substantial doubt regarding a restatementthe Company’s ability to continue as a correction of error on those financial statements.going concern. In our opinion, the information set forth in the accompanying condensed consolidated balance sheet as of June 30, 2022,2023, is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has continued to incur substantial losses, and net cash used in operating activities during the three months ended September 30, 2023; accordingly, as of the date of this report, the substantial doubt that the Company will continue as a going concern that was included our audit report dated October 27, 2023 has not been alleviated and is still outstanding. Management’s plans to address this substantial doubt are set forth in Note 2. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Review Results
These interim financial statements are the responsibility of the Company’s management. We conducted our review in accordance with the standards of the PCAOB. A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with standards of the PCAOB, the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
WWC, P.C.
Certified Public Accountants
PCAOB ID No. 1171
We have served as the Company’s auditor since 2022.
San Mateo, California
May 19,November 20, 2023 except for Note 11 for which the date is August 11, 2023.

ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED BALANCE SHEETS
| As of March 31, | As of June 30, | As of September 30, | As of June 30, | |||||||||||||
| 2023 | 2022 | 2023 | 2023 | |||||||||||||
| US$ | US$ | US$ | US$ | |||||||||||||
| (Unaudited) | (as restated) | (Unaudited) | ||||||||||||||
| Assets | ||||||||||||||||
| Current assets: | ||||||||||||||||
| Cash | 2,529,498 | 4,783,864 | 2,587,218 | 2,622,279 | ||||||||||||
| Prepaid expenses and other current assets, net | 457,302 | 221,847 | 194,090 | 163,709 | ||||||||||||
| Total current assets | 2,986,800 | 5,005,711 | 2,781,308 | 2,785,988 | ||||||||||||
| Equipment, net | 696,611 | 783,073 | 663,683 | 718,697 | ||||||||||||
| Right-of-use assets, net | 300,967 | 442,895 | 180,024 | 235,373 | ||||||||||||
| Intangible assets, net | 299,016 | 410,799 | 216,026 | 250,321 | ||||||||||||
| Deferred initial public offering (“IPO”) costs | 553,471 | 553,471 | ||||||||||||||
| Other non-current assets | 133,389 | 71,021 | 54,212 | 56,127 | ||||||||||||
| Total non-current assets | 1,429,983 | 1,707,788 | 1,667,416 | 1,813,989 | ||||||||||||
| TOTAL ASSETS | 4,416,783 | 6,713,499 | 4,448,724 | 4,599,977 | ||||||||||||
| Liabilities | ||||||||||||||||
| Current liabilities: | ||||||||||||||||
| Accounts payable, accruals, and other current liabilities | 1,732,426 | 2,753,035 | ||||||||||||||
| Accounts payable, accruals, and other current liabilities (including amount due to related parties of $1,969,685 as of September 30, 2023 and $1,656,479 as of June 30, 2023 (1)) | 3,463,556 | 3,059,016 | ||||||||||||||
| Lease payable - current | 228,370 | 236,677 | 179,544 | 216,567 | ||||||||||||
| Total current liabilities | 1,960,796 | 2,989,712 | 3,643,100 | 3,275,583 | ||||||||||||
| Lease payable – non-current | 70,849 | 232,191 | 480 | 18,806 | ||||||||||||
| Total non-current liabilities | 70,849 | 232,191 | 480 | 18,806 | ||||||||||||
| TOTAL LIABILITIES | 2,031,645 | 3,221,903 | 3,643,580 | 3,294,389 | ||||||||||||
| Commitments and contingencies | - | - | - | - | ||||||||||||
| Stockholders’ equity | ||||||||||||||||
| Common stock $0.00025 par value per share; as of March 31, 2023 and June 30, 2022; 500,000,000 and 500,000,000 shares authorized; 100,000,000 and 99,000,000 shares issued and outstanding, respectively* | 25,000 | 24,750 | ||||||||||||||
| Common stock $0.00025 par value per share; as of September 30, 2023 and June 30, 2023; 500,000,000 and 500,000,000 shares authorized; 100,000,000 and 100,000,000 shares issued and outstanding, respectively* | 100,000 | 100,000 | ||||||||||||||
| Additional paid-in capital | 13,656,757 | 12,657,007 | 13,593,365 | 13,593,365 | ||||||||||||
| Accumulated deficits | (11,511,225 | ) | (9,459,020 | ) | (13,963,113 | ) | (13,191,797 | ) | ||||||||
| Accumulated other comprehensive income | 214,606 | 268,859 | 1,074,892 | 804,020 | ||||||||||||
| Total stockholders’ equity | 2,385,138 | 3,491,596 | 805,144 | 1,305,588 | ||||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | 4,416,783 | 6,713,499 | 4,448,724 | 4,599,977 | ||||||||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
| (1) | See Note 8 for disclosure of related party amounts. |
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the three-month periods ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (169,185 | ) | (116,605 | ) | ||||
| General and administrative expenses | (271,060 | ) | (292,014 | ) | ||||
| Total operating expenses | (440,245 | ) | (408,619 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 14 | 443 | ||||||
| Other expense, net | (331,085 | ) | (740,538 | ) | ||||
| Total other expense, net | (331,071 | ) | (740,095 | ) | ||||
| Income before tax expense | (771,316 | ) | (1,148,714 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | (771,316 | ) | (1,148,714 | ) | ||||
| Other comprehensive income | ||||||||
| Foreign currency translation gain, net of taxes | 270,872 | 354,389 | ||||||
| Total comprehensive loss | (500,444 | ) | (794,325 | ) | ||||
| Loss per share: | ||||||||
| basic and diluted* | (0.03 | ) | (0.05 | ) | ||||
| Weighted average number of shares of common stock in computing net loss per share | ||||||||
| basic and diluted* | 25,000,000 | 24,791,667 | ||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
UNAUDITED INTERIM CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Common stock | ||||||||||||||||||||||||
No. of shares* | Amount | Additional paid-in capital | Accumulated other comprehensive income | Accumulated deficit |
Total | |||||||||||||||||||
| US$ | US$ | US$ | US$ | US$ | ||||||||||||||||||||
| Balance as of July 1, 2022 | 99,000,000 | 99,000 | 12,582,757 | 268,859 | (9,459,020 | ) | 3,491,596 | |||||||||||||||||
| Net loss | - | - | - | - | (1,148,714 | ) | (1,148,714 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 354,389 | - | 354,389 | ||||||||||||||||||
| Issuance of shares for cash | 1,000,000 | 1,000 | 1,010,608 | - | - | 1,011,608 | ||||||||||||||||||
| Balance as of September 30, 2022 | 100,000,000 | 100,000 | 13,593,365 | 623,248 | (10,607,734 | ) | 3,708,879 | |||||||||||||||||
| Balance as of July 1, 2023 | 100,000,000 | 100,000 | 13,593,365 | 804,020 | (13,191,797 | ) | 1,305,588 | |||||||||||||||||
| Net loss | - | - | - | - | (771,316 | ) | (771,316 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 270,872 | - | 270,872 | ||||||||||||||||||
| Issuance of shares for cash | - | - | - | - | - | - | ||||||||||||||||||
| Balance as of September 30, 2023 | 100,000,000 | 100,000 | 13,593,365 | 1,074,892 | (13,963,113 | ) | 805,144 | |||||||||||||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSSCASH FLOWS
| For the nine-month periods ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
(Unaudited) | (Unaudited) | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | (895,011 | ) | (1,045,243 | ) | ||||
| General and administrative expenses | (1,157,047 | ) | (246,606 | ) | ||||
| Total operating expenses | (2,052,058 | ) | (1,291,849 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 122 | 301 | ||||||
| Other expense, net | (269 | ) | (37,300 | ) | ||||
| Total other expense, net | (147 | ) | (36,999 | ) | ||||
| Income before tax expense | ||||||||
| Income tax expense | - | - | ||||||
| Net loss | (2,052,205 | ) | (1,328,848 | ) | ||||
| Other comprehensive income | ||||||||
| Foreign currency translation gain/ (loss), net of taxes | (54,253 | ) | 241,661 | |||||
| Total comprehensive loss | (2,106,458 | ) | (1,087,187 | ) | ||||
| Loss per share: | ||||||||
| basic and diluted* | (0.02 | ) | (0.02 | ) | ||||
| Weighted average number of shares of common stock in computing net loss per share | ||||||||
| basic and diluted* | 99,722,224 | 58,885,468 | ||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
For the three-month period ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Net Loss | (771,316 | ) | (1,148,714 | ) | ||||
| Adjustment: | ||||||||
| Depreciation and amortization | 89,309 | 164,508 | ||||||
| Interest Income | (14 | ) | (443 | ) | ||||
| Changes in operating assets: | ||||||||
| (Increase) decrease of prepaid expenses and other current assets | (30,379 | ) | (169,316 | ) | ||||
| Increase of accounts payable, accruals and other current liabilities | 91,333 | (944,177 | ) | |||||
| Lease obligations net cash | - | (27,660 | ) | |||||
| Other non-current assets | 1,915 | 21,097 | ||||||
| Interest received | 14 | 443 | ||||||
| Cash used in operating activities | (619,138 | ) | (2,104,262 | ) | ||||
| Amount due to related parties – related corporations | 313,206 | - | ||||||
| Payment of deferred initial public offering costs | - | (521,471 | ) | |||||
| Proceeds from issuance of shares | - | 1,011,608 | ||||||
| Cash provided by financing activities | 313,206 | 490,137 | ||||||
| Foreign currency effect | 270,871 | 354,390 | ||||||
| Net change in cash and cash equivalents | (35,061 | ) | (1,259,735 | ) | ||||
| Cash and cash equivalents as of beginning of the period | 2,622,279 | 4,783,864 | ||||||
| Cash and cash equivalents as of the end of the period | 2,587,218 | 3,524,129 | ||||||
| Net decrease in cash and cash equivalents | (35,061 | ) | (1,259,735 | ) | ||||
| Supplementary Cash Flow Information: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
| Common stock | ||||||||||||||||||||||||
| No. of shares* | Amount | Additional paid-in capital | Accumulated other comprehensive income | Accumulated deficit |
Total | |||||||||||||||||||
| US$ | US$ | US$ | US$ | US$ | ||||||||||||||||||||
| Balance as of July 1, 2021 | 58,885,468 | 14,721 | 6,569,905 | (177,086 | ) | (5,431,298 | ) | 976,242 | ||||||||||||||||
| Net loss | - | - | - | - | (1,328,848 | ) | (1,328,848 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 241,661 | - | 241,661 | ||||||||||||||||||
| Balance as of March 31, 2022 | 58,885,468 | 14,721 | 6,569,905 | 64,575 | (6,760,146 | ) | (110,945 | ) | ||||||||||||||||
| Balance as of July 1, 2022 | 99,000,000 | 24,750 | 12,657,007 | 268,859 | (9,459,020 | ) | 3,491,596 | |||||||||||||||||
| Net loss | - | - | - | (2,052,205 | ) | (2,052,205 | ) | |||||||||||||||||
| Foreign currency translation adjustment | - | - | - | (54,253 | ) | - | (54,253 | ) | ||||||||||||||||
| Issuance of shares for cash | 1,000,000 | 250 | 999,750 | - | - | 1,000,000 | ||||||||||||||||||
| Balance as of March 31, 2023 | 100,000,000 | 25,000 | 13,656,757 | 214,606 | (11,511,225 | ) | 2,385,138 | |||||||||||||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the nine-month periods ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| US $ | US $ | |||||||
| (unaudited) | (unaudited) | |||||||
| Net loss | (2,052,205 | ) | (1,328,848 | ) | ||||
| Adjustments: | ||||||||
| Depreciation and amortization | 198,245 | 12,248 | ||||||
| Changes in operating assets: | ||||||||
| (Increase)/decrease of prepaid expenses and other current assets, net | (235,455 | ) | 49,080 | |||||
| (Decrease)/increase of accounts payable, accruals and other current liabilities | (1,020,609 | ) | 2,056,401 | |||||
| Lease obligations net cash | (27,721 | ) | 67 | |||||
| Cash (used in)/provided by operating activities | (3,137,745 | ) | 788,948 | |||||
| Payments for security deposits and other non-current assets | (62,368 | ) | (205,215 | ) | ||||
| Purchase of property, plant and equipment | - | (602,220 | ) | |||||
| Cash used in investing activities | (62,368 | ) | (807,435 | ) | ||||
| Proceeds from issuance of shares | 1,000,000 | - | ||||||
| Cash provided by financing activities | 1,000,000 | - | ||||||
| Foreign currency effect | (54,253 | ) | 241,661 | |||||
| Net change in cash and cash equivalents | (2,254,366 | ) | 223,174 | |||||
| Cash and cash equivalents as of beginning of the year | 4,783,864 | 65,922 | ||||||
| Cash and cash equivalents as of the end of the year | 2,529,498 | 289,096 | ||||||
| Net (decrease) increase in cash and cash equivalents | (2,254,366 | ) | 223,174 | |||||
| Supplementary Cash Flow Information: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
The accompanying notes are an integral part of these unaudited interim condensed consolidated financial statements.
ADVANCED BIOMED INC. AND ITS SUBSIDIARIES
(UNAUDITED)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
On July 16, 2021, Advanced Biomed Inc. (the “Company”) was incorporated in the State of Nevada, as an investment holding company. The Company’s principal executive offices are located in Tainan City, Taiwan. The Company has no substantive operations and assets. It is holding Company that holds all of the issued and outstanding shares of Advanced Biomed HK Limited and Advanced Biomed Inc. (Taiwan).
| (1) | Establishment of Advanced Biomed HK Limited |
On August 10, 2021, the Company incorporated a wholly owned subsidiary, Advanced Biomed HK Limited, in Hong Kong to facilitate market development and commercialization of the Company’s oncology products for sale and distribution in the People’s Republic of China (the “PRC”).
| (2) | Acquisition of Shanghai Sglcell Biotech Co., Ltd. and its subsidiaries |
On January 1, 2022, Advanced Biomed HK Limited acquired 100% equity interest of Shanghai Sglcell Biotech Co., Ltd. Shanghai Sglcell Biotech Co., Ltd. was established in the PRC on April 12, 2019. It is engaged in the establishment and operation of medical clinics in the PRC.
Shanghai Sglcell Biotech Co., Ltd. owns 100% equity interest of two subsidiaries namely 1.) Shandong Sglcell Medical Devices Co., Ltd. and 2.) Nanjing Yitian Biotech Co., Ltd. Shandong Sglcell Medical Devices Co., Ltd. was incorporated in the PRC on July 8, 2021 to carry out the establishment of medical clinics, and the supply of medical products and services to clinics in the PRC. Nanjing Yitian Biotech Co., Ltd. was established in the PRC on January 6, 2017. Nanjing Yitian Biotech Co., Ltd. wholly owns a subsidiary, Beijing Yitan Jiarui Technology Co. Ltd., which was established in the PRC on October 20, 2017. Both were established to carry out marketing and clinical services in the PRC.
On June 8, 2023, Shanghai Sglcell Biotech Co., Ltd. transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to independent third-party individuals at aggregate consideration of CNY500,000 (approximately US$71,942) without any other obligations arising from the transfer. Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd. have not commenced business activity since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., was CNY498,587 (approximately US$71,739).
On June 9, 2023, Shandong Sglcell Biotech Co., Ltd., the wholly owned subsidiary of Shanghai Sglcell Biotech Co., Ltd., was transferred to independent third-party individuals at zero consideration without any other obligations arising from the transfer. Shandong Sglcell Biotech Co., Ltd. has been inactive since it was incorporated on July 8, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
| (3) | Subsidiary established under Advanced Biomed HK Limited |
Advanced Biomed HK Limited incorporated a wholly owned subsidiary, Sglcell (Huangshan) Biotech Co., Ltd., in the PRC on March 4, 2022; it was established for the expected future manufacturing of medical devices in the PRC.
On June 15, 2023, Sglcell (Huangshan) Biotech Co., Ltd., the wholly owned subsidiary of Advanced Biomed HK Limited, was transferred to an independent third-party corporation at zero consideration without any other obligations arising from the transfers. Sglcell (Huangshan) Biotech Co., Ltd. has been dormant since its incorporation date from March 4, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
| (4) | Reorganization of Advanced Biomed Inc. (Taiwan) |
Advanced Biomed Inc. (Taiwan) was established in Taiwan on September 1, 2014. It is primarily focused on mainly operates as a research and development of new center for technologies in the field of oncology to help efficiently and cost-effectively identify and diagnose cancer cells.
On date of incorporation, July 16, 2021, the Company issued 8,000,000 shares to Dr. Hung To Pau. On March 15, 2022, Dr. Hung To Pau transferred all of his 8,000,000 shares to Sglcell Ltd, an exempted company incorporated under the law of Cayman Islands, the sole shareholder of which is Dr. Hung To Pau for a total consideration of $8,000. The Company was dormant and has no substantive assets. On June 8, 2022, Sglcell Ltd transferred all of its 8,000,000 shares to Dr. Yi Lu for a total consideration of $8,000. In July 2022, the Company consummated a reorganization of Advanced Biomed Inc. (Taiwan) under share exchange arrangement of its then existing shareholders, who collectively owned all the equity interests of Advanced Biomed Inc. (Taiwan) prior to the reorganization, transferred their respective shares in Advanced Biomed Inc. (Taiwan) to the Company. Prior to the re-organization, Advanced Biomed Inc. (Taiwan) was directly owned and controlled by Dr. Yi Lu and Chen-Yi Lee with 99.93% and 0.07% beneficial ownership interest, respectively.
The Share Exchange
Pursuant to a share exchange agreement (the “Agreement”) dated July 11, 2022, Dr. Yi Lu and Chen-Yi Lee transferred their respective shares in Advanced Biomed Inc. (Taiwan) at the time of the Agreement, representing in aggregate 100% of the issued share capital of Advanced Biomed Inc. (Taiwan), to the Company. The consideration for the share transfers was satisfied by the allotment and issuance of an aggregate of 385,257 fully paid up shares of common stock to Dr. Yi Lu and Chen-Yi Lee. Following the completion of the share exchange and related issuances by and among the Company, Dr. Yi Lu and Chen-Yi Lee, Advanced Biomed Inc. (Taiwan) ultimately became a wholly-owned subsidiary of the Company, and Dr. Yi Lu and Chen-Yi Lee became the beneficial owners of the Company with percentage ownerships of 99.99% and 0.01% as of August 12, 2022.
Subsequently, on October 24, 2022, the Company issued 365,352 shares to Chen-Yi Lee, and on October 24, 2022, the Company also issued 2,730,000 shares to Advance On Ventures Limited, a company incorporated under the law of British Virgin Islands (the “Ventures Limited”), the beneficial owners of which are employees of Advanced Biomed Taiwan. The Company issued 4,405,625 shares, 2,193,750 shares, 2,060,000 shares, 1,511,250 shares, 1,243,750 shares, 1,230,000 shares respectively to Dr. Hung To Pau, Yimin Jin, Xiaoyuan Luo, Nanzhen Shen, Jian Wang and Qiang Chen pursuant to the Debt-For-Equity Exchange Agreement the Company entered into with the abovementioned shareholders on June 30, 2022 to settle debt of a total amount of NTD 174,020,033 and RMB 22,200,000 (approximately $9.04 million). On October 25, 2022, the Company issued 625,000 shares to Hanyu Assets Co. Ltd. pursuant to the Investment Agreement the Company entered into with Hanyu Assets Co. Ltd. on June 6, 2022, and the Company issued 250,000 shares to Newlink Technology Inc. pursuant to the Investment Agreement the Company entered into with Newlink Technology Inc. on June 6, 2022.
Upon completion of the reorganization, Dr. Yi Lu’s percentage of ownerships of the Company became 33.54% as of October 25, 2022.
On November 7, 2022, the Company obtained the approval of the Investment Commission of the Ministry of Economic Affairs (“Taiwan Investment Commission”) for the reorganization, and the issuance number of which is “經審一字第11100116890號”. Additionally, the Bureau of Economic Development of Tainan City Government has also approved the reorganization in accordance with the Taiwan Company Act on December 26, 2022.
Pursuant to this reorganization, the Company determined that Advanced Biomed Inc. (Taiwan) is the predecessor entity as the Company is an investment holding company with no business activities carried out and all of the business of Advanced Biomed Inc. (Taiwan) acquired formed substantially all of the business of the Company under Rule 405 of Regulation C. To reflect the real economic substance of the Company’s business under the reorganization, the accompanying unaudited interim consolidated financial statements were prepared assuming that the share exchange transaction, as disclosed above has been completed, and the Company exercises control of Advanced Biomed Inc. (Taiwan). The transaction detailed above has been accounted for as reverse takeover and recapitalization of the Company; whereby the Company (the legal acquirer) is considered the accounting acquiree, and Advanced Biomed Inc. (Taiwan)(the legal acquiree) is considered as the accounting acquirer. This transaction is deemed to be a continuation of the business of Advanced Biomed Inc. (Taiwan); therefore, no goodwill has been recorded for this transaction, and the Company’s historical financial information prior to the date of the recapitalization transaction is that of Advanced Biomed Inc. (Taiwan) and historical changes in shareholders’ deficit and its results of operations have been presented from the beginning of the first period presented.
The Company and its subsidiaries are in the table as follows:
| Percentage of effective ownership | ||||||||||||||||||||||||||||||||||||
| Name | Date of Incorporation | March 31, 2023 | June 30, 2022 | Place of incorporation | Principal Activities | Date of Incorporation | September 30, 2023 | June 30, 2023 | Place of incorporation | Principal Activities | ||||||||||||||||||||||||||
| Advanced Biomed Inc. | July 16, 2021 | - | - | Nevada | Investment holding | July 16, 2021 | - | - | Nevada | Investment holding | ||||||||||||||||||||||||||
| Advanced Biomed Inc. (Taiwan) | September 1, 2014 | 100 | % | 100 | % | Taiwan | Research and development of various advanced and innovative microfluidic biochip technologies, and provide the leading application of such technologies in precision oncology detection, diagnosis and treatment | September 1, 2014 | 100 | % | 100 | % | Taiwan | Research and development of various advanced and innovative microfluidic biochip technologies, and provide the leading application of such technologies in precision oncology detection, diagnosis and treatment | ||||||||||||||||||||||
| Advanced Biomed HK Limited | August 10, 2021 | 100 | % | 100 | % | Hong Kong | Market development | August 10, 2021 | 100 | % | 100 | % | Hong Kong | Market development | ||||||||||||||||||||||
| Shanghai Sglcell Biotech Co., Ltd. | April 12, 2019 | 100 | % | 100 | % | People’s Republic of China | Clinical-related business | April 12, 2019 | 100 | % | 100 | % | People’s Republic of China | Clinical-related business | ||||||||||||||||||||||
| Nanjing Yitian Biotech Co., Ltd. | January 6, 2017 | 100 | % | 100 | % | People’s Republic of China | Marketing and clinical-related business | |||||||||||||||||||||||||||||
| Beijing Yitan Jiarui Technology Co., Ltd. | October 20, 2017 | 100 | % | 100 | % | People’s Republic of China | Marketing and clinical-related business | |||||||||||||||||||||||||||||
| Shandong Sglcell Medical Devices Co., Ltd. | July 8, 2021 | 100 | % | 100 | % | People’s Republic of China | Marketing and clinical-related business | |||||||||||||||||||||||||||||
| Sglcell (Huangshan) Biotech Co., Ltd. | March 4, 2022 | 100 | % | 100 | % | People’s Republic of China | Medical devices manufacture | |||||||||||||||||||||||||||||
The accompanying unaudited interim condensed consolidated financial statements are presented assuming that the Company was in existence at the beginning of the first period presented.
2. LIQUIDITY AND GOING CONCERN
The accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP which contemplates continuation of the Company on a going concern basis. The going concern basis assumes that assets are realized, and liabilities are settled in the ordinary course of business at amounts disclosed in the consolidated financial statements. The Company’s ability to continue as a going concern depends upon its ability to develop, register and obtain regulatory approval for commercial sell of its products to generate positive operating cash flows. For the three-month period ended September 30, 2023, the Company reported net loss of $771,316. As of September 30, 2023, the Company’s working capital deficit was $861,792. In addition, the Company had net cash outflows of $619,138 from operating activities for the three-month period ended September 30, 2023. These conditions give rise to substantial doubt as to whether the Company will be able to continue as a going concern.
To sustain its ability to support the Company’s operating activities, the Company may have to consider supplementing its available sources of funds through the following sources:
| cash generated from upcoming operations; and | ||
| ● | financial support from the Company’s related party and shareholders as well as third parties. |
Management has commenced a strategy to raise debt and equity. However, there can be no certainty that these additional financings will be available on acceptable terms or at all. If management is unable to execute this plan, there would likely be a material adverse effect on the Company’s business. All of these factors raise substantial doubt about the ability of the Company to continue as a going concern. The unaudited interim condensed consolidated financial statements for the three-month period ended September 30, 2023 and 2022 have been prepared on a going concern basis and do not include any adjustments to reflect the possible future effects on the recoverability and classifications of assets or the amounts and classifications of liabilities that may result from the inability of the Company to continue as a going concern.
2.3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of presentation
The unaudited interim condensed consolidated financial statements do not include all the information and footnotes required by the U.S. GAAP for complete financial statements. Certain information and note disclosures normally included in the annual financial statements prepared in accordance with the U.S. GAAP have been condensed or omitted consistent with Article 10 of Regulation S-X. In the opinion of the Company’s management, the unaudited interim condensed consolidated financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, in normal recurring nature, as necessary for the fair statement of the Company’s financial position as of March 31,September 30, 2023, and results of operations and cash flows for the nine-monththree-month periods ended March 31,September 30, 2023 and 2022. The unaudited interim condensed consolidated balance sheet as of March 31,September 30, 2023 has been derived from the audited financial statements at that date but does not include all the information and footnotes required by the U.S. GAAP. Interim results of operations are not necessarily indicative of the results expected for the full fiscal year or for any future period. These financial statements should be read in conjunction with the audited consolidated financial statements as of and for the years ended June 30, 20222023 and 2021,2022, and related notes included in the Company’s audited consolidated financial statements.
(b) Consolidation
The unaudited interim condensed consolidated financial statements include the financial statements of the Company and its subsidiaries. All inter-company transactions, if any, and balances due to, due from, long-term investment subsidiary, and registered paid in capital have been eliminated upon consolidation.
Business combinations are accounted for using the acquisition method of accounting. Under the acquisition method, assets acquired, and liabilities assumed are recorded at their respective fair values as of the acquisition date in the Company’s unaudited interim condensed consolidated financial statements. Any excess fair value of consideration transferred over the fair value of the net assets acquired is recorded as goodwill. Contingent consideration obligations incurred in connection with the business combination are recorded at their fair values on the acquisition date and remeasured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded in the consolidated statements of operations.
When the Company determines that assets acquired do not meet the definition of a business under the acquisition method of accounting, acquired assets is expensed, no goodwill is recorded, and any contingent consideration is recognized only when it becomes payable or is paid.
(c) Use of estimates
The preparation of unaudited interim condensed financial statements in conformity with accounting principles generally accepted in the U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited interim condensed financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant estimates relate to useful lives for property, plant and equipment and intangible assets, fair value of financial instruments, assumptions used in assessing right of use assets, impairment of long-lived assets, property, plant and equipment, intangible assets and uncertain tax position. Actual results could vary from the estimates and assumptions that were used.
(d) Stock split
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now have 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis similar to share split or dividend pursuant to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split. The shares of common stock retain a par value of $0.001 per share. Accordingly, an amount equal to the par value of the increased shares resulting from the stock split was reclassified from “Additional paid-in capital” to “Common stock”.
(e) Risks and uncertainties
The main operations of the Company are located in Taiwan and mainland China. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by political, economic, and legal environments in Taiwan and mainland China, as well as by the general state of the economy in these two countries. The Company’s results may be adversely affected by changes in the political, regulatory and social conditions in these two countries.
In March 2020, the World Health Organization declared the outbreak of COVID-19 to be a pandemic. The COVID-19 pandemic is having widespread, rapidly evolving, and unpredictable impacts on global society, economies, financial markets, and business practices. During 2021, there was a wide distribution of several vaccinations and medicines to overcome the pandemic. The Company has shifted its operations to co-exist along with the pandemic, including encouragement of vaccinations to all of its employees worldwide.
The uncertainty to which the COVID-19 pandemic impacts the Company’s business, affects management’s judgment and assumptions relating to accounting estimates in a variety of areas that depend on these estimates and assumptions. COVID-19 did not have a material influence on these estimates and judgements since the Company do not have any products approved for sale, we have not generated any revenue from the sale of products, and we do not expect to generate revenue from the sale of our product candidates until we complete clinical development, submit regulatory filings and receive approvals from the applicable regulatory bodies for such product candidates, if ever.
The Company continues to face relative uncertainty as to the remaining intensity and duration of and the nature and timeline for recovery from the COVID-19 pandemic going forward and how all of that impacts the Company, including the extent to which potentially permanent changes clinical trial operations have been caused by the pandemic. The Company has taken the approach of managing the pandemic (to the extent that it continues to remain a significant factor) via strengthening its balance sheet and cash assets and avoiding debt while focusing on cost controls.
The Company’s business, financial condition and results of operations may also be negatively impacted by risks related to natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt the Company’s operations.
(e)(f) Foreign currency translation and transaction and convenience translation
The accompanying unaudited interim condensed consolidated financial statements are presented in the US Dollar (“US$”), which is the reporting currency of the Company. The functional currency of the Company is the US$. Advanced Biomed Inc. (Taiwan) use New Taiwan dollar (“NT$”) as its functional currency. Advanced Biomed HK Limited uses Hong Kong dollars (“HKD”) and Shanghai Sglcell Biotech Co., Ltd., Nanjing Yitian Biotech Co., Ltd., Beijing Yitan Jiarui Technology Co., Ltd., Shandong Sglcell Medical Devices Co., Ltd., Sglcell (Huangshan) Biotech Co., Ltd use Chinese Yuan Renminbi (“CNY”) as their functional currencies.
Assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Translation gains and losses are recognized in the unaudited interim condensed consolidated statements of income and comprehensive income as other comprehensive income or loss. Transactions in currencies other than the reporting currency are measured and recorded in the reporting currency at the exchange rate prevailing on the transaction date. The cumulative gain or loss from foreign currency transactions is reflected in the unaudited interim condensed consolidated statements of income and comprehensive income as other income (other expenses).
The value of foreign currencies including, the NT$ and CNY, may fluctuate against the US Dollar. Any significant variations of the aforementioned currencies relative to the US Dollar may materially affect the Company’s financial condition in terms of reporting in US Dollar. The following table outlines the currency exchange rates that were used in preparing the accompanying consolidated financial statements:
March 31, 2023 | June 30, 2022 | |||||||
| US$ to NT$ fiscal year end | 30.48 | 29.74 | ||||||
| US$ to NT$ average rate | 30.71 | 28.29 | ||||||
| US$ to CNY fiscal year end | 6.87 | 6.70 | ||||||
| US$ to CNY average rate | 6.93 | 6.46 | ||||||
September 30,2023 | June 30,2023 | |||||||
| US$ to NT$ fiscal year end | 32.24 | 31.14 | ||||||
| US$ to NT$ average rate | 31.70 | 30.71 | ||||||
| US$ to CNY fiscal year end | 7.30 | 7.25 | ||||||
| US$ to CNY average rate | 7.24 | 6.95 | ||||||
(f)(g) Fair value measurement
Accounting guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact, and it considers assumptions that market participants would use when pricing the asset or liability.
Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Accounting guidance establishes three levels of inputs that may be used to measure fair value:
| ● | Level 1 applies to assets or liabilities for which there are quoted prices, in active markets for identical assets or liabilities. |
| ● | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included within Level 1 that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical asset or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. | |
| ● | Level 3 applies to asset or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
Cash and cash equivalents, other current assets, leases payable, accounts payables, accruals and other current liabilities are financial assets and liabilities. Cash and cash equivalents, other current assets, accounts payable, accruals and other current liabilities are subject to fair value measurement; however, because of their being short term in nature management believes their carrying values approximate their fair value. Financial instruments are fair value financial assets that are marked to fair value and are accounted for under as Level 3 under the above hierarchy. The Company accounts for bank loans and lease payables at amortized cost and has elected NOT to account for them under the fair value hierarchy.
(g)(h) Related parties
We adopted ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions
(h)(i) Cash
Cash consists of cash on hand, the Company’s demand deposit placed with financial institutions, which have original maturities of less than three months and unrestricted as to withdrawal and use. Deposits are held at highly liquid and well capitalized financial institutions. Risk of loss is not expected by management.
(i)(j) Intangible assets, net
The Company’s intangible assets are stated at cost less accumulated amortization and impairment, if any, and amortized on a straight-line basis over the estimated useful lives of the assets.
| Category | Estimated useful lives | |
| Software | 3 years | |
| Patents | 6 years |
Patents represent the estimated fair value assigned to finite-lived intangible assets acquired in a transaction that is accounted for as an acquisition of assets rather than a business combination are initially recognized in accordance with other application GAAP. Any consideration transferred in excess of the fair value of the assets acquired is allocated to each asset acquired on a relative fair value basis. Amortization is computed using the straight-line method over the estimated useful lives of the respective finite-lived intangible assets, generally six years. Intangible assets are reviewed for impairment at least annually or more frequently if indicators of potential impairment exist. The Company reviews finite-lived intangible assets for impairment at least annually or more frequently if events or changes in circumstances indicate that the carrying value of the assets might not be recoverable. If the carrying value of an finite-lived intangible asset exceeds its fair value, then it is written down to its adjusted fair value. As of September 30, 2023 and June 30, 2023, the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell Biotech Co., Ltd. Impairment allowance made was approximately US$1.6 million for the years ended September 30, 2023 and June 30, 2023, respectively, as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
(j)(k) Property, plant and equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation and impairment, if any, and depreciated on a straight-line basis over the estimated useful lives of the assets. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its intended use. Estimated useful lives are as follows:
| Category | Estimated useful lives | |
| Lab equipment | 3 to 5 years | |
| Computer equipment | 3 years | |
| Furniture and fixtures | 5 years | |
| Leasehold improvements | 3 years |
Expenditure for repair and maintenance costs, which do not materially extend the useful lives of the assets, are charged to expenses as incurred, whereas the expenditure for major renewals and betterment that substantially extends the useful lives of property and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the costs, accumulated depreciation and impairment with any resulting gain or loss recognized in the unaudited interim condensed consolidated statements of operations and comprehensive loss.
(k)(l) Impairment of long-lived assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Company measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Company would recognize an impairment loss, which is the excess of carrying amount over the fair value of the assets, using the expected future discounted cash flows. No impairment of long-lived assets was recognized as of March 31, 2023 and June 30, 2022.
(l)(m) Commitments and contingencies
In the normal course of business, the Company is subject to commitments and contingencies, including operating lease commitments, legal proceedings and claims arising out of its business that relate to a wide range of matters, such as government investigations and tax matters. The Company recognizes a liability for such contingency if it determines it is probable that a loss will occur, and a reasonable estimate of the loss can be made. The Company may consider many factors in making these assessments on liability for contingencies, including historical and the specific facts and circumstances of each matter.
(m)(n) Research and development expenses
Research and development expenses include costs directly attributable to the conduct of research and development programs, including licensing fees, cost of salaries, share-based compensation expenses, payroll taxes and other employee benefits, subcontractors and materials used for research and development activities, including clinical trials, manufacturing costs and professional services. All costs associated with research and developments are expensed as incurred.
(n)(o) General and administrative expenses
General and administrative expenses mainly consist of staff cost, depreciation, office supplies and upkeep expenses, travelling and entertainment, legal and professional fees, property and related expenses, other miscellaneous administrative expenses.
(o)(p) Operating leases
Prior to the adoption of ASC 842 on January 1, 2019:
Leases, mainly leases of factory buildings, offices and employee dormitories, where substantially all the rewards and risks of ownership of assets remain with the lessor are accounted for as operating leases. Payments made under operating leases are recognized as an expense on a straight-line basis over the lease term. The Company had no finance leases for any of the periods stated herein.
Upon and hereafter the adoption of ASC 842 on January 1, 2019:
The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liability, and operating lease liability, non-current in the Company’s consolidated balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. When determining the lease term, the Company includes options to extend or terminate the lease when it is reasonably certain that it will exercise that option, if any. As the Company’s leases do not provide an implicit rate, the Company used an incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company has elected to adopt the following lease policies in conjunction with the adoption of ASU 2016-02: (i) for leases that have lease terms of 12 months or less and does not include a purchase option that is reasonably certain to exercise, the Company elected not to apply ASC 842 recognition requirements; and (ii) the Company elected to apply the package of practical expedients for existing arrangements entered into prior to January 1, 2019 to not reassess (a) whether an arrangement is or contains a lease, (b) the lease classification applied to existing leases, and (c) initial direct costs.
(p)(q) Loss per share
Loss per share is computed by dividing net earnings attributable to ordinary shareholders by the weighted average number of common stock outstanding during the year. Diluted earnings per share reflect the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock.
(q)(r) Recent accounting pronouncements
In May 2020, the Financial Accounting Standard Board (“FASB”) issued ASU 2020-05, which is an update to ASU Update No. 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments”, which introduced the expected credit losses methodology for the measurement of credit losses on financial assets measured at amortized cost basis, replacing the previous incurred loss methodology. The amendments in Update 2016-13 added Topic 326, Financial Instruments — Credit Losses, and made several consequential amendments to the Codification. Update 2016-13 also modified the accounting for available-for-sale debt securities, which must be individually assessed for credit losses when fair value is less than the amortized cost basis, in accordance with Subtopic 326-30, Financial Instruments — Credit Losses — Available-for-Sale Debt Securities. The amendments in this Update address those stakeholders’ concerns by providing an option to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost basis. For those entities, the targeted transition relief will increase comparability of financial statement information by providing an option to align measurement methodologies for similar financial assets. Furthermore, the targeted transition relief also may reduce the costs for some entities to comply with the amendments in Update 2016-13 while still providing financial statement users with decision-useful information. In November 2020, the FASB issued ASU No. 2020-10, which to update the effective date of ASU No. 2016-02 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. The Company is currently evaluating the impact of this new standard on Company’s unaudited interim condensed consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-08, Codification Improvements to Subtopic 310-20, Receivables — Nonrefundable Fees and Other Costs. The amendments in this Update represent changes to clarify the Codification. The amendments make the Codification easier to understand and easier to apply by eliminating inconsistencies and providing clarifications. ASU 2021-08 is effective for the Company for annual and interim reporting periods beginning July 1, 2021. Early application is not permitted. All entities should apply the amendments in this Update on a prospective basis as of the beginning of the period of adoption for existing or newly purchased callable debt securities. These amendments do not change the effective dates for Update 2017-08. The Company is currently evaluating the impact of this new standard on Company’s unaudited interim condensed consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The amendments in this Update represent changes to clarify the Codification or correct unintended application of guidance that are not expected to have a significant effect on current accounting practice or create a significant administrative cost to most entities. The amendments in this Update affect a wide variety of Topics in the Codification and apply to all reporting entities within the scope of the affected accounting guidance. ASU 2021-10 is effective for annual periods beginning after December 15, 2021 for public business entities. Early application is permitted. The amendments in this Update should be applied retrospectively. The Company does not expect the adoption of this standard to have a material impact on its unaudited interim condensed consolidated financial statements.
Except as mentioned above, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s unaudited interim condensed consolidated balance sheets, statements of operations and comprehensive loss and statements of cash flows.
3.4. PREPAID EXPENSES AND OTHER CURRENT ASSETS, NET
| March 31, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Prepaid for research and development materials | 2,593 | 27,771 | ||||||
| Tax refundable | 153,201 | 139,049 | ||||||
| Other receivables | 294,680 | 23,469 | ||||||
| Prepayment | 6,828 | 31,558 | ||||||
| Total prepaid expenses and other current assets | 457,302 | 221,847 | ||||||
| Less: allowance for credit losses | - | - | ||||||
| Total prepaid expenses and other current assets | 457,302 | 221,847 | ||||||
September 30, 2023 | June 30, 2023 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Tax refundable | 145,640 | 146,625 | ||||||
| Other receivables | 45,192 | 3,325 | ||||||
| Prepayment | 3,258 | 13,759 | ||||||
| Total prepaid expenses and other current assets | 194,090 | 163,709 | ||||||
| Less: allowance for credit losses | - | - | ||||||
| Total prepaid expenses and other current assets | 194,090 | 163,709 | ||||||
4.5. EQUIPMENT, NET
Property, plant and equipment, net, consists of the following:
| March 31, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Lab equipment | 1,514,933 | 1,311,818 | ||||||
| Computer equipment | 30,620 | 26,712 | ||||||
| Furniture and fixtures | 29,501 | 38,089 | ||||||
| Leasehold improvements | 7,874 | 212,078 | ||||||
| 1,582,929 | 1,588,697 | |||||||
| Less: accumulated depreciation | (886,318 | ) | (805,624 | ) | ||||
| Property, plant and equipment, net | 696,611 | 783,073 | ||||||
September 30, 2023 | June 30, 2023 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Lab equipment | 1,427,713 | 1,446,527 | ||||||
| Computer equipment | 30,844 | 31,135 | ||||||
| Furniture and fixtures | 35,264 | 35,412 | ||||||
| Leasehold improvements | 184,495 | 201,994 | ||||||
| 1,678,316 | 1,715,068 | |||||||
| Less: accumulated depreciation | (1,014,633 | ) | (996,371 | ) | ||||
| Property, plant and equipment, net | 663,683 | 718,697 | ||||||
Depreciation expenses were approximately US$86,46238,789 and US$12,248107,364 for the nine-monththree-month periods ended March 31,September 30 2023 and 2022, respectively.
5. ACQUISITION
On January 1, 2022, the Company entered into an agreement to acquire all of the equity interests of Shanghai Sglcell Biotech Co., Ltd. The primary assets purchased in the acquisition were intangible assets, equipment and net working capital. The Company concluded the assets acquired and liabilities assumed did not meet the definition of a business as a limited number of inputs were acquired but no substantive business processes or signs of output were acquired. As such, the acquisition was accounted for as an asset purchase. The purchase consideration was approximately RMB12,000,000 (approximately US$1,791,553) in cash and paid in July 2022. The estimated fair value of assets acquired, and liabilities assumed as of acquisition date are approximately US$1,791,553.
The Company allocated the purchase price of US$1.8 million between equipment of US$0.1 million, right-of-use asset of US$0.4 million, cash of US$0.2 million, prepaid and other current assets of US$0.9 million, assumed lease payable and accounts payable, accruals and other current liabilities of US$1.5 million and intangible assets related to patents registered or applied with the China Patent and Trademark Office of US$1.6 million, attributable to 8 patents on novel separation technologies for antibody-free and label-free enrichment of rare cell-based cancer biomarkers (such as CTC, CTC cluster, tumor marker expressed cells) from very dense blood cells, 6 patents on high throughput droplet microfluidic chips for nano-liter scale reagent preparation and single-cell, 3 patents for the device of water phase single-cell isolation and capturing for single cell applications, 6 patents on single-CTC detection/discrimination and delivery for single-CTC sorting and downstream single-CTC analysis, and 3 patents on target cells pre-concentration and enrichment.
Management of the Company is responsible for determining the fair value of assets acquired and liabilities assumed as of the acquisition date and considered a number of factors including valuations from independent appraiser. Acquisition-related costs incurred for the acquisitions were not material and have been expensed into general and administrative expense when incurred.
The following table summarizes the estimated fair value of assets acquired, and liabilities assumed as of acquisition date which represents the net purchase price allocation on the date of the acquisition of Shanghai Sglcell Biotech Co., Ltd and its subsidiaries, based on valuation performed by an independent valuation firm engaged by the Company and translated the fair value from RMB to USD using the exchange rate on 1 January 2022 at the rate of US$1.00 to CNY6.70:
6. INTANGIBLE ASSETS, NET
The following table summarizes the carrying amount of the Company’s finite-lived intangible assets:
| March 31, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Acquired technology | 413,767 | 422,536 | ||||||
| Less: accumulated amortization | (114,751 | ) | (11,737 | ) | ||||
| 299,016 | 410,799 | |||||||
| September 30, 2023 | June 30, 2023 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Acquired technology | 389,472 | 391,943 | ||||||
| Less: accumulated amortization | (173,446 | ) | (141,622 | ) | ||||
| 216,026 | 250,321 | |||||||
Finite-lived intangible assets are carried at cost less accumulated amortization. Amortization expense was approximately US$111,78334,295 and US$057,144 for the nine-monththree-month periods ended March 31,September 30, 2023 and 2022, respectively.
| March 31, 2023 | June 30, 2022 | September 30, 2023 | June 30, 2023 | |||||||||||||
| US $ | US $ | US $ | US $ | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| Acquired patents through acquisition | 1,617,974 | 1,617,974 | 1,617,974 | 1,617,974 | ||||||||||||
| Less: impairment | (1,617,974) | (1,617,974 | ) | (1,617,974 | ) | (1,617,974 | ) | |||||||||
| - | - | - | - | |||||||||||||
As of JuneSeptember 30, 2022,20223 the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell Biotech Co., Ltd. Impairment allowance of approximately US$1,617,974 was made management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable as of JuneSeptember 30, 2022.2023.
7. DEFERRED INITIAL PUBLIC OFFERING (“IPO”) COSTS
7.The Company complies with the requirement of the ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering”. Initial public offering expense directly attributable to offering of securities are deferred and would be charged against the gross proceeds of the offering, as a reduction in share capital. These deferred expenses mainly consist of underwriting, legal and other expenses incurred through the balance sheet date that are directly related to the intended IPO. Should the IPO prove to be unsuccessful, these deferred initial public offering costs, as well as additional expenses to be incurred, will be charged to operations. As of September 30, 2023 and June 30, 2023, the Company capitalized US$553 thousand of deferred initial public offering costs.
8. ACCOUNTS PAYABLE, ACCRUALS AND OTHER CURRENT LIABILITIES
Account Payable, accrued expenses and other liabilities consists of the following:
| March 31, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Accounts Payable | 1,112,379 | 762,202 | ||||||
| Payroll payable | 104,563 | 96,203 | ||||||
| Acquisition payables* | - | 1,791,553 | ||||||
| Other payables** | 515,484 | 103,077 | ||||||
| 1,732,426 | 2,753,035 | |||||||
September 30, 2023 | June 30, 2023 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Accounts Payable | 614,582 | 632,820 | ||||||
| Payroll payable | 64,344 | 85,561 | ||||||
| Amount due to related parties – major shareholders * | 1,142,613 | 1,052,677 | ||||||
| Amount due to related parties – related corporations** | 827,072 | 603,802 | ||||||
| Other payables# | 814,945 | 684,156 | ||||||
| 3,463,556 | 3,059,016 | |||||||
*Acquisition payables The amounts due to the Company’s major shareholders as of September 30, 2023 and June 30, 2022, was an amount of US$1,791,553 payable to previous shareholder of the acquired subsidiary, Shanghai Sglcell Biotech Co., Ltd. and the amount was subsequently settled in its entirety in July 2022.
**The balances were payables owed to third party creditors; the balances were2023 are non-trade, unsecured, interest-free and repayable on demand.
8.** The amounts due to related corporations, which the Company’s major shareholders have controlling equity interest in, as of September 30, 2023 and June 30, 2023 are non-trade, unsecured, interest-free and repayable on demand.
# The amount consists of payables owed to third party creditors, which are unsecured, interest-free and repayable on demand, and other payables due to operational use.
9. EQUITY
For the sake of undertaking a public offering of the Company’s common stocks, the Company has performed a series of re-organizing transactions resulting in 25,000,000 shares and 24,750,00025,000,000 of common stock outstanding as of March 31,September 30, 2023 and June 30, 2022.2023. The Company has accounted for these shares had they been issued and outstanding at the beginning of the first period presented. The Company only has one single class of common stock that is accounted for as permanent equity.
The above mentioned re-organizing transactions and equity is before the stock split on May 16, 2023.
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now have 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis similar to share split or dividend pursuant to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split. The shares of common stock retain a par value of $0.001 per share. Accordingly, an amount equal to the par value of the increased shares resulting from the stock split was reclassified from “Additional paid-in capital” to “Common stock”.
10. STOCK OPTION
Effective from March 30, 2023, the Stock Incentive Plan (the “2023 Plan”) was approved by the Company's Board of Directors. Under the 2023 Plan, the Board of Directors may grant options or purchase rights to purchase common stock to officers, employees, and other persons who provide services to the Company or any related company. The participants to whom awards are granted, the type of awards granted, the number of shares covered for each award, and the purchase or exercise price, conditions and other terms of each award are determined by the Board of Directors, except that the term of the options shall not exceed 10 years. A total of 15 million shares of our common stock are subject to the 2023 Plan and maybe either a qualified or non-qualified stock option. The shares issued for the 2023 Plan may be either treasury or authorized and unissued shares. As of September 30, 2023, the Company has granted no stock options to purchase any shares of the common stock under the 2023 Plan.
11. RELATED PARTY TRANSACTIONS
These related parties of the Company with whom transactions are reported in these financial statements are as follows:
| Name of Related Party | ||
| Yi Lu, Ph.D. | Chairman of the Board, Chief Science Officer, President and Shareholder of the Company | |
| Hung To Pau, Ph.D. | Chief Executive Officer, Director, Secretary and Shareholder of the Company | |
| Steven I-Fang Cheng, Ph.D. | Chief Technology Officer of the Company | |
| Chen-Yi Lee | Chen-Yi Lee is the sole director and the controlling person of Advance On Ventures Limited, which owns 10.92% equity interest in the Company and has sole voting and dispositive power over shares beneficially owned by Advance On Ventures Limited. Chen-Yi Lee is also the Shareholder of the Company | |
| Advance On Ventures Limited | Shareholder of the Company | |
| Well Fancy Development Ltd | CEO-Hung To Pau is the director and shareholder of the entity | |
| Shanghai Junfu Electronic Technology Co., Ltd. | CEO-Hung To Pau is the legal person and shareholder of the entity |
9.
In the ordinary course of business, during the three-month periods ended September 30, 2023 and 2022, the Company was involved in certain transactions, either at cost or current market prices, and on the normal commercial terms with related parties. The following table provides the transactions with these parties for the years as presented (for the portion of such period that they were considered related):
| As of September 30, 2023 | As of June 30, 2023 | |||||||
| US$ | US$ | |||||||
| Amount due to related parties – major shareholders | ||||||||
| Name of related party | ||||||||
| Hung To Pau, Ph.D. (1) | 1,027,077 | 933,471 | ||||||
| Yi Lu, Ph.D. (2) | 110,987 | 114,907 | ||||||
| Chen-Yi Lee (3) | 4,549 | 4,299 | ||||||
| 1,142,613 | 1,052,677 | |||||||
| Amount due to related parties – related corporations | ||||||||
| Name of related party | ||||||||
| Well Fancy Development Ltd (4) | 621,480 | 396,905 | ||||||
| Shanghai Junfu Electronic Technology Co., Ltd. (5) | 205,592 | 206,897 | ||||||
| 827,072 | 603,802 | |||||||
| 1. | Payments of IPO costs paid on behalf of Advanced Biomed Inc. |
| 2. | Advanced Biomed Inc.(Taiwan) entered into an unsecured, interest-free loan to Yi Lu amounting to NTD 3,578,212 (approximately US$110,987) for general working capital in January 2023. As of September 30, 2023 and June 30 2022, the loan balance due to Yi Lu amounted to US$110,987 and US$114,907, respectively. |
| 3. | Payments of expenses on behalf of Advanced Biomed Inc. (Taiwan). |
| 4. | Advanced Biomed Inc.(Taiwan) entered into four unsecured, interest-free loans to Well Fancy Development Ltd amounting to NTD 2,967,700 (approximately US$92,050), NTD 2,989,873 (approximately US$92,738), NTD 4,270,200 (approximately US$132,450), NTD 1,945,000 (approximately US$60,329), NTD 1,858,120 (approximately US$57,634), and NTD 2,912,200 (approximately US$90,329)for general working capital in August 2022, April 2023, May 2023, June 2023, July 2023, and August 2023 respectively. As of September 30, 2023 June 2023, the loan balance due to Well Fancy Development Ltd totally amounted to US$525,530 and amounted to US$95,950 for general working capital. As of June 2023, the loan balance due to Well Fancy Development Ltd totally amounted and US$396,905. |
| 5. | Shanghai Sglcell Biotech Co., Ltd entered into three unsecured, interest-free loans to Shanghai Junfu Electronic Technology Co., Ltd. amounting to RMB 500,000 (approximately US$68,530) for general working capital in April 2023, May 2023, and June 2023, respectively. As of June 30, 2023 and 2022, the loan balance due to Shanghai Sglcell Biotech Co., Ltd totally amounted to US$205,592 and US$206,897, respectively. |
12. CONCENTRATIONS AND RISKS
Credit Risk
Credit risk is the potential financial loss to the Company resulting from the failure of a customer or a counterparty to settle its financial and contractual obligations to the Company, as and when they fall due. As the Company does not hold any collateral, the maximum exposure to credit risk is the carrying amounts of other receivables (exclude prepayments) and cash and bank deposits presented on the consolidated balance sheets. The Company has no other financial assets which carry significant exposure to credit risk.
Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Company’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Company’s reputation.
Typically, the Company ensures that it has sufficient cash on demand to meet expected operational expenses for a period of 60 days, including the servicing of financial obligations; this excludes the potential impact of extreme circumstances that cannot reasonably be predicted, such as natural disasters.
Foreign currency risk
Foreign currency risk is the risk that the fair value or future cash flows of an exposure will fluctuate because of changes in foreign exchange rates. The company’s exposure to the risk of changes in foreign exchange rates relates primarily to the Company’s operating activities (when expense is denominated in a foreign currency) and the Company’s net investments in foreign subsidiaries.
Impact of Inflation
Inflation in Taiwan and PRC has not materially affected the Company’s profitability and operating results. However, the Company can provide no assurance that we will be unaffected by higher inflation rates in Singapore or globally in the future.
10.13. COMMITMENTS AND CONTINGENCIES
Contingencies
In the ordinary course of business, the Company may be subject to legal proceedings regarding contractual and employment relationships and a variety of other matters. The Company records contingent liabilities resulting from such claims, when a loss is assessed to be probable, and the amount of the loss is reasonably estimable. In the opinion of management, there were no pending or threatened claims and litigation as of March 31,September, 2023 and up through August 11,November 20, 2023, the issuance date of these interim condensed consolidated financial statements were available to be issued.
Lease commitment
The Company determines if a contract contains a lease at inception. US GAAP requires that the Company’s leases be evaluated and classified as operating or finance leases for financial reporting purposes. The classification evaluation begins at the commencement date and the lease term used in the evaluation includes the non-cancellable period for which the Company has the right to use the underlying asset, together with renewal option periods when the exercise of the renewal option is reasonably certain and failure to exercise such option which results in an economic penalty.
The right-of-use assets relate to leases of office premises and a dormitory for employees in the PRC and the laboratory in Taiwan.
The recognized operating lease ROU assets and lease liabilities as follows:
| September 30, 2023 | June 30, 2023 | |||||||
| US$ | US$ | |||||||
| (unaudited) | ||||||||
| Operating lease ROU asset | 180,024 | 235,373 | ||||||
| March 31, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
(unaudited) | ||||||||
| Operating lease ROU asset | 300,967 | 442,895 | ||||||
| March 31, 2023 | June 30, 2022 | September 30, 2023 | June 30, 2023 | |||||||||||||
| US$ | US$ | US$ | US$ | |||||||||||||
(unaudited) | (unaudited) | |||||||||||||||
| Operating lease liabilities | ||||||||||||||||
| Current portion | 228,370 | 236,677 | 179,544 | 216,567 | ||||||||||||
| Non-current portion | 70,849 | 232,191 | 480 | 18,806 | ||||||||||||
| Total | 299,219 | 468,868 | 180,024 | 235,373 | ||||||||||||
As of March 31,September 30, 2023, future minimum lease payments under the non-cancelable operating leases are as follows:
| Future payment | US$ | US$ | ||||||
| 2024 | 226,482 | 183,983 | ||||||
| 2025 | 73,703 | 481 | ||||||
| 2026 | - | - | ||||||
| 2027 | - | - | ||||||
| 2028 | - | - | ||||||
| Thereafter | - | - | ||||||
| Total future lease payment | 300,185 | 184,464 | ||||||
| Less: imputed interest | (966 | ) | (4,440 | ) | ||||
| Present value of operating lease liabilities | 299,219 | 180,024 | ||||||
| Operating lease liabilities, current portion | 228,370 | 179,544 | ||||||
| Operating lease liabilities, non-current portion | 70,849 | 480 | ||||||
The following summarizes other supplemental information about the Company’s operating lease as of March 31,September 30, 2023:
| Weighted average discount rate | % | |||
| Weighted average remaining lease term (years) |
11.14. SUBSEQUENT EVENTS
The Company has assessed all events from March 31,September 30, 2023 through August 11,November 20, 2023, which is the date that these unaudited interim condensed consolidated financial statements are available to be issued, unless as disclosed below, there are not any material subsequent events that require disclosure in these condensed consolidated financial statements other than events detailed below.
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now has 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis in accordance to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split.
On June 8, 2023, Shanghai Sglcell Biotech Co., Ltd. transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to independent third-party individuals at aggregate consideration of CNY500,000 (approximately US$72,780) without any other obligations arising from the transfer. Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd. have been inactive since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., was CNY500,000.
On June 9, 2023, Shandong Sglcell Medical Devices Co., Ltd., the wholly owned subsidiary of Shanghai Sglcell Biotech Co., Ltd., was transferred to independent third-party individuals at zero consideration without any other obligations arising from the transfer. Shanghai Sglcell Biotech Co., Ltd. has been inactive since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
On June 15, 2023, Sglcell (Huangshan) Biotech Co., Ltd., the wholly owned subsidiary of Advanced Biomed HK Limited, was transferred to an independent third-party corporation at zero consideration without any other obligations arising from the transfers. Sglcell (Huangshan) Biotech Co., Ltd. has been dormant since its incorporation date from March 4, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
F-16


REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
| To: | The Board of Directors and Stockholders of |
Advanced Biomed Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Advanced Biomed Inc. and its subsidiaries (collectively the “Company”) as of June 30, 20222023 and 2021,2022, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows in each of the years for the two-year period ended June 30, 2022,2023, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022,2023, and 2021,2022, and the results of its operations and its cash flows in each of the years for the two-year period ended June 30, 2022,2023, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph — Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2, the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Emphasis of Matter
As detailed in Note 1116 - Restatement, the Company has corrected an error in its application of the accounting principle for business combinations where the Company previously incorrectly accounted for an acquisition of an entity as a business combination; the Company has amended its consolidated financial statements for the year ended June 30, 2022 to account for the transaction as purchase of assets.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
WWC, P.C.
Certified Public Accountants
PCAOB ID No. 1171
We have served as the Company’s auditor since 2022.
San Mateo, California
May 19,October 27, 2023 except for Notes 8 and 11 for which the date is June 20, 2023 and Note 12 for which the date is August 11, 2023.

ADVANCED BIOMED INC. ANDINC.AND ITS SUBSIDIARIES
| As of June 30, | As of June 30, | |||||||||||||||
| 2022 | 2021 | 2023 | 2022 | |||||||||||||
| US $ | US$ | US$ | US$ | |||||||||||||
| (as restated) | (as restated) | |||||||||||||||
| Assets | ||||||||||||||||
| Current assets: | ||||||||||||||||
| Cash | 4,783,864 | 65,922 | 2,622,279 | 4,783,864 | ||||||||||||
| Prepaid expenses and other current assets | 221,847 | 847,575 | ||||||||||||||
| Prepaid expenses and other current assets, net | 163,709 | 221,847 | ||||||||||||||
| Total current assets | 5,005,711 | 913,497 | 2,785,988 | 5,005,711 | ||||||||||||
| Property, plant and equipment, net | 783,073 | 85,855 | ||||||||||||||
| Equipment, net | 718,697 | 783,073 | ||||||||||||||
| Right-of-use assets, net | 442,895 | 7,021 | 235,373 | 442,895 | ||||||||||||
| Intangible assets, net | 410,799 | - | 250,321 | 410,799 | ||||||||||||
| Deferred initial public offering (“IPO”) costs | 553,471 | - | ||||||||||||||
| Other non-current assets | 71,021 | - | 56,127 | 71,021 | ||||||||||||
| Total non-current assets | 1,707,788 | 92,876 | 1,813,989 | 1,707,788 | ||||||||||||
| TOTAL ASSETS | 6,713,499 | 1,006,373 | 4,599,977 | 6,713,499 | ||||||||||||
| Liabilities | ||||||||||||||||
| Current liabilities: | ||||||||||||||||
| Accounts payable, accruals and other current liabilities | 2,753,035 | 23,110 | ||||||||||||||
| Lease payable – current | 236,677 | 5,240 | ||||||||||||||
| Accounts payable, accruals, and other current liabilities | 3,059,016 | 2,753,035 | ||||||||||||||
| Lease payable - current | 216,567 | 236,677 | ||||||||||||||
| Total current liabilities | 2,989,712 | 28,350 | 3,275,583 | 2,989,712 | ||||||||||||
| Lease payable – non-current | 232,191 | 1,781 | 18,806 | 232,191 | ||||||||||||
| Total non-current liabilities | 232,191 | 1,781 | 18,806 | 232,191 | ||||||||||||
| TOTAL LIABILITIES | 3,221,903 | 30,131 | 3,294,389 | 3,221,903 | ||||||||||||
| Commitments and contingencies | - | - | - | - | ||||||||||||
| Stockholders’ equity | ||||||||||||||||
Common stock $0.00025 par value per share; as of June 30, 2022 and 2021, 500,000,000 and 500,000,000 shares- authorized; 99,000,000 and 58,885,468 shares issued and outstanding, respectively.* | 24,750 | 14,721 | ||||||||||||||
| Common stock $0.001 par value per share; as of June 30, 2023 and June 30, 2022; 500,000,000 and 500,000,000 shares authorized; 100,000,000 and 99,000,000 shares issued and outstanding, respectively* | 100,000 | 99,000 | ||||||||||||||
| Additional paid-in capital | 12,657,007 | 6,569,905 | 13,593,365 | 12,582,757 | ||||||||||||
| Accumulated deficits | (9,459,020 | ) | (5,431,298 | ) | (13,191,797 | ) | (9,459,020 | ) | ||||||||
| Accumulated other comprehensive income/(loss) | 268,859 | (177,086 | ) | |||||||||||||
| Accumulated other comprehensive income | 804,020 | 268,859 | ||||||||||||||
| Total stockholders’ equity | 3,491,596 | 976,242 | 1,305,588 | 3,491,596 | ||||||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | 6,713,499 | 1,006,373 | 4,599,977 | 6,713,499 | ||||||||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these consolidated financial statements.
ADVANCED BIOMED INC. ANDINC.AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the years ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| US $ | US$ | |||||||
| (as restated) | ||||||||
| Operating expenses: | ||||||||
| Research and development expenses | (1,619,531 | ) | (1,734,620 | ) | ||||
| General and administrative expenses | (754,840 | ) | (102,320 | ) | ||||
| Impairment of intangible assets | (1,617,974 | ) | - | |||||
| Total operating expenses | (3,992,345 | ) | (1,836,940 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 243 | 28 | ||||||
| Other expense, net | (35,620 | ) | (246 | ) | ||||
| Total other expense, net | (35,377 | ) | (218 | ) | ||||
| Income before tax expense | ||||||||
| Income tax expense | - | - | ||||||
| Net loss | (4,027,722 | ) | (1,837,158 | ) | ||||
| Other comprehensive income | ||||||||
| Foreign currency translation gain/ (loss), net of taxes | 445,945 | (197,530 | ) | |||||
| Total comprehensive loss | (3,581,777 | ) | (2,034,688 | ) | ||||
| Loss per share | ||||||||
| basic and diluted* | (0.05 | ) | (0.04 | ) | ||||
| Weighted average number of shares of common stock in computing net loss per share | ||||||||
| basic and diluted* | 70,919,160 | 57,312,564 | ||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these consolidated financial statements.
ADVANCED BIOMED INC. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(as restated)
| Common stock | ||||||||||||||||||||||||
No. of Shares* | Amount | Additional paid-in capital | Accumulated other comprehensive income (loss) | Accumulated deficit | Total | |||||||||||||||||||
| US$ | US$ | US$ | US$ | US$ | ||||||||||||||||||||
| Balance as of July 1, 2020 | 55,425,492 | 13,856 | 5,873,606 | 20,444 | (3,594,140 | ) | 2,313,766 | |||||||||||||||||
| Net loss | - | - | - | - | (1,837,158 | ) | (1,837,158 | ) | ||||||||||||||||
| Issuance of shares for cash | 3,459,976 | 865 | 696,299 | - | - | 697,164 | ||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | (197,530 | ) | - | (197,530 | ) | ||||||||||||||||
| Balance as of June 30, 2021 | 58,885,468 | 14,721 | 6,569,905 | (177,086 | ) | (5,431,298 | ) | 976,242 | ||||||||||||||||
| Balance as of July 1, 2021 | 58,885,468 | 14,721 | 6,569,905 | (177,086 | ) | (5,431,298 | ) | 976,242 | ||||||||||||||||
| Net loss | - | - | - | - | (4,027,722 | ) | (4,027,722 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 445,945 | - | 445,945 | ||||||||||||||||||
| Issuance of shares for cash | 40,114,532 | 10,029 | 6,087,102 | - | - | 6,097,131 | ||||||||||||||||||
| Balance as of June 30, 2022 | 99,000,000 | 24,750 | 12,657,007 | 268,859 | (9,459,020 | ) | 3,491,596 | |||||||||||||||||
| For the years ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| (as restated) | ||||||||
| Operating expenses: | ||||||||
| Research and development expenses | (1,383,181 | ) | (1,619,531 | ) | ||||
| General and administrative expenses | (1,688,042 | ) | (754,840 | ) | ||||
| Impairment of intangible assets | - | (1,617,974 | ) | |||||
| Total operating expenses | (3,071,223 | ) | (3,992,345 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | 21,402 | 243 | ||||||
| Other expense, net | (682,956 | ) | (35,620 | ) | ||||
| Total other expense, net | (661,554 | ) | (35,377 | ) | ||||
| Income before tax expense | ||||||||
| Income tax expense | - | - | ||||||
| Net loss | (3,732,777 | ) | (4,027,722 | ) | ||||
| Other comprehensive income | ||||||||
| Foreign currency translation gain, net of taxes | 535,161 | 445,945 | ||||||
| Total comprehensive loss | (3,197,616 | ) | (3,581,777 | ) | ||||
| Loss per share: | ||||||||
| basic and diluted* | (0.04 | ) | (0.05 | ) | ||||
| Weighted average number of shares of common stock in computing net loss per share | ||||||||
| basic and diluted* | 99,679,452 | 70,919,160 | ||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these consolidated financial statements.
ADVANCED BIOMED INC.AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ (DEFICIT) EQUITY
| Common stock | ||||||||||||||||||||||||
| No. of shares* | Amount* | Additional paid-in capital* | Accumulated other comprehensive income | Accumulated deficit |
Total | |||||||||||||||||||
| US$ | US$ | US$ | US$ | US$ | ||||||||||||||||||||
| Balance as of July 1, 2021 | 58,885,468 | 58,884 | 6,525,742 | (177,086 | ) | (5,431,298 | ) | 976,242 | ||||||||||||||||
| Net loss | - | - | - | - | (4,027,722 | ) | (4,027,722 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 445,945 | - | 445,945 | ||||||||||||||||||
| Issuance of shares for cash | 40,114,532 | 40,116 | 6,057,015 | - | - | 6,097,131 | ||||||||||||||||||
| Balance as of June 30, 2022 |
99,000,000 |
99,000 |
12,582,757 |
268,859 |
(9,459,020 |
) |
3,491,596 | |||||||||||||||||
| Balance as of July 1, 2022 | 99,000,000 | 99,000 | 12,582,757 | 268,859 | (9,459,020 | ) | 3,491,596 | |||||||||||||||||
| Net loss | - | - | - | - | (3,732,777 | ) | (3,732,777 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 535,161 | - | 535,161 | ||||||||||||||||||
| Issuance of shares for cash | 1,000,000 | 1,000 | 1,010,608 | - | - | 1,011,608 | ||||||||||||||||||
| Balance as of June 30, 2023 | 100,000,000 | 100,000 | 13,593,365 | 804,020 | (13,191,797 | ) | 1,305,588 | |||||||||||||||||
* Giving retroactive effect to the 4 for 1 share split effected on May 16, 2023.
The accompanying notes are an integral part of these consolidated financial statements.
ADVANCED BIOMED INC. ANDINC.AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the years ended June 30, | ||||||||
| 2022 | 2021 | |||||||
| US$ | US$ | |||||||
| (as restated) | ||||||||
| Net Loss | (4,027,722 | ) | (1,837,158 | ) | ||||
| Adjustment: | ||||||||
| Depreciation and amortization | 126,632 | 57,850 | ||||||
| Impairment of intangible assets | 1,617,974 | - | ||||||
| Loss from disposal of property, plant and equipment | 37,634 | - | ||||||
| Interest Income | (243 | ) | (28 | ) | ||||
| Changes in operating assets: | ||||||||
| Decrease of prepaid expenses and other current assets | 565,635 | 1,288,095 | ||||||
| Increase of accounts payable, accruals and other current liabilities | 912,424 | 17,238 | ||||||
| Lease obligations net cash | 25,973 | - | ||||||
| Interest received | 243 | 28 | ||||||
| Cash used in operating activities | (741,450 | ) | (473,975 | ) | ||||
| Purchase of intangible assets | (422,536 | ) | - | |||||
| Purchase of property, plant and equipment | (878,666 | ) | (25,442 | ) | ||||
| Disposal of property, plant and equipment | 28,919 | - | ||||||
| Prepayment for the purchase of property, plant and equipment | (10,928 | ) | - | |||||
| Acquisition of subsidiaries* (Note 5) | 199,527 | - | ||||||
| Cash used in investing activities | (1,083,684 | ) | (25,442 | ) | ||||
| Proceeds from issuance of shares | 6,097,131 | 697,164 | ||||||
| Cash provided by financing activities | 6,097,131 | 697,164 | ||||||
| Foreign currency effect | 445,945 | (197,530 | ) | |||||
| Net change in cash and cash equivalents | 4,717,942 | 217 | ||||||
| Cash and cash equivalents as of beginning of the period | 65,922 | 65,705 | ||||||
| Cash and cash equivalents as of the end of the period | 4,783,864 | 65,922 | ||||||
| Net increase in cash and cash equivalents | 4,717,942 | 217 | ||||||
| Supplementary Cash Flow Information: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Acquisition of Shanghai Sglcell Biotech Co., Ltd. and its subsidiaries with acquisition payables | 1,791,553 | - | ||||||
| For the years ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| US$ | US$ | |||||||
| (as restated) | ||||||||
| Net Loss | (3,732,777 | ) | (4,027,722 | ) | ||||
| Adjustment: | ||||||||
| Depreciation and amortization | 365,231 | 126,632 | ||||||
| Loss from disposal of subsidiaries | 150,207 | - | ||||||
| Impairment of intangible assets | - | 1,617,974 | ||||||
| Loss from disposal of property, plant and equipment | - | 37,634 | ||||||
| Interest Income | (21,402 | ) | (243 | ) | ||||
| Changes in operating assets: | ||||||||
| (Increase) decrease of prepaid expenses and other current assets | (116,460 | ) | 565,635 | |||||
| Increase of accounts payable, accruals and other current liabilities | 381,253 | 912,424 | ||||||
| Lease obligations net cash | (25,973 | ) | 25,973 | |||||
| Other non-current assets | 14,894 | - | ||||||
| Interest received | 21,402 | 243 | ||||||
| Cash used in operating activities | (2,963,625 | ) | (741,450 | ) | ||||
| Purchase of intangible assets | (1,640 | ) | (422,536 | ) | ||||
| Purchase of property, plant and equipment | (210,883 | ) | (878,666 | ) | ||||
| Disposal of property, plant and equipment | - | 28,919 | ||||||
| Prepayment for the purchase of property, plant and equipment | - | (10,928 | ) | |||||
| Disposal of subsidiaries (Note 7) | (50,486) | - | ||||||
| Acquisition of subsidiaries (Note 6) | - | 199,527 | ||||||
| Cash used in investing activities | (263,009 | ) | (1,083,684 | ) | ||||
| Payment of deferred initial public offering costs | (553,471 | ) | - | |||||
| Proceeds from issuance of shares | 1,011,608 | 6,097,131 | ||||||
| Cash provided by financing activities | 458,137 | 6,097,131 | ||||||
| Foreign currency effect | 606,912 | 445,945 | ||||||
| Net change in cash and cash equivalents | (2,161,585 | ) | 4,717,942 | |||||
| Cash and cash equivalents as of beginning of the period | 4,783,864 | 65,922 | ||||||
| Cash and cash equivalents as of the end of the period | 2,622,279 | 4,783,864 | ||||||
| Net (decrease) increase in cash and cash equivalents | (2,161,585 | ) | 4,717,942 | |||||
| Supplementary Cash Flow Information: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Disposal of Nanjing Yitian Biotech Co., Ltd. and its subsidiary with proceeds from disposal receivables | 71,942 | - | ||||||
| Acquisition of Shanghai Sglcell Biotech Co., Ltd. and its subsidiaries with acquisition payables | - | 1,791,553 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
ADVANCED BIOMED INC. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
On July 16, 2021, Advanced Biomed Inc. (the “Company”) was incorporated in the State of Nevada, as an investment holding company. The Company’s principal executive offices are located in Tainan City, Taiwan. The Company has no substantive operations.operations and assets. It is holding Company that holds all of the issued and outstanding shares of Advanced Biomed HK Limited and Advanced Biomed Inc. (Taiwan).
| (1) | Establishment of Advanced Biomed HK Limited |
On August 10, 2021, the Company incorporated a wholly owned subsidiary, Advanced Biomed HK Limited, in Hong Kong to facilitate market development and commercialization of the Company’s oncology products for sale and distribution in the People’s Republic of China (the “PRC”).
| (2) | Acquisition of Shanghai Sglcell Biotech Co., Ltd. and its subsidiaries |
On January 1, 2022, Advanced Biomed HK Limited acquired 100% equity interest of Shanghai Sglcell Biotech Co., Ltd. Shanghai Sglcell Biotech Co., Ltd. was established in the PRC on April 12, 2019. It is mainly engaged in the establishment and operation of medical clinics in the PRC.
Shanghai Sglcell Biotech Co., Ltd. owns 100% equity interest of two subsidiaries namely 1.) Shandong Sglcell Medical Devices Co., Ltd. and 2.) Nanjing Yitian Biotech Co., Ltd. Shandong Sglcell Medical Devices Co., Ltd. was incorporated in the PRC on July 8, 2021 to carry out the establishment of medical clinics, and the supply of medical products and services to clinics in the PRC. Nanjing Yitian Biotech Co., Ltd. was established in the PRC on January 6, 2017. Nanjing Yitian Biotech Co., Ltd. wholly owns a subsidiary, Beijing YitianYitan Jiarui Technology Co. Ltd., which was established in the PRC on October 20, 2017. Both were established to carryout marketing and mainly engage in futurecarry out marketing and clinical services in the PRC.
On June 8, 2023, Shanghai Sglcell Biotech Co., Ltd. transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to independent third-party individuals at aggregate consideration of CNY500,000 (approximately US$71,942) without any other obligations arising from the transfer. Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd. have not commenced business activity since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., was CNY498,587 (approximately US$71,739).
On June 9, 2023, Shandong Sglcell Biotech Co., Ltd., the wholly owned subsidiary of Shanghai Sglcell Biotech Co., Ltd., was transferred to independent third-party individuals at zero consideration without any other obligations arising from the transfer. Shandong Sglcell Biotech Co., Ltd. has been inactive since it was incorporated on July 8, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
| (3) | Subsidiary established under Advanced Biomed HK Limited |
Advanced Biomed HK Limited incorporated a wholly owned subsidiary, Sglcell (Huangshan) Biotech Co., Ltd., in the PRC on March 4, 2022; it was established for the expected future manufacturing of medical devices in the PRC.
On June 15, 2023, Sglcell (Huangshan) Biotech Co., Ltd., the wholly owned subsidiary of Advanced Biomed HK Limited, was transferred to an independent third-party corporation at zero consideration without any other obligations arising from the transfers. Sglcell (Huangshan) Biotech Co., Ltd. has been dormant since its incorporation date from March 4, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
F-22
| (4) | Reorganization of Advanced Biomed Inc. (Taiwan) |
Advanced Biomed Inc. (Taiwan) was established in Taiwan on September 1, 2014. It is primarily focused on mainly operates as a research and development of new center for technologies in the field of oncology to help efficiently and cost-effectively identify and diagnose cancer cells.
On thedate of incorporation, date, July 16, 2021, the Company issued 8,000,000 shares to Dr. Hung To Pau. On March 15, 2022, Dr. Hung To Pau transferred all of his 8,000,000 shares to Sglcell Ltd, an exempted company incorporated under the law of Cayman Islands, the sole shareholder of which is Dr. Hung To Pau for a total consideration of $8,000. The Company was dormant and has no substantive assets. On June 8, 2022, Sglcell Ltd transferred all of its 8,000,000 shares to Dr. Yi Lu for a total consideration of $8,000. In July 2022, the Company consummated a reorganization of Advanced Biomed Inc. (Taiwan) under share exchange arrangement of its then existing shareholders, who collectively owned all the equity interests of Advanced Biomed Inc. (Taiwan) prior to the reorganization.reorganization, transferred their respective shares in Advanced Biomed Inc. (Taiwan) to the Company. Prior to the re-organization, Advanced Biomed Inc. (Taiwan) was directly owned and controlled by Dr. Yi Lu and Chen-Yi Lee with 99.93% and 0.07% beneficial ownership interest, respectively.
The Share Exchange
Pursuant to a share exchange agreement (the “Agreement”) dated July 11, 2022, Dr. Yi Lu and Chen-Yi Lee transferred their respective shares in Advanced Biomed Inc. (Taiwan) at the time of the Agreement, representing in aggregate 100% of the issued share capital of Advanced Biomed Inc. (Taiwan), to the Company. The consideration for the share transfers was satisfied by the allotment and issuance of an aggregate of 385,257 fully paid up shares of common stock to Dr. Yi Lu and Chen-Yi Lee. Following the completion of the share exchange and related issuances by and among the Company, Dr. Yi Lu and Chen-Yi Lee, Advanced Biomed Inc. (Taiwan) ultimately became a wholly-owned subsidiary of the Company, and Dr. Yi Lu and Chen-Yi Lee became the beneficial owners of the Company with percentage ownerships of 99.99% and 0.01% as of August 12, 2022.
Subsequently, on October 24, 2022, the Company issued 365,352365,368 shares to Chen-Yi Lee, and on October 24, 2022, the Company also issued 2,730,000 shares to Advance On Ventures Limited, a company incorporated under the law of British Virgin Islands (the “Ventures Limited”), the beneficial owners of which are employees of Advanced Biomed Taiwan. The Company issued 4,405,625 shares, 2,193,750 shares, 2,060,000 shares, 1,511,250 shares, 1,243,750 shares, 1,230,000 shares respectively to Dr. Hung To Pau, Yimin Jin, Xiaoyuan Luo, Nanzhen Shen, Jian Wang and Qiang Chen pursuant to the Debt-For-Equity Exchange Agreement the Company entered into with the abovementioned shareholders on June 30, 2022 to settle debt of a total amount of NTD 174,020,033 and RMB 22,200,000 (approximately $9.04 million). On October 25, 2022, the Company issued 625,000 shares to Hanyu Assets Co. Ltd. pursuant to the Investment Agreement the Company entered into with Hanyu Assets Co. Ltd. on June 6, 2022, and the Company issued 250,000 shares to Newlink Technology Inc. pursuant to the Investment Agreement the Company entered into with Newlink Technology Inc. on June 6, 2022.
Upon completion of the reorganization, Dr. Yi Lu’s percentage of ownerships of the Company became 33.54% as of October 25, 2022.
On November 7, 2022, the Company obtained the approval of the Investment Commission of the Ministry of Economic Affairs (“Taiwan Investment Commission”) for the reorganization, and the issuance number of which is “經審一字第11100116890號”. Additionally, the Bureau of Economic Development of Tainan City Government has also approved the reorganization in accordance with the Taiwan Company Act on December 28,26, 2022.
Pursuant to this reorganization, the Company determined that Advanced Biomed Inc. (Taiwan) is the predecessor entity as the Company is an investment holding company with no business activities carried out and all of the business of Advanced Biomed Inc. (Taiwan) acquired formed substantially all of the business of the Company under Rule 405 of Regulation C. To reflect the real economic substance of the Company’s business under the reorganization, the accompanying unaudited interim consolidated financial statements were prepared assuming that the share exchange transaction, as disclosed above has been completed, and the Company exercises control of Advanced Biomed Inc. (Taiwan). The transaction detailed above has been accounted for as reverse takeover and recapitalization of the Company; whereby the Company (the legal acquirer) is considered the accounting acquiree, and Advanced Biomed Inc. (Taiwan)(the legal acquiree) is considered as the accounting acquirer. This transaction is deemed to be a continuation of the business of Advanced Biomed Inc. (Taiwan); therefore, no goodwill has been recorded for this transaction, and the Company’s historical financial information prior to the date of the recapitalization transaction is that of Advanced Biomed Inc. (Taiwan) and historical changes in shareholders’ deficit and its results of operations have been presented from the beginning of the first period presented. The above-mentioned equity is before the stock split on May 16, 2023.
The Company and its subsidiaries are in the table as follows:
| Percentage of effective ownership | ||||||||||||||||
| Name | Date of Incorporation | June 30, 2022 | June 30, 2021 | Place of Incorporation | Principal Activities | |||||||||||
| Advanced Biomed Inc. | July 16, 2021 | - | - | Nevada | Holding | |||||||||||
| Advanced Biomed Inc. (Taiwan) | September 1, 2014 | 100 | % | 100 | % | Taiwan | Research and development of various advanced and innovative microfluidic biochip technologies, and provide the leading application of such technologies in precision oncology detection, diagnosis and treatment | |||||||||
| Advanced Biomed HK Limited | August 10, 2021 | 100 | % | - | % | Hong Kong | Market development | |||||||||
| Shanghai Sglcell Biotech Co., Ltd. | April 12, 2019 | 100 | % | - | % | People’s Republic of China | Clinical-related business | |||||||||
| Nanjing Yitian Biotech Co., Ltd. | January 6, 2017 | 100 | % | - | % | People’s Republic of China | Marketing and clinical-related business | |||||||||
| Beijing Yitian Jiarui Technology Co., Ltd. | October 20, 2017 | 100 | % | - | % | People’s Republic of China | Marketing and clinical-related business | |||||||||
| Shandong Sglcell Medical Devices Co., Ltd. | July 8, 2021 | 100 | % | - | % | People’s Republic of China | Marketing and clinical-related business | |||||||||
| Sglcell (Huangshan) Biotech Co., Ltd. | March 4, 2022 | 100 | % | - | % | People’s Republic of China | Medical devices manufacture | |||||||||
| Percentage of effective ownership | ||||||||||||||||||
| Name | Date of Incorporation | June 30, 2023 | June 30, 2022 | Place of incorporation | Principal Activities | |||||||||||||
| Advanced Biomed Inc. | July 16, 2021 | - | - | Nevada | Investment holding | |||||||||||||
| Advanced Biomed Inc. (Taiwan) | September 1, 2014 | 100 | % | 100 | % | Taiwan | Research and development of various advanced and innovative microfluidic biochip technologies, and provide the leading application of such technologies in precision oncology detection, diagnosis and treatment | |||||||||||
| Advanced Biomed HK Limited | August 10, 2021 | 100 | % | 100 | % | Hong Kong | Market development | |||||||||||
| Shanghai Sglcell Biotech Co., Ltd. | April 12, 2019 | 100 | % | 100 | % | People’s Republic of China | Clinical-related business | |||||||||||
| Nanjing Yitian Biotech Co., Ltd. | January 6, 2017 | - | 100 | % | People’s Republic of China | Marketing and clinical-related business | ||||||||||||
| Beijing Yitan Jiarui Technology Co., Ltd. | October 20, 2017 | - | 100 | % | People’s Republic of China | Marketing and clinical-related business | ||||||||||||
| Shandong Sglcell Medical Devices Co., Ltd. | July 8, 2021 | - | 100 | % | People’s Republic of China | Marketing and clinical-related business | ||||||||||||
| Sglcell (Huangshan) Biotech Co., Ltd. | March 4, 2022 | - | 100 | % | People’s Republic of China | Medical devices manufacture | ||||||||||||
The accompanying consolidated financial statements are presented assuming that the Company was in existence at the beginning of the first period presented.
2. LIQUIDITY AND GOING CONCERN
The accompanying consolidated financial statements have been prepared in conformity with U.S. GAAP which contemplates continuation of the Company on a going concern basis. The going concern basis assumes that assets are realized, and liabilities are settled in the ordinary course of business at amounts disclosed in the consolidated financial statements. The Company’s ability to continue as a going concern depends upon its ability to develop, register and obtain regulatory approval for commercial sell of its products to generate positive operating cash flows. For the financial year ended June 30, 2023, the Company reported net loss of $3,732,777. As of June 30, 2023, the Company’s working capital deficit was $489,595. In addition, the Company had net cash outflows of $2,963,625 from operating activities for the financial year ended June 30, 2023. These conditions give rise to substantial doubt as to whether the Company will be able to continue as a going concern.
To sustain its ability to support the Company’s operating activities, the Company may have to consider supplementing its available sources of funds through the following sources:
| cash generated from upcoming operations; and | ||
| ● | financial support from the Company’s related party and shareholders as well as third parties. |
2.Management has commenced a strategy to raise debt and equity. However, there can be no certainty that these additional financings will be available on acceptable terms or at all. If management is unable to execute this plan, there would likely be a material adverse effect on the Company’s business. All of these factors raise substantial doubt about the ability of the Company to continue as a going concern. The consolidated financial statements for the financial years ended June 30, 2023 and 2022 have been prepared on a going concern basis and do not include any adjustments to reflect the possible future effects on the recoverability and classifications of assets or the amounts and classifications of liabilities that may result from the inability of the Company to continue as a going concern.
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and pursuant to the regulations of the Securities and Exchange Commission (“SEC”).
(b) Consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All inter-company transactions, if any, and balances due to, due from, long-term investment subsidiary, and registered paid in capital have been eliminated upon consolidation.
Business combinations are accounted for using the acquisition method of accounting. Under the acquisition method, assets acquired, and liabilities assumed are recorded at their respective fair values as of the acquisition date in the Company’s consolidated financial statements. Any excess fair value of consideration transferred over the fair value of the net assets acquired is recorded as goodwill. Contingent consideration obligations incurred in connection with the business combination are recorded at their fair values on the acquisition date and remeasured at their fair values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded in the consolidated statements of operations.
When the Company determines that assets acquired do not meet the definition of a business under the acquisition method of accounting, acquired assets is expensed, no goodwill is recorded, and any contingent consideration is recognized only when it becomes payable or is paid.
(c) Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant estimates relate to useful lives for property, plant and equipment and intangible assets, fair value of financial instruments, assumptions used in assessing right of use assets, impairment of long-lived assets, property, plant and equipment, intangible assets and uncertain tax position. Actual results could vary from the estimates and assumptions that were used.
(d) Stock split
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now have 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis similar to share split or dividend pursuant to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split. The shares of common stock retain a par value of $0.001 per share. Accordingly, an amount equal to the par value of the increased shares resulting from the stock split was reclassified from “Additional paid-in capital” to “Common stock”.
(e) Risks and uncertainties
The main operations of the Company are located in Taiwan and mainland China. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by political, economic, and legal environments in Taiwan and mainland China, as well as by the general state of the economy in these two countries. The Company’s results may be adversely affected by changes in the political, regulatory and social conditions in these two countries.
In March 2020, the World Health Organization declared the outbreak of COVID-19 to be a pandemic. The COVID-19 pandemic is having widespread, rapidly evolving, and unpredictable impacts on global society, economies, financial markets, and business practices. During 2021, there was a wide distribution of several vaccinations and medicines to overcome the pandemic. The Company has shifted its operations to co-exist along with the pandemic, including encouragement of vaccinations to all of its employees worldwide.
The uncertainty to which the COVID-19 pandemic impacts the Company’s business, affects management’s judgment and assumptions relating to accounting estimates in a variety of areas that depend on these estimates and assumptions. COVID-19 did not have a material influence on these estimates and judgements since the Company do not have any products approved for sale, we have not generated any revenue from the sale of products, and we do not expect to generate revenue from the sale of our product candidates until we complete clinical development, submit regulatory filings and receive approvals from the applicable regulatory bodies for such product candidates, if ever.
The Company continues to face relative uncertainty as to the remaining intensity and duration of and the nature and timeline for recovery from the COVID-19 pandemic going forward and how all of that impacts the Company, including the extent to which potentially permanent changes clinical trial operations have been caused by the pandemic. The Company has taken the approach of managing the pandemic (to the extent that it continues to remain a significant factor) via strengthening its balance sheet and cash assets and avoiding debt while focusing on cost controls.
The Company’s business, financial condition and results of operations may also be negatively impacted by risks related to natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt the Company’s operations.
(e)(f) Foreign currency translation and transaction and convenience translation
The accompanying consolidated financial statements are presented in the US Dollar (“US$”), which is the reporting currency of the Company. The functional currency of the Company is the US$. Advanced Biomed Inc. (Taiwan) use New Taiwan dollar (“NT$”) as its functional currency. Advanced Biomed HK Limited uses Hong Kong dollars (“HKD”) and Shanghai Sglcell Biotech Co., Ltd., Nanjing Yitian Biotech Co., Ltd., Beijing YitianYitan Jiarui Technology Co., Ltd., Shandong Sglcell Medical Devices Co., Ltd., Sglcell (Huangshan) Biotech Co., Ltd use Chinese Yuan Renminbi (“CNY”) as their functional currencies.
Assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Translation gains and losses are recognized in the consolidated statements of income and comprehensive income as other comprehensive income or loss. Transactions in currencies other than the reporting currency are measured and recorded in the reporting currency at the exchange rate prevailing on the transaction date. The cumulative gain or loss from foreign currency transactions is reflected in the consolidated statements of income and comprehensive income as other income (other expenses).
The value of foreign currencies including, the NT$ and CNY, may fluctuate against the US Dollar. Any significant variations of the aforementioned currencies relative to the US Dollar may materially affect the Company’s financial condition in terms of reporting in US Dollar. The following table outlines the currency exchange rates that were used in preparing the accompanying consolidated financial statements:
| June 30, 2022 | June 30, 2021 | |||||||
| US$ to NT$ fiscal year end | 29.74 | 27.91 | ||||||
| US$ to NT$ average rate | 28.29 | 28.47 | ||||||
| US$ to CNY fiscal year end | 6.70 | 6.46 | ||||||
| US$ to CNY average rate | 6.46 | 6.62 | ||||||
June 30, 2023 | June 30, 2022 | |||||||
| US$ to NT$ fiscal year end | 31.14 | 29.74 | ||||||
| US$ to NT$ average rate | 30.71 | 28.29 | ||||||
| US$ to CNY fiscal year end | 7.25 | 6.70 | ||||||
| US$ to CNY average rate | 6.95 | 6.46 | ||||||
(f)(g) Fair value measurement
Accounting guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact, and it considers assumptions that market participants would use when pricing the asset or liability.
Accounting guidance establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Accounting guidance establishes three levels of inputs that may be used to measure fair value:
| ● | Level 1 applies to assets or liabilities for which there are quoted prices, in active markets for identical assets or liabilities. |
| ● | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included within Level 1 that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical asset or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data. | |
| ● | Level 3 applies to asset or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
Cash and cash equivalents, other current assets, leases payable, accounts payables, accruals and other current liabilities are financial assets and liabilities. Cash and cash equivalents, other current assets, accounts payable, accruals and other current liabilities are subject to fair value measurement; however, because of their being short term in nature management believes their carrying values approximate their fair value. Financial instruments are fair value financial assets that are marked to fair value and are accounted for under as Level 3 under the above hierarchy. The Company accounts for bank loans and lease payables at amortized cost and has elected NOT to account for them under the fair value hierarchy.
(g)(h) Related parties
We adopted ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions
(h)(i) Cash
Cash consists of cash on hand, the Company’s demand deposit placed with financial institutions, which have original maturities of less than three months and unrestricted as to withdrawal and use. Deposits are held at highly liquid and well capitalized financial institutions. Risk of loss is not expected by management.
(i)(j) Intangible assets, net
The Company’s intangible assets are stated at cost less accumulated amortization and impairment, if any, and amortized on a straight-line basis over the estimated useful lives of the assets.
| Category | Estimated useful lives | |
| Software | 3 years | |
| Patents | 6 years |
Patents represent the estimated fair value assigned to finite-lived intangible assets acquired in a transaction that is accounted for as an acquisition of assets rather than a business combination are initially recognized in accordance with other application GAAP. Any consideration transferred in excess of the fair value of the assets acquired is allocated to each asset acquired on a relative fair value basis. Amortization is computed using the straight-line method over the estimated useful lives of the respective finite-lived intangible assets, generally six years. Intangible assets are reviewed for impairment at least annually or more frequently if indicators of potential impairment exist. The Company reviews finite-lived intangible assets for impairment at least annually or more frequently if events or changes in circumstances indicate that the carrying value of the assets might not be recoverable. If the carrying value of an finite-lived intangible asset exceeds its fair value, then it is written down to its adjusted fair value. As of June 30, 2023 and 2022, the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell Biotech Co., Ltd. Impairment allowance made was approximately US$1.6 million for the years ended June 30, 2023 and 2022, respectively, as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
(j)(k) Property, plant and equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation and impairment, if any, and depreciated on a straight-line basis over the estimated useful lives of the assets. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its intended use. Estimated useful lives are as follows:
| Category | Estimated useful lives | |
| Lab equipment | 3 to 5 years | |
| Computer equipment | 3 to 5 years | |
| Furniture and fixtures | 3 to 5 years | |
| Leasehold improvements | 3 years |
Expenditure for repair and maintenance costs, which do not materially extend the useful lives of the assets, are charged to expenses as incurred, whereas the expenditure for major renewals and betterment that substantially extends the useful lives of property and equipment are capitalized as additions to the related assets. Retirements, sales and disposals of assets are recorded by removing the costs, accumulated depreciation and impairment with any resulting gain or loss recognized in the consolidated statements of income.operations and comprehensive loss.
(k)(l) Impairment of long-lived assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may no longer be recoverable. When these events occur, the Company measures impairment by comparing the carrying value of the long-lived assets to the estimated undiscounted future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Company would recognize an impairment loss, which is the excess of carrying amount over the fair value of the assets, using the expected future discounted cash flows. No impairment of long-lived assets was recognized asAs of June 30, 2023 and 2022, the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell Biotech Co., Ltd. Impairment allowance made was approximately US$1.6 million for the years ended June 30, 2023 and 2021.2022, respectively, as management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.
(l)(m) Commitments and contingencies
In the normal course of business, the Company is subject to commitments and contingencies, including operating lease commitments, legal proceedings and claims arising out of its business that relate to a wide range of matters, such as government investigations and tax matters. The Company recognizes a liability for such contingency if it determines it is probable that a loss will occur, and a reasonable estimate of the loss can be made. The Company may consider many factors in making these assessments on liability for contingencies, including historical and the specific facts and circumstances of each matter.
(m)(n) Research and development expenses
Research and development expenses include costs directly attributable to the conduct of research and development programs, including licensing fees, cost of salaries, payroll taxes and other employee benefits, subcontractors and materials used for research and development activities, including clinical trials, manufacturing costs and professional services. All costs associated with research and developments are expensed as incurred.
The Company has already started the registration process and plans to start clinical trials in March 2024. The trials are expected to end in June 2025, and the company expects to obtain the required registration certificate by the end of 2026. As of June 30, 2023, the Company has not commenced sales of the products nor have any revenue-generating products and do not expect sales of revenue-generating product candidates until the Company has completed clinical development, submitted regulatory filings, and received applicable regulatory approvals for candidate products.
Hence, the Company incurred its research and development cost during the years ended June 30, 2023 and 2022, which is in compliance under ASC 730-10-25.
(n)(o) General and administrative expenses
General and administrative expenses mainly consist of staff cost, depreciation, office supplies and upkeep expenses, travelling and entertainment, legal and professional fees, property and related expenses, other miscellaneous administrative expenses.
(o)(p) Operating leases
The Company adoptedPrior to the adoption of ASC 842 on JulyJanuary 1, 2020. 2019:
Leases, mainly leases of factory buildings, offices and employee dormitories, where substantially all the rewards and risks of ownership of assets remain with the lessor are accounted for as operating leases. Payments made under operating leases are recognized as an expense on a straight-line basis over the lease term. The Company had no finance leases for any of the periods stated herein.
Upon and hereafter the adoption of ASC 842 on January 1, 2019:
The Company determines if an arrangement is a lease at inception. Operating leases are included in operating lease right-of-use (“ROU”) assets, operating lease liability, and operating lease liability, non-current in the Company’s consolidated balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. When determining the lease term, the Company includes options to extend or terminate the lease when it is reasonably certain that it will exercise that option, if any. As the Company’s leases do not provide an implicit rate, the Company used an incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company has elected to adopt the following lease policies in conjunction with the adoption of ASU 2016-02: (i) for leases that have lease terms of 12 months or less and does not include a purchase option that is reasonably certain to exercise, the Company elected not to apply ASC 842 recognition requirements; and (ii) the Company elected to apply the package of practical expedients for existing arrangements entered into prior to JulyJanuary 1, 20202019 to not reassess (a) whether an arrangement is or contains a lease, (b) the lease classification applied to existing leases, and(c)and (c) initial direct costs.
(p) Income taxes
The Group accounts for income taxes under ASC 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities.
The provisions of ASC 740-10-25, “Accounting for Uncertainty in Income Taxes,” prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures.
The Company did not accrue any liability, interest or penalties related to uncertain tax positions in its provision for income taxes line of its consolidated statements of income for the periods ended June 30, 2022 and 2021, respectively. The Company does not expect that its assessment regarding unrecognized tax positions will materially change over the next 12 months.
(q) Loss per share
Loss per share is computed by dividing net loss attributable to ordinary stockholdersshareholders by the weighted average number shares of common stock outstanding during the year. Diluted lossearnings per share reflectsreflect the potential dilution that could occur if securities or other contracts to issue shares of common stock were exercised or converted into common stock.
(r) Business combination
The purchase price of an acquired company is allocated between tangible and intangible assets acquired and liabilities assumed from the acquired business based on their estimated fair values, with the residual of the purchase price recorded as goodwill. Transaction costs associated with business combinations are expensed as incurred and are included in general and administrative expenses in the Company’s consolidated statements of operations. The results of operations of the acquired business are included in the Company’s operating results from the date of acquisition.
(s) Segment reporting
ASC 280, “Segment Reporting”, establishes standards for reporting information about operating segments on a basis consistent with the Company’s internal organizational structure as well as information about geographical areas, business segments and major clients in financial statements for detailing the Company’s business segments. Based on the criteria established by ASC 280, the Company’s chief operating decision maker (“CODM”) has been identified as the Chief Executive Officer, who reviews consolidated results when making decisions about allocating resources and assessing performance of the Company. As a whole and hence, the Company has only one reportable segment. The Company does not distinguish between markets or segments for the purpose of internal reporting. As the Company’s long-lived assets are substantially located in Taiwan, no geographical segments are presented.
(t)(r) Recent accounting pronouncements
The Company considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued. Under the Jump start Our Business Start-ups Act of 2012, as amended (the “JOBS Act”), the Company meets the definition of an emerging growth company, or EGC, and has elected the extended transition period for complying with new or revised accounting standards, which delays the adoption of these accounting standards until they would apply to private companies.
In May 2020, the Financial Accounting Standard Board (“FASB”) issued ASU 2020-05, which is an update to ASU Update No. 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments”, which introduced the expected credit losses methodology for the measurement of credit losses on financial assets measured at amortized cost basis, replacing the previous incurred loss methodology. The amendments in Update 2016-13 added Topic 326, Financial Instruments — Credit Losses, and made several consequential amendments to the Codification. Update 2016-13 also modified the accounting for available-for-sale debt securities, which must be individually assessed for credit losses when fair value is less than the amortized cost basis, in accordance with Subtopic 326-30, Financial Instruments — Credit Losses — Available-for-Sale Debt Securities. The amendments in this Update address those stakeholders’ concerns by providing an option to irrevocably elect the fair value option for certain financial assets previously measured at amortized cost basis. For those entities, the targeted transition relief will increase comparability of financial statement information by providing an option to align measurement methodologies for similar financial assets. Furthermore, the targeted transition relief also may reduce the costs for some entities to comply with the amendments in Update 2016-13 while still providing financial statement users with decision-useful information. In November 2020, the FASB issued ASU No. 2020-10, which to update the effective date of ASU No. 2016-02 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. The Company is currently evaluating the impact of this new standard on Company’s consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-08, Codification Improvements to Subtopic 310-20, Receivables — Nonrefundable Fees and Other Costs. The amendments in this Update represent changes to clarify the Codification. The amendments make the Codification easier to understand and easier to apply by eliminating inconsistencies and providing clarifications. ASU 2021-08 is effective for the Company for annual and interim reporting periods beginning July 1, 2021. Early application is not permitted. All entities should apply the amendments in this Update on a prospective basis as of the beginning of the period of adoption for existing or newly purchased callable debt securities. These amendments do not change the effective dates for Update 2017-08. The Company is currently evaluating the impact of this new standard on Company’s consolidated financial statements and related disclosures.
In October 2021, the FASB issued ASU 2021-10, Codification Improvements. The amendments in this Update represent changes to clarify the Codification or correct unintended application of guidance that are not expected to have a significant effect on current accounting practice or create a significant administrative cost to most entities. The amendments in this Update affect a wide variety of Topics in the Codification and apply to all reporting entities within the scope of the affected accounting guidance. ASU 2021-10 is effective for annual periods beginning after December 15, 2021 for public business entities. Early application is permitted. The amendments in this Update should be applied retrospectively. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
Except as mentioned above, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s consolidated balance sheets, statements of incomeoperations and comprehensive incomeloss and statements of cash flows.
3.4. PREPAID EXPENSES AND OTHER CURRENT ASSETS, NET
| June 30, 2022 | June 30, 2021 | |||||||
| US $ | US $ | |||||||
| Prepaid for research and development materials | 27,771 | 847,156 | ||||||
| Tax refundable | 139,049 | - | ||||||
| Prepayment | 31,558 | - | ||||||
| Other receivables | 23,469 | 419 | ||||||
| 221,847 | 847,575 | |||||||
| Less: allowance for credit losses | - | - | ||||||
| Total prepaid expenses and other current assets | 221,847 | 847,575 | ||||||
| June 30, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Prepaid for research and development materials | - | 27,771 | ||||||
| Tax refundable | 146,625 | 139,049 | ||||||
| Other receivables | 3,325 | 23,469 | ||||||
| Prepayment | 13,759 | 31,558 | ||||||
| Total prepaid expenses and other current assets | 163,709 | 221,847 | ||||||
| Less: allowance for credit losses | - | - | ||||||
| Total prepaid expenses and other current assets | 163,709 | 221,847 | ||||||
4.5. EQUIPMENT, NET
Property, plant and equipment, net, consists of the following:
| June 30, 2022 | June 30, 2021 | |||||||
| US $ | US $ | |||||||
| Lab equipment | 1,311,818 | 824,624 | ||||||
| Computer equipment | 26,712 | 3,540 | ||||||
| Furniture and fixtures | 38,089 | 7,479 | ||||||
| Leasehold improvements | 212,078 | - | ||||||
| 1,588,697 | 835,643 | |||||||
| Less: accumulated depreciation | (805,624 | ) | (749,788 | ) | ||||
| 783,073 | 85,855 | |||||||
| June 30, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Lab equipment | 1,446,527 | 1,311,818 | ||||||
| Computer equipment | 31,135 | 26,712 | ||||||
| Furniture and fixtures | 35,412 | 38,089 | ||||||
| Leasehold improvements | 201,994 | 212,078 | ||||||
| 1,715,068 | 1,588,697 | |||||||
| Less: accumulated depreciation | (996,371 | ) | (805,624 | ) | ||||
| Property, plant and equipment, net | 718,697 | 783,073 | ||||||
Depreciation expenses were approximately US$114,895228,807 and US$57,850114,895 for the years ended June 30, 20222023 and 2021,2022, respectively.
5.6. ACQUISITION
On January 1, 2022, the Company entered into an agreement to acquire all of the equity interests of Shanghai Sglcell Biotech Co., Ltd. The primary assets purchased in the acquisition were intangible assets, equipment and net working capital. The Company concluded the assets acquired and liabilities assumed did not meet the definition of a business as a limited number of inputs were acquired but no substantive business processes or signs of output were acquired. As such, the acquisition was accounted for as an asset purchase. The purchase consideration was approximately RMB12,000,000 (approximately US$1,791,553) in cash and paid in July 2022. The estimated fair value of assets acquired, and liabilities assumed as of acquisition date are approximately US$1,791,553.
The Company allocated the purchase price of US$1.8 million between equipment of US$0.1 million, right-of-use asset of US$0.4 million, cash of US$0.2 million, prepaid and other current assets of US$0.9 million, assumed lease payable and accounts payable, accruals and other current liabilities of US$1.5 million and intangible assets related to patents registered or applied with the China Patent and Trademark Office of US$1.6 million, attributable to 8 patents on novel separation technologies for antibody-free and label-free enrichment of rare cell-based cancer biomarkers (such as CTC, CTC cluster, tumor marker expressed cells) from very dense blood cells, 6 patents on high throughput droplet microfluidic chips for nano-liter scale reagent preparation and single-cell, 3 patents for the device of water phase single-cell isolation and capturing for single cell applications, 6 patents on single-CTC detection/discrimination and delivery for single-CTC sorting and downstream single-CTC analysis, and 3 patents on target cells pre-concentration and enrichment.
Management of the Company is responsible for determining the fair value of assets acquired and liabilities assumed as of the acquisition date and considered a number of factors including valuations from independent appraiser. Acquisition-related costs incurred for the acquisitions were not material and have been expensed into general and administrative expense when incurred.
The following table summarizes the estimated fair value of assets acquired, and liabilities assumed as of acquisition date which represents the net purchase price allocation on the date of the acquisition of Shanghai Sglcell Biotech Co., Ltd and its subsidiaries, based on valuation performed by an independent valuation firm engaged by the Company and translated the fair value from RMB to USD using the exchange rate on 1 January 2022 at the rate of US$1.00 to CNY6.70:
| US$ | ||||
| Cash | 199,527 | |||
| Prepaid expenses and other current assets | 904,603 | |||
| Right-of-use asset, net | 467,197 | |||
| Lease payable | (467,197 | ) | ||
| Accounts payable, accruals and other current liabilities | (1,000,131 | ) | ||
| Equipment | 69,580 | |||
| Intangible assets | 1,617,974 | |||
| Total consideration | 1,791,553 | |||
6.7. DISPOSAL OF SUBSIDIARIES
On June 8, 2023, Shanghai Sglcell Biotech Co., Ltd. transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to independent third-party individuals at aggregate consideration of CNY500,000 (approximately US$71,942). Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd. have not commenced its business activity since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., was CNY498,587 (approximately US$71,739).
On June 9, 2023, Shandong Sglcell Biotech Co., Ltd., the wholly owned subsidiary of Shanghai Sglcell Biotech Co., Ltd., was transferred to independent third-party individuals at zero consideration without any other obligations arising from the transfer. Shandong Sglcell Biotech Co., Ltd. has been inactive since it was incorporated on July 8, 2021 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
On June 15, 2023, Sglcell (Huangshan) Biotech Co., Ltd., the wholly owned subsidiary of Advanced Biomed HK Limited, was transferred to an independent third-party corporation at zero consideration without any other obligations arising from the transfers. Sglcell (Huangshan) Biotech Co., Ltd. has been dormant since its incorporation date from March 4, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
Effect of disposal subsidiaries on the financial position of the Company:
| US$ | ||||
| Cash | 50,486 | |||
| Prepaid expenses and other current assets | 246,540 | |||
| Equipment | 395 | |||
| Accounts payable, accruals and other current liabilities | (75,272 | ) | ||
| Net assets | 222,149 | |||
| Consideration * | 71,942 | |||
| Loss on disposal of subsidiaries | 150,207 | |||
* Debt forgiving as consideration based on the settlement agreement on June 10, 2023.
8. INTANGIBLE ASSETS, NET
The following table summarizes the carrying amount of the Company’s finite-lived intangible assets:
| June 30, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Acquired software OCR and clinical intelligent database system | 391,943 | 422,536 | ||||||
| Less: accumulated amortization | (141,622 | ) | (11,737 | ) | ||||
| 250,321 | 410,799 | |||||||
Finite-lived intangible assets are carried at cost less accumulated amortization. Amortization expense was approximately US$11,737136,424 and US$011,737 for the years ended June 30, 20222023 and 2021,2022, respectively.
| June 30, 2023 | June 30, 2022 | |||||||
| US $ | US $ | |||||||
| Acquired patents through acquisition | 1,617,974 | 1,617,974 | ||||||
| Less: impairment | (1,617,974) | (1,617,974 | ) | |||||
| - | - | |||||||
As of June 30, 2023 and 2022, the Company had finite-lived intangible assets of US$1.6 million of purchased patents from the acquisition of Shanghai Sglcell Biotech Co., Ltd. Impairment allowance made wasof approximately US$1,617,974 and nil for the years ended June 30, 2022 and 2021, respectively, aswas made management assessed that there were changes in circumstances indicate that the carrying value of the assets might not be recoverable.recoverable as of June 30, 2023 and 2022.
9. DEFERRED INITIAL PUBLIC OFFERING (“IPO”) COSTS
The Company complies with the requirement of the ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering”. Initial public offering expense directly attributable to offering of securities are deferred and would be charged against the gross proceeds of the offering, as a reduction in share capital. These deferred expenses mainly consist of underwriting, legal and other expenses incurred through the balance sheet date that are directly related to the intended IPO. Should the IPO prove to be unsuccessful, these deferred initial public offering costs, as well as additional expenses to be incurred, will be charged to operations. As of June 30, 2023 and 2022, the Company capitalized US$553 thousand and US$ nil of deferred initial public offering costs.
7.10. ACCOUNTS PAYABLE, ACCRUALS AND OTHER CURRENT LIABILITIES
Account Payable, accrued expenses and other liabilities consists of the following:
| June 30, 2022 | June 30, 2021 | |||||||
| US $ | US $ | |||||||
| Accounts Payable | 762,202 | 194 | ||||||
| Payroll payable | 96,203 | 19,319 | ||||||
| Acquisition payables* | 1,791,553 | - | ||||||
| Other payables** | 103,077 | 3,597 | ||||||
| Total | 2,753,035 | 23,110 | ||||||
| June 30, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Accounts Payable | 632,820 | 762,202 | ||||||
| Payroll payable | 85,561 | 96,203 | ||||||
| Amount due to related parties – major shareholders * | 1,052,677 | - | ||||||
| Amount due to related parties – related corporations** | 603,802 | - | ||||||
| Acquisition payables# | - | 1,791,553 | ||||||
| Other payables## | 684,156 | 103,077 | ||||||
| 3,059,016 | 2,753,035 | |||||||
*Acquisition The amount due to the Company’s major shareholders as of June 30, 2023 is non-trade, unsecured, interest-free and repayable on demand.
** The amount due to related corporations, which the Company’s major shareholders have controlling equity interest in, as of June 30, 2023 are non-trade, unsecured, interest-free and repayable on demand.
#Acquisition payables as of June 30, 2022, was an amount of US$1,791,553 payable to previous shareholder of the acquired subsidiary, Shanghai Sglcell Biotech Co., Ltd. and the amount was subsequently settled in its entirety.entirety in July 2022.
**## The balanceamount consists of US$103,077payables owed to third party creditors; the balance wascreditors, which are unsecured, interest-free and repayable on demand.demand, and other payables due to operational use.
8.11. EQUITY
For the sake of undertaking a public offering of the Company’s common stock,stocks, the Company has performed a series of re-organizing transactions resulting in 25,000,000 shares and 24,750,000 shares of common stock issued and outstanding as of June 30, 2023 and June 30, 2022. The Company has accounted for these shares had they been issued and outstanding at the beginning of the first period presented. The Company only has one single class of common stock that is accounted for as permanent equity.
The above mentioned re-organizing transactions and equity is before the stock split on May 16, 2023.
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now have 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis similar to share split or dividend pursuant to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split. The shares of common stock retain a par value of $0.001 per share. Accordingly, an amount equal to the par value of the increased shares resulting from the stock split was reclassified from “Additional paid-in capital” to “Common stock”.
12. STOCK OPTION
Effective from March 30, 2023, the Stock Incentive Plan (the “2023 Plan”) was approved by the Company's Board of Directors. Under the 2023 Plan, the Board of Directors may grant options or purchase rights to purchase common stock to officers, employees, and other persons who provide services to the Company or any related company. The participants to whom awards are granted, the type of awards granted, the number of shares covered for each award, and the purchase or exercise price, conditions and other terms of each award are determined by the Board of Directors, except that the term of the options shall not exceed 10 years. A total of 15 million shares of our common stock are subject to the 2023 Plan and maybe either a qualified or non-qualified stock option. The shares issued for the 2023 Plan may be either treasury or authorized and unissued shares. As of June 30, 2023, the Company has granted no stock options to purchase any shares of the common stock under the 2023 Plan.
13. RELATED PARTY TRANSACTIONS
These related parties of the Company with whom transactions are reported in these financial statements are as follows:
| Name of Related Party | Relationship to Us | |
| Yi Lu, Ph.D. | Chairman of the Board, Chief Science Officer, President and Shareholder of the Company | |
| Hung To Pau, Ph.D. | Chief Executive Officer, Director, Secretary and Shareholder of the Company | |
| Steven I-Fang Cheng, Ph.D. | Chief Technology Officer of the Company | |
| Chen-Yi Lee | Chen-Yi Lee is the sole director and the controlling person of Advance On Ventures Limited, which owns 10.92% equity interest in the Company and has sole voting and dispositive power over shares beneficially owned by Advance On Ventures Limited. Chen-Yi Lee is also the Shareholder of the Company | |
| Advance On Ventures Limited | Shareholder of the Company | |
| Well Fancy Development Ltd | CEO-Hung To Pau is the director and shareholder of the entity | |
| Shanghai Junfu Electronic Technology Co., Ltd. | CEO-Hung To Pau is the legal person and shareholder of the entity |
In the ordinary course of business, during the fiscal years ended June 30, 2023 and 2022, the Company was involved in certain transactions, either at cost or current market prices, and on the normal commercial terms with related parties. The following table provides the transactions with these parties for the years as presented (for the portion of such period that they were considered related):
| | June 30, 2023 | June 30, 2022 | ||||||
| US$ | US$ | |||||||
| Amount due to related parties – major shareholders | ||||||||
| Name of related party | ||||||||
| Hung To Pau, Ph.D. (1) | 933,471 | — | ||||||
| Yi Lu, Ph.D. (2) | 114,907 | — | ||||||
| Chen-Yi Lee (3) | 4,299 | — | ||||||
| Amount due to related parties – related corporations | ||||||||
| Name of related party | ||||||||
| Well Fancy Development Ltd (4) | 396,905 | — | ||||||
| Shanghai Junfu Electronic Technology Co., Ltd. (5) | 206,897 | — | ||||||
9.
| 1. | Payments of IPO costs paid on behalf of Advanced Biomed Inc. |
| 2. | Advanced Biomed Inc.(Taiwan) entered into an unsecured, interest-free loan to Yi Lu amounting to NTD 3,578,212 (approximately US$114,907) for general working capital in January 2023. As of June 30, 2023 and 2022, the loan balance due to Yi Lu amounted to US$114,907 and nil, respectively. |
| 3. | Payments of expenses on behalf of Advanced Biomed Inc. (Taiwan). |
| 4. | Advanced Biomed Inc.(Taiwan) entered into four unsecured, interest-free loans to Well Fancy Development Ltd amounting to NTD 2,967,700 (approximately US$95,302), NTD 2,989,873 (approximately US$96,014), NTD 4,270,200 (approximately US$137,129), and NTD 1,945,000 (approximately US$62,460) for general working capital in August 2022, April 2023, May 2023, and June 2023, respectively. As of June 30, 2023 and 2022, the loan balance due to Well Fancy Development Ltd totally amounted to US$396,905 and nil, respectively. |
| 5. | Shanghai Sglcell Biotech Co., Ltd entered into three unsecured, interest-free loans to Shanghai Junfu Electronic Technology Co., Ltd. amounting to RMB 500,000 (approximately US$68,965) for general working capital in April 2023, May 2023, and June 2023, respectively. As of June 30, 2023 and 2022, the loan balance due to Shanghai Sglcell Biotech Co., Ltd totally amounted to US$206,897 and nil, respectively. |
14. CONCENTRATIONS AND RISKS
Credit Risk
Credit risk is the potential financial loss to the Company resulting from the failure of a customer or a counterparty to settle its financial and contractual obligations to the Company, as and when they fall due. As the Company does not hold any collateral, the maximum exposure to credit risk is the carrying amounts of other receivables (exclude prepayments) and cash and bank deposits presented on the consolidated balance sheets. The Company has no other financial assets which carry significant exposure to credit risk.
Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Company’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Company’s reputation.
Typically, the Company ensures that it has sufficient cash on demand to meet expected operational expenses for a period of 60 days, including the servicing of financial obligations; this excludes the potential impact of extreme circumstances that cannot reasonably be predicted, such as natural disasters.
Foreign currency risk
Foreign currency risk is the risk that the fair value or future cash flows of an exposure will fluctuate because of changes in foreign exchange rates. The company’s exposure to the risk of changes in foreign exchange rates relates primarily to the Company’s operating activities (when expense is denominated in a foreign currency) and the Company’s net investments in foreign subsidiaries.
Impact of Inflation
Inflation in Taiwan and PRC has not materially affected ourthe Company’s profitability and operating results. However, wethe Company can provide no assurance that we will be unaffected by higher inflation rates in SingaporeTaiwan and PRC or globally in the future.
10.15. COMMITMENTS AND CONTINGENCIES
Contingencies
In the ordinary course of business, the Company may be subject to legal proceedings regarding contractual and employment relationships and a variety of other matters. The Company records contingent liabilities resulting from such claims, when a loss is assessed to be probable, and the amount of the loss is reasonably estimable. In the opinion of management, there were no pending or threatened claims and litigation as of June 30,202230, 2023 and up through August 11,October 27, 2023, the issuance date of these consolidated financial statements were available to be issued.
Lease commitment
The Company determines if a contract contains a lease at inception. US GAAP requires that the Company’s leases be evaluated and classified as operating or finance leases for financial reporting purposes. The classification evaluation begins at the commencement date and the lease term used in the evaluation includes the non-cancellable period for which the Company has the right to use the underlying asset, together with renewal option periods when the exercise of the renewal option is reasonably certain and failure to exercise such option which results in an economic penalty.
The right-of-use assets relate to leases of office premises and a dormitory for employees in the PRC and the laboratory in Taiwan.
The recognized operating lease ROU assets and lease liabilities as follows:
| June 30, 2022 | June 30, 2021 | |||||||
| US $ | US $ | |||||||
| Operating lease ROU asset | 442,895 | 7,021 | ||||||
| June 30, 2023 | June 30, 2022 | |||||||
| US$ | US$ | |||||||
| Operating lease ROU asset | 235,373 | 442,895 | ||||||
| June 30, 2023 | June 30, 2022 | |||||||||||||||
| June 30, 2022 | June 30, 2021 | US$ | US$ | |||||||||||||
| US $ | US $ | |||||||||||||||
| Operating lease liabilities | ||||||||||||||||
| Current portion | 236,677 | 5,240 | 216,567 | 236,677 | ||||||||||||
| Non-current portion | 232,191 | 1,781 | 18,806 | 232,191 | ||||||||||||
| Total | 468,868 | 7,021 | 235,373 | 468,868 | ||||||||||||
As of June 30, 2022,2023, future minimum lease payments under the non-cancellable operating leases are as follows:
| Future payment | US$ | US$ | ||||||
| 2023 | 244,287 | |||||||
| 2024 | 221,108 | 223,480 | ||||||
| 2025 | 18,426 | 19,014 | ||||||
| 2026 | - | - | ||||||
| 2027 | - | - | ||||||
| 2028 | - | |||||||
| Thereafter | - | - | ||||||
| Total future lease payment | 483,821 | 242,494 | ||||||
| Less: imputed interest | (14,953 | ) | (7,121 | ) | ||||
| Present value of operating lease liabilities | 468,868 | 235,373 | ||||||
| Operating lease liabilities, current portion | 236,677 | 216,567 | ||||||
| Operating lease liabilities, non-current portion | 232,191 | 18,806 | ||||||
The following summarizes other supplemental information about the Company’s operating lease as of June 30, 2022:2023:
| Weighted average discount rate | % | |||
| Weighted average remaining lease term (years) |
11.16. RESTATEMENT
The Company has revised the financial statements previously reported as of June 30, 2022 as the acquisition of all of the equity interests of Shanghai Sglcell Biotech Co., Ltd. and the assets acquired and liabilities assumed in Note 56 did not meet the definition of a business as a limited number of inputs were acquired but no substantive processes were acquired. As such, the acquisition was adjusted and accounted for as an asset purchase. Hence, we have adjusted the financial statements accordingly.
F-36
The following tables summarize the material impacts on the Group’s consolidated balance sheet as of June 30, 2022, and the related consolidated statements of operations and comprehensive loss, changes in stockholders’ equity for the year ended June 30, 2022.
| Impact of adjustments June 30, 2022 | ||||||||||||
| As previously reported | Adjustments | As restated | ||||||||||
| US $ | US $ | US $ | ||||||||||
| Goodwill | 1,617,974 | (1,617,974 | ) | - | ||||||||
| Intangible assets | 422,536 | 1,617,974 | 2,028,773 | |||||||||
| Less: Accumulated amortization | (11,737 | ) | - | (11,737 | ) | |||||||
| Less: Impairment allowance | - | (1,617,974 | ) | (1,617,974 | ) | |||||||
| Net | 410,799 | - | 410,799 | |||||||||
| Others | 6,713,499 | - | 6,713,499 | |||||||||
| Total assets | 8,331,473 | (1,617,974 | ) | 6,713,499 | ||||||||
| Accumulated deficits | (7,841,046 | ) | (1,617,974 | ) | (9,459,020 | ) | ||||||
| Others | 12,950,616 | - | 12,950,616 | |||||||||
| Total stockholders’ equity | 5,109,570 | (1,617,974 | ) | 3,491,596 | ||||||||
| Impairment of intangible assets | - | (1,617,974 | ) | (1,617,974 | ) | |||||||
| Net loss | (2,409,748 | ) | (1,617,974 | ) | (4,027,722 | ) | ||||||
17. SUBSEQUENT EVENTS
12. SUBSEQUENT EVENTS
The Company has assessed all events from June 30, 20222023 through August 11,October 27, 2023, which is the date that these consolidated financial statements are available to be issued, unless as disclosed below, there are not any material subsequent events that require disclosure in these consolidated financial statements other than events detailed below.
On September 13, 2022, the Company issued 250,000 shares at US$4.0 to Newlink Technology Inc. pursuant to the Investment Agreement the Company entered into with Newlink Technology Inc.
On May 16, 2023, the Company effected a forward stock split of all issued and outstanding shares of 25,000,000 shares at a ratio of 1-to-4. As a result of the forward split, the Company now has 100,000,000 common stock issued and outstanding as of the date hereof. The Company believed it is appropriate to reflect the above transactions on a retroactive basis in accordance to ASC 260. All references made to share or per share amounts in the accompanying consolidated financial statements and applicable disclosures have been retroactively adjusted to reflect the 4 for 1 share split.
On June 8, 2023, Shanghai Sglcell Biotech Co., Ltd. transferred its wholly owned subsidiary, Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., to independent third-party individuals at aggregate consideration of CNY500,000 (approximately US$72,780) without any other obligations arising from the transfer. Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd. have been inactive since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Nanjing Yitian Biotech Co., Ltd. and its subsidiary, Beijing Yitan Jiarui Technology Co., Ltd., was CNY500,000.
On June 9, 2023, Shandong Sglcell Medical Devices Co., Ltd., the wholly owned subsidiary of Shanghai Sglcell Biotech Co., Ltd., was transferred to independent third-party individuals at zero consideration without any other obligations arising from the transfer. Shanghai Sglcell Biotech Co., Ltd. has been inactive since it was acquired on January 1, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
On June 15, 2023, Sglcell (Huangshan) Biotech Co., Ltd., the wholly owned subsidiary of Advanced Biomed HK Limited, was transferred to an independent third-party corporation at zero consideration without any other obligations arising from the transfers. Sglcell (Huangshan) Biotech Co., Ltd. has been dormant since its incorporation date from March 4, 2022 and the Company believed the transfer will improve in operational efficiency. The consideration is determined by the Company according to the net assets appraisal report issued by an independent third-party appraisal company on May 31, 2023, as of the date of May 31, 2023, the net assets value of Shandong Sglcell Medical Devices Co., Ltd., was zero.
No dealer, salesperson or any other person is authorized to give any information or make any representations in connection with this offering other than those contained in this prospectus and, if given or made, the information or representations must not be relied upon as having been authorized by us. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any security other than the securities offered by this prospectus, or an offer to sell or a solicitation of an offer to buy any securities by anyone in any jurisdiction in which the offer or solicitation is not authorized or is unlawful.
Until , all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in an addition to the dealers obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
25,000,000 Shares of Common Stock
PROSPECTUS
Dated , 2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than underwriting commissions and the underwriter’s unaccountable expense allowance, to be paid in connection with the sale of the shares of common stock being registered, all of which we will pay. All amounts, other than the SEC registration fee, and the FINRA filing fee are estimates.
| SEC registration fee | $ | 16,722 | $ | 16,722 | ||||
| Printing/EDGAR expenses | $ | 25,000 | $ | 25,000 | ||||
| FINRA filing fee | $ | 23,261 | $ | 23,261 | ||||
| Nasdaq entry and listing fee | $ | 75,000 | $ | 75,000 | ||||
| Legal fees and expenses | $ | 550,000 | $ | 550,000 | ||||
| Accounting fees and expenses | $ | 180,000 | $ | 380,000 | ||||
| Miscellaneous | $ | 109,800 | $ | 164,800 | ||||
| Total | $ | 979,783 | $ | 1,234,783 |
Item 14. Indemnification of Directors and Officers
Nevada Law
Section 78.7502 of the Nevada Revised Statutes provides that a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation or he acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful.
Section 78.7502 further provides a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that he is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if he is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation or he acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation.
Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any non-derivative proceeding or any derivative proceeding, or in defense of any claim, issue or matter therein, the corporation shall indemnify him or her against expenses, including attorneys’ fees, actually and reasonably incurred in connection with the defense.
II-1
Further, Nevada law permits a Nevada corporation to purchase and maintain insurance or to make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him or her and liability and expenses incurred by him or her in his or her capacity as a director, officer, employee or agent, or arising out of his or her status as such, whether or not the corporation has the authority to indemnify him or her against such liability and expenses.
Charter Provisions
Pursuant to our Articles of Incorporation and Bylaws, we may indemnify an officer or director who is made a party to any proceeding, including a lawsuit, because of his position, if he acted in good faith and in a manner he reasonably believed to be in or not opposed to our best interest, provided, however, that (i) we will not indemnify such person against expenses incurred in connection with an action if he is threatened but does not become a party unless the incurring of such expenses was authorized by the board of directors and (ii) we will not indemnify against any amount paid in settlement unless our board of directors has consented to such settlement.
An officer or director is not entitled to indemnification against costs or expenses incurred in connection with any action, commenced by such person against us or any person who is or was a director, officer, fiduciary, employee or agent of our company unless and to the extent that the officer or directors is successful on the merits in any such proceeding as to which such person is to be indemnified, we must indemnify him against all expenses incurred, including attorney’s fees. With respect to a derivative action, indemnity may be made only for expenses actually and reasonably incurred in defending the proceeding, and if the officer or directors is judged liable, only by a court order. The indemnification is intended to be to the fullest extent permitted by the laws of the State of Nevada.
Item 15. Recent Sales of Unregistered Securities
During the past three years, we have issued the following securities which were not registered under the Securities Act. We believe that each of the following issuance was exempt from registration under the Securities Act in reliance on Regulation S under the Securities Act regarding sales by an issuer in offshore transactions. No underwriters were involved in these issuances of securities.
On July 16, 2021, we issued 8,000,000 shares to Dr. Hung To Pau. On March 15, 2022, Dr. Hung To Pau transferred all of his 8,000,000 shares to Sglcell Ltd, an exempted company incorporated under the law of Cayman Islands, the sole shareholder of which is Dr. Hung To Pau for a total consideration of $8,000. On June 8, 2022, Sglcell Ltd transferred all of its 8,000,000 shares to Dr. Yi Lu for a total consideration of $8,000.
Shares Issued to Advanced Biomed Taiwan Holders/Employees
On August 12, 2022, we issued additional 385,000 shares to Dr. Yi Lu, and 257 shares to Chen-Yi Lee as a consideration of Dr. Yi Lu and Chen-Yi Lee transferred 2,998,000 shares and 2,000 shares owned by them respectively in Advanced Biomed Inc. (Taiwan), representing in aggregate 100% of the issued share capital of Advanced Biomed Inc. (Taiwan), to the Company pursuant to the share exchange agreement the Company entered into with Dr. Yi Lu and Chen-Yi Lee.
On October 24, 2022, we issued 365,352 shares at nil consideration to Chen-Yi Lee , who is an employee of Advanced Biomed Taiwan, for consideration of past services to Advanced Biomed Taiwan. On October 24, 2022, we also issued 2,730,000 shares at nil consideration to Advance On Ventures Limited, a company incorporated under the law of British Virgin Islands (the “Ventures Limited”), the beneficial owners of which are employees of Advanced Biomed Taiwan for past services to Advanced Biomed Taiwan. We issued 4,405,625 shares, 2,193,750 shares, 2,060,000 shares, 1,511,250 shares, 1,243,750 shares, 1,230,000 shares respectively to Dr. Hung To Pau, Yimin Jin, Xiaoyuan Luo, Nanzhen Shen, Jian Wang and Qiang Chen pursuant to the Debt-For-Equity Exchange Agreement the Company entered into with the abovementioned shareholders on June 30, 2022 to settle debt of a total amount of NTD 174,020,033 and RMB 22,200,000 (approximately $9.04 million).
Shares Issued to Advanced Biomed Nevada Investors
On October 25, 2022, we issued 625,000 shares to Hanyu Assets Co. Ltd. pursuant to the Investment Agreement the Company entered into with Hanyu Assets Co. Ltd for a total consideration of $2,500,000. on June 6, 2022, and we issued 250,000 shares to Newlink Technology Inc. pursuant to the Investment Agreement the Company entered into with Newlink Technology Inc. on June 6, 2022 for a total consideration of $1,000,000.
As of the date of this prospectus, the securities we issued are as follows:
| Purchaser | Date of Issuance | Number of Securities | |||||
| Common Stock | |||||||
| Yi Lu | June 8, 2022&August 12, 2022 | 8,385,000 | |||||
| Chen-Yi Lee | August 12, 2022 & October 24, 2022 | 365,625 | |||||
| Hung To Pau | October 24, 2022 | 4,405,625 | |||||
| Advanced On Ventures Limited | October 24, 2022 | 2,730,000 | |||||
| Yimin Jin | October 24, 2022 | 2,193,750 | |||||
| Nanzhen Shen | October 24, 2022 | 1,511,250 | |||||
| Qiang Chen | October 24, 2022 | 1,230,000 | |||||
| Jian Wang | October 24, 2022 | 1,243,750 | |||||
| Hanyu Assets Co., ltd. | October 25, 2022 | 625,000 | |||||
| Newlink Technology Inc. | October 25, 2022 | 250,000 | |||||
| Xiaoyuan Luo | October 24, 2022 | 2,060,000 | |||||
II-2
Item 16. Exhibits Index
* Filed herewith
** Previously filed
*** To be filed by Amendment to this Registration Statement
Item 17. Undertakings
The Registrant hereby undertakes:
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
II-3
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) (§230.424(b) of this chapter) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
To provide to the underwriter at the closing specified in the underwriting agreements certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter); |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Tainan, Taiwan, on August 28,December 5, 2023.
| ADVANCED BIOMED INC. | ||
| By: | /s/ Hung To Pau | |
| Hung To Pau | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Yi Lu | Chairman of the Board and |
| ||
| Yi Lu | Chief Science Officer, and President | |||
| /s/ Mingze Yin | Chief Financial Officer |
| ||
| Mingze Yin | (Principal Financial and Accounting Officer) | |||
| /s/ Hung To Pau | Director and |
| ||
| Hung To Pau | Chief Executive Officer (Principal Executive Officer) |
II-5