
As filed with the Securities and Exchange Commission on January 10, 2020
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-3
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
ENDRA LIFE SCIENCES INC. | |
(Exact name of registrant as specified in its charter) |
Delaware | 26-0579295 | |
(State or other jurisdiction of | ( |
ENDRA Life Sciences Inc.
3600 Green Court, Suite 350
Ann Arbor, MI 48105
(734) 335-0468
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
_____________________
Francois Michelon
Chief Executive Officer
ENDRA Life Sciences Inc.
3600 Green Court, Suite 350
Ann Arbor, MI 48105
(734) 335-0468
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_____________________
Please send copies of all communications to:
Mark R. Busch
Coleman Wombwell
K&L Gates LLP
300 South Tryon Street, 47th Floor
Charlotte, North CarolinaNC 28202
(704) 331-7440
_____________________
Approximate date of commencement of proposed sale to the public:From time to time after the effective date of this registration statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. ☐
Indicate by check mark whetherif the registrant is a large accelerated filer, an accelerated filer,file, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act (check one):
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☒
| Title of Each Class of Securities to be Registered | Amount to be Registered (1)(2) | Proposed Maximum Offering Price Per Share (3) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee |
| Common stock, par value $0.0001 per share | 25,644,002 | $1.85 | $47,313,183.69 | $6,141.25 |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This Amendment No. 1 to the Registration Statement on Form S-3 of ENDRA Life Sciences Inc. (the “Company”) is being filed solely to (a) update the Incorporation by Reference sections of the prospectuses and prospectus supplement contained herein to incorporate by reference the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 28, 2024 (the “2023 Annual Report”), (b) update certain disclosures related to the Company’s business in the sections titled “The Company” in the prospectuses and prospectus supplement contained herein to align with the corresponding disclosure in the 2023 Annual Report and (c) revise Item 16. “Exhibits” to (i) include certain agreements relating to the shares of the Company’s common stock being registered for resale hereby, (ii) reference the filing fee table filed as Exhibit 107 hereto, and (iii) provide an updated consent of the Company’s independent registered public accounting firm.
Except as described above, no changes have been made to the Registration Statement.
i |
The information in this prospectus is not complete and may be changed. The selling stockholdersWe may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and the selling stockholders areit is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated January 10, 2020

PROSPECTUS

ENDRA Life Sciences Inc.
$50,000,000
Common Stock for sale by the Selling Stockholders
Preferred Stock
Debt Securities
Warrants
Units
This prospectus relates to the resale or other disposition from time to time of up to 25,644,002 shares of our common stock, par value $0.0001 per share (“Common Stock”), in connection with a private placementpreferred stock, debt securities, warrants and units that closed on December 11, 2019 (the “First Private Placement”) and a private placement that closed on December 23, 2019 (the “Second Private Placement”), by the persons described in this prospectus, whom we call the “Selling Stockholders,” identified in the section of this prospectus entitled “Selling Stockholders.” Of these shares, (i) 904,526 shares were issued in the First Private Placement; (ii) 7,191,873 shares are issuable upon conversion of the Company’s Series A Convertible Preferred Stock, par value $0.0001 per share (the “Series A Preferred Stock”), issued in the First Private Placement; (iii) 8,096,399 shares are issuable upon exercise of the warrants that were issued with the Series A Preferred Stock, including warrants issued to Lake Street Capital Markets, LLC (“Lake Street”) and its designees in connection with Lake Street’s services as placement agent in the First Private Placement (collectively, the “Series A Warrants”); (iv) an estimated 8,660,410 sharesENDRA Life Sciences Inc. may be issuable in respect of accrued and unpaid dividends on shares of Series A Preferred Stock; (v) 232,461 shares are issuable upon conversion of the Company’s Series B Convertible Preferred Stock, par value $0.0001 per share (the “Series B Preferred Stock”), issued in the Second Private Placement; (vi) 278,948 shares are issuable upon exercise of the warrants that were issued with the Series B Preferred Stock, including warrants issued to Lake Street and its designees in connection with Lake Street’s services as placement agent in the Second Private Placement (collectively, the “Series B Warrants”); and (vii) an estimated 279,385 shares may be issuable in respect of accrued and unpaid dividends on shares of Series B Preferred Stock (all such shares of Common Stock described in (i) through (vii) above, the “Securities”).
These securities may be sold directly by us, through privately negotiated transactionsdealers or agents designated from time to time, to or through underwriters or through a combination of these methods, at market prices prevailing atmethods. See “Plan of Distribution” in this prospectus. We may also describe the timeplan of saledistribution for any particular offering of these securities in any applicable prospectus supplement. If any agents, underwriters or at negotiated prices. The distribution of the Securities by the Selling Stockholders is not subject to any underwriting agreement. We will not receive any proceeds fromdealers are involved in the sale of the Securities by the Selling Stockholders, althoughany securities in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a prospectus supplement. The net proceeds we expect to receive the exercise price offrom any exercised Series A Warrants and Series B Warrants paid to us by the Selling Stockholders, whichsuch sale will also be used for working capital and general corporate purposes. We will bear all expenses of registration incurredincluded in connection with this offering, but all selling and other expenses incurred by the Selling Stockholders will be borne by them.
Our Common Stock is tradedcommon stock trades on the Nasdaq Capital Market under the symbol “NDRA.” On January 8, 2020,February 13, 2024, the last reported sale price for our Common Stockcommon stock was $1.81$1.12 per share. The warrants issued
As of February 14, 2024, the aggregate market value of our outstanding common stock held by non-affiliates was $27,200,435 based on 11,035,659 shares of outstanding common stock, of which 10,880,174 shares are held by non-affiliates, and the last reported sale price of our common stock of $2.50 per share on December 22, 2023. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities in a public primary offering with a value exceeding more than one-third of our May 2017 initial public offering are listed on the Nasdaq Capital Market under the symbol “NDRAW;” however, the Series A Warrants and Series B Warrants issuedfloat in the First Private Placement and Second Private Placement, respectively, are notany 12-month period so listed.
INVESTING IN OUR SECURITIES INVOLVES RISKS. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK FACTORS” CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT AND ANY RELATED FREE WRITING PROSPECTUS, AND UNDER SIMILAR HEADINGS IN OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS OR ANY SUCH PROSPECTUS SUPPLEMENT. SEE “RISK FACTORS” ON PAGE 6 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the Securitiesadequacy or determined ifaccuracy of this prospectus is truthful or complete.prospectus. Any representation to the contrary is a criminal offense.
The Selling Stockholders are offering to sell and seeking offers to buy the Securities only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus regardless of the time of delivery of this prospectus or of any sale of our Securities. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of any Securities in any jurisdiction where the offer is not permitted.
ii | |
Page | ||
1 | ||
2 | ||
4 | ||
7 | ||
7 | ||
7 | ||
8 | ||
9 | ||
10 | ||
18 | ||
19 | ||
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS | 20 | |
22 | ||
24 | ||
24 | ||
24 | ||
24 |
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, (the “SEC”)or the SEC, utilizing a “shelf” registration process. Under this shelf process, the Selling Stockholderswe may from time to time sell any combination of securities described in this prospectus in one or more offerings,offerings.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities under this shelf registration process, we will provide a prospectus supplement that will contain specific information about the sharesterms of Common Stock described in this prospectus.
When acquiring any securities discussed in this prospectus.
Unless otherwise stated or the context requires otherwise, references to “ENDRA”, the “Company,” “we,” “us” or “our” are to ENDRA Life Sciences Inc.
 | ||
 | ||
| Contents |
Certain information set forth in this prospectus or incorporated by reference in this prospectus may contain forward-looking statements within the meaning of Section 27A of | ||
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following:
· | our limited commercial experience, limited cash and history of losses; | |
· | our ability to obtain adequate financing to fund our business operations in the future; | |
· | our ability to achieve profitability; | |
· | delays and changes in regulatory requirements, policy and guidelines, including potential delays in submitting required regulatory applications or other submissions with respect to U.S. Food and Drug Administration (“FDA”) or other regulatory agency approval; | |
· | our ability to obtain CE mark certification and secure required FDA and other governmental approvals for our Thermo-Acoustic Enhanced Ultrasound (“TAEUS”) applications | |
· | our ability to develop a commercially feasible application based on our TAEUS technology; | |
· | market acceptance of our technology; | |
· | uncertainties associated with COVID-19 or other pandemics that may occur, including effects on our operations; | |
· | the effect of macroeconomic conditions on our business; | |
· | results of our human studies, which may be negative or inconclusive; | |
· | our ability to find and maintain development partners; | |
· | our reliance on third parties, collaborations, strategic alliances and licensing arrangements to complete our business strategy; | |
· | the amount and nature of competition in our industry; | |
· | our ability to protect our intellectual property; |
| 2 |
| Table of Contents |
· | potential changes in the healthcare industry or third-party reimbursement practices; | |
· | our ability to comply with regulation by various federal, state, local and foreign governmental agencies and to maintain necessary regulatory clearances or approvals; | |
· | our ability to maintain compliance with Nasdaq listing standards; | |
· | our dependence on our senior management team; and | |
· | the other risks and uncertainties described in the Risk Factors section of this prospectus, any prospectus supplement and our most recent Annual Form 10-K and subsequently filed Quarterly Reports on Form 10-Q, which filings are incorporated herein by reference. |
We urge you to obtain adequate financing to fund our business operations in the future;
| 3 |
| Table of Contents |
We were incorporated as a Delaware corporation in 2007. We are developing a next-generation enhanced ultrasound technology platform—Thermo Acoustic Enhanced Ultrasound, or TAEUS® in order to broaden patient access to the safe diagnosis and treatment of a number of significant medical conditions in circumstances where expensive X-ray computed tomography (“CT”), magnetic resonance imaging (“MRI”) technology, or other diagnostic technologies such as surgical biopsy, are unavailable or impractical.
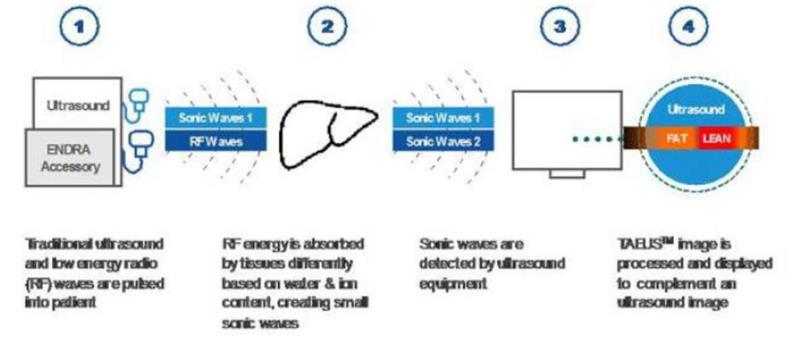
Our TAEUS technology uses radio frequency (“RF”) pulses to stimulate tissues, using a small fraction (less than 1%) of the amount of energy that would be transmitted into the body during an MRI scan. The use of RF energy allows our TAEUS technology to penetrate deep into tissue, enabling the imaging of human anatomy at depths equivalent to those of conventional ultrasound. The RF pulses are absorbed by tissue and converted into ultrasound signals, which are detected by an external ultrasound receiver and a digital acquisition system that is part of the TAEUS system. The detected ultrasound is processed into images and other forms of data using our proprietary software and algorithms and then displayed to complement conventional gray-scale ultrasound images. The TAEUS imaging concept is illustrated below:
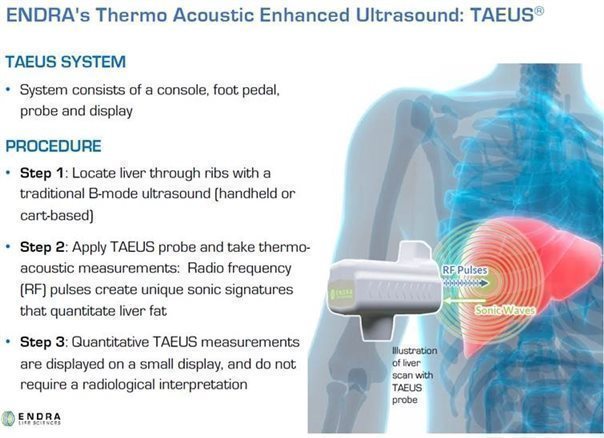
We believe that our TAEUS technology has the potential to add a number of new capabilities to conventional ultrasound, and other types of capital medical equipment such as interventional thermo-ablation systems, and thereby enhance the utility of those systems. Additionally, we believe that our technology can extend the use of ultrasound technology to indications and clinical situations that currently require the use of expensive CT or MRI imaging systems, where imaging is not practical using existing technology, or where other assessment tools such as surgical biopsy are required.
Our TAEUS platform is not intended to replace CT or MRI systems, both of which are versatile imaging technologies with capabilities and uses beyond the focus of our business. These systems, while versatile, are relatively expensive—a CT system can cost approximately $1 million and an MRI system can cost up to approximately $3 million. In addition, and in contrast to ultrasound systems, due to their limited number and the fact that they are usually fixed-in-place at major medical facilities, CT or MRI systems are frequently inaccessible to many patients. For example, CT or MRI systems are generally less accessible to primary care practices, rural clinics, economically developing markets, and patient bedsides.
| 4 |
| Table of Contents |
Ultrasound systems are more broadly available to patients than either CT or MRI systems. There are an estimated 1.6 million diagnostic ultrasound systems globally in use today. The global diagnostic ultrasound device market is anticipated to expand at a CAGR of 4.07% from 2022 to 2030, according to Grand View Research. Ultrasound systems are relatively inexpensive compared to CT and MRI systems, as smaller portable ultrasound systems can cost as little as approximately $5,000 and the price of new cart-based ultrasound systems can range from approximately $75,000 to $200,000. These numbers include both portable and cart-based ultrasound systems, and cover all types of diagnostic ultrasound procedures, including systems intended for cardiology, prenatal and abdominal use. We do not currently intend to address cart-based ultrasound systems focused on applications in prenatal care, nor certain portable ultrasound applications such as emergency room medicine, where we believe our TAEUS technology may not substantially impact patient care. Accordingly, we estimate the addressable market for one or more of our current or future TAEUS applications to include approximately 700,000 ultrasound systems currently in use throughout the world, in addition to other types of capital equipment.
To demonstrate the capabilities of our TAEUS platform, we have conducted various internal ex-vivo laboratory experiments and limited internal in-vivo large animal studies. In our ex-vivo and in-vivo testing, we have demonstrated that the TAEUS platform has the following capabilities and potential clinical applications:
· | Tissue Composition: Our TAEUS technology enables ultrasound to distinguish fat from lean tissue. This capability would enable the use of TAEUS-enhanced ultrasound for the early identification, staging and monitoring of NAFLD, a precursor to NASH, liver fibrosis, cirrhosis and liver cancer. | |
· | Temperature Monitoring: Our TAEUS technology enables traditional ultrasound to visualize changes in tissue temperature, in real time. This capability would enable the use of TAEUS-enhanced ultrasound to guide thermoablative therapy, which uses heat or cold to affect tissue, such as in the treatment of cardiac atrial fibrillation, or removal of cancerous liver and kidney lesions, with greater accuracy, and perform cosmetology procedures such as lipolysis of abdominal fat. | |
· | Vascular Imaging: Our TAEUS technology has the potential to enable visualization of blood vessels from any angle, using only a saline solution contrasting agent, unlike Doppler ultrasound, which requires precise viewing angles. This capability would enable the use of TAEUS-enhanced ultrasound to assist in identifying arterial plaques or malformed vessels. | |
· | Tissue Perfusion: Our TAEUS technology has the potential to image blood flow at the capillary level in a region, organ or tissue. This capability could be used to assist physicians in characterizing abnormalities in tissue perfusion symptomatic of damaged tissue, such as internal bleeding from trauma, or diseased tissue, such as certain cancers. |
The first TAEUS application we intend to commercialize is our NAFLD TAEUS application addressing liver tissue composition. Our initial target market for this application is the European Union (“EU”) and United Kingdom. In September 2019, we announced the completion and reported top-level findings of an initial healthy subject study and data collection of 50 subjects, which was included in our TAEUS liver device technical file submission for device CE mark. We received CE mark approval for our TAEUS FLIP (Fatty Liver Imaging Probe) application in March 2020. We have registered the product in each of our primary target European markets (i.e., Germany, France, and the United Kingdom).
In June 2020, we submitted a 510(k) Application to the FDA for our TAEUS Fatty Live Imaging Probe (“FLIP”) System. In February 2022, we announced that we would pursue FDA reclassification and clearance of our TAEUS FLIP System through the FDA’s “de novo” process. We subsequently voluntarily withdrew our 510(k) Application and submitted a de novo request for the TAEUS system to the FDA in the third quarter of 2023. In the fourth quarter of 2023, the FDA sent an Additional Information (“AI”) request related to our de novo application. Since we received the AI request, we have had several interactions with the FDA and have provided additional information. In order to fully respond to the FDA’s questions, we will need to compile additional clinical data, provide additional device test data, and respond to cybersecurity related questions in a new de novo submission. We have a scheduled in-person pre-submission meeting with the FDA in the second quarter of 2024. We currently anticipate completing the necessary clinical studies by the fourth quarter of 2024 and submitting the new de novo request to the FDA in the first half of 2025.
| 5 |
| Table of Contents |
After required regulatory approvals, our TAEUS technology can be added as an accessory to existing, commercially available ultrasound systems, helping to improve clinical decision-making on the front lines of patient care, without requiring substantially new clinical workflows or large capital investments. We are also developing TAEUS for possible incorporation into new medical equipment manufactured by original equipment manufacturers (“OEMs”), such as GE Healthcare and others, to enhance the utility of those OEM systems, as described more fully in our Annual Report on Form 10-K. Based on our design work and our understanding of the medical capital equipment market, we intend to price our initial liver TAEUS system at a price point of approximately $65,000, which we believe could enable clinical purchasers to recoup their investment in less than one year by performing a relatively small number of additional procedures, initially paid out-of-pocket by patients until government and private insurance reimbursement is secured for the TAEUS liver procedures.
Corporate Information
We were incorporated in Delaware in July 2007. Our corporate headquarters is located at 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105-1570. Our website can be accessed at www.endrainc.com. The telephone number of our principal executive office is (734) 335-0468. The information contained on, or that subsequent eventsmay be obtained from, our website is not, and developments will causeshall not be deemed to be, a part of this prospectus.
| 6 |
| Table of Contents |
Investing in our views to change.securities involves a high degree of risk. You should read this prospectuscarefully consider the risk factors described in our Annual Report on Form 10-K for our most recent fiscal year (together with any material changes thereto contained in subsequent filed Quarterly Reports on Form 10-Q) and those contained in our other filings with the documents referencedSEC, which are incorporated by reference in this prospectus and filed as exhibitsany accompanying prospectus supplement.
The prospectus supplement applicable to each type or series of securities we offer may contain a discussion of risks applicable to the registration statement,particular types of which thissecurities that we are offering under that prospectus issupplement. Prior to making a part, completely anddecision about investing in our securities, you should carefully consider the specific factors discussed under the caption “Risk Factors” in the applicable prospectus supplement, together with the understanding that our actual future results may be materially different from what we expect. Our forward-looking statements do not reflect the potential impact of any future acquisitions, merger, dispositions, joint ventures or investments we may undertake. We qualify all of the other information contained in the prospectus supplement or appearing or incorporated by reference in this prospectus. These risks could materially affect our forward-looking statements by these cautionary statements.
We will not receive anycurrently intend to use the estimated net proceeds from the sale of Common Stock by the Selling Stockholders. To the extent we receive proceeds from the exercise of Series A Warrants and Series B Warrants held by the Selling Stockholders, we will use those proceedsthese securities for working capital and other general corporate purposes.
DESCRIPTION OF SECURITIES WE MAY OFFER
We have agreed to bear the expenses (other than selling commissions or any legal expenses incurred by any Selling Stockholder) in connection with the registration of the shares of our Common Stock being offered for resale hereunder by the Selling Stockholders.
· | shares of common stock; | |
· | shares of preferred stock; | |
· | debt securities, which may include senior debt securities, subordinated debt securities and senior subordinated debt securities; | |
· | warrants for the purchase of debt securities, preferred stock or common stock; and | |
· | units consisting of two or more of the foregoing. |
Set forth below of up to 25,644,002 shares of Common Stock, which include:
We may sell the registration requirements of the Securities Act. Information concerning the Selling Stockholders may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly. We cannot give an estimate as to the number of shares of Common Stock that will actually be held by the Selling Stockholders upon termination of this offering because the Selling Stockholders may offer some or all of their Common Stock, as applicable, under the offering contemplated by this prospectus or may acquire additional shares of Common Stock. The aggregate total number of shares of Common Stock that may be sold hereunder will not exceed the number of shares of Common Stock offered hereby. Please read the section entitled “Plan of Distribution” in this prospectus.
| Name of Selling Security Holder | Shares of Common Stock Beneficially Owned Prior to this Offering | Shares of Common Stock Registered Hereby | Shares of Common Stock Beneficially Owned upon Completion of this Offering (1) | Percentage of Common Stock Beneficially Owned upon Completion of this Offering (2) |
| Anthony DiGiandomenico (3) | 391,058 | 201,006 | 190,049 | 2.3% |
| Dennis T. Whalen & Linda P. Whalen JTWROS (4) | 40,202 | 64,407 | - | * |
| Xavier Aguirre (5) | 20,102 | 32,205 | - | * |
| Carlo Alberici (6) | 147,450 | 236,229 | - | * |
| James R. Aldridge (7) | 50,252 | 80,509 | - | * |
| Howard Altshuler (8) | 51,202 | 64,407 | 11,000 | * |
| Patrick M. Barberich & Monica Barberich JTWROS (9) | 30,152 | 48,306 | - | * |
| Anthony J. Berni (10) | 40,202 | 64,407 | - | * |
| Chase Lankford Bice (11) | 30,152 | 48,306 | - | * |
| Mark W. Boyer (12) | 194,396 | 311,441 | - | * |
| Jeffrey Ronald Boyle (13) | 37,558 | 60,171 | - | * |
| John M. Brady (14) | 77,098 | 123,518 | - | * |
| Michael J. Burwell (15) | 194,396 | 311,441 | - | * |
| Matthew W. Cambi (16) | 77,098 | 123,518 | - | * |
| Donald Cameron (17) | 50,252 | 80,509 | - | * |
| Timothy L. Carpenter & Julie L. Carpenter JTWROS (18) | 30,152 | 48,306 | - | * |
| Peter A. Casey (19) | 20,102 | 32,205 | - | * |
| Catalytic Opportunity LLC Series A (20) | 2,010,052 | 3,220,301 | - | * |
| Catalytic Opportunity LLC Series A-1 (21) | 2,512,564 | 4,025,375 | - | * |
| Vaidyanthan Chandrashekhar (22) | 46,948 | 75,215 | - | * |
| Rich Chirico (23) | 50,252 | 80,509 | - | * |
| Ronald J. Ciasulli (24) | 56,336 | 90,256 | - | * |
| Mario Dell'Aera (25) | 244,646 | 391,947 | - | * |
| Mark R. Demich (26) | 97,198 | 155,721 | - | * |
| Paul G. Elie (27) | 97,198 | 155,721 | - | * |
| Michael Fahey (28) | 46,948 | 75,215 | - | * |
| Charles Joseph Finn (29) | 147,450 | 236,229 | - | * |
| Gregory G. Galdi (30) | 288,288 | 461,866 | - | * |
| Philip Garland & Cynthia Garland JTWROS (31) | 77,098 | 123,518 | - | * |
| Scott J. Gehsman (32) | 46,948 | 75,215 | - | * |
| TTEE Patrick John Gregory Revocable Trust DTD 6-26-1990 (33) | 67,048 | 107,417 | - | * |
| Scott Guasta (34) | 100,504 | 161,017 | - | * |
| Michael Harsh & Sandra E. Hansen Harsh JTWROS (35) | 56,044 | 35,425 | 33,932 | * |
| Kevin A. Healy (36) | 40,202 | 64,407 | - | * |
| Kevin J. Herzberg (37) | 50,252 | 80,509 | - | * |
| Dennis D. Howarter & Pamela J. Howarter JTWROS (38) | 194,396 | 311,441 | - | * |
| Donald L. Hulet (39) | 67,708 | 108,475 | - | * |
| Frank Ingriselli (40) | 46,948 | 75,215 | - | * |
| Joseph M. Janinski (41) | 40,202 | 64,407 | - | * |
| Anthony D. Johnston (42) | 30,152 | 48,306 | - | * |
| Robert Kastenschmidt (43) | 97,198 | 155,721 | - | * |
| Robert Richard Keehan (44) | 97,198 | 155,721 | - | * |
| Frederick M. Kelso (45) | 77,098 | 123,518 | - | * |
| Benjamin King (46) | 50,252 | 50,252 | - | * |
| John Klinge (47) | 46,948 | 75,215 | - | * |
| James M. Koch (48) | 70,352 | 112,711 | - | * |
| James P. Kolar (49) | 97,198 | 155,721 | - | * |
| John C. Koppin (50) | 46,948 | 75,215 | - | * |
| Kurtis Krentz (51) | 144,144 | 230,933 | - | * |
| Jeffrey E. Kuhlin (52) | 50,252 | 80,509 | - | * |
| Dennis Lam (53) | 30,152 | 48,306 | - | * |
| James C. Leslie (54) | 40,202 | 64,407 | - | * |
| Paul E. Linthorst (55) | 30,152 | 48,306 | - | * |
| Jose M. Martinez (56) | 46,948 | 75,215 | - | * |
| William E. Marx (57) | 50,252 | 80,509 | - | * |
| Thorne Joseph Brown Matteson (58) | 50,252 | 80,509 | - | * |
| Joseph Michalczyk (59) | 77,758 | 124,576 | - | * |
| Francois Michelon (60) | 450,811 | 16,104 | 440,759 | 5.2% |
| Jeffery L. Miller & Khristen N. Zar JTWROS (61) | 97,198 | 155,721 | - | * |
| Russell Moore (62) | 30,152 | 48,306 | - | * |
| Jorge Morazzani (63) | 46,948 | 75,215 | - | * |
| Edmond Allen Morrison (64) | 46,948 | 75,215 | - | * |
| Chester P. Mowrey, Jr. (65) | 46,948 | 75,215 | - | * |
| James Eric Nicely & Karen B. Nicely JTWROS (66) | 50,252 | 80,509 | - | * |
| Michael P. Niland & Jill Kathleen Niland JTWROS (67) | 50,252 | 80,509 | - | * |
| David B. O'Neill (68) | 147,450 | 236,229 | - | * |
| Jonathan Padnos (69) | 201,006 | 201,006 | - | * |
| Alan W. Page (70) | 87,148 | 139,620 | - | * |
| Michael A. Page (71) | 40,202 | 64,407 | - | * |
| Alexandre N. Palma (72) | 87,148 | 139,620 | - | * |
| David Petterson (73) | 164,244 | 263,136 | - | * |
| Laurence M. Pfeffer (74) | 50,252 | 80,509 | - | * |
| John D. Potter (75) | 46,948 | 75,215 | - | * |
| Michael P. Quackenbush Jr. (76) | 46,948 | 75,215 | - | * |
| Peter D. Raymond (77) | 50,252 | 80,509 | - | * |
| Erick E. Richardson (78) | 502,514 | 805,077 | - | * |
| Juan R. Rivero (79) | 40,202 | 64,407 | - | * |
| Thomas Michael Rooney (80) | 46,948 | 75,215 | - | * |
| Maj-Britt Rosenbaum (81) | 50,252 | 80,509 | - | * |
| Scott Joseph Schueller (82) | 77,098 | 123,518 | - | * |
| Donald P. Sesterhen (83) | 97,198 | 155,721 | - | * |
| Steven J. Shanker Living Trust DTD 4-9-1997 (84) | 147,450 | 236,229 | - | * |
| Kevin P. Smithson (85) | 50,252 | 80,509 | - | * |
| Michael Snow (86) | 97,198 | 155,721 | - | * |
| James Somers (87) | 164,244 | 263,136 | - | * |
| John Richard Stamm (88) | 46,948 | 75,215 | - | * |
| Gary Sterbinsky (89) | 37,558 | 60,171 | - | * |
| James Douglas Summa (90) | 30,152 | 48,306 | - | * |
| Ramesh Telang (91) | 30,152 | 48,306 | - | * |
| Oscar Teunissen (92) | 100,504 | 161,017 | - | * |
| Gary and Deborah Tillett Revocable Trust DTD 8-10-2012 (93) | 46,948 | 75,215 | - | * |
| Amaresh Tripathy (94) | 46,948 | 75,215 | - | * |
| Chetan R. Vagholkar (95) | 97,198 | 155,721 | - | * |
| Gerard J. Verweij (96) | 67,048 | 107,417 | - | * |
| Timothy S. Vitale (97) | 40,202 | 64,407 | - | * |
| Craig Watchmaker (98) | 46,948 | 75,215 | - | * |
| Jonathan Richard Worden (99) | 20,102 | 32,205 | - | * |
| Mohibullah Yousufani (100) | 46,948 | 75,215 | - | * |
| Jeffrey E. Zaleski (101) | 50,252 | 80,509 | - | * |
| Simon Mawson (102) | 100,504 | 161,017 | - | * |
| Mike Ruffer (103) | 70,352 | 112,711 | - | * |
| Dennis Scullin (104) | 48,247 | 74,605 | - | * |
| Jon E. Schmidt (105) | 173,685 | 268,569 | - | * |
| Daniel Goodman (106) | 48,247 | 74,605 | - | * |
| Eric Samuelson (107) | 192,983 | 298,410 | - | * |
| The Crudup Family Trust (108) | 48,247 | 74,605 | - | * |
| Jonathan E. Ansbacher (109) | 97,198 | 155,721 | - | * |
| Joseph C. Atkinson (110) | 50,252 | 80,509 | - | * |
| Keith Belote (111) | 37,558 | 60,171 | - | * |
| Kevin M. Borkowski (112) | 20,102 | 32,205 | - | * |
| Mark R Busch/Valerie Y Busch JTWROS (113) | 48,202 | 64,407 | 8,000 | * |
| Dennis L. Chesley (114) | 77,098 | 123,518 | - | * |
| Charles Christensen (115) | 147,450 | 236,229 | - | * |
| Michael L. Desautels Account 2 (116) | 50,252 | 80,509 | - | * |
| Daniel DiFilippo (117) | 100,504 | 161,017 | - | * |
| Emilio DiMatteo & Jessica DiMatteo JTWROS (118) | 50,252 | 80,509 | - | * |
| Miles E. Everson (119) | 97,198 | 155,721 | - | * |
| James M. Fiscus (120) | 30,152 | 48,306 | - | * |
| David A. Fitz (121) | 50,252 | 80,509 | - | * |
| Paul P. Frank III & Colleen B. Frank Joint Tenants by Entirety (122) | 50,252 | 80,509 | - | * |
| David J. Gilbertson (123) | 30,152 | 48,306 | - | * |
| Brian W. Hannan (124) | 46,948 | 75,215 | - | * |
| Keith Jackson (125) | 194,396 | 311,441 | - | * |
| Donald Kendall (126) | 244,646 | 391,947 | - | * |
| Samir Mammadov (127) | 46,948 | 75,215 | - | * |
| Benjamin L. Padnos (128) | 502,514 | 502,514 | - | * |
| Daniel P. Padnos (129) | 50,252 | 50,252 | - | * |
| Jeffrey and Margaret Padnos (130) | 301,508 | 301,508 | - | * |
| Jeffrey Padnos & Margaret Padnos 2010 Generation Trust (131) | 502,514 | 502,514 | - | * |
| Rich Shappard (132) | 100,504 | 161,017 | - | * |
| Jaivardhan Sinha (133) | 46,948 | 75,215 | - | * |
| Harold Wayne Smith Jr. (134) | 50,252 | 80,509 | - | * |
| John W. Stadtler (135) | 144,144 | 230,933 | - | * |
| Pierre Alain Nicholas P Sur (136) | 77,098 | 123,518 | - | * |
| Robert B. Taylor (137) | 30,152 | 48,306 | - | * |
| Ravjiv A. Thadani (138) | 87,148 | 139,620 | - | * |
| Laurie A. Vanraemdonck Trust DTD 4-7-2008 (139) | 46,948 | 75,215 | - | * |
| Stephen V. Zawoyski (140) | 46,948 | 75,215 | - | * |
| David R. Wells (141) | 99,786 | 32,205 | 79,684 | * |
| Kim E. Tobler (142) | 40,202 | 64,407 | - | * |
| Marc D. Silverman & Mitra Best Silverman JTWROS (143) | 50,252 | 80,509 | - | * |
| Henry A. Padinha & Terri A. Padinha JT COMM PROP (144) | 30,152 | 48,306 | - | * |
| Chad F. Mueller (145) | 100,504 | 161,017 | - | * |
| Adan Martinez (146) | 40,202 | 64,407 | - | * |
| Scott M. Curran (147) | 97,198 | 155,721 | - | * |
| Allan Michael Baccala (148) | 50,252 | 80,509 | - | * |
| Ballington Living Trust DTD 8-5-14 (149) | 97,198 | 155,721 | - | * |
The following is a briefsummary description of our capital stock. This summary does not purport to be complete in all respects. This descriptioncommon stock is subject to and qualified entirely bybased on the termsprovisions of our Fourth Amended and Restated Certificate of Incorporation, (the “Certificateas amended, our Amended and Restated Bylaws, and the applicable provisions of Incorporation”),Delaware law. This description may not contain all of the Series A Certificateinformation that is important to you and is subject to, and is qualified in its entirety by reference to, our charter, our bylaws and the applicable provisions of Designations, the Series B Certificate of Designations and our amended and restated bylaws,Delaware law. For information on how to obtain copies of whichour certificate of Incorporation and Bylaws, see “Where You Can Find More Information.”
Authorized Capital
We currently have been filed with the SEC and are also available upon request from us.
Voting Rights
Each holder of our common stock is entitled to one vote for each such share outstanding in the holder’s name. No holder of common stock is entitled to cumulate votes in voting for directors.
Dividend and Liquidation Rights
The holders of outstanding shares of Common Stockour common stock are entitled to such dividends as may be declared by our board of directors out of funds legally available for such purpose. The shares of Common Stockour common stock are neither redeemable nor convertible. Holders of Common Stockour common stock have no preemptive or subscription rights to purchase any of our securities.
We have never paid any cash dividends on our common stock.
Registration Rights
Under the terms of the Restricted Stock Agreement, dated as of November 30, 2023 (the “Restricted Stock Agreement”), by and between us and PatentVest, Inc. (“PatentVest”), we granted PatentVest rights with respect to the registration of 202,020 shares of restricted common stock issued to PatentVest (the “Restricted Stock”) pursuant to the Restricted Stock Agreement in connection with the Consulting Services, dated as of November 30, 2023 (“Services Agreement”), by and between the Company and PatentVest.
Pursuant to the Restricted Stock Agreement, the Company agreed to include the Restricted Stock in any registration statement subsequently filed by the Company, to the extent a shelf registration statement with respect to the Restricted Stock had not previously been filed.
Our shares of Common Stockcommon stock are listed on the Nasdaq Capital Market under the symbol “NDRA.”
| 8 |
| Table of Contents |
DESCRIPTION OF PREFERRED STOCK WE MAY OFFER
This section describes the general terms and provisions of the preferred stock we may offer. This information may not be complete in all respects and is qualified entirely by reference to our Fourth Amended and Restated Certificate of Incorporation, as amended, with respect to each series of preferred stock. The specific terms of any series will be described in a prospectus supplement. Those terms may differ from the terms discussed below. Any series of preferred stock we issue will be governed by our Fourth Amended and Restated Certificate of Incorporation, as amended, and by the certificate of designations relating to that series. We will file the certificate of designations with the SEC and incorporate it by reference as an exhibit to our registration statement at or before the time we issue any preferred stock of that series.
Authorized Preferred Stock
Our Certificate of Incorporation authorizes us to issue 10,000,000 shares of preferred stock, par value $0.0001per share. We have designated 10,000 shares of preferred stock as Series A Convertible Preferred Stock (“Series A Preferred Stock”), 1,000 shares of preferred stock as Series B Convertible Preferred Stock (“Series B Preferred Stock”) and 100,000 shares of preferred stock as Series C Preferred Stock (“Series C Preferred Stock”) and the remainder of 9,889,000 shares remain authorized but undesignated. As of February 13, 2024, we had 34.976 shares of Series A Preferred Stock and no shares of Series B Preferred Stock or Series C Preferred Stock issued and outstanding. Our authorized but unissued shares of preferred stock are available for issuance without further action by our stockholders, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded.
Our board of directors has the authority without further action by the stockholders, to issue up to 10,000,000 shares of Preferred Stockpreferred stock in one or more series and to fix the designations, powers, rights, preferences, qualifications, limitations and restrictions thereof. These designations, powers, rights and preferences could include voting rights, dividend rights, dissolution rights, conversion rights, exchange rights, redemption rights, liquidation preferences, and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of Common Stock.common stock. The issuance of Preferred Stockpreferred stock could adversely affect the voting power of holders of Common Stockcommon stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of Preferred Stockpreferred stock could have the effect of delaying, deferring or preventing change in our control or other corporate action.
Specific Terms of a Series A Convertible Preferred Stock.
The preferred stock we may offer under this prospectus will be issued in one or more series. A prospectus supplement will discuss the following features of the series of preferred stock to which it relates:
· | the designations and stated value per share; | |
· | the number of shares offered; | |
· | the amount of liquidation preference per share; | |
· | the public offering price at which the preferred stock will be issued; | |
· | the dividend rate, the method of its calculation, the dates on which dividends would be paid and the dates, if any, from which dividends would cumulate; | |
· | any redemption or sinking fund provisions; | |
· | any conversion or exchange rights; and | |
· | any additional voting, dividend, liquidation, redemption, sinking fund and other rights, preferences, privileges, limitations and restrictions. |
| 9 |
| Table of Contents |
DESCRIPTION OF DEBT SECURITIES WE MAY OFFER
General
The debt securities that we may issue will constitute debentures, notes, bonds or other evidences of indebtedness of ENDRA, to be issued in one or more series, which may include senior debt securities, subordinated debt securities and senior subordinated debt securities. The particular terms of any series of debt securities we offer, including the extent to which the general terms set forth below may be applicable to a Seriesparticular series, will be described in a prospectus supplement relating to such series.
Debt securities that we may issue will be issued under an indenture between us and a trustee qualified to act as such under the Trust Indenture Act of 1939. We have filed the form of the indenture as an exhibit to the registration statement of which this prospectus is a part. When we refer to the “indenture” in this prospectus, we are referring to the indenture under which the debt securities are issued as supplemented by any supplemental indenture applicable to the debt securities. We will provide the name of the trustee in any prospectus supplement related to the issuance of debt securities, and we will also provide certain other information related to the trustee, including describing any relationship we have with the trustee, in such prospectus supplement.
THE FOLLOWING DESCRIPTION IS A Issue PriceSUMMARY OF THE MATERIAL PROVISIONS OF THE INDENTURE. IT DOES NOT RESTATE THE INDENTURE IN ITS ENTIRETY. THE INDENTURE IS GOVERNED BY THE TRUST INDENTURE ACT OF 1939. THE TERMS OF THE DEBT SECURITIES INCLUDE THOSE STATED IN THE INDENTURE AND THOSE MADE PART OF THE INDENTURE BY REFERENCE TO THE TRUST INDENTURE ACT. WE URGE YOU TO READ THE INDENTURE BECAUSE IT, AND NOT THIS DESCRIPTION, DEFINES YOUR RIGHTS AS A HOLDER OF THE DEBT SECURITIES.
Information You Will Find in the Prospectus Supplement
The indenture provides that we may issue debt securities from time to time in one or more series and that we may denominate the debt securities and make them payable in foreign currencies. The indenture does not limit the aggregate principal amount of $1,000. Dividends accrue ondebt securities that can be issued thereunder. The prospectus supplement for a series of debt securities will provide information relating to the Series A Issue Priceterms of the series of debt securities being offered, which may include:
· | the title and denominations of the debt securities of the series; | |
· | any limit on the aggregate principal amount of the debt securities of the series; | |
· | the date or dates on which the principal and premium, if any, with respect to the debt securities of the series are payable or the method of determination thereof; | |
· | the rate or rates, which may be fixed or variable, at which the debt securities of the series shall bear interest, if any, or the method of calculating and/or resetting such rate or rates of interest; | |
· | the dates from which such interest shall accrue or the method by which such dates shall be determined and the duration of the extensions and the basis upon which interest shall be calculated; | |
· | the interest payment dates for the series of debt securities or the method by which such dates will be determined, the terms of any deferral of interest and any right of ours to extend the interest payment periods; | |
· | the place or places where the principal and interest on the series of debt securities will be payable; | |
· | the terms and conditions upon which debt securities of the series may be redeemed, in whole or in part, at our option or otherwise; | |
· | our obligation, if any, to redeem, purchase, or repay debt securities of the series pursuant to any sinking fund or other specified event or at the option of the holders and the terms of any such redemption, purchase, or repayment; |
| 10 |
| Table of Contents |
· | the terms, if any, upon which the debt securities of the series may be convertible into or exchanged for other securities, including, among other things, the initial conversion or exchange price or rate and the conversion or exchange period; | |
· | if the amount of principal, premium, if any, or interest with respect to the debt securities of the series may be determined with reference to an index or formula, the manner in which such amounts will be determined; | |
· | if any payments on the debt securities of the series are to be made in a currency or currencies (or by reference to an index or formula) other than that in which such securities are denominated or designated to be payable, the currency or currencies (or index or formula) in which such payments are to be made and the terms and conditions of such payments; | |
· | any changes or additions to the provisions of the indenture dealing with defeasance, including any additional covenants that may be subject to our covenant defeasance option; | |
· | the currency or currencies in which payment of the principal and premium, if any, and interest with respect to debt securities of the series will be payable, or in which the debt securities of the series shall be denominated, and the particular provisions applicable thereto in accordance with the indenture; | |
· | the portion of the principal amount of debt securities of the series which will be payable upon declaration of acceleration or provable in bankruptcy or the method by which such portion or amount shall be determined; | |
· | whether the debt securities of the series will be secured or guaranteed and, if so, on what terms; | |
· | any addition to or change in the events of default with respect to the debt securities of the series; | |
· | the identity of any trustees, authenticating or paying agents, transfer agents or registrars; | |
· | the applicability of, and any addition to or change in, the covenants currently set forth in the indenture; | |
· | the subordination, ranking or priority, if any, of the debt securities of the series and terms of the subordination; and | |
· | any other terms of the debt securities of the series which are not prohibited by the indenture. |
Holders of debt securities may present debt securities for exchange in the manner, at the places, and subject to the restrictions set forth in the debt securities, the indenture, and the prospectus supplement. We will provide these services without charge, other than any tax or other governmental charge payable in connection therewith, but subject to the limitations provided in the indenture, any board resolution establishing such debt securities and any applicable indenture supplement.
Senior Debt
We may issue senior debt securities under the indenture. Unless otherwise set forth in the applicable indenture supplement and described in a prospectus supplement, the senior debt securities will be senior unsecured obligations, ranking equally with all of our existing and future senior unsecured debt. The senior debt securities will be senior to all of our subordinated debt and junior to any secured debt we may incur as to the assets securing such debt.
Subordinated Debt
We may issue subordinated debt securities under the indenture. These subordinated debt securities will be subordinate and junior in right of payment, to the extent and in the manner set forth in the indenture and any applicable indenture supplement, to all of our senior indebtedness.
| 11 |
| Table of Contents |
If this prospectus is being delivered in connection with a series of subordinated debt securities, the accompanying prospectus supplement or the information incorporated by reference will set forth the approximate amount of senior indebtedness outstanding as of the end of the most recent fiscal quarter.
Senior Subordinated Debt
We may issue senior subordinated debt securities under the indenture. These senior subordinated debt securities will be, to the extent and in the manner set forth in the applicable indenture supplement, subordinate and junior in right of payment to all of our “senior indebtedness” and senior to our other subordinated debt. See the discussions above under “—Senior Debt” and “—Subordinated Debt” for a more detailed explanation of our senior and subordinated indebtedness.
Interest Rate
Debt securities that bear interest will do so at a fixed rate or a variable rate. We may sell, at a discount below the stated principal amount, any debt securities which bear no interest or which bear interest at a rate that at the time of 6.0% per annumissuance is below the prevailing market rate. The relevant prospectus supplement will describe the special United States federal income tax considerations applicable to:
· | any discounted debt securities; and | |
· | any debt securities issued at par which are treated as having been issued at a discount for United States federal income tax purposes. |
Registered Global Securities
We may issue registered debt securities of a series in the form of one or more fully registered global securities. We will deposit the registered global security with a depository or with a nominee for a depository identified in the prospectus supplement relating to such series. The global security or global securities will represent and will be in a denomination or aggregate denominations equal to the portion of the aggregate principal amount of outstanding registered debt securities of the series to be represented by the registered global security or securities. Unless it is exchanged in whole or in part for debt securities in definitive registered form, a registered global security may not be transferred, except as a whole in three cases:
· | by the depository for the registered global security to a nominee of the depository; | |
· | by a nominee of the depository to the depository or another nominee of the depository; and | |
· | by the depository or any nominee to a successor of the depository or a nominee of the successor. |
The prospectus supplement relating to a series of debt securities will describe the specific terms of the depository arrangement concerning any portion of that series of debt securities to be represented by a registered global security. We anticipate that the following provisions will generally apply to all depository arrangements.
Upon the issuance of a registered global security, the depository will credit, on its book-entry registration and transfer system, the principal amounts of the debt securities represented by the registered global security to the accounts of persons that have accounts with the depository. These persons are payablereferred to as “participants.” Any underwriters, agents or dealers participating in the distribution of debt securities represented by the registered global security will designate the accounts to be credited. Only participants or persons that hold interests through participants will be able to beneficially own interests in a registered global security. The depository for a global security will maintain records of beneficial ownership interests in a registered global security for participants. Participants or persons that hold through participants will maintain records of beneficial ownership interests in a global security for persons other than participants. These records will be the only means to transfer beneficial ownership in a registered global security.
The laws of some states may require that specified purchasers of securities take physical delivery of the securities in definitive form. These laws may limit the ability of those persons to own, transfer or pledge beneficial interests in global securities.
| 12 |
| Table of Contents |
So long as the depository, or its nominee, is the registered owner of a registered global security, the depository or its nominee will be considered the sole owner or holder of the debt securities represented by the registered global security for all purposes under the indenture. Except as set forth below, owners of beneficial interests in a registered global security:
· | may not have the debt securities represented by a registered global security registered in their names; | |
· | will not receive or be entitled to receive physical delivery of debt securities represented by a registered global security in definitive form; and | |
· | will not be considered the owners or holders of debt securities represented by a registered global security under the indenture. |
Accordingly, each person owning a beneficial interest in a registered global security must rely on the procedures of the depository for the registered global security and, if the person is not a participant, on the procedures of the participant through which the person owns its interests, to exercise any rights of a holder under the indenture applicable to the registered global security.
We understand that, under existing industry practices, if we request any action of holders, or if an owner of a beneficial interest in a registered global security desires to give or take any action which a holder is entitled to give or take under the indenture, the depository for the registered global security would authorize the participants holding the relevant beneficial interests to give or take the action, and the participants would authorize beneficial owners owning through the participants to give or take the action or would otherwise act upon the instructions of beneficial owners holding through them.
Payment of Interest on and Principal of Registered Global Securities
We will make principal, premium, if any, and interest payments on debt securities represented by a registered global security registered in the name of a depository or its nominee to the depository or its nominee as the registered owner of the registered global security. None of ENDRA, the trustee, or any paying agent for debt securities represented by a registered global security will have any responsibility or liability for:
· | any aspect of the records relating to, or payments made on account of, beneficial ownership interests in such registered global security; | |
· | maintaining, supervising, or reviewing any records relating to beneficial ownership interests; | |
· | the payments to beneficial owners of the global security of amounts paid to the depository or its nominee; or | |
· | any other matter relating to the actions and practices of the depository, its nominee or any of its participants. |
We expect that the depository, upon receipt of any payment of principal, premium or interest in respect of the global security, will immediately credit participants’ accounts with payments in amounts proportionate to their beneficial interests in the principal amount of a registered global security as shown on the depository’s records. We also expect that payments by participants to owners of beneficial interests in a registered global security held through participants will be governed by standing instructions and customary practices. This is currently the case with the securities held for the accounts of customers registered in “street name.” Such payments will be the responsibility of participants.
Exchange of Registered Global Securities
We may issue debt securities in definitive form in exchange for the registered global security if both of the following occur:
· | the depository for any debt securities represented by a registered global security is at any time unwilling or unable to continue as depository or ceases to be a clearing agency registered under the Exchange Act; and | |
· | we do not appoint a successor depository within 90 days. |
| 13 |
| Table of Contents |
In addition, we may, at any time, determine not to have any of the debt securities of a series represented by one or more registered global securities. In this event, we will issue debt securities of that series in definitive form in exchange for all of the registered global security or securities representing those debt securities.
Covenants by ENDRA
The indenture includes covenants by us, including among other things that we will make all payments of principal and interest at the times and places required. The supplemental indenture establishing each series of debt securities may contain additional covenants, including covenants which could restrict our right to incur additional indebtedness or liens and to take certain actions with respect to our businesses and assets.
Events of Default
Unless otherwise indicated in the applicable prospectus supplement, the following will be events of default under the indenture with respect to each series of debt securities issued under the indenture:
· | failure to pay when due any interest on any debt security of that series, continued for 30 days; | |
· | failure to pay when due the principal of, or premium, if any, on, any debt security of that series; | |
· | failure to perform any other covenant or agreement of ours under the indenture or the supplemental indenture with respect to that series or the debt securities of that series, continued for 90 days after written notice to us by the trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the series to which the covenant or agreement relates; | |
· | certain events of bankruptcy, insolvency or similar proceedings affecting us; and | |
· | any other event of default specified in any supplemental indenture under which such series of debt securities is issued. |
Except as to certain events of bankruptcy, insolvency or similar proceedings affecting us and except as provided in the applicable prospectus supplement, if any event of default shall occur and be continuing with respect to any series of debt securities under the indenture, either the trustee or the holders of Series A Preferred Stockat least 25% in aggregate principal amount of outstanding debt securities of such series may accelerate the maturity of all debt securities of such series. Upon certain events of bankruptcy, insolvency or similar proceedings affecting us, the principal, premium, if any, and interest on all debt securities of each series shall be immediately due and payable.
After any such acceleration, but before a judgment or decree based on acceleration has been obtained by the trustee, the holders of a majority in aggregate principal amount of each affected series of debt securities may waive all defaults with respect to such series and rescind and annul such acceleration if all events of default, other than the non-payment of accelerated principal, have been cured, waived or otherwise remedied.
No holder of any debt securities will have any right to institute any proceeding with respect to the indenture or for any remedy under the indenture, unless such holder shall have previously given to the trustee written notice of a continuing event of default and the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the relevant series shall have made written request and offered indemnity satisfactory to the trustee to institute such proceeding as whentrustee, and the trustee shall not have received from the holders of a majority in aggregate principal amount of the outstanding debt securities of such series a direction inconsistent with such request and shall have failed to institute such proceeding within 60 days. However, such limitations do not apply to a suit instituted by a holder of a debt security for enforcement of payment of the principal of and premium, if declared by our Board of Directors.
| 14 |
| Table of Contents |
Supplemental Indentures
We and similar transactions. Holdersthe trustee may, convert their shares of Series A Preferred Stock into Common Stock at any time and we have the rightfrom time to cause each holdertime, without prior notice to convert their shares of Series A Preferred Stock in the event that (i) the average of the daily volume-weighted average price of Common Stock over any 10 consecutive trading days is greater than $1.74 (as adjusted for stock splits, stock dividends and similar transactions) and (ii) there is then an effective registration statement registering under the Securities Act the resale of the shares of Common Stock issuable upon such conversion of Series A Preferred Stock.
· | to add guarantees to or secure any series of debt securities; | |
· | to provide for the succession of another person pursuant to the provisions of the indenture relating to consolidations, mergers and sales of assets and the assumption by such successor of our covenants, agreements, and obligations, or to otherwise comply with the provisions of the indenture relating to consolidations, mergers, and sales of assets; | |
· | to surrender any right or power conferred upon us under the indenture or to add to our covenants further covenants, restrictions, conditions or provisions for the protection of the holders of all or any series of debt securities; | |
· | to cure any ambiguity or to correct or supplement any provision contained in the indenture, in any supplemental indenture or in any debt securities that may be defective or inconsistent with any other provision contained therein; | |
· | to modify or amend the indenture in such a manner as to permit the qualification of the indenture or any supplemental indenture under the Trust Indenture Act; | |
· | to add to or change any of the provisions of the indenture to supplement any of the provisions of the indenture in order to permit the defeasance and discharge of any series of debt securities pursuant to the indenture, so long as any such action does not adversely affect the interests of the holders of debt securities of any series in any material respect; | |
· | to add to, change, or eliminate any of the provisions of the indenture with respect to one or more series of debt securities, so long as any such addition, change or elimination shall not apply to any debt securities of any series created prior to the execution of such supplemental indenture and entitled to the benefit of such provision; | |
· | to evidence and provide for the acceptance of appointment by a successor or separate trustee; and | |
· | to establish the form or terms of debt securities of any series and to make any change that does not adversely affect the interests of the holders of debt securities. |
With the consent of the Company based on their shares’ aggregate Series A Issue Price and accrued and unpaid dividends.
Notwithstanding our rights and the rights of the trustee to enter into one or more supplemental indentures with the consent of the holders of debt securities of the affected series as described above, no such supplemental indenture shall, without the consent of the holder of each outstanding debt security of the affected series, among other things:
· | change the final maturity of the principal of, or any installment of interest on, any debt securities; | |
· | reduce the principal amount of any debt securities or the rate of interest on any debt securities; | |
· | change the currency in which any debt securities are payable; | |
· | impair the right of the holders to conduct a proceeding for any remedy available to the trustee; | |
· | reduce the percentage in principal amount of any series of debt securities whose holders must consent to an amendment or supplemental indenture; | |
· | modify the ranking or priority of the securities; or | |
· | reduce any premium payable upon the redemption of any debt securities. |
| 15 |
| Table of Contents |
Satisfaction and Discharge of the Indenture; Defeasance
Except to the extent set forth in a supplemental indenture with respect to any series of debt securities, we, at our election, may discharge the indenture and the indenture shall generally cease to be of any further effect with respect to that series of debt securities if (a) we have delivered to the trustee for cancellation all debt securities of that series (with certain limited exceptions) or (b) all debt securities of that series not previously delivered to the trustee for cancellation shall have become due and payable, or are entitledby their terms to become due and payable within one year or are to be called for redemption within one year, and we have deposited with the trustee the entire amount sufficient to pay at maturity or upon redemption all such debt securities.
In addition, we have a “legal defeasance option” (pursuant to which we may terminate, with respect to the debt securities of a particular series, all of our obligations under such debt securities and the indenture with respect to such debt securities) and a “covenant defeasance option” (pursuant to which we may terminate, with respect to the debt securities of a particular series, our obligations with respect to such debt securities under certain specified covenants contained in the indenture). If we exercise our legal defeasance option with respect to a numberseries of votes equaldebt securities, payment of such debt securities may not be accelerated because of an event of default. If we exercise our covenant defeasance option with respect to a series of debt securities, payment of such debt securities may not be accelerated because of an event of default related to the specified covenants.
We may exercise our legal defeasance option or our covenant defeasance option with respect to the debt securities of a series only if we irrevocably deposit in trust with the trustee cash or U.S. government obligations (as defined in the indenture) for the payment of principal, premium, if any, and interest with respect to such debt securities to maturity or redemption, as the case may be. In addition, to exercise either of our defeasance options, we must comply with certain other conditions, including the delivery to the trustee of an opinion of counsel to the effect that the holders of debt securities of such series will not recognize income, gain or loss for Federal income tax purposes as a result of such defeasance and will be subject to Federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such defeasance had not occurred (and, in the case of legal defeasance only, such opinion of counsel must be based on a ruling from the Internal Revenue Service or other change in applicable Federal income tax law).
The trustee will hold in trust the cash or U.S. government obligations deposited with it as described above and will apply the deposited cash and the proceeds from deposited U.S. government obligations to the payment of principal, premium, if any, and interest with respect to the debt securities of the defeased series.
Mergers, Consolidations and Certain Sales of Assets
We may not:
· | consolidate with or merge into any other person or entity or permit any other person or entity to consolidate with or merge into us in a transaction in which we are not the surviving entity, or | |
· | transfer, lease or dispose of all or substantially all of our assets to any other person or entity, unless: |
o | the resulting, surviving or transferee entity shall be a corporation organized and existing under the laws of the United States or any state thereof and such resulting, surviving or transferee entity shall expressly assume, by supplemental indenture, executed and delivered in form satisfactory to the trustee, all of our obligations under the debt securities and the indenture; | |
o | immediately after giving effect to such transaction (and treating any indebtedness which becomes an obligation of the resulting, surviving or transferee entity as a result of such transaction as having been incurred by such entity at the time of such transaction), no default or event of default would occur or be continuing; and | |
o | we shall have delivered to the trustee an officers’ certificate and an opinion of counsel, each stating that such consolidation, merger or transfer and such supplemental indenture (if any) comply with the indenture. |
The phrase “substantially all” of our assets will likely be interpreted under applicable state law and will be dependent upon particular facts and circumstances. As a result, there may be a degree of uncertainty in ascertaining whether a sale or transfer of “substantially all” of our assets has occurred.
| 16 |
| Table of Contents |
Governing Law
The indenture and the debt securities will be governed by the laws of the State of New York.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, incorporator or stockholder of ENDRA, as such, shall have any liability for any obligations of ENDRA under the debt securities or the indenture or for any claim based on, in respect of, or by reason of, such obligations or their creation, solely by reason of his, her, or its status as director, officer, incorporator or stockholder of ENDRA. By accepting a debt security, each holder waives and releases all such liability, but only such liability. The waiver and release are part of the consideration for issuance of the debt securities. Nevertheless, such waiver may not be effective to waive liabilities under the federal securities laws and it has been the view of the SEC that such a waiver is against public policy.
Conversion or Exchange Rights
Any debt securities offered hereby may be convertible into or exchangeable for shares of our equity or other securities. The terms and conditions of such conversion or exchange will be set forth in the applicable prospectus supplement. Such terms may include, among others, the following:
· | the conversion or exchange price; | |
· | the conversion or exchange period; | |
· | provisions regarding our ability or that of the holder to convert or exchange the debt securities; | |
· | events requiring adjustment to the conversion or exchange price; and | |
· | provisions affecting conversion or exchange in the event of our redemption of such debt securities. |
Concerning the Trustee
The indenture provides that there may be more than one trustee with respect to one or more series of debt securities. If there are different trustees for different series of debt securities, each trustee will be a trustee of a trust under a supplemental indenture separate and apart from the trust administered by any other trustee under such indenture. Except as otherwise indicated in this prospectus or any prospectus supplement, any action permitted to be taken by a trustee may be taken by the trustee only with respect to the one or more series of debt securities for which it is the trustee under an indenture. Any trustee under the indenture or a supplemental indenture may resign or be removed with respect to one or more series of debt securities. All payments of principal of, premium, if any, and interest on, and all registration, transfer, exchange authentication and delivery (including authentication and delivery on original issuance of the debt securities) of, the debt securities of a series will be effected by the trustee with respect to such series at an office designated by the trustee.
The indenture contains limitations on the right of the trustee, should it become a creditor of ENDRA, to obtain payment of claims in certain cases or to realize on certain property received in respect of any such claim as security or otherwise. If the trustee acquires an interest that conflicts with any duties with respect to the debt securities, the trustee is required to either resign or eliminate such conflicting interest to the extent and in the manner provided by the indenture.
| 17 |
| Table of Contents |
DESCRIPTION OF WARRANTS WE MAY OFFER
We may issue warrants for the purchase of debt securities, preferred stock or common stock. Warrants may be issued independently or together with debt securities, preferred stock or common stock and may be attached to or separate from any offered securities. Any issue of warrants will be governed by the terms of the applicable form of warrant and any related warrant agreement which we will file with the SEC and they will be incorporated by reference to the registration statement of which this prospectus is a part on or before the time we issue any warrants.
The particular terms of any issue of warrants will be described in the prospectus supplement relating to the issue. Those terms may include:
· | the title of such warrants; | |
· | the aggregate number of such warrants; | |
· | the price or prices at which such warrants will be issued; | |
· | the currency or currencies (including composite currencies) in which the price of such warrants may be payable; | |
· | the terms of the securities purchasable upon exercise of such warrants and the procedures and conditions relating to the exercise of such warrants; | |
· | the price at which the securities purchasable upon exercise of such warrants may be purchased; | |
· | the date on which the right to exercise such warrants will commence and the date on which such right shall expire; | |
· | any provisions for adjustment of the number or amount of securities receivable upon exercise of the warrants or the exercise price of the warrants; | |
· | if applicable, the minimum or maximum amount of such warrants that may be exercised at any one time; | |
· | if applicable, the designation and terms of the securities with which such warrants are issued and the number of such warrants issued with each such security; | |
· | if applicable, the date on and after which such warrants and the related securities will be separately transferable; | |
· | information with respect to book-entry procedures, if any; and | |
· | any other terms of such warrants, including terms, procedures and limitations relating to the exchange or exercise of such warrants. |
The prospectus supplement relating to any warrants to purchase equity securities may also include, if applicable, a discussion of certain U.S. federal income tax and ERISA considerations.
Warrants for the purchase of preferred stock and common stock will be offered and exercisable for U.S. dollars only.
Each warrant will entitle its holder to purchase the principal amount of debt securities or the number of shares of Common Stock into which such holder’s sharespreferred stock or common stock at the exercise price set forth in, or calculable as set forth in, the applicable prospectus supplement.
After the close of Series A Preferred Stock are then convertible.
Prior to a liquidation preference in the event of any liquidation, dissolution or winding up of the Company based on their shares’ aggregate Series B Issue Price and accrued and unpaid dividends. Such liquidation preference of Series B Preferred Stock holders is on a
| 18 |
| Table of Contents |
As of February 13, 2024, warrants to purchase a total of 882,349 shares of Common Stock issuable upon the exercise of outstanding unregistered warrants,common stock at a weighted average exercise price of $2.83$1.58 per share;
DESCRIPTION OF UNITS WE MAY OFFER
We may issue units consisting of any combination of the other types of securities offered under our Incentive Plan.
The following description, together with the additional information included in any applicable prospectus supplement, summarizes the general features of the Notesunits that we may offer under this prospectus. You should read any prospectus supplement and each three month period thereafterany free writing prospectus that we may authorize to be provided to you related to the series of units being offered, as well as the complete unit agreements that contain the terms of the units. Specific unit agreements will contain additional important terms and on the Maturity Date.
If we offer any units, certain terms of that series of units will be described in the applicable prospectus supplement, including, without limitation, the following, as applicable:
· | the title of the series of units; | |
· | identification and description of the separate constituent securities comprising the units; | |
· | the price or prices at which the units will be issued; | |
· | the date, if any, on and after which the constituent securities comprising the units will be separately transferable; | |
· | a discussion of certain United States federal income tax considerations applicable to the units; and | |
· | any other terms of the units and their constituent securities. |
| 19 |
| Table of Contents |
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
The provisions of Delaware law, ourthe Certificate of Incorporation and the Bylaws could have the effect of delaying, deferring or discouraging another person from acquiring control of us. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our bylaws. This summary does not purportboard of directors. We believe that the benefits of increased protection of our potential ability to be complete and is qualifiednegotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in its entirety by reference to the corporate lawan improvement of their terms.
Delaware and our Certificate of Incorporation and bylaws.
We are subject to Section 203 of the Delaware General Corporation Law (the “DGCL”), an anti-takeover law. In general, Section 203 prohibits a Delaware corporation from engaging in any business combination (as defined below) with any interested stockholder (as defined below) for a period of three years following the date that the stockholder became an interested stockholder, unless:
· | prior to that date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; | |
· | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares of voting stock outstanding (but not the voting stock owned by the interested stockholder) those shares owned by persons who are directors and officers and by excluding employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or | |
· | on or subsequent to the time the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines “business combination” to include the following:
· | any merger or consolidation involving the corporation and the interested stockholder; | |
· | any sale, lease, exchange, mortgage, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; | |
· | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; | |
· | subject to limited exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or | |
· | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
Section 203 generally defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation, or who beneficially owns 15% or more of the outstanding voting stock of the corporation at any time within a three-year period immediately prior to the date of determining whether such person is an interested stockholder, and any entity or person affiliated with or controlling or controlled by any of these entities or persons.
| 20 |
| Table of Contents |
Certificate of Incorporation and Bylaw Provisions
The Certificate of Incorporation and the Bylaws include a number of provisions that may have the effect of discouraging, delayingcould deter hostile takeovers or preventing a changedelay or prevent changes in control or an unsolicited acquisition proposal that a stockholder might consider favorable, including a proposal that might result in the payment of a premium over the market price for the shares held by our stockholders.us. Certain of these provisions are summarized in the following paragraphs.
Effects of authorized but unissued common stock
. One of the effects of the existence of authorized but unissued common stock may be to enable our board of directors to make more difficult or to discourage an attempt to obtain control of our Company by means of a merger, tender offer, proxy contest or otherwise, and thereby to protect the continuity of management. If, in the due exercise of its fiduciary obligations, the board of directors were to determine that a takeover proposal was not in our best interest, such shares could be issued by the board of directors without stockholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover transaction by diluting the voting or other rights of the proposed acquirer or insurgent stockholder group, by putting a substantial voting block in institutional or other hands that might undertake to support the position of the incumbent board of directors, by effecting an acquisition that might complicate or preclude the takeover, or otherwise.Cumulative Voting
. Our Certificate of Incorporation does not provide for cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors.Vacancies
. Our Certificate of Incorporation provides that all vacancies may be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum.Special Meeting of Stockholders
. A special meeting of stockholders may only be called by the Chairman of the board of directors, the President, the Chief Executive Officer, or the board of directors at any time and for any purpose or purposes as shall be stated in the notice of the meeting, or by request of the holders of record of at least 20% of the outstanding shares of common stock. This provision could prevent stockholders from calling a special meeting because, unless certain significant stockholders were to join with them, they might not obtain the percentage necessary to request the meeting. Therefore, stockholders holding less than 20% of the issued and outstanding common stock, without the assistance of management, may be unable to propose a vote on any transaction that would delay, defer or prevent a change of control, even if the transaction were in the best interests of our stockholders.| 21 |
| Table of Contents |
We may sell the securities offered by this prospectus to one or more underwriters or dealers for public offering, through agents, directly to purchasers or through a combination of any such methods of sale. The validityname of any such underwriters, dealers or agents involved in the offer and sale of the securities, the amounts underwritten and the nature of its obligation to take the securities will be specified in the applicable prospectus supplement. We have reserved the right to sell the securities directly to investors on our own behalf in those jurisdictions where we are authorized to do so. The sale of the securities may be effected in transactions (a) on any national or international securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, (b) in the over-the-counter market, (c) in transactions otherwise than on such exchanges or in the over-the-counter market or (d) through the writing of options.
We and our agents and underwriters may offer and sell the securities at a fixed price or prices that may be changed, at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices. The securities may be offered on an exchange, which will be disclosed in the applicable prospectus supplement. We may, from time to time, authorize dealers, acting as our agents, to offer and sell the securities upon such terms and conditions as set forth in the applicable prospectus supplement. We may also sell the securities offered by any applicable prospectus supplement in “at-the-market offerings” within the meaning of Rule 415 of the Securities Act, to or through a market maker or into an existing trading market, on an exchange or otherwise.
If we use underwriters to sell securities, we will enter into an underwriting agreement with them at the time of the sale to them. In connection with the sale of the securities, underwriters may receive compensation from us in the form of underwriting discounts or commissions and may also receive commissions from purchasers of the securities for whom they may act as agent. Any underwriting compensation paid by us to underwriters or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers, will be set forth in the applicable prospectus supplement to the extent required by applicable law. Underwriters may sell the securities to or through dealers, and such dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions (which may be changed from time to time) from the purchasers for whom they may act as agents.
Dealers and agents participating in the distribution of the securities may be deemed to be underwriters, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions under the Securities Act. Unless otherwise indicated in the applicable prospectus supplement, an agent will be acting on a best efforts basis and a dealer will purchase debt securities as a principal, and may then resell the debt securities at varying prices to be determined by the dealer.
If so indicated in the prospectus supplement, we will authorize underwriters, dealers or agents to solicit offers by certain specified institutions to purchase offered securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. Such contracts will be subject to any conditions set forth in the applicable prospectus supplement and the prospectus supplement will set forth the commission payable for solicitation of such contracts. The underwriters and other persons soliciting such contracts will have no responsibility for the validity or performance of any such contracts.
Underwriters, dealers and agents may be entitled, under agreements entered into with us, to indemnification against and contribution towards certain civil liabilities, including any liabilities under the Securities Act.
To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise affect the price of the securities. These may include over-allotment, stabilization, syndicate short covering transactions and penalty bids. Over-allotment involves sales in excess of the offering size, which creates a short position. Stabilizing transactions involve bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Syndicate short covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. Penalty bids permit the underwriters to reclaim selling concessions from dealers when the securities originally sold by the dealers are purchased in covering transactions to cover syndicate short positions. These transactions may cause the price of the securities sold in an offering to be higher than it would otherwise be. These transactions, if commenced, may be discontinued by the underwriters at any time.
| 22 |
| Table of Contents |
Any securities other than our common stock issued hereunder may be new issues of securities with no established trading market. Any underwriters or agents to or through whom such securities are sold for public offering and sale may make a market in such securities, but such underwriters or agents will not be obligated to do so and may discontinue any market making at any time without notice. No assurance can be given as to the liquidity of the trading market for any such securities. The amount of expenses expected to be incurred by us in connection with any issuance of securities will be set forth in the applicable prospectus supplement. Certain of the underwriters, dealers or agents and their associates may engage in transactions with, and perform services for, us and certain of our affiliates in the ordinary course of business.
During such time as we may be engaged in a distribution of the securities covered by this prospectus we are required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes us, any affiliated purchasers, and any broker-dealer or other person who participates in such distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase, any security which is the subject of the distribution until the entire distribution is complete. Regulation M also restricts bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of our shares of common stock.
| 23 |
| Table of Contents |
The validity and legality of the securities offered hereby has beenand certain other legal matters will be passed upon for usthe Company by K&L Gates LLP, Charlotte, North Carolina. As described under “Selling Stockholders,” Mark R. Busch, a partner at K&L Gates
RBSM LLP, beneficially owns certain ofindependent registered public accounting firm, has audited our securities and is participating as a selling stockholder in this offering.
We file annual, reports, quarterly reports, currentand special reports, proxy statements and other information with the SEC under theSecurities and Exchange Act.Commission (the “SEC”). You can read our SEC filings, including the registration statement,inspect and copy these reports, proxy statements and other information at the SEC’s websitePublic Reference Room at www.sec.gov100 F Street, N.E., whichWashington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding issuers, that file electronically with the SEC.
We will provide, upon written or oral request, without charge to you, including any agreement that is filed as an exhibitbeneficial owner to the registration statement of whichwhom this prospectus is delivered, a part were made solely for the benefitcopy of any or all of the partiesdocuments incorporated herein by reference other than the exhibits to such agreement, including, in some cases,those documents, unless the exhibits are specifically incorporated by reference into the information that this prospectus incorporates. You should direct a request for the purpose of allocating risk among the partiescopies to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were made as of an earlier date. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC:
· | Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the SEC on March 28, 2024; and | |
· | The description of the Company’s common stock contained in the Company’s Registration Statement on Form 8-A (File No. 001-37969) initially filed with the SEC on December 16, 2016 pursuant to Section 12(g) of the Exchange Act, including any amendment or reports filed for the purpose of updating such description. |
We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made on or after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, until we file a post-effective amendment that indicates the termination of the offering of the Securities made by this prospectus and will become a part of this prospectus from the date that such documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference, including exhibits to these documents. You should direct any requests for documents to ENDRA Life Sciences Inc., 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105; Telephone: (734) 335-0468. Copies of the above reports may also be accessed from our websiteweb site at www.endrainc.com.www.endrainc.com. We have authorized no one to provide you with any information that differs from that contained in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date onof the front cover of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed to be modified superseded or replacedsuperseded for purposes of this prospectus to the extent that a statement contained in this prospectus or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this prospectus modifies or supersedes that statement. Any statement that is modified or replaces such statement.

| 24 |
| Table of Contents |

ENDRA Life Sciences Inc.
$50,000,000
Common Stock
Preferred Stock
Debt Securities
Warrants
Units
PROSPECTUS
, 2024
i |
The information in this prospectus supplement and the accompanying prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus supplement and the accompanying prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated March 28, 2024
PROSPECTUS SUPPLEMENT
(to Prospectus dated , 2024)

ENDRA Life Sciences Inc.
$6,200,000
Common Stock
ENDRA Life Sciences Inc. has entered into an At-The-Market Issuance Sales Agreement (the “Sales Agreement”) with Ascendiant Capital Markets, LLC (the “Sales Agent”) relating to the offer and sale of up to $6,200,000 of our common stock, par value $0.0001 per share (the “common stock”), offered by this prospectus supplement and the accompanying prospectus. Effective upon the effectiveness of the registration statement of which this prospectus supplement and accompanying prospectus form a part, we and the Sales Agent are terminating our previous At-The-Market Issuance Sales Agreement, dated June 21, 2021.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made in transactions that are deemed to be “at-the-market” offerings as defined in Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”), including sales made directly on or through the Nasdaq Capital Market (“Nasdaq”), the trading market for our common stock, or any other trading market in the United States for our common stock, sales made to or through a market maker other than on an exchange, directly to the Sales Agent as principal for its own account in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, in privately negotiated transactions, in block trades, or through a combination of any such methods of sale. The Sales Agent will act as sales agent on a commercially reasonable efforts basis consistent with its normal trading and sales practices. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
We will pay the Sales Agent a commission equal to 3.0% of the gross sales price per share of common stock issued by us and sold through the Sales Agent as our sales agent under the Sales Agreement. In connection with the sale of the common stock on our behalf, the Sales Agent will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of the Sales Agent will be deemed to be underwriting commissions or discounts.
Our common stock trades on the Nasdaq Capital Market under the symbol “NDRA.” On February 13, 2024, the last reported sale price for our common stock was $1.12 per share.
As of February 14, 2024, the aggregate market value of our outstanding common stock held by non-affiliates was $27,200,435 based on 11,035,659 shares of outstanding common stock, of which 10,880,174 shares are held by non-affiliates, and the last reported sale price of our common stock of $2.50 per share on December 22, 2023. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75,000,000. As of the date of this prospectus, we have sold securities with an aggregate market value of $2,258,746 pursuant to General Instruction 1.B.6 of Form S-3 during the 12-calendar month period that ends on and includes the date hereof.
ii |
INVESTING IN OUR SECURITIES INVOLVES RISKS. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK FACTORS” CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT AND ANY RELATED FREE WRITING PROSPECTUS, AND UNDER SIMILAR HEADINGS IN OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS OR ANY SUCH PROSPECTUS SUPPLEMENT. SEE “RISK FACTORS” ON PAGE 8 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.

The date of this prospectus supplement is , 2024.
iii |
Page | ||
1 | ||
2 | ||
4 | ||
7 | ||
8 | ||
10 | ||
11 | ||
11 | ||
12 | ||
13 | ||
13 | ||
13 | ||
13 |
| iv |
| Table of Contents |
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying base prospectus forms part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, utilizing a “shelf” registration process. Under this shelf process, we may from time to time sell any combination of securities described in this prospectus in one or more offerings.
In this prospectus supplement, the terms “ENDRA,” “we,” “us,” “our” and the “Company” refer to ENDRA Life Sciences Inc. unless otherwise stated or the context otherwise requires.
This prospectus supplement, and the information incorporated herein by reference, may add, update or change information in the accompanying prospectus and in any free writing prospectuses we may provide to you in connection with this offering. You should read both this prospectus supplement and the accompanying prospectus together with additional information described under the headings “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.” If there is any inconsistency between the information in this prospectus supplement and the accompanying prospectus, you should rely on the information in this prospectus supplement.
You may rely only on the information contained in or incorporated by reference in this prospectus supplement and the accompanying prospectus. Neither we nor the Sales Agent has authorized anyone to provide information different from that contained in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein. If anyone provides you with different or inconsistent information, you should not rely on it. The information in this prospectus supplement, the accompanying prospectus and in any free writing prospectuses we may provide to you in connection with this offering is accurate only as of their respective dates, regardless of time of delivery. Our business, financial condition, results of operations and prospects may have changed since those dates.
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the offering of the securities in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus supplement outside the United States. This prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
All references in this prospectus to our consolidated financial statements include, unless the context indicates otherwise, the related notes.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
This prospectus and the information incorporated by reference herein include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus are the property of their respective owners.
The industry and market data and other statistical information contained in this prospectus supplement, the accompanying prospectus and the documents we incorporate by reference are based on management’s own estimates, independent publications, government publications, reports by market research firms or other published independent sources, and, in each case, are believed by management to be reasonable estimates. Although we believe these sources are reliable, we have not independently verified the information. None of the independent industry publications used in this prospectus supplement, the accompanying prospectus or the documents we incorporate by reference were prepared on our or our affiliates’ behalf and none of the sources cited by us consented to the inclusion of any data from its reports, nor have we sought their consent.
| 1 |
| Table of Contents |
Certain information set forth in this prospectus supplement, the accompanying prospectus or incorporated by reference in this prospectus may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Exchange Act, that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “estimate,” “goal,” “anticipate,” “project” or other comparable terms. All statements other than statements of historical facts included in this prospectus supplement and accompanying prospectus regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding: estimates of the timing of future events and anticipated results of our development efforts, including the timing of submission for and receipt of required regulatory approvals and product launches; statements relating to future financial position and projected costs and revenue; expectations concerning our business strategy; and statements regarding our ability to find and maintain development partners.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside our control. Our actual results and financial condition may differ materially from those in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following:
· | our limited commercial experience, limited cash and history of losses; | |
· | our ability to obtain adequate financing to fund our business operations in the future; | |
· | our ability to achieve profitability; | |
· | delays and changes in regulatory requirements, policy and guidelines, including potential delays in submitting required regulatory applications or other submissions with respect to U.S. Food and Drug Administration (“FDA”) or other regulatory agency approval; | |
· | our ability to obtain CE mark certification and secure required FDA and other governmental approvals for our Thermo-Acoustic Enhanced Ultrasound (“TAEUS”) applications | |
· | our ability to develop a commercially feasible application based on our TAEUS technology; | |
· | market acceptance of our technology; | |
· | uncertainties associated with COVID-19 or other pandemics that may occur, including effects on our operations; | |
· | the effect of macroeconomic conditions on our business; | |
· | results of our human studies, which may be negative or inconclusive; | |
· | our ability to find and maintain development partners; |
| 2 |
| Table of Contents |
· | our reliance on third parties, collaborations, strategic alliances and licensing arrangements to complete our business strategy; | |
· | the amount and nature of competition in our industry; | |
· | our ability to protect our intellectual property; | |
· | potential changes in the healthcare industry or third-party reimbursement practices; | |
· | our ability to comply with regulation by various federal, state, local and foreign governmental agencies and to maintain necessary regulatory clearances or approvals; | |
· | our ability to maintain compliance with Nasdaq listing standards; | |
· | our dependence on our senior management team; and | |
· | the other risks and uncertainties described in the Risk Factors section of this prospectus supplement, any additional prospectus supplement and our most recent Annual Form 10-K and subsequently filed Quarterly Reports on Form 10-Q, which filings are incorporated herein by reference. |
We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their entirety by the applicable cautionary statements. We further caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
| 3 |
| Table of Contents |
We were incorporated as a Delaware corporation in 2007. We are developing a next-generation enhanced ultrasound technology platform—Thermo Acoustic Enhanced Ultrasound, or TAEUS® in order to broaden patient access to the safe diagnosis and treatment of a number of significant medical conditions in circumstances where expensive X-ray computed tomography (“CT”), magnetic resonance imaging (“MRI”) technology, or other diagnostic technologies such as surgical biopsy, are unavailable or impractical. Our TAEUS technology uses radio frequency (“RF”) pulses to stimulate tissues, using a small fraction (less than 1%) of the amount of energy that would be transmitted into the body during an MRI scan. The use of RF energy allows our TAEUS technology to penetrate deep into tissue, enabling the imaging of human anatomy at depths equivalent to those of conventional ultrasound. The RF pulses are absorbed by tissue and converted into ultrasound signals, which are detected by an external ultrasound receiver and a digital acquisition system that is part of the TAEUS system. The detected ultrasound is processed into images and other forms of data using our proprietary software and algorithms and then displayed to complement conventional gray-scale ultrasound images. The TAEUS imaging concept is illustrated below:
We believe that our TAEUS technology has the potential to add a number of new capabilities to conventional ultrasound, and other types of capital medical equipment such as interventional thermo-ablation systems, and thereby enhance the utility of those systems. Additionally, we believe that our technology can extend the use of ultrasound technology to indications and clinical situations that currently require the use of expensive CT or MRI imaging systems, where imaging is not practical using existing technology, or where other assessment tools such as surgical biopsy are required. Our TAEUS platform is not intended to replace CT or MRI systems, both of which are versatile imaging technologies with capabilities and uses beyond the focus of our business. These systems, while versatile, are relatively expensive—a CT system can cost approximately $1 million and an MRI system can cost up to approximately $3 million. In addition, and in contrast to ultrasound systems, due to their limited number and the fact that they are usually fixed-in-place at major medical facilities, CT or MRI systems are frequently inaccessible to many patients. For example, CT or MRI systems are generally less accessible to primary care practices, rural clinics, economically developing markets, and patient bedsides. |
| 4 |
| Table of Contents |
Ultrasound systems are more broadly available to patients than either CT or MRI systems. There are an estimated 1.6 million diagnostic ultrasound systems globally in use today. The global diagnostic ultrasound device market is anticipated to expand at a CAGR of 4.07% from 2022 to 2030, according to Grand View Research. Ultrasound systems are relatively inexpensive compared to CT and MRI systems, as smaller portable ultrasound systems can cost as little as approximately $5,000 and the price of new cart-based ultrasound systems can range from approximately $75,000 to $200,000. These numbers include both portable and cart-based ultrasound systems, and cover all types of diagnostic ultrasound procedures, including systems intended for cardiology, prenatal and abdominal use. We do not currently intend to address cart-based ultrasound systems focused on applications in prenatal care, nor certain portable ultrasound applications such as emergency room medicine, where we believe our TAEUS technology may not substantially impact patient care. Accordingly, we estimate the addressable market for one or more of our current or future TAEUS applications to include approximately 700,000 ultrasound systems currently in use throughout the world, in addition to other types of capital equipment. To demonstrate the capabilities of our TAEUS platform, we have conducted various internal ex-vivo laboratory experiments and limited internal in-vivo large animal studies. In our ex-vivo and in-vivo testing, we have demonstrated that the TAEUS platform has the following capabilities and potential clinical applications: | ||
· | Tissue Composition: Our TAEUS technology enables ultrasound to distinguish fat from lean tissue. This capability would enable the use of TAEUS-enhanced ultrasound for the early identification, staging and monitoring of NAFLD, a precursor to NASH, liver fibrosis, cirrhosis and liver cancer. | |
· | Temperature Monitoring: Our TAEUS technology enables traditional ultrasound to visualize changes in tissue temperature, in real time. This capability would enable the use of TAEUS-enhanced ultrasound to guide thermoablative therapy, which uses heat or cold to affect tissue, such as in the treatment of cardiac atrial fibrillation, or removal of cancerous liver and kidney lesions, with greater accuracy, and perform cosmetology procedures such as lipolysis of abdominal fat. | |
· | Vascular Imaging: Our TAEUS technology has the potential to enable visualization of blood vessels from any angle, using only a saline solution contrasting agent, unlike Doppler ultrasound, which requires precise viewing angles. This capability would enable the use of TAEUS-enhanced ultrasound to assist in identifying arterial plaques or malformed vessels. | |
· | Tissue Perfusion: Our TAEUS technology has the potential to image blood flow at the capillary level in a region, organ or tissue. This capability could be used to assist physicians in characterizing abnormalities in tissue perfusion symptomatic of damaged tissue, such as internal bleeding from trauma, or diseased tissue, such as certain cancers. | |
The first TAEUS application we intend to commercialize is our NAFLD TAEUS application addressing liver tissue composition. Our initial target market for this application is the European Union (“EU”) and United Kingdom. In September 2019, we announced the completion and reported top-level findings of an initial healthy subject study and data collection of 50 subjects, which was included in our TAEUS liver device technical file submission for device CE mark. We received CE mark approval for our TAEUS FLIP (Fatty Liver Imaging Probe) application in March 2020. We have registered the product in each of our primary target European markets (i.e., Germany, France, and the United Kingdom). In June 2020, we submitted a 510(k) Application to the FDA for our TAEUS Fatty Live Imaging Probe (“FLIP”) System. In February 2022, we announced that we would pursue FDA reclassification and clearance of our TAEUS FLIP System through the FDA’s “de novo” process. We subsequently voluntarily withdrew our 510(k) Application and submitted a de novo request for the TAEUS system to the FDA in the third quarter of 2023. In the fourth quarter of 2023, the FDA sent an Additional Information (“AI”) request related to our de novo application. Since we received the AI request, we have had several interactions with the FDA and have provided additional information. In order to fully respond to the FDA’s questions, we will need to compile additional clinical data, provide additional device test data, and respond to cybersecurity related questions in a new de novo submission. We have a scheduled in-person pre-submission meeting with the FDA in the second quarter of 2024. We currently anticipate completing the necessary clinical studies by the fourth quarter of 2024 and submitting the new de novo request to the FDA in the first half of 2025. | ||
| 5 |
| Table of Contents |
After required regulatory approvals, our TAEUS technology can be added as an accessory to existing, commercially available ultrasound systems, helping to improve clinical decision-making on the front lines of patient care, without requiring substantially new clinical workflows or large capital investments. We are also developing TAEUS for possible incorporation into new medical equipment manufactured by original equipment manufacturers (“OEMs”), such as GE Healthcare and others, to enhance the utility of those OEM systems, as described more fully in our Annual Report on Form 10-K. Based on our design work and our understanding of the medical capital equipment market, we intend to price our initial liver TAEUS system at a price point of approximately $65,000, which we believe could enable clinical purchasers to recoup their investment in less than one year by performing a relatively small number of additional procedures, initially paid out-of-pocket by patients until government and private insurance reimbursement is secured for the TAEUS liver procedures. June 2021 At-The-Market Program On June 21, 2021, the Company entered into an At-The-Market Issuance Sales Agreement with the Sales Agent to offer and sell, from time to time through the Sales Agent, shares of the Company’s common stock for aggregate gross proceeds of up to $20 million (the “June 2021 Sales Agreement”). As of February 13, 2024, the Company had sold 2,258,746 shares of common stock under the June 2021 Sales Agreement for proceeds of approximately $11.4 million, net of commissions paid, but excluding estimated transaction expenses. Effective upon the effectiveness of the registration statement of which this prospectus supplement and accompanying prospectus form a part, we and the Sales Agent are terminating the June 2021 Sales Agreement. Corporate Information We were incorporated in Delaware in July 2007. Our corporate headquarters is located at 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105-1570. Our website can be accessed at www.endrainc.com. The telephone number of our principal executive office is (734) 335-0468. The information contained on, or that may be obtained from, our website is not, and shall not be deemed to be, a part of this prospectus supplement or the accompanying prospectus. |
| 6 |
| Table of Contents |
Common stock offered by us | Shares of our common stock having an aggregate offering price of up to $6,200,000. | ||
Common stock to be outstanding after the offering | Up to 16,369,353 shares, after giving effect to the sale of $6.2 million of shares of our common stock at an assumed price of $1.12 per share, which was the closing price of our common stock on the Nasdaq Capital Market on February 13, 2024. The maximum number of shares that may be issued will vary depending on the price at which shares may be sold from time to time during this offering.(1) | ||
Manner of offering | “At the market offering,” as defined in Rule 415(a)(4) of the Securities Act, that may be made from time to time by the Sales Agent. We may also sell the shares of our common stock to the Sales Agent as principal for its own accounts, at a price per share agreed upon at the time of sale. If we sell shares to a Sales Agent as principal, we will enter into a separate terms agreement setting forth the terms of such transaction, and we will describe the agreement in a pricing supplement. See “Plan of Distribution” on page 12 of this prospectus supplement. | ||
Use of proceeds | We currently intend to use the estimated net proceeds from the sale of this offering for working capital and general corporate purposes, including to fund our product development efforts and to fund our commercialization activities for our TAEUS technology. See “Use of Proceeds” for additional information. | ||
Risk factors | Investing in our common stock involves significant risks. See the section entitled “Risk Factors” beginning on page 8 and the other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. | ||
The Nasdaq Capital Market symbol | “NDRA” | ||
(1) | The number of shares of our common stock to be outstanding after this offer is based on 11,035,659 shares of common stock outstanding as of February 13, 2024 and excludes the following: | ||
· | 882,349 shares of common stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $1.58 per share; | ||
· | 2,010 shares of common stock issuable upon the conversion of outstanding shares of Series A Convertible Preferred Stock; | ||
· | 624,107 shares of common stock issuable upon the exercise of outstanding stock options issued pursuant to our 2016 Omnibus Incentive Plan (the “Incentive Plan”) at a weighted average exercise price of $19.24 per share; and | ||
· | 698,062 shares of common stock reserved for future issuance under our Incentive Plan. | ||
| 7 |
| Table of Contents |
Investing in our securities involves a high degree of risk. You should carefully consider the risk factors described in our Annual Report on Form 10-K for our most recent fiscal year (together with any material changes thereto contained in subsequent filed Quarterly Reports on Form 10-Q) and those contained in our other filings with the SEC, which are incorporated by reference in this prospectus and any accompanying prospectus supplement.
The prospectus supplement applicable to each type or series of securities we offer may contain a discussion of risks applicable to the particular types of securities that we are offering under that prospectus supplement. Prior to making a decision about investing in our securities, you should carefully consider the specific factors discussed under the caption “Risk Factors” in the applicable prospectus supplement, together with all of the other information contained in the prospectus supplement or appearing or incorporated by reference in this prospectus. These risks could materially affect our business, results of operations or financial condition and cause the value of our securities to decline. You could lose all or part of your investment.
Risks Related to this Offering of Our Common Stock
Sales of our common stock in this offering, or the perception that such sales may occur, could cause the market price of our common stock to fall.
We may issue and sell shares of our common stock for aggregate gross proceeds of up to $6.2 million from time to time in connection with this offering. The issuance and sale from time to time of these new shares of common stock, or our ability to issue these new shares of common stock in this offering, could have the effect of depressing the market price of our common stock.
You will suffer immediate and substantial dilution in the net tangible book value per share of the common stock that you purchase in this offering.
The shares sold in this offering, if any, will be sold from time to time at various prices; however, the assumed public offering price of our common stock is substantially higher than the as-adjusted net tangible book value per share of our common stock. Therefore, investors purchasing shares of our common stock in this offering will pay a price per share that substantially exceeds the as-adjusted net tangible book value per share after this offering. Assuming that an aggregate of 5,535,714 shares of our common stock are sold at a public offering price of $1.12 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on February 13, 2024, for aggregate gross proceeds of $5.9 million, and after deducting commissions and estimated offering expenses payable by us, new investors in this offering will experience immediate dilution of $0.32 per share, representing the difference between the assumed public offering price and our as adjusted net tangible book value per share after giving effect to this offering. See “Dilution” for a more detailed discussion of the dilution you would incur if you purchase common stock in this offering.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be lower than the price per share paid by investors in this offering.
Our management will have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and our stockholders will not have the opportunity as part of their investment decisions to assess whether the net proceeds are being used appropriately. You may not agree with our decisions, and our use of the proceeds may not yield any return on your investment. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our failure to apply the net proceeds of this offering effectively could compromise our ability to pursue our growth strategy and we might not be able to yield a significant return, if any, in our investment of these net proceeds. You will not have the opportunity to influence our decisions on how to use our net proceeds from this offering.
| 8 |
| Table of Contents |
The common stock offered hereby will be sold in “at-the-market” offerings, and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and so may experience different outcomes in their investment results. We will have discretion, subject to market demand, to vary the timing, prices, and numbers of shares sold, and there is no minimum or maximum sales price. Investors may experience a decline in the value of their shares as a result of share sales made at prices lower than the prices they paid.
The actual number of shares we will issue under the Sales Agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the Sales Agreement and compliance with applicable law, we have the discretion to deliver a sales notice to the Sales Agent at any time throughout the term of the Sales Agreement. The number of shares that are sold by the Sales Agent after we deliver a sales notice will fluctuate based on the market price of the common stock during the sales period and limits we set with the Sales Agent. Because the price per share of each share sold will fluctuate based on the market price of our common stock during the sales period, it is not possible at this stage to predict the number of shares, if any, that will ultimately be issued.
Our stock price and trading volume has fluctuated in the past, has recently been volatile and may be volatile in the future for reasons unrelated to our operating performance or prospects, and as a result, investors in our common stock could incur substantial losses.
Our stock price has fluctuated in the past, has recently been volatile and may be volatile in the future. During 2024 to date, daily trading volume ranged from approximately 20,900 to approximately 334,800 shares. We may incur rapid and substantial decreases in our stock price in the foreseeable future that are unrelated to our operating performance or prospects. The stock market in general and the market for healthcare companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may experience losses on their investment in our common stock.
These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business, financial condition, results of operations and growth prospects. There can be no guarantee that our stock price will remain at current prices or that future sales of our common stock will not be at prices lower than those sold to investors.
Additionally, recently, securities of certain companies have experienced significant and extreme volatility in stock price due to a sudden increase in demand for stock resulting in aggregate short positions in the stock exceeding the number of shares available for purchase, forcing investors with short exposure to pay a premium to repurchase shares for delivery to share lenders. This is known as a “short squeeze.” These short squeezes have led to the price per share of those companies to trade at a significantly inflated rate that is disconnected from the underlying value of the company. Many investors who have purchased shares in those companies at an inflated rate face the risk of losing a significant portion of their original investment as the price per share declines steadily as interest in those stocks abates. While we have no reason to believe our shares would be the target of a short squeeze, there can be no assurance that they will not be in the future, and you may lose a significant portion or all of your investment if you purchase our shares at a rate that is significantly disconnected from their underlying value.
| 9 |
| Table of Contents |
We may issue and sell shares of our common stock having aggregate gross proceeds of up to $6.2 million from time to time. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions paid to the Sales Agent and proceeds to us, if any, are not determinable at this time. We estimate that the net proceeds from the sale of the shares of common stock that we are offering may be up to approximately $5.9 million, after deducting commissions payable to the Sales Agent and estimated offering expenses payable by us.
We currently intend to use the estimated net proceeds from the sale of this offering for working capital and general corporate purposes, including to fund our product development efforts and to fund our commercialization activities for our TAEUS technology. We have not yet determined the amount of net proceeds to be used specifically for any particular purposes or the timing of these expenditures. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds from the sale of these securities, and investors will be relying on our judgment regarding the application of the net proceeds from this offering.
Pending our use of the net proceeds from this offering, we intend to maintain the net proceeds as cash deposits or cash management instruments, such as U.S. government securities or money market mutual funds.
| 10 |
| Table of Contents |
We have not declared or paid any cash dividends on our capital stock. We currently intend to retain any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
If you purchase shares of common stock in this offering, you will experience dilution to the extent of the difference between the public offering price per share in this offering and our as adjusted net tangible book value per share after this offering.
Net tangible book value per share represents total tangible assets less total liabilities, divided by the number of shares of common stock outstanding. Our historical net tangible book value as of September 30, 2023 was approximately $5.2 million, or $0.62 per share of common stock. After giving effect to the sale of our common stock in the aggregate amount of $6.2 million at an assumed public offering price of $1.12 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on February 13, 2024, and after deducting commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2023 would have been approximately $11.1 million, or approximately $0.80 per share. This represents an immediate increase in net tangible book value of approximately $0.18 per share to existing stockholders and an immediate dilution in as adjusted net tangible book value of approximately $0.32 per share to new investors in this offering.
The following table illustrates this dilution on a per share basis. The as adjusted information is illustrative only and will adjust based on the actual public offering price, the actual number of shares sold and other terms of the offering determined at the time shares of our common stock are sold pursuant to this prospectus supplement. The as adjusted information assumes that all of our common stock in the aggregate amount of $6.2 million is sold at the assumed public offering price of $1.12 per share. The shares sold in this offering, if any, will be sold from time to time at various prices.
Assumed public offering price per share Net tangible book value per share as of September 30, 2023 Increase in net tangible book value per share attributable to this offering As adjusted net tangible book value per share, after giving effect to this offering Dilution per share to new investors in this offering $ 1.12 $ 0.62 $ 0.18 $ 0.80 $ 0.32
The shares sold in this offering, if any, will be sold from time to time at various prices. An increase of $0.12 per share in the price at which the shares are sold from the assumed offering price of $1.12 per share shown in the table above, assuming all of our common stock in the aggregate amount of $6.2 million is sold at that price, would increase our adjusted net tangible book value per share after the offering to $0.83 per share and would increase the dilution in net tangible book value per share to new investors in this offering to $0.41 per share, after deducting commissions and estimated aggregate offering expenses payable by us. A decrease of $0.12 per share in the price at which the shares are sold from the assumed offering price of $1.12 per share shown in the table above, assuming all of our common stock in the aggregate amount of $6.2 million is sold at that price, would decrease our adjusted net tangible book value per share after the offering to $0.76 per share and would decrease the dilution in net tangible book value per share to new investors in this offering to $0.24 per share, after deducting commissions and estimated aggregate offering expenses payable by us. This information is supplied for illustrative purposes only, and will adjust based on the actual offering prices, the actual number of shares that we offer and sell in this offering and other terms of each sale of shares in this offering.
The number of shares of our common stock to be outstanding after this offer is based on 8,411,777 shares of common stock outstanding as of September 30, 2023 and excludes the following:
· | 2,468,063 shares of common stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $1.51 per share; | |
· | 8,126 shares of common stock issuable upon the conversion of outstanding shares of Series A Convertible Preferred Stock; | |
· | 624,240 shares of common stock issuable upon the exercise of outstanding stock options issued pursuant to our 2016 Omnibus Incentive Plan (the “Incentive Plan”) at a weighted average exercise price of $19.25 per share; and | |
· | 697,929 shares of common stock reserved for future issuance under our Incentive Plan. |
| 11 |
| Table of Contents |
We have entered into an At-The-Market Issuance Sales Agreement with Ascendiant Capital Markets, LLC, as Sales Agent, under which we may issue and sell over a period of time, and from time to time, shares of our common stock having an aggregate offering price of up to $6.2 million through the Sales Agent acting as sales agent or directly to a Sales Agent acting as principal. This prospectus supplement relates to our ability to issue and sell over a period of time, and from time to time, shares of our common stock to or through the Sales Agent pursuant to the Sales Agreement. Sales of the shares to which this prospectus supplement and the accompanying prospectus relate, if any, may be made in transactions that are deemed to be “at-the-market” offerings as defined in Rule 415 under the Securities Act, including sales made directly on or through the Nasdaq Capital Market (“Nasdaq”), the trading market for our common stock, or any other trading market in the Unites States for our common stock, sales made to or through a market maker other than on an exchange, directly to the Sales Agent as principal for its account in negotiated transactions at market prices prevailing at the time of sale or at prices related to such prevailing market prices, in privately negotiated transactions, in block trades, or through a combination of any such methods of sale. To the extent required by Regulation M, the Sales Agent acting as our sales agent will not engage in any transactions that stabilize our common stock while the offering is ongoing under this prospectus supplement.
Upon written instructions from us, the Sales Agent will offer the shares of our common stock, subject to the terms and conditions of the Sales Agreement, on a daily basis or as otherwise agreed upon by us and the Sales Agent. We will designate the maximum amount of shares of our common stock to be sold through the Sales Agent on a daily basis or otherwise determine such maximum amount together with the Sales Agent, subject to certain limitations set forth by the SEC. Subject to the terms and conditions of the Sales Agreement, the Sales Agent will use commercially reasonable efforts to sell on our behalf all of the shares of our common stock so designated or determined. We may instruct the Sales Agent not to sell shares of our common stock if the sales cannot be effected at or above the price designated by us in any such instruction. The Sales Agent may also sell our common stock in negotiated transactions with our prior approval. We or the Sales Agent may suspend the offering of shares of our common stock being made under the Sales Agreement upon proper notice to the other party.
For their services as sales agent in connection with the sale of shares of our common stock that may be offered hereby, we will pay the Sales Agent an aggregate fee of 3.0% of the gross sales price per share for any shares sold through it acting as our sales agent. The remaining sales proceeds, after deducting any expenses payable by us and any transaction fees imposed by any governmental, regulatory or self-regulatory organization in connection with the sales, will equal our net proceeds for the sale of such shares. We have agreed to reimburse the Sales Agent for certain of its expenses in an amount not to exceed $10,000, and, thereafter, reasonable fees and expenses of the Sales Agent’s incurred in conjunction of performing legal services related to the Sales Agreement for the Company.
The Sales Agent will provide written confirmation to us no later than the opening of the trading day immediately following the day in which shares of common stock are sold by it on our behalf under the Sales Agreement. Each confirmation will include the number of shares sold on that day, the compensation payable by us to the Sales Agent and the proceeds to us net of such compensation.
Settlement for sales of our common stock will occur, unless the parties agree otherwise, on the second business day following the date on which any sales were made in return for payment of the proceeds to us net of compensation paid by us to the Sales Agent. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
Unless otherwise required, we will report at least quarterly the number of shares of common stock sold through the Sales Agent under the Sales Agreement, the net proceeds to us and the compensation paid by us to the Sales Agent in connection with the sales of common stock.
In connection with the sale of common stock on our behalf, the Sales Agent will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation paid to it will be deemed to be underwriting commissions or discounts. We have agreed, under the Sales Agreement, to provide indemnification and contribution to the Sales Agent against certain civil liabilities, including liabilities under the Securities Act.
In the ordinary course of its business, the Sales Agent and/or its affiliates may perform investment banking, broker-dealer, financial advisory or other services for us for which it may receive separate fees.
We estimate that the total expenses from this offering payable by us, excluding compensation payable to the Sales Agent under the Sales Agreement, will be approximately $80,000. Additionally, pursuant to the terms of the Sales Agreement, we agreed to reimburse the Sales Agent for the reasonable fees and expenses of its legal counsel incurred in connection with quarterly and annual bring-downs required under the Sales Agreement in an amount not to exceed $2,500 in the aggregate for each such bring-down.
The offering of common stock pursuant to the Sales Agreement will terminate upon the earlier of (1) the sale of shares of our common stock with an aggregate offering price of $6.2 million subject to the Sales Agreement, (2) February 14, 2027 and (3) the termination of the Sales Agreement, pursuant to its terms, by either the Sales Agent or us.
The Company and the Sales Agent may in the future agree to add one or more additional sales agents to the offering, in which case the Company will file a further prospectus supplement providing the name of such additional sales agents and any other required information.
| 12 |
| Table of Contents |
The validity and legality of the securities offered hereby and certain other legal matters will be passed upon for the Company by K&L Gates LLP, Charlotte, North Carolina.
RBSM LLP, independent registered public accounting firm, has audited our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, which is incorporated by reference into this prospectus and elsewhere in the registration statement of which this prospectus is a part. Our financial statements are incorporated by reference in reliance on RBSM LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission (the “SEC”). You can inspect and copy these reports, proxy statements and other information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding issuers, such as ENDRA Life Sciences Inc. (www.sec.gov). Our web site is located at www.endrainc.com. The information contained on, or that may be obtained from, our website is not, and shall not be deemed to be, a part of this prospectus.
We will provide, upon written or oral request, without charge to you, including any beneficial owner to whom this prospectus is delivered, a copy of any or all of the documents incorporated herein by reference other than the exhibits to those documents, unless the exhibits are specifically incorporated by reference into the information that this prospectus incorporates. You should direct a request for copies to ENDRA Life Sciences Inc., 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105; Telephone: (734) 335-0468.
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC:
· | Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the SEC on March 28, 2024; and | |
· | The description of the Company’s common stock contained in the Company’s Registration Statement on Form 8-A (File No. 001-37969) initially filed with the SEC on December 16, 2016 pursuant to Section 12(g) of the Exchange Act, including any amendment or reports filed for the purpose of updating such description. |
We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made on or after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, until we file a post-effective amendment that indicates the termination of the offering of the Securities made by this prospectus and will become a part of this prospectus from the date that such documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference, including exhibits to these documents. You should direct any requests for documents to ENDRA Life Sciences Inc., 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105; Telephone: (734) 335-0468. Copies of the above reports may also be accessed from our web site at www.endrainc.com. We have authorized no one to provide you with any information that differs from that contained in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date of the front cover of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this prospectus modifies or supersedes that statement. Any statement that is modified or superseded will not constitute a part of this prospectus, except as modified or superseded.
| 13 |
| Table of Contents |

ENDRA Life Sciences Inc.
$6,200,000
Common Stock
PROSPECTUS SUPPLEMENT
, 2024
i |
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated March 28, 2024
PROSPECTUS

ENDRA Life Sciences Inc.
202,020 Shares of Common Stock
This prospectus relates to 202,020 shares of common stock, par value $0.0001 per share (the “common stock”), of ENDRA Life Sciences Inc. that PatentVest, Inc. (“PatentVest” or the “Selling Stockholder”) may sell from time to time in one or more offerings on terms to be determined at the time of sale. You can find detailed information on PatentVest and the transaction in which it acquired our securities in the section entitled “Selling Stockholder” beginning on page 8 of this prospectus.
The Selling Stockholder may sell its shares of common stock from time to time at market prices prevailing at the time of sale, at prices related to the prevailing market price, or at privately negotiated prices. We will not receive any proceeds from the sale of common stock by the Selling Stockholder. We have agreed to bear all of the expenses incurred in connection with the registration of the common stock. The Selling Stockholder will pay or assume brokerage commission and similar charges, if any, incurred in the sale of the common stock.
Our common stock trades on the Nasdaq Capital Market under the symbol “NDRA.” On February 13, 2024, the last reported sale price for our common stock was $1.12 per share.
INVESTING IN OUR SECURITIES INVOLVES RISKS. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK FACTORS” CONTAINED IN THE APPLICABLE PROSPECTUS SUPPLEMENT AND ANY RELATED FREE WRITING PROSPECTUS, AND UNDER SIMILAR HEADINGS IN OTHER DOCUMENTS THAT ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS OR ANY SUCH PROSPECTUS SUPPLEMENT. SEE “RISK FACTORS” ON PAGE 6 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2024.
ii |
Page | ||
1 | ||
2 | ||
3 | ||
5 | ||
6 | ||
7 | ||
8 | ||
10 | ||
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS | 11 | |
13 | ||
13 | ||
13 | ||
13 |
iii |
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, utilizing a “shelf” registration process. Under this shelf process, the Selling Stockholder may from time to time sell up to 202,020 shares of our common stock in one or more offerings.
We may file one or more prospectus supplements or, if appropriate, post-effective amendments, to accompany this prospectus to add, update or change information contained in this prospectus. If the information varies between this prospectus and the accompanying prospectus supplement or post-effective amendment, if any, you should rely on the information in the accompanying prospectus supplement or post-effective amendment. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to the offering. You should read both this prospectus and the accompanying prospectus supplement or post-effective amendment, if any, and any free writing prospectus together with the additional information described under “Where You Can Find More Information.” You should also carefully consider, among other things, the matters discussed in the section entitled “Risk Factors” herein, and the accompanying prospectus supplement or post-effective amendment, if any, and any related free writing prospectus, and under similar headings in any other documents that are incorporated by reference into this prospectus, and the accompanying prospectus supplement or post-effective amendment, if any, and any related free writing prospectus.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
You should rely only on the information contained or incorporated by reference in this prospectus or in any prospectus supplement or post-effective amendment or free-writing prospectus we may authorize to be delivered or made available to you. Neither we nor the Selling Stockholder have authorized anyone to provide you with information different from that contained or incorporated by reference in this prospectus and any free writing prospectus we have prepared. We and the Selling Stockholder take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Offers to sell, and solicitations of offers to buy, shares of our common stock are being made only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since the date of this prospectus.
Unless otherwise stated or the context requires otherwise, references to “ENDRA”, the “Company,” “we,” “us” or “our” are to ENDRA Life Sciences Inc. and its subsidiaries.
| 1 |
| Table of Contents |
Certain information set forth in this prospectus or incorporated by reference in this prospectus may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Exchange Act, that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “estimate,” “goal,” “anticipate,” “project” or other comparable terms. All statements other than statements of historical facts included in this prospectus regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding: estimates of the timing of future events and anticipated results of our development efforts, including the timing of submission for and receipt of required regulatory approvals and product launches; statements relating to future financial position and projected costs and revenue; expectations concerning our business strategy; and statements regarding our ability to find and maintain development partners.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside our control. Our actual results and financial condition may differ materially from those in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following:
· | our limited commercial experience, limited cash and history of losses; | |
· | our ability to obtain adequate financing to fund our business operations in the future; | |
· | our ability to achieve profitability; | |
· | delays and changes in regulatory requirements, policy and guidelines, including potential delays in submitting required regulatory applications or other submissions with respect to U.S. Food and Drug Administration (“FDA”) or other regulatory agency approval; | |
· | our ability to obtain CE mark certification and secure required FDA and other governmental approvals for our Thermo-Acoustic Enhanced Ultrasound (“TAEUS”) applications | |
· | our ability to develop a commercially feasible application based on our TAEUS technology; | |
· | market acceptance of our technology; | |
· | uncertainties associated with COVID-19 or other pandemics that may occur, including effects on our operations; | |
· | the effect of macroeconomic conditions on our business; | |
· | results of our human studies, which may be negative or inconclusive; | |
· | our ability to find and maintain development partners; | |
· | our reliance on third parties, collaborations, strategic alliances and licensing arrangements to complete our business strategy; | |
· | the amount and nature of competition in our industry; | |
· | our ability to protect our intellectual property; | |
· | potential changes in the healthcare industry or third-party reimbursement practices; | |
· | our ability to comply with regulation by various federal, state, local and foreign governmental agencies and to maintain necessary regulatory clearances or approvals; | |
· | our ability to maintain compliance with Nasdaq listing standards; | |
· | our dependence on our senior management team; and | |
· | the other risks and uncertainties described in the Risk Factors section of this prospectus, any prospectus supplement and our most recent Annual Form 10-K and subsequently filed Quarterly Reports on Form 10-Q, which filings are incorporated herein by reference. |
We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their entirety by the applicable cautionary statements. We further caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
| 2 |
| Table of Contents |
We were incorporated as a Delaware corporation in 2007. We are developing a next-generation enhanced ultrasound technology platform—Thermo Acoustic Enhanced Ultrasound, or TAEUS® in order to broaden patient access to the safe diagnosis and treatment of a number of significant medical conditions in circumstances where expensive X-ray computed tomography (“CT”), magnetic resonance imaging (“MRI”) technology, or other diagnostic technologies such as surgical biopsy, are unavailable or impractical.
Our TAEUS technology uses radio frequency (“RF”) pulses to stimulate tissues, using a small fraction (less than 1%) of the amount of energy that would be transmitted into the body during an MRI scan. The use of RF energy allows our TAEUS technology to penetrate deep into tissue, enabling the imaging of human anatomy at depths equivalent to those of conventional ultrasound. The RF pulses are absorbed by tissue and converted into ultrasound signals, which are detected by an external ultrasound receiver and a digital acquisition system that is part of the TAEUS system. The detected ultrasound is processed into images and other forms of data using our proprietary software and algorithms and then displayed to complement conventional gray-scale ultrasound images. The TAEUS imaging concept is illustrated below:

We believe that our TAEUS technology has the potential to add a number of new capabilities to conventional ultrasound, and other types of capital medical equipment such as interventional thermo-ablation systems, and thereby enhance the utility of those systems. Additionally, we believe that our technology can extend the use of ultrasound technology to indications and clinical situations that currently require the use of expensive CT or MRI imaging systems, where imaging is not practical using existing technology, or where other assessment tools such as surgical biopsy are required.
Our TAEUS platform is not intended to replace CT or MRI systems, both of which are versatile imaging technologies with capabilities and uses beyond the focus of our business. These systems, while versatile, are relatively expensive—a CT system can cost approximately $1 million and an MRI system can cost up to approximately $3 million. In addition, and in contrast to ultrasound systems, due to their limited number and the fact that they are usually fixed-in-place at major medical facilities, CT or MRI systems are frequently inaccessible to many patients. For example, CT or MRI systems are generally less accessible to primary care practices, rural clinics, economically developing markets, and patient bedsides.
| 3 |
| Table of Contents |
Ultrasound systems are more broadly available to patients than either CT or MRI systems. There are an estimated 1.6 million diagnostic ultrasound systems globally in use today. The global diagnostic ultrasound device market is anticipated to expand at a CAGR of 4.07% from 2022 to 2030, according to Grand View Research. Ultrasound systems are relatively inexpensive compared to CT and MRI systems, as smaller portable ultrasound systems can cost as little as approximately $5,000 and the price of new cart-based ultrasound systems can range from approximately $75,000 to $200,000. These numbers include both portable and cart-based ultrasound systems, and cover all types of diagnostic ultrasound procedures, including systems intended for cardiology, prenatal and abdominal use. We do not currently intend to address cart-based ultrasound systems focused on applications in prenatal care, nor certain portable ultrasound applications such as emergency room medicine, where we believe our TAEUS technology may not substantially impact patient care. Accordingly, we estimate the addressable market for one or more of our current or future TAEUS applications to include approximately 700,000 ultrasound systems currently in use throughout the world, in addition to other types of capital equipment.
To demonstrate the capabilities of our TAEUS platform, we have conducted various internal ex-vivo laboratory experiments and limited internal in-vivo large animal studies. In our ex-vivo and in-vivo testing, we have demonstrated that the TAEUS platform has the following capabilities and potential clinical applications:
· | Tissue Composition: Our TAEUS technology enables ultrasound to distinguish fat from lean tissue. This capability would enable the use of TAEUS-enhanced ultrasound for the early identification, staging and monitoring of NAFLD, a precursor to NASH, liver fibrosis, cirrhosis and liver cancer. | |
· | Temperature Monitoring: Our TAEUS technology enables traditional ultrasound to visualize changes in tissue temperature, in real time. This capability would enable the use of TAEUS-enhanced ultrasound to guide thermoablative therapy, which uses heat or cold to affect tissue, such as in the treatment of cardiac atrial fibrillation, or removal of cancerous liver and kidney lesions, with greater accuracy, and perform cosmetology procedures such as lipolysis of abdominal fat. | |
· | Vascular Imaging: Our TAEUS technology has the potential to enable visualization of blood vessels from any angle, using only a saline solution contrasting agent, unlike Doppler ultrasound, which requires precise viewing angles. This capability would enable the use of TAEUS-enhanced ultrasound to assist in identifying arterial plaques or malformed vessels. | |
· | Tissue Perfusion: Our TAEUS technology has the potential to image blood flow at the capillary level in a region, organ or tissue. This capability could be used to assist physicians in characterizing abnormalities in tissue perfusion symptomatic of damaged tissue, such as internal bleeding from trauma, or diseased tissue, such as certain cancers. |
The first TAEUS application we intend to commercialize is our NAFLD TAEUS application addressing liver tissue composition. Our initial target market for this application is the European Union (“EU”) and United Kingdom. In September 2019, we announced the completion and reported top-level findings of an initial healthy subject study and data collection of 50 subjects, which was included in our TAEUS liver device technical file submission for device CE mark. We received CE mark approval for our TAEUS FLIP (Fatty Liver Imaging Probe) application in March 2020. We have registered the product in each of our primary target European markets (i.e., Germany, France, and the United Kingdom).
In June 2020, we submitted a 510(k) Application to the FDA for our TAEUS Fatty Live Imaging Probe (“FLIP”) System. In February 2022, we announced that we would pursue FDA reclassification and clearance of our TAEUS FLIP System through the FDA’s “de novo” process. We subsequently voluntarily withdrew our 510(k) Application and submitted a de novo request for the TAEUS system to the FDA in the third quarter of 2023. In the fourth quarter of 2023, the FDA sent an Additional Information (“AI”) request related to our de novo application. Since we received the AI request, we have had several interactions with the FDA and have provided additional information. In order to fully respond to the FDA’s questions, we will need to compile additional clinical data, provide additional device test data, and respond to cybersecurity related questions in a new de novo submission. We have a scheduled in-person pre-submission meeting with the FDA in the second quarter of 2024. We currently anticipate completing the necessary clinical studies by the fourth quarter of 2024 and submitting the new de novo request to the FDA in the first half of 2025.
After required regulatory approvals, our TAEUS technology can be added as an accessory to existing, commercially available ultrasound systems, helping to improve clinical decision-making on the front lines of patient care, without requiring substantially new clinical workflows or large capital investments. We are also developing TAEUS for possible incorporation into new medical equipment manufactured by original equipment manufacturers (“OEMs”), such as GE Healthcare and others, to enhance the utility of those OEM systems, as described more fully in our Annual Report on Form 10-K. Based on our design work and our understanding of the medical capital equipment market, we intend to price our initial liver TAEUS system at a price point of approximately $65,000, which we believe could enable clinical purchasers to recoup their investment in less than one year by performing a relatively small number of additional procedures, initially paid out-of-pocket by patients until government and private insurance reimbursement is secured for the TAEUS liver procedures.
Corporate Information
We were incorporated in Delaware in July 2007. Our corporate headquarters is located at 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105-1570. Our website can be accessed at www.endrainc.com. The telephone number of our principal executive office is (734) 335-0468. The information contained on, or that may be obtained from, our website is not, and shall not be deemed to be, a part of this prospectus.
| 4 |
| Table of Contents |
Investing in our securities involves a high degree of risk. You should carefully consider the risk factors described in our Annual Report on Form 10-K for our most recent fiscal year (together with any material changes thereto contained in subsequent filed Quarterly Reports on Form 10-Q) and those contained in our other filings with the SEC, which are incorporated by reference in this prospectus and any accompanying prospectus supplement.
The prospectus supplement applicable to each type or series of securities we offer may contain a discussion of risks applicable to the particular types of securities that we are offering under that prospectus supplement. Prior to making a decision about investing in our securities, you should carefully consider the specific factors discussed under the caption “Risk Factors” in the applicable prospectus supplement, together with all of the other information contained in the prospectus supplement or appearing or incorporated by reference in this prospectus. These risks could materially affect our business, results of operations or financial condition and cause the value of our securities to decline. You could lose all or part of your investment.
| 5 |
| Table of Contents |
We are filing this prospectus to permit the Selling Stockholder to resell the shares of common stock covered by this prospectus. We will not receive any proceeds from the sale of our common stock by the Selling Stockholder. The Selling Stockholder will determine at what price it may sell the shares of common stock offered by this prospectus, and such sales may be made at market prices prevailing at the time of sale, at prices related to the prevailing market price, or at privately negotiated prices.
| 6 |
| Table of Contents |
In the Restricted Stock Agreement (as defined below), we agreed to include the 202,020 shares of common stock to the Selling Stockholder issued pursuant to the Restricted Stock Agreement in any registration statement that we file after the date of the Restricted Stock Agreement for our own account, to the extent not included in a previously filed shelf registration statement.
This prospectus covers the resale of such 202,020 shares of common stock held by PatentVest (or hereafter may be held by its transferees, pledgees, donees or successors). The shares of common stock are subject to a vesting schedule pursuant to the Restricted Stock Agreement and the shares may not be sold, assigned, transferred, pledged, hypothecated, disposed of or otherwise encumbered prior to becoming vested. Should the Services Agreement (as defined below) be terminated at any point prior to the full vesting of the shares of common stock issued thereunder, all shares of common stock that have not vested or that do not then vest pursuant to the terms of the Restricted Stock Agreement shall cease to be outstanding and shall automatically be cancelled and PatentVest shall not have any rights thereunder or as a holder thereof.
The following table sets forth certain information regarding PatentVest and the shares of common stock beneficially owned by it, based on information available to us as of February 13, 2023. PatentVest may offer the shares under this prospectus from time to time and may elect to sell some, all or none of its shares. As a result, we cannot estimate the number of shares of common stock that PatentVest will beneficially own after the termination of sales under this prospectus. However, for the purpose of the table below, we have assumed that, after the completion of the offering, none of the shares covered by this prospectus will be held by PatentVest. For further information see the section of this prospectus entitled “Plan of Distribution” beginning on page 9.
The shares of common stock are owned by PatentVest, Inc., which is a wholly-owned subsidiary of MDB Capital Holdings, LLC (“MDB”). Christopher Marlett, Chief Executive Officer and Chairman of MDB, may be deemed to have voting and investment control over the shares of common stock owned by PatentVest, Inc. The address of each of Mr. Marlett and the Selling Stockholder is 14135 Midway Road, Suite G-150, Addison, TX 75001. Additionally, Anthony DiGiandomenico is the Chief of Transactions and a director of MDB and has been a member of our board of directors since 2013.
Selling Stockholder # of Shares Beneficially Owned Before Offering # of Shares Offered % of Shares Beneficially Owned Before Offering(1) # of Shares Beneficially Owned After Offering % of Shares Beneficially Owned After Offering PatentVest, Inc. 202,020 202,020 1.8 % -- % -- %
(1) | Based on 11,035,659 shares outstanding as of February 13, 2024. |
| 7 |
| Table of Contents |
We are registering the offer and sale of 202,020 shares of common stock previously issued to PatentVest to permit the resale of these shares of common stock by PatentVest from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the Selling Stockholders
The Selling Stockholder may sell all or a portion of the shares of common stock beneficially owned by it and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the Selling Stockholder will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. The Selling Stockholder will act independently of us in making decisions with respect to the timing, manner and size of each sale. These sales may involve cross trades or block transactions. These sales may be effected in transactions:
· | on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; | |
· | in the over-the-counter market; | |
· | in transactions otherwise than on these exchanges or systems or in the over-the-counter market; | |
· | through the writing of options, whether such options are listed on an options exchange or otherwise; | |
· | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
· | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
· | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
· | an exchange distribution in accordance with the rules of the applicable exchange; | |
· | privately negotiated transactions; | |
· | short sales; | |
· | through the distribution of the common stock by the Selling Stockholder to its partners, members or stockholders; | |
· | through one or more underwritten offerings on a firm commitment or best efforts basis; | |
· | broker-dealers may agree with the Selling Stockholder to sell a specified number of such shares at a stipulated price per share; | |
· | a combination of any such methods of sale; and | |
· | any other method permitted pursuant to applicable law. |
| 8 |
| Table of Contents |
The Selling Stockholder may also sell shares pursuant to Rule 144 under the Securities Act, or any other exemption from registration, if available, rather than under this prospectus.
If the Selling Stockholder effects such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the Selling Stockholder or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common stock or otherwise, the Selling Stockholder may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The Selling Stockholder may also sell shares of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The Selling Stockholder may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares.
The Selling Stockholder may pledge or grant a security interest in some or all of the shares of common stock owned by it and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
The Selling Stockholder and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the Selling Stockholder and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers. The Selling Stockholder may indemnify any broker-dealer that participates in transactions involving the sale of the shares of common stock against certain liabilities, including liabilities arising under the Securities Act.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that the Selling Stockholder will sell any or all of the shares of common stock registered pursuant to the registration statement, of which this prospectus forms a part.
The Selling Stockholder and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the Selling Stockholder and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We will pay all expenses of the registration of the shares of common stock pursuant to the Restricted Stock Agreement, including, without limitation, SEC filing fees and expenses of compliance with state securities or “Blue Sky” laws; provided, however, that the Selling Stockholder will pay all underwriting discounts and selling commissions, if any. We will indemnify the Selling Stockholder against liabilities, including some liabilities under the Securities Act, in accordance with the Restricted Stock Agreement, or the Selling Stockholder will be entitled to contribution. We may be indemnified by the Selling Stockholder against civil liabilities, including liabilities under the Securities Act, that may arise from any written information furnished to us by the Selling Stockholder specifically for use in this prospectus, in accordance with the Restricted Stock Agreement, or we may be entitled to contribution.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
The 202,020 shares of common stock previously issued to PatentVest will be available for sale in the public market following the vesting of such shares pursuant to the terms of the Restricted Stock Agreement. Until such shares of common stock vest, the shares may not be sold, assigned, transferred, pledged, hypothecated, disposed of or otherwise encumbered.
| 9 |
| Table of Contents |
The following summary description of our common stock is based on the provisions of our Fourth Amended and Restated Certificate of Incorporation, as amended, our Amended and Restated Bylaws, and the applicable provisions of Delaware law. This description may not contain all of the information that is important to you and is subject to, and is qualified in its entirety by reference to, our charter, our bylaws and the applicable provisions of Delaware law. For information on how to obtain copies of our certificate of Incorporation and Bylaws, see “Where You Can Find More Information.”
Authorized Capital
We currently have authority to issue 80,000,000 shares of our common stock, par value of $0.0001per share. As of February 13, 2024, 11,035,659 shares of our common stock were issued and outstanding, held of record by 25 stockholders. Our authorized but unissued shares of common stock are available for issuance without further action by our stockholders, unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded.
Voting Rights
Each holder of our common stock is entitled to one vote for each such share outstanding in the holder’s name. No holder of common stock is entitled to cumulate votes in voting for directors.
Dividend and Liquidation Rights
The holders of outstanding shares of our common stock are entitled to such dividends as may be declared by our board of directors out of funds legally available for such purpose. The shares of our common stock are neither redeemable nor convertible. Holders of our common stock have no preemptive or subscription rights to purchase any of our securities. In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to receive pro rata our assets, which are legally available for distribution, after payments of all debts and other liabilities. All of the outstanding shares of our common stock are fully paid and non-assessable.
We have never paid any cash dividends on our common stock.
Registration Rights
Under the terms of the Restricted Stock Agreement, dated as of November 30, 2023 (the “Restricted Stock Agreement”), 2020
Pursuant to the Restricted Stock Agreement, the Company agreed to include the Restricted Stock in any registration statement subsequently filed by the Company, to the extent a shelf registration statement with respect to the Restricted Stock had not previously been filed.
Our shares of common stock are listed on the Nasdaq Capital Market under the symbol “NDRA.”
| 10 |
| Table of Contents |
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
The provisions of Delaware law, the Certificate of Incorporation and the Bylaws could have the effect of delaying, deferring or discouraging another person from acquiring control of us. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
Delaware Law
We are subject to Section 203 of the Delaware General Corporation Law (the “DGCL”), an anti-takeover law. In general, Section 203 prohibits a Delaware corporation from engaging in any business combination (as defined below) with any interested stockholder (as defined below) for a period of three years following the date that the stockholder became an interested stockholder, unless:
· | prior to that date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; | |
· | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares of voting stock outstanding (but not the voting stock owned by the interested stockholder) those shares owned by persons who are directors and officers and by excluding employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or | |
· | on or subsequent to the time the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines “business combination” to include the following:
· | any merger or consolidation involving the corporation and the interested stockholder; | |
· | any sale, lease, exchange, mortgage, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; | |
· | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; | |
· | subject to limited exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; or | |
· | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
| 11 |
| Table of Contents |
Section 203 generally defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation, or who beneficially owns 15% or more of the outstanding voting stock of the corporation at any time within a three-year period immediately prior to the date of determining whether such person is an interested stockholder, and any entity or person affiliated with or controlling or controlled by any of these entities or persons.
Certificate of Incorporation and Bylaw Provisions
The Certificate of Incorporation and the Bylaws include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of us. Certain of these provisions are summarized in the following paragraphs.
Effects of authorized but unissued common stock. One of the effects of the existence of authorized but unissued common stock may be to enable our board of directors to make more difficult or to discourage an attempt to obtain control of our Company by means of a merger, tender offer, proxy contest or otherwise, and thereby to protect the continuity of management. If, in the due exercise of its fiduciary obligations, the board of directors were to determine that a takeover proposal was not in our best interest, such shares could be issued by the board of directors without stockholder approval in one or more transactions that might prevent or render more difficult or costly the completion of the takeover transaction by diluting the voting or other rights of the proposed acquirer or insurgent stockholder group, by putting a substantial voting block in institutional or other hands that might undertake to support the position of the incumbent board of directors, by effecting an acquisition that might complicate or preclude the takeover, or otherwise.
Cumulative Voting. Our Certificate of Incorporation does not provide for cumulative voting in the election of directors, which would allow holders of less than a majority of the stock to elect some directors.
Vacancies. Our Certificate of Incorporation provides that all vacancies may be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum.
Special Meeting of Stockholders. A special meeting of stockholders may only be called by the Chairman of the board of directors, the President, the Chief Executive Officer, or the board of directors at any time and for any purpose or purposes as shall be stated in the notice of the meeting, or by request of the holders of record of at least 20% of the outstanding shares of common stock. This provision could prevent stockholders from calling a special meeting because, unless certain significant stockholders were to join with them, they might not obtain the percentage necessary to request the meeting. Therefore, stockholders holding less than 20% of the issued and outstanding common stock, without the assistance of management, may be unable to propose a vote on any transaction that would delay, defer or prevent a change of control, even if the transaction were in the best interests of our stockholders.
| 12 |
| Table of Contents |
The validity and legality of the securities offered hereby and certain other legal matters will be passed upon for the Company by K&L Gates LLP, Charlotte, North Carolina.
RBSM LLP, independent registered public accounting firm, has audited our financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2022, which is incorporated by reference into this prospectus and elsewhere in the registration statement of which this prospectus is a part. Our financial statements are incorporated by reference in reliance on RBSM LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission (the “SEC”). You can inspect and copy these reports, proxy statements and other information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding issuers, such as ENDRA Life Sciences Inc. (www.sec.gov). Our web site is located at www.endrainc.com. The information contained on, or that may be obtained from, our website is not, and shall not be deemed to be, a part of this prospectus.
We will provide, upon written or oral request, without charge to you, including any beneficial owner to whom this prospectus is delivered, a copy of any or all of the documents incorporated herein by reference other than the exhibits to those documents, unless the exhibits are specifically incorporated by reference into the information that this prospectus incorporates. You should direct a request for copies to ENDRA Life Sciences Inc., 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105; Telephone: (734) 335-0468.
The SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus.
We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC:
· | Annual Report on Form 10-K for the fiscal year ended December 31, 2023 filed with the SEC on March 28, 2024; and | |
· | The description of the Company’s common stock contained in the Company’s Registration Statement on Form 8-A (File No. 001-37969) initially filed with the SEC on December 16, 2016 pursuant to Section 12(g) of the Exchange Act, including any amendment or reports filed for the purpose of updating such description. |
We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made on or after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, until we file a post-effective amendment that indicates the termination of the offering of the Securities made by this prospectus and will become a part of this prospectus from the date that such documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference, including exhibits to these documents. You should direct any requests for documents to ENDRA Life Sciences Inc., 3600 Green Court, Suite 350, Ann Arbor, Michigan 48105; Telephone: (734) 335-0468. Copies of the above reports may also be accessed from our web site at www.endrainc.com. We have authorized no one to provide you with any information that differs from that contained in this prospectus. Accordingly, you should not rely on any information that is not contained in this prospectus. You should not assume that the information in this prospectus is accurate as of any date other than the date of the front cover of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this prospectus modifies or supersedes that statement. Any statement that is modified or superseded will not constitute a part of this prospectus, except as modified or superseded.
| 13 |
| Table of Contents |

ENDRA Life Sciences Inc.
202,020 Shares of Common Stock
PROSPECTUS
, 2024
| 14 |
| Table of Contents |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 14. Other Expenses of Issuance and Distribution.
The following is an estimate (except for registration fees, which are actual)itemized statement of expenses of the approximate amount of the fees and expenses payable by usCompany in connection with the issuance and distributiondelivery of the Securities. The Selling Stockholderssecurities being registered hereby, other than underwriting discounts and commissions.
SEC registration fee Trustee Fees and Expenses * Accounting Fees and Expenses Legal Fees and Expenses Miscellaneous Total $ 2,985 $ 35,000 $ 40,000 $ 2,015 $ 80,000
* These fees will not be responsible for anydependent on the types of the expensessecurities offered and number of offerings and, therefore, cannot be estimated at this offering.
Item 15. Indemnification of Directors and Officers.
The following summary is qualified in its entirety by reference to the complete text of any statutes referred to below and the Fourth Amended and Restated Certificate of Incorporation of ENDRA Life Sciences Inc., a Delaware corporation.
Section 145 of the General Corporation Law of the State of Delaware (the “DGCL”) permits a Delaware corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful.
In the case of an action by or in the right of the corporation, Section 145 of the DGCL permits a Delaware corporation to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses that the Court of Chancery or such other court shall deem proper.
| 15 |
| Table of Contents |
Section 145 of the DGCL also permits a Delaware corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s status as such, whether or not the corporation would have the power to indemnify such person against such liability under Section 145 of the DGCL.
Article NINTH of our Fourth Amended and Restated Certificate of Incorporation states that our directors shall not be personally liable to us or to our stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability. Under Section 102(b)(7) of the DGCL, the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duty can be limited or eliminated except (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders; (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; (iii) under Section 174 of the DGCL (relating to unlawful payment of dividend or unlawful stock purchase or redemption); or (iv) for any transaction from which the director derived an improper personal benefit.
Article EIGHTH of our Fourth Amended and Restated Certificate of Incorporation provides that we shall indemnify (and advance expenses to) our officers and directors to the full extent permitted by the DGCL.
All of the Company’s directors and officers are covered by insurance policies maintained by the Company against specified liabilities for actions taken in their capacities as such, including liabilities under the Securities Act. Such insurance also insures us against losses which we may incur in indemnifying our officers and directors.
As permitted by the DGCL, we have entered into indemnification agreements with each of our directors and executive officers that require us to indemnify them against various actions including, but not limited to, third-party actions where such director or executive officer, by reason of his or her corporate status, is a party or is threatened to be made a party to an action, or by reason of anything done or not done by such director in any such capacity. We indemnify directors and executive officers against all costs, judgments, penalties, fines, liabilities, amounts paid in settlement by or on behalf of such directors or executive officers and for any expenses actually and reasonably incurred by such directors or executive officers in connection with such action, if such directors or executive officers acted in good faith and in a manner they reasonably believed to be in or not opposed to our best interests, and with respect to any criminal proceeding, had no reasonable cause to believe their conduct was unlawful. We also intend to advance to our directors and executive officers expenses (including attorney’s fees) incurred by or on behalf of such directors and executive officers in advance of the final disposition of any action after our receipt of a statement or statements from directors or executive officers requesting such payment or payments from time to time, provided that such statement or statements are preceded or accompanied by a written undertaking, by or on behalf of such directors or executive officers, to repay such amount if it shall ultimately be determined that they are not entitled to be indemnified against such expenses by us.
The indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification or advancement of expenses, including, among others, provisions about submitting a written request to us that includes such documentation and information as is reasonably available to the director or executive officer and is reasonably necessary to determine entitlement to indemnification and provisions.
| 16 |
| Table of Contents |
Item 16. Exhibits and Financial Statement Schedules.
| Incorporated by Reference | |||||||
| Exhibit Number | Exhibit Description | Filed Herewith | Form | Exhibit | Filing Date | Registration/File No. | |
| Specimen Certificate representing shares of common stock of the Registrant | S-1 | 4.1 | 11/21/16 | 333-214724 | |||
| Certificate of Designations of Series A Convertible Preferred Stock | 8-K | 4.1 | 12/11/19 | 001-37969 | |||
| Certificate of Designations of Series B Convertible Preferred Stock | 8-K | 4.1 | 12/26/19 | 001-37969 | |||
| Form of Series A Warrant | 8-K | 4.2 | 12/11/19 | 001-37969 | |||
| Form of Series B Warrant | 8-K | 4.2 | 12/26/19 | 001-37969 | |||
| Form of Securities Purchase Agreement dated December 5, 2019 | 8-K | 10.1 | 12/11/19 | 001-37969 | |||
| Form of Securities Purchase Agreement dated December 19, 2019 | 8-K | 10.1 | 12/26/19 | 001-37969 | |||
| Form of Registration Rights Agreement | 8-K | 10.2 | 12/11/19 | 001-37969 | |||
| Opinion of K&L Gates LLP | X | ||||||
| Consent of RBSM LLP, Independent Registered Public Accounting Firm | X | ||||||
| 23.2 | Consent of K&L Gates LLP (contained in Exhibit 5.1) | X | |||||
| 24.1 | Power of Attorney (included on signature page) | X | |||||
Incorporated by Reference Exhibit Number Exhibit Description Filed Herewith Form Exhibit Filing Date Registration/File No. 1.1 Form of Underwriting Agreement* Fourth Amended and Restated Certificate of Incorporation of the Company 8-K 3.2 05/12/2017 001-37969 Certificate of Amendment to the Fourth Amended and Restated Certificate of Incorporation 8-K 3.1 06/18/2020 001-37969 S-1/A 3.4 12/06/2016 333-214724 Specimen Certificate representing shares of common stock of the Company S-1 4.1 11/21/2016 333-214724 4.2 Form of certificate representing preferred stock* 4.3 Form of certificate of designations for preferred stock* 4.5 Form of Senior Debt Security* 4.6 Form of Subordinated Debt Security* 4.7 Form of Warrant* 4.8 Form of Unit Certificate* x Restricted Stock Agreement, dated November 30, 2023, by and between the Company and PatentVest, Inc. x Opinion of K&L Gates LLP as to legality of securities being registered† Consent of RBSM LLP, Independent Registered Public Accounting Firm x 25.1 Form T-1 Statement of Eligibility of Trustee under the Trust Indenture Act of 1939, as amended**
* To be filed, if necessary, with a Current Report on Form 8-K or a Post-Effective Amendment to the registration statement.
** To be filed, if necessary, pursuant to Section 305(b)(2) of the Trust Indenture Act of 1939.
† Previously filed
| 17 |
| Table of Contents |
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant, pursuant to the provisions described under Item 15 or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification by it is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities other(other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding,proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
(1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
(i) | to include any prospectus required by Section 10(a)(3) of the Securities Act; | |
(ii) | to reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of a prospectus filed with the SEC pursuant to Rule 424(b) under the Securities Act if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement); and | |
(iii) | to include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in this registration statement; |
provided
, however, that(2) | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(3) | To remove from registration, by means of a post-effective amendment, any of the securities being registered which remain unsold at the termination of the offering. |
(4) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser if the registrant is relying on Rule 430B: (A) each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and (B) each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
| 18 |
| Table of Contents |
(5) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use |
(6) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: (i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; (ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; (iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and (iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
(7) | That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in this registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(8) | That, for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective. |
(9) | That, for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(10) | To file an application for the purpose of determining the eligibility of the trustee to act under subsection (a) of Section 310 of the Trust Indenture Act in accordance with the rules and regulations prescribed by the Commission under Section 305(b)(2) of the Act. |
| 19 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ann Arbor, State of Michigan, on this 10th28th day of January 2020.
ENDRA Life Sciences Inc. | ||
/s/ Francois Michelon | ||
Francois Michelon Chief Executive Officer and Director (Principal Executive Officer) |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Dated: | /s/ Francois Michelon | |
Francois Michelon Chief Executive Officer and Director | ||
Dated: | /s/ | |
Irina Pestrikova Senior Director, Finance (Principal Financial and Accounting Officer) | ||
Dated: | *** | |
Louis J. Basenese Director | ||
Dated: | *** | |
Anthony DiGiandomenico Director | ||
Dated: March 28, 2024 | *** | |
Michael Harsh Director | ||
Dated: March 28, 2024 | *** | |
Alexander Tokman Director |
*** By: | /s/ | |
Francois Michelon | ||
Attorney-In-Fact |
| 20 |